UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended SeptemberJune 30, 20202021
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from __________ to ________________
Commission File Number: 000-55657
NEUROPATHIX, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 46-2645343 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
3805 Old Easton Road
Doylestown, PA18902
(Address of principal executive offices including Zip Code)
(858) 883-2642(215)695-6559
(Registrant’s telephone number, including area code)
KANNALIFE, INC.
(Former Name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| None | N/A | N/A |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
As of November 10, 2020,August 5, 2021, the issuerregistrant had 75,252,189 issued and outstanding shares of common stock, (parpar value $0.0001) outstanding.
$0.0001 per share.
| 1 |
Table of Contents
| Page | |
| PART I - FINANCIAL INFORMATION | |
| Item 1. Financial Statements | 3 |
| Unaudited Condensed Consolidated Balance Sheets | 3 |
| Unaudited Condensed Consolidated Statements of Operations | 4 |
| Unaudited Condensed Consolidated Statements of Stockholders' | 5 |
| Unaudited Condensed Consolidated Statements of Cash Flows | 6 |
| Notes to Unaudited Condensed Consolidated Financial Statements | 7 |
| Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
| Item 3. Quantitative and Qualitative Disclosures About Market Risk | |
| Item 4. Controls and Procedures | |
| PART II - OTHER INFORMATION | |
| Item 1. Legal Proceedings | |
| Item 1A. Risk Factors | |
| Item 2. Unregistered Sales of Equity Securities and Use of Proceeds | |
| Item 3. Defaults Upon Senior Securities | |
| Item 4. Mine Safety Disclosures | |
| Item 5. Other Information | |
| Item 6. Exhibits | |
| Signatures |
| 2 |
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements (unaudited)
NEUROPATHIX, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
Unaudited
| June 30, 2021 | December 31, 2020 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 51,029 | $ | 21,874 | ||||
| Prepaid expenses | 10,833 | 161,000 | ||||||
| Other receivables | 400 | 400 | ||||||
| Total Current Assets | 62,262 | 183,274 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Property and equipment, net | 81,285 | 59,266 | ||||||
| Security deposits | 17,121 | 17,121 | ||||||
| Total Non-Current Assets | 98,406 | 76,387 | ||||||
| TOTAL ASSETS | $ | 160,668 | $ | 259,661 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | 587,652 | $ | 721,308 | ||||
| Payroll and related liabilities | 424,324 | 379,241 | ||||||
| Loan payable | 900,000 | 950,000 | ||||||
| Loan payable - related party | 42,092 | 42,092 | ||||||
| Convertible notes payable, net of $65,069 debt discount | 253,931 | 198,127 | ||||||
| Convertible notes payable, net of $43,014 debt discount - related party | 106,986 | — | ||||||
| Capital lease obligations | 12,860 | 8,471 | ||||||
| Patent purchase liability | 53,135 | 53,135 | ||||||
| Due to related party, net | 25,496 | 55,258 | ||||||
| Derivative liabilities | 109,580 | 138,046 | ||||||
| Total Current Liabilities | 2,516,056 | 2,545,678 | ||||||
| LONG TERM LIABILITIES: | ||||||||
| Convertible notes payable - long term | 376,373 | 376,373 | ||||||
| Convertible notes payable - long term - related party | — | 70,000 | ||||||
| Capital lease obligation - long term | 27,194 | 19,293 | ||||||
| Patent purchase liability - long term | 204,826 | 264,826 | ||||||
| Derivative liabilities - long term | 227,904 | 178,143 | ||||||
| Total Long Term Liabilities | 836,297 | 908,635 | ||||||
| TOTAL LIABILITIES | 3,352,353 | 3,454,313 | ||||||
| Commitments and contingencies (Note 14) | — | — | ||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Preferred stock, $ par value, shares authorized | ||||||||
| Series A preferred stock, shares designated, issued and outstanding (Liquidation preference of $75,000) | — | — | ||||||
| Series B preferred stock, shares designated, issued and outstanding (Liquidation preference of $75,000) | — | — | ||||||
| Common stock, $ par value, authorized, and issued and outstanding, respectively | 8,797 | 7,767 | ||||||
| Additional paid-in capital | 11,912,576 | 9,830,944 | ||||||
| Accumulated deficit | (15,113,058 | ) | (13,033,363 | ) | ||||
| TOTAL STOCKHOLDERS' DEFICIT | (3,191,685 | ) | (3,194,652 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 160,668 | $ | 259,661 | ||||
| The accompanying notes are an integral part of these consolidated financial statements | ||||||||
| NEUROPATHIX, INC. | ||||||||
| (FORMERLY KNOWN AS KANNALIFE, INC. ) | ||||||||
| CONDENSED CONSOLIDATED BALANCE SHEETS | ||||||||
| Unaudited | ||||||||
| September 30, 2020 | December 31, 2019 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 14,450 | $ | 121,455 | ||||
| Prepaid expenses | 250,000 | 9,000 | ||||||
| Other receivables | 400 | 400 | ||||||
| Total Current Assets | 264,850 | 130,855 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Property and equipment, net | 63,299 | 75,401 | ||||||
| Security deposits | 17,121 | 17,121 | ||||||
| Total Non-Current Assets | 80,420 | 92,522 | ||||||
| TOTAL ASSETS | $ | 345,270 | $ | 223,377 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | 537,213 | $ | 389,195 | ||||
| Payroll and related liabilities | 351,797 | 243,208 | ||||||
| Loan payable | 771,456 | 620,000 | ||||||
| Loan payable - related party | 42,092 | 42,092 | ||||||
| Convertible notes payable, net of $249,244 debt discount | 147,756 | — | ||||||
| Capital lease obligations | 7,757 | 7,533 | ||||||
| Due to related party, net | 47,354 | 25,349 | ||||||
| Derivative liabilities | 186,721 | — | ||||||
| Total Current Liabilities | 2,092,146 | 1,327,377 | ||||||
| LONG TERM LIABILITIES: | ||||||||
| Loan payable - long term | 32,744 | — | ||||||
| Convertible notes payable - long term, net of $60,548 debt discount | 415,825 | 378,839 | ||||||
| Convertible notes payable - long term, net of $98,943 debt discount - related party | 51,507 | — | ||||||
| Capital lease obligation - long term | 21,974 | 27,764 | ||||||
| Derivative liabilities - long term | 467,393 | 183,451 | ||||||
| Total Long Term Liabilities | 989,443 | 590,054 | ||||||
| TOTAL LIABILITIES | 3,081,589 | 1,917,431 | ||||||
| Commitments and contingencies (Note 13) | — | — | ||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Preferred stock, $0.0001 par value, 5,000,000 shares authorized | ||||||||
| Series A preferred stock, 75 shares designated, 75 issued and outstanding (Liquidation preference of $75,000) | — | — | ||||||
| Series B preferred stock, 75 shares designated, 75 issued and outstanding (Liquidation preference of $75,000) | — | — | ||||||
| Common stock, $0.0001 par value, 200,000,000 authorized, 74,797,037 and 74,225,141 issued and outstanding, respectively | 7,480 | 7,422 | ||||||
| Additional paid-in capital | 8,729,879 | 6,794,612 | ||||||
| Accumulated deficit | (11,473,678 | ) | (8,496,088 | ) | ||||
| TOTAL STOCKHOLDERS' DEFICIT | (2,736,319 | ) | (1,694,054 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 345,270 | $ | 223,377 | ||||
| The accompanying notes are an integral part of these condensed consolidated financial statements | ||||||||
| 3 |
NEUROPATHIX, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Unaudited
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| NET REVENUES: | ||||||||||||||||
| Grant revenue | $ | — | $ | — | $ | 32,000 | — | |||||||||
| TOTAL NET REVENUES | — | — | 32,000 | — | ||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Research and development | 76,004 | 287,665 | 204,653 | 307,392 | ||||||||||||
| General and administrative | 585,415 | 1,202,307 | 1,572,604 | 1,566,414 | ||||||||||||
| TOTAL OPERATING EXPENSES | 661,419 | 1,489,972 | 1,777,257 | 1,873,806 | ||||||||||||
| LOSS FROM OPERATIONS | (661,419 | ) | (1,489,972 | ) | (1,745,257 | ) | (1,873,806 | ) | ||||||||
| OTHER INCOME (EXPENSE): | ||||||||||||||||
| Interest expense, net | (110,550 | ) | (399,204 | ) | (245,071 | ) | (614,367 | ) | ||||||||
| Change in fair value of derivative liabilities | 20,849 | 169,776 | (89,367 | ) | 121,389 | |||||||||||
| TOTAL OTHER INCOME (EXPENSE) | (89,701 | ) | (568,980 | ) | (334,438 | ) | (492,978 | ) | ||||||||
| NET LOSS BEFORE INCOME TAX | $ | (751,120 | ) | $ | (2,058,952 | ) | (2,079,695 | ) | (2,366,784 | ) | ||||||
| Income tax expense | — | — | ||||||||||||||
| NET LOSS | $ | (751,120 | ) | $ | (2,058,952 | ) | (2,079,695 | ) | (2,366,784 | ) | ||||||
| Loss per common share - basic and diluted | $ | (0.01 | ) | $ | (0.03 | ) | (0.02 | ) | (0.03 | ) | ||||||
| Weighted average common shares outstanding - basic and diluted | 87,978,445 | 74,240,141 | 85,392,456 | 74,232,600 | ||||||||||||
| The accompanying notes are an integral part of these consolidated financial statements | ||||||||||||||||
| NEUROPATHIX, INC. | ||||||||||||||||
| (FORMERLY KNOWN AS KANNALIFE, INC. ) | ||||||||||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||||||||||
| Unaudited | ||||||||||||||||
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| NET REVENUES: | ||||||||||||||||
| Grant revenue | $ | — | $ | 23,968 | $ | — | $ | 126,027 | ||||||||
| TOTAL NET REVENUES | — | 23,968 | — | 126,027 | ||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Research and development | 68,546 | 189,443 | 375,938 | 439,680 | ||||||||||||
| General and administrative | 737,123 | 601,567 | 2,303,537 | 1,511,334 | ||||||||||||
| TOTAL OPERATING EXPENSES | 805,669 | 791,010 | 2,679,475 | 1,951,014 | ||||||||||||
| LOSS FROM OPERATIONS | (805,669 | ) | (767,042 | ) | (2,679,475 | ) | (1,824,987 | ) | ||||||||
| OTHER INCOME (EXPENSE): | ||||||||||||||||
| Interest expense, net | (151,845 | ) | (17,794 | ) | (766,212 | ) | (49,754 | ) | ||||||||
| Net gains and losses recognized on marketable security | — | (301,661 | ) | — | (844,671 | ) | ||||||||||
| Change in fair value of derivative liabilities | 346,708 | — | 468,097 | — | ||||||||||||
| TOTAL OTHER INCOME (EXPENSE) | 194,863 | (319,455 | ) | (298,115 | ) | (894,425 | ) | |||||||||
| NET LOSS BEFORE INCOME TAX | $ | (610,806 | ) | $ | (1,086,497 | ) | $ | (2,977,590 | ) | $ | (2,719,412 | ) | ||||
| Income tax expense | — | — | — | — | ||||||||||||
| NET LOSS | $ | (610,806 | ) | $ | (1,086,497 | ) | $ | (2,977,590 | ) | $ | (2,719,412 | ) | ||||
| Net loss attributable to noncontrolling interests | — | (1,953 | ) | — | (6,828 | ) | ||||||||||
| Net loss attributable to Kannalife, Inc. | $ | (610,806 | ) | $ | (1,084,544 | ) | $ | (2,977,590 | ) | $ | (2,712,584 | ) | ||||
| Loss attributable to Kannalife, Inc. per common share - basic | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.04 | ) | $ | (0.04 | ) | ||||
| Loss attributable to Kannalife, Inc. per common share - diluted | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.04 | ) | $ | (0.04 | ) | ||||
| Weighted average common shares outstanding - basic | 74,258,962 | 70,934,271 | 74,241,419 | 70,218,141 | ||||||||||||
| Weighted average common shares outstanding - diluted | 74,258,962 | 70,934,271 | 74,241,419 | 70,218,141 | ||||||||||||
| The accompanying notes are an integral part of these condensed consolidated financial statements | ||||||||||||||||
NEUROPATHIX, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
Unaudited
| Series A Preferred Stock | Series B Preferred Stock | Common Stock | ||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Additional Paid-In Capital | Accumulated Deficit | Total Stockholders’ Deficit | ||||||||||||||||||||||||||||
| Balance at December 31, 2019 | 75 | $ | — | 75 | $ | — | 74,225,141 | $ | 7,422 | $ | 6,794,612 | $ | (8,496,088 | ) | $ | (1,694,054 | ) | |||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (307,832 | ) | (307,832 | ) | |||||||||||||||||||||||||
| Balance at March 31, 2020 | 75 | $ | — | 75 | $ | — | 74,225,141 | $ | 7,422 | $ | 6,794,612 | $ | (8,803,920 | ) | $ | (2,001,886 | ) | |||||||||||||||||||
| Issuance of common stock for acquisition of intellectual property | — | — | — | — | 25,000 | 25 | 13,225 | — | 13,250 | |||||||||||||||||||||||||||
| Stock based compensation | — | — | — | — | — | — | 1,182,019 | — | 1,182,019 | |||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,058,952 | ) | (2,058,952 | ) | |||||||||||||||||||||||||
| Balance at June 30, 2020 | 75 | — | 75 | $ | — | 74,250,141 | $ | 7,447 | 7,989,856 | $ | (10,862,872 | ) | $ | (2,865,569 | ) | |||||||||||||||||||||
| Balance at December 31, 2020 | 75 | $ | — | 75 | $ | — | 77,670,908 | $ | 7,767 | $ | 9,830,944 | $ | (13,033,363 | ) | $ | (3,194,652 | ) | |||||||||||||||||||
| Stock based compensation | — | — | — | — | — | — | 453,950 | — | 453,950 | |||||||||||||||||||||||||||
| Issuance of common stock for cash | — | — | — | — | 7,268,188 | 727 | 813,030 | — | 813,757 | |||||||||||||||||||||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | — | — | — | — | 988,069 | 99 | 89,632 | — | 89,731 | |||||||||||||||||||||||||||
| Issuance of common stock for services | — | — | — | — | 525,000 | 52 | 105,243 | — | 105,295 | |||||||||||||||||||||||||||
| Issuance of common stock in lieu of deferred compensation | — | — | — | — | 692,308 | 69 | 89,931 | — | 90,000 | |||||||||||||||||||||||||||
| Issuance of common stock due to settlement of accrued expenses | — | — | — | — | 520,000 | 52 | 103,948 | — | 104,000 | |||||||||||||||||||||||||||
| Issuance of common stock for payment of patent purchase liability | — | — | — | — | 313,972 | 31 | 59,969 | — | 60,000 | |||||||||||||||||||||||||||
| Reduction of derivative liability due to conversions | — | — | — | — | — | — | 68,072 | — | 68,072 | |||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (1,328,575 | ) | (1,328,575 | ) | |||||||||||||||||||||||||
| Balance at March 31, 2021 | 75 | $ | — | 75 | $ | — | 87,978,445 | $ | 8,797 | $ | 11,614,719 | $ | (14,361,938 | ) | $ | (2,738,422 | ) | |||||||||||||||||||
| Stock based compensation | 297,857 | — | 297,857 | |||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (751,120 | ) | (751,120 | ) | |||||||||||||||||||||||||
| Balance at June 30, 2021 | 75 | $ | — | 75 | $ | — | 87,978,445 | $ | 8,797 | $ | 11,912,576 | $ | (15,113,058 | ) | $ | (3,191,685 | ) | |||||||||||||||||||
| The accompanying notes are an integral part of these consolidated financial statements | ||||||||||||||||||||||||||||||||||||
| 5 |
NEUROPATHIX, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Unaudited
| Six Months Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (2,079,695 | ) | $ | (2,366,784 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 9,140 | 8,069 | ||||||
| Amortization of debt discount | 177,790 | 136,164 | ||||||
| Stock based compensation | 751,807 | 1,182,019 | ||||||
| Issuance of common stock for acquisition of intellectual property | — | 13,250 | ||||||
| Issuance of common stock for services | 157,295 | — | ||||||
| Non-cash interest expense | — | 429,470 | ||||||
| Change in fair value of derivative liabilities | 89,367 | (121,389 | ) | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses | 150,167 | 6,000 | ||||||
| Accounts payable and accrued expenses | (76,925 | ) | 61,476 | |||||
| Payroll and related liabilities | 135,083 | 61,418 | ||||||
| Due to related party, net | (29,762 | ) | 14,555 | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (715,733 | ) | (575,752 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of equipment | (14,622 | ) | — | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (14,622 | ) | — | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of common stock | 813,757 | — | ||||||
| Principal payments toward capital lease obligations | (4,247 | ) | (3,656 | ) | ||||
| Proceeds from loan payable | — | 84,200 | ||||||
| Proceeds from loan payable - related party | — | 150,000 | ||||||
| Proceeds from convertible notes payable | — | 372,150 | ||||||
| Repayment of convertible notes payable | (50,000 | ) | — | |||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 759,511 | 602,694 | ||||||
| Net increase (decrease) in cash | 29,155 | 26,942 | ||||||
| Cash and cash equivalents, beginning of period | 21,874 | 121,455 | ||||||
| Cash and cash equivalents, end of period | $ | 51,029 | $ | 148,397 | ||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | 798 | $ | — | ||||
| Cash paid for taxes | $ | — | $ | — | ||||
| NON-CASH ACTIVITIES: | ||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | $ | 89,731 | $ | — | ||||
| Issuance of common stock for payment of patent purchase liability | $ | 60,000 | $ | — | ||||
| Issuance of common stock in lieu of deferred compensation | $ | 90,000 | $ | — | ||||
| Property and equipment financed through capital leases | $ | 16,537 | $ | — | ||||
| Reduction of derivative liability | $ | 68,072 | $ | — | ||||
| Settlement of accrued expenses through issuance of common stock | $ | 52,000 | $ | — | ||||
| Debt discount upon the issuance of convertible note payable | $ | — | $ | 372,150 | ||||
| Debt discount upon the issuance of convertible note payable - related party | $ | — | $ | 150,000 | ||||
| The accompanying notes are an integral part of these consolidated financial statements | ||||||||
| NEUROPATHIX, INC. | ||||||||||||||||||||||||||||||||||||||||
| (FORMERLY KNOWN AS KANNALIFE, INC. ) | ||||||||||||||||||||||||||||||||||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||||||||||||||||||||||||||||||||||
| Unaudited | ||||||||||||||||||||||||||||||||||||||||
| Series A Preferred Stock | Series B Preferred Stock | Common Stock | ||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Additional Paid-In Capital | Accumulated Deficit | Non-controlling interest | Total Stockholders’ Equity (Deficit) | |||||||||||||||||||||||||||||||
| Balance at June 30, 2019 | 75 | $ | — | 75 | $ | — | 69,854,141 | $ | 6,985 | $ | 6,386,793 | $ | (6,680,091 | ) | $ | (10,631 | ) | $ | (296,944 | ) | ||||||||||||||||||||
| Issuance of stock options for services | — | — | — | — | — | — | 840 | — | — | 840 | ||||||||||||||||||||||||||||||
| Issuance of common stock for services | — | — | — | — | 1,750,000 | 175 | 174,825 | — | — | 175,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock to board members for services | — | — | — | — | 950,000 | 95 | 94,905 | — | — | 95,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | — | — | — | — | 1,500,000 | 150 | 149,850 | — | — | 150,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock for remaining share of non-controlling interest | — | — | — | — | 171,000 | 17 | (12,601 | ) | — | 12,584 | — | |||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | — | (1,084,544 | ) | (1,953 | ) | (1,086,497 | ) | |||||||||||||||||||||||||||
| Balance at September 30, 2019 | 75 | $ | — | 75 | $ | — | 74,225,141 | $ | 7,422 | $ | 6,794,612 | $ | (7,764,635 | ) | $ | — | $ | (962,601 | ) | |||||||||||||||||||||
| Balance at December 31, 2018 | 75 | $ | — | 75 | $ | — | 69,854,141 | $ | 6,985 | $ | 6,381,755 | $ | (5,052,051 | ) | $ | (5,756 | ) | $ | 1,330,933 | |||||||||||||||||||||
| Issuance of stock options for services | — | — | — | — | — | — | 5,878 | — | — | 5,878 | ||||||||||||||||||||||||||||||
| Issuance of common stock for services | — | — | — | — | 1,750,000 | 175 | 174,825 | — | — | 175,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock to board members for services | — | — | — | — | 950,000 | 95 | 94,905 | — | — | 95,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | — | — | — | — | 1,500,000 | 150 | 149,850 | — | — | 150,000 | ||||||||||||||||||||||||||||||
| Issuance of common stock for remaining share of non-controlling interest | — | — | — | — | 171,000 | 17 | (12,601 | ) | — | 12,584 | — | |||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,712,584 | ) | (6,828 | ) | (2,719,412 | ) | |||||||||||||||||||||||||||
| Balance at September 30, 2019 | 75 | $ | — | 75 | $ | — | 74,225,141 | $ | 7,422 | $ | 6,794,612 | $ | (7,764,635 | ) | $ | — | $ | (962,601 | ) | |||||||||||||||||||||
| Balance at June 30, 2020 | 75 | $ | — | 75 | $ | — | 74,250,141 | $ | 7,425 | $ | 7,989,878 | $ | (10,862,872 | ) | $ | — | $ | (2,865,569 | ) | |||||||||||||||||||||
| Stock based compensation | — | — | — | — | — | — | 306,596 | — | — | 306,596 | ||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | — | — | — | — | 46,896 | 5 | 20,595 | — | — | 20,600 | ||||||||||||||||||||||||||||||
| Issuance of common stock for services | — | — | — | — | 500,000 | 50 | 399,950 | — | — | 400,000 | ||||||||||||||||||||||||||||||
| Reduction of derivative liability | — | — | — | — | — | — | 12,860 | — | — | 12,860 | ||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (610,806 | ) | — | (610,806 | ) | ||||||||||||||||||||||||||||
| Balance at September 30, 2020 | 75 | $ | — | 75 | $ | — | 74,797,037 | $ | 7,480 | $ | 8,729,879 | $ | (11,473,678 | ) | $ | — | $ | (2,736,319 | ) | |||||||||||||||||||||
| Balance at December 31, 2019 | 75 | $ | — | 75 | $ | — | 74,225,141 | $ | 7,422 | $ | 6,794,612 | $ | (8,496,088 | ) | $ | — | $ | (1,694,054 | ) | |||||||||||||||||||||
| Stock based compensation | — | — | — | — | — | — | 1,488,615 | — | — | 1,488,615 | ||||||||||||||||||||||||||||||
| Issuance of common stock for acquisition of intellectual property | — | — | — | — | 25,000 | 3 | 13,247 | — | — | 13,250 | ||||||||||||||||||||||||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | — | — | — | — | 46,896 | 5 | 20,595 | — | — | 20,600 | ||||||||||||||||||||||||||||||
| Issuance of common stock for services | — | — | — | — | 500,000 | 50 | 399,950 | — | — | 400,000 | ||||||||||||||||||||||||||||||
| Reduction of derivative liability | — | — | — | — | — | — | 12,860 | — | — | 12,860 | ||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,977,590 | ) | — | (2,977,590 | ) | ||||||||||||||||||||||||||||
| Balance at September 30, 2020 | 75 | $ | — | 75 | $ | — | 74,797,037 | $ | 7,480 | $ | 8,729,879 | $ | (11,473,678 | ) | $ | — | $ | (2,736,319 | ) | |||||||||||||||||||||
| The accompanying notes are an integral part of these condensed consolidated financial statements | ||||||||||||||||||||||||||||||||||||||||
| NEUROPATHIX, INC. | ||||||||
| (FORMERLY KNOWN AS KANNALIFE, INC. ) | ||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||||
| Unaudited | ||||||||
| Nine Months Ended September 30, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (2,977,590 | ) | $ | (2,719,412 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 12,102 | 1,430 | ||||||
| Amortization of debt discount | 254,249 | — | ||||||
| Stock based compensation | 1,488,615 | 275,878 | ||||||
| Issuance of common stock for acquisition of intellectual property | 13,250 | — | ||||||
| Non-cash interest expense | 430,070 | — | ||||||
| Change in fair value of derivative liabilities | (468,097 | ) | — | |||||
| Net gains and losses recognized on marketable security | — | 301,661 | ||||||
| Changes in operating assets and liabilities: | — | |||||||
| Prepaid expenses | 9,000 | — | ||||||
| Other receivables | — | 90,392 | ||||||
| Accounts payable and accrued expenses | 150,018 | 32,698 | ||||||
| Payroll and related liabilities | 108,589 | 1,007 | ||||||
| Due to related party, net | 22,005 | 16,334 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (807,789 | ) | (2,000,012 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Proceeds from sale of marketable securities | — | 1,882,774 | ||||||
| Purchase of equipment | — | (41,950 | ) | |||||
| Purchase of other asset | — | (27,490 | ) | |||||
| NET CASH PROVIDED BY INVESTING ACTIVITIES | — | 1,813,334 | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Principal payments toward capital lease obligations | (5,566 | ) | — | |||||
| Proceeds from loan payable | 184,200 | — | ||||||
| Proceeds from convertible notes payable, net of OID | 372,150 | — | ||||||
| Proceeds from convertible notes payable - related party | 150,000 | 97 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 700,784 | 97 | ||||||
| Net decrease in cash | (107,005 | ) | (186,581 | ) | ||||
| Cash and cash equivalents, beginning of period | 121,455 | 307,131 | ||||||
| Cash and cash equivalents, end of period | $ | 14,450 | $ | 120,550 | ||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | 2,905 | $ | — | ||||
| Cash paid for taxes | $ | — | $ | — | ||||
| NON-CASH ACTIVITIES: | ||||||||
| Issuance of common stock for conversion of notes payable and accrued interest | $ | 20,000 | $ | 150,000 | ||||
| Issuance of common stock for services | $ | 250,000 | $ | — | ||||
| Issuance of common stock for remaining share of non-controlling interest | $ | — | $ | 12,584 | ||||
| Property and equipment financed through capital leases | $ | — | $ | 35,886 | ||||
| Debt discount upon the issuance of convertible note payable | $ | 372,150 | $ | — | ||||
| Debt discount upon the issuance of convertible note payable - related party | $ | 150,000 | $ | — | ||||
| Reduction of derivative liability | $ | 12,860 | $ | — | ||||
| The accompanying notes are an integral part of these condensed consolidated financial statements | ||||||||
| NEUROPATHIX, INC. |
| NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS |
| (UNAUDITED) |
Note 1 – Organization and Nature of OperationsORGANIZATION AND NATURE OF OPERATIONS
Neuropathix, Inc. (the “Company”) was incorporated under the laws of the state of Delaware on March 25, 2013 under the name TYG Solutions Corp. The Company consummated a share exchange transaction on July 25, 2018 with Kannalife Sciences, Inc. (“Kannalife”), a privately held Delaware corporation formed in 2010, the accounting acquirer. Upon completion of the share exchange transaction, Kannalife was treated as the surviving entity and accounting acquirer although the Company was the legal acquirer. Accordingly, the Company’s historical financial statements are those of Kannalife the surviving entity and accounting acquirer. All references that refer to (the “Company” or “we” or “us” or “our”) are Kannalife, unless otherwise differentiated. Kannalife is a phytomedical/pharmaceutical company that specializes in the research and development of synthetic molecules and therapeutic products derived from botanical sources, including the cannabis taxa. On November 9, 2018, the Company filed an amendment to its certificate of incorporation with the Delaware Secretary of State that changed its name to Kannalife, Inc.
Name Change – Neuropathix, Inc.
On November 4, 2020, the Company filed an amendment to its certificate of incorporation with the Delaware Secretary of State that changed its name to Neuropathix, Inc. The Company concurrently submitted a request to FINRA for approval of the name change as well as a ticker symbol change from “KLFE” to “NPTX.” The Company’s name change and ticker symbol change was reviewed and processed by FINRA and went effective November 6, 2020.
Unaudited Interim Financial Information
We have prepared the accompanying condensed consolidated financial statements pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”) for interim financial reporting. These condensed consolidated financial statements are unaudited and, in our opinion, include all adjustments, consisting of normal recurring adjustments and accruals necessary for a fair presentation of our balance sheets, operating results, and cash flows for the periods presented. Operating results for the periods presented are not necessarily indicative of the results that may be expected for 2020.2021. Certain information and footnote disclosures normally included in consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) have been omitted in accordance with the rules and regulations of the SEC. These condensed consolidated financial statements should be read in conjunction with our audited financial statements and accompanying notes for the year ended December 31, 2019,2020, included in the Company’s Annual Report on Form 10-K filed with the SEC on March 30, 2020.2021.
Note 2 - Summary of Significant Accounting PoliciesSUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The significant accounting policies used in the preparation of the condensed consolidated financial statements are as follows:
Basis of Presentation
The accompanying condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP.
Significant Risks and Uncertainties
The Company’s operations are subject to a number of factors that can affect its operating results and financial condition. Such factors include, but are not limited to: the results of clinical testing and trial activities of the Company’s products, the Company’s ability to obtain regulatory approval to market its products, competition from products manufactured and sold or being developed by other companies, the price of, and demand for, Company products, the Company’s ability to negotiate favorable licensing or other manufacturing and marketing agreements for its products, and the Company’s ability to raise capital.
The Company currently has no commercially approved products and there can be no assurance that the Company’s research and development will be successfully commercialized. Developing and commercializing a product requires significant time and capital and is subject to regulatory review and approval as well as competition from other biotechnology and pharmaceutical companies. The Company operates in an environment of rapid change and is dependent upon the continued services of its employees and consultants and obtaining and protecting intellectual property.
| 7 |
In December 2019, a novel strain of coronavirus, commonly known asAlthough there are signs that COVID-19 surfaced. The spread ofmay begin to taper off, COVID-19 around the world in 2020still has caused significant volatility in U.S. and international markets. There is significant uncertainty around the breadth and duration of business disruptions related to COVID-19, as well as itsan impact on worldwide economic activity, and the U.S. and international economies and, as such,ongoing effects of the Company is unable to determine if it will have a material impact to its operations. The Company’s operationsCOVID-19 pandemic has impacted (although not significantly as of SeptemberJune 30, 2020 have not been significantly affected, but2021), and may be affected in the future, by the ongoing outbreakcontinue to impact, certain aspects of COVID-19 which was declared a pandemic by the World Health Organization. The ultimate disruption which may be caused by the outbreak is uncertain; however, it may result in a material adverse impact on the Company’s financial position, operations and cash flows. Possible areas that may be affected include, but are not limited to, disruption to the Company’sour business such as our labor workforce, unavailability of products and supplies used in our operations, and thea potential decline in value of assets held by the Company. In response to the COVID-19 pandemic, many state, local, and foreign governments have put in place restrictions in order to control the spread of the disease. Such restrictions, or the perception that further restrictions could occur, have resulted in business closures, work stoppages, slowdowns and delays, work-from-home policies, travel restrictions, and cancellation or postponement of events, among other effects that impacted productivity and disrupted our operations and those of our partners, suppliers, and contractors.
The COVID-19 pandemic may also have the effect of heightening many of the other risks described in the “Risk Factors” section of our December 31, 2020 Annual Report on Form 10-K filed March 30, 2021. We may take further actions that alter our operations as may be required by federal, state, or local authorities, or which we determine are in our best interests. While some of our operations can be performed remotely, certain activities often require personnel to be on-site, and our ability to carry out these activities have been, and may continue to be negatively impacted if our employees or local personnel are not able to travel or be restricted to on-site access. In addition, for activities that may be conducted remotely, there is no guarantee that we will be as effective while working remotely because our team is dispersed and many employees and their families have been negatively affected, mentally or physically, by the COVID-19 pandemic. Decreased effectiveness and availability of our team could harm our business. In addition, we may decide to postpone or cancel planned investments in our business in response to changes in our business as a result of the spread of COVID-19, which may impact our rate of innovation, and may impact the start and/or completion of our studies and/or clinical trials, either of which could harm our business.
We do not yet know the full extent of potential delays or impacts on our business, operations, or the global economy as a whole. While there have recently been vaccines developed and administered, and certain government orders and restrictions in particular cities, counties, and states have been lifted as the spread of COVID-19 starts to get contained and mitigated, we cannot predict the timing of the vaccine roll-out globally or the efficacy of such vaccines, and we do not yet know how businesses, contractors, suppliers, or our partners will operate in a post COVID-19 environment, especially if additional or supplemental governmental orders, limitations, and restrictions are reinstated. There may be additional costs or impacts to our business and operations. In addition, there is no guarantee that a future outbreak of this or any other widespread epidemics will not occur, or that the global economy will recover, either of which could harm our business.
Furthermore, while the potential impact and duration of the COVID-19 pandemic on the economy and our business in particular may be difficult to assess or predict, the pandemic has resulted in, and may continue to result in, significant disruption of global financial markets, and may reduce our ability to access additional capital, which could negatively affect our liquidity in the future.
Use of Estimates
The preparation of consolidated financial statements and accompanying notes in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the dates of the consolidated financial statements, and the reported amounts of revenues and expenses during the periods. Actual results could differ from those estimates. Significant matters requiring the use of estimates and assumptions include, but are not necessarily limited to, establishing the fair value of marketable securities and periodically evaluating marketable securities for potential impairment, fair value of the Company’s stock, stock-based compensation, valuation of derivative liabilities and valuation allowance relating to the Company’s deferred tax assets. Management believes that its estimates and assumptions are reasonable, based on information that is available at the time they are made.
Basic net loss per share is calculated by dividing the net loss for the period by the weighted-average number of common shares outstanding during the period. Diluted net income per share is calculated by dividing income for the period by the weighted-average number of common shares outstanding during the period, increased by potentially dilutive common shares ("dilutive securities") that were outstanding during the period. Dilutive securities include stock options and warrants granted, convertible debt, and convertible preferred stock.
The weighted average number of common stock equivalents not included in diluted income per share, because the effects are anti-dilutive, was 12,642,869 and for the three and ninesix months ended SeptemberJune 30, 2020. The weighted average number of common stock equivalents not included in diluted income per share, because the effects are anti-dilutive, was 4,113,729 for the three2021 and nine months ended September 30, 2019.2020, respectively.
Research and Development
In accordance with FASB ASC 730, Research and Development (“ASC 730”) research and development (“R&D”) costs are expensed when incurred. R&D costs include supplies, clinical trial and related clinical manufacturing costs, contract and other outside service and facilities and overhead costs. Total R&D costs for the three months ended SeptemberJune 30, 2021 and 2020, were $76,004and 2019, were $68,546 and $189,443,$287,665, respectively. Total R&D costs for the ninesix months ended SeptemberJune 30, 2021 and 2020, were $204,653and 2019, were $375,938 and $439,680,$307,392, respectively.
Stock Based Compensation
The Company accounts for share-based compensation in accordance with the fair value recognition provision of FASB ASC 718, Compensation – Stock Compensation (“ASC 718”), prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense, which is included in the general and administrative expense in the consolidated financial statements based on the estimated grant date fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
Recently Issued Authoritative Guidance
In June 2016, the FASB issued ASU 2016-13, “Financial Instruments – Credit Losses” to improve information on credit losses for financial assets and net investment in leases that are not accounted for at fair value through net income. ASU 2016-13 replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses. In April 2019 and May 2019, the FASB issued ASU No. 2019-04, “Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments” and ASU No. 2019-05, “Financial Instruments-Credit Losses (Topic 326): Targeted Transition Relief” which provided additional implementation guidance on the previously issued ASU. In November 2019, the FASB issued ASU 2019-10, “Financial Instruments - Credit Loss (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842),” which defers the effective date for public filers that are considered small reporting companies (“SRC”) as defined by the Securities and Exchange Commission to fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. Since the Company is an SRC, implementation is not needed until January 1, 2023. The Company will continue to evaluate the effect of adopting ASU 2016-13 will have on the Company’s consolidated financial statements.
| 8 |
Note 3 – Going Concern and Management’s Liquidity PlansGOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
The Company’s condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. As reflected in our accompanying condensed consolidated financial statements, the Company has had a net loss from operations of $2,679,475$1,745,257 and $1,824,987$1,873,806 for the ninesix months ended SeptemberJune 30, 20202021 and 2019, respectively. The net cash used in operations were $807,789 and $2,000,012 for the nine months ended September 30, 2020, and 2019, respectively. Additionally, the Company had an accumulated deficit of $11,473,678$15,113,058 at SeptemberJune 30, 20202021 and has not yet established an adequate ongoing source of revenues sufficient to cover its operating costs and to allow it to continue as a going concern. These factors raise substantial doubt about its ability to continue as a going concern.
In order to continue as a going concern, the Company will need, among other things, additional capital resources. Management plans to raise additional capital through the sale of convertible debt securities offering. However, there are no assurances that such additional funding will be achieved or that management’s plans will be successful. The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 4 – Fair Value MeasurementsFAIR VALUE MEASUREMENTS
The Company follows FASB ASC 820, Fair Value Measurements and Disclosures (“ASC 820”) to measure and disclosure the fair value of its financial instruments. ASC 820 establishes a framework for measuring fair value in U.S. GAAP and expands disclosures about fair value measurements and establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The three levels of fair value hierarchy defined by ASC 820 are described below:
Level 1 - Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
Level 2 - Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
Level 3 - Pricing inputs that are generally unobservable inputs and not corroborated by market data.
| 9 |
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The carrying amounts reported in the Company’s condensed consolidated financial statements for cash, accounts payable and accrued expenses approximate their fair value because of the immediate or short-term nature of these financial instruments.
Transactions involving related parties cannot be presumed to be carried out on an arm's-length basis, as the requisite conditions of competitive, free-market dealings may not exist. Representations about transactions with related parties, if made, shall not imply that the related party transactions were consummated on terms equivalent to those that prevail in arm's-length transactions unless such representations can be substantiated.
The following table presents liabilities that are measured and recognized at fair value as of SeptemberJune 30, 20202021 and December 31, 2019,2020, on a recurring basis:
| September 30, 2020 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total Carrying Value | |||||||||||||
| Derivative liabilities | — | — | 654,114 | $ | 654,114 | |||||||||||
| Fair Value, Liabilities Measured on Recurring Basis | ||||||||||||||||
| June 30, 2021 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total Carrying Value | |||||||||||||
| Derivative liabilities | $ | 0 | $ | 0 | 337,484 | $ | 337,484 | |||||||||
| December 31, 2019 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total Carrying Value | |||||||||||||
| Derivative liabilities | — | — | 183,451 | $ | 183,451 | |||||||||||
| December 31, 2020 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total Carrying Value | |||||||||||||
| Derivative liabilities | $ | 0 | $ | 0 | 316,189 | $ | 316,189 | |||||||||
NOTE 5 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses at SeptemberJune 30, 20202021 and December 31, 20192020 consisted of the following:
September 30, 2020 | December 31, 2019 | |||||||
| Accounts payable and accrued expenses | $ | 382,096 | $ | 309,231 | ||||
| Accrued interest | 155,117 | 79,964 | ||||||
| Totals | $ | 537,213 | $ | 389,195 | ||||
| Accounts payable and accrued expenses | ||||||||
June 30, 2021 | December 31, 2020 | |||||||
| Accounts payable and accrued expenses | $ | 346,318 | $ | 538,527 | ||||
| Accrued interest | 241,334 | 182,781 | ||||||
| Totals | $ | 587,652 | $ | 721,308 | ||||
NOTE 6 – PAYROLL AND RELATED LIABILITIES
Payroll and related liabilities at SeptemberJune 30, 20202021 and December 31, 20192020 consisted of the following:
| Schedule of Payroll and Related Liabilities | ||||||||||||||||
September 30, 2020 | December 31, 2019 | June 30, 2021 | December 31, 2020 | |||||||||||||
| Payroll | $ | 119,291 | $ | — | $ | 181,555 | $ | 149,704 | ||||||||
| Payroll taxes | 232,506 | 243,208 | 242,769 | 229,537 | ||||||||||||
| Totals | $ | 351,797 | $ | 243,208 | $ | 424,324 | $ | 379,241 | ||||||||
As of the nine months period ended SeptemberJune 30, 2020 and the year ended December 31, 2019,2021, the Company has accrued payroll and payroll taxes in connection with salaries paid and accrued to four officers of the Company.Company which includes $120,000 accrued for the CEO, and $12,500 accrued for executive management. As of December 31, 2020, the Company has accrued payroll and payroll taxes in connection with salaries paid and accrued to four officers of the Company which includes $100,000 accrued for the CEO, and $12,500 accrued for executive management.
| 10 |
NOTE 7 – LOAN PAYABLE
| Schedule of Loans Payable | ||||||||||||||||||||||||
| September 30, 2020 | December 31, 2019 | June 30, 2021 | December 31, 2020 | |||||||||||||||||||||
| Loan payable at 8%, matures 12/31/2020 | * {a} | $ | 720,000 | $ | 620,000 | |||||||||||||||||||
| Loan payable at 1%, matures 4/23/2022 | * | 84,200 | — | |||||||||||||||||||||
| Loan payable at 8%, matures December 31, 2021 | * | $ | 850,000 | $ | 850,000 | |||||||||||||||||||
| Loan payable at 1%, matured June 11, 2021 | * | 50,000 | 100,000 | |||||||||||||||||||||
| Total | 804,200 | 620,000 | 900,000 | 950,000 | ||||||||||||||||||||
| Less: short term loans | 771,456 | 620,000 | 900,000 | — | ||||||||||||||||||||
| Total long-term loans | $ | 32,744 | $ | — | $ | — | $ | 950,000 | ||||||||||||||||
| {a} - On July 1, 2020, the Company extended the note to December 31, 2020 based on the same terms and conditions. On October 7, 2020, borrowed an additional $80,000 based on the same terms and conditions. | ||||||||||||||||||||||||
| * - unsecured note | ||||||||||||||||||||||||
Total interest expense on notes payable, amounted to $13,753$16,767 and $12,230$12,369 for the three months ended SeptemberJune 30, 20202021 and 2019,2020, respectively. Total interest expense on notes payable, amounted to $38,352$33,534 and $36,690$24,599 for the ninesix months ended SeptemberJune 30, 2021 and 2020, and 2019, respectivelyrespectively. Accrued interest related to these notes was $111,732$161,359 and $73,381$127,825 as of SeptemberJune 30, 20202021 and December 31, 2019,2020, respectively.
NOTE 8 – LOAN PAYABLE – RELATED PARTY
Prior to the share exchange agreement, the Company borrowed $25,822$25,822 and issued a promissory note with a maturity date of March 31, 2020 which was later extended to March 31, 2021.2022. Additionally, the note holder advanced the Company $16,270$16,270 for working capital, for a total of $42,092$42,092 – also see Note 16.
The loans represent working capital advances from shareholders, bear interest at 0.5% 0.5%, and grant a security interest in the Company’s assets as collateral. In March 2018, this note was amended, and the original note holder assigned the note to Kettner Investments, LLC, a significant shareholder. The note is now non-interest bearing. Accrued interest related to this note is $226$226 as of SeptemberJune 30, 20202021 and December 31, 2019,2020, respectively.
NOTE 9 – CAPITAL LEASE OBLIGATIONS
In September 2019, the Company entered into a lease agreement with Thermo Fisher Scientific to acquire equipment with 48 monthly payments of $941,$941, payable through September 1, 2023, with an effective interest rate of 12%12% per annum. The outstanding balance of this capital lease was $29,731,$23,653, secured by equipment with carrying value of $53,601,$22,457, as of SeptemberJune 30, 2020.2021.
In March 2021, the Company entered into another lease agreement with Thermo Fisher Scientific to acquire equipment with 36 monthly payments of $699, payable through February 29, 2024, with an effective interest rate of 13% per annum. The outstanding balance of this capital lease was $11,983, secured by equipment with carrying value of $23,404, as of June 30, 2021.
NOTE 10 – CONVERTIBLE NOTES PAYABLE
Prior to the Share Exchange, the Company issued a convertible note to an investor, face value of $500,000,$500,000, in exchange for $500,000$500,000 in cash. The note is unsecured, bears interest at the rate of 3%3% per annum and matures on February 16, 2030.2030. The note is convertible into common stock of the Company at $0.10$0.10 per share at any time at the option of the holder, subject to a 4.9% blocking provision which prohibits the holder from converting into common stock of the Company if such conversion results in the holder owning greater than 4.9% of the outstanding common stock of the Company after giving effect to such conversion. On September 26, 2019, the Company issued shares of common stock for the conversion of $123,627$123,627 convertible notes payable and $26,373$26,373 of related accrued interest. The outstanding balance on this convertible note after the conversion was $376,373.$376,373 as of June 30, 2021 and December 31, 2020.
In December 2019, the Company entered into a Securities Purchase Agreement with an investor pursuant to which the Company agreed to sell to the investor a $100,000$ convertible note bearing interest at 8%8% per annum (the “Note”). The Note matures two years from the date of issuance. The Note is convertible at the option of the holder at any time into shares of the Company’s common stock at an effective conversion price of 75% of the average closing price of the Company’s common stock on the fifteen days prior to conversion. The Company may not prepay this Note within the first six months. If, after the first six months until the maturity of the Note the Company:
| (a) | elects to repay the Note, it must do so at a premium of one hundred and twenty five percent (125%) of the face amount of the Note, together with all unpaid and accrued interest to the date of repayment. | |
| (b) | elects to involuntarily exercise conversion of this Note to the Holder, the Company must provide written notice to the Holder along with an executed copy of the Company’s Notice of Conversion, specifying that the Note shall be converted into shares of the Company’s Common Stock based upon at an effective conversion price of 75% of the average closing price of the Company’s common stock on the fifteen days prior to conversion. |
| 11 |
The embedded conversion feature of this Note was deemed to require bifurcation and liability classification, at fair value. Pursuant to the Securities Purchase Agreement, the Company also sold warrants to the investors to purchase up to an aggregate of 100,000 shares of common stock. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the Note (see Note 12) resulting in full discount of the Note. Note as of June 30, 2021 and December 31, 2020.
On March 12, 2020, the Company entered into securities purchase agreements with two different accredited investors (each an “Investor”, and together the “Investors”) pursuant to which each Investor purchased an 8% unsecured convertible promissory note (each a “8% Note”, and together the “8% Notes”) from the Company. The terms and conditions of each of the 8% Notes are substantially the same. Each 8% Note has a principal amount of $105,000$105,000 less a $5,000$5,000 original issue discount for a purchase price of $100,000,$100,000, with a maturity date of March 12, 2021.2021. This note is in default as of March 12, 2021, which may trigger cross defaults on other notes. The Company is in negotiations to extend the maturity date of the Note. All principal amounts and the interest thereon are convertible into shares of the Company’s common stock at the option of each Investor, after six (6) months from the date of the 8% Notes. These 8% Notes have a variable conversion price and the Company recorded embedded derivative liabilities. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the 8% Note (see Note 12) resulting in full discount of the 8%8% Note. On September 24, 2020,During the six months ended June 30, 2021, the Company issued 46,896 shares of common stock for the conversion of $18,000$85,000 convertible notes payable and $2,000$4,731 of related accrued interest. The outstanding balance on these convertible notes after the conversion was $192,000.$14,000.
On June 8, 2020, the Company entered into a securities purchase agreement, dated as of June 2, 2020 (the “Purchase Agreement”), with an accredited investor pursuant to which the investor purchased a 12% unsecured convertible promissory note (the “12% Note”) from the Company. The 12%12% Note has a principal amount of $165,000$165,000 less a $9,000$9,000 original issue discount (“OID”) for a purchase price of $156,000,$156,000, of which $52,000$52,000 was paid on June 8, 2020 less $3,100 in transaction fees (the “First Tranche”). The 12% Note matures 12 months from the effective date of each tranche. This note is in default as of June 8, 2021, which may trigger cross defaults on other notes. The Company is in negotiations to extend the maturity date of the Note. All principal amounts and the interest thereon are convertible into shares of the Company’s common stock at the option of the Investor, after six (6) months from the date of the 12% Note. All closings occurred following the satisfaction of customary closing conditions. The 12% Note is convertible at the option of the holder at any time into shares of the Company’s common stock at an effective conversion price of the lesser of (i) 68% multiplied by the lowest Trading Price (representing a discount rate of 32%) during the previous fifteen (15) trading day period ending on the latest complete trading day prior to the date of the 12% Note or (ii) the Variable Conversion Price. In connection with the Purchase Agreement and the 12% Note, the Company issued a common stock purchase warrant to purchase 36,666 shares of the Company’s common stock at $0.75 $per share (the “Warrant”) which may be exercised by cashless exercise, exercisable for a period of three years. The 12% Note has a variable conversion price and the Company recorded embedded derivative liabilities. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the 12% Note (see Note 12)13) resulting in full discount of the 12% Note.
On June 23, 2020, the Company entered into a securities purchase agreement, dated as of June 19, 2020, with an accredited investor pursuant to which the investor purchased a 12%12% convertible promissory note in the principal amount of $150,000,$150,000, less $20,750$20,750 in transaction-related, broker, legal and due diligence expenses. The note matures on June 19, 2021.2021. This note is in default as of June 19, 2021, which may trigger cross defaults on other notes. The Company is in negotiations to extend the maturity date of the Note. Principal payments on the note shall be made in six (6) installments, each in the amount of $25,000,$25,000, starting on December 19, 2020, and continuing thereafter each thirty (30) days for five (5) months. Notwithstanding the foregoing, the final payment of principal, and accrued and unpaid interest shall be due on the June 19, 2021. The investor is entitled to, at its option, convert all or any amount of the principal amount and any accrued but unpaid interest of the note into shares of the Company’s common stock, at any time upon an event of default, at a conversion price for each share of common stock equal to the lesser of (i) the lowest trading price during the previous five (5) trading day period ending on the latest complete trading day prior to the date of the note, or (ii) the Variable Conversion Price, subject to certain equitable adjustments. Furthermore, in connection with the securities purchase agreement and the note, the Company issued two common stock purchase warrants each to purchase 115,385 shares of the Company’s common stock at $1.30 $per share which may be exercised by cashless exercise, exercisable for a period of five years. One of the warrants only becomes exercisable upon default of the note. During the thirdfirst quarter of 2020,2021, the anti-dilution clause was triggered and the exercise price was reset to $0.40$0.09 resulting in the number of warrants to be increased to 378,694.. The note has a variable conversion price and the Company recorded embedded derivative liabilities. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the note (see Note 12)13) resulting in full discount of the note.
Total interest expense on convertible notes payable, inclusive of amortization of debt discount of $92,466$59,110 and $0,$73,562, amounted to $107,161$70,363 and $3,750$80,912 for the three months ended SeptemberJune 30, 20202021 and 2019,2020, respectively. Total interest expense on convertible notes payable, inclusive of amortization of debt discount of $195,616$140,804 and $0,$103,151, amounted to $226,240$164,554 and $11,250$113,434 for the ninesix months ended SeptemberJune 30, 2021 and 2020, and 2019, respectively.
Total accrued interest on convertible notes payable, as of SeptemberJune 30, 20202021 and December 31, 2019,2020, was $31,421$32,860 and $200,$26,105, respectively.
| 12 |
NOTE 11 – CONVERTIBLE NOTES PAYABLE – RELATED PARTY
In January 2020, the Company sold an additional $100,000,$100,000, to Kettner Investments, LLC, a significant shareholder, under the Note and sold warrants to purchase up to an aggregate of shares of common stock under the Securities Purchase Agreement. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the Note (see Note 12)13) resulting in full discount of the Note.
In February 2020, the Company sold an additional $50,000,$50,000, to the CEO of MJNA, a significant shareholder, under the Note and sold warrants to purchase up to an aggregate of shares of common stock under the Securities Purchase Agreement. The fair value of the derivative liability and warrants as of the date of issuance was in excess of the Note (see Note 12)13) resulting in full discount of the Note.
Total interest expense on convertible notes payable – related party, inclusive of amortization of debt discount of $18,493 $18,493 and $0,$73,562, amounted to $21,493 $21,493 and $0 $80,912 for the three months ended SeptemberJune 30, 20202021 and 2019,2020, respectively. Total interest expense on convertible notes payable – related party, inclusive of amortization of debt discount of $51,507 $36,986 and $0,$103,151, amounted to $59,685 $42,986 and $0 $113,434 for the ninesix months ended SeptemberJune 30, 2021 and 2020, and 2019, respectively.
Total accrued interest on convertible notes payable – related party, as of SeptemberJune 30, 20202021 and December 31, 2019,2020, was $8,178$17,178 and $0,$11,178, respectively.
The following is a schedule by year of future debt payments at June 30, 2021.
| Schedule of future debt payments | ||||||||||||||||||||
| Year Ending December 31, | Loan payable | Loan payable - related party | Convertible notes payable | Convertible notes payable - related party | Total | |||||||||||||||
| 2021 – remainder of the year | $ | 900,000 | $ | 42,092 | $ | 319,000 | $ | 100,000 | $ | 1,361,092 | ||||||||||
| 2022 | — | — | — | 50,000 | 50,000 | |||||||||||||||
| 2023 | — | — | — | — | — | |||||||||||||||
| 2024 | — | — | — | — | — | |||||||||||||||
| 2025 | — | — | — | — | — | |||||||||||||||
| Thereafter | — | — | $ | 376,373 | — | 376,373 | ||||||||||||||
| Total | $ | 900,000 | $ | 42,092 | $ | 695,373 | $ | 150,000 | $ | 1,787,465 | ||||||||||
NOTE 12 – PATENT PURCHASE LIABILITY
On December 17, 2020, the Company entered into an Intellectual Property Rights Purchase and Transfer Agreement (the “IP Purchase Agreement”) by and between Advanced Neural Dynamics (“AND”), Fox Chase, Dr. Douglas Brenneman (“Brenneman”) and the Company to acquire the IP Rights and concurrently entered into a Pharmaceutical Royalty Agreement with AND and Fox Chase.
Pursuant to the IP Purchase Agreement, the Company acquired the IP Assets for a $570,000 aggregate purchase price payable in restricted common stock of the Company to Fox Chase, Brenneman and AND, payable as follows:
| • | shares of restricted common stock of the Company were issued to Fox Chase at a price per share of $ for an aggregate of $270,000; and |
| • | $300,000 in common stock will be issued to AND/Brenneman in five annual installments which shall be calculated as $60,000 divided by the average ten day closing price prior to each installment date with the initial installment date occurring on January 5, 2021; provided, however, that for the initial installment issuance price only, the price per share shall not be below $ or above $per share. |
In addition, AND/Brenneman shall receive cash payments of $15,000 annually, payable in quarterly installments to offset against tax payments, netted out against actual tax costs incurred. In the event such payments are not made, there will be a 10% penalty assessed on said late tax offset payment.
The liabilities from the IP purchase agreement are recognized at the commencement date based on the present value of remaining payments over the payment term using the Company’s secured incremental borrowing rates or implicit rates, when readily determinable.
The Company’s IP purchase agreement does not provide an implicit rate that can readily be determined. Therefore, the Company uses an 8% discount rate based on our incremental borrowing rate, which is determined using the average interest rate of our long-term debt as of December 17, 2020.
| 13 |
| Schedule of patent purchase | ||||
| Maturity of Patent Purchase Liability | ||||
| Year Ending December 31, | ||||
| 2021 - remainder of the year | $ | 7,500 | ||
| 2022 | 75,000 | |||
| 2023 | 75,000 | |||
| 2024 | 75,000 | |||
| 2025 | 75,000 | |||
| Total undiscounted payments | 315,000 | |||
| Less: Imputed interest | (57,038 | ) | ||
| Present value of Patent Purchase liabilities | $ | 250,562 | ||
NOTE 1213 – DERIVATIVE LIABILITIES
The Company issued debts that consist of the issuance of convertible notes with variable conversion provisions. In addition, the Company issued warrants with variable conversion provisions. The conversion terms of the convertible notes and warrants are variable based on certain factors, such as the future price of the Company’s common stock. The number of shares of common stock to be issued is based on the future price of the Company’s common stock. The number of shares of common stock issuable upon conversion of the promissory note is indeterminate. Pursuant to ASC 815-15 Embedded Derivatives, the fair values of the variable conversion option and warrants and shares to be issued were recorded as derivative liabilities on the issuance date.
Based on the various convertible notes described in Note 10 and 11, the fair value of applicable derivative liabilities on notes, warrants and change in fair value of derivative liability are as follows for the ninesix months ended SeptemberJune 30, 2020:2021:
| Schedule of Derivative Liabilities | ||||||||||||||||||||||||
| Derivative Liability - Convertible Notes | Derivative Liability - Warrants | Total | Derivative Liability - Convertible Notes | Derivative Liability - Warrants | Total | |||||||||||||||||||
| Balance as of December 31, 2019 | $ | 61,430 | $ | 122,021 | $ | 183,451 | ||||||||||||||||||
| Balance as of December 31, 2020 | $ | 153,140 | $ | 163,049 | $ | 316,189 | ||||||||||||||||||
| Additions during the period | 597,841 | 353,779 | 951,620 | — | — | — | ||||||||||||||||||
| Change in fair value | (409,165 | ) | (58,932 | ) | (468,097 | ) | 24,512 | 64,855 | 89,367 | |||||||||||||||
| Change due to exercise / redemptions | (12,860 | ) | — | (12,860 | ) | (68,072 | ) | — | (68,072 | ) | ||||||||||||||
| Balance as of September 30, 2020 | $ | 237,246 | $ | 416,868 | $ | 654,114 | ||||||||||||||||||
| Balance as of June 30, 2021 | $ | 109,580 | $ | 227,904 | $ | 337,484 | ||||||||||||||||||
The fair value of the derivative liability – convertible notes is estimated using a Monte Carlo pricing model with the following assumptions:
| Schedule of share-based payment award, stock options, valuation assumptions | ||||||
| Market value of common stock | $ | 0.11 – 0.12 | ||||
| Expected volatility | % | |||||
| Expected term (in years) | ||||||
| Risk-free interest rate | % | |||||
The fair value of the derivative liability – warrants is estimated using a Monte Carlo pricing model with the following assumptions:
| Schedule of share-based payment award, stock options, valuation assumptions | ||||||
| Market value of common stock | $ | 0.11 – 0.12 | ||||
| Expected volatility | % | |||||
| Expected term (in years) | ||||||
| Risk-free interest rate | % | |||||
| 14 |
NOTE 1314 – COMMITMENTS AND CONTINGENCIES
Legal Proceedings
From time to time the Company may get involved in legal proceedings arising in the ordinary course of business. Other than as set forth in “Legal Proceedings” in Part II below, the Company believes there is no litigation pending that could have, individually or in the aggregate, a material adverse effect on its results of operations or financial condition.
Occupancy Leases
On April 1, 2014, the Company entered into a one year lease arrangement for office space, with the option to renew the lease annually. The lease has been renewed through April 2022. The monthly rent payment is $5,600 and the security deposit is $15,000.
On September 15, 2015, we entered into a one year lease arrangement for additional office space, the lease has been renewed is currently scheduled to expire on September 30, 2021. The monthly rent payment is $5,600$359, and we provided a security deposit of $183.
On July 1, 2018, we entered into a one year lease arrangement for additional office space, with the option to renew the lease annually. On September 1, 2018, we subleased this office space to a third party. The subleasee will pay 50% of the rent until expiration of lease on June 30, 2021. The monthly rent payment is $2,723, and we provided a security deposit of $2,121.
Royalties
On December 17, 2020, the Company entered into an Intellectual Property Rights Purchase and Transfer Agreement by and between AND, Fox Chase, Brenneman and the security deposit is $15,000.Company to acquire the IP Rights and concurrently entered into the “Royalty Agreement with AND and Fox Chase.
Pursuant to the Royalty Agreement, the following royalties and license fees are payable to Fox Chase and AND as well:
| • | 1% upfront sublicense fees per participant; and |
| • | 1% reversion rights to each participant (for 2% aggregate), which rights include future milestone payments. |
NOTE 1415 – STOCKHOLDERS’ DEFICIT
Series A Preferred Stock
Effective May 3, 2018, the Company’s Board of Directors authorized and designated 75 shares of the Company’s Preferred Stock as Series A Preferred Stock. Each share of the Series A Preferred Stock is entitled to a liquidation preference of $1,000 per share and is convertible into 1,000 shares of the Company’s common stock. The holders of a majority of the Series A Preferred Stock are entitled to elect up to four (4) directors to the Company’s board of directors and have preferential rights in regard to the election of Series A directors. In all other voting matters, the holders of Series A Preferred Stock are entitled to cast 1,000 votes per share.
Series B Preferred Stock
Effective May 3, 2018, the Company’s Board of Directors authorized and designated 75 shares of the Company’s Preferred Stock as Series B Preferred Stock. Each share of the Series B Preferred Stock is entitled to a liquidation preference of $1,000 per share and is convertible into 1,000 shares of the Company’s common stock. The holders of a majority of the Series B Preferred Stock are entitled to elect up to three (3) directors to the Company’s board of directors and have preferential rights in regard to the election of Series B directors. In all other voting matters, the holders of Series B Preferred Stock are entitled to cast 1,000 votes per share.
| 15 |
Common Stock
The Company is authorized to issue shares of common stock, par value of $0.0001$ per share. All common stock shares have equal voting rights, are non-assessable and have one vote per share. Voting rights are not cumulative and, therefore, the holders of more than 50% of the common stock could, if they choose to do so, elect all of the directors of the Company, subject to the rights of the preferred stockholders.
Equity Purchase Agreement with Cross & Company
On April 28,September 18, 2020, the Company executedentered into an intellectual property rightsEquity Purchase Agreement with Cross and Company. We have the right to “put,” or sell, up to 8,108,108 shares of our common stock to Cross. Unless terminated earlier, Cross’s purchase and transfer agreement whereby this agreement grants certain IPcommitment will automatically terminate on the earlier of the date on which Cross shall have purchased shares pursuant to the Company. In connection withEquity Purchase Agreement for an aggregate purchase price of $6,000,000 or September 18, 2023. The purchase price per share is calculated at a fifteen percent discount of the execution of this agreement, the Company issued 25,000 shareslowest trading price of the Company’s common stock at $0.53 a share toduring the research organization.ten days after Cross and Co. receives the shares.
On September 24, 2020,January 4, 2021, the Company issued 46,896 shares of common stock for the conversion of $18,000$10,000 convertible notes payable and $2,000$272 of related accrued interest.interest at $0.09 per share.
On September 25, 2020,January 12, 2021, the companyCompany issued 500,000 shares of the company’s common stock at $0.50 a share, which was a discount to the trading price of $0.80$0.18 per share in exchange for a settlement of accrued expenses.
On January 13, 2021, the Company issued shares of common stock for the conversion of $10,000 convertible notes payable and $97 of related accrued interest at $0.09 per share.
On January 14, 2021, the Company sold 258,559 shares of common stock at the purchase price of $ per share for a total purchase price of $.
On January 15, 2021, the Company issued shares of common stock at $ a share, to a consultant for investor relationbusiness development services. The discount was expensed at issuance and the rest of the expense will be amortized over the life of the agreement.
As of September 30, 2020 and December 31, 2019, there were 74,797,037 and 74,225,141 shares of common stock issued and outstanding, respectively.
Stock Options
On September 1, 2017,January 15, 2021, the Company entered into an agreement for consulting services. As compensation the Company granted options to purchase 100,000issued 313,972 shares of common stock at athe price of $2.00$0.19 per share for the purchase of intellectual property based on a five year installment sale. This compensation is included in research and are exercisabledevelopment on the consolidated statement of operations. The issuance was an error and was intended, as per agreement to be 200,000 shares at the floor price of $.30 per share. The Company and the recipient have discussed the cancellation of 113,972 shares which will occur in the second quarter of 2021.
On January 28, 2021, the Company sold shares of common stock at the purchase price of $ per share for five years. Thea total purchase price of $51,410.
On February 1, 2021, the Company issued shares of common stock option vestsfor the conversion of $45,000 convertible notes payable and $162 of related accrued interest at $0.09 per share.
On February 10, 2021, the Company issued shares of common stock for the conversion of $20,000 convertible notes payable and $4,200 of related accrued interest at $ per share.
On February 10, 2021, the Company issued shares of common stock in equal monthly installmentsexchange of 24 months. These options were valuedcash at $20,154 using$0.17 per share for a Black-Scholes options pricing model.total purchase price of $121,577.
On February 10, 2021, the Company issued shares of common stock in exchange of cash at $0.10 per share for a total purchase price of $350,000.
On February 22, 2021, the Company issued shares of common stock in exchange of cash at $ per share for a total purchase price of $115,617.
On February 26, 2021, the Company issued shares of common stock in exchange of cash at $ per share for a total purchase price of $90,146.
On March 2, 2021, the Company issued shares of common stock at the price of $ per share in exchange for a settlement of accrued expenses.
On March 4, 2021, the Company issued shares of common stock at $ a share, to a consultant for business development services.
On March 12, 2021, the Company issued its CEO shares of common stock at $0.13 a share in lieu of $90,000 of deferred salary.
On March 25, 2021, the Company issued shares of common stock in exchange of cash at $0.14 per share for a total purchase price of $56,437.
| 16 |
Stock Options
On May 4, 2020, the Company granted options to purchase shares of common stock at a price of $0.57$ per share to certain directors and employees of the Company (including our named executive officers) and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over thirty six (36) months starting January 1, 2020. These options were valued at $3,152,050$3,152,050 using a Black-Scholes Options Pricing Model.
On May 18, 2020, the Company granted options to purchase shares of common stock at a price of $ per share to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $34,260 using a Black-Scholes Options Pricing Model.
On September 14, 2020 and December 24, 2020, the Company granted options to purchase shares of common stock, respectively, at a price of $ and $ per share, respectively, to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $180,950 using a Black-Scholes Options Pricing Model.
On September 23, 2020, the Company granted options to purchase shares of common stock at a price of $ per share to a consultant, who is a related party, and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twenty four (24) months. These options were valued at $109,060 using a Black-Scholes Options Pricing Model.
On December 28, 2020, the Company granted options to purchase shares of common stock at a price of $ per share to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $26,720 using a Black-Scholes Options Pricing Model.
On March 12, 2021, the Company granted options to purchase shares of common stock at a price of $ per share to certain directors and employees of the Company (including our named executive officers) and are exercisable for ten years. One quarter of these options vested on the grant day, and the remainder of the options vest equally over thirty-six months starting March 12, 2021. These options were valued at $732,795 using a Black-Scholes Options Pricing Model.
On March 12, 2021, the Company granted options to purchase shares of common stock at a price of $per share to a certain member of the Company’s corporate advisory board, as governed under agreement. One quarter of these options vested on the grant day, and the remainder of the options vest equally over twenty four months thereafter. These options were valued at $19,940 using a Black-Scholes Options Pricing Model.
On April 2, 2021, the Company granted options to purchase 75,000 shares of common stock at a price of $0.51$0.16 per share to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $35,295$9,000 using a Black-Scholes Options Pricing Model.
On September 14, 2020, the Company granted options to purchase 250,000 shares of common stock at a price of $0.84 per share to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $192,975 using a Black-Scholes Options Pricing Model.
On September 23, 2020, the Company granted options to purchase 200,000 shares of common stock at a price of $0.84 per share to a consultant, who is a related party, and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twenty four (24) months. These options were valued at $141,840 using a Black-Scholes Options Pricing Model.
The remaining expense outstanding through December 31, 2022March 12, 2024 is $2,033,545.$.
For the threesix months ended SeptemberJune 30, 20202021 and 2019,2020, the Company recorded $267,521$ and $840,$, respectively, as stock based compensation which is included in the general and administrative expenses in the condensed consolidated statement of operations and $39,075$78,955 and $0$117,225, respectively, as research and development expense.
For the nine months ended September 30, 2020 and 2019, the Company recorded $1,215,090 and $5,878, respectively, as stock based compensation which is included in the general and administrative expenses in the condensed consolidated statement of operations and $273,525 and $0 respectively, as research and development expense.
The fair value of the options is estimated using a Black-Scholes Options Pricing Model with the following assumptions:
| Schedule of share-based payment award, stock options, valuation assumptions | ||||
| Market value of common stock on issuance date | $ | |||
| Exercise price | $ | |||
| Expected volatility | % | |||
| Expected term (in years) | ||||
| Risk-free interest rate | % | |||
| Expected dividend yields |
| 17 |
On August 14, 2019, the Board authorized the Company’s 2019 Equity Incentive Plan (the “2019 Plan”) in order to facilitate the grant of cash and equity incentives to directors, employees (including our named executive officers) and consultants of our company and certain of its affiliates and to enable our company and certain of its affiliates to obtain and retain services of these individuals, which is essential to our long-term success. Our 2019 Plan allows for the grant of a variety of equity vehicles to provide flexibility in implementing equity awards, including incentive stock options, non-qualified stock options, restricted stock grants, unrestricted stock grants and restricted stock units. A total of There were initially shares of Company common stock authorized for issuance under our 2019 Plan.
On May 4, 2020, the Company amended its 2019 Plan to increase the number of shares of Company common stock authorized for issuance thereunder to 11,500,000 shares. On March 12, 2021, the Company executed a second amendment to the 2019 Plan to (i) replace all references to “Kannalife, Inc.,” the Company’s former name, to “Neuropathix, Inc.,” and (ii) increase the number of shares of Company common stock authorized for issuance thereunder 20,000,000 shares (the “Second Plan Amendment”).
The Second Plan Amendment was approved by the Company’s Board of Directors on March 12, 2021. The Second Plan Amendment remains subject to shareholder approval, which the Company shall undertake to obtain as soon as reasonably practicable, but in no event later than one year from the amendment date. In the event that the Company does not obtain the requisite shareholder approval of the Second Plan Amendment within one year, the Second Plan Amendment shall not be effective.
As of June 30, 2021, there were authorized shares of Company common stock issued and outstanding under the 2019 Plan, which was amended to 11,500,000 shares of common stock, for which as of September 30, 2020 a total of 6,050,000 are outstanding.amended.
The following is a summary of outstanding and exercisable options:
Number of Shares |
Weighted Average Exercise Price | ||||||||
| Balance at December 31, 2019 | 100,000 | $ | 2.00 | ||||||
| Issued | 6,575,000 | 0.59 | |||||||
| Expired | — | — | |||||||
| Balance at September 30, 2020 | 6,675,000 | $ | 0.61 | ||||||
| Schedule of outstanding and exercisable options | ||||||||||||
| Numbers of Options | Weighted Avg Exercise Price | Weighted Avg Remaining Years | ||||||||||
| Outstanding as of December 31, 2020 | 7,125,000 | $ | 0.58 | |||||||||
| Granted | 7,625,000 | 0.13 | ||||||||||
| Exercised | 0 | 0 | — | |||||||||
| Forfeited | 0 | 0 | — | |||||||||
| Expired | 0 | 0 | — | |||||||||
| Outstanding as of June 30, 2021 | 14,750,000 | $ | 0.35 | 9.27 | ||||||||
| Outstanding as of June 30, 2021, vested | 6,918,750 | $ | 0.44 | |||||||||
At September 30, 2020, 2,908,594 options for common stock were exercisable and the intrinsic value of these options was $1,460,334 and the weighted average remaining contractual life for options outstanding was 9.51 years.
Warrants
In December 2019,January and February 2020, the Company entered into a Securities Purchase Agreement with an investorinvestors pursuant to which the Company agreed to sell the investorinvestors a $100,000$100,000 and $50,000 convertible note bearing interest at 8%8% per annum.annum, respectively. The Company also sold warrants to the investors to purchase up to an aggregate of and shares of common stock, respectively, with an exercise term of three (3)(3) years, at a per share purchase price of one hundred twenty five percent (125%) of the voluntary or involuntary conversion price of the Company’s 8% convertible note. The warrants were deemed as a derivative liability and was recorded as a debt discount at date of issuance. See Note 10.
In January and February 2020, the Company entered into a Securities Purchase Agreement with investors pursuant to which the Company agreed to sell the investors a $100,000 and $50,000 convertible note bearing interest at 8% per annum, respectively. The Company also sold warrants to the investors to purchase up to an aggregate of 100,000 and 50,000 shares of common stock, respectively, with an exercise term of three (3) years, at a per share purchase price of one hundred twenty five percent (125%) of the voluntary or involuntary conversion price of the Company’s 8% convertible note. The warrants were deemed as a derivative liability and was recorded as a debt discount at date of issuance. See Note 10.
On June 8, 2020, the Company entered into a Securities Purchase Agreement, dated as of June 2, 2020 (the “Purchase Agreement”) with an accredited investor pursuant to which the investor purchased a 12% unsecured convertible promissory note (the “12% Note”) from the Company. In connection with the Purchase Agreement and the 12%12% Note, the Company issued a common stock purchase warrant to purchase shares of the Company’s common stock at $0.75 per share which may be exercised by cashless exercise, exercisable for a period of3 three years. The warrants were deemed as a derivative liability and was recorded as a debt discount at date of issuance. See Note 10.
On June 23, 2020, the Company entered into a Securities Purchase Agreement, dated as of June 19, 2020 with an accredited investor pursuant to which the Investor purchased a 12%12% convertible promissory note from the Company. In connection with the securities purchase agreement and the note, the Company issued two common stock purchase warrants each to purchase shares of the Company’s common stock at $1.30$ per share which may be exercised by cashless exercise, exercisable for a period of five years. One of the warrants is to be issued only in the case of default on the note. During the thirdfirst quarter of 2020,2021, the anti-dilution clause was triggered and the exercise price was reset to $0.40$ resulting in the number of warrants to be increased to 378,694.. The warrants were deemed as a derivative liability and was recorded as a debt discount at date of issuance. See Note 10.
| 18 |
On February 10, 2021, the Company entered into a letter agreement with Lyons Capital, pursuant to which the Company agreed to issue and sell 3,500,000 shares of the Company’s common stock, par value $ per share, and two warrants to purchase an aggregate of additional shares of Common Stock, the terms of such warrants are further discussed below, for an aggregate purchase price of $1,207,500. The first warrant grants Lyons Capital the right to purchase up to 1,750,000 shares of common stock at an exercise price of $0.22 per share. The second warrant grants Lyons Capital the right to purchase up to an additional 1,750,000 shares of common stock at an exercise price of $0.27 per share. The warrants are exercisable immediately, will expire five years from the date of issuance, and contain customary provisions allowing for adjustment to the exercise price and number of shares of common stock issuable upon exercise in the event of any stock dividend, recapitalization, reorganization, reclassification, or similar transaction. Lyons Capital has the right to exercise the warrants at any time; provided, however, that subject to limited exceptions, Lyons Capital may not exercise any portion of the warrants if Lyons Capital, together with any of its affiliates, would beneficially own in excess of 4.99% of the number of shares of the Company’s common stock outstanding immediately after giving effect to such exercise.
The following is a summary of outstanding and exercisable warrants:
| Schedule of outstanding and exercisable warrants | ||||||||||||||||
Number of Shares |
Weighted Average Exercise Price | Number of Shares |
Weighted Average Exercise Price | |||||||||||||
| Balance at December 31, 2019 | 100,000 | $ | 3.26 | |||||||||||||
| Balance at December 31, 2020 | 1,289,343 | $ | 0.18 | |||||||||||||
| Issued | 565,360 | 0.92 | 4,194,161 | 0.22 | ||||||||||||
| Expired | — | — | 0 | 0 | ||||||||||||
| Balance at September 30, 2020 | 665,360 | $ | 0.56 | |||||||||||||
| Balance at June 30, 2021 | 5,483,504 | $ | 0.19 | |||||||||||||
At SeptemberJune 30, 2020, 665,3602021, 5,483,504 warrants for common stock were exercisable and the intrinsic value of these warrants was $130,233$62,618 and the weighted average remaining contractual life for warrants outstanding was 3.68 4.26 years.
Amendment to Registration Statement
On September 22, 2020, the Company filed a registration statement with the SEC on Form S-1 (File No. 333-248966) (the “Registration Statement”) covering the resale of up to 8,108,108 shares of the Company’s common stock, par value $0.0001 per share, that the selling stockholder identified in the Registration Statement may acquire pursuant to that Equity Purchase Agreement by and between the Company and Cross & Company, dated September 18, 2020. The Registration Statement was originally declared effective by the SEC on October 2, 2020.
On June 23, 2021, the Company filed a Post-Effective Amendment No. 1 to the Registration Statement (the “Post-Effective Amendment”) in order to (i) include information from the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, which was filed with the SEC on March 30, 2021, and the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2021, which was filed with the SEC on May 13, 2021; (ii) incorporate by reference all future filings that the Company makes with the SEC under Sections 13(a), 13(c), 14 or 15 of the Securities Exchange Act of 1934, as amended, subsequent to the filing date of the Post-Effective Amendment until the termination of the offering of the securities made under the prospectus therein (excluding any documents or information or portions of such documents that are deemed to be furnished and not filed with the SEC); and (iii) update certain other information in the Registration Statement.
No additional securities were registered under the Post-Effective Amendment. Accordingly, the Post-Effective Amendment covers only resales from time to time by the selling stockholder of up to 8,108,108 shares of the Company’s common stock registered under the Registration Statement.
NOTE 1516 – RELATED PARTY TRANSACTIONS
The Company’s Chief Executive Officer (“CEO”) shares the use of the leased office space for personal living quarters. The CEO reimburses the Company for 50% of the monthly rent, or $2,800$2,800 per month.
As of SeptemberJune 30, 2020,2021, the Company owes the CEO $47,354$160,000 for accrued compensation and $25,496 for expenses the CEO incurred on behalf of the Company.
During the six months ended June 30, 2021, the Company repaid $40,000 to its CEO in exchange for the discharge of a portion of his accrued expenses.
On March 12, 2021, the Company issued its CEO shares of common stock at $0.13 a share in lieu of $ of accrued compensation.
In May 2021, the Company repaid $10,000 to its CEO in exchange for the discharge of a portion of his accrued expenses.
See Notes 8, 11, 14, and 1417 for additional related party transactions.
NOTE 1617 – SUBSEQUENT EVENTS
On October 7, 2020, borrowed an additional $80,000 from a note holder based on July 13, 2021, the same terms and conditions. See Note 7.
On October 8, 2020, the company issued 350,000Company granted options to purchase shares of the company’s common stock at $0.48 a price of $ per share to a consultant and are exercisable for business development services.ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. provided, however, that there has not been a termination of service as of each such date. In no event will the option become exercisable for any additional option shares after a termination of service.
On October 13, 2020,July 7, 2021, the company put 250,000 shares of its common stock to Cross & Company with net proceeds of $8,287.50. On July 26, 2021, Cross & Company exercised its right of offset under the promissory note agreement with the Company. After the effect of the offset, the promissory note has a remaining balance of $91,712.50 with a maturity date of July 26, 2023. The note bears interest at a rate of 0.25% per annum.
On July 28, 2021, the Company issued 64,421agreed to convert $90,000 of Dean Petkanas’ accrued salary to 1,875,000 shares at a market closing price of common stock for the conversion of $18,000 convertible notes payable and $2,000 of related accrued interest.$0.048 per share.
On October 23, 2020, the Company issued 40,731 shares of common stock for the conversion of $10,000 convertible notes payable.
Name Change – Neuropathix, Inc.
On November 4, 2020, the Company filed an amendment to its certificate of incorporation with the Delaware Secretary of State that changed its name to Neuropathix, Inc. The Company concurrently submitted a request to FINRA for approval of the name change as well as a ticker symbol change from “KLFE” to “NPTX.” The Company’s name change and ticker symbol change was reviewed and processed by FINRA and went effective November 6, 2020.
| 19 |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this quarterly report. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” in our Form 10-K filed with the Securities and Exchange Commission on March 30, 2020.2021.
Forward-Looking Statements
This quarterly report on Form 10-Q contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act.(the “Exchange Act”). All statements other than statements of historical fact contained in this quarterly report, including statements regarding our future operating results, financial position and cash flows, our business strategy and plans and our objectives for future operations, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. This quarterly report on Form 10-Q also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “would,” “could,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this quarterly report are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, operating results, business strategy, short-term and long-term business operations and objectives. These forward looking statements speak only as of the date of this quarterly report and are subject to a number of risks, uncertainties and assumptions. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Business Developments
The Company was originally incorporated in the State of Delaware on March 25, 2013 under the name of TYG Solutions Corp. Our original business plan was to develop iPhone and Android smartphone apps for companies who need an app for their internal and external operations. We subsequently expanded our operations to offering corporate website design services.
On July 25, 2018, the Company entered into a Share Exchange Agreement with Kannalife Sciences, Inc., a Delaware corporation (“Kannalife”Kannalife Sciences”), and certain stockholders of Kannalife Sciences (the “Kannalife Sciences Stockholders”). Pursuant to the terms of the Share Exchange Agreement, the Company acquired nearlysubstantially all of the issued and outstanding shares of Kannalife Sciences by means of a share exchange with the Kannalife Sciences Stockholders in exchange for newly issued shares of the common stock of the Company (the “Share Exchange”). As a result of the Share Exchange, Kannalife Sciences became a wholly-owned99.7% owned subsidiary of the Company. The business operations of the Company shall continue uninterrupted,regarding iPhone and Android smartphone apps was reduced significantly to focus efforts on target therapeutics and drug discovery, and accordingly, by virtue of the Share Exchange, the Company acquired the business of Kannalife Sciences including all of its assets. The Share Exchange was accounted for as a reverse acquisition and change in reporting entity, whereby Kannalife Sciences was the accounting acquirer.
Kannalife was incorporated in the State of Delaware on August 11, 2010. Kannalife is a developmental stage phyto-medical/pharmaceutical and drug discovery company that specializes in the research, development of cannabinoid and cannabinoid-based therapeutic products derived from synthetic and botanical sources, including the Cannabis “taxa” (the word “taxa” is the plural of “taxon” which defines a group of one or more populations of an organism or organisms to form a unit.) On November 9, 2018, the Company filed an amendment to its certificate of incorporation with the Delaware Secretary of State that changedto change its name to Kannalife, Inc. The Company concurrently submitted a request to FINRA for approval of the name change as well as a ticker symbol change to “KLFE” and such action went effective on January 17, 2019.
Name Change – Neuropathix, Inc.
On November 4, 2020, the Company filed an amendment to its certificate of incorporation with the Delaware Secretary of State that changedto change its name to Neuropathix,“Neuropathix, Inc.” The Company concurrently submitted a request to FINRA for approval of the name change as well as a ticker symbol change from “KLFE” to “NPTX.” The Company’s name change and ticker symbol change was reviewed and processed by FINRA, and went effective November 6, 2020.
| 20 |
Kannalife Sciences was incorporated in the State of Delaware on August 11, 2010. Kannalife Sciences is a developmental stage phyto-medical/pharmaceutical and drug discovery company that specializes in the research, development of cannabinoid and cannabinoid-based therapeutic products derived from synthetic and botanical sources, including the Cannabis “taxa” (the word “taxa” is the plural of “taxon,” which defines a group of one or more populations of an organism or organisms to form a unit).
Business Overview
As a result of the Share Exchange, the Company’sour core businesses are comprised of the following:
| A drug development company focused on the research and development (R&D) of synthetic and phyto-medical products from: |
| o | naturally recurring sources, including but not limited to cannabis, hemp, and other similar species of |
| o | semi-synthetic sources; and |
| o | synthetic and bio-synthetic sources. |
The Company is primarily involved in the research and development of novel therapeutic agents for use in and as U.S. Food and Drug Administration (“FDA”) approved ethical pharmaceuticals (available by doctor prescription); FDA Monograph topical solutions; and Personal Care Products Council (“PCPC”) / International Nomenclature of Cosmetic Ingredients (“INCI”). The primary focus of the Company’s research and development revolves around its patented, proprietary cannabidiol-derived new chemical entities and cannabidiol. In preclinical testing, certain molecules under U.S. Patent 9,611,213 were screened for neuroprotection, and may have the potential mechanism of action for reducing inflammation and neuropathic pain. These molecules indicate that they are more soluble than cannabidiol, also deemed a neuroprotectant with potential anti-inflammatory properties. A molecule that is potentially more water soluble than cannabidiol in this regard may be good candidate(s) for use in topical applications.
The Company has been the only licensee from the National Institutes of Health (“NIH”) for the licensed use of the U.S. Government’s patent 6,630,507 – “Cannabinoids as Antioxidants and Neuroprotectants” (the “’507 Patent”) in the disease indications of hepatic encephalopathy (“HE”) and Chronic Traumatic Encephalopathy (“CTE”). Having been the only licensee to the ‘507 Patent has given the Company an early start in the research and development of cannabinoid therapeutics within this emerging market. The Company is the only company that has had use of the ‘507 Patent and corresponding licenses from NIH-OTT.
| Drug discovery platform to evaluate and potentially treat neurological and oxidative stress related disorders such as OHE, CTE and CIPN with high quality assured, quality controlled cGMP pharmaceutical grade semi-synthetic and synthetic cannabinoids, CBD, and CBD-like molecules. |
| • | Topical skincare pre-clinical program designed to some of our patented, proprietary CBD-derived NCEs, for use as topical solutions, ointments, and creams for disorders such as diabetic neuropathies, diabetic ulcers, and for use as an anti-pruritic. Anti-pruritics are known as anti-itch drugs and medications that inhibit the itching often associated with a variety of disorders and diseases. |
The jurisdictions in which the ‘507 Patent is valid are: the U.S., the U.K., Ireland, the E.U., and Australia. The patent life in these jurisdictions were good until April 21, 2019.
The Company believes that these licenses with the NIH have given the Company, through the years, the preclinical lead time to evaluate both HE and CTE without stress of competition. The Company also believes that such advances in preclinical have led to a drug development program regarding cannabidiol based therapeutics that focuses on neurodegenerative and oxidative stress related diseases described in the ‘507 Patent, and also the development of the Company’s own intellectual property underlying U.S. Patents 9,611,213 and 10,004,722.
Furthermore, it is on the Company’s belief and knowledge that while the U.S. Government patent 6,630,507 expired on April 21, 2019, there may be additional opportunities related to the original licensing of the ‘507 Patent in which the Company may engage with the NIH and certain collaborators of the aforementioned patent to enter into a Cooperative Research and Development Agreement (“CRADA”) with the NIH for one or more disease indications underlying the ‘507 Patent, including but not limited to HE and CTE. Moreover, the weight of the Company’s future success regarding its drug development program in relation to cannabidiol based therapeutics is not centered on the ‘507 Patent, but rather its own intellectual property underlying U.S. Patents 9,611,213 and 10,004,722.
We intend to study KLS-13019 in patients with chemotherapy induced neuropathic pain, and we intend to study KLS-13023 in patients with mild traumatic brain injury.
We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens.
We are still conducting pre-clinical studies and have not yet commenced our clinical program or tested KLS-13019 or KLS-13023 in humans. For KLS-13019, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in either the United States or Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for KLS-13023 in either the United States or Australia, subject to applicable regulatory approval. We plan to submit New Drug Applications (NDAs) for KLS-13019 and KLS-13023 to the FDA upon completion of all requisite clinical trials. We expect to initiate clinical trials for KLS-13019 and KLS-13023 in the first half of 2021.
We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for KLS-13019 in Australia, subject to applicable regulatory approval, and do not expect at this time to file an investigational new drug application, or IND, with the U.S. Food and Drug Administration, or the FDA, prior to the commencement of those clinical trials. We must file an IND with the FDA and receive approval from the U.S. Drug Enforcement Agency, or DEA, prior to commencement of any clinical trials in the United States.
We plan to seek orphan drug designation for KLS-13023 in Overt Hepatic Encephalopathy.
Cannabinoids are a class of molecules derived from Cannabis plants. The two primary cannabinoids contained in Cannabis are cannabidiol, or CBD, and D9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating refractory epilepsy, FXS and arthritis and THC has positive effects on treating pain. Interest in cannabinoid therapeutics has increased significantly over the past several years as preclinical and clinical data has emerged highlighting the potential efficacy and safety benefits of cannabinoid therapeutics. The cannabinoid therapeutics market is expected to grow significantly due to the potential benefits these products may provide over existing therapies. In addition to KLS-13019 and KLS-13023 may potentially offeroffering first-line therapies to patients suffering from chemotherapy induced peripheral neuropathy and mild traumatic brain injury, respectively.
KLS-13019’s advanced formulation is designed to improve on some of the limitations associated with CBD, including but not limited to CBD’s low bioavailability and limited drug like properties. However, KLS-13019 has not been reviewed or approved for patient use by the FDA or any other healthcare authority in the world. Our pre-clinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an in vitro study performed by us demonstrated that CBD is degraded to THC in an acidic environment such as the stomach.
In the past three years, our most recent research and development efforts have been centered on the use of KLS-13019 as a neuroprotectant and therapeutic agent to treat chronic and neuropathic pain. There is currently no FDA approved drug to treat CIPN. Our preclinical efforts in the research and development of treating CIPN with our lead compound KLS-13019 have been fostered by a successful study grant from NIH-NIDA that compared KLS-13019 to CBD in the prevention and reversal of neuropathic pain in animal models. As a result of the outcome of this and other preclinical studies, we believe there is strong evidence to support the use of KLS-13019 as a non-opioid solution to chronic and neuropathic pain in human clinical trials.
We intend to study KLS-13019 in patients with chemotherapy induced neuropathic pain, and we intend to study KLS-13023 in patients with mild traumatic brain injury. We believe that the claims made in the Pat. 9,611,213 and Pat. 10,004,722 sufficiently cover the use of the novel molecule KLS-13019 in the treatment of neuropathic pain, which is broadly defined and includes chemotherapy induced neuropathic pain (a/k/a: chemotherapy induced peripheral neuropathy).
| 21 |
To date, we have synthesized, pre-clinically tested and patented our proprietary CBD like NCEs, including KLS-13019, and also formulated a new CBD based molecule, KLS-13023. KLS-13023 is a target drug candidate that includes a synthetic CBD formulated in a gel capsule designed for potential use in humans.humans, which is intended to enable more effective delivery of CBD. The formulation of this product is proprietary and currently held as a trade secret of the Company. CBD is the primary non-psychoactive component of Cannabis.cannabis. KLS-13023 has undergone a manufacturing feasibility study to improve some of the limitations associated with CBD, including but not limited to CBD’s low bioavailability and limited drug like properties and improvement of the delivery of CBD through the first pass in the gut and into the circulatory system.
In addition toour preclinical animal studies, KLS-13023 the Company has developed a proprietary patented new chemical entity (NCE), KLS-13019. This NCE is a cannabidiol derived molecule which has undergone pre-clinical studies for the treatmentdemonstrated effective intervention of overt hepatic encephalopathy and chemotherapy induced peripheral neuropathy.
In pre-clinical studies, KLS-13019's advanced formulation is designed to improve on some of the limitations associated with CBD, including but not limited to CBD’s low bioavailability and limited drug like properties. However, KLS-13019 has not been reviewed or approved for patient use by the U.S. Food and Drug Administration or any other healthcare authorityneurodegeneration in the world.
These pre-clinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an OHE disease state. We intend to study KLS-13023 in vitro study performed by us demonstrated that CBD is degraded to THC in an acidic environment such as the stomach.
The Company has filed for orphan designationpatients with the U.S. Food and Drug Administration’s (“FDA”) Office of Orphan Products Development (the “OOPD”) for the use of CBD in the treatment of overt hepatic encephalopathy (“OHE”). The Company has received notice from the OOPD that its current application qualifies for a patient population of less than 200,000, but is currently in abeyance to resolve clinical use of CBD in this sub-set of hepatic encephalopathy. The Company has retained Coté Orphan to continue the process of responding to the OOPD’s abeyance letter. On November 5, 2018, the OOPD has granted the Company a one year extension to respond to the abeyance letter until November 30, 2019. On June 28, 2019, the Company sent its response to the OOPD's abeyance letter dated November 5, 2019. On August 28, 2019, the OOPD replied with certain objections associated with the Company's request for orphan drug designation. The OOPD has given the Company until August 28, 2020 to respond to the abeyance letter dated August 28, 2019.
KLS-13023 is a proprietary formulation containing CBD that intends to enable more effective delivery of CBD via a gel capsule.mild traumatic brain injury. In addition, we expect that KLS-13023 will be classified by the FDA as a new chemical entity, oran NCE. In our preclinical animal studies, KLS-13023 demonstrated effective intervention of neurodegeneration in the OHE disease state. Our key development programs
We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens.
We are still conducting pre-clinical studies and expected timelineshave not yet commenced our clinical program or tested KLS-13019 or KLS-13023 in humans. For KLS-13019, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in either the United States or Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for KLS-13023 in either the development ofUnited States or Australia, subject to applicable regulatory approval. We plan to submit NDAs for KLS-13019 and KLS-13023 are shownto the FDA upon completion of all requisite clinical trials. We expect to initiate clinical trials for KLS-13019 and KLS-13023 in the table below:first quarter of 2023.
We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for KLS-13019 in the U.S. or Australia, subject to applicable regulatory approval, and do not expect at this time to file an investigational new drug application, or IND, with the U.S. Food and Drug Administration, or the FDA, prior to the commencement of those clinical trials. We must file an IND with the FDA and receive approval from the U.S. Drug Enforcement Agency, or DEA, prior to commencement of any clinical trials in the United States.
Pharmacokinetic and Pharmacodynamic Comparison Between KLS-13019 and CBD
Results from PK and PD studies performed in evaluating CBD versus KLS-13019 have shown KLS-13019 to be superior in aqueous solubility (potential for drug absorption after oral administration); Log P (ratio which measures difference in solubility in two phases); bioavailability (proportion of the drug that enters the circulation); and C max at 10 mg/kg, p.o. (peak serum concentration).

Results from our pre-clinical efforts in the potential treatment of OHE and the potential treatment of CIPN have shown a marked improvement over 99.7% pure pharmaceutical grade synthetic CBD in side by side pre-clinical comparison. In a pre-clinical comparison for neuroprotection between CBD and KLS-13019, results indicated increased potency for the new molecule (KLS-13019) as determined by six assays, while both molecules exhibited efficacy in preventing oxidative stress-related toxicities back to control values.
| 22 |
Treatment with KLS-13019 alone, however, was 5-fold less toxic than CBD. Previous studies suggested that CBD targeted the Na+ Ca2+ (sodium-calcium) exchanger in mitochondria to regulate intracellular calcium levels, an important determinant of neuronal survival. After treatment with an inhibitor, the mNCX inhibitor (“CGP-37157”), no detectable neuroprotection from ethanol toxicity was observed for either CBD or KLS-13019. Furthermore, AM630 (a CB2 antagonist) significantly attenuated CBD-mediated neuroprotection, while having no detectable effect on KLS-13019 neuroprotection. Our studies indicated KLS-13019 was more potent and less toxic than CBD. Both molecules can act through mNCX. Based on these results, amongst other things, we believe that KLS-13019 may provide an alternative to CBD as a therapeutic candidate to treat disease associated with oxidative stress.
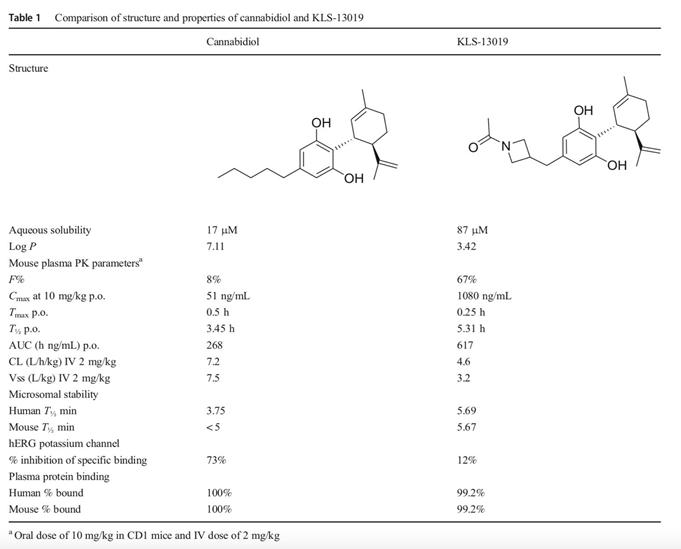

As previously noted, comparisons between CBD and KLS-13019 have been published in peer reviewed articles in ACS Medicinal Chemistry Letters (2016, 7, 424-428) and Journal of Molecular Neuroscience (14 August 2018).
Additional follow on studies recently published on May 10, 2019 in the Journal of Molecular Neuroscience have further advanced our studies on the mechanism of action for CBD and KLS-13019 in pre-clinical testing for the treatment of CIPN. The mechanism of action for CBD-and KLS-13019-mediated protection now has been explored with dissociated dorsal root ganglion (“DRG”) cultures using small interfering RNA (siRNA) to the mitochondrial Na+ Ca2+ exchanger-1 (“mNCX-1”). Treatment with this siRNA produced a 50–55% decrease in the immunoreactive (“IR”) area for mNCX-1 in neuronal cell bodies and a 72–80% decrease in neuritic IR area as determined with high-content image analysis. After treatment with 100 nM KLS-13019 and siRNA, DRG cultures exhibited a 75 ±5% decrease in protection from paclitaxel-induced toxicity, whereas siRNA studies with 10 μM CBD produced a 74± 3% decrease in protection. Treatment with mNCX-1 siRNA alone did not produce toxicity. The protective action of cannabidiol and KLS-13019 against paclitaxel-induced toxicity during a 5-h test period was significantly attenuated after a 4-day knockdown of mNCX-1 that was not attributable to toxicity. This data indicates that decreases in neuritic mNCX-1 corresponded closely with decreased protection after siRNA treatment. Pharmacological blockade of mNCX-1 with CGP-37157 produced complete inhibition of cannabinoid-mediated protection from paclitaxel in DRG cultures, supporting the observed siRNA effects on mechanism.
| 23 |
Sodium-Calcium Exchanger (“NCX”) (often denoted Na+/Ca2+ exchanger, NCX, or exchange protein) is an antiporter membrane protein that removes calcium from cells. The exchanger exists in many different cell types and animal species. The NCX is considered to be one of the most important cellular mechanisms for removing Ca2+ (calcium ions) from cells. The exchanger is usually found in the plasma membranes and the mitochondria and endoplasmic reticulum of excitable cells.
Mitochondria is a double-membrane-bound organelle found in most eukaryotic organisms. Mitochondria generate most of the cell’s supply of adenosine triphosphate (“ATP”), used as a source of chemical energy. ATP is a complex organic chemical that provides energy to drive many processes in living cells, including muscle contractions, nerve impulse propagation and chemical synthesis.
According to Fallon, et al. in the March/April 2006 edition of Clinical Medicine, pain is uncontrolled with opioid treatments in approximately 20% of patients with advanced cancer, or 420,000 people in the United States. There are currently no FDA approved non-opioid treatments for patients who do not respond to, or experience negative side effects with, opioid medications. We believe that KLS-13019 has the potential to address a significant unmet need in this large market by treating patients with a product that employs a differentiated non-opioid mechanism of action, and offers the prospect of pain relief without increasing opioid-related adverse side effects.
Clinical Timelines
As a result of the unprecedented effects of COVID-19, the Company haswe have updated itsour clinical timelines to give effect to the significant interruption to business and financial operations worldwide as a result of the COVID-19 crisis. The Companypandemic. We will continue to monitor the progress of the shutdowns currently in effect, and revise itsour clinical timelines accordingly.
Product Candidate | Target Indication | Delivery Method | Current Development Status | Expected Next Steps |
| KLS-13019 | Chemotherapy Induced | Oral Gel Capsule | Preclinical | |
| Peripheral Neuropathy | ||||
| Mild Traumatic Brain Injury | Oral Gel Capsule | Preclinical | ||
| KLS-13023 | Overt Hepatic Encephalopathy | Oral Gel Capsule | Preclinical | |
| Mild Traumatic Brain Injury | Oral Gel Capsule | Preclinical |
With respect to certain other proprietary compounds underlying U.S. PatentPat. 9,611,213, the Company planswe plan on pursuing topical solutions as potential relief creams and/or ointments for neuropathic pain, anti-inflammation, anti-pruritic and skin ulcers. The Company isWe are considering commercialization routes that include, but are not limited to, filing and FDA Monograph and/or pursing a path to the marketplace through INCI certification and registration with the PCPC. In preclinical testing, certain molecules under U.S. PatentPat. 9,611,213 were screened for neuroprotection and may have the potential mechanism of action for reducing inflammation and neuropathic pain. These molecules indicate that they are more soluble than cannabidiol,CBD, also deemed a neuroprotectant with potential anti-inflammatory properties. A molecule that is potentially more water soluble than cannabidiolCBD in this regard may be good candidate(s) for use in topical applications.
The Company believes it
We believe that we will be able to raise the sufficient capital to proceed forth with a Phase 1 human safety trial for the treatment of Chemotherapy Induced Peripheral Neuropathy. All preclinical work in this indication, including animal toxicity studies, are expected to be completed before the end of the second quarter 2021. The Company plans2022. We plan on entering into clinical trials sometime in the fourthfirst quarter 2021. of 2023.
Additionally, the Company believes itwe believe that we will be able to raise the sufficient capital to proceed forth with a Phase 1 human safety trial for the treatment of Overt Hepatic Encephalopathy. All preclinical work in this indication, including animal toxicity studies, are expected to be completed before the end of the second quarter 2021. 2023. We plan on entering into clinical trials sometime in the first quarter 2024.
The Company intends on seeking
We intend to seek additional capital to proceed forth with itsour business plan regarding additional drug pipeline opportunities.
| 24 |
Components of Results of Operations
Our net losses were $2,977,590$2,079,695 and $2,719,412$2,366,784 for the ninesix months ended SeptemberJune 30, 20202021 and 2019,2020, respectively. We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability.
Financial Operations Overview
The following discussion sets forth certain components of our statements of operations as well as factors that impact those items.
Revenues
Our revenues consist of state and federal research grants and fees received from research services for third-party product development. These revenues are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured.
Research and Development Expenses
Our research and development expenses consist of expenses incurred in development and preclinical studies relating to our product candidates, including:
| • | expenses associated with preclinical development; |
| • | personnel-related expenses, such as salaries, benefits, travel and other related expenses, including stock-based compensation; |
| • | payments to third-party contract research organizations, or CROs, contractor laboratories and independent contractors; and |
| • | depreciation, maintenance and other facility-related expenses. |
We expense all research and development costs as incurred. Preclinical development expenses for our product candidates are a significant component of our current research and development expenses. Product candidates in later stage clinical development generally have higher research and development expenses than those in earlier stages of development, primarily due to increased size and duration of the clinical trials. We track and record information regarding external research and development expenses for each grant, study or trial that we conduct. From time to time, we intend to use third-party CROs, and have used contractor laboratories and independent contractors in preclinical studies. We recognize the expenses associated with third parties performing these services for us in our preclinical studies based on the percentage of each study completed at the end of each reporting period.
We incurred research and development expenses of $204,653 and $307,392 for the six months ended June 30, 2021 and 2020, respectively.
We expect that our research and development expenses in 20202021 and for the next several years will be higher than in 20192020 as a result of the work needed for our expected initiation of our Phase 1 clinical trials of KLS-13019 and KLS-13023. These expenditures are subject to numerous uncertainties regarding timing and cost to completion. Completion of our preclinical development and clinical trials may take several years or more and the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:others:
| • | the number of sites included in the clinical trials; |
| • | the length of time required to enroll suitable patients; |
| • | the size of patient populations participating in the clinical trials; |
| • | the duration of patient follow-ups; |
| • | the development stage of the product candidates; and |
| • | the efficacy and safety profile of the product candidates. |
| 25 |
Due to the early stages of our research and development, we are unable to determine the duration or completion costs of our development of KLS-13019 and KLS-13023. As a result of the difficulties of forecasting research and development costs of KLS-13019 and KLS-13023 as well as the other uncertainties discussed above, we are unable to determine when and to what extent we will generate revenues from the commercialization and sale of an approved product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and other related costs, including stock-based compensation, for personnel serving in our executive, finance, accounting, legal and human resource functions. Our general and administrative expenses also include facility and related costs not included in research and development expenses, professional fees for legal services, including patent-related expenses, consulting, tax and accounting services, insurance and general corporate expenses. We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates.
We expect that our general and administrative expenses in 20202021 and for the next several years will be higher than in 20192020 as we increase our headcount. We also anticipate increased expenses relating to our operations as a public company, including increased costs for the hiring of additional personnel, and for payment to outside consultants, including lawyers and accountants, to comply with additional regulations, corporate governance, internal control and similar requirements applicable to public companies, as well as increased costs for insurance.
Interest Income (Expense), net
Interest expense consists of interest expense on our notes payable. Interest income consists primarily of interest earned on our money market bank account.
Income Taxes
We file income tax returns in the United States federal jurisdiction and in various state and local jurisdictions. In the normal course of business, we are subject to examination by taxing authorities. The tax years ending 2017 through 2020 remain subject to examination for federal tax purposes and remain subject to examination in significant state tax jurisdictions.
As of December 31, 2019,2020, we had $4,248,000$7.00 million of federal operating loss carryforwards. These operating loss carryforwards will begin to expire in 2031.2033. The Tax Reform Act of 1986, or the Act, provides for limitation on the use of net operating loss and research and development tax credit carryforwards following certain ownership changes (as defined by the Act) that could limit our ability to utilize these carryforwards. We may have experienced various ownership changes, as defined by the Act, as a result of past financings. Accordingly, our ability to utilize the aforementioned carryforwards may be limited. Additionally, U.S. tax laws limit the time during which these carryforwards may be applied against future taxes; therefore, we may not be able to take full advantage of these carryforwards for federal income tax purposes.
The closing of the Share Exchange transaction, together with private placements and other transactions that have occurred since our inception, may trigger, or may have already triggered, an "ownership change"“ownership change” pursuant to Section 382 of the Internal Revenue Code of 1986. If an ownership change is triggered, it will limit our ability to use some of our net operating loss carryforwards. In addition, since we will need to raise substantial additional funding to finance our operations, we may undergo further ownership changes in the future, which could further limit our ability to use net operating loss carryforwards. As a result, if we generate taxable income, our ability to use some of our net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could result in increased future tax liability to us.
| 26 |
Critical Accounting Policies and Use of Estimates
We have based our management's discussion and analysis of financial condition and results of operations on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to preclinical development expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe to be appropriate under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully discussed in Note 2 to our condensed consolidated financial statements appearing above, we believe that the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our financial statements.
Research and Development Expenses
We rely on third parties to conduct our preclinical studies and to provide services, including data management, statistical analysis and electronic compilation. Once our clinical trials begin, at the end of each reporting period, we will compare the payments made to each service provider to the estimated progress towards completion of the related project. Factors that we will consider in preparing these estimates include the number of patients enrolled in studies, milestones achieved and other criteria related to the efforts of our vendors. These estimates will be subject to change as additional information becomes available. Depending on the timing of payments to vendors and estimated services provided, we will record net prepaid or accrued expenses related to these costs.
Fair Value of Common Stock and Stock-Based Compensation
We account for grants of stock options and restricted stock to employees based on their grant date fair value, and recognize compensation expense over the vesting periods. We estimate the fair value of stock options as of the date of grant using the Black-Scholes option pricing model, and we estimate the fair value of restricted stock based on the fair value of the underlying common stock as determined by our board of directors or the value of the services provided, whichever is more readily determinable. We account for stock options and restricted stock awards to non-employees using the fair value approach. Stock options and restricted stock awards to non-employees are subject to periodic revaluation over their vesting terms.
InThe Company accounts for share-based compensation in accordance with the absencefair value recognition provision of a public trading marketFASB ASC 718, Compensation – Stock Compensation (“ASC 718”), prescribes accounting and reporting standards for our commonall share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the consolidated financial statements based on eachthe estimated grant date we developfair values. That expense is recognized over the period during which an estimateemployee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of FASB ASC 505, Equity–based Payments to Non-Employees (“ASC 505”). Measurement of share-based payment transactions with non-employees is based on the fair value of our common stock forwhichever is more reliably measurable: (a) the option and restricted stock grants based in part on input from an independent third-party valuation firm. We determinedgoods or services received; or (b) the equity instruments issued. The fair value of our common stock using methodologies, approaches and assumptions consistent with the AICPA Practice Guide, Valuation of Privately-Held Company Equity Securities Issued as Compensation. In addition, our board of directors considered various objective and subjective factors, along with input from management and an independent third-party valuation firm, to estimate the fair value of our common stock, including external market conditions affecting the pharmaceutical industry, trends within the pharmaceutical industry, the prices at which we sold shares of our different series of preferred stock, the superior rights and preferences of each series of preferred stock relative to our common stockshare-based payment transaction is determined at the timeearlier of each grant, our results of operations and financial position, the status of our research and development efforts and progress of our preclinical programs, our stage of development and business strategy, the lack of an active public market for our common and our preferred stock, and the likelihood of achieving a liquidity event.performance commitment date or performance completion date.
| 27 |
Results of Operations – For the Three Month Periods Ended SeptemberJune 30, 20202021 and 20192020
Revenues
Revenues for the three months ended SeptemberJune 30, 2020, was $0 compared to $23,9682021 as well as for the three months ended SeptemberJune 30, 2019. Our2020 was $0.
Research and Development Expenses
Research and development expenses decreased by $211,661 or 74%, to $76,004 for the three months ended June 30, 2021, from $287,665 for the three months ended June 30, 2020. The decrease was primarily due to less stock compensation through issuance of stock options to the officers and research and development companies for their services rendered during the three months ended June 30, 2021.
General and Administrative Expenses
General and administrative expenses decreased by $616,892 or 51%, to $585,415 for the three months ended June 30, 2021, from $1,202,307 for the three months ended June 30, 2020. This decrease was primarily due to a decrease in stock based compensation issued to employees and consultants.
Results of Operations – For the Six Month Periods Ended June 30, 2021 and 2020
Revenues
Revenues for the six months ended June 30, 2021, was $32,000 compared to $0 for the six months ended June 30, 2020. The revenue was the result of a decrease of grant revenue due to reachingreceived during the maximum amount of the grant.six months period ended June 30, 2021.
Research and Development Expenses
Research and development expenses decreased by $120,897$102,739 or 64%33%, to $68,546$204,653 for the threesix months ended SeptemberJune 30, 2020,2021, from $189,443$307,392 for the threesix months ended SeptemberJune 30, 2019.2020. The decrease was primarily the result of reduction in grant revenue used in research and development.issuing less stock options to employees.
General and Administrative Expenses
General and administrative expenses increased by $135,556$6,190 or 23%0.4%, to $737,123$1,572,604 for the threesix months ended SeptemberJune 30, 2020,2021, from $601,567$1,566,414 for the threesix months ended SeptemberJune 30, 2019. This increase was primarily due to an increase in stock based compensation issued to employees and consultants.2020.
Results of Operations – For the Nine Month Periods Ended September 30, 2020 and 2019
Revenues
Revenues for the nine months ended September 30, 2020, was $0 compared to $126,027 for the nine months ended September 30, 2019. Our decrease in revenue was the result of a decrease of grant revenue due to reaching the maximum amount of the grant.
Research and Development Expenses
Research and development expenses decreased by $63,742 or 15%, to $375,938 for the nine months ended September 30, 2020, from $439,680 for the nine months ended September 30, 2019. The decrease was primarily the result of reduction in grant revenue used in research and development.
General and Administrative Expenses
General and administrative expenses increased by $792,203 or 52%, to $2,303,537 for the nine months ended September 30, 2020, from $1,511,334 for the nine months ended September 30, 2019. This increase was primarily due to an increase in stock based compensation issued to employees and consultants.
Liquidity and Capital Resources
Since our inception in 2010, we have devoted most of our cash resources to research and development and general and administrative activities. We have financed our operations primarily with the proceeds from the sale of preferred stock and convertible promissory notes, state and federal grants and research services. To date, we have not generated any revenues from the sale of products, and we do not anticipate generating any revenues from the sales of products for the foreseeable future. We have incurred losses and generated negative cash flows from operations since inception. As of SeptemberJune 30, 2020,2021, our principal sources of liquidity were our cash and cash equivalents, which totaled $14,450.$51,029. Our working capital deficit was $(1,827,296)$2,501,398 as of SeptemberJune 30, 2020.2021.
Our sources of liquidity and cash flows are used to fund ongoing operations and research and development projects for our product candidates. In addition, as part of our business strategy, we occasionally evaluate potential acquisitions of businesses, products and technologies, and minority equity investments. Accordingly, a portion of our available cash may be used at any time for the acquisition of complementary products or businesses or minority equity investments. Such potential transactions may require substantial capital resources, which may require us to seek additional debt or equity financing. We cannot assure you that we will be able to successfully identify suitable acquisition or investment candidates, complete acquisitions or investments, integrate acquired businesses into our current operations, or expand into new markets. Furthermore, we cannot provide assurances that additional financing will be available to us in any required time frame and on commercially reasonable terms, if at all.
| 28 |
Equity Financings
For the ninesix months ended SeptemberJune 30, 2020,2021, and year ended December 31, 2019,2020, we received net proceeds of $706,350$0 and $100,000,$522,150, respectively, from the sale of convertible notes and promissory notes.
For the six months ended June 30, 2021, and year ended December 31, 2020, we received net proceeds of $813,757 and $0, respectively, from the sale of shares of our common stock.
Debt
We had the following schedule of debt as of SeptemberJune 30, 20202021 and December 31, 2019:2020:
September 30, 2020 | December 31, 2019 | June 30, 2021 | December 31, 2020 | |||||||||||||
| Outstanding Debt Obligations: | ||||||||||||||||
| Loan payable | $ | 804,200 | $ | 620,000 | $ | 900,000 | $ | 950,000 | ||||||||
| Loan payable - related party | 42,092 | 42,092 | 42,092 | 42,092 | ||||||||||||
| Convertible notes payable | 563,581 | 378,839 | 630,304 | 574,500 | ||||||||||||
| Convertible notes payable – related party | 51,507 | — | 150,000 | 70,000 | ||||||||||||
| Capital lease obligations | 29,731 | 35,297 | 40,054 | 27,764 | ||||||||||||
| Total All Debt Obligations | $ | 1,491,111 | $ | 1,076,228 | $ | 1,762,450 | $ | 1,664,356 | ||||||||
Future Capital Requirements
The Company isWe have not yet achieved profitability and anticipate that we will continue to incur net losses for the foreseeable future. As discussed in further detail below, we expect that our expenses will continue to grow and, as a result, we will need to generate significant product revenues to achieve profitability. We may never achieve profitability. As such, we are dependent on obtaining, and are continuing to pursue, the necessary funding from outside sources, including obtaining additional funding from the sale of securities in order to continue our operations. Without adequate funding, we may not be able to meet our obligations. We believe these conditions raise substantial doubt about our ability to continue as a going concern.
We are currently raising capital and we anticipate raising funds sufficient to commence a Phase 1 clinical trials for KLS-13019 for patients with chemotherapy induced peripheral neuropathy. We anticipate, based on current estimates, that costs associated Phase 1 clinical trials for KLS-13019 will be approximately $2.75 million.
Management of the Company believes that it will need to seek additional sources of capital to facilitate and carry out its business plan of proceeding forth with commencing a Phase 2 clinical trial for KLS-13019 for patients with chemotherapy induced peripheral neuropathy; commencing a Phase 1 clinical trial for KLS-13019 for patients suffering from the effects of mild traumatic brain injury; and commencing a Phase 1 clinical trial for KLS-13023 for patients suffering with overt hepatic encephalopathy. The cost of commencing and conducting these trials will likely be in the tens of millions of dollars.
Furthermore, it is difficult to predict our spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control.
Our expectations regarding future cash requirements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we make in the future. We have no current understandings, agreements or commitments for any material acquisitions or licenses of any products, businesses or technologies. We may need to raise substantial additional capital in order to engage in any of these types of transactions.
We expect to continue to incur substantial additional operating losses for at least the next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval, the eventual commercialization of our product candidates. If we obtain marketing approval for either of our product candidates, we will incur significant sales, marketing and outsourced manufacturing expenses. In addition, we expect to incur additional expenses to add operational, financial and information systems and personnel, including personnel to support our planned product commercialization efforts. We also expect to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to us as a public company.
| 29 |
Our future use of operating cash and capital requirements will depend on many forward-looking factors, including, without limitation, the following:
| • | the initiation, progress, timing, costs and results of preclinical studies and clinical trials for our product candidates; |
| • | the clinical development plans we establish for these product candidates; |
| • | the number and characteristics of product candidates that we develop or may in-license; |
| • | the terms of any collaboration agreements we may choose to execute; |
| • | the outcome, timing and cost of meeting regulatory requirements established by the DEA, the FDA, the EMA or other comparable foreign regulatory authorities; |
| • | the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
| • | the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us; |
| • | costs and timing of the implementation of commercial scale manufacturing activities; and |
| • | the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own. |
To the extent that our capital resources are insufficient to meet our future operating and capital requirements, we will need to finance our cash needs through public or private equity offerings, debt financings, collaboration and licensing arrangements or other financing alternatives. We have no committed external sources of funds. Additional equity or debt financing or collaboration and licensing arrangements may not be available on acceptable terms, if at all.
If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
Cash Flows
The following table summarizes our cash flows from operating, investing and financing activities for the ninesix months ended SeptemberJune 30, 2020,2021, and 2019.2020.
Nine Months Ended September 30, | Six Months Ended June 30, | |||||||||||||||
| 2020 | 2019 | 2021 | 2020 | |||||||||||||
| Statement of Cash Flows Data: | ||||||||||||||||
| Total net cash provided by (used in): | ||||||||||||||||
| Operating activities | $ | (807,789 | ) | $ | (2,000,012 | ) | $ | (715,733 | ) | $ | (575,752 | ) | ||||
| Investing activities | — | 1,813,334 | (14,622 | ) | — | |||||||||||
| Financing activities | 700,784 | 97 | 759,511 | 602,694 | ||||||||||||
| Decrease in cash | $ | (107,005 | ) | $ | (186,581 | ) | ||||||||||
| Increase in cash | $ | 29,155 | $ | 26,942 | ||||||||||||
| 30 |
Operating Activities
Net cash used in operating activities for the ninesix months ended SeptemberJune 30, 20202021 was $807,789,$715,733, including $1,730,189$1,185,399 of net non-cash expenses and a $289,612$178,562 net change in operating assets and liabilities. The net noncash expenses were primarily related to the stock based compensation and issuance of common stock shares of $751,808, amortization of debt discount of $177,790 and change in fair value of derivative liabilities of $89,367. The change in operating assets and liabilities was due to a $76,926 decrease in accounts payable and accrued expenses, a $135,083 increase in payroll and related liabilities, a $150,167 increase in prepaid expenses and a $29,762 decrease in due to related party and prepaid expenses.
Net cash used in operating activities for the six months ended June 30, 2020 was $575,752, including $1,647,583 of net non-cash expenses and a $143,449 net change in operating assets and liabilities. The net noncash expenses were predominantly related to the stock based compensation of $1,488,615,$1,182,019, non-cash interest expense of $430,070,$429,470, amortization of debt discount of $254,249$136,164 and change in fair value of derivative liabilities of $(468,097)$(121,389). The change in operating assets and liabilities was primarily due to a $150,018$61,476 increase in accounts payable and accrued expenses, a $108,589$61,418 increase in payroll and related liabilities and a $22,005$20,555 increase in due to related party and prepaid expenses.
Investing Activities
Net cash used in operating activities for the nine months ended September 30, 2019 was $2,000,012, including $578,969 of net non-cash expenses and a $140,431 net change in operating assets and liabilities. The net noncash expenses were predominantly related to the net gains and losses on marketable security of $301,661. The change in operating assets and liabilities was primarily due to a $90,392 decrease on other receivables and a $32,698 increase in accounts payable and accrued expenses.
Investing Activities
Net cash provided by investing activities for the ninesix months ended SeptemberJune 30, 20202021 was $0.
Cash provided by investing activities from$14,622 compared to $0 for the cash received fromsame period in the sale of marketable securitiesprio fiscal year. This increase was $1,882,774 offset by adue to the purchase of an asset and equipment for ($69,440), forin 2021.
Financing Activities
For the ninesix months ended SeptemberJune 30, 2019.
Financing Activities
For the nine months ended September 30, 2020,2021, cash provided by financing activities was $700,784$759,511 compared to $97$602,694 for the ninesix months ended SeptemberJune 30, 2019.2020. This increase was due to a significant increase of proceeds from convertible notes payablethe sale of common stock in 2021.
Inflation
We have not been affected materially by inflation during the periods presented, and notes payableno material effect is expected in 2020 from 2019.the near future.
Known Trends or Uncertainties
We have seen some consolidation in our industry during economic downturns. These consolidations have not had a negative effect on us to date; however, should consolidations and downsizing in the industry continue to occur, those events could adversely impact our future revenues and earnings, if any.
As discussed in this Quarterly Report on Form 10-Q, the world has been affected due to the COVID-19 pandemic. Until the pandemic has passed, there remains uncertainty as to the effect of COVID-19 on our business in both the short and long-term.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, except for operating leases, or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU 2016-13, “Financial Instruments – Credit Losses” to improve information on credit losses for financial assets and net investment in leases that are not accounted for at fair value through net income. ASU 2016-13 replaces the current incurred loss impairment methodology with a methodology that reflects expected credit losses. In April 2019 and May 2019, the FASB issued ASU No. 2019-04, “Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments” and ASU No. 2019-05, “Financial Instruments-Credit Losses (Topic 326): Targeted Transition Relief” which provided additional implementation guidance on the previously issued ASU. In November 2019, the FASB issued ASU 2019-10, “Financial Instruments - Credit Loss (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842),” which defers the effective date for public filers that are considered small reporting companies (“SRC”) as defined by the Securities and Exchange Commission to fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. Since the Company is an SRC, implementation is not needed until January 1, 2023. The Company will continue to evaluate the effect of adopting ASU 2016-13 will have on the Company’s condensed consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 4. Controls and Procedures.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
| 31 |
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(e) and 15d-15(e). Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of our internal controls over financial reporting based on the framework in “Internal Control Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based upon this evaluation, management has concluded that our internal control over financial reporting was ineffective as of SeptemberJune 30, 2020,2021, to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. Our reporting was ineffective due to a lack of sufficient resources to hire a support staff in order to separate duties between different individuals. The Company lacks the appropriate personnel to handle all the varying recording and reporting tasks on a timely basis. Steps that the Company believes it must undertake is to retain a consulting firm to, among other things, design and implement adequate systems of accounting and financial statement disclosure controls during the current fiscal year to comply with the requirements of the SEC. We believe that the ultimate success of our plan to improve our disclosure controls and procedures will require a combination of additional financial resources, outside consulting services, legal advice, additional personnel, further reallocation of responsibility among various persons, and substantial additional training of those of our officers, personnel and others, including certain of our directors such as our committee chairs, who are charged with implementing and/or carrying out our plan.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as required in Rule 13a-15(b). We are conducting an evaluation to design and implement adequate systems of accounting and financial statement disclosure controls. We expect to complete this review during 20202021 to comply with the requirement of the SEC. We believe that the ultimate success of our plan to improve our internal control over financial reporting will require a combination of additional financial resources, outside consulting services, legal advice, additional personnel, further reallocation of responsibility among various persons, and substantial additional training of our officers, personnel and others, including certain of our directors such as our Chairman of the Board and Chief Financial Officer, who are charged with implementing and/or carrying out our plan. It should also be noted that the design of any system of controls and procedures is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote.
Our management, including our chief executive officer and chief financial officer, does not expect that our disclosure controls or our internal control over financial reporting, or any system we design or implement in the future, will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
| 32 |
Changes in Internal Control
There have not been any changes in our internal control over financial reporting during the ninesix month period ended SeptemberJune 30, 2020,2021, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
On or about September 18, 2013, a lawsuit was filed by two individuals against the Company and the Company’s CEO. The plaintiffs allege that they provided business services to Kannalife Sciences, Inc. (“Kannalife”) in the amount of $150,000, including but not limited to providing strategic introductions to Kannalife and Mr. Petkanas and were seeking 17% of the issued and outstanding stock of Kannalife. The Company believed, at all times, that the allegations to be without merit and vigorously defended itself.
On or about September 30, 2013, Kannalife and Mr. Petkanas filed a motion to dismiss all five causes of action alleged against Kannalife and Mr. Petkanas.
On May 12, 2014, the court dismissed all five causes of action alleged by one plaintiff against Kannalife and Mr. Petkanas.
On March 27, 2015, the court granted permission to this plaintiff to replead his complaint (the “Repleading Plaintiff”).
On July 14, 2015, the court denied the Repleading Plaintiff’s motion to reargue, affirming the dismissal of all of the Repleading Plaintiff’s causes of action, which left three causes of action that remain open relating to the remaining plaintiff (the “Remaining Plaintiff”).
In December 2016, Kannalife and Mr. Petkanas filed a motion for summary judgment to seek the court’s decision in dismissing the remainder of the claims alleged by the Remaining Plaintiff.
On June 30, 2017, the motion for summary judgment made by Kannalife and Mr. Petkanas was granted. All remaining causes of action by the Remaining Plaintiff were dismissed.
On February 7, 2018, the Remaining Plaintiff, (the “Plaintiff-Appellant”) appealed from the June 30, 2017 decision and order of the lower court, which granted the Kannalife’s and Mr. Petkanas’ (Defendants-Respondents) motion for summary judgment dismissing all of Plaintiff-Appellant’s claims. In his amended complaint, Plaintiff-Appellant alleged the existence of an oral agreement between himself and Kannalife and Mr. Petkanas for the exchange of investments (including both money and services) from Plaintiff-Appellant in return for the transfer of 17% of Kannalife’s shares. However, Plaintiff-Appellant’s allegations consisted of nothing more than vague statements regarding what he promised to provide to Kannalife and to Mr. Petkanas in exchange for nearly one-fifth of Kannalife’s shares. And afterAfter years of litigation, including extensive depositions and document exchanges, the evidence elicited by both parties failed to clarify either the precise terms of the alleged oral agreement or that Plaintiff-Appellant actually made any investments as he allegedly promised to do. In the lower court, Kannalife and Mr. Petkanas moved for summary judgment dismissing Plaintiff-Appellant’s claims based on certain undisputed facts: that no evidence existed to show that Plaintiff-Appellant–or Stone Engineering, P.C., which is Plaintiff-Appellant’s S Corporation–made any investment at all in Kannalife; that even if Plaintiff-Appellant did make any investments, the alleged agreement is unenforceable pursuant to General Obligations Law § 5-701(a)(1) (the Statue of Frauds) because the terms cannot be completed within one year; and the contract is unenforceable as a matter of hornbook law because Plaintiff-Appellant’s own testimony establishes that he and Kannalife and Mr. Petkanas never reached a “meeting of the minds” with respect to the contours of Plaintiff-Appellant’s supposed offer of investments or the time period for transferring the shares to Plaintiff-Appellant.
| 33 |
On appeal, Plaintiff-Appellant argues the lower court’s decision was wrong because: (1) it was based upon an erroneous finding that Plaintiff-Appellant lacks standing to recover his shares in Kannalife; and (2) enforcement of the alleged contract is not barred by the Statute of Frauds because (a) its terms were capable of being performed within one year and (b) the alleged agreement constitutes a securities contract under UCC § 8-113 that does not require a writing to be enforceable. However, in Opposition Kannalife and Mr. Petkanas argued that the lower court’s decision should primarily be affirmed based upon an argument raised by Kannalife and Mr. Petkanas in their motion: the undisputed evidence shows that there was no meeting of the minds between Plaintiff-Appellant, and Kannalife and Mr. Petkanas regarding the terms of the alleged oral agreement. Moreover, the terms of the alleged agreement that Plaintiff-Appellant himself asserted–if they are assumed to be true for purposes of the motion and appeal–indicate that it was impossible for him to perform his obligations within one year; and a review of UCC § 8-113 along with interpretive case law requires a conclusion that the alleged agreement in this case does not constitute the type of securities contract that does not require a writing to be enforceable. Thus, to the extent an oral agreement between Plaintiff-Appellant, and Kannalife and Mr. Petkanas was ever actually created, then its enforcement is barred by the Statute of Frauds—and the lower court’s decision to dismiss Plaintiff-Appellant’s claim seeking enforcement of the alleged oral agreement was properly reached for these reasons. Accordingly, Kannalife and Mr. Petkanas believe that the 2nd Department will affirm the lower court’s decision and order entirely.
On September 28, 2018, in an attempt to correct fatal flaws in the Plaintiff-Appellant’s original case dismissed on June 30, 2017, the Plaintiff-Appellant filed a new lawsuit against Kannalife and Mr. Petkanas, alleging much, if not all of the same claims as in the original case filed by the Plaintiff-Appellant, a case which was dismissed on June 30, 2017. This new lawsuit now seeks, instead of the relief sought in the case previously dismissed, a sum of no less than $21,250,000.
Subsequently, Kannalife and Mr. Petkanas filed a motion to dismiss the new lawsuit on grounds of res judicata. The Plaintiff-Appellant filed a cross-motion for a stay.
On October 30, 2019, the Court ruled in favor of Kannalife and Mr. Petkanas, stating "The motion to dismiss is granted to the extent that the matter is dismissed on the ground of res judicata, and cross-motion for a stay is denied, in accordance with the reasons set forth in the Court's oral decision of October 30, 2019, following oral argument. This constitutes the Decision and Order of this Court".
On February 3, 2021, the 2nd Department entered a final decision and order with respect to the first case, affirming the lower court’s grant of the Company’s and Mr. Petkanas’ motion for summary judgment dismissing all of Plaintiff-Appellant’s remaining claims based on its finding that the parties never reached a “meeting of the minds” with respect to the contours of Plaintiff-Appellant’s supposed offer of investments or the time period for transferring the shares to Plaintiff-Appellant, and therefore no enforceable contract was formed.
To date, the Plaintiff-Appellant has not appealed the decision of the 2nd Department. However, if he does, we will continue to defend ourselves vigorously and believe that we will likely be successful in such defense.
Other than aforementioned, there are no pending legal proceeding relating to the Companyour company and its CEO to which we are a party, and to our knowledge there are no material proceedings to which any of our directors, executive officers or affiliates is a party adverse to us or which have a material interest adverse to us.
| 34 |
Item 1A. Risk Factors.
Please carefully consider the information set forth in this Quarterly Report on Form 10-Q and the risk factors discussed in Part I, Item I A. of our Annual Report on Form 10-K for the year ended December 31, 2019,2020, which could materially affect our business, financial condition or future results. In evaluating our business, you should carefully consider the risk factors discussed in our Annual Report on Form 10-K, as updated by our subsequent filings under the Exchange Act. The occurrence of any of the risks discussed in such filings, or other events that we do not currently anticipate or that we currently deem immaterial, could harm our business, prospects, financial condition and results of operations. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Our business may be subject to risks arising from pandemic, epidemic, or an outbreak of diseases, such as the outbreak of COVID-19.
The outbreak of the novel strain of coronavirus, or COVID-19, which has been declared by the World Health Organization to be a “pandemic,” has spread across the globe and is impacting worldwide economic activity. Our business may be negatively affected by a range of external factors related to COVID-19 that are not within our control. For example, numerous measuresThere have been implemented by governmental authorities acrossno material changes from the globe to contain the virus, including travel bans and restrictions, quarantines, shelter-in-place orders, restrictions and limitations of public gatherings, and business limitations and shutdowns.
The impacts of COVID-19 on our business, suppliers, employees, markets and financial results and condition are uncertain, evolving and dependent on numerous unpredictablerisk factors outside of our control, including:
While it is not possible at this time to estimate the impact that COVID-19 could have on our business, if we are not able to respond to and manage the impact of such events effectively, it may adversely impact our business, financial condition or results of operations.
COVID-19 may also have the effect of heightening many of the other risks described in the “Risk Factors” section of our Annual Report on Form 10-K. We will continue10-K for the year ended December 31, 2020, although we may disclose changes to evaluatesuch risk factors or disclose additional risk factors from time to time in our future filings with the natureSecurities and extent of the impact of COVID-19 may have on our business.Exchange Commission
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
The information set forth below relates to our issuances of securities without registration under the Securities Act of 1933 during the reporting period which were not previously included in an Annual Report on Form 10-K, Quarterly Report on Form 10-Q, or Current Report on Form 8-K.
On September 24, 2020,January 4, 2021, the Company issued 46,896109,098 shares of common stock for the conversion of $18,000$10,000 convertible notes payable and $2,000$272 of related accrued interest.interest at $0.09 per share.
On September 25, 2020,January 12, 2021, the companyCompany issued 500,000175,000 shares of the company’s common stock at $0.80the price of $0.18 per share in exchange for a settlement of accrued expenses.
On January 13, 2021, the Company issued 117,609 shares of common stock for the conversion of $10,000 convertible notes payable and $97 of related accrued interest at $0.09 per share.
On January 14, 2021, the Company sold 258,559 shares of common stock at the purchase price of $0.11 per share for a total purchase price of $28,571.
On January 15, 2021, the Company issued 29,167 shares of common stock at $0.23 a share, to a consultant for investor relationbusiness development services.
On January 15, 2021, the Company issued 313,972 shares of common stock at the price of $0.19 per share for the purchase of intellectual property based on a five year installment sale. This compensation is included in research and development on the consolidated statement of operations. The issuance was an error and was intended, as per agreement to be 200,000 shares at the floor price of $.30 per share. The Company and the recipient have discussed the cancellation of 113,972 shares which will occur in the second quarter of 2021.
On January 28, 2021, the Company sold 388,583 shares of common stock at the purchase price of $0.13 per share for a total purchase price of $51,410.
On February 1, 2021, the Company issued 517,674 shares of common stock for the conversion of $45,000 convertible notes payable and $162 of related accrued interest at $0.09 per share.
On February 10, 2021, the Company issued 243,688 shares of common stock for the conversion of $20,000 convertible notes payable and $4,200 of related accrued interest at $0.10 per share.
On February 10, 2021, the Company issued 697,714 shares of common stock in exchange of cash at $0.17 per share for a total purchase price of $121,577.
On February 10, 2021, the Company issued 3,500,000 shares of common stock in exchange of cash at $0.10 per share for a total purchase price of $350,000.
On February 22, 2021, the Company issued 715,893 shares of common stock in exchange of cash at $0.16 per share for a total purchase price of $115,617.
On February 26, 2021, the Company issued 1,050,045 shares of common stock in exchange of cash at $0.09 per share for a total purchase price of $90,146.
On March 2, 2021, the Company issued 520,000 shares of common stock at the price of $0.10 per share in exchange for a settlement of accrued expenses.
On March 4, 2021, the Company issued 320,833 shares of common stock at $0.23 a share, to a consultant for business development services.
On March 12, 2021, the Company issued its CEO 692,308 shares of common stock at $0.13 a share in lieu of $90,000 of deferred salary.
On March 25, 2021, the Company issued 657,394 shares of common stock in exchange of cash at $0.14 per share for a total purchase price of $56,437.
| 35 |
On March 12, 2021, the Company granted options to purchase 7,350,000 shares of common stock at a price of $0.13 per share to certain directors and employees of the Company (including our named executive officers) and are exercisable for ten years. One quarter of these options vested on the grant day, and the remainder of the options vest equally over thirty-six months starting March 12, 2021. These options were valued at $732,795 using a Black-Scholes Options Pricing Model.
In March 2021, the Company granted options to purchase 200,000 shares of common stock at a price of $0.13 per share to a certain member of the Company’s corporate advisory board, as governed under agreement. One quarter of these options vested on the grant day, and the remainder of the options vest equally over twenty four months thereafter. These options were valued at $19,940 using a Black-Scholes Options Pricing Model.
On April 2, 2021, the Company granted options to purchase 75,000 shares of common stock at a price of $0.16 per share to a consultant and are exercisable for ten years. One quarter of these options vest on the grant day, and the remainder of the options vest equally over twelve (12) months. These options were valued at $9,000 using a Black-Scholes Options Pricing Model.
No underwriters were involved in the transactions described above. All of the securities issued in the foregoing transactions were issued pursuant toby the Company in reliance upon the exemption from registration available under Section 4(a)(2) of the Securities Act, and/including Regulation D promulgated thereunder, in that the transactions involved the issuance and sale of the Company’s securities to financially sophisticated individuals or Rule 506 promulgated thereunder. The holders represented their intention to acquireentities that were aware of the Company’s activities and business and financial condition, and took the securities for investment onlypurposes and not with a view towards distribution.understood the ramifications of their actions. The investors were given adequate information about us to make an informed investment decision. WeCompany did not engage in any form of general solicitation or advertising.general advertising in connection with the transactions. The individuals or entities represented that they were each an “accredited investor” as defined in Regulation D at the time of issuance of the securities, and that each of such individuals or entities was acquiring such securities for their own account and not for distribution. All certificates, if such certificates were issued in certificated form, representing the securities issued have a legend imprinted on them stating that the shares have not been registered under the Securities Act and cannot be transferred until properly registered under the Securities Act or an exemption applies.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not Applicable.
Item 5. Other Information.
None.
| 36 |
Item 6. Exhibits.
EXHIBIT INDEX
| * | Filed herewith. |
| ** | These certifications are being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
| (1) | Incorporated by reference from an exhibit to our Current Report on Form 8-K filed on February 23, 2021 |
| (2) | Incorporated by reference from an exhibit to our Current Report on Form 8-K filed on March 17, 2021 |
| 37 |
SIGNATURES
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| NEUROPATHIX, INC. | ||
| Date: | By: | /s/ Dean Petkanas |
| Dean Petkanas | ||
Chief Executive Officer and Chairman (Principal Executive Officer) | ||
| Date: | By: | /s/ Mark Corrao |
| Mark Corrao | ||
Chief Financial Officer (Principal Financial and Accounting Officer) | ||
| 38 |