UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 10-Q
| QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended: September 30, 2017March 31, 2019
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______to______.
| XG SCIENCES, INC. | ||||
| (Exact name of registrant as specified in its charter) | ||||
| Michigan | 333-209131 | 20-4998896 | ||
| (State or other jurisdiction of incorporation or organization) | (Commission File No.) | (I.R.S. Employer Identification No.) |
3101 Grand Oak Drive
Lansing, MI 48911
(Address of principal executive offices) (zip code)
(517) 703-1110
(Issuer Telephone number)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes☐Yes☒ No ☒
(Note: Registrant is a voluntary filer of reports required to be filed by certain companies under Sections 13 and 15(d) of the Securities Exchange Act of 1934 and has filed all reports that would have been required during the preceding 12 months, had it been subject to such filing requirements.)¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes☒ No ☐¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act (Check one):
| Large accelerated filer | Accelerated filer | ||
| Non-accelerated filer | Smaller reporting company | ||
| (Do not check if a smaller reporting company) | Emerging growth company |
If an emerging growth company, indicate by checkmark if the registrant has not elected to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☑þ
Indicate by check mark whether the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Yes ☐ No☒
Securities registered pursuant to Section 12(b) of the Act: N/A
As of November 13, 2017,May 15, 2019, there were 2,213,3504,011,943 shares outstanding of the registrant’s common stock.stock outstanding.
XG SCIENCES, INC.
FORM 10-Q
March 31, 2019
INDEX
XG SCIENCES, INC.
FORM 10-Q
September 30, 2017
INDEX
FORWARD-LOOKING STATEMENTS
The information in this Quarterly Report on Form 10-Q contains “forward-looking statements” and information within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) relating to XG Sciences, Inc., a Michigan corporation and its subsidiary, XG Sciences IP, LLC, a Michigan limited liability company (collectively referred to as “we”, “us”, “our”, “XG Sciences”, “XGS”, or the “Company”), which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our strategy, future operations, future financial position, future revenue, projected costs, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve known and unknown risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the risks set forth on beginning on page 1412 under the section entitled “Risk Factors” in Post-Effective Amendment No. 5 (declared effective April 14, 2017) to our registration statementannual report on Form S-1 (File No. 333-209131)10-K/A as filed with the Securities and Exchange Commission (the “SEC”) on April 12, 2016, and originally declared effective on April 13, 2016 (the “Existing Registration Statement”).3, 2019.
XG SCIENCES, INC.
CONDENSED CONSOLIDATED
BALANCE SHEETS
(unaudited)
| September 30, 2017 | December 31, 2016 | |||||||
| (Restated) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 1,317,761 | $ | 1,785,343 | ||||
| Accounts receivable, less allowance for doubtful accounts of $10,000 at September 30, 2017 and December 31, 2016 | 378,686 | 99,078 | ||||||
| Inventory | 193,223 | 205,973 | ||||||
| Incentive refunds receivable | — | 165,635 | ||||||
| Other current assets | 194,864 | 174,495 | ||||||
| Total current assets | 2,084,534 | 2,430,524 | ||||||
| PROPERTY, PLANT AND EQUIPMENT, NET | 2,648,624 | 2,886,421 | ||||||
| RESTRICTED CASH FOR LETTER OF CREDIT | 195,718 | 195,499 | ||||||
| INTANGIBLE ASSETS, NET | 561,719 | 478,019 | ||||||
| TOTAL ASSETS | $ | 5,490,595 | $ | 5,990,463 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and other liabilities | $ | 667,605 | $ | 964,757 | ||||
| Deferred revenue | — | 6,428 | ||||||
| Current portion of capital lease obligations | 159,628 | 268,667 | ||||||
| Total current liabilities | 827,233 | 1,239,852 | ||||||
| LONG TERM LIABILITIES | ||||||||
| Long term portion of capital lease obligations | 31,311 | 115,106 | ||||||
| Long term debt | 3,814,703 | 1,862,120 | ||||||
| Derivative liability – warrants | — | 249,807 | ||||||
| Total long term liabilities | 3,846,014 | 2,227,033 | ||||||
| TOTAL LIABILITIES | 4,673,247 | 3,466,885 | ||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Series A convertible preferred stock, 3,000,000 shares authorized, 1,850,676 and 1,829,256 shares issued and outstanding, liquidation value of $22,208,112 and $21,951,072 at September 30, 2017 and December 31, 2016, respectively | 21,831,374 | 21,574,360 | ||||||
| Series B Preferred Stock, 1,500,000 shares authorized, 0 shares issued and outstanding, liquidation value of $0 at September 30, 2017 and December 31, 2016 | — | — | ||||||
| Common stock, no par value, 25,000,000 shares authorized, 2,162,725 and 1,885,175 shares issued and outstanding at September 30, 2017 and December 31, 2016, respectively | 17,562,267 | 15,647,839 | ||||||
| Additional paid in capital | 7,677,319 | 6,490,230 | ||||||
| Accumulated (deficit) | (46,253,612 | ) | (41,188,851 | ) | ||||
| Total stockholders’ equity | 817,348 | 2,523,578 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 5,490,595 | $ | 5,990,463 | ||||
See notes to unaudited condensed consolidated financial statements
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
| Three Months Ended September 30, | Nine Months ended September 30, | |||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| (Restated) | (Restated) | |||||||||||||||
| REVENUE | ||||||||||||||||
| Product sales | $ | 446,795 | $ | 88,856 | $ | 863,574 | $ | 230,635 | ||||||||
| Grants | 25,466 | 50,111 | 124,955 | 208,475 | ||||||||||||
| Licensing revenue | — | 25,000 | 50,000 | 75,000 | ||||||||||||
| Total revenue | 472,261 | 163,967 | 1,038,529 | 514,110 | ||||||||||||
| COST OF GOODS SOLD | ||||||||||||||||
| Direct costs | 266,462 | 44,344 | 476,774 | 101,397 | ||||||||||||
| Unallocated manufacturing expenses | 449,799 | 323,051 | 1,236,101 | 1,071,000 | ||||||||||||
| Total cost of goods sold | 716,261 | 367,395 | 1,712,875 | 1,172,397 | ||||||||||||
| GROSS LOSS | (244,000 | ) | (203,428 | ) | (674,346 | ) | (658,287 | ) | ||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | 215,949 | 231,312 | 706,575 | 866,668 | ||||||||||||
| Sales, general and administrative | 1,466,505 | 896,650 | 3,386,857 | 2,618,252 | ||||||||||||
| Total operating expenses | 1,682,454 | 1,127,962 | 4,093,432 | 3,484,920 | ||||||||||||
| OPERATING LOSS | (1,926,454 | ) | (1,331,390 | ) | (4,767,778 | ) | (4,143,207 | ) | ||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||
| Interest expense, net | (62,814 | ) | (55,816 | ) | (176,347 | ) | (240,588 | ) | ||||||||
| Gain (Loss) from change in fair value of derivative liability – warrants | (43,154 | ) | 26,738 | (46,612 | ) | 50,799 | ||||||||||
| Government incentives | — | 24,000 | (74,024 | ) | 72,000 | |||||||||||
| Loss on disposal of intangible assets | — | (18,609 | ) | — | (18,609 | ) | ||||||||||
| Total other income (expense) | (105,968 | ) | (23,687 | ) | (296,983 | ) | (136,398 | ) | ||||||||
| NET LOSS | $ | (2,032,422 | ) | $ | (1,355,077 | ) | $ | (5,064,761 | ) | $ | (4,279,605 | ) | ||||
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING –Basic and diluted | $ | 2,110,546 | $ | 914,648 | $ | 2,028,373 | $ | 959,904 | ||||||||
| NET LOSS PER SHARE – Basic and diluted | $ | (.96 | ) | $ | (1.48 | ) | $ | (2.50 | ) | $ | (4.46 | ) | ||||
| March 31, 2019 | December 31, 2018 | |||||||
| ASSETS | (unaudited) | |||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 2,844,498 | $ | 4,703,834 | ||||
| Accounts receivable, less allowance for doubtful accounts of $85,000 at March 31, 2019 and December 31, 2018 | 465,853 | 859,054 | ||||||
| Inventories | 705,905 | 660,217 | ||||||
| Other current assets | 111,177 | 114,453 | ||||||
| Total current assets | 4,127,433 | 6,337,558 | ||||||
| PROPERTY, PLANT AND EQUIPMENT, NET | 4,246,616 | 4,223,650 | ||||||
| RESTRICTED CASH FOR LETTER OF CREDIT | 190,210 | 190,140 | ||||||
| LEASE DEPOSIT | 20,156 | 20,156 | ||||||
| INTANGIBLE ASSETS, NET | 700,410 | 690,646 | ||||||
| RIGHT OF USE ASSET | 1,871,366 | — | ||||||
| TOTAL ASSETS | 11,156,191 | 11,462,150 | ||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | 838,069 | 1,102,910 | ||||||
| Other current liabilities | 381,260 | 429,573 | ||||||
| Deferred revenue | — | 832 | ||||||
| Current portion of long-term debt | 723,755 | 196,723 | ||||||
| Current portion of lease liabilities | 449,683 | 3,613 | ||||||
| Total current liabilities | 2,392,767 | 1,733,651 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Long-term portion of lease liabilities | 1,532,112 | 11,914 | ||||||
| Long term debt | 4,054,800 | 4,725,866 | ||||||
| Total long-term liabilities | 5,586,912 | 4,737,780 | ||||||
| TOTAL LIABILITIES | 7,979,679 | 6,471,431 | ||||||
| STOCKHOLDERS' EQUITY | ||||||||
| Series A convertible preferred stock, 3,000,000 shares authorized, 1,890,354 shares issued and outstanding, liquidation value of $22,684,248 at March 31, 2019 and December 31, 2018 | 22,307,480 | 22,307,480 | ||||||
| Common stock, no par value, 25,000,000 shares authorized, 3,811,518 and 3,760,268 shares issued and outstanding at March 31, 2019 and December 31, 2018, respectively | 30,682,476 | 30,268,476 | ||||||
| Additional paid-in capital | 8,190,211 | 8,101,923 | ||||||
| Accumulated deficit | (58,003,655 | ) | (55,687,160 | ) | ||||
| Total stockholders' equity | 3,176,512 | 4,990,719 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | 11,156,191 | 11,462,150 | ||||||
See notes to unaudited condensed consolidated financial statements
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
For the Three Months Ended March 31, | ||||||||
| 2019 | 2018 | |||||||
| REVENUE | ||||||||
| Product sales | $ | 857,278 | $ | 886,337 | ||||
| Grants | — | — | ||||||
| Licensing revenue | — | — | ||||||
| Total revenues | 857,278 | 886,337 | ||||||
| COST OF GOODS SOLD | ||||||||
| Direct costs | 681,153 | 468,191 | ||||||
| Unallocated manufacturing expenses | 493,469 | 746,583 | ||||||
| Total cost of goods sold | 1,174,622 | 1,214,774 | ||||||
| GROSS LOSS | (317,344 | ) | (328,437 | ) | ||||
| OPERATING EXPENSES | ||||||||
| Research and development | 385,245 | 277,063 | ||||||
| Sales, general and administrative | 1,420,922 | 1,186,679 | ||||||
| Total operating expenses | 1,806,167 | 1,463,742 | ||||||
| OPERATING LOSS | (2,123,511 | ) | (1,792,179 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense, net | (76,665 | ) | (85,169 | ) | ||||
| Government incentives, net | — | 3,253 | ||||||
| Total other expense | (76,665 | ) | (81,916 | ) | ||||
| NET LOSS | $ | (2,200,176 | ) | $ | (1,874,095 | ) | ||
| WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING – Basic and diluted | 3,774,879 | 2,454,314 | ||||||
| NET LOSS PER SHARE – Basic and diluted | $ | (0.58 | ) | $ | (0.76 | ) | ||
See notes to unaudited condensed consolidated financial statements
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(unaudited)
| Preferred stock (A) | Common stock | Additional paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | Total | ||||||||||||||||||||||
| Balances, December 31, 2016 (Restated)– see note 2 | 1,829,256 | $ | 21,574,360 | 1,885,175 | $ | 15,647,839 | $ | 6,490,230 | $ | (41,188,851 | ) | $ | 2,523,578 | |||||||||||||||
| Reclassification of warrant liabilities to equity | — | — | — | — | 296,419 | — | 296,419 | |||||||||||||||||||||
| Stock issued for cash | — | — | 267,550 | 2,140,400 | — | — | 2,140,400 | |||||||||||||||||||||
| Stock issuance fees and expenses | — | — | — | (245,972 | ) | — | — | (245,972 | ) | |||||||||||||||||||
| Warrants issued with Dow Financings | — | — | — | — | 145,800 | — | 145,800 | |||||||||||||||||||||
| Preferred stock issued to pay capital lease obligations | 21,420 | 257,014 | — | — | — | — | 257,014 | |||||||||||||||||||||
| Stock-based compensation | — | — | 10,000 | 20,000 | 744,870 | — | 764,870 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (5,064,761 | ) | (5,064,761 | ) | |||||||||||||||||||
| Balances, September 30, 2017 | 1,850,676 | $ | 21,831,374 | 2,162,725 | $ | 17,562,267 | $ | 7,677,319 | $ | (46,253,612 | ) | $ | 817,348 | |||||||||||||||
| Preferred stock (A) | Common stock | Additional paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | Total | ||||||||||||||||||||||
| Balances, December 31, 2018 | 1,890,354 | $ | 22,307,480 | 3,760,268 | $ | 30,268,476 | $ | 8,101,923 | $ | (55,687,160 | ) | $ | 4,990,719 | |||||||||||||||
| Stock issued for cash | — | — | 51,250 | 410,000 | — | — | 410,000 | |||||||||||||||||||||
| Stock issuance fees and expenses | — | — | — | (16,000 | ) | — | — | (16,000 | ) | |||||||||||||||||||
| Transition adjustment for adoption of new lease standard | — | — | — | — | — | (116,319 | ) | (116,319 | ) | |||||||||||||||||||
| Stock-based compensation | — | — | — | 20,000 | 88,288 | — | 108,288 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (2,200,176 | ) | (2,200,176 | ) | |||||||||||||||||||
| Balances, March 31, 2019 | 1,890,354 | $ | 22,307,480 | 3,811,518 | $ | 30,682,476 | $ | 8,190,211 | $ | (58,003,655 | ) | $ | 3,176,512 | |||||||||||||||
| Preferred stock (A) | Common stock | Additional paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | Total | ||||||||||||||||||||||
| Balances, December 31, 2017 | 1,857,816 | $ | 21,917,046 | 2,353,350 | $ | 19,116,012 | $ | 7,831,958 | $ | (47,767,544 | ) | $ | 1,097,472 | |||||||||||||||
| Stock issued for cash | — | — | 201,925 | 1,615,400 | — | — | 1,615,400 | |||||||||||||||||||||
| Stock issuance fees and expenses | — | — | — | (9,838 | ) | — | — | (9,838 | ) | |||||||||||||||||||
| Preferred stock issued to pay capital lease obligations | 7,140 | 85,671 | — | — | — | — | 85,671 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | 20,000 | 67,764 | — | 87,764 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (1,874,095 | ) | (1,874,095 | ) | |||||||||||||||||||
| Balances, March 31, 2018 | 1,864,956 | $ | 22,002,717 | 2,555,275 | $ | 20,741,574 | $ | 7,899,722 | $ | (49,641,639 | ) | $ | 1,002,374 | |||||||||||||||
| 6 |
XG Sciences, Inc.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
For the 3 Months Ended March 31, | ||||||||
| 2019 | 2018 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (2,200,176 | ) | $ | (1,874,095 | ) | ||
| Depreciation | 194,055 | 209,131 | ||||||
| Amortization of intangible assets | 15,858 | 12,934 | ||||||
| Stock-based compensation expense | 108,288 | 87,764 | ||||||
| Non-cash interest expense | 13,166 | 85,973 | ||||||
| Non-cash equipment rent expense | 53,082 | |||||||
| Changes in current assets and liabilities: | ||||||||
| Accounts receivable | 393,201 | (199,417 | ) | |||||
| Inventory | (45,688 | ) | (37,847 | ) | ||||
| Other current assets | (2,613 | ) | (75,809 | ) | ||||
| Accounts payable and other liabilities | (313,986 | ) | 467,765 | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,837,895 | ) | (1,270,519 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchases of property and equipment | (217,021 | ) | (874,357 | ) | ||||
| Purchases of intangible assets | (25,623 | ) | (15,574 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (242,644 | ) | (889,931 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayments of capital lease obligations | (15,527 | ) | (5,721 | ) | ||||
| Repayments of long-term loan debt | (157,200 | ) | 0 | |||||
| Proceeds from issuance of common stock | 410,000 | 1,615,400 | ||||||
| Common stock issuance fees and expenses | (16,000 | ) | (9,838 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 221,273 | 1,599,841 | ||||||
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | (1,859,266 | ) | (560,609 | ) | ||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH, BEGINNING OF PERIOD | 4,893,974 | 3,041,591 | ||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH, END OF PERIOD | 3,034,708 | 2,480,982 | * | |||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | 220 | |||||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Value of preferred stock issued for AAOF capital lease obligations | 85,671 | |||||||
*For reporting purposes, restricted cash was included with Cash and Cash Equivalents beginning in April 2018. It has been included in the 2018 Cash and Cash Equivalents amount for the first three months of 2018 for comparable purposes.
See notes to unaudited condensed consolidated financial statements
| 7 |
XG SCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2017 AND 2016 (unaudited)
| 2017 | 2016 | |||||||
| (Restated) | ||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (5,064,761 | ) | $ | (4,279,605 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 665,813 | 684,199 | ||||||
| Amortization of intangible assets | 31,770 | 26,856 | ||||||
| Loss on disposal of intangible assets | — | 18,609 | ||||||
| Stock-based compensation expense | 764,870 | 342,189 | ||||||
| Non-cash interest expense | 177,188 | 213,906 | ||||||
| Gain from change in fair value of derivative liability - warrants | 46,612 | (50,799 | ) | |||||
| Changes in current assets and liabilities: | ||||||||
| Accounts receivable | (279,608 | ) | (4,044 | ) | ||||
| Inventory | 12,750 | 2,434 | ||||||
| Other current and non-current assets | 145,047 | (101,216 | ) | |||||
| Accounts payable and other liabilities | (303,580 | ) | 480,164 | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (3,803,899 | ) | (2,667,307 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchases of property and equipment | (428,016 | ) | (84,187 | ) | ||||
| Purchases of intangible assets | (115,470 | ) | (89,264 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (543,486 | ) | (173,451 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Advances (repayments) of capital lease obligations, net | (14,625 | ) | 29,896 | |||||
| Repayments of short-term notes, net | — | (175,250 | ) | |||||
| Proceeds from long-term loan | 2,000,000 | — | ||||||
| Proceeds from issuance of common stock | 2,140,400 | 3,102,032 | ||||||
| Common stock issuance fees and expenses | (245,972 | ) | (538,640 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 3,879,803 | 2,418,038 | ||||||
| NET DECREASE IN CASH | (467,582 | ) | (422,720 | ) | ||||
| CASH AT BEGINNING OF PERIOD | 1,785,343 | 1,060,224 | ||||||
| CASH AT END OF PERIOD | $ | 1,317,761 | $ | 637,504 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | — | $ | 27,107 | ||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING: | ||||||||
| Value of preferred stock issued for AAOF capital lease obligations | $ | 257,014 | $ | 257,014 | ||||
| Property and equipment under capital leases | $ | — | $ | 38,998 | ||||
| Reclassification of derivative liability warrants to equity - ASU 2017-11 (see note 2) | $ | 7,650,442 | $ | — | ||||
| Reclassification of derivative liability warrants to equity - Series B Amendment | $ | 296,419 | $ | 51,418 | ||||
| Warrants issued with long and short-term financings | $ | 145,800 | $ | 24,060 | ||||
See notes to unaudited condensed consolidated financial statements
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
NOTE 1 -– NATURE OF BUSINESS AND BASIS OF PRESENTATION
XG Sciences, Inc., a Michigan company located in Lansing, Michigan and its subsidiary, XG Sciences IP, LLC (collectively referred to as “we”, “us”, “our”, or the “Company”) manufactures graphene nanoplatelets made from graphite, using two proprietary manufacturing processes to split natural flakes of crystalline graphite into very small and thin particles, which we sell as xGnP® graphene nanoplatelets. We sell our nanoparticlesnanoplatelets in the form of bulk powders or dispersions to other companies for use as additives to make composite and other materials with specially engineered characteristics. We also manufacture and sell integrated, value-added products containing these graphene nanoplatelets such as greases, composites, thin sheets, inks and coating formulations that we sell to other companies. Additionally, we licensehave licensed our technology to other companies in exchange for royalties and other fees.
Basis of Presentation
The accompanying interim condensed consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for interim financial information and the instructions to Form 10-Q and do not include all of the information and footnotes required by GAAP for complete financial statements. All intercompany transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in our annual audited consolidated financial statements and accompanying notes have been condensed or omitted in these interim condensed consolidated financial statements. Accordingly, the unaudited condensed consolidated financial statements included herein should be read in conjunction with the audited consolidated financial statements for the year ended December 31, 2016,2018, as filed with the Securities and Exchange Commission (“SEC”) on Form 10-K10-K/A on March 31, 2017.April 3, 2019.
The results of operations presented in this quarterly report are not necessarily indicative of the results of operations that may be expected for any future periods. In the opinion of management, these unaudited condensed consolidated financial statements include all adjustments and accruals, consisting only of normal recurring adjustments that are necessary for a fair statement of the results of all interim periods reported herein.
NOTE 2 -– SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Revenue Recognition
Revenues are recognized at a point in time, typically when control of the promised goods is transferred to customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods. The Company does not recognize revenue in cases where collectability is not probable, and defers the recognition until collection is probable or payment is received.
The Company generally expenses sales commissions when incurred because the amortization period would have been one year or less. These costs are recorded within selling, general and administrative expenses. Customer deposits, deferred revenue and other receipts are deferred and recognized when the revenue is realized and earned.
Revenue related to licensing agreements is recorded upon substantial performance of the terms of the licensing contract. In the case of licensing arrangements that involve up-front payments, revenue is recorded when management determines that the appropriate terms of the contract have been fulfilled. For example, this may occur when technology has been transferred via written documents or, if training is involved, whenever all contracted training has occurred. In the case of licenses where product delivery is also embedded in the deliverable, a portion of revenue would be recognized when products are delivered.
We have also out-licensed certain of our intellectual property to licensees under terms and conditions of license agreements that specify the intellectual property licensed, the territory, and the type of license. In exchange for these licenses, we have recorded revenues associated with the initial granting of the license and expect to receive royalties based on sales of products
produced under these licenses. License revenues are recorded to reflect our performance of requirements under these license agreements. In addition, we record royalty revenues from licensees at the time they are earned.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Grant contract revenue is recognized over the life of the contracts as the services are performed or as milestones are met.
Amounts received in excess of revenues earned are recorded as deferred revenue.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are stated at the amount management expects to collect from outstanding balances. Management provides for probable uncollectible amounts through a provision for bad debt expense and an adjustment to a valuation allowance based on their assessment of the current status of individual accounts. Balances that are still outstanding after management has used reasonable collection efforts are written off through a charge to the allowance account and a credit to accounts receivable.
Intangible Assets
We have entered into a license agreement with Michigan State University under which we have licensed certain intellectual property in the form of patents and patent applications and invention disclosures. We are responsible for managing the patent process and ongoing filings for this licensed intellectual property and for bearing the cost thereof. We capitalize all costs related to the acquisition and ongoing administration of this license agreement and we amortize these costs over 15 years or the remaining life of the license agreement, whichever is shorter.
In addition to the costs of managing in-licensed intellectual property, we also file for patent protection for inventions and other intellectual property generated by our employees. All patents are evaluated for filing in international markets on a case-by-case basis and are filed in the United States and in selected international markets as considered appropriate. All external legal and filing costs related to patent applications, patent filings, ongoing registrations, overseas filings, and legal opinions related thereto are capitalized as intangible assets at cost and amortized over a period of 15 years from the date incurred, or the remaining useful life of the associated patent, whichever is shorter.
The cost of royalties or minimum payments specified under the license agreement for in-licensed technology is expensed as incurred.
Liquidity
We have historically incurred recurring losses from operations and we may continue to generate negative cash flows as we implement our business plan. Our condensed consolidated financial statements are prepared using US GAAP as applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
In December
As of May 9, 2019, we had cash on hand of $2,686,494. We believe our cash is sufficient to fund our operations through May 31, 2020 after taking into account various sources of funding and cash received from continued commercial sales transactions. Our primary means for raising funds since 2016 we entered intohas been through our offering of shares of common stock at a fixed price of $8.00 per share to the general public in a self-underwritten offering (the “Offering” or our “IPO”) and under a draw loan note and agreement (the “Dow Facility”) with The Dow Chemical Company (“Dow”(the “Dow Facility”) to provide up to $10 million of secured debt financing at an interest rate of 5% per year, drawable at our request under certain conditions. We received $2 million at closing, $1 million on July 18, 2017, and $1 million on September 22, 2017. We currently have $1 million of additional funding available on or before December 1, 2017. On April 12, 2019, we completed the Offering, after selling 2,615,425 shares under the Dow Facility. After December 1, 2017, an additional $5 million becomes available under the Dow Facility if weRegistration Statement at a price of $8.00 per share for total proceeds of $20,923,400. We have raised $10 million of equity capital after October 31, 2016.
As of November 10, 2017, we had cash on hand of $1,226,776 and currently available funds of $1 million under the Dow Facility. Our financial projections show that we may need to raise an additional $6-8 million before we are capable of achieving sustainable free cash flow after capital expenditures. We intend that the primary means for raising such funds will be through our IPO, the additional $1 million of currently available funds under the Dow Facility, and up to an additional $5 million of proceeds from the Dow Facility in the event thatavailable to us, which we raise $10 millionintend to be our primary source of additional equity capital after October 31, 2016. Thus far, we have raised approximately $3 million through the sale of 376,078 shares ofliquidity at this time (See Note 3).
There has been no public market for our securities and a public market may never develop, or, if any market does develop, it may not be sustained. Our common stock between November 1, 2016 and September 30, 2017 towards the requirement to raise $10 million of additional equity capital in order to open up the remaining $5 million of availabilityis not currently quoted on the Dow Facility. There can be no assurance that we will be able to raise additional equity capital in the IPO or in subsequent equity offeringstraded on any exchange or that the terms and conditions ofon any future financings will be workable or acceptable to us and our stockholders.
over-the-counter market. In the event we are unable to fund our operations from existing cash on hand, operating cash flows, additional borrowings or raising equity capital, we may be forced to reduce our expenses, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
Use of Estimates
The preparation of our condensed consolidated financial statements in conformity with GAAP requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, together with amounts disclosed in the related notes to the financial statements. Actual results and outcomes may differ from our estimates, judgments and assumptions. Significant estimates, judgments and assumptions used in these condensed consolidated financial statements include, but are not limited to, those related to revenue, accounts receivable and related allowances, contingencies, useful lives and recovery of long-term assets, including intangible assets, income taxes, and the fair value of stock-based compensation. These estimates, judgments, and assumptions are reviewed periodically and the effects of material revisions in estimates are reflected in the financial statements prospectively from the date of the change in estimate.
Inventory
Inventory consists of raw materials and finished goods, all of which are valued at standard cost, which approximates average cost.
| The following amounts were included in inventory at the end of the period: | ||||||||||||||||
| September 30, | December 31, | March 31, | December 31, | |||||||||||||
| 2017 | 2016 | 2019 | 2018 | |||||||||||||
| Raw materials | $ | 48,450 | $ | 45,964 | $ | 67,711 | $ | 48,371 | ||||||||
| Consumables | 160,630 | 188,764 | ||||||||||||||
| Finished goods | 144,773 | 160,009 | 477,564 | 423,082 | ||||||||||||
| Total | $ | 193,223 | $ | 205,973 | $ | 705,905 | $ | 660,217 | ||||||||
Derivative Financial Instruments
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risk. The terms of convertible preferred stock and convertible notes that we issue are reviewed to determine whether or not they contain embedded derivative instruments that are required by ASC 815: “Derivatives and Hedging” to be accounted for separately from the host contract, and recorded at fair value. In addition, freestanding warrants are also reviewed to determine if they achieve equity classification. Certain stock warrants that we have issued did not meet the conditions for equity classification and were classified as derivative instrument liabilities measured at fair value. The fair values of these derivative liabilities were revalued at each reporting date, with the change in fair value recognized in earnings. See Note 5 for additional information.Recent Accounting Pronouncements
In July 2017,February 2016, the FASB issued Accounting Standards Update No. 2017-11,2016-02,Earnings Per Share (Topic 260), Distinguishing Liabilities From Equity (Topic 480), DerivativesLeases (“ASU 2016-02”). The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and Hedging (Topic 815) (“ASU 2017-11”). This update changesa lease liability on the classification analysis of certain equity-linked financial instrumentsbalance sheet for all leases with down-round features. When determining whether certain financial instruments shouldterms longer than 12 months. Leases will be classified as liabilitieseither finance or equity instrument, securitiesoperating, with anti-dilution features no longer preclude equity classification when assessing whetheraffecting the instrument is indexed to an entity’s own stock. Aspattern of expense recognition in the income statement. The new standard must be adopted using a result, freestanding equity-linked financial instruments (or embedded conversion features) would no longer be accounted for as derivative liabilities at fair value as a resultmodified retrospective transition and requires application of the existence of an anti-dilution feature. For freestanding equity classified financial instruments, ASU 2017-11 requires entities that present earnings per share in accordance with ASC Topic 260 to recognize the effect of the anti-dilution feature when it is triggered. That effect is treated as a dividend and as a reduction of income available to common shareholders in basic EPS. Thenew guidance in this Update is effective for fiscal years, and interim period within those fiscal years, beginning after December 15, 2018, with earlier adoption permitted. When adopted in an interim period, any adjustments are reflected as ofat the beginning of the fiscal year that includes that interim period.earliest comparative period presented. We elected to early adoptadopted ASU 2017-11 during the three months ended September 30, 2017 by applying the standard retrospectively to outstanding financial instruments with a down round feature by means of a cumulative-effect adjustment to the Company’s beginning accumulated deficit2016-02 as of January 1, 2016 (see note 2). There were 972,720, warrants indexed to Series A Preferred Stock which were originally recorded as derivative liabilities as a result of their anti-dilution features. We chose to early adopt ASU 2017-11 because it permitted these warrants to be recorded as equity rather than derivative liabilities. If ASU 2017-11 had been effective in 2016, it would have resulted in a decrease in the derivative liability and a corresponding decrease in the accumulated deficit of $7,582,158 as of September 30, 2016. The impact to the financial statements as of the three and nine-months ended September 30, 2016 is as follows:2019.
Adoption of Lease Accounting Policy
We applied ASU 2016-02 and all related amendments (“ASC 842”) using the modified retrospective method by recognizing the cumulative effect of adoption as an adjustment to the opening balance of retained earnings at January 1, 2019. Therefore, the comparative information has not been adjusted and continues to be reported under prior leasing guidance. As a result, in the first quarter of 2019 we recorded ROU assets of $1,871,366. We also recorded lease liabilities of $1,981,795. The decrease to retained earnings was $116,319, reflecting the cumulative impact of the accounting change. The standard did not have a material effect on consolidated net income or cash flows.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. As our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
We do not record a ROU asset or lease liability for leases with an expected term of 12 months or less. The comparative information has not been adjusted and continues to be reported under prior leasing guidance.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
| Three months ended September 30, 2016 | ||||||||
| As previously | As | |||||||
| reported | Adjusted | |||||||
| Operating loss | $ | (1,331,390 | ) | $ | (1,331,390 | ) | ||
| Other income (expense): | ||||||||
| Interest expense, net | (56,013 | ) | (55,816 | ) | ||||
| Gain from change in fair value of derivative warrants | 108,056 | 26,738 | ||||||
| Government incentives | 24,197 | 24,000 | ||||||
| Loss on disposal of intangible assets | (18,609 | ) | (18,609 | ) | ||||
| Total other income (expense) | 57,631 | (23,687 | ) | |||||
| Net loss | $ | (1,273,759 | ) | $ | (1,355,077 | ) | ||
| Nine months ended September 30, 2016 | ||||||||
| As previously | As | |||||||
| reported | Adjusted | |||||||
| Operating loss | $ | (4,143,207 | ) | $ | (4,143,207 | ) | ||
| Other income (expense): | ||||||||
| Interest expense, net | (241,011 | ) | (240,588 | ) | ||||
| Gain from change in fair value of derivative warrants | 340,669 | 50,799 | ||||||
| Government incentives | 72,423 | 72,000 | ||||||
| Loss on disposal of intangible assets | (18,609 | ) | (18,609 | ) | ||||
| Total other income (expense) | 153,472 | (136,398 | ) | |||||
| Net loss | $ | (3,989,735 | ) | $ | (4,279,605 | ) | ||
| The impact to the balance sheet as of December 31, 2016 is as follows: | ||||||||
| As previously | As | |||||||
| reported | Adjusted | |||||||
| Derivative liability-warrants | $ | 7,900,249 | $ | 249,807 | ||||
| Total long-term liabilities | $ | 9,877,475 | $ | 2,227,033 | ||||
| Total liabilities | $ | 11,117,327 | $ | 3,466,885 | ||||
| Series A convertible preferred stock | $ | 21,634,597 | $ | 21,574,360 | ||||
| Accumulated deficit | $ | (48,899,530 | ) | $ | (41,188,851 | ) | ||
Total stockholders’ (deficit) equity | $ | (5,126,864 | ) | $ | 2,523,578 | |||
| Total liabilities and stockholder’s deficit | $ | 5,990,463 | $ | 5,990,463 | ||||
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
NOTE 3 — WARRANTS AND FINANCING AGREEMENTS–FINANCING AGREEMENT
Dow LoanFacility
In December 2016, we entered into the Dow Facility which provides us with up to $10 million of secured debt financing at an interest rate of 5% per year, drawable at our request under certain conditions. We received $2 million at closing and an additional $1 million on July 18, 2017, and September 22, 2017 respectively. We currently have $1 million of additional funding available on or beforeand December 1,4, 2017, under the Dow Facility.respectively. After December 1, 2017, an additional $5 million becomesbecame available ifwhen we have raised $10 million of equity capital after October 31, 2016. As of September 30, 2018, we had raised in excess of $10,000,000 from our IPO since November 1, 2016, and thus have met this requirement. Therefore, the remaining $5 million under the Dow Facility is now available to us.
The Dow Facility is senior to most of our other debt and is secured by all of our assets (Dow is subordinate only to the capital leases with AAOF, see Note 9). The loan does not mature untilassets. It matures on December 1, 2021 (subject to certain mandatory prepayments based on our equity financing activities). When we raise a cumulative amount of equity capital exceeding $15 million, we are required to prepay an amount equal to 30% of the amount raised over $15 million, but less than $25 million. We began these prepayments on equity raised as of September 10, 2018. Interest iswas payable beginning January 1, 2017, although we may electhad elected, per the loan documents, to capitalize the interest as part of the outstanding debt through January 1, 2019. Beginning April 1, 2019, current interest is payable in cash on the first day of each quarter.
Dow received warrant coverage of one share of common stock for each $40 in loans received by us, equating to 20% warrant coverage, with an exercise price of $8.00 per share for the warrants issued at closing of the initial $2 million draw. After the initial closing, the strike price of future warrants issued areis subject to adjustment if we sell shares of common stock at a lower price. As of September 30, 2017,March 31, 2018, we had issued 100,000125,000 warrants to Dow, which are exercisable on or before the expiration date of December 1, 2023.
The aforementioned warrants meet the criteria for classification within stockholders’ equity. Proceeds were allocated between the debt and the warrants at their relative fair value. The total debt discount on the Dow Facility was approximately $372,000. The debt discount is being amortized to interest expense using the effective interest method over the term of the loans using an average effective interest rate of 7.68%. During the ninethree months ended September 30, 2017,March 31, 2019, we recognized $77,792 of amortization expense consisting of $98,384 was recognized$64,626 interest expenseand $13,166 of amortization from debt discount accretion related to the Dow Facility. We have repaid $157,200 of outstanding principal on the debt, resulting in a carrying value of $3,814,703$4,778,555 for the Dow LoanFacility as of September 30, 2017.March 31, 2019.
The Dow Facility entitles Dow to appoint an observer to our board of directors (the “Board”).Board. Dow will maintain theirthis observation right until the later of December 1, 2019 or when the amount of principal and interest outstanding under the Dow Facility is less than $5 million.
NOTE 4 — PRIVATE PLACEMENT AND PREEMPTIVE RIGHTS
Private Placement
In April 2015, we commenced a private placement offering of Series B Units consisting of shares of Series B Preferred Stock and warrants to purchase common stock at an offering price of $16.00 per Series B Unit. During the period April 2015 through December 2016, we sold 266,887 shares of Series B Convertible Preferred Stock and Warrants to purchase 222,262 shares of common stock, for aggregate gross proceeds of $4,270,192.
The private Series B Unit offering was terminated on February 25, 2016. As a result of our IPO and pursuant to certain exchange rights granted to participants in the Series B Unit offering, holders of Series B Preferred Stock received the right to exchange each share of Series B Preferred Stock they owned into two shares of common stock. As of December 31, 2016, all holders of Series B Preferred Stock had exercised their Series B exchange rights, and as a result we issued 539,974 shares of restricted common stock in exchange for the 269,987 shares of Series B Preferred Stock that had been previously outstanding. All of the previously issued Series B Preferred Stock was cancelled. Although the stock was cancelled all of the 224,897 warrants issued in connection with the Series B Units remain outstanding at September 30, 2017. Such warrants have an exercise price of $16.00 per share and expire between April 21 and June 30, 2022. These warrants were classified as derivative liabilities until September 30, 2017; at which time they were reclassified to equity (additional paid in capital). The reclassification was made on September 30, 2017 after determining that the exchange rights as defined in the Michigan “Certificate of Amendment – Corporation”, filed on August 19, 2016 no longer required liability classification (see Note 2).
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
NOTE 5 – DERIVATIVE LIABILITY WARRANTS
At inception, the Series A Convertible Preferred Stock warrants issued in conjunction with convertible notes issued in 2013 (subsequently converted into Series A Preferred Stock), equipment financing leases procured in 2013 and 2014, and certain other pre-emptive rights and the common stock warrants issued in connection with the 2015 Series B Unit offering were derivative liabilities which require re-measurement at fair value each reporting period.
As mentioned in Note 2, during the three months ended September 30, 2017, we chose to adopt ASU 2017-11 which changed the classification analysis of certain warrants with anti-dilution features. Since we chose to early adopt ASU 2017-11 in an interim period, the adjustments were reflected as of the beginning of the fiscal year as a cumulative-effect adjustment to the Company’s beginning accumulated deficit as of January 1, 2016. As a result of adopting ASU 2017-11, the Company no longer recognizes a liability related to 972,720 warrants, which were only classified as liabilities a result of having anti-dilution features.
As mentioned in Note 4, 224,897 warrants related to the Series B offering were reclassified from derivative liabilities on the balance sheet to equity at September 30, 2017 because the requirement to classify them as liabilities was removed when we amended the Series B Certificate of Designation in August of 2016.
The initial value of the stock warrants issued as consideration for the equipment financing leases in 2013 and 2014 was recorded as a reduction of the capital lease obligation and is being amortized as part of the effective interest cost on the capital lease obligation (see Note 8).
In 2014 when we entered into financing agreements with Samsung, AAOF and XGS II, we provided our shareholders with preemptive rights to purchase shares of Series A Convertible Preferred Stock for every two shares of Series A Convertible Preferred Stock or Common Stock owned by the shareholder. In addition, for every two shares of Series A Convertible Preferred Stock purchased by a shareholder, we issued such shareholder a warrant to purchase one additional share of Series A Convertible Preferred Stock with the same terms as the warrants issued to AAOF and XGS II.
Also, as part of our private placement of Series B Units in April 2015, shareholders and holders of our convertible notes were provided the right to purchase their pro rata share of any class of stock that the Company sells or issues. The sale of Series B Preferred Stock in the April 2015 offering triggered the preemptive rights resulting in the issuance of shares of Series B Preferred Stock and warrants. As of September 30, 2017, the total number of Stock Warrants issued due to the preemptive rights offerings was 58,689.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
The following table summarizes the fair value of the derivative liabilities as of September 30, 2017 and December 31, 2016:
| September 30, 2017 | December 31, 2016 | |||||||
| Warrants issued with Secured Convertible Notes | $ | — | $ | 6,554,160 | ||||
| Warrants issued with equipment financing leases | — | 655,418 | ||||||
| Warrants issued with preemptive rights | — | 443,790 | ||||||
| Warrants issued with April 2015 private placement of Series B Units | — | 246,881 | ||||||
| Adoption of accounting standard ASU 2017-11 | — | (7,650,442 | ) | |||||
| Total derivative liabilities | $ | — | $ | 249,807 | ||||
The Company estimated the fair value of their warrant derivative liabilities as of September 30, 2017 and December 31, 2016, using a lattice model and the following assumptions:
The value of the warrants is estimated using a binomial lattice model. Equivalent amounts reflect the net results of multiple modeling simulations that the lattice model applies to underlying assumptions. Because the Company is not publicly traded on a national exchange or to our knowledge, an over-the-counter market, the expected volatility of the Company’s stock was developed using historical volatility for a peer group for a period equal to the expected term of the warrants. The fair value of the warrants will be significantly influenced by the fair value of our common stock, stock price volatility, and the risk-free interest components of the lattice technique.
Changes in the fair value of Derivative Liabilities, carried at fair value, are reported as “Change in fair value of derivative liability — warrants” in the Statement of Operations. Comparative prior periods were prepared using the newly adopted ASU 2017-11 as follows:
| Three months ended September 30, | ||||||||
| 2017 | 2016 | |||||||
| Warrants issued with preemptive rights | $ | (506 | ) | $ | 26,423 | |||
| Warrants issued with April 2015 private placement of Series B Units | (42,648 | ) | 315 | |||||
| Total Derivative Gain (Loss) | $ | (43,154 | ) | $ | 26,738 | |||
| Nine months ended September 30, | ||||||||
| 2017 | 2016 | |||||||
| Warrants issued with preemptive rights | $ | (545 | ) | $ | 48,964 | |||
| Warrants issued with April 2015 private placement of Series B Units | (46,067 | ) | 577 | |||||
| Warrants issued with Bridge Financing | 1,258 | |||||||
| Total Derivative Gain (Loss) | $ | (46,612 | ) | $ | 50,799 | |||
13
| 11 |
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
Subsequent to the Company’s early adoption of ASU 2017-11, which effected 972,720 warrants related to Series A Preferred stock, and the Company’s reclassification of 224,897 warrants related to Series B Preferred stock on September 30, 2017 (from derivative liabilities to equity) we are no longer required to record the change in fair values for these instruments.
NOTE 64 – STOCK WARRANTS ACCOUNTED FOR AS EQUITY INSTRUMENTS
The following table summarizes the common stock warrants (including the warrants previously accounted for as derivatives) outstanding at September 30, 2017,March 31, 2019, which are accounted for as equity instruments, all of which are exercisable:
| Date Issued | Expiration Date | Indexed Stock | Exercise Price | Number of Warrants | ||||||||
| 07/01/2009 | 07/01/2019 | Common | $ | 8.00 | 6,000 | |||||||
| 10/08/2012 | 10/08/2027 | Common | $ | 12.00 | 5,000 | |||||||
| 01/15/2014 - 12/31/2014 | 01/15/2024 | Series A Convertible Preferred | $ | 6.40 | 972,720 | |||||||
| 04/30/2015- 05/26/2015 | 04/30/2022 | Common | $ | 16.00 | 218,334 | |||||||
| 06/30/2015 | 06/30/2022 | Common | $ | 16.00 | 6,563 | |||||||
| 12/31/2015 | 12/31/2020 | Common | $ | 8.00 | 20,625 | |||||||
| 03/31/2016 | 03/31/2021 | Common | $ | 10.00 | 10,600 | |||||||
| 04/30/2016 | 04/30/2021 | Common | $ | 10.00 | 895 | |||||||
| 12/14/2016 | 12/01/2023 | Common | $ | 8.00 | 50,000 | |||||||
| 07/18/2017 | 12/01/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 09/22/2017 | 12/01/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 12/04/2017 | 12/01/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 1,365,737 | ||||||||||||
Each warrant indexed to Series A Convertible Preferred Stock is currently exercisable and exchangeable into 1.875 shares of common stock.
NOTE 5 – STOCKHOLDERS’ EQUITY (DEFICIT)
Common Stock
The Company is authorized to issue 25,000,000 shares of common stock, no par value per share of which 3,811,518 and 3,760,268 shares were issued and outstanding as of March 31, 2019 and December 31, 2018, respectively.
During the three months ended March 31, 2019 the Company issued 51,250 shares of common stock pursuant to the Offering. During the three months ended March 31, 2018 the Company issued 201,925 shares of common stock pursuant to the Offering. Upon its completion on April 12, 2019, the Company had sold 2,615,425 shares of common stock in its IPO at a price of $8.00 per share for gross proceeds of $20,923,400.
Potentially dilutive securities consist of shares potentially issuable pursuant to stock options and warrants as well as shares that would result from full conversion of all outstanding convertible securities. These potentially dilutive securities were 3,013,987 and 2,903,987 as of March 31, 2019 and 2018, respectively, and are excluded from diluted net loss per share calculations because they are anti-dilutive.
Series A Convertible Preferred Stock
The Company is authorized to issue up to 3,000,000 shares of Series A Convertible Preferred Stock (the “Series A Preferred”). Each share of the Series A Preferred, which has a liquidation preference of $12.00 per share, is convertible at any time, at the option of the holder, into one share of common stock at the lower of: (a) $12.00 per share, or (b) 80% of the price at which the Company sells any equity or equity-linked securities in the future. The Series A Preferred also contains typical anti-dilution provisions that provide for adjustment of the conversion price to reflect stock splits, stock dividends, or similar events. The Series A Preferred is subject to mandatory conversion into common stock upon the listing of the Company’s common stock on a Qualified National Exchange. However, the Series A Preferred is not subject to the mandatory conversion until all outstanding convertible securities are also converted into common stock. The Series A Preferred ranks senior to all other equity or equity equivalent securities of the Company other than those securities which are explicitly senior or pari passu in rights and liquidation preference to the Series A Preferred and pari passu with the Company’s Series B Preferred Stock.
The Company issued 1,456,126 shares of Series A Preferred in connection with the conversion of certain convertible notes on December 31, 2015.
| Date Issued | Expiration Date | Exercise Price | Number of Warrants | |||||||
| 07/01/2009 | 07/01/2019 | $ | 8.00 | 6,000 | ||||||
| 10/08/2012 | 10/08/2027 | $ | 12.00 | 5,000 | ||||||
| 01/15/2014 - 12/31/2014 | 01/15/2024 | $ | 6.40 | 972,720 | ||||||
| 04/30/2015- 05/26/2015 | 04/30/2022 | $ | 16.00 | 218,334 | ||||||
| 06/30/2015 | 06/30/2022 | $ | 16.00 | 6,563 | ||||||
| 12/14/2016 | 12/01/2023 | $ | 8.00 | 50,000 | ||||||
| 07/18/2017 | 12/01/2023 | $ | 8.00 | 25,000 | ||||||
| 09/22/2017 | 12/01/2023 | $ | 8.00 | 25,000 | ||||||
| 1,308,617 | ||||||||||
In December 2015, the conversion price of the Series A Preferred was reduced from $12.00 to $6.40 (80% of $8.00), and thus, each share of Series A Preferred Stock is convertible into 1.875 shares of common stock. During the period from May 17, 2016 through December 31, 2018 the Company issued shares of Series A Preferred Stock to Aspen Advanced Opportunity Fund, LP (“AAOF”) as payment for lease financing obligations under the terms of a Master Leasing Agreement.
As of March 31, 2019, and December 31, 2018, the Company had 1,890,354 shares of Series A Preferred Stock issued and outstanding which is currently convertible into 3,544,414 shares of our common stock.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 76 – EQUITY INCENTIVE PLAN
We previously established the 2007 Stock Option Plan (the “2007 Plan”), which was scheduled to expire on October 30, 2017 and under which we granted key employees and directors options to purchase shares of our common stock at not less than fair market value as of the grant date. On May 4, 2017, the Board approved the 2017 Equity Incentive Plan (the “2017 Plan”) to replace the 2007 Stock Option Plan, which became effective upon the approval of the stockholders holding a majority of the voting power in the Company on July 18, 2017. The 2017 Plan replaces the 2007 Plan and authorizes us to issue awards (stock options and restricted stock) with respect of a maximum of 1,200,000 shares of our common stock, which equals the number of shares authorized under the 2007 Plan, as amended.Plan.
On July 24, 2017, certain stock options from the prior incentive stock option plan2007 Plan were cancelled and replacement stock options were awarded. The replacement stock option awards have an exercise price of $8.00 per share, a seven-year term, are vested 50% on date of grant with the remaining vesting over a 4 year4-year period from the date issued and are subject to certain other terms. Each option holder received options equal to 150% of the number of cancelled stock options. The cancellation and reissuance of the stock options were treated as a modification under ASC 718, Compensation-Stock Compensation. Incremental compensation cost of approximately$1,015,758 $1,015,758 was measured as the excess of the fair value of the modified award over the fair value of the original award immediately before the terms were modified. Compensation cost of approximately $501,071 was recorded on the date of cancellation for awards that were vested on the date of the modification. For unvested awards, compensation cost of approximately $514,687 will be recorded over the remaining requisite service period.
On September 30, 2018 and August 10, 2017, the Company granted each Board member 2,500 stock options and 2,500 shares of restricted stock for their Board services. The options were granted at a price of $8.00 per share and vest ratably over a four-year period beginning on the one-year anniversary. The options had an aggregate grant date fair value of $29,580 and $26,120 on September 30, 2018 and August 10, 2017, respectively. The restricted stock issued to the Board members has an aggregate fair value of $160,000 and vest ratably in arrears over four quarters on the last day of each fiscal quarter following the grant date. As of March 31, 2019, 17,500 of the 20,000 shares of restricted stock issued had vested, resulting in compensation expense of $20,000 for the period ended March 31, 2019.
During the three months ended March 31, 2019, the Company granted 7,500 employee stock options. The options were granted at a price of $8.00 per share and had an aggregate grant date fair value of $22,822. The options vest ratably over a four-year period beginning on the one-year anniversary. The fair valuesvalue of the replacement options granted werewas estimated on the datesdate of grant using the Black Scholes option-pricing model using the following assumptions: Stock price: $8.00, Exercise Price: $8.00, Expected Term: 3.51-4.78,4.75 years, Volatility: 34.78% - 36.87%41.07%, Risk free rate: 1.53% - 1.83%2.23%, Dividend rate: 0%.
On August 10,All options granted thus far under the 2017 the Company granted stock options and restricted stock to each of its board members as part of their compensation package. Each of the 4 board members received 2,500 stock options and 2,500 shares of restricted stock for their board services. The options were granted at aPlan have an exercise price of $8.00 per share and had an aggregate grant date fair valuevesting of $26,120. Thethe options vest ratably over a four-year periodranges from immediate to 25% per year, with most options vesting 25% per year beginning on the one-year anniversary. The restricted stock issued to the board members have an aggregate fair valueanniversary of $80,000 and vest ratably in arrears on the last day of each fiscal quarter following the grant date. The options expire seven years from the date of grant.
Stock-based compensation expense was $108,288and $87,764 for the three months ended March 31, 2019 and March 31, 2018, respectively. As of September 30,March 31, 2019, there was approximately $621,000 in unrecognized compensation cost related to the options granted under the 2017 2,500 sharesplan. We expect to recognize these costs over the remaining vesting terms, ranging from 3 to 4 years.
A summary of restrictedthe stock had vested resulting in compensation expenseoptions available as of $20,000.March 31, 2019 is as follows:
| Weighted | ||||||||
| Number | Average | |||||||
| Of | Exercise | |||||||
| Options | Price | |||||||
| Options outstanding at December 31, 2018 | 797,875 | $ | 8.00 | |||||
| Changes during the period: | ||||||||
| Expired | (0 | ) | 8.00 | |||||
| New Options Granted – at market price | 7,500 | 8.00 | ||||||
| Options outstanding at March 31, 2019 | 805,375 | $ | 8.00 | |||||
| Options exercisable at March 31, 2019 | 399,025 | $ | 8.00 | |||||
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
A summaryNOTE 7 – LEASES
Right of Use Asset and Leased Liability:
Estimated Lease Life – Lease term through December 2022
| Right-of-use lease assets- operating as of January 1, 2019 | $ | 1,982,739 | ||
| Less: Accumulated amortization | (111,373 | ) | ||
| Right-of-use lease assets- operating as of March 31, 2019 | $ | 1,871,366 | ||
| Lease liability-operating as of January 1, 2019 | $ | 2,094,958 | ||
| Less: Accumulated Amortization | (113,163 | ) | ||
| Lease liability operating-as of March 31, 2019 | $ | 1,981,795 | ||
| Operating lease expense for the three months ended March 31, 2019 | $ | 150,557 | ||
| Actual remaining lease payments | $ | 2,369,312 | ||
| Present value of remaining payments | $ | 1,981,795 |
Supplemental cash flow information related to leases:
| Leases | ||||
| Three months | ||||
| ended | ||||
| March 31, 2019 | ||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||
| Operating cash flows from operating leases | $ | 152,347 | ||
| Weighted average remaining lease term- operating leases ( in months) | 21.25 | |||
| Weighted average discount rate- operating leases (annual) | 9.98 | % | ||
| Maturities of leases liabilities were as follows: | ||||
| Year ending December 31, 2019 (excluding the three months ended March 31, 2019) | $ | 464,708 | ||
| Year ending December 31, 2020 | 622,878 | |||
| Year ending December 31, 2021 | 638,178 | |||
| Year ending December 31, 2022 | 643,548 | |||
| Total Lease payments | 2,369,312 | |||
| Less imputed interest | (387,517 | ) | ||
| Total | $ | 1,981,795 | ||
With the exception of the stock option activity for the nine months ended September 30, 2017 is as follows:
| Weighted | ||||||||
| Number | Average | |||||||
| Of | Exercise | |||||||
| Options | Price | |||||||
| Options outstanding at beginning of year | 369,750 | $ | 11.89 | |||||
| Changes during the year: | ||||||||
| Expired | (12,000 | ) | 12.00 | |||||
| Cancellation of existing options | (357,750 | ) | 12.00 | |||||
| Issuance of replacement options | 536,625 | 8.00 | ||||||
| New Options Granted – at market price | 108,000 | 8.00 | ||||||
| Exercised | — | — | ||||||
| Options outstanding at end of Period | 644,625 | 8.00 | ||||||
| Options exercisable at end of Period | 322,158 | 8.00 | ||||||
The fair values of options granted are estimated on the dates of grant using the Black Scholes option-pricing model. Vesting of the options granted range from immediatelystandards discussed above, we believe there have been no new accounting pronouncements effective or not yet effective which have significance, or potential significance, to 25% per year, with most of the replacement options vesting 50% on date of grant with the remaining vesting over a 4 year period from the date issued. The options expire in seven years from date of grant. our Consolidated Financial Statements.
NOTE 8 – CAPITAL LEASES
| 14 |
As of September 30, 2017 and December 31, 2016, we have capital lease obligations as follows:
| September 30, 2017 | December 31, 2016 | |||||||
| Capital lease obligations | $ | 214,191 | $ | 449,368 | ||||
| Unamortized warrant discount | (23,252 | ) | (65,595 | ) | ||||
| Net obligations | 190,939 | 383,773 | ||||||
| Short-term portion of obligations | (159,628 | ) | (268,667 | ) | ||||
| Long-term portion of obligations | $ | 31,311 | $ | 115,106 | ||||
Our AAOF capital lease obligations are four-year leases starting on January 1, 2014 and January 1, 2015. Our other capital leases expire at various dates in 2018, have average effective interest rates of 0% and contain bargain purchase options that allow us to purchase the leased property for a minimal amount upon the expiration of the lease term.
NOTE 9 —Customer, Supplier, country, and Product Concentrations
Grants and Licensing Revenue Concentration
For the three months ended September 30, 2017, one grantor accounted for 100% of the total grant revenue. During the nine months ended September 30, 2017, two grantors accounted for 25% and 75% of total grant revenue. During the three months ended September 30, 2016, two grantors accounted for 50% each of the total grant revenue, and for the nine months ended September 30, 2016, two grantors accounted for 12% and 88% of the total grant revenue in each period. There was no licensing revenue for the three months ended September 30, 2017. Licensing revenue for the nine months ended September 30, 2017, and for the three and nine months ended 2016, came from one licensor.
Product Concentration
Concentrations of product sales greater than 10% of total product sales are shown in the table below. We attempt to minimize the risk associated with product concentrations by continuing to develop new products to add to our portfolio of products offered.
| For the Three Months Ended September 30, | For the Nine Months Ended September 30, | |||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| Grade C-300 HP | * | * | 14% | * | ||||||||||||
| Grade C-500 | 66% | * | 41% | * | ||||||||||||
| Grade R-10 | * | 16% | * | 12% | ||||||||||||
| Grade M-15 | * | 16% | * | 14% | ||||||||||||
* Denotes less than 10% of product sales.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
Customer Concentration
During the three months ended September 30, 2017 we had two customers whose purchases accounted for 19% and 65% of product sales. During the three months ended September 30, 2016 we had three customers who accounted for 10%, 12% and 21% of product sales.
For the nine months ended September 30, 2017 we had two customers whose purchases accounted for 17%, and 36% of product sales. During the nine months ended September 30, 2016 we had two customers whose purchases accounted for 11% each of product sales.
At September 30, 2017, there were two customers who had an accounts receivable balance greater than 10% of our total outstanding receivable balance. At September 30, 2016, there were two customers who had an accounts receivable balance greater than 10% of our total outstanding receivable balance.
Country Concentration
We sell our products on a worldwide basis. All of these sales are denominated in U.S. dollars.
International sales for the three months ended September 30, 2017 were 33% of product sales as compared with 54% for the three months ended September 30, 2016. One country, China, accounted for 19% of product sales for the three months ended September 30, 2017 and three countries, China, the United Kingdom and South Korea, accounted for 10%, 14%, and 20%, respectively, of product sales for the three months ended September 30, 2016.
International sales for the nine months ended September 30, 2017 were 34% of product sales as compared with 66% for the nine months ended September 30, 2016. One country, China, accounted for approximately 17% of product sales for the nine months ended September 30, 2017 and two countries, the United Kingdom and South Korea, accounted for 10% and 30%, respectively, of product sales for the nine months ended September 30, 2016.
Suppliers
We buy raw materials used in manufacturing from several sources. These materials are available from a large number of sources. Thus, we believe a change in suppliers would have no material effect on our operations. We did not have any purchases from one supplier that were more than 10% of total purchases for the three months and nine months ended September 30, 2017 and 2016.
NOTE 10 -8 – RELATED PARTY TRANSACTIONS
We have a licensing agreement for exclusive use of patents and pending patents with Michigan State University (“MSU”), a shareholder of the Company via the MSU Foundation. During the three months ended September 30, 2017March 31, 2019 and 20162018 we recorded licensing expense of $12,500 per quarter. During the nine months ended September 30, 2017 and 2016 we recorded licensing expense of $37,500 in each period.
We have also entered into product licensing agreements with POSCO, a shareholder. See below for POSCO. Other than MSU and POSCO, there were nocertain other shareholders. No royalty revenue or expenses or revenuehave been recognized related to these agreements during the three or nine months ended September 30, 2017 and 2016.
The Company and POSCO, a shareholder ofMarch 31, 2019 or the Company, entered into a license agreement dated June 8, 2011, pursuant to which POSCO agreed to pay a minimum annual royalty of $100,000 per year if certain circumstances existed, among other things. The Company believed that this minimum annual royalty became due annually beginning on February 28, 2015, and up until June 30, 2017, recorded this royalty revenue at a rate of $25,000 per quarter. POSCO disputed its obligation to pay this minimum annual royalty, and did not pay the royalty in any prior year. We filed a demand for arbitration in the International Court of Arbitration onthree months ended March 9, 2016, in an effort to resolve the dispute. Pursuant to a confidential settlement, on November 3, 2017, the Company and POSCO agreed to settle the dispute and to dismiss the arbitration. Based on terms of the settlement, no allowance is considered necessary. At September 30, 2017 we have a balance of $175,000 reflected in other current assets on the condensed consolidated balance sheet. This represents an accrual of licensing revenue of $100,000 for three and a half years less 50% to reflect an estimate of the portion of 2017, 2016, 2015, and 2014 licensing fees we believed to be not collectible. At December 31, 2016 the accrued licensing fees and allowance netted together was $150,000.
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2017
On March 18, 2013, we entered into a series of agreements with two private investment funds: Aspen Advanced Opportunity Fund, LP (“AAOF”) and XGS II, LLC (“XGS II”), and pursuant to a Shareholders’ Agreement dated March 18, 2013 (as amended on February 26, 2016), a principal of each private fund serves as a member of our Board of Directors. These financing agreements were amended and restated on July 12, 2013 to provide for expanded financing commitments from AAOF and XGS II. Pursuant to these agreements, AAOF and XGS II agreed to provide $10 million of financing to the Company in the form of Secured Convertible Notes and AAOF agreed to provide an additional $1.0 million of lease financing arrangements. All of the principal and accrued interest on the Secured Convertible Notes issued to AAOF and XGS II were converted into Series A Preferred Stock in December 2016.2018.
During the three months ended September 30, 2017 and 2016March 31, 2019 we did not issue any Series A Preferred stock. For the three months ended March 31, 2018, we issued 7,140 shares per period of Series A Preferred Stockstock to AAOF as payment for lease financing obligations under the terms of the Master Lease Agreement, dated March 18, 2013. For the nine months ended September 30, 2017 and 2016 we issued a total of 21,420 shares per period as payment for lease obligations.
On August 10, 2017 restricted common stock in the amount of 2,500 shares, vesting at 25% or 625 shares on September 30, 2017, December 31, 2017, March 31, 2017, and June 30, 2017 was granted to each of four Board members: Steven C. Jones, Arnold Allemang, Dave Pendell, and Peifeng (Molly) Zhang. These awards were pursuant to the 2017 Equity Incentive Plan. In addition to the restricted stock, these Board members also received 2,500 stock options granted on August 10, 2017. These options vest equally over four years starting on the 1st anniversary of the date of grant. Both the restricted stock and the stock options have an exercise price of $8.00 per share. The options expire on the seventh anniversary of the date of grant.
NOTE 9 – SUBSEQUENT EVENTS
NOTE 11 – OTHER SUBSEQUENT EVENTS
During the period from OctoberApril 1 through November 13, 2017,its completion on April 12, 2019, we received common stock proceeds of $405,000$1,603,400 for the sale of 50,625 shares.200,425 shares of common stock in our IPO.
Item ITEM2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
In this Quarterly Report on Form 10-Q, unless otherwise indicated, the words “we”, “us”, “our”, “XG”, “XGS”, “XG Sciences” or the “Company” refer to XG Sciences, Inc. and its wholly owned subsidiary, XG Sciences IP, LLC, a Michigan limited liability company.
Introduction
The following discussion and analysis should be read in conjunction with the unaudited condensed consolidated financial statements, and the notes thereto included herein. The information contained below includes statements of the Company’s or management’s beliefs, expectations, hopes, goals and plans that, if not historical, are forward-looking statements subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. For a discussion on forward-looking statements, see the information set forth in the introductory note to this quarterly report on Form 10-Q under the caption “Forward-Looking Statements”, which information is incorporated herein by reference.
Overview of our Business
XG Sciences was formed in May 2006 for the purpose of commercializing certain technology to produce graphene nanoplatelets and integrated, value-added products containing graphene nanoplatelets. First isolated and characterized in 2004, graphene is a single layer of carbon atoms configured in an atomic-scale honeycomb lattice. Among many noted properties, monolayer graphene is harder than diamonds, lighter than steel but significantly stronger, and conducts electricity better than copper. Graphene nanoplatelets are particles consisting of multiple layers of graphene. Graphene nanoplatelets have unique capabilities for energy storage, thermal conductivity, electrical conductivity, barrier properties, lubricity and the ability to impart physical property improvements when incorporated into plastics, metals or other matrices.
We believe the unique properties of graphene and graphene nanoplatelets will enable numerous new product applications and the market for such products will quickly grow to be a significant market opportunity. Our business model is to design, manufacture and sell advanced materials we call xGnP®graphene nanoplatelets and value-added products incorporating xGnP® nanoplatelets. We currently have hundreds of customers trialing our products for numerous applications, including, but not limited to lithium ion batteries, lead acid batteries, thermally conductive adhesives, composites, thermal transfer fluids, thermal management and heat transfer, inks and coatings, printed electronics, construction materials, cement, and militaryin a range of other industrial uses. We believe our proprietary processes have enabled us to be a low-cost producer of high quality,high-quality, graphene nanoplatelets and value-added integrated products containing graphene nanoplatelets and that we are well positioned to address a wide range of end-use applications.
Our Customers
We sell products to customers around the world and have sold materials to over 1,0001,300 customers in 47 countries since 2008. Some of these customers are research organizations and some are commercial organizations. Our customers have included well-known automotive and OEM suppliers around the world (Ford, Johnson Controls, Magna, Honda Engineering) world-scale, global-scale lithium ion battery manufacturers in the US,U.S., South Korea and China (Samsung SDI, LG Chemical, Lishen, A123) and diverse specialty material companies (3M, BASF, Henkel, Dow Chemical, DuPont), as well as leading research centers such as Lawrence Livermore National Laboratory and Oakridge National Laboratory. We have also licensed some of our base manufacturing technology to other companies and we consider technology licensing a component of our business model.companies. Our licensees include POSCO, the fourthfifth largest steel manufacturer in the world by volume of2018 tonnage output, and Cabot Corporation (“Cabot”), a leading global specialty chemicals and performance materials company. These licensees further extend our technology through their customer networks. Ultimately, we believe we will benefit in terms of royalties on sales of xGnP® nanoplatelets produced and sold by our licensees. TheAs can be seen in the below bar charts showchart, the cumulative number of customers has steadily grown over the last ten years.
Cumulative Customers, By Year

We believe average order size is an early indicator of commercial traction. The majority of our customers are still ordering in smaller quantities consistent with their development and totalengineering qualification work. As can be seen in the chart below, our quarterly average order size was relatively modest until the second half of 2017, when a number of customers reached commercial status with different product applications. The data below represents orders fulfilledshipped in the respective quarter and exclude no charge orders targeted mainly for R&D purposes. The data shows that the average order size has increased steadily over the last two years, and we believe that it will continue to increase as more customers commercialize products using our materials. In the three months ending March 31, 2019 the average order size was $16,809, an increase of 6.2% from $15,827 for the three months ending March 31, 2018 and an increase of 11.9% from $15,024 for the three months ending December 31, 2018, which resulted from changes in customer and product mix in sequential quarters. In 2018 our customer shipments increased by yearover 210% to 59.9 metric tons (MT) of products from the 19.3 MT shipped in 2017. In the 3 months ending March 31, 2019 we shipped 11.1 MT of product, primarily in the form of dry powder, a decrease of 3% over the 3 months ending December 31, 2018
Average Order Size of Fulfilled Orders

Our Products
XG Sciences is a manufacturer of graphene nanoplatelets marketed under the brand xGnP® and value-added products that contain graphene nanoplatelets. The term “graphene” is used widely in the literature and the popular press to cover a variety of specific forms of the material. We generally think about two broad classes of graphene materials:
| 1. | One-atom thick films of carbon commonly referred to as monolayer graphene, manufactured typically from gases by assembling molecules to form relatively large, transparent sheets of material. These materials have been characterized by their performance attributes that differentiate them from other advanced materials and that may include: 200 times stronger than steel, flexible and able to stretch up to 25% of its original length, optically transparent, more electrically conductive than copper, more thermally conductive than any other known material and atomic-level barrier properties. XG Sciences does not manufacture these films and does not participate in the markets for these films and believes that in general, the markets for these films do not compete with those for graphene nanoplatelets. |
| 2. | Ultra-thin particles of carbon that consist of layers of graphene sheets ranging in thickness from a few layers to many layers – that are commonly referred to as graphene nanoplatelets (“GNP” or “GNPs”). Because GNPs are thin and can be manufactured in a range of diameters, they are useful for a wide variety of applications. XG Sciences manufactures GNPs that range in thickness from a few nanometers and up to 10-20 nanometers and with diameters ranging from less than 1 micron and up to 100 microns. The manufacture of these graphene particles is our main area of expertise, and their use in practical applications is the focus of our sales, marketing and development activities. |
The well-publicized isolation and characterization of graphene in 2004 at the University of Manchester, has spawned a new class of 2D materials based on actual purchaseslayers of ourcarbon atoms arranged in a hexagonal array and each carbon having lone pair electrons. The unique characterization and related performance of this new class of materials is derived from their two-dimensional nature and orderstheir composition of sp2carbon atoms arranged in a hexagonal array. The ability of any new material to be exploited in industrial applications will depend on its fit-for-performance. In the case of graphene nanoplatelets, the fit-for-performance is very much related to their aspect ratio (among other factors) such that the diameter is greater than the thickness and is what differentiates the material from bulk graphite of high crystallinity and purity. We classify nanoplatelets consisting of largely basel planes of carbon atoms packed in a hexagonal array (i.e., graphene) as graphene nanoplatelets so long as their aspect ratio may be classified as two-dimension and are thus in the form of platelets. Such a definition implies that the thickness is nanoscale – GNPs having a thickness in the range from generally 0.6 nanometers and up to many 10’s of nanometers. We have chosen to utilize the definitions as set out by the Carbon Journal editorial team (Carbon, volume 65, pp.1-6) and Fullerex (Bulk Graphene Pricing Report, 2018) which provides classification for free samples or materials used in joint development programs.

Our ProductsGraphene Product Thickness Definitions Based on Thickness
| Number of Layers | Product Description |
| 1 | Graphene (monolayer) |
| 1-3 | Very Few Layer Graphene (vFLG) |
| 2-5 | Few Layer Graphene (FLG) |
| 2-10 | Multilayer Graphene (MLG) |
| >10 | Graphene Nanoplatelets (GNP) |
Bulk MaterialsMaterials.. We target oursell bulk materials under the trademarked brand name of xGnP® nanoplatelets for usegraphene nanoplatelets. These materials are produced in a wide range of largevarious grades, which are analogous to average particle thickness, and growing end-use markets. Our proprietary manufacturing processes allow us to produce nanoplatelets with varying performance characteristics that can be tuned to specific end-use applications based on customer requirements. We currently offer fouraverage particle diameters. There are three commercial “grades” of bulk graphene nanoplatelet materials,grades (Grades H, M & R), each of which is offered in three standard particle sizes and a fourth, C Grade, which is offered in three standard surface areas. We also have access to other development grades (Grade T, for example), but which are not yet made available commercially and have been used internally for those products containing graphene nanoplatelets. These bulk materials, which normally ship in the form of a dry powder, are especially applicable for use as additives in polymeric or metallic composites, or in coatings or other formulations where particular electrical, thermal or barrier applications are desired by our customers. We also offer our material in the form of dispersions of nanoplatelets in liquids such as water, alcohol, or organic solvents, or mixed into resins or polymers such as thermoplastics or thermosets. We use two different commercial processes to produce these bulk materials:
Grade H/M/R/T materials are produced through chemical intercalation of natural graphite followed by thermal exfoliation using a proprietary process developed by us. The “grade” designates the thickness and surface characteristics of the material, and each grade is available in various average particle sizes,diameters. Surface area, calculated by the Brunauer, Emmet, and Teller (BET) Method, is used as a convenient proxy for thickness, so each grade of products produced through chemical intercalation is designated by its average surface area, which allows for surface areas rangingranges from 50 to 150 m2/g of material. We are able to extend the surface area higher (250 m2/g for T Grade) but are not yet producing these materials in metric-ton quantities. As the market need emerges for this class of materials, we will scale them as needed. For example, we introduced a new Grade of xGnP® powders, R-Grade, with improved electrical conductivity targeting use in applications for electrically and thermally conductive ink and composites and have scaled R-Grade to metric-ton quantities.
Grade C materials are produced through a high-shear mechanical exfoliation using a proprietary process and equipment that we invented, designed, constructed and patented. The Grade C materials are smaller particles than those grades produced through chemical exfoliation, and Grade C materials are designated by their BET surface area, which ranges from 300 to 800 square meters per gram of material depending on the product. Other gradesm2/g. We are able to produce other surface areas and may be mademake those available depending on the needs for specific applications. In addition, we sellcommercially as needed by our customers.
The following graphic depicts xGnP® graphene nanoplatelets in the form of pre-dispersed mixtures with water, alcohol, or other organic solvents and resins. In addition to selling bulk nanoplatelets, we also offer the following integrated, value-added products that contain our graphene nanoplatelets in various forms.
Energy Storage Materials. These consist of specialty advanced materials that have been formulated for specific applications in the energy storage segment. Chief among these is our proprietary, specially formulated silicon-graphene composite material (also referred to as “SiG” or “XG SiG®”) for use in lithium-ion battery anodes. XG SiG® targets the never-ending need for higher battery capacity and longer life. In several customer trials, our SiG material has demonstrated the potential to increase battery energy storage capacity by 3-5x what is currently available with conventional lithium ion batteries today. Additionally, we offer various bulk materials for use as conductive additives for cathodes and anodes in lithium-ion batteries, as an additive to anode slurries for lead-carbon batteries, as a componentfunction of both layer thickness and aspect ratio (thickness by diameter), two key parameters which will influence their performance in coatings for current collectors in lithium-ion batteriesa range of industrial applications.
XG Sciences’ Graphene Nanoplatelet Product Portfolio and we are investigating the use of our materials as part of other battery components.Versus Graphite
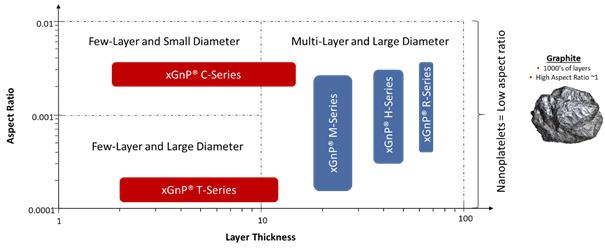
Composites.These consist of an aqueous-based compositioncompositions of specially designed xGnP®xGnP® graphene nanoplatelets formulated toin pre-dispersed mixtures that can be easily dispersedincorporated in concretevarious polymers. Our integrated composites portfolio includes pre-compounded resins derived from a range of thermoplastics as well as master batches of resins and targeted specificallyxGnP® nanoplatelets and their combination with resins and fibers for consumeruse in various end-use applications that may include industrial, automotive and industrial applications. Usesporting goods and which have demonstrated efficacy in standard injection molding, compression molding, blow molding and 3-D processes, to name but a few. Our current product portfolio of polymer resins containing various forms of our GNPxGnP® Concrete Additivegraphene nanoplatelets and in varying concentration includes polyurethane (XGPU), polypropylene (XGPP), polyethylene terephthalate (XGPET), vinyl ester (XGVE), polyetherimide (XGPEI) and high density polyethylene (XGHDPE). Others polymers may be added over time depending on the end-market and customer needs. In addition, we offer various bulk materials with demonstrated efficacy in plastic composites to impart improved physical performance to such matrices, which may be supplied as dry powders or as aqueous or solvent-based dispersions or cakes as described above. We have also targeted use of our graphene nanoplatelets as an additive in cement mixtures, which we believe results in improved barrier resistance, durability, toughness and corrosion protection. The graphene nanoplatelets promoteOur GNP®Concrete Additive promotes the formation of more uniform and smaller grain structure in the cement. This fine-grain and uniform structure gives the concrete improvements in flexural and compressive strength. In addition, the embedded graphene nanoplatelets will stop cracks from forming and retard crack propagation, should any cracks form – the combination of which will improve lifetime and durability. We intend to further extend our integrated composites portfolio to include pre-compounded resins derived from a rangedurability of thermoplastics as well as mother batches of resins and xGnP® nanoplatelets and their combination with resins and fibers for use various end-use applications. In addition, we offer various bulk materials with demonstrated efficacy in plastic composites to impart improved physical performance to such matrices, which may be supplied as dry powders or as aqueous or solvent-based dispersions or cakes.
Thermal Management Materials. These consist mainly of two types of products, our XG Leaf® sheet products and various thermal interface materials (“TIM”) in the form of custom greases or pastes. XG Leaf® is a family of sheet products ideally suited for use in thermal management in portable electronics, which may include cell phones, tablets and notebook PC’s. As these devices continue to adopt faster electronics, higher data management capabilities, brighter displays with ever increasing definition, they generate more and more heat. Managing that heat is a key requirement for the portable electronics market and our XG Leaf® product line is well suited to address the need. These sheets are made using special formulations of xGnP® graphene nanoplatelets as precursors, along with other materials for specific applications. There are several different types of XG Leaf® available in various thicknesses, depending on the end-use requirements for thermal conductivity, electrical conductivity, or resistive heating. Our custom XG TIM™ greases and pastes are also designed to be used in various high temperature environments. Additionally, we offer various bulk materials for use as active components in liquids, coatings and plastic composites to impart improved thermal management performance to such matrices.
19
cement.
Inks and Coatings. These consist of specially-formulated dispersions of xGnP®together with solvents, binders, and other additives to make electrically or thermally conductive products designed for printing or coating and which are showing promise in diverse customer applications such as advanced packaging, electrostatic dissipation and thermal management. We also offer a set of standardized ink formulations suitable for printing. These inks offer the capability to print electrical circuits or antennas or mightand may be suitable for other electrical or thermal applications. All of these formulations can be customized for specific customer requirements.
Energy Storage Materials. These consist of specialty advanced materials that have been formulated for specific applications in the energy storage segment. We offer various bulk materials for use as conductive additives for cathodes and anodes in lithium-ion batteries, as an additive to anode slurries for lead-carbon batteries, as a component in coatings for current collectors in lithium-ion batteries and we are investigating the use of our materials as part of other battery components.
Thermal Management Materials. These consist mainly of various thermal interface materials (“TIM”) in the form of custom greases or pastes. Ourcustom XG TIM®greases and pastes are also designed to be used in various high temperature environments. Additionally, we offer various bulk materials for use as active components in adhesives, liquids, coatings and plastic composites to impart improved thermal management performance to such matrices.
Our Focus Areas
We believe we are a “platform play” in advanced materials, because our proprietary processes allow us to produce varying grades of graphene nanoplatelets that can be mapped to a variety of applications and in many market segments. However, we are prioritizing our efforts in specific areas and with specific customers that we believe represent opportunities for either relatively near-term revenue or especially large and attractive markets. At this time, we are focused on three high priority areas: Energy Storage, Thermal Managementfour key vertical markets: Automotive, Sporting Goods, Packaging and Composites.Industrial. The following table showsgraphic provides examples of target applications within each of the types of applications we are pursuing, the expecting timing of revenue and the addressable market size of selected market opportunities.four key verticals where XG Sciences has either commercial sales or is in development with one or more customers.
XGS Market/Application Focus Areas & 2018 Market Size


Addressable Markets
20
The markets for our materials arelarge and growing. As one example, the 2019 North American packaging market for plastic bottles and containers is estimated to be more than $34 billion (Mordor Intelligence).Further,Mordor estimates the 2019 global market for PET water bottles at 543.8 billion units. XG Sciences is engaged in the commercial supply ofxGnP®graphene nanoplatelets for use in water bottles manufactured initially in North America and we are expanding our market activities into other geographies. If each water bottle produced in 2019 were to incorporate just 1milligram ofxGnP®graphene nanoplates, the total revenue available to XG Sciences may range from $200 to $300 million, depending on product form.
Commercialization Process
Because graphene is a new material, most of our customers are still developing applications that use our products. Commercialization is a process, the exact timing of which is often difficult to predict. It starts with our own internal R&D to validate performance for an identified market or customer-specific need. Our customers then validate the performance of our materials and determine whether our products can be incorporated into their manufacturing processes. This is initially done at pilot projectproduction scale levels. Our customers then have to introduce products that incorporate our materials to their own customers to validate performance. After their customers have validated performance, our customers will then move to commercial scale production. Every customer goes through the same process, but will do so at varying speeds, depending on the customer, the product application and the end-use market. Thus, we are not always able to predict when our customers will begin ordering commercial volumes of our materials or predict their expected volumes over time. However, as customers move through the process, we generally receive feedback and gain greater insights regarding their commercialization plans. TheAccording to our respective customers, the following are examples of where our products are providing value to our customers at levels that are either in commercial production or we believe will warrant their use on a commercial basis (see also Exhibit 99.1 to Post-Effective Amendment No. 5 to the Existing Registration Statement for our Summary Customer Pipeline validating the value of our products in various end-use markets and applications):
basis.
The process of “designing-in” new materials is relatively complex and involves the use of relatively small amounts of the new material in laboratory and engineering development for an extended period of time. Following successful development, customers that incorporate our materials into their products will then order much larger quantities of material to support commercial production. Although, our customers are under no obligation to report to us on the usage of our materials, some have indicated that they have introduced or will soon introduce commercial products that use our materials. Thus, while many of our customers are currently purchasing our materials in kilogram (one or two pound) quantities, some are now ordering at multiple ton quantities and we believe many will require tens of tons or even hundreds of tons of material whenas they commercialize products that incorporate our materials. We also believe that those customers already in production will increase their order volume as demand increases and others will begin to move into commercial volume production as they gain more experience in working with our materials and engage new customers. For example, we shipped a 1 metric ton order in the fourth quarter of 2016 to a customer who is currently moving into larger scale production and had previously used smaller quantities. In the first quarter of 2017 we shipped 1.619.3 metric tons of product for various end-use customers. In 2018, we shipped 59.9 metric tons of products comprising 48.3 metric tons of dry powder for various end-use customerspowders and another 1.8 metric tons in the second quarter. In the third quarter of 2017 we shipped over 5.7 tons and based on known customer demand we forecast shipping more than 15approximately 11.6 metric tons of additional product in the fourth quarterform of 2017.slurry, cake or other integrated products. This demand profile is further evidence that we are transitioning into higher-volume production, and we expect further, sizable increases in demand in 2018.commercial production.
We also believe average order size is an indicator of commercial traction. The majority of our customers are still ordering in smaller quantities consistent with their development and engineering qualification work. The average order sizes, by quarter from the first quarter of 2015 through third quarter of 2017 are given below. These data represent orders shipped in the respective quarter and exclude no charge orders targeted mainly for R&D purposes. The data show that the average order size has increased steadily over the last two years, and we believe that it will continue to increase in the fourth quarter of 2017 and in 2018 as more customers commercialize products using our materials.

2017 and 2018 RevenueCommercialization Trends
We are tracking the commercial and development status of more than 10075 different customer applications using our materials with some customers pursuing multiple applications. As of September 30, 2017,March 31, 2019, we had fifteeneighteen specific customer applications where our materials are incorporated into our customers’ products and such customers are actively promoting or selling these products to their own customers. In addition, we have another thirteentwenty customer applications where our customers have indicated that they expect to begin shipping product incorporating our materials in the next 3 – 6 months and we have another twelvetwenty-two customer applications where our customers have indicated an intent to commercialize in the next 6 – 9 months. We are also haveworking with numerous additional customers with whom we are working that have not yet indicated an exact date for commercialization, but we believe have the potential to contribute to revenue in 2018.2019. The following graphic demonstrates the trendcommercialization trends over the past 68 fiscal quarters as an increasing number of customers indicate their intent to commercialize applications and move into actively selling or promoting products for future sales. We anticipatebelieve that the average order size for these customers will increase in the fourth quarter of 2017 and throughout 20182019 as their demand grows. As a result, we believe we will begin shipping significantly greater quantities of our products, and thus begincontinue scaling revenue through 2019.
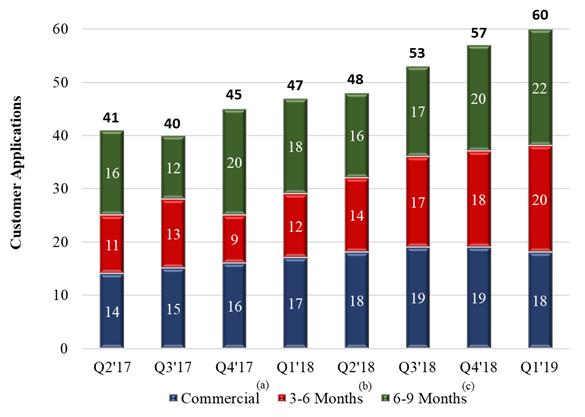
(a) Customer applications where our materials are used in the last quarter of 2017customer products and in 2018. Based on the status of current discussions withthey are actively selling them to their customers.
(b) Customer applications where our customers and their feedback on the performance ofare indicating that they expect to begin shipping products incorporating our materials in theirthe next 3-6 months.
(c) Customer applications where our customers are indicating an intent to commercialize in the next 6-9 months.
Additional 10’s of customers demonstrating efficacy and moving through qualification process.
Manufacturing Capacity
We completed the first phase of expansion in our newest 64,000 square-foot facility in the first half of 2018. The expansion has added 90 metric tons of graphene nanoplatelet production capacity, bringing the total capacity of the facility up to approximately 180 metric tons of dry powder. Phase two of the expansion was partially complete by year-end 2018 and resulted in up to ~270 metric tons of total graphene nanoplatelet output capacity at the facility. We expect to complete the last portion of this phase two expansion in the first half of 2019, resulting in up to ~400 metric tons of total graphene nanoplatelet output capacity. Our total graphene nanoplatelet output capacity across both of our manufacturing facilities, as of March 31, 2019, exceeded 300 metric tons per year and will increase to an approximate 450 metric tons by year-end. The expansions support our mission to continue commercializing the use of graphene in customer products across diverse industries. XG’s increasing capacity will support the growing demand for our products over the next several fiscal quarters. However, additional manufacturing capabilities for certain value-added products and certain bulk materials remain to be developed and may require the acquisition of additional facilities. In particular, the production processes for certain integrated products will require additional capital and may require additional facilities to meet expected future customer demand.
Some of the Company’s products are new products that have not yet been fully developed and for which manufacturing operations have not yet been fully scaled. Although we believe we will be ablecontinue to recognize approximately $15 – $30 million ofscale our production capability and revenue rapidly in 2018, although this cannot be assured.
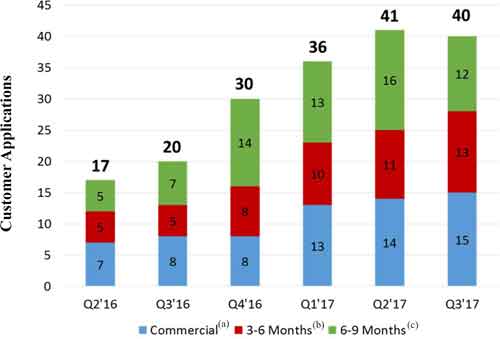
Addressable Markets
The markets that2019, we serve are large and rapidly growing. For example, as shownhave not yet demonstrated the capability to produce sufficient materials to generate the ongoing revenues necessary to sustain our operations in the figure below, Avicenne Energy (“The Rechargeable Battery Market, 2014 – 2025”, July 2015) estimates that the market for materials used in lithium ion battery anodes is currently approximately $1 billion, but is expected to approximately double over the next ten years. We believe our ability to address next generation anode materials represents a significant opportunity for us.
According to Prismark Partners, LLC, a leading electronics industry consulting firm specializing in advanced materials, the 2014 market for finished graphitic heat spreaders as sold to the OEM and EMS companies with adhesive, PET, and/or copper backing for selected portable applications was $600 million, and is expected to reach $900 million in 2018. The market is currently in a significant expansion period driven by the demand for portable devices. In a press release dated June 30, 2016, Gartner, Inc., a leading research organization, estimated the 2016 global smartphone market at 1.9 billion units and worldwide combined shipments of devices (PC’s, tablets, ultraphones and mobile devices were expected to reach 2.4 billion units in 2016). Every cell phone has some form of thermal management system, and we believe many of the new smart phones and other portable devices being developed can benefit from the thermal management properties of our XG Leaf® product line. In August 2016, International Data Corporation (IDC) in their Worldwide Quarterly Tablet Tracker, estimated the global shipment of tablets in 2016 at 183.4 million units. Thus, we believe our XG Leaf® product line is well positioned to address a very large and rapidly growing market.long-term.
Our Intellectual Property
Some of our proprietary manufacturing processes were developed at Michigan State University (MSU) and licensed to us in 2006. We license three U.S. patents and patent applications from MSU. However, overOn August 8, 2016, we signed an agreement acquiring an exclusive license to Metna’s background IP for use of graphene nanoplatelets as additives to concrete mixtures. For purposes of the agreement, Metna’s background IP relates to the U.S. Patent 8,951,343. Also, on August 8, 2016, we entered into a second agreement for an exclusive license related to all Metna’s background technology and foreground technology, including any jointly-owned foreground technology where the end use is known to be any graphite additive dispersed in concrete mixtures. Over time, our scientists and engineers have made many further discoveries and inventions that are embodied in the form of (as(and as of September 30, 2017)March 31, 2019): eighteleven (11) additional U.S. patents, 11sixteen (16) foreign patents, 17eleven (11) additional U.S. patent applications and numerous trade secrets. For each patent application filed in the U.S., we make a determination on the nature and value of the patent. For many of the applications filed in the U.S., additional filings are made in other countries such as the European Union, Japan, South Korea, China, Taiwan or other applicable countries. As of September 30, 2017,March 31, 2019, we maintained 36twenty-two (22) international patent applications. These filings and analyses are made on a case-by-case basis. Typically, patents that are defensive in nature are not filed abroad, while those that are protective of active XGS products or applications are filed in relevant countries abroad. Our general IP strategy is to keep as trade secrets those manufacturing processes that are difficult to enforce should they be disclosed and to seek patent coverage for other manufacturing processes, materials derived from those processes, unique combinations of materials and end uses of materials containing graphene nanoplatelets. We believe that the combination of our rights under the MSU license, our patents and patent applications, and our trade secrets create a strong intellectual property position.
Operating Segment
We have one reportable operating segment that manufactures xGnP® graphene nanoplatelets and value-added products produced therefrom, conducts research on graphene nanoplatelets and related products, and licenses our technology as appropriate. As of September 30, 2017,March 31, 2019, we shipped products on a worldwide basis, but all of our assets were located within the United States.
Results of Operations for the Three and Nine Months Ended September 30, 2017March 31, 2019 Compared with the Three and Nine Months Ended September 30, 2016March 31, 2018
| Summary Income Statement | For the Three Months Ended September 30, | For the Nine Months Ended September 30, | ||||||||||||||||||||||||||||||||||
| 2017 | 2016 | Change | 2017 | 2016 | Change | For the Three Months Ended March 31, | ||||||||||||||||||||||||||||||
| Total Revenue | $ | 472,261 | $ | 163,967 | $ | 308,294 | $ | 1,038,529 | $ | 514,110 | $ | 524,419 | ||||||||||||||||||||||||
| 2019 | 2018 | Change | ||||||||||||||||||||||||||||||||||
| Total Revenues | $ | 857,278 | $ | 886,337 | $ | (29,059 | ) | |||||||||||||||||||||||||||||
| Cost of Goods Sold | 716,261 | 367,395 | 348,866 | 1,712,875 | 1,172,397 | 540,478 | 1,174,622 | 1,214,774 | 40,152 | |||||||||||||||||||||||||||
| Gross Loss | (244,000 | ) | (203,428 | ) | (40,572 | ) | (674,346 | ) | (658,287 | ) | (16,059 | ) | (317,344 | ) | (328,437 | ) | 11,093 | |||||||||||||||||||
| Research & Development Expense | 215,949 | 231,312 | (15,363 | ) | 706,575 | 866,668 | (160,093 | ) | 385,245 | 277,063 | (108,182 | ) | ||||||||||||||||||||||||
| Sales, General & Administrative Expense | 1,466,505 | 896,650 | 569,855 | 3,386,857 | 2,618,252 | 768,605 | 1,420,922 | 1,186,679 | (234,243 | ) | ||||||||||||||||||||||||||
| Total Operating Expense | 1,682,454 | 1,127,962 | 554,492 | 4,093,432 | 3,484,920 | 608,512 | 1,806,167 | 1,463,742 | (342,425 | ) | ||||||||||||||||||||||||||
| Operating Loss | (1,926,454 | ) | (1,331,390 | ) | (595,064 | ) | (4,767,778 | ) | (4,143,207 | ) | (624,571 | ) | (2,123,511 | ) | (1,792,179 | ) | (331,332 | ) | ||||||||||||||||||
| Other Income (Expense) | (105,968 | ) | (23,687 | ) | (82,281 | ) | (296,983 | ) | (136,398 | ) | (160,585 | ) | ||||||||||||||||||||||||
| Other Expense | (76,665 | ) | (81,916 | ) | 5,251 | |||||||||||||||||||||||||||||||
| Net Loss | $ | (2,032,422 | ) | $ | (1,355,077 | ) | $ | (677,345 | ) | $ | (5,064,761 | ) | $ | (4,279,605 | ) | $ | (785,156 | ) | $ | (2,200,176 | ) | $ | (1,874,095 | ) | $ | (326,081 | ) | |||||||||
Revenue
RevenueRevenues for the three and nine months ended September 30, 2017March 31, 2019 and 2016,2018, by category, are shown below.
| Summary of Revenue | For the Three Months Ended September 30, | For the Nine Months Ended September 30, | ||||||||||||||||||||||
| 2017 | 2016 | Change | 2017 | 2016 | Change | |||||||||||||||||||
| Product Sales | $ | 446,795 | $ | 88,856 | $ | 357,939 | $ | 863,574 | $ | 230,635 | $ | 632,939 | ||||||||||||
| Grants | 25,466 | 50,111 | (24,645 | ) | 124,955 | 208,475 | (83,520 | ) | ||||||||||||||||
| Licensing | — | 25,000 | (25,000 | ) | 50,000 | 75,000 | (25,000 | ) | ||||||||||||||||
| Total Revenue | $ | 472,261 | $ | 163,967 | $ | 308,294 | $ | 1,038,529 | $ | 514,110 | $ | 524,419 | ||||||||||||
| For the Three Months Ended March 31, | ||||||||||||
| 2019 | 2018 | Change | ||||||||||
| Product Sales | $ | 857,278 | $ | 886,337 | $ | (29,059 | ) | |||||
| Licensing Revenues | — | — | — | |||||||||
| Total | $ | 857,278 | $ | 886,337 | $ | (29,059 | ) | |||||
Product sales consist of two broad categories: (1) material sold to customers for research or development purposes; and (2) production orders for customers. Typically, the order sizes for the first category are relatively small, however we expect orders in the second category to be much larger in the future. For the three months ended September 30, 2017,March 31, 2019, product sales increaseddecreased by $357,939,$29,059, or 403%3% from the comparable period in the prior year. For the nine months ended September 30, 2017, product sales increased by $632,939, or 274% from the comparable period in the prior year. The main reason for the increase in product sales was customersCustomers are moving through development programs towards commercialization, requiring larger quantities of our materials for advanced testing, pilot production and commercial-scale production activities. We believe that those customers already in production will increase their order volume as demand increases and other customersothers will begin to move into commercial volume production as they gain more experience in working with our materials and engage their own customers. As a result of this movement, we shipped 1.6 metric tons11.1 MT of bulk powdersproduct, primarily in the first quarterform of 2017 and 1.8 metric tons of bulk powdersdry powder in the second quarterthree months ended March 31, 2019 as compared to 10.4 MT of 2017. In the third quarter of 2017 wedry powder shipped over 5.7 metric tons and based on known customer demand we forecast shipping more than 15 metric tons of product in the fourth quarter of 2017.three months ended March 31, 2018.
We ship our products from our Lansing, MI manufacturing facilities to customers around the world. During the three months ended September 30, 2017,March 31, 2019, we shipped materials to customers in 1613 countries, as compared to 1612 countries during the same three-month period in 2016.2018. For the three months ended September 30, 2017,March 31, 2019, shipments to only one country, South Korea, accounted for more than 10% of product sales, China.sales. For the three months ended, September 30, 2016,March 31, 2018, there were no shipments to three countries, South Korea, China and the United Kingdomany one country that accounted for more than 10% of product sales.
During the first nine months ended September 30, 2017, we shipped materials to customers in 29 different countries, versus 24 countries the same nine-month period in 2016. For the nine months ended September 30, 2017, shipments to only once country, China, accounted for more than 10% of product sales. For the first nine months ended September 30, 2016, two countries, South Korea and the United Kingdom, accounted for more than 10% of product sales.
Order Summary
The table below shows a comparison of domestic and international orders fulfilled (note that this does not include orders for free samples). The table also includes the average order size for product sales. These numbers indicate that our customer base remains active with research and development projects that use our materials, but that the order size is increasing as more customers order for production purposes or approach commercial status with products using our materials. The average order size for the product revenue during the first ninethree months ended September 30, 2017March 31, 2019 increased by 259%6.2% as compared to the same period in 2016.2018. Although the average size of these orders is still relatively small, we have begun shipping in metric ton quantities to multiple customers.
| For the Three Months Ended September 30, | For the Nine Months Ended September 30, | |||||||||||||||||||||||
| 2017 | 2016 | Change | 2017 | 2016 | Change | |||||||||||||||||||
| Number of orders - domestic | 22 | 39 | (17 | ) | 89 | 91 | (2 | ) | ||||||||||||||||
| Number of orders - international | 37 | 29 | 8 | 125 | 114 | 11 | ||||||||||||||||||
| Number of orders - total | 59 | 68 | (9 | ) | 214 | 205 | 9 | |||||||||||||||||
| Average order size for product sales recorded in r Statement of Operations | $ | 7,573 | $ | 1,307 | $ | 6,266 | $ | 4,035 | $ | 1,125 | $ | 2,910 | ||||||||||||
| Percentage change | 480 | % | 259 | % | ||||||||||||||||||||
Grant Revenue
| For the Three Months Ended March 31, | Change | |||||||||||||||
| 2019 | 2018 | % | ||||||||||||||
| Number of orders – domestic | 25 | 38 | (13 | ) | (34.2) | |||||||||||
| Number of orders – international | 26 | 18 | 8 | 44.4 | ||||||||||||
| Number of orders – total | 51 | 56 | (5 | ) | (8.9) | |||||||||||
| Average order size for product sales recorded in Statement of Operations | $ | 16,809 | $ | 15,827 | 982 | 6.2 | ||||||||||
Grant revenue for the three and nine months September 30, 2017 and 2016 consisted of proceeds from sources as shown in the table below:
| For the Three Months Ended September 30 | For the Nine Months Ended September 30 | |||||||||||||||||||||||||||||||
| 2017 | % | 2016 | % | 2017 | % | 2016 | % | |||||||||||||||||||||||||
| Mercedes Benz North America | $ | 25,466 | 100 | % | $ | — | — | % | $ | 31,208 | 25 | % | $ | — | — | % | ||||||||||||||||
| Department of Energy | — | — | 25,111 | 50 | 93,747 | 75 | 183,475 | 88 | ||||||||||||||||||||||||
| Grand Valley State | — | — | 25,000 | 50 | — | 25,000 | 12 | |||||||||||||||||||||||||
| Total | $ | 25,466 | 100 | % | $ | 50,111 | 100 | % | $ | 124,955 | 100 | % | $ | 208,475 | 100 | % | ||||||||||||||||
Licensing Revenue
The Company and POSCO, a shareholder of the Company, entered into a license agreement dated June 8, 2011, pursuant to which POSCO agreed to pay a minimum annual royalty of $100,000 per year if certain circumstances existed, among other things. The Company believed that this minimum annual royalty became due annually beginning on February 28, 2015, and up until June 30, 2017, recorded this royalty revenue at a rate of $25,000 per quarter. POSCO disputed its obligation to pay this minimum annual royalty, and did not pay the royalty in any prior year. We filed a demand for arbitration in the International Court of Arbitration on March 9, 2016, in an effort to resolve the dispute. Pursuant to a confidential settlement, on November 3, 2017, the Company and POSCO agreed to settle the dispute and to dismiss the arbitration. Based on terms of the settlement, no allowance is considered necessary. At September 30, 2017 we have a balance of $175,000 reflected in other current assets on the condensed consolidated balance sheet. This represents an accrual of licensing revenue of $100,000 for three and a half years less 50% to reflect an estimate of the portion of 2017, 2016, 2015, and 2014 licensing fees we believed to be not collectible. At December 31, 2016 the accrued licensing fees and allowance netted together was $150,000.
Cost of Goods Sold
We use a standard cost system to estimate the direct costs of products sold. Direct costs include estimates of raw material costs, packaging, freight charges net of those billed to customers, and an allocation for direct labor and manufacturing overhead. Because of the nature of our production processes, there is a substantial fixed manufacturing expense requirement that represents the ongoing cost of maintaining production facilities that are not directly related to products sold, so we use a “full capacity” allocation of overhead based on an estimate of what product costs would be if the manufacturing facilities were operating on a full-time basis and producing products at the designed capacity. This estimate involves estimating both the level of expenses as well as production amounts as if the manufacturing facility were operating on a continuous 24 hour per day, 5 day per week production schedule.
Gross Profit Summary
| 25 |
The following table shows the relationship of direct costs to product sales for the three and nine months ended September 30, 2017March 31, 2019 and 2016:2018:
| Direct Margin and Gross Profit Summary | For the Three Months Ended March 31, | |||||||||||
| 2019 | 2018 | Change | ||||||||||
| Product Sales | $ | 857,278 | $ | 886,337 | $ | (29,059 | ) | |||||
| Direct Costs | 681,153 | 468,191 | (212,962 | ) | ||||||||
| Direct Cost Margin | 176,125 | 418,146 | (242,021 | ) | ||||||||
| % of Sales | 20.5 | % | 47.2 | % | (26.7 | )% | ||||||
| Unallocated Manufacturing Expense | 493,469 | 746,583 | (253,114 | ) | ||||||||
| Gross Loss on Product Sales | $ | (317,344 | ) | $ | (328,437 | ) | $ | 11,093 | ||||
| % of Sales | (37.0 | )% | (37.1 | )% | 0.1 | % | ||||||
(1) Gross Loss on Product Sales excludes any licensing or grant revenues.
| Gross Profit Summary | For the Three Months Ended September 30, | For the Nine Months Ended September 30, | ||||||||||||||||||||||
| 2017 | 2016 | Change | 2017 | 2016 | Change | |||||||||||||||||||
| Product Sales | $ | 446,795 | $ | 88,856 | $ | 357,939 | $ | 863,574 | $ | 230,635 | $ | 632,939 | ||||||||||||
| Direct Costs | 266,462 | 44,344 | 222,118 | 476,774 | 101,397 | 375,377 | ||||||||||||||||||
| Direct Cost Margin | $ | 180,333 | $ | 44,512 | $ | 135,821 | $ | 386,800 | $ | 129,238 | $ | 257,562 | ||||||||||||
| % of Sales | 40.4 | % | 50.1 | % | 44.8 | % | 56.0 | % | ||||||||||||||||
| Unallocated Manufacturing Expense | 449,799 | 323,051 | 126,748 | 1,236,101 | 1,071,000 | 165,101 | ||||||||||||||||||
| Gross Loss on Product Sales | $ | (269,466 | ) | $ | (278,539 | ) | $ | 9,073 | $ | (849,301 | ) | $ | (941,762 | ) | $ | 92,461 | ||||||||
We believe that the fluctuations in gross loss on product sales and direct cost from period to period are not indicative of future margins because of the relatively small size of our sales in comparison to our future expectations. Direct costs vary depending on the size of an order, the specific products being ordered, and other factors like shipping destination (which on small orders can represent a significant percentage of the cost).
Costs associated with grant revenue tend to be a mixture of facilities use, management time, labor from scientists, technicians and manufacturing personnel, and some supplies. Because of the difficulty of developing and maintaining an administrative system to gather direct costs for grants, together with the relatively small size of grant revenue, we do not track direct costs for grant revenue as a separate cost category. Therefore, we do not calculate direct cost margins associated with grant revenue but, rather, we view this revenue as being supported by indirect corporate expenses.
Costs associated with licensing revenue tend to be a mixture of IP costs as well as management and administrative expenses that are indirect in nature. As such, we do not assign direct costs to licensing revenue. Where revenue from a license agreement can be assigned to specific product revenue, we classify this revenue as product sales and, using our standard cost system, assign direct costs to those sales.
The remaining “non-direct” costs of operating our manufacturing facilities are recorded as unallocated manufacturing expenses. These expenses include personnel costs, rent, utilities, indirect supplies, depreciation, and related indirect expenses. Unallocated manufacturing expenses are expensed as incurred. We allocate these costs to direct product costs based on the proportion of these expenses that would be representative of direct product costs if we were operating our factory at full capacity.
For the three months ended September 30, 2017,March 31, 2019, unallocated manufacturing expenses increaseddecreased by 39%34% to $449,799$493,469 as compared to $323,051 during$746,583 in 2018. The decrease of $253,114 is largely due to increased operating efficiencies at the Mason, MI facility since opening in mid-2018 as well as an increase in inventory for the three months ended March 31, 2019 as compared to the same period in 2016, an increase of $126,748. Higher levels of manufacturing overhead expense will be incurred as we prepare for anticipated volume increases.
For the nine months ended September 30, 2017, unallocated manufacturing expenses increased by 15% to $1,236,101 as compared to $1,071,000 during the same period in 2016, an increase of $165,103. In the first three quarters of 2017 we have incurred some higher levels of manufacturing overhead expense as we prepare for anticipated volume increases.prior year.
Sales, General and Administrative Expenses
During the three months ended September 30, 2017March 31, 2019, we incurred selling, general and administrative (“SG&A”) expenses (SG&A) of $1,466,505.$1,420,921. This is an increase of $569,855approximately $234,243 or 64%19.7% from the same period in 2016, which was primarily the result2018. This increase in SG&A expense is related to both an increased hiring of a one-time, non-cash charge of $501,071 recorded in July 2017skilled personnel to account for the cancellationrespond to customer needs and replacement of certain stock options. See note 7 in the financial statements for further discussion of the modifications.to promote growth. As we continue to grow and gain traction in the marketplace, we expect that our SG&A expenses will fluctuate but should stabilize and become more fixed in nature as we achieve economies of scale.
During the nine months ended September 30, 2017 we incurred SG&A expenses of $3,386,857. This is an increase, of approximately $768,605 or 29% from the same period in 2016, which was primarily the result of a one-time, non-cash charge of $501,071 recorded in July 2017 to account for the cancellation and replacement of certain stock options. See note 7 in the financial statements for further discussion of the modifications. In addition, during the nine months ended September 30, 2016, we incurred a $182,146 reduction in SG&A expenses associated with reclassifying certain expenses related to the DOE Phase I SBIR grant into our research and development expenses. This resulted in a reduction of SG&A expenses in 2016 that did not occur in 2017. As we continue to grow and gain traction in the marketplace our SGA expenses will fluctuate but should stabilize and become more fixed in nature as we achieve economies of scale.
Research and Development Expenses
Research and development expenses for
During the three months ended September 30, 2017 were $215,949 as compared to $231,312 forMarch 31, 2019, we incurred research and development expenses (R&D) of $385,246. This is an increase of $108,183 or 39% from the same period in 2016. The decrease of $15,363 or 7%2018. This increase in R&D is largelyprimarily due to higher expensesadditional utilization of external testing services and other increased activities in 2016our research and development area as we continue to work oncommercialize and complete the DOE Phase I SBIR grant (which was completed by June 30, 2016).expand our sales reach.
Research and development expenses for the nine months ended September 30, 2017 were $706,575 as compared to $866,668 for the same period ended September 30, 2016. The decrease of $160,093 or 18% is largely due to higher expenses in 2016 to work on and complete the DOE Phase I SBIR grant.
| 26 |
Other Income (Expense)
The following table shows a comparison of other income and expense by major expense component for the three and nine months ended September 30, 2017March 31, 2019 and 2016:2018:
| For the Three Months Ended March 31, | ||||||||||||
| 2019 | 2018 | $ | ||||||||||
| Interest expense, net | $ | (76,665 | ) | $ | (85,169 | ) | 8,504 | |||||
| Government incentives, net | — | 3,253 | (3,253 | ) | ||||||||
| Total | $ | (76,665 | ) | $ | (81,916 | ) | 5,251 | |||||
| Other Income (Expense) | For the Three Months Ended September 30, | For the Nine Months Ended September 30, | ||||||||||||||||||||||
| 2017 | 2016 | Change | 2017 | 2016 | Change | |||||||||||||||||||
| Interest expense, net | $ | (62,814 | ) | $ | (55,816 | ) | $ | (6,998 | ) | $ | (176,347 | ) | $ | (240,588 | ) | $ | 64,241 | |||||||
| Gain (loss) from change in fair value of derivative liability - warrants | (43,154 | ) | 26,738 | (69,892 | ) | (46,612 | ) | 50,799 | (97,411 | ) | ||||||||||||||
| Government incentives, net | — | 24,000 | (24,000 | ) | (74,024 | ) | 72,000 | (146,024 | ) | |||||||||||||||
| Loss on disposal of intangible assets | — | (18,609 | ) | 18,609 | — | (18,609 | ) | 18,609 | ||||||||||||||||
| Total | $ | (105,968 | ) | $ | (23,687 | ) | $ | (82,281 | ) | $ | (296,983 | ) | $ | (136,398 | ) | $ | (160,585 | ) | ||||||
Interest expense, net of interest income in the three months ended September 30, 2017, increasedMarch 31, 2019, decreased by $6,998$8,504 compared to the same period in 2016. Net interest expense for the nine months ended September 30, 2017 decreased by $64,241 compared2018. The decrease is due to the same period in 2016. The decreasing balance in our lease financing agreements accounts for declining interesta decrease in the current year but this is offset by our draws onamount of indebtedness outstanding under the Dow Facility (see Note 2 in the financial statements).
Gain/(loss) from changes in the fair value of derivative liability warrants from the previous valuation period are characterized as other (expense)/other income on our Statement of Operations as a result of the GAAP requirement to use variable accounting for such instruments. These values fluctuate from period to period as a result of updating inputs used in the trinomial lattice model used to value such warrants, including risk free rate, volatility, remaining term of each warrant, and the underlying stock price assumption used in such calculations. On September 30, 2017 we reclassified 224,897 warrants related to Series B Preferred stock from derivative liabilities to equity and we are no longer required to record the change in fair values for these instruments.Facility.
Government incentives include accruals for incentive awards from state and local government entities, relatingthese incentives often relate to new hires during the period indicated, net of any true up of previous accruals to reflect actual payments. In 2016, we accrued $74,000 for expected incentive awards from the Michigan Economic Growth Authority (“MEGA”), based on our experience in receiving such incentive awards over the previous four years for our hiring practices. Upon review by MEGA in May 2017, our 2016 incentive was declined, because of our failure to meet a baseline assumed hiring threshold, which we missed by two full-time equivalent employees by December 31, 2016. Since 2016 was the final year for this incentive award program, we wrote off this previously accrued award in the three months ended June 30, 2017, and thus we recorded a loss of $74,024 in the nine month period ending September 30, 2017.
Cash Flow Summary
The following condensed cash flow statement compares cash flow from operating, investing, and financing activities for the nine months ended September 30, 2017 and 2016.
| For the Nine Months Ended September 30, | Change 2016 – 2017 | |||||||||||||||
| 2017 | 2016 | $ | % | |||||||||||||
| Cash, beginning of period | $ | 1,785,343 | $ | 1,060,224 | $ | 725,119 | 68 | % | ||||||||
| Net Cash provided by (used in): | ||||||||||||||||
| Operating activities | (3,803,899 | ) | (2,667,307 | ) | (1,136,592 | ) | 43 | |||||||||
| Investing Activities | (543,486 | ) | (173,451 | ) | (370,035 | ) | 213 | |||||||||
| Financing Activities | 3,879,803 | 2,418,038 | 1,461,765 | 60 | ||||||||||||
| Net increase (decrease) in cash | (467,582 | ) | (422,720 | ) | 44,862 | 11 | ||||||||||
| Cash, end of period | $ | 1,317,761 | $ | 637,504 | $ | 680,257 | 107 | % | ||||||||
Net cash used in operating activities for the nine months ended September 30, 2017 and 2016 was $3,803,900 and $2,667,307, respectively.
Investing activities for the nine months ended September 30, 2017 included net capital expenditures for the purchase of property and equipment of $428,015 and $115,470 for intellectual property as compared with $84,187 for property and equipment and $89,264 for intellectual property during the same period in 2016. These levels of capital expenditures are higher as we have begun to update and install equipment necessary to increase production capacity to meet anticipated customer orders for those customers who are moving into commercialization of products containing our materials.
Financing activities provided a net increase in cash of $3,879,803 and $2,418,038 for the nine months ended September 30, 2017 and 2016, respectively. For the first nine months ended September 30, 2017 gross proceeds from the issuance of common stock was $2,140,400 and stock issuance expenses were $245,972 as compared to proceeds from the issuance of common stock for the nine months ended September 30, 2016 of $3,102,032 and stock issuance expenses of $538,640. Financing activities in nine months ending September 30, 2017 also included $2,000,000 of loan proceeds from the Dow Facility (see Note 3 in the financial statements) versus zero in the nine months ending September 30, 2016.or job creation activities.
Liquidity and Capital Expenditures
Liquidity
We have historically incurred recurring losses from operations and we may continue to generate negative cash flows as we implement our business plan. Our condensed consolidated financial statements are prepared using US GAAP as applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
In December
As of May 9, 2019 , we had cash on hand of $2,686,494. We believe our cash is sufficient to fund our operations through May 31, 2020 after taking into account various sources of funding and cash received from continued commercial sales transactions. Our primary means for raising funds since 2016 we entered intohas been through our offering of shares of common stock at a fixed price of $8.00 per share to the general public in a self-underwritten offering (the “Offering” or our “IPO”) and under a draw loan note and agreement (the “Dow Facility”) with The Dow Chemical Company (“Dow”(the “Dow Facility”) to provide up to $10 million of secured debt financing at an interest rate of 5% per year, drawable at our request under certain conditions. We received $2 million at closing, $1 million on July 18, 2017, and $1 million on September 22, 2017. We currently have $1 million of additional funding available on or before December 1, 2017. On April 12, 2019, we completed the Offering, after selling 2,615,425 shares under the Dow Facility. After December 1, 2017, an additional $5 million becomes available under the Dow Facility if weRegistration Statement at a price of $8.00 per share for total proceeds of $20,923,400. We have raised $10 million of equity capital after October 31, 2016.
As of November 10, 2017, we had cash on hand of $1,226,776 and currently available funds of $1 million under the Dow Facility. Our financial projections show that we may need to raise an additional $6-8 million before we are capable of achieving sustainable free cash flow after capital expenditures. We intend that the primary means for raising such funds will be through our IPO, the additional $1 million of currently available funds under the Dow Facility, and up to an additional $5 million of proceeds from the Dow Facility inavailable to us, which we intend to be our primary source of liquidity at this time (See Note 3 to the event that we raise $10 million of additional equity capital after October 31, 2016. Thus far, we have raised approximately $3 million through the sale of 376,078 shares ofcondensed consolidated financial statements).
There has been no public market for our securities and a public market may never develop, or, if any market does develop, it may not be sustained. Our common stock between November 1, 2016 and September 30, 2017 towards the requirement to raise $10 million of additional equity capital in order to open up the remaining $5 million of availabilityis not currently quoted on the Dow Facility. There can be no assurance that we will be able to raise additional equity capital in the IPO or in subsequent equity offeringstraded on any exchange or that the terms and conditions ofon any future financings will be workable or acceptable to us and our stockholders.
over-the-counter market. In the event we are unable to fund our operations from existing cash on hand, operating cash flows, additional borrowings or raising equity capital, we may be forced to reduce our expenses, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Critical Accounting EstimatesPolicies
In preparing the condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), we have adopted various accounting policies. Our most significant accounting policies are disclosed in Note 2 to the consolidated financial statements included in our Form 10-K10-K/A for the year ended December 31, 2016.2018.
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the amounts reported in the condensed consolidated financial statements and accompanying notes. Our estimates and assumptions, including those related to inventories, intangible assets, property, plant and equipment, legal proceedings, research and development, warranty obligations, product liability, fair valued liabilities, sales returns and discounts, going concern, and income taxes are updated as appropriate, which in most cases is at least quarterly. We base our estimates on historical experience, or various judgements about the reported values of assets, liabilities, revenue and expenses. Actual results may materially differ from these estimates.
ItemITEM 3. Quantitative and Qualitative Disclosures about Market RiskQUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Smaller reporting companies are not required to provide this information.
ItemITEM 4. Controls and ProceduresCONTROLS AND PROCEDURES
(a)Evaluation of disclosure controls and procedures. We maintain disclosure controls and procedures designed to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives.
As required by SEC Rule 15d-15, our management carried out an evaluation, under the supervision and with the participation of our principal executive officer and principal financial officer, of the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of the end of the period covered by this report.
(b)Changes in internal controls. There were no changes in our internal control over financial reporting that occurred during the three months ended September 30, 2017March 31, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ItemITEM 1. Legal Proceedings.LEGAL PROCEEDINGS.
The Company and POSCO, a shareholder of the Company, entered into a license agreement dated June 8, 2011, pursuant to which POSCO agreed to pay a minimum annual royalty of $100,000 per year if certain circumstances existed, among other things. The Company believed that this minimum annual royalty became due annually beginning on February 28, 2015, and up until June 30, 2017, recorded this royalty revenue at a rate of $25,000 per quarter. POSCO disputed its obligation to pay this minimum annual royalty, and did not pay the royalty in any prior year. We filed a demand for arbitration in the International Court of Arbitration on March 9, 2016, in an effort to resolve the dispute. Pursuant to a confidential settlement, on November 3, 2017, the Company and POSCO agreed to settle the dispute and to dismiss the arbitration. Based on terms of the settlement, no allowance is considered necessary.None.
ItemITEM 1A. Risk Factors.RISK FACTORS.
Smaller reporting companies are not required to provide this information.
ItemITEM 2. Unregistered Sales of Equity Securities and Use of Proceeds.UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
None.
During the three months ended September 30, 2017 and 2016 we issued 7,140 shares per period of Series A Preferred Stock to Aspen Advanced Opportunity Fund, LP as payment for lease financing obligations under the terms of the Master Lease Agreement, dated March 18, 2013. For the nine months ended September 30, 2017 and 2016 we issued a total of 21,420 shares per period as payment for lease obligations.
On August 10, 2017, we granted 2,500 shares of restricted stock to each of the following independent Board members as Board compensation: Steven C. Jones, Arnold Allemang, Dave Pendell, and Peifeng (Molly) Zhang. These shares vest 25% on the last day of each fiscal quarter on September 30, 2017, December 31, 2017, March 31, 2018, and June 30, 2017. Such shares were issued pursuant to the 2017 Equity Incentive Plan.
ItemITEM 3. Defaults Upon Senior Securities.DEFAULTS UPON SENIOR SECURITIES.
None.
ItemITEM 4. Mine Safety Disclosures.MINE SAFETY DISCLOSURES.
None.
ItemITEM 5. Other Information.OTHER INFORMATION.
None
None.
ItemITEM 6. Exhibits.EXHIBITS.
| EXHIBIT NUMBER | DESCRIPTION | LOCATION | ||
| Certifications of the Chief Executive Officer | Filed herewith | |||
| 31.2 | Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | Filed herewith | ||
| 32.1 | ||||
| Certification Pursuant To 18 U.S.C. Section 1350, As Adopted Pursuant | Filed herewith | |||
| 32.2 | Certification Pursuant To 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | Filed herewith | ||
| 101. INS | XBRL Instance Document | Filed herewith | ||
| 101. CAL | XBRL Taxonomy Extension Calculation Link base Document | Filed herewith | ||
| 101. DEF | XBRL Taxonomy Extension Definition Link base Document | Filed herewith | ||
| 101. LAB | XBRL Taxonomy Label Link base Document | Filed herewith | ||
| 101. PRE | XBRL Extension Presentation Link base Document | Filed herewith | ||
| 101. SCH | XBRL Taxonomy Extension Scheme Document | Filed herewith |
31
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended,this report has been signed below by the following persons on behalf of the registrant has duly caused this Quarterly Reportand in the capacities and on Form 10-Q to be signed on its behalf by the undersigned, thereunto duly authorized.
XG SCIENCES, INC.dates indicated.
Dated: | By: | /s/ Philip L. Rose | |
| Name: | Philip L. Rose | ||
| Title: | Chief Executive Officer | ||
| Dated: | By: | /s/ | |
| Name: | |||
| Title: |
| 31 |
32