SECURITIES AND EXCHANGE COMMISSION
____________________________
_____________________________
(Mark One)
| ☒
| | | | |
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2017March 31, 2024
Or
| ☐
| | | | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-37524
_____________________________
(Exact name of registrant as specified in its charter)
_____________________________
| | | | | |
| Delaware | | 47-3916571 |
(State or other jurisdiction of
incorporation or organization)
| | (I.R.S. Employer
Identification No.)
|
| | |
4170 Mendenhall Oaks Pkwy
3980 Premier Dr, Suite 310 High Point, NC | | 27265 |
(Address of principal executive offices) | | (Zip Code) |
(Registrant’s telephone number, including area code)
(Former name, former address and former fiscal year, if changed since last report)
_____________________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Class A common stock, par value $0.01 per share | VTVT | NASDAQ Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒x No ☐o Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒x No ☐o Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer,
or a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated
filer”filer,” “smaller reporting company,” and
“smaller reporting“emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ☐
o | | Accelerated filer | ☐
o |
| | | |
Non-accelerated filer | ☒
o | | Smaller reporting company | ☐
x |
| | | |
Emerging growth company | ☒
o | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒o Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐o No ☒x | | | | | | | | |
| Class of Stock | | Shares Outstanding as of November 1, 2017 | May 9, 2024 |
Class A common stock, par value $0.01 per share | | 2,432,857 | | 9,693,254
| |
Class B common stock, par value $0.01 per share | | 577,349 | | 23,119,246
| |
vTv THERAPEUTICS INC. AND SUBSIDIARIES
FOR THE QUARTER
ENDED September 30, 2017MARCH 31, 2024 | | | | | | | | |
| | PAGE
NUMBER |
| | | |
| | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | 18
|
| | | | |
| | | | 27
|
| | | | |
| | | | 28
|
| | | |
| | |
| | | | |
| | | | 28
|
| | | | |
| | | | 28
|
| | | | |
| | | | 30
|
| | | | |
| | | | 30
|
| | | | |
| | | | 30
|
| | | | |
| | | | 30
|
| | |
| | | | |
| Item 6.
| | Exhibits
| 31
|
| | | | |
| | | | 32
|
PART I –
FINANCIALFINANCIAL INFORMATIONThe financial statements and other disclosures contained in this report include those of vTv Therapeutics Inc. (“we”, the “Company” or the “Registrant”), which is the registrant, and those of vTv Therapeutics LLC (“vTv LLC”), which is the principal operating subsidiary of the Registrant. Unless the context suggests otherwise, references in this Quarterly Report on Form 10-Q to the “Company”, “we”, “us” and “our” refer to vTv Therapeutics Inc. and its consolidated subsidiaries.
Condensed Consolidated Balance Sheets
(in thousands, except number of shares and per share data)
| September 30, | | | December 31, | |
| 2017 | | | 2016 | |
| (Unaudited) | | | | | |
| March 31,
2024 | | | March 31,
2024 | | December 31,
2023 |
| (Unaudited) | |
| Assets | |
| Assets | |
Assets | | | | | | | |
Current assets: | | | | | | | |
| Current assets: | |
| Current assets: | |
Cash and cash equivalents | $ | 20,488 | | | $ | 51,505 | |
Restricted cash | | 281 | | | | — | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Accounts receivable | |
| Prepaid expenses and other current assets | | 725 | | | | 612 | |
| Prepaid expenses and other current assets | |
| Prepaid expenses and other current assets | |
| Current deposits | |
Total current assets | | 21,494 | | | | 52,117 | |
Property and equipment, net | | 310 | | | | 444 | |
Other long-term assets | | 2,251 | | | | 1,934 | |
| Operating lease right-of-use assets | |
| Total assets | $ | 24,055 | | | $ | 54,495 | |
Liabilities, Redeemable Noncontrolling Interest and Stockholders’ Deficit | | | | | | | |
| Total assets | |
| Total assets | |
| Liabilities, Redeemable Noncontrolling Interest and Stockholders’ Equity (Deficit) | |
| Current liabilities: | |
| Current liabilities: | |
Current liabilities: | | | | | | | |
Accounts payable and accrued expenses | $ | 10,120 | | | $ | 11,413 | |
Deferred revenue | | — | | | | 21 | |
| Accounts payable and accrued expenses | |
| Accounts payable and accrued expenses | |
| Current portion of operating lease liabilities | |
| Current portion of contract liabilities | |
Current portion of notes payable | | 2,083 | | | | — | |
Total current liabilities | | 12,203 | | | | 11,434 | |
Notes payable | | 17,228 | | | | 11,058 | |
Other liabilities | | 285 | | | | 433 | |
| Contract liabilities, net of current portion | |
| Operating lease liabilities, net of current portion | |
| Warrant liability, related party | |
Total liabilities | | 29,716 | | | | 22,925 | |
Commitments and contingencies | | | | | | | | Commitments and contingencies | | | |
Redeemable noncontrolling interest | | 130,642 | | | | 122,515 | |
Stockholders’ deficit: | | | | | | | |
Class A Common Stock, $0.01 par value; 100,000,000 shares authorized, 9,693,254 shares outstanding as of September 30, 2017 and December 31, 2016 | | 97 | | | | 97 | |
Class B Common Stock, $0.01 par value; 100,000,000 shares authorized, 23,119,246 shares outstanding as of September 30, 2017 and December 31, 2016 | | 232 | | | | 232 | |
| Stockholders’ equity (deficit): | |
| Class A common stock, $0.01 par value; 200,000,000 shares authorized, 2,432,857 and 2,084,973 shares outstanding as of March 31, 2024 and December 31, 2023, respectively | |
| Class A common stock, $0.01 par value; 200,000,000 shares authorized, 2,432,857 and 2,084,973 shares outstanding as of March 31, 2024 and December 31, 2023, respectively | |
| Class A common stock, $0.01 par value; 200,000,000 shares authorized, 2,432,857 and 2,084,973 shares outstanding as of March 31, 2024 and December 31, 2023, respectively | |
| Class B common stock, $0.01 par value; 100,000,000 shares authorized, and 577,349 outstanding as of March 31, 2024 and December 31, 2023 | |
| | Additional paid-in capital | |
| Additional paid-in capital | |
Additional paid-in capital | | 127,036 | | | | 124,212 | |
Accumulated deficit | | (263,668 | ) | | | (215,486 | ) |
Total stockholders’ deficit attributable to vTv Therapeutics Inc. | | (136,303 | ) | | | (90,945 | ) |
Total liabilities, redeemable noncontrolling interest and stockholders’ deficit | $ | 24,055 | | | $ | 54,495 | |
| Total stockholders’ equity (deficit) attributable to vTv Therapeutics Inc. | |
| Noncontrolling interest | |
| Total stockholders’ equity (deficit) | |
| Total liabilities, redeemable noncontrolling interest and stockholders’ equity (deficit) | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Condensed Consolidated Statements of Operations - Unaudited
(in thousands, except number of shares and per share data)
| Three Months Ended | | | Nine Months Ended | |
| September 30, | | | September 30, | |
| 2017 | | | 2016 | | | 2017 | | | 2016 | |
| Three Months Ended
March 31, | |
| Three Months Ended
March 31, | |
| Three Months Ended
March 31, | |
| 2024 | |
| 2024 | |
| 2024 | |
| Revenue | |
| Revenue | |
Revenue | $ | 15 | | | $ | 38 | | | $ | 58 | | | $ | 596 | |
Operating expenses: | | | | | | | | | | | | | | | |
| Operating expenses: | |
| Operating expenses: | |
Research and development | | 8,989 | | | | 10,636 | | | | 29,572 | | | | 33,854 | |
Research and development - related party | | — | | | | 529 | | | | — | | | | 795 | |
| Research and development | |
| Research and development | |
| General and administrative | |
| General and administrative | |
General and administrative | | 2,567 | | | | 2,401 | | | | 8,396 | | | | 7,654 | |
Total operating expenses | | 11,556 | | | | 13,566 | | | | 37,968 | | | | 42,303 | |
| Total operating expenses | |
| Total operating expenses | |
Operating loss | | (11,541 | ) | | | (13,528 | ) | | | (37,910 | ) | | | (41,707 | ) |
Other income (loss), net | | — | | | | 2 | | | | — | | | | 2 | |
| Operating loss | |
| Operating loss | |
| Other income, net | |
| Other income, net | |
| Other income, net | |
| Other expense– related party | |
| Other expense– related party | |
| Other expense– related party | |
Interest income | | 35 | | | | 21 | | | | 95 | | | | 66 | |
Interest expense | | (849 | ) | | | — | | | | (2,240 | ) | | | (3 | ) |
| Interest income | |
| Interest income | |
| | Loss before income taxes and noncontrolling interest | |
| | Loss before income taxes and noncontrolling interest | |
| Loss before income taxes and noncontrolling interest | | (12,355 | ) | | | (13,505 | ) | | | (40,055 | ) | | | (41,642 | ) |
Income tax provision | | — | | | | — | | | | — | | | | — | |
| Income tax provision | |
| Income tax provision | |
| Net loss before noncontrolling interest | |
| Net loss before noncontrolling interest | |
Net loss before noncontrolling interest | | (12,355 | ) | | | (13,505 | ) | | | (40,055 | ) | | | (41,642 | ) |
Less: net loss attributable to noncontrolling interest | | (8,705 | ) | | | (9,512 | ) | | | (28,222 | ) | | | (29,340 | ) |
| Less: net loss attributable to noncontrolling interest | |
| Less: net loss attributable to noncontrolling interest | |
Net loss attributable to vTv Therapeutics Inc. | $ | (3,650 | ) | | $ | (3,993 | ) | | $ | (11,833 | ) | | $ | (12,302 | ) |
Net loss per share of vTv Therapeutics Inc. Class A Common Stock, basic and diluted | $ | (0.38 | ) | | $ | (0.41 | ) | | $ | (1.22 | ) | | $ | (1.30 | ) |
Weighted-average number of vTv Therapeutics Inc. Class A Common Stock, basic and diluted | | 9,693,254 | | | | 9,691,362 | | | | 9,693,254 | | | | 9,495,926 | |
| Net loss attributable to vTv Therapeutics Inc. | |
| Net loss attributable to vTv Therapeutics Inc. | |
| Net loss attributable to vTv Therapeutics Inc. common shareholders | |
| Net loss attributable to vTv Therapeutics Inc. common shareholders | |
| Net loss attributable to vTv Therapeutics Inc. common shareholders | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Weighted average number of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Weighted average number of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Weighted average number of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
| (*) Adjusted retroactively for reverse stock split | |
| (*) Adjusted retroactively for reverse stock split | |
| (*) Adjusted retroactively for reverse stock split | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Condensed Consolidated Statement of Changes in Redeemable Noncontrolling Interest and Stockholders’
DeficitEquity (Deficit) - Unaudited
(in thousands, except number of shares)
| | | | | Class A Common Stock | | | Class B Common Stock | | | | | | | | | | | | | |
| Redeemable Noncontrolling Interest | | | Shares | | | Amount | | | Shares | | | Amount | | | Additional Paid-in Capital | | | Accumulated Deficit | | | Total Stockholders' Deficit | |
Balances at December 31, 2016 | | 122,515 | | | | 9,693,254 | | | | 97 | | | | 23,119,246 | | | | 232 | | | | 124,212 | | | | (215,486 | ) | | | (90,945 | ) |
Net loss | | (28,222 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,833 | ) | | | (11,833 | ) |
Share-based compensation | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,657 | | | | — | | | | 2,657 | |
Issuance of warrants to purchase Class A Common Stock | | — | | | | — | | | | — | | | | — | | | | — | | | | 167 | | | | — | | | | 167 | |
Change in redemption value of noncontrolling interest | | 36,349 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (36,349 | ) | | | (36,349 | ) |
Balances at September 30, 2017 | $ | 130,642 | | | | 9,693,254 | | | $ | 97 | | | | 23,119,246 | | | $ | 232 | | | $ | 127,036 | | | $ | (263,668 | ) | | $ | (136,303 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the three months ended March 31, 2024 |
| | | | Class A Common Stock | | Class B Common Stock | | | | | | | | | | |
| Redeemable
Noncontrolling
Interest | | | Shares | | Amount | | Shares | | Amount | | Additional
Paid-in
Capital | | Accumulated Deficit | | Total vTv Therapeutics Inc Stockholders’ Equity (Deficit) | | Noncontrolling
Interest | | Total Stockholders'
Equity (Deficit) |
| Balances at December 31, 2023 | $ | 6,131 | | | | 2,084,973 | | | $ | 21 | | | 577,349 | | | $ | 6 | | | $ | 256,335 | | | $ | (281,042) | | | $ | (24,680) | | | $ | — | | | $ | (24,680) | |
| Net loss attributable to vTv Therapeutics Inc. | — | | | | — | | | — | | | — | | | — | | | — | | | (4,865) | | | (4,865) | | | — | | | (4,865) | |
Net loss attributable to redeemable noncontrolling interest(*) | (1,085) | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Change in redemption value of redeemable noncontrolling interest | 214 | | | | — | | | — | | | — | | | — | | | — | | | (214) | | | (214) | | | — | | | (214) | |
| Reclassification of redeemable noncontrolling interest to permanent equity (See Note 7) | (5,260) | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,260 | | | 5,260 | |
| Share-based compensation | — | | | | — | | | — | | | — | | | — | | | 220 | | | — | | | 220 | | | — | | | 220 | |
| Issuance of Class A common stock and pre-funded warrants, net offering costs | — | | | | 347,884 | | | 3 | | | — | | | — | | | 50,332 | | | — | | | 50,335 | | | — | | | 50,335 | |
| Net loss attributable to noncontrolling interest | — | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (69) | | | (69) | |
| Balances at March 31, 2024 | $ | — | | | | 2,432,857 | | | $ | 24 | | | 577,349 | | | $ | 6 | | | $ | 306,887 | | | $ | (286,121) | | | $ | 20,796 | | | $ | 5,191 | | | $ | 25,987 | |
| (*) Allocation of NCI net loss was a result from the reclassification to permanent equity on February 27, 2024 (See Note 7) | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the three months ended March 31, 2023 |
| | | | Class A Common Stock | | Class B Common Stock | | | | | | |
| Redeemable
Noncontrolling
Interest | | | Shares(*) | | Amount(*) | | Shares(*) | | Amount(*) | | Additional
Paid-in
Capital(*) | | Accumulated Deficit | | Total Stockholders' Deficit |
| Balances at December 31, 2022 | $ | 16,579 | | | | 2,084,973 | | | $ | 21 | | | 577,349 | | | $ | 6 | | | $ | 254,757 | | | $ | (265,524) | | | $ | (10,740) | |
| Net loss | (1,275) | | | | — | | | — | | | — | | | — | | | — | | | (4,499) | | | (4,499) | |
| Share-based compensation | — | | | | — | | | — | | | — | | | — | | | 343 | | | — | | | 343 | |
| Change in redemption value of noncontrolling interest | 4,296 | | | | — | | | — | | | — | | | — | | | — | | | (4,296) | | | (4,296) | |
| Balances at March 31, 2023 | $ | 19,600 | | | | 2,084,973 | | | $ | 21 | | | 577,349 | | | $ | 6 | | | $ | 255,100 | | | $ | (274,319) | | | $ | (19,192) | |
| (*) Adjusted retroactively for reverse stock split | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
��
Condensed Consolidated Statements of Cash Flows - Unaudited
| | Nine Months Ended September 30, | |
| | 2017 | | | 2016 | |
Cash flows from operating activities: | | | | | | | | |
Net loss before noncontrolling interest | | $ | (40,055 | ) | | $ | (41,642 | ) |
Adjustments to reconcile net loss before noncontrolling interest to net cash used in operating activities: | | | | | | | | |
Gain on disposal of property and equipment, net | | | (11 | ) | | | (2 | ) |
Depreciation expense | | | 152 | | | | 216 | |
Share-based compensation expense | | | 2,657 | | | | 1,988 | |
Amortization of debt discount | | | 753 | | | | — | |
Changes in assets and liabilities: | | | | | | | | |
Accounts receivable | | | — | | | | 69 | |
Change in restricted cash | | | (281 | ) | | | — | |
Prepaid expenses and other assets | | | (113 | ) | | | 56 | |
Employee loans receivable – related party | | | — | | | | 46 | |
Other long-term assets | | | (317 | ) | | | (268 | ) |
Accounts payable and accrued expenses | | | (1,293 | ) | | | 3,417 | |
Accounts payable and accrued expenses – related party | | | — | | | | (474 | ) |
Deferred revenue | | | (21 | ) | | | (198 | ) |
Other liabilities | | | 19 | | | | 9 | |
Net cash used in operating activities | | | (38,510 | ) | | | (36,783 | ) |
Cash flows from investing activities: | | | | | | | | |
Proceeds from sale of assets | | | 32 | | | | 4 | |
Purchases of property and equipment | | | (39 | ) | | | (87 | ) |
Net cash used in investing activities | | | (7 | ) | | | (83 | ) |
Cash flows from financing activities: | | | | | | | | |
Proceeds from debt issuance | | | 7,500 | | | | — | |
Debt issuance costs | | | — | | | | (50 | ) |
Repayment of long-term obligations | | | — | | | | (29 | ) |
Net cash provided by (used in) financing activities | | | 7,500 | | | | (79 | ) |
Net decrease in cash and cash equivalents | | | (31,017 | ) | | | (36,945 | ) |
Cash and equivalents, beginning of period | | | 51,505 | | | | 88,003 | |
Cash and equivalents, end of period | | $ | 20,488 | | | $ | 51,058 | |
| | | | | | | | |
Non-cash activities: | | | | | | | | |
Change in redemption value of noncontrolling interest | | $ | 36,349 | | | $ | 26,120 | |
Exchange of vTv Therapeutics Inc. Class B Common Stock and vTv Therapeutics, LLC member units for vTv Therapeutics Inc. Class A Common Stock | | $ | — | | | $ | 3,164 | |
| | | | | | | | | | | |
| Three Months Ended March 31, |
| 2024 | | 2023 |
| Cash flows from operating activities: | | | |
| Net loss before noncontrolling interest | $ | (6,019) | | | $ | (5,774) | |
| Adjustments to reconcile net loss before noncontrolling interest to net cash used in operating activities: | | | |
| Depreciation expense | 22 | | | 22 | |
| Loss from promissory note early redemption | — | | | 313 | |
| Non-cash interest income | — | | | (100) | |
| Share-based compensation expense | 220 | | | 343 | |
| Change in fair value of investments | — | | | (2,104) | |
| Change in fair value of warrants, related party | 371 | | | 238 | |
| Changes in assets and liabilities: | | | |
| Accounts receivable | (879) | | | 173 | |
| Prepaid expenses and other current assets | 473 | | | 691 | |
| Other assets | 28 | | | — | |
| Accounts payable and accrued expenses | (1,511) | | | 1,032 | |
| | | |
| Other liabilities | (40) | | | — | |
| Net cash used in operating activities | (7,335) | | | (5,166) | |
| | | |
| | | |
| | | |
| Cash flows from financing activities: | | | |
| Proceeds from sale of Class A common stock and pre-funded warrants, net of offering costs | 50,335 | | | — | |
| Proceeds from promissory note early redemption related to sale of Class A common stock to collaboration partner | — | | | 12,030 | |
| | | |
| Repayment of notes payable | (191) | | | (224) | |
| Net cash provided by financing activities | 50,144 | | | 11,806 | |
| Net increase in cash and cash equivalents | 42,809 | | | 6,640 | |
| Total cash and cash equivalents, beginning of period | 9,446 | | | 12,126 | |
| Total cash and cash equivalents, end of period | $ | 52,255 | | | $ | 18,766 | |
| | | |
| Non-cash activities: | | | |
| Change in redemption value of noncontrolling interest | $ | (214) | | | $ | 4,296 | |
| Reclassification of noncontrolling interest to additional paid-in capital | $ | 5,260 | | | — | |
| | | |
| | | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Notes to Condensed Consolidated Financial Statements – Unaudited
(dollar amounts are in thousands, unless otherwise noted)
Note 1:
| Description of Business, Basis of Presentation and Going Concern
|
Note 1: Description of Business
and Basis of Presentation
Description of Business
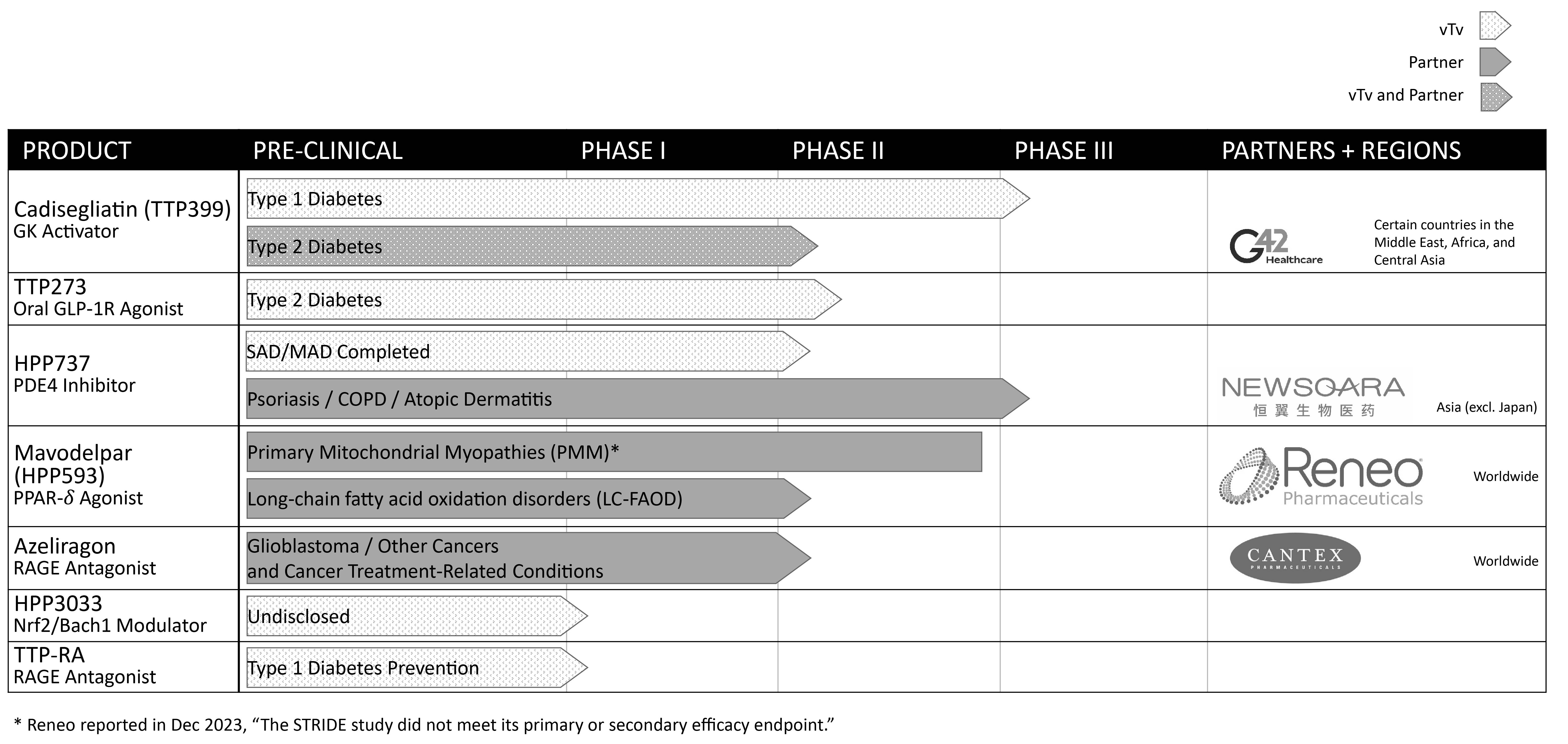
vTv Therapeutics Inc. (the “Company,” the “Registrant,” “we” or “us”) was incorporated in the state of Delaware in April 2015. The Company
was formedis a clinical stage pharmaceutical company focused on treating metabolic diseases to
discover and develop orally administered small molecule drug candidates to fill significant unmet medical needs.minimize their long-term complications through end-organ protection.
Principles of Consolidation
vTv Therapeutics Inc. is a holding company, and its principal asset is a controlling equity interest in vTv Therapeutics LLC (“vTv LLC”), the Company’s principal operating subsidiary, which is a
clinical-stage biopharmaceuticalclinical stage pharmaceutical company engaged in the discovery and development of orally administered small molecule drug candidates to fill significant unmet medical needs.
The Company has determined that vTv LLC is a variable-interest entity (“VIE”) for accounting purposes and that vTv Therapeutics Inc. is the primary beneficiary of vTv LLC because (through its managing member interest in vTv LLC and the fact that the senior management of vTv Therapeutics Inc. is also the senior management of vTv LLC) it has the power and benefits to direct all of the activities of vTv LLC, which include those that most significantly impact vTv LLC’s economic performance. vTv Therapeutics Inc. has therefore consolidated vTv LLC’s results pursuant to Accounting Standards Codification Topic 810, “Consolidation” in its Condensed Consolidated Financial Statements. AsThe assets and liabilities of September 30, 2017, variousvTv LLC represent substantially all of the Company's consolidated assets and liabilities with the exception of the Warrants.
Various holders own non-voting interests in vTv LLC, representing a 70.5%19.2% economic interest in vTv LLC, effectively restricting vTv Therapeutics Inc.’s interest to 29.5%80.8% of vTv LLC’s economic results, subject to increase in the future, should vTv Therapeutics Inc. purchase additional non-voting common units (“vTv Units”) of vTv LLC, or should the holders of vTv Units decide to exchange such units (together with shares of the Company’s Class B Common Stock)common stock, par value $0.01 (“Class B common stock”)) for shares of Class A Common Stockcommon stock (or cash) pursuant to the Exchange Agreement (as defined in Note 7)8). vTv Therapeutics Inc. has provided financial and other support to vTv LLC in the form of its purchase of vTv Units with the net proceeds of the Company’s initial public offering (“IPO”) in 2015, its registered direct offering in March 2019, and its agreeing to be a co-borrower under the Venture Loan and Security Agreement (the “Loan Agreement”) with Horizon Technology Finance Corporation and Silicon Valley Bank (together, the “Lenders”) which was entered into in 2016. vTv Therapeutics Inc. entered into the letter agreements with MacAndrews and Forbes Group LLC (“M&F Group”), a related party and an affiliate of MacAndrews & Forbes Incorporated (together with its affiliates “MacAndrews”) in December 2017, July 2018, December 2018, March 2019, September 2019, and December 2019 (each a “Letter Agreement” and collectively, the “Letter Agreements”). vTv Therapeutics Inc. entered into a common stock purchase agreement with G42 Investments AI Holding RSC Ltd (“G42 Investments”) (the “G42 Purchase Agreement”), the common stock and warrant purchase agreement with CinPax, LLC and CinRx, LLC, respectively (the “CinRx Purchase Agreement”). In addition vTv Therapeutics Inc also entered into a Securities Purchase Agreement with Private Placement Investors and the sales agreement with Cowen and Company, LLC (“TD Cowen”) (“TD Cowen Sales Agreement”). vTv Therapeutics Inc. will not be required to provide financial or other support for vTv LLC outside of its obligations pertaining to the Loan Agreement as a co-borrower. LLC. However, vTv Therapeutics Inc. will control its business and other activities through its managing member interest in vTv LLC, and its management is the management of vTv LLC. The creditors of vTv LLC do not have any recourse to the general credit of vTv Therapeutics Inc. except as allowed under the provisions of the Loan Agreement. Nevertheless, because vTv Therapeutics Inc. will have no material assets other than its interests in vTv LLC, any financial difficulties at vTv LLC could result in vTv Therapeutics Inc. recognizing a loss.Going Concern and
To date, the Company has not generated any product revenue and has not achieved profitable operations. The continuing development of our drug candidates will require additional financing. From its inception through September 30, 2017,March 31, 2024, the Company has funded its operations primarily through a combination of private placements of common and preferred equity, research collaboration agreements, upfront and milestone payments for license agreements, debt financingand equity financings and the completion of its IPO in August 2015. As of September 30, 2017,March 31, 2024, the Company hashad an accumulated deficit of $263.7$286.1 million and has generated net losses in each year of its existence. Management estimates thatAs of March 31, 2024, the Company’s liquidity sources included cash and cash equivalents balance as of September 30, 2017$52.3 million.
Based on the Company’sour current operating plan, management believeswe believe that theour current cash and cash equivalents will allow us to meet our liquidity requirements for at least the next twelve months.
On February 27, 2024, we entered into a securities purchase agreement (the “Securities Purchase Agreement”) with certain institutional accredited investors (the “Private Placement Investors”), pursuant to which we agreed to issue and sell to the Private Placement Investors in a private placement (the “Private Placement”) (i) an aggregate of 464,377 shares (the “Private Placement Shares”) of our Class A common stock, at a purchase price of $11.81 per share, and (ii) pre-funded warrants (the “Private Placement Pre-Funded Warrants”) to purchase up to an aggregate of 3,853,997 shares of our Class A common stock (the “Private Placement Warrant Shares”) at a purchase price of $11.80 per Private Placement Pre-Funded Warrant (representing the $11.81 per Private Placement Share purchase price less the exercise price of $0.01 per Private Placement Warrant Share). We received aggregate gross proceeds from the Private Placement of approximately $51.0 million, before deducting offering expenses payable by us. The Private Placement Pre-Funded Warrants are exercisable at any time after their original issuance and will not expire.
On March 5, 2024, the Company entered into a letter agreement with the Private Placement Investors pursuant to meet its liquidity requirementswhich the Private Placement Investors agreed to exchange an aggregate of 116,493 Private Placement Shares for an aggregate of 116,590 Private Placement Pre-Funded Warrants.
On February 28, 2024, we entered into a sales agreement (the “TD Cowen Sales Agreement”) with Cowen and Company, LLC (“TD Cowen”), pursuant to which we may offer and sell, from time to time, through or to TD Cowen, as sales agent or principal, shares of our Class A common stock, having an aggregate offering price of up to $50.0 million (the “TD Cowen ATM Offering”). Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities registered on the receiptregistration statement relating to the TD Cowen ATM Offering with a value exceeding more than one-third of top-line results for Subpart A of its STEADFAST Studyour public float in early 2018. In addition to available cash and cash equivalents,any 12-month period so long as our public float remains below $75.0 million. Under the Company is seeking possible partnering opportunities for our GKA, GLP-1r and other drug candidates which it believes may provide additional cash for use in its operations and the continuationterms of the clinical trials for its drug candidates. The Company is also pursuingTD Cowen Sales Agreement, we will pay TD Cowen a commission of 3.0% of the aggregate proceeds from the sale of shares and reimburse certain legal fees or other sources of interim financing to provide flexibility to its operating plan. The timing and occurrence of such interim financing is not yet known. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.disbursements.
The Company’s financial statements have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. The Condensed Consolidated Financial Statements do not include adjustments to reflect the possible future effects on the recoverability and classification of recorded assets or the amounts of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 2:
| Note 2: Summary of Significant Accounting Policies |
Unaudited Interim Financial Information
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The accompanying Condensed Consolidated Balance Sheet as of
September 30, 2017,March 31, 2024, Condensed Consolidated Statements of Operations for the three
and nine months ended
September 30, 2017March 31, 2024 and
2016,2023, Condensed Consolidated Statement of Changes in Redeemable Noncontrolling Interest and Stockholders’
DeficitEquity (Deficit) for the
ninethree months ended
September 30, 2017March 31, 2024 and 2023 and Condensed Consolidated Statements of Cash Flows for the
ninethree months ended
September 30, 2017March 31, 2024 and
20162023 are unaudited. These unaudited financial statements have been prepared in accordance with the rules and regulations of the United States Securities and Exchange Commission (“SEC”) for interim financial information. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. These financial statements should be read in conjunction with the audited financial statements and the accompanying notes for the year ended December 31,
20162023, contained in the Company’s Annual Report on Form 10-K. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments (consisting of normal recurring adjustments) necessary to state fairly the Company’s financial position as of
September 30, 2017,March 31, 2024, the results of operations for the three
and nine months ended
September 30, 2017March 31, 2024 and
20162023 and cash flows for the
ninethree months ended
September 30, 2017March 31, 2024 and
2016.2023. The December 31,
20162023 Condensed Consolidated Balance Sheet included herein was derived from the audited financial statements but does not include all disclosures or notes required by GAAP for complete financial statements.
The financial data and other information disclosed in these notes to the financial statements related to the three
and nine months ended
September 30, 2017March 31, 2024 and
20162023 are unaudited. Interim results are not necessarily indicative of results for an entire year.
The Company does not have any components of other comprehensive income recorded within its Condensed Consolidated Financial Statements, and, therefore, does not separately present a statement of comprehensive income in its Condensed Consolidated Financial Statements.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
On an ongoing basis, the Company evaluates its estimates, including those related to the grant date fair value of equity awards, the fair value of
warrants to purchase shares of its Class A common stock, the
fair value of its Class B
Common Stock,common stock, the useful lives of property and equipment
the fair value of derivative liabilities, and the fair value of the Company’s debt, among others. The Company bases its estimates on historical experience and on various other assumptions that it believes to be reasonable, the results of which form the basis for making judgments about the carrying value of assets and liabilities.
Concentration of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist principally of cash on deposit with multiple financial institutions. The
balancesbalance of
thesethe cash
accountsaccount frequently
exceedexceeds insured limits.
There were no accounts receivable balances outstanding as The associated risk of September 30, 2017concentration for cash and December 31, 2016.
cash equivalents is mitigated by transferring a majority of our cash to a AAA rated money market account with a creditworthy institution.
One and two customerscustomer represented 100% of the revenue earned during the three and nine months ended September 30, 2017 and 2016, respectively.March 31, 2024. The Company did not have any revenue during the three months ended March 31, 2023.
Cash and Cash Equivalents
The Company considers any highly liquid investments with an original maturity of three months or less to be cash and cash equivalents.
Restricted Cash and Cash Equivalents
Restricted cash and cash equivalents as of September 30, 2017 reflect cash and cash equivalents that have been received through a research, development and commercialization agreement with JDRF International (“JDRF”) (the “JDRF Agreement”) but which have not yet been utilized to fund the development activities required under the JDRF Agreement.
Collaboration Agreements
Calithera
The majority of the Company’s collaboration revenue recognized
Investments
Investments in the three and nine months ended September 30, 2016 is related to an exclusive global license agreement (the “License Agreement”),entities in which the Company entered intohas no control or significant influence, is not the primary beneficiary, and have a readily determinable fair value are classified as equity investments with readily determinable fair value. The investments are measured at fair value based on March 6, 2015 with Calithera Biosciences, Inc. (“Calithera”)a quoted market price per unit in active markets multiplied by the number of units held without consideration of transaction costs (Level 1). Gains and losses are recorded in other income (expense), granting Calithera exclusive world-widenet on the Condensed Consolidated Statements of Operations.
Equity investments without readily determinable fair value include ownership rights to research, develop and commercialize the Company’s portfolio of hexokinase II inhibitors. Under the terms of the License Agreement, Calithera paidthat do not provide the Company an initial license fee of $0.6 millionwith control or significant influence and potential development and regulatory milestone payments totaling upthese investments do not have readily determinable fair values. The Company has elected to $30.5 millionmeasure its equity investments without readily determinable fair values at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the first licensed product, an additional $77.0 million in potential sales-based milestones, as well as royalty payments, based on tiered sales of the first commercialized licensed product. In addition, the Company recognized a total of $0.3 million for the three and nine months ended September 30, 2016 for the costs associated with up to four full-time employees for the Company to develop additional hexokinase inhibitors. If Calithera develops additional licensed products, after achieving regulatory approval of the first licensed product, Calithera would owe additional regulatory milestone payments and additional royalty payments based on sales of such additional licensed products.JDRF
In August 2017, the Company entered into the JDRF Agreement to support the funding of a Phase 2 Proof of Concept study to explore the effects of TTP399, an orally administered, liver-selective glucokinase activator (“GKA”), in type 1 diabetics. According to the terms of the JDRF Agreement, JDRF will provide research funding of up to $3.0 million based on the achievement of research and development milestones, with the total funding not to exceed approximately one-half of the total cost of the project. Additionally, the Company has the obligation to make certain milestone payments to JDRF upon the commercialization, licensing, saleidentical or transfer of TTP399 as a treatment for Type 1 Diabetes.
Payments that the Company receives from JDRF under this agreement will be recorded as restricted cash and current liabilities, and recognized as an offset to research and development expense, based on the progress of the project, and only to the extent that the restricted cash is utilized to fund such development activities. As of September 30, 2017, the Company had received funding under this agreement of $0.3 million, and research and development costs were offset by an insignificant amount. As of September 30, 2017, the Company has recognized restricted cash of $0.3 million related to this agreement.
similar investment
. The Company uses the revenue recognition guidance established by ASC Topic 605, “Revenue Recognition.”606, Revenue From Contracts With Customers (“ASC 606”). When an agreement falls under the scope of other standards, such as ASC 808, Collaborative Arrangements (“ASC 808”), the Company will apply the recognition, measurement, presentation, and disclosure guidance in ASC 606 to the performance obligations in the agreements if those performance obligations are with a customer. Revenue recognized by analogizing to ASC 606, is recorded as collaboration revenue on the statements of operations.
The majority of the Company’s revenue results from its license and collaboration agreements associated with the development of investigational drug products. The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance and collectability of consideration is probable. For each contract meeting these criteria, the Company identifies the performance obligations included within the contract. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. The Company then recognizes revenue when: 1) persuasive evidence of an arrangement exists; 2)under each contract as the service has been provided torelated performance obligations are satisfied.
The transaction price under the
customer; 3) collection of the feecontract is
reasonably assured; and 4) the amount of the fee to be paid by the customer is fixed or determinable. In determining the accounting for collaboration and alliance agreements, the Company follows the provisions of ASC Topic 605, Subtopic 25, “Multiple-Element Arrangements” (“ASC 605-25”) and ASC 808 (“Collaborative Arrangements”). ASC 605-25 provides guidance on whether an arrangement that involves multiple revenue-generating activities or deliverables should be divided into separate units of accounting for revenue recognition purposes and, if division is required, how the arrangement consideration should be allocated among the separate units of accounting. If a deliverable has value on a stand-alone basis, the Company treats the deliverable as a separate unit of accounting. If the arrangement constitutes separate units of accounting according to the separation criteria of ASC 605-25, the consideration received is allocated among the separate units of accounting and the applicable revenue recognition criteria is applied to each unit. The Company determines how to allocate amounts received under agreements among the separate unitsdetermined based on the
respectivevalue of the consideration expected to be received in exchange for the transferred assets or services. Development, regulatory and sales milestones included in the Company’s collaboration agreements are considered to be variable consideration. The amount of variable consideration expected to be received is included in the transaction price when it becomes probable that the milestone will be met. For contracts with multiple performance obligations, the contract’s transaction price is allocated to each performance obligation using the Company’s best estimate of the standalone selling price of each
unit. Ifdistinct good or service in the
arrangement constitutes a single unit of accounting,contract. The primary method used to estimate standalone selling price is the
revenue recognition policy must be determined for the entire arrangement and the consideration receivedexpected cost plus margin approach. Revenue is recognized over the
related period
of inception throughover which the
dateCompany expects the
last deliverable within the single unit of accounting is expectedservices to be
delivered.
Collaboration research and development revenue is earned and recognized as research is performed and related expenses are incurred. Non-refundable upfront fees are recorded as deferred revenue and recognized into revenue as license fees and milestones from collaborations onprovided using a proportional performance model or a straight-line basismethod of recognition if there is no discernible pattern over the estimated period of the Company’s substantive performance obligations. If the Company does not have substantive performance obligations, it recognizes non-refundable upfront fees into revenue ratably over the period during which the product deliverable is provided to the customer.
Revenue for non-refundable payments based on the achievementservices will be provided.
Table of milestone events under collaborative arrangements is recognized in accordance with ASC Topic 605, Subtopic 28, “Milestone Method” (“ASC 605-28”). Milestone events under the Company’s collaboration agreements may include research, development, regulatory, commercialization, and sales events. Under ASC 605-28, a milestone payment is recognized as revenue when the applicable event is achieved if the event meets the definition of a milestone and the milestone is determined to be substantive. ASC 605-28 defines a milestone event as an event having all of the following characteristics: (1) substantive uncertainty regarding achievement of the milestone event exists at the inception of the arrangement; (2) the event can only be achieved based, in whole or in part, on either the Company’s performance or a specific outcome resulting from the Company’s performance; and (3) if achieved, the event will result in additional payment due to the Company. The Company also treats events that can only be achieved based, in whole or in part, on either a third party’s performance or a specific outcome resulting from a third party’s performance as milestone events if the criteria of ASC 605-28 are otherwise satisfied.Research and development costs that are reimbursable under collaboration agreements are recorded in accordance with ASC Topic 605, Subtopic 45, “Principal-Agent Considerations.” Amounts reimbursed under a cost-sharing arrangement are reflected as reductions of research and development expense.
Contents
Major components of research and development costs include cash
and share-based compensation, depreciation expense on research and development property and equipment, costs of preclinical studies, clinical trials and related clinical manufacturing, costs of drug development, costs of materials and supplies, facilities
costs,cost, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on the Company’s behalf. Research and development costs are expensed as incurred.
The Company records accruals based on estimates of the services received, efforts expended and amounts owed pursuant to contracts with numerous contract research
and manufacturing organizations. In the normal course of business, the Company contracts with third parties to perform various clinical study activities in the ongoing development of potential products. The financial terms of these agreements are subject to negotiation and variation from contract to contract and may result in uneven payment flows. Payments under the contracts depend on factors such as the achievement of certain events and the completion of portions of the clinical study or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses in its financial statements to the actual services received and efforts expended. As such, expense accruals related to clinical studies are recognized based on the Company’s estimate of the degree of completion of the event or events specified in the specific clinical study.
The Company records nonrefundable advance payments it makes for future research and development activities as prepaid expenses. Prepaid expenses are recognized as expense in the Condensed Consolidated Statements of Operations as the Company receives the related goods or services.
Share-Based Compensation
Compensation
Research and development costs that are reimbursed under a cost-sharing arrangement are reflected as a reduction of research and development expense.
Recently Issued Accounting Pronouncements Not Yet Adopted
Segment Reporting: In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): “Improvements to Reportable Segment Disclosures” (ASU 2023-07). The ASU expands public entities' segment disclosures by requiring disclosures of significant segment expenses that are regularly provided to the CODM and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment's profit or loss and assets. Early adoption is permitted. Since the Company only has one segment, the Company does not expect an impact when adopting this ASU on the Company's Consolidated Financial Statements and disclosures.
Income Taxes: In December 2023, the FASB issued ASU 2023-09: “Improvements to Income Tax Disclosures” (“ASU 2023-09”). The ASU is intended to enhance the transparency and decision usefulness of income tax disclosures. The amendments in the ASU address investor requests for enhanced income tax information primarily through changes to the rate reconciliation and income taxes paid information. ASU 2023-09 will be effective for us in the annual period beginning January 1, 2025, though early adoption is permitted. The Company is currently evaluating the presentational effect that ASU 2023-09 will have on the Company's Consolidated Financial Statements and disclosures, and we expect considerable changes to our income tax disclosures.
Note 3: Collaboration Agreements
G42 Purchase Agreement and Cogna Collaborative and License Agreement
The Company and G42 Investments AI Holding RSC Ltd, a private limited company (“G42 Investments”), entered into a Common Stock Purchase Agreement (the “G42 Purchase Agreement”), pursuant to which the Company sold to G42 Investments 259,657 shares of the Company’s Class A common stock at a price per share of approximately $96.40, for an aggregate purchase price of $25.0 million, which was paid (i) $12.5 million in cash at the closing and (ii) $12.5 million in the form of a promissory note of G42 Investments to be paid at May 31, 2023 (the “G42 Promissory Note”). On February 28, 2023, the Company and G42 Investments amended the G42 Purchase Agreement and modified the G42 Promissory Note to accelerate the payment due under the note. Pursuant to the amendment, on February 28, 2023, the Company received $12.0 million, which reflected the original amount due under the G42 Promissory Note less a 3.75% discount, in full satisfaction of the note, resulting in a loss of $0.3 million and was recognized as a component of other income, net in the Company’s Condensed Consolidated Statements of Operations.
G42 Investments has agreed to certain transfer restrictions (including restrictions on short sales or similar transactions) and restrictions on further acquisitions of shares, in each case subject to specified exceptions. Following the expiration of a lock up period, from the period May 31, 2022 until December 31, 2024 (or if earlier, the date of receipt of U.S. Food and Drug Administration (“FDA”) approval in the U.S. for cadisegliatin (TTP399), the Company has granted to G42 Investments
certain shelf and piggyback registration rights with respect to those shares of Class A common stock issued to G42 Investments pursuant to the G42 Purchase Agreement, including the ability to conduct an underwritten offering to resell such shares under certain circumstances. The registration rights include customary cooperation, cut-back, expense reimbursement, and indemnification provisions.
Contemporaneously with the G42 Purchase Agreement, effective on May 31, 2022, the Company entered into a collaboration and license agreement (the “Cogna Agreement”) with Cogna Technology Solutions LLC, an affiliate of G42 Investments (“Cogna”), which requires Cogna to work with the Company in performing clinical trials for share-based compensation awardscadisegliatin (TTP399) as well as jointly creating a global development plan to develop, market, and commercialize cadisegliatin in certain countries in the Middle East, Africa, and Central Asia (the “Partner Territory”). Under the terms of the Cogna Agreement, Cogna will obtain a license under certain intellectual property controlled by the Company to enable it to fulfill its obligations and exercise its rights under the Cogna agreement, including to develop and commercialize cadisegliatin in the Partner Territory, but will not have access to the various intellectual property (“IP”) related to the license and cadisegliatin. Specifically, the Company will share various protocols with Cogna related to conducting the clinical trials and will provide the patient dosages and placebo of cadisegliatin needed to conduct the trials.
Under the Cogna Agreement, Cogna has the right to develop and commercialize the cadisegliatin in the Partner Territory at its own cost once restrictions on the use of the IP have been lifted by the Company. The Cogna Agreement determined which specific countries in the Partner Territory that Cogna may pursue development and commercialization and provides the Company with the ability to determine when Cogna can benefit from this IP through the powers granted to the Company to approve the global development plan. Further, the Company may supply at cost, or Cogna may manufacture, cadisegliatin for commercial sale under terms to be agreed upon by the parties at a later date.
Separately, the Company will conduct its clinical trials for cadisegliatin outside of the Partner Territory at its own cost. The results of each party’s clinical trials will be combined by the Company to seek FDA approval in the United States for cadisegliatin. On December 21, 2022, G42 Healthcare Technology Solutions LLC (formerly known as Cogna Technology Solutions LLC) novated its rights and obligation under the Cogna agreement to G42 Healthcare Research Technology Projects LLC (“G42 Healthcare”), an affiliate of G42 Investments. As a result of the novation, all reference to Cogna herein shall be deemed to refer to G42 Healthcare.
The G42 Purchase Agreement also provides for, following the receipt of the cadisegliatin FDA Approval, at the option of G42 Investments, either (a) the issuance of the Company’s Class A common stock (the “Milestone Shares”) having an aggregate value equal to $30.0 million or (b) the payment by the Company of $30.0 million in cash (the “Milestone Cash Payment”). The issuance of the Milestone Shares or the payment of the Milestone Cash Payment, as applicable, is basedconditioned upon receipt of the cadisegliatin FDA Approval and subject to certain limitations and conditions set forth in the G42 Purchase Agreement. There can be no assurance that the cadisegliatin FDA Approval will be granted or as to the timing thereof.
Once commercialization takes place in the Partner Territory, the Company will receive royalties in the single digits from Cogna on the net sales of cadisegliatin for a period of at least ten years after the first commercial sale of cadisegliatin in the Partner Territory.
Common stock is generally recorded at fair value at the date of issuance. In determining the fair value of the award atClass A common stock issued to G42 Investments, the dateCompany considered the closing price of grant, and compensation expense is recognizedthe common stock on the effective date. The Company did not make an adjustment to the fair value for those awards earned oversale restrictions on the service period. The grant datestock in accordance with guidance recently adopted in ASU 2022-03. See the “Recently Issued Accounting Guidance” in this quarterly report on Form 10-Q for details of the ASU. Accordingly, the Company determined that cash consideration of $5.7 million should be recorded as fair value of the Class A common stock option awardsat the effective date, utilizing the Class A common stock closing price of $22.04 at the effective date.
A premium was paid on the Class A common stock by G42 Investments of $18.7 million, net of a note receivable discount of $0.6 million. This premium is estimated usingdetermined to be the Black-Scholes option pricing formula. Due totransaction price for all remaining obligations under the lackagreements, which will be accounted for under ASC 808 or ASC 606 based on determination of sufficient historical trading information with respect to its own shares,the unit of account.
The Company determined that certain commitments under the agreements are in the scope of ASC 808 as both the Company estimates expected volatility based on a portfolioand Cogna are active participants in the clinical trials of selected stocks of companies believedcadisegliatin, and both are exposed to have marketsignificant risks and economic characteristics similar to its own. The risk-free rate isrewards based on the U.S. Treasury yield curvesuccess of the clinical trials and subsequent FDA approval. Cogna is determined to be a vendor of the Company during the clinical trial phase, working on the Company’s behalf to complete research and development activities, and not in effect ata customer capacity. The Company accounted for the timecommitments related to the clinical trials, which includes transfer of grant.trial protocols, supply of clinical trial dosages, and collaboration on the joint development committee (“JDC”) as an ASC 808 unit of account, applying the recognition and measurement principles of ASC 606 by analogy. The fair valueCompany
will recognize collaboration revenue for its development activities under ASC 808 over time based on the market valueestimated period of our Class A Common Stockperformance.
By applying the principals in ASC 606 by analogy, the Company identified the performance obligation and considered the timing of satisfaction of the obligation to account for the pattern of revenue recognition. In order to recognize collaboration revenue, generally, the Company would begin satisfying its performance obligation and Cogna would need to be able to use and benefit from delivery of the assets or services. The performance obligation under the agreements that fall within the 808 unit of account are concentrated in the clinical trials. As of March 31, 2024, the clinical trials had not commenced. Accordingly, no collaboration revenue was recognized for the ASC 808 unit of account during the three months ended March 31, 2024.
The Company identified certain commitments that are in the scope of ASC 606 as Cogna’s relationship is that of a customer for these commitments. The significant performance obligations that are in the scope of ASC 606 are (1) the development, commercialization and manufacturing license of the IP once restrictions on the dateuse of grant.the IP have been lifted by the Company and (2) a potential material right to a commercial supply agreement. The Company also estimateswill recognize revenue from the numberdevelopment, commercial and manufacturing license at a point in time when the Company releases the restrictions on the use of share-based awards that arethe IP, which is expected to be forfeited based on historical employee turnover rates. Recently Issued Accounting Pronouncements
In May 2014,after
cadisegliatin is approved by the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue From Contracts With Customers”, that outlines a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance, including industry-specific
guidance.FDA. The ASU is based on the core principle that an entity shouldCompany will recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires disclosures sufficient to enable users to understand the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers, including qualitative and quantitative disclosures about contracts with customers, significant judgments and changes in judgments, and assets recognized from the costsmaterial right related to obtain or fulfill a contract. Entities haveCogna’s ability to purchase the option of using either a full retrospective or a modified retrospective approach forcommercial supply at cost as Cogna purchases the adoptioncommercial supply from the Company, which will occur after the completion of the new standard. In addition, in March, April, and May 2016,initial clinical trials (if Cogna decides to purchase the FASB issued final amendments to clarifyclinical supply from the implementation guidance for principal versus agent considerations, identifying performance obligations and the accounting for licenses of intellectual property, and narrow-scope improvements and practical expedients, respectively. This ASU is effective for fiscal years beginning after December 15, 2017 including interim periods within that reporting period. The Company plans to adopt this guidance using the modified retrospective transition method. To date,Company). As a result, the Company has not generatedrecognized any revenue from drug sales and its ability to recognize revenue from its collaboration and licensing agreements is contingent upon its ability to enter into such agreements inunder the future orASC 606 unit of account during the clinical success and subsequent approval by the United States Food and Drug Administration of investigational drug products subject to its current agreements. As such,three months ended March 31, 2024.
On February 28, 2023, the Company will continueand G42 Investments amended the G42 Purchase Agreement and modified the G42 Promissory Note to evaluateaccelerate the effect this standard will havepayment due under the note. Pursuant to the amendment, on February 28, 2023, the Company received $12.0 million, which reflected the original amount due under the G42 Promissory Note less a 3.75% discount, in full satisfaction of the note, resulting in a loss of $0.3 million and was recognized as a component of other income,net in the Company’s Condensed Consolidated Financial Statements based onof Operations. The G42 Promissory Note receivable was classified and accounted for under ASC 310 “Receivables“ (“ASC 310”) and was initially measured at its potential futurefair value of $11.9 million. The Company also recorded the $18.7 million as deferred revenue sources.In February 2016, the FASB issued ASU No. 2016-02, “Lease (Topic 842)” (“ASU 2016-02”), which increases transparency and comparability among companies accounting for lease transactions. The most significant change of this update will require the recognition by a lessee of lease assets and liabilities on its balance sheet for operating lease arrangements with lease terms greater than 12 months. This update will require a modified retrospective application which includes a number of optional practical expedients related to the identification and classification of leases commenced before the effective date. This ASU is effective for fiscal years and interim periods within those fiscal years, beginning after December 18, 2018. The adoption of this guidance will result in the recognitionCondensed Consolidated Balance Sheets, as none of additional assetsthe underlying performance obligations had been satisfied as of and liabilitiesfor the three months ended March 31, 2024.
Newsoara License Agreement
The Company is party to a license agreement with Newsoara Biopharma Co., Ltd., (“Newsoara”) (the “Newsoara License Agreement”) under which Newsoara obtained an exclusive and sublicensable license to develop and commercialize the Company’s phosphodiesterase type 4 inhibitors (“PDE4”) program, including the compound HPP737, in China, Hong Kong, Macau, Taiwan and other pacific rim countries (collectively, the “Newsoara License Territory”). Additionally, under the Newsoara License Agreement, the Company obtained a non-exclusive, sublicensable, royalty-free license to develop and commercialize certain Newsoara patent rights and know-how related to the Company’s operating leases within its Condensed Consolidated Balance Sheets.In March 2016, the FASB issued ASU No. 2016-09, “Compensation – Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting” (“ASU 2016-09”), which simplifies several aspectsPDE4 program for therapeutic uses in humans outside of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. Newsoara License Territory.
The Company
adoptedhas fully allocated the transaction price to the license and the technology transfer services which represents a single performance obligation because they were not capable of being distinct on their own. The Company recognized revenue for this
guidance inperformance obligation using the straight-line method over the transfer service period. The revenue for this performance obligation has been fully recognized as of March 31, 2024. In the first quarter of
fiscal 2017 on2024, the transaction price for this performance obligation was increased by $1.0 million due to the satisfaction of a
prospective basisdevelopment milestone under the Newsoara License Agreement. This amount was fully recognized as revenue during the three months ended March 31, 2024, as the related performance obligation was fully satisfied. No revenue related to this performance obligation was recognized and
will continue to estimate forfeitures of outstanding awards throughout the requisite service period. The adoption of this guidance did not have a material impact on the Company’s Condensed Consolidated Financial Statements.In November 2016, the FASB issued ASU No. 2016-18, “Statement of Cash Flows (Topic 230): Restricted Cash” (“ASU2016-18”), which clarifies the classification and presentation of changes in restricted cash on the statement of cash flows. The update requires beginning-of-period and end-of-period total amounts shown on the statement of cash flows to include cash and cash equivalents as well as restricted cash and restricted cash equivalents. This ASU is effective for fiscal years beginning after December 15, 2017, including interim reporting periods within those fiscal years. The Company’s adoption of this guidance will change the presentation of restricted cash and restricted cash equivalents within its Condensed Consolidated Statements of Cash Flows, but is not expected to have a material impact on the Company’s Condensed Consolidated Financial Statements.
In May 2017, the FASB issued ASU No. 2017-09, “Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting” (“ASU 107-09”), which clarifies thethere were no changes to terms or conditions of a share-based payment award that require an entity to apply modification accounting. ASU 2017-09 is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2017. Early application is permitted and prospective application is required. The Company does not expect that the adoption of this guidance will have a material impact ontransaction price during the Company's Condensed Consolidated Financial Statements.
three months ended March 31, 2023.Note 3:
| Note 4: Share-Based Compensation |
During the three and nine months ended September 30, 2017, the
The Company has issued non-qualified stock option awards to management, other key employees, consultants, and restricted stock units to certain employeesnon-employee directors. These option awards generally vest ratably over a three-year period and directorsthe option awards expire after a term of ten years from the Company.date of grant. As of September 30, 2017,March 31, 2024, the Company had total unrecognized stock-based compensation expense for its outstanding stock option awards of approximately $5.0$1.3 million, which is expected to be recognized over a weighted average period of 1.72.1 years. The weighted average grant date fair value of option grantsoptions granted during the ninethree months ended September 30, 2017 and 2016March 31, 2023 was $4.17 and $3.94$0.80 per option, respectively.option. There were no options granted during the three months ended March 31, 2024. The aggregate intrinsic value of the stock optionin-the-money awards outstanding at September 30, 2017March 31, 2024 was $0.2 million.de minimis.
On February 23, 2024, in connection with the Private Placement, several directors resigned as members of the Company’s Board of Directors, effective on the closing of the Private Placement. As a result of their resignations, 14,340 stock options to purchase shares of common stock were modified to increase the time period to exercise the options and 7,590 stock options to purchase shares of common stock were modified to accelerate vesting at the termination date. All the unvested options were modified to be fully vested as of the posting of the Private Placement which resulted in a reduction in their fair value. The Company usesincurred $0.1 million reduction in stock compensation expense for the Black-Scholes option pricing model to calculatemodifications for the fair value of stock options granted. The fair value of stock options granted was estimated using the following assumptions:three months ended March 31, 2024. | For the Nine Months Ended September 30, | |
| 2017 | | | 2016 | |
Expected volatility | 76.88% - 85.93% | | | 81.57% - 87.23% | |
Expected life of option, in years | 5.8 - 6.0 | | | 5.0 - 6.0 | |
Risk-free interest rate | 1.87% - 2.24% | | | 1.22% - 1.42% | |
Expected dividend yield | | 0.00% | | | | 0.00% | |
The following table summarizes the activity related to the stock option awards for the ninethree months ended September 30, 2017:
March 31, 2024: | Number of Shares | | | Weighted- Average Exercise Price | |
Awards outstanding at December 31, 2016 | | 1,096,101 | | | $ | 10.68 | |
Granted | | 872,500 | | | | 5.78 | |
Forfeited | | (16,202 | ) | | | 6.78 | |
Awards outstanding at September 30, 2017 | | 1,952,399 | | | $ | 8.52 | |
Options exercisable at September 30, 2017 | | 653,756 | | | $ | 11.23 | |
Weighted average remaining contractual term | 8.0 Years | | | | | |
Options vested and expected to vest at September 30, 2017 | | 1,862,429 | | | $ | 8.63 | |
Weighted average remaining contractual term | 8.6 Years | | | | | |
| | | | | | | | | | | |
| Number of Shares | | Weighted
Average Exercise Price |
| Awards outstanding at December 31, 2023 | 249,247 | | | $ | 77.53 | |
| | | |
| Forfeited | (625) | | | 28.80 | |
| Awards outstanding at March 31, 2024 | 248,622 | | | $ | 77.65 | |
| Options exercisable at March 31, 2024 | 163,336 | | | $ | 102.02 | |
| Weighted average remaining contractual term | 6.4 Years | | |
| Options vested and expected to vest at March 31, 2024 | 220,524 | | | $ | 83.88 | |
| Weighted average remaining contractual term | 7.0 Years | | |
The following table summarizes the activity related to the awards of RSUs for the nine months ended September 30, 2017:
| Number of Shares | | | Weighted- Average Grant Date Fair Value | |
Awards outstanding at December 31, 2016 | | — | | | $ | — | |
Granted | | 35,000 | | | | 5.81 | |
Awards outstanding at September 30, 2017 | | 35,000 | | | $ | 5.81 | |
RSUs vested and expected to vest at September 30, 2017 | | 34,002 | | | $ | 5.81 | |
As of September 30, 2017, the Company had total unrecognized stock-based compensation expense for its outstanding RSU awards of approximately $0.2 million, which is expected to be recognized over a weighted-average period of 2.4 years. The aggregate intrinsic value of the RSUs outstanding at September 30, 2017 was $0.2 million.
Compensation expense related to the grants of stock options and RSUs is included in research and development and general and administrative expense as follows (in thousands):
| Three Months Ended September 30, | | | Nine Months Ended September 30, | |
| 2017 | | | 2016 | | | 2017 | | | 2016 | |
| Three Months Ended March 31, | |
| Three Months Ended March 31, | |
| Three Months Ended March 31, | |
| 2024 | |
| 2024 | |
| 2024 | |
| Research and development | |
| Research and development | |
Research and development | $ | 405 | | | $ | 252 | | | $ | 1,077 | | | $ | 735 | |
General and administrative | | 554 | | | | 429 | | | | 1,580 | | | | 1,253 | |
| General and administrative | |
| General and administrative | |
Total share-based compensation expense | $ | 959 | | | $ | 681 | | | $ | 2,657 | | | $ | 1,988 | |
| Total share-based compensation expense | |
| Total share-based compensation expense | |
Note 4:Notes Payable
Notes payable consist of the following (in thousands):
| September 30, 2017 | | | December 31, 2016 | |
Notes payable under the Loan Agreement | $ | 20,000 | | | $ | 12,500 | |
Less: Debt discount | | (689 | ) | | | (1,442 | ) |
Total notes payable | | 19,311 | | | | 11,058 | |
Less: Current portion | | (2,083 | ) | | | — | |
Total notes payable, net of current portion | $ | 17,228 | | | $ | 11,058 | |
On October 28, 2016, the Company and vTv LLC entered into the Loan Agreement under which the Company and vTv LLC could borrow up to $25.0 million in three tranches of $12.5 million, $7.5 million and $5.0 million, respectively.
The Company borrowed the first tranche of $12.5 million upon closing of the transaction on October 28, 2016 and the second tranche of $7.5 million on March 24, 2017. The availability of the third tranche of $5.0 million expired unused on June 30, 2017.
Each loan tranche bears interest at a floating rate equal to 10.5% plus the amount by which the one-month London Interbank Offer Rate (“LIBOR”) exceeds 0.5%.
The Company has agreed to repay the first tranche of $12.5 million on an interest only basis monthly until May 1, 2018 followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date for the first tranche loan on May 1, 2020. In addition, a final payment for the first tranche loan equal to $0.8 million will be due on May 1, 2020, or such earlier date specified in the Loan Agreement. The Company has agreed to repay the second tranche of $7.5 million on an interest only basis monthly until October 1, 2018 followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date for the second tranche loan on October 1, 2020. In addition, a final payment for the second tranche loan equal to $0.5 million will be due on October 1, 2020, or such earlier date specified in the Loan Agreement.
If the Company repays all or a portion of the loan prior to the applicable maturity date, it will pay the Lenders a prepayment penalty fee, based on a percentage of the then outstanding principal balance equal to 4.0% during the first 18 months following the funding of the second tranche and 2.0% thereafter.
The Company’s obligations under the Loan Agreement are secured by a first priority security interest in substantially all of its assets other than its intellectual property. Subject to certain conditions related to the Company’s Phase 3 clinical trial of azeliragon, the Company may be required to grant a security interest in its intellectual property. The Company has agreed not to pledge or otherwise encumber its intellectual property assets, subject to certain exceptions.
The Loan Agreement includes customary affirmative and restrictive covenants, including, but not limited to, restrictions on the payment of dividends or other equity distributions and the incurrence of debt or liens upon the assets of the Company or its subsidiaries. The Loan Agreement does not contain any financial maintenance covenants. The Loan Agreement includes customary events of default, including payment defaults, covenant defaults, and material adverse change default. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5% will be applied to the outstanding loan balances, and the Lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement.
In connection with the Loan Agreement, the Company issued to the Lenders warrants to purchase shares of the Company’s Class A Common Stock (the “Warrants”). On October 28, 2016, the Company issued Warrants to purchase 152,580 shares of its Class A Common Stock at a per share exercise price of $6.39 per share, which aggregate exercise price represents 6.0% of the principal amount borrowed under the first tranche of the Loan Agreement and 3.0% of the amount available under the second tranche of the Loan Agreement. Additionally, upon funding of the second tranche on March 24, 2017, the Company issued Warrants to purchase 38,006 shares of its Class A Common Stock at a per share exercise price of $5.92 per share, which aggregate exercise price represents 3.0% of the amount available under the second tranche of the Loan Agreement. In each instance, the Warrants have an exercise price equal to the lower of (a) the volume weighted average price per share of the Company’s Class A Common Stock, as reported on the principal stock exchange on which the Company’s Class A Common Stock is listed, for 10 trading days prior to the issuance of the applicable Warrants or (b) the closing price of a share of the Company’s Class A Common Stock on the trading day prior to the issuance of the applicable Warrants. The Warrants will expire seven years from their date of issuance.
The Company incurred $0.7 million of costs in connection with the Loan Agreement. These costs, along with the allocated fair value of the Warrants issued for the first and second tranches which were treated as a debt discount, and are offset against the carrying value of the notes payable in the Company’s Consolidated Balance Sheet as of September 30, 2017 and December 31, 2016 and will be recognized as interest expense over the term of each applicable tranche using the effective interest method. The final payments for the first and second loan tranches will be accrued as additional interest expense, using the effective interest method, over the term of the relevant loan tranche.
The fair value of the Company’s notes payable is considered to approximate its carrying value because it bears interest at a variable interest rate.
Note 5:
| Note 5: Commitments and Contingencies |
From time to time, the Company is involved in various legal proceedings arising in the normal course of business. If a specific contingent liability is determined to be probable and can be reasonably estimated, the Company accrues and discloses the amount. The Company is not currently a party to any material legal proceedings.
Columbia University Agreement
In May 2015, the Company entered into a worldwide exclusive agreement with Columbia University (“Columbia”) to license certain intellectual property from Columbia. Under the agreement, the Company is obligated to pay to Columbia (1) an annual fee of $0.1 million from 2015 through 2021, (2) a potential regulatory milestone payment of $0.8 million and (3) potential royalty payments at a single digit royalty rate based on net sales of licensed products as defined in the agreement.
In February 2007, the Company entered into an Agreement (the “Novo License Agreement”) Concerning Glucokinase Activator Project with Novo Nordisk A/S (the “Novo License Agreement”Nordisk”) whereby wethe Company obtained an exclusive, worldwide, sublicensable license under certain Novo Nordisk intellectual property rights to discover, develop, manufacture, have manufactured, use and commercialize products for the prevention, treatment, control, mitigation or palliation of human or animal diseases or conditions. As part of this license grant, the Company obtained certain worldwide rights to Novo Nordisk’s GKA program, including rights to preclinical and clinical compounds such as TTP399. This agreement was amended in May 2019 to create milestone payments applicable to certain specific and non-specific areas of therapeutic use. Under the terms of the amended Novo License Agreement, the Company has additional potential developmental and regulatory milestone payments totaling up to $9.0 million for approval of a product for the treatment of type 1 diabetes, $50.5 million for approval of a product for the treatment of type 2 diabetes, or $115.0 million for approval of a product.product in any other indication. The Company may also be obligated to pay an additional $75.0 million in potential sales-based milestones, as well as royalty payments, at mid-single digit royalty rates, based on tiered sales of commercialized licensed products. Note 6:
| Redeemable Noncontrolling Interest
|
Note 6: Leases
In August 2019, the Company leased office space for its headquarters location under an operating lease. This lease commenced in November 2019 after the completion of certain tenant improvements made by the lessor. The lease includes an
option to renew for a five-year term as well as an option to terminate after three years, neither of which have been recognized as part of its related right of use assets or lease liabilities as their election is not considered reasonably certain. In November 2022, the Company entered into a second amendment to the lease, (i) to reduce the square footage and (ii) to extend the lease term, which constituted a modification event under ASC 842 and, the lease classification for the asset remains as an operating lease. As a result of the remeasurement of the associated lease liabilities, the Company recognized additional right of use assets and corresponding lease liabilities of $0.1 million. Further, the second amendment to the lease does not include any material residual value guarantee or restrictive covenants.
At each of March 31, 2024 and December 31, 2023, the weighted average incremental borrowing rate for the operating leases held by the Company was 9.5%. At March 31, 2024 and December 31, 2023, the weighted average remaining lease terms for the operating leases held by the Company were 1.7 years and 1.9 years, respectively.
Maturities of lease liabilities for the Company’s operating leases as of March 31, 2024 were as follows (in thousands):
| | | | | |
| 2024 (remaining nine months) | $ | 145 | |
| 2025 | 177 | |
| 2026 | — | |
| 2027 | — | |
| 2028 | — | |
| Thereafter | — | |
| Total lease payments | 322 | |
| Less: imputed interest | (24) | |
| Present value of lease liabilities | $ | 298 | |
Operating lease cost and the related operating cash flows for the three months ended March 31, 2024 was $0.1 million and was immaterial for the three months ended March 31, 2023.
Note 7: Noncontrolling Interest
The Company is subject to the Exchange Agreement with respect to the vTv Units representing the
70.5%19.2% noncontrolling interest in vTv LLC outstanding as of
September 30, 2017March 31, 2024 (see Note
7)9). The Exchange Agreement requires the surrender of an equal number of vTv Units and Class B
Common Stockcommon stock for (i) shares of Class A
Common Stockcommon stock on a one-for-one basis or (ii) cash (based on the fair market value of the Class A
Common Stockcommon stock as determined pursuant to the Exchange Agreement), at the Company’s option (as the managing member of vTv LLC), subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications. The exchange value is determined based on a
20 day20-day volume weighted average price of the Class A
Common Stock
common stock as defined in the Exchange Agreement, subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications.
On February 27, 2024, in connection with the Private Placement financing, the Investor Rights Agreement altered M&F TTP Holdings Two LLC (“M&F”) governance rights such that directors designated by M&F no longer comprised a majority of the Company’s Board of Directors (see Note 9). The redeemable noncontrolling interest redemption feature to exchange vTv Units for cash rather than shares of Class A common stock is recognizeda contingent event that is now within control of the Company through the Company’s independent Board of Directors. As a result, $5.3 million representing the fair value of redeemable noncontrolling interest on February 27, 2024, was reclassified from temporary equity in the mezzanine section of the Condensed Consolidated Balance Sheets to noncontrolling interest as a component of permanent equity.
Prior to February 27, 2024, the Company recorded redeemable noncontrolling interest at the higher of (1) theits initial fair value plus accumulated earnings/losses associated with the noncontrolling interest or (2) the redemption value as of the balance sheet date. At September 30, 2017 and December 31, 2016,2023, the redeemable noncontrolling interest was recorded based on the redemption value as of $130.6the balance sheet date of $6.1 million.
Changes in the Company’s ownership interest in vTv LLC while the Company retains its controlling interest in vTv LLC are accounted for as equity transactions, and the Company is required to adjust noncontrolling interest and equity for
such changes. The following is a summary of net income attributable to vTv Therapeutics Inc. and transfers to noncontrolling interest:
| | | | | | | | | | | | | | | |
| For the Three Months Ended March 31, | | |
| 2024 | | 2023 | | | | |
| Net loss attributable to vTv Therapeutics Inc. common shareholders | $ | (4,865) | | | $ | (4,499) | | | | | |
| Increase in vTv Therapeutics Inc. stockholders' equity (deficit) for sale of vTv Units as a result of common stock issuances | 9,564 | | | 1,345 | | | | | |
| Change from net loss attributable to vTv Therapeutics Inc. common shareholders and transfers to noncontrolling interest | $ | 4,699 | | | $ | (3,154) | | | | | |
Note 8: Stockholders’ Equity (Deficit)
Amendment to Certificate of Incorporation
On May 4, 2021, the Company filed an amendment to its Amended and Restated Certificate of Incorporation (the “Charter Amendment”) to increase the number of shares of Class A common stock that the Company is authorized to issue from 100,000,000 shares of Class A common stock to 200,000,000 shares of Class A common stock, representing an increase of 100,000,000 shares of authorized Class A common stock, with a corresponding increase in the total authorized common stock, which includes Class A common stock and Class B common stock, from 200,000,000 to 300,000,000, and a corresponding increase in the total authorized capital stock, which includes common stock and preferred stock, from 250,000,000 shares to 350,000,000 shares.
On November 20, 2023, the Company filed an amendment to its Amended and Restated Certificate of Incorporation as amended, to effect a reverse stock split at a ratio of 1-for-40 (the "Reverse Stock Split"). Pursuant to the Reverse Stock Split, every 40 shares of the Company’s Class A common stock was combined into one issued and outstanding share of Class A Common Stock and every 40 shares of the Company’s Class B common stock was combined into one issued and outstanding share of Class B Common Stock. The Reverse Stock Split did not reduce the number of authorized shares of Class A and Class B common stock, which remained at 200,000,000 and 100,000,000 respectively and did not change the par value of the common stock, which remained at $0.01 per share. The Reverse Stock Split did not have any effect on the number of authorized shares of the Company’s preferred stock, par value of $0.01 per share, which would remain at 50,000,000 shares. Currently no shares of preferred stock are outstanding.
Common Stock and Pre-funded Warrants
In February 2024, the Company entered into a Securities Purchase Agreement with certain Private Placement Investors, pursuant to which we agreed to issue and sell to the Private Placement Investors in a private placement (i) an aggregate of 464,377 shares of our Class A common stock, at a purchase price of $11.81 per share and (ii) issued pre-funded warrants to purchase an aggregate of 3,853,997 shares of the Company’s Class A common stock at a price of $11.80 per pre-funded warrant. The pre-funded warrants were immediately exercisable, have an exercise price of $0.01 and may be exercised at any time after the date of issuance. A holder of pre-funded warrants may not exercise the warrant if the holder, together with its affiliates, would beneficially own more than 9.99% of the number of shares of the Company’s common stock outstanding immediately after giving effect to such exercise. A holder of the pre-funded warrants may increase or decrease this percentage not in excess of 19.99% by providing at least 61 days’ prior notice to the Company. As of March 31, 2024, there were pre-funded warrants to purchase an aggregate of 3,970,587 shares of the Company’s common stock that remained available for exercise.
The pre-funded warrants were classified as a component of permanent equity in the Company's Condensed Consolidated Balance Sheet as they are freestanding financial instruments that are immediately exercisable, do not embody an obligation for the Company to repurchase its own shares and permit the holders to receive a fixed number of shares of common stock upon exercise. All of the shares underlying the pre-funded warrants have been included in the weighted-average number of shares of common stock used to calculate net loss per share attributable to common stockholders because the shares may be issued for little or no consideration, are fully vested and are exercisable after the original issuance date of the pre-funded warrants.
On March 5, 2024, the Company entered into a letter agreement with the Private Placement Investors pursuant to which the Private Placement Investors agreed to exchange an aggregate of 116,493 Private Placement Shares for an aggregate of 116,590 Private Placement Pre-Funded Warrants.
G42 Investments Transaction
On May 31, 2022, the Company and G42 Investments entered in to the G42 Purchase Agreement (see Note 3), pursuant to which the Company agreed to sell to G42 Investments 259,657 shares of the Company's Class A common stock at a price per share of approximately $96.40, for an aggregate purchase price of $25.0 million, consisting of (i) $12.5 million in cash at the closing of the transaction and (ii) $12.5 million in the form of a promissory note of G42 Investments to be paid at the one-year anniversary of the execution of the G42 Purchase Agreement (the “G42 Promissory Note”). On February 28, 2023, the Company and G42 Investments amended the G42 Purchase Agreement and modified the G42 Promissory Note to accelerate the payment due under the note. Pursuant to the amendment, on February 28, 2023, the Company received $12.0 million, which reflected the original amount due under the G42 Promissory Note less a 3.75% discount, in full satisfaction of the note, resulting in a loss of $0.3 million and $122.5was recognized as a component of other income, net in the Company’s Condensed Consolidated Statements of Operations.
CinPax and CinRx Transaction
On July 22, 2022 (the “Transaction Date”), the Company entered into the CinRx Purchase Agreement with CinPax, LLC (“CinPax”) and CinRx-Pharma, LLC (“CinRx”), pursuant to which the Company agreed to sell to CinPax 103,864 shares of the Company’s Class A common stock at a price per share of approximately $96.40, for an aggregate purchase price of $10.0 million,
respectively.which was paid (i) $6.0 million in cash at the closing of the transaction and (ii) $4.0 million in the form of a non-interest-bearing promissory note with CinPax and was paid to the Company on November 22, 2022. Common stock is generally recorded at fair value at the date of issuance. In determining the fair value of the Class A common stock issued to CinPax, the Company considered the closing price of the common stock on the Transaction Date. The Company did not make an adjustment to the fair value for sale restrictions on the stock in accordance with guidance recently adopted in ASU 2022-03. See the “Recently Issued Accounting Guidance” in this quarterly report on Form 10-Q for details of the ASU.Accordingly, the Company determined that cash consideration of $3.0 million should be recorded as fair value of the Class A common stock at the effective date, utilizing the Class A common stock closing price of $28.80 at the effective date.
The CinRx Purchase Agreement also provides CinRx warrants to purchase up to 30,000 shares of common stock at an initial exercise of price of approximately $28.80 per share (the “CinRx Warrants”). The CinRx Warrants were initially measured at fair value of $0.4 million using the Black-Scholes option model at the time of issuance and will be recorded in Warrant liability related party in the Condensed Consolidated Balance Sheets and will be subsequently remeasured at fair value through earnings on a recurring basis. (see Note 12)
The CinRx Warrants will become exercisable by CinRx only if (i) the Company receives approval from the U.S. Food and Drug Administration (“FDA Approval”) to market and distribute the pharmaceutical product containing the Company’s proprietary candidate, cadisegliatin (the “Product”), or (ii) the Company is acquired by a third party, sells all or substantially all of its assets related to the Product to a third party or grants a third party an exclusive license to develop, commercialize and manufacture the Product in the United States. If neither of these events happen within five years of the date of the issuance of the CinRx Warrants, the CinRx Warrants will expire and not be exercisable by CinRx. The exercise price of the CinRx Warrants and the number of shares issuable upon exercise of the CinRx Warrants are subject to adjustments in accordance with the terms of the CinRx Warrants.
Additionally, in conjunction with the CinRx Purchase Agreement the Company and CinRx entered into a Master Service Agreement (“CinRx MSA”) whereby CinRx provides the Company with consulting, preclinical and clinical trial services, as enumerated in project proposals negotiated between the Company and CinRx from time to time. (see Note 9)
The Company did not identify any other promises in the CinRx Purchase Agreement (aside from the issuance of common shares and the CinRx Warrants) and determined since there is no value ascribed to the CinRx MSA, the right to appoint a member and observer to the board of directors, that the remaining unallocated amount meets the definition of contributed equity and represents the amount in excess of par. The Company, CinPax and CinRx subsequently amended the CinRx Purchase Agreement on February 27, 2024, in connection with the Private Placement. The CinRx Purchase Agreement provides CinPax the right for two years following the Closing to designate a board observer, which has been subsequently approved by the Company’s board.
ATM Offering
On February 28, 2024, we entered into a sales agreement (the “TD Cowen Sales Agreement”) with Cowen and Company, LLC (“TD Cowen”), pursuant to which we may offer and sell, from time to time, through or to TD Cowen, as sales agent or principal, shares of our Class A common stock, having an aggregate offering price of up to $50.0 million (the
“TD Cowen ATM Offering”). Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities registered on the registration statement relating to the TD Cowen ATM Offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million. Under the terms of the TD Cowen Sales Agreement, we will pay TD Cowen a commission of 3.0% of the aggregate proceeds from the sale of shares and reimburse certain legal fees or other disbursements.
Note 7:
| Note 9: Related-Party Transactions |
PharmaCore, Inc.
Prior to its acquisition by Cambrex Corporation in October 2016, certain controlling shareholders of the Company also controlled PharmaCore, Inc. (“PharmaCore”) and PharmaCore was therefore considered to be a related party. The Company purchased chemistry and Good Manufacturing Practices manufacturing services from PharmaCore. Total purchases from PharmaCore for the three and nine months ended September 30, 2016 were $0.5 million and $0.8 million, respectively.
MacAndrews & Forbes Incorporated
As of September 30, 2017, subsidiaries and affiliates of
MacAndrews
& Forbes Incorporated (collectively “MacAndrews”) held 23,084,267directly or indirectly controls 577,108 shares of
the Company’s Class B
Common Stock and 2,565,666common stock. Further, as of March 31, 2024, MacAndrews directly or indirectly holds 912,982 shares of the Company’s Class A
Common Stock.common stock. As a result, MacAndrews’ holdings represent approximately
78.2%49.5% of the combined voting power of the Company’s outstanding common stock.
The Company has entered into several agreements with MacAndrews or its affiliates as partfurther detailed below:
Letter Agreements
The Company had previously entered into the Letter Agreements with MacAndrews. Under the terms of the
Reorganization Transactions as further detailed below:ExchangeLetter Agreements, during the one year commitment period beginning on the date of each Letter Agreement,
The the Company had the right to sell to MacAndrews shares of its Class A common stock at a specified price per share, and MacAndrews arehad the right (exercisable up to three times) to require the Company to sell to it shares of Class A common stock at the same price. The commitment period of each of the Letter Agreements has now expired. In addition, in connection with and as a commitment fee for the entrance into certain of these Letter Agreements, the Company also issued MacAndrews warrants (the “Letter Agreement Warrants”) to purchase additional shares of the Company’s Class A common stock.
The Letter Agreement Warrants have been recorded as warrant liability, related party within the Company’s Condensed Consolidated Balance Sheets based on their fair value. The issuance of the Letter Agreement Warrants was considered to an exchange agreement (the “Exchange Agreement”) pursuantbe a cost of equity recorded as a reduction to whichadditional paid-in capital.
Exchange Agreement
Pursuant to the terms of the Exchange Agreement, but subject to the Amended and Restated LLC Agreement of vTv Therapeutics LLC, the vTv Units (along with a corresponding number of shares of the Class B
Common Stock)common stock) are exchangeable for (i) shares of the
Company’s Class A
Common Stockcommon stock on a one-for-one basis or (ii) cash (based on the fair market value of the
Company’s Class A
Common Stockcommon stock as determined pursuant to the Exchange Agreement), at the
Company’s option
of vTv Therapeutics Inc. (as the managing member of vTv
Therapeutics LLC), subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications. Any decision to require an exchange for cash rather than shares of Class A
Common Stockcommon stock will ultimately be determined by the entire
boardBoard of
directors of vTv Therapeutics Inc. (the “Board of Directors”).Directors. As of
September 30, 2017,March 31, 2024, MacAndrews had not exchanged any shares under the provisions of
this agreement.the Exchange Agreement.
The Company and MacAndrews are party to a tax receivable agreement (the “Tax Receivable Agreement”), which provides for the payment by the Company to M&F TTP Holdings Two LLC (“M&F”), as successor in interest to vTv Therapeutics Holdings, LLC (“vTv Therapeutics Holdings”), and M&F TTP Holdings LLC (or certain of its transferees or other assignees) of 85% of the amount of cash savings, if any, in U.S. federal, state and local income tax or franchise tax that the Company actually realizes (or, in some circumstances, the Company is deemed to realize) as a result of (a) the exchange of Class B
Common Stock,common stock, together with the corresponding number of vTv Units, for shares of the Company’s Class A
Common Stockcommon stock (or for cash), (b) tax benefits related to imputed interest deemed to be paid by the Company as a result of the Tax Receivable Agreement and (c) certain tax benefits attributable to payments under the Tax Receivable Agreement.
As no shares have been exchanged by MacAndrews pursuant to the Exchange Agreement (discussed above), the Company has not recognized any liability, nor has it made any payments pursuant to the Tax Receivable Agreement as of September 30, 2017.
March 31, 2024.
Investor Rights Agreement
The Company is party to an investor rights agreement with M&F, as successor in interest to vTv Therapeutics Holdings (the “Investor Rights Agreement”). The Investor Rights Agreement provides M&F with certain demand, shelf, and piggyback registration rights with respect to its shares of Class A Common Stockcommon stock and also provides M&F with certain governance rights, depending on the size of its holdings of Class A Common Stock.common stock. Under the Investor Rights Agreement,
M&F was initially entitled to nominate a majority of the members of the Board of Directors and designate the members of the committees of the Board of Directors. The Investor Rights Agreement was amended on February 27, 2024 to alter M&F governance rights that now entitles M&F the right to designate two members of our Board of Directors, and as part of the Private Placement, the Private Placement Investors have rights to designate three members of our Board of Directors, making it more difficult for a third party to acquire control of our Board. The agreement with the Private Placement Investors also provides that five of our directors must approve certain actions including any acquisition by a third party, which makes it more difficult for our Board of Directors to approve such a transaction.
Note 8:
| Note 10: Income Taxes |
The Company is subject to U.S. federal income taxes as well as state taxes. As a result of theThe Company’s operating losses, theincome tax provision for three months ended March 31, 2024 was $0.1 million representing foreign withholding taxes accrued in connection with revenue recorded under license agreements with foreign entities. The Company did not record an income tax expenseprovision for the three and nine months ended September 30, 2017 and 2016. March 31, 2023.
Management has evaluated the positive and negative evidence surrounding the realization of its deferred tax assets, including the Company’s history of losses, and under the applicable accounting standards determined that it is more-likely-than-not that the deferred tax assets will not be realized. The difference between the effective tax rate of the Company and the U.S. statutory tax rate of
34%21% on March 31, 2024, is due to the valuation allowance against the Company’s expected net operating losses.
As discussed in Note
7,9, the Company is party to a tax receivable agreement with a related party which provides for the payment by the Company to
vTv Therapeutics HoldingsM&F (or certain of its transferees or other assignees) of 85% of the amount of cash savings, if any, in U.S. federal, state and local income tax or franchise tax that the Company actually realizes (or, in some circumstances, the Company is deemed to realize) as a result of certain transactions. As
there have been no transactions
which are probable to occurhave occurred which would trigger a liability under this agreement, the Company has not recognized any liability related to this agreement as of
September 30, 2017.March 31, 2024.Note 9:
| Net Loss per Share
|
Note 11: Net Loss per Share
Basic loss per share is computed by dividing net loss attributable to vTv Therapeutics Inc. by the
weighted-averageweighted average number of shares of Class A
Common Stockcommon stock outstanding during the period. Diluted loss per share is computed giving effect to all potentially dilutive shares. Diluted loss per share for all periods presented is the same as basic loss per share as the inclusion of potentially issuable shares would be antidilutive.
A reconciliation of the numerator and denominator used in the calculation of basic and diluted net loss per share of Class A
Common Stockcommon stock is as follows
(in(amounts in thousands, except
share and per share amounts):
| For the Three Months Ended September 30, | | | For the Nine Months Ended September 30, | |
| 2017 | | | 2016 | | | 2017 | | | 2016 | |
| For the Three Months Ended March 31, | |
| For the Three Months Ended March 31, | |
| For the Three Months Ended March 31, | |
| 2024 | |
| 2024 | |
| 2024 | |
| Numerator: | |
| Numerator: | |
Numerator: | | | | | | | | | | | | | | | |
Net loss | $ | (12,355 | ) | | $ | (13,505 | ) | | $ | (40,055 | ) | | $ | (41,642 | ) |
| Net loss | |
| Net loss | |
Less: Net loss attributable to noncontrolling interests | | (8,705 | ) | | | (9,512 | ) | | | (28,222 | ) | | | (29,340 | ) |
Net loss attributable to vTv Therapeutics Inc., basic and diluted | $ | (3,650 | ) | | $ | (3,993 | ) | | $ | (11,833 | ) | | $ | (12,302 | ) |
| Less: Net loss attributable to noncontrolling interests | |
| Less: Net loss attributable to noncontrolling interests | |
| Net loss attributable to common shareholders of vTv Therapeutics Inc., basic and diluted | |
| Net loss attributable to common shareholders of vTv Therapeutics Inc., basic and diluted | |
| Net loss attributable to common shareholders of vTv Therapeutics Inc., basic and diluted | |
Denominator: | | | | | | | | | | | | | | | |
Weighted-average vTv Therapeutics Inc. Class A Common Stock, basic and diluted | | 9,693,254 | | | | 9,691,362 | | | | 9,693,254 | | | | 9,495,926 | |
Net loss per share of vTv Therapeutics Inc. Class A Common Stock, basic and diluted | $ | (0.38 | ) | | $ | (0.41 | ) | | $ | (1.22 | ) | | $ | (1.30 | ) |
| Denominator: | |
| Denominator: | |
Weighted average vTv Therapeutics Inc. Class A common stock, basic and diluted (1)(*) | |
Weighted average vTv Therapeutics Inc. Class A common stock, basic and diluted (1)(*) | |
Weighted average vTv Therapeutics Inc. Class A common stock, basic and diluted (1)(*) | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
Net loss per share of vTv Therapeutics Inc. Class A common stock, basic and diluted (*) | |
| (*) Adjusted retroactively for reverse stock split | |
| (*) Adjusted retroactively for reverse stock split | |
| (*) Adjusted retroactively for reverse stock split | |
| | | | | | | | |
| (1) | The shares underlying the pre-funded warrants to purchase shares of the Company’s common stock have been included in the calculation of the weighted-average number of shares outstanding, basic and diluted, for the three months ended March 31, 2024. |
Potentially dilutive securities not included in the calculation of
diluteddilutive net loss per share are as follows:
| September 30, 2017 | | | September 30, 2016 | |
Class B Common Stock (1) | | 23,119,246 | | | | 23,119,246 | |
Common stock options | | 1,952,399 | | | | 1,099,434 | |
Restricted stock units | | 35,000 | | | | — | |
Common stock warrants | | 190,586 | | | | — | |
Total | | 25,297,231 | | | | 24,218,680 | |
| | | | | | | | | | | |
| March 31, 2024 | | March 31, 2023 |
Class B common stock (1)(*) | 577,349 | | | 577,349 | |
Common stock options granted under the Plan (*) | 248,622 | | | 227,372 | |
Common stock warrants (*) | 75,595 | | | 80,359 | |
Total (*) | 901,566 | | | 885,080 | |
| (*) Adjusted retroactively for reverse stock split | | | |
___________________________
| | | | | | | | |
| (1) | (1)
| Shares of Class B Common Stockcommon stock do not share in the Company’s earnings and are not participating securities. Accordingly, separate presentation of loss per share of Class B Common Stockcommon stock under the two-class method has not been provided. Each share of Class B Common Stockcommon stock (together with a corresponding vTv Unit) is exchangeable for one share of Class A Common Stock. common stock. |
Note 12: Fair Value of Financial Instruments
The carrying amount of certain of the Company’s financial instruments, including cash and cash equivalents, net accounts receivable, note receivable, accounts payable and other accrued liabilities approximate fair value due to their short-term nature.
The Company measures the value of its equity investments without readily determinable fair values at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level in which to classify them for each reporting period. This determination requires significant judgments. The following table summarizes the conclusions reached regarding fair value measurements as of March 31, 2024 and December 31, 2023 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Balance at March 31, 2024 | | Quoted Prices in Active
Markets for Identical Assets
(Level 1) | | Significant Other Observable
Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Liabilities: | | | | | | | |
Warrant liability, related party (1) | $ | 481 | | | $ | — | | | $ | — | | | $ | 481 | |
| Total | $ | 481 | | | $ | — | | | $ | — | | | $ | 481 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2023 | | Quoted Prices in Active
Markets for Identical Assets
(Level 1) | | Significant Other Observable
Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Liabilities: | | | | | | | |
Warrant liability, related party (1) | $ | 110 | | | $ | — | | | $ | — | | | $ | 110 | |
| Total | $ | 110 | | | $ | — | | | $ | — | | | $ | 110 | |
_____________________________
| ITEM 2.
| | | | |
| (1) | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Fair value determined using the Black-Scholes option pricing model. Expected volatility is based on the historical volatility of the Company’s common stock over the most recent period. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of valuation. |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Changes in Level 3 instruments for the three months ended March 31, |
| Balance at January 1 | | Net Change in
fair value included in
earnings | | Purchases /
Issuance | | Sales /
Repurchases | | Balance at March 31, |
| 2024 | | | | | | | | | |
| Warrant liability, related party | $ | 110 | | | $ | 371 | | | $ | — | | | $ | — | | | $ | 481 | |
| Total | $ | 110 | | | $ | 371 | | | $ | — | | | $ | — | | | $ | 481 | |
| | | | | | | | | |
| 2023 | | | | | | | | | |
| Warrant liability, related party | $ | 684 | | | $ | 238 | | | $ | — | | | $ | — | | | $ | 922 | |
| Total | $ | 684 | | | $ | 238 | | | $ | — | | | $ | — | | | $ | 922 | |
There were no transfers into or out of level 3 instruments and/or between level 1 and level 2 instruments during the three months ended March 31, 2024. Gains and losses recognized due to the change in fair value of the warrant liability, related party are recognized as a component of other expense, related party in the Company’s Condensed Consolidated Statements of Operations.
The fair value of the Letter Agreement Warrants was determined using the Black-Scholes option pricing model or option pricing models based on the Company’s current capitalization. Expected volatility is based on the historical volatility of the Company’s common stock over the most recent period. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of valuation. Significant inputs utilized in the valuation of the Letter Agreement Warrants as of March 31, 2024 and December 31, 2023, were:
| | | | | | | | | | | | | | | | | |
| March 31, 2024 | | December 31, 2023 |
| Range | Weighted Average | | Range | Weighted Average |
| Expected volatility | 93.31% - 142.10% | 101.45% | | 79.96% - 89.61% | 81.55% |
| Risk-free interest rate | 4.45% - 5.25% | 4.64% | | 4.01% - 4.87% | 4.15% |
The fair value of the CinRx Warrants was determined using the Black-Scholes option pricing model. Expected volatility is based on the historical volatility of the Company’s common stock over the most recent period. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of valuation. Significant inputs utilized in the valuation of the CinRx Warrants as of March 31, 2024, were:
| | | | | |
| Expected volatility | 91.6 | % |
| Expected life of options in years | 3.3 |
| Risk-free interest rate | 4.4 | % |
| Expected dividend yield | — | % |
The weighted average expected volatility and risk-free interest rate was based on the relative fair values of the warrants.
Changes in the unobservable inputs noted above would impact the amount of the liability for the Letter Agreement Warrants and CinRx Warrants. Increases (decreases) in the estimates of the Company’s annual volatility would increase (decrease) the liability and an increase (decrease) in the annual risk-free rate would increase (decrease) the liability.
Note 13: Subsequent Events
The Company evaluated subsequent events through May 9, 2024 and determined that there have been no events that have occurred that would require adjustments to our disclosures or the unaudited condensed consolidated financial statements.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
As used in this Quarterly Report on Form 10-Q, the “Company”, the “Registrant”, “we” or “us” refer to vTv Therapeutics Inc. and “vTv LLC” refers to vTv Therapeutics LLC. The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this report. In addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, assumptions and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this report under “Part II, Other Information—Item 1A, Risk Factors.” Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies and operations, financing plans, potential growth opportunities, potential market opportunities, potential results of our drug development efforts or trials, and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management’s plans, estimates, assumptions and beliefs only as of the date of this report. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
We are a clinical-stage biopharmaceuticalclinical stage pharmaceutical company engaged infocused on treating metabolic and inflammatory diseases to minimize their long-term complications and improve the discovery and developmentlives of orally administeredpatients. We have an innovative pipeline of first-in-class small molecule clinical and preclinical drug candidates to fill significant unmet medical needs. We have a pipeline of clinical drug candidates, led by our programs for the treatment of Alzheimer’s disease (“AD”) and diabetes.candidates. Our drug candidate for the treatment of AD, azeliragonlead program is cadisegliatin (TTP488)TTP399), is an orally administered, small molecule, antagonist targeting the receptor for advanced glycation endproducts (“RAGE”), for which we have successfully completed the enrollment of patients in both sub-studies in our Phase 3 clinical trial (the “STEADFAST Study”) under a Food and Drug Administration (“FDA”) agreed Special Protocol Assessment (“SPA”). Our type 2 diabetes drug candidates include TTP399, an orally administered, liver-selective glucokinase activator (“GKA”) as an adjunctive therapy to insulin for the treatment of type 1 diabetes (“T1D”).
Recent Developments
Private Placement of Class A Common Shares and Pre-Funded Warrants
On February 27, 2024, we entered into a securities purchase agreement (the “Securities Purchase Agreement”) with certain institutional accredited investors (the “Private Placement Investors”),
forpursuant to which we
completedagreed to issue and sell to the Private Placement Investors in a
Phase 2b clinical trialprivate placement (the
“AGATA Study”“Private Placement”)
in August 2016,(i) an aggregate of 464,377 shares (the “Private Placement Shares”) of our Class A common stock, at a purchase price of $11.81 per share, and
TTP273,(ii) pre-funded warrants (the “Private Placement Pre-Funded Warrants”) to purchase up to an
orally administered, non-peptide agonist that targetsaggregate of 3,853,997 shares of our Class A common stock (the “Private Placement Warrant Shares”) at a purchase price of $11.80 per Private Placement Pre-Funded Warrant (representing the
glucagon-like peptide-1 receptor (“GLP-1r”), for which we completed a Phase 2 clinical trial (the “LOGRA Study”) in December 2016.In August 2017,$11.81 per Private Placement Share purchase price less the exercise price of $0.01 per Private Placement Warrant Share). We received aggregate gross proceeds from the Private Placement of approximately $51.0 million, before deducting offering expenses payable by us. The Private Placement Pre-Funded Warrants are exercisable at any time after their original issuance and will not expire.
On March 5, 2024, the Company entered into a letter agreement with the JDRF AgreementPrivate Placement Investors pursuant to supportwhich the fundingPrivate Placement Investors agreed to exchange an aggregate of 116,493 Private Placement Shares for an adaptive Phase 1b/2 study to explore the effectsaggregate of TTP399, an orally administered, liver-selective glucokinase activator116,590 Private Placement Pre-Funded Warrants.
ATM
On February 28, 2024, we entered into a sales agreement (the “TD Cowen Sales Agreement”) with Cowen and Company, LLC (“GKA”TD Cowen”), pursuant to which we may offer and sell, from time to time, through or to TD Cowen, as sales agent or principal, shares of our Class A common stock, having an aggregate offering price of up to $50.0 million (the “TD Cowen ATM Offering”). Pursuant to General Instruction I.B.6 of Form S-3, in type 1 diabetics. We planno event will we sell securities registered on the registration statement relating to initiate this trialthe TD Cowen ATM Offering with a value exceeding more than one-third of our public float in the fourth quarter of 2017. According toany 12-month period so long as our public float remains below $75.0 million. Under the terms of the JDRFTD Cowen Sales Agreement, JDRFwe will provide research fundingpay TD Cowen a commission of up to $3.0 million based on the achievement of research and development milestones, with the total funding not to exceed approximately one-half3% of the total costaggregate proceeds from the sale of the project. Additionally, the Company has the obligation to makeshares and reimburse certain milestone payments to JDRF upon the commercialization, licensing, salelegal fees or transferother disbursements.
The following table summarizes our current drug candidates, their partnership status and their respective stages of development:

Our Type 1 Diabetes Program – Cadisegliatin (TTP399)
The U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation in 2021 for cadisegliatin as an adjunctive therapy to insulin for the treatment of type 1 diabetes. The Breakthrough Therapy designation provides a sponsor with added support and the potential to expedite development and review timelines for a promising new investigational medicine. The Breakthrough Therapy designation for cadisegliatin in T1D was supported by the positive results from the Phase 2 SimpliciT-1 Study, a multi-center, randomized, double-blind, adaptive study assessing the safety and efficacy of cadisegliatin as an adjunct to insulin therapy in adults with T1D. In this trial, treatment with cadisegliatin resulted in a statistically significant improvement in HbA1c relative to placebo and a clinically meaningful decrease (40%) in the frequency of severe and symptomatic hypoglycemia. Cadisegliatin demonstrated a favorable safety profile, in which abnormal levels of serum or urine ketones were detected less frequently in patients taking cadisegliatin than those taking placebo.
In May of 2023, the FDA issued new draft guidance on "Diabetes Mellitus: Efficacy Endpoints for Clinical Trials Investigating Antidiabetic Drugs and Biological Products" which, for the first time, permitted the use of hypoglycemia as an endpoint to support a label claim. Consistent with this guidance and with input from the FDA, we have initiated a Phase 3 double-blind controlled trial to assess the effect of cadisegliatin on reducing the frequency of Level 2 hypoglycemia (blood glucose levels are less than 54 mg/dL or 3 mmol/L, regardless of symptoms) and Level 3 hypoglycemia ("severe" hypoglycemia e.g., requiring assistance of another person) in 150 patients with type 1 diabetes. Participants in the trial will be randomized to two doses of cadisegliatin or placebo. Reduction in glycated hemoglobin (HbA1c), a traditional efficacy endpoint in diabetes trials, is a key secondary endpoint to assess the potential of cadisegliatin to reduce hyperglycemia, Following the initial assessment of efficacy after six months of treatment, participants will remain on trial for another six months to assess the durability of potential beneficial effects and safety. The study protocol was submitted to the FDA on February 29, 2024. The trial will be conducted in the U.S. only and is expected to complete enrollment by the fourth quarter in 2024 providing top line 1-year data by the first quarter in 2026.
Concurrently, we will be working on the design for two international registrational studies for cadisegliatin in type 1 diabetes,which we expect to start in 2026.
In addition, we continue to work with our partner, G42 Investments AI Holding RSC Ltd. (“G42”), to initiate a double-blind, randomized, controlled Phase 2 trial in the Middle East region in 450 insulin-using patients with type 2 diabetes. We expect that trial to begin in 2024.
Holding Company Structure
vTv Therapeutics Inc. is a holding company and its principal asset is a controlling equity interest in vTv Therapeutics LLC (“vTv LLC”), the principal operating subsidiary.
The Company hasWe have determined that vTv LLC is a variable-interest entity (“VIE”) for accounting purposes and that vTv Therapeutics Inc. is the primary beneficiary of vTv LLC because (through its managing member interest in vTv LLC and the fact that the senior management of vTv Therapeutics Inc. is also the senior management of vTv LLC) it has the power to direct all of the activities of vTv LLC, which include those that most significantly impact vTv LLC’s economic performance. vTv Therapeutics Inc. has therefore consolidated vTv LLC’s results under the VIE accounting model in its consolidated financial statements.
To date, we have devoted substantially all of our resources to our research and development efforts relating to our drug candidates, including conducting clinical trials with our drug candidates, providing general and administrative support for these operations and protecting our intellectual property. We do not have any products approved for sale and have not generated any revenue from drug sales. From our inception through September 30, 2017, we have funded our operations primarily through a combination of private placements of preferred equity, research collaboration agreements, upfront and milestone payments for license agreements, debt financing and the completion of our initial public offering (“IPO”) in August 2015.
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially as we:
continue the development of our lead drug candidate, azeliragon, for the treatment of AD;
seek to obtain regulatory approvals for azeliragon;
prepare for the potential commercialization of azeliragon;
begin outsourcing the commercial manufacturing of azeliragon;
expand our research and development activities and advance our clinical programs, including our type 2 diabetes programs, TTP399 and TTP273; and
maintain, expand and protect our intellectual property portfolio.
We do not expect to generate revenue from drug sales unless and until we successfully complete development and obtain marketing approval for one or more of our drug candidates, which we expect will take a number of years and will be subject to significant uncertainty. Accordingly, we anticipate that we will need to raise additional capital in addition to the net proceeds of the IPO and the Loan Agreement prior to the commercialization of azeliragon or any of our other drug candidates. Until such time that we can generate substantial revenue from product sales, we expect to finance our operating activities through a combination of equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing
arrangements. Nevertheless, we may be unable to raise additional funds or enter into such other arrangements when needed, on favorable terms or at all, which would have a negative impact on our liquidity and financial condition and could force us to delay, reduce the scope or eliminate one or more of our research and development programs or commercialization efforts. Failure to receive additional funding could cause us to cease operations, in part or in full.
Financial Overview
To date, we have not generated any revenue from drug sales.
All of ourOur revenue
to date has been primarily derived from
milestone payments, up-front proceeds and research fees under collaboration and license
agreements and government grants.agreements.
In the future, we may generate revenue from a combination of product sales, license fees, milestone payments and royalties from the sales of products developed under licenses of our intellectual property. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the timing and amount of license fees, milestone and other payments, and the amount and timing of payments that we receive upon the sale of our products, to the extent any are successfully commercialized. If we fail to complete the development of our drug candidates in a timely manner or obtain regulatory approval for them, our ability to generate future revenue and our results of operations and financial position will be materially adversely affected.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for our drug candidates. We recognize research and development expenses as they are incurred. Our direct research and development expenses consist primarily of external costs such as fees paid to investigators, consultants, central laboratories and clinical research organizations
(“CRO”s) in connection with our clinical trials, and costs related to acquiring and manufacturing clinical trial materials. Our indirect research and development costs consist primarily of
salaries,cash and share-based compensation costs, the cost of employee benefits and related overhead expenses for personnel in research and development
functions and depreciation of leasehold improvements, laboratory equipment and computers.functions. Since we typically use our employee and infrastructure resources across multiple research and development programs such costs are not allocated to the individual projects.
From our inception, including our predecessor companies, through September 30, 2017, we have incurred approximately $531.8 million in research and development expenses.
Our research and development expenses by project for the three and nine months ended September 30, 2017March 31, 2024 and 20162023 were as follows (in thousands):
| Three Months Ended September 30, | | | Nine Months Ended September 30, | |
| Three Months Ended March 31, | |
| Three Months Ended March 31, | |
| Three Months Ended March 31, | |
| 2024 | |
| 2024 | |
| 2024 | |
| Direct research and development expense: | |
| Direct research and development expense: | |
| Direct research and development expense: | |
| Cadisegliatin | |
| Cadisegliatin | |
| Cadisegliatin | |
| HPP737 | |
| HPP737 | |
| HPP737 | |
| 2017 | | | 2016 | | | 2017 | | | 2016 | |
Direct research and development expense: | | | | | | | | | | | | | | | |
Azeliragon | $ | 6,163 | | | $ | 6,287 | | | $ | 20,948 | | | $ | 21,372 | |
TTP399 | | 60 | | | | 749 | | | | 199 | | | | 2,396 | |
TTP273 | | 42 | | | | 1,228 | | | | 323 | | | | 3,151 | |
| Other projects | |
| | Other projects | |
| Other projects | | 290 | | | | 685 | | | | 942 | | | | 1,139 | |
Indirect research and development expense | | 2,434 | | | | 2,216 | | | | 7,160 | | | | 6,591 | |
| Indirect research and development expense | |
| Indirect research and development expense | |
Total research and development expense | $ | 8,989 | | | $ | 11,165 | | | $ | 29,572 | | | $ | 34,649 | |
| Total research and development expense | |
| Total research and development expense | |
We plan to continue to incur significant research and development expenses for the foreseeable future as we continue the development of azeliragoncadisegliatin and further advance the development of our other drug candidates, subject to the availability of additional funding. The successful development of our clinical and preclinical drug candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our clinical or preclinical drug candidates or the period, if any, in which material net cash inflows
from these drug candidates may commence. This is due to the numerous risks and uncertainties associated with the development of our drug candidates, including:
•the uncertainty of the scope, rate of progress and expense of our ongoing, as well as any additional, clinical trials and other research and development activities;
•the potential benefits of our candidates over other therapies;
•our ability to market, commercialize and achieve market acceptance for any of our drug candidates that we are developing or may develop in the future;
| •
| our ability to market, commercialize and achieve market acceptance for any of our drug candidates that we are developing or may develop in the future;
|
•future clinical trial results;
•our ability to enroll patients in our clinical trials;
•the timing and receipt of any regulatory approvals, if any;approvals;
•our ability to secure sufficient capital and
cash resources, including access to available debt and equity financing and revenues from operations, to satisfy all of our short-term and longer-term cash requirements and other cash needs, at the times and in the amounts needed;•legislation and regulatory actions and changes in laws or regulations; and
•the filing, prosecuting, defending and enforcing of patent claims and other intellectual property rights, and the expense of doing so.
A change in the outcome of any of these variables with respect to the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of clinical development of a drug candidate, or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time with respect to the development of that drug candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and related costs for employees in executive, finance, corporate development,
and human resources and administrative support functions. Other significant general and administrative expenses include accounting and legal services, expenses associated with obtaining and maintaining patents, cost of various consultants, occupancy costs and information systems.
We expect that
Interest Income
Interest income represents noncash interest income related to the imputed interest from the G42 Promissory Note receivable using the effective interest method, which ended in 2023, and cash interest income from dividends and interest from our
general and administrative expenses will continue to increase as we operate as a public company and commercializemoney market account, all of which are recognized in our
drug candidates. We also expect to incur additional costs in future periods as we continue to establish our investor relations function, implement a systemCondensed Consolidated Statement of
internal control over financial reporting and a system of disclosure controls and procedures that are compliant with applicable requirements and with corporate governance requirements and other rules of the stock exchange on which we are listed and other similar requirements applicable to public companies.Interest Expense
Beginning in October 2016, interest expenseOperations.
Other (Expense)/Income, net
Other expense/income primarily consists of cash and non-cash interest expense relatedunrealized gains or losses attributable to our Loan Agreement. Cash interest on the Loan Agreement is recognized at a floating interest rate equal to 10.5% plus the amount by which the one-month London Interbank Offer Rate (“LIBOR”) exceeds 0.5%. Non-cash interest expense represents the amortizationchanges in fair value of the costs incurredequity investments in connection withReneo Pharmaceuticals, Inc ("Reneo"), the Loan Agreement, the allocatedrecognition of changes in fair value of the warrants to purchase shares of our Class A Common Stock issued in connection with the Loan Agreement (the “Warrants”)common stock held by related parties and the accretionloss from the G42 promissory note early redemption on February 28, 2023.
Results of Operations
Comparison of the three months ended
September 30, 2017March 31, 2024 and
20162023
The following table sets forth certain information concerning our results of operations for the periods shown:
| (dollars in thousands) | Three Months Ended September 30, | | (dollars in thousands) | Three Months Ended March 31, |
Statement of operations data: | 2017 | | | 2016 | | | Change | | Statement of operations data: | 2024 | | 2023 | | Change |
Revenue | $ | 15 | | | $ | 38 | | | $ | (23 | ) | Revenue | $ | 1,000 | | $ | — | | $ | 1,000 |
Operating expenses: | | | | | | | | | | | |
| Research and development | |
| Research and development | |
Research and development | | 8,989 | | | | 11,165 | | | | (2,176 | ) | 2,649 | | 3,942 | | (1,293) |
General and administrative | | 2,567 | | | | 2,401 | | | | 166 | | General and administrative | 3,978 | | 3,485 | | 493 |
Total operating expenses | | 11,556 | | | | 13,566 | | | | (2,010 | ) | Total operating expenses | 6,627 | | 7,427 | | (800) |
Operating loss | | (11,541 | ) | | | (13,528 | ) | | | 1,987 | | Operating loss | (5,627) | | (7,427) | | 1,800 |
Interest income | | 35 | | | | 21 | | | | 14 | | Interest income | 79 | | 100 | | (21) |
Interest expense | | (849 | ) | | | — | | | | (849 | ) | Interest expense | — | | — |
Other income (expense), net | | — | | | | 2 | | | | (2 | ) |
Loss before income taxes | | (12,355 | ) | | | (13,505 | ) | | | 1,150 | |
| Other (expense) income, net | | Other (expense) income, net | (371) | | 1,553 | | (1,924) |
| Loss before income taxes and noncontrolling interest | | Loss before income taxes and noncontrolling interest | (5,919) | | (5,774) | | (145) |
Income tax provision | | — | | | | — | | | | — | | Income tax provision | 100 | | — | | 100 |
Net loss before noncontrolling interest | | (12,355 | ) | | | (13,505 | ) | | | 1,150 | | Net loss before noncontrolling interest | (6,019) | | (5,774) | | (245) |
Less: net loss attributable to noncontrolling interest | | (8,705 | ) | | | (9,512 | ) | | | 807 | | Less: net loss attributable to noncontrolling interest | (1,154) | | (1,275) | | 121 |
Net loss attributable to vTv Therapeutics Inc. | $ | (3,650 | ) | | $ | (3,993 | ) | | $ | 343 | | Net loss attributable to vTv Therapeutics Inc. | $ | (4,865) | | $ | (4,499) | | $ | (366) |
Revenue
was insignificant for the three months ended
September 30, 2017 and 2016.March 31, 2024, includes a $1.0 million increase to the transaction price for the license performance obligation under the Newsoara License Agreement due to the satisfaction of a development milestones. There was no revenue for the three months ended March 31, 2023.
Research and Development Expenses
Research and development expenses were $9.0$2.6 million and $11.2$3.9 million for the three months ended September 30, 2017March 31, 2024 and 2016,2023, respectively. The decrease in research and development expenses during thethis period of $2.2$1.3 million or 19.5%32.8%, was primarily driven by i) lower spending on cadisegliatin of $1.5 million, due to:A decreaseto decreases in toxicity study costs and drug manufacturing related costs partially offset by increases in clinical trial start-up costs, of $0.1 million for azeliragon which was mainly driven by a decrease of $0.6 million related to the timing of expenses for our ongoing STEADFAST study, offset byand ii) an increase in indirect costs and other projects of $0.5 million related to the costs for our open-label extension (“OLE”) trial as patients completing the STEADFAST Study elect to roll into the OLE trial;
Costs related to TTP399 in the third quarter of 2017 decreased $0.7 million from the three months ended September 30, 2016, due to the completion of the AGATA study in August 2016;
A decrease in clinical trial costs of $1.2 million for TTP273 in the third quarter of 2017 as compared with the third quarter of 2016, due to the completion of the LOGRA study in December 2016.
General and Administrative Expenses
Expense
s General and administrative expenses were
$2.6$4.0 million and
$2.4$3.5 million for the three months ended
September 30, 2017March 31, 2024 and
2016,2023, respectively. The increase in general and administrative expenses during
thethis period of
$0.2$0.5 million, or
6.9%14.1%, was primarily
attributable todriven by i) increases
in the expense related to share-based awards due to the granting of
additional awards in fiscal 2017.Interest Expense
Interest expense was $0.8 million in payroll related costs, ii) increases of $0.3 million in legal expenses, partially offset by iii) decreases of $0.5 million in other general and an insignificant amountadministrative costs, and iv) decreases of $0.1 million in share-based expense.
Interest Income
Interest income for the three months ended September 30, 2017 and 2016, respectively.March 31, 2024, of $0.1 million, is related to dividend income from our money market account. Interest expense recognized in 2017 relates toincome for the cash and non-cash interest for our Loan Agreement which was finalized in late October 2016 and which bears interest at 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%.
Comparison of the ninethree months ended September 30, 2017 and 2016
The following table sets forth certain information concerning our resultsMarch 31, 2023, of operations for
$0.1 million, is related to imputed interest on the periods shown:promissory notes. (dollars in thousands) | Nine Months Ended September 30, | |
Statement of operations data: | 2017 | | | 2016 | | | Change | |
Revenue | $ | 58 | | | $ | 596 | | | $ | (538 | ) |
Operating expenses: | | | | | | | | | | | |
Research and development | | 29,572 | | | | 34,649 | | | | (5,077 | ) |
General and administrative | | 8,396 | | | | 7,654 | | | | 742 | |
Total operating expenses | | 37,968 | | | | 42,303 | | | | (4,335 | ) |
Operating loss | | (37,910 | ) | | | (41,707 | ) | | | 3,797 | |
Interest income | | 95 | | | | 66 | | | | 29 | |
Interest expense | | (2,240 | ) | | | (3 | ) | | | (2,237 | ) |
Other income (expense), net | | — | | | | 2 | | | | (2 | ) |
Loss before income taxes | | (40,055 | ) | | | (41,642 | ) | | | 1,587 | |
Income tax provision | | — | | | | — | | | | — | |
Net loss before noncontrolling interest | | (40,055 | ) | | | (41,642 | ) | | | 1,587 | |
Less: net loss attributable to noncontrolling interest | | (28,222 | ) | | | (29,340 | ) | | | 1,118 | |
Net loss attributable to vTv Therapeutics Inc. | $ | (11,833 | ) | | $ | (12,302 | ) | | $ | 469 | |
Revenue
Revenue
Other expense was insignificant for the nine months ended September 30, 2017 and was $0.6$0.4 million for the ninethree months ended September 30, 2016. The revenue earned during the nine months ended September 30, 2016March 31, 2024, and was primarily attributabledriven by losses related to the global license agreement that we entered into with Calithera Biosciences, Inc.change in the fair value of the outstanding warrants to purchase shares of our own stock issued to related parties (“Calithera”Related Party Warrants”) in March 2015.Research and Development Expenses
Research and development expenses were $29.6 million and $34.6. Other income was $1.6 million for the ninethree months ended September 30, 2017 March 31, 2023,
and 2016, respectively. The decrease in research and development expenses during the period of $5.1 million, or 14.7%, was primarily due to:A decrease in clinical trial costs of $0.4 million for azeliragon for the nine months ended September 30, 2017 which was driven by a $1.8 million decrease in costsan unrealized gain related to our investment in Reneo, losses related to the drug-drug interaction study which is now complete and a decrease of $1.2 million for compound manufacturing costs due to timing of activities. These decreases were offset by increaseschange in the costfair value of the STEADFASToutstanding warrants to purchase shares of our stock issued to related parties and OLE studies of $1.0 million each, due to higher enrollment in these trials during the nine months ended September 30, 2017;
Costs related to TTP399 in the nine months ended September 30, 2017 decreased $2.2 millionloss from the nine months ended September 30, 2016, due to the completionG42 promissory note early redemption.
A decrease in clinical trial costs of $2.8 million for TTP273 for the nine months ended September 30, 2017, due to the completion of the LOGRA study in December 2016.
General and Administrative Expenses
General and administrative expenses were $8.4 million and $7.7 million for the nine months ended September 30, 2017 and 2016, respectively. The increase in general and administrative expenses during the period of $0.7 million, or 9.7%, was primarily attributable to increases in compensation expense, including the expense for share-based awards.
Interest Expense
Interest expense was $2.2 million and an insignificant amount for the nine months ended September 30, 2017 and 2016, respectively. Interest expense recognized in 2017 relates to the cash and non-cash interest for our Loan Agreement which was finalized in late October 2016 and which bears interest at 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%.
Liquidity and Capital Resources
Liquidity
and Going ConcernAs of September 30, 2017,March 31, 2024, we havehad an accumulated deficit of $263.7 million as well as$286.1 million. Since our inception, we have experienced a history of negative cash flows from operating activities. We anticipate that we will continue to incur losses and negative cash flow from operations for the foreseeable future as we continue our clinical trials. Further, we expect that we will need additional capital to continue to fund our operations. Our currently available sourcesAs of liquidity include ourMarch 31, 2024, we had cash and cash equivalents of $20.5$52.3 million.
On February 27, 2024, the Company closed a private placement financing of up to $51.0 million at September 30, 2017. Based on our current operating plan, we believe that our current cash and cash equivalentsadditionally granting investors the right to purchase up to an additional $30.0 million of common stock 18 months following the closing of the private placement financing. The financing raised will allow usthe Company to meet our liquidity requirements through the receipt of top-line resultsfurther advance its lead program for Subpart A of our STEADFAST Study in early 2018. cadisegliatin.
In addition to available cash and cash equivalents
and available funds discussed above, we are seeking possible
additional partnering opportunities for our GKA, GLP-1r and other drug candidates which we believe may provide additional cash for use in our operations and the continuation of the clinical trials for our drug candidates. We
will also pursue other sourcesare evaluating several financing strategies to fund our planned and ongoing clinical trials, including direct equity investments and future public offerings of
interim financing to provide flexibility to our
operating plan.common stock. The timing and
occurrenceavailability of such
interimadditional financing
isare not yet known.
These factors raise substantial doubt regarding our ability to continue as a going concern. Debt Transaction
In October 2016,
ATM Offering
TD Cowen Sales Agreement
On February 28, 2024, we and vTv LLC entered into the Loan Agreement, undera sales agreement (the "TD Cowen Sales Agreement") with Cowen and Company, LLC (“TD Cowen”) pursuant to which we have borrowed $20.0 million. Each loan tranche bears interest at a floating rate equalmay offer and sell, from time to 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%. We borrowed the first tranche of $12.5 million upon the close of the Loan Agreement in October 2016. Payments with respecttime, through or to the first tranche are payable on an interest only basis monthly until May 1, 2018, followed by equal monthly payments ofTD Cowen, as sales agent or principal, plus accrued interest through the scheduled maturity date on May 1, 2020. In addition, a final payment for the first tranche loan equal to $0.8 million will be due on May 1, 2020, or such earlier date specified in the Loan Agreement. We borrowed the second tranche of $7.5 million in March 2017. Payments with respect to the second tranche are payable on an interest only basis monthly until October 1, 2018, followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date on October 1, 2020. In addition, a final payment for the second tranche loan equal to $0.5 million will be due on October 1, 2020, or such earlier date specified in the Loan Agreement. The availability of the third tranche of $5.0 million expired unused on June 30, 2017.
If we repay all or a portion of the loan prior to the applicable maturity date, we will pay the Lenders a prepayment penalty fee, based on a percentage of the then outstanding principal balance equal to 4.0% during the first 18 months following the funding of the second tranche and 2.0% thereafter.
In connection with the Loan Agreement, we have issued to the Lenders warrants to purchase shares of our Class A common stock. On October 28, 2016, we issued Warrants to purchase 152,580 shares of our Class A common stock at a per share exercisehaving an aggregate offering price of $6.39 per share, which aggregate exercise price represents 6.0%up to $50.0 million, although we may only offer and sell under the TD Cowen ATM Offering up to one-third of the principal amount borrowed under the first tranche of the Loan Agreement and 3.0% of the amount available under the second tranche of the Loan Agreement. On March 24, 2017, in connection with the funding of the second tranche, we issued Warrants to purchase 38,006 sharesaggregate market value of our Class A common stock at a per share exercise priceheld by non-affiliates during any 12 calendar month period pursuant to
General Instruction I.B.6 of $5.92 per share, which aggregate exercise price represents 3.0%Form S-3. We are not obligated to sell any shares under the TD Cowen Sales Agreement. Under the terms of the principal amountTD Cowen Sales Agreement, we will pay TD Cowen a commission of 3% of the second tranche. In each instance,aggregate proceeds from the Warrants have an exercise price equal to the lowersale of (a) the volume weighted average price per share of our Class A common stock, as reported on the principal stock exchange on which our Class A common stock is listed, for 10 trading days prior to the issuance of the applicable Warrants or (b) the closing price of a share of our Class A common stock on the trading day prior to the issuance of the applicable Warrants. The Warrants will expire seven years from their date of issuance.The Loan Agreement includes customary affirmativeshares and restrictive covenants, including, but not limited to, restrictions on the payment of dividendsreimburse certain legal fees or other equity distributions and the incurrence of debt or liens upon the assets of the Company or its subsidiaries. The Loan Agreement does not contain any financial maintenance covenants. The Loan Agreement includes customary events of default, including payment defaults, covenant defaults and material adverse change default. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5% will be applied to the outstanding loan balances, and the Lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement.
disbursements.
Cash Flows
| | Nine Months Ended | |
| | September 30, | |
| | 2017 | | | 2016 | |
(dollars in thousands) | | | | | | | | |
Net cash used in operating activities | | $ | (38,510 | ) | | $ | (36,783 | ) |
Net cash used in investing activities | | | (7 | ) | | | (83 | ) |
Net cash provided by (used in) financing activities | | | 7,500 | | | | (79 | ) |
Net decrease in cash and cash equivalents | | $ | (31,017 | ) | | $ | (36,945 | ) |
| | | | | | | | | | | |
| Three Months Ended March 31, |
| 2024 | | 2023 |
| (dollars in thousands) | | | |
| Net cash used in operating activities | $ | (7,335) | | | $ | (5,166) | |
| | | |
| Net cash provided by financing activities | 50,144 | | | 11,806 | |
| Net increase in cash and cash equivalents | $ | 42,809 | | | $ | 6,640 | |
For the ninethree months ended September 30, 2017,March 31, 2024, our net cash used in operating activities increased $1.7by $2.2 million from the ninethree months ended September 30, 2016.March 31, 2023. The increased use ofsignificant contributor to the change in cash used during the year was primarily driven by a higher usage of existing working capital. capital changes.
There were no cash flows from investing activities for the three months ended March 31, 2024 and 2023.
Financing Activities
For the
ninethree months ended
September 30, 2017 and 2016,March 31, 2024, net
cash used in investing activities was insignificant. Financing Activities
For the nine months ended September 30, 2017, our cash provided by financing activities was driven primarilyby sales of our Class A common stock and proceeds from pre-funded warrants of $51.0 million from the Private Placement financing. For the three months ended March 31, 2023, net cash provided by financing activities was driven by the borrowingreceipt of proceeds of $12.0 million from the $7.5 million second tranche available to us under our Loan Agreement.
G42 promissory note early redemption.
Future Funding Requirements
To date, we have not generated any revenue from drug product sales. We do not know when, or if, we will generate any revenue from drug product sales. We do not expect to generate
significant revenue from drug sales unless and until we obtain regulatory approval of and commercialize
azeliragon or any of our
other drug candidates. At the same time, we expect our expenses to continue
or to increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our drug candidates. In addition, subject to obtaining regulatory approval of any of our drug candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations.
We will also continue to use cash to fund expenses related to our compliance with requirements applicable to us as a listed public company.Based on our current operating plan, we believe that our current cash and cash equivalents will allow us to meet our liquidity requirements for at least the next twelve months. We plan to finance our operations into the first quarter of 2026 through the receiptuse of top-line results for Subpart A of our STEADFAST Study in early 2018. In addition to available cash and cash equivalents and based on current operating plans, we are seeking possible partnering opportunities for our GKA, GLP-1revaluating several financing strategies to fund the ongoing and future clinical trials of cadisegliatin, including direct equity investments and the potential licensing and monetization of other drug candidates whichCompany programs. The timing of any such transactions is not certain, and we believe may provide additional cash for use innot be able to complete such transactions on acceptable terms, or at all. Even if we are able to complete such transactions, they may contain restrictions on our operations and the continuation of the clinical trials for our drug candidates. We will also pursue other sources of interim financing to provide flexibilityor cause substantial dilution to our operating plan. The timing and occurrence of such interim financing is not yet known.stockholders. We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development of our drug candidates. Additionally, we may rely on our ability to sell shares of our Class A common stock pursuant to the ATM Offering. However, the ability to use this source of capital is dependent on a number of factors, including the prevailing market price of and the volume of trading in the Company’s Class A common stock.
Our future capital requirements will depend on many factors, including:
•the progress, costs, results and timing of our planned trials to evaluate cadisegliatin as a potential adjunctive therapy for the STEADFAST Study, and the clinical developmenttreatment of azeliragon;
•the willingness of the FDA to accept the STEADFAST Study, as well asrely upon our other completed and planned clinical and preclinical studies and other work, as the basis for review and approval of azeliragon;
•our ability to maintain control over our costs in line with our budget to complete the Phase 3 clinical trial for our lead product candidate, cadisegliatin;
•the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals;
•the number and characteristics of drug candidates that we pursue, including our drug candidates in preclinical development;
•the ability of our drug candidates to progress through clinical development successfully;
•our need to expand our research and development activities;
•the costs associated with securing, establishing and maintaining commercialization capabilities;
| •
| the costs associated with securing, establishing and maintaining commercialization capabilities;
|
•the costs of acquiring, licensing or investing in businesses, products, drug candidates and technologies;
•our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights;
•our need and ability to hire additional management, and scientific, and medical personnel;
•the effect of competing technological and market developments;
•our need to implement additional internal systems and infrastructure, including financial and reporting systems;
•the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future; and
•the amount of any payments we are required to make to M&F TTP Holdings Two LLC in the future under the Tax Receivable Agreement.
Until such time, if ever, as we can generate substantial revenue from drug sales, we expect to finance our cash needs through a combination of equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We do not currently have any committed external source of funds. arrangements.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants that will further limit or restrict our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, future revenue streams or drug candidates or grant licenses on terms that may not be favorable to us.
If we are unable to obtain additional funding, we could be forced to delay, reduce or eliminate our research and development programs or commercialization efforts, which could adversely affect our business prospects.Disclosures About Contractual Obligations and Commitments
The following table summarizes our contractual obligations at September 30, 2017 (in thousands):
| | | | | Three months ended December 31, | | | Years ended December 31, | |
| Total | | | 2017 | | | 2018 - 2020 | | | 2021 - 2022 | | | 2023 and thereafter | |
Principal payments under Loan Agreement | $ | 20,000 | | | $ | — | | | $ | 20,000 | | | $ | — | | | $ | — | |
Interest on Loan Agreement (1) | | 5,256 | | | | 568 | | | | 4,688 | | | | — | | | | — | |
Operating lease commitments | | 810 | | | | 88 | | | | 722 | | | | — | | | | — | |
Total contractual obligations | $ | 26,066 | | | $ | 656 | | | $ | 25,410 | | | $ | — | | | $ | — | |
| (1)
| Interest payments associated with the Loan Agreement are projected based on interest rates in effect as of September 30, 2017 assuming no variable rate fluctuations going forward. An increase in the interest rates applicable to our Loan Agreement by 1% would result in an additional $0.2 million of annual cash interest expense. In addition to the estimated monthly cash interest payments, the projected interest payments stated above also include the 6% final interest payment to be paid upon the maturity of the debt obligation.
|
Off-Balance Sheet Arrangements
During the periods presented,
As of March 31, 2024, we did not have
nor do we currently have,outstanding any off-balance sheet arrangements as defined under SEC rules.
Discussion of Critical Accounting Policies
and Estimates
For a discussion of our critical accounting policies and estimates, please refer to Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2016.2023. There have been no material changes to our critical accounting policies and estimates in 2017.2024.
Forward-Looking Statements
This quarterly report includes certain forward-looking statements within the meaning of the federal securities laws regarding, among other things, our management’s intentions, plans, beliefs, expectations, or predictions of future events, which are considered forward-looking statements. You should not place undue reliance on those statements because they are subject to numerous uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control. Forward-looking statements include information concerning our possible or assumed future results of operations, including descriptions of our business strategy. These statements often include words such as
“anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,
” “plans,” “potential,” “predicts,” “projects,” “should,” “will,”
“should,” “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate”“would” or similar
expressions.expressions and the negatives of those terms. These statements are based upon assumptions that we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors that we believe are appropriate under the circumstances. As you read this quarterly report, you should understand that these statements are not guarantees of performance or results. They involve known and unknown risks, uncertainties, and assumptions, including those described under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K and under Item 1A of Part II of this Quarterly Report on Form 10-Q. Although we believe that these forward-looking statements are based upon reasonable assumptions, you should be aware that many factors, including those described under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K and under Item 1A of Part II of this Quarterly Report on Form 10-Q, could affect our actual financial results or results of operations and could cause actual results to differ materially from those in the forward-looking statements.
Our forward-looking statements made herein are made only as of the date of this quarterly report. We expressly disclaim any intent, obligation or undertaking to update or revise any forward-looking statements made herein to reflect any change in our expectations with regard thereto or any change in events, conditions, or circumstances on which any such statements are based. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained in this quarterly report.
Effect of Recent Accounting Pronouncements
See discussion of recent accounting pronouncements in Note 2, “Summary of Significant Accounting Policies”, to the Condensed Consolidated Financial Statements in this Form 10-Q.
ITEM 3.
|
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Risk
Our Loan Agreement bears interest at a floating rate equal to 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%. A one percent increase in the variable rate of interest on the Loan Agreement would increase interest expense by approximately $0.2 million annually based on the amounts currently outstanding.
We do not currently
hedge ourhave any material interest rate exposure.
Our exposure to market risk is limited to our cash and cash equivalents, all of which have maturities of one year or less. The goals of our investment strategy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, we maintain
a portfolio ofcash and cash equivalents
and investments in a variety of securitieswith multiple financial institutions that management believes to be of high credit quality.
The securities in our investment portfolio are not leveraged and are, due to their short-term nature, subject to minimal interest rate risk. Because of the short-term maturities of our investments, we do not believe that an increase in market rates would have a material negative impact on the value of our investment portfolio.We do not have any material foreign currency exposure.
ITEM 4.
| CONTROLS AND PROCEDURES
|
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our Chief Executive Officer
(our Principal Executive Officer) and Chief Financial Officer
(our Principal Financial Officer), management has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) of the Securities Exchange Act of 1934) as of
September 30, 2017.March 31, 2024. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of
September 30, 2017,March 31, 2024, our disclosure controls and procedures were effective in causing material information relating to us (including our consolidated subsidiaries) to be recorded, processed, summarized, and reported by management on a timely basis and to ensure the quality and timeliness of our public disclosures pursuant to SEC disclosure obligations.
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, with the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error and mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of controls.
The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may become inadequate because of changes in conditions or because the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Changes to Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting
other than those described aboveduring our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Website Availability of Reports and other Corporate Governance Information
The Company maintains a comprehensive corporate governance program, including Corporate Governance Guidelines for its Board of Directors, Board Guidelines for Assessing Director Independence, and charters for its Audit Committee, Nominating and Corporate Governance Committee and Compensation Committee. The Company maintains a corporate investor relations website, www.vtvtherapeutics.com, where stockholders and other interested persons may review, without charge, among other things, corporate governance materials and certain SEC filings, which are generally available on the same business day as the filing date with the SEC on the SEC’s website
http://www.sec.gov.www.sec.gov. The contents of our website are not made a part of this Quarterly Report on Form 10-Q.
PART II – OTHER INFORMATION
ITEM 1.
|
ITEM 1. LEGAL PROCEEDINGS |
We are not currently a party to any material legal proceedings.
ITEM 1A.
|
ITEM 1A. RISK FACTORS |
In addition to the
Our risk
factor listed below and other information in this report, investors should carefully consider the risk factors
are set forth under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K for the year ended December 31,
2016.
We will need additional capital2023. There have been no material changes to complete the STEADFAST Study and to complete the development and commercialization of azeliragon and our other drug candidates and there is a substantial doubt about our ability to continue as a going concern. If we are unable to raise sufficient capital for these purposes, we would be forced to delay, reduce or eliminate our product development programs.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is expensive. We expect our research and development expenses to increase in connection with our ongoing activities, particularly as we continue the STEADFAST Study, undertake additional clinical trials of our other drug candidates and continue to work on our other research programs. Our current capital will not be sufficient for us to complete the STEADFAST Study and the development of our other drug candidates. As such, we will need to raise substantial additional capital to complete the development and commercialization of azeliragon. We are seeking possible partnering opportunities for our GKA, GLP-1r and other drug candidates which we believe may provide additional cash for userisk factors from those previously disclosed in our operations andAnnual Report on Form 10-K for the continuation of the clinical trials for our drug candidates. We will also pursue other sources of interim financing to provide flexibility to our operating plan. The timing and occurrence of such interim financing is not yet known.
If the FDA or other regulators require that we perform additional studies beyond those we currently expect, or if there are any delays in completing our clinical trials or the development of any of our drug candidates, our expenses could increase beyond what we currently anticipate and the timing of any potential product approval may be delayed. We have no commitments or arrangements for any additional financing to fund our research and development programs. We also will need to raise substantial additional capital in the future to complete the development and commercialization of azeliragon for additional indications and for developing our other drug candidates. Because successful development of our drug candidates is uncertain, we are unable to estimate the actual funds required to complete research and development and commercialize and license our products under development.
Until we can generate a sufficient amount of revenue from our drug candidates, if ever, we expect to finance future cash needs through equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. If worldwide economic conditions and the international equity and credit markets deteriorate and return to depressed states, it will be more difficult for us to obtain additional equity or credit financing, when needed.
Our recurring losses, accumulated deficit and our current levels of cash and cash equivalents raise substantial doubt about our ability to continue as a going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and it is likely that investors will lose all or a part of their investment. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all. Further, if adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs.
Our future capital requirements will depend on many factors, including:
the progress, costs, results and timing of the STEADFAST Study, and the clinical development of azeliragon;
the willingness of the FDA to accept the STEADFAST Study, as well as our other completed and planned clinical and preclinical studies and other work, as the basis for review and approval of azeliragon;
the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals;
the number and characteristics of drug candidates that we pursue, including our drug candidates in preclinical development;
the ability of our drug candidates to progress through clinical development successfully;
our need to expand our research and development activities;
the costs associated with securing, establishing and maintaining commercialization capabilities;
the costs of acquiring, licensing or investing in businesses, products, drug candidates and technologies;
our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights;
our need and ability to hire additional management and scientific and medical personnel;
the effect of competing technological and market developments;
| •
| our need to implement additional internal systems and infrastructure, including financial and reporting systems; and
|
the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future;
the amount of any payments we are required to make to M&F TTP Holdings Two LLC in the future under the Tax Receivable Agreement.
ITEM 2.
|
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS |
None.
On February 27, 2024, we entered into a securities purchase agreement (the “Securities Purchase Agreement”) with certain institutional accredited investors (the “Private Placement Investors”), pursuant to which we agreed to issue and sell to the Private Placement Investors in a private placement (the “Private Placement”) (i) an aggregate of 464,377 shares (the “Private Placement Shares”) of our Class A common stock, at a purchase price of $11.81 per share, and (ii) pre-funded warrants (the “Private Placement Pre-Funded Warrants”) to purchase up to an aggregate of 3,853,997 shares of our Class A common stock (the “Private Placement Warrant Shares”) at a purchase price of $11.80 per Private Placement Pre-Funded Warrant (representing the $11.81 per Private Placement Share purchase price less the exercise price of $0.01 per Private Placement Warrant Share). We received aggregate gross proceeds from the Private Placement of approximately $51.0 million, before deducting offering expenses payable by us. The Private Placement Pre-Funded Warrants are exercisable at any time after their original issuance and will not expire.
On March 5, 2024, the Company entered into a letter agreement with the Private Placement Investors pursuant to which the Private Placement Investors agreed to exchange an aggregate of 116,493 Private Placement Shares for an aggregate of 116,590 Private Placement Pre-Funded Warrants.
The Private Placement Shares and the Private Placement Pre-Funded Warrants were issued pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act.
ITEM 3.
|
ITEM 3. DEFAULTS UPON SENIOR SECURITIES |
ITEM 4.
|
ITEM 4. MINE SAFETY DISCLOSURES |
ITEM 5.
|
ITEM 5. OTHER INFORMATION |
None.
Rule 10b5-1 Trading Plans
During the first fiscal quarter ended March 31, 2024, none of our directors or executive officers adopted or terminated any contract, instruction or written plan for the purchase or sale of our securities to satisfy the affirmative defense conditions of Rule 10b5-1(c) or any “non-Rule 10b5-1 trading arrangement.”
| | | | | | | | |
Exhibit
Number | | Description |
| | |
31.1
31.1* | | |
| | |
31.2
31.2* | | |
| | |
32.1
32.1* | | |
| | |
32.2
32.2* | | |
| | |
101.INS | | Inline XBRL Instance Document |
| | |
101.SCH | | Inline XBRL Taxonomy Extension Schema |
| | |
101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase |
| | |
101.DEF | | Inline XBRL Taxonomy Extension Definition Document |
| | |
101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase |
| | |
101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase |
| | |
| 104 | | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
___________________________
* Filed herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date:
November 1, 2017May 9, 2024 | | | | | | | | |
| | VTV THERAPEUTICS INC. |
| | (Registrant) |
| | |
| By: | | /s/ Paul J. Sekhri |
| By:
| /s/ Stephen L. Holcombe
Paul J. Sekhri |
| | Stephen L. Holcombe
|
| | President and Chief Executive Officer |
| | |
| By: | | /s/ Steven Tuch |
| By:
| /s/ Rudy C. Howard
Steven Tuch |
| | Rudy C. Howard
|
| | Chief Financial Officer |
32