17. Restructuring
On February 13, 2023, the Company announced the topline results for the Phase 2b study of FX-322 (FX-322-208) which failed to achieve its primary efficacy endpoint of an improvement in speech perception. As a result, the Company also announced that it would be discontinuing the FX-322 and FX-345 hearing development programs and focusing resources on its remyelination in MS development program. This restructuring resulted in a 55% reduction in the Company's workforce.
The Company expects to incur a total of approximately $4,329 in restructuring-related expenses, of which $3,970 was expensed in the three months ended March 31, 2023. The total estimated restructuring charges consist of accelerated depreciation expense of $360, accelerated hearing program expense of $129, and severance and other benefit-related costs for allof $3,840, of which $3,481 was expensed in the joint activities in future periodsthree months ended March 31, 2023 and reimbursements received from Astellasthe remaining $359 will be recognized as an offset to research and development expense onexpensed in the consolidated statementsecond quarter of operations during2023.
During the development period.
23
Frequency Therapeutics, Inc.
Notes to unaudited consolidated financial statements – (continued)
(Amounts in thousands, except share and per share amounts)
14. Net loss per share
Basic and diluted net loss per share attributable to common stockholders was calculated as follows:
|
| Three Months Ended September 30, |
|
| Nine Months Ended September 30, |
| ||||||||||
(in thousands, except share and per share amounts) |
| 2019 |
|
| 2018 |
|
| 2019 |
|
| 2018 |
| ||||
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders |
| $ | (1,589 | ) |
| $ | (5,136 | ) |
| $ | (14,267 | ) |
| $ | (13,721 | ) |
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares of common stock outstanding- basic and diluted |
|
| 2,163,289 |
|
|
| 1,575,728 |
|
|
| 1,990,106 |
|
|
| 1,476,678 |
|
Net loss per share attributed to common stockholders-basic and diluted |
| $ | (0.73 | ) |
| $ | (3.26 | ) |
| $ | (7.17 | ) |
| $ | (9.29 | ) |
The weighted-average number of shares of common stock outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same.
The Company excludedthree months ended March 31, 2023, the following potential sharesrestructuring-related charges were included in the Consolidated Statement of common stock fromOperations:
| Severance and other benefit-related costs |
|
| Accelerated depreciation charges |
|
| Accelerated hearing program charges |
|
| Total |
| ||||
Research and development | $ | 1,932 |
|
| $ | 360 |
|
| $ | 129 |
|
| $ | 2,421 |
|
General and administrative |
| 1,549 |
|
|
| — |
|
|
| — |
|
|
| 1,549 |
|
Total | $ | 3,481 |
|
| $ | 360 |
|
| $ | 129 |
|
| $ | 3,970 |
|
At March 31, 2023, the computation of diluted net loss per share attributable to common stockholders because including them would have had an anti-dilutive effect.liability for the restructuring is classified as current and included in accrued expenses in the Consolidated Balance Sheets.
|
| Three Months Ended September 30, |
|
| Nine Months Ended September 30, |
| ||||||||||
|
| 2019 |
|
| 2018 |
|
| 2019 |
|
| 2018 |
| ||||
Unvested Restricted Common Stock |
|
| 156,202 |
|
|
| 409,099 |
|
|
| 156,202 |
|
|
| 409,099 |
|
Series C Preferred (as converted to common stock) |
|
| 5,863,365 |
|
|
| — |
|
|
| 5,863,364 |
|
|
| — |
|
Series B Preferred (as converted to common stock) |
|
| 9,257,256 |
|
|
| — |
|
|
| 6,257,256 |
|
|
| — |
|
Series A Preferred (as converted to common stock) |
|
| 9,283,307 |
|
|
| 9,283,307 |
|
|
| 9,283,307 |
|
|
| 9,283,307 |
|
Conversion of Frequency Japan preferred stock |
|
| 637,605 |
|
|
| — |
|
|
| 637,605 |
|
|
| — |
|
Outstanding stock options (as converted to common stock) |
|
| 4,787,897 |
|
|
| 2,052,218 |
|
|
| 4,787,897 |
|
|
| 2,052,218 |
|
Total |
|
| 29,985,632 |
|
|
| 11,744,624 |
|
|
| 26,985,631 |
|
|
| 11,744,624 |
|
| Accelerated hearing program charges |
|
| Severance and other benefit-related costs |
|
| Total |
| |||
Liability balance as of December 31, 2022 | $ | - |
|
| $ | - |
|
| $ | - |
|
Net charges |
| 129 |
|
|
| 3,329 |
|
|
| 3,458 |
|
Liability balance as of March 31, 2023 | $ | 129 |
|
| $ | 3,329 |
|
| $ | 3,458 |
|
15.
18. Subsequent events
The Company has evaluated subsequent events for recognition, remeasurement and remeasurementdisclosure purposes through November 15, 2019,May 12, 2023, the date that these interim unauditedwhich the consolidated financial statements were available to be issued and through November 18, 2019 for disclosure purposes.issued. The identified subsequent events are discussed below:event is as follows:
In October 2019,
On April 3, 2023, the company completedCompany prepaid the IPO, see discussion in Note 1.
In November 2019,remaining $11,667 due under the company signed a lease amendment for its Woburn, Massachusetts facility. The lease amendment increasedterm loan. Upon prepayment of the office space under lease by 7,550 square feet and extendedterm loan, the final payment of $150, which the Company had been accruing over the term of the leaseloan, was also paid. The
21
Company was not subject to any prepayment premium as the prepayment occurred after the second anniversary of the closing date. Refer to Note 7, "Debt".
On April 4, 2023, the Company's termination of the MEE License Agreement became final. No termination costs were incurred as a result of terminating this license agreement. Refer to Note 14, "License agreements".
On April 6, 2023, the Company provided written notice of termination of the MIT License Agreement. Pursuant to the terms of the agreement, the Company has the right to terminate for any reason upon a 3-month prior written notice. No termination costs will be incurred as a result of terminating this license agreement. Refer to Note 14, "License agreements".
On April 14, 2023, the facilityAstellas Agreement was terminated in its entirety. No termination costs will be incurred as a result of terminating this collaboration agreement. Refer to February 2025. The annual rent is approximately $545.0 withNote 13, "Collaboration agreement".
On April 28, 2023, the Company's termination of the CALIBR License Agreement became final. No termination costs were incurred as a provision for an annual increaseresult of 3%terminating this license agreement. Refer to Note 14, "License agreements".
22
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Quarterly Report on Form 10-Q.10-Q and with our Annual Report on Form 10-K for the year ended December 31, 2022, or the 2022 Annual Report, including the audited consolidated financial statements and notes thereto contained in our 2022 Annual Report. Some of the information contained in this discussion and analysis contains forward-looking statements that involve risks and uncertainties. You should review the section titled “Risk factors” in this Quarterly Report on Form 10-Q for a discussion of important factors that could cause actual results to differ materially from the results described below.
Overview
We are pioneering a clinical-stage biotechnology company focused on harnessingnew category in regenerative medicine that aims to restore human function by developing therapeutics that activate a person's innate regenerative potential through the body’s innate biology to potentially repairactivation of progenitor cells. We believe our progenitor cell activation, or reverse damage caused byPCA, approach can impact a broadwide range of degenerative diseases. Our proprietary approach, called Progenitor Cell Activation, or PCA, uses combinations of small moleculeslead preclinical program is designed to activate progenitoroligodendrocyte precursor cells withinwith the bodygoal of inducing remyelination and functional recovery for individuals living with multiple sclerosis (MS).
We are using our PCA approach to create functional tissue. Further, these progenitor cells,develop therapeutics to remyelinate neurons in individuals with MS. MS, which are closely related to stem cells, are already residentinduces demyelination, stripping axons of the myelin sheaths that support axonal signal conduction and survival, affects approximately 1 million people in the targeted locationUnited States. MS patients experience blurred vision, loss of balance, poor coordination, slurred speech, tremors, numbness, fatigue, problems with memory and concentration, paralysis, and blindness. Currently available immunomodulatory treatments can reduce the rate of relapses, but cannot repair demyelination. We are developing a novel therapy to restore myelin and potentially restore function designed to be used in conjunction with existing immunomodulatory treatments.
Previously discovered remyelination agents, including anti-LINGO antibody and clemastine, an anti-muscarinic antagonist, induce modest improvements in electrophysiological and functional measures in MS patients. These results indicate that MS patients may benefit from remyelinating agents and suggest that a more efficacious therapeutic may generate meaningful patient benefit.
We have identified a novel target relevant to myelination and generated a range of New Chemical Entities (NCEs) that outperform previous approaches in head-to-head preclinical studies. Modulation of this target induced robust oligodendrocyte differentiation and expression of myelin proteins in vitro. Frequency NCEs induce oligodendrocyte formation at a greater level than the published comparator approaches in vivo, as shown in Figure 1 below. We believe our compounds may be sufficient to drive meaningful patient benefit.
Figure 1: Frequency compounds induce greater levels of oligodendrocyte differentiation than comparator approaches in a mouse model. New oligodendrocytes shown in red.
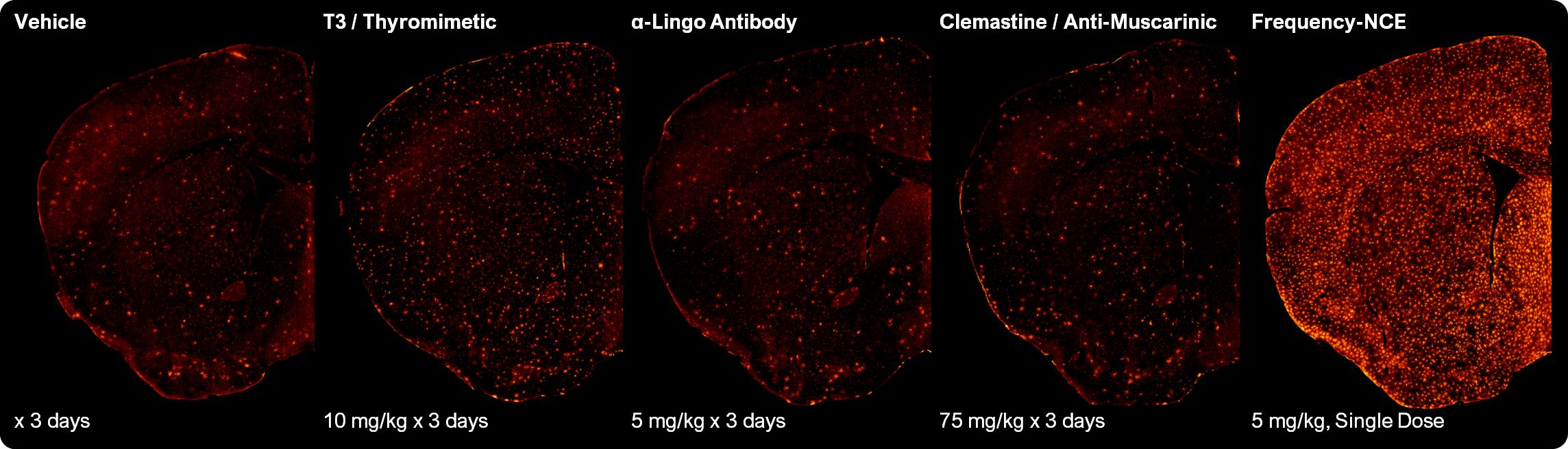
MS patients may require not only new oligodendrocytes but also new myelin. We tested Frequency and comparator compounds in the bodychronic cuprizone model of demyelination. In preclinical studies, a single oral dose of one of our compounds yielded more remyelination than up to 10 doses of comparator compounds, as shown in Figure 2 below.
23
Figure 2: Frequency compound induce higher levels of remyelination than comparator approaches in a mouse model of demyelination. Myelin basic protein shown in green.
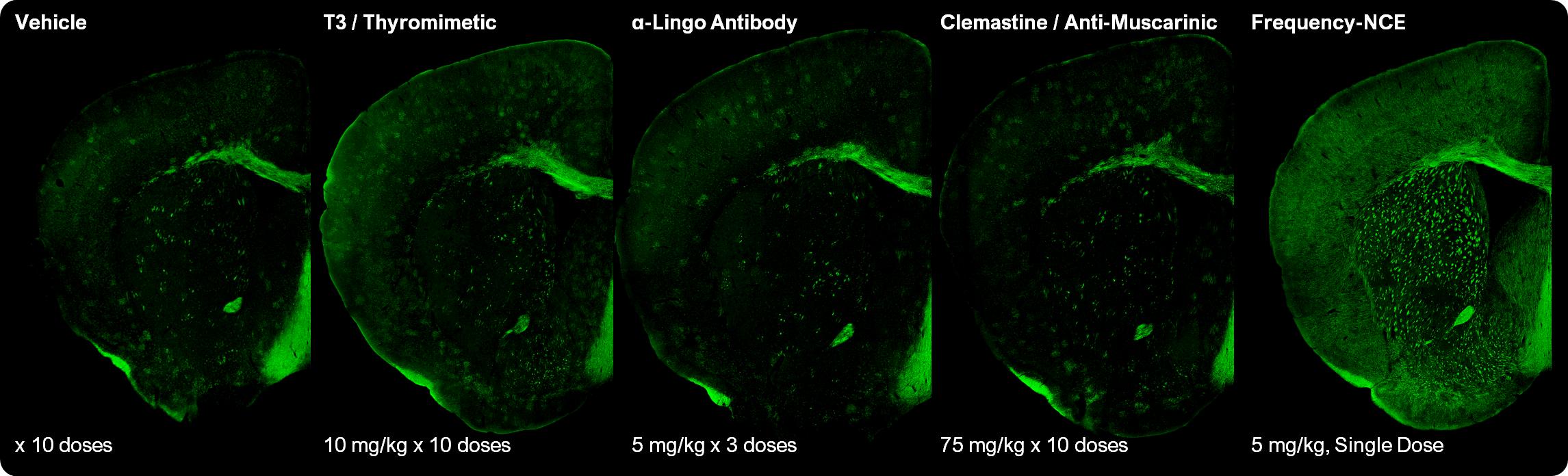
Frequency’s NCEs drive oligodendrocyte differentiation in evolutionarily distinct species including mice, rats, and programmedpigs. Further, independently conducted preclinical studies of our NCEs have shown statistical improvements in MRI measurements of white matter volume, which is an important clinical endpoint. We are advancing candidates through toxicology studies and plan to develop and differentiate into specific cell types within an organ.begin our clinical program for remyelination in MS in the first half of 2024.
Our initial therapeutic
We first applied our PCA approach for the restoration of the cochlea, with a focus ison treating sensorineural hearing loss, or SNHL, which isSNHL. Beginning in 2019, we ran five clinical studies of FX-322 aimed at understanding safety as well as severities and etiologies that FX-322 might treat and the most prevalent type of hearing loss. We are developing FX-322 to treat the underlying cause of SNHL, which is the loss of hair cells. FX-322 is designed to regenerate hair cells through the activation of progenitor cells already present in the ear.appropriate dose regime. In 2021, we commenced our Phase 1/2 clinical trial evaluating FX-322 in 23 patients with stable SNHL, we observed a statistically significant improvement in word recognition, a key measure of hearing function, and FX-322 was observed to be well-tolerated. We commencedsixth study, a Phase 2a2b clinical trial of FX-322 (FX-322-208), and introduced a second hearing program, FX-345, which we believed might expand the opportunity to treat different types of SNHL. In February 2023, we announced that the FX-322-208 study failed to achieve its primary endpoint of an improvement in patientsspeech perception. We decided to discontinue the FX-322 development program and, given the similarities between the mechanisms of FX-322 and FX-345, we decided to discontinue the FX-345 development program as well.
The MS development program is independent of the hearing program, with SNHLa distinct molecular mechanism, progenitor cell population, and small molecule drug candidates. Further, a well-defined clinical path with objective biomarkers such as visual evoked potential (VEP) and magnetic resonance imaging (MRI) exist for studying the performance of remyelination therapies in October 2019MS patients.
On April 8, 2022, we announced a reduction in force of approximately 30% of our workforce to better align our workforce with the near-term needs of our business and anticipate reporting top-line results from thisfocus more of our capital resources on our research and development programs for hearing and remyelination in MS. On February 13, 2023, in connection with the discontinuation of the hearing program, we announced a restructuring of our business including a downsizing of personnel by approximately 55%. We believe these changes will generate sufficient cost savings to enable us to complete a first clinical trial in the second half of 2020. We are also working to identify a product candidate for the treatment of multiple sclerosis, or MS. This program focuses on activating progenitor cells in the central nervous system to repair the myelin sheath that protects nerves and may have the potential to reverse damage done by the disease. We intend to submit an investigational new drug application or, IND, to the U.S. Federal Drug Administration, or the FDA, for our MS product candidate in the second half of 2021.development program.
In October 2019, we completed the initial public offering of our common stock, or the IPO. In the IPO, we issued and sold 6,325,000 shares of our common stock at a price to the public of $14.00 per share, inclusive of the underwriters’ exercise in part of their over-allotment option. We received approximately $79.2 million in net proceeds, after deducting underwriting discounts and commissions and other offering expenses. In July 2019, we closed our Series C convertible preferred stock financing in which we issued and sold 39,492,960 shares of our Series C convertible preferred stock for aggregate gross proceeds of approximately $62.0 million. In July 2019, we also entered into a License and Collaboration Agreement, or the Astellas Agreement, with Astellas Pharma, Inc., or Astellas, pursuant to which Astellas paid us an upfront payment of $80.0 million.
Since our formation in 2014, we have devoted substantially all our resources to developing our PCA platform,approach, conducting research and development activities, including product candidate development, recruiting skilled personnel, establishing our intellectual property portfolio, and providing general and administrative support for these operations. We have financed our operations primarily through proceeds from the sale of convertible notesprivate and of our convertible preferred stock, our IPOpublic securities financings, a term loan, and the Astellas Agreement. To date, we have raised approximately $316.5 million throughamounts received under a combination of convertible notes, convertible preferred stock financings, the upfront payment under the Astellas Agreement, grants and our IPO.collaboration agreement.
Since our formation,inception, we have incurred significant operating losses and have not generated any revenue from the sale of products. Our ability to generate any product revenue or product revenue sufficient to achieve profitability will depend on the successful development and eventual commercialization of one or more of our product candidates. Our net losses were $20.2 million, $19.2$19.5 million and $13.3$81.6 million for the yearsthree months ended March 31, 2023 and year ended December 31, 2017 and 2018 and the nine months ended September 30, 2019,2022, respectively. As of September 30, 2019,March 31, 2023, we had an accumulated deficit of $63.4$281.2 million.
We expect our
Our operating expenses to increase substantiallydiscussed in connection with the expansion ofthis section reflect our product development programs around FX-322, FX-345, MS and capabilities.any future programs as well as our operations as a public company. We will not generate revenue from product sales unless
24
and until we successfully complete clinical development, obtain regulatory approval for, and successfully commercialize our product candidates, or until our collaborators do so, which could result in milestone payments or royalties to us.candidates. If we obtain regulatory approval for any of our product candidates, we expect to incur significant expenses related to developing our commercialization capability to support product sales, marketing and distribution. In addition, we expect to continue to incur additional costs associated with operating as a public company.
As a result of these anticipated expenditures, we will need additional financing to support our continuing operations. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our cash needs through a combination of public or private equity or debt financings and other sources, which may include current and new collaborations with third parties. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We cannot be sure that we will ever generate sufficient revenue to achieve profitability.
Because of the numerous risks and uncertainties associated with the development of therapeutics, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we can generate revenue from product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be required to raise additional capital on terms that are unfavorable to us or we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
License and collaboration agreements
Astellas Pharma Inc.
In July 2019, we entered into a license and collaboration agreement, or the Astellas Agreement with Astellas Pharma, Inc., or Astellas, under which we granted Astellas an exclusive, royalty-bearing, sub-licensable, nontransferable license to certain patent rights to research, develop, manufacture, have manufactured, use, seek, and secure regulatory approval for, commercialize, offer for sale, sell, have sold and import, and otherwise exploit licensed products containing both a GSK-3 inhibitor and an HDAC inhibitor, or the Astellas licensed products, including our product candidate FX-322, outside of the United States. We and Astellas have agreed to jointly develop the Astellas licensed products, including carrying out joint studies. Each party has agreed to use commercially reasonable efforts to carry out development activities assigned to it under an agreed-upon development plan. Astellas has agreed to use commercially reasonable efforts to obtain regulatory approval for at least one Astellas licensed product in SNHL and in age-related hearing loss, in each case in one major Asian country and one major European country. We have agreed to use commercially reasonable efforts to obtain regulatory approval for at least one Astellas licensed product in the United States. Astellas has the sole right to commercialize the Astellas licensed products outside of the United States and we have the sole right to commercialize the Astellas licensed products in the United States. Astellas has agreed to use commercially reasonable efforts to commercialize Astellas licensed products in a major Asian country and a major European country following receipt of regulatory approval in such countries.
As consideration for the licensed rights underOn April 14, 2023, the Astellas Agreement Astellas agreedwas terminated in its entirety. We are not subject to pay usany payments or costs as a result of this termination.
Massachusetts Institute of Technology
In December 2016, we entered into an upfront payment of $80.0 million, which we received in July 2019, and potential development milestone payments up to $230.0 million. If the Astellas licensed products are successfully commercialized, we would be eligible for up to $315.0 million in potential commercial milestone payments and also tiered royalties at rates ranging from low- to mid-teen percentages. Pursuant to our Exclusive Patent License Agreement, or the MIT License, with the Massachusetts Institute of Technology, or MIT, we are required to pay MIT a royalty on sublicense revenues and as of September 30, 2019, we have accrued $16.0 million related to the $80.0 million upfront payment received. The $16.0 million royalty was expensed in the three months ended September 30, 2019.The $80.0 million upfront payment received from Astellas in July 2019 has been recorded as deferred revenue and will be recognized as revenue, using the input method, over the period in which we will be conducting Phase 2a clinical trials for FX-322, which is estimated to be the period from execution of the Astellas Agreement into the fourth quarter of 2020. In the three months ended September 30, 2019, we recognized $24.2 million of the $80.0 million as revenue.
Massachusetts Institute of Technology
In December 2016, we entered into the MIT License, with MIT, under which we received an exclusive, worldwide, royalty-bearing license to certain patent rights to develop, make, have made, use, sell, offer to sell, lease, and import products or the MIT licensed products, and to develop and perform processes or the MIT licensed processes, whichthat incorporate the licensed technology for the treatment of disease, including but not limited to the prevention and remediation of hearing loss. We are required to use diligent efforts to develop and commercialize the MIT licensed products or processes, and to make such products or processes reasonably available to the public. We are also subject to certain development obligations with regards to a first MIT licensed product. We have satisfied certain obligations related to preclinical studies and the filing of an IND for a first MIT licensed product with our development activities related to FX-322. Our future development obligations are: (i) to commence a Phase 2 clinical trial for such product within two years of the IND filing for such product, (ii) to commence a Phase 3 clinical trial for such product within five years of the IND filing for such product, (iii) to file a New Drug Application, or NDA, or equivalent with the FDA or comparable European regulatory agency for such product within nine years of the IND filing for such product, and (iv) to make a first commercial sale of such product within 11 years of the IND filing for such product. We also have certain development obligations with regards to a second MIT licensed product.
Upon entering into the MIT License, we paid a $50 thousand license fee payment and issued shares of our common stock equal to 5% of our then-outstanding capital stock to MIT. We arewere required to pay certain annual license maintenance fees ranging from $30 thousand to $0.1 million per year prior to first commercial saleMIT under the MIT License. On April 6, 2023, we sent MIT a notice stating that we would be terminating the MIT License Agreement effective as of a MIT licensed product and an annual license maintenance fee of $0.2 million every year afterwards, which may be credited to running royalties during the same calendar year, if any.July 6, 2023. We are also requirednot subject to make potential milestoneany payments in an aggregate amountor costs as a result of up to $2.9 million on each MIT licensed product or process. In addition, we agreed to pay a low single-digit royalty on the MIT licensed products and processes and a low-twenties royalty on sub-license revenues.this termination.
Massachusetts Eye and Ear Infirmary(Formerly Massachusetts Eye and Ear Infirmary)
In February 2019, we entered into an Non-Exclusive Patent License Agreement, or the MEEIMEE License, with the Massachusetts Eye and Ear, Infirmary, or MEEI,MEE, under which we received a non-exclusive, non-sub-licensable, worldwide, royalty-bearing license to certain patent rights to develop, make, have made, use, sell, offer to sell, lease, and import products, and to develop and perform processes that incorporate the licensed technology for the treatment or prevention of hearing loss, or the MEEI licensed products.loss. We are obligated to use diligent efforts to develop and commercialize the MEEI licensed products. We are also subject to milestone timeline obligations to dose a first patient in a Phase II trial by December 31, 2020 and to dose a first patient in a Phase 3 trial by December 31, 2024.
Upon entering into the MEEI License, we made a $20 thousand license fee payment. We arewere obligated to pay certain annual license maintenance fees between $5 thousand and $7.5 thousand per each MEEIMEE patent family case number included in the licensed MEEIMEE patent rights prior to first commercial sale of an MEEI licensed product.under the MEE License. On April 4, 2023, the MEE License was terminated in its entirety. We are also obligatednot subject to payany payments or costs as a minimum annual royalty paymentresult of $15 thousand per each MEEI patent family case number included in the licensed MEEI patent rights after first commercial sale of an MEEI licensed product. We are also obligated to make milestone payments up to $350 thousand on each product or process that incorporates the licensed patent rights. In addition, we have agreed to pay a low single-digit royalty on products and processes that incorporate the licensed patent rights.this termination.
The Scripps Research Institute (California Institute for Biomedical Research)
In September 2018, we entered into a license agreement, or the CALIBR License, with the California Institute for Biomedical Research, or CALIBR, a division of the Scripps Research Institute, or Scripps, under which we received an exclusive, worldwide, royalty-bearing license to certain patent rights to make, have made, use, sell, offer to sell, and import products or the CALIBR licensed products, whichthat incorporate licensed technology for the treatment of MS. We have agreed to use commercially reasonable efforts to develop, manufacture, and sell at least one CALIBR licensed product. We are also subject to certain milestone timeline obligations to: (i) submit an IND (or equivalent) for a CALIBR licensed product by the 30th month after the effective date ofOn April 28, 2023, the CALIBR License (ii) initiate a Phase 2 clinical trial (or equivalent) for a CALIBR licensed product by the fourth anniversary of the effective date of the CALIBR License, and (iii) initiate a Phase 3 clinical trial (or equivalent) for a CALIBR licensed product by the sixth anniversary of the effective date of the CALIBR License.
Upon entering into the CALIBR License, we made a $1.0 million license fee payment, and are required to make milestone payments in an aggregate amount of up to $26.0 million for each category of CALIBR licensed products. Category 1 is any CALIBR licensed products containing a compound that modulates any muscarinic receptor, and Category 2 is any CALIBR licensed products not included in Category 1 that could differentiate oligodendrocyte precursor cells from in vitro studies and/or are active in animal models relevant to MS.was terminated. We are also requirednot subject to payany payments or costs as a mid-single-digit royalty on CALIBR licensed products and a royalty on sub-license revenues ranging from a low-teen percentage to 50%.result of this termination.
The Scripps Research Institute
In September 2018, we entered into a Research Funding and Option Agreement, or the Scripps option agreement, with Scripps, under which we were granted an exclusive option to acquire an exclusive, sublicensable, worldwide license under certain intellectual property related to the treatment of MS. As consideration for the Scripps option agreement, we are required to make funding payments totaling $0.7 million to Scripps to support its research activities. Scripps has agreed to use reasonable efforts to perform the research program pursuant to the Scripps option agreement.
25
Components of our results of operations
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from product sales in the foreseeable future. In July 2019, we entered into the Astellas Agreement and received an upfront license fee payment of $80.0 million. In accordance with ASC 606, we are recognizing the $80.0 million as revenue over the period that research and development services and the Phase 2a clinical study for FX-322 are being provided using the input method. This is estimated to be the period from execution of the Astellas Agreement into the fourth quarter of 2020. In the three months ended September 30, 2019, we recognized $24.2 million of the $80.0 million upfront fee as revenue. This reflects revenue of $16.0 million equal to the royalty expense under the MIT License as well as $8.2 million from applying the input method to the remaining $64.0 million of the upfront payment.
Royalty
We are required to pay royalties under the MIT License on the receipt of fees for the sublicense of the MIT intellectual property. We expensed the $16.0 million royalty due MIT on the $80.0 million license fee pursuant to the Astellas Agreement in the third quarter of 2019.
Research and development expenses
Research and development expenses presented in this section consist primarily of costs incurred for our researchrelated to activities including our discovery effortslargely focused on hearing restoration and for the development of our product candidate, FX-322.MS. These expenses include the following:
salaries, benefits and other related costs, including stock-based compensation expense, for personnel engaged in research and development functions;
expenses incurred under agreements with third parties, including contract research organizations, or CROs, and other third parties that conduct preclinical research and development activities and clinical trials on our behalf;
costs to manufacture our clinical trial material for use in our preclinical studies and clinical trials;
costs of outside consultants, including their fees and related travel expenses;
costs of laboratory supplies and acquiring, developing and manufacturing preclinical study and clinical trial materials;
option and license payments made to third parties, including MIT, Scripps,CALIBR, and MEEI,MEE for intellectual property used in research and development activities; and
facility-related expenses, which include direct depreciation costs and expenses for rent and maintenance of facilities and other operating costs if specifically, identifiable to research activities.
We expense research and development costs as incurred.
We track external research and development costs, including the cost of services, outsourced research and development, clinical trials, contract manufacturing, laboratory equipment and maintenance, and certain other development costs, by product candidate when the costs are specifically identifiable to a product candidate. Internal and external costs associated with infrastructure resources, other research and development costs, facility-related costs, and depreciation and amortization that are not identifiable to a specific product candidate are included in the platform development, early-stage research, and unallocated expenses category in the table below.
The following table summarizes our research and development expenses by product candidate or development program for the three and nine month period ended September 30, 2019 and 2018:category.
|
| Three Months Ended September 30, |
|
| Increase |
|
| Nine Months Ended September 30, |
|
| Increase |
| ||||||||||||
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
| ||||||
Direct research and development expenses by product candidate: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FX-322 external development costs |
| $ | 1,574 |
|
| $ | 639 |
|
| $ | 935 |
|
| $ | 3,190 |
|
| $ | 1,711 |
|
| $ | 1,479 |
|
Platform development, early-stage research and unallocated expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee-related costs |
|
| 2,344 |
|
|
| 987 |
|
|
| 1,357 |
|
|
| 5,743 |
|
|
| 3,270 |
|
|
| 2,473 |
|
Laboratory supplies |
|
| 138 |
|
|
| 75 |
|
|
| 63 |
|
|
| 294 |
|
|
| 232 |
|
|
| 62 |
|
Outsourced research and development |
|
| 726 |
|
|
| 1,633 |
|
|
| (907 | ) |
|
| 2,332 |
|
|
| 2,979 |
|
|
| (647 | ) |
Facility-related costs |
|
| 315 |
|
|
| 156 |
|
|
| 159 |
|
|
| 710 |
|
|
| 587 |
|
|
| 123 |
|
Depreciation and amortization |
|
| 124 |
|
|
| 60 |
|
|
| 64 |
|
|
| 319 |
|
|
| 180 |
|
|
| 139 |
|
Total research and development expenses |
| $ | 5,221 |
|
| $ | 3,550 |
|
| $ | 1,671 |
|
| $ | 12,588 |
|
| $ | 8,959 |
|
| $ | 3,629 |
|
We expect that our research and development expenses will continue to increase substantially for the foreseeable future and will comprise a larger percentage of our total expenses as we continue and complete our Phase 2a clinical trial and initiate additional clinical trials for FX-322 and continue to discover and develop additional product candidates. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to increased scale and duration.
We cannot determine with certainty the duration and costs of future clinical trials of FX-322 or any other product candidate we may develop or if, when, or to what extent we will generate revenue from the commercialization and sale of any product candidate for which we obtain marketing approval. The duration, costs, and timing of clinical trials and development of FX-322 and any other product candidate we may develop will depend on a variety of factors, including:
the scope, rate of progress, expense and results of clinical trials of FX-322, as well as of any future clinical trials of other product candidates and other research and development activities that we may conduct;
the actual probability of success for our product candidates, including their safety and efficacy, early clinical data, competition, manufacturing capability, and commercial viability;
significant and changing government regulation and regulatory guidance;
the timing and receipt of any marketing approvals;
the progress of the development efforts of parties with whom we may enter into collaboration agreements;
our ability to secure manufacturing supply through relationships with third parties;
the commercialization of our product candidates, if and when approved;
raising additional funds necessary to complete preclinical and clinical development of our product candidates; and
the expense of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights.
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate.
26
General and administrative expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in our executive, finance, business development, and administrative functions. General and administrative expenses also include legal fees relating to intellectual property and corporate matters; professional fees for accounting, auditing, tax and consulting services; investor and public relations costs; insurance costs; travel expenses; and facility-related expenses, which include direct depreciation costs and expenses for rent and maintenance of facilities, and other operating costs that are not specifically attributable to research and development activities.
We expect that our general and administrative expenses will increase in the future as we increase our personnel headcount to support our continued research activities and development of product candidates. We also expect to continue to incur increased expenses associated with being a public company, including costs of accounting, audit, legal, regulatory, and tax-related services associated with maintaining compliance with the requirements of The Nasdaq Stock Market and the SEC; director and officer insurance costs; and investor and public relations costs.
Interest income
Interest income consists of interest earned on cash equivalents and short-term investments.marketable securities.
Interest expense
Interest expense consists of interest paid on convertible notes payable which were converted in our Series B convertible preferred stock financing in 2018.term loan.
Realized gain
Other income, net
Other income, net consists of amortization expense and accretion income on investments as well as sublease income.
Realized gain
Income taxes
Our total provision is based on investments represents the gain realizedUnited States statutory rate of 21%, increased by state taxes and reduced by a full valuation allowance on our marketable securities indeferred tax assets. The income tax expense for the three and nine months ended September 30, 2019.March 31, 2023 and 2022 represents state taxes on interest income earned by our subsidiary, Frequency Therapeutics Securities Corporation, a Massachusetts Securities Corporation.
LossASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on extinguishment of debt
In 2018, we issued notes payable, which were convertible at a 5% discount to the price of shares issued in a qualified financing. In October 2018, the notes payable converted into Series B convertible preferred stock at a 5% discount to the priceweight of the Series B convertible preferred stock, whichevidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, we have recorded as a $0.3 millionvaluation allowance against our deferred tax assets at December 31, 2022 because we have determined that it is more likely than not that we will not recognize the benefits of our federal and state deferred tax assets primarily due to our cumulative loss on extinguishment of debt.
Foreign exchange gain (loss)
Foreign exchange gain (loss) represents the gain or loss recordedposition and, as a result, of remeasuring the financial statements of our foreign subsidiaries.a valuation allowance has been established.
Income taxes
Since our inception in 2014, we have generated cumulative federal and state net operating loss and research and development credit carryforwards for which we have not recorded any net tax benefit due to uncertainty around utilizing these tax attributes within their respective carryforward periods.
As of December 31, 2018, we had federal net operating loss carryforwards, or NOLs, of approximately $39.3 million and Massachusetts state NOLs of approximately $31.7 million which may be available to offset future taxable income. Our federal NOLs include $22.4 million available to reduce future taxable income through 2037 and approximately $16.9 million of NOLs that do not expire and are available to reduce future taxable income indefinitely. The state NOLs are available to offset future taxable income through 2038. As of December 31, 2018, we also had federal and Massachusetts research and development tax credit carryforwards of $0.7 million and $0.4 million, respectively, which are available to offset federal and state tax liabilities through 2038 and 2033, respectively.
Realization of future tax benefits is dependent on many factors, including our ability to generate taxable income within the NOL period. NOL and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50%, as provided under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, as well as under similar state provisions. These ownership changes may limit the amount of NOLs that can be utilized annually to offset future taxable income. In general, an ownership change, as defined under Section 382 of the Code, or Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% over a three-year period. We have completed several financings and have conducted a study to assess whether a change of control has occurred or whether there have been multiple changes of control since inception and have determined that an ownership change did occur in March 2017. Accordingly, utilization of $12.4 million of the federal NOLs that were incurred prior to March 2017 (pre-ownership change) is limited under Section 382. After the Section 382 limitations, we may utilize approximately $10.8 million of our pre-ownership change NOLs based upon an annual usage of approximately $1.6 million for each of the next five years after the ownership change and approximately $0.2 million for each of the 15 years thereafter. The remaining pre-ownership change NOLs of approximately $1.6 million were written off due to expiration under limitation. The limitation has been determined by first multiplying the value of our stock at the time of the ownership change by the applicable long-term tax-exempt rate. These NOLs may be subject to further annual limitations under Section 382 in the event of future changes in ownership.
In December 2017, the Tax Cuts and Jobs Act was signed into U.S. law. The Tax Cuts and Jobs Act included a number of changes to the tax law, including, among other things, a permanent reduction in the federal corporate income tax rate from a top marginal tax rate of 35% to a flat rate of 21%, which went into effect on January 1, 2018, as well as a limitation of the deduction for NOLs to 80% of annual taxable income, and elimination of net operating loss carrybacks, in each case, for losses arising in taxable years beginning after December 31, 2017 (though any such NOLs may be carried forward indefinitely). The federal tax rate change resulted in a reduction in the gross amount of our deferred tax assets and liabilities recorded as of December 31, 2017, and a corresponding reduction in our valuation allowance. As a result, no income tax expense or benefit was recognized as of the enactment date of the Tax Cuts and Jobs Act.
Results of operations
Comparison of three months ended September 30, 2019March 31, 2023 and 20182022
The following table summarizes our results of operations for the three months ended September 30, 2019March 31, 2023 and 2018:2022:
|
| Three Months Ended |
| |||||||||
|
| 2023 |
|
| 2022 |
|
| Increase |
| |||
|
| (in thousands) |
| |||||||||
Operating expenses: |
|
|
|
|
|
|
|
|
| |||
Research and development |
|
| 11,355 |
|
|
| 13,781 |
|
|
| (2,426 | ) |
General and administrative |
|
| 9,156 |
|
|
| 9,477 |
|
|
| (321 | ) |
Total operating expenses |
|
| 20,511 |
|
|
| 23,258 |
|
|
| (2,747 | ) |
Loss from operations |
|
| (20,511 | ) |
|
| (23,258 | ) |
|
| 2,747 |
|
Interest income |
|
| 523 |
|
|
| 95 |
|
|
| 428 |
|
Interest expense |
|
| (284 | ) |
|
| (178 | ) |
|
| (106 | ) |
Other income (expense), net |
|
| 753 |
|
|
| (33 | ) |
|
| 786 |
|
Loss before income taxes |
|
| (19,519 | ) |
|
| (23,374 | ) |
|
| 3,855 |
|
Income tax |
|
| (24 | ) |
|
| (12 | ) |
|
| (12 | ) |
Net loss |
| $ | (19,543 | ) |
| $ | (23,386 | ) |
| $ | 3,843 |
|
27
|
| Three Months Ended September 30, |
|
| Increase |
| ||||||
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
| |||
Revenue |
| $ | 24,238 |
|
| $ | — |
|
| $ | 24,238 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Royalty |
|
| 16,000 |
|
|
| — |
|
|
| 16,000 |
|
Research and development |
|
| 5,221 |
|
|
| 3,550 |
|
|
| 1,671 |
|
General and administrative |
|
| 4,269 |
|
|
| 1,507 |
|
|
| 2,762 |
|
Total operating expenses |
|
| 25,490 |
|
|
| 5,057 |
|
|
| 20,433 |
|
Loss from operations |
|
| (1,252 | ) |
|
| (5,057 | ) |
|
| 3,805 |
|
Interest income |
|
| 624 |
|
|
| — |
|
|
| 624 |
|
Interest expense |
|
| — |
|
|
| (63 | ) |
|
| 63 |
|
Realized gain on investments |
|
| 62 |
|
|
| — |
|
|
| 62 |
|
Foreign exchange gain (loss) |
|
| (9 | ) |
|
| (16 | ) |
|
| 7 |
|
Net loss |
| $ | (575 | ) |
| $ | (5,136 | ) |
| $ | 4,561 |
|
Revenue
We had no revenueResearch and development expenses
|
| Three Months Ended |
| |||||||||
|
| 2023 |
|
| 2022 |
|
| Increase |
| |||
|
| (in thousands) |
| |||||||||
Direct research and development expenses by therapeutic area and product candidate: |
|
|
|
|
|
|
|
|
| |||
FX-322 |
| $ | 1,081 |
|
|
| 2,702 |
|
| $ | (1,621 | ) |
FX-345 |
|
| 647 |
|
|
| 1,029 |
|
|
| (382 | ) |
Multiple Sclerosis |
|
| 1,795 |
|
|
| 997 |
|
|
| 798 |
|
Platform development, early-stage research |
|
|
|
|
|
|
|
|
| |||
Employee-related costs |
|
| 5,437 |
|
|
| 6,119 |
|
|
| (682 | ) |
Laboratory supplies |
|
| 54 |
|
|
| 119 |
|
|
| (65 | ) |
Outsourced research and development |
|
| 50 |
|
|
| 335 |
|
|
| (285 | ) |
Facility-related costs |
|
| 1,437 |
|
|
| 1,774 |
|
|
| (337 | ) |
Depreciation and amortization |
|
| 733 |
|
|
| 439 |
|
|
| 294 |
|
Other research and development costs |
|
| 121 |
|
|
| 267 |
|
|
| (146 | ) |
Platform development, early-stage research and unallocated expenses total |
|
| 7,832 |
|
|
| 9,053 |
|
|
| (1,221 | ) |
Total research and development expenses |
| $ | 11,355 |
|
| $ | 13,781 |
|
| $ | (2,426 | ) |
The $1.1 million of costs related to FX-322 incurred for the three months ended September 30, 2018. In July 2019, we entered into the Astellas Agreement and received an upfront license fee paymentMarch 31, 2023 consisted of $80.0 million. In accordanceclinical costs associated with ASC 606, we are recognizing the $80.0 million as revenue over the period that research and development services and the Phase 2a2b clinical study fortrial (FX-322-208) which concluded in the first quarter 2023. The $2.7 million of costs related to FX-322 are being provided using the input method. Inincurred in the three months ended September 30, 2019, we recognized $24.2March 31, 2022 consisted primarily of $2.5 million of clinical costs associated with the $80.0 million as revenue. This reflects revenue of $16.0 million equal to the royalty expense under the MIT License as well as $8.2 million from applying the input method to the remaining $64.0Phase 2b clinical trial (FX-322-208) and $0.2 million of the upfront payment.drug development and manufacturing costs.
Royalty
We are requiredThe $0.6 million of costs related to pay royalties under the MIT License on the receipt of fees for the sublicense of the MIT intellectual property. In the three months ended September 30, 2019, we expensed the $16.0 million royalty due MIT on the $80.0 million upfront license fee received from Astellas.
Research and development expenses
|
| Three Months Ended September 30, |
|
| Increase |
| ||||||
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
| |||
Direct research and development expenses by product candidate: |
|
|
|
|
|
|
|
|
|
|
|
|
FX-322 external development costs |
| $ | 1,574 |
|
| $ | 639 |
|
| $ | 935 |
|
Platform development, early-stage research and unallocated expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Employee-related costs |
|
| 2,344 |
|
|
| 987 |
|
|
| 1,357 |
|
Laboratory supplies |
|
| 138 |
|
|
| 75 |
|
|
| 63 |
|
Outsourced research and development |
|
| 726 |
|
|
| 1,633 |
|
|
| (907 | ) |
Facility-related costs |
|
| 315 |
|
|
| 156 |
|
|
| 159 |
|
Depreciation and amortization |
|
| 124 |
|
|
| 60 |
|
|
| 64 |
|
Total research and development expenses |
| $ | 5,221 |
|
| $ | 3,550 |
|
| $ | 1,671 |
|
Research and development expensesFX-345 incurred for the three months ended September 30, 2019 were $5.2March 31, 2023 consisted of clinical trial costs associated with the planned Phase 1b trial (FX-345-101), which was discontinued in the first quarter of 2023. The $1.0 million comparedof costs related to $3.5 millionFX-345 incurred for the three months ended September 30, 2018. The increase of $1.7 million was primarily due to an increaseMarch 31, 2022 consisted of $0.9 million in FX-322 externalof drug development and manufacturing costs and $0.1 million related to preclinical safety costs.
The $1.8 million of costs related to the MS development program incurred for the three months ended March 31, 2023 consisted primarily of $0.9 million of chemistry and compound characterization costs, $0.2 million of drug development and manufacturing costs, and $0.6 million of preclinical safety costs. Similarly, the $1.0 million of costs related to the MS development program incurred for the three months ended March 31, 2022 consisted primarily of $0.6 million of chemistry and compound characterization costs, $0.2 million of preclinical safety costs, and $0.1 million of drug development and manufacturing costs.
The $7.8 million of platform development, early-stage research and developmentunallocated expenses and an increaseincurred for the three months ended March 31, 2023, consisted primarily of $5.4 million in employee-related costs, including $0.8 million of stock-based compensation expense, and $1.4 million in facility-related costs. The decrease in platform development, early-stage research and unallocated expenses. expenses of $1.2 million during the three months ended March 31, 2023, compared to the three months ended March 31, 2022, is primarily attributable to a decrease of $0.7 million in employee-related costs, including salary, bonus, and stock-based compensation costs, as a result of the reduction in force implemented in April 2022, as well as a $0.3 million decrease in facility-related costs due to a combination of reduced headcount and the initiation of cost sharing with SalioGen Therapeutics, Inc. in connection with our sublease.
General and administrative expenses
The $1.6$9.2 million of FX-322 external development costs incurredgeneral and administrative expenses for the three months ended September 30, 2019March 31, 2023 consisted primarily of approximately $1.1$6.1 million in employee-related costs, including $2.6 million of clinical developmentstock-based compensation, $1.4 million in facility-related costs, for the Phase 2a clinical trial, including manufacturing$1.4 million in professional services costs, for FX-322 to be usedand $0.3 million in clinical trials,depreciation expense. General and approximately $0.2administrative expenses decreased by $0.3 million of outside consulting costs. The $0.6 million of FX-322 external development costs incurred infrom the three months ended September 30, 2018 consistedMarch 31, 2022 due primarily of approximatelyto a $0.2 million of preclinicaldecrease in professional services costs for safety testing andas we reduced reliance on third-party consulting and approximately $0.4 million related to conducting the Phase 1 clinical trial. FX-322 external development costs for the three months ended September 30, 2019 are presented net of approximately $0.2 million in research and development tax credits received from the Australian government related to our Phase 1 clinical trial conducted in Australia.public relations vendors.
Platform development, early-stage research and unallocated expenses were $3.628
Interest income
Interest income was $0.5 million for the three months ended September 30, 2019,March 31, 2023 compared to $2.9interest income of $0.1 million for the three months ended September 30, 2018. TheMarch 31, 2022. This increase of $0.7 million was primarilyis due to $1.4 million of increased employee-related costs associated with increased headcount to support our preclinical and clinical development of FX-322 and research on our PCA platform development and $.3 of increased facility and supply costs. Thethe increase in employee-related costs includes an increase ofinterest rates from the previous year.
Interest expense
Interest expense was $0.3 million in compensation related to stock options. The $0.7 increase in the 2019 period is net of a $1.0 million expense in the three months ended September 30, 2018 for the upfront license fee to Scripps for the MS program.
General and administrative expenses
General and administrative expenses were $4.3 million for the three months ended September 30, 2019,March 31, 2023 compared to $1.5interest expense of $0.2 million for the three months ended September 30, 2018. General and administrative expenses increased by $2.8 millionMarch 31, 2022. This increase is due to increased business development activities, increasesthe increase in interest rates from the infrastructure necessary to manage research and development and fundraising activities, an increase of $0.9 million in audit, tax and consulting fees, $0.2 million in legal and patent filing fees incurred to file and defend intellectual property and $1.7 million in employee-related costs as we increased our general and administrative headcount to manage our growth, including an increase of $0.5 in compensation related to stock options.previous year.
Interest
Other income, net
InterestOther income, net was $0.6$0.8 million for the three months ended September 30, 2019 dueMarch 31, 2023 as compared to an increase in cash equivalents and short-term investments. We did not have interest income in the three months ended September 30, 2018.
Interestother expense,
Interest expense was $63 net of $33 thousand for the three months ended September 30, 2018.March 31, 2022. This represents interest on convertible notes payable issued in 2018 and converted into Series B convertible preferred stock in 2018. We did not incur interest expensesincrease is due to the sublease income generated in the three months ended September 30, 2019.March 31, 2023.
Realized gain on investments
Realized gain on investmentsIncome tax provision
Income tax provision was $62$24 thousand for the three months ended September 30, 2019 dueMarch 31, 2023 as compared to our purchasing short-term marketable securities in 2019. We held no investments in the three months ended September 30, 2018.
Foreign exchange gain (loss)
Foreign exchange loss was $9income tax provision of $12 thousand for the three months ended September 30, 2019, compared to a foreign exchange loss of $16 thousand for the three months ended September 30, 2018. The decrease of $7 thousand was due to differences in foreign exchange remeasurement of the financial statements of our foreign subsidiaries.
Comparison of nine months ended September 30, 2019 and 2018March 31, 2022.
The following table summarizes our results of operations for the nine months ended September 30, 2019 and 2018:
|
| Nine Months Ended September 30, |
|
| Increase |
| ||||||
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
| |||
Revenue |
| $ | 24,238 |
|
| $ | — |
|
| $ | 24,238 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Royalty |
|
| 16,000 |
|
|
| — |
|
|
| 16,000 |
|
Research and development |
|
| 12,588 |
|
|
| 8,959 |
|
|
| 3,629 |
|
General and administrative |
|
| 9,837 |
|
|
| 4,660 |
|
|
| 5,177 |
|
Total operating expenses |
|
| 38,425 |
|
|
| 13,619 |
|
|
| 24,806 |
|
Loss from operations |
|
| (14,187 | ) |
|
| (13,619 | ) |
|
| (568 | ) |
Interest income |
|
| 842 |
|
|
| — |
|
|
| 842 |
|
Interest expense |
|
| — |
|
|
| (95 | ) |
|
| 95 |
|
Realized gain on investments |
|
| 88 |
|
|
| — |
|
|
| 88 |
|
Foreign exchange gain (loss) |
|
| 4 |
|
|
| (7 | ) |
|
| 11 |
|
Net loss |
| $ | (13,253 | ) |
| $ | (13,721 | ) |
| $ | 468 |
|
Revenue
We had no revenue for the nine months ended September 30, 2018. In July 2019, we entered into the Astellas Agreement and received an upfront license fee payment of $80.0 million. In accordance with ASC 606, we are recognizing the $80.0 million as revenue over the period that research and development services and the Phase 2a clinical study for FX-322 are being provided using the input method. This is estimated to be into the fourth quarter of 2020. In the nine months ended September 30, 2019, we recognized $24.2 million of the $80.0 million as revenue. This reflects revenue of $16.0 million equal to the royalty expense under the MIT License as well as $8.2 million applying the input method to the remaining $64.0 million of the upfront payment.
Royalty Expense
We are required to pay royalties under the MIT License on the receipt of fees for the sublicense of the MIT intellectual property. In the nine months ended September 30, 2019, we expensed the $16.0 million royalty due MIT on the $80.0 million upfront license fee received from Astellas.
Research and development expenses
|
| Nine Months Ended September 30, |
|
| Increase |
| ||||||
|
| 2019 |
|
| 2018 |
|
| (Decrease) |
| |||
Direct research and development expenses by product candidate: |
|
|
|
|
|
|
|
|
|
|
|
|
FX-322 external development costs |
| $ | 3,190 |
|
| $ | 1,711 |
|
| $ | 1,479 |
|
Platform development, early-stage research and unallocated expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Employee-related costs |
|
| 5,743 |
|
|
| 3,270 |
|
|
| 2,473 |
|
Laboratory supplies |
|
| 294 |
|
|
| 232 |
|
|
| 62 |
|
Outsourced research and development |
|
| 2,332 |
|
|
| 2,979 |
|
|
| (647 | ) |
Facility-related costs |
|
| 710 |
|
|
| 587 |
|
|
| 123 |
|
Depreciation and amortization |
|
| 319 |
|
|
| 180 |
|
|
| 139 |
|
Total research and development expenses |
| $ | 12,588 |
|
| $ | 8,959 |
|
| $ | 3,629 |
|
Research and development expenses for the nine months ended September 30, 2019 were $12.6 million, compared to $9.0 million for the nine months ended September 30, 2018. The increase of $3.6 million was primarily due to an increase of $1.5 million in FX-322 external research and development expenses and an increase of $2.1 million in platform development, early-stage research and unallocated expenses. The $3.2 million of FX-322 external development costs incurred for the nine months ended September 30, 2019 consisted primarily of approximately $1.9 million of clinical development costs for the Phase 1 and 2a clinical trials of FX-322, including manufacturing costs for FX-322 to be used in clinical trials, and approximately $0.6 million of outside consulting costs. The $1.7 million of FX-322 external development costs incurred in the nine months ended September 30, 2018 consisted primarily of approximately $0.2 million of preclinical costs for safety testing and consulting, and approximately $0.9 million related to conducting the Phase 1 clinical trial. FX-322 external development costs for the nine months ended September 30, 2018 are presented net of approximately $0.2 million in research and development tax credits received from the Australian government related to our Phase 1 clinical trial conducted in Australia.
Platform development, early-stage research and unallocated expenses were $9.4 million for the nine months ended September 30, 2019, compared to $7.2 million for the nine months ended September 30, 2018. The increase of $2.2 million was primarily due to a $0.4 million increase in outsourced research and development spending on our development programs, excluding our FX-322 program, and $2.5 million of increased employee-related costs associated with increased headcount to support our preclinical and clinical development of FX-322 and research on our PCA platform development. The increase in employee-related costs includes an increase of $1.0 million in compensation related to stock options. Platform development, early-stage research and unallocated expenses in the nine months ended September 30, 2018 includes an expense of $1.0 million for the upfront license fee to Scripps for the MS program.
General and administrative expenses
General and administrative expenses were $9.8 million for the nine months ended September 30, 2019, compared to $4.7 million for the nine months ended September 30, 2018. General and administrative expenses increased by $5.1 million due to increased business development activities, increases in the infrastructure necessary to manage research and development and fundraising activities, an increase of $1.3 million in audit, tax and consulting fees, $0.8 million in legal and patent filing fees incurred to file and defend intellectual property, $0.2 million in corporate legal fees and $2.0 million in employee-related costs as we increased our general and administrative headcount to manage our growth, including an increase of $1.1 million in compensation related to stock options.
Interest income
Interest income was $0.8 million for the nine months ended September 30, 2019 due to an increase in cash equivalents and short-term investments. We did not have interest income in the nine months ended September 30, 2018.
Interest expense
Interest expense was $95 thousand for the nine months ended September 30, 2018. This represents interest on convertible notes payable issued in 2018 and converted into Series B convertible preferred stock in 2018. We did not incur interest expenses in the nine months ended September 30, 2019.
Realized gain on investments
Realized gain on investments was $88 thousand for the nine months ended September 30, 2019 due to our purchasing short-term marketable securities in 2019. We held no investments in the nine months ended September 30, 2018.
Foreign exchange gain (loss)
Foreign exchange gain was $4 thousand for the nine months ended September 30, 2019, compared to a foreign exchange loss of $7 thousand for the nine months ended September 30, 2018. The decrease of $11 thousand was due to differences in foreign exchange remeasurement of the financial statements of our foreign subsidiaries.
Liquidity and capital resources
Since our inception, we have incurred significant operating losses. We expect to continue to incur significant expenses and operating losses for the foreseeable future as we advance the preclinical and clinical development of our product candidates. We expect that our research and development and general and administrative costs will continue to increase, includingfluctuate, in connection with conducting preclinical studies and clinical trials for our product candidates, contracting with contract manufacturing organizations, or CMOs, to support preclinical studies and clinical trials, expanding our intellectual property portfolio, and providing general and administrative support for our operations particularly as a public company.operations. As a result, we will need additional capital to fund our operations, which we may obtain from additional equity or debt financings, collaborations, licensing arrangements or other sources.
We do not currently have any approved products and have never generated any revenue from product sales. We have financed our operations primarily through proceeds from the sale of our convertible notes, convertible preferred stock, our IPOprivate and the Astellas Agreement.public securities financings, a term loan, and amounts received under a collaboration agreement. To date, we have raised approximately $316.5$378.3 million, through a combination of convertible notes, convertible preferred stock, the upfront payment under the Astellas Agreement,including from grants and our IPO.option exercises. Our cash, cash equivalents and short-term investmentsmarketable securities totaled $165.3$66.7 million as of SeptemberMarch 31, 2023. As of March 31, 2023, we had $11.7 million of current debt.
In December 2020, we entered into a Loan and Security Agreement with a commercial bank for a term loan with a principal balance of $15.0 million. We made monthly interest only payments through November 30, 2019. 2022. On April 3, 2023, we prepaid the remaining $11.7 million due under the term loan. We were not subject to any prepayment premium as the prepayment occurred after the second anniversary of the closing date.
In October 2019,December 2021, we completedentered into an IPOEquity Distribution Agreement with Oppenheimer & Co. Inc., or Oppenheimer, to sell shares of our common stock, and issued andhaving an aggregate offering price of up to $125.0 million, from time to time, through an “at the market” equity offering program under which Oppenheimer will act as sales agent and/or principal, or the ATM Program. During the year ended December 31, 2022, we sold 6,325,00012,767 shares of common stock at a public offering of $14.00 per share, inclusive ofunder the exercise in part by the underwriters of their option to purchase additional shares, resulting inATM Program for net proceeds of $79.2 million after deducting underwriting discountsapproximately $50 thousand and commissions and offering expenses. We had no indebtedness as of September 30, 2019. In July 2019, we closed our Series C convertible preferred stock financing,paid $2 thousand to Oppenheimer in which we issued 39,492,960sales agent fees. No shares of our Series C convertible preferredcommon stock for aggregate gross proceedshave been sold under the ATM Program in 2023.
On April 8, 2022, we announced a reduction in force of approximately $62.0 million. In30% of our workforce to better align our workforce with the needs of our business and focus more of our capital resources on our research and development programs. The total costs related to this reduction in force are approximately $1.0 million in research and development expense and $0.2 million in general and administrative expense, primarily related to severance costs and related expenses.
On February 13, 2023, in connection with our receiptthe discontinuation of the upfront payment of $80.0 million under the Astellas Agreement,FX-322 and FX-345 hearing programs, we announced a restructuring that included downsizing personnel by approximately 55%. These changes are expected to
29
preserve capital, ensuring that we are obligatedappropriately resourced to pay $16.0complete a first clinical trial of our MS development program. The total costs related to this restructuring are approximately $4.3 million, of which $3.8 million is related to MIT under the MIT License in satisfaction of a royalty owedseverance costs and related expenses, $0.4 million is related to MIT upon receipt of such upfront payment.accelerated depreciation, and $0.1 million is related to accelerated hearing program costs.
Cash flows
The following table summarizes our sources and uses of cash flows for the nine months ended September 30, 2019 and 2018:periods presented:
|
| Nine Months Ended September 30, |
| |||||
|
| 2019 |
|
| 2018 |
| ||
Net cash provided by (used in) operating activities |
| $ | 63,958 |
|
| $ | (12,305 | ) |
Net cash used in investing activities |
|
| (23,690 | ) |
|
| (197 | ) |
Net cash provided by financing activities |
|
| 59,817 |
|
|
| 6,842 |
|
Increase (decrease) in cash and cash equivalents |
| $ | 100,085 |
|
| $ | (5,660 | ) |
|
| Three Months Ended |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| (in thousands) |
| |||||
Net cash used in operating activities |
| $ | (14,132 | ) |
| $ | (17,343 | ) |
Net cash provided by (used in) investing activities |
|
| 5,499 |
|
|
| (12,662 | ) |
Net cash (used in) provided by financing activities |
|
| (2,468 | ) |
|
| 200 |
|
Decrease in cash and cash equivalents |
| $ | (11,101 | ) |
| $ | (29,805 | ) |
Cash flows for the ninethree months ended September 30, 2019 and 2018March 31, 2023
Operating activities
Net cash provided by operating activities for the nine months ended September 30, 2019 was $64.0 million, primarily consisting of a net loss of $13.3 million as we incurred expenses associated with our FX-322 program, platform development and early-stage research, and general and administrative expenses. In addition, we had non-cash charges of $3.0 million for depreciation, stock-based compensation expense and deferred lease incentives. Net cash provided by operating activities was also impacted by a net $71.8 million change in operating assets and liabilities, including an increase of $55.8 million in deferred revenue, $16.0 million in royalty payable, $1.7 million in accounts payable and $1.4 million in accrued expenses, which were partially offset by an increase of $3.1 million in grants receivable, prepaid expenses and other assets.
Net cash used in operating activities for the ninethree months ended September 30, 2018March 31, 2023 was $12.3$14.1 million, primarily consisting of a net loss of $13.7 million as we incurred expenses associated with our FX-322 program, platform development and early-stage research, and general and administrative expenses.$19.5 million. In addition, we had non-cash charges of $0.6$4.9 million for depreciation, stock-based compensation expense, non-cash lease expense, and deferred lease incentives.non-cash gain on investments. Net cash used in operating activities was also impacted by a net $0.8$3.6 million changedecrease in operating assets and liabilities, including an increase of $1.1a $2.1 million decrease in accounts payable, and an increase of $0.3 million in accrued expenses, which were partially offset by an increase of $0.6 million in grants receivable, prepaid expenses and other current assets.assets, a $0.5 million decrease in lease liabilities, a $0.4 million decrease in accrued expenses, and a $0.7 million decrease in accounts payable.
The decrease in net cash used in operating activities for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to a $3.9 million decrease in net loss. This decrease was partially offset by a $1.8 million decrease in stock based compensation expense and various changes in operating assets and liabilities quarter over quarter.
Investing activities
Net cash provided by investing activities for the three months ended March 31, 2023 was $5.5 million, which was primarily attributable to $7.6 million in redemptions of marketable securities, partially offset by $2.0 million purchases of marketable securities.
The increase in net cash provided by investing activities for the three months ended March 31, 2023 compared to the three months ended March 31, 2022 was primarily due to a $18.8 million decrease in the purchase of marketable securities partially offset by a $0.6 million decrease in redemptions of marketable securities.
Financing activities
Net cash used in financing activities for the three months ended March 31, 2023 was $2.5 million, primarily attributable to term loan repayments. This represents an increase in net cash used in financing activities when compared to the three months ended March 31, 2022 as no such repayments were made in the three months ended March 31, 2022.
Cash flows for the three months ended March 31, 2022
Operating activities
Net cash used in operating activities for the three months ended March 31, 2022 was $17.3 million, consisting of a net loss of $23.4 million. In addition, we had non-cash charges of $6.9 million for depreciation, stock-based compensation expense, non-cash lease expense, and non-cash interest expense. Net cash used in operating activities was also impacted by a net $3.6 million decrease in operating assets and liabilities, including a decrease in accrued expenses of $1.4 million, a $0.4 million decrease in accounts payable, and a $1.4 million decrease in prepaid expenses and other current assets.
30
Investing activities
Net cash used in investing activities for the ninethree months ended September 30, 2019March 31, 2022 was $23.7$12.7 million, which was attributable to $0.6$20.8 million of purchases of property and equipment and $23.0marketable securities, partially offset by $8.1 million of net purchasesin redemptions of marketable securities.
Net cash used in investing activities for the nine months ended September 30, 2018 was $0.2 million, which was attributable to purchases of property and equipment.
Financing activities
Net cash provided by financing activities for the ninethree months ended September 30, 2019March 31, 2022 was $59.8primarily attributable to $0.2 million consisting of netin proceeds from the issuance of our Series Ccommon stock and Series B convertible preferred stockthe sale of $62.0 million and $0.3 million of proceeds from the exercise of stock options.
Net cash provided by financing activities for the nine months ended September 30, 2018 was $6.8 million, consisting of $5.0 million in proceeds received upon the issuance of convertible notes payable and $70 thousand of proceeds from the exercise of stock options. We also issued shares of preferredcommon stock in Frequency Therapeutics Japan KK,under the Employee Stock Purchase Plan.
Funding requirements
We expect our majority-owned subsidiary, for proceeds of $1.8 million, which has been recorded as a liability.
Funding requirements
Ourfuture operating expenses increased substantially in the nine months ended September 30, 2019, and are expected to increase substantially in the future in connection withreflect our ongoing activities, particularly as we advance FX-322 through clinical trials and as we research and develop additional product candidates includingdevelopment for our MS development program, preclinical activities, studies for INDs, applications, and initiation of human clinical trials. In addition, we expect to continue to incur additionalmaintain general and administrative costs associated withto manage the requirements of operating as a public company.
Specifically, our costs and expenses will increase if and as we:
advance the clinical development of FX-322;
pursue the preclinical and clinical development of a candidate for remyelination in MS and any other product candidates using our PCA platform, including our program for the treatment of MS;
in-license or acquire the rights to other products, product candidates or technologies;
maintain, expand, and protect our intellectual property portfolio;
hire additional personnel in research, manufacturing, and regulatory and clinical development, as well as management personnel; and
expand our operational, financial, and management systems and increase personnel, including personnel to support our operations as a public company.
As a result, we expect to incur substantial operating losses and negative operating cash flows for the foreseeable future.
Based on our current operating plan, weWe believe that the net proceeds from our IPO of $79.2 million, together with our existing cash, cash equivalents, and short-term investments,marketable securities will enable us to fund our operating expenses and capital expenditure requirements into 2022.2025. We have based this estimate on assumptions that may prove to be incorrect, and we could utilize our available capital resources sooner than we expect.
Because of the numerous risks and uncertainties associated with the research, development, and commercialization of therapeutics, it is difficult to estimate with certainty the amount of our working capital requirements. Our future funding requirements will depend on many factors, including:
the progress, costs, and results of our clinical development and clinical trials for FX-322;
the progress, costs, and results of our additional research and preclinical development programs, including our program for the treatment ofremyelination in MS;
the outcome, timing and cost of meeting regulatory requirements established by the FDA and comparable foreign regulatory authorities, if applicable, for our product candidates;
business and operations interruptions resulting from public health emergencies;
our ability to establish and maintain strategic collaborations, licensing or other agreements and the financial terms of such agreements;
the scope, progress, results, and costs of any product candidates that we may derive from our PCA platformapproach or any other product candidates we may develop alone or with collaborators;
the extent to which we in-license or acquire rights to other products, product candidates, or technologies;
additions or departures of key scientific or management personnel;
the costs and timing of preparing, filing, and prosecuting patent applications, maintaining and protecting our intellectual property rights, and defending against any intellectual property-related claims; and
the costs and timing of future commercialization activities, including product manufacturing, marketing, sales, and distribution for any product candidates for which we or our collaborators obtain marketing approval.
Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our cash needs through a combination of public or private equity or debt financings and other sources, which may include current and new collaborations with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, yourour existing stockholders’ ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect yourthe rights as aof our common stockholder.stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take
31
specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through other sources, such as collaboration agreements, strategic alliances, licensing arrangements or marketing and distribution arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, product development, and research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce, or terminate our product development or future commercialization efforts or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves.
Contractual obligations and commitments
There have been no material changes from the contractual commitments and obligations previously disclosed in our final prospectus, dated October 2, 2019, filed with the SEC on October 4, 2019 pursuant to Rule 424(b) relating to our Registration Statement on Form S-1 (File No. 333-233652).:
|
| Payments Due by Period |
| |||||||||||||||||
|
| Total |
|
| Less Than 1 Year |
|
| 1 - 3 Years |
|
| 3 - 5 Years |
|
| More Than 5 years |
| |||||
Operating lease obligation(1) |
| $ | 2,740 |
|
| $ | 505 |
|
| $ | 1,716 |
|
| $ | 249 |
|
| $ | — |
|
|
|
We have not included future milestone payments under our collaboration and license agreements in the table above since the payment obligations under these agreements are contingent upon future events, such as the achievement of specified product development milestones or generating product sales, and we are unable to estimate the timing or likelihood of achieving these milestones or generating future product sales. We are also required to spend certain minimum amounts on research and development of licensed products or processes under the MIT License, and may have certain funding obligations under the Scripps option agreement, which are not included in the table above.
We also enter into contracts in the normal course of business with CROs, CMOs, universities, and other third parties for preclinical research studies, clinical trials and testing and manufacturing services. These contracts do not contain minimum purchase commitments and are cancelable by us upon prior written notice. Payments due upon cancellation consist only of payments for services provided or expenses incurred, including noncancelable obligations of our service providers, up to the date of cancellation. These payments are not included in the table above as the amount and timing of such payments are not known.
Critical accounting policies and use of estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our unaudited consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events, and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in more detail in the notes to our unaudited consolidated financial statements included elsewhere in this Quarterly Report on Form 10-Q. During the three months ended September 30, 2019,March 31, 2023, there were no material changes to our critical accounting policies from those described in our final prospectus dated October 2, 2019 for our IPOAnnual Report on Form 10-K filed with the SEC on October 4, 2019 pursuant to Rule 424(b)(4) relating to our Registration Statement on Form S-1 (File No. 333-233652).March 10, 2023.
Recent accounting pronouncements
A description of recent accounting pronouncements that may potentially impact our financial position, results of operations, or cash flows is disclosed in Note 2 to our unaudited consolidated financial statements included elsewhere in this Quarterly Report on Form 10-Q.
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Emerging growth company status
The Jumpstart Our Business Startups Act of 2012 permits an “emerging growth company,” such as us, to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have elected to take advantage of this extended transition period.
32
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
WeThis item is not required as we are exposed to market risks in the ordinary course of our business. These risks primarily include interest rate sensitivities. Our interest-earning assets consist of cash, cash equivalents and short-term investments, which are denominated in U.S. dollars. We had cash, cash equivalents and short-term investments of $165.3 million, or 96.7% of our total assets, at September 30, 2019. Interest income earned on these assets was $0.8 million for the nine months ended September 30, 2019. Our interest income is sensitive to changes in the general level of interest rates, primarily U.S. interest rates. Such interest-earning instruments carrycurrently considered a degree of interest rate risk; however, a change by 10% in interest rates would not have a material impact on our financial position or results of operations. We had no debt outstanding as of September 30, 2019.small reporting company.
Item 4. Controls and Procedures.
Limitations on Effectiveness of Controls and Procedures
Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their control objectives.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Vice President of Finance and Operations (our principal executive officer and principal financial officer, respectively), evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of September 30, 2019.March 31, 2023. Based on thethat evaluation, of our disclosure controls and procedures as of September 30, 2019, our Chief Executive Officer and Vice President of Finance and Operations concluded that, as of such date,March 31, 2023, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15(d)-15(f) under the Exchange Act) that occurred during the period covered by this Quarterly Report on Form 10-Q that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
33
We are not subject to any material legal proceedings.
See Note 15, “Commitments and contingencies – Legal Contingencies”, for more information.
Item 1A. Risk Factors.
Our future operating results could differ materially from the results described in this Quarterly Report on Form 10-Q due to the risks and uncertainties described below. You should consider carefully the following information about risks below in evaluating our business. If any of the following risks actually occur, our business, financial conditions, results of operations and future growth prospects would likely be materially and adversely affected. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operationsoperations. In these circumstances, the market price of our common stock would likely decline. In addition, we cannot assure investors that our assumptions and expectations will prove to be correct. Important factors could cause our actual results to differ materially from those indicated or implied by forward-looking statements. See “Forward Looking Statements” for a discussion of some of the forward-looking statements that are qualified by these risk factors. Factors that could cause or contribute to such differences include those factors discussed below.
Risks related to our financial position and need for additional capital
We have incurred significant losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline.
We are a clinical-stagepreclinical-stage biotechnology company with a limited operating history. As a result, we are not profitable and have incurred significant losses since our formation. We had net losses of $20.2 million, $19.2$19.5 million and $13.3$81.6 million for the yearsthree months ended March 31, 2023 and the year ended December 31, 2017 and 2018 and the nine months ended September 30, 2019,2022, respectively. As of September 30, 2019,March 31, 2023, we had an accumulated deficit of $63.4$281.2 million. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to gain regulatory approval and become commercially viable. We have not commercialized any products and have never generated revenue from the commercialization of any product. To date, we have devoted most of our financial resources to licensing technologies and research and development, including our preclinical platform development activities and clinical trials.
We expect to incur significant additional operating losses for the next several years, at least, as we advance FX-322a potential therapeutic candidate for multiple sclerosis, or MS, and any other product candidate through clinical development, complete clinical trials, seek regulatory approval and commercialize FX-322an MS product candidate or any other product candidate, if approved. The costs of advancing product candidates into each clinical phase tend to increase substantially over the duration of the clinical development process. Therefore, the total costs to advance any product candidate to marketing approval in even a single jurisdiction are substantial. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to begin generating revenue from the commercialization of any product candidates or achieve or maintain profitability. Our expenses will also increase substantially if and as we:
continue to develop and commence clinical trials of our Phase 2a trial of FX-322remyelination program in sensorineural hearing loss, or SNHL;
expand our development programs based on our progenitor cell activation, or PCA, platform, including our program for a treatment for multiple sclerosis, or MS, and develop other product candidates;
continue to develop our PCA platform;
seek regulatory approvals for FX-322an MS product candidate and any other product candidates;
expand the target indications and patient population for FX-322;
secure a commercial manufacturing source and supply chain capacity sufficient to produce commercial quantities of any product candidate for which we obtain regulatory approval;
establish a sales, marketing and distribution infrastructure to commercialize FX-322 for the treatment of SNHL,an MS product candidate, if approved, and for any other product candidates for which we may obtain marketing approval;
maintain, expand, and protect our intellectual property portfolio;
andhire additional clinical, scientific, and commercial personnel;
add operational, financial, and management personnel, including personnel to support our product development and planned future commercialization efforts, as well as to support operations as a public company; and
acquire or in-license other product candidates or technologies.
34
Furthermore, our ability to successfully develop, commercialize and license any product candidates and generate product revenue is subject to substantial additional risks and uncertainties, as described under “—Risks related to development, clinical testing, manufacturing, and regulatory approval” and “—Risks related to commercialization.” As a result, we expect to continue to incur net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. The amount of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If we are unable to develop and commercialize one or more product candidates, either alone or through collaborations, or if revenues from any product that receives marketing approval are insufficient, we will not achieve profitability. Even if we successfully commercialize FX-322,an MS product candidate or any other product candidates, we may continue to incur substantial research and development and other expenses to identify and develop other product candidates. Even if we do achieve profitability, we may not be able to sustain profitability or meet outside expectations for our profitability. If we are unable to achieve or sustain profitability or to meet outside expectations for our profitability, the value of our common stock will be materially adversely affected.
We will require additional capital to fund our operations, and if we fail to obtain necessary financing, we may not be able to complete the development and commercialization of FX-322 andan MS product candidate or explore additional product candidates.
We expect to spend substantial amounts to complete the development of, seek regulatory approvals for and, if approved, commercialize FX-322.an MS product candidate and any other product candidates. These expenditures include and will include, as the case may be, preclinical development costs and costs related to the Phase 2a trial of FX-322 for the treatment of SNHL, and, if the Phase 2a trial is supportive, a planned Phase 2b trial of FX-322, and any additional trials we conduct to support the development of FX-322. In addition, we are obligated to make milestonean MS product candidate and royalty payments in connection with the sale of resulting products and licensing revenues under our license agreements with Massachusetts Institute of Technology, or MIT, and the Scripps Research Institute, or Scripps. We also expect to spend substantial amounts to identify and develop newany other product candidates based on our PCA platform.candidates.
We will require additional capital to enable us to develop additionalan MS product candidates based on our PCA platform,candidate, which we may acquire through equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or other sources. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative effect on our financial condition and our ability to pursue our business strategy. In addition, attempting to secure additional financing may divert the time and attention of our management from day-to-day activities and harm our development efforts.
Based upon our current operating plan, and as a result of the realignment of the Company to focus on the MS development program, including cost saving measures such as our reduction in force during the first half of 2023, we believe that our existing cash, and cash equivalents, including theand marketable securities of $66.7 million, $55.0 million net proceeds from our IPO of $79.2 million, and short-term investmentsdebt, will enable us to fund our operating expenses and capital expenditure requirements into 2022.2025. This estimate and our expectation regarding the sufficiency of our current financial resources to advance the clinical development of FX-322an MS product candidate and any other product candidates are based on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect, or our clinical trials, includingplanned Phase 1 study in our Phase 2a trial of FX-322program for the treatment of SNHL,remyelination in MS may be more expensive, time consuming or difficult to design or implement than we currently anticipate. Changing circumstances, including any unanticipated expenses, could cause us to consume capital significantly faster than we currently anticipate, and we may need to spend more than currently expected because of circumstances beyond our control. Because the length of time and scope of activities associated with successful development of FX-322an MS product candidate or any product candidate we may develop is highly uncertain, we are unable to estimate the actual funds we will require for development and any marketing and commercialization activities. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
the initiation, progress, timing, costs and results of our clinical trials through all phases of development, including the planned Phase 2a trial for FX-3221 study in our MS development program, and the development of any other product candidates;
the outcome, timing and cost of meeting regulatory requirements established by the U.S. Food and Drug Administration, or the FDA, and other comparable foreign regulatory authorities, including any additional clinical trials required by the FDA or other comparable foreign regulatory authorities;
the willingness of the FDA and other comparable foreign regulatory authorities to accept our clinical trial designs, as well as data from our completed and planned clinical trials and preclinical studies, as the basis for review and approval of FX-322an MS product candidate and any other product candidates;
the cost of filing, prosecuting, defending, and enforcing our patent claims and other intellectual property rights;
the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us;
the effect of competing technological and market developments;
35
the cost and timing of completion of commercial-scale manufacturing activities;
the costs of operating as a public company;
the cost of making royalty, milestone or other payments under current and any future in-license agreements;
the extent to which we in-license or acquire other product candidates or technologies;
the cost of establishing sales, marketing and distribution capabilities for our product candidates, if approved;
andour ability to maintain our collaboration with Astellas Pharma Inc., or Astellas on favorable terms and establish new collaborations; and
the initiation, progress, and timing of our commercialization of FX-322,an MS product candidate, if approved, or anyour other product candidate.
Depending on our business performance, the economic climate and market conditions, we may be unable to raise additional funds through any sources. Market volatility resulting from the COVID-19 global pandemic, international geopolitical conflict, global supply chain issues, and increased inflation could also adversely impact our ability to access capital as and when needed. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of FX-322an MS product candidate or any other product candidate,candidates, or potentially discontinue operations.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, asAdditionally, we can generate substantial revenues, we may financemaintain the majority of our cash needs through a combinationand cash equivalents in accounts with major U.S. and multi-national financial institutions, and our deposits at certain of equity offerings, debt financings, marketingthese institutions exceed insured limits. Market conditions can impact the viability of these institutions. In the event of failure of any of the financial institutions where we maintain our cash and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or other sources. We do not currently have any committed external source of funds. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believecash equivalents, there can be no assurance that we have sufficientwould be able to access uninsured funds for our currentin a timely manner or future operating plans.
To the extent that we raise additional capital through the sale of equityat all. Any inability to access or convertible debt securities, our existing stockholders’ ownership interests will be diluted, and the terms ofdelay in accessing these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. In addition, debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, intellectual property, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. Furthermore, any capital raising efforts may divert our management from their day-to-day activities, which maycould adversely affect our ability to advance research programs, product development activities or product candidates. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate product candidate development or future commercialization efforts.business and financial position.
We have a limited operating history and no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
We were established and began operations in 2014. Our operations to date have been limited to financing and staffing our company, licensing technologies, developing our PCA platform,approach, developing and conducting preclinical and clinical studies of FX-322 for the treatment of SNHL, and developing a pipeline of preclinical and research programs, including our remyelination program for the treatment ofin MS. We have not yet demonstrated the ability to successfully complete a large-scale, pivotal clinical trial, obtain marketing approval, manufacture a commercial-scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
In addition, as a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown challenges. Our FX-322 Phase 2b results (FX-322-208), for example, showed no statistically significant difference at day 90 between those administered FX-322 versus those receiving placebo in the proportion of individuals that demonstrated an improvement in speech perception. We will eventually need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition and, as a result, our business may be adversely affected.
As we continue to build our business, we expect our financial condition and operating results may fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, the results of any particular quarterly or annual period are not necessarily indicative of future operating performance.
Our ability to use our net operating loss carryforwards to offset future taxable income, or tax credit carryforwards to offset future income tax liabilities, may be subject to certain limitations.
As of December 31, 2018,2022, we had net operating loss carryforwards, or NOLs, of $39.3$174.1 million for federal income tax purposes and $31.7$141.3 million for state income tax purposes, which may be available to offset our future taxable income, if any. Our NOLs begin to expire in various amounts in 2036,through 2042, provided that federal NOLs generated in taxable years beginning after December 31, 2017 will not be subject to expiration. As of December 31, 2018,2022, we also had federal and state research and development and other tax credit carryforwards of approximately $0.7$8.2 million and $0.4$3.6 million, respectively, available to reduce future income tax liabilities. Our tax credit carryforwards expire at various dates through 2038.2042. These NOLs and tax credit carryforwards could expire unused, to the extent subject to expiration, and be unavailable to offset future taxable income or income tax liabilities.liabilities, as applicable. In addition, in general, under Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to use its pre-change NOLs and tax credit carryforwards to offset future taxable income. For these purposes, an ownership change generally occurs where the aggregate stock ownership of one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points
36
over its lowest ownership percentage within a specified testing period. Similar rules may apply under state tax laws. We believe we have experienced an ownership changechanges in the past,2017 and 2019 and may experience ownership changes in the future as a result of future transactions in our stock, some of which may be outside our control. As a result of the ownership changes in 2017 and 2019, $0.01 million and $0.04 million of NOL carryforwards are limited under Section 382 of the Code. If we undergo anotheradditional ownership change,changes, our ability to use our NOLs and tax credit carryforwards could be further limited. For these reasons, we may not be able to use a material portion of our NOLs or tax credit carryforwards, even if we attain profitability. Furthermore, federal NOLs generated in taxable years beginning after December 31, 2017 may be carried forward indefinitely but may only be used to offset 80% of our taxable income in years beginning after December 31, 2020, which may require us to pay federal income taxes in future years despite generating federal NOLs in prior years. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future tax benefitsbenefit of such assets. The reduction of the corporate tax rate under the Tax Cuts and Jobs Act of 2017, or TCJA, from 35% to 21% may cause a reduction in the economic benefit of our NOLs and other deferred tax assets available to us. Furthermore, under the TCJA, although the treatment of tax losses generated before December 31, 2017 has generally not changed, tax losses generated in calendar year 2018 and beyond will only be able to offset 80% of taxable income. This change may require us to pay federal income taxes in future years despite generating a loss for federal income tax purposes in prior years.
Risks related to development, clinical testing, manufacturing, and regulatory approval
We are heavily dependent on the success of FX-322, our lead product candidate,MS development program, which is still under clinical development, and if FX-322an MS product candidate does not receive regulatory approval or is not successfully commercialized, our business will be materially adversely harmed.
To date, we have invested a significant portion of our efforts and financial resources in the development of FX-322 for the treatment of SNHL. We recently discontinued our FX-322 and FX-345 development programs following the results of our FX-322 Phase 2b study which showed no statistically significant difference at day 90 between those administered FX-322 versus those receiving placebo in the proportion of individuals that demonstrated an improvement in speech perception. Our future success is substantially dependent on our ability to successfully complete clinical development for, obtain regulatory approval for, and successfully commercialize FX-322,an MS product candidate, which may never occur. We currently have no products that are approved for commercial sale and may never be able to develop a marketable product. We expect that a substantial portion of our efforts and expenditures over the next few years will be devoted to FX-322,our MS development program, which will require additional clinical development, management of clinical and manufacturing activities, regulatory approval, establishing commercial scale manufacturing, and significant sales, marketing, and distribution efforts before we can generate any revenues from any commercial sales. We cannot be certain that we will be able to successfully complete any of these activities or that, even if it receives regulatory approval, FX-322a remyelinating therapeutic will be as effective as anticipated at treating SNHL.MS.
The research, testing, manufacturing, labeling, approval, sale, packaging, marketing, and distribution of drug products are subject to extensive regulation by the FDA and comparable regulatory authorities in other countries. We are not permitted to market FX-322 in the United Statesan MS product candidate until we receive regulatory approval of a New Drug Application, or NDA, from the FDA or in any foreign countries until our collaborator, Astellas, receives the requisite approval from such countries. We have not submitted an NDA to the FDA and Astellas has not submitted comparable applications to other regulatory authorities for FX-322. We or Astellasin other countries, and we may not be in a position to do so for several years, if ever. If we or Astellas are unable to obtain the necessarynever receive such regulatory approval for FX-322 in a particular country, we or Astellas will not be able to commercialize FX-322 for the treatment of SNHL in that country.approval. As a result, our financial position will be materially adversely affected, and we may not be able to generate sufficient revenue to continue our business.
We utilize our PCA platformapproach to develop product candidates that are designed to activate progenitor cells, which is a new approach to therapeutic intervention and, as a result, successful development, approval, and commercialization of ourany product candidates, including FX-322,an MS product candidate, is uncertain.
We utilize our PCA platformapproach to develop product candidates, including FX-322, for the treatment of SNHL.in our MS development program. Our PCA platformapproach is designed to identify pathways to activate progenitor cells already present in the body to treat conditions or diseases through cellular regeneration. We have not, nor to our knowledge has any other company, received regulatory approval utilizing this mechanism of cellular regeneration. Given the novelty of our approach, we could encounter a longer than expected regulatory review process, increased development costs, or unexpected delays in, or even prevention of, the regulatory approval and commercialization of our product candidates, and we cannot be certain that our approach will lead to the development of any approvable or marketable products. For example, the FDA-approved treatment options available for patients with SNHL are hearing aids and cochlear implants. Unlike FX-322, which is a therapeutic that targets the underlying biology of SNHL, these treatment options are medical devices that are designed to target the symptoms of SNHL. As a result, these treatment options are not directly comparable to FX-322, and FDA requirements for marketing authorization of these treatment options may not be relevant for FX-322. While we are developing what we believe are appropriate measurements of efficacy for FX-322, we cannot be certain that the FDA will agree with our measurements or that they will be sufficient for approval. If we were to encounter any of the foregoing, our business and financial prospects could be materially harmed.
Clinical trials are expensive, time consuming, and difficult to design and implement, and involve an uncertain outcome. The results of preclinical studies and early clinical trials are not always predictive of future results. Any product candidate that we advance into clinical trials may not achieve favorable results in later clinical trials, if any, or receive marketing approval.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and completed clinical trials are not necessarily predictive of future results, and any product candidates we develop may not be further developed or may have favorableadditional unfavorable results in later studies or trials. Clinical trial failure may result from a multitude of factors, including, but not limited to, flaws in study design, dose selection, placebo effect, patientsubject enrollment criteria, selection of patientssubjects based
37
on patientsubject misrepresentations, and failure to demonstrate favorable safety or efficacy traits. As such, failure in clinical trials can occur at any stage of testing. A number of companiestesting, which may results in the pharmaceutical industry have suffered setbacks in the advancement of their drug candidates intodevelopment or a determination to no longer pursue a particular product candidate or indication. For example, later-stage clinical trials duein our hearing program failed to lack of efficacy or adverse safety profiles, notwithstanding favorablemeet their primary end points despite promising results infrom earlier preclinical studies or clinical trials. Basedtrials and, as a result, we ended our hearing program. Further, based upon negative or inconclusive results or a need for additional information, we may decide, or regulatory authorities may require us, to conduct additional clinical trials or preclinical studies.
We may experience delays in initiating and completing any clinical trials that we intend to conduct, and we do not know whether our clinical trials will begin on time, need to be redesigned, enroll patientssubjects on time, or be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays related to:
the FDA or comparable foreign regulatory authorities disagreeing as to the design or implementation of our clinical studies;
obtaining regulatory approval to commence a trial;
reaching an agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
obtaining Institutional Review Board, or IRB, approval at each site within the United States, or Independent Ethics Committee, or IEC, approval at sites outside the United States;
business interruptions resulting from public health emergencies, such as the COVID-19 pandemic;
having patientssubjects complete a trial or return for post-treatment follow-up;
imposition of a clinical hold by regulatory authorities, including as a result of unforeseen safety issues or side effects or failure of trial sites or investigators to adhere to regulatory requirements or follow trial protocols;
clinical sites deviating from the trial protocol or dropping out of a trial;
addressing patientsubject safety concerns that arise during the course of a trial;
adding a sufficient number of clinical trial sites; or
manufacturing sufficient quantities of a product candidate for use in clinical trials.
We could also encounter delays if a clinical trial is suspended or terminated by us, the IRBs or IECs of the institutions in which such trials are being conducted, the FDA or other regulatory authorities, or recommended for termination by a Data and Safety Monitoring Board, or DSMB, for such trial. Such authorities may impose a suspension or termination or recommend an alteration due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions, or lack of adequate funding to continue the clinical trial.
Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and, while we have agreements governing their committed activities, we have limited influence over their actual performance, as described in the section titled “—Risks related to our dependence on third parties.”
Our lead product candidate, FX-322,MS development program is still in development and will require the successful completion of one, and possibly more, Phase 3several trials before we are prepared to submit an NDA for regulatory approval by the FDA. We cannot predict with any certainty if or when we might complete the development of FX-322 and submit an NDA for regulatory approval by the FDA of FX-322 or whether any such NDA will be approved by the FDA.
If we experience delays in the commencement or completion of any clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of any product candidate we develop could be harmed, and our ability to generate revenues may be delayed. In addition, any delays in our clinical trials could increase our costs, slow the development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may materially harm our business, financial condition, and results of operations. In addition, many of the factors that may cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA. The FDA may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of a clinical trial. The FDA may therefore question the integrity of the data generated at the applicable clinical trial site, and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of marketing approval of a product candidate.38
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time- consuming, and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for FX-322an MS product candidate, or any other product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during a product candidate’s clinical development and may vary among jurisdictions. The approval process may also be delayed by changes in government regulation, future legislation or administrative action. We have not obtained regulatory approval for any product candidate and it is possible that we will never obtain regulatory approval for any product candidate. We are not permitted to market any of our product candidates in the United States until we receive approval of an NDA from the FDA.
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, we must demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or comparable foreign regulatory authority, that such product candidates are safe and effective for their intended uses. In addition, data obtained from preclinical trials and clinical trials are susceptible to varying interpretations, and regulatory authorities may not interpret our data as favorably as we do, which may further delay, limit, or prevent development efforts, clinical trials, or marketing approval. Furthermore, as more competing drug candidates within a particular class of drugs proceed through clinical development to regulatory review and approval, the amount and type of clinical data that may be required by regulatory authorities may increase or change. Even if we believe the preclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other comparable regulatory authorities.
The FDA or any foreign regulatory authority can delay, limit, or deny approval of FX-322 or any otheran MS product candidatescandidate that we develop or require us to conduct additional preclinical or clinical testing or abandon a program for many reasons, including:
the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials;
we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication;
serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates, or other products containing an active ingredient in our product candidates;
negative or ambiguous results from our clinical trials or results that may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval;
we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials;
the data collected from clinical trials of our product candidates may not be acceptable or sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere, and we may be required to conduct additional clinical trials;
the FDA’s or the applicable foreign regulatory authority’s disagreement regarding the formulation, the labeling, and/or the specifications of our product candidates;
the FDA or comparable foreign regulatory authorities may fail to approve or find deficiencies with the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and
the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
Of the large number of drugs in development, only a small percentage successfully complete the regulatory approval processes and are commercialized. This lengthy approval process, as well as the unpredictability of future clinical trial
39
results, may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations, and prospects.
In addition, the FDA or the applicable foreign regulatory authority also may approve a product candidate for a more limited indication or patient population than we originally requested, and the FDA or applicable foreign regulatory authority may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing circumstances could materially harm the commercial prospects for our product candidates and our business.
Enrollment and retention of patientsindividuals in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patientssubjects who remain in the study until its conclusion. We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patientssubjects to complete any of our clinical trials, and even once enrolled, we may be unable to retain a sufficient number of patientssubjects to complete any of our trials.
PatientSubject enrollment and retention in clinical trials depends on many factors, including:
the patientsubject eligibility criteria defined in the protocol;
the size of the patientsubject population required for analysis of the trial’s primary endpoints;
the nature of the trial protocol;
the existing body of safety and efficacy data with respect to the product candidate;
the proximity of patientssubjects to clinical sites;
our ability to recruit clinical trial investigators with the appropriate competencies, motivation and experience;
clinicians’ and patients’subjects’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs or medical devices that may be approved for the indications we are investigating;
competing clinical trials being conducted by other companies or institutions;
our ability to obtain and maintain patientsubject consents; and
the risk that patientssubjects enrolled in clinical trials will drop out of the trials before completion.
In addition, our clinical trials will compete with other clinical trials for product candidates and medical devices that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patientssubjects available to us, because some patientspeople who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Furthermore, any negative results we may report in clinical trials of any product candidate may make it difficult or impossible to recruit and retain patientspeople in other clinical trials of that same product candidate. Delays or failures in planned patientsubject enrollment or retention may result in increased costs or program delays, which could have a harmful effect on our ability to develop a product candidate or could render further development impossible.
Results of preclinical studies, clinical trials, or analyses may not be indicative of results that may be obtained in later trials.trials or preclinical studies.
The results of preclinical studies, clinical trials, or analyses of the results from such trials including our prospective and post hoc analyses of the data from the Phase 1/2 trial of FX-322 for the treatment of SNHL, may not be predictive of the results of later preclinical studies or clinical trials. Product candidates in later clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and prior clinical trials or having shown promising results based on analyses of data from earlier trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding earlier promising results. Our FX-322 Phase 2b results (FX-322-208), for example, showed no statistically significant difference at day 90 between those administered FX-322 versus those receiving placebo in the proportion of individuals that demonstrated an improvement in speech perception. In addition, conclusions based on promising data from analyses of clinical results, such as the prospective and post hoc analysis of resultsdata from our Phase 1/2 clinical trial of FX-322 for the treatment of SNHL (FX-322-201), may be shown to be incorrect in subsequent clinical trials that have pre-specified end points or may not be considered adequate by regulatory authorities. Further, we have in the past and may in the future abandon product candidates that we initially advanced for development based on positive preclinical results due to
40
unfavorable results from additional preclinical studies. For example, we recently discontinued our FX-322 and FX-345 development programs after the results of the FX-322-208 clinical trial. Even if we complete later clinical trials as planned, we cannot be certain that their results will support the safety and efficacy requirements sufficient to obtain regulatory approval, and, as a result, our clinical development plans may be materially harmed.
Interim and preliminary “top-line” data from our clinical trials that we announce or publish from time to time may change as more patientsubject data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publishpublicly disclose interim, top-line or preliminary “top-line” data from our clinical studies.trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the top-line or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Top-line or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the top-line or preliminary data we previously published. As a result, top-line and preliminary data should be viewed with caution until the final data are available.
From time to time, we may also disclose interim data from our preclinical studies and clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patientsubject enrollment continues and more patientsubject data become available. PreliminaryAdverse differences between interim data also remain subject to audit and verification procedures that mayfinal data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the final data being materially different from the preliminary data previously published. In addition, we may report interim or preliminary analysesprice of only certain endpoints rather than all endpoints. Furthermore, the information we choose to publicly disclose regarding a particular study or clinical trial is based on more extensive information, and you orour common stock.
Further, others, may not agree with what we determine is the material or otherwise appropriate information to disclose. Any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities, or otherwise regarding a particular drug, drug candidate, or our business. Others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular programs,program, the approvability or commercialization of the particular product candidates,candidate or product and our businesscompany in general. As a result, interim and preliminary data and analyses should be viewed with caution. Adverse differences between preliminary or interim data and final data or changes in what is materialIn addition, the information we choose to publicly disclose regarding the results from a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If the interim, top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could significantly harm our clinical development and business, operating results, prospects and cause volatility in the priceor financial condition.
Any of our common stock.
Anyproduct candidates or component of a product candidate that we develop or the administration thereof, may cause serious adverse events or undesirable side effects, which may halt their clinical development, delay or prevent marketing approval, or, if approved, require them to be taken off the market, include safety warnings, or otherwise limit their sales.
Serious adverse events or undesirable side effects caused by anyour product candidates or component of a product candidate we develop could cause us or regulatory authorities to interrupt, delay, or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. Results of any clinical trial we conduct could reveal a high and unacceptable severity and prevalence of side effects. To date, subjects treated with FX-322 have experienced adverse events that include ear discomfort and ear pain that are considered to be associated with the intratympanic injection procedure.
If unacceptable side effects arise in the development of any product candidate, we, the FDA, or the IRBs or IECs at the institutions in which our studies are conducted, or the DSMB, if constituted for our clinical trials, could recommend a suspension or termination of our clinical trials, or the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of a product candidate for any or all targeted indications. In addition, drug-related side effects could affect patientsubject recruitment or the ability of enrolled patientssubjects to complete a trial or result in potential product liability claims. In addition, theseThese side effects also may not be appropriately recognized or managed by the treating medical staff. We may have to train medical personnel regarding the proper administration protocol for our product candidates and to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patientsubject injury or death. Any of these occurrences may harm our business, financial condition, and prospects significantly.
Additionally, if FX-322an MS product candidate or any other product candidatecandidates we develop receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
41
regulatory authorities may suspend, withdraw, or limit approvals of such product, or seek an injunction against its manufacture or distribution;
regulatory authorities may require us to recall a product or we may decide to initiate a voluntary recall of a product;
regulatory authorities may require additional warnings on the label, such as a “black box” warning or contraindication;
additional restrictions may be imposed on the marketing of the product or the manufacturing processes for the product or any component thereof;
we may be required to implement a Risk Evaluation and Mitigation Strategy, or REMS, or create a medication guide outlining the risks of such side effects for distribution to patients;
we may be required to conduct post-market studies or agree to post marketing commitments;
we could be sued and held liable for harm caused to patients;
the product may become less competitive; and
our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of a product candidate, if approved, and could significantly harm our business, results of operations, and prospects.
Changes in funding for
Disruptions at the FDA and other government agencies caused by funding shortages, changes in the federal administration or global health concerns could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical employees and stop critical activities. If a prolonged government shutdown occurs or the FDA experiences other delays, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Separately, in response to the COVID-19 pandemic, the FDA postponed most inspections of foreign and domestic manufacturing facilities at various points. Even though the FDA has since resumed standard inspection operations of domestic facilities where feasible, the FDA has continued to monitor and implement changes to its inspectional activities to ensure the safety of its employees and those of the firms it regulates as it adapts to the evolving COVID-19 pandemic, and any resurgence of the virus or emergence of new variants may lead to further inspectional delays.
Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic or issue guidance materially affecting the conduct of clinical trials. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns or delays could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
We may not be successful in our efforts to identify additional product candidates. Due to our limited resources and access to capital, we must prioritize development of certain product candidates, the choice of which may prove to be wrong and adversely affect our business.
Although we intend to explore additional product candidates based on our PCA platform,approach, we may fail to identify viable new product candidates for clinical development for several reasons. If we fail to identify additional potential product candidates, our business could be materially harmed.
42
Research programs to develop additional product candidates based on our PCA platformapproach require substantial technical, financial, and human resources whether or not they are ultimately successful. Our research programs may initially show promise in identifying potential indications or product candidates, yet fail to yield results for clinical development for several reasons, including:
the research methodology used may not be successful in identifying potential indications or product candidates;
potential product candidates may, after further study, be shown to have harmful or unexpected adverse effects or other characteristics that indicate they are unlikely to be effective drugs; or
it may take greater human and financial resources than we possess to identify additional therapeutic opportunities for our product candidates or to develop suitable potential product candidates through internal research programs, thereby limiting our ability to develop, diversify, and expand our product portfolio.
Because we have limited financial and human resources, we intend to initially focus on research programs and product candidates for a limited set of indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that could have greater commercial potential or a greater likelihood of success. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities.
Accordingly, there can be no assurance that we will ever be able to identify additional therapeutic opportunities for our product candidates or to develop suitable potential product candidates through internal research programs, which could materially adversely affect our future growth and prospects. For example, we may encounter delays in the process of selecting a product candidate for the treatment of MS and we may not achieve the time linetimeline we currently anticipate for submitting an IND.IND or comparable foreign equivalent. We may focus our efforts and resources on potential product candidates or other potential programs that ultimately prove to be unsuccessful.
The market opportunities for FX-322, if approved, may be smaller than we anticipate and, as a result, our commercial opportunity may be limited.
We expect to initially seek approval of FX-322 for the treatment of SNHL. Our projections of the number of eligible patients are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, patient foundations, and market research, and may prove to be incorrect. Further, new sources may reveal a change in the estimated number of eligible patients, and the number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our current programs or future product candidates may be limited. For example, even if we obtain FDA approval for FX-322, it may be approved for a target population that is more limited than what we currently anticipate. Even if we obtain significant market share for any product candidate, if approved, if the potential target populations are smaller, we may never achieve profitability without obtaining marketing approval for additional indications.
We have never obtained marketing approval for a product candidate and we may be unable to obtain, or may be delayed in obtaining, marketing approval for any product candidate.
We have never obtained marketing approval for a product candidate. It is possible that the FDA may refuse to accept for substantive review any NDAs that we submit for our product candidates or may conclude after review of our data that our applications are insufficient to obtain marketing approval of our product candidates. We believe our approach of activating progenitor cells to treat conditions or diseases through cellular regeneration is novel and, as a result, the process for, and the outcome of, FDA approval is especially uncertain. If the FDA does not accept or approve our NDAs for our product candidates, it may require that we conduct additional clinical, preclinical, or manufacturing validation studies and submit that data before it will reconsider our applications. Depending on the extent of these or any other FDA-required studies, approval of any NDA that we submit may be delayed or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve our NDAs.
Any delay in obtaining, or an inability to obtain, marketing approvals would prevent us from commercializing our product candidates, generating revenues, and achieving and sustaining profitability. If any of these outcomes occur, we may be forced to abandon our development efforts for our product candidates, which could significantly harm our business.
Even if we obtain FDA approval for a product candidate in the United States, we or our collaborators may never obtain approval for or commercialize the product candidate in any other jurisdiction, which would limit our ability to realize its full market potential.
In order to market any product in a particular jurisdiction, we or our collaborators must establish and comply with numerous and varying regulatory requirements regarding safety and efficacy on a country-by-country basis. Approval by the FDA in the United States does not ensure approval by comparable regulatory authorities in other countries or jurisdictions. However, the failure to obtain approval in one jurisdiction may negatively impact our or our collaborators’ ability to obtain approval elsewhere. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country.
Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and increased costs for us and require additional preclinical studies or clinical trials which could be costly and time- consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our products in those countries. We do not have any product candidates approved for sale in any jurisdiction, including in international markets, and we do not have experience in obtaining regulatory approval in international markets. If we or our collaborators fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are
43
delayed, our target market will be reduced and we will be unable to realize the full market potential of any product we develop.
Even if we obtain regulatory approval for any product candidate, we will still face extensive and ongoing regulatory requirements and obligations, which may result in significant additional expense, and any product candidates, if approved, may face future development and regulatory difficulties.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, packaging, distribution, adverse event reporting, storage, recordkeeping, export, import, and advertising and promotional activities for such product, among other things, will be subject to extensive and ongoing requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, establishment registration and drug listing requirements, continued compliance with current Good Manufacturing Practice, or cGMP, requirements relating to manufacturing, quality control, quality assurance, and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping and Good Clinical Practice, or GCP, and requirements for any clinical trials that we conduct post-approval.
Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product candidate may be marketed or to the conditions of approval, including a requirement to implement a REMS. If a product candidate receives marketing approval, the accompanying label may limit the approved indicated use of the product, which could limit sales of the product. The FDA may also require costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use, and if we market our products for uses beyond their approved indications, we may be subject to enforcement action for off-label marketing. Violations of the Federal Food, Drug, and Cosmetic Act, or FDCA, relating to the promotion of prescription drugs, may lead to FDA enforcement actions and investigations alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers, or manufacturing processes or failure to comply with regulatory requirements, may yield various results, including:
restrictions on manufacturing such products;
restrictions on the labeling or marketing of products;
restrictions on product distribution or use;
requirements to conduct post-marketing studies or clinical trials;
warning letters or untitled letters;
refusal to approve pending applications or supplements to approved applications that we submit;
recalls or market withdrawals of products;
fines, restitution, or disgorgement of profits or revenues;
suspension or withdrawal of marketing approvals;
refusal to permit the import or export of our products;
product seizure; or
injunctions, consent decrees, or the imposition of civil or criminal penalties.
Further, the FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of a product candidate. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects, and ability to achieve or sustain profitability.
We also cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. The policies of the FDA and of other comparable regulatory authorities may change and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of a product candidate. For example, certain policies of the current presidential administration may impact our business and industry. Namely, the current presidential administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. These executive actions and other policies of the current administration may impact the FDA’s ability to exercise its regulatory authority, though the extent to which they will impact the development of FX-322 or any other product candidates we develop is unknown. If these executive actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained,be subject to
44
enforcement action, and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition, and results of operations. Furthermore, noncompliance by us or any collaborator with regulatory requirements, including safety monitoring or pharmacovigilance, may also result in significant financial penalties, which would adversely affect our business.
We may seek Fast Track designation by the FDA for our product candidates, but we might not receive such a designation and even if we do, such designation may not lead to a faster development or regulatory review or approval process.
In October 2019, FX-322 received Fast Track designation by the FDA. If a drug is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address an unmet medical need for this condition, a drug sponsor may qualify for FDA Fast Track designation. Fast Track designation provides increased opportunities for sponsor meetings with the FDA during preclinical and clinical development, in addition to the potential for rolling review once a marketing application is filed. The FDA has broad discretion whether to grant Fast Track designation, and we may not receive such a designation for all of the product candidates for which we may request it. Moreover, even if we receive Fast Track designation, Fast Track designation does not ensure that we will receive marketing approval or that approval will be granted within any particular time frame. We may not experience a faster development or regulatory review or approval process with Fast Track designation compared to conventional FDA procedures. In addition, the FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast Track designation alone does not guarantee qualification for the FDA’s priority review procedures.
We may seek a Breakthrough Therapy designation for our product candidates, but we might not receive such designation, and even if we do, such designation may not lead to a faster development or regulatory review or approval process.
We may seek a Breakthrough Therapy designation for FX-322 if future results support such designation or other product candidates we may develop. A Breakthrough Therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs designated as breakthrough therapies by the FDA may also be eligible for priority review if supported by clinical data at the time the NDA is submitted to the FDA.
Designation as a Breakthrough Therapy is within the discretion of the FDA. Accordingly, even if we believe that a product candidate meets the criteria for designation as a Breakthrough Therapy, the FDA may disagree and instead determine not to make such a designation. Even if we receive Breakthrough Therapy designation, the receipt of such designation may not result in a faster development or regulatory review or approval process compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if a product candidate qualifies as a Breakthrough Therapy, the FDA may later decide that it no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Potential product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
The use of any product candidate we may develop in clinical trials and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated adverse effects. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
impairment of our business reputation and significant negative media attention;
withdrawal of participants from our clinical trials;
significant costs to defend the litigation;
distraction of management’s attention from our primary business;
substantial monetary awards to patients or other claimants;
inability to commercialize a product candidate;
product recalls, withdrawals or labeling, marketing or promotional restrictions;
decreased market demand for any product; and
loss of revenue.
The product liability insurance we currently carry, and any additional product liability insurance coverage we acquire in the future, may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any product candidate, we intend to acquire insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. A successful product liability claim, or series of claims, brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operation and business, including preventing or limiting the commercialization of any product candidates we develop.
The COVID-19 pandemic has caused and could continue to cause disruptions to our business, including our preclinical studies, clinical trials and operations and could adversely impact our financial condition and results of operations.
The global outbreak of COVID-19 continues to rapidly evolve and continues to have indeterminable adverse effects on general commercial activity and the world economy. Due to the uncertain nature of the effects of the outbreak, particularly in the United States, enrollment, participation and retention in our planned trials may be reduced, and for a number of the clinical sites, halted for an unknown period of time. Any reduction in enrollment, participation and retention and any halts may delay our planned clinical trials and our development plans for an MS product candidate and any other product candidates, which could have an adverse impact on our business and results of operations.
If COVID-19 or its variants again spread in the United States and worldwide, and measures to mitigate the ongoing effects of the pandemic, such as stay home orders and/or advisories persist or are reintroduced, we may continue to experience disruptions and other effects on our business that could severely impact our business, operations, preclinical studies and clinical trials, including:
45
The extent to which COVID-19 may continue to impact our business, preclinical studies, planned clinical trials and operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence. In addition, if we or any of the third parties with whom we engage were to experience shutdowns or additional business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted, which could have a material adverse effect on our business and our financial results. The COVID-19 pandemic has resulted in a widespread health crisis that has adversely affected the economies and financial markets worldwide, resulting in an economic downturn that could continue to significantly impact our business, financial condition and results of operations. To the extent the COVID-19 pandemic adversely affects our business, financial condition and results of operations, it may also have the effect of heightening many of the other risks described in this “Risk Factors” section.
Risks related to commercialization
We face significant competition from biotechnology pharmaceutical, and medical devicepharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biotechnology pharmaceutical, and medical devicepharmaceutical industries are highly competitive and subject to significant and rapid technological change. Our success is highly dependent on our ability to acquire, develop, and obtain marketing approval for new products on a cost-effective basis and to market them successfully. If a product candidate we develop is approved, we will face intense competition from a variety of businesses, including large, fully-integratedfully integrated pharmaceutical companies, specialty pharmaceutical companies, and early-stage companies, particularly if the early-stage company has a collaborative arrangement with a large and established company. We are aware of several companies developing productsprograms with research and development efforts to treat SNHLMS through the regeneration of hair cells, and we also anticipate that new companies will enter the SNHL market in the future.myelin. If we successfully develop and, if approved, commercialize FX-322 for the treatment of SNHL,an MS product candidate, it may compete, or potentially be used in conjunction with, currently marketed devices, including the hearing aids and cochlear implants currently available and the next generation of improved hearing aids and cochlear implants,therapeutics and any new therapiestherapeutics that may become available in the future.
Competition could render any product candidate we develop obsolete, less competitive, or uneconomical. Our competitors may, among other things:
have significantly greater name recognition and financial, manufacturing, marketing, product development, technical, and human resources than we do, with mergers and acquisitions in the biotechnology pharmaceutical, and medical devicepharmaceutical industries resulting in even more resources being concentrated in our competitors;
more effectively recruit and retain qualified scientific and management personnel;
more effectively establish clinical trial sites and patientsubject registration;
46
develop and commercialize products that are safer, more effective, less expensive, more convenient, or easier to administer, or have fewer or less severe side effects;
obtain quicker regulatory approval;
better protect their patents and intellectual property or acquire technologies that are complementary to, or necessary for, our programs;
implement more effective approaches to sales, marketing, pricing, coverage, and reimbursement; or
form more advantageous strategic alliances or collaborations.
If we are not able to effectively compete for any of the foregoing reasons, our business will be materially harmed.
The successful commercialization of any product candidate we develop will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage, reimbursement levels, and pricing policies. Failure to obtain or maintain coverage and adequate reimbursement for our product candidates, if approved, could limit our or our collaborators’ ability to market those products and decrease our or our collaborators’ ability to generate revenue.
The availability and adequacy of coverage and reimbursement by governmental healthcare programs such as Medicare and Medicaid, private health insurers, and other third-party payors are essential for most patients to be able to afford prescription medications. Our ability to achieve acceptable levels of coverage and reimbursement for products or procedures using our products by governmental authorities, private health insurers and other organizations will influence our ability to successfully commercialize any product candidates we develop. Obtaining adequate coverage and reimbursement for any product candidate we develop that is administered under the supervision of a physician, which is what we anticipate for FX-322, may be particularly difficult because of the higher prices associated with such products. In addition, we believe that FX-322 is a novel approach to treating hearing loss and, as a result, availability of coverage and reimbursement by payors is highly uncertain. A decision by a third-party payor not to cover or separately reimburse for our products or procedures using our products could reduce physician utilization of our products once approved. Assuming we obtain coverage for ourany product candidates or procedures using our products by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. We cannot be sure that coverage and reimbursement in the United States or elsewhere will be available for any product we commercialize, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and the current presidential administration and Congress have introduced several proposals related to drug pricing. Many third-party payors may refuse to provide coverage and reimbursement for particular drugs or biologics when an equivalent generic drug, biosimilar, or a less expensive therapy is available. Although there are currently no FDA approved drugs for the treatment of SNHL,MS through the regeneration of myelin, it is possible that a third-party payor may consider FX-322such a drug as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy, pricing of existing drugs may limit the amount we will be able to charge for any product we commercialize. Payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable us to realize a satisfactory return on our investment in our product candidates. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on our product candidates. Additionally, our ability to obtain a satisfactory financial return depends on what, if any, proposals related to drug pricing may be implemented and, if implemented, when they might take effect.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models in the United States for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
No uniform policy for coverage and reimbursement for products exists among third-party payors in the United States. Therefore, coverage and reimbursement for products can differ significantly from payor to payor, and one third-party payor’s decision to cover a particular product does not ensure that other payors will also provide similar coverage. Additionally, the process for determining whether a third-party payor will provide coverage for a product is typically separate from the process for setting the price of such product or establishing the reimbursement rate that the payor will pay for the product once coverage is approved. As a result, the determination of coverage and reimbursement is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
47
Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely.
Moreover, increasing efforts by governmental and third-party payors in the United States to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for any product we commercialize. We expect to experience pricing pressures in connection with the sale of ourany product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations, and additional legislative, administrative, or regulatory changes. The downward pressure on healthcare costs in general, particularly prescription drugs and biologics and surgical procedures and other treatments, has become intense. As a result, increasingly high barriers are being erected to the entry of new products.
We or our collaborators may also be subject to extensive governmental price controls and other market regulations outside of the United States, and we believe the increasing emphasis on cost-containment initiatives in other countries have and will continue to put pressure on the pricing and usage of medical products. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we or our collaborators are able to charge for products we or our collaborators commercialize. Accordingly, in markets outside the United States, the reimbursement for products we or our collaborators commercialize may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Even if a product candidate we develop receives marketing approval, it may fail to achieve market acceptance by physicians, patients, third-party payors, or others in the medical community necessary for commercial success.
If a product candidate we develop receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors, and others in the medical community. For example, current MS treatments are well established in the medical community, and physicians may continue to rely on these treatments to the exclusion of ours. In addition, physicians, patients, and third-party payors may prefer other novel products to ours. If ita product candidate does not achieve an adequate level of acceptance, we may not generate significant product revenues or become profitable. The degree of market acceptance of our product candidates, if approved, will depend on several factors, including, but not limited to:
the efficacy and potential advantages compared to alternative treatments;
effectiveness of sales and marketing efforts;
the cost of treatment in relation to alternative treatments, including any similar generic treatments;
our ability to offer our products for sale at competitive prices;
the convenience and ease of administration compared to alternative treatments;
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
the strength of marketing and distribution support;
the availability of third-party coverage and adequate reimbursement;
the prevalence and severity of any side effects; and
any restrictions on the use of our product together with other medications.
Because we expect sales of our product candidates, if approved, to generate substantially all our revenues for the foreseeable future, the failure of our product candidates to find market acceptance would harm our business and could require us to seek additional financing.
If we are unable to establish sales and marketing capabilities either on our own or in collaboration with third parties, we may not be successful in commercializing any product candidate we develop, if approved.
In order to market and successfully commercialize any product candidate we develop, if approved, we must build our sales and marketing capabilities or enter into collaborations with third parties for these services. We currently have no sales, marketing or distribution capabilities and as a company have no experience in marketing products. We intend to directly market and commercialize FX-322 for the treatment of SNHL,an MS product candidate, if approved, in the United States by developing our own sales and marketing force, targeting ear, nose, and throat doctors and audiologists. There are significant expenses and risks involved with establishing our own sales and marketing capabilities, including our ability to hire, train, retain, and appropriately incentivize a sufficient number of qualified individuals, generate sufficient sales leads and provide our sales and marketing team with adequate access to physicians who may prescribe our product, effectively manage a geographically dispersed sales and marketing team, and other unforeseen costs and expenses. Any failure or delay in the development of a product candidate that affects the expected timing of commercialization of the product candidate or results in the failure of the product candidate to be commercialized could result in us having prematurely or unnecessarily incurred costly commercialization expenses. Our investment would be lost if we are unable to retain or reposition our sales and marketing personnel.
We may also enterentering into collaborations for the sales and marketing of our product candidates, if approved. To the extent that we depend on collaborators for sales and marketing activities, any revenues we receive will depend upon the success of those collaborators’ sales and marketing teams and the collaborators’
48
prioritization of our product and compliance with applicable regulatory requirements, and there can be no assurance that the collaborators’ efforts will be successful. For example, under the License and Collaboration Agreement with Astellas, or the Astellas Agreement, we will depend on Astellas to sell and market FX-322 for the treatment of SNHL, if approved, outside of the United States, and we can have no assurance that it will be successful in its efforts or devote sufficient resources to the sale and marketing of FX-322.
If we are unable to build our own sales and marketing team or enter into a collaboration for the commercialization of product candidates we develop, if approved, we may be forced to delay the commercialization of our product candidates or reduce the scope of our sales or marketing activities, which would have an adverse effect on our business, operating results and prospects.
A variety of risks associated with operating internationally could materially adversely affect our business.
Our business strategy includes potentially expanding internationally if any of our product candidates receive regulatory approval. Doing business internationally involves several risks, including, but not limited to:
multiple, conflicting, and changing laws and regulations, such as data privacy and security laws and regulations, tax laws, export and import restrictions, economic sanctions laws and regulations, employment laws, regulatory requirements, and other governmental approvals, permits, and licenses;
failure by us to obtain and maintain regulatory approvals for the use of our products in various countries;
additional potentially relevant third-party patent rights;
complexities and difficulties in obtaining protection and enforcing our intellectual property;
difficulties in staffing and managing foreign operations;
complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems;
limits in our ability to penetrate international markets;
financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products, and exposure to foreign currency exchange rate fluctuations;
natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions;
certain expenses, including, among others, expenses for travel, translation, and insurance; and
regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, its books and records provisions, or its anti-bribery provisions, as well as other applicable laws and regulations prohibiting bribery and corruption.
Any of these factors could significantly harm any future international expansion and operations and, consequently, our results of operations.
Risks related to our dependence on third parties
The Astellas Agreement is important to our business. If we or Astellas fail to adequately perform under the Astellas Agreement, or if we or Astellas terminate the Astellas Agreement, the development and commercialization of FX-322 for SNHL outside the United States would be materially delayed and our business would be adversely affected.
Under the Astellas Agreement, Astellas is responsible for the development and commercialization of FX-322 outside of the United States and we are responsible for development and commercialization in the United States. We and Astellas are jointly responsible for conducting global clinical studies and coordinating commercial launch activities. We have received an upfront payment from Astellas of $80.0 million and we may also receive up to an additional $545.0 million based on development and commercial milestones, as well as double-digit royalties on any future product sales in the licensed territory.
Termination of the Astellas Agreement could cause significant delays in our development and commercialization efforts for FX-322 for the treatment of SNHL outside of the United States. If the Astellas Agreement is terminated, we would need to expand our internal capabilities or enter into another agreement to compensate for the loss in funding and clinical development support from Astellas. Any suitable alternative agreement would take considerable time to negotiate and could also be on less favorable terms to us. Whether or not we identify another suitable collaborator, we may need to seek additional financing to continue the development of FX-322, or we may be forced to discontinue development of FX-322, either of which could have a material adverse effect on our business.
We intend to continue to collaborate with third parties for the development and commercialization of ourany product candidates. We may not succeed in establishing and maintaining collaborations, which may significantly limit our ability to successfully develop and commercialize our other product candidates, if at all.
We have entered into the Astellas Agreement for the development and commercialization of FX-322 for the treatment of SNHL outside the United States and may seek collaborations for the development and commercialization of otherany product candidates. The process of establishing and maintaining collaborative relationships is difficult, time-consuming, and involves significant uncertainty, such as:
a collaborator may shift its priorities and resources away from our product candidates due to a change in business strategies, or a merger, acquisition, sale, or downsizing;
a collaborator may seek to renegotiate or terminate its relationships with us due to unsatisfactory clinical results, manufacturing issues, a change in business strategy, a change of control or other reasons;
a collaborator may cease development in therapeutic areas which are the subject of our collaboration;
a collaborator may not devote sufficient capital or resources towards our product candidates, or may fail to comply with applicable regulatory requirements;
49
a collaborator may change the success criteria for a product candidate, thereby delaying or ceasing development of such candidate;
a significant delay in initiation of certain development activities by a collaborator will also delay payment of milestones tied to such activities, thereby impacting our ability to fund our own activities;
a collaborator could develop a product that competes, either directly or indirectly, with our product candidate;
a collaborator with commercialization obligations may not commit sufficient financial resources or personnel to the marketing, distribution, or sale of a product;
a collaborator with manufacturing responsibilities may encounter regulatory, resource, or quality issues and be unable to meet demand requirements;
a collaborator may terminate a strategic alliance;
a dispute may arise between us and a collaborator concerning the research, development, or commercialization of a product candidate resulting in a delay in milestones or royalty payments or termination of the relationship and possibly resulting in costly litigation or arbitration, which may divert management’s attention and resources; and
a collaborator may use our products or technology in such a way as to invite litigation from a third party.
If any collaborator fails to fulfill its responsibilities in a timely manner, or at all, our research, clinical development, manufacturing, or commercialization efforts related to that collaboration could be delayed or terminated, or it may be necessary for us to assume responsibility for expenses or activities that would otherwise have been the responsibility of our collaborator. If we are unable to establish and maintain collaborations on acceptable terms or to successfully transition away from terminated collaborations, we may have to delay or discontinue further development of one or more of our product candidates, undertake development and commercialization activities at our own expense, or find alternative sources of capital, which would have a material adverse impact on our clinical development plans and business.
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization of our product candidates, could engage in misconduct, including intentional, reckless, or negligent conduct or unauthorized activities that violate applicable laws, rules, and regulations including: the laws and regulations of the FDA or other similar regulatory requirements of other authorities, including those laws that require the reporting of true, complete, and accurate information to such authorities; manufacturing standards; data privacy, security, fraud and abuse, and other healthcare laws and regulations; or laws that require the reporting of true, complete, and accurate financial information and data. Specifically, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Activities subject to these or other laws could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, creation of fraudulent data in preclinical studies or clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Additionally, we are subject to the risk that a person or government agency could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us or them and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal, and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid, other U.S. federal healthcare programs or healthcare programs in other jurisdictions, individual imprisonment, other sanctions, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations.
50
We currently intend to rely on third-party contract manufacturing organizations, or CMOs, for the production of clinical supply of FX-322 and intend to rely on CMOs for the production of commercial supply of FX-322, if approved, and for clinical and commercial supply of our future product candidates, as well as to supply raw materials necessary to produce our product candidates. Our dependence on CMOs may impair the development of our product candidates and may impair their commercialization, which would adversely impact our business and financial position.
We do not own facilities for manufacturing FX-322 or any product candidate. Instead, we rely on and expect to continue to rely on CMOs for the supply of cGMP grade clinical trial materials of FX-322 and any product candidates we develop and, in future, for commercial quantities. Reliance on CMOs may expose us to more risk than if we were to manufacture our product candidates ourselves. If any CMO we engage is unable to provide sufficient supply of any product candidate we develop, we may be unable to arrange for an alternative supply or to do so on commercially reasonable terms or in a timely manner, which could delay any clinical trials, the commercial launch of our product candidates, if approved, or, regarding any commercial supply, result in a shortage in supply that could negatively impact our revenues. For example, we are substantially dependent on the CMO that supplies us with the proprietary glycogen synthase kinase 3, or GSK3, inhibitor that is a key component of FX-322 and the CMO that lyophilizes FX-322 into a powder. While there are other CMOs who are able to supply the GSK3 inhibitor or lyophilize FX-322, manufacture of the GSK3 inhibitor and the lyophilization process require proprietary knowledge or specialized capabilities that only a limited number of CMOs have. As a result, transitioning to a new CMO for either the supply of the GSK3 inhibitor or to conduct the lyophilization process would be particularly time consuming and costly. We have not engaged any other CMOs as back-up for the manufacture and supply of FX-322. As a result, if any of the CMOs involved in the manufacture and supply of FX-322 experience a delay or disruption, we may not have sufficient quantities of FX-322 for our planned activities and may not be able to transition to a new CMO in a timely or cost-effective manner, or at all, which would negatively impact our ability to develop and potentially commercialize FX-322.
The facilities used to manufacture ourany product candidates we develop must be inspected by the FDA and comparable foreign regulatory authorities. While we provide oversight of manufacturing activities, we do not and will not control the execution of manufacturing activities by, and are or will be dependent on, our CMOs for compliance with cGMP requirements for the manufacture of ourany product candidates. As a result, we are subject to the risk that ourany product candidates may have manufacturing defects that we have limited ability to prevent. If a CMO cannot successfully manufacture material that conforms to our specifications and the regulatory requirements, we will not be able to secure or maintain regulatory approval for the use of our product candidates in clinical trials, or for commercial distribution of ourany product candidates, if approved. While we have engaged independent auditors to assess the compliance with the protocol that we co-developed with our CMOs regarding the manufacturing process for FX-322, in general, weWe have limited control over the ability of our CMOs to maintain adequate quality control, quality assurance, and qualified personnel, and we were not involved in developing our CMOs’ policies and procedures.
If the FDA or comparable foreign regulatory authority finds deficiencies with or does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval or finds deficiencies in the future, we may need to find alternative manufacturing facilities, which would delay our development program and significantly impact our ability to develop, obtain regulatory approval for, or commercialize our product candidates, if approved. In addition, any failure to achieve and maintain compliance with laws, regulations, and standards related to manufacturing could subject us to risks, including the risk that we may have to suspend the manufacture of our product candidates, that obtained approvals could be revoked, and that the FDA or another governmental regulatory authority may take enforcement actions, including untitled letters, warning letters, seizures, injunctions, or product recalls. Furthermore, CMOs may breach existing agreements they have with us because of factors beyond our control. They may also terminate or refuse to renew their agreement at a time that is costly or otherwise inconvenient for us. If we were unable to find an adequate CMO or another acceptable solution in time, our clinical trials could be delayed, or our commercial activities could be harmed.
We contract for the supply of the active pharmaceutical ingredient, or API, and other raw material necessary to produce FX-322 and we may contract in the future for the supply of API and other raw material for any other product candidatecandidates we develop. Supplies of API or other raw material could be interrupted from time to time and we cannot be certain that alternative supplies could be obtained within a reasonable time frame, at an acceptable cost, or at all. The extent to which the COVID-19 pandemic impacts our ability to procure sufficient supplies for the development of our products and product candidates will depend on the severity and duration of the spread of the virus, and the actions undertaken to mitigate the spread of COVID-19 or treat its effects and may cause delays. In addition, a disruption in the supply of API or other raw material could delay the commercial launch of our product candidates, if approved, or result in a shortage in supply, which would impair our ability to generate revenues. Growth in the costs and expenses of API or other raw material may also impair our ability to cost-effectively manufacture our product candidates. In addition, there may be a limited number of suppliers for API or other raw material that we may use to manufacture our product candidates, and we cannot be certain that we will be able to engage such suppliers in a timely manner or at all. If we are unable to do so, clinical development of our product candidates, commercialization for any approved product, or our business could be adversely affected.
Finding new CMOs or third-party suppliers involves additional cost and requires our management’s time and focus. In addition, there is typically a transition period when a new CMO commences work. Although we have not, and do not intend to, begin a clinical trial unless we believe we have on hand, or will be able to obtain, a sufficient supply of our product candidates to complete the clinical trial, any significant delay in the supply of our product candidates or the raw materials needed to produce our product candidates, could considerably delay conducting our clinical trials and potential regulatory approval of our product candidates.
As part of their manufacture of our product candidates, our CMOs and third-party suppliers are expected to comply with and respect the proprietary rights of others. If a CMO or third-party supplier fails to acquire the proper licenses or otherwise infringes the proprietary rights of others in the course of providing services to us, we may have to find alternative
51
CMOs or third-party suppliers or defend against claims of infringement, either of which would significantly impact our ability to develop, obtain regulatory approval for, or commercialize our product candidates, if approved.
We intend to rely on third parties to conduct, supervise, and monitor our clinical trials. If those third parties do not successfully carry out their contractual duties, or if they perform in an unsatisfactory manner, it may harm our business.
We rely,have relied, and will continue to rely, on CROs, CRO-contracted vendors, and clinical trial sites to ensure the proper and timely conduct of our clinical trials, including our planned Phase 2a trial1 study in our MS development program, and any future clinical trials of FX-322 for the treatment of SNHL.other product candidates. Our reliance on CROs and clinical trial sites for clinical development activities limits our control over these activities and we were not involved in developing our CRO’stheir policies and procedures, but we remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol and legal, regulatory, and scientific standards.
We and our CROs will be required to comply with the Good Laboratory Practice requirements for our preclinical studies and GCP requirements for our clinical trials, which are regulations and guidelines enforced by the FDA and are also required by comparable foreign regulatory authorities. Regulatory authorities enforce GCP requirements through periodic inspections of trial sponsors, principal investigators, and clinical trial sites. If we or our CROs fail to comply with GCP requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. Accordingly, if our CROs fail to comply with these requirements, we may be required to repeat clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and we do not control whether they devote sufficient time and resources to our clinical trials. Our CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities, which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs do not successfully carry out their contractual duties or obligations, or fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationship with any CROs terminates, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management’s time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. While the COVID-19 pandemic and government measures taken in response have had a significant impact on our CROs and their ability to conduct clinical trials, there is potential they will face disruption in the future, which may affect our ability to initiate and complete our clinical trials. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition, and prospects.
Risks related to healthcare laws and other legal compliance matters
Enacted and future healthcare legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates, if approved, and may affect the prices we may set.
In the United States and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes, and additional proposed changes, to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of health care. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, was enacted, which substantially changed the way healthcare is financed by both governmental and private insurers. Among the provisions of the ACA, those of greatest importance to the biotechnology and pharmaceutical industries include the following:
52
an annual, non-deductible fee payable by any entity that manufactures or imports certain branded prescription drugs and biologic agents;
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs and biologics that are inhaled, infused, instilled, implanted, or injected;
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and
establishment of a Center for Medicare and Medicaid Innovation at the Centers for Medicare & Medicaid Services, or CMS, to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
Since its enactment, there have been judicial challenges to certain aspects of the ACA, and we expect there will be additional challengesACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA inbrought by several states without specifically ruling on the future. For example, in December 2018, a U.S. District Court judge in the Northern District of Texas ruled that the individual mandate is a critical and inseverable featureconstitutionality of the ACA, and therefore, because it was modified as partACA. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of the TCJA, the remaining provisions ofobtaining health insurance coverage through the ACA are invalid as well. While the current presidential administration, Department of Healthmarketplace. The executive order also instructed certain governmental agencies to review and Human Services,reconsider their existing policies and CMS have indicatedrules that the ruling will have no immediate effect, itlimit access to healthcare. It is unclear how this decision, subsequent appeals, or other efforts to repeal and amend some or all aspects of the ACAhealthcare reform measures, if any, will impact the law. Additionally, the current presidential administration will likely continue to seek to modify, repeal, use executive actions to change the implementation of or otherwise invalidate all or certain provisions of the ACA. This includes enactment of the TCJA which, amongour business.
In addition, other things, modified penalties for not complying with the ACA’s individual mandate to carry health insurance. The extent to which any such changes may impact our business or financial condition is uncertain.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example,In March 2021, the Budget ControlAmerican Rescue Plan Act of 2011 resulted in aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2027 unless additional action is taken by Congress. In January 2013, the American Taxpayer Relief Act of 20122021 was signed into law, which, among other things, further reduced Medicare payments to several typeseliminated the statutory cap on drug manufacturers’ Medicaid Drug Rebate Program rebate liability, effective January 1, 2024. Under current law enacted as part of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statuteACA, drug manufacturers’ Medicaid Drug Rebate Program rebate liability is capped at 100% of limitations periodthe average manufacturer price for the government to recover overpayments to providers from three to five years.a covered outpatient drug. These new laws or any other similar laws introduced in the future may result in additional reductions in Medicare and other healthcare funding which could negativelyand otherwise affect our potential customers and accordingly, our financial operations.the prices we may obtain.
Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been administration efforts, Congressional inquiries and proposed federal and state legislation designed to bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient assistance programs and reform government program reimbursement methodologies for drugs. For example, the current presidential administration’s fiscal year 2019 budget proposal contains further drug price control measures which could be implemented in future legislation or through rulemakings or administrative or executive actions. These measures include, for example, allowing some states to negotiate drug prices under Medicaid and eliminating cost sharing for generic drugs for low-income patients. Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. Most recently, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. For that and other reasons, it is currently unclear how the IRA will be effectuated. We expect that additional U.S. federal healthcare reform measures will be implemented in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Individual states in the United States have also become increasingly aggressiveactive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, measures designed to encourage importation from other countries and bulk purchasing. Legally-mandatedLegally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition, and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices. These reforms could reduce the ultimate demand for our product candidates or put pressure on our product pricing.
In markets outside of the United States, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. We cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative action in the United States or any other jurisdiction. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance,
53
our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability.
Our business operations and current and future relationships with contractors, investigators, healthcare professionals, consultants, third-party payors, patient organizations, customers, and others will be subject to applicable healthcare regulatory laws, which could expose us to penalties.
Our business operations and current and future arrangements with contractors, investigators, healthcare professionals, consultants, third-party payors, patient organizations, and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell, and distribute our product candidates, if approved. Such laws include:
the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving, or providing any remuneration (including any kickback, bribe, or certain rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order, or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under U.S. federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. The U.S. federal Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other hand;
the U.S. federal false claims, and civil monetary penalties laws, including the civil False Claims Act, or FCA, which, among other things, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. federal government claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. A claim includes “any request or demand” for money or property presented to the federal government. In addition, pharmaceutical manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims;
the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation:
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose, among other things, specified requirements relating to privacy, security and breaches of individually identifiable health information by covered entities subject to the rule, such as health plans, healthcare clearinghouses and healthcare providers as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information. HITECH created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA, as amended by HITECH, and seek attorneys’ fees and costs associated with pursuing federal civil actions;
the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics, and medical devices;
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products;
the U.S. federal legislation commonly referred to as the Physician Payments Sunshine Act, enacted as part of the ACA, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics, and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to the government information related to certain payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician practitioners (physician assistants, nurse practitioners, clinical nurse specialists, certified nurse
54
|
analogous U.S. state laws and regulations, including: state anti-kickback and false claims laws, which may apply to our business practices, including but not limited to, research, distribution, sales, and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; and state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; and state laws governing privacy, security, and breaches of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts;
similar healthcare laws and regulations in the European Union, or EU, and other jurisdictions, including reporting requirements detailing interactions with and payments to healthcare providersproviders; and laws governing the privacy and security of personal information, such as, where applicable, the General Data Protection Regulation, or GDPR, which imposes obligations and restrictions on the collection, use, and disclosure of personal data relating to individuals located in the EU and the European Economic Area, or EEA, (including health data); and
laws and regulations prohibiting bribery and corruption such as the FCPA, which, among other things, prohibits U.S. companies and their employees and agents from authorizing, promising, offering, or providing, directly or indirectly, corrupt or improper payments or anything else of value to foreign government officials, employees of public international organizations or foreign government-owned or affiliated entities, candidates for foreign public office, and foreign political parties or officials thereof.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that some of our business activities, including our consulting agreements and other relationships with healthcare providers, some of whom receive stock or stock options as compensation for their services, could be subject to challenge under one or more of such laws. Ensuring that our current and future internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance, or case law involving applicable fraud and abuse or other healthcare laws and regulations.
If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to actions including the imposition of civil, criminal, and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid, and other federal healthcare programs, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements, or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs and imprisonment, which could affect our ability to operate our business. Further, defending against any such actions can be costly, time consuming, and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
Actual or perceived failures to comply with applicable data protection, privacy and security laws, regulations, standards and other requirements could adversely affect our business, results of operations, and financial condition.
The global data protection landscape is rapidly evolving, and we are or may become subject to numerous state, federal and foreign laws, requirements and regulations governing the collection, use, disclosure, retention, and security of personal data, such as information that we may collect in connection with clinical trials in the U.S. and abroad. Implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future, and we cannot yet determine the impact future laws, regulations, standards, or perception of their requirements may have on our business. This evolution may create uncertainty in our business, affect our ability to operate in certain jurisdictions or to collect, store, transfer use and share personal information, necessitate the acceptance of more onerous obligations in our contracts, result in liability or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase in the future. Any failure or perceived failure by us to comply with federal, state or foreign laws or regulation, our internal policies and procedures or our contracts governing our processing of personal information could result in negative publicity, government investigations and enforcement actions, claims by third parties and damage to our reputation, any of which could have a material adverse effect on our operations, financial performance and business.
55
As our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. In the U.S., HIPAA imposes, among other things, certain standards relating to the privacy, security, transmission and breach reporting of individually identifiable health information. We may obtain health information from third parties (including research institutions from which we obtain clinical trial programs, marketing, or research collaborations in the European Economic Area will subject us to the GDPR.
The GDPR applies to companies established in the EEA, as well as to companiesdata) that are subject to privacy and security requirements under HIPAA. While we do not established inbelieve that we are currently acting as a covered entity or business associate under HIPAA and thus are not directly regulated under HIPAA, any person may be prosecuted under HIPAA’s criminal provisions either directly or under aiding-and-abetting or conspiracy principles. Consequently, depending on the EEAfacts and circumstances, we could be subject to significant penalties if we violate HIPAA. Certain states have also adopted comparable privacy and security laws and regulations, some of which collectmay be more stringent than HIPAA. Such laws and use personal data in relation to (i) offering goods or services to, or (ii) monitoring the behavior of, individuals located in the EEA. If we conduct clinical trial programs in the EEA (whether the trials are conducted directly by us or through a clinical vendor or collaborator), or enter into research collaborations involving the monitoring of individuals in the EEA, or market our products to individuals in the EEA, weregulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners.
Further, we may also be or become subject to other state laws governing the GDPR. The GDPR puts in place stringent operational requirements for processorsprivacy, processing and controllersprotection of personal data, including,information. For example, the California Consumer Privacy Act, or CCPA, went into effect on January 1, 2020. The CCPA creates individual privacy rights for example, high standards for obtaining consent from individuals to process theirCalifornia consumers and increases the privacy and security obligations of entities handling certain personal data (or reliance on another appropriate legal basis), the provision of robust and detailed disclosures to individuals about how personal data is collected and processed (in a
concise, intelligible and easily accessible form), a comprehensive individual data rights regime (including access, erasure, objection, restriction, rectification and portability), maintaining a record of data processing, data export restrictions governing transfers of data from the EEA, short timelines for data breach notifications to be given to data protection regulators or supervisory authorities (and in certain cases, affected individuals) of significant data breaches, and limitations on retention of information. The GDPR also puts in place increased requirements pertaining to health data and other special categories of personal data,CCPA provides for civil penalties for violations, as well as a definitionprivate right of pseudonymized (i.e., key-coded) data.action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and many similar laws have been proposed at the federal level and in other states. Further, the California Privacy Rights Act, or CPRA, passed in California and it significantly amends the CCPA. It will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions went into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. Similar laws have passed in Virginia, Connecticut, Utah, Colorado, and Iowa and have been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. The enactment of such laws could have potentially conflicting requirements that would make compliance challenging. In the event that we are subject to or affected by HIPAA, the CCPA, the CPRA, or other domestic privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.
Our operations abroad may also be subject to increased scrutiny or attention from data protection authorities. For example, in Europe, the EU General Data Protection Regulation, or GDPR, provideswent into effect in May 2018 and imposes strict requirements for processing the personal data of individuals within the EEA. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States; in July 2020, the Court of Justice of the EU, or CJEU, limited how organizations could lawfully transfer personal data from the EU/EEA member states may establish their own lawsto the United States by invalidating the Privacy Shield for purposes of international transfers and regulations limitingimposing further restrictions on the processinguse of genetic, biometric,standard contractual clauses, or healthSCCs. In March 2022, the US and EU announced a new regulatory regime intended to replace the invalidated regulations; however, this new EU-US Data Privacy Framework has not been implemented beyond an executive order signed by President Biden on October 7, 2022 on Enhancing Safeguards for United States Signals Intelligence Activities. European court and regulatory decisions subsequent to the CJEU decision of July 16, 2020 have taken a restrictive approach to international data which could limit our ability to collect, use, and share suchtransfers. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the SCCs cannot be used, and/or start taking enforcement action, we could cause oursuffer additional costs, to increase. In addition, there are certain obligationscomplaints and/or regulatory investigations or fines, and/or if we contract third-party processorsare otherwise unable to transfer personal data between and among countries and regions in connection withwhich we operate, it could affect the processingmanner in which we provide our services, the geographical location or segregation of personal data. If our orrelevant systems and operations, and could adversely affect our collaborators’ or service providers’ privacy or data security measures failfinancial results.
Further, from January 1, 2021, companies have had to comply with the GDPR requirements,and also the United Kingdom GDPR, or the UK GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, i.e., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how United Kingdom data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. As we continue to expand into other foreign countries and jurisdictions, we may be subject to litigation, regulatory investigations, enforcement notices requiringadditional laws and regulations that may affect how we conduct business.
56
Although we work to comply with applicable laws, regulations and standards, our contractual obligations and other legal obligations, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another, and may conflict with one another or other legal obligations with which we must comply. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to change the way we use personal data,comply with such requirements or fines of upadequately address privacy and security concerns, even if unfounded, could result in additional cost and liability to 20 million Euros or up to 4% ofus, damage our total worldwide annual revenue of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, including class-action type litigation, negative publicity, reputational harmreputation, and a potential loss ofadversely affect our business and goodwill.results of operations.
We are subject to environmental, health and safety laws and regulations, and we may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities.
Our operations, including our development, testing and manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release, and disposal of and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds, and compounds that have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions.
As with other companies engaged in activities similar to ours, we face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of or exposure to hazardous or biological materials. Environmental, health and safety laws and regulations are becoming more stringent. We may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, the production efforts of our third-party manufacturers or our development efforts may be interrupted or delayed.
Risks related to our intellectual property
If we are unable to obtain, maintain, enforce and protect patent protection for our technology and product candidates or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully develop and commercialize our technology and product candidates may be adversely affected.
Our success depends in large part on our ability to obtain and maintain protection of the intellectual property we may own solely and jointly with others, or may license from others, particularly patents, in the United States and other countries with respect to any proprietary technology and product candidates we develop. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our technologies and product candidates that are important to our business and by in-licensing intellectual property related to such technologies and product candidates. If we are unable to obtain or maintain patent protection with respect to any proprietary technology or product candidate, our business, financial condition, results of operations and prospects could be materially harmed.
The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, defend, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing, and prosecution of patent applications, or to maintain, enforce, and defend the patents, covering technology that we license from third parties. Therefore, these in-licensed patents, and applications may not be prepared, filed, prosecuted, maintained, defended, and enforced in a manner consistent with the best interests of our business.
The patent position of pharmaceutical and biotechnology companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the scope of patent protection outside of the United States is uncertain and laws of foreign countries may not protect our rights to the same extent as the laws of the United States or vice versa. For example, European patent law restricts the patentability of methods of treatment of the human body more than U.S. law does. With respect to both owned and in-licensed patent rights, we cannot predict whether the patent applications we and our licensors are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors. Further, we may not be aware of all third-party intellectual property rights potentially relating to our product candidates. In
addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not published at all. Therefore, neither we nor our licensors can know with certainty whether either we or our licensors were the first to make the inventions claimed in the patents and patent applications we own or in-license now or in the future, or that either we or our licensors were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability, and commercial value of our owned and in-licensed patent rights are uncertain. Moreover, our owned and in-licensed pending and future patent applications may not result in patents being issued whichthat protect our technology and product candidates, in whole or in part, or whichthat effectively prevent others from commercializing competitive technologies and
57
products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents and our ability to obtain, protect, maintain, defend, and enforce our patent rights, narrow the scope of our patent protection and, more generally, could affect the value or narrow the scope of our patent rights.
Moreover, we or our licensors may be subject to a third-party pre-issuance submission of prior art to the United States Patent and Trademark Office, or USPTO, or become involved in opposition, derivation, revocation, reexamination, inter partesreview, post-grant review, or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or product candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. If the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Additionally, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if our owned and in-licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us, or otherwise provide us with any competitive advantage. The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our owned and in-licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and product candidates. Such proceedings also may result in substantial cost and require significant time from our management and employees, even if the eventual outcome is favorable to us. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. Furthermore, our competitors may be able to circumvent our owned or in-licensed patents by developing similar or alternative technologies or products in a non-infringing manner. As a result, our owned and in-licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing technology and products similar or identical to any of our technology and product candidates.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the naturalexpiration of a patent is generally 20 years from its earliest United States non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
If we are unable to obtain licenses from third parties on commercially reasonable terms or fail to comply with our obligations under such agreements, our business could be harmed.
It may be necessary for us to use the patented or proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license from these third parties. If we are unable to license such technology, or if we are forced to license such technology on unfavorable terms, our business could be materially harmed. If we are unable to obtain a necessary license, we may be unable to develop or commercialize the affected product candidates, which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales or an obligation on our part to pay royalties and/or other forms of compensation. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us.
If we are unable to obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may be required to expend significant time and resources to redesign our technology, product candidates, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop or commercialize the affected technology and product candidates, which could significantly harm our business, financial condition, results of operations, and prospects significantly.prospects.
58
Additionally, if we fail to comply with our obligations under license agreements, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market, or may be forced to cease developing, manufacturing or marketing, any product that is covered by these agreements or may face other penalties under such agreements. Such an occurrence could materially adversely affect the value of the product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements, or restrictions on our ability to freely assign or sublicense our rights under such agreements when it is in the interest of our business to do so, may result in our having to negotiate new or reinstated agreements with less favorable terms, cause us to lose our rights under these agreements, including our rights to important intellectual property or technology, or impede, or delay or prohibit the further development or commercialization of, one or more product candidates that rely on such agreements.
If we do not obtain patent term extension in the United States under the Hatch-Waxman Act and in foreign countries under similar legislation, thereby potentially extending the term of our marketing exclusivity for any product candidates we may develop, our business may be materially harmed.
In the United States, the patent term of a patent that covers an FDA-approved drug may be eligible for limited patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. In addition, only one patent applicable to an approved drug may be extended, and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar provisions aremay be available in Europe and certain other non-United States jurisdictions to extend the term of a patent that covers an approved drug. While, in the future, if and when our product candidates receive FDA approval, we expect to apply for patent term extensions on patents covering those product candidates, there is no guarantee that the applicable authorities will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such extensions. We may not be granted patent term extension either in the United States or in any foreign country because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than we request. If we are unable to obtain any patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following the expiration of our patent rights, and our business, financial condition, results of operations, and prospects could be materially harmed.
It is possible that we will not obtain patent term extension under the Hatch-Waxman Act for a United States patent covering any of our product candidates that we may identify even where that patent is eligible for patent term extension, or if we obtain such an extension, it may be for a shorter period than we had sought. Further, for our licensed patents, we may not have the right to control prosecution, including filing with the USPTO, of a petition for patent term extension under the Hatch-Waxman Act. Thus, if one of our licensed patents is eligible for patent term extension under the Hatch-Waxman Act, we may not be able to control whether a petition to obtain a patent term extension is filed, or obtained, from the USPTO.
Also, there are detailed rules and requirements regarding the patents that may be submitted to the FDA for listing in the Approved Drug Products with Therapeutic Equivalence Evaluations, or the Orange Book. We may be unable to obtain patents covering our product candidates that contain one or more claims that satisfy the requirements for listing in the Orange Book. Even if we submit a patent for listing in the Orange Book, the FDA may decline to list the patent, or a manufacturer of generic drugs may challenge the listing. If one of our product candidates is approved and a patent covering that product candidate is not listed in the Orange Book, a manufacturer of generic drugs would not have to provide advance notice to us of any abbreviated new drug application filed with the FDA to obtain permission to sell a generic version of such product candidate.
Changes to patent laws in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes in either the patent laws or interpretation of patent laws in the United States, including patent reform legislation such as the Leahy-Smith America Invents Act, or the Leahy-Smith Act, could increase the uncertainties and costs surrounding the prosecution of our owned and in-licensed patent applications and the maintenance, enforcement, or defense of our owned and in-licensed issued patents. The Leahy-Smith Act includes a number of significant changes to United States patent law. These changes include provisions that affect the way patent applications are prosecuted, redefine prior art, provide more efficient and cost-effective avenues for competitors to challenge the validity of patents, and enable third-party submission of prior art to the USPTO during patent prosecution, and additional procedures to attack the validity of a patent at USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith Act, the United States transitioned to a first-to-file system in which, assuming that the other statutory requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. As such, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future.
Although we or our licensors are not currently involved in any litigation, we may become involved in lawsuits to protect or
enforce our patent or other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
Competitors and other third parties may infringe, misappropriate or otherwise violate our or our licensors’ issued patents or other intellectual property. As a result, we or our licensors may need to file infringement, misappropriation or other intellectual property related claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke such parties to assert counterclaims against us alleging that we infringe, misappropriate, or otherwise violate their intellectual property. In addition, in a patent infringement proceeding, such parties could counterclaim that the patents we or our licensors have asserted are invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement.
59
Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may institute such claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). The outcome following legal assertions of invalidity and unenforceability is unpredictable.
An adverse result in any such proceeding could put one or more of our owned or in-licensed patents at risk of being invalidated or interpreted narrowly and could put any of our owned or in-licensed patent applications at risk of not yielding an issued patent. A court may also refuse to stop the third party from using the technology at issue in a proceeding on the grounds that our owned or in-licensed patents do not cover such technology. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information or trade secrets could be compromised by disclosure during this type of litigation. Any of the foregoing could allow such third parties to develop and commercialize competing technologies and products and have a material adverse impact on our business, financial condition, results of operations, and prospects.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Our defense of litigation or interference or derivation proceedings may fail and, even if successful, may result in substantial costs, and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
Third parties may initiate legal proceedings alleging that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing, misappropriating or otherwise violating the intellectual property and proprietary rights of third parties. There is considerable patent and other intellectual property litigation in the pharmaceutical and biotechnology industries. We may become party to, or threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our technology and product candidates, including interference proceedings, post grant review, inter partes review, and derivation proceedings before the USPTO and similar proceedings in foreign jurisdictions such as oppositions before the European Patent Office. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our technologies or product candidates that we may identify may be subject to claims of infringement of the patent rights of third parties.
The legal threshold for initiating litigation or contested proceedings is low, so that even lawsuits or proceedings with a low probability of success might be initiated and require significant resources to defend. Litigation and contested proceedings can also be expensive and time-consuming, and our adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can. The risks of being involved in such litigation and proceedings may increase if and as our product candidates near commercialization and as we gain the greater visibility associated with being a public company. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of merit. We may not be aware of all such intellectual property rights potentially relating to our technology and product candidates and their uses, or we may incorrectly conclude that third party intellectual property is invalid or that our activities and product candidates do not infringe such intellectual property. Thus, we do not know with certainty that our technology and product candidates, or our development and commercialization thereof, do not and will not infringe, misappropriate or otherwise violate any third party’s intellectual property.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment
60
related to the discovery, use or manufacture of the product candidates that we may identify or related to our technologies. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that the product candidates that we may develop may be found to infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Moreover, as noted above, there may be existing patents that we are not aware of or that we have incorrectly concluded are invalid or not infringed by our activities. If any third-party patents were held by a court of competent jurisdiction to cover, for example, the manufacturing process of the product candidates that we may develop, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize the product candidates that we may identify. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products, or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We may choose to take a license or, if we are found to infringe, misappropriate or otherwise violate a third party’s intellectual property rights, we could also be required to obtain a license from such third party to continue developing, manufacturing and marketing our technology and product candidates. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us and could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease developing, manufacturing and commercializing the infringing technology or product. In addition, we could be found liable for significant monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right and could be forced to indemnify our customers or collaborators. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. In addition, we may be forced to redesign our product candidates, seek new regulatory approvals, and indemnify third parties pursuant to contractual agreements. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar material adverse effect on our business, financial condition, results of operations, and prospects.
Intellectual property litigation or other legal proceedings relating to intellectual property could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and may also have an advantage in such proceedings due to their more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of intellectual property litigation or other proceedings could compromise our ability to compete in the marketplace.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance, renewal and annuity fees and various other government fees on any issued patent and pending patent application must be paid to the USPTO and foreign patent agencies in several stages or annually over the lifetime of our owned and in-licensed patents and patent applications. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we rely on our licensing partners to pay these fees to, or comply with the procedural and documentary rules of, the relevant patent agency. With respect to our patents, we rely on an annuity service, outside firms, and outside counsel to remind us of the due dates and to make payment after we instruct them to do so. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable
61
rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. Under certain circumstances, we may be unable to comply with requirements. For example, due to the sanctions imposed by the United States on Russia as a result of the conflict in Ukraine, it is not possible to pay fees on Russian patents and the future of such patents is uncertain. In such an event, potential competitors might be able to enter the market with similar or identical products or technology. If we or our licensors fail to maintain the patents and patent applications covering our product candidates, it would have a material adverse effect on our business, financial condition, results of operations, and prospects.
Changes to patent laws in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes in either the patent laws or interpretation of patent laws in the United States, including patent reform legislation such as the Leahy-Smith America Invents Act, or the Leahy-Smith Act, could increase the uncertainties and costs surrounding the prosecution of our owned and in-licensed patent applications and the maintenance, enforcement, or defense of our owned and in-licensed issued patents. The Leahy-Smith Act includes a number of significant changes to United States patent law. These changes include provisions that affect the way patent applications are prosecuted, redefine prior art, provide more efficient and cost-effective avenues for competitors to challenge the validity of patents, and enable third-party submission of prior art to the USPTO during patent prosecution, and additional procedures to attack the validity of a patent at USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith Act, the United States transitioned to a first-to-file system in which, assuming that the other statutory requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. As such, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future.
We may not be able to protect our intellectual property and proprietary rights throughout the world.
Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States, and even where such protection is nominally available, judicial and governmental enforcement of such intellectual property rights may be lacking. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection or licenses, but enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally. In addition, certain jurisdictions do not protect, to the same extent or at all, inventions that constitute new methods of treatment.
In addition, geo-political actions in the United States and in foreign countries could increase the uncertainties and costs surrounding the prosecution or maintenance of our patent applications or those of any current or future licensors and the maintenance, enforcement or defense of our issued patents or those of any current or future licensors. For example, the
62
United States and foreign government actions related to Russia’s conflict in Ukraine may limit or prevent filing, prosecution, and maintenance of patent applications in Russia. Government actions may also prevent maintenance of issued patents in Russia. These actions could result in abandonment or lapse of our patents or patent applications, resulting in partial or complete loss of patent rights in Russia. If such an event were to occur, it could have a material adverse effect on our business. In addition, a decree was adopted by the Russian government in March 2022, allowing Russian companies and individuals to exploit inventions owned by patentees from the United States without consent or compensation. Consequently, we would not be able to prevent third parties from practicing our inventions in Russia or from selling or importing products made using our inventions in and into Russia. Accordingly, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
Proceedings to enforce our intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
Finally, Europe is implementing a Unified Patent Court that may present uncertainties for our ability to protect and enforce our patent rights in Europe and the ability of third parties to do the same. In 2012, regulations were passed with the goal of providing a pan-European Unitary Patent and a new European Unified Patent Court, or UPC, for litigation involving European patents. Implementation is currently scheduled for June 1, 2023. Under the UPC, all European patents granted in countries that have ratified the UPC Agreement, including those patents issued prior to the UPC, will automatically fall under the jurisdiction of the UPC. The UPC will provide our competitors with a new forum to centrally revoke European patents, and allow for the possibility of a competitor to obtain injunctions in multiple European countries in a single UPC action. It will be several years before we will understand the scope of patent rights that will be recognized and the strength of patent remedies that will be provided by the UPC. Note that we will have the option to opt our patents out of the UPC over the first seven years of the court’s existence, but doing so may preclude us from realizing the benefits of the new unified court.
If we fail to comply with our obligations in our intellectual property licenses and funding arrangements with third parties, or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are party to license and funding agreements that impose, and we may enter into additional licensing and funding arrangements with third parties that may impose, diligence, development, and commercialization timelines, milestone payment, royalty, insurance and other obligations on us. Under our existing licensing and funding agreements, we are obligated to pay royalties on net product sales of product candidates or related technologies to the extent they are covered by the agreements. If we fail to comply with such obligations under current or future license and funding agreements, our counterparties may have the right to terminate these agreements or require us to grant them certain rights. Such an occurrence could materially adversely affect the value of any product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology, which would have a material adverse effect on our business, financial condition, results of operations, and prospects.
Disputes may arise regarding intellectual property subject to a licensing agreement, including:
the scope of rights granted under the license agreement and other interpretation related issues;
the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
the sublicensing of patent and other rights under our collaborative development relationships;
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
63
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
the priority of invention of patented technology.
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected technology and product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
Our current or future licensors may have relied on third-party consultants or collaborators or on funds from third parties such that our licensors are not the sole and exclusive owners of the patents and patent applications we in-license. If other third parties have ownership rights to patents and/or patent applications we in-license, they may be able to license such patents to our competitors, and our competitors could market competing products and technology. This could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
In spite of our best efforts, our licensors might conclude that we have materially breached our license agreements and might therefore terminate the license agreements, thereby removing our ability to develop and commercialize product candidates and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying intellectual property fails to provide the intended exclusivity, competitors would have the freedom to seek regulatory approval of, and to market, products and technologies identical to ours. This could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
We may not be able to protect our intellectual property and proprietary rights throughout the world.
Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States, and even where such protection is nominally available, judicial and governmental enforcement of such intellectual property rights may be lacking. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection or licenses but enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally. In addition, certain jurisdictions do not protect, to the same extent or at all, inventions that constitute new methods of treatment.
Proceedings to enforce our intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we or our licensors may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or our or our licensors’ ownership of our owned or in-licensed patents, trade secrets, or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our product candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be subject to claims by third parties asserting that our employees, consultants or contractors have wrongfully used or disclosed confidential information of third parties, or we have wrongfully used or disclosed alleged trade secrets of their current or former employers, or claims asserting we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees, consultants and contractors were previously employed at universities or other pharmaceutical or biotechnology companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims.
In addition, while it is our policy to require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our intellectual property assignment agreements with them may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could have a material adverse effect on our competitive business position and prospects. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products, which license may not be available on commercially reasonable terms, or at all, or such license may be non-exclusive. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our management and employees.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets and confidentiality agreements to protect our unpatented know-how, technology, and other proprietary information, to maintain our competitive position. We seek to protect our trade secrets and other proprietary technology, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. We cannot guarantee that we have entered into such agreements with each party that may have or has had access to our trade secrets or proprietary technology. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Detecting the disclosure or misappropriation of a trade secret and enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside of the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position would be materially and adversely harmed.
64
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered and unregistered trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trade names or trademarks that incorporate variations of our unregistered trade names or trademarks. Over the long term, if we are unable to successfully register our trade names and trademarks and establish name recognition based on our trade names and trademarks, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trade names and trademarks may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
we, or our license partners or current or future collaborators, might not have been the first to file patent applications covering certain of our or their inventions;
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or in-licensed intellectual property rights;
it is possible that our owned and in-licensed pending patent applications or those we may own or in-license in the future will not lead to issued patents;
issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors;
our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
we cannot ensure that any of our pending patent applications, if issued, or those of our licensors, will include claims having a scope sufficient to protect our product candidates;
we cannot ensure that any patents issued to us or our licensors will provide a basis for an exclusive market for our commercially viable product candidates or will provide us with any competitive advantages;
we cannot ensure that our commercial activities or product candidates will not infringe upon the patents of others;
we cannot ensure that we will be able to successfully commercialize our product candidates on a substantial scale, if approved, before the relevant patents that we own, or license expire;
we may not develop additional proprietary technologies that are patentable;
the patents of others may harm our business; and
we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations, and prospects.
65
Risks related to our employees, managing our growth and our operations
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
We are highly dependent on the expertise of David L. Lucchino, our President and Chief Executive Officer, as well as the other principal members of our management scientific, and clinicalscientific teams. Although we have employment agreements, offer letters or consulting agreements with our executive officers, these agreements do not prevent them from terminating their services at any time.
If we lose one or more of our executive officers or key employees, our ability to implement our business strategy successfully could be seriously harmed. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize product candidates successfully. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel on acceptable terms given the competition among numerous biotechnology and pharmaceutical companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. We may also decide not to replace an executive officer, which may have an adverse effect on our operations. For example, we do not have a Chief Financial Officer and do not currently intend to fill that position, which could adversely affect our financial reporting and operations.
In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be engaged by other companies or organizations and may have commitments that limit their availability. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize our product candidates will be limited.
Our recent reduction in force undertaken to better align our workforce with the needs of our business and focus more of our capital resources on our pre-clinical program for remyelination in MS.
In February 2023, we implemented a reduction in force affecting approximately 55% of our workforce to better align our workforce with the needs of our business and focus more of our capital resources on our pre-clinical program for remyelination in MS. We expectbelieve these changes will preserve capital, ensuring that we are appropriately resourced to expandcomplete a first clinical trial of our MS development regulatory,program. In connection with these actions, we will incur termination costs, which include severance costs and salesrelated expenses, which are estimated to be approximately $4.0 million in future cash outlays.
The reduction in force may result in unintended consequences and marketing capabilities,costs, such as the loss of institutional knowledge and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth inexpertise, attrition beyond the intended number of employees, decreased morale among our remaining employees, and the scope of our operations, particularly in the areas of clinical development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities or acquire new facilities, and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth,risk that we may not be ableachieve the anticipated benefits of the reduction in force. In addition, while positions have been eliminated certain functions necessary to effectively manage the expansion of our operations remain, and we may be unsuccessful in distributing the duties and obligations of departed employees among our remaining employees. The reduction in workforce could also make it difficult for us to pursue, or recruitprevent us from pursuing, new opportunities and traininitiatives due to insufficient personnel, or require us to incur additional qualified personnel. The expansionand unanticipated costs to hire new personnel to pursue such opportunities or initiatives. If we are unable to realize the anticipated benefits from the reduction in force, or if we experience significant adverse consequences from the reduction in force, our business, financial condition, and results of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.be materially adversely affected.
We may engage in transactions that could disrupt our business, cause dilution to our shareholders or reduce our financial resources.
In the future, we may enter into transactions to acquire or in-license rights to product candidates, products or technologies, or to acquire other businesses. If we do identify suitable candidates, we may not be able to enter into such transactions on favorable terms, or at all. Any such acquisitions or in-licenses may not strengthen our competitive position, and these transactions may be viewed negatively by analysts, investors, customers, or other third parties with whom we have relationships. We may decide to incur debt in connection with an acquisition, or in-license or issue our common stock or other equity securities as consideration for the acquisition, which would reduce the percentage ownership of our existing stockholders. We could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by the indemnification we may obtain from the sellers of the acquired business. In addition, we may not be able to successfully integrate the acquired personnel, technologies, and operations into our existing business in an effective, timely, and nondisruptive manner. Such transactions may also divert management attention from day-to-day responsibilities, increase our expenses, and reduce our cash available for operations and other uses. We cannot predict the number, timing or size of future acquisitions or in-licenses or the effect that any such transactions might have on our operating results.
66
Our business and operations would suffer in the event of security breaches or information technology system failures.
Despite the implementation of security measures, our computer systems, as well as those of our CROs and other contractors and consultants, are vulnerable to damage from computer viruses, unauthorized access, natural and manmade disasters (including hurricanes), terrorism, war, and telecommunication and electrical failures. While we do not believe that we have experienced any such system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our or their operations, it could result in delays and/or material disruptions of our research and development programs. For example, the loss of preclinical or clinical trial data from completed, ongoing, or planned trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we currently rely on third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability, and the development of our product candidates could be delayed.
Our proprietary or confidential information may be lost or we may suffer security breaches.
The U.S. federal and various state and foreign governments have enacted or proposed requirements regarding the collection, distribution, use, security and storage of personally identifiable information and other data relating to individuals. In the ordinary course of our business, we and third parties with which we have relationships will continue to collect and store sensitive data, including clinical trial data, proprietary business information, personal data and personally identifiable information of our clinical trial subjects and employees, in data centers and on networks. The secure processing, maintenance and transmission of this information is critical to our operations. Despite our and our collaborators’the implementation of security measures, our computer systems, as well as those of our CROs and other contractors and consultants, are vulnerable to attack, damage and interruption from computer viruses and malware (e.g. ransomware), malicious code, unauthorized access, malfeasance, natural and manmade disasters (including hurricanes), terrorism, war, and telecommunication, electrical failures, hacking, cyberattacks, phishing attacks and other social engineering schemes, employee theft of misuse, human error, fraud, denial or degradation of service attacks, sophisticated nation-state and nation-state-supported actors or unauthorized access or use by persons inside our organization, or persons with access to systems inside our organization.
Attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and infrastructureintensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. For instance, companies have experienced an increase in phishing and social engineering attacks from third parties in connection with COVID-19 global pandemic, and the recent hostilities between Russia and Ukraine may result in increased attacks that could either directly or indirectly impact us. We may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be vulnerableunable to attacks by hackersanticipate these techniques or internal bad actors,implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. Even if identified, we may be unable to adequately investigate or remediate incidents or breaches due to employee error, technical vulnerabilities, malfeasance,attackers increasingly using tools and techniques that are designed to circumvent controls, to avoid detection, and to remove or obfuscate forensic evidence.
We and certain of our service providers are from time to time subject to cyberattacks and security incidents. While we do not believe that our network has experienced any significant system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in delays and/or material disruptions of our research and development programs. For example, the loss of preclinical or clinical trial data from completed, ongoing, or planned trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we currently rely and intend to rely in the future on third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. If a security breach or other disruptions. A numberincident were to result in the unauthorized access to or unauthorized use, disclosure, release or other processing of proposed and enacted federal, state and international laws and regulations obligate companiespersonal information, it may be necessary to notify individuals, ofgovernmental authorities, supervisory bodies, the media and other parties pursuant to privacy and security breaches involving particular personally identifiable information, which could result from breaches experienced by us or by third parties, including collaborators, vendors, contractors, or other organizations with which we have formed strategic relationships. Although, to our knowledge, neither we nor any such third parties have experienced any material security breach, and even though we may have contractual protections with such third parties, any such breach could compromise our or their networks and the information stored therein could be accessed, publicly disclosed, lost or stolen.laws. Any such access, disclosure, notifications, follow-up actions related to such a security breach or other loss of information could result in legal claims or proceedings, liability under data protection laws, that protect the privacy of personal information, and significant costs, including regulatory penalties, fines, and legal expenses, and such an event could disrupt our operations, cause us to incur remediation costs, damage our reputation, and cause a loss of confidence in us and our or such third parties’ ability to conduct clinical trials, which could adversely affect our reputation and delay the clinical development of our product candidates.
Our employees work remotely and in a shared office with our sublessor, and we may be subject to heightened operational, confidentiality and cybersecurity risks.
Many of our employees work remotely from home at times. In addition, when in the office, our employees share an open, undivided office space with our sublessor. This subjects us to heightened operational risks. For example, technologies in our employees’ homes may not be as robust and could cause the networks, information systems, applications, and other tools available to employees to be more limited or less reliable, and we may be subject to increased cybersecurity risk which could expose us to risks of data or financial loss. In our office, there are risks that individuals accessing our shared office space who are not associated with us may have access to confidential data, including from our clinical trials. There is no guarantee that the security and privacy safeguards we will put in place both for remote work and for our shared office space will be completely effective or that we will not encounter risks associated with unauthorized access to our data and information. If any of these risks were to occur, our business and operations could be materially adversely affected.
67
Risks related to our common stock
The market price of our common stock may behas been volatile and fluctuated and may in future fluctuate substantially, which could result in substantial losses for our stockholders.
The market price of our common stock is likely to behas been highly volatile and may be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this section titled “Risk factors” and elsewhere in this prospectus,Quarterly Report on Form 10-Q, these factors include:
any delay in the enrollment or ultimate completion of the planned Phase 1 study in our Phase 2a trial of FX-322 for the treatment of SNHL;
the results of the planned Phase 2a trial1 study in our MS development program, or any future clinical trials of FX-322 for the treatment of SNHLour MS development program or clinical trials of our competitors for the same indication;
our ability to develop our remyelination program in MS, or any additional product candidates based on our PCA platform, including MS;
any delay in submitting a regulatory filing and any adverse development or perceived adverse development with respect to the regulatory review of such filing;
failure to successfully develop and commercialize FX-322 for the treatment of SNHLan MS product candidate or any future product candidate;
inability to obtain additional funding;
regulatory or legal developments in the United States and other countries applicable to our PCA platformapproach or any product candidate:
adverse regulatory decisions;
changes in the structure of healthcare payment systems;
adverse developments concerning our CMOs or CROs;
inability to obtain adequate product supply for our other product candidates, or the inability to do so at acceptable prices;
introduction of new products, services or technologies by our competitors;
our ability to effectively manage our growth;
failure to meet or exceed financial projections we provide to the public;
failure to meet or exceed the estimates and projections of the investment community;
changes in the market valuations of companies similar to us;
market conditions in the biotechnology and pharmaceutical sectors, and the issuance of new or changed securities analysts’ reports or recommendations;
announcements of significant acquisitions, strategic collaborations, joint ventures or capital commitments by us or our competitors;
the termination of a collaboration agreement, licensing agreement or other strategic arrangement, or the inability to establish additional collaboration arrangements that we need on favorable terms, or at all;
significant lawsuits and their outcomes, including patent or shareholder litigation, and disputes or other developments relating to our proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our product candidates and PCA platform;
additions or departures of key scientific or management personnel;
sales of our common stock by us or our shareholders in the future;
trading volume of our common stock; and
68
general economic, industry and market conditions.
In addition, the trading prices for common stock markets have experienced extreme price and volume fluctuations that have affected andof biopharmaceutical companies continue to affectbe highly volatile as a result of the COVID-19 pandemic and general market pricesconditions, among other reasons. The COVID-19 pandemic and fluctuations in the global economy continue to evolve and remain unpredictable. The extent to which a public health emergency, such as the COVID-19 pandemic, may impact our business and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence.
Our failure to meet the continued listing requirements of the Nasdaq Stock Market LLC, or Nasdaq, could result in a delisting of our common stock.
Our common stock is currently listed on the Nasdaq Stock Market. Nasdaq requires listed companies to meet certain listing criteria including total number of stockholders, corporate governance requirements, minimum closing bid price, total value of public float, and in some cases total stockholders’ equity securities of many companies. These fluctuations have often been unrelated or disproportionateand market capitalization requirements. If we fail to satisfy the continued listing standards, including with respect to the operating performancemaintenance of those companies. Broad marketa minimum share price, or if Nasdaq, in its discretion, determines that a condition exists that makes further dealings of our company on the exchange unwarranted, Nasdaq may issue a non-compliance letter or initiate delisting proceedings.
For example, on March 28, 2023, we received a notification letter from the Listing Qualifications Department of Nasdaq notifying us that, for the last 30 consecutive business days, the bid price for our common stock had closed below the $1.00 per share minimum bid price requirement for continued inclusion on the Nasdaq Global Select Market pursuant to Nasdaq Listing Rule 5450(a)(1), or the Bid Price Requirement. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have been provided an initial compliance period of 180 calendar days from receipt of the notification letter, or until September 25, 2023, to regain compliance with the Bid Price Requirement. To regain compliance, the closing bid price for our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days prior to September 25, 2023, unless Nasdaq exercises its discretion to extend this period pursuant to Nasdaq Listing Rule 5810(c)(3)(H). If we do not regain compliance with the Bid Price Requirement by September 25, 2023, we may be eligible for an additional 180 calendar day compliance period. There can be no assurance we will be able to regain compliance or that Nasdaq will extend the compliance period.
If for any reason our common stock does not maintain eligibility for listing on Nasdaq, we may list our common stock elsewhere, such as one of the OTC markets, which are generally considered less liquid and industry factors,more volatile than a national securities exchange, and could mean that certain institutional investors could no longer hold or purchase our stock, and as well as general economic, political, regulatory, and market conditions, may negatively affect the market pricea result, a purchaser of our common stock regardlessmay find it more difficult to dispose of, our actual operating performance.
We could be subjector to securities class action litigation.
Inobtain accurate quotations as to the past, securities class action litigation has often been brought against companies following a decline in the market price of their securities.shares. This risk is especially relevant for us because biotechnologycould materially and pharmaceutical companies have experienced significant share price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversionadversely affect the liquidity of management’s attention and resources, which could harm our business.common stock.
Our directors, executive officers and shareholders affiliated with our directors and executive officers own a significant percentage of our common stock and, if they choose to act together, will be able to exert significant influence over matters subject to shareholder approval.
Our directors, executive officers, and shareholders affiliated with our directors and executive officers exert significant influence on us. As of OctoberMarch 31, 2019,2023, these holders beneficially ownowned approximately 17.1%16.9% of the voting power of our outstanding common stock. As a result, these holders, acting together, have significant influence over all matters that require approval of our stockholders, including the election of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transactions. The interests of these holders may not always coincide with our corporate interests or the interests of other shareholders, and they may act in a manner with which our shareholders may not agree or that may not be in the best interests of our other shareholders.
If securities or industry analysts issue an adverse or misleading opinion regarding our common stock, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our clinical studies and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be the sole source of gain for our stockholders.
We have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, our loan and security agreement with Silicon Valley Bank currently prohibits us from paying dividends on our equity securities, and any future debt agreements may likewise preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be the sole source of gain on an investment in our common stock for the foreseeable future.
Sales of a substantial number of shares of our common stock, or the perception that substantial sales might occur, could cause the price of our common stock to fall.
Sales of a substantial number of shares of our common stock , or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. The shares of common stock that were sold in the initial public offering of our common stock are freely transferable without restrictions or further registration under the Securities Act of 1933, as amended, or the Securities Act, except for any shares acquired by our affiliates, as defined in Rule 144 under the Securities Act. The remaining shares of our common stock that are outstanding are restricted as a result of securities laws, lock-up agreements or other contractual restrictions that restrict, subject to certain exceptions, transfers for 180 days after the date of our initial public offering. We or certain of the underwriters of our initial public offering may release certain stockholders from the lock-up agreements or other contractual restrictions prior to the end of the 180-day period. In addition, there are shares of common stock that are either subject to outstanding options or reserved for future issuance under our existing equity incentive plans and may become eligible for future sale subject to vesting, the lock-up agreements, and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.69
Based on the shares of capital stock outstanding as of September 30, 2019, after giving effect to the conversion of shares of convertible preferred stock into 22,079,913 shares of common stock and our initial public offering, the holders of shares of our common stock are entitled to rights with respect to the registration of their shares under the Securities Act, subject to the 180-day lock-up agreements and other contractual restrictions described above Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
We will continue to incur increased costs as a result of operating as a public company, and our management will be requiredcontinue to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we no longer qualify as an emerging growth company, we will continue to incur significant legal, accounting and other expenses that we did not incur previously.expenses. The Sarbanes-Oxley Act of 2002, or SOX, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Stock Market LLC, and other applicable securities rules and regulations impose various requirements on U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our board of directors. In addition, these rules and regulations are often subject to varying interpretations, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of SOX, or Section 404, as a public company, we will beare required to furnish a report by our senior management on our internal control over financial reporting, and our independent registered public accounting firm will beis required to provide an attestation report on our internal control over financial reporting. However, while we remain an emerging growth company, weour independent registered public accounting firm will not be required to provide the attestation report. To ensure compliance with Section 404, we are engagedcontinue to engage in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction on the price of our common stock in the market due to a loss of confidence in the reliability of our financial statements. Furthermore, if we are unable to conclude that our internal control over financial reporting is effective, our investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could decline.
We are an “emerging growth company” and a “smaller reporting company” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and a “smaller reporting company” as defined under the rules promulgated under the Securities Act. As an emerging growth company and a smaller reporting company we may follow reduced disclosure requirements and do not have to make all of the disclosures that public companies that are not emerging growth companies or smaller reporting companies do. We will remain an emerging growth company until the earlier of (a) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (b) the last day of the fiscal year following the fifth anniversary of the date of the completion of our initial public offering; (c) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (d) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or SEC, which means the market value of our voting and non-voting common stock that is held by non-affiliates exceeds $700 million as of the last business day of our second fiscal quarter. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
not being required to comply with the auditor attestation requirements of Section 404;
an exemption from compliance with the requirement of the Public Company Accounting Oversight Board regarding the communication of critical audit matters in the auditor’s report on the financial statements;
progressively adding to the number of years of audited financial statements required to be included in our periodic reports; and
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation, stockholder approval of any golden parachute payments not previously approved and having to disclose the ratio of the compensation of our chief executive officer to the median compensation of our employees.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the
70
adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period for complying with new or revised accounting standards; and as a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.