UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended: December 31, 20222023
or
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-38355
| Nemaura Medical Inc. |
| (Exact name of registrant as specified in its charter) |
| nevada | 46-5027260 | |||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||
57 West 57th Street Manhattan, NY 10019 | ||||
| (Address of Principal Executive Offices) (Zip Code) | ||||
| 646-416-8000 | ||||
| (Registrant’s Telephone Number, Including Area Code) | ||||
| N/A | ||||
| (Former name, former address and former fiscal year, if changed since last report) | ||||
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | |
| Common Stock | NMRD |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer", "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | |
Non-accelerated Filer ☒
| Smaller reporting company☒
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No☒
The number of shares of common stock, par value $0.001 per share, outstanding as of February 23, 202312, 2024, was .
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). All statements, other than statements of historical fact, included in this Quarterly Report on Form 10-Q regarding development of our strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management are forward-looking statements. Forward-looking statements may include, but are not limited to, statements about:
The words "believe," "anticipate," "design," "estimate," "plan," "predict," "seek," "expect," "intend," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements reflect our current views with respect to future events, are based on assumptions and are subject to risks and uncertainties. We cannot guarantee that we actually will achieve the plans, intentions or expectations expressed in our forward-looking statements and you should not place undue reliance on these statements. There are a number of important factors that could cause our actual results to differ materially from those indicated or implied by forward-looking statements. These factors and the other cautionary statements made in this Quarterly Report on Form 10-Q should be read as being applicable to all related forward-looking statements whenever they appear herein. Except as required by law, we do not assume any obligation to update any forward-looking statement. We disclaim any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
NEMAURA MEDICAL INC.
TABLE OF CONTENTS
| 2 |
PART I – FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
| NEMAURA MEDICAL INC. |
| Condensed Consolidated Balance Sheets |
December 31, (Unaudited) | March 31, | |||||||||||||||
December 31, 2022 (Unaudited) | March 31, 2022
| 2023 | 2023 | |||||||||||||
| ASSETS | ||||||||||||||||
| Current assets: | ||||||||||||||||
| Cash | $ | 7,340,840 | $ | 17,749,233 | ||||||||||||
| Cash and cash equivalents | $ | 137,416 | $ | 10,105,135 | ||||||||||||
| Inventory | 3,671,533 | 1,754,852 | ||||||||||||||
| Prepaid expenses and other receivables | 1,217,237 | 750,167 | 169,174 | 357,934 | ||||||||||||
| Accounts receivable - related party | 25,320 | 101,297 | ||||||||||||||
| Inventory | 2,352,407 | 1,487,771 | ||||||||||||||
| VAT receivable | 247,788 | 409,648 | ||||||||||||||
| Deposit on foreign exchange contract | 146,434 | 909,666 | ||||||||||||||
| Total current assets | 10,935,804 | 20,088,468 | 4,372,345 | 13,537,235 | ||||||||||||
| Property and equipment, net of accumulated depreciation | 581,903 | 532,508 | 558,697 | 641,906 | ||||||||||||
| Intangible assets, net of accumulated amortization | 1,443,991 | 1,480,980 | 238,033 | 384,092 | ||||||||||||
| Total other assets | 2,025,894 | 2,013,488 | ||||||||||||||
| Total assets | $ | 12,961,698 | $ | 22,101,956 | $ | 5,169,075 | $ | 14,563,233 | ||||||||
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | ||||||||||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||||||
| Current liabilities: | ||||||||||||||||
| Accounts payable | $ | 171,207 | $ | 136,310 | $ | 352,483 | $ | 326,641 | ||||||||
| Other liabilities and accrued expenses | 390,858 | 558,426 | 281,055 | 130,678 | ||||||||||||
| Foreign currency contract | 1,075,692 | 440,196 | ||||||||||||||
| Notes payable, current portion | 11,512,711 | 19,188,724 | 19,643,038 | 16,942,500 | ||||||||||||
| Deferred revenue | 69,681 | 259,256 | ||||||||||||||
| Payable to related parties | 800,403 | 920,780 | ||||||||||||||
| Deferred revenue, current portion | 1,184,412 | 123,640 | ||||||||||||||
| Foreign exchange contract derivative liability | 242,295 | 731,730 | ||||||||||||||
| Warrant liability | 492,000 | 3,092,000 | ||||||||||||||
| Total current liabilities | 13,220,149 | 20,582,912 | 22,995,686 | 22,267,969 | ||||||||||||
| Notes payable, net of current portion | 8,557,548 | — | ||||||||||||||
| Deferred revenue, net of current portion | 1,042,710 | 1,052,960 | ||||||||||||||
| Notes payable, non-current portion | — | 3,087,651 | ||||||||||||||
| Deferred revenue, non-current portion | — | 1,021,811 | ||||||||||||||
| Total liabilities | 22,820,407 | 21,635,872 | 22,995,686 | 26,377,431 | ||||||||||||
| Commitments and contingencies | — | — | ||||||||||||||
| Stockholders’ (deficit) equity: | ||||||||||||||||
| Stockholders’ deficit: | ||||||||||||||||
| Common stock, $ par value, shares authorized and and shares issued and outstanding at December 31, 2022 and March 31, 2022 | 24,103 | 24,103 | ||||||||||||||
| Common stock, $ par value, shares authorized and shares issued and outstanding at December 31, 2023 and March 31, 2023 | 28,899 | 28,899 | ||||||||||||||
| Additional paid-in capital | 38,296,198 | 38,295,775 | 40,991,377 | 40,991,377 | ||||||||||||
| Accumulated deficit | (47,192,364 | ) | (37,731,476 | ) | (57,843,297 | ) | (51,875,211 | ) | ||||||||
| Accumulated other comprehensive loss | (986,646 | ) | (122,318 | ) | (1,003,590 | ) | (959,263 | ) | ||||||||
| Total stockholders’ (deficit) equity | (9,858,709 | ) | 466,084 | |||||||||||||
| Total liabilities and stockholders’ (deficit) equity | $ | 12,961,698 | $ | 22,101,956 | ||||||||||||
| Total stockholders’ deficit | (17,826,611 | ) | (11,814,198 | ) | ||||||||||||
| Total liabilities and stockholders’ deficit | $ | 5,169,075 | $ | 14,563,233 | ||||||||||||
See notes to the unaudited condensed consolidated financial statements.
|
| Condensed Consolidated Statements of Operations and Comprehensive Loss |
(Unaudited)
|
Three Months Ended December 31, | Nine Months Ended December 31, | Three Months Ended December 31, | Nine Months Ended December 2023, | |||||||||||||||||||||||||||||
| 2022 | 2021 | 2022 | 2021 | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||
| Sales | $ | 3,017 | $ | 183,628 | $ | 77,044 | $ | 183,628 | $ | — | $ | 3,017 | $ | — | $ | 77,044 | ||||||||||||||||
| Cost of Sales | (2,971 | ) | (172,393 | ) | (75,327 | ) | (172,393 | ) | — | (2,971 | ) | — | (75,327 | ) | ||||||||||||||||||
| Gross Profit | 46 | 11,235 | 1,717 | 11,235 | — | 46 | — | 1,717 | ||||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||||||
| Research and development | 393,747 | 412,341 | 980,862 | 987,711 | 291,104 | 393,747 | 1,332,664 | 980,862 | ||||||||||||||||||||||||
| General and administrative | 239,628 | 1,391,278 | 4,329,306 | 4,151,380 | 1,250,149 | 1,230,160 | 4,317,358 | 1,509,095 | ||||||||||||||||||||||||
| Total operating expenses | 633,375 | 1,803,619 | 5,310,168 | 5,139,091 | 1,541,253 | 1,623,907 | 5,650,022 | 2,489,957 | ||||||||||||||||||||||||
| Loss from operations | (633,329 | ) | (1,792,384 | ) | (5,308,451 | ) | (5,127,856 | ) | (1,541,253 | ) | (1,623,907 | ) | (5,650,022 | ) | (2,488,240 | ) | ||||||||||||||||
| Other income (expense) | ||||||||||||||||||||||||||||||||
| Interest expense | (1,082,949 | ) | (1,639,184 | ) | (4,152,437 | ) | (5,141,701 | ) | (1,925,678 | ) | (1,082,949 | ) | (3,407,499 | ) | (4,152,437 | ) | ||||||||||||||||
| Change in fair value of warrant liability | 1,003,000 | — | 2,600,000 | — | ||||||||||||||||||||||||||||
| Change in fair value of foreign exchange contract derivative liability | 302,453 | 990,532 | 489,435 | (2,820,211 | ) | |||||||||||||||||||||||||||
| Net loss | (1,716,278 | ) | (3,431,568 | ) | (9,460,888 | ) | (10,269,557 | ) | (2,161,478 | ) | (1,716,278 | ) | (5,968,086 | ) | (9,460,888 | ) | ||||||||||||||||
| Other comprehensive loss: | ||||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | 556,080 | (25,065 | ) | (864,328 | ) | (142,922 | ) | 204,828 | (556,080 | ) | (44,327 | ) | (864,328 | ) | ||||||||||||||||||
| Comprehensive loss | $ | (1,160,198 | ) | $ | (3,456,633 | ) | $ | (10,325,216 | ) | $ | (10,412,479 | ) | $ | (1,956,650 | ) | $ | (4,831,171 | ) | $ | (6,012,413 | ) | $ | (10,325,216 | ) | ||||||||
| Net loss per share, basic and diluted | $ | (0.07 | ) | $ | (0.15 | ) | $ | (0.39 | ) | $ | (0.44 | ) | $ | ) | $ | ) | $ | ) | $ | ) | ||||||||||||
| Weighted average number of shares outstanding, basic and diluted | 24,103,196 | 23,313,629 | 24,102,976 | 23,244,345 | ||||||||||||||||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
NEMAURA MEDICAL INC.
Condensed Consolidated Statements of Changes in Stockholders’ (Deficit) EquityDeficit
Three and Nine Months Ended December 31, 20222023 and 20212022 (Unaudited)
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount ($) | Additional Paid-in Capital ($) | Accumulated Deficit ($) | Accumulated Other Comprehensive (Loss) Income ($) | Total Stockholders’ Equity (Deficit) ($) | |||||||||||||||||||
| Balance at September 30, 2022 | 24,102,866 | 24,103 | 38,295,775 | (45,476,086 | ) | (1,542,726 | ) | (8,698,934 | ) | |||||||||||||||
| Shares issued under ATM facility | 330 | — | 423 | — | — | 423 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | 556,080 | 556,080 | ||||||||||||||||||
| Net loss | — | — | — | (1,716,278 | ) | — | (1,716,278 | ) | ||||||||||||||||
| Balance at December 31, 2022 | 24,103,196 | 24,103 | 38,296,198 | (47,192,364 | ) | (986,646 | ) | (9,858,709 | ) | |||||||||||||||
| Balance at September 30, 2021 | 23,308,049 | 23,308 | 35,007,626 | (30,682,660 | ) | 17,710 | 4,365,984 | |||||||||||||||||
| Shares issued under ATM facility | 22,524 | 23 | 114,386 | — | — | 114,409 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (25,065 | ) | (25,065 | ) | ||||||||||||||||
| Net loss | — | — | — | (3,431,568 | ) | — | (3,431,568 | ) | ||||||||||||||||
| Balance at December 31, 2021 | 23,330,573 | 23,331 | 35,122,012 | (34,114,228 | ) | (7,355 | ) | 1,023,760 | ||||||||||||||||
| Common Stock | Additional Paid-in | Accumulated | Accumulated Other Comprehensive | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | (Loss) Income | (Deficit) | |||||||||||||||||||
| Balance at September 30, 2023 | 28,899,402 | $ | 28,899 | $ | 40,991,377 | $ | (55,681,819 | ) | $ | (1,208,418 | ) | $ | (15,869,961 | ) | ||||||||||
| Foreign currency translation adjustment | — | — | — | — | 204,828 | 204,828 | ||||||||||||||||||
| Net loss | — | — | — | (2,161,478 | ) | — | (2,161,478 | ) | ||||||||||||||||
| Balance at December 31, 2023 | 28,899,402 | $ | 28,899 | $ | 40,991,377 | $ | (57,843,297 | ) | $ | (1,003,590 | ) | $ | (17,826,611 | ) | ||||||||||
| Balance at March 31, 2023 | 28,899,402 | $ | 28,899 | $ | 40,991,377 | $ | (51,875,211 | ) | $ | (959,263 | ) | $ | (11,814,198 | ) | ||||||||||
| Foreign currency translation adjustment | — | — | — | — | (44,327 | ) | (44,327 | ) | ||||||||||||||||
| Net loss | — | — | — | (5,968,086 | ) | — | (5,968,086 | ) | ||||||||||||||||
| Balance at December 31, 2023 | 28,899,402 | $ | 28,899 | $ | 40,991,377 | $ | (57,843,297 | ) | $ | (1,003,590 | ) | $ | (17,826,611 | ) | ||||||||||
| Common Stock | Additional Paid-in | Accumulated | Accumulated Other Comprehensive | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | (Loss) Income | (Deficit) | |||||||||||||||||||
| Balance at September 30, 2022 | 24,102,866 | $ | 24,103 | $ | 38,295,775 | $ | (45,476,086 | ) | $ | (1,542,726 | ) | $ | (8,698,934 | ) | ||||||||||
| Shares issued under ATM facility | 330 | 423 | 423 | |||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | 556,080 | 556,080 | ||||||||||||||||||
| Net loss | — | — | — | (1,716,278 | ) | — | (1,716,278 | ) | ||||||||||||||||
| Balance at December 31, 2022 | 24,103,196 | $ | 24,103 | $ | 38,296,198 | $ | (47,192,364 | ) | $ | (986,646 | ) | $ | (9,858,709 | ) | ||||||||||
| Balance at March 31, 2022 | 24,102,866 | $ | 24,103 | $ | 38,295,775 | $ | (37,731,476 | ) | $ | (122,318 | ) | $ | 466,084 | |||||||||||
| Shares issued under ATM facility | 330 | 423 | 423 | |||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (864,328 | ) | (864,328 | ) | ||||||||||||||||
| Net loss | — | — | — | (9,460,888 | ) | — | (9,460,888 | ) | ||||||||||||||||
| Balance at December 31, 2022 | 24,103,196 | $ | 24,103 | $ | 38,296,198 | $ | (47,192,364 | ) | $ | (986,646 | ) | $ | (9,858,709 | ) | ||||||||||
See notes to the unaudited condensed consolidated financial statements.
NEMAURA MEDICAL INC.
Condensed Consolidated Statements of Changes in Stockholders’ Equity (deficit)
Nine Months Ended December 31, 2022 and 2021 (Unaudited)
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount ($) | Additional Paid-in Capital ($) | Accumulated Deficit ($) | Accumulated Other Comprehensive (Loss) Income ($) | Total Stockholders’ Equity (Deficit) ($) | |||||||||||||||||||
| Balance at March 31, 2022 | 24,102,866 | 24,103 | 38,295,775 | (37,731,476 | ) | (122,318 | ) | 466,084 | ||||||||||||||||
| Shares issued under ATM facility | 330 | — | 423 | — | — | 423 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (864,328 | ) | (864,328 | ) | ||||||||||||||||
| Net loss | — | — | — | (9,460,888 | ) | — | (9,460,888 | ) | ||||||||||||||||
| Balance at December 31, 2022 | 24,103,196 | 24,103 | 38,296,198 | (47,192,364 | ) | (986,646 | ) | (9,858,709 | ) | |||||||||||||||
| Balance at March 31, 2021 | 22,941,157 | 22,941 | 32,044,335 | (23,844,671 | ) | 135,567 | 8,358,172 | |||||||||||||||||
| Shares issued under ATM facility | 22,524 | 23 | 114,386 | — | — | 114,409 | ||||||||||||||||||
| Exercise of warrants | 366,892 | 367 | 2,963,291 | — | — | 2,963,658 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (142,922 | ) | (142,922 | ) | ||||||||||||||||
| Net loss | — | — | — | (10,269,557 | ) | — | (10,269,557 | ) | ||||||||||||||||
| Balance at December 31, 2021 | 23,330,573 | 23,331 | 35,122,012 | (34,114,228 | ) | (7,355 | ) | 1,023,760 | ||||||||||||||||
|
| NEMAURA MEDICAL INC. |
| Condensed Consolidated Statements of Cash Flows |
| (Unaudited) |
| Nine Months Ended December 31, | ||||||||||||||||
| 2022 | 2021 | Nine Months Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net loss | $ | (9,460,888 | ) | $ | (10,269,557 | ) | $ | (5,968,086 | ) | $ | (9,460,888 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
| Depreciation and amortization | 268,595 | 139,751 | 309,684 | 268,595 | ||||||||||||
| Inventory write down | 104,449 | — | ||||||||||||||
| Amortization of debt discount | 4,152,437 | 5,141,701 | 1,803,126 | 4,152,437 | ||||||||||||
| Change in fair value of foreign currency contract | 635,494 | 199,522 | ||||||||||||||
| Changes in assets and liabilities: | ||||||||||||||||
| Prepaid expenses and other receivables | (467,070 | ) | 797,155 | |||||||||||||
| Addition of PIK monitoring fee to note payable | 488,022 | — | ||||||||||||||
| Change in fair value of foreign exchange contract derivative liability. | (489,435 | ) | 635,494 | |||||||||||||
| Change in fair value of warrant liability | (2,600,000 | ) | — | |||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||
| Prepaid expenses and other receivables, VAT receivable and deposit on foreign exchange deposit | 1,113,853 | (467,070 | ) | |||||||||||||
| Inventory | (864,636 | ) | (533,656 | ) | (2,021,130 | ) | (864,636 | ) | ||||||||
| Accounts payable | 34,897 | (77,075 | ) | 25,842 | 34,897 | |||||||||||
| Due to (from) related parties | 75,977 | (301,387 | ) | |||||||||||||
| Other liabilities and accrued expenses | (167,568 | ) | 264,786 | |||||||||||||
| Receivable/payable to related parties | (120,378 | ) | 75,977 | |||||||||||||
| Accrued expense and other liabilities | 150,377 | (167,568 | ) | |||||||||||||
| Deferred revenue | (297,419 | ) | 285,266 | — | (297,419 | ) | ||||||||||
| Net cash used in operating activities | (6,090,181 | ) | (4,353,494 | ) | (7,203,676 | ) | (6,090,181 | ) | ||||||||
| Cash Flows From Investing Activities: | ||||||||||||||||
| Capitalized patent costs | (135,168 | ) | (60,241 | ) | — | (135,168 | ) | |||||||||
| Capitalized software development costs | (27,879 | ) | (460,466 | ) | — | (27,879 | ) | |||||||||
| Purchase of property and equipment | (275,758 | ) | (359,301 | ) | (76,807 | ) | (275,758 | ) | ||||||||
| Net cash used in investing activities | (438,805 | ) | (880,008 | ) | (76,807 | ) | (438,805 | ) | ||||||||
| Cash Flows From Financing Activities: | ||||||||||||||||
| Proceeds from issuance of common stock | 696 | 118,791 | — | 696 | ||||||||||||
| Equity issuance cost paid | (273 | ) | (4,382 | ) | — | (273 | ) | |||||||||
| Proceeds from issuance of notes payable | 4,700,000 | — | ||||||||||||||
| Proceeds from warrant exercise | — | 2,963,658 | ||||||||||||||
| Repayments of note payable | (7,974,282 | ) | (6,500,000 | ) | ||||||||||||
| Proceeds from issuance of note payable | 6,500,000 | 4,700,000 | ||||||||||||||
| Principal payments on notes payable | (9,178,261 | ) | (7,974,282 | ) | ||||||||||||
| Net cash used in financing activities | (3,273,859 | ) | (3,421,933 | ) | (2,678,261 | ) | (3,273,859 | ) | ||||||||
| Effect of exchange rate changes on cash | (605,548 | ) | (163,658 | ) | ||||||||||||
| Net decrease in cash | (10,408,393 | ) | (8,819,093 | ) | ||||||||||||
| Net decrease in cash and restricted cash | (9,958,745 | ) | (9,802,845 | ) | ||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | (8,975 | ) | (605,548 | ) | ||||||||||||
| Cash and cash equivalent at beginning of period | 10,105,135 | 17,749,233 | ||||||||||||||
| Cash, cash equivalent at end of period | $ | 137,416 | $ | 7,340,840 | ||||||||||||
| Cash at beginning of period | 17,749,233 | 31,865,371 | ||||||||||||||
| Cash at end of period | 7,340,840 | 23,046,278 | ||||||||||||||
| Cash paid for: | ||||||||||||||||
| Interest | $ | 921,000 | $ | 1,522,372 | ||||||||||||
| Supplemental disclosure of non-cash financing activities: | ||||||||||||||||
| Release of prepayment from equity compensation | — | 50,000 | ||||||||||||||
| Monitoring fees related to notes payable | 1,522,372 | — | ||||||||||||||
| Supplemental schedule of non-cash transactions: | ||||||||||||||||
| Debt discount recognized upon issuance of notes payable | $ | 1,310,000 | $ | — | ||||||||||||
See notes to the unaudited condensed consolidated financial statements.
NEMAURA MEDICAL INC.
Notes to Condensed Consolidated Financial Statements
For the Nine Months Ended December 31, 2023 and 2022
(Unaudited)
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTIVITIESSUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nemaura Medical Inc. (“Nemaura” or the “Company”), through its operating subsidiaries, performs medical device research and manufacturing of a continuous glucose monitoring system (“CGM”), named sugarBEAT®. The sugarBEAT® device is a non-invasive, wireless device for use by persons with Type I and Type II diabetes and may also be used to screen pre-diabetic patients.patients and support obesity and weight-loss programs. The sugarBEAT® device extracts analytes, such as glucose, to the surface of the skin in a non-invasive manner where it is measured using unique sensors and interpreted using a unique algorithm.
Nemaura isGoing Concern
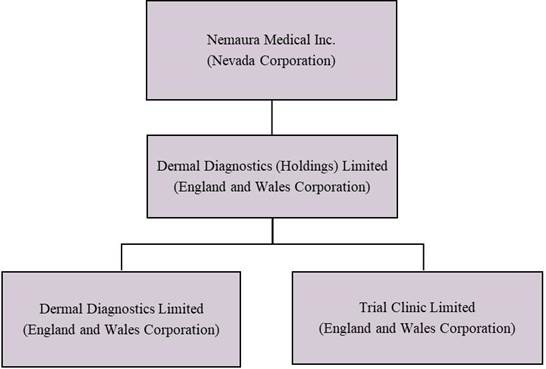
The accompanying unaudited financial statements have been prepared on a Nevada holding company organizedgoing concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in 2013. Nemaura owns 100%the normal course of business. As reflected in the accompanying unaudited financial statements, for the nine months ended December 31, 2023, the Company recorded a net loss of $5,968,086and used cash in operations of $7,203,676. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year of the stockdate that the financial statements are issued. In addition, the Company’s independent registered public accounting firm in Dermal Diagnostic (Holdings) Limited, an England and Wales corporation (“DDHL”) formed on December 11, 2013, which in turn owns 100% of Dermal Diagnostics Limited, an England and Wales corporation formed on January 20, 2009 (“DDL”), and 100% of Trial Clinic Limited, an England and Wales corporation formed on January 12, 2011 (“TCL”).
DDL is a diagnostic medical device company headquartered in Loughborough, Leicestershire, England, and is engaged in the discovery, development, and commercialization of diagnostic medical devices. The Company’s initial focus has beenits report on the development of the sugarBEAT® device, which consists of a disposable patch containing a sensor, and a non-disposable miniature wireless transmitter with a re-chargeable power source, which is designed to enable trending or tracking of blood glucose levels. All ofCompany’s March 31, 2023 financial statements, raised substantial doubt about the Company’s operations and assets are located in England.
During the fiscal year ended March 31, 2021, the Board of Directors assessed the adequacy of the group’s organizational structure and concluded that the intermediate holding company that sat below Nemaura Medical Inc., Region Green Limited (a British Virgin Islands corporation), was no longer required as the entity had been effectively dormant since inception and no longer represented a requirementability to be maintained. It was therefore determined that Region Green Limited should be unwound, with the intention that the assets held by Region Green Limited be transferred up to Nemaura Medical Inc. following which Region Green Limited would be dissolved.
The transfer of assets took place on March 5, 2021 and Region Green Limited was formally dissolved as of April 23, 2021.
The following diagram illustrates Nemaura’s corporate structure as of December 31, 2022:

The Company was incorporated in 2013 and has reported recurring losses from operations to date and an accumulated deficit of $47,192,364 as of December 31, 2022. These operations have resulted in the successful completion of clinical programs to support a CE mark (European Union (“EU”) approval of the product) approval, as wellcontinue as a De Novo 510(k) medical device application togoing concern. The financial statements do not include any adjustments that might be necessary if the U.S. Food and Drug Administration (“FDA”) submission.
The Company expectsis unable to continue to incur losses from operations until revenues are generated through licensing fees or product sales. However, given the completion of the requisite clinical programs, these losses are expected to decrease over time. Management has entered into licensing, supply, or collaboration agreements with unrelated third parties relating to the United Kingdom (“UK”), Europe, Qatar, and all countries in the Gulf Cooperation Council.
Going Concern
As identified under Item 1A, included in the Company’s Annual Report on Form 10-K for the year ended March 31, 2022, as filed with the SEC, management is aware of the need to raise additional funds in order to finance the ongoing commercialization of sugarBEAT®. The Company had $7,340,840 of cash at December 31, 2022. The Company has debt on its balance sheet which will reach maturity in July 2024.a going concern.
In evaluating the going concern position of the Company,company, management has considered the ability of the Company to raise additional funding in combination with one or more of the different funding options available to it at this time. Based on current and ongoing engagement with potential funding providers managementand believes that there is a reasonable expectation that fundingfinancing to fund future operations could be provided by one, equity and/or more,debt financing. There can be no assurance that funding would be available, or that the terms of the following options:
Equitysuch funding –would be on favorable terms if available. Even if the Company has immediate accessis able to funds throughobtain additional financing, it may contain undue restrictions on our operations, in the ATM facility that is currentlycase of debt financing, or cause substantial dilution for our stockholders, in place; in addition to this, there are various alternative mechanisms available to the Company similar to those used previously e.g. direct sale of shares to interested third parties, as well as other mechanisms to sell common stock via an underwritten agreement or the further exercise of warrants by the current warrant holders etc. The Company completed a Registered Direct Offering and concurrent Private Placement in January 2023 which has increased cash by $7,655,974.
Debt funding – the Company continues to be in ongoing discussions with third party debt providers, including the incumbent, to enable the existing debt facility to be restructured or renewed, should management feel that this route offers a more attractive option compared to the salecase of equity that is dependent on the current market conditions.
Alternative funding as used in the past such as the sale of licenses. As product development is now at a significant more advanced stage then it was, it is management’s belief that the sufficient funding could be provided through the sale of licenses or a large-scale partnership that could bring in additional funds and infrastructure to support the commercial growth ambitions of the company.
However, as a consequence of this funding requirement being triggered without the funding bridge having been put in place by the filing date of these unaudited condensed consolidated financial statements, Financial Accounting Standards Board’s (the “FASB”) Accounting Standards Codification (“ASC”) 205-40: “Going Concern”, requires that management recognize and disclose this point as an event which creates a substantial doubt as to the Company’s ability to continue as a going concern for at least one year from the date of filing of these unaudited condensed consolidated financial statements.
Following the receipt of the CE mark approval in the EU, and in support of our plans for similar certification with the FDA in the U.S., our plan is to utilize the cash on hand to continue establishing commercial manufacturing operations for the commercial supply of the sugarBEAT® device and sensor patches in our target markets.
Management's strategic plans include the following:
financing.
NOTE 2 – BASIS OF PRESENTATION
(a)Basis of presentationPresentation
The accompanying unaudited condensed consolidated financial statements have been prepared pursuant to the rules and regulations of the SEC, and do not include all of the information and footnotes required by U.S. generally accepted accounting principles (“U.S. GAAP”) for complete financial statements. However, such information reflects all adjustments consisting of normal recurring accruals which are, in the opinion of management, necessary for a fair statementpresentation of the financial condition and results of operations for the interim periods. The results for the three-three and nine-nine months ended December 31, 20222023 are not indicative of annual results. The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. These unaudited condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the year ended March 31, 2022, as filed with the SEC.2023.
The accompanying unaudited condensed consolidated financial statements include the accounts of the Company and the Company’s subsidiaries. References to “we”, “us”, “our”, or the “Company” refer to Nemaura Medical Inc. and its consolidated subsidiaries. The unaudited condensed consolidated financial statements are prepared in accordance with U.S. GAAP, and all significant intercompany balances and transactions have been eliminated in consolidation.
The functional currency for the majority of the Company’s operations is the Great Britain Pound Sterling (“GBP”), and the reporting currency is the U.S. Dollar (“USD”). Financial statements for foreign subsidiaries are translated into USD using period end exchange rates for assets and liabilities and average exchange rates for each period for revenue, costs and expenses.
Reclassification - We have reclassified certain amounts as previously disclosed within the March 31, 2022 consolidated balance sheets to conform to our current period presentation. The reclassification of $440,196 from Other liabilities and accrued expenses to Foreign currency contract at March 31, 2022 has no impact to prior year net loss, current quarter net loss or year-to-date net loss.
(b) – Summary of Significant Accounting Policies
Use of Estimates
The preparation of theconsolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts reported inof assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and accompanying notes. Actual results could differ materially from those estimates. The Company’s most significantthe reported amounts of revenues and expenses during the periods presented. Significant estimates include the useful lifeassumptions used in the accrual for potential liabilities, the net realizable value of intangible assets,inventory, the valuation of foreign currency contractdebt and equity instruments, the fair value of derivative liabilities, valuation allowance onof stock options issued for services, and deferred tax assets.
Our estimates are often based on complex judgments, probabilities and assumptions that management believes to be reasonable, but that are inherently uncertain and unpredictable. It is also possible that other professionals, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts. For a complete discussion of our critical accounting policies, see the “Critical Accounting Policies” section of the Management’s Discussion & Analysis in our March 31, 2022 Form 10-K.
Cash and Cash Equivalents
Cash includes cash deposited in major financial institutions in the United Kingdom. The Company’s cash balances exceed amounts covered by the Financial Services Compensation scheme. The Company has never suffered a loss due to such excess balances.
The Company considers highly liquid investments with maturities of three months or lessvaluation allowances. Actual results may differ from the date of purchase to be cash equivalents. These investments are carried at cost, which approximates fair value. As of December 31, 2022 and March 31, 2022, the Company had no cash equivalents.those estimates.
Revenue Recognition
The Company recognizes revenue in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”). The underlying principle of ASC 606 is to recognize revenue to depict the transfer of goods or services to customers at the amount expected to be collected. ASC 606 creates a five-step model that requires entities to exercise judgment when obligations underconsidering the terms of acontract(s), which include (1) identifying the contract or agreement with a customer, are satisfied; generally this occurs with(2) identifying our performance obligations in the transfer of controlcontract or access ofagreement, (3) determining the Company’s licenses or performance of services. Revenue is measured astransaction price, (4) allocating the amount of consideration the company expects to receive in exchange for transferring goods or providing services. A performance obligation is a promise in a contract to transfer a distinct good or servicetransaction price to the customer,separate performance obligations, and is the unit of account in the contract. A contract’s transaction price is allocated to(5) recognizing revenue as each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied.
Deferred Revenues
Contracts
In March 2014, the Company executed an Exclusive Marketing Rights agreement with customers consistDallas Burston Pharma (“DB Pharma”)(now known as MySugarWatch Limited “MSW”), a Jersey (Channel Island) based company for the exclusive right to sell the Company’s SugarBEAT® device in the UK and Republic of licensing arrangementsIreland, both direct to consumer and through prescriptions by general practitioners. The agreement has a term of five years and automatically renewed for another five years unless terminated by either party. As part of the agreement, the Company received a non-refundable upfront fee of £1 million ($1.6 million). Pursuant to current accounting guidelines, the Company recorded the upfront fee of £1 million as a lesser extent,deferred revenue (i.e. liability) and is being amortized to revenues based upon the corresponding sale of the Company’s SugarBEAT devices. As of December 31, 2023 and March 31, 2023, the outstanding deferred revenues amounted to $1,184,412 and $1,145,451, respectively or approximately £875,000GBP.
The agreement is scheduled to expire in March 2024, however, the Company expects that it will be renewed for another five years based upon the ongoing relationship with MSW.
Cash and cash equivalents
Cash and cash equivalents consists primarily of cash deposits maintained in the United Kingdom (“UK”). We maintain cash balances in U.S. Dollar (“USD”), Great Britain Pound Sterling (“GBP”), and the Euro. The following table, reported in USD, disaggregates our cash balances by currency denomination:
| Schedule of cash and cash equivalents | ||||||||
| December 31, 2023 | March 31, 2023 (audited) | |||||||
| Cash denominated in: | ||||||||
| USD | $ | 13,169 | $ | 5,606,972 | ||||
| GBP | 65,925 | 4,446,720 | ||||||
| Euro | 58,322 | 51,443 | ||||||
| Total | $ | 137,416 | $ | 10,105,135 | ||||
Inventory
As of December 31, 2023 and March 31, 2023, inventory consisted of the following:
| Schedule of inventory | ||||||||
December 31, 2023 | March 31, 2023, (audited) | |||||||
| Raw materials | $ | 3,553,811 | $ | 1,586,777 | ||||
| Finished goods | 117,722 | 168,075 | ||||||
| Total Inventories | $ | 3,671,533 | $ | 1,754,852 | ||||
Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out (“FIFO”) basis. For the nine months ended December 31, 2023, there were additional general write-downs of inventory of approximately $104,000.
Research and development expenses
The Company charges research and development expenses to operations as incurred. Research and development expenses primarily consist of salaries and related expenses for personnel and outside contractor and consulting services. Revenues from licensingOther research and royalty feesdevelopment expenses include the costs of materials and supplies used in research and development, prototype manufacturing, clinical studies, related information technology and an allocation of facilities costs.
Basic loss per share is computed by dividing the loss available to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted loss per common share reflects the potential dilution that could occur if convertible debentures, options and warrants were to be exercised or converted or otherwise resulted in the issuance of common stock that then shared in the earnings of the entity.
Since the effects of outstanding options and warrants are receivedanti-dilutive for the nine months ended December 31, 2023 and 2022, shares of common stock underlying these instruments have been excluded from the grantingcomputation of exclusive sales, marketing, manufacturingloss per common share.
The following sets forth the number of shares of common stock underlying outstanding options and distribution rights associated with the Company’s functional intellectual property (IP). The Company’s performance obligation is satisfied at a point in time (upon delivery to the customer), where the Company has no remaining obligation to support or maintain the intellectual property licensed to the customer. The Company typically requires a non-refundable license fee, paid upfront.warrants as of December 31, 2023 and 2022:
| Schedule of common stock underlying outstanding options | ||||||||
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Stock Warrants | 5,233,551 | 1,573,098 | ||||||
| Stock options | 40,000 | 40,000 | ||||||
| 5,273,551 | 1,613,098 | |||||||
Revenue from license fees are recognized at a point in time when the Company transfers the functional IP to the customer as long as management believes the total consideration owed by the customer for the license fee is probable of being received.Stock-Based Compensation
The Company’s contracts do not include multiple performance obligationsCompany periodically issues share-based awards to employees and non-employees and consultants for services rendered. Stock options vest and expire according to terms established at the issuance date of each grant. Stock grants are measured at the grant date fair value. Stock-based compensation cost is measured at fair value on the grant date and is generally recognized as a charge to operations ratably over the requisite service, or variable consideration. Sincevesting, period. Recognition of compensation expense for non-employees is in the same period and manner as if the Company had paid cash for the services.
The Company values its equity awards using the Black-Scholes option-pricing model, and accounts for forfeitures when they occur. Use of the Black-Scholes option pricing model requires the input of subjective assumptions, including expected volatility, expected term, and a risk-free interest rate. The expected volatility is based on the historical volatility of the Company’s revenuecommon stock, calculated utilizing a look-back period approximately equal to the contractual life of the stock option being granted. The expected life of the stock option is generated from a small number of customer contracts,calculated as the Company does not have material contract assets or liabilities.mid-point between the vesting period and the contractual term (the “simplified method”). The risk-free interest rate is estimated using comparable published federal funds rates.
Fair valueValue of financial instrumentsFinancial Instruments
In accordance
The authoritative guidance with the FASB ASC 820, “Fair Value Measurements and Disclosures,” the Company determines therespect to fair value of financial instruments with the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishesestablished a fair value hierarchy based onthat prioritizes the level of independent, objective evidence surrounding the inputs to valuation techniques used to measure fair value. Avalue into three levels and requires that assets and liabilities carried at fair value be classified and disclosed in one of three categories, as presented below. Disclosure as to transfers in and out of Levels 1 and 2, and activity in Level 3 fair value measurements, is also required. Fair value of a financial instrument’s categorization withininstrument is defined as the amount at which the instrument could be exchanged in a current transaction between willing parties.
The three levels of the fair value hierarchy isare as follows:
Level 1 - Valuations based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1: Applies to assets or liabilities for which there areon unadjusted quoted prices in active markets for identical assets or liabilities.liabilities that the entity has the ability to access.
Level 2: Applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as - Valuations based on quoted prices for similar assets or liabilities, in active markets; quoted prices for identical assets or liabilities in markets with insufficient volumethat are not active, or infrequent transactions (less active markets); or model-derived valuations in which significantother inputs that are observable or can be derived principally from, or corroborated by observable market data.
Level 3: Applies todata for substantially the full term of the assets or liabilities for which thereliabilities.
Level 3 - Valuations based on inputs that are unobservable, inputs to the valuation methodologysupported by little or no market activity and that are significant to the measurement of the fair value of the assets or liabilities.
Intangible Assets
The Foreign exchange contract derivative liability is valued using Level 2 fair values while the warrant liability is valued using Level 3 fair values.
The following table sets forth by level, within the fair value hierarchy, the Company’s intangiblefinancial assets consistand liabilities at fair value as of patents relatingDecember 31, 2023 and March 31, 2023:
| Schedule of assets and liabilities at fair value | ||||||||||||||||
| December 31, 2023 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets | ||||||||||||||||
| Total assets | $ | — | $ | — | $ | — | $ | — | ||||||||
| Liabilities | ||||||||||||||||
| Foreign exchange contract derivative liability | $ | — | $ | 242,295 | $ | — | $ | 242,295 | ||||||||
| Warrant derivative liability | — | — | 492,000 | 492,000 | ||||||||||||
| Total liabilities | $ | — | $ | 242,295 | $ | 492,000 | $ | 734,295 | ||||||||
| March 31, 2023 (audited) | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets | ||||||||||||||||
| Total assets | $ | — | $ | — | $ | — | $ | — | ||||||||
| Liabilities | ||||||||||||||||
| Foreign exchange contract derivative liability | $ | — | $ | 731,730 | $ | — | $ | 731,730 | ||||||||
| Warrant derivative liability | — | — | 3,092,000 | 3,092,000 | ||||||||||||
| Total liabilities | $ | — | $ | 731,730 | $ | 3,092,000 | $ | 3,823,730 | ||||||||
The following table provides a roll-forward of the warrant derivative liability measured at fair value on a recurring basis using unobservable level 3 inputs for the nine months ended December 31, 2023:
| Schedule of warrant derivative liability measured at fair value on a recurring basis | ||||
| Warrant derivative liability | ||||
| Balance as of beginning of period – March 31, 2023 | $ | 3,092,000 | ||
| Change in fair value of warrant derivative liability | (2,600,000 | ) | ||
| Balance as of end of period – December 31, 2023 | $ | 492,000 | ||
As of December 31, 2023 and March 31, 2023, the Company’s outstanding warrants were treated as derivative liabilities and changes in the fair value were recognized in earnings (see Note 3).
The Company believes the carrying amounts of certain financial instruments, including cash, accounts receivable, and accounts payable and accrued liabilities, approximate fair value due to the sensorshort-term nature of such instruments and algorithm that are granted in some territories, and pending still in others. The Company also plans to file further patents asexcluded from the opportunity arises. The cost of issued patents is capitalized and amortized over the life of the patents which is 20 years. The costs of patents in development are expensed as incurred. Any unamortized costs previously capitalized associated with patents that have expired or have been abandoned are written off as an impairment loss. The company has also capitalized certain software development costs which are regularly reviewed to ensure that if development has been abandoned, costs are written off as an impairment loss.
fair value tables above.
Share-Based PaymentsInflation
The Company measures the cost of services received in exchange for an award of equity instrumentsdoes not believe that inflation has had a material effect on its operations to employees and nonemployees baseddate, other than its impact on the grant date fair value of the award, whichgeneral economy. However, there is recognized as compensation expense over the vesting term.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
The Company recognizes deferred tax assets to the extent that management believes these assets are more likely than not to be realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If we determinerisk that the Company would be ableCompany’s operating costs could become subject to realize deferred tax assetsinflationary and interest rate pressures in the future, which would have the effect of increasing the Company’s operating costs (including, specifically, clinical trial costs in excess of their net recorded amount,countries where the Company would make an adjustmentis applying to the deferred tax asset valuation allowance,sell its products), and which would reduceput additional stress on the provision for income taxes.Company’s working capital resources.
(c) Recently adoptedRecent accounting pronouncements
Accounting standard updatesManagement believes that any recently issued, but not yet added were assessed and determined to be eithereffective, accounting pronouncements, if currently adopted, would not applicable or not expected to have a material impacteffect on ourthe Company’s unaudited condensed consolidated financial statements.
NOTE 3 – LICENSING AGREEMENTS
United Kingdom and the Republic of Ireland, the Channel Islands, and the Isle of Man
In March 2014, the Company entered into an Exclusive Marketing Rights Agreement (the “Marketing Rights Agreement”) with an unrelated third party (the “Licensee”), that granted to the Licensee the exclusive right to market and promote the sugarBEAT® device and related patches under its own brand in the UK and the Republic of Ireland, the Channel Islands, and the Isle of Man. The Company received a non-refundable, up-front cash payment of GBP 1,000,000 (approximately $1.20 million and $1.31 million as of December 31, 2022 and March 31, 2022, respectively), upon signing the Marketing Rights Agreement. The upfront payment received from the Marketing Rights Agreement has been deferred and will be recorded as income over the term of the Marketing Rights Agreement, which commenced upon the first delivery of the sugarBEAT® device to the Licensee in December 2021. Consequently, approximately $70,000, and $259,000 is included in deferred revenue classified as a current liability as of December 31, 2022 and March 31, 2022, respectively, with the remainder being shown in the non-current portion of deferred revenue.
NOTE 42 – RELATED PARTY TRANSACTIONS
DDL has a service agreement with Nemaura Pharma Limited (“Pharma”), an entity controlled by the Company’s President and Chief Executive officer, to provide development, manufacture, and regulatory approval process under Pharma’s ISO13485 accreditation. Pharma invoices DDL for these services on a cost-plus basis.
The table below provides a summary of activity between the Company and Pharma for the nine months ended December 31, 2023 and 2022.
| Schedule of related party transactions | ||||||||
Nine Months Ended December 31, 2023 (unaudited) | Nine Months Ended December 31, 2022 (unaudited) | |||||||
| Due to (from) related parties at beginning of period | $ | 920,782 | $ | (101,297 | ) | |||
| Amounts invoiced by Pharma to DDL, NM and TCL, primarily relating to research and development expenses | 4,211,705 | 2,833,546 | ||||||
| Amounts invoiced by DDL to Pharma | — | (3,159 | ) | |||||
| Amounts received from Pharma | — | 4,452 | ||||||
| Amounts paid by DDL to Pharma | (4,311,770 | ) | (2,789,939 | ) | ||||
| Foreign exchange differences | (20,314 | ) | 31,077 | |||||
| Due to (from) related parties at end of period | $ | 800,403 | $ | (25,320 | ) | |||
NOTE 3 – DERIVATIVE LIABILITIES
Warrant liability
In January 2023, the Company completed an equity offering, which included the issuance of 4,796,206 warrants. Upon the occurrence of certain transactions (“Fundamental Transactions,” as defined), the warrants provide for a value determined using a Black Scholes model with inputs calculated as described in the warrant agreement which includes a 100% floor on the volatility input to be utilized. The Company has determined that this provision introduces leverage to the holders of the warrants that could result in a value that would be greater than the settlement amount of a fixed-for-fixed option on the Company’s own equity shares. Accordingly, pursuant to ASC 815, the Company has classified the fair value of the warrants as a liability to be re-measured at the end of every reporting period with the change in value reported in the statement of operations.
The warrant liability was valued at the following dates using a Black-Scholes model with the following assumptions:
| Schedule of warrant liability | ||||||||
December 31, 2023 | March 31, 2023 | |||||||
| Warrant liability: | ||||||||
| Stock price | $ | 0.22 | $ | 0.90 | ||||
| Risk-free interest rate | % | % | ||||||
| Expected volatility | % | % | ||||||
| Expected life (in years) | ||||||||
| Expected dividend yield | ||||||||
| Fair value of Warrant liability | $ | 492,000 | $ | 3,092,000 | ||||
The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of measurement commensurate with expected life of the warrants. Expected volatility was determined based on the historical volatility data of the Company, and the expected term of the warrants granted are determined based on the duration of time the warrants are expected to be outstanding. The dividend yield on the Company’s warrants is assumed to be zero as the Company has not historically paid dividends.
Foreign exchange contract liability
The Company is exposed to the impact of foreign currency exchange fluctuations as a significant proportion of its expenses are denominated in GBP, and the Company’s cash is in USD and GBP. In February 2021, the Company entered into a forward contract to sell USD and buy GBP. The contract meets the definition of a derivative subject to the guidance of ASC 815, does not qualify for hedge accounting, and accordingly is recognized at fair value, with changes in fair value recognized in earnings.
The term of the contract is 25 months, beginning July, 2022, and 2021,ending August, 2024. The contract initially had a maximum notional amount of $6,250,000 (and a maximum leveraged amount equal to two times the notional amount, or $12,500,000). $250,000 of the contractual notional amount is settled (expires) each month through August 2024. On each monthly settlement date, if the USD/GBP spot rate is above $1.359, the Company has the right to convert $250,000 USD into GBP at a fixed rate of $1.359. If the spot rate is between $1.359 and $1.319 on the settlement date, the Company has no obligations, but can convert $250,000 USD into GBP at the spot rate. Finally, if the spot rate is below $1.319 on the monthly settlement date, the Company is obligated to convert $500,000 USD (the settlement date leveraged amount) into GBP at the fixed rate of $1.359. Alternatively, instead of selling $500,000 USD, the Company can pay the difference in the spot rate and the year ended$1.359 exchange rate for $500,000 USD (net settle) to the counterparty.
At December 31, 2023 and March 31, 2022.2023, the fair value of the foreign currency contract liability was valued as follows:
| Schedule of fair value of the foreign currency contract liability | ||||||||
December 31, 2023 | March 31, 2023 | |||||||
| Notional Amount | $ | 2,000,000 | $ | 4,250,000 | ||||
| Leveraged amount (used to determine fair value of contract liability) | $ | 4,000,000 | $ | 8,500,000 | ||||
| Expected remaining term (in months) | 8 | 17 | ||||||
| Fair Value: | ||||||||
| Foreign currency contract liability | $ | 242,295 | $ | 731,730 | ||||
| Schedule of related party transactions | ||||||||||||
Nine Months Ended December 31, 2022 (unaudited) | Nine Months Ended December 31, 2021 (unaudited) | Year Ended March 31, 2022 | ||||||||||
| Due to (from) related parties at beginning of period | $ | (101,297 | ) | $ | 148,795 | $ | 148,795 | |||||
| Amounts invoiced by Pharma to DDL | 2,833,546 | 2,114,801 | 3,245,985 | |||||||||
| Amounts invoiced by DDL to Pharma | (3,159 | ) | (2,495 | ) | (2,495 | ) | ||||||
| Amounts paid by DDL to Pharma | (2,785,487 | ) | (2,316,544 | ) | (3,492,962 | ) | ||||||
| Foreign exchange differences | 31,077 | (97,149 | ) | (620 | ) | |||||||
| Due to (from) related parties at end of period | $ | (25,320 | ) | $ | (152,592 | ) | $ | (101,297 | ) | |||
The Company’s foreign currency forward contracts are measured at fair value on a recurring basis and are classified as Level 2 fair value measurement. As of December 31, 2023, and March 31, 2023, the Company has deposited $146,434, and $909,666, respectively, as collateral with the counterparty related to the foreign currency forward contract and recorded as part of prepaid expenses and other receivables in the accompanying balance sheet.
NOTE 54 – NOTES PAYABLE
| Schedule of notes payable | ||||||||
December 31, 2023 (unaudited) | March 31, 2023 | |||||||
| Note Payable Agreement 2 | $ | 13,551,346 | $ | 14,772,293 | ||||
| Note Payable Agreement 3 | 6,365,649 | 6,024,941 | ||||||
| Note Payable Agreements 4 and 5 | — | — | ||||||
| Total notes payable | 19,916,995 | 20,797,234 | ||||||
| Unamortized debt discount | (273,957 | ) | (767,083 | ) | ||||
| Notes payable, net of note discounts | 19,643,038 | 20,030,151 | ||||||
| Current portion | (19,643,038 | ) | (16,942,500 | ) | ||||
| Non-current portion | $ | — | $ | 3,087,651 | ||||
At October 5, 2023, the Company had four note payable agreements (Notes #2, #3, #4, and #5) outstanding. Effective October 5, 2023, the Company entered into standstill agreements for Notes #2 and #3, pursuant to which the investors would not seek repayment of any portion of the notes during the period from October 5, 2023 to October 31, 2023. In consideration, the Company agreed to pay a standstill fee of $1,300,000, that was added to the note principal of Notes #2 and #3.
On October 5, 2023, the Company entered into termination agreements to terminate and cancel Notes #4 and #5, which had an aggregate balance of principal and accrued interest of $7,940,657. In consideration, a principal payment of $3,000,000 was made, and $4,940,657 was added to the principal of Notes #2 and #3.
NOTE PURCHASEPAYABLE AGREEMENT 12
On April 15, 2020,February 8, 2021, the Company entered intoissued a note purchase agreement (the “Note Purchase Agreement 1”payable (“Note 2”) to a third-party investor. The note was for $24,015,000, originally matured on February 9, 2023 (see below), and is secured by and amongall the assets of the Company. Beginning in March 2023, the monthly principal payments are $1,000,000 per month. In addition, the Company DDL, TCL andis required to accrue a third-party investor (the “Investor”).
Pursuantmonthly PIK fee equal to 0.833% of the outstanding balance, which is in substance interest at an annual rate of approximately 10%, that is added to the terms ofnote principal each month. In October 2022 Note Purchase Agreement2 was amended to extend the maturity from February 9, 2023 to July 1, 2024. In consideration, the Company agreed to issue and sellpay aggregate fees of $2,304,539 to the Investor,investor which were added to the principal balance of Note 2.
As of March 31, 2023, outstanding balance of note payable amounted to $14,772,293. On October 5, 2023, $3,143,134 was added to the principal of Note 2 related to the termination of Notes 4 and 5 and addition of the Investor agreedstandstill fee (see above). During the nine months ended December 31, 2023, principal payments of $4,364,081 were made. As of December 31, 2023, outstanding balance of note payable amounted to purchase from$13,551,346.
NOTE PAYABLE AGREEMENT 3
On May 20, 2022, the Company issued a secured promissory note (the “2020 Secured Note”) in the original principal amount ofpayable (“Note 3) to a third-party investor. The note was for $6,015,000. In consideration thereof,, matures on April 15, 2020, (i) the Investor (a) paid $1,000,000 in cash, (b) issued to the Company (1) Investor Note #1 in the principal amount of $2,000,000 (“Investor Note #1”),May 20, 2024, and (2) Investor Note #2 in the principal amount of $2,000,000 (“Investor Note #2” and together with Investor Note #1, the “2020 Investor Notes”), and (ii) the Company delivered the 2020 Secured Note on behalf of the Company, to the Investor, against delivery of the 2020 Purchase Price. For these purposes, the “2020 Purchase Price” means the Investor’s initial cash purchase price, together with the sum of the initial principal amounts of the Investor Notes.
The 2020 Secured Note is secured by all the Collateral (as hereinafter defined).assets of the Company. The 2020 Secured Note carriesCompany received cash proceeds of $4,700,000, resulting in a discount of $1,315,000 made up of an original issue discount (“OID”) of $1,000,000 (, commission of $16.7300,000%). that was paid from proceeds, and $15,000 to cover transaction expenses. In addition, the Company agreedis required to pay $15,000 to the Investor to cover the Investor’s legal fees, accounting costs, due diligence, monitoring and other transaction costs incurred in connection with the purchase and sale of the 2020 Secured Note (the “Transaction Expense Amount”). In addition to this,accrue a payment of $325,000 was made to Ascendiant Capital Markets, LLC (“Ascendiant”) for structuring the agreement between both parties. The 2020 Purchase Price for the 2020 Secured Note is $4,675,000, computed as follows: $6,015,000 original principal balance, less: OID, Transaction Expense Amount, and commission paid.
The borrowing period is 24 months, and the Company shall pay the outstanding balance and all fees on maturity. A monitoringmonthly PIK fee equal to 0.833%0.833% of the outstanding balance, will automatically bewhich is in substance interest at an annual rate of approximately 10%, that is added to the outstanding balance on the first day of each month. The debt less the discount and transaction expenses will be accreted over the term of the 2020 Secured Note using the effective interest method.
Security Agreement
On April 15, 2020, the Company entered into the Security Agreement by the Company, DDL and TCL, in favor of the Investor (the “2020 Security Agreement”). Pursuant to the terms of the 2020 Security Agreement, the Company granted the Investor a first-priority security interest in all rights, title, interest, claims and demands of the Company in and to all of the Company’s patents and all other proprietary rights, and all rights corresponding to the Company’s patents throughout the world, now owned and existing, and all replacements, proceeds, products, and accessions thereof (the “Collateral”).
Note Purchase Agreement 1 was settled in full on April 22, 2022.
NOTE PURCHASE AGREEMENT 2
On February 8, 2021, the Company entered into an additional note purchase agreement (“Note Purchase Agreement 2”) with the Investor. Pursuant to the terms of Note Purchase Agreement 2, the Company agreed to issue and sell to the Investor, and the Investor agreed to purchase from the Company, a secured promissory note (the “Secured Note 2”) in the original principal amount of $24,015,000. The Secured Note 2 carries an OID of $4,000,000 (16.7%), and the Company agreed to pay $15,000 to the Investor to cover the Investor’s transaction expenses. In addition to this, a commission of $1,200,000 was also payable to Ascendiant.
In consideration thereof, on February 9, 2021, (i) the Investor paid $20,000,000 in cash to the Company, and (ii) the Company delivered Secured Note 2 on behalf of the Company, to the Investor, against the delivery of the 2021 Purchase Price. For these purposes, the “2021 Purchase Price” means the Investor’s initial cash purchase price. After adjusting for transaction expenses of $1,200,000, cash proceeds received were $18,800,000.
The borrowing terms for Note Purchase Agreement 2 were originally consistent with those of Note Purchase Agreement 1, with the borrowing period being 24 months from the date of the agreement, the Company being required to pay the outstanding balance and all fees on maturity, and a monitoring fee equal to 0.833% of the outstanding balance being automatically added to the outstanding balance on the first day of each month. The debt less discount and transaction expenses will be accreted over the term of the Secured Note 2note using the effective interest rate method.
On October 21, 2022, the Company entered into an amendment to Note Purchase Agreement 2. Pursuant to the terms of the amendment, the Company and Investor agreed to extend the maturity date of Note Purchase Agreement 2 to July 1, 2024. In consideration thereof, the Company agreed to pay to the Investor an extension fee in the amount of 5% ofAt March 31, 2023, the outstanding balance of Note Purchase Agreement 2, which resulted in $813,834 being added onto the liability due to the Investor.
The Company and the Investor previously agreed to reduce the maximum monthly redemption amount from $2,000,000 to $500,000 from June 2022 to February 2023, which reduction remains in force. Pursuant to the terms of the amendment, the Company and Investor agreed to reduce the maximum monthly redemption amount during the period beginning March 2023 until Note Purchase Agreement 2 is paid in full from $2,000,000 to $1,000,000; provided, however, that upon the occurrence of an event of default under the Note Purchase Agreement 2, the maximum monthly redemption amount will automatically be increased back to $2,000,000.
Security Agreement
On February 8, 2021, the 2020 Security Agreement3 was extended to include Note Purchase Agreement 2, which is also secured against all of the Company’s assets owned as of February 9, 2021 and extends to any assets acquired at any time that the Company’s obligations under Secured Note 2 are outstanding.
NOTE PURCHASE AGREEMENT 3
On May 20, 2022, the Company entered into a new note purchase agreement (“Note Purchase Agreement 3”) by and among the Company, DDL, TCL and a third-party investor.
Pursuant to the terms of the Note Purchase Agreement 3, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company a secured promissory note (the “Secured Note”) in the original principal amount of $6,015,000. In consideration thereof, on May 20, 2022 (the closing date), (i) the Investor paidOn October 5, 2023, $5,000,000 in cash, and (ii) the Company delivered the Secured Note on behalf of the Company, to the Investor, against delivery of the Purchase Price. For these purposes, the “Purchase Price” means the Investor’s initial cash purchase price.
The Secured Note is secured by the Collateral (as hereinafter defined). The Secured Note carries an original issue discount (“OID”) of $1,000,000 (16.7%). In addition, the Company agreed to pay $15,000 to the Investor to cover the Investor’s legal fees, accounting costs, due diligence, monitoring and other transaction costs incurred in connection with the purchase and sale of the Secured Note (the “Transaction Expense Amount”). In addition to this, a payment of $300,0002,164,829 was made to Ascendiant Capital Markets, LLC, (the “Commission”) for structuring the agreement between both parties. The Purchase Price for the Secured Note is $4,700,000, computed as follows: $6,015,000 original principal balance, less: OID, Transaction Expense Amount, and commission paid.
The borrowing period is 24 months, and the Company shall pay the outstanding balance and all fees on maturity. A monitoring fee equal to 0.833% of the outstanding balance will automatically be added to the outstanding balance onprincipal of Note 3 related to the first daytermination of each month. The debt less the discountNotes 4 and transaction expenses will be accreted over the term5 and addition of the Note usingstandstill fee (see above). During the effective interest method.
Security Agreement
On May 20, 2022, the Company entered into the Security Agreement by the Company, DDL and TCL, in favornine months ended December 31, 2023, principal payments of the Investor (the “Security Agreement”). Pursuant to the terms of the Security Agreement, the Company granted the Investor a first-priority security interest in all rights, title, interest, claims and demands of the Company in and to all of the Company’s patents and all other proprietary rights, and all rights corresponding to the Company’s patents throughout the world, now owned and existing, and all replacements, proceeds, products, and accessions thereof.$1,814,180 were made. As of December 31, 2022, long-term debt matures as follows:
| ||||||
NOTE 6 – STOCKHOLDERS’ (DEFICIT) EQUITY
During2023, the three month period ended December 31, 2022, shares were sold under the ATM Equity Distribution Agreement in place with H.C. Wainwright & Co., for total gross proceedsoutstanding balance of Note 3 was $696, with associated costs of $2736,365,649. other shares were issued during the nine month period ended December 31, 2022.
During the nine month period ended December 31, 2021, 366,892 warrants were exercised generating gross proceeds2023, debt discount amortization of $2,963,658. There were a total of 1,573,098493,125 warrants outstanding at this date. During the three month period endedwas recorded. At December 31, 2021, shares were sold under the ATM Equity Distribution Agreement in place with H.C. Wainwright & Co., for total gross proceeds of $118,791, with associated costs of $4,382. other shares were issued during the three and nine month periods ended December 31, 2021.
Loss per share
The following table sets forth the computation of basic and diluted loss per share for the periods indicated.
| Schedule of earnings (loss) per share | ||||||||||||||||
Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
| (in Dollars, except Share Amounts) | (in Dollars, except Share Amounts) | |||||||||||||||
| Net loss attributable to common stockholders | (1,716,278 | ) | (3,431,568 | ) | (9,460,888 | ) | (10,269,557 | ) | ||||||||
| Weighted average basic and diluted shares outstanding | 24,103,196 | 23,313,629 | 24,102,976 | 23,244,345 | ||||||||||||
| Basic and diluted loss per share: | (0.07 | ) | (0.15 | ) | (0.39 | ) | (0.44 | ) | ||||||||
The Company excludes warrants outstanding, which are anti-dilutive given the Company is in a loss position, from the basic and diluted loss per share calculation.
Basic loss per share is computed by dividing loss available to common stockholders by the weighted-average number of common shares outstanding during the period. For the three and nine month periods ended December 31, 2022, warrants to purchase 1,573,098 shares of common stock and a unit purchase option to purchase 9,710 shares of common stock, as well as warrants to purchase 9,710 shares of common stock, were considered anti-dilutive and were excluded from the calculation of diluted loss per share. For the three and nine month periods ended December 31, 2021, warrants to purchase 1,573,098 shares of common stock and a unit purchase option to purchase 9,710 shares of common stock, as well as warrants to purchase 9,710 shares of common stock, were considered anti-dilutive and were also excluded from the calculation of diluted loss per share.
NOTE 7 – OTHER ITEMS
The outbreak of COVID-19 in December 2019 has since rapidly increased its exposure globally. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. We continue to monitor the impact of COVID-19 on our own operations and are working with our employees, suppliers and other stakeholders to mitigate the risks posed by its spread, but COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers have not been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities and respond favorably to the challenges faced during the outbreak. We have also seen a surge in the uptake of technologies for remote monitoring of patients and patient self-monitoring, which potentially enhances the prospects for the Company, its CGM product and its planned digital healthcare offering.
NOTE 8 – SUBSEQUENT EVENTS
Management has evaluated subsequent events and transactions for potential recognition or disclosure in the financial statements through February 23, 2023, the date these financial statements were available to be issued.
The Company commenced a Registered Direct Offering and concurrent Private Placement on January 27, 2023 with two healthcare-focused U.S. institutional investors to sell shares of its common stock, pursuant to a registered direct offering and warrants to purchase up to 4,796,206 shares in a concurrent private placement. The combined purchase price for one share and one warrant was $. The warrants have an exercise price of $2.00 per share and are initially exercisable at the later of shareholder approval or six months following the date of issuance and will expire 5.5 five and a half years from January 31, 2023, the closing date.unamortized debt discount was $273,958.
NOTE PAYABLE AGREEMENTS 4 and 5
In August 2023, the Company issued two notes payable to two third party investors (“Notes 4 and 5”) , with a face value of $7,810,000 in exchange for cash of $6,500,000 or an original issue discount of $1,310,000. The aggregate gross proceeds fromnotes were secured by all tangible and intangible assets of the Registered Direct OfferingCompany and will mature in 24 months or in August 2025. The notes did not bear any interest; however, the implied annual interest rate is 9.5% based upon the OID rate of 19% and annual monitoring fee of 9.9%. As a result, the Company recorded a debt discount of $1,310,000 to account the note's original issue discount, commission and direct costs computed which is being amortized over interest expense over the term of the note payable.
On October 2023, the Company and the concurrent Private Placement werenoteholders amended the two notes payable. As part of the amendment, the Company paid the noteholder $8.43 million before deducting placement agentin principal and the remaining balance of $4,810,000 and accrued interest of $130,657, was transferred and added to the outstanding principal balance of Note 2 for $2,775,828 and Note 3 for $2,164,829, issued in May 2022 and October 2022, respectively. In addition, the Company also incurred additional fees of $367,306 as part of the amendment of notes payable 4 and other estimated offering expenses.5, which was added to Note 2. In addition, the Company also expensed the entire debt discount of $1,310,000. As of result of these amendments, notes payable 4 and 5 were extinguished and cancelled by the noteholder.
LINE OF CREDIT
In November 2023, the Company executed a line of credit (LOC) with a third party financing company, Streeterville Capital LLC. Pursuant to the LOC agreement, the Company can loan up to $10 million at a rate of 10% per annum and a 20% original issue discount for a period of one year. The LOC is secured by all tangible and intangible assets of the Company. The Company received net proceeds of $7,655,974 after costs.has not yet made advances or drawdowns against the LOC.
ITEM 2: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion in conjunction with the Unaudited Condensed Consolidated Financial Statements and accompanying notes included elsewhere in this Quarterly Report on Form 10-Q. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements. The matters discussed in these forward-looking statements are subject to risks, uncertainties, and other factors that could cause actual results to differ materially from those made, projected, or implied in the forward-looking statements. See "Cautionary Statement Concerning Forward-Looking Statements" below, and "Item 1A. Risk Factors" in our Annual Report on Form 10-K for the fiscal year ended March 31, 2022,2023, as filed with the Securities and Exchange Commission, as the same may be updated from time to time, for a discussion of the uncertainties, risks and assumptions associated with these statements.
Overview
Business Review and Outlook
WeIt is management’s view that the Company made good progress during the nine month period ended December 31, 2023, and some of the key developments are a medical technologylisted as follows:
| 1. | The Company continued to support its UK licensee with its endeavours to obtain reimbursement for the sensors in the UK. |
| 2. | Advanced development of the Company’s BEATdiabetes offering in readiness for a commercial launch in due course. |
| 3. | Continued development of its consumer metabolic health platform and potential deployment as a bolt-on service into existing metabolic and wellness programs. |
| 4. | Received approval from the Saudi Arabia Food and Drug Agency for marketing of sugarBEAT in the Kingdom of Saudi Arabia (KSA), with support from the Company’s licensee in the region, TP MENA. |
| 5. | Used feedback from the Company’s pre-diabetes and consumer metabolic health program with the UK National Health Service, to commence plans for a commercial launch of the program in various territories with partners, in due course. |
Management is working towards fulfilling the remainder of the UK licensees’ initial orders and supporting MSW’s UK launch plans, and for potential supplies to fulfill the provisional purchase order for the KSA from TPMENA. The company developing sugarBEAT®, a non-invasive, affordable,continues to also develop capabilities to develop and flexible continuous glucose monitoring system for adjunctiveservice new channels of business across other geographic markets via the use by persons with diabetes, as well as for consumersof our BEAT platform. To this end the company is now actively planning product launch in other territories that accept the CE mark registration. In addition, the company is seeking to exploit its product platform in the non-diabetes space for monitoring metabolic health and general health and wellness. sugarBEAT® consists of a disposable adhesive skin-patch connected to a rechargeable wireless transmitter that displays glucose readings at regular five-minute intervals via a mobile app. sugarBEAT® works by extracting glucose from the skin into a chamber in the patch that is in direct contact with an electrode-based sensor. The transmitter sends the raw data to a mobile app where it is processed by an algorithm and displayed as a glucose reading after calibration, with the ability to track and trend the data over 14 hours for each sensor wear period. Sensors can be worn as frequently as a user chooses. While sugarBEAT® requires once per day calibration by the patient using a blood sample obtained by a finger stick, we believe sugarBEAT® will be adopted by non-insulin dependent persons with diabetes alongside insulin-injecting persons with diabetes, who all perform multiple daily finger sticks to manage their disease. In the consumer application, or non-medical applications, the relative glucose fluctuations are used and therefore finger-prick calibrations are not required.space.
CE approval was granted by the European Notified Body BSI in May 2019, allowing the product to be made available for commercial sale. This approval is subject to an annual review of the underlying ISO 13485 accredited Quality Management System. The accreditation was successfully renewed in November 2021 and 2022. In conjunction with the UK Licensee, the Company commenced a phase 1 launch whereby devices were made available to limited cohorts of users to gauge their feedback so that any fine-tuning could be completed prior to a mass market launch. The UK Licensee has also confirmed that it will undertake two Key Opinion Leader (“KOL”) studies in the UK for its white-labelled service offering that is supported by sugarBEAT®. The KOL studies are intended to provide additional support for the UK Licensee’s broader ongoing marketing plans and more specifically to support potential reimbursement for the device and the licensees overall diabetes management offering(s)
The UK Licensee placed an initial order for sugarBEAT® in April 2021 and provided a forecast for its post-launch volume expectations, which the Company has used to establish both a short and medium term view to inform the Company’s commercial operational requirements. In line with this view, the Company has taken the following actions sinceduring and after the initial order was received:
nine month period ended December 31, 2023:
| Increased headcount of production operatives; this will be phased in line with the volume forecasts currently available, however the Company has also factored in an ability to scale further and faster should this be required. |
| • | Moved forward with placing phased orders for raw materials to ensure future product availability to support both our UK Licensee while also providing for capacity to flex up further as other routes to market materialize in line with management’s commercialization program. |
In July 2020, Nemaura filed a PMA application with the FDA to use sugarBEAT® as an adjunct to finger prick testing for blood glucose trending. We, along with other applicants, were then informed by the FDA that the approval process was currently subject to delays as a result of the FDA’s Center for Devices and Radiological Health (“CDRH”) being actively engaged in responding to the current pandemic caused by COVID-19 which resulted in staff being reallocated to other approval requests associated with COVID-19. During April 2021 the FDA confirmed that they would recommence their review of the PMA application and this is now ongoing and in-progress. In December 2021 the FDA’s Bio-monitoring research division conducted an audit of the clinical program submitted in support of the PMA application. A single 483 observation was raised, and the Company submitted a full response in January 2022. The FDA subsequently scheduled a pre-market inspection for during the second calendar quarter of 2022, intended to cover the FDA’s Quality System/Current Good Manufacturing Practice regulations for Medical Devices (21 CFR Part 820). This audit was conducted in the first quarter of the fiscal year ending March 31, 2023. The Company reported that a single 483 observation was raised to which the Company responded in a timely manner, and Dialogue with the FDA continues with respect to the PMA application.
In addition to this, Nemaura established that proBEAT™, which is based on the sugarBEAT® platform, can be classified under the Wellness guidance when it is used according to the FDA Wellness guidance notes, to provide prompts and educate users on factors affecting their blood sugar profiles. Nemaura launched proBEAT™ in the U.S. in December 2020, as part of a diabetes prevention and reversal program branded BEATdiabetes.life, in the form of pilot studies. During the quarter ended December 31, 2020, Nemaura licensed a clinically validated weight loss program for the management of diabetes from Healthimation, LLC, which was originally developed at the Joslin Diabetes Center, an affiliate of Harvard Medical School. This program, together with proBEAT™, originally formed the basis of the BEATdiabetes.life program that is currently being developed for commercialization in the U.S. The program is under further refinement based on feedback gathered to date. Further KOL studies are planned to provide additional marketing support of the program in preparation for a broader U.S.-wide roll-out. While still in the relatively early stages, we are pleased with initial results and feedback received from these user-groups and one key outcome has been that the company is now looking to provide proBEAT as a standalone plug-in for existing diabetes management programs therefore potentially allowing the company to accelerate commercialization as well as reduce the cost burden that could be associated with running its own program.
We believe there are additional applications for sugarBEAT® and the underlying BEAT technology platform, which may include:
| Engaged with external third-party manufacturers with the ability to provide significant scale up services for product manufacture moving forward. Specifically, this quarter the company has engaged with a |
During this period of product development, the Company has experienced recurring losses and negative cash flows from operations. As of December 31, 2022, the Company had cash balances of $7,340,840, a working capital deficit of ($2,309,666), an accumulated deficit of $47,192,364 and a deficiency in total stockholders' equity of $9,858,709.
While the Company expects to continue to incur losses from operations for the near-term and these losses could be significant as product development, regulatory activities, clinical trials, and other commercial and product development related expenses are incurred, the Company reached a significant milestone during the three month period ended December 31, 2021, as the Company commenced commercial delivery of its sugarBEAT® device to its UK Licensee, allowing the UK Licensee to continue studies dedicated to developing user based feedback and evidence that could potentially support reimbursement in the UK .
Management's strategic assessment continues to include the following potential options:
Recent Developments
On September 24, 2021,October 3rd 2023 the Company entered intoallowed its FDA PMA application to lapse in favor of submitting a License, Supplyrevised application based on a 24-hour sensor life in place of the current 14-hour sensor life, in particular in light of improvements that had been made to sensor performance and Distribution Agreementmanufacture which out-date the original application. The Company has selected the Modular route for this submission.
In a traditional PMA, the applicant submits all PMA data, as outlined in 21 CFR 814.20, at the same time, regardless of when testing is completed. FDA begins its review only upon receipt of all the required information. In 1998, however, as part of CDRH’s reengineering effort, FDA issued the above mentioned guidance. In these documents, FDA described a new policy whereby applicants could submit “Modular PMAs.” The goal of FDA’s 1998 guidance was to increase the efficiency of the PMA review process by allowing applicants to submit discrete sections (modules) of the PMA to FDA for review soon after completing the testing and analysis.
Guidance notes were revised on November 3, 2023 (https://www.fda.gov/files/medical%20devices/published/Premarket-Approval-Application-Modular-Review---Guidance-for-Industry-and-FDA-Staff.pdf).
In accordance with ‘MySugarWatch DuoPack Limited’ (“MSW-DP”),the guidelines Nemaura submitted its Proposal on October 30, 2023 and has now commenced the process of compiling the dossier for staged submission over the coming months.
Furthermore, the Company reported that it completed a sister company100 patient study, collecting over 30,000 glucose measurements from the sugarBEAT device paired with venous blood samples over an extended duration of MSW, whereby MSW-DP will provide CGM sensors free24 hours and reported interim data suggesting that 24 hour in-use sensor life was viable.
Affiliated Company Relationships
Nemaura Pharma was incorporated in November 2005. Through October 2013, all technology development and related transactions were incurred by Pharma. As new technology platforms were invented and developed, additional companies were set up to contain these new technology platforms, and to aid in the process of charge with certain medications that are widely prescribedraising further investments to persons with Type 2 diabetes. These medications areprogress the development of these subsequent technologies. However, due to come off patent in the fourth calendar quartersmall size of 2022 in Europethe operations, low number of employees and laboratory and office space required, initially, certain costs were borne by Pharma and charged to DDL as required. On April 4, 2018, a service agreement was put into place between Pharma and DDL which covered the UK,development of sugarBEAT® under Pharma’s ISO13485 Accreditation. In lieu of these services, Pharma invoices DDL on a periodic basis for said services. Services are provided at cost plus a service surcharge amounting to less than 10% of the total costs incurred. This agreement includes all aspects of the development, registration and 2023 inmanufacture of sugarBEAT®.
Full legal title and beneficial ownership of the U.S. The agreed sale price of sensors to MSW-DPCE mark and all related intellectual property remains with Nemaura Medical under the terms of the agreement is $20 per box of 5 sensors forservice contract.
Dr. D.F.H. Chowdhury, the U.S. market,Company’s Chief Executive Officer, President and in Europe and the UK 12.50 Euros in the first 12 months from product launch and 10 Euros thereafter per box of 5 sensors. Nemaura’s anticipated cost of goods per sensor on large-scale production is $1 per sensor once economies of scale are achieved. As of January 2022, there were over 2 million prescriptions written for these medications each month in the combined key EU and UK territories. The Company believes this will provide an opportunity for rapid market penetration in the use of its CGM sensors, at a scale that can enable the targeted lower cost of goods to be achieved and thereby support both revenue and margin growth into the future.
Management is now focused on fulfilling the UK licensees’ initial orders based on MSW’s UK launch plans, which is gradually evolving with a clear focus on aiming to generate data to support product reimbursement, while also developing the capabilitiesChairman of the Company to developBoard, and service new channels of business across other geographic markets via the use of our BEAT platform. This includes expansion of the consumer metabolic health offering Miboko, for which the beta registrations was launched in late 2021, to employers and insurers across the U.S.
ATM Offering
In July 2021, the Company entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (the “Agent”) pursuant to which the Company may offer and sell from time to time to or through the Agent sharesMr. Bashir Timol, a member of the Company’s common stock. On April 1, 2022,Board of Directors, are officers of Pharma. The current management at DDL, including Dr. D. F. H. Chowdhury allocate 15% - 20% of their time to oversee the Companycurrent operations at Pharma and Agent entered intowill in due course implement a new management team in Pharma, and provide ongoing support in an amendment (the “Amendment”)advisory role. Pharma is a drug delivery company, which means that its activities are entirely related to the ATM Agreement, pursuant to which the parties agreed to expand the meaningadministration of the defined term “Registration Statement” in the ATM Agreement to include, for the period from April 1, 2022 and thereafter, a new shelf registration statement (File Number 333-263618) on Form S-3 (“New Registration Statement”) that was filed on March 16, 2022 with the SEC and declared effective by the SEC on March 28, 2022. No other changesdrugs to the ATM Agreement werebody of a human or animal subject. DDL is a diagnostic company, which means it is entirely focused on extracting molecules from the human or animal subject and analyzing it to make a diagnosis or to monitor the level of a particular molecule such as glucose. These are two independent businesses engaged in different activities, therefore there is no conflict of interest between the two and management does not see any conflicts arising from the allocations of some of DDL management time to overseeing the operations of Pharma.
Payments made by the Amendment.
The offersolely for work that Dr. D. F. H. Chowdhury performs for Pharma in his capacity as manager are not charged to Nemaura Medical Inc. and sale of shares of common stock through the Agent will be made pursuant to the New Registration Statement, and a related prospectus supplement filed with the SEC pursuant to which the Company is offering shares of its common stock having an aggregate offering price of up to $3,000,000.
Termination of Chief Financial Officer
Effective July 1, 2022, the Company terminated its Company’s Chief Financial Officer and has commenced a search for a U.S. based replacement. Until a replacement has been selected, the Company’s President and Chief Executive Officer will act as principal financial and accounting officer of the Company, and the Company’s finance team will continue to support the Company with respect to its accounting and financial reporting compliance requirements. The Company is currently reviewing a large number of applicants for the role, advertised recently in the US.
Preliminary agreement with EVERSANA
On September 27, 2022, the Company entered into a preliminary agreement with EVERSANA to collaborate on the launch strategy of the Company’s BEATdiabetes program.
Amendment of Uptown Capital Secured Promissory Note
On February 8, 2021, the Company, Dermal Diagnostics Limited, a wholly owned subsidiary of the Company (“Dermal Diagnostics”), and Trial Clinic Limited, a wholly owned subsidiary of the Company (“Trial Clinic” and collectively with the Company and Dermal Diagnostics) issued to Uptown Capital, LLC (“Uptown”) a secured promissory note (the “Uptown Note”) in the original principal amount of $24,015,000. The Uptown Note carried an original issue discount of $4,000,000. In addition, the Company agreed to pay $15,000 to Uptown to cover Uptown’s legal fees, accounting costs, due diligence, monitoring and other transaction costs incurred in connection with the purchase and sale of the Uptown Note, all of which amount wasare not included in the initial principal balance of the Uptown Note. The purchase price of the Uptown Note, therefore, was $20,000,000. The original maturity date of the Uptown Note was 24 months after the date the purchase price for the Uptown Note was delivered.our consolidated financial statements.
Inflation
The Company does not believe that inflation has had a material effect on its operations to date, other than its impact on the general economy. However, there is a risk that the Company’s operating costs could become subject to inflationary and Uptown previously agreedinterest rate pressures in May 2022 to reduce the maximum monthly redemption amount from $2,000,000 to $500,000 from June 2022 to February 2023,future, which reduction remainswould have the effect of increasing the Company’s operating costs (including, specifically, clinical trial costs in force. Pursuant to the terms of the Amendment,countries where the Company is applying to sell its products), and Uptown agreed to reducewhich would put additional stress on the maximum monthly redemption amount duringCompany’s working capital resources.
Nasdaq Compliance Deficiencies
As previously disclosed, on April 3, 2023, the Company received a written notice from the Nasdaq Listing Qualification Department (the “Nasdaq Staff”) indicating that the Company was not in compliance with the $35 million minimum market value of listed securities (“MVLS”) requirement set forth in Nasdaq Listing Rule 5550(b)(2) for continued listing on The Nasdaq Capital Market. Accordingly, the Company was granted a grace period beginning Marchthat expired on October 2, 2023. In addition, on April 6, 2023, until the Uptown Note is paidCompany received a written notice that the Company was not in full from $2,000,000 to $1,000,000; provided, however,compliance with the $1 bid price (“Bid Price”) requirement for continued listing set forth in Listing Rule 5550(a)(2) and was granted a grace period that upon the occurrence of an event of default under the Uptown Note, the maximum monthly redemption amount will automatically be increased back to $2,000,000.expired on October 3, 2023.
On October 21, 2022,3 and 4, 2023, respectively, the Company entered into an amendment to Secured Promissory Note, dated as of October 21, 2022, by and amongreceived written notices from the Nasdaq Staff indicating that the Company Dermal Diagnostics, Trial Clinichad not regained compliance with the MVLS and Uptown. PursuantBid Price requirements, and that the Company’s common stock would be subject to delisting from The Nasdaq Capital Market unless the Company timely requests a hearing before a Nasdaq Hearings Panel (the “Panel”).
Accordingly, the Company timely requested a hearing before the Panel. The hearing request automatically stayed any suspension or delisting action pending the hearing and the expiration of any additional extension period granted by the Panel following the hearing. In that regard, pursuant to the termsNasdaq Listing Rules, the Panel granted an extension not to exceed April 1, 2024. However following further attrition to its share price, and the need for substantial dilution to existing shareholders as part of the amendment, the Company and Uptown agreedcompanies plan to extend the maturity date of the Uptown Note to July 1, 2024. In consideration thereof, the Company agreed to pay to Uptown an extension feeregain compliance with Nasdaq rules, management did not see it in the amountbest interest of 5% ofshareholders to effect such dilution and chose to de-list the outstanding balance ofcompany from the Uptown Note which results in $813,834 being added onto the liability due to Uptown.
Restatements
On February 17, 2023, the management and the Audit Committee of the Board of Directors of the Company concluded that the following financial statements should be restated and should no longer be relied upon:
The following errors impacted the Filings: (i) not translating correctly the foreign currency balance for a mark-to-market contract; and (ii) not including certain debt issuance costs in the computation of the effective interest rate for a loan note.
The Company determined that the reporting effects of the above errors had a material impactNasdaq to the Company’s unaudited condensed consolidated financial statementsOver The Counter market, with a view to strengthening the commercialization roadmap and partnering as a means of growing the Company for the three months ended June 30, 2022, as reported in the Q1 2022 10-Q,company and for the three and six months ended September 30, 2022, as reported in the Q2 2022 10-Q. As a result, the unaudited condensed consolidated financial statements for the three months ended June 30, 2022 and the unaudited condensed consolidated financial statements for the three and six months ended September 30, 2022 have been restated, and the Company has filed an amendment to each of the Q1 2022 10-Q and the Q2 2022 10-Q with the SEC.
The errors had the effects as shown below:
The errors do not impact the Company’s previously reported sales, gross profit, or cash positions in any period.
The Company’s management concluded that in light of the errors mentioned above, a material weakness existed in the Company’s internal control over financial reporting related to accounting for debt issuance costs using the effective interest rate method and translating the mark-to-market liability as of June 30, 2022 and September 30, 2022, and the Company’s disclosure controls and procedures were not effective as of such dates. The Company’s remediation plan with respect to such material weakness has been described in more detail in the amendment filed to each of the Q1 2022 10-Q and the Q2 2022 10-Q on February 23, 2023. These errors relate to the material weakness as previously identified and detailed in the Q1 2022 10-Q and the Q2 2022 10-Q related to lack of financial expertise over complex transactions and lack of resources to review out of the ordinary transactions and arrangements of the Company.
generating revenues.
COVID-19 Pandemic
The outbreak of COVID-19 in December 2019 has since rapidly increased its exposure globally. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. We continue to monitor the impact of COVID-19 on our own operations and are working with our employees, suppliers, and other stakeholders to mitigate the risks posed by its spread, but COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers have not always been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities and respond favorably to the challenges faced during this period. We also recognize that one of the consequences of this pandemic has been a surge in the uptake of technologies for remote monitoring of patients and patient self-monitoring, which potentially enhances the prospects for the Company, its CGM product and its planned digital healthcare offering.
Results of Operations
Comparative Results for the NineThree Months Ended December 31, 20222023 and 20212022
Revenue
There was no revenue generated in the three month period ended December 31,2023.
The Company recognized revenuegenerated revenue of $77,044$3,017 in the ninethree month period ended December 31, 2022, relating to deliveries of sugarBEAT®sugarBEAT® to MSW pursuant to the initial order placed in April 2021. A portion also related to the recognition of the GBP 1 million (approximately $1.20$1.12 million), that was previously received and held within deferred revenue relating to the exclusive Marketing Rights Agreement that was signed with MSW.
Revenue recognized in the comparative nine month period ended December 30, 2021 was $183,628 relating to the delivery of goods and including a small portion of the recognition of the deferred revenue relating to the exclusive Marketing Rights Agreement that was signed with MSW.
Research and Development Expenses
Research and development (“R&D”) expenses were $980,862$291,104 and $987,711$393,747 for the ninethree months ended December 31, 20222023 and 2021,2022, respectively. This amount consisted primarily of expenditures on wages and sub-contractor activities incurred for improvements made to the sugarBEAT® device.
General and Administrative Expenses
General and administrative expenses were $4,329,306$1,250,149 and $4,151,380$1,230,160 for the ninethree months ended December 31, 20222023 and 2021,2022, respectively. These expenses consisted of fees for legal, professional, consultancy, audit services, investor relations, insurance, advertising and general and operational wages. Non-cash charges of $635,494 and $199,522 were recorded in the nine-month period ended December 31, 2022 and 2021 as a result of the mark-to-market adjustments on the Company’s outstanding foreign currency forward contracts.
As the Company continues to scale up to service its existing order book, it is expected that general and administrative expenses will continue to increase in a similar way moving forward, as the business transitions to a more operational focused base that will encompass an increase in functional expenses relating to production, sales, marketing, customer service, as well as enhancements to other existing functions.
Other Income (Expense)
Other income (expense) was $302,161 and $92,417 for the three months ended December 31, 2023 and 2022, respectively. These expenses consist of interest expense, change in fair value of foreign exchange and change in fair value of warrant liability. There was a significant decrease in the fair value of the Company’s warrant liability which resulted in a gain of approximately $1 million as a result of the decrease in the stock price of the Company, which is an input in the fair value computation every reporting period.
Other Comprehensive Loss
For the nine monthsthree month periods ended December 31, 20222023 and 2021,2022 other comprehensive loss was $864,328income saw gains of $204,829 and $142,922, respectively. Currently all transactions recorded through other comprehensive loss arise$556,080, respectively, arising from fluctuations in the USD:GBP exchange rate and the impact that this has on consolidation of the Company’s non-USD denominated assets and liabilities.foreign currency translation adjustments.
Comparative Results for the ThreeNine Months Ended December 31, 20222023 and 20212022
Revenue
Revenue of $3,017There was recognizedno revenue generated in the threenine month period ended December 31, 2023.
The Company generated revenue of $77,044 in the nine month period ended December 31, 2022, relating to deliveries of sugarBEAT®sugarBEAT® to MSW pursuant to the initial order placed in April 2021. A portion also related to the recognition of the GBP 1 million (approximately $1.12 million), that was previously received and held within deferred revenue relating to the exclusive Marketing Rights Agreement that was signed with MSW.
The comparative revenue recognized in the three month period ended December 31, 2021 was $183,628.
Research and Development Expenses
R&D expenses were $393,747$1,332,664 and $412,341$980,862 for the three month periodsnine months ended December 31, 20222023 and 2021,2022, respectively. This continues to be largely composedamount consisted primarily of expenditures on wages and sub-contractor activities incurred in finalizingfor improvements made to the product design for the sugarBEAT®device, in order to enable scaling of the production ability combined withand costs associated with new pipeline products as they move through their respective development phases.the 24-hour sensor study performed on 100 subjects in the Middle East.
General and Administrative Expenses
General and administrative expenses were $239,628$4,317,358 and $1,391,278$1,509,095 for the three month periodsnine months ended December 31, 2023 and 2022, and 2021, respectively. Given the nature of the Company’s activities has remained unchanged, the cost drivers in this area have also remained consistent and are largely representativeThese expenses consisted of fees for legal, professional, consultancy, audit services, investor relations, insurance, advertising and wages. Non-cash credits of $990,531general and $70,878 were recorded for the mark-to-market adjustments on the Company’s outstanding foreign currency forward contracts.operational wages.
We anticipateAs the Company continues to scale up to service its existing order book, it is expected that general and administrative expenses will continue to increase in a similar way moving forward, as the business transitions to a more operational focused base that will encompass an increase in functionsfunctional expenses associated withrelating to production, sales, marketing, customer service, as well as enhancements to other existing functions.
Other Income (Expense)
Other Expense was ($318,064) and ($6,972,648) for the nine months ended December 31, 2023 and 2022, respectively. These expenses consist of interest expense, change in fair value of foreign exchange and change in fair value of warrant liability. There was a significant decrease in the fair value of the Company’s warrant liability which resulted in a gain of approximately $2.6 million as a result of the decrease in the stock price of the Company, which is an input in the fair value computation every reporting period.
Other Comprehensive Loss
For the three monthsnine month periods ended December 31, 20222023 and 2021,2022 other comprehensive income was $556,080losses of $44,326 and ($25,065) loss, respectively. Currently all transactions recorded through other comprehensive loss ariselosses of $864,328, respectively, arising from fluctuations in the USD:GBP exchange rate and the impact that this has on consolidation of the Company’s non-USD denominated assets and liabilities.foreign currency translation adjustments.
| 16 |
Liquidity and Capital Resources
We have experiencedAs reflected in the accompanying unaudited financial statements, for the nine months ended December 31, 2023, the Company recorded a net lossesloss of $5,968,086 and negativeused cash flows fromin operations since our inception. Weof $7,203,676. In addition, we have sustained cumulative losses of $47,192,364 through$57,843,297 as of December 31, 2022.2023. We have historically financed our operations through a combination of debt and equity funding.
As of December 31, 2022,2023, the Company had a deficiency in net working capital deficiency of $2,309,666,$18,623,341, which included total cash balances of $7,340,840. $137,416 and current notes payable of $19,643,038.
Going Concern
The accompanying unaudited financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. As reflected in the accompanying financial statements, for the nine months ended December 31, 2023, the Company reportedrecorded a net loss for the threeof $5,968,086 and nine month periods ended December 31, 2022used cash in operations of $1,716,278 and $9,460,888, respectively. This loss is after taking account of interest and debt discount charges arising from the note purchase agreements for the three- and nine- month periods ended December 31, 2022 of $1,082,949and $4,152,437, respectively.
Having reviewed$7,203,676. These factors raise substantial doubt about the Company’s forward looking cashflow requirements in relationability to the cash balance held at December 31, 2022, management is awarecontinue as a going concern within one year of the needdate that the financial statements are issued. In addition, the Company’s independent registered public accounting firm in its report on the Company’s March 31, 2023 financial statements, raised substantial doubt about the Company’s ability to raise additional funds in ordercontinue as a going concern. The financial statements do not include any adjustments that might be necessary if the Company is unable to finance the ongoing commercialization of sugarBEAT®. The Company had $7,340,840 of cash at December 31, 2022. The Company has debt on its balance sheet which will reach maturity in July 2024, followingcontinue as a restructure in October 2022.going concern.
In evaluating the going concern position of the Company,company, management has considered the ability of the Company to raise additional funding in combination with one or more of the different funding options available to it at this time. Based on current and ongoing engagement with potential funding providers managementand believes that there is a reasonable expectation that fundingfinancing to fund future operations could be provided by one, equity and/or more, of the following options:
Equity funding – the Company has immediate access to funds through the ATM facility that is currently in place; in addition to this, there are various alternative mechanisms available to the Company similar to those used previously e.g. direct sale of shares to interested third parties, as well as other mechanisms to sell common stock via an underwritten agreement or the further exercise of warrants by the current warrant holders etc. The Company completed a Registered Direct Offering and concurrent Private Placement in January 2023 which has increased cash by $7,655,974.
Debt funding – the Company continues to be in ongoing discussions with third party debt providers, including the incumbent, to enable the existing debt facility to be restructured or renewed, should management feel that this route offers a more attractive option compared to the sale of equity that is dependent on the current market conditions.
Alternative funding as used in the past such as the sale of licenses. As product development is now at a significant more advanced stage then it was, it is management’s belief that the sufficient funding could be provided through the sale of licenses or a large-scale partnership that could bring in additional funds and infrastructure to support the commercial growth ambitions of the company.
financing. There can be no assurance that any such equity, debt or alternative funding willwould be available, or that the terms of such funding would be on favorable terms acceptableif available. Even if the Company is able to obtain additional financing, it may contain undue restrictions on our operations, in the case of debt financing, or cause substantial dilution for our stockholders, in the case of equity financing.
Cash Flows
Net cash used in operating activities for the nine months ended December 31, 2023 was $7,203,676, reflecting a net loss of $5,968,086, and includes accretion of debt discount expense and accrued interest totaling $2,291,148, change in fair value of warrant liability of $2,600,000, the mark-to-market charge booked in relation to the Company, or at all. Asrevaluation of the foreign currency forward contracts of $489,435 and the depreciation and amortization charge of $309,684.
Cash was also impacted by increases in inventory of $2,021,130, which was directly driven as a consequenceresult of this funding requirement being triggered withoutcommercial scale up.
Prepayments dropped by $1,113,851, which was a result of the funding bridge having been putdecrease in placedeposit to Hamilton Court, our forward contract provider, plus by reduction on other prepayments.
There was a $25,842 increase in accounts payable during the nine months ended December 31, 2023 as well as an increase in other liabilities and accrued expenses of $150,377. The related party payable balance increased to $120,378 as of December 31, 2023.
Net cash used in investing activities for the nine months ended December 31, 2023, was $76,807, which was from the purchase of property and equipment driven by the filing date of these unaudited condensed consolidated financial statements, ASC 205-40 requires that management recognize and disclose this point as an event which creates a substantial doubt asprocurement to support the Company’s abilitytransition to continue as a going concern for at least one year from the date of filing of these unaudited condensed consolidated financial statements.
Cash Flowsoperational production.
Net cash used in operating activities for the nine months ended December 31, 2022 was $6,090,181, reflecting a net loss of $9,460,888, adjusted for the add back of thewhich includes accretion of debt discount expense ofand accrued interest totaling $4,152,437, the mark-to-market charge booked in relation to the revaluation of the foreign currency forward contracts of $635,494 and the depreciation and amortization charge of $268,595.
Cash was also impacted by increases in inventory of $864,636 as of December 31, 2022, which was directly driven as a result of commercial scale up.
Prepayments increaseddecreased by $467,070, which was a result of an increasethe amounts in amount paid to Hamilton Court, (approximately $876,000), our forward contract provider partially offset by savingsplus movements on other prepayments, including a reduction in prepaid insurance.prepayments.
There was a $34,897 increase in accounts payable during the nine months ended December 31, 2022 withbut decreases seen in other liabilities and accrued expenses of $167,568 and deferred revenue of $297,419. The related party payable balance increased by $75,977.
Net cash used$75,977 in operating activities for the nine months ended December 31, 2021 was $4,353,494, reflecting a net loss of $10,269,557, adjusted for the add back of the non-cash amortization of debt discount expense of $5,141,701, the mark-to-market charge recorded in relation to the foreign currency forward contracts of $199,522 and the depreciation and amortization charge of $139,751. Cash was also impacted by increases in inventory of $533,656, which was directly driven as a result of the commercial scale up.
Prepayments increased by $797,155, which was also a direct result of commercial scale up with prepayments for raw materials being pre-purchased to ensure that inventory would be on-hand to support the Company’s shift to commercial operations. The decrease was partly offset by an increase in accounts receivable – related party, of $301,387, which relates to additional advance purchasing of inventory that is completed through a related party company.
There was also a reduction in accounts payable of $77,075 with increases seen in both other liabilities and accrued expenses and deferred revenue.2023.
Net cash used in investing activities for the nine months ended December 31, 2022, was $438,805, which included patent filing costs of $135,168 and the purchase of property and equipment of $275,758($208,945) driven by the procurement to support the transition to operational production.production, and patent filing costs of $144,343.
Net cash used in investingfinancing activities was $880,008 for the nine months ended December 31, 2021, which reflected patent filing costs2023 was $2,918,086, for the scheduled re-payment of $60,241, the purchasenotes payable of property and equipment of $359,301 driven$9,418,086 offset by the procurement of cleanroom facilities and injection molding tooling to support the operational production steps taken$6,500,000 proceeds from notes payable issued in advance of product delivery to the UK licensee. In addition to this, $460,466 was invested in software development costs relating to the digital health program in the US and recent Beta launch of our consumer health program, Miboko, in the UK.August 2023.
Net cash used in financing activities for the nine months ended December 31, 2022 was $3,273,859, comprised ofcomprising $4,700,000 from proceeds from the issuance of notes payable,long term debt offset by $7,974,282 for the repayments of notes payable.
Net cash used in financing activities for the nine months ended December 31, 2021 was $3,421,933, comprised of $2,963,658 of proceeds from the exercise of warrants, $114,409 of proceeds (net of costs) from the sale of common stock through the ATM facility, offset by $6,500,000 for the scheduled repayments of notes payable.payable, also including proceeds of $696 from issuance of common stock offset by $273 for equity issuance cost paid.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies and Estimates
When we prepare our unaudited condensed consolidated financial statements and accompanying notes in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”), we must make estimates and assumptions about future events that affect the amounts we report. Certain of these estimates result from judgements that can be subjective and complex. As a result of that subjectivity and complexity, and because we continuously evaluate these estimates and assumptions based on a variety of factors, actual results could materially differ from our estimates and assumptions if changes in one or more factors require us to make accounting adjustments. We believe our critical accounting policies affect our more significant judgments and estimates used in the preparation of the unaudited condensed consolidated financial statements. For further discussion of our critical accounting policies, see Note 2.
During the three and nine month periodsperiod ended December 31, 2022,2023, we have made no material changes or additions with regard to such policies and estimates.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Dr. Dewan F.H. Chowdhury, our Chief Executive Officer and Interim Chief Financial Officer, has evaluated the effectiveness of ourWe have established disclosure controls and procedures as ofto ensure that the end ofinformation required to be disclosed by the period covered by this Quarterly Report on Form 10-Q. Based on this evaluation as of December 31, 2022,Company in the Company’s Chief Executive Officer and interim Chief Financial Officer has concludedreports that as of December 31, 2022, the Company’s disclosure controls and procedures (as defined in Rules 13a-15it files or submits under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission and that such information is accumulated and communicated to the officers who certify the Company's financial reports and to other members of senior management and the Board of Directors as appropriate to allow timely decisions regarding required disclosure.
The Company’s Chief Executive Officer / Chief Financial Officer has evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2023. Based on his evaluation, the Chief Executive Officer / Chief Financial Officer have concluded that as of December 31, 2023 our disclosure controls and procedures were not effective due to aas of December 31, 2023. As of December 31, 2023, management’s assessment identified the following material weaknessweaknesses in the Company’s internal control over financial reporting.reporting:
As of December 31, 2022,We continue to have a material weakness in our management, with the participation of our Chief Executive Officer, who is also serving as our Interim Chief Financial Officer, evaluated our internal control over financial reporting. As a result of our assessment, management identified the following material weaknesses in internal control over financial reporting as disclosed in the March 31, 2023 Form 10-K, in that the Company did not design and maintain effective controls over (i) accounting for the foreign currency balance for a mark-to-market contract; and (ii) accounting for certain debt issuance costs in the computation of December 31, 2022:
Management has identified that there isthe effective interest rate for a loan note mainly due to lack of adequate financial expertise relatedtechnical expertise. Management is developing and implementing remediation plans to the assessment of complex transactions and a lack of adequate resources to review out of the ordinary transactions and arrangements of the Company, which created a deficiency in the design and implementation of our review control. Due to the deficiency in our design and implementation of our review control, material errors in the financial statements may not be identified as part of the review process. Based on these factors, we concluded that the deficiency rises to the level of a material weakness.
Remediation of Material Weaknesses
We are in the process of implementing improvements and remedial measures in response toaddress the material weakness. We are currently in the process of hiring a replacement CFO with US public company experience and technical expertise. In addition we are engaging with additional consultants with U.S. reporting expertise. This weakness has yet to be remediated. weaknesses.
Changes in Internal Control overOver Financial Reporting
There have been no changes in the Company’sour internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15 under the Exchange Act during the fiscal quarterour most recent nine months ended December 31, 20222023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
None.
ITEM 1A. RISK FACTORS
Except as set forth below, thereThere have been no material changes to the risk factors disclosed in the Company’s Annual Report on Form 10-K for the year ended March 31, 2022, filed with the SEC on June 29, 2022,2023, as the same may be updated from time to time.amended.
The following risk factors are in addition to the risk factors disclosed in the Company’s Annual Report on Form 10-K for the year ended March 31, 2022, filed with the SEC on June 29, 2022, as the same may be updated from time to time:
The restatement of certain of our financial statements may subject us to risks and uncertainties, including the increased possibility of legal proceedings.
On February 17, 2023, the management and the Audit Committee of the Company’s Board of Directors concluded that the following financial statements should be restated and should no longer be relied upon:
The following errors impacted the Filings: (i) not translating correctly the foreign currency balance for a mark-to-market contract; and (ii) not including certain debt issuance costs in the computation of the effective interest rate for a loan note.
The Company determined that the reporting effects of the above errors had a material impact to the Company’s unaudited condensed consolidated financial statements of the Company for the three months ended June 30, 2022, as reported in the Q1 2022 10-Q, and for the three and six months ended September 30, 2022, as reported in the Q2 2022 10-Q. As a result, the Company determined that the unaudited condensed consolidated financial statements for the three months ended June 30, 2022 and the unaudited condensed consolidated financial statements for the three and six months ended September 30, 2022 should be restated, and the Company should file an amendment to the Q1 2022 10-Q and the Q2 2022 10-Q with the SEC.
As a result of the restatements, we may become subject to additional risks and uncertainties, including, among others, the increased possibility of legal proceedings or a review by the SEC and other regulatory bodies. The costs of defending against such legal proceedings or administrative actions could be significant. In addition, we could face monetary judgments, penalties or other sanctions that could have a material adverse effect on our business, financial condition and operating results. In addition, the restatements:
The occurrence or continued occurrence of any of the foregoing could have a material adverse effect on our business, results of operations and financial condition.
We have identified a material weakness in our internal control over financial reporting which could, if not remediated, adversely impact the reliability of our financial statements, result in material misstatements in our financial statements and cause current and potential stockholders to lose confidence in our financial reporting, which in turn could adversely affect the trading price of our common stock.
As discussed in the Explanatory Note to this Amendment No. 1 and Note 9-Restatement, included in the interim financial statements, the Company has restated certain information contained in its previously issued unaudited interim condensed financial statements for the three month period ended June 30, 2022 and for the three and six months ended September 30, 2022.
We have concluded that there is a material weakness in our internal control over financial reporting. For additional information on the material weakness identified and our remedial efforts, see “Item 9A, Controls and Procedures.” The material weakness resulted in the restatement of certain of our financial statements and related disclosures, as discussed in the Explanatory Note to this Amendment No. 1 and Note 9-Restatement. Thus, management has determined that our disclosure controls and procedures and internal control over financial reporting were not effective as of June 30, 2022, September 30, 2022 and December 31, 2022. Under Public Company Accounting Oversight Board standards, a material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a misstatement of our consolidated annual or interim financial statements will not be prevented or detected on a timely basis. The existence of this issue could adversely affect us, our reputation or investor perceptions of us. We will take measures to remediate the underlying cause of the material weakness noted above. As we continue to evaluate and work to remediate the material weakness, we may determine to take additional measures to address the control deficiencies.
Although we plan to complete this remediation process as quickly as possible, we cannot provide any assurance as to when the remediation process will be complete, and our measures may not prove to be successful in remediating the material weakness. If our remedial measures are insufficient to address the material weakness, or if additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain misstatements and we could be required to restate our financial results. In addition, if we are unable to successfully remediate the material weakness or if we are unable to produce accurate consolidated financial statements in the future, our stock price, liquidity and access to the capital markets may be adversely affected and we may be unable to maintain compliance with applicable stock exchange listing requirements and debt covenant requirements. Further, because of its inherent limitations, even our remediated and effective internal control over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in our conditions, or that the degree of compliance with our policies or procedures may deteriorate.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
| (a) | None |
None
| (b) | There have been no material changes to the procedures by which security holders may recommend nominees to the Company’s Board of Directors since the Company last provided disclosure in response to the requirements of Item 407(c)(3) of Regulation S-K. |
| (c) | During the quarter ended December 31, 2023, no director or officer of the Company adopted or terminated a contract, instruction or written plan for the purchase or sale of securities of the Company intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) and/or a non-Rule 10b5-1 trading arrangement. |
| 19 |
ITEM 6. EXHIBITS
The exhibits listed on the Exhibit Index below are filed as part of this report.
| Exhibit No. | Document Description |
| 31.1* | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2* | Certification of the Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1** | Certification of the Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 101.INS* | XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH* | Inline XBRL Taxonomy Extension Schema |
| 101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase |
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Document |
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase |
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase |
| 104* | Cover Page Interactive Data File – the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
* Filed herewith.
** Furnished herewith.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| NEMAURA MEDICAL INC. | ||
| Date: February | By: | /s/ Dewan F.H. Chowdhury |
Dewan F.H. Chowdhury Chief Executive Officer, Interim Chief Financial Officer, and President (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | ||