As filed with the Securities and Exchange Commission on February 21, 202320, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 20-F
(Mark one)
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20222023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report . . . . . . . . . . . . . . . . . . .
For the transition period from ___________________________ to ___________________________
Commission file number 001-05146-01
KONINKLIJKE PHILIPS NV
(Exact name of Registrant as specified in its charter)
ROYAL PHILIPS
(Translation of Registrant's name into English)
The Netherlands
(Jurisdiction of incorporation or organization)
Philips Center, Amstelplein 2, 1096 BC Amsterdam, The Netherlands
(Address of principal executive offices)
Marnix van Ginneken, Chief ESG & Legal Officer
+31 2059 77232, marnix.van.ginneken@philips.com, Philips Center, Amstelplein 2, 1096 BC Amsterdam, The Netherlands
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Shares - par value | PHG | New York Stock Exchange | ||
| Euro (EUR) 0.20 per share |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
(Title of class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of class)
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report.
| Class | Outstanding at December 31, | |
| KONINKLIJKE PHILIPS NV | ||
| Common Shares par value EUR 0.20 per share |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☒Yes ☐ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒ No
Note - Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer," "accelerated filer,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large Accelerated Filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the
effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C.
7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
Contents
- 1Introduction
- 2Forward-looking statements
- 3Form 20-F cross reference table
- 4Message from the CEO
- 5Board of Management and Executive Committee
- 6Strategy and Businesses
- 7Financial performance
- 8Environmental, Social and Governance
- 9Risk management
- 10Supervisory Board
- 11Supervisory Board report
- 12Corporate governance
- 12.1Introduction
- 12.2Board of Management and Executive Committee
- 12.3Supervisory Board
- 12.4Other Board-related matters
- 12.5General Meeting of Shareholders
- 12.6Annual financial statements and external audit
- 12.7Stichting Preferente Aandelen Philips
- 12.8Major shareholders
- 12.9Corporate information
- 12.10Additional information
- 13Group financial statements
- 13.1Controls and Procedures
- 13.2Reports of the independent auditor
- 13.3Independent auditor’s report on internal control over financial reporting
- 13.4Independent auditor’s report on the consolidated financial statements
- 13.5Consolidated statements of income
- 13.6Consolidated statements of comprehensive income
- 13.7Consolidated balance sheets
- 13.8Consolidated statements of cash flows
- 13.9Consolidated statements of changes in equity
- 13.10Notes to the Consolidated financial statements
- 14Further information
- 15Exhibits
1Introduction
This document contains information required for the Annual Report on Form 20-F for the year ended December 31, 20222023 of Koninklijke Philips N.V. (the 20222023 Form 20-F). Reference is made to the Form 20-F cross reference table herein. Only (i) the information in this document that is referenced in the Form 20-F cross reference table, (ii) this introduction and the cautionary statement “forward-looking statements” on the next two pages and (iii) the Exhibits shall be deemed to be filed with the Securities and Exchange Commission for any purpose. Any additional information in this document which is not referenced in the Form 20-F cross reference table, or the Exhibits themselves, shall not be deemed to be so incorporated by reference, shall not be part of the 20222023 Form 20-F and is furnished to the Securities and Exchange Commission for information only.
References to Philips
References to the Company or company, to Philips or the (Philips) Group or group, relate to Koninklijke Philips N.V. and its subsidiaries, as the context requires. Royal Philips refers to Koninklijke Philips N.V.
IFRS based information
The audited consolidated financial statements as of December 31, 20222023 and 2021,2022, and for each of the years in the three-year period ended December 31, 2022,2023, included in the 20222023 Form 20-F have been prepared in accordance with International Financial Reporting Standards (IFRS) as endorsed by the European Union (EU). All standards and interpretations issued by the International Accounting Standards Board (IASB) and the IFRS Interpretations Committee effective 20222023 have been endorsed by the EU; consequently, the accounting policies applied by Philips also comply with IFRS as issued by the IASB. These accounting policies have been applied by group entities.
Use of non-IFRS information
In presenting and discussing the Philips financial position, operating results and cash flows, management uses certain financial measures that are not measures of financial performance or liquidity under IFRS (‘non-IFRS’). These non-IFRS measures should not be viewed in isolation as alternatives to the equivalent IFRS measure and should be used in conjunction with the most directly comparable IFRS measures. Non-IFRS measures do not have standardized meaning under IFRS and therefore may not be comparable to similar measures presented by other issuers. A reconciliation of these non-IFRS measures to the most directly comparable IFRS measures is contained in this document. Reference is made in Reconciliation of non-IFRS information.
Third-party market share data
Statements regarding market share, contained in this document, including those regarding Philips’ competitive position, are based on outside sources such as specialized research institutes, industry and dealer panels in combination with management estimates. Where full year information regarding 20222023 is not yet available to Philips, market share statements may also be based on estimates and projections prepared by management and/or based on outside sources of information. Management's estimates of rankings are based on order intake or sales, depending on the business.
Documents on display
Philips’ SEC filings are publicly available through the SEC’s website at www.sec.gov. The SEC website contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Philips’ internet address is www.philips.com/investor. The contents of any websites referred to herein shall not be considered a part of or incorporated by reference into this document.
For definitions and abbreviations reference is made in Definitions and abbreviations
Due to rounding, amounts may not add up precisely to the totals provided in this report.
2Forward-looking statements
Pursuant to provisions of the United States Private Securities Litigation Reform Act of 1995, Philips is providing the following cautionary statement.
This document, including the information referred to in the Form 20-F cross reference table, contains certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items, in particular, among other statements, certain statements in Item 4 “Information on the Company” with regard to management objectives, market trends, market standing, product volumes, business risks, the statements in Item 5 “Operating and financial review and prospects” with regards to trends in results of operations, margins overall, market trends, risk management, exchange rates, the statements in Item 8 “Financial Information” relating to legal proceedings and goodwill and statements in Item 11 “Quantitative and qualitative disclosure about market risks” relating to risk caused by derivative positions, interest rate fluctuations and other financial exposure are forward-looking in nature. Forward-looking statements can be identified generally as those containing words such as “anticipates”, “assumes”, “believes”, “estimates”, “expects”, “should”, “will”, “will likely result”, “forecast”, “outlook”, “projects”, “may” or similar expressions. By their nature, these statements involve risk and uncertainty because they relate to future events and circumstances and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these statements.
These factors include but are not limited to: Philips’ ability to gain leadership in health informatics in response to developments in the health technology industry; Philips’ ability to transform its business model tokeep pace with the changing health technology solutions and services;environment; macroeconomic and geopolitical changes; integration of acquisitions and their delivery on business plans and value creation expectations; securing and maintaining Philips’ intellectual property rights, and unauthorized use of third-party intellectual property rights; ability to meet expectations with respect to ESG-related matters; failure of products and services to meet quality or security standards, adversely affecting patient safety and customer operations; breach of cybersecurity; challenges in connection with Philips’ strategy to improve executionsimplifying our organization and other business performance initiatives;our ways of working; the resilience of Philips'our supply chain; attracting and retaining personnel; COVID-19 and other pandemics; challenges to drivein driving operational excellence and speed in bringing innovations to market; compliance with regulations and standards including quality, product safety and (cyber) security; compliance with business conduct rules and regulations including privacy and upcoming ESG disclosure and due diligence requirements; treasury and financing risks; tax risks; reliability of internal controls, financial reporting and management process; global inflation.
As a result, Philips’ actual future results may differ materially from the plans, goals and expectations set forth in such forward-looking statements. For a discussion of factors that could cause future results to differ from such forward-looking statements, reference is made to the information in Risk factors.
3Form 20-F cross reference table
Only (i) the information in this document that is referenced in the Form 20-F cross reference table, (ii) the Introduction and the cautionary statements concerning forward-looking statements of this report on pages 5-6,6-7, and (iii) the Exhibits shall be deemed to be filed with the Securities and Exchange Commission for any purpose. The content of Philips’ websites and other websites referenced herein should not be considered to be a part of or incorporated into the 20222023 Form 20-F. Any additional information which is not referenced in the Form 20-F cross reference table or the Exhibits themselves shall not be deemed to be so incorporated by reference, shall not be part of the 20222023 Form 20-F and is furnished to the Securities and Exchange Commission for information only.
The table below sets out the location in this document of the information required by SEC Form 20-F. The exact location is included in the column ‘Location in this document’. The column “Page”page number refers to the starting page of the section for reference only (and is not intended to refer to the starting page of the specific subsection, if applicable).
4Message from the CEO
Dear Stakeholder,
The challenges of a world in turmoil amplify the sense of urgency I feel to make sure Philips isdelivers on its purpose and becomes an even stronger force for good, so people everywhere can look after their health and well-being and access the care they need, with us focusing on where we can help, from the hospital to the home.
In January 2023, we announced our multi-year plan to create value with sustainable impact. Throughout the year, Philips teams around the world worked relentlessly, in a company withvolatile environment, to deliver on the first phase of that plan, laying a strong market leadership positions, an extensive customer base, strong innovation portfolio, talented employees,foundation for sustained future success.
Our products and a global purpose-driven brand. Yet, asservices reached 1.9 billion people in 2023, including 221 million in underserved communities – taking us closer to our 2022goal of improving 2.5 billion lives per year by 2030, including 400 million in underserved communities.
Our improved operational performance underlines, we are not extractingwas driven by significant progress on the full valuethree pillars of our businesses and have disappointed many stakeholders.
My priority as CEO isplan to address operational challenges, improve performance, and drive progressivecreate value creation through a strategy offor all our stakeholders: 1) focused organic growthgrowth; 2) scalable people- and an innovation model shiftpatient-centric innovation; and 3) focus on execution to increase the impact of patient- and people-centric innovation at scale. Execution will be the key value driver, with three clear priorities around improvingenhance patient safety and quality, creating more reliablestrengthen our supply chain reliability, and resilient supply chains,establish a simplified, agile operating model.
We achieved our raised 2023 outlook with strong sales growth, improved profitability, and simplifyingstrong cash flow, despite the way weuncertainties brought about by an increasingly turbulent geopolitical environment. While the order book remains strong in absolute terms, order intake was down, for which the necessary improvement actions are under way. There is still a lot of work so we are more agile and competitive.
Addressing priority challenges – improved execution as key value driver
Our first priority is to rebuild Philips’ reputation around patient safety and quality. The recall of specific Respironics sleep therapy devices and ventilators let down the patients who depended on them, and the doctors caring for those patients. We apologize deeply for that and are working hard to restore trust with all stakeholders. By year-end, following the substantial ramp-up of capacity, Philips Respironics had completed around 90% of the production required for the delivery of replacement devices to patients.
In consultation with regulators around the world, we have also been conducting a comprehensive test and research program to better understand the potential health risks associated with the use of affected devices. I am very conscious that 18 months is long, but this work had to be done, thoroughly.but 2023 represents a good start, and it reinforces our confidence in delivering on our three-year plan.
Patient safety and quality
Resolving the consequences of the Respironics recall for our patients and customers is a key focus area, and I am encouraged by the test resultsapologize for the first-generation DreamStation devices, that account fordistress caused. Globally, over two thirds99% of the registered affected devices:sleep therapy device registrations that are complete and actionable have been remediated, while the prevalence of visible foam degradation is low, and the emissionremediation of the detected volatile organic compounds and particulates are within the applicable safety limits and not expected to result in appreciable harm to health in patients.ventilator devices remains ongoing.
We are fully committed to completingcomplying with the terms of the consent decree agreed with the US Department of Justice (DOJ), representing the US Food and Drug Administration (FDA), which primarily focuses on Philips Respironics recallin the US. The proposed consent decree will provide Philips Respironics with a roadmap of defined actions, milestones, and testing program in 2023. Wedeliverables to demonstrate compliance with regulatory requirements and to restore the business. Further details will also implementbecome available once the consent decree has been finalized and submitted to the relevant US court for approval.
As well as implementing all measures agreed with the US Food & Drug Administration (FDA)FDA and US Department of Justice, including a consent decree, andDOJ in connection with the Respironics recall, we will continue to rebuild tiesrelations with the FDA and other national regulators. We have putIn October, Philips Respironics received preliminary court approval for the leadershipclass action settlement that would resolve all or nearly all private economic loss claims in the US related to the recall. The settlement does not include or constitute any admission of liability, wrongdoing, or fault by any of the Philips parties. The previously disclosed litigation, including the personal injury and end-to-end organization in placemedical monitoring claims, and have invested significantly in doing so. Acrossinvestigation by the company, we have assignedDOJ related to the highest priority to making the necessary step-up inRespironics recall are ongoing.
Driving progress on priorities
With patient safety and quality managementthe number one priority, oversight now resides at Executive Committee level, and we have elevated leadershipa new organization in place, with stronger processes and more effective early warning systems in the businesses. We are pro-actively addressing quality improvements and first-time-right design. One of the most inspiring events of the year took place in October – a company-wide Timeout for Patient Safety and Quality. All 70,000 employees came together in their teams to discuss how we are moving forward on patient safety and quality, and how to Executive Committee level.take it further.
An integral aspect of quality is the abilityIn our drive to deliver and install equipment on time and to the required specifications. To this end, we are taking decisive action to make our supply chaincreate more reliable and predictable, by securing near-termresilient supply redesigningchains, we have significantly reduced our high-risk components and pruning our portfolio,inventories, and moving fromthe actions we have taken continued to have a ‘one size fits all’positive impact on our sales and service levels. We continue to strengthen further through regionalization of the supply chain structurebase and manufacturing capability to better respond to local requirements with a more agile, tailoredshorter value chain model per business, with dedicated and upgraded domain expertise. This will secure more deliveries, drive faster order-book conversion and build down inventory.chain.
We are also simplifyingstarted the way we workshift to drive accountability and agility, with the aim of unlocking significant productivity and margin gains. This simplificationour new, simplified operating model – with end-to-end businesses with single accountability and more focused targets, supported by a much leaner enterprise layer, strong regions and a reinvigorated impact culture – and completed the realignment of workforce roles and reporting lines. This included the difficult but necessary reduction of approximately 8,000 roles to date, out of the planned reduction of 10,000 roles by 2025.
Reflecting our changing culture of patient-people- and people-centricity,patient-centricity, accountability and impact, our Executive Committee was strengthened with the arrival of four new members in 2023, each bringing valuable experience and skills to the work of our leadership team. We also welcome the decision by Exor to take a 15% minority stake in our company – a sign of confidence in our plan, our people, and our future. And we marked the 100th anniversary of Philips in China, a remarkable achievement.
The opportunity to deliver better care for more people
Today, millions of people around the world have little or no access to basic healthcare, and climate change is impacting both environmental and human health. Healthcare is simply not working as it needs to. There are not enough doctors and nurses to address the growing demand for care. In parallel, rising costs are stretching financial budgets to the limit.
That’s why we are advocating for systemic change, driven by all ecosystem players, that addresses technology, clinical practice, financing and regulation as a whole. Change that delivers better, more productive healthcare that works for everyone. Without this change, communities all around the world will increasingly face challenges to get the care they need.
Focusing our efforts on where we can make a difference, we want to help more healthcare providers help more patients, in a sustainable way, and empower more people to take care of their health and well-being – by applying our combined capabilities in innovation, impactdesign and clear accountability –sustainability.
There is a primary enablerlot of work to drive flawless execution.be done, but we see the potential for a future where health systems run smoothly, efficiently and sustainably, with doctors and nurses seeing the patient at the right time and at the right point of care. Where they can be confident that the right choice is also the easy choice, with simpler workflows enabling them to give patients the best care and best experience.
The set of measures we have taken includesWith real-time and predictive insights supporting collaboration across the very difficult, yet necessary decisions announcedpatient journey. And AI being used in October 2022a responsible manner to optimize workflows and January 2023improve efficiency, so that clinical staff get the time and space to reduce our workforce by 4,000 employeesfocus on what matters and then a further 6,000 respectively, as we drive a major step-up in productivity. We will strive to implement these reductions with due respectwhat they do best: caring for every employee affected and in line with all local rules and regulations.their patients.
We believesee a future where it is also easier for people everywhere to look after their health and well-being. For example, with solutions helping more parents and babies in the first 1,000 days and supporting the connection between good oral care and good overall health.
Recognizing that together, these measureshuman health and environmental health go hand in hand, we are collaborating with our customers and suppliers to decarbonize healthcare and so create a more sustainable and resilient industry.
Looking ahead
While realistic about the challenging economic environment, geopolitical risks, and uncertainties around ongoing litigation, I am confident we will help us establish the culture, capabilities and infrastructure neededcontinue to consistently execute and deliver as a reliable patient- and people-centric health technology company.
Focused organic growth in Diagnosis & Treatment, Connected Care and Personal Health
As well as restoringon our reputation as a responsible patient- and people-centric innovation leader in health technology, we urgently need to get back on coursemulti-year plan to create value with sustainable impact. To do this, we will drive organic growth through scaleimpact – helping consumers lead healthy lives and leadership:
Focusing investmentshealthcare providers deliver efficient, high-quality care toaccelerate growthpatients inImage Guided Therapy, Ultrasound and Monitoring, where we have strong #1 or #2 positions, and expand our leadership position in Personal HealthScaling our new Enterprise Informatics businessDriving margin improvement in Diagnostic ImagingRestoring the Sleep & Respiratory Care business.
We will leverage our distinctive market positions, especially our strong presence in North America and many international markets, while further localizing to support our leadership position in China.
Patient- and people-centric innovation at scale
We will continue to invest significantly in innovation, but are making a number of important changes to increase the impact of our patient- and people-driven innovation. Focusing our resources on fewer, better-resourced and more impactful projects, we will concentrate a higher proportion of our R&D resources in the businesses to ensure that innovation is done closer to our customers. We will scale and accelerate innovations, driven by the business and supported by rightsized corporate research, with patient safety, quality and sustainability at the core of innovation design. The technological and business model innovation that Philips brings to healthcare across care settings – often as part of long-term partnerships – is critical, making care delivery more convenient and sustainable.
2022 performance
Looking back on last year, sales increased nominally to EUR 17.8 billion, while several factors weighed down on profitability. Performance was impacted by our efforts to mitigate supply chain and inflationary pressures and the revenue and cost consequences of the Philips Respironics sleep recall, whilst at the same time dealing with global challenges such as the COVID situation in China, volatile demand and supply, and the war in Ukraine. As we worked through the operational challenges, we progressedsustainable way. Based on our ongoing actions to enhance execution priorities in the fourth quarter and saw initial signs of improvement.
I find it greatly encouraging that, despite our recent difficulties, Philips’ purpose, strategy and solutions resonate strongly with customers, as evidenced by the around 100 long-term strategic partnerships we entered into with hospitals and health systems around the world in 2022, and by the continued strength of our order book.
Delivering on our ESG commitments
Environmental, Social & Governance (ESG) are three key dimensions defining our approach to doing business responsibly and sustainably. In 2022, we reached 1.81 billion people with our products and services, including 202 million in underserved communities – taking us a step closer to our goal of improving 2 billion lives per year by 2025, including 300 million in underserved communities.
We continued to work hard to deliver on our other key ESG commitments. For example, our updated carbon reduction targets were approved by the Science Based Targets initiative (SBTi), and we were included in CDP’s climate action ‘A-List’ for the 10th year in a row. We see increasing momentum within the healthcare industry and on the part of our customers to reduce their environmental impact, and we are well placed – with innovations such as our BlueSeal magnet for helium-free-for-life MR and our Circular portfolio – to support that trend and help create a sustainable infrastructure for the future of healthcare.
Looking ahead
We remain cautious in light of the subdued economic outlook for the year, staffing and inflationary pressures facing our customers, geopolitical risks, supply and demand volatility, and uncertainties around ongoing consent decree negotiations, litigation and Department of Justice investigations. Nevertheless, we expect that, by prioritizingdriving patient safety and quality, tightening our focus on innovationincreasing supply chain reliability, and strengthening our category leadership areas, while at the same time improving execution and taking a disciplined approach to capital,further simplifying how we will be able to progressively create value with sustainable impact. work – we expect further performance improvement in 2024.
Against this background, and reflecting the importance we attach to dividend stability, we propose to maintain the dividend at EUR 0.85 per share, to be distributed fully in shares.
On behalf of the Executive Committee, I would like to acknowledge, once again, that 2022 has been very disappointing and we carry accountability for the plan to bring Philips back to where it belongs. I want to thank our consumers, our customers and their patients, for their understanding – and our suppliers and ecosystem partners for their support, – over this past year.and the Supervisory Board for their support and guidance. I appreciatealso want to express my deep gratitude to our employees' hard workemployees for their dedication to improving people’s lives and willingnessour company’s performance, and to embrace change and drive performance improvement. And I wish to thank our shareholders and other stakeholders for their continued support in these challenging times.support.
As I am honored to have been tasked with leading our company and am heartened by the support I have encountered from our employees and customers, investors and other stakeholders.look ahead, I am realistic about the challenges we face, butoptimistic about building on the momentum we have full confidence increated, and excited about delivering on our planpurpose – for the benefit of actionpatients, customers and am firm in my resolve to lead Philips back to a position of strength in aconsumers the world that needs meaningful innovation.over.
Roy Jakobs
Chief Executive Officer
5Board of Management and Executive Committee
Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties. The Board of Management is entrusted with the management of the company. The other members of the Executive Committee have been appointed to support the Board of Management in the fulfilment of its managerial duties. Please also refer to Board of Management and Executive Committee within the chapter Corporate governance.
Members of the Board of Management
Roy Jakobs
Chairman of the Board of Management and the Executive Committee (since October
Roy Jakobs joined Philips in 2010 and has held various global leadership positions across the company, starting as Chief Marketing & Strategy Officer for Philips Lighting. In 2012, he became Market Leader for Philips Middle East & Turkey, leading the Healthcare, Consumer, and Lighting businesses out of Dubai. Subsequently, he became Business Leader of Domestic Appliances, based in Shanghai, in 2015. In 2018, Roy joined the Executive Committee as Chief Business Leader of the Personal Health businesses and in early 2020 he started as Chief Business Leader of Connected Care. Prior to his career at Philips, he held various management positions at Royal Dutch Shell and Reed Elsevier.
Abhijit Bhattacharya
Born 1961, Indian
Executive Vice President
Member of the Board of Management (since December 20152015)
Chief Financial Officer
Abhijit Bhattacharya first joined Philips in 1987 and has held multiple senior leadership positions across various businesses and functions in Europe, Asia Pacific and the US. Between 2010 – 2014, he was the Head of Investor Relations of Philips, and subsequently, CFO of Philips Healthcare, Philips’ largest sector at the time. Prior to 2010, Abhijit was Head of Operations & Quality at ST-Ericsson, the joint venture of ST Microelectronics and Ericsson, and he was CFO of NXP’s largest business group.
Marnix van Ginneken
Born 1973, Dutch
Executive Vice President
Member of the Board of Management (since November 20172017)
Chief ESG & Legal Officer
Marnix van Ginneken joined Philips in 2007 and became Head of Group Legal in 2010. In 2014, Marnix became Chief Legal Officer of Royal Philips and Member of the Executive Committee. In 2017 he was appointed to the Board of Management. He is responsible for ESG/Sustainability,driving ESG efforts across the company, including Sustainability. He is also responsible for Legal, Intellectual Property & Standards and Government & Public affairs. Since January 1, 2024 he is Chairman of the Board of the Philips Foundation. In 2011, he is alsowas appointed Professor of International Corporate Governance at the Erasmus School of Law in Rotterdam. Before joining Philips, Marnix worked for Akzo Nobel and as an attorney in a private practice.
Other members of the Executive Committee
as of December 31, 2022
Willem Appelo
Chief Operations Officer
Andy Ho
Chief Market Leader of Philips Greater China
Deeptha Khanna
Chief Business Leader Personal Health
Bert van Meurs
Chief Business Leader Image Guided Therapy and
Edwin Paalvast
Chief Market Leader of International
Shez Partovi
Chief Innovation & Strategy Officer
Vitor Rocha Jeff DiLullo
Chief Market Leader of Philips North America
Daniela SeabrookHeidi Sichien
Chief
Kees WesdorpSteve C de Baca
Chief Patient Safety and Quality Officer
Julia Strandberg
Chief Business Leader
This page reflects the composition of the Executive Committee as per December 31, 2022. As announced on December 8, 2022, Kees Wesdorp left the company on January 1, 2023, with Bert van Meurs (Chief Business Leader for the Image Guided Therapy businesses) temporarily expanding his role to include the leadership of the Precision Diagnosis businesses. As announced on January 30, 2023, Steve C. de Baca and Jeff DiLullo joined the Executive Committee, effective February 6, 2023, as Chief Patient Safety & Quality Officer and Chief Market Leader of Philips North America, respectively. As such, Mr DiLullo succeeds Vitor Rocha, who left the company effective as per the same date. Philips expects to announce new leaders for its Connected Care businesses (which was the responsibility of Roy Jakobs until his appointment as CEO) as well as for its Precision Diagnosis businesses, in 2023. For a current overview of the Executive Committee members, see also https://www.philips.com/a-w/about/executive-committee.html
6Strategy and Businesses
6.1Our strategic focus
A strategy of focused organic growth, founded on clear choices in business and innovation, and improved execution
OverToday, most healthcare systems are struggling to keep up with the past 10 years, Philips has undergone a transformation to reshape its portfolio and become a focused health technology company. As a result, we are active in highly attractive segments that offer significant potential for growth and margin expansion.
These markets are attractive due to the underlying growth of demand for access to healthcare from an aging and growing population. This in turn fuels theever-rising need for, meaningful innovation to address the risingand cost of, healthcare, spending andwhile systemic staff shortages and makefinancial resource constraints increase the pressure. Climate change is impacting both environmental and human health, compounding the stress on our healthcare more efficientsystems and productive, while driving better outcomes.
influencing consumer behavior. At Philips, we view the provision and collection of data from patient monitors, imaging devices, and Electronic Medical Records as the foundation upon which Artificial Intelligence (AI) propositions can be built to turn clinical data into actionable insights for patients, providers, and consumers. In addition to providing clinical insights, the same system, informatics and service solutions also provide improved operational forecasting – something our customers have been requesting since COVID-19 to help them improve productivity.
When we perform all of the above for a particular health condition, such as cardiac disease, we establish domain expertise across various sites of care for that disease state. Our healthcare customers are asking for integrated innovations that enable them to care for patientstime, in both in the hospital and in outpatient settings. In parallel we continue to provide impactful consumer health propositions
Creating value with sustainable impact
2022 was a difficult year for Philips as its business and financial performance suffered due to challenges in execution, quality and supply, and a complex operating model. Going forward, Philips will address these operational challenges, improve performance, and drive progressive value creation through a strategy of a) focused organic growth, b) scalable patient- and people-centric innovation, and c) focus on reliable execution, prioritizing patient safety and quality, supply chain reliability, and a simplified operating model. All supported by a reinvigorated culture of accountability, empowerment and strengthened health technology talent and capabilities.
Focused organic growth
Having transformed to become a health technology company in recent years, we will now focus on extracting the full value of our strong portfolio with leading positions.
We will focus investments to accelerate growth and margin expansion in areas – Image Guided Therapy, Ultrasound, Monitoring, and Personal Health – where we have strong #1 or #2 positions. In 2022, approximately 70% of our sales were generated by businesses with such leadership positions in the hospital and the home. We will also scalehome, emerging technologies and AI are affecting our new Enterprise Informatics business, drive margin improvement in Diagnostic Imaging, and restore Sleep & Respiratory Care.lives like never before.
Scalable patient- and people-centric innovation
Philips’At Philips, our purpose –is to improve people’s health and well-being through meaningful innovation. As such, we see huge opportunities to make a difference through innovation, design, and sustainability – is at the centerpartnering with our healthcare customers to increase productivity and deliver better care for more people through our innovation platforms of everything we do. This core principle has never beenmonitoring, imaging, interventional and enterprise informatics. And empowering more relevant than it is in these challenging times. people to take care of their health and well-being through our personal health propositions.
Our plan to create value with sustainable impact
As a leading health technology company, we believe that – viewedPhilips is committed to driving progressive value creation through the lensa strategy of customer needs –focused organic growth, scalable patient- and people-centric innovation, can improve people’s health and healthcare outcomes, as well as makingfocus on reliable execution.
Philips has significant strengths to build on. We have a portfolio of patient- and people-centric innovations in hardware, software, AI and services, supporting care more convenient and sustainable, both in the hospital and at home.
Given our global presence, strong enterprise informatics platforms, (ambulatory) monitoring and imaging data, as well as our capabilities to support care across settings, we believe Philips is well positioned to do this, and – leveraging our strong clinical, consumer and Environmental, Social & Governance (ESG) franchise, and our strong brand – do it in a convenient and sustainable way.
In the consumer domain, we develop innovative solutions that support healthier lifestyles, prevent disease, and help people to live well with chronic illness, also in the home and community settings.
In clinics and hospitals,home. And we are teaming up with healthcare providers to innovatethe preferred strategic and transforminnovation partner for many customers across the way care is delivered. globe.
A strategy of focused growth
We listen closely tooperate in growing market segments, where attractive margins provide a foundation for sustainable value creation. To deliver on our customers’ needs and togetherstrategy, we co-create solutions that help our customers improve outcomes, patient and staff experience and productivity.make clear portfolio choices. We are embedding AIconcentrating our resources on areas where we have strong positions and data science in our propositionscan accelerate growth and expand margins more quickly – for instance, applying the power of predictive data analyticsImage Guided Therapy, Monitoring, Ultrasound, and artificial intelligence at the point of care – to leverage the value of data in the clinical and operational domains, aiding clinical decision making and improving the quality and efficiency of healthcare services. Increasingly, we are working together with our health systems customers in novel business models, including outcome-oriented payment models, that align their interests and ours in long-term partnerships.
Going forward,Personal Health. In doing so, we will focus our innovation on where we see customer needs evolving. To improve outcomes, we willto support clinical workflows in areas where we have domain leadership, e.g.such as cardiology, and that build on our deep strength in the ICU. ToIntensive Care Unit (ICU) and Cath Lab.
In Diagnostic Imaging, our goal is to help healthcare providers who need to do more with less. We will do so by leveraging our differentiating, AI-enabled innovations to increase their imaging workflow productivity, departmental efficiency and financial sustainability and, by doing so, improve our margins and drive uptake of our services supporting care pathways.
We help our customers to unlock actionable insights from pools of medical imaging data, vital signs (patient monitoring) data and insights generated with the support of artificial intelligence (AI) to optimize care delivery across the patient journey. With the scaling of our end-to-end multi-vendor Enterprise Informatics business, we aim to grow our platforms such as radiology, pathology and remote care delivery across health systems and care settings, while building long-term customer relationships, generating recurring revenue and enabling the hardware business to maintain a competitive advantage.
Additionally, we remain committed to rebuilding our position in Sleep & Respiratory Care while continuing to resolve the effects of the Respironics recall.
Scalable patient- and people-centric innovation
At Philips, we’ve been innovating to improve lives for over 130 years. People’s needs are at the very heart of how we innovate and design for sustainable impact with a system having‘safety and quality first’ mindset.
Innovation is our core strength and will continue to contend with high patient volumes, staff shortagesbe our core differentiator. Recent industry trends have accelerated the adoption of technology within healthcare. We are embracing these trends and rising costs, we will enhance care pathways and operational workflows through integrated technology infrastructure, and we will leverageshifting our (enterprise) informatics and hardware innovation to lower costsa more patient- and reduce the burden on staff. To improve the delivery of care outside the hospital,people-centric model closer to our customers. This starts with asking: What do people – in our case, patients and clinicians, nurses and technicians, consumers – really need? And how can we will utilize our consumer/home experience and our strength in data and informatics to connect andbest support care for patients,healthcare professionals with better outcomes, across settings.their workflow?
In doing so, we will leverage leading technologies across our portfolio. To name just a handful, by way of example: our Ingenia Ambition MR system with BlueSeal magnet that offers helium-free-for-life operation; our Azurion suite of interventional cardiology solutions; our IntelliVue MX750 and MX850 patient monitors and MCOT ambulatory cardiac ePatch offering comprehensive monitoring capabilities across sites of care; and our multi-vendor, multi-modality, multi-site Radiology Operations Command Center virtualized imaging solution.
While we continue to invest significantly in innovation, we are making a number of important changes to increase the impact of our patient- and people-driven innovation and generate better returns. Moving forward, we will focus our resources on a smaller number of projects and products with greater potential for impact. We will scale and accelerate innovations, driven by the business and supported by rightsized Group research, with patient safety, quality and sustainability at the core of design. By bringing our central innovation activities into the heart of the businesses, we are bringingfocusing our systemefforts and software innovation closerresources on fewer projects offering greater scale and impact on patient outcomes and care providers’ clinical, operational and sustainability challenges. We do this by balancing new, breakthrough innovations and continuous optimized lifecycle management, through upgrades and services, of Philips products and systems already deployed in care settings. We bring together expertise across the product lifecycle, from research through serviceability, to ensure our customers.innovations drive maximum impact for our customers and consumers – delivering a superior experience and value, with minimum environmental impact.
Focus on improvedExecution as the value driver
Enabled by a culture of patient- and people-centricity, accountability and impact, supported by strong health technology capabilities, we see effective execution
The as the key value driver of our plan and a key driver for change. We are focusing on:
- Patient safety and quality – remains our
performance improvement is improved execution grounded in three decisive actions:- highest priority
- End-to-end supply chain resilience
- A simplified operating model with an agile way of working
First, patient safety and quality: putting thisquality is at the heart of (business) innovation,everything we do. We have stepped up accountability for patient safety and quality, for example, by elevating oversight to the function to Executive Committee level, and embedding itcreating a new organization, with stronger processes and more effective early warning systems in our culture, e.g. bythe businesses, as well as giving all employees dedicated patient safety and quality objectives;
Second, we are re-shaping our supply chain reliability: movingset-up so we can ensure reliable delivery of products and services and deliver our order book. We have moved away from being organized around central functions to a centralized ‘one size fits all’structure where we align procurement and supply chain to our businesses. A more regionalized structure combined with dual sourcing that can work effectively even when volatile conditions emerge in different parts of the world. We are pruning our product portfolio, which includes a long tail of smaller product lines and older generations of our products. We also have a dedicated team redesigning products and components to aincrease our resilience to more volatile demand.
Finally, we are implementing our simplified operating model to enable us to better serve patients, customers and consumers, as well as ensuring that our cost of organization remains competitive in an inflationary and cost-driven environment, and that we are more agile dedicated end-to-end set-up per business,in responding to changes in the market. Prime accountability has been assigned to the businesses, supported by lean Functions and pruning and redesigning products;
Driving impact for people and accountable leadershipplanet
Building on our strong heritage in environmental sustainability and social impact, we have operationalized our purpose by adopting a fully integrated approach to doing business responsibly and sustainably. We are partnering with empowered teams.stakeholders to drive environmental, social and governance (ESG) priorities and make a global impact. For example:
- We aim to improve the lives of 2.5 billion people a year, including 400 million in underserved communities, by 2030
- We will continue to operate carbon-neutrally and are partnering with customers and suppliers on reducing emissions across the full value chain in line with science-based targets
- We aim to increase circular revenues from 15% of sales in 2020 to 25% of sales by 2025
Our ambitionDelivering on our plan
With our global reach, market leadership positions, deep clinical and technological insights, and customer-centric, patient- and people-focused innovation, capability, we believe Philips is well placedpositioned to create further value inhelp deliver real change across healthcare. Fueled by our purpose and supported by a changingreinvigorated culture of accountability and empowerment, as well as strengthened health and care world.
Wetechnology capabilities, we aim to improve the lives of 2 billion people a year by 2025, including 300 million in underserved communities, rising to 2.5 billion and 400 million respectively by 2030. This is one of the comprehensive set of commitments we have deployed across all the Environmental, Social and Governance (ESG) dimensions that help guide the execution of our strategy and support our contribution to UN Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages), 12 (Ensure sustainable consumption and production patterns) and 13 (Take urgent action to combat climate change and its impacts).
We strive to deliver superior, long-termprogressively create value to patients, customers, consumers and shareholders, while acting responsibly towards our planet and society, in partnership with our stakeholders. We believe that, executed with rigor, discipline and quality, the strategic imperatives outlined above, in combination with a relentless focus on execution, will put us back on track for a future of progressive value creation with sustainable impact.
6.2How we create value with sustainable impact
BasedThe overview below is based on the International Integrated Reporting Council framework we useand includes resource inputs, value outcomes and societal impact across various resources to create value with sustainable impact for our stakeholders.financial and Environmental, Social and Governance (ESG) dimensions.
Resource inputs
Human
- Employees
77,233,69,656, 120-plus nationalities, 39% female Philips University 1,344,956Training 3,670,963 courses,1,880,4162,987,260 hours,1,009,4593,578,199 training completions32,74230,558 employees ingrowthGrowth geographies- Focus on Inclusion & Diversity
Intellectual
- Invested in R&D EUR
2.11.9 billion(Green(Green/EcoDesigned Innovation EUR168142 million) - Employees in R&D
11,69010,833
Financial
- Equity EUR
13.312.1 billion - Net debt*) EUR
7.05.8 billion
Manufacturing
- Employees in production
39,74235,281 - Industrial sites 23, cost of materials used EUR
4.34.6 billion - Total assets EUR
3129 billion - Capital expenditures on property, plant and equipment EUR
444345 million
Natural
- Energy used in manufacturing
338.1 gigawatt322,532 megawatt hours - Water used
677,632661,076 m3 - 'Closing the loop' on all our professional medical equipment by 2025
Social
- Philips Foundation
- Stakeholder engagement
- Volunteering policy
Value outcomes
Human
- Employee Engagement Index
77%73% favorable - Sales per employee EUR
230,817260,840 - Safety 172 Total Recordable Cases
Intellectual
- New patent filings
920795 - Royalties EUR
419.0434.2 million 171160 design awards for the Philips brand
Financial
- Comparable sales growth*)
(2.8)%6.0% - Adjusted EBITA*) as a % of sales
7.4%10.6% - Free cash flow*) EUR
(961)1,582 million
Manufacturing
- EUR
12.112.4 billion revenues from goods sold
Natural
71.7%70.5% Green/EcoDesigned Revenues18%20.0% revenues from circular propositions- Net CO2 emissions from own operations down to zero kilotonnes
62,000107,000 tonnes (estimated)materialsfrom products, parts and packaging used to put products on the market- Waste
22,80219,375 tonnes, of which 91%repurposedrecirculated
Social
- Brand value USD
12.811.2 billion (Interbrand) - Partnerships with UNICEF, Red Cross, Amref and Ashoka
Societal impact
Human
- Employee benefit expenses EUR
6,9526,903 million, all staff paid at least a Living Wage - Appointed
71%81% of our senior positions from internal sources 30%31% of Leadership positions held by women
Intellectual
- Around
55%48% of revenues from new products and solutions introduced in the last three years - Approximately 70% of sales from leadership positions
Financial
- Market capitalization EUR
1219 billion at year-end - Long-term credit rating
A-BBB+1, Baa12, BBB+3**) - Dividend EUR
741749 million
Manufacturing
- 100% electricity from renewable sources
Natural
- Environmental impact of Philips operations up to EUR
128261 million - All 23/23 industrial sites 'Zero Waste to Landfill' at year-end
20222023 - Updated CO2 reductions approved by the Science Based Targets initiative
Social
1.811.88 billion Lives Improved, of which202221 million in underserved communities (including2.21.4 million via Philips Foundation)459,000723,000 employees impacted at suppliers participating in the 'Beyond Auditing' program- Total tax contribution EUR
3,4693,051 million (taxes paid/withheld) Income tax benefit EUR 113 million; the effectiveCorporate income taxrate is 6.5%paid EUR 152 million
6.3Double Materiality analysisAssessment
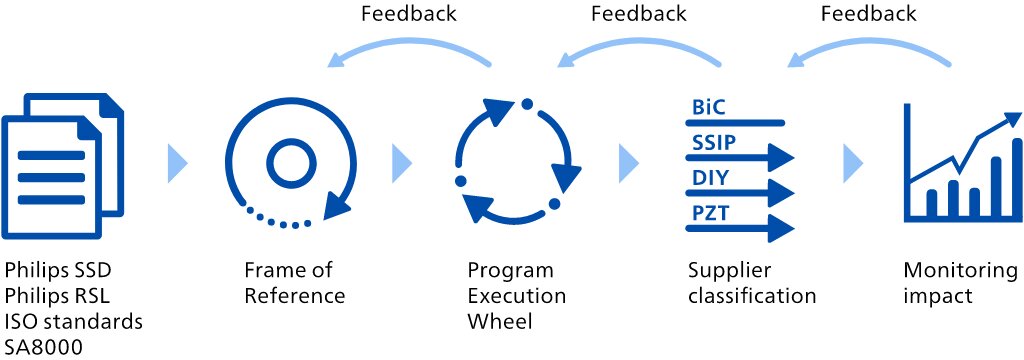
We identify the Environmental, Social and Governance topics which we believe have the greatest impact on our business and the greatest level of concern to stakeholders along our value chain, for instance patient safety and quality. We do this through a multi-stakeholder process. Please refer to Working with stakeholders and advocacy for more information. Assessing these topics enables us to prioritize and focus upon the most material topics and effectively address these in our policies, programs, targets and targets.actions. We do this with reference to the GRI standard and identify and assess impacts on an ongoing basis, for example through discussions with our customers, suppliers, investors, employees, peer companies, social partners, regulators, NGOs, and academics. We also conduct a benchmark exercise, carry out trend analysis and run media searches to provide input for our materiality analysis. GRI has not yet published a sector standard for the healthcare industry. Philips’ impact on society at large is covered through our Lives Improved metric and the Environmental Profit & Loss account, as well as a number of other KPIs addressed in Environmental, Social and Governance. The result of our impact materiality assessment you will find below.

Similar to 2021,2022, we used an evidence-based approach to materiality analysis,assessment, powered by a third-party AI-based application. The application allows automated sifting and analysis of millions of data points from publicly available sources, including corporate reports, mandatory regulations and voluntary initiatives, as well as news. In our 2022 materiality analysis, we identified a list of topics that are material to our businesses. With this data-driven approach to materiality analysisassessment we have incorporated a wider range of data and stakeholders than was ever possible before and managed to get an evidence-based perspective on regulatory, strategic and reputational risks and opportunities. Topics were prioritized through a survey sent to a large and diverse set of internal and external stakeholders, combined with input from the application.
Public health risks emerged as a new material topic in 2020, as a result of the COVID-19 pandemic, and it was assessed as a material topic in 2022 as well.
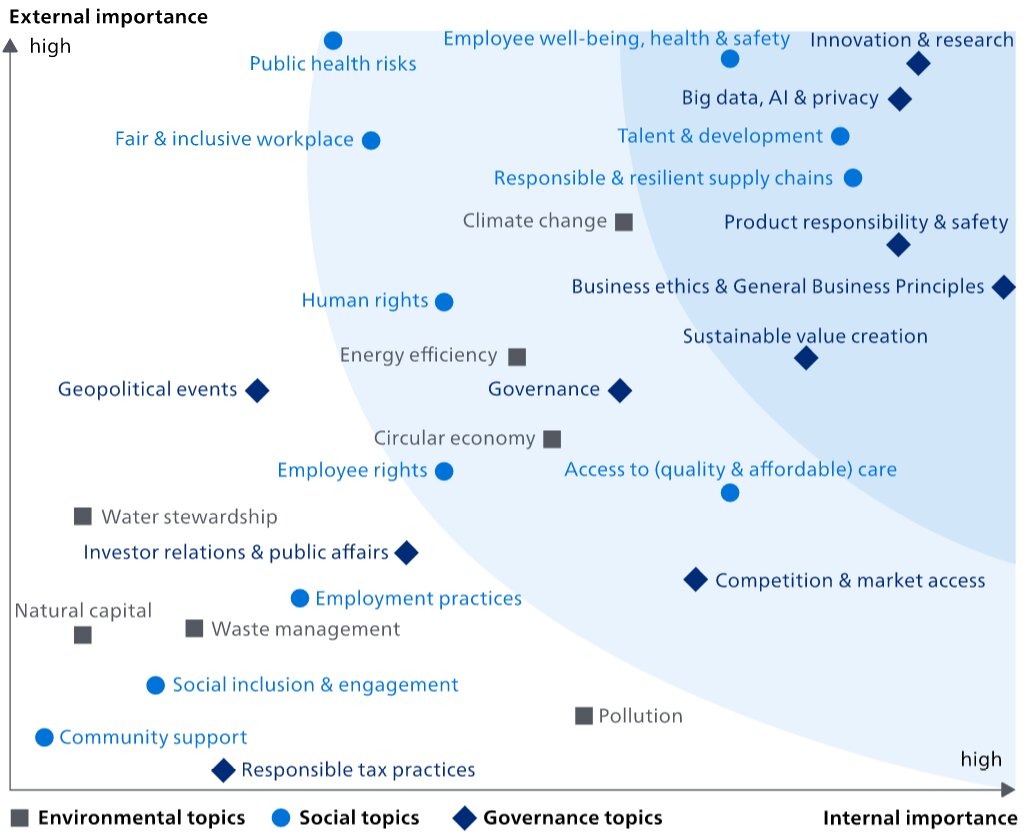
Changes in 20222023
On theboth external and internal importance axis, the most significant increases compared to 20212022 were Sustainable value creation, Geopolitical events, ResponsibleWaste management and Resilient Supply Chains, TalentSocial inclusion & development, and Energy efficiency.engagement. Innovation & research went down on both axes. On the internal importance axis, there werewas a significant increasesdecrease on Pollution, Governance, Access to (quality and affordable) care, Competition & market access, and Talent & development.Pollution.
Double materiality
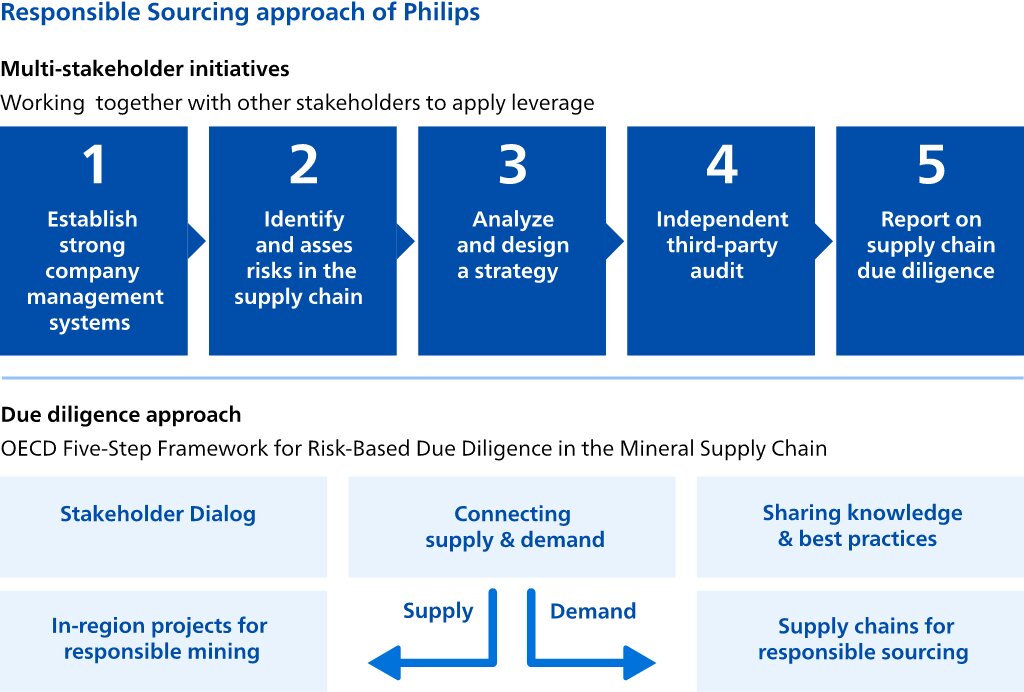
After completing the regular impact materiality analysis,assessment, we completed a preliminary 'double materiality' analysis,assessment, in preparation for the upcoming requirements of the EU Corporate Sustainability Reporting Directive (CSRD). The double materiality analysisassessment addresses both financial materiality (the impact of society on Philips) as well as impact materiality (the impact of Philips on society): we only included the high and medium material topics listed above.from impact materiality and/or financial materiality. The data sources used for the financial materiality include corporate reports, mandatory regulations with sanctions, voluntary initiatives by e.g. central banks, and Sustainability Accounting Standards Board (SASB) accounting metrics. For impact materiality, we included sustainability data from corporate reports or sustainability reports, coverage in the news and voluntary initiatives and regulation. We calibrated the financial and impact materiality with a team of internal experts from Enterprise Risk Management, Group Control, Internal Audit, Insurance and Risk Management and Sustainability and aligned with our Enterprise Risk Management assessment. After this calibration the financial impact of Product responsibility & safety, Geopolitical events, and Big data, AI & Cybersecurity were increased. The results of the double materiality analysis are depicted below.
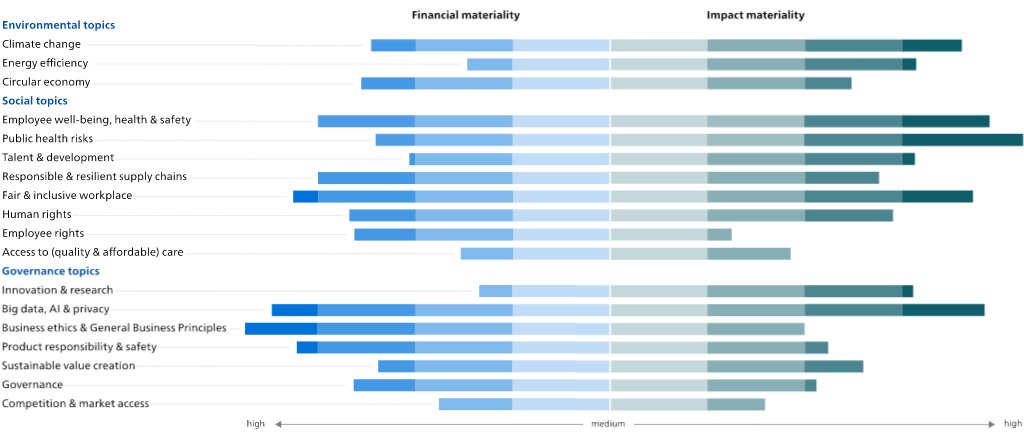

From the financial materiality analysis,assessment, the topics that ranked highest were: (1) from the environmental topics, Circular economy, and Climate change; (2) from the social topics, Fair & inclusive workplace, Employee well-being, healthProduct responsibility & safety, and Responsible & resilient supply chains;(2) Geopolitical events, and (3) from the governance topics,Big data, AI & Cybersecurity, as well as Business ethics & General Business Principles, Big data & privacy, and Product responsibility & safety.business principles.
From the impact materiality analysis,assessment, the topics that ranked the highest were: (1) from the environmentalEnvironmental topics, Climate change, and Energy efficiency; (2) from the socialSocial topics, Public health risks and Employee well-being, health & safety, and FairAccess to (quality & inclusive workplace;affordable) care; and (3) from the governanceGovernance topics, Big data, AI & privacyCybersecurity, and InnovationCompetition & research.market access. These topics are all covered in more detail in the Annual Report 20222023 and monitored regularly.
The outcome of the double materiality assessment did not result in any significant changes in the material topics identified.identified from impact materiality.
The results of our materiality assessment have been reviewed and approved by the Philips ESG CommitteeBoard of Management and will be used to prepare for the upcoming EU legislation.
6.4Our businessesbusiness structure
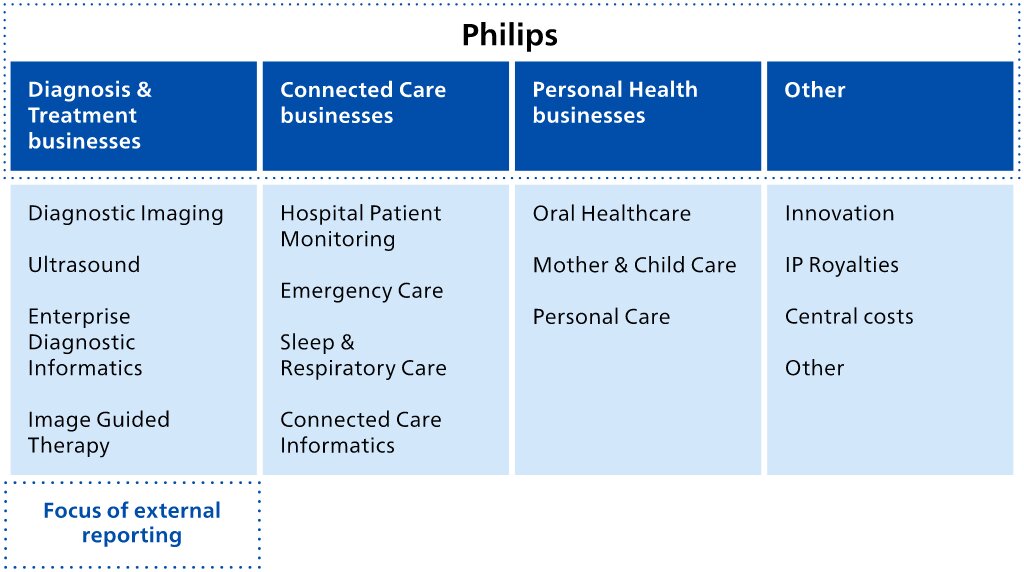
Our reporting structure in 2022
Koninklijke Philips N.V. (Royal Philips) is the parent company of the Philips Group. In 2022,As announced on January 30, 2023, Philips has changed its operating model to end-to-end businesses with single accountability in order to make the reportablecompany more agile in its drive to create value with sustainable impact. The segments were Diagnosis & Treatment, businesses, Connected Care businesses, and Personal Health businesses,are each having been responsible for the management of itstheir business worldwide.activity worldwide, and are made up of the six businesses shown below. Additionally, Royal Philips identifies the segment Other.

Philips Group
Total sales by reportable segment
| Diagnosis & Treatment | |
| Connected Care | |
| Personal Health | 20% |
| Other |
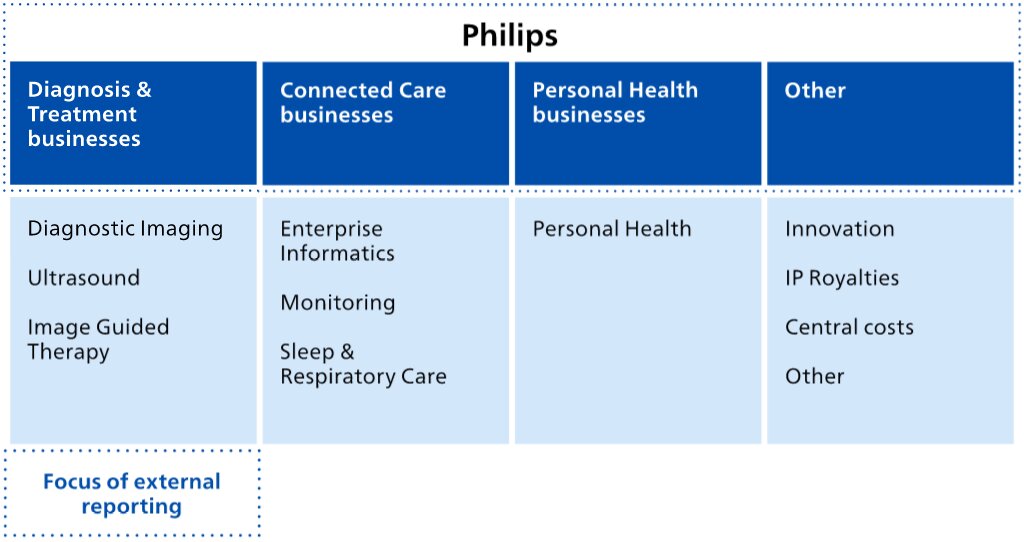
Our reporting structure in 2023 and beyond
As announced on January 30, 2023, Philips is changing its operating model to end-to-end businesses with single accountability. In 2023, the businesses will be as follows.
6.4.1Diagnosis & Treatment businesses in 2022segment
Our Diagnosis & Treatment businesses create value through their unique portfolio of innovative solutions – consisting of systems, smart devices, software and services, powered by AI-enabled informatics –solutions that support precision diagnosis and minimally invasive treatment in therapeutic areas such as cardiology, peripheral vascular, neurology, surgery, and oncology. With these solutions, we enable our customers to realize the full potential of the Quadruple Aim – better health outcomes, improved patient and staff experience, and lower cost of care.
Serving diagnostic enterprise imaging markets globally, weour goal is to improve customer performance in the radiology/imaging workflow. We see significant opportunity to enable precision diagnosis while at the same time supporting adjacent needs for care orchestration across care pathways and increasing departmental productivity. We do this through smart diagnostic systems, connected workflow solutions, and integrated AI-supported diagnostics and pathway informatics, drivinginformatics. These drive enterprise-wide operational efficiency and helpinghelp clinicians to provide an early and definitive diagnosis, enabling them to select tailored care pathways with predictable outcomes for every patient, both inside and outside the hospital.
We also provide integrated solutions combining imaging systems, and diagnostic monitoring data and therapeutic devices, which optimize interventional procedures to deliver more effective treatment, better outcomes and higher productivity. Building upon our leading-edge Image Guided Therapy System – Azurion system, we continue to innovate, optimizing clinical and operational lab performance through advances in workflow and integration for routine procedures, and expanding the role of image-guided interventions to treat new groups of patients such as those with complex diseases including various cardiovascular conditions, stroke and lung cancer and spine disorders.cancer. We are also innovating the way we engage with our customers, using new business models across different care settings, including out-of-hospital settings such as office-based labs and ambulatory surgical centers, which offer clear clinical, financial and operational benefits.
In 2022, Philips completed the acquisition of Vesper Medical Inc. a US-based medical technology company that develops minimally-invasive peripheral vascular devices. Vesper Medical will further expand Philips’ portfolio of diagnostic and therapeutic devices with an advanced venous stent portfolio for the treatment of deep venous disease.
In 2022,2023, the Diagnosis & Treatment segment consisted of the following areas of business:businesses:
- Precision Diagnosis, consisting of:
- Diagnostic
Imaging:Imaging:- Diagnostic X-ray business unit – systems with associated software to optimize diagnostic imaging quality and improve efficiency and productivity for the hospital
- Magnetic Resonance Imaging
(MRI), withbusiness unit – helium-free-for-life operations, bundled with associated AI-enabled software to streamline workflows, optimize diagnostic quality, and improve patientexperience; X-ray systems, together with associated software to streamline workflows and optimize diagnostic quality;experience - Computed Tomography AMI business unit – advanced and efficient
Computed Tomography (CT)systems and software, including detector-based Spectral CT and molecular and hybrid imaging solutions for nuclear medicine
- Ultrasound
:business unit – echography solutions focused on diagnosis, treatment planning and guidance for cardiology, general imaging, obstetrics/gynecology, and point-of-care applications, as well as proprietary AI-enabled and intelligent software capabilities to enable early, advanced diagnostics and timely interventions, and remote capabilities to enable tele-ultrasound operations and training Enterprise Diagnostic Informatics: a suite of integrated multivendor products and services that deliver a comprehensive platform designed to connect clinical data and optimize workflows around every step in the patient’s journey across a range of diagnostic (radiology, point-of-care, laboratory) and clinical (oncology, cardiology, neurology) service lines
- Diagnostic
- Image Guided
Therapy:Therapy, consisting of:- Image Guided Therapy Systems business unit – integrated interventional systems that combine information from imaging systems, interventional devices, navigation tools, monitoring patient data and patient health records, supported by AI, to provide interventional staff with the control and information they need to perform procedures
efficiently;efficiently - Image Guided Therapy Devices business unit – interventional diagnostic and therapeutic devices to treat coronary artery and peripheral vascular disease
- Image Guided Therapy Systems business unit – integrated interventional systems that combine information from imaging systems, interventional devices, navigation tools, monitoring patient data and patient health records, supported by AI, to provide interventional staff with the control and information they need to perform procedures
Diagnosis & Treatment
Total sales by business
| Image Guided Therapy |
Revenue is predominantly earned through the sale of products, leasing, customer services fees, recurring per-procedure fees for disposable devices, and software license fees. For certain offerings, per-study fees or outcome-based fees are earned over the contract term.
Sales channels are a mix of a direct sales force, especially in all the larger markets, third-party distributors and an online sales portal. This varies by product, market and price segment. Our sales organizations have an intimate knowledge of technologies and clinical applications, as well as the solutions necessary to solve problems for our customers.
Under normal circumstances, salesSales at Philips’ Diagnosis & Treatment businesses are generally higher in the second half of the year, largely due to the timing of customer spending patterns.
At year-end 2022,2023, Diagnosis & Treatment had around 33,00028,397 employees worldwide.
2022 business2023 highlights
At RSNA23, Philips received FDA clearance to marketannounced a raft of new innovations, including next-generation EPIQ Elite 10.0 and Affiniti ultrasound systems that increase diagnostic confidence and workflow efficiency, BlueSeal MRI Mobile, the world’s first and only mobile MRI system with helium-free operations, and new AI-enabled cloud solutions that enhance radiology efficiency and clinical confidence.
Philips launched its new MR 7700 3.0T MR system, which features an enhanced gradient system for Philips’ highest image qualitydesigned to deliver outstanding imaging results and speed to support confident diagnosis for every patient.
Five top hospitals in Shanghai, with a precision diagnosis. Philips also received FDA clearance for its SmartSpeed MR acceleration software, adding AI data collection algorithms tototal of more than 10,000 beds, installed Philips’ existing Compressed SENSE MR engine for higher image resolution with three times faster scan times and virtually no loss in image quality.
By combining theadvanced Spectral CT 7500 scanner with the Azurion with FlexArm image-guided therapyimaging system, Philips has developed a fully integrated hybrid angio CT suite solution for single-room, single-sessionhelping physicians deliver first-time-right diagnosis and treatment in areas such as oncology, stroke, and trauma care.
In radiotherapy, the AI-enabled Philips MR for Calculating Attenuation (MRCAT) Head and Neck radiotherapy application expands the range of MR-only workflows for cancer patients, advancing comprehensive and personalized cancer care through precision oncology solutions.fast, low-dose X-ray scans.
Philips further expanded its leading ultrasound portfolio with the FDA market clearance for its newlaunch of the Ultrasound 5000 Compact system to deliver5500 CV, which delivers cart-based premium image quality in compact formultrasound exams for point-of-care, cardiology generaland vascular patients at the bedside.
Philips IntraSight Mobile received approval from the Chinese regulatory authority, paving the way for its commercial introduction in the Chinese market. IntraSight Mobile combines imaging and obstetricsphysiology applications on a mobile system for peripheral and gynecology applications.coronary artery disease therapy.
Building on Philips’ leading position in interventional cardiology solutions,Philips expanded its image-guided therapy portfolio with the companylaunch of Philips Zenition 10, which provides a cost-effective imaging solution to guide high-volume routine surgery, as well as complex orthopedic and trauma procedures. Philips also launched the latest versionZenition 30 Image Guided Therapy Mobile C-arm. Its workflow-enhancing features and excellent image quality enable surgeons to deliver enhanced care to more patients, helping alleviate the staff shortages faced by many hospitals.
Through the WE-TRUST study, Philips is driving innovation in stroke treatment. The trial examines the impact of its EchoNavigator image-guidance tool, which integrates live ultrasound, interventional X-ray imagingthe direct-to-angio treatment pathway on clinical outcomes, facilitated by a helical scan in the angio suite developed by Philips to reduce time to treatment. With 16 leading stroke centers and advanced 3D heart modelshospitals around the world involved in the trial and 100 patients enrolled, the WE-TRUST study has already achieved the scale and momentum needed to help interventional teams treat structural heart disease with greater ease and efficiency.
To improve outcomesdeliver a reliable evidence base on the potential benefits of the Direct to Angio Suite pathway for patients undergoing endovascularsuspected of having a large vessel occlusion (LVO) ischemic stroke – a treatment physicians now have accesspathway that focuses squarely on addressing the fact that the faster a patient is treated, the more likely they are to advanced new 3D image-guidance capabilities through Philips’ Zenition mobile C-arm system, which offers enhanced clinical accuracy and efficiency.
Philips is successfully expanding into interventional oncology withrecover. Another significant milestone was the installationpublication of its innovative lung cancer diagnosis and treatment solution Lung Suite in hospitals in Belgium, France, Israel, and the UK. Based on Philips Azurion, this solution enhances the accuracy of biopsy procedures and provides a therapy option for immediate treatment of early-stage lung cancer patients.
Inferior Vena Cava (IVC) filters are used to treat patients with venous thromboembolism, in which blood clots formhealth economics analysis results in the deep veinsJournal of the leg and groin andNeuroInterventional Surgery, demonstrating that this new stroke care pathway can travel through the circulatory system, but research has shown that they may have long-term complications. In the United States, the first patients were successfully treated for Inferior Vena Cava (IVC) filter removal using Philips' CavaClear solution – the only FDA-cleared solution for advanced IVC filter removal.save over USD 3,000 per patient.
6.4.2Connected Care businessessegment
With technology constantly advancing and becoming increasingly pervasive in 2022
Thehealthcare, the Connected Care businesses aim to connect and elevate care for all. Philips connects patients and caregivers across care settings, delivering clinical, operational and therapeutic solutions that help our customers address the Quadruple Aim ofdeliver better health outcomes, improvedimprove the patient and staff experience, and lower the cost of care. Aftercare across care settings. In 2023, the years of the COVID pandemic, which has accelerated the digital transformation of healthcare, in 2022 the volatile global economic situation continued to put additional pressure on customer budgets and worsened trends such as staff shortages, as well as increasing the need for solutions that enable more effective, sustainable and convenient care in hospital, clinics and the home.home – especially enabled by strong informatics and AI.
Philips’The Sleep & Respiratory Care business in particular facedcontinued to face multiple operational regulatory and supply-chainregulatory challenges in 2022,2023, but action has been taken to address these throughimprove the decision to establishability of the Sleep & Respiratory Care as an organization with end-to-end accountability, spanning product creation through to customer fulfillment (pending the outcome of consultation with workers councils in a number of countries). Therecorrectly assess potential patient safety or quality issues. Philips has been a reset toreaffirmed its core activities, which put patient safety front and center in everything we do, and we believe that the implementation of a new simplified organization, which for Sleep & Respiratory Care began in 2022, will help to achieve this, as well as to improve productivity and increase agility. In the course of the year, Sleep & Respiratory Care gradually returned to the market for sleep therapy devices outside the US. For information about the Philips Respironics recall and related remediation effort, please refer to Quality & Regulatory and patient safety.
With clinical depth and discovery, Philips Connected Care technologies help to cultivate a more accurate and complete view of the patient that drives better health and care. The combination of advanced technological solutions and a consultativeco-creation approach allows Philips to be an effective partner to its customers in their digital transformation, both across the enterprise and at the level of the individual clinician, nurse and patient. The roleWe help our customers to unlock actionable insights from pools of Connected Care is to collect, connect, analyzemedical imaging data, patient monitoring data, and communicate data to provide insights and clinical decision support that helpthrough the use of advanced AI, to improve outcomes and drive productivity.
To help enable care delivery across the health continuum and help our customers embrace healthcare’s digital transformation, the Connected Care businesses continue to step up platform investments that span three key domains:
Acute Patient Management: in-hospital continuous monitoring and workflow solutions fueled by advanced interoperability and patient data insightsAmbulatory Patient Care Management: ambulatory and home-based monitoring and diagnosis solutions and services supporting the patient journeyEnterprise Informatics Management: turning data insights into decision support and productivity tools.
Philips’ open, interoperable platforms aggregate and leverage information from clinical devices, patient and historical data to support care providers in patient engagement, diagnostics, (ambulatory)and patient monitoring in the hospital, ambulatory and (clinical) therapy solutions.home settings.
In January 2022, Philips completed the acquisition of Cardiologs, a France-based medical technology company focused on transforming cardiac diagnostics using artificial intelligence (AI) and cloud technology. Cardiologs is already further strengthening Philips’ cardiac monitoring and diagnostics offering with innovative software technology, electrocardiogram (ECG) analysis and reporting services.
In 2022,2023, the Connected Care segment consisted of the following areas of business:businesses:
- Monitoring – in-hospital, ambulatory and home-based monitoring and diagnosis solutions and services supporting the patient journey; as well as continuous monitoring and workflow solutions fueled by advanced interoperability and patient data insights.
- The Hospital Patient Monitoring
: Thisbusiness unit delivers acute patient management solutions to improve clinical and patient outcomes and achieve operational and economic efficiencies. Leveraging a strong presence in the operating theater and intensive care unit (ICU), Hospital Patient Monitoring solutions enhance customers’ experience and improve patient outcomes with seamless patient data pulled from over 1,000 vendor-neutral devices monitoring from admission to discharge, and by turning that patient data into clinical insights that are actionable at the right time and specific to targeted care settings. - The Ambulatory Monitoring & Diagnostics business unit provides patient care management in ambulatory and home care settings through a suite of cardiac diagnostic and monitoring solutions to identify heart rhythm disorders plus other disease states supported by AI algorithms that orchestrate workflows and services across care settings to provide care virtually anywhere.
- The Emergency Care
: Emergency Carebusiness unit’s propositions play a critical role in connected acute care management, both inside and outside the hospital, including cardiac resuscitation (e.g.AEDs)Automated External Defibrillators) and emergency care solutions (devices, services, and digital/data solutions) for professional and consumer applications.
- The Hospital Patient Monitoring
- Sleep & Respiratory Care
:– Working closely with clinical partners and Durable/Home Medical Equipment providers, Philips Respironics provides sleep and respiratory solutions to customers, clinicians and patients. This extends from ambulatory patient care solutions for obstructive sleep apnea, to solutions encompassing diagnostics, people-centric therapy, cloud-based connected propositions and care management services for patients with COPD (Chronic Obstructive Pulmonary Disease) and respiratory conditions. Hospital Respiratory Care provides invasive and non-invasive ventilators for acute and sub-acute hospital environments; Home Respiratory Care supports chronic care management in the home.
Connected Care Informatics:Enterprise InformaticsComprised of three core– By combining our informatics propositions into one end-to-end businessunits, thissegment, Philips can scale its software business,focuses on clinical analyticsproviding vendor-agnostic, integrated workflow solutions thatprovide actionable insightsconvert data from our imaging and monitoring systems into clinical and operational.- The Radiology Informatics business unit enables enterprise imaging across sites, specialties and technologies to
optimizesimplify medical image management, facilitate effective collaboration and enhance patientdata.care. - The
fully integrated Electronic Medical RecordEMR & Care Management businessenables centralized management of clinical, organizationalunit provides electronic medical record (EMR), acute care, andoperational processes,TeleICU solutions to deliver intelligent informatics propositions to connect people, data, andvirtualtechnology across caredelivery propositions, including remote patient management and real-time monitoring in acute care. The Tele-ICU program continues to play a pivotal role, enabling clinicians and nursing staff to remotely monitor a scalable number of ICU beds from a central monitoring facility with predictive analytics, enabled by Philips’ HealthSuite Platform.settings. - The Clinical
Data ServicesIntegration & Insights business– formerly Capsule Technologies, acquired by Philips in early 2021 –unit offers medical device and data integration across the enterprise for continuous, vendor-neutral data capture from more than 1,000 device models supported by insightful clinical decision support and analysis.
Our Ambulatory Monitoring - The Cardiovascular Informatics business unit offers solutions that empower caregivers across the enterprise to make fast, informed clinical decisions to care for cardiac patients, providing support in managing increased volume and complexity of clinical and administrative data, wherever care is delivered.
- The Clinical Insight &
DiagnosticsInformatics business– comprisedunit delivers clinical decision support in the domains ofBioTelemetry, which Philips acquired in 2021 – provides industry-leading patient caredigital pathology, advanced visualization and specific disease managementin ambulatory and home care settings in North America and beyond through a suite of cardiac diagnostic and monitoring solutions to identify heart rhythm disorders supported by AI algorithms that orchestrate workflows and services across care settings. The acquisition of Cardiologs complements this capability with a vendor-neutral heart disorder screener and ECG analysis applications, based on machine learning algorithms.solutions.
- The Radiology Informatics business unit enables enterprise imaging across sites, specialties and technologies to
Connected Care
Total sales by business
| Sleep & Respiratory Care | |
In most of the Connected Care businesses, revenue is earned through the sale of products and solutions, customeras well as services fees and software license fees.licenses. Where bundled offerings result in solutions for our customers, or offerings are based on the number of people being monitored, we see more usage-based earnings models. In the area of patient care management businesses (Ambulatory Monitoring & Diagnostics business unit and Sleep & Respiratory Care)Care business), revenue is generated through clinical services, product sales and through rental models, whereby revenue is generated over time.
Sales channels include a mix of a direct salesforce,sales force, partly paired with an online sales portal and distributors (varying by product, market and price segment). Sales are mostly driven by a direct salesforcesales force with an intimate knowledge of the procedures that use our integrated solutions’ smart devices, systems, softwareclinical settings and services.patient-specific diagnosis and treatment. Philips workscollaborates with customers and partners to co-create solutions, drive commercial innovation and adapt to new models such as monitoring-as-a-service and software-as-a-service.
Sales at Philips’ Connected Care businesses are generally higher in the second half of the year, largely due to customer spending patterns. However, the Philips Respironics voluntary recall notification in the Sleep & Respiratory Care business in June 2021 had(as further discussed in Quality & Regulatory and patient safety) continued to have a negative impact on sales throughout 2022.2023.
At year-end 2022,2023, Connected Care had around 17,00017,549 employees worldwide.
2022 business2023 highlights
Philips' offerings improving clinical workflowPhilips signed a 10-year, EUR 100 million Enterprise Monitoring as a Service agreement with one of the largest health systems in the US, covering 20 hospitals with over 3,000 beds. The agreement provides the health system with constant access to the latest technology, including software and alarm management in critical care environments, as well as its contributions to a quieter healing environment in intensive care units, resonated well with customers.services, while lowering initial investments.
Philips expanded its Advanced Life Support activities across international markets and Greater China.NYU Langone Health announced an 8-year strategic partnership valued up to USD 115 million and aimed at enhancing patient care through further innovation. The partnership includes digital pathology, clinical informatics, and innovative AI-enabled diagnostics, with an Enterprise Monitoring as a Service model. With these new technologies, NYU Langone clinicians can collaborate in real time, sharing pathology, imaging studies or patient data to support diagnostic confidence and tailor individualized care plans.
In Greater China, Philips partnered to drive localizationHighlighting the strength of its EMR Tasy offering in order to be locally relevant for the China market.
Philips continues to successfully expand into ambulatory care. Newly published research validated that Philips Mobile Cardiac Outpatient Telemetry (MCOT) is crucial in detecting arrhythmias and providing data that allows care teams to intervene quickly and decisively to provide the optimal patient treatment.
Underlining the clinical and economic value of remote cardiaccomprehensive patient monitoring offering, Philips announced a multi-year partnership with Northwell Health to standardize and centralize patient monitoring across the hospital, allowing caregivers to see what is happening at each bedside.
Philips announced new research demonstrating increased atrial fibrillation detectioninteroperability capabilities that offer a comprehensive view of patient health for improved monitoring and significant cost savings using Philips’ mobile cardiac outpatient telemetry monitoring.care coordination. This is realized through the interoperability of Philips Capsule Medical Device Information Platform (MDIP) with Philips Patient Information Center iX (PIC iX) with streaming, vendor-neutral data that supports care delivery and collaboration.
Philips expandedintroduced the cloud-based Philips HealthSuite Imaging PACS on Amazon Web Services. This cloud-based enterprise imaging solution, which includes advanced AI-enabled applications, has been designed to enhance image access speed, reliability, and data orchestration for clinicians across the imaging workflow, while reducing costs for healthcare organizations.
Philips launched its remote cardiacambulatory monitoring portfoliooffering in Japan, combining Philips ePatch Holter monitors with a patch-based, clinical-grade ECG analysis through AI and advanced algorithms. This innovative approach aims to reduce clinician workload and improve the patient recruitment, compliance and retention for clinical trials.experience.
6.4.3Personal Health businesses in 2022segment
Our Personal Health businesses playbusiness plays an important role serving people's needs in the areas ofenabling healthy living, preventionindividual care routines with technology and home care – delivering compelling value propositions to enable people to live a healthy lifesolutions that support people’s long-term health and proactively manage their own health.well-being.
We aim to drive profitable growth through a focus on innovation across three key areas:
- Reaching more people through consumer-driven product and solutions innovation
Accelerating online growthEnsuring the highest quality of consumer experience from pre-purchase consideration through to purchase andengaging more people through an end-to-end digital approachunboxing, all the way to end-of-use recycling- Expanding our ecosystem through partnerships with leading retailers and scaling new business models, such as try-and-buy and subscription services
The Personal Health segment consists of the Personal Health business, which comprises the following areas of business:business units:
- Oral Healthcare
:business unit – power toothbrushes for a range of price segments, from entry-level battery-operated toothbrushes for a young audience to premiumintuitivepower toothbrushes connected to the Sonicare app with in-app coaching; brush heads, which are also available as a subscription service; products for interdental cleaning and for teeth whitening - Mother & Child Care
:business unit – products to support parents and babies in the first 1,000 days, including infant feeding (breast pumps, baby bottles and sterilizers), connected baby monitors and digital parental and women’s health solutions (Pregnancy+ and Baby+ apps) - Personal Care
:business unit – grooming and beauty products ranging from entry-level to premium. The grooming portfolio includes shavers, OneBlade, groomers, trimmers and hair clippers, as well as premium solutions with SkinIQ technology, in-app coaching for a personalized shave, and blade subscriptions. The beauty portfolio includes devices to support skin care, hair care and hair removal, including Lumea premium IPL hair removal devices and solutions with the latest SenseIQ technology that sense and adapt for personalized care; also available through subscription models.
Personal Health
Total sales by business
Through our Personal Health businesses,business, we offer a broad range of solutions in various consumer price segments to support people in proactively managing their health and well-being. Depending on the market, we offer an additional portfolio of locally relevant innovations and adjust our range to increase accessibility. A notable aspect of our commercial strategy is driving increased direct-to-consumer relationships and sales through our consumer communities and online store. About half of our Personal Health sales worldwide now take place online.
We are leveraging connectivity to offer new business models, partnering with other players in the health ecosystem, e.g. insurance companies and healthcare professionals, with the goal of extending opportunities for people to live healthily and prevent or manage disease. We are engaging consumers in their health journey in new and impactful ways through social media and digital innovation.
In Personal Health, improving lives also means caring for the world, with a key focus on environmental sustainability. For example, in 2023 we strongly believelaunched an initiative in Germany, Philips Refurb Editions, to give products a second life, with the connection between good oral care and good overall health –same two-year guarantee as a belief underpinned by the World Health Organization (WHO), which in 2021 adopted a stronger resolution on oral healthcare asnew product. This is part of the drive towards universal health coverage. Good oral care is important for everyone. And since everyone is different, oral healthcare should also be personalizedPersonal Health’s commitment to each userdriving a more circular economy, and we believe we need to garner the best health outcome. Philips Sonicare, which celebrated its 30th anniversary in 2022, offers a wide range of solutions for complete oral care: from intelligent and intuitive power toothbrusheskeep finding innovative ways to interdental cleaning solutions and apps that help userssupport consumers with greater choices to manage their complete oral care on a daily basis and give the option to share brushing data with their dental practitioners, putting personalized guidance at their fingertips.live sustainably.
We also offer mobile solutions to support parents and parents-to-be for a more informed, more connected and healthier journey to parenthood. The Pregnancy+ app and Baby+ app offer parents supportive content at every stage of their first 1,000-day journey. Pregnancy+ also offers state-of-the-art, photo-realistic and interactive 3D fetal models to make the experience even more exciting, with new, personalized content for each day of the pregnancy. As of year-end 2022, theThe Philips Pregnancy+ app and Baby+ app combined have more than 68 million downloads,was ranked among the best pregnancy apps of 2023 by Forbes*). It has more than 1.5 million daily active users and areis available in 22 languages.
The company’s wide portfolio of connected consumer health platforms leverages Philips HealthSuite Platform, a cloud-enabled connected health ecosystem of devices, apps and digital tools that support personalized health and continuous care.
The revenue model is mainly based on product sale at the point in time the products are delivered to retailers and online platforms. We continue to increase revenue model diversity by expanding our new business models, including direct-to-consumer, subscriptions, try-and-buy offerings and services.
The Personal Health businesses experiencebusiness experiences seasonality, with higher sales around key national and international events and holidays.
At year-end 2022,2023, Personal Health employed around 9,0009,085 people worldwide.
2022 business2023 highlights
BuildingPhilips successfully launched the Sonicare DiamondClean 7900 Series electric toothbrush in China on major online shopping channels Alibaba and JD.com. Highlighting increasing customer demand, it claimed the successful strengtheningnumber-one position in the high-end toothbrush category on Alibaba’s Tmall.
In partnership with JD.com, Philips launched the premium 7 Series Shaver in China, debuting as the #1 shaver on this major online shopping channel. Additionally, Philips’ DiamondClean 9000 premium electric toothbrush has become the best-selling high-end oral healthcare product on Alibaba.
To improve oral care habits among children, Philips introduced Sonicare for Kids 'Design a Pet Edition' with an entry price point designed to give more parents access to an electric toothbrush for their children.
Philips OneBlade packaging was named the 2023 Red Dot Communication Design Best of the company’s innovative power toothbrushes portfolio, ranging from entry-level to premium propositions, as well as targeted advertising and promotion campaigns, Philips Oral Healthcare recorded continued market share gainsBest in North America.
Philips' locally developed China power interdental cleaning innovation launched in Q1 contributed to our leadership position in overall market share (source: GfK).
Building on its successful OneBlade platform, Philips introduced in Europe the new OneBlade 360, which leverages a new blade that adjusts to the curves of the face to enhance shaving comfort. The global roll-out is expected to start in 2023.
Philips completed the global introductionrecognition of its new Philips Shaver S9000paper-based model, illustrating how the use of less material, fewer parts, and less volume can go hand in hand with SkinIQ with its launch in Japan, resulting in accelerated sales growth for this categoryiconic presence and a 4.9 (out of 5) consumer rating and review score within the first month.best user experience.
Philips continues the integration of SkinIQ technology by expanding into the S5000-S7000 ranges, increasing access to Philips proprietary technology that senses pressure and movement to adapt and guides the users for a more efficient shave.
In China, Philips launched its first premium portable shaver, which garnered 4.7-star ratings/reviews (source: Taobao) within'Better than New' campaign in Germany, repositioning refurbished innovations and underscoring the first month.company's commitment to circularity and sustainability. The campaign led to a significant year-on-year increase in sales revenue of refurbished Lumea and refurbished shaving products.
Philips announced Babybell Maternal & Child Supplies as the exclusive distributor for Philips Avent OneFeeding in China; the partnership aims to accelerate growth of the Philips Avent brand in the Chinese maternal health industry. The partnership combines the power of Philips’ latest innovations with Babybell's rich understanding of the local market and robust retail network to deliver on a faster innovation pace and expand market share.
6.4.4Segment Other
In our external reporting on Other we report on the items Innovation & Strategy, IP Royalties, Central costs, and other small items. At year-end 2022, around 18,0002023, 14,626 people worldwide were working in these areas.
About segment Other
Innovation & Strategy
Innovation & Strategy supports all businesses and markets withinAt Philips, in developing anwe have set up our innovation roadmap and strategiesteams to deliver on our customers’ needs and achieve our growth and profitability ambitions.
We innovatebe as close to help our customers and consumers overcome clinical challenges,as possible. The majority of our Research & Development (R&D) experts work in one of our business units, which allows them to directly hear customer and consumer needs and work closely with other stakeholders to improve healthcare. We helpturn innovations into actual products. Innovation at Philips is organized to encourage innovation anywhere along the value chain and not just at the product ideation stage.
The remaining R&D experts are part of our businesses to enable and accelerate innovation by providing deeply specialized expertise. This starts with strategy and entails cooperation between research, development, design, medical affairs, professional services, marketing and businesses in a multi-disciplinary fashion, from early exploration to first-of-a-kind offerings.
We do so in the following ways:
We actively participate in open innovation through relationships with academic, clinical, industrial partners and start-ups, as well as via public-private partnerships. We do so to increase innovation speed and improve agility, to capture and generate new ideas, and in some cases to leverage third-party capabilities.We drive continuous improvement in the Philips product and solution portfolios.central Innovation & Strategyimproves the efficiency and effectivenessorganization. The main job ofinnovation through Centers of Excellence, such as Platform Modularity & Re-use, Data Science, Artificial Intelligence (AI) and Internet of Things.We strive for breakthrough innovations that can help drive fundamental shift in the healthcare industry, thereby supporting businessesthese experts is to focus onselected must-win battles.We drive adoptionindustry-shifting ideas that advance a core product to fulfill the needs ofdigital architecturea broad new customer segment. Innovation & Strategy focuses on enabling andplatforms, movingaccelerating innovation across our business units in different ways:Strategy supports the business units in shaping their strategy to
cloud-based Software-as-a-Servicecreate a competitive advantage. The Enterprise Strategy team focuses on overall corporate strategy, and the Market Analysis & Forecasting team analyzes customer segments and market growth trends. Strategy also partners closely with the Mergers & Acquisitions and Finance teams to ensure our M&A activity is aligned with the business units and our enterprise strategic direction.Research helps to define the future of healthcare by unlocking opportunities that have the potential to disrupt the healthcare industry. Breakthrough Innovation Teams (BRITE) and Exploratory Innovations Teams (XITE) are two ways Philips nurtures long-term ‘big bets’ in innovation and enables the growth of an overarching entrepreneurial mindset across all of Philips.
Experience Design plays an important role in making sure that the voice of the customer is heard and included in innovation. This means linking product development from inception with a patient and consumer view and ensuring that the highest product and experience performance requirements are embedded throughout all innovation projects. In 2023, the Philips brand won 160 awards for
informatics offerings,design excellence.Innovation Engineering teams bring innovations to life by providing Philips business units with a central team of experienced, talented individuals with capabilities in software, hardware engineering, and
excellenceAI. Innovation Engineering teams also enable business units to scale through shared platforms.Innovation Excellence helps our business units to be the best they can in
Data Scienceinnovation by offering them an outside-in view andAI, as well as software engineering. Industry best practices include creating and maintaining application-level software, modular and configurable system design and model-based system engineering.We drive our HealthSuite Architecture to help unlock the power ofdeveloping competencies, processes, data andenable healthcare professionals, patientstools they need to excel at innovation.Innovation Effectiveness teams help to measure the return on innovation investments and
consumers. Its modular set of re-usable digital capabilities liberate, integrate and enable actionable insights on dataguide enterprise-wide innovation initiatives.Innovation & Strategy works from
disparate systems within a secure environment.We help secure patient safety and quality by participating in stage gate reviews from the start of the idea-to-market (I2M) process. This is intended to ensure that patient safety and quality aspects of Philips products and solutions match the expectations and criteria of the end-users in the field. We engage with professional medical societies during regular and ad hoc advisory boards to help us understand the criteria on patient safety and quality related to medical devices. Together with the Regulatory function, we contribute to clinical evaluation during pre-market risk assessments, post-market surveillance, and support any health hazards evaluations with expert advice.We leverage the knowledge and expertise of our community of medical professionals. Activities include strategic guidance built on clinical and scientific knowledge, building and nurturing customer partnerships and growth opportunities, fostering peer-to-peer relationships in relevant medical communities, driving co-innovation with customers, liaising with medical regulatory bodies, and supporting clinical and economic evidence development.We deploy our engineering capabilities to realize innovations that deliver on our customers’ needs, advancing the Quadruple Aim of better health outcomes, improved patient and staff experience, and lower cost of care.We drivefour main innovationeffectiveness and enable locally relevant solution creation at four established innovation hubs:sites – Eindhoven (Netherlands), Cambridge (USA), Bangalore (India) and Shanghai (China). These four hubs form a global network, together with the other– and smaller innovation and research sites intheir respective regions,the Regions. Our global footprint enables us toprovide accessunderstand, anticipate and react toeach other’s capabilities to serve businesses,local markets andcustomers globally.We ensure that the user experiences of our innovations are inspiring, meaningful, people-focused, and locally relevant. A key enabler for this is an engaging and differentiating design language system (DLS) that is embedded in software, hardware, and services across our businesses. In 2022, Philips received a total of 171 awards for design excellence.
During 2022, Innovation & Strategy started to refocus R&D to deliver a greater return on investments by being selective in our choice of innovations in which to invest. We stopped projects and reduced the workforce by 5%. As part of the strategy to create value with sustainable impact, resources will shift to the businesses to innovate closer to, and with, customers.needs.
IP Royalties
Philips Intellectual Property & Standards (IP&S) proactively pursues the creation of new Intellectual Property (IP) in close co-operation with Philips’ operating businesses and Innovation & Strategy. IP&S is a leading industrial IP organization providing world-class IP solutions to Philips’ businesses to support their growth, competitiveness and profitability.
Royal Philips’ IP portfolio currently consists of 56,000approximately 53,000 patent rights, 33,00031,500 trademarks, 114,000135,000 design rights and 3,2003,300 domain names. Philips filed 920795 new patents in 2022,2023, with a strong focus on the growth areas in health technology services and solutions.
Philips earns substantial annual income from license fees and royalties.
Philips believes its business as a whole is not materially dependent on any particular third-party patent or license, or any particular group of third-party patents and licenses.
Central costsCosts
We recharge the directly attributable part of the functional costs to the businesses. The remaining part is accounted for as 'central costs', and includes costs related to the Executive Committee and Group functionsFunctions such as Strategy, Legal and Audit fees.
Real estate
Other small items
Philips is present in 75 countries globallyOther small items refer to remaining items for intra-group services and has its corporate headquarters in Amsterdam, Netherlands. Our real estate sites are spread around the globe, with key manufacturing and R&D sites in Europe, the Americas and Asia.
In 2022, we relocated key offices in Budapest (Hungary), Carlsbad (USA) and Haifa (Israel), and manufacturing operations in Zhuhai (China). We invested in, amongst others, our R&D and manufacturing sites in Bangalore (India), Pune (India), Plymouth (USA), Pittsburg (USA), Shenzhen (China) and Suzhou (China)legacy items relating to create an engaging work environment that fosters the attraction and retention of the best talent. We have continued to drive productivity by optimizing our footprint globally and reducing the number of sites through post-acquisition integration programs, as well as by implementing our Future of Work concepts to support hybrid working. We also announced that Philips' headquarters will be moving to a new location in Amsterdam in 2025.
In line with our Environmental ESG commitments towards 2025, we continue to actively optimize our real estate portfolio. Having met our goal of bringing our site-related CO₂ emissions under 35 kilotons per year in 2020, we further reduced our CO₂ emissions to 25 kilotons in 2022. In addition, we reached 77% renewable energy in 2022, already exceeding our target of 75% by 2025. Anticipating the higher cost of energy for 2023, we redoubled our efforts on energy-saving measures. Combined with portfolio optimization, this resulted in a 5.3% reduction in 2022 total energy consumption compared to 2020 and 2021.
Over 75% of our locations are leased properties, and we manage vacancy closely to ensure the right level of space efficiency and flexibility to support our business dynamic. Our current facilities are adequate to meet the requirements of our present and foreseeable future operations. As expected, occupancy rates in our offices continued to be low in the first half of 2022 in the aftermath of COVID-19. In the second half of 2022 we saw occupancy stabilizing and we are currently evaluating options to right-size our office footprint, to further adopt task-based working principles, and to cater for meaningful presence in inspiring layout and workplace solutions. The net book value of our land and buildings as of December 31, 2022, represented EUR 1,336 million; construction in progress represented EUR 23 million.previously disposed businesses.
6.5Our geographiesgeographic structure
6.5.1Our MarketsRegions
We address North America, Western Europe and other mature geographies, as well as Greater China and other growth geographies, viaGeographically, our business is organized in three market groups –Regions: North America, Greater China and International Markets – which are active in more than 100 countries worldwide.
The Markets’ core objectiveRegion (the latter made up of Europe and Growth groupings). Within our Regions, we further organize the business by Zones and Countries. Their primary accountability is to understandmanage customer intimacy, relationships and understanding of their needs, (strategic) account management, service delivery, and indirect partner management. They are also accountable for government relations, local market/customer needs,infrastructure needed to createsupport Philips’ presence in a country (license to operate) and activate the local marketing plans,for statutory, fiscal & compliance duties, safety, sustainability and labor relations to develop and manage the relationship with existing and new customers, and to deliver orders. They act as the voice of the customersecure compliant operations in the creationRegion/Zone/Country.
For financial reporting purposes, we recognize four geographic areas: Western Europe, North America, Other mature geographies, and Growth geographies. Western Europe, North America and Other mature geographies are collectively recognized as Mature geographies in reporting on sales. This reflects the grouping of the suite of solutions strategy, bring relevant products and solutions to market, and ensure local (solution) delivery and service execution, as well as managing the (integral) go-to-market approaches to our key customers and indirect channels – all with the aim of maximizing long-term customer value and gaining market share.countries based on similar economic characteristics.
6.5.2Macro-economic landscape in 2022
In 2022, global economic activity slowed down compared to 2021, when the global economy rebounded strongly from a COVID-induced recession. Several factors were at play. Firstly, the re-opening of the economy for most of the world in 2021 has disrupted global supply chains. Secondly, previous loose monetary policy, combined with supply chain issues, resulted in strong inflationary pressures commencing towards the end of 2021. Thirdly, to combat high inflation, central banks around the globe have embarked on aggressive monetary policy tightening cycles. Consequently, global real GDP is estimated to have grown by 3.0% in 2022, compared with the 6.0% estimated in 2021 for 2022. Looking ahead, Oxford Economics expects mild recessions for advanced economies in 2023 with full-year global real GDP growth expected at just 1.3%.
6.5.32022 highlights from our Market GroupsRegions
North America
In North America, Philips continues to expand its leadership inLeading health systems such as Northwell, TriHealth, and Atrium Health, have extended their long-term strategic partnerships (LSPs) with Philips to include enterprise informatics and precision diagnostics. In Canada, eHealth Saskatchewan elected to extend their enterprise informatics relationship with Philips, reinforcing the value of the partnership helpingto clinicians and patients across the province.
The Enterprise Monitoring as a Service model (EMaaS) also drove innovation, giving health systems like TriHealth, PrismaNYU Langone Health, and the University Health System of San AntonioCalifornia Irvine and Children’s Hospital of Orange County a predictable, scalable business model that enables them to address interoperability challenges and standardize care across their networks. This includes entering into a 7-year agreement with Northwell Health, the largest healthcare provider in the state of New York, to help standardize patient monitoring drive interoperability,platform across the enterprise. In addition to adopting EMaaS for their monitoring solutions, NYU Langone Health is also partnering with Philips to advance patient safety, quality and layimprove patient outcomes through digital innovation, including integrating patient data across the foundationnetwork, AI-enabled diagnostic imaging and digital pathology for a future-proof, enterprise-wide platform. Moreover, Philips has signed multi-year agreements to continue to expand virtual monitoringprecision diagnosis and care with the US Department of Defense.treatment.
Philips continues to innovate in its personal health business and has started selling Philips Avent breast pumps via Durable Medical Equipment (DME) providers to give parentsSonicare remains the ability to receive breastfeeding equipment and supplies that may be covered by their health insurance. Celebrating 30 years in business in 2022, Philips Sonicare leads theleading electric rechargeable toothbrush market in the USUnited States and Canada, and isas well as the most-recommended rechargeable toothbrush brand in the US.United States. Additionally, Philips Norelco remains the leading electric male grooming brand in the US and Canada, reaching the next generation of young men with our new OneBladeOne Blade multi-purpose shaver.
Philips continues to belead the way with innovation in its efforts to help address health disparities and maternal health access specifically – partnering with the state of Michigan to tailor the Philips Avent Pregnancy+ app, making it easier for moms within the state to find resources available to them, such as home-visiting nurses. During the first year, Pregnancy+ reached over 32,000 Michigan families, helping them get access to vital resources. The app was recognized by Forbes as the best pregnancy app of 2023. Forbes also recognized Philips for its Inclusion and& Diversity efforts in North America, including being recognized by Forbes as one of theirnaming the company among the Best Employers for Diversity and Best Employers for Women.Women for the second year in a row.
Greater China
In 20222023, we continued to provide innovative health technology solutions indeliver on our commitment to support of China’s national health strategy, supplying national top hospitals, primary hospitals and private hospitals with tailor-made solutions for their clinical and research needs.
We partnered with national top hospitals Shanghai Ruijin Hospitalneeds, and Sichuan Huaxi Hospital on clinical research that leverages our cutting-edgeempowering consumers to manage their health technologies, and helped the 1st Affiliated Hospital of Guangzhou Medical University establish the largest sleep center in South China by providing consulting services, key equipment and systems. We also provided Zhongshan-Jinshan Diabetic Foot Center with an integrated solution comprised of laser ablation and ultrasound screening technology, and supplied Hainan Dongfang People’s hospital with high-end patient monitors and defibrillators for use in acute and critical care. And we provided Suzhou Kowloon Hospital with a radiology solution that included Ingenia Elition, Ingenia Ambition, Spectral CT and Azurion 7, and delivered a cardiology and smart hospital solution to Jiangxi Cihuai Cardiovascular and Cerebrovascular Hospital.
In the consumer space, in line with the consistent ‘Professional, Young and Premium’ positioning, we continue to accelerate local innovation to address the specific needs of local consumers. In 2022, locally initiated products generated 20% of revenue. In addition, Philips was recognized as a ‘gold brand’ (most favored brand of consumers) in the Personal Health category for the third consecutive year by China Business Weekly.well-being.
With the aim of better serving the Chinese market, we established three Philips Innovation Centers inare committed to our ‘In China, to focusFor China’ strategy, which focuses on ‘local-for-local'local innovation, in systems, productsmanufacturing, services and software, andpartnership. We continue to drive ‘made in China’ fulfillment and create more locally relevant solutions by leveraging local ecosystems to serve both professional and consumer markets.
In the professional market, we have expanded our cooperation with local customers by providing cutting-edge imaging systems, informatics solutions and other products in support of delivering better care to patients in terms of precision diagnosis, interventional treatment, and smart hospital development. Key customers include many top hospitals: Huaxi Hospital in Sichuan Province, Renji Hospital, Xinhua Hospital, the Sixth People's Hospital, the 10th People's Hospital and Children’s Hospital in Shanghai, Jishuitan Hospital and Anzhen Hospital in Beijing, the First Affiliated Hospital of Dalian Medical University, the First Affiliated Hospital of Zhengzhou University, and Regional Imaging Center of Jiangxi Province, to name just a few.
In the consumer market, in line with our consistent ‘Professional, Young and Premium’ positioning, Philips’ brand strength increased in 2023, despite an overall weak consumer market. We leveraged new online and offline channels, including Healthy Living Lab, TikTok, O2O instant retail platforms like Meituan, JD to home, and Ele.me to engage with young consumers and grow business. Local innovation drove significant growth in Male Grooming and Oral Healthcare, which continue to solidify their leadership positions in China. Philips was recognized as a ‘gold brand’ (most favored consumer brand) in the Personal Health category for professional medical equipment.the fourth consecutive year by China Business Weekly.
2023 marked the 100th anniversary of Philips in China. This achievement stands as a testament to Philips’ commitment and dedication to improving people’s lives through meaningful innovation and fostering strong partnerships in China.
International Markets
In our International MarketsRegion we strive to execute on a shared global vision whilst meeting the unique local needs and circumstances of our customers. Our goal is to elevate customer relationships and move from being a trusted supplier of equipment, services and software to a transformational partner directly contributing to our customers’ long-term success. To support this vision we have made great progress on leveling up our go-to-market model, developing scalable solutions and software, expanding fit-for-future capabilities, reinvesting revenue to enable new business models, and establishing new partnerships.
In International Markets,Region, our Personal Health showed top-line resiliencebusiness plays an important role in 2022. Growth was strongest in the Middle East, Turkey & Africa, Indiaenabling healthy individual care routines with technology and Japan,solutions that support people’s long-term health and overall our growth markets delivered double-digit growth. A major driver of growth in Middle East, Turkey & Africa came from activating GenZ consumers via social media and influencer marketing campaigns on TikTok and other platforms, focusing on relevant GenZ propositions such as OneBlade and Hair Care. In Japan, we successfully launched our latest Shaver series 9000 with SkinIQ technology via a cut-through advertising campaign in Hokkaido and Kanto prefectures. This campaign targeted younger audiences and succeeded in increasing market shares and distribution points.well-being.
Philips entered into many new customer partnerships in 2023, including the following:
International – Europe
Philips entered into partnerships with healthcare providersPatients at Martini Hospital were the first in the UKNetherlands to use Philips' ePatch wearable sensor to diagnose cardiac arrhythmias. The hospital uses the sensor and GermanyCardiologs software to deliverdetect atrial fibrillation after patients have had a stroke. The patch is designed to replace traditional Holter monitors, which are more cumbersome and can only be worn for a day. The new sensor is expected to improve detection of heart rhythm disorders and provide more personalized care, as well as reducing workload and lowering costs.
Philips and Gibraltar Health Authority announced a 16-year strategic partnership to transform patient imaging and cardiac care for local patients. The partnership will provide local coronary angiography and angioplasty services in a new interventional cardiac suite equipped with the latest diagnostic technology. The announcement represents a major reform in service delivery, improving the region’s access to life-saving interventions while reducing environmental impact, with patients no longer needing to travel abroad for treatment.
Developed by Dr David Tscholl and Dr Christoph Nöthiger, consulting anesthesiologists at University Hospital Zurich, the Visual Patient Avatar is a new approach to patient monitoring: patient data is translated into a simple visual design, reducing the time needed in the operating room to check and interpret vital signs. Together with Philips, this idea was further developed into a commercial solution that is now being implemented at University Hospital Bonn, the first hospital in Europe to use this type of display for faster decision support.
Philips is partnering with Assistance Publique-Hôpitaux de Paris, Hôpitaux Civils de Lyon and Incepto (a PACS AI application platform) to make Artificial Intelligence more accessible to radiologists. Philips has also joined forces with Hôpital Saint-Joseph (Paris) and Hôpital Marie-Lannelongue (Hauts-de-Seine) to improve personalized cancer care by integrating digital pathology into the imaging workflow. And Philips has opened its vendor-neutral Radiology Operations Command Center, which enables remote collaboration between technologists, radiologistsnew healthcare innovation center, Health Innovation Paris (HIP).
Philips' innovative technologies feature across the Polish hospital network. In 2023, the first Incisive CT scanner in Central & Eastern Europe was installed to diagnose patients with cardiac disease at a private cardiology network. In addition, a state-of-the-art hybrid room was created at the University Hospital in Bydgoszcz. Longstanding cooperation with the American Heart of Poland has resulted in further contracts, including the installation of a monitoring network.
Philips and imaging operations teams across multiple sites,Norwegian Vestre Viken Health Trust deployed AI-enabled clinical care to help increase productivity and expandradiologists improve patient care. The large-scale deployment provides access to MR-an AI-based bone fracture radiology application that will serve the needs of around half a million people across 22 Norwegian municipalities.
International – Growth
Philips Japan officially launched the Turbo-Power laser atherectomy catheter, which debulks lesions in a single step and CT-based diagnosis. offers remote automatic rotation for precise directional control – a powerful tool for the treatment of peripheral vascular diseases. The Philips MR7700 3.0T imaging system with SmartSpeed AI was installed for the first time in Japan, at Hamamatsu University Hospital. The MR7700 achieves high image quality, while SmartSpeed utilizes the Compressed SENSE speed engine to reduce scan time.
Philips launched the Spectral CT 7500 imaging system with an event for the top 100 radiologists in India. This system performs low-dose scans without compromising speed, power or field of view. We also received an order for 28 Philips Incisive CT systems from a single state. This system combines operator and design efficiencies to improve patient and staff experience and support clinical decision-making. Philips Innovation Campus opened its new site in Bengaluru, home to over 5,000 engineers, scientists, business developers, and clinical experts.
In Germany,Australia, Philips signed a 10-year7-year partnership agreement with the municipal hospital Städtisches Klinikum Braunschweig, one of the country’s largest care providers,Queensland Government and Cairns and Hinterland Hospital and Health Service (CHHHS) to provide monitoring solutionsa turnkey solution including Vue PACS, Reporting & VNA Philips Software, and alarm management. In an interview withInfrastructure as a German healthcare magazine, Dr. Andreas Goepfert, CEO Braunschweig Clinic, commented: “QualityService (IaaS) across a remote and large geography. The Philips solution will enable clinicians to access a complete imaging health record of care is an important aspect that is safeguarded by suchtheir patients and provide a partnership. Nowadays, it is difficult to imagine successful economic operation in the healthcare sector in the medium and long term without technology partners.” In the Netherlands, Philips signed a long-term agreement with the Rijnstate hospital to deliver a wide range of advanced ultrasound devices for 17 different departments at multiple locations of the hospital. The agreement involves ultrasound devices and services for cardiological, vascular or radiological examinations, OB/GYN, as well as mobile devicesplatform for the emergency department. integration of all image data across the CHHHS enterprise, greatly enhancing the radiology workflow.
Demonstrating our commitment to high-quality, sustainable healthcare, Philips undertook multiple initiatives to expand helium-free MR operations in Brazil. Besides Brazil, this expansion reached Mexico, Panama, Puerto Rico, Colombia, Chile, Argentina and Ecuador, making a significant mark on the region's healthcare landscape. We are also working to localize the production of BlueSeal MRI magnets in Brazil. Other notable ventures included the Brazilian Company of Hospital Services (EBSERH) installing 14 Incisive CT imaging systems at Federal University Hospitals.
In Spain, we provided computed tomography, magnetic resonance and image-guided therapy solutions for several Spanish public hospitals as part of INVEAT, an impact initiative driving investment in high-technology equipment in the Spanish national health system. In Finland,Turkey, Philips signed a 10-year agreement with Oulu University Hospital to deliver the latest Azurion image-guided therapy solutions, as well as maintenance, consultancy and financing services. In the SDA Imaging Center in Chelm, Poland, Philips installed an MR 5300 scanner with Ambient Experience technology, which combines images, sound and light to create an atmosphere that puts patients at ease and reduces the need to redo scans. In Romania, Philips is the trusted partner and supplier ofhas supplied high-grade medical equipment to the new Gaziantep City Hospital. The public city hospital complex, with 1,875 new beds, will serve Gaziantep and solutionssurrounding cities, adding much-needed healthcare capacity to Transylvania Hospital,the region, which was hit by a private medical initiative launcheddevastating earthquake in September 2022. The medical technology we provided includes an Azurion 7 biplane angiograph, an Ingenia 3T MR system, and an Affiniti 50 Doppler ultrasound. In Turkey, asFebruary 2023.
As part of a project supported bydeal with Egypt’s Ministry of Health, Philips unveiled the European Bankfirst Mobile MRI Truck for Reconstructionthe Middle East, Turkey & Africa region, to enhance healthcare delivery in remote, difficult-to-access and Development (EBRD), we installed 3,400 hospital patient monitors and 437 ultrasound systemsunderserved locations. In just 3 months after implementation, more than 1,100 patients across 250 different hospitals within a 4-month window. The Philips team also trained over 3,000 clinicians and monitored usage.Egypt had already benefited from this initiative.
In Central Asia, we suppliedKazakhstan, Philips provided advanced medical equipment to the National Coordination Center for Kazakhstan’s National Research OncologyEmergency HealthCare in Astana and the Hematology and Cardiology Center and twoin Ust-Kamenogorsk. Both are multi-modality projects while in Uzbekistan we wonand have a project to equip Tashkent International Medical Clinic (TIMC) with advanced clinical technology solutions. In a 10-year partnership deal withhigh social importance, as the Cloud Nine hospital group in India, we connected 257 beds across 26 tele-ICU locations. In addition, some 2,000 Zenition C-arms and 500 Affiniti ultrasound systems were shipped from our manufacturing plant in Chakan.
In Japan, Philips signed a 10-year agreement with a large university hospitalEmergency Center will be the flagship center for the expansion of its eICU program for centralized, remote surveillance of high-risk ICU patients. PhilipsNational Stroke Program in Kazakhstan, and Thanh Vu Medic Hospital Vietnam signed a 10-year strategic partnership agreement for which Philips is providing state-of-the-art imaging technology, informatics connectivity, 10-year comprehensive servicethe Hematology and 5-year structured financing. In Fiji, Philips and Aspen Medical signed a 12-year strategic partnership agreement to supply and integrate diagnostic imaging equipment and services for use in two public hospitals.
In Brazil, Philips´ joint venture to provide and operate imaging diagnostics in the state of Bahia via a Public-Private Partnership model continues to expand access to quality diagnostics and care for underserved populations, e.g. through the provision of a new reporting center and 12 imaging units placed in 12 hospitals. Also in Brazil, a two-year Electronic Medical Record implementation at Fundação Hospitalar do Estado de Minas Gerais (FEHMIG) will integrate 23 public hospitals in the state of Minas Gerais. In Argentina, Philips successfully participated in a tender for a turnkey solution providing high-end imaging equipment for 17 public hospitals. In Mexico, Philips secured a dealCardiology Canter treats patients with Grupo Angeles to provide an extensive range of Diagnostic Imaging and Image Guided Therapy solutions.serous blood diseases.
6.6Supply chain and procurement
6.6.1Integrated Supply Chain
Philips runs an Integrated Supply Chain (ISC), which encompasses supplier selection and management through procurement, manufacturing across all the industrial sites, logistics and warehousing operations, customer installation, as well as demand/supply orchestration.
When selecting and evaluating partners, we consider not only business metrics such as quality, on-time delivery performance and cost, but also environmental, social and governance factors. We use supplier classification models to identify critical suppliers, including those supplying materials, components and services that could influence the safety and performance of our products and solutions.
The Philips Supplier Quality Manual outlines Philips’ quality, regulatory, product, process and customer requirements. The standards outlined in this manual underpin agreements between suppliers and Philips, and guide compliance with Philips’ quality standards.
Addressing immediate challenges
2022 continued to test the resilience of supply chains globally. The Russia-Ukraine war continues to put severe pressure on the commodity landscape and supply chains, and has contributed to the sharp rise in energy and food prices and extreme inflation rates. This came on top of a pre-war environment of low inventories and long lead times, with the accompanying build-up of backlog orders. In addition, the Chinese government’s zero-COVID policy in 2022 again led to outages and supply chain issues. Furthermore, Philips has been hampered by an aging product portfolio with older technology (component designs) and a fragmented supplier landscape. As a result, our lead times to customers suffered, for which we are sorry and have defined corrective actions.
Under these market circumstances, the ISC function’s priority was to endeavor to safeguard continuity of supply, with dedicated Procurement teams by modalities and types of commodities, so that Philips could continue to provide critical healthcare equipment and solutions to our customers all over the world. For example, we have placed non-cancellable semiconductor orders for a 12-month horizon to ensure our place in the queue. At the same time, we have intensified spot buys and alternate parts qualifications in partnership with Research & Development. In parallel, we continue our advocacy towards the industry and governments on prioritizing supply for life-saving equipment.
In 2022, extremely high energy costs, especially in Europe, and increasing labor costs driven by inflation led to a significant rise in production/operational cost in our supply base. In the second half of the year, these same drivers led to a slowdown of demand, and spot prices for commodities and energy started to come off from their historical peaks in the second quarter. However, costs for production and materials remained at historical elevated levels. Specifically for electronic components, although the supply crunch has receded from its peaks, distributor data suggest that shortages will continue into 2023.
For information about the Philips Respironics recall and remediation effort, please refer to Quality & Regulatory and patient safety.
Driving end-to-end supply chain reliability and agility
As part of our plan to create value with sustainable impact, the supply chain plays an important role in improving our performance and delivering to our customers and consumers as promised. In 2023, we initiated multiple interventions and have longer-term programs planned to improve our execution capabilities and become more resilient in navigating volatility.
In 2023, we focused on restoring the stability and reliability of our supply chain, including safeguarding material flows and de-risking in a sustainable manner. For example, we accelerated the redesign of printed circuit board assemblies (PCBAs) to replace older e-components with more modern and widely available e-components. We also reduced our purchases of high-risk parts by applying our supplier risk management framework, which assesses suppliers for factors such as strategic fit, financial stability, operational performance, quality, sustainability, compliance and location. We aim to maintain close relationships with our suppliers and conduct an ongoing dialogue with respect to our forecast.
Over the past year, we have re-aligned our end-to-end supply chain organization, with dedicated teams by Business and Region allowing us to tailor to specific challenges and implement solutions that address different customer and consumer needs. Whereas Philips’ supply chain organization historically delivered efficiencies through a functional orientation, the above-mentioned factors required much greater agility fornew operating model has sped up decision-making and better supports the businesses in achieving their short-, mid- and long-term goals.
Under the new set-up, initial investments have been made to improve our businesses, each having their own specific requirements. We are moving to a business and customer-centric orientation to address end-to-end visibility and improve reliability and outcomesplanning tools by implementing solutions that are tailoreddigitizing our priority information flows.
We continue to specific business needs.
Much like the rest of the industry, we remain exposed to inflation and the continued geopolitical tensions around the world. All of these challenges have reinforceddeploy our strategy for a more ‘regionalregional vs global’global approach to our end-to-end network design.
Philips has continued to progress the consolidation of its manufacturing footprintdesign, taking into versatile ‘multi-modality’ manufacturing sites that produce multiple product categories and are located within or near the regions they serve. We do this for enhanced scale, efficiency andaccount factors such as customer proximity, and to reduceleveraging manufacturing capabilities, our environmental footprint. Whilefootprint, and efficiency. We are using our site count has continued to decrease, the number of locations equipped to make the same product is increasing. Philips is using its multi-modality sites, in combination with contract manufacturing partners, to regionally ‘multi-source’ many of itsour products. This willis intended to increase the resilience of our supply chain to manage future, unplanned disruptions and to ensure access to public healthcare investment where ‘local’ requirements exist in our largest markets.
We continueLike the rest of the industry, we remain exposed to make progresscontinued geopolitical tensions around the world. Labor costs remain a concern due to the persistent inflation in transforming our warehousing2023 and distribution operations into a more customer-centric and agile network. In 2022, we reduced our warehousing footprint by 31% compared to 2021, essentially through consolidation and servicing of multiple businesses from a single location.
show an upward trend entering 2024. On the logistics front, we have established long-term contracts with suppliers, withother hand, overall macroeconomics show improved availability of materials. As a result, the aimcost of increasing reliabilityraw materials and energy, as well as secured costsinflation, show a downward trend compared to 2022. We believe that our interventions, in combination with the improvement in macroeconomic trends, put us on the right track in our journey to build a reliable, predictable and availability on contracted lanes. We continue to explore and implement solutions to diversify transportation options to increase reliability while reducing carbon emission and cost.efficient supply chain.
Philips Group
Supplier spend analysis per regiongeographic area
in %
| Western Europe | |
| North America | |
| Other mature geographies | 6% |
| Growth geographies | |
| Philips Group | 100% |
6.6.26.7Supplier sustainability
Real EstatePhilips is present in 75 countries globally and has its corporate headquarters in Amsterdam, Netherlands. Our real estate locations are spread around the globe, with key manufacturing and R&D sites in Europe, the Americas and Asia.
In 2023, we consolidated five different R&D locations into a new R&D Hub in Bangalore, India, which will host some 5,000 employees. We continued our right-sizing program through our Future of Work concepts to support hybrid working. The project to move Philips’ purposeheadquarters to improve people’s lives applies throughouta new location in Amsterdam in 2025 is progressing as planned.
We also continue to optimize our value chain. An important areareal estate portfolio in line with our Environmental ESG commitments towards 2025. Having met our goal of focus for the Integrated Supply Chain is sustainability,bringing our site-related CO₂ emissions under 35 kilotons per year in 2020, we further reduced our CO₂ emissions to 22 kilotons in 2023. In addition, we reached 78% renewable energy in 2023, already exceeding our target of 75% by 2025. Energy consumption decreased by 7.8% compared to 2022.
Over 75% of our locations are leased properties, and we manage vacancy closely to ensure the right level of space efficiency and flexibility to support our business dynamic. Our current facilities are actively working on this together withadequate to meet the requirements of our partners, whether these be component suppliers or energy or logistics providers. Close cooperation with our suppliers not only helps us deliver health technology innovations, it also supports new approaches that help us minimize our environmental impactpresent and maximize the social and economic value we create.
Since 2003, our sustainability strategy has included dedicated supplier sustainability programs. We have a direct (tier 1) business relationship with approximately 5,300 product and component suppliers and 17,100 service providers. In many cases, social issues deeperforeseeable future operations. As expected, occupancy rates in our supply chain require usoffices continued to intervene beyond tier 1stabilize in the first half of the chain.
2023. We wantcontinue to make a difference through sustainable supply managementevaluate options to right-size our office footprint, to further adopt task-based working principles, and responsible sourcing. This is more than just managing compliance – it is about collaborating with our supply partners to make a positivecater for meaningful presence in inspiring layout and lasting impact. Therefore, the sustainability performanceworkplace solutions. The net book value of our suppliers is fully embeddedland and buildings as of December 31, 2023, represented EUR 1,282 million; construction in our procurement strategy and way of working.
In 2022, we focused on further maximizing our positive impact deeper in the supply chain, strengthening our maturity-based approach to drive continuous improvement. Through the Supplier Sustainability Performance program, we improved the lives of approximately 459,000 workers in our supply chain (2021: 430,000). We also launched new ways to engage our suppliers, performing deep-dives at second-tier suppliers and actively supporting our strategic partners to become more effective in their own supply chain engagement approaches.
In addition, our improvement program has been adopted by the Responsible Business Alliance under the name Responsible Factory Initiative. This program enables other companies to work on continuously improving their suppliers’ sustainability performance through the same methodology as Philips.
Managing our large and diverse supply chain in a socially and environmentally responsible way requires a structured and innovative approach, while being transparent and engaging with a wide variety of stakeholders. In 2022, our programs focused specifically on improving suppliers’ sustainability performance, responsible sourcing of minerals, and reducing the environmental footprint of our supply base by driving the adoption of Science Based Targets.progress represented EUR 32 million.
7Financial performance
7.1Performance review
The year 2023
- Sales amounted to EUR 18.2 billion, an increase of 2% on a nominal basis. On a comparable basis*), sales increased 6%, driven by supply chain improvements. Comparable sales*) showed 11% growth in the Diagnosis & Treatment segment, 1% growth in the Connected Care segment, and 3% growth in the Personal Health segment.
- Net income amounted to a loss of EUR 463 million, driven by higher earnings offset by a EUR 575 million Respironics litigation provision. Net income for 2022 was a loss of 1.6 billion, which included a charge of EUR 1.5 billion related to goodwill and R&D impairments.
- As of December 31, 2023, over 99% of the sleep therapy device registrations that are actionable had been remediated, while the remediation of the ventilator devices remains ongoing. In October 2023, Philips Respironics received preliminary court approval for the class action settlement that would resolve all or nearly all private economic loss claims in the US.
- Adjusted EBITA*) amounted to EUR 1,921 million, or 10.6% of sales, compared to 7.4% of sales in 2022. All segments showed an increase in Adjusted EBITA*) margin, mainly driven by increased sales and pricing & productivity measures, partly offset by cost inflation.
- Supported by significant change management efforts, by year-end 2023 Philips had reduced its workforce by approximately 8,000 roles, out of 10,000 roles in total planned by 2025.
- Net cash flows from operating activities amounted to EUR 2,136 million; free cash flow*) amounted to EUR 1,582 million.
- Philips cancelled approximately 15.1 million shares acquired under its 2021 share buyback program for capital reduction purposes.
- In 2023, Philips issued EUR 500 million of fixed-rate notes under the company’s EMTN program which mature in 2031 and used the proceeds for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in the fourth quarter of 2022.
The year 2022
- Sales amounted to EUR 17.8 billion, an increase of 4% on a nominal basis. On a comparable basis*), sales declined 3%, due to operational and supply challenges, the COVID situation in China, the consequences of the Respironics
field action,recall, and the Russia-Ukraine war. Comparable sales*) showed a 1% decline in the Diagnosis & Treatment businesses, an11%9% decline in the Connected Care businesses, and flat growth in the Personal Health businesses. - Net income amounted to a loss of EUR 1,605 million in 2022, a decrease of EUR 4.9 billion compared to 2021, mainly due to a charge of EUR 1.5 billion related to goodwill and R&D impairments in 2022 and the EUR 2.5 billion gain on the sale of Domestic Appliances in 2021.
- In
2021, our subsidiary, Philips Respironics, initiated a voluntary recall notification in the United States and field safety notice outside the United States for certain sleep and respiratory care products. In2022,as we took steps to accelerate the remediation program,we recorded an additional provision of EUR 250million. By year-end 2022, around 90% ofmillion for remediation related to theproduction required for the delivery of replacement devices to patients had been completed.Respironics recall. - Due to revisions to the financial forecast of Philips Respironics, Philips recorded a EUR 1.3 billion non-cash charge in the third quarter for the impairment of goodwill of this business.
- Adjusted EBITA*) amounted to EUR 1,318 million, or 7.4% of sales, compared to 12.0% of sales in 2021. The Diagnosis & Treatment, Connected Care and Personal Health businesses showed a decline in Adjusted EBITA*) margin, mainly due to the sales decline and cost inflation, partly offset by pricing and productivity measures.
Philips has initiated several productivity actions, including simplifying the organization to streamline ways of working, increase agility and reduceNet cash provided by operatingexpenses. Additionally, Philips is implementing several actions to enhance performance and productivity in the supply chain, R&D and quality.Operating cash flowactivities amounted to an outflow of EUR 173 million; free cash flow*) amounted to an outflow of EUR 961 million.To further strengthen its liquidity position and optimize its debt maturity profile, Philips secured a EUR 1 billion credit facility and conducted a liability management exercise, which reduced the debt repayment profile for the period 2023 to 2025 from EUR 3.2 to 2.0 billion and increased the average maturity on bonds by 1.3 years to 7.9 years.
The year 2021
2021 saw strong growth in orders and sales in the Diagnosis & Treatment and Personal Health segments, with a decline in Connected Care following the extraordinary growth in 2020 and the impact of the Philips Respironics voluntary recall notification. Increases in component and transportation costs, along with shortages of key components due to capacity constraints and delays in transport routes, impacted Philips' sales and profitability.Sales amounted to EUR 17.2 billion, a decline of 1% on a nominal and comparable basis. Comparable sales growth*)in the Diagnosis & Treatment businesses was 8% and in the Personal Health businesses 9% on a comparable basis. However, this was more than offset by a 23% decline in the Connected Care businesses. This was largely due to the Respironics recall but also the high comparable base in 2020. Nevertheless, we ended the year with our highest-ever order book, 18% above 2020.In Q3 2021, Philips completed the divestment of Domestic Appliances as planned, resulting in a EUR 2.5 billion gain after tax and transaction-related costs; reported in Discontinued Operations.Net income amounted to EUR 3.3 billion, an increase of EUR 2.1 billion compared to 2020, mainly driven by the gain on the sale of the Domestic Appliances business. Net income is not allocated to segments, as certain income and expense line items are recorded on a centralized basis.Adjusted EBITA*)amounted to EUR 2.1 billion, or 12.0% of sales. Productivity programs delivered annual savings of approximately EUR 279 million. This included approximately EUR 140 million procurement savings, led by the Design for Excellence (DfX) program, and approximately EUR 139 million savings from other productivity programs. While the Diagnosis & Treatment and Personal Health businesses delivered improved profit expansion, the Connected Care businesses showed a decline in Adjusted EBITA*)margin, primarily due to the decline in sales and the impact of the Philips Respironics voluntary recall notification in the Sleep & Respiratory Care business.Operating cash flow amounted to EUR 1.6 billion, and Free cash flow*)amounted to EUR 0.9 billion.
Philips Group
Key data
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Sales | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | 1.0% | (0.9)% | 3.9% | (0.9)% | 3.9% | 1.9% |
| Comparable sales growth1) | 2.9% | (1.2)% | (2.8)% | (1.2)% | (2.8)% | 6.0% |
| Impairment of goodwill | (144) | (15) | (1,357) | (15) | (1,357) | (8) |
| Income from operations | 1,264 | 553 | (1,529) | 553 | (1,529) | (115) |
| as a % of sales | 7.3% | 3.2% | (8.6)% | 3.2% | (8.6)% | (0.6)% |
| Financial expenses, net | (44) | (39) | (200) | (39) | (200) | (314) |
| Investments in associates, net of income taxes | (9) | (4) | (2) | (4) | (2) | (98) |
| Income tax expense | (212) | 103 | 113 | |||
| Income tax (expense) benefit | 103 | 113 | 73 | |||
| Income from continuing operations | 999 | 612 | (1,618) | 612 | (1,618) | (454) |
| Discontinued operations, net of income taxes | 196 | 2,711 | 13 | 2,711 | 13 | (10) |
| Net income | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Adjusted EBITA1) | 2,277 | 2,054 | 1,318 | 2,054 | 1,318 | 1,921 |
| as a % of sales | 13.2% | 12.0% | 7.4% | 12.0% | 7.4% | 10.6% |
| Income from continuing operations attributable to shareholders2) per common share (in EUR) - diluted | 1.08 | 0.67 | (1.84) | 0.64 | (1.76) | (0.50) |
| Adjusted income from continuing operations attributable to shareholders2) per common share (in EUR) - diluted1) | 1.74 | 1.65 | 0.96 | 1.58 | 0.92 | 1.25 |
7.1.17.2Factors impacting performance
In 2022,The introduction of a simplified operating model, workforce reduction, an improved global economic activity slowed down comparedsupply chain, and the geopolitical environment contributed to 2021, when the global economy rebounded strongly from a COVID-induced recession. Global real GDP is estimated to have grown by 3.0% in 2022, compared with the 6.0% estimated in 2021 for 2022.
The company’s business and results in 2022 were impacted by global and industry-wide challenges, including global supply chain constraints, COVID lockdown measures in China, inflationary pressures and the Russia-Ukraine war.2023. Where relevant, the impact of these factors and the resulting uncertainties on the company’s results, balance sheet and cash flows have been considered and are reflected in amounts reported. Comparable sales*) declined
Macro-economic landscape 2023
In 2023, global economic growth is estimated to have slowed compared to 2022, marked by 3%, mainly due to operationaleasing price pressures and supply chain challenges,stress while major central banks were tightening their respective monetary policies. Global real GDP is estimated to have grown by 2.7% in 2023, compared with 3.1% in 2022. On the consumer side, households were dipping into their savings accumulated during the COVID situationperiod to maintain their spending levels and to cushion against the inflation surge since late 2021. However, consumer spending momentum is not expected to sustain due to the depletion of savings and expected labor market softening because of tighter financial conditions. The delayed effect of higher benchmark interest rates is expected to manifest further in China and2024, leading to a further slowdown in global economic growth. Oxford Economics expects world real GDP growth of 2.3% in 2024.
In 2022, global economic activity slowed down compared to 2021, when the Russia-Ukraine war. We aim to offsetglobal economy had rebounded strongly from a COVID-induced recession. Several factors were at play. Firstly, the operational andre-opening of the economy for most of the world in 2021 had disrupted global supply challengeschains. Secondly, previous loose monetary policy, combined with specific programs to increase supply chain resilience, improveissues, resulted in strong inflationary pressures commencing towards the end of 2021. Thirdly, to combat high inflation, central banks around the globe embarked on aggressive monetary policy tightening cycles.
Simplified operating model
On January 30, 2023, Philips announced its plan to create value with sustainable impact, which is based on focused organic growth to deliver patient- and people-driven innovation at scale, with improved execution as a key value driver, prioritizing patient safety and quality, supply chain reliability and a simplified operating model. The simplified operating model aims to simplify the organization to increase agility and structurally lower the cost base.base by giving end-to-end accountability to the segments. Operating model productivity savings, procurement savings and other productivity programs contributed positively to the results of operations.
GlobalWorkforce reduction
In addition to the reduction of its workforce by 4,000 roles announced in October 2022, in 2023 Philips announced plans to reduce its workforce by an additional 6,000 roles globally by 2025, in line with relevant local regulations and processes. These reductions are focused on Corporate and Functions optimization and non-core activities, and amounted to approximately 8,000 roles by year-end 2023. Workforce-related restructuring charges were EUR 196 million in 2023 and EUR 136 million in 2022.
Supply chain resilience
In 2023, following significant actions to increase supply chain constraintsresilience and mitigate the impact of disruptions, our sales benefited from improved material availability and resolved shortages in components.
Limited availability and delays in the supply of certain components and products internationally – partly a consequence of the COVID pandemic and the Russia-Ukraine war – impacted the company’sour results in 2022. In addition, the supply chain constraints resulted in an increase in overall working capital, balances, in particular inventories. Inventories increased compared to 2021, as work-in-process inventories could not be converted into finished goods available for sale due to the scarcity of certain components. Improved component supplies contributed to a comparable sales*) increase in the fourth quarter of 2022.
Geopolitical environment
In response, we continue to drive significant actions to increase supply chain resilience and mitigate the impact of disruptions. We are: engaging with senior government officials, strategic suppliers and foundries to prioritize healthcare supplies; directly working on component issues across all tiers of suppliers; diversifying sourcing of high-risk components, with almost 400 alternate components certified to date. Lastly, we are also redesigning our printed circuit boards to qualify alternate sources of supply.
COVID situation in China
COVID continued to affect the company’s results, balance sheet and cash flows presented in these consolidated financial statements, in particular due to the lockdowns in China. Production in several of our factories, as well as those of our suppliers in China, was suspended periodically, which exacerbated the global supply chain and cost challenges. The China lockdowns impacted the results of operations due to lower sales and factory under-utilization.
COVID did not result in any material adjustments to the carrying amounts of assets and liabilities during 2022. In addition, there were no material changes to treasury and other financial risks directly related to the pandemic.
Cost inflation
Global inflation and cost headwinds, including higher energy prices, resulted in an increase in cost levels and negatively impacted gross margin in 2022.
In response, we have been raising pricing by low- to mid-single-digits since the beginning of 2022. In the Personal Health businesses, the higher sales prices contributed to a gross increase in sales of around 3% compared to 2021. In the Diagnosis & Treatment and Connected Care businesses, due to the longer equipment order book cycles, the price increases take longer to be fully realized in the profit and loss account.
Russia-Ukraine war
Since February 2022, Philips hasHaving substantially reduced its activitiesour operations in Russia. This includes stopping shipments of our consumer health products toRussia in 2022, the country (except for certain child care products), the suspension of marketing activities, and winding down of R&D activities. We are focusing our remaining activities in Russiawere focused on the delivery of medical systems, devices, and spare parts to healthcare providers to the extent possible underproviders. In 2023, increased sanctions and export controls and sanctions. Philips'led to a further reduction in sales activity. Philips’ operations in Russia and Ukraine on a combined basis represented less than 2% of group sales in both 20212022 and 2022.2023. The asset value of the activities in Russia and Ukraine, mainly working capital, was less than 1% of the consolidated total assets as of December 31, 20212022 and 2022. There have been no significant asset write-downs to date, but we continue to closely monitor developments in this regard.2023. The Russia-Ukraine war continues to put severe pressure on the global commodity landscape and supply chains, and has contributedcontribute to higher levels of cost inflation.
The company’s global operations are exposed to geopolitical and macroeconomic changes (refer to Risk management and internal control). The current situation in the sharp riseMiddle East further increases economic and political uncertainty. Philips is present in energyIsrael with several subsidiaries, mainly in Diagnosis & Treatment and food pricesConnected Care, that are primarily involved in manufacturing and high cost inflation, as further discussed above.research and development activities.
Climate-related matters
In preparing the consolidated financial statements, management has considered the impact of climate change, specifically the financial impact of Philips meeting its internal and external climate-related aims, the potential impact of climate-related risks, and the costs incurred to pro-actively manage such risks. These considerations did not have a material impact on the financial reporting judgments, estimates or assumptions. The financial impacts considered include specific climate mitigation measures, such as the use of lower carbonlower-carbon energy sources, the cost of developing more sustainable product offerings, and expenses incurred to mitigate against the impact of extreme weather conditions. To meet its long-term Science Based Targets and reduce its full value chain emissions in line with a 1.5 °C1.5°C global warming scenario, Philips has entered into a number of power purchase agreements. Some of these contracts have a fixed price structure, which in 2022 helped to mitigate the impact of increased electricity prices.
Actions in response
In 2022, Philips took several actions to enhance performance and productivity in the supply chain (e.g. dual sourcing, supplier consolidation, warehouse footprint rationalization), R&D (e.g. shifting the focus to fewer, high-impact projects in the innovation pipeline) and quality (e.g. enhancing processes, increasing capabilities and product management). As a result, Philips recorded non-cash portfolio realignment impairments and charges of EUR 282 million in 2022, consisting of R&D project impairments of EUR 134 million, Connected Care portfolio realignment charges of EUR 109 million and asset impairments in Sleep & Respiratory Care of EUR 39 million.
As announced in October 2022, Philips has initiated general productivity actions, including simplifying the organization to streamline the way of working and reduce operating expenses. This includes an immediate reduction of around 4,000 positions globally across the organization, subject to consultation with the relevant workers councils and social partners, with severance and termination-related costs of EUR 80 million incurred in 2022 and an additional EUR 50 million expected in 2023.
On January 30, 2023, Philips announced plans to create value with sustainable impact, which is based on focused organic growth to deliver patient- and people-driven innovation at scale, with improved execution as a key value driver, prioritizing patient safety and quality, supply chain reliability and a simplified operating model. In addition to the reduction of its workforce by 4,000 roles announced in October 2022, Philips plans to reduce its workforce by an additional 6,000 roles globally by 2025, of which 3,000 will be implemented in 2023, in line with relevant local regulations and processes. These reductions are focused on Corporate and Functions optimization and non-core activities, for which charges in 2023 are expected to be approximately EUR 470 million.
7.1.27.3Results of operations
Sales
Sales
The composition of sales growth in percentage terms in 2022,2023, compared to 20212022 and 2020,2021, is presented in the following table.
Philips Group
Sales
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Diagnosis & Treatment businesses | 8,175 | 8,635 | 9,168 | |||
| Diagnosis & Treatment | 7,825 | 8,290 | 8,818 | |||
| Nominal sales growth | (3.7)% | 5.6% | 6.2% | 5.9% | 6.4% | |
| Comparable sales growth1) | (2.3)% | 8.1% | (0.7)% | 8.3% | (0.8)% | 11.1% |
| Connected Care businesses | 5,543 | 4,573 | 4,403 | |||
| Connected Care | 5,371 | 5,268 | 5,138 | |||
| Nominal sales growth | 18.6% | (17.5)% | (3.7)% | (14.9)% | (1.9)% | (2.5)% |
| Comparable sales growth1) | 21.6% | (22.6)% | (10.8)% | (19.0)% | (9.1)% | 1.1% |
| Personal Health businesses | 3,199 | 3,429 | 3,626 | |||
| Personal Health | 3,429 | 3,626 | 3,602 | |||
| Nominal sales growth | (9.0)% | 7.2% | 5.7% | 7.2% | 5.7% | (0.7)% |
| Comparable sales growth1) | (6.2)% | 8.8% | 0.1% | 8.8% | 0.1% | 3.2% |
| Other | 396 | 519 | 629 | 530 | 643 | 612 |
| Philips Group | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | 1.0% | (0.9)% | 3.9% | (0.9)% | 3.9% | 1.9% |
| Comparable sales growth1) | 2.9% | (1.2)% | (2.8)% | (1.2)% | (2.8)% | 6.0% |
Group sales in 2023 amounted to EUR 18,169 million, 1.9% higher than in 2022 on a nominal basis. Considering a 4.1% negative currency effect and consolidation impact, comparable sales growth*) was 6.0%. The negative currency effect was mainly due to depreciation of currencies against the euro, and affected all segments. In addition, provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, had a negative impact of 1%.
Comparable order intake decreased 5% in 2023, compared to a 3% decline in 2022. The order book (which covers around 40% of Group sales) remains strong, and we are taking the necessary actions to improve order intake by shortening lead times from order to delivery and building on the positive impact we are making with our innovations, for example in predictive data analytics and artificial intelligence across our portfolio, to help improve the quality and efficiency of care delivery. The order book remains strong and is expected to continue to support growth.
Group sales amounted to EUR 17,827 million in 2022, 3.9% higher than in 2021 on a nominal basis. Considering a 6.7% positive effect from currency and consolidation, comparable sales*) decreased by 2.8%. This was driven by a positive currency effect, mainly due to appreciation of currencies against the euro, and affected all segments.
The order book at year-end 2022 was 10% higher than at the end of 2021, ensuring a higher coverage for sales in 2023. The increase mainly relates to the Diagnosis & Treatment businesses driven by Diagnostic Imaging. Comparable order intake decreased 3%, compared to 4% growth in 2021.
Diagnosis & Treatment
In the fourth quarter of 2022, lower demand for COVID-19-related products compared to 2021 and company actions to improve the order book margin profile contributed to this decrease.
Group2023, sales amounted to EUR 17,1568,818 million, in 2021, 0.9% lower6.4% higher than in 20202022 on a nominal basis. Considering a 0.3% positive4.7% negative currency effect from currency and consolidation impact, comparable sales*) decreasedincreased by 1.2%11.1%. While the currency effectThis was negative, mainly due to depreciation of currencies against the euro,double-digit growth in Ultrasound and affected all business segments, this was more than offset by a positive consolidation impact from new acquisitions.Image-Guided Therapy and high-single-digit growth in Diagnostic Imaging, due to supply chain improvements.
Diagnosis & Treatment businesses
In 2022, sales amounted to EUR 9,1688,290 million, 6.2%5.9% higher than in 2021 on a nominal basis. Considering a 6.9%6.7% positive currency effect and consolidation impact, comparable sales*) decreased by 0.7%0.8%. This was due to mid-single-digit growth in Image-Guided Therapy and low-single-digit growth in Enterprise Diagnostic Informatics, which was more than offset by a decline in Ultrasound and in Diagnostic Imaging due to specific electronic component shortages.
Connected Care
In 2021,2023, sales amounted to EUR 8,6355,138 million, 5.6% higher2.5% lower than in 20202022 on a nominal basis. Considering a 2.5%3.6% negative currency effect and consolidation impact, comparable sales*) increased by 8.1%1.1%. This growth was mainly driven by double-digit growth in Image-Guided Therapy and mid-single-digit growthMonitoring, partly offset by a decline in Diagnostic Imaging and Ultrasound, reflecting demand for Philips' portfolio and positive market conditions.
ConnectedSleep & Respiratory Care businesses
due to the consequences of the Respironics recall. In addition, sales were impacted by provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, which had a negative impact of 3.4%. In 2022, sales amounted to EUR 4,4035,268 million, 3.7%1.9% lower than in 2021 on a nominal basis. Considering a 7.1%7.2% positive currency effect and consolidation impact, comparable sales*) decreased by 10.8%9.1%. This was mainly due to the consequences of the Respironics field actionrecall and the impact of supply chain headwinds.
Personal Health
In 2021,2023, sales amounted to EUR 4,5733,602 million, 17.5%0.7% lower than in 20202022 on a nominal basis. Considering a 5.1% positive3.9% negative currency effect and consolidation impact, comparable sales*) decreasedincreased by 22.6%, following the high COVID-19-generated demand3.2%. This was mainly driven by high-single-digit growth in 2020 and the impact of the Respironics recallPersonal Care, partly offset by a decline in 2021.Oral Healthcare.
Personal Health businesses
In 2022, sales amounted to EUR 3,626 million, 5.7% higher than in 2021 on a nominal basis. Considering a 5.6% positive currency effect and consolidation impact, comparable sales*) increased by 0.1%, consisting of a global increase of 2.5%, offset by a 2.4% decline in sales attributable to Russia due to the war with Ukraine. Oral Healthcare and Mother & Child Care recorded mid-single-digit growth, which was offset by a mid-single-digit decline in Personal Care.
Other
In 2021,2023, sales amounted to EUR 3,429612 million, 7.2% higher thancompared to EUR 643 million in 2020 on a nominal basis. Considering a 1.6% negative currency effect and consolidation impact, comparable sales*) increased by 8.8%. This2022. The decrease was driven by robust customer demand for new product introductions acrossmainly due to the world.discontinuation of innovation consultancy activities provided to other companies until 2023.
Other
In 2022, sales amounted to EUR 629643 million, compared to EUR 519530 million in 2021. The increase was mainly due to additional royalty income and supplies to the divested Domestic Appliances business.
In 2021, sales amounted to EUR 519 million, compared to EUR 396 million in 2020. The increase was mainly driven by supplies to a divested business and higher royalty income.
Performance by geographic area
Philips Group
Sales by geographic area
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Western Europe | 3,702 | 3,645 | 3,603 | 3,645 | 3,603 | 3,819 |
| North America | 6,884 | 6,781 | 7,588 | 6,781 | 7,588 | 7,562 |
| Other mature geographies | 1,750 | 1,694 | 1,643 | 1,694 | 1,643 | 1,626 |
| Total mature geographies | 12,336 | 12,120 | 12,833 | |||
| Mature geographies | 12,120 | 12,833 | 13,007 | |||
| Nominal sales growth | 2% | (2)% | 6% | (2)% | 6% | 1% |
| Comparable sales growth1) | 3% | (3)% | (1)% | (3)% | (1)% | 4% |
| Growth geographies | 4,977 | 5,036 | 4,993 | 5,036 | 4,993 | 5,162 |
| Nominal sales growth | (3)% | 1% | (1)% | 1% | (1)% | 3% |
| Comparable sales growth1) | 3% | (7)% | 3% | (7)% | 10% | |
| Philips Group | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | (0.9)% | 3.9% | 1.9% | |||
| Comparable sales growth1) | (1.2)% | (2.8)% | 6.0% | |||
Sales in Mature geographies in 2023 were 1% higher than in 2022 on a nominal basis and 4% higher on a comparable basis*). Sales in Western Europe were 6% higher year-on-year on a nominal basis and 7% higher on a comparable basis*), with double-digit growth in the Diagnosis & Treatment segment, mid-single-digit growth in the Personal Health segment, and low-single-digit decline in the Connected Care segment. Sales in North America were flat year-on-year on a nominal basis and 3% higher on a comparable basis*), as high-single-digit growth in the Diagnosis & Treatment segment was offset by a low-single-digit decline in the Personal Health segment. Sales in Other mature geographies decreased by 1% on a nominal basis and increased by 7% on a comparable basis*), with high-single-digit comparable sales growth*) in the Connected Care segment and mid-single-digit growth in the Diagnosis & Treatment and Personal Health segments.
Sales in mature geographies in 2022 were 6% higher than in 2021 on a nominal basis and 1% lower on a comparable basis*). Sales in Western Europe were 1% lower year-on-year on a nominal basis and 3% lower on a comparable basis*), with a double-digit decline in the Connected Care businesses, a low-single-digit decline in the Diagnosis & Treatment businesses, and flat growth in the Personal Health businesses. Sales in North America were 12% higher year-on-year on a nominal basis and were flat on a comparable basis*), as double-digit growth in the Personal Health businesses and low-single-digit growth in the Diagnosis & Treatment businesses were offset by a mid-single-digit decline in the Connected Care businesses, mainly due to the Sleep & Respiratory Care business. Sales in other mature geographies decreased by 3% on a nominal basis and 1% on a comparable basis*), with high-single-digit comparable sales growth*) in the Personal Health businesses more than offset by a high-single-digit decline in the Connected Care businesses and a low-single-digit decline in the Diagnosis & Treatment businesses.
Sales in matureGrowth geographies in 2021 were 2% lower than in 20202023 increased by 3% on a nominal basis and 3% lower on a comparable basis*). Sales in Western Europe were 2% lower year-on-year on a nominal basis and 3% lower10% on a comparable basis*), with a double-digit decline in the Connected Care businesses, partly offset by high-single-digit growth in the Diagnosis & Treatment businesses and mid-single-digit growth in the Personal Health businesses. Sales in North America were 1% lower year-on-year on a nominal basis and decreased 3% on a comparable basis*), as double-digit growth in the Diagnosis & Treatment businessesand Connected Care segments and low-single-digit growth in the Personal Health businesses were largely offset by asegment. The double-digit declinegrowth in the Connected Care businesses. Sales in other mature geographies decreased by 3% on a nominal basis and were in line with 2020 on a comparable basis*). High-single-digit comparable sales growth*) in the Personal Health businesseswas driven by China, Middle East & Turkey and mid-single-digit comparable sales growth*) in the Diagnosis & Treatment businesses was partly offset by a double-digit decline in the Connected Care businesses.Latin America.
Sales in growth geographies in 2022 decreased by 1% on a nominal basis and 7% on a comparable basis*), with a double-digit decline in the Connected Care and Personal Health businesses and a low-single-digit decline in the Diagnosis & Treatment businesses. The high-single-digit decline in comparable sales growth*) was due to a double-digit decline in China and Russia & Central Asia, partly offset by double-digit growth in Middle East, Turkey & Africa.
Sales in growth geographies in 2021 increased by 1% on a nominal basis and 3% on a comparable basis*), with double-digit growth in the Personal Health businesses and high-single-digit growth in the
Diagnosis & Treatment businesses, partly offset by a double-digit decline in the Connected Care businesses. The low-single-digit comparable sales growth*) was driven by double-digit growth in India, high-single-digit growth in Russia & Central Asia, and mid-single-digit growth in Central & Eastern Europe and Latin America.Diagnosis & Treatment businesses
Diagnosis & Treatment businesses
Sales by geographic area
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Western Europe | 1,589 | 1,743 | 1,707 | 1,553 | 1,521 | 1,743 |
| North America | 2,931 | 3,088 | 3,514 | 2,664 | 3,019 | 3,172 |
| Other mature geographies | 835 | 849 | 825 | 805 | 782 | 766 |
| Total mature geographies | 5,355 | 5,681 | 6,046 | |||
| Mature geographies | 5,022 | 5,322 | 5,681 | |||
| Growth geographies | 2,820 | 2,954 | 3,122 | 2,803 | 2,968 | 3,137 |
| Sales | 8,175 | 8,635 | 9,168 | 7,825 | 8,290 | 8,818 |
| Nominal sales growth | (4)% | 6% | 6% | 6% | 6% | |
| Comparable sales growth1) | (2)% | 8% | (1)% | 8% | (1)% | 11% |
Sales in Growth geographies increased by 6% on a nominal basis in 2023, and on a comparable basis*) showed double-digit growth, which was mainly driven by China and Middle East & Turkey. Sales in Mature geographies increased by 7% on a nominal basis and showed double-digit growth on a comparable basis*), which was driven by double-digit growth in Western Europe and high-single-digit growth in North America.
Sales in growth geographies increased by 6% on a nominal basis in 2022, and on a comparable basis*) showed a low-single-digit decline, which was mainly due to China. Sales in mature geographies increased by 6% on a nominal basis and were flat year-on-year on a comparable basis*).
Sales in growth geographies increased by 5% on a nominal basis in 2021, and on a comparable basis*) showed high-single-digit growth, driven by double-digit growth in Latin America, India and Central & Eastern Europe and mid-single-digit growth in China. Sales in mature geographies increased by 6% on a nominal basis and showed high-single-digit growth on a comparable basis*). Comparable sales*) increased, with double-digit growth in North America and high-single-digit growth in Western Europe.
Connected Care businesses
Connected Care businesses
Sales by geographic area
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Western Europe | 1,106 | 764 | 646 | 949 | 828 | 798 |
| North America | 2,876 | 2,602 | 2,741 | 3,019 | 3,227 | 3,132 |
| Other mature geographies | 722 | 605 | 544 | 649 | 587 | 573 |
| Total mature geographies | 4,704 | 3,971 | 3,931 | |||
| Mature geographies | 4,618 | 4,642 | 4,503 | |||
| Growth geographies | 839 | 602 | 472 | 753 | 626 | 635 |
| Sales | 5,543 | 4,573 | 4,403 | 5,371 | 5,268 | 5,138 |
| Nominal sales growth | 19% | (17)% | (4)% | (15)% | (2)% | (2)% |
| Comparable sales growth1) | 22% | (23)% | (11)% | (19)% | (9)% | 1% |
Sales in Growth geographies increased by 1% on a nominal basis in 2023, and on a comparable basis*) grew 7%, which was driven by Latin America and China. Sales in Mature geographies decreased by 3% on a nominal basis and were flat year-on-year on a comparable basis*), as growth in Other mature geographies was offset by Western Europe. In addition, provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, had a negative impact of 3.4%.
Sales in growth geographies decreased by 22%17% on a nominal basis in 2022, and on a comparable basis*) showed a double-digit decline, with a double-digit decline across most regions, mainly due to the consequences of the Respironics field action and the COVID situation in China.China. Sales in mature geographies decreasedincreased by 1% on a nominal basis and showed a high-single-digit decline on a comparable basis*), with a double-digit decline in Western Europe and a mid-single-digit decline in North America.
Sales in growth geographies decreased by 28% on a nominal basis in 2021, and on a comparable basis*) showed a double-digit decline, with a double-digit decline across most regions. Sales in mature geographies decreased by 16% on a nominal basis and showed a double-digit decline on a comparable basis*), with a double-digit decline in Western Europe and North America and a mid-single-digit decline in Japan.
Personal Health businesses
Personal Health businesses
Sales by geographic area
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Western Europe | 859 | 894 | 902 | 894 | 902 | 961 |
| North America | 937 | 939 | 1,209 | 939 | 1,209 | 1,144 |
| Other mature geographies | 190 | 198 | 211 | 198 | 211 | 207 |
| Total mature geographies | 1,986 | 2,032 | 2,322 | |||
| Mature geographies | 2,032 | 2,322 | 2,312 | |||
| Growth geographies | 1,213 | 1,398 | 1,304 | 1,398 | 1,304 | 1,290 |
| Sales | 3,199 | 3,429 | 3,626 | 3,429 | 3,626 | 3,602 |
| Nominal sales growth | (9)% | 7% | 6% | 7% | 6% | (1)% |
| Comparable sales growth1) | (6)% | 9% | 0% | 9% | 0% | 3% |
Sales in Growth geographies decreased by 1% on a nominal basis in 2023, and on a comparable basis*) showed mid-single-digit growth, which was mainly driven by Middle-East & Turkey and China, partly offset by a decline in Russia & Central Asia. Sales in Mature geographies were flat on a nominal basis, and on a comparable basis*) showed low-single-digit growth, driven by Western Europe.
Sales in growth geographies decreased by 7% on a nominal basis in 2022, and on a comparable basis*) showed a double-digit decline, which was mainly attributable to China. Sales in mature geographies increased by 14% on a nominal basis, and on a comparable basis*) showed high-single-digit growth, driven by double-digit growth in North America.
Sales in growth geographies increased by 15% on a nominal basis in 2021, and on a comparable basis*) showed double-digit growth, which was attributable to double-digit growth in Central & Eastern Europe, Russia & Central Asia and Latin America and mid-single-digit growth in China. Sales in mature geographies increased by 2% on a nominal basis, and on a comparable basis*) showed mid-single-digit growth, driven by mid-single-digit growth in Western Europe and low-single-digit growth in North America.
Cost of sales
Philips Group
Cost of sales components
in millions of EUR unless otherwise stated
| 2020 | as a % of sales | 2021 | as a % of sales | 2022 | as a % of sales | 2021 | as a % of sales | 2022 | as a % of sales | 2023 | as a % of sales | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs of materials used | 4,221 | 24.4% | 4,142 | 24.1% | 4,320 | 24.2% | 4,142 | 24.1% | 4,320 | 24.2% | 4,626 | 25.5% |
| Salaries and wages | 2,316 | 13.4% | 2,245 | 13.1% | 2,462 | 13.8% | 2,245 | 13.1% | 2,462 | 13.8% | 2,381 | 13.1% |
| Depreciation and amortization | 591 | 3.4% | 479 | 2.8% | 535 | 3.0% | 479 | 2.8% | 535 | 3.0% | 461 | 2.5% |
| Other manufacturing costs | 2,364 | 13.7% | 3,123 | 18.2% | 3,316 | 18.6% | 3,123 | 18.2% | 3,316 | 18.6% | 3,252 | 17.9% |
| Cost of sales | 9,493 | 54.8% | 9,988 | 58.2% | 10,633 | 59.6% | 9,988 | 58.2% | 10,633 | 59.6% | 10,721 | 59.0% |
Cost of sales includes only expenses directly or indirectly attributable to the production process,sale of products or services, such as cost of materials used, salaries and wages, depreciation and amortization of assets used in manufacturing, and other manufacturing costs (such as repair and maintenance costs related to production, expenses incurred for shipping and handling of internal movements of goods, and other expenses related to manufacturing).
Philips’ cost of sales increased by EUR 88 million to EUR 10,721 million in 2023, and decreased as a percentage of sales, compared to EUR 10,633 million in 2022, mainly due to an increase in Cost of materials used by EUR 306 million in 2023, mainly due to increased sales volume and cost inflation, partly offset by productivity measures and a favorable foreign currency impact. Other key factors influencing cost of sales were as follows:
- Salaries and wages decreased by EUR 81 million, driven by productivity measures and a favorable foreign currency impact, partly offset by cost inflation.
- Depreciation and amortization decreased by EUR 74 million in 2023, mainly due to a favorable foreign currency impact.
- Other manufacturing costs decreased by EUR 64 million in 2023, driven by productivity measures and a favorable foreign currency impact, partly offset by cost inflation.
Philips’ cost of sales increased by EUR 645 million to EUR 10,633 million in 2022, compared to EUR 9,988 million in 2021, mainly due to increased expenses of EUR 217 million in salaries and wages, driven by an unfavorable foreign currency impact and wage inflation, partly offset by productivity measures. Other key factors influencing cost of sales were as follows:
- Cost of materials used increased by EUR 178 million in 2022, mainly due to an unfavorable foreign currency impact and cost inflation, partly offset by the reduced field action provision and productivity measures.
- Depreciation and amortization increased by EUR 56 million in 2022, mainly due to an unfavorable foreign currency impact.
- Other manufacturing costs increased by EUR 193 million in 2022, mainly driven by an unfavorable foreign currency impact and cost inflation, partly offset by the lower field action provision and productivity measures.
Gross margin
In 2023, Philips’ costgross margin was EUR 7,448 million, or 41.0% of sales, compared to EUR 7,194 million, or 40.4% of sales, in 2022. Gross margin increased by EUR 495254 million to EUR 9,988 million in 2021, compared to EUR 9,493 million in 2020, mainly due to the field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification in the Sleep & Respiratory Care business reflected in other manufacturing costs. Other key factors influencing cost of sales were as follows:
Costs of materials used decreased by EUR 79 million in 2021, mainlyyear-on-year, driven by increased sales and price & productivitysavings and a positivemeasures, partly offset by cost inflation, an unfavorable foreign currency impact,partly offset by the impact of increases in procurementandsupply chain costs.Salarieshigher restructuring, acquisition-related andwages decreased by EUR 71 million in 2021, driven by productivity and restructuring savings, partly offset by acquisitions.Depreciation and amortization decreased by EUR 112 million in 2021, mainly due to lower impairments of technology assets of EUR 55 million compared to EUR 92 million in 2020.
Gross margin
other charges. In 2022, Philips’ gross margin was EUR 7,194 million, or 40.4% of sales, compared to EUR 7,168 million, or 41.8% of sales, in 2021. Gross margin was flat year-on-year due to cost inflation and a decrease in sales, which was offset by a favorable foreign currency impact, a decrease in restructuring, acquisition-related and other charges, and productivity and pricing measures.
In 2021, Philips’ gross margin was EUR 7,168 million, or 41.8% of sales, compared to EUR 7,822 million, or 45.2% of sales, in 2020. The year-on-year decrease in gross margin was mainly driven by the field action provision of EUR 719 million (representing 4.2% of sales) in connection with the Philips Respironics voluntary recall notification in the Sleep & Respiratory Care business.
Selling expenses
Selling expenses amounted to EUR 4,6094,524 million, or 24.9% of sales, in 2023, compared to EUR 4,621 million, or 25.9% of sales, in 2022. The year-on-year decrease in selling expenses of EUR 97 million was mainly driven by a favorable foreign currency impact, partly offset by higher restructuring, acquisition-related and other charges.
Selling expenses amounted to EUR 4,621 million, or 25.9% of sales, in 2022, compared to EUR 4,258 million, or 24.8% of sales, in 2021. The year-on-year increase in selling expenses of EUR 351363 million was mainly due to an unfavorable foreign currency impact and an increase in restructuring, acquisition-related and other charges.
General and administrative expenses
SellingGeneral and administrative expenses amounted to EUR 4,258608 million, or 24.8%3.3% of sales, in 2021,2023, compared to EUR 4,056671 million, or 23.4%3.8% of sales, in 2020.2022. The year-on-year increase in selling expensesdecrease of EUR 20463 million was mainly driven by the acquisitions of BioTelemetry and Capsule Technologies and higher investments in advertising and promotion, partly offset by a positivefavorable foreign currency impact and lower restructuring, costs. Selling expenses include restructuring, acquisition-related and other charges of EUR 140 million in 2021, compared to EUR 133 million in 2020.charges.
General and administrative expenses
General and administrative expenses amounted to EUR 671 million, or 3.8% of sales, in 2022, compared to EUR 599 million, or 3.5% of sales, in 2021. The year-on-year increase of EUR 72 million was mainly driven by higher restructuring, acquisition-related and other charges.
General
Research and administrativedevelopment expenses amounted to
Research and development costs were EUR 5991,890 million, or 3.5%10.4% of sales, in 2021,2023, compared to EUR 6302,091 million, or 3.6%11.7% of sales, in 2020.2022. The year-on-year decrease of EUR 31201 million in general and administrative expenses was mainly driven by lower restructuring, acquisition-related and other charges.
Researchcharges and development expenses
a favorable foreign currency impact. 2022 included R&D project impairment charges.Research and development costs were EUR 2,1032,091 million, or 11.8%11.7% of sales, in 2022, compared to EUR 1,806 million, or 10.5% of sales, in 2021. The year-on-year increase of EUR 297285 million was mainly driven by higher restructuring, acquisition-related and other charges in relation to R&D project impairments and an unfavorable foreign currency impact.
Research and development costs were EUR 1,806 million, or 10.5% of sales, in 2021, compared to EUR 1,822 million, or 10.5% of sales, in 2020. The year-on-year decrease of EUR 16 million was mainly driven by lower restructuring, acquisition-related and other charges. 2021 includes EUR 101 million of restructuring, acquisition-related and other charges, compared to EUR 131 million in 2020.
Philips Group
Research and development expenses
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 891 | 910 | 1,124 | 762 | 894 | 827 |
| Connected Care | 547 | 543 | 637 | 670 | 822 | 663 |
| Personal Health | 190 | 200 | 190 | 200 | 197 | |
| Other | 194 | 163 | 142 | 184 | 175 | 203 |
| Philips Group | 1,822 | 1,806 | 2,103 | 1,806 | 2,091 | 1,890 |
| As a % of sales | 10.5% | 11.8% | 10.5% | 11.7% | 10.4% | |
Impairment of goodwill
In addition to the annual goodwill-impairment tests for Philips, trigger-based impairment tests were performed during the years 2023, 2022 2021 and 2020.2021. As a result of the tests, recorded goodwill impairments were recorded of EUR 1,357 million in 2022, and EUR 15 million in 2021, and EUR 144 million in 2020.2021. The goodwill impairment of EUR 1,331 million in 2022 was recorded in the Sleep & Respiratory Care business and was due to revisions to the expected future cash flows. In addition, in 2023 a EUR 8 million goodwill impairment was recognized for a business held for sale, whereas in 2022 a EUR 27 million goodwill impairment was recognized in the Precision Diagnosis Solutions business.
During 2022, EUR 1,331 million of goodwillGoodwill impairment charges were recordedEUR 8 million in the Sleep & Respiratory Care business, due to revisions to the expected future cash flows. In addition, a2023 and EUR 271,357 million goodwill impairment was recognized in the Precision Diagnosis Solutions business.2022. For further information refer to Goodwill.
Net income, Income from operations (EBIT) and Adjusted EBITA*)
Net income amounted to a loss of EUR 463 million in 2023, an improvement of EUR 1.1 billion compared to 2022, which included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Higher earnings in 2023 were offset by a EUR 575 million litigation provision in connection with the Respironics recall. Net income in 2022 included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Net income is not allocated to segments, as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
The following overview shows Income from operations and Adjusted EBITA*) by segment.
Philips Group
Income from operations and Adjusted EBITA1)
in millions of EUR unless otherwise stated
| Income from operations | as a % of sales | Adjusted EBITA1) | as a % of sales | |
|---|---|---|---|---|
| 2023 | ||||
| Diagnosis & Treatment | 720 | 8.2% | 1,026 | 11.6% |
| Connected Care | (1,199) | (23.3)% | 369 | 7.2% |
| Personal Health | 552 | 15.3% | 597 | 16.6% |
| Other | (188) | (71) | ||
| Philips Group | (115) | (0.6)% | 1,921 | 10.6% |
| 2022 | ||||
| Diagnosis & Treatment | 538 | 6.5% | 788 | 9.5% |
| Connected Care | (2,347) | (44.6)% | 111 | 2.1% |
| Personal Health | 515 | 14.2% | 538 | 14.8% |
| Other | (235) | (119) | ||
| Philips Group | (1,529) | (8.6)% | 1,318 | 7.4% |
| 2021 | ||||
| Diagnosis & Treatment | 948 | 12.1% | 1,028 | 13.1% |
| Connected Care | (716) | (13.3)% | 553 | 10.3% |
| Personal Health | 576 | 16.8% | 590 | 17.2% |
| Other | (255) | (117) | ||
| Philips Group | 553 | 3.2% | 2,054 | 12.0% |
Income from operations amounted to a loss of EUR 115 million, or (0.6)% of sales, in 2023, compared to a loss of EUR 1,529 million, or (8.6)% of sales, in 2022, which included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Higher earnings in 2023 were offset by a EUR 575 million Respironics litigation provision. Adjusted EBITA*) increased to EUR 1,921 million and the margin improved to 10.6%, compared to EUR 1,318 million and a margin of 7.4% in 2022, mainly driven by increased sales and pricing & productivity measures.
Amortization and goodwill impairment charges were EUR 298 million in 2023. This includes amortization charges of EUR 290 million and goodwill impairment charges of EUR 8 million. Amortization and goodwill impairment charges in 2022 were EUR 1,720 million. This included a charge of EUR 1,331 million related to an impairment of goodwill in the Sleep & Respiratory Care business, EUR 363 million amortization charges and a EUR 27 million goodwill impairment in the Precision Diagnosis Solutions business.
Restructuring, acquisition-related and other charges were EUR 1,739 million in 2023. This includes: a EUR 575 million Respironics litigation provision, EUR 363 million in connection with the proposed Respironics consent decree, and EUR 224 million Respironics field-action running remediation costs. In addition, it includes EUR 285 million restructuring charges, mainly related to workforce reduction, and charges in relation to quality remediation actions of EUR 175 million. 2022 charges were EUR 1,127 million and included: restructuring charges of EUR 185 million; EUR 148 million portfolio realignment impairments and charges; R&D project impairment charges of EUR 134 million; EUR 250 million for the Respironics field-action provision; EUR 210 million Respironics field-action running remediation costs; an approximately EUR 60 million provision for public investigations into tender irregularities; and EUR 59 million for provisions for quality actions in Connected Care.
Income from continuing operations attributable to shareholders per common share (in EUR) - diluted, was EUR (0.50) in 2023, compared to EUR (1.76) in 2022. Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted*) was EUR 1.25, compared to EUR 0.92 in 2022.
Net income amounted to a loss of EUR 1,605 million in 2022, a decrease of EUR 4.9 billion compared to 2021, mainly due to a charge of EUR 1.5 billion related to goodwill and R&D impairments in 2022 and a gain of EUR 2.5 billion on the sale of the Domestic Appliances business in 2021. Net income is not allocated to segments, as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
The following overview shows Income from operations and Adjusted EBITA*) by segment.
Philips Group
Income from operations and Adjusted EBITA1)
in millions of EUR unless otherwise stated
| Income from operations | as a % of sales | Adjusted EBITA1) | as a % of sales | |
|---|---|---|---|---|
| 2022 | ||||
| Diagnosis & Treatment | 404 | 4.4% | 774 | 8.4% |
| Connected Care | (2,246) | (51.0)% | 95 | 2.2% |
| Personal Health | 515 | 14.2% | 538 | 14.8% |
| Other | (202) | (89) | ||
| Philips Group | (1,529) | (8.6)% | 1,318 | 7.4% |
| 2021 | ||||
| Diagnosis & Treatment | 941 | 10.9% | 1,071 | 12.4% |
| Connected Care | (722) | (15.8)% | 497 | 10.9% |
| Personal Health | 576 | 16.8% | 590 | 17.2% |
| Other | (242) | (105) | ||
| Philips Group | 553 | 3.2% | 2,054 | 12.0% |
| 2020 | ||||
| Diagnosis & Treatment | 497 | 6.1% | 818 | 10.0% |
| Connected Care | 704 | 12.7% | 1,191 | 21.5% |
| Personal Health | 362 | 11.3% | 433 | 13.5% |
| Other | (300) | (165) | ||
| Philips Group | 1,264 | 7.3% | 2,277 | 13.2% |
Income from operations in 2022 amounted to a loss of EUR 1,529 million, or (8.6)% of sales, compared to EUR 553 million, or 3.2% of sales, in 2021, mainly impacted by a charge of EUR 1.5 billion related to goodwill and R&D impairments. Adjusted EBITA*) in 2022 was EUR 1,318 million and the margin amounted to 7.4%, compared to EUR 2,054 million and a margin of 12.0% in 2021, primarily due to the sales decline and cost inflation, partly offset by pricing and productivity measures.
Amortization and goodwill impairment charges in 2022 were EUR 1,720 million. This includes a charge of EUR 1,331 million related to an impairment of goodwill in the Sleep & Respiratory Care business, a EUR 27 million goodwill impairment in the Precision Diagnosis Solutions business, and amortization charges of EUR 22 million related to an impairment of a technology asset. In 2021, amortization and goodwill impairment charges were EUR 337 million and included a charge of EUR 13 million related to an impairment of goodwill and amortization charges of EUR 55 million related to an impairment of a technology asset.
Restructuring, acquisition-related and other charges in 2022 were EUR 1,127 million. This includes: restructuring charges of EUR 185 million; EUR 282 million portfolio realignment impairments and charges; EUR 250 million for the Respironics field-action provision; EUR 210 million Respironics field-action running remediation costs; a EUR 60 million provision for public investigations tender irregularities; and EUR 59 million for provisions for quality actions in Connected Care. 2021 charges were EUR 1,164 million and included: a field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification; provisions for quality actions of EUR 94 million and other matters of EUR 53 million in Connected Care; restructuring charges of EUR 80 million; acquisition-related charges of EUR 102 million partly offset by a EUR 87 million gain related to the re-measurement of contingent consideration liabilities; a loss of EUR 76 million related to a divestment; and separation costs of EUR 64 million related to the Domestic Appliances business. 2021 also included a release of a legal provision of EUR 38 million, a gain of EUR 33 million related to a minority participation, and a benefit from the re-measurement of environmental liabilities of EUR 22 million.
Income from continuing operations attributable to shareholders per common share (in EUR) - diluted, was EUR (1.84) in 2022, compared to EUR 0.67 in 2021. Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted*) was EUR 0.96 in 2022, compared to EUR 1.65 in 2021.
Diagnosis & Treatment
Net incomeIncome from operations increased to EUR 720 million in 2021 increased by EUR 2.1 billion2023, compared to 2020,EUR 538 million in 2022. This was mainly driven by the gain on the sale of the Domestic Appliances business,increased sales and pricing & productivity measures, partly offset by the EUR 719 million field action provision.
Income from operationscost inflation. These factors also resulted in 2021 amounted to EUR 553 million, or 3.2% of sales, compared to EUR 1,264 million, or 7.3% of sales,an increase in 2020, mainly impacted by the EUR 719 million field action provision. Adjusted EBITA*) to 11.6% of sales in 2021 was EUR 2,054 million and the margin amounted to 12.0%, compared to EUR 2,277 million and a margin of 13.2%, due to a decline in sales and the impact of supply chain headwinds, partly offset by productivity measures.2023.
Amortization and goodwill impairment charges in 20212023 were EUR 337 million. This includes a charge of98 million and include EUR 1389 million related to an impairment of goodwill and amortization charges and EUR 8 million goodwill impairment charges. 2022 charges were EUR 115 million and included EUR 22 million of EUR 55 millioncharges related to an impairment of a technology asset. In 2020, amortization and goodwill impairment charges were EUR 521 million and included a charge of EUR 144 million related to an impairment of goodwillasset in the Connected Care segment, as well as amortization charges of EUR 92 million related to an impairment of a technology asset.Image-Guided Therapy.
Restructuring, acquisition-related and other charges in 20212023 were EUR 1,164 million. This includes a field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification, provisions for quality actions of EUR 94210 million and other matters ofinclude EUR 5381 million charges in the Connected Care businesses,relation to quality remediation actions and EUR 73 million restructuring charges, mainly related to workforce reduction. 2022 charges amounted to EUR 136 million and included R&D project impairment charges of EUR 8073 million acquisition-related charges of EUR 102 million partly offset byand a EUR 87 million gain related to the re-measurement of contingent consideration liabilities, a loss of EUR 76 million related to a divestment, and separation costs of EUR 64 million related to the Domestic Appliances business. 2021 also includes a release of a legal provision of approximately EUR 3860 million a gain of EUR 33 million related to a minority participation, and a benefit from the re-measurement of environmental liabilities of EUR 22 million. 2020 charges were EUR 494 million and included EUR 200 million of restructuring charges, EUR 95 million of acquisition-related charges offset by a EUR 101 million gain related to the re-measurement of a contingent consideration liability, EUR 31 million related to impairments of capitalized development costs, EUR 43 million of charges due to changes in ventilator demand, EUR 42 million of separation costs related to the Domestic Appliances business, a EUR 38 million provision related to legal matters, and EUR 21 million related to pension liability de-risking in the US.for public investigations into tender irregularities.
Income from continuing operations attributable to shareholders per common share (in EUR) - diluted, was EUR 0.67 in 2021, compared to EUR 1.08 in 2020. Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted*) was EUR 1.65 in 2021, compared to EUR 1.74 in 2020.
Diagnosis & Treatment businesses
Income from operations in 2022 decreased to EUR 404 million, compared to EUR 941 million in 2021. This was mainly due to cost inflation, partly offset by productivity measures. These factors also resulted in a decrease in Adjusted EBITA*) to 8.4% of sales in 2022.
Amortization and goodwill impairment charges in 2022 were EUR 170115 million and includeincluded EUR 22 million of charges related to an impairment of a technology asset in Image-Guided Therapy and a goodwill impairment of EUR 27 million in Precision Diagnosis Solutions.Therapy. 2021 charges were EUR 155144 million and included EUR 55 million of charges related to an impairment of a technology asset in Image-Guided Therapy.
Restructuring, acquisition-related and other charges in 2022 were EUR 201 million and include EUR 120 million portfolio realignment impairments and charges and a provision of EUR 60 million for public investigations tender irregularities. 2021 charges amounted to a gain of EUR 25 million and included: restructuring charges of EUR 44 million; acquisition-related charges of EUR 48 million offset by a EUR 85 million gain related to the re-measurement of contingent consideration liabilities; and the release of a legal provision of EUR 38 million.
Connected Care
Income from operations in 2021 increased to EUR 941(1,199) million in 2023, compared to EUR 497(2,347) million in 2020. This2022, which included a charge of EUR 1.3 billion related to goodwill impairment. 2023 was primarily due tomainly impacted by the consequences of the Respironics field action, in particular the EUR 575 million provision in connection with the Respironics litigation, partly offset by increased sales growth and productivity measures. These factors also resulted in an increased Adjusted EBITA*), which was 12.4% improved to 7.2% of sales in 2021.and was also impacted by cost inflation.
Amortization and goodwill impairment charges in 20212023 were EUR 155178 million and include EUR 55178 million of charges related to an impairment of a technology asset in Image-Guided Therapy. 2020amortization charges. 2022 charges were EUR 2091,583 million and included EUR 921,331 million impairment of chargesgoodwill related to anthe Sleep & Respiratory Care business and a goodwill impairment of a technology assetEUR 27 million in Image-Guided Therapy.Precision Diagnosis Solutions.
Restructuring, acquisition-related and other charges in 2021 amounted to a gain of2023 were EUR 251,390 million and include restructuringinclude: charges of EUR 44575 million acquisition-relatedRespironics litigation provision, EUR 363 million in connection with the proposed Respironics consent decree, and EUR 224 million Respironics field-action running remediation costs. In addition, it includes EUR 64 million restructuring charges, mainly related to workforce reduction, and charges in relation to quality remediation actions of EUR 48 million offset by a EUR 85 million gain related to the re-measurement of contingent consideration liabilities, and a release of a legal provision of EUR 3894 million. 20202022 charges were EUR 112875 million and includedincluded: EUR 57250 million of restructuring charges,for the Respironics field action provision; EUR 73210 million of acquisition-related charges offset by aRespironics running remediation costs; EUR 101148 million gain related to the re-measurement of a contingent consideration liability,portfolio realignment impairments and charges; and EUR 3859 million related to legal matters, and a EUR 31 million impairment of capitalized development costs.
provisions for quality actions in Connected Care businesses
Care.Income from operations in 2022 decreased to EUR (2,246) million, compared to EUR (722) million in 2021. This was mainly due to the EUR 1.3 billion goodwill impairment, the sales decline, the consequences of the Respironics field action and cost inflation. Adjusted EBITA*) was 2.2% of sales in 2022 and was also impacted by the sales decline and cost inflation, partly offset by productivity measures.
Amortization and goodwill impairment charges in 2022 were EUR 1,530 million and include EUR 1,331 million impairment of goodwill related to the Sleep & Respiratory Care business.business and a goodwill impairment of EUR 27 million in Precision Diagnosis Solutions. 2021 charges were EUR 161 million and included a EUR 13 million impairment of goodwill related to the divested Personal Emergency Response Services (PERS) and Senior Living business.
Restructuring, acquisition-related and other charges in 2022 were EUR 811 million and include: EUR 250 million for the Respironics field action provision; EUR 210 million Respironics running remediation costs; EUR 160 million portfolio realignment impairments and charges; and EUR 59 million provisions for quality actions in Connected Care. 2021 charges were EUR 1,058 million and included: a field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification; EUR 93 million of restructuring and acquisition-related charges; provisions for quality actions of EUR 94 million and other matters of EUR 53 million; and a gain of EUR 33 million related to a minority participation.
Personal Health
Income from operations in 2021 decreasedincreased to EUR (722)552 million in 2023, compared to EUR 704515 million in 2020.2022. This was mainly due to the decline indriven by increased sales and the impact of the Respironics recall on the Sleeppricing & Respiratory Care business.productivity measures. These factors also impactedresulted in an increase in Adjusted EBITA*), which was 10.9% to 16.6% of sales in 2021.sales.
Amortization and goodwill impairment charges in 20212023 were EUR 16114 million and include EUR 13 million impairment of goodwillamortization charges related to the divested Personal Emergency Response Services (PERS) and Senior Living business. 2020intangible assets in Mother & Child Care. 2022 charges were EUR 27815 million and included a EUR 144 million impairment of goodwillamortization charges related to the Population Health Management business.intangible assets in Mother & Child Care.
Restructuring, acquisition-related and other charges in 20212023 were EUR 1,05831 million and include a field action provisionEUR 23 million investment re-measurement loss and restructuring costs mainly related to workforce reduction of EUR 719 million in connection with the Philips Respironics voluntary recall notification, EUR 93 million of restructuring and acquisition-related charges, provisions for quality actions of EUR 94 million and other matters of EUR 53 million, and a gain of EUR 33 million related to a minority participation. 20209 million. 2022 charges were EUR 209 million and included restructuring charges of EUR 76 million, acquisition-related charges of EUR 22 million, and charges of EUR 43 million due to changes in ventilator demand.not material.
Personal Health businesses
Income from operations in 2022 decreased to EUR 515 million, compared to EUR 576 million in 2021. This was mainly driven by cost inflation and an adverse foreign currency impact, partly offset by pricing and productivity measures. These factors also resulted in a decrease in Adjusted EBITA*) to 14.8% of sales.
Amortization charges in 2022 were EUR 15 million and include amortization charges related to intangible assets in Mother & Child Care. 2021 charges were EUR 15 million and included amortization charges related to intangible assets in Mother & Child Care.
Restructuring, acquisition-related and other charges in 2022 and 2021 were not material.
Income from operations in 2021 increased to EUR 576 million, compared to EUR 362 million in 2020. This was mainly driven by sales growth and productivity measures, partly offset by higher investments in advertising & promotion. These factors also resulted in an increased Adjusted EBITA*), which was 17.2% of sales.
Amortization charges in 2021 were EUR 15 million and include amortization charges related to intangible assets in Mother & Child Care. 2020 charges were EUR 16 million and included amortization charges related to intangible assets in Mother & Child Care.
Restructuring, acquisition-related and other charges in 2021 were not material. 2020 charges were EUR 55 million and included restructuring charges of EUR 31 million.
Other
In Other we report on the items Innovation & Strategy, IP Royalties, Central costs and Other.
Income from operations amounted to a loss of EUR 188 million in 2023, compared to a loss of EUR 235 million in 2022. Adjusted EBITA*) amounted to a loss of EUR 71 million, compared to a loss of EUR 119 million in 2022. The increase in Adjusted EBITA*) was mainly due to cost savings, partly offset by lower royalty income.
Restructuring, acquisition-related and other charges in 2023 were EUR 108 million and include EUR 139 million restructuring charges mainly related to workforce reduction and a gain of EUR 35 million due to a divestment. 2022 charges were EUR 108 million and included restructuring charges of EUR 61 million and a EUR 21 million impairment of intangible assets.
Income from operations in 2022 amounted to a loss of EUR 202 million, compared to a loss of EUR 242 million in 2021. Adjusted EBITA*)EBITA* in 2022 amounted to a loss of EUR 89 million, compared to a loss of EUR 105 million in 2021. Adjusted EBITA*) increased, mainly due to higher royalty income, partly offset by an adverse currency impact and investment in Quality & Regulatory.
Restructuring, acquisition-related and other charges in 2022 were EUR 107 million and include restructuring charges of EUR 61 million and a EUR 21 million impairment of intangible assets. 2021 charges were EUR 131 million and included a loss of EUR 76 million related to a divestment and EUR 64 million of separation costs related to the Domestic Appliances business, partly offset by a benefit from the re-measurement of environmental liabilities of EUR 22 million.
Income from operations in 2021 was EUR (242) million, compared to EUR (300) million in 2020. Adjusted EBITA*) in 2021 was EUR (105) million, compared to EUR (165) million in 2020. Income from operations and Adjusted EBITA*) increased, mainly due to higher royalty income and lower charges related to environmental provisions, partly offset by investments, mainly in IT and Quality & Regulatory affairs.
Restructuring, acquisition-related and other charges in 2021 were EUR 131 million and include a loss of EUR 76 million related to a divestment and EUR 64 million of separation costs related to the Domestic Appliances business, partly offset by a benefit from the re-measurement of environmental liabilities of EUR 22 million. 2020 charges were EUR 118 million and included restructuring charges of EUR 37 million, EUR 42 million of separation costs related to the Domestic Appliances business, and EUR 21 million related to pension liability de-risking in the US.
Financial income and expenses
A breakdown of financial income and expenses is presented in the following table.
Philips Group
Financial income and expenses
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Interest expense, net | (160) | (141) | (210) | (141) | (210) | (230) |
| Sale of securities | 2 | - | - | |||
| Net change in fair value of financial assets through profit or loss | 129 | 95 | 9 | 95 | 9 | (26) |
| Net foreign exchange gains (losses) | - | 9 | (23) | |||
| Other | (15) | 6 | 2 | 6 | (8) | (34) |
| Financial income and expenses | (44) | (39) | (200) | (39) | (200) | (314) |
Financial income and expenses resulted in a net expense of EUR 314 million in 2023, compared to a net expense of EUR 200 million in 2022. 2023 includes higher interest expense, fair value losses on minority investments and net foreign exchange losses compared to 2022. For further information, refer to Financial income and expenses.
Financial income and expenses resulted in a net expense of EUR 200 million, compared to a net expense of EUR 39 million in 2021. 2022 includes lower gains on the value of Philips' minority participations and higher interest expense, primarily due to financial charges related to early redemption of EUR and USD bonds and issuance of new EUR bonds in April 2022, compared to 2021. For further information, refer to Financial income and expenses.
Income taxes
FinancialIncome tax expense increased by EUR 40 million year-on-year. The income tax benefit in 2023 is mainly driven by the negative income before tax, recognition of tax credits and expenses resulted in a net expense of EUR 39 million in 2021, compared to a net expense of EUR 44 million in 2020. 2021 includes gainstax incentives, partly offset by the tax effect on the value of Philips' minority participationseconomic loss class-action settlement provision relating to the Respironics recall. The income tax benefit in 2022 was mainly driven by the negative income before tax and higher net interest income. For further information, refer to Financial income and expenses.tax incentives, partly offset by non-tax-deductible goodwill impairment.
Income taxes
Income tax expense decreased by EUR 10 million year-on-year,in 2022 compared to 2021, mainly due to lower income, partly offset by a non-deductible goodwill impairment in the Sleep & Respiratory Care business in 2022 and a one-off benefit relating to the recognition of tax assets due to a business transfer in 2021.
Investments in associates
Income taxes amountedResults related to investments in associates declined from a loss of EUR 2 million in 2022 to a benefitloss of EUR 10398 million in 2021. The effective income tax rate in 2021 was (20.0)%, compared to 17.6% in 2020, mainly due to the impact from the recognition2023. 2023 includes impairments of tax assetsEUR 58 million and other tax benefits as a resultshare of a business transfer during the year.results of associates of EUR 40 million.
Investments in associates
Results related to investments in associates improved from a loss of EUR 4 million in 2021 to a loss of EUR 2 million in 2022. In 2022, Philips recorded an impairment of EUR 66 million in relation to its interest in Candid Care Co. As part of the acquisition of Affera, Inc. by Medtronic plc in August 2022, the company sold its investment in Affera to Medtronic and recorded a gain of EUR 84 million on the sale.
Results related to investments in associates improved from a loss of EUR 9 million in 2020 to a loss of EUR 4 million in 2021. The number of associates increased compared to 2020. Although gains were recorded in a number of investments in associates, these were more than offset by losses in the remainder.
Discontinued operations
Philips Group
Discontinued operations, net of income taxes
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Domestic Appliances | 206 | 2,698 | 3 | 2,698 | 3 | (2) |
| Other | (10) | 13 | 10 | 13 | 10 | (7) |
| Net income of Discontinued operations | 196 | 2,711 | 13 | 2,711 | 13 | (10) |
In 2023 and 2022, Discontinued operations consisted primarily of the Domestic Appliances business and certain other divestments that were reported as discontinued operations. In 2021, the sale of the Domestic Appliance business resulted in an after-tax gain of EUR 2.5 billion.
For further information, refer to Discontinued operations and assets classified as held for sale.
Non-controlling interests
Net income attributable to non-controlling interests decreased from EUR 3 million in 2022 to EUR 2 million in 2023.
Net income attributable to non-controlling interests decreased from EUR 4 million in 2021 to EUR 3 million in 2022.
Net income attributable to non-controlling interests decreased from EUR 8 million in 2020 to EUR 4 million in 2021.
7.1.47.5Acquisitions and divestments
Acquisitions
In 2023, Philips completed one acquisition involving a total net cash outflow of EUR 53 million (total equity price and settlement of debt). The acquisition is subject to final purchase price allocation procedures, which are expected to be finalized in the second quarter of 2024.
In 2022, Philips completed three acquisitions. The acquisition of Vesper Medical Inc., completed on January 11, 2022, was the most notable. Acquisitions in 2022 and prior years led to acquisition and post-merger integration charges of EUR 6570 million in the Connected Care businesses.segment.
In 2021, Philips completed two acquisitions: BioTelemetry, which was completed on February 9, 2021, and Capsule Technologies, which was completed on March 4, 2021. Acquisitions in 2021 and prior years led to acquisition and post-merger integration charges of EUR 51 million in the Connected Care businesses.
Divestments
In 2020,2023, Philips completed three acquisitions, with Intact Vascular being the most notable. Acquisitions in 2020 and prior years led to acquisition and post-merger integration charges resulting insix divestments for a gaincash consideration of EUR 2880 million, notably Philips Pharma Solutions in the Diagnosis & Treatment businesses and charges of EUR 22 million in the Connected Care businesses.US.
Divestments
In 2022, Philips completed one divestment, which was not material.
Inn 2021, Philips completed three divestments. On September 1, 2021, Philips sold its Domestic Appliances business to a global investment firm, Hillhouse Investment, resulting in a EUR 2.5 billion gain after tax and transaction-related costs; reported in Discontinued Operations.
In addition, Philips completed the divestment of the Personal Emergency Response Services (PERS) and Senior Living business on June 30, 2021, and September 17, 2021, respectively, as well as completing the divestment of a small business in segment Other. As part of the PERS divestment, Philips acquired shares in the buyer, Connect America Investment Holdings, LLC, with a value of EUR 40 million. The investment is classified as a financial asset measured at Fair Value through Other Comprehensive Income (FVTOCI) and is reported as part of Other non-current financial assets. The divestment resulted in a loss of EUR 76 million, which is included in Other business expenses in our Consolidated statements of income.
Philips did not complete any divestments in 2020.
For details, please refer to Acquisitions and divestments.
7.1.57.6Cash flows
The movements in cash and cash equivalents balance for the years ended December 31, 2020, 2021, 2022 and 20222023 are presented and explained in the following table.
Philips Group
Condensed consolidated cash flows
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Beginning cash and cash equivalents balance | 1,425 | 3,226 | 2,303 | 3,226 | 2,303 | 1,172 |
| Net cash flows from operating activities | 2,511 | 1,629 | (173) | 1,629 | (173) | 2,136 |
| Net cash flows from investing activities | ||||||
| Net capital expenditures | (876) | (729) | (788) | (729) | (788) | (554) |
| Other cash flows from investing activities | (391) | (2,943) | (698) | (2,943) | (698) | (82) |
| Net cash flows from financing activities | ||||||
| Treasury shares transactions | (297) | (1,613) | (174) | (1,613) | (174) | (662) |
| Changes in debt | 783 | (251) | 1,092 | (251) | 1,092 | (181) |
| Dividend paid to shareholders of the company | (1) | (482) | (412) | (482) | (412) | (2) |
| Other cash flow items | (57) | 62 | 34 | 62 | 34 | (81) |
| Net cash flows from discontinued operations | 129 | 3,403 | (12) | 3,403 | (12) | 123 |
| Ending cash and cash equivalents balance | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
Net cash flows from operating activities
Net cash flows from operating activities amounted to an inflow of EUR 2,136 million in 2023, compared to an outflow of EUR 173 million in 2022. This increase is mainly due to higher cash earnings and lower working capital, and includes a EUR 141 million payment related to the previously announced resolution of the economic loss class action in the US. Free cash flow*) amounted to a cash inflow of EUR 1,582 million in 2023, compared to an outflow of EUR 961 million in 2022.
Net cash flows from operating activities amounted to an outflow of EUR 173 million in 2022, compared to an inflow of EUR 1,629 million in 2021. This decrease is mainly due to lower cash earnings, increased working capital and cash costs related to the Philips Respironics field action. Free cash flow*flow*) amounted to a cash outflow of EUR 961 million in 2022, compared to an inflow of EUR 900 million in 2021.
In 2021, net cash flows from operating activities amounted to EUR 1,629 million, compared to EUR 2,511 million in 2020. This decrease is mainly due to increased working capital and consumption of provisions, partly offset by lower income tax paid. Free cash flow*flow*) amounted to EUR 900 million in 2021, compared to EUR 1,635 million in 2020.
In 2020, net cash flows from operating activities amounted to EUR 2,511 million, and Free cash flow*) amounted to EUR 1,635 million.
Net cash flows from investing activities
Net cash flows from investing activities consist of net capital expenditures and other cash flows from investing activities.
In 2023, other cash flows from investing activities amounted to a cash outflow of EUR 82 million, mainly due to a new business acquisition and minority investments, partly offset by divestment proceeds.
In 2022, other cash flows from investing activities amounted to a cash outflow of EUR 698 million, mainly due to the acquisitions of Vesper Medical and Cardiologs, amounting to EUR 414 million, and new minority investments.
In 2021, other cash flows from investing activities amounted to a cash outflow of EUR 2,943 million, mainly due to the acquisitions of BioTelemetry and Capsule Technologies amounting to EUR 2.8 billion.
In 2020, other cash flows from investing activities amounted to a cash outflow of EUR 391 million, mainly due to the acquisition of Intact Vascular for EUR 241 million and investments in other non-current financial assets.
Net cash flows from financing activities
Net cash flows from financing activities consist of treasury shares transactions, changes in debt, dividend paid and other cash flow items.
In 2023, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 662 million net cash outflow. Changes in debt mainly includes new bonds issued of EUR 500 million and loan repayments amounting to EUR 500 million. The dividend was distributed fully in shares.
In 2022, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 174 million net cash outflow. Changes in debt mainly includesincluded new bonds issued of EUR 2 billion and a new term loan issued of EUR 500 million, partly offset by bond repayments of EUR 1.2 billion. Philips’ shareholders received a total dividend of EUR 741 million, including costs, of which the cash portion amounted to EUR 412 million.
In 2021, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 1,613 million net cash outflow. Changes in debt mainly relates to short-term debt and lease repayments. Philips’ shareholders received a total dividend of EUR 773 million, including costs, of which the cash portion amounted to EUR 482 million.
In 2020, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 297 million net cash outflow. Changes in debt included EUR 991 million cash inflow from the issuance of two new bonds under the EMTN program, partly offset by outflows related to lease payments. The 2019 dividend was distributed fully in shares in July 2020.
Net cash flows from discontinued operations
In 2023, net cash provided by discontinued operations was EUR 123 million, mainly related to a refund received of one-off advance tax payments of a previously disposed business.
In 2022, net cash used for discontinued operations was EUR 12 million, mainly related to previously disposed businesses.
In 2021, net cash provided by discontinued operations was EUR 3,403 million and consisted primarily of the net cash inflow of EUR 3,319 million from the sale of the Domestic Appliances business on September 1, 2021.
In 2020, net cash provided by discontinued operations mainly related to the Domestic Appliances business, partly offset by advance income tax payments amounting to EUR 78 million.
7.1.67.7Financing
Condensed consolidated balance sheets for the years 2020,as of December 31, 2021, 2022 and 20222023 are presented in the following table:
Philips Group
Condensed consolidated balance sheets
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Intangible assets | 11,012 | 14,287 | 13,764 | 14,287 | 13,764 | 13,067 |
| Property, plant and equipment | 2,682 | 2,699 | 2,638 | 2,699 | 2,638 | 2,483 |
| Investments and financial assets | 781 | 1,121 | 1,334 | 1,121 | 1,334 | 1,050 |
| Deferred tax assets | 1,820 | 2,216 | 2,449 | 2,216 | 2,449 | 2,627 |
| Inventories | 2,993 | 3,450 | 4,049 | 3,450 | 4,049 | 3,491 |
| Receivables | 4,537 | 4,191 | 4,616 | 4,191 | 4,616 | 4,146 |
| Other assets | 663 | 693 | 665 | 693 | 665 | 672 |
| Payables | (3,854) | (3,784) | (3,635) | (3,784) | (3,635) | (3,886) |
| Provisions | (1,980) | (2,313) | (2,115) | (2,313) | (2,115) | (2,498) |
| Contract liabilities | (1,643) | (1,936) | (2,210) | (1,936) | (2,210) | (2,278) |
| Other liabilities | (1,402) | (1,473) | (1,244) | (1,473) | (1,244) | (993) |
| Net asset employed | 15,609 | 19,151 | 20,311 | |||
| Net assets employed | 19,151 | 20,311 | 17,881 | |||
| Cash and cash equivalents | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
| Debt | (6,934) | (6,980) | (8,201) | (6,980) | (8,201) | (7,689) |
| Net debt1) | (3,708) | (4,676) | (7,028) | (4,676) | (7,028) | (5,820) |
| Non-controlling interests | (31) | (36) | (34) | (36) | (34) | (33) |
| Shareholders' equity | (11,870) | (14,438) | (13,249) | (14,438) | (13,249) | (12,028) |
| Financing | (15,609) | (19,151) | (20,311) | (19,151) | (20,311) | (17,881) |
7.1.77.8Debt position
Total debt outstanding at the end of 20222023 was EUR 8,2017,689 million, compared with EUR 6,9808,201 million at the end of 2021.2022.
Philips Group
Balance sheet changes in debt
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| New lease liabilities | 128 | 164 | 104 | 164 | 104 | (233) |
| New borrowings long-term debt | 1,065 | 76 | 2,516 | 76 | 2,516 | (544) |
| Repayments long-term debt incl. leases | (298) | (302) | (1,472) | (302) | (1,472) | 754 |
| New borrowings (repayments) short-term debt | 16 | (25) | 47 | (25) | 47 | (29) |
| Forward contracts entered (matured) | 793 | (48) | (76) | (48) | (76) | 462 |
| Currency effects, consolidation changes and other | (217) | 180 | 101 | 180 | 101 | 102 |
| Changes in debt | 1,487 | 46 | 1,221 | 46 | 1,221 | 512 |
In 2022,2023, total debt increaseddecreased by EUR 1,221512 million compared to 2021.2022. The increasedecrease mainly comes from maturing forward contracts related to the issuanceshare buyback program and long-term incentive and employee stock purchase plans, and repayments of EUR 2 billion Notes in April 2022,long-term debt including leases, partly offset by new borrowings. In 2023, Philips issued EUR 500 million of fixed rate notes under the early redemption of approximately EUR 1.2 billion Notes originally duecompany’s EMTN program that mature in 2023, 2024, 20252031 and 2026 and byused the utilizationproceeds for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in Octoberthe fourth quarter of 2022. Changes in payment obligations from forward contracts are related to the maturity in 20222023 of EUR 83481 million of share buyback forwards (as announced in July 2021) and EUR 57125 million of forwards relating to long-term incentive and employee stock purchase plans (as announced in January 2020)March 2020 and May 2021), partially offset by EUR 63138 million of forwards entered into relating to long-term incentive and employee stock purchase plans (as announced in June 2022)2023).
In April 2022, Philips announced a series of Liability Management transactions to optimize its debt maturity profile. The transactions included the issuance of three series of Notes under its EMTN program for a total of EUR 2 billion with maturities in 2027, 2029 and 2033. Part of the proceeds were used to tender certain of Philips’ outstanding US Dollar denominated bonds due 2025 and 2026 and Euro-denominated bonds due 2023, 2024 and 2025, as well as make-whole and fully redeem the Euro-denominated bonds due 2023 and 2024 that were not purchased as part of the Euro tender offer. Philips issued Commercial Paper of EUR 200 million in September 2022 and EUR 101 million in October 2022. These tranches were repaid throughout the fourth quarter of 2022. In addition, in October 2022 Philips entered into a EUR 1 billion credit facility that cancould be used for general corporate purposes. The credit facility matureswas fully repaid in October 2023 and has a 12-month extension option at Philips discretion.2023. Per year-end 2022, EUR 500 million was utilized and outstanding under the credit facility.
In 2021, total debt increased by EUR 46 million compared to 2020. The increase mainly comes from currency effects and consolidation changes, partly offset by net lease repayments and forward settlements. Repayments of long-term debt amounted to EUR 302 million. In February 2021, Philips entered into two bilateral loans amounting to a total of EUR 500 million that were repaid in September 2021. In addition, Philips issued commercial paper of EUR 300 million in May 2021 and EUR 150 million in July 2021 that was repaid in September 2021. Changes in payment obligations from forward contracts are mainly related to the forward contracts entered into of EUR 731 million relating to the EUR 1.5 billion share buyback program announced on July 26, 2021, and EUR 90 million relating to the long-term incentive and employee stock purchase plans announced on May 19,2021.19, 2021. In addition, a total amount of EUR 745 million of forward contracts matured in 2021, which completed the settlement of the EUR 1.5 billion share buyback program announced on January 29, 2019, and a total amount of EUR 123 million of forward contracts matured in 2021 relating to the long-term incentive and employee stock purchase plans announced on October 22, 2018 and January 29, 2020. These payment obligations are recorded as financial liabilities under long-term debt. Other changes, mainly resulting from currency effects, led to an increase of EUR 175 million.
In 2020,At the end of 2023, long-term debt as a proportion of the total debt increased by EUR 1,487 millionstood at 91.5% with an average remaining term (including current portion) of 6.0 years, compared to 2019. New borrowings88.6% and 6.1 years respectively at the end of long-term debt included the net proceeds of EUR 991 million from the issuance of two new bonds under the EMTN program in 2020. Repayments of long-term debt amounted to EUR 298 million, mainly due to the repayment of leases. Changes in payment obligations from forward contracts mainly related to the forward contracts entered into of EUR 745 million to complete the remainder of the EUR 1.5 billion share buyback program announced on January 29, 2019. In addition, Philips entered into forward contracts for a total amount of EUR 174 million in 2020 related to the long-term incentive and employee stock purchase plans announced on January 29, 2020, and a total amount of EUR 126 million of forward contracts matured relating to the company's long-term incentive and employee stock purchase plans announced on October 22, 2018. These payment obligations are recorded as financial liabilities under long-term debt. Other changes, mainly resulting from currency effects, led to a decrease of EUR 221 million.2022.
At the end of 2022, long-term debt as a proportion of the total debt stood at 88.6% with an average remaining term (including current portion) of 6.1 years, compared to 92.7% and 6.0 years respectively at the end of 2021.
At the end of 2021, long-term debt as a proportion of the total debt stood at 92.7% with an average remaining term (including current portion) of 6.0 years, compared to 82.3% and 6.3 years respectively at the end of 2020.
At the end of 2020, long-term debt as a proportion of the total debt stood at 82.3% with an average remaining term (including current portion) of 6.3 years, compared to 91% and 8.0 years respectively at the end of 2019.
For further information, please refer to Debt.
7.1.87.9Liquidity position
As of December 31, 2023, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility, the Philips Group had access to available liquidity of EUR 2,883 million, compared with gross debt (including short and long-term) of EUR 7,689 million.
As of December 31, 2022, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility and the EUR 500 million undrawn portion of the credit facility entered into in October 2022, the Philips Group had access to available liquidity of EUR 2,704 million, compared with gross debt (including short and long-term) of EUR 8,201 million.
As of December 31, 2021, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility, the Philips Group had access to available liquidity of EUR 3,370 million, compared with debt (including short and long-term) of EUR 6,980 million.
As of December 31, 2020, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility, the Philips Group had access to available liquidity of EUR 4,243 million, compared with gross debt (including short and long-term) of EUR 6,934 million.
Philips Group
Liquidity position
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Cash and cash equivalents | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
| Listed equity investments at fair value1) | 17 | 67 | 32 | 67 | 32 | 14 |
| Committed revolving credit facility | 1,000 | 1,000 | 1,000 | 1,000 | ||
| Credit facility | 500 | 500 | ||||
| Liquidity | 4,243 | 3,370 | 2,704 | 3,370 | 2,704 | 2,883 |
| Short-term debt | (1,229) | (506) | (931) | (506) | (931) | (654) |
| Long-term debt | (5,705) | (6,473) | (7,270) | (6,473) | (7,270) | (7,035) |
| Debt | (6,934) | (6,980) | (8,201) | (6,980) | (8,201) | (7,689) |
| Net available liquidity resources | (2,691) | (3,609) | (5,497) | (3,609) | (5,497) | (4,806) |
Philips has a EUR 1 billion committed revolving credit facility which was signed in April 2017 and refinanced in March 2022, which will expire in March 2027. In 2023, Philips extended the maturity of the facility to 2028 and has one 1-year extension option remaining. The facility can be used for general group purposes, such as a backstop of its Commercial Paper Program. In addition, Philips entered into a EUR 1 billion credit facility in October 2022 which can be used for general corporate purposes, of which EUR 500 million is undrawn by year-end 2022.
Philips' Commercial Paper Program amounts to USD 2.5 billion, under which commercial paper can be issued up to 364 days in tenor, both in the US and in Europe, in any major freely convertible currency. As of December 31, 2022,2023, Philips had no commercial paper outstanding. During the year 2020, Philips established a Euro Medium Term Note (EMTN) program which facilitates the issuance of notes for a total amount of up to EUR 10.0 billion. In 20222023, Philips issued three new tranchesEUR 500 million fixed rate notes due 2031 under the programprogram. The proceeds were used for a totalgeneral corporate purposes, including the repayment of EUR 2 billion, while also early redeeming its500 million that was outstanding 2023 and 2024 Notes and completing a tender offer onunder the outstanding 2025 and 2026 Notes.credit facility entered into in the fourth quarter of 2022.
In terms of liquidity, the company has access to various sources. The company’s liquidity risk management procedures have not changed significantly during 2022.2023. The access to existing lines of credit remains intact. These lines of credit, along with other financial risks to which Philips is exposed, are disclosed in Details of treasury and other financial risks. Further, with respect to potential claims related to the Respironics field action,recall, please refer to Contingencies. The management continues to monitor the risks associated with such potential claims and its impact on liquidity position, if any.
Philips’ existing long-term debt is rated A-BBB+ (with stable outlook) by Fitch, Baa1 (with negative outlook) by Moody’s, and BBB+ (with negative outlook) by Standard & Poor’s. As part of our capital allocation policy, our net debt*) position is managed with the intention of retaining our strong investment grade credit rating. Ratings are subject to change at any time and there is no assurance that Philips will be able to achieve this goal. Philips' aim when managing the net debt*) position is dividend stability and a pay-out ratio of 40% to 50% of adjusted income from continuing operations attributable to shareholders*). Philips’ outstanding long-term debt and credit facilities do not contain financial covenants. Adverse changes in the company’s ratings will not trigger automatic withdrawal of committed credit facilities or any acceleration in the outstanding long-term debt (provided that the USD-denominated bonds issued by Philips in March 2008 and 2012 contain a ‘Change of Control Triggering Event’ and the EUR-denominated bonds contain a ‘Change of Control Put Event’). A description of Philips’ credit facilities can be found in Debt.
Philips Group
Credit rating summary
| long-term | short-term | outlook | |
| Fitch | Stable | ||
| Moody's | Baa1 | P-2 | Negative |
| Standard & Poor's | BBB+ | A-2 | Negative |
Philips pools cash from subsidiaries to the extent legally and economically feasible. Cash not pooled remains available for local operational needs or general purposes. The company faces cross-border foreign exchange controls and/or other legal restrictions in a few countries, which could limit its ability to make these balances available on short notice for general use by the group.
Philips believes its current liquidity and direct access to capital markets is sufficient to meet its present financing needs.
7.1.107.11Cash obligations
Contractual cash obligations
The following table presents a summary of the Group’sGroup's fixed contractual cash obligations and commitments as of December 31, 2022.2023. These amounts are an estimate of future payments, which could change as a result of various factors such as a change in interest rates, foreign exchange, contractual provisions, as well as changes in our business strategy and needs. Therefore, the actual payments made in future periods may differ from those presented in the following table:
| payments due by period | payments due by period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| total | less than 1 year | 1-3 years | 3-5 years | after 5 years | total | less than 1 year | 1-3 years | 3-5 years | after 5 years | |
| Long-term debt | 8,168 | 842 | 1,760 | 1,809 | 3,757 | 7,615 | 533 | 1,934 | 1,431 | 3,717 |
| Short-term debt | 89 | 122 | ||||||||
| Interest on debt | 1,683 | 159 | 304 | 264 | 956 | 1,704 | 180 | 328 | 285 | 911 |
| Derivative liabilities | 210 | 208 | 2 | 39 | 38 | 1 | ||||
| Purchase obligations3) | 782 | 336 | 412 | 21 | 12 | 668 | 355 | 286 | 27 | |
| Trade and other payables | 1,968 | 1,917 | ||||||||
| Contractual cash obligations | 12,901 | 3,603 | 2,478 | 2,094 | 4,725 | 12,065 | 3,145 | 2,549 | 1,716 | 4,655 |
Included in debt are remaining forward contracts of EUR 648167 million related to the EUR 1.5 billion share buyback program announced in July 2021 and EUR 211224 million relating to the repurchase of shares to cover long-term incentive and employee stock purchase plans. In 2022,2023, Philips entered into a total amount of EUR 63138 million of forward contracts relating to the repurchase of up to 3.27.1 million shares to cover long-term incentive and employee stock purchase plans. In addition, in 20222023 there were maturities of a total of EUR 83481 million of forward contracts for 13.0 million shares related to the EUR 1.5 billion share buyback program announced in July 2021, as well as maturities of a total of EUR 57125 million of forward contracts to repurchase shares to cover long-term incentive and employee stock purchase plans. Philips intends to cancel all of the shares acquired under the share buyback program and has canceled 15.1 million shares acquired in 2023, as the program was initiated for capital reduction purposes.
Philips offers voluntary supply chain finance programs with third parties, which provide participating suppliers with the opportunity to factor their trade receivables at the sole discretion of both the suppliers and the third parties. Philips continues to recognize these liabilities as trade payables and settles them accordingly on the invoice maturity date based on the terms and conditions of these arrangements. As of December 31, 2022,2023, approximately EUR 151114 million (2021:(2022: EUR 139151 million) of the Philips accounts payable were transferred under these arrangements.
Other cash commitments
The company and its subsidiaries sponsor post-employment benefit plans in many countries in accordance with legal requirements, customs and the local situation in the countries involved. For a discussion of the plans and expected cash outflows, please refer to Post-employment benefits.
The company had EUR 140 million restructuring-relatedvarious provisions by the end of 2022, of2023 which EUR 134 million isare expected to result in cash outflows in 2022.2024. Refer to Provisions for details of restructuring provisions..
Philips has contracts with investment funds where it committed itself to make, under certain conditions, capital contributions to these funds of an aggregated remaining amount of EUR 127153 million (2021:(2022: EUR 116127 million). Capital contributions already made to these investment funds are recorded as non-current financial assets.
Please refer to Dividend for information on the proposed dividend distribution.
Please refer to Equity for information on other Long-term incentive and employee stock purchase plans.
Guarantees
Philips’ policy is to provide guarantees and other letters of support only in writing. Philips does not provide other forms of support. The total fair value of guarantees recognized on the balance sheet amounts to EUR nil million for both 20212023 and 2022. Remaining off-balance-sheet business-related guarantees on behalf of third parties and associates amount to EUR 2 million as of December 31, 20222023 (December 31, 2021:2022: EUR 2 million).
7.1.117.12Dividend
Dividend policy
Philips’ dividend policy is aimed at dividend stability and a pay-out ratio of 40% to 50% of adjusted income from continuing operations attributable to shareholders*).
Proposed distribution
A proposal will be submitted to the Annual General Meeting of Shareholders, to be held on May 9, 2023,7, 2024, to declare a distribution of EUR 0.85 per common share, in common shares, against retained earnings.
If the above dividend proposal is adopted, the shares will be traded ex-dividend as of May 11, 20239, 2024 at the New York Stock Exchange and Euronext Amsterdam. In compliance with the listing requirements of the New York Stock Exchange and Euronext Amsterdam, the dividend record date will be May 12, 2023.10, 2024.
The number of share dividend rights entitled to one new common share will be determined based on the volume-weighted average price of all traded common shares of Koninklijke Philips N.V. at Euronext Amsterdam on May 11, 129, 10 and 15, 2023.13, 2024. The company will calculate the number of share dividend rights entitled to one new common share (the ratio), such that the gross dividend in shares will be approximately equal to EUR 0.85. The ratio and the number of shares to be issued will be announced on May 17, 2023.15, 2024. Distribution of the dividend (up to EUR 751770 million) and delivery of new common shares, with settlement of any fractions in cash, will take place from May 18, 2023.16, 2024.
| ex-dividend date | record date | distribution from | |
| Euronext Amsterdam | May | May | May |
| New York Stock Exchange | May | May | May |
Further details will be given in the agenda with explanatory notes for the 20232024 Annual General Meeting of Shareholders. The proposed distribution and all dates mentioned remain provisional until then.
Dividend in shares distributed out of retained earnings is subject to 15% dividend withholding tax, but only in respect of the par value of the shares (EUR 0.20 per share). Shareholders are advised to consult their tax advisor on the applicable situation with respect to taxes on the dividend received.
In June 2022,May 2023, Philips settled a dividend of EUR 0.85 per common share, representing a total value of EUR 741749 million (including costs). Shareholders could elect for a cashThe dividend or a share dividend. Approximately 45%was distributed in the form of the shareholders elected for a share dividend,shares only, resulting in the issuance of 14,174,56839,334,938 new common shares, leading to a 1.6%4.5% dilution. For more information refer to Shareholders’ equity. The settlement of the cash dividend involved an amount of EUR 411 million (including costs).
Dividends and distributions per common share
The following table sets forth in euros the gross dividends on the common shares in the fiscal years indicated (from prior-year profit distribution) and such amounts as converted into US dollars and paid to holders of shares of the New York Registry:
Philips Group
Gross dividends on the common shares
| 20181) | 20191) | 20202) | 20211) | 20222) | 20191) | 20201) | 20212) | 20221) | 20232) | |
|---|---|---|---|---|---|---|---|---|---|---|
| in EUR | 0.80 | 0.85 | 0.85 | 0.85 | 0.85 | |||||
| in USD | 0.94 | 0.96 | 0.95 | 1.03 | 0.90 | 0.96 | 0.95 | 1.03 | 0.90 | 0.93 |
7.1.12Outlook
We remain cautious in light of the subdued economic outlook for the year, staffing and inflationary pressures facing our customers, geopolitical risks, supply and demand volatility, and uncertainties around ongoing consent decree negotiations, litigation and Department of Justice investigations. Nevertheless, we expect that, by prioritizing patient safety and quality, tightening our focus on innovation and strengthening our category leadership areas, while at the same time improving execution and taking a disciplined approach to capital, we will be able to progressively create value with sustainable impact.
7.2Taxation
Dutch taxation
The statements below are only a general summary of certain material Dutch tax consequences for holders of common shares that are non-residents of the Netherlands based on Dutch tax laws, presently in force, and the Tax Convention of December 18, 1992, as amended by the protocol that entered into force on December 28, 2004, between the United States of America and the Kingdom of the Netherlands (the US Tax Treaty) and are not to be read as extending by implication to matters not specifically referred to herein. As to individual tax consequences, investors in common shares should consult their own professional tax advisor.
With respect to a holder of common shares that is an individual who receives income or derives capital gains from common shares and this income received or capital gains derived are attributable to past, present or future employment activities of such holder, the income of which is taxable in the Netherlands, the Dutch tax position is not discussed in this summary.
Dividend withholding tax
In general, a distribution to shareholders by a company resident in the Netherlands (such as the company) is subject to a withholding tax imposed by the Netherlands at a rate of 15%. Share dividends paid out of the company’s paid-in share premium recognized for Dutch tax purposes are not subject to the abovementioned withholding tax. Share dividends paid out of the company’s retained earnings are subject to dividend withholding tax on the nominal value of the shares issued.
Relief at source is available to certain qualifying corporate holders of common shares if such common shares are attributable to a business carried out in the Netherlands. Relief at source is available for dividend distributions to certain qualifying corporate holders of common shares resident in EU/EEA member states, and to certain qualifying corporate holders of common shares resident in non-EU/EEA states with which the Netherlands has concluded a tax treaty that includes a dividend article, unless such holder holds the common shares of the company with the primary aim or one of the primary aims to avoid the levy of Dutch dividend withholding tax from another person and the shareholding is put in place without valid commercial reasons that reflect economic reality.
Upon request and under certain conditions, certain qualifying non-resident individual and corporate holders of common shares resident in EU/EEA member states or in a qualifying non-EU/EEA state may be eligible for a refund of Dutch dividend withholding tax to the extent that the withholding tax levied is higher than the personal and corporate income tax which would have been due if they were resident in the Netherlands. However, this refund is not applicable when, based on the US Tax Treaty, the Dutch dividend withholding tax can be fully credited in the United States by the US holder.
Pursuant to the provisions of the US Tax Treaty, a reduced rate may be applicable in respect of dividends paid by the company to a beneficial owner holding directly 10% or more of the voting power of the company, if such owner is a company resident in the United States (as defined in the US Tax Treaty) and entitled to the benefits of the US Tax Treaty.
Pursuant to Dutch anti-dividend stripping legislation, a holder of common shares who is the recipient of dividends will generally not be considered the beneficial owner of the dividends if (i) as a consequence of a combination of transactions, a person other than the recipient benefits, in full or in part, directly or indirectly, from the dividends; (ii) whereby such other person retains, directly or indirectly, an interest similar to that in the common shares on which the dividends were paid; and (iii) that other person is entitled to a credit, reduction or refund of dividend withholding tax that is less than that of the recipient.
Dividends paid to qualifying exempt US pension trusts and qualifying exempt US organizations are, under certain conditions, exempt from Dutch withholding tax under the US Tax Treaty. Qualifying exempt US pension trusts normally remain subject to withholding at the rate of 15% and are required to file for a refund of the tax withheld. Only if certain conditions are fulfilled, such pension trusts may be eligible for relief at source upon payment of the dividend. However, for qualifying exempt US organizations no relief at source upon payment of the dividend is currently available; such exempt US organizations should apply for a refund of the 15% withholding tax withheld. Further, under certain circumstances, certain exempt organizations (e.g. pension funds) may be eligible for a refund of Dutch withholding tax upon their request pursuant to Dutch tax law. Under Dutch tax law (not yet entered into force), provided certain conditions are met, such (US) organizations may be eligible for relief at source upon request.
The company may, with respect to certain dividends received from qualifying non-Dutch subsidiaries, credit taxes withheld from those dividends against the Dutch withholding tax imposed on certain qualifying dividends that are redistributed by the company, up to a maximum of the lesser of:
3% of the amount of qualifying dividends redistributed by the company; and3% of the gross amount of certain qualifying dividends received by the company.
The reduction is applied to the Dutch dividend withholding tax that the company must pay to the Dutch tax authorities and not to the Dutch dividend withholding tax that the Company must withhold.
Income and capital gains
Income and capital gains derived from the common shares by a non-resident individual or non-resident corporate shareholder are generally not subject to Dutch income or corporation tax, unless (i) such income and gains are attributable to a (deemed) permanent establishment or (deemed) permanent representative of the shareholder in the Netherlands; or (ii) the shareholder is entitled to a share in the profits of an enterprise or (in the case of a non-resident corporate shareholder only) a co-entitlement to the net worth of an enterprise that is effectively managed in the Netherlands (other than by way of securities) and to which enterprise the common shares are attributable; or (iii) such income and capital gains are derived from a direct, indirect or deemed substantial participation in the share capital of the company (such substantial participation not being a business asset), and, in the case of a non-resident corporate shareholder only, it is being held with the primary aim or one of the primary aims to avoid the levy of income tax from another person and is put in place without valid commercial reasons that reflect economic reality; or (iv) in the case of a non-resident corporate shareholder, such shareholder is a resident of Aruba, Curacao or Saint Martin with a permanent establishment or permanent representative in Bonaire, Eustatius or Saba to which the common shares are attributable and certain conditions are met; or (v) in the case of a non-resident individual, such individual derives income or capital gains from the common shares that are taxable as benefits from ‘miscellaneous activities’ in the Netherlands (resultaat uit overige werkzaamheden, as defined in the Dutch Income Tax Act 2001), which includes the performance of activities with respect to the common shares that exceed regular portfolio management.
In general, a holder of common shares has a substantial participation if he holds either directly or indirectly and either independently or jointly with his partner (as defined in the Dutch Income Tax Act 2001), the ownership of, or certain other rights over, at least 5% of the total issued share capital or total issued particular class of shares of the company or rights to acquire direct or indirect shares, whether or not already issued, that represent at any time 5% or more of the total issued capital (or the total issued particular class of shares) or the ownership of certain profit participating certificates that relate to 5% or more of the annual profit or to 5% or more of the liquidation proceeds. A shareholder will also have a substantial participation in the company if one or more of certain relatives of the shareholder hold a substantial participation in the company. A deemed substantial participation amongst others exists if (part of) a substantial participation has been disposed of, or is deemed to have been disposed of, on a nonrecognition basis.
Estate and gift taxes
No estate, inheritance or gift taxes are imposed by the Netherlands on the transfer or deemed transfer of common shares by way of gift by or on the death of a shareholder if, at the time of the death of the shareholder or the gift of the common shares (as the case may be), such shareholder is not a (deemed) resident of the Netherlands.
Inheritance or gift taxes (as the case may be) are due, however, if such shareholder:
has Dutch nationality and has been a resident of the Netherlands at any time during the ten years preceding the time of their death or gift; ordoes not have Dutch nationality but has been a resident of the Netherlands at any time during the twelve months preceding the time of the gift (for Netherlands gift taxes only).
United States Federal Taxation
This section describes the material United States federal income tax consequences to a US holder (as defined below) of owning common shares. It applies only if the common shares are held as capital assets for United States federal income tax purposes. This discussion addresses only United States federal income taxation and does not discuss all of the tax consequences that may be relevant to a US holder in light of its individual circumstances, including foreign, state or local tax consequences, estate and gift tax consequences, and tax consequences arising under the Medicare contribution tax on net investment income or the alternative minimum tax. This section does not apply to a member of a special class of holders subject to special rules, including:
a dealer in securities,a trader in securities that elects to use a mark-to-market method of accounting for securities holdings,a tax-exempt organization,a life insurance company,a person that actually or constructively owns 10% or more of the combined voting power of our voting stock or of the total value of our stock,a person that holds common shares as part of a straddle or a hedging or conversion transaction,a person that purchases or sells common shares as part of a wash sale for tax purposes, ora person whose functional currency is not the US dollar.
This section is based on the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations, published rulings and court decisions, all as currently in effect, as well as on the US Tax Treaty. These authorities are subject to change, possibly on a retroactive basis.
If an entity or arrangement that is treated as a partnership for United States federal income tax purposes holds the common shares, the United States federal income tax treatment of a partner will generally depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding the common shares should consult its tax advisor with regard to the United States federal income tax treatment of an investment in the common shares.
A US holder is defined as a beneficial owner of common shares that is, for United States federal income tax purposes::
a citizen or resident of the United States,a domestic corporation,an estate whose income is subject to United States federal income tax regardless of its source, ora trust if a United States court can exercise primary supervision over the trust’s administration and one or more United States persons are authorized to control all substantial decisions of the trust.
A US holder should consult its own tax advisor regarding the United States federal, state and local tax consequences of owning and disposing of common shares in its particular circumstances.
The tax treatment of common shares will depend in part on whether or not we are classified as a passive foreign investment company, or PFIC, for United States federal income tax purposes. Except as discussed below under “—PFIC Rules”, this discussion assumes that we are not classified as a PFIC for United States federal income tax purposes.
Taxation of Distributions
Under the United States federal income tax laws, the gross amount of any distribution paid in stock or cash out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes), other than certain pro-rata distributions of our common shares, will be treated as a dividend that is subject to United States federal income taxation. For a non-corporate US holder, dividends paid that constitute qualified dividend income will be taxable at the preferential rates applicable to long-term capital gains, provided that the non-corporate US holder holds the common shares for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and provided it meets other holding period requirements. Dividends paid with respect to the common shares generally will be qualified dividend income provided that, in the year in which the dividend is received, the common shares are readily tradable on an established securities market in the United States. Our common shares are listed on the New York Stock Exchange and we therefore expect that dividends will be qualified dividend income. A US holder must include any Dutch tax withheld from the dividend payment in this gross amount even though it does not in fact receive it. The dividend is taxable to a US holder when it receives the dividend, actually or constructively. The dividend will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations. For dividend payments made in euro, the amount of the dividend distribution that a US holder must include in its income will be the US dollar value of the euro payments made, determined at the spot euro/US dollar rate on the date the dividend is distributed, regardless of whether the payment is in fact converted into US dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend is distributed to the date a US holder converts the payment into US dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. Distributions in excess of current and accumulated earnings and profits, as determined for United States federal income tax purposes, will be treated as a non-taxable return of capital to the extent of a US holder’s basis in the common shares and thereafter as capital gain. However, we do not expect to calculate earnings and profits in accordance with United States federal income tax principles. Accordingly, US holders should expect to generally treat distributions we make as dividends.
Subject to certain limitations (including, but not limited to, those described in this paragraph), the Dutch tax withheld in accordance with the US Tax Treaty and paid over to the Netherlands will be creditable or deductible against a US holder’s United States federal income tax liability. However, under recently finalized Treasury regulations, it is possible that the Dutch withholding tax may not be creditable unless a US holder is eligible for and elect to apply the benefits of the US Tax Treaty. Even in such case, the Dutch withholding tax may not be creditable or deductible to the extent that we reduce (as described above under “Dutch taxation - Dividend withholding tax”) the amount of withholding tax paid over to the Netherlands by crediting taxes withheld from certain dividends received by us. In addition, special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the preferential tax rates. To the extent reduction or refund of the tax withheld is available under Dutch law, or under the US Tax Treaty, the amount of tax withheld that could have been reduced or that is refundable will not be eligible for credit against United States federal income tax liability. Dividends will generally be income from sources outside the United States, and will generally be “passive” income for purposes of computing the foreign tax credit allowable to the holder. In addition, to the extent an amount of Dutch tax withheld is contingent on the availability of a credit against the amount of income tax owed to another country, that amount of Dutch tax withheld will not be eligible for a credit against the US holder’s United States federal income tax liability.
Taxation of Capital Gains
A US holder that sells or otherwise disposes of its common shares will recognize capital gain or loss for United States federal income tax purposes equal to the difference between the US dollar value of the amount that it realizes and its tax basis, determined in US dollars, in its common shares. Capital gain of a non-corporate US holder is generally taxed at preferential rates where the property is held more than one year. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Passive Foreign Investment Company Rules
We believe that the common shares should currently not be treated as stock of a PFIC for United States federal income tax purposes, and we do not expect to become a PFIC in the foreseeable future. However, this conclusion is a factual determination that is made annually and thus may be subject to change. It is therefore possible that we could become a PFIC in a future taxable year. If we are treated as a PFIC, gain realized on the sale or other disposition of the common shares would in general not be treated as capital gain. Instead, unless a US holder elects to be taxed annually on a mark-to-market basis with respect to the common shares, a US holder would generally be treated as if it had realized such gain and certain “excess distributions” ratably over the holding period for the common shares and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, in addition to which an interest charge in respect of the tax attributable to each such year would apply. Any dividends received by a US holder will not be eligible for the special tax rates applicable to qualified dividend income if we are treated as a PFIC with respect to such US holder either in the taxable year of the distribution or the preceding taxable year, but instead will be taxable at rates applicable to ordinary income and subject to the excess distribution regime described above.
8Environmental, Social and Governance
Environmental, Social & Governance (ESG) are three key dimensions within which a company’s approach to doing business responsibly and sustainably, and its overall societal impact, are defined. They give expression to an increasingly widely held view – that companies that hold themselves accountable to their stakeholders and increase transparency will be more viable, and valuable, in the long term.
Philips is a purpose-driven company aiming to improve the health and well-being of 2.5 billion people annually by 2030. We believe that private-sector companies like ours have a vital role to play in collaborating with other partners across our supply chain, and with private and public organizations in society, to address the major challenges the world is facing.
Taking a multi-stakeholder approach, we draw inspiration from the societal impact we can have through our products and solutions, and through how we operate in the world. Our company is very conscious of our responsibility and our contribution to society and the environment. We are also witnessing growing interest in ESG on the part of our customers, who are increasingly turning to technology companies for support in addressing their sustainability objectives and are including ESG-related considerations in their procurement policies and criteria.
We aim to be a front-runner in the area of ESG and have been recognized as leading the way in, for example, sustainability, corporate governance practices and tax transparency.
Our reporting is aligned with the comprehensive and integrated Environmental, Social & Governance (ESG) commitments we have adopted for the period 2020-2025.
We have excluded the data from Domestic Appliances from the ESG information wherever possible. In a limited number of cases, for example for road logistics emissions, we have used proxies. If Domestic Appliances information was not available for past years, and could therefore not be excluded, we have indicated this in the respective section. The Employee Engagement Index (EEI) and General Business Principles (GBP) results have not been restated.
8.1ESG reporting framework
Building on our extensive experience of environmental and social impact measurement and of providing transparency on governance, Philips has taken an active role – in collaboration with, in particular, the International Financial Reporting Standards (IFRS) Foundation, the World Economic Forum (WEF) and the European Union – to help drive the evolution towards a standard ESG reporting framework.
In 2007, Philips signed up to the United Nations Global Compact, to advance ten universal principles in the areas of human rights, labor, the environment and anti-corruption. In 2017, at the WEF Annual Meeting in Davos, we signed the Compact for Responsive and Responsible Leadership – an initiative (initiated by WEF and Philips) to promote and align the long-term sustainability of corporations and the long-term goals of society, with an inclusive approach for all stakeholders. The WEF secured a commitment from over 140 CEOs to align their corporate values and strategies with the United Nations’ Sustainable Development Goals (SDGs).
In 2020, the WEF’s International Business Council (IBC) published its core set of Stakeholder Capitalism Metrics and disclosures. These can be used by companies to align their mainstream reporting on performance against environmental, social and governance (ESG) indicators and track their contributions towards the SDGs on a consistent basis. Thus far, 135 companies reported in line with this framework. Based where possible on existing standards, the full set is comprised as follows:
Core metrics: A set of 21 more‑established or critically important metrics and disclosures that focus primarily on activities within an organization’s own boundaries.
Expanded metrics: A set of 34 metrics and disclosures that tend to be less well‑established in existing practice and have a wider value chain scope or convey impact in a more sophisticated or tangible way, e.g. in monetary terms.
The recommended metrics are organized under four pillars that are aligned with the SDGs and principal ESG domains: Principles of Governance, Planet, People and Prosperity. There is no intention to replace industry- or company-specific metrics (like our Lives Improved metric). Companies are encouraged to report against as many of the core and expanded metrics as they find material and appropriate, on the basis of ‘disclose or explain’.
In section 5.6 of this Annual Report, we show how Philips performed in 2022 on the above-mentioned 21 Core metrics, mapped to the three dimensions of our ESG commitments, as well as a number of additional Philips-specific metrics that we consider fundamental to the strategy and operation of our business.
Philips is also contributing to the IFRS Foundation’s endeavors to drive standardization of non-financial reporting as well as the development of sustainability standards by the European Union by EFRAG.
EU taxonomy framework
The aim of the European Taxonomy Regulation (EU 2020/852), including the delegated acts adopted thereunder, is to provide companies, investors and policymakers with appropriate criteria for determining which economic activities can be considered environmentally sustainable, and it requires companies to report on how and to what extent their activities are associated with such ‘taxonomy-eligible activities’. The Taxonomy Regulation is relatively new and there are after the first year of reporting (2021) still significant uncertainties around its phased implementation. It is expected, however, that the EU Taxonomy will develop into a comprehensive and detailed framework over the coming years.
The Taxonomy Regulation provides certain conditions for taxonomy alignment. Among others, the relevant activity must substantially contribute to one or more of the following six environmental objectives (while not significantly harming any of the others):
Climate change mitigationClimate change adaptationThe sustainable use and protection of water and marine resourcesThe transition to a circular economyPollution prevention and controlThe protection and restoration of biodiversity and ecosystems
The delegated acts adopted under the Taxonomy Regulation will provide technical screening criteria which must also be met to constitute taxonomy alignment. On the date of this Annual Report 2022, only one relevant delegated act has been adopted, concerning activities significantly contributing to climate change mitigation and adaptation.
The taxonomy framework provisions effective on the date of this Annual Report 2022 require Philips to disclose the proportion of its taxonomy-eligible activities (described in any delegated act adopted to date) and non-eligible economic activities in its total turnover, capital and operational expenditure, as well as certain qualitative information. We used the delegated act ((EU) 2021/2139) to identify activities that are eligible. However, none of our revenue-generating activities were included as this delegated act only applies to sectors with very high CO2 emissions. As a result, Philips’ core activities are not within the scope of this delegated act and consequently none of Philips' revenues were eligible under this delegated act during 2022 (0%). All revenues were non-eligible (100%). We used delegated act (EU) 2021/2178 for the definition and calculation of the taxonomy-eligible percentages. Revenue is calculated based on ’Sales’ as per Consolidated statements of income. Philips expects to be eligible and report its taxonomy-eligible revenues under additional environmental objectives as further delegated acts with applicable technical screening criteria are adopted.
Philips Group
Proportion of turnover from products or services associated with Taxonomy aligned economic activities 2022
in millions of EUR unless otherwise stated
| Economic activities | Absolute Turnover | Proportion of turnover |
|---|---|---|
| A. ELIGIBLE ACTIVITIES | ||
| Turnover of eligible Taxonomy-aligned activities (A.1) | 0 | 0% |
| Turnover of eligible not Taxonomy-aligned activities (A.2) | 0 | 0% |
| Total (A.1 + A.2) | 0 | 0% |
| B. Taxonomy-non-eligible activities | ||
| Turnover of Taxonomy-non-eligible activities (B) | 17,827 | 100% |
| Total (A + B) | 17,827 | 100% |
Some other (enabling) Philips activities are included in the delegated act ((EU) 2021/2139) and are eligible for capital expenditures for the objective of climate change mitigation and climate change adaptation. We therefore screened (EU) 2021/2139, assessed our capital expenditure and identified relevant activities mainly related to our real estate portfolio. For these activities, capital expenditures are determined based on the 2022 additions to property, plant and equipment, intangible assets, and additions to right-of-use assets, excluding any re-assessments (refer to Property, plant and equipment and Intangible assets excluding goodwill).
Reportable taxonomy-eligible capital expenditures in 2022 amounted to EUR 8 million, or 1% of total capital expenditure (non-eligible capital expenditures 99%), and mainly related to energy efficiency improvement measures in our buildings (installation, maintenance and repair of energy efficiency equipment), such as energy efficient heating, ventilation, and air conditioning (HVAC) in various locations around the world. Next, we invested in onsite renewable electricity generation (installation, maintenance and repair of renewable energy technologies) by installing PV panels in one of our factories in Asia.
We assessed compliance with the criteria set out in Article 3 of Regulation (EU) 2020/852 and the associated technical screening criteria on a project basis.
Philips Group
Proportion of CapEx from products or services associated with Taxonomy aligned economic activities 2022
in EUR unless otherwise stated
| Substantial contribution criteria | DNSH criteria ('Do No Significant Harm') | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Economic activities | Absolute CapEx | Proportion of CapEx | Climate change mitigation | Climate change adaption | Water and marine resources | Circular economy | Pollution | Biodiversity and ecosystems | Climate change mitigation | Climate change adaption | Water and marine resources | Circular economy | Pollution | Biodiversity and ecosystems | Minimum safeguards | Taxonomy-aligned proportion of CapEx 2022 | Taxonomy-aligned proportion of CapEx 2021 | Category (enabling activity or transitional activity) | |
| % | % | % | % | % | % | % | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | % | % | E/T | |||
| A. ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1 Eligible Taxonomy-aligned activities | |||||||||||||||||||
| 4.16 Installation and operation of electric heat pumps | 234,000 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 0 | NA | E | ||
| 7.2 Renovation of existing buildings | 121,000 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 0 | NA | T | ||
| 7.3 Installation, maintenance and repair of energy efficient equipment | 7,720,000 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 1 | NA | E | ||
| 7.4 Installation, maintenance and repair of charging stations for electric vehicles | 61,000 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 0 | NA | E | ||
| 7.6 Installation, maintenance and repair of renewable energy technologies | 240,000 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 0 | NA | E | ||
| CapEx of eligible Taxonomy-aligned activities (A.1) | 8,376,000 | ||||||||||||||||||
| A.2. Eligible not Taxonomy aligned activities | |||||||||||||||||||
| No eligible not Taxonomy aligned activiites identified | |||||||||||||||||||
| CapEx of eligible not Taxonomy-aligned activities (A.2) | 0 | ||||||||||||||||||
| Total (A.1 + A.2) | 8,376,000 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | Y | Y | Y | Y | Y | Y | 100 | NA | E | ||
| B. Taxonomy-non-eligible activities | |||||||||||||||||||
| CapEx of Taxonomy-non-eligible activities (B) | 591,600,000 | 99 | |||||||||||||||||
| Total (A+B) | 600,000,000 | 100 | |||||||||||||||||
Similar to capital expenditures, we screened (EU) 2021/2139, assessed for relevant operational expenditures activities and have not identified any eligible operational expenditure. Total operational expenditures are determined based on the 2022 non-capitalized costs that relate to research and development, building renovation, short-term lease, maintenance and repair, and any other direct expenditures relating to day-to day servicing of property, plant and equipment.
In 2022, we did not record reportable taxonomy-eligible operational expenditures (0%), as, for example, the sourcing of renewable energy was not included in the Taxonomy. Non-eligible operational expenditures were 100%.
Philips Group
Proportion of OpEx from products or services associated with Taxonomy aligned economic activities 2022
in millions of EUR unless otherwise stated
| Economic activities | Absolute OpEx | Proportion of OpEx |
|---|---|---|
| A. ELIGIBLE ACTIVITIES | ||
| OpEx of eligible Taxonomy-aligned activities (A.1) | 0 | 0% |
| OpEx of eligible not Taxonomy-aligned activities (A.2) | 0 | 0% |
| Total (A.1 + A.2) | 0 | 0% |
| B. Taxonomy-non-eligible activities | ||
| OpEx of Taxonomy-non-eligible activities (B) | 2,276 | 100% |
| Total (A + B) | 2,276 | 100% |
We followed the same accounting principles as in our financial statements.
We will continue to monitor legislative developments and adapt our disclosures where needed.
8.2Philips' ESG commitments
In September 2020, Philips reinforced its commitments as a purpose-driven company with the announcement of an enhanced and fully integrated approach to doing business responsibly and sustainably. Philips’ framework comprises a comprehensive set of key commitments across all the Environmental, Social and Governance (ESG) dimensions that guide execution of the company’s strategy. It includes ambitious targets and detailed plans of action.
As a leading health technology company today, our purpose is to improve people’s health and well-being through meaningful innovation, positively impacting 22.5 billion lives per year by 2025.2030. We aim to grow Philips responsibly and sustainably, and we therefore continuously set ourselves challenging environmental and social targets, and highest standards of governance. Acting responsibly towards the planet and society is part of our DNA. We believe that this is the best way for us to create superior, long-term value for Philips’ multiple stakeholders.
Our key ESG commitments
Environmental
We act responsibly towards our planet in line with UN SDGs 12 and 13.
- We will maintain carbon neutrality and use 75% renewable energy in our operations by 2025.
While maintaining carbon neutrality in our operations, weWe will reduceCO2CO₂ emissions in our entire value chain in line with a 1.5 °C global warming scenario (based on Science Based Targets).We will actively partner with our suppliers and our customers to achieve this.- We will generate 25% of our revenue from
circularproducts, services and solutions contributing to circularity, and offera trade-inresponsible take-back on all professional medical equipmentso that we can take care of responsible repurposingby 2025. - We will embed circular practices at our sites and put zero waste to landfill by 2025.
AllWe will design all new product introductionswill fulfillin line with our EcoDesign requirements by 2025, with ‘EcoHeroes’ accounting for 25% of hardware revenues.- We work with our suppliers to reduce the environmental footprint of our supply chain in line with a 1.5 °C global warming scenario (based on Science Based Targets).
- We engage with our stakeholders and other companies to drive sustainability efforts addressing the United Nations Sustainable Development Goals.
Social
Our purpose is to improve people’s health and wellbeingwell-being through meaningful innovation, in line with UN SDG 3. We act responsibly towards society and partner with our stakeholders
- We aim to improve the health and well-being of 2 billion people per year by 2025, including 300 million people in underserved communities.
- It is our strategy to lead with innovative solutions along the health continuum – helping our customers deliver
on the Quadruple Aim (betterbetter health outcomes, a better experience for patients and staff, lower cost ofcare)care, and helping people take better care of their health. - We aim to be the best place to work for our employees, providing opportunities for learning and development, embracing diversity and inclusion, and assuring a safe and healthy work environment. We pay at least a living wage and aim for employee engagement above the high-performance norm.
- Through our supplier development program we will improve the lives of 1,000,000 workers in our supply chain by 2025.
- We actively engage with and support the communities in which we operate, e.g. through volunteering, internships, STEM (Science, Technology, Engineering, Mathematics) initiatives.
- We contribute to the Philips Foundation, an independent foundation (stichting) organized under Dutch law, which aims to provide access to quality healthcare for disadvantaged communities.
- We consider our tax payments as a contribution to the communities in which we operate, as part of our social value creation.
Governance
We aim to deliver superior long-term value for our customers and shareholders, and we live up to the highest standards of ethics and governance in our culture and practices
- Our management structure and governance combines responsible leadership and independent supervision.
The Philips Business System is ourOur integrated operatingmodel. Itmodel defines how we work together to delight our customers and achieve our company goals, leveraging our global scale and capabilities.- We are committed to delivering the highest-quality products, services and solutions compliant with all applicable laws and standards.
- Our remuneration policy is designed to encourage employees to deliver on our purpose and strategy and create stakeholder value, and to motivate and retain them. Our executive long-term incentive plan includes environmental and social commitments.
- We ensure ethical behavior through our General Business Principles, with a strong compliance and reporting framework.
- Our risk management is designed to provide reasonable assurance that strategic and operational objectives are met, legal requirements complied with, and the integrity of the company’s reporting and related disclosures safeguarded.
- We are transparent about our plans, activities, results and contributions to society (e.g. Country activity and Tax report), and engage with shareholders, customers, business partners, governments and regulators through a variety of platforms.
8.38.2Environmental performance
In September 2020, we launched our ESG commitments, with ambitious targets to be achieved by the end of 2025. Besides our social impact, focusing on SDG 3, described in the Social performance section, weWe have an environmental impact through our global operations (including our supply chain), but even more so through our products and solutions. This is where we contribute to SDG 12 (Ensure sustainable consumption and production patterns) and SDG 13 (Take urgent action to combat climate change and its impacts).
EnvironmentalMeasuring our environmental impact
Philips has been performing Life-Cycle Assessments (LCAs) since 1990. LCAs provide insight into the lifetime environmental impact of our products. They are used to steer our EcoDesign efforts by reducing the environmental impact during the lifetime of our products and to grow our Green/EcoDesigned/EcoHero and Circular Solutions portfolio. As a next step, for the sixthseventh year, we have measured our environmental impact on society at large via a so-called Environmental Profit & Loss (EP&L) account, which includes the hidden environmental costs associated with our activities and products. It provides insights into the main environmental hotspots and innovation areas to reduce the environmental impact of our products and solutions.
The EP&L account is based on LCA methodology, in which the environmental impacts are expressed in monetary terms using conversion factors developed by CE Delft. These conversion factors are subject to further refinement and are expected to change over time. We used expert opinions and estimates for some parts of the calculations. The figures reported are Philips’ best possible estimates. As we gain new insights and retrieve more and better data, we will enhance the methodology, use-cases and accuracy of results in the future. For more information and details we refer to our methodology document.
The definition of the use-case scenarios has a significant impact on the result, especially for consumer products, which have large sales volumes, long lifetimes and frequently high energy consumption.
The current EP&L account only includes the hidden environmental costs. It does not yet include the benefits to society that Philips generates by improving people’s health and well-being through our products and solutions. We have a well-established methodology to calculate the number of lives we positively touch with our products and solutions. We aim to look into valuing these societal benefits in monetary terms in the future.
The Philips products subject to the Respironics recall were evaluated as part of the 20222023 EP&L calculation. In accordance with the EP&L methodology, products replaced during the recall by new products with lifetime guarantees were included in the 20222023 EP&L calculation for all life cycle stages. Refurbished products and repair kits were not included.
Results 20222023
InCompared to the adjusted 2022 Philips'EP&L impact of EUR 4.38 billion, Philips reduced its environmental impact amountedin 2023 to EUR 1.63 billion, compared4.21 billion. This is mainly due to EUR 2.16 billiondifferences in 2021. This reductionsales mix (including the impact of the Respironics recall).
The increase in the 2022 baseline was mainly driven by updated energy use cases for hair dryers (causing a reduction of around EUR 450 million) and a changed product mix (causing a EUR 250 million reduction), but was mitigatedmainly driven by the update to the EcoInvent 3.83.9.1 database using ReCiPe 2016 (our Life Cycle Inventory database containing environmental impacts of products and services, causing around EUR 75 million increase)services) from the EcoInvent 3.8 database using ReCiPe 2008, and further granulation of the data, includingupdate to the application of country2023 CE Delft prices for EU27 from the 2017 CE Delft prices for Dutch territory only. Philips updates the EcoInvent database used on a yearly basis to utilize recent emission factors (causing aroundand in this case, to utilize the current ReCiPe 2016 methodology. Additionally, the CE Delft prices for EU27 were more representative of a global manufacturing company, like Philips.
To understand the changes to the 2022 EP&L and have a comparable baseline for the 2023 reporting, please refer to the following chart:

The majority of this increase can be attributed to the increase in the emission factors and/or prices for the following environmental impact categories on the lifecycle stages included in the 2022 EP&L:
- Climate change
- Human toxicity, mainly linked to electricity generation
- Particulate matter formation
Additionally, the environmental impact categories associated with biodiversity and ecosystem services were included, which amounted to approximately EUR 10061 million. Therefore, the total increase attributed to methodology changes to the 2022 EP&L with the existing 2022 lifecycle stages is EUR 2.48 billion. Additionally, to compare the 2022 EP&L with the 2023 EP&L, the Raw Material Processing and Raw Material Waste lifecycle stages should be included (adding some EUR 302 million increase)to the 2022 EP&L). With the inclusion of data quality improvements and corrections performed in 2023, the 2022 EP&L would be approximately EUR 4.38 billion.
The most significant environmental impact, 63%51% of the total, is related to the usage of our products, which is due to electricity consumption. Human toxicity, particulate matter formation, and climate change are other important impacts. The environmental costs include the environmental impact of the full lifetime of the products that we put on the market in 2022,2023, e.g. 10 years in the case of a MRI or 5 years of usage in the case of a Sonicare toothbrush. Products identified as rentals are the only exception, with an energy consumption of one year. As we expand our EcoDesign activities, with a target to have all our products EcoDesigned by 2025, we expect anto better report on its environmental impact in the years to come.
Of the total 20222023 impact, just EUR 128261 million (7%(6%) is directly caused by Philips’ own operations, mainly driven by outbound logistics, followed by business travel. Compared to EUR 106128 million in 2021,2022, this is almost a 21%two times increase, mainly due to more granular data on our operations and updating the emission factors from EcoInvent 3.43.8 to EcoInvent 3.8,3.9.1 and the prices to the 2023 CE Delft prices for EU27, mitigating the downward trend in logistics emissions as presented in Sustainable Operations.
Our materials and components supply chain, including raw materials supply, raw materials processing, raw materials waste, and packaging currently has an environmental impact of some EUR 421 million,1.80 billion, which is 26%43% of our total environmental impact. The main contributors are the electronic components (including printed circuit boards), cables and metals used in our products. Through our Circular Economy and Supplier Sustainability programs we will continue to focus on reducing the environmental impact caused by the materials we source and apply in our products. We will also include the impact on biodiversity and ecosystem services in the future.

In order to deliver on our carbon neutrality commitment, we have set ambitious reduction targets. In 2018, we were the first health technology company to have its 2020-2040 targets (including purchased goods and services and the use-phaseuse of oursold products) approved by the Science Based Targets initiative – a collaboration between CDP (formerly Carbon Disclosure Project),showing our commitment to drive climate action across the United Nations Global Compact (UNGC),value chain, from suppliers to customers, and ensuring that we contribute to the World Resources Institute (WRI) and the World Wide Fund for Nature (WWF) aimed at driving ambitious corporate climate action. Approval confirms that Philips’ long-term targets are in line with the level of decarbonization required to keep the global temperature increase below 2 °C. As a next step in our journey to reduce our environmental impact, and part of our ESG commitments launched in September 2020, we have committed to reduce our full value chain emissionsstay in line with a 1.5 °C global warming scenario.scenario, as agreed in the Paris Agreement. Together with the insights gained through the EP&L we will optimize our climate impact by providing our businesses with actionable insights. For more information on our climate performance please refer to Climate Action.
For more information on our efforts to reduce emissions in the supply chain, please refer to Supplier indicatorssustainability.
For more information on our efforts to reduce emissions in the customer use-phase, please refer to Green/EcoDesigned Innovation and Green/EcoDesigned and EcoHero Revenues.
8.3.18.2.1Green/EcoDesigned InnovationClimate Action
Carbon footprint and energy efficiency
At Philips, we see climate change as a serious threat. Research from the Potsdam Institute for Climate Impact researchResearch shows that over 4% of global CO2 emissions are caused by the Healthcarehealthcare sector. Therefore, we are taking action to rethink our business models and decouple economic growth from the impact we have on the environment. We believe large corporates should lead the transition to a low-carbon economy. This will not only benefit the environment, but will also positively impact social and economic aspects.
Operational carbon footprint
During the COP21 United Nations Climate Conference in Paris in 2015, we committed to become carbon-neutral in our operations, pursue all efforts to reduce our operational emissions, source all our electricity from 100% renewable sources, and offset all unavoidable emissions by year-end 2020. We delivered on a comprehensive program that included energy-efficiency improvements, on-site renewables, and Power Purchase Agreements, as well as business travel improvements and transport mode shifts to low-carbon-emitting alternatives. As a result, we have significantly reduced our operational carbon footprint.
Since 2020, Philips has been carbon-neutral in its operations (scope 1, scope 2, and scope 3 - business travel and transportation & distribution). Although we prioritize carbon reduction, our comprehensive carbon offsetting program is still necessary to ensure carbon neutrality in our own operations.
Philips Group
Net operational carbon footprint
in kilotonnes CO2 -equivalent

Driving emission reductions across the value chain
Having achieved our 2020 carbon neutrality target, we have raised the bar and set ambitious emission reduction targets to ensure we help limit the impact of global warming throughout our value chain – collaborating with suppliers and customers to amplify our impact. Philips is committed to addressing climate change by establishing ambitious long-term emission reduction targets, officially approved by the Science Based Targets initiative (SBTi). We have added the emissions from our (scope 3 categories) Purchased Goods & Services and Use of Sold Products retrospectively to define a baseline (2020). With these additions, we cover approximately 96% of our value chain emissions.
For all of our SBTi-approved and 1.5 °C-aligned targets, baselines and performance, please refer to the following table. These targets follow the cross-sector guidance of the SBTi. Philips was the first health technology company to have its targets approved by the SBTi.
Philips Group
Science Based Targets
reduction % compared to baseline
| Scope coverage | 2025 | 2030 | 2040 | ||
|---|---|---|---|---|---|
| Absolute Contraction Approach (ACA) emission reduction targets | Scope 1 & 2 (Baseline 2015) | 100% | -75% | -90% | |
| Scope 3 (Baseline 2020) | 96% | -42% |
In establishing our Greenhouse Gas (GHG) emissions baseline, the selection of the base year is guided by several considerations. More precisely, it is driven by historical data availability, the stability of operations during that period, and the desire to capture a representative snapshot of our emissions profile. In particular, we consider factors such as significant changes in business operations, facility expansions, or the implementation of emission reduction initiatives.
Despite the unprecedented challenges brought about by the COVID-19 pandemic, the year 2020 stands out as a significant year for Philips, marked by a level of relative stability in both customer base and emissions profile. In contrast, the year 2015 was selected as the baseline for scope 1 and 2 emissions due to it being the earliest feasible date for measurement and target-setting in alignment with the Paris Agreement. Should enhancements in data quality or methodological changes lead to an emission deviation exceeding 5% compared to our current baseline emissions, we are committed to restating the baseline in accordance with the Science Based Targets initiative.
How we will drive emission reduction across the value chain
By joining forces with customers and suppliers, we can reduce our shared carbon footprint and create a sustainable and more resilient healthcare industry. To deliver, we will focus on the following four objectives, in order of magnitude:
Designing energy-efficient products and collaborating with our customers to reduce emissions during the use-phase
More and more, customers – both in healthcare and retail – are seeking solutions that are less impactful on the environment. To address that demand, we are continuously reducing the climate impact of our products by increasing the energy efficiency of our existing installed base and future product introductions. We see improving energy efficiency as a huge lever to deliver on our value chain emission reductions. More information can be found on our sustainability website.
Collaborating with our suppliers to reduce emissions in our supply chain
There is a pressing need for industry and business to manage and reduce CO2-e emissions across the entire value chain – including at supplier level. To this end, we have invited many of our largest suppliers – first-tier manufacturing and transportation-related suppliers – to report their climate performance and strategy as part of the Carbon Disclosure Project (CDP) Supply Chain program. Additionally, we engage with these suppliers to reduce their emissions as part of our Supplier Sustainability program. More information can be found on our sustainability website.
Minimizing our climate impact by adopting circular economy principles
From a climate perspective, applying circular business models can lead to a significant emission reduction. As the value of materials is retained, the need for virgin resources is significantly reduced, and consequently, the need for e.g. energy to produce those virgin materials, leading to reduced emissions. This is also part of our Circular Economy program. More information can be found on our sustainability website.
Transitioning to lower carbon-emitting energy at our sites
By continuing to phase out fossil fuels at our sites and increase our global renewable energy share, we will be able to achieve our long-term emission targets (scope 1 and 2). This entails, for example, moving towards geothermal and renewable district heating and cooling solutions where available. More information can be found on our sustainability website.
Recognition
Our efforts are acknowledged by CDP (formerly known as the Carbon Disclosure Project), a global NGO that assesses the greenhouse gas (GHG) emission performance and management of reporting companies. In 2023, we were ranked on the CDP Climate Change ’A’ List for our continued climate performance and transparency for the 11th consecutive year. None of our peers can claim the same.
Actions related to the achievement of our targets are governed by our Environmental policy, which incorporates input from Philips' regulatory, design, sustainability, supply chain, and operations stakeholders, as well as the voice of our customers to minimize their environmental footprint.
Philips reports all its emissions in line with the Greenhouse Gas Protocol (GHGP).
8.2.2Circular Economy
A circular economy aims to decouple economic growth from the consumption of natural resources and ecosystems by optimizing their use, eliminating waste and pollution, and circulating products and materials for as long as possible, while giving natural systems the opportunity to regenerate themselves. The way we take, make and use materials has a significant impact on both climate and nature, as 45% of global GHG emissions come from the way products are made and used, and more than 90% of biodiversity loss stems from extraction and processing. Bringing this back to Philips’ impact on the planet, our use of materials accounts for over 40% of our environmental impact based on our EP&L methodology, which includes raw material supply, processing, waste and packaging. Therefore, in addition to the use of renewable resources and energy efficiency, the transition to a circular economy will be essential to meet our global climate goals.
Our Circular Economy program
The Circular Economy program at Philips ran for the 11th year in 2023, building on more than 30 years' experience of applying resource efficiency through our sustainability programs. Our ambition is to help our customers to ‘do more with less’ and drive the circular transformation across the value chain together with our partners. We apply Philips’ circularity principles ‘use less, use longer and use again’ across five strategic areas:
- Circular design of software and hardware
- Circular manufacturing and supply
- Circular delivery and financing models
- Circular service in use-phase
- Circular end-of-use management
Philips Group
Progress towards Philips' 2025 circularity targets
| Metrics | Unit | 2020 Baseline | 2022 Results | 2023 Results | 2025 Targets | Key actions to deliver on 2025 targets |
|---|---|---|---|---|---|---|
| Resource inflows & outflows (products) | ||||||
| Circular revenues | % | 15 | 18.2 | 20.0 | 25 | Grow sales from products, services and solutions that use less virgin materials, optimize and extend product lifetime, recirculate materials |
| Resource inflows & outflows (waste) | ||||||
| Zero waste to landfill | % | 2.6 | 0.0 | 0.0 | less than 0.5 | Minimize waste to landfill |
| Circular materials management | % | 90 | 91 | 91 | 95 | Avoid waste by increasing the recirculation of discarded materials |
| Resource inflows (products) | ||||||
| Close the loop on medical equipment | # | Achieved for large medical equipment | Extend to small medical equipment | Adopt policy ensuring responsible end-of-use management |
Philips has committed to voluntary circularity targets to be delivered by 2025 as part of our externally communicated 2025 Sustainability Commitments. Key actions to deliver on these are stated in the above table. In 2023, Philips increased its circular revenues by 1.8% compared to the previous year, mainly driven by circular design of software and hardware. We implemented sharpened circular revenue requirements to further align with developments on circularity metrics and reporting disclosures. For example, external trends on metrics led to further sharpening of the definition of our software contributions. We also brought our circular revenue reporting more in line with our circular strategy on design and closing the loop.
In 2023, Philips achieved 91% circular materials management, comparable to 2022. We continued the emphasis on our Zero Waste to Landfill KPI, achieving 0.0% waste to landfill, compared to 0.0% in 2022.
We reclaimed more than 11,500 systems or pieces of equipment in 2023. The main driver was our take-back program for patient monitors.
8.2.3Biodiversity and Ecosystem Services
Philips recognizes the importance of healthy ecosystems and biodiversity for our company, our employees, and society. Therefore, Philips has developed the Natural Capital program, which is an addition to existing sustainability programs. This program is dedicated to reducing Philips’ impacts on natural capital, focusing on our chemicals footprint, water consumption, and improving biodiversity and ecosystem services. By systematically quantifying and reducing the environmental impact of our operations, supply chain and the use-phase of our products, we actively aim to protect and restore biodiversity. Philips acknowledges its dependency and impact on natural capital and aims to iteratively improve its understanding to drive regenerative decisions.
Philips aims to restore and enhance biodiversity and ecosystem services (BES) at our industrial sites and to actively promote ecosystem restoration activities through partnerships with, among others, NGOs, local communities, and governments. The Natural Capital program is focused, taking our 23 manufacturing sites as a starting point; Philips has created a BES community and trained employees on all these sites in ecosystem services. As a result, the ecosystem services of Philips' global manufacturing sites have been mapped and quantified. Based on this data, Philips evaluated the total area and ecological value of each manufacturing site and established the first BES data baseline to measure BES improvements by 2024.Together with our partners, we are working to develop more advanced BES metrics suitable for industrial areas.
In 2022, our manufacturing sites delivered some 80 potential measures to enhance biodiversity on-site. Philips implemented 30% of the biodiversity improvement measures selected for the short term at a number of sites in 2023, e.g. planting native trees in India, creating flower gardens in China, and creating habitats for endangered bee species in Central America. Furthermore, we have published our first Taskforce on Nature-related Financial Disclosures (TNFD) report and aim to set ourselves Science Based Targets for Nature (SBTN) in the future.
Philips aims to expand BES improvements in 2024 and track BES performance at our manufacturing sites with a new ecosystem services mapping according to our Environmental Policy. Improving BES at our manufacturing sites, and thereby also improving the working environment, is a contributor to making Philips the ’best place to work’, one of the ESG commitments Philips announced in 2020. Furthermore, healthy ecosystems support our efforts to mitigate climate risks assessed in our TCFD report for our sites.
As can be derived from our Environmental Profit & Loss (EP&L) account, the environmental impact of Philips’ sites is limited, as they are not very energy-intensive, are 100%-powered with electricity from renewable sources, do not emit large quantities of high-impact substances, and are not water-intensive. At the same time, Philips is aware that the total environmental impact of the full value chain is substantial, especially upstream in the mining industry. Philips considers improving biodiversity on its own land as a first important step towards reducing biodiversity impact over the full value chain.
Having become carbon-neutral in our operations by year-end 2020, and with our drive to send zero waste to landfill, focus on circular materials management, and enhance BES, the environmental impact of our sites will be further optimized in the years to come.
8.2.4Green/EcoDesigned Innovation
At Philips, we recognize that human health and environmental health go hand in hand. In 2022, the United Nations declared the ability to live in a clean, healthy and sustainable environment a human right.
Climate change poses a threat to health and is expected to cause some 250,000 additional deaths per year globally, according to the World Health Organization. It creates a pressing need – together with global resource constraints, growing and aging populations, and the rise of chronic diseases – for resilient and sustainable healthcare models.
We see a growing demand from our customers, including hospitals, to reduce their environmental impact, reduce waste and decarbonize healthcare. Our Green/EcoDesigned Innovation – the Research & Development spend related to the development of new generations of Green/EcoDesigned products and solutions and Green technologies, addressing SDG 12 (Ensure sustainable consumption and production patterns) – is focused on addressing that impact.
Sustainable Innovation is the Research & Development spend related to the development of new generations of products and solutions that address the United Nations’ Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages) or 12.
In 2022,2023, Philips invested EUR 168142 million in Green/EcoDesigned Innovation, a reductiondecrease compared to 20212022 due to the completionmore demanding EcoDesign criteria, a growing share of a number of sizeable innovation projectsspend in the course of 2022.software, for which reporting processes still need to be further implemented, and reduced R&D investments at Philips. We expect thisGreen/EcoDesigned Innovation spend to increase again in the years to come. In 2022, overcome, as one of our 2025 ESG commitments is to design all our new product introductions in line with our EcoDesign requirements by 2025. Over EUR 1.81.5 billion was invested in Sustainable Innovation.
As the current EU Taxonomy delegated act only applies to sectors with highest CO2 emissions, Philips’ activities are not within the scope of this delegated act and consequently none of Philips' R&D investments were eligible under this taxonomy during 2022.Innovation in 2023.
Philips Group
GreenGreen/EcoDesigned Innovation per segment
in millions of EUR

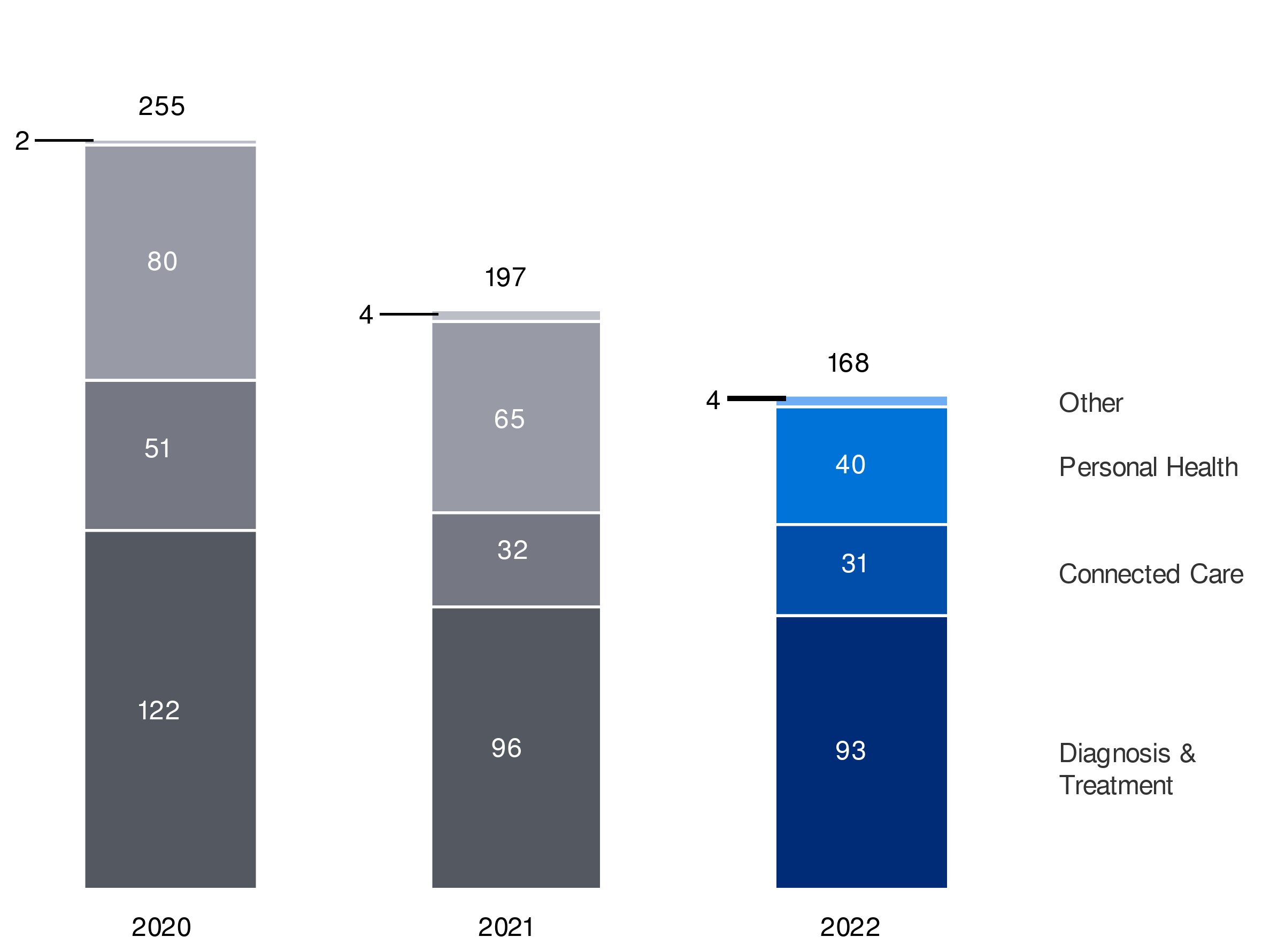
Diagnosis & Treatment businesses
Philips develops innovative diagnosis and treatment solutions that support precision diagnosis and effective, minimally invasive interventions and therapy, while respecting the limits of natural resources. Investments in Green Innovation in 20222023 amounted to EUR 78 million, a reduction compared to EUR 93 million comparable to EUR 96 million in 2021.2022.
All Philips EcoDesign/Green Focal Areas are taken into account as weWe aim to reduce environmental impact over the total lifecycle.lifecycle and our Green/EcoDesign innovations focus on four areas: Energy, Substances, Circularity, and Packaging. Energy efficiency is an area of focus, especially for our large imaging systems such as MRI. Through circular-readycircular design, Philips also pays particular attention to enabling the reduction in use of virgin materials, for example, through designing for low weight and enabling the upgrading and reuse pathways, sore-use of our products. As a result, our customers can, for example, benefit from enhancements in workflow, dose management and imaging quality and availability of re-used service parts with the equipment they already own.quality. In addition, we are reducing the amountsubstances of hazardous substanceconcern and improving our packaging. We continued to actively partner with multiple leading care providers to investigate innovative ways to reduce the environmental impact of healthcare, for example by maximizing energy-efficient use of medical equipment (by for example(e.g. by introducing EcoModes) and optimizing lifecyclelifetime value. Philips aims to closeclosed the loop on all medicallarge equipment that becomes available to us by the end of 2025. To achieve this target, we actively drive trade-ins2020, by structurally embedding a responsible take-back policy into its customer trade-in offers. This means that for all equipment that a customer is willing to trade-in at end of use, Philips will take it back for refurbishment and parts recovery where feasible, or locally recycle it in markets where de-install, trade-ina certified way to ensure it does not end up in landfill. Sustainable design and reverse logistics capabilities are in place,innovation help to further increase the value created and build these capabilities in countries that do not yet have them.decrease the environmental impact Philips can deliver from returned systems.
Connected Care businesses
Philips’ connected health IT solutions integrate, collect, combine and deliver quality data for actionable insights to help improve access to quality care, while respecting the limits of natural resources. It is our belief that well-designed e-health solutions can reduce the travel-related carbon footprint of healthcare, increase efficiency in hospitals, reduce waste, and improve access to care and outcomes. This hasFor example, our Philips Radiology Operations Command Center enables real-time collaboration and virtual imaging operations and can decrease staff travel time and costs. The value and adoption of e-health solutions also becomebecame apparent during the COVID-19 crisis. Green/EcoDesigned Innovation investments in 20222023 amounted to EUR 3129 million, in line with EUR 3231 million in 2021. Green2022. Over the coming years, Green/EcoDesigned Innovation projects in 2022 will deliver, the coming years, among other things, new EcoDesigned patient monitors with lower environmental footprints, reflecting all the Philips EcoDesign/Green Focal Areas. Energy efficiency, material reduction, less hazardous substances and closing the loop activities are the main areas of focus.footprints.
Personal Health businesses
R&D investments at our Personal Health businessessegment amounted to EUR 33 million in 2023, compared with EUR 40 million in 2022, compared with EUR 65 million in 2021, as some larger innovation projects were finalized in the course of 2022. The Personal Health businesses continued theirits work on improving the energy efficiency of theirits products, closing the materials loop (e.g. by using recycled materials in products and packaging), and the voluntary phase-out of polyvinyl chloride (PVC), brominated flame retardants (BFR), Bisphenol A (BPA) and phthalates from, among others, food contact and childcare products. More specifically, asNew hairdryers have been launched that are more energy-efficient, with an improvement of more than 10% compared to the 2020 baseline. Personal Health also continues to increase circularity by, for example, using recycled materials in products and packaging. For packaging, we are increasingly shifting away from single use plastic materials. As part of our Fit for Future Packaging program, we have launched the first plastic free,additional paper-based, mailbox-ready packaging solutionsolutions in our Grooming and& Beauty portfolio, for an online One Blade shaver,including OneBlades and plastic free packaging in the Female Depilation and Hairstyling portfolio. Philips also launched a foldable, more energy-efficient hairdryer containing recycled plastic.hairdryers.
Other
The segment Other invested EUR 42 million in Green/EcoDesigned Innovation, spread over projects focused on global challenges relating to water, air, energy, food, circular economy, and access to affordable healthcare.
Circular economy
For a sustainable world, the transition from a linear to a circular economy is essential. A circular economy aims to decouple economic growth from the use of natural resources and ecosystems by using these resources more effectively. It is a driver of innovation in the areas of material, component and product re-use, as well as new business models such as system solutions and services. At Philips, we have set ambitious targets to guide this journey. In 2020, as we announced our ESG commitments, we aimed, among other things, to generate 25% of our revenues from circular products and services, to extend our ‘closing the loop’ practices across all our medical products, and to further embed circular practices at our sites and send zero waste to landfill in our own operations.
8.3.28.2.5Green/EcoDesigned and EcoHero Revenues
Green/EcoDesigned Revenues are generated through products and solutions that offer a significant environmental improvement in one or more Green Focal Areas – Energy efficiency, Packaging, Hazardous substances, Weight,Substances, and Circularity and Lifetime reliability – and thereby deliver a contribution to SDG 12 (Ensure sustainable consumption and production patterns). Green/EcoDesigned Revenues amounted to EUR 12.8 billion in 2022,2023, or 71.7%70.5% of sales, (70.5%comparable to 2022 (71.7% in 2021)2022). This increase is mainly attributable to higher Green/EcoDesigned revenues in the Precision Diagnosis and Personal Health businesses.
As the currentfirst EU Taxonomy delegated act, addressing Climate Change Adaptation and Climate Change Mitigation, only applies to sectors with the highest CO2 emissions, Philips’ activities are not within the scope of this delegated act and consequently none of Philips' revenues were eligible under this taxonomy during 2022.2023.
Philips Group
GreenGreen/EcoDesigned Revenues per segment
in millions of EUR unless otherwise stated

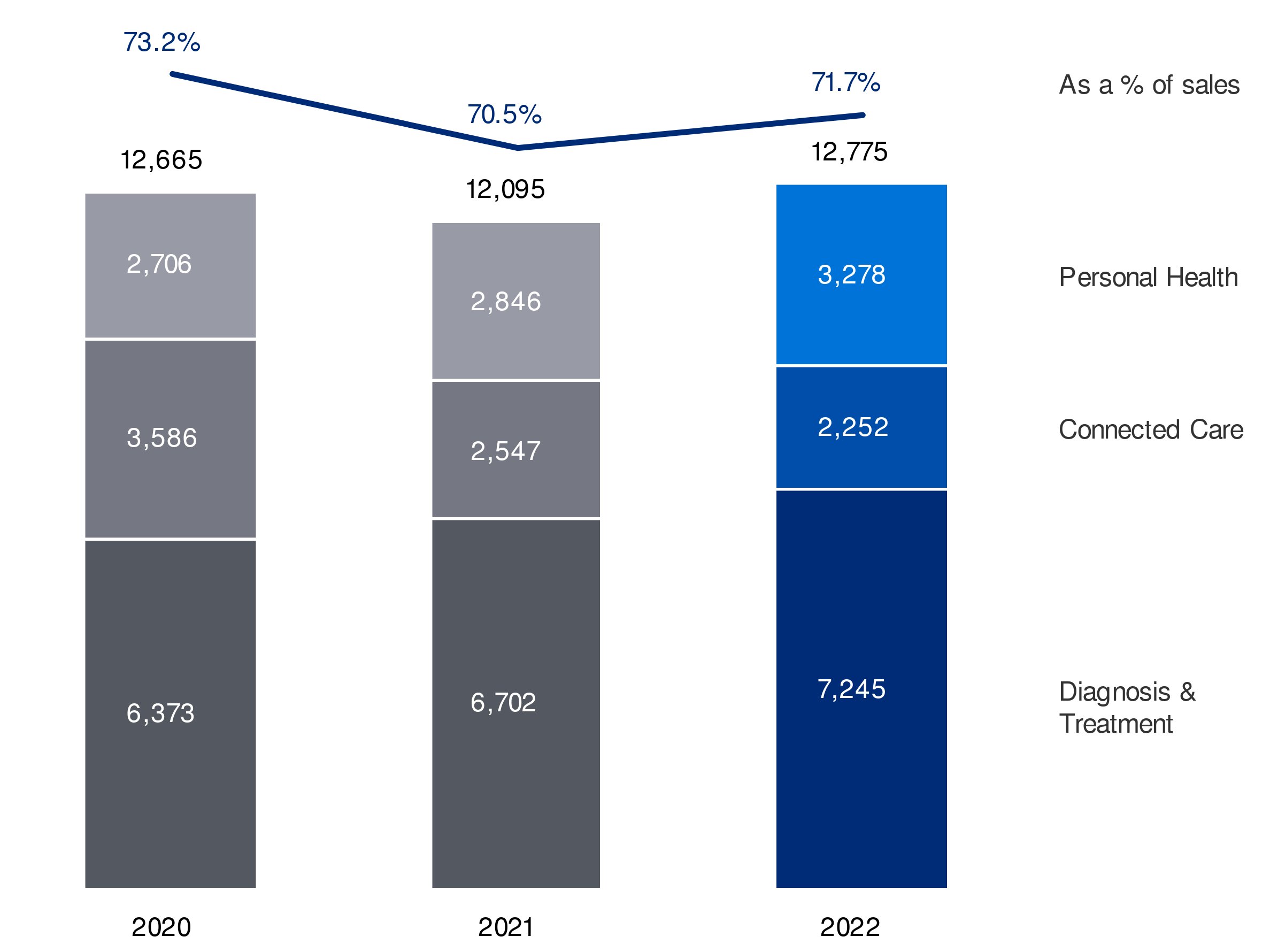
Through our EcoDesign process we aim to create products and solutions that have significantly less impact on the environment over their whole lifecycle. Overall, the most significant improvements have been in energy efficiency and lower weight (thus less resources), although increased attention was also given to hazardous substances of concern, packaging and circular design, in particular design for recyclability, in all segments in 2021,2023.
By 2025, 25% of hardware revenues should come from EcoHeroes, which meet the latter drivenEcoDesign requirements and outperform in at least one of the focal areas compared to their predecessor or relevant benchmarks, supported by our Circular Economy initiatives.a sustainability claim. In 2023, Philips achieved 15.9% in EcoHero Revenues, with most contributions from improvements in energy use.
Diagnosis & Treatment businesses
In 2022,2023, a number of main platforms were launched in our Diagnosis & Treatment businesses. CT7500 and various redesigns of current platforms have been launched offering further environmental improvements.launched. Specific attention was paid to preparing for future EcoDesigned product launches.
Connected Care businesses
After several launches of newNew Green/EcoDesigned products in 2020, no major new launches took place in 2021 and 2022 except for the VS20 monitor which has good performance on all EcoDesign focal areas. New EcoDesigned ProductsConnected Care are expected in 20232024 and beyond, with improvements onin all EcoDesign focal areas.
Personal Health businesses
In our Personal Health businesses,business, the focus is on Green/EcoDesigned Products and Solutions that meet or exceed our minimum requirements in the areas of energy consumption, packaging, substances of concern, and application of recycled plastics. Green/EcoDesigned Revenues in 2022 amounted to 90% of total sales, compared to 85% in 2021. We continue to make progress in developing PVC/BFR-free products. More than 90% of our consumer product sales consist of PVC/BFR-free products, with the exception of power cords, for which there are not yet economically viable alternatives available. In our Oral Healthcare portfolio, we introduced the first brush heads containing 75% bio-based materials. In our Mother & Child Care portfolio, we launched the first baby monitor with recycled plastic. And we implemented recycled plastic in the interior parts of a significant proportion of the Male Grooming portfolio.
8.3.38.2.6Sustainable Operations
Philips’ Sustainable Operations programs focus on the main contributors to climate change, with the aim of reducing, re-using and/or recycling of waste, reduction ofreducing water consumption, and reductionreducing emissions.
Water
Annually, we undertake thorough assessments of emissions.
Carbon footprint and energy efficiency
At Philips, we see climate change as a serious threat. Therefore, we are taking action to rethink our business models and decouple economic growth from the impact we have on the environment. We believe large corporates should lead the transition to a low-carbon economy. This will not only benefit the environment, but will also positively impact social and economic aspects.
During the COP 21 United Nations Climate Conference in Paris in 2015, we committed to become carbon-neutral in our operations, pursue all efforts to reduceboth our operational emissions, source all our electricity from 100% renewable sources,sites and offset all unavoidable emissions by year-end 2020. Since 2020, Philips has been carbon-neutral in its operations. We delivered on this commitment as a result of a comprehensive program that included energy-efficiency improvements, on-site renewables, Power Purchase Agreements, as well as business travel reduction and transport mode shifts to low-carbon emitting alternatives, and finally a carbon offset program.
Our efforts are acknowledged by the CDP (formerly known as the Carbon Disclosure Project), a global NGO that assesses the greenhouse gas (GHG) emission performance and management of reporting companies. In 2022, we were ranked on the CDP Climate Change 'A' List for our continued climate performance and transparency for the 10th consecutive year.
Having achieved our 2020 carbon neutrality target, we have raised the bar and set ambitious emission reduction targets to ensure we help limit the impact of global warming, not only in our operations, but throughout our value chain – collaborating with suppliers and customers to amplify our impact. That is why Philips has set new long-term emission reduction targets, which have been assessed and approved by the Science Based Targets initiative (SBTi) – locking down our commitment to drive climate action across the value chain, fromstrategic suppliers to customers, and ensuring that we contribute to the decarbonization required to keep the global temperature increase below 1.5 °C. At COP 26, we announced our plan to step up our acclaimed supplier sustainability program with the goal of having at least 50% of our suppliers (based on spend) committing to science-based targets (SBTs) for CO₂-e emissions reduction by 2025.
address potential water-related risks. We stepped up our commitment to reduce our scope 3 carbon emissions in line with the 1.5 °C global warming scenario (Paris agreement). This commitment has been reviewed and approved by the Science Based Targets initiative (SBTi) in 2022, after we sold the Domestic Appliances business in 2021. The latter had a material downward impact on our scope 3 emissions, requiring a new assessment by the SBTi.
In 2022, our net operational carbon footprint resulted in zero kilotonnes carbon dioxide-equivalent (CO2-e), mainly driven by continued use of 100% electricity from renewable sources and a continuing reduction in air freight. A total of 438 kilotonnes carbon dioxide-equivalent (CO2-e) were compensated via carbon offsets.
Philips reports all its emissions in line with the Greenhouse Gas Protocol (GHGP).
Philips Group
Net operational carbon footprint
in kilotonnes CO2 -equivalent
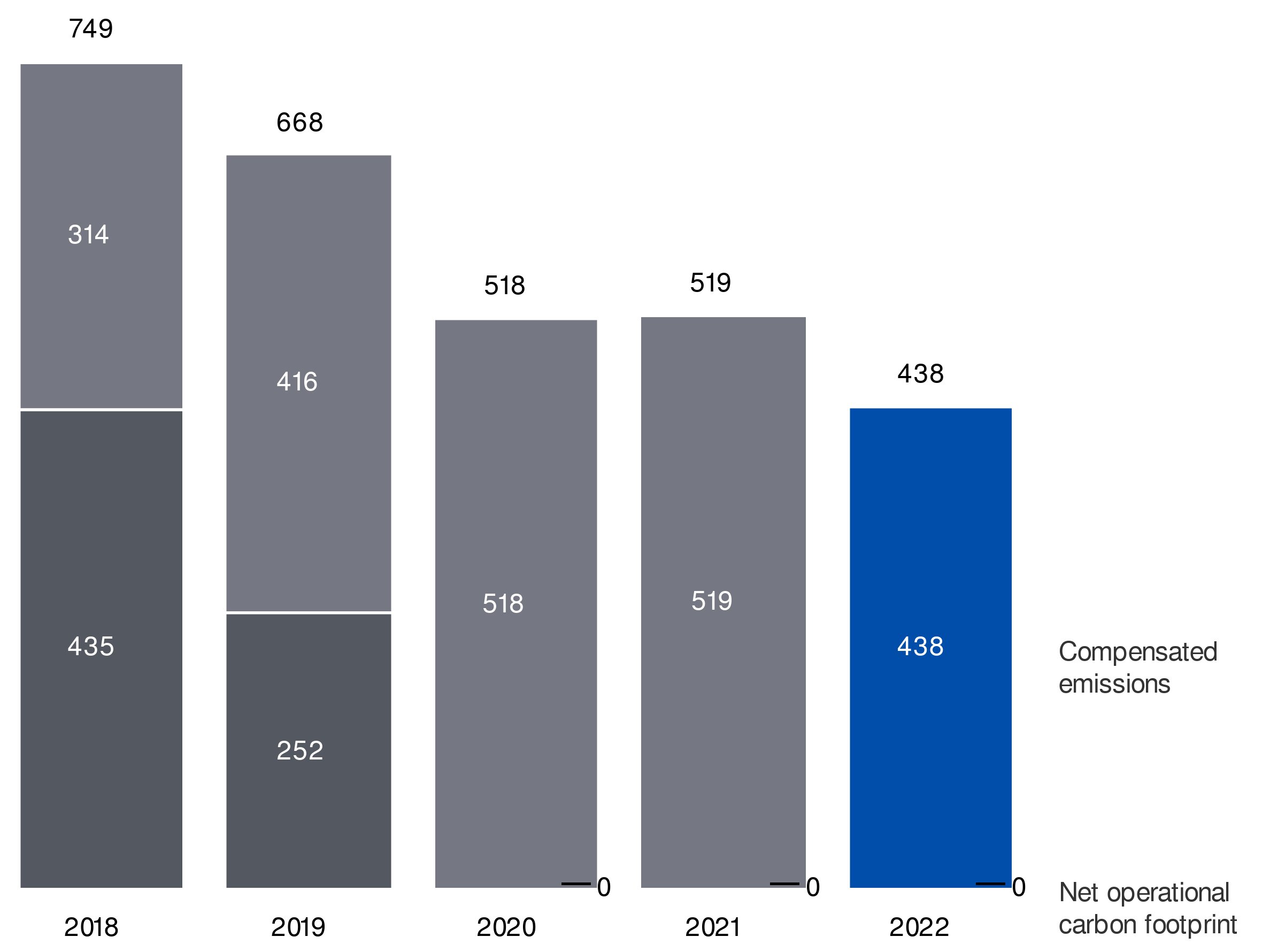
Scope 1
In our sites, we reduced our scope 1 (direct) CO2-e emissions by 16% compared to 2021. Scope 1 emissions cover the emissions from our direct fuel consumption and the use of refrigerants that have a global warming potential. The reduction in scope 1 emissions is mainly driven by our continued energy efficiency measures, our program to phase out fossil fuels, working from home, and mild winters. As the consumption of natural gas is still the main source of our scope 1 emissions, we will continue to drive down our overall consumption and find alternative renewable sources to heat our buildings.
Scope 2
In 2022, our indirect scope 2 (market-based method) CO2-e emissions declined by 33% compared to 2021. Scope 2 (market-based) emissions cover the emissions of non-renewable electricity and purchased (city/district) heating and cooling. As we have already been sourcing 100% renewable electricity since 2020, the remaining emissions are associated with purchasing (city/district) heating and cooling, which we leverage as a low-carbon alternative to natural gas to heat our buildings. Moving forward, we will continue to increase the renewable energy share of our (city/district) heating and cooling that we purchase.
To secure long-term delivery and quality of our renewable electricity, we have multiple Power Purchase Agreements (PPAs) in place. For instance, the Los Mirasoles wind farm in the US and the Krammer and Bouwdokken wind farms in the Dutch province of Zeeland. We closed the latter agreements with our renewable electricity purchasing consortium with Nouryon, DSM and Google, powering all our operations in the Netherlands. Combined with the Los Mirasoles wind farm, this covers 49% of our total electricity demand. In December 2020, Philips announced its next Power Purchase Agreement that will become operational during the summer of 2023, again in a purchasing consortium with Heineken, Nouryon and Signify, to power most of the remaining European sites with renewable electricity for the long term.
In 2022, our indirect scope 2 (location-based method) CO2-e emissions declined by 6% compared to 2021. Scope 2 (location-based method) emissions cover the emissions of electricity (excluding the renewable share) and purchased (city/district) heating and cooling. Emissions are calculated using average grid emission factors, ignoring the renewable electricity share of the reporting entity. This method indicates the efforts to reduce energy.
Our operational energy efficiency improved by 9%, from 0.031 GWh/millions EUR sales in 2021 to 0.028 GWh/millions EUR sales in 2022.
Our continued efforts to reduce our energy consumption, eliminate refrigerants with a high global warming potential (GWP), and increase our renewable energy share led to a 16% reduction in (scope 1 and scope 2 market-based) emissions in 2022 compared to 2021. Overall, we are making good progress, increasing our renewable energy share to 77% in 2022, from 74% in 2021. We are already overachieving our 2025 ambition to source 75% of our energy from renewable sources and delivering on our 2025 scope 1 and scope 2 (market-based method) ambition. Even though we have already achieved our 2025 SBTi targets, we will continue to accelerate our efforts to phase out fossil fuels (mainly natural gas) consumption from our operations by driving down overall consumption and finding alternative renewable sources, making sure we remain well on track to deliver on our long-term (2040) science-based targets.
Scope 3
In our operational carbon footprint, we include two scope 3 (indirect) emission categories – not included in scope 2 – that occur in the value chain, namely business travel and transportation & distribution. Together with our scope 1 and scope 2 (market-based method) emissions, these comprise our operational carbon footprint.
Our business travel emissions, covering emissions from air travel, lease cars and rental cars, increased by 20% compared to 2021. This is mainly due to the fact that more of our employees are traveling to meet customers and are using their lease cars again post-COVID-19. The remaining effects of COVID-19 also continued to keep these emissions low compared to pre-COVID-19 levels. Moving forward, we continue to electrify our lease fleet and to promote online collaboration post-COVID-19 to limit air travel, as well as increasing our efforts to move travelers to rail transport for shorter distances.
In 2022, we recorded a 22% decrease in emissions from our transportation & distribution compared to 2021. The scope of these emissions covers the CO2-e emitted by air freight, ocean freight, road freight and parcel shipments. As air freight accounts for most of our operational carbon footprint, we have taken several measures,utilize publicly accessible tools such as the Corridor Project, where we shifted air freight shipmentsAqueduct Water Risk Atlas by WRI and WWF Water Risk Filter to ocean freight for several lanes.define and respond to these risks. This helped to reduce our air freight emissions by 15% compared to 2021. CO2-e emissions from ocean freight decreased by 43% in 2022 compared to 2021. Most of these reductions can be attributed tocomprehensive process evaluates the fact that the Domestic Appliances businesses have now been fully disentangled and (combined) shipment data for ocean freight now fully excludes all their related shipments. To quantify our ocean freight emissions by leveraging carrier-trade-lane specific emission factors, we use data from the Smart Freight Center – Clean Cargo (formerly known as the CCWG). This improved approach was implemented in 2021, allowing us to quantify our ocean freight emissions more accurately. This approach has been implemented for 2020, 2021 and 2022.
Emissions from parcel shipments decreased by 10%, as the number of shipments increased but was mitigated by shorter average distances per shipment. The emissions from road transport decreased by 51%, mainly driven by a reduction of shipments and the average weight per shipment. The emission reductions in road freight are also impacted by the inclusion of combined shipments of Domestic Appliances and Philips in 2021. Historically, we were not able to exclude all the Domestic Appliances businesses' shipments from our shipment data.
Moving forward, we will continue to drive efforts to further reduce emissions from air freight and are exploring options to source sustainable fuel alternatives for shipments, which will help us to reach our long-term emission reduction targets.
Although reduction is key to achieving carbon neutrality, unavoidable carbon emissions required offsetting to gradually drive down our emissions to zero by year-end 2022. We did this by financing projects in emerging regions that have a strong link with UN Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages) and 12 (Ensure sustainable consumption and production patterns). In 2022, we decreased offsets to 438 kilotonnes, equivalent to the annual uptake of approximately 13 million medium-sized oak trees. This covers the total emissionssusceptibility of our entire operations,sites to various risks including all CO2-e emissions from our sites, all business travel,water availability, wastewater quality, workplace water accessibility, and all transportation & distribution. We do this by financing carbon reduction projects through long-term carbon offsets in emerging regions that drive social, economicgroundwater replenishment, encompassing physical, regulatory, and additional environmental progress for the local communities, such as:
Providing access to safe drinking water while reducing wood consumption
This carbon-emission reduction project will provide millions of liters of safe drinking water in Uganda and will reduce the mortality risk from water-borne diseases. Additionally, less wood will be required for boiling water, leading to less indoor air pollution and slowing down the deforestation rate. To ensure quality, all offsets are verified under the Gold Standard.
Replanting degraded land while providing education on health matters
Planting trees will improve livelihoods and address issues such as deforestation, biodiversity loss, and adaptation to climate change and provide support and education including on HIV and malaria. To ensure quality, all offsets are verified under the VCS standard.
Protecting forests through sustainable production
Deforestation is reduced through promotion of sustainable businesses to protect the forest. Unsustainable harvest of fuelwood is reduced. The forest supports the supply of water to other parts of Ethiopia and neighboring countries. It is also the habitat of diverse and, in some cases, rare species. To ensure quality, all offsets are verified under the VCS standard.
Increasing employment through provision of sustainable energy
The energy supply gap is reduced by providing access to clean energy and related employment through wind generation in India. This enables an improvement in livelihoods. To ensure quality, all offsets are verified under the VCS standard.
Improving respiratory health and reducing deforestation through provision of clean cookstoves
By supporting a range of cookstove technologies across Ghana and Kenya, the projects improve respiratory health, reduce fuel costs and reduce deforestation for fuel. This also enables more time for paid work, thus improving prospects. To ensure quality, all offsets are verified under the Gold Standard.
Operational carbon footprint
Philips Group
Operational carbon footprint by scopereputational concerns.
in kilotonnes CO2-equivalent unless otherwise stated
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Scope 1 | 36 | 32 | 30 | 27 | 23 |
| Scope 2 (market-based) | 26 | 14 | 3 | 3 | 2 |
| Scope 2 (location-based) | 200 | 196 | 173 | 177 | 167 |
| Scope 3 | 687 | 622 | 485 | 489 | 413 |
| Scope 3 - Transportation & Distribution | 540 | 470 | 415 | 417 | 327 |
| Scope 3 - Business Travel | 147 | 152 | 70 | 72 | 86 |
| Total (scope 1, 2 (market-based), and 3)1) | 749 | 668 | 518 | 519 | 438 |
| Emissions compensated by carbon offset projects | 314 | 416 | 518 | 519 | 438 |
| Net operational carbon emissions | 435 | 252 | - | - | - |
| Operational CO2e efficiency in tonnes CO2e/mln EUR sales | 47.2 | 39.0 | 29.9 | 30.3 | 24.6 |
In 2022, we updated our emission factors to the latest available sources to reflect the most accurate results. Historical emissions of our discontinued Domestic Appliances business have been excluded for all years, except for some combined ocean and road freight shipments in 2021 as described above. Where available, actual emission allocations were applied. Where business-specific emission data were not available, a spend allocation key was applied. Philips reports all its emissions in line with the Greenhouse Gas Protocol (GHGP).
Energy consumption
Philips Group
Energy consumption1)
in gigawatt hours (GWh) unless otherwise stated
2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Electricity consumption | 421.6 | 403.5 | 381.6 | 389.1 | 382.1 |
| Renewable electricity | 374.6 | 382.0 | 381.3 | 389.1 | 382.1 |
| In-contract renewable electricity | 146.8 | 95.5 | 63.1 | 56.7 | 39.6 |
| Power Purchase Agreement (PPA) | 45.7 | 160.9 | 186.2 | 168.7 | 187.4 |
| Purchased renewable electricity certificates | 181.1 | 124.5 | 130.0 | 161.3 | 152.3 |
| Renewable electricity generated and consumed on-site | 1.0 | 1.1 | 2.1 | 2.4 | 2.7 |
| Fuel consumption | 146.1 | 134.7 | 133.8 | 120.6 | 102.7 |
| Natural gas | 137.0 | 127.3 | 126.4 | 116.3 | 97.7 |
| Other non-renewable fuel | 9.1 | 7.4 | 7.4 | 4.3 | 5.0 |
| Purchased heat, steam and cooling | 17.2 | 17.8 | 12.4 | 14.4 | 11.9 |
| Total energy consumption | 584.9 | 556.1 | 527.9 | 524.1 | 496.7 |
| Renewable energy consumption | 374.6 | 382.0 | 381.3 | 389.1 | 382.1 |
| Renewable energy share | 64% | 69% | 72% | 74% | 77% |
| Renewable electricity share | 89% | 95% | 100% | 100% | 100% |
| Non-renewable energy consumption | 210.3 | 174.0 | 146.5 | 135.0 | 114.7 |
| Non-renewable energy share | 36% | 31% | 28% | 26% | 23% |
| Sales to thirds in millions of EUR | 15,878 | 17,147 | 17,313 | 17,156 | 17,827 |
| Operational energy efficiency in GWh/millions EUR sales | 0.037 | 0.032 | 0.030 | 0.031 | 0.028 |
Our high-level plan to deliver on Science Based Targets
Philips has set long-term CO2-e emission targets approved by the Science Based Targets initiative (SBTi) for all three scopes. The approval confirms that Philips’ targets across our value chain are in line to limit global warming to below 1.5 °C. By joining forces with our customers and suppliers, we can reduce our shared carbon footprint and help create a sustainable and more resilient healthcare industry.
Together with our customers and suppliers, we intend to continue to reduce our collective need for fossil fuels by using renewable and energy-efficient alternatives. To deliver, we will focus on the following four objectives:
Collaborating with our suppliers to reduce emissions in our supply chain
With growing global concerns about the impact of climate change, there is a pressing need for industry and business to manage and reduce CO2-e emissions across the entire value chain – including at supplier level. To this end, we have invited many of our largest suppliers – first-tier manufacturing and transportation-related suppliers – to report their climate performance and strategy as part of the Carbon Disclosure Project (CDP) Supply Chain program. Additionally, we engage with these suppliers to reduce their emissions as part of our Supplier Sustainability program. In October 2021, during COP26, we announced our ambition to have at least 50% of our suppliers (based on spend) committed to science-based targets for carbon reduction by 2025. At year-end 2022 already 41% of our suppliers (based on spend) had committed. Please refer toSupplier indicatorsfor more details.Minimizing our climate impact in our supply chain by adopting circular economy principles
From a climate perspective, applying circular business models leads to a significant emission reduction in our supply chain. As the value of materials is retained, the need for new abiotic resources is significantly reduced, and consequentially, the need for energy to produce those new resources/materials, leading to reduced emissions. This is also part of our Circular Economy program.Transitioning to lower carbon emitting energy in our sitesBy continuing to phase out fossil fuels at our sites, we will be able to achieve our long-term emission targets. This entails, for example, moving towards geothermal and district heating and cooling solutions where available.Designing energy-efficient products and collaborating with our customers to reduce emissions during the use-phase
More and more, our customers – both in healthcare and retail – are seeking solutions that are less impactful to the environment. To address that demand, we are continuously reducing the climate impact of our products by increasing energy efficiency, increasing the use of recycled plastics and other recyclable materials, and ensuring we make our packaging easier to re-use and recycle. We see improving energy efficiency as a huge lever to deliver on our value chain emission reductions. In 2022, we performed an initial assessment of our scope 3 category Use of Sold Products by estimating the lifetime energy consumption and applying the Life-Cycle Assessment (LCA) methodology on a country-by-country basis. Initial results indicate that the emissions from the use of sold products are 3,898 kilotonnes CO2-e, approximately 9 times more than our entire operational carbon footprint. This emphasizes the need to drive energy efficiency efforts under our EcoDesign program and collaborate with our customers to magnify our impact.
Taskforce on Climate-related Financial Disclosures (TCFD)
Philips recognizes the importance of identifying, assessing and mitigating climate-related risks to ensure business continuity and resilience. This 2022 integrated financial, social and environmental report aims to follow the recommendations of the TCFD.
In 2022, relevant risks and opportunities have been quantified by applying Philips’ internal risk assessment methodology. This ensures alignment with the risk management team, increasing cross-business comparability and integration with already existing risk screening procedures. Moreover, physical risk factors were evaluated on a site-specific level by exploring 25 of our financially material sites in more detail. Transition risks on the other hand, were assessed on a company level and by subject matter experts. The reason for this differentiation is because physical risks vary on a regional level while transition forces generally apply on a global scale.
The site-specific analysis leveraged both the external Munich RE NATHAN tool and internal site experts. While RE NATHAN uses scientific models to determine how exposed different regions are to climate risk factors, the site-specific experts have access to specialized knowledge on the climate change preparedness of the sites. Combining both internal and external expertise ensured we have a holistic view that considers both regional implications and Philips specific implications. RE NATHAN assessed which of the following hazards are most threatening in the medium-term accounting for four global warming scenarios (RCP 1.9, RCP 2.6, RCP 4.5, and RCP 8.5): drought, heat stress, precipitation, river flood, and tropical cyclones. In case one or multiple risk factors seemed impactful in the future we then asked site specific experts to provide us with a more detailed impact and control measure evaluation. This thereby provided us with a good overview on how exposed we currently are to extreme or chronic weather conditions and highlighted key action points.
We also further assessed internal and external forces pushing Philips to a low carbon future considering three global warming scenarios. In our 1.5 and 2 degrees model (RCP 1.9 and 2.6) we assumed that strong cross sector pressures exist. Governments enforce strict environmental rules, society is environmentally conscious, and the private sector invests in collaborative innovations. In contrast, the 4ºC global warming scenario (RCP 4.5) assumed short-sighted governments focused on protectionism, customers with a cost orientation, and a private sector focused on product innovation. For each scenario, experts were then consulted to determine the potential likelihood of the predefined transition risks/opportunities becoming material. We, furthermore, assessed the potential impact of the risks/opportunities unraveling and to what extent we can control the underpinning risk or exploit the opportunity.
Through our ambition to reduce CO2 emissions in our entire value chain in line with a 1.5 °C global warming scenario, we are reducing our exposure to transition risks, such as changing legislation, changing customer demands and carbon pricing. Nonetheless, strong government policies in line with the Paris Agreement could result in higher carbon pricing impacts for Philips, its supply chain, and its customers. Furthermore, a global financial downturn could also promote inertia in the field of environmentally friendly innovations. Hence, our Science Based Targets are a key factor in mitigating the risk associated with the changing legislation, customer preferences and preventing inertia.
In 2023, we plan to further assess the impact of climate change on our value chain and continue to standardize our assessment process.
Water
Philips is not a water-intense company. However, a numberwater-intensive organization, this practice ensures the uninterrupted continuity of our manufacturingoperations and the provision of high-quality Water, Sanitation and Hygiene (WASH) services at all our sites and those of our strategic suppliers. Among our facilities, five locations have been identified as exposed to substantive financial and strategic risks related to water. These sites are locatedsituated either in water-stressed regionsareas highly vulnerable to coastal flooding or extremely high-water stress (>80%), with three in for example, USA (California), IndiaChina, one in Indonesia, and Israel. Withone in the help of the WRI Aqueduct tool, the water withdrawn from areas with high baseline water stress was identified across all Philips' industrial operations. It shows that around 13% of the industrial sites are located in Extremely High (>80%) baseline water stress areas. However, the impact from these operational sites is very limited, only amountingUSA. In response, we have deployed engineers and experts to 4% of Philips' total water withdrawal. conduct further investigations, accurately identify risk exposure, anticipate potential losses, and implement proactive measures to mitigate property loss and business interruption.
We were includedare proud to have received an 'A' score for disclosure transparency on water security in the CDP "A-list" forEurope 2023, demonstrating our ongoing commitment to water in the 2022 ranking, achieving a 'double-A' score when combined with our Climate Change results.risk management and sustainability practices.
Total water withdrawal in 20222023 was 677,632661,076 m3, a 4%3% decrease compared to 20212022 and a 5%7% reduction compared to 2019 (pre-COVID level). Water consumption in 2020 and 2021 was impacted by the government-mandated lockdowns and the working-from-home protocol – resulting in a significant reduction in water intake at several sites (mainly in China).
Diagnosis & Treatment, which consumes 46%49% of total water usage, recorded an 8% decrease,a 5% increase, mainly caused by lower construction activity and effective processes, mitigated byamounts of reused water in a site expansion in India.North America. Personal Health recorded a 4% increase.7% decrease. In 2022, one of our manufacturing sites in Asia experienced a water leakage which resulted in higher water intake in that year. This leakage was mainly due to the construction of a new factory in China, mitigated by decreased production volume at a water-intensive manufacturing site in Asia.remedied. Connected Care showed a decrease of 7%11%, due to the decreased production volumevolumes at a site in Asia, mitigated by construction activity at a site in North America.Asia.
Philips Group
Water withdrawal
in thousands of m3
| 2018 | 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 288 | 295 | 286 | 337 | 310 | 295 | 286 | 337 | 310 | 324 |
| Connected Care | 161 | 150 | 116 | 119 | 111 | 150 | 116 | 119 | 111 | 99 |
| Personal Health | 238 | 265 | 221 | 247 | 257 | 265 | 221 | 247 | 257 | 238 |
| Philips Group | 687 | 710 | 623 | 703 | 678 | 710 | 623 | 703 | 678 | 661 |
In 2022, 99.7%2023, 99.6% of water was purchased and 0.3%0.4% was extracted from groundwater wells.
Waste
In 2022,2023, our manufacturing sites generated 22,80219,375 tonnes of waste, an increasea decrease of 3%15% compared to 2021,2022, mainly driven by the high impact of ourlower construction activities in different locations across the globe, lower paper/cardboard and changes inplastic waste due to reduced production volumes at some sites and more efficient waste management. The reported re-used materials were 9% of the operations.total waste.
The Diagnosis & Treatment businesses increaseddecreased waste by 7%12%, mainly driven by a strong increase in construction-related reused material in Best (see below),the decreased volume of one-time construction related re-used materials and lower construction activity, which was partially offset by the operational changes and lower construction activity on the other sites. The reported reused materials now constitute 22% of total waste. Thechanges. Connected Care businesses increaseddecreased waste by 5%21% due to the significant decrease in shipment packaging materials related to the recall, increased volume of reusedre-used materials and operational changes. The reported reused materials are 24% of the total waste. Personal Health decreasedreduced waste by 3%17% due to operational changes, lower construction activityproduction volumes and changes in production.the start of a smart warehouse, that significantly reduced amounts of wood pallets and cardboard wastes by using reusable plastic trays.
Re-using temporary offices
Philips Environmental Policy addresses the waste hierarchy stating that Philips drives action by ensuring circular manufacturing and supply to house refugees
In the past, Philips in Best (Netherlands) decided to purchase temporary offices to resolve office space shortages,increase circular practices at our sites and after many years these temporary offices became redundant. Since the temporary offices were still of good quality, Philips made every effort to find a sustainable solution for the building and found a partner in COA (Centraal Orgaan opvang asielzoekers, the Dutch national organization helping asylum seekers). These units were completely refurbished for their new purpose: a COA location for people seeking asylum in the Netherlands. The 'new' building is located in Zeist. By re-using the offices, we are contributingresponsible waste management according to the provision of good housing for asylum seekers and to a circular society. waste hierarchy.
Philips Group
Total waste
in tonnes
| 2018 | 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 8,368 | 9,675 | 19,703 | 9,974 | 10,694 | 9,675 | 19,703 | 9,974 | 10,694 | 9,422 |
| Connected Care | 3,962 | 4,095 | 3,475 | 2,753 | 2,899 | 4,095 | 3,475 | 2,753 | 2,899 | 2,276 |
| Personal Health | 8,820 | 8,758 | 7,929 | 9,477 | 9,209 | 8,758 | 7,929 | 9,477 | 9,209 | 7,677 |
| Philips Group | 21,150 | 22,528 | 31,107 | 22,204 | 22,802 | 22,528 | 31,107 | 22,204 | 22,802 | 19,375 |
Until 2020, total waste consisted of waste that is delivered for landfill, incineration, waste to energy or recycling. We extended the scope with materials sent for reusere-use and other recovery as of 2021. Total waste does not include waste prevented.
Materials delivered for reuse,re-use, other recovery or recycling via an external contractor amounted to 20,40617,446 tonnes, which equals 89%90% of the total waste. OfMaterials delivered to incineration and landfill amounted to 1,929 tonnes, which equals 10% of the 11% remainingtotal waste, 77%of which 74% comprised non-hazardous waste and 23%26% hazardous waste. We recorded 1,4841,531 tonnes of waste prevented in our own activities in 2022,2023, compared to 1,5251,484 tonnes in 2021.2022. Philips did not produce any radioactive waste in 2023.
Philips Group
Total waste by destination
in tonnes
| Waste generated | Hazardous waste | Non-hazardous waste | Total waste generated | Hazardous waste | Non-hazardous waste | |
|---|---|---|---|---|---|---|
| Reuse | 3,382 | 11 | 3,371 | |||
| Re-use | 1,651 | 1 | 1,650 | |||
| Recycling | 16,978 | 1,582 | 15,396 | 15,762 | 1,536 | 14,226 |
| Other recovery | 46 | 0 | 46 | 33 | - | 33 |
| Waste diverted from disposal by recovery operation | 20,406 | 1,593 | 18,813 | 17,446 | 1,537 | 15,909 |
| Incineration (with energy recovery) | 1,802 | 156 | 1,646 | 1,480 | 199 | 1,281 |
| Incineration (without energy recovery) | 412 | 383 | 29 | 298 | 294 | 4 |
| Landfilling | 182 | 5 | 177 | |||
| Landfill | 1511) | 4 | 147 | |||
| Waste directed to disposal by disposal operation | 2,396 | 544 | 1,852 | 1,929 | 497 | 1,432 |
| Total waste generated | 22,802 | 2,137 | 20,665 | 19,375 | 2,034 | 17,341 |
The total waste destinations are fully categorized above. There is no waste generated that is destined for other disposal methods. Our sites addressed both the Circular MaterialMaterials Management percentage as well as waste sent to landfill, as part of our ESG commitments.commitments; refer to Definitions and abbreviations for the definition of Circular Materials Management.
The Circular MaterialMaterials Management percentage has replaced the recycling percentage and includes circular measures suchin 2021. In 2023, it remained at 91%, the same level as waste prevented, reuse and other recovery, but excludes waste delivered to landfill and incineration (with and without energy recovery) due to regulatory requirements. The Circular Material Management percentage was 91% in 2022, compared to 87% in 2021.2022.
Our Zero Waste to Landfill KPI excludes one-time-only waste and waste delivered to landfill due to regulatory requirements. According to this definition, in 20222023 we reported 1 tonne2.7 tonnes of waste sent to landfill, a significant reductionsmall increase compared to 19 tonnes1 tonne in 2021.2022. All our 23 industrial sites achieved Zero Waste to Landfill status at the end of 2022.2023.
Philips Group
Total waste by composition
in tonnes
| Waste generated | Waste diverted from disposal | Waste directed to disposal | |
|---|---|---|---|
| Wood | 4,413 | 4,356 | 57 |
| Paper/cardboard | 4,122 | 4,117 | 5 |
| Metal scrap | 3,490 | 3,440 | 49 |
| Plastic waste | 2,891 | 2,533 | 358 |
| General waste | 2,308 | 1,266 | 1,042 |
| Demolition scrap | 2,216 | 2,163 | 53 |
| Chemical waste | 2,117 | 1,570 | 547 |
| Other | 1,245 | 961 | 285 |
| Waste generated | Waste diverted from disposal | Waste directed to disposal | |
|---|---|---|---|
| Wood waste | 4,140 | 4,104 | 36 |
| Paper/cardboard waste | 3,527 | 3,522 | 5 |
| Metal waste | 3,338 | 3,291 | 48 |
| Plastic waste | 2,381 | 2,237 | 144 |
| Municipal (mixed) waste | 2,136 | 1,156 | 980 |
| Chemical waste | 2,020 | 1,532 | 487 |
| Electrical and electronic waste | 626 | 622 | 4 |
| Other | 1,208 | 983 | 225 |
8.4.18.3.1Improving people’s lives
Lack of access to affordable, quality care is one of the most pressing issues of our time. Climate change is exacerbating this situation and putting the lives of millions of people at risk. At Philips, we are conscious of our responsibilities towards society and the planet. It is our purpose to improve people’s health and well-being through meaningful innovation. As such, we aim to improve the lives of 2.5 billion people a year by 2030. To ensure we remain on track to achieve this goal, we have developed an integrated approach that tells us how many lives have been improved by our products and solutions in a given year. We call this our Lives Improved model.
The Lives Improved model helps us to track our performance on a country-to-countrycountry-by-country basis in line with UN Sustainable Development Goal 3, allowing us to shape strategies to ensure healthy lives and promote well-being for all at all ages.
In 2022,2023, Philips improved 1.811.88 billion lives, an increase of around 13567 million compared to 2021.2022. This increase was driven by a steady growth ofacross all segments, improved statistics and the inclusionaddition of our Picture Archivingthe Ambulatory Monitoring & Diagnostics (AM&D) and Communication System (PACS) productsClinical Data Services (CDS) business units in the Lives Improved model. PACS is an image-management software within our Enterprise Diagnostic Informatics business. From a marketzone perspective, we saw significant growth mainly in Latin America, North America, Asia Pacific, Iberia,Japan, Indian Subcontinent, Middle East & Turkey, and Africa.
Philips believes that improving access to healthcare requires meaningful innovation. It also requires a deep understanding of the relationship between all stakeholders and their specific needs in underserved communities to truly make a difference and help improve access to healthcare.communities. We have an additional commitment to improve the lives of 300 million people in underserved communities with our health-related products by 2025, rising to 400 million by 2030. This commitment allows us to increase our focus on those populations where we can make a positive impact by providing access to effective and affordable healthcare for those in greatest need. By combining the strengths of Philips, Philips Ventures, Philips Foundation, and its partners, we can provide better healthcare and improve health outcomes for all. In 2022,2023, our health-related solutions improved the lives of 202221 million people in underserved marketscommunities (an increase of 35some 20 million compared to 2021)2022).
For more information, please refer to our Lives Improved methodology document.
Lives Improved per marketregion/zone
The following table shows the number of Lives Improved per market.region/zone.
Philips Group
Lives improved per marketregion/zone
| Market | Lives Improved (million)1) | Population (million)2) | Saturation rate (as % of population) | |||
|---|---|---|---|---|---|---|
| Africa | 29 | 1,340 | 2% | |||
| ASEAN & Pacific | 125 | 976 | 13% | |||
| Lives Improved (million) | Population (million) | Saturation rate (as % of population) | ||||
| APAC | 132 | 1,023 | 13% | |||
| Benelux | 26 | 30 | 87% | 26 | 30 | 86% |
| Central & Eastern Europe | 79 | 164 | 48% | |||
| Germany, Austria & Switzerland | 84 | 101 | 83% | |||
| Central Eastern Europe | 79 | 166 | 48% | |||
| DACH | 87 | 101 | 86% | |||
| France | 44 | 68 | 64% | 46 | 69 | 66% |
| Greater China | 496 | 1,442 | 34% | 506 | 1,442 | 35% |
| Iberia | 47 | 58 | 81% | 48 | 58 | 83% |
| IIG | 47 | 81 | 58% | |||
| Indian Subcontinent | 92 | 1,610 | 6% | 99 | 1,628 | 6% |
| Italy, Israel & Greece | 47 | 81 | 58% | |||
| Japan | 48 | 125 | 38% | 49 | 125 | 39% |
| Latin America | 158 | 654 | 24% | |||
| Middle East & Turkey | 72 | 378 | 19% | |||
| Latam | 169 | 650 | 26% | |||
| META | 113 | 1,763 | 6% | |||
| Nordics | 19 | 28 | 68% | 21 | 28 | 74% |
| North America | 360 | 369 | 98% | 363 | 372 | 98% |
| Russia & Central Asia | 50 | 252 | 20% | |||
| Russia, Central Asia | 52 | 253 | 21% | |||
| UK & Ireland | 41 | 73 | 56% | 42 | 73 | 58% |
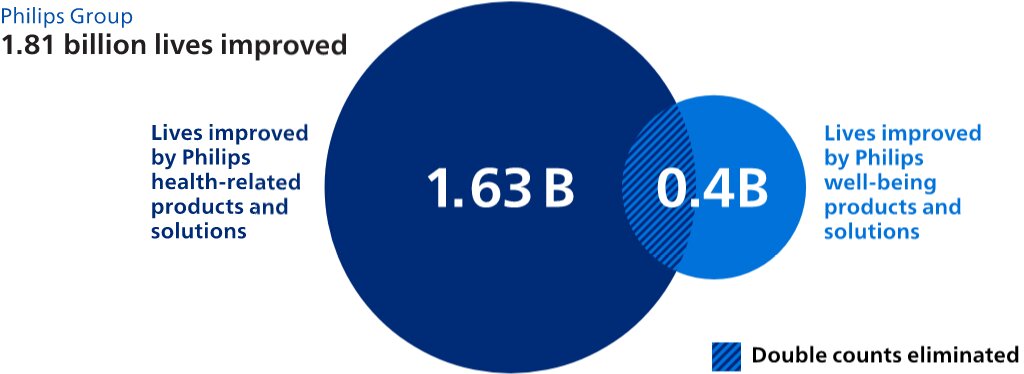

8.4.28.3.2Our organization, people and culture
2023 has been a year of change, focused on building the foundation to deliver greater impact in all we do by addressing challenges and shifting to our new operating model, whilst continuing to deliver for patients, customers and consumers. We laid the groundwork for our updated People strategy and the key pillars of growing our people, igniting our culture, simplifying how we work, and bringing oxygen to the organization through simple adaptive people processes and a focus on well-being and inclusion.
Aligned with these pillars, we continued driving the broader Talent agenda, with a strong focus on Executive Committee successor identification and development. This resulted in two internal executive appointments, our new Chief People Officer and Market Leader North America, and the appointment of two critical external executive female talents, Chief Business Leader Monitoring & Connected Care and Chief Medical Officer, further strengthening our MedTech expertise. We continued to support the development of our internal talents resulting in 33% internal mobility (vs. target 30%) and further improved our diversity ambitions with over 31% women in senior leadership. Overall, employee turnover is slightly up to 17.6% from 2022 (17.5%) and top talent has seen an increase to 12.3% from 10.2% in 2022.
In 2023, we began reinvigorating our culture using a phased approach to emphasize action over words, laying the groundwork for scaling this work in 2024. This included working on our culture through our most pressing challenges with the Philips Leadership Team and how to understand our disabling patterns as an organization so that we can begin to disrupt them. Deeper culture work was started in the businesses with specific needs, and preparations to ignite, embed and embody our culture at scale are under way. This culture work will run in parallel with our priority of scaling development for all 7,500 people leaders. Building on 2023 progress, we will enable leaders to model the skills and behaviors that align with our refreshed culture and position us to deliver results through our teams.
As a result of the shift in the operating model, we have made progress in simplifying how we work and are trending below the target headcount (69,656 actual vs. 72,295 target). Despite the amount of change in the organization, we saw an improvement in engagement scores between March and October 2023 by 5% to 73% indicating that the organization is starting to recover from the change, although the number is still below 2022 (78%) and benchmark levels.
In 2023, we gained momentum towards our strategy of becoming a more people-centric organization and we have put in place the foundations that will help us to deliver value with sustainable impact through our people.
Our culture
Our culture is crucial to meeting our goal of improving the lives of 2.5 billion people by 2030. The way we act and behave shapes our shared understanding of what is important and how we deliver. As a leader in health technology, we are on a journey to reinvigorate our culture so that we become even more people-focused, reduce complexity, and drive greater accountability to meet the needs of our patients, consumers, and customers more consistently.
We have launched a new global People strategy to accelerate this mission and significantly shape our organizational culture. This strategy rests on three pillars. Firstly, we simplify and enhance our core operations to empower employees to address pressing needs efficiently, all to prioritize patient safety and customer experience. Secondly, we focus on nurturing our workforce's skills and capabilities, adapting to the ever-evolving health technology industry. Thirdly, we instill a culture of inclusivity and belonging by integrating health, well-being, and diversity into our work practices, ensuring that every employee feels valued and connected to our shared mission. These pillars form the foundation of our talent ecosystem and reflect our commitment to fostering innovation and excellence on a global scale.
But our culture is more than words; it is also shaped by how our organization is structured. Our new operating model, implemented in 2023, defines how we work in a regulated environment to safely develop innovations that improve people's health and well-being responsibly and sustainably. We prioritize patient safety, quality, compliance, and integrity in everything we do. With this new structure, our culture-defining initiatives and programs are expected to have a greater impact as we focus on consistency and tangible action.
For example, in October we held a global Timeout for Patient Safety and Quality, where our entire company took time to reflect on how our daily work affects patient safety and quality, where we can commit to taking personal and collective responsibility, and how to create a culture centered on patients and people. Initiatives like this, along with targeted learning activities like our Clinical and Medical Learning Hub, which provides world-class clinical training and resources, contribute to making our culture ‘real’ and deliver a sense of pride for our people.
Considering the ever-changing economic, political, and health environment, we also remain flexible in our approach to how we work. We continue to adapt our hybrid model for more flexibility and collaboration, with a stronger focus on policies and programs that prioritize people and are tech-enabled for easier access and efficiency. This means we continue to:
- Embrace flexibility, making innovative choices about how and where we work, giving employees more autonomy.
- Be at our best, investing in and growing our Talent through on-going learning and development.
- Collaborate impactfully, simplifying how we work to bring additional efficiency into the organization and create opportunities to come together, build strong teams, and solve problems together.
This comprehensive and people-centric strategy – combined with our operating model and targeted interventions – is designed to transform Philips into an even more agile, high-performing organization with a thriving culture at the core.
8.3.3Workforce of the Future
In 2022, transformingThrough 2023, our organizationStrategic Priority recruitment team continued to focus on delivery of the roles most critical to the delivery of our strategy. Together, the Strategic Priority team delivered 1,427 hires within R&D, Q&R and Clinical roles at Corporate Grade 70 and above, and all Informatics roles. To further enhance our workforce forof the future, remainedTalent Acquisition delivered 20.2% of external candidates with MedTech experience.
Early Career Talent
Our Philips-wide Graduate Development Program (GDP) continues to perform well and increased from 40 participants in 2021 to 240 in 2023. The GDP lasts two years and includes two job rotations, as well as offering the graduates a key pillar of our People strategy. We are operating in a fast-changing landscape and adapting to changes in the nature of work accelerated by the pandemic. Moreover, at the end of 2022, a company-wide change initiative was launched. This requires us to continuously evolve capabilities in support of our business transformation. Our focus on the Workforce of the Future helps us attract, onboard, develop and retain a workforce that is fit for today and future with the skills and capabilities to successfully deliver on our strategic imperatives.
We staff our positions based on assessed behavior, potential and capabilities. In 2022, we filled 71% of our Director-level and more senior positions from within the company. We ensure our candidates are high performers with strong potential – more than 69% of all internal vacancies were filled by appointing top performers. We supplement this internal growth with targeted external hiring, bringing in employees with the behaviors and capabilities we require for our Workforce of the Future.
Strategic Capability Building
We apply an enterprise-wide Strategic Workforce Planning approach, which all businesses, markets and functions adopt as part of the strategic planning cycle, to identify and develop the capabilities needed to realize our ambitions as a health technology company. This approach recognizes that capabilities are complex, with people, processes and systems being developed holistically. In 2022, we strengthened our focus on strategic priorities and top talent and used the lens of strategic enterprise capabilities to streamline our talent attraction, onboarding,comprehensive learning and development initiatives.track, and access to career centers to help guide future steps. Philips also gave meaningful work experience to 1,802 interns, offering 321 of them permanent employment after their internship.
Total Workforce Strategy
We continue our Total Workforce Strategy, which considers all sources of skills, capabilities, locations and changes in the labor market in order to deliver the Workforce of the Future. Our Right Shoring & Sourcing methodology is used to implement this strategy. This methodology steers improvements in workforce composition towards the ‘right shore’ (onshore, nearshore and offshore) and the ‘right source’ (employees, contingent workers and outsourced)external services). The program has delivered € 20EUR 11.4 million in savings in 2022.2023.
We continue workingIn 2023 we started with the Freelance Management System, which covers India,implementation of our external workforce strategy. In addition, we have been looking at how we are attracting contingent workforce talent. Direct sourcing has been expanded to 32% in the Netherlands, Germanyto 14% in the United States, and has been rolled out to India. To strengthen the USA. By advertising opportunities for freelancers onway we directly source contingent workforce talent, our own career site alongside employee jobs, in 2022 we filled 48% of all our freelancer roles without havingPhilips employer value proposition is utilized for the different Functions to go through staffing agencies.
Our Philips-wide Graduate Development Program (GDP) continues to perform well and has increased from 40 participants in 2021 to 285 in 2022. The GDP lasts two years and includes three job rotations, as well as offering the graduates a comprehensive learning and development track and access to career centers to help guide future steps. We continue focusing on campus hiring, with 901 campus hires in 2022. Philips also offered meaningful work experience to 1,822 interns in 2022, and they formed a critical source of our graduate hires – with 55% of all graduate hires having been an intern with us prior.attract these contingent workers.
8.4.38.3.4Diversity, Inclusion & Diversityand Well-Being
As a health technology leader, we attach great importance to the health and well-being of our workforce and to creating an environment of inclusion and belonging, where all employees feel psychologically safe. Our company’s success depends on our employees feeling valued, respected, and empowered to contribute fully. We are a diverse team made up of some 77,000approximately 70,000 individuals across over 100 countries, all with different backgrounds, perspectives, and experiences. We fully value and leverage these differences to ensure that creativity and innovation can flourish. Philips’ commitment towards Inclusion & Diversity is reflected in our General Business Principles and the company-wide Inclusion & Diversity Policy and Fair Employment Policy.
Representation
We continue to put in place measures to enhance representation of diverse talent at all levels within the organization, and to ensure that representation at senior management levels reflects the diversity of our stakeholders, including consumers, our customers and their patients.
To this end, in 2022, Philips restated its commitment to having 35% of senior management positions held by women, by the end of 2025. Senior management positions (including senior directors and executives) amount to approximately 1,300 employees.1,155 employees, 363 females (31.4%), 792 males (68.6%) at the end of 2023. As of year-end 2022,2023, we had reached our initial goal (set in 2020) of a 30% representation of women in senior management.
Our Supervisory Board has adopted the Diversity Policy for the Supervisory Board, Board of Management and Executive Committee, which also includes the Supervisory Board’s aim that at least one-third of the members of each of the Board of Management and the Executive Committee are women and at least one-third are men. For more information on the Diversity Policy, please refer to Report of the Corporate Governance and Nomination & Selection Committee.
At year-end, none of the three members of the Board of Management were women, and tworemained male, with three out of the other nine10 members of the Executive Committee were women. Thesebeing female. The Executive Committee numbers reflect a slight declineincrease compared to previous years (2021: 32022, where there were two women out of 13; 2020: 3 out of 15),twelve in total, pending expected announcements of new leaders. The company generally seeks to fill vacancies by considering candidates that bring a diversity of (amongst others) gender, and it is noted that thegender. The selection of candidates is based on merit and there have been and may be pragmatic reasons – such as other relevant selection criteria and the availability of suitable candidates – that have impacted the achievement of our gender diversity goals.
Long-term Inclusion & Diversity ambitions are embedded in our People strategy. In our ongoing effort to increase transparency and accountability, we are sharingshare data on the representation of women throughout our businesses, marketsBusinesses, Regions and functions,Functions, including a monthly review with the Executive Committee. We closely monitor the inflow, advancement and outflow of talent, which makes it possible to customize goals and intervene where appropriate. We continue variousimportant initiatives aroundthat address unconscious bias, health and well-being, inclusion and development of underrepresented talent.
Philips Group
Generation diversity
in %
| 2023 | |
|---|---|
| Generation Z (1997 and onwards) | 11% |
| Generation Y / Millennials (1981 - 1996) | 48% |
| Generation X (1965 - 1980) | 33% |
| Baby Boomers (1946 - 1964) | 8% |
Philips Group
Employees by age group
| Employee age group | Under 30 | 30 to 50 | Above 50 | Total |
|---|---|---|---|---|
| % | 18 | 59 | 23 | 100 |
At Philips, we remain committed to a multi-generational workforce, as the presence of generational diversity is a key factor to foster creativity, productivity and innovation. The different life experiences of a multi-generational workforce support collaborative decision making and the inclusion of different beliefs and points of view.
Global Diversity Council
Our Global Diversity Council is comprised of 10 senior leaders representing our businesses, marketsBusinesses, Regions and functions.Functions. The Council provides governance and oversight on diversity efforts, promotes company-wide behavior change, and communicates on progress. Additionally, every Council member is an Executive Sponsor to one of our Employee Resource Groups.
Employee Resource Groups
Since 2016, Employee Resource Groups (ERGs) provide an inclusive space for employees to support and care for one another, develop skills, experience meaningful cultural connections, expand their knowledge, all while strengthening relationships among the Philips community.
Philips currently has 1311 ERGs globally, with over 7,00010,000 employees participating: Able & Allies; Asian Employee Resource Group; Black Employee Resource Group; #BeTheChange Network; Caregivers Network; Future Leaders and Rising Employees; Latinx Employee Resource Group; Middle Eastern Employee Resource Group; Philips Empowering Parents; Philips Women Lead; Pride Network; Veterans and Family Coalition; and Neurodiversity Network.
Health & Well-being
In 2022,2023, we embeddedevolved our (mental) health and well-being framework further acrossto incorporate two additional pillars of well-being – career and environmental – enhancing our businesses, marketsholistic approach and functions.integrating global and local programs. We continued to address mental health by further rolling out the Employee Assistance Program (EAP), extending the service to a further 25 countries, including crisis support for Ukraine and Poland..
We grew our Mental Health Champion program to 180250 Champions across the globe, providing accredited training for peer-to-peer confidential support. We developed Compassionate leadership training for all our people managers, as well as encouraging dialogues around self-care, building trust and resilience. We also encouraged leader-led dialogues on mental health, to remove stigmalaunched a Manager Mental Health toolkit, complemented by training sessions.
Along with International Women’s Day and help engender a sense of psychological safety.
Our efforts culminated inPRIDE, Philips also recognized World Health Day and World Mental Health Day, with a variety of virtual mental well-being sessions and self-care tipsresilience practices that engaged employees from across our markets.Regions. In collaboration with Philips University, the Philips Energy Management well-being program was further extended across the organization.
Building
Capability
In 2022,2023, we deployed bias training to all recruiters, continued the deployment of Unconscious Bias traininglearning journey for employees with a focus on emotional wellness, and launched a learning series for all employees called Learn With Us, where we featured Diversity, Inclusion and Well-being best practices from across the organization while focusing new content on Allyship, Resilience and Psychological Safety. In North America, we launched four mandatory e-learnings, reaching our 20,000 employees in this market.globe.
External awards
Many stakeholders, including customers and potential partners and employees, view third-party assessments as objective indications of how well we are demonstrating the strength of our commitment. Awards received in 2022 included:2023 included Forbes Best Employers for Women;Women, Forbes Best Place to WorkEmployers for Diversity, and Forbes Best Employers in America; Forbes World Best Employers; and 100% Human Rights Campaign’s Corporate Equality Index.Texas.
8.4.4Our culture
Culture is foundational to achieving our strategic ambitions. Our behaviors create a shared understanding of how we all need to act in order to live up to our purpose of improving the lives of people around the world. All Philips employees are expected to commit to living our behaviors – customers first, patient safety, quality and integrity always, team up to win, take ownership to deliver fast, and be eager to improve and inspire – every step of the way. As we evolve our culture, we will drive patient- and people-centricity, accountability and empowerment, transparency and execution rigor in order to become an industry-leading player in HealthTech.
External partnerships
As we continue strengtheningto focus on integrating inclusion, belonging and equity into the employee experience, we see value in partnering with diverse professional network organizations and job boards to sharpen our position as a focused leader in health technology, leading with open, respectfulfocus on the development and caring communication is critical. We foster a culture within Philips that will help us achieve operational excellence and extend our solutions capability to address our customers’ unmet needs. Patient safety and quality are at the heartretention of our purpose. To further strengtheninternal diverse talent and increasing representation of diverse talent across our patient- and people-centric culture,organization. In 2023, we launched in 2022renewed a company-wide ‘Accelerating Patient Safety & Quality’ culture program. We also foster an inclusive and psychologically safe environment where our people feel valued for who they are and for their contributions. We do this through our rich Well-being offering, as well as a ‘Speak Up!’ campaign in 2022. As a health technology leader, the health and well-being of our people is imperative for success.
In the wake of the evolving external economic, geopolitical, and global health situation, we remain flexible in our ways of working, making use of learnings developed through the COVID-19 pandemic. We have embraced a hybrid working model that offers greater flexibility and improved collaboration across teams. Our new ways of working are defined by three goals:
Embracing flexibility: Making innovative choices for how and where to work, allowing more autonomy for our employees.Being at our best: Caring for ourselves, each other and our customers, patients and consumers. This means prioritizing our own well-being, as well as making time for personal growth and development.Impactful collaboration: Creating moments to come together, supporting employees’ sense of connection and belonging, so we can build strong teams, generate ideas and solve problems.
All of the above underpins how we lead, engage, hire and develop our employees. We have been focusing on well-being, deepening our leadership asks into the organization and supporting our culture shift as a leading innovative, customer-focused health technology company.
We are building an organization that is fit for today and the futurepartnership with the skillsNational Black MBA Association and capabilities needed to successfully deliver on our strategic imperatives. We attract, onboardintroduced partnerships with the National Sales Network, Circa and retain the best talent to accelerate our business transformation.Mogul.
8.4.58.3.5Employee engagement
We continue to keep a close pulse on our employee sentiment through our quarterlybi-annual Employee Engagement Survey. Amidst the changes across the company, our Employee Engagement Survey (EES) saw an extremely high response rate of 73%, which means that almost 50,000 employees participated.
In 2022,2023, average employee engagement scores remained high at 77% in line withdecreased to 73% – lower than the Fortune 500 benchmark. However thereThe decrease in employee engagement scores, specifically in the first half of the year, did not come as a surprise, as we announced employee reductions and organizational changes in January and many of our employees were (pre-) informed of how they would be impacted. There was a declinesignificant improvement (5 percentage points) in overall engagement levelsthe EES scores in the second half of 2022. This feedback does not comethe year, as a surprise given the recent challenges thatemployees settled within the company has encountered and the announcement of productivity measures.new organization structure.
Philips Group
Employee Engagement indexIndex
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Favorable | 79% | 77% | 79% | 77% | 73% | |
| Neutral | 14% | 15% | 14% | 15% | 17% | |
| Unfavorable | 7% | 8% | 7% | 8% | 10% | |
In a challenging businesschanging environment, we listened actively to our employees to provide them with greater clarity on future direction and enable them to proactively deal with change to meet our customer and patient needs. In 2023, we introduced specific sessions around Patient Safety and Quality. We saw that our employees feel comfortable reporting a patient safety or quality concern, while some employees feel that we can learn more from reviewing quality and regulatory events, and that there is an opportunity to improve training and recognition on these topics. This is critical given the culture of people- and patient-centricity that we aspire to.
Using the Customer Experience Index, we look at how well employees think we orient ourselves tofocus on customer needs. These inputs are actively exchanged with the customer experience team to design and work on related programs.Customer Experience team.
Our employee engagement is primarily driven by how prouda clear understanding of our customer needs and delivering on commitments that we make to each other. The results of the EES indicate that employees feel to work for Philips, as well as feeling that they can be themselves and have trusting relationships at work. Another significant factor driving engagement is our high scores on the Inclusion & Diversity index, which staysremains above the Industry Benchmark.industry benchmark.
8.4.68.3.6Employment
The total number of Philips Group employees was 69,656 at the end of 2023, compared to 77,233 at the end of 2022, compared to 78,189 at the end of 2021, a decrease of 956 FTE.7,577 FTEs.
TogetherOn January 30, 2023, we launched a multi-year plan to create value with the announcementsustainable impact. Part of this plan is to improve performance and simplify our Q3 results in October 2022, we hadway of working to takeimprove our agility and productivity. This includes the difficult, decision to reduce our workforce by approximately 4,000 roles globally. This was followed in January 2023 by the announcement of a furtherbut necessary reduction of our workforce by an additionalaround 6,000 roles globally.globally by 2025. This is in addition to the 4,000 reductions announced in 2022. As we gowent through this change, we dodid it with the utmost care and respect for our people, with a strong focus on supporting themthose who were directly impacted by the reductions in finding a new role.
Subject to local country legislation, our support offers include:
- Social Plan or respective severance policy
- Outplacement services and support through our Employee Assistance
programProgram - Work placement agency, where applicable, for
anEmployment-to-Employment support - Redeployment – where possible – as applicable by local legislation and in the context of the hiring restrictions
Philips Group
Employees per segment
in FTEs at year-end
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 32,193 | 32,390 | 32,904 | 29,094 | 30,335 | 28,397 |
| Connected Care | 15,866 | 17,751 | 16,673 | 21,047 | 19,241 | 17,549 |
| Personal Health | 10,253 | 10,134 | 9,319 | 10,134 | 9,319 | 9,085 |
| Other | 16,689 | 17,913 | 18,337 | 17,913 | 18,337 | 14,626 |
| Philips Group | 75,001 | 78,189 | 77,233 | 78,189 | 77,233 | 69,656 |
Philips Group
Employment
in FTEs
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Balance as of January 1 | 73,311 | 75,001 | 78,189 | 75,001 | 78,189 | 77,233 |
| Consolidation changes: | ||||||
| Acquisitions | 72 | 2,594 | 87 | 2,594 | 87 | 27 |
| Divestments | (744) | (33) | (744) | (33) | (353) | |
| Other changes | 1,618 | 1,338 | (1,010) | 1,338 | (1,010) | (7,251) |
| Balance as of December 31 | 75,001 | 78,189 | 77,233 | 78,189 | 77,233 | 69,656 |
Geographic footprint
Approximately 56% (2022: 58% (2021: 59%) of the Philips workforce is located in matureMature geographies and 44% (2022: 42% (2021: 41%) in growthGrowth geographies. In 2022,2023, the number of employees in matureMature geographies decreased by 1,774.5,392. The number of employees in growthGrowth geographies increaseddecreased by 819.2,184.
Philips Group
Employees per geographic clusterarea
in FTEs at year-end
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Western Europe | 19,925 | 19,775 | 19,297 | 19,775 | 19,297 | 16,900 |
| North America | 21,118 | 21,807 | 20,618 | 21,807 | 20,618 | 18,094 |
| Other mature geographies | 4,664 | 4,683 | 4,576 | 4,683 | 4,576 | 4,105 |
| Mature geographies | 45,707 | 46,265 | 44,491 | 46,265 | 44,491 | 39,099 |
| Growth geographies | 29,294 | 31,923 | 32,742 | 31,923 | 32,742 | 30,558 |
| Philips Group | 75,001 | 78,189 | 77,233 | 78,189 | 77,233 | 69,656 |
Employee turnover
In 2022,2023, employee turnover amounted to 17.5%17.6%, of which 11.1%9.5% was voluntary, compared to 17.6% (10.0%17.5% (11.1% voluntary) in 2021.2022. External benchmarks show that our voluntary employee turnover remains in line with similar-sized companies, and that we are reasonably successful in retaining our employees.
Philips Group
Employee turnover 2023
in number of employees
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 2,935 | 2,261 | 237 | 19 | 5,452 |
| I choose not to self-identify | 1 | 1 | |||
| Male | 2,583 | 3,676 | 527 | 50 | 6,836 |
| Philips Group | 5,518 | 5,938 | 764 | 69 | 12,289 |
2022Philips Group
Employee turnover 2023
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 23.2% | 16.7% | 14.6% | 22.4% | 19.6% |
| Male | 19.5% | 14.5% | 14.5% | 17.5% | 16.2% |
| Philips Group | 21.3% | 15.3% | 14.5% | 18.7% | 17.5% |
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 23.1% | 16.8% | 17.9% | 26.8% | 19.7% |
| I choose not to self-identify | 6.3% | 3.9% | |||
| Male | 18.7% | 14.5% | 18.3% | 23.4% | 16.1% |
| Philips Group | 20.8% | 15.2% | 18.1% | 24.3% | 17.6% |
Philips Group
Voluntary turnover 2023
2022
| Staff | Professionals | Management | Executives | Total | Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 12.9% | 11.8% | 9.2% | 11.8% | 12.2% | 11.9% | 9.6% | 7.5% | 9.9% | 10.6% |
| I choose not to self-identify | 6.3% | 3.9% | ||||||||
| Male | 11.8% | 9.9% | 8.2% | 7.1% | 10.4% | 11.1% | 7.8% | 7.1% | 6.1% | 8.8% |
| Philips Group | 12.3% | 10.5% | 8.5% | 8.2% | 11.1% | 11.5% | 8.4% | 7.2% | 7.0% | 9.5% |
The rate of employee turnover reported, is calculated in headcount as a monthly average across the reporting period.
8.4.78.3.7Equal opportunities and equal pay
As a company, Philips is committed to ensuringremunerating equal pay for equal work. In the Netherlands,We ensure that all employees are compensated fairly and without inequity based on gender, race, or any other characteristic protected by law.
A pay equity review is a crucial process that reflects our commitment to fostering fairness, equality, and transparency within our organization. Annually, Philips was certifiedconducts a comprehensive analysis of its compensation structure to ensure that all employees are remunerated fairly for Gender Equality by Economic Dividends for Gender Equality (EDGE) in 2021.their skills, experience, and contributions, regardless of human characteristics that can lead to potential biases. The study did not find asystematic measurement of gender pay gapgaps within Philips is an integral aspect of our operational practices. This commitment ensures that exceedswe not only provide equal opportunities, but also uphold fairness in compensation for the threshold as setwork undertaken by EDGE. Philips continuesour diverse workforce. This dedication to study gender pay parity using EDGE methodology. Manytransparency and proactive correction reflects our overarching commitment to fostering an environment of equality and inclusivity within the company.
In alignment with our global ethos, many countries in which Philips operates have already undertakenembraced the practice of regular pay equity reviews for example inover several years. Notable examples include Australia, UK,the United Kingdom, Sweden, India, and certain US states. Instates within the US,United States. This demonstrates our unwavering dedication to upholding the principles of equity and inclusivity on a global scale. When an unexplained gap is brought to light, immediate actions are taken to rectify the situation. Furthermore, Philips will be executingis committed to understanding the underlying factors contributing to these unexplained gaps, and formulating proactive measures to prevent such occurrences in the future.
Specifically focusing on the United States, Philips embarked on a significant initiative in the form of a company-wide Pay Equity & Transparency Project starting in 2022 and culminating in 2023. This project builds upon the successful groundwork laid during 2023, originally scheduledpay equity reviews at the state level in the US. The objective has been to create a comprehensive and standardized approach to pay equity and pay transparency, ensuring that all employees across the US are remunerated fairly, in accordance with their skills and responsibilities. All US employees have the right to request pay transparency and understand their position against peers, which is over and above the current legislative requirements.
The Pay Equity Project is a testament to our commitment to not only meeting but exceeding legal and regulatory requirements. By proactively addressing pay equity concerns, we aim to create an inclusive work environment that fosters diversity and promotes equal opportunities for 2022, building on work completed at US state level.everyone within the organization. This initiative reinforces our belief that fair compensation is not only a legal obligation but a fundamental ethical responsibility that contributes to the overall success and sustainability of our company.
Through this proactive approach, we strive to set an industry standard for pay equity, demonstrating that a commitment to fairness and equality is not only beneficial for our employees but also the long-term success and reputation of Philips as a global leader in innovation and healthcare solutions.
8.4.88.3.8Living wage
Philips can only achieve its aim to improve the lives of 2.5 billion people per year by 2030 if we support and empower our people, so they can be their best and perform effectively. To this end, we conducted a living wage analysis for the fourth year in a row on the lowest salaries in every country in which we currently operate.
The living wage is a concept defined by Anker and Anker (2017) as “Remuneration received by a worker in a particular place sufficient to afford a decent standard of living for the worker and her or his family. Elements of a decent standard of living include food, water, housing, education, healthcare, transport, clothing, and other essential needs, including provision for unexpected events”. We combined forces with Valuing Nature, several local NGOs, WageIndicator and other global corporates to develop living wage standards that are complete and have a reliable geographical scope.
Based on the living wageLiving Wage analysis conducted in 2022,2023, all Philips employees received wages and benefits that are consistent with at least the minimum Living Wage standard for an individual. Furthermore, 99%approximately 97% of Philips employees received wages and benefits that are consistent with at least the minimum Living Wage standard for a family (based on reference data and guidance from WageIndicator)Wage Indicator). Assuming no significant changes in reference data, it is expected that the wages of the 1% of employees currently below the family standard will be within that standard in the course of 2023.
8.4.98.3.9Health and Safety
In 2022,2023, the safety of our employees remained paramount. However, asAs the COVID-19 pandemic enteredcontinued the endemic phase, the centralized controls put in place during the pandemic were relaxed in line with local governments’ advice. Control was gradually returned from the Group Crisis Operations Team to the local Crisis Management Teams. As Philips started to resumeresumed normal operations, office occupancy started to rise, and business travel restarted. However, critical control measures were maintained, including maintaining safety stocks of PPE, and the internal website containing guidance was updated regularly. Campaigns and advice concerning the importance of vaccinations was promoted widely.maintained. Philips has emerged from the pandemic with a good record of management and control that restricted the impact of the pandemic on employees and the wider business operations.
At Philips, we strive for an injury-free and illness-free work environment. Since 2016, the Total Recordable Cases (TRC) rate has been defined as a Key Performance Indicator (KPI). A TRC is a case where an injured employee is unable to work for one or more days, has medical treatment, or sustains an industrial illness. We set yearly TRC targets for the company, businesses and industrial sites.
We recorded 172 TRCs in 2022, a 19% decrease compared to 2132023, the same as in 2021.2022. While our workforce continued to expanddecreased in 2022,2023, the TRC rate decreasedincreased from 0.290.23 per hundred FTEs in 20212022 to 0.230.24 in 2022.2023.
In 20222023 we recorded 8190 Lost Workday Injury Cases (LWIC). These are occupational injury cases where an injured person is unable to work for one or more days after the injury. This represents a 29% decrease9% increase compared with 11481 in 2021.2022. The LWIC rate decreasedincreased to 0.110.12 per 100 FTEs in 2022,2023, compared with 0.160.11 in 2021.2022. The number of Lost Workdays caused by injuries decreased by 2161,471 days (5%(37%) to 4,0202,549 days in 2022.2023.
8.4.108.3.10Human rights
Philips strongly believes that companies have both the responsibility to respect human rights and the ability to protect them. Philips’ Human Rights Policy, General Business Principles, Supplier Sustainability Declaration and other relevant policies detail how Philips respects human rights, in line with the International Bill of Human Rights and the International Labor Organization’s Declaration on Fundamental Principles and Rights at Work.
In this regard, Philips follows the guidance given in the UN Guiding Principles on Business and Human Rights and the Organization for Economic Co-operation and Development (OECD) Guidelines for Multinational Enterprises. Philips has also been a signatory to the UN Global Compact since 2007. Philips’ Board of Management is responsible for strategy and oversight of all company activities across the three ESG dimensions, including human rights. The Board also monitors progress and takes corrective action where needed.
In addition, a cross-functional project team, comprised of a Human Rights Manager and professionals from several business functions, is in place to drive several human rights initiatives. The project team is overseen by the Human Rights Steering Committee, consisting of senior leaders from Operations, Legal, Human Resources and Sustainability.
In 2023, we continued to develop our due diligence strategy by conducting Human Rights Impact Assessments (HRIA). Philips conducted Human Rights Impact Assessments (HRIAs) at its sites in Pune (India) and Varginha (Brazil), living up to its commitment of conducting HRIAs at 100% of its at-risk sites by 2023. Philips intends to monitor the progress and findings from these sites and take them on a continuous improvement journey regarding human rights topics.
Although the Human Rights Impact Assessment of selected sites is primarily focused on Philips own operations, a derived deep-dive approach for certain suppliers has been rolled out since 2022. For 8 suppliers, a focused assessment on human rights was conducted, compared with the broader Supplier sustainability assessment approach which covers sustainability more holistically and is detailed in Supplier sustainability.
Our Human Rights Report contains detailed information regarding our progress, targets, and plans for continuous improvement.
8.3.11Supplier sustainability
Philips’ purpose to improve people’s health and well-being extends throughout our value chain. At Philips, we have a direct business relationship with approximately 4,900 product and component suppliers and 16,100 service providers. Our supply chain sustainability strategy is evaluated annually through a structured process, combined with multi-stakeholder dialogues. From this, we have developed multiple ESG programs aimed at driving sustainable improvement. These programs cover compliance with our policies, improvement of our suppliers’ sustainability performance, our approach towards responsible sourcing of minerals, and reducing the environmental impact of our supply base. Supplier engagement in these programs is driven by screening ESG opportunities and risks, evaluating materiality and impact along the lines of material, industry, and geographical characteristics.
Procurement and supplier information sessions are scheduled on an ongoing basis. During these sessions, our supplier ESG expectations are shared and clarified. Training courses are organized to support suppliers in meeting those expectations. In addition, suppliers are supported in improving their ESG performance via individual training. Where data is available, suppliers are informed on their performance compared to industry peers, and best practices are shared, and their adoption encouraged. During the sourcing process, supplier ESG indicators are evaluated. In addition to minimum requirements set out in our Code of Conduct, suppliers with a better ESG performance are considered favorably.
Supplier sustainability compliance
Two core policy documents form the basis of our supplier sustainability compliance approach: the Supplier Sustainability Declaration and the Regulated Substances List.
Supplier Sustainability Declaration (SSD)
The SSD sets out the standards and behaviors Philips requires from its suppliers. The SSD is based on the Responsible Business Alliance (RBA) Code of Conduct, in combination with several additional Philips-specific expected behaviors. The Code is in alignment with the UN Guiding Principles on Business and Human Rights and key international human rights standards, including the ILO Declaration on Fundamental Principles and Rights at Work and the UN Universal Declaration of Human Rights. It covers topics such as Labor, Health & Safety, Environment, Ethics, and Management Systems. The RBA is the world’s largest industry coalition dedicated to responsible business conduct in global supply chains. As a Regular member of the RBA, Philips is required to commit publicly to the RBA Code of Conduct and actively pursue conformance to the Code and its standards, which must be regarded as a total supply chain initiative.
Regulated Substances List (RSL)
The RSL specifies the chemical substances regulated by legislation. Suppliers are required to follow all the requirements stated in the RSL. Substances are marked as restricted or declarable.
All suppliers are required to commit to the SSD and RSL. Through integration of a Sustainability Agreement in our purchasing agreements, suppliers declare compliance to both the SSD and RSL. Upon request, they provide additional information and evidence.
Supplier Sustainability Performance (SSP) – 'Beyond Auditing'
In 2016, Philips first piloted its 'Beyond Auditing' approach to engage suppliers on ESG matters, with a focus on:
- a systematic approach to improve the sustainability of our supply chain through continuous improvement against a set of recognized and global references
- collaboration, increased transparency, clear commitments, and ensuring suppliers meet the agreed targets
- encouraging our suppliers, industry peers and cross-industry peers to adopt our approach
This systematic approach is shown in the figure below and is a high-level representation of the SSP program.

First, a set of references, international standards, and Philips requirements are used to develop the Frame of Reference, which covers management systems, environment, health & safety, business ethics, and human rights. For each, the maturity level of suppliers is identified in the Program Execution Wheel, which assesses suppliers against the Plan–Do–Check–Act (PDCA) cycle. Suppliers are then categorized through the Supplier Classification model, which differentiates on the basis of supplier maturity, resulting in supplier-specific proposals for improvement. The SSP process is monitored and adjusted through continuous feedback loops. The outcome of the SSP assessment is a supplier sustainability score ranging from 0 to 100. This score is based on supplier performance in environmental management, health & safety, business ethics, and human rights.
Supplier classification
Supplier selection for the program is based on significance. Significance of suppliers is determined through an assessment of the supplier’s associated ESG risks and opportunities, including material, industry, and geographical characteristics, as well as annual spend. In 2023, 152 of our suppliers were considered significant. After this initial assessment, the engagement strategy is tailored based on the suppliers’ current performance in terms of sustainability.
Philips Group
Significant suppliers - tier 1
| 2023 | |
|---|---|
| Number of suppliers | 152 |
| Spend as percentage of total | 20% |
There are four different engagement approaches: BiC (Best in Class), SSIP (Supplier Sustainability Improvement Plan), DIY (Do It Yourself) and PZT (Potential Zero Tolerance). The PZT status is a temporary status and requires immediate attention and action. Depending on the categorization, suppliers are engaged in different ways to improve their sustainability performance through agreed improvement plans.
If a (Potential) Zero Tolerance is identified, immediate action is taken. If the requested additional information and evidence lead to the conclusion that there is no structural Zero Tolerance, the supplier’s status will be changed and the supplier will go back to the original track in the program. If the conclusion gives rise to a structural Zero Tolerance, the supplier is required to:
- propose a corrective action plan to mitigate and/or resolve the identified Zero Tolerance(s)
- commit to structurally resolving the Zero Tolerance
- provide regular updates and evidence
- avoid quick-fixing
Philips defines six Zero Tolerances:
- Fake or falsified records
- Child and/or forced labor
- Immediate threats to the environment
- Immediate threats to worker health and safety
- Failure to comply with regulatory and/or Philips requirements
- Workers’ monthly income (covering salary for regular hours and overtime, tax deductions, social insurance) failing to meet regulatory requirements
For more details on the SSP process, refer to the SSP brochure.
Our 2023 results
In 2023, five zero tolerances were found across the following categories: health and safety, labor, and environmental impact. All five cases were successfully closed in 2023 after confirmation of completion of the corrective action plan. One zero tolerance, found in the last quarter of 2022, has also been closed during 2023.
Philips measures the impact of SSP engagements through the number of lives improved in the supply chain. This is derived from the improvements that suppliers make in their performance. To determine improvements, we calculate the pro rata change in performance from one year to the next.
Philips Group
Lives improved in the supply chain
in thousands of Lives
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Lives improved in the supply chain | 430 | 459 | 723 |
In 2023, the overall year-on-year improvement in performance was 37% for suppliers that received their first re-assessment in 2023. The number of employees impacted (first and second tier) suppliers participating in the SSP program was approximately 723,000. This figure includes suppliers assessed in the last three years, for which the supplier has communicated their number of employees via the self-assessment questionnaire, which was validated during the on-site assessment. For those workers, labor conditions improved, the risk of serious injury reduced, and the negative environmental impact of suppliers was brought down. This includes the workers at suppliers of the Domestic Appliances business, for which Philips continued the sustainability engagement. For a detailed break-down of percentage improvements realized by active suppliers in the past year, by comparing the assessment in 2023 to their previous assessment, refer to the following table.
Philips Group
SSP 2023 performance: pro-rata improvements
in %
| Topics | Policy | Procedures | Implementation | Management responsibility | Communication | Risk control | Target-setting & tracking | Corrective action approach | Supplier management |
|---|---|---|---|---|---|---|---|---|---|
| Environment | 2% | 7% | -2% | 10% | 2% | 23% | 15% | 10% | -8% |
| Health and Safety | 11% | 11% | 16% | 0% | 6% | 10% | 11% | 21% | 4% |
| Business Ethics | 11% | 20% | 9% | -4% | 26% | 21% | 33% | 26% | 1% |
| Human Capital | 13% | 13% | 19% | 11% | 7% | 4% | 10% | 12% | -2% |
Categories which showed the biggest improvement are:
- Risk control and Target setting and tracking of Environmental topics: improving the audit process to periodically assess conformance, including compliance with applicable laws and regulations pertaining to environmental topics, as well as the target setting and tracking on topics such as Greenhouse Gas Emissions.
- Implementation and Corrective action approach of Health & Safety topics: improving working conditions of workers in the value chain, including corrective action approaches where applicable.
- Business Ethics, especially in the domains of communication, target setting and corrective actions.
- Implementation for Human Capital: improving the approach to implement policies and procedures into formal records for the supplier's human capital system.
In 2023, 158 suppliers were added to the SSP program (compared to 47 added in 2022). Of the population of suppliers that entered the program in the years before 2023 and have been assessed at least once in the past three years, 392 suppliers were still active in 2023 (compared to 249 in 2022). The combined group represents 77% of our significant suppliers who are in the program.
As part of our commitment to improve the lives of 1 million workers in the supply chain by 2025, we increased our engagement with second-tier suppliers. By teaming up with Tier 1 suppliers in conducting the assessment, Philips has supported in building ESG supplier management skills. In 2023, 110 second-tier suppliers entered the program, resulting in a total number of 138 second-tier suppliers engaged with in the last three years.
Additional progress made in 2023
Philips is actively applying the latest insights in data science and machine learning methods to make the SSP program more efficient. Through the use of reference data collected over 1,600 assessments in the past years, Philips is working towards integrating maturity and improvement predictions in the program. This is expected to support us in determining the sustainability maturity of suppliers, while also increasing the effectiveness of our supplier improvement approach.
On an annual basis, Philips experts organize quality trainings in the sustainability area for suppliers in the scope of the SSP program.
Responsible sourcing of minerals
The supply chains for minerals are long and complex. Philips does not source minerals directly from mines as there are typically 7+ tiers between end-user companies like Philips and the mines where the minerals are extracted. The extraction of minerals can take place in conflict-affected and high-risk regions, where mining is often informal and unregulated, and carried out at artisanal small-scale mines (ASM). These ASMs are vulnerable to exploitation by armed groups and local traders. Within this context, there is an increased risk of severe human rights violations (forced labor, child labor or widespread sexual violence), unsafe working conditions or environmental concerns.
Philips addresses the complexities of minerals supply chains through a continuous due diligence process, combined with active participation in multi-stakeholder initiatives to promote the responsible sourcing of minerals.
Conflict minerals due diligence
Each year, Philips investigates its supply chain to identify smelters of tin, tantalum, tungsten and gold in its supply chain and we have committed to not purchasing raw materials, sub-assemblies, or supplies found to contain conflict minerals.
Philips applies collective cross-industry leverage through active engagement via the Responsible Minerals Initiative (RMI). RMI identifies smelters that can demonstrate, through an independent third-party audit, that the minerals they procure are conflict-free. In 2023, Philips continued to actively direct its supply chain towards these smelters.
The Philips Conflict Minerals Due Diligence framework, measures and outcomes are described in the Conflict Minerals Report that we file annually to the US Securities and Exchange Commission (SEC). The conflict minerals report is also publicly available on Philips’ website. Philips fully supports and complies with the OECD Due Diligence Guidance for Responsible Supply Chains of Minerals from Conflict-Affected and High-Risk Areas (OECD Guidance).
Each year, we work with our suppliers on the quality of their due diligence reporting by setting minimum criteria for the Conflict Minerals Reporting Templates (CMRT). For the 2023 Conflict Minerals Report, Philips significantly strengthened the acceptance criteria for CMRTs as it intensified the required due diligence performed by suppliers towards the use of smelters of high concern. In addition, we strive to reduce the number of non-identified smelters. As a result, the percentage of CMRTs that satisfied minimum acceptance criteria has decreased by 13 percentage points.
Philips Group
Conflict Minerals Due Diligence results
| Key performance indicator | 2021 | 2022 | 2023 |
|---|---|---|---|
| Response rate of suppliers (%) | 99% | 95% | 95% |
| CMRTs that reached minimum acceptance criteria (%) | 84% | 78% | 65% |
| Non-listed smelters in our supply chain (#) | 0 | 0 | 0 |

Cobalt
Philips has performed due diligence on cobalt since 2019. We use cobalt predominantly in lithium-ion batteries. As part of this initiative, we engaged suppliers that provide materials containing cobalt. In 2023, we again reached a 100% response rate (2022: 100%).
Multi-stakeholder initiatives for responsible sourcing of minerals
We believe that multi-stakeholder collaboration in the responsible sourcing of minerals is the most viable approach for addressing the complexities of minerals value chains.
European Partnership for Responsible Minerals (EPRM)
Philips is a founding partner of EPRM and has been a strategic member since its inception in May 2016. EPRM is a multi-stakeholder partnership between governments, companies, and civil society actors working toward more sustainable minerals supply chains. The goal of EPRM is to create better social and economic conditions for mine workers and local mining communities by increasing the number of mines that adopt responsible mining practices in Conflict-Affected and High-Risk Areas (CAHRAs).
EPRM is an accompanying measure to the EU Conflict Minerals Regulation dedicated to making real change ‘on the ground’. Through EPRM, Philips supports activities to improve responsible mining practices in mining areas in CAHRAs and shares our knowledge and practice in conducting due diligence. Since 2018, Philips has actively participated in several working groups focused on strengthening the responsible production of minerals, as well as improving responsible sourcing practices.
Supplier decarbonization program
Since 2003, Philips has looked at ways to improve the environmental performance of its suppliers. When it comes to climate change, we have adopted a multi-pronged approach: reducing the environmental impact of our products, committing to carbon neutrality in our own operations, and engaging with our supply chain to reduce their carbon footprint. Through initiatives such as the CDP supply chain program, Philips motivates its suppliers to disclose emissions, embed board responsibility on climate change, and actively work on reduction activities.
In October 2021, during COP26, Philips announced its target to have at least 50% of its suppliers (based on spend) committed to science-based targets for carbon reduction by 2025.
Philips Group
% of suppliers committed to science-based targets
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| % of suppliers committed to Science Based Targets | 28% | 41% | 46% |
We consider suppliers to have committed to science-based targets when this is communicated via the Science Based Targets initiative (SBTi), the suppliers' CDP disclosures, or public websites and announcements (on a 'Science Based Target', 'Net Zero Target', or equivalent). Multiple activities have been deployed to help us achieve this climate target. We consider spend to be relevant if it relates to product and component suppliers and relevant service providers, like logistics and information technology suppliers.
CDP engagement: Since 2011 we have been partnering with CDP Supply Chain, through which we invite suppliers to disclose their environmental performance and carbon intensity. In 2023, there was a response rate of 93% (2022: 85%). With 500 of our biggest suppliers included in the CDP engagement program in 2023, CDP confirmed Philips is in the top tier in terms of its supplier engagement coverage.
Of the group that responded, 60% engaged in emission-reduction initiatives (2022: 59%). In addition, 48% committed to carbon emission targets (2022: 47%). In the 2023 survey, our suppliers reported 14 million metric tonnes CO2 savings from improvement projects undertaken in 2023.
Philips Group
Supplier response rate to CDP questionnaire
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Supplier response rate to CDP questionnaire | 87% | 85% | 93% |
Data-driven insights: Through accurate data insights, Philips’ buyers are enabled to consider climate action in their supplier selection. In 2023, 46% of our purchases (in spend) were made at suppliers that have committed to science-based CO2 reduction targets.
Capability building: We support suppliers in advancing their company approach to climate action, offering guidance that is tailored to their climate action maturity. In 2023, we further grew the offering of tailored feedback and guidance for 80% of our suppliers to support their growth in capabilities and help improve their approach.
Opportunities for decarbonization: Through on-site assessments we identify energy efficiency opportunities that enable our suppliers to make cost-effective carbon reductions. Our team calculates for the supplier what the cost impact would be, and also the return. In 2023, 19 on-site assessments took place, which resulted in tailored plans for improvement.
8.3.12Total tax contribution
To fulfill our company purpose, a responsible tax approach is required. We fully acknowledge our societal role when it comes to paying taxes in the geographies where value is created. We consider our tax payments as a contribution to the communities in which we operate, and part of our social value creation.
Our Approach to Tax sets the standard for our conduct, by which individual employees, the company and its subsidiaries must abide. We consider tax in the context of the broader society, inspired by our stakeholder dialogues, global initiatives of the Organization for Economic Cooperation and Development and United Nations, human rights, international tax laws and regulations, and relevant codes of conduct.
Under the ultimate responsibility of the Board of Management, the Chief Financial Officer annually reviews, evaluates, approves and where necessary adjusts Philips’ approach to tax. Part of our approach is to acknowledge the importance of transparency in respect of our tax contributions. Philips supports and participates in transparency initiatives such as the Dow Jones Sustainability Index (DJSI) and the Tax Transparency Benchmark of the Dutch Association of Investors for Sustainable Development (VBDO). The Tax Transparency Benchmark is a study conducted by the VBDO on tax transparency practices among Dutch and European listed companies. The 2023 benchmark assessed the tax transparency practices of 51 Dutch companies and 65 listed companies from Belgium, Denmark, France, Germany, Italy, Spain, and Sweden. Philips scored the maximum achievable 40 points. The jury noted our comprehensive Country Activity and Tax Report 2022, which explicitly linked to the GRI 207 Tax Standard and included information on environmental and social factors. Furthermore, the jury commended Philips for the clear description of the role taxes play within its value creation model. In addition, Philips scored a top score (100 out of 100) in the Tax Strategy section of the 2023 Dow Jones Sustainability Index.
Since 2020, we have been providing certain voluntary disclosures about taxes paid and collected in the countries in which we operate. The 2023 Country Activity and Tax Report is published on our website, in addition to, and simultaneously with the disclosures on tax included in this Annual Report.
Philips has a Tax Control Framework in place that forms part of its standard set of Internal Controls over Financial Reporting (ICFR). Philips' tax position is therefore reflected in its financial statements and covered by the Board of Management's report on ICRF and its conclusion regarding the effectiveness thereof, as referred to in the section Risk management and internal control.
Philips also endorses the ambitions expressed in the Tax Governance Code published by Dutch employers' organization VNO-NCW. We comply with the principles prescribed in the Code, available at www.vno-ncw.nl/taxgovernancecode, and we have touched upon the elements of this code in our Country Activity and Tax Report.
In 2023, Philips contributed to the communities where we operate through taxes paid (e.g. corporate income tax) and taxes collected (e.g. VAT). Philips' total tax contribution in 2023, amounting to EUR 3,051 million, is presented by tax type in the following table. Please refer to our 2023 Country Activity and Tax Report for more details.
Philips Group
Total contribution 2023 per tax type
in millions of EUR
| Corporate income tax paid | Customs duties | VAT1) | Payroll tax | Other taxes | Total | |
|---|---|---|---|---|---|---|
| Western Europe | (17) | 9 | 206 | 844 | 66 | 1,107 |
| North America | (31) | 38 | 123 | 721 | 8 | 859 |
| Other mature geographies | 35 | 3 | 77 | 116 | 1 | 232 |
| Growth geographies | 65 | 77 | 332 | 340 | 40 | 853 |
| Philips Group | 52 | 127 | 737 | 2,021 | 115 | 3,051 |
8.3.13Philips Foundation
Stichting Philips Foundation, an independent foundation organized under Dutch law, is a registered charity established in 2014. In 2022,2023, Royal Philips supported the Philips Foundation with a contribution of EUR 6.7 million and provided the operating staff as well as the expert assistance of skilled employeesvolunteers in the execution of the Foundation’s programs.
The Philips Foundation’s mission is to reduce healthcare inequality by providing access to quality healthcare for underserved communities through meaningful innovation. It does this through the provision and application of Philips’ healthcare expertise, innovation power, talent and resources and by financial support. Together with key partners around the globe (including respected NGOs such as Red Cross organizations, UNICEF, Amref and Save the Children)(NGOs, academic partners, entrepreneurs), the Philips Foundation seeks to identify challenges where a combination of Philipshealthcare technology expertise and partner experience can be used to create meaningful solutions that have ana positive impact on people’s lives.
Philips Foundation works in projects (grant-based) and through impact investments (loans and equity). The instrument depends on the status and self-sustainability of the respective healthcare technology in serving the more disadvantaged communities.
8.4.118.3.14Working with stakeholders and advocacy
Our stakeholder engagement is closely aligned with the company’s purpose to improve people’s health and well-being through meaningful innovation. One of our key ESG commitments is to be transparent about our plans, activities, targets, results and contributions to society, and to engage with shareholders, customers, business partners, governments and regulators through a variety of platforms. The purpose of our engagement efforts is to pursue and foster an open, meaningful, effective, and informed dialogue regarding our activities and our internal and external stakeholders’ needs, concerns and expectations. Please refer to the Philips Stakeholder Engagement Policy available at our website.
The purpose of our advocacy efforts is to contribute to policy development and legislative processes and to support business opportunities in the areas relevant to Philips and its businesses, for example: health system resilience policies and investment plans; ESG, in particular on climate, circularity and green procurement; and Digital Health, such as AI, data protection, interoperability, cybersecurity, and technological sovereignty.
In organizing ourselves around customers and markets, we conduct dialogues with our diverse stakeholders in order to explore common ground for addressing societal challenges, building partnerships and jointly developing supporting ecosystems for our innovations around the world. We derive significant value from our stakeholders across all our activities and engage with, listen to and learn from them. Working in partnerships is crucial to delivering on our purpose to improve people’s health and well-being through meaningful innovation. We incorporate feedback on specific areas of our business into our planning, actions, targets, policies and disclosures. In addition, we participate in meetings and task forces as a member of organizations including the World Economic Forum, WBCSD, Responsible Business Alliance (RBA), EFRAG, Dutch Sustainable Growth Coalition, the Ellen MacArthur Foundation, European Round Table for Industry, Platform for Accelerating the Circular Economy (PACE) and the European Partnership for Responsible Minerals.
Furthermore, we engage with the leading Dutch labor union (FNV) and a number of NGOs, including Enough, GoodElectronics, the Chinese Institute of Public and Environmental Affairs, UNICEF, Amnesty International, Greenpeace, Friends of the Earth, and WageIndicator. We also engage with a variety of investors, analysts, institutional advisory and other organizations, such as Eumedion, ISS, Glass Lewis, VEB and VBDO. Please also refer to Investor information.
In addition to our many stakeholder engagement sessions, our sustainability e-mail account (philips.sustainability@philips.com) enables stakeholders to share their issues, comments and questions, also about this Annual Report. The following table provides a non-exhaustive overview of our stakeholder engagement, which is also used for our materiality analysis.
| Stakeholder engagement overview (non-exhaustive) | |||
| Stakeholders | Processes | Results | |
| Employees |
| Regular meetings, quarterly Employee Survey, employee development process, quarterly update webinars. For more information, refer to Social performance Regular mail updates, team meetings, webinars | Engaged and informed employees, action plans, policies |
| Customers |
| Joint (research) projects, business development, Lean value chain projects, strategic partnerships, consumer panels, Net Promoter Scores, Philips Customer Experience Centers, Philips Customer Care centers, Training centers, social media | New technologies and processes, Frustration Free Packaging solutions, green consumer propositions, Life Cycle Analysis of products, EU Product Environmental Footprint pilots |
| Suppliers |
| Supplier development activities (including topical training sessions), supplier forums, supplier website, participation in industry working groups like COCIR and RBA. For more information, refer to Supplier sustainability. | Supplier improvement projects, supplier commitments to Science Based Targets to reduce CO2 emissions, joint projects |
| Governments, municipalities, etc. |
| Topical meetings, research projects, policy and legislative developments, business development, multi-stakeholder projects. | Feedback on proposed legislation, investment plans, transition plans to a circular and low carbon society |
| NGOs |
| Topical meetings, multi-stakeholder projects, joint (research) projects, innovation challenges, renewables projects, social investment program and Philips Foundation. | Projects to increase access to care in underserved communities, action plans, policies |
| Investors |
| Webinars, roadshows, capital markets day, Investor relations and Sustainability accounts | Green and Sustainability Innovation Bonds, visits to Philips Customer Experience Centers |
8.58.4Governance
8.5.18.4.1Corporate governance structure
Koninklijke Philips N.V. (Royal Philips), a company organized under Dutch law, is the parent company of the Philips group. Its shares have been listed on the Amsterdam stock exchange (Euronext Amsterdam) since 1912. Furthermore, its shares have been traded in the United States since 1962 and have been listed on the New York Stock Exchange since 1987.
Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties. The Board of Management is entrusted with the management of the company. The other members of the Executive Committee have been appointed to support the Board of Management in the fulfilment of its managerial duties. See the chapter Corporate governance of this Annual Report, where the company addresses the main elements of its corporate governance structure, reports on how it applies the principles and best practices of the Dutch Corporate Governance Code, and provides other information required under Dutch law.
Under the chairmanship of the President/Chief Executive Officer (CEO) and supported by the other members of the Executive Committee, the members of the Board of Management drive the company’s management agenda and share responsibility for the continuity of the Philips group, focusing on sustainable long-term value creation. In fulfilling their duties, the members of the Board of Management and Executive Committee are guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders.
The company is governed by Dutch corporateSupervisory Board supervises the policies, management and securities laws, its Articlesgeneral affairs of Association,Philips, and the Rules of Procedure ofassists the Board of Management and the Executive Committee with advice on general policies related to the activities of the company, including setting and executing the strategy of the Philips Group.
Philips’ strategy, and the way it has been developed by the Board of Management under the supervision of the Supervisory Board, respectively. Its corporate governance framework is also basedclearly integrates the company’s impact in the field of sustainability, including the effects on people and the Dutch Corporate Governance Code (dated December 8, 2016) and US laws and regulations applicable to Foreign Private Issuers. Additionally, theenvironment. The Board of Management has implementedregularly convenes on ESG matters with other Executive Committee members (the Chief Operating Officer, the Philips GeneralChief Strategy & Innovation Officer, the Chief Human Resources Officer, the Chief Business Principles (GBP) and underlying policies, as well as separate codes of ethics that apply to employees working in specific areas of our business, i.e. the Financial Code of EthicsLeader Precision Diagnosis and the Procurement Code of Ethics. ManyChief International Markets Market Leader) and certain functional executives. Together they define Philips’ ESG strategy, commitments, programs, targets and policies, they monitor and evaluate progress and take corrective action where needed. Progress on ESG is reported on a quarterly basis to the Audit Committee of the documents referred to are published onSupervisory Board, which assist the company’s websiteSupervisory Board in fulfilling its oversight responsibilities for the integrity and more information can be found in Our approach to risk management.
Please also refer to Corporate governance where the main elementsquality of the company’s corporate governance structure have been addressed.sustainability statements,
ESG is also embedded in our core business processes, like innovation (EcoDesign), sourcing (Supplier Sustainability Program), manufacturing (Sustainable Operations), logistics (Green Logistics) and programs like the Circular Economy initiative.
8.5.28.4.2Philips Business SystemOperating Model
Our operating model – the Philips Business System (PBS) – integrates key aspects of how we operate – from our strategy, structure & governance, organizational design,policies, processes, and systems & data, to our people and team practices, and our& culture and performance management.
Towards the end of 2022In 2023 we initiatedcontinued the process of simplifying the way we work to drive clear accountability and agility, and to unlock significant productivity and margin gains. This simplification – with end-to-end accountable businesses supported by a much leaner Group layer and strong Regions, together with a strengthened culture of patientpatient- and people centricity,people-centricity, innovation impact and clear accountability – is a primary enabler to drive flawless execution.create value with sustainable impact.
It is designed to help us to fulfill our purpose of improving the health and well-being of billions of people and ensure the highest standards of quality and integrity in everything we do.
8.5.38.4.3Quality & RegulatoryRisk management and patient safetyinternal control
Enabling the delivery of patient-centric, safeThe company’s risk management and high-quality care – the essence of patient safety and quality – is inextricably linked to Philips’ purpose to improve the health and well-being of people through meaningful innovation. Patient safety and quality management represents the very foundation of our license to operate as a health technology company. Compliance with quality and regulatory standards is a pre-requisite for ensuring patient safety, which is Philips’ highest priority.
Philips’ reputation – and ultimately our long-term business continuity and success – fully depends on the quality and safety of our products, services and solutions for patients, customers and consumers, and on our compliance with global regulations and standards. This has never been more crucial than in this last year as we continued to remediate the devices included in the Philips Respironics recall: see section below, ‘Philips Respironics voluntary recall notification’.
Acting with due urgency, in 2022 we accelerated our focus on patient safety and quality, with the goal of achieving and maintaining the highest level of quality. We upgraded the Quality & Regulatory leadership team with emphasis on medical technology expertise; over 90% of the renewed team has direct industry experience. We further strengthened our Post Market Surveillance global complaint handling organization and improved ways of working; this represents a significant milestone toward improving investigation and issues reporting and moving away from transactional elements of complaint handling. In addition, Philips continued to focus on harmonizing processes and enhancing the quality culture across the enterprise. Activities include training approximately 77,000 employees throughout the world on key process changes and refreshers on quality and regulatory topics.
As a global business, we must ensure compliance with various and evolving regulations and standards. In the dynamic medical technology industry, we also must stay ahead of innovation and trends such as data privacy and cybersecurity. This involves increased levels of investment to meet the competitive demands and evolving regulatory compliance activities in such areas as secure electronic transmission and storage solutions for protected personal information, protected health information, financial information, intellectual property, and other sensitive information related to our customers, consumers, patients, and workforce. For information on how Philips manages cybersecurity risk, please refer to Operational risks.
Quality
Quality isinternal control framework forms an integral part of the leadershipPhilips business planning and culture at Philips.performance review cycle. The purpose of our risk management is to identify and analyze the risks Philips faces in executing its strategy and activities, to set the risk appetite of the company, to take appropriate risk responses and to monitor its effectiveness. The objective of internal control is committed to deliveringmaintain integrated management control of the highest quality products, servicescompany’s operations, reporting, and solutions, which are compliantsafeguarding compliance with all applicable laws and qualityregulations. As part of its internal control framework, Philips has implemented a standard set of Internal Controls over Financial Reporting (ICFR). Key elements of our framework include (but are not limited to) our General Business Principles, our corporate governance, authorization structures, our policy framework and safety standards. We continuously striveinternal reporting structures. Furthermore, it comprises various frameworks to raisehelp manage and control risk in line with our performance in ensuring quality, which is demonstrated by the continued, substantial investmentrisk appetite. These frameworks include (but are not limited to) our Enterprise Risk Management framework (refer to embed quality through standardizationRisk management), and adoption of industry best practices throughout our Quality Management Systemsother management systems and enhanced capabilities.
Through quality system improvement program activities, our aim is to enhance consistency in how we work, collaborate, and make decisions. Our critical Accelerating Patient Safety & Quality program initially focused on awareness and compliance improvements, triaging, and process design. Examples of improvements include reducing and consolidating our Quality Management Systems from 107 to 75 by year-end 2022, with further reductions planned. In 2022 we harmonized and improved consistency for a significant number of processes across Philips to enhance our best practices and implemented standard education programs tailoredframeworks relevant to specific roles plus many mandatory all-employee, quality-related courses for capability building and to demonstrate compliance. The program is now focused on further strengthening design and product reliability, andrisk areas such as patient safety and quality, culturehealth & safety, environmental, tax, business continuity, information security and competencies, while continuing efforts reducing complexity. This is an ongoing journeyprivacy. Please refer to Risk management for a more detailed description of continuous improvement and we expect our plansPhilips’ approach to yield demonstrable progress starting in 2023.
In 2022, we updated our Quality Policy, which expresses our overall intention and direction with respect to quality. Established by management with executive responsibility, it states our objectives for, and commitment to, quality. Everyone at Philips is responsible for understanding, implementing, and maintaining the Quality Policy, and all employees now have patient safety and quality as one of five key KPIs. Underscoring leadership's continued commitment, all Philips business leaders are held accountable for patient safety and quality, and performance on Quality metrics will be part of the remuneration of all Philips Executives.
Regulatory compliance
As required by global regulatory requirements, Philips actively maintains Quality Management Systems that establish procedures, processes and documentation to ensure quality at each stage of the product lifecycle. These requirements outline actions from product design and pre-market submissions, production, operations, distribution, servicing and post-marketrisk management and oversight in every market we serve. These requirements include those from national government regulatory authorities (e.g.more information on the US Food and Drug Administration and China National Medical Products Administration), Notified Bodies, and National Competent Authorities in the EU.
Productsrisk factors that we introduce to the market often must undergo pre-market regulatory review (e.g. pre-market approval (PMA), pre-market notification (510(k), or de novo authorization) before they can be marketed and sold in the USA as an FDA-regulated device, subjected to Notified Body review in the EU for a CE Mark, and subjected to review in China by the National Medical Products Association. If the regulatory body reviewing the submission determines that the required supporting data has nothave been provided, further data may be required to obtain the clearance or approval, which could prolong the process to market the product. During the lifecycle of a cleared/approved device, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, may require a further regulatory submission and review. Regulatory bodies require each manufacturer to determine whether the proposed change requires a submission, but can review any such decision and disagree with a manufacturer’s determination. If the regulatory body disagrees with a manufacturer’s determination regarding whether a new submission is required for the modification of an existing device, they can require the manufacturer to cease marketing and/or recall the modified device until the relevant approval/clearance is obtained. In addition, in these circumstances, significant regulatory fines or other penalties may be imposed.
We also must comply with the EU’s Waste from Electrical and Electronic Equipment (WEEE), Restriction of Hazardous Substances (RoHS), and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), Energy-using Products (EuP), and other product safety regulations.
Post-market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. In the USA these include:
establishment of registration and device listing with the FDA;Quality System Regulation (QSR) requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;labeling regulations and restrictions, including, for example, prohibitions against the promotion of investigational products, the promotion of “off-label” uses of cleared or approved products, or the use of false, misleading or unsubstantiated claims or statements;requirements related to promotional activities;clearance or approval of product modifications to 510(k)-cleared or,de novo-authorized devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices, and approval of certain modifications to PMA-approved devices;medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctionedidentified, and thedevice or a similar devicerisk responses thatit markets would be likelyhelp tocause or contribute to a death or serious injury, if the malfunction were to recur;correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;the FDA’s notification and recall authority, whereby the agency can order a device manufacturer to take certain actions if the FDA determines the manufacturer’s device presents an unreasonable risk of substantial harm to public health,manage suchas: provide notice to users and other affected stakeholders; submit a plan for the repair, replacement or refund of devices; or recall the device from the market;post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device.
Our manufacturing processes are required to comply with the applicable portions of the QSR and/or ISO13485, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file and complaint files. As a manufacturer, we will be subject to periodic scheduled or unscheduled inspections by the FDA, Notified Bodies or other relevant regulatory bodies. Our failure to maintain compliance with the QSR or ISO 13485 requirements could result in the shut-down of, or the imposition of restrictions on, our manufacturing operations, imposition of an import alert, or the recall or seizure of our products, which would have a material adverse effect on our business. The discovery of previously unknown problems with any of our products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
In the USA, the FDA has broad regulatory compliance and enforcement powers, which it can impose on its own or in coordination with the Department of Justice (DoJ), which has separate enforcement authority. If the FDA determines that we failed to comply with applicable regulatory requirements, it may lead to any of the following sanctions:
warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;orders to issue notifications to users and other stakeholders, or to submit to the FDA a plan for the repair, replacement or refund of devices;recalls, withdrawals or administrative detention or seizure of our products;operating restrictions or partial suspension or total shutdown of production;refusing or delaying requests for 510(k) marketing clearance,de novoauthorization, or PMA approvals of new products or modified products;withdrawing 510(k) clearances,de novoauthorizations, or PMA approvals that have already been granted;refusal to grant export approvals for our products; orcriminal prosecution.
European Union Medical Device Regulation
The European Union Medical Device Regulation (EU-MDR) passed its date of application (May 26, 2021). For a portion of the portfolio, we used the available grace period, where products that were placed on the market under the predecessor of the EU-MDR, the European Union Medical Device Directive (EU-MDD), can continue to be placed on the market if meeting a subset of EU-MDR requirements in addition to the EU-MDD requirements. Reasons for this include stock depletion management and Notified Body capacity limitations. Throughout 2022, we made progress in transitioning some of the portfolio to become EU-MDR-compliant. We also started registering our entities and medical devices in the European Database for Medical Devices (EUDAMED) on a voluntary basis.
As the global regulatory environment continues to evolve, we are working to address the impact on cost, time and resources needed to obtain future approvals, and our ability to maintain existing approvals for our products, services, and solutions.
Philips Respironics voluntary recall and consent decree
On June 14, 2021, Philips’ subsidiary, Philips Respironics, initiated a voluntary recall notification in the United States, and field safety notice outside the United States, for certain sleep and respiratory care products to address identified potential health risks related to the polyester-based polyurethane (PE-PUR) sound abatement foam in these devices.
This recall let down the patients who depended on them, as well as their caregivers, and we are deeply sorry for that. We are treating this matter with the highest possible seriousness and are working to address this issue as efficiently and thoroughly as possible.
Following the substantial ramp-up of production, service and repair capacity in 2021 and 2022, by year-end 2022 around 90% of the production required for the delivery of replacement devices to patients had been completed. In order to expedite the completion of the recall, Philips Respironics will increase the proportion of new replacement devices.Working with five certified, independent testing laboratories in the US and Europe and other third-party qualified experts and an external medical panel, we have been conducting a comprehensive test and research program on the PE-PUR foam to better assess and scope the potential patient health risks related to possible emissions of particulate from degraded foam and Volatile Organic Compounds related to the first-generation DreamStation devices. We provided an update to healthcare providers, patients, and other stakeholders in June 2022 and December 2022,outlining encouraging test results for the first-generation DreamStation (DS1), which accounts for over two thirds of the sleep therapy devices subjected to the recall.The company developed and began executing a comprehensive plan to replace the PE-PUR sound abatement foam used in earlier-generation devices, with the new material used in next-generation products such as DreamStation 2, which was cleared by the US FDA and approved by many competent authorities around the world. Philips Respironics has regularly been communicating progress to regulators and competent authorities around the world, as well as customers, clinicians, and patients, to complete the needed repairs and replacements associated with this recall. In certain circumstances, the products in question may be replaced or financially compensated rather than repaired.
While third-party lab and internal testing efforts are ongoing, the company will continue with the remediation activities for all devices and continues to communicate with customers through a variety of channels.Philips Respironics will also continue to monitor complaints received following the recall/field safety notice via our Quality Management System,in accordance with themedical devices regulations and laws in the markets that we serve.
Following the FDA’s inspection of a Philips Respironics manufacturing facility in connection with the recall and the subsequent inspectional observations, the US DoJ, acting on behalf of the FDA, began discussions with Philips in July 2022 regarding the terms of a consent decree to resolve the identified issues. Philips is engaged in ongoing discussions with FDA and DoJ on the proposed consent decree. For more information, see Note Contingencies.
Consent decree – ECR
In October 2017, Philips North America LLC reached agreement on a consent decree with the US Department of Justice, representing the Food and Drug Administration (FDA), related to compliance with current good manufacturing practice requirements arising from inspections conducted in 2015 and prior, focusing primarily on Philips’ Emergency Care & Resuscitation (ECR) business operations in Andover, Massachusetts, and Bothell, Washington.
Following a successful inspection in Bothell, Washington, in April 2020, the FDA determined that Philips had met the conditions for resuming manufacturing and distribution of defibrillators in the US. The consent decree remains in effect for several years, during which the Emergency Care (formerly Emergency Care & Resuscitation) business will be subject to a series of annual assessments by an independent expert. Hospital Patient Monitoring (formerly Monitoring & Analytics), also named in the consent decree, is also under a heightenedrelevant level of scrutiny over the same period.risk appetite.
Substantial progress continues to be made inTogether with Philips’ established accounting procedures, our compliance efforts. In August 2021, the FDA inspected Emergency Care in Bothell as a consent decree follow-up. Three observations (Form 483) were issued and subsequently remediated and reported to the FDA. The FDA later presented Emergency Care with four Establishment Inspection Reports dating back to 2015, signaling the closure of the four open inspections. There was a consent decree follow-up inspection in October 2022, resulting in three observations (Form 483). These will soon be reported as fully remediated.
We cannot predict the outcome of this matter, and the consent decree authorizes the FDA, in the event of any violations in the future, to order us to cease manufacturing and distributing Emergency Care or Hospital Patient Monitoring devices, recall products, pay liquidated damages, and take other actions. We cannot currently predict whether additional monetary investment will be incurred to resolve this matter or the matter’s ultimate impact on our business.
8.5.4Remuneration policy
Our remuneration policyICFR is designed to encourage employeesprovide reasonable assurance that assets are safeguarded, that the books and records properly reflect transactions necessary to deliver on our purposepermit preparation of financial statements, that policies and strategyprocedures are carried out by qualified personnel, and create stakeholder value,that published financial statements are properly prepared and do not contain any material misstatements. With respect to motivatefinancial reporting, a structured self-assessment and retain them. Our executive long-term incentive plan includes environmentalmonitoring process is used company-wide to assess, document, review and social commitments. A descriptionmonitor compliance with ICFR.
Each year, management’s accountability for ICFR is evidenced through the formal certification statement sign-off. Any deficiencies noted in the design and operating effectiveness of the composition of the remuneration of the individual members ofICFR that were not completely remediated are evaluated at year-end by the Board of Management and the outcome reported to the Supervisory Board. The Board is included in Reportof Management’s report on ICFR, including its conclusions regarding the effectiveness thereof and its statement on compliance with section 404 of the Remuneration Committee.US Sarbanes-Oxley Act, can be found in this report in the section Management’s annual report on internal control over financial reporting.
8.5.58.4.4Philips General Business Principles (GBP)
While pursuing our business objectives, we aim to be a responsible partner in society, acting with integrity towards our employees, customers, patients, business partners and shareholders, as well as the wider community in which we operate. Everyone at Philips is expected to always act with integrity, and Philips rigorously enforces compliance of its General Business Principles (GBP) throughout the company.
In the highly regulated world of healthcare, integrity requires in-depth knowledgeTo that end, our GBP – part of the applicable rulesPhilips Operating Model – and regulations and a sensitivity to healthcare-specific issues. The GBPtheir underlying policies incorporate and represent the fundamental principles by which all Philips businesses and employees around the globe must abide. They set the minimum standard for our business conduct bothas a health technology company, for our individual employees and for our subsidiaries, and Philips rigorously enforces compliance of its GBP throughout the company and our subsidiaries.company. Our GBP also serve as a reference for the business conduct, we expect from all our business partners.
The GBP also include principles whichof doing business with integrity at work, integrity in the market and professional integrity outside work. They also set our integrity standard on inside information, aiming to prevent trading on or disclosure of non-public information, the publication of which would be likely to have a significant influence on the trading price of Philips securities or securities of companies that Philips is seeking to acquire. More specifically, Philips has adopted Rules of Conduct with respect to trading in Philips securities to promote compliance with applicable insider trading and other market abuse laws, rules and regulations, in particular the EU Market Abuse Regulation. The Rules of Conduct apply to all employees, the members of the Board of Management and the Supervisory Board of Royal Philips.
The GBP form an integral part of labor contracts in virtually every country in which Philips operates. Translations of the GBP text are available in 30 languages, allowing almost every employee to read the GBP in their native language. Detailed underlying policies, manuals, training, and tools are in place to give employees practical guidance on how to apply and uphold the GBP in their daily work environment. Details can be found at www.philips.com/gbp. Each year, employees reconfirm their commitment to the code of conduct after completing their GBP e-learning, and there is an additional annual sign-off for Executives. A similar sign-off is in place for Finance and Procurement staff for their respective codes of conduct.
The Executive Committee is responsible for the effective deployment of the GBP and for generally promoting a culture of compliance and ethics within the company. Furthermore, each quarter all our key Regions convene market compliance committees dealing with GBP-related matters in the local context. They are also responsible for the design and execution of localized compliance plans that are tailored to their market-specific risks and organizational set-up, and regularly review the relevant compliance metrics for their respective market through dashboards delivered by the legal compliance monitoring team. The GBP Program Office, together with a worldwide network of GBP Compliance Officers, supports the implementation of GBP initiatives.
As part of our continuous effort to raise GBP awareness and foster dialogue throughout the organization, each year a global GBP communications and training plan is deployed, including structured dialogues led by managers where quality, integrity and speaking up are discussed. This is part of a company-wide initiative aimed at reinforcing a culture of dialogue using ethical dilemma case studies that are relevant to our workforce. All functions at risk also receive annual training which includes content on, amongst others, antibribery and anticorruption and healthcare compliance via tailored case studies. Almost 60,000 (94%) of our assigned employees completed their yearly GBP e-learning. Specifically in 2023, we again focused on increasing awareness on integrity and on the importance of speaking up, through and following the deployment of our biennial Business Integrity Survey. Through this survey, more than 22,500 employees trusted us with their views and opinions on integrity within Philips. Ninety-four percent of employees expressed the belief that we act with integrity with Philips. To gain deeper insights into the results of the Business Integrity Survey, we execute deep-dive initiatives amongst our employees.
A key control to measure implementation of our GBP is the GBP monitoring and reporting program, which is part of our Internal Control framework. In addition, we continue to expand the capabilities of our legal compliance monitoring team, serving our business customers as well as our compliance networks with actionable data, thus further improving our compliance control framework.
The GBP are supported by established mechanisms with the aim of ensuring standardized reporting and enabling employees and third parties to escalate concerns 24/7. Concerns raised are registered consistently in a single database hosted outside of Philips servers to ensure confidentiality and security of identity and information. Encouraging people to speak up through the available channels if they have a concern will continue to be a cornerstone of our GBP communications and awareness campaigns. At least twice a year, the Executive Committee and Audit Committee of the Supervisory Board are informed on relevant GBP metrics, cases, trends and learnings.
In 2022,2023, a total of 706764 concerns were reported via Philips Speak Up (Ethics Line) and through our network of GBP Compliance Officers. This represents an increase of 16%8% from the total of 610706 concerns in the previous reporting period (2021)(2022).
While this This is a continuation of a year-on-year upward trend.
Through the upward trend, the increase is flattening. Specifically in 2022, we once more focused on increasing awareness on Integrity and on the importance of speaking up, following up on the conclusionsAudit Committee of the deep-dives executed after our 2021 biennial Business Integrity Survey. We believeSupervisory Board, the upward trendcompany also has procedures in reporting remains in line with our multi-year effortsplace for the receipt, retention and treatment of complaints specifically relating to accounting, internal accounting controls, or auditing matters, enabling the confidential, anonymous submission of complaints.
8.4.5Remuneration policy
Our remuneration policy is designed to encourage our employees to express their concerns, whilst realizing that the extraordinary business conditions in the past few years make it imprudentdeliver on our purpose and strategy and create stakeholder value, and to draw any specific conclusions from these numbers.
More information on the Philips GBP can be found in Risk management.
8.5.6Risk management approach
Risk managementmotivate and control forms an integral partretain them. Our executive long-term incentive plan includes environmental and social commitments. A description of the Philips business planning and performance review cycle. The company’s risk management policy and framework are designed to provide reasonable assurance that its strategic and operational objectives are met, that legal requirements are complied with, and that the integritycomposition of the company’s financial reporting and its related disclosures is safeguarded. Please refer to Risk management for a more detailed description of Philips’ approach to risk management (including Internal Control over Financial Reporting), risk categories and factors, and certain specific risks that have been identified.
With respect to financial reporting, a structured self-assessment and monitoring process is used company-wide to assess, document, review and monitor compliance with Internal Control over Financial Reporting. On the basisremuneration of the outcome of this process, the Board of Management confirms that: (i) the management report (within the meaning of section 2:391 of the Dutch Civil Code) provides sufficient insights into any failings in the effectiveness of the internal risk management and control systems; (ii) such systems provide a reasonable level of assurance that the financial reporting does not contain any material inaccuracies; (iii) based on the current state of affairs, it is justified that the financial reporting is prepared on a going concern basis; and (iv) the management report states those material risks and uncertainties that are relevant to the expected continuity of the company for a period of 12 months after the preparation of the report. The financial statements fairly represent the financial condition and result of operations of the company and provide the required disclosures.
In view of the above, the Board of Management believes that it is in compliance with best practice provision 1.4.2 of the Dutch Corporate Governance Code. It should be noted that the above does not imply that the internal risk management and control systems provide certainty as to the realization of operational and financial business objectives, nor can they prevent all misstatements, inaccuracies, errors, fraud or non- compliances with rules and regulations. The above statement on internal control should not be construed as a statement in response to the requirements of section 404 of the US Sarbanes-Oxley Act. The statement as to compliance with section 404 is set forth in Management's annual report on internal control over financial reporting.
8.5.7Total tax contribution
To fulfil our company purpose, a responsible tax approach is required. We fully acknowledge our societal role when it comes to paying taxes in the geographies where value is created. We consider our tax payments as a contribution to the communities in which we operate, as part of our social value creation.
Our Approach to Tax sets the standard for our conduct, by which individual employees, the company and its subsidiaries must abide. We consider tax in the context of the broader society, inspired by our stakeholder dialogues, global initiatives of the Organization for Economic Cooperation and Development and United Nations, human rights, international tax laws and regulations and relevant codes of conduct.
Under the ultimate responsibilitymembers of the Board of Management and the Supervisory Board is included in the Report of the Remuneration Committee.
8.4.6Quality & Regulatory and patient safety
Enabling the delivery of patient-centric, safe, and high-quality care – the essence of patient safety and quality – is foundational to Philips' purpose to improve the health and well-being of people through meaningful innovation. This year we formed the Patient Safety and Quality organization, which brings together the Quality, Regulatory Affairs, and Clinical and Medical functions as one unified team. Positioned to support and enable the entire Philips organization and to foster the quality culture at Philips, the Patient Safety and Quality team is instrumental in ensuring we have the capabilities, processes, and tools required for operating in a highly regulated healthcare technology industry.
Throughout 2023, we continued to accelerate our work in crucial areas, with the goal of achieving and maintaining the highest level of patient safety and quality. Specific areas of focus included: preparation and manufacturing site readiness for audits and inspections; a review of quality records; new ways of undertaking product quality reviews; planning for IT and data enhancements; and simplification of our process framework and Quality Management Systems.
This year, we created the new role of Chief Patient Safety and Quality Officer, who is a member of the Philips Executive Committee and reports directly to the Chief Executive Officer. Additionally, we hired a new Chief Medical Officer to lead the team that is focused on clinical research, medical safety, and medical support for our global businesses.
Early in 2023, we organized CAPA management, Quality Management Systems, compliance training, internal quality audits, and other crucial areas for patient safety and quality into a Compliance and Quality shared service team. We set up a Transformations team, responsible for project management of Patient Safety and Quality programs. Regulatory Affairs formed new teams that are responsible for expanding our engagement with external stakeholders and regulators, and for the delivery of effective tools, processes, and services to facilitate timely and compliant market access. We appointed a new leader for Product Safety and Surveillance, Corrections and Removals, and strengthened our post-market processes and ways of working.
Across Philips, teams in all Businesses, Regions, and Functions continued to foster a quality culture and mindset, where all employees are encouraged to speak up and share ideas for improving the safety and efficacy of our products. In October, our employees took part in a Timeout for Patient Safety and Quality to solidify commitment and planning.
Quality
We strive to continuously raise our performance to deliver safe and high-quality products, services, and solutions, which are compliant with quality and safety standards and all applicable laws. In 2023, as part of our plan to create value with sustainable impact, we introduced a new operating model that enables us to simplify how we work and improve accountability and ownership. We are strengthening our engineering capabilities for new product development in areas such as quality systems engineering, reliability and software design.
We further reduced the number of Quality Management Systems in which we operate to increase focus, reduce complexity, and minimize risk. This year we closed nine QMS for a total of 66 Quality Management Systems by year-end 2023, with further significant reductions planned for 2024.
In 2023, our Accelerating Patient Safety & Quality program instituted an improved approach for management of skills and launched a training program on patient safety and quality topics. In collaboration with business units and Innovation & Strategy, we established programs to improve product design and reliability.
All Philips businesses are accountable for patient safety and quality. This year, we instituted a new Patient Safety and Quality performance review meeting with each business and at the Philips level in aggregate. We set Patient Safety and Quality key performance indicators for the company, and Quality performance metrics are part of the remuneration of all Philips Executives. Additionally, every employee has a patient safety and quality goal as part of people performance management.
Regulatory Affairs
Under Philips' new operating model, Regulatory Affairs sits on the leadership team of each Business and Region as a key partner. In 2023, we established internal governance and requirements for engagements with national government regulatory authorities (e.g. the US Food and Drug Administration (FDA), European Medicines Agency (EMA), China National Medical Products Administration, Notified Bodies, and National Competent Authorities in the European Union.
As a global business in a dynamic regulatory environment, we must ensure compliance with evolving regulations related to innovations in areas such as Artificial Intelligence, and healthcare informatics and software design. This year, we increased levels of investment in regulatory science and policy and in enterprise informatics. Teams are working on global harmonization of requirements, safe and innovative applications of AI, and secure transmission and storage of protected health information. Sought as strategic partners, the Regulatory Affairs team participated in international consensus standards groups alongside regulators and engaged with international regulators as invited experts and speakers at the International Medical Device Regulators Forum, Global Harmonization Working Party, and other meetings. We are working with the National Institutes of Health to establish ethical applications of Artificial Intelligence in medical devices.
In 2023, we also increased our collaborations with organizations supporting regulatory science like the Food, Drug, and Law Institute and the Regan-Udall Foundation for the FDA. In partnership with the Boston Globe and the Washington Post, we hosted an industry and customer panel on Transforming Healthcare with AI: Harnessing Technology to Promote Safety and Quality for Patients. Regulatory Affairs leaders moderated panel discussions among key international regulators and were featured speakers at events such as MedTech Europe and the AdvaMed annual conference.
Throughout 2023, we continued transitioning our portfolio to become European Union Medical Device Regulation (EU-MDR)-compliant. In March 2023, the grace period was extended until year-end 2027 or 2028, under strict conditions and depending on risk class. We continue to use this available grace period for placing a portion of our portfolio on the market under the European Union Medical Device Directive (EU-MDD).
Regulatory Affairs deployed a digital regulatory information management tool to all Regions in 2023, with continued deployments planned to Businesses in 2024. This tool will be a single source for regulatory data that will help increase speed to market.
Medical Office
In 2023, we formed a centralized medical office with expertise in clinical research, medical safety, healthcare economics, and a breadth of care specialties. This team is focused on supporting the global business, navigating the intricacies of addressing patients' and customers’ unmet needs across a variety of ecosystems, and looking across the entire product lifecycle to help teams develop solutions that are safe, effective, and relevant for patients, providers, and payers.
In 2023, we hosted our second Patient Safety Advisory Board, where external leaders from across the world, from a variety of professional backgrounds, participated in intensive workshops focused on themes identified as key to improving the safety and efficacy of our solutions. Experts from outside and within Philips exchanged ideas ranging from the role of innovation in human interface models, patient safety and clinical education programs and capability building at Philips, and other topics that are key to our overall way forward in innovation and safety. This year, Philips also introduced an extensive Clinical and Medical curriculum, available to all employees.
The team continued our advocacy and investment in topics such as radiation safety, medical device testing, and improved access to physician and staff training. At Harbor-UCLA Lundquist Institute, we host a facility for R&D pre-clinical testing, clinical and medical education proctoring, and fellows training. Our team continues to design, generate, and disseminate clinical and economic evidence to show the value of our solutions in improving patient outcomes.
Our Health Economics and Outcomes Research team continued to contribute economic evidence to support innovation and expand access to high-quality care, authoring 12 publications and 18 studies during 2023. The Ambulatory Monitoring & Diagnostics business unit increased in-network access to ambulatory patient monitoring for an additional 35 million patients in the United States through payer contracting initiatives. Our Image Guided Therapy-Devices team advocated with the Centers for Medicare and Medicaid (CMS) to improve Medicare payment to ambulatory surgery centers for cardiovascular and peripheral vascular treatments, ensuring patients have access to care in the setting that their providers determine is most appropriate.
Philips Respironics voluntary recall
On June 14, 2021, Philips’ subsidiary, Philips Respironics, initiated a voluntary recall notification in the United States, and field safety notice outside the US, for certain sleep and respiratory care products related to the polyester-based polyurethane (PE-PUR) sound abatement foam in these devices.
Since June 2021, together with five independent, certified testing laboratories and third-party experts, Philips Respironics has conducted extensive testing. Based on the results to date, Philips Respironics and the third-party experts concluded that use of the sleep therapy devices is not expected to result in any appreciable harm to health in patients. Further testing remains ongoing. Following ongoing communications, Philips Respironics agreed in October 2023 to the FDA’s recommendations to implement additional testing on the sleep and respiratory care devices to supplement current test data. Philips Respironics is in discussions with the FDA on the details of that further testing. Further testing remains ongoing. Since the start of the test and research program, Philips Respironics has endeavored to work cooperatively with the FDA on the program and to publish regular test updates as agreed with the FDA.
Following the FDA’s inspection of a Philips Respironics manufacturing facility in the US and the subsequent inspectional observations, the US Department of Justice (DOJ), acting on behalf of the FDA, began discussions with Philips in July 2022 regarding the terms of a proposed consent decree. On January 29, 2024, as part of the announcement of Philips’ fourth quarter and full year 2023 financial results, the company provided an update stating that Philips agrees on the terms of a consent decree with the DOJ and FDA. The consent decree primarily focuses on Philips Respironics’ business operations in the US. The consent decree is being finalized and will be submitted to the relevant US court for approval. The decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business.
In the US, Philips Respironics will continue to service sleep and respiratory care devices already with healthcare providers and patients, and supply accessories (including patient interfaces), consumables (including patient circuits), and replacement parts (including repair kits). Until the relevant requirements of the consent decree are met, Philips Respironics will not resume selling new CPAP or BiPAP sleep therapy devices or other respiratory care devices in the US. Outside the US, Philips Respironics will continue to provide new sleep and respiratory care devices, accessories, consumables, replacement parts and services, subject to certain requirements. Further details will become available once the proposed Respironics consent decree has been finalized and submitted to the relevant US court for approval. For more information, refer to Contingencies.
In 2023, we also maintained manufacturing capacity to produce the necessary devices and rework kits required for remediation of the Respironics recall. As of December 31, 2023, over 99% of the sleep therapy device registrations that are actionable had been remediated, while the remediation of the ventilator devices remains ongoing. We expect to continue such remediation activities in 2024.
Philips Respironics regularly communicates on product remediation and testing associated with the recall with global regulators and customers through a variety of channels. Philips Respironics continues to monitor complaints received following the recall/field safety notice via our Quality Management Systems, in accordance with the medical devices regulations and laws.
Consent decree – Emergency Care & Resuscitation
In October 2017, Philips North America LLC reached agreement on a consent decree with the DOJ, representing the FDA, related to compliance with current good manufacturing practice requirements arising from inspections conducted in 2015 and prior. The consent decree focuses primarily on Philips' Emergency Care and Resuscitation (ECR) business operations in Andover, Massachusetts, and Bothell, Washington.
Following a successful inspection in Bothell, Washington, in April 2020, the FDA determined Philips had met the conditions for resuming manufacturing and distribution of defibrillators in the US. The consent decree remains in effect for several years, during which the Emergency Care (formerly Emergency Care and Resuscitation) business unit will be subject to a series of annual assessments by an independent expert. Hospital Patient Monitoring (formerly Monitoring & Analytics), also named in the consent decree, is also under a heightened level of scrutiny over the same period.
We continue to make substantial progress in our compliance efforts. In October 2022, the FDA inspected Emergency Care in Bothell as a consent decree follow-up. Three observations (Form 483) were issued and subsequently remediated and reported to the FDA. In June 2023, Emergency Care in Bothell received the October 2022 Establishment Inspection Report marking closure. In late October 2023, the FDA conducted a follow-up inspection to the 2022 inspection that resulted in a 483 with two observations, which were rapidly addressed in formal responses and subsequently acknowledged by the FDA the first week of January 2024 as closed. Efforts in 2024 are focused on ensuring the sustained demonstrated state of substantial compliance to support seeking formal relief in the first half of 2025, which is the earliest time allowed in the consent decree.
We cannot predict the outcome of this matter, and the consent decree authorizes the FDA, in the event of any violations in the future, to order us to cease manufacturing and distributing Emergency Care or Hospital Patient Monitoring devices, recall products, pay liquidated damages, and take other actions. We cannot predict whether additional monetary investment will be incurred to resolve this matter or the matter’s ultimate impact on our business.
8.4.7Cybersecurity
As a health technology company operating in a highly competitive industry, Philips’ brand reputation depends on the safety, quality and security of our products and services. Failure to meet cybersecurity standards may cause patient harm, negatively impact customer operations and their ability to provide healthcare or provide unauthorized access to patient records and medical devices. Philips furthermore relies on information technology to operate and manage its businesses, as well as store and process confidential data (relating to patients, employees, customers, intellectual property, suppliers and other partners). For a discussion of cybersecurity risks facing our business, see “Products and services may fail quality or security standards, which could adversely affect patient safety or customer operations" and “Philips could be exposed to a significant enterprise cybersecurity breach” in section Operational risks. As of the date of this Annual Report, there are no breaches of cybersecurity or other related risk threats that have, or are reasonably likely to, materially affect our business.
Security risk management is part of our broader risk management processes, and its aim is to protect the confidentiality, integrity, and availability of Philips’ products and services. To this end, the company has established a Group Security function and implemented security management processes, requirements and controls for the assessment, identification and management of material risks from, amongst others, cybersecurity threats. Our Head of Group Security, reporting to our Chief Financial Officer, annually reviews, evaluates, approvesleads the Group Security function in supporting the Board of Management in evaluating and setting the Group’s security strategy, issuing security policies and evaluating the progress and effectiveness of the deployment of the company’s security management framework. Our Chief Information Security Officer, reporting to our Head of Group Security, has nearly 26 years of technology and information security management experience in the industry, including prior roles with the Dutch Government and multinationals in the Consumer goods, Manufacturing, Chemical and Food processing industries, in various roles ranging from Chief Information Security Officer to IT security officer and Security Architect. Our Chief Information Security Officer is informed of and monitors the prevention, detection, mitigation and remediation of cybersecurity incidents through the Global Security Operations Center.
The company’s security management framework, including its cybersecurity policies and procedures, is maintained by the Group Security function and is designed to implement security requirements into all applicable business processes, information processing systems and infrastructure pertaining to our products and services and our supporting and enabling functions, during the entire lifecycle. The framework includes risk, vulnerability and penetration assessments, mandatory yearly security training for all employees, (including new hires Regular Phishing simulations for all employees three times a year), monitoring and response activities for vulnerabilities identified in products, services and infrastructure.
The Group Security function is also responsible for addressing security risks, including monitoring cybersecurity threats and responding to cybersecurity incidents. Philips’ Global Security Operations Center monitors the prevention, detection, mitigation and remediation of cybersecurity incidents on global enterprise systems, supported by certain external services and periodic/intermittent assessments. The severity and materiality of incidents are assessed through a dedicated security incident reporting process and, if necessary, incidents are escalated through central crisis management and (potentially) to the Philips Disclosure Committee, which assesses the need for public disclosure of (material) incidents. Incidents, where necessary adjusts Philips’ Approachneeded, are further escalated to Tax. Part ofGlobal Crisis Management.
Additionally, in order to address the security risks associated with our approach is to acknowledge the importance of transparency in respect of our tax contributions. Philips supports and participates in transparency initiatives such as the Dow Jones Sustainability Index (DJSI)suppliers and the Tax Transparency Benchmarkservices they provide, security controls are embedded in our procurement and supplier management processes, covering due diligence when engaging with new suppliers, contracting, monitoring and managing existing supplier relationships, and terminating supplier relationships. These security controls check for existing security certificates and assurances reports for the services in scope, validate suppliers’ answers to security questionnaires in due diligence, and ensure that security schedules are part of the Dutch Associationsigned contracts.
The Board of InvestorsManagement is ultimately responsible for Sustainable Development (VBDO)Philips’ cybersecurity management, which is overseen by the Supervisory Board (and specifically its Audit Committee). Since 2020, we have been providing certain voluntary disclosures about taxes paidAs part of this process, quarterly reports on cybersecurity risks and collectedincidents are prepared by the IT Audit & Risk Committee (consisting of representatives from the Group Security and Group IT functions, Philips Internal Audit and the external auditor and submitted to the Board of Management and the Supervisory Board. This reporting includes the overall risk level, relevant changes in the countriesrisk environment, challenges in which we operate. The 2022 Country Activityreaching and/or maintaining current risk levels and Tax Report is published on our website, in addition to, and simultaneously with the disclosures on tax included in this Annual Report.
Philips also endorses the ambitions expressedactual risk responses in the Tax Governance Code published by Dutch employers' organization VNO-NCW. We comply with the principles prescribed in the Code, available at www.vno-ncw.nl/taxgovernancecode,form of actions and we have touched upon the elements on this code in our Country Activity and Tax Report.owners.
In 2022, Philips contributed to the communities where we operate through taxes paid (e.g., corporate income tax) and taxes collected (e.g., VAT). Philips' total tax contribution in 2022, amounting to EUR 3,469 million, is presented by tax type in the following table. Please refer to our 2022 Country Activity and Tax Report for more details.
Philips Group
Total Contribution 2022 per Tax Type
in millions of EUR
| Corporate income tax paid | Customs duties | VAT1) | Payroll Tax | Other Taxes | Total | |
|---|---|---|---|---|---|---|
| Western Europe | 224 | 10 | 183 | 848 | 68 | 1,333 |
| North America | 80 | 45 | 102 | 846 | 8 | 1,081 |
| Other mature geographies | 35 | 3 | 63 | 134 | 1 | 236 |
| Growth geographies | 22 | 86 | 317 | 345 | 48 | 818 |
| Philips Group | 362 | 144 | 664 | 2,174 | 124 | 3,469 |
8.68.5Philips' ESG performance at a glance
Below we show how Philips performed in 20222023 on the 21 Core metrics of the WEF ESG reporting framework, mapped to the three dimensions of our ESG commitments, as well as a number of additional Philips-specific metrics that we consider fundamental to the strategy and operation of our business.
Environmental
- Green House Gas (GHG) emissions100% electricity from renewable sources0 kilotonnes CO2-equivalent (net operational carbon footprint)
- Taskforce on Climate-related Financial Disclosures (TCFD) implementationUpdated 1.5, 2 and 4 °C global warming scenarios and assessed their impact on our supply chain, Philips and customers (disclosed in separate report)
- Land use and ecological sensitivity
1 tonne2.7 tonnes waste sent to landfillAll 23/23 industrial sites 'Zero Waste to Landfill' at year-end - Water consumption and withdrawal in water-stressed areas
677,632661,076 m3 total water intake224,627211,063 m3 in water-stressed areas - Circular revenues *)
18.1%20.0% of revenues - Closing the loop *)
Closed the loop for over 3,400We reclaimed more than 11,500 systemsreturned to usor pieces of equipment in 2023
Social
- Lives Improved *)
1.811.88 billion, of which202221 million in underserved communities - Diversity & Inclusion
30%31.4% gender diversity in senior management positions39% gender diversity in total workforce77%73% Employee Engagement Index Score *) - Pay equality
EDGE-certified for Gender Equality in the NetherlandsUSUS Nationwide Pay Equity projectscheduled forcompleted in 2023 - Wage levelEUR
6,9526,903 million employee benefit expensesPhilips pays all employees at least a living wage - Risk for incidents of child, forced or compulsory laborAddressed in Philips GBP, Supplier Sustainability Declaration and Supplier Sustainability program
- Health & Safety
0.230.24 Total Recordable Case rate per 100 FTEs172 Total Recordable Cases - Training provided
1,880,4162,987,260 training hours in Philips University1,009,4593,578,199 training completions - Absolute number and rate of employment
77,23369,656 employees18%17.6% turnover - Supplier development program *)
296392 companies459,000723,000 employees impacted - Volunteering *)
2917 new projects in20222023 reaching26.012.0 million people
Governance
- Setting purposePhilips’ purpose is to improve the health and well-being of people through meaningful innovation
- Governance body compositionPhilips has a Board of Management and an independent Supervisory Board
- Material issues impacting stakeholdersDetailed double Materiality Analysis performed
- Anti-corruption
62,00060,000 employees completed General Business Principles training - Protected ethics advice and reporting mechanismsWhistleblower mechanism in place
- Integrating risk and opportunity in business processesIncluded in Risk Management section
- Economic contributionEUR
17,82718,169 million revenuesEUR741749 million dividend declaredEUR 6.7 million contribution to Philips FoundationEUR10395 million government grants - Financial investment contributionEUR
2,6382,483 million total tangible assetsEUR444345 million capital expenditures on property, plant and equipment - Total R&D expensesEUR
2.11.9 billion invested in R&D(11.8%(10.4% of revenues) - Total tax contributionEUR
3,4693,051 million
9Risk management
9.1Our approach to risk management
Vision and objectives
Philips approaches risk management as a value-creating activity that is integral to innovation and entrepreneurship. As such, it is part of the Philips Business System (PBS). KeyOperating Model. The key elements areof our risk management governance, Risk appetite, the Risk management process standard, the Philips Business Control Framework, and our General Business Principles (GBP), whichcontrol system are further described in this chapter. There can be no absolute assurance that our risk management will avoid or mitigate all risks that Philips faces. The material risks are described in the section Risk factors.
Risk management governance
The Executive Committee identifiesBoard of Management (BoM) is ultimately responsible for identifying, analyzing and managesmanaging the risks Philips faces in realizingexecuting its objectives. It definesstrategy and activities, for setting the risk appetite providesof the company, and for the design, implementation and maintenance of our risk management framework, and monitorscontrol system, including the effectiveness thereof. monitoring of its effectiveness. As described below, the Executive Committee (ExCo), several experts, Enterprise functions and committees support the BoM in the discharge of its responsibilities.
The ExCo is primarily responsible for identifying and mitigating materials risks to Philips. The ExCo is supported by the Risk Management Support Team, consisting of experts on various categories of risk, supports the Executive Committee through regular analysis of the enterprise risk profile and enhancement of the risk management framework. ManagementIn addition, management across the company is responsible for identifying critical risks and implementing appropriate risk responses within their areas of responsibility.
Various Enterprise functions (such as Internal Control,(e.g. Legal & ESG, Patient Safety & Quality, & Regulatory, Legal,Finance and Group Security) support the ExCo and management with the process of risk identification, risk management, and monitoring of key risk areas. With the support of these functions certain designated frameworks and activities to structurally manage specific risk areas.areas are maintained and deployed, such as:
- Deploying the Philips General Business Principles (GBP), which set the minimum standard for our business conduct as a health technology company for our individual employees and for our subsidiaries, and generally promoting a culture of compliance and ethics within the company.
- Maintaining Quality Management Systems (QMS) with the aim of ensuring the quality and safety of product design, manufacturing, distribution, and servicing in compliance with regulation from various government and regulatory agencies, e.g., FDA (US), EMA (Europe), NMPA (China). Through specialist teams at the global, regional or local level, standards and requirements are defined and continuously improved, deployed, and monitored to ensure our employees are aware of and comply with these requirements.
- Maintaining integrated management control of the company’s operations, reporting, and compliance with applicable laws, including the deployment of the Philips standard for Internal Controls over Financial Reporting (ICFR).
- Managing security (including cybersecurity) risks and evaluating the Group’s security strategy, issuing security policies and evaluating the progress and effectiveness of the deployment of the Security Management Framework (SMF).
- Developing the Philips ESG strategy, setting ESG policies, disclosures and planning of programs and activities in relation to our ESG commitments and obligations.
For further details refer to the section Governance.
To ensure clarity and alignment on the status of, and to make recommendations on key risk areas these functions have recurring items on the BoM meeting agenda. The BoM discusses these topics with participation from relevant ExCo members and other senior executives and subject matter experts. Furthermore, dedicated reports on these key risk areas are shared and discussed with the Supervisory Board and external auditors in the relevant Audit & Risk Committees facilitated by Internal Audit.
The Internal Audit function assesses the quality of risk management and controls through the execution of a risk-based audit plan, as approved by the Audit Committee of the Supervisory Board. LeadershipThe BoM and leadership from the Executive Committee, Businesses, MarketsRegions/Zones and key Functions meet quarterly with Internal Audit in Audit &and Risk Committees to discuss strengths and weaknesses of risk management and controls – as evaluated by internal and external auditors and by means of other (self) assessments – and take corrective action where necessary.
The Disclosure Committee oversees the company’s disclosure activities and assists the Board of ManagementBoM in fulfilling its responsibilities in this respect. The Disclosure Committee ensuresseeks to ensure that the company implements and maintains internal procedures for the timely collection, evaluation, and disclosure of information potentially subject to public disclosure under the legal, regulatory and stock exchange requirements to which the company is subject.
The GBP Review Committee is responsible for the effective deployment of the Philips General Business Principles (GBP) and for generally promoting a culture of compliance and ethics within the company. For more information see below under ‘Philips General Business Principles’.
The Security Steering Committee (SSC) and the Group Security function manage security (including cybersecurity) risks. The SSC evaluates and sets the Group’s security strategy, issues security policies and evaluates progress and effectiveness. Dedicated security reports are shared with the Executive Committee, the Supervisory Board and external auditors. On a quarterly basis, briefings on cybersecurity risks are provided to the IT Audit & Risk Committee.
The Environmental, Social and Governance (ESG) Committee initiates, drives and coordinates ESG strategy development, policy setting, disclosures and planning of programs and activities in relation to our ESG commitments and obligations. It administers ESG reporting, monitors progress, assesses risks in relation to ESG and makes recommendations to the Executive Committee on our ESG endeavors.
Philips actively maintains Quality Management Systems (QMS) with the aim of ensuring the quality and safety of product design, manufacturing, distribution, and servicing in compliance with regulation from various government and regulatory agencies, e.g., FDA (US), EMA (Europe), NMPA (China). Our Quality & Regulatory function closely monitors developments in the regulatory landscape. Through specialist teams at the global, regional or local level, standards and requirements are defined and continuously improved, deployed, and monitored to ensure our employees are aware of and comply with these requirements. Next to continuous improvement a program runs with the aim to accelerate patient safety and quality. A formal quality audit program assesses our organization’s compliance with our QMS. Quality & safety is a standard item in personal goal setting and evaluation of all Philips’ employees.
The Supervisory Board oversees Philips’ risk management including the identifiedidentification of material risks, in relation to the Riskrisk appetite of the company, the response measures put in place and the effectiveness thereof. The Audit Committee and the Quality & Regulatory Committee of the Supervisory Board assist the full Supervisory Board in fulfilling its risk management oversight responsibilities. The Audit Committee reviews the quality of risk management and controls, and the reported findings of internal and external audits. The Quality & Regulatory Committee’s role particularly relates to the quality and regulatory compliance of the company’s products (including software), services and systems throughout their lifecycle.
The chapter Corporate governance chapter of this report addresses the main elements of the company’s corporate governance structure, reports on how it applies the principles and best practice provisions of the Dutch Corporate Governance Code and provides other information relevant to risk management governance.
Risk appetite
The Executive Committee and management seekPhilips seeks to manage risks consistently within theits risk appetite. Risk appetite is set by the Executive CommitteeBoM, reviewed at least annually and capturedpublished in the Philips risk management policy. It is effectuated through our PBS,Operating Model, of which various elements – such as our strategy, Philips General Business Principles (GBP) and behaviors, authority schedules, policies, process standards and performance management systems – include or reflect risk-taking guidance.
Philips’ risk appetite differs depending on the type of risk, ranging from an averse to a seeking approach. Philips operates within the dynamics of the health technology industry and aims to take the risks needed to ensure we continually revitalize our offerings and the way we work. At the same time, Philips is committed to always act with integrity always and is averse to risks impacting our GBP, which include (but are not limited to) the Philips behavior ‘Patient safety, Quality, and Integrity always’. Our employees are expected to ensure compliance with our GBP, laws, and regulations, and to acttake action in the case of concerns or violations to our GBP, pleaseGBP. Please refer to the GBP section belowPhilips General Business Principles (GBP) for more information. Philips’ Riskrisk appetite for the main risk categories is visualized below. Philips does not classify these risk categories in order of importance.
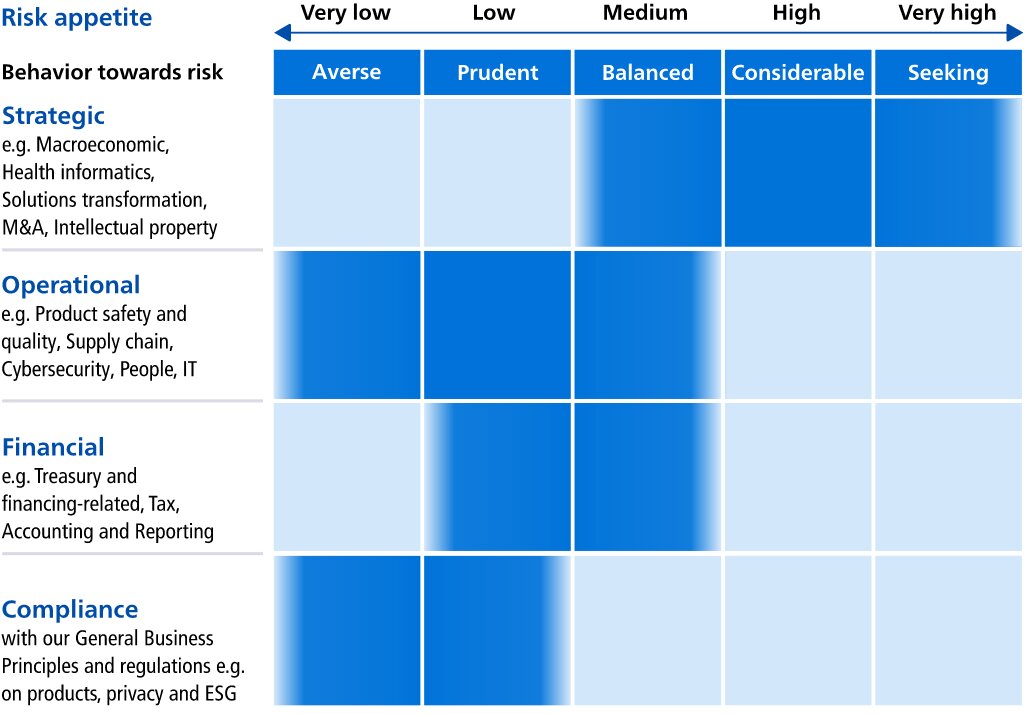

Risk management process
To provide a comprehensive view of Philips’ risks, structured risk assessments take place according to the Philips risk management process standard, applying a top-down and bottom-up approach. Our process standard is designed based on the Enterprise‘Enterprise Risk Management Framework:Management: Integrating with Strategy and PerformancePerformance’ (2017) from the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and on ISO 31000 - Risk Management.
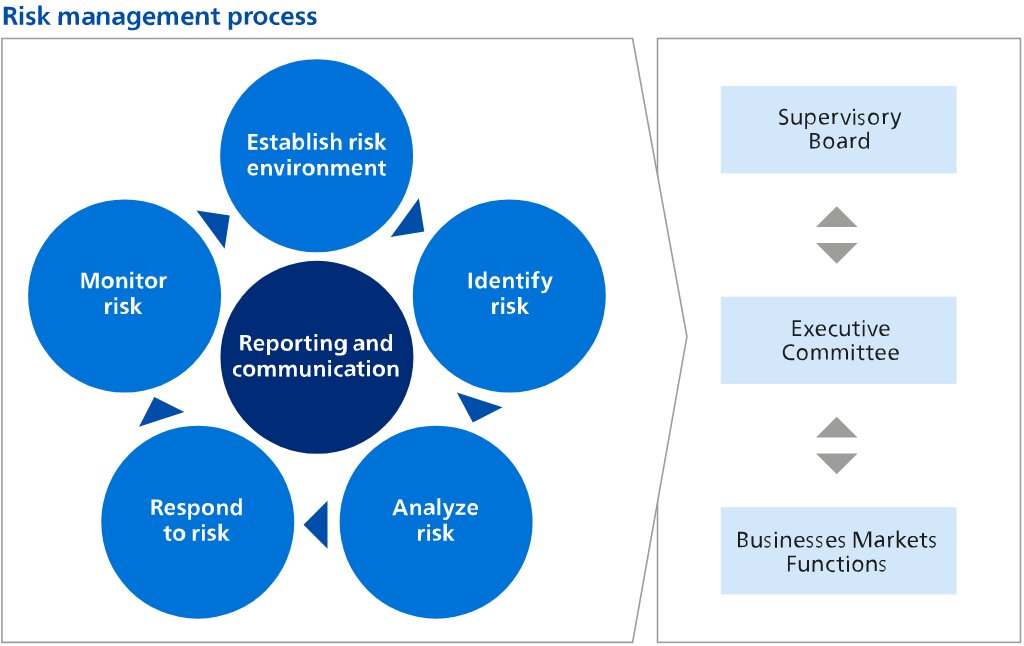

Key elements of the Philips risk management process are:
- Management of Businesses,
MarketsZones/Regions and key Functions perform a risk assessment at least once a year, withupdatesthe update ofthetheir strategicplan,plans, to identify and prioritize risks, assign ownership, and implement appropriate risk responses. Risk workshops areconductedfacilitated by Internal Audit with senior management across the company tofacilitatefurther support these risk assessments, and during20222023 several of such risk workshops were held. - Senior management discusses and monitors the risk profile and risk response effectiveness at least quarterly in its performance reviews and during Audit & Risk Committees, which cover all Businesses,
MarketsZones/Regions and selectedFunctions and at Group level.Functions. - Developments in the enterprise risk profile and management’s initiatives to improve risk responses are discussed and monitored during the quarterly
meetingAudit & Risk update session of theAudit Committee of the Supervisory Board.BoM. - As an integral part of the strategy review, each year the
Executive CommitteeExCo assesses the enterprise risk profile and the potential risk impact versusGroupEnterprise risk appetite. The assessment also covers the effectiveness of the risk management framework and potential improvements thereto. - The Philips risk profile and the risk management framework, including the outcomes of the annual ExCo risk workshop, are presented to the full Supervisory Board. The underlying risks and response plans are discussed at
least once athe end of the year with the Audit Committee of the Supervisory Board.
Examples ofThe measures taken during 20222023 to further strengthen risk management:management include:
In addition to the continuous improvementImproved clarity and efficiency of ourQMS we run an enterprise-wide program Accelerating Patient Safetyrisk management governance by streamlining the reviews andQuality. This program was initiated in 2021 following the Respironics voluntary recall to evaluate and further improve our QMS, our oversight & performance management and our culture where necessary. For more information, refer to theQuality & Regulatory and patient safety.decision making on several key risk areas.An enterprise-wide analysis is conducted regarding detection and reporting of product defects/failures, applying the lessons learned from the Respironics field action to each Philips business.VariousImplemented various improvements to our risk management process standards in several risk areas, for exampleenterprise risk, productfor compliance risk andsupplier-operational risk management.- Strengthened our capabilities via upgrades to our risk management tooling and
supply-chain-related risk.use of data analytics. - Conducted various analytics to further increase knowledge about risks within the company, via deep dives, risk interdependency analysis, and risk velocity analysis using statistical scenario modelling.
- Further standardization and alignment of controls in Philips process standards.
- Further developed our regulatory landscape monitoring to enhance foresight in and
the embedding in the global Philips standard process framework.internal communication on upcoming regulatory change to enable a more proactive response. Risk & Compliance (R&C) community building to drive continuous improvement, knowledge sharing, capability building and risk transparency across various R&C areas.StrengtheningContinued strengthening the risk dialogue as an integrated part of regular performance and strategy execution dialogues.Continued improvement of our risk management capabilities in security (including cyber, product and supply chain).Extension ofFurther improved our supplier risk management,to deeper tiers,anddiversifieddiversification of critical-component sourcingof high-risk componentsto further reduce supply dependencies.AnalysisContinued analysis of global warming and weather scenarios on the geographical footprint of our facilities as well as suppliers’, in line with the recommendations of the Task Force on Climate-Related Financial Disclosures.Ongoing exploration and capturing of opportunities to use data analytics and automation in controls monitoring, and the ongoing deployment of our governance, risk and compliance IT solutions.
Philips Business Control Framework
The Philips Business Control Framework (PBCF) sets the standard for internal control at Philips. The objective of the PBCF is to maintain integrated management control of the company’s operations and reporting, as well as safeguard compliance with applicable laws and regulations. Philips has designed its PBCF based on the COSO Internal Control-Integrated Framework (2013).
As part of the PBCF, Philips has implemented a standard set of Internal Controls over Financial Reporting (ICFR). Together with Philips’ established accounting procedures, this standard set of internal controls is designed to provide reasonable assurance that assets are safeguarded, that the books and records properly reflect transactions necessary to permit preparation of financial statements, that policies and procedures are carried out by qualified personnel, and that published financial statements are properly prepared and do not contain any material misstatements. In each reporting unit, management is responsible for customizing the controls set for their business, risk profile and operations.
Each year, management’s accountability for ICFR is evidenced through the formal certification statement sign-off. Any deficiencies noted in the design and operating effectiveness of ICFR that were not completely remediated are evaluated at year-end by the Board of Management. The Board of Management’s report, including its conclusions regarding the effectiveness of ICFR, can be found in this report in the section Management's annual report on internal control over financial reporting.
Philips General Business Principles (GBP)
The GBP – part of the Philips Business System – incorporate and represent the fundamental principles by which all Philips businesses and employees around the globe must abide. They set the minimum standard for our business conduct as a health technology company, for our individual employees and for our subsidiaries. The GBP form an integral part of labor contracts in virtually every country in which Philips operates, and translations are available in 30 languages. Each year, employees reconfirm their commitment to the code of conduct after completing their GBP e-learning, and there is an additional annual sign-off for Executives. A similar sign-off is in place for Finance and Procurement staff for their respective codes of conduct. Detailed underlying policies, manuals, training, and tools are in place to give employees practical guidance on how to apply and uphold the GBP in their daily work.
The GBP Review Committee is responsible for the effective deployment of the GBP and for generally promoting a culture of compliance and ethics within the company. The Committee is chaired by the Chief ESG & Legal Officer, and its members include the Chief Financial Officer, Chief Human Resources Officer and the Chief of International Markets. Furthermore, each quarter all our key markets convene market compliance committees, which act as local satellites of the GBP Review Committee, dealing with GBP-related matters in the local context. They are also responsible for the design and execution of localized compliance plans that are tailored to their market-specific risks and organizational set-up, and regularly review the relevant compliance metrics for their respective market through dashboards delivered by the legal compliance monitoring team. The Secretariat of the GBP Review Committee, together with a worldwide network of GBP Compliance Officers, supports the organization with the implementation of GBP initiatives.
As part of our continuous effort to raise GBP awareness and foster dialogue throughout the organization, each year a global GBP communications and training plan is deployed, including structured dialogues led by managers where quality, integrity and speaking up are discussed. This is part of a company-wide initiative aimed at reinforcing a culture of dialogue using ethical dilemma case studies that are relevant to our workforce. A key control to measure implementation of our GBP is the GBP monitoring and reporting program, which is part of our Internal Control framework. In addition, we continue to expand the capabilities of our legal compliance monitoring team, serving our business customers as well as compliance networks with actionable data, thus further improving our compliance control framework.
The GBP are supported by established mechanisms with the aim of ensuring standardized reporting and enable employees and third parties to escalate concerns 24/7. Concerns raised are registered consistently in a single database hosted outside of Philips servers to ensure confidentiality and security of identity and information. Encouraging people to speak up through the available channels if they have a concern will continue to be a cornerstone of our GBP communications and awareness campaigns. At least twice a year, the GBP Review Committee, as well as the Executive Committee and Audit Committee of the Supervisory Board, are informed on relevant GBP metrics, cases, trends and learnings.
Through the Audit Committee of the Supervisory Board, the company also has procedures in place for the receipt, retention and treatment of complaints specifically relating to accounting, internal accounting controls or auditing matters, which enable the confidential, anonymous submission of complaints.
More information on the Philips GBP can be found in Environmental, Social and Governance. The GBP and underlying policies, including the Financial and Procurement Code of Ethics, are published on the company website, at https://www.philips.com/a-w/about/investor-relations/governance/business-principles.html .
9.2Risk factors
Philips believes the risks set out below are the material risks that, individually or in combination, could impact our ability to achieve our objectivesaffecting Philips and to live up to the expectations of our customers and stakeholders.its securities. These risk factors may not, however, include all the risks that ultimately may affect Philips. Some risks not yet known to Philips, or currently believed not to be material, may ultimately have a major impact on Philips’ business, revenue, income, assets, liquidity, capital resources, reputation and/or ability to achieve its business and ESG objectives. Please note that this section is not intended to describe riskrisks that have materialized, as these are addressed in other sections and referenced to where relevant. Philips defines risks in four main categories: Strategic, Operational, Compliance and Financial. Philips presents the risk factors within each category in order of our current view of their expected significance. Compared to the previous year we have further prioritized risk factors relating to patient safety and quality, management, addressing the Respironics voluntary recallsupply chain, and the regulatory and legal processes connected to this, geopolitical and macro-economic factors, and to our supply chain operations.simplification of how we work. Although still relevant, we have de-emphasized risk factors related to pandemics. This does not mean that a lower-listed risk factor may not have a material and adverse impact on Philips’ business, revenue, income, assets, liquidity, capital resources, reputation, and/or ability to achieve its business and ESG objectives. Furthermore, other risk factors not listed below may ultimately prove to have more significant adverse consequences than the listed risk factors.
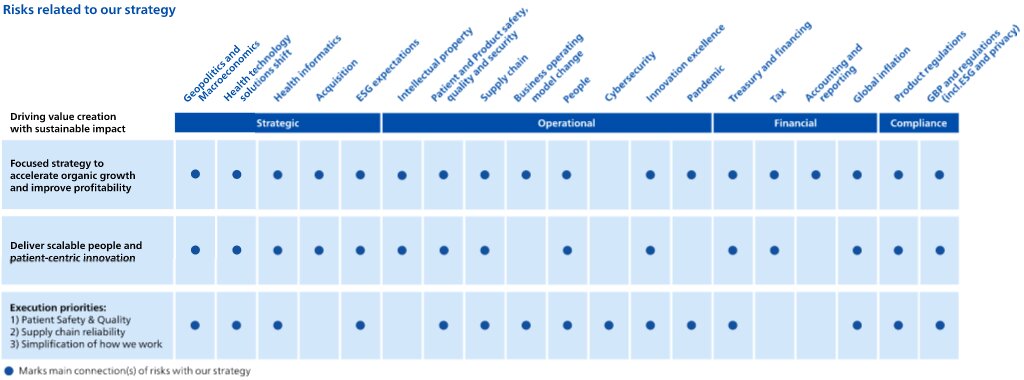

9.3Strategic risks
Philips’ global operations are exposed to geopolitical and macroeconomic changes
Philips’ business environment can be adversely impacted by macroeconomic and geopolitical conditions in global and individual markets. In 2023, Mature geographies accounted for 72% of Philips’ revenue, while Growth geographies accounted for the remaining 28%. Mature economies are currently the main source of Philips’ revenues, while growth economies are an increasing source of revenues. Philips produces, sources, and designs its products and services mainly from the United States (US), the European Union (EU) (primarily the Netherlands) and China, and the majority of Philips’ assets are located in these geographies. Changes in politics and monetary, trade and tax policies in the US, the EU and China may trigger reactions and countermeasures and may also have an adverse impact on other economies and international markets in which Philips is active. Philips continues to expect global market conditions to remain highly uncertain and volatile due to geopolitical and macroeconomic factors, whether or not they are related to or caused by the Russia-Ukraine war.war and/or the current situation in Israel and the larger Middle East region.
Philips observes a trend of geopolitical tensions and deglobalization which intensifies protectionism. Examples of protectionism measures are trade policies, on trade, tariffs, sanctions, local value creation and production requirements to obtain market access, custom duties, taxation, technology and data restrictions, cyberattacks, import or export controls, talent mobility restrictions, nationalization of assets, restrictions on repatriation of returns from foreign investments, andinvestments. In addition, there is general uncertainty on the development of local regulations and compliance thereto. Philips observes this trend in the major markets in which it operates and has a particular concern on the development of the US-China relationship and China’s drive to expand its global political footprint and become self-sufficient in critical technologies, including health-related ones.
If this trend continues, geopolitical relations deteriorate, and economies decouple, it is expected that existing global trade and investment restrictions will remain, and further regulatory and compliance challenges for doing business globally may emerge, resulting in continued pressure on market growth and investments.
Uncertainty and challenges regarding various global macroeconomic factors continue to persist. Examples of general factors are an overall weakening economy, declines in economic growth projections in the US, the EU and China (which collectively account for around two-thirds of Philips’ sales),outlook, reduced government spending, declining customer and consumer confidence and spending, risinghigh inflation and interest rates, and the emergence of economic impacts related to the climate crisis. Although the ability to manage pandemics (for example, resurgences of COVID-19 or mutations thereof) has improved, pandemics may continue to affect Philips’ operations in the future. Examples of healthcare-specific potential factors include rising uncertainty over the future direction of public healthcare policy and the risk of declining public investment in healthcare ecosystems.
The Russia-Ukraine war has increased global economic and political uncertainty. Governments in the US, the UK, the EU, Canada, and Japan have each imposed export controls on certain products and sanctions on certain industry sectors and institutions in Russia, and additional controls and sanctions could be enacted in the future.
Similarly, the conflict in Israel will further increase economic and political uncertainty and may affect the company’s results of operations, financial position and cash flows. Philips is present in Israel with several subsidiaries, mainly in Diagnosis & Treatment and Connected Care, that are primarily involved in manufacturing and research and development (R&D) activities. Upcoming elections in the US, the UK and the EU could also have an impact on the course of these conflicts. The Russia-Ukraine warongoing conflicts may heighten the impact of other risks factors described herein, including but not limited to: volatility in prices for transportation, energy, commodities and other raw materials; disruptions in the global supply chain; decreased customer and consumer confidence and spending; increased cyberattacks; intensified protectionism; political and social instability; increased exposure to foreign currency fluctuations; rising inflation and interest rates; and constraints, volatility or disruptions in the credit and capital markets. It is possible that the conflict in Ukraine may escalate or expand and current or future sanctions and resulting geopolitical and macroeconomic disruptions could be significant. We cannot predict the impact the conflict may have on the global economy in the future.
Changes in geopolitical and macroeconomic conditions are difficult to predict, and the factors described above, or other factors, may lead to adverse impacts on global trade levels and flows, economic growth, and financial market and political stability, all of which could adversely affect the demand for, and supply of, Philips’ products and services. This may result in a material adverse impact on Philips’ business, financial condition, and operating results. These factors could also make it more difficult to budget and to make reliable financial forecasts or could have a negative impact on Philips’ access to funding.
Philips may be unable to shift tokeep pace with the changing health technology solutions and services business modelenvironment
With Philips’ focus on health technology, our business model is transforming from transactional, product-focused business models to customer- and patient-centric, outcome-oriented business models, with multi-year customer partnerships enabled by a portfolio of innovative devices, solutions, platforms, insights and value-added services. If this transformation is made too slowly or is not successful, Philips may not meet the expectations of patients and other stakeholders in the Health Technologyhealth technology business environment. ItWe may face a loss of customer relevance, fail to capture growth, and lose market share. In addition, because of our health technology focus, Philips may have a reduced ability to offset potential negative impacts (including, but not limited to, impacts on sales, operating results, liabilities, compliance, and financing) on its health technology business by other businesses through a more diversified portfolio. As a result of the shift to aits focus on health technology, Philips is deepening customer engagement and entering into long-term solutions and services business model, Philipsarrangements and, as a result, is becoming more dependent on a number of key customers for long-term recurring revenues, thus increasing the risk that the loss of, or a significant reduction in, orders from one or more of our key customers could cause a significant decline in our revenues. As Philips looks to increase our use of indirect sales channels, Philips will increasingly rely on successfully leveraging new and existing partners to support end customers and patients. Any of these factors may have a material adverse impact on Philips’ brand value and reputation, business, financial condition, and operating results. More specific Health Technologyhealth technology risks and their potential impacts are included in the Operational, Financial and Compliance risk sections below as well as in the Notenote Contingencies.
Philips may be unable to gain leadership in health informatics
New digital technologies and ways of conducting business are fundamentally changing the health technology industry, and thus our competitive business environment. A key trend, started in radiology, is the application of artificial intelligence (AI) and machine learning (ML) to drive quality and efficiency in clinical and operational workflows. Customers need workflow-aware solutions that convert data from our imaging and monitoring systems into actionable insights. Another trend, accelerated by the pandemic, is the shift toward cloud-based Software as a Service (SaaS) business models and remotely upgradable and serviceable systems with suites of apps. These new types of offerings are enabled by hybrid cloud/on-premise digital platforms. Our informatics and systems businesses may fall behind established and new ‘born digital’ competitors if Philips fails to, in a timely way, develop the requisite capabilities, adjust its business models, and find ways to globally commercialize new products and services at scale. This could result in an inability to satisfy customer and patient needs, thereby missing out on revenue and margin growth opportunities, which may have a material adverse impact on Philips’ business, financial condition and operating results.
Acquisitions could fail to deliver on Philips’ business plans and value creation expectations, and we may not be able to successfully integrate acquired operations
SelectedAlthough Philips focusses on organic growth to deliver patient and people driven innovation at scale, selected acquisitions have been, and are expected to remain part of Philips’ growth strategy. We may not be able to integrate acquisitions successfully or efficiently integrate new acquisitions with our existing operations, culture and systems, which may expose Philips to risks in areas such as sales and service, logistics, quality, regulatory compliance, legal claims, information technology, and finance. Integration challenges may adversely impact the realization of value creation expectations. Transactions may incur significant costs, result in unforeseen operating difficulties, divert management attention from other business priorities, and may ultimately be unsuccessful. Cost savings expected to be implemented, or other assumptions underlying the business case relating to a particular acquisition, may not be realized. If we are unable to accomplish any of our objectives in respect of any of our new acquisitions, we may not realize the anticipated benefits of such acquisitions and we may experience lower than anticipated profits, or even incur losses. Acquisitions may also lead to a substantial increase in long-lived assets, including goodwill, which may later be subject to write-down if an acquired business does not perform as expected, which may have a material adverse effect on Philips’ earnings.
Philips may be unable to meet internal or external aims or expectations with respect to ESG-related matters
Environmental, Social and Governance (ESG) factors may directly and indirectly impact the business environment in which Philips operates. Philips may, from time to time, disclose ESG-related initiatives or aims in connection with the conduct of its business and operations (for example, with respect to reducing greenhouse gas emissions in its supply chain). However, there is no guarantee that Philips will be able to implement such initiatives or meet such aims within anticipated timeframes, or at all. In addition, there is an increasing focus from Philips’ stakeholders – including customers, employees, regulators, and investors – on ESG matters, and those stakeholders may also have ESG-related expectations with respect to Philips’ business and operations. For example, customers may focus on ESG-related criteria in buying our products, and any inability by Philips to address concerns about ESG-related matters could negatively impact sentiment towards Philips and our products and brands. There are an increasing number of regulatory and legislative initiatives in the EU and other jurisdictions to address ESG issues, which will or may (if(once implemented) require Philips to significantly increase the scope of mandatory ESG disclosures.disclosures, including the Corporate Sustainability Reporting Directive (CSRD), European Sustainability Reporting Standards (ESRS) and the SEC’s proposed climate disclosure rules. They will introduce or may (if implemented) requireextend a duty of care, requiring Philips to identify and act on adverse environmental and human rights impacts across the organization and potentially the entire value chain, beyond or different from our current efforts. These regulatory and legislative initiatives, in turn, could also affect how customers or other stakeholders perceive our products or business operations. If our products or business operations do not meet the criteria for sustainability according to, for example, the EU Taxonomy Regulation (including the related delegated regulations) or any other similar regulations, this may negatively affect how customers or other stakeholders view Philips. Philips may fail to fulfill internal or external ESG-related initiatives, aims or expectations, or be perceived to do so, or we may fail adequately or accurately to report performance or developments adequately or accurately with respect to such initiatives, aims or expectations. In addition, Philips could be criticized or held responsible for the scope of its initiatives or goals regarding ESG matters. Any of these factors may have an adverse impact on Philips’ reputation and brand value, or on Philips’ business, financial condition and operating results.
Philips may be unable to secure and maintain intellectual property rights for its products and services or may infringe others’ intellectual property rights
Philips is dependent on its ability to obtain and maintain licenses and other intellectual property (IP) rights covering its products and services and its design and manufacturing processes. The IP portfolio is the result of an extensive IP generation process that could be influenced by a number of factors, including innovation and acquisitions. The value of the IP portfolio is dependent on the successful promotion and market acceptance of standards (co-)developed by Philips. This is particularly applicable to the segment ‘Other’, where licenses from Philips to third parties generate IP royalties and are important to Philips’ results of operations. The timing of licenses from Philips to third parties and associated revenues from IP royalties are uncertain and may vary significantly from period to period. Additionally, royalties are often based on sales by third parties, creating an exposure to macroeconomic effects and continuity of these third parties. A loss or impairment in connection with such licenses to third parties could have a material adverse impact on Philips’ financial condition and operating results. Philips is also exposed to the risk that a third party may claim to own IP rights to technology applied in Philips’ products and services. If any such claims of infringement of these IP rights are successful, Philips may be required to pay damages to such third parties or may incur other costs or losses.
9.4Operational risks
Products and services may fail quality or security standards, which maycould adversely affect patient safety andor customer operations
The safety of patients and our brand reputation depends on the safety and quality of our products and services. Our products and services, either new and/or in field use by our customers, may failFailure to meet product quality or product security standards. In particular, Philips is exposed to the ongoing impact of the Respironics voluntary recall and related matters. Please refer to the section Quality & Regulatory and patient safety and the Note Contingencies. If products fail to meet product quality and/or security standards this may cause (patient)patient harm, negatively impact customer operations and their ability to provide healthcare, provide unauthorized access to patient records and medical devices through cybersecurity incidents, or generally cause customer dissatisfaction. Given Philips’ focus onand damage Philips' reputation and brand.
As a health technology innovator, our products and services often requiremust comply with rules and regulations that govern our operations, processes, and ways of working. Risks associated with non-compliance with quality, regulatory, approvals, including approval ofand security assessments apply to pre-market activities (such as product design, production and supplier quality activities) and benefit/risk prior to market introduction.post-market activities. There are risks involving hardware, software and human error, spanning across the lifecycle, and involving third-party suppliers and components. Many of our products also have multiple third-party software components, which may be exposed to security threats, including potentially in the eventthreats. We are subject to risk from known issues, and emerging potential issues. Potential consequences of obsolescence or insufficient maintenance. Issues with the quality or security of our products and services can occur as a result of various factors, including product design, production, suppliers, materials used, installation, or newly emerging and rapidly evolving cybersecurity threats. These (and other) issues could cause events that need to be actively addressed, which may lead to (amongst others) higher costs of design, market de-activation, stop use, field recalls and repairs, financial claims and liabilities,these risks include damage to our brand reputation, competitive disadvantage, regulatory non-compliance (referconsent decrees (for example, the proposed Respironics consent decree described in note Subsequent events to the section Compliance risks)Consolidated financial statements), consent decrees orand losing our licenselicenses to operate for products or access to markets. Anyin specific markets, all of thesewhich may have a material adverse impact on Philips’ business, financial condition and operating results.
Notwithstanding the proliferation of technology and technology-based control systems to detect defects or other errors in our products before they are released, our business ultimately relies on people as our greatest resource, and, from time to time, they make mistakes or engage in violations of applicable policies, laws, rules or procedures. These events are not always caught immediately by our technological processes or by our controls and other procedures, which are intended to prevent and detect such errors or violations. In addition to human error, our quality controls are also subject to overriding, as well as resource or technical constraints. As such, these quality controls and preventative measures may not be effective in detecting all defects or errors in our products before they have been released into the marketplace. In such an event, the technological reliability and safety of our products could be below our standards, and our reputation, brand and sales could be adversely affected. In addition, we could be required to, or may find it necessary to, offer a refund for the product or service, suspend the availability or sale of the product or service, or expend significant resources to cure the defect or error. Any of these factors may have an adverse impact on Philips’ reputation and brand value, or on Philips’Philips' business, financial condition, and operating results.
Philips may be unable to ensure a resilient supply chain
Most of Philips’ operations are conducted internationally, which exposes Philips to supply chain challenges and uncertainties. Philips produces and procures products and parts in various countries globally, including Asian countries. Disruption to production in, and shipping from, Asian countries could have a disproportionate impact on our business compared to disruptions in other markets.globally. The production and shipping of products and parts, whether from Philips or from third parties, could be interrupted and may face increasing costs by various external factors, such as geopolitics (for example, US-China relations and protectionist measures taken in other markets), regional conflicts (e.g. the Middle East region), natural disasters, or extreme weather events (the effects of which may be exacerbated by climate change), container imbalances, port congestions, and continued uncertainty relatedgeopolitics.
While macro trends around materials availability have improved in 2023, Philips’ medical systems stay in production for longer periods than the lifecycle of their semi-conductors and require continuous rejuvenation of their electronic components. Philips may fail to COVID-19 measures (particularly in China). Throughout 2022 we experienced supply chain headwindstimely obtain or replace such components from existing supplies, and expect these to continue throughout 2023. Currently, components are scarce. Global supply constraints and cost impacts as a result of worldwide economic disruptions, electronic component shortages, fear of future or ongoing pandemics, inflation, and geopolitical events, including the war in Ukraine, are impacting our ability to procure components. Obtaining alternative sources of components could involve significant costs and regulatory challenges and may not be available to us on reasonable terms, if at all. As a health technology company, Philips is dependent on the availability of components, including semiconductors. Semiconductors have been subject to an ongoing global supply shortage. At the same time our product design may include obsolescent semiconductors and other components. If semiconductor shortage continues, we may experience delays, production interruptions, increased costs, the need to make engineering design changes or the inability to fulfill customer demand, any of which could adversely affectaffecting our business and financial performance. Philips, our customers, our
Our suppliers and our third-party service providers may also be exposed to labor shortages and potentially as a result of COVID-19.worsening macroeconomic and geopolitical trends. These factors may cause increased lead times and adversely impact our production capacity, which may negatively impactaffect the delivery of products and services to customers, for example the postponement of equipment installations in hospitals. If Philips is not able to respond swiftly to those factors, this may result in an inability to deliver on customer needs, ultimately resulting in loss of revenue and margin.
A general shortage of energy, materials, (sub-)components or means of transportation may drive fluctuations in price. Philips purchases raw materials, including rare-earth metals, copper, steel, aluminum, noble gases and oil-related products. ThereWhile the macroeconomic trend of improved materials availability also positively impacts the raw materials and energy cost compared to 2022, there is no assurance that these raw materials will be available for purchase in the future. The actions by the governments in the US, UK, and the EU in response to the war between Russia and Ukraine, among other factors, have had an adverse impact on the cost of the raw materials that we purchase. future or available at current costs.
Commodities have been subject to volatile markets, and such volatility is expected to continue and costs to increase. Costs may also increase as a result of stricter climate-change-related laws and regulations. Such legislation could require investments in technology to reduce energy use and greenhouse gas emissions, beyond what we expect in our existing plans, or could result in additional and increased carbon pricing. If Philips is not able to compensate for increased costs of energy, (sub-)components, (raw) materials, and transportation – either by reducing reliance thereon or passing on increased costs to customers – then price increases could have a material adverse impact on Philips’ business, financial condition, and operating results.
Philips may increase its dependency on a concentration of external suppliers, as a result of the continuing process of creating a leaner supply base and launching initiatives to replace internal capabilities with less costly outsourced products and services. These initiatives also need to be balanced with local-market value-creation requirements, including those relating to local manufacturing and data storage.
Although Philips works closely with its suppliers to avoid supply discontinuities, there can be no assurance that Philips will not encounter future supply issues, causing disruptions or unfavorable conditions. Furthermore, while the materials supply has improved in 2023, the challenges in our capability for the planning and synchronization of supply with demand continue, which combined with a drive for inventory reduction and cash flow improvements, can lead to further materials running out of stock, which could have a material adverse impact on Philips’ business, financial condition and operating results.
Philips may face challenges in connection with its strategy to improve executionsimplifying the organization and other business performance initiativesthe ways of working
As announced in January 2023, Philips has prioritizeda simplified, more agile operating model is a priority to improve the further strengtheningexecution of our patient safety and quality management, our supply chain operations, and the simplification of the organization and the ways we work.strategy. If we do not effectively managesimplify the necessaryorganization and our ways of working, which changes including any upgradesinclude, but are not limited to, Philips’changes in governance, roles, processes, and IT landscape and architecture, this may result in us not realizinglimiting our ability to fully realize our business ambitions with respect to growth, safety, quality, operational excellence, productivitydelivering sustainable impact, meeting critical patient and solutions delivery, amongst others,customer needs, delivering integral value proposition, growing the business, and/or may causemaintaining business discontinuities. There can be no assurance that the recently announced changes incontinuity. While Philips has implemented a new operating model will be successful in supporting Philips’ strategy or improving Philips’ resultsto simplify the organization and improve its ways of operations, andworking, Philips may need to undertake further restructuringschanges and related restructuring in the future. Iffuture if the recently announced restructuringoperating model ultimately proves to be wholly or any future restructurings ultimately prove unsuccessful or have a material adverse effect on Philips’ reputationpartly unsuccessful.
To simplify ways of working and brand value, Philips’ business, financial condition, and operating results could be materially adversely affected.
improve performance, Philips continuallycontinuously seeks to create a more open, standardized, and cost-effective IT landscape. Approaches include further outsourcing, offshoring, commoditization,integration, and ongoing reduction in the numberconsolidation of IT systems. These changes createmay elevate third-party dependency risks regarding the delivery of IT services, the availability of IT systems, and the functionality offered by IT systems. Although Philips has sought to strengthen security measures and quality controls relatingrelated to these systems, these measures may prove to be insufficient or unsuccessful, which may lead to a material adverse impact on Philips’ business, financial condition, and operating results.
Philips is dependent on its people for leadership and specialized skills and may be unable to attract and retain such personnel
In October 2022 and January 2023, Philips announced a series of reductions in workforce. These restructuring measures may negatively impact Philips’ reputation and its ability to attract and retain employees whose skills and experience are important for its business. Layoffs of skilled employees may subject Philips to potential employment lawsuits and benefit Philips’ competitors. Philips’ restructuring measures may also pose operational challenges and place a substantial strain on remaining management and employees. The reduction in workforce may adversely affect the pace and breadth of Philips’ research and development efforts. The diversion of management time to planning and implementing any restructuring measures may also cause disruptions to Philips’ business.
The attraction and retention of talented employees is critical to Philips’ success, and the loss of employees with specialized skills could result in business interruptions. There is fiercestrong competition for talent in key capability segments, and there is a heightened expectation of attrition post-pandemic. The announced organizational restructuring may also impact employee engagement. These factors may affect Philips’ abilityPhilips needs to attract and retain critical talent. Post-COVID-19 adjustments such as hybrid working may continue to present challenges to team interactions and the onboarding of new people. If employees perceive our post-COVID-19 approachthe workload following the recent operating model transformation and workforce reduction to working to be inadequate, overly burdensome or prefer the safety or convenience ofmore flexibility than offered by our hybrid working from home,policies, to mention two, employees may choose to terminate their employment with us, productivityus. In this case, efficiencies in workflow may decline,be impacted, or we may experience employee unrest, slowdowns, stoppages or other demands.demands, such as overburden of the remaining employees. Philips is competing for the best talent and most sought-after skills, and there is no assurance of succeeding compared to other companies in attracting and retaining the highly qualified employees needed in the future. Wage inflation is increasing the competition for talent andas well as the cost of labor. This may negatively impact our ability to deliver onrealize our strategic imperatives,plan for creating value with sustainable impact, and if we are unable to offset the increased costs of labor through higher selling prices and increased productivity, then rising costs could also have a material adverse impact on Philips’ business, financial condition and operating results.
Philips could be exposed to a significant enterprise cybersecurity breach
Philips relies on information technology to operate and manage its businesses, andas well as store and process confidential data (relating to patients, employees, customers, intellectual property, suppliers and other partners). Philips’ products, solutions and services increasingly contain sophisticated and complex information technology. The healthcare industry is subject to strict privacy, security and safety regulations with regard to a wide range of health information. At the same time, geopolitical conflicts and criminal activity continue to drive increases in the number, severity, and sophistication of cyberattacks globally. Considering the general increase in cybercrime, our customers and other stakeholders are becoming more demanding regarding the cybersecurity of our products and services. As a global health technology company, Philips is inherently and increasingly exposed to the risk of cyberattacks and potential impact of attacks on our suppliers. Information systems may be damaged, disrupted (including the provision of services to customers), or shut down due to cyberattacks. In addition, breaches in the security of our systems (or the systems of our customers, suppliers, or other partners) could result in the misappropriation, destruction, or unauthorized disclosure of confidential information (including intellectual property) or personal data belonging to us or our employees, customers, suppliers or other partners. These risks are particularly significant with respect to patient medical records. Cyberattacks may result in substantial costs and other negative consequences, which may include, but are not limited to, lost revenues, reputational damage, remediation and enhancement costs, penalties, and other liabilities to regulators, customers and other partners. Philips has not encountered any material breaches or other significant cybersecurity incidents in 2022.2023. While Philips deals with the operational threat of cybercrime on a continuous basis and has so far been able to prevent significant damage or significant monetary cost in taking corrective action, there can be no assurance that future cyberattacks will not result in significantmaterial or other consequences than as described above, which may result in a material adverse impact on Philips’ business, financial condition and operating results.
Philips may face challenges to drive excellence and speed in bringing innovations to market
To gain sustainable competitive advantage and create value with sustainable impact, Philips aims to deliver on our purposescalable, people-centric, and the Quadruple Aim (better health outcomes, improved patient experience, improved staff experience and lower cost of care), itpatient-centric innovations. It is important that Philips continues to innovateinnovates and delivers these innovations to the marketin close collaboration with its customers on a timely basis.basis and at scale. The emergence of new low-cost competitors, particularly in Asia, the rise of artificial intelligence (AI) and data driven solutions, and the increasing importance of product and cyber security, further underlines the importance of improvements in the innovation process. Success in launching innovations depends on a number of factors, including development of value propositions, architecture and platform creation, product development, market acceptance, production, and delivery ramp-up. It is also dependent on addressing potential quality issues or other defects in the early stages of introduction, and on attracting and retaining skilled employees. Costs of developing new products and solutions may partially be reflected on Philips’ balance sheet and may be subject to write-down or impairment depending on the performance of such products or services. The significance and timing of such write-downs or impairments are uncertain, as is the ultimate commercial success of new product introductions. Accordingly, Philips cannot determine in advance the ultimate effect that innovations will have on its financial condition and operating results. If Philips fails to create and commercialize its innovations at scale, it may lose market share and competitiveness, which could have a material adverse effect on its financial condition and operating results.
Pandemics could have an adverse effect on Philips’ operations and employees
Although the ability to manage pandemics (for example, resurgences of COVID-19 or mutations thereof) has improved, pandemics may continue to affect Philips’ operations and results in 2023 and Philips expects uncertainty and volatility related to the impact of pandemics and the local response policies thereto, in China in particular given our footprint in China and recent developments in China to loosen restrictions and countervailing measures imposed by other countries. This is driven by, among other things, the extent and depth of government policies to restrict the spread of viruses, the effectiveness of vaccination programs, the appearance of mutations, and the emergence of new viruses that may cause new pandemics. COVID-19 and other pandemics may continue to impact delivery on our triple duty of care in various ways: the health and safety of our employees (in our various working environments); meeting critical customer needs (for example, our production capacity and our ability to deliver, install and provide services); and business continuity (for example, our functional operations, supply chain, and commercial processes). In 2022, we have gradually reopened our offices mostly applying a hybrid schedule. For further discussion or the risks related to hybrid working, see the risk factor “Philips is dependent on its people for leadership and specialized skills and may be unable to attract and retain such personnel”. The expectation remains that responses to the risks of COVID-19 continue to require effort and expense and may negatively affect Philips’ business, financial conditions, and results of operations. In addition, Philips’ customers may not yet be fully focused on making new investments in medical equipment while recovering from COVID-19 disruptions, or they may be facing liquidity issues caused by COVID-19, which may adversely impact Philips’ revenue and cash flow generation.
9.5Financial risks
Philips is exposed to a variety of treasury and financing risks, including liquidity, currency, credit and country risk
Negative developments impacting the liquidity of global capital markets could affect Philips’ ability to raise or re-finance debt in the capital markets or could lead to significant increases in the cost of such borrowing in the future. If the markets expect a downgrade by the rating agencies, or if such a downgrade has actually taken place, this could increase the cost of borrowing, reduce our potential investor base and adversely affect our business.
Philips’ financing and liquidity position may also impact its ability to implement or complete any share-buyback program or distribute any dividends in accordance with its dividend policy or at all. Any announced share-buyback program or dividend policy may also be amended, suspended or terminated at any time, including at Philips’ discretion or as a result of applicable law, regulation or regulatory guidance, and any such amendment, suspension or termination could negatively affect the trading price of, increase trading price volatility of, or reduce the market liquidity of Philips’ shares or other securities. Additionally, any share-buyback program or distribution of dividend could diminish Philips’ cash or other reserves, which may impact its ability to finance future growth and to pursue potential future strategic opportunities. Any share-buyback program or dividend payment will depend on factors such as availability of financing, liquidity position, business outlook, cash flow requirements and financial performance, the state of the market and the general economic climate, and other factors, including tax and other regulatory considerations. Philips and its subsidiaries may also be subject to limitations on the distribution of shareholders’ equity under applicable law.
Philips operates in over 100 countries and its reported earnings and equity are therefore inevitably exposed to fluctuations in the exchange rates of foreign currencies against the euro. Philips’ sales and net investments in its foreign subsidiaries are sensitive in particular to movements in the US dollar, Japanese yen, Chinese renminbi, and a wide range of other currencies from developed and emerging economies. Philips’ sourcing and manufacturing spend is concentrated in the EU, the US and China. Income from operations is particularly sensitive to movements in currencies of countries where Philips has no or very small-scale manufacturing/local sourcing activities but significant sales of its products or services, such as Japan, Canada, Australia, the United Kingdom, and a range of emerging markets, such as South Korea, Indonesia, India and Brazil. Philips’ operations in all segments were scaled back in Russia and Ukraine in 2022, which together represented less than 2% of group sales in 2021 and in 2022. The asset value of the activities in Russia and Ukraine were less than 1% of the consolidated total assets of the group as of December 31, 2022. While there have been no significant asset write-downs to date in Russia and Ukraine, we continue to closely monitor developments in this regard.
In view of the long lifecycle of health technology solution sales and long-term strategic partnerships, the financial risk of counterparties with outstanding payment obligations creates exposure risks for Philips, particularly in relation to accounts receivable from customers, liquid assets, and the fair value of derivatives and insurance contracts with financial counterparties. A default by counterparties in such transactions can have a material adverse effect on Philips’ financial condition and operating results.
Contingent liabilities may have a significant impact on the company’s consolidated financial position, results of operations and cash flows. For an overview of current cases please refer to the Notenote Contingencies.
Philips is exposed to tax risks which could have a significant adverse financial impact
Philips is exposed to tax risks whichthat could result in double taxation, penalties and interest payments. The source of the risks could originate from local tax ruleslaws and regulations as well as international and EU regulatory frameworks. These include transfer pricing risks on internal cross-border deliveries of goods and services, as well as tax risks relating to changes in the transfer pricing model. Examples of initiatives that may result in changing tax rules and regulations include, but are not limited to, the plans adopted by the Dutch parliament to abolish the tax exemption for dividend withholding tax on share buy backs with effect from 2025 and the OECD/G20 Inclusive Framework to address the allocation of income to user markets (“Pillar One”)(Pillar One) and a 15 per cent.15% minimum corporate income tax rate (“Pillar Two”)(Pillar Two). The formal adoption of Council Directive (EU) 2022/2523 (the “PillarPillar Two Directive”) perDirective) in December 2022 aims to achieve a coordinated implementation of Pillar Two in EU Member States, which is expected to have an effect onmember states. The Dutch government adopted the draft Dutch legislative proposal for the proposed Minimum Tax Rate Act 2024 (the “MTR Act”)(MTR Act) in December 2023 and the Pillar Two legislation will be applicable in local law with effect from 2024. As for Pillar One, it is too early to assess the potential impact. Philips is closely monitoring thesefollowing the developments but does notof this initiative.
As Philips maintains substance in the form of relevant assets and personnel in the countries in which it operates, and with the recently provided transitional safe harbor rules (based on Country-by-Country report) enacted by OECD, Philips currently expect that it will be affected byexpects to have limited exposure to taxation under Pillar One implementing measures (subject to clarity on final regulations). However, Philips may be affected by the “MTR Act” following its implementation, which is expected to occur on 1 January 2024, and other regulations and rules that have been, or will be, enacted to implementTwo. Pillar Two (for example, any implementing acts in EU Member States in respect of the “Pillar Two Directive”). This may still impose an additional tax burden on a jurisdiction-by-jurisdiction basis (which do not meet the transitional safe harbor rules) and increase Philips’ tax compliance requirements. burden significantly.
Furthermore, Philips is exposed to tax risks related to acquisitions and divestments, permanent establishments, tax loss, interest and tax credits carried forward, and potential changes in tax law that could result in higher tax expenses and payments. The risks may have a significant impact on local financial tax results, which in turn, could adversely affect Philips’ financial condition and operating results. The value of the deferred tax assets, such as tax losses carried forward, is subject to the availability of sufficient taxable income within the tax loss-carry-forward period. It is also subject to the availability of sufficient taxable income within the foreseeable future, in the case of tax losses carried forward with an indefinite carry-forward period. The ultimate realization of the company’s deferred tax assets is uncertain. Accordingly, there can be no absolute assurance that all deferred tax assets, such as (net) tax losses and credits carried forward, will be realized.
Flaws in internal controls could adversely affect our financial reporting and management process
Accurate disclosures provide investors and other market professionals with significant information for a better understanding of Philips’ businesses. Failures in internal controls or other issues with respect to Philips’ public disclosures, including disclosures with respect to cybersecurity risks and incidents, could create market uncertainty regarding the reliability of the information (including financial data) presented. This could have a negative impact on the price of Philips securities. In addition, the reliability of revenue and expenditure data is key for steering the businesses and for managing top-line and bottom-line growth. The long lifecycle of health technology solution sales, from order acceptance to accepted installation and servicing, together with the complexity of the accounting rules recognizing revenue in the accounts, presents a challenge in terms of ensuring consistent and correct application of the accounting rules throughout Philips’ global business. Significant changes in the way of working, such as hybrid workingthe changes made to our operating model, restructurings, and shifting processes to remote Global Business Services locations, may have an adverse impact on the control environment under which controls are executed, monitored, reviewed, and tested. Any flaws in internal controls, or regulatory or investor actions in connection with flaws in internal controls, could have a material adverse effect on Philips’ business, financial condition, operation results, and reputation and brand.
Global inflation could materially adversely impact our business and results of operations
Changes in macroeconomic conditions, supply chain constraints, labor shortages, the conflict in Ukraine, and steps taken by governments and central banks, particularlyincluding in response to the COVID-19 pandemic as well as otherrecent stimulus and spending programs, have led to higher inflation, which is likely, in turn, to lead to increased interest rates and adverse changes in the availability and cost of capital. These inflationary pressures could affect our manufacturing costs, operating expenses (including wages), and other expenses. We may not be able to compensate for increased costs by driving productivity to reduce costs and by passing these cost increases on through price measures in a timely manner, if at all, which could have an impact on our gross margins and profitability. Our business also operates in certain countries that have experienced hyperinflation, including Argentina and Turkey, and hyperinflationary conditions in any of the markets in which we operate may have a material adverse effect on our business, result of operations and financial condition. Inflation may also cause our customers to reduce or delay orders for our products, which could have a material adverse effect on our business, results of operations, and cash flows.
9.6Compliance risks
Philips isproducts and services may be exposed to risksthe risk of non-compliance of its products and services with various regulations and standards includinginvolving quality, product safety, and security
Our reputation and license to operate depends on our compliance with global regulations and standards. In particular, Philips is exposed to the ongoing impact of the Respironics voluntary recall and related matters. Please refer to the section Quality & Regulatory and patient safety and the Note Contingencies. Philips operatesOperating in a highly regulated health-technology product safety and quality environment and itsindustry, our products and services, including parts orand materials from suppliers, are subject to regulation by various government and regulatory agencies, e.g., FDA (US), EMA (Europe), NMPA (China), MHRA (UK), ASNM (France), BfArM (Germany), and IGZ (the Netherlands). In the EU, the Medical Device Regulation (EU MDR) became effective in May 2021 and imposes significant additional pre-market and post-market requirements. Examples of other product-related regulations are the EU’s Waste from Electrical and Electronic Equipment (WEEE), Restriction of Hazardous Substances (RoHS), Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and Energy-using Products (EuP) regulations. We are subject to various European, United States, and domestic, EU, US and foreign environmental laws and regulations, which are continuing to develop. Any failure to comply with such laws and regulations could jeopardize product quality, safety, and security and/or expose us to lawsuits, administrative penalties, and civil remedies, all of which may have a material adverse impact on Philips’ business, financial condition, and operating results.
Philips has observed an increase in safety and security requirements in a variety of new and upcoming legislation dealing with market access of consumer goods, medical devices, information and communication technology products, (cloud)Cloud services, and specific areas such as data protection, cybersecurity, AI, and supply chain.
Both regulators and customers require us to demonstrate legal compliance and adequate security management using national and international standards and associated certifications. Non-compliance with conditions imposed by regulatory authorities, including in connection with the proposed Respironics consent decree relating to the Respironics recall or any similar regulatory undertakings, could result in product recalls, a temporary ban on products, stoppages at production facilities, remediation costs, fines, disgorgements of profits, and/or claims for damages. Product safety incidents or user concerns could jeopardize patient safety and/or trigger inspections by the FDA or other regulatory agencies, which, if failed,depending on the results of such inspections, could trigger the impacts described above, as well as other consequences. These issues could adversely impact Philips’ financial condition or operating result through lost revenue and cost of any required remedial actions, penalties or claims for damages. They could also negatively impact Philips’ reputation, brand, relationship with customers and market share. In particular, Philips is exposed to the ongoing impact of the Respironics voluntary recall/field action and related matters. Please refer to the section Quality & Regulatory and patient safety and the note Contingencies.
Philips is exposed to the risks of non-compliance with business conduct rules and regulations, including privacy and upcoming ESG disclosure and due diligence requirements.requirements
In the execution of its strategy, Philips could be exposed to the risk of non-compliance with business conduct rules and regulations and our General Business Principles, including, but not limited to, patient safety, quality, anti-bribery, healthcare compliance, privacy and data protection, as well as upcoming ESG disclosure requirements and due diligence requirements. This risk is heightened in growthGrowth geographies, as the legal and regulatory environment is less developed compared to matureMature geographies. Examples of compliance risk areas include commission payments to third parties and remuneration payments to agents, distributors, consultants and similar entities, as well as the acceptance of gifts, which may be considered in some markets to be normal local business practice. The ongoing digitalization of Philips’ products and services, including its processing of personal data, increases the importance of compliance with privacy, data protection and similar laws. These risks could adversely affect Philips’ financial condition, reputation and brand and trigger the additional risk of exposure to governmental investigations, inquiries and legal proceedings and fines. In various jurisdictions, ESG disclosure requirements are currently being drafted. In Europe, the Corporate Sustainability Reporting Directive has been approved.and European Sustainability Reporting Standards (ESRS) will be adopted in 2023 andhave been approved. The latter will significantly increase the scope of mandatory ESG disclosures. Also,Philips needs to report over FY 2024 in line with the requirements of the CSRD and the ESRS. In addition, the proposed European Corporate Sustainability Due Diligence Directive and similar regulations and directives or other rules will (if implemented) require companies to identify and act on adverse environmental and human rights impacts across their organization – and potentially their entire value chain. Failure to meet these requirements could trigger the additional risk of exposure to inquiries from supervisory bodies and adversely affect Philips’ reputation and brand or could adversely impact Philips’ financial condition or operating result through lost revenue and cost of any required remedial actions, penalties or claims for damages.
For further details, please refer to the sub-section Legal proceedings within the Notenote Contingencies.
10Supervisory Board
In the two-tier corporate structure under Dutch law, the Supervisory Board is a separate body that is independent of the Board of Management and the company. The Supervisory Board supervises the policies, management and general affairs of Philips, and assists the Board of Management and the Executive Committee with advice. Please also refer to Supervisory Board within the chapter Corporate governance.
Feike Sijbesma2)3)
Chairman of the Corporate Governance and Nomination & Selection Committee
Member of the Supervisory Board since 2020; first term expires in 2024
Chua Sock Koong1)
Former Group CEO of Singapore Telecommunications Limited and currently member of the Board of Directors of Prudential plc, Bharti Airtel Limited, Bharti Telecom Limited and Ayala Corporation. Member of the Council of Presidential Advisors of Singapore, Deputy Chairman of the Public Service Commission of Singapore.
Liz Doherty1)
Member of the Supervisory Board since 2019;
Marc Harrison4)
Peter Löscher1)4)
Indra Nooyi3)
Former CFO, andPresident, Chairman and CEO of PepsiCo. Currently member of the Board of Directors and Chair of the Audit Committee of Amazon, Inc. Member of the International Board of Advisors of Temasek, member of the Board of Trustees of the Memorial Sloan Kettering Hospital.Hospital, trustee of the national gallery of art.
Sanjay Poonen1)
Former Chief Operating Officer at VMware and President at SAP. Currently CEO and President of Cohesity and member of the Board of Directors of Snyk.
David Pyott2)4)
Member of the Supervisory Board since 2015;
Paul Stoffels2)3)
Member of the Supervisory Board since 2018; second term expires in 2026
Herna Verhagen2)
Currently CEO of PostNL, member of the Supervisory Board of ING Groep N.V., member of the Supervisory Board of Het Concertgebouw N.V., and member of the Advisory Board of Goldschmeding Foundation and member of the executive committee and general board of VNO/NCW (Confederation of Netherlands Industry and Employers).Foundation.
11Supervisory Board report
Letter from the Chairman of the Supervisory Board
Dear Stakeholder,
20222023 was a challenging as well as an extremely challengingencouraging year for Philips, whichas the company started to deliver on its three-year plan to create value with sustainable impact. Improved operational performance was reflected indriven by a disappointing set of results.strong focus on execution to enhance patient safety and quality, strengthen supply chain reliability, and establish a simplified operating model. The company faced significant issues, includingsucceeded in achieving its raised 2023 outlook with strong sales growth, improved profitability, and strong cash flow. All despite the consequences ofuncertainties brought about by an increasingly volatile geopolitical environment. That said, order intake and the Philips Respironics recall, supply chain and inflationary pressures, the war in Ukraineincluding litigation and the COVID situation in China, which all contributed toinvestigation by the below-par business and financial performance. These developments had a significant impact on our shareholders and employees. In that context, we greatly appreciate the trust our customers show in us, as reflected in our order book.US Department of Justice (DOJ), remain key areas of attention.
Mindful of the seriousness of the situation, theThe Supervisory Board isremains fully committed to supportingits responsibilities to supervise and advise management in leading the company out of its current difficulties and towards a future of progressive value creation with sustainable impact. As we explain in our Report, the Supervisory Boardwe spent many sessions in 20222023 engaging with the Board of Management and closely and actively reviewing key priority issues and actions to putbuild further momentum and keep Philips back on a value creation track for its stakeholders.
In the course of the year, our succession planning – during which we extensively evaluated internal and external candidates – resulted in the appointment of Roy Jakobs as CEO of Philips. The Board recognizes the portfolio transformation of Philips over the last decade into a focused, global solutions leader in health technology, which needs further performance improvement on several aspects. Our Board is convinced that Roy is the right CEO to take Philips to the next level of performance, by driving execution of the strategic plan and the firm measures announced in the October and January releases. Our Board focus is fully aligned with the company’s priorities: driving quality, completing the recall, improving supply chain and business performance, and simplifying the organization. We continue to offer the leadership team our support wherever applicable.
The Supervisory Board knows that addressing the Philips Respironics recall and strengtheningmain topics discussed were patient safety and quality, supply chain strengthening, the workforce reduction, the new operating model and strategy for future business growth, as well as the composition of the Board of Management and Supervisory Board, the remuneration of the Board of Management, and the succession slate/bench across the organization.
Strengthening patient safety and quality across Philips is Philips’ first priority.the highest priority, and resolving the consequences of the Respironics recall for our patients and customers is a key focus area. We feel encouraged by the most recent update aroundprogress made on the recall, as the company completes remediation of the sleep devices and strives to finalize remediation of the ventilator devices. Philips is fully committed to complying with the terms of the consent decree agreed with the DOJ, representing the US Food and testing. We fully understand the impact this issue has hadDrug Administration (FDA), which primarily focuses on patients, clinicians, care givers, as well on regulators and investors. We are pleased to note that by year-end 2022, following the substantial ramp-up of capacity, Philips Respironics had completed around 90%in the US. The consent decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the production required forbusiness. In 2023, the deliverySupervisory Board spent time on further anchoring patient safety and quality in the organization, and it remains actively involved in the further outstanding steps which are expected to follow in respect of replacement devices to patients. We are also encouragedlitigation and the investigation by the complete set of test results for the first-generation DreamStation (DS1), which accounts for over two thirds of the sleep therapy devices subjectedDOJ. For more information, please refer to the recall.Quality & Regulatory and patient safety.
The Supervisory BoardWe also discussed developments around the supply chain situation frequently and in depth in 2022 – both the external situation and the further improvements neededplanned internally to improve business and financial performance. The progress made in this area has been a strong driver of the company's increased growth.
The Supervisory Board supportsendorses the simplification of Philips’ organizational structure, where the businessesBusinesses are leading, supported by the regionsRegions and global functions,Functions, with more focused KPIs. The workforce reductions announced in October 2022reduction of approximately 8,000 roles to date, out of the planned reduction of 10,000 roles by 2025, was an impactful and January 2023 were difficult, yet necessary, measuresmeasure as the company as drives a major step-up in productivity, including focusing its R&D activities on fewer, yet more impactful projects. The impact of this reduction became visible in the P&L in 2023. Philips will strive to implement thesethe remaining reductions with due respect for every employee affected and in line with all local rules and regulations.
Changes
We welcome the decision by Exor, the Netherlands-based diversified holding company, to become a long-term investor in Philips, supporting the compositioncompany’s strategy. Their purchase of a 15% shareholding underlines their confidence in Philips’ growth and value potential, and their trust in the leadership, and provides for Exor to nominate one member to our Supervisory Board
AtBoard. We are pleased with the Annualavailability of Mr. Benoît Ribadeau-Dumas as Exor nominee, and we will propose his appointment at the upcoming 2024 General Meeting of Shareholders held in May 2022,Shareholders.
We appreciate the support expressed by the shareholders for the current management and direction of Philips. The Supervisory Board was strengthened byhas every confidence in CEO Roy Jakobs and his leadership team, as they focus on implementing the addition of Herna Verhagenplan to create value with sustainable impact for our shareholders and Sanjay Poonen as new members. With her proven track record in driving a customer-first company culture and a background in e-commerce logistics, Herna Verhagen has brought valued and new perspectives to the Supervisory Board, while Sanjay Poonen’s extensive experience in enterprise IT and cloud-enabled business models has further strengthened the Supervisory Board’s digital competencies. I also wish to thank Neelam Dhawan, who stepped down at the end of the 2022 AGM, for her long-term counsel and support.all other stakeholders.
Together with my fellow members of the Supervisory Board, members, I look forward to providing furthercontinued oversight of Philips as the company addresses the key priorities forcontinues its recoveryvalue creation trajectory and at the same time continues to deliverdelivers on its purpose of improving people’s health and well-being through meaningful innovation.
Feike Sijbesma
Chairman of the Supervisory Board
Introduction Supervisory Board report
The Supervisory Board supervises, advises and challenges the Board of Management in performing their management tasks as well as setting and executing the strategy of the Philips Group. AsThe members of the Supervisory Board we act in the interests of Royal Philips, its businesses and all its stakeholders. This report includes a more specific description of the Supervisory Board’s activities during the financial year 20222023 and other relevant information on its functioning.
20222023 focus areas and activities of the Supervisory Board
In 2022, Philips’ performance continued to be impacted byAgainst the Philips Respironics voluntary recall and operational and supply challenges, such a shortage of electronic components, longer shipping timelines, and disruptions at suppliers caused by the COVID-19 pandemic, which also affected Philips’ manufacturing sites in China. The company also faced other headwinds, such as inflationary pressure and the Russia-Ukraine war. These headwinds negatively impacted the conversion of the company’s strong order book into sales and the 2022 margin. Furthermore, performance continued to be negatively impacted by the consequences of the Philips Respironics voluntary recall notificationbackground set forth in the Sleep & Respiratory Care business in June 2021.
Against this background,Chairman’s letter above, the Supervisory Board was regularly updated by management on the company’s performance and outlook, and theoutlook. The Supervisory Board engaged in discussions with management on improving performance among othersand increasing productivity and agility, by addressing the enhancement of patient safety and accelerating ourthe focus on quality, resilience and quality ofstrengthening the supply chain operationsreliability and simplifyingestablishing a simplified operating model at Philips. Progress, as well as the ways of working at Philips to improve performance and increase productivity and agility. Near termnear-term and longer-term actions to strengthen the supply chain resilience, as proposed by management,on those three priorities were reviewed and monitored by the Supervisory Board.
In this context, the Supervisory Board and management also discussed the external environment in which the company operates, and the impact that the macro-economic outlook has on its performance.
In 2022,2023, the Supervisory Board devoted considerable time to the Philips Respironics voluntary recall, as a recurring agenda item for each of its (regular) meetings. The Supervisory Board discussed and trackedwas kept apprised of the progress made with the repair and replacement program, as well asand in particular discussed and tracked the comprehensive test and research approachprogram for the affected CPAP, BiPAP and mechanical ventilator devices affected.devices. Putting the interest of patients first, the Supervisory Board askedchallenged management to keepremain focused on keeping patients regularly updated on the status of the repair or replacement of their devices and to accelerate the repair and replacement program where possible, despite operational and supply challenges.
The Supervisory Board was also regularly updated on other aspects of the recall, such asrecall. This includes, amongst other things, the negotiations and preliminary approval of the economic loss class settlement, the ongoing engagements with the US Food and Drug Administration (FDA)FDA and other competent authorities globally, and the discussions with the US Department of Justice (DOJ),DOJ, acting on behalf of the FDA, regarding thea proposed consent decree, as well asdecree. It also includes the criminal and civil investigation opened by the DOJ'sDOJ’s Consumer Protection Branch and Civil Fraud Section, and the US Attorney’s Office for the Eastern District of Pennsylvania to which Philips Respironics is subject and the ongoing class-action lawsuits and individual personal injury claims in which Philips Respironics is a defendant.and other entities are defendants. The Supervisory Board specifically engaged with management on the potential impact of the consent decree as well as the litigation and investigations on the Philips Respironics business, both in North America and the rest of the world, and reviewed its plans to keep on serving patients with affected devices until market re-entry.
Recognizing the importance of patient safety and quality of products and solutions sold by the Philips Group generally, significant time was spent in 20222023 on reviewing and tracking progress of the company-wide program launched in 2021 (‘Accelerating Patient Safety and Quality’)Quality to improve and foster a culture, behaviors and a mindset that puts quality and patient safety first. In the context of this program, the Supervisory Board also discussed the process framework for product design and production controls in the company.
The Supervisory Board carefully considered the CEO succession planning and ran an extensive selection and evaluation process, supported by an external executive search firm, during which various scenarios were considered to ensure the best outcome. Following the completion of this process in which both internal and external candidates were considered and evaluated, the Supervisory Board unanimously concluded that Mr Roy Jakobs was the best candidate. The Supervisory Board subsequently nominated Mr Jakobs as the new CEO/President of the company effective October 15, 2022, to allow for him to take full ownership of theAs presented on January 30, 2023, budget and business plan. The Supervisory Board is very pleased that Philips' shareholders appointed Mr Jakobs at the Extraordinary General Meeting of Shareholders (with 99.77% of our shareholders voting favor) held on September 30, 2022. Since the appointment of Mr Jakobs, the Supervisory Board has been working closely with him on his key priorities to further improve and strengthen Philips’ performance as a leading health technology company, which priorities include: (i) further deepening the patient safety and quality capability across the company, which includes the completion of the Philips Respironics voluntary recall; (ii) leading the Philips Group to resume its profitable growth trajectory by addressing current headwinds, including strengthening the supply chain resilience as noted above; and (iii) simplification of the organization to improve performance and productivity.
Following Mr Jakobs' appointment, the Supervisory Board and the Board of Management together with the Executive Committee interacted on the company’s overall strategy to extend its leadership as a health technology company and its plan to create value with sustainable impact towards 2025 and beyond, based on focused organic growth and scalable innovation with improved execution as the key value driver, as presented on January 30, 2023.driver. This plan is designed to restore Philips' value creation based upon sales growth and improvement of profitability includingand cash generation by strong focus on execution. It includes, amongst other things, the strategic plans and priorities of each of the segments Diagnosis & Treatment, Connected CareSegments and Personal Health.Regions and at Enterprise level. The Supervisory Board engaged in multiple deep dive sessions on the strategy. These interactions led to more detailed strategy plans that were reviewed and signed-off by the Supervisory Board. Each strategic plan is supplemented with milestones and an execution plan to achieve the company’s ambition and productivity initiatives,ambitions. Specific attention was given to the role of China in the strategy moving forward given the dynamics of the China market.
Our oversight over the company’s overall strategy also included the restructuring and other actions designed to improve its supplythe operations and performance, as well as its plans to invest in quality, to simplify ways of working, to remove organizational complexity by putting businesses with single accountability in the lead enabled by strong regionsRegions and lean functions,Functions, and to reduce operatingoperational expenses as publicly announced by management on October 24, 2022 and January 30, 2023. Furthermore, the number of key performance indicators that is used to track the company’s performance will be significantly reduced. In this context, the Supervisory Board is also pleasedin close alignment with the strengthening ofrespective works councils and unions and with respect for the Executive Committee with the appointments of Steve C. de Bacaimpacted employees and Jeff DiLullo as members of the Executive Committee, in their roles as Chief Patient Safety & Quality Officer and Chief Market Leader of Philips North America respectively. This includes the immediate reduction of around 4,000 roles globally across the organization announced on October 24, 2022 and the further reduction of the company’s workforce by around 6,000 roles globally by 2025 announced on January 30, 2023.colleagues.
The overview below indicates other key matters that were reviewed and/or discussed during one or more meetings in the course of 2022:2023:
- Capital allocation, including the dividend policy and pay-out and the M&A framework, and specifically the company’s flexibility under its capital structure and credit ratings to pay dividends and to fund capital investments, including share repurchases and other corporate finance initiatives.
- The company’s liquidity position and leverage, including the measures taken to strengthen it in light of the financial
underperformanceperformance of the company. These measures includesecuring athe issue of bonds through the Euro Medium Term Note (EMTN) program to repay the EUR1 billion credit facility and executing the settlement of the forward contracts (entered into as part of the share repurchase program announced on July 26, 2021) at the original settlement dates500 million term loan in2023 and 2024 instead of in 2022 as announced on April 28, 2022.August 2023. - Geopolitical developments and their impact on Philips’ business, in particular the impact of the
Russia-Ukrainewar in Ukraine, the situation in Russia, and the Israel/Hamas conflict on Philips’ employees and the (potential) implications on continuity of Philips’ business in these countries. - Regular review of the dashboard, tracking the performance of the
20222023 key performance indicators for the Executive Committee versus target. - Philips’ annual management commitments, including the
20232024 key performance indicators for the Executive Committee, the20232024 targets for such key performance indicators, and the annual operating plan for2023.2024. - Quality & Regulatory compliance, systems and processes. The Supervisory Board was regularly updated on past and upcoming FDA inspections at various company sites, including the preparations for and outcomes of such inspections. Also,
referreferred to the Report of the Quality & Regulatory Committee. - Oversight of the adequacy of the company’s Internal Control over Financial Reporting.
- Enterprise risk management, including updates on and improvements to the relevant processes; the outcome of the annual risk assessment dialogue with the Executive Committee; and an update of the top risks faced by the Philips Group, including the possible impact of such risks, as well as control and mitigation measures. Refer to Our approach to risk
management.management. EngagementThe terms and conditions of the Relationship Agreement between the company and long-term minority investor Exor.- Engagements with shareholders and institutional advisory firms on the remuneration for the Board of Management
followingand the negativeadvisoryshareholder vote against the2021 Remuneration Reportdischarge of the Board of Management at the20222023 Annual General Meeting of Shareholders.SeeRefer to the Letter from the Remuneration Committee Chair below for more information. SuccessionThe revised Remuneration Policy for the Board of Management and the revised Remuneration Policy for the Supervisory Board, to be proposed for adoption at the upcoming 2024 Annual General Meeting of Shareholders. Refer to the Letter from the Remuneration Committee Chair below for more information.- Continuous succession planning for the Supervisory Board, as well as for the Board of Management and Executive Committee, including the re-appointment of Abhijit Bhattacharya as member of the Board of Management for a 2-year term at the 2023 Annual General Meeting of Shareholders, and the appointments of
Wim Appelo,SteveC.C de Baca,andJeff DiLullo, Julia Strandberg and Heidi Sichien as members of the Executive Committee. - The company’s People strategy and priorities, employee engagement and retention of employees, review of talent management, leadership and talent development, leadership culture, inclusion and diversity.
Following best practices, an evaluation of the CEO succession process, with a satisfactory outcome.- Evaluation of the Board of Management and the Executive Committee based on the achievement of specific group and individual targets approved by the Supervisory Board at the beginning of the
year.year, as well as the assessment of the main findings and conclusions of the evaluation and the related follow-up. - Significant civil litigation claims against, and public investigations into, Philips.
- Philips’ Environmental, Social and Governance (ESG) approach, comprising an update on progress made with respect to the 2025 ESG key programs and sustainability commitments and aims (including circular revenues) and Philips’ aim to improve the health and well-being of 2.5 billion people per year by 2030 through meaningful innovation. The Supervisory Board was also educated on sustainability reporting requirements and requirements related to sustainability-related financial disclosures, as well as European Union regulatory developments in this context. These include but are not limited to education on the European Union Corporate Sustainability Reporting Directive and European Union Sustainability Reporting Standards and the impact thereof on reporting by the Philips Group.
- The agenda for the
20222023 Annual General Meeting of Shareholders (held on May10, 2022) and the Extraordinary General Meeting of Shareholders (held on September 30, 2022)9, 2023) and the proposed agenda for the2023upcoming 2024 Annual General Meeting of Shareholders (to be held on May9, 2023)7, 2024). - The
re-appointment, at the 2022 Annual General Meeting of Shareholders, of Ernst & Young Accountants LLP as the company’s external auditor for a term of one year, starting on January 1, 2023. The proposedre-appointment of Ernst & Young Accountants LLP as the company’s external auditor for a term of one year, starting on January 1, 2024,andas well as theproposedappointment ofPricewaterhouseCoopers Accountants N.V.Price Waterhouse Coopers LLP as the company’snewexternalaccountant,auditor for a term of 4 years starting on January 1, 2025,for a term of four years. Both proposals will be submitted to the shareholders for their approvalboth at the 2023 Annual General Meeting of Shareholders.- The market environment for global M&A activities that
has slowed downoffered limited opportunities inthe second half of 20222023 driven by growing macro-economic challenges, inflationary pressure and rising interest rates, as well as the company’s selective approach towards M&A going forward and the (business) performance of companies previously acquired by the company.
The Supervisory Board also conducted 'deep dives'four dedicated dialogues on Philips' positioning in the market and versus its competitors. Subsequently, the Supervisory Board ’deep dived’ into the overall Philips strategy and the strategy and performance of:
- The
Image Guided TherapyDiagnostic Imaging, Enterprise Informatics, Personal Health businesses; and - Philips
North America and Philips International Markets,Greater China Region, including market trends, business performance and key strategic and transformation initiatives and priorities.
The Supervisory Board also reviewed Philips’ annual and interim financial statements, including information related to ESG, prior to publication.
Supervisory Board meetings and attendance
In 2022,2023, the members of the Supervisory Board convened for seven regular meetings and four extraordinary meetings. Moreover, the Supervisory Board members collectively and individually interacted with members of the Board of Management, with members of the Executive Committee and with senior management outside the formal Supervisory Board meetings. The Chairman of the Supervisory Board and the CEO met regularly forfrequently had bilateral discussions about the company’s progress on a variety of matters. Herna Verhagen and Sanjay Poonen were appointed to the Supervisory Board with effect from May 10, 2022. They followed an induction program and interacted with the members of the Board of Management and various Executive Committee members for deep dives on strategy, finance and investor relations, governance and legal affairs.
The Supervisory Board meetings were well attended in 2022.2023. All Supervisory Board members were present during the Supervisory Board meetings in 2022.2023. The committees of the Supervisory Board also convened regularly (see the separate reports of the committees below) and the committees reported back on their activities to the full Supervisory Board. In addition, to the formal meetings of theSupervisory Board and its committees, the Board and Committee membersCommittees held private meetings. The members of the Supervisory Board concluded that they devoted sufficient time to engage (proactively if the circumstances so required) in their supervisory responsibilities.
In May 2022, someMarch 2023, a Supervisory Board member visited the European Congress of Radiology in Vienna, Austria. In June 2023, the Supervisory Board members visited Philips’ Personal HealthImage Guided Therapy-Devices site in Drachten, the Netherlands and some Supervisory Board members participated in an innovation tour at the Philips site at the High Tech Campus in Eindhoven.Plymouth, Minnesota, USA. In the course of 2022,2023, various Supervisory Board members visited Philips’ Diagnosis & Treatment manufacturing site in Best, the Netherlands, including a visit to the Customer Experience Center. Furthermore, in June 2022, the Supervisory Board visited the headquarters of Philips North America in Cambridge, Massachusetts, US, where the North American Research & Development Center of the company is based and met with several key members of the North American management team.Netherlands.
Supervisory Board: composition, diversity and self-evaluation
The Supervisory Board is a separate corporate body that is independent of the Board of Management and the company. Its independent character is also reflected in the requirement that members of the Supervisory Board can be neither a member of the Board of Management nor an employee of the company. The Supervisory Board considers all its members (i.e. 100%) to be independent under the Dutch Corporate Governance Code. Furthermore, the members of its Audit Committee are independent under the rules of the US Securities and Exchange Commission, applicable to the Audit Committee.
The Supervisory Board currently consists of 10 members. In 2022, there were a number of changes to the composition of the Supervisory Board, all effectiveEffective as per (the end of) the 20222023 Annual General Meeting of Shareholders. Herna Verhagen and Sanjay Poonen were each appointed to the Supervisory BoardShareholders, David Pyott was re-appointed for a term of four years. Paul Stoffelstwo years and Marc Harrison were eachLiz Doherty was re-appointed for a term of four years. The termagenda for the upcoming 2024 Annual General Meeting of Shareholders will include proposals to re-appoint Feike Sijbesma and Peter Löscher as members of the Supervisory Board. The agenda will also include the proposal to appoint Benoît Ribadeau-Dumas as new member of the Supervisory Board, who will be nominated pursuant to the commitment of Exor N.V. to be a long-term minority investor in Philips and its right to propose one member to the Supervisory Board. We are very pleased with the availability of Mr. Benoît Ribadeau-Dumas and welcome him as new member of our Board (subject to his appointment of Neelam Dhawan expired. at the 2024 AGM).
The Supervisory Board attaches great value to diversity in its composition and has adopted a Diversity Policy for the Supervisory Board, Board of Management and Executive Committee. For more information on the Diversity Policy, please refer to Report of the Corporate Governance and Nomination & Selection Committee. The Supervisory Board spent time in 20222023 considering its composition, as well as the composition of the Executive Committee (including the Board of Management), taking into account the criteria set forth in the Diversity Policy.
The composition of the Supervisory Board furthermore follows its profile (which was updated in early 2023), as included in the Rules of Procedure of the Supervisory Board. The profile which aims for an appropriate combination of knowledge and experience among the members of the Supervisory Board, encompassing general management, international business, environmental, social and governance (ESG) and sustainability, (consumer) health and medical technology, quality and regulatory, finance and accounting, human resources, manufacturing and supply chain, information technology and digital, marketing, and governmental and public affairs, all in relation to the global character of Philips’ businesses. The Supervisory Board also aims for having members with different nationalities and (cultural) backgrounds, working experiences or otherwise diverse qualities, as well as one or more members with an executive or similar position in business or society no more than five years ago. The composition of the Supervisory Board shall furthermore be in accordance with the Dutch Corporate Governance Code best practice provisions on independence, and each member of the Supervisory Board shall be capable of assessing the broad outline of the overall policy of the company. The size of the Supervisory Board may vary as it considers appropriate to support its profile.
Effective 2022,Any (re-)appointments of members of the Supervisory Board must meet the gender quota, in accordance with Dutch law, requiring that at least one-third of the supervisory board members are women and at least one-third are men. (For calculation purposes, a total number of board members that cannot be divided by three, must be rounded up to the next number that can be divided by three.) Currently, the statutory quota is met, as out of ten Supervisory Board members, four members are female and six members are male.
In 2022,2023, each member of the Supervisory Board completed a questionnaire to verify compliance with the applicable corporate governance rules and the Rules of Procedure of the Supervisory Board. The outcome of this survey was satisfactory.
An independent external party facilitated the 20222023 self-evaluation process for the Supervisory Board and its committees. This included drafting and submitting relevant questionnaires, interviewing members of the Supervisory Board and aggregating and reporting on the results. The members of the Board of Management also provided their input. The questionnaires covered various topics such as the composition, size, skills and experience, geographical coverage and diversity of the Supervisory Board, stakeholder oversight, strategic oversight, the management, dynamics and focus of the meetings of the Supervisory Board, the effectiveness of the Supervisory Board’s oversight of various aspects of the company’ssuch as strategy, business performance, risk management, succession planning and people, oversight, the CEO succession process, theand engagement with management andmanagement. All members of the Supervisory Board were invited to share recommendations to improve the Supervisory Board’s functioning and ways of working going forward. Furthermore, the performance of the Chairman, the other Supervisory Board members individually, and of the Supervisory Board’s committees was evaluated separately.
The reports on the results of the evaluation were discussed in a meeting of the Supervisory Board. The results of the evaluationself-evaluation indicated that the Supervisory Board continues to beis a well-functioning team as also demonstrated during the expedited CEO succession process in 2022. The results demonstrated that the Supervisory Board is of appropriate size andthat benefits from different expertise, diversity, and international geographical representation. SuggestionsThe meetings and meeting dynamics were rated positively with good interactions, discussions and feedback and a strong relationship with management, and suggestions were made to further strengthenimprove meeting efficiency. Board members also noted the importance of being challenged constructively and of the need to maintain a collective understanding of priorities and options for any relevant matters. The composition of the Supervisory Board and its succession plan is considered adequate. Already anticipating the expiry of the third term of appointment of Mr. Pyott in 2025, the Supervisory Board is tasked with identifying a candidate with equivalent MedTech expertise. In addition, attracting candidates with expertise in artificial intelligence will be part of the Supervisory Board’s succession planning. Supervisory Board members further noted the potential benefit from getting external views from various stakeholders, such as Exor, regulators, suppliers, employees, patients, and investors, to enhance its oversight and decision-making. Finally, the Supervisory Board members confirmed a number of current focus areas, such as Patient Safety and Quality, senior executive succession planning, strategy execution and value creation. Early 2024, the Chairman of the Supervisory Board had several meetings with individual members of the Supervisory Board to discuss ways to further enhance the functioning of the Supervisory Board and its Committeesindividual members going forward. The Supervisory Board stresses the importance of going deep in some important matters, in which the Committees play a key role too. This is in full alignment with the current focus of management on patient safety and quality, supply chain reliability and performance and simplification of the organization, with the aim to enhance organic growth and people and patient centric innovation. Early 2023, the Chairman of the Supervisory Board also discussed the results of the self-evaluation with each of the individual members of the Supervisory Board; the Chairman also discussed the evaluation of his own functioning with the Vice -Chairman.
Key topics that the Supervisory Board and its Committees will focus on in 2023 include tracking progress on certain aspects of the Philips Respironics voluntary recall notification (including but not limited to the repair and replace program and the testing program), the internal Accelerating Patient Safety and Quality program launched in 2021, with respect to improving the resilience of the supply chain and the company’s performance and cash flow generation. Furthermore, in 2023, the Supervisory Board will focus on the company’s liquidity position and financial headroom and prepare updates to the remuneration policies for the Supervisory Board and the Board of Management that will be submitted to the 2024 Annual General Meeting of Shareholders, and track the progress made with the simplification of the company’s operating model with the aim of reducing complexity and clarifying accountabilities and tracking the reduction of roles as announced by the company on October 24, 2022 and January 30, 2023 respectively.
The periodic use of an external facilitator to measure the functioning of the Supervisory Board will continue to be considered in the future.Vice-Chairman.
Supervisory Board composition
| Feike Sijbesma | Paul Stoffels | Chua Sock Koong | Liz Doherty | Marc Harrison | Peter Löscher | Indra Nooyi | Sanjay Poonen1) | David Pyott | Herna Verhagen1) | Feike Sijbesma | Paul Stoffels | Chua Sock Koong | Liz Doherty | Marc Harrison | Peter Löscher | Indra Nooyi | Sanjay Poonen | David Pyott | Herna Verhagen | |
| Year of birth | 1959 | 1962 | 1957 | 1964 | 1957 | 1955 | 1969 | 1953 | 1966 | 1959 | 1962 | 1957 | 1964 | 1957 | 1955 | 1969 | 1953 | 1966 | ||
| Gender | Male | Male | Female | Male | Male | Female | Male | Male | Female | Male | Female | Male | Female | Male | Female | |||||
| Nationality | Dutch | Belgian | Singaporean | British/Irish | American | Austrian | American | American | British/American | Dutch | Dutch | Belgian | Singaporean | British/Irish | American | Austrian | American | British/American | Dutch | |
| Initial appointment date | 2020 | 2018 | 2021 | 2019 | 2018 | 2020 | 2021 | 2022 | 2015 | 2022 | 2020 | 2018 | 2021 | 2019 | 2018 | 2020 | 2021 | 2022 | 2015 | 2022 |
| Date of (last) (re-)appointment | n/a | 2022 | n/a | 2022 | n/a | n/a | n/a | 2019 | n/a | n/a | 2022 | n/a | 2023 | 2022 | n/a | 2023 | n/a | |||
| End of current term | 2024 | 2026 | 2025 | 2023 | 2026 | 2024 | 2025 | 2026 | 2023 | 2026 | 2024 | 2026 | 2025 | 2027 | 2026 | 2024 | 2025 | 2026 | 2025 | 2026 |
| Independent | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||
| Committee memberships2) | RC & CGNSC | RC & CGNSC | AC | QRC | AC & QRC | CGNSC | AC3) | RC & QRC | RC4) | |||||||||||
| Committee memberships1) | RC & CGNSC | RC & CGNSC | AC | QRC | AC & QRC | CGNSC | AC | RC & QRC | RC | |||||||||||
| Attendance at Supervisory Board meetings | (11/11) | (11/11) | (11/11) | (11/11) | (11/11) | (11/11) | (8/8) | (11/11) | (8/8) | (11/11) | (9/11) | (11/11) | (10/11) | (11/11) | (10/11) | (11/11) | ||||
| Attendance at committee meetings | RC (7/7) CGNS (9/9) | RC (7/7) CGNSC (9/9) | AC (7/7) | QRC (6/6) | AC (7/7) QRC (6/6) | CGNSC (8/9) | AC (4/4) | RC (7/7) QRC (6/6) | RC (5/5) | RC (8/8) CGNSC (6/6) | AC (6/6) | QRC (4/4) | AC (6/6) QRC (4/4) | CGNSC (6/6) | AC (6/6) | RC (7/8) QRC (4/4) | RC (8/8) | |||
| General management | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||
| International business | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||
| ESG & sustainability | yes | yes | yes | yes | yes | yes | yes | |||||||||||||
| (Consumer) health and medical technology | yes | yes | yes | yes | yes | yes | yes | yes | yes | |||||||||||
| Patient safety, quality & regulatory and product development | yes | yes | yes | yes | ||||||||||||||||
| Patient safety, quality & regulatory and product development | yes | yes | yes | |||||||||||||||||
| Finance and accounting | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||
| Human Resources | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | |||||||||
| Manufacturing and supply chain | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||||
| Information technology and digital | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||||||||||
| Marketing | yes | yes | yes | yes | yes | yes | yes | yes | yes | |||||||||||
| Governmental and public affairs | yes | yes | yes | yes | yes | yes | yes | yes | yes | |||||||||||
Supervisory Board committees
While retaining overall responsibility, the Supervisory Board has assigned certain of its tasks to the three long-standing committees, also referred to in the Dutch Corporate Governance Code: the Corporate Governance and Nomination & Selection Committee, the Remuneration Committee and the Audit Committee. In 2015, the Supervisory Board also established the Quality & Regulatory Committee. The separate reports of these committees are part of this Supervisory Board report and are published below.
The function of all of the Supervisory Board’s committees is to prepare the decision-making of the full Supervisory Board, and the committees currently have no independent or assigned powers. The full Supervisory Board retains overall responsibility for the activities of its committees.
Financial statements 20222023
The financial statements of the company for 2022,2023, as presented by the Board of Management, have been audited by Ernst & Young Accountants LLP, the independent external auditor appointed by the General Meeting of Shareholders. We have approved these financial statements, and all individual members of the Supervisory Board have signed these documents (as did the members of the Board of Management).
We recommend to shareholders that they adopt the 2022 financial statements. We likewise recommend to shareholders that they adopt the proposal of the Board of Management to make a distribution of declare a dividend of EUR 0.85 per common share, against retained earnings, and to distribute such dividend in shares.
Finally, we would like to express our thanks to the members of the Board of Management, the Executive Committee and all other employees for their continued contribution throughout 2022.
February 21, 20232023.
The Supervisory Board
Feike Sijbesma
Paul Stoffels
Chua Sock Koong
Liz Doherty
Marc Harrison
Peter Löscher
Indra Nooyi
Sanjay Poonen
David Pyott
Herna Verhagen
Further information
To gain a better understanding of the responsibilities of the Supervisory Board and the internal regulations and procedures governing its functioning and that of its committees, please refer to Corporate governance and to the following documents published on the company’s website:
Articles of AssociationRules of Procedure of the Supervisory Board, including the Charters of the Supervisory Board committeesDiversity Policy for the Supervisory Board, Board of Management and Executive Committee
11.1Report of the Corporate Governance and Nomination & Selection Committee
The Corporate Governance and Nomination & Selection Committee is chaired by Feike Sijbesma. Its other members are Paul Stoffels and Indra Nooyi. The Committee is responsible for the review of the overall corporate governance, the selection criteria and appointment procedures for the Board of Management, the Executive Committee, certain other key management positions, as well as the Supervisory Board.
In 2022,2023, the Corporate Governance and Nomination & Selection Committee held ninesix meetings and all Committee members attended these meetings, with the exception of one member unable to attend the meeting in August 2022. Furthermore, the Committee had numerous additional special meetings in 2022, in particular on the topic of the CEO succession process, which were attended by all Committee members.meetings.
The Committee devoted time to the appointment or reappointment of candidates to fill current and future vacancies on the Supervisory Board. Following those consultations, it prepared decisions and advised the Supervisory Board, on candidates for appointment. Thiswhich resulted in the appointmentre-appointments of Herna VerhagenDavid Pyott and Sanjay PoonenLiz Doherty as members of the Supervisory Board at the 20222023 Annual General Meeting of Shareholders. This also resulted in the proposals to re-appoint Feike Sijbesma and Peter Löscher as members of the Supervisory Board, and to appoint Exor nominee Benoît Ribadeau-Dumas as new member of the Supervisory Board at the upcoming 2024 Annual General Meeting of Shareholders.
Under its responsibility for the selection criteria and appointment procedures for Philips’ senior management, the Committee reviewed the functioning of the Board of Management and its individual members, the Executive Committee succession plans and emergency candidates for key roles in the company. The review and evaluation consists of periodical performance review meetings with the individual members of the Board of Management and the Executive Committee, and evaluation of the results of these meetings by the Committee. The main findings and conclusions from these reviews were also shared with the Supervisory Board and the Remuneration Committee, and were taken into account in the performance evaluation of the Board of Management and Executive Committee members and the selection of succession candidates. Reference is made to 20222023 Annual Incentive, setting out the performance review of the Board of Management and the Executive Committee members by the Remuneration Committee.
In 2022, the Committee devoted ample time to the selection and appointment of the new CEO/President of the company as discussed above in the report of the Supervisory Board. This resulted in the appointment of Mr Roy Jakobs as President/CEO and member of the Board of Management at the Extraordinary General Meeting of Shareholders on September 30, 2022. Furthermore, theThe Committee devoted time in 20222023 to the selection and/or appointment of candidates to fill other current and future vacancies on the Board of Management and the Executive Committee. This resulted in:in the appointmentre-appointment, at the 2023 Annual General Meeting of Willem AppeloShareholders, of Abhijit Bhattacharya for a two-year’s term to his tenure as CFO that started in 2015, thereby ensuring continuity and enabling a membersmooth succession process in parallel. The Committee’s work furthermore resulted in appointments of four new members of the Executive Committee in his role as Chief Operations Officer (succeeding Sophie Bechu who stepped down from the Executive Committee), effective September 2022; the appointment ofCommittee: Steve C. de Baca as a member of the Executive Committee in his roleand Jeff DiLullo were appointed, effective February 6, 2023, as Chief Patient Safety & Quality Officer effective February 6, 2023; and the appointment of Jeff DiLullo as a member of the Executive Committee in his role as Chief Market Leader of Philips North America, (succeeding Vitor Rocha who leftrespectively. Furthermore, Julia Strandberg was appointed as the company), alsoChief Business Leader of the Connected Care segment, effective February 6,April 24, 2023, and Heidi Sichien was appointed as Chief People Officer, effective August 1, 2023. As announced on December 8, 2022, Kees Wesdorp leftPhilips expects to announce a new leader for its Precision Diagnosis business in 2024, which business is currently under the company on January 1, 2023, withtemporarily extended leadership of Bert van Meurs (Chief Business Leader for the Image Guided Therapy businesses) temporarily expanding his role to include the leadership of the Precision Diagnosis businesses.business).
With respect to corporate governance matters, the Committee discussed relevant developments and legislative changes, includingaround the revised Dutch Corporate Governance Code, relevant legislation under consideration in the Netherlands and the regulatory regimes around disclosure requirements related to ESG. Finally, the Committee reviewed the Charter of the Corporate Governance and Nomination and Selection Committee and concluded it remains appropriate.
With respect to the productivity initiatives and other actions to improve the company’s performance (including the unfortunate but necessary reduction of roles), the Committee was updated by management on the impact on employees and the phased deployment approach and reviewed the simplification of the organization.
Diversity
The Diversity Policy for the Supervisory Board, Board of Management and Executive Committee was adopted in 2017 and revised in early 2023, and is published on the company website. The Committee periodically assesses the Diversity Policy and the size and composition of the Supervisory Board and makes recommendations, if relevant, relating to the profile for the Supervisory Board.
The criteria in the Diversity Policy aim to ensure that the Supervisory Board, the Board of Management and the Executive Committee have a sufficient diversity of views and the expertise needed for a good understanding of current affairs and longer-term risks and opportunities related to the company’s business. The nature and complexity of the company’s business is taken into account when assessing optimal diversity, as well as the social and environmental context in which the company operates.
Pursuant to the Diversity Policy, the selection of candidates for appointment to the Supervisory Board, Board of Management and Executive Committee is based on merit. With due regard to the criteria set forth in the Diversity Policy, the company shall seek to fill vacancies by considering candidates that represent a diversity of (among others) ages, gender, identities and educational and professional backgrounds. Please refer to the Supervisory Board report for more information on the diversity of the Supervisory Board.
The Diversity Policy includes the Supervisory Board’s aim that the Board of Management and the Executive Committee comprise members with different nationalities and (cultural) backgrounds, working experiences or otherwise diverse qualities. Effective 2022, Dutch law requires listed companies to set appropriate and ambitious gender diversity targets for the Board of Management and for a management level of a seniority to be determined by the company. To this end, the Diversity Policy includes the Supervisory Board’s aim that at least one-third of the members of each of the Board of Management and the Executive Committee are women and at least one-third are men. For more information, please refer to Diversity, Inclusion & Diversityand Well-Being.
11.2Report of the Remuneration Committee
11.2.1Letter from the Remuneration Committee Chair
Dear Stakeholder,
On behalf of the Remuneration Committee, I am pleased to report on the Committee’s activities in 20222023 and to present the 20222023 Remuneration Report, on behalfproviding a comprehensive overview of the remuneration paid and owed to the individual members of the Board of Management and the Supervisory Board.Board, respectively, in the financial year 2023.
Company performance in 2023 and incentive plan realization
2023 was a challenging as well as an encouraging year for Philips, as the company started to deliver on its three-year plan to create value with sustainable impact. Improved operational performance was driven by a strong focus on execution to enhance patient safety and quality, strengthen supply chain reliability, and establish a simplified operating model. The company succeeded in achieving its raised 2023 outlook with strong sales growth, improved profitability, and strong cash flow. All despite the uncertainties brought about by an increasingly volatile geopolitical environment. That said, order intake and the Respironics recall, including litigation and the investigation by the US Department of Justice, remain key areas of attention. Please refer to Financial performance and Environmental, Social and Governance of our 2023 Annual Report for a detailed review of the company’s financial performance and its ESG performance in the year 2023.
TheFor the awards granted under our Long-Term Incentive Plan in 2021, the company performance resulted in a realization significantly above target for the sustainability objectives. For the relative TSR and adjusted EPS metrics in our Long-Term Incentive Plan, however, there was a below-threshold realization based on the performance since the start of the performance period in 2021. In respect of the financial metrics 2023 Annual Incentive, performance was also significantly above target. Nevertheless, to acknowledge the decrease in order intake in 2023, the Supervisory Board decided (upon the proposal of the Remuneration Committee) to lower the Annual Incentive payout. Please refer to our 2023 Remuneration Report for more details.
Other remuneration matters prepared by the Remuneration Committee has been very mindful of
Considering the fact that during2022 had been a disappointing year for Philips, the Annual General Meeting of Shareholders (AGM) held in 2022, a majority ofSupervisory Board followed the advisory votes were cast against the 2021 Remuneration Report. We have taken this negative advisory vote very seriously and that is why we reached out to the company’s shareholders immediately after the 2022 AGM, and further engaged with our shareholders in the second half of 2022. I, as the Chairmanproposal of the Remuneration Committee together with Investor Relations, held discussions with thirteen of our larger shareholders (in aggregate representing approximately 45% of the issued share capital) and with three of the most representative institutional advisory organizations.
Feedback received from our shareholders
Most of our shareholders understand that under certain circumstances the Supervisory Board should be able to adjust the Annual Incentive (AI) and Long-Term Incentive (LTI) payouts, but they did express their specific concern regarding the adjustments madenot apply any base salary increases for the members of the Board of Management over 2021 alsoduring the 2023 compensation review in viewApril 2023.
During the Annual General Meeting of the impact the year had onShareholders held in May 2023, our shareholders. Our shareholders, however, did understand the discretionary adjustments made for the wider employee workforce, particularlyChief Financial Officer Abhijit Bhattacharya was re-appointed, adding a two-year’s term to address retention risks. Furthermore, they requested us to be more transparenthis tenure as CFO that started in the way we disclose our individual performance realization, especially given the above-target realization over 2021 for the CEO. Finally, they requested us to be transparent about the way the 2021 adjustments would be reflected2015, thereby ensuring continuity and enabling a smooth succession process in theparallel. The company performance targets set for the 2022 AI.
How have we addressed this feedback
Naturally the AI and LTI pay-outMr Bhattacharya entered into a new service agreement that was impactedprepared by the low company performance. As explained in our 2021 Remuneration ReportCommittee and during our engagements ahead of the 2022 AGM we have applied the adjustments in the best interest of the company and employees to address retention risks in view of the challenging circumstances our people had and still have to work in. However, in discussions with our shareholders after the 2022 AGM, we concluded that in making adjustments for the members of the Board of Management, a stronger alignment with the interest of our shareholders should be applied. Therefore, the Supervisory Board reconsideredpublished on the company’s long standing practice, and decided to no longer automatically apply a uniform AI and LTI adjustment methodologywebsite.
Proposed 2024 Remuneration Policies for the entire company and effectively de-couple the remuneration approaches for the members of the Board of Management and for the broader workforce.Supervisory Board
We still haveStarting in May 2023, the opinion that it is good to haveRemuneration Committee carried out a strong alignment in remuneration between membersreview of the Board of ManagementRemuneration Policy and our broader workforce, but we realize that in certain circumstances addressing the retention risks of our own people can result in a disalignment between the remuneration of the members ofLong-Term Incentive Plan for the Board of Management, and the interestRemuneration Policy for the Supervisory Board. Dutch law requires the renewal of our policies at least every four years, and we also considered this a good opportunity to test the shareholders. Therefore we have adjustedalignment of our approach.
A decision we have taken – already priorpolicies with our company’s strategy, to the 2022 AGM –review how they compare to increase clarity on potential adjustmentsmarket practice and reward for performance, is to set targets going forward, startingensure our compliance with the 2022 AI basedupdated regulatory and corporate governance requirements. Building on our adjusted EBITA*) metric reported externallystakeholder engagements during the past years, we engaged with stakeholders through a dedicated remuneration roadshow and as such apply a well-definedother interactions to solicit their feedback on, and disclosed set of adjustments (please refer to Reconciliation of non-IFRS information for an exact definition of the performance metric).
In the context of our company’s performance in 2022 and to align with the shareholder experience, the Supervisory Board and Board of Management have jointly concluded that it was appropriate to waive any 2022 AI pay-out and any vesting of the 2020 LTI grant of the current members of the Board of Management. Specifically, this means that an amount of EUR 236,957 of the AI and an amount of EUR 188,994 of the LTI was waived.
For transparency purposes, we provided an enhanced disclosure of the individual performance realization. While there would have been a payout based on the individual performance realization, there was no AI payoutsupport for the financial performance criteria becauseproposals. This process resulted in the realized performance is below the respective thresholds. For the avoidance of doubt we confirm that the financial targets that were setproposals to adopt an amended Remuneration Policy for 2022 took into account the adjustments made in relation to the 2021 remuneration in a way that the members of the Board of Management would not benefit twiceand an amended Remuneration Policy for the Supervisory Board, respectively, that will be submitted for adoption at the upcoming Annual General Meeting of Shareholders to be held on May 7, 2024. Upon convocation of the 2024 AGM (in March 2024), the proposals will be published on our website and the main changes following from these adjustments.
Other feedback received during these (and future) shareholder engagements will also be taken into account when preparing for a renewal of our shareholders’ mandate on our remuneration policies (to be voted on during our 2024 AGM). As I have mentioned in my letter last year, it is our purpose at Philipsproposals, compared to improve people’s health and well-being through meaningful innovation. As a Remuneration Committee we want to assure that our remuneration policy supports this purpose.
CEO remuneration
Per October 15, 2022, Roy Jakobs was appointed as CEOeach of the company. The annual base compensation of Mr Jakobs was set at EUR 1,200,000, belowcurrent 2020 Remuneration Policies, as well as other relevant information will be explained in the base salary of his predecessor, and in line with Philips’ remuneration policy, but just belowexplanatory notes to the median of our Quantum Peer Group. Upon his appointment, Mr Jakobs received performance shares with a grant value of EUR 314,137, which equals his 2022 CEO LTI grant value pro-ratedrelevant agenda items. Please note that, subject to their adoption, the 2024 Remuneration Policies will have retrospective effect for the timefull year 2024, and for that reason our 2023 Remuneration Report includes certain ex-ante disclosures in role in 2022. The 2022 LTI grant that Mr Jakobs received as partrespect of the remuneration, in his previous role, was likewise pro-ratedperformance metrics for the time in role2024 Annual Incentive and until he took over the role as CEO. Our 2022 Remuneration Report also includes a description of the remuneration (to be) received by the former CEO after his succession under his services agreement terminating on April 30, 2023. All payments are in line with contractual obligations.2024 Long-Term Incentive.
The composition of the Remuneration Committee and its activities
The Remuneration Committee is chaired by Paul Stoffels. Its other members are David Pyott, Herna Verhagen and Feike Sijbesma. The Committee is responsible for preparing decisions of the Supervisory Board on the remuneration of individual members of the Board of Management and the Executive Committee, as well as the policies governing this remuneration. In performing its duties and responsibilities, the Remuneration Committee is assisted by an external consultant and an in-house remuneration expert. For a full overview of the responsibilities of the Committee, please refer to the Charter of the Remuneration Committee, as set forth in Chapter 3 of the Rules of Procedure of the Supervisory Board (which are published on the company’s website). Our annual Remuneration Committee cycle enables us to have an effective decision-making process supporting the determination, review and implementation of the Remuneration Policy. The Committee met seveneight times in 2022.2023. All Committee members were present during these meetings.
I look forward to presenting thisour 2023 Remuneration report and our proposals for the renewed 2024 Remuneration Policies at our annualupcoming Annual General Meeting of Shareholders.
On behalf of the Remuneration Committee,
Paul Stoffels
Chairman of the Remuneration Committee
11.2.2Remuneration report 20222023
In this Remuneration Report, the Supervisory Board provides a comprehensive overview, in accordance with article 2:135b of the Dutch Civil Code, of the remuneration paid and owed to the individual members of the Board of Management and the Supervisory Board, respectively, in the financial year 2022.2023. The report will also be published as a stand-alone document on the company’s website after the 20232024 Annual General Meeting of Shareholders, the agenda of which will include an advisory vote on this Remuneration Report.
Board of Management
Summary of 2020 Remuneration Policy
The Remuneration Policy and Long-Term Incentive Plan for the Board of Management have been adopted and approved, respectively, by the Annual General Meeting of Shareholders 2020, which took place on April 30, 2020.
The objectives of the Remuneration Policy for the Board of Management are: to focus them on delivering on our purpose and strategy, to motivate and retain them, and to create stakeholder value.
Thus, the Remuneration Policy:
- Supports improving the company’s overall performance and enhancing the long-term value of the company;
- Directly supports our purpose by:
a) linking a part of remuneration to achieving our strategic imperatives through the criteria and targets included in the Annual and Long-Term Incentives;
b) offering market competitive compensation compared to a peer group of business competitors and companies we compete with for executive talent;
c) enabling us to motivate, retain and attract world-class talent in order to support our purpose of improving people’s health and well-being through meaningful innovation and our goal of addressing our customers’ healthcare challenges (delivering on the Quadruple Aim);
d) stimulating share ownership to create alignment with shareholders and encourage employees to act as stewards and ambassadors of the company; - Encourages the company and its employees to act responsibly and sustainably;
- Delivers value for our stakeholders, such as shareholders, customers, consumers and employees, by continuously engaging with them and make a positive contribution to society at large;
- Leads to fair and internally consistent pay levels by taking into account internal pay ratios.
Main elements of the Remuneration Policy
Compensation element | Purpose and link to strategy | Operation | Policy Level |
|---|---|---|---|
Total Direct Compensation | To support the Remuneration Policy’s objectives, the Total Direct Compensation includes a significant variable part in the form of an Annual Incentive (cash bonus) and Long-Term Incentive in the form of performance shares. As a result, a significant proportion of pay is ‘at risk’. | The Supervisory Board ensures that a competitive remuneration package for Board-level executive talent is maintained and benchmarked. The positioning of Total Direct Compensation is reviewed against benchmark data on an annual basis and is recalibrated if and when required. To establish this benchmark, data research is carried out each year on the compensation levels in the Quantum Peer Group. | Total direct remuneration is aimed at or close to, the median of the Quantum Peer Group. |
Annual Base Compensation | Fixed cash payments intended to attract and retain executives of the highest caliber and to reflect their experience and scope of responsibilities. | Annual Base Compensation levels and any adjustments made by the Supervisory Board are based on factors including the median of Quantum Peer Group data and performance and experience of the individual member. The annual review date for the base salary is typically before April 1. | The individual salary levels are shown in this Remuneration Report. |
Annual Incentive | Variable cash bonus incentive of which achievement is tied to specific financial and non-financial targets derived from the company’s annual strategic plan. These targets are set at challenging levels and are partly linked to the results of the company (80% weighting) and partly to the contribution of the individual member (20% weighting). | The payout in any year relates to the achievements of the preceding year. Metrics are disclosed ex-ante in the Remuneration Report and there will be no retroactive changes to the selection of metrics used in any given year once approved by the Supervisory Board and disclosed. | President & CEO Other BoM members |
Long-Term Incentive | Our Long-Term Incentives form a substantial part of total remuneration, with payouts contingent on achievement of challenging EPS targets, relative TSR performance against a | The annual award size is set by reference to a multiple of base salary. The actual number of performance shares to be awarded is determined by reference to the average of the closing price of the Royal Philips share on the day of publication of the first quarterly results and the four subsequent trading days. Dependent upon the achievement of the performance conditions, cliff-vesting applies three years after the date of grant. During the vesting period, the value of dividends will be added to the performance shares in the form of shares. These dividend-equivalent shares will only be delivered to the extent that the award actually vests. | President & CEO Other BoM members |
Mandatory share ownership and holding requirement | To further align the interests of executives to those of stakeholders and to motivate the achievement of sustained performance. | The guideline for members of the Board of Management is to hold at least a minimum shareholding in the company. Until this level has been reached the members of the Board of Management are required to retain all after-tax shares derived from any Long-Term Incentive Plan. All Board of Management members have reached the required share ownership level. The shares granted under the Long-Term Incentive Plan shall be retained for a period of at least 5 years or until at least the end of their contract period if this period is shorter. | The minimum shareholding requirement is 400% of annual base compensation for the CEO and 300% for other members of the Board of Management. |
Pension | Pension plan and pension contribution intended to result into an appropriate level at retirement. |
| |
Additional arrangements | To aid retention and remain competitive within the marketplace | Additional arrangements include expense and relocation allowances, medical insurance, accident insurance and company car arrangements, which are in line with other Philips executives in the Netherlands. The members of the Board of Management also benefit from coverage under the company’s Directors & Officers (D&O) liability insurance. The company does not grant personal loans to members of the Board of Management. |
Peer Groups
We use a Quantum Peer Group for remuneration benchmarking purposes, and therefore we aim to ensure that it includes business competitors, with an emphasis on companies in the healthcare, technology-related or consumer products area, and other companies we compete with for executive talent. The Quantum Peer Group consists of predominantly Dutch and other European companies, plus a minority (up to 25%) of US-based global companies, of comparable size, complexity and international scope. As of 2023, the Supervisory Board has decided to replace Atos with Baxter in the Quantum Peer Group.
Philips Group
Quantum Peer Group
20222023
| European companies | Dutch companies | US companies | |
|---|---|---|---|
| Alcon | Reckitt Benckiser | Ahold Delhaize | Baxter |
| BAE Systems | Roche | AkzoNobel | Becton Dickinson |
| Boston Scientific | |||
| Safran | Heineken | ||
| Siemens Healthineers | |||
| Smith & Nephew | |||
| Stryker | |||
| Nokia | |||
| Thales | |||
In addition, we use a TSR Performance Peer Group to benchmark our relative Total Shareholder Return performance for LTI purposes and against our business peers in the health technology market and other markets in which we compete. The companies we have selected for this peer group include predominantly US-based healthcare companies. Given that a substantial number of relevant competitors are US-headquartered, the weighting of US-based healthcare companies is more notable than for the Quantum Peer Group.
Philips Group
TSR Performance Peer Group
20222023
| US companies | European companies | Japanese companies |
|---|---|---|
| Alcon | Canon | |
| Elekta | Terumo | |
| Fresenius Medical Care | ||
| Danaher | Getinge | |
| Hologic | ||
| Johnson & Johnson | ||
| Medtronic | ||
| Resmed | ||
| Stryker |
The Remuneration Policy and the LTI Plan allow changes to the peer groups to be made by the Supervisory Board without further approval from the General Meeting of Shareholders in respect of up to three companies on an annual basis (for instance: following a delisting of a company or, a merger of two peer companies), or six companies in total during the four years following adoption and approval of the Remuneration Policy and the LTI Plan respectively (or, if earlier, until the adoption or approval of a revised Remuneration Policy or revised LTI Plan). Since the adoption of the current Remuneration Policy in 2020, the divestment of the Domestic Appliances business in 2021 led to the decision of the Supervisory Board to remove Electrolux, Essity and Henkel from the Quantum Performance Peer Group and replace them with Alcon, GlaxoSmithKline and Stryker. No changes were made to the TSR Peer Group during 2022. However, as Cerner has been delisted after its acquisition by Oracle in 2022, the Supervisory Board has selected Baxter to replace Cerner for the 2023 LTI grant. In addition, following the initial public offering of GE Healthcare, GE Healthcare is included in the TSR Performance Peer Group for the 2023 LTI grant, replacing General Electric.
Services agreements
The members of the Board of Management are engaged by means of a services agreement (overeenkomst van opdracht). Termination of the contract by either party is subject to six months’ notice period. The severance payment is set at a maximum of one year’s annual base compensation. No severance payment is due if the agreement is terminated early on behalf of the Board of Management member or in the case of urgent cause (dringende reden) as defined in article 7:678 and further of the Dutch Civil Code. The term of the services agreement is aligned with the term for which the relevant member has been appointed by the General Meeting of Shareholders (which is a maximum period of four years, it being understood that this period expires no later than at the end of the Annual General Meeting of Shareholders (AGM) held in the fourth year after the year of appointment).
Philips Group
Contract terms for current members
20222023
| end of term | |
|---|---|
| Roy Jakobs | AGM 2026 |
| Abhijit Bhattacharya | AGM |
| Marnix van Ginneken | AGM 2025 |
11.2.3Remuneration of the Board of Management in 20222023
The Supervisory Board has determined the 20222023 pay-outs and awards to the members of the Board of Management, upon the proposal of the Remuneration Committee, in accordance with the 2020 Remuneration Policy and the 2020 LTI Plan. In addition, the Supervisory Board has determined the 2022 pay-out2023 vesting of the 20202021 LTI Plan,grant, of which the performance period ended on December 31, 2022.2023. This was done in accordance with the LTI Plan as approved during the 2020 Annual General Meeting of Shareholders.
The Remuneration Committee annually conducts a scenario analysis. This includes the calculation of remuneration under different scenarios, whereby different Philips performance assumptions and corporate actions are examined. The Supervisory Board concluded that the relationship between the strategic objectives and the chosen performance criteria for the 20222023 Annual Incentive, as well as for the 20202021 LTI, were adequate.
However, in the context of our company’s performance in 2022 and to align with the shareholder experience, the Supervisory Board and Board of Management have jointly concluded that it was appropriate to waive any 2022 AI pay-out and any vesting of the 2020 LTI grant of the members of the Board of Management. The partial 2022 AI pay-out and partial vesting of the 2020 LTI grant was not waived by the former CEO, consequently the company will comply with its contractual obligations in this regard.
This 2022 Remuneration Report also includes a description of the remuneration (to be) received by the former CEO of the company in respect of the period after October 15, 2022 (the date on which he was succeeded by Mr Jakobs) pursuant to and in line with the terms of his services agreement that was concluded and published on the company’s website and presented to the AGM in view of his appointment in 2019 and which will terminate on April 30, 2023 (reference is made to ‘Remuneration former CEO’).
Annual Base Compensation
The annual base compensation of Roy Jakobs as new CEO was set at EUR 1,200,000 (below the base salary of his predecessor of EUR 1,325,000), in line with Philips’ remuneration policy, following market practice and considering the complexity of the role. The annual base compensation of the other members of the Board of Management has been reviewed as part of the regular remuneration review. No increase was applied to acknowledge the disappointing company performance in 2022 and to reflect the limited budget available for the annual compensation review for the wider population. As a result, the annual base compensation of Roy Jakobs, Abhijit Bhattacharya and Marnix van Ginneken has been increased per April 1, 2022, from EUR 795,000 to EUR 810,000 and EUR 615,000 to EUR 630,000, respectively. This increase was made to move the total compensation level closer to the market median level, as well as to reflect internal relativities.remained unchanged in 2023.
20222023 Annual Incentive
The Annual Incentive performance has been assessed based on company financial results as well as individual results. Details are as follows:
Company financial results (80% weighting)
In line with the Remuneration Policy, the company sets financial targets in advance of the year for all members of the Board of Management. For the year 2022,2023, the financial targets set at Group level cover Comparable Sales Growth*), Adjusted EBITA*) and Free Cash Flow*). The realized performance regrettably did not reachfor all three metrics was above target. Realized performance levels presented in the thresholdtable below include the financial impact connected with the Respironics consent decree, and excluding this impact the Comparable Sales Growth*) and Adjusted EBITA*) would have been 7.0% and 10.5% respectively. Reviewing these performance levels, the Supervisory Board decided to apply two downward adjustments. First, to acknowledge the decrease in comparable order intake reported over 2023, the Supervisory Board lowered the payout on the Comparable Sales Growth metric from 200% to 175% of target . Second, to account for the upward effect on anyAdjusted EBITA resulting from excluding the financial impact connected with the proposed Respironics consent decree, the Supervisory Board also lowered the payout from 180% to 175% of these three criteria.target for this metric (corresponding to an Adjusted EBITA performance of 10.5% which excludes the financial impact referred to).
| Financial performance criteria | Weighting as % of target Annual Incentive | Assessment of performance | Weighted pay-out as % of target Annual Incentive | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| threshold performance | target performance | maximum performance | realized performance | resulting payout as % of target | ||||||||||
| Financial performance metric | Weighting as % of target Annual Incentive | Assessment of performance | Weighted pay-out as % of target Annual Incentive | |||||||||||
| threshold performance | target performance | maximum performance | realized performance | resulting payout as % of target | ||||||||||
| Comparable Sales Growth1) | 30% | 1.8% | 4.8% | 6.8% | (2.8)% | 0.0% | 0% | 30% | 0.0% | 1.5% | 3.5% | 6.0% | 175.0% | 52.5% |
| Adjusted EBITA1) | 30% | 9.7% | 12.7% | 14.7% | 7.4% | 0.0% | 0% | |||||||
| Adjusted EBITA margin1) | 30% | 7.5% | 9.0% | 11.0% | 10.6% | 175.0% | 52.5% | |||||||
| Free Cash Flow1) | 20% | 400 | 700 | 1,000 | (961) | 0.0% | 0% | 20% | 571 | 871 | 1,171 | 1,582 | 200.0% | 40.0% |
| Total | 80% | 0% | 80% | 145.0% | ||||||||||
Individual targets based on area of responsibility (20% weighting)
In the context of our company’s performance in 2022 and to align with the shareholder experience, the members of the Supervisory Board and Board of Management jointly concluded that it was appropriate to waive any 2022 AI pay-out of the current members of the Board of Management, despite a positive realization on their individual performance criteria. Specifically, this means that aggregately an amount of EUR 236,957 (including an amount of EUR 35,881 related to the AI for Roy Jakobs in his role as Chief Business Leader Connected Care for the period January 1, 2022 up and until October 14, 2022) was waived.
For the sake of transparency, theThe individual performance criteria and assessment targets set at the beginning of the year have been disclosed in the table below.following table. To determine the payout levels for the individual goals, the Supervisory Board typically applies a holistic assessment as to the performance against the set goals as well as the relative weighting of the goal categories. These relative weightings are not in all cases equal, but such that any goal category remains relevant and aligned with the strategic priorities for the year.
| Board of Management Member | Individual Performance criteria | Assessment of performance | Weighted pay-out as% of target Annual Incentive |
|---|---|---|---|
| Roy Jakobs | Strategy execution |
| (fully waived) |
Quality & operational excellence |
| ||
People & organization |
| ||
Customer results |
| ||
ESG/Sustainability |
| ||
| Abhijit Bhattacharya | Strategy execution |
| (fully waived) |
| Quality & operational excellence |
| ||
| People & organization |
| ||
| Customer results |
| ||
| ESG/Sustainability |
| ||
| Marnix van Ginneken | Strategy execution |
| (fully waived) |
| Quality & operational excellence |
| ||
| People & organization |
| ||
| |||
| ESG/Sustainability |
|
Overall, this leads to the following total Annual Incentive realization and no payout:realization:
Annual Incentive realization 20222023
in EUR unless otherwise stated
| Annual incentive opportunity | Realized annual incentive | Annual incentive opportunity | Realized annual incentive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target as a % of base compensation | Target Annual Incentive | Financial performance (weighted pay-out %) | Individual performance (weighted pay-out %) | Payout as % of target Annual Incentive1) | Realized annual incentive | Payout of annual incentive | Target as a % of base compensation | Target Annual Incentive | Financial performance (weighted pay-out %) | Individual performance (weighted pay-out %) | Payout as % of target Annual Incentive1) | Realized annual incentive | |
| Roy Jakobs2) | 100% | 256,438 | 0% | 69% | 14% | 35,260 | 0 | ||||||
| Roy Jakobs | 100% | 1,200,000 | 145.0% | 22.0% | 167.0% | 2,004,480 | |||||||
| Abhijit Bhattacharya | 80% | 648,000 | 0% | 63% | 13% | 81,648 | 0 | 80% | 648,000 | 145.0% | 21.0% | 166.0% | 1,075,939 |
| Marnix van Ginneken | 80% | 504,000 | 0% | 84% | 17% | 84,168 | 0 | 80% | 504,000 | 145.0% | 23.0% | 168.0% | 846,922 |
20232024 Annual Incentive
The Annual Incentive criteria consist of:This section presents incentive performance metrics under the proposed 2024 Remuneration Policy for the Board of Management. In the event that the proposed 2024 Remuneration Policy would not be adopted by the 2024 AGM, the current 2020 Remuneration Policy would continue to apply.
In the proposed 2024 Remuneration Policy, the weighting of the non-financial element has increased to 30% (from 20%) and, correspondingly, the weighting of the financial element has decreased to 70% (from 80%). This change reflects the increased relative importance of factors relating to strategic priorities (such as patient safety and quality, supply chain reliability, and a simplified operating model), as well as our Environmental, Social and Governance (ESG) performance.
Financial criteria (80%element (70% weighting):
For the year 2023,2024, the following financial indicators of the company’s resultsperformance metrics are selected to ensure alignment with the key (strategic) priorities in the year:
Profit/margin25% weighting: Comparable Sales Growth*)Revenue/growth25% weighting: Adjusted EBITA*) margin- 20% weighting: Free Cash
flowFlow*)
Individual criteria (20%Non-Financial element (30% weighting):
The contributionAt the start of the individual member is assessed based on areas of responsibility, for which annuallyeach year, two to a maximum of fivefour performance categories are selected for each Board of Management member from the following list:list, whereby each selected category receives an equal weighting:
Customer resultsPatient Safety & QualityQuality & operational excellenceCustomer- Strategy
executionand Execution People & organizationESG/SustainabilityESG
For each selected category, one or more performance objectives are determined at the start of the year for each of the members of the Board of Management.
For the year 2023,2024, the following performance categories and objectives are selected to ensure alignment with the key (strategic) priorities in the year:
| Applicable for | Weighting | Measurement description | ||
|---|---|---|---|---|
Patient Safety & Quality | Drive Patient Safety & Quality as highest priority in the organization | All members of Board of Management | 7.5% | This objective measures delivery on our company-wide program to strengthen our Patient Safety & Quality culture, capabilities and performance. Additionally, we measure the progress on the Respironics recall and delivery of the proposed consent decree commitments. |
Customer | Improve customer experience | Roy Jakobs; Abhijit Bhattacharya | 7.5% | This objective is measured by the improvement of the customer NPS. |
| Improve supply chain reliability | Roy Jakobs |
| ||
Improve financial forecasting | Abhijit Bhattacharya | Deliver Reliable Forecast as per plan. | ||
Manage legal issues | Marnix van Ginneken | Develop and manage litigation strategy and potential liabilities. | ||
Strategy | Drive focused strategy to win in the market | Roy Jakobs | 7.5% | This objective measures delivery on our value creation plan and market share gain. |
| Abhijit Bhattacharya |
| |||
| Marnix van Ginneken |
| |||
Establish simplified, more agile operating model | All members of Board of Management | This objective measures delivery on the operating model simplification and our headcount reduction plan. | ||
| ESG | Deliver on ESG Commitments | All members of Board of Management | 7.5% | This objective measures: - Performance on our ESG index (which includes various elements such as emission- and diversity targets) - Our employee engagement score - Talent and succession development of senior roles in the organization |
20202021 Long-Term Incentive
The 3-year performance period of the 20202021 LTI grant, consisting of performance shares, ended on December 31, 2022.2023. The realization of this grant is based on TSR achievement, adjusted EPS growth and sustainability objectives.
In the context of our company’s The following performance in 2022achievement and to align with the shareholder experience,vesting levels have been determined by the Supervisory Board and Board of Management jointly concluded that it was appropriate to waive any vestingin respect of the 2020 LTI2021 grant of the current members of the Board of Management, despite a positive performance achievement of the sustainability objectives. Specifically, this means that an amount of EUR 188,994 was waived.shares:
Philips Group
Performance achievement and vesting levels
| achievement | weighting | vesting level | adjusted vesting level (waived) | achievement | weighting | vesting level | |
|---|---|---|---|---|---|---|---|
| TSR | 0% | 50% | 0% | 0% | 0.0% | 50.0% | 0.0% |
| EPS | 0% | 40% | 0% | 0% | 0.0% | 40.0% | 0.0% |
| Sustainability objectives | 180% | 10% | 18% | 0% | 175.0% | 10.0% | 17.5% |
| Total | 18% | 0% | 17.5% |
TSR (50% weighting)
A ranking approach to TSR applies with Philips itself included in the TSR Performance Peer Group. TSR scores are calculated based on a local currency approach and by taking a 3-month averaging period prior to the start and end of the 3-year performance period. The performance incentive pay-out zone is outlined in the following table, which results in zero vesting for performance below the 40th percentile and 200% vesting for performance levels above the 75th percentile. The incentive zone range has been constructed such that the average pay-out over time is expected to be approximately 100%.
Philips Group
Performance-incentive zone for TSR
in %
| Position | 20-14 | 13 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 5-1 | 20-14 | 13 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 5-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Payout | 0 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 190 | 200 | ||||||||||
| Vesting % | 0 | 60 | 80 | 90 | 100 | 120 | 140 | 160 | 180 | 200 | ||||||||||
The TSR achieved by Philips during the performance period was -63.66%-51.10%, using a start date of October 20192020 and end date of December 2022.2023. This resulted in Philips being positioned at rank 20 in the TSR performance peer group shown in the following table, resulting in a TSR achievement of 0%.
Following Oracle’s acquisition of Cerner (completed June 2022), the Supervisory Board adopted the approach of recognizing Cerner’s performance through the delisting date. As a proxy for future performance, reinvestment in an index of the remaining 19 peer companies was assumed (effectively retaining a peer group of 20 companies).
TSR results LTI Plan 20202021 grant: (63.66%(51.10%)
| total return | rank number | total return | rank number | |
|---|---|---|---|---|
| Canon | 119.99% | 1 | ||
| General Electric | 109.08% | 2 | ||
| Boston Scientific | 48.10% | 3 | ||
| Siemens Healthineers | 35.00% | 4 | ||
| Stryker | 28.06% | 5 | ||
| Getinge | 18.79% | 6 | ||
| Alcon | 16.73% | 7 | ||
| Johnson & Johnson | 12.57% | 8 | ||
| Cerner | 11.12% | 9 | ||
| Becton Dickinson | 10.29% | 10 | ||
| Terumo | 8.71% | 11 | ||
| Danaher | 85.47% | 1 | 7.33% | 12 |
| Hitachi | 74.64% | 2 | ||
| Hologic | (1.37)% | 13 | ||
| Reckitt Benckiser | (11.46)% | 14 | ||
| Elekta | (20.67)% | 15 | ||
| ResMed | 56.08% | 3 | (21.05)% | 16 |
| Getinge | 44.14% | 4 | ||
| Hologic | 43.04% | 5 | ||
| Johnson & Johnson | 37.70% | 6 | ||
| Siemens Healthineers | 24.07% | 7 | ||
| De Longhi | 15.22% | 8 | ||
| Terumo | 14.05% | 9 | ||
| Stryker | 13.15% | 10 | ||
| Cerner | 7.70% | 11 | ||
| Boston Scientific | 3.48% | 12 | ||
| Becton Dickinson | (1.36)% | 13 | ||
| General Electric | (3.63)% | 14 | ||
| Medtronic | (20.68)% | 15 | (24.84)% | 17 |
| Smith & Nephew | (35.25)% | 16 | (27.58)% | 18 |
| Groupe SEB | (39.46)% | 17 | ||
| Elekta | (48.80)% | 18 | ||
| Fresenius Medical | (51.91)% | 19 | (45.30)% | 19 |
| Philips | (63.66)% | 20 | (51.10)% | 20 |
Adjusted EPS growth (40% weighting)
The LTI Plan EPS payouts and targets set at the beginning of the performance period were as follows:
Philips Group
LTI Plan EPS payouts
| Below threshold | Threshold | Target | Maximum | Actual | Below threshold | Threshold | Target | Maximum | Actual | |
|---|---|---|---|---|---|---|---|---|---|---|
| LTI plan EPS (euro) | <1.28 | 1.28 | 1.50 | 1.71 | (1.43) | <1.38 | 1.38 | 1.54 | 1.72 | 0.26 |
| Payout | 0% | 40% | 100% | 200% | 0% | |||||
| Vesting % | 0% | 40% | 100% | 200% | 0% | |||||
In respect of the 20202021 LTI grant, the LTI plan EPS is calculated based on a reported net income attributable to shareholders divided by the number of common shares outstanding (after deduction of treasury shares) on the day prior to the beginning of the performance period (to eliminate the impact of any share buyback, stock dividend, etc.), resulting in an EPS of EUR (1.82)(0.50). Furthermore, as per the 2020 LTI Plan, the LTI Plan EPS includes adjustments to account for events that were not planned when targets were set or were outside management’s control such as the profit and loss impact of acquisitions and divestitures (positive adjustment)divestments (balance is neutral), the profit and loss impact of portfolio restructuringunhedged foreign exchange variations versus plan (positive impact),adjustment) and the profit and loss impact of legacy legal chargesproceedings (positive impact) and impact of foreign exchange variations versus plan (positive adjustment). Overall, this resulted in an LTI Plan EPS of EUR (1.43)0.26 based on adjusted net income from continuing operations, leading to a realization of 0% of target.
Philips Group
LTI Plan EPS realization
in millions of EUR unless otherwise stated
Net income | EPS (euro) | |
|---|---|---|
Income from continuing operations attributable to shareholders | (456) | (0.50) |
Profit and loss impact of: | ||
- Acquisitions and divestitures 1) | 1 | 0.00 |
- Foreign exchange variations versus plan 2) | 60 | 0.07 |
- Legacy legal proceedings 3) | 628 | 0.69 |
Adjusted net income from continuing operations | 234 | 0.26 |
Sustainability objectives (10% weighting)
In order to further align the remuneration package for the Board of Management with our purpose and our ESG commitment, a sustainability criterion was introduced in the 2020 LTI Plan. Philips believes that ESG performance will improve the company’s performance as a whole and, therefore, that it should be explicitly linked to (long-term) remuneration. The criteria are based on three Sustainable Development Goals (SDGs) as defined by the United Nations that are included in Philips’ strategy on sustainability (no. 3, 12 and 13). These three SDGs are translated in five underlying objectives, which are measured against a specific target range.
At the beginning of the performance period, challenging target ranges are set for each of the five objectives. Based on a point-to-point method, performance achievement is measured at the end of the performance period (i.e., 3 years) versus the beginning of the performance period. The pay-outvesting level is determined based on the following scheme:
| No. of measures achieved within or above target zone | Pay-out % | |
|---|---|---|
| No. of measures achieved on or above target | Vesting % | |
| 1 | 0% | 0% |
| 2 | 0% | 0% |
| 3 | 50%-100% | 50%-100% |
| 4 | 100%-150% | 100%-150% |
| 5 | 150%-200% | 150%-200% |
The realized performance is described in the following table. As five out of five objectives are achieved within or abovebetter than target zone,range, the payoutvesting % lies between 150% and 200% of target. Based on the overall performance of the five objectives, the Supervisory Board has assessed that a vesting level of 180%175% would reflect an appropriate positioning within the target range. However, as explained above, any vesting of the 2020 LTI grant of the Board of Management was waived, including vesting relating to the achieved sustainability objectives. While the strong performance on the sustainability objectives is therefore not resulting in any vesting for the current members of the Board of Management, it is celebrated by the company as it contributes to our purpose and our ESG commitment.
For more information on the realized performance on all five objectives please refer to our Environmental, Social and Governance.
| Sustainability category | Underlying objective | Target range | realized performance | |
|---|---|---|---|---|
| Ensure healthy lives and promote well-being for all at all ages (SDG3) Lives Improved | Targeted # of Lives Improved in year 31) | |||
| Ensure sustainable consumption and production patterns (SDG12) Circularity | Targeted circular revenue in year 32) | |||
| Targeted waste to landfill in year 33) | ||||
| Targeted closing the loop in year 34) | ||||
| Take urgent action to combat climate change and its impacts (SDG13) Carbon footprint | Targeted CO2 equivalent (in Kilo Tonnes) in year 3 | |||
20232024 Long-Term Incentive
This section presents incentive performance metrics under the proposed 2024 Remuneration Policy for the Board of Management. In the event that the proposed 2024 Remuneration Policy will not be adopted by the 2024 AGM, the current 2020 Remuneration Policy will continue to apply.
The vesting of the 20232024 Long-Term Incentive grant consistingremains to consist of 100% performance shares of which vesting is subject to performance over a period of 3 yearsyears. We have broadened the sustainability perspective to the full Environmental, Social and basedGovernance ('ESG') spectrum, and subsequently increased the weighting of the ESG performance metric from 10% to 20%. By doing so, we aim to reflect the importance of ESG to our company and its increasing relevance to our stakeholders (as a strategic matter and in the context of our risk management), and to incentivize management’s focus on two financial criteriaour policy objective to deliver superior, long-term value to our stakeholders, while acting responsibly towards our planet and one non-financial criterion:society. As a result of this, the weighting of the relative TSR metric has been slightly reduced to 40% (from 50%) to keep a balanced weighting among the three LTI performance metrics. Lastly, the weighting of the adjusted EPS growth metric remains unchanged, resulting in the following performance metrics and weighting:
50%40% weighting: Relative Total Shareholder Return (‘TSR’)- 40% weighting: Adjusted Earnings per Share growth*) (‘EPS’)
10%20% weighting: ESG performance
ESG Performance (20% weighting)
At the start of each performance year, we select four ESG objectives in line with our long-term strategic priorities. There is no exhaustive list of objectives that can be selected. To ensure that all objectives are material, auditable and measurable, we only select objectives which are reported in our Annual Report (in preparation for the Corporate Sustainability objectives
Please referReporting Directive) and therefore are subject to our external auditor’s reasonable assurance. Furthermore, we make sure that in any measurement year, the Long-Term Incentive Plan publishedESG objectives do not overlap with our non-financial performance objectives for the Annual Incentive.
The objectives selected for the 2024 LTI grant are shown in the following table, including the rationale for selecting these objectives and more details on the company’s website for more information.measurement approach.
2024-2026
ESG objective | Weighting | Rationale | Measurement approach |
|---|---|---|---|
Targeted # of Lives Improved in year 3 1) | 5.0% | Ensure healthy lives and promote well-being for all at all ages (SDG3) Lives Improved | We have a Lives Improved calculation methodology, which follows a three-step approach. 1) We first determine the installed base of our health- and well-being solutions, 2) We determine the number of touchpoints per product per year, and 3) To avoid double-counting, we eliminate all direct- and indirect double-counts between products and solutions. |
Targeted circular revenue in year 3 2) | 5.0% | Ensure sustainable consumption and production patterns (SDG12) Circularity | Revenues generated through products and solutions that meet specific Circular Economy requirements (e.g. refurbished, reconditioned and remanufactured components). |
Targeted CO2 equivalent (in Kilotonnes) in year 3 | 5.0% | Take urgent action to combat climate change and its impacts (SDG13) Carbon footprint | Total greenhouse gas emissions caused by Philips, expressed in kilotonnes CO2-equivalent. Operational carbon footprint on a half-year basis, incl. industrial sites, non-industrial sites, business travel and logistics. |
Targeted Employee Engagement Score in year 3 | 5.0% | Retain an engaged workforce Employee Engagement Score | The Employee Engagement Score (EES) is the single measure of the overall level of employee engagement at Philips, measured on a bi-yearly basis. |
Pension
The following pension arrangement is in place for the members of the Board of Management working under a services agreement governed by Dutch law:
- Flex ES Pension Plan in the Netherlands, which is a Collective Defined Contribution plan with a fixed contribution of (currently) 30.3% (including an own contribution of 2%) of the maximum pensionable salary of EUR
114,866128,810 (effective January 1,2022)2023) minus the offset. The Flex ES Plan has a target retirement age of 68 and a target accrual rate of 1.85%; - A gross Pension Allowance equal to 25% of the base compensation exceeding EUR
114,866;128,810; - A temporary gross Transition Allowance, for a maximum period of 8 years (first 5 years in full; year 6: 75%; year 7: 50%, year 8: 25%) for members of the Board of Management who were participants of the former Executive Pension Plan. The level of the allowance is based on the age and salary of the Board member on December 31, 2014.
Total remuneration costs in 20222023
The following table gives an overview of the costs incurred by the company in 20222023 and 20212022 in relation to the remuneration of the Board of Management. Costs related to performance shares are based on accounting standards (IFRS), which prescribe that costs for each LTI grant are recognized over the full (multi-year) vesting period, proportionate to the relevant fiscal year. Therefore, the costs for any year reflect costs of multiple LTI grants, as opposed to the actual value for the holder of an LTI grant at the vesting date. Hence, the waiving of the 2020 LTI grant by the current members of the Board of Management is not apparent in this table. Please refer to section 20202021 Long-Term Incentive for more details on the actual vesting of the performance shares.
Philips Group
Remuneration Board of Management1)
in EUR
| Accounting costs in the year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| reported year | annual base compensation2) | base compensation | realized annual incentive | performance shares3) | pension allowances4) | pension scheme costs | other compensation5) | total cost | Fixed-variable remuneration6) | |
| R. Jakobs7) | 2022 | 1,200,000 | 256,438 | waived | 112,7378) | 57,973 | 6,012 | 11,507 | 444,667 | 75%-25% |
| F.A. van Houten7) | 2022 | 1,325,000 | 1,041,849 | 208,370 | 2,930,068 | 444,051 | 22,121 | 42,533 | 4,688,992 | 33%-67% |
| 2021 | 1,325,000 | 1,325,000 | 850,915 | 2,626,295 | 565,403 | 27,462 | 57,224 | 5,452,299 | 36%-64% | |
| A. Bhattacharya | 2022 | 810,000 | 806,250 | waived | 763,1408) | 237,250 | 28,133 | 61,308 | 1,896,081 | 60%-40% |
| 2021 | 795,000 | 790,000 | 360,103 | 1,172,533 | 233,857 | 27,462 | 68,908 | 2,652,864 | 42%-58% | |
| M.J. van Ginneken | 2022 | 630,000 | 626,250 | waived | 585,4908) | 141,622 | 28,133 | 35,343 | 1,416,837 | 59%-41% |
| 2021 | 615,000 | 605,000 | 317,192 | 886,035 | 150,755 | 27,462 | 42,610 | 2,029,054 | 41%-59% | |
| Total | 2022 | 2,730,788 | 208,370 | 4,391,434 | 880,896 | 84,398 | 150,691 | 8,446,577 | 46%-54% | |
| 2021 | 2,720,000 | 1,528,210 | 4,684,863 | 950,015 | 82,386 | 168,742 | 10,134,217 | 39%-61% | ||
| Accounting costs in the year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| reported year | annual base compensation2) | base compensation | realized annual incentive | performance shares3) | pension allowances4) | pension scheme costs | other compensation5) | total cost | Fixed-variable remuneration6) | |
| R. Jakobs | 2023 | 1,200,000 | 1,200,000 | 2,004,480 | 968,922 | 267,798 | 31,891 | 109,256 | 4,582,347 | 35%-65% |
| 2022 | 1,200,000 | 256,438 | waived | 112,737 | 57,973 | 6,012 | 11,507 | 444,667 | 75%-25% | |
| A. Bhattacharya | 2023 | 810,000 | 810,000 | 1,075,939 | 793,429 | 197,133 | 31,891 | 94,516 | 3,002,907 | 38%-62% |
| 2022 | 810,000 | 806,250 | waived | 763,1407) | 237,250 | 28,133 | 61,308 | 1,896,081 | 60%-40% | |
| M.J. van Ginneken | 2023 | 630,000 | 630,000 | 846,922 | 614,840 | 125,298 | 31,891 | 53,446 | 2,302,397 | 37%-63% |
| 2022 | 630,000 | 626,250 | waived | 585,4907) | 141,622 | 28,133 | 35,343 | 1,416,837 | 59%-41% | |
| Total | 2023 | 2,640,000 | 3,927,341 | 2,377,191 | 590,228 | 95,673 | 257,218 | 9,887,650 | 36%-64% | |
| 2022 | 1,688,938 | waived | 1,461,367 | 436,845 | 62,278 | 108,158 | 3,757,585 | 61%-39% | ||
Remuneration former CEO
Per October 15, 2022, Frans van Houten, the former CEO, was succeeded by Roy Jakobs as CEO of the company.
In view of a proper handover, and pursuant to the contractual obligations of his services agreement (published on the company’s website at the time of his re-appointment in 2019 and filed as Exhibit 4(e) hereto), the former CEO’s services agreement will terminate on April 30, 2023 in line with the applicable conditions as laid down in such services agreement. Until this time, the former CEO remains available for advisory services.
Up to the termination date of April 30, 2023, the former CEO will be receiving the base compensation, pension arrangement and other allowances following from the termination of his 2019 services agreement. For the period October 15, 2022 up and until December 31, 2022, the base compensation, pension expenditures and other compensation represent a value of EUR 283,151, EUR 126,695 and EUR 11,774 respectively. The former CEO did not waive the partial 2022 AI pay-out and partial vesting of the 2020 LTI grant, consequently the Company will comply with its contractual obligations in this regard. Therefore, the former CEO received an AI payment of EUR 265,000 for the year 2022 and his 2020 LTI grant vested at 18% of target in line with the 2020 LTI plan realization.
For the year 2023, the base compensation, pension expenditures and other compensation represent a value of EUR 435,616, EUR 194,986 and EUR 18,087 (expected) respectively. In respect of the remainder of his services agreement during 2023, the former CEO will be eligible for a prorated AI payment based on the actual 2023 financial performance and his individual performance at target according to the contractual obligations. At target this prorated AI represents a value of EUR 435,616. The former CEO will not receive an LTI grant for the year 2023. In accordance with the relevant provisions of his services agreement, the former CEO will receive a severance payment equal to one-year annual base compensation (amounting to EUR 1,325,000).
The former CEO’s LTI grants with a vesting date after April 30, 2023 (granted in 2021 and 2022) will continue to vest at their regular vesting dates (April 30, 2024, and April 29, 2025 respectively) subject to the predetermined performance conditions. The termination of the services agreement with the former CEO did not trigger a tax expense for the company based on Article 32bb of the Dutch Wage Tax Act.
5-year development of CEO and BoM versus average employee remuneration costs compared to company performance
Internal pay ratios are a relevant input factor for determining the appropriateness of the implementation of the Remuneration Policy, as recognized in the Dutch Corporate Governance Code. For the 20222023 financial year, the ratio between the annual total compensation for the CEO and the average annual total compensation for an employee was 55:46:1. The ratio decreased from 63:55:1 in 2021.2022. Further details on the development of these amounts and ratios over time can be found in the following table. The average employee remuneration costs and company financial performance have been adjusted retroactively such that the Domestic Appliances business is excluded from the figures. Please note that the amounts presented in the following table reflect total remuneration costs to the company which differ from the actual payout to the members of the Board of Management.
Philips Group
Remuneration costcosts
in EUR
| 2018 | 2019 | 2020 | 2021 | 2022 | 2019 | 2020 | 2021 | 2022 | 2023 | |
| Remuneration | ||||||||||
| CEO Total Remuneration Costs (A)1) | 5,391,265 | 5,260,111 | 6,153,067 | 5,452,299 | 5,133,659 | 5,260,111 | 6,153,067 | 5,452,299 | 5,133,659 | 4,582,347 |
| CFO Total Remuneration Costs | 2,595,688 | 2,602,606 | 3,007,990 | 2,652,864 | 1,896,081 | 2,602,606 | 3,007,990 | 2,652,864 | 1,896,081 | 3,002,907 |
| CLO Total Remuneration Costs | 1,861,200 | 1,856,426 | 2,203,160 | 2,029,054 | 1,416,837 | 1,856,426 | 2,203,160 | 2,029,054 | 1,416,837 | 2,302,397 |
| Average Employee (FTE) Total Remuneration Costs (B)2) | 89,843 | 92,645 | 91,455 | 86,853 | 93,373 | 92,645 | 91,455 | 86,853 | 93,373 | 99,870 |
| Ratio A versus B3) | 60:1 | 57:1 | 67:1 | 63:1 | 55:1 | 57:1 | 67:1 | 63:1 | 55:1 | 46:1 |
| Company performance | ||||||||||
| Annual TSR4) | 1.2% | 25.6% | 6.2% | (14.5)% | (60.0)% | 25.6% | 6.2% | (14.5)% | (60.0)% | 42.9% |
| Comparable Sales Growth%5) | 4.9% | 4.5% | 2.9% | (1.2)% | (2.8)% | 4.5% | 2.9% | (1.2)% | (2.8)% | 6.0% |
| Adjusted EBITA%5) | 13.3% | 13.2% | 12.0% | 7.4% | 13.2% | 12.0% | 7.4% | 10.6% | ||
| Free Cash Flow5) | 990 | 923 | 1,635 | 900 | (961) | 923 | 1,635 | 900 | (961) | 1,582 |
Historical LTI grants and holdings
Number of performance shares (holdings)
Under the LTI Plan the current members of the Board of Management were granted 153,891236,622 performance shares in 2022.2023. The following table provides an overview at end December 20222023 of performance share grants.
Philips Group
Number of performance shares (holdings)
in number of shares unless otherwise stated
| grant date | number of shares originally granted | value at grant date | vesting date | end of holding period | unvested opening balance at Jan. 1, 2022 | number of shares awarded in 2022 | (dividend) shares awarded | number of shares vested in 20221) | value at vesting date in 2022 | unvested closing balance at Dec. 31, 2022 | grant date | number of shares originally granted | value at grant date | vesting date | end of holding period | unvested opening balance at Jan. 1, 2023 | number of shares awarded in 2023 | (dividend) shares awarded | number of shares vested in 20231) | value at vesting date in 2023 | unvested closing balance at Dec. 31, 2023 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R. Jakobs | 5/6/2019 | 21,5922) | 810,000 | 06/05/2022 | 06/05/2022 | 22,979 | - | - | 8,717 | 216,060 | - | 4/30/2020 | 17,7042) | 706,250 | 4/30/2023 | 4/30/2025 | 19,073 | waived | 0 | |||
| 4/30/2020 | 17,7042) | 706,250 | 30/04/2023 | 30/04/2025 | 18,399 | - | 674 | - | - | 19,073 | 4/30/2021 | 15,8122) | 750,000 | 4/30/2024 | 4/30/2026 | 16,696 | 747 | 17,443 | ||||
| 4/30/2021 | 15,8122) | 750,000 | 30/04/2024 | 30/04/2026 | 16,105 | - | 590 | - | - | 16,696 | 4/29/2022 | 37,6302) | 930,000 | 4/29/2025 | 4/29/2027 | 39,009 | 1,745 | 40,754 | ||||
| 4/29/2022 | 37,6302) | 930,000 | 29/04/2025 | 29/04/2027 | - | 37,630 | 1,379 | - | - | 39,009 | 10/28/2022 | 24,279 | 314,137 | 10/28/2025 | 10/28/2027 | 24,279 | 1,086 | 25,365 | ||||
| 10/28/2022 | 24,279 | 314,137 | 28/10/2025 | 28/10/2027 | - | 24,279 | - | - | - | 24,279 | 4/28/2023 | 124,538 | 2,400,000 | 4/28/2026 | 4/28/2028 | 0 | 124,538 | 5,571 | 130,109 | |||
| F.A. van Houten3) | 5/6/2019 | 70,640 | 2,650,000 | 06/05/2022 | 06/05/2024 | 75,177 | - | - | 28,567 | 708,078 | - | |||||||||||
| 4/30/2020 | 66,431 | 2,650,000 | 30/04/2023 | 30/04/2025 | 69,037 | - | 2,530 | - | - | 71,567 | ||||||||||||
| 4/30/2021 | 55,868 | 2,650,000 | 30/04/2024 | 30/04/2026 | 56,905 | - | 2,086 | - | - | 58,991 | ||||||||||||
| 4/29/2022 | 107,227 | 2,650,000 | 29/04/2025 | 29/04/2027 | - | 107,227 | 3,930 | - | - | 111,157 | ||||||||||||
| A. Bhattacharya | 5/6/2019 | 31,388 | 1,177,500 | 06/05/2022 | 06/05/2024 | 33,404 | - | - | 12,693 | 314,626 | - | 4/30/2020 | 29,518 | 1,177,500 | 4/30/2023 | 4/30/2025 | 31,800 | waived | 0 | |||
| 4/30/2020 | 29,518 | 1,177,500 | 30/04/2023 | 30/04/2025 | 30,676 | - | 1,124 | - | - | 31,800 | 4/30/2021 | 25,141 | 1,192,500 | 4/30/2024 | 4/30/2026 | 26,547 | 1,187 | 27,734 | ||||
| 4/30/2021 | 25,141 | 1,192,500 | 30/04/2024 | 30/04/2026 | 25,608 | - | 939 | - | - | 26,547 | 4/29/2022 | 49,162 | 1,215,000 | 4/29/2025 | 4/29/2027 | 50,964 | 2,280 | 53,244 | ||||
| 4/29/2022 | 49,162 | 1,215,000 | 29/04/2025 | 29/04/2027 | - | 49,162 | 1,802 | - | - | 50,964 | 4/28/2023 | 63,047 | 1,215,000 | 4/28/2026 | 4/28/2028 | 0 | 63,047 | 2,820 | 65,867 | |||
| M.J. van Ginneken | 5/6/2019 | 22,9912) | 862,500 | 06/05/2022 | 06/05/2024 | 24,467 | - | - | 9,298 | 230,456 | - | 4/30/2020 | 22,373 | 892,500 | 4/30/2023 | 4/30/2025 | 24,103 | waived | 0 | |||
| 4/30/2020 | 22,373 | 892,500 | 30/04/2023 | 30/04/2025 | 23,251 | - | 852 | - | - | 24,103 | 4/30/2021 | 19,448 | 922,500 | 4/30/2024 | 4/30/2026 | 20,535 | 919 | 21,454 | ||||
| 4/30/2021 | 19,448 | 922,500 | 30/04/2024 | 30/04/2026 | 19,809 | - | 726 | - | - | 20,535 | 4/29/2022 | 38,237 | 945,000 | 4/29/2025 | 4/29/2027 | 39,638 | 1,773 | 41,412 | ||||
| 4/29/2022 | 38,237 | 945,000 | 29/04/2025 | 29/04/2027 | - | 38,237 | 1,401 | - | - | 39,638 | 4/28/2023 | 49,037 | 945,000 | 4/28/2026 | 4/28/2028 | 0 | 49,037 | 2,194 | 51,231 | |||
Number of stock options (holdings)
The tables below give an overview of the stock options held by the members of the Board of Management.
Philips Group
Stock options (holdings)
in number of shares unless otherwise stated
| grant date | vesting date | exercise price (in EUR) | expiry date | opening balance at January 1, 2022 | number of stock options awarded in 2021 | number of stock options exercised in 2021 | share price on exercise date | number of stock options expired in 2021 | closing balance at December 31, 2022 | |
|---|---|---|---|---|---|---|---|---|---|---|
| F.A. van Houten1) | 23/04/2012 | 23/04/2015 | 14.82 | 23/04/2022 | 75,000 | - | - | - | - | - |
| 29/01/2013 | 29/01/2014 | 22.43 | 29/01/2023 | 55,000 | - | - | - | - | 55,000 | |
| A. Bhattacharya | 30/01/2012 | 30/01/2014 | 15.24 | 30/01/2022 | 20,000 | - | - | - | - | - |
| 23/04/2012 | 23/04/2015 | 14.82 | 23/04/2022 | 16,500 | - | - | - | - | - | |
| M.J. van Ginneken | 30/01/2012 | 30/01/2014 | 15.24 | 30/01/2022 | 10,000 | - | 10,000 | 28.35 | - | - |
| 23/04/2012 | 23/04/2015 | 14.82 | 23/04/2022 | 8,400 | - | - | - | - | - |
Remuneration of the Supervisory Board in 20222023
Summary of the Remuneration Policy
Please find below a brief summary of the Remuneration Policy for the Supervisory Board, as adopted at the Annual General Meeting of Shareholders 2020. The fee levels in this Remuneration Policy are the same as the Supervisory Board fee levels as determined by our shareholders at the 2018 Extraordinary General Meeting of Shareholders.
The overarching objective of the 2020 Remuneration Policy for the Supervisory Board is to enable its members to fulfill their duties, acting independently: supervising the policies, management and the general affairs of Philips, and supporting the Board of Management and the Executive Committee with advice. Also, the members of the Supervisory Board are guided by the company’s long-term interests, with due observance of the company’s purpose and strategy, taking into account the interests of shareholders and all other stakeholders.
To support the objectives mentioned above, the 2020 Remuneration Policy is aimed at attracting and retaining international Supervisory Board members of the highest caliber and with experience and expertise relevant to our health technology businesses.
In compliance with the Dutch Corporate Governance Code, the 2020 Remuneration Policy provides that the remuneration for the members of the Supervisory Board is not dependent on the results of the company and does not include any shares (or rights to shares). Nevertheless, members of the Supervisory Board are encouraged to hold shares in the company for the purpose of long-term investment to reflect their confidence in the future course of the company. The company does not grant personal loans to members of the Supervisory Board.
The Supervisory Board reviews fee levels in principle every three years, in order to monitor and take account of market developments and manage expectations of our key stakeholders. The levels are aimed at broadly median market levels (and around the 25th percentile market level for the Chairman) paid in the Quantum Peer Group (as used in the 2020 Remuneration Policy for the Board of Management).
The following table provides an overview of the current remuneration structure:
Philips Group
Remuneration Supervisory Board
in EUR
| Chair | Vice Chair | Member | |
|---|---|---|---|
| Supervisory Board | 155,000 | 115,000 | 100,000 |
| Audit Committee | 27,000 | n.a. | 18,000 |
| Remuneration Committee | 21,000 | n.a. | 14,000 |
| Corporate Governance and Nomination & Selection Committee | 21,000 | n.a. | 14,000 |
| Quality & Regulatory Committee | 21,000 | n.a. | 14,000 |
| Attendance fee per inter-European trip | 2,500 | 2,500 | 2,500 |
| Attendance fee per intercontinental trip | 5,000 | 5,000 | 5,000 |
| Entitlement to Philips product arrangement | 2,000 | 2,000 | 2,000 |
| Annual fixed net expense allowance | 11,345 | 2,269 | 2,269 |
| Other travel expenses | As reasonably incurred | ||
The members of the Supervisory Board benefit from coverage under the company’s Directors and Officers (D&O) liability insurance.
Remuneration of the Supervisory Board in 20222023
The individual members of the Supervisory Board received, by virtue of the positions they held, the following remuneration in 2022:2023:
Philips Group
Remuneration of the Supervisory Board
in EUR
| membership | committees | other compensation1) | total | membership | committees | other compensation1) | total | |
|---|---|---|---|---|---|---|---|---|
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 | 155,000 | 35,000 | 16,345 | 206,345 |
| P.A.M. Stoffels | 115,000 | 35,000 | 27,269 | 177,269 | 115,000 | 35,000 | 22,269 | 172,269 |
| N. Dhawan | 35,616 | 6,411 | 5,808 | 47,836 | ||||
| D.E.I. Pyott | 100,000 | 35,000 | 17,269 | 152,269 | 100,000 | 35,000 | 19,769 | 154,769 |
| A.M. Harrison | 100,000 | 14,000 | 12,269 | 126,269 | 100,000 | 14,000 | 19,769 | 133,769 |
| M.E. Doherty | 100,000 | 27,000 | 24,769 | 151,769 | 100,000 | 27,000 | 27,269 | 154,269 |
| P. Löscher | 100,000 | 32,000 | 24,769 | 156,769 | 100,000 | 32,000 | 17,269 | 149,269 |
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 | 100,000 | 14,000 | 17,269 | 131,269 |
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 | 100,000 | 18,000 | 22,269 | 140,269 |
| H. Verhagen | 100,000 | 14,000 | 7,269 | 121,269 | 100,000 | 14,000 | 7,269 | 121,269 |
| S. Poonen | 100,000 | 18,000 | 17,269 | 135,269 | 100,000 | 18,000 | 19,769 | 137,769 |
| Total | 1,105,616 | 248,411 | 192,574 | 1,546,602 | 1,070,000 | 242,000 | 189,266 | 1,501,266 |
11.3Report of the Audit Committee
The Audit Committee is chaired by Liz Doherty. Its other members are Peter Löscher, Chua Sock Koong and Sanjay Poonen (who joined in the course of 2022).Poonen. Feike Sijbesma and Herna Verhagen also regularly attendsattend Audit Committee meetings. The Committee assists the Supervisory Board in fulfilling its supervisory responsibilities, including ensuring the integrity of the company’s financial statements, reviewing the company’s internal controls and overseeing the enterprise risk management process.
In 2022,2023, the Audit Committee held five regular meetings and twoone extraordinary meetings,meeting, which all Audit Committee members attended.
The CEO, CFO, Chief ESG & Legal Officer, Head of Internal Audit, Chief Accounting Officer and external auditor (Ernst & Young Accountants LLP) were invited to and attended all regular meetings.
The Committee together with the Chief ESG & Legal Officer, also met separately in private sessions with the CEO, CFO, Head of Internal Audit and external auditor after every regular quarterly meeting of the Committee. Prior to the Committee meetings, the Audit Committee chair met one-on-one with the Group Treasurer as well as with each of the management who regularly attend the Audit Committee meetings (as set out in the previous paragraph) and with the external auditor (Ernst & Young Accountants LLP).
The following overview highlights matters that were reviewed and/or discussed during Committee meetings in the course of, or in respect of, the financial year 2022:2023:
- The company’s
20222023 annual and interim financial statements and non-financial information, prior to publication. This review included theincreaselegal provision of EUR165575 million in relation to thefieldUS economic loss class action provision recorded in Q12022 in connection2023, the charges of EUR 363 million connected with thePhilipsproposed Respironicsvoluntary recall notification related toconsent decree, thesound abatement foam in certain sleeprestructuring provision, the FCO provisions, the goodwill impairment tests andrespiratory care products (announced on June 14, 2021), to cater forthehigher expected volume of devices eligible for remediation, higher communication costs and potential higher cost of execution and to ensure the speed of the program in a volatile environment. The Committee also reviewed the increase of EUR 85 million in such provision recorded in Q4 2022, resulting from the increased proportion of new replacement devices in order to expedite the completion of the Philips Respironics voluntary recall.legal matters. In each of the regular quarterly meetings of the Committee, the Committee reviewed the draft of the press release on the company’s annual or interim financial statements. - Matters relating to accounting policies, financial risks, reporting and compliance with accounting standards. Key accounting judgments were discussed in-depth, and treatments were challenged, as were quality of earnings. Compliance with statutory and legal requirements and regulations, particularly in the financial domain, was also reviewed. Important findings, Philips’ top and emerging areas of risk (including the internal auditor’s reporting thereon, and the Chief ESG & Legal Officer’s review of litigation and other claims, as well as material
investigations)investigations, including those related to the Philips Respironics voluntary recall), and follow-up actions and appropriate measures were examined thoroughly. - The company’s cash flow generation, liquidity and financing headroom, and its ability under its capital structure and credit ratings to pay dividends and to fund capital investments, including share repurchases and other corporate finance initiatives. The Committee also monitored ongoing goodwill impairment indicators, in particular in the Sleep & Respiratory Care
business, which resulted in a EUR 1.3 billion non-cash charge in Q3 2022 for the impairment of goodwill of thisbusiness.The non-cash charge of EUR 168 million that was recorded in Q3 2022 in connection with the initiative to enhance the productivity in Research & Development (among others, by discontinuing certain Research & Development projects) has also been reviewed by the Committee.Furthermore, the Committee reviewed the goodwill impairment tests performed in the fourth quarter, risk management, legal compliance, and developments in regulatory investigations, as well as legal proceedings, including antitrust investigations and related provisions. - The quarterly Internal Audit reports in which the Head of Internal Audit highlighted key findings of internal audits and fraud investigations by the Internal Audit
Functionfunction in the previous quarter. The Committee discussed the adequacy of the remediation actions agreed with management and accountabilities for executing on these actions. In each meeting the Head of Internal Audit also presented the audit schedule for the upcoming quarter. - Specific finance topics,
share repurchases, and in particular the settlement of forward contracts entered into as part of the share repurchase program announced on July 26, 2021, (at the original settlement dates in 2023 and 2024, instead of in 2022 as earlier announced),capital spending and the company’s debt financing strategy (including the issuance of bonds through the Euro Medium Term Note (EMTN) program to repay the EUR1 billion credit facility the company entered into as announced on October 24, 2022)500 million Term Loan in August 2023). - A post-investment review of projects in the areas of Information Technology, Research & Development, Real Estate, Operations and Restructuring, and assessment of the actual spend and timing of such projects against the original budget and timing.
- Review and approval of the revised Internal Audit charter, annual audit plan and budget, audit scope, and its coverage in relation to the scope of the external audit, as well as the staffing, independence, performance and organizational structure of the Internal Audit
Function.function. - The performance of the external auditor in conducting the group and statutory audits as required by the Auditor Policy and the results of the
20212022 EY service quality review program for Philips. Taking into account this performance review, the Committee evaluated the proposal for re-appointment of Ernst & Young Accountants LLP. Subsequently, Ernst & Young Accountants LLP was re-appointed at the20222023 Annual General Meeting of Shareholders as external auditor for a term of one year, starting on January 1,2023.2024. Later inThe Committee reviewed theyear, the Committee also evaluated the auditor tender process which resulted in the Committee’s recommendationtransition plan as proposed by PricewaterhouseCoopers Accountants N.V. tothe Supervisory Board to submit to the 2023 Annual General Meeting of Shareholders proposals to re-appointtake over from Ernst & Young Accountants LLPas the company’s external auditor for a term of one year, starting on January 1, 2024 and to appoint PricewaterhouseCoopers Accountants N.V.as the company’s new external auditor, starting on January 1, 2025 for a term of four years.- The proposed
20222023 external audit scope, including key audit areas, approach and fees, and non-audit services provided by the external auditor in conformity with the Philips Auditor Policy. - Review and challenge of the independence as well as the professional fitness and good standing of the external auditor and its engagement partners. For information on the fees of the Group auditor, please refer to Audit fees in the note Income from operations.
- The company’s policy on business controls, legal compliance and the General Business Principles (including deployment). The Committee reviewed, discussed and monitored closely the company’s internal control certification processes, and in particular, compliance with section 404 of the US Sarbanes-Oxley Act and its requirements regarding assessment, review and monitoring of internal controls. The Committee also reviewed the status of previously reported significant deficiencies and progress made with respect to the remediation thereof. It also discussed on a regular basis the developments in, and findings relating to, conduct resulting from investigations into alleged violations of the General Business Principles and, if required, any measures taken.
- The company’s structure and system on export controls and sanctions for compliance with the international sanctions and export controls.
Furthermore,
In February 2023,2024, the Committee reviewed, together with the other members of the Supervisory Board, the draft of the Annual Report 2023, as well as the key audit matters and the critical audit matters identified by the external auditor in relation to the 20222023 financial statements included in the Annual Report 20222023 and the Annual Report on Form 20-F, respectively as well as the draft of the Annual Report 2022.respectively. In February 2023,2024, the Committee also reviewed the draft of the company’s 20222023 Country Activity and Tax Report.
During each regular quarterly Audit Committee meeting, the Committee reviewed the quarterly report from the external auditor, in which the auditor set forth its findings and attention points during the relevant period. Apart from the Audit Committee meetings, the external auditor also attended all private sessions with the Audit Committee, where their observations were, if necessary, further discussed. The Annual Audit Letter was circulated to the full Supervisory Board, and planned actions to address the items raised were discussed with management in the subsequent Audit Committee meetings as well as in private sessions with management.
Finally, the Committee reviewed the Audit Committee Charter and concluded it remains appropriate.
11.4Report of the Quality & Regulatory Committee
The Quality & Regulatory Committee was established in view of the importance of patient safety and the quality of the company’s products, systems, services and solutions. The Committee provides broad oversight of compliance with the regulatory requirements that govern the development, manufacturing, marketing and servicing of the company’s products, systems, services and solutions. The Quality & Regulatory Committee assists the Supervisory Board in fulfilling its oversight responsibilities in these areas. It is chaired by David Pyott and its members are Marc Harrison and Peter Löscher.
In 2022,2023, the Quality & Regulatory Committee held sixfour meetings and all Committee members attended these meetings. The Quality & Regulatory Committee convened less frequently in 2023 (compared to 2022), as the quality related matters were a regular item on the agenda of the Supervisory Board meeting. The Chief Executive Officer, the Chief ESG & Legal Officer, the Chief Operations Officer and the Chief Quality & Regulatory Officer were present during these meetings.
The following overview indicates some of the matters that were discussed during meetings in the course of 2022:2023:
- The company’s Quality & Regulatory strategy, focusing on patients and customers to ensure the safety and efficacy of the company’s products and solutions and the status and progress of the company’s Accelerating Patient Safety and Quality
program.phase 2 program, including enhancing the engagement with regulators, ensuring sustainability and predictable performance, Patient Safety & Quality Culture Intervention, and the Quality Management System (QMS) transformation to drive process simplification in a tailored manner and Product Quality Reviews (PQRs) to ensure the installed base meets patient safety and design control standards as well as compliance requirements. For more information, please refer to Quality & Regulatory and patient safety. - The Philips Respironics voluntary recall notification related to the sound abatement foam in certain sleep and respiratory care products (announced on June 14, 2021) in the company’s Sleep & Respiratory Care business. Management regularly updated the Committee on the trend of the number of devices registered for remediation and on the progress of the repair and replace program for the affected devices, as well as actions taken to accelerate the remediation. The Committee reviewed aspects of this issue, such as the program governance to enable effective execution, ongoing engagements with the FDA and the DOJ,
amongamongst others, with respect to the 518(a) Notification order issued by the FDA on March 10, 2022, the investigation initiated by the DOJ to which Philips Respironics is subject, and the proposed consent decree that is currently under discussion with theUS Department of Justice (DOJ),DOJ, acting on behalf of the FDA, and engagements with other regulatory authorities globally. Furthermore, the Committee reviewed and discussed with management the engagement with and communication efforts to patients, physicians, customers and durable medical equipment providers, the testing program and its outcomes, and health hazard evaluations. The Committee also discussed theincreases in thelevel of field actionprovision of EUR 165 million and EUR 85 million,provisions, respectively, as set out in more detail in the report of the Audit Committee above.Finally, management updated the Committee on two problems detected in corrected Trilogy 100/200 ventilators that had already been repaired, as announced by the company on November 21, 2022; the potential root causes of these issues were discussed between the Committee and management. - Management updated the Committee regularly with respect to other quality issues (other than the Philips Respironics voluntary recall notification mentioned in the previous bullet), and the Committee reviewed the progress made with solving and closing such other
issues, including but not limited to the quality issue with respect to the pads of the HeartStart 1 devices (for which the company issued a Field Safety Notice on February 21, 2022).issues. - Review of progress in the transformation of the company’s Quality & Regulatory function, aimed at further strengthening expertise and capabilities within the company’s Quality & Regulatory
function.function, including upscaling Patient Safety & Quality talent at mid-level leadership positions. - Review of the progress made with global initiatives around the transformation, standardization and simplification of the company’s structure and organizational processes relating to
QualityQMSs (the reduction of the current QMSs to one third by the end of 2024), Management Systems,Management Systems andregulated manufacturing sites (Legal Manufacturers)., CAPA and Complaint Management. - Review the implementation of a Patient Safety & Quality IT roadmap and ensure adoption of the IT and data enhancements.
- The status and outcome of Quality & Regulatory-related investigations and inspections by regulatory authorities and Notified Bodies globally across the organization.
This in particular covered findings, related matters and follow-up actions taken by the company to address these findings and includes the progress made with respect to closing the open warning letter from the FDA in relation to the company’s Hospital Respiratory Care business.Management also regularly provided the Committee with an overview of upcoming scheduled inspections across company sites by the FDA, other regulatory authorities and Notified Bodies, and the actions taken to prepare for such inspections. - Review of the
2022product risk per business based on a product assessment approach and remediation across the company, including findings resulting from internal audits. - Review of the 2023 dashboard of Quality & Regulatory key performance indicators, showing the trend of performance. The Committee also reviewed the Quality & Regulatory key performance indicators for
2023.2024.
12Corporate governance
12.1Introduction
Koninklijke Philips N.V. (Royal Philips), a company organized under Dutch law, is the parent company of the Philips group. Its shares have been listed on the Amsterdam stock exchange (Euronext Amsterdam) since 1912. Furthermore, its shares have been traded in the United States since 1962 and have been listed on the New York Stock Exchange since 1987.
Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties.
The company is governed by Dutch corporate and securities laws, its Articles of Association, and the Rules of Procedure of the Board of Management and the Executive Committee and of the Supervisory Board, respectively. Its corporate governance framework is also based on the Dutch Corporate Governance Code (dated December 8, 2016)20, 2022) and US laws and regulations applicable to Foreign Private Issuers. Additionally, the Board of Management has implemented the Philips General Business Principles (GBP) and underlying policies, as well as separate codes of ethics that apply to employees working in specific areas of our business, i.e., the Financial Code of Ethics and the Procurement Code of Ethics. Many of the documents referred to are published on the company’s website and more information can be found in Our approach to risk management.
In this section of the Annual Report, the company addresses the main elements of its corporate governance structure, reports on how it applies the principles and best practices of the Dutch Corporate Governance Code, and provides the information required by the Dutch governmental Decree on Corporate Governance (Besluit inhoud bestuursverslag) and governmental Decree on Article 10 Takeover Directive (Besluit artikel 10 overnamerichtlijn). When deemed necessary in the interests of the company, the company may deviate from aspects of the company’s corporate governance structure, and any such deviations will be disclosed in the company’s corporate governance report.
In compliance with the Dutch Corporate Governance Code, other parts of the management report (within the meaning of article 2:391 of the Dutch Civil Code) included in the Annual Report address the strategy and culture of Philips aimed at sustainable long-term value creation. As described in more detail in Our strategic focus , Philips’ strategy of focused organic growth scalable patient- and people-centric innovation, and focus on reliable execution, is driven by our purposepurpose: to improve people’s health and well-being through meaningful innovation, as described in more detail in Strategy and Businesses.innovation. The Message from the CEOexplains how the company’sthis strategy was executed in 2022; in this regard, please2023; refer also to Financial performance. Furthermore, reference is made to the Philips Business System, an interdependent, collaborative operating model that covers all aspects of how we operate – strategy, governance, processes, people, culture and performance management. As set out in Our cultureOperating Model, we setwhich among others includes standards for behaviors, quality and integrity within Philips that will help achieve operational excellencePhilips.
Philips’ strategy, and extend our solutions capability to address our customers’ unmet needs. Finally, refer tothe way it has been developed by the Board of Management under the supervision of the Supervisory Board, clearly integrates the company’s impact in the field of sustainability, including the effects on people and the environment. In How we create value with sustainable impact we report on resource inputs, value outcomes and societal impact across various financial and Environmental, Social and Governance (ESG) dimensions. We engage with our stakeholders and use a double materiality analysis to identify the ESG topics that we believe have the greatest impact: those having financial materiality (the impact of society on Philips) as well as those having impact materiality (the impact of Philips on society); refer to Working with stakeholders and advocacy for more information onand Double Materiality Assessment. The materiality analysis underpins the relevance of our fully integrated approach to doing business responsibly and sustainably, including a comprehensive set of key commitments across all the ESG dimensions that guide execution of our strategy; refer to Philips' ESG commitments. As one of these commitments, Philips considers its tax payments as a significant contribution to the communities in which it operates, and our overall societal impact.an integral part of its social value creation; refer to Total tax contribution.
12.2Board of Management and Executive Committee
Introduction
The Board of Management is entrusted with the management of the company. Certain key officers have been appointed to support the Board of Management in the fulfilment of its managerial duties. The members of the Board of Management and these key officers together constitute the Executive Committee. In this Corporate governance report, wherever the Executive Committee is mentioned, this also includes the members of the Board of Management, unless the context requires otherwise. Please refer to Board of Management and Executive Committee for an overview of the current members of the Board of Management and the Executive Committee.members.
Under the chairmanship of the President/Chief Executive Officer (CEO), and supported by the other members of the Executive Committee, the members of the Board of Management drive the company’s management agenda and share responsibility for the continuity of the Philips group, focusing on sustainable long-term value creation. Please refer to the Rules of Procedure of the Board of Management and the Executive Committee, which are published on the company’s website, for a description of further responsibilities and tasks, as well as procedures for meetings, resolutions, and minutes.
In fulfilling their duties, the members of the Board of Management and Executive Committee shall beare guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders. The Board of Management and the Executive Committee have adopted a division of responsibilities based on the functional and business areas, each of which is monitored and reviewed by the individual members. The Board of Management is accountable for the actions and decisions of the Executive Committee and has ultimate responsibility for the company’s external reporting (including reporting to the shareholders of the company).
The Board of Management and the Executive Committee are supervised by the Supervisory Board. Members of the Board of Management and the Executive Committee will be present in the meetings of the Supervisory Board, if so invited. In addition, the CEO and other members of the Board of Management (and if needed, the other members of the Executive Committee) meet on a regular basis with the Chairman and other members of the Supervisory Board. The Board of Management and the Executive Committee are required to keep the Supervisory Board informed of all facts and developments concerning Philips that the Supervisory Board may need to be aware of in order to function as required and to properly carry out its duties.
Certain important decisions of the Board of Management require Supervisory Board approval, including decisions concerning: the operational and financial objectives of the company and the strategy designed to achieve these objectives; the issue, repurchase or cancellation of shares; and major acquisitions or divestments.
Appointment and composition
Members of the Board of Management, including the CEO, are appointed by the General Meeting of Shareholders upon a binding recommendation drawn up by the Supervisory Board after consultation with the CEO. This binding recommendation may be overruled by a resolution of the General Meeting of Shareholders adopted by a simple majority of the votes cast and representing at least one-third of the issued share capital. If a simple majority of the votes cast is in favor of the resolution to overrule the binding recommendation, but such majority does not represent at least one-third of the issued share capital, a new meeting may be convened, at which the resolution may be passed by a simple majority of the votes cast, regardless of the portion of the issued share capital represented by such majority. In the event that a binding recommendation has been overruled, a new binding recommendation shall be submitted to the General Meeting of Shareholders. If such second binding recommendation has been overruled, the General Meeting of Shareholders shall be free to appoint a board member.
The CEO and the other members of the Board of Management are appointed for a (maximum) term of four years, it being understood that this term expires at the closing of the General Meeting of Shareholders to be held in the fourth calendar year after the year of their appointment or, if applicable, at a later retirement date or other contractual termination date in the fourth year, unless the General Meeting of Shareholders resolves otherwise. The same applies in the case of re-appointment, which is possible for consecutive terms of (a maximum of) four years. A (re-)appointment schedule for the Board of Management is published on the company’s website.
Pursuant to Dutch law, the members of the Board of Management are engaged by means of a services agreement (overeenkomst van opdracht). The term of the services agreement is aligned with the term for which the relevant member has been appointed by the General Meeting of Shareholders. In casethe event of termination of the services agreement by the company, severance payment is limited to a maximum of one year’s base salary. The services agreements provide no additional termination benefits.
Members of the Board of Management may be suspended by the Supervisory Board and by the General Meeting of Shareholders, and members of the Board of Management may be dismissed by the General Meeting of Shareholders (in each case in accordance with the Articles of Association). A shareholders’ resolution to suspend or dismiss a member of the Board of Management, other than a resolution proposed by the Board of Management or the Supervisory Board, may only be adopted by a simple majority of the votes cast, representing at least one third of the issued share capital. The other members of the Executive Committee are appointed, suspended and dismissed by the CEO, subject to approval by the Supervisory Board.
12.3Supervisory Board
Introduction
The Supervisory Board supervises the policies, management and general affairs of Philips, and assists the Board of Management and the Executive Committee with advice on general policies related to the activities of the company. In fulfilling their duties, the members of the Supervisory Board shall be guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders.
In the two-tier corporate structure under Dutch law, the Supervisory Board is a separate body that is independent of the Board of Management and the company. Its independent character is also reflected in the requirement that members of the Supervisory Board can be neither a member of the Board of Management nor an employee of the company. TheCurrently, the Supervisory Board considers all its members to be independent under the Dutch Corporate Governance Code. Furthermore, the members of its Audit Committee are independent under the rules of the US Securities and Exchange Commission, applicable to the Audit Committee.
The Supervisory Board must approve certain important decisions of the Board of Management, including decisions concerning the operational, business and financial objectives of the company and the strategy designed to achieve these objectives, the issue, repurchase or cancellation of shares and major acquisitions or divestments. The Supervisory Board and its individual members each have a responsibility to request from the Board of Management, the Executive Committee and the external auditor all information that the Supervisory Board needs in order to be able to carry out its duties properly as a supervisory body.
Please refer to the Rules of Procedure of the Supervisory Board, which are published on the company’s website, for a description of further responsibilities and tasks, as well as procedures for meetings, resolutions and minutes.
In its report (included in the company’s Annual Report), the Supervisory Board describes the composition and functioning of the Supervisory Board and its committees, their activities in the financial year, the number of committee meetings held and the main items discussed. Please refer to Supervisory Board report. Please also refer to Supervisory Board for an overview of the current members of the Supervisory Board.
Appointment and composition
Members of the Supervisory Board are appointed by the General Meeting of Shareholders upon a binding recommendation drawn up by the Supervisory Board. This binding recommendation may be overruled by a resolution of the General Meeting of Shareholders adopted by a simple majority of the votes cast and representing at least one-third of the issued share capital. If a simple majority of the votes cast is in favor of the resolution to overrule the binding recommendation, but such majority does not represent at least one-third of the issued share capital, a new meeting may be convened. At this new meeting the resolution may be passed by a simple majority of the votes cast, regardless of the portion of the issued share capital represented by such majority. In the event that a binding recommendation has been overruled, a new binding recommendation shall be submitted to the General Meeting of Shareholders. If such second binding recommendation has been overruled, the General Meeting of Shareholders shall be free to appoint a board member.
The term of appointment of members of the Supervisory Board expires at the closing of the General Meeting of Shareholders to be held after a period of four years following their appointment. There is no age limit requiring the retirement of board members.
In line with the Dutch Corporate Governance Code, members of the Supervisory Board are eligible for re-appointment for a fixed term of four years once, and may subsequently be re-appointed for a period of two years, which appointment may be extended by at most two years. The report of the Supervisory Board must state the reasons for any re-appointment beyond an eight-year period.
A (re-)appointment schedule for the Supervisory Board is published on the company’s website.
Members of the Supervisory Board may be suspended or dismissed by the General Meeting of Shareholders in accordance with the Articles of Association. A resolution to suspend or dismiss a member of the Supervisory Board, other than a resolution proposed by the Supervisory Board, may only be adopted by a simple majority of the votes cast, representing at least one third of the issued share capital.
Candidates for appointment to the Supervisory Board are selected taking into account the company’s Diversity Policy, which is published on the company’s website. The Supervisory Board’s composition furthermore follows the profile included in the Rules of Procedure of the Supervisory Board, and the size of the board may vary as it considers appropriate to support its profile. Please refer to Supervisory Board report by the Supervisory Board. Typically, newly appointed members of the Supervisory Board follow an induction program and interact with Executive Committee members for deep-dives on matters such as strategy, finance and investor relations, quality, governance, legal, sustainability and digitization.
Effective 2022, Dutch law provides a mandatory gender quota, requiring that at least one-third of the Supervisory Board members are women and at least one-third men (for calculation purposes, a total number of board members that cannot be divided by three must be rounded up to the next number that can be divided by three). The quota is applicable to (i) the appointment of new Supervisory Board members, and (ii) the re-appointment of acting board members after eight years following their initial appointment. Except in certain exceptional circumstances, any appointment or re-appointment resulting in a Supervisory Board composition whichthat does not meet (or no longer meets) the quota, will be invalid (null and void).
As announced on August 14, 2023, Philips and Exor N.V. entered into a relationship agreement on August 13, 2023, as a result of which Exor bought a 15% shareholding in the company. The relationship agreement with Exor includes Exor’s commitment to be a long-term minority investor and it's right to propose one member to the Supervisory Board. In this context, it is noted that, for as long as Exor has such nomination right pursuant to the relationship agreement, the independence exception of best practice provision 2.1.7(iii) of the Dutch Corporate Governance Code is deemed to apply to any Exor nominee that has been appointed upon such nomination in accordance with the Relationship Agreement. It is expected that the Supervisory Board will, upon Exor’s exercise of its right, submit a proposal for the appointment of the relevant nominee at the upcoming 2024 Annual General Meeting of Shareholders.
Supervisory Board committees
The Supervisory Board, while retaining overall responsibility, has assigned certain tasks to four committees: the Corporate Governance and Nomination & Selection Committee, the Remuneration Committee, the Audit Committee, and the Quality & Regulatory Committee. Each committee reports to the full Supervisory Board. Please refer to the charters of the respective committees, which are published on the company’s website as part of the Rules of Procedure of the Supervisory Board, for a description of their responsibilities, composition, meetings and working procedures.
The Corporate Governance and Nomination & Selection Committee is responsible for preparing selection criteria and appointment procedures for members of the Supervisory Board, the Board of Management and the Executive Committee. The Committee makes proposals to the Supervisory Board for the (re)appointment of such members, and periodically assesses their functioning. The Committee also periodically assesses the Executive Committee succession planning and the Diversity Policy, and supervises the policy of the Executive Committee on the selection criteria and appointment procedures for Philips executives. At least once a year, the Committee reviews the corporate governance principles applicable to the company, and advises the Supervisory Board on any changes to these principles that it deems appropriate.
The Remuneration Committee is responsible for preparing decisions of the Supervisory Board on the remuneration of individual members of the Board of Management and the Executive Committee. The Committee prepares an annual remuneration report, which is published on the company’s website by the Supervisory Board ahead of the Annual General Meeting of Shareholders. In performing its duties and responsibilities, the Remuneration Committee is assisted by an external consultant and an in-house remuneration expert.
The Audit Committee assists the Supervisory Board in fulfilling its oversight responsibilities for: the integrity of the company’s financial statements; the financial and non-financial (ESG) reporting process;processes; the effectiveness (also in respect of the financial reporting process) of the system ofrisk management and internal controls and risk management;framework; the internal and external audit process; the internal and external auditor’s qualifications, independence and performance; as well as the company’s process for monitoring compliance with laws and regulations and the GBP (including related manuals, training and tools). It reviews the company’s annual and interim financial statements, including non-financial information, prior to publication and advises the Supervisory Board on the adequacy and appropriateness of internal control policies and internal audit programs and their findings. The Committee furthermore supervises the internal auditInternal Audit function, maintains contact with and supervises the external auditor and prepares the nomination of the external auditor for appointment by the General Meeting of Shareholders.
The composition of the Audit Committee meets the relevant requirements under Dutch law and the applicable US rules. All of the members are considered to be independent and financially literate, and the Audit Committee as a whole has the competence relevant to the sector in which the company is operating. In addition, Liz Doherty is designated as an Audit Committee financial expert, as defined under the regulations of the US Securities and Exchange Commission. The Supervisory Board considers the expertise and experience available in the Audit Committee, in conjunction with the possibility to take advice from internal and external experts and advisors, to be sufficient for the fulfillment of the tasks and responsibilities of the Audit Committee.
The Quality & Regulatory Committee has been established by the Supervisory Board in view of the central importance of the quality and (patient) safety of the company’s products, systems, services and software as well as the development, testing, manufacturing, marketing and servicing thereof, and the regulatory requirements relating thereto. The Quality & Regulatory Committee assists the Supervisory Board in fulfilling its oversight responsibilities in this area, whilstwhile recognizing that the Audit Committee assists the Supervisory Board in its oversight of other areas of regulatory, compliance and legal matters.
12.6Annual financial statements and external audit
The annual financial statements are prepared by the Board of Management and reviewed by the Supervisory Board upon the advice of its Audit Committee, taking into account the report of the external auditor. Upon approval by the Supervisory Board, the accounts are signed by all members of both the Board of Management and the Supervisory Board and are published together with the opinion of the external auditor. The Board of Management is responsible, under the supervision of the Supervisory Board, for the quality and completeness of such publicly disclosed financial reports. The annual financial statements are presented for discussion and adoption at the Annual General Meeting of Shareholders, to be convened subsequently.
The external auditor is appointed by the General Meeting of Shareholders in accordance with the Articles of Association. Philips’ current external auditor, Ernst & Young Accountants LLP, was appointed by the General Meeting of Shareholders held on May 7, 2015, for a term of four years starting January 1, 2016, was re-appointed at the Annual General Meeting of Shareholders held on May 9, 2019 for a term of three years starting January 1, 2020, and was re-appointed at the Annual General Meeting of Shareholders held on May 10, 2022 for a term of one year starting January 1, 2023.2023, and was re-appointed at the Annual General Meeting of Shareholders held on May 9, 2023 for a term of one year starting January 1, 2024.
PricewaterhouseCoopers Accountants N.V. was appointed at the Annual General Meeting of Shareholders held on May 9, 2023 as the company’s new external auditor for a term of four years starting January 1, 2025.
European and Dutch law requires the separation of audit and certain non-audit services. The external auditor may only provide audit and audit-related services and is prohibited from providing any other services. This is reflected in the Auditor Policy, which is published on the company’s website. The policy is also in line with (and in some ways stricter than) applicable US rules, under which the appointed external auditor must be independent from the company both in fact and appearance.
The Auditor Policy specifies certain audit services and audit-related services (also known as assurance services) that will or may be provided by the external auditor, and includes rules for the pre-approval by the Audit Committee of such services. Audit services must be pre-approved on the basis of the annual audit services engagement agreed with the external auditor. Proposed audit-related services may be pre-approved at the beginning of the year by the Audit Committee (annual pre-approval) or may be pre-approved during the year by the Audit Committee inwith respect ofto a particular engagement (specific pre-approval). The annual pre-approval is based on a detailed, itemized list of services to be provided, which is designed to ensure that there is no management discretion in determining whether a service has been approved, and to ensure that the Audit Committee is informed of each of the services it is pre-approving. Unless pre-approval with respect to a specific service has been given at the beginning of the year, each proposed service requires specific pre-approval during the year. Any annually pre-approved services where the fee for the engagement is expected to exceed pre-approved cost levels or budgeted amounts will also require specific pre-approval. The term of any annual pre-approval is 12 months from the date of the pre-approval unless the Audit Committee states otherwise. During 2022,2023, there were no services provided to the company by the external auditor whichthat were not pre-approved by the Audit Committee.
12.7Stichting Preferente Aandelen Philips
Stichting Preferente Aandelen Philips, a Foundation (stichting) organized under Dutch law, has been granted the right to acquire preference shares in the capital of Royal Philips, as stated in the company’s Articles of Association. In addition, the Foundation has the right to file a petition with the Enterprise Chamber of the Amsterdam Court of Appeal to commence an inquiry procedure within the meaning of article 2:344 of the Dutch Civil Code.
The object of the Foundation is to represent the interests of Royal Philips, the enterprises maintained by the company and its affiliated companies within the company’s group, in such a way that the interests of the company, these enterprises and all parties involved with them are safeguarded as effectively as possible, and that they are afforded maximum protection against influences which, in conflict with those interests, may undermine the autonomy and identity of Philips and those enterprises, and also to do anything related to the above ends or conducive to them. ThisThe Foundation's object includes the protection of Philips against (an attempt at) an unsolicited takeover or other attempt to exert (de facto) control of the company. The arrangement will allow Philips to determine its position in relation to the relevant third party (or parties) and its (their) plans, to seek alternatives and to defend the company’s interests and those of its stakeholders.
The mere notification that the Foundation exercises its right to acquire preference shares will result in such shares being effectively issued. The Foundation may exercise this right for as many preference shares as there are common shares in the company outstanding at that time. No preference shares have been issued as of December 31, 2022.2023.
The members of the self-electing Board of the Foundation are Messrs J.P. de Kreij, J.V. Timmermans, J. van der Veer and P.N. Wakkie. No Philips Supervisory Board or Board of Management members or Philips officers are represented on the board of the Foundation.
Other protective measures
Other than the arrangements made with the Foundation referred to above, the company does not have any measures whichthat exclusively or almost exclusively have the purpose of defending against unsolicited public offers for shares in the capital of the company. It should be noted that the Board of Management and the Supervisory Board remain under all circumstances authorized to exercise all powers vested in them to promote the interests of Philips.
The company has issued certain corporate bonds, the provisions of which contain a ‘Change'Change of Control Triggering Event’Event' or a ‘Change'Change of Control Put Event’. Upon the occurrence of such events, the company might be required to offer to redeem or purchase any outstanding bonds at certain pre-determined prices. Please also refer to Debt. Furthermore, the Relationship Agreement entered into between the company and its long-term minority investor Exor N.V. (published on the company’s website) includes certain temporary lock-up obligations for Exor which fall away when any third party has ‘Acquired’ an ‘Interest’ of fifty percent (50%) or more in the company.
12.9Corporate information
The company began as a limited partnership with the name Philips & Co in Eindhoven, the Netherlands, in 1891, and was converted into the company with limited liability N.V. Philips’ Gloeilampenfabrieken on September 11, 1912. The company’s name was changed to Philips Electronics N.V. on May 6, 1994, to Koninklijke Philips Electronics N.V. on April 1, 1998, and to Koninklijke Philips N.V. on May 15, 2013.
The majority of the shares in Royal Philips are held through the system maintained by the Dutch Central Securities Depository (Euroclear Nederland). In the past, Philips has also issued (physical) bearer share certificates ('Share Certificates'). A limited number of Share Certificates have not been surrendered yet, although the holders of Share Certificates are still entitled to a corresponding number of shares in Royal Philips. It is noted that, as a result of Dutch legislation that became effective perin July 2019, the relevant shares were registered in the name of Royal Philips by operation of law per January 1, 2021. Owners of Share Certificates will continue to be entitled to a corresponding number of shares, but may not exercise the rights attached to such shares until they surrender their Share Certificates. Owners of Share Certificates may come forward to do so and to receive a corresponding number of shares until January 1, 2026, at the latest. As per January 2, 2026, entitlements attached to the Share Certificates not surrendered will expire by operation of law. For more information, please contact the Investor Relations department by email (investor.relations@philips.com) or telephone (+31-20-59 77222).
The statutory seat of the company is Eindhoven, the Netherlands, and the statutory list of all subsidiaries and affiliated companies, prepared in accordance with the relevant legal requirements (Dutch Civil Code, Book 2, articles 379 and 414), forms part of the notes to the consolidated financial statements and is deposited at the office of the Commercial Register in Eindhoven, the Netherlands (file no. 17001910). The executive offices of the company are located at the Philips Center, Amstelplein 2, 1096 BC Amsterdam, the Netherlands, telephone +31-20-59 77777.
12.10Additional information
Articles of association
Set forth below is a summary of certain provisions of the Articles of Association of the company, applicable Dutch law and related company policies. This summary does not constitute legal advice regarding those matters and should not be regarded as such.
Object and purpose
The objects of the company are to establish, participate in, administer and finance legal entities, companies and other legal forms for the purpose of the manufacture and trading of electrical, electronic, mechanical or chemical products, the development and exploitation of technical and other expertise, including software, or for the purpose of other activities, and to do everything pertaining thereto or connected therewith, including the provision of security in particular for commitments of business undertakings which belong to its group, all this in the widest sense, as may also be conducive to the proper continuity of the collectivity of business undertakings, in the Netherlands and abroad, which are carried on by the company and the companies in which it directly or indirectly participates. These objects can be found in Article 2 of the Articles of Association.
Share Capital
On December 31, 2022,2023, the issued share capital amounted to EUR 177,863,016.40182,703,193.20 divided into 889,315,082913,515,966 common shares and no preference shares.
Voting rights
All issued and outstanding shares carry voting rights and each share confers the right to cast one vote in a shareholders’ meeting. Pursuant to Dutch law, no votes may be cast at a General Meeting of Shareholders in respect of shares which are held by the company. There are no special statutory rights attached to the shares of the company and no restrictions on the voting rights of the company’s shares exist. Major shareholders do not have different voting rights than other shareholders.
Dividends
A dividend will first be declared on preference shares out of net income. The Board of Management has the power to determine what portion of the net income shall be retained by way of reserve, subject to the approval of the Supervisory Board. The remainder of the net income, after reservations made, shall be available for distribution to holders of common shares subject to shareholder approval after year-end.
Liquidation rights
In the event of the dissolution and liquidation of the company, the assets remaining after payment of all debts and liquidation expenses are to be distributed in the following order of priority: to the holders of preference shares, the amount paid thereon; and the remainder to the holders of the common shares.
Preemptive rights
Shareholders have a pro rata preferential right of subscription to any common share issuance unless the right is restricted or excluded. If designated by the General Meeting of Shareholders, the Board of Management has the power to restrict or exclude the preferential subscription rights. A designation of the Board of Management will be effective for a specified period of up to five years and may be renewed. Currently, the Board of Management has been granted the power to restrict or exclude the preferential right of subscription up to and including November 9, 2023.8, 2024. If the Board of Management has not been designated, the General Meeting of Shareholders has the power to restrict or exclude such rights, upon the proposal of the Board of Management, which proposal must be approved by the Supervisory Board. Resolutions by the General Meeting of Shareholders referred to in this paragraph require approval of at least two-thirds of the votes cast if less than half of the issued share capital is represented at the meeting.
The foregoing provisions also apply to the issuance of rights to subscribe for shares.
General Meeting of Shareholders
The Annual General Meeting of Shareholders shall be held each year not later than the thirtieth day of June and, at the Board of Management’s option, in Eindhoven, Amsterdam, The Hague, Rotterdam, Utrecht or Haarlemmermeer (including Schiphol airport); the notice convening the meeting shall inform the shareholders accordingly. Without prejudice to applicable laws and regulations, the Board of Management may resolve to give notice to holders of its listed and traded via a stock exchange shares via the company’s website and/or by other electronic means representing a public announcement, which announcement remains directly and permanently accessible until the General Meeting of Shareholders. Holders of registered shares shall be notified by letter, unless the Board of Management resolves to give notice to holders of registered shares by electronic means of communication by sending a legible and reproducible message to the address indicated by the shareholder to the company for such purpose provided the relevant shareholder has agreed hereto.
In principle, all shareholders are entitled to attend a General Meeting of Shareholders, to address the meeting and to vote, except for shares held in treasury by the company. They may exercise the aforementioned rights at a meeting only for the common shares which on the record date are registered in their name. The record date is published in the above announcement and is, pursuant to Dutch law, set as the 28th day prior to the day of the relevant meeting. Holders of registered shares must advise the company in writing of their intention to attend the General Meeting of Shareholders. Holders of shares listed and traded via a stock exchange who either in person or by proxy wish to attend the General Meeting of Shareholders, should notify ABN AMRO Bank N.V., which is acting as agent for the company. They must submit a confirmation by a participating institution, in which administration they are registered as holders of the shares, that such shares are registered and will remain registered in its administration up to and including the record date, whereupon the holder will receive an admission ticket for the General Meeting of Shareholders. Holders of shares who wish to attend by proxy have to submit the proxy at the same time. A participating institution is a bank or broker which, according to the Dutch Securities Depository Act (Wet giraal effectenverkeer), is an intermediary (intermediair) of the Dutch Central Securities Depository (Euroclear Nederland).
In connection with the General Meeting of Shareholders, the company does not solicit proxies within the United States.
The Articles of Association of the company provide that there are no quorum requirements to hold a General Meeting of Shareholders. Subject to certain exceptions provided by Dutch law and/or the Articles of Association, resolutions of the General Meeting of Shareholders are passed by an absolute majority of votes cast and do not require a quorum.
Limitations on right to hold or vote Common Shares
There are no limitations imposed by Dutch law or by the Articles of Association on the right of non-resident owners to hold or vote the Common Shares.
Exchange controls
Cash dividends paid in euros on Dutch registered shares and bearer shares may be officially transferred from the Netherlands and converted into any other currency without Dutch legal restrictions, except that for statistical purposes such payments and transactions must be reported to the Dutch Central Bank. Furthermore, no payments, including dividend payments, may be made to jurisdictions subject to sanctions adopted by the government of the Netherlands and implementing resolutions of the Security Council of the United Nations.
The Articles of Association of the company provide that cash distributions on New York Registry Shares shall be paid in US dollars, converted at the rate of exchange on the stock market of Euronext Amsterdam at the close of business on the day fixed and announced for that purpose by the Board of Management.
Significant differences in corporate governance practices
The corporate governance rules established by the New York Stock Exchange (NYSE) allow Foreign Private Issuers, like Royal Philips, to follow home country practices on most corporate governance matters instead of those that apply to US domestic issuers, provided that they disclose any significant ways in which their corporate governance practices differ from those applying to listed US domestic issuers under the NYSE listing standards. The following paragraphs summarize what we believe to be the significant differences between certain Dutch practices on corporate governance matters and the corporate governance provisions applicable to US domestic issuers under the NYSE listing standards.
Dutch corporate governance code
The company is a company organized under Dutch law, with its Common Shares listed on Euronext Amsterdam, and is subject to the Dutch Corporate Governance Code of December 8, 201620, 2022 (the Dutch Corporate Governance Code). Philips’ New York Registry Shares, representing Common Shares of the company, are listed on the NYSE.
Board structure
The NYSE listing standards prescribe regularly scheduled executive sessions of non-executive directors. The company has a two-tier corporate structure consisting of a Board of Management consisting of executive directors under the supervision of a Supervisory Board consisting exclusively of non-executive directors. Members of the Board of Management and other officers and employees cannot simultaneously act as member of the Supervisory Board. The Supervisory Board must approve specified decisions of the Board of Management.
Independence of members of our Supervisory Board
The Dutch Corporate Governance Code sets forth certain best practices limiting the number of non-independent members of the Supervisory Board, and its committees. The Supervisory Board considers all its members to be independent under the Dutch Corporate Governance Code. The definitions of independence under the Dutch Corporate Governance Code, however, differ in their details from the definitions of independence under the NYSE listing standards. In some cases the Dutch requirements are stricter than the NYSE listing standards, and in other cases the NYSE listing standards are the stricter of the two. The members of the Audit Committee of the Supervisory Board are also independent under the NYSE listing standards.
Committees of our Supervisory Board
The company has established four committees, consisting of members of the Supervisory Board only: the Audit Committee, the Remuneration Committee, the Corporate Governance and Nomination & Selection Committee and the Quality & Regulatory Committee. The roles, responsibilities and composition of these committees reflect the requirements of the Dutch Corporate Governance Code, the company’s Articles of Association and Dutch law, which differ from the NYSE listing standards in these respects. The role of each committee is to advise the Supervisory Board and to prepare the decision-making of the Supervisory Board. In principle, the entire Supervisory Board remains responsible for its decisions even if such decisions were prepared by one of the Supervisory Board’s committees.
The NYSE requires that, when an audit committee member of a listed US domestic issuer serves on four or more audit committees of public companies, the listed company should disclose (either on its website or in its Annual Report on Form 10-K) that the board of directors has determined that this simultaneous service would not impair the director’s service to the listed company. Dutch law does not require the company to make such a determination.
In accordance with the procedures laid down in the Philips Auditor Policy and as mandatorily required by Dutch law, the external auditor of the company is appointed by the General Meeting of Shareholders on the proposal of the Supervisory Board, after the latter has been advised by the Audit Committee and the Board of Management.
Equity compensation plans
The company complies with Dutch legal requirements regarding shareholder approval of equity compensation plans for the members of the Board of Management. Dutch law does not require shareholder approval of certain equity compensation plans for which the NYSE listing standards would require such approval. The company is subject to a Dutch requirement to seek shareholder approval for equity compensation plans for its members of the Board of Management.
Code of business conduct
The listing standards of the NYSE prescribe certain parameters for listed company codes of business conduct and ethics. The company has implemented the Philips General Business Principles, which are applicable to all employees, and a Financial Code of Ethics, which is applicable to all employees performing an accounting or financial function. Waivers granted to Senior (Financial) Officers (as defined in our Financial Code of Ethics) must be disclosed. In 20222023 the company did not grant any waivers of the Financial Code of Ethics.
Related party transactions
The NYSE listing standards require certain transactions with related parties to be reviewed by a company’s audit committee or another independent body of the board of directors for potential conflicts of interest, and for the audit committee or other independent body to prohibit such a transaction if it determines it to be inconsistent with the interests of the company and its shareholders. However, foreign private issuers can rely on home country practice with respect to review and approval of related party transactions. Philips has internal procedures in place to confirm that related party transactions are entered into at arm’s length and, if and to the extent required under Dutch law, to enable the Supervisory Board to assess the terms of significant related party transactions.
New York Registry Shares
Certain common shares of the company are registered in the register maintained by Deutsche Bank Trust Company Americas, as the New York transfer agent, registrar and dividend disbursing agent (the “New York Transfer Agent”), pursuant to a Transfer Agent Agreement, dated July 16, 2018, between the New York Transfer Agent and the company (such common shares, “New York Registry Shares”). As soon as practicable after receipt from the company, the New York Transfer Agent will provide holders of New York Registry Shares with a notice of any meeting or solicitation of consents or proxies with a notice prepared by the company stating (a) such information as is contained in such notice of meeting and any solicitation materials (or a summary thereof in English provided by the company), (b) that each registered holder at the close of business on the record date set by the company therefor will be entitled, subject to any applicable provisions of Dutch law and the Articles of Association, to exercise the voting rights pertaining to the New York Registry Shares, and (c) the manner in which such voting rights may be exercised. The New York Transfer Agent may, to the extent not prohibited by applicable law or by the requirements of the New York Stock Exchange, in lieu of distribution of the materials provided to it in connection with any meeting of, or solicitation of consents or proxies from, holders of common shares, distribute to the registered holders of New York Registry Shares a notice that provides such holders with, or otherwise publicizes to such holders, instructions on how to retrieve such materials or receive such materials upon request (i.e. by reference to a website containing the materials for retrieval or a contact for requesting copies of the materials).
Major shareholders as filed with SEC
On February 5, 2020, BlackRock Inc. filed a Schedule 13G with the SEC indicating that, as of December 31, 2019, it beneficially owned 9.2% (82,571,656 shares) of the company’s common shares. On January 27, 2020, Wellington Management Group LLP, Wellington Group Holdings LLP, Wellington Investment Advisors Holdings LLP and Wellington Management Company LLP jointly filed a Schedule 13G with the SEC indicating that, as of December 31, 2019, Wellington Management Group LLP, Wellington Group Holdings LLP and Wellington Investment Advisors Holdings LLP each beneficially owned 7.17% (64,327,165 shares) of the company’s common shares and Wellington Management Company LLP beneficially owned 6.80% (60,988,928 shares) of the company’s common shares.
On January 29, 2021, BlackRock Inc. filed a Schedule 13G with the SEC indicating that, as of December 31, 2020, it beneficially owned 8.5% (77,552,149 shares) of the company’s common shares. On February 3, 2021, Wellington Management Group LLP, Wellington Group Holdings LLP, and Wellington Investment Advisors Holdings LLP jointly filed a Schedule 13G with the SEC indicating that, as of December 31, 2020, Wellington Management Group LLP, Wellington Group Holdings LLP and Wellington Investment Advisors Holdings LLP each beneficially owned 1.85% (16,883,298 shares) of the company’s common shares.
On January 28, 2022, Blackrock Inc. filed a Schedule 13G with the SEC indicating that, as of December 31, 2021, it beneficially owned 7.2% (63,499,693 shares) of the company’s common shares.
On January 25, 2023, Blackrock Inc. filed a Schedule 13G with the SEC indicating that, as of December 31, 2022, it beneficially owned 8.8% (78,533,730 shares) of the company’s common shares.
Please also refer to Major shareholders.
Group financial statements contents
- 13.1Controls and Procedures
- 13.2Reports of the independent auditor
- 13.3Independent auditor’s report on internal control over financial reporting
- 13.4Independent auditor’s report on the consolidated financial statements
- 13.5Consolidated statements of income
- 13.6Consolidated statements of comprehensive income
- 13.7Consolidated balance sheets
- 13.8Consolidated statements of cash flows
- 13.9Consolidated statements of changes in equity
- 13.10Notes to the Consolidated financial statements
- 1General information to the Consolidated financial statements
- 2Information by segment and main country
- 3Discontinued operations and assets classified as held for sale
- 4Acquisitions and divestments
- 5Interests in entities
- 6Income from operations
- 7Financial income and expenses
- 8Income taxes
- 9Earnings per share
- 10Property, plant and equipment
- 11Goodwill
- 12Intangible assets excluding goodwill
- 13Other financial assets
- 14Other assets
- 15Inventories
- 16Receivables
- 17Equity
- 18Debt
- 19Provisions
- 20Post-employment benefits
- 21Accrued liabilities
- 22Other liabilities
- 23Cash flow statement supplementary information
- 24Contingencies
- 25Related-party transactions
- 26Share-based compensation
- 27Information on remuneration
- 28Fair value of financial assets and liabilities
- 29Details of treasury and other financial risks
- 30Subsequent events
13Group financial statements
Introduction
Statutory financial statements
This section ‘Group financial statements’ and the section 'Company Financial Statements' together contain the statutory financial statements of the company. These statements are subject to adoption by the company’s shareholders at the upcoming 2023 Annual General Meeting of Shareholders.
Management report and statement
13.1Controls and Procedures
13.1.1Disclosure controls and procedures
The Company’s Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of the design and operation of the company’s disclosure controls and procedures (as defined in Rules 13a15(e) and 15d15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by the Annual Report. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that these disclosure controls and procedures are effective as of December 31, 2022.2023.
13.1.2Management's annual report on internal control over financial reporting
The Board of Management of Koninklijke Philips N.V. (Royal Philips) is responsible for establishing and maintaining an adequate system of internal control over financial reporting (as such term is defined in Rule 13a-15 (f) under the US Securities Exchange Act). Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with IFRS as issued by the IASB.
Internal control over financial reporting includes maintaining records that, in reasonable detail, accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of company assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Also, projections of any evaluation of the effectiveness of internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Board of Management conducted an assessment of Royal Philips' internal control over financial reporting based on the “Internal Control Integrated Framework (2013)” established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Based on the Board of Management’s assessment of the effectiveness of Royal Philips' internal control over financial reporting as of December 31, 2022,2023, it has concluded that, as of December 31, 2022,2023, Royal Philips' internal control over Group financial reporting is considered effective.
13.1.3Attestation report of the registered public accounting firm
The effectiveness of the Royal Philips’ internal control over financial reporting as of December 31, 2022,2023, as included in this section Group financial statements, has been audited by Ernst & Young Accountants LLP, an independent registered public accounting firm, as stated in their report which follows hereafter.
13.1.4Changes in internal control over financial reporting
There were no changes in our internal control over financial reporting during 20222023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
13.2Reports of the independent auditor
Management’s report on internal control over financial reporting is set out on Management's annual report on internal control over financial reporting. The report set out on Independent auditor’s report on internal control over financial reporting, is provided in compliance with standards of the Public Company Accounting Oversight Board in the US and includes an opinion on the effectiveness of internal control over financial reporting as at December 31, 2022, based on COSO criteria.
Ernst & Young Accountants LLP (PCAOB ID: 1396) has also issued a report on the consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board in the US, which is set out on Independent auditor’s report on the consolidated financial statements.
13.3Independent auditor’s report on internal control over financial reporting
Report of Independent Registered Public Accounting Firm
To: The Supervisory Board and Shareholders of Koninklijke Philips N.V.
Opinion on Internal Control over Financial Reporting
We have audited Koninklijke Philips N.V.’s internal control over financial reporting as of December 31, 2022,2023, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Koninklijke Philips N.V. (the company)Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022,2023, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the companyCompany as of December 31, 20222023 and 2021,2022, the related consolidated statements of income, comprehensive income, cash flows and changes in equity for each of the three years in the period ended December 31, 2022,2023, and the related notes and our report dated February 21, 202320, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
The company’sCompany’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying section ‘Management’s report on internal control’, of this Annual Report. Our responsibility is to express an opinion on the company’sCompany’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the companyCompany in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young Accountants LLP
Amsterdam, the Netherlands
February 21, 202320, 2024
13.4Independent auditor’s report on the consolidated financial statements
Report of Independent Registered Public Accounting Firm
To: The Supervisory Board and Shareholders of Koninklijke Philips N.V.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Koninklijke Philips N.V. (Philips, or the Company) as of December 31, 20222023 and 2021,2022, the related consolidated statements of income, comprehensive income, cash flows and changes in equity for each of the three years in the period ended December 31, 2022,2023, and the related notes (collectively referred to as the group“group financial statements)statements”). In our opinion, the group financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20222023 and 2021,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022,2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2022,2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013“(2013 framework)”, and our report dated February 21, 202320, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
These group financial statements are the responsibility of the Company‘s management. Our responsibility is to express an opinion on the Company‘s group financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the US federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the group financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the group financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the group financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the group financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical audit matters
The critical audit matters communicated below are matters arising from the current period audit of the group financial statements that were communicated or required to be communicated to the Audit Committee of the Supervisory Board and that: (1) relate to accounts or disclosures that are material to the group financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the group financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
| Revenue recognition – | |
| Description of the Matter |
|
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of the Company’s controls that address the risks of material misstatement relating to measurement for
|
| Valuation of Goodwill for Cash Generating Unit Sleep & Respiratory Care | |
| Description of the Matter |
|
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over management’s goodwill impairment review process related to the CGU S&RC.
|
| Measurement and disclosure of the Respironics field action provision related to Sleep & Respiratory Care products | |
| Description of the Matter | The Company recognized a provision based on management’s best estimate of the costs to replace, repair, or refund devices subject to the Respironics field action, initiated in 2021. As more fully described in Note 19, Provisions, the Respironics field action provision amounted to EUR 334 million as of December 31, 2023. |
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of the Company’s controls relating to the Respironics field action recall provision calculation and utilization. For example, we tested the management review controls over the completeness, the utilization and mathematical accuracy of the provision.
|
Measurement of provisions and disclosures for legal claims, litigations and contingent liabilities | |
| Description of the Matter | The Company and certain of its group companies and former group companies are involved as a party in legal proceedings, including regulatory and other governmental proceedings, as well as being investigated by governmental authorities for alleged non-compliance with laws and regulations. As more fully described in Note 19, Provisions, and Note 24, Contingencies, this includes legal claims and litigation related to the Respironics field action, and discussions with and information provided to the Securities and Exchange Commission (SEC) and Department of Justice
The Company recognizes provisions for legal claims and litigation when it has a present obligation, it is probable that an outflow of economic benefits will be required to settle the obligation and the amount can be estimated reliably.
|
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of the Company’s
|
| Description of the Matter | As more fully described in Note
|
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design, and tested the operating effectiveness of
|
/s/ Ernst & Young Accountants LLP
We have served as the Company‘s auditor since 2016.
Amsterdam, the Netherlands
February 21, 202320, 2024
13.5Consolidated statements of income
Philips Group
Consolidated statements of income
in millions of EUR
For the year ended December 31
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Sales6 | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
| Cost of sales | (9,493) | (9,988) | (10,633) | (9,988) | (10,633) | (10,721) |
| Gross margin | 7,820 | 7,168 | 7,194 | 7,168 | 7,194 | 7,448 |
| Selling expenses | (4,054) | (4,258) | (4,609) | (4,258) | (4,621) | (4,524) |
| General and administrative expenses | (630) | (599) | (671) | (599) | (671) | (608) |
| Research and development expenses | (1,822) | (1,806) | (2,103) | (1,806) | (2,091) | (1,890) |
| Impairment of goodwill11 | (144) | (15) | (1,357) | (15) | (1,357) | (8) |
| Other business income6 | 122 | 186 | 127 | 186 | 127 | 112 |
| Other business expenses6 | (29) | (123) | (109) | (123) | (109) | (645) |
| Income from operations6 | 1,264 | 553 | (1,529) | 553 | (1,529) | (115) |
| Financial income7 | 158 | 149 | 58 | 149 | 58 | 63 |
| Financial expenses7 | (202) | (188) | (258) | (188) | (258) | (376) |
| Investments in associates, net of income taxes | (9) | (4) | (2) | (4) | (2) | (98) |
| Income before taxes | 1,211 | 509 | (1,731) | 509 | (1,731) | (526) |
| Income tax expenses8 | (212) | 103 | 113 | |||
| Income tax (expense) benefit8 | 103 | 113 | 73 | |||
| Income from continuing operations | 999 | 612 | (1,618) | 612 | (1,618) | (454) |
| Discontinued operations, net of income taxes3 | 196 | 2,711 | 13 | 2,711 | 13 | (10) |
| Net income | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Attribution of net income: | ||||||
| Net income attributable to shareholders of Koninklijke Philips N.V. | 1,187 | 3,319 | (1,608) | 3,319 | (1,608) | (466) |
| Net income attributable to non-controlling interests | 8 | 4 | 3 | 4 | 3 | 2 |
Philips Group
Earnings per common share attributable to shareholders of Koninklijke Philips N.V.
in EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Basic earnings per common share attributable to shareholders of Koninklijke Philips N.V. | ||||||
| Basic earnings per common share attributable to shareholders of Koninklijke Philips N.V. 1) | ||||||
| Income from continuing operations | 1.09 | 0.67 | (1.84) | 0.64 | (1.76) | (0.50) |
| Net income | 1.31 | 3.67 | (1.82) | 3.52 | (1.75) | (0.51) |
| Diluted earnings per common share attributable to shareholders of Koninklijke Philips N.V. | ||||||
| Diluted earnings per common share attributable to shareholders of Koninklijke Philips N.V. 1) | ||||||
| Income from continuing operations | 1.08 | 0.67 | (1.84) | 0.64 | (1.76) | (0.50) |
| Net income | 1.29 | 3.65 | (1.82) | 3.50 | (1.75) | (0.51) |
Amounts may not add up due to rounding.
13.6Consolidated statements of comprehensive income
Philips Group
Consolidated statements of comprehensive income
in millions of EUR
For the year ended December 31
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net income for the period | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Pensions and other-post employment plans:20 | ||||||
| Remeasurement, before tax | 51 | 134 | 101 | 134 | 101 | (26) |
| Income tax effect on remeasurements8 | (12) | (21) | (20) | (21) | (20) | 3 |
| Financial assets fair value through OCI: | ||||||
| Net current-period change, before tax | - | (39) | (32) | (39) | (32) | (20) |
| Income tax effect on net current-period change | - | 1 | 1 | 1 | 3 | |
| Total of items that will not be reclassified to Income Statement | 39 | 74 | 49 | 74 | 49 | (40) |
| Currency translation differences: | ||||||
| Net current period change, before tax | (1,040) | 1,078 | 748 | 1,078 | 748 | (579) |
| Income tax effect on net current-period change8 | 1 | (5) | 2 | (5) | 2 | - |
| Reclassification adjustment for (gain) loss realized | 36 | - | 36 | - | (26) | |
| Reclassification adjustment for (gain) loss realized, in discontinued operations | 69 | 69 | ||||
| Cash flow hedges: | ||||||
| Net current-period change, before tax | 69 | (52) | (29) | (52) | (29) | 29 |
| Income tax effect on net current-period change8 | (17) | 18 | (10) | 18 | (10) | (2) |
| Reclassification adjustment for (gain) loss realized | (6) | (14) | 63 | (14) | 63 | (19) |
| Total of items that are or may be reclassified to Income Statement | (992) | 1,129 | 774 | 1,129 | 774 | (597) |
| Other comprehensive income for the period | (953) | 1,203 | 823 | 1,203 | 823 | (637) |
| Total comprehensive income for the period | 242 | 4,527 | (782) | 4,527 | (782) | (1,100) |
| Total comprehensive income attributable to: | ||||||
| Total comprehensive income (loss) attributable to: | ||||||
| Shareholders of Koninklijke Philips N.V. | 235 | 4,520 | (786) | 4,520 | (786) | (1,101) |
| Non-controlling interests | 6 | 7 | 4 | 7 | 4 | 1 |
Amounts may not add up due to rounding.
13.7Consolidated balance sheets
Amounts may not add up due to rounding.
Philips Group
Consolidated balance sheets
in millions of EUR unless otherwise stated
As of December 31
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Non-current assets | ||||
| Property, plant and equipment 102 | 2,699 | 2,638 | 2,638 | 2,483 |
| Goodwill112 | 10,637 | 10,238 | 10,238 | 9,876 |
| Intangible assets excluding goodwill122 | 3,650 | 3,526 | 3,526 | 3,190 |
| Non-current receivables16 | 224 | 279 | 279 | 193 |
| Investments in associates5 | 426 | 537 | 537 | 381 |
| Other non-current financial assets13 | 630 | 660 | 660 | 619 |
| Non-current derivative financial assets28 | 2 | 4 | 4 | 3 |
| Deferred tax assets8 | 2,216 | 2,449 | 2,449 | 2,627 |
| Other non-current assets14 | 129 | 98 | 98 | 93 |
| Total non-current assets | 20,613 | 20,429 | 20,429 | 19,466 |
| Current assets | ||||
| Inventories15 | 3,450 | 4,049 | 4,049 | 3,491 |
| Other current financial assets13 | 2 | 11 | 11 | 3 |
| Other current assets14 | 493 | 490 | 490 | 500 |
| Current derivative financial assets28 | 61 | 123 | 123 | 45 |
| Income tax receivable | 180 | 222 | 222 | 220 |
| Current receivables2516 | 3,787 | 4,115 | 4,115 | 3,733 |
| Assets classified as held for sale3 | 71 | 77 | 77 | 79 |
| Cash and cash equivalents29 | 2,303 | 1,172 | 1,172 | 1,869 |
| Total current assets | 10,347 | 10,259 | 10,259 | 9,940 |
| Total assets | 30,961 | 30,688 | 30,688 | 29,406 |
| Equity17 | ||||
| Shareholders' equity | 14,438 | 13,249 | 13,249 | 12,028 |
| Common shares | 177 | 178 | 178 | 183 |
| Capital in excess of par value | 4,646 | 5,025 | 5,025 | 5,827 |
| Reserves | 748 | 1,488 | 1,488 | 879 |
| Other | 8,868 | 6,558 | 6,558 | 5,139 |
| Non-controlling interests17 | 36 | 34 | 34 | 33 |
| Group equity | 14,475 | 13,283 | 13,283 | 12,061 |
| Non-current liabilities | ||||
| Long-term debt 18 | 6,473 | 7,270 | 7,270 | 7,035 |
| Non-current derivative financial liabilities28 | 119 | 4 | 4 | 3 |
| Long-term provisions2019 | 1,315 | 1,097 | 1,097 | 1,035 |
| Deferred tax liabilities8 | 83 | 91 | 91 | 71 |
| Non-current contract liabilities22 | 446 | 515 | 515 | 469 |
| Non-current tax liabilities 8 | 544 | 435 | 435 | 390 |
| Other non-current liabilities22 | 56 | 60 | 60 | 54 |
| Total non-current liabilities | 9,037 | 9,471 | 9,471 | 9,058 |
| Current liabilities | ||||
| Short-term debt 18 | 506 | 931 | 931 | 654 |
| Current derivative financial liabilities28 | 83 | 207 | 207 | 40 |
| Income tax payable | 128 | 40 | 40 | 83 |
| Accounts payable25 | 1,872 | 1,968 | 1,968 | 1,917 |
| Accrued liabilities21 | 1,784 | 1,626 | 1,626 | 1,887 |
| Current contract liabilities22 | 1,491 | 1,696 | 1,696 | 1,809 |
| Short-term provisions2019 | 998 | 1,018 | 1,018 | 1,463 |
| Dividend payable | - | 11 | ||
| Liabilities directly associated with assets held for sale | 1 | - | - | 9 |
| Other current liabilities22 | 587 | 448 | 448 | 414 |
| Total current liabilities | 7,450 | 7,934 | 7,934 | 8,287 |
| Total liabilities and group equity | 30,961 | 30,688 | 30,688 | 29,406 |
13.8Consolidated statements of cash flows
Amounts may not add up due to rounding.
13.8Consolidated statements of cash flows
Philips Group
Consolidated statements of cash flows
in millions of EUR
For the year ended December 31
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Cash flows from operating activities | ||||||
| Net income (loss) | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Results of discontinued operations, net of income tax | (196) | (2,711) | (13) | (2,711) | (13) | 10 |
| Adjustments to reconcile net income to net cash provided by (used for) operating activities: | ||||||
| Depreciation, amortization, and impairment of assets | 1,462 | 1,323 | 1,602 | 1,323 | 1,602 | 1,261 |
| Impairment of goodwill | 144 | 15 | 1,357 | 15 | 1,357 | 8 |
| Share-based compensation | 112 | 108 | 95 | 108 | 95 | 88 |
| Net loss (gain) on sale of assets | (1) | 55 | (115) | 55 | (115) | (71) |
| Interest income | (13) | (18) | (25) | (18) | (25) | (46) |
| Interest expense on debt, borrowings, and other liabilities | 159 | 152 | 226 | 152 | 226 | 255 |
| Investments in associates, net of income taxes | 9 | 4 | 112 | 4 | 112 | 107 |
| Income taxes | 212 | (103) | (113) | (103) | (113) | (71) |
| Decrease (increase) in working capital | (98) | (401) | (862) | (401) | (862) | 913 |
| Decrease (increase) in receivables and other current assets | 92 | (39) | (342) | (39) | (342) | 298 |
| Decrease (Increase) in inventories | (578) | (581) | (572) | (581) | (572) | 257 |
| Increase (decrease) in accounts payable, accrued and other current liabilities | 387 | 219 | 52 | 219 | 52 | 358 |
| Decrease (increase) in non-current receivables and other assets | (9) | (46) | 1 | (46) | 1 | (33) |
| Increase (decrease) in other liabilities | 50 | 33 | (84) | 33 | (84) | (38) |
| Increase (decrease) in provisions19 | (91) | 427 | (199) | 427 | (199) | 422 |
| Other items | 96 | (164) | (39) | (164) | (39) | 129 |
| Interest received | 13 | 17 | 15 | 17 | 15 | 53 |
| Interest paid | (148) | (151) | (205) | (151) | (205) | (250) |
| Dividends received from investments in associates | 4 | 14 | 12 | 14 | 12 | 13 |
| Income taxes paid | (390) | (249) | (333) | (249) | (333) | (152) |
| Net cash provided by (used for) operating activities | 2,511 | 1,629 | (173) | 1,629 | (173) | 2,136 |
| Cash flows from investing activities | ||||||
| Net capital expenditures | (876) | (729) | (788) | (729) | (788) | (554) |
| Purchase of intangible assets | (114) | (107) | (105) | (107) | (105) | (96) |
| Expenditures on development assets | (296) | (259) | (257) | (259) | (257) | (203) |
| Capital expenditures on property, plant and equipment | (485) | (397) | (444) | (397) | (444) | (345) |
| Proceeds from sales of property, plant and equipment | 19 | 33 | 18 | 33 | 18 | 90 |
| Net proceeds from (cash used for) derivatives and current financial assets23 | (13) | 48 | (72) | 48 | (72) | (46) |
| Purchase of other non-current financial assets23 | (131) | (124) | (116) | (124) | (116) | (92) |
| Proceeds from other non-current financial assets23 | 65 | 124 | 78 | 124 | 78 | 48 |
| Purchase of businesses, net of cash acquired45 | (317) | (3,098) | (712) | (3,098) | (712) | (73) |
| Net proceeds from sale of interests in businesses, net of cash disposed | 4 | 107 | 124 | 107 | 124 | 80 |
| Net cash provided by (used for) for investing activities | (1,267) | (3,672) | (1,487) | (3,672) | (1,487) | (636) |
| Cash flows from financing activities | ||||||
| Proceeds from issuance (payments on) short-term debt18 | 16 | (25) | 47 | |||
| Principal payments on current portion of long-term debt18 | (298) | (302) | (1,472) | |||
| Proceeds from issuance of long-term debt18 | 1,065 | 76 | 2,516 | |||
| Proceeds from issuance (payments on) short-term debt1823 | (25) | 47 | 29 | |||
| Principal payments on current portion of long-term debt1823 | (302) | (1,472) | (754) | |||
| Proceeds from issuance of long-term debt1823 | 76 | 2,516 | 544 | |||
| Re-issuance of treasury shares | 46 | 23 | 12 | 23 | 12 | |
| Purchase of treasury shares | (343) | (1,636) | (187) | |||
| Purchase of treasury shares17 | (1,636) | (187) | (662) | |||
| Dividends paid to shareholders of Koninklijke Philips N.V. | (1) | (482) | (412) | (482) | (412) | (2) |
| Dividends paid to shareholders of non-controlling interests | (2) | (6) | (2) | (6) | (3) | |
| Net cash provided by (used for) financing activities | 483 | (2,347) | 500 | (2,347) | 500 | (848) |
| Net cash provided by (used for) continuing operations | 1,727 | (4,390) | (1,160) | (4,390) | (1,160) | 652 |
| Net cash provided by (used for) discontinued operations3 | 129 | 3,403 | (12) | 3,403 | (12) | 123 |
| Net cash provided by (used for) continuing and discontinued operations | 1,856 | (986) | (1,172) | (986) | (1,172) | 776 |
| Effect of changes in exchange rates on cash and cash equivalents | (55) | 65 | 41 | 65 | 41 | (79) |
| Cash and cash equivalents at the beginning of the period | 1,425 | 3,226 | 2,303 | 3,226 | 2,303 | 1,172 |
| Cash and cash equivalents at the end of the period | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
Amounts may not add up due to rounding.
13.9Consolidated statements of changes in equity
Philips Group
Consolidated statements of changes in equity
in millions of EUR
For the year ended December 31
Common shares | Capital in excess of par value |
Amounts may not add up due to rounding. 13.10Notes to the Consolidated financial statements1General information to the Consolidated financial statementsReporting entity and its operationsKoninklijke Philips N.V. (‘Royal Philips’), incorporated and domiciled in the Netherlands, is a public limited liability company organized under Dutch Law. Philips is headquartered in Amsterdam, the Netherlands and has its registered address at High Tech Campus 52, 5656 AG Eindhoven, the Netherlands. The consolidated financial statements of Royal Philips as of December 31, Basis of preparationThe Consolidated financial statements are:
Accounting estimates and judgmentsThe preparation of financial statements requires management to make a number of estimates and judgments that affect the application of accounting policies and the reporting amounts of assets and liabilities, revenues and expenses, and the disclosure of contingent assets and liabilities. Amounts recognized are based on factors that are by default associated with uncertainty. Actual results may therefore differ from estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revision to estimates are recognized prospectively. Where applicable, the estimates and judgments of specific financial statement items are described in the respective note to the consolidated financial statements. The areas involving a higher degree of judgment and complexity in applying accounting principles and for which changes in the assumptions and estimates could result in significantly different results than those recorded in the consolidated financial statements are the following:
The company regularly updates its significant assumptions and estimates to support the reported amounts of assets, liabilities, income and expenses. In preparing the consolidated financial statements management has considered the impact of climate change, specifically the financial impact of Philips meeting its internal and external climate related aims, the potential impact of climate related risks and the costs incurred to pro-actively manage such risks. These considerations did not have a material impact on the financial reporting judgments, estimates or assumptions. The specific financial impacts considered include, for example: specific climate mitigation measures, such as the use of lower carbon energy sources, the costs of developing more sustainable product offerings and expenses incurred to mitigate against the impact of extreme weather conditions. Philips uses 100% electricity from renewable sources, mainly through long-term Power Purchase Agreements thereby mitigating the impact of carbon taxes. The development of more sustainable products are covered through our EcoDesign program and already included in our R&D expenses. The physical risk related to climate change on our sites resulting from our TCFD-assessment is currently considered limited.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sales | sales including intercompany | depreciation and amortization1) | Adjusted EBITA | |||||
|---|---|---|---|---|---|---|---|---|
| 2023 | ||||||||
| Diagnosis & Treatment | 8,818 | 9,253 | (306) | 1,026 | ||||
| Connected Care | 5,138 | 5,149 | (445) | 369 | ||||
| Personal Health | 3,602 | 3,685 | (115) | 597 | ||||
| Other | 612 | 428 | (394) | (71) | ||||
| Inter-segment eliminations | (346) | |||||||
| Philips Group | 18,169 | (1,261) | 1,921 | |||||
| sales | sales including intercompany | depreciation and amortization1) | Adjusted EBITA | |||||
| 2022 | ||||||||
| Diagnosis & Treatment | 9,168 | 9,471 | (559) | 774 | 8,290 | 8,576 | (417) | 788 |
| Connected Care | 4,403 | 4,441 | (514) | 95 | 5,268 | 5,280 | (646) | 111 |
| Personal Health | 3,626 | 3,684 | (132) | 538 | 3,626 | 3,684 | (132) | 538 |
| Other | 629 | 596 | (397) | (89) | 643 | 736 | (407) | (119) |
| Inter-segment eliminations | (366) | (449) | ||||||
| Philips Group | 17,827 | (1,602) | 1,318 | 17,827 | (1,602) | 1,318 | ||
| 2021 | ||||||||
| Diagnosis & Treatment | 8,635 | 8,846 | (459) | 1,071 | 7,825 | 8,023 | (363) | 1,028 |
| Connected Care | 4,573 | 4,617 | (382) | 497 | 5,371 | 5,388 | (472) | 553 |
| Personal Health | 3,429 | 3,462 | (131) | 590 | 3,429 | 3,462 | (131) | 590 |
| Other | 519 | 531 | (350) | (105) | 530 | 636 | (357) | (117) |
| Inter-segment eliminations | (299) | (353) | ||||||
| Philips Group | 17,156 | (1,323) | 2,054 | 17,156 | (1,323) | 2,054 | ||
| 2020 | ||||||||
| Diagnosis & Treatment | 8,175 | 8,289 | (536) | 818 | ||||
| Connected Care | 5,543 | 5,620 | (414) | 1,191 | ||||
| Personal Health | 3,199 | 3,198 | (145) | 433 | ||||
| Other | 396 | 481 | (368) | (165) | ||||
| Inter-segment eliminations | (275) | |||||||
| Philips Group | 17,313 | (1,462) | 2,277 | |||||
The term Adjusted EBITA is used to evaluate the performance of Philips and its segments. Adjusted EBITA represents Income from operations excluding amortization and impairment of acquired intangible assets and impairment of goodwill. Adjusted EBITA represents EBITAgoodwill (EBITA) and excluding gains or losses from restructuring costs, acquisition-related charges and other items.
Adjusted EBITA is not a recognized measure of financial performance under IFRS. Presented in the following table is a reconciliation of Adjusted EBITA to the most directly comparable IFRS measure, Net income, for the years indicated. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Philips Group
Reconciliation from net income to Adjusted EBITA
In millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | ||||||||||
| 2023 | ||||||||||
| Net Income | (1,605) | (463) | ||||||||
| Discontinued operations, net of income taxes | (13) | 10 | ||||||||
| Income tax expense | (113) | |||||||||
| Income taxes | (73) | |||||||||
| Investments in associates, net of income taxes | 2 | 98 | ||||||||
| Financial expenses | 258 | 376 | ||||||||
| Financial income | (58) | (63) | ||||||||
| Income from operations | (1,529) | 404 | (2,246) | 515 | (202) | (115) | 720 | (1,199) | 552 | (188) |
| Amortization and impairment of acquired intangible assets | 363 | 143 | 199 | 15 | 7 | 290 | 89 | 178 | 14 | 9 |
| Impairment of goodwill | 1,357 | 27 | 1,331 | 8 | - | |||||
| EBITA | 192 | 573 | (716) | 531 | (196) | 183 | 816 | (1,020) | 567 | (179) |
| Restructuring and acquisition-related charges | 202 | 21 | 108 | 11 | 61 | 381 | 118 | 115 | 9 | 140 |
| Other items: | 925 | 180 | 703 | (4) | 46 | 1,358 | 92 | 1,275 | 22 | (32) |
| Respironics field-action provision | 250 | 250 | ||||||||
| Respironics litigation provision | 575 | 575 | ||||||||
| Respironics field-action connected to the proposed consent decree | 363 | 363 | ||||||||
| Respironics field-action running remediation costs | 210 | 210 | 224 | 224 | ||||||
| R&D project impairments | 134 | 120 | 12 | 3 | ||||||
| Portfolio realignment charges | 109 | 109 | ||||||||
| Impairment of assets in S&RC | 39 | 39 | ||||||||
| Provision for public investigations tender irregularities | 60 | |||||||||
| Provisions for quality actions in Connected Care | 59 | 59 | ||||||||
| Quality remediation actions | 175 | 81 | 94 | |||||||
| Provision for a legal matter | 31 | 31 | ||||||||
| Investment re-measurement loss | 23 | 23 | ||||||||
| Gain on divestment of business | (35) | (35) | ||||||||
| Remaining items | 63 | - | 24 | (6) | 46 | 2 | 11 | (12) | (1) | 3 |
| Adjusted EBITA | 1,318 | 774 | 95 | 538 | (89) | 1,921 | 1,026 | 369 | 597 | (71) |
Philips Group
Reconciliation from net income to Adjusted EBITA
In millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | ||||||||||
| 2022 | ||||||||||
| Net Income | 3,323 | (1,605) | ||||||||
| Discontinued operations, net of income taxes | (2,711) | (13) | ||||||||
| Income tax expense | (103) | |||||||||
| Income taxes | (113) | |||||||||
| Investments in associates, net of income taxes | 4 | 2 | ||||||||
| Financial expenses | 188 | 258 | ||||||||
| Financial income | (149) | (58) | ||||||||
| Income from operations | 553 | 941 | (722) | 576 | (242) | (1,529) | 538 | (2,347) | 515 | (235) |
| Amortization and impairment of acquired intangible assets | 322 | 153 | 148 | 15 | 6 | 363 | 115 | 226 | 15 | 8 |
| Impairment of goodwill | 15 | 2 | 13 | 1,357 | 1,357 | |||||
| EBITA | 890 | 1,097 | (562) | 591 | (236) | 192 | 652 | (764) | 531 | (227) |
| Restructuring and acquisition-related charges | 95 | 7 | 93 | (1) | (5) | 202 | 3 | 125 | 11 | 62 |
| Other items: | 1,069 | (32) | 965 | - | 136 | 925 | 133 | 750 | (4) | 46 |
| Respironics field-action provision | 719 | - | 719 | - | ||||||
| Respironics field-action connected to the proposed consent decree | 250 | 250 | ||||||||
| Respironics field-action running remediation costs | 94 | 94 | 210 | 210 | ||||||
| Provisions for quality actions in Connected Care | 94 | 94 | ||||||||
| Loss on divestment of business | 76 | 76 | ||||||||
| R&D project impairments | 134 | 73 | 59 | 3 | ||||||
| Portfolio realignment charges | 109 | 109 | ||||||||
| Provision for public investigations tender irregularities | 60 | |||||||||
| Quality remediation actions | 59 | 59 | ||||||||
| Impairments of assets in S&RC | 39 | 39 | ||||||||
| Remaining items | 87 | (32) | 58 | - | 61 | 63 | - | 24 | (6) | 46 |
| Adjusted EBITA | 2,054 | 1,071 | 497 | 590 | (105) | 1,318 | 788 | 111 | 538 | (119) |
Philips Group
Reconciliation from net income to Adjusted EBITA
In millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | ||||||||||
| 2021 | ||||||||||
| Net Income | 1,195 | 3,323 | ||||||||
| Discontinued operations, net of income taxes | (196) | (2,711) | ||||||||
| Income tax expense | 212 | |||||||||
| Income taxes | (103) | |||||||||
| Investments in associates, net of income taxes | 9 | 4 | ||||||||
| Financial expenses | 202 | 188 | ||||||||
| Financial income | (158) | (149) | ||||||||
| Income from operations | 1,264 | 497 | 704 | 362 | (300) | 553 | 948 | (716) | 576 | (255) |
| Amortization and impairment of intangible assets | 377 | 209 | 134 | 16 | 18 | |||||
| Amortization and impairment of acquired intangible assets | 322 | 142 | 158 | 15 | 6 | |||||
| Impairment of goodwill | 144 | - | 144 | 15 | 2 | 13 | ||||
| EBITA | 1,784 | 706 | 982 | 378 | (282) | 890 | 1,092 | (545) | 591 | (248) |
| Restructuring and acquisition-related charges | 195 | 29 | 97 | 31 | 37 | 95 | (30) | 130 | (1) | (5) |
| Other items | 299 | 83 | 112 | 24 | 81 | |||||
| Other items: | 1,069 | (35) | 968 | - | 136 | |||||
| Respironics field-action connected to the proposed consent decree | 719 | 719 | ||||||||
| Respironics field-action running remediation costs | 94 | 94 | ||||||||
| Quality remediation actions | 94 | 94 | ||||||||
| Loss on divestment of business | 76 | 76 | ||||||||
| Remaining items | 87 | (35) | 61 | - | 61 | |||||
| Adjusted EBITA | 2,277 | 818 | 1,191 | 433 | (165) | 2,054 | 1,028 | 553 | 590 | (117) |
Transactions between the segments are mainly related to components and parts included in the product portfolio of the other segments. The pricing of such transactions was at cost or determined on an arm’s length basis. Philips has no single external customer that represents 10% or more of sales.
Philips Group
Main countries
in millions of EUR
| sales1) | tangible and intangible assets2) | |||
|---|---|---|---|---|
| 2023 | ||||
| Netherlands | 2,390 | 1,624 | ||
| United States | 7,178 | 11,410 | ||
| China | 1,408 | 234 | ||
| Japan | 941 | 407 | ||
| Germany | 573 | 348 | ||
| Other countries | 5,679 | 1,527 | ||
| Total main countries | 18,169 | 15,550 | ||
| sales1) | tangible and intangible assets2) | |||
| 2022 | ||||
| Netherlands | 540 | 1,746 | 2,021 | 1,746 |
| United States | 7,246 | 12,087 | 7,226 | 12,087 |
| China | 2,193 | 290 | 1,239 | 260 |
| Japan | 1,077 | 436 | 1,011 | 436 |
| Germany | 821 | 323 | 642 | 323 |
| United Kingdom | 463 | 527 | ||
| France | 400 | 249 | ||
| Other countries | 5,085 | 744 | 5,688 | 1,550 |
| Total main countries | 17,827 | 16,402 | 17,827 | 16,402 |
| 2021 | ||||
| Netherlands | 570 | 1,934 | 1,860 | 1,934 |
| United States | 6,420 | 12,615 | 6,403 | 12,615 |
| China | 2,335 | 283 | 1,400 | 258 |
| Japan | 1,073 | 480 | 1,068 | 480 |
| Germany | 839 | 305 | 970 | 305 |
| France | 446 | 49 | ||
| India | 431 | 85 | ||
| United Kingdom | 481 | 567 | 426 | 567 |
| France | 397 | 49 | ||
| Other countries | 5,040 | 753 | 4,150 | 693 |
| Total main countries | 17,156 | 16,986 | 17,156 | 16,986 |
| 2020 | ||||
| Netherlands | 404 | 1,926 | ||
| United States | 6,580 | 9,080 | ||
| China | 2,319 | 313 | ||
| Japan | 1,113 | 511 | ||
| Germany | 980 | 302 | ||
| United Kingdom | 509 | 545 | ||
| Italy | 383 | 111 | ||
| Other countries | 5,024 | 906 | ||
| Total main countries | 17,313 | 13,694 | ||
3Discontinued operations and assets classified as held for sale
Accounting policies
Assets classified as held-for-sale
Non-current assets (or disposal groups) are classified as held-for-sale if their carrying amounts are expected to be recovered through a sale transaction rather than through continuing use. Non-current assets (or disposal groups) classified as held-for-sale are measured at the lower of their carrying amount or the fair value less costs of disposal. Depreciation or amortization of an asset ceases when it is classified as held-for-sale. When non-current assets (or disposal groups) are classified as held-for-sale, comparative balances prior to such date are not represented in the Consolidated balance sheets.
Discontinued operations
A discontinued operation is a component of the company that has either been disposed of or is classified as held-for-sale and represents a separate major line of business or geographical area of operations or is a part of a single coordinated plan to dispose of a separate major line of business or geographical area of operations. Any gain or loss from disposal, together with the results of these operations until the date of disposal, are reported separately as discontinued operations in the Consolidated statements of income.
The financial information of discontinued operations is excluded from the respective captions in the Consolidated financial statements and related notes for all periods presented. Comparatives are re-presented for presentation of discontinued operations in the Consolidated statements of income and Consolidated statements of cash flows.
Accounting estimates and judgments
The determination of the fair value less costs of disposal involves the use of estimates and assumptions that tend to be uncertain. Circumstances to which these adjustments may relate include resolution of uncertainties that arise from the terms of the disposal transaction, such as the resolution of purchase price adjustments and indemnifications, resolution of uncertainties that arise from and are directly related to the operations of the component before its disposal, such as environmental and assurance-type product warranty obligations retained by the company, and the settlement of employee benefit plan obligations provided that the settlement is directly related to the disposal transaction.
In 2020, 20212023 and 2022 Discontinueddiscontinued operations consist of certain costs related to other divestments, which were previously reported as discontinued operations. In 2021 discontinued operations consist primarily of the Domestic Appliances business. The following table summarizes the results of discontinued operations, net of income taxes, reported in the consolidated statements of income.
Philips Group
Discontinued operations, net of income taxes
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Domestic Appliances | 206 | 2,698 | 3 | 2,698 | 3 | (2) |
| Other | (10) | 13 | 10 | 13 | 10 | (7) |
| Discontinued operations, net of income taxes | 196 | 2,711 | 13 | 2,711 | 13 | (10) |
Discontinued operations: Domestic Appliances
In 2022, net results from discontinued operations for Domestic Appliances was EUR 3 million.
On March 25, 2021, Philips signed an agreement to sell its Domestic Appliances business to global investment firm Hillhouse Investment. Since the first quarter of 2021, the Domestic Appliances business is presented as a discontinued operation, and comparative results have been restated to reflect the treatment of the Domestic Appliances business as a discontinued operation, because the sale of the Domestic Appliances business constitutes the discontinuance of a major line of business from the Personal Health segment.
The following table summarizes the results of Domestic Appliances included in the Consolidatedconsolidated statements of income as a discontinued operation.
Philips Group
Results of Domestic Appliances
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Sales | 2,222 | 1,516 | 6 | 1,516 | 6 | - |
| Costs and expenses | (1,944) | (1,322) | (2) | (1,322) | (2) | (2) |
| Income from operations | 279 | 194 | 4 | 194 | 4 | (2) |
| Result on the sale of discontinued operations | 3,241 | 1 | 3,241 | 1 | (1) | |
| Income before tax | 279 | 3,435 | 5 | 3,435 | 5 | (3) |
| Income tax expense1) | (72) | 6 | (2) | |||
| Income tax benefit (expense)1) | 6 | (2) | 1 | |||
| Income tax related the sale of discontinued operations | (743) | (743) | ||||
| Results from discontinued operations | 206 | 2,698 | 3 | 2,698 | 3 | (2) |
Costs of EUR 64 million incurred in relation to the separation of the Domestic Appliances business in 2021 have been accounted for in continuing operations, because these costs reflect expenses incurred by Royal Philips in the divestment process and are not considered representative of the core business results of the Domestic Appliances business.
On September 1, 2021, the company completed the sale of the Domestic Appliances business and recognized a transaction gain before tax of EUR 3,241 million. Philips received consideration of EUR 4,041 million, which is based on an enterprise value of EUR 3,850 million, increased by an amount of EUR 191 million for closing adjustments related to working capital and net indebtedness. The transaction gain before tax is the net effect of (i) the EUR 4,041 million consideration (ii) less the derecognition of net assets employed of EUR 715 million (iii) less transaction related costs of EUR 16 million, (iv) less the release of cumulative translation losses of EUR 69 million included in Other comprehensive income. The income tax charges related to the divestment process was EUR 743 million, resulting in an after-tax transaction gain of EUR 2,499 million. The income tax charge represents the consolidated tax expense resulting from asset transactions completed as part of the disentanglement of the business in anticipation of its sale, a significant portion of which relates to taxes payable in the Netherlands. In addition, Philips and the buyer entered into a 15-year brand license agreement with future annual payments that represents an estimated net present value of approximately EUR 0.7 billion, which will be received and recognized over time.
Discontinued operations: Other
Certain costs related to other divestments, which were previously reported as discontinued operations, resulted in a net loss of EUR (7) million in 2023, a net gain of EUR 10 million in 2022 and a net gain of EUR 13 million in 2021 and a net loss of EUR 10 million in 2020.2021.
Discontinued operations cash flows
The following table presents the net cash provided by (used for) discontinued operations reported in the Consolidated statements of cash flows.
Net cash provided by (used for) discontinued operations
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net cash provided by (used for) operating activities | 129 | 85 | (27) | 85 | (27) | 123 |
| Net cash provided by (used for) investing activities | 3,319 | 15 | 3,319 | 15 | ||
| Net cash provided by (used for) discontinued operations | 129 | 3,403 | (12) | 3,403 | (12) | 123 |
In 2023, net cash provided by discontinued operations was EUR 123 million and consisted primarily of a refund received of one-off advance tax payments related to a previously divested business.
In 2022, net cash used for discontinued operations was EUR (12) million and consisted primarily of cash flows related to the tax claims from thea previously divested business.
In 2021, net cash provided for discontinued operations was EUR 3,403 million and consisted primarily of the net cash inflow of EUR 3,319 million from the sale of the Domestic Appliances business on September 1, 2021.
In 2020, net cash provided for discontinued operations was EUR 129 million and consisted primarily of cash flows provided by operating activities of the Domestic Appliances business, partly offset by advance income tax payments amounting to EUR 78 million
Assets classified as held for sale
As of December 31, 2023, assets held for sale primarily consisted of assets and liabilities directly associated with a business held for sale.
As of December 31, 2022, assets held for sale consists of property, plant and equipment mainly related to the APAC Center Singapore building. The sale was finalized in January 2023.
As of December 31, 2021, assets held for sale consists of property, plant and equipment mainly related to the APAC Center Singapore building.
4Acquisitions and divestments
Accounting policies
Acquisitions
The company accounts for business combinations using the acquisition method when control is transferred to the group. The consideration transferred in the acquisition is generally measured at fair value, as are the identifiable net assets acquired and the liabilities assumed. Transaction costs are expensed as incurred. Any contingent consideration is measured at fair value at the acquisition date and is initially presented in Long-term provisions. When the timing and amount of the consideration become more certain, it is reclassified to Accrued liabilities. If the contingent consideration that meets the definition of a financial instrument is classified as equity, it is not remeasured and settlement is accounted for within equity. Otherwise, subsequent changes in the fair value of the contingent consideration are recognized in the Consolidated statements of income.
Changes to the initial fair value of the acquired assets and liabilities, based on new information about the circumstances at the acquisition date, can be made up to twelve months after the acquisition date.
Divestments
Upon loss of control, the company derecognizes the assets and liabilities of the subsidiary, any non-controlling interests and the other components of equity related to the subsidiary. Any surplus or deficit arising from the loss of control is recognized in the Consolidated statements of income. If the company retains any interest in the previous subsidiary, such interest is measured at fair value at the date the control is lost. Subsequently it is accounted for as either an equity-accounted investee (associate) or as a financial asset, depending on the level of influence retained. Further information on loss of control can be found in Discontinued operations and assets classified as held for sale.
Accounting estimates and judgments
Intangible assets acquired in a business acquisition and the financial liability related to non-controlling interest are measured at fair value at the date of the acquisition.
To determine the fair value of intangible assets at the acquisition date, estimates and assumptions are required. The valuation of the identifiable intangible assets involves estimates of expected sales, earnings and/or future cash flows and require use of key assumptions such as discount rate, royalty rate and growth rates.
Estimates are also applied when determining the fair value of legal cases and tax positions in the acquired entity. The fair value is based on estimates of the likelihood, the expected timing and the amount of the potential cash outflow. Provisions for legal cases and non-income tax positions are recognized at fair value even if it is not probable that an outflow will be required to settle the obligation. After initial recognition and until the liability is settled, cancelled or expired, the liability is measured at the higher of the amount that would be recognized in accordance with IAS 37 'Provisions, contingent liabilities and contingent assets' and the initial liability amount. For income tax positions, the company applies IAS 12 'Income Taxes', which requires recognition of provisions only when the likelihood of cash outflow is considered probable.
2023
Acquisitions
On May 5, 2023, Philips completed one acquisition within Ultrasound business unit to accelerate the growth of its Diagnosis & Treatment segment. The total equity purchase price and the settlement of debt, net of acquired cash, involved an amount of EUR 53 million and a contingent consideration of EUR 6 million at fair value, the latter recognized as a Long-term provision. Upon acquisition, the company recognized Goodwill of EUR 24 million, Other intangible assets of EUR 40 million and deferred tax asset and liability of EUR 5 million and EUR 2 million, respectively. The acquisition is subject to final purchase price allocation procedures, which is expected to be finalized in the second quarter of 2024. The primary provisional accounts subject to change are related to acquired intangible assets and goodwill.
Since the acquisition date through December 31, 2023, the contribution to sales to third parties and net income of the acquiree was not material. The sales and net income would not differ materially if the acquisition date had been January 1, 2023. Acquisition-related costs were recognized in General and administrative expenses and were not material.
Divestments
During 2023 Philips completed six divestments for net cash consideration of EUR 80 million and a gain of EUR 50 million, which is included in Other business income of the Consolidated statements of income. The most notable was the sale of Philips Pharma Solutions in the US. The divestments were not material.
2022
Acquisitions
In 2022 Philips completed three acquisitions. The acquisitions involved aggregated net cash outflow of EUR 359 million andmillion. Including final purchase price adjustments processed in the course of 2023, the company recognized contingent consideration of EUR 96 million measured at fair value. Upon acquisition, the company recognizedvalue, aggregated Goodwill of EUR 307 million, Other intangible assets of EUR 179 million, Deferred tax assets of EUR 20 million and Deferred tax liabilities generated from the intangible assets of EUR 43 million.
Vesper Medical Inc. (Vesper) was the most notable acquisition and is discussed below. The remaining two acquisitions involved aggregated net cash outflow of EUR 139 million andmillion. Including final purchase price adjustments processed in the course of 2023, the company recognized contingent consideration of EUR 61 million measured at fair value. The two acquisitions resulted invalue, aggregated Goodwill of EUR 130 million, Other intangible assets of EUR 95 million and Deferred tax liabilities of EUR 23 million.
The opening balance sheet positions reflect the preliminary determination of the fair value of identifiable assets acquired and liabilities assumed with the acquisitions. The final determination of the fair values will be completed in 2023. As of December 31, 2022, the valuation studies necessary to determine the fair value of the intangible assets and the valuation of goodwill are preliminary.
Since the respective acquisition dates through December 31, 2022, the contribution to sales to third parties and net income of the three acquired entities was not material. The sales and net income of the combined entities would not differ materially from these amounts if the acquisition date had been January 1, 2022. Acquisition-related costs were not material.
Vesper
On January 11, 2022, Philips acquired all shares of Vesper for an amount of EUR 227 million in cash and EUR 34 million contingent consideration at fair value. Vesper, headquartered in Wayne, Pennsylvania, US, is a medical technology company that develops minimally-invasive peripheral vascular devices. The company is developing the Vesper DUO Venous Stent System®, commercialization of which is estimated to start after approval by the US Food and Drug Administration (FDA), expected in 2024. The Vesper DUO Venous Stent System® consists of venous stents intended to treat deep venous obstruction. It provides physicians with a modular portfolio to customize therapy, restore venous flow, and resolve the painful symptoms of deep venous disease for the broad range of patients suffering from chronic venous insufficiency. As of the acquisition date, Vesper forms part of the Image-Guided Therapy business portfolio of the Diagnosis & Treatment segment.
The condensed opening balance sheet of Vesper was as follows:
Philips Group
Opening balance sheet
in millions of EUR
| At acquisition date | |
|---|---|
| Vesper Medical Inc, | |
| Assets | |
| Intangible assets excluding goodwill | 84 |
| Deferred tax assets | 15 |
| Cash | 7 |
| Total Assets | 106 |
| Liabilities | |
| Accounts payable and other payables | (1) |
| Deferred tax liabilities | (20) |
| Total Liabilities | (21) |
| Total identifiable net assets at fair value | 85 |
| Goodwill arising on acquisition | 177 |
| Total purchase consideration | 262 |
| Of which: | |
| Purchase consideration transferred | 227 |
| Contingent consideration | 34 |
Goodwill recognized in the amount of EUR 177 million mainly represents revenue synergies expected from the combination of Philips’ peripheral vascular portfolio and Vesper's venous stenting solution to address the root cause of chronic deep venous disease (DVD). Strong clinical synergies between Vesper’s innovative stenting solution and Philips' existing peripheral vascular offering will help to better support clinicians to decide, guide, treat and confirm during the procedure, thereby enhancing patient care. Vesper Goodwillgoodwill is not tax-deductible.
The majority of the Intangibleintangible assets balance relates to capitalized development costs, the fair value of which is determined using the multi-period excess earnings method, which is a valuation technique that estimates the fair value of an asset based on market participants’ expectations of the cash flows associated with that asset over its remaining useful life. The fair value of capitalized development costs is based on an estimate of positive future cash flows associated with incremental profits related to excess earnings, discounted at a rate of 12.0%. Capitalized development costs are tested for impairment on an annual basis until FDA approval is obtained and the asset is reclassified to an intangible asset that is depreciated over its economical useful life.
The contingent consideration arrangement requires Philips to pay the former owners of Vesper up to a maximum undiscounted amount of EUR 44 million contingent upon FDA approval of the Vesper DUO Venous Stent System. The fair value of the contingent consideration arrangement of EUR 34 million has been estimated by calculating the present value of the future expected cash flows. The estimate is based on a discount rate of 12% and assumed probability adjusted likelihood of FDA approval at a certain point in time.
Divestments
During 2022 Philips completed two divestments that were not material.
2021
Acquisitions
In 2021 Philips completed two acquisitions, BioTelemetry, Inc. and Capsule Technologies, Inc., that involved aggregated net cash outflow of EUR 2,824 million. Including final purchase price adjustment processed in the course of 2022, the company recognized aggregated Goodwill of EUR 2,113 million, Other intangible assets of EUR 840 million and related Deferred tax liabilities of EUR 206 million.
The condensed opening balance sheets of BioTelemetry and Capsule Technologies were as follows:
Opening balance sheet
in millions of EUR
| At acquisition date | ||
| BioTelemetry | Capsule Technologies | |
| Assets | ||
| Intangible assets excluding goodwill | 623 | 217 |
| Property, plant and equipment | 42 | 11 |
| Other non-current assets | 48 | - |
| Deferred tax assets | 77 | 17 |
| Inventories | 11 | 11 |
| Receivables and other current assets | 75 | 97 |
| Cash | 205 | 19 |
| Total Assets | 1,082 | 371 |
| Liabilities | ||
| Accounts payable and other payables | (278) | (98) |
| Deferred tax liabilities | (160) | (46) |
| Long-term liabilities | (82) | (11) |
| Acquired provision for contingent considerations | (16) | |
| Total Liabilities | (536) | (155) |
| Total identifiable net assets at fair value | 547 | 217 |
| Goodwill arising on acquisition | 1,790 | 322 |
| Purchase consideration transferred | 2,337 | 539 |
BioTelemetry
On February 9, 2021, Philips successfully completed a tender offer to acquire all issued and outstanding shares of BioTelemetry, Inc. for USD 72 per share. As a result, BioTelemetry shares were delisted from NASDAQ. The total equity purchase price and the settlement of stock option rights, including BioTelemetry’s cash and debt, involved an amount of EUR 2,132 million and EUR 172 million equity awards consideration paid to employees after the acquisition day.
BioTelemetry, headquartered in Malvern, Pennsylvania, is a leading US-based provider of remote cardiac diagnostics and monitoring solutions. BioTelemetry offers a complete range of clinically validated ambulatory cardiac diagnostics and monitoring services: Short term Holter monitoring services, Long-term Holter monitoring services, Event recorder services, and Mobile Cardiac Outpatient Telemetry (MCOT) services. The acquisition of BioTelemetry is a strong fit with Philips’ cardiac care portfolio, and its strategy to transform the delivery of care along the health continuum with integrated solutions. BioTelemetry, forms part of the Connected Care segment.
Goodwill recognized in the amount of EUR 1,790 million mainly represents revenue synergies expected from the combination of Philips’ cardiac care portfolio and its strategy to transform the delivery of care along the health continuum with integrated solutions, and BioTelemetry complete range of clinically validated ambulatory cardiac diagnostics and monitoring services. BioTelemetry Goodwill is not tax-deductible.
The majority of the Intangible assets balance relates to the Customer relationships asset, the fair value of which is determined using the multi-period excess earnings method, which is a valuation technique that estimates the fair value of an asset based on market participants’ expectations of the cash flows associated with that asset over its remaining useful life. The fair value of the Customer relationships asset is based on an estimate of positive future cash flows associated with incremental profits related to excess earnings, discounted at a rate of 10.0%. The amortization period of the Customer relationships asset is 14 years. Receivables and other current assets reflect the best estimate at the acquisition date of the contractual cash flows expected to be received.
Since the acquisition date through December 31, 2021, the contribution to sales to third parties and net income of BioTelemetry was EUR 387 million and EUR 32 million loss, respectively. The sales and net income would not differ materially from these amounts if the acquisition date had been on January 1, 2021.
In 2021, acquisition-related costs of EUR 40 million were mainly recognized in General and administrative expenses.
Capsule Technologies
On March 4, 2021, Philips acquired all shares of Capsule Technologies, Inc. for an amount of EUR 520 million in cash. Capsule Technologies, headquartered in Andover, Massachusetts, is a leading provider of medical device integration and data technologies for hospitals and healthcare organizations. Capsule Technologies offers a leading vendor-neutral Medical Device Information Platform with a software-as-a-service business model. The acquisition of Capsule Technologies is a strong fit with Philips’ strategy to transform the delivery of care along the health continuum with integrated solutions. Capsule Technologies, forms part of the Connected Care segment.
Goodwill recognized in the amount of EUR 322 million mainly represents revenue synergies expected from the combination of Philips’ industry-leading portfolio of real-time patient monitoring, therapeutic devices, telehealth, informatics and interoperability solutions and Capsule’s leading Medical Device Information Platform, connected through Philips’ secure vendor-neutral cloud-based HealthSuite digital platform. Capsule Technologies Goodwill is not tax-deductible.
The majority of the Intangible assets balance relates to the Customer relationships asset, the fair value of which is determined using the multi-period excess earnings method, which is a valuation technique that estimates the fair value of an asset based on market participants’ expectations of the cash flows associated with that asset over its remaining useful life. The fair value of the Customer relationships asset is based on an estimate of positive future cash flows associated with incremental profits related to excess earnings, discounted at a rate of 12.0%. The amortization period of the Customer relationships asset is 17 years.
Receivables and other current assets reflect the best estimate at the acquisition date of the contractual cash flows expected to be received.
Since the acquisition date through December 31, 2021, the contribution to sales to third parties and net income of Capsule was EUR 75 million and EUR 10 million loss, respectively. The sales and net income would not differ materially from these amounts if the acquisition date had been on January 1, 2021.
In 2021, acquisition-related costs of EUR 11 million were mainly recognized in General and administrative expenses.
Divestments
During 2021 Philips completed three divestments. On September 1, 2021, Philips sold its Domestic Appliances business to global investment firm Hillhouse Investment. For further details on this transaction, refer to note Discontinued operations and assets classified as held for sale.
In addition, the company completed the divestment of the PERS business on June 30, 2021 and completed the divestment of a small business of segment Other on September 17, 2021. As part of PERS divestment, Philips acquired shares in the buyer Connect America Investment Holdings, LLC with a value of EUR 40 million. The investment is classified as a financial asset measured at Fair Value through Other Comprehensive Income (FVTOCI) and is reported as part of Other non-current financial assets. The divestment resulted in a loss of EUR 75 million, which is included in Other Business Expenses in the Statement of Income.
5Interests in entities
Accounting policies
Associates are all entities over which the company has significant influence, but not control or joint control. Significant influence is presumed with a shareholding of between 20% and 50% of the voting rights.
Investments in associates are accounted for using the equity method of accounting and are initially recognized at cost. The carrying amount of an investment in associate includes the carrying amount of goodwill identified on acquisition. An impairment loss on such investment is allocated to the investment as a whole.
The company’s share of the net income of these associates is included in Investments in associates, net of income taxes, in the Consolidated statements of income, after adjustments to align the accounting policies with those of the company. Dilution gains and losses arising from investments in associates are recognized in the Consolidated statements of income as part of Investments in associates, net of income taxes. Impairment losses and gains or losses on sale of investments are recorded in the Consolidated statements of income, more specifically on the line item ’Investments in associates, net of income taxes’.
When the company’s share of losses exceeds its interest in an associate, the carrying amount of that interest is reduced to zero and recognition of further losses is discontinued except to the extent that the company has an obligation or made payments on behalf of the associate.
The nature of the company’s interests in its consolidated entities and associates, and the effects of those interests on the company’s financial position and financial performance are discussed below.
Group companies
Below is a list of material subsidiaries as of December 31, 20222023 representing greater than 5% of either the consolidated group Sales, Income from operations or Income from continuing operations (before any intra-group eliminations) of Group legal entities. All of the entities are fully consolidated in the groupGroup financial statements.
Philips Group
Interests in group companies
in alphabetical order by country
20222023
| Legal entity name | Principal country of business |
| Philips (China) Investment Company, Ltd. | China |
| Philips Medizin Systeme Böblingen GmbH | Germany1) |
| Philips Japan, Ltd. | Japan |
| Philips Consumer Lifestyle B.V. | Netherlands |
| Philips | |
| United States | |
| Philips North America LLC | United States |
| Philips | United States |
Information related to non-controlling interests
As of December 31, 2022,2023, four consolidated subsidiaries are not wholly owned by Philips (December 31, 2021:2022: four). In 2022,2023, Sales to third parties and Net income for these subsidiaries in aggregate are EUR 472492 million (December 31, 2021:2022: EUR 522472 million) and EUR 2827 million (December 31, 2021:2022: EUR 3928 million), respectively.
Investments in associates
Philips has investments in a number of associates. During 2022,2023, Philips purchased eightmade two investments in associates for a total amount of EUR 2563 million. The most notable investment was a EUR 172 million investment in B-SOFT Co, Ltd, a China-based IT supplier for
Due to the medical and health sectors, listed on the stock exchange in Shenzhen. Philips acquired only a 10% interest, but determined that it is able to exerciseloss of significant influence amongst others duein Candid Care during 2023, Philips reclassified the investment to its representation on B-SOFT’s BoardOther non-current financial asset at FVTOCI (Level 3). On reclassification Philips recorded a loss of Directors. NoneEUR 23 million in Other business expenses. For more information about Other non-current financial asset at FVTOCI, refer to Other financial assets and Fair value of these investments are regarded as individually material from the point of view of the consolidated financial statements. assets and liabilities.
In 2022,2023, Philips recorded anits share in negative results of associates of EUR 51 million and impairment of EUR 66 million58 million. The impairment losses mainly related to HALO Dx (EUR 33 million) and were recorded within the Investments in relation to its interest in Candid Care Co. As partassociates, net of the acquisition of Affera, Inc. by Medtronic plc in August 2022, the company sold its investment in Affera to Medtronic and recorded a gain of EUR 84 million on the sale.income taxes line item.
Cumulative translation adjustments related to investments in associates were EUR 22(21) million as of December 31, 2022 (2021:2023 (2022: EUR 32(22) million).
Involvement with unconsolidated structured entities
Philips founded three Philips Medical Capital (PMC) entities, in the United States,US, France and Germany, in which Philips holds a minority interest. Philips Medical Capital, LLC in the United StatesUS is the most significant entity. PMC entities provide healthcare equipment financing and leasing services to Philips customers for diagnostic imaging equipment, patient monitoring equipment, and clinical IT systems.
The company concluded that it does not control, and therefore should not consolidate the PMC entities. In the United States,US, PMC operates as a subsidiary of De Lage Landen Financial Services, Inc. The same structure and treatment is applied to the PMC entities in the other countries, with other majority shareholders. Operating agreements are in place for all PMC entities, whereby acceptance of sales and financing transactions resides with the respective majority shareholder. After acceptance of a transaction by PMC, Philips transfers control and does not retain any obligations towards PMC or its customers, from the sales contracts.
As of December 31, 2022,2023, Philips’ shareholding in Philips Medical Capital, LLC had a carrying value of EUR 2927 million (December 31, 2021:2022: EUR 2729 million).
The company does not have any material exposures to losses from interests in unconsolidated structured entities other than the invested amounts.
6Income from operations
Accounting policies
Revenue recognition
The company recognizes revenue when it transfers control over a good or service to a customer, in an amount that reflects the consideration (i.e., transaction price) to which the company expects to be entitled to in exchange for the good or service. The consideration expected by the company may include fixed and/or variable amounts which can be impacted by sales returns, trade discounts and volume rebates. The company adjusts the consideration for the time value of money if the period between the transfer of the promised goods or services to the customer and payment by the customer exceeds six months.
Transfer of control varies depending on the individual terms of the contract of sale. For consumer-type products in the segment Personal Health businesses,segment, control is transferred when the product is shipped and delivered to the customer and title and risk have passed to the customer (depending on the delivery conditions) and acceptance of the product has been obtained.
Revenues from transactions relating to distinct goods or services are accounted for separately based on their relative stand-alone selling prices. The stand-alone selling price is the price that would be charged for the goods or service in a separate transaction under similar conditions to similar customers. The transaction price is determined (considering variable considerations) and allocated to performance obligations based on their relative stand-alone selling prices. These transactions mainly occur in the segments Diagnosis & Treatment businesses and Connected Care businesses and include arrangements that require subsequent installation and training activities to make distinct goods operable for the customer. As such, the related installation and training activities are part of equipment sales rather than separate performance obligations. Revenue is recognized when the performance obligation is satisfied, i.e., when the installation has been completed and the equipment is ready to be used by the customer in the way contractually agreed.
Variable consideration is included in the transaction price to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur once associated uncertainties are resolved. Such assessment is performed on each reporting date to check whether it is constrained. For products for which a right of return exists during a defined period, revenue recognition is determined based on the historical pattern of actual returns, or in cases where such information is not available, revenue recognition is postponed until the return period has lapsed. Return policies are typically based on customary return arrangements in local markets. A provision is recognized for assurance-type product warranty at the time of revenue recognition and reflects the estimated costs of replacement and free-of-charge services that will be incurred by the company with respect to the products sold. For certain products, the customer has the option to purchase the warranty separately, which is considered a separate performance obligation on top of the assurance-type product warranty. For such warranties which provide distinct service, revenue recognition occurs on a straight-line basis over the extended warranty contract period. Occasionally, the company may offer a full or partial refund of consideration previously paid, for example as part of the resolution to warranty related matters. In such instances, a provision is recognized for the amounts expected to be refunded to customers, and remeasured at each reporting date to reflect changes in the estimated refunds, with a corresponding adjustment to revenue.
In the case of loss under a sales agreement, the loss is recognized immediately.
Sale of goods
Revenues are recognized at a point in time when control of the goods passes to the buyer, based on the allocation of the transaction price to the performance obligation.
Revenue from services
Revenues are recognized over time as the company transfers control of the services to the customer which is demonstrated by the customer simultaneously receiving and consuming the benefits provided by the company. The amount of revenues is measured by reference to the progress made towards complete satisfaction of the performance obligation, which in general is evenly over time. Service revenue related to repair and maintenance activities for goods sold is recognized ratably over the service period or as services are rendered.
Income from royalties
Royalty income from brand license arrangements and from intellectual property rights, such as technology licenses or patents, is recognized on an accrual basis in accordance with the substance of the relevant agreement.
Shipping and handling
Expenses incurred for shipping and handling are mainly recorded as cost of sales. When shipping and handling are part of a project and billed to the customer, then the related expenses are recorded as cost of sales. Shipping and handling related to sales to third parties are partly recorded as selling expenses. When shipping and handling billed to customers are considered a distinct and separate performance obligation, the fees are recognized as revenue and costs included in cost of sales.
Other business income (expenses)
Other business income (expenses) includes gains and losses on the sale of property, plant and equipment, gains and losses on the sale of businesses as well as other gains and losses not related to the company’s operating activities.
Government grants
Grants from governments are recognized at their fair value when there is a reasonable assurance that the grant will be received and the company will comply with the conditions. Grants related to costs are deferred in the consolidated balance sheet and recognized in the consolidated statement of income as a reduction of the related costs that they are intended to compensate. Grants related to assets are deducted from the cost of the asset and presented net in the consolidated balance sheets.
Accounting estimates and judgments
Sales-related accruals
The company has sales promotions-related agreements with distributors and retailers designed to promote the sale of products. Among the programs are arrangements under which rebates and discounts can be earned by the distributors and retailers by attaining agreed upon sales levels, or for participating in specific marketing programs. Management estimates the sales-related accruals associated with these arrangements based on a combination of historical patterns and future expectations regarding which promotional targets are expected to be met by distributors and retailers. Accrued customer rebates are presented as other current liabilities, unless there is a right to offset against the respective accounts receivable.
A breakdown by nature of the income (loss) from operations is as follows:
Philips Group
Sales and costs by nature
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Sales | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
| Costs of materials used | (4,221) | (4,142) | (4,320) | (4,142) | (4,320) | (4,626) |
| Employee benefit expenses | (6,289) | (6,246) | (6,952) | (6,246) | (6,952) | (6,903) |
| Depreciation and amortization1) | (1,462) | (1,323) | (1,602) | (1,323) | (1,602) | (1,261) |
| Impairment of goodwill | (144) | (15) | (1,357) | (15) | (1,357) | (8) |
| Shipping and handling | (554) | (645) | (756) | (645) | (756) | (668) |
| Advertising and promotion | (696) | (752) | (739) | (752) | (739) | (700) |
| Lease expenses | (34) | (19) | (39) | (19) | (39) | (51) |
| Other operational costs | (2,741) | (3,524) | (3,609) | (3,524) | (3,609) | (3,535) |
| Other business income (expenses) | 92 | 63 | 18 | 63 | 18 | (533) |
| Income from operations | 1,264 | 553 | (1,529) | 553 | (1,529) | (115) |
Sales composition and disaggregation
For information related to sales on a segment and geographical basis, refer to Information by segment and main country.
Philips Group
Sales composition
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Goods | 12,491 | 11,981 | 12,139 | 11,981 | 12,139 | 12,419 |
| Services | 4,058 | 4,374 | 4,878 | 4,374 | 4,878 | 4,926 |
| Royalties | 301 | 383 | 419 | 383 | 419 | 434 |
| Total sales from contracts with customers | 16,851 | 16,738 | 17,435 | 16,738 | 17,435 | 17,779 |
| Sales from other sources | 462 | 418 | 391 | 418 | 391 | 390 |
| Total sales | 17,313 | 17,156 | 17,827 | 17,156 | 17,827 | 18,169 |
Sales of goods include provisions of EUR 174 million that were recognized as a reduction of sales, primarily for (partial) refunds to customers in connection with the proposed Respironics consent decree. Refer to Provisions.
Total sales from other sources mainly relates to operating leases including sublease income from right-of-use assets and related services ofEUR 234 million (2022: EUR 258 million (2021:2021: EUR 293 million 2020: EUR 325 million). Sales represent revenue from external customers.
As of December 31, 2022,2023, the aggregate amount of the transaction price allocated to remaining performance obligations from a sale of goods and services was EUR 16.5715,571 million. The company expects to recognize approximately 50%47% of the remaining performance obligations within 1 year. Revenue expected to be recognized beyond 1 year is mostly related to longer term customer service and software contracts.
Sales over time represent services and Other also includes royalties over time (2022:(2023: EUR 283 million 2022: EUR 292 million 2021: EUR 220 million 2020: EUR 211 million).
Sales per geographic area are reported based on country of destination.
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2022 | 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Diagnosis & Treatment | 5,565 | 3,547 | 9,112 | 56 | 9,168 | 5,762 | 2,978 | 8,742 | 76 | 8,818 |
| Connected Care | 2,803 | 1,266 | 4,068 | 335 | 4,403 | 2,970 | 1,854 | 4,824 | 314 | 5,138 |
| Personal Health | 3,615 | 11 | 3,626 | 3,626 | 3,586 | 16 | 3,602 | 3,602 | ||
| Other | 279 | 348 | 629 | - | 629 | 251 | 361 | 612 | - | 612 |
| Philips Group | 12,263 | 5,172 | 17,435 | 391 | 17,827 | 12,569 | 5,210 | 17,779 | 390 | 18,169 |
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2021 | 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Diagnosis & Treatment | 5,408 | 3,177 | 8,583 | 52 | 8,635 | 5,285 | 2,950 | 8,234 | 55 | 8,290 |
| Connected Care | 3,116 | 1,090 | 4,207 | 366 | 4,573 | 3,079 | 1,853 | 4,932 | 336 | 5,268 |
| Personal Health | 3,423 | 6 | 3,429 | 3,429 | 3,615 | 11 | 3,626 | 3,626 | ||
| Other | 194 | 323 | 518 | - | 519 | 284 | 357 | 643 | - | 643 |
| Philips Group | 12,142 | 4,596 | 16,738 | 418 | 17,156 | 12,263 | 5,172 | 17,435 | 391 | 17,827 |
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2020 | 2021 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Diagnosis & Treatment | 5,133 | 2,997 | 8,129 | 46 | 8,175 | 5,120 | 2,656 | 7,776 | 50 | 7,825 |
| Connected Care | 4,183 | 943 | 5,126 | 417 | 5,543 | 3,399 | 1,604 | 5,002 | 369 | 5,371 |
| Personal Health | 3,195 | 4 | 3,199 | 3,199 | 3,423 | 6 | 3,429 | 3,429 | ||
| Other | 69 | 327 | 396 | - | 396 | 200 | 330 | 530 | - | 530 |
| Philips Group | 12,580 | 4,271 | 16,851 | 462 | 17,313 | 12,142 | 4,596 | 16,738 | 418 | 17,156 |
Philips Group
Disaggregation of Sales per geographical clustergeographic area
in millions of EUR
| 2022 | 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Western Europe | 2,387 | 1,183 | 3,572 | 31 | 3,603 | 2,552 | 1,221 | 3,770 | 49 | 3,819 |
| North America | 4,889 | 2,612 | 7,502 | 86 | 7,588 | 4,859 | 2,608 | 7,470 | 92 | 7,562 |
| Other mature geographies | 972 | 399 | 1,369 | 274 | 1,643 | 980 | 398 | 1,378 | 248 | 1,626 |
| Total mature geographies | 8,248 | 4,194 | 12,443 | 390 | 12,833 | |||||
| Mature geographies | 8,392 | 4,227 | 12,618 | 389 | 13,007 | |||||
| Growth geographies | 4,015 | 978 | 4,992 | 1 | 4,993 | 4,177 | 984 | 5,161 | 1 | 5,162 |
| Sales | 12,263 | 5,172 | 17,435 | 391 | 17,827 | 12,569 | 5,210 | 17,779 | 390 | 18,169 |
Philips Group
Disaggregation of Sales per geographical clustergeographic area
in millions of EUR
| 2021 | 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Western Europe | 2,537 | 1,087 | 3,624 | 21 | 3,645 | 2,387 | 1,183 | 3,572 | 31 | 3,603 |
| North America | 4,427 | 2,268 | 6,695 | 86 | 6,781 | 4,889 | 2,612 | 7,502 | 86 | 7,588 |
| Other mature geographies | 1,000 | 386 | 1,386 | 309 | 1,694 | 972 | 399 | 1,369 | 274 | 1,643 |
| Total mature geographies | 7,964 | 3,741 | 11,705 | 415 | 12,120 | |||||
| Mature geographies | 8,248 | 4,194 | 12,443 | 390 | 12,833 | |||||
| Growth geographies | 4,178 | 856 | 5,033 | 3 | 5,036 | 4,015 | 978 | 4,992 | 1 | 4,993 |
| Sales | 12,142 | 4,596 | 16,738 | 418 | 17,156 | 12,263 | 5,172 | 17,435 | 391 | 17,827 |
Philips Group
Disaggregation of Sales per geographical clustergeographic area
in millions of EUR
| 2020 | 2021 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | Sales at a point in time | Sales over time | Total sales from contracts with customers | Sales from other sources | Total sales | |
| Western Europe | 2,747 | 936 | 3,682 | 19 | 3,702 | 2,537 | 1,087 | 3,624 | 21 | 3,645 |
| North America | 4,654 | 2,135 | 6,789 | 95 | 6,884 | 4,427 | 2,268 | 6,695 | 86 | 6,781 |
| Other mature geographies | 1,035 | 373 | 1,408 | 342 | 1,750 | 1,000 | 386 | 1,386 | 309 | 1,694 |
| Total mature geographies | 8,435 | 3,444 | 11,879 | 457 | 12,336 | |||||
| Mature geographies | 7,964 | 3,741 | 11,705 | 415 | 12,120 | |||||
| Growth geographies | 4,145 | 828 | 4,972 | 5 | 4,977 | 4,178 | 856 | 5,033 | 3 | 5,036 |
| Sales | 12,580 | 4,271 | 16,851 | 462 | 17,313 | 12,142 | 4,596 | 16,738 | 418 | 17,156 |
Costs of materials used
Cost of materials used represents the inventory recognized in cost of sales.
Employee benefit expenses
Philips Group
Employee benefit expenses
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Salaries and wages excluding share-based compensation | 5,085 | 5,014 | 5,594 | 5,014 | 5,594 | 5,635 |
| Share-based compensation | 119 | 115 | 104 | 115 | 104 | 97 |
| Post-employment benefit costs | 418 | 396 | 439 | 396 | 439 | 402 |
| Other social security and similar charges: | ||||||
| Required by law | 556 | 529 | 590 | 529 | 590 | 567 |
| Voluntary | 111 | 192 | 225 | 192 | 225 | 202 |
| Employee benefit expenses | 6,289 | 6,246 | 6,952 | 6,246 | 6,952 | 6,903 |
The employee benefit expenses relate to employees who are working on the payroll of Philips, both with permanent and temporary contracts.
For further information on post-employment benefit costs, refer to Post-employment benefits.
For details on the remuneration of the members of the Board of Management and the Supervisory Board, refer to Information on remuneration.
Employees
The average number (full-time equivalents, or FTEs) of employees by category is summarized as follows:
Philips Group
Employees by category
in FTEs
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Production | 35,482 | 38,618 | 39,742 | 38,618 | 39,742 | 35,281 |
| Research & development | 10,812 | 10,751 | 11,690 | 10,751 | 11,690 | 10,833 |
| Other | 22,474 | 22,543 | 23,019 | 22,543 | 23,019 | 23,001 |
| Employees | 68,769 | 71,912 | 74,451 | 71,912 | 74,451 | 69,115 |
| Third party workers | 4,998 | 4,533 | 4,086 | 4,533 | 4,086 | 3,149 |
| Philips Group | 73,767 | 76,445 | 78,538 | 76,445 | 78,538 | 72,264 |
Employees consist of those persons working on the payroll of Philips and whose costs are reflected in employee benefit expenses. Other consists of employees in commercial, general and administrative functions. Third party workers consist of personnel hired on a per-period basis, via external companies.
Philips Group
Employees by geographical location
in FTEs
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Netherlands | 11,146 | 11,142 | 11,180 | 11,142 | 11,180 | 9,794 |
| Other countries | 62,621 | 65,303 | 67,357 | 65,303 | 67,357 | 62,471 |
| Philips Group | 73,767 | 76,445 | 78,538 | 76,445 | 78,538 | 72,264 |
Depreciation and amortization
Depreciation of property, plant and equipment and amortization of intangible assets, including impairments, are as follows:
Philips Group
Depreciation and amortization1)
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Depreciation of property, plant and equipment | 691 | 630 | 711 | 630 | 711 | 689 |
| Amortization of software | 76 | 88 | 117 | 88 | 117 | 98 |
| Amortization of other intangible assets | 377 | 322 | 363 | 322 | 363 | 290 |
| Amortization of development costs | 319 | 284 | 411 | 284 | 411 | 184 |
| Depreciation and amortization | 1,462 | 1,323 | 1,602 | 1,323 | 1,602 | 1,261 |
Depreciation of property, plant and equipment is mainly included in cost of sales. Amortization of software is mainly included in general and administration expenses. Amortization of other intangible assets is included in selling expenses for brand names and customer relationships and is included in cost of sales for technology based and other intangible assets. Amortization of development costs is included in research and development expenses.
Impairment of goodwill
In 2023 a goodwill charge of EUR 8 million was recorded for the partial impairment of goodwill allocated to a business that was classified as held-for-sale as of December 31, 2023. During 2022, EUR 1,331 million of goodwill impairment charges were recorded in the Sleep & Respiratory Care business, due to revisions to the expected future cash flows. In addition, a EUR 27 million goodwill impairment was recognized in the Precision Diagnosis Solutions business. For further information refer to note Goodwill.
Shipping and handling
Shipping and handling costs are included in cost of sales and selling expenses in the Consolidated statements of income.
Advertising and promotion
Advertising and promotion costs are included in selling expenses in the Consolidated statements of income.
Lease expense
Lease expense relates to short-term and low value leases.
Other operational costs
Other operational costs contain items which are dissimilar in nature and individually insignificant in amount to disclose separately. These costs contain among others expenses for outsourcing services, mainly in Information Technology and Human Resources, third party workers, consultants, warranty, patents, costs for travelling and external legal service. Government grants of EUR 10395 million were recognized as cost reduction in 2022 (2021:2023 (2022: EUR 104103 million 2020:2021: EUR 98104 million). The grants mainly relate to research and development activities and business development. The increase in other operational costs 2021 versus 2020 is mainly due to the Respironics field action provision. For more details refer to Provisions .
Audit and audit-related fees
The following table shows the fees attributable to the fiscal years 2020, 2021, 2022 and 20222023 for services rendered by the external auditors.
Philips Group
Audit and audit-related fees
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | |
| Audit fees | 9.0 | 5.6 | 14.6 | 10.3 | 5.4 | 15.7 | 8.9 | 5.5 | 14.4 | 10.3 | 5.4 | 15.7 | 9.5 | 5.6 | 15.2 | 9.7 | 5.1 | 14.7 |
| consolidated financial statements | 9.0 | 2.9 | 11.9 | 10.3 | 2.7 | 13.0 | 8.9 | 3.0 | 11.9 | 10.3 | 2.7 | 13.0 | 9.5 | 3.1 | 12.6 | 9.7 | 2.6 | 12.3 |
| statutory financial statements | 2.7 | 2.7 | 2.7 | 2.7 | 2.5 | 2.5 | 2.7 | 2.5 | 2.5 | |||||||||
| Audit-related fees2) | 2.2 | 0.5 | 2.7 | 0.6 | 0.3 | 0.9 | 0.7 | 0.2 | 0.9 | 0.6 | 0.3 | 0.9 | 0.8 | 0.2 | 1.0 | 0.9 | 0.2 | 1.0 |
| divestment | 1.5 | 0.2 | 1.7 | |||||||||||||||
| sustainability assurance | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.8 | 0.8 | ||||||
| other | 0.2 | 0.3 | 0.5 | 0.1 | 0.3 | 0.4 | 0.1 | 0.2 | 0.3 | 0.1 | 0.3 | 0.4 | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 |
| Tax fees | - | |||||||||||||||||
| All other fees | ||||||||||||||||||
| Fees | 11.2 | 6.1 | 17.3 | 10.9 | 5.7 | 16.6 | 9.6 | 5.7 | 15.3 | 10.9 | 5.7 | 16.6 | 10.3 | 5.8 | 16.2 | 10.6 | 5.2 | 15.8 |
Other business income (expenses)
Other business income (expenses) consists of the following:
Philips Group
Other business income (expenses)
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Result on disposal of businesses: | ||||||
| income | - | 4 | - | 4 | 50 | |
| expenses | - | (75) | - | (75) | - | - |
| Result on disposal of fixed assets: | ||||||
| income | 2 | 24 | 3 | 24 | 3 | 12 |
| expenses | - | (5) | (1) | (5) | (1) | (1) |
| Result on other remaining businesses: | ||||||
| income | 120 | 161 | 121 | 161 | 121 | 49 |
| expenses | (30) | (43) | (109) | (43) | (109) | (643) |
| Other business income (expenses) | 92 | 63 | 18 | 63 | 18 | (533) |
| Total other business income | 122 | 186 | 127 | 186 | 127 | 112 |
| Total other business expenses | (29) | (123) | (109) | (123) | (109) | (645) |
The result on disposal of businesses mainly relates to divestment of non-strategic businesses. For more information refer to Acquisitions and divestments.
The result on disposal of fixed assets mainly relates to the sale of real estate assets.
The result on other remaining businesses mainly relates to the revaluation of contingent consideration and various legal matters. In 2023 Philips Respironics recorded a EUR 575 million provision in connection with the anticipated resolution of the economic loss class action. For more information on contingent consideration, refer to Provisions.
In 2023 a loss of EUR 23 million related to a minority participation was recognized in Other business expenses. For more information refer to Interests in entities.
7Financial income and expenses
Accounting policies
Financial income and expenses are recognized on the accrual basis in the consolidated statements of income. Interest income and expense are measured using the effective interest method. Dividend income is recognized in the consolidated statements of income on the date that the company’s right to receive payment is established, which in the case of quoted securities is normally the ex-dividend date.
Philips Group
Financial income and expenses
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Interest income | 13 | 18 | 25 | 18 | 25 | 46 |
| Interest income from loans and receivables | 8 | 7 | 7 | 7 | 13 | |
| Interest income from cash and cash equivalents | 5 | 11 | 18 | 11 | 18 | 33 |
| Dividend income from financial assets | 3 | 2 | 3 | 2 | 3 | 2 |
| Net gains from disposal of financial assets | 2 | - | - | - | - | |
| Net change in fair value of financial assets through profit or loss | 129 | 95 | 9 | 95 | 9 | |
| Other financial income | 12 | 33 | 20 | 33 | 20 | 15 |
| Financial income | 158 | 149 | 58 | 149 | 58 | 63 |
| Interest expense | (173) | (159) | (235) | (159) | (235) | (277) |
| Interest expense on debt and borrowings | (130) | (126) | (200) | (126) | (200) | (229) |
| Finance charges under lease contract | (29) | (25) | (25) | (25) | (27) | |
| Interest expense on pensions | (13) | (8) | (10) | (8) | (10) | (21) |
| Provision-related accretion expenses | (10) | (5) | (9) | (5) | (9) | (29) |
| Net foreign exchange gains (losses) | 4 | - | 9 | - | 9 | (23) |
| Net change in fair value of financial assets through profit or loss | (26) | |||||
| Other financial expenses | (23) | (24) | (24) | (24) | (21) | |
| Financial expenses | (202) | (188) | (258) | (188) | (258) | (376) |
| Financial income and expenses | (44) | (39) | (200) | |||
| Financial income and expenses, net | (39) | (200) | (314) | |||
In 2023, financial income and expenses, net increased by EUR 114 million year-on-year, mainly due to fair value losses and net foreign exchange losses in 2023, compared to gains in 2022. The fair value losses mainly relate to power purchase agreements for renewable energy, limited-life funds (mainly Gilde Healthcare) and other investments recognized at fair value through profit and loss. Furthermore, provision-related accretion expenses and net interest expense were higher in 2023 compared to 2022. Net interest expense in 2023 was EUR 21 million higher than in 2022, mainly due the issuance of new debt in 2022 and 2023 and the impact of increasing interest rates.
In 2022, Financial income and expenses increased by EUR 161 million year-on-year, mainly due to higher interest expense and lower fair value gains. The lower fair value gains compared to 2021 are mainly from investments in limited-life funds (mainly Gilde Healthcare) and other investments recognized at fair value through profit or loss compared with in 2021.loss. Net interest expense in 2022 was EUR 69 million higher than in 2021, mainly due to the financial charges related to early redemption of EUR and USD bonds and the issuance of new EUR bonds issued in 2022. The decrease in 2022 compared to 2021 in other financial income is mainly due to higher interest income on tax in 2021.
In 2021, Financial income and expenses decreased by EUR 5 million year-on-year, mainly due to higher other financial income and lower interest expense, offset by lower fair value gain. Fair value gains of EUR 95 million are from investments in limited-life funds (mainly Gilde Healthcare) and other investments recognized at fair value through profit or loss. Net interest expense in 2021 was EUR 19 million lower than in 2020, mainly due to lower interest expense on borrowings and provisions, and interest expense on pensions. The increase in other financial income is mainly due to higher interest income on tax.
8Income taxes
Accounting policies
Income taxes comprise of current, non-current and deferred tax. Income tax is recognized in the Consolidated statements of income except to the extent that it relates to items recognized directly within equity or in other comprehensive income. Current tax is the expected taxes payable on the taxable income for the year, using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
In cases where it is concluded it is not probable that tax authorities will accept a tax treatment, the effect of the uncertainty is reflected in the recognition and measurement of tax assets and liabilities or, alternatively, a provision is made for the amount that is expected to be settled, where this can be reasonably estimated. This assessment relies on estimates and assumptions and may involve a series of judgments about future events. New information may become available that causes the company to change its judgment regarding the adequacy of existing tax assets and liabilities. Such changes to tax assets and liabilities will impact the income tax expense in the period during which such a determination is made.
Deferred tax assets and liabilities are recognized, using the consolidated balance sheetssheet method, for the expected tax consequences of temporary differences between the carrying amounts of assets and liabilities and the amounts used for taxation purposes. Deferred tax is not recognized for the following temporary differences: (a) the initial recognition of goodwill; or (b) the initial recognition of assets and liabilitiesan asset or liability in a transaction thatwhich: (i) is not a business combination, and that(ii) at the time of transaction, affects neither accounting profit nor taxable profit;profit (tax loss), (iii) at the time of the transaction, does not give rise to equal amounts of taxable and deductible differences; or (c) differences relating to investments in subsidiaries, joint ventures and associates where the reversal of the respective temporary difference can be controlled by the company and it is probable that it will not reverse in the foreseeable future. Deferred taxes are measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity or on different taxable entities, but the company intends to settle current tax liabilities and assets on a net basis or their tax assets and liabilities will be realized simultaneously.
A deferred tax asset is recognized for unused tax losses, tax credits and deductible temporary differences to the extent that it is probable that there will be future taxable profits against which they can be utilized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in the countries where the deferred tax assets originated and during the periods when the deferred tax assets become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment.
Deferred tax liabilities for withholding taxes are recognized for subsidiaries in situations where the income is to be paid out as dividend in the foreseeable future and for undistributed earnings of unconsolidated companies to the extent that these withholding taxes are not expected to be refundable or deductible. Changes in tax rates and tax laws are reflected in the period when the change was enacted or substantively enacted by the reporting date.
Any subsequent adjustment to a tax asset or liability that originated in discontinued operations and for which no specific arrangements were made at the time of divestment, due to a change in the tax base or its measurement, is allocated to discontinued operations (i.e. backwards tracing). Examples are a tax rate change or change in retained assets or liabilities directly relating to the discontinued operation. Any subsequent change to the recognition of deferred tax assets is allocated to the component in which the taxable gain is or will be recognized. The above principles are applied to the extent the ‘discontinued operations’ are sufficiently separable from continuing operations.
Consistent with the IAS 12 amendment regarding Pillar Two taxation as issued by the IASB and adopted by the EU, Philips does not recognize and disclose deferred taxes arising from tax laws that implement Pillar Two model rules published by the Organisation for Economic Co-operation and Development. Furthermore, Philips will recognize and disclose the impact (if any) from Pillar Two income taxes on current tax effective from 2024.
Accounting estimates and judgments
Deferred tax recoverability
Deferred tax assets are recognized to the extent that it is probable that there will be future taxable profits against which these can be utilized. Significant judgment is involved in determining whether such profits are probable. Management determines this on the basis of expected taxable profits arising from the reversal of recognized deferred tax liabilities, appropriate tax planning opportunities to support business goals and on the basis of forecasts.
Uncertain tax positions
Uncertain tax positions are recognized as liabilities if and to the extent it is probable that additional tax will be due and the amount can be reliably measured. Significant judgment is involved in determining these positions.
The income tax benefit of continuing operations amounts to EUR 73 million (2022: EUR 113 million (2021:tax benefit, 2021: EUR 103 million tax benefit, 2020: EUR 212 million tax expense)benefit).
The components of income before taxes and income tax expense are as follows:
Philips Group
Income tax expense
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Income before taxes | 1,211 | 509 | (1,731) | 509 | (1,731) | (526) |
| Investments in associates, net of income taxes | (9) | (4) | (2) | (4) | (2) | (98) |
| Income before taxes and Investment in associates | 1,220 | 513 | (1,729) | 513 | (1,729) | (429) |
| Current tax (expense) benefit | (380) | (298) | (97) | (298) | (97) | (201) |
| Deferred tax (expense) benefit | 167 | 401 | 210 | 401 | 210 | 274 |
| Income tax (expense) of continuing operations | (212) | 103 | 113 | |||
| Income tax (expense) benefit of continuing operations | 103 | 113 | 73 | |||
Income tax benefit of continuing operations excludes the tax benefit of the discontinued operations of EUR 9 million (2022: EUR 18 million (2021:benefit, 2021: EUR 737 million expense, 2020: EUR 81 million expense), mainly related to the release of provisions.
The components of income tax expense of continuing operations are as follows:
Philips Group
Current income tax expense
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Current year tax (expense) benefit | (390) | (291) | (111) | (291) | (111) | (211) |
| Prior year tax (expense) benefit | 10 | (7) | 14 | (7) | 14 | 10 |
| Current tax (expense) benefit | (380) | (298) | (97) | (298) | (97) | (201) |
Philips Group
Deferred income tax expense
In millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |||
|---|---|---|---|---|---|---|---|---|
| Recognition of previously unrecognized tax loss and credit carryforwards | 6 | 138 | 2 | 138 | 2 | 72 | ||
| Unrecognized tax loss and credit carryforwards | (10) | (13) | (10) | (13) | (41) | |||
| Changes to recognition of temporary differences | 19 | (1) | (4) | (1) | (4) | (112) | ||
| Prior year tax (expense) benefit | (8) | 20 | (1) | 20 | (1) | (2) | ||
| Tax rate changes | 12 | 10 | (18) | 10 | (18) | 4 | ||
| Origination and reversal of temporary differences, tax losses and tax credits | 137 | 245 | 244 | 245 | 244 | 353 | ||
| Deferred tax (expense) benefit | 167 | 401 | 210 | 401 | 210 | 274 |
Philips’ operations are subject to income taxes in various foreign jurisdictions. The statutory income tax rate varies per country, which results in a difference between the weighted average statutory income tax rate and the Netherlands’ statutory income tax rate of 25.8% (2021: 25.0% 2020:(2022: 25.8% 2021: 25.0%).
A reconciliation of the weighted average statutory income tax rate to the effective income tax rate of continuing operations is as follows:
Philips Group
Effective income tax rate
in %
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Weighted average statutory income tax rate in % | 25.2 | 22.7 | 23.6 | 22.7 | 23.6 | 22.0 |
| Recognition of previously unrecognized tax loss and credit carryforwards | (0.5) | (26.9) | 0.1 | (26.9) | 0.1 | 16.8 |
| Unrecognized tax loss and credit carryforwards | 0.0 | 1.9 | (0.7) | 1.9 | (0.7) | (9.6) |
| Changes to recognition of temporary differences | (1.6) | 0.3 | (0.2) | 0.3 | (0.2) | (26.2) |
| Non-taxable income and tax incentives | (12.9) | (40.6) | 5.8 | (40.6) | 5.8 | 22.8 |
| Non-deductible expenses | 7.0 | 19.3 | (22.9) | 19.3 | (22.9) | (10.7) |
| Withholding and other taxes | 0.6 | 7.2 | (1.4) | 7.2 | (1.4) | (5.1) |
| Tax rate changes | (1.0) | (1.9) | (1.0) | (1.9) | (1.0) | 0.9 |
| Prior year tax | (0.2) | (2.4) | 0.7 | (2.4) | 0.7 | 1.9 |
| Tax expense (benefit) due to change in uncertain tax treatments | 1.2 | 4.4 | 2.8 | 4.4 | 2.8 | 2.3 |
| Others, net | (0.2) | (4.0) | (0.2) | (4.0) | (0.2) | 1.9 |
| Effective income tax rate | 17.6 | (20.0) | 6.5 | (20.0) | 6.5 | 17.0 |
The effective income tax rate is lower than the weighted average statutory income tax rate in 20222023 mainly due to the recognition of previously unrecognized tax loss and credit carryforwards, which is mainly related to a non-deductible goodwill impairment in the Sleep & Respiratory Care businessone-off recognition of tax credits and other non-deductible expenses such as share based compensation expenses, partly offset bynon-taxable income and tax incentives which includes recurring favorable tax incentives related to R&D investments, the innovation box regime in the Netherlands and export activities. This is partly offset by the changes to recognition of temporary differences, which mostly represents deferred tax assets not fully recognized in United States.
Due to the loss position in 2022,2023, items such as non-deductible expense lead to a decrease of the effective income tax rate and items such as tax incentives lead to an increase in the effective income tax rate.
Global minimum tax (Pillar Two)
In December 2021, the OECD released model rules to introduce a global minimum corporate income tax rate of 15% applicable to multinational enterprise groups with global revenue over EUR 750 million (“Pillar Two”). The formal adoption of Directive (EU) 2022/2523 in December 2022 aims to achieve a coordinated implementation of Pillar Two in the EU Member States. The Dutch implementation of Pillar Two, the so-called Minimum Tax Rate Act 2024 (the “MTR Act”), was enacted in December 2023 and will apply to Philips from the financial year ending December 31, 2024 and onwards. Under this legislation, Philips may be required to pay top-up taxes on profits if the related Pillar Two jurisdictional effective tax rate is less than 15%.
Philips will be affected by the “MTR Act” as well as the implementation of Pillar Two per local law in other jurisdictions and has performed an assessment of the Group’s potential exposure to the Pillar Two legislation.
This assessment indicates potential exposure from the constituent entities in Hong Kong and the United Arab Emirates, where the Pillar Two effective tax rate is below 15%. This would potentially have resulted in top-up taxes had Pillar Two legislation been effective in 2023. The assessment of the potential exposure to top-up taxes is based on the profits and tax expenses determined as part of the preparation of Philips’ consolidated financial statement, most recent tax fillings and country-by-country reporting. The Pillar Two effective tax rate is lower in these jurisdictions due to exempted income and domestic tax rates either below or close to 15%.
The group effective tax rate, had Pillar Two legislation been effective from 2023, would have been 16.4% which is 0.6% lower than the reported effective tax rate of 17% under IFRS. The decrease in the effective tax rate can be attributed to the reduction in the income tax benefit of continuing operations resulting from the inclusion of potential top-up tax exposure (expense).
Deferred tax assets and liabilities
Deferred tax assets are recognized for temporary differences, unused tax losses, and unused tax credits to the extent that realization of the related tax benefits is probable. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in the countries where the deferred tax assets originated and during the periods when the deferred tax assets become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment.
Net deferred tax assets relate to the following underlying assets and liabilities and tax loss carryforwards (including tax credit carryforwards) and their movements during the years 20222023 and 20212022 respectively are presented in the following tables.
The net deferred tax assets of EUR 2,3582,556 million (2021:(2022: EUR 2,1342,358 million) consist of deferred tax assets of EUR 2,4492,627 million (2021:(2022: EUR 2,2162,449 million) and deferred tax liabilities of EUR 9171 million (2021:(2022: EUR 8391 million). Of the total deferred tax assets of EUR 2,4492,627 million as of December 31, 2022 (2021:2023 (2022: EUR 2,2162,449 million), EUR 1,4531,676 million (2021:(2022: EUR 121,453 million) is recognized in respect of entities in various countries where there have been tax losses in the current or preceding period.period, primarily the United States (US). The increase is mainly related to the United StatesUS where there has been a tax loss in 2022,2023, among others due to the consequencesPhilips Respironics’ business operations. Philips assessed the recoverability of the Respironics field action. Management'stax losses and recognized the related deferred tax asset only to the extent future tax profits are considered probable. For the recoverability assessment, the income projections were determined using similar methodology as used for goodwill impairment testing (for more information please refer to note Goodwill). The company evaluated multiple risk-adjusted scenarios which support the assumption that it is probable that the results of future operations will generate sufficient taxable income to utilize the tax losses as well the deductible temporary differences. The projections include forward-looking assumptions whereby the most recent available information was used to determine the expected period of recovery of the deferred tax assets. Relevant developments potentially impacting the period and probability of recovery will be monitored closely.
As of December 31, 20222023 the temporary differences associated with investments, including potential income tax consequences on dividends, for which no deferred tax liabilities are recognized, aggregate to EUR 355444 million (2021:(2022: EUR 298355 million).
Philips Group
Deferred tax assets and liabilities
in millions of EUR
| Balance as of January 1, 2022 | recognized in income statement | other1) | Balance as of December 31, 2022 | Assets | Liabilities | Balance as of January 1, 2023 | recognized in income statement | other1) | Balance as of December 31, 2023 | Assets | Liabilities | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intangible assets | 587 | 63 | (20) | 630 | 783 | (152) | 630 | 61 | (12) | 679 | 826 | (147) |
| Property, plant and equipment | 29 | (33) | 2 | (2) | 49 | (52) | (2) | 18 | (103) | (88) | 44 | (132) |
| Inventories | 372 | 75 | 17 | 464 | 473 | (8) | 464 | (26) | (78) | 360 | 363 | (2) |
| Other assets | 68 | (16) | (8) | 44 | 98 | (55) | 44 | 20 | 120 | 184 | 233 | (48) |
| Pensions and other employee benefits | 180 | 6 | (32) | 153 | 175 | (22) | 153 | 69 | (29) | 193 | 204 | (11) |
| Other liabilities | 499 | (34) | 17 | 483 | 560 | (77) | 483 | (56) | 69 | 496 | 521 | (25) |
| Deferred tax assets on tax loss carryforwards | 398 | 149 | 38 | 586 | 586 | 188 | (44) | 730 | ||||
| Set-off deferred tax positions | (275) | 275 | (294) | 294 | ||||||||
| Net deferred tax assets | 2,134 | 210 | 14 | 2,358 | 2,449 | (91) | 2,358 | 274 | (77) | 2,556 | 2,627 | (71) |
Philips Group
Deferred tax assets and liabilities
in millions of EUR
| Balance as of January 1, 2021 | recognized in income statement | other1) | Balance as of December 31, 2021 | Assets | Liabilities | Balance as of January 1, 2022 | recognized in income statement | other1) | Balance as of December 31, 2022 | Assets | Liabilities | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intangible assets | 240 | 535 | (188) | 587 | 716 | (130) | 587 | 63 | (20) | 630 | 783 | (152) |
| Property, plant and equipment | 32 | 13 | (16) | 29 | 55 | (26) | 29 | (33) | 2 | (2) | 49 | (52) |
| Inventories | 313 | 31 | 28 | 372 | 381 | (9) | 372 | 75 | 17 | 464 | 473 | (8) |
| Other assets | 97 | (30) | 1 | 68 | 112 | (43) | 68 | (16) | (8) | 44 | 98 | (55) |
| Pensions and other employee benefits | 245 | (45) | (21) | 180 | 182 | (2) | 180 | 6 | (32) | 153 | 175 | (22) |
| Other liabilities | 384 | 91 | 25 | 499 | 584 | (84) | 499 | (34) | 17 | 483 | 560 | (77) |
| Deferred tax assets on tax loss carryforwards | 449 | (194) | 143 | 398 | 398 | 149 | 38 | 586 | ||||
| Set-off deferred tax positions | (211) | 211 | (275) | 275 | ||||||||
| Net deferred tax assets | 1,761 | 401 | (28) | 2,134 | 2,216 | (83) | 2,134 | 210 | 14 | 2,358 | 2,449 | (91) |
The company has available tax loss and credit carryforwards, which expire as follows:
Philips Group
Expiry years of net operating loss and credit carryforwards
in millions of EUR
| Total Balance as of December 31, 2021 | Unrecognized balance as of December 31, 2021 | Total Balance as of December 31, 2022 | Unrecognized balance as of December 31, 2022 | Total balance as of December 31, 2022 | Unrecognized balance as of December 31, 2022 | Total balance as of December 31, 2023 | Unrecognized balance as of December 31, 2023 | |
|---|---|---|---|---|---|---|---|---|
| Within 1 year | 1,593 | 1,592 | 4 | 3 | 4 | 3 | 17 | 15 |
| 1 to 2 years | 6 | - | 10 | 5 | 10 | 5 | 20 | 16 |
| 2 to 3 years | 9 | - | 9 | 3 | 9 | 3 | 7 | 2 |
| 3 to 4 years | 7 | - | 13 | 4 | 13 | 4 | 9 | 5 |
| 4 to 5 years | 18 | - | 38 | 3 | 38 | 3 | 38 | 16 |
| Later | 751 | 21 | 812 | 93 | 812 | 93 | 808 | 81 |
| Unlimited | 1,567 | 934 | 2,301 | 920 | 2,301 | 920 | 2,997 | 1,231 |
| Total | 3,951 | 2,547 | 3,187 | 1,032 | 3,187 | 1,032 | 3,896 | 1,366 |
The increase in the unrecognized balance as of December 31, 2023 is mainly explained by the US.
As of December 31, 2022,2023, the amount of deductible temporary differences for which no deferred tax asset has been recognized in the balance sheet was EUR 125 million (2022: EUR 45 million (2021: EUR 33 million). The unrecognized balance as of December 31, 2021 (expiring within 1 year, EUR 1,592 million) which were partly utilized and the remainder expired unutilized.
Tax risks
Philips is exposed to tax risks and uncertainty over tax treatments. For particular tax treatments that are not expected to be accepted by tax authorities, Philips either recognizes a liability or reflects the uncertainty in the recognition and measurement of its current and deferred tax assets and tax attributes. For the measurement of the uncertainty, Philips uses the most likely amount or the expected value of the tax treatment. The expected liabilities resulting from the uncertain tax treatments are included in non-current tax liabilities (2022:(2023: EUR 435390 million, 2021:2022: EUR 544435 million, decrease due to release of liabilities, in combination with higher tax losses or similar tax carryforwards that can be used if uncertain tax treatments were settled for the presumed amount at balance sheet date). The positions include, among others, the following:
Transfer pricing risks
Philips has issued transfer pricing directives, which are in accordance with international guidelines such as those of the Organization of Economic Co-operation and Development. In order to reduce the transfer pricing uncertainties, monitoring procedures are carried out by Group Tax to safeguard the correct implementation of the transfer pricing directives. However, tax disputes can arise due to inconsistent transfer pricing regimes and different views on "at arm's length" pricing.
Tax risks on general and specific service agreements and licensing agreements
Due to the centralization of certain activities (such as research and development, IT and group functions), costs are also centralized. As a consequence, these costs and/or revenues must be allocated to the beneficiaries, i.e. the various Philips entities. For that purpose, service contracts such as intra-group service agreements and licensing agreements are signed with a large number of group entities. Tax authorities review these intra-group service and licensing agreements, and may reject the implemented intra-group charges. Furthermore, buy in/out situations in the case of (de)mergers could affect the cost allocation resulting from the intragroup service agreements between countries. The same applies to the specific service agreements.
Tax risks due to disentanglements and acquisitions
When a subsidiary of Philips is disentangled, or a new company is acquired, tax risks may arise. Philips creates merger and acquisition (M&A) teams for these disentanglements or acquisitions. In addition to representatives from the involved business, these teams consist of specialists from various group functions and are formed, among other things, to identify tax risks and to reduce potential tax claims.
Tax risks due to permanent establishments
A permanent establishment may arise when a Philips entity has activities in another country, tax claims could arise in both countries on the same income.
10Property, plant and equipment
Accounting policies
Owned assets
The cost of property, plant and equipment comprise all directly attributable costs (including the cost of material and direct labor).
Depreciation is generally calculated using the straight-line method over the useful life of the asset. Land and assets under construction are not depreciated. When assets under construction are ready for their intended use, they are transferred to the relevant asset category and depreciation starts. All other property, plant and equipment items are depreciated over their estimated useful lives to their estimated residual values.
The estimated useful lives of property, plant and equipment are as follows:
Philips Group
Useful lives of property, plant and equipment
| Buildings | from 5 to 50 years |
| Machinery and installations | from 3 to 20 years |
| Other equipment | from 1 to 10 years |
Property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the assets concerned may not be recoverable. An impairment loss is recognized for the amount by which the asset's book value exceeds their recoverable amount. Impairments are reversed if and to the extent that the impairment no longer exists. The recoverable amount is defined as the higher of the asset’s fair value less costs of disposal and its value in use.
Gains and losses on the sale of property, plant and equipment are included in other business income. Costs related to repair and maintenance activities are expensed in the period in which they are incurred unless they extend the asset's original lifetime or capacity.
Right-of-use assets
The company leases various items of real estate, vehicles and other equipment. The company determines whether an arrangement constitutes or contains a lease based on the substance of the arrangement at the lease inception. The arrangement constitutes or contains a lease if fulfillment is dependent on the use of a specific asset and the arrangement conveys a right to use the asset, even if that asset is not explicitly specified in the arrangement.
Company as a lessee
The company recognizes right-of-use assets and lease liabilities for leases with a term of more than twelve months if the underlying asset is not of low value. Payments for short-term and low-value leases are expensed over the lease term. Extension options are included in the lease term if their exercise is reasonably certain. Right-of-use assets are measured at cost less accumulated depreciation and impairment losses, adjusted for any remeasurements. Right-of-use assets are depreciated using the straight-line method over the shorter of the lease term and the useful life of the underlying assets.
Company as a lessor
When the company acts as a lessor, it determines at lease inception whether a lease is a finance lease or an operating lease. Leases in which the company does not transfer substantially all the risks and rewards incidental to ownership of an asset are classified as operating leases. The company recognizes lease payments received under operating leases as income on a straight-line basis over the lease term in the Consolidated statement of income.
Accounting estimates and judgments
Impairment of owned and right-of-use assets
Judgments are required, not only to determine whether there is an indication that an asset may be impaired, but also whether indications exist that impairment losses previously recognized may no longer exist or may have decreased (impairment reversal). After indications of impairment have been identified, estimates and assumptions are used in the determination of the recoverable amount of a fixed asset. These involve estimates of expected future cash flows (based on future growth rates and remaining useful life) and residual value assumptions, as well as discount rates to calculate the present value of the future cash flows.
Owned assets
Estimates are required to determine the (remaining) useful lives of fixed assets. Useful lives are determined based on an asset's age, the frequency of its use, repair and maintenance policy, technology changes in production and expected restructuring. The company estimates the expected residual value per asset item. The residual value is the higher of the asset's expected sales price (based on recent market transactions of similar sold items) and its material scrap value.
Right-of-use assets
Significant judgmentJudgment is required to determine the lease term. The assessment of whether the company is reasonably certain to exercise extension options impacts the lease term, which could affect the amount of lease liabilities and right-of-use assets recognized.
Property, plant and equipment are fixed assets that are owned or right-of-use assets under a lease agreement.
Philips Group
Property, plant and equipment
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Owned assets | 1,641 | 1,718 | 1,718 | 1,565 |
| Right-of-use assets | 1,058 | 919 | 919 | 919 |
| Total | 2,699 | 2,638 | 2,638 | 2,483 |
Philips Group
Property, plant and equipment - owned assets
in millions of EUR
| Land and buildings | Machinery and installations | Other equipment | Assets under construction | Total | Land and buildings | Machinery and installations | Other equipment | Assets under construction | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2022 | ||||||||||
| Balance as of January 1, 2023 | ||||||||||
| Cost | 1,097 | 1,585 | 1,382 | 208 | 4,273 | 1,135 | 1,779 | 1,454 | 309 | 4,676 |
| Accumulated depreciation | (591) | (1,074) | (967) | (2,632) | (621) | (1,291) | (1,046) | (2,958) | ||
| Book value | 506 | 511 | 415 | 208 | 1,641 | 514 | 488 | 408 | 309 | 1,718 |
| Additions | 1 | 102 | 77 | 314 | 494 | 1 | 115 | 77 | 239 | 433 |
| Assets available for use | 34 | 69 | 111 | (220) | (6) | 20 | 90 | 144 | (262) | (8) |
| Depreciation | (56) | (215) | (176) | - | (447) | (56) | (196) | (167) | (420) | |
| Impairments | (3) | (20) | (18) | (1) | (42) | (5) | (23) | (17) | - | (45) |
| Transfer (to) from AHFS | (3) | - | (3) | (1) | (45) | (46) | ||||
| Reclassifications | 18 | 14 | (5) | 2 | 29 | 15 | 2 | (17) | (5) | (6) |
| Translation differences and other | 16 | 26 | 2 | 5 | 50 | (14) | (22) | (19) | (7) | (62) |
| Total change | 8 | (23) | (8) | 100 | 78 | (39) | (35) | (45) | (35) | (154) |
| Balance as of December 31, 2022 | ||||||||||
| Balance as of December 31, 2023 | ||||||||||
| Cost | 1,135 | 1,779 | 1,454 | 309 | 4,676 | 1,114 | 1,731 | 1,404 | 274 | 4,521 |
| Accumulated depreciation | (621) | (1,291) | (1,046) | (2,958) | (638) | (1,278) | (1,041) | (2,957) | ||
| Book value | 514 | 488 | 408 | 309 | 1,718 | 476 | 453 | 363 | 274 | 1,565 |
Philips Group
Property, plant and equipment - right-of-use assets
in millions of EUR
| Land and buildings | Machinery and installations | Other equipment | Total | Land and buildings | Other equipment | Total | |
|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2022 | |||||||
| Balance as of January 1, 2023 | |||||||
| Cost | 1,332 | 176 | 216 | 1,724 | 1,365 | 206 | 1,571 |
| Accumulated depreciation | (418) | (139) | (109) | (666) | (543) | (108) | (651) |
| Book value | 914 | 37 | 107 | 1,058 | 822 | 98 | 919 |
| Additions | 52 | - | 54 | 106 | 175 | 62 | 236 |
| Assets available for use | 5 | 1 | 6 | 2 | 6 | 8 | |
| Depreciation | (155) | (2) | (58) | (214) | (150) | (51) | (201) |
| Impairments | (8) | - | - | (9) | (23) | - | (23) |
| Transfer (to) from AHFS | 3 | 3 | (2) | (2) | |||
| Reclassifications | (19) | (13) | - | (32) | - | 4 | |
| Translation differences and other | 31 | (23) | (6) | 1 | (18) | (5) | (23) |
| Total change | (92) | (37) | (9) | (139) | (16) | 15 | (1) |
| Balance as of December 31, 2022 | |||||||
| Balance as of December 31, 2023 | |||||||
| Cost | 1,365 | - | 206 | 1,571 | 1,425 | 216 | 1,641 |
| Accumulated depreciation | (543) | (108) | (651) | (619) | (104) | (722) | |
| Book value | 822 | - | 98 | 919 | 806 | 112 | 919 |
Philips Group
Property, plant and equipment - owned assets
in millions of EUR
| Land and buildings | Machinery and installations | Other equipment | Assets under construction | Total | Land and buildings | Machinery and installations | Other equipment | Assets under construction | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2021 | ||||||||||
| Balance as of January 1, 2022 | ||||||||||
| Cost | 1,076 | 1,506 | 1,572 | 261 | 4,415 | 1,097 | 1,585 | 1,382 | 208 | 4,273 |
| Accumulated depreciation | (539) | (1,028) | (1,185) | (2,752) | (591) | (1,074) | (967) | (2,632) | ||
| Book value | 537 | 478 | 387 | 261 | 1,663 | 506 | 511 | 415 | 208 | 1,641 |
| Additions | 9 | 62 | 77 | 261 | 409 | 1 | 102 | 77 | 314 | 494 |
| Assets available for use | 72 | 110 | 117 | (305) | (5) | 34 | 69 | 111 | (220) | (6) |
| Acquisitions | - | 9 | 43 | 53 | ||||||
| Depreciation | (53) | (144) | (158) | (355) | (56) | (215) | (176) | - | (447) | |
| Impairments | (1) | (6) | (11) | - | (18) | (3) | (20) | (18) | (1) | (42) |
| Transfer (to) from AHFS | (87) | (16) | (46) | (20) | (170) | (3) | - | (3) | ||
| Reclassifications | 6 | 2 | (10) | 1 | - | 18 | 14 | (5) | 2 | 29 |
| Translation differences and other | 23 | 14 | 16 | 10 | 65 | 16 | 26 | 2 | 5 | 50 |
| Total change | (31) | 33 | 29 | (53) | (22) | 8 | (23) | (8) | 100 | 78 |
| Balance as of December 31, 2021 | ||||||||||
| Balance as of December 31, 2022 | ||||||||||
| Cost | 1,097 | 1,585 | 1,382 | 208 | 4,273 | 1,135 | 1,779 | 1,454 | 309 | 4,676 |
| Accumulated depreciation | (591) | (1,074) | (967) | (2,632) | (621) | (1,291) | (1,046) | (2,958) | ||
| Book value | 506 | 511 | 415 | 208 | 1,641 | 514 | 488 | 408 | 309 | 1,718 |
Philips Group
Property, plant and equipment - right-of-use assets
in millions of EUR
| Land and buildings | Machinery and installations | Other equipment | Assets under construction | Total | Land and buildings | Machinery and installations | Other equipment | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2021 | |||||||||
| Balance as of January 1, 2022 | |||||||||
| Cost | 1,147 | 199 | 213 | 1 | 1,560 | 1,332 | 176 | 216 | 1,724 |
| Accumulated depreciation | (310) | (144) | (86) | (540) | (418) | (139) | (109) | (666) | |
| Book value | 837 | 55 | 126 | 1 | 1,020 | 914 | 37 | 107 | 1,058 |
| Additions | 150 | 21 | 44 | 215 | 52 | - | 54 | 106 | |
| Assets available for use | 2 | 3 | 5 | 5 | 1 | 6 | |||
| Acquisitions | 43 | 43 | |||||||
| Depreciation | (157) | (32) | (63) | (252) | (155) | (2) | (58) | (214) | |
| Impairments | 1 | (5) | - | (4) | (8) | - | - | (9) | |
| Transfer (to) from AHFS | (7) | (1) | (8) | 3 | 3 | ||||
| Reclassifications | 2 | (1) | 1 | (19) | (13) | - | (32) | ||
| Translation differences and other | 44 | (2) | (4) | 39 | 31 | (23) | (6) | 1 | |
| Total change | 77 | (18) | (20) | (1) | 38 | (92) | (37) | (9) | (139) |
| Balance as of December 31, 2021 | |||||||||
| Balance as of December 31, 2022 | |||||||||
| Cost | 1,332 | 176 | 216 | 1,724 | 1,365 | - | 206 | 1,571 | |
| Accumulated depreciation | (418) | (139) | (109) | (666) | (543) | (108) | (651) | ||
| Book value | 914 | 37 | 107 | 1,058 | 822 | - | 98 | 919 | |
Lease related notes
Below are the references with respect to year-end disclosures as lessee:
- Short-term and low-value leases, are disclosed in Income from operations;
- Disclosures regarding interest expenses on lease liabilities, are disclosed in Financial income and expenses;
- For disclosure on leasing related cash outflow and the split between interest and principal payments, refer to Cash flow statement supplementary information;
- For disclosure on sale and leaseback transactions, refer to Details of treasury and other financial risks;
- For disclosure on lease liabilities and maturity analysis, refer to Debt;
Other qualitative and quantitative disclosures regarding the nature of lessee’s leasing activities and future lease obligations, refer to Debt.
Below are the references with respect to year-end disclosures as lessor:
- For disclosures on lease income and sublease income, refer to Details of treasury and other financial risks;
- Other qualitative disclosures regarding the nature of lessors leasing activities and risk management, refer to Details of treasury and other financial risks.
11Goodwill
Accounting policies
The measurement of goodwill at initial recognition is described in the Acquisitions and divestments note. Goodwill is subsequently measured at cost less accumulated impairment losses.
Goodwill is not amortized but is instead tested for impairment annually and wheneverin the fourth quarter, or more frequently if indicators of potential impairment indicators require.exist. Internal orand external sources of information are considered to assess if there are indicators that an asset or a CGUgroups of cash-generating units (CGUs) may be impaired. In most casesGoodwill is allocated to groups of CGUs and tested for impairment at the company identifies its cash-generating units for goodwill at onebusiness level (one level below that of an operating segment. Cash flows atsegment), as this level are substantially independent from other cash flows and this isrepresents the lowest level at which goodwill is monitored by the Executive Committee.for internal management purposes. An impairment loss is recognized in the Consolidated statements of income whenever and to the extent that the carrying amount of a cash-generating unitgroup of CGUs exceeds the unit’s recoverable amount for the group of CGUs, whichever is the greater, its value in use or its fair value less cost of disposal. Value in use is measured as the present value of future cash flows expected to be generated by the asset. Fair value less cost of disposal is measured as the amount obtained from the sale of an asset in an arm’s length transaction, less costs of disposal.
Accounting estimates and judgments
The cash flow projections used in the value in use calculations for goodwill impairment testing contain various judgments and estimations as described in the key assumptions sections below.‘key assumptions’ section.
The changes in 20212022 and 20222023 were as follows:
Philips Group
Goodwill
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Balance as of January 1 | ||||
| Cost | 9,094 | 11,793 | 11,793 | 12,747 |
| Impairments | (1,080) | (1,156) | (1,156) | (2,509) |
| Book value | 8,014 | 10,637 | 10,637 | 10,238 |
| Acquisitions | 2,095 | 317 | ||
| Acquisitions1) | 317 | 24 | ||
| Impairments | (15) | (1,357) | (1,357) | (8) |
| Divestments and transfers to assets classified as held for sale | (189) | |||
| Divestments and transfers to assets classified as held for sale2) | (8) | |||
| Translation differences and other | 732 | 641 | 641 | (370) |
| Total change | 2,622 | (399) | (399) | (362) |
| Balance as of December 31 | ||||
| Cost | 11,793 | 12,747 | 12,747 | 12,133 |
| Impairments | (1,156) | (2,509) | (2,509) | (2,256) |
| Book value | 10,637 | 10,238 | 10,238 | 9,876 |
In 2022, goodwill decreased by EUR 399 million, primarily as a result of goodwill impairments of EUR 1,357 million partially offset by translation differences of EUR 641 million and acquisitions of EUR 317 million (which includes changes in the provisional opening balance sheet position for certain 2021 acquisitions, refer1)Refer to Acquisitions and divestments2)Refer to Discontinued operations and assets classified as held for sale
Effective from April 1, 2023, Philips implemented a simplified operating model (refer to relevant sections of General information to the Consolidated financial statements).
In 2021, goodwill increased by EUR 2,622 million, primarily as As a result, the level at which goodwill is monitored has changed to align with the revised governance under the new operating model. Prior to April 1, 2023, goodwill was monitored at the business unit level. From April 1, 2023, goodwill impairment testing is performed at the business level (one level below segment), as this represents the lowest level at which goodwill is monitored for internal management purposes. The changes in the monitoring and management structure for recognized goodwill did not otherwise prevent recognition of provisional goodwill recognized on new acquisitions of BioTelemetry (EUR 1,776 million) and Capsule Technologies of (EUR 325 million), and translation differences of EUR 732 million. This was partially offset by EUR 15 million ofan impairment losses primarily relatedthat existed prior to the PERS CGU and EUR 189 million divestedchange, nor did it result in the period, mostly relating to the Domestic Appliances business. For details on the impactrecognition of new acquisitions and the divestment of the Domestic Appliances business, refer to Acquisitions and divestments. an impairment charge.
Goodwill reallocations in 2022impairment testing
For impairment testing, goodwill is allocated to groups of CGUs and 2021
In 2022 and 2021 there were changes to the CGU structure following internal reorganizations. These resulted in a goodwill reallocation across certain CGUs, none of which had a significant impact on headroom or led to goodwill impairments. These reallocations were performed using a relative value approach. In addition there were also certain CGU movements and/or combinations within businesses that did not result in a reallocation of goodwill, but resulted in changes totested for impairment at the business structure. This did not havelevel (one level below segment level), which represents the lowest level at which the goodwill is monitored internally for management purposes. Goodwill is tested for impairment annually in the fourth quarter, or more frequently if indicators of potential impairment exist.
An impairment trigger assessment is performed on a significant impactquarterly basis to determine whether there is an indication based on headroomeither internal or lead to goodwill impairments.
Impairments
external sources of information, that a group of CGUs may be impaired. During 2022 goodwill2023, interim impairment charges of EUR 1,357 milliontests were recognized. This relates to the third quarter impairment charge of EUR 1,331 million incompleted for the Sleep & Respiratory Care (S&RC) CGU of the Connected Care segment. In addition, as a result of the annual impairment testing a goodwill impairment charge of EUR 27 million was recognized in relation to the Precision Diagnosis Solutions (PDS) CGU which is part of the Diagnosis & Treatment segment. The value in use methodology was used to estimate the recoverable amount for the PDS CGU.
During 2021 an impairment charge of EUR 15 million was recognized. The majority of this related to the PERS CGU which was classified as an asset held for sale as of Q4 2020. The PERS CGU was divested as of June 30, 2021. Prior to the divestment a goodwill impairment of EUR 13 million was recorded to reflect a decrease in the recoverable amount of the CGU, this reduced the goodwill balance of the CGU to zero. The fair value less cost of disposal methodology was used to estimate the recoverable amount for the PERS CGU, this was based on Level 3 inputs. Key assumptions and inputs used in the calculation included the signed purchase agreement for the PERS divestment. The impairment of EUR 13 million was recorded in the Connected Care segment.
Interim goodwill impairment testing
As explained in the accounting policy above, goodwill is tested for impairment annually and whenever impairment indicators require. In the third quarter of 2022, an impairment indicator was noted in relation to the S&RC CGU as a consequence ofbusiness mainly following revisions to the expected future cashflows of the CGU. The drivers of the revised forecast (which form the basis for the future cashflow assumptions) were current assumptions regarding the estimated impact of athe proposed Respironics consent decree, that is currently under discussion with the US Department of Justice (DoJ), acting on behalf of the FDA, along with updates to expected business performance and changes to the pre-tax discount rate. AnThe interim goodwill impairment test was performedtests did not result in orderan impairment.
Goodwill allocated to determine if the businesses (groups of cash-generating units) as of December 31, 2023, is presented in the following table:
Philips Group
Goodwill by business
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Monitoring1) | 4,110 | 3,964 |
| Image-Guided Therapy | 3,154 | 3,044 |
| Precision Diagnosis | 1,429 | 1,363 |
| Sleep & Respiratory Care | 731 | 687 |
| Personal Health | 488 | 483 |
| Enterprise Informatics | 327 | 336 |
| Book value | 10,238 | 9,876 |
The carrying amount of the cash-generating unit exceeded the unit’s recoverable amount, which was determined on a value in use basis. As a resulteach group of this test a goodwill impairment charge of EUR 1,331 million was recognized. Following the impairment charge, the estimated recoverable amount, based on the CGU’s value in use, for the S&RC CGU was EUR 1,001 million and equalCGUs is compared to its carrying value.
The assumptions used to determine the recoverable amount of the CGUgroup of CGUs. Unless otherwise noted, the recoverable amount for each group of CGUs is based on value in use calculations. Value in use is measured as the present value of future cash flows expected to be generated from the continuing use of the assets. In the 2023 annual goodwill impairment test, these cash flow projections were determined using Royal Philips managements’ internal forecasts that cover an initial forecast period from 2024 to 2026. Projections were extrapolated using the growth rates disclosed in the following table for an extrapolation period of 4 years (2027-2030), after which a terminal value was calculated per 2031. For the terminal value calculation, growth rates were capped at a historical long-term average growth rate. The company uses scenarios in the interim testing datebusiness forecasting process and the most reasonable and supportable assumptions which represent management’s best estimate are presented below:used as the basis for the value in use calculations.
Philips Group
Key assumptions
Key assumptions used in the value in use calculations were compound sales growth rates, EBITA*) in the terminal value and the rates used for discounting the projected cash flows.
- Interim impairment testing
| compound sales growth rate1) | ||||
|---|---|---|---|---|
| initial forecast period | extrapolation period2) | used to calculate terminal value3) | pre-tax discount rates | |
| Sleep & Respiratory Care | 1.5% | 4.3% | 2.5% | 9.5% |
In addition to the above assumptions, assumptions were made regarding the estimated impact of a consent decree on the business. These assumptions included the expected financial impact of the scope and duration of a consent decree, as well as expected additional costs. These assumptions were determined by management based on discussions held in relation to the consent decree and other available sources of information.
Annual goodwill impairment testing
For impairment testing, goodwill is allocated to cash generating units (typically one level below segment level, i.e. at the business level), which represent the lowest level at which the goodwill is monitored internally for management purposes.
Goodwill allocated to the cash generating units Ambulatory Monitoring & Diagnostics, Hospital Patient Monitoring and Image-Guided Therapy is considered to be significant in comparison to the total book value of goodwill for the Group as of December 31, 2022. The amounts associated as of December 31, 2022 are presented in the following table:
Philips Group
Goodwill allocated to the cash-generating units
in millions of EUR
| 2021 | 2022 | |
|---|---|---|
| Ambulatory Monitoring & Diagnostics | 1,897 | 2,215 |
| Hospital Patient Monitoring | 1,663 | 1,806 |
| Image-Guided Therapy | 2,802 | 3,154 |
| Sleep & Respiratory Care | 2,031 | 731 |
| Other (units carrying a non-significant goodwill balance) | 2,245 | 2,332 |
| Book value | 10,637 | 10,238 |
Unless otherwise noted, the basis of the recoverable amount used in the annual impairment tests for the units disclosed further in this note is the value in use. The fair value less cost of disposal methodology was used as a basis for the recoverable amount in the annual impairment test when greater than the value-in-use test. Refer to the ‘key assumptions- general’ section for further detail on the methodology.
Key assumptions - general
Key assumptions used in the value-in-use impairment tests for the units were sales growth rates, EBITA*) in the terminal value and the rates used for discounting the projected cash flows. These cash flow projections were determined using Royal Philips managements’ internal forecasts that cover an initial forecast period from 2023 to 2025. Projections were extrapolated with stable or declining growth rates for an extrapolation period of 4 years (2026-2029), after which a terminal value was calculated per 2030. For the terminal value calculation, growth rates were capped at a historical long-term average growth rate. In the case of the Ambulatory Monitoring & Diagnostics CGU management's internal forecasts were used in the value in use test for a period of 5 years (2023-2027). calculation.
The compound sales growth rates and EBITA*) used to estimate cash flows are based on past performance, external market growth assumptions and industry long-term growth averages. EBITA*) in all units mentioned in this notefor each group of CGUs is expected to increase over the projection period as a result of volume growth and cost efficiencies.
In 2022 By their nature, these assumptions involve risk and uncertainty because they relate to future events and circumstances and there continuedare many factors that could cause actual results and developments to be uncertaintydiffer materially from the plans, goals and volatility related to global, industry-wide macroeconomic challenges including global supply chain constraints, COVID lockdown measuresexpectations set forth in China, inflationary pressures and the Russia-Ukraine war. Where relevant, and to the extent possible, the estimated impact of these factors and the resulting uncertainties have been reflected in the forecasts used for the value-in-use calculations. As was the case in 2021, the company uses scenarios in the business forecasting process and the most reasonable and supportable assumptions which represent management’s best estimate are used as the basis for the value-in-use tests.assumptions.
The rates used for discounting the projected cash flows in goodwill impairment testing is based on a business weighted cost of capital (WACC), which in turn is based on business-specific inputs along with other inputs as mentioned below. The WACC is based on post-tax cost of equity and cost of debt, and is further calculated based on market data and inputs to accurately capture changes to the time value of money, such as the risk-free interest rate, the beta factor and country risk premium. In order to properly reflect the different risk-profiles of different businesses, a WACC is determined for each business. As such, the beta factor is determined based on a selection of peer companies, which can differ per business. Different businesses have different geographical footprints, resulting in business-specific inputs for variables like country risk. Philips performs the value in use calculationcalculations using post-tax cashflows and discount rate, the implicit pre-tax rate discount rate is derived from an iterative calculation for disclosure purposes.
In 2022 the pre-tax discount rates increased for all CGUs primarily dueThe values assigned to the impact on the WACC of higher interest rates. As explained above, for S&RC this increased pre-tax discount rate contributed to the impairment charge recognized in the third quarter of 2022.
Key assumptions and sensitivity analysis relating to cash-generating units to which a significant amount of goodwill is allocated
In 2022 cash flow projections of Ambulatory Monitoring & Diagnostics, Hospital Patient Monitoring, Image-Guided Therapy and Sleep & Respiratory Care are based on the key assumptions included in the following table, which were used in the annual impairment test performed in the fourth quarter.
Philips Group
Key assumptions
2022
| compound sales growth rate1) | ||||
|---|---|---|---|---|
| initial forecast period | extrapolation period2) | used to calculate terminal value3) | pre-tax discount rates | |
| Ambulatory Monitoring & Diagnostics | 15.4% | 9.5% | 2.5% | 8.5% |
| Hospital Patient Monitoring | 4.8% | 3.4% | 2.5% | 8.5% |
| Image-Guided Therapy | 8.7% | 5.0% | 2.5% | 10.6% |
| Sleep & Respiratory Care | 10.0% | 5.0% | 2.5% | 9.9% |
The assumptions used for the 2021 cash flow projectionsvalue in use calculations were as follows:
Philips Group
Key assumptions
20212023
| compound sales growth rate1) | compound sales growth rate | |||||||
|---|---|---|---|---|---|---|---|---|
| initial forecast period | extrapolation period2) | used to calculate terminal value3) | pre-tax discount rates | initial forecast period | extrapolation period | used to calculate terminal value | pre-tax discount rates | |
| Ambulatory Monitoring & Diagnostics | 24.5% | 11.9% | 2.5% | 7.3% | ||||
| Hospital Patient Monitoring | 5.4% | 3.4% | 2.5% | 7.8% | ||||
| Monitoring | 8.2% | 5.5% | 2.5% | 9.5% | ||||
| Image-Guided Therapy | 10.2% | 5.4% | 2.5% | 8.9% | 7.9% | 5.2% | 2.5% | 10.7% |
| Precision Diagnosis | 3.8% | 3.4% | 2.5% | 10.4% | ||||
| Sleep & Respiratory Care | 9.2% | 5.0% | 2.5% | 9.2% | 9.5% | 9.3% | 2.5% | 10.8% |
| Personal Health | 5.0% | 4.6% | 2.5% | 10.3% | ||||
| Enterprise Informatics | 5.3% | 5.8% | 2.5% | 9.0% | ||||
The assumptions used for the 2022 value in use calculations for cash-generating units to which a significant amount of goodwill was allocated were as follows:
Philips Group
Key assumptions
2022
| compound sales growth rate | ||||
|---|---|---|---|---|
| initial forecast period | extrapolation period | used to calculate terminal value | pre-tax discount rates | |
| Ambulatory Monitoring & Diagnostics1) | 15.4% | 9.5% | 2.5% | 8.5% |
| Hospital Patient Monitoring1) | 4.8% | 3.4% | 2.5% | 8.5% |
| Image-Guided Therapy | 8.7% | 5.0% | 2.5% | 10.6% |
| Sleep & Respiratory Care | 10.0% | 5.0% | 2.5% | 9.9% |
The S&RC compound sales growth rate is the annualized steady nominal growth rate over the forecast period 2)Also referred to later in the text as compound long-term sales growth rate3)The historical long-term growth rate is only applied to the first year afterduring the extrapolation period after which no furtherincreased from 5.0% in 2022 to 9.3% in 2023. The growth rate percentage is assumedcalculated with reference to the last year of the initial forecast period. Although the absolute forecasted sales during the extrapolation period used in 2023 decreased compared to that used in 2022, the larger decline in the forecasted sales of the respective reference year results in a higher calculated growth rate percentage compared to 2022.
Impairment losses
Goodwill impairment charges for the terminal value calculationyears ended December 31, 2023 and 2022, were EUR 8 million and EUR 1,357 million, respectively.
Impairment tests are performed based on forward looking assumptions, usingThe 2023 charge relates to the most recent available information. By their nature, these assumptions involve riskpartial impairment of goodwill allocated to a business that was classified as held-for-sale as of December 31, 2023. At the time of classification as held-for-sale, goodwill totaling EUR 16 million was allocated from the Precision Diagnosis business to the held-for-sale business, of which EUR 8 million was subsequently impaired. For further information refer to Discontinued operations and uncertainty because theyassets classified as held for sale.
The 2022 charges relate to future eventsthe impairment charge of EUR 1,331 million in the S&RC CGU within the Connected Care segment and circumstances and there are many factors that could cause actual results and developmentsa charge of EUR 27 million recognized in relation to differ materially from the plans, goals and expectations set forthPrecision Diagnosis Solutions CGU, which was formerly part of the Diagnosis & Treatment segment but is now included in these assumptions.the Enterprise Informatics business within the Connected Care segment.
Sensitivity to changes in assumptions
In performing the value-in-use testcalculations for the S&RC CGU, it was necessary for management to make assumptions regarding the estimated impact of a consent decree on the business.business of the proposed Respironics consent decree. These assumptions includedinclude, amongst others, the expected financial impact of the scope of products, geography, and duration of athe proposed consent decree, as well as expected additional costs. These assumptions were determined by management based on discussions held in relation to the proposed Respironics consent decree and other available sources of information. There have been no significant changes to these assumptions since
The value-in-use of the interim goodwill testing in the third quarter of 2022 (see Interim Goodwill impairment testing section above).
For the Sleep & Respiratory Care CGU, based on the annual goodwill impairment testing performed by management during the fourth quarter of 2022 in accordance with the methodology discussed above, no additional impairment charge was warranted. However, following the interim impairment charge, the annual impairment test indicates that the value in use of theS&RC CGU remains sensitive to the assumptions set out above. This means that there is a higher risk that deviations in the mentioned key assumptions could cause the recoverable amount to fall below the level of its carrying value. There continues to be significant uncertainty associated with the initiated voluntary recall notification in the United StatesUS and field safety notice outside the United StatesUS for certain sleep and respiratory care products the associated legal matters and the outcomeimpact on the business of athe proposed Respironics consent decree. The legal matters are described in further detail in Contingencies.
Based on the annual impairment test, the estimated recoverable amount of Sleep & Respiratory Care, itthe S&RC CGU exceeds the carrying value by EUR 326 million. It was noted that an increase of 40190 basis points in the pre-tax discount rate, a 160690 basis points decline in the compound long-term sales growth rate during the extrapolation period or a 7%27% decrease in terminal value would, individually, cause its recoverable amount to fall to the level of its carrying value. Additionally, any significant adverse changes to the assumptions related to the expected financial impact of athe proposed Respironics consent decree could cause the recoverable amount of the CGU to fall below its carrying value, resulting in impairment.
The results of the annual impairment tests of the Ambulatory Monitoring, & Diagnostics CGU indicate that the value in use of the CGUs is sensitive to the assumptions set out above. This means that there is a higher risk that deviations in the mentioned key assumptions could cause the recoverable amount to fall below the level of its carrying value. Based on the annual impairment test of Ambulatory Monitoring & Diagnostics, it was noted that an increase of 40 basis points in the pre-tax discount rate, a 210 basis points decline in the compound long-term sales growth rate or a 8% decrease in terminal value would, individually, cause its recoverable amount to fall to the level of its carrying value.
The results of the annual impairment test of Hospital Patient Monitoring and Image-Guided Therapy, Precision Diagnosis, Personal Health and Enterprise Informatics indicate that a reasonably possible change in key assumptions would not cause the value in use to fall to the level of the carrying value.
Additional information relating to cash-generating units to which a non-significant amount relative to the total goodwill is allocated
The results of the annual impairment tests of the Emergency Care CGU indicate that the value in use of the CGU is sensitive to the assumptions set out above. This means that there is a higher risk that deviations in the mentioned key assumptions could cause the recoverable amount to fall below the level of its carrying value. Based on the annual impairment test of Emergency Care, it was noted that an increase of 190 basis points in the pre-tax discount rate, a 900 basis points decline in the compound long-term sales growth rate or a 26% decrease in terminal value would, individually, cause its recoverable amount to fall to the level of its carrying value.
With the exception of those described above, for the cash generating units to which a non-significant amount relative to the total goodwill is allocated, any reasonable change in assumptions would not cause the value in use to fall to the level of the carrying value.
12Intangible assets excluding goodwill
Accounting policies
Acquired finite-lived intangible assets are amortized using the straight-line method over their estimated useful life. The useful lives are evaluated annually. Intangible assets are initially capitalized at cost, with the exception of intangible assets acquired as part of a business combination, which are capitalized at their acquisition date fair value.
The company expenses all research costs as incurred. Expenditure on development activities, whereby research findings are applied to a plan or design for the production of new or substantially improved products and processes, is capitalized as an intangible asset if the product or process is technically and commercially feasible, the company has sufficient resources and the intention to complete development and can measure the attributable expenditure reliably.
The capitalized development expenditure comprises of all directly attributable costs (including the cost of materials and direct labor). Other development expenditures and expenditures on research activities are recognized in the Consolidated statements of income. Capitalized development expenditure is stated at cost less accumulated amortization and impairment losses. Amortization of capitalized development expenditure is charged to the Consolidated statements of income on a straight-line basis over the estimated useful lives of the intangible assets.
The expected useful lives of the intangible assets excluding goodwill are as follows:
Philips Group
Expected useful lives of intangible assets excluding goodwill
in years
| Brand names | 2-20 |
| Customer relationships | 2-25 |
| Technology | 3-20 |
| Other | 1-10 |
| Software | 1-10 |
| Product development | 3-10 |
The weighted average expected remaining life of brand names, customer relationships, technology and other intangible assets is 9.49.3 years as of December 31, 2022 (2021: 9.62023 (2022: 9.4 years).
Impairment of intangible assets not yet ready for use
Intangible assets not yet ready for use are not amortized but are tested for impairment annually and whenever impairment indicators require. In the case of intangible assets not yet ready for use, either internal or external sources of information are considered to assess if there are indicators that an asset or a CGU may be impaired.
Impairment of non-financial assets other than goodwill, intangible assets not yet ready for use, inventories and deferred tax assets
Non-financial assets other than goodwill, intangible assets not yet ready for use, inventories and deferred tax assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is assessed by a comparison of the carrying amount of an asset with the greater of its value in use and fair value less cost of disposal. Value in use is measured as the present value of future cash flows expected to be generated by the asset. Fair value less cost of disposal is measured as the amount obtained from a sale of an asset in an arm’s length transaction, less costs of disposal. If the carrying amount of an asset is deemed not recoverable, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the recoverable amount. The review for impairment is carried out at the level where cash flows occur that are independent of other cash flows.
Impairment losses recognized in prior periods for Intangible assets other than goodwill are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if and to the extent that there has been a change in the estimates used to determine the recoverable amount. The loss is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been recognized. Reversals of impairment are recognized in the Consolidated statements of income.
Accounting estimates and judgments
The cash flow projections used in the value in use calculations for intangible assets excluding goodwill contain various judgments and estimations. For intangible assets excluding goodwill, estimates are required to determine the (remaining) useful lives.
Philips Group
Intangible assets excluding goodwill
in millions of EUR
| brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2022 | ||||||||||||||||
| Balance as of January 1, 2023 | ||||||||||||||||
| Cost | 644 | 2,590 | 2,605 | 2,701 | 505 | 754 | 146 | 9,944 | 647 | 2,735 | 2,947 | 2,605 | 648 | 869 | 152 | 10,602 |
| Amortization / impairments | (481) | (1,447) | (1,605) | (2,102) | (91) | (467) | (101) | (6,294) | (507) | (1,665) | (1,845) | (2,212) | (146) | (589) | (113) | (7,077) |
| Book value | 162 | 1,143 | 1,000 | 599 | 414 | 287 | 44 | 3,650 | 140 | 1,070 | 1,102 | 393 | 502 | 280 | 39 | 3,526 |
| Additions | (3) | - | 51 | - | 257 | 109 | 1 | 416 | 33 | - | 214 | 70 | - | 317 | ||
| Assets available for use | 118 | (118) | 157 | (157) | ||||||||||||
| Acquisitions | 1 | 3 | 177 | - | - | 180 | 40 | - | - | 40 | ||||||
| Amortization | (24) | (141) | (140) | (206) | (1) | (100) | (3) | (614) | (20) | (137) | (131) | (169) | (97) | (1) | (556) | |
| Impairments | - | (6) | (46) | (123) | (81) | (17) | (2) | (276) | - | - | - | (7) | (7) | (1) | - | (16) |
| Transfers to assets classified as held for sale | (1) | (20) | (8) | (2) | (32) | |||||||||||
| Translation differences and other | 4 | 71 | 59 | 5 | 31 | 1 | (2) | 0 | (1) | (37) | (30) | (38) | 1 | 18 | - | (87) |
| Total change | (22) | (74) | 102 | (206) | 88 | (7) | (6) | (125) | (22) | (195) | (89) | (57) | 42 | (13) | (1) | (335) |
| Balance as of December 31, 2022 | ||||||||||||||||
| Balance as of December 31, 2023 | ||||||||||||||||
| Cost | 647 | 2,735 | 2,947 | 2,605 | 648 | 869 | 152 | 10,602 | 629 | 2,593 | 2,908 | 2,432 | 635 | 929 | 139 | 10,265 |
| Amortization / impairments | (507) | (1,665) | (1,845) | (2,212) | (146) | (589) | (113) | (7,077) | (511) | (1,718) | (1,895) | (2,096) | (91) | (662) | (101) | (7,075) |
| Book Value | 140 | 1,070 | 1,102 | 393 | 502 | 280 | 39 | 3,526 | 118 | 875 | 1,013 | 336 | 544 | 267 | 38 | 3,190 |
Philips Group
Intangible assets excluding goodwill
in millions of EUR
| brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2021 | ||||||||||||||||
| Balance as of January 1, 2022 | ||||||||||||||||
| Cost | 556 | 2,036 | 2,434 | 2,519 | 480 | 723 | 135 | 8,883 | 644 | 2,590 | 2,605 | 2,701 | 505 | 754 | 146 | 9,944 |
| Amortization / impairments | (437) | (1,385) | (1,565) | (1,897) | (83) | (427) | (91) | (5,886) | (481) | (1,447) | (1,605) | (2,102) | (91) | (467) | (101) | (6,294) |
| Book value | 120 | 651 | 869 | 622 | 398 | 295 | 44 | 2,997 | 162 | 1,143 | 1,000 | 599 | 414 | 287 | 44 | 3,650 |
| Additions | 9 | 1 | 261 | 117 | 2 | 392 | (3) | - | 51 | - | 257 | 109 | 1 | 416 | ||
| Assets available for use | 247 | (247) | - | - | - | 118 | (118) | - | - | - | ||||||
| Acquisitions | 62 | 544 | 235 | - | - | 841 | 1 | 3 | 177 | - | - | - | 180 | |||
| Amortization | (21) | (126) | (114) | (219) | - | (85) | (3) | (568) | (24) | (141) | (140) | (206) | (1) | (100) | (3) | (614) |
| Impairments | (3) | (57) | (51) | (15) | - | - | (126) | - | (6) | (46) | (123) | (81) | (17) | (2) | (276) | |
| Transfers to assets classified as held for sale | (10) | (3) | (11) | (17) | (6) | (34) | (82) | |||||||||
| Translation differences and other | 12 | 80 | 69 | 17 | 23 | (7) | 1 | 195 | 4 | 71 | 59 | 5 | 31 | 1 | (2) | 169 |
| Total change | 42 | 492 | 131 | (22) | 17 | (8) | 1 | 653 | (22) | (74) | 102 | (206) | 88 | (7) | (6) | (125) |
| Balance as of December 31, 2021 | ||||||||||||||||
| Balance as of December 31, 2022 | ||||||||||||||||
| Cost | 644 | 2,590 | 2,605 | 2,701 | 505 | 754 | 146 | 9,944 | 647 | 2,735 | 2,947 | 2,605 | 648 | 869 | 152 | 10,602 |
| Amortization / impairments | (481) | (1,447) | (1,605) | (2,102) | (91) | (467) | (101) | (6,294) | (507) | (1,665) | (1,845) | (2,212) | (146) | (589) | (113) | (7,077) |
| Book Value | 162 | 1,143 | 1,000 | 599 | 414 | 287 | 44 | 3,650 | 140 | 1,070 | 1,102 | 393 | 502 | 280 | 39 | 3,526 |
Acquisitions in 20222023 involved Intangibleintangible assets of EUR 18040 million in aggregate (2021:(2022: EUR 841180 million). For more information, refer to Acquisitions and divestments.
Impairments in 20222023 were EUR 27616 million (2021:(2022: EUR 126276 million) and mainly relate to technology (EUR 46 million) and product development (EUR 204 million), including product development construction in progress. In the third quarter of 2022 an initiative was undertaken to enhance productivity in R&D, specifically to shift the focus to fewer, high-impact projects in the innovation pipeline. As a result of this initiative EUR 132 million ofprogress and product development (including product development construction in progress) asset impairments were recognized.development.
The most notable impairments in 2022, recognized as part of the above productivity initiative, were in the Diagnosis & Treatment segment, for product development assets in Precision Diagnosis (PD) of EUR 36 million and Image Guided Therapy-Systems (IGT Systems) of EUR 41 million (EUR 16 million of which was product development construction in progress). The basis of the recoverable amount used in these tests was the value-in-use. After the impairment charge the recoverable amount of the related intangible assets is EUR 0 million.
In 2022 there continued to be uncertainty and volatility related to by global, industry-wide macroeconomic challenges including global supply chain constraints, COVID lockdown measures in China, inflationary pressures and the Russia-Ukraine war. Where relevant, and to the extent possible, the estimated impact of these factors and the resulting uncertainties have been reflected in the forecasts used for the VIU calculations. As was the case in 2021, the company uses scenarios in the business forecasting process and the most reasonable and supportable assumptions which represent management’s best estimate are used as the basis for the value-in-use testscalculations.
The amortization and impairment of intangible assets is further specified in Income from operations.
The most notable intangible assets as of December 31, 2023 relate to the BioTelemetry customer relationships and technology with a carrying value of EUR 327 million and EUR 123 million and a remaining amortization period of 13 years and 9 years, respectively and Spectranetics customer relationships and technology with a carrying value of EUR 261 million and EUR 175 million and a remaining amortization period of 14 years and 9 years, respectively. The most notable intangible assets as of December 31, 2022 relate to the BioTelemetry customer relationships and technology with a carrying value of EUR 385 million and EUR 150 million and a remaining amortization period of 14 years and 10 years, respectively and Spectranetics customer relationships and technology with a carrying value of EUR 291 million and EUR 203 million and a remaining amortization period of 15 years and 10 years, respectively. The most notable intangible assets as of December 31, 2021 relate to the BioTelemetry customer relationships and technology with value of EUR 391 million and EUR 162 million and a remaining amortization period of 15 years and 11 years, respectively and Spectranetics customer relationships and technology with a carrying value of EUR 292 million and EUR 210 million and a remaining amortization period of 16 years and 11 years, respectively.
13Other financial assets
Accounting policies
Classification and measurement of financial assets
The classification of financial assets at initial recognition depends on the financial asset’s contractual cash flow characteristics and the company’s business model for managing them.
The company initially measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss, transaction costs.
For the purposes of subsequent measurement, financial assets are classified into four categories:
- Financial assets at amortized cost (debt instruments).
- Financial assets at fair value through other comprehensive income (OCI) with recycling of cumulative gains and losses (debt instruments).
- Financial assets designated at fair value through OCI with no recycling of cumulative gains and losses upon derecognition (equity instruments).
- Financial assets at fair value through profit or loss (debt instruments and equity instruments).
Impairment of financial assets
The company recognizes a loss allowance for expected credit losses for trade receivables, contract assets, lease receivables, debt investments carried at amortized cost and fair value through other comprehensive income (FVTOCI).
At each balance sheet date, the company assesses whether there is objective evidence that a financial asset or a group of financial assets is impaired and recognizes a loss allowance for expected credit losses for financial assets measured at either amortized costs or at fair value through other comprehensive income. If, at the reporting date, the credit risk on a financial instrument has not increased significantly since initial recognition, the company measures the loss allowance for the financial instrument at an amount equal to 12 months of expected credit losses. If, at the reporting date, the credit risk on a financial instrument has increased significantly since initial recognition, the company measures the loss allowance for the financial instrument at an amount equal to the lifetime-expected credit losses. For all trade receivables, contract assets and lease receivables the company measures the loss allowance at an amount equal to lifetime-expected credit losses.
Accounting estimates and judgments
The determination of fair value is subject to estimates for investments that are not publicly traded. Refer to Fair value of financial assets and liabilities
Financial assets classified at amortized cost and at fair value through OCI are subject to impairment assessment. The calculation of expected credit losses requires the company to apply significant judgment and make estimates and assumptions that involve significant uncertainty at the time they are made. Changes to these estimates and assumptions can result in significant changes to the timing and amount of expected credit losses to be recognized.
Other current financial assets
In 2022,2023, Other current financial assets decreased from EUR 11 million to EUR 3 million (2022: increased from EUR 2 million to EUR 11 million (2021: increased from EUR nil million to EUR 2 million).
Other non-current financial assets
The company’s investments in Other non-current financial assets mainly consist of investments in common shares of companies in various industries and investments in limited life funds. The changes during 20222023 and 20212022 were as follows:
Philips Group
Other non-current financial assets
in millions of EUR
| Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | |
|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2022 | 283 | 300 | 47 | 630 | ||||
| Balance as of January 1, 2023 | 322 | 284 | 54 | 660 | ||||
| Changes: | ||||||||
| Acquisitions/additions | 114 | 18 | 150 | 71 | 14 | 20 | 105 | |
| Sales/redemptions/reductions | (75) | (3) | (8) | (86) | (33) | (14) | (11) | (58) |
| Impairments | (3) | (1) | (5) | |||||
| Value adjustment through OCI | - | (35) | (35) | - | (17) | (17) | ||
| Value adjustment through P&L | 5 | - | 5 | (39) | - | (39) | ||
| Translation differences and other | (2) | 5 | (1) | 2 | (29) | (14) | (1) | (44) |
| Reclassifications | 1 | (2) | (1) | (2) | (8) | 5 | 15 | 12 |
| Balance as of December 31, 2022 | 322 | 284 | 54 | 660 | ||||
| Balance as of December 31, 2023 | 284 | 258 | 77 | 619 | ||||
Philips Group
Other non-current financial assets
in millions of EUR
| Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | |
|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2021 | 248 | 146 | 37 | 430 | ||||
| Balance as of January 1, 2022 | 283 | 300 | 47 | 630 | ||||
| Changes: | ||||||||
| Acquisitions/additions | 54 | 59 | 10 | 123 | 114 | 18 | 150 | |
| Sales/redemptions/reductions | (122) | - | (3) | (126) | (75) | (3) | (8) | (86) |
| Impairments | (3) | (1) | (5) | |||||
| Value adjustment through OCI | (43) | - | (43) | - | (35) | (35) | ||
| Value adjustment through P&L | 95 | - | 95 | 5 | - | 5 | ||
| Translation differences and other | 8 | 19 | 2 | 29 | (2) | 5 | (1) | 2 |
| Reclassifications | (1) | 120 | 2 | 122 | 1 | (2) | (1) | (2) |
| Balance as of December 31, 2021 | 283 | 300 | 47 | 630 | ||||
| Balance as of December 31, 2022 | 322 | 284 | 54 | 660 | ||||
As of December 31, 2022,2023, equity investments of EUR 259231 million (2021:(2022: EUR 273259 million) are accounted under the FVTOCI category based on the company's election at initial recognition mainly because such investments are neither held for trading purposes nor primarily for their increase in value and the elected presentation is considered to reflect the nature and purpose of the investment.
14Other assets
Accounting policies
The company recognizes contract assets for revenue earned from installation services because the receipt of consideration is conditional on successful completion of the installation. Upon completion of the installation and acceptance by the customer, the amount recognized as contract assets is reclassified to trade receivables.
Other assets are measured at amortized cost minus any impairment losses.
Other non-current assets
Other non-current assets as of December 31, 20222023 were EUR 9893 million (2021:(2022: EUR 12998 million). These are mainly related to prepaid expenses.
Other current assets
Other current assets as of December 31, 20222023 of EUR 490500 million (2021:(2022: EUR 493490 million) included contract assets of EUR 292297 million (2021:(2022: EUR 290292 million), accrued income of EUR 246 million (2021:(2022: EUR 3124 million) and prepaid expenses of EUR 174197 million (2021:(2022: EUR 172174 million) mainly related to Diagnosis & Treatment businesses and Connected Care businesses.
15Inventories
Accounting policies
Inventories are stated at the lower of cost or net realizable value. The cost of inventories comprises all costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. The costs of conversion of inventories include direct labor and fixed and variable production overheads, considering the stage of completion and the normal capacity of production facilities. Costs of idle facility and abnormal waste are expensed. The cost of inventories is determined using the first-in, first-out (FIFO) method. The write-down of inventories to net realizable value is included in cost of sales.
Accounting estimates and judgments
Inventory is reduced for the estimated losses due to obsolescence. This reduction is determined for groups of products based on sales in the recent past and/or expected future demand.
Inventories are summarized as follows:
Philips Group
Inventories
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Raw materials and supplies | 1,143 | 1,541 | 1,541 | 1,309 |
| Work in process | 646 | 648 | 648 | 552 |
| Finished goods | 1,660 | 1,860 | 1,860 | 1,629 |
| Inventories | 3,450 | 4,049 | 4,049 | 3,491 |
In 2023, overall global inventories have operationally decreased in all categories due to the deployment of strategic management of aging and unhealthy inventory accompanied by a more optimized tracking in both production and commercial inventories.
The write-down of inventories to net realizable value was EUR 215339 million in 2022 (2021:2023 (2022: EUR 177215 million). The write-down is included in cost of sales.
In 2022, the limited availability and delays in the supply of certain components and products internationally, resulted in an increase in inventories compared to December 31, 2021, as work in process inventories could not be translated to finished goods available for sale due, including EUR 82 million related to the scarcity of certain components. While there was an increase in inventories, this has not resulted in a significant write-down of inventories, as the expectation is that such components will become available in the near future.proposed Respironics consent decree (refer to Provisions).
16Receivables
Accounting policies
Receivables are held by the company to collect the related cash flows. These receivables are measured at fair value and subsequently measured at amortized cost minus any impairment losses.
Receivables are derecognized when the company has transferred substantially all risks and rewards, which includes transactions in which the company enters into factoring transactions, or if the company does not retain control over the receivables.
Accounting estimates
Receivables are subject to impairment assessment, which involves estimating expected credit losses. Refer to Other financial assets for accounting policies on impairment of financial assets.
Non-current receivables
Non-current receivables are associated mainly with customer financing in the Diagnosis & Treatment businesses amounting to EUR 70102 million (2021:(2022: EUR 4469 million), for Signify indemnification amounting to EUR 264 million (2021:(2022: EUR 4626 million), an income tax receivable amounting to EUR 12612 million (which includes an interest receivable of EUR 103 million) for which Philips expects to get a refund (2021:(2022: EUR 78126 million) and insurance receivables in Other in the US amounting to EUR 3033 million (2021:(2022: EUR 3730 million).
Current receivables
Current receivables of EUR 4,1153,733 million (2021:(2022: EUR 3,7874,115 million) as of December 31, 20222023 included trade accounts receivable (net of allowance) of EUR 3,8323,546 million (2021:(2022: EUR 3,5593,832 million), accounts receivable other of EUR 228170 million (2021:(2022: EUR 188228 million) and accounts receivable from investments in associates of EUR 5518 million (2021:(2022: EUR 4055 million).
The trade accounts receivable, net, per segment are as follows:
Philips Group
Trade accounts receivable, net
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Diagnosis & Treatment | 1,759 | 2,013 | 1,795 | 1,688 |
| Connected Care | 980 | 1,114 | 1,332 | 1,105 |
| Personal Health | 575 | 479 | 479 | 576 |
| Other | 245 | 226 | 226 | 177 |
| Trade accounts receivable, net | 3,559 | 3,832 | 3,832 | 3,546 |
The aging analysis of trade accounts receivable, net, representing current and overdue but not fully impaired receivables, is as follows:
Philips Group
Aging analysis
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Current | 3,075 | 3,280 | 3,280 | 3,132 |
| Overdue 1-30 days | 160 | 169 | 169 | 117 |
| Overdue 31-180 days | 245 | 282 | 282 | 234 |
| Overdue more than 180 days | 79 | 101 | 101 | 63 |
| Trade accounts receivable, net | 3,559 | 3,832 | 3,832 | 3,546 |
The changes in the allowance for doubtful accounts receivable are as follows:
Philips Group
Allowance for accounts receivable
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Balance as of January 1 | 195 | 190 | 190 | 226 |
| Additions charged to expense | 4 | 66 | 66 | 27 |
| Deductions from allowance1) | (17) | (51) | (51) | (26) |
| Transfer to assets held for sale | (8) | (1) | ||
| Other movements | 16 | 21 | 21 | (10) |
| Balance as of December 31 | 190 | 226 | 226 | 216 |
The allowance for doubtful accounts receivable has been primarily established for receivables that are past due. The allowance presented also includes the allowance for Non-current customer finance receivables of EUR 8 million (2022: EUR 7 million). Other movements in the current period are mainly related to foreign currency valuations.
Included in the above balances as of December 31, 20222023 are allowances for individually impaired receivables of EUR 210 million (2022: EUR 222 million (2021: EUR 188 million).
17Equity
Accounting policies
Common shares are classified as equity. Incremental costs directly attributable to the issuance of shares are recognized as a deduction from equity. Where the company repurchases the company’s equity share capital (treasury shares), the consideration paid, including any directly attributable incremental transaction costs (net of income taxes), is deducted from shareholders’ equity until such treasury shares are cancelled or reissued.
Where such treasury shares are subsequently reissued, any consideration received, net of any directly attributable incremental transaction costs and the related income tax effects, is included in shareholders’ equity.
Call options on own shares are treated as equity instruments.
Dividends are recognized as a liability in the period in which they are declared and approved by shareholders. The income tax consequences of dividends are recognized when a liability to pay the dividend is recognized.
Common shares
As of December 31, 2022,2023, authorized common shares consist of 2 billion shares (December 31, 2021:2022: 2 billion; December 31, 2020:2021: 2 billion) and the issued and fully paid share capital consists of 889,315,082913,515,966 common shares, each share having a par value of EUR 0.20 (December 31, 2021: 883,898,969;2022: 889,315,082; December 31, 2020: 911,053,001)2021: 883,898,969).
Preference shares
As a means to protect the company against (an attempt at) an unsolicited takeover or other attempt to exert (de facto) control of the company, the ‘Stichting Preferente Aandelen Philips’ has been granted the right to acquire preference shares in the company. As of December 31, 2022,2023, no such right has been exercised and no preference shares have been issued. Authorized preference shares consist of 2 billion shares as of December 31, 20222023 (December 31, 2021:2022: 2 billion; December 31, 2020:2021: 2 billion).
Options, restricted and performance shares
Under its share-based compensation plans, the company granted stock options on its common shares up to 2013 and other conditional rights to receive common shares in the future such as restricted shares and performance shares (refer to Share-based compensation).
Treasury shares
In connection with the company’s share repurchase programs, shares which have been repurchased and are held in Treasury for the purpose of (i) delivery under share-based compensation plans upon exercise of options, or vesting of restricted or performance shares, and (ii) capital reduction, are accounted for as a reduction of shareholders’ equity. Treasury shares are recorded at cost, representing the market price on the acquisition date. When treasury shares are delivered by the company under its share-based compensation plans, such shares are removed from treasury shares on a first-in, first-out (FIFO) basis.
When treasury shares are delivered by the company upon exercise of options, (granted to employees up to 2013), the difference between the cost and the cash received is recorded in retained earnings. When treasury shares are delivered by the company upon vesting of restricted shares or performance shares (granted under the company’s share-based compensation plans), the difference between the market price of the shares and the cost is recorded in retained earnings, and the market price is recorded in capital in excess of par value.
The following table shows the movements in the outstanding number of shares over the last three years:
Philips Group
Outstanding number of shares
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Balance as of January 1 | 890,973,790 | 905,128,293 | 870,182,445 | 905,128,293 | 870,182,445 | 881,480,527 |
| Dividend distributed | 18,080,198 | 6,345,968 | 14,174,568 | 6,345,968 | 14,174,568 | 39,334,938 |
| Purchase of treasury shares | (8,669,622) | (45,486,392) | (5,080,693) | (45,486,392) | (5,080,693) | (15,964,445) |
| Delivery of treasury shares | 4,695,170 | 4,194,577 | 2,204,207 | 4,194,577 | 2,204,207 | 1,552,136 |
| Issuance of new shares | 48,757 | |||||
| Balance as of December 31 | 905,128,293 | 870,182,445 | 881,480,527 | 870,182,445 | 881,480,527 | 906,403,156 |
The following table reflects transactions that took place in relation to former and current share-based compensation plans:
Philips Group
Transactions related to share-based compensation plans
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Shares acquired | 5,351,411 | 3,996,576 | 2,142,445 | 3,996,576 | 2,142,445 | 3,000,000 |
| Average market price | EUR 33.81 | EUR 36.15 | EUR 31.76 | EUR 36.15 | EUR 31.76 | EUR 41.59 |
| Amount paid | EUR 181 million | EUR 144 million | EUR 68 million | EUR 144 million | EUR 68 million | EUR 125 million |
| Shares delivered | 4,695,170 | 4,194,577 | 2,204,207 | 4,194,577 | 2,204,207 | 1,552,136 |
| Average price (FIFO) | EUR 34.35 | EUR 34.14 | EUR 35.16 | EUR 34.14 | EUR 35.16 | EUR 34.59 |
| Cost of delivered shares | EUR 161 million | EUR 143 million | EUR 77 million | EUR 143 million | EUR 77 million | EUR 54 million |
| Total shares in treasury at year-end | 5,924,708 | 5,726,708 | 5,664,946 | 5,726,708 | 5,664,946 | 7,112,810 |
| Total cost | EUR 199 million | EUR 201 million | EUR 191 million | EUR 201 million | EUR 191 million | EUR 262 million |
The following transactions took place for capital reduction purposes:
Philips Group
Transactions related to capital reduction
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Shares acquired | 3,318,211 | 41,489,816 | 2,938,248 | 41,489,816 | 2,938,248 | 12,964,445 |
| Average market price | EUR 39.21 | EUR 36.22 | EUR 36.61 | EUR 36.22 | EUR 36.61 | EUR 37.25 |
| Amount paid | EUR 130 million | EUR 1,503 million | EUR 108 million | EUR 1,503 million | EUR 108 million | EUR 483 million |
| Cancellation of treasury shares (shares) | 3,809,675 | 33,500,000 | 8,758,455 | 33,500,000 | 8,758,455 | 15,134,054 |
| Cancellation of treasury shares (EUR) | EUR 152 million | EUR 1,216 million | EUR 299 million | EUR 1,216 million | EUR 299 million | EUR 566 million |
| Total shares in treasury at year-end | 7,989,816 | 2,169,609 | 7,989,816 | 2,169,609 | ||
| Total cost | EUR 287 million | EUR 83 million | EUR 287 million | EUR 83 million |
Share purchase transactions related to employee option and share plans, as well as transactions related to the reduction of share capital, involved a cash outflow of EUR 187 million. A cash inflow662 million in 2023. In 2023, the settlement of forward contracts resulted in a withholding tax liability for an amount of EUR 1266 million from treasury shares mainly relatesrelating to the exercisedividend distribution. As of employee stock options (granted until 2013).December 31, 2023, the remaining liability to be settled amounted to EUR 11 million.
Share repurchase methods for share-based remuneration plans and capital reduction purposes
Philips uses different methods to repurchase shares in its own capital: (i) share buyback repurchases in the open market via an intermediary; (ii) repurchase of shares via forward contracts for future delivery of shares; and (iii) the unwinding of call options on own shares. During 2022,2023, Philips used methods (i)method (ii) to repurchase shares for capital reduction purposes and methods (ii) and (iii) to repurchase shares for share-based compensation plans.
Forward contracts to repurchase shares
For share-based compensation plans
On June 14, 2023, Royal Philips announced that it will repurchase up to 7.1 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchases plans. Under this program, Philips entered into one forward contract for an amount of EUR 138 million to acquire 7.1 million shares with settlement dates varying between November 2024 and November 2025 and a weighted average forward price of EUR 19.43.
On June 13, 2022, Royal Philips announced that it will repurchase up to 3.2 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchases plans. Under this program, Philips entered into one forward contract for an amount of EUR 63 million to acquire 3.2 million shares with settlement dates in November 2024 and December 2024 and a weighted average forward price of EUR 19.75.
On May 19, 2021, Royal Philips announced that it will repurchase up to 2 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchase plans. Under this program, Philips entered into one forward contract for an amount of EUR 90 million to acquire 2 million shares with settlement dates in October 2023 and November 2023 and a weighted average forward price of EUR 44.85. As of December 31, 2023, all shares under this program were acquired (settled in the fourth quarter of 2023). This resulted in a EUR 90 million increase in retained earnings against treasury shares.
On January 29, 2020, Philips announced that it will repurchase up to 6 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchase plans. Under this program, Philips entered into three forward contracts to acquire in total 5 million for an amount of EUR 174 million to acquire with settlement dates varying between October 2021 and November 2022 and a weighted average forward price of EUR 34.85. On October 26, 2022, the original settlement date of two tranches entered into under this program (in total 1.75 million shares) has been extended from November 23, 2022 to November 2023, and November 2024, respectively. As of December 31, 2022,2023, a total of 3.34.3 million shares (December 31, 2021: 1.5 million)2022: 3.3 million shares) under this program were acquired (settled in the fourth quarter of 2021, 2022, and 2022, respectively)2023). This resulted in a EUR 5735 million (December 31, 2021:(2022: EUR 6157 million) increase in retained earnings against treasury shares.
As of December 31, 2022,2023, the remaining forward contracts to cover obligations under share-based remunerationcompensation plans related to 7.011.1 million shares (December 31, 2021: 5.5 million)2022: 7.0 million shares) and amounted to EUR 211224 million (December 31, 2021:2022: EUR 203211 million).
For capital reduction
On July 26, 2021, Philips announced a share buyback program for share cancellation purposes for an amount of up to EUR 1.5 billion. Consequently, in the third quarter of 2021 Philips entered into three forward contracts for an amount of EUR 731 million to acquire 20 million shares with settlement dates in 2022, 2023 and 2024 and a weighted average forward price of EUR 37.36. Philips executed the remainder of the program through open market purchases by an intermediary in the fourth quarter of 2021 (acquiring 21 million shares) and January 2022 (acquiring 0.8 million shares). This resulted in a EUR 781 million increase in retained earnings against treasury shares. As of December 31, 2022,2023, a total of 15.1 million (December 31, 2022: 2.2 millionmillion) shares under this program were acquired (in the fourth quarter of 2022)2022 and third and fourth quarters of 2023). This resulted in EUR 83483 million (including dividend adjustment) increase in retained earnings against treasury shares.shares (2022: EUR 83 million).
As of December 31, 2022,2023, the remaining forward contracts entered into for capital reduction purposes relate to 17.44.4 million shares (December 31, 2021: 19.6 million)2022: 17.4 million shares) and amounted to EUR 648167 million (December 31, 2021:2022: EUR 731648 million).
Share call options
In 2016, Philips purchased EUR-denominated and USD-denominated call options on its own shares to hedge options granted to employees up to 2013.
InOn December 31, 2022, the company unwound 239,880there were 55,750 EUR-denominated and 152,565 USD-denominated call options against the transfer of the same number of its own shares (392,445 shares) and an additional EUR 6 million cash payment to the buyer of the call options.
outstanding. As of December 31, 2022, the remaining2023, all outstanding EUR-denominated call options related to 55,750 shares while there are no remaining USD-denominated call options.have expired.
Shares cancellation
In June 2022,December 2023, Philips completed the cancellation of 8.815.1 million of its common shares (with a cost price of EUR 299566 million). The cancelled shares were acquired as part of the Philips’ EUR 1.5 billion share repurchase programsprogram announced on July 26, 2021.
Dividend distribution
2023
In May 2023, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 749 million (including costs). The dividend was distributed in the form of shares only, resulting in the issuance of 39,334,938 new common shares. Per share calculations have been adjusted retrospectively for all periods presented to reflect the issuance of shares for the share dividend in respect of 2022. Further reference is made to Earnings per share.
A proposal will be submitted to the 2024 Annual General Meeting of Shareholders to pay a dividend of EUR 0.85 per common share, in common shares only, against retained earnings for 2023.
2022
In May 2022, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 741 million (including costs). Shareholders could elect for a cash dividend or a share dividend. Approximately 45% of the shareholders elected for a share dividend, resulting in the issuance of 14,174,568 new common shares. The settlement of the cash dividend involved an amount of EUR 411 million (including costs).
A proposal will be submitted to the 2023 Annual General Meeting of Shareholders to pay a dividend of EUR 0.85 per common share, in common shares only, against retained earnings for 2022.
2021
In June 2021, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 773 million (including costs). Shareholders could elect for a cash dividend or a share dividend. Approximately 38% of the shareholders elected for a share dividend, resulting in the issuance of 6,345,968 new common shares. The settlement of the cash dividend involved an amount of EUR 482 million (including costs).
2020
In July 2020, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 758 million (including costs). The dividend was distributed in the form of shares only resulting in the issuance of 18,080,198 new common shares.
Limitations in the distribution of shareholders’ equity
As of December 31, 2022,2023, pursuant to Dutch law, certain limitations exist relating to the distribution of shareholders’ equity of EUR 3,0542,435 million. Such limitations relate to common shares of EUR 183 million, as well as to legal reserves required by Dutch law included under retained earnings of EUR 990 million and unrealized currency translation differences of EUR 1,263 million. The unrealized gain related to cash flow hedges of EUR 6 million and unrealized loss related to fair value through OCI financial assets of EUR 390 million qualify as revaluation reserves and reduce the distributable amount due to the fact that these reserves are negative.
The legal reserves required by Dutch law of EUR 990 million included under retained earnings relates to any legal or economic restrictions on the ability of affiliated companies to transfer funds to the parent company in the form of dividends.
As of December 31, 2022, these limitations in distributable amounts were EUR 3,054 million and related to common shares of EUR 178 million, as well as to legal reserves required by Dutch law included under retained earnings of EUR 1,010 million and unrealized currency translation differences of EUR 1,866 million. The unrealized loss related to cash flow hedges of EUR 2 million and unrealized losslosses related to fair value through OCI financial assets of EUR 376 million qualify as revaluation reserves and reduce the distributable amount due to the fact that these reserves are negative.
The legal reserves required by Dutch law of EUR 1,010 million included under retained earnings relates to any legal or economic restrictions on the ability of affiliated companies to transfer funds to the parent company in the form of dividends.
As of December 31, 2021, these limitations in distributable amounts were EUR 1,947 million and related to common shares of EUR 177 million, as well as to legal reserves required by Dutch law included under retained earnings of EUR 654 million and unrealized currency translation differences of EUR 1,117 million. The unrealized losses related to fair value through OCI financial assets of EUR 344 million and unrealized loss related to cash flow hedges of EUR 252 million qualify as a revaluation reserve and reduce the distributable amount due to the fact that this reserve is negative.
Non-controlling interests
Non-controlling interests relate to minority stakes held by third parties in consolidated group companies.
Capital management
Philips manages capital based upon the IFRS measures, net cash provided by operating activities and net cash used for investing activities as well as the non-IFRS measure net debt. The definition of this non-IFRS measure and a reconciliation to the IFRS measure is included below.
Net debt is defined as the sum of long and short-term debt minus cash and cash equivalents. Group equity is defined as the sum of shareholders’ equity and non-controlling interests. This measure is used by Philips Treasury management and investment analysts to evaluate financial strength and funding requirements. The Philips net debt position is managed with the intention of retaining the current strong investment grade credit rating. Furthermore, Philips’ aim when managing the net debt position is dividend stability and a pay-out ratio of 40% to 50% of Adjusted income from continuing operations attributable to shareholders (reconciliation to the most directly comparable IFRS measure, Net income, is provided at the end of this note).
Philips Group
Composition of net debt and group equity
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Long-term debt | 5,705 | 6,473 | 7,270 | 6,473 | 7,270 | 7,035 |
| Short-term debt | 1,229 | 506 | 931 | 506 | 931 | 654 |
| Total debt | 6,934 | 6,980 | 8,201 | 6,980 | 8,201 | 7,689 |
| Cash and cash equivalents | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
| Net debt | 3,708 | 4,676 | 7,028 | 4,676 | 7,028 | 5,820 |
| Shareholders' equity | 11,870 | 14,438 | 13,249 | 14,438 | 13,249 | 12,028 |
| Non-controlling interests | 31 | 36 | 34 | 36 | 34 | 33 |
| Group equity | 11,901 | 14,475 | 13,283 | 14,475 | 13,283 | 12,061 |
| Net debt and group equity ratio | 24:76 | 35:65 | 24:76 | 35:65 | 33:67 | |
Adjusted income from continuing operations attributable to shareholders is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted income from continuing operations attributable to shareholders to the most directly comparable IFRS measure, Net income for 20222023 is included in the following table.
Philips Group
Adjusted income from continuing operations attributable to shareholders
1) in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net income | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (196) | (2,711) | (13) | (2,711) | (13) | 10 |
| Income from continuing operations | 999 | 612 | (1,618) | 612 | (1,618) | (454) |
| Income from continuing operations attributable to non-controlling interests | (8) | (4) | (3) | (4) | (3) | (2) |
| Income from continuing operations attributable to shareholders1) | 991 | 608 | (1,622) | 608 | (1,622) | (456) |
| Adjustments for: | ||||||
| Amortization and impairment of acquired intangible assets | 377 | 322 | 363 | 322 | 363 | 290 |
| Impairment of goodwill | 144 | 15 | 1,357 | 15 | 1,357 | 8 |
| Restructuring costs and acquisition-related charges | 195 | 95 | 202 | 95 | 202 | 381 |
| Other items: | 299 | 1,069 | 925 | 1,069 | 925 | 1,358 |
| Respironics field-action provision | 719 | 250 | ||||
| Respironics litigation provision | 575 | |||||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 | |||
| Respironics field-action running remediation cost | 94 | 210 | 94 | 210 | 224 | |
| Quality remediation actions | 94 | 59 | 175 | |||
| R&D project impairments | 134 | 134 | ||||
| Portfolio realignment charges | 109 | 109 | ||||
| Impairment of assets in S&RC | 39 | 39 | ||||
| Provision for public investigations tender irregularities | 60 | 60 | ||||
| Provisions for quality actions in Connected Care | 94 | 59 | ||||
| Loss on divestment of business | 76 | |||||
| Provision for a legal matter | 31 | |||||
| Investment re-measurement loss | 23 | |||||
| Loss (gain) on divestment of business | 76 | (35) | ||||
| Remaining items | 299 | 87 | 63 | 87 | 63 | 2 |
| Net finance income/expenses | (125) | (84) | (4) | (84) | (4) | 18 |
| Tax impact of adjusted items and tax only adjusting items | (285) | (527) | (376) | (527) | (376) | (450) |
| Adjusted Income from continuing operations attributable to shareholders1) | 1,594 | 1,497 | 845 | 1,497 | 845 | 1,148 |
18Debt
Accounting policies
Debt
Debt is initially measured at fair value net of directly attributable transaction costs. Subsequently, debt is measured at amortized cost using the effective interest rate method. Amortized cost is calculated by taking into account any discount or premium on acquisition and fees or costs that are an integral part of the effective interest rate. Debt is derecognized when the obligation under the liability is discharged, cancelled or has expired.
Lease liabilities
Lease liabilities are measured at the present value of the lease payments due over the lease term, generally discounted using the incremental borrowing rate. Lease liabilities are subsequently measured at amortized cost using the effective interest method. Lease liabilities are remeasured in case of modifications or reassessments of the lease.
Philips has a USD 2.5 billion Commercial Paper Program and a EUR 1 billion committed standby revolving credit facility that can be used for general group purposes, such as a backstop of its Commercial Paper Program. As of December 31, 2022,2023, Philips did not have any loans outstanding under either facility. These facilities do not have a material adverse change clause, have no financial covenants and no credit-rating-related acceleration possibilities. Philips issued commercial paper of EUR 200 million in September 2022 and EUR 101 million in October 2022, that was repaid throughout the fourth quarter of 2022. In addition, Philips secured a EUR 1 billion credit facility in the fourth quarter of 2022 that can be used for general corporate purposes. As of December 31, 2022, Philips had EUR 500 million outstanding under the credit facility. The facility does not have a material adverse change clause, has no financial covenants and no credit-rating-related acceleration possibilities. As per March 9, 2020, Philips established a Euro Medium-Term Note (EMTN) program, a framework that facilitates the issuance of notes for a total amount up to EUR 10 billion. In 2022As of December 31, 2023, Philips has EUR 3.3 billion outstanding under this program of which EUR 500 million fixed rate notes were issued three new tranches under the program forin August 2023 with a total of EUR 2 billion, while also redeeming its outstanding 2023 and 2024 Notes and issuing a tender offer on the outstanding 2025 and 2026 Notes.maturity date in 2031.
The provisions applicable to all USD-denominated corporate bonds issued by the company in March 2008 and March 2012 (due 2038 and 2042) contain a ‘Change of Control Triggering Event’. If the company would experience such an event with respect to a series of corporate bonds the company might be required to offer to purchase the bonds that are still outstanding at a purchase price equal to 101% of their principal amount, plus accrued and unpaid interest, if any. Furthermore, the conditions applicable to the EUR-denominated corporate bonds issued insince 2018 2019, 2020 and 2022 (due 2025, 2026, 2027, 2028, 2029, 2030 and 2033) contain a similar provision (‘Change of Control Put Event’). Upon the occurrence of such an event, the company might be required to redeem or purchase any of such bonds at their principal amount together with interest accrued. Philips’ outstanding long-term debt do not contain financial covenants.
In April2023, Philips issued EUR 500 million of fixed rate notes under the company’s EMTN program that mature in 2031 and used the proceeds for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in the fourth quarter of 2022. In 2023, Philips entered into a total amount of EUR 138 million forward contracts relating to the company’s long-term incentive plans. These forwards mature in the fourth quarters of 2024 (EUR 61m) and 2025 (EUR 77m). In addition, a total of EUR 125 million forward contracts relating to the long-term incentive and employee stock purchase plans and EUR 481 million of forwards related to the share buyback program announced in 2021 matured throughout 2023.
In 2022, Philips announced a series of Liability Management transactions to optimize its debt maturity profile. The transactions included the issuance of three series of Notes under its EMTN program for a total of EUR 2 billion with maturities in 2027, 2029 and 2033. Part of the proceeds were used to tender certain of Philips’ outstanding US Dollar denominated bonds due 2025 and 2026 and Euro-denominated bonds due 2023, 2024 and 2025, as well as make-whole and fully redeem the Euro-denominated bonds due 2023 and 2024 that were not purchased as part of the Euro tender offer. Philips issued Commercial Paper of EUR 200 million in September 2022 and EUR 101 million in October 2022. These tranches were repaid throughout the fourth quarter of 2022. In addition, in October 2022 Philips entered into a EUR 1 billion credit facility that cancould be used for general corporate purposes. The credit facility maturesmatured in October 2023 and hashad a 12-month extension option at Philips discretion. Per year-end 2022, EUR 500 million was utilized and outstanding under the credit facility. In 2022, Philips entered into a total amount of EUR 63 million forward contracts relating to the company’s long-term incentive and employee stock purchase plans. A total of EUR 57 million forward contracts relating to the long-term incentive and employee stock purchase plans as announced in 2020 and EUR 83 million of forwards related to the share buyback program announced in 2021 matured throughout 2022.
In February 2021, Philips entered into two new bilateral loans amounting to a total of EUR 500 million (EUR 250 million each) with a tenor of up to one year, that were repaid in September 2021. In 2021, Philips also entered into a total amount of EUR 731 million of forward contracts relating to the EUR 1.5 billion share buyback program announced on July 26, 2021, with maturity dates in 2022, 2023 and 2024. A total amount of EUR 745 million of forward contracts matured in 2021, which completed the settlement of the EUR 1.5 billion share buyback program announced on January 29, 2019. In addition, Philips entered into a total amount of EUR 90 million of forward contracts in 2021 relating to the long-term incentive and employee stock purchase plans announced on May 19, 2021, with maturity dates in 2023, and a total amount of EUR 123 million of forward contracts matured in 2021 relating to the company's long-term incentive and employee stock purchase plans announced on October 22, 2018 and January 29, 2020.
Long-term debt
The following tables present information about the long-term debt outstanding, its maturity and average interest rates in 20222023 and 2021.2022.
Philips Group
Long-term debt
in millions of EUR unless otherwise stated
| 2022 | 2023 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| amount outstanding | Current portion | Non-current portion | Between 1 and 5 years | amount due after 5 years | average remaining term (in years) | average rate of interest | amount outstanding | Current portion | Non-current portion | Between 1 and 5 years | amount due after 5 years | average remaining term (in years) | average rate of interest | |
| USD bonds | 1,378 | 1,378 | 250 | 1,128 | 14.3 | 6.3% | 1,325 | 1,325 | 240 | 1,085 | 13.3 | 6.3% | ||
| EUR bonds | 4,061 | 4,061 | 1,836 | 2,225 | 5.7 | 1.7% | 4,569 | 4,569 | 2,335 | 2,234 | 5.1 | 2.0% | ||
| Forward contracts | 858 | 606 | 252 | 252 | 1.0 | 396 | 321 | 76 | 0.8 | 1.4% | ||||
| Lease liabilities | 1,082 | 230 | 852 | 504 | 348 | 3.9 | 2.4% | 1,074 | 211 | 864 | 505 | 358 | 3.9 | 3.1% |
| Bank borrowings | 705 | 2 | 702 | 702 | 1.9 | 1.7% | 203 | 1 | 201 | 1.2 | 4.2% | |||
| Other long-term debt | 28 | 4 | 24 | 17 | 6 | 8.9 | 2.9% | - | 7.4 | 1.2% | ||||
| Long-term debt | 8,111 | 842 | 7,270 | 3,562 | 3,706 | 6.1 | 2.4% | 7,568 | 532 | 7,035 | 3,357 | 3,678 | 6.0 | 2.9% |
Philips Group
Long-term debt
in millions of EUR unless otherwise stated
| 2021 | 2022 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| amount outstanding | Current portion | Non-current portion | Between 1 and 5 years | amount due after 5 years | average remaining term (in years) | average rate of interest | amount outstanding | Current portion | Non-current portion | Between 1 and 5 years | amount due after 5 years | average remaining term (in years) | average rate of interest | |
| USD bonds | 1,313 | 1,313 | 255 | 1,058 | 15.1 | 6.3% | 1,378 | 1,378 | 250 | 1,128 | 14.3 | 6.3% | ||
| EUR bonds | 3,233 | 3,233 | 2,242 | 991 | 4.4 | 1.0% | 4,061 | 4,061 | 1,836 | 2,225 | 5.7 | 1.7% | ||
| Forward contracts | 934 | 196 | 738 | 738 | 1.6 | 858 | 606 | 252 | 1.0 | |||||
| Lease liabilities | 1,220 | 257 | 963 | 580 | 383 | 4.2 | 2.1% | 1,082 | 230 | 852 | 504 | 348 | 3.9 | 2.4% |
| Bank borrowings | 203 | 1 | 202 | 202 | 3.2 | 0.1% | 705 | 2 | 702 | 1.9 | 1.7% | |||
| Other long-term debt | 30 | 5 | 26 | 18 | 8 | 8.6 | 3.5% | 28 | 4 | 24 | 17 | 6 | 8.9 | 2.9% |
| Long-term debt | 6,933 | 459 | 6,473 | 4,034 | 2,439 | 6.0 | 2.1% | 8,111 | 842 | 7,270 | 3,562 | 3,706 | 6.1 | 2.4% |
Bonds
The following table presents the amount outstanding and effective rate of bonds.
Philips Group
Unsecured Bonds
in millions of EUR unless otherwise stated
| effective rate | 2021 | 2022 | effective rate | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Unsecured EUR Bonds | ||||||
| Due 06/09/2023; 1/2% | 0.634% | 500 | ||||
| Due 02/05/2024; 3/4% | 0.861% | 500 | ||||
| Due 30/03/2025; 1 3/8% | 1.509% | 346 | 346 | |||
| Due 22/05/2026; 1/2% | 0.608% | 750 | 750 | 0.608% | 750 | 750 |
| Due 05/05/2027; 1 7/8% | 2.049% | 750 | 750 | |||
| Due 02/05/2028; 1 3/8% | 1.523% | 500 | 500 | 1.523% | 500 | 500 |
| Due 30/03/2025; 1 3/8% | 1.509% | 500 | 346 | |||
| Due 05/11/2029; 2 1/8% | 2.441% | 650 | 650 | |||
| Due 30/03/2030; 2% | 2.128% | 500 | 500 | 2.128% | 500 | 500 |
| Due 05/05/2027; 1 7/8% | 2.049% | 750 | ||||
| Due 05/11/2029; 2 1/8% | 2.441% | 650 | ||||
| Due 08/09/2031; 4 2/8% | 4.330% | 500 | ||||
| Due 05/05/2033; 2 5/8% | 2.710% | 600 | 2.710% | 600 | 600 | |
| Unsecured USD Bonds | ||||||
| Due 15/05/2025; 7 3/4% | 7.429% | 56 | 51 | 7.429% | 51 | 49 |
| Due 15/05/2025; 7 1/8% | 6.794% | 78 | 75 | |||
| Due 01/06/2026; 7 1/5% | 6.885% | 120 | 119 | 6.885% | 119 | 114 |
| Due 15/05/2025; 7 1/8% | 6.794% | 74 | 78 | |||
| Due 11/03/2038; 6 7/8% | 7.210% | 641 | 683 | |||
| Due 03/11/2038; 6 7/8% | 7.210% | 683 | 657 | |||
| Due 15/03/2042; 5% | 5.273% | 441 | 470 | 5.273% | 470 | 452 |
| Adjustments1) | (37) | (57) | (57) | (47) | ||
| Unsecured Bonds | 4,545 | 5,439 | 5,439 | 5,894 | ||
Leases
The following table presents a reconciliation between the total of future minimum lease payments and their present value.
Philips Group
Lease liabilities
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| future minimum lease payments | interest | present value of minimum lease payments | future minimum lease payments | interest | present value of minimum lease payments | future minimum lease payments | interest | present value of minimum lease payments | future minimum lease payments | interest | present value of minimum lease payments | |
| Less than one year | 280 | 22 | 257 | 251 | 21 | 230 | 251 | 21 | 230 | 239 | 28 | 211 |
| Between one and five years | 636 | 56 | 580 | 554 | 49 | 505 | 554 | 49 | 505 | 572 | 67 | 505 |
| More than five years | 417 | 34 | 383 | 376 | 28 | 348 | 376 | 28 | 348 | 388 | 30 | 358 |
| Lease liabilities | 1,333 | 113 | 1,220 | 1,180 | 98 | 1,082 | 1,180 | 98 | 1,082 | 1,200 | 125 | 1,074 |
Short-term debt
Philips Group
Short-term debt
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Short-term bank borrowings | 47 | 89 | 89 | 122 |
| Current portion of long-term debt | 459 | 842 | 842 | 532 |
| Short-term debt | 506 | 931 | 931 | 654 |
During 2022,2023, the weighted average interest rate on the bank borrowings was 8.6% (2022: 5.7% (2021: 1.2%). This increase was mainly driven by financial market conditionshigher interest rate environments across various countries globally.
19Provisions
Accounting policies
A provision is a liability of uncertain timing or amount. Provisions are recognized if, as a result of a past event, the company has a present legal or constructive obligation, it is probable that an outflow of economic benefits will be required to settle the obligation and the amount can be estimated reliably. Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax discount rate that reflects current market assessments of the time value of money. The increase in the provision due to passage of time (accretion) is recognized as interest expense.
Restructuring-related provisions
Provisions for severance and termination benefits are recognized for those costs only when the company has a detailed formal plan for the restructuring and has raised a valid expectation with those affected that it will carry out the restructuring by starting to implement that plan or announcing its main features to those affected by it. Before a provision is established, the company recognizes any impairment loss on the assets associated with the restructuring.
Accounting estimates and judgments
By their nature, the recognition of provisions requirerequires estimates and assumptions regarding the timing and the amount of outflow of resources. The main estimates include:
- Respironics field-action provision – the provision requires management to make estimates and assumptions about items such as quantities and the portion of products to be remediated through replacement, repair or
repair.(partial) refund. - Product warranty provisions – the provisions for assurance-type product warranty reflect the estimated costs of replacement and free-of-charge services that will be incurred by the company with respect to products sold and include costs to execute field change orders.
- Environmental provisions – provisions for environmental remediation can change significantly due to the emergence of additional information regarding the extent or nature of the contamination, the need to utilize alternative technologies, actions by regulatory authorities as well as changes in judgments and discount rates.
- Legal provisions – provisions for legal claims and investigations reflect the best estimate of the outflow of resources, supported by internal and external legal counsel, when it is probable that such outflow of resources will be required to settle an obligation.
- Contingent consideration provisions – the provision for contingent consideration reflects the fair value of the expected payment to former shareholders of an acquired company for the exchange of control if specified future events occur or conditions are met, such as the achievement of certain regulatory milestones or the achievement of certain commercial milestones. The provision for contingent consideration can change significantly due to changes in the estimated achievement of milestones and changes in discount rates. Changes in fair value of the contingent consideration liability are reflected in other business income (expenses).
Philips Group
Provisions
in millions of EUR
| 2021 | 2022 | |||||
|---|---|---|---|---|---|---|
| long-term | short-term | total | long-term | short-term | total | |
| Post-employment benefits1) | 659 | 659 | 546 | 546 | ||
| Respironics field-action provision | 52 | 525 | 577 | 23 | 366 | 390 |
| Product warranty provisions | 32 | 207 | 238 | 57 | 287 | 344 |
| Environmental provisions | 99 | 26 | 124 | 83 | 20 | 104 |
| Restructuring-related provisions | 8 | 58 | 66 | 6 | 134 | 140 |
| Legal provisions | 53 | 39 | 91 | 14 | 74 | 89 |
| Contingent consideration provisions | 156 | 52 | 208 | 89 | 23 | 113 |
| Other provisions | 257 | 92 | 349 | 279 | 112 | 390 |
| Provisions | 1,315 | 998 | 2,313 | 1,097 | 1,018 | 2,115 |
| Post-employment benefits | Respironics field-action | Product warranty | Environmental | Restructuring- related | Legal | Contingent consideration | Other | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 366 | 287 | 20 | 134 | 74 | 23 | 112 | 1,018 | |
| Non-current | 546 | 23 | 57 | 83 | 6 | 14 | 89 | 279 | 1,097 |
| Balance as of December 31, 2022 | 546 | 390 | 344 | 104 | 140 | 89 | 113 | 390 | 2,115 |
| Additions | 112 | 240 | 313 | 18 | 263 | 644 | 24 | 223 | 1,836 |
| Utilizations | (91) | (285) | (268) | (14) | (219) | (235) | (20) | (134) | (1,266) |
| Releases | (10) | (20) | (2) | (67) | (10) | (7) | (45) | (159) | |
| Accretion | 5 | 23 | 1 | (3) | 25 | ||||
| Acquisitions | 6 | 6 | |||||||
| Changes in discount rate | (6) | (6) | |||||||
| Translation differences and other | - | (10) | (12) | (3) | (2) | (23) | (2) | (1) | (53) |
| Total change | 12 | (55) | 13 | (2) | (24) | 399 | 2 | 39 | 383 |
| Current | 331 | 293 | 22 | 102 | 477 | 57 | 181 | 1,463 | |
| Non-current | 558 | 3 | 64 | 80 | 14 | 10 | 58 | 248 | 1,035 |
| Balance as of December 31, 2023 | 558 | 334 | 357 | 102 | 116 | 487 | 115 | 429 | 2,498 |
Philips Group
Provisions
in millions of EUR
| Post-employment benefits | Respironics field-action | Product warranty | Environmental | Restructuring -related | Legal | Contingent consideration | Other | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 525 | 207 | 26 | 58 | 39 | 52 | 92 | 998 | |
| Non-current | 659 | 52 | 32 | 99 | 8 | 53 | 156 | 257 | 1,315 |
| Balance as of December 31, 2021 | 659 | 577 | 238 | 124 | 66 | 91 | 208 | 349 | 2,313 |
| Additions | 61 | 250 | 320 | 15 | 154 | 89 | 160 | 1,049 | |
| Utilizations | (185) | (486) | (224) | (17) | (61) | (100) | (105) | (95) | (1,274) |
| Releases | (1) | (2) | (18) | (3) | (35) | (59) | |||
| Accretion | 4 | - | (3) | 2 | |||||
| Acquisitions | 4 | 96 | 99 | ||||||
| Changes in discount rate | (27) | (27) | |||||||
| Fair value changes | (86) | (86) | |||||||
| Translation differences and other | 12 | 49 | 9 | 7 | (1) | 7 | 14 | 97 | |
| Total change | (113) | (187) | 105 | (21) | 74 | (3) | (95) | 41 | (198) |
| Current | 366 | 287 | 20 | 134 | 74 | 23 | 112 | 1,018 | |
| Non-current | 546 | 23 | 57 | 83 | 6 | 14 | 89 | 279 | 1,097 |
| Balance as of December 31, 2022 | 546 | 390 | 344 | 104 | 140 | 89 | 113 | 390 | 2,115 |
Respironics field action provision
On June 14, 2021, Philips’ subsidiary, Philips Respironics initiated a voluntary recall notification in the United States and field safety notice outside the United StatesUS for certain sleep and respiratory care products related to the polyester-based polyurethane (PE-PUR) sound abatement foam in these devices.
The repair and replacement programremediation is under wayprogressing globally. Because of the prioritization of the repair and replace program, Philips is currently not taking new orders for sleep therapy systems, while masks and other consumables continue to be sold. As of December 31, 2022, approximately 90% of2023, the production required for the delivery of replacement devices to patients has been completed. The timesubstantially completed and the total number of units expected to completebe remediated remained stable during the program is impacted by the dependency on supply of materials, including from China,year at 5.6 million devices (specific CPAP, BiPAP and global logistics capacity. Philips Respironics is also conducting a test and research program with independent laboratories. mechanical ventilator devices), excluding certain end-of-life-devices which are expected to be retired.
Philips has recognized a provision based on Philips'Philips’ best estimate of the costs to repair, replace or replacerefund devices, subject to the Respironics field action. The provision is related to the cost to repair, and/replace or replacerefund affected devices and includes, amongst others, the costs for the remaining production, the cost of intensified communication with physicians and patients, material costs, labor cost and logistics.logistics, as well as costs relating to the (partial) refunds provided to customers under the field action. The provision does not include any product liability costs or other claims. Movements during the year were as follows:
Philips Group
Respironics field-action provision
in millions of EUR
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | - | 577 |
| Additions | 719 | 250 |
| Utilizations | (175) | (486) |
| Translation differences | 33 | 49 |
| Balance as of December 31 | 577 | 390 |
AdditionsThe additions for the year primarily reflect updated expectationsthe impact of the revised remediation approach in relation to the volume ofmechanical ventilator devices eligible for remediation as well as additional costs relatedsubject to the accelerationrecall, following the agreed terms of the program. As of December 31, 2022, Philips Respironics expectsproposed consent decree (see below). The revised approach, which includes a revised repair program and assumes (partial) refunds to remediate a total of around 5.6 million devices (specific CPAP, BiPAP and mechanical ventilator devices) globally, excluding certain end-of-life devices that are expectedcustomers (refer to be retired. In 2022, following Philips Respironics’ comprehensive patient and customer communication outreach and based on current insights, the total expected units to be remediated have increased by approximately 0.4 million, primarilyIncome from operations), resulted in an increase in the US. Furthermore, efforts to acceleratecosts associated with the program resulted in a shift towards replacement, which increased the replacement share to 60% (compared to 46% asremediation of December 31, 2021) and as a result further reduced repair quantities.these devices. Utilizations for the year reflect the costs incurred in executing the repair and replace programremediation during the year.
The completion of the field action continues to be subject to significant uncertainties,uncertainty, which requirerequires management to make estimates and assumptions about items such as quantities and the portion to be replaced, or repaired. Asrepaired and refunded. An increase in the assumption for the refund portion by 10 percentage points, could have the effect of December 31, 2022,increasing the impact of changes in these main assumptions and estimates, holding other assumptions constant, on the field action provision are as follows:
Philips Group
Main assumptions
in millions ofby an estimated EUR unless otherwise stated
| Increase (decrease) in provision | ||
|---|---|---|
| Assumption | Increase individual assumption by 10% | Decrease individual assumption by 10% |
| Total quantity of devices remaining | 26 | (26) |
| Replacement share | 12 | (12) |
19 million. Actual outcomes in future periods may differ from these estimates and affect the company'scompany’s results of operations, financial position and cash flows.
In addition,Further to the above, running remediation costs of EUR 210224 million (2021:(2022: EUR 94210 million) related to the remediation, such as testing, external advisory and regulatory response and additional right-of-return and warranty provisions, have been incurred.
Following the FDA’sUS Food and Drug Administration (FDA) inspection of certain of Philips Respironics’Respironics' facilities in the US in 2021 and the subsequent inspectional observations, the US Department of Justice, acting on behalf of the FDA, in July 2022 started discussions with Philips regarding the terms of a consent decree to resolve the identified issues. Atissues, which Philips has now agreed. As a consequence of addressing the endconsent decree, the company recorded charges of EUR 363 million in December 2022,2023, mainly consisting of EUR 240 million addition to the discussionsRespironics field action provision, EUR 82 million inventory write-down (refer to Inventories), EUR 31 million onerous contract provision and EUR 6 million fixed asset impairment.
In addition to the above, Philips and its affiliates are ongoing. Furthermore, Philips is a defendantdefendants in a number of consumer class action lawsuits from users of the affected devices and a number of individual personal injury and other compensation claims. To date no provisions have been recorded for the litigation and investigations associated with the Respironics field action. For legal matters including claims refer to the legal provisions section of this note as well as Contingencies.
Product warranty provisions
The provisions for assurance-type product warranty reflect the estimated costs of replacement and free-of-charge services that will be incurred by the company with respect to products sold, and include costs to execute field change orders. The field action provision in connection with the Philips Respironics voluntary recall notification is shown separately above.
Additions in 2023 include quality remediation actions of EUR 81 million in the Diagnosis & Treatment segment.
The company expects the provisions to be utilized mainly within the next year.
Philips Group
Provisions for assurance-type product warranty
in millions of EUR
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | 167 | 238 |
| Additions | 364 | 320 |
| Utilizations | (265) | (224) |
| Transfer to liabilities associated with assets held for sale | (37) | |
| Translation differences and other | 10 | 9 |
| Balance as of December 31 | 238 | 344 |
Additions in 2022 include quality actions of EUR 108 million in the Connected Care segment, mainly for the following matters:
Pads Cartridges
In February 2022, Philips issued a field safety notice notifying customers of a potential issue with the Adult SMART Pads Cartridge (M5071A) and the Infant/Child SMART Pads Cartridge (M5072A) for use specifically with the HeartStart HS1 Automated External Defibrillator (AED) devices. Philips has identified that for affected pads the HS1 AED could deliver less effective or ineffective therapy. Philips is actively working on replacing these pads and has commenced the replacement program in 2022.
V60 35V
In March 2022, Philips Respironics issued a voluntary recall notification/field safety notice to customers of its V60, V60 Plus and V680 ventilators, regarding a potential issue that could affect the main electrical circuit (“35V Rail”) powering the ventilator and alarm. This notification was updated in April 2022 with additional customer instructions. In June 2022, Philips issued a further update to this notification, regarding the projected correction for this matter. To address the issue with the 35V Rail, Philips Respironics has commenced the remediation program in 2022.
Environmental provisions
The environmental provisions include accrued costs recorded with respect to environmental remediation in various countries. In the United States,US, subsidiaries of the company have been named as potentially responsible parties in state and federal proceedings for the clean-up of certain sites.
Provisions for environmental remediation can change significantly due to the emergence of additional information regarding the extent or nature of the contamination, the need to utilize alternative technologies, actions by regulatory authorities as well as changes in judgments and discount rates.
Approximately EUR 73 million of the long-term provision is expected to be utilized after one to five years, with the remainder after five years. For more details on the environmental remediation refer to Contingencies.
Philips Group
Environmental provisions
in millions of EUR
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | 183 | 124 |
| Additions | 18 | 15 |
| Utilizations | (15) | (17) |
| Releases | (64) | (2) |
| Changes in discount rate | (10) | (27) |
| Accretion | 3 | 4 |
| Translation differences and other | 9 | 7 |
| Balance as of December 31 | 124 | 104 |
The additions and the releases of the provisions originate from additional insights in relation to factors like the estimated cost of remediation, changes in regulatory requirements and efficiencies in completion of various site work phases.
BasedApproximately EUR 63 million of the long-term provision is expected to be utilized after one to five years, with the remainder after five years. For more details on the progressive insight with respectenvironmental remediation refer to site remediation experience, technological progress and risk-based clean-up strategies, the estimated remaining duration of remediation activities for environmental liabilities for infinite environmental sites was revised in 2021 from 60 years to 30 years. The resulting release was EUR 55 million of which EUR 33 million is recorded in continuing operations and EUR 22 million in discontinued operations.Contingencies.
Restructuring-related provisions
Philips Group
Restructuring-related provisions
in millions of EUR
| January 1, 2022 | additions | utilizations | releases | other changes | December 31, 2022 | December 31, 2022 | December 31, 2023 | |
|---|---|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 26 | 58 | (27) | (8) | 0 | 49 | 42 | 36 |
| Connected Care | 17 | 34 | (13) | (3) | (1) | 34 | 42 | 18 |
| Personal Health | 9 | (7) | (2) | 0 | 10 | 10 | 7 | |
| Other | 14 | 52 | (14) | (5) | 0 | 47 | 47 | 56 |
| Philips Group | 66 | 154 | (61) | (18) | (1) | 140 | 140 | 116 |
InFurther to the workforce reduction in 2022, Philips initiated general productivity actions aimed at simplifyingmeasures were announced on January 30, 2023 that primarily focus on the organization to streamline the way of working and reduce operating expenses. This includes an immediate reduction of around 4,0006,000 positions globally acrossby 2025. In 2023, the organization, subject to consultation with the relevant workers councils and social partners, with severance and termination-relatedrestructuring costs, expected to be approximatelynet of releases, for these measures were EUR 130 million in aggregate, of which EUR 80 million was recorded in 2022.140 million.
In addition, restructuring projects were executed during the year, of which the most significant impacted Diagnosis & TreatmentConnected Care and Other and mainly took place in the US and Netherlands. The restructuring mainly comprised product portfolio rationalization and the reorganization of global support functions. The company expects the provisions to be utilized mainly within the next year.
2021
In 2021,2022, Philips initiated general productivity actions aimed at simplifying the most significant restructuring projects impacted Diagnostic & Treatmentorganization to streamline the way of working and Connected Care businessesreduce operating expenses. This includes an immediate reduction of around 4,000 positions globally across the organization, with severance and mainly took placetermination-related costs of EUR 80 million recorded in the Netherlands and US. 2022.
The movements in the provisions for restructuring in 2021 are presented by segment as follows:
Philips Group
Restructuring-related provisions
in millions of EUR
| January 1, 2021 | additions | utilizations | releases | other changes | December 31, 2021 | |
|---|---|---|---|---|---|---|
| Diagnosis & Treatment | 33 | 23 | (19) | (13) | 1 | 26 |
| Connected Care | 17 | 16 | (12) | (4) | - | 17 |
| Personal Health | 28 | 6 | (21) | (6) | 2 | 9 |
| Other | 38 | 10 | (21) | (16) | 4 | 14 |
| Philips Group | 117 | 55 | (73) | (39) | 6 | 66 |
Legal provisions
The company and certain of its group companies and former group companies are involved as a party in legal proceedings, including regulatory and other governmental proceedings.
Philips Group
Legal provisions
in millions of EUR
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | 72 | 91 |
| Additions | 43 | 89 |
| Acquisitions | 38 | 4 |
| Utilizations | (17) | (100) |
| Releases | (48) | (3) |
| Accretion | 1 | - |
| Translation differences and other | 3 | 7 |
| Balance as of December 31 | 91 | 89 |
The majority of the movements in the above schedule are: Additions mainly relate to a provision recognized for alleged tender irregularities as disclosedEUR 575 million in note Contingenciesconnection with the anticipated resolution of the economic loss class action in the US. The final cost of the settlement may vary based on, among other things, how many patients and provisions recognized for CRT matters. other settlement class members participate in the settlement.
Utilizations mainly relate to the settlement of investigationsthe company reached with the US Securities and Exchange Commission (SEC) to resolve the SEC inquiry regarding alleged tender irregularities in the Connected Care businesses (unrelatedmedical device industry in China, for which the company had recorded a provision of approximately EUR 60 million in 2022. The settlement reached was in line with the amount provided for and EUR 58 million was subsequently paid in 2023. In addition the company funded an amount of USD 155 million (EUR 141 million) into the Qualified Settlement Fund in relation to the Philips Respironics voluntary recall notification).economic loss class action settlement announced on September 7, 2023.
For details of other legal matters, including regulatory and other governmental proceedings, refer to Contingencies.
The company expects the provisions to be utilized mainly within the next three years.
Contingent consideration provisions
Philips Group
Contingent consideration provisions
in millionsIn 2023, the addition of EUR
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | 318 | 208 |
| Acquisitions | 16 | 96 |
| Utilizations | (48) | (105) |
| Fair value changes | (78) | (86) |
| Balance as of December 31 | 208 | 113 |
24 million is largely offset by utilizations of EUR 20 million. The provision for contingent consideration reflects the fair valueacquisition of a business within Ultrasound resulted in a EUR 6 million increase.
Approximately EUR 28 million of the expected payment to former shareholders of an acquiree for the exchange of control if specified future events occur or conditions are met, such as the achievement of certain regulatory milestones or the achievement of certain commercial milestones. Thelong-term provision for contingent consideration can change significantly due to changes in the estimated achievement of milestones and changes in discount rates. Changes in fair value of the contingent consideration liability are reflected in other business income.
In 2021 and 2022, the fair value changes mainly related to EPD. In 2022, the decrease of EUR 61 million in the fair value of the contingent consideration comprised of EUR 30 million due to the revisions to EPD’s forecast due to more severe short-term impacts of COVID-19 and the competitive environment, and EUR 31 million due to delays in achievement of certain milestones. In 2021, the decrease of EUR 45 million in the fair value of the contingent consideration comprised of EUR 14 million due to the revisions to EPD’s forecast due to more severe short-term impacts of COVID-19 and the competitive environment, and EUR 31 million due to delays in achievement of certain milestones.
The company expects the provisionsis expected to be utilized mainly within the next three years, with the remainder after four years.
Other provisions
The main elements of other provisions are:
Philips Group
Other provisions
in millions of EUR unless otherwise stated
| 2021 | 2022 | |
|---|---|---|
| Balance as of January 1 | 372 | 349 |
| Additions | 89 | 160 |
| Utilizations | (87) | (95) |
| Releases | (29) | (35) |
| Accretion | (5) | (3) |
| Translation differences and other | 9 | 14 |
| Balance as of December 31 | 349 | 390 |
| 2022 | 2023 | |
|---|---|---|
| Employee jubilee funds | 83 | 77 |
| Self-insurance | 57 | 63 |
| Non-income taxes / social security | 46 | 51 |
| Rights of return | 36 | 39 |
| Decommissioning costs | 33 | 34 |
| Onerous contracts | 38 | 76 |
| Remaining | 97 | 89 |
| Balance as of December 31 | 390 | 429 |
The main elements of other provisions are:
provisions for employee jubilee funds EUR 83 million (2021: EUR 94 million);self-insurance provisions of EUR 57 million (2021: EUR 43 million);provisions for non-income taxes/social security of EUR 46 million (2021: EUR 37 million);provisions for rights of return of EUR 36 million (2021: EUR 40 million);provisions for decommissioning costs of EUR 33 million (2021: EUR 33 million);provisions for onerousOnerous contractsof EUR 38 million (2021: EUR 12 million), reflectingreflect non-cancellable commitments on supplies for which no future demand or alternative usage has been identified,primarily caused by volatilityincluding EUR 31 million indemand due to COVID-19.- connection with the
remainingproposed Respironics consent decree as of December 31, 2023.Remaining provisions relate to a variety of positions, for example provision for disability of employees and provision for royalty obligations.
the releasesReleases in
20212022 and20222023 are due to the reassessment of the positions in other provisions throughout the year.
The company expects the other provisions to be utilized mainly within the next five years, except for:years.
provisions for employee jubilee funds of which half is expected to be utilized after five years;provisions for decommissioning costs of which half is expected to be utilized after five years;provisions for rights of return to be utilized mainly within the next year.
20Post-employment benefits
Accounting policies
Defined contribution plans
A defined contribution plan is a post-employment benefit plan for which the company pays fixed contributions into a separate entity and will have no legal or constructive obligation to pay further amounts. Obligations for contributions to defined contribution pension plans are recognized as an employee benefit expense in the Consolidated statements of income in the periods during which services are rendered by employees.
Defined Benefitbenefit plans
A defined benefit plan is a post-employment benefit plan that is not a defined contribution plan. Defined benefit plans define an amount of pension benefit that an employee will receive after retirement. That pension benefit typically depends on several factors such as years of service, age and salary.
The net pension asset or liability recognized in the Consolidated balance sheets in respect of defined benefit plans is the fair value of plan assets less the present value of the projected defined benefit obligation at the Consolidated balance sheetssheet date. The defined benefit obligation is calculated annually by qualified actuaries using the projected unit credit method. Recognized assets are limited to the present value of any reductions in future contributions or any future refunds. The net pension liability is presented as a long-term provision; no distinction is made for the short-term portion.
For the company’s major plans, a full discount rate curve of high-quality corporate bonds is used to determine the defined benefit obligation, where available. The curves are based on the Mercer Yield Curve methodology, which uses data of corporate bonds rated AA or equivalent. For the other plans the Mercer Yield Curve/Mercer Methodology has also been used taking into account the cash flows as much as possible in case there is a deep market in corporate bonds. For plans in countries without a deep corporate bond market, the discount rate is based on government bonds and the plan’s maturity.
Pension costs in respect of defined benefit plans primarily represent the increase of the actuarial present value of the obligation for post-employment benefits based on employee service during the year and the interest on the net recognized asset or liability in respect of employee service in previous years.
Remeasurements of the net defined benefit asset or liability comprise actuarial gains and losses, the return on plan assets (excluding interest) and the effect of the asset ceiling (excluding interest). The company recognizes all remeasurements in Other comprehensive income.
Past service costs arising from the introduction of a change to the benefit payable under a plan or a significant reduction of the number of employees covered by a plan (curtailment) are recognized in full in the Consolidated statements of income.
Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided. The company recognizes a liability and an expense for bonuses and incentives based on a formula that takes into consideration the profit attributable to the company’s shareholders after certain adjustments.
The company’s net obligation in respect of other long-term employee benefits is the amount of future benefit that employees have earned in return for their service in the current and prior periods, such as jubilee entitlements. That benefit is discounted to determine its present value. Remeasurements are recognized in the Consolidated statements of income in the period in which they arise.
Further information on other employee benefits can be found in Provisions in the Other provisions section.
Accounting estimates and judgments
To make the actuarial calculations for the valuation of defined benefit obligations, assumptions are needed for interest rates, healthcare cost increases, future pension increases, life expectancy and employee turnover rates. The actuarial calculations are made by external actuaries based on inputs from observable market data, such as corporate bond returns and yield curves to determine the discount rates to apply, mortality tables to determine life expectancy and inflation rates to determine future salary and pension growth assumptions.
Employee post-employment benefit plans have been established in many countries in accordance with the legal requirements, customs and the local practice in the countries involved. The larger part of post-employment benefits are company pension plans, of which some are funded and some are unfunded. All funded post-employment benefit plans are considered to be related parties.
Most employees that take part in a company pension plan are covered by defined contribution (DC) pension plans. The main DC plans are in the Netherlands and the United States. The company also sponsors a number of defined benefit (DB) pension plans. The benefits provided by these plans are based on employees’ years of service and compensation levels.
The company also sponsors a limited number of DB retiree medical plans. The benefits provided by these plans typically cover a part of the healthcare costs after retirement. None of these plans are individually significant to the company and are therefore not further separately disclosed.
The larger funded DB and DC plans are governed by independent Trustees who have a legal obligation to protect the interests of all plan members and operate under the local regulatory framework.
The DB plans in Germany and the United StatesUS make up most of the defined benefit obligation (DBO) and the net position. The company also has DB plans in the rest of the world; however these are individually not significant to the company and do not have a significantly different risk profile that would warrant separate disclosure.
The adjacent table provides a break-down of the present value of the funded and unfunded DBO, the fair value of plan assets and the net position in Germany, the United StatesUS and in Other Countries. The table also provides the value of reimbursement rights.
Philips Group
Post-employment benefits
in millions of EUR
| Germany | United States | Other Countries | Total | Germany | United States | Other Countries | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Present value of funded DBO | (606) | (489) | (558) | (440) | (206) | (179) | (1,370) | (1,108) | (489) | (511) | (440) | (404) | (179) | (182) | (1,108) | (1,097) |
| Present value of unfunded DBO | (316) | (249) | (149) | (128) | (135) | (136) | (600) | (513) | (249) | (253) | (128) | (118) | (136) | (137) | (513) | (508) |
| Total present value of DBO | (921) | (738) | (708) | (568) | (341) | (315) | (1,970) | (1,621) | (738) | (764) | (568) | (522) | (315) | (319) | (1,621) | (1,605) |
| Fair value of plan assets | 572 | 477 | 623 | 474 | 185 | 171 | 1,380 | 1,122 | 477 | 481 | 474 | 442 | 171 | 166 | 1,122 | 1,089 |
| Net position | (349) | (261) | (84) | (94) | (157) | (144) | (590) | (499) | (261) | (283) | (94) | (80) | (144) | (153) | (499) | (516) |
| Value of reimbursement rights | 6 | 6 | 6 | 8 | 6 | 8 | ||||||||||
The classification of the net position is as follows:
Philips Group
Classification net position
in millions of EUR
| Germany | United States | Other Countries | Total | Germany | United States | Other Countries | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Total asset for plans in a surplus | 3 | 9 | 65 | 34 | 1 | 4 | 69 | 46 | 9 | - | 34 | 39 | 4 | 2 | 46 | 41 |
| Total liability for plans in a deficit | (352) | (270) | (149) | (128) | (157) | (148) | (659) | (546) | (270) | (283) | (128) | (118) | (148) | (156) | (546) | (558) |
| Provisions for post-employment benefit plans under AHFS | ||||||||||||||||
| Net position | (349) | (261) | (84) | (94) | (157) | (144) | (590) | (499) | (261) | (283) | (94) | (80) | (144) | (153) | (499) | (516) |
Germany
The company has several DB plans in Germany, which for the largest part are partially unfunded, meaning that after retirement the company is responsible for the benefit payments to retirees.
Due to the relatively high level of social security in Germany, the company’s pension plans mainly provide benefits for the higher earners. The plans are open for future pension accrual. Indexation is mandatory due to legal requirements. Some of the German plans have a DC design, but are accounted for as DB plans due to a legal minimum return requirement.
Company pension commitments in Germany are partly protected against employer bankruptcy via the “Pensions-Sicherungs-Verein” which charges a fee to all German companies providing pension promises.
Philips is one of the sponsors of Philips Pensionskasse VVaG in Germany, which is a multi-employer plan. The plan is classified and accounted for as a DC plan.
The United States
The US DB pension plans are closed plans without future pension accrual. For the funding of any deficit in the US plan the Group adheres to the minimum funding requirements of the US Pension Protection Act.
The assets of the US funded pension plans are in Trusts governed by fiduciaries. The non-qualified pension plans that cover accrual above the maximum salary of the funded qualified plan are unfunded.
The company’s qualified pension commitments in the United StatesUS are covered via the Pension Benefit Guaranty Corporation which charges a fee to US companies providing DB pension plans. The fee is also dependent on the amount of unfunded vested liabilities.
Risks related to DB plans
DB plans expose the company to various demographic and economic risks such as longevity risk, investment risks, currency and interest rate risk and in some cases inflation risk. The latter plays a role in the assumed wage increase but more importantly in some countries where indexation of pensions is mandatory.
The company has an active de-risking strategy in which it constantly looks for opportunities to reduce the risks associated with its DB plans. Liability-driven investment strategies, lump sum cash-out options, buy-ins, buy-outs and a change to DC are examples of the strategy.
Investment policy in the largest pension plans
Pension fund trustees are responsible for and have full discretion over the investment strategy of the plan assets. The plan assets of the Philips pension plans are invested in well diversified portfolios. The interest rate sensitivity of the fixed income portfolio is closely aligned to that of the plan’s pension liabilities for most of the plans. Any contributions from the sponsoring company are used to further increase the fixed income part of the assets. As part of the investment strategy, any improvement in the funded ratio over time is used to further decrease the interest rate mismatch between the plan assets and the pension liabilities.
Summary of pre-tax costs for post-employment benefits and reconciliations
The adjacent table contains the total of current and past service costs, administration costs and settlement results as included in Income from operations and the interest cost as included in Financial expenses.
Philips Group
Pre-tax costs for post-employment benefits
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Defined benefit plans | 74 | 36 | 50 | 36 | 50 | 47 |
| - included in income from operations | 59 | 28 | 39 | 28 | 39 | 25 |
| - included in financial expense | 13 | 8 | 10 | 8 | 10 | 21 |
| - included in Discontinued operations | 1 | 1 | ||||
| Defined contribution plans | 366 | 375 | 400 | 375 | 400 | 376 |
| - included in income from operations | 358 | 368 | 400 | 368 | 400 | 376 |
| - included in Discontinued operations | 8 | 7 | 7 | |||
| Post-employment benefits costs | 440 | 411 | 449 | 411 | 449 | 423 |
Summary of the reconciliations for the DBO and plan assets
The adjacent tables contain the reconciliations for the DBO and plan assets.
Philips Group
Defined benefit obligations
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Balance as of January 1 | 2,153 | 1,970 | 1,970 | 1,621 |
| Service cost | 36 | 32 | 32 | 32 |
| Interest cost | 33 | 36 | 36 | 71 |
| Employee contributions | 7 | 4 | 4 | 3 |
| Actuarial (gains) / losses | ||||
| - demographic assumptions | 3 | 2 | 2 | |
| - financial assumptions | (86) | (366) | (366) | 48 |
| - experience adjustment | (6) | 12 | 12 | 2 |
| (Negative) past service cost | (5) | 16 | 16 | (9) |
| Settlements | (90) | - | 2 | |
| Benefits paid from plan | (95) | (95) | (95) | (104) |
| Benefits paid directly by employer | (33) | (41) | (41) | (39) |
| Translation differences and other | 52 | 52 | 52 | (22) |
| Balance as of December 31 | 1,970 | 1,621 | 1,621 | 1,605 |
Philips Group
Plan assets
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Balance as of January 1 | 1,403 | 1,380 | 1,380 | 1,122 |
| Interest income on plan assets | 25 | 26 | 26 | 49 |
| Admin expenses paid | (1) | (1) | (1) | (1) |
| Return on plan assets excluding interest income | 44 | (254) | (254) | 23 |
| Employee contributions | 7 | 4 | 4 | 3 |
| Employer contributions | 33 | 17 | 17 | 14 |
| Settlements | (86) | 0 | ||
| Benefits paid from plan | (96) | (95) | (95) | (104) |
| Translation differences and other | 50 | 45 | 45 | (17) |
| Balance as of December 31 | 1,380 | 1,122 | 1,122 | 1,089 |
The past service cost in 2023 and 2022 mainly relatesrelate to the retiree medical plans in Brazil. The settlement amounts of 2021 mainly relate to the transfer of the provident fund plan into the government provident fund in India.
Plan assets allocation
The asset allocation in the company’s DB plans as of December 31, was as follows:
Philips Group
Plan assets allocation
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Assets quoted in active markets | ||||
| - Debt securities | 790 | 560 | 560 | 513 |
| - Equity securities | ||||
| - Other | 195 | 203 | 203 | 182 |
| Assets not quoted in active markets | ||||
| - Debt securities | 1 | |||
| - Equity securities | 122 | 101 | 101 | 31 |
| - Other | 272 | 258 | 258 | 363 |
| Total assets | 1,380 | 1,122 | 1,122 | 1,089 |
The plan assets in 20222023 contain 36% (2022: 32% (2021: 29%) unquoted plan assets. Plan assets in 20222023 do not include property occupied by or financial instruments issued by the company.
Assumptions
The mortality tables used for the company’s largest DB plans are:
Germany: Heubeck-Richttafeln 2018 Generational, assuming 93% of mortality rates for male retirees between age 60 and 85
US: PRI-2012 Generational with MP2021 improvement scale + white collar adjustment
The weighted averages of the assumptions used to calculate the DBO as of December 31, were as follows:
Philips Group
Assumptions used for defined benefit obligations in Germany, the United States and the rest of the world
in %
| Germany | United States | Other Countries | Total | Germany | United States | Other Countries | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Discount rate | 1.1% | 4.1% | 2.6% | 5.2% | 2.1% | 4.9% | 1.8% | 4.7% | 4.1% | 3.7% | 5.2% | 5.0% | 4.9% | 4.9% | 4.7% | 4.3% |
| Inflation rate | 1.8% | 2.0% | 2.2% | 2.3% | 2.0% | 2.6% | 2.0% | 2.2% | 2.0% | 2.0% | 2.3% | 2.3% | 2.6% | 2.5% | 2.2% | 2.2% |
| Salary increase | 2.5% | 2.8% | 0.0% | 0.0% | 2.9% | 3.3% | 2.6% | 2.9% | 2.8% | 2.8% | 0.0% | 0.0% | 3.3% | 4.3% | 2.9% | 3.0% |
Sensitivity analysis
The following table illustrates the approximate impact on the DBO from movements in key assumptions. The DBO was recalculated using a change in the assumptions of 1% which overall is considered a reasonably possible change. The impact on the DBO because of changes in discount rate is normally accompanied by offsetting movements in plan assets, especially when using matching strategies.
The average duration in years of the DBO of the DB plans is 8 years10 (Germany: 9,11, United States: 8, and Other countries: 8)10) as of December 31, 2022 (2021: 11 years)2023 (2022: 8).
Philips Group
Sensitivity of key assumptions
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Increase | ||||
| Discount rate (1% movement) | (196) | (122) | (122) | (123) |
| Pension increase (1% movement) | 99 | 57 | 57 | 60 |
| Salary increase (1% movement) | 19 | 12 | 12 | 12 |
| Longevity1) | 48 | 32 | 32 | 32 |
| Decrease | ||||
| Discount rate (1% movement) | 241 | 145 | 145 | 147 |
| Pension increase (1% movement) | (83) | (49) | (49) | (52) |
| Salary increase (1% movement) | (18) | (11) | (11) | (11) |
Cash flows and costs in 20232024
Cash outflows in relation to post-employment benefits are estimated to amount to EUR 464434 million in 2023,2024, consisting of:
- EUR
1917 million employer contributions tofundedDB plans (Germany: EUR78 million,United States:US: EUR 0 million, Other Countries: EUR129 million); - EUR
4342 million cash outflows in relation tounfundedDB plans (Germany: EUR 20 million,United States:US: EUR1110 million, Other Countries: EUR 12 million); and - EUR
402375 million employer contributions to DC plans (Netherlands: EUR186174 million,United States:US: EUR153136 million, Other Countries: EUR6365 million).
The service and administration cost for 20232024 is expected to amount to EUR 2930 million for DB plans. The net interest cost for 20232024 for the DB plans is expected to amount to EUR 21 million. The cost for DC pension plans in 20232024 is equal to the expected DC cash flow.
21Accrued liabilities
Accounting policies
Accrued liabilities are initially measured at fair value and subsequently at amortized cost and are derecognized when the obligation under the liability is discharged, cancelled or has expired.
Accrued liabilities are summarized as follows:
Philips Group
Accrued liabilities
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Personnel-related costs: | ||||
| - Salaries and wages | 566 | 490 | 490 | 791 |
| - Accrued holiday entitlements | 127 | 97 | 97 | 96 |
| - Other personnel-related costs | 108 | 101 | 101 | 93 |
| Fixed-asset-related costs: | ||||
| - Gas, water, electricity, rent and other | 33 | 46 | 46 | 43 |
| Communication and IT costs | 82 | 64 | 64 | 61 |
| Distribution costs | 122 | 110 | 110 | 99 |
| Sales-related costs: | ||||
| - Commission payable | 7 | 8 | 8 | 12 |
| - Advertising and marketing-related costs | 175 | 127 | 127 | 133 |
| - Other sales-related costs | 20 | 20 | 20 | 20 |
| Material-related costs | 130 | 132 | 132 | 138 |
| Interest-related accruals | 52 | 71 | 71 | 76 |
| Other accrued liabilities | 362 | 361 | 361 | 324 |
| Accrued liabilities | 1,784 | 1,626 | 1,626 | 1,887 |
22Other liabilities
Accounting policies
Other liabilities are initially measured at fair value and subsequently at amortized cost and are derecognized when the obligation under the liability is discharged, cancelled or has expired.
The company recognizes contract liabilities if a payment is received or a payment is due (whichever is earlier) from a customer before the company transfers the related goods or services. Contract liabilities are recognized as revenue when the company performs under the contract (i.e., transfers control of the related goods or services to the customer).
Other non-current liabilities
Non-current liabilities were EUR 6054 million as of December 31, 20222023 (December 31, 2021:2022: EUR 5660 million).
Non-current liabilities are associated mainly with indemnification and non-current accruals.
Other current liabilities
Other current liabilities are summarized as follows:
Philips Group
Other current liabilities
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Accrued customer rebates | 280 | 213 | 213 | 186 |
| Other taxes including social security premiums | 190 | 115 | 115 | 129 |
| Other liabilities | 116 | 120 | 120 | 98 |
| Other current liabilities | 587 | 448 | 448 | 414 |
Contract liabilities
Non-current contract liabilities were EUR 515469 million as of December 31, 20222023 (December 31, 2021:2022: EUR 446515 million) and current contract liabilities were EUR 1,6961,809 million as of December 31, 20222023 (December 31, 2021:2022: EUR 1,4911,696 million).
The current contract liabilities increased by EUR 205113 million, which is mainly driven by an increase in deferred balances for customer service contracts.
The current contract liabilities as of December 31, 20212022 resulted in revenue recognized of EUR 1,4911,696 million in 2022.2023.
23Cash flow statement supplementary information
Accounting policies
Cash and cash equivalents
Cash and cash equivalents include all cash balances, certain money market funds and short-term highly liquid investments with an original maturity of three months or less that are readily convertible into known amounts of cash. Bank overdrafts are included in borrowings in current liabilities.
Cash flow statements
The cash flow statement is prepared using the indirect method. Cash flows related to interest and tax are included in operating activities. Assets and liabilities acquired as part of a business combination are included in investing activities (net of cash acquired). Dividends paid to shareholders are included in financing activities. Dividends received are included in operating activities.
Cash flows arising from transactions in a foreign currency are translated into the company’s functional currency using the exchange rate at the date of the cash flow. Cash flows from derivative instruments that are accounted for as cash flow hedges are classified in the same category as the cash flows from the hedged items. Cash flows from other derivative instruments are classified as investing cash flows.
Income taxes
Income taxes in 2023 include EUR 2 million of interest related to uncertain tax positions.
Cash paid for leases
In 2022,2023, gross lease payments of EUR 271 million (2022: EUR 316 million (2021:million; 2021: EUR 308 million; 2020: EUR 325 million) included interest of EUR 2527 million (2021:(2022: EUR 25 million; 2020:2021: EUR 2925 million).
Net cash used for derivatives and current financial assets
In 2022,2023, a total of EUR 7246 million cash was paid with respect to foreign exchange derivative contracts related to activities for liquidity management (2021:(2022: EUR 72 million outflow; 2021: EUR 48 million inflow; 2020: EUR 13 million outflow)inflow).
Purchase and proceeds from non-current financial assets
In 2023, the net cash outflow is EUR 44 million. In 2022, the net cash outflow is EUR 38 million.
In 2021, the net cash flow is EUR 0 million.
In 2020, the net cash outflow of EUR 66 million was mainly the cash outflow due to investment in DC Health amounting to EUR 45 million in China.
Reconciliation of liabilities arising from financing activities
Certain items in the statements of cash flows do not correspond to the differences between the balance sheet amounts for the respective items, principally because of the effects of translation differences and consolidation changes.
Philips Group
Reconciliation of liabilities arising from financing activities
in millions of EUR
| Balance as of December 31, 2021 | Cash flow | Currency effects and consolidation changes | Other1) | Balance as of December 31, 2022 | Balance as of December 31, 2022 | Cash flow | Currency effects and consolidation changes | Other1) | Balance as of December 31, 2023 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Long term debt2) | 6,933 | 1,045 | 107 | 27 | 8,111 | 8,111 | (210) | (96) | (238) | 7,567 |
| EUR bonds | 3,233 | 827 | 4,061 | 4,061 | 497 | 11 | 4,569 | |||
| USD bonds | 1,313 | (20) | 85 | 1,378 | 1,378 | (53) | 1,325 | |||
| Leases | 1,220 | (260) | 17 | 105 | 1,082 | 1,082 | (200) | (42) | 235 | 1,074 |
| Forward contracts3) | 934 | (76) | 858 | 858 | (462) | 396 | ||||
| Bank borrowings | 203 | 498 | 4 | 705 | 705 | (502) | 203 | |||
| Other long-term debt | 30 | (1) | 1 | (1) | 28 | 28 | (5) | (1) | (22) | |
| Short term debt2) | 47 | 47 | (6) | 1 | 89 | 89 | 29 | 3 | 122 | |
| Short-term bank borrowings | 47 | 47 | (6) | 1 | 89 | 89 | 46 | (14) | 122 | |
| Other short-term loans | (17) | 17 | ||||||||
| Forward contracts3) | ||||||||||
| Equity | (1,410) | (593) | 869 | (1,133) | (1,133) | (666) | 1,143 | (656) | ||
| Dividend payable | (418) | 418 | (4) | 4 | ||||||
| Forward contracts3) | (934) | 76 | (858) | (858) | 465 | (394) | ||||
| Treasury shares | (476) | (174) | 375 | (275) | ||||||
| Treasury shares4) | (275) | (662) | 675 | (262) | ||||||
| Total | 500 | (848) | ||||||||
Philips Group
Reconciliation of liabilities arising from financing activities
in millions of EUR
| Balance as of December 31, 2020 | Cash flow | Currency effects and consolidation changes | Other1) | Balance as of December 31, 2021 | Balance as of December 31, 2021 | Cash flow | Currency effects and consolidation changes | Other1) | Balance as of December 31, 2022 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Long term debt2) | 6,857 | (226) | 200 | 101 | 6,933 | 6,933 | 1,045 | 107 | 27 | 8,111 |
| EUR bonds | 3,229 | 4 | 3,233 | 3,233 | 827 | 4,061 | ||||
| USD bonds | 1,210 | 103 | 1,313 | 1,313 | (20) | 85 | 1,378 | |||
| Leases | 1,216 | (239) | 98 | 145 | 1,220 | 1,220 | (260) | 17 | 105 | 1,082 |
| Forward contracts3) | 982 | (48) | 934 | 934 | (76) | 858 | ||||
| Bank borrowings | 205 | (1) | 203 | 203 | 498 | 4 | 705 | |||
| Other long-term debt | 16 | 14 | 30 | 30 | (1) | 1 | (1) | 28 | ||
| Short term debt2) | 76 | (25) | (5) | 47 | 47 | 47 | (6) | 1 | 89 | |
| Short-term bank borrowings | 76 | (24) | (5) | 47 | 47 | 47 | (6) | 1 | 89 | |
| Other short-term loans | 1 | (1) | ||||||||
| Forward contracts3) | ||||||||||
| Equity | (1,181) | (2,096) | 1,868 | (1,410) | (1,410) | (593) | 869 | (1,133) | ||
| Dividend payable | (484) | 484 | (418) | 418 | ||||||
| Forward contracts3) | (982) | 48 | (934) | (934) | 76 | (858) | ||||
| Treasury shares | (199) | (1,613) | 1,336 | (476) | (476) | (174) | 375 | (275) | ||
| Total | (2,347) | 500 | ||||||||
24Contingencies
Accounting policies
Contingent liabilities
A contingent liability is a liability of uncertain timing and amount. Contingencies are not recognized in the balance sheet because they are dependent on the occurrence or non-occurrence of one or more uncertain future events not wholly within the control of the company or because the risk of loss is estimated to be possible but not probable or because the amount cannot be measured reliably. Pursuant to IAS 37, Provisions, Contingent Liabilities and Contingent Assets, certain information is not disclosed for legal proceedings for which the company concludes that disclosure can be expected to seriously prejudice the outcome of the matter.
Contingent assets
Contingent assets are disclosed if the inflow of economic benefits is probable, but not virtually certain. If the inflow of economic benefits becomes virtually certain, the asset would be considered no longer contingent and its recognition appropriate. Contingent assets are assessed continually and require management to apply judgment, especially to estimate the likelihood of the inflow of economic benefits.
Financial guarantees
Philips’ policy is to provide guarantees and other letters of support only in writing. Philips does not stand by other forms of support. The company recognizes a liability at the fair value of the obligation at the inception of a financial guarantee contract. The guarantee is subsequently measured at the higher of the best estimate of the obligation or the amount initially recognized less, when appropriate, cumulative amortization.
Accounting estimates and judgments
Significant judgment is required to determine the likelihood of a potential outflow of resources. In addition, judgment is involved in determining whether the amount of an obligation can be measured with sufficient reliability. Contingencies involve inherent uncertainties including, but not limited to, court rulings, negotiations between affected parties, governmental actions, tax and environmental remediation.
Contingent assets
As of December 31, 2022, the company had no material contingent assets.
Guarantees
The total fair value of guarantees recognized on the balance sheet amounts to EUR nil million for both 20222023 and 2021.2022. Remaining off-balance-sheet business related guarantees on behalf of third parties and associates to EUR 2 million in 20222023 (December 31, 2021:2022: EUR 2 million).
Environmental remediation
The company and its subsidiaries are subject to environmental laws and regulations. Under these laws, the company and/or its subsidiaries may be required to remediate the effects of certain manufacturing activities on the environment.
Legal proceedings
The company and certain of its group companies and former group companies are involved as a party in legal proceedings, regulatory and other governmental proceedings, including discussions on potential remedial actions, relating to such matters as competition issues, commercial transactions, product liability, participations, and environmental pollution.
While it is not feasible to predict or determine the outcome of all pending or threatened legal proceedings, regulatory and governmental proceedings, the company is of the opinion that the cases described below may have, or have had in the recent past, a significant impact on the company’s consolidated financial position, results of operations and cash flows.
Public Investigations
TheIn May 2023, the company is engaged in discussionsreached a settlement with and has provided information to, the US Securities and Exchange Commission (SEC) and US Department of Justice (DoJ)to resolve the SEC inquiry regarding alleged tender irregularities in the medical device industry in China, for which the company had recorded a provision of approximately EUR 60 million in 2022. The settlement reached was in line with the amount provided for. In addition, the previously disclosed SEC inquiry regarding alleged similar conduct in Brazil and Bulgaria has been discontinued. The US Department of Justice (DOJ) has closed its parallel inquiry into these matters.
In February 2023, the company received a statement of objections from the French Competition Authority (FCA) initiating a formal investigation to verify whether the company and certain jurisdictions. These interactions are primarily focused onother manufacturers of small domestic appliances breached antitrust rules in France in the period 2009-2014 through the alleged exchange of commercially sensitive information. The company filed its response to the statement of objections denying such allegations in May 2023 and is continuing to defend itself. The FCA is expected to organize a numberhearing and issue its decision in 2024. It is the company’s assessment that it is possible but not probable that this matter could lead to an outflow of compliance findings thateconomic resources. Given the uncertain outcome of the investigation and subsequent proceedings, the company is addressing in Brazil, China and Bulgaria. In connection with these discussions and their status, the company recorded a provision in the amount of EUR 60 million.
Given the significant uncertainty regarding the nature of the relevant events and obligations, Philips is not currently able to reliably estimate the full financial effectimpact, if any, and no provision has been recognized as of a range of possible outcomes in connection with the abovementioned discussions with the SEC and DoJ beyond the recorded provision. The outcomes of these matters could have a material impact on the company’s consolidated financial position, results of operations and cash flows.December 31, 2023.
Respironics field action
On June 14, 2021, Philips’ subsidiary Philips RS North America LLC (Philips Respironics) issued a voluntary recall notification in the United States and field safety notice outside the United States for specific Philips Respironics CPAP, Bi-Level PAP, and mechanical ventilator devices (the “Recalled Devices”).
Consent decree
On August 26, 2021, the US Food and Drug Administration (FDA) commenced an inspection of the Philips Respironics manufacturing facility in Murrysville, Pennsylvania and provided Philips Respironics with its preliminary inspectional observations on November 9, 2021. Philips Respironics responded to the FDA’sFDA's inspectional observations in December 2021, which described the actions already taken by the company, as well as additional planned actions. Philips Respironics is also providing periodic updates to the FDA on its progress for the planned actions. In July 2022, Philips started discussions with the DoJDOJ, acting on behalf of the FDA on a consent decree that would, among other things, address compliance requirements for future sales, the resolution of the inspectional findings and the completion of the recall. AtOn January 29, 2024, Philips announced that it agrees on the endterms of December 2022,a consent decree with the discussions are ongoing.DOJ, representing the FDA. For further details please see Subsequent events.
DoJDOJ investigation
On April 8, 2022, Philips Respironics and certain of Philips’Philips' subsidiaries in the US received a subpoena from the US DoJDOJ to provide information related to events leading to the Respironics recall. The relevant subsidiaries are cooperating with the investigation. The criminal and civil investigation is being conducted by the US DoJ’sDOJ's Consumer Protection Branch and Civil Fraud Section, and the US Attorney’s Office for the Eastern District of Pennsylvania. Given the early stages of the investigation, the company is not able to reliably estimate the financial impact, if any.
Product liability claims
Following the voluntary recall notification, a number of civil complaints have been filed in several jurisdictions against Philips Respironics and certain of its affiliates (including the company) generally alleging economic loss, personal injury and/or the potential for personal injury allegedly caused by devices subject to the recall.Recalled Devices.
In the United States, consumer and commercial class action lawsuits have been filed alleging economic loss and medical monitoring claims. Individual personal injury lawsuits have also been filed. On October 8, 2021, a Multi-District Litigation (MDL) in the US District Court for the Western District of Pennsylvania was formed, and most of these class action and personal injury lawsuits have been consolidated in the MDL for pre-trial proceedings. As of December 31, 2022,2023, plaintiffs have filed a consolidated economic loss class action complaint on behalf of device users, hospitals, and insurers and other third-party payers, a consolidated medical monitoring class action complaint on behalf of device users, and over 300600 individual personal injury complaints. The company anticipates that the number of individual personal injury complaints will continue to increase in 2024.
On September 7, 2023, Philips Respironics reached agreement on a class action settlement in relation to the economic loss class action complaint, for which the company recorded a EUR 575 million provision in the first quarter of 2023. Under the agreement, which was preliminarily approved by the US District Court for the Western District of Pennsylvania on October 10, 2023, the Philips defendants will provide predefined cash awards to all eligible participants in the US depending on the type of device, extended warranties on all remediated devices provided as part of Respironics’ recall program, and an additional cash award if they return the Recalled Device to Philips Respironics. The settlement also provides for compensation for individuals who acquired replacement devices in the market after the recall and prior to the announcement of the settlement. The settlement also provides for compensation to private insurers and other third-party payers. The final cost of the settlement may vary based on, among other things, how many patients and other settlement class members participate in the settlement. The final approval hearing is scheduled for April 11, 2024.
In September 2022, the MDL court established a voluntary, court-approved census registry, and associated tolling, for potential claimants who have not filed claims, but may file claims in the future, relating to the Recalled Devices. The census registry replacesreplaced the private tolling agreement that had been in effect before the establishment of the census registry. At the time of termination, approximately 60,000 individuals had entered into the private tolling agreement. In the event these individuals wish to pursue or preserve their claims, they will need to file a lawsuit or register on the census registry. By December 31, 2022,2023, approximately 13,50057,000 individuals had joined the census registry. The company anticipates that the number of individuals on the census registry will increase in 2023.2024. To better assess the claimed injuries and their relation, if any, to use of the Recalled Devices, Philips Respironics is working to require census registrants to supplement the information they are required to submit in the census registry established by the MDL court.
In Australia, a consumer class action lawsuit alleging personal injury was filed against the company’s subsidiary Philips Electronics Australia Ltd on October 4, 2021. In the course of 2022, the plaintiff in the case has sought leave of the court to discontinue the class action citing that there is insufficient evidence to warrant the continuation of the class action and that since the issue of proceedings, Philips Respironics has been repairing, replacing, or (partially) refunding the devices which are the subject of the recall, meaning that any compensation relating to financial loss would be relatively confined. It is expected thatDuring the process for withdrawal of the case, will be discontinueda new lead plaintiff came forward in the firstsecond half of 2023.2023 and is now continuing the class action.
Philips Respironics and certain of its affiliates (including the company) are also defendants in consumer class action lawsuits in Canada and Israel and collective actions in Chile, France and the Netherlands alleging economic loss and/or personal injury. In Canada, where various class actions had been filed, the court issued a decision on a carriage motion in April 2023, deciding that a class action filed in British Columbia may continue as a nationwide class action while defendants are seeking for all other class actions to be stayed.
While the company believes it is probable that these lawsuits will in the aggregate lead to an outflow of economic resources for Philips Respironics or other Philips entities, given the significant uncertainty regarding the nature of the relevant events and potential obligations, the company is not currently able to reliably estimate the amount of the obligation associated with these various lawsuits. The final outcome of the lawsuits and the cost to resolve them cannot currently be determined due to a number of variables, including uncertainty regarding the ultimate number of claimants and their allegations. Moreover, Philips Respironics has not yet completed its test and research program, for all ofincluding the categories ofadditional testing requested by the FDA, for the Recalled Devices.
For the United States specifically, the relative early stage of the census registry, and lack of clarity around the nature of the specific injury each claimantcensus registrant is claiming and its relation, if any, to use of the Recalled Devices contribute to the uncertainty. In addition, the MDL court has not yet decided several significant motions, including motions to dismiss all of the complaints, and plaintiffs have not yet filed their motionsmotion for class certification in the economic loss and medical monitoring actions.action. Further, while document discovery is still in its early stages, andhas progressed, expert discovery has not yet begun. Moreover, Philips Respironicsbegun, and the Court has not yet completed its test and research program for allbeen asked to decide the question of whether any of the categoriesclaimed injuries could have been caused by use of the Recalled Devices. An adverse outcome with respect to any or all of these lawsuits and/or any future claims could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
The company has product liability insurance in place that it expects to partially cover product liability-related cash outflows. Based on ongoing discussions with certain insurance carriers that took place during 2023, management of the company concluded that the likelihood of cash inflows changed to probable, but (consistent with prior periods) not virtually certain. Given the uncertainties associated with the cash outflows of the above claims and the applicable conditions of insurance coverage, no reliable estimate can be made or disclosed in relation to the expected insurance recovery.
Securities claims
On August 16, 2021, a securities class action complaint was filed against the company, its former CEO and its CFO in the United StatesUS District Court for the Eastern District of New York alleging violations of the Securities Exchange Act of 1934 causing damage to investors. On January 3, 2022, the lead plaintiff in the case filed its amended complaint seeking to represent individuals that purchased Philips shares between February 23, 2016, through November 12, 2021. Following the filing and briefing of the company’s motion to dismiss in the first half of 2022, plaintiff filed a second amended complaint on November 30, 2022, in whichnaming an additional defendant and expanding the alleged damage period was expanded to include certain share price declines that were allegedly based on disclosures made in 2022. The second amended complaint now focuses on share price declines that allegedly occurred as a result of various disclosures starting on April 26, 2021 through October 2022. The company’scompany's motion to dismiss the second amended complaint is duewas filed in the first quarter of 2023. As of December 31, 2023, that motion is still pending with the Court.
OnIn the Netherlands, in addition to the September 11, 2022 the company received a letter from shareholders representative organization European Investors-VEB, ("VEB"). The VEB holds Philipsholding the company and its (former) managing and supervisory directors liable for – inter alia – allegedly failingan alleged failure to make timely disclose price-sensitive informationdisclosures in relation to shareholders regarding indicationsthe Respironics recall, the company received letters from two other parties with similar allegations. As of potential (severe) health risks from the use of Recalled Devices, failing to exercise proper oversight over Philips Respironics and implement and ensure a proper information and risk management structure; providing incorrect or incomplete informationDecember 31, 2023, no formal claims have been filed in the company’s financial disclosures.this respect.
It is the company’s assessment that it is possible but not probable that these cases could lead to a certain outflow of economic resources. The company is not able to reliably estimate the financial impact, if any. An adverse outcome of these cases could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
Other claims
On October 12, 2021, SoClean, a company offering ozone-based cleaning products for sleep devices, filed a lawsuit against the company and certain of its affiliates alleging that the defendants’ statements about the potential adverse effect ozone cleaning may have on the recalled devicesRecalled Devices has significantly damaged its business. Philips believes that the claim is without merit and will vigorously defend itself. MotionsIn November 2023, the Court ruled on one of the motions to dismiss the case were filed in Novemberby defendants and December 2022.partially dismissed some of SoClean’s claims. On January 4, 2024, Philips and its affiliates filed their answer and counterclaims against SoClean and one of its affiliates.
In addition, some of Philips Respironics’ business partners such as distributors and durable medical equipment providers have filed or threatened to file claims alleging economic losses suffered as a consequence of the voluntary recall. In particular, Philips Respironics is engaging with certain of its business partners on the level of compensation they allege to be entitled to under Philips Respironics’ replacement program of the Recalled Devices.
It is the company’s assessment that it is possible but not probable that these cases could lead to a certain outflow of economic resources. The company is not able to reliably estimate the financial impact, if any. In the event of an adverse outcome, these matters could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
To date, other than for the economic loss class action settlement, no provisions have been recorded for the litigation and investigations associated with the Respironics field action.
Other
In the second half of 2023, Electro Medical Systems S.A., a manufacturer of among others medical devices for dental prophylaxis, filed a lawsuit against the company alleging that the company materially breached its duties under a cooperation agreement entered into between the parties in 2016, claiming damages in excess of EUR 300 million, alleging loss of profit and lost increase in brand value. Philips disagrees with the allegations and will vigorously defend itself.
Miscellaneous
For details on other contractual obligations, please refer to liquidity risk in Details of treasury and other financial risks.
27Information on remuneration
Remuneration of the Executive Committee
In 2022,2023, the total remuneration costs relating to the members of the Executive Committee (consisting of 1416 members throughout the year, including the members of the Board of Management) amounted to EUR 32.8 million (2022: EUR 25.6 million (2021:million; 2021: EUR 33.4 million; 2020: EUR 33.2 million) consisting of the elements in the following table.
Philips Group
Remuneration costs of the Executive Committee1)
in EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Base salary/Base compensation | 9,299,794 | 9,598,588 | 9,528,279 | 9,598,588 | 9,528,279 | 8,729,458 |
| Annual incentive2) | 6,726,768 | 5,250,408 | 208,370 | 5,250,408 | 208,370 | 11,405,130 |
| Performance shares3) | 13,153,975 | 12,610,073 | 11,242,581 | 12,610,073 | 11,242,581 | 7,272,815 |
| Stock options | 13,358 | |||||
| Restricted share rights3) | 288,372 | 1,380,644 | 1,191,529 | 1,380,644 | 1,191,529 | 1,907,511 |
| Pension allowances4) | 2,054,570 | 2,107,953 | 1,949,204 | 2,107,953 | 1,949,204 | 1,346,937 |
| Pension scheme costs | 382,513 | 306,694 | 288,179 | 306,694 | 288,179 | 260,554 |
| Other compensation5) | 1,264,908 | 2,104,044 | 1,216,163 | 2,104,044 | 1,216,163 | 1,900,224 |
| Total | 33,170,901 | 33,358,405 | 25,624,305 | 33,358,405 | 25,624,305 | 32,835,987 |
As of December 31, 2022,2023, the members of the Executive Committee (including the members of the Board of Management) held 0 stock options (2021: 184,900; 2020: 193,300)(2022: 0; 2021: 184,900).
Remuneration of the Board of Management
In 2022,2023, the total remuneration costs relating to the members of the Board of Management amounted to EUR 9.9 million (2022: EUR 8.4 million (2021:million; 2021: EUR 10.3 million; 2020: EUR 11.4 million), see the following table.
Philips Group
Remuneration costs of individual members of the Board of Management
in EUR
| base compensation/salary | annual incentive1) | performance shares2) | restricted share rights2) | pension allowances3) | pension scheme costs | other compensation | total costs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2023 | ||||||||||||||||
| R. Jakobs | 1,200,000 | 2,004,480 | 968,922 | 267,798 | 31,891 | 109,256 | 4,582,347 | |||||||||
| A. Bhattacharya | 810,000 | 1,075,939 | 793,429 | 197,133 | 31,891 | 94,516 | 3,002,907 | |||||||||
| M.J. van Ginneken | 630,000 | 846,922 | 614,840 | 125,298 | 31,891 | 53,446 | 2,302,397 | |||||||||
| 2,640,000 | 3,927,341 | 2,377,191 | 590,228 | 95,673 | 257,218 | 9,887,650 | ||||||||||
| base compensation/salary | annual incentive1) | performance shares2) | restricted share rights2) | pension allowances3) | pension scheme costs | other compensation | total costs | |||||||||
| 2022 | ||||||||||||||||
| R. Jakobs4) | 256,438 | 112,737 | - | 57,973 | 6,012 | 11,507 | 444,667 | 256,438 | 112,737 | 57,973 | 6,012 | 11,507 | 444,667 | |||
| F.A. van Houten4) | 1,041,849 | 208,370 | 2,930,068 | - | 444,051 | 22,121 | 42,533 | 4,688,992 | 1,041,849 | 208,370 | 2,930,068 | 444,051 | 22,121 | 42,533 | 4,688,992 | |
| A. Bhattacharya | 806,250 | - | 763,140 | - | 237,250 | 28,133 | 61,308 | 1,896,081 | 806,250 | 763,140 | 237,250 | 28,133 | 61,308 | 1,896,081 | ||
| M.J. van Ginneken | 626,250 | - | 585,490 | - | 141,622 | 28,133 | 35,343 | 1,416,837 | 626,250 | 585,490 | 141,622 | 28,133 | 35,343 | 1,416,837 | ||
| 2,730,788 | 208,370 | 4,391,434 | - | 880,896 | 84,398 | 150,691 | 8,446,577 | 2,730,788 | 208,370 | 4,391,434 | 880,896 | 84,398 | 150,691 | 8,446,577 | ||
| 2021 | ||||||||||||||||
| F.A. van Houten | 1,325,000 | 850,915 | 2,626,295 | - | 565,403 | 27,462 | 57,224 | 5,452,299 | 1,325,000 | 850,915 | 2,626,295 | 565,403 | 27,462 | 57,224 | 5,452,299 | |
| A. Bhattacharya | 790,000 | 360,103 | 1,172,533 | - | 233,857 | 27,462 | 68,908 | 2,652,864 | 790,000 | 360,103 | 1,172,533 | 233,857 | 27,462 | 68,908 | 2,652,864 | |
| M.J. van Ginneken | 605,000 | 317,192 | 886,035 | - | 150,755 | 27,462 | 42,610 | 2,029,054 | 605,000 | 317,192 | 886,035 | 150,755 | 27,462 | 42,610 | 2,029,054 | |
| 2,720,000 | 1,528,211 | 4,684,863 | - | 950,014 | 82,387 | 168,742 | 10,134,217 | 2,720,000 | 1,528,211 | 4,684,863 | 950,014 | 82,387 | 168,742 | 10,134,217 | ||
| 2020 | ||||||||||||||||
| F.A. van Houten | 1,325,000 | 1,298,500 | 2,874,467 | - | 565,922 | 27,001 | 62,176 | 6,153,067 | ||||||||
| A. Bhattacharya | 785,000 | 596,600 | 1,295,996 | - | 233,126 | 27,001 | 70,267 | 3,007,990 | ||||||||
| M.J. van Ginneken | 580,000 | 437,920 | 952,453 | - | 158,800 | 27,001 | 46,986 | 2,203,160 | ||||||||
| 2,690,000 | 2,333,020 | 5,122,916 | - | 957,849 | 81,004 | 179,428 | 11,364,217 | |||||||||
The accumulated annual pension entitlements and the pension costs of individual members of the Board of Management are as follows:
Philips Group
Accumulated annual pension entitlements and pension-related costs
in EUR unless otherwise stated
| age at December 31, 2022 | accumulated annual pension as of December 31, 2022 | total pension related costs | age at December 31, 2023 | accumulated annual pension as of December 31, 2023 | total pension related costs | |
|---|---|---|---|---|---|---|
| R. Jakobs | 48 | 53,175 | 63,985 | 49 | 56,383 | 299,689 |
| A. Bhattacharya | 61 | 37,446 | 265,383 | 62 | 40,324 | 229,024 |
| M.J. van Ginneken | 49 | 50,614 | 169,755 | 50 | 53,769 | 157,189 |
| Pension costs | 965,294 | 685,901 | ||||
When pension rights are granted to members of the Board of Management, necessary payments (if insured) and all necessary provisions are made in accordance with the applicable accounting principles. In 2022,2023, no (additional) pension benefits were granted to former members of the Board of Management.
Remuneration of the Supervisory Board
The remuneration of the members of the Supervisory Board amounted to EUR 1.5 million (2021:(2022: EUR 1.31.5 million; 2020:2021: EUR 1.3 million). Former members received no remuneration.
The members of the Supervisory Board do not receive any share-based remuneration. Therefore, as of December 31, 20222023 the members of the Supervisory Board held no stock options, performance shares or restricted shares.
The individual members of the Supervisory Board received, by virtue of the positions they held, the following remuneration:
Philips Group
Remuneration of the Supervisory Board
in EUR
| membership | committees | other compensation1) | total | |||||
|---|---|---|---|---|---|---|---|---|
| 2023 | ||||||||
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 | ||||
| P.A.M. Stoffels | 115,000 | 35,000 | 22,269 | 172,269 | ||||
| D.E.I. Pyott | 100,000 | 35,000 | 19,769 | 154,769 | ||||
| A.M. Harrison | 100,000 | 14,000 | 19,769 | 133,769 | ||||
| M.E. Doherty | 100,000 | 27,000 | 27,269 | 154,269 | ||||
| P. Löscher | 100,000 | 32,000 | 17,269 | 149,269 | ||||
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 | ||||
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 | ||||
| H. Verhagen | 100,000 | 14,000 | 7,269 | 121,269 | ||||
| S. Poonen | 100,000 | 18,000 | 19,769 | 137,769 | ||||
| membership | committees | other compensation1) | total | 1,070,000 | 242,000 | 189,266 | 1,501,266 | |
| 2022 | ||||||||
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 | 155,000 | 35,000 | 16,345 | 206,345 |
| P.A.M. Stoffels | 115,000 | 35,000 | 27,269 | 177,269 | 115,000 | 35,000 | 27,269 | 177,269 |
| N. Dhawan | 35,616 | 6,411 | 5,808 | 47,836 | 35,616 | 6,411 | 5,808 | 47,836 |
| D.E.I. Pyott | 100,000 | 35,000 | 17,269 | 152,269 | 100,000 | 35,000 | 17,269 | 152,269 |
| A.M. Harrison | 100,000 | 14,000 | 12,269 | 126,269 | 100,000 | 14,000 | 12,269 | 126,269 |
| M.E. Doherty | 100,000 | 27,000 | 24,769 | 151,769 | 100,000 | 27,000 | 24,769 | 151,769 |
| P. Löscher | 100,000 | 32,000 | 24,769 | 156,769 | 100,000 | 32,000 | 24,769 | 156,769 |
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 | 100,000 | 14,000 | 17,269 | 131,269 |
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 | 100,000 | 18,000 | 22,269 | 140,269 |
| H. Verhagen | 100,000 | 14,000 | 7,269 | 121,269 | 100,000 | 14,000 | 7,269.0 | 121,269 |
| S. Poonen | 100,000 | 18,000 | 17,269 | 135,269 | 100,000 | 18,000 | 17,269 | 135,269 |
| 1,105,616 | 248,411 | 192,574 | 1,546,602 | 1,105,616 | 248,411 | 192,574 | 1,546,602 | |
| 2021 | ||||||||
| J. van der Veer | 53,507 | 12,082 | 3,916 | 69,505 | 53,507 | 12,082 | 3,916 | 69,505 |
| C.A. Poon | 39,699 | 16,915 | 783 | 57,397 | 39,699 | 16,915 | 783 | 57,397 |
| N. Dhawan | 100,000 | 18,000 | 2,269 | 120,269 | 100,000 | 18,000 | 2,269 | 120,269 |
| O. Gadiesh | 34,521 | 4,833 | 783 | 40,137 | 34,521 | 4,833 | 783 | 40,137 |
| D.E.I. Pyott | 100,000 | 36,370 | 2,269 | 138,639 | 100,000 | 36,370 | 2,269 | 138,639 |
| P.A.M. Stoffels | 109,863 | 27,808 | 4,769 | 142,440 | 109,863 | 27,808 | 4,769 | 142,440 |
| A.M. Harrison | 100,000 | 14,000 | 2,269 | 116,269 | 100,000 | 14,000 | 2,269 | 116,269 |
| M.E. Doherty | 100,000 | 27,000 | 4,769 | 131,769 | 100,000 | 27,000 | 4,769 | 131,769 |
| P. Löscher | 100,000 | 32,000 | 4,769 | 136,769 | 100,000 | 32,000 | 4,769 | 136,769 |
| F. Sijbesma | 141,301 | 27,808 | 8,237 | 177,346 | 141,301 | 27,808 | 8,237 | 177,346 |
| I. Nooyi | 100,000 | 14,000 | 2,269 | 116,269 | 100,000 | 14,000 | 2,269 | 116,269 |
| S.K. Chua | 65,753 | 11,836 | 1,492 | 79,081 | 65,753 | 11,836 | 1,492 | 79,081 |
| 1,044,644 | 242,652 | 38,595 | 1,325,891 | 1,044,644 | 242,652 | 38,595 | 1,325,891 | |
| 2020 | ||||||||
| J. van der Veer | 155,000 | 35,000 | 11,345 | 201,345 | ||||
| C.A. Poon | 115,000 | 49,000 | 7,269 | 171,269 | ||||
| P. Löscher | 66,667 | 21,333 | 1,513 | 89,513 | ||||
| F. Sijbesma | 76,667 | 9,333 | 1,513 | 87,513 | ||||
| N. Dhawan | 100,000 | 18,000 | 7,269 | 125,269 | ||||
| O. Gadiesh | 100,000 | 14,000 | 2,269 | 116,269 | ||||
| D.E.I. Pyott | 100,000 | 42,000 | 12,269 | 154,269 | ||||
| P.A.M. Stoffels | 100,000 | 9,333 | 9,769 | 119,102 | ||||
| A.M. Harrison | 100,000 | 14,000 | 2,269 | 116,269 | ||||
| M.E. Doherty | 100,000 | 24,000 | 9,769 | 133,769 | ||||
| 1,013,333 | 236,000 | 65,254 | 1,314,587 | |||||
Supervisory Board members’ and Board of Management members’ interests in Philips shares
Members of the Supervisory Board and of the Board of Management are prohibited from writing call and put options or similar derivatives of Philips securities.
| December 31, 2021 | December 31, 2022 | December 31, 2022 | December 31, 2023 | |
|---|---|---|---|---|
| R. Jakobs | 101,156 | 109,422 | 109,422 | 126,809 |
| F.A. van Houten | 525,761 | 578,840 | ||
| A. Bhattacharya | 148,365 | 169,517 | 169,517 | 177,088 |
| M.J. van Ginneken | 110,528 | 123,914 | 123,914 | 129,447 |
| P. Stoffels | - | 17,000 | 17,000 | 17,759 |
| S. Poonen | - | 3,000 | 3,000 | 3,133 |
| I. Nooyi | - | 3,100 | 3,100 | 3,238 |
| D. Pyott | - | 19,000 | 19,000 | 19,848 |
| S.K. Chua | - | 2,000 | 2,000 | 2,089 |
| F. Sijbesma | - | 12,500 | 12,500 | 25,000 |
| M. Harrison | - | 1,500 | 1,500 | 1,567 |
| P. Löscher | - | 20,732 | 20,732 | 21,658 |
28Fair value of financial assets and liabilities
Accounting policies
Fair value hierarchy
For financial reporting purposes, financial instruments are categorized into Level 1, 2 or 3, based on the degree to which the inputs to the fair value measurements are observable and the significance of the inputs to the fair value measurement in its entirety, which are as follows:
- Level 1 – inputs are quoted prices (unadjusted) for identical assets or liabilities in active markets that the company can access at the measurement date.
- Level 2 – all significant inputs (other than quoted prices included within Level 1) are observable for the asset or liability, either directly (as prices) or indirectly (derived from prices).
- Level 3 – one or more of the significant inputs are not based on observable market data, such as third-party pricing information without adjustments, for the asset or liability.
Transfers between levels of the fair value hierarchy are recognized at the end of the reporting period during which the change has occurred.
Offsetting and master netting agreements
Financial assets and liabilities are offset and the net amount is reported in the balance sheet when, and only when, the company has currently a legally enforceable right to set-off the amounts and the group intends either to settle them on a net basis or to realize the asset and settle the liability simultaneously.
Accounting estimates and judgments
Determining the fair value of financial instruments requires the use of estimates according to the method applied for each type of financial asset of liability. The estimated fair value of financial instruments has been determined by the company using available market information and appropriate valuation methods. The estimates presented are not necessarily indicative of the amounts that will ultimately be realized by the company upon maturity or disposal. The use of different market assumptions and/or estimation methods may have a material effect on the estimated fair value amounts.
Specific valuation techniques used to value financial instruments include:
Level 1
Instruments included in level 1 are comprised primarily of listed equity investments classified as financial assets carried at fair value through profit or loss or carried at fair value through other comprehensive income. The fair value of financial instruments traded in active markets is based on quoted market prices at the balance sheet date. A market is regarded as active if quoted prices are readily and regularly available from an exchange, dealer, broker, industry group, pricing service, or regulatory agency, and those prices represent actual and regularly occurring market transactions on an arm’s length basis.
Level 2
The fair value of financial instruments that are not traded in an active market (for example, over-the-counter derivatives or convertible bond instruments) is determined by using valuation techniques. These valuation techniques maximize the use of observable market data where it is available and rely as little as possible on entity-specific estimates. If all significant inputs required to fair value an instrument are based on observable market data, the instrument is included in level 2. The fair value of derivatives is calculated as the present value of the estimated future cash flows based on observable interest yield curves, basis spread and foreign exchange rates. The valuation of convertible bond instruments uses observable market quoted data for the options and present value calculations using observable yield curves for the fair value of the bonds.
Level 3
If one or more of the significant inputs are not based on observable market data, such as third-party pricing information without adjustments, the instrument is included in level 3.
The fair value of debt is estimated on the basis of the quoted market prices for certain issuances, or on the basis of discounted cash flow analysis using market rates plus Philips’ spread for the particular tenors of the borrowing arrangement. Accrued interest is not included within the carrying amount or estimated fair value of debt.
Level 3
If one or more of the significant inputs are not based on observable market data, such as third-party pricing information without adjustments, the instrument is included in level 3.
The fair value of contingent consideration is dependent on the terms of the respective acquisition agreement that may require Philips to pay additional consideration to former shareholders if specified future events occur or conditions are met, such as the achievement of certain regulatory milestones or the achievement of certain commercial milestones. The fair value of the contingent consideration provision is generally determined using a probability-weighted and a risk-adjusted approach to estimate the achievement of future regulatory and commercial milestones, respectively. The discount rates used in the risk adjusted approach reflect the inherent risk related to achieving the commercial milestones. Both regulatory and commercial milestones are discounted for the time value of money at risk-free rates. The fair value measurement is based on management’s estimates and assumptions and hence classified as Level 3 in the fair value hierarchy.
The following tables show the carrying amounts and fair values of financial assets and financial liabilities, including their levels in the fair value hierarchy. Fair value information for financial assets and financial liabilities not carried at fair value is not included if the carrying amount is a reasonable approximation of fair value.
Philips Group
Fair value of financial assets and liabilities
in millions of EUR
| carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| December 31, 2022 | ||||||||||
| December 31, 2023 | ||||||||||
| Financial assets | ||||||||||
| Carried at fair value: | ||||||||||
| Debt instruments | 232 | 232 | 232 | 226 | 226 | |||||
| Equity instruments | 4 | 4 | 1 | 2 | 2 | 2 | ||||
| Other financial assets | 86 | 86 | 35 | 51 | 56 | 34 | 22 | |||
| Financial assets carried at FVTP&L | 322 | 322 | 1 | 35 | 285 | 284 | 34 | 250 | ||
| Debt instruments | 25 | 25 | 25 | 27 | 26 | |||||
| Equity instruments | 259 | 259 | 30 | 229 | 231 | 14 | 217 | |||
| Current financial assets | 9 | 9 | 9 | 3 | 3 | |||||
| Receivables - current | 26 | 26 | 26 | 32 | 32 | |||||
| Financial assets carried at FVTOCI | 319 | 319 | 30 | 25 | 264 | 293 | 14 | 26 | 253 | |
| Derivative financial instruments | 127 | 127 | 127 | 48 | 48 | |||||
| Financial assets carried at fair value | 768 | 768 | 32 | 187 | 549 | 624 | 14 | 108 | 503 | |
| Carried at (amortized) cost: | ||||||||||
| Cash and cash equivalents | 1,172 | 1,869 | ||||||||
| Loans and receivables: | ||||||||||
| Current loans receivables | 2 | - | ||||||||
| Other non-current loans and receivables | 54 | 77 | ||||||||
| Receivables - current | 4,088 | 3,701 | ||||||||
| Receivables - non-current | 279 | 193 | ||||||||
| Financial assets carried at (amortized) cost | 5,596 | 5,840 | ||||||||
| Total financial assets | 6,364 | 6,465 | ||||||||
| Financial liabilities | ||||||||||
| Carried at fair value: | ||||||||||
| Contingent consideration | (113) | (113) | (113) | (115) | (115) | |||||
| Financial liabilities carried at FVTP&L | (113) | (113) | (113) | (115) | (115) | |||||
| Derivative financial instruments | (211) | (211) | (211) | (43) | (43) | |||||
| Financial liabilities carried at fair value | (324) | (324) | (211) | (113) | (158) | (43) | (115) | |||
| Carried at (amortized) cost: | ||||||||||
| Accounts payable | (1,968) | (1,917) | ||||||||
| Interest accrual | (71) | (76) | ||||||||
| Debt (Corporate bonds and leases) | (6,520) | (6,083) | (5,001) | (1,082) | (6,969) | (6,798) | (5,724) | (1,074) | ||
| Debt (excluding corporate bonds and leases) | (1,680) | (721) | ||||||||
| Financial liabilities carried at (amortized) cost | (10,240) | (9,682) | ||||||||
| Total financial liabilities | (10,564) | (9,840) | ||||||||
Philips Group
Fair value of financial assets and liabilities
in millions of EUR
| carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| December 31, 2021 | ||||||||||
| December 31, 2022 | ||||||||||
| Financial assets | ||||||||||
| Carried at fair value: | ||||||||||
| Debt instruments | 233 | 233 | 233 | 232 | 232 | |||||
| Equity instruments | 4 | 4 | 4 | 4 | 1 | 2 | ||||
| Other financial assets | 46 | 46 | 34 | 12 | 86 | 35 | 51 | |||
| Financial assets carried at FVTP&L | 283 | 283 | 4 | 34 | 245 | 322 | 1 | 35 | 285 | |
| Debt instruments | 27 | 27 | 27 | 25 | 25 | |||||
| Equity instruments | 273 | 273 | 63 | 210 | 259 | 30 | 229 | |||
| Current financial assets | - | - | 9 | 9 | ||||||
| Receivables - current | 68 | 68 | 68 | 26 | 26 | |||||
| Financial assets carried at FVTOCI | 368 | 368 | 63 | 27 | 278 | 319 | 30 | 25 | 264 | |
| Derivative financial instruments | 63 | 63 | 63 | 127 | 127 | |||||
| Financial assets carried at fair value | 714 | 714 | 67 | 124 | 523 | 768 | 32 | 187 | 549 | |
| Carried at (amortized) cost: | ||||||||||
| Cash and cash equivalents | 2,303 | 1,172 | ||||||||
| Loans and receivables: | ||||||||||
| Current loans receivables | 2 | 2 | ||||||||
| Other non-current loans and receivables | 47 | 54 | ||||||||
| Receivables - current | 3,720 | 4,088 | ||||||||
| Receivables - non-current | 224 | 279 | ||||||||
| Financial assets carried at (amortized) cost | 6,296 | 5,596 | ||||||||
| Total financial assets | 7,010 | 6,364 | ||||||||
| Financial liabilities | ||||||||||
| Carried at fair value: | ||||||||||
| Contingent consideration | (208) | (208) | (208) | (113) | (113) | |||||
| Financial liabilities carried at FVTP&L | (208) | (208) | (208) | (113) | (113) | |||||
| Derivative financial instruments | (202) | (202) | (202) | (211) | (211) | |||||
| Financial liabilities carried at fair value | (410) | (410) | (202) | (208) | (324) | (211) | (113) | |||
| Carried at (amortized) cost: | ||||||||||
| Accounts payable | (1,872) | (1,968) | ||||||||
| Interest accrual | (52) | (71) | ||||||||
| Debt (Corporate bonds and leases) | (5,765) | (6,396) | (5,177) | (1,220) | (6,520) | (6,083) | (5,001) | (1,082) | ||
| Debt (excluding corporate bonds and leases) | (1,214) | (1,680) | ||||||||
| Financial liabilities carried at (amortized) cost | (8,904) | (10,240) | ||||||||
| Total financial liabilities | (9,314) | (10,564) | ||||||||
The following table shows the reconciliation from the beginning balance to the end balance for Level 3 fair value measurements.
Philips Group
Reconciliation of Level 3 fair value measurements
in millions of EUR
| Financial assets | Financial liabilities | Financial assets | Financial liabilities | |
|---|---|---|---|---|
| Balance as of January 1, 2022 | 523 | 208 | ||
| Balance as of January 1, 2023 | 549 | 113 | ||
| Acquisitions | 96 | 6 | ||
| Purchase | 131 | 85 | ||
| Sales | (76) | (56) | ||
| Utilizations | (105) | (20) | ||
| Recognized in profit and loss: | ||||
| other business income | (85) | 16 | ||
| financial income and expenses1) | 7 | (8) | (43) | 1 |
| Recognized in other comprehensive income2) | 8 | (40) | (2) | |
| Receivables held to collect and sell | (41) | 6 | ||
| Reclassification | 5 | 1 | ||
| Balance as of December 31, 2022 | 549 | 113 | ||
| Balance as of December 31, 2023 | 503 | 115 | ||
Philips Group
Reconciliation of Level 3 fair value measurements
in millions of EUR
| Financial assets | Financial liabilities | Financial assets | Financial liabilities | |
|---|---|---|---|---|
| Balance as of January 1, 2021 | 411 | 318 | ||
| Balance as of January 1, 2022 | 523 | 208 | ||
| Acquisitions | 16 | 96 | ||
| Purchase | 113 | 131 | ||
| Sales | (122) | (76) | ||
| Utilizations | (48) | (105) | ||
| Recognized in profit and loss: | ||||
| other business income | (87) | (85) | ||
| financial income and expenses | 98 | 1 | 7 | (8) |
| Recognized in other comprehensive income1) | 12 | 9 | 8 | |
| Receivables held to collect and sell | (25) | (41) | ||
| Reclassification from associates | 36 | 5 | ||
| Balance as of December 31, 2021 | 523 | 208 | ||
| Balance as of December 31, 2022 | 549 | 113 | ||
Offsetting and master netting agreements
Transactions in derivatives are subject to master netting and set-off agreements. In the case of certain termination events, under the terms of the master agreement, Philips can terminate the outstanding transactions and aggregate their positive and negative values to arrive at a single net termination sum (or close-out amount). This contractual right is subject to the following:
- The right may be limited by local law if the counterparty is subject to bankruptcy proceedings.
- The right applies on a bilateral basis.
Philips Group
Financial assets subject to offsetting, enforceable master netting arrangements or similar agreements
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Derivatives | ||||
| Gross amounts of recognized financial assets | 63 | 127 | 127 | 48 |
| Gross amounts of recognized financial liabilities offset in the balance sheet | ||||
| Net amounts of financial assets presented in the balance sheet | 63 | 127 | 127 | 48 |
| Related amounts not offset in the balance sheet | ||||
| Financial instruments | (47) | (54) | (54) | (34) |
| Net amount | 17 | 73 | 73 | 13 |
Philips Group
Financial liabilities subject to offsetting, enforceable master netting arrangements or similar agreements
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Derivatives | ||||
| Gross amounts of recognized financial liabilities | (202) | (211) | (211) | (43) |
| Gross amounts of recognized financial assets offset in the balance sheet | ||||
| Net amounts of financial liabilities presented in the balance sheet | (202) | (211) | (211) | (43) |
| Related amounts not offset in the balance sheet | ||||
| Financial instruments | 47 | 54 | 54 | 34 |
| Net amount | (155) | (157) | (157) | (9) |
29Details of treasury and other financial risks
Accounting policies
Derivative financial instruments, including hedge accounting
The company uses derivative financial instruments principally to manage its foreign currency risks and, to a more limited extent, interest rate and commodity price risks. All derivative financial instruments are accounted for at the trade date and classified as current or non-current assets or liabilities based on the maturity date or the early termination date. The company measures all derivative financial instruments at fair value that is derived from the market prices of the instruments, calculated on the basis of the present value of the estimated future cash flows based on observable interest yield curves, basis spread, credit spreads and foreign exchange rates, or derived from option pricing models, as appropriate. Gains or losses arising from changes in fair value of derivatives are recognized in the Consolidated statements of income, except for derivatives that are highly effective and qualify for cash flow or net investment hedge accounting.
Changes in the fair value of foreign exchange forward contracts attributable to forward points and changes in the time value of the option contracts are deferred in the cash flow hedges reserve within equity. The deferred amounts are recognized in the Consolidated statements of income against the related hedged transaction when it occurs.
Changes in the fair value of a derivative that is highly effective and that is designated and qualifies as a cash flow hedge are recorded in OCI until the Consolidated statements of income are affected by the variability in cash flows of the designated hedged item. To the extent that the hedge is ineffective, changes in the fair value are recognized in the Consolidated statements of income.
The company formally assesses, both at the hedge’s inception and on an ongoing basis, whether the derivatives that are used in hedging transactions are highly effective in offsetting changes in fair values or cash flows of hedged items. When it is established that a derivative is not highly effective as a hedge or that it has ceased to be a highly effective hedge, the company discontinues hedge accounting prospectively. When hedge accounting is discontinued because it is expected that a forecasted transaction will not occur, the company continues to carry the derivative on the Consolidated balance sheets at its fair value, and gains and losses that were accumulated in OCI are recognized immediately in the same line item as they relate to in the Consolidated statements of income.
Foreign currency differences arising upon retranslation of financial instruments designated as a hedge of a net investment in a foreign operation are recognized directly in the currency translation differences reserve through OCI, to the extent that the hedge is effective. To the extent that the hedge is ineffective, such differences are recognized in the Consolidated statements of income.
Accounting estimates and judgments
Financial assets are subject to impairment assessment, which involves estimating expected credit losses. Refer to Other financial assets for accounting policies on impairment of financial assets.
Philips is exposed to several types of financial risks which are further analyzed below. Philips does not purchase or hold derivative financial instruments for speculative purposes. Information regarding financial instruments is included in Fair value of financial assets and liabilities.
Liquidity risk
Liquidity risk is the risk that an entity will encounter difficulty in meeting obligations associated with financial liabilities.
Liquidity risk for the group is monitored through the Treasury liquidity committee, which tracks the development of the actual cash flow position for the group and uses input from a number of sources in order to forecast the overall liquidity position on both a short and longer term basis. Philips invests surplus cash in short-term deposits with appropriate maturities to ensure sufficient liquidity is available to meet liabilities when due and in money market funds.
The rating of the company’s debt by major rating agencies may improve or deteriorate. As a result, Philips’ future borrowing capacity may be influenced and its financing costs may fluctuate. Philips has various sources to mitigate the liquidity risk for the group. As of December 31, 2022,2023, Philips had EUR 1,1721,869 million in cash and cash equivalents (2021:(2022: EUR 2,3031,172 million), within which short-term deposits of EUR 4821,399 million (2021:(2022: EUR 1,357482 million). Cash and cash equivalents include all cash balances, money market funds and short-term highly liquid investments with an original maturity of three months or less that are readily convertible into known amounts of cash. Philips pools cash from subsidiaries to the extent legally and economically feasible; cash not pooled remains available for the company’s operational or investment needs.
Philips faces cross-border foreign exchange controls and/or other legal restrictions in a few countries that could limit its ability to make these balances available on short notice for general use by the group.
Philips has a USD 2.5 billion Commercial Paper Program and a EUR 1 billion committed standby revolving credit facility that can be used for general group purposes, such as a backstop for its Commercial Paper Program. As of December 31, 2022,2023, Philips did not have any loans outstanding under either facility. These facilities do not have a material adverse change clause, have no financial covenants and no credit-rating-related acceleration possibilities. Philips issued commercial paper of EUR 200 million in September 2022 and EUR 101 million in October 2022, that was repaid throughout the fourth quarter of 2022. In addition, Philips secured a EUR 1 billion credit facility in the fourth quarter of 2022 that can be used for general corporate purposes. As of December 31, 2022, Philips had EUR 500 million outstanding under the credit facility. The facility does not have a material adverse change clause, has no financial covenants and no credit-rating-related acceleration possibilities. As per March 9, 2020, Philips established a Euro Medium-Term Note (EMTN) program, a framework that facilitates the issuance of notes for a total amount up to EUR 10 billion. In 2022,As of December 31, 2023, Philips has EUR 3.3 billion outstanding under this program of which EUR 500 million fixed rates notes were issued three new tranches under the program for a total of EUR 2 billion, while also redeeming its outstandingin August 2023 and 2024 Notes and issuing a tender offer on the outstanding 2025 and 2026 Notes.with maturity date in 2031. For a description of Philips’ credit facilities, refer to Debt.
In addition to cash and cash equivalents, as of December 31, 2022,2023, Philips also held EUR 3214 million of listed (level 1) equity investments at fair value (classified as other non-current financial assets).
The following table presents a summary of the Group’s fixed contractual cash obligations and commitments as of December 31, 2022.2023. These amounts are an estimate of future payments which could change as a result of various factors such as a change in interest rates, foreign exchange, contractual provisions, as well as changes in business strategy and needs. Therefore, the actual payments made in future periods may vary from those presented in the following table:
| payments due by period | payments due by period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| total | less than 1 year | 1-3 years | 3-5 years | after 5 years | total | less than 1 year | 1-3 years | 3-5 years | after 5 years | |
| Long-term debt | 8,168 | 842 | 1,760 | 1,809 | 3,757 | 7,615 | 533 | 1,934 | 1,431 | 3,717 |
| Short-term debt | 89 | 122 | ||||||||
| Interest on debt | 1,683 | 159 | 304 | 264 | 956 | 1,704 | 180 | 328 | 285 | 911 |
| Derivative liabilities | 210 | 208 | 2 | 39 | 38 | 1 | ||||
| Purchase obligations3) | 782 | 336 | 412 | 21 | 12 | 668 | 355 | 286 | 27 | |
| Trade and other payables | 1,968 | 1,917 | ||||||||
| Contractual cash obligations | 12,901 | 3,603 | 2,478 | 2,094 | 4,725 | 12,065 | 3,145 | 2,549 | 1,716 | 4,655 |
Philips has contracts with investment funds where it committed itself to make, under certain conditions, capital contributions to these funds of an aggregated remaining amount of EUR 127153 million (2021:(2022: EUR 116127 million). As of December 31, 20222023 capital contributions already made to these investment funds are recorded as non-current financial assets.
Philips offers voluntary supply chain finance programs with third parties which provide participating suppliers the opportunity to factor their trade receivables at the sole discretion of both the suppliers and the third parties. Philips continues to recognize these liabilities as trade payables and settles them accordingly on the invoice maturity date based on the terms and conditions theseof those arrangements. As of December 31, 20222023 approximately EUR 114 million (2022: EUR 151 million (2021: EUR 139 million)of the Philips account payable were transferred under these arrangements.
With respect to the Respironics field action, please refer to Contingencies. The management continues to monitor the risks associated with such potential claims and its impact on liquidity position, if any.
Leasing activities
The company leases various items of real estate, vehicles and other equipment where it acts as a lessee. The company has multiple extension and termination options in a number of lease contracts. These are used to maximize operational flexibility in terms of managing the assets used in the company's operations. The options considered reasonably certain are part of lease liabilities. However, the options not considered reasonably certain are not part of lease liability, which exposes the company to potential future cash outflows amounting to EUR 400 million. In addition, the company is committed to leases not yet commenced to EUR 93128 million. The company's lease contracts do not contain financial covenants.
The company enters into sale-and-leaseback transactions primarily for its Sleep & Respiratory Care businesses. These transactions are accounted for at market value. The payments for these leases are considered in determining lease liabilities. Principal repayments are part of cash flows used for financing activities and interest payments are part of cash flows used for operating activities. The cash inflows arising from the sales transactions are part of cash flows provided by financing activities. Lease payments under sale-and-leaseback arrangements for 20222023 were EUR 7255 million (2021:(2022: EUR 8572 million). The remaining minimum payment under sale-and-leaseback arrangements included in lease obligations above are as follows:
Philips Group
Remaining minimum payments under sale-and-leaseback arrangements
in millions of EUR
| 2023 | 55 | |
| 2024 | 38 | 43 |
| 2025 | 23 | 30 |
| 2026 | 14 | 21 |
| 2027 | 5 | 12 |
| 2028 | 5 | |
| Thereafter | 18 | 26 |
Philips has leasing activities where it acts as lessor. In such arrangements, Philips provides the customer with a right to use of medical equipment in exchange for a series of payments. Residual values of assets under lease form an insignificant part of the carrying amount of those assets. Residual values are influenced by asset market prices and are therefore subject to management estimation. Residual values are at least reassessed on an annual basis, or more often when necessary. Reassessments are based on a combination of realization of assets sold, expert knowledge and judgment of local markets. For lease receivables, the value of unguaranteed residual values as of December 31, 20222023 was EUR 0.60.0 million (2021:(2022: EUR 0.20.6 million). In order to reduce residual value risk exposures there may be residual value guarantees or purchase options embedded in the customer contract. Credit risk for lease receivables is reviewed regularly and mitigated, for example, by retaining a security interest in the leased asset.
Currency risk
Currency risk is the risk that reported financial performance or the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. Philips operates in many countries and currencies and therefore currency fluctuations may impact Philips’ financial results. Philips is exposed to currency risk in the following areas:
- Transaction exposures, related to anticipated sales and purchases and on-balance-sheet receivables/payables resulting from such transactions
- Translation exposure of foreign-currency intercompany and external debt and deposits
- Translation exposure of net income in foreign entities
- Translation exposure of foreign-currency-denominated equity invested in consolidated companies
- Translation exposure to equity interests in non-functional-currency investments in associates and other non-current financial assets.
It is Philips’ policy to reduce the potential year-on-year volatility caused by foreign-currency movements on its net earnings by hedging the anticipated net exposure of foreign currencies resulting from foreign-currency sales and purchases. In general, net anticipated exposures for the Group are hedged during a period of 15 months in layers of 20% up to a maximum hedge of 80%. Philips’ policy requires significant committed foreign currency exposures to be fully hedged, generally using forwards. However, not every foreign currency can or shall be hedged as there may be regulatory barriers or prohibitive hedging cost preventing Philips from effectively and/or efficiently hedging its currency exposures. As a result, hedging activities cannot and will not eliminate all currency risks for anticipated and committed transaction exposures.
The following table outlines the estimated nominal value in millions of EUR for committed and anticipated transaction exposure and related hedges for Philips’ most significant currency exposures consolidated as of December 31, 2022:2023:
Philips Group
Estimated transaction exposure and related hedges
in millions of EUR
| Sales/Receivables | Purchases/Payable | Sales/Receivables | Purchases/Payable | |||||
|---|---|---|---|---|---|---|---|---|
| exposure | hedges | exposure | hedges | exposure | hedges | exposure | hedges | |
| Balance as of December 31, 2022 | ||||||||
| Balance as of December 31, 2023 | ||||||||
| Exposure currency | ||||||||
| USD | 1,754 | (1,530) | (979) | 936 | 1,793 | (1,449) | (888) | 805 |
| JPY | 479 | (289) | (9) | 9 | 547 | (319) | (14) | 14 |
| GBP | 303 | (188) | (7) | 7 | 312 | (196) | (12) | 12 |
| CNY | 346 | (259) | (80) | 79 | 439 | (304) | (98) | 95 |
| CAD | 203 | (138) | 249 | (161) | (1) | 1 | ||
| PLN | 65 | (62) | 91 | (103) | ||||
| AUD | 139 | (92) | (1) | 1 | 226 | (137) | ||
| CHF | 132 | (56) | (3) | 2 | 103 | (63) | (1) | 1 |
| CZK | 48 | (50) | 63 | (70) | ||||
| SEK | 55 | (17) | (1) | 1 | 33 | (18) | (3) | 3 |
| RUB | 192 | (192) | (129) | 129 | ||||
| EUR | 233 | (232) | (114) | 113 | ||||
| Others | 64 | (46) | (259) | 162 | 198 | (134) | (216) | 130 |
| Total 2023 | 4,287 | (3,185) | (1,346) | 1,173 | ||||
| Total 2022 | 3,779 | (2,920) | (1,468) | 1,326 | 3,779 | (2,920) | (1,468) | 1,326 |
| Total 2021 | 5,131 | (3,363) | (1,559) | 1,322 | ||||
Philips uses foreign exchange spot and forward contracts, as well as zero cost collars in hedging the exposure. The derivatives related to transactions are, for hedge accounting purposes, split into hedges of on-balance-sheet accounts receivable/ payable and forecasted sales and purchases. Changes in the value of on-balance-sheet foreign-currency accounts receivable/payable, as well as the changes in the fair value of the hedges related to these exposures, are reported in the income statement under costs of sales. The RUB as shown in the table above was hedged for part of the year till Q2 2022. Hedges related to forecasted transactions, where hedge accounting is applied, are accounted for as cash flow hedges. The results from such hedges are deferred in other comprehensive income within equity to the extent that the hedge is effective. As of December 31, 2022,2023, a lossgain of EUR 26 million was deferred in equity as a result of these hedges (2021:(2022: EUR 252 million loss). The result deferred in equity will be released to earnings mostly during 20232024 at the time when the related hedged transactions affect the income statement. During 2022,2023, nil (2022: EUR 1 million (2021: EUR nil million net gain) was recorded in the consolidated statement of income as a result of ineffectiveness on certain anticipated cash flow hedges. Ineffectiveness arises when anticipated exposures are no longer expected to be highly probable. During 2022,2023, a lossgain of EUR 4219 million included in the cash flow hedges reserve in equity pertaining to changes in fair value of foreign exchange forward contracts attributable to forward points and changes in the time value of option contracts was released to income statement.
The total net fair value of hedges related to transaction exposure as of December 31, 2022,2023, was an unrealized gain of EUR 610 million. The estimated impact of a 10% increase of value of the EUR is estimated to be EUR 114116 million. The following table contains an overview of the instantaneous 10% increase in the value of EUR against major currencies.
Philips Group
Estimated impact of 10% increase of value of the EUR on the fair value of hedges
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| USD | 78 | 68 | 68 | 64 |
| JPY | 13 | 15 | 15 | 15 |
| GBP | 14 | 16 | 16 | 16 |
| CHF | 5 | 4 | 4 | 5 |
| PLN | 3 | 2 | 2 | 1 |
| RUB | 10 | 0 | - |
The EUR 114116 million increase includes a gain of EUR 4140 million that would impact the income statement, which would largely offset the opposite revaluation effect on the underlying accounts receivable and payable, and the remaining gain of EUR 7377 million would be recognized in equity to the extent that the cash flow hedges were effective.
Foreign exchange exposure also arises as a result of inter-company loans and deposits. Where the company enters into such arrangements, the financing is generally provided in the functional currency of the subsidiary entity. The currency of the company’s external funding and liquid assets is matched with the required financing of subsidiaries, either directly through external foreign currency loans and deposits, or synthetically by using foreign exchange derivatives, including cross currency interest rate swaps and foreign exchange forward contracts. In certain cases where group companies may also have external foreign currency debt or liquid assets, these exposures are also hedged through the use of foreign exchange derivatives. Changes in the fair value of hedges related to this exposure are recognized within financial income and expenses in the statements of income. When such loans would be considered part of the net investment in the subsidiary, net investment hedging would be applied.
Translation exposure of foreign-currency equity invested in consolidated entities is generally not hedged. If a hedge is entered into, it is accounted for as a net investment hedge. Net current-period change, before tax, of the currency translation reserve of negative EUR 748579 million mainly relates to the development of the USD versus the EUR. As of December 31, 2022,2023, a weakening of USD by 10% versus the EUR would result in a decrease in the currency translation reserve in equity of approximately EUR 1,1321,146 million, while a strengthening of USD by 10% versus the EUR would result in an increase in the currency translation reserve in equity of approximately EUR 1,3841,400 million. Refer to the country risk paragraph for countries with significant foreign currency denominated equity invested.
As of December 31, 2023, external bond funding for a nominal value of USD 1,474 million (liability at book value: EUR 1,325 million) was designated as a net investment hedge of financing investments in foreign operations for an equal amount. During 2023 a total loss of EUR 2 million was recognized in the income statement as ineffectiveness on net investment hedges, arising from counterparty and own credit risk.
An instantaneous 10% increase in the value of the EUR against all currencies would lead to an decrease of EUR 52 million in the value of the derivatives, including a EUR 11 million increase related to the USD.
As of December 31, 2022, cross-currency interest rate swaps for a nominal value of USD 500 million (liability at fair value: EUR 147 million) and external bond funding for a nominal value of USD 1,490 million (liability at book value: EUR 1,378 million) were designated as a net investment hedgeshedge of financing investments in foreign operations for an equal amount. During 2022 a total lossgain of EUR 1.1 million was recognized in the income statement as ineffectiveness on net investment hedges, arising from counterparty and own credit risk.
The total net fair value of financing derivatives as of December 31, 2022, was a liability of EUR 147 million. An instantaneous 10% increase in the value of the EUR against all currencies would lead to an increase of EUR 192 million in the value of the derivatives, including a EUR 191 million increase related to the USD.
As of December 31, 2021, cross-currency interest rate swaps for a nominal value of USD 500 million (liability at fair value: EUR 116 million) and external bond funding for a nominal value of USD 1,473 million (liability at book value: EUR 1,313 million) were designated as net investment hedges of financing investments in foreign operations for an equal amount. During 2021 a total gain of EUR 1.1 million was recognized in the income statement as ineffectiveness on net investment hedges, arising from counterparty and own credit risk.
The total net fair value of financing derivatives as of December 31, 2021, was a liability of EUR 116 million. An instantaneous 10% increase in the value of the EUR against all currencies would lead to an increase of EUR 40 million in the value of the derivatives, including a EUR 40 million increase related to the USD.
Philips does not currently hedge the foreign exchange exposure arising from equity interests in non-functional-currency investments in associates and other non-current financial assets.
Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. As of December 31, 2022,2023, Philips had outstanding debt of EUR 8,2017,689 million (2021:(2022: EUR 6,9808,201 million), which constitutes an inherent interest rate risk with potential negative impact on financial results. At year-end,As of December 31, 2023, Philips held EUR 1,1721,869 million in cash and cash equivalents (2021:(2022: EUR 2,3031,172 million), and had total long-term debt of EUR 7,2707,035 million (2021:(2022: EUR 6,4737,270 million) and total short-term debt of EUR 931654 million (2021:(2022: EUR 506931 million). As of December 31, 2022,2023, Philips had a ratio of fixed-rate long-term debt to total outstanding debt of approximately 80%89% compared to 90%80% one year earlier. Philips debt has a long maturity profile with an average tenor of long-term debt of 6.16.0 years with maturities up to 2042.
The following table provides the impact of a 1% increase/decrease of interest rates on the fair value of the debt and the annualized net interest expenses.
Philips Group
Net debt1) and interest rate sensitivity
in millions of EUR
| 2021 | 2022 | 2022 | 2023 | |
|---|---|---|---|---|
| Impact 1% interest increase on the fair value of the fixed-rate long-term debt2)3) | (297) | (274) | (274) | (283) |
| Impact 1% interest decrease on the fair value of the fixed-rate long-term debt2)3) | 298 | 274 | 274 | 284 |
| Impact 1% interest increase on the annualized net interest expense4) | 20 | 4 | 4 | 15 |
Global regulators and central banks have been driving international efforts to reform key benchmark interest rates (Interbank Offered Rate or IBOR rates). The market has transitioned to alternative risk-free reference rates (RFRs) that are transaction-based. LIBOR has been discontinued for most currencies and maturities after December 31, 2021, except for the US-dollar for which certain maturities are expected to be phased out in 2023. The company has no interest rate hedging relationships which get affected by the reform and does not expect any significant impact on existing contracts due to change in the interest rates. The company implemented new alternative risk-free rates from January 1, 2022 and the impact upon transition was EUR 1 million financial expense.
Equity price risk
Equity price risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in equity prices.
Philips is a shareholder in some publicly listed companies and as a result is exposed to potential financial loss through movements in their share prices. The aggregate equity price exposure in such financial assets amounted to approximately EUR 3214 million as of December 31, 2022 (2021:2023 (2022: EUR 6732 million). Philips does not hold derivatives in the above-mentioned listed companies. Philips also has shareholdings in several privately-owned companies amounting to EUR 229219 million, mainly consisting of minority stakes in companies in various industries. As a result, Philips is exposed to potential value adjustments.
Commodity price risk
Commodity price risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in commodity prices.
Philips is a purchaser of certain base metals, precious metals and energy. Philips may hedge certain commodity price risks using derivative instruments to minimize significant, unanticipated earnings fluctuations caused by commodity price volatility. As of December 31, 20222023 and 2021,2022, respectively, Philips did not have any significant outstanding financial commodity derivatives.
Credit risk
Credit risk represents the loss that would be recognized at the reporting date, if counterparties failed completely to perform their payment obligations as contracted. Credit risk is present within Philips trade receivables and contract assets. To have better insights into the credit exposures, Philips performs ongoing evaluations of the financial and non-financial condition of its customers and adjusts credit limits when appropriate. In instances where the creditworthiness of a customer is determined not to be sufficient to grant the credit limit required, there are a number of mitigation tools that can be utilized to close the gap, including reducing payment terms, cash on delivery, pre-payments and pledges on assets.
Philips invests available cash and cash equivalents with various financial institutions and is exposed to credit risk with these counterparties. Philips is also exposed to credit risks in the event of non-performance by financial institutions with respect to financial derivative instruments. Philips actively manages concentration risk and on a daily basis measures the potential loss under certain stress scenarios, should a financial institution default. These worst-case scenario losses are monitored and limited by the company.
The company does not enter into any financial derivative instruments to protect against default by financial institutions. However, where possible the company requires all financial institutions with which it deals in derivative transactions to complete legally enforceable netting agreements under an International Swap Dealers Association master agreement or otherwise prior to trading, and whenever possible, to have a strong credit rating. Philips also regularly monitors the development of the credit risk of its financial counterparties. Wherever possible, cash is invested and financial transactions are concluded with financial institutions with strong credit ratings or with governments or government-backed institutions.
The following table shows the number of financial institutions with credit rating A- and above with which Philips has cash at hand and short-term deposits above EUR 10 million as of December 31, 2022.2023.
Philips Group
Credit risk with number of counterparties
for deposits above EUR 10 million
| 10-100 million | 100-500 million | 500 million and above | 10-100 million | 100-500 million | 500 million and above | |
|---|---|---|---|---|---|---|
| AAA rated bank counterparties | 4 | |||||
| AA- rated bank counterparties | 1 | 0 | 1 | |||
| A+ rated bank counterparties | 3 | 1 | 0 | 2 | 3 | |
| A rated bank counterparties | 0 | 1 | 0 | 2 | 1 | |
| A- rated bank counterparties | 1 | 0 | 1 | |||
| 5 | 3 | 0 | 6 | 8 | ||
For an overview of the overall maximum credit exposure related to debt instruments, derivatives and loans and receivables, refer to Fair value of financial assets and liabilities.
Country risk
Country risk is the risk that political, legal, or economic developments in a single country could adversely impact performance. The country risk per country is defined as the sum of the equity of all subsidiaries and associated companies in country cross-border transactions, such as intercompany loans, accounts receivable from third parties and intercompany accounts receivable. The country risk is monitored on a regular basis.
As of December 31, 2022,2023, the company had country risk exposure of EUR 14.013.3 billion in the United States, EUR 1.4 billion in the Netherlands, EUR 1.3 billion in China (including Hong Kong). Other countries higher than EUR 500 million are Germany EUR 808786 million, United Kingdom EUR 766731 million, and Japan EUR 639614 million. Other country which havecountries with significant exposure isare Singapore EUR 206202 million and Israel EUR 214 million. The degree of risk of a country is taken into account when new investments are considered. The company does not, however, use financial derivative instruments to hedge country risk.
The impact of hyperinflation is also routinely assessed and was not material for the periods presented.
Other insurable risks
Philips is insured for a broad range of losses by global insurance policies in the areas of property damage/business interruption, general and product liability, transport, directors’ and officers’ liability, employment practice liability, crime and cybersecurity. The counterparty risk related to the insurance companies participating in the above-mentioned global insurance policies is actively managed. As a rule, Philips only selects insurance companies with a financial strength of at least A-. Throughout the year the counterparty risk is monitored on a regular basis.
To lower exposures and to avoid potential losses, Philips has a global Risk Engineering program in place. The main focus of this program is on property damage and business interruption risks including company interdependencies. Regular on-site assessments take place at Philips locations and business-critical suppliers by risk engineers of the insurer in order to provide an accurate assessment of the potential loss and its impact. The results of these assessments are shared across the company’s stakeholders. On-site assessments are carried out against the predefined Risk Engineering standards, which are agreed between Philips and the insurers. Recommendations are made in a Risk Improvement report and are monitored centrally. This is the basis for decision-making by the local management of the business as to which recommendations will be implemented.
For all policies, deductibles are in place, which vary from EUR 0.30 million to EUR 10 million per occurrence and this variance is designed to differentiate between the existing risk categories within Philips. Above a first layer of working deductibles, Philips operates its own re-insurance captive, which during 20222023 retained EUR 25 million per claim and EUR 50 million in the annual aggregate for general, product, professional liability, and marine cargo claims.claims and EUR 15 million aggregate for cyber.
New contracts were signed effective December 31, 2022,2023, for the coming year, whereby the re-insurance captive retentions remained the same.
30Subsequent events
Philips Respironics consent decree
On January 30, 2023,29, 2024, Philips announced plansthat it has agreed on the terms of a consent decree with the US Department of Justice (DOJ), representing the US Food and Drug Administration (FDA). The proposed consent decree primarily focuses on Philips Respironics’ business operations in the US. The proposed consent decree is being finalized and will be submitted to create valuethe relevant US court for approval. The decree will provide Philips Respironics with sustainable impact,a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business.
- In the US, Philips Respironics will continue to service sleep and respiratory care devices already with healthcare providers and patients, and supply accessories (including patient interfaces), consumables (including patient circuits), and replacement parts (including repair kits). Until the relevant requirements of the proposed consent decree are met, Philips Respironics will not sell new CPAP or BiPAP sleep therapy devices or other respiratory care devices in the US.
- Outside the US, Philips Respironics will continue to provide new sleep and respiratory care devices, accessories (including patient interfaces), consumables (including patient circuits), replacement parts (including repair kits) and services, subject to certain requirements.
As a consequence of addressing this proposed consent decree, which is based on focused organic growtha multi-year plan, Philips recorded charges of EUR 363 million in December 2023 that relate to deliver patient-remediation activities, inventory write-downs and people-driven innovation at scale with improved execution as key value driver, prioritizing patient safety and quality, supply chain reliability and a simplified operating model. In additiononerous contract provisions. Refer to the reduction of its workforce by 4,000 roles announced in October 2022, Philips plans to reduce its workforce by an additional 6,000 roles globally by 2025, of which 3,000 will be implemented in 2023 in line with the relevant local regulations and processes. These reductions are focused on Corporate and Functions optimization and non-core activities, for which charges in 2023 are expected to be approximately EUR 470 million.Provisions.
14Further information
14.1Reconciliation of non-IFRS information
In this Annual Report Philips presents certain financial measures when discussing Philips’ performance that are not measures of financial performance or liquidity under IFRS (‘non-IFRS’). These non-IFRS measures (also known as non-GAAP or alternative performance measures) are presented because management considers them important supplemental measures of Philips���Philips’ performance and believes that they are widely used in the industry in which Philips operates as a means of evaluating a company’s operating performance and liquidity. Philips believes that an understanding of its sales performance, profitability, financial strength and funding requirements is enhanced by reporting the following non-IFRS measures:
- Comparable sales growth;
- EBITA;
- Adjusted EBITA;
- Adjusted EBITDA;
- Adjusted income from continuing operations attributable to shareholders;
- Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted (Adjusted EPS);
Adjusted EBITDA;- Free cash flow;
- Net debt : group equity ratio; and
- Organic Return on Invested Capital (ROIC)
Non-IFRS measures do not have standardized meanings under IFRS and not all companies calculate non-IFRS measures in the same manner or on a consistent basis. As a result, these measures may not be comparable to measures used by other companies that have the same or similar names. Accordingly, undue reliance should not be placed on the non-IFRS measures contained in this Annual Report and they should not be considered as substitutes for sales, net income, net cash provided by operating activities or other financial measures computed in accordance with IFRS.
This chapter contains the definitions of the non-IFRS measures used in this Annual Report as well as reconciliations from the most directly comparable IFRS measures. The non-IFRS measures discussed in this Annual Report are cross referenced to this chapter. These non-IFRS measures should not be viewed in isolation or as alternatives to equivalent IFRS measures and should be used in conjunction with the most directly comparable IFRS measures.
The non-IFRS financial measures presented are not measures of financial performance or liquidity under IFRS, but measures used by management to monitor the underlying performance of Philips’ business and operations and, accordingly, they have not been audited or reviewed by Philips’ external auditors.
Additionally, Philips provides forward-looking targets for comparable sales growth, adjusted EBITA margin improvement, free cash flow and organic ROIC, which are non-IFRS financial measures. Philips has not provided a quantitative reconciliation of these targets to the most directly comparable IFRS measures because certain information needed to reconcile these non-IFRS financial measures to the most comparable IFRS financial measures are dependent on specific items or impacts which are not yet determined, are subject to uncertainty and variability in timing and amount due to their nature, are outside of Philips’ control, or cannot be predicted, including items and impacts such as currency exchange rates, acquisitions and disposals, legal and tax gains and losses and pension settlements, charges and costs such as impairments, restructuring and acquisition-related charges, amortization of intangible assets and net capital expenditures. Accordingly, reconciliations of these non-IFRS forward looking financial measures to the most directly comparable IFRS financial measures are not available without unreasonable effort. Such unavailable reconciling items could significantly impact the results of operations and financial condition.
Comparable sales growth
Comparable sales growth represents the period-on-period growth in sales excluding the effects of currency movements and changes in consolidation. As indicated in General information to the Consolidated financial statements, foreign currency sales and costs are translated into Philips’ presentation currency, the euro, at the exchange rates prevailing at the respective transaction dates. As a result of significant foreign currency sales and currency movements during the periods presented, the effects of translating foreign currency sales amounts into euros could have a material impact on the comparability of sales between periods. Therefore, these impacts are excluded when presenting comparable sales in euros by translating the foreign currency sales of the previous period and the current period into euros at the same average exchange rates. In addition, the years presented were affected by a number of acquisitions and divestments, as a result of which various activities were consolidated or deconsolidated. The effect of consolidation changes has also been excluded in arriving at the comparable sales. For the purpose of calculating comparable sales, when a previously consolidated entity is sold or control is lost, relevant sales forof that entity offor the corresponding prior year period are excluded. Similarly, when an entity is acquired and consolidated, relevant sales forof that entity offor the current year period are excluded.
Comparable sales growth is presented for the Philips Group, operating segments and geographic area. Philips’ believes that the presentation of comparable sales growth is meaningful for investors to evaluate the performance of Philips’ business activities over time. Comparable sales growth may be subject to limitations as an analytical tool for investors, because comparable sales growth figures are not adjusted for other effects, such as increases or decreases in prices or quantity/volume. In addition, interaction effects between currency movements and changes in consolidation are not taken into account.
Philips Group
Sales growth composition by segment
in %
| nominal growth | consolidation changes | currency effects | comparable growth | nominal growth | consolidation changes | currency effects | comparable growth | |
|---|---|---|---|---|---|---|---|---|
| 2023 versus 2022 | ||||||||
| Diagnosis & Treatment | 6.4 | 0.2 | 4.5 | 11.1 | ||||
| Connected Care | (2.5) | 0.3 | 3.3 | 1.1 | ||||
| Personal Health | (0.7) | 0.0 | 3.9 | 3.2 | ||||
| Philips Group | 1.9 | 0.2 | 3.9 | 6.0 | ||||
| 2022 versus 2021 | ||||||||
| Diagnosis & Treatment | 6.2 | 0.0 | (6.8) | (0.7) | 5.9 | 0.0 | (6.7) | (0.8) |
| Connected Care | (3.7) | (0.1) | (7.0) | (10.8) | (1.9) | 0.0 | (7.2) | (9.1) |
| Personal Health | 5.7 | 0.0 | (5.7) | 0.1 | 5.7 | 0.0 | (5.7) | 0.1 |
| Philips Group | 3.9 | (0.2) | (6.5) | (2.8) | 3.9 | (0.3) | (6.4) | (2.8) |
2021 versus 2020 | ||||||||
| Diagnosis & Treatment | 5.6 | 0.0 | 2.5 | 8.1 | 5.9 | (0.1) | 2.5 | 8.3 |
| Connected Care | (17.5) | (7.2) | 2.2 | (22.6) | (14.9) | (6.3) | 2.2 | (19.0) |
| Personal Health | 7.2 | 0.0 | 1.6 | 8.8 | 7.2 | 0.0 | 1.6 | 8.8 |
| Philips Group | (0.9) | (2.5) | 2.2 | (1.2) | (0.9) | (2.5) | 2.3 | (1.2) |
2020 versus 2019 | ||||||||
| Diagnosis & Treatment | (3.7) | (1.0) | 2.3 | (2.3) | ||||
| Connected Care | 18.6 | 0.7 | 2.3 | 21.6 | ||||
| Personal Health | (9.0) | 0.0 | 2.8 | (6.2) | ||||
| Philips Group | 1.0 | (0.5) | 2.4 | 2.9 | ||||
Philips Group
Sales growth composition by geographic area
in %
| nominal growth | consolidation changes | currency effects | comparable growth | nominal growth | consolidation changes | currency effects | comparable growth | |
|---|---|---|---|---|---|---|---|---|
| 2023 versus 2022 | ||||||||
| Western Europe | 6.0 | 0.3 | 6.6 | |||||
| North America | (0.3) | 0.2 | 2.7 | 2.5 | ||||
| Other mature geographies | (1.0) | 0.1 | 8.2 | 7.3 | ||||
| Mature geographies | 1.4 | 0.2 | 2.7 | 4.2 | ||||
| Growth geographies | 3.4 | 0.2 | 6.9 | 10.5 | ||||
| Philips Group | 1.9 | 0.2 | 3.9 | 6.0 | ||||
| 2022 versus 2021 | ||||||||
| Western Europe | (1.2) | (1.3) | (0.4) | (2.8) | (1.2) | (1.3) | (0.4) | (2.8) |
| North America | 11.9 | 0.2 | (12.4) | (0.3) | 11.9 | 0.2 | (12.4) | (0.3) |
| Other mature geographies | (3.0) | 0.0 | 2.5 | (0.5) | (3.0) | 0.0 | 2.5 | (0.5) |
| Total mature geographies | 5.9 | (0.3) | (6.7) | (1.1) | ||||
| Mature geographies | 5.9 | (0.3) | (6.7) | (1.1) | ||||
| Growth geographies | (0.8) | - | (6.0) | (6.9) | (0.8) | (0.1) | (5.9) | (6.9) |
| Philips Group | 3.9 | (0.2) | (6.5) | (2.8) | 3.9 | (0.3) | (6.4) | (2.8) |
2021 versus 2020 | ||||||||
| Western Europe | (1.5) | (1.3) | (0.4) | (3.2) | (1.5) | (1.3) | (0.4) | (3.2) |
| North America | (1.5) | (5.5) | 3.6 | (3.4) | (1.5) | (5.5) | 3.6 | (3.4) |
| Other mature geographies | (3.2) | (0.1) | 3.6 | 0.3 | (3.2) | (0.1) | 3.6 | 0.3 |
| Total mature geographies | (1.8) | (3.5) | 2.4 | (2.8) | ||||
| Mature geographies | (1.8) | (3.5) | 2.4 | (2.8) | ||||
| Growth geographies | 1.2 | - | 1.8 | 3.0 | 1.2 | (0.3) | 2.1 | 3.0 |
| Philips Group | (0.9) | (2.5) | 2.2 | (1.2) | (0.9) | (2.5) | 2.3 | (1.2) |
2020 versus 2019 | ||||||||
| Western Europe | 11.2 | (1.1) | 0.1 | 10.2 | ||||
| North America | (0.3) | 1.9 | 1.3 | |||||
| Other mature geographies | (3.0) | (0.5) | 0.4 | (3.1) | ||||
| Total mature geographies | 2.5 | (0.6) | 1.1 | 3.0 | ||||
| Growth geographies | (2.6) | (0.2) | 5.4 | 2.6 | ||||
| Philips Group | 1.0 | (0.5) | 2.4 | 2.9 | ||||
EBITA and Adjusted EBITA
The term Adjusted EBITA is used to evaluate the performance of Philips and its segments. EBITA represents Income from operations excluding amortization and impairment of acquired intangible assets and impairment of goodwill. Adjusted EBITA represents EBITA excluding gains or losses from restructuring costs, acquisition-related charges and other items.
Restructuring costs are defined as the estimated costs of initiated reorganizations, the most significant of which have been approved by the Executive Committee, and which generally involve the realignment of certain parts of the industrial and commercial organization.
Acquisition-related charges are defined as costs that are directly triggered by the acquisition of a company, such as transaction costs, purchase accounting related costs and integration-related expenses.
Other items are defined as any individual item with an income statement impact (loss or gain) that is deemed by management to be both significant and incidental to normal business activity. This includes the following: litigation costs and settlements in favor of (or against) the company, gains (or losses) on sale of businesses or assets, remediation costs, impairment of assets, portfolio realignment charges, environmental charges and other items which are individually above an amount of EUR 20 million in a quarter, or an individual item which is above EUR 40 million across multiple quarters. Refer to Net income, Income from operations (EBIT) and Adjusted EBITA within the Results of operations section of Financial performance.
Philips considers the use of Adjusted EBITA appropriate as Philips uses it as a measure of segment performance and as one of its strategic drivers to increase profitability through re-allocation of its resources towards opportunities offering more consistent and higher returns. This is done with the aim of making the underlying performance of the businesses more transparent.
EBITA excludes amortization and impairment of acquired intangible assets (andand impairment of goodwill),goodwill, which primarily relates to brand names, customer relationships and technology, as Philips believes that such amounts are inconsistent in amount and frequency, are significantly impacted by the timing and/or size of acquisitions and do not factor into its decisions on allocation of its resources across segments. Although we exclude amortization and impairment of acquired intangible assets from the Adjusted EBITA measure, Philips believes that it is important for investors to understand that these acquired intangible assets contribute to revenue generation.
Philips believes Adjusted EBITA is useful to evaluate financial performance on a comparable basis over time by factoring out restructuring costs, acquisition-related charges and other incidental items which are not directly related to the operational performance of Philips Group or its segments.
Adjusted EBITA may be subject to limitations as an analytical tool for investors, as it excludes restructuring costs, acquisition-related charges and other incidental items and therefore does not reflect the expense associated with such items, which may be significant and have a significant effect on Philips’ net income.
Adjusted EBITA margin refers to Adjusted EBITA divided by sales expressed as a percentage.
Adjusted EBITA is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted EBITA to the most directly comparable IFRS measure, Net income, for the years indicated is presented in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Adjusted EBITDA
Adjusted EBITDA is defined as Income from operations excluding amortization and impairment of intangible assets, impairment of goodwill, depreciation and impairment of property, plant and equipment, restructuring costs, acquisition-related charges and other items.
Philips understands that Adjusted EBITDA is broadly used by analysts, rating agencies and investors in their evaluation of different companies because it excludes certain items that can vary widely across different industries or among companies within the same industry. Philips considers Adjusted EBITDA useful when comparing its performance to other companies in the HealthTech industry. However, Adjusted EBITDA may be subject to limitations as an analytical tool because of the range of items excluded and their significance in a given reporting period. Furthermore, comparisons with other companies may be complicated due to the absence of a standardized meaning and calculation framework. Philips management compensates for the limitations of using Adjusted EBITDA by using this measure to supplement IFRS results to provide a more complete understanding of the factors and trends affecting the business rather than IFRS results alone. In addition to the limitations noted above, Adjusted EBITDA excludes items that may be recurring in nature and should not be disregarded in the evaluation of performance. However, we believe it is useful to exclude such items to provide a supplemental analysis of current results and trends compared to other periods. This is because certain excluded items can vary significantly depending on specific underlying transactions or events. Also, the variability of such items may not relate specifically to ongoing operating results or trends and certain excluded items, while potentially recurring in future periods and may not be indicative of future results. Net income, for the years indicated is included in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | ||||||||||
| 2023 | ||||||||||
| Net Income | (1,605) | (463) | ||||||||
| Discontinued operations, net of income taxes | (13) | 10 | ||||||||
| Income tax expense | (113) | |||||||||
| Income taxes | (73) | |||||||||
| Investments in associates, net of income taxes | 2 | 98 | ||||||||
| Financial expenses | 258 | 376 | ||||||||
| Financial income | (58) | (63) | ||||||||
| Income from operations | (1,529) | 404 | (2,246) | 515 | (202) | (115) | 720 | (1,199) | 552 | (188) |
| Amortization and impairment of acquired intangible assets | 363 | 143 | 199 | 15 | 7 | 290 | 89 | 178 | 14 | 9 |
| Impairment of goodwill | 1,357 | 27 | 1,331 | 8 | - | |||||
| EBITA | 192 | 573 | (716) | 531 | (196) | 183 | 816 | (1,020) | 567 | (179) |
| Restructuring and acquisition-related charges | 202 | 21 | 108 | 11 | 61 | 381 | 118 | 115 | 9 | 140 |
| Other items: | 925 | 180 | 703 | (4) | 46 | 1,358 | 92 | 1,275 | 22 | (32) |
| Respironics field-action provision | 250 | 250 | ||||||||
| Respironics litigation provision | 575 | 575 | ||||||||
| Respironics field-action connected to the proposed consent decree | 363 | 363 | ||||||||
| Respironics field-action running remediation costs | 210 | 210 | 224 | 224 | ||||||
| R&D project impairments | 134 | 120 | 12 | 3 | ||||||
| Portfolio realignment charges | 109 | 109 | ||||||||
| Impairment of assets in S&RC | 39 | 39 | ||||||||
| Provision for public investigations tender irregularities | 60 | |||||||||
| Provisions for quality actions in Connected Care | 59 | 59 | ||||||||
| Quality remediation actions | 175 | 81 | 94 | |||||||
| Provision for a legal matter | 31 | 31 | ||||||||
| Investment re-measurement loss | 23 | 23 | ||||||||
| Gain on divestment of business | (35) | (35) | ||||||||
| Remaining items | 63 | - | 24 | (6) | 46 | 2 | 11 | (12) | (1) | 3 |
| Adjusted EBITA | 1,318 | 774 | 95 | 538 | (89) | 1,921 | 1,026 | 369 | 597 | (71) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 971 | 217 | 267 | 101 | 385 | |||||
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (47) | (4) | (14) | (30) | ||||||
| Adjusted EBITDA | 2,845 | 1,239 | 623 | 698 | 284 | |||||
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | ||||||||||
| 2022 | ||||||||||
| Net Income | 3,323 | (1,605) | ||||||||
| Discontinued operations, net of income taxes | (2,711) | (13) | ||||||||
| Income tax expense | (103) | |||||||||
| Income taxes | (113) | |||||||||
| Investments in associates, net of income taxes | 4 | 2 | ||||||||
| Financial expenses | 188 | 258 | ||||||||
| Financial income | (149) | (58) | ||||||||
| Income from operations | 553 | 941 | (722) | 576 | (242) | (1,529) | 538 | (2,347) | 515 | (235) |
| Amortization and impairment of acquired intangible assets | 322 | 153 | 148 | 15 | 6 | 363 | 115 | 226 | 15 | 8 |
| Impairment of goodwill | 15 | 2 | 13 | 1,357 | 1,357 | |||||
| EBITA | 890 | 1,097 | (562) | 591 | (236) | 192 | 652 | (764) | 531 | (227) |
| Restructuring and acquisition-related charges | 95 | 7 | 93 | (1) | (5) | 202 | 3 | 125 | 11 | 62 |
| Other items: | 1,069 | (32) | 965 | - | 136 | 925 | 133 | 750 | (4) | 46 |
| Respironics field-action provision | 719 | - | 719 | - | ||||||
| Respironics field-action connected to the proposed consent decree | 250 | 250 | ||||||||
| Respironics field-action running remediation costs | 94 | 94 | 210 | 210 | ||||||
| Provisions for quality actions in Connected Care | 94 | 94 | ||||||||
| Loss on divestment of business | 76 | 76 | ||||||||
| R&D project impairments | 134 | 73 | 59 | 3 | ||||||
| Portfolio realignment charges | 109 | 109 | ||||||||
| Impairments of assets in S&RC | 39 | 39 | ||||||||
| Provision for public investigations tender irregularities | 60 | |||||||||
| Quality remediation actions | 59 | 59 | ||||||||
| Remaining items | 87 | (32) | 58 | - | 61 | 63 | - | 24 | (6) | 46 |
| Adjusted EBITA | 2,054 | 1,071 | 497 | 590 | (105) | 1,318 | 788 | 111 | 538 | (119) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 1,239 | 302 | 420 | 117 | 400 | |||||
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (252) | (83) | (136) | (3) | (30) | |||||
| Adjusted EBITDA | 2,305 | 1,008 | 394 | 652 | 250 | |||||
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | ||||||||||
| 2021 | ||||||||||
| Net Income | 1,195 | 3,323 | ||||||||
| Discontinued operations, net of income taxes | (196) | (2,711) | ||||||||
| Income tax expense | 212 | |||||||||
| Income taxes | (103) | |||||||||
| Investments in associates, net of income taxes | 9 | 4 | ||||||||
| Financial expenses | 202 | 188 | ||||||||
| Financial income | (158) | (149) | ||||||||
| Income from operations | 1,264 | 497 | 704 | 362 | (300) | 553 | 948 | (716) | 576 | (255) |
| Amortization and impairment of acquired intangible assets | 377 | 209 | 134 | 16 | 18 | 322 | 142 | 158 | 15 | 6 |
| Impairment of goodwill | 144 | - | 144 | 15 | 2 | 13 | ||||
| EBITA | 1,784 | 706 | 982 | 378 | (282) | 890 | 1,092 | (545) | 591 | (248) |
| Restructuring and acquisition-related charges | 195 | 29 | 97 | 31 | 37 | 95 | (30) | 130 | (1) | (5) |
| Other items | 299 | 83 | 112 | 24 | 81 | |||||
| Other items: | 1,069 | (35) | 968 | - | 136 | |||||
| Respironics field-action connected to the proposed consent decree | 719 | 719 | ||||||||
| Respironics field-action running remediation costs | 94 | 94 | ||||||||
| Quality remediation actions | 94 | 94 | ||||||||
| Loss on divestment of business | 76 | 76 | ||||||||
| Remaining items | 87 | (35) | 61 | - | 61 | |||||
| Adjusted EBITA | 2,277 | 818 | 1,191 | 433 | (165) | 2,054 | 1,028 | 553 | 590 | (117) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 1,001 | 221 | 314 | 116 | 351 | |||||
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (70) | (4) | (68) | - | 2 | |||||
| Adjusted EBITDA | 2,985 | 1,245 | 799 | 706 | 235 | |||||
Adjusted income from continuing operations attributable to shareholders
The term Adjusted income from continuing operations attributable to shareholders represents income from continuing operations less continuing operations non-controlling interests, amortization and impairment of acquired intangible assets, impairment of goodwill, excluding gains or losses from restructuring costs and acquisition-related charges, other items, adjustments to net finance expenses, adjustments to investments in associates and adjustments to tax expense. Shareholders refers to shareholders of Koninklijke Philips N.V.
Restructuring costs, acquisition-related charges and other items are all defined in the EBITA and Adjusted EBITA section above.
Net finance expenses are defined as either the financial income or expense component of an individual item already identified to be excluded as part of the Adjusted income from continuing operations, fair value movements of equity investments in limited life funds recognized at fair value through profit or loss or a financial income or expense component with an income statement impact (gain or loss) that is deemed by management to be both significant and incidental to normal business activity.
The adjustments to tax expense include the tax impact of the adjustments to income from continuing operations as well as tax only adjusting items, and uses the Weighted Average Statutory Tax Rate plus any recurring tax costs or benefits.
Philips considers the use of Adjusted income from continuing operations attributable to shareholders appropriate as Philips uses it as the basis for the Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted, a non-IFRS measure.
Adjusted income from continuing operations attributable to shareholders may be subject to limitations as an analytical tool for investors, as it excludes certain items and therefore does not reflect the expense associated with such items, which may be significant and have a significant effect on Philips’ net income. Net income, for the years indicated is included in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Adjusted income from continuing operations attributable to shareholders is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted income from continuing operations attributable to shareholders to the most directly comparable IFRS measure, Net income, for the years indicated is included in the following table.
Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted (Adjusted EPS)
Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted is calculated by dividing the Adjusted income from continuing operations attributable to shareholders by the diluted weighted average number of shares (after deduction of treasury shares) outstanding during the period, as defined in General information to the Consolidated financial statements, earnings per share section.
Philips considers the use of Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted appropriate as it is a measure that is useful when comparing its performance to other companies in the HealthTech industry. However, it may be subject to limitations as an analytical tool for investors, as it uses Adjusted income from continuing operations attributable to shareholders which has certain items excluded.
Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted is not a recognized measure of financial performance under IFRS. The most directly comparable IFRS measure, income from continuing operations attributable to shareholders per common share (in EUR) - diluted for the years indicated, is included in the following table.
Philips Group
Adjusted income from continuing operations attributable to shareholders1)
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net income | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (196) | (2,711) | (13) | (2,711) | (13) | 10 |
| Income from continuing operations | 999 | 612 | (1,618) | 612 | (1,618) | (454) |
| Income from continuing operations attributable to non-controlling interests | (8) | (4) | (3) | (4) | (3) | (2) |
| Income from continuing operations attributable to shareholders1) | 991 | 608 | (1,622) | 608 | (1,622) | (456) |
| Adjustments for: | ||||||
| Amortization and impairment of acquired intangible assets | 377 | 322 | 363 | 322 | 363 | 290 |
| Impairment of goodwill | 144 | 15 | 1,357 | 15 | 1,357 | 8 |
| Restructuring costs and acquisition-related charges | 195 | 95 | 202 | 95 | 202 | 381 |
| Other items: | 299 | 1,069 | 925 | 1,069 | 925 | 1,358 |
| Respironics field-action provision | 719 | 250 | ||||
| Respironics litigation provision | 575 | |||||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 | |||
| Respironics field-action running remediation costs | 94 | 210 | 94 | 210 | 224 | |
| Quality remediation actions | 94 | 59 | 175 | |||
| R&D project impairments | 134 | 134 | ||||
| Portfolio realignment charges | 109 | 109 | ||||
| Impairment of assets in S&RC | 39 | 39 | ||||
| Provision for public investigations tender irregularities | 60 | 60 | ||||
| Provisions for quality actions in Connected Care | 94 | 59 | ||||
| Loss on divestment of business | 76 | |||||
| Provision for a legal matter | 31 | |||||
| Investment re-measurement loss | 23 | |||||
| Loss (gain) on divestment of business | 76 | (35) | ||||
| Remaining items | 299 | 87 | 63 | 87 | 63 | 2 |
| Net finance income/expenses | (125) | (84) | (4) | (84) | (4) | 18 |
| Tax impact of adjusted items and tax only adjusting items | (285) | (527) | (376) | (527) | (376) | (450) |
| Adjusted Income from continuing operations attributable to shareholders1) | 1,594 | 1,497 | 845 | 1,497 | 845 | 1,148 |
| Earnings per common share: | ||||||
| Income from continuing operations attributable to shareholders1) per common share (in EUR) - diluted | 1.08 | 0.67 | (1.84) | 0.64 | (1.76) | (0.50) |
| Adjusted income from continuing operations attributable to shareholders1) per common share (in EUR) - diluted | 1.74 | 1.65 | 0.96 | 1.58 | 0.92 | 1.25 |
Adjusted EBITDA
Adjusted EBITDA is defined as Income from operations excluding amortization and impairment of intangible assets, impairment of goodwill, depreciation and impairment of property, plant and equipment, restructuring costs, acquisition-related charges and other items.
Philips understands that Adjusted EBITDA is broadly used by analysts, rating agencies and investors in their evaluation of different companies because it excludes certain items that can vary widely across different industries or among companies within the same industry. Philips considers Adjusted EBITDA useful when comparing its performance to other companies in the HealthTech industry. However, Adjusted EBITDA may be subject to limitations as an analytical tool because of the range of items excluded and their significance in a given reporting period. Furthermore, comparisons with other companies may be complicated due to the absence of a standardized meaning and calculation framework. Philips management compensates for the limitations of using Adjusted EBITDA by using this measure to supplement IFRS results to provide a more complete understanding of the factors and trends affecting the business rather than IFRS results alone. In addition to the limitations noted above, Adjusted EBITDA excludes items that may be recurring in nature and should not be disregarded in the evaluation of performance. However, we believe it is useful to exclude such items to provide a supplemental analysis of current results and trends compared to other periods. This is because certain excluded items can vary significantly depending on specific underlying transactions or events. Also, the variability of such items may not relate specifically to ongoing operating results or trends and certain excluded items, while potentially recurring in future periods and may not be indicative of future results. A reconciliation from net income to Adjusted EBITDA is provided in the following table. Net income, for the years indicated is included in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Philips Group
Reconciliation of Net income to Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2022 | |||||
| Net Income | (1,605) | ||||
| Discontinued operations, net of income taxes | (13) | ||||
| Income tax expense | (113) | ||||
| Investments in associates, net of income taxes | 2 | ||||
| Financial expenses | 258 | ||||
| Financial income | (58) | ||||
| Income from operations | (1,529) | 404 | (2,246) | 515 | (202) |
| Depreciation, amortization and impairment of fixed assets | 1,602 | 559 | 514 | 132 | 397 |
| Impairment of goodwill | 1,357 | 27 | 1,331 | ||
| Restructuring and acquisition-related charges | 202 | 21 | 108 | 11 | 61 |
| Other items: | 925 | 180 | 703 | (4) | 46 |
| Respironics field-action provision | 250 | 250 | |||
| Respironics field-action running remediation costs | 210 | 210 | |||
| R&D project impairments | 134 | 120 | 12 | 3 | |
| Portfolio realignment charges | 109 | 109 | |||
| Impairment of assets in S&RC | 39 | 39 | |||
| Provision for public investigations tender irregularities | 60 | 60 | |||
| Provisions for quality actions in Connected Care | 59 | 59 | |||
| Remaining items | 63 | - | 24 | (6) | 46 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related changes and Other items | (252) | (135) | (84) | (3) | (30) |
| Adjusted EBITDA | 2,305 | 1,055 | 326 | 652 | 272 |
Philips Group
Reconciliation of Net income to Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2021 | |||||
| Net Income | 3,323 | ||||
| Discontinued operations, net of income taxes | (2,711) | ||||
| Income tax expense | (103) | ||||
| Investments in associates, net of income taxes | 4 | ||||
| Financial expenses | 188 | ||||
| Financial income | (149) | ||||
| Income from operations | 553 | 941 | (722) | 576 | (242) |
| Depreciation, amortization and impairment of fixed assets | 1,323 | 459 | 382 | 131 | 350 |
| Impairment of goodwill | 15 | 2 | 13 | ||
| Restructuring and acquisition-related charges | 95 | 7 | 93 | (1) | (5) |
| Other items: | 1,069 | (32) | 965 | - | 136 |
| Respironics field-action provision | 719 | - | 719 | - | |
| Respironics field-action running remediation costs | 94 | 94 | |||
| Provisions for quality actions in Connected Care | 94 | 94 | |||
| Loss on divestment of business | 76 | 76 | |||
| Remaining items | 87 | (32) | 58 | - | 61 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related changes and Other items | (70) | (21) | (51) | 2 | |
| Adjusted EBITDA | 2,985 | 1,358 | 680 | 706 | 241 |
Philips Group
Reconciliation of Net income to Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2020 | |||||
| Net Income | 1,195 | ||||
| Discontinued operations, net of income taxes | (196) | ||||
| Income tax expense | 212 | ||||
| Investments in associates, net of income taxes | 9 | ||||
| Financial expenses | 202 | ||||
| Financial income | (158) | ||||
| Income from operations | 1,264 | 497 | 704 | 362 | (300) |
| Depreciation, amortization and impairment of fixed assets | 1,462 | 536 | 414 | 145 | 368 |
| Impairment of goodwill | 144 | 0 | 144 | ||
| Restructuring and acquisition-related charges | 195 | 29 | 97 | 31 | 37 |
| Other items | 299 | 83 | 112 | 24 | 81 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related changes and Other items | (102) | (35) | (64) | 1 | (4) |
| Adjusted EBITDA | 3,262 | 1,111 | 1,407 | 563 | 180 |
Free cash flow
Free cash flow is defined as net cash flows from operating activities minus net capital expenditures. Net capital expenditures are comprised of the purchase of intangible assets, expenditures on development assets, capital expenditures on property, plant and equipment and proceeds from sales of property, plant and equipment.
Philips discloses free cash flow as a supplemental non-IFRS financial measure, as Philips believes it is a meaningful measure to evaluate the performance of its business activities over time. Philips understands that free cash flow is broadly used by analysts, rating agencies and investors in assessing its performance. Philips also believes that the presentation of free cash flow provides useful information to investors regarding the cash generated by the Philips operations after deducting cash outflows for purchases of intangible assets, capitalization of product development, expenditures on development assets, capital expenditures on property, plant and equipment and proceeds from disposal of property, plant and equipment. Therefore, the measure gives an indication of the long-term cash generating ability of the business. In addition, because free cash flow is not impacted by purchases or sales of businesses and investments, it is generally less volatile than the total of net cash provided by (used for) operating activities and net cash provided by (used for) investing activities.
Free cash flow may be subject to limitations as an analytical tool for investors, as free cash flow is not a measure of cash generated by operations available exclusively for discretionary expenditures and Philips requires funds in addition to those required for capital expenditures for a wide variety of non-discretionary expenditures, such as payments on outstanding debt, dividend payments or other investing and financing activities. In addition, free cash flow does not reflect cash payments that may be required in future for costs already incurred, such as restructuring costs.
Philips Group
Composition of free cash flow
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net cash flows provided by operating activities | 2,511 | 1,629 | (173) | 1,629 | (173) | 2,136 |
| Net capital expenditures: | (876) | (729) | (788) | (729) | (788) | (554) |
| Purchase of intangible assets | (114) | (107) | (105) | (107) | (105) | (96) |
| Expenditures on development assets | (296) | (259) | (257) | (259) | (257) | (203) |
| Capital expenditures on property, plant and equipment | (485) | (397) | (444) | (397) | (444) | (345) |
| Proceeds from disposals of property, plant and equipment | 19 | 33 | 18 | 33 | 18 | 90 |
| Free cash flow | 1,635 | 900 | (961) | 900 | (961) | 1,582 |
Net debt : group equity ratio
Net debt : group equity ratio is presented to express the financial strength of Philips. Net debt is defined as the sum of long- and short-term debt minus cash and cash equivalents. Group equity is defined as the sum of shareholders’ equity and non-controlling interests. This measure is used by Philips Treasury management and investment analysts to evaluate financial strength and funding requirements. This measure may be subject to limitations because cash and cash equivalents are used for various purposes, not only debt repayment. The net debt calculation deducts all cash and cash equivalents whereas these items are not necessarily available exclusively for debt repayment at any given time.
Philips Group
Composition of net debt to group equity
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Long-term debt | 5,705 | 6,473 | 7,270 | 6,473 | 7,270 | 7,035 |
| Short-term debt | 1,229 | 506 | 931 | 506 | 931 | 654 |
| Total debt | 6,934 | 6,980 | 8,201 | 6,980 | 8,201 | 7,689 |
| Cash and cash equivalents | 3,226 | 2,303 | 1,172 | 2,303 | 1,172 | 1,869 |
| Net debt | 3,708 | 4,676 | 7,028 | 4,676 | 7,028 | 5,820 |
| Shareholders' equity | 11,870 | 14,438 | 13,249 | 14,438 | 13,249 | 12,028 |
| Non-controlling interests | 31 | 36 | 34 | 36 | 34 | 33 |
| Group equity | 11,901 | 14,475 | 13,283 | 14,475 | 13,283 | 12,061 |
| Net debt : group equity ratio | 24:76 | 35:65 | 24:76 | 35:65 | 33:67 | |
Organic Return on Invested Capital
Organic Return on Invested Capital (ROIC) is defined as organic return which includes income from operations for the year excluding the impact of: Income or Loss from operations of businesses acquired in the five year period prior to the measurement date; certain tax gains and losses determined by management to be material in nature and require separate disclosure and; certain other items; and tax effects of the other adjustments (calculated at group effective tax rate) divided by average of the Net operating capital at the end of each of the five quarters ending on the relevant measurement date excluding the average net operating capital at the end of each of the five quarters ending on the relevant measurement date of the businesses acquired in the five year period prior to the measurement date, expressed as a percentage.
Net operating capital is defined as tangible fixed assets, intangible fixed assets, including goodwill, inventories and receivable balances, minus payable balances and provisions, all as further defined below. Net operating capital is adjusted to exclude assets and liabilities of businesses acquired in the five year period prior to the relevant measurement date, and adjustments determined by management to be necessary for comparability.
Other items are defined as material in nature and require separate disclosure and have the same nature as the items excluded from Adjusted EBITA. In the years 2020-2022 these other items included legal provisions, pension settlements, results of divestments, remediation costs, impairment of assets and portfolio realignment charges. Refer to Net income, Income from operations (EBIT) and Adjusted EBITA within the Results of operations section of Financial performance. Organic ROIC is calculated after taxes.
The term Organic Return on Invested Capital (ROIC) is used by management to evaluate Philips’ efficiency at allocating the capital under its control to profitable investments and how well the company uses capital to generate returns. Philips believes that Organic ROIC provides useful information to investors because it excludes the impact of recently acquired businesses, giving a more accurate representation of how the Philips Business System is leveraged to drive operational excellence and removes irregularity caused by various operating models of recently acquired businesses. Philips also believes that excluding certain items determined by management to be material in nature and requiring separate disclosure enhances comparability across several periods. Organic ROIC may be subject to limitations as an analytical tool for investors, as it excludes Income or Loss from operations of acquired businesses and tax gains and losses and certain other items, which may have a significant effect on ROIC. Organic ROIC is not a recognized measure of financial performance under IFRS.
The most comparable IFRS measure to Organic ROIC is Return on total assets, calculated as Income from operations for the year divided by total assets as of the end of the year. Return on total assets as of the balance sheet date for the years ended December 31, 2020, 2021 and 2022 is included in the following table.
Philips Group
Return on total assets
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Income from operations | 1,264 | 553 | (1,529) | 553 | (1,529) | (115) |
| Total assets | 27,713 | 30,961 | 30,688 | 30,961 | 30,688 | 29,406 |
| Return on total assets (%) | 4.6% | 1.8% | (5.0)% | 1.8% | (5.0)% | (0.4)% |
The reconciliation of Average Net operating capital and the reconciliation of Net income to Organic ROIC for the years ended December 31, 2020, 2021 and 2022 are included in the following tables.
Philips Group
Reconciliation of Average Net operating capital1)
in millions of EUR
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Tangible fixed assets | 2,799 | 2,716 | 2,715 | 2,716 | 2,715 | 2,553 |
| Intangible assets (including goodwill) | 11,789 | 13,454 | 14,684 | 13,454 | 14,684 | 13,475 |
| Inventories | 3,056 | 3,248 | 3,999 | 3,248 | 3,999 | 3,984 |
| Receivable balances2) | 5,010 | 4,648 | 5,043 | 4,648 | 5,043 | 4,981 |
| Payable balances3) | (6,520) | (6,627) | (7,129) | (6,627) | (7,129) | (6,810) |
| Provisions4) | (2,066) | (2,178) | (2,313) | (2,178) | (2,313) | (2,420) |
| Group Average Net operating capital | 14,068 | 15,261 | 16,999 | 15,261 | 16,999 | 15,763 |
| Net operating capital of businesses acquired | (3,176) | (5,511) | (5,739) | (5,511) | (5,739) | (4,081) |
| Average Net operating capital | 10,892 | 9,750 | 11,260 | 9,750 | 11,260 | 11,681 |
Philips Group
Reconciliation of Net Income to Organic ROIC
in millions of EUR unless otherwise stated
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Net Income | 1,195 | 3,323 | (1,605) | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (196) | (2,711) | (13) | (2,711) | (13) | 10 |
| Income tax expense | 212 | (103) | (113) | |||
| Income taxes | (103) | (113) | (73) | |||
| Investments in associates, net of income taxes | 9 | 4 | 2 | 4 | 2 | 98 |
| Financial expenses | 202 | 188 | 258 | 188 | 258 | 350 |
| Financial income | (158) | (149) | (58) | (149) | (58) | (36) |
| Income from operations | 1,264 | 553 | (1,529) | 553 | (1,529) | (115) |
| Loss from operations of businesses acquired | 265 | 124 | 178 | 124 | 178 | 253 |
| Tax gains and losses | (22) | (197) | (169) | (197) | (169) | (140) |
| Goodwill impairment | 144 | 15 | 1,357 | 15 | 1,357 | 8 |
| Other items: | 59 | 872 | 802 | 872 | 802 | 1,181 |
| Respironics field-action provision | 719 | 250 | ||||
| Respironics litigation provision | 575 | |||||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 | |||
| Respironics field-action running remediation costs | 94 | 210 | 94 | 210 | 224 | |
| R&D project impairments | 134 | 134 | ||||
| Portfolio realignment charges | 109 | 109 | ||||
| Impairment of assets in S&RC | 39 | 39 | ||||
| Loss on divestment of business | 76 | |||||
| Provision for specified legal matters | 38 | (17) | 60 | (17) | 60 | 31 |
| Pension liability derisking | 21 | |||||
| Income tax expense | (212) | 103 | 113 | |||
| Investment re-measurement loss | 23 | |||||
| Loss (gain) on divestment of business | 76 | (35) | ||||
| Income taxes | 103 | 113 | 73 | |||
| Tax effects of other adjustments | 30 | (33) | (45) | (33) | (45) | (56) |
| Organic return | 1,528 | 1,437 | 707 | 1,437 | 707 | 1,204 |
| Average Net operating capital | 10,892 | 9,750 | 11,260 | 9,750 | 11,260 | 11,681 |
| Organic ROIC (%) | 14.0% | 14.7% | 6.3% | 14.7% | 6.3% | 10.3% |
14.2Other Key Performance Indicators
In addition to monitoring the IFRS and non-IFRS financial measures discussed under Financial performance, Philips’ management also uses the following other key performance indicators to monitor the performance of the business and to manage the business. Comparative results have been restated to reflect the treatment of the Domestic Appliances business as a discontinued operation (for more information, please refer to Discontinued operations and assets classified as held for sale).
Philips Group
Other Key Performance Indicators
| 2020 | 2021 | 2022 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|
| Lives improved, in billions | 1.53 | 1.67 | 1.81 | 1.67 | 1.81 | 1.88 |
| Operational carbon footprint, in kilotonnes CO2-equivalent | 518 | 519 | 438 | 519 | 438 | 418 |
| Circular revenues | 15% | 16% | 18% | |||
| Circular revenue | 16.0% | 18.1% | 20.0% | |||
| Waste to landfill | 2.6% | 0.1% | 0.0% | 0.1% | 0.0% | 0.0% |
| Closing the Loop1) | N/A | 34% | 35% | |||
| Closing the Loop | 34.0% | 35.3% | 20.5% | |||
| Comparable order intake | 9% | 4% | (3)% | 4% | (3)% | (5)% |
Lives Improved
The purpose of Philips is to improve people’s health and well-being through meaningful innovation and we aim to improve the lives of 2 billion people a year by 2025, including 300 million in underserved communities, rising to 2.5 billion and 400 million respectively by 2030. We use Lives Improved as a measurement of our societal impact. In the course of 2021 we changed the definition of ‘lives improved’ (effective January 2021) to align more closely with our purpose. The new definition includes only products or solutions that contribute to people’s health and well-being, and no longer includes the contribution from our Green Products and Solutions that support a healthy ecosystem. Additionally, as we discontinued our Domestic Appliances business, we have removed the impact of this business from the Lives Improved results. The combined impact of these changes resulted in an overall drop of 223 million lives improved in 2021. We calculate Lives Improved as the number of individual interactions for each product sold (based on market intelligence and statistical data) and multiply by the number of those products delivered in a year (eliminating double counting for multiple different product touches per individual). See Improving people’s lives for more information on Lives Improved.
Operational Carbon Footprint
We aim to minimize our environmental impact and we use the Operational Carbon Footprint as one of the measurements of our impact. We define Operational Carbon Footprint as the total greenhouse gas emissions caused by an organization, event, product or person; expressed in kilotonnes CO2-equivalent. We calculate our Operational Carbon Footprint on a monthly basis and include industrial sites (manufacturing and assembly sites), non-industrial sites (offices, warehouses, IT centers and R&D facilities), business travel (lease and rental cars and airplane travel) and logistics (air, sea and road transport) See Sustainable Operations for more information on our Operational Carbon Footprint.
Circular RevenuesAs a company committed to the transition to a circular economy, we aim to decouple economic growth from the use of natural resources and ecosystems by using those resources more effectively. We define Circular Revenues asare revenues generated throughfrom Philips products, services and solutions that meet specific Circular Economycontribute to circular practices. Propositions that qualify for circular revenues must comply with the requirements (including performance and access-based business models, refurbished, reconditioned and remanufactured products and systems, refurbished, reconditioned and remanufactured components, upgrades or refurbishment on site or remote, andfor at least one of the circular revenue categories. These include, among others, products with low weight or containing a minimum threshold of recycled or bio-based plastics, content of >25% post-consumer recycled plasticsas-a-service models, software running in the cloud, telehealth, upgrades, lifetime extensions, and refurbished equipment or >30% post-industrial/postconsumer recycled plastics by total weight of eligible plastics). We calculate Circular Revenues as annual revenues attributable to products and solutions that meet the Circular Economy requirements.components.
Waste to Landfill
At Philips, as a responsible company, we strive to reduce our environmental impact. We define Waste to Landfill as total waste that is delivered for landfill and exclude one-time-only waste and waste delivered to landfill due to regulatory requirements. We calculate Waste to Landfill in kilotonnes per year. See Sustainable Operations for more information on Waste to Landfill.
Closing the Loop At Philips,Closing the loop means we are committedembedding a policy to offer a trade-in onresponsibly take back all our professional medical equipment andsold directly to take carecustomers as part of responsible repurposinga trade-in offer or as a service at customer request. As part of such trade-in systems.the policy, we will ensure that equipment coming back to us is, where feasible, made available for refurbishment and/or parts recovery, or locally recycled in a certified way to ensure it does not end up in landfill. We callmonitor the impact of our policies by measuring the amount of equipment that we collect from our customers. We report on this “Closing the Loop”. We calculate Closing the Loop as Process Adherence (%) multiplied by Reclaim (%)‘reclaimed equipment’. Process adherence (%) is defined as the % of won Replacement Philips deals which are associated with a trade in request in our CRM system. Reclaim (%) is defined as the % of won Replacement Philips deals with a customer accepted trade in request in our CRM system and a repurposing strategy that fulfills our reclaim requirements.
Philips believes that the five other key performance indicators described above (Lives Improved, Operational Carbon Footprint, Circular Revenues, Waste to Landfill and Closing the Loop) provide important information to investors and are important to understanding the long-term performance and prospects of the business. In addition, these other key performance indicators are also used for management compensation purposes. Members of the Board of Management are eligible for grants of performance shares under the Long-Term Incentive (LTI) Plan, and the vesting of the performance shares is subject to performance over a period of 3 years and based on certain criteria, including a 10% weighting for Sustainability Objectives, which Philips defines as the five other key performance indicators described above: Lives Improved, Carbon Footprint, Circular Revenues, Waste to Landfill and Closing the Loop. Philips believes that including these other key performance indicators in our remuneration policy encourages management to act responsibly and sustainably, supporting the company’s overall performance and enhancing the long-term value of the company. See Remuneration of the Board of Management in 20222023 for more information on the Philips’ Long-Term Incentive (LTI) Plan.
Philips currently intends to propose a 2024 Remuneration Policy for the Board of Management which would, among other things, provide for the vesting of performance shares subject to performance over a period of 3 years and based on certain criteria, including a 20% weighting for Sustainability Objectives, which would be defined under that plan as: Lives Improved, Carbon Footprint, Circular Revenues and Employee Engagement Score. The 2024 Remuneration Policy is subject to the approval of Philips shareholders at the 2024 AGM. See Remuneration of the Board of Management in 2023, starting on page 115 for more information on the Philips’ Long-Term Incentive (LTI) Plans under the 2020 Remuneration Policy and the currently proposed 2024 Remuneration Policy.
Comparable order intake
Comparable order intake represents the period-on-period growth, expressed as a percentage, in order intake excluding the effects of currency movements and changes in consolidation. Comparable order intake is reported for equipment and software in the Diagnoses & Treatment and Connected Care businesses,segments, and is defined as the total contractually committed value of equipment and software to be delivered within a specified timeframe, and is an approximation of expected future revenue growth in the respective businesses. Comparable order intake does not derive from the financial statements and a quantitative reconciliation is thus not provided. In 2023, comparable order book was tracked for businesses that represented approximately 40% of 2023 sales.
Philips has simplified its order intake policy by aligning horizons for all modalities to 18 months to revenue. Order intake for software contracts corresponds to the same 18 months to revenue horizon, meaning that only the next 18 months conversion to revenue under the contract is recognized. Philips believes this policy eliminates major variances in order intake growth and better reflects expected revenue in the short term from order intake booked in the reporting period.
Philips uses comparable order intake as an indicator of business activity and performance. Comparable order intake is not an alternative to revenue and may be subject to limitations as an analytical tool due to differences in amount and timing between booking orders and revenue recognition. Due to divergence in practice, other companies may calculate this or a similar measure (such as order backlog) differently and therefore comparisons between companies may be complicated.
14.3Taxation
Dutch taxation
The statements below are only a general summary of certain material Dutch tax consequences for holders of common shares that are non-residents of the Netherlands based on Dutch tax laws, presently in force, and the Tax Convention of December 18, 1992, as amended by the protocol that entered into force on December 28, 2004, between the United States of America and the Kingdom of the Netherlands (the US Tax Treaty) and are not to be read as extending by implication to matters not specifically referred to herein. As to individual tax consequences, investors in common shares should consult their own professional tax advisor.
With respect to a holder of common shares that is an individual who receives income or derives capital gains from common shares and this income received or capital gains derived are attributable to past, present or future employment activities of such holder, the income of which is taxable in the Netherlands, the Dutch tax position is not discussed in this summary.
Dividend withholding tax
In general, a distribution to shareholders by a company resident in the Netherlands (such as the company) is subject to a withholding tax imposed by the Netherlands at a rate of 15%. Share dividends paid out of the company’s paid-in share premium recognized for Dutch tax purposes are not subject to the abovementioned withholding tax. Share dividends paid out of the company’s retained earnings are subject to dividend withholding tax on the nominal value of the shares issued.
Relief at source is available to certain qualifying corporate holders of common shares if such common shares are attributable to a business carried out in the Netherlands, provided that such holder demonstrates that it is the beneficial owner of the dividend. Relief at source is available for dividend distributions to certain qualifying corporate holders of common shares resident in EU/EEA member states, and to certain qualifying corporate holders of common shares resident in non-EU/EEA states with which the Netherlands has concluded a tax treaty that includes a dividend article, provided that such holder demonstrates that it is the beneficial owner of the dividend unless such holder holds the common shares of the company with the primary aim or one of the primary aims to avoid the levy of Dutch dividend withholding tax from another person and the shareholding is put in place without valid commercial reasons that reflect economic reality.
Upon request and under certain conditions, certain qualifying non-resident individual and corporate holders of common shares resident in EU/EEA member states or in a qualifying non-EU/EEA state may be eligible for a refund of Dutch dividend withholding tax to the extent that the withholding tax levied is higher than the personal and corporate income tax which would have been due if they were resident in the Netherlands. However, this refund is not applicable when, based on the US Tax Treaty, the Dutch dividend withholding tax can be fully credited in the United States by the US holder.
Pursuant to the provisions of the US Tax Treaty, a reduced rate may be applicable in respect of dividends paid by the company to a beneficial owner holding directly 10% or more of the voting power of the company, if such owner is a company resident in the United States (as defined in the US Tax Treaty) and entitled to the benefits of the US Tax Treaty.
Pursuant to Dutch anti-dividend stripping legislation, a holder of common shares who is the recipient of dividends will in any case not be considered the beneficial owner of the dividends if (i) as a consequence of a combination of transactions, a person other than the recipient benefits, in full or in part, directly or indirectly, from the dividends; (ii) whereby such other person retains, directly or indirectly, an interest similar to that in the common shares on which the dividends were paid; and (iii) that other person is entitled to a credit, reduction or refund of dividend withholding tax that is less than that of the recipient.
Dividends paid to qualifying exempt US pension trusts and qualifying exempt US organizations are, under certain conditions, exempt from Dutch withholding tax under the US Tax Treaty. Qualifying exempt US pension trusts normally remain subject to withholding at the rate of 15% and are required to file for a refund of the tax withheld. Only if certain conditions are fulfilled, such pension trusts may be eligible for relief at source upon payment of the dividend. However, for qualifying exempt US organizations no relief at source upon payment of the dividend is currently available; such exempt US organizations should apply for a refund of the 15% withholding tax withheld. Further, under certain circumstances, certain exempt organizations (e.g. pension funds) may be eligible for a refund of Dutch withholding tax upon their request pursuant to Dutch tax law. From 1 January 2024 onwards, , provided certain conditions are met, such (US) organizations may be eligible for relief at source upon request.
The company may, with respect to certain dividends received from qualifying non-Dutch subsidiaries, credit taxes withheld from those dividends against the Dutch withholding tax imposed on certain qualifying dividends that are redistributed by the company, up to a maximum of the lesser of:
- 3% of the amount of qualifying dividends redistributed by the company; and
- 3% of the gross amount of certain qualifying dividends received by the company.
The reduction is applied to the Dutch dividend withholding tax that the company must pay to the Dutch tax authorities and not to the Dutch dividend withholding tax that the Company must withhold.
From 1 January 2024 onwards, in addition to Dutch dividend withholding tax, Dutch conditional withholding tax may apply at a statutory rate of 25.8% on dividends and other (deemed) distributions to certain affiliated (gelieerde) entities of the company for the purpose of the Dutch Withholding Tax Act 2021 (Wet bronbelasting 2021).
The Dutch conditional withholding tax only applies on dividends and other (deemed) distributions to entities that are resident (gevestigd), or have a permanent establishment to which the dividend or distribution is attributable, in a jurisdiction that is listed in the Dutch Regulation on low-taxing states and non-cooperative jurisdictions for tax purposes (Regeling laagbelastende staten en niet-coöperatieve rechtsgebieden voor belastingdoeleinden), and in certain deemed abusive situations.
An entity is generally affiliated within the meaning of the Dutch Withholding Tax Act 2021 if there is a controlling relationship between such entity and the distributing company.
Income and capital gains
Income and capital gains derived from the common shares by a non-resident individual or non-resident corporate shareholder are generally not subject to Dutch income or corporation tax, unless (i) such income and gains are attributable to a (deemed) permanent establishment or (deemed) permanent representative of the shareholder in the Netherlands; or (ii) the shareholder is entitled to a share in the profits of an enterprise or (in the case of a non-resident corporate shareholder only) a co-entitlement to the net worth of an enterprise that is effectively managed in the Netherlands (other than by way of securities) and to which enterprise the common shares are attributable; or (iii) such income and capital gains are derived from a direct, indirect or deemed substantial participation in the share capital of the company (such substantial participation not being a business asset), and, in the case of a non-resident corporate shareholder only, it is being held with the primary aim or one of the primary aims to avoid the levy of income tax from another person and is put in place without valid commercial reasons that reflect economic reality; or (iv) in the case of a non-resident corporate shareholder, such shareholder is a resident of Aruba, Curacao or Saint Martin with a permanent establishment or permanent representative in Bonaire, Eustatius or Saba to which the common shares are attributable and certain conditions are met; or (v) in the case of a non-resident individual, such individual derives income or capital gains from the common shares that are taxable as benefits from ‘miscellaneous activities’ in the Netherlands (resultaat uit overige werkzaamheden, as defined in the Dutch Income Tax Act 2001), which includes the performance of activities with respect to the common shares that exceed regular portfolio management.
In general, a holder of common shares has a substantial participation if he holds either directly or indirectly and either independently or jointly with his partner (as defined in the Dutch Income Tax Act 2001), the ownership of, or certain other rights over, at least 5% of the total issued share capital or total issued particular class of shares of the company or rights to acquire direct or indirect shares, whether or not already issued, that represent at any time 5% or more of the total issued capital (or the total issued particular class of shares) or the ownership of certain profit participating certificates that relate to 5% or more of the annual profit or to 5% or more of the liquidation proceeds. A shareholder will also have a substantial participation in the company if one or more of certain relatives of the shareholder hold a substantial participation in the company. A deemed substantial participation amongst others exists if (part of) a substantial participation has been disposed of, or is deemed to have been disposed of, on a nonrecognition basis.
Estate and gift taxes
No estate, inheritance or gift taxes are imposed by the Netherlands on the transfer or deemed transfer of common shares by way of gift by or on the death of a shareholder if, at the time of the death of the shareholder or the gift of the common shares (as the case may be), such shareholder is not a (deemed) resident of the Netherlands.
Inheritance or gift taxes (as the case may be) are due, however, if such shareholder:
- has Dutch nationality and has been a resident of the Netherlands at any time during the ten years preceding the time of their death or gift; or
- does not have Dutch nationality but has been a resident of the Netherlands at any time during the twelve months preceding the time of the gift (for Netherlands gift taxes only).
United States Federal Taxation
This section describes the material United States federal income tax consequences to a US holder (as defined below) of owning common shares. It applies only if the common shares are held as capital assets for United States federal income tax purposes. This discussion addresses only United States federal income taxation and does not discuss all of the tax consequences that may be relevant to a US holder in light of its individual circumstances, including foreign, state or local tax consequences, estate and gift tax consequences, and tax consequences arising under the Medicare contribution tax on net investment income or the alternative minimum tax. This section does not apply to a member of a special class of holders subject to special rules, including:
- a dealer in securities,
- a trader in securities that elects to use a mark-to-market method of accounting for securities holdings,
- a tax-exempt organization,
- a life insurance company,
- a person that actually or constructively owns 10% or more of the combined voting power of our voting stock or of the total value of our stock,
- a person that holds common shares as part of a straddle or a hedging or conversion transaction,
- a person that purchases or sells common shares as part of a wash sale for tax purposes, or
- a person whose functional currency is not the US dollar.
This section is based on the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations, published rulings and court decisions, all as currently in effect, as well as on the US Tax Treaty. These authorities are subject to change, possibly on a retroactive basis.
If an entity or arrangement that is treated as a partnership for United States federal income tax purposes holds the common shares, the United States federal income tax treatment of a partner will generally depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding the common shares should consult its tax advisor with regard to the United States federal income tax treatment of an investment in the common shares.
A US holder is defined as a beneficial owner of common shares that is, for United States federal income tax purposes:
- a citizen or resident of the United States,
- a domestic corporation,
- an estate whose income is subject to United States federal income tax regardless of its source, or
- a trust if a United States court can exercise primary supervision over the trust’s administration and one or more United States persons are authorized to control all substantial decisions of the trust.
A US holder should consult its own tax advisor regarding the United States federal, state and local tax consequences of owning and disposing of common shares in its particular circumstances.
The tax treatment of common shares will depend in part on whether or not we are classified as a passive foreign investment company, or PFIC, for United States federal income tax purposes. Except as discussed below under “—PFIC Rules”, this discussion assumes that we are not classified as a PFIC for United States federal income tax purposes.
Taxation of Distributions
Under the United States federal income tax laws, the gross amount of any distribution paid in stock or cash out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes), other than certain pro-rata distributions of our common shares, will be treated as a dividend that is subject to United States federal income taxation. For a non-corporate US holder, dividends paid that constitute qualified dividend income will be taxable at the preferential rates applicable to long-term capital gains, provided that the non-corporate US holder holds the common shares for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and provided it meets other holding period requirements. Dividends paid with respect to the common shares generally will be qualified dividend income provided that, in the year in which the dividend is received, the common shares are readily tradable on an established securities market in the United States. Our common shares are listed on the New York Stock Exchange and we therefore expect that dividends will be qualified dividend income. A US holder must include any Dutch tax withheld from the dividend payment in this gross amount even though it does not in fact receive it. The dividend is taxable to a US holder when it receives the dividend, actually or constructively. The dividend will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations. For dividend payments made in euro, the amount of the dividend distribution that a US holder must include in its income will be the US dollar value of the euro payments made, determined at the spot euro/US dollar rate on the date the dividend is distributed, regardless of whether the payment is in fact converted into US dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend is distributed to the date a US holder converts the payment into US dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. Distributions in excess of current and accumulated earnings and profits, as determined for United States federal income tax purposes, will be treated as a non-taxable return of capital to the extent of a US holder’s basis in the common shares and thereafter as capital gain. However, we do not expect to calculate earnings and profits in accordance with United States federal income tax principles. Accordingly, US holders should expect to generally treat distributions we make as dividends.
Subject to certain limitations (including, but not limited to, those described in this paragraph), the Dutch tax withheld in accordance with the US Tax Treaty and paid over to the Netherlands will be creditable or deductible against a US holder’s United States federal income tax liability. However, the Dutch withholding tax may not be creditable or deductible to the extent that we reduce (as described above under “Dutch taxation - Dividend withholding tax”) the amount of withholding tax paid over to the Netherlands by crediting taxes withheld from certain dividends received by us. In addition, special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the preferential tax rates. To the extent reduction or refund of the tax withheld is available under Dutch law, or under the US Tax Treaty, the amount of tax withheld that could have been reduced or that is refundable will not be eligible for credit against United States federal income tax liability. Dividends will generally be income from sources outside the United States, and will generally be “passive” income for purposes of computing the foreign tax credit allowable to the holder. In addition, to the extent an amount of Dutch tax withheld is contingent on the availability of a credit against the amount of income tax owed to another country, that amount of Dutch tax withheld will not be eligible for a credit against the US holder’s United States federal income tax liability.
Taxation of Capital Gains
A US holder that sells or otherwise disposes of its common shares will recognize capital gain or loss for United States federal income tax purposes equal to the difference between the US dollar value of the amount that it realizes and its tax basis, determined in US dollars, in its common shares. Capital gain of a non-corporate US holder is generally taxed at preferential rates where the property is held more than one year. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Passive Foreign Investment Company Rules
We believe that the common shares should currently not be treated as stock of a PFIC for United States federal income tax purposes, and we do not expect to become a PFIC in the foreseeable future. However, this conclusion is a factual determination that is made annually and thus may be subject to change. It is therefore possible that we could become a PFIC in a future taxable year. If we are treated as a PFIC, gain realized on the sale or other disposition of the common shares would in general not be treated as capital gain. Instead, unless a US holder elects to be taxed annually on a mark-to-market basis with respect to the common shares, a US holder would generally be treated as if it had realized such gain and certain “excess distributions” ratably over the holding period for the common shares and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, in addition to which an interest charge in respect of the tax attributable to each such year would apply. Any dividends received by a US holder will not be eligible for the special tax rates applicable to qualified dividend income if we are treated as a PFIC with respect to such US holder either in the taxable year of the distribution or the preceding taxable year, but instead will be taxable at rates applicable to ordinary income and subject to the excess distribution regime described above.
14.4Investor information
14.3.214.4.2Financial calendar
Financial calendar
| Annual General Meeting of Shareholders | |
| Record date | April |
| May | |
| Quarterly reports1) | |
| First quarter results | April |
| Second quarter results | July |
| Third quarter results | October |
| Fourth quarter results |
20232024 Annual General Meeting of Shareholders
The Agenda and the explanatory notes to the Agenda for the Annual General Meeting of Shareholders on May 9, 2023,7, 2024, will be published on the company’s website.
For the 20232024 Annual General Meeting of Shareholders, a record date of April 11, 20239, 2024 will apply. Those persons who, on that date, hold shares in the company, and are registered as such in one of the registers designated by the Board of Management for the Annual General Meeting of Shareholders, will be entitled to participate in, and vote at, the meeting.
14.3.314.4.3Investor contact
Shareholder services
Shareholders and other interested parties can make inquiries about the Annual Report 20222023 to:
Royal Philips
Annual Report Office
Philips Center
P.O. Box 77900
1070 MX Amsterdam, The Netherlands
E-mail: annual.report@philips.com
The Annual Report on Form 20-F is filed electronically with the US Securities and Exchange Commission.
Holders of shares listed on Euronext Amsterdam
Communications concerning share transfers, share certificates, dividends and change of address should be directed to:
ABN AMRO Bank N.V.
Department Equity Capital Markets/Corporate Broking and Issuer Services HQ7212
Gustav Mahlerlaan 10,
1082 PP Amsterdam, The Netherlands
Telephone: +31-20-628-6070
E-mail: corporate.broking@nl.abnamro.com
Holders of New York Registry shares
Communications concerning share transfers, share certificates, dividends and change of address should be directed to:
Deutsche Bank Trust Company Americas
C/O ASTEquiniti Trust Company LLC6201 15th Avenue Brooklyn,Peck Slip Station, PO Box 2050, New York NY 1121910272-2050
Telephone (toll-free US): +1-866-706-8374
Telephone (outside of US): +1-718-921-8137
Website: www.astfinancial.comwww.equiniti.com
E-mail: db@astfinancial.comadr@equiniti.com
International direct investment program
Royal Philips offers a Dividend Reinvestment and Direct Stock Purchase Plan designed for the US market. This program provides existing shareholders and interested investors with an economical and convenient way to purchase and sell Philips New York Registry shares (listed at the New York Stock Exchange) and to reinvest cash dividends. Deutsche Bank (the registrar of Philips NY Registry shares) has been authorized to implement and administer both plans for registered shareholders of and new investors in Philips NY Registry shares. Philips does not administer or sponsor the Program and assumes no obligation or liability for the operation of the plan. For further information on this program and for enrollment forms, contact:
Deutsche Bank Global Direct Investor ServicesTrust Company Americas
C/O Equiniti Trust Company LLC
PO Box 10027, Newark NJ 07101
Telephone (toll-free(toll free US): +1-866-706-8374
Telephone (outside of US): +1-718-921-8137Monday through Friday 8:00 AM EST through 8:00 PM ESTWebsite: www.equiniti.com
Website www.astfinancial.com
E-mail: db@astfinancial.com
or write to:
Deutsche Bank Trust Company Americasadr@equiniti.com
IC/O AST
6201 15th Avenue Brooklyn, NY 11219
Analysts’ coverage
Royal Philips is covered by approximately 20 analysts. For a list of our current analysts, please refer to: www.philips.com/a-w/about/investor/stock-info/analyst-coverage.html
How to reach us
Investor Relations contact
Royal Philips
Philips Center
P.O. Box 77900
1070 MX Amsterdam, The Netherlands
Telephone: +31-20-59 77222
Website: www.philips.com/investor
E-mail: investor.relations@philips.com
Leandro Mazzoni
Head of Investor Relations
Telephone: +31-20-59 77222
Derya Guzel
Investor Relations Director
Telephone: +31-20-59 77222
Dorin Danu
Investor Relations Director
Telephone: +31-20-59 7722277055
Global Sustainability contact
Royal Philips
High Tech Campus 51, 1st floor
5656 AG Eindhoven, The Netherlands
Telephone: +31-40-27 83651
Website: www.philips.com/sustainability
E-mail: philips.sustainability@philips.com
Global Press Office contact
Royal Philips
Philips Center
Amstelplein 2
1096 BC Amsterdam, The Netherlands
E-mail: group.communications@philips.com
For media contacts please refer to:
https://www.philips.com/a-w/about/news/contacts.html
Registered address
High Tech Campus 52, 5656 AG Eindhoven, The Netherlands
14.414.5Definitions and abbreviations
Actionable
In the context of the Respironics recall, actionable registrations are those that contain the necessary information needed to complete the remediation and are not awaiting further information, including from patient registrants.
Artificial Intelligence (AI)
Philips embraces the following formal definition of AI (source: European Commission High-Level Expert Group definition AI): Artificial intelligence (AI) systems are software (and possibly also hardware) systems designed by humans that, given a complex goal, act in the physical or digital dimension by perceiving their environment through data acquisition, interpreting the collected structured or unstructured data, reasoning on the knowledge, or processing the information, derived from this data and deciding the best action(s) to take to achieve the given goal.
AI systems can either use symbolic rules or learn a numeric model, and they can also adapt their behavior by analyzing how the environment is affected by their previous actions.
As a scientific discipline, AI includes several approaches and techniques, such as machine learning (of which deep learning and reinforcement learning are specific examples), machine reasoning (which includes planning, scheduling, knowledge representation and reasoning, search, and optimization), and robotics (which includes control, perception, sensors and actuators, as well as the integration of all other techniques into cyber-physical systems).
See also Philips AI Principles.
Brominated flame retardants (BFR)
Brominated flame retardants are a group of chemicals that have an inhibitory effect on the ignition of combustible organic materials. Of the commercialized chemical flame retardants, the brominated variety are most widely used.
Business/Business unit
In the Philips Operating Model, our three operating segments are made up of six businesses, which are in turn comprised of 18 business units. See also the entry under Segment.
CO2-equivalent
CO2-equivalent or carbon dioxide equivalent is a quantity that describes, for a given mixture and amount of greenhouse gas, the amount of CO2 that would have the same global warming potential (GWP), when measured over a specified timescale (generally 100 years).
Circular economy
A circular economy aims to decouple economic growth from the use consumption of natural resources by optimizing their use, eliminating waste and ecosystems by using those resources more effectively. By definition itpollution, and circulating products and materials for as long as possible, while giving natural systemsthe opportunity to regenerate themselves.
Circular Materials Management
Circular Materials Management is a driverKPI for innovationpromoting an increase in the areasproportion of material, componentwaste treated using waste management hierarchy levels that are circular: prevention, re-use, and product reuse, as well as new business models such as solutions and services. In arecycling. Circular Economy,Materials Management % is the more effective useproportion of materials makes it possiblemanaged circularly in comparison to create more value,the total used materials baseline. The total used materials baseline is the total of both circular and linear waste, excluding linear disposal of waste that is required by cost savings and by developing new markets or growing existing ones.
law. Circular MaterialMaterials Management
Circular Material Management has replaced the includes recycling, percentage at Philips, as we endeavor to reduce total material use. The Circular Material Management percentage includes circular measures such as waste prevented, reusere-use, prevention and other recovery but(e.g. repurposing). It excludes all linear disposal, which is classified as waste delivered to landfillenergy, incineration and incineration (with and without energy recovery) due to regulatory requirements.landfill.
Circular Revenues
Circular Revenues are defined by revenues generated throughfrom Philips products, services and solutions that meet specific Circular Economy requirements.contribute to circular practices. Propositions that qualify for circular revenues must comply with the requirements for at least one of the circular revenue categories. These include, performance and access-based business models, refurbished, reconditioned and remanufactured products and systems, refurbished, reconditioned and remanufactured components, upgrades or refurbishment on site or remote, andamong others, products with low weight or containing a minimum threshold of recycled or bio-based plastics, contentas-a-service models, software running in the cloud, telehealth, upgrades, lifetime extensions, and refurbished equipment.
Closing the Loop / reclaimed equipment
Closing the loop means we are embedding a policy to responsibly take back all professional medical equipment sold directly to customers as part of >25% post-consumera trade-in offer or as a service at customer request. As part of the policy, we will ensure that equipment coming back to us is, where feasible, made available for refurbishment and/or parts recovery, or locally recycled plastics or >30% post-industrial/post-consumer recycled plasticsin a certified way to ensure it does not end up in landfill. We monitor the impact of our policies by total weightmeasuring the amount of eligible plastics.equipment that we collect from our customers. We report on this as ‘reclaimed equipment’.
Dividend yield
The dividend yield is the annual dividend payment divided by Philips’ market capitalization. All references to dividend yield are as of December 31 of the previous year.
EcoHeroes
Philips’ ‘EcoHeroes’ concept aims to drive innovation beyond our EcoDesign requirements, delivering solutions that are demonstrably setting the pace in terms of environmental impact. EcoHeroesAn EcoHero product meets all EcoDesign requirements applicable to new product introductions and outperform the relevant benchmarks in at least one of the focal areas; any comparative sustainability claim is underpinned by a quantitative analysis. Our target is to have 25%areas of total hardware revenue coming from EcoHeroes by 2025.EcoDesign (Energy, Packaging, Substances and Circularity).
Employee Engagement Index (EEI)
The Employee Engagement Index (EEI) is the single measure of the overall level of employee engagement at Philips. It is a combination of perceptions and attitudes related to employee satisfaction, commitment and advocacy.
Energy-using Products (EuP)
An energy-using product is a product that uses, generates, transfers or measures energy (electricity, gas, fossil fuel). Examples include boilers, computers, televisions, transformers, industrial fans and industrial furnaces.
Functions
In the Philips Operating Model, Philips' businesses are supported by lean Functions. The Functions deliver cost-effective services, ensure legal & regulatory requirements are deployed, and propose Enterprise policies, standards, guidance and infrastructure, as well as providing functional capabilities and expertise (e.g. via Centers of Excellence).
Full-time equivalent employee (FTE)
Full-time equivalent is a way to measure a worker’s involvement in a project. An FTE of 1.0 means that the person is equivalent to a full-time worker, while an FTE of 0.5 signals that the worker works half-time.
Global Reporting Initiative (GRI)
The Global Reporting Initiative (GRI) is a network-based organization that pioneered the world’s most widely used sustainability reporting framework. GRI is committed to the framework’s continuous improvement and application worldwide. GRI’s core goals include the mainstreaming of disclosure on environmental, social and governance performance.
Green/EcoDesigned Innovation
Green/EcoDesigned Innovation comprises all R&D activities directly contributing to the intended development of Green/EcoDesigned Products or Green/EcoDesigned Technologies. Innovation projects are characterized as Green/EcoDesigned based on the innovation brief; this designation is not revised during the project lifetime.Products.
Green/EcoDesigned Products
A Green/EcoDesigned Products offer a significant environmental improvementProduct must comply with all applicable legal requirements, Philips policies, and all stated EcoDesigned Product requirements in one or more Green Focal Areas:our four focal areas: Energy, efficiency, Packaging, Hazardous substances, Weight,Substances, Circularity and Lifetime reliability.Packaging. The life cycle approachaim is used to determine a product’s overall environmental improvement. It calculatesimprove the environmental impactenergy efficiency of a product over its total life cycle (raw materials, manufacturing, productour products, use less resources and disposal). Green/EcoDesigned Products needmore recycled content, avoid the use of hazardous substances, design for circularity, and make our packaging easier to prove leadership in at least one Green Focal Area compared to industry standards, which is defined by a segment-specific peer group. This is done either by outperforming reference products (which can be a competitor or predecessor product in the particular product family) by at least 10%, by outperforming product-specific eco-requirements or by being awarded with a recognized eco-performance label. Because of different product portfolios, businesses have specified additional criteria for Green/EcoDesigned Products, including product specific minimum requirements where relevant.recycle and re-use.
Green/EcoDesigned Revenues
Green/EcoDesigned Revenues are generated through products and solutions which offer a significant environmental improvement in one or more ofthat meet the Green Focal Areas: Energy efficiency, Packaging, Hazardous substances, Weight, Circularity, and Lifetime reliability. Green/EcoDesigned Revenues are determined by classifying the environmental impact of the product or solution over its total life cycle. Philips uses Green/EcoDesigned Revenues as a measure of social and economic performance in addition to its environmental results. The use of this measure may be subject to limitations as it does not have a standardized meaning and similar measures could be determined differently by other companies. A product or solution that has been determined to contribute to Green/EcoDesigned Revenues will continue to do so until it is decommissioned.Products definition.
Growth geographies
Growth geographies areconsists of the grouping 'Growth', which comprises the developing geographies comprising of Asia Pacific (excluding Japan, South Korea, Australia and New Zealand), Latin America, Central & Eastern Europe, Middle East & Turkey (excluding Israel) and Africa.
Hazardous substances
Hazardous substances are generally defined as substances posing imminent and substantial danger to public health and welfare or the environment.
Income from operations (EBIT)
Income from operations as reported on the IFRS consolidated statement of income. The term EBIT (earnings before interest and tax) has the same meaning as Income from operations.
Income from continuing operations
Income from continuing operations as reported on the IFRS consolidated statement of income, which is net income from continuing operations, or net income excluding discontinued operations.
Large medical equipment
MRI systems, CT scanners, NM systems, DXR equipment, and IGT Fixed systems. This includes all Main Article Groups (MAGs) in the portfolio of these business units, except for the MAGs that represent non-life-extending upgrades: 'T82', 'Q72', 'I66', 'X19', 'Q71', 'W62', 'P10', 'S08', 'S14', 'Q74', 'S47', 'S33', 'Z44', 'S66', 'Q76', 'BI9'.
Lean
The basic insight of Lean thinking is that if every person is trained to identify wasted time and effort in their own job and to better work together to improve processes by eliminating such waste, the resulting enterprise will deliver more value at less expense.
Lives improved by Philips
To calculate how many lives we are improving, market intelligence and statistical data on the number of people touched by the products contributing to the social or ecological dimension over the lifetime of a product are multiplied by the number of those products delivered in a year. After elimination of double counts – multiple different product touches per individual are only counted once – the number of lives improved by our innovative solutions is calculated.
Long-term strategic partnership
Multi-year contractual agreement that represents a partnership to enable long-term collaboration.
Market/Market Group
A Market consists of one or more countries operating as a single organization under a Market Leader. Our 17 Market organizations are organized in three market groups: North America, Greater China and International Markets.
Mature geographies
Mature geographies are the highly developed markets comprising ofconstituting three geographic areas: Western Europe, North America, and Other mature (including Japan, South Korea, Israel, Australia and New Zealand.Zealand).
Net Promoter Score
Net Promoter Score®, or NPS®, measures customer experience and predicts business growth. NPS is calculated by taking the answer to a key question on a 0-10 scale: How likely is it that you would recommend [brand] to a friend or colleague?
Respondents are grouped as follows:
- Promoters (score 9-10) are loyal enthusiasts who will keep buying and refer others, fueling growth.
- Passives (score 7-8) are satisfied but unenthusiastic customers who are vulnerable to competitive offerings.
- Detractors (score 0-6) are unhappy customers who can damage the brand and impede growth through negative word-of-mouth.
Subtracting the percentage of Detractors from the percentage of Promoters yields the Net Promoter Score, which can range from a low of -100 (if every customer is a Detractor) to a high of 100 (if every customer is a Promoter).
Operational carbon footprint
A carbon footprint is the total set of greenhouse gas emissions caused by an organization, event, product or person; usually expressed in kilotonnes CO2-equivalent. Philips' operational carbon footprint is calculated on a monthly basis and includes industrial sites (manufacturing and assembly sites), non-industrial sites (offices, warehouses, IT centers and R&D facilities), business travel (lease and rental cars and airplane travel) and logistics (air, sea and road transport).
Philips Lighting/Signify
References to 'Signify' in this Annual Report relate to Philips' former Lighting segment (prior to deconsolidation as from the end of November 2017 and when reported as discontinued operations), Philips Lighting N.V. (before or after such deconsolidation) or Signify N.V. (after its renaming in May 2018), as the context requires.
Polyvinyl chloride (PVC)
Polyvinyl chloride, better known as PVC or vinyl, is an inexpensive plastic so versatile it has become completely pervasive in modern society.
Quadruple Aim
At Philips, we make value-based care principles actionable by addressing the Quadruple Aim – better health outcomes, improved patient experience, improved staff experience, and lower cost of care.
REACH
Registration, Evaluation, Authorization and Restriction of Chemicals (REACH)(REACH;Regulation (EC) No 1907/2006) is a European Union regulation that addresses the production and use (e.g. in products) of chemical substances, and their potential impact on both human health and the environment. This regulation is covered in the Philips Regulated Substances List.
Regulated Substance List
Philips Regulated Substances List (RSL) combines legal, industry, and voluntary Philips requirements regarding chemical substances used in Philips products and their packaging, either on a homogenous material level or present in the product as such. The RSL contains restricted and declarable substances.
Respironics recall
The voluntary recall notification in the United States and field safety notice outside the United States for certain sleep and respiratory care products initiated by Philips Respironics in 2021.
Responsible Business Alliance (RBA)
The Responsible Business Alliance (formerly known as The Electronic Industry Citizenship Coalition (EICC)) was established in 2004 to promote a common code of conduct for the electronics and information and communications technology (ICT) industry. EICC now includes more than 100 global companies and their suppliers.
Restriction on Hazardous Substances (RoHS)
The RoHS Directive prohibits all new electrical and electronic equipment placed on the market in the European Economic Area from containing lead, mercury, cadmium, hexavalent chromium, poly-brominated biphenyls (PBB) or polybrominated diphenyl ethers (PBDE)and four phthalates (DEHP, DBP, BBP and DiHP), except in certain specific applications, in concentrations greater than the values decided by the European Commission. These values have been established as 0.01% by weight per homogeneous material for cadmium and 0.1% for the other fivenine substances. This regulation is covered in the Philips Regulated Substances List.
Segment
The Philips Operating Model identifies three operating segments – Diagnosis & Treatment, Connected Care and Personal Care – comprised of six businesses and 18 business units, as well as segment Other. Other includes Innovation & Strategy, IP Royalties, Central Costs, and other small items. See also the entry under Business/Business unit.
Solution
A combination of Philips (and 3rd-party) systems, devices, software, consumables and services, configured and delivered in a way to solve customer (segment)-specific needs and challenges.
Sustainable Development Goals
The Sustainable Development Goals (SDGs) are a collection of 17 global goals set by the United Nations. The broad goals are interrelated though each has its own targets. The SDGs cover a broad range of social and economic development issues. These include poverty, hunger, health, education, climate change, water, sanitation, energy, environment and social justice.
Sustainable Innovation
Sustainable Innovation is the Research & Development spend related to the development of new generations of products and solutions that address the United Nations Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages) or 12 (Ensure sustainable consumption and production patterns). This includes all Diagnosis & Treatment and Connected Care innovation spend. In addition, innovation spend that contributes to Green Products and healthy living at Personal Health is included. Finally, innovation spend at Other that addresses the SDGs 3 and 112 is included.
VOC
Volatile organic compounds (VOCs) are organic chemicals that have a high vapor pressure at ordinary room temperature. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and enter the surrounding air, a trait known as volatility.
Voluntary turnover
Voluntary turnover covers all employees who resigned of their own volition.
Waste Electrical and Electronic Equipment (WEEE)
The Waste Electrical and Electronic Equipment Directive (WEEE Directive) is the European Community directive on waste electrical and electronic equipment setting collection, recycling and recovery targets for all types of electrical goods. The directive imposes the responsibility for the disposal of waste electrical and electronic equipment on the manufacturers of such equipment.
Weighted Average Statutory Tax Rate (WASTR)
The reconciliation of the effective tax rate is based on the applicable statutory tax rate, which is a weighted average of all applicable jurisdictions. This weighted average statutory tax rate (WASTR) is the aggregation of the result before tax multiplied by the applicable statutory tax rate without adjustment for losses, divided by the group result before tax.
15Exhibits
Index of exhibits
| Exhibit 1 | English translation of the Articles of Association of the company (incorporated by reference to Exhibit 1 to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 27, 2019) |
| Exhibit 2 (a) | Description of securities registered under Section 12 of the Exchange Act (Incorporated by reference to Exhibit 2 to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 25, 2020) |
| Exhibit 2 (b) | Amended and Restated Trust Deed Related to a €10,000,000,000 Euro Medium Term Note Programme between the company and Philips agrees to furnish copies of any or all |
| Exhibit 4 | Material Contracts. |
| Exhibit 4 (a) | Services contract between the company and R.W.O. Jakobs |
| |
| Exhibit 4 (b) | Services contract between the company and A. Bhattacharya |
| Exhibit 4 (c) | Services contract between the company and M.J. van Ginneken |
| Exhibit 4 (d) | Global Philips Performance Share Plan applicable to the Board of Management of Koninklijke Philips N.V. |
| Exhibit 4 (e) | Services contract between the company and F.A. van Houten (Incorporated by reference to Exhibit 4 (a) to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 25, 2020) |
| Exhibit 4 (f) | Relationship Agreement between the company and Exor N.V. |
| Exhibit 8 | List of Subsidiaries. |
| Exhibit 12 (a) | Certification of R.W.O. Jakobs filed pursuant to 17 CFR 240. 13a-14(a). |
| Exhibit 12 (b) | Certification of A. Bhattacharya filed pursuant to 17 CFR 240. 13a-14(a). |
| Exhibit 13 (a) | Certification of R.W.O. Jakobs furnished pursuant to 17 CFR 240. 13a-14(b). |
| Exhibit 13 (b) | Certification of A. Bhattacharya furnished pursuant to 17 CFR 240. 13a-14(b). |
| Exhibit 15 (a) | EY Consent of independent registered public accounting firm. |
| Exhibit 97 | Clawback policy |
| 101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH | XBRL Taxonomy Extension Schema Document. |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
KONINKLIJKE PHILIPS N.V. (Registrant) | |
/s/ R.W.O. Jakobs (Chief Executive Officer, Chairman of the Board of Management and the Executive Committee) | /s/ A. Bhattacharya (Chief Financial Officer, Member of the Board of Management and the Executive Committee) |
| Date: February | |