2
EXPLANATORY NOTEFORWARD-LOOKING STATEMENTS
XG Sciences, Inc., a Michigan corporation (the “Company”) is filingThe information in this Amendment No. 1 (this “Amendment”) to its AnnualQuarterly Report on Form 10-Q forcontains “forward-looking statements” and information within the quarter ended March 31, 2020 (the “Original 10-Q”), as originally filed withmeaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Exchange Commission (the “SEC”) on June 17, 2020, solely to disclose that the Company had filed the Original 10-Q after the May 14, 2020 deadline applicable to the Company for the filing of a Form 10-Q in reliance on the 45-day extension provided by an order issued by the SEC pursuant to Section 3621E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) (Release No. 34-88465 dated March 25, 2020)relating to XG Sciences, Inc., a Michigan corporation and its subsidiary, XG Sciences IP, LLC, a Michigan limited liability company (collectively referred to as “we”, “us”, “our”, “XG Sciences”, “XGS”, or the “Company”), which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our strategy, future operations, future financial position, future revenue, projected costs, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve known and unknown risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the risks set forth beginning on page 12 under the section entitled “Risk Factors” in our annual report on Form 10-K as filed with the Securities and Exchange Commission (the “Order”“SEC”). on April 29, 2020.
3
| XG SCIENCES, INC. |
| CONDENSED CONSOLIDATED BALANCE SHEETS |
| (unaudited) |
| June 30, 2020 | December 31, 2019 | |||||||
| ASSETS | (Unaudited) | |||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 667,664 | $ | 1,129,702 | ||||
| Accounts receivable, less allowance for doubtful accounts of $157,965 at June 30, 2020 and $179,600 at December 31, 2019 | 108,125 | 72,227 | ||||||
| Inventories | 866,792 | 891,587 | ||||||
| Other current assets | 289,590 | 334,493 | ||||||
| Total current assets | 1,932,171 | 2,428,009 | ||||||
| Property, Plant and Equipment, Net | 3,268,725 | 3,676,142 | ||||||
| Lease Deposit | 59,440 | 77,544 | ||||||
| Intangible Assets, Net | 763,215 | 753,862 | ||||||
| Right of Use Asset | 1,325,549 | 1,606,443 | ||||||
| TOTAL ASSETS | 7,349,100 | 8,542,000 | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | 815,067 | 634,564 | ||||||
| Other current liabilities | 272,323 | 238,554 | ||||||
| Deferred revenue | 12,757 | — | ||||||
| Current portion of lease liabilities | 484,430 | 520,197 | ||||||
| Total current liabilities | 1,584,577 | 1,393,315 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Long-term portion of lease liabilities | 962,482 | 1,183,872 | ||||||
| Convertible notes | 247,104 | — | ||||||
| SBA Loan payable | 802,069 | — | ||||||
| Long term debt - Dow | 9,396,644 | 8,111,610 | ||||||
| Total long-term liabilities | 11,408,299 | 9,295,482 | ||||||
| TOTAL LIABILITIES | 12,992,876 | 10,688,797 | ||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Series A convertible preferred stock, 3,000,000 shares authorized, 1,890,354 shares issued and outstanding, liquidation value of $22,684,248 at June 30, 2020 and December 31, 2019 | 22,307,480 | 22,307,480 | ||||||
| Series B Preferred stock, 1,500,000 shares authorized, 137,043 and zero shares issued and outstanding, liquidation value of $1,096,344 at June 30, 2020 and zero at December 31, 2019 | 1,079,400 | — | ||||||
| Common stock, no par value, 25,000,000 shares authorized, 3,887,400 and 4,024,443 shares issued and outstanding at June 30, 2020 and December 31, 2019, respectively | 31,305,532 | 32,351,876 | ||||||
| Additional paid-in capital | 9,421,443 | 8,774,975 | ||||||
| Accumulated deficit | (69,757,631 | ) | (65,581,128 | ) | ||||
| Total stockholders’ equity (deficit) | (5,643,776 | ) | (2,146,797 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | 7,349,100 | 8,542,000 | ||||||
On April 23, 2020,See notes to unaudited condensed consolidated financial statements.
4
| XG SCIENCES, INC. |
| CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited) - USD ($) |
| For the Three Months Ended June 30, | For the Six Months Ended June 30, | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| REVENUE | ||||||||||||||||
| Product Sales | 81,146 | 247,069 | 248,209 | 1,104,346 | ||||||||||||
| Total Revenues | $ | 81,146 | $ | 247,069 | $ | 248,209 | $ | 1,104,346 | ||||||||
| COST OF GOODS SOLD | ||||||||||||||||
| Direct costs | 41,828 | 196,411 | 145,398 | 877,564 | ||||||||||||
| Unallocated manufacturing expenses | 412,802 | 599,745 | 1,071,715 | 1,093,214 | ||||||||||||
| Cost of Goods Sold | 454,630 | 796,156 | 1,217,113 | 1,970,778 | ||||||||||||
| GROSS LOSS | (373,484 | ) | (549,087 | ) | (968,904 | ) | (866,432 | ) | ||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research & Development Expense | 184,527 | 384,767 | 470,254 | 770,013 | ||||||||||||
| Sales, General & Administrative Expense | 1,076,265 | 1,486,380 | 2,368,400 | 2,907,301 | ||||||||||||
| Total Operating Expenses | 1,260,792 | 1,871,147 | 2,838,654 | 3,677,314 | ||||||||||||
| OPERATING LOSS | (1,634,276 | ) | (2,420,234 | ) | (3,807,558 | ) | (4,543,746 | ) | ||||||||
| OTHER EXPENSE | ||||||||||||||||
| Interest expense, net | (190,800 | ) | (78,277 | ) | (368,808 | ) | (154,941 | ) | ||||||||
| Noperating Expense | (136 | ) | — | (136 | ) | — | ||||||||||
| Total Other Expense | (190,936 | ) | (78,277 | ) | (368,944 | ) | (154,941 | ) | ||||||||
| Net Loss | $ | (1,825,212 | ) | $ | (2,498,511 | ) | $ | (4,176,502 | ) | $ | (4,698,687 | ) | ||||
| WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING - | ||||||||||||||||
| Basic and diluted (in shares) | 4,001,778 | 3,990,038 | 4,013,111 | 3,883,053 | ||||||||||||
| NET LOSS PER SHARE - Basic and diluted (in dollars per share) | $ | (0.46 | ) | $ | (0.63 | ) | $ | (1.04 | ) | $ | (1.21 | ) | ||||
See notes to unaudited condensed consolidated financial statements.
5
| XG SCIENCES, INC. |
| CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY |
| For the Six Months Ended June 30, 2019 - USD ($) | ||||||||||||||||||||||||||||||||||||
| Preferred stock A | Preferred stock B | Common stock | Additional paid-in capital | Accumulated deficit | ||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Amount | Amount | Total | ||||||||||||||||||||||||||||
| Balances, December 31, 2018 | 1,890,354 | $ | 22,307,480 | 3,760,268 | $ | 30,268,476 | $ | 8,101,923 | $ | (55,687,160 | ) | $ | 4,990,719 | |||||||||||||||||||||||
| Stock issued for cash | 51,250 | 410,000 | 410,000 | |||||||||||||||||||||||||||||||||
| Stock issuance fees and expenses | (16,000 | ) | (16,000 | ) | ||||||||||||||||||||||||||||||||
| Transition adjustment for adoption of new lease standard | (116,319 | ) | (116,319 | ) | ||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 20,000 | 88,288 | 108,288 | |||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,200,176 | ) | (2,200,176 | ) | |||||||||||||||||||||||||
| Balances, March 31, 2019 | 1,890,354 | $ | 22,307,480 | — | $ | — | 3,811,518 | $ | 30,682,476 | $ | 8,190,211 | $ | (58,003,655 | ) | $ | 3,176,512 | ||||||||||||||||||||
| Stock issued for cash | 200,425 | 1,603,400 | 1,603,400 | |||||||||||||||||||||||||||||||||
| Stock issuance fees and expenses | (4,000 | ) | (4,000 | ) | ||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 20,000 | 90,368 | 110,368 | |||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,498,511 | ) | (2,498,511 | ) | |||||||||||||||||||||||||
| Balances, June 30, 2019 | 1,890,354 | $ | 22,307,480 | — | $ | — | 4,011,943 | $ | 32,301,876 | $ | 8,280,579 | $ | (60,502,166 | ) | $ | 2,387,769 | ||||||||||||||||||||
| For the Six Months Ended June 30, 2020 - USD ($) | ||||||||||||||||||||||||||||||||||||
| Preferred stock A | Preferred stock B | Common stock | Additional paid-in capital | Accumulated deficit | ||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Amount | Amount | Total | ||||||||||||||||||||||||||||
| Balances, December 31, 2019 | 1,890,354 | $ | 22,307,480 | $ | — | $ | — | 4,024,443 | $ | 32,351,876 | $ | 8,774,975 | $ | (65,581,128 | ) | $ | (2,146,797 | ) | ||||||||||||||||||
| Stock-based compensation expense | 25,000 | 85,276 | 110,276 | |||||||||||||||||||||||||||||||||
| Warrants issued with Dow financing | 71,276 | 71,276 | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | $ | (2,351,291 | ) | (2,351,291 | ) | ||||||||||||||||||||||||
| Balances, March 31, 2020 | 1,890,354 | $ | 22,307,480 | — | $ | — | 4,024,443 | $ | 32,376,876 | $ | 8,931,527 | $ | (67,932,419 | ) | $ | (4,316,536 | ) | |||||||||||||||||||
| Common stock exchanged for Series B stock | 137,043 | 1,096,344 | (137,043 | ) | (1,096,344 | ) | — | |||||||||||||||||||||||||||||
| Stock issuance fees and expenses | (16,944 | ) | (16,944 | ) | ||||||||||||||||||||||||||||||||
| Value of exchange rights | 268,431 | 268,431 | ||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 25,000 | 221,485 | 246,485 | |||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (1,825,212 | ) | (1,825,212 | ) | |||||||||||||||||||||||||
| Balances, June 30, 2020 | 1,890,354 | $ | 22,307,480 | 137,043 | $ | 1,079,400 | 3,887,400 | $ | 31,305,532 | $ | 9,421,443 | $ | (69,757,631 | ) | (5,643,776 | ) | ||||||||||||||||||||
See notes to unaudited condensed consolidated financial statements
6
| XG Sciences, Inc. |
| CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS |
| (unaudited) |
| For the Six Months Ended June 30, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Net loss | $ | (4,176,502 | ) | $ | (4,698,687 | ) | ||
| Depreciation | 432,026 | 388,110 | ||||||
| Amortization of intangible assets | 36,093 | 31,716 | ||||||
| Provision for bad debts | 0 | 26,000 | ||||||
| Stock-based compensation expense | 356,760 | 218,656 | ||||||
| Non-cash interest expense | 369,840 | 39,584 | ||||||
| Changes in current assets and liabilities: | ||||||||
| Accounts receivable | (35,898 | ) | 563,515 | |||||
| Inventory | 24,795 | (305,361 | ) | |||||
| Other current and non-current assets | 86,744 | (46,002 | ) | |||||
| Accounts payable and other liabilities | 227,028 | (353,563 | ) | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (2,679,114 | ) | (4,136,032 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchases of property and equipment | (24,609 | ) | (287,746 | ) | ||||
| Purchases of intangible assets | (45,445 | ) | (74,127 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (70,054 | ) | (361,873 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of common stock | — | 2,013,400 | ||||||
| Common stock issuance fees and expenses | — | (20,000 | ) | |||||
| Proceeds from (repayment of) long term debt- Dow | 1,000,000 | (638,220 | ) | |||||
| Proceeds from convertible notes | 550,000 | — | ||||||
| Convertible notes issuance fees and expenses | (63,801 | ) | — | |||||
| Proceeds from SBA Loan and EIDL Grant | 800,795 | — | ||||||
| Repayments of capital lease obligations | — | (15,528 | ) | |||||
| Exchange Rate value | 136 | |||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,287,130 | 1,339,652 | ||||||
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | (462,038 | ) | (3,158,253 | ) | ||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH, BEGINNING OF PERIOD | 1,129,702 | 4,893,974 | ||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH, END OF PERIOD | 667,664 | 1,735,721 | ||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | 77 | 64,626 | ||||||
| Warrants issued with Dow financing | 71,276 | — | ||||||
See notes to unaudited condensed consolidated financial statements
7
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – NATURE OF BUSINESS AND BASIS OF PRESENTATION
XG Sciences, Inc., a Michigan company located in Lansing, Michigan and its subsidiary, XG Sciences IP, LLC (collectively referred to as “we”, “us”, “our”, or the Company“Company”) manufactures graphene nanoplatelets made from graphite, using two proprietary manufacturing processes to split natural flakes of crystalline graphite into very small and thin particles, which we sell as xGnP® graphene nanoplatelets. We sell our nanoplatelets in the form of bulk powders or dispersions to other companies for use as additives to make composite and other materials with specialty engineered characteristics.
Basis of Presentation
The accompanying interim condensed consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for interim financial information and the instructions to Form 10-Q and do not include all of the information and footnotes required by GAAP for complete financial statements. All intercompany transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in our annual audited consolidated financial statements and accompanying notes have been condensed or omitted in these interim condensed consolidated financial statements. Accordingly, the unaudited condensed consolidated financial statements included herein should be read in conjunction with the audited consolidated financial statements for the year ended December 31, 2019, as filed a Current Reportwith the Securities and Exchange Commission (“SEC”) on Form 8-K10-K on April 29, 2020.
The results of operations presented in this quarterly report are not necessarily indicative of the results of operations that may be expected for any future periods. In the opinion of management, these unaudited condensed consolidated financial statements include all adjustments and accruals, consisting only of normal recurring adjustments that are necessary for a fair statement of the results of all interim periods reported herein.
Use of Estimates
The preparation of our condensed consolidated financial statements in conformity with GAAP requires us to indicate its intention to rely onmake estimates, judgments and assumptions that affect the Order for such extension. Consistentreported amounts of assets, liabilities, revenue, and expenses, together with the Company’s statements madeamounts disclosed in the Form 8-K, the Company was unable to file the Original 10-Q priorrelated notes to the prescribedfinancial statements. Actual results and outcomes may differ from our estimates, judgments, and assumptions. Significant estimates, judgments and assumptions used in these condensed consolidated financial statements include, but are not limited to, those related to revenue, accounts receivable and related allowances, inventory valuations, contingencies, useful lives, and recovery of long-term assets, including intangible assets, income taxes, and the fair value of stock-based compensation. These estimates, judgments, and assumptions are reviewed periodically and the effects of material revisions in estimates are reflected in the financial statements prospectively from the date of the change in estimate.
8
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Liquidity
We have historically incurred losses from operations, and we may continue to generate negative cash flows as we implement our business plan. Our consolidated financial statements are prepared using US GAAP as applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
As of May 14, 2020 filing date because17, 2021, we had cash on hand of $x,xxx,xxx. Due to lower-than-expected revenue in 2019 and the Company’s operations have been impacted by the novel coronavirus disease 2019 ( “COVID-19”). Because of thecurrent COVID-19 global pandemic impacting both the Company’sour operations and that of itsour customers, management was required to devote additional time and resources toManagement has taken several steps to ensure that the Company waswe are able to fund its operations. At the time the , the Company was engaged in restructuring itsour operations from existing cash on hand, operating cash flows, additional borrowings, and restructured debt obligations.
In March of 2020, we restructured our organization by reducing headcount by 45%53%, by furloughing substantially all manufacturing employees, and by implementing temporary salary reductions ranging from 15-20%. Although we have re-employed key employees since the reductions and furloughs, annual payroll and related costs have been reduced by 38%. In April 2020, we furloughed additional employees in our R&D and Engineering departments. Salary reductions remained in place at the discretion of the Compensation Committee of the Board of Directors until February 1, 2021.
In early April of 2020, we applied for relief under the Coronavirus Aid, Relief and Economic Security Act (CARES) by applying with the Small Business Administration (SBA) for an Economic Injury Disaster Loan (“EIDL”) and by submitting an application to an SBA lender bank, PNC, for a Paycheck Protection Plan (“PPP”) loan. On April 18, 2020, we received an approved and fully executed PPP Term Note for $825,200 with a term of two years, a six-month repayment deferral period, and an annual rate of interest of 1%, with a potential for some or all of the loan to be forgiven, dependent upon use of the loan proceeds. On April 20, 2020, we received the $825,200 of proceeds under the PPP loan which was subsequently reduced by $34,405 to $790,795 due to additional SBA guidance regarding 1099 income paid. On October 6, 2020, the Paycheck Protection Flexibility Act of 2020 extended the deferral expiration period for loan repayments to either the date that the SBA remits the borrower’s loan forgiveness or to 24 weeks after the receipt of proceeds plus 10 months or July 3, 2021. We plan to apply for loan forgiveness before the end of the deferred expiration date. The outstanding principal that is not forgiven converts to an amortizing term loan. On May 17, 2020, the SBA issued us a decline letter for the EIDL for which reconsideration has not been granted.
On April 23, 2020, we entered into an amended and in seeking additional financingrestated Draw Loan Note and Agreement and related transaction documents (collectively, the “Amended Dow Facility”) with the Dow Chemical Company to amend the terms of our current loan facility to allow us to structure a private placement of units (“Units”) comprised (in part) of subordinated, secured convertible notes (“Convertible Notes” and such offering, the “Unit Offering”), to support continuing operations. The reduction in staffing had an impactongoing cash needs. In the Amended Dow Facility, the Company and Dow agreed to 1) extend the term of such loan facility by two years to December 1, 2023, 2) significantly reduce any required prepayment to Dow from the proceeds of new equity or equity-linked financings from the current 30-50% prepayment requirement on the pre-existing Dow Facility to a 10% prepayment requirement in the Amended Dow Facility, which does not begin until after we have raised an additional $7 million in equity or equity-linked capital from the date of the amendment, 3) capitalize all interest payable until such time as we have recorded GAAP revenue of at least $2 million for two consecutive calendar quarters, 4) increase the rate of interest to 6.5% per annum from 5% in the pre-existing Dow Facility, and 5) allow for a subordinated security interest to be granted to new investors in the Unit Offering.
Immediately after the execution of the transaction documents related to the Amended Dow Facility, we commenced the Unit Offering in a private placement to accredited investors. The Unit Offering is comprised of the Convertible Notes and a right to exchange two shares of previously issued Common Stock of the Company for two shares of Series B Convertible Preferred Stock of the Company (“Series B Preferred Stock”) for every $8.00 invested in the Unit Offering (the “Exchange Rights”). The Convertible Notes are secured by a junior security interest in all the assets of the Company, bear an interest rate of 7.5% per annum and mature on December 31, 2024. Each investor’s Exchange Rights are exercisable for a period of thirty (30) days after acceptance by the Company of a fully executed subscription agreement.
At the option of each holder, the Convertible Notes are convertible into either i) Series B Preferred Stock at a conversion price of $8.00/share; or ii) any other form of preferred or common stock (“Subsequent Stock”) issued by the Company at a conversion price per share equal to 80% of the purchase price per share at which such Subsequent Stock is sold (or if the value per share is fixed, 125% of the number of shares that might otherwise be issuable).
9
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
If and when we raise at least $15 million of equity capital (excluding capital raised in this Unit Offering), the Convertible Notes will be automatically converted into whichever of the following equity securities would result in the greatest number of shares of Common Stock being issued to the holders on an “as-if-converted” basis at such time: (i) Series B Preferred Stock at a note conversion price of $8.00/share; or (ii) Subsequent Stock at a note conversion price per share equal to 80% of the purchase price per share at which such Subsequent Stock is sold (or if the value per share is fixed, 125% of the number of shares that might otherwise be issuable);
Each share of Series B Preferred Stock has an original issue price of $8.00 per share (the “Series B Original Issue Price”) and a liquidation preference of $8.00 per share, with both the Series B Original Issue Price and the liquidation preference per share subject to adjustment for stock splits, recapitalizations, and the like. The Series B Preferred Stock will be senior to the Company’s businessCommon Stock and its abilitypari-passu with the Series A Preferred Stock in terms of right of repayment in a liquidation.
The Series B Preferred Stock has full ratchet antidilution protection that provides that each share of Series B Preferred Stock outstanding may be converted by an Investor at any time into that number of shares of Common Stock determined by dividing the then current Series B Original Issue Price by the applicable Conversion Price (as defined below) with the resulting fraction equal to timely file its annual reportthe “Series B Conversion Rate”. The total number of shares of Common Stock issuable will be equal to the number of shares of Series B Preferred Stock being converted multiplied by the Series B Conversion Rate. The “Conversion Price” is, at any time, the price per share equal to the lesser of a) the Series B Original Issue Price per share and b) the lowest price per share at which the Company has sold equity or equity-linked securities (other than customary exclusions) at any future date while any shares of the Series B Preferred Stock remain outstanding. The Series B Original Issue Price and Conversion Price in effect at any time are also subject to proportional adjustment for share splits, share dividends, recapitalizations, and the yearlike.
On April 23, 2020, certain members of our Board of Directors and their affiliates purchased $550,000 of convertible notes. In addition, these Board members and their affiliates purchased $800,000, $1,801,504, and $515,016 of convertible notes in the quarters ended September 30, 2020, December 31, 2020 and March 31, 2021. In total, the Board members and their affiliates purchased a total of $3,666,520 of convertible notes through March 31, 2021 from the Company. Non-Board affiliated third parties purchased $700,160 and $2,073,600 of convertible notes in the quarters ended December 31, 2020 (the “10-K”)and March 31, 2021 from the Company for a total purchase of $2,773,760. Total proceeds from the sale of Convertible Notes as of March 31, 2021 was $6,440.280.
Taking into consideration our current cash on hand, we estimate that we will need to raise approximately $500,000 - $1,000,000 of additional capital to continue our operations for the next twelve months in a minimal to no revenue growth environment. Our continuation as a going concern is dependent upon continued financial support from our shareholders, our ability to obtain necessary equity and/or debt financing to continue operations, and growth in revenue from operations. These factors raise substantial doubt regarding our ability to continue as a going concern. We cannot make any assurances that additional financings will be available to us and, if available, completed on a timely basis, on acceptable terms or at all. If we are unable to complete an equity or debt offering, or otherwise obtain sufficient financing when and if needed, it would negatively impact our business and operations. It could also lead to the reduction or suspension of our operations and ultimately force us to cease our operations.
There has been no public market for our securities and a public market may never develop, or, if any market does develop, it may not be sustained. Our Common Stock is not currently quoted on or traded on any exchange or on any over-the-counter market. In the event we are unable to fund our operations from existing cash on hand, operating cash flows, additional borrowings or raising equity capital, we may be forced to reduce our expenses, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
10
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Inventory
The following amounts were included in inventory at the end of the period:
| June 30, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Raw Materials | $ | 54,461 | $ | 68,784 | ||||
| Consumables | 70,103 | 70,103 | ||||||
| Finished Goods | 742,228 | 752,700 | ||||||
| Total | $ | 866,792 | $ | 891,587 | ||||
Recent Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, “Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes,” which simplifies the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of and simplify GAAP for other areas of Topic 740 by clarifying and amending existing guidance. ASU 2019-12 is effective for us for annual periods beginning January 1, 2021. We are currently reviewing the provisions of this new pronouncement, and the impact, if any, the adoption of this guidance has on our financial position and results of operations.
In January 2020, the FASB issued ASU 2020-01, “Investments—Equity Securities (Topic 321)”, “Investments—Equity Method and Joint Ventures (Topic 323)”, and “Derivatives and Hedging (Topic 815): Clarifying the Interactions between Topic 321, Topic 323, and Topic 815”, which clarifies that an entity should consider observable transactions when either applying or discontinuing the equity method of accounting for the purposes of applying the measurement alternative in similarlyaccordance with Topic 321. ASU 2020-01 clarifies that for certain forward contracts or purchased options to fileacquire investments, an entity should not consider whether, upon settlement of the Original 10-Qforward contract or exercise of the purchased option, the underlying securities would be accounted for under the equity method or the fair value option. ASU 2020-01 is effective for us for annual periods beginning January 1, 2021. Early adoption is permitted. We are currently reviewing the provisions of this new pronouncement and the impact, if any, the adoption of this guidance has on our financial position and results of operations.
In March 2020, the FASB issued ASU 2020-04, “Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting,” which provides optional guidance and expedients for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. The amendments are intended to ease the potential burden in accounting for (or recognizing the effects of) reference rate reform on financial reporting. The amendments in this update are elective and are effective upon issuance. We are currently assessing whether and how we will elect to apply ASU 2020-04.
Except for the standards discussed above, we believe there have been no new accounting pronouncements effective or not yet effective that have significance, or potential significance, to our Consolidated Financial Statements.
11
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 –FINANCING AGREEMENT
Dow Facility
In December 2016, we entered into the Dow Facility which provided us with up to $10 million of secured debt financing at an interest rate of 5% per year, drawable at our request under certain conditions. Under the original terms, the loan matured on December 1, 2021. In addition, after we raised a cumulative amount of equity capital exceeding $15 million, we were required to prepay an amount equal to 30% of the amount raised over $15 million, but less than $25 million. We began these prepayments on equity raised as of September 10, 2018. Interest was payable beginning January 1, 2017 although we elected, per the loan documents, to capitalize the interest as part of the outstanding debt through January 1, 2019. Beginning April 1, 2019, current interest was payable in cash on the first day of following the quarter. Dow received warrant coverage of one share of common stock for each $40 in loans received by us, equating to 20% warrant coverage, with an exercise price of $8.00 per share for the warrants issued at closing of the initial $2 million draw. After the initial closing, the strike price of future warrants issued is subject to adjustment if we sell shares of common stock at a lower price. As of June 30, 2020, we have issued 250,000 warrants to Dow, which are exercisable on or before the expiration date of December 1, 2023.
On April 23, 2020, we entered into an amended and restated Draw Loan Note and Agreement and related transaction documents (collectively, the “Amended Dow Facility”) whereby the Company and Dow agreed to 1) extend the term of such loan facility by two years to December 1, 2023, 2) reduce required prepayment to Dow from the proceeds of new equity or equity-linked financings from the current 30-50% prepayment requirement on the pre-existing Dow Facility to a 10% prepayment requirement in the Amended Dow Facility, which does not begin until after we have raised an additional $7 million in equity or equity-linked capital from the date of the amendment, 3) capitalize all interest payable until such time as we have recorded GAAP revenue of at least $2 million for two consecutive calendar quarters, 4) increase the rate of interest to 6.5% per annum from 5% in the pre-existing Dow Facility, and 5) allow for a subordinated security interest to be granted to new investors in the Unit Offering.
The warrants meet the criteria for classification within stockholders’ equity. Proceeds were allocated between the debt and the warrants at their relative fair value on the date of issue. The total debt discount on the Dow Facility was approximately $747,000. This debt discount is being amortized to interest expense using the effective interest method over the term of the loans using an average effective interest rate of 7.9%. During the six months ended June 30, 2020, we recognized $366,309 of amortization expense consisting of $264,835 of interest expense accrued and $91,474 of amortization from debt discount accretion related to the Dow Facility warrants. As of June 30, 2020, the Dow Facility has a carrying value of $9,396,645.
The Dow Facility entitles Dow to appoint an observer to our Board. Dow will maintain this observation right until the amount of principal and interest outstanding under the Dow Facility is less than $5 million.
12
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – STOCK WARRANTS ACCOUNTED FOR AS EQUITY INSTRUMENTS
The following table summarizes the warrants (including the warrants previously accounted for as derivatives) outstanding on June 30, 2020, which are accounted for as equity instruments, all of which are exercisable:
| Date Issued | Expiration Date | Indexed Stock | Exercise Price | Warrants | ||||||||
| 10/8/2012 | 10/8/2027 | Common | $ | 12.00 | 5,000 | |||||||
| 01/15/2014 - 12/31/2014 | 1/15/2024 | Series A Convertible Preferred | $ | 12.00 | 972,720 | |||||||
| 04/30/2015 - 05/26/2015 | 4/30/2022 | Common | $ | 16.00 | 218,334 | |||||||
| 6/30/2015 | 6/30/2022 | Common | $ | 16.00 | 6,563 | |||||||
| 12/31/2015 | 12/31/2020 | Common | $ | 8.00 | 20,625 | |||||||
| 3/31/2016 | 3/31/2021 | Common | $ | 10.00 | 10,600 | |||||||
| 4/30/2016 | 4/30/2021 | Common | $ | 10.00 | 895 | |||||||
| 12/14/2016 | 12/1/2023 | Common | $ | 8.00 | 50,000 | |||||||
| 7/18/2017 | 12/1/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 9/22/2017 | 12/1/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 12/4/2017 | 12/1/2023 | Common | $ | 8.00 | 25,000 | |||||||
| 7/8/2019 | 12/1/2023 | Common | $ | 8.00 | 50,000 | |||||||
| 11/11/2019 | 12/1/2023 | Common | $ | 8.00 | 50,000 | |||||||
| 2/12/2020 | 10/1/2023 | Common | $ | 8.00 | 25,000 | |||||||
| Total | 1,484,737 | |||||||||||
Each warrant indexed to Series A Convertible Preferred Stock is currently exercisable and exchangeable into 1.875 shares of common stock. On a common stock equivalent basis, the exercise price would equal $6.40/common share.
NOTE 5 – STOCKHOLDERS’ EQUITY (DEFICIT)
Series B Convertible Preferred Stock
As of June 30, 2020, and December 31, 2019, the Company was authorized to issue up to 1,500,000 shares of Series B Preferred Stock, of which 137,500 and zero were issued and outstanding as of June 30, 2020 and December 31, 2019. On March 31, 2021, the Company was authorized to issue up to 3,750,000 shares of Series B Preferred Stock. Exchange rights received with the purchase of each unit of the Convertible Notes Unit offering dated April 23, 2020, allow for an exchange of two shares of Common Stock for two shares of Series B Preferred Stock. Exchange rights exercised resulted in the issuance of Series B Preferred stock as listed below.
| Series B | ||||
| For the three | Preferred | |||
| months ended | Stock Issued | |||
| June 30, 2020 | 137,043 | |||
| September 30, 2020 | 200,457 | |||
| December 31, 2020 | 309,540 | |||
| March 31, 2021 | 854,354 | |||
| Total | 1,501,394 | |||
Each share of the Series B Preferred, is convertible at any time, at the option of the holder, into one share of common stock. As of March 31, 2021, conversion of the Promissory Notes into Series B Preferred Stock would result in an additional 1,501,394 shares of Series B Preferred stock.
The Series B Preferred also contains typical anti-dilution provisions that provide for adjustment of the conversion price to reflect stock splits, stock dividends, or similar events. Each share of Series B Preferred is subject to mandatory conversion into common stock at the then-effective Series B conversion rate upon the public listing by the Company of its common stock on a Qualified National Exchange. However, the Series B Preferred is not subject to the mandatory conversion until all outstanding Convertible Securities are also converted into common stock. The Series B Preferred ranks senior to all other equity or equity equivalent securities of the Company other than those securities which are explicitly senior or pari passu in rights and liquidation preference to the Series B Preferred and pari passu with the Company’s Series A Preferred.
On April 27, 2020, the Company filed the Second Amended and Restated Certificate of Designations of the Series B Preferred Stock in connection with the Unit Offering and the Amended Dow Facility, lowering the liquidation preference from $16.00 per share to $8.00 per share, and lowering the conversion price from $16.00 per share to $8.00 per share, among other things. The Company also adjusted certain provisions to harmonize the rights of the Series B Preferred with the rights in the Series A Certificate of Designations. On December 28, 2020, the Board of Directors and a majority of the holders of Series A and Series B Preferred Stock consented to Amendments to the Second Amended and Restated Certificate of Designation of the Series A Convertible Preferred Stock and the Series B Convertible Preferred Stock by adapting the Third Amended and Restated Certificate of Designations of Series A and of Series B Convertible Preferred Stock. The Third Amended and Restated Certificate of Designations continue to allow for liquidation preference prior and in preference to any distribution of any of the assets of the Corporation to the holders of junior securities and, as amended, after the payment of all preferential amounts required to be paid to the holders of the Series A Preferred Stock, Series B Preferred Stock and other senior securities, the remaining assets of the corporation available for distribution to its stockholders shall be distributed among the holders of junior securities, including the Common Stock. On March 31, 2021, subsequent to consent by the Board of Directors and a majority of the holders of Series B Preferred Stock, the Company filed the Fourth Amended and Restated Certificate of Designation of the Series B Convertible Preferred Stock increasing the number of authorized shares from 1,250,000 to 3,750,000.
The Series A Preferred and Series B Preferred are not redeemable for cash and the Company concluded that they are more akin to equity-type instruments than debt-type instruments. Accordingly, the embedded conversion option in each agreement is clearly and closely related to an equity-type host and the conversion option does not require classification and measurement as a derivative financial instrument. Therefore, the securities meet the conditions for stockholders’ equity classification.
13
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – EQUITY INCENTIVE PLAN
On September 30, 2019 and September 30, 2018, the Company granted each Board member 2,500 stock options and 2,500 shares of restricted stock for their Board services. The options were granted at a price of $8.00 per share and vest ratably over a four-year period beginning on the one-year anniversary. The options had an aggregate grant date fair value of $38,295 and $29,580 on September 30, 2019 and September 30, 2018, respectively. The restricted stock issued to the Board members has an aggregate fair value of $260,000 and vests ratably in arrears over four quarters on the last day of each fiscal quarter following the grant date. As of June 30, 2020, and 2019, 32,500 and 20,000 of the 32,500 shares of restricted stock issued had vested, resulting in compensation expense of $25,000 and $20,000 for the three months ended June 30, 2020 and June 30, 2019, respectively.
During the three months ended June 30, 2020, the Company granted 138,734 employee stock options. The options were granted at a price of $8.00 per share and had an aggregate grant date fair value of $329,850. The options vest one-third immediately upon the grant date and one-third on each the first and second anniversary dates. The fair value of the options granted was estimated on the date of the grant using the Black Scholes option-pricing model using the following assumptions: Stock price $8.00, Expected Term: 4.51 years, Volatility: 35.36%, Risk free rate: .29%, Dividend rate: 0%.
The following table shows the stock options activity as of June 30, 2020 is as follows:
| Weighted | ||||||||
| Number | Average | |||||||
| Of | Exercise | |||||||
| Options | Price | |||||||
| Options outstanding at March 31, 2020 | 774,625 | $ | 8.00 | |||||
| Changes during the period: | ||||||||
| Expired | (42,500 | ) | 8.00 | |||||
| New Options Granted – at market price | 138,734 | 8.00 | ||||||
| Options outstanding at June 30, 2020 | 870,859 | $ | 8.00 | |||||
| Options exercisable at June 30, 2020 | 600,881 | $ | 8.00 | |||||
14
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – LEASES
Right of Use Asset and Leased Liability:
Estimated Lease Life – Lease term through December 2022
| Three Months | Three Months | |||||||
| Ended June 30, | Ended June 30, | |||||||
| 2019 | 2020 | |||||||
| Right-of-use lease assets- operating as of prior qtr-end | $ | 1,871,366 | $ | 1,486,112 | ||||
| Less: Accumulated amortization | (104,097 | ) | (160,563 | ) | ||||
| Right-of-use lease assets- operating as of current qtr-end | $ | 1,767,269 | $ | 1,325,549 | ||||
| Lease liability-operating as of prior qtr-end | $ | 1,981,795 | $ | 1,579,531 | ||||
| Less: Accumulated Amortization | (108,443 | ) | (132,619 | ) | ||||
| Lease liability operating-as of current qtr-end | $ | 1,873,352 | $ | 1,446,912 | ||||
| Operating lease expense for the three months ended current qtr-end date | $ | 150,557 | $ | 160,072 | ||||
| Actual remaining lease payments, current qtr-end date | $ | 2,289,714 | $ | 1,638,479 | ||||
| Present value of remaining payments, current qtr-end date | $ | 1,873,453 | $ | 1,446,912 | ||||
| Leases Three | Leases Three | |||||||
| Supplemental cash flow information related to leases: | Months Ended | Months Ended | ||||||
| June 30, 2019 | June 30, 2020 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows from operating leases | $ | 154,903 | $ | 122,206 | ||||
| Maturities of leases liabilities were as follows: | ||||||||
| Year ending December 31, 2019 | $ | 325,341 | ||||||
| Year ending December 31, 2020 | 660,387 | $ | 226,755 | |||||
| Year ending December 31, 2021 | 660,438 | 737,947 | ||||||
| Year ending December 31, 2022 | 643,548 | 673,777 | ||||||
| Total Lease payments | $ | 2,289,714 | $ | 1,638,479 | ||||
| Less imputed interest | (416,261 | ) | (191,567 | ) | ||||
| Total | $ | 1,873,453 | $ | 1,446,912 | ||||
| Weighted average remaining lease term- operating leases ( in months) | 40.0 | 24.7 | ||||||
| Weighted average discount rate- operating leases (annual) | 9.98 | % | 9.98 | % | ||||
15
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – RELATED PARTY TRANSACTIONS
Effective June 23, 2020, we signed a Restated and Amended Non-Exclusive License Agreement with Michigan State University (“MSU”), in which we converted our exclusive rights to non-exclusive rights in exchange for changes in the financial terms while adding additional technologies to the license. During the three and six months ended June 30, we recorded licensing expenses of zero in 2020 and we recorded licensing expense of $12,500 per quarter in 2019.
During the three months ended June 30, 2020 we did not issue any Series A Preferred stock and we issued 137,043 shares of Series B Preferred stock in exchange for 137,043 shares of Common Stock, as per the terms of the April 23, 2020 Unit Offering. The Units were purchased by Company Directors Allemang, Jones and Pendell with proceeds of $250,000, $250,000, and $50,000, respectively. Interest accrued on these Notes during the three months ended June 30, 2020, was $7,792.
NOTE 9 - DERIVATIVE LIABILITY
On April 23, 2020, the Company entered into an Amended and Restated Draw Notice and Agreement with the Dow Chemical Company (“Amended Dow Facility”) to allow us to structure an offering of secured, subordinated convertible notes to support ongoing cash needs (the “Unit Offering”). In the Amended Dow Facility, the Company and Dow agreed to 1) extend the term of the loan facility by two years to December 1, 2023, 2) significantly reduce any required prepayment to Dow from the proceeds of new equity or equity-linked financings from the current 30-50% prepayment requirement on the pre-existing Dow Facility to a 10% prepayment requirement in the Amended Dow Facility, which does not begin until after we have raised an additional $7 million in equity or equity-linked capital from the date of the amendment, 3) capitalize all interest payable until such time as we have recorded GAAP revenue of at least $2 million for two consecutive calendar quarters 4) increase the rate of interest to 6.5% per annum and 5) allow for a subordinated security interest to be granted to new investors in the Unit Offering.
We evaluated the accounting for the amendment and concluded it met the conditions to be a troubled debt restructure. The future cash flows on the restructured debt were approximately $2.7 million greater than the carrying value of the debt which was accounted for prospectively through the effective interest rate.
Immediately after executing the Amended Dow Facility, we commenced a Unit Offering in a private placement to accredited investors. The Unit Offering consists of (i) an offering of up to $10 million in Convertible Notes (“Notes”) in $8 units, and (ii) an assignable or transferrable right to exchange two shares of previously issued common stock for two shares of Series B Convertible Preferred Stock for every $8.00 invested in the Unit Offering (“Exchange Rights”). As of June 30, 2020, we have raised $550,000 in the Unit Offering through the sale of 68,750 Units and 137,043 shares of Common Stock have been exchanged for 137,043 shares of Preferred Series B stock.
The Convertible Notes pay interest at 7.5% per year, compounded quarterly, which is payable on the maturity date unless the Company has recorded GAAP revenue of at least $4.0 million for two consecutive quarters at which time the holder may elect to receive all accrued interest in cash on a quarterly basis. The maturity date of the Notes is December 31, 2024. The Notes are convertible at the holder’s option into either) Series B Preferred Stock at a conversion price of $8.00/share; or ii) any other form of preferred or common stock (“Subsequent Stock”) issued by the Company at a conversion price per share equal to 80% of the purchase price per share at which such Subsequent Equity is sold, or if the value per share is fixed, 125% of the number of shares that might otherwise be issuable, provided that if a Qualified Capital Event occurs within 120 days after the initial funding, then the conversion price will be 90% of the purchase price per share at which the Subsequent Equity is sold, or if the value per share is fixed, 110% of the number of shares that would otherwise be issuable. No Qualified Capital Event has occurred within 120 days after the initial funding on April 23, 2020.
If we raise at least $15 million of equity capital, excluding equity raised in this offering, we may choose to convert the outstanding amount due and payable under the Note into whichever form of conversion shares would result in the greatest number of shares of Common Stock being issued to the holder in an “as-if-converted” into Common Stock basis at the conversion price then in effect.
In the event the Notes are outstanding on the date our shares of Common Stock are listed on a Qualified National Exchange, then the Notes will automatically be converted first into Series B Preferred Stock at a price of $8.00 per share and then into shares of Common Stock at the Series B Conversion Rate in effect at the time. If there is a change in control, all principal and accrued interest will paid in cash or can be converted according to the terms of the Note, at the holder’s option.
16
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
The Notes are secured by a junior security interest in all the assets of the Company. The Notes may not be prepaid prior to the maturity date, but they may be transferred at any time to i) any entity controlled by the holder, ii) any investors in the holder or iii) any other accredited investor only in compliance with the Securities Act of 1933 and other state laws.
After the original issue date but prior to the date we raise $15 million of cumulative equity capital, excluding capital raised in this Offering, if we consummate a financing transaction with a third-party using any other form of convertible debt security, then the holder has the right to exchange their outstanding obligations for a new security with the same terms as the new convertible debt security offered to the third party. This provision is viewed as a redemption option.
Embedded features in the Notes were analyzed under ASC 815 to determine if they required bifurcation as derivative instruments. To be a derivative, one of the criteria is that the embedded component must be net settleable. The Company’s common stock is not publicly traded, there is no mechanism outside the notes that would permit the holder to achieve net settlement and the underlying shares are not readily convertible to cash. Accordingly, the embedded derivatives, including the embedded conversion feature, do not meet the definition of a derivative, and therefore, do not require bifurcation from the host instrument. Certain default put provisions, the change in control repayment provision and the redemption option were not considered to be clearly and closely related to the host instrument but we concluded that the value of these provisions was de minim us at inception. We reconsider the value of these provisions each reporting period to determine if the value becomes material to the financial statements.
For every $8.00 invested in the Unit Offering, we provided investors in the Unit Offering, the one-time right to exchange two shares of previously issued Common Stock for two shares of Series B Preferred Stock of the Company within 30 days of the closing (the “Exchange Right”). The Exchange Right is fully transferable and assignable to any other party during the 30-day period if they are an accredited investor and executed a joinder to become bound by the terms of the Agreement. The Exchange Right is a freestanding financial instrument, and its fair value is estimated based on the incremental fair value between the exchanged Common Stock and the issued Preferred Stock. In this instance, the Exchange Right was included as part of the Unit Financing in conjunction with the issuance of the Notes, therefore, the proceeds from the financing were allocated between the Notes and the Exchange Right based on their relative fair values. The difference between the fair value of the Exchange Right and the relative fair value assigned to the Exchange Right is recognized immediately as Nonoperating expense. As of June 30, 2020, Nonoperating expense of $136 had been recorded related to the Exchange Rights.
A convertible financial instrument includes a beneficial conversion feature (“BCF”)if the effective conversion price is less than the company’s market price of their stock on the commitment date. The BCF for the three months ended June 30, 2021 was $122,230
The sale of Convertible Notes through the quarter ended March 31, 2020.2021, raised total proceeds of $6,440,280.
| For the three months | ||||
| ended | Proceeds | |||
| June 30, 2020 | $ | 550,000 | ||
| September 30, 2020 | 800,000 | |||
| December 31, 2020 | 2,501,664 | |||
| March 31, 2021 | 2,588,616 | |||
| Total | $ | 6,440,280 | ||
The proceeds received in the Unit Offering for the three months ended June 30, 2020 were allocated as follows:
| For the three | ||||
| months ended | ||||
| June 30, 2020 | ||||
| Convertible Notes | 403.934 | |||
| Additional Paid In Capital – Exchange Rights | 146.066 | |||
| Proceeds | 550,000 | |||
| Convertible Notes | 403,934 | |||
| Additional Paid In Capital – BCF | (122,230 | ) | ||
| Convertible Notes - Accrued Interest | 12,256 | |||
| Convertible Notes - Cost of Debt Offering | (46,857 | ) | ||
| Convertible Notes Balance | 247,104 | |||
The value of exchange rights, the BCF and the debt issuance costs are being amortized using the effective interest method over the term of the Note. The Company recognized interest expense of $12,256 associated with the Note for the three months ended June 30, 2020. As of June 30, 2020, the carrying value of the Convertible Notes was engaged in finalizing$247,104
17
XG SCIENCES, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – SUBSEQUENT EVENTS
Financing
We continued sales of the 10-K immediately prior to May 14,Unit Offering throughout the calendar year 2020 and subsequently filed the Original 10-Q on June 17, 2020.first quarter of 2021. See Derivatives Liability write-up in this Note 9.
In accordanceFebruary of 2021, we applied for continued relief under the Coronavirus Aid, Relief and Economic Security Act (CARES) by applying to an SBA lender bank, PNC, for a second Paycheck Protection Plan (“PPP”) loan. On February 16, 2021, we received an approved and fully executed PPP Term Note for $536,700 with Rule 12b-15a term of five years, a repayment deferral period which is 10 months plus 24 weeks from the date that the funds were disbursed or the date any forgiven amount of the Facility is remitted by the SBA to the Bank, and an annual rate of interest of 1%, with a potential for some or all of the loan to be forgiven, dependent upon use of the loan proceeds. Funds were disbursed to us on February 17, 2021.
Equity Preference Rights in the Event of Liquidation
On December 28, 2020, the Board of Directors consented to Amendments to the Second Amended and Restated Certificate of Designation of the Series A Convertible Preferred Stock and the Series B Convertible Preferred Stock by adapting the Third Amended and Restated Certificate of Designations of Series A and of Series B Convertible Preferred Stock. The Third Amended and Restated Certificate of Designations continue to allow for liquidation preference prior and in preference to any distribution of any of the assets of the Corporation to the holders of Junior Securities and, as amended, after the payment of all preferential amounts required to be paid to the holders of the Series A Preferred Stock, Series B Preferred Stock and other Senior Securities, the remaining assets of the corporation available for distribution to its stockholders shall be distributed among the holders of Junior Securities.
Related Party Transactions
As of March 31, 2021, the Board of Directors and their affiliates had purchased $3,666,520 of Convertible Notes.
18
ITEM 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
In this Quarterly Report on Form 10-Q, unless otherwise indicated, the words “we”, “us”, “our”, “XG”, “XGS”, “XG Sciences” or the “Company” refer to XG Sciences, Inc. and its wholly owned subsidiary, XG Sciences IP, LLC, a Michigan limited liability company.
Introduction
The following discussion and analysis should be read in conjunction with the unaudited condensed consolidated financial statements, and the notes thereto included herein. The information contained below includes statements of the Company’s or management’s beliefs, expectations, hopes, goals and plans that, if not historical, are forward-looking statements subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. For a discussion on these risk factors, please refer to the Company’s Annual Report on Form 10-K as filed April 29, 2020. For a discussion on forward-looking statements, see the information set forth in the introductory note to this quarterly report on Form 10-Q under the caption “Forward-Looking Statements”, which information is incorporated herein by reference.
Overview of our Business
XG Sciences was formed in May 2006 for the purpose of commercializing certain technology to produce graphene nanoplatelets and integrated, value-added products containing graphene nanoplatelets. First isolated and characterized in 2004, graphene is a single layer of carbon atoms configured in an atomic-scale honeycomb lattice. Among many noted properties, monolayer graphene is harder than diamonds, lighter than steel but significantly stronger, and conducts electricity better than copper. Graphene nanoplatelets are particles consisting of multiple layers of graphene. Graphene nanoplatelets have unique capabilities for energy storage, thermal conductivity, electrical conductivity, barrier properties, lubricity and the ability to impart physical property improvements when incorporated into plastics, metals or other matrices.
We believe the unique properties of graphene and graphene nanoplatelets will enable numerous new product applications and the market for such products will quickly grow to be a significant market opportunity. Our business model is to design, manufacture and sell advanced materials we call xGnP® graphene nanoplatelets and value-added products incorporating xGnP® nanoplatelets. We currently have many customers trialing our products for numerous applications, including, but not limited to lithium ion batteries, lead acid batteries, thermally conductive adhesives, composites, thermal management and heat transfer, inks and coatings, printed electronics, construction materials, cement, and in a range of other industrial uses. We believe our proprietary processes have enabled us to be a low-cost producer of high-quality, graphene nanoplatelets and value-added integrated products containing graphene nanoplatelets and that we are well positioned to address a wide range of end-use applications.
Our Customers
We sell products to customers around the world and have sold materials to over 1,500 customers in 47 countries since 2008. Some of these customers are research organizations and some are commercial organizations. Our customers have included well-known automotive and OEM suppliers around the world (Ford, Johnson Controls, Magna, Honda Engineering), global-scale lithium-ion battery manufacturers in the U.S., South Korea and China (Samsung SDI, LG Chemical, Lishen, A123) and diverse specialty material companies (3M, BASF, Henkel, Dow Chemical, DuPont), as well as leading research centers such as Lawrence Livermore National Laboratory and Oakridge National Laboratory. The majority of our customers are still ordering in smaller quantities consistent with their development and engineering qualification work. We expect our average order size to increase in the second half of 2020 into early 2021 as many of our customers have expressed their intention of beginning to order commercial quantities of product from us.
19
Our Products
XG Sciences is a manufacturer of graphene nanoplatelets marketed under the brand xGnP® and value-added products that contain graphene nanoplatelets. The term “graphene” is used widely in the literature and the popular press to cover a variety of specific forms of the material. We generally think about two broad classes of graphene materials:
| 1. | One-atom thick films of carbon commonly referred to as monolayer graphene, manufactured typically from gases by assembling molecules to form relatively large, transparent sheets of material. These materials have been characterized by their performance attributes that differentiate them from other advanced materials and that may include: strength up to 200 times that of steel, flexible and able to stretch up to 25% of its original length, optically transparent, more electrically conductive than copper, more thermally conductive than any other known material and atomic-level barrier properties. XG Sciences does not manufacture these films and does not participate in the markets for these films and believes that in general, the markets for these films do not compete with those for graphene nanoplatelets. |
| 2. | Ultra-thin particles of carbon that consist of layers of graphene sheets ranging in thickness from a few layers to many layers – that are commonly referred to as graphene nanoplatelets (“GNP” or “GNPs”). Because GNPs are thin and can be manufactured in a range of diameters, they are useful for a wide variety of applications. XG Sciences manufactures GNPs that range in thickness from a few nanometers and up to 10-20 nanometers and with diameters ranging from less than 1 micron and up to 100 microns. The manufacture of these graphene particles is our main area of expertise, and their use in practical applications is the focus of our sales, marketing and development activities. |
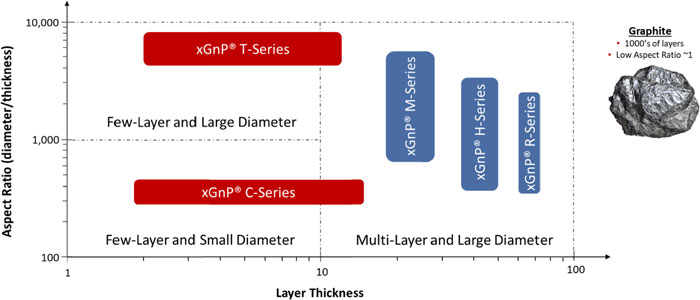
The well-publicized isolation and characterization of graphene in 2004 at the University of Manchester, has spawned a new class of two-dimensional materials based on layers of carbon atoms arranged in a hexagonal array and each carbon having lone pair electrons. The unique characterization and related performance of this new class of materials is derived from their two-dimensional nature and their composition of sp2 carbon atoms arranged in a hexagonal array. The ability of any new material to be exploited in industrial applications will depend on its fit-for-performance. In the case of graphene nanoplatelets, the fit-for-performance is very much related to their aspect ratio (among other factors) in that the diameter is typically significantly greater than the thickness. This is what differentiates the material from bulk graphite of high crystallinity and purity. We classify nanoplatelets consisting of largely basel planes of carbon atoms packed in a hexagonal array (i.e., graphene) as graphene nanoplatelets so long as their aspect ratio may be classified as two-dimension and are thus in the form of platelets. Such a definition implies that the thickness is nanoscale – GNPs having a thickness in the range from generally 0.6 nanometers and up to many 10’s of nanometers. For context one nanometer is one billionth of a meter, and the average thickness of a human hair is about 75,000 nanometers. We have chosen to utilize the definitions as set out by the Carbon Journal editorial team (Carbon, volume 65, pp.1-6) and Fullerex (The Graphene Report, March 2020 Edition, the Graphene Council) which provides classification for the various material types which provide meaningful descriptions of commercially available graphene.
Graphene Product Thickness Definitions Based on Thickness
| Number of Layers | Product Description |
| 1 | Graphene (monolayer) |
| 1-3 | Very Few Layer Graphene (vFLG) |
| 2-5 | Few Layer Graphene (FLG) |
| 2-10 | Multilayer Graphene (MLG) |
| >10 | Graphene Nanoplatelets (GNPs) |
Bulk Materials. We sell bulk materials under the trademarked brand name of xGnP® graphene nanoplatelets. These materials are produced in various grades, which are analogous to average particle thickness, and average particle diameters. There are three commercial grades (Grades H, M & R), each of which is offered in three standard particle sizes and a fourth, Grade C, which is offered in three standard surface areas. We also have access to other development grades (Grade T, for example), but which are not yet made available commercially and have been used internally for those products containing graphene nanoplatelets. These bulk materials, which normally ship in the form of a dry powder, are especially applicable for use as additives in polymeric or metallic composites, or in coatings or other formulations where particular electrical, thermal or barrier applications are desired by our customers. We also offer our material in the form of dispersions of nanoplatelets in liquids such as water, alcohol, or organic solvents, or mixed into resins or polymers such as thermoplastics or thermosets. We use two different commercial processes to produce these bulk materials:
20
Grade H/M/R/T materials are produced through chemical intercalation of natural graphite followed by thermal exfoliation using a proprietary process developed by us. The “grade” designates the thickness and surface characteristics of the material, and each grade is available in various average particle diameters. Surface area, calculated by the Brunauer, Emmet, and Teller (BET) Method, is used as a convenient proxy for thickness, so each grade of products produced through chemical intercalation is designated by its average surface area, which ranges from 50 to 150 m2/gram of material. We are able to extend the surface area higher (250 m2/gram for T Grade) but are not yet producing these materials in metric-ton quantities. As the market need emerges for this class of materials, we may scale them as needed. For example, we introduced a Grade of xGnP® powders, R-Grade, with improved electrical conductivity targeting use in applications for electrically and thermally conductive ink and composites and have scaled R-Grade to metric-ton quantities.
Grade C materials are produced through high-shear mechanical exfoliation using a proprietary process and equipment that we invented, designed, constructed and patented. The Grade C materials are smaller particles than those grades produced through chemical exfoliation, and Grade C materials are designated by their BET surface area, which ranges from 300 to 800 m2/gram. We are able to produce other surface areas and may make those available commercially as needed by our customers.
The following graphic depicts xGnP® graphene nanoplatelets as a function of both layer thickness and aspect ratio (diameter by thickness), two key parameters which will influence their performance in a range of industrial applications.
XG Sciences’ Graphene Nanoplatelet Product Portfolio and Versus Graphite
21
Composites. These consist of compositions of specially designed xGnP® graphene nanoplatelets formulated in pre-dispersed mixtures that can be easily incorporated in various polymers. Our integrated composites portfolio includes pre-compounded resins derived from a range of thermoplastics as well as master batches of resins and xGnP® nanoplatelets and their combination with resins and fibers for use in various end-use applications that may include industrial, automotive, packaging and sporting goods and which have demonstrated efficacy in standard injection molding, compression molding, blow molding and 3-D processes, to name but a few. Our current product portfolio of polymer resins containing various forms of our xGnP® graphene nanoplatelets and in varying concentration includes polyurethane (XGPU), polypropylene (XGPP™), polyethylene terephthalate (XGPET™), nylon (XGNylon) and high-density polyethylene (XGHDPE™). Other polymers may be added over time depending on the end-market and customer needs. In addition, we offer various bulk materials with demonstrated efficacy in plastic composites to impart improved physical performance to such matrices, which may be supplied as dry powders, or as aqueous or solvent-based dispersions or cakes as described above. We have also targeted use of our graphene nanoplatelets as an additive in cement mixtures, which we believe results in improved barrier resistance, durability, toughness and corrosion protection. Our XGConcrete™ Additive promotes the formation of more uniform and smaller grain structure in cement. This fine-grain and uniform structure gives the concrete improvements in flexural and compressive strength. In addition, the embedded graphene nanoplatelets will stop cracks from forming and retard crack propagation, should any cracks form – the combination of which will improve lifetime and durability of cement.
Inks and Coatings. These consist of specially-formulated dispersions of xGnP® together with solvents, binders, and other additives to make electrically or thermally conductive products designed for printing or coating and which are showing promise in diverse customer applications such as advanced packaging, electrostatic dissipation and thermal management. We also offer a set of standardized ink formulations suitable for printing. These inks offer the capability to print electrical circuits or antennas and may be suitable for other electrical or thermal applications. All of these formulations can be customized for specific customer requirements.
Energy Storage Materials. These consist of specialty advanced materials that have been formulated for specific applications in the energy storage segment. We offer various bulk materials for use as conductive additives for cathodes and anodes in lithium-ion batteries, as an additive to anode slurries for lead-carbon batteries, as a component in coatings for current collectors in lithium-ion batteries and we are investigating the use of our materials as part of other battery components.
Thermal Management Materials. These consist mainly of various thermal interface materials (“TIM”) in the form of custom greases or pastes. Our custom XG TIM® greases and pastes are also designed to be used in various high temperature environments. Additionally, we offer various bulk materials for use as active components in adhesives, liquids, coatings and plastic composites to impart improved thermal management performance to such matrices.
22
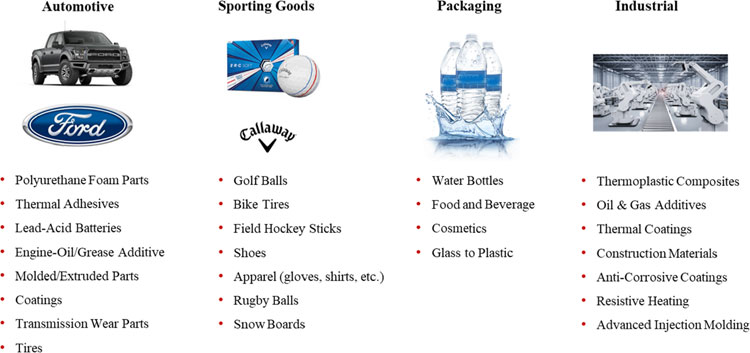
Our Focus Areas
We believe we are a platform play in advanced materials, because our proprietary manufacturing processes allow us to produce varying grades of graphene nanoplatelets that can be mapped to a variety of applications and in many market segments. However, we are prioritizing our efforts in specific areas and with specific customers that we believe represent opportunities for either relatively near-term revenue or especially large and attractive markets. At this time, we are focused on four key vertical markets: Automotive, Sporting Goods, Packaging and Industrial. The following graphic provides examples of target applications within each of the four key verticals where XG Sciences has either commercial sales or is in development with one or more customers.
XGS Market/Application Focus Areas
Addressable Markets
The markets for our materials are large and growing. As one example, the 2019 North American packaging market for plastic bottles and containers is estimated to be more than $34 billion (Mordor Intelligence). Further, Mordor estimates the 2019 global market for PET water bottles at 543.8 billion units. XG Sciences is engaged in the supply of xGnP® graphene nanoplatelets for use in water bottles manufactured initially in North America and we are expanding our market activities into other geographies. If each water bottle produced in 2019 were to incorporate just 1 milligram of xGnP® graphene nanoplatelets, the total revenue available to XG Sciences may range from $200 to $300 million, depending on product form. As a second example, the 2019 Global market for rubber used in tires is estimated at 30 million metric tons (European Rubber Journal, January 9, 2019 and according to the International Rubber Study Group). Graphene nanoplatelets have been shown to provide performance improvements when incorporated into tires. If graphene nanoplatelets were used in just 1 weight percent of all rubber used in tires and used in only 20% of the available tire market, then there would exist a total global market for use of graphene nanoplatelets in tires of 60,000 metric tons on a dry powder basis.
Commercialization Process
Because graphene is a new material, most of our customers are still developing applications that use our products. Commercialization is a process, the exact timing of which is often difficult to predict. It starts with our own internal R&D to validate performance for an identified market or customer-specific need. Our customers then validate the performance of our materials and determine whether our products can be incorporated into their manufacturing processes. This is initially done at pilot production scale levels. Our customers then have to introduce products that incorporate our materials to their own customers to validate performance. After their customers have validated performance, our customers will then move to commercial scale production. Every customer goes through the same process, but will do so at varying speeds, depending on the customer, the product application and the end-use market. Thus, we are not always able to predict when our customers will begin ordering commercial volumes of our materials or predict their expected volumes over time. However, as customers move through the process, we generally receive feedback and gain greater insights regarding their commercialization plans. According to our respective customers, the following are examples where our products are providing value to our customers at levels that are either in commercial production or we believe will warrant their use on a commercial basis.
23
| ● | In 2018, Callaway Golf Company introduced new dual-core Chrome Soft and Chrome Soft X golf balls incorporating our xGnP® graphene nanoplatelets into the outer core, resulting in a new class of golf balls that enable higher driving speeds, greater distance and increased control, allowing Callaway to command a premium price for their golf balls in the marketplace. In 2019, Callaway expanded the use of our technology to incorporate our xGnP® into the E·R·C Soft line of golf balls. In 2020, Callaway continued to use our products in the newly launched Chrome Soft line of golf balls. The 2020 version of the Chrome Soft X is based on a solid core, which no longer benefited from use of our products; |
| ● | The Ford Motor Company, after having demonstrated a 17 percent reduction in noise, a 20 percent improvement in mechanical properties and a 30 percent improvement in heat endurance properties compared with that of polyurethane foam used without graphene, began incorporating our graphene nanoplatelets for polyurethane based foam parts in over ten under hood components on the Ford F-150 and Mustang in 2018. In late 2019, our products were qualified for use in engine covers in all Ford and Lincoln light truck and passenger car platforms. To meet this new demand, we began an expanded level of production in early 2020; |
| ● | International specialty materials company formulating our products into an adhesive used in production by a European automotive manufacturer to improve thermal performance. In 2018 we shipped them 900 Kgs of our products. In 2019 their demand increased by 200% to 1,800 Kilograms. In 2020, their initial forecasts were for a 166% increase in demand to 3,000 Kilograms. We shipped just over 900 Kgs in the first half of 2020, less than their original forecast due to COVID-19; |
| ● | Lead acid battery manufacturers incorporating our materials in the commercial supply of batteries demonstrated improvements in measured cycle life, capacity and charge acceptance; |
| ● | In 2019, we also announced the use of our products in an engine oil additive supplied by Hella, an innovative family-owned company serving the automotive and industrial markets with annual revenue of approximately 7 billion Euros; |
| ● | A US-based construction company has demonstrated a 40% improvement in strength when our products are incorporated into composite panels, and are currently used in commercial production; |
| ● | Large plastics packaging company having demonstrated more than a 2x improvement in strength upon adoption of our xGnP graphene nanoplatelets and while maintaining low color and optical transparency, are now targeting commercial introduction impacting recycling and light weighting; and |
| ● | Several customers are currently in initial scale production using our products for applications including resistive heating, additives for use in the oil and gas industry, coatings to improve load-bearing and thermal conductivity, chemical resistance and barrier. We expect that demand for our products will grow as new applications gain market momentum. |
The process of “designing-in” new materials is relatively complex and involves the use of relatively small amounts of the new material in laboratory and engineering development for an extended period of time. Following successful development, customers that incorporate our materials into their products will then order much larger quantities of material to support commercial production. Although, our customers are under no obligation to report to us on the usage of our materials, some have indicated that they have introduced or will soon introduce commercial products that use our materials. Thus, while many of our customers are currently purchasing our materials in kilogram (one or two pound) quantities, some are now ordering at multiple ton quantities and we believe many will require tens of tons or even hundreds of tons of material as they commercialize products that incorporate our materials. We also believe that those customers already in production will increase their order volume as demand increases and others will begin to move into commercial volume production as they gain more experience in working with our materials and engage new customers.
24
Manufacturing Capacity
We completed the first phase of expansion in our newest 64,000 square-foot facility in the first half of 2018. The expansion has added 90 metric tons of graphene nanoplatelet production capacity, bringing the total capacity of the facility up to approximately 180 metric tons of dry powder. Phase two of the expansion was partially complete by year-end 2018 and resulted in up to ~270 metric tons of total graphene nanoplatelet output capacity at the facility. We completed the last portion of this phase two expansion in the second half of 2019, resulting in up to ~400 metric tons of total graphene nanoplatelet output capacity. Our total graphene nanoplatelet output capacity across both of our manufacturing facilities, as of June 30, 2020, is an approximate 450 metric tons per year on a dry powder basis. The expansions support our mission to continue commercializing the use of graphene in customer products across diverse industries. XG’s increasing capacity will support our expected growth in demand for our products over the next several fiscal quarters. However, additional manufacturing capabilities for certain value-added products and certain bulk materials remain to be developed and may require the acquisition of additional facilities. In particular, the production processes for certain integrated products will require additional capital and may require additional facilities to meet expected future customer demand.
Some of the Company’s products are new products that have not yet been fully developed and for which manufacturing operations have not yet been fully scaled. Although we believe we will continue to scale our production capability, we have not yet demonstrated the capability to produce sufficient materials to generate the ongoing revenues necessary to sustain our operations in the long-term.
Our Intellectual Property We have acquired a non-exclusive license for certain inventions from Michigan State University. These rights are embodied in a Technology Licensing Agreement between XGS and Michigan State University dated July 27, 2007, amended on May 24, 2010 and May 27, 2011, and amended and restated on June 23, 2020 which provides us the non-exclusive worldwide rights to the patents as defined in the Agreement for application in the Composite, Energy Storage, Specialty Liquids, and other fields which may be applicable. In exchange for the rights to the technology covered by this Agreement, we paid MSU an initial fee and agreed to pay ongoing royalties on material sold by us in future years. Under a previous version of the Licensing Agreement, we also awarded a small amount of common stock to MSU. As of June 30, 2020, the following technologies were included in the MSU License Agreements with XGS: U.S. Patent 7,550,529, U.S. Patent 8,501,858, U.S. Patent 8,834,959, U.S. Patent 9,080,122 and U.S. Patent 9,776,874. On August 8, 2016, we signed an agreement acquiring an exclusive license with Metna Co. (Metna) to Metna’s background IP for use of graphene nanoplatelets as additives to concrete mixtures. For purposes of the agreement, Metna’s background IP relates to the U.S. Patent 8,951,343. Also, on August 8, 2016, we entered into a second agreement for an exclusive license related to all Metna’s background technology and foreground technology, including any jointly-owned foreground technology where the end use is known to be any graphite additive dispersed in concrete mixtures. On April 25, 2019 we signed an Intellectual Property License, Joint Development and Commercialization Agreement with Niagara Bottling acquiring exclusive license to certain of Niagara’s patents and applications relating to the use of graphene nanoplatelets in packaging applications. For purposes of the agreement, Niagara’s IP relates to five (5) U.S. patents and patent applications and two (2) international applications and future registered intellectual property directed at the field. Over time, our scientists and engineers have made many further discoveries and inventions that are embodied in the form of (and as of June 30, 2020): nineteen (19) additional U.S. patents, twenty-five (25) foreign patents, eleven (11) additional U.S. patent applications and numerous trade secrets. For many of the applications filed in the U.S., additional filings are made in other countries such as the European Union, Japan, South Korea, China, Taiwan or other applicable countries. As of June 30, 2020, we maintained fourteen (14) international patent applications. These filings and analyses are made on a case-by-case basis. Typically, patents that are defensive in nature are not filed abroad, while those that are protective of active XGS products or applications are filed in relevant countries abroad. Our general IP strategy is to keep as trade secrets those manufacturing processes that are difficult to enforce should they be disclosed and to seek patent coverage for other manufacturing processes, materials derived from those processes, unique combinations of materials and end uses of materials containing graphene nanoplatelets. We believe that the combination of our rights under various licenses, our patents and patent applications, and our trade secrets create a strong intellectual property position. |
25
Operating Segment
We have one reportable operating segment that manufactures xGnP® graphene nanoplatelets and value-added products produced therefrom, conducts research on graphene nanoplatelets and related products, and licenses our technology as appropriate. As of June 30, 2020, we shipped products on a worldwide basis, however, all of our assets were located within the United States.
Results of Operations for the Three and Six Months Ended June 30, 2020
compared with the Three and Six Months Ended June 30, 2019
| For the Three Months Ended June 30, | Change 2020 to 2019 | For the Six Months Ended June 30, | Change 2020 to 2019 | |||||||||||||||||||||||||||||
| 2020 | 2019 | $ | % | 2020 | 2019 | $ | % | |||||||||||||||||||||||||
| Total Revenues | 81,146 | 247,069 | (165,923 | ) | -67.2 | % | 248,209 | 1,104,346 | (856,137 | ) | -77.5 | % | ||||||||||||||||||||
| Cost of Goods Sold | 454,630 | 796,156 | 341,527 | 42.9 | % | 1,217,114 | 1,970,778 | 753,665 | 38.2 | % | ||||||||||||||||||||||
| Gross Loss | (373,484 | ) | (549,087 | ) | 175,603 | 32.0 | % | (968,904 | ) | (866,432 | ) | (102,472 | ) | -11.8 | % | |||||||||||||||||
| Research & Development Expense | 184,527 | 384,767 | 200,240 | 52.0 | % | 470,254 | 770,013 | 299,759 | 38.9 | % | ||||||||||||||||||||||
| Sales, General & Administrative Expense | 1,076,265 | 1,486,380 | 410,115 | 27.6 | % | 2,368,399 | 2,907,301 | 538,901 | 18.5 | % | ||||||||||||||||||||||
| Total Operating Expenses | 1,260,792 | 1,871,147 | 610,355 | 32.6 | % | 2,838,654 | 3,677,314 | 838,660 | 22.8 | % | ||||||||||||||||||||||
| Operating Loss | (1,634,276 | ) | (2,420,234 | ) | 785,958 | 32.5 | % | (3,807,558 | ) | (4,543,746 | ) | 736,188 | 16.2 | % | ||||||||||||||||||
| Other Expense | (190,936 | ) | (78,277 | ) | 112,659 | -143.9 | % | (368,945 | ) | (154,941 | ) | 214,003 | -138.1 | % | ||||||||||||||||||
| Net Loss | (1,825,211 | ) | (2,498,511 | ) | 673,299 | 26.9 | % | (4,176,503 | ) | (4,698,687 | ) | 522,184 | 11.1 | % | ||||||||||||||||||
Revenue
| For the Three Months Ended June 30, | Change 2020 to 2019 | For the Six Months Ended June 30, | Change 2020 to 2019 | |||||||||||||||||||||||||||||
| 2020 | 2019 | $ | % | 2020 | 2019 | $ | % | |||||||||||||||||||||||||
| Product Sales | $ | 81,146 | $ | 247,069 | $ | (165,923 | ) | (67.2 | ) | $ | 248,209 | $ | 1,104,346 | $ | (856,137 | ) | (77.5 | ) | ||||||||||||||
| Total Revenues | $ | 81,146 | $ | 247,069 | $ | (165,923 | ) | (67.2 | ) | $ | 248,209 | $ | 1,104,346 | $ | (856,138 | ) | (77.5 | ) | ||||||||||||||
For the three months ending June 30, 2020 we reported revenue of $81,146, a 67.2% decrease from the $247,069 reported for the three months ending June 30, 2019. Since we are in the business of developing new materials and for use in new ways, accurately forecasting new product adoption can be a challenge for our customers. The second quarter year-on-year decrease is due primarily to the vagaries of unpredictable demand and inaccurate forecasts. Also, many of our customers were impacted by COVID-19 in the second quarter and either delayed commercial adoption of our products or did not order replenishment products. For the six months ending June 30, 2020 we reported revenue of $248,209, a 77.5% decrease from the $1,104,346 reported for the six months ending June 30, 2019. We have a strong customer pipeline and continue to establish a robust baseline of customers who are nearing commercial status. We believe that once these customers reach commercial status with products that incorporate our products, each will begin to order from us on a regular basis, which will help to mitigate the quarter-to-quarter revenue variability. However, until such time, we expect to see variations in quarterly revenue resulting from variable order patterns from our customers.
26
Product sales consist of two broad categories: (1) material sold to customers for research or development purposes; and (2) production orders for customers. Typically, the order sizes for the first category are relatively small, however we expect orders in the second category to be much larger in the future. In addition, other customers are moving through development programs towards commercialization, requiring larger quantities of our materials for advanced testing, pilot production and commercial-scale production activities. We believe that those customers already in production will increase their order volume as demand increases and others will begin to move into commercial volume production as they gain more experience in working with our materials and engage their own customers.
We ship our products from our Michigan manufacturing facilities to customers around the world. During the three months ended June 30, 2020, we shipped materials to customers in 14 countries, as compared to 13 countries during the same period in 2019. For the three months ended June 30, 2020, sales to two countries, Switzerland and Turkey, accounted for more than 42% of revenue. For the three months ended June 30, 2019, shipments to South Korea accounted for more than 10% of product sales.
Order Summary
The table below shows a comparison of domestic and international orders fulfilled (excluding orders for free samples). The table also includes the average order size for product sales. The average order size for product revenue during the three months ended June 30, 2020 decreased by 87%, as compared to the same period in 2019. The relatively small average order size means that the majority of our customer base remains active with research and development projects that only use limited amounts of our material. We expect our average order size to increase significantly as more customers reach commercial status.
| Order Summary | For the Three Months Ended June 30, | |||||||||||
| (unaudited) | 2020 | 2019 | Change | |||||||||
| Number of orders – domestic | 20 | 33 | (13 | ) | ||||||||
| Number of orders – international | 21 | 26 | (5 | ) | ||||||||
| Number of orders – total | 41 | 59 | (18 | ) | ||||||||
| Average order size for product sales recorded in our Statement of Operations | $ | 1,962 | $ | 15,018 | $ | (13,056 | ) | |||||
| % change | (87 | %) | ||||||||||
Cost of Goods Sold
We use a standard cost system to estimate the direct costs of products sold. Variances between the standard costs and the actual costs are then properly capitalized to the inventories and expensed to cost of goods sold. Direct costs include estimates of raw material costs, packaging, freight charges net of those billed to customers, and an allocation for direct labor and manufacturing overhead. Because of the nature of our production processes, there is a substantial fixed manufacturing expense requirement that represents the ongoing cost of maintaining production facilities that are not directly related to products sold, so we use a “full capacity” allocation of overhead based on an estimate of what product costs would be if the manufacturing facilities were operating on a full-time basis and producing products at the designed capacity.
The following table shows the relationship of direct costs to product sales for the three months ended June 30, 2020 and 2019:
| Gross Loss Summary | For the Three Months Ended June 30, | Change 2020 to 2019 | ||||||||||||||
| 2020 | 2019 | $ | % | |||||||||||||
| Product Sales | $ | 81,146 | $ | 247,069 | $ | (165,923 | ) | (67.2 | ) | |||||||
| Direct Costs | 41,828 | 196,411 | (154,583 | ) | (78.7 | ) | ||||||||||
| Direct Cost Margin | $ | 39,318 | $ | 50,658 | $ | (11,340 | ) | (22.4 | ) | |||||||
| % of Sales | 48.5 | % | 20.5 | % | ||||||||||||
| Unallocated Manufacturing Expense | 412,802 | 599,745 | (186,943 | ) | (31.2 | ) | ||||||||||
| Gross Loss on Product Sales | $ | (373,484 | ) | $ | (549,088 | ) | $ | 175,603 | 32.0 | |||||||
27
We believe that the fluctuations in gross loss on product sales and direct cost from period to period are not indicative of future margins because of the relatively small size of our sales in comparison to our future expectations. Direct costs vary depending on the size of an order, the specific products being ordered, and other factors like shipping destination (which on small orders can represent a significant percentage of the cost).
The remaining “non-direct” costs of operating our manufacturing facilities are recorded as unallocated manufacturing expenses. These expenses include personnel costs, rent, utilities, indirect supplies, depreciation, and related indirect expenses. Unallocated manufacturing expenses are expensed as incurred. We allocate these costs to direct product costs based on the proportion of these expenses that would be representative of direct product costs if we were operating our factory at full capacity.
For the three months ended June 30, 2020, unallocated manufacturing expenses decreased by 31.2% to $412,802 as compared to $599,745 for the same period in 2019. The decrease of $186,943 is largely due to furloughs and headcount reductions as well as lower variable overhead costs due to the discontinuance of production at the Legion Drive site in late June of 2019 until sales rebound.
Sales, General and Administrative Expenses
During the three months ended June 30, 2020, we incurred selling, general and administrative expenses (SG&A) of $1,076,265. This is a decrease of $410,115 or 27.6% from the same period in 2019. This decrease in SG&A expense is primarily related to decreased personnel costs as the Company began taking measures in anticipation of slower demand and delays in customer adoption caused by COVID-19.
Research and Development Expenses
During the three months ended June 30, 2020, we incurred research and development expenses (R&D) of $184,527. This is a decrease of $200,240 or 52% from the same period in 2019. This decrease in R&D is primarily related to decreased personnel costs as the Company began taking measures in anticipation of slower demand due to COVID-19.
Other Expense
Other income and expense consist primarily of Interest expense on the Dow Facility and the Convertible Notes. Following shows a comparison of other income and expense for the three months ended June 30, 2020 and 2019:
| For the Three Months Ended | ||||||||||||
| June 30, | ||||||||||||
| 2020 | 2019 | Change $ | ||||||||||
| Interest Expense, Net | (190,800 | ) | (78,277 | ) | (112,523 | ) | ||||||
| Nonoperating Expense | (136 | ) | — | (136 | ) | |||||||
| Total Other Expense | (190,936 | ) | (78,277 | ) | (112,659 | ) | ||||||
28
Fair Value Measurements
ASB ASC 820: “Fair Value Measurements and Disclosures” defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices included within Level 1 which are either directly or indirectly observable.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Our derivative liabilities are classified as Level 3 within the fair value hierarchy because they were valued using other unobservable inputs. The valuation technique used to measure fair value of the derivative liabilities is based on a lattice model with significant assumptions and inputs determined by the Company. A lattice model was used to estimate the fair value of the derivative liabilities because management believes it reflects all of the assumptions that market participants would likely consider including early exercise of the warrants. The fair value of the derivative liabilities will be significantly influenced by the fair value of our common stock, stock price volatility and the risk-free interest components of the lattice technique.
Derivative Financial Instruments
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risk. The terms of the Unit Offering issued April 23, 2020 were reviewed to determine whether or not they contain embedded derivative instruments that are required by ASC 815: “Derivatives and Hedging” to be accounted for separately from the host contract and recorded at fair value. The anti-dilution provision of the Series B Preferred Stock, the redemption rights, the conversion rights and the participation rights issued with the Unit Offering met the conditions to be accounted for separately from the host contract and are classified as derivative instrument liabilities measured at fair value. The fair values of these derivative liabilities are revalued at each reporting date, with the change in fair value recognized in earnings.
Liquidity and Capital Expenditures
Liquidity
We have historically incurred losses from operations, and we may continue to generate negative cash flows as we implement our business plan. Our consolidated financial statements are prepared using US GAAP as applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
As of May 17, 2021, we had cash on hand of $x,xxx,xxx. Due to lower-than-expected revenue in 2019 and the current COVID-19 global pandemic impacting both our operations and that of our customers, Management has taken several steps to ensure we are able to fund our operations from existing cash on hand, operating cash flows, additional borrowings, and restructured debt obligations.
In March of 2020, we restructured our organization by reducing headcount by 53%, by furloughing substantially all manufacturing employees, and by implementing temporary salary reductions ranging from 15-20%. Although we have re-employed key employees since the reductions and furloughs, annual payroll and related costs have been reduced by 38%. In April 2020, we furloughed additional employees in our R&D and Engineering departments. Salary reductions remained in place at the discretion of the Compensation Committee of the Board of Directors until February 1, 2021.
In early April of 2020, we applied for relief under the Coronavirus Aid, Relief and Economic Security Act (CARES) by applying with the Small Business Administration (SBA) for an Economic Injury Disaster Loan (“EIDL”) and by submitting an application to an SBA lender bank, PNC, for a Paycheck Protection Plan (“PPP”) loan. On April 18, 2020, we received an approved and fully executed PPP Term Note for $825,200 with a term of two years, a six-month repayment deferral period, and an annual rate of interest of 1%, with a potential for some or all of the loan to be forgiven, dependent upon use of the loan proceeds. On April 20, 2020, we received the $825,200 of proceeds under the PPP loan which was subsequently reduced by $34,405 to $790,795 due to additional SBA guidance regarding 1099 income paid. On October 6, 2020, the Paycheck Protection Flexibility Act of 2020 extended the deferral expiration period for loan repayments to either the date that the SBA remits the borrower’s loan forgiveness or to 24 weeks after the receipt of proceeds plus 10 months or July 3, 2021. We plan to apply for loan forgiveness before the end of the deferred expiration date. The outstanding principal that is not forgiven converts to an amortizing term loan. On May 17, 2020, the SBA issued us a decline letter for the EIDL for which reconsideration has not been granted.
29
On April 23, 2020, we entered into an amended and restated Draw Loan Note and Agreement and related transaction documents (collectively, the “Amended Dow Facility”) with the Dow Chemical Company to amend the terms of our current loan facility to allow us to structure a private placement of units (“Units”) comprised (in part) of subordinated, secured convertible notes (“Convertible Notes” and such offering, the “Unit Offering”), to support ongoing cash needs. In the Amended Dow Facility, the Company and Dow agreed to 1) extend the term of such loan facility by two years to December 1, 2023, 2) significantly reduce any required prepayment to Dow from the proceeds of new equity or equity-linked financings from the current 30-50% prepayment requirement on the pre-existing Dow Facility to a 10% prepayment requirement in the Amended Dow Facility, which does not begin until after we have raised an additional $7 million in equity or equity-linked capital from the date of the amendment, 3) capitalize all interest payable until such time as we have recorded GAAP revenue of at least $2 million for two consecutive calendar quarters, 4) increase the rate of interest to 6.5% per annum from 5% in the pre-existing Dow Facility, and 5) allow for a subordinated security interest to be granted to new investors in the Unit Offering.
Immediately after the execution of the transaction documents related to the Amended Dow Facility, we commenced the Unit Offering in a private placement to accredited investors. The Unit Offering is comprised of the Convertible Notes and a right to exchange two shares of previously issued Common Stock of the Company for two shares of Series B Convertible Preferred Stock of the Company (“Series B Preferred Stock”) for every $8.00 invested in the Unit Offering (the “Exchange Rights”). The Convertible Notes are secured by a junior security interest in all the assets of the Company, bear an interest rate of 7.5% per annum and mature on December 31, 2024. Each investor’s Exchange Rights are exercisable for a period of thirty (30) days after acceptance by the Company of a fully executed subscription agreement.
At the option of each holder, the Convertible Notes are convertible into either i) Series B Preferred Stock at a conversion price of $8.00/share; or ii) any other form of preferred or common stock (“Subsequent Stock”) issued by the Company at a conversion price per share equal to 80% of the purchase price per share at which such Subsequent Stock is sold (or if the value per share is fixed, 125% of the number of shares that might otherwise be issuable).
If and when we raise at least $15 million of equity capital (excluding capital raised in this Unit Offering), the Convertible Notes will be automatically converted into whichever of the following equity securities would result in the greatest number of shares of Common Stock being issued to the holders on an “as-if-converted” basis at such time: (i) Series B Preferred Stock at a note conversion price of $8.00/share; or (ii) Subsequent Stock at a note conversion price per share equal to 80% of the purchase price per share at which such Subsequent Stock is sold (or if the value per share is fixed, 125% of the number of shares that might otherwise be issuable);
Each share of Series B Preferred Stock has an original issue price of $8.00 per share (the “Series B Original Issue Price”) and a liquidation preference of $8.00 per share, with both the Series B Original Issue Price and the liquidation preference per share subject to adjustment for stock splits, recapitalizations, and the like. The Series B Preferred Stock will be senior to the Company’s Common Stock and pari-passu with the Series A Preferred Stock in terms of right of repayment in a liquidation.
The Series B Preferred Stock has full ratchet antidilution protection that provides that each share of Series B Preferred Stock outstanding may be converted by an Investor at any time into that number of shares of Common Stock determined by dividing the then current Series B Original Issue Price by the applicable Conversion Price (as defined below) with the resulting fraction equal to the “Series B Conversion Rate”. The total number of shares of Common Stock issuable will be equal to the number of shares of Series B Preferred Stock being converted multiplied by the Series B Conversion Rate. The “Conversion Price” is, at any time, the price per share equal to the lesser of a) the Series B Original Issue Price per share and b) the lowest price per share at which the Company has sold equity or equity-linked securities (other than customary exclusions) at any future date while any shares of the Series B Preferred Stock remain outstanding. The Series B Original Issue Price and Conversion Price in effect at any time are also subject to proportional adjustment for share splits, share dividends, recapitalizations, and the like.
On April 23, 2020, certain members of our Board of Directors and their affiliates purchased $550,000 of convertible notes. In addition, these Board members and their affiliates purchased $800,000, $1,801,504, and $515,016 of convertible notes in the quarters ended September 30, 2020, December 31, 2020 and March 31, 2021. In total, the Board members and their affiliates purchased a total of $3,666,520 of convertible notes through March 31, 2021 from the Company. Non-Board affiliated third parties purchased $700,160 and $2,073,600 of convertible notes in the quarters ended December 31, 2020 and March 31, 2021 from the Company for a total purchase of $2,773,760. Total proceeds from the sale of Convertible Notes as of March 31, 2021 was $6,440.280.
Taking into consideration our current cash on hand, we estimate that we will need to raise approximately $500,000 - $1,000,000 of additional capital to continue our operations for the next twelve months in a minimal to no revenue growth environment. Our continuation as a going concern is dependent upon continued financial support from our shareholders, our ability to obtain necessary equity and/or debt financing to continue operations, and growth in revenue from operations. These factors raise substantial doubt regarding our ability to continue as a going concern. We cannot make any assurances that additional financings will be available to us and, if available, completed on a timely basis, on acceptable terms or at all. If we are unable to complete an equity or debt offering, or otherwise obtain sufficient financing when and if needed, it would negatively impact our business and operations. It could also lead to the reduction or suspension of our operations and ultimately force us to cease our operations.
30
There has been no public market for our securities and a public market may never develop, or, if any market does develop, it may not be sustained. Our Common Stock is not currently quoted on or traded on any exchange or on any over-the-counter market. In the event we are unable to fund our operations from existing cash on hand, operating cash flows, additional borrowings or raising equity capital, we may be forced to reduce our expenses, slow down our growth rate, or discontinue operations. Our condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Smaller reporting companies are not required to provide this information.
ITEM 4. CONTROLS AND PROCEDURES
(a) Evaluation of disclosure controls and procedures. We maintain disclosure controls and procedures designed to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized, and reported within the Companytime periods specified in the SEC rules and forms, and that such information is accumulated and communicated to our management, including in this Amendment certifications from itsour principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required by Rule 13a-14(a) or Rule 15d-14(a)disclosure. In designing and evaluating our disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the Exchange Act as exhibits to this Amendment. Because no financial statements have been included in this Amendment and this Amendment does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4 and 5 of the certifications have been omitted. Similarly, we are not including the certifications under Section 906 of the Sarbanes-Oxley Act of 2002 as no financial statements are being filed with this Amendment.desired control objectives.
Except as described above, this Amendment does not amend, modify or updateAs required by SEC Rule 15d-15, our management carried out an evaluation, under the information in, or exhibits to, the Original 10-Q. Furthermore, this Amendment does not change any previously reported financial results nor does it reflect events occurring after the filing of the Original 10-Q. This Amendment should be read in conjunction with the Original 10-Qsupervision and with the Company’s other filings made with the SEC subsequent to the filingparticipation of our principal executive officer and principal financial officer, of the Originaleffectiveness of our disclosure controls and procedures as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of the end of the period covered by this report.
(b) Changes in internal controls. There were no changes in our internal control over financial reporting that occurred during the three months ended June 30, 2020 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
31
PART II - OTHER INFORMATION
None.
Smaller reporting companies are not required to provide this information. Please refer to the risk factors as set forth beginning on page 12 under the section entitled “Risk Factors” in our annual report on Form 10-K as filed with the Securities and Exchange Commission (the “SEC”) on April 29, 2020.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
The following sets forth information regarding all unregistered securities sold from July 1, 2019 through June 30, 2020:
| (1) | We granted stock options to purchase an aggregate of 12,500 shares at an exercise prices of $8.00 per share to Board members, consultants and directors under the 2017 Equity Plan; |
| (2) | We granted an aggregate of 12,500 shares of restricted stock with an aggregate fair value of $100,000 under the 2017 Incentive Plan. |
The issuances of securities described in paragraphs (1) and (2) above were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 promulgated under the Securities Act as transactions by an issuer not involving a public offering or under benefit plans and contracts relating to compensation as provided under Rule 701.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES.
None.
ITEM 4. MINE SAFETY DISCLOSURES.
None.
On April 27, 2020, the Company amended and restated the Certificate of Designation of its Series B Preferred Stock, a copy of which is filed as Exhibit 3.1 hereto. On December 20, 2020, the Company again amended and restated the Certificate of Designation of its Series A and Series B Preferred Stock, a copy of which is filed as Exhibit 3.2 hereto. On March 31, 2021, the Company again amended and restated the Certificate of Designation of its Series B Preferred Stock, a copy of which is filed as Exhibit 3.3 hereto.
32
| 32.1 | Certification Pursuant To 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act Of 2002* | Filed herewith | ||
| 32.2 | Certification Pursuant To 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | Filed herewith | ||
| 101. INS | XBRL Instance Document | Filed herewith | ||
| 101. CAL | XBRL Taxonomy Extension Calculation Link base Document | Filed herewith | ||
| 101. DEF | XBRL Taxonomy Extension Definition Link base Document | Filed herewith | ||
| 101. LAB | XBRL Taxonomy Label Link base Document | Filed herewith | ||
| 101. PRE | XBRL Extension Presentation Link base Document | Filed herewith | ||
| 101. SCH | XBRL Taxonomy Extension Scheme Document | Filed herewith |
33
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Dated: | By: | /s/ | |
| Name: | |||
| Title: | Chief Executive Officer | ||
| Dated: | By: | /s/ Jacqueline M. Lemke | |
| Name: | Jacqueline M. Lemke | ||
| Title: | Chief Financial Officer |
34