MARCH 31, 2024
| Page No. | ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
September 30, December 31, 2017 2016 ASSETS CURRENT ASSETS Cash and cash equivalents $ 13,162 $ 47,748 Marketable securities 42,587 15,754 Accounts receivable - grant 2,452 1,215 Prepaid expenses and other current assets 1,318 1,707 Total current assets 59,519 66,424 Property and equipment, net 844 599 Other non-current assets 139 40 TOTAL ASSETS $ 60,502 $ 67,063 LIABILITIES AND STOCKHOLDERS’ EQUITY CURRENT LIABILITIES Accounts payable $ 615 $ 168 Deferred revenue 50 71 Accrued and other current liabilities 4,212 3,726 Total current liabilities 4,877 3,965 Other non-current liabilities 117 132 TOTAL LIABILITIES 4,994 4,097 Commitments and Contingencies (Notes 9 and 11) STOCKHOLDERS’ EQUITY Preferred stock, $0.0001 par value; 10,000,000 shares authorized as of September 30, 2017 and December 31, 2016; no shares issued and outstanding as of September 30, 2017 and December 31, 2016 — — Common stock, $0.0001 par value; 500,000,000 shares authorized as of September 30, 2017 and December 31, 2016; 16,472,831 shares and 13,430,833 shares issued and outstanding as of September 30, 2017 and December 31, 2016, respectively 2 1 Additional paid-in capital 121,568 108,246 Accumulated other comprehensive loss (32 ) (4 ) Accumulated deficit (66,030 ) (45,277 ) TOTAL STOCKHOLDERS’ EQUITY 55,508 62,966 TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY $ 60,502 $ 67,063 Three Months Ended Nine Months Ended September 30, September 30, 2017 2016 2017 2016 Revenues: Grant $ 1,261 $ 1,149 $ 3,723 $ 3,381 Operating expenses: Research and development Research and development Research and development $ 6,239 $ 5,385 $ 17,024 $ 13,402 General and administrative 3,020 2,065 7,749 6,342 Total operating expenses 9,259 7,450 24,773 19,744 Loss from operations (7,998 ) (6,301 ) (21,050 ) (16,363 ) Other income (expense): Interest income 136 72 332 172 Other expense (12 ) (9 ) (35 ) (24 ) Total other income 124 63 297 148 Net loss $ (7,874 ) $ (6,238 ) $ (20,753 ) $ (16,215 ) Net loss per share, basic and diluted $ (0.48 ) $ (0.47 ) $ (1.42 ) $ (1.89 ) Weighted-average common shares outstanding, basic and diluted 16,409,871 13,326,093 14,641,082 8,598,268 Three Months Ended Nine Months Ended September 30, September 30, 2017 2016 2017 2016 Net loss $ (7,874 ) $ (6,238 ) $ (20,753 ) $ (16,215 ) Other comprehensive income (loss): Unrealized (loss) gain on marketable securities (3 ) 2 (28 ) 4 Total comprehensive loss $ (7,877 ) $ (6,236 ) $ (20,781 ) $ (16,211 ) Changes in Nine Months Ended September 30, 2017 2016 CASH FLOWS FROM OPERATING ACTIVITIES Net loss $ (20,753 ) $ (16,215 ) Adjustments to reconcile net loss to net cash used in operating activities: Depreciation and amortization 183 96 Purchase discount (premium) on marketable securities, net 2 (146 ) Amortization of premium on marketable securities, net 82 51 Stock-based compensation 1,794 963 Research and development services settled with convertible preferred stock 15 80 Other, net (15 ) (16 ) Changes in operating assets and liabilities: Accounts receivable - grant (1,237 ) 126 Prepaid expenses and other assets 275 (1,261 ) Accounts payable 447 347 Deferred revenue (21 ) 181 Accrued and other liabilities 486 1,309 Net cash used in operating activities (18,742 ) (14,485 ) CASH FLOWS FROM INVESTING ACTIVITIES Purchases of property and equipment (428 ) (91 ) Purchases of marketable securities (62,115 ) (20,390 ) Proceeds from maturities of marketable securities 35,170 5,766 Decrease in restricted cash — 80 Net cash used in investing activities (27,373 ) (14,635 ) CASH FLOWS FROM FINANCING ACTIVITIES Proceeds from issuance of common stock in public offering, net of offering costs 11,380 49,294 Proceeds from employee stock plan purchases and stock option exercises 149 76 Net cash provided by financing activities 11,529 49,370 NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS (34,586 ) 20,250 CASH AND CASH EQUIVALENTS Beginning of period 47,748 29,294 End of period $ 13,162 $ 49,544 Supplemental Disclosure of Non-Cash Investing and Financing Information: Conversion of Series A convertible preferred stock to common stock upon initial public offering $ — $ 13,573 Conversion of Series B convertible preferred stock to common stock upon initial public offering $ — $ 44,738 Waltham, Massachusetts. $485.0 million. SEC on February 29, 2024 and amended on March 1, 2024. Company's Annual Report. The Company September 30, 2017 Amortized Cost Gross Unrealized Gains Gross Unrealized Losses Estimated Fair Value Cash equivalents: Money market funds $ 3,959 $ — $ — $ 3,959 Reverse repurchase agreements 7,250 — — 7,250 Total cash equivalents 11,209 — — 11,209 Marketable securities: U.S. treasury securities 2,509 (2 ) 2,507 U.S. government securities 40,110 — (30 ) 40,080 Total marketable securities $ 42,619 $ — $ (32 ) $ 42,587 December 31, 2016 Amortized Cost Gross Unrealized Gains Gross Unrealized Losses Estimated Fair Value Cash equivalents: Money market funds $ 4,584 $ — $ — $ 4,584 Reverse repurchase agreements 39,250 — — 39,250 Total cash equivalents 43,834 — — 43,834 Marketable securities: U.S. government securities 15,758 — (4 ) 15,754 Total marketable securities $ 15,758 $ — $ (4 ) $ 15,754 2023. September 30, December 31, 2017 2016 Due in one year or less $ 26,619 $ 15,754 Due in 1 - 2 years 15,968 — Total marketable securities $ 42,587 $ 15,754 September 30, December 31, 2017 2016 Accrued compensation $ 1,468 $ 1,270 Accrued contracted research and development costs 1,958 1,749 Accrued professional and consulting fees 553 480 Accrued other and other current liabilities 233 227 Total accrued and other current liabilities $ 4,212 $ 3,726 benefit of the Series B Preferred Stock, regardless of whether any of the foregoing actions will be by means of amendment to the Certificate of Incorporation or by merger, consolidation, recapitalization, Employee Stock Purchase Plan Three Months Ended Nine Months Ended September 30, September 30, 2017 2016 2017 2016 Research and development $ 277 $ 190 $ 672 $ 400 General and administrative 451 244 1,122 563 Total stock-based compensation expense $ 728 $ 434 $ 1,794 $ 963 Three Months Ended Nine Months Ended September 30, September 30, 2017 2016 2017 2016 Equity Incentive Plans Expected term 5.82 6.07 5.91 5.99 Expected volatility 85 % 81 % 86 % 87 % Risk-free interest 1.88 % 1.16 % 1.99 % 1.28 % Dividend yield 0 % 0 % 0 % 0 % 2016 ESPP Expected term 0.50 0.50 0.50 0.45 Expected volatility 79 % 81 % 79 % 82 % Risk-free interest 1.23 % 0.57 % 0.90 % 0.49 % Dividend yield 0 % 0 % 0 % 0 % September 30, 2017 Level 1 Level 2 Level 3 Total Financial Assets Money market funds $ 3,959 $ — $ — $ 3,959 Reverse repurchase agreements — 7,250 — 7,250 U.S. treasury securities 2,507 — — 2,507 U.S. government securities — 40,080 — 40,080 Total financial assets $ 6,466 $ 47,330 $ — $ 53,796 December 31, 2016 Level 1 Level 2 Level 3 Total Financial Assets Money market funds $ 4,584 $ — $ — $ 4,584 Reverse repurchase agreements — 39,250 — 39,250 U.S. government securities — 15,754 — 15,754 Total financial assets $ 4,584 $ 55,004 $ — $ 59,588 The Company The Company has generated a net loss for all periods presented, therefore diluted net loss per share is the same as basic net loss per share since the inclusion of potentially dilutive securities would be anti-dilutive. Three Months Ended Nine Months Ended September 30, September 30, 2017 2016 2017 2016 Series A convertible preferred stock — — — 816,677 Series B convertible preferred stock — — — 1,879,553 Unvested restricted common stock 52,882 95,415 63,364 105,996 Options to purchase common stock 2,294,012 1,361,135 1,846,593 1,017,620 You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our unaudited condensed consolidated financial statements and related notes included in Part I, Item 1 of this Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2024 (this "Quarterly Report") as well as the audited consolidated financial statements and notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations, included in our Annual Report on Form 10-K for the year ended December 31, the risks associated with our portfolio, see Item 1A, “Risk Factors” included in our Annual Report. Research and development expenses Series B Preferred Stock (convertible on a 40 to 1 basis), par value $0.0001 per share, for $1,480 per share for an aggregate purchase price of $180.0 million (collectively, the “March 2024 PIPE”). compensation expense; estimated future cash flows used in calculating the impairment of right-of-use lease assets; and estimated cost to complete performance obligations related to revenue recognition. The consideration transferred in acquiring IPR&D in connection with the acquisition of Pre-Merger Spyre was comprised of shares of our Common Stock and shares of our Series A non-voting convertible preferred stock, par value $0.0001 per share ("Series A Preferred Stock"). To determine the fair value of the equity transferred, we considered the per share value of the private placement we closed in June 2023, which was an over-subscribed financing event involving a group of accredited investors. Our significant accounting policies are more fully described in Note 2 to our condensed consolidated financial statements appearing elsewhere in this Quarterly Report. 2023 Three Months Ended September 30, Dollar 2017 2016 Change % Change (dollars in thousands) Revenues: Grant $ 1,261 $ 1,149 $ 112 10 % Operating expenses: Research and development $ 6,239 $ 5,385 $ 854 16 % General and administrative 3,020 2,065 955 46 % Total operating expenses 9,259 7,450 1,809 24 % Loss from operations (7,998 ) (6,301 ) (1,697 ) 27 % Interest income 136 72 64 89 % Other expense, net (12 ) (9 ) (3 ) 33 % Net loss $ (7,874 ) $ (6,238 ) $ (1,636 ) 26 % PEACE Phase 3 trial and BLA package. General and Administrative Expenses. General and administrative expenses increased by Nine Months Ended September 30, Dollar 2017 2016 Change % Change (dollars in thousands) Revenues: Grant $ 3,723 $ 3,381 $ 342 10 % Operating expenses: Research and development $ 17,024 $ 13,402 $ 3,622 27 % General and administrative 7,749 6,342 1,407 22 % Total operating expenses 24,773 19,744 5,029 25 % Loss from operations (21,050 ) (16,363 ) (4,687 ) 29 % Interest income 332 172 160 93 % Other expense, net (35 ) (24 ) (11 ) 46 % Net loss $ (20,753 ) $ (16,215 ) $ (4,538 ) 28 % Liquidity and Capital Resources revenue from the sale of any products. There can be no assurance that profitable operations will ever be achieved, and, if achieved, whether profitability can be sustained on a continuing basis. Flows Nine Months Ended September 30, 2017 2016 Net cash and cash equivalents (used in) provided by: Operating activities $ (18,742 ) $ (14,485 ) Investing activities (27,373 ) (14,635 ) Financing activities 11,529 49,370 Net (decrease) increase in cash and cash equivalents $ (34,586 ) $ 20,250 Operating Activities driven by timing of payments. $0.6 million for depreciation and amortization. (Used in) Provided by Investing Activities Financing Activities bankruptcy. We are exposed to market risks in the ordinary course of our business. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of Changes in Internal Control over Financial Reporting Issuer Purchases of Equity Securities Period Total number of shares purchased Average price paid per share Total number of shares purchased as part of publicly announced plans or programs Maximum number of shares that may yet be purchased under the plans or programs July 1, 2017 - July 31, 2017 — — — — August 1, 2017 - August 31, 2017 419 (1) $ 0.00105 — — September 1, 2017 - September 30, 2017 — — — — Total 419 $ 0.00105 — — 32.1(1) Inline XBRL Instance Document 101.SCH Inline XBRL Taxonomy Extension Schema Document 101.CAL Inline XBRL Taxonomy Extension Calculation Linkbase Document 101.DEF Inline XBRL Taxonomy Extension Definition Linkbase Document 101.LAB Inline XBRL Taxonomy Extension Label Linkbase Document 101.PRE Inline XBRL Taxonomy Extension Presentation Linkbase Document Chief Financial Officer (quarterly reportQuarterly Report on Form 10-Q for the quarter ended March 31, 2024 (this “Quarterly Report”) contains forward-looking statements.statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and Section 27A of the Securities Act of 1933, as amended (the "Securities Act"). All statements contained in this Quarterly Report other than statements of historical fact, are “forward-looking statements” for purposesincluding statements regarding stockholder approval of this Quarterly Report on Form 10-Q. These forward-looking statements may include, but are not limitedthe conversion rights of our Series B preferred stock, par value $0.0001 (the "Series B Preferred Stock"); any future payouts under our contingent value rights ("CVRs") issued in connection with the acquisition of Spyre Therapeutics, Inc. ("Pre-Merger Spyre") (the "Asset Acquisition"); our ability to statements regardingachieve the expected benefits or opportunities and related timing with respect to the Asset Acquisition or to monetize our legacy assets, our future results of operations and financial position, business strategy, the length of time that we believe our existing cash resources will fund operations, market size, potential growth opportunities, preclinical and future clinical development activities, efficacy and safety profile of our product candidates, our ability to maintainpotential therapeutic benefits and recognize the benefitseconomic value of certain designations received byour product candidates, use of net proceeds from our public offerings, the timing and results of preclinical studies and clinical trials, the expected impact of macroeconomic conditions, including inflation, increasing interest rates and volatile market conditions, current or potential bank failures, as well as global events, including the ongoing military conflict in Ukraine, conflict in Israel and surrounding areas, and geopolitical tensions in China on our operations, and the receipt and timing of potential regulatory approvaldesignations, approvals and commercialization of product candidates.candidates, are forward-looking statements. The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “predict,” “target,” “intend,” “could,” “would,” “should,” “project,” “plan,” “expect,” and similar expressions that convey uncertainty of future events or outcomes are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.quarterly report.Quarterly Report. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the forward-looking events and circumstances discussed in this quarterly reportQuarterly Report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.As You should read this Quarterly Report with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.on Form 10-Q, the terms “Aeglea,“Spyre,” "Aeglea BioTherapeutics, Inc.," “the Company,” “we,” “us,” and “our” refer to Aeglea BioTherapeutics,Spyre Therapeutics, Inc., a Delaware corporation, and where appropriate, its consolidated subsidiaries unlesstaken as a whole. “Spyre” and all product candidate names are our common law trademarks. This Quarterly Report contains additional trade names, trademarks and service marks of other companies, which are the context indicates otherwise.property of their respective owners. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, these other companies.FINANCIAL INFORMATIONFinancial InformationAeglea BioTherapeutics,(Unaudited)InUnaudited, in thousands, except share and per share amounts)March 31,
2024March 31,
2024December 31,
2023ASSETS CURRENT ASSETS CURRENT ASSETS CURRENT ASSETS Cash and cash equivalents Cash and cash equivalents Cash and cash equivalents Marketable securities Prepaid expenses and other current assets Prepaid expenses and other current assets Prepaid expenses and other current assets Total current assets Restricted cash Other non-current assets Other non-current assets LIABILITIES AND STOCKHOLDERS’ EQUITY LIABILITIES AND STOCKHOLDERS’ EQUITY CURRENT LIABILITIES CURRENT LIABILITIES Accounts payable Accounts payable CVR liability CVR liability CVR liability Accrued and other current liabilities Accrued and other current liabilities Related party accounts payable and other current liabilities Non-current CVR liability TOTAL LIABILITIES TOTAL LIABILITIES Commitments and Contingencies (Note 6) Commitments and Contingencies (Note 6) Series B non-voting convertible preferred stock, $0.0001 par value; 271,625 and 150,000 shares authorized as of March 31, 2024 and December 31, 2023, respectively; 271,625 and 150,000 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively Series A non-voting convertible preferred stock, $0.0001 par value; 1,086,341 shares authorized as of March 31, 2024 and December 31, 2023; 437,037 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively Series A non-voting convertible preferred stock, $0.0001 par value; 1,086,341 shares authorized as of March 31, 2024 and December 31, 2023; 437,037 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively Series A non-voting convertible preferred stock, $0.0001 par value; 1,086,341 shares authorized as of March 31, 2024 and December 31, 2023; 437,037 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively Preferred stock, $0.0001 par value; 8,642,034 shares and 8,763,659 shares authorized as of March 31, 2024 and December 31, 2023, respectively; no shares issued and outstanding as of March 31, 2024 and December 31, 2023 Common stock, $0.0001 par value; 400,000,000 shares authorized as of March 31, 2024 and December 31, 2023; 36,629,680 shares and 36,057,109 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively Accumulated other comprehensive (loss) income TOTAL LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (Unaudited)InUnaudited, in thousands, except share and per share amounts) Revenue: Revenue: Revenue: Development fee and royalty Development fee and royalty Development fee and royalty Total revenue Total revenue Total revenue Operating expenses: Operating expenses: (1)(1)(1)General and administrative General and administrative Total operating expenses Total operating expenses Loss from operations Loss from operations Other income (expense): Other income (expense): Interest income Interest income Other expense Other expense Total other income (expense) Total other income (expense) Total other income (expense) Loss before income tax expense Loss before income tax expense Loss before income tax expense Income tax (expense) benefit Income tax (expense) benefit Income tax (expense) benefit Net loss Net loss Net loss per share, basic and diluted Net loss per share, basic and diluted Weighted-average common shares outstanding, basic and diluted Weighted-average common shares outstanding, basic and diluted (Unaudited)InUnaudited, in thousands)Three Months Ended
March 31,Three Months Ended
March 31,Three Months Ended
March 31,2024 2024 2024 Net loss Net loss Other comprehensive (loss) income: Other comprehensive (loss) income: Other comprehensive (loss) income: Foreign currency translation adjustment Foreign currency translation adjustment Foreign currency translation adjustment Unrealized (loss) gain on marketable securities Unrealized (loss) gain on marketable securities Total comprehensive loss Total comprehensive loss Cash Flows(Unaudited)InUnaudited, in thousands)Three Months Ended March 31, 2024 Series A Non-Voting
Convertible Preferred StockCommon Stock Shares Amount Shares Amount Shares Amount Balances - December 31, 2023 150 $ 84,555 437 $ 184,927 36,057 $ 10 $ 763,191 $ 302 $ (764,414) $ 184,016 Issuance of Series B non-voting convertible preferred stock in connection with private placement, net of financing costs 122 168,850 — — — — — — — — Issuance of common stock in connection with exercise of stock options and employee stock purchase plan — — — — 572 — 4,390 — — 4,390 Stock-based compensation expense — — — — — — 8,385 — — 8,385 Foreign currency translation adjustment — — — — — — — 16 — 16 Unrealized gain on marketable securities — — — — — — — (681) — (681) Net loss — — — — — — — — (43,857) (43,857) Balances - March 31, 2024 272 $ 253,405 437 $ 184,927 36,629 $ 10 $ 775,966 $ (363) $ (808,271) $ 152,269 Three Months Ended March 31, 2023 Series B Non-Voting
Convertible Preferred StockSeries A Non-Voting
Convertible Preferred StockCommon Stock Shares Amount Shares Amount Shares Amount Balances - December 31, 2022 — $ — — $ — 2,614 $ 6 $ 475,971 $ (48) $ (425,624) $ 50,305 Issuance of common stock in connection with employee stock purchase plan — — — — 2 — 18 — — 18 Stock-based compensation expense — — — — — — 1,709 — — 1,709 Foreign currency translation adjustment — — — — — — — 10 — 10 Unrealized gain on marketable securities — — — — — — — 32 — 32 Net loss — — — — — — — — (18,422) (18,422) Balances - March 31, 2023 — $ — — $ — 2,616 $ 6 $ 477,698 $ (6) $ (444,046) $ 33,652 Aeglea BioTherapeutics, Three Months Ended
March 31, 2024 2023 CASH FLOWS FROM OPERATING ACTIVITIES Net loss $ (43,857) $ (18,422) Adjustments to reconcile net loss to net cash used in operating activities: Stock-based compensation 13,835 1,709 Change in fair value of CVR liability 430 — Net accretion of discount on marketable securities (2,423) (107) Depreciation and amortization — 384 Amortization of operating lease assets — 164 Other — 2 Changes in operating assets and liabilities: Accounts payable 2,210 1,384 Accrued and other liabilities 8,151 (3,164) Related party payable (6,507) — Prepaid expenses and other assets (381) 622 Deferred revenue — (53) Development receivables — 45 Operating lease liabilities — (198) Net cash used in operating activities (28,542) (17,634) CASH FLOWS FROM INVESTING ACTIVITIES Purchases of marketable securities (152,713) — Proceeds from maturities and sales of marketable securities 47,750 17,750 Net cash (used in) and provided by investing activities (104,963) 17,750 CASH FLOWS FROM FINANCING ACTIVITIES Proceeds from issuance of Series B non-voting convertible preferred stock in connection with private placement, net of placement and other offering costs 169,205 — Payments related to contingent value rights liability (1,430) — Proceeds from employee stock plan purchases and stock option exercises 4,390 18 Principal payments on finance lease obligation — (8) Net cash provided by financing activities 172,165 10 Effect of exchange rate on cash, cash equivalents, and restricted cash (4) 11 NET INCREASE IN CASH, CASH EQUIVALENTS, AND RESTRICTED CASH 38,656 137 CASH, CASH EQUIVALENTS, AND RESTRICTED CASH Beginning of period 189,215 36,416 End of period $ 227,871 $ 36,553 Supplemental Disclosure of Non-Cash Investing and Financing Information: Unpaid amounts related to issuance of Series B non-voting convertible preferred stock in connection with private placement $ 355 $ — Aeglea”Spyre” or the “Company”), is a clinical-stagepreclinical stage biotechnology company committed tofocused on developing enzyme-basednext generation therapeutics in the field of amino acid metabolism to treat rare genetic diseases and cancer.for patients living with inflammatory bowel disease. The Company was formed as a Limited Liability Company (LLC)("LLC") in Delaware on December 16, 2013 under the name Aeglea BioTherapeutics Holdings, LLC and was converted from a Delaware LLC to a Delaware corporation (the “LLC Conversion”) on March 10, 2015. On November 27, 2023, the Company completed its corporate rebranding, changing the name of the Company to Spyre Therapeutics, Inc. The Company operates in one segment and has its principal offices in Austin, Texas.OfferingsInitial Public OfferingInat a ratio of 1-for-25 (the “Reverse Split”). Except as indicated otherwise, all share numbers related to the Company's Common Stock disclosed in these financial statements have been adjusted on a post-Reverse Split basis.2016,12, 2023, based on the review of the inconclusive interim results from the Company's Phase 1/2 clinical trial of pegtarviliase for the treatment of Classical Homocystinuria and other business considerations, the Company announced that it had initiated a process to explore strategic alternatives to maximize stockholder value and engaged an independent exclusive financial advisor to support this process. As a result, in April 2023, the Company implemented a restructuring plan resulting in an approximate 83% reduction of the Company’s Registration Statement on Form S-1 (File No. 333-205001) relatingexisting headcount.initial public offering (“IPO”treatment of inflammatory bowel disease through a research and development option agreement ("Paragon Agreement") of its common stock was declared effective by the Securities and Exchange Commission (“SEC”with Paragon Therapeutics ("Paragon"). The IPO closedasset acquisition was accomplished through a two-step reverse triangular merger whereby a wholly owned subsidiary of the Company merged with and into Pre-Merger Spyre, which existed at the time the Acquisition Agreement was entered into, became a wholly owned subsidiary of the Company in accordance with the terms of the Acquisition Agreement. Immediately following this merger, Pre-Merger Spyre merged with an into a second wholly subsidiary of the Company (“Merger Sub”) in accordance with the terms of the Acquisition Agreement and Pre-Merger Spyre ceased to exist. Subsequently, Aeglea BioTherapeutics, Inc. was renamed Spyre Therapeutics, Inc. and is a different entity than Pre-Merger Spyre, which ceased to exist upon merging with Merger Sub. The transaction was structured as a stock-for-stock transaction pursuant to which all of Pre-Merger Spyre's outstanding equity interests were exchanged based on April 12, 2016, and 5,481,940a fixed exchange ratio of 0.5494488 to 1 for consideration from the Company of 517,809 shares of common stock, were sold at a public offering price of $10.00 per share, including 481,940 shares of common stock issued upon the partial exercise by the underwriters of their option to purchase additional shares. The Company received $47.3 million in aggregate cash proceeds, net of underwriting discounts and commissions of $3.8 million and offering costs of $3.7 million incurred by the Company.Immediately prior to the closing of the IPO, all shares of outstanding convertible preferred stock were automatically converted, at a ratio of one share of common stock for each share of convertible preferred stock, into 7,172,496 shares of common stock with the related carrying value of $58.3 million reclassified to common stock and additional paid-in capital.In connection with the IPO, the Company amended its Restated Certificate of Incorporation to change the authorized capital stock to 510,000,000 shares of which 500,000,000 shares are designated as common stock and 10,000,000 shares are designated as preferred stock, all with a par value of $0.0001 per share. There are noshare ("Common Stock"), and 364,887 shares of Series A non-voting convertible preferred stock, par value of $0.0001 per share ("Series A Preferred Stock") (convertible on a 40 to 1 basis), in addition to the assumption of outstanding and unexercised stock options to purchase 2,734 shares of Common Stock from the Amended and Restated Spyre 2023 Equity Incentive Plan (the "Asset Acquisition"). The Common Stock and Series A Preferred Stock related to the Asset Acquisition were issued to the Pre-Merger Spyre stockholders on July 7, 2023.September 30, 2017.Follow-on Public OfferingInthe close of business on July 3, 2023 (the "Legacy Stockholders"), but was not distributed to the holders of shares of Common Stock or Series A Preferred Stock issued to the former stockholders of Pre-Merger Spyre or the June 2017,2023 Investors in the Transactions. Holders of the CVRs will be entitled to receive cash payments from proceeds received by the Company issued and sold 3,000,000for a three-year period related to the disposition or monetization of its legacy assets for a period of one-year following the closing of the Asset Acquisition.commonCommon Stock and Series B non-voting convertible preferred stock, in an underwritten public offering pursuantpar value of $0.0001 per share ("Series B Preferred Stock") (convertible on a 40 to 1 basis) (the “December 2023 PIPE”) to a shelf registration statement on Form S-3 at a public offeringgroup of investors. The Company sold an aggregate of 6,000,000 shares of Common Stock and 150,000 shares of Series B Preferred Stock for an aggregate purchase price of $4.10 per share. The net proceeds toapproximately $180.0 million before deducting approximately $10.9 million of placement agent and other offering expenses.from this publiccompleted a private placement of Series B Preferred Stock (convertible on a 40 to 1 basis) (the “March 2024 PIPE”) to a group of investors. The Company sold 121,625 shares of Series B Preferred Stock for a purchase price of $180.0 million before deducting approximately $11.2 million of placement agent and other offering was approximately $11.4 million, after deducting underwriting discountscosts.commissions of $615,000due to its significant research and offering costs of $306,000. As of September 30, 2017,development expenditures, the Company did not have any offering costs recorded as an outstanding liability on the balance sheet.LiquidityAs of September 30, 2017, the Company had working capital of $54.6 million, an accumulated deficit of $66.0 million,has generated operating losses since its inception and cash, cash equivalents, and marketable securities of $55.7 million. The Company has not generated any product revenues and has not achieved profitable operations.revenue from the commercial sale of any products. There iscan be no assurance that profitable operations will ever be achieved, and, if achieved, couldwhether profitability can be sustained on a continuing basis. In addition, development activities, clinicalnonclinical testing,through March 31, 2024, the Company has funded our operations by raising an aggregate of approximately $1.1 billion of gross proceeds from the sale and issuance of convertible preferred stock and common stock, pre-funded warrants, the collection of grant proceeds, and the licensing of its product rights for commercialization of pegzilarginase in Europe and certain countries in the Company’s products will require significant additional financing.The Company is subject to a numberMiddle East. As of risks similar to other life science companies, including, but not limited to, risks related to the successful discoveryMarch 31, 2024, Spyre had an accumulated deficit of $808.3 million, and developmentcash, cash equivalents, marketable securities and restricted cash of product candidates, raising additional capital, development of competing drugs and therapies, protection of proprietary technology and market acceptance of the Company’s products. As a result of these and other factors and the related uncertainties, there can be no assurance of the Company’s future success.upon the Company’son current operating plan,plans, the Company believes that it has sufficient resources to fund operations through September 30, 2019for at least one year from the issuance date of these financial statements with its existing cash, cash equivalents, and marketable securities. The CompanySpyre will need to secure additional fundingfinancing in the future in order to carry out all of its plannedfund additional research and development, activities.and before a commercial drug can be produced, marketed and sold. If the Company is unable to obtain additional financing or generate license or product revenue, the lack of liquidity could have a material adverse effect on the Company’s future prospects.Company.
Basis of PresentationdocumentQuarterly Report on Form 10-Q are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and reflect, in the opinion of management, all adjustments of a normal and recurring nature that are necessary for a fair statement of the Company’s financial position as of September 30, 2017,March 31, 2024, and its results of operations for the three and nine months ended September 30, 2017,March 31, 2024 and 2023, changes in convertible preferred stock and stockholders’ equity for the three months ended March 31, 2024 and 2023, and cash flows for the ninethree months ended September 30, 2017March 31, 2024 and 2016.2023. The results of operations for ninethe three months ended September 30, 2017March 31, 2024, are not necessarily indicative of the results to be expected for the year ending December 31, 20172024 or for any other future annual or interim period. The December 31, 20162023 balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States (“U.S. GAAP”).GAAP. These financial statements should be read in conjunction with the audited financial statements included in the Company’s Form 10-K for the year ended December 31, 20162023 (the "Annual Report") as filed with the SEC.UseEstimatesThe preparationPresentation” and "2. Summary of Significant Accounting Policies” of the Company's Annual Report.conformityaccordance with U.S. GAAP requires management to make estimates and assumptions that affectSEC instructions for interim financial information, and should be read in conjunction with the amounts reportedCompany's Annual Report. Significant accounting policies and other disclosures normally provided have been omitted since such items are disclosed in the consolidated financial statements and accompanying notes. Such management estimates include those related to accruals of research and development related costs, fair values of preferred and common stock, stock-based compensation, and certain company income tax related items. Management bases its estimates on historical experience and on various other market-specific and relevant assumptions that management believes to be reasonable under the circumstances. Actual results could differ significantly from those estimates.Cash and Cash Equivalentsconsiders all highly liquid investments with original maturities ofuses the same accounting policies in preparing quarterly and annual financial statements.less frompotential significance to the dateCompany.purchase to be cash equivalents. Cash equivalents consistthe Company’s financial assets and liabilities at fair value on a recurring basis based on the three-tier fair value hierarchy (in thousands):March 31, 2024 Level 1 Level 2 Level 3 Total Financial Assets: Money market funds $ 225,797 $ — $ — $ 225,797 U.S. government treasury securities 85,045 — — 85,045 U.S. government agency securities — 55,818 — 55,818 Commercial paper — 74,792 — 74,792 Corporate bonds — 41,434 — 41,434 Total financial assets $ 310,842 $ 172,044 $ — $ 482,886 Liabilities: Parapyre Option Obligation $ — $ 5,449 $ — $ 5,449 CVR liability — — 41,700 41,700 Total liabilities $ — $ 5,449 $ 41,700 $ 47,149 December 31, 2023 Level 1 Level 2 Level 3 Total Financial Assets: Money market funds $ 150,648 $ — $ — $ 150,648 U.S. government treasury securities 32,843 — — 32,843 U.S. government agency securities — 16,257 — 16,257 Commercial paper — 104,141 — 104,141 Corporate bonds — 33,064 — 33,064 Total financial assets $ 183,491 $ 153,462 $ — $ 336,953 Liabilities: CVR liability $ — $ — $ 42,700 $ 42,700 Total liabilities $ — $ — $ 42,700 $ 42,700 debt securitiescorporate bonds, and are statedvalued based on quoted prices for similar assets in active markets and inputsfair value.Marketable SecuritiesAll investments have been classifiedthe end of each reporting period. There were no transfers between Level 1 and Level 2 during the periods presented.available-for-sale and are carried at estimatedthere would be no obligation for the 2024 grant had the Company exercised or terminated all of the options under the Paragon Agreement prior to December 31, 2023. The service inception period for the grant precedes the grant date, with the full award being vested as of the grant date with no post-grant date service requirement. Accordingly, a liability related to the Parapyre Option Obligation is recorded pursuant to the Paragon Agreement during interim periods. On December 31, 2023, the Company settled its 2023 obligation under the Parapyre Option Obligation by issuing Parapyre 684,407 warrants to purchase the Company's Common Stock, with a $21.52 per share exercise price for each warrant.based upon quoted market prices or pricing models for similar securities. Management determinesusing the appropriate classificationprobability weighted discounted cash flow method to estimate future cash flows associated with the sale of its investmentsthe legacy assets. Analogous to a dividend being declared/approved in debt securitiesone period and paid out in another, the liability was recorded at the timedate of purchase. The Company may or may not hold securities with stated maturities greater than one year until maturity. All available-for-sale securities are considered availableapproval, June 22, 2023, as a Common Stock dividend, returning capital to support current operations and are classified as current assets.Unrealized gains and losses are excluded from earnings and are reportedthe Legacy Stockholders. Changes in fair value of the liability will be recognized as a component of accumulated comprehensive loss. Realized gains and losses and declines in fair value judged to be other than temporary, if any, on available-for-sale securities are included in otherOther income(expense). The cost of securities sold is based on the specific-identification method. There were no realized gains or losses on marketable securities for the nine months ended September 30, 2017. Interest on marketable securities is included in interest income.Concentration of Credit RiskFinancial instruments that potentially subject the Company to a concentration of credit risk consist of cash, cash equivalents, and marketable securities. The Company’s investment policy limits investments to high credit quality securities issued by the U.S. government, U.S. government-sponsored agencies and highly rated banks, subject to certain concentration limits and restrictions on maturities. The Company’s cash, cash equivalents, and marketable securities are held by financial institutions in the United States that management believes areconsolidated statement of high credit quality. Amounts on deposit may at times exceed federally insured limits. The Company has not experienced any losses on its deposits of cashoperations and cash equivalents and its accounts are monitored by management to mitigate risk. The Company is exposed to credit risk in the event of default by the financial institutions holding its cash and cash equivalents and bond issuers.Property and equipment are stated at cost, net of accumulated depreciation and amortization. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets. Repairs and maintenance that do not extend the life or improve an asset are expensed as incurred. Upon retirement or sale, the cost of disposed assets and their related accumulated depreciation and amortization are removed from the balance sheet. Any gain orcomprehensive loss is credited or charged to operations.The useful lives of the property and equipment are as follows:Laboratory equipment5 yearsFurniture and office equipment5 yearsComputer equipment3 yearsSoftware3 yearsLeasehold improvementsShorter of remaining lease term or estimated useful lifeImpairment of Long-Lived AssetsLong-lived assets are reviewed for indications of possible impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparison of the carrying amounts to the future undiscounted cash flows attributable to these assets. An impairment loss is recognized to the extent an asset group is not recoverable, and the carrying amount exceeds the projected discounted future cash flows arising from these assets. There were no impairments of long-lived assets for the nine months ended September 30, 2017.Accrued Research and Development CostsThe Company records the costs associated with research nonclinical studies, clinical trials, and manufacturing development as incurred. These costs are a significant component of the Company’s research and development expenses, with a substantial portion of the Company’s on-going research and development activities conducted by third-party service providers, including contract research and manufacturing organizations.The Company accrues for expenses resulting from obligations under agreements with contract research organizations (“CROs”), contract manufacturing organizations (“CMOs”), and other outside service providers for which payment flows do not match the periods over which materials or services are provided to the Company. Accruals are recorded based on estimates of services received and efforts expended pursuant to agreements established with CROs, CMOs, and other outside service providers. These estimates are typically based on contracted amounts applied to the proportion of work performed and determined through analysis with internal personnel and external service providers as to the progress or stage of completion of the services. The Company makes significant judgments and estimates in determining the accrual balance in each reporting period. InThe liability value is based on significant inputs not observable in the event advance payments are made to a CRO, CMO, or outside service provider, the payments will be recorded as a prepaid asset which will be amortized as the contracted services are performed. As actual costs become known, the Company adjusts its accruals. Inputs,market such as the services performed, the numberestimated cash flows, estimated probabilities of patients enrolled, or the study duration, may vary from the Company’s estimates, resulting in adjustments to researchregulatory success, and development expense in future periods. Changes in these estimates that result in material changes to the Company’s accruals could materially affect the Company’s results of operations. The Company has not experienced any material deviations between accrued and actual research and development expenses.LeasesThe Company entered into lease agreements for its office and laboratory facilities. The leases are classified as operating leases. The Company records rent expense ondiscount rates, which represent a straight-line basis over the term of the leases and, accordingly records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. Incentives granted under the Company’s facilities leases, including allowances to fund leasehold improvements, are deferred and are recognized as adjustments to rental expense on a straight-line basis over the term of the lease.Fair Value of Financial InstrumentsThe Company uses fair value measurements to record fair value adjustments to certain financial and non-financial assets and liabilities and to determine fair value disclosures. The accounting standards define fair value, establish a framework for measuring fair value, and require disclosures about fair value measurements. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at theLevel 3 measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the principal or most advantageous market in which the Company would transact are considered along with assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance.The accounting standard for fair value establishes a fair value hierarchy based on three levels of inputs, the first two of which are considered observable and the last unobservable, that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that ishierarchy.to the fair value measurement.The three levels of inputs that may be used to measure fair value are as follows:Level 1:Observable inputs, such as quoted prices in active markets for identical assets or liabilities.Level 2:Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data for substantiallyestimate the full term of the assets or liabilities.Level 3:Valuations based on unobservable inputs to the valuation methodology and including data about assumptions that market participants would use in pricing the asset or liability based on the best information available under the circumstances.Financial instruments carried at fair value include cash, cash equivalents, and marketable securities. The carrying amount of accounts receivable, accounts payable and accrued liabilities approximate fair value due to their relatively short maturities.Revenue RecognitionThe Company’s sole source of revenue is grant revenue related to a $19.8 million research grant received from the Cancer Prevention and Research Institute of Texas (“CPRIT”), covering a four-year period from June 1, 2014 through May 31, 2018. Grant revenue is recognized when qualifying costs are incurred and there is reasonable assurance that the conditions of the award have been met for collection. Proceeds received prior to the costs being incurred or the conditions of the award being met are recognized as deferred revenue until the services are performed and the conditions of the award are met (see Note 5).Research and Development CostsResearch and development costs are expensed as incurred. Research and development costs include, but are not limited to, salaries, benefits, travel, share-based compensation, consulting costs, contract research service costs, laboratory supplies, contract manufacturing costs, and costs paid to other third parties that conduct research and development activities on the Company’s behalf. Amounts incurred in connection with license agreements are also included in research and development expense.Certain research and development costs incurred were settled contractually by the Company issuing a variable number of the Company’s shares determined by dividing the fixed monetary amount of costs incurred by the issuance-date fair value of the issuable shares. CVR liability were as follows:March 31, 2024 Estimated cash flow dates 02/28/25 - 06/22/26 Estimated probability of success 39% - 100% Estimated reimbursement rate compared to reimbursement agent 81% - 100% Risk-adjusted discount rates 6.32% - 6.65% Company recorded researchchange in fair value between December 31, 2023 and development expense for these costsMarch 31, 2024 was a $0.4 million increase, and accruedwas primarily driven by changes in the risk-adjusted discount rates and the time value of money.fixed monetary amount as an accrued liability as the services were rendered until the amount was settled. In June 2015, the remaining Company obligation to settle these costs with Company shares was converted to a cash-based payment through a contract amendment with the service provider.Advance payments for goods or services to be rendered in the future for use in research and development activities are recorded as a prepaid asset and expensed as the related goods are delivered or the services are performed.The Company recognizes the cost of stock-based awards granted to employees based on the estimated grant-date fair values of the awards. The value of the award is recognized as expense ratably over the requisite service period. The Company recognizes the compensation costs for awards that vest over several years on a straight-line basis over the vesting period. Forfeitures are recognized when they occur, which may result in the reversal of compensation costs in subsequent periods as the forfeitures arise. The Company recognizes the cost of stock-based awards granted to nonemployees at their then-current fair values as services are performed, and are remeasured through the counterparty performance date.Income TaxesEffective January 1, 2015, the Company, for tax purposes, converted from a partnership to a corporation and continues to serve as a holding company for seven wholly-owned subsidiary corporations. Beginning with the year ended December 31, 2015, the Company filed a consolidated corporate federal income tax return. The Company and its subsidiaries use the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statements and the tax bases of assets and liabilities. A valuation allowance is established against the deferred tax assets to reduce their carrying value to an amount that is more likely than not to be realized. The deferred tax assets and liabilities are classified as noncurrent along with the related valuation allowance. Due to a lack of earnings history, the net deferred tax assets have been fully offset by a valuation allowance.The Company recognizes benefits of uncertain tax positions if it is more likely than not that such positions will be sustained upon examination based solely on the technical merits, as the largest amount of benefits that is more likely than not to be realized upon the ultimate settlement. The Company’s policy is to recognize interest and penalties related to the unrecognized tax benefits as a component of income tax expense.Comprehensive LossComprehensive loss is the change in stockholders’ equity from transactions and other events and circumstances other than those resulting from investments by stockholders and distributions to stockholders. The Company’s other comprehensive income (loss) is currently comprised of changes in unrealized gains and losses on available-for-sale securities.ReclassificationCertain reclassifications have been made to prior period amounts to conform to current period presentation. These reclassifications did not have an impact on the Company’s results of operations or financial position as of September 30, 2017 and December 31, 2016.Recent Accounting PronouncementsIn February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842), which establishes a comprehensive new lease accounting model. The new standard: (a) clarifies the definition of a lease; (b) requires a dual approach to lease classification similar to current lease classifications; and, (c) causes lessees to recognize leases on the balance sheet as a lease liability with a corresponding right-of-use asset for leases with a lease-term of more than twelve months. The new standard is effective for fiscal years and interim periods beginning after December 15, 2018 and requires modified retrospective application. Early adoption is permitted. The Company is currently evaluating the impact that the adoption of ASU 2016-02 will have on its consolidated financial statements, but expect the impact to be limited to the operating lease agreements for the office and laboratory spaces in Austin, Texas.In May 2017, the FASB issued ASU No. 2017-09, Compensation (Topic 718), which provides clarity and reduces both the diversity in practice and cost and complexity when applying the guidance in Topic 718, Compensation—Stock Compensation, to a change to the terms or conditions of a share-based payment award. The amendments in this update provide guidance on which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. The amendments in this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, and the amendment should be applied prospectively to an award modified on or after the adoption date. The Company does not expect the adoption of the amendment to have a material impact on its consolidated financial statements.CVR Liability Beginning balance as of December 31, 2023 $ 42,700 Changes in the fair value of the CVR liability 430 Payments (1,430) Ending Balance as of March 31, 2024 $ 41,700 3.March 31, 2024 March 31, 2024 Cash equivalents: Money market funds Money market funds Money market funds Total cash equivalents Total cash equivalents Marketable securities: Marketable securities: Commercial paper Commercial paper Commercial paper Corporate bonds U.S. government treasury securities U.S. government agency securities December 31, 2023 Cash equivalents: Money market funds $ 150,648 $ — $ — $ 150,648 Commercial paper 24,950 5 — 24,955 U.S. government treasury securities 10,965 1 — 10,966 Total cash equivalents $ 186,563 $ 6 $ — $ 186,569 Marketable securities: Commercial paper $ 79,124 $ 62 $ — $ 79,186 Corporate bonds 32,984 81 (1) 33,064 U.S. government treasury securities 21,846 31 — 21,877 U.S. government agency securities 16,147 110 — 16,257 Total marketable securities $ 150,101 $ 284 $ (1) $ 150,384 reverse repurchase agreements are settled in cash nightly, and as such are classified as cash equivalents.As of September 30, 2017 and December 31, 2016,following table summarizes the Company held 24 and nine debtavailable-for-sale securities respectively, that were in an unrealized loss position for less than one year. The aggregate fair value of debt securities inwhich an unrealized loss positionallowance for credit losses has not been recorded as of September 30, 2017March 31, 2024 and December 31, 2016 were $36.8 million2023, aggregated by major security type and $15.8 million, respectively, with no individual securitieslength of time in a significantcontinuous unrealized loss position. position:March 31, 2024 12 Months or Longer Fair Value Fair Value Fair Value Commercial paper $ 30,027 $ (23) $ — $ — $ 30,027 $ (23) Corporate bonds 30,737 (74) — — 30,737 (74) U.S. government treasury securities 77,707 (209) — — 77,707 (209) U.S. government agency securities 44,742 (145) — — 44,742 (145) Total marketable securities $ 183,213 $ (451) $ — $ — $ 183,213 $ (451) December 31, 2023 12 Months or Longer Fair Value Fair Value Fair Value Corporate bonds $ 9,907 $ (1) $ — $ — $ 9,907 $ (1) U.S. government treasury securities 4,831 — — — 4,831 — Total marketable securities $ 14,738 $ (1) $ — $ — $ 14,738 $ (1) other-than-temporary impairmentcredit losses and considered the decline in market value for the securities to be primarily attributable to current economic and market conditions and would not to a credit loss or other factors. Additionally, the Company does not intend to sell the securities in an unrealized loss position and does not expect it will be required to sell the securities before recovery of the amortizedunamortized cost basis. Based on this analysis,As of March 31, 2024 and December 31, 2023, an allowance for credit losses had not been recognized. Given the Company's intent and ability to hold such securities until recovery, and the lack of significant change in credit risk of these investments, the Company does not consider these marketable securities were not considered to be other-than-temporarily impaired as of September 30, 2017March 31, 2024 and December 31, 2016.tablestable summarizes the contractual maturities of the Company’s marketable securities at estimated fair value (in thousands):March 31,
2024March 31,
2024December 31,
2023March 31,
2024March 31,
2024December 31,
2023Accrued compensation Accrued compensation Accrued other 5. Grant RevenuesJune 2015,connection with the Asset Acquisition, the Company entered into a Cancer Research Grant Contract (“Grant Contract”)assumed the rights and obligations of Pre-Merger Spyre under the Paragon Agreement. Under the Paragon Agreement, Spyre is obligated to compensate Paragon for its services performed under each research program based on the actual costs incurred with CPRIT, under which CPRIT awarded a grant notmark-up costs pursuant to exceed $19.8 million for use in developing cancer treatments by exploiting the metabolism of cancer cells. The Grant Contract covers a four-year period from June 1, 2014 through May 31, 2018.Upon commercialization of the product, the terms of the Grant Contract requireParagon Agreement. Spyre is also obligated under the CompanyParagon Agreement to pay tiered royaltiesissue Parapyre annual equity grants of warrants in accordance with the low to mid-single digit percentages. Such royalties reduce to less than one percent after a mid-single-digit multiple of the grant funds have been paid to CPRIT as royalties.The Company recognized grant revenue of $1.3 million and $1.2 million inParapyre Option Obligation.September 30, 2017March 31, 2024, the Company recognized expenses related to services provided by Paragon subsequent to the Asset Acquisition totaling $17.1 million, which included $5.4 million of stock-based compensation expense, and 2016, respectively,were recorded as Research and $3.7 million and $3.4 milliondevelopment expenses in the nine months ended September 30, 2017 and 2016, respectively, for qualified expenditures under the grant.consolidated statements of operations. As of September 30, 2017March 31, 2024 and December 31, 2016,2023, $15.5 million and $16.6 million, respectively, was unpaid and was included in Related party accounts payable and other current liabilities on the Company's consolidated balance sheets.had outstanding grant receivablesmade payments totaling $18.2 million to Paragon.Three Months Ended
March 31,Financial Statement Line Item 2024 2023 Reimbursable costs under the Paragon Agreement $ 11.7 $ — Research and development $1.2December 31, 2024, to purchase 1% of the then outstanding shares of the Company's Common Stock, on a fully diluted basis, during the term of the Paragon AgreementMarch 31,
2024December 31,
2023Reimbursable costs under the Paragon Agreement $ 10.1 $ 16.6 Parapyre warrants liability 5.4 — Total related party accounts payable $ 15.5 $ 16.6 respectively,in stock-based compensation expense related to Mr. McKenna's consulting agreement. There was no such expense for the grant expenditures that were paid but had not been reimbursedthree months ended March 31, 2023.deferred revenue of $50,000Stockholders’ Equity$71,000, respectively, for proceeds received but for whichMay 2022, the costs had not been incurred orCompany issued pre-funded warrants to purchase the conditionsCompany’s Common Stock in underwritten public offerings at the offering price of the award hadCommon Stock, less the $0.0025 per share exercise price of each warrant. The warrants were recorded as a component of stockholders’ (deficit) equity within additional paid-in capital and have no expiration date. Per the terms of the warrant agreements, the outstanding warrants to purchase shares of Common Stock may not be exercised if the holder’s ownership of the Company’s Common Stock would exceed 4.99% (“Maximum Ownership Percentage”), or 9.99% for certain holders. By written notice to the Company, each holder may increase or decrease the Maximum Ownership Percentage to any other percentage (not in excess of 19.99% for the majority of such warrants). The revised Maximum Ownership Percentage would be effective 61 days after the notice is received by the Company.Issue Date Expiration Date Exercise Price Number of Warrants Outstanding May 20, 2022 None $ 0.0025 250,000 Total pre-funded warrants 250,000 met.6. Stock-Based CompensationThe 2016 Equity Incentive Plan (“2016 Plan”exercised.providesin connection with the Asset Acquisition and the June 2023 PIPE.an automatic annualthe benefit of the Series A Preferred Stock, regardless of whether any of the foregoing actions will be by means of amendment to the Certificate of Incorporation or by merger, consolidation, recapitalization, reclassification, conversion or otherwise, (b) issue further shares of Series A Preferred Stock or increase inor decrease (other than by conversion) the number of authorized shares available for issuance thereunder,of Series A Preferred Stock, (c) prior to be added on the first day of each fiscal year, beginning on January 1, 2017 and continuing through 2023, up to 4%stockholder approval of the conversion of the Series A Preferred Stock into shares of Common Stock in accordance with Nasdaq Stock Market Rules (the “Series A Conversion Proposal”) or at any time while at least 30% of the originally issued Series A Preferred Stock remains issued and outstanding, consummate (x) any Fundamental Transaction (as defined in the Series A Certificate of Designation) or (y) any merger or consolidation of the Company with or into another entity or any stock sale to, or other business combination inCompany’s common stockCompany completed a private placement of 721,452 shares of Series A Preferred Stock in exchange for gross proceeds of approximately $210.0 million, or net proceeds of $197.3 million, after deducting placement agent and other offering costs.December 31 immediately prior torelated forward contract liability.date of increase, provided that an increase is only effective if the Company’s board of directors either confirmed the automatic increase orCompany's stockholders approved the increaseSeries A Conversion Proposal, among other matters, at a special meeting of a lesser number of shares prior to January 1 of each relevant year.stockholders. As a result of thisthe approval of the Series A Conversion Proposal, all conditions that could have required cash redemption of the Series A Preferred Stock were satisfied. Since the Series A Preferred Stock is no longer redeemable, the associated balances of the Series A Preferred Stock were reclassified from mezzanine equity to permanent equity during the fourth quarter of 2023. In addition, 649,302 shares of Series A Preferred Stock automatically converted to 25,972,080 shares of Common Stock; 437,037 shares of Series A Preferred Stock did not automatically convert and remain outstanding as of March 31, 2024 due to beneficial ownership limitations. This conversion was recorded as a reclassification between Series A Preferred Stock and Common Stock based on the historical per-share contributed capital amount, inclusive of any forward-contract valuation adjustments, of the Series A Preferred Stock.on January 1, 2017, an additional 537,233 shares became availableof, or add any provision to, the Company’s Certificate of Incorporation or its Bylaws, or file any articles of amendment, certificate of designations, preferences, limitations and relative rights of any series of preferred stock, if such action would adversely alter or change the preferences, rights, privileges or powers of, or restrictions provided for issuance under the 2016 Plan.grantedhas agreed to use its best efforts to obtain stockholder approval of the conversion of all issued and outstanding Series B Preferred Stock into shares of Common Stock in accordance with the Nasdaq Stock Market Rules (the "Series B Conversion Proposal") at its 2024 annual meeting of stockholders (the "2024 Annual Meeting"), which the Company expects to hold on May 13, 2024. The Series B Preferred Stock is recorded outside of stockholders’ equity awards underbecause, if conversion to Common Stock is not approved by the stockholders, the Series B Preferred Stock will be redeemable at the option of the holders for cash equal to the closing price of the Common Stock per share of Common Stock underlying the Series B Preferred Stock, on the last trading day prior to the holder’s redemption request. As of March 31, 2024, the redemption value of the Company's outstanding Series B Preferred Stock was $412.1 million based on the closing stock price of the Company's Common Stock on March 31, 2024 of $37.93 per share. The Company has determined that the Series B Preferred Stock did not contain any embedded derivatives and therefore the conversion and redemption features did not require bifurcation.awardsoptions that remain outstanding under the 2015 Plan. In connection withCompany’s adoption of2015 Plan and will become available under the 2016 Equity Incentive Plan (“2016 Plan”) to the extent the options are forfeited or lapse unexercised.allthe Company may grant stock options, stock appreciation rights, restricted stock awards, restricted stock units, performance awards, and stock bonuses. The 2016 Plan, as amended, provides for an automatic increase in the number of shares reserved for issuance thereunder on January 1 of each year for the remaining term of the plan equal to (a) 5.0% of the number of issued and outstanding shares of Common Stock (including such shares issuable pursuant to the exercise or conversion, as applicable, of any outstanding pre-funded warrants and nonvoting convertible preferred stock) on December 31 of the immediately preceding year, or (b) a lesser amount as approved by the board each year (the “Evergreen Provision”). As a result of the Evergreen Provision, on January 1, 2024 and 2023, an additional 3,023,650 and 104,561 shares, respectively, became available for issuance under the 2016 Plan.transferredsubject to outstanding option awards and restricted unit awards.2016 Plan.DuringAsset Acquisition, the Company assumed the Amended and Restated Spyre 2023 Equity Incentive Plan and its outstanding and unexercised stock options, which were converted to options to purchase 2,734 shares of Common Stock. The acquisition-date fair value of these grants will be recognized as an expense on a pro-rata basis over the vesting period.September 30, 2017 and 2016,March 31, 2024, $5.4 million was recognized as stock compensation expense related to the Company issued an aggregateParapyre Option Obligation. There was no similar expense for the three months ended March 31, 2023. As of 735,600 and 11,562 optionsMarch 31, 2024, the unamortized expense related to purchase commonthe Parapyre Option Obligation was $16.5 million.respectively,awards granted under its equity incentiveall plans for an aggregate fair valueeach of $1.7 million and $42,000, respectively.During the nine months ended September 30, 2017 and periods indicated:Three Months Ended March 31, 2024 2023 Grants Weighted Average Grant Date Fair Value Grants Weighted Average Grant Date Fair Value Stock options 1,044,658 $ 26.50 177,620 $ 11.00 the Company issued an aggregate of 1,753,300 and 731,779 options to purchase common stock, respectively, under its equity incentive plans for an aggregate fair value of $6.6 million and $3.7 million, respectively.20,9902,330 and 1,793 shares for aggregate cash proceeds of $53,000 during the three months ended September 30, 2017March 31, 2024 and 39,174 shares forMarch 31, 2023, respectively. The aggregate cash proceeds of $131,000 during the nine months ended September 30, 2017. There were 19,061 shares issued and sold under the 2016 ESPPdi minimis for aggregate cash proceeds of $76,000 during the three and nine months ended September 30, 2016.both periods.
Stock-based Compensation Expenserelated torecognized from the Company’s equity incentive plans, and2018 Plan, 2016 ESPP and Parapyre Option Obligation during the periods presented was as follows (in thousands):Three Months Ended
March 31,Three Months Ended
March 31,Three Months Ended
March 31,2024 2024 2024 General and administrative General and administrative Total stock-based compensation expense Total stock-based compensation expense Three Months Ended
March 31,Three Months Ended
March 31,Three Months Ended
March 31,2024 2024 2024 Stock Options Granted Stock Options Granted Stock Options Granted Expected term (in years) Expected term (in years) Expected term (in years) Expected volatility Expected volatility Expected volatility Risk-free interest Risk-free interest Risk-free interest Dividend yield Dividend yield Dividend yield 2016 ESPP 2016 ESPP 2016 ESPP Expected term (in years) Expected term (in years) Expected term (in years) Expected volatility Expected volatility Risk-free interest Dividend yield Dividend yield 7. Fair Value Measurementsmeasuresdid not recognize any revenue under the Immedica Agreement for the three months ended March 31, 2024. For the three months ended March 31, 2023, the Company recognized $0.2 million of development fee revenue in connection with the Immedica Agreement, which was attributable to the PEACE Phase 3 trial and reports certain financial instruments asBLA package for pegzilarginase.at fair value on the Company's balance sheets. The Company recognizes license and development receivables based on billed services, which are derecognized upon reimbursement. When consideration is received, or such consideration is unconditionally due, from a recurring basis. The following tables sets forthcustomer prior to transferring goods or services to the fair valuecustomer under the terms of a contract, a contract liability is recorded. Contract liabilities are recognized as revenue after control of the Company’s financial assetsgoods or services is transferred to the customer and liabilities at fair value on a recurring basis based on the three-tier fair value hierarchy (in thousands):all revenue recognition criteria have been met.measures the fair valuedid not have any contract assets or liabilities as of money market funds on quoted prices in active markets for identical asset or liabilities. The Level 2 assets include reverse repurchase agreementsMarch 31, 2024 and U.S. government securities and are valued based on quoted prices for similar assets in active markets and inputs other than quoted prices that are derived from observable market data.The Company evaluates transfers between levels at the end of each reporting period. There were no transfers between Level 1 and Level 2 during the periods presented.8. Attributable to Common Stockholderscomputedcomputes net loss attributable per common stockholder using the two-class method required for participating securities through the date of the IPO. Immediately prior to the IPO, all outstanding convertible preferred stock was converted into common stock (see Note 1).securities. The Company considered convertibleconsiders convertible. preferred stock to be participating securities. In the event that the Company had paid out distributions, holders of convertible preferred stock would have participatedparticipate in the distribution.common stockCommon Stock and participating security considering a participating security’s rights to undistributed earnings (loss) as if all such earnings (loss) had been distributed during the period. The convertible preferred stock didholders of Series A Preferred Stock and Series B Preferred Stock do not have an obligation to fund losses and are therefore the Series A Preferred Stock and the Series B Preferred Stock were excluded from the calculation of basic net loss per share. The Company’s Series A and B convertible preferred stock were entitled to receive noncumulative dividends and in preference to any dividends on shares of the Company’s common stock.attributable to common stockholders is computed by dividing the net loss attributable to common stock by the weighted-average number of common stockCommon Stock and pre-funded warrants outstanding during the period.period, without consideration of potential dilutive securities. The pre-funded warrants are included in the computation of basic net loss per share as the exercise price is negligible and they are fully vested and exercisable. For periods in which the Company generated a net loss, the Company does not include the potential impact of dilutive securities in diluted net loss per share, as the impact of these items is anti-dilutive.Three Months Ended
March 31,2024 2023 Options to purchase common stock 3,200,918 459,425 Unvested restricted stock units 61,253 766 Outstanding Parapyre warrants 684,407 — 9. Research and License AgreementsUniversity Research AgreementIn December 2013, the Company entered intoresearch agreement with the University of Texas at Austin (the “University”). Under the terms of this research agreement, the Company engaged the University to perform certain nonclinical research activities related to the systemic depletion of amino acids for cancer and rare disease therapy.Under the research agreement, the Company was required to pay the University an annual amount not to exceed $386,000 during the one-year termreconciliation of the agreement fromshares used as the effective date. Pursuant to subsequent amendments to the research agreement, the term and maximum expenditure limitation were extended and increased through August 31, 2018 for a combined amount of $2.2 million (See Note 12). The Company did not pay the University any fees under the research agreementdenominator for the three months ended September 30, 2017calculation of basic and paid $188,000 for the three months ended September 30, 2016. For the nine months ended September 30, 2017 and 2016, the Company paid $375,000 and $645,000, respectively, to the University under the research agreement.License AgreementsIn December 2013, two of the Company’s wholly owned subsidiaries, AECase, Inc. (“AECase”) and AEMase, Inc. (“AEMase”), entered into license agreements with the University under which the University granted to AECase and AEMase exclusive, worldwide, sublicenseable licenses. The University granted the AECase license under a patent application relating to the right to use technology related to the Company’s AEB3103 product candidate. The University granted the AEMase license under a patent relating to the right to use technology related to the Company’s AEB2109 product candidate.Three Months Ended
March 31,2024 2023 Weighted average Common Stock 36,262,662 2,614,843 Weighted average pre-funded warrants 250,000 1,155,663 Total basic and diluted weighted average shares 36,512,662 3,770,506 In January 2017,Amended and Restated Patent License Agreementexchange agreement with Fairmount Healthcare Fund II L.P. (the “Restated License”“Stockholder”), pursuant to which the Stockholder agreed to exchange an aggregate of 90,992 shares of Series A Preferred Stock for an aggregate of 3,639,680 shares of Common Stock (the “April 2024 Exchange”). The Common Stock issued in connection with the University which consolidatedApril 2024 Exchange was issued without registration under the two license agreements, revised certain obligations, and licensed additional patent applications and invention disclosures toSecurities Act of 1933, as amended (the “Securities Act”) in reliance on the Company. Pursuant to the termsexemption from registration contained in Section 3(a)(9) of the Restated License, the Company may be required to pay the University up to $6.4 million milestone payments basedSecurities Act. The April 2024 Exchange closed on the achievementApril 25, 2024.certain development milestones, including clinical trialsFinancial Condition and regulatory approvals, the majorityResults of which are due upon the achievement of later development milestones, including a $5.0 million payment due on regulatory approval of a product and a $500,000 payment payable on final regulatory approval of a product for a second indication. In addition, the Company is required to pay the University a low single-digit royalty on worldwide-net sales of products covered under the Restated License, together with a revenue share on non-royalty consideration received from sublicensees. The rate of the revenue share ranges from 6.5% to 25% depending on the date the sublicense agreement is signed.10. Related Party TransactionsThe spouse of the Company’s former Chief Executive Officer previously provided consulting services to the Company. No payments were made to the spouse in the nine months ended September 30, 2017. For the three and nine months ended September 30, 2016, the Company paid $65,000 and $389,000, respectively, to the spouse in consulting fees, which were recorded in Research and Development expenses. As of September 30, 2017 and December 31, 2016, the Company had no outstanding liability to the related party.One of the founders, a non-employee member of the Company’s board of directors, entered into a consulting agreement with the Company in 2014 under which the founder would receive $50,000 per year for a fixed number of hours of consulting and advisory services and receive equity incentive shares, which converted into 43,290 restricted stock awards and 13,852 stock options upon the LLC Conversion, with the vesting contingent on time and performance milestones being achieved. The Company paid $12,500 and $25,000 during each of the three months ended September 30, 2017 and 2016 and $37,500 and $50,000 during the nine months ended September 30, 2017 and 2016, respectively, to the founder under the consulting agreement. As of September 30, 2017 and December 31, 2016, the Company had no outstanding liability to the related party.11. Commitments and ContingenciesThe Company leases office space and laboratory facilities in Austin, Texas under operating leases that commenced in January 2015 and February 2017, respectively. The office space lease was amended in September 2016 and extended to December 31, 2020. The laboratory facility lease will expire on December 31, 2017. 12. Subsequent EventIn October 2017, the Company signed an amendment to the research agreement with the University. Under the amendment, the Company extended the research agreement with the University through August 31, 2018 for an annual amount not to exceed $375,000 during the term of the agreement.
Operations20162023 filed with the SEC on March 23, 2017.February 29, 2024. This discussion and other parts of this Quarterly Report contain forward-looking statements that involve risks and uncertainties, such as statements regarding our expected results, outcomes, and the timing of ourthese results and outcomes, plans, objectives, expectations and intentions. Our actual results and outcomes could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section of this reportQuarterly Report entitled “Risk Factors.”OverviewWe are As used in this Quarterly Report, unless the context suggests otherwise, “we,” “us,” “our,” “the Company,” "Aeglea BioTherapeutics, Inc." or “Spyre” refers to Spyre Therapeutics, Inc. and its consolidated subsidiaries, including Spyre Therapeutics, LLC, taken as a whole.committedthat was incorporated on April 28, 2023 under the direction of Peter Harwin, a Managing Member of Fairmount, for the purpose of holding rights to certain intellectual property being developed by Paragon. Fairmount is a founder of Paragon.enzyme-basednext generation therapeutics for patients living with IBD, including ulcerative colitis ("UC") and Crohn's disease ("CD"). Through the Paragon Agreement, our portfolio of novel and proprietary monoclonal antibody product candidates has the potential to address unmet needs in IBD care by improving efficacy, safety, and/or dosing convenience relative to products currently available or product candidates in development. We have engineered our product candidates with the aim to bind potently and selectively to their target epitopes and to exhibit extended pharmacokinetic half-lives through modifications in the field of amino acid metabolism to treat rare genetic diseases and cancer. Our engineered human enzymesFc domain, which modifications are designed to degradeincrease affinity to human FcRn and increase antibody recycling. We anticipate that half-life extension will enable less frequent administration as compared to marketed or development-stage mAbs that do not incorporate half-life extension modifications. In addition to the development of our product candidates as potential monotherapies, we plan to investigate combinations of our proprietary antibodies in preclinical and clinical studies in order to evaluate whether combination therapy (co-administration or co-formulation of multiple monoclonal antibodies) can lead to greater efficacy, as compared toamino acidsdelivery mechanism or technology has not been selected given our early stage.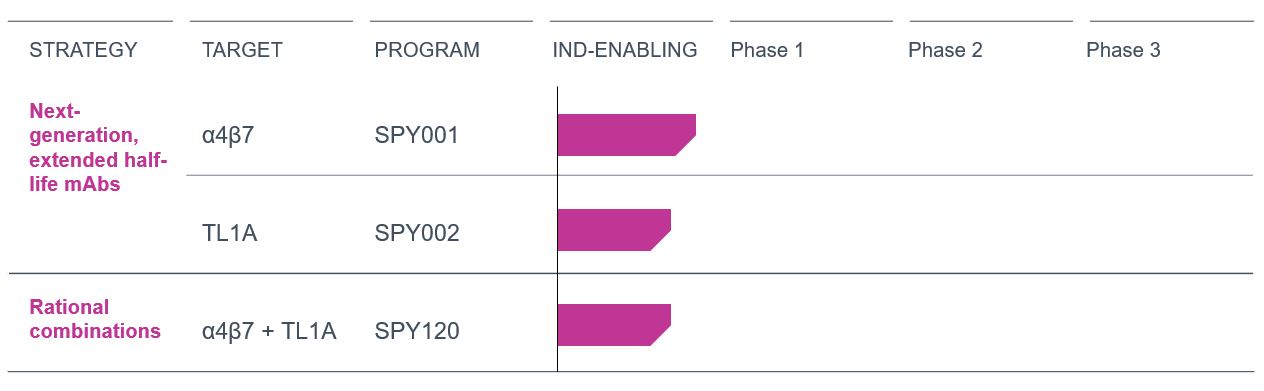
bloodsecond quarter of 2024. We continue to target these diseases. In inborn errors of metabolism, or IEM, a subset of rare genetic diseases,hold the Option to license similar rights from Paragon for certain other programs. We expect the SPY003 license to be restricted to IBD, and we are seeking to reduce the toxic levels of amino acids in patientsexpect other potential program licenses related to the normal range. In oncology,Option to be indication agnostic. We additionally have an exclusive option under the agreement for a discovery stage program targeting a novel MOA that also incorporates half-life extension (SPY004). See the section titled “Paragon Agreement” for more information on the Paragon Agreement, including the Option.are seekinghold the Option to reduce amino acid blood levels belowacquire intellectual property license rights related to the normal range whereSPY003 and SPY004 programs, such Option remains unexercised.believehave not yet tested any product candidate in humans. Notwithstanding our efforts to develop safe and effective monotherapies and combination therapies, there can be no guarantee that we will be able to exploitdevelop product candidates that will be found to be safe and effective so as to obtain the dependencenecessary regulatory approvals to market our product candidates.certain cancers on specific amino acids.leadmost advanced product candidate, AEB1102 (pegzilarginase),SPY001, is engineereda highly potent, highly selective, and humanized monoclonal immunoglobulin G1 antibody designed to degradebind selectively to the amino acid arginine and isα4β7 integrin being developed to treat two extremes of arginine metabolism, including arginine excess in patients with Arginase 1 Deficiency, an IEM, as well as some cancers which have been shown to have a metabolic dependence on arginine. The United States Adopted Names Council and World Health Organization have recently adopted pegzilarginase as the unique nonproprietary, or generic, name for AEB1102. AEB1102 has demonstrated clinical proof-of-mechanism in both scenarios. In a Phase 1 clinical trial for the treatment of patientsIBD (UC and CD). The α4β7 integrin is a protein found on the surface of immune cells known as lymphocytes. This integrin regulates the migration of lymphocytes to the gut where they contribute to the inflammatory process in IBD. By selectively binding to the α4β7 integrin, SPY001 is designed to prevent the interaction of these lymphocytes with ArginaseMAdCAM-1, a molecule expressed on endothelial cells lining the blood vessels in the gut. This interaction is responsible for guiding lymphocytes from the bloodstream into the gut tissue, where they cause inflammation. By blocking the interaction between α4β7 integrin and MAdCAM-1, SPY001 aims to reduce the recruitment of lymphocytes to the gut, leading to a decrease in inflammation. Since it specifically targets the gut immune system, SPY001 is designed to minimize systemic immunosuppressive effects unrelated to IBD pathology.Deficiency,n=6 replicates per group, study completed in August 2023). It also incorporates a dose-proportional reductionhalf-life extending modification resulting in plasma arginine levels was observedan increase in two patients. A reductionhalf-life of >three-fold in blood arginine levels wasTg276 transgenic mice that express human FcRn (n=5 per group, studies completed in August 2023) and an increase in half-life of >three-fold in NHPs (n=6 per group, studies completed in December 2023), compared to vedolizumab (see Figure 2).observedcomplete. Initiation of the FIH study in the second quarter of 2024 remains on track, pending health agency approval. Interim data from the Phase 1 healthy volunteer study are expected by the end of 2024. If successful, SPY001 would then advance to Phase 2 clinical trialsstudies and, pending further success, Phase 3 clinical studies to support global regulatory submissions and commercial approval.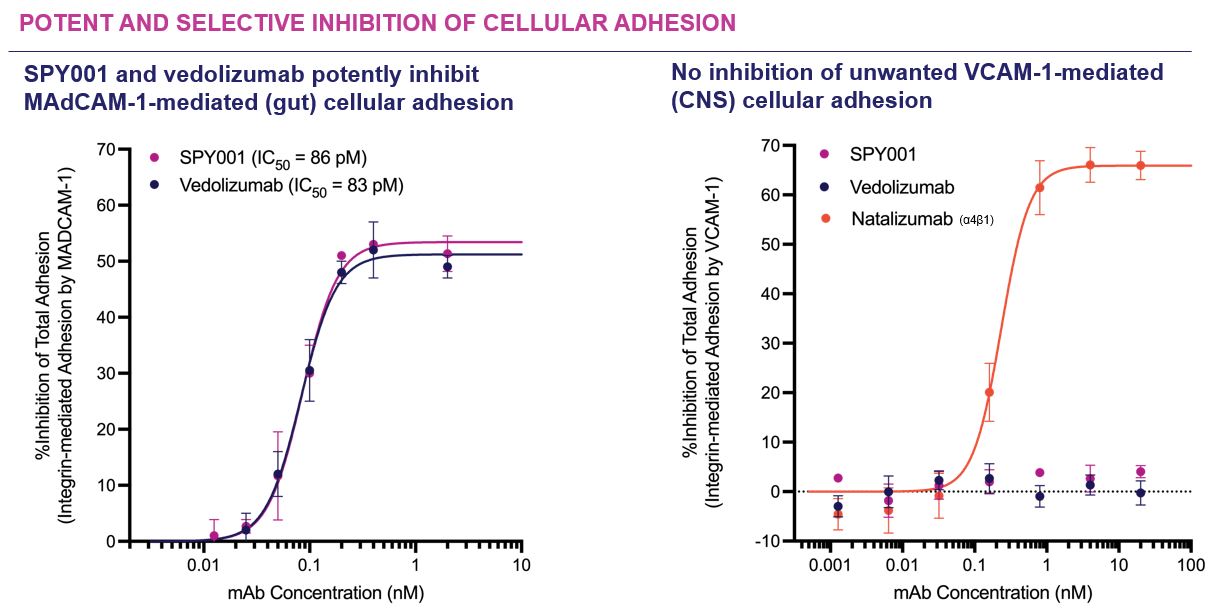
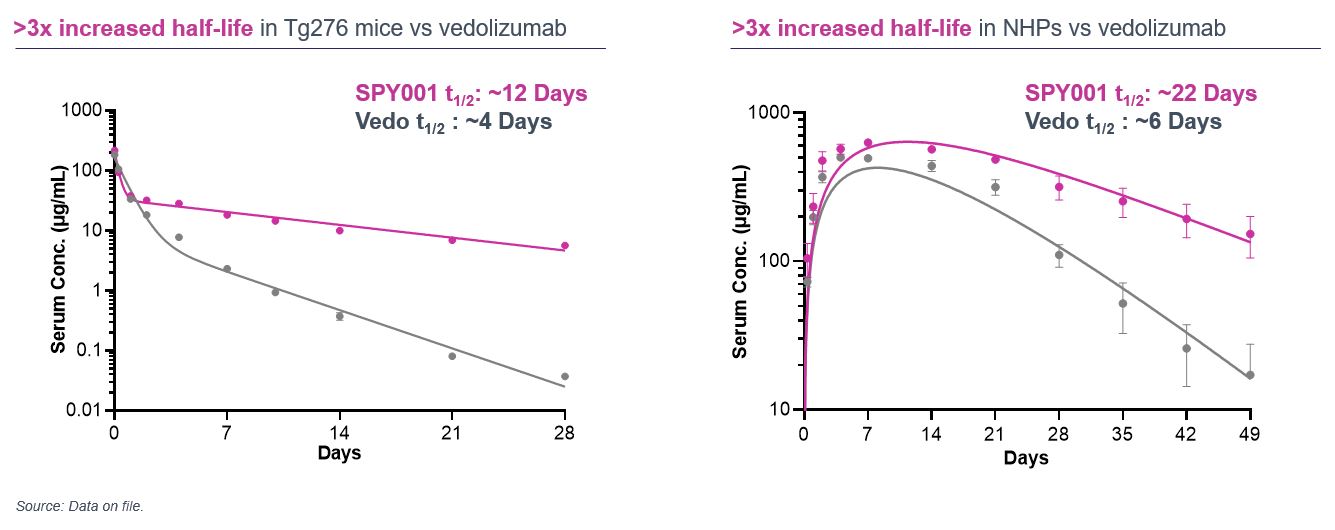
advanced solid tumorssupport from third party vendors. Our extensive discovery campaign has identified two lead candidates which bind TL1A monomers and the hematological malignancies relapsed refractory acute myeloid leukemia,trimers and have subnanomolar potency in cellular assays (see Figure 3, n=4 replicates per group per study, studies completed in Q42023 and Q12024). The candidates also exhibited extended pharmacokinetic half-lives of greater than two to three-fold relative to competitor molecules in clinical development that do not incorporate half-life extending modifications, based on head-to-head preclinical studies in NHPs (see Figure 4, n=5 per group, studies completed in Q42023 and Q12024). SPY002 candidates are currently progressing through IND-enabling studies (CMC scale-up ongoing) and we expect to submit an IND or AML,equivalent foreign regulatory submission and myelodysplastic syndrome, or MDS. These preliminary results support its potential use asenter a therapeutic of both Arginase 1 Deficiency and certain cancers associated with abnormal amino acid metabolism.We are conducting three clinical trials for AEB1102, consisting of one Phase 1/2 clinical trial for the treatment of Arginase 1 Deficiency and two Phase 1 clinical trials for the treatment of certain cancers.Arginase 1 Deficiency. In November 2016, we submitted a protocol amendment to broaden the scope of our Phase 1 trial to a Phase 1/2 trial. The amended protocol includes dosing of pediatric patients (two and older) and weekly repeat dosing, with the intent to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical response of AEB1102FIH study in patients with this IEM. In the first quarter of 2017, we received approval for the Phase 1/2 protocol for the treatment of patients with Arginase 1 Deficiency at multiple Institutional Review Boards. In March 2017, we received an information request from the FDA and began discussions with the agency to support the inclusion of pediatric patients in our Phase 1/2 trial. We are working to resolve a difference in opinion with the FDA on data required to support inclusion of pediatric patients, which has delayed our planned initiation of dosing in pediatric patients in the United States. We are continuing our dialogue with the FDA on this topic and have initiated activities to reach more patients in Canada and Europe with this rare disease. In September 2017, we dosed two adults in the repeat dose part of our Phase 1/2 trial. These two adult patients had previously received single ascending doses of AEB1102 which demonstrated that AEB1102 was well tolerated and reduced arginine levels in the blood. We expect topline data to be releasedhealthy volunteers in the second half of 2018.Hematological Malignancies. In July 2016, we initiated a Phase 1 clinical trial in patients2024, with the hematological malignancies AMLone or both of our SPY002 candidates pending additional preclinical data and MDS in the United States and Canada. Consistent with the trial for patients with advanced solid tumors, this trial has demonstrated proof-of-mechanism. We expect to announce results ofpending health agency approval. Interim data from the Phase 1 dose escalation trial in patients with AML and MDShealthy volunteer study are expected in the fourth quarterfirst half of 2017 or2025. If successful, one SPY002 candidate would then advance to Phase 2 clinical studies and, pending further success, Phase 3 clinical studies to support global regulatory submissions and commercial approval.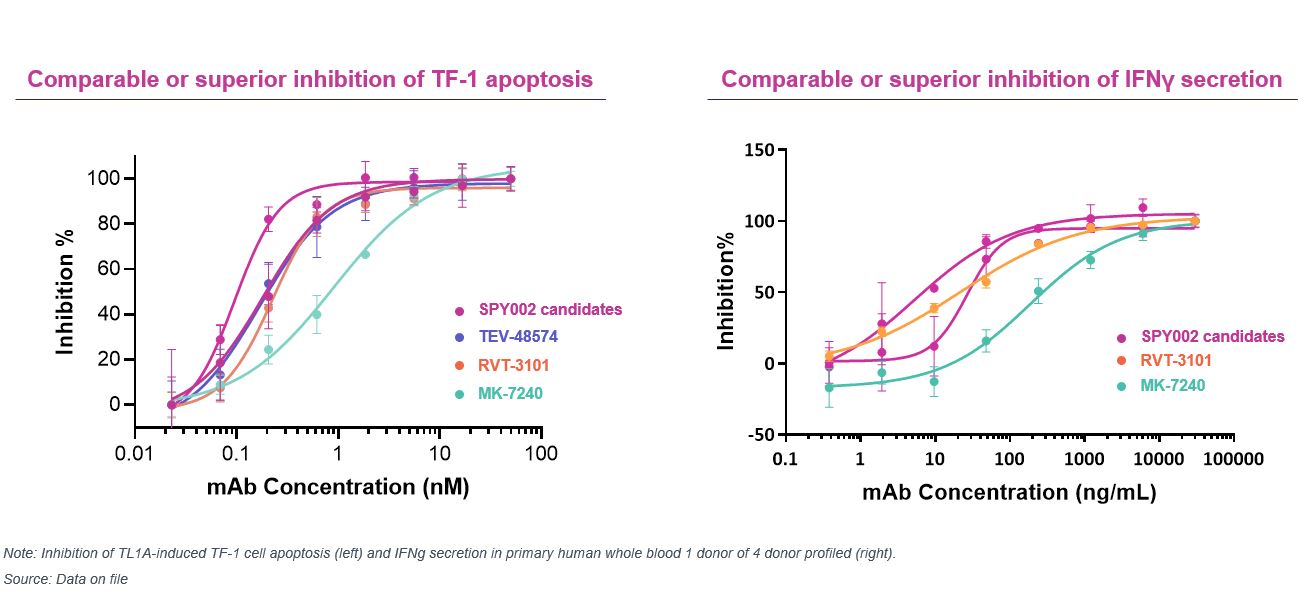
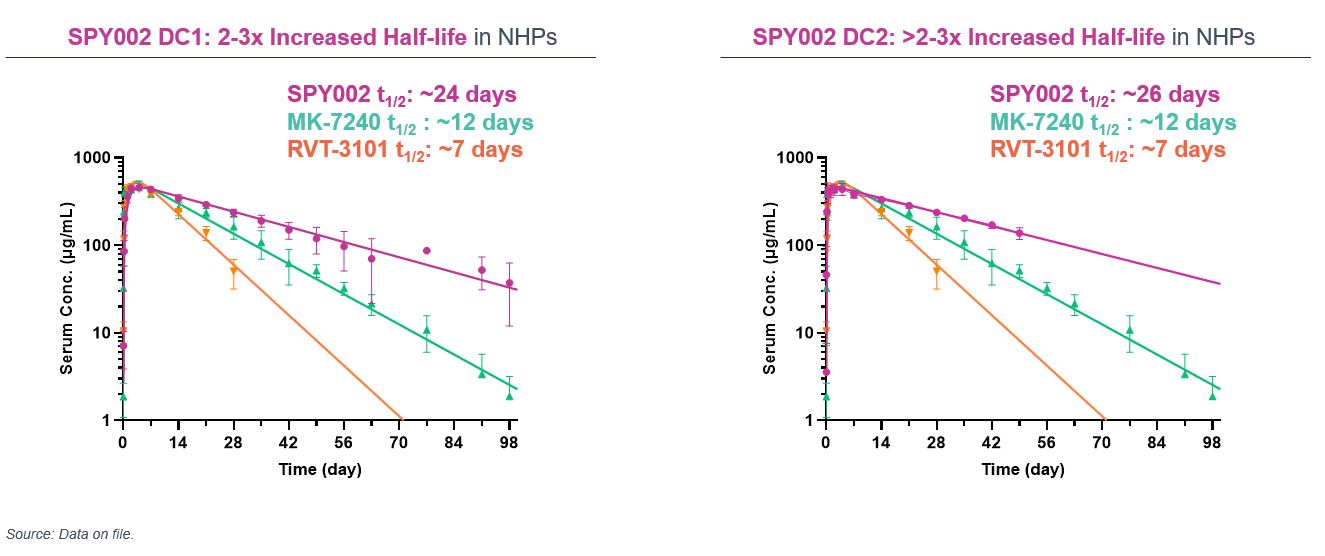
first quartersurvival, expansion, and activity of 2018.We areTh17 cells. Th17 cells produce inflammatory cytokines, such as IL-17, which contribute to the inflammation seen in IBD. IL-23 also building a pipelinehelps in the recruitment and activation of additional product candidates targeting key amino acids and other metabolites, including homocystine, a target for another IEMimmune cells, such as well as cysteine, and its oxidized form cystine, and methionine, for cancer indications. Since inception, we have devoted substantially all of our efforts and resourcesneutrophils, which further contribute to identifying and developing product candidates, conducting nonclinical studies, initiating and conducting clinical trials, recruiting personnel and raising capital.tissue damage in the gut. To date, we have financedidentified several promising clones that meet our operations primarily through private placementstarget product profile, and we are in the process of narrowing down the potential clones to select a development candidate based on pharmacokinetic performance and CMC developability. We are continuing our preferred stock,preclinical development efforts with the initial public offering, or IPO,SPY003 program and expect to nominate a development candidate in mid-2024, move into IND-enabling studies in the second half of 2024 and initiate FIH studies in the first half of 2025. Upon development candidate nomination, we intend to exercise our common stock, which closed on April 12, 2016, a follow-on public offering of our common stock in June 2017 and collection of a research grant.We have not recorded revenue from product sales and all of our revenueOption to date has been grant revenue. Since our inception, and through September 30, 2017, we have raised an aggregate of $122.1 million to fund our operations through the sale and issuance of convertible preferred and common equity securities and collected $12.0 million in grant proceeds. As of September 30, 2017, we had cash, cash equivalents, and marketable securities of $55.7 million.We have incurred net losses in each year since inception. Our net losses were $20.8 million and $16.2 millionacquire intellectual property rights for the nine months ended September 30, 2017SPY003 program pursuant to the Paragon Agreement. We expect the license to be restricted to IBD.2016, respectively,incorporates half-life extension modifications. Upon development candidate nomination, we intend to exercise our Option to acquire intellectual property rights for the SPY004 program pursuant to the Paragon Agreement.have resulted from costs incurredanti-TL1A mAbsconnection with our researchthird-party clinical trials targeting non-overlapping sites of action. We are currently evaluating SPY120 in preclinical studies, and development programs and from general and administrative expenses associated with our operations. As of September 30, 2017, we had an accumulated deficit of $66.0 million.plan to initiate combination toxicology studies in 2024. We expect to continueinitiate clinical studies for SPY120 in 2025, pending approval of an IND or equivalent foreign regulatory submission anticipated in 2025.incur significant expensesaddress overlapping and operating losses overnon-overlapping triggers of inflammation. Wenext several years. Our net losses may fluctuate significantly from quarterCompany's capital stock through their respective holdings of the Company's Common Stock. Fairmount Funds Management LLC ("Fairmount") beneficially owns more than 5% of the Company's capital stock on an as-converted basis, has two seats on our board of directors (the "Board") and beneficially owns more than 5% of Paragon, which is a joint venture between Fairmount and Fair Journey Biologics. Fairmount appointed Paragon's board of directors and has the contractual right to quarter and from year to year. We anticipate that our expenses will increase significantly as we continue our clinical and diagnostic development activities for our lead product candidate, AEB1102; concurrently develop our pipeline product candidates; expand and protect our intellectual property portfolio; and hire additional personnel. In addition, we have incurred and expect to continue to incur additional costs associated with operatingapprove the appointment of any executive officers. Parapyre is an entity formed by Paragon as a public company.Componentsvehicle to hold equity in Spyre in order to share profits with certain employees of Operating ResultsRevenueTo date,Paragon.have recognized revenue solely fromassumed the rights and obligations of Pre-Merger Spyre under the Paragon Agreement, including the obligation to issue Parapyre an annual equity grant of warrants, on the last business day of each of the years ended December 31, 2023 and December 31, 2024, to purchase 1% of the then outstanding shares of the Company's Common Stock, on a fully diluted basis, during the term of the Paragon Agreement (the "Parapyre Option Obligation"). Pursuant to the Paragon Agreement, on a research grant fromprogram-by-research program basis following the Cancer Prevention and Research Institute of Texas, or CPRIT, and have not generated any revenue from the sale of any of our product candidates. Our ability to generate product revenues, which we do not expect will occur for several years, if ever, will depend heavily on the successful development, regulatory approval and eventual commercialization of our product candidates.In June 2015, we entered into a grant agreement with CPRIT, or the Grant Contract, for $19.8 million for use in developing cancer treatments by exploiting the metabolism of cancer cells. The Grant Contract covers a four year period from June 1, 2014 through May 31, 2018. The grant allows us to receive funds in advance of costs and allowable expenses being incurred. We record the revenue as qualifying costs are incurred and there is reasonable assurance that the conditionsfinalization of the award have been metresearch plan for collection. Proceeds received prior to the costs being incurred or the conditions of the award being met are recognized as deferred revenue until the services are performed and the conditions of the award are met.On a quarterly basis,each respective research program, we are required to submitpay Paragon a financial reporting package outliningnonrefundable fee in cash of $0.8 million.nature and extent of reimbursable costs paid and requesting reimbursement under the grant. At the end of each period, qualifying costs paid prior to reimbursement resultthree months ended March 31, 2024, we recognized $17.1 million, in the recognition of a grant receivable.Research and development expensesconsist primarilythat are due to Paragon under the Paragon Agreement. As of costs incurred forMarch 31, 2024, $15.5 million was unpaid and owed to Paragon under the discoveryParagon Agreement.development ofDecember 14, 2023, we exercised our product candidates, most notably, our lead product candidate AEB1102. Since we currently do not have internal manufacturing capabilities, we contractOption available under the Paragon Agreement with external providers for manufacturing services. In addition, while we opened an internal research laboratory in February 2017, we continue to contract with external providers for nonclinical studies and clinical trials. Our research and development expenses include:costs from acquiring clinical trial materials and services performed for contracted services with a contract manufacturing organization;fees paid to clinical trial sites, clinical research organizations, contract research organizations, contract manufacturing organizations, nonclinical research companies, and academic institutions; andemployee and consultant-related expenses incurred, which include salaries, benefits, travel and stock-based compensation.Research and development costs are expensed as incurred. Advance payments for goods or services to be rendered in the future for use in research and development activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed.Research and development expenses have historically represented the largest component of our total operating expenses. We plan to increase our research and development expenses for the foreseeable future as we continue the development of our product candidates.Our expenditures on current and future nonclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. The duration, costs, and timing of clinical trials and development of our product candidates will depend on a variety of factors, including:the scope, rate of progress, and expenses of our ongoing research activities as well as any additional clinical trials and other research and development activities;future clinical trial results;uncertainties in clinical trial enrollment rates or drop-out or discontinuation rates of patients;potential safety monitoring or other studies requested by regulatory agencies;significant and changing government regulation; andthe timing and receipt of regulatory approvals, if any.The process of conducting the necessary clinical research to obtain FDA and other regulatory approval is costly and time consuming and the successful development of our product candidates is highly uncertain. The risks and uncertainties associated with our research and development projects are discussed more fully in Part II, Item 1A of this Quarterly Report titled “Risk Factors.” As a result of these risks and uncertainties, we are unable to determine with any degree of certainty the duration and completion costs of our research and development projects, or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates.General and administrative expensesGeneral and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in executive, finance, accounting, operations, and human resources functions. Other significant costs include legal fees relating to corporate matters and fees for insurance, accounting, consulting, and recruiting services.We expect that our general and administrative expenses will increase in the future to support our continued research and development activities, and the potential commercialization of our product candidates. These increases will likely include higher costs relatedrespect to the hiring of additional personnelSPY001 and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we have incurredSPY002 research programs, respectively, and expect to enter into the SPY001 License Agreement and the SPY002 License Agreement. Our Option available under the Paragon Agreement with respect to the SPY003 and SPY004 programs remains unexercised.incur increased costs associatedprovide consulting services as an independent contractor to the Company, with being a public company, including expenses relatedan effective date of August 1, 2023 (the “Vesting Commencement Date”). As compensation for Mr. McKenna’s consulting services, on November 22, 2023, he was granted non-qualified stock options to services associatedpurchase 477,000 shares of the Company’s Common Stock under the Company's equity incentive plan with maintaining compliance with NASDAQ listing rules and SEC requirements, insurance and investor relations costs.Interest incomeInterest income consistsan exercise price of interest earned on our cash, cash equivalents, and marketable securities.Income taxesWe serve$10.39 per share, which vest as a holding company for our seven wholly-owned subsidiary corporations and file consolidated corporate federal income tax returns. We use the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statements and the tax bases of assets and liabilities. A valuation allowance is established against the deferred tax assets to reduce their carrying value to an amount that is more likely than not to be realized. The deferred tax assets and liabilities are classified as noncurrent along with the related valuation allowance. Due to our lack of earnings history, the net deferred tax assets have been fully offset by a valuation allowance.We recognize benefits of uncertain tax positions if it is more likely than not that such positions will be sustained upon examination based solely25% on the technical merits, asone year anniversary of the largest amount of benefits that is more likely than notVesting Commencementbe realized upon the ultimate settlement. Our policy is to recognize interest and penalties relatedMr. McKenna’s continued service to the unrecognized tax benefits asCompany through each applicable vesting date.componentsecurities purchase agreement with certain accredited investors, pursuant to which the Company agreed to issue and sell, in a private placement, 121,625 shares of income tax expense.U.S. generally accepted accounting principles or GAAP.in the United States. The preparation of these condensed consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs and expenses, and related disclosures. These estimates form the basis for judgments we make about the carrying values of our assets, liabilities and liabilities,equity and the amount of revenues and expenses, which are not readily apparent from other sources. We base our estimates and judgments on historical experience and on various other assumptions that we believe are reasonable under the circumstances. On an ongoing basis, we evaluate our estimates and assumptions. Our actual results may differ materially from these estimates under different assumptions or conditions.We believeThe most significant estimates and assumptions that management considers in the assumptions and estimates associated withpreparation of our most critical accounting policies are those relatingfinancial statements relate to accrued research and development costscosts; the valuation of consideration transferred in acquiring IPR&D; the discount rate, probabilities of success, and timing of estimated cash flows in the valuation of the CVR liability; inputs used in the Black-Scholes model for stock-based compensation.into our critical accounting policies and estimates as compared to the critical accounting policies and estimates disclosed in Management’s"Management’s Discussion and Analysis of Financial Condition and OperationsOperations" included in our Annual Report on Form 10-K for the year ended December 31, 2016.Report.September 30, 2017March 31, 2024 and 2016September 30, 2017March 31, 2024 and 2016,2023, together with the changes in those items in dollars and as a percentage:Three Months Ended
March 31,Dollar
Change% Change 2024 2023 (in thousands) Revenue: Development fee and royalty $ — $ 198 $ (198) (100) % Total revenue — 198 (198) (100) % Operating expenses: Research and development 34,928 13,776 21,152 154 % General and administrative 12,846 5,228 7,618 146 % Total operating expenses 47,774 19,004 28,770 * Loss from operations (47,774) (18,806) (28,968) * Other income (expense): Interest income 4,432 420 4,012 * Other expense (483) (72) (411) * Total other income (expense) 3,949 348 3,601 Loss before income tax expense (43,825) (18,458) (25,367) * Income tax (expense) benefit (32) 36 (68) * Net loss $ (43,857) $ (18,422) $ (25,435) * Grant Revenues. Grant revenues increased by $0.1 million, or 10%, to $1.3 million forSeptember 30, 2017 from $1.2 million forMarch 31, 2024, we did not recognize any revenue in connection with our now terminated exclusive license and supply agreement with Immedica Pharma AB, dated March 21, 2021 (the "Immedica Agreement"). For the three months ended September 30, 2016. The increase was primarily due to additional research andMarch 31, 2023, we recognized $0.2 million of development costs associatedfee revenue in connection with the clinical trials for AEB1102 in patients with advanced solid tumors and the hematological malignancies AML and MDS, forImmedica Agreement, which we recognized grant revenue pursuantwas attributable to the Grant Contract. $0.9$21.2 million, or 16%154%, to $6.2$34.9 million for the three months ended September 30, 2017March 31, 2024, from $5.4$13.8 million for the three months ended September 30, 2016. The changeMarch 31, 2023. Our Research and development expenses incurred during the three months ended March 31, 2024 primarily related to $26.9 million in costs associated with preclinical development and manufacturing costs associated with advancing our IBD pipeline, and $5.4 million in stock-based compensation expenses associated with the Parapyre Option Obligation, partially offset by a $9.7 million decrease in costs related to the Company's legacy rare disease pipeline and a $1.5 million decrease related to lower research and development headcount.wasinclude costs associated with third parties contracted to conduct research and development activities on behalf of the Company, including through Paragon, CROs, CMOs, and third-party laboratories. For the three months ended March 31, 2024 and 2023, external research and development costs accounted for $31.3 million and $7.9 million, respectively. The increase in external research and development expenses is primarily due to:to increases in costs associated with our IBD pipeline candidates and stock compensation expense related to the Parapyre Option Obligation, partially offset by a decrease in activities associated with the Legacy Assets.Higher personnel-relatedwhich increased by $1.0 million asis primarily due to a result of additional employee headcount to expanddecrease in costs associated with our internal regulatory,on-premises research laboratory and clinical development capabilities;Higher manufacturing expenses, which increased by $0.8 million as a result of scaling-up the development and clinical manufacturing for AEB1102 and manufacturing activities for pipeline development; andLower nonclinical expenses, which decreased by $0.9 million as a result of completing toxicology studies in 2016 for the preparation of multi-dose clinical trials related to AEB1102 for patients with Arginase 1 Deficiency.$1.0$7.6 million, or 46%146%, to $3.0$12.8 million for the three months ended September 30, 2017March 31, 2024, from $2.1$5.2 million for the three months ended September 30, 2016.March 31, 2023. The increase in general and administrative expenses was primarily due to ana $6.0 million increase in employeestock-based compensation expense and facilities costs.Interest Income. Thea $1.5 million increase in interestprofessional services and legal fees.to $136,000(expense). Other income for the three months ended September 30, 2017 from $72,000 forMarch 31, 2024, totaled $3.9 million primarily driven by $4.4 million of interest earned on the three months ended September 30, 2016 was primarily dueCompany's cash and marketable securities, partially offset by a $0.4 million expense related to the investment of additional funds received as a result of our IPOchange in April 2016 and our follow-on public offering in June 2017.Comparisonfair value of the Nine Months Ended September 30, 2017 and 2016The following table summarizes our results of operations for the nine months ended September 30, 2017 and 2016, together with the changes in those items in dollars and as a percentage:Grant Revenues. Grant revenues increased by $0.3 million, or 10%, to $3.7 million for the nine months ended September 30, 2017 from $3.4 million for the nine months ended September 30, 2016. The increase was primarily due to additional research and development costs associated with the clinical trials for AEB1102 in patients with advanced solid tumors and the hematological malignancies AML and MDS, for which we recognized grant revenue pursuant to the Grant Contract. Research and Development Expenses. Research and development expenses increased by $3.6 million, or 27%, to $17.0 million for the nine months ended September 30, 2017 from $13.4 million for the nine months ended September 30, 2016. The change in research and development expenses was primarily due to:Higher personnel-related expenses, which increased by $2.2 million as a result of additional employee headcount to expand our internal regulatory, research laboratory, and clinical development capabilities;Higher manufacturing expenses, which increased by $2.2 million as a result of scaling-up the development and clinical manufacturing for AEB1102 and manufacturing activities for pipeline development;Higher clinical development expenses, which increased by $0.6 million as a result of initiating our Phase 1 dose escalation trial for AEB1102 in patients with hematological malignancies in July 2016, development of our natural history study for patients with Arginase 1 Deficiency, and our preparation for three solid tumor single agent expansion arms; andLower nonclinical expenses, which decreased by $1.4 million as a result of completing toxicology studies in 2016 for the preparation of multi-dose clinical trials related to AEB1102 for patients with Arginase 1 Deficiency.General and Administrative Expenses. General and administrative expenses increased by $1.4 million, or 22%, to $7.7 million for the nine months ended September 30, 2017 from $6.3 million for the nine months ended September 30, 2016. The increase in general and administrative expenses was primarily due to an increase in employee compensation and office rent.Interest Income. The increase in interest income to $332,000 for the nine months ended September 30, 2017 from $172,000 for the nine months ended September 30, 2016 was primarily due to the investment of additional funds received as a result of our IPO in April 2016 and our follow-on public offering in June 2017.Sources of liquidityclinical-stagepreclinical stage biotechnology company with a limited operating history, and due to our significant research and development expenditures, we have generated operating losses since our inception and have not generated anySeptember 30, 2017,March 31, 2024, we have funded our operations by raising an aggregate of $122.1 millionapproximately $1.1 billion of gross proceeds from the sale and issuance of convertible preferred stock and common equity securitiesstock, pre-funded warrants, the collection of grant proceeds, and collecting $12.0 million in grant proceeds. Additionally, we entered into an agreement with a contract manufacturing organization, or CMO, in 2013 whereby we issued convertible preferred shares to the CMO in exchange for services performed, with the obligation fully satisfied in June 2015.In April 2016, we completed our IPO and sold 5,481,940 shares of common stock for aggregate proceeds of $47.3 million, net of underwriting discounts and commissions and offering expenses.In May 2017, we filed a shelf registration statement on Form S-3 with the SEC for the offering, issuance and sale by us of up to $150.0 millionlicensing of our common stock, preferred stock, debt securities, warrants to purchase common stock, preferred stockproduct rights for commercialization of pegzilarginase in Europe and debt securities, subscription rights to purchase common stock and units consisting of all or some of these securities.In June 2017, we sold an aggregate of 3,000,000 shares of common stockcertain countries in an underwritten public offering pursuant to the shelf registration statement for gross proceeds of $12.3 million, resulting in net proceeds of $11.4 million after deducting underwriting discounts and commissions and offering expenses.Middle East. As of September 30, 2017,March 31, 2024, we do not have any offering costs ashad an outstanding liability on the balance sheet.In addition, common stock with an aggregate offering priceaccumulated deficit of up to $20.0 million may be issued and sold pursuant to an at-the-market sales agreement with JonesTrading Institutional Services LLC. As of September 30, 2017, no sales had been made under this at-the-market sales agreement and $20.0 million of common stock remained available to be sold, subject to certain conditions as specified in the sales agreement.In June 2015, we entered into the Grant Contract with CPRIT, under which CPRIT agreed to provide up to $19.8 million in grant funding to fund our development of AEB1102. Through September 30, 2017, we have collected $12.0 million in grant proceeds with $7.8 million available for future collection under the grant contract. As of September 30, 2017, we have a grant receivable outstanding of $2.5$808.3 million.lead product candidate, AEB1102.candidates, and advance our pipeline. This includes both the research and development costs and the general and administrative expenses required to support those operations. Since we are a clinical-stagepreclinical stage biotechnology company, we have incurred significant operating losses since our inception and we anticipate such losses, in absolute dollar terms, to increase as we continuepursue clinical development of our clinical trials in AEB1102product candidates, prepare for the potential commercialization of our product candidates, and expand our development efforts in our pipeline of nonclinical candidates.As of September 30, 2017, we had available cash, cash equivalents, and marketable securities of $55.7 million. Under our Based on current operating plan, we believe thatplans, we have sufficient resources to fund our operations through September 30, 2019for at least one year from the issuance date of the financial statements included in this Quarterly Report with our existing cash, cash equivalents, and marketable securities.Future funding requirements and operational planOur operational plan for We will need to secure additional financing in the near future is to continue clinical trials for our lead product candidate AEB1102 in three separate indications: Arginase 1 Deficiency, advanced solid tumors, and the hematological malignancies AML and MDS, and to expand development for at least onefund additional product candidate. As such, we plan to increase our research and development, expenditures forand before a commercial drug can be produced, marketed and sold. If the foreseeable future with nonclinical studies, clinical trials, manufacturing and an integrated biomarker strategy. We expect our principal expenditures during this time periodCompany is unable to include expenses for the following:funding the continuing development of AEB1102;funding the advancement ofobtain additional product candidates; andfunding working capital, including general operating expenses.We anticipate that we will continue tofinancing or generate losses into the foreseeable future as we develop our product candidates, seek regulatory approval of those candidates and begin to commercialize any approved products. Until such time as we can generate substantiallicense or product revenue, the lack of liquidity could have a material adverse effect on the Company.expectsold 430,107 shares of common stock and pre-funded warrants to finance our cash needs throughpurchase up to 694,892 shares of common stock in a combinationregistered direct offering for gross proceeds of equity or debt financings, research grants, collaborations, or$45.0 million, resulting in net proceeds of $42.9 million after deducting placement agent fees and offering costs.sources. We currently have no debt or debt facility or additional committed capital. To the extent thatoffering expenses.raise additional equity, the ownership interestsold 6,000,000 shares of our shareholders will be diluted.Due to our significant researchCommon Stock and development expenditures,150,000 shares of convertible Series B Preferred Stock for gross proceeds of $180.0 million before deducting approximately $10.9 million of placement agent and other offering expenses.have generated substantial losses in each period since inception. We have an accumulated deficitsold 121,625 shares of $66.0convertible Series B Preferred Stock for gross proceeds of $180.0 million asbefore deducting approximately $11.2 million of September 30, 2017. We expect to incur substantial losses in the future as we expand our researchplacement agent and development capabilities. Based on those plans, we expect that our existing cash, cash equivalent, and marketable securities will enable us to fund our operating expenses and capital expenditure requirements at least through September other offering expenses. 2019. We have based this estimate on assumptions that may prove to be incorrect, however, and we could deplete our capital resources sooner than we expect.flowsThree Months Ended
March 31,Three Months Ended
March 31,2024 2024 2023 Net cash, cash equivalents, and restricted cash (used in) provided by: Operating activities Operating activities Effect of exchange rate on cash, cash equivalents, and restricted cash Net increase in cash, cash equivalents, and restricted cash usedUsed in operating activitiesninethree months ended September 30, 2017March 31, 2024 was $18.7$28.5 million and reflected a net loss of $20.8 million. Our$43.9 million and $2.4 million in net loss wasaccretion of discount on marketable securities, partially offset in part by non-cash expenses of $1.8 million for stock-based compensation of $13.8 million and $0.2a $3.5 million for depreciation and amortization with nodecrease in net change in operating assets and liabilities during the nine months ended September 30, 2017.ninethree months ended September 30, 2016March 31, 2023 was $14.5$17.6 million and reflected a net loss of $16.2 million. Our net loss was offset in part by non-cash expenses of $1.0$18.4 million for stock-based compensation. The change in operating assets and liabilities was primarily due to an increase in accounts payable of $0.3 million and accrued and other liabilities of $1.3 million driven by an increase in accrued research and development costs offset by a $1.4 million increase in prepaid expensesnet operating assets and other assets resulting from the prepaymentliabilities, partially offset by non-cash expense of manufacturing costs, clinical development costs$1.7 million for stock-based compensation and insurance premiums.used in investing activitiesninethree months ended September 30, 2017March 31, 2024 was $27.4$105.0 million and primarily consisted of $62.1$152.7 million in purchases of marketable securities, and $0.4 million in purchases of property and equipment primarily to develop an internal research laboratory,partially offset by $35.2$47.8 million in maturities and sales of marketable securities.used inprovided by investing activities for the ninethree months ended September 30, 2016March 31, 2023 was $14.7$17.8 million from maturities and primarily consisted of $20.4 million in purchasessales of marketable securities and $0.1 million in purchases of property and equipment offsetsecurities.$5.8 million in maturities of marketable securities.Cash provided by financing activitiesninethree months ended September 30, 2017March 31, 2024 was $11.5$172.2 million, which primarily consisted of $12.3the net proceeds from the issuance of the Series B Preferred Stock in the March 2024 PIPE of $169.2 million and $4.4 million from the follow-on public offering of our common stock in June 2017, offset by $0.6 million in underwriting discounts and commissions and $0.3 million of offering costs, and $0.1 million in proceeds received from stock option exercises and sales of Common Stock under our Employee Stock Purchase Plan.common stockCommon Stock under our 2016 Employee Stock Purchase Plan.Cash provided by financing activitiesnine months ended September 30, 2016 was $49.4respective research program. Upon license execution, we expect to be obligated to pay Paragon up to $22.0 million which consistedbased on specific development, regulatory and clinical milestones for each licensed research program. As of $54.8 million from the IPO in April 2016, offset by $3.8 million in underwriting discounts and commissions and $1.7 million in offering costs, and $0.1 million in sale of common stock under our 2016 Employee Stock Purchase Plan.In September 2016, we amended our operating lease agreement for office space in Austin, Texas. The amended lease increased the office space and extended the lease term through DecemberMarch 31, 2020. The total estimated rent payments over the remaining term2024, none of the lease$22.0 million obligation was accrued for since the related license agreements are still being negotiated. As of the date of the filing of this Quarterly Report, the Option remains unexercised with respect to the two remaining research programs under the Paragon Agreement. Should the Option for these research programs be exercised and upon entry into licenseSeptember 30, 2017 is approximately $0.9 million.In February 2017, we entered into a separate lease agreement for laboratory space in Austin, Texas, which will expire on December 31, 2017. The total estimated rent payments over the remaining term ofdate hereof, the lease as of September 30, 2017 is approximately $15,000.In October 2017, we amended our research agreement with the University. The scope and term under the agreement were extended through August 31, 2018 with a $375,000 increase in the maximum expenditure limitation. The research agreement, as amended, expires on August 31, 2018 with no remaining payment obligations after such date.Contingent contractual obligationsThefollowing summarizes other key terms of the Grant Contract require that we pay CPRIT tiered royaltiesexpect to be included in the low-such agreements:mid-single digit percentages on revenues from sales and license of products or services that are based upon, utilize, are developed from or materially incorporate the intellectual property resulting from the grant-funded activities for AEB1102. Such royalties reduce to less than one percent after a mid-single digit multiple of the grant funds have been repaid to CPRIT in royalties. Such royalties are payable for so long as we have marketing exclusivity orits patents covering the applicable productrelated antibody, the method of use and its method of manufacture.service (or twelve yearsanti-TL1A monospecific antibodies for at least 5 years.commercial saleParagon.product or service in certain countries1/3rd if there is no such exclusivityParagon patent in effect during the royalty term.protection).On December 24, 2013, twodirected to a derived antibody or (ii) 12 years from the date of our wholly owned subsidiaries, AECase, Inc., or AECase,first sale of a Spyre product. AEMase, Inc., or AEMase, entered into license agreements with the University under which the University granted to AECase and AEMase exclusive, worldwide, sublicenseable licenses. The University granted to AECase a license under a patent application relating to the right to use technology related to our AEB3103 product candidate. The University granted to AEMaseextent permitted by law, on a license under a patent relating to the right to use technology related to our AEB2109 product candidate. On January 31, 2017, we entered into an Amended and Restated Patent License Agreement,party’s insolvency or the Restated License, with the University which consolidated the two license agreements dated December 24, 2013, revised certain obligations, and licensed additional patent applications and invention disclosures to Aeglea.eachthe SPY002 License Agreement only, on a product candidate covered by the Restated License, we could be required toproduct basis, Spyre will pay the UniversityParagon sublicensing fees of up to $6.4approximately $20.0 million in milestone payments based on the achievement of certain development milestones, including clinical trials and regulatory approvals, the majority of which are due upon the achievement of later development milestones, includingmostly commercial milestones.$5.0 million payment due on regulatory approval of a product and a $500,000 payment payable on final regulatory approval of a product for a second indication. In addition, we are required to pay the University a low single digit royalty on worldwide-net sales of products covered under the Restated License, together with a revenue share on non-royalty consideration received from sublicensees. The rate of the revenue share ranges from 6.5% to 25% dependingmaterial effect on the date the sublicense agreement is signed. The University may terminate the agreement under certain circumstances, including for a breach by us that is not cured within 30Company’s financial position or 60 daysresults of notice (depending on the type of breach), or if we or any of our affiliates or sublicensees participate in any proceeding to challenge the licensed patent rights (unless, with respect to sublicensees, we terminate the applicable sublicense).Off Balance Sheet ArrangementsThrough September 30, 2017, we do not have any off balance sheet arrangements, as defined by applicable SEC regulations.Recent Accounting PronouncementsIn February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842), which establishes a comprehensive new lease accounting model. The new standard: (a) clarifies the definition of a lease; (b) requires a dual approach to lease classification similar to current lease classifications;(c) causes lessees to recognize leases on the balance sheet as a lease liability with a corresponding right-of-use asset for leases with a lease-term of more than twelve months. The new standard is effective for fiscal years and interim periods beginning after December 15, 2018 and requires modified retrospective application. Early adoption is permitted. We are currently evaluating the impact that the adoption of ASU 2016-02 will have on our consolidated financial statements, but expect the impact to be limited to the operating lease agreement for office and laboratory space in Austin, Texas.In May 2017, the FASB issued ASU No. 2017-09, Compensation (Topic 718), which provides clarity and reduces both the diversity in practice and cost and complexity when applying the guidance in Topic 718, Compensation—Stock Compensation, to a change to the terms or conditions of a share-based payment award. The amendments in this update provide guidance on which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. The amendments in this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, and the amendment should be applied prospectively to an award modified on or after the adoption date. We do not expect the adoption of the amendment to have a material impact on our consolidated financial statements.U.S.United States interest rates, particularly because our investments are in marketable securities. Our marketable securities are subject to interest rate risk and could fall in value if market interest rates increase. However, we believe that our exposure to interest rate risk is not significant as the majority of our investments are short-term in duration and due to thehave a low risk profile of our investments, aprofile. A hypothetical 10% change in interest rates wouldis not expected to have a material effect on the total market value of our investment portfolio. We have the ability to hold our marketable securities until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degreematerially impacted by the effect of a change in market interest rates on our investments.September 30, 2017,March 31, 2024, we held $55.7 million$485.0 in cash, cash equivalents, and marketable securities, and restricted cash, predominately all of which was denominated in U.S. dollar assets,dollars, and consistingconsisted primarily of investments in reverse repurchase agreementsmoney market funds, commercial paper, U.S. government obligations, and U.S government securities.corporate bonds.
We are also exposed to market risk related to changes in foreign currency exchange rates as a result of our entering into transactions denominated in currencies other than U.S. dollars. Due to the uncertain timing ofItem 4. our principal financial officer, evaluated, as of the end of the period covered by this Quarterly Report on Form 10-Q, the effectiveness of our disclosure controls and procedures. Based on thatthe foregoing evaluation of our disclosure controls and procedures, as of September 30, 2017,March 31, 2024, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date arewere effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.have beenwere no changes in our internal control over financial reporting during our fiscalthe quarter ended September 30, 2017March 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.OTHER INFORMATIONOther InformationInvesting in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information in this quarterly report on Form 10-Q, including our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before investing in our common stock. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks occur, our business, operating results and prospects could be materially harmed. In that event, the price of our common stock could decline, and you could lose part or all of your investment.Risks Related to Our Business and IndustryOur limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.We are a clinical-stage biotechnology company. We began operations as a limited liability company in December 2013 and converted to a Delaware corporation in March 2015. Our operations to datelimited to organizing and staffing our company, business planning, raising capital, acquiring and developing our technology, identifying potential product candidates, undertaking nonclinical studies, and preparing for, commencing and conducting initial clinical trials of our most advanced product candidate, AEB1102 (pegzilarginase).We have not yet demonstrated our ability to successfully complete any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Products, on average, take ten to 15 years to be developed from the time they are discovered to the time they are approved and available for treating patients. Although we have recruited a team that has experience with clinical trials, as a company we have little experience in conducting clinical trials. In part because of this lack of experience, we cannot be certain that planned or ongoing clinical trials will begin or be completed on time, if at all. Consequently, any predictions you make about our future success or viability based on our short operating history to date may not be as accurate as they could be if we had a longer operating history or an established track record in commercializing products or conducting clinical trials.In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.We have no source of product revenue and we have incurred significant losses since inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.We have a limited operating history. We have no approved products and have only begun clinical development of AEB1102. Our ability to generate revenue and become profitable depends upon our ability to successfully complete the development of any of our product candidates, including AEB1102, for any of our target indications and to obtain necessary regulatory approvals. To date, we have recognized revenue solely from a government grant and have not generated any product revenue. Even if we receive regulatory approval for any of our product candidates, we do not know when these product candidates will generate revenue for us, if at all.In addition, since inception, we have incurred significant operating losses. For the three and nine months ended September 30, 2017, we reported a net loss of $7.9 million and $20.8 million, respectively. For the years ended December 31, 2016 and 2015, we reported a net loss of $21.7 million and $11.3 million, respectively. As of September 30, 2017, we had an accumulated deficit of $66.0 million. We have financed our operations primarily through private placements of our preferred stock, the initial public offering, or IPO, of our common stock, which closed on April 12, 2016, a follow-on public offering of our common stock in June 2017 and collection of a research grant. We have devoted substantially all of our efforts to research and development. We have only initiated clinical development for AEB1102 for the treatment of advanced solid tumors, Arginase 1 Deficiency and the hematological malignancies AML and MDS. We have not initiated clinical development of our other product candidates and expect that it will be many years, if ever, before we have a product candidate ready for commercialization. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, and the net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:continue our research, nonclinical and clinical development of our product candidates;seek to identify additional product candidates;conduct additional nonclinical studies and initiate clinical trials for our product candidates;seek marketing approvals for any of our product candidates that successfully complete clinical trials, including pivotal trials;ultimately establish a sales, marketing and distribution infrastructure to commercialize any product candidates for which we may obtain marketing approval;maintain, expand and protect our intellectual property portfolio;hire additional executive, clinical, quality control and scientific personnel;add operational, financial and management information systems and personnel, including personnel to support our product development; andacquire or in-license other product candidates and technologies.We are unable to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability because of the numerous risks and uncertainties associated with product development. In addition, our expenses could increase significantly beyond expectations if we are required by the FDA, EMA, MHRA or other relevant regulatory authorities to modify protocols of our clinical trials or perform studies in addition to those that we currently anticipate. Even if AEB1102, or any of our other product candidates, is approved for commercial sale, we anticipate incurring significant costs associated with the commercial launch of any product candidate.To become and remain profitable, we must develop and eventually commercialize a product candidate or product candidates with significant market potential. This will require us to be successful in a range of challenging activities, including completing nonclinical testing, initiating and completing clinical trials of one or more of our product candidates, obtaining marketing approval for these product candidates, manufacturing, marketing and selling those product candidates for which we obtain marketing approval and satisfying any post-marketing requirements. We may never succeed in these activities and, even if we do, we may never generate revenues that are significant or large enough to achieve profitability. We are currently only in the nonclinical development stages for most of our product candidates, and have only initiated clinical development for AEB1102 for the treatment of advanced solid tumors, Arginase 1 Deficiency and the hematological malignancies AML and MDS. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the company and could impair our ability to raise capital, maintain or expand our research and development efforts, expand our business or continue our operations. A decline in the value of our company would also cause you to lose part or even all of your investment.We may not be successful in advancing the clinical development of our product candidates, including AEB1102.In order to execute on our strategy of advancing the clinical development of our product candidates, we are conducting three clinical trials for AEB1102, consisting of one Phase 1/2 clinical trial for the treatment of Arginase 1 Deficiency and two Phase 1 clinical trials for the treatment of patients with advanced solid tumors and the hematological malignancies AML and MDS. We have designed the planned expansion portion of our Phase 1 trial of AEB1102 for the treatment of advanced solid tumors to study cutaneous melanoma, uveal melanoma and small cell lung cancer, all of which have been shown in published literature and preclinical studies to demonstrate a dependence on arginine. If ourproduct candidate fails to work as we expect, our ability to assess the therapeutic effect, seek regulatory approval or otherwise begin or further clinical development, could be compromised. This may result in longer development times, larger trials and a greater likelihood of terminating the trial or not obtaining regulatory approval.In addition, as we pursue oncology-related applications of our product candidates, because the natural history of different tumor types is variable, we will need to study our product candidates, including AEB1102, in clinical trials specific for a given tumor type and this will result in increased time and cost. Even if our product candidate demonstrates efficacy in a particular tumor type, we cannot guarantee that any product candidate, including AEB1102, will behave similarly in all tumor types, and we will be required to obtain separate regulatory approvals for each tumor type we intend a product candidate to treat. If any of our ongoing or planned clinical trials are unsuccessful, our business will suffer.We or third parties may not be successful in developing companion diagnostic assays for our product candidates.In developing a product candidate, we expect that if we use a biomarker-based test to identify and only enroll patients in clinical trials with tumors that express the biomarker, the FDA will require the development and regulatory approval of a companion diagnostic assay as a condition to approval of the product candidate. We do not have experience or capabilities in developing or commercializing these companion diagnostics and plan to rely in large part on third parties to perform these functions. Companion diagnostic assays are subject to regulation by the FDA as medical devices and require separate regulatory approval prior to the use of such diagnostic assays with a therapeutic product candidate. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostic assays for use with our product candidates, or experience delays in development, we may be unable to identify patients with the specific profile targeted by our product candidates for enrollment in our clinical trials. Accordingly, further investment may be required to further develop or obtain the required regulatory approval for the relevant companion diagnostic assay, which would delay or substantially impact our ability to conduct further clinical trials or obtain regulatory approval. In addition, if a companion diagnostic is necessary for any of our product candidates, the delay or failure to obtain regulatory approval of the companion diagnostic would delay or prevent the approval of the therapeutic product candidate. EMA, MHRA or comparable foreign regulatory authorities may also require the development and regulatory approval of a companion diagnostic assay as a condition to approval of the product candidate.We will need substantial additional funding. If we are unable to raise capital when needed, we would be compelled to delay, reduce or eliminate our product development programs or commercialization efforts.We expect our expenses to increase in parallel with our ongoing activities, particularly as we continue our discovery and nonclinical development to identify new clinical candidates and initiate and continue clinical trials of, and seek marketing approval for, our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution. Furthermore, we expect to continue to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, reduce or eliminate our discovery and nonclinical development programs or any future clinical development or commercialization efforts.Based upon our planned use of our cash, cash equivalents, and marketable securities as of September 30, 2017, we estimate such funds will be sufficient for us to fund our ongoing Phase 1/2 clinical trial for the treatment of patients with Arginase 1 Deficiency, our ongoing Phase 1 clinical trial for the treatment of patients with the hematological malignancies AML and MDS, our ongoing Phase 1 clinical trial for the treatment of patients with advanced solid tumors, including our three anticipated single agent expansion arms in cutaneous melanoma, uveal melanoma, and small cell lung cancer, as well as with the combination clinical trial of AEB1102 with Merck’s anti-PD-1 therapy for the treatment of patients with small cell lung cancer. Our future capital requirements will depend on many factors, including:the costs associated with the scope, progress and results of compound discovery, nonclinical development, laboratory testing and clinical trials for our product candidates;the costs related to the extent to which we enter into partnerships or other arrangements with third parties in order to further develop our product candidates;the costs and fees associated with the discovery, acquisition or in-license of product candidates or technologies;our ability to establish collaborations on favorable terms, if at all;revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive marketing approval; andthe costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims.Our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of product candidates that we do not expect to be commercially available for many years, if at all. Accordingly, we will continue to rely on additional financing to achieve our business objectives, which may not be available to us on acceptable terms, or at all.Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity or equity-linked offerings, debt financings, grants from research organizations and license and collaboration agreements. We do not have any committed external source of funds other than our grant agreement with the Cancer Prevention and Research Institute of Texas. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may rank senior to our common stock and include liquidation or other preferences, covenants or other terms that adversely affect your rights as a common stockholder. Further, any future sales of our common stock by us or resale of our common stock by our existing stockholders could cause the market price of our common stock to decline. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us and/or that may reduce the value of our common stock.We depend heavily on the success of our most advanced product candidate, AEB1102. All of our product candidates, other than AEB1102, are still in nonclinical development or nonclinical testing, and for AEB1102, the early stages of clinical development. Existing and future clinical trials of our product candidates, including AEB1102, may not be successful. If we are unable to commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed.We have invested a significant portion of our efforts and financial resources in the nonclinical and clinical development and testing of our most advanced product candidate, AEB1102, for the treatment of patients with Arginase 1 Deficiency, advanced solid tumors and the hematological malignancies AML and MDS. Our ability to generate product revenues, which we do not expect will occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of AEB1102. The success of AEB1102 and our other product candidates will depend on many factors, including the following:successful enrollment of patients in, and the completion of, our ongoing and planned clinical trials;receiving required regulatory approvals for the development and commercialization of our product candidates;establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers;obtaining and maintaining patent and trade secret protection and non-patent exclusivity for our product candidates and their components;enforcing and defending intellectual property rights and claims;achieving desirable therapeutic properties for our product candidates’ intended indications;launching commercial sales of our product candidates, if and when approved, whether alone or in collaboration with third parties;•acceptance of our product candidates, if and when approved, by patients, the medical community and third-party payors;effectively competing with other therapies; andmaintaining an acceptable safety profile of our product candidates through clinical trials and following regulatory approval.If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business.Clinical drug development involves a lengthy and expensive process with an uncertain outcome. We may experience delays in completing, or ultimately be unable to complete, the development and commercialization of any of our product candidates.We have initiated clinical trials of our lead product candidate AEB1102, and the risk of failure for all of our product candidates is high. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete nonclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans for the respective target indications. Clinical testing is expensive, difficult to design and implement and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Further, the results of nonclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials that will likely differ in design and size from early-stage clinical trials, and interim results of a clinical trial do not necessarily predict final results. For example, while we have observed a reduction in blood arginine due to AEB1102 in patients with Arginase 1 Deficiency, advanced solid tumors, and the hematological malignancies AML and MDS, this data may not necessarily be predictive of the final results of all patients intended to be enrolled in these ongoing clinical trials or in future trials. Furthermore, our ongoing Phase 1/2 clinical trial for the treatment of patients with Arginase 1 Deficiency and our Phase 1 clinical trials for the treatment of advanced solid tumors and the hematological malignancies AML and MDS will evaluate the safety of our product candidates, and we will not be evaluating the efficacy of our product candidates in these early trials. Moreover, nonclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in nonclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval.We may experience delays in our ongoing and planned clinical trials and we do not know whether planned clinical trials will begin or enroll subjects on time, whether they will need to be redesigned or whether they will be able to be completed on schedule, if at all. There can be no assurance that the FDA, EMA, MHRA or any similar foreign regulatory agency will allow us to begin clinical trials or that they will not put any of the trials for any of our product candidates that enter or have entered clinical development on clinical hold in the future. We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our product candidates. Clinical trials may be delayed, suspended or prematurely terminated because costs are greater than we anticipate or for a variety of reasons, such as:delay or failure in reaching agreement with the FDA, EMA, MHRA or a comparable foreign regulatory authority on a trial design that we are able to execute;delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial;delays in reaching, or failure to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with planned trial sites;modifications to our ongoing and planned clinical trial protocols due to regulatory requirements or decisions made by regulatory authorities;reports of safety issues, side effects or dose-limiting toxicities, or any additional or more severe safety issues in addition to those observed to date;inability, delay, or failure in identifying and maintaining a sufficient number of trial sites, many of which may already be engaged in other clinical programs;delay or failure in recruiting and enrolling suitable subjects to participate in one or more clinical trials;clinical sites and investigators deviating from the trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial;a clinical hold for any of our ongoing or planned clinical trials, including for AEB1102, where a clinical hold in a trial in one indication could result in a clinical hold for clinical trials in other indications;clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct more clinical trials than we anticipate or abandon product development programs;the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or insufficient or participants may drop out of these clinical trials at a higher rate than we anticipate;we may experience delays or difficulties in the enrollment of patients with Arginase 1 Deficiency or patients with tumors or hematological malignancies, including the identification of patients with Arginase 1 Deficiency or development or identification of a test, if needed, to screen for those cancer patients;our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;we may have difficulty partnering with experienced CROs that can screen for patients with tumors or hematological malignancies dependent on arginine that AEB1102 is designed to target and with CROs that can run our clinical trials effectively;regulators may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; orthere may bematerial changes in governmental regulations or administrative actions.If we are required to modify our ongoing clinical trial protocols, conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully initiate or complete clinical trials of our product candidates or other testing, if the results of these trials or tests do not demonstrate sufficient clinical benefit or if our product candidates do not have an acceptable safety profile, we may:be delayed in obtaining marketing approval for our product candidates;not obtain marketing approval at all;cease development of our product candidates;obtain approval for indications or patient populations that are not as broad as intended or desired;obtain approval with labeling that includes significant use or distribution restrictions or safety warnings that would reduce the potential market for our product candidates or inhibit our ability to successfully commercialize our product candidates;be subject to additional post-marketing restrictions and/or testing requirements; orhave the product removed from the market after obtaining marketing approval.We do not know whether any of our planned or current nonclinical studies, or ongoing or planned clinical trials, will need to be restructured or will be completed on schedule, or at all. For example, in November 2016, we submitted a protocol amendment to broaden the scope of our Phase 1 clinical trial for the treatment of Arginase 1 Deficiency to a Phase 1/2 trial. The amended protocol includes dosing of pediatric patients (two and older) and weekly repeat dosing, with the intent to assess the safety and tolerability, pharmacokinetics, pharmacodynamics, and clinical response of AEB1102 in patients with Arginase 1 Deficiency. In March 2017, we received an information request from the FDA and began discussions with the agency to support the inclusion of pediatric patients. We are working to resolve a difference in opinion with the FDA on data required to support inclusion of pediatric patients, which has delayed our planned initiation of dosing in pediatric patients in the United States. The FDA may require additional information or studies to beconducted, or impose conditions that could further delay or restrict our planned clinical activities. Significant nonclinical or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidates and may materially harm our business and results of operations.We may not be able to submit INDs, or foreign equivalents outside of the United States, to commence clinical trials for product candidates on the timeframes we expect, and even if we are able to, the FDA, EMA, MHRA or comparable foreign regulatory authorities may not permit us to proceed with planned clinical trials.We are currently conducting nonclinical development of our product candidates other than our clinical trials for AEB1102 for the treatment of patients with advanced solid tumors, Arginase 1 Deficiency and the hematological malignancies AML and MDS. Progression of any candidate into clinical trials is inherently risky and dependent on the results obtained in nonclinical programs, and other potential results such as the results of other clinical programs and results of third-party programs. If results are not available when expected or problems are identified during therapy development, we may experience significant delays in clinical development. This may also impact our ability to achieve certain financial milestones and the expected timeframes to market any of our product candidates. Failure to submit or have effective INDs, CTAs or other comparable foreign equivalents and commence clinical programs will significantly limit our opportunity to generate revenue.Our engineered human enzyme product candidates for our oncology indications represent a novel approach to cancer treatment, which could result in heightened regulatory scrutiny, delays in clinical development, or delays in our ability to achieve regulatory approval or commercialization of our product candidates.Engineered human enzyme products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the manufacturing and quality control standards required to be met by regulators, the number of patients the FDA, EMA, MHRA or another applicable regulatory authority will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of engineered human enzyme products, or that the data generated in these trials will be acceptable to the FDA or another applicable regulatory authority to support marketing approval.We have only initiated early-stage clinical trials for AEB1102 for the treatment of patients with advanced solid tumors, Arginase 1 Deficiency, and the hematological malignancies AML and MDS. We have not dosed any of our other product candidates in humans. Our existing and future planned clinical trials may reveal significant adverse events, toxicities or other side effects not seen in our nonclinical studies and may result in a safety profile that could inhibit regulatory approval or market acceptance of any of our product candidates.In order to obtain marketing approval for any of our product candidates, we must demonstrate the safety and efficacy of the product candidate for the relevant clinical indication or indications through nonclinical studies and clinical trials as well as additional supporting data. If our product candidates are associated with undesirable side effects in nonclinical studies or clinical trials or have characteristics that are unexpected, we may need to interrupt, delay or abandon their development or limit development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective.We are currently conducting clinical trials for AEB1102 for the treatment of patients with advanced solid tumors, Arginase 1 Deficiency and the hematological malignancies AML and MDS. Given the nature of the patient population enrolled in these trials, we have observed and expect to continue to observe serious adverse events that could be related or unrelated to AEB1102. In two separate Phase 1 trials for AEB1102 for the treatment of patients with advanced solid tumors and the hematological malignancies AML and MDS, we have observed serious adverse events in some patients, including death. To date, those serious adverse events that were considered possibly or probably related to the administration of AEB1102 include nausea, diarrhea, vomiting, dehydration, dizziness, encephalopathy manifest as acute agitation, failure to thrive, fatigue, hypertension, and intracranial hemorrhage. In our Phase 1/2 trial of AEB1102 for the treatment of patients with Arginase 1 Deficiency, we have not observed serious adverse events to date. Subjects in our ongoing and planned clinical trials may suffer minor, significant, serious, or even life-threatening adverse events, including those that are drug-related. Subjects in our ongoing and planned clinical trials may also suffer side effects not yet observed in any of our prior and ongoing clinical or nonclinical studies, including, but not limited to, toxicities to the nervous system, liver, heart, kidney, blood or immune system. We have not dosed any of our other product candidates in humans.Testing in animals, such as our primate studies for AEB1102, may not uncover all side effects in humans or any observed side effects in animals may be more severe in humans. For example, it is possible that patients’ immune systems may recognize our engineered human enzymes as foreign and trigger an immune response. This risk is heightened in patients who lack the target enzyme, as is the case with patients with Arginase 1 Deficiency that we are treating in our Phase 1/2 trial and our future trials for this IEM. In addition, our product candidates such as AEB1102 break down target amino acids such as arginine, thereby releasing metabolites such as ornithine into the bloodstream. Some patients may be sensitive to these metabolites, increasing the risk of an adverse reaction due to treatment, which risk may not be able to be mitigated through dosing. Finally, although our engineered human enzyme product candidates such as AEB1102 are engineered from the human genome, AEB1102 is produced in E. coli. This manufacturing process could lead AEB1102 to be more likely to trigger an immune response than we expect.To the extent significant adverse events or other side effects are observed in any of our clinical trials, we may have difficulty recruiting patients to the clinical trial, patients may drop out of our trial, or we may be required to abandon the trial or our development efforts of that product candidate altogether. Some potential therapeutics developed in the biotechnology industry that initially showed therapeutic promise in early-stage studies have later been found to cause side effects that prevented their further development. Even if the side effects do not preclude the drug from obtaining or maintaining marketing approval, undesirable side effects may inhibit market acceptance of the approved product due to its tolerability versus other therapies. Any of these developments could materially harm our business, financial condition and prospects.Further, toxicities associated with our product candidates may also develop after regulatory approval and lead to the withdrawal of the product from the market. We cannot predict whether our product candidates will cause organ or other injury in humans that would preclude or lead to the revocation of regulatory approval based on nonclinical studies or early stage clinical testing.If we experience delays or difficulties in the enrollment of patients in our ongoing or planned clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.We may not be able to initiate or continue our ongoing or planned clinical trials if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA, EMA, MHRA or other foreign regulatory bodies. More specifically, many of our product candidates, including AEB1102, initially target indications that may be characterized as orphan markets, which can prolong the clinical trial timeline for the regulatory process if sufficient patients cannot be enrolled in a timely manner. Arginase 1 Deficiency is the least common of the urea cycle disorders, with a reported incidence of 1:350,000 to 1:1,000,000 live births. Urea cycle disorders are the IEM resulting from defects in the enzymes of the urea cycle, the process by which the human body detoxifies ammonia, a natural byproduct of protein metabolism. While there is currently a neonatal blood test to screen for Arginase 1 Deficiency, it has only been in broad use in the United States since 2006 and is not commonly used in Europe. To date, the Urea Cycle Disorder Consortium and one national urea cycle disorder patient group have together identified approximately 40 treating physicians with over 50 patients with Arginase 1 Deficiency in the United States and Europe. Because neonatal blood testing for this disorder did not become common in the United States until 2006, we believe that approximately half of those individuals identified in the United States are younger than 18. However, due to screening requirements and enrollment restrictions in our amended clinical trial protocol, or any additional restrictions that may be imposed by regulatory agencies, not all pediatric patients, if any at all, may be eligible for inclusion in our Phase 1/2 trial in the United States.Delays in patient enrollment could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology or termination of the clinical trials altogether.Patient enrollment is affected by factors including:the severity of the disease under investigation;the design of the clinical trial protocol;the novelty of the product candidate and acceptance by physicians;the patient eligibility criteria for the study in question;the size of the total patient population;the design of the clinical trials;the availability and efficacy of competing therapies and clinical trials;our payments for conducting clinical trials;the patient referral practices of physicians;the ability to monitor patients adequately during and after treatment with the product candidate; andthe proximity and availability of clinical trial sites for prospective patients.In addition, some patients with Arginase 1 Deficiency suffer from heightened levels of ammonia, or hyperammonemia. Horizon Pharma plc has gained approval for its product RAVICTI (glycerol phenylbutyrate) to treat patients with urea cycle disorders suffering from hyperammonemia. Some patients who may be eligible for our ongoing or planned clinical trials may instead pursue treatment for this effect of their condition by taking RAVICTI (glycerol phenylbutyrate) or through dietary protein restriction. Our inability to enroll a sufficient number of patients for any of our clinical trials could result in significant delays and could require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidates and in delays to commercially launching our product candidates, if approved, which would cause the value of our company to decline and limit our ability to obtain additional financing.The safety or efficacy profile of AEB1102 may differ in combination therapy with other existing or future drugs, and therefore may preclude its further development or approval, which would materially harm our business.From time to time, our commercialization strategy may include the combination of our product candidates with third-parties’ products or product candidates. For example, we recently announced our clinical collaboration agreement with Merck to evaluate the combination of AEB1102 with Merck’s anti-PDF-1 therapy, KEYTRUDA (pembrolizumab), for the treatment of patients with small cell lung cancer. These combination studies involve additional risks due to their reliance on circumstances outside our control, such as those relating to the availability and marketability of the third-party product involved in the study. Although Merck has agreed to provide KEYTRUDA in connection with our planned combination trial, we may be unable to secure and maintain a sufficient supply of such third-party products when needed on commercially reasonably terms. Any such shortages could cause us to delay or terminate our combination trials.It is also difficult to predict the way in which AEB1102 will interact with third-party products used in combination clinical trials. As a result, such combination trials may demonstrate reduced efficacy, increase or exacerbate side effects that have been seen with AEB1102 alone, or result in new side effects that have not previously been identified with AEB1102 alone. In addition, data obtained from any combination trials may be subject to a variety of interpretations. For instance, positive data may not guarantee the ability to move forward due to changes in the landscape for the treatment of targeted indications, and failure to achieve our primary endpoints may not necessarily preclude a viable commercial path. Any undesirable side effects, lack of efficacy seen in combination trials, differing interpretation of clinical data or other unforeseen circumstances may affect our ability to continue with and obtain regulatory approval for the combination therapy, as well as our ability to continue with and obtain regulatory approval for AEB1102 monotherapy.Further, to evaluate AEB1102 in combination with other products in clinical development may require us to establish collaborations, licensing arrangements or alliances with third parties. There is no assurance that we will be able to enter into such arrangements on favorable terms, or at all.Even though we have obtained orphan drug designation for AEB1102 in the United States and Europe for the treatment of hyperargininemia, we may not obtain or maintain orphan drug exclusivity for AEB1102 and we may not obtain orphan drug designation or exclusivity for any of our other product candidates or indications.Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs or biologics for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug or biologic intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. Similarly, the European Commission may designate a product as an orphan drug under certain circumstances.Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA or the EMA from approving another marketing application for the same drug for that time period. The applicableperiod is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.In March 2015, we obtained orphan drug designation in the United States for AEB1102 for the treatment of patients with hyperargininemia, also known as Arginase 1 Deficiency. In July 2016, we also received orphan drug designation in Europe for AEB1102 for the treatment of patients with Arginase 1 Deficiency. A company that first obtains FDA or EMA approval for a designated orphan drug for the specified rare disease or condition receives orphan drug marketing exclusivity for that drug for a period of seven years in the United States or ten years in the European Union, respectively. This orphan drug exclusivity prevents the FDA or EMA from approving another application, including a Biologics License Application, or BLA, in the United States or a MAA in the European Union, to market a drug containing the same principal molecular structural features for the same orphan indication, except in very limited circumstances, including when the FDA or the EMA concludes that the later drug is safer, more effective or makes a major contribution to patient care. In addition, a designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation.Even though we have received orphan drug designation for AEB1102 for the treatment of Arginase 1 Deficiency, we may not be the first to obtain marketing approval for the orphan-designated indication due to the uncertainties associated with developing pharmaceutical product candidates. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition or a drug with the same principal molecular structural features can be approved for a different indication. Orphan drug designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process. In addition, even if we intend to seek orphan drug designation for other product candidates or indications, we may never receive such designations or obtain orphan drug exclusivity.If the market opportunities for our product candidates are smaller than we believe they are, our future product revenues may be adversely affected and our business may suffer.Our understanding of both the number of people who suffer from conditions such as Arginase 1 Deficiency or who have advanced solid tumors or hematological malignancies dependent on arginine, as well as the potential subset of those who have the potential to benefit from treatment with our product candidates such as AEB1102, are based on estimates. These estimates may prove to be incorrect and new studies may reduce the estimated incidence or prevalence of these diseases. The number of patients in the United States, Europe or elsewhere may turn out to be lower than expected, may not be otherwise amenable to treatment with our product candidates or patients may become increasingly difficult to identify and access, all of which would adversely affect our business, financial condition, results of operations and prospects.Further, there are several factors that could contribute to making the actual number of patients who receive our potential product candidates less than the potentially addressable market. These include the lack of widespread availability of, and limited reimbursement for, new therapies in many underdeveloped markets.Even if any of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.Even if any of our product candidates receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success. For example, current cancer treatments like chemotherapy and radiation therapy are well established in the medical community, and physicians may continue to rely on these treatments instead of adopting the use of AEB1102 for the treatment of patients with arginine dependent cancers. In addition, many new drugs have been recently approved and many more are in the pipeline to treat patients with cancer. Additionally, current treatments for Arginase 1 Deficiency include dietary protein restriction and, in some instances, nitrogen-scavenging drugs such as RAVICTI (glycerol phenylbutyrate). If our product candidates do not achieve an adequate level of acceptance, we may never generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:our ability to offer them for sale at competitive prices;their convenience and ease of administration compared to alternative treatments;the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;the strength of marketing and distribution support;the availability of third-party coverage and adequate reimbursement for our product candidates;the prevalence and severity of their side effects;any restrictions on the use of our product candidates together with other medications;interactions of our product candidates with other products patients are taking; andinability of patients with certain medical histories to take our product candidates.We expect to expand our development and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and, as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of product candidate development, regulatory affairs and, if any of our product candidates receives marketing approval, sales, marketing and distribution.We currently do not have a marketing or sales team for the marketing, sales and distribution of any of our product candidates that are potentially able to obtain regulatory approval. In order to commercialize any product candidates, we must build on a territory-by-territory basis marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. If our product candidates receive regulatory approval, we intend to establish an internal sales or marketing team with technical expertise and supporting distribution capabilities to commercialize our product candidates, which will be expensive and time consuming and will require significant attention of our executive officers to manage. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of any of our product candidates that we obtain approval to market. With respect to the commercialization of all or certain of our product candidates, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If we are unable to enter into such arrangements when needed on acceptable terms, or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a public company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.We face significant competition from other biotechnology and pharmaceutical companies and our operating results will suffer if we fail to compete effectively.The biotechnology and pharmaceutical industries are intensely competitive. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies, universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations and well-established sales forces. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors maysucceed in developing, acquiring or licensing, on an exclusive basis, product candidates that are more effective or less costly than any product candidate that we are currently developing or that we may develop.We face intense competition from companies developing products to address urea cycle disorders. For example, Horizon Pharma plc has gained approval for its drug RAVICTI (glycerol phenylbutyrate), which is used to treat patients with urea cycle disorders suffering from hyperammonemia, which may sometimes include patients suffering from Arginase 1 Deficiency. Patients with Arginase 1 Deficiency may also benefit from taking RAVICTI (glycerol phenylbutyrate). Erytech Pharma is currently advancing its eryminase program into preclinical development for the treatment of Arginase 1 Deficiency. We also face intense competition from companies developing products and therapies to treat cancer. For example, Polaris Pharmaceuticals is conducting numerous clinical trials of ADI-PEG 20, an enzyme derived from mycoplasma, which degrades arginine in the blood.Our ability to compete successfully will depend largely on our ability to leverage our experience in product candidate discovery and development to:discover and develop product candidates that are superior to other products in the market;attract qualified scientific, product development and commercial personnel;obtain and maintain patent and/or other proprietary protection for our product candidates and technologies;obtain required regulatory approvals; andsuccessfully collaborate with research institutions or pharmaceutical companies in the discovery, development and commercialization of new product candidates.The availability and price of our competitors’ products could limit the demand, and the price we are able to charge, for any of our product candidates, if approved. We will not achieve our business plan if acceptance is inhibited by price competition or the reluctance of physicians to switch from existing drug products or other therapies to our product candidates, or if physicians switch to other new drug products or choose to reserve our product candidates for use in limited circumstances.Established biotechnology companies may invest heavily to accelerate discovery and development of products that could make our product candidates less competitive. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA or non-U.S. regulatory approval or discovering, developing and commercializing product candidates before we do, which would have a material adverse impact on our business. Many of our competitors have greater resources than we do and have established sales and marketing capabilities, whether internally or through third parties. We will not be able to successfully commercialize our product candidates without establishing sales and marketing capabilities internally or through strategic partners.The insurance coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current product candidates could limit our ability to market those product candidates and decrease our ability to generate revenue.The availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of any of our product candidates that receive marketing approval will depend substantially, both in the United States and internationally, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new products are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services since CMS decides whether and to what extent a new product will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for novel products such as ours since there is no body of established practices and precedents for these new products.Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United States and have not been approved for reimbursement in certain European countries.Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of therapeutics such as our product candidates. In many countries, particularly the countries of the European Union, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. In general, the prices of products under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for products, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits.Moreover, increasing efforts by governmental and third-party payors, in the United States and internationally, to cap or reduce healthcare costs may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. The U.S. Congress and the new Trump administration have similarly expressed concerns over the pricing of pharmaceutical products and there can be no assurance as to how this scrutiny will impact future pricing of pharmaceutical products generally. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products into the healthcare market.In addition to CMS and private payors, professional organizations such as the National Comprehensive Cancer Network and the American Society of Clinical Oncology can influence decisions about reimbursement for new products by determining standards for care. In addition, many private payors contract with commercial vendors who sell software that provide guidelines that attempt to limit utilization of, and therefore reimbursement for, certain products deemed to provide limited benefit to existing alternatives. Such organizations may set guidelines that limit reimbursement or utilization of our product candidates.Furthermore, some of our target indications, including for Arginase 1 Deficiency for AEB1102, are orphan indications where patient populations are small. In order for therapeutics that are designed to treat smaller patient populations to be commercially viable, the reimbursement for such therapeutics must be higher, on a relative basis, to account for the lack of volume. Accordingly, we will need to implement a coverage and reimbursement strategy for any approved product candidate that accounts for the smaller potential market size. If we are unable to establish or sustain coverage and adequate reimbursement for any future product candidates from third-party payors, the adoption of those products and sales revenue will be adversely affected, which, in turn, could adversely affect the ability to market or sell those product candidates, if approved, and ultimately our financial results.Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.We are a clinical-stage biotechnology company with a limited operating history, and, as of September 30, 2017, had only 45 employees, including six executive officers. We are highly dependent on the research and development, clinical and business development expertise of our executive officers, as well as the other principal members of our management, scientific and clinical team. Any of our management team members may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees.Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills andexperience required to successfully develop, facilitate regulatory approval of and commercialize product candidates. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. For instance, we have recently appointed Dr. Anthony Quinn, a member of our board of directors, as interim Chief Executive Officer, and are currently in the process of searching for a permanent Chief Executive Officer. There is no assurance that a qualified individual will be found timely or engaged on acceptable terms. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions.In addition, we rely on consultants and advisors, including scientific and clinical advisors such as our scientific advisory board, to assist us in formulating our discovery and nonclinical development and commercialization strategy. Our consultants and advisors, including members of our scientific advisory board, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.Our product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.With the enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as interchangeable based on its similarity to an existing reference product. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the original branded product is approved under a BLA. On March 6, 2015, the FDA approved the first biosimilar product under the BPCIA. However, the law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when the processes intended to implement BPCIA may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.We believe that if any of our product candidates are approved as a biological product under a BLA, it should qualify for the 12-year period of exclusivity. However, there is a risk that the FDA will not consider any of our product candidates to be reference products for competing products, potentially creating the opportunity for biosimilar competition sooner than anticipated. Additionally, this period of regulatory exclusivity does not apply to companies pursuing regulatory approval via their own traditional BLA, rather than via the abbreviated pathway. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products that may be approved in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.Our business and operations would suffer in the event of system failures.Despite the implementation of security measures, our internal computer systems and those of our strategic partners and third-parties on whom we rely are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Furthermore, we have little or no control over the security measures and computer systems of third parties including the University of Texas at Austin and any CROs we may work with in the future. While we and, to our knowledge, our third-party strategic partners have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, the operations of our strategic partner the University of Texas at Austin, our other third-party strategic partners, or our manufacturers or suppliers, it could result in a material disruption of our product candidate development programs. For example, the loss of research data by University of Texas at Austin could delay development of our product candidates and the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts, and we may incur substantial costs to attempt to recover or reproduce the data. If any disruption or security breach resulted in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability or the further development of our product candidates could be delayed.Risks Related to Our Reliance on Third PartiesWe will rely on third parties to conduct our ongoing and future planned clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.We currently rely and will continue to rely on third parties to provide manufacturing and clinical development capabilities. For example, we rely on a contract manufacturing organization, KBI BioPharma, Inc., or KBI, to manufacture and supply nonclinical and clinical trial quantities of the biological substance of our lead product candidate, AEB1102 and pipeline product candidates. We also expect to rely on KBI to manufacture and supply commercial quantities of AEB1102.We will rely on third-party CROs to conduct our ongoing and future planned clinical trials of AEB1102. We do not plan to independently conduct clinical trials of our other product candidates. These agreements might terminate for a variety of reasons, including a failure to perform by the third parties. If we need to enter into alternative arrangements, that would delay our product development activities.Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our ongoing and future planned clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with regulatory standards, commonly referred to as good clinical practices for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Other countries’ regulatory agencies also have requirements for clinical trials with which we must comply. We also will be required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our ongoing and future planned clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to complete our clinical trials, obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our product candidates, producing additional losses and depriving us of potential product revenue.We contract with third parties for the manufacture of our product candidates for nonclinical studies and our ongoing and future planned clinical testing and expect to continue to do so for commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates at an acceptable cost and quality, which could delay, prevent or impair our development or commercialization efforts.We do not own or operate facilities for the manufacture of our product candidates, and we do not have any manufacturing personnel. We currently have no plans to build our own clinical or commercial scale manufacturing capabilities. We rely, and expect to continue to rely, on third parties, including KBI and Lyophilization Services of New England, Inc., for the manufacture of our product candidates for nonclinical studies and for our existing and future planned clinical trials. We also expect to rely on third parties, including KBI, for commercial manufacture if any of our product candidates receive marketing approval. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts.Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply or a source for bulk drug substance. Currently, KBI is supplying, and is expected to continue to supply, the drug substance requirements for our ongoing and planned clinical trials with AEB1102. If KBI cannot supply us with sufficient amounts, pursuant to product requirements as agreed, we may be required to identify alternative manufacturers, which would lead us to incur added costs and delays in identifying and qualifying any replacement.The formulation used in early studies is not a final formulation for commercialization. If we are unable to demonstrate that our commercial scale product is comparable to the product used in clinical trials, we may not receive regulatoryapproval for that product without additional clinical trials. We have contracted with KBI for certain studies related to potential commercial scale manufacturing of AEB1102 at a separate KBI facility, but there is no guarantee that such studies, the transfer of technology to or any potential manufacturing at such facility, will be completed successfully, on time, or at all. We also cannot guarantee that we will be able to make any required modifications within currently anticipated timeframes or that such modifications, if and when made, will obtain regulatory approval or that the new processes or modified processes will be successfully implemented by or transferred to any third-party contract suppliers within currently anticipated timeframes. These may require additional studies, and may delay our clinical trials and/or commercialization.We expect to rely on third-party manufacturers, including KBI, or third-party strategic partners for the manufacture of commercial supply of any product candidates for which our strategic partners or we obtain marketing approval. We may be unable to establish any additional agreements with third-party manufacturers, including KBI, or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers on acceptable terms, such third-party manufacturers may have limited experience manufacturing pharmaceutical drugs for commercialization, and reliance on third-party manufacturers for the commercial supply of our products may expose us to various risks, including:possible noncompliance by the third party with regulatory requirements and quality assurance;the possible breach of the manufacturing agreement by the third party;the possible misappropriation of our proprietary information, including our trade secrets and know-how; andthe possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us.Third-party manufacturers may not be able to comply with current good manufacturing practices, or cGMP, or similar regulatory requirements outside the United States. Although we do not have day-to-day control over third-party manufacturers’ compliance with these regulations and standards, we are responsible for ensuring compliance with such regulations and standards. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates, operating restrictions and criminal prosecutions, any of which would significantly and adversely affect supplies of our product candidates and our business.In addition, the process of manufacturing and administering our product candidates is complex and highly regulated. As a result of the complexities, our manufacturing and supply costs are likely to be higher than those at more traditional manufacturing processes and the manufacturing process is less reliable and more difficult to reproduce.We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our product candidates, producing additional losses and depriving us of potential product revenue.Our product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to commercialize any product candidates that receive marketing approval on a timely and competitive basis.Failure of any future third-party collaborators to successfully commercialize companion diagnostics developed for use with our therapeutic product candidates for oncology indications could harm our ability to commercialize these product candidates.We do not plan to develop companion diagnostics internally and, as a result, we are dependent on the efforts of our third-party strategic partners to successfully commercialize any needed companion diagnostics. Our strategic partners:may not perform their obligations as expected;may encounter production difficulties that could constrain the supply of the companion diagnostics;may have difficulties gaining acceptance of the use of the companion diagnostics in the clinical community;may elect not to continue or renew commercialization programs based on changes in the strategic partners’ strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;may not commit sufficient resources to the marketing and distribution of such companion diagnostic product candidates; andmay terminate their relationship with us.If companion diagnostics needed for use with our therapeutic product candidates in oncology fail to gain market acceptance, our ability to derive revenues from sales of these therapeutic product candidates could be harmed. If our strategic partners fail to commercialize these companion diagnostics, it could adversely affect and delay the development or commercialization of our therapeutic product candidates.We may not be successful in finding strategic partners for continuing development of certain of our product candidates or successfully commercializing or competing in the market for certain indications.We may seek to develop strategic partnerships for developing certain of our product candidates, due to capital costs required to develop the product candidates or manufacturing constraints. We may not be successful in our efforts to establish such a strategic partnership or other alternative arrangements for our product candidates because our research and development pipeline may be insufficient, our product candidates may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy. In addition, we may be restricted under existing collaboration agreements from entering into future agreements with potential strategic partners. We cannot be certain that, following a strategic transaction or license, we will achieve an economic benefit that justifies such transaction.If we are unable to reach agreements with suitable strategic partners on a timely basis, on acceptable terms or at all, we may have to curtail the development of a product candidate, reduce or delay its development program, delay its potential commercialization, reduce the scope of any sales or marketing activities or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our product candidates and our business, financial condition, results of operations and prospects may be materially and adversely affected.Our employees may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable non-U.S. regulatory authorities, provide accurate information to the FDA or comparable non-U.S. regulatory authorities, comply with manufacturing standards we have established, comply with the Foreign Corrupt Practices Act and federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable non-U.S. regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not usethe proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used orfactors disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims.In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.We and our strategic partners that we rely on may be adversely affected by natural disasters, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.Natural disasters could severely disrupt our operations or the operations of KBI’s manufacturing facilities and have a material adverse effect on our business, financial condition, results of operations and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as KBI’s manufacturing facilities, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently are limited and may not prove adequate in the event of a serious disaster or similar event. Substantially all of our current supply of product candidates are located at KBI’s manufacturing facilities, and we do not have any existing back-up facilities in place or plans for such back-up facilities. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business, financial condition, results of operations and prospects.Risks Related to Government RegulationIf we are not able to obtain, or if there are delays in obtaining, required regulatory approvals in the United States or in foreign jurisdictions, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.Our product candidates must be approved by the FDA pursuant to a BLA in the United States, and by the EMA pursuant to a MAA, and by other comparable regulatory authorities outside the United States prior to commercialization. The process of obtaining marketing approvals, both in the United States and internationally, is expensive and takes many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval in Europe or another non-U.S. jurisdiction may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or our third-party strategic partners may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our product candidates in any market.Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market any of our product candidates from regulatory authorities in any jurisdiction. We have no experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party CROs to assist us in this process. Securing marketing approval requires the submission of extensive nonclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketingapproval or prevent or limit commercial use. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional nonclinical, clinical or other studies. In addition, varying interpretations of the data obtained from nonclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may also cause delays in or prevent the approval of an application.Approval of our product candidates may be delayed or refused for many reasons, including the following:the FDA, EMA, MHRA or other comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials;we may be unable to demonstrate to the satisfaction of the FDA, EMA, MHRA or other comparable foreign regulatory authorities that our product candidates are safe and effective for any of their proposed indications;the results of clinical trials may not meet the level of statistical significance required by the FDA, EMA, MHRA or other comparable foreign regulatory authorities for approval;we may be unable to demonstrate that our product candidates’ clinical and other benefits outweigh their safety risks;the FDA, EMA, MHRA or other comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical programs or clinical trials;the data collected from clinical trials of our product candidates may not be sufficient to the satisfaction of the FDA, EMA, MHRA or other comparable foreign regulatory authorities to support the submission of a BLA, MAA or other comparable submission in other jurisdictions or to obtain regulatory approval in the United States or elsewhere;the facilities of the third-party manufacturers with which we partner may not be adequate to support approval of our product candidates; andthe approval policies or regulations of the FDA, EMA or other comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.New products for the treatment of cancer frequently are initially indicated only for patient populations that have not responded to an existing therapy or have relapsed. If any of our product candidates receives marketing approval, the approved labeling may limit the use of our product candidates in this way, which could limit sales of the product.Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable. If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.Any Fast Track Designation by the FDA, even if granted for any of our product candidates, may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval.We have received Fast Track Designation from the FDA for our lead product candidate AEB1102 for the treatment of hyperargininemia secondary to Arginase 1 Deficiency, and may seek such designation for some or all of our product candidates. If a drug or biologic is intended for the treatment of a serious or life-threatening condition and the drug or biologic demonstrates the potential to address unmet medical needs for this condition, the drug or biologic sponsor may apply for FDA Fast Track Designation. The FDA has broad discretion whether or not to grant this designation. Even if we believe a particular product candidate is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even though we have received Fast Track Designation for AEB1102 for the treatment of hyperargininemia secondary to Arginase 1 Deficiency, and even if we receive Fast Track Designation for other product candidates or indications in the future, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program. Many drugs or biologics that have received Fast Track Designation have failed to obtain approval.We may also seek accelerated approval for products that have obtained fast track designation. Under the FDA’s accelerated approval program, the FDA may approve a drug or biologic for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. For drugs or biologics granted accelerated approval, post-marketing confirmatory trials are required to describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. These confirmatory trials must be completed with due diligence and, in some cases, the FDA may require that the trial be designed and/or initiated prior to approval. Moreover, the FDA may withdraw approval of our product candidate or indication approved under the accelerated approval pathway if, for example:the trial or trials required to verify the predicted clinical benefit of our product candidate fail to verify such benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with the drug;other evidence demonstrates that our product candidate is not shown to be safe or effective under the conditions of use;we fail to conduct any required post-approval trial of our product candidate with due diligence; orwe disseminate false or misleading promotional materials relating to the relevant product candidate.A Breakthrough Therapy Designation by the FDA, even if granted for any of our product candidates, may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval.We do not currently have Breakthrough Therapy Designation for any of our product candidates, but may seek such designation. A Breakthrough Therapy is defined as a drug or biologic that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biologic may demonstrate substantial improvement over existing therapies with respect to one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs or biologics that have been designated as Breakthrough Therapies, interaction and communication between the FDA and the sponsor can help to identify the most efficient path for development.Designation as a Breakthrough Therapy is within the discretion of the FDA. Accordingly, even if we believe, after completing early clinical trials, that one of our product candidates meets the criteria for designation as a Breakthrough Therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a Breakthrough Therapy designation for a product candidate may not result in a faster development process, review or approval compared to drugs or biologics considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as Breakthrough Therapies, the FDA may later decide that such product candidates no longer meet the conditions for qualification.Any product candidate for which we obtain marketing approval will be subject to extensive post-marketing regulatory requirements and could be subject to post-marketing restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any of them are approved.Our product candidates and the activities associated with their development and commercialization, including their testing, manufacture, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP, requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, including periodic inspections by the FDA and other regulatory authorities, requirements regarding the distribution of samples to physicians and recordkeeping.The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of any approved product. The FDA closely regulates the post-approval marketing and promotion of drugs and biologics to ensure drugs and biologics are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding use of their products and if we promote our product candidates beyond their approvedindications, we may be subject to enforcement action for off-label promotion. Violations of the Federal Food, Drug, and Cosmetic Act relating to the promotion of prescription drugs may lead to investigations alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws.In addition, later discovery of previously unknown adverse events or other problems with our product candidates, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:restrictions on such product candidates, manufacturers or manufacturing processes;restrictions on the labeling or marketing of a product;restrictions on product distribution or use;requirements to conduct post-marketing studies or clinical trials;warning or untitled letters;withdrawal of any approved product from the market;refusal to approve pending applications or supplements to approved applications that we submit;recall of product candidates;fines, restitution or disgorgement of profits or revenues;suspension or withdrawal of marketing approvals;refusal to permit the import or export of our product candidates;product seizure; orinjunctions or the imposition of civil or criminal penalties.Non-compliance with European requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with Europe’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any product candidates for which we obtain marketing approval. Restrictions under applicable U.S. federal and state healthcare laws and regulations include the following:the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;federal law requires applicable manufacturers of covered drugs to report payments and other transfers of value to physicians and teaching hospitals, which includes annual data collection and reporting obligations. The information was made publicly available on a searchable website in September 2014 and will be disclosed on an annual basis; andanalogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers.Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of product candidates from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved product candidates. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.More recently, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.Among the provisions of the ACA of importance to our potential product candidates are the following:an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents;an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;a Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices;extension of manufacturers’ Medicaid rebate liability to manage care initiation;expansion of eligibility criteria for Medicaid programs;expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;requirements to report financial arrangements with physicians and teaching hospitals;a requirement to annually report drug samples that manufacturers and distributors provide to physicians; anda Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. More recently, President Trump has suggested that he plans to seek repeal of all or portions of the ACA, and he has indicated that he wants Congress to replace the ACA with new legislation. We cannot predict whether these challenges will continue or other proposals will be made or adopted, or what impact these efforts may have on us.We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product candidates.Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.Although we maintain workers’ compensation insurance that we believe is consistent with industry norms to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, we cannot assure you that it will be sufficient to cover our liability in such cases. We do not maintain insurance forenvironmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our discovery, nonclinical development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.Risks Related to Our Intellectual PropertyIf we are unable to obtain and maintain intellectual property protection for our technology and product candidates, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and product candidates similar or identical to ours, and our ability to successfully commercialize our technology and product candidates may be impaired.We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our technology and product candidates.In particular, our success depends in large part on our ability, and our licensors’ ability, to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and product candidates, including any companion diagnostic developed by us or a third-party strategic partner. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and product candidates, and rely on our licensors to obtain patent protection for our licensed intellectual property. Our patent portfolio includes patents and patent applications own or we exclusively license from the University of Texas at Austin. This patent portfolio includes issued patents and pending patent applications covering compositions of matter and methods of use.The patent prosecution process is expensive and time-consuming, and we may not be able to file, prosecute, maintain, enforce or license all necessary or desirable patent applications at a reasonable cost or in a timely manner, or in all jurisdictions. We may choose not to seek patent protection for certain innovations and may choose not to pursue patent protection in certain jurisdictions, and under the laws of certain jurisdictions, patents or other intellectual property rights may be unavailable or limited in scope. It is also possible that we will fail to identify patentable aspects of our discovery and nonclinical development output before it is too late to obtain patent protection. Moreover, the risks pertaining to our patents and intellectual property rights also apply to the intellectual property rights that we license from third parties. In some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. We may also require the cooperation of our licensors in order to enforce the licensed patent rights, and such cooperation may not be provided. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business and the rights we have licensed may be reduced or eliminated.The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. The U.S. Patent and Trademark Office, or U.S. PTO, has not established a consistent policy regarding the breadth of claims that it will allow in biotechnology patents. In addition, the laws of foreign jurisdictions may not protect our rights to the same extent as the laws of the United States. For example, India and China do not allow patents for methods of treating the human body. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions, nor can we know whether those from whom we license patents were the first to make the inventions claimed or were the first to file. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our technology or product candidates, in whole or in part, or which effectively prevent others from commercializing competitive technologies and product candidates. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, during prosecution of any patent application, the issuance of any patents basedAnnual Report on the application may depend upon our ability to generate additional preclinical or clinical data that supports the patentability of our proposed claims. We may not be able to generate such data on a timely basis, to the satisfaction of the U.S. PTO, or at all.Moreover, we may be subject to a third-party preissuance submission of prior art to the U.S. PTO or patent offices in foreign jurisdictions, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or product candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize product candidates without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or product candidates in a non-infringing manner.The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and product candidates, or limit the duration of the patent protection of our technology and product candidates. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after the first non-provisional filing in the patent family. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.Any inability on our part to adequately protect our intellectual property may have a material adverse effect on our business, operating results and financial position.Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the U.S. PTO and various governmental patent agencies outside the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to non-U.S. patent agencies. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, in some cases we rely on licensors to effect such payments with respect to the patents and patent applications that we in-license. Moreover, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.Our commercial success depends upon our ability, and the ability of our collaborators, to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates and technology, including interference or derivation proceedings before the U.S. PTO and similar bodies in other jurisdictions. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future.It is also possible that we have failed to identify relevant third-party patents or applications. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Moreover, it is difficult for industry participants, including us, toidentify all third-party patent rights that may be relevant to our product candidates and technologies because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. We may fail to identify relevant patents or patent applications or may identify pending patent applications of potential interest but incorrectly predict the likelihood that such patent applications may issue with claims of relevance to our technology. In addition, we may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of a current or future product candidate, or we may incorrectly conclude that a third-party patent is invalid, unenforceable or not infringed by our activities. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our technologies, our products or the use of our products.If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our product candidates and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information or trade secrets of third parties or that our employees, consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of former or other employers.Many of our employees, independent contractors and consultants, including our senior management, have been previously employed or retained by universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Further, many of our consultants are currently retained by other biotechnology or pharmaceutical companies and may be subject to conflicting obligations to these third parties. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of third parties in their work for us, and do not perform work for us that is in conflict with their obligations to another employer or any other entity, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise improperly used or disclosed confidential information, including trade secrets or other proprietary information, of a former employer or other third parties. We may also be subject to claims that an employee, advisor, consultant, or independent contractor performed work for us that conflicts with that person's obligations to a third party, such as an employer, and thus, that the third party has an ownership interest in the intellectual property arising out of work performed for us. We are not aware of any threatened or pending claims related to these matters, but in the future litigation may be necessary to defend against such claims.In addition, while it is our policy to require our employees, independent contractors and consultants who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in timely obtaining such an agreement with each party who in fact develops intellectual property that we regard as our own. Even if timely obtained, such agreements may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable personnel or intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. As a result, we may also elect to enter into license agreements in order to settle patent infringement claims or to resolve disputes prior to litigation, and any such license agreements may require us to pay royalties and other fees that could be significant. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.Any lawsuits relating to infringement of intellectual property rights necessary to defend ourselves or enforce our rights will be costly and time consuming, and could be unsuccessful.Because competition in our industry is intense, competitors may infringe or otherwise violate our issued patents, patents of our licensors or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging, among other claims, that we infringe theirpatents. In addition, in a patent infringement proceeding there are many grounds upon which a party may assert invalidity or unenforceability of a patent, and a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. Litigation is uncertain and we cannot predict whether we would be successful in any such litigation. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial, managerial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial, managerial and other resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our business. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure.Intellectual property disputes could cause us to spend substantial resources and distract our personnel from their normal responsibilities.Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and/or management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common stock. In some cases, we may choose not to pursue litigation against those that have infringed on our patents, or used them without authorization, due to the associated expenses and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operationsWe may not be successful in obtaining or maintaining necessary rights for our development pipeline through acquisitions and in-licenses.Presently we have rights to intellectual property to develop our product candidates, including patents and patent applications we own or exclusively license from the University of Texas at Austin. Because our programs may involve additional product candidates that may require the use of proprietary rights held by third parties, the growth of our business may depend in part on our ability to acquire, in-license or use these proprietary rights. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify as necessary for our product candidates. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.If we are not able to prevent disclosure of our trade secrets and other proprietary information, the value of our technology and product candidates could be significantly diminished.We rely on trade secret protection to protect our interests in proprietary know-how and in processes that are unpatentable or for which patents are difficult to obtain or enforce. We may not be able to protect our trade secrets adequately. We have a policy of requiring our consultants, advisors and strategic partners to enter into confidentiality agreements and our employees to enter into invention, non-disclosure and non-compete agreements. However, no assurance can be given that we have entered into appropriate agreements with all parties that have had access to our trade secrets, know-how or other proprietary information, or that such agreements will provide for a meaningful protection of our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of information. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Even if we are successful in prosecuting such claims, any remedy awarded may be insufficient to fully compensate us for the improper disclosure or misappropriation. Furthermore, although we seek to preserve the integrity and confidentiality of our data, trade secrets and know-how by maintaining physical security of our premises and physical and electronic security of our information technology systems, it is also possible that our trade secrets, know-how or other proprietary information could be obtained by third parties as a result of breaches of such systems.Any disclosure of confidential information into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us. In addition, others may independently discover or develop our trade secrets and proprietary information or substantially equivalent techniques. Any action to enforce our rights is likely to be time consuming and expensive, and may ultimately be unsuccessful, or may result in a remedy that is not commercially valuable. These risks are accentuated in foreign countries where laws or law enforcement practices may not protect proprietary rights as fully as in the United States or Europe. Any unauthorized disclosure of our trade secrets or confidential information could harm our competitive position.We may not be able to protect our intellectual property rights throughout the world.Filing, prosecuting and defending patents on all of our product candidates throughout the world would be prohibitively expensive, and our patent rights in some countries outside the United States can be less extensive than those in the United States. The requirements for patentability may differ in certain countries, particularly developing countries. For example, unlike other countries, China has a heightened requirement for patentability and specifically requires a detailed description of medical uses of a claimed therapeutic. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.As part of ordinary course prosecution and maintenance activities, we determine whether to seek patent protection outside the United States and in which countries. This also applies to patents we have acquired or in-licensed from third parties. In some cases, this means that we, or our predecessors in interest or licensors of patents within our portfolio, have sought patent protection in a limited number of countries for patents covering our product candidates. Competitors may use our technologies in jurisdictions where we have not pursued and obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as in the United States. These products may compete with our products in jurisdictions where we do not have any issued patents and, even in jurisdictions where we have or are able to obtain issued patents, our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing. Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, certain countries in Europe and certain developing countries, including India and China, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, we may have limited remedies if our patents are infringed or if we are compelled to grant a license to our patents to a third party, which could materially diminish the value of those patents. In addition, there may be patent law reforms in foreign jurisdictions that could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents in those foreign jurisdictions. This could limit our potential revenue opportunities.Accordingly, our efforts to obtain, register, and enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we own or license. Moreover, patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.If we breach any of the agreements under which we license patent rights to use, develop and commercialize our product candidates or our technologies from third parties or, in certain cases, we fail to meet certain development deadlines, we could lose license rights that are important to our business.We are a party to a number of license agreements under which we are granted rights to intellectual property that are important to our business and we expect that we may need to enter into additional license agreements in the future. In particular, we partner with the University of Texas at Austin, which is a U.S. academic institution, in order to accelerate our discovery and nonclinical development work under a Sponsored Research Agreement. Under the Sponsored Research Agreement, we made payments of $375,000 and $645,000 for the nine months ended September 30, 2017 and 2016,respectively, to sponsor research in the laboratory of our director, Dr. George Georgiou, at the University of Texas at Austin on the engineering, optimization and initial animal validation of human enzymes to determine the systemic depletion of amino acids for cancer therapy and to analyze enzyme replacement for the treatment of patients having inborn metabolic defects.The University of Texas at Austin has provided us with an option to negotiate a royalty-bearing, exclusive license to any invention or discovery that is conceived or reduced to practice during the term of the Sponsored Research Agreement. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue a program based on that technology.In December 2013, our wholly-owned subsidiaries AECase, Inc. and AEMase, Inc. each entered into an exclusive, worldwide license agreement, including the right to grant sublicenses, with the University of Texas at Austin for certain intellectual property owned by the University of Texas at Austin related to our product candidates AEB3103 and AEB2109. On January 31, 2017, we and the University of Texas at Austin entered into an Amended and Restated Patent License Agreement which consolidated the two license agreements, revised certain obligations, and licensed additional patent applications and invention disclosures to us, or the Restated License. The intellectual property licensed under the Restated License includes an invention that was made with U.S. government support. The U.S. government therefore has certain rights in such inventions under the applicable funding agreements and under applicable law. In addition, we are subject to a requirement that the products covered by the applicable patents that are sold or used in the United States must be manufactured substantially in the United States unless a written waiver is obtained in advance from the U.S government. The Restated License obligates us to make certain payments at the achievement of certain milestones and at regular intervals throughout the life of the license. The University of Texas at Austin may terminate the Restated License under certain circumstances, including for a breach by us that is not cured within 30 or 60 days of notice (depending on the type of breach), or if we or any of our affiliates or sublicensees participate in any proceeding to challenge the licensed patent rights (unless, with respect to sublicensees, we terminate the applicable sublicense). Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Any other licenses or other intellectual property agreements we may enter into may impose various diligence, milestone payment, royalty and other obligations on us. If disputes arise between us and our licensor or if we fail to comply with our obligations under current or future intellectual property agreements, potentially giving our counterparties the right to terminate these agreements, we might not be able to develop, manufacture or market any product that is covered by the agreement or face other penalties under the agreement. Such an occurrence could materially adversely affect the value of the product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.The loss of any one of our current licenses, or any other license we may acquire in the future, could prevent or impair our ability to successfully develop and commercialize the affected product candidates and thus materially harm our business, prospects, financial condition and results of operations.Intellectual property rights do not necessarily address all potential threats to our competitive advantage.The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, provide a barrier to entry against our competitors or potential competitors, or permit us to maintain our competitive advantage. Moreover, if a third party has intellectual property rights that cover the practice of our technology or product candidates, we may not be able to fully exercise or extract value from our intellectual property rights. The following examples are illustrative:others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of the patents that we own or license;we or our licensors or collaborators might not have been the first to make the inventions covered by an issued patent or pending patent application that we own or license;we or our licensors or collaborators might not have been the first to file patent applications covering an invention;others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing or misappropriating our intellectual property rights;issued patents that we own or license may not provide us with any competitive advantages, or may be narrowly construed or held invalid or unenforceable, as a result of legal challenges by our competitors;our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;we may not develop or in-license additional proprietary technologies that are patentable; andthe patents of others may have an adverse effect on our business.Any of these events could significantly harm our business, results of operations and prospects.Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products, and recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.As is the case with other biotechnology companies, our success is heavily dependent on patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.For our U.S. patent applications containing a claim not entitled to priority before March 16, 2013, there is a greater level of uncertainty in the patent law. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, which affect both the way patent applications will be prosecuted and potentially patent litigation. The U.S. PTO has promulgated regulations and developed procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act (in particular, the first to file provisions) did not come into effect until March 16, 2013. Accordingly, it is not yet clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.An important change introduced by the Leahy-Smith Act is that, as of March 16, 2013, the United States transitioned to a "first-to-file" system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the U.S. PTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Furthermore, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our technology and the prior art allow our technology to be patentable over the prior art. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our patents or patent applications.Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and that allow third parties to challenge any issued patent, whether issued before or after March 16, 2013, in the U.S. PTO. Because of a lower evidentiary standard in U.S. PTO proceedings compared to the evidentiary standard in United States federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a U.S. PTO proceeding sufficient for the U.S. PTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the U.S. PTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.If we do not obtain patent term extensions in the United States under the Hatch-Waxman Act and in foreign countries under similar legislation, thereby potentially extending the term of our marketing exclusivity for our product candidates, our business may be materially harmed.Depending upon the timing, duration and specifics of FDA marketing approval of our product candidates, if any, one of the U.S. patents covering each of such approved product(s) or the use thereof may be eligible for up to five years of patent term restoration under the Hatch-Waxman Act. The Hatch-Waxman Act allows a maximum of one patent to be extended per FDA-approved product. Patent term extension also may be available in certain foreign countries upon regulatory approval of our product candidates. Nevertheless, we may not be granted patent term extension either in the United States or in any foreign country because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than we request. In addition, if a patent we wish to extend is owned by another party and licensed to us, we may need to obtain approval and cooperation from our licensor to request the extension.If we are unable to obtain patent term extension or restoration, or the term of any such extension is less than we request, the period during which we will have the right to exclusively market our product will be shortened and our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.Risks Related to Our Common StockOur executive officers, directors and principal stockholders, if they choose to act together, will continue to have the ability to control all matters submitted to stockholders for approval.We have a concentrated stockholder base and our executive officers and directors, combined with our stockholders who, to our knowledge, each owned more than 5% of our outstanding common stock, in the aggregate, beneficially own shares representing a majority of our capital stock as of September 30, 2017. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of ownership control may:delay, defer or prevent a change in control;entrench our management and the board of directors; orimpede a merger, consolidation, takeover or other business combination involving us that other stockholders may desire or may result in you obtaining a premium for your shares.Our internal control over financial reporting does not currently meet the standards required by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business and stock price.We are not currently required to comply with the rules of the Securities and Exchange Commission that implement Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose until our annual reportForm 10-K for the year ended December 31, 2017. Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting2023 and the preparation of financial statements in accordance with generally accepted accounting principles in the United States. We are not currently in compliance with, and we cannot be certain when we will be able to implement the requirements of Section 404(a). We may encounter problems or delays in implementing any changes necessary to make a favorable assessment of our internal control over financial reporting. If we cannot favorably assess the effectiveness of our internal control over financial reporting, or if our independent registered public accounting firm is unable to provide an unqualified attestation report on our internal controls when required, investors could lose confidence in our financial information and the price of our common stock could decline.Additionally, the existence of any material weakness or significant deficiency would require management to devote significant time and incur significant expense to remediate any such material weaknesses or significant deficiencies and management may not be able to remediate any such material weaknesses or significant deficiencies in a timely manner. The existence of any material weakness in our internal control over financial reporting could also result in errors in our financial statements that could require us to restate our financial statements causing us to fail to meet our reporting obligations and cause stockholders to lose confidence in our reported financial information, all of which could materially and adversely affect us.Provisions in our corporate charter documents and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.Provisions in our certificate of incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:establish a classified board of directors such that only one of three classes of directors is elected each year;allow the authorized number of our directors to be changed only by resolution of our board of directors;limit the manner in which stockholders can remove directors from our board of directors;establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors;require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;limit who may call stockholder meetings;authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; andrequire the approval of the holders of at least two-thirds of the votes that all our stockholders would be entitled to cast to amend or repeal specified provisions of our certificate of incorporation or bylaws.Moreover, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.Any of these provisions of our charter documents or Delaware law could, under certain circumstances, depress the market price of our common stock.Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, employees or agents.Our amended and restated certificate of incorporation provides that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, employees or agents to us or our stockholders, any action asserting a claim arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws or any action asserting a claim that is governed by the internal affairs doctrine, in each case subject to the Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein and the claim not being one which isvested in the exclusive jurisdiction of a court or forum other than the Court of Chancery or for which the Court of Chancery does not have subject matter jurisdiction. Any person purchasing or otherwise acquiring any interest in any shares of our capital stock shall be deemed to have notice of and to have consented to this provision of our amended and restated certificate of incorporation. This choice of forum provision may limit our stockholders’ ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, employees or agents, which may discourage such lawsuits against us and our directors, officers, employees and agents even though an action, if successful, might benefit our stockholders. Stockholders who do bring a claim in the Court of Chancery could face additional litigation costs in pursuing any such claim, particularly if they do not reside in or near Delaware. The Court of Chancery may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments or results may be more favorable to us than to our stockholders. Alternatively, if a court were to find this provision of our amended and restated certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could have a material adverse effect on our business, financial condition or results of operations.The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our common stock.Our stock price is volatile. The stock market in general and the market for smaller biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price for our common stock may be influenced by many factors, including:the success or failure of competitive products or technologies;results of ongoing or planned clinical trials of our product candidates or those of our competitors;regulatory or legal developments in the United States and other countries;developments or disputes concerning patent applications, issued patents or other proprietary rights;the recruitment or departure of key personnel;the level of expenses related to any of our product candidates or clinical development programs;the results of our efforts to discover, develop, acquire or in-license additional product candidates or products;actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;operating results that fail to meet expectations of securities analysts that cover our company;variations in our financial results or those of companies that are perceived to be similar to us;changes in the structure of healthcare payment systems;market conditions in the pharmaceutical and biotechnology sectors;general economic and market conditions; andthe other factors described in this “Risk Factors” section.We may be subject to securities litigation, which is expensive and could divert management attention.Our stock price is volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to an increased incidence of securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our stock price and trading volume could decline.The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our stock price would likely decline. If one or more of these analysts ceasecoverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline. We have broad discretion in the use of the net proceeds from our public offerings and may not use them effectively.Our management has broad discretion in the application of the net proceeds from our public offerings, and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Our management could spend the net proceeds from our public offerings in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from our public offerings in a manner that does not produce income or that loses value.Future sales of our common stock in the public market could cause the market price of our common stock to drop significantly, even if our business is doing well.Sales of a substantial number of shares of our common stock in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock and make it more difficult for you to sell your common stock at a time and price that you deem appropriate.Certain holders of our common stock have rights, subject to conditions, to require us to file registration statements covering their shares or to include their shares in Securities Act registration statements that we may file for ourselves or other stockholders. Once we register these shares, they can be freely sold in the public market. Moreover, we have also registered under the Securities Act shares of common stock that we may issue under our equity compensation plans.In addition, on May 1, 2017, we filed a shelf registration statement on Form S-3 for the potential offering, issuance and sale by us of up to $150.0 million of our common stock, preferred stock, debt securities, warrants to purchase our common stock, preferred stock and debt securities, subscription rights to purchase our common stock, preferred stock and debt securities, and units consisting of all or some of these securities. The shelf registration statement was declared effective by the SEC on May 30, 2017. In June 2017, we sold 3,000,000 shares of our common stock in an underwritten public offering pursuant to the shelf registration statement for aggregate gross proceeds of $12.3 million. In addition, common stock with an aggregate offering price of up to $20.0 million may be issued and sold pursuant to an “at-the-market” offering of our common stock pursuant to a sales agreement between us and JonesTrading Institutional Services LLC, or JonesTrading. Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver a placement notice to JonesTrading at any time throughout the term of the sales agreement, which has a term equal to the term of the registration statement on Form S-3 unless otherwise terminated earlier by us or JonesTrading pursuant to the terms of the sales agreement. The number of shares that are sold by JonesTrading after delivering a placement notice will fluctuate based on the market price of our common stock during the sales period and limits we set with JonesTrading. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued. Issuances of such shares pursuant to the sales agreement will have a dilutive effect on our existing stockholders. Further, if we sell common stock, preferred stock, convertible securities and other equity securities in other transactions pursuant to our shelf registration statement on Form S-3, existing investors may be materially diluted by such subsequent sales and new investors could gain rights superior to our existing stockholders.In addition, in the future, we may issue additional shares of common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. Any such issuance could result in substantial dilution to our existing stockholders and could cause our stock price to decline.We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure;not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting of Section 404(b) of the Sarbanes-Oxley Act;not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;reduced disclosure obligations regarding executive compensation; andexemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of these accounting standards until they would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.We will continue to incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.As a public company, and particularly after we are no longer an emerging growth company, we incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Global Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain and maintain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors.We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.Pursuant to Section 404, we will first be required to furnish a report by our management on our internal control over financial reporting for the year ending December 31, 2017. As discussed above, if we cease to be an emerging growth company, we will be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm as required by Section 404(b). To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our consolidated financial statements.Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards, or NOLs, and other pre-change tax attributes (such as research tax credits) to offset its post-change income or taxes may be limited. It is possible that we may have triggered an “ownership change” limitation. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership (some of which are outside of our control). As a result, if we earn net taxable income, our ability to use our pre-change NOLs and other pre-change tax attributes to offset U.S. federal taxable income or taxes may be subject to limitations, which could potentially result in increased future tax liability to us. Our NOLs and other tax attributes arising before our conversion from a Delaware limited liability company to a Delaware corporation in 2015 also may be limited by the Separate Return Limitation Year rule, which could increase our U.S. federal tax liability. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.Since we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, stock price appreciation, if any, will be your sole source of gain.We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, appreciation, if any, in the market price of our common stock will be your sole source of gain for the foreseeable future.Recent Sales of Unregistered Equity SecuritiesThe following table provides information about the acquisition of our common stock by us during the third quarter of 2017:(1)Represents unvested restricted shares that were repurchased from a former employee upon termination of employment in accordance with the terms of the employee’s stock restriction agreement.On April 6, 2016, our Registration Statement on Form S-1 (File No. 333-200501) relating to the IPO of our common stock was declared effective by the SEC. There has been no material change in our planned use of the net proceeds from the IPO, as described in our final prospectus filed with the SEC on April 7, 2016.Not applicable.None.Exhibit
NumberDescription Form File No Date of Filing Exhibit
No.Filed
Herewith2.1 S-1 333-276251 12/22/2023 2.1 3.1 S-1 333-276251 12/22/2023 3.1 3.2 S-1/A 333-276251 02/05/2024 3.2 3.3 S-1 333-276251 12/22/2023 3.3 3.4 S-1 333-276251 12/22/2023 3.4 3.5 8-K 001-37722 03/18/2024 3.2 4.1 8-K 001-37722 03/18/2024 10.2 4.2 X 10.1 S-1/A 333-276251 02/05/2024 10.19 10.2+ S-1/A 333-276251 02/05/2024 10.4 10.3 8-K 001-37722 03/18/2024 10.1 10.4 10-K 001-37722 02/29/2024 10.20 10.5 8-K 001-37722 4/25/2024 10.1 10.6 X 31.1 X Exhibit
NumberDescription Form File No Date of Filing Exhibit
No.Filed
HerewithNumberDescription10.1*Terms of Resignation between the Registrant and Dr. David G. Lowe.10.2Offer Letter, dated August 31, 2017, issued by the Registrant to Dr. Anthony Quinn.10.3†31.131.2X X 32.2(1)101.INSX X X X X X *104 Incorporated by reference to Exhibit 10.1 to the Registrant’sThe cover page from this Quarterly Report on Form 10-Q for the quarter ended June 30, 2017, filed with the SEC on August 9, 2017.formatted in Inline XBRL and contained in Exhibit 101
+ Indicates management contract or compensatory plan.†Portions of this exhibit have been omitted based on an application for confidential treatment submitted to the SEC. The omitted portions of this exhibit have been filed separately with the SEC.
# Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.(1)The certifications on Exhibit 32 hereto are deemed not “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that Section. Such certifications will not be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act.
(1)The certifications on Exhibit 32 hereto are deemed furnished and not “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that Section. Such certifications will not be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act. of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.November 7, 2017May 9, 2024AEGLEA BIOTHERAPEUTICS, INC.Spyre Therapeutics, Inc. By: /s/ Scott Burrows By:/s/ Anthony G. Quinn, M.B Ch.B. Ph.D.Anthony G. Quinn, M.B Ch.B. Ph.D.Interim Chief Executive Officer and Director(Principal Executive Officer)Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.Date: November 7, 2017AEGLEA BIOTHERAPEUTICS, INC.By:/s/ Charles N. York IICharles N. York II and Vice PresidentPrincipal Accounting Officer and Principal Financial Officer and duly Authorized Signatory)64