UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31,September 30, 2021
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _ to _
Commission File Number: 001-38753
Moderna, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | | | | |
| Delaware | | 81-3467528 |
| (State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
| | | |
| 200 Technology Square | | |
| Cambridge, | Massachusetts | | 02139 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(617) 714-6500
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.0001 per share | MRNA | The NASDAQNasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | | Accelerated filer o | | Non-accelerated filer o | | Smaller reporting company | ☐ |
| | | | | | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No x☒
As of April 30,October 29, 2021, there were 401,527,789405,449,527 shares of the registrant’s common stock, par value $0.0001 per share, outstanding.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q (“Form 10-Q”), including the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains express or implied forward-looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this Form 10-Q include, but are not limited to, statements about:
•our activities with respect to our COVID-19 vaccine, and our plans and expectations regarding future generations of our COVID-19 vaccine, including boosters, that we may develop in response to variants of the SARS-CoV-2 virus, ongoing clinical development, manufacturing and supply, pricing, commercialization, if approved, regulatory matters (including dosage for vaccines and authorization or approval for boosters) and third-party and governmental arrangements and potential arrangements;
•our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately, particularly with respect to the timely production and delivery of our COVID-19 vaccine;vaccine, including any variant booster vaccine candidate, if authorized;
•our ability and the ability of third parties with whom we contract to successfully manufacture our commercial products at scale, as well as drug substances, delivery vehicles, development candidates, and investigational medicines for preclinical and clinical use;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our commercial products, investigational medicines and technology;
•the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs;
•the ultimate impact of the current coronavirus pandemic, or the COVID-19 pandemic, or any other health epidemic, on our business, manufacturing, clinical trials, research programs, supply chain, regulatory review, healthcare systems or the global economy as a whole;
•risks related to the direct or indirect impact of the COVID-19 pandemic or any future large-scale adverse health event, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays in diagnoses, initiation or continuation of treatment for diseases that may be addressed by our development candidates and investigational medicines, or in patient enrollment in clinical trials, potential clinical trials, regulatory review or supply chain disruptions, and other potential impacts to our business, the effectiveness or timeliness of steps taken by us to mitigate the impact of the pandemic, and our ability to execute business continuity plans to address disruptions caused by the COVID-19 pandemic or future large-scale adverse health event;
•our anticipated next steps for our development candidates and investigational medicines that may be slowed down due to the impact of the COVID-19 pandemic, including our resources being significantly diverted towards our COVID-19 vaccine efforts, particularly if the federal government seeks to require us to divert such resources;
•our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop development candidates and investigational medicines, including by applying learnings from one program to our other programs and from one modality to our other modalities;
•the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and investigational medicines;
•our ability to obtain and maintain regulatory approval of our investigational medicines;
•our ability to successfully commercialize any future products, if approved;
•the pricing and reimbursement of our investigational medicines, if approved;
•the implementation of our business model, and strategic plans for our business, investigational medicines, and technology;
•estimates of our future expenses, revenues, capital requirements, and our needs for additional financing;
•the potential benefits of strategic collaboration agreements, our ability to enter into strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise;
•future agreements with third parties in connection with the commercialization of our investigational medicines, if approved;
•the size and growth potential of the markets for our investigational medicines, and our ability to serve those markets;
•our financial performance;
•the rate and degree of market acceptance of our investigational medicines;
•legal and regulatory developments in the United States and foreign countries;
•our ability to produce our products or investigational medicines with advantages in turnaround times or manufacturing cost;
•the success of competing therapies or treatments that are or may become available;available as an alternative to our products;
•our ability to attract and retain key scientific or management personnel;
•the impact of laws and regulations;
•developments relating to our competitors and our industry; and
•other risks and uncertainties discussed in this Form 10-Q.industry.
In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “could,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” and elsewhere in this Form 10-Q. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those expressed or implied by the forward-looking statements. No forward-looking statement is a promise or a guarantee of future performance.
The forward-looking statements in this Form 10-Q represent our views as of the date of this Form 10-Q. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Form 10-Q.
This Form 10-Q includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We have not independently verified the information contained in such sources.
NOTE REGARDING COMPANY REFERENCES
Unless the context otherwise requires, the terms “Moderna,” “the Company,” “we,” “us,” and “our” in this Form 10-Q refer to Moderna, Inc. and its consolidated subsidiaries.
ADDITIONAL INFORMATION
Our website, www.modernatx.com, including the Investor Relations section, www.investors.modernatx.com; and corporate blog www.modernatx.com/moderna-blog; as well as our social media channels: Facebook, www.facebook.com/modernatx; Twitter, www.twitter.com/modernatx; and LinkedIn, www.linkedin.com/company/modernatx; contain a significant amount of information about us, including financial and other information for investors. We encourage investors to visit these websites and social media channels as information is frequently updated and new information is shared. Information contained on our website and social media channels shall not be deemed incorporated into, or be a part of this Form 10-Q.
Table of Contents
| | | | | | | | |
PART I. | | Page |
| Item 1. | | |
| | |
| | |
| | |
| | |
| | |
| | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| PART II. | | |
| Item 1. | | |
| Item 1A. | | |
| Item 6. | | |
| | |
Item 1. Financial Statements
MODERNA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited, in millions, except per share data)
| | | March 31, | | December 31, | | September 30, | | December 31, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Assets | Assets | | | | Assets | | | |
| Current assets: | Current assets: | | Current assets: | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 5,442 | | | $ | 2,624 | | Cash and cash equivalents | $ | 5,550 | | | $ | 2,624 | |
| Investments | Investments | 2,293 | | | 1,984 | | Investments | 3,356 | | | 1,984 | |
| Accounts receivable | Accounts receivable | 3,210 | | | 1,391 | | Accounts receivable | 3,142 | | | 1,391 | |
| Inventory | Inventory | 494 | | | 47 | | Inventory | 965 | | | 47 | |
| Prepaid expenses and other current assets | Prepaid expenses and other current assets | 264 | | | 252 | | Prepaid expenses and other current assets | 412 | | | 252 | |
| Total current assets | Total current assets | 11,703 | | | 6,298 | | Total current assets | 13,425 | | | 6,298 | |
| Investments, non-current | Investments, non-current | 468 | | | 639 | | Investments, non-current | 6,442 | | | 639 | |
| Property and equipment, net | Property and equipment, net | 372 | | | 297 | | Property and equipment, net | 845 | | | 297 | |
| Right-of-use assets, operating leases | Right-of-use assets, operating leases | 89 | | | 90 | | Right-of-use assets, operating leases | 115 | | | 90 | |
| Restricted cash, non-current | Restricted cash, non-current | 11 | | | 11 | | Restricted cash, non-current | 11 | | | 11 | |
| Deferred tax assets | Deferred tax assets | 50 | | | 0 | | Deferred tax assets | 81 | | | — | |
| Other non-current assets | Other non-current assets | 1 | | | 2 | | Other non-current assets | 4 | | | 2 | |
| Total assets | Total assets | $ | 12,694 | | | $ | 7,337 | | Total assets | $ | 20,923 | | | $ | 7,337 | |
| Liabilities and Stockholders’ Equity | Liabilities and Stockholders’ Equity | | | | Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | Current liabilities: | | Current liabilities: | |
| Accounts payable | Accounts payable | $ | 8 | | | $ | 18 | | Accounts payable | $ | 87 | | | $ | 18 | |
| Accrued liabilities | Accrued liabilities | 753 | | | 470 | | Accrued liabilities | 1,076 | | | 470 | |
| Deferred revenue | Deferred revenue | 7,531 | | | 3,867 | | Deferred revenue | 7,977 | | | 3,867 | |
| Income taxes payable | | Income taxes payable | 565 | | | — | |
| Other current liabilities | Other current liabilities | 149 | | | 34 | | Other current liabilities | 252 | | | 34 | |
| Total current liabilities | Total current liabilities | 8,441 | | | 4,389 | | Total current liabilities | 9,957 | | | 4,389 | |
| Deferred revenue, non-current | Deferred revenue, non-current | 179 | | | 177 | | Deferred revenue, non-current | 498 | | | 177 | |
| Operating lease liabilities, non-current | Operating lease liabilities, non-current | 96 | | | 97 | | Operating lease liabilities, non-current | 105 | | | 97 | |
| Financing lease liabilities, non-current | Financing lease liabilities, non-current | 138 | | | 110 | | Financing lease liabilities, non-current | 238 | | | 110 | |
| Other non-current liabilities | Other non-current liabilities | 2 | | | 3 | | Other non-current liabilities | 1 | | | 3 | |
| Total liabilities | Total liabilities | 8,856 | | | 4,776 | | Total liabilities | 10,799 | | | 4,776 | |
| Commitments and contingencies | 0 | | 0 | |
| Commitments and contingencies (Note 12) | | Commitments and contingencies (Note 12) | 0 | | 0 |
| Stockholders’ equity: | Stockholders’ equity: | | Stockholders’ equity: | |
Preferred stock, par value $0.0001; 162 shares authorized as of March 31, 2021 and December 31, 2020; 0 shares issued or outstanding at March 31, 2021 and December 31, 2020 | 0 | | | 0 | | |
| Common stock, par value $0.0001; 1,600 shares authorized as of March 31, 2021 and December 31, 2020; 401 and 399 shares issued and outstanding as of March 31, 2021 and December 31, 2019, respectively | 0 | | | 0 | | |
| Preferred stock, par value $0.0001; 162 shares authorized as of September 30, 2021 and December 31, 2020; no shares issued or outstanding at September 30, 2021 and December 31, 2020 | | Preferred stock, par value $0.0001; 162 shares authorized as of September 30, 2021 and December 31, 2020; no shares issued or outstanding at September 30, 2021 and December 31, 2020 | — | | | — | |
| Common stock, par value $0.0001; 1,600 shares authorized as of September 30, 2021 and December 31, 2020; 405 and 399 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | | Common stock, par value $0.0001; 1,600 shares authorized as of September 30, 2021 and December 31, 2020; 405 and 399 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | — | | | — | |
| Additional paid-in capital | Additional paid-in capital | 4,860 | | | 4,802 | | Additional paid-in capital | 5,003 | | | 4,802 | |
| Accumulated other comprehensive income | Accumulated other comprehensive income | 1 | | | 3 | | Accumulated other comprehensive income | 31 | | | 3 | |
| Accumulated deficit | (1,023) | | | (2,244) | | |
| Retained earnings (accumulated deficit) | | Retained earnings (accumulated deficit) | 5,090 | | | (2,244) | |
| Total stockholders’ equity | Total stockholders’ equity | 3,838 | | | 2,561 | | Total stockholders’ equity | 10,124 | | | 2,561 | |
| Total liabilities and stockholders’ equity | Total liabilities and stockholders’ equity | $ | 12,694 | | | $ | 7,337 | | Total liabilities and stockholders’ equity | $ | 20,923 | | | $ | 7,337 | |
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited, in millions, except per share data)
| | | | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | | | 2021 | | 2020 | | 2021 | | 2020 | | 2021 | | 2020 |
| Revenue: | Revenue: | | | | | | | Revenue: | | | | | | | | |
| Product sales | Product sales | | | | $ | 1,733 | | | $ | 0 | | Product sales | | $ | 4,810 | | | $ | — | | | $ | 10,740 | | | $ | — | |
| Grant revenue | Grant revenue | | | | 194 | | | 4 | | Grant revenue | | 140 | | | 145 | | | 473 | | | 187 | |
| Collaboration revenue | Collaboration revenue | | | | 10 | | | 4 | | Collaboration revenue | | 19 | | | 12 | | | 47 | | | 45 | |
| Total revenue | Total revenue | | | | 1,937 | | | 8 | | Total revenue | | 4,969 | | | 157 | | | 11,260 | | | 232 | |
| Operating expenses: | Operating expenses: | | | | | | | Operating expenses: | | | | | | | | |
| Cost of sales | Cost of sales | | | | 193 | | | 0 | | Cost of sales | | 722 | | | — | | | 1,665 | | | — | |
| Research and development | Research and development | | | | 401 | | | 115 | | Research and development | | 521 | | | 344 | | | 1,343 | | | 611 | |
| Selling, general and administrative | Selling, general and administrative | | | | 77 | | | 24 | | Selling, general and administrative | | 168 | | | 48 | | | 366 | | | 109 | |
| Total operating expenses | Total operating expenses | | | | 671 | | | 139 | | Total operating expenses | | 1,411 | | | 392 | | | 3,374 | | | 720 | |
| Income (loss) from operations | Income (loss) from operations | | | | 1,266 | | | (131) | | Income (loss) from operations | | 3,558 | | | (235) | | | 7,886 | | | (488) | |
| Interest income | Interest income | | | | 4 | | | 8 | | Interest income | | 4 | | | 6 | | | 11 | | | 21 | |
| Other expense, net | Other expense, net | | | | (10) | | | (1) | | Other expense, net | | (10) | | | (3) | | | (22) | | | (6) | |
| Income (loss) before income taxes | Income (loss) before income taxes | | | | 1,260 | | | (124) | | Income (loss) before income taxes | | 3,552 | | | (232) | | | 7,875 | | | (473) | |
| Provision for income taxes | Provision for income taxes | | | | 39 | | | 0 | | Provision for income taxes | | 219 | | | 1 | | | 541 | | | 1 | |
| Net income (loss) | Net income (loss) | | | | $ | 1,221 | | | $ | (124) | | Net income (loss) | | $ | 3,333 | | | $ | (233) | | | $ | 7,334 | | | $ | (474) | |
| | Earnings (loss) per share | | | | |
| Earnings (loss) per share: | | Earnings (loss) per share: | |
| Basic | Basic | | | | $ | 3.05 | | | $ | (0.35) | | Basic | | $ | 8.27 | | | $ | (0.59) | | | $ | 18.25 | | | $ | (1.26) | |
| Diluted | Diluted | | | | $ | 2.84 | | | $ | (0.35) | | Diluted | | $ | 7.70 | | | $ | (0.59) | | | $ | 17.00 | | | $ | (1.26) | |
| | Weighted average common shares used in calculation of earnings (loss) per share | | | | |
| Weighted average common shares used in calculation of earnings (loss) per share: | | Weighted average common shares used in calculation of earnings (loss) per share: | |
| Basic | Basic | | | | 400 | | | 353 | | Basic | | 404 | | | 395 | | | 402 | | | 376 | |
| Diluted | Diluted | | | | 430 | | | 353 | | Diluted | | 434 | | | 395 | | | 431 | | | 376 | |
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(Unaudited, in millions)
| | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, | |
| | 2021 | | 2020 | | | 2021 | | 2020 | | 2021 | | 2020 | |
| Net income (loss) | Net income (loss) | $ | 1,221 | | | $ | (124) | | Net income (loss) | | $ | 3,333 | | | $ | (233) | | | $ | 7,334 | | | $ | (474) | | |
| Other comprehensive loss: | | |
| Unrealized loss on available-for-sale debt securities, net of tax of $0 and $0, for the three months ended March 31, 2021 and 2020, respectively | (2) | | | (8) | | |
| Other comprehensive income (loss), net of tax: | | Other comprehensive income (loss), net of tax: | | |
| Available-for-sales securities: | | Available-for-sales securities: | | |
| Unrealized (losses) gains on available-for-sale debt securities | | Unrealized (losses) gains on available-for-sale debt securities | | (3) | | | (3) | | | (10) | | | 2 | | |
| Less: net realized (gains) losses on available-for-sale securities reclassified in net income (loss) | | Less: net realized (gains) losses on available-for-sale securities reclassified in net income (loss) | | (1) | | | — | | | (2) | | | 1 | | |
| Net (decrease) increase from available-for-sale debt securities | | Net (decrease) increase from available-for-sale debt securities | | (4) | | | (3) | | | (12) | | | 3 | | |
| Cash flow hedges: | | Cash flow hedges: | | |
| Unrealized gains on derivative instruments | | Unrealized gains on derivative instruments | | 30 | | | — | | | 51 | | | — | | |
| Less: net realized (gains) on derivative instruments reclassified in net income | | Less: net realized (gains) on derivative instruments reclassified in net income | | (11) | | | — | | | (11) | | | — | | |
| Net increase from derivatives designated as hedging instruments | | Net increase from derivatives designated as hedging instruments | | 19 | | | — | | | 40 | | | — | | |
| Total other comprehensive income (loss) | | Total other comprehensive income (loss) | | 15 | | | (3) | | | 28 | | | 3 | | |
| Comprehensive income (loss) | Comprehensive income (loss) | $ | 1,219 | | | $ | (132) | | Comprehensive income (loss) | | $ | 3,348 | | | $ | (236) | | | $ | 7,362 | | | $ | (471) | | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
FOR THE THREE MONTHS AND NINE MONTHS ENDED MARCH 31,SEPTEMBER 30, 2021 AND 2020
(Unaudited, in millions)
| | | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Accumulated Deficit | | Total Stockholders’ Equity | | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Retained Earnings | | Total Stockholders’ Equity |
| | Shares | | Amount | | | Shares | | Amount | |
| Balance at December 31, 2020 | 399 | | | $ | 0 | | | $ | 4,802 | | | $ | 3 | | | $ | (2,244) | | | $ | 2,561 | | |
| Balance at June 30, 2021 | | Balance at June 30, 2021 | 403 | | | $ | — | | | $ | 4,931 | | | $ | 16 | | | $ | 1,757 | | | $ | 6,704 | |
| | Exercise of options to purchase common stock | Exercise of options to purchase common stock | 2 | | | — | | | 28 | | | — | | | — | | | 28 | | Exercise of options to purchase common stock | 2 | | | — | | | 32 | | | — | | | — | | | 32 | |
| | Stock-based compensation | Stock-based compensation | — | | | — | | | 30 | | | — | | | — | | | 30 | | Stock-based compensation | — | | | — | | | 40 | | | — | | | — | | | 40 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (2) | | | — | | | (2) | | |
| Other comprehensive income, net of tax | | Other comprehensive income, net of tax | — | | | — | | | — | | | 15 | | | — | | | 15 | |
| Net income | Net income | — | | | — | | | — | | | — | | | 1,221 | | | 1,221 | | Net income | — | | | — | | | — | | | — | | | 3,333 | | | 3,333 | |
| Balance at March 31, 2021 | 401 | | | $ | 0 | | | $ | 4,860 | | | $ | 1 | | | $ | (1,023) | | | $ | 3,838 | | |
| Balance at September 30, 2021 | | Balance at September 30, 2021 | 405 | | | $ | — | | | $ | 5,003 | | | $ | 31 | | | $ | 5,090 | | | $ | 10,124 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income (loss) | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2019 | 337 | | | $ | 0 | | | $ | 2,670 | | | $ | 2 | | | $ | (1,497) | | | $ | 1,175 | |
| Proceeds from public offering of common stock, net of issuance costs of $1 | 30 | | | — | | | 550 | | | — | | | — | | | 550 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 3 | | | — | | | 28 | | | — | | | — | | | 28 | |
| Stock-based compensation | — | | | — | | | 20 | | | — | | | — | | | 20 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (8) | | | — | | | (8) | |
| Net loss | — | | | — | | | — | | | — | | | (124) | | | (124) | |
| Balance at March 31, 2020 | 370 | | | $ | 0 | | | $ | 3,268 | | | $ | (6) | | | $ | (1,621) | | | $ | 1,641 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at June 30, 2020 | 393 | | | $ | — | | | $ | 4,677 | | | $ | 8 | | | $ | (1,738) | | | $ | 2,947 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 2 | | | — | | | 27 | | | — | | | — | | | 27 | |
| | | | | | | | | | | |
| Stock-based compensation | — | | | — | | | 22 | | | — | | | — | | | 22 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (3) | | | — | | | (3) | |
| Net loss | — | | | — | | | — | | | — | | | (233) | | | (233) | |
| Balance at September 30, 2020 | 395 | | | $ | — | | | $ | 4,726 | | | $ | 5 | | | $ | (1,971) | | | $ | 2,760 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Retained Earnings (Accumulated Deficit) | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2020 | 399 | | | $ | — | | | $ | 4,802 | | | $ | 3 | | | $ | (2,244) | | | $ | 2,561 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 6 | | | — | | | 91 | | | — | | | — | | | 91 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 5 | | | — | | | — | | | 5 | |
| Stock-based compensation | — | | | — | | | 105 | | | — | | | — | | | 105 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 28 | | | — | | | 28 | |
| Net income | — | | | — | | | — | | | — | | | 7,334 | | | 7,334 | |
| Balance at September 30, 2021 | 405 | | | $ | — | | | $ | 5,003 | | | $ | 31 | | | $ | 5,090 | | | $ | 10,124 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Accumulated Deficit | | Total Stockholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2019 | 337 | | | $ | — | | | $ | 2,670 | | | $ | 2 | | | $ | (1,497) | | | $ | 1,175 | |
| Proceeds from public offering of common stock, net of issuance costs of $2 | 48 | | | — | | | 1,853 | | | — | | | — | | | 1,853 | |
| | | | | | | | | | | |
| Exercise of options to purchase common stock | 10 | | | — | | | 133 | | | — | | | — | | | 133 | |
| Purchase of common stock under employee stock purchase plan | — | | | — | | | 3 | | | — | | | — | | | 3 | |
| Stock-based compensation | — | | | — | | | 67 | | | — | | | — | | | 67 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 3 | | | — | | | 3 | |
| Net loss | — | | | — | | | — | | | — | | | (474) | | | (474) | |
| Balance at September 30, 2020 | 395 | | | $ | — | | | $ | 4,726 | | | $ | 5 | | | $ | (1,971) | | | $ | 2,760 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited, in millions)
| | | Three Months Ended March 31, | | Nine Months Ended September 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Operating activities | Operating activities | | | | Operating activities | | | |
| Net income (loss) | Net income (loss) | $ | 1,221 | | | $ | (124) | | Net income (loss) | $ | 7,334 | | | $ | (474) | |
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | |
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |
| Stock-based compensation | Stock-based compensation | 30 | | | 20 | | Stock-based compensation | 105 | | | 67 | |
| Depreciation and amortization | Depreciation and amortization | 15 | | | 7 | | Depreciation and amortization | 154 | | | 24 | |
| Amortization/accretion of investments | Amortization/accretion of investments | 5 | | | 1 | | Amortization/accretion of investments | 33 | | | 5 | |
| | Deferred income taxes | Deferred income taxes | (50) | | | 0 | | Deferred income taxes | (89) | | | — | |
| Changes in assets and liabilities: | Changes in assets and liabilities: | | Changes in assets and liabilities: | |
| Accounts receivable | Accounts receivable | (1,819) | | | (2) | | Accounts receivable | (1,751) | | | (185) | |
| Prepaid expenses and other assets | Prepaid expenses and other assets | (12) | | | (4) | | Prepaid expenses and other assets | (186) | | | (68) | |
| Inventory | Inventory | (448) | | | 0 | | Inventory | (918) | | | — | |
| Right-of-use assets, operating leases | Right-of-use assets, operating leases | 2 | | | (14) | | Right-of-use assets, operating leases | (25) | | | (13) | |
| Accounts payable | Accounts payable | (15) | | | 2 | | Accounts payable | 26 | | | 14 | |
| Accrued liabilities | Accrued liabilities | 285 | | | (12) | | Accrued liabilities | 600 | | | 132 | |
| Deferred revenue | Deferred revenue | 3,666 | | | (1) | | Deferred revenue | 4,431 | | | 1,240 | |
| Income taxes payable | | Income taxes payable | 565 | | | — | |
| Operating lease liabilities | Operating lease liabilities | (2) | | | 15 | | Operating lease liabilities | 8 | | | 14 | |
| Other liabilities | Other liabilities | 93 | | | 6 | | Other liabilities | 23 | | | 7 | |
| Net cash provided by (used in) operating activities | 2,971 | | | (106) | | |
| Net cash provided by operating activities | | Net cash provided by operating activities | 10,310 | | | 763 | |
| Investing activities | Investing activities | | Investing activities | |
| Purchases of marketable securities | Purchases of marketable securities | (726) | | | (621) | | Purchases of marketable securities | (10,279) | | | (2,326) | |
| Proceeds from maturities of marketable securities | Proceeds from maturities of marketable securities | 339 | | | 269 | | Proceeds from maturities of marketable securities | 1,075 | | | 748 | |
| Proceeds from sales of marketable securities | Proceeds from sales of marketable securities | 242 | | | 42 | | Proceeds from sales of marketable securities | 1,983 | | | 140 | |
| Purchases of property and equipment | Purchases of property and equipment | (35) | | | (6) | | Purchases of property and equipment | (164) | | | (44) | |
| Net cash used in investing activities | Net cash used in investing activities | (180) | | | (316) | | Net cash used in investing activities | (7,385) | | | (1,482) | |
| Financing activities | Financing activities | | | | Financing activities | | | |
| Proceeds from public offerings of common stock, net of issuance costs | Proceeds from public offerings of common stock, net of issuance costs | 0 | | | 550 | | Proceeds from public offerings of common stock, net of issuance costs | — | | | 1,853 | |
| Proceeds from issuance of common stock through equity plans, net | Proceeds from issuance of common stock through equity plans, net | 28 | | | 28 | | Proceeds from issuance of common stock through equity plans, net | 96 | | | 136 | |
| | Changes in financing lease liabilities | Changes in financing lease liabilities | (2) | | | 0 | | Changes in financing lease liabilities | (96) | | | — | |
| Net cash provided by financing activities | Net cash provided by financing activities | 26 | | | 578 | | Net cash provided by financing activities | — | | | 1,989 | |
| Net increase in cash, cash equivalents and restricted cash | Net increase in cash, cash equivalents and restricted cash | 2,817 | | | 156 | | Net increase in cash, cash equivalents and restricted cash | 2,925 | | | 1,270 | |
| Cash, cash equivalents and restricted cash, beginning of year | Cash, cash equivalents and restricted cash, beginning of year | 2,636 | | | 248 | | Cash, cash equivalents and restricted cash, beginning of year | 2,636 | | | 248 | |
| Cash, cash equivalents and restricted cash, end of period | Cash, cash equivalents and restricted cash, end of period | $ | 5,453 | | | $ | 404 | | Cash, cash equivalents and restricted cash, end of period | $ | 5,561 | | | $ | 1,518 | |
| Non-cash investing and financing activities | Non-cash investing and financing activities | | | | Non-cash investing and financing activities | | | |
| Purchases of property and equipment included in accounts payable and accrued liabilities | Purchases of property and equipment included in accounts payable and accrued liabilities | $ | 21 | | | $ | 7 | | Purchases of property and equipment included in accounts payable and accrued liabilities | $ | 66 | | | $ | 13 | |
| Right-of-use assets obtained through finance lease modifications and reassessments | Right-of-use assets obtained through finance lease modifications and reassessments | $ | 51 | | | $ | 0 | | Right-of-use assets obtained through finance lease modifications and reassessments | $ | 364 | | | $ | 46 | |
| Right-of-use assets obtained in exchange for financing lease liabilities | | Right-of-use assets obtained in exchange for financing lease liabilities | $ | 126 | | | $ | — | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MODERNA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Description of the Business
Moderna, Inc. (collectively, with its consolidated subsidiaries, any of Moderna, we, us, our, or the Company) was incorporated in Delaware on July 22, 2016. We are the successor in interest to Moderna LLC, a limited liability company formed under the laws of the State of Delaware in 2013. Our principal executive office is located at 200 Technology Square, Cambridge, MA.
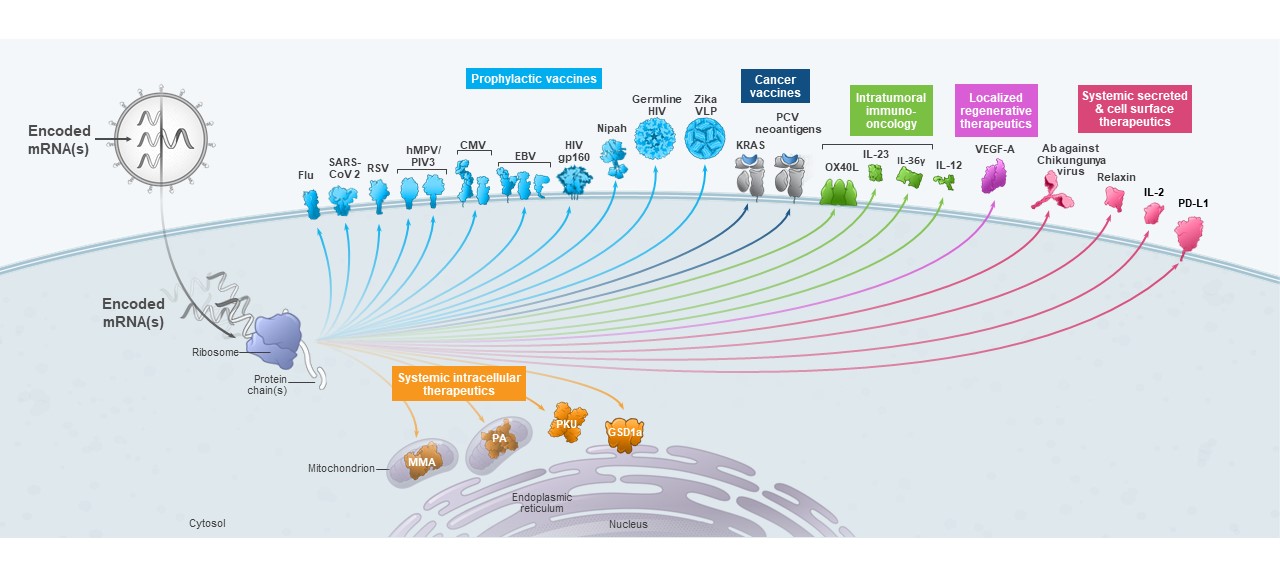
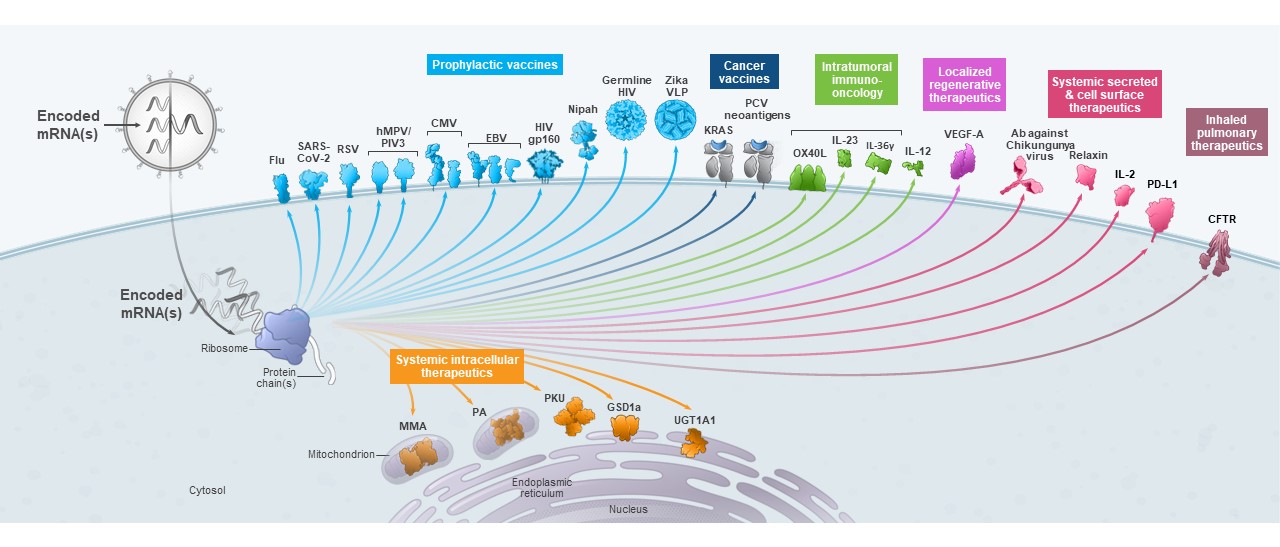
We are a biotechnology company creating a new generation of transformative medicines based on messenger RNA (mRNA), to improve the lives of patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane, or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology, and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing vaccines and therapeutics for infectious diseases, immuno-oncology, rare diseases, autoimmune and cardiovascular diseases, independently and with our strategic collaborators.
On December 18, 2020, we received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the emergency use of the Moderna COVID-19 Vaccine (also referred to as mRNA-1273)mRNA-1273 and marketed under the brand name Spikevax) in individuals 18 years of age or older. We have also received authorization for our COVID-19 vaccine from health agencies in Canada, Israel, the European Union, the United Kingdom, Switzerland, Singapore, Qatar, Taiwan, and the Philippines,more than 60 countries and from the World Health Organization. Additional authorizations are currently under review in other countries. In addition, we have received authorization for our COVID-19 vaccine for use in adolescents in the United Kingdom, European Union, Japan, Canada, Switzerland, Taiwan, Saudi Arabia, Australia, and the Philippines, and have pending applications for authorization to administer the vaccine to adolescents with regulatory agencies in the United States and other countries.
The FDA has approved an update to the EUA for the Moderna COVID-19 vaccine to include a third dose at the 100 µg level for immunocompromised individuals 18 years of age or older in the United States, as well as the administration of 50 µg booster doses for individuals age 65 and older, people aged 18 to 64 who are at high risk of severe COVID-19, and people aged 18 to 64 with frequent institutional or occupational exposure to SARS-CoV-2. In October 2021, the U.S. Advisory Committee on Immunization Practices (ACIP) also endorsed recommending the Moderna COVID-19 Vaccine as a booster, regardless of the original vaccine received by an individual in their primary series. The European Medicines Agency (EMA) has also authorized a third dose of the Moderna COVID-19 vaccinegiven at least 28 days after the second dose to severely immunocompromised individuals 12 years of age or older, as well as the administration of 50 µg booster doses for individuals 18 years of age and older. In August 2021, we completed the rolling submission process with the FDA for a Biologics License Application (BLA) for our COVID-19 vaccine, which is subject to Priority Review.
As of March 31,September 30, 2021, we had 2437 mRNA development programs in our portfolio with 1322 having entered the clinic. In the third quarter of 2021, we refined the way we track our development programs and now separately track each indication of our COVID-19 and RSV vaccine candidates, which resulted in an increase in the number of our development programs. We have incurred significant expenses in connection with the discovery, development and commercialization of our products, and we expect to continue to incur significant expenses for the foreseeable future. We anticipate that our expenses will increase significantly in connection with the ongoing development and commercialization of our COVID-19 vaccine and ongoing activities to support our platform research, drug discovery and clinical development, including development of any new generations of boosters and vaccines against variants of SARS-CoV-2 and vaccines against other respiratory diseases, infrastructure and Research Engine and Early Development Engine (which includes our Moderna Technology Center), digital infrastructure, creation of a portfolio of intellectual property, and administrative support. We may finance our future cash needs that exceed our operating costs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, strategic alliances and marketing, manufacturing, distribution and licensing arrangements. We may be unable to raise additional funds or enter into such other agreements on favorable terms, or at all.
We believe that our cash, cash equivalents, and investments as of March 31,September 30, 2021 will be sufficient to enable us to fund our projected operations through at least the next 12 months from the issuance of ourthese financial statements. We are subject to numerous risks and uncertainties associated with pharmaceutical development and commercialization, and we are unable to predict the timing or amount of expenses or if we will be able to maintain profitability. If we are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
2. Summary of Basis of Presentation and Recent Accounting Standards
Basis of Presentation and Principles of Consolidation
The accompanying unaudited condensed consolidated financial statements that accompany these notes have been prepared in accordance with U.S. generally accepted accounting principles (GAAP) and applicable rules and regulations of the Securities and Exchange Commission (SEC) for interim financial reporting, consistent in all material respects with those applied in our Annual Report on Form 10-K for the year ended December 31, 2020 (2020 Form 10-K). Any reference in these notes to applicable guidance is meant to refer to the authoritative accounting principles generally accepted in the United States as found in the Accounting Standard Codification (ASC) and Accounting Standards Update (ASU) of the Financial Accounting Standards Board (FASB). This report should be read in conjunction with the consolidated financial statements in our 2020 Form 10-K.
The condensed consolidated financial statements include the CompanyModerna, Inc. and its subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
The significant accounting policies used in preparation of these condensed consolidated financial statements for the three and nine months ended March 31,September 30, 2021 are consistent with those described in our 2020 Form 10-K.10-K, except for “Derivative financial instruments” disclosed within Note 6.
Use of Estimates
We have made estimates and judgments affecting the amounts reported in our condensed consolidated financial statements and the accompanying notes. We base our estimates on historical experience and various relevant assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods that are not readily apparent from other sources. Significant estimates relied upon in preparing these financial statements include, but are not limited to, critical accounting policies or estimates related to revenue recognition, research and development expenses, income tax provisions, stock-based compensation, leases, fair value of financial instruments, derivative financial instruments, inventory, and useful lives of property and equipment, income taxes and our valuation allowance on our deferred tax assets. The actual results that we experience may differ materially from our estimates.
Comprehensive Income (Loss)
Comprehensive income (loss) includes net income (loss) and other comprehensive lossincome (loss) for the period. Other comprehensive lossincome (loss) consists of unrealized gains/losses and gains/losses on our investments.investments and derivatives designated as hedging instruments. Total comprehensive income (loss) for all periods presented has been disclosed in the condensed consolidated statements of comprehensive income (loss).
The components of accumulated other comprehensive income for the three and nine months ended March 31,September 30, 2021 arewere as follows (in millions):
| | | | | |
| Unrealized Loss on Available-for-Sale Debt Securities |
Accumulated other comprehensive income, balance at December 31, 2020 | $ | 3 | |
Other comprehensive loss | (2) | |
Accumulated other comprehensive income, balance at March 31, 2021 | $ | 1 | |
| | | | | | | | | | | | | | | | | |
| Unrealized Loss on Available-for-Sale Debt Securities | | Net Unrealized Gains on Derivatives Designated As Hedging Instruments | | Total |
| Accumulated other comprehensive income, balance at December 31, 2020 | $ | 3 | | | $ | — | | | $ | 3 | |
| Other comprehensive loss | (2) | | | — | | | (2) | |
| Accumulated other comprehensive income, balance at March 31, 2021 | 1 | | | — | | | 1 | |
| Other comprehensive (loss) income | (6) | | | 21 | | | 15 | |
| Accumulated other comprehensive income, balance at June 30, 2021 | $ | (5) | | | $ | 21 | | | $ | 16 | |
| Other comprehensive (loss) income | (4) | | | 19 | | | 15 | |
| Accumulated other comprehensive income, balance at September 30, 2021 | $ | (9) | | | $ | 40 | | | $ | 31 | |
| | | | | |
Restricted Cash
We include our restricted cash balance in the cash, cash equivalents and restricted cash reconciliation of operating, investing and financing activities in the condensed consolidated statements of cash flows.
The following table provides a reconciliation of cash, cash equivalents and restricted cash in the condensed consolidated balance sheets that sum to the total of the same such amounts shown in the condensed consolidated statements of cash flows (in millions):
| | | March 31, | | September 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 5,442 | | | $ | 392 | | Cash and cash equivalents | | $ | 5,550 | | | $ | 1,506 | |
| Restricted cash | Restricted cash | | 0 | | | 1 | | Restricted cash | | — | | | 1 | |
| Restricted cash, non-current | Restricted cash, non-current | | 11 | | | 11 | | Restricted cash, non-current | | 11 | | | 11 | |
Total cash, cash equivalents and restricted cash shown in the condensed consolidated
statements of cash flows | Total cash, cash equivalents and restricted cash shown in the condensed consolidated
statements of cash flows | | $ | 5,453 | | | $ | 404 | | Total cash, cash equivalents and restricted cash shown in the condensed consolidated
statements of cash flows | | $ | 5,561 | | | $ | 1,518 | |
Recently Issued Accounting Standards Not Yet Adopted
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our condensed consolidated financial statements and disclosures.
3. Product Sales
In December 2020, we began selling our COVID-19 vaccine to the U.S. Government and international governments. Under the supply agreements with these governments, we received or billed for upfront deposits for our future vaccine supply, which are initially recorded as deferred revenue. We recognize revenue based on the fixed price per dose when control of the product has transferred and customer acceptance has occurred as applicable, unless such acceptance provisions are deemed perfunctory.
Product sales by customer geographic location was as follows (in millions):
| | | | | | | | |
| | Three Months Ended March 31, 2021 |
United States | | $ | 1,358 | |
Rest of world | | 375 | |
Total | | $ | 1,733 | |
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended
September 30, 2021 | | | | Nine Months Ended
September 30, 2021 | | |
| United States | | $ | 1,197 | | | | | $ | 4,648 | | | |
| Rest of world | | 3,613 | | | | | 6,092 | | | |
| Total | | $ | 4,810 | | | | | $ | 10,740 | | | |
There were no product sales for the three and nine months ended March 31,September 30, 2020. As of March 31,September 30, 2021, we had oneour COVID-19 vaccine was our only commercial product authorized for use, our COVID-19 vaccine.use.
As of March 31,September 30, 2021 and December 31, 2020, we had deferred revenue of $7.5$8.3 billion and $3.8 billion, respectively, related to customer deposits, classified as currentdeposits. We expect $7.9 billion of our deferred revenue related to customer deposits as of September 30, 2021 to be realized in our consolidated balance sheet.less than one year. Timing of product manufacturing, delivery, and receipt of marketing approval will determine the period in which revenue is recognized.
4. Grant Revenue
In September 2020, we entered into an agreement with the Defense Advanced Research Projects Agency (DARPA) for an award of up to $56 million to fund development of a mobile manufacturing prototype leveraging our existing manufacturing technology that is capable of rapidly producing vaccines and therapeutics. As of March 31,September 30, 2021, the committed funding, net of revenue earned, was $3$4 million. An additional $43 million with an additional $51 millionof funding will be available if DARPA exercises additional contract options.
In April 2020, we entered into an agreement with the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), for an award of up to $483 million to accelerate development of mRNA-1273, our vaccine candidate against the novel coronavirus.COVID-19. In July 2020, we amended our agreement with BARDA to provide for an additional commitment of up to $472 million to support late-stage clinical development of mRNA-1273, including the execution of a 30,000 participant Phase 3 study in the U.S. We further amended the agreement in March 2021 to provide for an additional commitment of $63 million to further support late-stage clinical development, including Phase 2/3 mRNA-1273 pediatric studies. As of March 31, 2021, the maximum award from BARDA, inclusive of the March 2021 amendment, was approximately $1.0 billion. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. All contract options have been exercised. As of March 31, 2021, the remaining available funding net of revenue earned was $317 million. Subsequent to the end of the quarter, onIn April 18, 2021, we entered into a further amendment to the BARDA agreement, increasing the amount of potential reimbursements by $236 million in connection with costs associated with the Phase 3 clinical trials for mRNA-1273 and pharmacovigilance efforts. In June 2021, the agreement with BARDA was further amended to award additional funding of $144 million to support pediatric clinical trials for mRNA-1273. The maximum award from BARDA, inclusive of the 2020 and 2021 amendments, was approximately $1.4 billion. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. All contract options have been exercised. As of September 30, 2021, the remaining available funding, net of revenue earned, was $441 million.
In September 2016, we received from BARDA an award of up to $126 million, from BARDA,subsequently adjusted to $117 million in 2021, to help fund our Zika vaccine program. NaN of the 4 contract options have been exercised. As of March 31,September 30, 2021, the remaining available funding, net of revenue earned, was $69$55 million, with an additional $8 million available if the final contract option is exercised.
In January 2016, we entered a global health project framework agreement with the Bill and Melinda Gates Foundation (Gates Foundation) to advance mRNA-based development projects for various infectious diseases, including human immunodeficiency virus (HIV). As of March 31,September 30, 2021, the available funding, net of revenue earned, was $11$7 million, with up to an additional $80 million available if additional follow-on projects are approved.
The following table summarizes grant revenue as of and for the periodperiods presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| BARDA | $ | 128 | | | $ | 143 | | | $ | 454 | | | $ | 183 | |
| Other grant revenue | 12 | | | 2 | | | 19 | | | 4 | |
| Total grant revenue | $ | 140 | | | $ | 145 | | | $ | 473 | | | $ | 187 | |
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | | | 2021 | | 2020 |
| BARDA | | | | | $ | 192 | | | $ | 3 | |
| Other grant revenue | | | | | 2 | | | 1 | |
| Total grant revenue | | | | | $ | 194 | | | $ | 4 | |
| | | | | | | |
5. Collaboration Agreements
We have entered into collaboration agreements with strategic collaborators to accelerate the discovery and advancement of potential mRNA medicines across therapeutic areas. As of March 31,September 30, 2021 and December 31, 2020, we had collaboration agreements with AstraZeneca plc (AstraZeneca), Merck & Co., Inc (Merck), Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Europe) Limited (together, Vertex), and Chiesi Farmaceutici S.P.A. (Chiesi).others. Please refer to our 2020 Form 10-K under the heading “Third-Party Strategic Alliances” and Note 5 to our consolidated financial statements for further description of each of thethese collaboration agreements.
The following table summarizes our total consolidated revenue from our strategic collaborators for the periods presented (in millions):
| | | | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| Collaboration Revenue by Strategic Collaborator: | Collaboration Revenue by Strategic Collaborator: | | | 2021 | | 2020 | Collaboration Revenue by Strategic Collaborator: | 2021 | | 2020 | | 2021 | | 2020 |
| AstraZeneca | AstraZeneca | | | $ | 0 | | | $ | 1 | | AstraZeneca | $ | 3 | | | $ | — | | | $ | 7 | | | $ | 17 | |
| Merck | Merck | | | 0 | | | 1 | | Merck | 7 | | | 6 | | | 11 | | | 18 | |
| Vertex | Vertex | | | 9 | | | 2 | | Vertex | 5 | | | 6 | | | 23 | | | 10 | |
| Other | Other | | | 1 | | | 0 | | Other | 4 | | | — | | | 6 | | | — | |
| Total collaboration revenue | Total collaboration revenue | | | $ | 10 | | | $ | 4 | | Total collaboration revenue | $ | 19 | | | $ | 12 | | | $ | 47 | | | $ | 45 | |
The following table presents changes in the balances of our receivables and contract liabilities related to our strategic collaboration agreements during the threenine months ended March 31,September 30, 2021 (in millions):
| | | December 31, 2020 | | Additions | | Deductions | | March 31, 2021 | | December 31, 2020 | | Additions | | Deductions | | September 30, 2021 |
| Contract Assets: | Contract Assets: | | | | | | | | | Contract Assets: | | | | | | | | |
| Accounts receivable | Accounts receivable | | $ | 6 | | | $ | 5 | | | $ | (2) | | | $ | 9 | | Accounts receivable | | $ | 6 | | | $ | 21 | | | $ | (21) | | | $ | 6 | |
| Contract Liabilities: | Contract Liabilities: | | Contract Liabilities: | |
| Deferred revenue | Deferred revenue | | $ | 240 | | | $ | 5 | | | $ | (10) | | | $ | 235 | | Deferred revenue | | $ | 240 | | | $ | 23 | | | $ | (45) | | | $ | 218 | |
As of March 31,September 30, 2021, the aggregated amount of the transaction price allocated to performance obligations under our collaboration agreements that are unsatisfied or partially unsatisfied was $314$310 million.
6. Financial Instruments
Cash and Cash Equivalents and Investments
The following tables summarize our cash and available-for-sale securities by significant investment category at March 31,September 30, 2021 and December 31, 2020 (in millions):
| | | March 31, 2021 | | September 30, 2021 |
| | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities | | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 5,442 | | | $ | 0 | | | $ | 0 | | | $ | 5,442 | | | $ | 5,442 | | | $ | 0 | | | $ | 0 | | Cash and cash equivalents | | $ | 5,550 | | | $ | — | | | $ | — | | | $ | 5,550 | | | $ | 5,550 | | | $ | — | | | $ | — | |
| Available-for-sale: | Available-for-sale: | | Available-for-sale: | |
| Certificates of deposit | Certificates of deposit | | 413 | | | 0 | | | 0 | | | 413 | | | 0 | | | 413 | | | 0 | | Certificates of deposit | | 85 | | | — | | | — | | | 85 | | | — | | | 85 | | | — | |
| U.S. treasury securities | | 374 | | | 0 | | | 0 | | | 374 | | | 0 | | | 374 | | | 0 | | |
| Debt securities of U.S. government agencies and corporate entities | | 1,972 | | | 3 | | | (1) | | | 1,974 | | | 0 | | 1,506 | | | 468 | | |
| $ | 8,201 | | | $ | 3 | | | $ | (1) | | | $ | 8,203 | | | $ | 5,442 | | | $ | 2,293 | | | $ | 468 | | |
| U.S. treasury bills | | U.S. treasury bills | | 95 | | | — | | | — | | | 95 | | | — | | | 95 | | | — | |
| U.S. treasury notes | | U.S. treasury notes | | 6,557 | | | 1 | | | (5) | | | 6,553 | | | — | | | 2,046 | | | 4,507 | |
| Corporate debt securities | | Corporate debt securities | | 2,956 | | | 1 | | | (4) | | | 2,953 | | | — | | | 1,118 | | | 1,835 | |
| Government debt securities | | Government debt securities | | 113 | | | — | | | (1) | | | 112 | | | — | | | 12 | | | 100 | |
| Total | | Total | | $ | 15,356 | | | $ | 2 | | | $ | (10) | | | $ | 15,348 | | | $ | 5,550 | | | $ | 3,356 | | | $ | 6,442 | |
| | | December 31, 2020 | | December 31, 2020 |
| | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities | | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | Estimated Fair Value | | Cash and
Cash
Equivalents | | Current
Marketable
Securities | | Non-
Current
Marketable
Securities |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 2,624 | | | $ | 0 | | | $ | 0 | | | $ | 2,624 | | | $ | 2,624 | | | $ | 0 | | | $ | 0 | | Cash and cash equivalents | | $ | 2,624 | | | $ | — | | | $ | — | | | $ | 2,624 | | | $ | 2,624 | | | $ | — | | | $ | — | |
| Available-for-sale: | Available-for-sale: | | Available-for-sale: | |
| Certificates of deposit | Certificates of deposit | | 239 | | | 0 | | | 0 | | | 239 | | | 0 | | | 215 | | | 24 | | Certificates of deposit | | 239 | | | — | | | — | | | 239 | | | — | | | 215 | | | 24 | |
| U.S. treasury securities | | 492 | | | 0 | | | 0 | | | 492 | | | 0 | | | 492 | | | 0 | | |
| Debt securities of U.S. government agencies and corporate entities | | 1,888 | | | 4 | | | 0 | | | 1,892 | | | 0 | | | 1,277 | | | 615 | | |
| $ | 5,243 | | | $ | 4 | | | $ | 0 | | | $ | 5,247 | | | $ | 2,624 | | | $ | 1,984 | | | $ | 639 | | |
| U.S. treasury bills | | U.S. treasury bills | | 492 | | | — | | | — | | | 492 | | | — | | | 492 | | | — | |
| U.S. treasury notes | | U.S. treasury notes | | 87 | | | — | | | — | | | 87 | | | — | | | 38 | | | 49 | |
| Corporate debt securities | | Corporate debt securities | | 1,788 | | | 4 | | | — | | | 1,792 | | | — | | | 1,239 | | | 553 | |
| Government debt securities | | Government debt securities | | 13 | | | — | | | — | | | 13 | | | — | | | — | | | 13 | |
| Total | | Total | | $ | 5,243 | | | $ | 4 | | | $ | — | | | $ | 5,247 | | | $ | 2,624 | | | $ | 1,984 | | | $ | 639 | |
The amortized cost and estimated fair value of marketable securities by contractual maturity at March 31,September 30, 2021 and December 31, 2020 arewere as follows (in millions):
| | | March 31, 2021 | | September 30, 2021 |
| | Amortized Cost | | Estimated Fair Value | | Amortized
Cost | | Estimated
Fair Value |
| Due in one year or less | Due in one year or less | | $ | 2,291 | | | $ | 2,293 | | Due in one year or less | | $ | 3,356 | | | $ | 3,356 | |
| Due after one year through five years | Due after one year through five years | | 468 | | | 468 | | Due after one year through five years | | 6,450 | | | 6,442 | |
| Total | Total | | $ | 2,759 | | | $ | 2,761 | | Total | | $ | 9,806 | | | $ | 9,798 | |
| | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Amortized
Cost | | Estimated
Fair Value |
| Due in one year or less | | $ | 1,981 | | | $ | 1,984 | |
| Due after one year through five years | | 638 | | | 639 | |
| Total | | $ | 2,619 | | | $ | 2,623 | |
In accordance with our investment policy, we place investments in investment grade securities with high credit quality issuers, and generally limit the amount of credit exposure to any one issuer. We evaluate securities for impairment at the end of each reporting period. Impairment is evaluated considering numerous factors, and their relative significance varies depending on the situation.
Factors considered include whether a decline in fair value below the amortized cost basis is due to credit-related factors or noncredit-relatednon-credit-related factors, the financial condition and near-term prospects of the issuer, and our intent and ability to hold the investment to allow for an anticipated recovery in fair value. Any impairment that is not credit related is recognized in other comprehensive loss, net of applicable taxes. A credit-related impairment is recognized as an allowance on the balance sheet with a corresponding adjustment to earnings. We did 0tnot recognize any impairment charges related to available-for-sale securities for the three and nine months ended March 31,September 30, 2021 and 2020. We did not recognize any credit losses relatedcredit-related allowance to available-for-sale securities as of March 31,September 30, 2021 and December 31, 2020.
As of March 31,September 30, 2021 and December 31, 2020, we did not have material gross unrealized losses. We neither intend to sell these investments, nor do we believe that we are more-likely-than-not to conclude we will have to sell them before recovery of their carrying values. We also believe that we will be able to collect both principal and interest amounts due to us at maturity.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used to value the assets and liabilities:
•Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
•Level 2: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; or
•Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
The following tables summarize our financial assets and liabilities measured at fair value on a recurring basis as of March 31,September 30, 2021 and December 31, 2020 (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | Fair Value at
March 31, 2021 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Money market funds | | $ | 537 | | | $ | 537 | | | $ | 0 | |
| Certificates of deposit | | 413 | | | 0 | | | 413 | |
| U.S. treasury securities | | 374 | | | 0 | | | 374 | |
| Debt securities of U.S. government agencies and corporate entities | | 1,974 | | | 0 | | | 1,974 | |
| Derivative instruments (Note 7) | | 1 | | | 0 | | | 1 | |
| Total | | $ | 3,299 | | | $ | 537 | | | $ | 2,762 | |
| Liabilities: | | | | | | |
| Derivative instruments (Note 7) | | $ | 1 | | | $ | 0 | | | $ | 1 | |
| | | | | | | | | | | | | | | | | | | | |
| | Fair value at September 30, 2021 | | Fair Value Measurement Using |
| | | Level 1 | | Level 2 |
| Assets: | | | | | | |
| Money market funds | | $ | 4,606 | | | $ | 4,606 | | | $ | — | |
| Certificates of deposit | | 85 | | | — | | | 85 | |
| U.S. treasury bills | | 95 | | | — | | | 95 | |
| U.S. treasury notes | | 6,553 | | | — | | | 6,553 | |
| Corporate debt securities | | 2,953 | | | — | | | 2,953 | |
| Government debt securities | | 112 | | | — | | | 112 | |
| Derivative instruments (Note 7) | | 51 | | | — | | | 51 | |
| Total | | $ | 14,455 | | | $ | 4,606 | | | $ | 9,849 | |
| Liabilities: | | | | | | |
| Derivative instruments (Note 7) | | $ | 1 | | | $ | — | | | $ | 1 | |
| | | Fair Value at December 31, 2020 | | Fair Value Measurement Using | | Fair value at December 31, 2020 | | Fair Value Measurement Using |
| | Level 1 | | Level 2 | | Level 1 | | Level 2 |
| Assets: | Assets: | | | | | | | Assets: | | | | | | |
| Money market funds | Money market funds | | $ | 621 | | | $ | 621 | | | $ | 0 | | Money market funds | | $ | 660 | | | $ | 660 | | | $ | — | |
| Certificates of deposit | Certificates of deposit | | 239 | | | 0 | | | 239 | | Certificates of deposit | | 239 | | | — | | | 239 | |
| U.S. treasury securities | | 492 | | | 0 | | | 492 | | |
| Debt securities of U.S. government agencies and corporate entities | | 1,892 | | | 0 | | | 1,892 | | |
| U.S. treasury bills | | U.S. treasury bills | | 492 | | | — | | | 492 | |
| U.S. treasury notes | | U.S. treasury notes | | 87 | | | — | | | 87 | |
| Corporate debt securities | | Corporate debt securities | | 1,792 | | | — | | | 1,792 | |
| Government debt securities | | Government debt securities | | 13 | | | — | | | 13 | |
| Total | Total | | $ | 3,244 | | | $ | 621 | | | $ | 2,623 | | Total | | $ | 3,283 | | | $ | 660 | | | $ | 2,623 | |
As of March 31,September 30, 2021 and December 31, 2020, we did not have non-financial assets or liabilities measured at fair value on a recurring basis.basis and did not have any Level 3 financial assets or financial liabilities.
7. Derivative Financial Instruments
We transact business in various foreign currencies and have international sales and expenses denominated in foreign currencies. Therefore, we are exposed to certain risks arising from both our business operations and economic conditions. Our risk management strategy includes the use of derivative financial instruments to hedgehedge: (1) forecasted product sales that are denominated in foreign currencies and (2) foreign currency exchange rate fluctuations on monetary assets or liabilities denominated in foreign currencies. We do not enter into derivative financial contracts for speculative or trading purposes. We do not believe that we are exposed to more than a nominal amount of credit risk in our foreign currency hedges, as counterparties are large, global and well-capitalized financial institutions. We classify cash flows from our derivative transactions as cash flows from operating activities in our condensed consolidated statements of cash flows.
Cash Flow Hedges
We mitigate the foreign exchange risk arising from the fluctuations in foreign currency denominated product sales in Euro through a foreign currency cash flow hedging program, using forward contracts and foreign currency options that do not exceed 15 months in duration. We hedge these cash flow exposures to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. To receive hedge accounting treatment, all hedging relationships are formally documented at the inception of the hedge, and the hedges must be highly effective in offsetting changes to future cash flows on hedged transactions. The derivative assets or liabilities associated with our hedging activities are recorded at fair value in other current assets or other current liabilities, respectively, in our condensed consolidated balance sheets. The gains or losses resulting from changes in the fair value of these hedges are initially recorded as a component of accumulated other comprehensive income (AOCI) in stockholders’ equity and subsequently reclassified to product sales in the period during which the hedged transaction affects earnings. In the event the underlying forecasted transaction does not occur, or it becomes probable that it will not occur, within the defined hedge period, we reclassify the gains or losses on the related cash flow hedge from AOCI to other expense, net, in our condensed consolidated statements of operations. We evaluate hedge effectiveness at the inception of the hedge prospectively, and on an on-going basis both retrospectively and prospectively. If we do not elect hedge accounting, or the contract does not qualify for hedge accounting treatment, the changes in fair value from period to period are recorded as a component of other expense, net, in our condensed consolidated statements of operations. As of September 30, 2021, we had net deferred gains of $48 million on our foreign currency forward contracts included in AOCI that are expected to be recognized into product sales within the next 12 months.
Balance Sheet Hedges
OurWe enter into foreign currency forward contracts to hedge fluctuations associated with foreign currency denominated monetary assets and liabilities, primarily accounts receivable in Euro and lease liabilities in Swiss Franc, that are not designated for hedge accounting treatment. Therefore, these forward contracts are accounted for as derivatives whereby the fair value of the contracts are reported as other current assets or other current liabilities onin our condensed consolidated balance sheets, and gains and losses resulting from changes in the fair value are recorded as a component of other expense, net, in our condensed consolidated statements of operations. The gains and losses on these foreign currency forward contracts generally offset the gains and losses in the underlying foreign currency denominated assets and liabilities, which are also recorded to other expense, net, in our condensed consolidated statements of operations.
Total gross notional amount and fair value forof our foreign currency derivatives that are not designated as hedging instruments are accounted forwere as follows (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | March 31, 2021 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability (2) |
| Derivatives not designated as hedging instruments | | | | | | |
| Foreign currency forward contracts | | $ | 1,367 | | | $ | 1 | | | $ | 1 | |
| Total | | $ | 1,367 | | | $ | 1 | | | $ | 1 | |
| | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability (2) |
| Derivatives not designated as hedging instruments | | | | | | |
| Foreign currency forward contracts | | $ | 368 | | | $ | 0 | | | $ | 0 | |
| Total | | $ | 368 | | | $ | 0 | | | $ | 0 | |
_________
(1) As presented in the condensed consolidated balance sheet within other current assets.
(2) As presented in the condensed consolidated balance sheet within other current liabilities.
The effect of foreign currency forward contracts not designated as hedging instruments in our condensed consolidated statements of operations for the three months ended March 31, 2021 was as follows (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | September 30, 2021 |
| | Notional Amount | | Fair Value |
| | | Asset (1) | | Liability (2) |
| Derivatives designated as cash flow hedging instruments: | | | | | | |
| Foreign currency forward contracts | | $ | 1,436 | | | $ | 48 | | | $ | — | |
| | | | | | |
| Derivatives not designated as hedging instruments: | | | | | | |
| Foreign currency forward contracts | | 556 | | | 3 | | | 1 | |
| Total derivatives | | $ | 1,992 | | | $ | 51 | | | $ | 1 | |
| | | | | | | | | | | | | | | | | | | | |
| | Statement of Operations ClassificationDecember 31, 2020 |
| | Notional Amount | | Three Months Ended
March 31, 2021Fair Value |
| | | Asset (1) | | Liability (2) |
Derivatives not designated as hedging instrumentsinstruments: | | | | | | |
| Foreign currency forward contracts | | Other (expense) income, net | | $ | 35368 | |
Total | | | | $ | 35— | | | $ | — | |
| Total derivatives | | $ | 368 | | | $ | — | | | $ | — | |
_________
(1) As presented in the condensed consolidated balance sheets within prepaid expenses and other current assets.
(2) As presented in the condensed consolidated balance sheets within other current liabilities.
Gains on our foreign currency derivatives, net of tax, recognized in our condensed consolidated statements of comprehensive income (loss) for the three and nine months ended September 30, 2021 were as follows (in millions):
| | | | | | | | | | | | | | |
| | Three Months Ended
September 30, 2021 | | Nine Months Ended
September 30, 2021 |
| Derivatives in cash flow hedging relationships: | | | | |
| Foreign currency forward contracts | | $ | 30 | | | $ | 51 | |
The effect of our foreign currency derivatives in our condensed consolidated statements of operations for the three and nine months ended September 30, 2021 was as follows (in millions):
| | | | | | | | | | | | | | | | | | | | |
| | Statement of Operations Classification | | Three Months Ended
September 30, 2021 | | Nine Months Ended
September 30, 2021 |
| Derivatives in cash flow hedging relationships: | | | | | | |
| Foreign currency forward contracts | | | | | | |
| Net gain reclassified from AOCI into income | | Product sales | | $ | (11) | | | $ | (11) | |
| | | | | | |
| Derivatives not designated as hedging instruments: | | | | | | |
| Foreign currency forward contracts | | | | | | |
| Net realized and unrealized gain (loss) | | Other expense, net | | $ | 3 | | | $ | (16) | |
There were no hedging activities for the three and nine months ended March 31,September 30, 2020.
8. Inventory
Inventory as of March 31,September 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | March 31, | | December 31, | | | September 30, | | December 31, | |
| | 2021 | | 2020 | | | 2021 | | 2020 | |
| Raw materials | Raw materials | | $ | 430 | | | $ | 37 | | | Raw materials | | $ | 605 | | | $ | 37 | | |
| Work in progress | Work in progress | | 52 | | | 9 | | | Work in progress | | 233 | | | 9 | | |
| Finished goods | Finished goods | | 12 | | | 1 | | | Finished goods | | 127 | | | 1 | | |
| Total inventory | Total inventory | | $ | 494 | | | $ | 47 | | | Total inventory | | $ | 965 | | | $ | 47 | | |
9. Property and Equipment, Net
Property and equipment, net, as of March 31,September 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | March 31, | | December 31, | | September 30, | | December 31, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Laboratory equipment | Laboratory equipment | | $ | 125 | | | $ | 121 | | Laboratory equipment | | $ | 152 | | | $ | 121 | |
| Leasehold improvements | Leasehold improvements | | 184 | | | 180 | | Leasehold improvements | | 231 | | | 180 | |
| Furniture, fixtures and other | Furniture, fixtures and other | | 5 | | | 5 | | Furniture, fixtures and other | | 8 | | | 5 | |
| Computer equipment and software | Computer equipment and software | | 15 | | | 13 | | Computer equipment and software | | 15 | | | 13 | |
| Internally developed software | Internally developed software | | 7 | | | 7 | | Internally developed software | | 9 | | | 7 | |
| Right-of-use asset, financing | | 108 | | | 56 | | |
| Right-of-use asset, financing (Note 11) | | Right-of-use asset, financing (Note 11) | | 546 | | | 56 | |
| Construction in progress | Construction in progress | | 63 | | | 35 | | Construction in progress | | 158 | | | 35 | |
| 507 | | | 417 | | |
| Total | | Total | | 1,119 | | | 417 | |
| Less: Accumulated depreciation | Less: Accumulated depreciation | | (135) | | | (120) | | Less: Accumulated depreciation | | (274) | | | (120) | |
| Property and equipment, net | Property and equipment, net | | $ | 372 | | | $ | 297 | | Property and equipment, net | | $ | 845 | | | $ | 297 | |
Depreciation and amortization expense for the three months ended March 31,September 30, 2021 and 2020 was $15$70 million and $7$8 million, respectively. Depreciation and amortization expense for the nine months ended September 30, 2021 and 2020 was $154 million and $24 million, respectively.
10. Other Balance Sheet Components
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets, as of March 31,September 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | March 31, | | December 31, |
| | 2021 | | 2020 |
| Down payments to manufacturing vendors | | $ | 224 | | | $ | 217 | |
| Other prepaid expenses | | 22 | | | 16 | |
| Tenant incentives receivables | | 10 | | | 10 | |
| Interest receivable on marketable securities | | 8 | | | 9 | |
| Prepaid expenses and other current assets | | $ | 264 | | | $ | 252 | |
| | | | | | | | | | | | | | |
| | September 30, | | December 31, |
| | 2021 | | 2020 |
| Down payments to manufacturing vendors | | $ | 156 | | | $ | 217 | |
| Other prepaid expenses | | 105 | | | 7 | |
| Value added tax receivable | | 57 | | | 7 | |
| Derivative assets | | 51 | | | — | |
| Other current assets | | 43 | | | 21 | |
| Prepaid expenses and other current assets | | $ | 412 | | | $ | 252 | |
Accrued Liabilities
Accrued liabilities, as of March 31,September 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | September 30, | | December 31, |
| | 2021 | | 2020 |
| Clinical trials | | $ | 166 | | | $ | 98 | |
| Raw materials | | 141 | | | 78 | |
| Royalties | | 168 | | | — | |
| Development operations | | 125 | | | 29 | |
| Manufacturing | | 196 | | | 53 | |
| Other external goods and services | | 87 | | | 92 | |
| Compensation-related | | 105 | | | 95 | |
| Other | | 88 | | | 25 | |
| | | | |
| | | | |
| Accrued liabilities | | $ | 1,076 | | | $ | 470 | |
| | | | | | | | | | | | | | |
| | March 31, | | December 31, |
| | 2021 | | 2020 |
| Clinical trials | | $ | 181 | | | $ | 98 | |
| Raw materials | | 185 | | | 78 | |
| Royalties | | 84 | | | 0 | |
| Development operations | | 71 | | | 29 | |
| Manufacturing | | 119 | | | 53 | |
| Other external goods and services | | 58 | | | 92 | |
| Compensation-related | | 33 | | | 95 | |
| Property and equipment | | 17 | | | 18 | |
| Commercial | | 5 | | | 7 | |
| Accrued liabilities | | $ | 753 | | | $ | 470 | |
Other current liabilities, as of September 30, 2021 and December 31, 2020 consists of the following (in millions):
| | | | | | | | | | | | | | |
| | September 30, | | December 31, |
| | 2021 | | 2020 |
| Lease liabilities - financing (Note 11) | | $ | 218 | | | $ | 24 | |
| Lease liabilities - operating (Note 11) | | 22 | | | 6 | |
| | | | |
| Other | | 12 | | | 4 | |
| Other current liabilities | | $ | 252 | | | $ | 34 | |
Deferred Revenue
The following table summarizes the activities in deferred revenue for the the threenine months ended March 31,September 30, 2021 (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | Additions | | Deductions | | March 31, 2021 |
| Product sales | | $ | 3,799 | | | $ | 4,467 | | | $ | (796) | | | $ | 7,470 | |
| Grant revenue | | 5 | | | 2 | | | (2) | | | 5 | |
| Collaboration revenue | | 240 | | | 5 | | | (10) | | | 235 | |
| Total deferred liabilities | | $ | 4,044 | | | $ | 4,474 | | | $ | (808) | | | $ | 7,710 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | Additions | | Deductions | | September 30, 2021 |
| Product sales | | $ | 3,799 | | | $ | 9,615 | | | $ | (5,163) | | | $ | 8,251 | |
| Grant revenue | | 5 | | | 20 | | | (19) | | | 6 | |
| Collaboration revenue | | 240 | | | 23 | | | (45) | | | 218 | |
| Total deferred revenue | | $ | 4,044 | | | $ | 9,658 | | | $ | (5,227) | | | $ | 8,475 | |
11. Leases
We have entered into various long-term non-cancelable lease arrangements for our facilities and equipment expiring at various times through 2032.2035. Certain of these arrangements have free rent periods or escalating rent payment provisions. We recognize lease cost under such arrangements on a straight-line basis over the life of the leases. We have 2 campuses in Massachusetts, our Cambridge facility and our Moderna Technology Center, (MTC), located in Norwood. We also lease other office spaces globally for our business operations.
Operating Leases
Cambridge facility
We occupy a multi-building campus in Technology Square in Cambridge, Massachusetts with a mix of offices and research laboratory space totaling approximately 175,000261,000 square feet. Our Cambridge facility leases have expiry ranges from 20202022 to 2029.
Finance Leases
Moderna Technology Center manufacturing facility (MTC South)
We have an industrial technology center in Norwood, Massachusetts, our Moderna Technology Center (MTC), which comprises 3 buildings, MTC South, MTC North, and MTC East.
In August 2016, we entered into a lease agreement for approximately 200,000 square feet of office, laboratory, and light manufacturing space MTC South, in Norwood, Massachusetts.(MTC South). The lease will expire in September 2032. We have the option to extend the term for 2 extension periods of ten years each at market-based rents. The base rent is subject to increases over the term of the lease.
Moderna Technology Center North (MTC North)
In February 2019, we entered into a lease agreement for office and laboratory space of approximately 200,000 square feet MTC North, located in Norwood, Massachusetts.(MTC North). The lease commenced in the second quarter of 2019 and had an initial expiration date of 2031. We have the option to extend the lease for up to 4 additional five-year terms. In May 2020, we entered into an amendment to the lease whereby we exercised an option available in the original lease to receive a tenant improvement allowance in the amount of $22 million to be paid back over the term of the lease with interest and extend the term of the lease to 2035.
In April 2021, we entered into a lease agreement for a 240,000 square foot building located on the same campus for expansion of our commercial and clinical activities (MTC East). The lease will expire in February 2034. We have the option to extend the term for 2 extension periods of five years each at market-based rents.
Embedded Leases
We have entered into multiple contract manufacturing service agreements with third parties which contain embedded leases within the scope of ASC 842. As of March 31,September 30, 2021 and December 31, 2020, we had lease liabilities of $73$218 million and $24 million, respectively, related to the embedded leases. As of March 31,September 30, 2021 and December 31, 2020, we had right-of-use assets of $44$238 million and 0,zero, as certain embedded leases dedicated to our COVID-19 vaccine program were deemed to have no alternative use prior to the EUA from the FDA in December 2020.
Operating and financing lease right-of-use assets and lease liabilities as of March 31,September 30, 2021 and December 31, 2020 were as follows (in millions):
| | | March 31, | | December 31, | | September 30, | | December 31, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Assets: | Assets: | | | | | Assets: | | | | |
Right-of-use assets, operating, net (1) (2) | Right-of-use assets, operating, net (1) (2) | | $ | 89 | | | $ | 90 | | Right-of-use assets, operating, net (1) (2) | | $ | 115 | | | $ | 90 | |
Right-of-use assets, financing, net (3) (4) | Right-of-use assets, financing, net (3) (4) | | 99 | | | 55 | | Right-of-use assets, financing, net (3) (4) | | 416 | | | 55 | |
| Total | Total | | $ | 188 | | | $ | 145 | | Total | | $ | 531 | | | $ | 145 | |
| | Liabilities: | Liabilities: | | Liabilities: | |
| Current: | Current: | | Current: | |
Operating lease liabilities (5) | Operating lease liabilities (5) | | $ | 7 | | | $ | 6 | | Operating lease liabilities (5) | | $ | 22 | | | $ | 6 | |
Financing lease liabilities (5) | Financing lease liabilities (5) | | 45 | | | 24 | | Financing lease liabilities (5) | | 218 | | | 24 | |
| Total current lease liabilities | Total current lease liabilities | | 52 | | | 30 | | Total current lease liabilities | | 240 | | | 30 | |
| Non-current: | Non-current: | | Non-current: | |
| Operating lease liabilities, non-current | Operating lease liabilities, non-current | | 96 | | | 97 | | Operating lease liabilities, non-current | | 105 | | | 97 | |
| Financing lease liabilities, non-current | Financing lease liabilities, non-current | | 138 | | | 110 | | Financing lease liabilities, non-current | | 238 | | | 110 | |
| Total non-current lease liabilities | Total non-current lease liabilities | | $ | 234 | | | $ | 207 | | Total non-current lease liabilities | | $ | 343 | | | $ | 207 | |
| Total | Total | | $ | 286 | | | $ | 237 | | Total | | $ | 583 | | | $ | 237 | |
_______
(1) These assets are real estate related assets, which include land, office, and laboratory spaces.
(2) Net of accumulated depreciation.
(3) These assets are real estate assets related to the MTC South, MTC North, and MTC SouthEast leases as well as assets related to contract manufacturing service agreements.
(4) Included in property and equipment in the condensed consolidated balance sheets, net of accumulated depreciation.
(5) Included in other current liabilities in the condensed consolidated balance sheets.
Future minimum lease payments under our non-cancelable lease agreements at March 31,September 30, 2021, are as follows (in millions):
| | Fiscal Year | Fiscal Year | Operating Leases (1) | | Financing Leases (1) | Fiscal Year | Operating Leases (1) | | Financing Leases (1) |
| 2021 | 2021 | (remainder of the year) | $ | 12 | | | $ | 46 | | | 2021 | (remainder of the year) | $ | 7 | | | $ | 66 | | |
| 2022 | 2022 | | 16 | | | 48 | | | 2022 | | 31 | | | 173 | | |
| 2023 | 2023 | | 16 | | | 12 | | | 2023 | | 25 | | | 19 | | |
| 2024 | 2024 | | 16 | | | 12 | | | 2024 | | 16 | | | 19 | | |
| 2025 | 2025 | | 17 | | | 13 | | | 2025 | | 17 | | | 19 | | |
| Thereafter | Thereafter | 94 | | | 428 | | | Thereafter | 94 | | | 600 | | |
| Total minimum lease payments | Total minimum lease payments | 171 | | | 559 | | | Total minimum lease payments | 190 | | | 896 | | |
| Less amounts representing interest or imputed interest | Less amounts representing interest or imputed interest | (68) | | | (376) | | (2) | Less amounts representing interest or imputed interest | (63) | | | (440) | | (2) |
| Present value of lease liabilities | Present value of lease liabilities | $ | 103 | | | $ | 183 | | | Present value of lease liabilities | $ | 127 | | | $ | 456 | | |
______
(1) Includes optional extensions in the MTC South, MTC North, and MTC SouthEast lease terms, which represent a total of $339$445 million un-discountedundiscounted future lease payments.
(2) MTC South interest is based on an imputed interest rate of 17.2%. MTC North, MTC East, and the embedded lease interest is based upon incremental borrowing rates of 8.2%, 3.7%, and 0.6%, respectively.
12. Commitments and Contingencies
Strategic Collaborations
Under our strategic collaboration agreements, we are committed to perform certain research, development, and manufacturing activities. As part of our PCVpersonalized cancer vaccine (PCV) Agreement and PCV/SAV Agreement (which also relates to shared neoantigen mRNA cancer vaccine) with Merck, we are committed to perform certain research, development and manufacturing activities related to PCV products through an initial Phase 2 clinical trial up to a budgeted amount of $243 million for both periods as of March 31,September 30, 2021 and December 31, 2020. Please refer to Note 5 for our consolidated financial statements in our 2020 Form 10-K Note 5 to our consolidated financial statements.10-K.
Legal Proceedings
We are not currently a party to any material legal proceedings.
Indemnification Obligations
As permitted under Delaware law, we indemnify our officers, directors, and employees for certain events, occurrences while the officer, or director is, or was, serving at our request in such capacity. The term of the indemnification is for the officer’s or director’s lifetime.
We have standard indemnification arrangements in our leases for laboratory and office space that require us to indemnify the landlord against any liability for injury, loss, accident, or damage from any claims, actions, proceedings, or costs resulting from certain acts, breaches, violations, or non-performance under our leases.
We enter into indemnification provisions under our agreements with counterparties in the ordinary course of business, typically with business partners, contractors, clinical sites and customers. Under these provisions, we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited.
Through the three and nine months ended March 31,September 30, 2021 and the year ended December 31, 2020, we had not experienced any losses related to these indemnification obligations, and no material claims were outstanding. We do not expect significant claims related to these indemnification obligations and, consequently, concluded that the fair value of these obligations is negligible, and no related reserves were established.
Purchase Commitments and Purchase Orders
We enter into agreements in the normal course of business with vendors and contract manufacturing organizations (CMOs) for raw materials and manufacturing services and with vendors for preclinical research studies, clinical trials and other goods or services. As of March 31,September 30, 2021, we had$1.0 $2.3 billion of non-cancelable purchase commitments related to raw materials and manufacturing agreements, including the Lonza agreement, which are expected to be paid through 2022.2024. As of March 31,September 30, 2021, we had $30$69 million o off non-cancelable purchase commitments related to clinical services and other goods and services which are expected to be paid through 2024.2026. These amounts represent our minimum contractual obligations, including termination fees.
In addition to purchase commitments, we have agreements with third parties for various services, including services related to clinical operations and support and contract manufacturing, for which we are not contractually able to terminate for convenience and avoid any and all future obligations to the vendors. Certain agreements provide for termination rights subject to termination fees or wind down costs. Under such agreements, we are contractually obligated to make certain payments to vendors, mainly, to reimburse them for their unrecoverable outlays incurred prior to cancellation. At March 31,September 30, 2021 and December 31, 2020, we had cancelable open purchase orders of $993 million$1.3 billion and $897 million, respectively, in total under such agreements for our significant clinical operations and support and contract manufacturing. These amounts represent only our estimate of those items for which we had a contractual commitment to pay at March 31,September 30, 2021 and December 31, 2020, assuming we would not cancel these agreements. The actual amounts we pay in the future to the vendors under such agreements may differ from the purchase order amounts.
Licenses to Patented Technology
On June 26, 2017, we entered into sublicense agreements with Cellscript, LLC and its affiliate, mRNA RiboTherapeutics, Inc. to sublicense certain patent rights. Pursuant to each agreement, we are required to pay certain license fees, annual maintenance fees, minimum royalties on future net sales and milestone payments contingent on achievement of certain development, regulatory and commercial milestones for specified products, on a product-by-product basis. The development and regulatory milestone payments, up to $2 million for therapeutic and prophylactic products and up to $1 million for diagnostic products will be recognized as a cost of the asset acquired upon resolution of the associated contingency and will be capitalized or expensed depending on the nature of the associated asset as of the date of recognition. Conversely, commercialCommercial milestone payments, up to $24 million, and royalties based on annual net sales of licensed products for therapeutic and prophylactic products will beare accounted for as additional expense of the related product sales in the period in which the corresponding sales occur. WeFor the three and nine months ended September 30, 2021, we recognized $84$168 million and $400 million, respectively, of royalty expenses associated with our product sales, in the first quarter of 2021, which was recorded to cost of sales in our condensed consolidated statements of operations. We did not recognize any such royalties infor the first quarter ofthree and nine months ended September 30, 2020 as we did not have product sales in that quarter.during the period.
Additionally, we have other in-license agreements with third parties which require us to make future development, regulatory and commercial milestone payments for specified products associated with the agreements. The achievement of these milestones was not deemed probable as of March 31,September 30, 2021.
Moderna Science Center
In September 2021, we announced an investment in our Moderna Science Center (MSC), in Cambridge, Massachusetts. MSC will integrate scientific and non-scientific spaces, including our principal executive offices, and is being built to support our growth as we continue to advance our pipeline of mRNA medicines. In relation to the investment, we entered into a lease agreement for approximately 462,000 square feet and will undergo an approximately two-year building project. Following the building project, the lease term is 15 years, subject to our right to extend the lease for up to 2 additional seven-year terms. Pursuant to this lease agreement, we are committed to approximately $1.1 billion non-cancellable rent payments for the initial lease term. Construction at the location has begun, and we expect to begin a phased move-in process in 2023.
13. Stock-Based Compensation
As of March 31,September 30, 2021, we had a total of 6258 million shares reserved for future issuance under our Equity Plans, of which 3531 million shares were reserved for equity awards previously granted, and 27 million shares were available for future grants under the 2018 Equity Plan.Plan.
Options
The following table summarizes our option activity during the threenine months ended March 31,September 30, 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options (in millions) | | Weighted- Average Exercise Price per Share | | Weighted- Average Grant Date Fair Value per Share | | Weighted- Average Remaining Contractual Term | | Aggregate Intrinsic Value (1) (in millions) |
| Outstanding at Outstanding at Outstanding at December 31, 2020 | | 34.06 | | | $ | 17.14 | | | $ | 9.12 | | | 6.7 years | | $ | 2,976 | |
| Granted | | 0.87 | | | 171.07 | | | 75.13 | | | | | |
| Exercised | | (1.89) | | | 14.80 | | | 8.16 | | | | | |
| Canceled/forfeited | | (0.09) | | | 23.92 | | | 11.41 | | | | | |
| Outstanding at March 31, 2021 | | 32.95 | | | 21.32 | | | 10.91 | | | 6.6 years | | 3,649 | |
| Exercisable at March 31, 2021 | | 17.65 | | | 11.61 | | | 6.04 | | | 5.4 years | | 2,107 | |
| Expected to vest at March 31, 2021 | | 15.30 | | | 32.51 | | | 16.52 | | | 8.0 years | | 1,542 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Number of Options (in millions) | | Weighted-
Average
Exercise
Price per
Share | | Weighted-
Average
Grant Date Fair
Value per
Share | | Weighted-
Average
Remaining
Contractual
Term | | Aggregate Intrinsic Value (1) (in millions) |
| Outstanding at December 31, 2020 | | 34.06 | | | $ | 17.14 | | | $ | 9.12 | | | 6.7 years | | $ | 2,976 | |
| Granted | | 1.28 | | | 195.93 | | | 86.02 | | | | | |
| Exercised | | (5.69) | | | 15.77 | | | 8.89 | | | | | |
| Canceled/forfeited | | (0.86) | | | 26.97 | | | 14.51 | | | | | |
| Outstanding at September 30, 2021 | | 28.79 | | | 25.09 | | | 12.43 | | | 6.1 years | | 10,362 | |
| Exercisable at September 30, 2021 | | 16.84 | | | 12.68 | | | 6.56 | | | 5.0 years | | 6,266 | |
| Expected to vest at September 30, 2021 | | 11.95 | | | 42.56 | | | 20.71 | | | 7.6 years | | 4,096 | |
_______
(1)Aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the fair value of common stock for those options in the money as of March 31,September 30, 2021.
The total intrinsic value of options exercised was $250 million$1.2 billion for the threenine months ended March 31,September 30, 2021. The aggregate intrinsic value represents the difference between the exercise price and the selling price received by option holders upon the exercise of stock options during the period. The total consideration recorded as a result of stock option exercises was approximately $28$91 million for the threenine months ended March 31,September 30, 2021.
Restricted Common Stock Units (RSUs) and Performance Stock Units (PSUs)
The following table summarizes our RSU and PSU activity during the threenine months ended March 31,September 30, 2021:
| | | Units
(in millions) | | Weighted-Average
Fair Value
per Unit | | Units
(in millions) | | Weighted-Average Fair Value per Unit |
| Outstanding, non-vested at December 31, 2020 | Outstanding, non-vested at December 31, 2020 | 2.19 | | | $ | 30.85 | | Outstanding, non-vested at December 31, 2020 | 2.19 | | | $ | 30.85 | |
| Issued | Issued | 0.46 | | | 165.19 | | Issued | 0.63 | | | 198.19 | |
| Vested | Vested | (0.24) | | | 24.77 | | Vested | (0.48) | | | 27.41 | |
| Canceled/forfeited | Canceled/forfeited | (0.02) | | | 32.39 | | Canceled/forfeited | (0.10) | | | 43.72 | |
| Outstanding, non-vested at March 31, 2021 | 2.39 | | | 57.28 | | |
| Outstanding, non-vested at September 30, 2021 | | Outstanding, non-vested at September 30, 2021 | 2.24 | | | 78.43 | |
The total fair value of restricted stock units vested during the threenine months ended March 31,September 30, 2021 was $6$13 million. The total intrinsic value of restricted stock units vested during the threenine months ended March 31,September 30, 2021 was $35$108 million.
During the first quarter of 2021, we granted PSUs to certain senior executives with vesting that is contingent upon the achievement of specified preestablished goals over the performance period, generally three years. The actual number of common shares ultimately
issued is calculated by multiplying the number of PSUs by a payout percentage ranging from 0% to 200%. The estimated fair value of PSUs is based on the grant date fair value.
2018 Employee Stock Purchase Plan (ESPP)
There were 0We sold an immaterial number of shares sold under the ESPP during the threenine months ended March 31,September 30, 2021. As of March 31,September 30, 2021, 4 million shares were available for future issuance under the ESPP.
The following table presents the components and classification of stock-based compensation expense for the three and nine months ended March 31,September 30, 2021 and 2020 as follows (in millions):
| | | | | | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | | | | | 2021 | | 2020 | | 2021 | | 2020 | | 2021 | | 2020 |
| Options | Options | | | | | $ | 22 | | | $ | 18 | | Options | | $ | 27 | | | $ | 18 | | | $ | 73 | | | $ | 57 | |
| RSUs and PSUs | RSUs and PSUs | | | | | 7 | | | 2 | | RSUs and PSUs | | 12 | | | 3 | | | 29 | | | 8 | |
| ESPP | ESPP | | | | | 1 | | | 0 | | ESPP | | 1 | | | 1 | | | 3 | | | 2 | |
| Total | Total | | | | | $ | 30 | | | $ | 20 | | Total | | $ | 40 | | | $ | 22 | | | $ | 105 | | | $ | 67 | |
| | Cost of sales | Cost of sales | | | | | $ | 4 | | | $ | 0 | | Cost of sales | | $ | 1 | | | $ | — | | | $ | 13 | | | $ | — | |
| Research and development | Research and development | | | | | 14 | | | 12 | | Research and development | | 25 | | | 13 | | | 54 | | | 40 | |
| Selling, general and administrative | Selling, general and administrative | | | | | 12 | | | 8 | | Selling, general and administrative | | 14 | | | 9 | | | 38 | | | 27 | |
| Total | Total | | | | | $ | 30 | | | $ | 20 | | Total | | $ | 40 | | | $ | 22 | | | $ | 105 | | | $ | 67 | |
As of March 31,September 30, 2021, there was $337$348 million of total unrecognized compensation cost related to unvested stock-based compensation with respect to options, RSUs and PSUs granted. That cost is expected to be recognized over a weighted-average period of 3.13.0 years at March 31,September 30, 2021.
Share Repurchase Program
On August 2, 2021, our Board of Directors authorized a Share Repurchase Program of our common stock, which expires on August 2, 2023. Pursuant to the Share Repurchase Program, we may repurchase up to $1.0 billion of our outstanding common stock. The timing and actual number of shares repurchased depend on a variety of factors, including price, general business and market conditions, and other investment opportunities, and shares may be repurchased through open market purchases through the use of trading plans intended to qualify under Rule 10b5-1 under the Exchange Act.
During the three and nine months ended September 30, 2021, we did not make any repurchases under the Share Repurchase Program.
14. Income Taxes
We are subject to U.S. federal and state and foreign income taxes. For the three and nine months ended March 31,September 30, 2021, and 2020, we recorded provisions for income taxes of $39$219 million and an immaterial amount, respectively.$541 million, respectively, compared to $1 million in each of the same periods in 2020. Our effective tax rate for the three and nine months ended March 31,September 30, 2021 was 6% and 7%, respectively, and was lower than the U.S. statutory rate primarily due to the benefit of the foreign derived intangible income deduction, the benefit related to the release of the valuation allowance on the majority of our tax attributes and other deferred tax assets, the benefit of the foreign derived intangible income deduction, as well as a discrete item for excess tax benefits related to stock-based compensation. Our effective tax rate for the three and nine months ended March 31,September 30, 2020 was lower than the U.S. statutory rate primarily due to the valuation allowance.
We recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities. These differences are measured using the enacted statutory tax rates that are expected to be in effect for the years in which differences are expected to reverse. On a periodic basis, we reassess any valuation allowances that we maintain on our deferred tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. ForIn the three months ended March 31,first quarter of 2021, we reassessed the valuation allowance noting the increase in positive evidence, including significant revenue growth, expectations regarding future profitability, and successful supply chain and manufacturing capabilities to meet global product demand. After assessing both the positive evidence and negative evidence, we determined it was more likely than not that we will realize the majority of our deferred tax assets. Therefore, we determined we should reverse the majority of our valuation allowance through the annual effective tax rate (AETR) with respect to amounts we expect to realize through current year income. In addition, for the nine months ended September 30, 2021, we have recorded a discrete benefit of $49 million related to the release of the valuation allowance on deferred tax assets that we expect to utilize in future years. We will maintain a valuation allowance on certain state tax attributes that we expect will expire prior to the utilization.
In March 2020, the Coronavirus Aid, Relief and Economic Security Act (CARES Act) was signed into law. The CARES Act includes provisions relating to several aspects of corporate income taxes. We do not currently expect the CARES Act to have a significant impact on our provision for income taxes.
We have reviewed the tax positions taken, or to be taken, in our tax returns for all tax years currently open to examination by a taxing authority. Unrecognized tax benefits represent the aggregate tax effect of differences between tax return positions and the benefits recognized in the financial statements. As of March 31, 2021 and December 31, 2020, we had an immaterial amount of gross unrecognized tax benefits, which would affect our tax rate if recognized. We do not expect that our unrecognized tax benefits will materially increase within the next twelve months. We accrue interest and penalties related to unrecognized tax benefits as a component of our provision for income taxes. We did not recognize any material interest or penalties related to uncertain tax positions during the three months ended March 31, 2021 and 2020.
We file U.S. federal income tax returns and income tax returns in various state, local and foreign jurisdictions. We are not currently subject to any tax assessment from an income tax examination in the United States or any other major taxing jurisdiction since inception.
15. Earnings (Loss) per Share
The computation of basic earnings (loss) per share (EPS) is based on the weighted-average number of our common shares outstanding. The computation of diluted EPS is based on the weighted-average number of our common shares outstanding and potential dilutive common shares outstanding during the period as determined by using the treasury stock method.
Basic and diluted EPS for the three and nine months ended March 31,September 30, 2021 and 2020 arewere calculated as follows (in millions, except per share data):
| | | | | | | Three Months Ended March 31, | | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | | | | | 2021 | | 2020 | | | 2021 | | 2020 | | 2021 | | 2020 |
| Numerator: | Numerator: | | | | | | | | | Numerator: | | | | | | | | |
| Net income (loss) | Net income (loss) | | | | | $ | 1,221 | | | $ | (124) | | | Net income (loss) | | $ | 3,333 | | | $ | (233) | | | $ | 7,334 | | | $ | (474) | |
| Denominator: | Denominator: | | | | | | | | | Denominator: | | | | | | | | |
| Basic weighted-average common shares outstanding | Basic weighted-average common shares outstanding | | | | | 400 | | | 353 | | | Basic weighted-average common shares outstanding | | 404 | | | 395 | | | 402 | | | 376 | |
| Effect of dilutive securities | Effect of dilutive securities | | | | | 30 | | | 0 | | | Effect of dilutive securities | | 30 | | | — | | | 29 | | | — | |
| Diluted weighted-average common shares outstanding | Diluted weighted-average common shares outstanding | | | | | 430 | | | 353 | | | Diluted weighted-average common shares outstanding | | 434 | | | 395 | | | 431 | | | 376 | |
| | Basic EPS | Basic EPS | | | | | $ | 3.05 | | | $ | (0.35) | | | Basic EPS | | $ | 8.27 | | | $ | (0.59) | | | $ | 18.25 | | | $ | (1.26) | |
| Diluted EPS | Diluted EPS | | | | | $ | 2.84 | | | $ | (0.35) | | | Diluted EPS | | $ | 7.70 | | | $ | (0.59) | | | $ | 17.00 | | | $ | (1.26) | |
The following common stock equivalents, presented based on amounts outstanding as of March 31,September 30, 2020 were excluded from the calculation of diluted net loss per share attributable to common stockholders for the periods indicated because their inclusion would have been anti-dilutive (in millions):
| | | | | | | | | |
| | | March 31,September 30, |
| | | 2020 |
| Stock options | | | 4537 | |
| | | |
| Restricted common stock units | | | 2 | |
| Total | | | 4739 | |
For the three and nine months ended September 30, 2021, we had an immaterial number of securities that were not included in the diluted earnings per share calculation because the effect would have been anti-dilutive.
16. Subsequent Events
Subsequent to March 31,September 30, 2021, we have entered into additional commitments and supply agreements with customers to provide up to 4758 million doses of our COVID-19 vaccine and our updated variant booster vaccine candidate based on the initial confirmed volume, subject to modifications.
Subsequent to March 31, 2021, we have entered into additional binding purchase commitments with third-party contractual manufacturing organizations for our COVID-19 vaccine under existing or newly executed agreements. We are currently committed to minimum non-cancelable purchase obligations of $284 million related to these agreements, which are expected to be paid through 2022.
Additionally, on April 18, 2021, we entered into a further amendment to our existing agreement with BARDA, pursuant to which BARDA has agreed to fund the advancement of mRNA-1273 to potential licensure, including funding for clinical studies and manufacturing scale-up. The amendment increases the amount of potential reimbursements by $236 million in connection with costs associated with the Phase 3 clinical trials for mRNA-1273 and pharmacovigilance efforts, bringing the total BARDA support to approximately $1.3 billion.
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our unaudited financial information and related notes included in this Form 10-Q and our consolidated financial statements and related notes and other financial information in our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the SEC on February 26, 2021 (the “2020 Form 10-K”). Some of the information contained in this discussion and analysis or set forth elsewhere in this Form 10-Q, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in Part II, Item 1A - Risk Factors in this Form 10-Q, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines to improve the lives of patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane, or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases. Our platform builds on continuous advances in basic and applied mRNA science, delivery technology, and manufacturing, providing us the capability to pursue in parallel a robust pipeline of new development candidates. We are developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, autoimmune diseases and cardiovascular diseases, independently and with our strategic collaborators.
Within our platform, we develop technologies that enable the development of mRNA medicines for diverse applications. When we identify technologies that we believe could enable a new group of potential mRNA medicines with shared product features, we call that group a ““modality.”modality.” While the programs within a modality may target diverse diseases, they share similar mRNA technologies, delivery technologies, and manufacturing processes to achieve shared product features. The programs within a modality will also generally share similar pharmacology profiles, including the desired dose response, the expected dosing regimen, the target tissue for protein expression, safety and tolerability goals, and pharmaceutical properties. Programs within a modality often have correlated technology risk, but, because they pursue diverse diseases, they often have uncorrelated biology risk. We have created sixseven modalities to date:
•prophylactic vaccines;
•systemic secreted and cell surface therapeutics;
•cancer vaccines;
•intratumoral immuno-oncology;
•localized regenerative therapeutics; and
•systemic intracellular therapeutics; and
•inhaled pulmonary therapeutics.
We have designated our prophylactic vaccines and systemic secreted and cell surface therapeutics modalities as our “core modalities”.modalities.” In these core modalities, our strategy is to invest in additional development candidates using our accumulated innovations in technology, our process insights and our preclinical and clinical experience. Our exploratory modalities continue to be a critical part of advancing our strategy to maximize the application of our potential mRNA medicines.
Business Highlights
Moderna COVID-19 Vaccine
On December 18, 2020, we received an Emergency Use Authorization (EUA) from the U.S. FDA authorizedFood and Drug Administration (FDA) for the emergency use of the Moderna COVID-19 Vaccine (also referred to as mRNA-1273 and marketed under the brand name Spikevax) in individuals 18 years of age or older. We have also received authorization for our COVID-19 vaccine from health agencies in Canada, Israel, the European Union, the United Kingdom, Switzerland, Singapore, Qatar, Taiwan, and the Philippines,more than 50 countries and from the World Health Organization.Organization (WHO). Additional authorizations are currently under review in other countriescountries. In addition, we have received authorization for our COVID-19 vaccine for use in adolescents in the United Kingdom, European Union, Japan, Canada, Switzerland, Taiwan, Saudi Arabia, Australia and the Philippines, and have pending applications for authorization to administer the vaccine to adolescents with regulatory agencies in the United States and other countries.
The FDA has approved an update to the EUA for the Moderna COVID-19 Vaccine to include a third dose for immunocompromised individuals 18 years of age or older in the United States, as well as the administration of 50 µg booster doses for individuals age 65 and older, people aged 18 to 64 who are at high risk of severe COVID-19, and people aged 18 to 64 with frequent institutional or occupational exposure to SARS-CoV-2. In October 2021, the U.S. Advisory Committee on Immunization Practices (ACIP) also endorsed recommending the Moderna COVID-19 Vaccine as a booster, regardless of the original vaccine received by an individual in their primary series. The European Medicines Agency (EMA) has also authorized a third dose of the World Health Organization. We planModerna COVID-19 vaccine given at least 28 days after the second dose to initiateseverely immunocompromised individuals 12 years of age or older, as well as the administration of 50 µg booster doses for individuals 18 years of age and older. In August 2021, we completed the rolling submission of data toprocess with the U.S. FDA for a Biologics License Application (BLA) for our COVID-19 vaccine, which is subject to Priority Review.
On October 29, 2021, the FDA informed us that the agency will require additional time to evaluate recently conducted international analyses of the risk of myocarditis (inflammation of the heart muscle) after vaccination in May 2021.connection with our requested EUA to administer the Moderna COVID-19 Vaccine in adolescents (12-17 years of age). The FDA notified us that this review may not be completed before January 2022. An increased risk of myocarditis has been described for mRNA COVID-19 vaccines, including the Moderna COVID-19 vaccine, particularly in young men and following the second dose. The U.S. Centers for Disease Control (CDC) and Prevention and the WHO have stated that myocarditis following vaccination with mRNA vaccines has been rare and generally mild. We are committed to conducting our own careful review of new external analyses as they become available. We do not yet have access to data from some recent international analyses. We currently expect that we will also delay filing a request for EUA of our COVID-19 vaccine at the 50 µg dose level in the pediatric population (6-11 years of age) while the FDA completes its review of the adolescent EUA request.
We have entered into supply agreements with the U.S. Government, and several other governments outside the United States and with UNICEF (on behalf of the COVAX Facility) and the African Union for the supply of our COVID-19 vaccine. The agreements are generally subject to receipt of authorization or approval for the use and distribution of the vaccine from the relevant regulatory authority in each jurisdiction. Under these agreements, we are entitled to upfront deposits for our COVID-19 vaccine supply, initially recorded as deferred revenue. As of March 31,September 30, 2021, we had approximately $7.5$8.3 billion in deferred revenue in connection with the supply agreements with the U.S. Government and other governments,customers, which will be recognized as revenue when revenue recognition criteria have been met.
For the firstthird quarter of 2021, we delivered approximately 8873 million doses of our COVID-19 vaccine to the U.S. governmentGovernment and approximately 14136 million doses to other governments, and recognized $1.7$4.8 billion in product sales. For the nine months ended September 30, 2021, we delivered approximately 287 million doses of our COVID-19 vaccine to the U.S. Government and approximately 222 million doses to other governments, and recognized $10.7 billion in product sales.
In the fourthsecond quarter of 2020, we delivered approximately 17 million doses of our COVID-19 vaccine to the U.S. government. Through April 12, 2021, we cumulatively delivered approximately 132 million doses globally, including approximately 117 million doses to the U.S. government and approximately 15 million doses delivered to other governments from our ex-U.S. supply chain. We remain on track to deliver the second 100 million doses to the U.S. government by end of May 2021 followed by another 100 million additional doses by end of July 2021.
On April 29, 2021, we announced additional investments to facilitate the increased supply of our COVID-19 vaccine from our own and partnered manufacturing facilities. We anticipate these investments will increasefacilities, and an expansion of the Moderna Technology Center (MTC) in Norwood, Massachusetts, to more than double our global 2022 capacity for the vaccinefacility space to uphelp transform it from a production and lab space to 3 billion doses, depending upon the mix between the COVID-19 vaccine at the current 100 µg dose level and potentially lower doses of our variant booster candidates and pediatric vaccines, which are subject to further regulatory approval and authorization.an industrial technology center. These investments are expected to facilitate a doubling of drug substance manufacturing from Lonza’s Switzerland-based facility, a more than doubling of formulation, fill and fill/finish and drug substance manufacturing at Rovi’s Spain-based facility, as well as a 50% increase of drug substance at Moderna’s facilities in the U.S. When completed, the investments are expected to also result in an increase in safety stock of raw materials and finished product used to deliver committed volumes. These forecasted increases to our supply are subject in part to performance by our manufacturing partners, which will require ramping-up capabilities at their own facilities and the hiring of qualified manufacturing personnel. On April 29,
We currently anticipate that we will be supply between 700 million and 800 million doses of our COVID-19 vaccine in 2021, at the 100 µg dose.Key variables impacting output include longer delivery lead times for international shipments and exports that may shift deliveries to early 2022, temporary impact from expansion of fill/finish capacity and ramp up of product release to market. The ultimate number of doses that we also announced an increaseanticipate delivering in our projected supply2022 is subject to a number of factors, including demand for the vaccine as the COVID-19 pandemic shifts to an anticipated endemic phase, demand for presentations of our vaccine, the dosage for 2021 to between 800 millionthe vaccine (including different dose amounts for primary series, booster series and 1 billion doses.pediatric vaccines), and other factors.
Moderna COVID-19 Vaccine Clinical Studies
An updated reviewThe final analysis of adjudicated cases has identified over 900 cases of COVID-19 infrom the Phase 3 clinical trial for mRNA-1273, which we refer to as the COVE study, asStudy, demonstrated efficacy of April 9, 2021, including over 100 cases of severe COVID-19, as defined in the protocol, with a median follow-up of approximately 693% through six months after the second dose. Vaccine efficacy starting two weeks followingdose of the second dose and based on the updated adjudicated cases remains consistent with prior updates, includingvaccine. The final analysis also demonstrated greater than 90% efficacy against all cases of COVID-19, and greater than 95%98% efficacy against severe cases of COVID-19.COVID-19 and 100% efficacy against death caused by COVID-19 in the per protocol cohort. The COVE study is ongoingfinal analysis also demonstrated consistency in our subgroup analysis, including analyses by gender, by race and reported results remain preliminary.by preexisting medical conditions. The safety profile for mRNA-1273 continues to be consistent with the Phase 3 data over the longer period of safety follow up and across population subgroups.
The Phase 2/3 TeenCOVE study of mRNA-1273 in adolescents ages 12-17 years has completed enrollment in the U.S.United States. An initial analysis of 3,2353,732 participants randomized 2:1 in the TeenCOVE study showed a vaccine efficacy rate of 96%93% in seronegative participants who received at least one injection. The analysis included 12 cases starting 14 days after the first dose and based on the CDC definition of COVID-19. Because the incidence rate of COVID-19 is lowerinjection (modified intent-to-treat cohort) in adolescents, the case definition is less stringent than for the Phase 3 COVE study, resulting in vaccine efficacy against milder disease.a secondary analysis. The median duration for follow-up in this initial analysis was 3553 days following the second dose. mRNA-1273 was generally well tolerated. The majority of adverse events were mild or moderate in severity. No serious safety concerns have been identified to date. The most common solicited local adverse event was injection site pain. The most common solicited systemic adverse events after the second dose of mRNA-1273 were headache, fatigue, myalgia and chills. We are continuing to collect datahave received authorization for our COVID-19 vaccine for use in adolescents in the TeenCOVE studyUnited Kingdom, European Union, Japan, Canada, Switzerland, Taiwan, Saudi Arabia, Australia and arethe Philippines. We have filed for an EUA for adolescents with the FDA and a determination is pending, as described in discussions with regulators about a potential amendment to our regulatory filings.further detail above.
The Phase 2/3 KidCOVE study of mRNA-1273 in the pediatric population ages 6 months-11months to 11 years is currently enrolling.ongoing. We expect to enroll 6,750more than 12,000 healthy pediatric participants in the U.S. and Canada into this two-part, dose escalation study. In Part 1, each participant agesData from the KidCOVE study two weeks after the first dose of mRNA-1273 at the 50 µg dose level showed a vaccine efficacy of 100%, using the Phase 3 COVE study primary case definition for COVID-19. Additionally, for asymptomatic infection two weeks after the first dose, vaccine efficacy was 65% (95% CI: .16, .85). For SARS-CoV-2 infection regardless of symptoms, vaccine efficacy was 80% (95% CI: .62, .90) two weeks after the first dose. On October 24, the Company announced positive top line top line data from the Phase 2/3 study of mRNA-1273 in children 6 to 11 years of age. GMR comparing the response in children to the response in young adults from the Phase 3 COVE study was 1.5 (95% CI: 1.3, 1.8), with a seroresponse rate of 99.3%. Two 50 μg doses of mRNA-1273 were generally well tolerated. The Company plans to submit results to the U.S. FDA, EMA and regulatory agencies around the world. The age groups are 6 years to <12 years, 2 years to less than 12<6 years may receiveand 6 months to <2 years. In part 1, each age group tests one of two dose levels (50 µg or 100 µg). Also in Part 1, each participant ages six months to less than 2 years may receive one of three dose levels (25 µg, 50 µg and 100 µg). Anlevels. Following an interim analysis, will be conducted to determine whicha dose will be used in Partselected for part 2, thea placebo-controlled expansion portion of the study.study, which is expected to enroll 4,000 participants. We are in part 1 of the study for the two other age groups, 2 years to <6 years and 6 months to <2 years old. Dose selection studies are underway for the 2 to <6 years and 6 months to <2 years age groups.
The Phase 1 study of mRNA-1283 is fully enrolled and ongoing.An interim analysis of data from the Phase 1 study of mRNA-1283 at three dose levels indicates that a lower dose of mRNA-1283 achieved similar neutralizing antibody responses compared to a primary series of mRNA-1273. mRNA-1283 had an acceptable tolerability profile. The Company is preparing to begin a Phase 2 booster study of mRNA-1283.mRNA-1283 is a next-generation vaccine candidate against COVID-19 that encodes for the portions of the SARS-CoV-2 spike protein critical for neutralization, specifically the Receptor Binding Domain (RBD) and N-terminal Domain (NTD). The encoded mRNA-1283 antigen is shorter than mRNA-1273 and is being developed as a potential refrigerator-stable mRNA vaccine that will facilitate easier distribution and administration by healthcare providers.
Variant-Specific Booster Candidates
OnIn February 24,2021, we announced that we had completed manufacturing of clinical trial material for our variant-specific vaccine candidate, mRNA-1273.351, against the SARS-CoV-2 variant known as the Beta variant (or B.1.351, (firstfirst identified in the Republic of South Africa) and that this vaccine had been shipped to the National Institutes of Health (NIH) for a Phase 1 clinical trial to be led and funded by the NIH’s National Institute of Allergy and Infectious Diseases. We are also developing a multivalent booster candidate, mRNA-1273.211, which combines mRNA-1273 (Moderna’s authorized vaccine against ancestral strains) and mRNA-1273.351the Beta variant in a single vaccine.
Initial data
Data from our Phase 2 study showed that a single 50 µg dose of mRNA-1273, mRNA-1273.351 or mRNA-1273.351mRNA-1273.211 given as a booster to previously vaccinated individuals (n=20 per group) increased neutralizing antibody titer responses against SARS-CoV-2 B.1.351 and P.1 (another variantimportant variants of concern, including the Gamma variant (or P.1, first identified in Brazil), the Beta variant, and the Delta variant (B.1.617.2). A booster doseNeutralizing antibody levels following the boost approached those observed after primary vaccination with two doses of mRNA-1273.351, the Company’s strain-matched booster, achieved higher neutralizing antibody titers against the B.1.351 variant of concern than a booster dose100 µg of mRNA-1273. These data have been published in Nature Medicine. Safety and tolerability profiles following third dose booster injections of 50 µg of mRNA-1273, mRNA-1273.351 or mRNA-1273.351mRNA-1273.211 were generally comparable to those observed after the second dose of mRNA-1273 in the previously reported Phase 2 and Phase 3 studies. Our Phase 2 study to evaluate three approaches to boosting is ongoing. We are also in the process of developing a booster tailored to the Delta variant (mRNA-1273.617), and anticipate developing a multivalent booster (referred to as mRNA-1273.213) that combines mRNA-1273.617 with another COVID-19 candidate.
In preclinical studies,Other Business Updates
On November 1, 2021, we announced that, as part of our commitment to sustainability, we will work to achieve net-zero carbon emissions from our operations globally by 2030. These efforts to reduce our environmental impact are expected to include implementing key initiatives to reduce the carbon footprint from both mRNA-1273.351our existing and mRNA-1273.211 demonstrated increased neutralizing titers against SARS-CoV-2 variants of concern future facilities, encouraging green transportation and working with our suppliers as they move toward their own carbon reduction goals.
On November 2, 2021, we announced that we have entered into a strategic research and development collaboration with Metagenomi, Inc., focused on advancing novel gene editing technologies for in mice. Specifically, this preclinical data confirms improved neutralizing titersvivo human therapeutic applications. The collaboration will utilize Metagenomi’s next generation gene editing tools and leverage Moderna’s mRNA platform, as well as LNP delivery technologies, with the mRNA-1273.351 vaccine primary series. The multi-valent vaccine provided the broadest levelgoal of immunity in mice. A boost at 6 monthsdeveloping curative therapies for patients with mRNA-1273.351 closed the neutralizing titer gap for the variants of concern. Following the mRNA-1273.351 boost, neutralizing titers were comparable between the ancestral strain and the new B.1.351 variant.serious genetic diseases.
Key Updates for our Other Development Candidates
•CMVCytomegalovirus (CMV) vaccine (mRNA-1647): Our vaccine against cytomegalovirus, or CMV (mRNA-1647) is a vaccine combining six mRNAs in a single vial, which encode for two antigens located on the surface of CMV: five mRNAs encoding the subunits that form the membrane-bound pentamer complex and one mRNA encoding the full-length membrane-bound glycoprotein B (gB). Both the pentamer and gB are essential for CMV to infect barrier epithelial surfaces and gain access to the body. mRNA-1647 is designed to produce an immune response against both the pentamer and gB for the prevention of CMV infection.
In April 2021, we announced seven-month data from the Phase 2 study of mRNA-1647 at the 50 μg, 100 μg and 150 μg dose levels. mRNA-1647 was generally well tolerated. The most common solicited local adverse reaction (AR) was injection site pain and the most common solicited systemic ARs were headache, fatigue, myalgia, arthralgia and chills. Rates of Grade 3 solicited ARs after the third vaccination were similar to, or lower than the rates of Grade 3 solicited ARs after the second vaccination. In CMV-seronegative participants in mRNA-1647 treatment groups after the third vaccination, neutralizing antibody geometric mean titers (GMTs) against epithelial cell infection were at least 20-fold higher than the baseline GMT of the CMV-seropositive group, and neutralizing antibody GMTs against fibroblast infection approximated the baseline GMT of the CMV-seropositive group. In CMV positive participants in mRNA-1647 treatment groups after the third vaccination, neutralizing antibody GMTs against epithelial cell infection increased to at least 6.8-fold over baseline, and neutralizing antibody GMTs against fibroblast infection increased to approximately 2-fold over baseline.
Based on the interim analysis of the Phase 2 study, which we announced in April 2021, the 100 μg dose has been chosen for the Phase 3 pivotal study for mRNA-1647, known as the CMVictory study, which will evaluate the prevention of primary CMV infection in seronegative women ages 16-40 years. We plan to enroll approximatelyup to a total of 8,000 participants, including 6,900 women of child-bearing age from approximately 150 sites across the U.S., Europe and Asia-Pacific into the Phase 3 study, which is expected to beginand the first participant was dosed in the Phase 3 study on October 26, 2021. Moderna owns worldwide commercial rights for mRNA-1647.
•Pediatric respiratoryRespiratory syncytial virus (RSV) vaccine (mRNA-1345): mRNA-1345 is a vaccine against RSV encoding for a prefusion F glycoprotein, which elicits a superior neutralizing antibody response compared to the postfusion state. The Phase 1 study of mRNA-1345 to evaluate the tolerability, reactogenicity and reactogenicityimmunogenicity of mRNA-1345 in younger adults, older adults, women of child-bearing age and children is ongoing. All four cohorts of younger adultsadult (ages 18-49 years) and older adult (ages 65-79 years) cohorts are fully enrolled. Dosing in the older adult cohort (ages 65-79 years) is ongoing. Phase 1 interim data from the older adult cohort showed that a single mRNA-1345 vaccination at 50 µg, 100 µg or 200 µg boosted neutralizing antibody titers against RSV-A by approximately 14-fold and against RSV-B by approximately 10-fold. We are preparing for a global Phase 2/3 study with approximately 34,000 participants, which will test the 50 µg dose and we expect the trial to begin by the end of 2021. The age range of toddlers in this de-escalation Phase 1 study is 12-59 months.
In April 2021, we announced the first interim analysis of the Phase 1 study of mRNA-1345, through 1-month post-vaccination, of the younger adult cohorts. A single mRNA-1345 vaccination of 50 μg (N=19) or 100 μg (N=20) was generally well-tolerated in younger adults (ages 18-49 years). Ten participants received placebo. The most common local solicited adverse reaction was injection site pain,months and the most common systemic solicited adverse reactions were headache, fatigue and myalgia.pediatric cohort is ongoing, as are the cohorts of women of child-bearing potential. The majorityFDA has granted Fast Track designation for mRNA-1345 in adults older than 60 years of solicited adverse reactions occurred within 1-3 days after vaccination and resolved after 1-4 days. There were no deaths, no severe adverse events, no study discontinuations due to adverse events, and no adverse events that led to a study pause. mRNA-1345 was shown to increase RSV neutralizing antibodies in seropositive younger adults. Neutralizing antibodies were confirmed to be present at baseline in all participants, as expected. A single vaccination of mRNA-1345 at the 50 or 100 μg dose level boosted neutralizing antibody titers against both serotypes of RSV-A and RSV-B with no apparent dose response. At month 1, the geometric mean fold rise in neutralizing antibody relative to baseline was at least 20.5 for RSV-A and at least 11.7 for RSV-B. At the 100 μg dose level, the geometric mean fold rise (95% CI) in RSV-A neutralizing antibody titer at month 1 relative to baseline was 2.7 (2.1, 3.4) with mRNA-1777, our previous RSV vaccine candidate, compared to 21.0 (13.9, 31.8) with mRNA-1345. Both mRNA-1777 and mRNA-1345 encode for the prefusion RSV-F. We also intend to evaluate the potential of combinations of mRNA-1345 with our vaccines against other respiratory pathogens in children and separately in older adults.age. There is no approved vaccine for RSV. Moderna owns worldwide commercial rights to mRNA-1345.
•Seasonal influenza (flu) (mRNA-1010, mRNA-10202 and mRNA-1030): In January 2021, we announced our plans to test three development candidates for a seasonal influenza vaccine (mRNA-1010, mRNA-1020 and mRNA-1030), which will allow us to identify a candidate to take forward into pivotal efficacy studies. The vaccine will be administered as a single dose and will aim to elicit protection from all seasonal influenza viruses. Our first-generation. In July 2021, the first participants were dosed in the Phase 1/2 study of mRNA-1010, our first generation flu program, will evaluate multiple candidates comprising multiple antigen combinations againstand the four seasonal viruses recommended byPhase 1 portion of the World Health Organization. InPhase1/2 study is fully enrolled. Preparation for the future we also plan to evaluatePhase 2 portion of the combination ofstudy are ongoing. mRNA-1010 is a quadrivalent seasonal influenza vaccine with vaccines against othercandidate targeting WHO recommendations including A H1N1, H3N2 and influenza B Yamagata and Victoria lineages. Our vision is to develop a respiratory viruses with similar epidemiology. We also planvaccine for the adult and elderly populations combining seasonal flu, a COVID-19 variant booster and RSV. Moderna owns worldwide commercial rights to explore potential combination vaccines against flu, SARS-CoV-2, RSV and humanmRNA-1010.
•Human metapneumovirus (hMPV). and parainfluenza type 3 (PIV3) vaccine (mRNA-1653): We expect to begin aare enrollingseropositive pediatric participants (12-36 months of age) in the Phase 1 clinical trial for the seasonal influenza programstudy of hMPV/PIV3 study (mRNA-1653). The first cohort in 2021.this study is fully enrolled. Moderna owns worldwide commercial rights to mRNA-1653.
•Epstein-Barr virus (EBV)Pediatric RSV and hMPV combination vaccine (mRNA-1189)(mRNA-1365): mRNA-1189mRNA-1365 is a vaccine against EBV containing five mRNAsRSV encoding for the prefusion F glycoprotein and the hMPV F protein. Moderna owns worldwide commercial rights to mRNA-1365.
•Zika vaccine (mRNA-1893): mRNA-1893 is a vaccine against ZIKV. After administration of the vaccine, the mRNA is translated as a polyprotein and processed inside the cell to make a virus-like particle (VLP). This process mimics the response of the cell after natural infection. This program is funded by BARDA. In 2020, we announced positive Phase 1 data showing that encode viral proteins (gp350, gB, gp42, gHmRNA-1893 was well-tolerated at all dose levels, and gL) in EBV. Similarsafety and tolerability did not appear to our CMV vaccine (mRNA-1647), the viral proteins in mRNA-1189 are expressed in their native membrane-bound form for recognitionbe influenced by the immune system.serostatus of the participants at baseline. All dose levels of mRNA-1893 induced a strong neutralizing ZIKV-specific antibody response in baseline flavivirus seronegative participants. Geometric mean titers (GMTs) post-dose two were comparable to those in a small panel of Zika convalescent sera collected during the epidemic. In participants with pre-existing flavivirus antibodies, neutralizing antibody titers were boosted with a single dose of the vaccine as shown by the GMTs and the seroconversion rates. We are planning to beginhave started a Phase 12 study of mRNA-1189that is expected to enroll approximately 800 participants in 2021.the United States and Puerto Rico. There is no approved vaccine for EBV. Moderna owns worldwide commercial rightsZika.
•Human immunodeficiency virus (HIV) (mRNA-1644 and mRNA-1574): In January 2021, we announced two vaccine programs against HIV. mRNA-1644 is a novel approach to mRNA-1189.HIV vaccine strategy in humans designed to elicit broadly neutralizing HIV-1 antibodies (bNAbs) and is being developed in collaboration with the International AIDS Vaccine Initiative (IAVI) and the Gates Foundation. A Phase 1 study for mRNA-1644 will use iterative human testing to validate the approach and antigens and multiple novel antigens will be used for germline-targeting and immuno-focusing. A second approach, mRNA-1574, is being evaluated in collaboration with the National Institutes of Health (NIH) and includes multiple native-like trimer antigens.
•Propionic acidemia (PA) (mRNA-3927):The first participant has been dosed in Enrollment for the Phase 11/2 clinical trial for mRNA-3927, our therapy for the treatment of propionic acidemia, or PA.PA, is ongoing and the first cohort is fully enrolled. The Phase 11/2 study is designed to evaluate the safety and tolerability of mRN-3927mRNA-3927 in patients with PA. PA, is a rare, life-threatening, inherited metabolic disorder due to a defect in the mitochondrial enzyme propionyl-CoA carboxylase or PCC.(PCC). It primarily affects the pediatric population. There is no approved therapy for PA, including no approved enzyme replacement therapy. We have received Rare Pediatric Disease Designation and Orphan Drug Designation from the FDA and Orphan Drug Designation from the European Commission for the PA program. The FDA has also granted Fast Track designation to mRNA-3927. We implemented a novel design for the Phase 1/2 study in 2020 to identify the optimal dose in the most efficient manner and to make the study less burdensome on patients, their families and clinical partners. This is the first development candidate to enter the clinic in our intracellular therapeutics modality.
•hMPV/PIV3 vaccine (mRNA-1653)Methylmalonic acidemia (MMA) (mRNA-3705): Enrollment for the Phase 1/2 clinical trial for mRNA-3705, our therapy for the treatment of methylmalonic acidemia, or MMA, is ongoing and the first patient has been dosed. The first 10 subjectsPhase 1/2 study is designed to evaluate the safety and tolerability of mRNA-3705 in patients with MMA. MMA is a rare, life-threatening, inherited metabolic disorder that is primarily caused by a defect in the mitochondrial enzyme methylmalonyl-coenzyme A mutase, or MUT. It primarily affects the pediatric population. There is no approved therapy that addresses the underlying disorder, including no approved enzyme replacement therapy, due to the complexity of the protein and its mitochondrial localization.
•Glycogen storage disease type 1a (GSD1a) (mRNA-3745): The FDA has granted mRNA-3745 Orphan Drug Designation and completed its review of the IND application allowing it to proceed to clinic. Individuals with GSD1a have a deficiency in glucose-6-phosphatase resulting in pathological blood glucose imbalance.mRNA-3745 is an IV-administered mRNA encoding human G6Pase enzyme, designed to restore the deficient or defective intracellular enzyme activity in patients with GSD1a. Moderna owns worldwide commercial rights to mRNA-3745.
•Crigler-Najjar Syndrome Type 1 (CN-1)(mRNA-3351): mRNA-3351 encodes for the human UGT1A1 and is designed to restore the missing or dysfunctional proteins that causes Crigler-Najjar Syndrome Type 1. mRNA-3351 has been granted Rare Pediatric Disease designation by the FDA. We are providing investigational mRNA-3351 to the nonprofit Institute for Life Changing Medicines (ILCM) free of charge. ILCM will be responsible for the clinical development of mRNA-3351 and plans to initiate clinical studies of mRNA-3351 in 2022.
•IL-2 Mutein (mRNA-6231): mRNA-6231 is an mRNA-encoded IL-2 modified for the expansion of regulatory T cells. A Phase 1b age de-escalation clinical1 study in healthy volunteers has dosed its first participants. It is also our first subcutaneously administered therapeutic program.
•Intratumoral Immuno-Oncology: The Phase 1 trial of mRNA-1653 were enrolled and dosed prior to a COVID-19 related pause. The first two cohorts in the Phase 1b trial are fully enrolledevaluating Triplet (mRNA-2752), which includes OX40L and two furtherproinflammatory cytokines, IL-23, and IL-36γ, encapsulated in our proprietary LNP, is ongoing. New expansion cohorts are also enrolling.
•Human immunodeficiency virus (HIV) (mRNA-1644Personalized cancer vaccine (mRNA-4157): Our personalized cancer vaccine, or PCV, is currently being evaluated in a Phase 1 and mRNA-1574)Phase 2 study. The randomized, placebo-controlled Phase 2 study investigating a 1 mg dose of mRNA-4157 in combination with Merck’s pembrolizumab (KEYTRUDA®), compared to pembrolizumab alone, for the adjuvant treatment of high-risk resected melanoma is fully enrolled (n=150). The primary endpoint of the Phase 2 study is recurrence-free survival at 12 months. The Phase 1 in multiple cohorts is ongoing and the expanded head and neck cohort is recruiting additional patients. Moderna shares worldwide commercial rights to mRNA-4157 with Merck.
•Cystic Fibrosis (CF) (VXc-522): In January 2021,VXc-522 is an mRNA therapeutic that we announced two vaccine programs against HIV. mRNA-1644 is a novel approach to HIV vaccine strategy in humans designed to elicit broadly neutralizing HIV-1 antibodies (bNAbs) and is being developedare designing in collaboration with Vertex Pharmaceuticals. VXc-522 is designed to treat the International AIDS Vaccine Initiative (IAVI)underlying cause of CF by enabling cells in the lungs to produce functional cystic fibrosis transmembrane conductance regulator (CFTR) protein for the treatment of the 10% of patients who do not produce any CFTR protein. IND-enabling studies are underway and the Bill and Melinda Gates Foundation (BMGF). A Phase 1 studyVertex expects to submit an IND for mRNA-1644 will use iterative human testing to validate the approach and antigens and multiple novel antigens will be used for germline-targeting and immuno-focusing. A second approach, mRNA-1574,this program in 2022. VXc-522 is being evaluated in collaboration with the National Institutes of Health (NIH) and includes multiple native-like trimer antigens. We currently expect to begin phase 1 clinical trials for both mRNA-1644 and mRNA-1574 in 2021.advanced by Vertex.
•Nipah virus (mRNA-1215): In January 2021, we announced a program to develop a vaccine against the Nipah virus, a zoonotic virus transmitted to humans from animals, contaminated food, or through direct human-to-human transmission and causes a range of illnesses including fatal encephalitis. The vaccine is being co-developed with the NIH’s Vaccine Research Center. We intend to initiate a Phase 1 clinical trial of mRNA-1215 in 2021, and anticipate the study will be sponsored by the NIH.
Our Pipeline
The following chart shows our current pipeline of 2437 development programs, grouped into modalities—first our two core modalities where we believe we have reduced the technology risk, followed by our four exploratory modalities in which we are continuing to investigate the clinical use of mRNA medicines. In the third quarter of 2021, we refined the way we track our development programs and now separately track each indication of our COVID-19 and RSV vaccine candidates, which resulted in an increase in the number of our development.
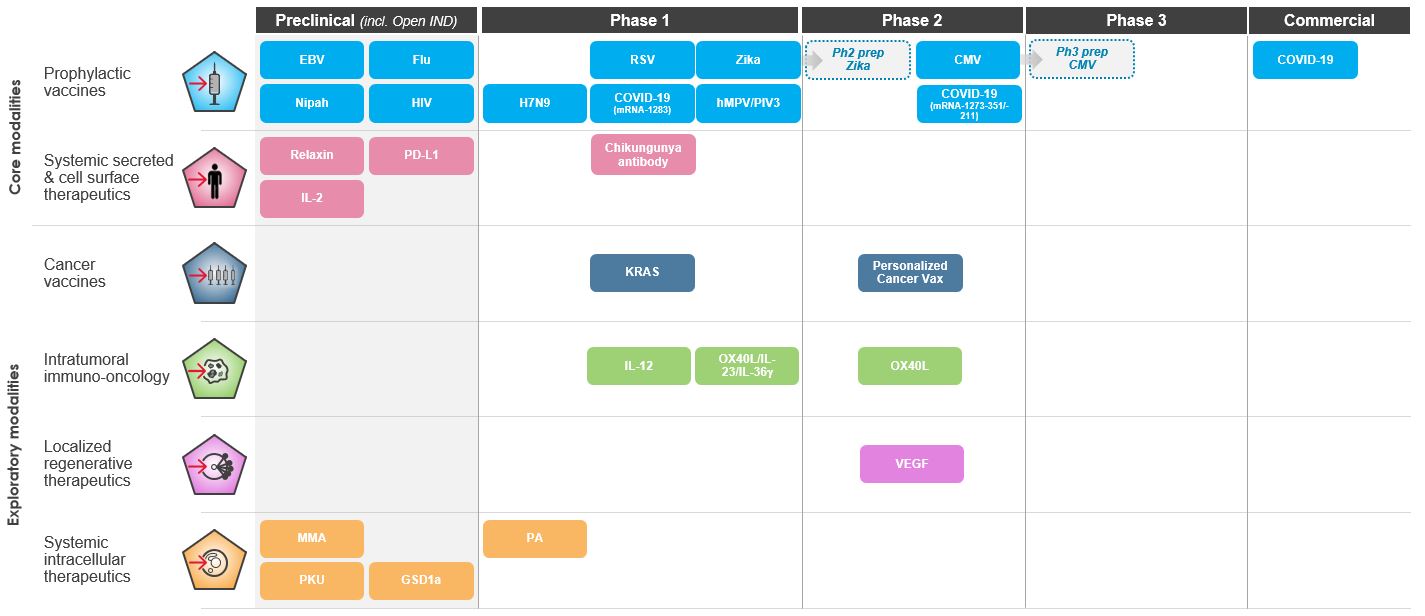
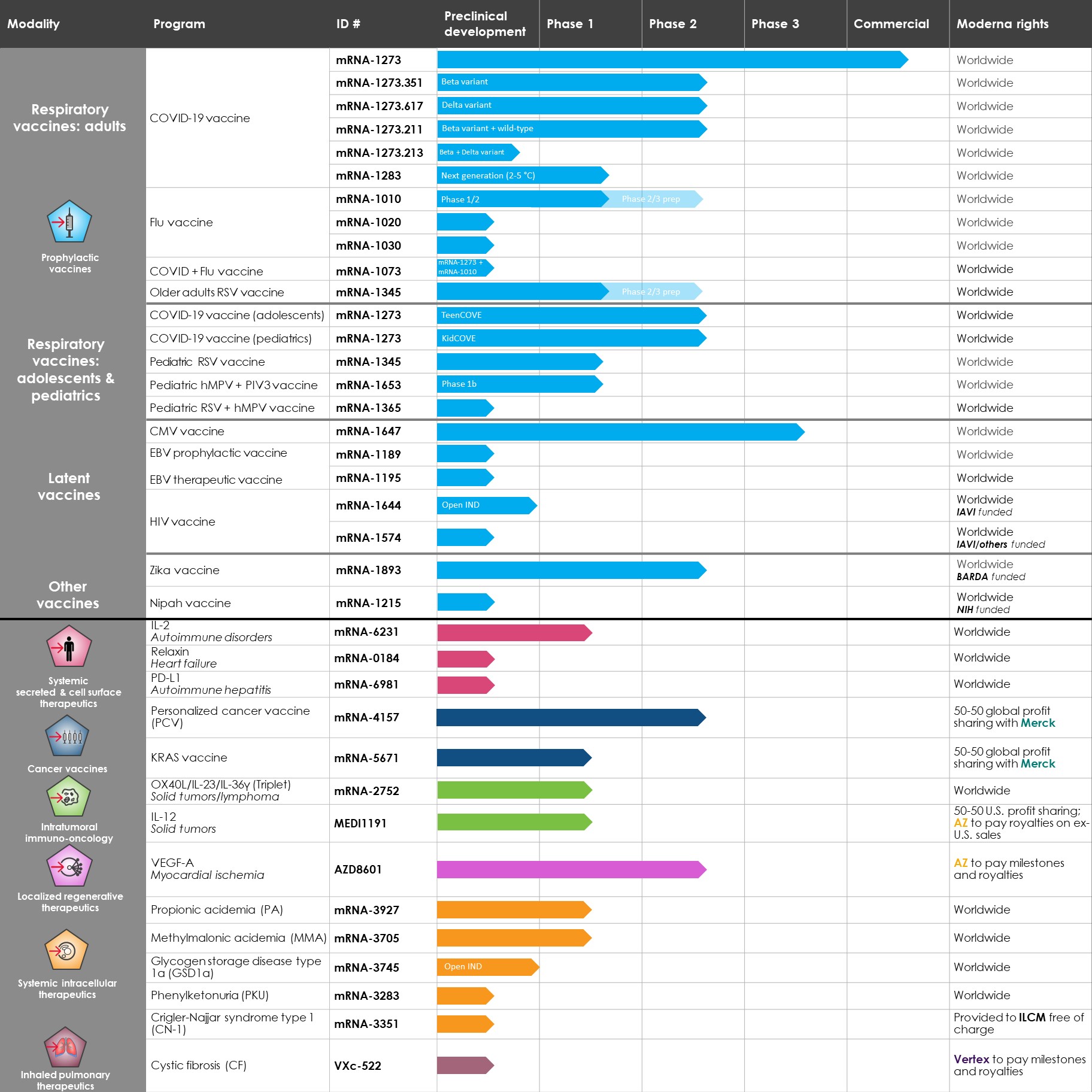
Abbreviations: GSD1a, Glycogen storage disease type 1a; H7N9, H7N9 influenza vaccine;AZ, AstraZeneca; BARDA, Biomedical Advanced Research and Development Authority; CMV, Cytomegalovirus; DARPA, Defense Advanced Research Projects Agency; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; hMPV, human metapneumovirus; IAVI, International AIDS Vaccine Initiative; ILCM, Institute for Life Changing Medicines; IL-2, Interleukin 2:interleukin 2; IL-12, interleukin 12; IL-23, interleukin 23; IL-36γIL-36γ, interleu;interleukin-36 gamma; NIH, National Institutes of Health; OX40L, wildtype OX40 ligand; PD-L1, programmed death-ligand-1;RSV, respiratory syncytial virus; VEGF-A, vascular endothelial growth factor A.
The breadth of biology addressable using mRNA technology is reflected in our current development pipeline of 2437 programs. The diversity of proteins made from mRNA within our development pipeline is shown in the figure below.
We have developed sixseven modalities, which are summarized as follows:
•Prophylactic vaccines: Our prophylactic vaccines modality currently includes ten23 development programs, six13 of which have entered into clinical trials and demonstrated desired pharmacology, in the form of immunogenicity, in positive Phase 1 clinical trials: H7N9 vaccine (mRNA-1851), RSV vaccine (mRNA-1777), human metapneumovirus (hMPV)/parainfluenza virus type 3 (PIV3) vaccine (mRNA-1653), Zika vaccine (mRNA-1893), CMV vaccine (mRNA-1647) and COVID-19 vaccine (mRNA-1273).trials. We have ongoing Phase 1 trials for theour RSV vaccine in pediatrics and older adults (mRNA-1345), flu vaccine (mRNA-1010), and hMPV/PIV3 vaccine (mRNA-1653). We have ongoing Phase 2 studies for our Zika vaccine (mRNA-1893), pediatric RSV and CMV vaccine (mRNA-1345), hMPV/PIV3 vaccine (mRNA-1653) and Merck is conducting a(mRNA-1647). The Phase 13 trial for an additional RSVour CMV vaccine (mRNA-1172), which will be transitioned to Moderna after completion.commenced in October 2021. Our COVID-19 vaccine (mRNA-1273) is described in detail above. TwoOur ten preclinical programs within our prophylactic vaccines modality are for our COVID-19 variant- specific candidate (mRNA-1273.213), our combined COVID-19 and flu vaccine and flu vaccine (mRNA-1073), our seasonal flu vaccines (mRNA-1020 and mRNA-1030), our combination pediatric RSV and hMPV vaccine (mRNA-1365), Epstein-Barr virus (EBV) (mRNA-1189), EBV therapeutic vaccine (mRNA-1195), Nipah virus (mRNA-1215) and HIV (mRNA-1644 and mRNA-1574). Three other vaccines being developed as part of public health programs―programs have had positive Phase 1 readouts―H10N8 vaccine (mRNA-1440), H7N9 flu vaccine (mRNA-1851), and Chikungunya vaccine (mRNA-1388)―also produced positive Phase 1 clinical trial results, but are not being further developed without government or other funding. Our four pre-clinical programs within our prophylactic vaccines modality are for Epstein-Barr virus (mRNA-1189), seasonal influenza (mRNA-1010, mRNA-1020 and mRNA-1030), Nipah virus (mRNA-1215) and HIV (mRNA-1644 and mRNA-1574).
•Systemic secreted and cell surface therapeutics: We have four systemic secreted and cell surface therapeutics development candidates in our pipeline. Our secreted programs include our antibody against Chikungunya virus (mRNA-1944), Relaxin (mRNA-0184) for cardiac disorders, PD-L1 (mRNA-6981) for autoimmune hepatitis and IL-2 (mRNA-6231) for autoimmune disorders. Our antibody against Chikungunya virus (mRNA-1944) has had positive Phase 1 readouts to date.date, but we do not have plans to advance to a Phase 2 study. Our IL-2 program (mRNA-6231) is currently in a Phase 1 study, and is our first autoimmune therapeutic candidate to enter the clinic. The remaining programs for Relaxin (mRNA-0184), and PD-L1 (mRNA-6981) and IL-2 (mRNA-6231) are currently in preclinical development.
•Cancer vaccines: We are currently developing two programs within our cancer vaccines modality. Our personalized cancer vaccine program mRNA-4157 is being developed in collaboration with Merck and is in a multiple-arm Phase 1 trial and a randomized Phase 2 trial.trial, which is fully enrolled. Our second program within this modality, mRNA-5671, is a KRAS vaccine. Our strategic collaborator Merck has a Phase 1 clinical trial ongoing for mRNA-5671.
•Intratumoral immuno-oncology: We have threetwo programs in this modality. TheOur first program in this modality, OX40L (mRNA-2416), was designed to overcome technological challenges in advancing this modality, including engineering the mRNA sequence to minimize off-target effects, utilizing our proprietary lipid nanoparticles (LNPs) to enhance safety and tolerability, and to demonstrate expression of a membrane protein in patients. OX40L (mRNA-2416) is currently being evaluated in an ongoing Phase 1/2 trial in the United States and Israel, and protein expression has been demonstrated in a number of patients. Our second program, OX40L/IL-23/IL-36γ (Triplet) (mRNA-2752), is currently in a Phase 1 study that is designed as an open-label, multicenter study of intratumoral injections of Triplet (mRNA-2752) alone or in combination with durvalumab (anti-PD-L1) with sites in the United States and Israel.. Our thirdsecond program, IL-12 (MEDI1191), is being developed in collaboration with AstraZeneca. AstraZeneca is currently enrolling an open-label multicenter Phase 1 clinical trial of intratumoral injections of MEDI1191 alone and in combination with the checkpoint inhibitor, durvalumab.
•Localized regenerative therapeutics: Our localized VEGF-A program, AZD8601, which is being developed by AstraZeneca, has completed a Phase 1a/b trial to describe its safety, tolerability, protein production, and activity in diabetic patients. The study has met its primary objectives of describing safety and tolerability and secondary objectives of demonstrating protein production and changes in blood flow post AZD8601 administration. We believe these data provide clinical proof of mechanism for our mRNA technology outside of the vaccine setting. AstraZeneca has initiated aThe Phase 2a study of AZD8601 VEGF-A, which is being developed for VEGF-A forpatients with ischemic heart disease in patients undergoing coronary artery bypass grafting (CABG) surgery with moderately impaired systolic function, andled by AstraZeneca, has completed recruitment after enrollment of the trial is ongoing.low dose cohort (n=11). Moderna has licensed worldwide commercial rights to AZD8601 to AstraZeneca.
•Systemic intracellular therapeutics: We have fourfive systemic intracellular therapeutics development candidates in our pipeline. Our intracellular programs address propionic acidemia, or PA (mRNA-3927), methylmalonic acidemia or MMA(MMA) (mRNA-3705), phenylketonuria or PKU(PKU) (mRNA-3283), and glycogen storage disorder type 1a or GSD1a(GSD1a) (mRNA-3745) and Crigler-Najjar Syndrome Type 1 (CN-1) (mRNA-3351). The first patient has been dosed in theWe have an ongoing Phase 1 clinical trial of mRNA-3927, our intracellular program to address PA. We plan to file new INDtrials for PA (mRNA-3927) and CTA applications for our next-generation MMA candidate, mRNA-3705. mRNA-3705 received a Breakthrough Designation from the FDA.(mRNA-3705). PKU (mRNA-3283), GSD1a (mRNA-3745) and GSD1a (mRNA-3745)CN-1 (mRNA-3351) are currently in preclinical development. The FDA has granted Orphan Drug Designation for mRNA-3745 and has completed its review of the IND application allowing it to proceed to clinic. We have entered into a collaboration agreement with the Institute for Life Changing Medicines (ILCM) to license mRNA-3351 to ILCM with no upfront fees, and without any downstream payments. ILCM will be responsible for the clinical development of mRNA-3351.
•Inhaled pulmonary therapeutics: We have one inhaled pulmonary therapeutic development candidate in our pipeline. Our program addresses cystic fibrosis, or CF (VXc-522), in collaboration partnership with Vertex Pharmaceuticals. VXc-522 is an mRNA therapeutic designed to treat the underlying cause of CF by enabling cells in the lungs to produce functional cystic fibrosis transmembrane conductance regulator (CFTR) protein for the treatment of the 10% of patients who do not produce any CFTR protein. IND-enabling studies are underway and Vertex expects to submit an IND for this program in 2022. Moderna has licensed worldwide commercial rights to VXc-522 to Vertex.
Financial Operations Overview
Revenue
The following table summarizes revenue for each period presented (in millions):
| | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | 2021 | | 2020 | | 2021 | | 2020 | | 2021 | | 2020 |
| Revenue: | Revenue: | | | | Revenue: | | | | | | | | |
| Product sales | Product sales | $ | 1,733 | | | $ | — | | Product sales | | $ | 4,810 | | | $ | — | | | $ | 10,740 | | | $ | — | |
| Grant revenue | Grant revenue | 194 | | | 4 | | Grant revenue | | 140 | | | 145 | | | 473 | | | 187 | |
| Collaboration revenue | Collaboration revenue | 10 | | | 4 | | Collaboration revenue | | 19 | | | 12 | | | 47 | | | 45 | |
| Total revenue | Total revenue | $ | 1,937 | | | $ | 8 | | Total revenue | | $ | 4,969 | | | $ | 157 | | | $ | 11,260 | | | $ | 232 | |
We began to record product sales for our COVID-19 vaccine subsequent to its authorization for emergency use by the FDA and Health Canada in December 2020. For the three months ended March 31,September 30, 2021, we recognized $1.7$4.8 billion of product sales from our COVID-19 vaccine, of which $1.4$1.2 billion was generated in the United States and $375 million$3.6 billion was generated from the rest of the world. For the nine months ended September 30, 2021, we recognized $10.7 billion of product sales from our COVID-19 vaccine, of which $4.6 billion was generated in the United States and $6.1 billion was generated from the rest of the world.
Other than product sales, our revenue has been primarily derived from government-sponsored and private organizations including BARDA, DARPA and the Gates Foundation and from strategic alliances with AstraZeneca, Merck and Vertex to discover, develop, and commercialize potential mRNA medicines.
Grant revenue was comprised as follows for the periods presented (in millions):
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | | | 2021 | | 2020 |
| Grant revenue: | | | | | | | |
BARDA (1) | | | | | $ | 192 | | | $ | 3 | |
| Other | | | | | 2 | | | 1 | |
| Total grant revenue | | | | | $ | 194 | | | $ | 4 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Grant revenue: | | | | | | | |
BARDA (1) | $ | 128 | | | $ | 143 | | | $ | 454 | | | $ | 183 | |
| Other | 12 | | | 2 | | | 19 | | | 4 | |
| Total grant revenue | $ | 140 | | | $ | 145 | | | $ | 473 | | | $ | 187 | |
_______
(1) For the three months ended March 31,September 30, 2021, $190$124 million of BARDA grant revenue was related to our mRNA-1273 program and $2$4 million was related to our Zika vaccine program. For the nine months ended September 30, 2021, $447 million of BARDA grant revenue was related to our mRNA-1273 program and $7 million was related to our Zika vaccine program.
CollaborativeCollaboration revenue from our strategic alliances was comprised as follows for the periods presented (in millions):
| | | | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | | | 2021 | | 2020 | | 2021 | | 2020 | | 2021 | | 2020 |
| Collaboration revenue: | Collaboration revenue: | | | | | | Collaboration revenue: | | | | | | | |
| AstraZeneca | AstraZeneca | | | $ | — | | | $ | 1 | | AstraZeneca | $ | 3 | | | $ | — | | | $ | 7 | | | $ | 17 | |
| Merck | Merck | | | — | | | 1 | | Merck | 7 | | | 6 | | | 11 | | | 18 | |
| Vertex | Vertex | | | 9 | | | 2 | | Vertex | 5 | | | 6 | | | 23 | | | 10 | |
| Other | Other | | | 1 | | | — | | Other | 4 | | | — | | | 6 | | | — | |
| Total collaboration revenue | Total collaboration revenue | | | $ | 10 | | | $ | 4 | | Total collaboration revenue | $ | 19 | | | $ | 12 | | | $ | 47 | | | $ | 45 | |
We expect our product sales to significantly increase in 2021 compared to 2020. As of March 31,September 30, 2021, we had signed supply agreements of approximately $17.3$25.1 billion for the future supply of our COVID-19 vaccine through 2023 and had deferred revenue of $7.5$8.3 billion associated with customer deposits received or billable under these agreements. Additional supply agreements have been agreed upon since March 31,September 30, 2021, and others are under discussion for 2021 and 2022 deliveries. In addition, we expect to continue to receive funding from our contract with BARDA. As of March 31,September 30, 2021, the remaining available funding, net of revenue, earned was $317 million. On April 18, 2021, we signed an amendment tounder our agreement with BARDA to increase total reimbursements by up to an additional $236for the development of our mRNA-1273 vaccine was $441 million. To the extent that existing or potential future products generate revenue, our revenue may vary due to many uncertainties in the independent development of our mRNA medicines and pursuant to our strategic alliances and other factors.
Cost of sales
Cost of Salessales includes raw materials, personnel and facility and other costs associated with manufacturing our commercial product. These costs include production materials, production costs at our manufacturing facilities, third-party manufacturing costs, and final formulation and packaging costs. Cost of Salessales also includes shipping costs and royalties payable to third parties based on sales of our products.
Research and development expenses
The nature of our business and primary focus of our activities generate a significant amount of research and development costs.
Research and development expenses represent costs incurred by us for the following:
•cost to develop our platform;
•discovery efforts leading to development candidates;
•preclinical, nonclinical, and clinical development costs for our programs;
•cost to develop our manufacturing technology and infrastructure; and
•digital infrastructure costs.
The costs above comprise the following categories:
•personnel-related expenses, including salaries, benefits, and stock-based compensation expense;
•expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations (CROs) that conduct our preclinical studies and clinical trials, and in-licensing arrangements;
•expenses associated with developing manufacturing capabilities and acquiring materials for preclinical studies and clinical trials, including both internal manufacturing and third-party contract manufacturing organizations (CMOs);
•expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process; and
•facilities, depreciation, and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
We use our employee and infrastructure resources for the advancement of our platform, and for discovering and developing programs. Due to the number of ongoing programs and our ability to use resources across several projects, indirect or shared operating costs incurred for our research and development programs are generally not recorded or maintained on a program- or modality-specific basis. The following table reflects our research and development expenses, including direct program-specific expenses summarized by modality and indirect or shared operating costs summarized under other research and development expenses during the three and nine months ended March 31,September 30, 2021 and 2020 (in millions):
| | | | | Three Months Ended March 31, | | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | | | 2021 | | 2020 | | 2021 | | 2020 | | 2021 | | 2020 |
| Program expenses by modality: | Program expenses by modality: | | | | | | Program expenses by modality: | | | | | | | | |
| Prophylactic vaccines | Prophylactic vaccines | | | $ | 248 | | | $ | 9 | | Prophylactic vaccines | | $ | 271 | | | $ | 163 | | | $ | 760 | | | $ | 208 | |
| Cancer vaccines | Cancer vaccines | | | 17 | | | 4 | | Cancer vaccines | | 11 | | | 8 | | | 35 | | | 24 | |
| Intratumoral immuno-oncology | Intratumoral immuno-oncology | | | 6 | | | 2 | | Intratumoral immuno-oncology | | 6 | | | 4 | | | 19 | | | 7 | |
| Systemic secreted and cell surface therapeutics | Systemic secreted and cell surface therapeutics | | | 1 | | | 1 | | Systemic secreted and cell surface therapeutics | | 3 | | | 1 | | | 5 | | | 2 | |
| Systemic intracellular therapeutics | Systemic intracellular therapeutics | | | 5 | | | 7 | | Systemic intracellular therapeutics | | 6 | | | 2 | | | 17 | | | 15 | |
Total program-specific expenses by modality (1) | Total program-specific expenses by modality (1) | | | 277 | | | 23 | | Total program-specific expenses by modality (1) | | 297 | | | 178 | | | 836 | | | 256 | |
| Other research and development expenses: | Other research and development expenses: | | | Other research and development expenses: | |
| Discovery programs | Discovery programs | | | 13 | | | 10 | | Discovery programs | | 15 | | | 16 | | | 43 | | | 37 | |
| Platform research | Platform research | | | 25 | | | 22 | | Platform research | | 28 | | | 24 | | | 80 | | | 64 | |
| Technical development and unallocated manufacturing expenses | Technical development and unallocated manufacturing expenses | | | 33 | | | 29 | | Technical development and unallocated manufacturing expenses | | 79 | | | 80 | | | 165 | | | 138 | |
| Shared discovery and development expenses | Shared discovery and development expenses | | | 39 | | | 19 | | Shared discovery and development expenses | | 77 | | | 33 | | | 165 | | | 76 | |
| Stock-based compensation | Stock-based compensation | | | 14 | | | 12 | | Stock-based compensation | | 25 | | | 13 | | | 54 | | | 40 | |
| Total research and development expenses | Total research and development expenses | | | $ | 401 | | | $ | 115 | | Total research and development expenses | | $ | 521 | | | $ | 344 | | | $ | 1,343 | | | $ | 611 | |
__________
(1)IncludeIncludes a total of 3034 and 2423 development candidates at March 31,September 30, 2021 and 2020, respectively. Program-specific expenses include external costs and allocated manufacturing costs of pre-launch inventory, mRNA supply and consumables, and are reflected as of the beginning of the period in which the program was internally advanced to development or removed if development was ceased.
A “modality” refers to a group of programs with common product features and the associated combination of enabling mRNA technologies, delivery technologies, and manufacturing processes. The program-specific expenses by modality summarized in the table above include expenses we directly attribute to our programs, which consist primarily of external costs, such as fees paid to outside consultants, central laboratories, investigative sites, and CROs in connection with our preclinical studies and clinical trials, CMOs, and allocated manufacturing costs of inventory, mRNA supply and consumables. Costs to acquire and manufacture inventory, mRNA supply for preclinical studies and clinical trials are recognized and included in unallocated manufacturing expenses when incurred, and subsequently allocated to program-specific manufacturing costs after completion of the program-specific production. The timing of allocating manufacturing costs to the specific program varies depending on the program development and production schedule. We generally do not allocate personnel-related costs, including stock-based compensation, costs associated with our general platform research, technical development, and other shared costs on a program-specific basis. These costs were therefore excluded from the summary of program-specific expenses by modality. Our newest modality, for inhaled pulmonary therapeutics, was added subsequent to the end of the third quarter of 2021.
Discovery program expenses are costs associated with research activities for our programs in the preclinical discovery stage, and primarily consist of external costs for CROs and lab services, and allocated manufacturing cost of preclinical mRNA supply and consumables.
Platform research expenses are mainly costs to develop technical advances in mRNA science, delivery science, and manufacturing process design. These costs include personnel-related costs, computer equipment, facilities, preclinical mRNA supply and consumables, and other administrative costs to support our platform research. Technology development and unallocated manufacturing expenses are primarily related to non-program-specific manufacturing process development and manufacturing costs.
Shared discovery and development expenses are research and development costs such as personnel-related costs and other costs, which are not otherwise included in development programs, discovery programs, platform research, technical development and unallocated manufacturing expenses, stock-based compensation, and other expenses.
The largest component of our total operating expenses has historically been our investment in research and development activities, including preclinical and clinical development of our product candidates, development of our platform, mRNA technologies, and manufacturing technologies. We expense research and development costs as incurred and cannot reasonably estimate the nature, timing, and estimated costs required to complete the development of the development candidates and investigational medicines we are currently developing or may develop in the future. There are numerous risks and uncertainties associated with the research and development of such development candidates and investigational medicines, including, but not limited to:
•scope, progress, and expense of developing ongoing and future development candidates and investigational medicines;
•entry in and completion of related preclinical studies;
•enrollment in and completion of subsequent clinical trials;
•safety and efficacy of investigational medicines resulting from these clinical trials;
•changes in laws or regulations relevant to the investigational medicines in development;
•receipt of the required regulatory approvals; and
•commercialization, including establishing manufacturing and marketing capabilities.
As we continue to progress mRNA-1273 through the development process toward a Biologics License Application approval, indication expansion of mRNA-1273 and potential development of variant-specific vaccine candidates during the current pandemic, we expect to continue to incur significant additional expenses. At this time, the magnitude of these potential expenditures is not known. In connection with the BARDA agreement to accelerate development of mRNA-1273, significant grant revenue and expenses are expected in 2021. BARDA'sBARDA’s funding is expected to offset those expenses that are covered under the BARDA agreement, subject to our obtaining reimbursement from BARDA. As of March 31,September 30, 2021, the remaining available funding, net of revenue earned was $317$441 million. On April 18, 2021, we signed an amendment to our agreement with BARDA, increasing the potential reimbursements thereunder by up to an additional $236 million in connection with the conduct of the Phase 3 clinical trial for mRNA-1273 and pharmacovigilance efforts.
Changes in expectations or outcomes of any of the known or unknown risks and uncertainties may materially impact our expected research and development expenditures. Continued research and development is central to the ongoing activities of our business. Investigational medicines in later stages of clinical development, such as our CMV vaccine (mRNA-1647) and our COVID-19 vaccine, generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect our research and development costs to continue to increase in the foreseeable future as our investigational medicines progress through the development phases and identify and develop additional programs. There are numerous factors associated with the successful commercialization of any of our investigational medicines, including future trial
design and various regulatory requirements, many of which cannot be determined with accuracy at this time due to the early stage of development of our investigational medicines. Moreover, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
Selling, general and administrative expenses
We started to incur sales and marketing expenses in the fourth quarter of 2020 to prepare for commercial operations in connection with the sale of our COVID-19 vaccine. Selling, general and administrative expenses consist primarily of personnel-related costs, including stock-based compensation, for executives, finance, legal, human resources, business development and other administrative and operational functions, professional fees, accounting and legal services, sales and marketing, information technology and facility-related costs, and expenses associated with obtaining and maintaining intellectual property, or IP. These costs relate to the operation of the business, unrelated to the research and development function, or any individual program.
We anticipate selling, general and administrative expenses will increase as we continue to expand the number of programs in development and to establish our commercial activities both within and outside the United States. We have already incurred additional expenses related to building out a regulatory, sales and marketing team to support the sale, marketing and distribution of our COVID-19 vaccine. If we obtain regulatory approval for any of our other investigational medicines, and do not enter into one or more third-party commercialization collaboration and manufacturing arrangements, we will incur significant additional expenses related to building out these functions.
We have a broad IP portfolio covering our development and commercialization of mRNA vaccine and therapeutic programs, including those related to mRNA design, formulation, and manufacturing platform technologies. We regularly file patent applications to protect innovations arising from our research and development. We also hold trademarks and trademark applications in the United States and foreign jurisdictions. Costs to secure and defend our IP are expensed as incurred and are classified as selling, general and administrative expenses.
Interest income
Interest income consists of interest generated from our investments in cash and cash equivalents, money market funds, and high-quality fixed income securities.
Other expense, net
Other expense, net consists of interest expense, gains (losses) from the sale of investments in marketable securities, foreign currency transaction and remeasurement gains (losses), gains (losses) on foreign currency balance sheet hedges, and other income and expense unrelated to our core operations. Interest expense is primarily derived from our finance leases related to our Moderna Technology Center, manufacturing facility (MTC South), Moderna Technology Center North (MTC North), and certain contract manufacturing service agreements.
We expect to continue to incur significant expenses as we continue our research and development and commercialization efforts. We expect our programs to mature and advance to later stage clinical development, and we expect expenses to increase as we seek regulatory approvals for our investigational medicines and commercialize any approved mRNA medicines. If we fail to sustain profitability on a continuing basis, we may incur losses in the future.
Critical accounting policies and significant judgments and estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these condensed consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues, and expenses and the disclosure of contingent assets and liabilities in our condensed consolidated financial statements. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts, and experience. The effects of material revisions in estimates, if any, are reflected in the condensed consolidated financial statements prospectively from the date of change in estimates.
There have been no material changes in our critical accounting policies and estimates in the preparation of our condensed consolidated financial statements during the three months ended March 31,September 30, 2021 compared to those disclosed in our Annual Report on Form 10-K for the year ended December 31, 2020, or 2020 Form 10-K.
Recently issued accounting pronouncements
We have reviewed all recently issued standards and have determined that such standards will not have a material impact on our financial statements or do not otherwise apply to our operations.
Results of operations
The following table summarizes our condensed consolidated statements of operations for each period presented (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
| Revenue: | | | | | | | |
| Product revenue | $ | 4,810 | | | $ | — | | | $ | 4,810 | | | 100% |
| Grant revenue | 140 | | | 145 | | | (5) | | | (3)% |
| Collaboration revenue | 19 | | | 12 | | | 7 | | | 58% |
| Total revenue | 4,969 | | | 157 | | | 4,812 | | | 3,065% |
| Operating Expenses: | | | | | | | |
| Cost of sales | 722 | | | — | | | 722 | | | 100% |
| Research and development | 521 | | | 344 | | | 177 | | | 51% |
| Selling, general and administrative | 168 | | | 48 | | | 120 | | | 250% |
| Total operating expenses | 1,411 | | | 392 | | | 1,019 | | | 260% |
| Income (loss) from operations | 3,558 | | | (235) | | | 3,793 | | | 1,614% |
| Interest income | 4 | | | 6 | | | (2) | | | (33)% |
| Other expense, net | (10) | | | (3) | | | (7) | | | 233% |
| Income (loss) before income taxes | 3,552 | | | (232) | | | 3,784 | | | 1,631% |
| Provision for income taxes | 219 | | | 1 | | | 218 | | | 21,800% |
| Net income (loss) | $ | 3,333 | | | $ | (233) | | | $ | 3,566 | | | 1,530% |
| | | Three Months Ended March 31, | | Change 2021 vs. 2020 | | Nine Months Ended September 30, | | Change 2021 vs. 2020 |
| | 2021 | | 2020 | | $ | | % | | 2021 | | 2020 | | $ | | % |
| Revenue: | Revenue: | | | | | | | | Revenue: | | | | | | | |
| Product revenue | Product revenue | $ | 1,733 | | | $ | — | | | $ | 1,733 | | | 100% | Product revenue | $ | 10,740 | | | $ | — | | | $ | 10,740 | | | 100% |
| Grant revenue | Grant revenue | 194 | | | 4 | | | 190 | | | 4750% | Grant revenue | 473 | | | 187 | | | 286 | | | 153% |
| Collaboration revenue | Collaboration revenue | 10 | | | 4 | | | 6 | | | 150% | Collaboration revenue | 47 | | | 45 | | | 2 | | | 4% |
| Total revenue | Total revenue | 1,937 | | | 8 | | | 1,929 | | | 24113% | Total revenue | 11,260 | | | 232 | | | 11,028 | | | 4,753% |
| Operating Expenses: | Operating Expenses: | | Operating Expenses: | |
| Cost of sales | Cost of sales | 193 | | | — | | | 193 | | | 100% | Cost of sales | 1,665 | | | — | | | 1,665 | | | 100% |
| Research and development | Research and development | 401 | | | 115 | | | 286 | | | 249% | Research and development | 1,343 | | | 611 | | | 732 | | | 120% |
| Selling, general and administrative | Selling, general and administrative | 77 | | | 24 | | | 53 | | | 221% | Selling, general and administrative | 366 | | | 109 | | | 257 | | | 236% |
| Total operating expenses | Total operating expenses | 671 | | | 139 | | | 532 | | | 383% | Total operating expenses | 3,374 | | | 720 | | | 2,654 | | | 369% |
| Income (loss) from operations | Income (loss) from operations | 1,266 | | | (131) | | | 1,397 | | | (1066)% | Income (loss) from operations | 7,886 | | | (488) | | | 8,374 | | | 1,716% |
| Interest income | Interest income | 4 | | | 8 | | | (4) | | | (50)% | Interest income | 11 | | | 21 | | | (10) | | | (48)% |
| Other expense, net | Other expense, net | (10) | | | (1) | | | (9) | | | 900% | Other expense, net | (22) | | | (6) | | | (16) | | | 267% |
| Income (loss) before income taxes | Income (loss) before income taxes | 1,260 | | | (124) | | | 1,384 | | | (1116)% | Income (loss) before income taxes | 7,875 | | | (473) | | | 8,348 | | | 1,765% |
| Provision for income taxes | Provision for income taxes | 39 | | | — | | | 39 | | | 100% | Provision for income taxes | 541 | | | 1 | | | 540 | | | 54,000% |
| Net income (loss) | Net income (loss) | $ | 1,221 | | | $ | (124) | | | $ | 1,345 | | | (1085)% | Net income (loss) | $ | 7,334 | | | $ | (474) | | | $ | 7,808 | | | 1,647% |
Revenue
Total revenue increased by $1.9$4.8 billion for the three months ended March 31,September 30, 2021, compared to the same period in 2020, due to increases in product sales. Product revenue was $4.8 billion for the three months ended September 30, 2021 from sales of our COVID-19 vaccine. For the three months ended September 30, 2021, we delivered approximately 73 million doses to the U.S. Government and approximately 136 million doses to other governments of our COVID-19 vaccine. We did not have product sales until December 2020.
Total revenue increased by $11.0 billion for the nine months ended September 30, 2021, compared to the same period in 2020, due to increases in product sales and grant revenue. Product revenue was $1.7$10.7 billion for the threenine months ended March 31,September 30, 2021 from sales of our COVID-19 vaccine. For the threenine months ended March 31,September 30, 2021, we delivered approximately 88287 million doses to the U.S. governmentGovernment and approximately 14222 million doses to other governments. We did not have product sales until December 2020. Grant revenue increased by $190$286 million for the threenine months ended March 31,September 30, 2021, compared to the same period in 2020, mainlyprimarily driven by an increase in revenue from BARDA related to our mRNA-1273 vaccine development.
Operating expenses
Cost of sales
We began capitalizing our COVID-19 vaccine inventory costs in December 2020, in connection with an EUA from the FDA, and based upon our expectation that these costs would be recoverable through commercialization of our COVID-19 vaccine. Prior to the capitalization of our COVID-19 vaccine inventory costs, such costs were recorded as research and development expenses in the period incurred. We expensed $242 million of pre-launch inventory costs in 2020. Our cost of sales were $193was $722 million, or 11%15%, of our product sales, for the three months ended March 31,September 30, 2021, including third-party royalties of $84$168 million. Our cost of sales was $1.7 billion, or 16%, of our product sales, for the nine months ended September 30, 2021, including third-party royalties of $400 million. A portion of the inventory costs associated with our productsproduct sales for the threenine months ended March 31,September 30, 2021 was expensed previously. At the end of the first quarter of 2021, we had substantially utilized our zero-cost COVID-19 vaccine inventory. If inventory sold for the threenine months ended March 31,September 30, 2021 was valued at cost, our cost of sales for the period would have been $377 million,$1.9 billion, or 22%17%, of our product sales. As of March 31, 2021, we had substantially utilized our zero-cost COVID-19 vaccine inventory. We expect that in future periods our cost of sales as a percentage of our product sales will increase, reflectingremain at a similar level for the full costremainder of manufacturing.2021.
Research and development expenses
Research and development expenses increased by $286$177 million, or 249%51%, for the three months ended March 31,September 30, 2021, compared to the same period in 2020. The increase was primarily attributable to an increase in clinical trial expenses of $219$155 million and an increase in manufacturingpersonnel-related costs of $40 million.
Research and development expenses relatedincreased by $732 million, or 120%, for the nine months ended September 30, 2021, compared to ourthe same period in 2020. The increase was primarily attributable to an increase in clinical trialstrial expenses of $25$591 million, an increase in personnel-related costs of $25$77 million, and an increase in consulting and outside services of $21$45 million.
These increases for both the three-month periodthree and nine month periods in 2021 were largely attributable todriven by increased mRNA-1273 clinical development and headcount.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by $53$120 million, or 221%250%, for the three months ended March 31,September 30, 2021, compared to the same period in 2020. The increase was mainly due to an increase in consulting and outside services of $30 million, an increase in distributor fees of $29 million, an increase in marketing expenses of $23 million, and an increase in personnel-related costs of $17 million.
Selling, general and administrative expenses increased by $257 million, or 236%, for the nine months ended September 30, 2021, compared to the same period in 2020. The increase was mainly due to an increase in consulting and outside services of $80 million, an increase in personnel-related costs of $11$48 million, an increase in marketing and other expenses of $10 million, an increase in legal and other licensing expenses of $7$41 million, and an increase in stock-based compensationdistributor fees of $4$32 million.
These increases for both the three-month periodthree and nine month periods in 2021 were primarily attributable to increased headcount anddriven by our COVID-19 vaccine commercialization-related activities.activities and increased headcount.
Interest income
Interest income decreased by $4$2 million, or 50%33%, for the three months ended March 31,September 30, 2021, compared to the same period in 2020. Interest income decreased by $10 million, or 48%, for the nine months ended September 30, 2021, compared to the same period in 2020. The decreasedecreases in interest income from our investments in marketable securities for both the three-month periodthree and nine month periods in 2021 waswere mainly driven by an overall lower interest rate environment.environment, partially offset by increased investment balances.
Other expense, net
The following table summarizes other expense, net for each period presented (in millions):
| | | Three Months Ended March 31, | | Change 2021 vs. 2020 | | Three Months Ended September 30, | | Change 2021 vs. 2020 |
| | 2021 | | 2020 | | $ | | % | | 2021 | | 2020 | | $ | | % |
| | Interest expense | Interest expense | (3) | | | (1) | | | (2) | | | 200% | Interest expense | (4) | | | $ | (2) | | | (2) | | | 100% |
| Other expense, net | Other expense, net | (7) | | | — | | | (7) | | | 100% | Other expense, net | (6) | | | (1) | | | (5) | | | 500% |
| Total other expense, net | Total other expense, net | $ | (10) | | | $ | (1) | | | $ | (9) | | | 900% | Total other expense, net | $ | (10) | | | $ | (3) | | | $ | (7) | | | 233% |
| | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, | | Change 2021 vs. 2020 |
| 2021 | | 2020 | | $ | | % |
| Gain on investments | $ | 2 | | | $ | 1 | | | $ | 1 | | | 100% |
| Interest expense | (12) | | | $ | (6) | | | (6) | | | 100% |
| Other expense, net | (12) | | | (1) | | | (11) | | | 1,100% |
| Total other expense, net | $ | (22) | | | $ | (6) | | | $ | (16) | | | 267% |
Total other expense, net increased by $9 million, or 900%,was immaterial for each of the three months ended March 31,September 30, 2021 and 2020. Total other expense, net increased by $16 million, or 267%, for the nine months ended September 30, 2021, compared to the same period in 2020. The increase in other expense, net for the nine-month period in 2021 was primarily due to losses inrelated to our balance sheet hedging activities, partially offset by gains on foreign currency transactions and remeasurements, partially offset by our balance sheet hedge activities.remeasurements. Our interest expense is primarily related to our finance leases.
Income taxes
Our provision for income taxes for the three and nine months ended March 31,September 30, 2021 was $39$219 million as and $541 million, respectively, compared to an insignificant amount$1 million for each of the same periodperiods in 2020. Our effective tax rate for the three and nine months ended March 31,September 30, 2021 was lower than the U.S. statutory rate primarily due to the benefit of the foreign derived intangible income deduction, the benefit related to the release of the valuation allowance on the majority of our tax attributes and other deferred tax assets, the benefit of the foreign derived intangible income deduction, as well as a discrete item for excess tax benefits related to stock-based compensation. Our effective tax rate for the three and nine months ended March 31,September 30, 2020 was lower than the U.S. statutory rate primarily due to the valuation allowance.
On a periodic basis, we reassessesreassess any valuation allowances that we maintain on our deferred tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. In the first quarter of 2021, we reassessed the valuation allowance noting the increase in positive evidence, including significant revenue growth, expectations regarding future profitability, and successful supply chain and manufacturing capabilities to meet global product demand. After assessing both the positive evidence and negative evidence, we determined it was more likely than not that we will realize the majority of our deferred tax assets. Therefore, in the first quarter of 2021, we released our valuation allowance on the majority of our federal and state net operating losses and other deferred tax assets through the annual effective tax rate (AETR) as income is earned, resulting in a reduction in the AETR. In addition, we have recorded a discrete benefit of $49 million related to the deferred tax assets that we expect to utilize in future years. As of March 31,September 30, 2021, we continue to maintain a valuation allowance on certain state tax attributes.
Liquidity and capital resources
As of March 31,September 30, 2021, we had cash, cash equivalents and investments of $8.2$15.3 billion. Cash, cash equivalents and investments are invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Investments, consisting primarily of government and corporate debt securities, are stated at fair value. As of March 31,September 30, 2021, we had current and non-current investments of approximately $2.3$3.4 billion and $468 million,$6.4 billion, respectively.
We historically funded our operations primarily from the sale of equity instruments and from proceeds from certain strategic alliance arrangements and grant agreements. Starting in August 2020, we have entered into supply agreements with the U.S. Government and several governments outside the United States for the supply of our COVID-19 vaccine and receive upfront deposits. As of March 31,September 30, 2021, we had $7.5$8.3 billion in deferred revenue related to customer deposits received or billable. In addition, as of March 31,September 30, 2021, BARDA has committed to fund up to $1.0$1.4 billion to acceleratefor the clinical development and manufacturing process scale-up of our COVID-19 vaccine. Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure and the scale-up of manufacturing processes.processes of our COVID-19 vaccine. As of March 31,September 30, 2021, the remaining available funding from BARDA, net of revenue earned, was $317 million. On April 18, 2021, we signed an amendment to our agreement with BARDA to increase total reimbursements by up to an additional $236$441 million.
We continue to work toward the large-scale technical development, manufacturing scale-up in several countries and larger scale deployment of our COVID-19 vaccine. To support the scale-up, we have expended and will need to continue to expend significant resources and capital.
Cash flow
The following table summarizes the primary sources and uses of cash for each period presented (in millions):
| | | Three Months Ended March 31, | | Nine Months Ended September 30, |
| | 2021 | | 2020 | | 2021 | | 2020 |
| Net cash provided by (used in): | Net cash provided by (used in): | | | | Net cash provided by (used in): | | | |
| Operating activities | Operating activities | $ | 2,971 | | | $ | (106) | | Operating activities | $ | 10,310 | | | $ | 763 | |
| Investing activities | Investing activities | (180) | | | (316) | | Investing activities | (7,385) | | | (1,482) | |
| Financing activities | Financing activities | 26 | | | 578 | | Financing activities | — | | | 1,989 | |
| Net increase in cash, cash equivalents and restricted cash | Net increase in cash, cash equivalents and restricted cash | $ | 2,817 | | | $ | 156 | | Net increase in cash, cash equivalents and restricted cash | $ | 2,925 | | | $ | 1,270 | |
Operating activities
We derive cash flows from operations primarily from cash collected from customer deposits and accounts receivable related to our COVID-19 vaccine supply agreements, as well as certain government-sponsored and private organizations and strategic alliances. Our cash flows from operating activities are significantly affected by our use of cash for operating expenses and working capital to support the business. Prior to 2020, we historically experienced negative cash flows from operating activities as we have invested in mRNA technologies, development pipeline, digital infrastructure, manufacturing technology and infrastructure.
Net cash provided by operating activities for the threenine months ended March 31,September 30, 2021 was $3.0$10.3 billion and consisted of net income of $1.2$7.3 billion and non-cash adjustments of $203 million, plus a net change in assets and liabilities of $1.8$2.8 billion. Non-cash items included depreciation and amortization of $154 million, stock-based compensation of $105 million, deferred income taxes of $50$89 million, and amortization of investment premium and discount of $33 million. The net change in assets and liabilities was mainly due to an increase in deferred revenue of $4.4 billion, an increase in accrued liabilities of $600 million, an increase in income taxes payable of $565 million, and an increase in accounts payable of $26 million, partially offset by an increase in accounts receivable of $1.8 billion, an increase in inventory of $918 million, an increase in prepaid expenses and other assets of $186 million, and an increase in operating lease right-of-use assets of $25 million.
Net cash provided by operating activities for the nine months ended September 30, 2020 was $763 million and consisted of net loss of $474 million and non-cash adjustments of $96 million, plus a net change in assets and liabilities of $1.1 billion. Non-cash items primarily included stock-based compensation of $30$67 million, depreciation and amortization of $15$24 million, and amortization of investment premium and discount of $5 million. The net change in assets and liabilities was mainly due to an increase in deferred revenue of $3.7$1.2 billion and an increase in accrued liabilities of $285 million, and an increase in other liabilities of $93$132 million, partially offset by an increase in accounts receivable of $1.8 billion, an increase in inventory of $448 million, an decrease in accounts payable of $15 million, and an increase in prepaid expenses and other assets of $12$185 million.
Net cash used in operating activities for the three months ended March 31, 2020 was $106 million and consisted of net loss of $124 million and non-cash adjustments of $28 million, minus a net change in assets and liabilities of $10 million. Non-cash items primarily included stock-based compensation of $20 million and depreciation and amortization of $7 million. The net change in assets and liabilities was primarily due to an increase of right-of-use assets relating to operating leases of $14 million and a decrease in accrued liabilities of $12 million, partially offset by an increase in operating lease liabilities of $15 million.
Investing activities
Our primary investing activities consist of purchases, sales, and maturities of our investments and capital expenditures for leasehold improvements, manufacturing, laboratory, computer equipment and software.
Net cash used in investing activities for the nine months ended September 30, 2021 was $7.4 billion, which included purchases of marketable securities of $10.3 billion and purchases of property and equipment of $164 million, partially offset by proceeds from sales of marketable securities of $2.0 billion and proceeds from maturities of marketable securities of $1.1 billion.
Net cash used in investing activities for the threenine months ended March 31, 2021September 30, 2020 was $180 million,$1.5 billion, which included purchases of marketable securities of $726 million$2.3 billion and purchases of property and equipment of $35$44 million, partially offset by proceeds from maturities of marketable securities of $339$748 million and proceeds from sales of marketable securities of $242 million.
Net cash used in investing activities for the three months ended March 31, 2020 was $316 million, which included purchases of marketable securities of $621 million and purchases of property and equipment of $6 million, partially offset by proceeds from maturities of marketable securities of $269 million and proceeds from sales of marketable securities of $42$140 million.
Financing activities
We generatedThere was no net cash fromprovided by financing activities of $26 million for the threenine months ended March 31, 2021,September 30, 2021.
Net cash provided by financing activities for the nine months ended September 30, 2020 was $2.0 billion, primarily from net proceeds from the issuanceequity offerings of common stock in connection with the exercise of stock options under our equity plans of $28 million.
We generated cash from financing activities of $578 million for the three months ended March 31, 2020, from net proceeds from an equity offering of $550 million$1.9 billion, and net proceeds from the issuance of common stock in connection with the exercise of stock options under our equity plans of $28$136 million.
Operation and funding requirements
From our inception to the end of 2020, we incurred significant losses and negative cash flows from operations due to our significant research and development expenses. We generated net income in the first quarternine months of 2021 in connection with our product sales. We have an accumulated deficitretained earnings of $1.0$5.1 billion as of March 31,September 30, 2021. We expect our expenses to increase in connection with our ongoing activities, particularly as we continue research and development of our development candidates and clinical activities for our investigational medicines. We also expect our expenses to increase associated with manufacturing costs, including our arrangements with our international supply and manufacturing partners. Our ongoing work on mRNA-1273, including development of any new generations of boosters and vaccines against variants of SARS-CoV-2, will require significant cash outflows during 2021, most of which may not be reimbursed or otherwise paid for by our partners or collaborators.
We believe that our cash, cash equivalents, and investments as of March 31,September 30, 2021, together with cash expected to be generated from operations, will be sufficient to enable us to fund our projected operations, capital expenditures and stock repurchases through at least the next 12 months from the issuance of ourthese financial statements. We are subject to all the risks related to the development and commercialization of novel medicines, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors including expenses related to the ongoing coronavirus pandemic, which may adversely affect our business. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
If we are unable to sustain profitability on a continuing basis, we may be required to finance future cash needs through a combination of public or private equity offerings, structured financings and debt financings, government funding arrangements, potential future strategic alliances from which we receive upfront fees, milestone payments, and other forms of consideration, and marketing, manufacturing, distribution and licensing arrangements. If we are required to finance future cash needs, additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back, or discontinue the development or commercialization of one or more of our investigational medicines, or slow down or cease work on one or more of our programs. If we raise additional funds through the issuance of additional equity or debt securities, it could result in dilution to our existing stockholders or increased fixed payment obligations, and any such securities may have rights senior to those of our common stock. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise funds through strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs, or investigational medicines or grant licenses on terms that may not be favorable to us. Any of these events could significantly harm our business, financial condition, and prospects.
Contractual Obligations
As of March 31,September 30, 2021, other than disclosed atwithin Note 11 and Note 12 to our condensed consolidated financial statements, there have been no material changes to our contractual obligations and commitments from those described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our 2020 Form 10-K.
Off balance sheet arrangements
As of March 31,September 30, 2021, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
As of March 31,September 30, 2021 and December 31, 2020, we had cash, cash equivalents, and investments in marketable securities of $8.2$15.3 billion and $5.2 billion, respectively. Our investment portfolio is comprised of money market funds and marketable debt securities (including U.S. Treasury securities, debt securities of U.S. government agencies and corporate entities, and commercial paper), which are classified as available-for-sale securities. Our primary investment objectives are the preservation of capital and the maintenance of liquidity and our investment policy defines allowable investments based on quality of the institutions and financial instruments designed to minimize risk exposure. Our exposure to interest rate sensitivity is affected by changes in the general level of U.S. interest rates. Our available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase. We generally hold investments in marketable debt securities to maturity to limit our exposure to interest rate risk. Due to the short-term maturities and low risk profiles of our investments, we do not anticipate a significant exposure to interest rate risk. If market interest rates were to increase immediately and uniformly by one percentage point from levels at March 31,September 30, 2021, the net fair value of our interest sensitive marketable securities would not experience a material change.decrease by approximately $144 million.
Foreign Currency Risk
Our revenue generating activities and operations have been primarily denominated in U.S. dollars. As we expand internationally, our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. To help manage the exposure to foreign currency exchange rate fluctuations, we have implemented acash flow hedging and balance sheet hedging program.programs.
Cash Flow Hedging Activities
We hedge foreign currency product sales denominated in Euros, including the use of foreign exchange forward contracts or purchased options. We hedge our cash flow exposures to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. These transactions are designated and qualify as cash flow hedges. Our foreign exchange contracts at September 30, 2021, carried at fair value, had maturities of up to 12 months.
Balance Sheet Hedging Activities
We use foreign currency forward contracts to mitigate foreign currency exchange risk associated with foreign currency-denominated monetary assets and liabilities. Notwithstanding our efforts to mitigate some foreignThese contracts reduce the impact of currency exchange risks, there can be no assurance thatrate movements on our assets and liabilities. As of September 30, 2021, our outstanding balance sheet hedging activities will adequately protect us againstderivatives, carried at fair value, had maturities of less than three months.
We enter into these foreign exchange contracts to hedge forecasted revenue in the risks associated with foreign currency fluctuations.normal course of business and accordingly, they are not speculative in nature. We believe the counterparties to our foreign currency forward contracts are creditworthy multinational commercial banks. While we believe the risk of counterparty nonperformance is not material, a sustained decline in the financial stability of financial institutions as a result of disruption in the financial markets could affect our ability to secure creditworthy counterparties for our foreign currency hedging programs.
Notwithstanding our efforts to mitigate some foreign currency exchange risks, there can be no assurance that our hedging activities will adequately protect us against the risks associated with foreign currency fluctuations. As of March 31,September 30, 2021, a hypothetical adverse movement of 10 percent in foreign currency exchange rates compared to the U.S. dollars across all maturities would have resulted in potential declines in the fair value on our foreign currency forward contracts used in cash flow hedging of approximately $138 million. As of September 30, 2021, a hypothetical adverse movement of 10 percent in foreign currency exchange rates compared to the U.S. dollars across all maturities would have resulted in potential declines in the fair value on our foreign currency forward contracts used in balance sheet hedging of approximately $136$28 million. We expect that any increase or decrease in the fair value of the balance sheet hedging portfolio would be substantially offset by increases or decreases in the underlying exposures being hedged.
Item 4. Controls and Procedures
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of March 31,September 30, 2021. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 or the(the Exchange Act,Act) means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms.
Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of March 31,September 30, 2021, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended March 31,September 30, 2021, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by the collusion of two or more people or by a management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
PART II
Item 1. Legal Proceedings
From time to time, we may be subject to legal proceedings and claims in the ordinary course of business. We are not currently a party to any material legal proceedings.
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. Information regarding risk and uncertainties related to our business appears in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the Securities and Exchange Commission, or the SEC, on February 26, 2021. There have been no material changes from the risk factors previously disclosed in the Annual Report on Form 10-K. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this Quarterly Report on Form 10-Q, including “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the condensed consolidated financial statements and the related notes. If any of the risks actually occur, it could harm our business, prospects, operating results and financial condition and future prospects. In such event, the market price of our common stock could decline and you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. This Quarterly Report on Form 10-Q also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Quarterly Report.
Item 6. Exhibits
The Exhibits listed below are filed or incorporated by reference as part of this Form 10-Q.
| | | | | | | | |
| Exhibit No. | | Exhibit Index |
| | |
| 10.1*† | | |
| 10.2*† | | |
10.2*† | | |
10.3*† | | |
10.4*† | | |
| | |
| 31.1* | | |
| 31.2* | | |
| 32.1+ | | |
| 101.INS* | | XBRL Instance Document - The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
| 101.SCH* | | XBRL Taxonomy Extension Schema Document |
| 101.CAL* | | XBRL Taxonomy Extension Calculation Document |
| 101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB* | | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE* | | XBRL Taxonomy Extension Presentation Link Document |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101.*) |
| | | | | |
| † | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission. |
+
| The certification furnished in Exhibit 32.1 hereto is deemed to accompany this Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certification will not be deemed to be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates it by reference. |
† | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission. |
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | | MODERNA, INC. |
| | | |
| Date: | | By: | /s/ Stéphane Bancel |
May 6,November 4, 2021 | | | |
| | | Stéphane Bancel |
| | | Chief Executive Officer and Director |
| | | (Principal Executive Officer) |
| | | |
| Date: | | By: | /s/ David W. Meline |
May 6,November 4, 2021 | | | |
| | | David W. Meline |
| | | Chief Financial Officer |
| | | (Principal Financial Officer) |
| | | |