For the fiscal year ended December 31,
As filed with the Securities and Exchange Commission on March 3, 2006
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 20-F
| Registration statement pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934 | |
| OR | |
| Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, | |
| OR | |
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| OR | |
| SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 1-15170
GlaxoSmithKline plc
(Exact name of Registrant as specified in its charter)
| Title of Each Class | Name of Each Exchange On Which Registered |
| American Depositary Shares, each representing 2 Ordinary Shares, Par value 25 pence | New York Stock Exchange |
Securities registered or to be registered to Section 12(g) of the Act:
None
(Title of class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
![]() Yes
Yes ![]() No
No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
![]() Yes
Yes ![]() No
No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the Registrantregistrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrantregistrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
![]() Yes
Yes 
![]() No
No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (check one):
Large accelerated filer ![]() Acccelerated filer
Acccelerated filer ![]() Non-accelerated filer
Non-accelerated filer ![]()
Indicate by check mark which financial statement item the Registrantregistrant has elected to follow.
![]() Item 17
Item 17 
![]() Item 18
Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
![]() Yes
Yes ![]() No
No

GlaxoSmithKline plc is an English public limited company. Its shares are listed on the London Stock Exchange and the New York Stock Exchange.
GlaxoSmithKline plc acquired Glaxo Wellcome plc and SmithKline Beecham plc on 27th December 2000 by way of a scheme of arrangement for the merger of the two companies which became effective on 27th December 2000.
This report is the Annual Report of GlaxoSmithKline plc for the year ended 31st December 2002. It comprises in a single document the Annual Report of the company in accordance with United Kingdom requirements and the Annual Report on Form 20-F to the Securities and Exchange Commission in the United States of America.
A summary report on the year, the Annual Review 2002, intended for the investor not needing the full detail of the Annual Report, is produced as a separate document. The Annual Review includes the joint statement by the Chairman and the Chief Executive Officer, a summary review of operations, summary financial statements and a summary remuneration report.
The Annual Review is issued to all shareholders. The Annual Report is issued to shareholders who have elected to receive it. Both documents are available on GlaxoSmithKline’s corporate website – at www.gsk.com.
WebsiteGlaxoSmithKline’s website, www.gsk.com gives additional information on the Group. Information made available on the website does not constitute part of this Annual Report.
 | |
 | |
| Do more, feel better, live longer | |

JP Garnier (left) and Sir Christopher Gent (right)
“Thanks to the efforts of our employees around the world,
2005 was a very successful year for GSK. Not only
was it our best year ever from a financial standpoint,
we also made substantial progress with our pipeline
of innovative medicines and vaccines.”
JP Garnier, Chief Executive Officer
An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer
2005: a year of success and progress
GSK delivered an excellent financial performance in 2005. Turnover of £21.7 billion grew by 7% at constant exchange rates (CER). Earnings per share (EPS) were 82.6p, with growth of 18% at CER, putting GSK in the top tier of global pharmaceutical companies in terms of performance.
“These figures confirm the excellent growth of our key products and the efficiency of our global operations,” says JP.
GSK’s performance was driven by sales of key pharmaceutical products. “Sales ofSeretide/Advair,Avandia,Coreg,LamictalandValtrexall continued their impressive growth,” says JP. “We also saw good performance from a number of newer products, includingAvodartfor enlarging prostate,Boniva/Bonvivafor osteoporosis andRequipfor Restless Legs Syndrome, which all show great promise for the future, both for patients and GSK.’
“Looking into 2006, the strong growth seen from key products and from our vaccines business is expected to continue and we anticipate an EPS growth of around 10% at CER.”
Pipeline progress
GSK continues to meet the challenge of increasing Research & Development (R&D) productivity to discover new medicines faster and more economically. The company’s pipeline is one of the largest and most promising in the industry, with 149 projects in clinical development (as at the end of February 2006), including 95 new chemical entities (NCEs), 29 product line extensions (PLEs) and 25 vaccines.
“In 2006, we anticipate further good news on GSK’s late-stage pipeline, which is developing at a fast pace. Eight major new assets are scheduled to enter phase III in 2006, doubling our late-stage pipeline,” says JP.
Year of the vaccine
2005 was a landmark year for GSK’s vaccines business. Sales increased by 15% and the company made a number of significant strategic acquisitions. “The acquisition of ID Biomedical was an important move for GSK,” says JP, “which strengthened our position in the global flu vaccine market, and increased our ability to prepare for and respond to a potential flu pandemic.”
“The pharmaceutical industry is making a
positive improvement to people’s lives. It
has a noble purpose. It develops medicines
and vaccines that save lives and make
people feel better.”
Sir Christopher Gent, Chairman
“We also acquired a plant in Marietta, Pennsylvania which will give us access to tissue culture technology in our vaccine manufacturing. The acquisition of Corixa gives us valuable adjuvant technology, enabling us to boost human immune response to our vaccines.”
GSK also made good progress on its pipeline of new vaccines. “We expect five major vaccine launches in the next five years,” says JP. “Perhaps most exciting isCervarixfor cervical cancer, which we expect to file for approval in Europe in March 2006 and in the USA by the end of the year.”
Improving access to medicines
GSK continues to seek new ways of improving access to its medicines for people who need them, but are least able to obtain them. This challenge is particularly acute in the developing world, where GSK has been offering many of its medicines and vaccines at not-for-profit prices for some years.
GSK Annual Report 2005 01 |
However, addressing this challenge is something GSK cannot do alone. The work of GSK with organisations such as the Bill & Melinda Gates Foundation highlights the benefits of public-private partnerships. They provide a way for companies such as GSK and the private sector to work together. Typically, GSK provides the R&D, technology, manufacturing and distribution expertise, while other partners and governments help fund the development and delivery costs.
In 2005, GSK entered three groundbreaking public-private partnerships to develop vaccines against the biggest causes of death in the developing world today – AIDS, malaria and tuberculosis.
“Public-private partnerships use the
respective strengths of the partners
and bring out the best of each. Most
importantly, it is a model that works.”
Reaching out to patients
In 2005, GSK introduced and strengthened a number of initiatives aimed at improving patients’ understanding of GSK’s medicines, and programmes to help gain access to them. These initiatives include GSK’s pioneering Clinical Trial Register, which was expanded to contain 2,125 summaries of clinical trials by the end of 2005.
In the USA, GSK is placing more emphasis on education and the patient in direct-to-consumer advertising, and providing people with advice on GSK’s programmes and the industry’s Partnerships for Prescriptions Assistance which help people gain access to the medicines they need.
“Through these and other initiatives, we are seeking to differentiate GSK as a company finding solutions to the healthcare challenges that society faces. I believe we are well on the way to achieving that,” says Sir Christopher.
A broader contribution
GSK's global community investment activities in 2005 were valued at £380 million, equivalent to 5.6% of Group profit before tax.
The year saw a number of natural disasters, including the Asian tsunami, the Guatemalan hurricane, the New Orleans floods and the earthquake that struck parts of India and Pakistan. GSK was quick to respond to help victims of these tragedies. “My thanks go to our employees for their response to these crises. It makes me proud to lead an organisation with such committed and compassionate people, who can respond so effectively to help people in real need,” says JP.
For these disasters alone, GSK contributed more than £3 million in cash and donated medicines and vaccines valued at over £14 million towards the relief efforts.
“The tragedies during the year brought home to me the extent to which the pharmaceutical industry is making a positive improvement to people’s lives,” says Sir Christopher. “It has a noble purpose. It develops medicines and vaccines that save lives and make people feel better.”
Being human
We continue to meet the challenges of improving productivity in R&D and ensuring patients have access to medicines, even in the poorest parts of the world. This Report highlights some of the work we have done to implement our strategies to meet these challenges. Behind each one is a human story.
We thank all our employees for their efforts in 2005. Their commitment and passion, both individually and through their teamwork, have helped us make GSK the success it is today. We also appreciate the great support our employees receive from their families for the work they are doing at GSK.
We are grateful for the significant contribution of Tachi Yamada, Chairman of R&D and Executive Director, who is to retire in June 2006, and we welcome Moncef Slaoui, who will succeed Tachi with effect from 1st June 2006. We would also like to thank Jack Ziegler, President of GSK Consumer Healthcare, who retired from the company in January 2006, and welcome his successor, John Clarke. We also thank Dr Lucy Shapiro, who is to retire as a Non-Executive Director at the company’s Annual General Meeting in May 2006, and we welcome Tom de Swaan, who joined the Board in January 2006 as a new Non-Executive Director.
 |  |
| Sir Christopher Gent | JP Garnier |
| Chairman | Chief Executive Officer |
| GSK Annual Report 2005 |
| 02 |
| Contents |
| Report of the Directors | |
| Financial summary | 4 |
| Description of business | 5 |
| Corporate governance | 27 |
| Remuneration Report | 37 |
| Operating and financial review and prospects | 55 |
| Financial statements | |
| Directors’ statements of responsibility | 82 |
| Independent Auditors’ report | 83 |
| Consolidated income statement | 84 |
| Consolidated balance sheet | 85 |
| Consolidated cash flow statement | 86 |
| Consolidated statement of recognised income and expense | 88 |
| Notes to the financial statements | 89 |
| Investor information | |
| Financial record | 166 |
| Shareholder information | 176 |
| Taxation information for shareholders | 180 |
| Glossary of terms | 181 |
| Cross reference to Form 20-F | 182 |
| The Annual Report was approved by the Board of Directors on | |
| GSK Annual Report 2005 |
| 03 |
| Financial summary | ||||||||
| 2001 | Increase | |||||||
| 2002 | (restated) | |||||||
| Statutory results | £m | £m | £% | CER% | ||||
| Sales | 21,212 | 20,489 | 4 | 7 | ||||
| Trading profit | 5,662 | 4,697 | 21 | 26 | ||||
| Profit before taxation | 5,506 | 4,517 | 22 | 28 | ||||
| Earnings/Net income | 3,915 | 3,053 | 28 | 35 | ||||
| Basic earnings per share | 66.2 | p | 50.3 | p | 32 | 38 | ||
| Dividends per share | 40.0 | p | 39.0 | p | ||||
| Merger, restructuring and disposal of subsidiaries | ||||||||
| Trading profit | (1,032 | ) | (1,356 | ) | ||||
| Profit before taxation | (1,011 | ) | (1,652 | ) | ||||
| Earnings/Net income | (712 | ) | (1,330 | ) | �� | |||
| Business performance | ||||||||
| Sales | 21,212 | 20,489 | 4 | 7 | ||||
| Trading profit | 6,694 | 6,053 | 11 | 15 | ||||
| Profit before taxation | 6,517 | 6,169 | 6 | 11 | ||||
| Adjusted earnings/Net income | 4,627 | 4,383 | 6 | 11 | ||||
| Adjusted earnings per share | 78.3 | p | 72.3 | p | 8 | 13 | ||
| Financial summary |
| Growth | ||||||||
| 2005 | 2004 | |||||||
| £m | £m | CER% | £% | |||||
| Turnover | 21,660 | 19,986 | 7 | 8 | ||||
| Operating profit | 6,874 | 5,756 | 16 | 19 | ||||
| Profit before taxation | 6,732 | 5,779 | 13 | 16 | ||||
| Profit after taxation for the year | 4,816 | 4,022 | 17 | 20 | ||||
| Profit attributable to minority interests | 127 | 114 | ||||||
| Profit attributable to shareholders | 4,689 | 3,908 | ||||||
| Earnings per share | 82.6 | p | 68.1 | p | 18 | 21 | ||
| Diluted earnings per share | 82.0 | p | 68.0 | p | ||||
| Dividends per share | 44 | p | 42 | p | ||||
| Net cash inflow from operating activities | 5,958 | 4,944 | ||||||
| Net assets | 7,570 | 5,937 | ||||||
Business performance, whichHistory and development of the company
GlaxoSmithKline plc is a public limited company incorporated on 6th December 1999 under English law. Its shares are listed on the primary performance measure used by management, is presented after excluding merger items, integration and restructuring costsLondon Stock Exchange and the disposalNew York Stock Exchange. On 27th December 2000 the company acquired Glaxo Wellcome plc and SmithKline Beecham plc, both English public limited companies, by way of businesses. Management believes that exclusiona scheme of these non-recurring items provides a better comparison of business performancearrangement for the periods presented. Statutory results include these non-recurring items. This information is provided asmerger of the two companies. Both Glaxo Wellcome and SmithKline Beecham were major global healthcare businesses.
GSK plc and its subsidiary and associated undertakings constitute a supplement to that includedmajor global healthcare group engaged in the consolidated statementcreation, discovery, development, manufacture and marketing of profitpharmaceutical and loss on pages 76consumer health-related products.
GSK has its corporate head office in London. It also has operational headquarters in Philadelphia and 77 preparedResearch Triangle Park, USA, and operations in accordancesome 119 countries, with products sold in over 130 countries. The principal research and development (R&D) facilities are in the UK, GAAP.the USA, Japan, Italy, Spain and Belgium. Products are currently manufactured in some 37 countries.
The major markets for the Group’s products are the USA, France, Japan, the UK, Italy, Germany and Spain.
| Business segments GSK operates principally in two industry segments: | |
| • | Pharmaceuticals (prescription pharmaceuticals and vaccines) |
| • | Consumer Healthcare (over-the-counter medicines, oral care and nutritional healthcare). |
The Group, as a multinational business, operates in many countries and earns revenues and incurs costs in many currencies. The results of the Group, as reported in sterling, are therefore affected by movements in exchange rates between sterling and overseas currencies. The Group uses the averageAverage exchange rates prevailing during the yearperiod are used to translate the results and cash flows of overseas companiessubsidiary and associated undertakings and joint ventures into sterling. Period end rates are used to translate the net assets of those undertakings. The currencies which most influence these translations are the US dollar, the Euro and the Japanese Yen. During 2002 average sterling exchange rates were stronger against the US dollar and the Japanese Yen by four per cent and seven per cent, respectively, and weaker against the Euro by one per cent, compared with 2001.
In order to illustrate underlying performance, it is the Group’s practice to discuss its results in terms of constant exchange rate (CER) growth. This represents growth calculated as if the exchange rates used to determine the results of overseas companies in sterling had remained unchanged from those used in the previous year. The discussion in this report is therefore in terms of CER unless otherwise stated.
£% represents growth at actual exchange rates. CER% represents growth at constant exchange rates.
During 2002 FRS 19 ‘Deferred tax’ has been implemented by the Group. This FRS requires deferred tax to be accounted for on a full provision basis, rather than a partial provision basis as in 2001 and earlier years. This change has been accounted for as a prior year adjustment and comparative information has been restated as necessary.
£% represents growth at actual exchange rates.
Cautionary statement regarding forward-looking statements
The Group's reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group's current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. The Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Forward-looking statements involve inherent risks and uncertainties. The Group cautions investors that a number of important factors, including those in this document, could cause actual results to differ materially from those contained in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Risk factors’ on pages 64 and 6571 to 74 of this Annual Report.
| GSK Annual Report 2005 |
| 04 |
Joint statement by the Chairman and the Chief Executive Officer

The purpose of GlaxoSmithKline is to deliver medicines that have a positive impact on the quality of human life. We have chosen this fundamental and challenging objective as the theme of this year’s Annual Review.
We are pleased to report that 2002 was a year of significant progress in establishing GlaxoSmithKline as one of the world’s leading pharmaceutical companies. We achieved strong financial results in 2002, despite the entry of generic competition in the USA to Augmentin, one of our major products.
Our progress stems from the Group’s key strengths: a broadly based product portfolio, strong financial capability and a promising early stage pipeline of products. We have built on each of these core attributes in 2002 and we are confident that they will help GlaxoSmithKline to continue to deliver success in the future.
While achieving business success it is essential that we demonstrate to all our stakeholders, around the world, how we conduct our business with integrity and continue to make a positive contribution to society.
Good financial performanceWe delivered a very solid financial performance in 2002 in a challenging operating environment. Global pharmaceutical sales grew eight per cent to nearly £18 billion and US pharmaceutical sales grew 13 per cent, despite generic competition to Augmentin. The Group demonstrated continued financial strength with total sales up seven per cent and business performance trading profit up 15 per cent. There were strong performances from our key therapy areas including central nervous system, respiratory, anti-virals and vaccines.
Our business performance earnings per share grew by 13 per cent, delivering on our guidance and demonstrating the continuing financial strength that will provide the Group with a sound platform for the future.
GlaxoSmithKline has made good progress with its merger and manufacturing restructuring plans and we remain on track to deliver forecast total annual merger and manufacturing restructuring savings of at least £1.8 billion by 2003. We are not stopping there; our continuous improvement programme, Operational Excellence, is delivering additional savings and will continue to do so.
New product growth drives commercial strengthThe success of our new products is providing the fuel for future growth, with new products now representing 27 per cent of total pharmaceutical sales, up 36 per cent in 2002. Sales of Seretide/Advair for asthma, now our second largest product, continued to grow impressively, up 96 per cent to £1.6 billion. We recently launched Avandamet for type 2 diabetes and Avodart for benign prostatic hyperplasia, as well as important line extensions of Augmentin and Paxil. During 2003-2004 we look forward to launching 12 new compounds and line extensions. These include Levitra, a new treatment for erectile dysfunction, which we are co-promoting with Bayer, and Wellbutrin XL, a new and improved version of our successful anti-depressant.
Creating the most productive R&D organisationAt the outset of the merger we rethought the way R&D was carried out at GlaxoSmithKline, with the aim of creating the most productive R&D organisation in the industry. We established six therapeutically focused Centres of Excellence for Drug Discovery (CEDDs). The CEDDs are nimble and entrepreneurial with the range of skills and scale of resources required to drive mid-stage development projects through to their key decision point, proof of concept, before large-scale phase III clinical trials.
After two years of activity by the new R&D organisation, we are seeing significant progress as we advance our promising early stage pipeline of pharmaceutical products through clinical development. GlaxoSmithKline has 123 projects in clinical development, of which 61 are new chemical entities in a number of therapy areas, and 23 new vaccines. The number of new chemical entities starting phase II clinical trials has more than doubled since the merger. We are confident that, as these and our phase I pipeline move through development, we will build the best late stage pharmaceutical pipeline in the industry. We plan to provide a detailed update on progress in R&D towards the end of 2003.
Success as partner of choiceThe size and quality of our global R&D organisation, together with the strength of our sales and marketing teams, have enabled GlaxoSmithKline to become the partner of choice in the industry. We have signed an unprecedented 24 major external collaborations in the last two years which has helped to boost our product portfolio. It has also provided some exciting new opportunities in a number of areas of unmet medical need such as erectile dysfunction, obesity and HIV.
Patent challengesOver the last year there have been a number of developments involving the patents on some of our key products.
In July, in the USA, the first generic version of Augmentin was launched. This followed a ruling by a federal judge that our Augmentin patents were invalid. We are appealing against this decision, in the firm belief that our patents are valid. Meanwhile, we have already offset some of the impact of generics with recent successful launches of new improved versions of Augmentin - the ES and XR formulations.
GlaxoSmithKline is also involved in litigation over the patents on Wellbutrin SR and Zyban in the USA. We are awaiting the outcome of our appeal against a judgement last year in favour of Andrx Corporation, which has applied to market generic versions of the products.
| Description of business | |
Seroxat/Paxil continues to be subject to threat of generic competition, particularly in the USA.
A federal judge in Chicago recently ruled that GlaxoSmithKline’s patent in the USA covering the hemihydrate form ofPaxil was valid but not infringed by generics company Apotex’s product. We believe our patent to be infringed by Apotex’s product and will appeal against the ruling. Also, we will continue to pursue litigation for infringement of other patents relating to Paxil against Apotex and other generics companies in the USA.
As a result of these pending matters, the possible timing of generic competition to Paxil in the USA is unclear. Consequently, GlaxoSmithKline’s published earnings guidance for 2003 remains as previously stated. The guidance is for high single digit percentage growth in business performance earnings per share at constant exchange rates, assuming there is no generic competition to Paxil in the USA. If a generic launch of paroxetine hydrochloride became imminent, GlaxoSmithKline would reassess this guidance.
Uptake of Paxil CR, our enhanced form of the antidepressant launched in 2002, has been excellent and it now represents over 30 per cent of Paxil’s new prescriptions in the USA. We also have patent challenges to a number of other products such as Zofran and Lamictal. These cases illustrate an industry-wide trend in which generics companies are filing more patent challenges earlier. We will obviously defend our intellectual property vigorously.
Contribution to societyMissionThe responsible behaviour of all types of organisations, including multinational companies, governments and charities, is high on the public agenda. Last year, in our first report of corporate and social responsibility, we set out our commitment to reflecting ethical, social and environmental concerns in our business decisions. Our second report, updating our activities in 2002, is being published at the same time as this Annual Report and covers the issues that have generated significant interest from stakeholders.
The Corporate and Social Responsibility Report also includes some indicators to show our progress in addressing these issues.
Corporate responsibility is an integral part of our business and inherent in our mission. GlaxoSmithKline makes a significant positive contribution to society around the world, through the medicines, vaccines and healthcare products that we research, develop, manufacture and sell.
Our products must improve people’s lives and ensure a profitable and sustainable future for our business. We also understand that stakeholders, including employees, want to know how we make this profit, and need to be reassured of the sound ethical basis for our business.
Our focus on making a contribution to improving healthcare and alleviating suffering in the developing world has never been greater. Significant progress has been made towards tackling the enormous challenge of HIV/AIDS. By the end of 2002, we had secured some 120 arrangements to supply preferentially-priced HIV/AIDS medicines to 50 of the world’s poorest countries. Shipments of these medicines to the developing world continue to grow significantly year on year. In September 2002, we further reduced the preferential prices of our HIV/AIDS medicines by up to 33 per cent.
Positive Action, our international programme of HIV/AIDS education, care and support has now been established for ten years backing international programmes in 32 countries.
GlaxoSmithKline is a key partner in the global effort to eliminate lymphatic filariasis. This disabling and disfiguring disease currently affects 120 million people and threatens a further one billion in some of the poorest nations of the world. To date, GlaxoSmithKline has donated 145 million tablets as part of our 20-year commitment to eradicate this disease.
The Guardian newspaper’s ‘Giving List’ recently recognised that GlaxoSmithKline’s total global community expenditure in 2001 was greater than that of any other British company. We increased our comprehensive programme of social investment in 2002, investing £239 million in support of global community programmes, product donations and charitable contributions.
Corporate governanceCorporate governance continues to be a high profile issue with the publication of the Higgs Review of the role and effectiveness of Non-Executive Directors and Sir Robert Smith’s Report on audit committees. In the USA, the Sarbanes-Oxley Act became law in July 2002 and will have an impact on GlaxoSmithKline in relation to certification of the Annual Report on Form 20-F, disclosure processes, our relationship with external auditors, internal controls and a number of governance issues. GlaxoSmithKline regularly undertakes thorough reviews of the Group’s internal control systems and is committed to remaining a leader in governance processes and structure.
AcknowledgementsOur business is to discover effective medicines and healthcare products for people throughout the world and, as a result, create shareholder value. We are in a great position to build on the success of the last year, to build the best pipeline in the industry and launch further new products. We extend our thanks to all our employees who are so committed to making this happen.
Bob Ingram, Chief Operating Officer and President, Pharmaceutical Operations, retired at the end of December but will continue to work part-time as Vice Chairman of Pharmaceuticals and special advisor to the Group. We would like to express our appreciation for his contribution to the company and in particular for his significant role in making the merger a success.
On behalf of the Board and the Corporate Executive Team, we also thank you, our shareholders, for your support and hope that you share our enthusiasm for our company and look forward to its continued success in 2003.

The business
History and development of the company
GlaxoSmithKline plc, and its subsidiary and associated undertakings, constitute a major global healthcare group engaged in the creation and discovery, development, manufacture and marketing of pharmaceutical and consumer health-related products.
GlaxoSmithKline has its corporate head office in the London area at:
GlaxoSmithKline also has operational headquarters in Philadelphia, PA and Research Triangle Park, NC, USA, and operations in some 102 countries, with products sold in over 150 countries. The principal research and development (R&D) facilities are in the UK, the USA, Japan, Italy and Belgium. Products are currently manufactured in some 38 countries.
The major markets for the Group’s products are the USA, Japan, France, Germany, the UK and Italy.
GlaxoSmithKline plc is a public limited company incorporated on 6th December 1999 under English law. On 27th December 2000 the company acquired Glaxo Wellcome plc and SmithKline Beecham plc, both English public limited companies, by way of a scheme of arrangement for the merger of the two companies. Both Glaxo Wellcome and SmithKline Beecham were major global healthcare businesses.
On 1st October 2001 Glaxo Wellcome plc changed its name to GlaxoSmithKline Services plc and on 28th March 2002 became GlaxoSmithKline Services Unlimited. Historical references to Glaxo Wellcome plc in this document have not been changed.
Business segmentsGlaxoSmithKline operates principally in two industry segments:
| live longer. | ||
| Our Spirit |
| We undertake our quest with the enthusiasm of entrepreneurs, excited by the constant search for innovation. We value performance achieved with integrity. We will attain success as a world class global leader with each and every one of our people contributing with passion and an unmatched sense of urgency. | ||||
Annual Report and Review
This report is the Annual Report of GlaxoSmithKline plc for the year ended 31st December 2005, prepared in accordance with United Kingdom requirements.
A summary report on the year, the Annual Review 2005, intended for the investor not needing the full detail of the Annual Report, is produced as a separate document.
The Annual Review includes the joint statement by the Chairman and the Chief Executive Officer, a summary review of operations, summary financial statements and a summary remuneration report.
The Annual Review is issued to all shareholders. The Annual Report is issued to shareholders who have elected to receive it. Both documents are available on GlaxoSmithKline’s corporate website at www.gsk.com.
The Description of business discusses the strategy, activities, resources and operating environment of the business and identifies developments and achievements in 2005, under the following headings:
| Strategy and business drivers | ||||
| Business drivers | ||||
| Build the best product pipeline in the industry | ||||
| Achieve commercial and operational excellence | 14 | |||
| Improve access to medicines | 15 | |||
| Be the best place for the best people to do their best work | 16 | |||
| Global manufacturing and supply | 17 | |||
| Corporate responsibility and community investment | 18 | |||
| Products and competition | ||||
| Pharmaceutical | ||||
| Consumer Healthcare | ||||
| Regulatory environment | ||||
| Regulation | ||||
| Intellectual property | ||||
| Responsibility for environment, health and safety | ||||
Discussion of the Group’s management structures and corporate governance procedures is set out in Corporate governance (pages 27 to 36).
The Remuneration Report gives details of the Group’s policies on Directors’ remuneration and the amounts earned by Directors and senior management in 2005 (pages 37 to 54).
Discussion of the Group’s operating and financial performance and financial resources is given in the Operating and financial review and prospects (pages 55 to 80).
In this report:
‘GlaxoSmithKline’, the ‘Group’ or ‘GSK’ means GlaxoSmithKline plc and its subsidiary undertakings.
The ‘company’ means GlaxoSmithKline plc.
‘GlaxoSmithKline share’ means an Ordinary Share of GlaxoSmithKline plc of 25p.
American Depositary Share (ADS) represents two GlaxoSmithKline shares.
Throughout this report, figures quoted for market size, market share and market growth rates relate to the 12 months ended 30th September 2005 (or later where available). These are GSK’s estimates based on the most recent data from independent external sources, valued in sterling at relevant exchange rates. Figures quoted for product market share reflect sales by GSK and licensees.
Brand names appearing in italics throughout this report are trademarks either owned by and/or licensed to GlaxoSmithKline or associated companies, with the exception ofBaycolandLevitra, trademarks of Bayer,Boniva/Bonviva, a trademark of Roche,Entereg, a trademark of Adolor Corporation in the USA,Hepsera, a trademark of Gilead Sciences in some countries including the USA,Integrilin, a trademark of Millennium Pharmaceuticals,Micropump, a trademark of Flamel Technologies,Natrecor, a trademark of Scios and Janssen,Navelbine, a trademark of Pierre Fabre Médicament,Nicoderm, a trademark of Sanofi-Aventis, Elan, Novartis or GlaxoSmithKline in certain countries,Pritor, a trademark of Boehringer Ingelheim andVesicare, a trademark of Yamanouchi Pharmaceuticals, and in Japan and South Korea a trademark of Astellas Pharmaceuticals, all of which are used in certain countries under license by the Group.
05 | ||||
| Description of business |
| Strategy and business drivers |
GlaxoSmithKline is addressing the key challenges that face both the pharmaceutical industry and society as a whole:
| • | improving productivity in research and development |
| • | ensuring patients have access to new medicines |
The strategies to meet these challenges focus on several business drivers:
Build the best product pipeline in the industry
The Group is aiming to create the best product pipeline in the industry for the benefit of patients, consumers and society. This includes developing a focused portfolio strategy to support the pipeline and manage the full life cycle of compounds from their launch as prescription medicines through to becoming over-the-counter products where appropriate. This strategy includes selective in-licensing and efficient execution of development, commercialisation and the supply chain processes.
GSK’s R&D organisation measures productivity by the number and innovation of the products it creates, and also by the commercial value of these products and their ability to address the unmet needs of all consumers. This includes patients, healthcare professionals, budget holders and regulators, each with their own perspective on what constitutes a valuable new product.
Further details are given on pages 7 to 13.
Achieve commercial and operational excellence
GSK links research and commercial operations closely in order to maximise the value of the portfolio. As compounds are developed and tested, marketing campaigns and sales efforts are planned. Where appropriate within markets, the Group aims to build strong relationships with patients and consumers as the ultimate users of its medicines.
Common approaches to management processes and business functions are used by an internationally diverse and talented management team in order to create and sustain competitive advantage in all markets. Further details are given on page 14.
Improve access to medicines
GSK has created extensive programmes designed to improve the healthcare of people who have limited access to medicines both in the developed and developing world. These are set out in the ‘Improve access to medicines’ section of this report (page 15).
Be the best place for the best people to do their best work
The single greatest source of competitive advantage of any organisation is its people. The Group’s ambition is to be the place where great people apply their energy and passion to make a difference in the world. Their skills and intellect are key components in the successful implementation of the Group’s strategy. The work environment supports an informed, empowered and resilient workforce, in which the Group values and draws on the diverse knowledge, perspectives, experience, and styles of the global community. Further details are given on page 16.
Corporate Responsibility
In working to meet these challenges and implement these business drivers, GSK recognises that it has a responsibility to support the delivery of better healthcare and education in under-served communities and to connect business decisions to ethical, social and environmental concerns. GSK’s commitment to these is outlined on pages 18 to 19, with more information available in the Corporate Responsibility Report, which is available on the website at www.gsk.com
| GSK Annual Report 2005 |
| 06 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
Research and Development – Pharmaceuticals
GSK’s strategic intent is to become the indisputable leader in the industry. This success depends on the bedrock of the Group’s business – a vibrant and productive Research and Development (R&D) function that develops new ways to help patients while supporting existing products.
Focus on the Patient
R&D’s focus on the patient involves seeking the views of patients and their families for an understanding of the most important aspects of their disease and the impact it has on their lives. This information, in conjunction with discussions with key opinion leaders, is then used to shape drug development programmes so that new medicines are likely to benefit patients.
Finding candidate compounds
Two components are needed in the early stages of finding new medicines – targets that can be shown to affect mechanisms of important pathological processes in human disease and compounds able to modulate the behaviour of specific targets.
Many diseases arise through complex interactions between gene variants and environmental factors. Within GSK, Genetics Research aims to take advantage of this by identifying genes which influence common diseases with large unmet medical needs and major patient burdens. These insights help in the search for targets with known relevance to the disease, and hence a greater chance of delivering benefit to the patients.
Discovery Research (DR) produces the lead compounds that may influence targets which form the basis of drug discovery efforts in GSK’s Centres of Excellence for Drug Discovery (CEDDs). In 2005, DR performed over 90 million assays and provided the CEDDs with 50 high-quality new lead compounds. Investment in DR has been focused on increasing the quality and quantity of the lead compounds available.
Selecting the best candidate molecules
The fundamental steps in turning a lead compound into a drug candidate are optimising it for potency, efficacy and safety and then demonstrating the validity of the therapeutic hypothesis through early clinical trials of the resulting candidate.
These steps are helped by rapid, informed decision making and creative solutions to the issues that inevitably arise in this phase of development. GSK has designed the CEDDs, which are focused on specific disease areas, to be nimble and entrepreneurial. There are seven CEDDs, based in Europe and the USA:
| • | Biopharmaceuticals – Stevenage, UK |
| • | Cardiovascular & Urogenital Diseases – Upper Merion, USA |
| • | Metabolic & Viral Diseases – Research Triangle Park, USA |
| • | Microbial, Musculoskeletal & Proliferative Diseases, including cancer –Upper Providence, USA |
| • | Neurology & Gastrointestinal Diseases – Harlow, UK |
| • | Psychiatry – Verona, Italy |
| • | Respiratory and Inflammation – Stevenage, UK. |
Each CEDD is responsible for assessing the safety and other development characteristics of lead compounds in preclinical screens, some of which may involve using animals. This allows the selection of the best candidate for a new medicine. Once this is achieved, the CEDDs are responsible for demonstrating that the compound has satisfied a proof of therapeutic concept during mid-stage clinical trials.
A decision is then made on whether the information available justifies the compound’s progression into late-stage drug development, where large-scale clinical trials are conducted to register and commercialise the product.
During 2005 18 compounds entered clinical trials for the first time.
A GSK research facility focusing on new therapies in the treatment of neurodegenerative illnesses, such as Alzheimer’s disease, was opened in Singapore in 2005.
The application of experimental medicine is a major opportunity for the industry. An important tool in this field is clinical imaging, which enables visualisation of changes in the body made in response to the administration of a new medicine. In 2005 world-class imaging experts were recruited from both the USA and UK, as GSK prepared to open the Clinical Imaging Centre at the Hammersmith Hospital in London in 2006. In addition, R&D has established global collaborations with academic imaging centres that make it a leader in application of imaging for drug discovery and development.
Converting candidates to medicines
Preclinical Development (PCD) includes a wide range of activities throughout the entire drug development process. It is also involves the enhancement of existing products by devising more convenient formulations. Early in the development process, the metabolism and safety of compounds are evaluated in laboratory animals before testing in humans. The testing required in animals is highly regulated (see Animals and research, page 10).
PCD researchers investigate appropriate dosage forms (for example, tablets or inhalers) and develop formulations to enhance a drug’s effectiveness and ease of use by the patient. Processes and supporting analytical methods for drug synthesis and product formulation and delivery are scaled up to meet increasing supply requirements. This leads to the technical transfer of the processes and methods to manufacturing. The New Product Supply process, a partnership between R&D and Global Manufacturing and Supply, ensures that a robust product is developed for large-scale commercial manufacturing and launch.
To provide focus for the development process, all the major functional components of clinical, medical, biomedical data, regulatory and safety are integrated into a single management organisation, Worldwide Development (WWD).
GSK’s Medicine Development Centres (MDCs), which provide a focus for late-stage development, are responsible for creating value through the delivery of full product development plans, managing the day-today operational activities for the late-stage development portfolio and ensuring strong partnerships with the CEDDs and Global Commercial Strategy (GCS).
| GSK Annual Report 2005 |
| 07 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| Continued |
The MDCs are based at the major USA and UK sites and are aligned with the following therapeutic areas:
| • | Cardiovascular/Metabolic |
| • | Infectious Diseases including Diseases of the Developing World (DDW) |
| • | Musculoskeletal/Inflammation/Gastrointestinal/Urology |
| • | Neuroscience (Psychiatry/Neurology) |
| • | Oncology |
| • | Respiratory. |
These teams are responsible for maximising the worldwide development opportunities for each product within their remit so that all the information needed to support the registration, safety programmes, pricing and formulary negotiations is available when needed. Commercial input from Global Commercial Strategy ensures that regional marketing needs are integrated into any development plans at an early stage.
In addition, R&D is investigating new ways of operating to enable it to respond to the variety of external pressures on the industry, such as increasing regulatory stringency, so that it is positioned to ensure that effective new medicines reach patients as soon as possible.
GSK believes that pharmacogenetic research, which correlates genetic data with response to medicine, will help to reduce pipeline attrition and improve productivity. R&D is collecting DNA samples in clinical studies to identify pharmacogenetic information that can help predict a patient’s response. This information is intended to define patient groups likely to gain benefit from treatment, or to suffer a side effect, as the compound progresses through development in the clinic. Ultimately, pharmacogenetics promises to provide physicians with information to help them select the medicine and dose most likely to benefit their patient.
During 2005, R&D has taken several approaches to improving productivity in clinical trials, including an increasing use of countries outside Western Europe and the USA and the introduction of direct electronic data capture in most new clinical trials. These improvements in productivity will continue going forward.
All clinical trials sponsored by GSK, irrespective of where they take place, are conducted according to international standards of good clinical practice and applicable laws and regulations. The protocols are reviewed by the external regulatory agencies in the relevant countries where required and all protocols are considered by an Ethics Review Committee, whose remit covers the site where the study will take place. Safety data is routinely collected throughout development programmes and is reported to national and regional regulatory agencies in line with applicable regulations.
The GSK Global Safety Board is responsible internally for approving pivotal studies and investigating any issues related to patient safety arising during the development programme. During 2005, GSK took a further step in making information from its clinical trials widely and easily available by extending its Clinical Trial Register, a public website on which clinical trials data are published. Regulatory authorities will continue to be informed of the data generated so they may be reassured of the safety and efficacy of GSK’s products. The Clinical Trial Register will enhance the ability of clinicians to make informed clinical judgements to benefit their patients.
Extending the use of existing products
Once a product is launched, it is important to establish additional ways in which patients can be helped. This can be through investigating whether any other illnesses may be treated with the product or by the development of additional, more convenient dosage forms. Some developments reflect feedback from patients and the medical professions, while others are the result of continuing research into disease and its causes.
Examples of the importance of lifecycle management to GSK include the new indication of restless leg syndrome forRequipand monthly dosing ofBonivato simplify its administration for prevention of osteoporosis. Line extensions add significant value to the product portfolio. Recent examples, such asAugmentin ES/XR,Seroxat/Paxil CRandWellbutri n XL, achieved sales of £888 million in 2005.
Productivity
The challenge of increasing R&D productivity continued in 2005. Programmes to identify associations between diseases and genes have helped point to areas of research more likely to produce new ways of helping patients. Increased automation in screening has provided higher quality lead compounds more quickly.
Progress of the portfolio is communicated to investors and the media at regular intervals during the year. A major presentation on the vaccine portfolio was held in June and on the oncology and supportive care portfolio in November 2005. Details of GSK’s product development pipeline are given on pages 11 to 13.
Managing the portfolio
With improved productivity, more compounds are progressed into later phases of development. This progress, however, puts demands on our R&D resources and it is important to look objectively at the portfolio. Key projects reaching significant milestones are reviewed each month by the Product Management Board (PMB), which is responsible for determining if an asset has met criteria for passing into the next phase of development.
GSK continues to identify compounds from other companies that would enhance the portfolio and to create innovative collaborations to ensure that the Group is regarded as the partner of choice for large and small companies.
In 2005 a specific Centre of Excellence for External Drug Discovery was created. This small internal management team is responsible for delivering compounds with clinical proof of concept by establishing and managing long-term strategic collaborations with biotechnology companies, small- and mid-sized pharmaceutical companies, and academic institutions. The Group has committed funding for two years to these collaborations, with an option to renew for an additional three years.
In-licensing
In-licensing or co-marketing/co-promotion agreements concluded in 2005 were:
| • | The development and commercialisation of Vertex Pharmaceuticals Inc.’s VX-409, Nav1.8 Na-channel blocker plus back-up molecules for pain (preclinical) |
| • | The development and promotion of Allergan Inc.’s Botox in Japan and China |
| • | The development and commercialisation of a renin inhibitor program (preclinical) with Vitae Pharmaceuticals Inc. |
| GSK Annual Report 2005 |
| 08 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| Continued |
| • | The exercise of an option for Theravance Inc.’s inhaled muscarinic antagonist / beta 2 agonist programme (preclinical) |
| • | The exercise of options for Human Genome Science Inc.’s LymphoStat B (completed Phase IIa) for rheumatoid arthritis and systematic lupus erythematosus and mapatumumab TRAIL R1 monoclonal antibody for various cancer indications (Phase II). |
Discontinuations
All R&D carries a risk of failure. Lead compounds showing positive activity against a validated target may prove insufficiently safe to introduce to humans or impossible to manufacture on a commercial scale. Also, compounds may not show the expected benefits in patients in large scale clinical testing. These discontinuations occur despite extensive predictive testing.
Late-stage projects terminated during 2005 in Phase III included aplaviroc (873140) and 695634, both for HIV,Avandiafor psoriasis andLamictal XRfor schizophrenia.
Research and development – GSK vaccines
The majority of GSK’s vaccine R&D activities are conducted at its biologicals headquarters in Rixensart, Belgium. These include clinical development, regulatory strategy, commercial strategy, scaling up, vaccine production, packaging and all other support functions. Over 1,500 scientists are devoted to developing new vaccines and more cost-effective and convenient combination vaccines to prevent infections that cause serious medical problems worldwide. GSK is also targeting therapeutic vaccines that may prevent relapse in cancer patients.
Vaccine discovery involves many collaborations with academia and the biotech industry worldwide and allows identification of new vaccine antigens which are then expressed in yeast, bacteria or mammalian cells and purified to a very high level.
This is followed by formulation of the clinical lots of the vaccine. This may involve mixing antigens with selected novel proprietary adjuvants, which are designed to stimulate a good immune response. The first step is to evaluate the safety and efficacy of the candidate vaccine in a preclinical setting, usually involving an animal model. The candidate vaccine is then tested in clinical trials in healthy individuals to evaluate safety and effectiveness in inducing an immune response to protect the body from infection encountered later in a natural setting (Phase I/II). Large-scale field trials in healthy individuals follow to establish safety and efficacy in a cross section of the population (Phase III).
The results obtained during clinical trials and data regarding the development of a quality and large-scale production process and facilities are then combined into a regulatory file which is submitted to the authorities in the various countries where the vaccine is to be made available.
After launch, post marketing studies of considerable size are set up to assess vaccination programmes’ impact and to monitor vaccine safety (Phase IV).
Vaccine manufacturing is particularly complex as it requires the use of living micro-organisms. Sophisticated quality assurance and quality control procedures are in place to ensure both quality and safety of the vaccines and this commonly includes animal use. Due to their biological nature, health authorities may subject vaccines to a second control to guarantee the highest quality standards.
In 2005, GSK made a number of investments that strengthen its vaccine capabilities:
| • | a significant increase in flu vaccine manufacturing and development capacity by: | |
| – | acquiring ID Biomedical, a North American developer of vaccines for infectious diseases and producer of influenza vaccines with sites in Canada and the USA, for £874 million | |
| – | investing over £64 million in extending its German vaccine facility | |
| – | purchasing a vaccine R&D and manufacturing site in the USA | |
| • | acquiring US based Corixa Corporation, a developer of innovative vaccine adjuvants, for approximately £150 million | |
| • | entering into three groundbreaking public-private partnerships to develop vaccines against the three biggest killers in the developing world, AIDS, malaria and tuberculosis. | |
| GSK expects to launch five major new vaccines within the next five years: | ||
| • | a human papilloma virus vaccine preventing cervical cancer | |
| • | the USA and EU launch of a vaccine against rotavirus induced gastroenteritis and the strengthening of its presence in international markets | |
| • | a vaccine against pneumococcal disease | |
| • | an improved vaccine for influenza | |
| • | vaccine combinations against meningitis. | |
The strength of GSK’s vaccine pipeline is expected to provide opportunities for GSK to deliver new vaccines for many years to come.
Research and development – Consumer Healthcare
R&D has aligned itself closely with the new Consumer Healthcare operating model and structure. For the Global brands, it now mirrors the commercial structure with R&D teams paired with commercial teams and located in the principal centres for Consumer Healthcare R&D at Weybridge in the UK and in Parsippany in the USA; with this co-location, these sites are now termed Innovation Centres. The focus of R&D is on the identification and rapid development of novel products that bring benefits to consumers in the over-the-counter (OTC), oral care and nutritional healthcare markets.
Diseases of the developing world
Continued investment in research into diseases that disproportionately affect the developing world is essential if there is to be a long-term improvement in the health of people who live in these regions. As part of GSK’s response to this challenge, it operates a drug discovery unit, dedicated to finding new medicines for these diseases, based at Tres Cantos, Spain. The work undertaken in Tres Cantos focuses on malaria and tuberculosis which, together with work elsewhere in the Group on HIV/AIDS and vaccines, means GSK is addressing the prevention and treatment of all three of the World Health Organization’s (WHO) top priority diseases.
| GSK Annual Report 2005 |
| 09 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| Continued |
GSK currently has 14 clinical programmes of relevance to the developing world, eight of which are aimed at producing vaccines and medicines for diseases that disproportionately affect developing countries.
Public/private partnerships (PPP) remain essential to fund research where there is no commercially viable market for a potential product. GSK is a leader in working with PPP and continues to collaborate closely with many governments, academic centres, United Nations’ agencies and other global funding bodies in this area, to maximise expertise and knowledge. This has the dual benefit of encouraging research and development and accelerating access to the medicines in the developing world. For example, in 2005, GSK announced partnerships with the Global Alliance for TB Drug Development, the Aeras Global TB Vaccine Foundation and the International AIDS Vaccine Initiative. GSK’s malaria ‘falcipain inhibitors’ project was chosen for the Medicines for Malaria Venture ‘project of the year’ award.
Animals and research
For ethical, regulatory and scientific reasons, research using animals remains a small but vital part of research and development of new medicines and vaccines. GSK only uses animals where there is no alternative and only in the numbers required for each test. The Group strives to exceed regulatory standards in the care and use of the animals it uses and undergoes internal and external review to assure these standards.
The vast majority of the experimental methods do not use animals. GSK is actively engaged in research to develop and validate more tests that either avoid the use of animals in research or reduce the numbers needed. When animals are used in research unnecessary pain or suffering is scrupulously avoided.
GSK understands that use of animals for research purposes commands a high level of public interest. The GlaxoSmithKline Public Policy Position ‘The care and ethical use of animals in research’, and further information and reports, are available on the website, www.gsk.com, or from Secretariat.
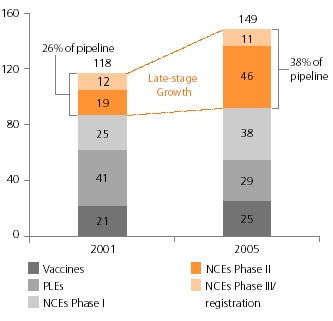
GSK’s pipeline
The chart on the right shows new chemical entities (NCE) and product line extensions (PLE) for projects in the clinic in 2001 and 2005. At the end of February 2006, GSK had nearly 200 pharmaceutical and vaccine projects in development. Of these, 149 are in the clinic comprising 95 NCEs, 29 PLEs and 25 vaccines, compared with 118 in 2001. Since 2001 the number of projects in the late stages of development has increased from 31 to 57.
This maturity in the late stage pipeline is expected to lead to an increase in registrations in the coming years. The content of the drug development portfolio will change over time as new compounds progress from discovery to development and from development to the market. Owing to the nature of the drug development process, many of these compounds, especially those in early stages of investigation, may be terminated as they progress through development. Phase I NCEs with multiple indications are counted only once. NCEs in later phases are counted by each indication. For competitive reasons, new projects in pre-clinical development have not been disclosed and some project types may not have been identified.
GSK’s submissions to the regulatory authorities in the USA and EU for the first time and approvals during 2005 were:
| USA | Europe | |||
| Submission | 5 | 7 | ||
| Approval | 6 | 6 | ||
| 11 | 13 | |||
In 2006, the late-stage pipeline is expected to expand further with eight major assets anticipated to enter phase III development. Also, in 2006, GSK anticipates seven products will be approved and/or launched and seven product filings are planned. For further details of these developments expected in 2006 see the GSK outlook on page 71.
GSK’s policy is to obtain patent protection on all significant products discovered or developed through its R&D activities. Patent protection for new active ingredients is available in all significant markets. Protection can also be obtained for new pharmaceutical formulations and manufacturing processes, and for new medical uses and special devices for administering products.
| Key | |
| (v) | Vaccine |
| (p) | Pharmaccine |
| * | Compounds in Shionogi-GlaxoSmithKline Pharmaceuticals LLC joint |
| venture | |
| † | In-license or other alliance relationship with third party |
| S | Date of first submission |
| A | Date of first regulatory approval (for MAA, this is the first EU |
| approval letter) | |
| AL | Approvable letter indicates that ultimately approval can be given |
| subject to resolution of deficiencies | |
| MAA | Marketing authorisation application (Europe) |
| NDA | New drug application (USA) |
| Phase I | Evaluation of clinical pharmacology, usually conducted in volunteers |
| Phase II | Determination of dose and initial evaluation of efficacy, conducted in a small number of patients |
| Phase III | Large comparative study (compound versus placebo and/or established treatment) in patients to establish clinical benefit and safety |
| GSK Annual Report 2005 |
| 10 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| continued |
| Estimated filing dates | ||||||||||
| Compound/Product | Type | Indication | Phase | MAA | NDA | |||||
| Cardiovascular & Metabolic | ||||||||||
| 256073 | high affinity nicotinic acid receptor (HM74A) agonist | dyslipidaemia | I | |||||||
| 681323 | p38 kinase inhibitor | atherosclerosis (also rheumatoid arthritis & chronicobstructive pulmonary disease, COPD) | I | |||||||
| 813893 | factor Xa inhibitor | prevention of stroke in atrial fibrillation | I | |||||||
| 856553 | p38 kinase inhibitor | atherosclerosis (also rheumatoid arthritis & COPD) | I | |||||||
| rilapladib† | lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor | atherosclerosis | I | |||||||
| 501516† | peroxisome proliferator-activator receptor(PPAR) delta agonist | dyslipidaemia | II | |||||||
| 590735 | PPAR alpha agonist | dyslipidaemia | II | |||||||
| odiparcil† | indirect thrombin inhibitor | prevention of thrombotic complications of cardiovascular disease | II | |||||||
| darapladib† | Lp-PLA2 inhibitor | atherosclerosis | ll/III | |||||||
| Arixtra | synthetic factor Xa inhibitor | treatment of acute coronary syndrome | III | 2006 | 2006 | |||||
| Coreg CR† | beta blocker | hypertension & congestive heart failure – once-daily | Submitted | N/A | S:Dec05 | |||||
| Metabolic projects | ||||||||||
| 625019 | PPAR pan agonist | type 2 diabetes | I | |||||||
| 716155† | glucagon-like peptide 1 agonist | type 2 diabetes | I | |||||||
| 856464 | melanin concentrating hormone antagonist | obesity | I | |||||||
| radafaxine | noradrenaline/dopamine re-uptake inhibitor | obesity (also fibromyalgia, neuropathic pain & depression) | I | |||||||
| 189075† | sodium dependent glucose transport (SGLT2) inhibitor | type 2 diabetes | II | |||||||
| 677954 | PPAR pan agonist | type 2 diabetes | II | |||||||
| 869682† | SGLT2 inhibitor | obesity | II | |||||||
| denagliptin | dipeptidyl peptidase lV (DPP IV) inhibitor | type 2 diabetes | II | |||||||
| solabegron | beta3 adrenergic agonist | type 2 diabetes (also overactive bladder) | II | |||||||
| Avandamet XR | PPAR gamma agonist + metformin | type 2 diabetes – extended release | III | 2007 | ||||||
| Avandia+ simvastatin | PPAR gamma agonist + statin | type 2 diabetes | III | 2007 | ||||||
| Avandaryl† | PPAR gamma agonist + sulphonylurea | type 2 diabetes – fixed dose combination | Approved | S:May05 | A:Dec05 | |||||
| Infectious Diseases | ||||||||||
| 565154 | oral pleuromutilin | treatment of bacterial infections | I | |||||||
| 742510 | oral pleuromutilin | treatment of bacterial infections | I | |||||||
| 270773† | phospholipid anti-endotoxin emulsion | sepsis | II | |||||||
| farglitazar | PPAR gamma agonist | hepatic fibrosis | II | |||||||
| sitamaquine | 8-aminoquinoline | treatment of visceral leishmaniasis | II | N/A | ||||||
| chlorproguanil, dapsone + | antifolate + artemisinin | treatment of uncomplicated malaria | IIl | 2007 | N/A | |||||
| artesunate (CDA)† | ||||||||||
| Etaquine† | 8-aminoquinoline | malaria | III | |||||||
| Altabax(retapamulin) | topical pleuromutilin | bacterial skin infections | Submitted | 2006 | S:Nov05 | |||||
| Antivirals | ||||||||||
| 825780† | DNA antiviral vaccine | HIV infection | I | |||||||
| brecanavir† | aspartyl protease inhibitor | HIV infection | II | |||||||
| Relenza† | neuraminidase inhibitor | influenza prophylaxis | Submitted | S:Nov05 | S:Nov05 | |||||
| Musculoskeletal, Inflammation, Gastrointestinal & Urology | ||||||||||
| 221149 | oxytocin antagonist | threatened pre-term labour | I | |||||||
| 232802 | 3G-selective oestrogen receptor modulator | treatment of menopausal symptoms | I | |||||||
| 267268 | vitronectin integrin antagonist | age-related macular degeneration | I | |||||||
| 366074† | potassium channel opener | overactive bladder | I | |||||||
| relacatib† | cathepsin K inhibitor | osteoporosis & osteoarthritis (also bone metastases) | I | |||||||
| 751689† | calcium antagonist | osteoporosis | I | |||||||
| 768974† | parathyroid hormone agonist | osteoporosis | I | |||||||
| 786034 | tyrosine kinase inhibitor | psoriasis | I | |||||||
| 842470† | PDE IV inhibitor (topical) | atopic dermatitis | I | |||||||
| 876008† | corticotrophin releasing factor (CRF1) antagonist | irritable bowel syndrome (also depression & anxiety) | I | |||||||
| dutasteride + testosterone | 5-alpha reductase inhibitor + testosterone | hypogonadism – fixed dose combination | I | |||||||
| solabegron | beta3 adrenergic agonist | overactive bladder (also type 2 diabetes) | I | |||||||
| 270384 | endothelial cell adhesion molecule inhibitor | inflammatory bowel disease | II | |||||||
| 274150 | selective iNOS inhibitor | rheumatoid arthritis (also migraine) | II | |||||||
| 681323 | p38 kinase inhibitor | rheumatoid arthritis (also atherosclerosis & COPD) | II | |||||||
| 683699† | dual alpha4 integrin antagonist (VLA4) | inflammatory bowel disease (also multiple sclerosis) | II | |||||||
| 856553 | p38 kinase inhibitor (oral) | rheumatoid arthritis (also atherosclerosis & COPD) | II | |||||||
| casopitant | NK1 antagonist | overactive bladder (also depression & anxiety, chemotherapy induced & postoperative nausea & vomiting) | II | |||||||
| mepolizumab | anti-IL5 monoclonal antibody | eosinophilic esophagitis (also asthma & nasal polyposis) | II | |||||||
| rosiglitazone XR | PPAR gamma agonist | rheumatoid arthritis (also Alzheimer’s disease) | II | |||||||
| Avodart+ alpha blocker | 5-alpha reductase inhibitor + alpha blocker | benign prostatic hyperplasia – fixed dose combination | III | 2007 | 2007 | |||||
| Avodart | 5-alpha reductase inhibitor | reduction in the risk of prostate cancer | III | |||||||
| Entereg/Entrareg† | peripheral mu-opioid antagonist | opioid induced GI symptoms | III | 2007 | 2007 | |||||
| mepolizumab | anti-IL5 monoclonal antibody | hypereosinophilic syndrome (also asthma & nasal polyposis) | III | 2006 | 2006 | |||||
| Entereg/Entrareg† | peripheral mu-opioid antagonist | post operative ileus | Approvable | 2007 | AL:Jul05 | |||||
| Boniva/Bonviva† | bisphosphonate | treatment of postmenopausal osteoporosis | Approved | S:Apr05 | A:Jan06 | |||||
| – i.v. injection | ||||||||||
| GSK Annual Report 2005 |
| 11 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| continued |
| Estimated filing dates | ||||||||||
| Compound/Product | Type | Indication | Phase | MAA | NDA | |||||
| Neurosciences | ||||||||||
| 163090 | presynaptic mixed 5HT1 antagonist | depression & anxiety | I | |||||||
| 189254 | histamine H3 antagonist | dementia | I | |||||||
| 234551† | endothelin A antagonist | stroke | I | |||||||
| 406725 | gap junction blocker | migraine, epilepsy & neuropathic pain | I | |||||||
| 644784 | dual-acting COX-2 inhibitor | acute & chronic pain conditions (including neuropathic pain) & schizophrenia | I | |||||||
| 737004† | endothelin A antagonist | stroke | I | |||||||
| 823296 | NK1 antagonist | depression & anxiety | I | |||||||
| 842166 | non-cannabinoid CB2 agonist | inflammatory pain | I | |||||||
| 876008† | CRF1 antagonist | depression & anxiety (also irritable bowel syndrome) | I | |||||||
| radafaxine | noradrenaline/dopamine re-uptake inhibitor | fibromyalgia & neuropathic pain (also obesity) | I | |||||||
| 274150 | selective iNOS inhibitor | migraine (also rheumatoid arthritis) | II | |||||||
| 372475† | triple (5HT/noradrenaline/dopamine) re-uptake inhibitor | depression and attention deficit hyperactivity disorder | II | |||||||
| 468816 | glycine antagonist | smoking cessation | II | |||||||
| 683699† | dual alpha4 integrin antagonist (VLA4) | multiple sclerosis (also inflammatory bowel disease) | II | |||||||
| 705498 | transient receptor potential vanilloid-1 (TRPV1) antagonist | acute migraine | II | |||||||
| 742457 | 5HT6 antagonist | dementia | II | |||||||
| 773812 | mixed 5HT/dopaminergic antagonist | schizophrenia | II | |||||||
| casopitant | NK1 antagonist | depression & anxiety (also overactive bladder, chemotherapy induced & postoperative nausea & vomiting) | II | |||||||
| radafaxine | noradrenaline/dopamine re-uptake inhibitor | depression (also obesity) | II | |||||||
| rosiglitazone XR | PPAR gamma agonist | Alzheimer's disease (also rheumatoid arthritis) | II | |||||||
| talnetant | NK3 antagonist | schizophrenia | II | |||||||
| vestipitant + paroxetine | NK1 antagonist + selective serotonin re-uptake inhibitor | depression & anxiety | II | |||||||
| 406381 | dual-acting COX-2 inhibitor | acute & chronic pain | III | |||||||
| Lamictal | sodium channel inhibitor | bipolar disorder – acute treatment | III | N/A | 2006 | |||||
| Lamictal XR | sodium channel inhibitor | epilepsy – once-daily | III | 2006 | ||||||
| Requipextended release | non-ergot dopamine agonist | restless legs syndrome | III | 2006 | ||||||
| Requip Modutab/XL | non-ergot dopamine agonist | Parkinson’s disease – once-daily controlled release | Submitted | S:Dec05 | 2006 | |||||
| 24 hour† | formulation | |||||||||
| Trexima† | 5HT1 agonist + naproxen | migraine – fixed dose combination | Submitted | N/A | S:Aug05 | |||||
| Wellbutrin XL† | noradrenaline/dopamine re-uptake inhibitor | seasonal affective disorder | Submitted | S:Dec04 | ||||||
| Wellbutrin XL† | noradrenaline/dopamine re-uptake inhibitor | depression | Approved | 2006 | A:Aug03 | |||||
| Oncology | ||||||||||
| 559448† | thrombopoietin agonist | thrombocytopaenia | I | |||||||
| 743921† | kinesin spindle protein (KSP) inhibitor | cancer | I | |||||||
| elacridar | oral bioenhancer | cancer | I | |||||||
| relacatib† | cathepsin K inhibitor | bone metastases (also osteoporosis & osteoarthritis) | I | |||||||
| casopitant | NK1 antagonist | postoperative nausea & vomiting (also overactive bladder, depression & anxiety) | II | 2007 | 2007 | |||||
| casopitant | NK1 antagonist | chemotherapy induced nausea & vomiting (also overactive bladder, depression & anxiety) | II | |||||||
| ethynylcytidine† | selective RNA polymerase inhibitor | solid tumours | II | |||||||
| iboctadekin† | recombinant human IL18 immunomodulator | immunologically-sensitive cancers (melanoma & renal cell) | II | |||||||
| ispinesib† | KSP inhibitor | non-small cell lung cancer & other tumours | II | |||||||
| pazopanib | vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor | solid tumours | II | |||||||
| vestipitant | NK1 antagonist | postoperative nausea & vomiting | II | |||||||
| eltrombopag† | thrombopoietin agonist | thrombocytopaenia | III | 2006/07 | 2006/07 | |||||
| Hycamtin | topo-isomerase I inhibitor | ovarian cancer first-line therapy | III | 2007 | 2007 | |||||
| Hycamtin | topo-isomerase I inhibitor | small cell lung cancer second-line therapy – oral formulation | III | 2007 | 2007 | |||||
| Tykerb/Tycerb | ErbB-2 and epidermal growth factor receptor (EGFR) dual kinase inhibitor | breast cancer (also renal, head & neck cancers) | III | 2006/07 | 2006/07 | |||||
| Hycamtin | topo-isomerase I inhibitor | cervical cancer second-line therapy | Submitted | 2006 | S:Dec05 | |||||
| Arranon | guanine arabinoside prodrug | acute lymphoblastic leukaemia & lymphomas | Approved | 2006 | A:Oct05 | |||||
| Hycamtin | topo-isomerase I inhibitor | small cell lung cancer second-line therapy | Approved | A:Jan06 | A:Nov98 | |||||
| GSK Annual Report 2005 |
| 12 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Build the best product pipeline in the industry |
| continued |
| Estimated filing dates | ||||||||||
| Compound/Product | Type | Indication | Phase | MAA | NDA | |||||
| Respiratory | ||||||||||
| 256066 | PDE IV inhibitor (inhaled) | asthma, COPD & allergic rhinitis | I | |||||||
| 656398† | muscarinic acetylcholine antagonist | COPD | I | |||||||
| 856553 | p38 kinase inhibitor (oral) | COPD (also atherosclerosis & rheumatoid arthritis) | I | |||||||
| 870086 | novel glucocorticoid agonist | asthma | I | |||||||
| 961081† | muscarinic antagonist, beta2 agonist | COPD | I | |||||||
| 159797† | long-acting beta2 agonist | COPD, also COPD & asthma in combination with a glucocorticoid agonist | II | |||||||
| 159802† | long-acting beta2 agonist | COPD, also COPD & asthma in combination with a glucocorticoid agonist | II | |||||||
| 233705 | muscarinic acetylcholine antagonist | COPD | II | |||||||
| 597901† | long-acting beta2 agonist | COPD, also COPD & asthma in combination with a glucocorticoid agonist | II | |||||||
| 642444† | long-acting beta2 agonist | COPD, also COPD & asthma in combination with a glucocorticoid agonist | II | |||||||
| 678007† | long-acting beta2 agonist | COPD, also COPD & asthma in combination with a glucocorticoid agonist | II | |||||||
| 681323 | p38 kinase inhibitor (oral) | COPD (also rheumatoid arthritis & atherosclerosis) | II | |||||||
| 685698 | glucocorticoid agonist | asthma & COPD in combination with a long-acting beta2 agonist (also allergic rhinitis) | II | |||||||
| 799943 | glucocorticoid agonist | asthma & COPD in combination with a long-acting beta2 agonist | II | |||||||
| mepolizumab | anti-IL5 monoclonal antibody | asthma & nasal polyposis (also hypereosinophilic syndrome & eosinophilic esophagitis) | II | |||||||
| Avamys/Allermist | glucocorticoid agonist | allergic rhinitis | III | 2006 | 2006 | |||||
| Seretide/Advair | beta2 agonist/inhaled corticosteroid | COPD – mortality claim | III | 2006 | 2006 | |||||
| Seretide | beta2 agonist/inhaled corticosteroid | asthma – initial maintenance therapy | Submitted | S:Aug04 | N/A | |||||
| Ariflo | PDE IV inhibitor (oral) | COPD | Approvable | AL:Oct03 | ||||||
| Seretide/Advair | beta2 agonist/inhaled corticosteroid | asthma – non-CFC inhaler | Approved | A:Jun00 | AL:Oct01 | |||||
| & Oct02 | ||||||||||
| Paediatric Vaccines | ||||||||||
| Hib-MenCY-TT | conjugated | Neisseria meningitis groups C & Y disease & Haemophilus influenzae type b disease prophylaxis | II | |||||||
| MenACWY-TT | conjugated | Neisseria meningitis groups A, C, W & Y disease prophylaxis | II | |||||||
| Globorix | conjugated | diptheria, tetanus, pertussis, hepatitis B, Haemophilus influenzae type b disease, Neisseria meningitis groups A & C disease prophylaxis | lll | 2006 | ||||||
| Streptorix† | conjugated | S.pneumoniae disease prophylaxis for children | lll | 2007 | ||||||
| Priorix-Tetra | live attenuated | measles, mumps, rubella & varicella prophylaxis | Submitted | S:Apr04 | ||||||
| Rotarix† | live attenuated – oral | rotavirus induced gastroenteritis prophylaxis | Submitted | S:Dec04 | ||||||
| Menitorix | conjugated | Neisseria meningitis group C disease & Haemophilus influenzae type b disease prophylaxis | Approved | A:Dec05 | ||||||
| Other Vaccines | ||||||||||
| HIV | recombinant | HIV infection prophylaxis | l | |||||||
| S. pneumoniae elderly† | recombinant | S. pneumoniae disease prophylaxis | l | |||||||
| S. pneumoniae paediatric | recombinant | S. pneumoniae disease prophylaxis | l | |||||||
| (PGCvax) | ||||||||||
| Varicella Zoster virus | recombinant | Varicella Zoster prevention | l | |||||||
| Tuberculosis† | recombinant | tuberculosis prophylaxis | I/II | |||||||
| Dengue fever† | attenuated tetravalent vaccine | Dengue fever prophylaxis | ll | |||||||
| Epstein-Barr virus† | recombinant | EBV infection prophylaxis | ll | |||||||
| Flu improved | inactivated split-adjuvanted | influenza prophylaxis | ll | |||||||
| Flu intranasal (FluINsure) | inactivated split-adjuvanted | influenza prophylaxis | ll | |||||||
| Hepatitis E virus | recombinant | hepatitis E prophylaxis | ll | |||||||
| Mosquirix† | recombinant | malaria prophylaxis | ll | |||||||
| Cervarix† | recombinant | human papilloma virus infection prophylaxis | lll | 2006 | 2006 | |||||
| Fluviral | inactivated split | influenza prophylaxis | lll | 2006 | ||||||
| Simplirix | recombinant | genital herpes prophylaxis | lll | |||||||
| Flu pandemic | inactivated whole-aluminium salt adjuvant | influenza prophylaxis | Submitted | S:Dec05 | ||||||
| Pharmaccines | ||||||||||
| P501 | recombinant | treatment of prostate cancer | l | |||||||
| Her2 | recombinant | treatment of breast cancer | l/II | |||||||
| MAGE-3† | recombinant | treatment of non-small cell lung cancer & melanoma | ll | |||||||
| GSK Annual Report 2005 |
| 13 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Achieve commercial and operational excellence |
GSK undertakes a range of activities to maximise the commercial potential of its intellectual property, by introducing innovative products into as many markets as possible, accelerating the process of bringing new products to market, increasing brand recognition and ensuring that patients have access to new medicines. Both the pharmaceutical and consumer healthcare businesses focus on ways to improve existing performance through commercial and operational excellence initiatives. Some of these are:
Worldwide pharmaceutical sales force excellence
GSK’s sales force has always ranked high on surveys with healthcare professionals. Worldwide sales force excellence (WSFE) aims to improve customer satisfaction even further.
The time available for physicians to learn about new medicines and clinical studies is precious. Through the WSFE initiative, sales representatives strengthen product knowledge and learn to deliver patient-specific treatment options more efficiently and more effectively. Research shows that a sales visit is highly effective when a representative engages the physician in dialogue around patient types and supports the message with visual aids that illustrate clinical results.
The Group has introduced a single global sales call model that focuses on treating the patient through a dialogue about ”when“ a GSK medicine is appropriate, “why” it is effective and “how” to administer it safely. All field people in the Group’s key markets had been trained in the new “When? Why? How?” approach. The entire sales organisation is now involved in WSFE to bring about a cultural change that raises ethical standards and helps build long-term, trusting relationships with the healthcare community.
Pharmaceutical marketing excellence
Large numbers of patients suffering the effects of their disease continue to be unable to benefit from innovative medicines and treatments. One of GSK’s goals is to provide accurate and balanced information on the Group’s products to allow as many people as possible to benefit from GSK’s medical advances. For example within Europe, around 50% of patients suffering from Chronic Obstructive Pulmonary Disease (COPD) are diagnosed and, of those, only 60% receive regular maintenance drug therapy. GSK’s marketing initiative implements programmes to overcome the barriers to proper diagnosis and treatment. As these programmes begin to show effects, the societal costs of disease will decrease. To the extent that a GSK product is chosen for patients’ treatment, the Group will benefit as well.
Marketing codes
GSK is committed to ethical, responsible and patient-centred marketing. The Group’s Pharmaceutical Marketing and Promotional Activity policy governs marketing activities and apply to all employees, suppliers, contractors and agents. This policy requires that all marketing and promotional activities are based on valid scientific evidence, and comply with applicable laws and regulations.
This policy is supported by regional marketing practices codes in Europe, GSK’s International region, Japan and the USA. These codes apply the same ethical standards but reflect differences in market structures, national healthcare systems and regulations. They incorporate the principles of industry codes of practice such as the European Federation of Pharmaceutical Industries Associations, the International Federation of Pharmaceutical Manufacturers Associations, Japan Pharmaceutical Manufacturers Association and Pharmaceutical Research and Manufacturers of America marketing codes.
Consumer Healthcare marketing excellence
The structure of this business was redesigned in 2004 in order to focus on brands and their growth opportunities. For those brands that have sales in multiple markets a new team called the Future group has been created to develop a global approach to support these global brands. For those brands that are large and marketed in several territories, but generally with one lead market, one anchor market team leads development of these lead market brands. The remaining valuable local brands are managed through a new model, which retains local responsibility for the brand, communications and innovation. These local enterprise brands are also supported globally and regionally to ensure the application of best practice and cross pollination of innovation.
Maintaining high standards
GSK expects employees to meet high ethical standards in all aspects of business by conducting activities with honesty and integrity, adhering to corporate responsibility principles and complying with applicable laws and regulations. GSK audits its operations to ensure relevant standards expected, such as those in marketing practices, are reached or exceeded.
Commitment to the GSK Code of Conduct is reinforced each year by a senior management certification programme, and in 2005 over 12,000 managers certified they had complied with “Performance with Integrity” principles.
Patient advocacy
The Patient advocacy initiative has demonstrated significant progress since its inception in 2002. The rationale for the strategy centres on enhancing access for the Group’s medicines by connecting with patient groups to ensure that they are informed of disease treatments, as well as improving GSK’s reputation as a patient-centric group.
Initially launched as a US programme, it is now a critical initiative in strategic plans throughout the world. Patient advocacy teams in the USA and Europe have shared best practices and established processes to optimise interaction with patient groups. In 2005, Patient advocacy Leaders Summits were held in the USA, Europe and Canada, with over 1,000 patient advocates attending GSK sponsored meetings throughout the world. Two diabetes summits were held with minority legislative groups in the USA in the hopes of developing a base for future legislation and awareness activities.
Vision Factory
GSK introduced the Vision Factory initiative in Global Manufacturing and Supply which is identifying improvements in productivity and cost reduction. This will increase operational excellence in the manufacturing operations to ensure product quality and patient safety are paramount.
Procurement
GSK non-production operations are supported by a number of third party purchases; worldwide this covers all areas including media, travel, R&D, IT and marketing. These purchases are managed by procurement, on behalf of their internal customers, and covers assurance of supply, service, quality, cost and innovation. Widely recognised by industry analysts as a global best practice leader, procurement works collaboratively with the business to develop and implement sourcing strategy that ensures GSK receives best value when buying goods and services.
| GSK Annual Report 2005 |
| 14 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Improve access to medicines |
Products – Pharmaceuticals (by therapeutic area)Access to healthcare in the developing world
Access to healthcare in developing countries remains a major challenge to the global community. The problem, which is rooted in poverty and a lack of political will, continues to demand a significant mobilisation of resources and a true spirit of partnership. GSK continues to play a vital role, through its commitment to R&D into diseases particularly prevalent in the developing world, through its programme of preferential pricing for its anti-retrovirals (ARVs), anti-malarials and vaccines, through its community investment programmes and through its willingness to seek innovative solutions, such as voluntary licencing arrangements.
Preferential pricing programme
GSK has offered its vaccines to key organisations for vaccination programmes in developing countries at preferential prices for over 20 years. The Group also sets a single not-for-profit price for each of its ARVs and anti-malarials to a wide range of customers in the Least Developed Countries (UN definition) and sub-Saharan Africa, as well as Country Coordinating Mechanism-projects fully funded by the Global Fund to Fight AIDS, TB, and Malaria and the US President’s Emergency Plan for AIDS Relief (PEPFAR).
GSK is committed to contributing to health improvements in a sustainable manner. The prices for its ARVs and anti-malarials are therefore set at levels at which no profit is made, but direct costs are covered, allowing supply to be sustained for as long as required. During 2005, GSK shipped to developing countries over 45 million tablets of preferentially-pricedCombivirand over 81 million tablets of preferentially-pricedEpivir.
The offer of not-for-profit prices requires a sustainable framework, combining GSK’s commitment to preferential pricing with commitments from governments of the developed world to avoid price referencing against preferentially priced medicines and to help prevent product diversion. GSK has taken steps to minimise the threat of diversion.Retrovirsyrup,Epivirsolution,Combivir,Epivirtablet andTrizivirare now available in special access packs in more than 50 countries. Differentiated red (as opposed to traditional white)Combivi randEpivirtablets are now registered across a number of International markets. GSK is the only company to have registered its ARVs under the European Union’s Anti-Diversion Regulation. During 2005, it also continued to encourage other countries to take the necessary steps to ensure the introduction and strict enforcement of appropriate anti-diversion measures.
Innovative solutions
GSK has shown industry leadership in granting voluntary licences to seven generic companies for the manufacture and supply of ARVs to both the public and private sectors in sub-Saharan Africa.
Looking ahead
GSK will continue to build on its products, pricing and partnership commitments to help improve healthcare in the developing world. However, a significant increase in funding from the global community is still needed. It is also important to maintain incentives for R&D through protection of intellectual property.
While much was achieved in 2005, sustainable progress will only occur if the significant barriers that stand in the way of better access to healthcare are tackled as a shared responsibility by all sectors of global society – governments, international agencies, charities, academic institutions, the pharmaceutical industry and others.
Access to medicines in the developed world
Programmes in the USA
GSK is working to provide meaningful access to medicines for people with limited financial resources and without prescription drug insurance. In 2005, GSK’s US patient assistance programs provided $464 million worth of medicines, valued at wholesale acquisition cost, to 565,000 qualifying low income US residents.
For uninsured Americans who do not qualify for Medicare or Medicaid, GSK and 11 other pharmaceutical companies created Together Rx Access, a programme for qualified individuals offering reductions in the usual pharmacy cost on more than 275 medicines. Launched in 2005, there are over 353,000 Together Rx Access cardholders, who saved about $10.1 million in 2005.
GSK participates in the Partnership for Prescription Assistance (PPA), the largest national programme dedicated to helping people in need access prescription medicines. PPA has matched more than one million US patients in need to programs providing significant help. GSK and other US pharmaceutical companies launched the program in 2005 in partnership with healthcare, physician and patient advocacy organisations.
Programmes in other countries
The Group has also introduced Orange Cards providing discounts on certain GSK prescription medicines for eligible patients in Bulgaria, Lithuania and Ukraine. The nature of the discounts varies between countries, depending on the needs of the patient and the way in which the healthcare system operates.
Preparing for a flu pandemic
The Group is committed to doing everything it can to support governments and health authorities around the world in planning responses to a possible global influenza pandemic. GSK was the first company to submit a “mock-up” dossier to the EMEA to apply for a pandemic influenza vaccine marketing authorisation in the EU, which allows for an accelerated final registration once a pandemic is declared. GSK is also developing an H5N1 prototype pandemic vaccine and clinical trials testing of this vaccine against the H5N1 flu strain are taking place in 2006. To increase the performance of its prototype pandemic vaccine, GSK has developed an innovative adjuvant that may allow lower amounts of antigen to be used, which is essential for manufacturing large number of doses in the event of a pandemic.
| GSK Annual Report 2005 |
| 15 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Be the best place for the best people to do their best work |
GlaxoSmithKline people
GlaxoSmithKline is committed to creating the best place for the best people to do their best work to deliver the Group’s business strategy. The Group employs over 100,000 people in over 116 countries.
Recruitment, talent management and leadership development
Attracting the best people in the industry is critical to enhancing and sustaining GSK’s performance. The Group’s recruiters in the USA and UK are focussed on pro-active identification of talented external candidates for key jobs, acting as an internal headhunting function.
The annual performance and development planning (PDP) process ensures that employees set objectives aligned with corporate strategies, set behavioural goals and create a development plan. PDPs are reviewed throughout the year, culminating with an end of year review that is factored into compensation decisions.
The annual talent management cycle identifies the highest performing people in each business and function. Individuals are given feedback on development needs and key talent is developed through exceptional management and leadership programmes (for more detail see the Group’s Corporate Responsibility Report), exposure to top management through programmes such as the Chief Executive Forum and via stretch assignments. A pool of successors is identified for all Vice-President positions and other critical roles in the organisation.
Performance and reward
Reward systems are designed to support a culture of high performance and to attract and retain the best people. Performance based pay, share awards and share options align employee interests with the accomplishment of business targets.
Business ethics and reputation
Performance with Integrity is central to operating at GSK. The most recent Global Leadership Survey showed over 90% believe that “people in their department show commitment to performance with integrity”. To enhance managers’ and leaders’ skills a programme on ethical decision making was run in 2005, attended by 479 people. Further training in this area is planned for 2006.
The PDP process includes an assessment of how well employees have implemented the GSK Spirit – the principles used to define the Group’s culture. This can have a significant impact on bonus payments, potentially reducing them to zero if an employee is found not to have followed the Spirit, and can also affect future career development. In this way the Group holds employees accountable for delivering performance with high standards of integrity to protect and enhance GSK’s reputation.
Diversity
The GSK diversity initiative focuses on improving performance by responding to the diverse needs of employees, customers and external stakeholders. At the third annual Multicultural Marketing and Diversity Awards, 60 entrants from the USA, UK and Continental Europe highlighted innovative activities that demonstrated business impact. In 2005, the global management population was 64.5% male and 35.5% female. For more details on diversity measures, see the Employment Practices section of the Corporate Responsibility report.
The Group is committed to employment policies free from discrimination against potential or existing staff on the grounds of age, race, ethnic and national origin, gender, sexual orientation, faith or disability. GSK is committed to offering people with disabilities access to the full range of recruitment and career opportunities. Every effort is made to retain and support employees who become disabled while working with the Group.
Communication and employee involvement
Good internal communication is important in achieving GSK’s business objectives as well as creating an open and inclusive work environment. There are a range of communication channels to keep employees up-to-date with GSK’s news and enable them to give feedback. These include:
| • | myGSK, the global intranet site, provides news and updates and a Q&A section where employees put questions directly to the Chief Executive Officer and other senior executives. Up to 100 questions are answered each month. Behind the News, a section of the GSK intranet, gives the Group’s position on important issues linked to press stories about GSK |
| • | Spirit, GSK’s internal magazine, reaches around 50,000 employees throughout the world four times a year |
| • | confidential feedback mechanisms enable employees to raise concerns. These include GSK’s integrity helpline. |
The Group conducts a Global Leadership Survey (GLS) every two years. The last GLS was conducted in 2004 among more than 10,000 managers to gauge opinion on critical issues such as culture and confidence in the Group’s future. Results showed significant improvement on 29 of 31 items compared with 2002 results. Compared with global benchmarks, managers rate highly on fostering alignment between personal goals and the GlaxoSmithKline mission and fostering an environment of ethics and integrity. In the survey, 80% of managers were “proud to be part of GlaxoSmithKline” and would “gladly refer a friend or family member to work for GSK”.
Between Leadership Surveys many business areas conduct surveys of all employees to gauge levels of engagement, satisfaction and motivation. Each business and function has developed action plans to address areas for improvement based on results from the GLS and these other surveys.
The Group also consults employees on changes that affect them and discusses developments in the businesses with the European Employee Forum and similar committees in countries where this is national practice.
Health and well-being
Healthy employees and healthy ways of working contribute to GSK’s sustained performance. Global policies on Employee Health are supported by mandatory standards that integrate employee health and safety and environmental requirements. These standards are applied to all the Group’s facilities and operations worldwide.
A commitment to flexible working through flexi-time, tele-conferencing, remote working and flexible work schedules, recognises that employees work best in an environment that helps them integrate their work and personal lives. During 2005 the Group’s Employee Health Management function won Personnel Today’s Managing Health at Work award in the UK in recognition of its impact in promoting a healthy workplace.
| GSK Annual Report 2005 |
| 16 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Global manufacturing and supply |
GSK has a large portfolio of products, ranging from tablets and toothpaste to inhalers and complex capsules, in over 28,000 different pack sizes and presentations.
Manufacture of medicines begins with the development of a therapeutic active ingredient (bulk active) in a selected formulation. Global Manufacturing and Supply (GMS) develops manufacturing processes for full scale volume production of active compounds at primary manufacturing sites. Converting active compounds into a finished dosage formulation is the responsibility of the secondary manufacturing sites.
GMS operates as a single global network of 80 sites in 37 countries. Each year GMS produces around 6,000 tonnes of bulk actives and over four billion packs, which are packaged and delivered for sale in over 160 countries. Throughout the world it also supports about 2,000 new product and line extension launches a year.
By adopting leading edge practices and developing its people GMS expects to derive benefits from:
| • | a secure source of supply of high quality products |
| • | compliance with regulatory requirements and customer expectations |
| • | best in class cost. |
Organisation
Supply divisions
There are four supply divisions, with sites grouped together based upon common business drivers, areas of expertise and the commercial activities that they support. These four divisions are described below:
Primary supply and Antibiotics
Primary supply and Antibiotics focuses on ensuring the supply of high quality and competitively priced bulk actives and on driving improvements in primary technologies and processes. It also supports the delivery of maximum value from the antibiotics franchise through a combined primary and secondary approach to cost competitive supply and response to market opportunities and customer needs. There are 17 sites in eight countries in Primary supply and Antibiotics.
Consumer Healthcare supply
Consumer Healthcare supply focuses on delivering high quality, competitively produced products and offering the capability for rapid new product introduction in a highly innovative and competitive business which has far shorter time frames than pharmaceuticals. New technologies have become a fundamental platform for lowering costs and providing flexibility in operations. There are 24 sites in 17 countries in Consumer Healthcare supply.
Regional pharma supply
Regional pharma supply focuses on several key activities, the supply of products that are key in one or more regions, the supply of products that are important in a particular market and the tailoring of packaging to meet specific local requirements. A key focus for the regional pharma supply team is on reducing costs so that GSK can compete more effectively in all its markets. There are 31 sites in 23 countries in Regional pharma supply.
New product and global supply
New product and global supply focuses on ensuring that the appropriate technical competencies exist to support rapid and successful new product introduction. It works closely with R&D’s development team to do this. It also ensures secure supply of the key brands that are sold across many markets and have global distribution. This division is the focal point for developing and introducing new secondary manufacturing technologies for GMS. It co-ordinates with Primary supply operations to ensure alignment between the two divisions and a full value stream approach to introducing new products. There are eight sites in six countries in New product and global supply.
Operational excellence
GMS has developed a set of measures and a uniform way of working to drive business improvement. These activities are mainly focused on increasing the quality of products supplied to customers. Extensive leadership education has been carried out to reinforce a culture of continuous improvement, with staff involved in solving problems in a rigorous, controlled and structured way. All this has provided the capability to improve significantly performance, and to accelerate delivery of benefits across the manufacturing network.
Since the formation of GSK, merger rationalisation and operational excellence initiatives have reduced the number of manufacturing sites by 35 (30%).
External suppliers
Manufacturing spends over £2 billion with many external suppliers every year, including on the purchase of active ingredients, chemical intermediates and part-finished and finished products. GMS takes appropriate steps to protect its supply chains from any disruption resulting from interrupted external supply through appropriate stock levels, contracting and alternative registered suppliers.
Vaccines supply chain
In Europe, vaccine manufacturing is located primarily at Rixensart and Wavre in Belgium, with three other sites in France, Germany and Hungary. In 2005, GSK strengthened its global production network in North America through three major acquisitions: US based Corixa Corporation, which produces an important component in many of GSK’s vaccines under development, a vaccine production site in Marietta, Pennsylvania and ID Biomedical with flu vaccine manufacturing facilities in Canada. In Asia, new vaccine production facilities are being built in India and Singapore. GSK’s vaccine division also has two joint ventures in China and Russia. Managing the vaccine supply chain involves anticipating market needs and using a flexible approach to be able to meet fluctuations in demand. These are based on forecasts from the different markets and firm orders from health authorities for mass vaccination campaigns.
Bulk, filling and packaging are carefully balanced and stocking of vaccines helps manage short-term increases in demand. Such increases result from disease outbreaks or increased demand from the public owing to disease awareness campaigns.
| GSK Annual Report 2005 |
| 17 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Corporate responsibility and community investment |
Commit to corporate responsibility
GSK is committed to connecting business decisions to ethical, social and environmental concerns. Thus, corporate responsibility is an integral and embedded part of the way GSK does business.
In 2003, GSK published a set of Corporate Responsibility principles to provide guidance on the standards to which the Group is committed. This sets out the approach to ten areas: standards of ethical conduct, research and innovation, products and customers, access to medicines, employment practices, human rights, community investment, caring for the environment, leadership and advocacy, and engagement with stakeholders. The Group reports annually on progress in upholding these principles in its Corporate Responsibility Report, which is available on the website at www.gsk.com.
Partnership success
GSK works as a partner with under-served communities in the developed and developing world. It supports programmes that are innovative and sustainable and that bring real benefits to these communities. The Group engages with numerous external stakeholders, funds community-led initiatives around the world and donates medicines to support humanitarian efforts and community based healthcare.
Community investment
GSK’s global community investment activities in 2005 were valued at £380 million, equivalent to 5.6% of Group profit before tax. This comprised product donations of £296 million, cash giving of £61 million, other in-kind donations of £2 million and costs of £21 million to manage and deliver community programmes in more than 100 countries.
Product donations in 2005 were as follows:
1. Product donations
GSK’s cash giving was targeted primarily at health and education initiatives.
2. Breakdown of cash giving
In the UK, GSK contributed £4 million in 2005 to its continuing corporate programme of charitable activities supporting over 80 organisations in health, medical research, science education, the arts and the environment. In addition, Group companies in the UK provided a further £8 million for charitable purposes.
Corporate programmes in North America focused on improving public education and access to better healthcare for children and seniors with funding of almost £8 million. In addition, the Group’s US-based businesses donated £14 million to regional community activities.
GSK does not operate a single charitable foundation for its community investment programmes, but has a number of country based foundations. The grants made by these foundations in 2005 are included in the investment total.
Global Health Programmes
Eliminating lymphatic filariasis
The Group’s effort to help rid the world of the disabling disease, lymphatic filariasis (LF), continued in close partnership with the governments of countries where the disease is endemic, the WHO and over 40 partner organisations. GSK is committed to donate as much of the anti-parasitic drug albendazole as required to treat the one billion people at risk in 80 countries by 2020. In 2005, 136 million albendazole treatments, worth over £14 million at wholesale acquisition cost, were donated to 36 countries. Since the global elimination programme started in 2000, a cumulative total of 442 million albendazole treatments have been donated and the programme is now reaching over 100 million people. During 2005, GSK opened a new $3 million manufacturing facility in Cape Town, South Africa to produce albendazole.
Positive Action on HIV/AIDS
Positive Action is GSK’s pioneering global programme working with communities affected by AIDS. Started in 1992, it supports community-based organisations to deliver effective HIV and AIDS education, prevention and healthcare services. During 2005, Positive Action worked with 29 partners to support programmes in 30 countries. The programme also supported the participation of community involvement at regional and international AIDS conferences.
The GlaxoSmithKline African Malaria Partnership
Since 2002, this partnership has supported three behavioural development programmes working in eight African countries. The programmes are targeting nearly two million people and focus particularly on young children and pregnant women, encouraging effective prevention measures, prompt treatment and antenatal malaria management. Extending this programme in 2005, the Group announced a three-year grant of £900,000 to the Malaria Consortium for a new initiative ‘Mobilising for Malaria’. Through increased and sustained advocacy activities in the UK, Europe and African countries, the programme aims to increase awareness of malaria and mobilise resources.
PHASE
The PHASE initiative (Personal Hygiene And Sanitation Education), initiated by GSK in 1998, is now providing education to thousands of school children in Kenya, Uganda, Zambia, Nicaragua and Peru to improve their health and hygiene to fight infectious diseases. In 2005 the Group committed three year funding of £300,000 to extend the programme to Bangladesh in partnership with Save the Children, USA.
| GSK Annual Report 2005 |
| 18 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Corporate responsibility and community investment |
| continued |
Humanitarian product donations
During 2005, GSK donated essential products, such as antibiotics, through non-profit partners including AmeriCares, MAP International and Project HOPE, to support humanitarian relief efforts and community healthcare. In December 2004, medicines donated by the Group were among the first to be shipped to support the south Asia tsunami relief efforts. In 2005, GSK continued to donate these lifesaving medicines to tsunami-affected countries and to those affected by other disasters, including hurricanes in the USA.
In 2005 the total value of the Group’s international humanitarian product donations was £27 million. This excludes albendazole donated as part of the Group’s commitment to the lymphatic filariasis elimination programme. Product donations are valued at wholesale acquisition cost which is the wholesale list price, not including discounts, and is a standard industry method.
Community initiatives
GSK is dedicated to strengthening the fabric of communities where we live and work through providing health and education initiatives and support for local civic and cultural institutions that improve the quality of life.
GSK’s contribution to improve healthcare includes a new grant of $2.65 million over three years to the Children’s Health Fund to expand their Referral Management Initiative (RMI) to sites in Philadelphia, including the Delaware Valley Community Health Center. The RMI ensures continuity of specialist medical care for high-risk children who are often homeless.
The annual Impact Awards recognise excellence in the work of non-profit community health organisations across the UK and in the Greater Philadelphia area of the USA. Over 20 charities receive unrestricted awards for their work dealing with diverse issues such as domestic and community violence, sexual health services for young people and bereavement and counselling services.
To further medical research, over £470,000 was provided to four UK medical charities, The Alzheimer’s Research Trust, The British Liver Trust, Meningitis UK and The Samantha Dickson Research Trust for childhood brain tumours.
As part of GSK’s support for the arts, the Group sponsored the popular ‘Gardens of Glass: Chihuly at Kew‘, an innovative exhibition of the work of Dale Chihuly, the contemporary glass artist, at the Royal Botanic Gardens, Kew near London.
Education initiatives
GSK’s efforts to improve public and science education included a three-year grant of $300,000 to the National Board for Professional Teaching Standards to increase the number of science teachers pursuing certification in the North Carolina and Philadelphia areas.
During 2005 GSK led a group of companies to come together to create the US Business Education Network (BEN). BEN is a new business coalition staffed by the Center for Corporate Citizenship of the US Chamber of Commerce, and is dedicated to harnessing the power of the business community to address issues facing the US education system.
GSK continued to support the Innovative Scheme for Post-docs in Research and Education (INSPIRE), developed in partnership with Imperial College London and the Specialist Schools and Academies Trust, with a £1 million donation over four years. INSPIRE places post-doctoral researchers in specialist science schools to assist with science teaching.
‘Science in the Summer’, a free library-based science education programme in the Philadelphia area teaching basic scientific concepts, continued to receive support with a grant of $300,000. Science Across the World is an award-winning international education programme that uses web-based resources to promote discussion of science issues between 3,600 teachers, 100,000 children and schools in more than 115 countries. A further grant of £110,000 was made in 2005 bringing GSK’s total contribution to this programme to £670,000 over five years.
Employee involvement
GSK employees are encouraged to contribute to their local communities through employee volunteering schemes. Support varies around the world, but includes employee time, cash donations to charities where employees volunteer and a matching gifts programme.
In 2005 in the USA, the Group matched more than 20,000 employee and retiree gifts at a value of over $5 million. The Group also matched more than $1.3 million of employee donations to GSK’s annual United Way campaign. GSK’s GIVE program provided grants of over $300,000 to more than 350 organisations where US employees have volunteered.
GSK’s Making a Difference programme in the UK provided grants of almost £300,000 to over 440 non-profit organisations and registered charities based on employee involvement.
| GSK Annual Report 2005 |
| 19 |
| REPORT OF THE DIRECTORS |
| Description of business |
Products and competition |
Pharmaceutical products
GlaxoSmithKline’s principal pharmaceutical products are presentlycurrently directed to 10nine therapeutic areas. An analysis of sales by these therapeutic areas, and a description of the principal products, are set out below:
| Turnover by therapeutic area | 2005 | 2004 | 2003 | |||||||||
| £m | £m | |||||||||||
| 2002 | 2001 | 2000 | ||||||||||
| Sales by therapeutic area | £m | £m | £m | |||||||||
| Respiratory | 5,054 | 4,394 | 4,390 | |||||||||
| Central nervous system | 4,511 | 4,007 | 3,279 | 3,219 | 3,462 | 4,446 | ||||||
| Respiratory | 3,987 | 3,537 | 2,789 | |||||||||
| Anti-virals | 2,299 | 2,128 | 1,899 | 2,598 | 2,359 | 2,345 | ||||||
| Anti-bacterials/anti-malarials | 2,210 | 2,604 | 2,472 | 1,519 | 1,547 | 1,800 | ||||||
| Metabolic and gastro-intestinal | 1,429 | 1,480 | 1,232 | |||||||||
| Metabolic | 1,495 | 1,251 | 1,077 | |||||||||
| Vaccines | 1,080 | 948 | 842 | 1,389 | 1,194 | 1,121 | ||||||
| Oncology and emesis | 977 | 838 | 710 | 1,016 | 934 | 1,000 | ||||||
| Cardiovascular | 655 | 591 | 463 | |||||||||
| Arthritis | 23 | 156 | 210 | |||||||||
| Others | 824 | 916 | 1,086 | |||||||||
| Divested products | – | – | 447 | |||||||||
| Cardiovascular and urogenital | 1,331 | 932 | 770 | |||||||||
| Other | 1,040 | 1,027 | 1,165 | |||||||||
| 17,995 | 17,205 | 15,429 | 18,661 | 17,100 | 18,114 | |||||||
Sales in 2005 were 8% higher in CER terms and 9% in sterling terms than in 2004.
Products and all their formulations may not be approved for all indications in all markets where they are available.
Respiratory
Seretide/Advair, a combination ofSereventandFlixotide, offers a long-acting bronchodilator and an anti-inflammatory in a single inhaler. It is approved for the treatment of asthma and COPD.
Flixotide/FloventandBecotide/Becloventare inhaled steroids for the treatment of inflammation associated with asthma and COPD.
Sereventis a long-acting bronchodilator used to treat asthma and COPD, andVentolinis a selective short-acting bronchodilator used to treat bronchospasm.
Flixonase/FlonaseandBeconaseare steriod intra-nasal preparations for the treatment of perennial and seasonal rhinitis.
Central nervous system (CNS)
Seroxat/Paxilis a selective serotonin re-uptake inhibitor (SSRI) approved for the treatment of depression, panic, obsessive compulsive disorder, post traumatic stress disorder, social anxiety disorder, premenstrual dysphoric disorder and generalgeneralised anxiety disorder.Paxil CR, a controlled release version, was launched in the USA in 2002.
Wellbutrinis an anti-depressant, available in the USA and some international markets in normal, or sustained release tabletsustained-release (SR) and once daily formulations.
Imigran/Imitrexis a 5HT1 5HT1 receptor agonist used for the treatment of severe or frequent migraine and cluster headache and has become the reference product in this sector.Naramig/Amergeis a newer migraine product.
Lamictalis, a well established treatment for epilepsy. Used alone or in combination with other products, it has achieved penetration of this mature market through successful treatment of severe cases.epilepsy, is now also indicated for bipolar disorder.
Requipis a specific dopamine D2-likeD2/D3 receptor agonist indicated for the treatment of Parkinson’s disease.
Zybandisease and is a nicotine-free prescription medicine, available as a sustained-release tablet,the first approved product for treating the problem of smoking addiction.
RespiratorySeretide/Advair, a combination ofSereventandFlixotide, offers a long-acting bronchodilator and an anti-inflammatory in a single inhaler.
Sereventis a long-acting bronchodilator andVentolinis a selective short-acting bronchodilator.
Flixotide/FloventandBecotide/Becloventare inhaled steroids for the treatment of inflammation associated with bronchial asthma and chronic bronchitis.
Flixonase/FlonaseandBeconaseare intra-nasal preparations for the treatment of perennial and seasonal rhinitis.Restless Leg Syndrome (RLS).
Anti-virals
Combivir, a combination ofRetrovirandEpivir, has consolidated the position of these two reverse transcriptase inhibitors as the cornerstone of many multiple anti-HIV product regimens. Physician acceptance has clearly demonstrated the value placed on minimising the ‘pill burden’pill burden faced by patients.
Ziagenis a reverse transcriptase inhibitor. The product’s potency, ease of use and resistance profile allow it to play a significant role in a variety of highly active, well tolerated and simplified HIV treatment regimens.
Triziviris a combination ofCombivirandZiagen, combining three anti-HIV therapies in one tablet, for twice daily administration.
AgeneraseEpzicom/Kivexa, approved for use in the USA and Europe, is a combination ofEpivirandZiagenthat is taken as one tablet with once-daily dosing for HIV/AIDS in combination with at least one other anti-HIV drug.
Lexiva/Telziris a protease inhibitor for the treatment of HIV the first medicine of this class to be brought to the market by GlaxoSmithKline.that is well tolerated and more convenient thanAgenerasehas awhich it supersedes.Lexivamay be taken twice daily dosing regimen and no significant food or drink restrictions.once daily when boosted with ritonavir.
Zeffixhas been approved for marketing in the USA, Europe, China and other markets for the treatment of chronic hepatitis B.
Valtrexis a treatment for chicken pox, zoster (shingles), cold sores and episodic genital herpes as well as the long term suppression and reduction of transmission of genital herpes.herpes, zoster (shingles), cold sores and chicken pox.ValtrexsupersedesZovirax, which is also widely used to treat herpes infections.
Anti-bacterials and anti-malarials
Augmentinis a broad-spectrum antibiotic suitable for the treatment of a wide range of common bacterial infections and is particularly effective against respiratory tract infections.Augmentin ES-600is an extra strength suspension specifically designed to treat children with recurrent or persistent middle ear infections.
Augmentin XRis an extra strength tablet form for adults to combat the growing problem of bacterial resistance in the community.difficult to treat infections.
Zinnatis an oral antibiotic used primarily for community-acquired infections of the lower respiratory tract.Fortumis used in the hospital-based injectable antibiotics market.
Malaroneis an oral anti-malarial used for the treatment and prophylaxis of malaria caused by Plasmodium falciparum.
Lapdapis an effective and well tolerated therapy for the treatment of malaria, which has been developed through a public/private collaboration.
| GSK Annual Report 2005 |
| 20 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Products and competition |
| continued |
Metabolic and gastro-intestinal
Avandiais a potent insulin sensitising agent which acts on the underlying pathophysiology of type 2 diabetes.
Avandametis a combination ofAvandiaand metformin HCI; it is the first medicine that targets insulin resistance and decreases glucose production in one convenient pill.
ZantacAvandaryl,is a fixed-dosed combination ofAvandiaand Amaryl, a Sanofi-Aventis product.
Bonviva/Bonivais a once-monthly oral bisphosphonate for the treatment of peptic ulcer diseaseosteoporosis. It was launched in the USA and a range of gastric acid related disorders, continues to play a major roleseveral EU markets in a number of markets, even where patent protection has been lost.2005.
VaccinesGlaxoSmithKlineGSK markets aover 25 vaccines worldwide. In GSK’s hepatitis vaccines range, of hepatitis vaccines.Havrixprotects against hepatitis A andEngerix-Bagainst hepatitis B.
Twinrixis athe only available combined hepatitis A and B vaccine, protecting against both diseases with one vaccine and available in both adult and paediatric strengths. In 2005, GSK received European approval forFendrix, a vaccine to prevent hepatitis B in patients with renal insufficiency including high-risk groups such as pre-haemodialysis and haemodialysis patients, from 15 years of age onwards.
Fluarixis indicated for prevention of certain types of influenza. It is distributed in 79 countries and was approved in the USA in 2005.Fluarixis the first vaccine to receive FDA approval under the agency’s accelerated approval regulations.
Infanrixis aGSK’s range of paediatric vaccine combinations.Infanrixprovides protection against diphtheria, tetanus and pertussis (whooping cough).Infanrix PeNta/PeNta/Pediarixprovides additional protection against hepatitis B and polio, andInfanrix HeXahexafurther adds protection against haemophilusHaemophilus influenzae type b, which causesis a cause of meningitis. In 2005, GSK launchedBoostrixin the USA, a vaccine that adds protection against pertussis (whooping cough) to the routine tetanus/di ptheria booster administered to teenagers.
Additionally, GlaxoSmithKlineGSK also marketsPriorix, a measles, mumps and rubella vaccine,Typherix, a vaccine for protection against typhoid fever, andVarilrix, a vaccine against varicella or chicken pox. In addition, the Group markets a range of vaccines to prevent meningitis under the umbrella nameMencevax. GSK recently received approval in the UK for a new Hib-MenC vaccine,Menitorix. GSK’s meningitis vaccine portfolio wil be complimented by new meningitis conjugate vaccines in the near future.
As part of its paediatric franchise, GSK has also developed a vaccine against rotavirus induced gastroenteritis. Since its launch in Mexico in 2005,Rotarixhas been licensed in several additional countries worldwide among them a number of Latin American countries including Brazil, with the Philippines and Singapore being the first Asian countries.
Oncology and emesis
Zofranis used to prevent nausea and vomiting associated with chemotherapy and radiotherapy for cancer, and is available in both oral and injectable forms. It is also approved for use in the prevention and treatment of post-operative nausea and vomiting.
Hycamtinis a second line treatment both for ovarian cancer and for small cell lung cancer.
NavelbineBexxaris approved as first linea treatment of non-small cell lung cancer in combinationfor patients with cisplatin or as a single agent.CD20 follicular, non-Hodgkin’s lymphoma with and without transformation whose disease is refractory to rituximab and who have relapsed following chemotherapy.
Cardiovascular and urogenital
Coregis an alpha/beta blocker which has been proven to be effective in treating patients with mild, moderate and severe heart failure. GlaxoSmithKlinefailure, heart attack or hypertension. GSK has sole marketing rights in the USA.USA and Canada. Generic versions of the product are available in Canada.
ArthritisRelafenLevitrais a non-steroidal anti-inflammatory drugPDE-5 inhibitor indicated for male erectile dysfunction. GSK has co-promotion rights in the USA and more than 20 other markets.
Avodartis a 5-ARI inhibitor currently indicated for benign prostatic hyperplasia. A large clinical outcome study is underway examining its efficacy in the prevention of prostate cancer.
ArixtraandFraxiparinewere acquired in 2004 as part of the divestitures required for the merger of Sanofi and Aventis.
Arixtra, a selective Factor Xa inhibitor, is indicated for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism, in hip fracture surgery, knee replacement, hip replacement surgery and abdominal surgery. It is also indicated for the treatment of arthritis.deep vein thrombosis and pulmonary embolism.
Fraxiparineis a low-molecular weight heparin indicated for prophylaxis of thromboembolic disorders (particularly deep vein thrombosis and pulmonary embolism) in general surgery and in orthopedic surgery, treatment of deep vein thrombosis and prevention of clotting during hemodialysis.
Integrilinis a GP IIb-IIIa inhibitor, approved in the EU for the prevention of early myocardial infarction in patients with unstable angina or non-Q-wave MI.
OtherThe otherThis category includes the Group’s principal dermatological products;Betnovate, the higher potencyDermovateand the newerCutivate, which are anti-inflammatory steroid products used to treat skin diseases such as eczema and psoriasis.psoriasis,Relafen
Divested productsIn accordance with agreements, a non-steroidal anti-inflammatory drug for regulatory approvalsthe treatment of the merger between Glaxo Wellcomearthritis, and SmithKline Beecham, the productsKytrilZantac, for the treatment of chemotherapy –peptic ulcer disease and radiotherapy–induced nausea and vomiting, andFamvir, an anti-viral for the treatmenta range of shingles and herpes, were divested in December 2000.gastric acid related disorders.
Products – Consumer Healthcare
GlaxoSmithKline’s principal consumer healthcare products are presently directed to three major areas. An analysis of sales by these areas is set out below:
| 2002 | 2001 | 2000 | ||||
| £m | £m | £m | ||||
| Over-the-counter medicines | 1,586 | 1,603 | 1,454 | |||
| Oral care | 1,052 | 1,106 | 642 | |||
| Nutritional healthcare | 579 | 575 | 535 | |||
| Divested products | – | – | 19 | |||
| 3,217 | 3,284 | 2,650 | ||||
At constant exchange rates 2002 sales were two per cent higher than 2001.
| GSK Annual Report 2005 | |
21 | |
Over-the-counter medicinesThe leading products arePanadol, a widely available paracetamol/acetominophen analgesic;Nicorettegum; theNicoderm, NiQuitin CQandNicabaterange of smoking control patches;Tums, a calcium based antacid;Citrucellaxative;Contacfor the treatment of colds and influenza;Abtei, a natural medicines and vitamin range;Oxyacne treatment andZoviraxandAbrevafor the treatment of cold sores.
In 2002, GlaxoSmithKline Consumer Healthcare introduced two new prescription to over-the-counter switches:
Oral careThe leading oral care products are toothpastes and mouthwashes under theAquafresh,Sensodyne,MacleansandOdolbrand names, and a range of toothbrushes sold under theAquafresh,SensodyneandDr Bestnames. In addition, denture care products are available principally under thePolident,PoligripandCoregabrand names.
Nutritional healthcareThe leading products in this category areLucozadeglucose energy and sports drinks,Ribenablackcurrant-based juice drink rich in vitamin C, andHorlicks, a range of milk-based malted food and chocolate drinks.
| Description of business | |
Products and competition | |
| continued | |
Operating environment
Competition – Pharmaceuticals competition
The pharmaceutical industry is highly competitive.GlaxoSmithKline’s GSK’s principal competitors arerange from small to large international pharmaceutical companies with substantial resources. Some of these companies and their major products are mentioned below.
Pharmaceuticals may be subject to competition from other products during the period of patent protection and, once off patent, from generic versions. The manufacturers of generic products typically do not bear significant research and development or education and marketing development costs and consequently are able to offer their products at considerably lower prices than the branded competitors. A research and development based pharmaceutical company will normally seek to achieve a sufficiently high profit margin and sales volume during the period of patent protection to repay the original investment, which is generally substantial, and to fund research for the future. Competition from generic products generally occurs as patents in major markets expire. Increasingly patent challenges are made prior to patent expiry.expiry, claiming that the innovator patent is not valid and/or that it is not infringed by the generic product. Following loss of patent protection, generic products rapidly capture a large share of the market, particularly in the USA.
GlaxoSmithKline undertakes a range of activities to maximise the value of its intellectual property, including introducing innovative products into as many markets as possible, accelerating the process to bring new products to market and increasing brand recognition among customers.
GlaxoSmithKlineGSK believes that itsremaining competitive position is dependent upon the discovery and development of new products, together with effective marketing of existing products. Within the pharmaceutical industry, the introduction of new products and processes by competitors may affect pricing levels or result in changing patterns of product replacement.use. There can be no assurance that products maywill not become outmoded, notwithstanding patent or trade marktrademark protection. In addition, increasingincreased government and other pressurepressures for physicians and patients to use generic pharmaceuticals, rather than brand-name medicines, may increase competition for products that are no longer protected by patent.
CNS disordersRespiratory
GSK’s respiratory franchise is driven by the growth ofSeretide/Advair, gaining patients from competitor products and the cannibalisation ofSereventandFlixotide/Flovent. Major respiratory competitors toPaxilare Singulair from Merck, especially in the USA selective serotonin re-uptake inhibitor (SSRI) market and in Europe, Symbicort from AstraZeneca and Spiriva from Pfizer/ Boehringer Ingelheim.
CNS disorders
Major competitors in the USA toPaxilare its generic forms, as well as generic fluoxetine, the generic form of Eli Lilly’s Prozac, Zoloft from Pfizer, Forest Laboratories’ Celexa and Lexapro.Lexapro, and Effexor from Wyeth. The principal competitors in the USA forWellbutrinare generic forms of bupropion, the generic forms of SSRIs and Effexor XR, a Wyeth product. The successPaxil CRand the once-dailyWellbutrin XLhelp to retain a strong presence in the anti-depressant market, given the availability of both generic paroxetine and bupropion in the USA. Generic competition forSeroxat/PaxilandWellbutrinhas made them a target for generic manufacturers, against whom GlaxoSmithKline continues to respond appropriately (see Note 30 to the Financial statements, ‘Legal proceedings’).
Imigranhas grown to be one of GlaxoSmithKline’s leading products through addressing the previously unmet needs of migraine sufferers. Although other companies have launched competing products, newer formulations ofImigran,such as the nasal spray, and the introduction ofNaramighave helped the Group to retain its lead over its competitorsalso commenced in the migraine marketUK and maintain growth.a number of other markets.
RespiratoryAnti-viralsGlaxoSmithKline’s respiratory franchiseGSK is driven by the growth ofSeretide/Advair, gaining patients from competitor products and the cannibalisation ofSereventandFlixotide.VentolinandBecotidehave faced generic competition for some years but have maintained significant sales.
Major respiratory competitors are Singulair from Merck, especially in the USA, Symbicort from AstraZeneca, primarily in the European Union and Spiriva from Pfizer/Boehringer Ingelheim, primarily in Europe.
Anti-viralsThe major competitorsa pioneer in the HIV market, are Bristol Myers Squibb, Mercklaunching AZT (Retrovir)in 1987 and Pfizer amongst others.
GlaxoSmithKline has a pioneering role in the HIV market, withRetrovirandEpiviracting as the cornerstone of combination therapy, andin 1995, which today are available asCombivirin a single tablet.tablet, a cornerstone of HIV combination therapy. The launches ofZiagen,Ageneraseand,Trizivir,LexivaandEpzicomhave broadened the Group’s portfolio of HIV products. Major competitors in the HIV market include Gilead, Bristol Myers Squibb, Abbott, Merck and Pfizer.
Valtrexhas helped strengthenstrengthened the Group’s position in the anti-herpes area, althoughwhere GSK’sValtrexandZoviraxcompete with Novartis’ Famvir.Valtrexis the market leader, whilstZoviraxfaces competition from generic aciclovir. Bothacyclovir. In the hepatitis B market, GSK’sValtrexandZoviraxcompete with Novartis’ Famvir.Zeffixwas the first anti-viral on the market to treat Hepatitis B.market. Gilead’s Hepsera iswas the second and was approved by the US Food and Drug Administration (FDA) second. The Group has secured marketing rights toHepserain September 2002.some key markets.
Anti-bacterials and anti-malarialsIn 2002 genericGeneric versions of bothAugmentinandCeftin/Zinnatwere introducedare available in the USA, following successful legal challenges by generic manufacturers (see Note 30 to the Financial statements, ‘Legal proceedings’).USA.Augmentinhas already lost patent protectionalso faces generic competition in various countries in Europe. The recently launchedEuropean countries.Augmentin XR, andAugmentin EScompete against a broad range of other branded and generic antibiotics. MalaroneMalar one’s’s safety profile and convenient dosing regimen have helped put this product in a strong position versus mefloquine following its recent launch for malaria prophylaxis.
Metabolic and gastro-intestinal
The major competitor forAvandiais Takeda Chemical’s Actos, which is co-promoted with Eli Lilly in the USA.
MonthlyBoniva/Bonvivacompetes with Merck’s weekly Fosamax and Proctor & Gamble/Sanofi-Aventis’s weekly Actonel. Generic Fosamax (alendronate) is available in a few markets such as the UK and Canada.
VaccinesGlaxoSmithKline’sThe vaccine market is dominated by four key players. GSK’s major competitors in the vaccine market include AventisSanofi Pasteur (AP)(SP), Merck and Wyeth. In the hepatitis market,Engerix-BandHavrixcompete with vaccines produced by APSP and Merck – respectively Comvax and Recombivax HB for hepatitis B, and Vaqta and Avaxim for hepatitis A. Within the paediatric vaccine field,Infanrix’smajormain competitor is AP’sSP’s range of DTPa-based combination vaccines.vaccines, although theInfanrix hexacombination is the only available hexavalent paediatric combination in Europe.
Oncology and emesis
Zofranpresently provides GSK with a leadership position in the anti-emetic market where competitor companies include Roche, Sanofi-Aventis and more recently MGI and Merck. Major competitors in the diverse cytotoxic market include Bristol Myers Squibb, Sanofi-Aventis, Pfizer and Novartis. GSK’s cytotoxic portfolio, led byHycamtin, currently holds a relatively small market position.
Cardiovascular and urogenital
GSK marketsCoregin the USA where its major competitors are Toprol XL and generic betablockers.Avodartcompetes directly with Merck’s Proscar within the BPH market. The Group has co-promotion rights in the USA forLevitra, which faces competition from Pfizer’s Viagra and Lilly/Icos’ Cialis.
| GSK Annual Report 2005 |
| 22 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Products and competition |
| continued |
Consumer Healthcare products
GlaxoSmithKline’s principal consumer healthcare products are in three major areas. An analysis of sales by these areas is set out below:
| 2005 | 2004 | 2003 | ||||
| £m | £m | £m | ||||
| OTC medicines | 1,437 | 1,400 | 1,472 | |||
| Oral care | 943 | 913 | 915 | |||
| Nutritional healthcare | 619 | 573 | 569 | |||
| 2,999 | 2,886 | 2,956 | ||||
In 2005 sales were 2% higher in CER terms and 4% higher in sterling terms than in 2004.
Major products, which are not necessarily sold in all markets, are:
| Category | Product |
| Over-the-counter medicines | |
| Analgesics | Panadol |
| Dermatologicals | |
| Abreva | |
| Gastro-intestinal | Tums |
| Citrucel | |
| Respiratory tract | Contac |
| Beechams | |
| Smoking control | Commit |
| Nicorette | |
| NicoDerm CQ | |
| NiQuitin CQ | |
| Nicabate CQ | |
| Natural wellness support | Abtei |
| Oral care | Aquafresh |
| Dr Best | |
| Macleans | |
| Odol | |
| Odol Med 3 | |
| Polident | |
| Poligrip | |
| Sensodyne | |
| Nutritional healthcare | Lucozade |
| Ribena | |
| Horlicks | |
Over-the-counter medicines
The leading products arePanadol, a widely available paracetamol/ acetominophen analgesic,Nicorettegum in the USA, theNicoDerm,NiQuitin CQandNicabaterange of smoking control products,Tums, a calcium-based antacid,Citrucellaxative,Contacfor the treatment of colds,Abtei, a natural medicines and vitamin range, andZoviraxandAbrevafor the treatment of cold sores.
Nutritional healthcare
The leading products in this category areLucozadeglucose energy and sports drinks,Ribena, a blackcurrant juice-based drink rich in vitamin C, andHorlicks, a range of milk-based malted food and chocolate drinks.
Consumer Healthcare competition
GSK holds leading global positions in all its key consumer product areas. Worldwide it is the third largest in Oral care and in OTC medicines. In Nutritional healthcare it holds the leading position in the UK, India and Ireland.
The environment in which the Consumer Healthcare business operates has become ever more challenging:
| • | consumers are demanding better quality, better value and improved performance |
| • | retailers have consolidated and globalised which has strengthened their negotiation power |
| • | competitors are finding conditions equally challenging and competing more aggressively across all elements of the marketing mix |
| • | cycle times for innovation have been reduced. |
Competition – Consumer Healthcare
The main competitors in the Group’s Consumer Healthcare markets include the major international companies Colgate-Palmolive, Johnson & Johnson, Pfizer, Procter & Gamble, Unilever and Wyeth. In addition, there are many other companies that compete with GlaxoSmithKlineGSK in selectedcertain markets.
The major competitor products in over-the-counter (OTC)OTC medicines are:
| • | in the USA: Metamucil (laxative), |
| • | in the UK: Lemsip (cold remedy), Nurofen and Anadin (analgesics), and Nicorette and Nicotinell (smoking control |
In Oral care the major competitors are Colgate-Palmolive’s Colgate and Procter & Gamble’s Crest.
In Nutritional healthcare the major competitors toHorlicksare Ovaltine and Milo malted food and chocolate drinks. The competitors toRibenaare primarily local fruit juice products, whileLucozadecompetes with other energy drinks.
GlaxoSmithKline holds leading global positions in all its key consumer product areas. Worldwide it is the second largest in Oral care and the third largest in OTC medicines. In Nutritional healthcare it holds the leading position in the UK, India and Ireland.
| GSK Annual Report 2005 |
| 23 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Regulatory environment |
Regulation – Pharmaceuticals
The international pharmaceutical industry isGSK operates within a highly regulated. National regulatory authorities administer a panoply ofregulated environment. Regional and country-specific laws and regulations governingdefine the data required to show safety and efficacy of pharmaceutical products, as well as govern testing, approval, manufacturing, labelling and marketing of drugs and also review the safety and efficacy of pharmaceutical products.drugs. These regulatory requirements are a major factor in determining whether a substance canmarketable product may be successfully developed into a marketable product and the amount of time and expense associated with suchthis development.
Of particular importance isIn Europe, pharmaceutical firms and regulators are managing a transition following the requirementimplementation of new medicines legislation at the end of 2005. Significant changes are being implemented in many countries that products be authorised or registered prior toa number of areas, including approval procedures, post marketing requirements, manufacturing controls (on active ingredients and that such authorisation or registration be maintained subsequently.excipients), labelling requirements, pharmacovigilance processes and an increased emphasis in involvement and availability of information for patients in the EU.
The national regulatory authoritiesclimate of change will continue, with the expectation that a new Paediatric Regulation will be finalised in many jurisdictions, including2006, stimulating industry research into paediatric indications, via intellectual property incentives.
The European Medicines Agency (EMEA) has published the USA,final version of its ‘Road Map’, a strategic plan to 2010. This will be an additional driver for change, covering areas such as new technologies, innovative development approaches and enhanced provision of agency advice during the European Union, Japan and Australia, have high standards of technical appraisal and consequently the introduction of new pharmaceutical products generally entails a lengthy approvaldevelopment process.
In the European Union, thereUSA, safety issues of prescription drugs are two proceduresa primary focus of the FDA and congressional oversight committees since the recent withdrawal of several products from the market for obtaining marketing authorisations for medicinal products:
Grant of a marketing authorisation affords the Group a protection period during which a competitor cannot rely on confidential data in the regulatory file as a basis for its own marketing authorisation. The data protection period begins on the datecurrent development programmes. As in Europe, evaluation of benefit and risk continues to be an authorisation is first granted in the European Union and expires after ten years for authorisations granted via the Centralised Procedure, or ten or six years for authorisations granted via the Mutual Recognition procedure, depending on the country concerned.
In the USA, the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman) established the current frameworkimportant consideration for approval of generic drugs, including related patent and data protection provisions. Under Hatch-Waxman,a new drug by the sponsor of an Abbreviated New Drug Application (ANDA) can receive marketing approval without submitting any safety or efficacy data. It can rely on the pioneer company’s extensive pre-clinical and clinical development data, provided the proposed generic drug has been demonstrated to be bioequivalent to the pioneer product. However, generic drug approvals are subject to data protection periods of five years for new chemical entities and three years for any modifications supported by new clinical studies. Moreover, under the provisions of Hatch-Waxman, the filing of an ANDA can trigger procedures that may allow patent holders to initiate patent infringement litigation with the significant procedural advantage of being assured that the FDA’s approval of the proposed generic product will be stayed for up to 30 months, pending resolution of the litigation. These procedures have generated litigation and controversy, particularly because, as currently applied, they have resulted in multiple, non-concurrent 30-month stays for some proposed generic products. FDA.
The FDA has proposed changesintroduced a new focus called the Critical Path Initiative. This is intended to its regulations to address certain aspects of the procedures that have generated litigationfacilitate innovation in drug development, hopefully allowing for more rapid development and controversy, and reform legislation has also been proposed in the US Congress.
In the USA, the second reauthorisation of the Prescription Drug User Fee Act came into effect on 1st October 2002 (PDUFA III).
A recent General Accounting Office report to the Senate Committee on Health, Education, Labor and Pensions, has noted a gradual increase in the median time to gain approval for drugs with a standard review designation. Since 1995, the approval time for priority review drugs, judged by the FDA to represent a significant therapeutic advance, has remained constant, with a median time for approval of six months.
There has been an increasing gap between the approval times for priority and standard applications. The approval time for drugs designated as a standard review reached a low of about 13 months in 1998 before rising to about 20 months in 2000 and 2001.
It remains to be seen if the substantial additional resources funded under PDUFA IIIneeded medicines. This initiative will result in a reduction of overall approval times for all drugs and biologics. The review times for certain biologics, other than vaccines, may be substantially impacted by a recently announced consolidation of the review activities to the Center for Drug Evaluation and Research. These activities had previously been carried out by the Center for Biologics Evaluation and Research.
Over the last decade, regulatory authorities have focused significant attention on measures to manage the risk associated withinvestigate the use of prescription products. This is particularly noticeable in the USA, wherepharmacogenomics and surrogate markers of efficacy, among other things, such as manufacturing innovations, as tools for rapidly developing and producing safe and effective drugs for unmet medical needs. The pharmaceutical industry, including GSK, are collaborating with the FDA and National Institutes of Health in a number of these areas, including the pharmaceutical industry haveuse of biomarkers.
A new health information source has been subjectedlaunched by the US government that includes electronic labelling of all approved prescription drugs, posted within one day of an FDA approval action, for immediate access by physicians and patients. GSK is now providing labelling to intense public scrutiny of the adequacy of measures taken to protect the public health. Substantial funding for the FDA to strengthen post market surveillance activities was included under PDUFA III. Under performance goals associated withfor all products in this new electronic format. New regulations from the legislation, drug manufacturers are encouraged to submit voluntarily proposed Risk Management Plans as partFDA will be implemented mid-2006 that will completely change the format of applications for marketing new drugs. These recent developments represent an additional challenge to the market registration processprescribing information in the USA.
On 7th June 2002,GSK is well placed to manage effectively these changes in the FDA announced approval of a supplemental New Drug Application (sNDA) that allows restricted marketing ofLotronex(alosetron hydrochloride) to treat only women with severe diarrhoea-predominant irritable bowel syndrome (IBS). The November 2002 reintroduction followed an advisory committee review and implementation of a FDA-GlaxoSmithKline agreed risk management programme intended to ensure patients and physicians are fully informed of possible risks and benefits ofLotronex.
Across International markets, countries outside the USA and Europe, theexternal regulatory environment continues to be extremely varied and challenging. GlaxoSmithKline anticipates that the introduction of new products will continue to require substantial effort, time and expense to comply with regulatory requirements.environment.
Price controls
In many countries the prices of pharmaceutical products are controlled by law. Governments may also influence prices through their control of national healthcare organisations, which may bear a large part of the cost of supplying products to consumers.
Recent government healthcare reforms in countries such as France, Spain and Germany may restrict pricing and reimbursement.
In the USA, debate overrecent legislation on healthcare reform, cross-border trade, the reformacceleration of the healthcare system hasgenerics to market and increased patient contributions have further increased the focus on pricing. Currently, there are no government price controls over private sector purchases, but federal legislationlaw requires pharmaceutical manufacturers to pay prescribed rebates on certain drugs in order to be eligible for reimbursement under Medicaid and other federal healthcare programmes.
Medicare
In Europe, historically affected2006, the US Medicare program, a federally funded healthcare insurance program benefiting senior citizens and certain disabled Americans, included coverage for prescription medicines. This is a new benefit under the Medicare program and the most dramatic change in the program since its inception in the 1960s. The coverage is voluntary, includes brand-name and generic drugs and is open to the 41 million Americans with Medicare coverage.
A number of competing private organisations provide the new benefit with premiums subsidised by the government. Benefits must satisfy a minimum standard outlined in federal law. While the law provides incentives for manufacturers to negotiate prices with private plans, it does not provide for government regulation in pricingprice controls. The government provides additional help to more than 14 million people on Medicare with limited incomes and reimbursement, the pharmaceutical industry continues to experience pressure on its prices through a range of measures, including across-the-board cuts, linking to low-cost countries, price referencingresources. Those qualifying beneficiaries pay no or reduced premiums and delays in agreeing reimbursement. There is an increasing pressuredeductibles, and low copayments for generic substitution and cross-border imports from low-priced markets may exert a commercial pressure on in-country pricing.their prescriptions.
In Japan, discussions are ongoing as to which new price and reimbursement controls the government will introduce.
During 2002, the worldwide pharmaceutical market continued to experience increasing pressure on pricing and reimbursement from governments and healthcare providers, though it is non-price factors, new products and higher volumes, which are principally driving the growth of pharmaceutical expenditure.
Value for money
It is becoming increasingly necessary to demonstrate the value for money of new products, inproducts. In particular, the impact uponon drug budget expenditure and the burden of the disease that will be treated.treated must be apparent.
In some markets, the needthis requirement to satisfy healthcare purchasers as to value for money is becoming an additional hurdle for product acceptance over and above the regulatory tests of safety, efficacy and quality. This canmay delay bringing effective and improved medicines to the market and reduce their effective patent protection time.
In many markets, especially in the USA and Europe, it is becoming increasinglymore difficult for even a significantly improved therapy to obtain a premium price over existing medication. Philosophies founded on value-basedValue-based pricing aremay be difficult to followapply in such circumstances, although in the USA it is still possible to price products to reflect their value.
It is not possible to predict whether, and to what extent, the Group’s business maywill be affected by future legislative and regulatory developments relating to specific pharmaceutical products or their price.
Regulation – Consumer Healthcare
The consumer healthcare industry is subject to national regulation for the testing, approval, manufacturing, labelling and marketing of products. In many countries, high standards of technical appraisal entailinvolve a lengthy approval process before a new product is launched.
National regulatory authorisation is also required to approve the switch of products from prescription to OTC. The requirements include long-term experience of the quality, safety and efficacy of the product in a wide patient population and data to confirm that the relevant condition is both self-limiting and can easily be diagnosed by the consumer.
Operating activities
Marketing and distribution
GlaxoSmithKline sells its products worldwide through an extensive network of subsidiaries, licensees and distributors.
The gross profit margins earned on sales of pharmaceutical products are generally higher than those earned on sales of consumer products, reflecting the many risks and uncertainties inherent in developing and marketing pharmaceuticals. These risks include the high level of research and development expenditure required to discover, test and obtain patent protection for new products and the competition from new and generic products.
GlaxoSmithKline’s worldwide operation is subject to a number of risks inherent in conducting business in certain countries, including possible nationalisation, expropriation and other restrictive government actions such as capital regulation. In addition, currency fluctuations and other changes in economic conditions may occur, which can have either a favourable or unfavourable effect on trading income. The Group does not regard these factors as deterrents to further expansion of its international operations. However, it closely reviews its methods of operation, particularly in developing countries, and develops strategies to respond to changing economic and political conditions.
Marketing and distribution – Pharmaceuticals
An analysis of total pharmaceutical sales, including in 2000 divested products, by geographic region is set out below:
| 2002 | 2001 | 2000 | ||||
| Sales by geographic region | £m | £m | £m | |||
| USA | 9,797 | 9,037 | 7,705 | |||
| Europe | 4,701 | 4,561 | 4,268 | |||
| International: | ||||||
| Asia Pacific | 1,177 | 1,119 | 1,049 | |||
| Japan | 712 | 741 | 832 | |||
| Latin America | 606 | 790 | 682 | |||
| Middle East, Africa | 575 | 539 | 511 | |||
| Canada | 427 | 418 | 382 | |||
| 17,995 | 17,205 | 15,429 | ||||
GlaxoSmithKline sells its prescription medicines primarily to wholesale drug distributors, independent and chain retail pharmacies, physicians, hospitals, clinics, government entities and other institutions. These products are ordinarily dispensed to the public by pharmacies through prescriptions written by doctors in hospitals or in doctors’ surgeries.
In the USA, the world’s largest pharmaceutical market, the pressure to contain healthcare costs has encouraged the growth of managed care organisations and pharmacy benefit managers. These intermediaries use a range of methods to lower costs, including the substitution of generic products or other cheaper therapies for branded products prescribed by doctors. Because of its increasing importance as a supplier of healthcare to the community, GlaxoSmithKline contracts with the managed care sector through a small number of wholesalers.
In each market, GlaxoSmithKline deploys salesforces of representatives and supporting medical staff to promote its prescription products to medical prescribers and healthcare purchasers through personal visits.
Promotion of GlaxoSmithKline’s products is supplemented by scientific seminars, advertising in medical and other journals, television advertising, provision of samples, direct mailing and information contained on the Group’s website.
Direct-to-consumer (DTC) advertising is a major component of product marketing in the USA. DTC advertisements are now the primary source of information for patients requesting specific brand name products from their physicians in the USA.
Outside the USA, DTC is either prohibited or has a more limited role in informing patients. In the European Union, DTC of prescription-only products is currently prohibited. In Australia, the government allows DTC advertising of pharmacy-only products subject to certain safeguards. In New Zealand, DTC is allowed and self-regulated by the industry in collaboration with the Advertising Standards Agency. Other markets allow DTC, but to date the impact has been more limited.
In addition to the direct marketing of products by its subsidiaries GlaxoSmithKline has entered into agreements with other pharmaceutical companies for the co-marketing and co-promotion of their products in many markets, for exampleLevitrawith Bayer.
Marketing and distribution – Consumer Healthcare
The principal markets for Consumer Healthcare’s OTC medicines are the USA, the UK, Germany, Australia, Argentina, Italy, Mexico, Japan, Canada and France. The principal markets for the Oral care products are the USA, Germany and the UK. The nutritional drinks business is particularly strong in the UK, Ireland and India, although the range of products is available in other markets.
OTC and Oral care products are primarily distributed through pharmacy or mass market outlets either directly or through wholesalers. Nutritional healthcare products are distributed through a similar but more extensive retail and wholesale network.
GlaxoSmithKline has a large portfolio of products, ranging from tablets and toothpaste to inhalers and complex capsules, in over 36,000 different pack sizes and presentations.
Manufacture of medicines begins with the development of a therapeutic active ingredient (bulk active) in a selected formulation. Global Manufacture & Supply (GMS) develops manufacturing processes for full scale volume production of active compounds at 'primary' manufacturing sites. Converting active compounds into a finished dosage formulation is the responsibility of the 'secondary' manufacturing sites.
GMS operates as a single global network of 95 sites in 38 countries. Each year GMS produces around 5,900 tonnes of bulk actives and over four billion packs, which are packaged and delivered for sale in over 150 countries. Throughout the world it also supports approximately 2,000 new product and line extension launches a year.
GMS is focused on delivering:
OrganisationGMS operations are structured into Supply Chains and Regions.
Primary supply chainThis is a global organisation with 13 sites, spread across 6 countries, where a broad range of active ingredients for antibiotic and non-antibiotic products are manufactured and packaged. The sites are located in Australia, India, Ireland, Singapore, the UK and the USA. The majority of the active ingredients manufactured by the primary supply chain are supplied to the secondary pharmaceutical sites in Europe, North America and International.
Secondary supply chainEuropean regionThere are 17 sites in the European region spread across eight countries. Between them the European sites manufacture nearly all of the major pharmaceutical products marketed globally by GlaxoSmithKline in a wide variety of finished dosage forms.
North America regionThere are six pharmaceutical sites in the North America region located in Puerto Rico, Canada and the USA.
International regionThe International region comprises 32 manufacturing sites, in 19 countries, spread across six distinct areas. There are five sites in Middle East/Africa, 17 sites spread across the Asia Pacific area, four sites in China, one in Japan and five in Latin America.
GlaxoSmithKline integrationThis long-term, integrated change programme implemented at the time of the merger is called the Global Supply Network (GSN) and is structured to deliver benefits through five major streams of activity:
As part of the network rationalisation plan, production ceased in 2002 at 12 sites in countries which included Argentina, India, Japan, Kenya, Mexico, South Africa, Taiwan, Venezuela and the USA. The disposal or closure of further sites were announced in the year.
External suppliersProcurement is a global function supporting all functions and areas of the GlaxoSmithKline business. Manufacturing is one of the largest areas with over £2 billion spent with many external suppliers every year, including the purchase of active ingredients, chemical intermediates, part-finished and finished products. GMS has taken appropriate steps to protect its supply chains from any disruption resulting from interrupted external supply through appropriate stock levels, contracting and alternative registered suppliers.
Vaccines supply chainVaccine production is located principally at Rixensart, Belgium, with six other sites worldwide. Managing the vaccine supply chain involves anticipating market needs and using a flexible approach to be able to meet fluctuations in demand. These are based on forecasts from the different markets and firm orders from health authorities for mass vaccination campaigns. Bulk, filling and packaging is carefully balanced and stocking of vaccines helps manage short-term increases in demand. Such increases are prompted by disease outbreaks or increased demand from the public owing to disease awareness campaigns.
Consumer Healthcare supply chainThere are 27 Consumer Healthcare manufacturing sites spread across 16 countries. The Consumer Healthcare supply chain is diverse and includes the manufacturing and supply of OTC medicines, Oral care, Nutritional healthcare and Smoking control products. As well as internal facilities, over 230 contract suppliers are used worldwide.
Research and development – PharmaceuticalsThe global biological and pharmaceutical Research and Development (R&D) function in GlaxoSmithKline is responsible for discovering, developing, registering, commercialising and supporting effective marketing of innovative prescription medicines, vaccines and delivery systems for the treatment and prevention of human disease.
Fundamental to this goal is a thorough understanding of the diseases under investigation, involving pioneering work in genetics and predictive medicine, as well as more traditional research disciplines. In addition to the work to create new medicines and vaccines, extensive efforts are made to gain a clear understanding of the unmet needs of patients and of healthcare providers and payers as a guide to the overall direction of R&D.
In 2002 GlaxoSmithKline invested over £2.6 billion in pharmaceuticals R&D. R&D is an organisation that benefits from the insights of top scientists around the world and employs over 15,000 staff in biological and pharmaceutical R&D activities, at more than 20 sites worldwide, including:
During 2002, R&D continued to deliver a range of products to the market and accelerated progress in the early stages of development. The extensive in-licensing activity begun in 2001 has continued and both the late-stage and the earlier pipeline have been significantly enhanced. Practical prioritisation and management of the portfolio of compounds in development has also been a focus, ensuring that GlaxoSmithKline R&D invests its resource to achieve the optimum value and deliver new medicines to patients.
| REPORT OF THE DIRECTORS |
| Description of business | ||
| Regulatory environment | ||
Product development pipelineThe product development pipeline set out below shows considerable breadth and depth: at the end of 2002 GlaxoSmithKline had 177 pharmaceutical and vaccine projects in development, of which 123 are in the clinic.
| Compound | Type | Indication | Phase | MAA | NDA | |
| Cardiovascular, Urogenital & Metabolic | ||||||
| 249417 | anti-Factor IX monoclonal antibody | stroke | I | |||
| 427353 | beta3 adrenergic agonist | type 2 diabetes | I | |||
| 473178 | thrombin inhibitor | prevention of thrombotic complications | I | |||
| of cardiovascular disease | ||||||
| 501516 | peroxisome proliferator-activator | dyslipidaemia | I | |||
| receptor (PPAR) agonist | ||||||
| 590735 | PPAR agonist | dyslipidaemia | I | |||
| 677954 | PPAR agonist | type 2 diabetes | I | |||
| 843362 (NIN-058)** | oral insulin analogue | type 2 diabetes | I | |||
| 181771 | CCK-A agonist | obesity | II | |||
| 424323** | indirect thrombin inhibitor | prevention of thrombotic complications | II | |||
| of cardiovascular disease | ||||||
| 480848 | Lp-PLA2 inhibitor | atherosclerosis | II | |||
| 876167 (BVT933)** | 5HT2c agonist | obesity | II | |||
| piboserod (207266) | 5HT4 antagonist | atrial fibrillation | II | |||
| Avandia + sulphonylurea | PPAR gamma agonist plus sulphonylurea | type 2 diabetes | III | |||
| Avodart | 5-alpha reductase inhibitor | prostate cancer prevention | III | |||
| nesiritide** | recombinant beta-type natriuretic peptide | acute heart failure | Submitted | S:Sep02 | N/A | |
| Levitra (vardenafil)** | PDE-V inhibitor | erectile dysfunction | Submitted | S:Jan02 | AL:Jul02 | |
| Avandia | PPAR gamma agonist | type 2 diabetes – in combination with insulin | Submitted | AL:Feb01 | ||
| Avandamet | PPAR gamma agonist plus metformin | type 2 diabetes | Approved | S:Oct02 | A:Oct02 | |
| (Avandia + metformin) | combination tablet | |||||
| Infectious Diseases | ||||||
| 275833 | topical pleuromutilin | bacterial skin infections | I | 2005 | 2005 | |
| Lapdap + artesunate | antifolate + artemisinin | treatment of uncomplicated malaria | I | 2005 | N/A | |
| 270773** | phospholipid anti-endotoxin emulsion | sepsis | II | |||
| Augmentin (granules)** | beta lactam antibiotic | respiratory tract infections (incl. penicillin-resistant | II | 2004 | N/A | |
| S. pneumoniae) – modified release granule formulation | ||||||
| Augmentin - ES Chewable | beta lactam antibiotic | acute otitis media (incl. penicillin-resistant | III | N/A | 2003 | |
| S. pneumoniae) – high-dose chewable tablet | ||||||
| Augmentin XR | beta lactam antibiotic | treatment of acute exacerbation of chronic bronchitis | III | 2003 | 2003 | |
| (AECB), including complicated AECB | ||||||
| oxibendazole | polymerase inhibitor | treatment of adult & paediatric helminth | III | 2004 | N/A | |
| intestinal infections | ||||||
| sitamaquine | unknown | treatment of visceral leishmaniasis | III | 2003 | N/A | |
| tafenoquine (252263)** | 8-aminoquinoline | malaria prophylaxis (adults) | III | 2005 | 2005 | |
| Lapdap | antifolate | treatment of uncomplicated malaria | Submitted | S:Oct02 | N/A | |
| Anti-virals | ||||||
| 640385** | aspartyl protease inhibitor | HIV infections | I | |||
| 695634 | non-nucleoside reverse transcriptase inhibitor | HIV infections | I | |||
| 810781 (S-1360)* | HIV integrase inhibitor | HIV infections | II | |||
| Ziagen/Epivir** | reverse transcriptase inhibitors | HIV infection – combination tablet | III | 2004 | 2003 | |
| 433908** | protease inhibitor; amprenavir pro-drug | HIV infection | Submitted | S:Dec02 | S:Dec02 | |
| Valtrex | nucleoside analogue | HSV suppression in immunocompromised patients | Submitted | N/A | S:Sep02 | |
| Valtrex/Zelitrex | nucleoside analogue | prevention of Herpes simplex virus (HSV) transmission | Submitted | S:Nov02 | S:Oct02 | |
| Compound | Type | Indication | Phase | MAA | NDA | ||
| Neurology & Gastrointestinal | |||||||
| 234551** | endothelin A antagonist | stroke | I | ||||
| 271046 | 5HT6 antagonist | Alzheimer’s disease & schizophrenia | I | ||||
| 683699 (T-0047)** | dual alpha4 integrin antagonist (VLA4) | multiple sclerosis (MS) & inflammatory bowel | I | ||||
| disease (IBD) (also asthma & rheumatoid arthritis (RA)) | |||||||
| 723620** | corticotropin releasing factor (CRF-R1) | irritable bowel syndrome (IBS) (also anxiety | I | ||||
| antagonist | & depression) | ||||||
| 406381 | COX-2 inhibitor (second generation) | pain | II | ||||
| 493838 | adenosine A1 agonist | neuropathic pain | II | ||||
| 737004 (S-0139)* | endothelin A antagonist | stroke | II | ||||
| 737552 (S-8510)* | benzodiazepine inverse agonist | Alzheimer’s disease & vascular dementia | II | ||||
| carabersat (204269) | benzopyran | migraine prophylaxis & epilepsy | II | ||||
| Imigran/Imitrex | 5HT1 agonist | migraine – needle-free injection | II | 2005 | 2005 | ||
| talnetant (223412) | tachykinin (NK3) antagonist | IBS (also schizophrenia) | II | 2005 | 2005 | ||
| alvimopan (ADL 8-2698)** | peripheral mu-opioid antagonist | post operative ileus | III | 2004 | 2003 | ||
| Imigran/Imitrex | 5HT1 agonist | migraine – fast dissolving tablet | III | 2003 | 2003 | ||
| Lamictal | sodium channel inhibitor | neuropathic pain | III | N/A | 2004 | ||
| Requip** | non-ergot dopamine agonist | Parkinson’s disease – controlled release formulation | III | 2005 | 2005 | ||
| Requip | non-ergot dopamine agonist | restless leg syndrome | III | 2003 | 2003 | ||
| Imigran/Imitrex | 5HT1 agonist | adolescent migraine – nasal formulation | Submitted | S:Sep02 | AL:Dec00 | ||
| Oncology, Musculoskeletal & Inflammation | |||||||
| 251353 | Groß-T CXC chemokine | prevention of chemotherapy-induced cytopaenias | I | ||||
| 462795 | cathepsin K inhibitor | osteoporosis & osteoarthritis | I | ||||
| 485232** | recombinant human interleukin-18 | immunologically-sensitive cancers (melanoma & | I | ||||
| immunomodulator | renal cell) | ||||||
| 497115** | thrombopoietin agonist | chemoprotection | I | ||||
| 681323 | p38 alpha kinase inhibitor | rheumatoid arthritis (also chronic obstructive | I | ||||
| pulmonary disease) | |||||||
| 715992** | kinesin inhibitor | breast & ovarian cancers | I | ||||
| 786034 | vascular epidermal growth factor 2 tyrosine | solid tumours | I | ||||
| kinase inhibitor | |||||||
| topotecan + 120918 | topo-isomerase l inhibitor + bioenhancer | cancer | I | ||||
| 572016 | ErbB-2 and EGFR dual kinase inhibitor | solid tumours (breast & colorectal cancers) | II | 2004 | 2004 | ||
| ethynylcytidine (596168)** | selective RNA polymerase inhibitor | solid tumours | II | ||||
| Hycamtin | topo-isomerase I inhibitor | small cell lung cancer first line therapy | II | 2004 | 2004 | ||
| repifermin** | keratinocyte Growth Factor-2 | mucositis (also wound care & inflammatory | II | ||||
| bowel disease) | |||||||
| Hycamtin | topo-isomerase I inhibitor | non-small cell lung cancer second line therapy | III | 2005 | N/A | ||
| Hycamtin | topo-isomerase I inhibitor | small cell lung cancer second line therapy | III | 2003 | 2003 | ||
| – oral formulation | |||||||
| Hycamtin | topo-isomerase I inhibitor | ovarian cancer first line therapy | III | 2004 | 2004 | ||
| ibandronate** | bisphosphonate | treatment & prevention of postmenopausal | III | 2004 | 2004 | ||
| osteoporosis – monthly oral dosing | |||||||
| ibandronate** | bisphosphonate | treatment & prevention of postmenopausal | III | 2004 | 2004 | ||
| osteoporosis – quarterly i.v. dosing | |||||||
| Navelbine** | vinca alkaloid | non-small cell lung cancer – oral therapy | III | N/A | 2003 | ||
| Bexxar** | I131 radiolabelled anti-B1 monoclonal | non-Hodgkin's lymphoma | Submitted | N/A | S:Sep00 | ||
| antibody | |||||||
| Hycamtin | topo-isomerase I inhibitor | small cell lung cancer second line therapy | Submitted | S:Nov02 | N/A | ||
| ibandronate** | bisphosphonate | treatment & prevention of postmenopausal | Submitted | S:Jun02 | S:Jul02 | ||
| osteoporosis – daily oral regimen | |||||||
| Psychiatry | |||||||
| 271046 | 5HT6 antagonist | schizophrenia (& Alzheimer’s disease) | I | ||||
| 353162 | noradrenaline/dopamine re-uptake inhibitor | depression & bipolar disorder | I | ||||
| 468816 | glycine antagonist | smoking cessation | I | ||||
| 679769 | NK1 antagonist | depression & anxiety | I | ||||
| 723620** | corticotropin releasing factor (CRF-R1) | anxiety & depression (also IBS) | I | ||||
| antagonist | |||||||
| 597599 | NK1 antagonist | depression & anxiety | II | ||||
| talnetant (223412) | tachykinin (NK3) antagonist | schizophrenia (also for IBS) | II | ||||
| vilazodone (659746) | selective serotonin re-uptake inhibitor (SSRI) | depression | II | 2005 | 2004 | ||
| (EMD 68843)** | + 5HT1a partial agonist | ||||||
| Lamictal | sodium channel inhibitor | bipolar disorder – acute treatment | III | N/A | 2006 | ||
| Paxil CR** | SSRI | premenstrual dysphoric disorder (PMDD), intermittent | III | N/A | 2003 | ||
| treatment – controlled release formulation | |||||||
| Lamictal | sodium channel inhibitor | bipolar disorder – long-term prophylaxis | Submitted | S:Aug02 | S:Jun02 | ||
| Paxil CR** | SSRI | PMDD continuous treatment – controlled | Submitted | N/A | S:Jun02 | ||
| release formulation | |||||||
| Paxil CR** | SSRI | social anxiety disorder | Submitted | N/A | S:Dec02 | ||
| Wellbutrin XL** | noradrenaline/dopamine reuptake inhibitor | depression – controlled release formulation, | Submitted | S:Aug02 | |||
| once daily dosing | |||||||
| Compound | Type | Indication | Phase | MAA | NDA | ||
| Respiratory | |||||||
| 159797 (TD 3327)** | beta2 agonist | asthma, chronic obstructive pulmonary disease (COPD) | I | ||||
| 274150 | selective iNOS inhibitor | asthma, COPD | I | ||||
| & allergic rhinitis | |||||||
| 597901 | beta2 agonist | asthma & COPD | I | ||||
| 681323 | p38 alpha kinase inhibitor | COPD (also RA) | I | ||||
| 683699 (T-0047)** | dual alpha4 integrin antagonist (VLA4) | asthma & RA (also MS & IBD) | I | ||||
| 766994 | chemokine receptor 3 antagonist | asthma & allergic rhinitis | I | ||||
| 559090 | alpha4 integrin antagonist | asthma & allergic rhinitis | II | ||||
| 685698 | glucocorticoid receptor agonist | asthma, COPD & allergic rhinitis | II | ||||
| 842470 (AWD 12-281)** | PDE IV inhibitor | asthma, COPD & allergic rhinitis | II | ||||
| mepolizumab (240563) | anti-IL5 monoclonal antibody | asthma & atopic dermatitis | II | ||||
| Ariflo | PDE IV inhibitor | COPD | Submitted | 2004 | S:Dec02 | ||
| Non-CFC Metered Dose Inhaler propellants (106642) | |||||||
| Serevent | beta2 agonist | asthma & COPD | III | 2004 | N/A | ||
| Flixotide/Flovent | inhaled corticosteroid | asthma & COPD | Approved | A:Apr97 | AL:Dec02 | ||
| Seretide/Advair | beta2 agonist/inhaled corticosteroid | asthma | Approved | A:Jun00 | AL:Oct01 | ||
| & Oct02 | |||||||
| Diskus/Accuhaler (dry powder inhaler) | |||||||
| Seretide/Advair | beta2 agonist/inhaled corticosteroid | adult & paediatric asthma – once daily dosing | III | 2005 | 2005 | ||
| Seretide/Advair | beta2 agonist/inhaled corticosteroid | COPD | Submitted | S:Sep01 | AL:Mar02 | ||
| & Dec02 | |||||||
| Serevent | beta2 agonist | COPD | Approved | 2003 | A:Mar02 | ||
| Hepatitis Vaccines | |||||||
| Hepatitis E | recombinant | hepatitis E prophylaxis | II | ||||
| Extra strength hepatitis B | recombinant | extra strength hepatitis B prophylaxis | III | 2003 | TBD | ||
| Twinrix 2 doses | recombinant | combined hepatitis A and B prophylaxis | Approved | A:Sep02 | 2003 | ||
| (child/adolescent) | |||||||
| Paediatric Vaccines | |||||||
| Rotarix | live attenuated – oral | rotavirus prophylaxis | II | 2005 | |||
| N. meningitidis | conjugated | meningitis prophylaxis | II | 2004 | |||
| Meningitis B (Cuba) | subunit | meningitis B prophylaxis | II | TBD | |||
| S. pneumoniae paediatric | conjugated | S. pneumoniae disease prophylaxis for children | III | 2005 | |||
| MMR-varicella | live attenuated | measles, mumps, rubella and varicella prophylaxis | III | 2005 | |||
| Infanrix/PediarixPeNta- | recombinant | diphtheria, tetanus, pertussis, hepatitis B | Approved | A:Oct00 | A:Dec02 | ||
| HepB-IPV | and inactivated polio prophylaxis | ||||||
| InfanrixHeXa-Hep B- IPV/Hib | conjugated/recombinant | diphtheria, tetanus, pertussis, hepatitis B | Approved | A:Oct00 | TBD | ||
| and inactivated polio prophylaxis and | |||||||
| Haemophilus influenzae type B prophylaxis | |||||||
| Other Vaccines | |||||||
| Dengue fever | attenuated tetravalent vaccine | prophylactic use | I | ||||
| HIV | recombinant | HIV prophylaxis | I | ||||
| New influenza | subunit | influenza prophylaxis – new delivery | I | ||||
| S. pneumoniae elderly | conjugated | S. pneumoniae disease prophylaxis | I | ||||
| Staphylococcal antibodies** | monoclonal antibody | prevention of staphylococcal infections | I | ||||
| Epstein-Barr virus (EBV) | recombinant | EBV prophylaxis | II | ||||
| Human papillomavirus (HPV) | recombinant | prophylaxis of HPV infections | II | ||||
| Malaria | recombinant | malaria prophylaxis | II | ||||
| Boostrix | subunit | adolescent/adult booster for diphtheria, tetanus | Approved | A:Oct00 | 2004 | ||
| and pertussis | |||||||
| Boostrix IPV | subunit | adolescent/adult booster for diphtheria, tetanus, | III | 2003 | |||
| pertussis and inactivated polio | |||||||
| Simplirix | recombinant | genital herpes prophylaxis | III | ||||
| Pharmaccines | |||||||
| GSK/PowderJect** | recombinant | hepatitis B treatment | I | ||||
| 249553 | recombinant | treatment of lung cancer/melanoma | II | ||||
The content of the portfolio will change over time as new compounds progress from research to development and from development to the market. Owing to the nature of the drug development process, it is not unusual for some compounds, especially those in early stage of investigation, to be terminated as they progress through development.
For competitive reasons, new projects in pre-clinical development have not been disclosed and some project types may not have been identified.
Compounds progressed into Phase I clinical development in 2002
During 2002 several discovery projects, listed in the table below, progressed through non-clinical safety testing and into early (Phase I) clinical development. These compounds are continuing their rigorous non-clinical, clinical and commercial assessments, leading to proof of concept decisions over the next 12–24 months.
Significant regulatory submissions in 2002
Product approvals
In 2002, approvals were received for a number of new products, including several significant new indications and formulations for existing products, as summarised in the table below.
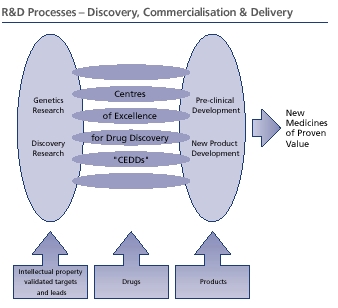
R&D processesIntellectual property
In line with GlaxoSmithKline's strategic intent to become the indisputable leader in the industry, R&D has set itself the goal of becoming the industry’s most productive R&D organisation. Steps to achieve this have included initiatives to both reduce the time taken in all phases of the discovery and development chain; and also to gain earlier understanding of candidate molecules, increasing the probability of making a new medicine available to treat patients as soon as possible.
R&D measures this productivity not just by the number and innovation of the products it creates, but also by the commercial value of the product's ability to address the unmet needs of healthcare customers including patients, healthcare professionals, budget holders and regulators; each with their own perspective on what constitutes a valuable new product. R&D is now positioned to ensure that it generates the right safety, efficacy and quality information to respond to these different perspectives through data demonstrating the overall social benefits of the new medicine; increased length or quality of life, and increased workplace productivity.
One of the historical contradictions in the pharmaceutical industry has been the need to lever the advantages of a large organisation without losing the creative spirit of the research environment. In GlaxoSmithKline, R&D has been structured to balance the areas that benefit from large scale with those that take advantage of being small to enhance their productivity. The key areas that benefit from being large are those that are capital intensive or high throughput activities such as compound screening; those that require scarce skills; and those that are highly regulated, mainly at the later end of the development chain. Other areas flourish to their best advantage if the structural unit remains small: the units can respond quickly to the changing environment; the opportunity for scientists to interact is optimised; and the need for return on investment is focussed through the fostering of an entrepreneurial, accountable culture.
In R&D the power of smaller units is manifest in the Centres of Excellence for Drug Discovery (CEDD). They ensure the most efficient and rapid validation of lead candidates through preclinical testing against proof of concept criteria, before handing over the compound to the New Product Development organisation for large scale clinical trials.
The New Product Development function integrates the clinical, regulatory and commercial activities necessary to bring a new medicine to the marketplace. Similarly, the New Product Supply organisation bridges the traditional divide between development and manufacturing, ensuring that robust manufacturing processes are developed.
Significant progress has also been made in the integration of the Group’s R&D in Japan with the global development and commercialisation processes in order to eliminate duplication and to speed up regulatory filings. During 2002 the discovery and development portfolio in Japan was reviewed and prioritised in the context of the global R&D pipeline.
Crucial to the success of R&D is its capacity to embrace and develop new technologies to streamline the drug discovery process. The technology development organisation keeps abreast of emerging technologies that may advance the creation of new medicines, evaluates them and provides the investment and knowledge required to develop selected technologies appropriately. As R&D generates and modifies technologies, it will not only focus them on the Group’s internal goals but also maximise the return on R&D assets through sales, spin-outs and out-licensing.
Early research and the role of geneticsThe early stages of finding new medicines requires essentially two components; targets that can be shown to affect mechanisms of important pathological processes in human disease and compounds, typically small molecules but also including macromolecules, protein therapeutics and vaccines, able to modulate the behaviour of specific targets.
As part of this target validation process, GlaxoSmithKline aims to identify the genes most relevant to common diseases with large unmet medical needs, such as asthma, non-insulin dependent diabetes, osteoarthritis, chronic obstructive pulmonary disease, early onset heart disease and Alzheimer’s disease. Many diseases arise through complex interactions between a number of gene variants and environmental factors, so the challenge involved is significant. Identifying the genes that predispose patients to a particular disease and understanding their role in its progression lead to finding new ways to intervene in these diseases.
In 2002, a programme to identify tractable targets that are genetically associated with human diseases of interest was initiated. This enables the validation of targets associated with these diseases prior to extensive investigation.
The practical application of genetics has moved forward during the year. Several opportunities have been identified where knowledge of specific markers for efficacy or susceptibility to adverse events is enhancing the ability to focus development of new medicines on patients who will be most likely to benefit from them, ultimately providing reassurance to both the prescriber and the patient.
Discovery researchThe purpose of Discovery Research (DR) is to identify lead compounds that may form the basis of drug discovery efforts in the CEDDs. Investment in DR is focused on improving productivity in both quality and quantity. In 2002, R&D completed construction of new automation facilities at Tres Cantos, in Spain, and continued work on facilities at Upper Providence and Harlow.
In parallel with the development of the ability to generate efficiently large numbers of high quality new compounds, there has been substantial progress in implementing methods to evaluate them using high throughput biology. This discipline, with its integration of knowledge from both animal and human biology, is starting to deliver highly predictive models to forecast efficacy of compounds and to extend understanding of human disease.
Centres of Excellence for Drug DiscoveryThe two crucial steps in converting lead compounds into drug candidates are (i) optimising the lead for potency, efficacy, safety and other intrinsic characteristics of the molecule, and (ii) demonstrating the validity of the therapeutic hypothesis through early clinical trials of the resulting candidate. The CEDDs are focused on specific disease areas and designed to be nimble and entrepreneurial with the range of skills and resources required to drive mid-stage development projects from lead optimisation through to their key decision-point, demonstration of proof of concept, before major investments are made to fund large-scale clinical trials.
There are six CEDDs, three based in the USA and three in Europe:
Each CEDD is responsible for identifying the optimal drug candidate for the desired biological effect and then assessing its safety and other development characteristics in preclinical screens. Once this is achieved, the CEDDs are responsible for proving that the compound is safe and efficacious in patients in small-scale clinical trials – the proof of concept decision point.
A decision is then made on whether the information available to date justifies the compound’s progression into late-stage drug development where the necessary large-scale clinical trials are conducted to register and commercialise the product.
In 2002, the CEDDs progressed significantly more compounds through both first dosing in humans and initial evaluation of efficacy in patients than in 2001. See table of compounds progressed into Phase I on page 18. In order to progress highly promising medicines yet more rapidly without compromising safety, selected projects are currently piloting a process that involves running some activities in parallel, rather than sequentially.
As part of GlaxoSmithKline’s major response to the challenges of diseases affecting the developing world, the Microbial, Musculoskeletal & Proliferative Diseases CEDD has responsibility for a drug discovery unit, based at Tres Cantos, that is dedicated to finding new medicines for these diseases. Research projects at Tres Cantos focus on malaria and TB and, together with work elsewhere in the Group on HIV/AIDS and vaccines, address the prevention and treatment of all three of the World Health Organization’s (WHO) top priority diseases. The Group also works with numerous external partners worldwide in the search for new treatment for Diseases of the Developing World (DDW).
Preclinical developmentPreclinical Development (PCD) participates in a wide range of activities within the drug development process from optimising the selection of compounds for potential development through launch to the marketplace and enhancement of existing products by devising more convenient formulations. Early in the development process, the metabolic fate and safety of compounds are evaluated in laboratory animals prior to testing in humans. The testing required in both animals and humans is mandated and is highly regulated by governmental agencies.
PCD researchers investigate dosage form (e.g. tablet or inhaled) and develop formulations to enhance the drug’s effectiveness. PCD is also responsible for the development of drug formulations used in clinical trials. Processes and supporting analytical methods for drug synthesis and product formulation and delivery are scaled up to meet increasing supply requirements, ultimately leading to the technical transfer of the processes and methods to manufacturing. The New Product Supply Process, a partnership between R&D and Global Manufacturing and Supply, ensures that a robust product is developed for large scale commercial manufacturing and launch.
PCD is pursuing novel technologies to enhance R&D productivity by lowering the rate of project failure, reducing cycle time and enhancing product value. Predictive toxicology, an integrated multi-disciplinary collaboration between PCD, DR and Genetics Research, has been established to improve the quality of candidate selections and reduce late-stage attrition due to toxicity.
Other key technology areas that provide ways to improve R&D’s productivity include drug delivery systems, predictive technologies, particle engineering and process innovation. The use of particle engineering and process innovations enhances the ability to manufacture efficiently consistently high-quality products.
New product developmentTo provide focus for the development and commercialisation process, which must proceed in unison, all the major functional components clinical, medical, biomedical data, regulatory, safety and commercial strategy, have been integrated into this single management organisation, New Product Development (NPD). There are six cross-functional Therapeutic Area Strategy Teams, each covering one of the following groups of diseases:
These matrix teams are responsible for maximising the worldwide development opportunities for each product within their remit. They ensure that at an early stage regional marketing needs are fully integrated into any development plans so that all information needed to support the registration, safety programmes, pricing and formulary negotiations is available. Careful prioritisation across all phases of development ensures that a high potential and integrated portfolio is achieved.
The teams collaborate at an early stage with the CEDDs to define target product profiles for new molecules and with integrated technical development and manufacturing functions to ensure rapid, effective launch and delivery of the product. Innovative clinical programmes for lead molecules from the CEDDs are developed using cross-functional project teams.
During 2002 a new group, Translational Medicine & Technology, was established within NPD to optimise the use of a variety of technologies to reduce risk and cost across development.
Cross-functional input extends to focused lifecycle management for products to deliver new indications and new presentations after the initial regulatory approval and commercial launch. Examples of lifecycle management include the extended release formulation,Augmentin XR, and development programmes designed to deliver new indications such as the use ofSeretide/Advairfor chronic obstructive pulmonary disease (COPD).
A new initiative,Gold Pass, was implemented in 2002. This designation, agreed between R&D, regional markets and manufacturing,Intellectual property is a key component of the portfolio and resource prioritisation and management process, to ensure that the resources placed behind key emerging assets yield the optimum commercial benefit as well as the maximum medical benefit to patients.Gold Passassets are of high value and strategic importance to GlaxoSmithKline and require specific organisational visibility and urgency to meet patients’ needs. Consequently, only a small number of assets receiveGold Passstatus at any one time, enabling the full focus of the organisation to be aligned.
In-licensing and research collaborationsGlaxoSmithKline has continued to identify compounds that would enhance the portfolio and to create innovative collaborations to ensure that the Group is regarded as the partner of choicebusiness asset for both large and small companies. Compounds that were the subject of in-licensing or co-promotion deals during 2002 and in January 2003 were:
A strategic alliance was formed with Nobex Corporation for the development and commercialisation of orally administered insulin products. The first product to be developed collaboratively is 843362 (NIN-058), a novel modified oral insulin, in Phase I for type 2 diabetes.
An alliance was also formed with Exelixis, Inc for the development by Exelixis of small molecule compounds. Using its gene-to-drug-discovery technology platform these will be delivered in Phase II for full development and exclusive global commercialisation and manufacturing rights. Exelixis retains co-promotion rights in North America.
The existing collaborative agreement with Tanabe Seiyaku Co Ltd was extended to facilitate the acceleration to candidate selection by Tanabe of GlaxoSmithKline hits from screening.
In addition, GlaxoSmithKline has already entered into a number of agreements with third parties to co-develop and then co-market certain compounds. These arrangements range from milestone payments to third parties to acquire rights to their intellectual property, to joint ventures to develop and commercialise specified compounds. Under many of these agreements the Group has obligations to make payments in the future if specified milestones are achieved. These financial commitments are summarised in Note 26 to the Financial statements, ‘Commitments’.
DiscontinuationsAll research and development carries a risk of failure commensurate with the extension of scientific knowledge of a compound and its effects. Not all lead compounds that are identified to possess positive activity against a validated target will prove to be safe enough to introduce to humans or feasible to manufacture on a commercial scale. GlaxoSmithKline R&D endeavours to ensure that as far as possible these risks are ameliorated by extensive predictive testing as early as possible in the development process. Despite these efforts, the ultimate test for a product remains the point at which it is administered to large numbers of patients with the disease.
In 2002, GlaxoSmithKline and Korean company LG Chem Investments (LGCI) reviewed the status of the joint development programme for the quinolone antibiotic Factive (gemifloxacin). As a result, the companies agreed that Factive’s value could be better realised within LGCI’s portfolio. LGCI has taken full worldwide responsibility for the future commercialisation of the product, including regulatory activities following a transition period.
Other late-stage projects terminated during 2002 were the development of 237376 for cardiac arrhythmia, 660511 for hypertension in Phase II and a once-daily formulation of Augmentin.
Vaccines R&DAs part of the Pharmaceuticals sector worldwide, vaccines R&D is conducted in GlaxoSmithKline’s centre in Rixensart, Belgium, together with other activities related to vaccines including clinical development, regulatory, scaling up, production, packaging and all support functions. Over 1,000 research scientists are employed who are devoted to discovering new vaccines and developing more cost-effective and convenient combination products to prevent infections which cause serious medical problems worldwide. Discovery work for new vaccines is performed, then potential candidate vaccines are expressed in yeast, bacteria or mammalian cells and purified to a very high level. This is followed by formulation of the vaccine which involves mixing antigens with selected adjuvants to stimulate a good immune response in humans. The next step is to evaluate safety and efficacy of the candidate vaccine in in-vivo models. Once preclinical proof of concept has been established, the next stage is to test the candidate vaccine in clinical trials in healthy individuals, to evaluate safety and how effective the vaccine is in inducing an immune response to protect the body from disease encountered later in a natural setting. Large-scale field trials in healthy individuals follow to establish safety and efficacy in a cross section of the population. The results obtained during clinical trials and the development of a quality production process and facilities are then combined into a regulatory file which is submitted to the authorities in the various countries where the Group intends to launch the vaccine.
Animals and researchFor ethical, regulatory and scientific reasons, research using animals remains a vital part of the research and development of new medicines and vaccines. Animals are only used where no alternative is available and GlaxoSmithKline constantly aims to reduce the numbers used. The Group strives to exceed industry standards in the care and welfare of the animals it uses: laboratory animals are usually bred specifically for research and are well cared for throughout their lives by qualified, trained staff.
When animals are used in research unnecessary pain or suffering is scrupulously avoided. GlaxoSmithKline is actively engaged in research to develop and validate experimental methods that can provide more and better alternatives to the use of animals in research.
GlaxoSmithKline acknowledges that use of animals for research purposes is a subject that rightly commands a high level of public interest. The full GlaxoSmithKline Public Policy Position 'The care and ethical use of animals in research' is available on the website, www.gsk.com, or from the Secretariat.
Research and development – Consumer Healthcare
The principal centres for Consumer Healthcare R&D are in the UK and in the USA. The focus of R&D is on the identification and rapid development of novel products that bring benefits to consumers in the OTC, Oral care and Nutritional healthcare markets. Consumer Healthcare liaises closely with Pharmaceuticals to maximise the Group’s assets, where prescription products can also find application in the OTC marketplace.
Operating resources
Intellectual property
GlaxoSmithKline regards its intellectual property as a key business asset.GSK. The effective legal protection of intellectual property is critical in ensuring an effectivea reasonable return on investment in R&D. Intellectual property can be protected by patents, trade marks,trademarks, registered designs, copyrights and domain name registrations. Patent and trade marktrademark rights are regarded as particularly valuable.
In many cases generic manufacturers launch, or attempt to launch, generic versions of patented drugs prior to normal patent expiry, arguing that the relevant patents are invalid and/or are not infringed by their product. Significant litigation concerning these challenges is summarised in Note 41 to the financial statements, ‘Legal proceedings’.
PatentsGlaxoSmithKline’sGSK’s policy is to obtain patent protection on all significant products discovered or developed through its R&D activities. Patent protection for new active ingredients is available in all significant markets. Protection can also be obtained for new pharmaceutical formulations and manufacturing processes, and for new medical uses and special devices for administering products.
The patent position with respect to the active ingredients in significant products is as follows:
Augmentin.AvandiaIn the USA patents on the key active ingredient, potassium clavulanate, extending to 2018, were held invalid by decisions of a federal court in 2001 and 2002. These decisions are under appeal. In other markets, the patents on potassium clavulanate have expired, except in Italy (2006c).
Avandia and Avandamet.Avandamet. The basic patent on the active ingredient rosiglitazone in these products is not due to expire until 20112012a,cin the USA(USA) and 2013 in Europe.b(Europe). Patents on the commercial form of the active ingredient rosiglitazone maleate are not due to expire until 2015 (USA) and 2014b(Europe). Litigation challenging the validity of the patents protecting these products is ongoing in the USA and 2014b ein Europe..
Avodart.AvodartPatents. The patent on the active ingredient dutasteride have a normal expiry of 2013 (USA) and 2014 (Europe). Requests for extension of term of these patents are pending and are expectedis not due to extend the terms of these patents toexpire until 2015ain the USA(USA) and 2017bin Europe.(Europe).
Combivir.Combivir. The patentspatent on the specific combination of lamivudine and zidovudine areis not due to expire until 2012 in the USA(USA) and 2013bin Europe.(Europe).
Coreg. GlaxoSmithKlineGSK is the exclusive licensee under the US patent on the active ingredient carvedilol, which is not due to expire until 2007a,c.
Epivir.Epivir. The patents on the active ingredient lamivudine are not due to expire until 2009a in the USA and 2011b in Europe.
Flixotide/FloventandFlixonase/Flonase.In the USA, the patent on the active ingredient fluticasone propionate expires in 2003, but protection is expected to be extended by virtue of paediatric exclusivity until May 2004. In most European countries protectionlamivudine is not due to expire until 2005.2010a,c(USA) and 2011b(Europe).
Flixotide/FloventandFlixonase/Flonase. The patents on fluticasone propionate have expired in the EU and USA. Generic competition toFlixonaseexists in the EU and the FDA recently approved a generic version ofFlonasein the USAe.
Imigran/Imitrex. The patentspatent on the active ingredient sumatriptan areis not due to expire until 2009c(USA) and generally 2006b(Europe, except 2008b(Italy)). Litigation challenging the validity of the patent protecting this product is ongoing in the USA and 2006b ein Europe, (2010c Italy).
Lamictal.Lamictal. The patentspatent on the active ingredient lamotrigine areis not due to expire until 20082009a,c(USA). Litigation challenging the validity of this patent in the USA (2009 by virtue of paediatric exclusivity)has been settlede. In Europe, the corresponding patent has expired and 2005b in Europe. GlaxoSmithKline has initiated legal action in the USA against a generic manufacturer that is attempting to launch its own version of the product prior to this patent expiry.competition exists.
Levitrad. GlaxoSmithKlineGSK has co-promotion rights under the US patent on the active ingredient vardenafil which is not due to expire until 2018 in the USA.
Lexiva/Telzir. GSK is the exclusive licensee under the patent on fosamprenavir, which is not due to expire until 2017 (USA) and 2019b(Europe).
Paxil/Seroxat. The patent protectingon the commercial form ofPaxil/Seroxat paroxetine is not due to expire until 2007c(USA) and 2006 (Europe). Litigation relating to the validity and infringement of the patents protecting this product is ongoing in most major markets, until 2006. GlaxoSmithKlinethe USAe. Generic competition has initiated patent infringement litigationcommenced in the USA, Europe and severalcertain other markets againstmarkets.Paxil CRis protected by a numberformulation patent that is not due to expire until 2012. A generic manufacturer has applied for FDA approval of a generic manufacturers who are attempting to launch their own versionsform of the product prior toPaxilCR asserting non-infringement of this patent expiry.e.
Retrovir.Requip. The patent on ropinirole is not due to expire until 2007a(USA) and 2008b(Europe). A patent relating to the use of ropinirole in Parkinson’s disease is not due to expire until 2008 (USA) and 2011b(Europe). Litigation challenging the validity of these patents is ongoing in the USAe.
Retrovir. There are no patents on the active ingredient zidovudine. Patents covering pharmaceutical formulations containing zidovudine and their medical use are not due to expire until 2005have expired in the USA and will expire in 2006 in Europe.
Seretide/Advair.The patentspatent on the specific combination of active ingredients salmeterol xinafoate and fluticasone propionate areis not due to expire until 2010 in the USA(USA) and 2013b in Europe. A(Europe). An application for re-issue of the US patent challenge has been made tofiled by GSKewith the combinationUS Patent and Trademark Office (USPTO). In January 2006, the USPTO issued a final office action rejecting this application. GSK will seek reconsideration of this rejectione. The UK patent has been revoked by the UK courts. Patents on the individual ingredients have expired in the UK. In the USA, the patent on salmeterol xinafoate does not expire until 2008.
SereventSerevent.. PatentsThe patent on the active ingredient salmeterol xinafoate areis not due to expire until 2005b in most of Europe (2008b in France and 2009c in Italy) and until 2008 in the USA. In Europe, the patent has expired, except France (2008b) and Italy (2009b).
Trizivir.The patent on the method of treatment using a combination of lamivudine, zidovudine and abacavir does not expire until 2016 (USA) and 2016 (Europe).
Valtrex.The patentspatent on the active ingredient valaciclovir areis not due to expire until 2009a(USA) and 2009b(Europe). Litigation challenging the validity of the patent protecting this product is ongoing in the USA and 2009be in Europe..
Wellbutrin SR, Wellbutrin XLandZyban.PatentsThe patent on the basic active ingredient havehas expired. VariousThere is now generic competition for the sustained release (SR) and instant release (IR) forms in the USA. In Europe, regulatory data exclusively provides protection until 2009 in some markets. In the USA,Wellbutrin XLis protected by formulation patents protectthat expire in 2018. Litigation relating to the currently marketedvalidity and infringement of these patents is ongoing in the USAe.
SRZiagen.(sustained release) formulations, the latest of whichThe patent on abacavir is not due to expire in the USA until 2013. These patents are under legal challenge in the US courts. In Europe, regulatory data exclusivity provides protection until at least 2005, and until 2009 in some countries.
Ziagen.The patents on the active ingredient abacavir are not due to expire until 20112012a,cin the USA(USA) and 2014b in Europe.(Europe).
Zofran.The patentspatent on the active ingredient ondansetron are not due to expire until 2005has expired in the USA and 2005Europe, (except France (2007b in Europe,) and Italy (2010cb Italy))). PatentsA patent on use in treating emesis expireexpires in 2006. GlaxoSmithKline has initiated legal action under these patents against generic manufacturersLitigation challenging the validity of the emesis use patent is ongoing in the USA.USAe.
| a) | Including patent term restoration under the Hatch-Waxman Act |
| b) | Including extension of term by national or European supplementary protection certificates |
| c) | Including granted or pending extension of term for paediatric exclusivity |
| d) | A registered trademark of Bayer AG |
| e) | See Note 41 to financial statements ‘Legal proceedings’. |
| GSK Annual Report 2005 |
| 25 |
| REPORT OF THE DIRECTORS |
| Description of business |
| Regulatory environment |
| continued |
TrademarksTrade marks
All of GlaxoSmithKline’sGSK’s pharmaceutical products are protected by registered trade markstrademarks in major markets. In general, the same mark is used for a product in each market around the world, but thereThere may be local variations. Forvariations, for example, in the USA the trade marktrademarkPaxilis used instead ofSeroxatandAdvairis used instead ofSeretide.
Trade markTrademark protection may generally be extended for as a long as the trade marktrademark is used by renewing it when necessary. GlaxoSmithKline’s trade marksGSK’s trademarks on pharmaceutical products generally assume an increasing importance whenare important for maintaining the patent for that product has expired in a particular country and generic versionsbrand identity of the product become available.upon expiration of the patent.
In theThe Consumer Healthcare business trade markstrademarks are particularly important, as the business is very brand orientated and many products do not have patent protection.
GlaxoSmithKline is routinely engaged in legal disputes in defence of intellectual property rights on many of its products (see Note 30 to the Financial statements, ‘Legal Proceedings’).
Information technologyResponsibility for environment, health and safety
Information technology (IT) plays three strategic roles in GlaxoSmithKline:
In addition to computer infrastructure, hardwareEnvironment, health and software, the IT organisation is responsible for voice and video technologies, monitoring business and technology trends that could have an IT impact on GlaxoSmithKline and preparing the Group for the risks associated with modern information technology.
Integrating business systems from the two legacy companies has remained a top priority for IT. This has been achieved while avoiding any significant disruption to critical business systems. The Group’s IT function has a strong focus on improving business processes and has adopted new, rapid and cost effective methods to do this.
Enhancing business performanceVirtually all GlaxoSmithKline’s major business processes rely heavily on the use of information technology. There are major programmes to capture at source key information in electronic form and make it available wherever required.
Improving the quality and potential value of the molecules that move from discovery to development is a key aim of R&D. IT has developed web based tools that provide scientists with the information they need on candidate medicines. In this way, early phase R&D teams can draw up shortlists of molecules for consideration as possible treatments for specific diseases faster than before and with more confidence in the qualities of the shortlist. Other areas in R&D where IT is playing an important role are high-throughput biology, laboratory automation, imaging, electronic data capture, document knowledge and clinical data management.
Work has continued to extend the Manufacturing Enterprise Resources Planning Solution to ensure that there are compliant systems with common processes in place. Standard transactions and middleware are being used to enable efficient movement across the supply chain whilst at the same time allowing for independent optimisation of commercial units at a regional or functional level as well as manufacturing.
The ability to consolidate critical operations reflects the growing availability and reliability of global data networks. Employees, such as US sales representatives, are benefiting from the ability to connect to systems via a virtual private network when away from the office.
Transforming and extending business activitiesInsights gained from genomics and proteomics are transforming the way that disease targets are identified and validated. Information obtained from a variety of external sources needs to be integrated with internally generated information in a rapid and flexible manner that relies heavily on information technology support. The analysis of these databases also requires significant amounts of processing power, taking full advantage of advances in computer technology. New technological approaches, such as grid computing, whereby computers are linked to use their processing power more fully, are being investigated.
Access to information for regulatory agencies, clinical opinion leaders, healthcare professionals, patients and the public has been enhanced in a number of markets. Steps have been taken to reduce reliance on paper based processes for clinical trials and registration of new medicines through use of wireless, handheld technologies as well as the internet.
Collaborating and assessing informationThe importance to GlaxoSmithKline of the internet and the internal intranet continues to grow. Internal websites allow information to be shared across the Group on a global basis and are supported by search engines analogous to those used externally on the internet. The ability to provide shared access to information has enabled the growing use of virtual teams, which work collaboratively, spanning multiple geographies and time zones. GlaxoSmithKline has adopted a strategy, which enables employees to choose and receive the information they most need.
GlaxoSmithKline project teams and departments are using their computers to collaborate effectively. A standard collaboration product suite is being deployed across the Group; included in this is a new specialist tool that permits information to be shared with external colleagues, securely and quickly.
As part of an overall internet technologies initiative, significant savings have been achieved, for example via global learning management. Information is exchanged electronically with a broad array of suppliers, customers and partners. Protection against unauthorised access to information assets and the growing risks posed by computer viruses is a major issue. This is being addressed through rigorous security management processes.
The telephone and video conferences that are a familiar aspect of business life are being complemented by computer-based collaborative working and screen-sharing tools that help teams respond to the practical challenges posed by operating in a global organisation. Enabling GlaxoSmithKline’s knowledge workers to be more productive is a key goal for IT. A standard desktop has been adopted globally, which will assist IT in supporting employees’ use of software more efficiently.
GlaxoSmithKline people are fundamental to the success of the business. Their skills and intellect are key components in the successful implementation of sound business strategy. This is the human capital that maximises the potential of the Group’s scientific, commercial and financial assets. The outcome of effective human resources policy is GlaxoSmithKline’s solid reputation as an international employer of choice.
To achieve this, the Group initiated Candidate Care – the commitment to seeking and acquiring the best employment candidates who reflect a diversity of background, experience and perspective and who can contribute most to the success of GlaxoSmithKline.
Performance and rewardThe importance of people must translate into employment practices that demonstrate the value of each individual. Compensation and benefit packages (GlaxoSmithKline’s Total Reward) aim to be competitive and innovative and are either global or local in orientation, depending on what best drives business performance and rewards individual contribution.
Compensation philosophy and programme development underscore GlaxoSmithKline’s commitment to a performance culture. Performance based pay, both base and variable, share awards, share options, performance and development planning and evaluation contribute to retention of key talent, superior performance and accomplishment of business targets.
A commitment to flexible working through flexi-time, teleconferencing, remote working and flexible work schedules, recognises that employees work best in an environment that helps them integrate their work and personal lives.
Communication and involvementAn extensive programme of open, two-way communications stimulates employee engagement in GlaxoSmithKline’s strategy and day-to-day operations. This includes the publication of regular summary reports from Corporate Executive Team meetings, a Chief Executive Officer’s home page featuring presentations and a Q&A area, a Group-wide magazine, town hall meetings and video conferences. In 2002, there was a satellite broadcast involving 60 sites in 31 countries, and watched by an employee audience of around 30,000. Live video streaming and video on demand options are being developed as additional means of ensuring employees have access to the most senior levels of management, and as powerful tools for building culture and driving alignment across common goals.
Share ownership schemes encourage participation as owners of the business, increasing awareness of short and long term business objectives. Global and local employee opinion surveys allow employees the opportunity to express their views and perspectives on important Group issues.
DiversityThe GlaxoSmithKline Diversity Strategy focuses on creating an inclusive work environment. The approach aims to enhance employee innovation and productivity by valuing and drawing on the differing knowledge, perspectives, experiences and styles resident in the global community. The Corporate Executive Team leads the Diversity Initiative with its key objective: to create and implement diversity strategies that measurably improve employee attraction, development and retention. Tailored initiatives are in progress to embed inclusive behaviours into GlaxoSmithKline’s culture and practices.
GlaxoSmithKline is committed to employment policies free from discrimination against potential or existing staff on the grounds of age, race, ethnic and national origin, gender, sexual orientation, faith or disability.
In particular GlaxoSmithKline is committed to offering people with disabilities access to the full range of recruitment and career opportunities. Every effort is made to retain and support staff who become disabled while working for the Group.
Talent management and leadership developmentDevelopment planningsafety (EHS) is a key element in performance planning for all employees each year. Reviews are conducted in each business and function to ensure that a diverse talent pool is fully developed to meet future business needs, and that successors are identified for key positions.
Comprehensive leadership development opportunities are available to managers at all levels. These opportunities are targeted to help leaders to meet the challenges they face in a global organisation. They ensure leadership motivates and enables teams and individuals to do their best work. Development opportunities are innovative, based on peer interaction and idea exchange, and contribute to strategy deployment.
Human Resources services and information systemsGlaxoSmithKline’s human resource delivery strategy is designed to make the most of technology. Human Resources services and information are delivered through low cost, highly effective channels that make it easy for job candidates, employees, and retirees to access information about employment, compensation and benefits, policies and programmes. These include intuitive personalised web-based tools, available to employees in many locations.
Property, plant and equipment
GlaxoSmithKline has operating establishments in some 102 countries. The geographical spread of the Group‘s activities is indicated in Note 38 to the Financial statements, ‘Principal Group companies’. GlaxoSmithKline conducts research and development at more than 20 sites and manufactures product at more than 95 sites in 38 countries. Refer to ‘Research and development – Pharmaceuticals’ (page 14) and ‘Manufacture and supply’ (page 13).
GlaxoSmithKline has invested over £4 billion in its property, with a carrying value in the Financial statements of almost £3 billion, with a further £3.6 billion, at carrying value, invested in plant and equipment and assets in construction. In 2002, GlaxoSmithKline invested £1 billion in new and renewal property, plant and equipment. This is mainly related to a large number of projects for the improvement and expansion of facilities at various worldwide sites. Property is mainly held freehold. New investment is financed from Group liquid resources. At 31st December 2002, the Group had capital contractual commitments for future expenditure of some £382 million and in 2003 operating lease commitments of £168 million.
GlaxoSmithKline’s business is science-based, technology-intensive and highly regulated by governmental authorities. It allocates significant financial resources to the renewal and maintenance of its property, plant and equipment to minimise risks of interruption of production and to achieve compliance with regulatory standards. The research and development and manufacture of active pharmaceutical ingredients require the use of chemicals and hazardous materials. GlaxoSmithKline observes stringent procedures and uses specialist skills to manage environmental risks from these activities. Environmental issues, sometimes dating from operations now modified or discontinued, are referenced under ‘Responsibility for Environment, Health and Safety’ (page 26) and in Note 30 to the Financial statements, ‘Legal proceedings’. GlaxoSmithKline believes that its facilities are adequate for its current needs. The integration of Glaxo Wellcome and SmithKline Beecham operations has involved a series of announcements of rationalisation and potential disposal of a number of sites and properties. It is considered that there will be further changes.
The business and the community
Corporate and socialcorporate responsibility
This year GlaxoSmithKline has again produced a separate report on social and environmental issues. This covers the issues that are of primary concern to stakeholders. These include medicines for the developing world, community investment, R&D, the environment and health and safety. While metrics for environmental performance have been reported for many years, during 2002 the Group developed indicators for other issues that will enable it to show progress in addressing these. The report is available from the Secretariat and on the website at www.gsk.com.
Responsibility for Environment, Health and Safety
Environment Health and Safety (EHS) is a key issue for the Group and has a high priority. Responsibility for EHS is at the highest level. There is a corporate group reporting to the General Counsel that has overall responsibility for providing governance and servicesleadership on EHS issues. The head of this group makes regular reports to the Corporate Executive Team (CET) and the Audit and Corporate Responsibility Committees of the Board of Directors. Within the businesses, operations line managers are responsible for EHS and are supported by site-based EHS and medical professionals.occupational health staff.
Environment, HealthEHS strategy and Safety managementplanGlaxoSmithKlineGSK has a strategic planning process for EHS that looks forward 10 years but is reviewed every year. The plan is aligned with the GSK business drivers and includes both management and performance measures and targets. Progress has been made in all areas of the plan, with particular success in incorporating EHS into the selection and management of contract manufacturers and key suppliers, in developing and maintaining an open and effective dialogue with external stakeholders, in providing EHS data for decision making on new products and processes and in ensuring safety and health concerns are properly addressed at GSK’s facilities to minimise risk and avoid disruption of product supply. Some areas for additional focus are driver safety, occupational chemical exposure, machine guarding, pharmaceuticals in the environment from patient excretion, energy conservation and the use of hazardous chemicals in manufacturing.
Strategic focus in 2005
The plan provides an area of special focus each year. In 2005, the focus was on completing core programmes. These programmes are essential to prevent injury or illness or harm to the environment and to ensure the continuity of GSK’s business. Some of them will be common to all operating locations. Operations with different risks may have different core needs and therefore different core programmes. For a programme to be complete it must have a management system in place, acceptable audit scores and acceptable progress against the EHS targets.
There is a need to operate and maintain the programmes, monitor their performance and continually look for improvements. Progress in this strategic focus area may be seen in the audit scores and progress to targets.
EHS management
GSK takes a systematic approach to managing EHS risks and impacts. A framework of information and programmes based on a set of universalthe global EHS Standardsstandards guides the management of these issueskey aspects, impacts and risks throughout the organisation.
Environment, Health and SafetyEHS audits
As part of its governance responsibility, GlaxoSmithKlineGSK conducts EHS audits of its sites, contract manufacturers and key suppliers. The audit protocols developed and introduced during 2002 were derived fromassessing performance against the EHS Standards. A new scoring system was tested during the yearstandards and will be fully implemented in 2003.
A pilot process has begun, with Global Manufacturing and Supply, to investigate obtaining Group wide certification to the international standards on EHS. This involves review by a third party registrar of GlaxoSmithKline’s EHS Standards and auditing procedures and completion of a number of certification audits of Group sites. The aim is for the registrar to gain confidence in the corporate auditing process as well as in theassigning quantitative performance of representative sites against the international standards to proceed with a full certification based on a sample of sites. The remaining sites will be subject to audits by the third party registrar as part of obtaining certification.
scores. In 2002, 212005, when 36 sites were audited, and seven follow-up reviews were performed.70% of these achieved audit scores of 70% or better. As part of the continuous improvement process, progress was monitored on actions arising from issues raised on all audits. A web based tool to assist this process was developed and will be launched in 2003.
As part of the commitment to corporate social responsibility and the pro-active management of the GlaxoSmithKlineGSK manufacturing and supply base, 16 of the key contract manufacturers and41 suppliers were also assessed.assessed, representing about 20% of priority suppliers. This process evaluated the management of key EHS risks and impacts, as well as human rights issues, based on the Group’s EHS requirements for contract manufacturers. Good performance was identified and recommendationspriority suppliers. Recommendations were made for improvements where improvements were needed.
Objectives andEHS targetsObjectives for 2002 focused on progressing toward full implementation of EHS management systems. These systems are meant to ensure on-going compliance with legislation and regulations as well as internal standards. Sites analysed how well their programmes met the requirementsAs part of the EHS Standardsplan, targets are set every five years and then developed plans to achieve any requirements that are not currently met in full. Assistance from2005 is the corporate EHS group is provided inend of the form of information materials, an intranet system to support EHS programmes and an awards programme to encourage innovative solutions.
first five-year target period. Targets for EHS improvements were set in 2001 that are to be accomplished over five years. Thefor 10 environmental measures and for one measure of occupational health and safety target is a reduction in lost time injury and illness rate by 15 per cent persafety.
Progress towards meeting these targets has been tracked every year. EnvironmentalFinal data for 2005 showing the level of achievement of targets include reductions in energy usage and associated greenhouse gas emissions, reductions in waste and wastewater disposed and increase in waste recycled.
Performance improvement measuresGlaxoSmithKline measures its impactwill be published on the health and safetywebsite www.gsk.com. Significant progress has been made towards achieving eight of people who work at our sites and its impact on the environment. The measure10 EHS targets with some of impact on people is the lost time injury and illness rate. This is the number of injuries and illnesses serious enoughprogress due to result in lost time per 100,000 hours worked. The impacts on air, water and land are measured as metric tonnes of material emitted,outsourcing some processes to contract manufacturers. For hazardous waste disposed and the impact on natural resources is measured as cubic metresproportion of water usedwaste recycled, the targets have not been achieved. The targets have not been achieved because of products transferred to facilities without appropriate recycling systems in place, other recycling systems that were down for maintenance and gigajoules of energy consumed.new products coming into manufacturing.
GlaxoSmithKlineGSK selects its measures of performance improvement based on the risk. Risks are determined, in part, through evaluation of impacts. The impacts considered were those with the potential for adverse impact on people or the environment, business continuity or business reputation. Most of the measures selected are similar to those reported by other companies and are recommended by the Global Reporting Initiative, a long-term, multi-stakeholder, international undertaking to develop and disseminate globally applicable sustainability reporting guidelines.
Product stewardshipSustainabilityGlaxoSmithKline has a global standard for product stewardship that establishes requirements for responsible and ethical management of EHS aspects of products throughout their life-cycles. Product stewardship provides a systematic way to identify product or process risks early, so that they may be mitigated and managed. Integrating product stewardship into business activities protects people andIn the environment, enhances compliance with local regulatory requirements and avoids interruption of product supply.
Environmental sustainabilityThe concept of sustainable development is central to the Group’s environmental programmes. Work has startedwork towards eventual sustainability, GSK is addressing economic, environmental sustainabilityand social issues in research, manufacturing, sales and distribution of its medicines. Sustainability starts with healthcare solutions found by mitigating environmental impactsR&D and looking at ways to improve production efficiency. Thecontinues with sustainable solutions in manufacturing and sales. R&D is considering improving operational efficiency for new products. In the future, the EHS plan for excellence proposes investigating the use of renewable raw materialsresources and the overall balance of its impact on society and the consumption of resources with the generation of waste will be investigated in the future.environment. The Group has a standardseeks dialogue with external stakeholders and considers their views when developing approaches to sustainable development. More information on sustainable development that definesEHS programmes and performance may be found on the approach from discovery through manufacturing to sales. Environmental sustainability starts with R&D. As part of the support for R&D, a toolkit has been developed to assist in the selection of “green” chemistries and processes.website.
| GSK Annual Report 2005 |
| 26 |
| Corporate governance |
This section discusses GlaxoSmithKline’s management structures and governance procedures.
It contains the company’s reporting disclosures on corporate governance required by the Combined Code on Corporate Governance of the Financial Reporting Council (Combined Code), including the required statement of compliance.
Further, the company reports on compliance with the US laws and regulations that apply to it.
| The Board | 28 |
| Corporate Executive Team | 29 |
| Governance and policy | 30 |
| Dialogue with shareholders | 31 |
| Annual General Meeting | 32 |
| Internal control framework | 33 |
| Committee reports | 34 |
| The Combined Code | 35 |
| US law and regulation | 36 |
Access to healthcare in the developing world
Access to healthcare in developing countries presents a unique challenge to the global community. The problem, which is rooted in poverty, demands a significant mobilisation of resources, an unprecedented sense of urgency and a new spirit of partnership. It must be tackled as a shared responsibility by all sectors of global society. The Group does not have the mandate, expertise or resources to address the underlying problems that exist. However, GlaxoSmithKline is playing a vital role. There are three key areas in which it makes innovative, responsible and, above all, sustainable contributions to improving healthcare in the developing world:
R&D for diseases of the developing worldContinued investment in R&D into new drugs and vaccines for diseases that affect the developing world is essential to long-term improvement in the health of people in these regions, not least because of challenges such as the development of resistance to current treatments and poor patient adherence to complex treatment regimens.
The Group believes GlaxoSmithKline has the industry’s most extensive portfolio of products and R&D projects for diseases of the developing world, and that it is the only Group undertaking R&D into the prevention and treatment of all three of the World Health Organisation’s (WHO) priority diseases in the developing world -HIV/AIDS, tuberculosis and malaria. The Group currently has over 20 R&D projects and programmes of relevance to the developing world, ten of which are aimed at producing vaccines and medicines for diseases that disproportionally affect developing countries. GlaxoSmithKline is increasingly involved in public-private partnerships to enable a wider range of projects to be undertaken.
In addition to the R&D on HIV/AIDS, an R&D group dedicated to Diseases of the Developing World has been created to ensure a focus on these diseases. Projects are prioritised primarily on their socio-economic and public health benefits rather than on their commercial returns.
Preferential pricing arrangementsGlaxoSmithKline has offered its vaccines to public health programmes at significant discounts for over 20 years. The Group sets a single, sustainable, preferential price for each of its ARVs and anti-malarials to a wide range of customers in the Least Developed Countries and sub-Saharan Africa - a total of 63 countries. GlaxoSmithKline is committed to contributing to health improvements in a sustainable manner. Preferential prices for its ARVs and anti-malarials are therefore set at levels on which no profit is made, but that cover direct costs, so that supply can be sustained for as long as required. There has been notable progress in expanding access through preferential pricing. The Group has some 120 arrangements, covering 50 of the world’s poorest countries, to supply ARVs at preferential prices. Customers include governments, non-governmental organisations (NGOs), hospitals, academic institutions and private employers.
In 2002, evidence was uncovered that some of the company’s ARVs that had been sold to Africa at not-for-profit prices were being illegally re-imported into the European Union for sale at a higher price. The victims of this trade are HIV/AIDS patients in Africa and the only beneficiaries are the illegal importers. This diversion threatens GlaxoSmithKline’s ability to provide preferential prices to the developing world. The offer of not-for-profit prices requires a sustainable framework, combining the Group’s commitment to preferential pricing with commitments from others to put in place ways to prevent product diversion and to avoid price referencing against preferentially priced medicines.
GlaxoSmithKline has taken steps to address the problem and from a regulatory perspective, it is now able to supply 31 countries with Combivir in a special, tri-lingual ‘access’ pack to provide a barrier to diversion. However, this alone will not fully deter illegal traders who are experts in the repackaging of medicines. Stricter regulations and enforcement to counter this illegal trade will be required.
Success through partnershipDuring 2002, GlaxoSmithKline continued to engage with stakeholders working on improving access to healthcare in the developing world. The Group has a long history of supporting community investment programmes and has a wide range of partnerships to support delivery of better health and education to under-served communities around the world. The Group also consulted and worked with governments of both the developed and developing world, the United Nations, the WHO, NGOs and with the investment community and will continue constructive dialogue with organisations that share its aim of trying to improve access to healthcare in the developing world.
GlaxoSmithKline is making a vital contribution to improving healthcare in the developing world. The Group will continue with its efforts, improving its initiatives by applying lessons learned and looking for opportunities to do more. For example, in September 2002 the Group further reduced its preferential prices for ARVs by up to 33 per cent. It looks to other stakeholders also to go further and play their part through embracing partnership, showing political will and, above all, committing significant new funding. This is critical if an improvement in healthcare and quality of life across the developing world is to be achieved.
GlaxoSmithKline’s community investment in 2002 totalled £239 million of which £112 million was related to the Group’s patient assistance programmes in the USA. This was equivalent to 4.3 per cent of Group profit before tax. Many of the programmes are long-term commitments that help bring about sustainable change. The Group’s community investment activities are focused on health and education and include:
Patient assistance programmesThe patient assistance programmes provide access to GlaxoSmithKline’s medicines for the most needy US patients who do not have prescription drug insurance. In 2002, over 410,000 patients received medicines through the Group’s patient assistance programmes, at a value of $168 million.
Public health programmes
The Global Alliance to Eliminate Lymphatic FilariasisGlaxoSmithKline is an active and involved member of the Global Alliance to Eliminate Lymphatic Filariasis (LF); a unique partnership which includes the WHO, the ministries of health in endemic countries, non-governmental organisations, community based organisations, academic institutions, international organisations and the private sector - all committed to eliminating one of the world’s most disabling diseases.
GlaxoSmithKline has committed as much of its anti-parasitic drug albendazole as is required to eliminate LF over the anticipated 20 year life of the programme. In 2002, the fourth year of the programme, 66 million tablets, worth £8.7 million at wholesale acquisition cost were donated to 31 countries. These numbers will expand as the programme extends to the one billion people at risk in 80 countries. In addition the Group gave grants to support the Global Alliance to Eliminate LF totalling £750,000.
Positive Action on HIV/AIDSIn 2002 Positive Action - the Group’s international programme of HIV education, care and community support – marked its tenth anniversary. Through the programme GlaxoSmithKline works in partnership with networks of people living with HIV/AIDS, community groups, international agencies, NGOs and governments to intensify community responses to HIV/AIDS.
During 2002 Positive Action supported 25 international programmes in 32 countries. Programmes included a grant of $250,000 over three years to the International Center for Research on Women, to investigate the underlying factors that cause HIV/AIDS-related stigma and discrimination and to develop interventions to minimise the barriers limiting access to healthcare. The project is being conducted in Ethiopia, Tanzania and Zambia.
Following consultation with the conference community committee, Positive Action contributed over £90,000 to support attendance and participation of community representatives from under-resourced regions at the 14th International AIDS Conference, held in Barcelona in July 2002.
African Malaria PartnershipIn April 2002 GlaxoSmithKline launched the African Malaria Partnership to fund three behavioural development programmes in Africa to help combat a disease that kills more than a million people every year.
In November 2002, it was announced that three programmes had been selected to share grants totalling £1 million over three years. The programmes will benefit nearly two million people in seven countries.
Regional community initiatives
United KingdomGlaxoSmithKline made corporate contributions of £4.1 million to UK charities. More than 350 projects in science education and medical research, healthcare, the arts and the environment were funded. In addition GlaxoSmithKline companies in the UK provided a further £8.5 million for community investment purposes, giving a combined total of £12.6 million in support of projects in the UK.
Almost £500,000 in total was provided for medical research to The Foundation for the Study of Infant Deaths, Multiple Sclerosis Society, Action Research, Primary Immunodeficiency Association and The Stroke Association.
GlaxoSmithKline gave an unrestricted gift of £5 million to Imperial College London. The gift will be used to support biomedical research in an effort to identify and develop new treatments for disease.
The Group announced renewed support for science education by investing £1 million over four years in INSPIRE (INnovative Scheme for Post-docs in Research and Education), in partnership with the Department for Education and Skills, Imperial College London, and the Specialist Schools Trust.
Other 2002 education programmes included £100,000 for Science Across The World, an international educational programme encouraging communication and shared learning across different cultures, and sponsorship of The Royal Institution Christmas Lectures, which provide an opportunity for young people to learn from eminent scientists.
The International Impact Awards (UK) recognise excellence in the work of voluntary community healthcare organisations across the UK. This years’ ten winners each received an unrestricted award of £25,000. £100,000 was donated to the Royal National Institute for the Blind, in support of their new Low Vision Unit in London. The Group is also supporting the Shaw Trust’s Pain Management project in Wales with a donation of over £36,000.
The Group sponsored the exhibition ‘Art in the Making – Underdrawings in Renaissance Paintings’ at the National Gallery, London. It is supporting Earthwatch Institute’s environmental awards for primary school teachers for three years with a donation of £150,000.
EuropeProgrammes in Europe in 2002 focused on children’s health with total funding of £1.1 million supporting a range of long-term programmes, including £335,000 for Zippy’s Friends; a programme run by Partnership for Children to teach coping skills to children in Denmark and Lithuania.
Barretstown Gang in Ireland and L’Envol in France, both of which provide therapeutic recreation for seriously ill children from across Europe, received £350,000 and £100,000, respectively.
Working with the charity HealthProm and the Azerbaijan Health Ministry, GlaxoSmithKline invested £92,000 in 2002 as part of a four year programme to benefit nearly 250,000 refugees in Azerbaijan with a new safe childbirth initiative.
North AmericaProgrammes in North America focused on improving access to better healthcare. Funding of $13.1 million was allocated through the North America Community Partnerships team. A further $93.7 million was donated to regional community activities.
The SHARE Awards foster healthy ageing across cultures by recognising community-based programmes that meet the needs of older people from racially, ethnically and culturally diverse backgrounds. Over the past four years, GlaxoSmithKline’s grants of $6.5 million have supported SHARE awards for 51 organisations, enabling them to improve healthcare access and delivery.
The International Impact Awards (USA) acknowledge and reward excellence in the non-profit healthcare community, in the Greater Philadelphia area. Ten winners each received $40,000 as an unrestricted award.
| REPORT OF THE DIRECTORS |
| Corporate governance | ||
| continued | ||
GlaxoSmithKline gave unrestricted gifts of $10 million to the University of Pennsylvania and $5 million to Duke University. The gifts will be used to support their discovery research efforts to identify and develop new treatments for disease.
Science in the Summer is a library-based science education programme in the Philadelphia area offering hands-on courses taught by certified teachers. A GlaxoSmithKline grant of $400,000 to the American Association for the Advancement of Science supports this programme.
In North Carolina, Duke University Medical Center received a $250,000 grant over two years to expand their adult diabetes education programme’s outreach to minority and underserved populations.
With a $250,000 contribution over five years, the Rescue Missions Ministry will set up a GlaxoSmithKline educational scholarship endowment for formerly homeless people.
The University of North Carolina at Chapel Hill received $250,000 as part of an overall $1.25 million grant for a travelling science and technology bus to help improve science teaching and to encourage and advance the science careers of underserved and ethnic minority students.
InternationalGlaxoSmithKline’s International Community Partnerships programmes addressed health education and mobilisation, providing partnership funding of £1.1 million in 2002. Programmes included:
£320,000 to support its PHASE initiative (Personal Hygiene And Sanitation Education) in Kenya, Uganda, Nicaragua and Peru. PHASE targets school children and aims to reduce diarrhoea-related disease and death.
£100,000 as part of a three-year commitment to fund an HIV/AIDS clinic in the Masoyi tribal area of Mpumalanga, South Africa.
The Group extended its Rural Nursing Excellence programme in Thailand, which sponsors female high school graduates from rural areas to complete four-year nursing degrees. GlaxoSmithKline has donated another £100,000 to train a further 50 nurses.
In Ethiopia GlaxoSmithKline provided £100,000 for the Integrated Management of Childhood Illnesses (IMCI) in partnership with the WHO and UNICEF. The goal is to contribute to the global reduction of mortality and morbidity in children under the age of five from pneumonia, diarrhoea, malaria, measles and malnutrition.
In China, £100,000 of GlaxoSmithKline funding is supporting the development of a community-based HIV/AIDS programme in collaboration with the Red Cross Movement in China, British Red Cross and Australian Red Cross.
Product donationsGlaxoSmithKline donates essential products for humanitarian relief efforts. Donations are made at the request of governments and major charitable organisations and are generally manufactured specifically to meet these requests. NGOs complete a needs assessment and then order the product needed in their international communities. This ensures that the right product reaches the right person at the right time.
In 2002, the total value of the Group’s international product donations, excluding the lymphatic filariasis programme, was $23.4 million, at wholesale acquisition cost. This is GlaxoSmithKline’s wholesale list price, not including discounts and is a standard industry method of valuing product donations.
Employee involvementGlaxoSmithKline employees are encouraged to contribute to their local communities through employee volunteering schemes. Support for this varies around the world but includes employee time, donations to charities where employees have completed voluntary work and a matching gifts programme. In 2002, in the USA, the Group matched more than 9,000 employee gifts, at a value of $3.1 million.
GlaxoSmithKline also matched contributions by employees to the United Way campaigns at a value of $1.6 million. This was further supplemented by GlaxoSmithKline’s three year grant of $555,000 to the United Way of Southeastern Pennsylvania which provides specific capacity building grants and creates more effective healthcare delivery at the United Way’s 91 member agencies.
GlaxoSmithKline’s Investment in Volunteer Excellence (GIVE) provided $500 grants to qualifying US non-profit organisations based on employee or partner volunteer time. The GIVE grants totalled $145,000 and reflect over 34,000 employee volunteer hours.
FoundationsThe Group does not operate a single charitable foundation for its corporate programmes but has a number of country-based foundations including:
The GlaxoSmithKline France Foundation supports programmes to improve HIV/AIDS prevention education, training and care, primarily in Africa. As a result, over 200,000 people are expected to access care and support services by the end of 2005.
The North Carolina GlaxoSmithKline Foundation is an endowed, self-funding organisation which operates as a separate entity. The foundation publishes its own annual report, which is available on request, and uses its asset base to support math, science and health education in North Carolina. In 2002, this foundation made donations totalling $2.2 million. This figure is not included in the Group’s total community investment figure.
| |
The Board
The Directors listed below were appointed on 23rd May 2000 and have served since that date.
Sir Christopher Hoggbdfh (Aged 66)Gent (Aged 57)Non-ExecutiveAppointed on 1st June 2004. Chairman. Sir Christopher was formerly a Non-Executive Directorthe Chief Executive Officer of SmithKline Beecham plc. He is Non-Executive Chairman of Reuters Group PLC and a member of the Supervisory Board of Air Liquide S.A. and Chairman of The Royal National Theatre.
Sir Roger Hurndhj (Aged 64)Non-Executive Deputy Chairman. Sir Roger was appointed a Non-Executive Director of Glaxo WellcomeVodafone plc, until his retirement in 1996 and Deputy Chairman in 1997.July 2003. He is a Non-Executive Director of Cazenove Group plc. He is alsoLehman Brothers Holdings Inc, a member of the Financial Reporting Council, a Senior Adviser at Bain& Co. and Chairman of the Courtadvisory board of Governors of Henley College.Reform.
Dr Jean-Pierre Garnierd (Aged 55)58)
Appointed on 23rd May 2000. Chief Executive Officer. Dr Garnier was appointed an Executive Director of SmithKline Beecham plc in 1992, and became Chief Executive Officer in April 2000. He is a Non-Executive Director of United Technologies Corporation and a member of the Board of Trustees of the Eisenhower Exchange Fellowships. He holds a PhD in pharmacology from the University of Louis Pasteur in France and an MBA from Stanford University in the USA.
John Coombed (Aged 57)Lawrence Culp (Aged 42)
Appointed on 1st July 2003. Non-Executive Director. Mr Culp is President and Chief Executive Officer of Danaher Corporation. Prior to joining Danaher, he held positions in Accenture, previously Andersen Consulting.
Sir Crispin Davis (Aged 56)
Appointed on 1st July 2003. Non-Executive Director. Sir Crispin is Chief Executive of Reed Elsevier PLC. Prior to that, he was Chief Executive of Aegis Group plc, which he joined from Guinness plc, where he was a member of the main board and Group Managing Director of United Distillers. He spent his early career with Procter & Gamble.
Julian Heslop (Aged 52)
Appointed on 1st April 2005. Chief Financial Officer. Mr CoombeHeslop joined Glaxo Wellcome as Financial Controller in April 1998. In January 2001, following the merger, he was appointed Senior Vice President, Operations Controller. Prior to joining Glaxo Wellcome, he held senior finance roles at Grand Metropolitan PLC.
Sir Deryck Maughan (Aged 58)
Appointed on 1st June 2004. Non-Executive Director. Sir Deryck is a Managing Director of Kohlberg Kravis Roberts & Co. He was formerly an Executive DirectorChairman and CEO of Glaxo Wellcome plc where he was responsible for FinanceCitigroup International and Investor Relations. He is a member of the Supervisory Board of Siemens AG, the UK Accounting Standards Board and the Code Committee of the UK Takeover Panel.
Paul Allairedi (Aged 64)Non-Executive Director. Mr Allaire was formerly a Non-Executive Director of SmithKline Beecham plc.Salomon Brothers Inc. He is a Non-Executive Director of Lucent TechnologiesReuters Group plc, as well as serving on the Boards of Directors of Carnegie Hall, Lincoln Center and NYU Medical Center. He is also an International Advisory Board member of British American Business Inc. and priceline.com Inc. He is Chairman of The Ford Foundation.
Dr Michèle Barzachdf j (Aged 59)Non-Executive Director. Dr Barzach was formerly a Non-Executive Director of Glaxo Wellcome plc. She is aBoard member of the International Cooperation High Council,Trilateral Commission. He served as Vice Chairman of the Board of Equilibres et Populations and Director of the Board of Project Hope. International consultant in health strategy, she was formerly French Minister of Health and Family.New York Stock Exchange from 1996 to 2000.
Sir Peter Jobbdj (Aged 61)Non-Executive Director. Sir Peter was formerly a Non-Executive Director of Glaxo Wellcome plc. He is a Non-Executive Director of Schroders plc, Shell Transport and Trading Company plc, TIBCO Software Inc, Instinet Group Inc. and Multex.com Inc. He is also a member of the Supervisory Boards of Deutsche Bank AG and Bertelsmann AG.
John McArthurdhj (Aged 68)Non-Executive Director. Mr McArthur was formerly a Non-Executive Director of Glaxo Wellcome plc. He is a Non-Executive Director of BCE Inc., BCE Emergis Inc., Cabot Corporation, HCA Corporation, Koc Holdings A.S., Rohm and Haas Company, Telsat Canada and The AES Corporation. He is also Senior Advisor to the President of the World Bank.
Donald McHenry (Aged 66)Non-Executive Director. Mr McHenry was formerly a Non-Executive Director of SmithKline Beecham plc. He is a Distinguished Professor in the Practice of Diplomacy at the School of Foreign Service at Georgetown University and President of the IRC Group, LLC. His other Non-Executive directorships include The Coca-Cola Company, FleetBoston Financial Corporation, International Paper Company and AT&T Corporation. He previously served as Ambassador and US Permanent Representative to the United Nations.
Sir Ian Prosser (Aged 62)bdg (Aged 59)Non-ExecutiveAppointed on 23rd May 2000. Senior Independent Director. Sir Ian was formerly a Non-Executive Director of SmithKline Beecham plc. He iswas Chairman and Chief Executive of Bass plc and ultimately Chairman of Six Continents PLC andthe demerged InterContinental Hotels Group plc. He was Chairman of the World Travel &and Tourism Council and the London Stock Exchange Listed Advisory Council. He is Non-Executive Deputy Chairman of BP plc. He isplc, a Non-Executive Director of Sara Lee Corporation and a member of the CBI President’s Committee.
Dr Ronaldo Schmitz (Aged 67)ad (Aged 64)
Appointed on 23rd May 2000. Non-Executive Director. Dr Schmitz was formerly a Non-Executive Director of Glaxo Wellcome plc. He is a Non-Executive Director of Legal & General Group plc and a member of the Board of Directors of Rohm and Haas Company and Cabot Corporation.
Dr Lucy Shapiro (Aged 65)df (Aged 62)
Appointed on 23rd May 2000. Non-Executive Director. Dr Shapiro was formerly a Non-Executive Director of SmithKline Beecham plc. She is Ludwig Professor of Cancer Research in the Department of Developmental Biology and Director of the Beckman Center for Molecular and Genetic Medicine at the Stanford University School of Medicine.Medicine and a Non-Executive Director of Anacor Pharmaceuticals, Inc. She holds a PhD in molecular biologybiology.
Tom de Swaan (Aged 59)
Appointed on 1st January 2006. Non-Executive Director. Mr de Swaan is a member of the Managing Board of ABN AMRO, of which he was Chief Financial Officer until 31st December 2005. He will retire from Albert Einstein Collegethe Board of Medicine.ABN AMRO on 1st May 2006. He is a Non-Executive Director of the Financial Services Authority, a member of the Board of the Institute of International Finance, Chairman of the Board of the Netherlands Opera and a member of the Board of the Royal Concertgebouw Orchestra.
MembershipSir Robert Wilson (Aged 62)
Appointed on 1st November 2003. Non-Executive Director. Sir Robert is Non-Executive Chairman of BG Group plc and the Economist Group and was previously Executive Chairman of Rio Tinto.
Dr Tachi Yamada (Aged 60)
Appointed on 1st January 2004. Retiring on 1st June 2006. Chairman, Research & Development. Dr Yamada was a Non-Executive Director, and subsequently an Executive Director, of SmithKline Beecham plc. Prior to joining SmithKline Beecham, he was Chairman of the Department of Internal Medicine at the University of Michigan Medical School and Physician-in-Chief of the University of Michigan Medical Center. He is a Trustee of the Rockefeller Brothers Fund and a member of the Advisory Board committees is indicated byof Quaker BioVentures, Inc.
Moncef Slaoui (Aged 46)
Chairman Designate, Research & Development. Dr Slaoui, Senior Vice President, Worldwide Business Development, has been appointed to the following symbols:Board with effect from 17th May 2006, and will succeed Dr Yamada as Chairman, Research & Development on 1st June 2006. Dr Slaoui joined GSK Biologicals in 1988 where he engineered the development of a robust vaccines pipeline. He has a PhD in Molecular Biology and Immunology from Université Libre de Bruxelles.
Other Directors
Mr John Coombe, formerly Chief Financial Officer, retired from the Board on 31st March 2005.
Details of the terms of referencemembership of the Board Committees may be found on page 34.
Other DirectorsSir Richard Sykes, Non-Executive Chairman, Sir Peter Walters, Non-Executive Deputy Chairman and Mr John Young, Non-Executive Director, all retired from the Board on 20th May 2002.31.
| GSK Annual Report 2005 |
| 28 |
| REPORT OF THE DIRECTORS |
| Corporate governance |
Corporate Executive Team (CET)
JP Garnier
Chief Executive Officer
As Chief Executive Officer, Dr Garnier is responsible for the link betweenmanagement of the Board and staff, andGroup. He oversees all operational aspects of the Group, including establishing policies, objectives and initiatives, and directinghe directs long-term strategy. Dr GarnierHe was formerly Chief Executive Officer of SmithKline Beecham, having joined the Group in 1990.
Rupert Bondy
Senior Vice President and General Counsel
Mr Bondy is responsible for legal matters across the Group, together with environmental, health and safety issues, insurance and security. He was a lawyer in private practice before joining SmithKline Beecham in 1995 as Senior Counsel.1995.
Ford Calhoun
Chief Information Officer
Dr Calhoun is responsible for information technology, a global function that enables key business processes across all parts of the Group. With doctoral and post-doctoral training in microbiology, genetics, biomathematics and computer science, Dr Calhounhe joined Smith Kline & French in 1984.
John CoombeClarke
President, Consumer Healthcare
Mr Clarke succeeded Mr Ziegler as President, Consumer Healthcare on 31st January 2006. He joined Beecham in 1976 and progressed through roles in Australasia, South Africa, The Far East, Japan, Canada and the UK. From 1998 to 2003, John was President, Consumer Healthcare Europe, and in 2004, appointed President, Futures Group.
Marc Dunoyer
President, Pharmaceuticals Japan
Mr Dunoyer was appointed President, Pharmaceuticals Japan in March 2003. He joined the Group in 1999 and was Senior Vice President and Regional Director, Japan until his current appointment.
Russell Greig
President, Pharmaceuticals International
Dr Greig leads the pharmaceutical operations outside the USA, Japan and most of Europe, covering more than 100 countries. He joined the Group in 1980 and was Senior Vice President, Worldwide Business Development for R&D prior to his current appointment in March 2003.
Julian Heslop
Chief Financial Officer
Mr Heslop became Chief Financial Officer on 1st April 2005. As head of the finance function Mr CoombeHeslop is responsible for activities such as financial reporting and control, tax and treasury, investor relations, finance systems, internal audit and real estate. He joined Glaxo in 1986Wellcome as Group Financial Controller and was appointed Group Finance Director in 1992.April 1998.
Dan Phelan
Senior Vice President, Human Resources
Mr Phelan is responsible for benefits, compensation, recruitment, organisation development, leadership development and succession planning, human resource information systems and employee health management. He joinedwas a lawyer in private practice before joining Smith Kline & French in 1981 and in 1994 was appointed Senior Vice President and Director, Human Resources, SmithKline Beecham.1981.
Howard PienPresidentPharmaceuticals InternationalMr Pien leads the pharmaceutical operations outside the USA and most of Europe, covering more than 100 countries that account for over 82 per cent of the world’s population. He joined SmithKline Beecham in 1991 and in 1998 was appointed President, Pharmaceuticals.
David Pulman
President,
Global Manufacturing &and SupplyAppointed to the post in December 2002, Dr Pulman is responsible for the global manufacturingGlobal Manufacturing and supply chain network.Supply Organisation and Global Procurement. He joined Glaxo in 1978 and prior to his most recent posting was responsible for the North American supply network, manufacturing strategy and logistics.logistics until his current appointment in 2002.
David Stout
President,
Pharmaceutical Operations
Mr Stout was President of US Pharmaceuticals until he was appointed to his current position in January 2003. He is responsible for all pharmaceuticals and vaccines operations worldwide, including the global pharmaceuticals business as well as the global vaccines business.USA, Europe, International, Japan and Global Manufacturing and Supply. He joined SmithKline Beecham in 1996 as head of itsand was President, US Sales and Marketing function, andPharmaceuticals, until his current appointment in 1998 became President, Pharmaceuticals, North America.January 2003.
Chris Viehbacher
President,
US PharmaceuticalsResponsible for European pharmaceuticals operations until the end of 2002, Mr Viehbacher took over theis responsible for US pharmaceuticals operations in January 2003.Pharmaceuticals. He joined Wellcome in 1988 and became Director, Continental Europe, at Glaxo Wellcomewas responsible for GSK’s European Pharmaceuticals business before his current appointment in 1999.2003.
Andrew Witty
President,
Pharmaceuticals Europe
Mr Witty is responsible for the Group’s pharmaceuticals operations in Europe, a post he took up in January 2003 when he was appointed to the CET. Mr WittyEurope. He joined Glaxo in 1985 and at GlaxoSmithKline was Senior Vice President, Asia Pacific until his current post.appointment in 2003.
Tachi Yamada
Chairman,
Research & Development
Dr Yamada leads the Group’s complex business of drug discovery and development, - creating new medicines through research. He joined SmithKline Beecham in 1994 as a Non-Executive member of the BoardDirector and became Chairman, R&D Pharmaceuticals in 1999.
Jennie Younger
Senior Vice President,
Corporate Communications & Community Partnerships
Mrs Younger is responsible for the Group’s internal and external communications, its image and partnerships with communities of the world.global communities. She joined Glaxo Wellcome in 1996 as Director of Investor Relations.Relations and was appointed to her current position in 2001.
Jack ZieglerMoncef Slaoui
Chairman Designate, Research & Development
Dr Slaoui will succeed Dr Yamada as Chairman, Research & Development on 1st June. He will join the CET on 17th May. He joined the Group in 1988 and is currently Senior Vice President, Worldwide Business Development.
Other membersConsumer HealthcareMr Combe retired as Chief Financial Officer on 31st March 2005.
Mr Ziegler isretired as head of the global Consumer Healthcare business, which produces oral healthcare, over-the-counter medicines and nutritional healthcare products. He joined SmithKline Beecham in 1991 and in 1998 was appointed President of the Consumer Healthcare business.business on 31st January 2006.
Other membersDr Palmer and Mr Tyson left the Group on 1st December 2002 to pursue other roles in the pharmaceutical industry. Mr Ingram retired on 31st December 2002, but will continuecontinues to work part-time as Vice Chairman of Pharmaceuticals, acting as a special advisor to the Group and will attendattending CET meetings in that capacity.
| GSK Annual Report 2005 |
29 |
| Corporate governance |
| continued |
Governance and policy
The Board and Corporate Executive Team
The Directors are listed under ‘The Board’ (page 32)28).
The Board of GlaxoSmithKline plc is responsible for the Group’s system of corporate governance and is ultimately accountable for the Group'sGroup’s activities, strategy and financial performance.
The Chief Executive Officer (CEO) is responsible for executive management of the Group and is assisted by the CET. The CET meets 11 times per year and otherwise as necessary. The members and their responsibilities are listed under “Corporate Executive Team” (page 29).
The Board comprises three Executive and nine Non-Executive Directors. The role of Non-Executive Directors is to bring independent judgement to Board deliberations and decisions. TheWhilst the Board considers each of theall its Non-Executive Directors to be independent in character and judgement, it has determined that one Non-Executive Director, Dr Shapiro, should not be considered as ’independent’ under the Combined Code. Dr Shapiro is not considered to be independent due to the remuneration that she receives from the Group as a member of the GlaxoSmithKline Scientific Advisory Board. When Sir Christopher Gent was appointed to the Board as Deputy Chairman, he was determined by the Board to be independent. Upon taking up the chairmanship of the Board on 1st January 2005, in accordance with the Combined Code, he was excluded from the determination of whether at least half the Board are independent Non-Executive Directors. Neither Dr Shapiro nor Sir Christopher Gent hold positions on a Board Committee where independence is required under the Combined Code.
The Board considers that Mr Culp, Sir Crispin Davis, Sir Deryck Maughan, Sir Ian Prosser, Dr Schmitz, Mr de Swaan and Sir Robert Wilson are independent in accordance with the recommendations of the Combined Code.
At the date of publication and throughout 2005, a majority of the Board members, excluding the Chairman, were independent Non-Executive Directors.
Sir Christopher Gent succeeded Sir Christopher Hogg on 1st January 2005 and was appointed Non-Executive Chairman following the retirement of Sir Richard Sykes on 20th May 2002, andthroughout 2005. Dr Jean-Pierre Garnier is Chief Executive Officer. Sir Roger Hurn is Non-Executive DeputyCEO. The Chairman leads the Board, and represents the Board to the CEO and other CET members as necessary between Board meetings. The CEO manages the Group and implements the strategy and policies adopted by the Board. The Chairman and the chairmen of Board Committees communicate regularly with the CEO and other CET members. The division of responsibilities between the role of Chairman and the CEO has been set out in writing, agreed by the Board and appears in full on the website.
Sir Ian Prosser was Senior Independent Director.Director (SID) throughout 2005.
Board process
The Board has the authority, and is accountable to shareholders, for ensuring that the company is appropriately managed and achieves the strategic objectives it sets. The Board discharges those responsibilities through an annual programme of meetings which includes the approval of overall budgetary planning and business strategy. The Board reviews the company’s internal controls and risk management policies and approves its governance structure and code of ethics.
The Board appraises and approves major financing, investment and contractual decisions in excess of defined thresholds. In addition, the Board evaluates and monitors the performance of the Group as a whole. This includes:
| • | engaging at Board meetings with the CEO, the other Executive Directors and members of the CET as appropriate, on the financial and operating performance of GSK and external issues material to the Group’s prospects |
| • | evaluating progress toward the achievement of the Group’s financial and business objectives and annual plans |
| • | monitoring, through reports received directly or from various committees, the significant risks facing the Group. |
The Board has overall responsibility for succession planning for the CEO and the other Executive Directors. The Board has given the CEO broad authority to operate the business of the Group, and the CEO is accountable for, and reports to the Board on, business performance.
CET members make regular presentations to the Board on their areas of responsibility, and the Board meets with all the CET members on an annual basis to discuss collectively the Group’s strategy. A primary element of the induction process for new Non-Executive Directors is undertaken by members of the CET, and all Non-Executive Directors are encouraged to have separate informal discussions at leasttheir discretion with any CET members.
The Board met six times in 2005, with each member attending as follows:
| Number of meetings | Number of | ||
| Name | held whilst a Board member | meetings attended | |
| Sir Christopher Gent | 6 | 6 | |
| Dr JP Garnier | 6 | 6 | |
| Mr J Heslop | 5 | 5 | |
| Dr T Yamada | 6 | 6 | |
| Mr L Culp | 6 | 5 | |
| Sir Crispin Davis | 6 | 6 | |
| Sir Deryck Maughan | 6 | 6 | |
| Sir Ian Prosser | 6 | 6 | |
| Dr R Schmitz | 6 | 6 | |
| Dr L Shapiro | 6 | 6 | |
| Sir Robert Wilson | 6 | 6 | |
| Mr J Coombe | 1 | 1 | |
In addition to the six scheduled meetings, the Board also met on a year. It hasquorate basis on two occasions.
Business environment development
To ensure that the Board is kept up-to-date on important matters, including legal, governance and regulatory developments, presentations are made on a formal schedule of matters reserved to it for decision but otherwise delegates specific responsibilities to Board committees, as described below. The Board works to an agreed agenda in reviewing the key activities of the business,regular basis by both external and receives papers and presentations to enable it to do so effectively. The Board considers and reviews the work undertaken by its Committees.internal advisers.
Independent advice
The Board recognises that there may be occasions when one or more of the Directors feel it is necessary to take independent legal and/or financial advice.advice at the company’s expense. There is an agreed procedure to enable them to do so. This is explained in the Corporate Governance section of the company’s website.
| GSK Annual Report 2005 |
30 |
| REPORT OF THE DIRECTORS |
| Corporate governance |
| continued |
Indemnification of Directors
Qualifying third party indemnity provisions (as defined in section 309B(1) of the Companies Act 1985) are in force for the benefit of the Directors and former Directors who held office during 2005.
Company Secretary
The Company Secretary is responsible to the Board and is available to individual Directors in respect of Board procedures. The Company Secretary is Simon Bicknell, who was appointed in May 2000. He is a barrister and joined the Group in 1984. He is secretary to all the Board Committees.
Board committeesCommittees
The Board has established a number of Committees and provides sufficient resources to enable them to undertake their duties. Executive Directors are not members of the Audit, Remuneration, Nominations or Corporate Responsibility Committees, although they may be invited to attend meetings. Each Director is a member of the Corporate Administration & Transactions and Financial Results Committees. Membership of these Committees is shown in the table below.
| Corporate | |||||||
| Audit | Remuneration | Nominations | Responsibility | ||||
| Sir Christopher Gent | – | – | C | C | |||
| Mr L Culp | – | M | – | – | |||
| Sir Crispin Davis | – | M | – | – | |||
| Sir Deryck Maughan | M | – | – | – | |||
| Sir Ian Prosser | M | – | M | M | |||
| Dr R Schmitz* | C | M | M | – | |||
| Dr L Shapiro | – | – | – | M | |||
| Mr de Swaan* | M | – | – | – | |||
| Sir Robert Wilson | M | C | – | – | |||
| * | Mr de Swaan will succeed Dr Schmitz as Chairman of the Audit Committee from September 2006. |
| Key: C = Chairman. M = Member. | |
The following Committees eachis a summary of which has its own writtenthe role and terms of reference:reference of each Committee. The current full terms of reference of each Committee may be obtained from the Company Secretary or the Corporate Governance section of the company’s website.
Audit Committee
The Audit Committee reviews the financial and internal reporting process, the system of internal control andcontrols, the management of risks and the external and internal audit process. The Committee also proposes to the shareholders the appointment of the external auditors and is directly responsible for their remuneration and oversight of their work. The Committee consists entirely of independent Non-Executive Directors. It meets at least four times a year and otherwise as necessary. The Audit Committee Report is on pages 34 and 35.
Remuneration Committee
The Remuneration Committee determines the terms of service and remuneration of the Executive Directors and members of the CET and, with the Chief Executive Officer (CEO),assistance of external independent advisors, it evaluates and makes recommendations to the Chief Financial Officer (CFO),Board on overall executive remuneration policy. The Committee consists entirely of independent Non-Executive Directors. It meets at least four times a year and otherwise as necessary. Information on the General Counsel,remuneration of Directors is given in the heads of global internal audit and corporate compliance, and representativesRemuneration Report on pages 37 to 54. The Chairman of the company and the CEO are responsible for evaluating and making recommendations to the Board on the remuneration of the Non-Executive Directors.
Nominations Committee
The Nominations Committee reviews the structure, size and composition of the Board and the appointment of members of the Board and the CET, and makes recommendations to the Board as appropriate. The Committee also monitors the planning of succession to the Board and Senior Management. The Committee consists entirely of Non-Executive Directors, of whom a majority are independent, and meets at least once a year and otherwise as necessary. The Nominations Committee Report is given on page 35.
Corporate Responsibility Committee
The Corporate Responsibility Committee consists entirely of Non-Executive Directors and provides a Board-level forum for the regular review of external auditors in attendance.issues that have the potential for serious impact upon the Group’s business and for the oversight of reputation management. The Committee is also responsible for governance oversight of the Group’s worldwide donations and community support. The Committee meets formally three times a year and otherwise as necessary.
Financial Results Committee
The Financial Results Committee reviews and approves, on behalf of the Board, the Annual Report onand Form 20-F, andthe Annual Review and the convening of the Annual General Meeting, together with the preliminary and quarterly statements of trading results. Each Director is a member of the Committee and the quorum for a meeting is any three members. To be quorate, each meeting must include the Chairman or the Chairman of the Audit Committee and the CEO or the CFO. It meets as necessary.
Remuneration CommitteeThe Remuneration Committee determines the terms of service and remuneration of the Executive Directors and Corporate Executives and with the assistance of external independent advisors it evaluates and makes recommendations to the Board on the remuneration of Non-Executive Directors.
Chief Financial Officer (CFO). The Committee consists entirely of Non-Executive Directors. It meets four times a year and otherwise as necessary. The Chairman and CEO attend the meetings except when their own remuneration is being considered. The Senior Vice President, Human Resources also attends each meeting.
Nominations CommitteeThe Nominations Committee reviews the structure, size and composition of the Board and the appointment of Corporate Executives and new Board members and makes recommendations to the Board as appropriate. The Committee will also review the management’s succession plan to ensure its adequacy. The Committee consists entirely of Non-Executive Directors and meets at least once a year to consider succession planning and otherwise as necessary.
Corporate Administration & Transactions Committee
The Corporate Administration & Transactions Committee reviews and approves matters in connection with the administration of the Group’s business, and of certain corporate transactions. The Committee consists of the Directors, Corporate Executive TeamCET members and the Company Secretary. The Committee meets as necessary.
Corporate Social Responsibility CommitteeEvaluation of the Board, Board Committees and Directors
The Corporate Social Responsibility Committee consists entirely of Non-Executive Directors and provides a Board level forum for the regular review of external issues that have the potential for serious impact upon the Group’s business and reputation. The Committee is also responsible for annual governance oversightperformance evaluation of the Group’s worldwide donationsBoard, its Committees and community support. TheDirectors during 2005 was undertaken by the Chairman and implemented in collaboration with the Committee meets formally twice a year and has further meetings and consultations as required.
Corporate Executive TeamThe executive managementChairmen, with the support of the Group isCompany Secretary. The Board considered the responsibilityreview conclusions at its meeting in December 2005 and agreed a number of minor improvements to its procedures and operating methodology.
The Senior Independent Non-Executive Director, Sir Ian Prosser, undertook the performance evaluation of the CEO and other senior managers, who formChairman through a discussion with the Corporate Executive Team (CET) which meets 11 times per year. The members and their responsibilities are listed under 'Corporate Executive Team' (page 33).Directors, excluding the Chairman, in December 2005.
Remuneration of DirectorsInformation on the remuneration of Directors is given in the Remuneration report on pages 39 to 50.
Financial results are announced quarterly.
The company reports formally to shareholders twice a year, when its half-year and full-year results are announced. The full-year results are included in the company’s Annual Report and Annual Review, which are issued to shareholders. The company’s half-year results are published in a national newspaper shortly after release. The CEO and CFO give presentations on the final year endfull-year results to institutional investors, analysts and the media in London and in New York. In addition, theremedia.
| GSK Annual Report 2005 |
31 |
| REPORT OF THE DIRECTORS |
| Corporate governance |
| continued |
There are webcast teleconferences after the release of the first, second and third quarter results for institutional investors, analysts and the media. These presentations may also be accessed viaThe Annual Report, Annual Review and quarterly results are available on the www.gsk.comcompany’s website.
The Annual General Meeting (AGM) takes place in London, and formal notification is sent to shareholders at least one month in advance. At the Meeting, a business presentation is made to shareholders and all Directors able to attend are available, formally during the Meeting,AGM, and informally afterwards, for questions. Committee Chairmen ordinarily attend the AGM to respond to shareholders’ questions. Mr Culp was unable to attend the company’s AGM in May 2005 due to other commitments. All resolutions at the AGM are decided on a poll as required by the company’s Articles of Association. The results of the poll are announced to the London Stock Exchange and posted on the company’s website. Details of the 2003 Annual General Meeting2006 AGM are set out in the section 'Annual‘Annual General Meeting'Meeting’ (see this page).
To ensure that the Non-Executive Directors are aware of and understand the views of major shareholders about the company, the Board has in place a process focusing on sector-specific issues, as well as general shareholder preferences. At its meeting in July, the Board received an external review of shareholder opinion.
The CEO and CFO maintain a dialogue with institutional shareholders on performance, plans and objectives through a programme of regular meetings. They both speak regularly at external conferences and presentations.
The Group’s Investor Relations department, with offices in London and Philadelphia, acts as a focal point for contact with investors throughout the year.
The Chairman meets regularly with institutional investors to hear their views and discuss issues of mutual importance.
The Chairman of the Remuneration Committee meets with major shareholders to discuss executive remuneration policy. All Non-Executive Directors, including new appointees, are available to meet with major shareholders if requested.
The company’s website www.gsk.com, gives access to current financial and business information about the Group.
Share buy-back programmeIn October 2002, following the completionA total of the £4£6.5 billion share buy-back programme announced in 2001,has been spent by the company announced planson buying its own shares for a new £4cancellation or to be held as Treasury shares, of which £1 billion share buy-back programme.
was spent in 2005. The programme covers purchases by the company of shares for cancellation or to be held as Treasury shares, in accordance with the authority given by shareholders at the Annual General Meetingscompany’s AGM in 2001 and 2002.2005.
In total £2.2 billion was spent during 2002. In May 20022005, the company was authorised to purchase a maximum of 617586.4 million shares. During 2005, 72.8 million shares, (623 million shares in May 2001) and 156 million sharesrepresenting 1.2% of the issued share capital, were purchased for cancellation during 2002; details are given inand held as Treasury shares (see Note 2731 to the Financialfinancial statements, ‘Share capital and share premium account’).
The exact amount and timing of future purchases, and the extent to which repurchased shares will be held as Treasury shares rather than being cancelled, will be determined by the company and is dependent on market conditions and other factors.
Donations to Political Organisations and EU Political Expenditure
At the Annual General MeetingsAGM in May 2001, and 2002, shareholders first authorised the company to make donations to EU Political Organisations and to incur EU Political Expenditure, under the provisions of the Political Parties, Elections and Referendums Act 2000, of up to £100,000 each year. This authority has since been renewed annually. Although the company does not make and does not intend to make such payments or donations to political parties, within the normal meaning of that expression, the definition in the legislation of ’EU Political Organisation’ is wide. It may extend to bodies, which the company and its subsidiaries might wish to support including those concerned with policy review, law reform, the representation of the business community and special interest groups, such as those concerned with the environment. No donations were made to EU Political Organisations during 2005. The Group made donations to non-EU Political Organisations totalling £554,000£320,000 during 2002. No donations2005 (£291,000 in 2004).
Donations of £301,000 were made in the USA and £19,000 in Canada. The USA is the largest recipient of political donations, and this reflects the US political system, where candidates are sponsored solely by donations from individuals, NGOs, companies and other parties.
In line with US law, the corporate donations by GSK are not made at a federal level, but only to EUcandidates and political parties at the state and local levels. Donations are accepted practice in the USA, and as a major employer in a heavily regulated industry, it is important for GSK to engage fully in the political process. Donations are one of the ways of doing this. GSK supports those candidates who seek an environment that appropriately rewards high-risk, high-investment industries and who believe in free market principles and intellectual property rights.
The situation is similar in Canada, and donations follow the same guidelines. In the rest of the world donations are very rare and of low value.
There is also a GSK Political Organisations.Action Committee (PAC) in the USA which gives political donations. PAC’s are employee organisations which allow employees to contribute to a fund for political donations. Employees decide upon the recipients of the PAC donations. In 2005, a total of £282,000 was donated to political organisations by the GSK PAC.
Annual General Meeting
The Annual General MeetingAGM will be held at 2.30pm on Monday, 19thWednesday, 17th May 20032006 at The Queen Elizabeth II Conference Centre, Broad Sanctuary, Westminster, London SW1P 3EE.
DirectorsSir Christopher Hogg, Dr Garnier, Sir Roger Hurn, Mr Coombe, Sir Peter Job, Mr McArthur, Mr McHenry, Sir Ian Prosser, Dr Schmitz and Dr Shapiro The business to be transacted at the meeting will each retire and offer themselves for re-election to the Board under article 93 of the company's Articles of Association. Biographical details for each of them are given under 'The Board' (page 32).
Remuneration reportThe Remuneration report on pages 39 to 50 sets out the remuneration policies operated by GlaxoSmithKline and disclosures on Directors’ remuneration including those required by the Companies Act 1985, Schedule 7A, of the Directors’ Remuneration Report Regulations 2002. A resolution will be proposed to approve the Remuneration report.
AuditorsFollowing the transfer of PricewaterhouseCoopers’ business into a limited liability partnership, PricewaterhouseCoopers LLP, on 1st January 2003, PricewaterhouseCoopers resigned as auditors of the company. The Audit Committee proposed, and the Board subsequently appointed, PricewaterhouseCoopers LLP as auditors of the company to fill the casual vacancy created by the resignation.
Resolutions will be proposed to re-appoint PricewaterhouseCoopers LLP as auditors and to authorise the Audit Committee to determine their remuneration.
Special businessThe company will seek to renew its authority to:include:
| • | Receiving and adopting GlaxoSmithKline's 2005 Annual Report |
| • | Approving the 2005 Remuneration Report |
| The Remuneration Report on pages 37 to 54 sets out the remuneration policies operated by GlaxoSmithKline and disclosures on Directors’ remuneration, including those required by the Companies Act 1985 and the Directors’ Remuneration Report Regulations 2002. A resolution will be proposed to approve the Remuneration Report. |
| GSK Annual Report 2005 |
| 32 |
| REPORT OF THE DIRECTORS |
| Corporate governance |
| continued |
| • | Retirement, election and re-election of Directors | |
| Dr Slaoui and Mr de Swaan have been appointed Directors since the 2005 AGM and will offer themselves for election to the Board. Mr Culp, Sir Crispin Davis and Dr Schmitz will retire and offer themselves for re-election to the Board under article 93 of the company’s Articles of Association. Dr Shapiro will retire at the conclusion of the AGM and will not offer herself for re-election. | ||
| • | Re-appointment and remuneration of Auditors | |
| Resolutions will be proposed to re-appoint PricewaterhouseCoopers LLP as auditors and to authorise the Audit Committee to determine their remuneration. | ||
| • | Special business | |
| The company will seek authority to: | ||
| • | make donations to EU Political Organisations and incur EU Political Expenditure | |
| • | allot Ordinary Shares in the company | |
| • | give the Directors authority to | |
Accountability, audit and internalInternal control framework
The Board recognises its responsibility to present a balanced and understandable assessment of the Group’s position and prospects. The structure of accountability and audit operated in GlaxoSmithKlineGSK is as follows:follows.
Audit Committee and the Board
The Audit Committee of the Board has accountability for reviewing and approving the adequacy and effectiveness of internal controls operated by the Group, including financial, operational and compliance controls and risk management. The Board has delegated responsibility for reviewing, on behalf of the Board, the effectiveness of the system of internal control, management of risks, the external and internal audit process, and the process for monitoring compliance with laws, regulations, and ethical codes of practice. An internal control framework has been in operation for the whole of the year undersuch review and continues to operate up to the date of approval of this report.
The Audit Committee receives regular reports on areas of significant risk to the Group and on related internal controls. Following consideration of these reports, the Committee reports annually to the Board on the effectiveness of controls. Such controls may mitigate but cannot eliminate risks. In addition, there are areas of the Group’s business where it is necessary to take risks to achieve a satisfactory return for shareholders. In these cases it is the company’s objective to apply its expertise in the prudent management rather than elimination of risk. The Directors’ review relates to the company and its subsidiaries and does not extend to material associated undertakings, joint ventures or other investments.
Having considered the Audit Committee, which receives reports from those individuals identified in the Committee’s Report on pages 34 and 35. It is the effectivenessresponsibility of controls,management, through the CET, to implement Board believes that the system of internal controls provides reasonable although not absolute assurance against material misstatement or loss. The process accords with the guidance on internal control issued by the Turnbull Committee in 1999.
The Audit Committee also keeps under review the scope and results of the external audit and the independence and objectivity of the external auditors. The Committee reviewed the nature and extent of non-audit services the external auditors provided during 2002 to ensure that the services were not so significant as to call into question the auditors’ independence from the Group. With effect from 1st January 2003 the Committee will pre-approve all non-audit services to be provided by the external auditors.
The Corporate Social Responsibility Committee of the Board reviews, amongst other matters, external issues that have the potential for serious impact upon the Group’s business and reputation and as such forms part of the internal control framework.
Management structureThe Board has overall responsibility for ensuring that the Group is appropriately managed and achieves the strategic objectives agreed by the Board. To enable it to exercise this responsibility, the Board requires from management information concerning the business, including relevant informationpolicies on risk exposures, internal controls and external developments.control. The CEO reports to the Board andCET is responsible for identifying, approving, monitoring and enforcing key policies that go to the managementheart of how the Group. To assist him in this task, the CEO has established the CET, which is not a Committee of the Board. Key functional activities and management sectors are represented on the CET.
Group conducts business. The internal control framework includes central direction, resource allocation and risk management of the key activities of research and development, manufacturing, marketing and sales, legal, human resources, information systems and financial practice. As part of this framework, there is a comprehensive planning system with an annual budget approved by the Board. The results of operating units are reported monthly and compared to the budget. Forecasts are prepared regularly during the year.
Extensive financial controls, procedures, self-assessment exercises and risk mitigation activities are reviewed by the Group’s internal auditors. Commercial and financial responsibility, however, is clearly delegated to local business units, supported by a regional management structure. These principles are designed to provide an environment of central leadership coupled with local operating autonomy as the framework for the exercise of accountability and control within the Group.
The Group also attaches importance to clear principles and procedures designed to achieve appropriate accountability and control. A corporateGroup policy, ‘Risk Management and Legal Compliance’, mandates that business units establish processes for managing and monitoring risks significant to their businesses and the Group.
The internal control framework also relies on the following for overseeing and reporting risk and compliance issues.
Risk Oversight and Compliance Council (ROCC)
The ROCC is a council of senior executives authorised by the Board to assist the Audit Committee oversee the risk management and internal control activities of the Group. Membership comprises several CET members and some of the heads of departments with internal control, risk management, audit and compliance responsibilities.
The ROCC meets on a regular basis to review and assess significant risks and their mitigation plans. The ROCC, responding to the Group policy referred to above, has provided the business units with a framework for risk management and upward reporting of significant risks. Mitigation planning and identification of a manager with overall responsibility for management of any given risk is a requirement.
Risk Management and Compliance Boards (RMCBs)
Risk Management and Compliance Boards (RMCBs) have been established in each of the major business units. Membership often comprises members of the senior executive team of the respective business unit, augmented by specialists where appropriate. The RMCBs oversee management of all risks that are considered important for their respective business units, including those risks that are designated as significant to GlaxoSmithKline as a whole, thus increasing the number of risks that are actively managed across the Group.
Each RMCB regularly reports the status regarding its significant risks to the ROCC.
Compliance functions
In a number of risk areas, specific standards that meet or exceed requirements of applicable law have been established. Specialist audit and compliance groupsfunctions (for example Corporate Environment, Health and& Safety, Global Quality Assurance and Worldwide Regulatory Compliance) assist in the dissemination, implementation and implementation of and carry out auditsaudit of these standards.
Risk Oversight andCorporate Ethics & Compliance Council (ROCC)(CEC)
The ROCC is a council of senior executives authorised by the Board to oversee the risk management and internal control activities of the Group and to ensure that business units have designated managers to manage significant risks. Membership comprises several members of the CET and the heads of departments with internal control, risk management, audit, or compliance responsibilities. The ROCC’s responsibilities also include ensuring that regular analysis is carried out to identify gaps in internal controls and providing reports to the CET and Audit Committee in addition to the separate reports provided by individual internal control, audit, and compliance departments. A direct reporting line to the Audit Committee provides a mechanism for bypassing the executive management if irregularities are ever identified.
The internal control framework relies on the ROCC, as well as sector and other business unit Risk Management and Compliance Boards (RMCBs), to help identify risks and to provide guidance to the risk management and compliance initiatives at the corporate and business unit levels. The ROCC meets regularly to review and assess significant risks and mitigation plans directed against those risks. The ROCC has developed the corporate policy referred to above and provided the business units with a framework for risk management and for reporting risks to management and the ROCC. Mitigation planning and identification of a manager with overall responsibility for management of any given risk is a requirement. While the ROCC oversees many of the risks deemed significant to GlaxoSmithKline, each RMCB oversees risks important to its business or function, thus increasing the number of risks that are actively managed across the Group. The ROCC is supported by the Corporate Ethics & Compliance department.
Corporate Ethics & ComplianceThe Corporate Ethics & Compliance department which is responsible for supporting the development and implementation of practices that facilitate employees’ compliance with laws and Group policy.
The thrust of the Group’s compliance effort is due diligence in preventing and detecting misconduct and non-compliance with law or regulation by promoting ethical behaviour, compliance with all laws and regulations, corporate responsibility at all levels and effective compliance systems.
The CEC is managed by the Corporate Compliance officers supportOfficer, who reports directly to the Group’s main operating sectors of R&D, Manufacturing, Pharmaceuticals, and Consumer Healthcare.CEO. The Corporate Compliance Officer chairs the ROCC coordinates some of the risk management activities among the various compliance and audit functions across the Group, and provides summary reports on the ROCC’s activities and the Group’s significant risks to the CET and the Audit Committee on a regular basis. The Corporate Compliance Officer’s direct reporting line to the Audit Committee provides a mechanism for bypassing the executive management should the need ever arise.
Areas of potentially significant risks
Areas of potentially significant risk that are subject to regular reporting to and by the ROCC include the following. Further
For details of the risks affecting the Group, may be found insee Note 3041 to the Financialfinancial statements, ‘Legal proceedings’ and in ‘Risk factors’ on pages 64 and 65.71 to 74.
| GSK Annual Report 2005 |
| 33 |
| REPORT OF THE DIRECTORS |
| Corporate governance |
| continued |
Human resourcesEffectiveness of controls
The legal requirements regarding discriminationinternal control framework has been in operation for the whole of the year under review and harassment,continues to operate up to the date of approval of this report. The system of internal controls is designed to manage rather than eliminate the risk of not achieving business objectives, and can only provide reasonable and not absolute assurance against material misstatement or loss.
The Audit Committee receives reports on areas of significant risk to the Group and on related internal controls. Following consideration of these reports, the Audit Committee reports annually to the Board on the effectiveness of controls. Such controls may mitigate but cannot eliminate risks. In addition, there are areas of the Group’s business where it is necessary to take risks to achieve a satisfactory return for shareholders, such as investment in R&D and in acquiring new products or businesses. In these cases, it is the Group’s objective to apply its expertise in the prudent management rather than elimination of risk. The Directors’ review relates to the company and its subsidiaries and does not extend to material associated undertakings, joint ventures or other investments.
The Board, through the Audit Committee, has reviewed the assessment of risks and the internal control framework that operates in GlaxoSmithKline and has considered the effectiveness of the system of internal control in operation in the Group for the year covered by this report and up to the date of its approval by the Board. The process followed by the Board in reviewing the system of internal controls accords with the guidance on internal control issued by the Turnbull Committee in 1999.
Committee reports
Audit Committee Report
The Audit Committee’s role flows directly from the Board’s oversight function and it is authorised by the Board to investigate any activity within its terms of reference. The Committee has written terms of reference which have been approved by the Board. The Committee reports regularly to the Board on the performance of the activities it has been assigned. The Committee’s main responsibilities include reviewing the corporate accounting and financial reporting process, monitoring the integrity of the workforce, including pre-employment screening,financial statements, evaluating the system of internal control and the controlmanagement of risks, overseeing activities of each of the Group’s compliance audit functions and useoverseeing compliance with laws, regulations and ethical codes of contractorspractice. The Committee’s oversight role requires it to address regularly the relationships between management and temporary staffthe internal and external auditors, and understand and monitor the reporting relationships and tiers of accountability between them. The Committee receives regular reports from members of the CET and senior managers covering the key compliance activities of the Group, including those concerning R&D, manufacturing, sales and marketing and EHS.
Committee members bring considerable financial and accounting experience to the Committee’s work. Members have past employment experience in either finance or accounting roles or comparable experience in corporate activities.
In respect of 2005, the Board had determined that the combined qualifications and experience of the Committee members, when taken together with its modus operandi, gave the Committee collectively the financial expertise necessary to discharge its responsibilities.
Accordingly, the Board chose not to nominate any one committee member as having recent and relevant financial experience as defined by the Combined Code, or as an Audit Committee Financial Expert as defined by Sarbanes-Oxley.
In arriving at its conclusion, the Board considered the following points. Dr Schmitz has been the Chairman of the Committee since April 2001. Prior to his appointment as a Non-Executive Director of the company, he was a Non-Executive Director of Glaxo Wellcome plc, where he served on the Audit Committee. Dr Schmitz has also been a member of the Executive Board of Directors of Deutsche Bank AG. He retired from that Board in 2000 having been in charge of investment banking. Dr Schmitz was formerly a member of the Executive Board of Directors of BASF from 1980 to 1990, including CFO from 1985 to 1990. He holds an MBA from Insead. Sir Ian Prosser was CFO and later CEO of Bass PLC and is a member of the Institute of Chartered Accountants in England and Wales. Sir Robert Wilson began his professional career as an economist. He is Chairman of BG Group plc. He held senior management positions at Rio Tinto plc culminating in his appointment as Executive Chairman, from which he retired in 2003.
Sir Deryck Maughan was appointed a member of the Committee on 21st January 2005. He is Managing Director of Kohlberg Kravis Roberts & Co (KKR) and Chairman of KKR Asia. He was Chairman and CEO of Citigroup International and Vice Chairman of Citigroup Inc. Prior to the creation of Citigroup, he was Chairman and Co-Chief Executive Officer of Salomon Smith Barney. He was also Chairman and Chief Executive Officer of Salomon Brothers.
When appointing Mr de Swaan to the Committee with effect from 1st January 2006, the Board determined that he had recent and relevant financial experience in accordance with the Combined Code. In coming to this conclusion, the Board paid particular attention to Mr de Swaan’s role as Chief Financial Officer of ABN AMRO, from which he retired on 31st December 2005. The Board also considers Mr de Swaan to be an Audit Committee Financial Expert as defined by Sarbanes-Oxley.
The Committee is supported by the Company Secretary, who attends the Committee’s meetings, and it has available to it financial resources to take independent professional advice when considered necessary. Meetings of the Committee are risks inherent inattended by the Chairman, CEO, CFO, General Counsel, Head of Global Internal Audit (GIA), Corporate Compliance Officer and the external auditors.
In 2005, the Committee worked to a Groupstructured programme of activities, with over 100,000 employees.standing items that the Committee is required to consider at each meeting together with other matters focused to coincide with key events of the annual financial reporting cycle:
| • | the external auditors reported to the Committee on all critical accounting policies and practices used by the company, alternative accounting treatments which had been discussed with management and the resultant conclusion by the external auditors, material written communications with management and any restrictions on access to information |
| • | the CFO reported on the financial performance of the company and on technical financial and accounting matters |
| • | the General Counsel reported on material litigation |
| • | the Company Secretary reported on corporate governance |
| GSK Annual Report 2005 |
| 34 |
| Corporate governance |
| continued |
| • | the Heads of each of the Group’s compliance and audit groups reported on their audit scope, annual coverage, audit resources and on the results of audits conducted throughout the year |
| • | the Corporate Compliance Officer reported on the activities undertaken by the ROCC |
| • | the Company Secretary, as Chairman of the Disclosure Committee, reported on matters that affected the quality and timely disclosure of financial and other material information to the Board, to the public markets and to shareholders. This enabled the Committee to review the clarity and completeness of the disclosures in the published annual financial statements, interim reports, quarterly and preliminary results announcements and other formal announcements relating to financial performance prior to their release by the Board. |
Research and developmentSafety of marketed products is a potentially significant risk and a matter of great concern to GlaxoSmithKline, as is the conduct of laboratory and clinical practices in R&D. These must be in accordance with applicable laws and regulations as well as with corporate standards that may exceed such requirements. All pharmaceutical products bring with them benefits and risks, including potential side effects. Pre-clinical and clinical trials are conducted during the development of potential products to determine the safety and efficacy of products for use by humans following approval by regulatory bodies. In spite of these efforts, when drugs are introduced into the marketplace, unanticipated side effects may become evident. The Group views the use of animals and human tissue in the testing required to develop new products as another risk.
Marketing and salesThe Group operates globally in complex legal and regulatory environments that often vary among jurisdictions. The Group’s policy is to conduct marketing in accordance with applicable laws and regulations as well as with corporate standards that may exceed such requirements. Any failure to observe applicable marketing codes, rules regarding government pricing,Audit Committee, management, of samples, and legal restrictions on sale and marketing practices may create significant risks to the commercial sectorsinternal auditors and the Group. Failurefull Board work together to comply may result in legal proceedings.
Legalensure the quality of the company’s corporate accounting and intellectual propertyProduct liability, intellectual property, antitrust and government investigations, and related private litigation are potential risks to GlaxoSmithKline,financial reporting. The Committee serves as the primary link between the Board and the Group is involved in various legalexternal and administrative proceedings in these areas. internal auditors. This facilitates the necessary independence from management and encourages the external and internal auditors to communicate freely and regularly with the Committee. In 2005, the Committee met both collectively and separately with the external auditors and the Head of GIA, without members of management being present.
The outcome of these proceedings cannot be predicted with any level of certainty.
There is alsoCommittee has primary responsibility for making a potential risk that third parties may allege thatrecommendation to shareholders on the marketingappointment, reappointment and removal of the Group’s own products will infringeexternal auditors by annually assessing the intellectual property rightsqualifications, expertise, resources and independence of those third parties.the external auditors and the effectiveness of the audit process.
FinanceThereIn making its assessment, the Committee considers papers which detail the relevant regulatory requirements relating to external auditors and evaluates reports from the external auditors on their compliance with the requirements. Where the external auditors provide non-audit services, the Committee ensures that auditor objectivity and independence are potential risks surroundingsafeguarded by a policy requiring pre-approval by the Group’s ability to forecast the futureAudit Committee for such services. Expenditure on audit and thus uncertainty about its ability to meet financial targetsnon-audit services is set out on pages 95 and 96.
The guidelines set out in its budgeting process. The Group investsthe company’s policy on engaging the external auditors to provide non-audit services include ascertaining that: the skills and experience of the external auditors make them a suitable supplier of the non-audit services; adequate safeguards are in new productsplace so that the objectivity and ventures based on assumptions aboutindependence of the success of those efforts that may prove to be inaccurate. In addition, thereaudit are potential risks around the Group’s treasury operations including tax liabilities, transfer pricing,not compromised; and the possibility of trading losses and counterparty fraud. Compliance with evolving financial disclosures and other legal reporting requirements constitute risks. The Group’s pension liabilities represent a further area of potential risk. Further discussion may be found in Note 33fee levels relative to the Financial statements, ‘Employee costs’.annual audit fee are within the limits set by the Committee.
The company also has well-established policies, including a Code of Ethics, which is available on its website, and a help-line facility for the reporting and investigation of unlawful conduct. No waivers to the Code were made in 2005.
The Committee met in full session five times in 2005 and five times on a quorate basis. Each full session was attended by all members except Sir Robert Wilson, who was unable to attend one meeting.
Nominations Committee ReportManufacturingMaintaining supplyThe Nominations Committee’s terms of key GlaxoSmithKline products isreference include responsibility for proposing the appointment of Board and Committee members. During 2005, the Committee made recommendations to the Board on the appointment of Mr de Swaan as a potentially significant risk. The Group’s policy is to take reasonable measures to ensure uninterrupted supply of product, including manufacturing in accordance with applicable laws and regulations as well as with corporate standards that may exceed such requirements. The Group takes efforts to minimise the single sourcing of key products. Rationalising the supply chain and balancing manufacturing capacity present other risks that could potentially disrupt the supply of important products.Non-Executive Director.
Information technologyProtecting information technology assets is an increasing riskThe Committee also recommended to the Board the appointment of Sir Deryck Maughan to the Audit Committee in January 2005 and Dr Schmitz to the Remuneration Committee in May 2005. In February 2006, the Committee recommended to the Board that Dr Moncef Slaoui, succeed Dr Yamada as businesses extend networks, systemsChairman, Research & Development on his retirement from the company on 1st June 2006.
In addition, the Committee recommended to the Board that Dr Schmitz should serve a further term of three years as a Non-Executive Director and datathat he should remain Chairman of the Audit Committee until September 2006. The Committee also made a recommendation to third parties,the Board that Dr Ralph Horwitz be appointed a Non-Executive Director. Following the announcement of Dr Horwitz’s appointment, a potential conflict of interest was disclosed, and Dr Horwitz decided not to take up his appointment as dependencya Non-Executive Director of the company.
When recruiting Non-Executive Directors, the Committee considers the particular skills, knowledge and experience that would benefit the Board most significantly for each appointment. Broad selection criteria are used which focus on achieving a balance between the Internet for communications increases. Ensuring proper systems validationrepresentation of European, UK and electronic recordsUS markets, and signatureshaving individuals with CEO experience and skills developed in various sectors and specialities. During 2005, particular focus was placed upon recruiting a new Non-Executive Director with recent and relevant financial expertise, to join the Audit Committee. Professional search agencies are key regulatory issues and mattersengaged specialising in the recruitment of high calibre Non-Executive Directors. Dossiers of potential risknon-executive appointees are provided to the Committee and candidates are short-listed for interview after considering their relevant qualifications.
A customised induction process is conducted for each of the new Non-Executive Directors focusing on their particular experience and taking account of their different backgrounds. This process includes meeting members of the CET and other senior executives and visiting particular operational facilities of the Group.
The Committee continued to keep under review the succession planning for senior executive positions, including that of the CEO and Chairman, Research & Development.
When appointing new Executive Directors, the Committee considers the skills, knowledge and experience required for the Group. Web systems accessibleparticular executive position. The Committee will consider potential external and internal candidates before recommending to the public must comply with legalBoard to approve the new appointment. All new Directors offer themselves for election at the company’s next AGM. Their appointments are announced publicly.
The Committee met once during 2005 in full session and regulatory requirements and represent potential risks. Other potential risks include use of personally identifiable information, electronic record retention, outsourced business applications, and susceptibilitytwice on a quorate basis. All members were present at the full meeting.
Remuneration Report
The Remuneration Report can be found on pages 37 to viruses and outside incursions. With much of the Group’s business dependent upon electronic means, disaster recovery also poses a potential risk.54.
Security, environment and safetyThreats to the security and well being of our employees, property and the environment present significant risks for which appropriate safeguards and precautions are continually reviewed and upgraded. Employee injury, changes in health due to occupational conditions and plant management and the potential impact of plants on the environment are potential risks the Group addresses through a process that sets targets and provides guidance on how results may be achieved.
TheThroughout 2005, the company seeks to uphold, and to report on compliance with, best practice in corporate governance. The Board is reviewing the recommendations from Derek Higgs’ ‘Review of the role and effectiveness of non-executive directors’ and Sir Robert Smith’s Report on ‘Audit Committees, Combined Code Guidance’ and intends to ensure that the Group will continue to complycomplied with the Listing Rule requirement in relation to ‘The Combined Code – Principles of Good Governance and Code of Best Practice’ (the Combined Code) which is issued by the UK Listing Authority. The Combined Code comprises recommendations as to best practice in terms of the control and reporting functions of the board of a company. The Combined Code sets out principles under the headings of:
Specifically the provisions require directors to report in the Annual Report on:
ComplianceThe Directors’ report on compliance with the Combined Code and other corporate governance requirements, and their reports in accordance with the provisions of the Combined Code, are set out under ‘Directors’ statementsexcept as follows:
| GSK Annual Report 2005 |
| 35 |
| Corporate governance |
| continued |
| • | C.3.1 – The Board should satisfy itself that at least one member of the Audit Committee has recent and relevant financial experience. The company’s position is explained on page 34. See page 34 for the position from 1st January 2006. |
| • | |
| D.2.3 – The Chairman should arrange for the Chairmen of the Audit, Remuneration and Nominations Committees to be available to answer questions at the AGM and for all Directors to attend. The company’s position is explained on pages 31 and 32. |
US law and regulation
A number of provisions of US law and regulation apply to GSK because the company’s shares are quoted on the New York Stock Exchange (NYSE) in the form of ADSs.
NYSE rulesThe In general, the NYSE rules permit the company to follow UK corporate governance practices instead of those applied in the USA, provided that the company explains any significant variations. This explanation is on the company’s website. NYSE rules that came into effect in 2005 require the company to file annual and interim written affirmations concerning the Audit Committee and the company’s statement on significant differences in corporate governance.
Sarbanes-Oxley Act of 2002
Following a number of corporate and accounting scandals in the USA, Congress passed the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley) which took effect on 30th July 2002.. Sarbanes-Oxley establishesestablished new or enhanced standards for corporate accountability for companies listed in the USA. A number of provisions of Sarbanes-Oxley apply to GlaxoSmithKline because the company is quoted on the New York Stock Exchange in the form of ADSs. Although the company’s corporate governance structure iswas believed to be robust and in line with best practice, certain changes were necessary to ensure compliance with Sarbanes-Oxley.
As recommended by the Securities and Exchange Commission (SEC), GlaxoSmithKlineGSK has established a Disclosure Committee. The Committee reports to the CEO, the CFO and to the Audit Committee. It is chaired by the Company Secretary and the members consist of senior managers from finance, legal, compliance, and publiccorporate communications and investor relations.
External legal counsel and the external auditors are invited to attend its meetings periodically. It has responsibility for considering the materiality of information and, on a timely basis, determination ofdetermining the disclosure and treatment of materialthat information. The Committee alsoIt has responsibility for the timely filing of reports with the SEC and the formal review of the contents of GlaxoSmithKline’s Annual Report onand Form 20-F. In 2005, the Committee met eleven times.
Sarbanes-Oxley requires that the Annual Report contains a statement as to whether a member of the company’s Audit Committee is an audit committee financial expert.
For an explanation and details of the basis for the Board’s judgement on this matter, refer to page 34.
For accounting periods ending on or after 15th July 2006, Sarbanes-Oxley requires that the company’s Form 20-F contain a report stating the responsibility of management for establishing and maintaining adequate internal control over financial reporting and assessing the effectiveness of the company’s internal control over financial reporting.
Although the company is not required to report compliance in its 2005 Form 20-F, management has undertaken a process to ensure that it will be in a position to report compliance by the due date.
Sarbanes-Oxley also introduced a requirement for the CEO and the CFO to complete formal certifications, confirming that:
| |
| • | they have each reviewed the Annual Report |
| • | based on their knowledge, it contains no material misstatements or omissions |
| • | based on their knowledge, the |
| • | they are responsible for establishing and maintaining disclosure controls and procedures that ensure that material information is made known to them, |
| • | they have disclosed, based on their most recent evaluation of internal control over financial reporting, to the external auditors and the Audit Committee all significant deficiencies and material weaknesses in the design or operation of internal |
The CEO and CFO have completed these certifications, which will be filed with the SEC in the USA as part of the Group’s Form 20-F.
Evaluation of disclosure controlsControls and proceduresWithin the 90 days prior to the date of this report, theThe Group carried out an evaluation under the supervision and with the participation, of the Group’s management, including the CEO and CFO, of the effectiveness of the design and operation of the Group’s disclosure controls and procedures.procedures as at 31st December 2005. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures.
Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon and as of the date of the Group’s evaluation, the CEO and CFO have concluded that, as at 31st December 2005, the disclosure controls and procedures arewere effective in all material respects to ensureprovide reasonable assurance that information required to be disclosed in the reports the Group files and submits under the US Securities Exchange Act of 1934, as amended, is recorded, processed, summarised and reported as and when required.required and that it is accumulated and communicated to management, including the CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
Related party transactions
GlaxoSmithKline held a 23 per cent interestThere have been no changes in Quest Diagnostics Inc. throughout 2002. This holding was reduced to 21 per cent in February 2003 following Quest’s acquisition of Unilab Corporation. In December 2002 GlaxoSmithKline and Quest amended the terms of their Global Trials Agreement, which is a long-term contractual relationship under which Quest is the primary provider of clinical laboratory testing to support the Group’s clinical trials testing requirements worldwide.
In February 2002, Mr Ingram, then a member ofinternal control over financial reporting during 2005 that have materially affected, or are reasonably likely to affect materially, the CET, purchased a portion of land being part of a residential property owned by the Group that was adjacent to Mr Ingram’s own residence. The total sale price was $16,500 based on an independent valuation of the land. The Group subsequently determined that retention of the residential property no longer served its business needs and listed the property for sale. An independent valuation of the property on 3rd June 2002 valued it at $1,050,000 and the property was offered for sale through an estate agent. Mr Ingram made the highest offer for the property and purchased it from the Group for total consideration of $1,070,000.
Documents on display
Documents referred to in this Annual Report are available for inspection at the Registered Office of the company.Group’s internal control over financial reporting.
| GSK Annual Report 2005 |
| 36 |
| REPORT OF THE DIRECTORS | |
| Remuneration Report |
2002The Remuneration Report sets out the remuneration policies operated by GSK in respect of the Directors and Corporate Executive Team (CET) members, together with disclosures on Directors’ remuneration reportincluding those required by The Directors’ Remuneration Report Regulations 2002 (the Regulations). In accordance with the Regulations, the following sections of the Remuneration Report are subject to audit: Annual remuneration; Non-Executive Directors’ remuneration; Share options; Incentive plans; performance criteria on Performance Share Plans and share options; and Pensions. The remaining sections are not subject to audit nor are the pages referred to from within the audited sections.
This reportReport is submitted to shareholders by the Board for approval at the Annual General Meeting, as referenced in the Chairman’s letter and notice of Annual General Meeting, which has been sent to all shareholders.Meeting.
This report describesThroughout the background to and outlinesRemuneration Report the Group’s remuneration policy. It also gives required information about the earnings ofExecutive Directors and senior management in 2002,CET members are referred to as well as about Directors’ interests in the shares of GlaxoSmithKline and in contracts.‘Executives’.
References to GlaxoSmithKline shares and ADSs mean, respectively, Ordinary Shares of GlaxoSmithKline plc of 25p and American Depositary sharesDepository Shares of GlaxoSmithKline plc. Each ADS represents two GlaxoSmithKline shares.
| Introduction | 38 |
| Remuneration policy | 38 |
| Executive Director terms, conditions and remuneration | 42 |
| Non-Executive Director terms, conditions and fees | 44 |
| Directors and Senior Management remuneration | 44 |
| Annual remuneration | 45 |
| Non-Executive Directors’ remuneration | 46 |
| Directors’ interests | 48 |
| Share options | 49 |
| Incentive plans | 51 |
| Pensions | 53 |
| Directors and Senior Management | 54 |
| Directors’ interests in contracts | 54 |
| GSK Annual Report 2005 |
| 37 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
The Remuneration CommitteeIntroductionIn reviewing governance arrangements, the Board decided during the year to separate the roles of the former Remuneration and Nominations (R&N) Committee in order to give a separate individual Board focus to both functions. Accordingly a Remuneration Committee, with terms of reference revised to take into account latest governance standards, assumed the remuneration responsibilities of the previous R&N Committee in October 2002. The members of the Remuneration Committee, and previously the R&N Committee, are and have been at all times when the matters relating to the directors’ remuneration for 2002 have been considered: Mr Allaire (Chairman), Dr Barzach, Sir Roger Hurn, Mr McArthur, Mr McHenry and Mr Young until his retirement. Mr Allaire succeeded Mr Young as chairman of the R&N Committee on 20th May 2002. Sir Peter Job was appointed a member of the Committee on 7th February 2003. The Chairman and the Chief Executive Officer (CEO) are invited to attend the Committee’s meetings except when their own remuneration is being considered. The Board will review the current terms of reference of all its committees in the light of the Higgs’ Review of the role and effectiveness of non-executive directors and consequent changes to the Combined Code. Any material changes to the Remuneration Committee’s terms of reference will be reported in next year’s report.
Remit of the Remuneration Committee
The Remuneration Committee considers and regularly reviews the Group’s policy on Executive remuneration(or ‘Committee’) is responsible for approval bymaking recommendations to the Board on the company’s remuneration policy and, determineswithin the terms of the agreed policy, determining the total individual remuneration packages of the members of the Corporate Executive Team.Executives.
The remuneration policy set byout in this Report was finalised after undertaking an extensive consultation process with shareholders and institutional bodies during the course of 2003 and 2004.
The Chairman of the Remuneration Committee shapescontinues to have regular dialogue with institutional investors regarding GSK’s remuneration at other levels within the Group affecting the arrangements for the management population as a whole (approximately 11,000 individuals, representing over 10 per cent of the employee population).policy.
Towers Perrin, a leading firm of remuneration and benefits consultants, provides strategic advice to GlaxoSmithKline on general remuneration and benefits planning and also provides market data. Towers Perrin also advises the Remuneration Committee, under a separate mandate, with regard to the remuneration of senior executive management and the Non-Executive Directors. Changes to the remuneration of the Non-Executive Directors are determined by the GlaxoSmithKline Board itself on advice from the Remuneration Committee.
In 2003, the Remuneration Committee engaged Deloitte & Touche to conduct an additional independent review of GlaxoSmithKline’s current remuneration policy including its competitiveness,is designed to establish a framework for remuneration that is consistent with the company’s scale and to advisescope of operations, meets the Committee as to the shaping and development of future remuneration policy in the context of GlaxoSmithKline’s corporate base in the UK and the competitiverecruitment needs of the business from a global point of view.
BackgroundGlaxoSmithKlineand is one ofclosely aligned with UK shareholder guidelines. As at 31st December 2005, the world’s leading research-based pharmaceutical and healthcare companies. As such, it has to be global in outlook and operations. The Group employs over 100,000 people in over 100 countries. Over 90 per cent of its sales are generated outsidecompany was the UK.
The USA is thesecond largest pharmaceutical marketcompany in the world by revenue, with operations on five continents with products sold in over 130 countries and is fundamental to GlaxoSmithKline’s success and profitability. More than 50 per centwith around 50% of its pharmaceutical sales arebeing generated in the USA. The Chief Executive Officer is based there, along with another eight
Remuneration Committee
Sir Robert Wilson has been Chairman of the thirteen person Corporate Executive Team (CET).Committee since 17th May 2004. Sir Crispin Davis and Mr Culp were members of the Committee throughout 2005. Dr Schmitz was appointed to the Committee in May 2005. The CET has considerable global experienceBoard deemed all of the members of the Committee to be independent Non-Executive Directors in accordance with over half the group having worked outside their home country.Combined Code.
The qualityCommittee met five times during 2005 with each member attending as follows:
| Name | Number of meetings held whilst a Committee member | Number of meetings attended by Committee member | ||
| Sir Robert Wilson | 5 | 5 | ||
| Mr L Culp | 5 | 5 | ||
| Sir Crispin Davis | 5 | 5 | ||
| Dr Ronaldo Schmitz | 4 | 4 | ||
Three quorate meetings were held to approve the formal grant of share options and motivationperformance share awards to give effect to the Committee’s decisions.
With the exception of management matter enormouslythe Company Secretary, no employees of the company were involved in the conduct of Committee meetings. Dr Garnier (CEO) and the Senior Vice President, Human Resources, were invited to attend part of some meetings of the Committee as required.
Deloitte & Touche LLP (Deloitte) have been appointed by the Committee to provide it with independent advice on executive remuneration.
Deloitte provided other consulting services to GSK during the year, but did not provide advice on executive remuneration matters other than to the Committee.
Towers Perrin provides market data and data analysis to the Committee.
Remuneration policy
Principles
The four core principles which underpin the remuneration policy for GlaxoSmithKline are:
| • | securing outstanding executive talent |
| • | pay for performance and only for performance |
| • | robust and transparent governance structures |
| • | a commitment to be a leader of good remuneration practice in the pharmaceutical industry. |
In formulating the policy, the Committee also decided that:
| • | the remuneration structure must support the needs of the business in a very competitive market place |
| • | UK shareholder guidelines will be followed to the maximum extent consistent with the needs of the business and the company would maintain a regular dialogue with shareholders |
| • | global pharmaceutical companies are the primary pay comparator group |
| • | performance conditions would be based on the measurable delivery of strong financial performance and the delivery of superior returns to shareholders as compared with other pharmaceutical companies |
| • | a high proportion of the total remuneration opportunity will be based on performance-related remuneration which will be delivered over the medium to long term |
| • | remuneration would be determined using the projected value method (see ‘Benchmarking’ below) |
| • | there would be one remuneration structure for Executive Directors and the CET with the same performance conditions, applying equally to their long-term incentive awards |
| • | no ex-gratia payments will be made |
| • | pay structures would be as simple as is consistent with the business needs. |
Overall, the policy is intended to provide median total remuneration for median performance. Poor performance will result in total remuneration significantly below the pay comparator group median, with the opportunity to earn upper quartile total remuneration for exceptional performance.
This strong alignment with performance is demonstrably in the interests of shareholders and provides the Executives with unambiguous signals about the importance of delivering success to the company’s shareholders.
Commitment
The Committee will apply this policy on a very largeconsistent and complex company like GlaxoSmithKline. A considerable corporate effort is requiredtransparent basis. Any significant changes in the measures used to recruit, develop and motivate talented people, andassess performance will be discussed with shareholders. In the use of comparators for pay benchmarking, the Committee will use its discretion to ensure that managers at each level haveremuneration levels are reasonable, and if it believes that changes may cause concern amongst shareholders, the necessary breadth of experience, knowledge and leadership skills.
The pharmaceutical industry is international and highly specialised. It is characterised by a handful of global companies which compete as intensely for talent as they do for business. The industry’s top managers and scientists are very much in demand, widely known in the industry and are internationally and corporately mobile. The way all managers and scientists in GlaxoSmithKline are rewarded and developed therefore hasposition will be discussed with shareholders prior to be industry-competitive. It is crucialimplementation.
| GSK Annual Report 2005 |
| 38 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
Pay and performance comparators
The following table sets out the companies used for pay and performance comparison:
| Company | Country | Market Cap 31.12.05 £m | ||
| Abbott Laboratories | USA | 35,561 | ||
| AstraZeneca | UK | |||
| Bristol-Myers Squibb | USA | 26,140 | ||
| Eli Lilly | USA | 37,396 | ||
| GlaxoSmithKline | UK | 85,497 | ||
| Johnson & Johnson | USA | 103,950 | ||
| Merck | USA | 40,440 | ||
| Novartis | Switzerland | 80,419 | ||
| Pfizer | USA | |||
| Roche Holdings | Switzerland | 61,334 | ||
| Sanofi-Aventis | France | 70,997 | ||
| Schering-Plough | USA | 17,915 | ||
| Takeda Pharmaceutical Company | Japan | 27,949 | ||
| Wyeth | USA | 35,952 | ||
At 31st December 2002 the market capitalisation of GlaxoSmithKline was £71,809 million, compared to the average market capitalisation of the group of £51,673 million.
The majority of the competitor panel are US based companies which operate globally. These companies are competing for the same talent and any perceived shortfall in GlaxoSmithKline’s competitive position could lead to a loss of key talent.
GlaxoSmithKline’s remuneration policy was set out at the time of the merger, endorsed by shareholders then, and has made a major contribution to the success of the merger.
Remuneration policyGlaxoSmithKline’s remuneration policy is to pay at industry competitive levels with a heavy emphasis on pay for performance and ‘at risk’ remuneration. The policy is designed to:
The Remuneration Committee believe that both individual and team performance are directly linked to organisational success and are, therefore, critical to GlaxoSmithKline’s future.
Global job evaluation for management level employees is monitored centrally to ensure consistency and the interlinking of performance objectives from top to bottom of the management chain and throughout the Group.
GlaxoSmithKline’s Executive remuneration consists of four components:
The relative importance of the fixed and variable elements of pay for the Executive Directors is illustrated in the table below:
These components areThe merger of Aventis and Sanofi-Synthelabo during 2004 reduced the size of the comparator group to 13 companies and GlaxoSmithKline. The Committee subsequently determined that for a number of reasons, including focus of operation and market capitalisation, there was no other suitable company to add to the group.
Benchmarking
For benchmarking purposes, total remuneration incorporates base salary, annual bonus and long-term incentives. When setting pay, the Committee has due regard to the Executives’ pension arrangements.
The global pharmaceutical industry is used as the primary pay comparator for the Executives, as it is the appropriate marketplace for the company’s most senior executive talent. In the first instance, pay is benchmarked to publicly available remuneration data for these companies.
To provide context to the above information, reference is made to the Towers Perrin annual global pharmaceutical pay survey for the Pharmaceutical Human Resources Association (PHRA). To ensure that the global pharmaceutical industry benchmark is subject to regularscrutiny and review, the Committee also considers pay data from other global businesses primarily in the consumer and the manufacturing sectors.
Prior to make suredetermining the annual long-term incentive opportunity, the Committee considers a range of vesting levels that may be achieved based on different assumptions, such as share price growth, performance levels etc. For performance in line with expectations, total remuneration remains competitiveis targeted at the median of the comparator group and challenging. the long-term incentive opportunity is set in a way which provides for positioning of total remuneration at the median.
To ensure that a stable benchmark is developed and to reduce the impact of short-term fluctuations, incentive policies for other global pharmaceutical companies are assessed over a number of years.
Valuation method
The following sections provide greater detailprojected value method is used to benchmark total remuneration. This method projects the future value of the remuneration package under different performance scenarios, whilst moderating the impact of market fluctuations in the short term and strengthening the focus on performance.
Following the independent review in 2003, the Committee made a deliberate and conscious decision to use the projected value method for pay benchmarking purposes as it enables a comparison of packages with different structural characteristics and provides an insight into the value gearing of different equity instruments.
Individual elements of remuneration
The balance between the fixed (base salary) and variable (annual bonus and long-term incentive) elements of remuneration changes with performance. The chart below shows the anticipated normal range of the mix between fixed and variable pay at different levels of performance for the CEO and the typical case for the other Executive Directors (“ED”). In some years, the ranges may be higher or lower, depending on the performance conditions above.
In relating total remuneration opportunity to that available for comparable roles within the competitor panel, the Committee’s policy is to provide the opportunity to earn total remuneration within a range targeted between the median and 65th percentile of that available among those comparable roles. These opportunities only crystallise where individual, team and corporate performance have met strategic, financial and related business objectives. To provide appropriate incentives for exceptional performance, the Committee’s policy is to provide market referenced opportunities beyond this for truly outstanding performance.
However, the Committee is aware that current levels of long term incentives do not deliver this policy. Independent market data demonstrates that GlaxoSmithKline’s top management remuneration is currently uncompetitive with regard to long-term incentives. As a result their total remuneration opportunity for 2002 was well below the industry median.
The Remuneration Committee will continue to monitor closely the quantum and trend of the competitor panel’s awards and will consider what should be done in the best interests of the company and its shareholders.the individual.
Salary
Base salary
Base salaries are establishedset by reference to the median for the relevant market (in most casesmarket. For Executives, this is the competitor panel)pharmaceutical pay comparator group. Actual salary levels are reviewed annually and willmay vary baseddepending on an executive’sExecutive’s experience, responsibility and market value. AdjustmentsAny changes usually take effect from 1st April. Following a market data review, base salaries for Dr Garnier and Dr Yamada were increased by 5.1% to $1,600,000 and 6.9% to $775,000, respectively, with effect from 1st April 2005 in line with stated policy in relation to base salariessalary positioning. The base salary for Mr Coombe prior to his retirement on 31st March 2005 was £509,850. The base salary on appointment for Mr Julian Heslop, who succeeded Mr Coombe, was £320,000. Following a market data review, undertaken in February 2006, the base salary for Dr Garnier and Dr Yamada was increased by 8% to $1,730,000 and by 3% to $800,000, respectively. Mr Heslop’s base salary was increased by 25% to £400,000 following the Committee’s review of his performance as CFO since his appointment to a position are basedthe role on performance. Salaries are typically reviewed on an annual basis.1st April 2005. Salary increases take effect 1st April 2006.
PerformanceAnnual bonusThis is based
All bonuses are determined on the basis of a formal review of annual performance by business teams against demandingstretching financial targets based on profit before interest and ontax and are subject to detailed assessment of individual, accomplishmentsbusiness unit and Group achievements against objectives. No bonus is payable if financial performance is less than 96% of the target performance. The individual performance against objectives can increase or decrease the bonus level by a factor which can range from zero to 1.5. Bonuses are subject to upper limits, derived from practice acrosswhich for the competitor panel. The highest of these limits is 200 per centExecutives other than the CEO, range between 100% and 200% of base salary. On targetThe CEO’s limit is 200%.
An annual bonus paid on the basis of on-target business performance brings totaltogether with base salary provides annual cash remuneration intoin line with the competitor panel. Annual cash remuneration rises ifmedian of the target performance is exceeded, but falls well belowpay comparator group.
In the levelcase of competitors if thesethe CEO, the bonus targets are not achieved. Executives may invest their bonus in GlaxoSmithKline shares, in which caseset by the bonus is enhanced by ten per cent butBoard. In setting the shares must be held for a minimum of three years.
Long-term incentivesThese comprise share options and participation in a Performance Share Plan that links reward to shareholder value over the long and medium term respectively.
Performance conditions over the relevant measurement periodsobjectives for the different plans were designed to provide a competitive remuneration packageCEO, the Board takes into account the strategies that as a whole, focuses Executive Directorshave been developed by the company, and are set out on meetingpage 6 of the Group’s business objectives.
The design of plans is reviewed to ensure that they evolve to meet the needs of a changing competitive environment, both in terms of maintaining the competitive position in the global market and ensuring a focus on current business issues.Annual Report.
| GSK Annual Report 2005 |
| 39 |
| Remuneration Report |
| continued |
The objectives set for 2005 focussed in particular on building the best product pipeline in the industry, delivering commercial and operational excellence and, in addition, formulating and updating the strategic plan for the vaccines business.
For reasons of commercial sensitivity, the specific objectives set against the strategic business drivers are kept confidential. Following the end of the financial year, the Board reviews the CEO’s performance generally and against the set objectives, and the Committee then determines the bonus payable. The CEO makes recommendations to the Committee regarding the performance level achieved against objectives for the other Executives. These recommendations are then considered by the Committee to determine the resultant bonus.
In determining bonus awards for 2005, the Committee took into account the excellent financial performance during the year and the encouraging progress in building the pipeline of new products.
In light of the low take up levels and in response to concerns expressed by institutional investors in relation to the 1 for 10 non-performance related match provided under the Annual Incentive Plan (AIP), the Committee decided to discontinue the AIP. Under the AIP, and its US equivalent, eligible employees could elect to invest their bonus in GSK shares or ADSs for a minimum period of three years. At the end of the three-year holding period, participants (including Executives) are entitled to a matching award of 10% of their deferred shareholding. The match is not subject to further performance conditions. This AIP was open to approximately 700 senior executives who all participated on the same terms. The last deferral elections under the AIP were made in respect to bonuses earned during 2005. Although the AIP has now closed, GSK will continue to manage the ongoing administration of subsisting awards as required by the AIP rules.
Long-term incentives
Executives are eligible for performance share awards and share options. The remuneration policy provides that annual long-term incentive (LTI) awards will normally be made up of a performance share award and a share option award.
The Committee considers that performance shares provide a stronger alignment to shareholder value, and therefore the remuneration policy places greater emphasis on the use of performance shares. LTI awards are determined such that for on-target performance more than half of the long-term incentive reward is derived from performance shares.
The annual grant of LTI awards using more than one plan is consistent with the practice of the pay comparator group and other leading UK companies. LTIs for the CET are provided on the same basis as the Executive Directors. The level of the annual LTI opportunity is considered carefully year-on-year by the Committee in the context of market practice.
To align the award cycles more closely with GSK’s financial year and budgeting process, the Committee decided to change the annual grant date for LTI awards for all eligible employees from the fourth quarter of each year to the first quarter of each year.
This change took effect from 2005 and thus LTI awards that would otherwise have been made in the fourth quarter of 2005 were made instead in February 2006. This change in award cycle does not affect the performance period.
Historically, the performance period for awards made in the fourth quarter started on 1st January following the date of award. For LTI awards made in 2006 and thereafter, the performance period starts on 1st January of the year of award (i.e. 1st January 2006 for awards made in February 2006).
No compensation was provided for the change in the awards cycle.
Full disclosure of LTI awards made to the Executive Directors in February 2006 will be made in the Remuneration Report for 2006. The summary details of the LTI awards made to the Executive Directors in February 2006 are set out on page 51 of the Remuneration Report.
Performance share awards and share options are delivered to US resident executives in the form of ADSs. Awards are delivered in the form of Ordinary Shares to executives resident in the UK and other countries. All awards are made under plans which incorporate dilution limits consistent with the guidelines provided by the Association of British Insurers, the National Association of Pension Funds and other shareholder representative bodies. Current estimated dilution from existing awards under all GlaxoSmithKline employee share schemes made since the merger is approximately 5% of the company’s share capital at 31st December 2005.
In 2005, the Committee, assisted by Deloitte, undertook a review of the current performance measures used under the GSK LTI plans. After extensive and careful consideration, the Committee concluded that the measures currently used under the LTI plans remain appropriate and relevant, although in the case of the Share Option Plan, it was agreed that the annualised growth in EPS to achieve 100% vesting for the awards granted in 2006, would be increased from RPI + 5% to RPI +6%.
a) Performance shares
For the Executives, the level of performance shares vesting is based on the company’s Total Shareholder Return (TSR) relative to the performance comparator group (see page 39) over a three-year measurement period. TSR was chosen as the most appropriate comparative measure since it focuses on the return to shareholders, is a well-understood and tested mechanism to measure performance and allows comparison between companies operating in different countries.
TSR is measured in sterling over the performance period and represents the change in the value of a share together with the value of reinvested dividends paid. In order to remove the impact of the varying tax treatments of dividends in different jurisdictions, all dividends are reinvested gross.
As a result of the change in the LTI award cycle for all eligible employees, no performance share awards were made in 2005 to the Executives. In respect of the performance share awards granted in December 2004 and in February 2006, with the performance periods of 1st January 2005 to 31st December 2007 and 1st January 2006 to 31st December 2008, respectively, if GSK is ranked at position seven (the mid-point) of the performance comparator group, 35% of the shares will vest. Any ranking below this point will result in no shares vesting. Only if GSK is one of the top two companies will all of the shares vest. When determining vesting levels, the Committee has regard for the company’s underlying financial performance.
| GSK Annual Report 2005 |
| 40 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
| TSR rank with 13 companies & | Percentage of | |
| GlaxoSmithKline | award vesting* | |
| 1 | 100% | |
| 2 | 100% | |
| 3 | 87% | |
| 4 | 74% | |
| 5 | 61% | |
| 6 | 48% | |
| 7 | 35% | |
| Below 7 | 0% | |
| * |
To provide a closer link between shareholder returns and payments to the Executives, notional dividends are reinvested and paid out in proportion to the vesting of the award. The receipt of dividends has been incorporated into the benchmarking of award levels. In addition, performance shares earned by the Executives cannot be sold, except to meet related tax liabilities, for a further two years following the end of the vesting period. The Committee believes that this further aligns the interests of the Executives with the long-term interests of shareholders.
The vesting table for the performance share awards granted in December 2003, with the performance period 1st January 2004 to 31st December 2006, is given on page 52.
b) Share options
Share options allow thea holder to buy shares at a future date at athe share price determined by reference to the open market price of sharesprevailing at the time of grant. Share options are granted to more than 11,00012,000 managers at GlaxoSmithKline, including the Executive Directors.Executives. The vesting of the share options granted to Executive Directorsthe Executives is subjectlinked to the achievement of compound annual EPS growth over the performance period.
The Committee considered that EPS was the key measure of the performance of the business and was also fully reflected through the business measures extended throughout the Group, ensuring organisational alignment.
When setting EPS targets, the Committee considers the company’s internal projections and analysts’ forecasts for GlaxoSmithKline’s EPS performance, as well as analysts’ forecasts for the pharmaceutical industry.
The following key principles govern the use of EPS as a performance measure:
| • | adjustments will only be considered for major items |
| • | adjustments will be for the judgement of the Committee |
| • | the purpose of the adjustments is to ensure that the performance measurement is fair and reasonable to both participants and shareholders |
| • | any discretion exercised by the Committee will be disclosed to shareholders in the Annual Report. |
The Committee will set out the basis of its decision if it considers it appropriate to make any adjustment.
Following the introduction of International Financial Reporting Standards (IFRS) on 1st January 2005, the Committee considered what EPS measurement basis, either IFRS or UK GAAP, should be used for share options and performance share plan awards (prior to 2003 see page 50), with EPS performance conditions having performance periods that straddled the IFRS conversion date. The Committee agreed that for the purpose of measuring EPS growth in determining whether vesting targets had been achieved, UK GAAP would be used for the 2002 grant (performance period: 1st January 2003 to 31st December 2005) as two out of three years would be reported under UK GAAP. This would require the 2005 CER growth to be restated on a UK GAAP basis. IFRS would be used for the 2003 grant (performance period: 1st January 2004 to 31st December 2006) as two out of the three years would be reported under IFRS.
As a result of the change in the LTI award cycle for all eligible employees, no share options were granted to Executives in 2005.
For share option grants in 2006, vesting increases on a straight-line basis for EPS performance between the hurdles set out in the following graph.
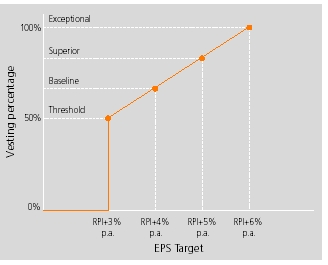 |
This performance condition is substantially consistent with UK shareholder guidelines and expectations and is demanding when compared with those operated by other global pharmaceutical companies. This is consistent with the policy of providing pay for performance and only for performance.
Performance is measured over periods of three financial years, which commence on the basis set out on page 40. There is no performance retesting, so if the performance condition that business performance earnings per share growth, excluding currency and exceptional items, should beis not met after the three-year period, the options will lapse.
Pensions
The Executives participate in GlaxoSmithKline senior executive pension plans. The pension arrangements are structured in accordance with the plans operated for executives in the country in which the executives are likely to retire. Benefits are normally payable at least nine percentage points more thanage 60. Details of individual arrangements for the increaseExecutive Directors are set out on page 43. In response to the future pensions regime in the UK, Retail Prices Index over any three-year measurement period. With respectthe Committee carefully considered the impact of the change in legislation and has decided the following:
| GSK Annual Report 2005 |
| 41 |
Back to future grants,Contents
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
| • | the company will continue to fulfil its obligations under existing pension arrangements |
| • | no compensation will be provided if participants are adversely affected by the new pension regime. |
In coming to these decisions, the Remuneration Committee reviews performance conditions annuallytook account of the following:
| • | new executive hires benefit from a 15%, plus 4% match opportunity, of base pay under the defined contribution plan in the UK, and a contribution equal to 5% of base salary plus under the bonus cash balance plan in the USA |
| • | in the UK, legacy final salary plans were grandfathered for existing employees and no new entrants have been allowed. For capped employees, benefits in excess of the cap are currently all provided through unfunded arrangements |
| • | for capped employees in the USA, benefits above the cap are provided by a non-qualified plan. |
Share ownership requirements
To align the interests of executives with those of shareholders, executives are required to maintain significant holdings of shares in GlaxoSmithKline. These requirements are an important part of aligning the lightinterests of market conditions. However, it currently considers that this performance condition achievesexecutives with shareholders. The CEO is required to hold shares to the value of four times base salary. Other Executive Directors are required to build a balance betweenshareholding to the expectationsvalue of UK institutional investor guidelines andthree times base salary. Members of the pressuresCET are required to build a shareholding to a value of responding to market practice within the competitor panel.
Performance Share PlanParticipations in the Performance Share Plan are granted to approximatelytwo times base salary. The other top 700 top executives in the Group includingare required to build a shareholding to a value of one times base salary. Executives are required to continue to satisfy these shareholding requirements for a minimum of twelve months following retirement from the company.
In order for shares to qualify for these share ownership requirements they must be held personally by the Executive Directors, designating a target numberor their spouse or minor children or have been earned but deferred under one of shares for each participant. Vesting of awards under the plan is subject to two performance conditions which apply during a three year measurement period. No provision for re-testing operates inshare programmes operated by the event that the performance conditionscompany. Unexercised share options are not satisfied overincluded in this period.
The first condition, which applies to halfcalculation. As at 31st December 2005, Dr Garnier’s holding was 225,896 ADSs, Dr Yamada’s was 67,512 ADSs and Mr Heslop’s was 18,885 ordinary shares. Dr Garnier’s and Dr Yamada’s holdings were in excess of the award, compares GlaxoSmithKline’s Total Shareholder Return (TSR) overshare ownership requirements. Mr Heslop has until December 2008 to build his holding to the period with the TSRvalue of companiesthree times base salary. Mr Coombe's shareholding at 31st December 2005, was in the UK FTSE 100 Index over the same period. If GlaxoSmithKline delivers returns which would rank in the top 20excess of the FTSE 100 basedshare ownership requirements following his retirement from the Board on TSR performance, then all of the shares, in this part of the award, will vest. For the 50th position in the FTSE 100, 40 per cent of the shares will vest. If GlaxoSmithKline is ranked below 50th position, none of the shares, subject to this part of the award, will vest. Between the 20th and 50th positions, vesting will occur on a sliding scale.31st March 2005.
The second condition, which applies to the balance of the award, requires GlaxoSmithKline business performance earnings per share growth, excluding currency and exceptional items, to be at least nine percentage points more than the increase in the UK Retail Prices Index over the three-year performance period. If this condition is met then all of the shares, subject to this performance condition, will vest. If this condition is not met, then none of the shares, subject to this part of the performance condition, will vest. The extent to which awards vest will be reported upon on completion of each performance period.
This combination of an earnings based measure and one based on shareholder return compared to the FTSE 100 Index was decided on at the time of the merger following consultation. At that time, a number of shareholders with a UK equity mandate requested a performance measure tied to a UK equity index.
Even if the performance conditions are met, the vesting of all Performance Share Plan awards for the CET is subject to final approval by the Remuneration Committee, which will consider whether the final vesting is consistent with the underlying financial performance of the Group.
BenefitsOther remuneration elements
The Executive DirectorsExecutives participate in GlaxoSmithKline’s senior executive pension plans (see page 50 for details). Mr Coombe participates in a defined benefit plan in the UK and Dr Garnier in cash balance plans in the USA. Benefits are payable at age 60.
Executive Directors participate invarious legacy Glaxo Wellcome and SmithKline Beecham all employeeall-employee share plans in either the UK or the USA and in the GlaxoSmithKline plans that replaced them. Under
The Sharesave plan and the US Retirement Savings Plan Dr Garnier received four per cent of his basic salary inShareReward plan are Inland Revenue-approved plans open to all UK employees on the form of GlaxoSmithKline shares.
Under the UK arrangements,same terms. Mr CoombeHeslop is a member of the Sharesave plan, andinto which he contributes £250 per montha month. This provides him with the option to buy shares at the end of the three yearthree-year savings period at a 20 per cent discountin line with the opportunity available to the market price ruling at the start of the savings period. all UK employees.
Mr Coombe isHeslop also a member of the ShareReward plan, contributingcontributes £125 per month to buy shares.shares under the ShareReward plan. The company matches the number of shares bought each month is matched by the company. Both the Sharesave plan and ShareReward plan are Inland Revenue approved plans open to all UK employees on the same terms.month.
The Executive DirectorsExecutives also receive the followingother benefits theincluding healthcare (medical and dental), personal financial advice and life assurance. The cash value of whichthe benefits received by the Executive Directors in 2005 is shown on page 46:45.
Executive Director terms, conditions and remuneration
Executive Director contracts
The policy regarding the Executive Directors’ contracts was the subject of extensive review and change during 2003. The policy provides the framework for contracts for Executive Directors appointed since and going forward.
The key aspects of GlaxoSmithKline’s contractual framework are:
| Aspect | Policy | ||
| Notice period on | 12 calendar months | ||
| termination by the | |||
| employing company | |||
| or executive | |||
| Termination payment | – | 1x annual salary and | |
| – | No mitigation required2 | ||
| Benefits | Governed by benefits policy, including: | ||
| – | healthcare | ||
| – | personal financial advice | ||
| – | life assurance contributions | ||
| Vesting of long-term | |||
| incentives | plan3 | ||
| Pension | Based on existing arrangements and | ||
| terms of the relevant pension plan | |||
| Non-compete clause | 12 months from termination | ||
| notice date2 | |||
Share ownership guidelinesTo align executive interest with that of shareholders, executives are required to hold shares in the company. The Chief Executive Officer is required to hold shares to the value of four times basic salary. Other Executive Directors are required to hold shares to the value of three times basic salary. Members of the CET are required to hold shares to the value of two times basic salary and other executives, who participate in the Performance Share Plan, one times salary. For the purposes of these requirements, shares and ADSs held in the GlaxoSmithKline Annual Investment Plan and the SmithKline Beecham bonus deferral plans, and vested but deferred awards under the SmithKline Beecham Mid-term Incentive Plan are included. As at the year end Dr Garnier’s total shareholding on this basis was 171,019 ADSs and Mr Coombe’s was 172,537 shares. As a result both Directors exceeded the share ownership guidelines.
Performance graphThe new regulations covering Directors’ remuneration require that a graph be presented showing the company’s total shareholder return (TSR) against the TSR performance of a broad equity market index. The following graph shows GlaxoSmithKline’s TSR performance against the FTSE 100, which has been chosen because it is the principal index in which the company’s shares are quoted and against the competitor panel set out on page 40 which indicates GlaxoSmithKline’s relative performance against its peers.
| 1 | Dr Garnier’s target bonus is 100% of salary, Dr Yamada’s is 85% of salary and Mr Heslop’s is 75% of salary. |
| 2 | The imposition of a 12-month non-compete period on the Executives is considered vitally important by the company in order to protect the Group’s intellectual property. In light of the non-compete clause and competitor practice, the Committee believes that it would not be appropriate to provide for mitigation in the contracts. When reviewing the level of severance payments, the Committee considered investor and DTI guidance. However, it determined that in line with competitive practice it is appropriate to provide for the payment of salary and target bonus on termination. |
| 3 | As approved by shareholders of GlaxoSmithKline, Glaxo Wellcome and SmithKline Beecham, as appropriate. |
| GSK Annual Report 2005 |
| 42 |
Performance graph
Notes
1.The TSR graph starts at the beginning of the first accounting year following the formation of GlaxoSmithKline and uses as a base the share price on 31st December 2000. Calculations for the graph are based on spot prices at the beginning and end of each year as required by the Directors Remuneration Report Regulations 2002, whereas GlaxoSmithKline’s performance conditions under the Performance Share Plan (PSP) use average prices over a period of a year. Therefore the above graph should not be taken as an indication of the likely vesting of awards granted under the PSP. The average price method was selected for the PSP because it smoothes out volatility and reduces the impact of any particularly large temporary price movements at either the beginning or end of the performance period.
2. Past performance should not be taken as a guide to future performance.
Executive Directors’ terms and conditionsThe following section sets out the date and unexpired term of each Executive Director’s contract, and details of other provisions necessary to enable shareholders to estimate the liability of the company in the event of early termination.
In determining its overall policy in respect of service contracts, the Committee aims to balance the costs associated with any early termination provisions with the need to protect GlaxoSmithKline’s intellectual property rights. The Committee maintains a close watch, through its advisors, on trends in contractual terms amongst other companies in the competitor panel and in the wider market place. It is committed to ensuring that, in achieving this balance, its processes are fair, while limiting as far as possible the scope for ‘rewarding failure’. The Committee has considered the recent guidance produced by the Association of British Insurers and the National Association of Pension Funds in the UK. It will take this into account, alongside market practice, when reviewing contractual terms.
Executive Directors are employed on service contracts under which the employing company is required to give 24 calendar months’notice of termination and the Executive Directors are required to give 12 calendar months’ notice.
Executive Directors’ service contracts contain ‘garden leave’, non-competition, non-solicitation and confidentiality clauses.
The Remuneration Committee currently believes that one year contracts would not be in the best interest of GlaxoSmithKline with regard to offering a globally competitive overall remuneration package and securing maximum protection for its intellectual property rights.
The Remuneration Committee believes that the current termination payments due under Executive Director’s contracts are justified because they represent fair and reasonable compensation in the event that the contracts are terminated, given market practice and the associated restrictions arising from the need to protect intellectual property.
Dr J P GarnierDr Garnier has a service agreement with SmithKline Beecham Corporation dated 2nd December 1999. The agreement expires on 31st October 2007, being the last day of the month in which Dr Garnier reaches his 60th birthday. Dr Garnier’s contract specifies the compensation to be paid on termination of his employment.
Dr Garnier’s current basic salary is US$1,450,000 and will be increased to US$1,522,500 on 1st April 2003.
Dr Garnier may terminate the agreement on giving 12 calendar months’ written notice, following which he is credited with an extra three years’ pension contributions and he will be treated as if he is three years older than his actual age.
SmithKline Beecham Corporation may terminate the agreement on giving 24 calendar months notice. On termination by SmithKline Beecham Corporation, other than for cause, Dr Garnier is entitled to receive, within 30 days, a lump sum representing his salary and bonus for the notice period. The bonus is calculated on the basis of ‘on target’ performance which gives a bonus payout of 100 per cent of basic salary.
In addition, for the first 12 months of the notice period Dr Garnier is entitled to receive share entitlements, under stock and share incentive plans available to senior executives in the USA, except to the extent of any part of that period that would fall beyond his retirement date, and he can be awarded only one annual share option grant and Performance Share Plan grant after the date of the termination notice.
For pension purposes, he is provided with pension benefits such that he is treated, by way of additional pension contributions or otherwise, as if his employment had ended three years after the actual termination date, or three years after the expiry of his service agreement in the event that Dr Garnier’s employment continues until the expiry of the service agreement. In addition, Dr Garnier and his spouse will be treated as if they are both three years older than their actual ages on the termination (other than for cause) or expiry of the service agreement, as applicable, for the purpose of calculating annuity rates on which the pension will be based.
He would also receive outplacement counselling and financial planning and advice for two years following termination, but this shall be limited to $20,000 per year and he can choose to have life assurance cover which will provide a benefit of two times his salary until his 65th birthday.
Dr Garnier will continue to receive his benefits, or their cash value, during the notice period. If Dr Garnier’s agreement is terminated by reason of disability he will be treated as if still employed for the purposes of his pension benefits until his retirement date.
In addition, if any payment or distribution to or for the benefit of Dr Garnier would be subject to excise tax, or any interest or penalties are incurred, Dr Garnier is entitled to receive an additional cash payment so that he is in the same after-tax position as if no such additional tax had been imposed.
In the event of retirement on the expiry of his service agreement or in the event of termination of his employment by SmithKline Beecham Corporation (other than for cause) or in the event of Dr Garnier not being elected or retained as a Director of GlaxoSmithKline (or any merged company), or as a result of a change of control of GlaxoSmithKline (provided that such resignation occurs on or within 30 days after the first anniversary of such change in control), then (a) all share option grants will vest immediately and will remain exercisable until the expiry of the option period as if Dr Garnier had still been employed by SmithKline Beecham Corporation and all performance and time conditions shall be deemed to have been satisfied, and (b) final awards under the Performance Share Plan will be determined after the end of the full performance period originally specified for the relevant participation without any proportionate reduction because of such retirement, termination or resignation. In respect of Dr Garnier’s participation in the SmithKline Beecham Senior Executive Bonus Investment Plan, provided that his agreement is terminated other than for cause, any deferred amount and any income, gains and losses, are automatically distributed as soon as administratively practicable after his termination. If Dr Garnier resigns, retires or the termination is for cause then any deferred amount is not distributed until the end of a minimum three year deferral period.
Mr J D CoombeMr Coombe has a service agreement with Glaxo Wellcome plc, now GlaxoSmithKline Services Unlimited, dated 14th February 2000. The agreement expires on 31st March 2005, being the last day of the month in which Mr Coombe reaches his 60th birthday.
Mr Coombe’s current basic salary is £475,000, and will be increased to £495,000 on 1st April 2003.
Mr Coombe may terminate the agreement on giving 12 calendar months’ written notice.
GlaxoSmithKline Services Unlimited may terminate the agreement on 24 calendar months’ written notice or without notice in the event of Mr Coombe’s gross misconduct, wilful neglect, dishonesty, bankruptcy or conviction of a criminal offence affecting his position as a senior executive.
Mr Coombe’s agreement specifies that the compensation in cash to be paid in the event of redundancy will be as follows:
| Following the independent review of remuneration undertaken in 2003, Dr Garnier, Mr Coombe and Dr Yamada agreed to changes in their previous contractual terms without compensation to come broadly in line with the new contractual framework, including the reduction of contractual notice period from 24 to 12 calendar months. However, in order to honour certain aspects of their ‘old’ contractual terms, there are a number of individual features which have been retained. | |
| In Dr Garnier’s case, these include the entitlement to reimbursement of excise tax on change of control related payments, life insurance benefit funded by the company to age 65 and the following provisions relating to the | |
In such circumstances, Mr Coombe’s pension entitlement will also be augmented by an amount equal in value to the pension which would have accrued to him over the period of 24 months commencing on the date of termination of his employment.
After a period of 12 consecutive months incapacity or after Mr Coombe becomes entitled to a disablement pension, he is entitled to an amount equivalent to the amount he would have received had he worked for a period of 24 months from the first day of his absence less any amounts actually received during that period.
In addition, if Mr Coombe leaves employment through incapacity before the age of 60, the pension he has already accrued becomes payable from the date of his incapacity and may be augmented by the trustees of the pension plan to the amount to which he would have been entitled had he been employed in full service until 60.
In the event that notice of termination is given, other than in the case of redundancy, Mr Coombe is required to mitigate any loss of earnings resulting thereafter.
In the event of Mr Coombe’s early retirement as a result of termination by GlaxoSmithKline Services Unlimited (other than for cause or redundancy), all outstanding options granted under the GlaxoSmithKline share option plan must be exercised within 48 months from the date of grant. All outstanding options granted under the Glaxo Wellcome share option plans must be exercised within 12 months from cessation of employment or by the end of the option period, if earlier, where such options are more than three years old and outstanding options less than three years old must be exercised within 42 months from the date of grant. In each case, the Remuneration Committee may use its discretion to allow a longer period of exercise.
In the case of awards under the Performance Share Plan, if Mr Coombe’s employment contract is terminated for redundancy, retirement or his employing company ceases to be a member of the GlaxoSmithKline Group then the Remuneration Committee will determine the percentage of each award that will vest under the Plan rules after the end of the financial year in which the cessation of employment occurred. This will ordinarily be calculated by reference to the performance period which has elapsed and the extent to which the performance condition has been satisfied over that period. If his employment ceases for any other reason before the end of the awards performance period, the awards will lapse unless the Committee determines otherwise.
In respect of Mr Coombe’s participation in the Glaxo Wellcome Long Term Incentive Plan (LTIP) special rules apply when a participant’s employment ends. However all awards made to Mr Coombe under the LTIP have now vested on the completion of the measurement period.
Other entitlementsIn addition to the contractual provisions outlined above in the event that Dr Garnier or Mr Coombe’s service agreements are terminated by their employing company they would be entitled to:
| • | Pre-2003 awards On termination by the |
| • | 2003 and thereafter Awards for the above provisions apply, but options will be subject to performance testing in all circumstances, and any options or performance share awards made 12 months prior to the termination notice date will lapse. |
| In addition, Dr Garnier and Dr Yamada are entitled to receive one year’s worth of pension contributions on termination. | |
| Dr Garnier’s contract was executed on 3rd March 2004 and took effect from 1st January 2004. His contract will expire on 31st October 2007 being the last day of the month in which he will reach his 60th birthday. Dr Yamada’s contract was executed on 27th July 2004 and took effect from 1st January 2004. Dr Yamada will retire from the Board and the company on 1st June 2006. Mr Coombe’s contract was executed on 3rd March 2004, took effect from 1st January 2004 and expired on 31st March 2005. | |
| Mr Heslop’s contract was executed on 16th March 2005 and took effect from 1st April 2005. Mr Heslop’s contract will expire on 31st January 2014, being the last day of the month in which he reaches his 60th birthday. | |
| No termination payments will be made in respect of | |
| Individual pension arrangements The UK plan provides for a pension based on two-thirds of final salary at age 60. The US cash balance plan provides for an annual contribution and | |
| GlaxoSmithKline makes annual contributions of 15% of Dr Garnier’s annual salary and bonus and 18% of Dr Yamada’s annual salary and bonus. The fund increases at an interest rate based on the yield on 30-year treasury bonds. The company has no liability beyond making these annual contributions. | |
| Prior to 1999 all US employees, including Dr Garnier and Dr Yamada, were moved from a final salary pension arrangement to the current cash balance structure. For all employees in the US, cash balance plan contributions are based on combined annual salary and annual bonus. | |
| Mr Heslop participates in the Glaxo Wellcome defined benefit plan with an accrual rate of 1/30th of final pensionable salary per annum. | |
| In 2000 all benefits accrued under the Glaxo Wellcome UK pension arrangements were augmented by the Trustees of the plans by 5% to reflect a distribution of surplus. This augmentation will apply to that | |
In addition to the contractual provisions outlined above, in the event that Executive Directors service agreements are terminated by their employing company, the following would apply: | |
| • | in the case of awards under the GlaxoSmithKline Annual Investment Plan, provided that their agreement is terminated other than for cause, any deferred amount, |
| • | in line with the policy applicable to US senior executives, Dr Garnier and Dr Yamada are entitled to receive continuing medical and dental insurance |
| • | following the merger, those participants in the legacy share option schemes who elected to exchange their legacy options for options over GlaxoSmithKline shares will receive an additional cash benefit equal to 10% of the grant price of the original option. This additional benefit is triggered when the new option is exercised or lapses. To qualify for this additional cash benefit, participants had to retain their options until at least the second anniversary of the effective date of the merger. |
| Outside appointments for Executive Directors Any outside appointments must be approved by the Chairman on behalf of the Board. It is the company’s policy that remuneration earned from such appointments may be kept by the individual Executive Director. | |
| GSK Annual Report 2005 |
| 43 |
| Remuneration Report |
| continued |
Non-Executive Directors
Director terms, conditions and fees
Non-Executive Directors of GlaxoSmithKline do not have service contracts but instead have letters of appointment. The company aims to provide Non-Executive Directors with fees that are competitive with other companies of equivalent size and complexity. The fee structure for the Non-Executive Directors is as follows:
| Per annum | |
| Standard annual cash retainer fee | £60,000 |
| Supplemental fees | |
Senior Independent Director, the Audit CommitteeChairmanand Scientific/Medical Experts | £30,000 |
| Chairmen of the Remuneration and Corporate Responsibility Committees | £20,000 |
| Non-Executive Director undertaking intercontinental travel to meetings | £5,000 per meeting |
| Automatic share allocation To enhance the link between Directors and shareholders GlaxoSmithKline requires Non-Executive Directors to receive a significant part of their fees in the form of shares. With effect from 1st October 2004, at least 25% of the Non-Executive Directors’ total fees, excluding the Chairman, are paid in the form of shares and allocated to a share account. The Non-Executive Directors may also take the opportunity to invest part or all of the balance of their fees into the same share account. | |
| Exchange rate Fees that are paid in US Dollars are converted at a rate of £1/US$1.8162, being the exchange rate that applied on 29th July 2004 when the new fee arrangements were approved by the Board. | |
Non-Executive Directors are not entitled to compensation if their appointment is terminated.
To enhance the link between Directors and shareholders and as set out in the table below, GlaxoSmithKline requires Non-Executive Directors to receive a significant part of their fees in the form of shares allocated to a share account and offers the opportunity to invest part or all of the balance of fees in a share account. These shares are not paid out until the Director’s retirement from the Board, or at a later date, on the basis of dividends being reinvested in the interim.
The Chairman, the Deputy Chairman and the chairmen of the Board Committees receive higher fees.
Terms and conditionsChairman
Sir Richard Sykes
Sir Richard SykesChristopher Hogg retired as Chairman and as a Non-Executive Director on 20th May 2002.with effect from 31st December 2004. Sir Richard’sChristopher Gent’s letter of appointment to the Board was dated 20th June 2000,26th May 2004, under which it was agreed that he serve the company as Deputy Chairman until 31st December 2004 and from 1st January 2005 as Chairman until the conclusion of the Annual General Meeting following the third anniversary of his appointment. This may be extended for a further term of three years by mutual agreement. He received fees at the rate of £240,000 per annum plus an allocation of GlaxoSmithKline shares to the value of £60,000 per annum whilst Deputy Chairman, and receives £400,000 per annum plus an allocation of GlaxoSmithKline shares to the value of £100,000 per annum as Chairman.
Other Non-Executive Directors
On appointment, each Non-Executive Director is provided with a letter of appointment under which it is agreed that they serve the company as a Non-Executive Director until the conclusion of the Annual General Meeting following the third anniversary of histheir appointment. This could have been extended for a further term of three years by mutual agreement.
Sir Richard received fees of £300,000 per annum together with an allocation of 6,000 shares under the Non-Executive Directors’ Share Arrangements.
Following his retirement from the Board, and in recognition of his services to the company, the Board decided to make an augmentation payment to the pension plan of £300,000 in respect of Sir Richard. It was also agreed that for a period of two years, from 1st June 2002, Sir Richard be appointed Senior Advisor to the company, at a salary of £49,000 per annum.
The company has agreed to procure that Sir Richard Sykes’ pension from the age of 60 will be calculated on the basis of his salary as at 31st December 2000 and as if he had remained in full-time employment until his 60th birthday.
Sir Christopher HoggSir Christopher Hogg’s letter of appointment to the Board was dated 19th June 2000, under which it was agreed that he serve the company as a Non-Executive Director until the conclusion of the Annual General Meeting following the third anniversary of his appointment. ThisIn each case this can be extended for a further term of three years by mutual agreement. No Directors serve a term longer than three years without offering themselves for re-election by the shareholders.
Sir Christopher’sThe following table shows the date of the letter of appointment was amended on 1st September 2002 to record his appointment asof each Non-Executive Chairman with effect from 20th May 2002. On becoming Chairman, Sir Christopher’s fees were increased from £45,000 per annum plus an allocation of 1,000 shares per annum under the Non-Executive Directors’ Share Arrangements, to £300,000 per annum plus an allocation of 6,000 shares per annum.
Sir Peter Walters and Mr John YoungSir Peter Walters retired as Deputy Chairman and as a Non-Executive Director, and Mr Young retired as a Non-Executive Director, with effect from 20th May 2002. Sir Peter’s and Mr Young’s letters of appointment were both dated 19th June 2000 and in both cases it was agreed that they serve the company as Non-Executive Directors until the conclusion of the Annual General Meeting following the third anniversary of their appointment. In both cases this could have been extended for a further term of three years by mutual agreement. Sir Peter received fees of £80,000 per annum together with an allocation of 3,000 ordinary shares under the Non-Executive Directors’ Share Arrangements. Mr Young received fees of $88,000 per annum together with an allocation of 500 American Depositary Shares made under the Non-Executive Directors’ Share Arrangements.
Sir Roger Hurn, Mr Paul Allaire, Dr Michèle Barzach, Sir Peter Job, Mr John McArthur, Mr Donald McHenry, Sir Ian Prosser, Dr Ronaldo Schmitz and Dr Lucy Shapiro
The letters of appointment for all of the above Directors were dated 19th June 2000 and in all cases it was agreed that they serve the company as Non-Executive Directors until the conclusion of the Annual General Meeting following the third anniversary of their appointment. In all cases this could be extended for a further term of three years by mutual agreement. The fees payable and the share allocations under the Non-Executive Directors’ Share Arrangements for each of these directors is as follows:Director:
| Non-Executive | ||
Mr L Culp | 09.06.03 | |
| Sir | ||
| Sir | ||
| Sir Ian Prosser | ||
| Mr T de Swaan | ||
| Sir Robert Wilson | 09.06.03 | |
Mr Allaire succeeded Mr Young as ChairmanTSR performance graph
The following graph sets out the performance of the R&N Committeecompany relative to the FTSE 100 index of which the company is a constituent and to the performance comparator group since the merger on 20th May 200227th December 2000. The graph has been prepared in accordance with the Regulations and his fees were increased to $88,000 per annum from that date. Mr McHenry succeeded Sir Christopher Hogg as Chairmanis not an indication of the Corporate Social Responsibility Committee on 7th February 2003 and his fees were increased to $88,000 per annum from that date. Sir Ian Prosser succeeded Sir Christopher Hogg as Chairmanlikely vesting of awards granted under any of the Nominations Committee on 7th February 2003 and his fees were increased to £55,000 per annum from that date.company’s incentive plans.
Directors and Senior Management Remuneration
remuneration
The following tables set out for the Directors of GlaxoSmithKline plc the remuneration earned in 2002;2005, their interestinterests in shares of GlaxoSmithKline plc;plc, their interests in share options and incentive plans and their pension benefits. The members of the Corporate Executive Team (CET)CET and the Company Secretary, known as the Senior Management, also participate in the same remuneration plans as the Executive Directors and the aggregate remuneration and interests of the Directors and Senior Management are also provided.
| GSK Annual Report 2005 |
| 44 |
| Remuneration |
| continued |
Annual remuneration
| 2002 | 2001 | ||||||||||||||||
| Total | Annual and | Total | |||||||||||||||
| Fees and | Other | Annual | annual | Fees and | Other | deferred | annual | ||||||||||
| salary | benefits | bonus | remuneration | salary | benefits | bonus | remuneration | ||||||||||
| Footnote | £000 | £000 | £000 | £000 | £000 | £000 | £000 | £000 | |||||||||
| Executive Directors | |||||||||||||||||
| Dr J P Garnier | a,c | 967 | 132 | 1,353 | 2,452 | 932 | 101 | 2,417 | 3,450 | ||||||||
| Mr J D Coombe | b,c | 475 | 15 | 457 | 947 | 475 | 3 | 848 | 1,326 | ||||||||
| Total | 1,442 | 147 | 1,810 | 3,399 | 1,407 | 104 | 3,265 | 4,776 | |||||||||
| Non-Executive Directors | |||||||||||||||||
| Sir Richard Sykes | d,e | 154 | 8 | – | 162 | 411 | 3 | – | 414 | ||||||||
| Sir Christopher Hogg | 252 | – | – | 252 | 63 | – | – | 63 | |||||||||
| Sir Roger Hurn | 121 | – | – | 121 | 135 | – | – | 135 | |||||||||
| Sir Peter Walters | e | 51 | 2 | – | 53 | 135 | 1 | – | 136 | ||||||||
| Mr P A Allaire | 68 | – | – | 68 | 68 | – | – | 68 | |||||||||
| Dr M Barzach | f | 100 | – | – | 100 | 102 | – | – | 102 | ||||||||
| Mr D C Bonham | g | – | 5 | – | 5 | 29 | – | – | 29 | ||||||||
| Sir Peter Job | 59 | – | – | 59 | 63 | – | – | 63 | |||||||||
| Mr J H McArthur | f | 62 | – | – | 62 | 73 | – | – | 73 | ||||||||
| Mr D F McHenry | 62 | – | – | 62 | 68 | – | – | 68 | |||||||||
| Sir Ian Prosser | 59 | – | – | 59 | 63 | – | – | 63 | |||||||||
| Dr R Schmitz | 69 | – | – | 69 | 70 | – | – | 70 | |||||||||
| Dr L Shapiro | h | 62 | – | – | 62 | 68 | – | – | 68 | ||||||||
| Mr J A Young | e | 29 | 2 | – | 31 | 80 | – | – | 80 | ||||||||
| Total | 1,148 | 17 | – | 1,165 | 1,428 | 4 | – | 1,432 | |||||||||
| Total remuneration | 2,590 | 164 | 1,810 | 4,564 | 2,835 | 108 | 3,265 | 6,208 | |||||||||
| 2005 | 2004 | ||||||||||||||||||||||||||||
| Total | Total | ||||||||||||||||||||||||||||
| Fees and | Other | Annual | Deferred | annual | Fees and | Other | Annual | annual | |||||||||||||||||||||
| salary | benefits | bonus | bonus | remuneration | salary | benefits | bonus | remuneration | |||||||||||||||||||||
| Footnote | 000 | 000 | 000 | 000 | 000 | 000 | 000 | 000 | 000 | ||||||||||||||||||||
| Current Executive Directors | |||||||||||||||||||||||||||||
| Dr JP Garnier | a,b,c | $ | 1,582 | $ | 641 | $ | 2,812 | $ | 1,556 | $ | 6,591 | $ | 1,523 | $ | 786 | $ | 2,250 | $ | 4,559 | ||||||||||
| Mr J Heslop | £ | 240 | £ | 9 | £ | 280 | – | £ | 529 | – | – | – | – | ||||||||||||||||
| Dr T Yamada | a,b,c | $ | 763 | $ | 739 | $ | 1,110 | $ | 698 | $ | 3,310 | $ | 725 | $ | 577 | $ | 1,001 | $ | 2,303 | ||||||||||
| Total Current Executive Directors | £ | 1,528 | £ | 767 | £ | 2,436 | £ | 1,238 | £ | 5,969 | £ | 1,228 | £ | 745 | £ | 1,777 | £ | 3,750 | |||||||||||
| Former Executive Director | |||||||||||||||||||||||||||||
| Mr J Coombe | b,c,d | £ | 139 | £ | 32 | – | – | £ | 171 | £ | 506 | £ | 9 | – | £ | 515 | |||||||||||||
| Total Executive Directors | £ | 1,667 | £ | 799 | £ | 2,436 | £ | 1,238 | £ | 6,140 | £ | 1,734 | £ | 754 | £ | 1,777 | £ | 4,265 | |||||||||||
| Current Non-Executive Directors | |||||||||||||||||||||||||||||
| Mr L Culp | $ | 136 | – | – | – | $ | 136 | $ | 97 | – | – | $ | 97 | ||||||||||||||||
| Sir Crispin Davis | £ | 70 | – | – | – | £ | 70 | £ | 57 | – | – | £ | 57 | ||||||||||||||||
| Sir Christopher Gent | £ | 500 | – | – | – | £ | 500 | £ | 175 | – | – | £ | 175 | ||||||||||||||||
| Sir Deryck Maughan | $ | 146 | – | – | – | $ | 146 | $ | 57 | – | – | $ | 57 | ||||||||||||||||
| Sir Ian Prosser | £ | 100 | – | – | – | £ | 100 | £ | 65 | – | – | £ | 65 | ||||||||||||||||
| Dr R Schmitz | £ | 95 | – | – | – | £ | 95 | £ | 72 | – | – | £ | 72 | ||||||||||||||||
| Dr L Shapiro | e | $ | 230 | – | – | – | $ | 230 | $ | 182 | – | – | $ | 182 | |||||||||||||||
| Sir Robert Wilson | £ | 90 | – | – | – | £ | 90 | £ | 66 | – | – | £ | 66 | ||||||||||||||||
| Total Current Non-Executive Directors | £ | 1,137 | – | – | – | £ | 1,137 | £ | 618 | – | – | £ | 618 | ||||||||||||||||
| Former Non-Executive Directors | |||||||||||||||||||||||||||||
| Dr M Barzach | f | £ | 58 | – | – | – | £ | 58 | £ | 78 | – | – | £ | 78 | |||||||||||||||
| Sir Christopher Hogg | – | – | – | – | – | £ | 369 | £ | 1 | – | £ | 370 | |||||||||||||||||
| Sir Roger Hurn | – | £ | 5 | – | – | £ | 5 | – | – | – | – | ||||||||||||||||||
| Sir Peter Job | – | £ | 5 | – | – | £ | 5 | £ | 57 | – | – | £ | 57 | ||||||||||||||||
| Mr J McArthur | – | – | – | – | – | $ | 42 | $ | 18 | – | $ | 60 | |||||||||||||||||
| Mr D McHenry | – | – | – | – | – | $ | 42 | – | – | $ | 42 | ||||||||||||||||||
| Sir Richard Sykes | – | £ | 1 | – | – | £ | 1 | – | £ | 1 | – | £ | 1 | ||||||||||||||||
| Total Former Non-Executive Directors | £ | 58 | £ | 11 | – | – | £ | 69 | £ | 550 | £ | 12 | – | £ | 562 | ||||||||||||||
| Total Non-Executive Directors | £ | 1,195 | £ | 11 | – | – | £ | 1,206 | £ | 1,168 | £ | 12 | – | £ | 1,180 | ||||||||||||||
| Total Remuneration | £ | 2,862 | £ | 810 | £ | 2,436 | £ | 1,238 | £ | 7,346 | £ | 2,902 | £ | 766 | £ | 1,777 | £ | 5,445 | |||||||||||
| Remuneration for Directors on | |
| c) | In 2001, following the merger, Dr Garnier, Mr Coombe and Dr Yamada were awarded a one-off special deferred bonus |
| GSK Annual Report 2005 |
| 45 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
| d) | Mr Coombe waived his prorated 2005 bonus of £106,870 and his 2004 annual bonus of £650,370. The company made a contribution to the |
| Dr Shapiro is a member of GlaxoSmithKline’s Scientific Advisory Board for which she received fees |
Where the Directors above have received part or all of their remuneration in currencies other than sterling, the average rates of exchange for the year have been used. None of the above Directors received expenses during the year requiring separate disclosure as required by schedule 7A.
| f) | |
| None of the above Directors received expenses during the year requiring separate disclosure as required by the Regulations. | |
| Mr de Swaan joined the Board as a Non-Executive Director on 1st January 2006. No remuneration is shown for him in the table above. | |
| Mr de Swaan joined the Board as a Non-Executive Director on 1st January 2006. No remuneration is shown for him in the table above. | |
Non-Executive DirectorsDirectors’ remuneration’ share arrangements
| 2005 | 2004 | |||||||||||||||||
| Total | Cash | Shares/ADSs | Total | Cash | Shares/ADSs | |||||||||||||
| Fees and salary | 000 | 000 | 000 | 000 | 000 | 000 | ||||||||||||
| Current Non-Executive Directors | ||||||||||||||||||
| Mr L Culp | $ | 136 | – | $ | 136 | $ | 97 | – | $ | 97 | ||||||||
| Sir Crispin Davis | £ | 70 | – | £ | 70 | 57 | – | £ | 57 | |||||||||
| Sir Christopher Gent | £ | 500 | £ | 400 | £ | 100 | £ | 175 | £ | 140 | £ | 35 | ||||||
| Sir Deryck Maughan | $ | 146 | – | $ | 146 | $ | 57 | – | $ | 57 | ||||||||
| Sir Ian Prosser | £ | 100 | £ | 50 | £ | 50 | £ | 65 | £ | 28 | £ | 37 | ||||||
| Dr R Schmitz | £ | 95 | £ | 57 | £ | 38 | £ | 72 | £ | 38 | £ | 34 | ||||||
| Dr L Shapiro | $ | 145 | $ | 109 | $ | 36 | $ | 97 | $ | 75 | $ | 22 | ||||||
| Sir Robert Wilson | £ | 90 | £ | 68 | £ | 22 | £ | 66 | £ | 52 | £ | 14 | ||||||
| Former Non-Executive Directors | ||||||||||||||||||
| Dr M Barzach | – | – | – | £ | 22 | £ | 19 | £ | 3 | |||||||||
| Sir Christopher Hogg | – | – | – | £ | 369 | £ | 150 | £ | 219 | |||||||||
| Sir Peter Job | – | – | – | £ | 57 | – | £ | 57 | ||||||||||
| Mr J McArthur | – | – | – | $ | 42 | $ | 37 | $ | 5 | |||||||||
| Mr D McHenry | – | – | – | $ | 42 | $ | 37 | $ | 5 | |||||||||
| Total | £ | 1,090 | £ | 635 | £ | 455 | £ | 1,066 | £ | 508 | £ | 558 | ||||||
The table above sets out the remuneration received as Non-Executive Directors areof GlaxoSmithKline. Accordingly, it does not include Dr Barzach’s fees received from GlaxoSmithKline France for healthcare consultancy provided, or Dr Shapiro’s fees received as a member of GlaxoSmithKline’s Scientific Advisory Board.
From the formation of GSK, the Non-Executive Directors, have been required to receivetake at least a part of their total fees in the form of shares and ADSs which are detailed below together with their valueallocated to a share account. From 1st October 2004, at least 25% of Non-Executive Directors fees, except those of the dates of award. They may alsoChairman, see page 44 for further details, must be taken under the fee allocation arrangement. Non-Executive Directors can then elect to receive parteither all or allpart of the balance of their feesremaining cash payment in the form of further shares andor ADSs. The shares allocated to their accounts, which are not transferred to them until retirement, are also included in Directors’ interests.
| 2002 | 2001 | |||||||||||||||||
| Total value | ||||||||||||||||||
| of holdings | Allocated | Elected | Allocated | Elected | ||||||||||||||
| at 31.12.02 | ||||||||||||||||||
| £ | Shares | ADSs | £ | £ | Shares | ADSs | £ | £ | ||||||||||
| Sir Richard Sykes | – | 1,500 | – | 24,535 | – | 6,000 | – | 110,995 | – | |||||||||
| Sir Christopher Hogg | 111,141 | 4,063 | – | 50,713 | 37,500 | 1,000 | – | 18,499 | – | |||||||||
| Sir Roger Hurn | 128,827 | 3,000 | – | 40,733 | 40,000 | 3,000 | – | 55,498 | 30,000 | |||||||||
| Sir Peter Walters | – | 750 | – | 12,268 | 10,000 | 3,000 | – | 55,498 | 30,000 | |||||||||
| Mr P A Allaire | 34,946 | – | 500 | 13,720 | – | – | 500 | 18,457 | – | |||||||||
| Dr M Barzach | 24,338 | 1,000 | – | 13,578 | – | 1,000 | – | 18,499 | – | |||||||||
| Mr D C Bonham | – | – | – | – | – | 250 | – | 4,539 | – | |||||||||
| Sir Peter Job | 87,128 | 1,000 | – | 13,578 | 45,000 | 1,000 | – | 18,499 | 33,750 | |||||||||
| Mr J H McArthur | 35,827 | – | 500 | 13,720 | – | – | 500 | 18,457 | 18,750 | |||||||||
| Mr D F McHenry | 34,946 | – | 500 | 13,720 | – | – | 500 | 18,457 | – | |||||||||
| Sir Ian Prosser | 67,254 | 1,000 | – | 13,578 | 22,500 | 1,000 | – | 18,499 | 16,875 | |||||||||
| Dr R Schmitz | 54,830 | 1,000 | – | 13,578 | 22,000 | 1,000 | – | 18,499 | 16,170 | |||||||||
| Dr L Shapiro | 53,666 | – | 500 | 13,720 | – | – | 500 | 18,457 | – | |||||||||
| Mr J A Young | – | – | 125 | 3,886 | 14,667 | – | 500 | 18,457 | 45,833 | |||||||||
The total value of holdingsthese shares and ADSs as at 31st December 2002 representsthe date of award, together with the cash payment, forms their total fees, which are included within the Annual remuneration table under ‘Fees and salary’. The table above sets out the value of thosetheir fees received in the form of cash and shares and ADSs.
The shares and ADSs are notionally awarded in 2002to the Non-Executive Directors and prior years,allocated to their interest accounts and are included within the Directors’ interests tables on page 48. The accumulated balance of these shares and ADSs, together with notional dividends subsequently reinvested, are not paid out to the value of dividends re-invested.Non-Executive Directors until retirement. Upon retirement, the Non-Executive Directors will receive either the shares and ADSs or a cash amount equal to the value of the shares and ADSs as at the date of retirement.
| GSK Annual Report 2005 |
| 46 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
The table below sets out the accumulated number of shares and ADSs held by each Non-Executive Director in relation to their fees received as Board members as at 31st December 2005, together with the movements in their account over the year.
| Number of shares and ADSs | ||||||||||||
| Non-Executive Directors’ share arrangements | Dividends | |||||||||||
| Footnote | At 31.12.04 | Elected | reinvested | Paid out | At 31.12.05 | |||||||
| Current Non-Executive Directors | ||||||||||||
| Shares | ||||||||||||
| Sir Crispin Davis | 7,333 | 5,192 | 233 | – | 12,758 | |||||||
| Sir Christopher Gent | 2,921 | 7,349 | 116 | – | 10,386 | |||||||
| Sir Ian Prosser | 12,520 | 3,728 | 342 | – | 16,590 | |||||||
| Dr R Schmitz | 10,771 | 2,810 | 317 | – | 13,898 | |||||||
| Dr L Shapiro | 1,676 | 47 | – | – | 1,723 | |||||||
| Sir Robert Wilson | 1,337 | 1,665 | 45 | – | 3,047 | |||||||
| ADSs | ||||||||||||
| Mr L Culp | 3,348 | 2,769 | 110 | – | 6,227 | |||||||
| Sir Deryck Maughan | 1,248 | 2,947 | 47 | – | 4,242 | |||||||
| Dr L Shapiro | 2,608 | 752 | 66 | – | 3,426 | |||||||
| Former Non-Executive Directors | ||||||||||||
| Shares | ||||||||||||
| Sir Christopher Hogg | a | 48,000 | – | – | 48,000 | – | ||||||
| Sir Roger Hurn | 11,305 | – | 309 | 1,330 | 10,284 | |||||||
| Sir Peter Job | a | 17,638 | – | – | 17,638 | – | ||||||
Dividends are notionally reinvested at the end of the financial year in which payment is made.
The table below sets out the settlement of former Non-Executive Directors’ share arrangements on their leaving the Board:
| Value of awards | Value of awards | Payments | |||||||||||||
| Date of leaving | on allocation | on leaving | in 2005 | ||||||||||||
| Prior years | |||||||||||||||
| Sir Christopher Hogg | a,b | 31.12.04 | £ | 565,857 | £ | 586,559 | £ | 586,559 | |||||||
| Sir Roger Hurn | c | 05.06.03 | £18,198 | ||||||||||||
| Sir Peter Job | a,b | 31.12.04 | £ | 225,360 | £ | 215,538 | £ | 215,538 | |||||||
| a) | Awards to Sir Christopher Hogg and Sir Peter Job under the Non-Executive Directors’ share arrangements were settled in full, with a transfer of shares in January 2005. |
| b) | The change in value of awards between allocation and leaving is attributable to dividends re-invested and the change in share price between the dates of award and the dates of leaving. |
| c) | On leaving the Board, Sir Roger Hurn elected to receive the settlement of his Non-Executive Directors share arrangements in 40 quarterly cash payments. |
| GSK Annual Report 2005 |
| 47 |
| REPORT OF THE DIRECTORS |
| Remuneration Report |
| continued |
Directors’ interests
The following beneficial interests of the Directors of the company are shown in the register maintained by the company in accordance with the Companies Act 1985:
| Shares | ADSs | ||||||||||||
| 3rd March | 31st December | 31st December | 3rd March | 31st December | 31st December | ||||||||
| Footnote | 2003 | 2002 | 2001 | 2003 | 2002 | 2001 | |||||||
| Dr J P Garnier | – | – | – | 55,639 | 55,010 | 53,735 | |||||||
| Mr J D Coombe | a,b | 173,243 | 172,537 | 150,836 | – | – | – | ||||||
| Sir Christopher Hogg | d | 13,714 | 13,714 | 6,216 | – | – | – | ||||||
| Sir Roger Hurn | d | 21,695 | 21,695 | 15,519 | – | – | – | ||||||
| Mr P A Allaire | d | – | – | – | 7,192 | 7,192 | 6,660 | ||||||
| Dr M Barzach | d | 3,028 | 3,028 | 1,990 | – | – | – | ||||||
| Sir Peter Job | d | 9,537 | 9,531 | 5,023 | – | – | – | ||||||
| Mr J H McArthur | d | – | – | – | 6,314 | 6,281 | 5,631 | ||||||
| Mr D F McHenry | c,d | – | – | – | 4,345 | 4,345 | 3,795 | ||||||
| Sir Ian Prosser | d | 7,047 | 7,047 | 4,255 | – | – | – | ||||||
| Dr R Schmitz | d | 4,600 | 4,600 | 1,878 | 2,840 | 2,840 | 3,840 | ||||||
| Dr L Shapiro | d | 1,570 | 1,570 | 1,518 | 3,399 | 3,399 | 2,218 | ||||||
One GlaxoSmithKline ADS represents two GlaxoSmithKline shares.
The interests of the above-mentioned Directors at
Share options
For those options outstanding at 31st December
The lapse dates for Mr Coombe’s options
The Directors hold these options under the various share option plans referred to in Note Following the merger, each of the Directors above elected to exchange their outstanding options in the legacy share option plans for options over Prior to 2003, only those share options granted to the then Executive Directors were subject to a performance condition. In order for the options to vest in full, business performance EPS growth, excluding currency and exceptional items, had on average to be at least three percentage points per annum more than the increase in the UK Retail Prices Index over any three-year performance period. For share options granted in 2003 and 2004 vesting increases on a straight-line basis for EPS performance between the hurdles set out in the following table.
In respect of the 2003 grant, if the performance condition is not met after the three-year measurement period, the performance will be measured again over the four financial years following the date of grant of the options. If the performance condition is not met at the end of four years, the option will lapse. The options granted to the Executive Directors in 2004 were subject to the same performance condition as set in 2003, but to the extent that the performance conditions have not been met at the end of the three-year performance period, the option will lapse with no retesting being permitted.
At the average exchange rate for the year, the above gain made by Dr Garnier amounted to £1,115,143. An EOI benefit of $174,472 (£95,864) was paid to Dr Garnier on exercise of these options. This benefit has been included in the table on page 45. At the average rate for the year, the above gain made by Dr Yamada amounted to £1,145,017. An EOI benefit of $167,405 (£91,981) was paid to Dr Yamada on the exercise Mr Coombe did not exercise any share options during 2005 or 2004. The highest and lowest closing prices during the year ended 31st December
Incentive plans
At the average exchange rate for the year, the above gains by Dr Garnier and Dr Yamada amounted to £897,500 and £256,429, respectively. The PSP is a medium-term incentive scheme introduced during 2001. The PSP replaces the Under the terms of the PSP the number of shares actually vesting is determined following the end of the relevant The measurement period commences on the Prior to the performance period beginning 1st January 2004, awards were in two parts: half can be earned by reference to GSK’s TSR performance compared to the FTSE 100, of which the company is a constituent, and the other half of the award will
The following vesting table applies to the awards with performance periods from 1st January 2004 to 31st December
The Mid-Term Incentive Plan (MTIP) was a share award scheme operated by SmithKline Beecham. The plan closed to new entrants upon completion of the merger and no further participations have been granted.
All SARs held by Dr Dr Shapiro is a member of GlaxoSmithKline’s Scientific Advisory Board (SAB). Dr Shapiro was a member of SmithKline Beecham’s SAB from 1993 until the completion of the merger with Glaxo Wellcome. Along with other members of the SAB, she received annual grants of SmithKline Beecham SARs which, in general, vested three years from the date of grant and will expire 10 years from the date of grant. Grants of SARs to SAB members ceased in 1999. SARs entitle the holder to a cash sum at a future date based on share price growth between the date of grant and the date of exercise. Full provision is made in the financial statements for accrued gains on SARs from the date of grant. In connection with the merger, all previously granted SARs became immediately exercisable.
Pensions
Dr Garnier and Dr Yamada are members of the all employee US cash balance pension plan, under which GlaxoSmithKline makes annual contributions calculated as a percentage of the employee’s base salary and bonus. The fund increases at an interest rate set annually in advance based on the 30-year treasury bond rate to provide a cash sum at retirement. This cash sum is used to purchase a pension at retirement based on the annuity rates applicable at that time. Neither has entitlement to a spouse’s pension or to pension increases, other than by reducing their own initial pension. The normal retirement age under this plan is 65 years of age. Dr Garnier’s pension arrangements have been brought into line with the terms of his service agreement and the assumed retirement age reduced to 60. Similarly Dr Yamada’s assumed retirement age had been reduced to 62. The transfer value, The transfer value, or cash sum, of Dr Yamada’s plan has increased by $458,737 over the year as a result of the further accumulation of interest and contributions paid by the company. Dr Garnier
Mr Heslop’s transfer value
Mr Coombe’s transfer value has been calculated on the basis of actuarial advice in accordance with Actuarial Guidance Note GN11. The transfer value represents the present value of future payments to be made under the pension plan. Mr Coombe’s transfer value increased by £289,211 but his accrued benefit fell by £8,201. This decline is due to Mr Coombe opting to receive a lumpsum on retirement. Mr Coombe waived his 2005 bonus of £106,870. The company made a contribution to the
Directors and Senior Management For US reporting purposes, it is necessary to provide information on compensation and interests of Directors and Senior Management as a group (‘the group’). For the purposes of this disclosure, the group is defined as the Directors, members of the CET and the Company Secretary. In respect of the financial year Directors’ Except as described The Directors’ Remuneration Report has been approved by the Board of Directors and
Sir Christopher Gent
The Operating and financial review and prospects discusses the operating and financial performance, Group. The results for each year, which have been prepared under IFRS, as adopted for use in the European Union, are compared primarily with the results for the preceding
Accounting presentation
Data for market share and market growth rates are GSK estimates based on the most recent data from independent external sources, and where appropriate, are valued in sterling
In order to illustrate underlying performance, it is the Group’s practice to discuss its results in terms of constant exchange rate (CER) growth. This represents growth calculated as if the exchange rates used to determine the results of overseas companies in sterling had remained unchanged from those used in the previous year. CER% represents growth at constant exchange rates. £% represents growth at actual exchange rates.
The gearing ratio is calculated as net debt as a percentage of Exchange rates Average exchange rates prevailing during the period are used to translate the results and cash flows of overseas subsidiary and associated undertakings and joint ventures into sterling. Period end rates are used to translate the net
World economy
GDP growth in China again exceeded expectations at 9.9% and was also robust in India, where continued expansion in services such as information technology remained strong. Global trade arrangements, including those with China, were again in the The Oil prices and higher commodity prices slowed growth in
Exchange The currencies that most influence the Group’s results are the US
World market – pharmaceuticals Global pharmaceutical sales increased by
Pharmaceutical All growth rates included in the review of turnover are at constant exchange rates (CER) unless otherwise stated. The sterling growth rates may be found in the tables of pharmaceutical turnover by therapeutic area on page 59 and by geographic region on page 60. Total pharmaceutical Within the Group’s portfolio,
Pharmaceutical
both cases compared with 2004. Sales of diabetes treatments were also strong, with
Other fast growing products wereLamictalfor epilepsy/bipolar disorder, up 24% (£0.8 billion),Valtrexfor herpes, up 21% (£0.7 billion),Coregfor heart disease, up 32% (£0.6 billion) and vaccines, up 15% (£1.4 billion). In addition, in 2005 there has been a rapid uptake of a number of high potential products such asRequip, for restless legs syndrome (sales up 34% to £156 million),Avodartfor benign prostatic hyperplasia (sales doubled to £129 million) andBoniva/Bonvivafor the treatment of osteoporosis, which was launched in 2005 and captured a 10% share of new prescriptions for oral bisphosphonates in the US market. Respiratory Central nervous system (CNS) CNS sales declined 8% to £3.2 billion. Sales declined in the USA and Europe, with a small gain in International. TotalPaxilsales fell 42% to £615 million, due to generic competition and the interruption in supply toPaxil CRduring the year. See ‘Product supply’ on page 61. Partially mitigating this decline was the strong performance ofPaxilin Japan, up 17% to £197 million. TotalWellbutrinturnover fell 2% to £739 million.Wellbutrin IRand
Anti-virals Anti-bacterials Metabolic Boniva/Bonviva, a new once-monthly oral bisphosphonate for the treatment of osteoporosis, which was developed with Roche, had a strong launch in the USA and in February 2006 had a 10% share of new prescriptions for oral bisphosphonates.Bonivainjection, the first-ever quarterly treatment for osteoporosis, was approved in the USA in January Vaccines In July, GSK acquired Corixa Corporation for Also in December, GSK submitted a “mock-up” dossier to the EMEA for accelerated approval of a potential pandemic influenza vaccine. GSK expects to begin clinical trials in the coming weeks on its H5N1 prototype pandemic vaccine using two different adjuvants: “alum” and a newly developed adjuvant. The Group is in discussions with governments around the world on plans to “prime” populations and stockpile the vaccine. GSK expects to complete its filing in Europe in 2006.
Pharmaceutical turnover by therapeutic area 2005
CER% represents growth at constant exchange rates. £% represents growth at actual exchange rates. Turnover by quarter is given in the Financial record on pages 166 to 171.
Oncology and emesis
Other therapeutic areas Regional analysis Pharmaceutical turnover by geographic region in 2005 on an
Individual governments determine the pricing of medicines in most countries within Europe, which can result in wide price variations for the same product. Parallel trade occurs when third parties exploit this price differential by purchasing products in markets where low prices are enforced and selling them to governments and other purchasers in those markets where higher prices have been agreed. This parallel trade is permitted under the single market rules in the European Union. GSK does not derive any benefit from the profit on resale at the higher price. As a result, management believes that within the European region, turnover by market, on an invoiced basis as presented above, does not properly represent the consumption of the products within each market. GSK employees based in each market are instrumental in the promotion of the Group’s products within their market, thereby creating a product sale and final consumption in that market. The following table gives the adjustments made in order to restate the turnover for markets within Europe on a turnover created basis. Pharmaceutical turnover for Europe region in 2005 on a turnover created basis
These adjustments are GSK’s estimates based on the most recent data from independent external sources, valued in sterling at relevant exchange rates. Management believes that this turnover created basis of reporting turnover by market provides a better reflection of the performance of the businesses in each market within Europe. The total turnover for the Europe region is unaffected by this restatement. Parallel trade occurs occasionally elsewhere in the world, but it is not sufficiently material to affect significantly the turnover data by market presented on an invoiced basis. Pharmaceutical turnover by geographic region in 2005 on a turnover created basis
USA
Sales of
Sales in the anti-virals therapeutic area grew 10% with HIV products up 2%.Valtrex, for herpes, grew 26% driven by patients switching to suppression therapy. Sales ofAvandia/Avandametincreased by 14%. Anti-bacterial sales declined Vaccines grew 26% reflecting the good performance of Europe The Europe region contributed 30% of pharmaceutical turnover and grew 8%, which reflected strong growth in a number of countries and the
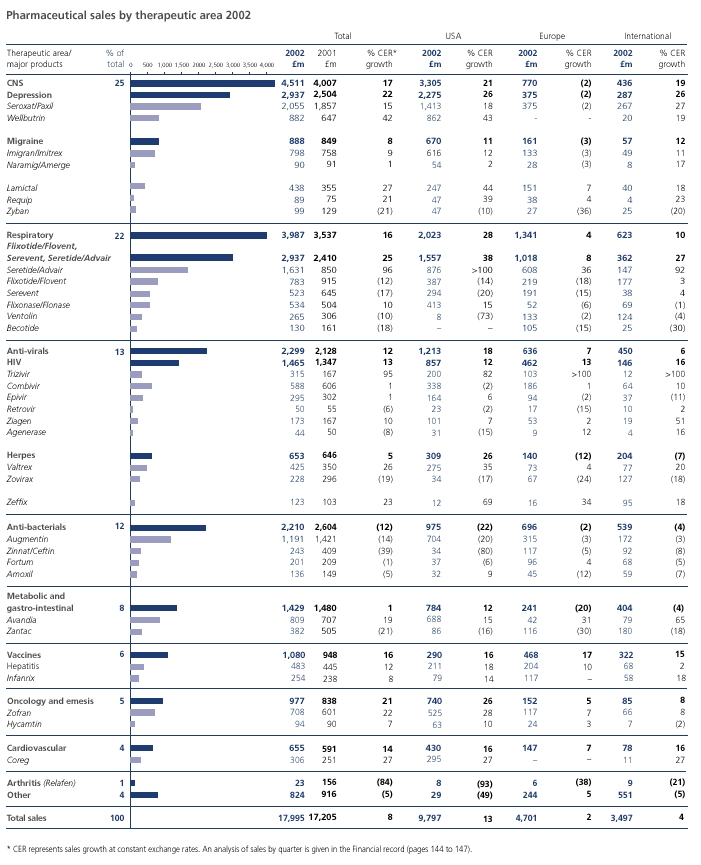
Major growth drivers wereSeretide,
Seroxatsales were down 26%, reflecting generic competition in the majority of markets in the region. Anti-bacterial sales increased 3%, due to a stronger than normal flu season in a number of Southern European markets. International
Product supply
In June 2005, the Group began re-supplying the US and other markets with bothPaxil CRandAvandamet. The sales of
The growth in Consumer Healthcare sales of
OTC medicines On 23rd January 2006, an FDA Advisory Committee recommended that Oral care Nutritional healthcare Operating profit
Cost of sales Selling, general and administration This was due to lower charges related to legal matters, equal to a 2% reduction in total SG&A, and lower share-based payment charges, equal to a 1% decrease in total SG&A, partly offset by higher costs related to programmes to deliver future cost savings equal to a 1% increase in total SG&A. Research and development
Other operating income
Profit before taxation The
Share of profits/(losses) of joint ventures and associated undertakings Disposal of interest in associates
The integrated nature of the Group’s worldwide operations, involving significant investment in research and strategic manufacture at a limited number of locations, with consequential cross-border supply routes into numerous end-markets, gives rise to complexity and delay in negotiations with revenue authorities as to the profits on which individual Group companies are liable to tax. Disagreements with, and between, revenue authorities as to intra-Group transactions, in particular the price at which goods should be transferred between Group companies in different tax jurisdictions, can produce conflicting claims from revenue authorities as to the profits
The Group had total current tax payable liabilities at 31st December 2005 of
Profit for the year
At actual rates of exchange,
Dividend Under IFRS interim dividends are only recognised in the accounts when paid and not when declared. GSK normally pays a dividend two quarters after the quarter to which it relates and one quarter after it is declared. Consequently, the 2005 financial statements recognise the dividends paid in 2005, namely the third and fourth interim dividends for 2004 and the first and second interim dividends for 2005 totalling £2,390 million.
Critical accounting policies The consolidated
The Group’s largest business is US pharmaceuticals, and the US market has the most complex arrangements for rebates, discounts and allowances. The following briefly describes the nature of the arrangements in existence in the Group’s US pharmaceuticals business.
A reconciliation of gross turnover to net turnover for the US pharmaceuticals business is as follows:
The increase in customer returns in 2005 arose from product recalls following the manufacturing issues at the Cidra plant and increased generic competition. The total accruals for rebates, discounts, allowances and returns in the US pharmaceuticals business at 31st December 2005 and 31st December 2004 were as follows:
A monthly process is operated to monitor inventory levels at wholesalers for any abnormal movements. This process uses gross sales volumes, prescription volumes based on third party data sources and information received from key wholesalers. The aim of this is to maintain inventories at a consistent level from year to year based on the pattern of consumption. On this basis, US pharmaceutical inventory levels at wholesalers and in other distribution channels at 31st December 2005 were estimated to amount to less than one month of turnover. This calculation uses third party information, the accuracy of which cannot be totally verified, but which is believed to be sufficiently reliable for this purpose. Taxation The Group has open tax issues with a number of revenue authorities, principally in relation to transfer pricing disputes. GSK uses the best advice in determining its transfer pricing methodology and in seeking to manage transfer pricing issues to a satisfactory conclusion and, on the basis of external professional advice, continues to believe that it has made adequate provision for the liabilities likely to arise from open assessments. However, there continues to be a wide difference of views where open issues exist. The ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of litigation proceedings and negotiations with the relevant tax authorities. Legal and other disputes Impairment of fixed assets Intangible assets
Pensions and Product rights and goodwill
equipment
GSK’s business is science-based, technology-intensive and highly regulated by governmental authorities. The Group allocates significant financial resources to the renewal and maintenance of its property, plant and equipment to minimise risks of interruption of production and to achieve compliance with regulatory standards. A number of its processes use chemicals and hazardous materials. The Group observes stringent procedures and uses specialist skills to manage environmental risks from these activities. Environmental issues, sometimes dating from operations now modified or discontinued, are reported under ‘Responsibility for environment, health and safety’ (page 26) and in Note 41 to the financial statements, ‘Legal proceedings’. GSK believes that its facilities are adequate for its current needs. Other intangible assets Investments
Provisions Provision has been made for tax, legal and other disputes, indemnified disposal liabilities and the costs of manufacturing restructuring and merger integration to the extent that at the balance sheet date an actual or constructive obligation Pensions and other post-employment benefits
Net debt
Net debt
At 31st December In 2005, GSK repurchased £1 billion of shares as Treasury shares and expects to Commitments and contingent liabilities Amounts provided for pensions and post-retirement benefits, restructuring and integration plans and legal, environmental and other disputes are set out in Note Contractual obligations and commitments
The Group has entered into a number of research collaborations to develop new compounds with other pharmaceutical companies. The terms of these arrangements can include up-front fees, equity investments, loans and commitments
Contingent liabilities The following table sets out contingent liabilities, comprising discounted bills, performance guarantees and other items arising in the normal course of business and when they are expected to expire.
In the normal course of business the Group has provided various indemnification guarantees in respect of business disposals in which legal and other disputes have subsequently arisen. A provision is made where a reasonable estimate can be made of the likely outcome of the dispute and this is included in Note It is the Group’s policy to provide for the settlement costs of asserted claims and environmental disputes when a reasonable estimate may be made. Prior to this point no liability is recorded. Legal and environmental costs are discussed in ‘Risk factors’ on pages
A summary of
The net cash inflow from
Free cash flow was £4,664 million, an increase of 26% over 2004. Free cash flow is the amount of cash generated by the business after meeting its obligations for interest, tax and dividends paid to minority interests, and after capital expenditure on non-current tangible and intangible assets. Free cash flow is used by GSK’s management for planning and reporting purposes and in discussions with and presentations to investment analysts. GSK’s free cash flow is presented on a basis other than in accordance with IFRS. This measure may not be directly comparable with similarly described measures used by other companies. A reconciliation of net cash inflow from operating activities, which is the closest equivalent IFRS measure, to free cash flow is shown below. Reconciliation of free cash flow
Reconciliation of net cash flow to movement in net debt
Investment appraisal Capital expenditure
Future
74. The Group may from time to time have additional demands for finance, such as for acquisitions. The Group has access to other sources of liquidity from banks and other financial institutions, in addition to the
Payment policies Group companies are responsible for monitoring and managing their working capital. The terms of sales collections and supplier payments In the UK, the company and each of its UK subsidiaries have policies to ensure that suppliers are paid on time. In particular, the UK companies seek:
The policy includes arrangements for accelerated payment of small suppliers. Payment performance Treasury policies GlaxoSmithKline plc The role of Corporate Treasury in GSK maintains treasury control systems and procedures to monitor foreign exchange, interest rate, liquidity, credit and other financial risks. Liquidity Operating
and short-term investments. The Group also has Treasury operations
GSK balances the use of borrowings and liquid assets having regard to: the cash flow from operating activities and the currencies in which it is earned; the tax cost of intra-Group distributions; the currencies in which business assets are denominated; and the post-tax cost of borrowings compared to the post-tax return on liquid assets. Liquid assets surplus to the immediate operating requirements of Group companies are generally invested and managed centrally by Corporate Treasury. Requirements of Group companies for operating finance are met whenever possible from central resources. External borrowings, mainly managed centrally by Corporate Treasury, comprise a portfolio of long and medium-term instruments and short-term finance.
Funding, maturity and counterparty risk The Group manages its net borrowing The Group’s
Foreign exchange risk management A significant proportion of Group borrowings, including the commercial paper programme, is in US dollars, to benefit from the liquidity of US dollar denominated capital markets. Certain of these and other borrowings are swapped into other currencies as required for Group purposes. The Group seeks to denominate borrowings in the currencies of its principal Borrowings denominated in, or swapped into, foreign currencies Based on the composition of net debt at 31st December Interest rate risk management The Group uses a limited number of interest rate swaps to redenominate external borrowings into the interest rate coupon required for Group purposes. The duration of these swaps matches the duration of the principal instruments. The Group manages centrally the short-term cash surpluses or borrowing requirements of subsidiary companies and uses forward contracts to hedge future repayments back into the originating currency. Sensitivity analysis considers the sensitivity of the Group’s net debt to hypothetical changes in market rates and assumes that all other variables remain constant. Based on the composition of net debt and financing arrangements at 31st December Equity risk management Financial assets and liabilities The Group continues to benefit from strong positive The financial assets and liabilities at 31st December
Outlook
Typically, sales of existing products decline dramatically when generic competition is introduced either on Group’s patent. GlaxoSmithKline is engaged in legal proceedings regarding the validity and infringement of the Group’s patents relating to many of its
The Group has net debt of There are risks and uncertainties inherent in the business Risk factors There are risks and uncertainties relevant to the Group’s business. The factors listed below are among those that the Group thinks could cause the Group’s actual results to differ materially from expected and historical results. Risk that R&D will not deliver commercially successful new products New product candidates may appear promising in development but, after significant investments, fail to reach the market or have only limited commercial success as a result of efficacy or safety concerns, inability to obtain necessary regulatory approvals, difficulty or excessive costs to manufacture, infringement of patents or other intellectual property rights of others or inability to differentiate the product adequately from those Risk of loss or expiration of patents or marketing exclusivity Generic drug manufacturers are seeking to market generic versions of many of the Group’s most important products, includingAvandia,Zofran, Wellbutrin XL, Imitrex,Lamictal,ValtrexandPaxil CR, prior to the expiration of the Group’s patents, and have exhibited a readiness to do so for other products in the future. Generic products competitive withPaxil IRandWellbutrin SRwere launched in the USA in 2003 and 2004, respectively, and had a significant impact on the Group’s overall turnover and earnings. Weakness of intellectual property protection in certain countries Any loss of patent protection, including abrogation of patent rights or compulsory licensing, is likely to affect adversely the Group’s operating results in those national markets but is not expected to be material to the Group overall. Absence of adequate patent protection could limit the opportunity to look to such markets for future sales growth.
Risk of substantial adverse outcome of litigation and government investigations Recent insurance loss experience, including pharmaceutical product liability exposures, has increased the cost of, and narrowed the coverage afforded by, insurance for pharmaceutical companies generally, including the Group. In order to contain insurance costs in recent years the Group has continued to adjust its coverage profile, accepting a greater degree of un-insured exposure. In addition, where claims are made under insurance policies, insurers may reserve the right to deny coverage on various grounds. If denial of coverage is ultimately upheld on these claims, this could result in material additional charges to the Group’s earnings. Product liability litigation Anti-trust litigation Sales, marketing and regulation Risks of competition, price controls and limitations on sales Among these products isAugmentin IR, with respect to which the Group already has generic competition, andZofran,Imitrex, Valtrex, AvandiaandWellbutrin XL, with respect to which the Group’s intellectual property rights in the USA are currently the subject of litigation, andFlonase, for which the FDA approved the first generic version in February 2006.
If these or any of the Group’s other major products were to become subject to a problem such as loss of patent protection, unexpected side effects, regulatory proceedings, publicity affecting doctor or patient confidence or pressure from competitive products, or if a new, more effective treatment should be introduced, the adverse impact on the Group’s revenues and operating results could be significant. In particular, the Group faces intense competition from manufacturers of generic pharmaceutical products in all of its major markets. Generic products often enter the market upon expiration of patents or data exclusivity periods for the Group’s products. Introduction of generic products typically leads to a dramatic loss of sales and reduces the Group’s revenues and margins for its proprietary products. The expiration dates for patents for the Group’s major products are set out
The Group cannot predict whether existing controls will increase or new controls will be introduced that will reduce the Group’s margins or affect adversely its ability to introduce new products profitably. For example, in the USA, where the Group has its highest margins and most sales Regulatory controls Stricter regulatory controls also heighten the risk of withdrawal by regulators on the basis of In addition, in some cases the Group may voluntarily cease marketing a product (for example the withdrawal ofLotronexin 2000 shortly after its initial launch in the USA) or face declining sales based on concerns about efficacy or safety, whether or not scientifically justified, even in the absence of regulatory action.
Risk of interruption of product supply
While the Group
Risk from concentration of sales to wholesalers Environmental liabilities
Reliance on information technology Taxation Disruption from pandemic influenza Global political and economic conditions The Group has no control over changes in inflation and interest rates, foreign currency exchange rates and controls or other economic factors affecting its businesses or the possibility of political unrest, legal and regulatory changes or nationalisation in jurisdictions in which the Group operates. These factors could materially affect the Group’s future
Accounting standards Human resources
In accordance with US SEC disclosure requirements, the following discussion compares results for the year to 31st December All growth rates are at constant exchange rates (CER) unless otherwise stated. The sterling growth rates for turnover by product may be found in the table of pharmaceutical sales by therapeutic area on page 77. Exchange
The pound hit its highest level against the dollar for more than four years, climbing to $1.92 at the year-end, and the Euro gained 1% against sterling and World market – pharmaceuticals Global pharmaceutical sales increased by 9% in 2004 to £284 billion.
Growth in the US market has slowed but remains in double digits and now represents 44% of the global prescription pharmaceutical market compared to 30% a decade ago. At 30th September 2004, GSK held second position in the world pharmaceutical market with
Pharmaceutical All growth rates included in the review of turnover are at constant exchange rates (CER) unless otherwise stated. The sterling growth rates may be found in the tables of pharmaceutical turnover by therapeutic area on page 77. Total pharmaceutical
Pharmaceutical
In all, 12 GSK products each had sales of Respiratory
In the USA,Advairsales grew 20% to £1.3 billion. Growth ofSeretidein Europe was also strong (up 19% to £882 million). International sales grew 15%, reflecting good growth in all geographic areas. The older respiratory productsVentolinandBecotidecontinued to decline as patients converted to newer products.
Anti-virals HIV performance was enhanced by the launch ofEpzicom, a new combination product (Epivir/Ziagen) in the USA in August 2004. Sales of the herpes treatmentValtrexexceeded £500 million for the first time in 2004 (up 24% to £571 million). Performance was driven by the USA (up 30% to £369 million) where the product is the clear market leader in treatments for genital herpes. Anti-bacterials Metabolic Sales in the USA grew 26% to £852 million. Encouragingly,Avandia/ Avandametalso grew very strongly in Europe and International markets with sales up 52% and 62%, respectively. Strong performance in these markets was driven by the growing acceptance amongst opinion leaders and physicians of the benefits of these new products in improving control for diabetic patients. Vaccines Oncology and emesis Cardiovascular and urogenital Other therapeutic areas USA Advairmaintained its strong growth with sales of £1,330 million, up 20%. However, this adversely affected sales of its constituent products,FloventandSerevent,which both showed declines.Flonase, indicated for the treatment of perennial rhinitis, grew by 9%. Sales ofWellbutrinproducts fell 12% to £735 million.Wellbutrin IRandSRsales fell 65% to £270 million as a result of generic competition. The impact was partially offset, however, by the exceptionally strong performance ofWellbutrin XL, the new once-daily product, which achieved sales of £465 million in its first full year on the market. Total sales ofPaxilwere down 51% to £519 million as a result of generic competition toPaxil IR(sales of which declined 82% to £131 million). Mitigating this decline was the performance ofPaxil CR,which generated sales of £388 million, up 13%. Sales in the anti-virals therapeutic area grew 12%, with HIV products up 4%.Valtrex, for herpes, grew 30% driven by patients switching to suppression therapy. Sales ofAvandia/Avandametincreased by 26%. Anti-bacterial sales declined 24% as a result of generic competition that began in the third quarter of 2002.Coregsales increased 37% as it continued to benefit from its wide range of indications. Vaccines grew 6% reflecting the good performance ofPediarix. Europe Europe region contributed 30% of pharmaceutical turnover. Although overall turnover growth in the region was only 2%, good growth was recorded in Spain and Southern and Eastern Europe. Government healthcare reforms, including pricing and reimbursement restrictions, adversely affected turnover in France, Italy and Germany. Seretide, GSK’s largest selling product in Europe, grew 19% and reported notable growth in Spain and the UK.Seretideand its constituent productsSereventandFlixotidegrew 9%. The decline in sales of the herpes franchise was mainly as a result of generic competition forZovirax, partially offset by patients switching to the newer product,Valtrex. Seroxatsales were down 31%, reflecting generic competition in the UK and France. Anti-bacterial sales declined 6% due to generic competition throughout the region Vaccines grew by 7% driven by the hepatitis franchise andInfanrix. International Japan recorded turnover growth of 5%, despite routine government price reductions being implemented in 2004.Paxil, up 25%,Serevent, up 74% andValtrex, up 16% performed particularly well, offsetting small declines inZantacandZovirax. Across all markets in International, the key products driving growth wereSeretide, which grew 15% to record sales of £229 million,Avandia/Avandamet, which grew 62% to £161 million and the vaccines franchise, which recorded growth of 21% and achieved sales of £405 million.
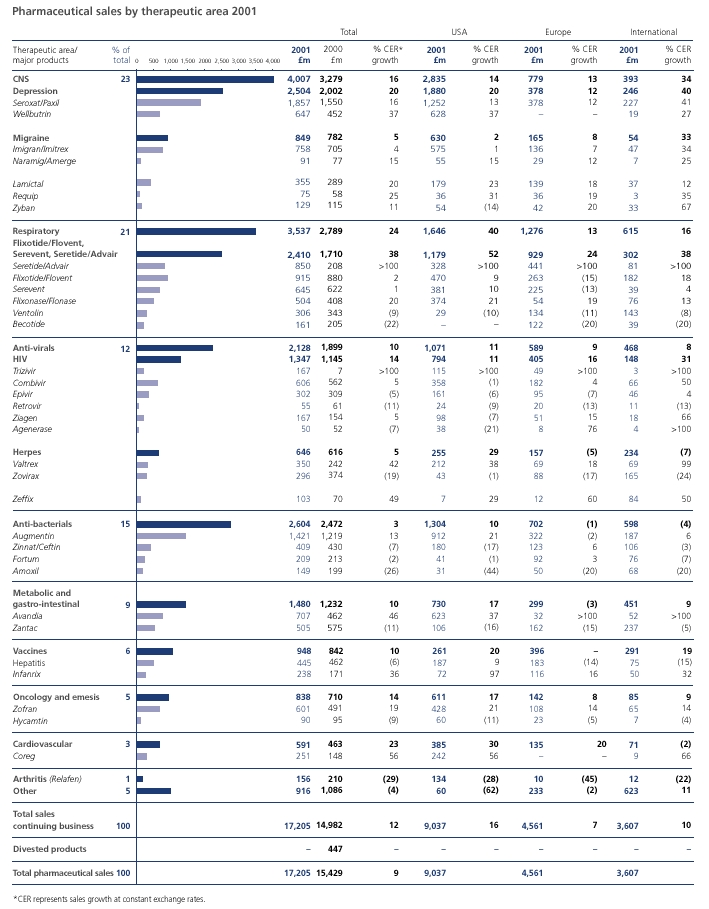
Pharmaceutical turnover by therapeutic area 2004
CER% represents turnover growth at constant exchange rates. £% represents growth at actual exchange rates.
The growth in OTC medicines
In
Nutritional healthcare
Cost of sales
Selling, general and administration Selling, general and administration (SG&A) costs Research and development
Other operating income
Share of after tax profits/(losses) of associates and joint ventures The share of profits of associates
Profit before taxation
The charge for taxation on
The integrated nature of the Group’s worldwide operations, involving significant investment in research and strategic manufacture at a limited number of locations, with consequential cross-border supply routes into numerous end-markets, gives rise to complexity and delay in negotiations with revenue authorities as to the profits on which individual Group companies are liable to tax. Disagreements with, and between, revenue authorities as to intra-Group transactions, in particular the price at which goods should be transferred between Group companies in different tax jurisdictions, can produce conflicting claims from revenue authorities as to the profits
For the latest position on taxation see ‘Taxation’ in the 2005 Year Operating and Financial review and prospects on page 63.
Profit for the year
Profit for the year was £4,022 million, an increase of 4% (7% decline in sterling terms). Net of profits attributable to minority interests, profit attributable to shareholders was £3,908 million, an increase of 4% (7% decline in sterling terms). EPS in 2004 was 68.1 pence compared with 72.3 pence in 2003. The sterling based decline in EPS of 6% reflected the significant weakening of the dollar. Excluding the effects of currency, statutory EPS grew 6% reflecting the completion of the Group’s merger and restructuring programmes in 2003 as well as underlying business growth, partly offset by a higher tax rate. Dividend
This section comprises the Directors’ statements of responsibility, the Independent Auditors’ report on the financial statements and the consolidated financial statements consisting of the principal financial statements and supporting notes prepared under IFRS as adopted for use in the European Union. Also presented is the balance sheet of GlaxoSmithKline plc, which has been prepared under UK GAAP.
Directors’ statement of responsibility in relation to the consolidated financial statements The Directors are responsible for:
The financial statements for the year ended 31st December 2005, comprising principal statements and supporting notes, are set out in ‘Financial statements’ on pages 84 to 164 of this report. The Directors confirm that suitable accounting policies have been consistently applied in the preparation of the financial statements, supported by reasonable and prudent judgements and estimates as necessary. The responsibilities of the auditors in relation to the financial statements are set out in the Independent Auditors’ report (page 83 opposite). The financial statements for the year ended 31st December 2005 are included in the Annual Report 2005, which is published in hard-copy printed form and made available on the website. The Directors are responsible for the maintenance and integrity of the Annual Report on the website in accordance with UK legislation governing the preparation and dissemination of financial statements. Access to the website is available from outside the UK, where comparable legislation may be different. Directors’ remuneration Going concern basis Internal control The Combined Code As required by the Listing Rules of the Financial Services Authority, the auditors have considered the Directors’ statement of compliance in relation to those points of the Combined Code which are specified for their review. Annual Report Sir Christopher Gent
To the Board of Directors and Shareholders of GlaxoSmithKline plc: In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, of recognised income and expense and of cash flows present fairly, in all material respects, the financial position of GlaxoSmithKline plc and its subsidiaries at 31 December 2005 and 31 December 2004, and the results of their operations and their cash flows for each of the three years in the period ended 31 December 2005 in conformity with International Financial Reporting Standards as adopted by the European Union. These financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion. International Financial Reporting Standards as adopted by the European Union vary in certain significant respects from accounting principles generally accepted in the United States of America. Information relating to the nature and effect of such differences is presented in Note 38 to the consolidated financial statements. As discussed in Note 1 to the financial statements, the Group changed the manner in which it accounts for financial instruments as of 1 January 2005. PricewaterhouseCoopers LLP
Approved by the Board on 1st March 2006 Sir Christopher Gent
1Presentation of the financial statements Description of business Compliance with applicable law and IFRS For GSK, there are no differences between IFRS as adopted for use in the European Union and full IFRS as published by the International Accounting Standards Board. Financial period Composition of the Group Composition of financial statements
Additional information in accordance with the requirements of US generally accepted accounting principles (US GAAP) is included in the notes to the financial statements. In Note 38 a statement of differences, and reconciliations of net income and shareholders’ equity, between IFRS and US GAAP are provided. Accounting convention Accounting principles and policies The financial statements have been prepared in accordance with the Group’s accounting policies approved by the Board and described in Note 2. Conversion to IFRS IFRS 1, First-Time Adoption of international Financial Reporting Standards, permits those companies adopting IFRS for the first time to take some exemptions from the full requirements of IFRS in the transition period. GlaxoSmithKline has adopted the following key exemptions:
See Note 40 for further details. 2 Accounting policies Consolidation
The financial statements of entities consolidated are made up to 31st December. Entities over which the Group has the ability to exercise control are accounted for as subsidiaries; where the Group has the ability to exercise joint control, they are accounted for as joint ventures; and where the Group has the ability to exercise significant influence, they are accounted for as associates. Interests acquired in entities are consolidated from the effective date of acquisition and interests sold are consolidated up to the date of disposal.
2Accounting policiescontinued Transactions and balances between subsidiaries are eliminated; no profit before tax is taken on sales between subsidiaries or on sales to joint ventures and associates until the products are sold to customers outside the Group. Deferred tax relief on unrealised intra-Group profit is accounted for only to the extent that it is considered recoverable. Goodwill arising on the acquisition of interests in subsidiaries, joint ventures and associates, representing the excess of the purchase consideration over the Group’s share of the fair values of the identifiable assets, liabilities and contingent liabilities acquired, is capitalised as a separate item in the case of subsidiaries and as part of the cost of investment in the case of joint ventures and associates. Goodwill is denominated in the currency of the operation acquired. In the case of acquisitions prior to 1998, goodwill was written off directly to equity; on a subsequent disposal of assets from such acquisitions, any related goodwill remains in equity and is not charged to the consolidated income statement. Business combinations have not been restated in 2004 and 2003. The results and assets and liabilities of associates and joint ventures are incorporated into the consolidated financial statements using the equity method of accounting. Assets and liabilities, including related goodwill, of overseas subsidiaries, associates and joint ventures, are translated into sterling at rates of exchange ruling at the balance sheet date. The results and cash flows of overseas subsidiaries, associates and joint ventures are translated into sterling using average rates of exchange. Exchange adjustments arising when the opening net assets and the profits for the year retained by overseas subsidiaries, associates and joint ventures are translated into sterling, less exchange differences arising on related foreign currency borrowings which hedge the Group’s net investment in these operations, are taken to a separate component of equity. When translating into sterling the assets, liabilities, results and cash flows of overseas subsidiaries, associates and joint ventures which are reported in currencies of hyper-inflationary economies, adjustments are made to reflect current price levels. Any loss on net monetary assets is charged to the consolidated income statement. Foreign currency transactions Revenue Expenditure Research and development Environmental expenditure Pensions and other post-employment benefits Pension scheme assets are measured at fair value at the balance sheet date. Actuarial gains and losses, differences between the expected and actual returns, and the effect of changes in actuarial assumptions are recognised in the statement of recognised income and expense in the year in which they arise. The Group’s contributions to defined contribution plans are charged to the income statement as incurred. The costs of other post-employment liabilities are calculated in a similar way to defined benefit pension schemes and spread over the period during which benefit is expected to be derived from the employees’ services, in accordance with the advice of qualified actuaries. Legal and other disputes
2Accounting policiescontinued In respect of product liability claims related to products where there is sufficient history of claims made and settlements, an “incurred but not reported” (IBNR) actuarial technique is used to determine a reasonable estimate of the Group’s exposure to unasserted claims for those products and a provision is made on that basis. No provision is made for other unasserted claims or where an obligation exists under a dispute but it is not possible to make a reasonable estimate. Costs associated with claims made by the Group against third parties are charged to the income statement as they are incurred. Employee share plans The Group provides finance to ESOP Trusts to purchase company shares on the open market to meet the obligation to provide shares when employees exercise their options or awards. Costs of running the ESOP Trusts are charged to the income statement. Shares held by the ESOP Trusts are deducted from other reserves and held at the value of the proceeds receivable from employees on exercise. If there is deemed to be a permanent impairment in value this is reflected by a transfer to retained earnings. Property, plant and equipment Depreciation is calculated to write off the cost of PP&E, excluding freehold land, using the straight-line basis over its expected useful life. The normal expected useful lives of the major categories of PP&E are reviewed annually and are:
On disposal of PP&E, the cost and related accumulated depreciation and impairments are removed from the financial statements and the net amount, less any proceeds, is taken to the income statement. Leases Goodwill Where the fair value of the interest acquired in an entity’s assets, liabilities and contingent liabilities exceeds the consideration paid, this excess is recognised immediately as a gain in the income statement. Intangible assets Licences, patents, know-how and marketing rights separately acquired or acquired as part of a business combination are amortised over their estimated useful lives from the time they are available for use. The estimated useful lives for determining the amortisation charge are reviewed annually, and take into account the estimated time it takes to bring the compounds or products to market. Any development costs incurred by the Group and associated with acquired licences, patents, know-how or marketing rights are written off to the income statement when incurred, unless the criteria for recognition of an internally generated intangible asset are met. Brands are valued independently as part of the fair value of businesses acquired from third parties where the brand has a value which is substantial and long-term and where the brands can be sold separately from the rest of the businesses acquired. Brands are amortised over their estimated useful lives, except where it is considered that the useful economic life is indefinite. Prior to 1998, acquired minor brands and similar intangibles were eliminated in the Group balance sheet against reserves in the year of acquisition. The costs of acquiring and developing computer software for internal use and internet sites for external use are capitalised as intangible fixed assets where the software or site supports a significant business system and the expenditure leads to the creation of a durable asset. ERP systems software is amortised over seven years and other computer software over three to five years. Impairment of non-current assets Investments in associates and joint ventures Available-for-sale investments
2Accounting policiescontinued Purchases and sales of equity investments are accounted for on the In 2004 and 2003 equity investments are recorded at cost. Inventories Taxation Deferred tax is provided in full, using the liability method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. Deferred tax assets are recognised to the extent that it is probable that future taxable profits will be available against which the temporary differences can be utilised. Deferred tax is provided on temporary differences arising on investments in subsidiaries, associates and joint ventures, except where the timing of the reversal of the temporary difference can be controlled and it is probable that the temporary difference will not reverse in the foreseeable future. Deferred tax is provided using rates of tax that have been enacted or substantively enacted by the balance sheet date. Deferred tax liabilities and assets are not discounted. Derivative financial instruments and hedging (2005) Derivative financial instruments are initially recognised in the balance sheet at cost and then remeasured at subsequent reporting dates to fair value. Hedging derivatives are classified on inception as fair value hedges, cash flow hedges or net investment hedges. Changes in the fair value of derivatives designated as fair value hedges are recorded in the income statement, with the changes in the fair value of the hedged asset or liability. Changes in the fair value of derivatives designated as cash flow hedges are recognised in equity. Amounts deferred in equity are transferred to the income statement in line with the hedged forecast transaction. Hedges of net investments in foreign entities are accounted for in a similar way to cash flow hedges. Changes in the fair value of any derivative instruments that do not qualify for hedge accounting are recognised immediately in the income statement. Derivative financial instruments and hedging (2004 and 2003) Derivative contracts are treated from inception as an economic hedge of the underlying financial instrument with matching accounting treatment and cash flows. Derivative instruments no longer designated as hedges are restated at market value and any future changes in value are taken directly to the profit and loss account. Currency swaps and forward exchange contracts used to fix the value of the related asset or liability in the contract currency and at the contract rate are accrued to the profit and loss account over the life of the contract. Gains and losses on foreign exchange contracts designated as hedges of forecast foreign exchange transactions are deferred and included in the measurement of the related foreign currency transactions in the period they occur. Gains and losses on balance sheet hedges are accrued and are taken directly to reserves except that forward premiums/discounts are recognised as interest over the life of the contracts. Interest differentials under interest swap agreements are recognised in the profit and loss account by adjustment of interest expense over the life of the agreement. 3New accounting policies and future requirements The following IFRS and IFRIC interpretation have been issued by the IASB and are likely to affect future Annual Reports. IFRS 7 ‘Financial instruments: disclosures’ was issued in August 2005 and is required to be implemented by GSK from 1st January 2007. This new standard incorporates the disclosure requirements of IAS 32, which it supersedes, and adds further quantitative and qualitative disclosures in relation to financial instruments. IFRIC 4 ‘Determining whether an arrangement contains a lease’ was issued in December 2004 and is required to be implemented by GSK from 1st January 2006. The interpretation requires arrangements which may have the nature, but not the legal form, of a lease to be accounted for in accordance with IAS 17 ‘Leases’. This interpretation is not expected to have a material impact on the Group. 4Exchange rates The Group uses the average of exchange rates prevailing during the period to translate the results and cash flows of overseas subsidiaries, joint ventures and associated undertakings into sterling and period end rates to translate the net assets of those undertakings. The currencies which most influence these translations, and the relevant exchange rates, were:
5Segment information The Group’s primary segment reporting is by business sector with geographical reporting being the secondary format. The business sectors consist of Pharmaceuticals (prescription pharmaceuticals and vaccines) and Consumer Healthcare (oral care, OTC medicines and nutritional healthcare). The geographical sectors of the USA, Europe and International (other Rest of World markets) reflect the Group’s most significant regional markets and are consistent with the Group’s regional market management reporting structure. Business sector data includes an allocation of corporate costs to each sector on an appropriate basis. There are no sales between business sectors.The Group’s activities are organised on a global basis. The geographical sector figures are therefore influenced by the location of the Group’s operating resources, in particular manufacturing and research, and by variations over time in intra-Group trading and funding arrangements. Turnover is shown by business sector and by location of customer. Other geographic information is given by location of subsidiary. The UK segment information gives turnover by location of customer and location of subsidiary. The UK operating profit, total assets and net assets are also shown. Where the Group co-promotes a product and the third party records the sale, the Group records its share of revenue as co-promotion income within turnover. The nature of co-promotion activities is such that the Group records no costs of sales. Pharmaceutical turnover includes co-promotion revenue of £112 million (2004 – £65 million, 2003 – £35 million).
5Segment informationcontinued
5Segment informationcontinued UK Segment
6Other operating income
Royalties are principally a core of recurring income from the out-licensing of intellectual property. Asset disposal profits include product divestments and disposals of equity investments, intellectual property and tangible property. Other income includes equity investment carrying value adjustments arising from stock market changes and fair value adjustments arising on the Quest Collar and Theravance put and call options. 7Operating profit
7Operating profitcontinued
Included within audit fees above is a fee of £10,700 (2004 – £10,000, 2003 – £10,000) relating to the company audit of GlaxoSmithKline plc. Included within other non-statutory assurance services are amounts related to the Group’s preparation for the adoption of International Financial Reporting Standards. Other services include human resources advisory, compliance and treasury related services. At 31st December 2005, the amount due to PricewaterhouseCoopers for fees yet to be invoiced was £3.0 million, comprising statutory audit £2.1 million, further assurance £0.7 million and taxation services of £0.2 million. 8Employee costs
The Group provides benefits to employees, commensurate with local practice in individual countries, including, in some markets, healthcare insurance, subsidised car schemes and personal life assurance.
The average number of Group employees excludes temporary and contract staff. The numbers of Group employees at the end of each financial year are given in the Financial record on page 174.The average number of persons employed by GlaxoSmithKline plc in 2005 was nil (2004 – nil). The compensation of the Directors, the CET and the Company Secretary, in aggregate, was as follows:
Information on Directors’ remuneration is given in the Remuneration Report on pages 37 to 54.
9 Finance income
10 Finance costs
11 Associates and joint ventures
Summarised income statement information in respect of the Group’s associates is set out below:
12 Taxation
12Taxationcontinued
The Group operates in countries where the tax rate differs from the UK tax rate. Profits arising from certain operations in Singapore, Puerto Rico, Ireland and Belgium are accorded special status and are taxed at reduced rates compared with the normal rates of tax in these territories. The effect of this reduction in the taxation charge increased earnings per share by 2.7p in 2005, 3.6p in 2004 and 3.9p in 2003. The Group is required under IFRS to create a deferred tax asset in respect of unrealised intercompany profit arising on stock held by the Group at the year end by applying the tax rate of the country in which the stock is held (rather than the tax rate of the country where the profit was originally made and tax paid, which is the practice under UK and US GAAP). The Group tax rate was increased by 1.0% in 2005 (2004 – 0.3%, 2003 – 0.4% decrease) as a result of reductions in work-in-progress and finished goods. The integrated nature of the Group’s worldwide operations, involving significant investment in research and strategic manufacture at a limited number of locations, with consequential cross-border supply routes into numerous end-markets, gives rise to complexity and delay in negotiations with revenue authorities as to the profits on which individual Group companies are liable to tax. Disagreements with, and between, revenue authorities as to intra-Group transactions, in particular the price at which goods should be transferred between Group companies in different tax jurisdictions, can produce conflicting claims from revenue authorities as to the profits to be taxed in individual territories. Resolution of such issues is a continuing fact of life for GSK. The Group has open issues with the revenue authorities in the USA, UK, Japan and Canada; by far the largest relates to Glaxo heritage products, in respect of which the US Internal Revenue Service (IRS) and HM Revenue & Customs (HMRC) in the UK have made competing and contradictory claims. GSK has attempted to settle the US dispute, first through direct discussion with the IRS and subsequently through discussions between the US and UK On 2nd April 2004 the Group filed a petition in the US Tax Court disputing the IRS claim and
GSK is in continuing discussions with HMRC in respect of UK transfer pricing and other matters which are in dispute for the years 1995 to date. However little progress has been made over the past year and consequently these matters may become subject to litigation in due course.
12Taxationcontinued GSK uses the best advice in determining its transfer pricing methodology and in seeking to manage transfer pricing issues to a satisfactory conclusion and, on the basis of external professional advice, continues to believe that it has made adequate provision for the liabilities likely to arise from open assessments.
Except as shown in At 31st December
Deferred taxation provided
Earnings per share
Shares held by the 14 Dividends
Under IFRS interim dividends are only recognised in the financial statements when paid and not when declared. GSK normally pays a dividend two quarters after the quarter to which it relates and one quarter after it is declared. The 2005 financial statements recognise those dividends paid in 2005, namely the third and fourth interim dividends for 2004 and the first and second interim dividends for 2005. The amounts recognised in each year are as follows:
15Property, plant and equipmentcontinued The net book value at 31st December 2005 of the Group’s land and buildings comprises freehold properties £2,635 million (2004 – £2,556 million), properties with leases of 50 years or more £155 million (2004 – £143 million) and properties with leases of less than 50 years £55 million (2004 – £56 million). Included in land and buildings at 31st December 2005 are leased assets with a cost of £165 million (2004 – £155 million), accumulated amortisation of £49 million (2004 – £46 million) and a net book value of £116 million (2004 – £109 million). Included in plant, equipment and vehicles at 31st December 2005 are leased assets with a cost of £153 million (2004 – £93 million), accumulated amortisation of £57 million (2004 – £25 million) and a net book value of £96 million (at 1st January 2005 – £68 million). The impairment losses principally arise from decisions to rationalise facilities and are calculated based on either fair value less costs to sell or value in use. The value in use calculations determine the net present value of the projected risk-adjusted, post-tax cash flows of the relevant asset or cash generating unit, applying a discount rate of the Group post-tax weighted average cost of capital of 8%, adjusted where appropriate for country specific risks. This approximates to applying a pre-tax discount rate to pre-tax cash flows. These losses have been charged through Cost of sales, £16 million, Research and development, £2 million, and Selling, general and administration, £13 million. 16 Goodwill
The additions for the year comprise £357 million on the acquisition of ID Biomedical Corporation and £26 million on the acquisition of Corixa Corporation. See Note 34 for further details. Goodwill is not amortised but is tested for impairment at least annually. Value in use calculations are generally utilised to calculate recoverable amount. Value in use is calculated as the net present value of the projected risk-adjusted, post-tax cash flows of the cash generating unit in which the goodwill is contained, applying a discount rate of the Group post-tax weighted average cost of capital of 8%, adjusted where appropriate for country specific risks. This approximates to applying a pre-tax discount rate to pre-tax cash flows.
17Other intangible assets continued Amortisation and impairment has been charged through Research and development, and Selling, general and administration. At 31st December 2005, the net book value of computer software included £24 million that had been internally generated. The additions through business combinations in the year of £816 million comprise £701 million from the acquisition of ID Biomedical Corporation and £115 million from the acquisition of Corixa Corporation (see Note 34). Other additions to licences and patents Brands
Each of these brands is considered to have an
The main assumptions include future sales prices and volumes, product contribution and the future development expenditure required to maintain the products marketability and registration in the relevant jurisdiction and the product’s life. These assumptions are reviewed as part of management’s budgeting and strategic planning cycle for changes in market conditions and product erosion, through generic competition. 18 Investments in associates and joint ventures
The principal associated undertaking is Quest Diagnostics Inc., a US clinical laboratory business listed on the New York Stock Exchange. The investment At 31st December
18Investments in associates and joint venturescontinued
Investments in joint ventures comprise £17 million share of gross assets (2004 – £16 million) and £3 million share of gross liabilities (2004 – £2 million). These principally arise from 50% interests in two joint ventures, Shionogi-GlaxoSmithKline Holdings, L.P., which is developing specified chemical compounds, and GlaxoSmithKline Shire BioChem, which primarily co-marketsCombivir,TrizivirandEpivirin certain territories.
Other investments comprise non-current equity investments which are available-for-sale investments that are recorded at fair value at each balance sheet date. For investments traded in an active market, the fair value is determined by reference to the relevant stock exchange quoted bid price. For other investments, the fair value is estimated by reference to the current market value of similar instruments or by reference to the discounted cash flows of the underlying net assets. The Group holds a number of equity investments, frequently in entities where the Group has entered into research collaborations. Equity investments are recorded as non-current assets unless they are expected to be sold within one year, in which case they are recorded as current assets. Non-current equity investments include listed investments of £268 million (2004 – £270 million) that offer the Group the opportunity for return through dividend income and fair value gains. On disposal investments fair value movements are reclassified from reserves to the income statement based on average cost. The impairment losses recorded in the tables above have been recognised in the income statement for the year within other operating income, together with amounts recycled from the fair value reserve (Note 32) on recognition of the impairments. These impairments initially result from prolonged or significant declines in the fair value of the equity investments below acquisition cost, subsequent to which any further declines in fair value are immediately taken to the income statement. 20Other non-current assets Other non-current assets comprise of sundry receivables which are due in more than one year, including insurance recovery receivables which have been discounted using risk-free rates of return and derivative financial instruments.
Trade receivables include £2 million (2004 – £7 million) due from associates and joint ventures, and are shown after deducting provisions for bad and doubtful debts of £140 million (2004 – £128 million).
Cash and cash equivalents include highly liquid investments with maturities of three months or less.
Customer return and rebate accruals are provided for by the Group at the point of sale in respect of the estimated rebates, discounts or allowances payable to customers, principally in the USA. Provisions are made at the time of sale but the actual amounts paid are based on claims made some time after the initial recognition of the sale. Because the amounts are estimated they may not fully reflect the final outcome and the amounts are subject to change dependent upon, amongst other things, the types of buying group and product sales mix. The level of provision is reviewed and adjusted quarterly in the light of historical experience of actual rebates, discounts or allowances given and returns made and any changes in arrangements. Future events could cause the assumptions on which the provisions are based to change, which could affect the future results of the Group.
GSK entities operate pension arrangements which cover the Group’s material obligations to provide pensions to retired employees. These arrangements have been developed in accordance with local practices in the countries concerned. Pension benefits can be provided by state schemes; by defined contribution schemes, whereby retirement benefits are determined by the value of funds arising from contributions paid in respect of each employee, or by defined benefit schemes, whereby retirement benefits are based on employee pensionable remuneration and length of service. Some ‘hybrid’ defined benefit schemes also include defined contribution sections. Contributions to defined benefit schemes are determined in accordance with the advice of independent, professionally qualified actuaries. Pension costs of defined benefit schemes for accounting purposes have been assessed in accordance with independent actuarial advice, using the projected unit method. In certain countries pension benefits are provided on an unfunded basis, some administered by trustee companies. Liabilities are generally assessed annually in accordance with the advice of independent actuaries. Formal, independent, actuarial valuations of the Group’s main plans are undertaken regularly, normally at least every three years. The assets of funded schemes are generally held in separately administered trusts or are insured. Assets are invested in different classes in order to maintain a balance between risk and return. Investments are diversified to limit the financial effect of the failure of any individual investment. During 2005, the target asset allocations for the UK schemes were 65% equities and 35% bonds and for the US scheme were 77% equities, 20% bonds and 3% property. The longer term aim is to increase the property element to 10% with a consequent reduction in equities. Actuarial movements in the year are recognised in full through the statement of recognised income and expense. The UK discount rate is based on the iBoxx over 15 year AA index and the US discount rate is based on Moody’s Aa index. The expected return on bonds reflects the portfolio mix of index-linked, government and corporate bonds. An equity risk premium of between 3% and 4% is added to this for equities. Projected inflation rate and pension increases are long term predictions based on the yield gap between long term index-linked and fixed interest Gilts. In the UK, mortality rates are calculated using the PA92 standard mortality tables projected to 2006. Plan obligations are then increased by between 3% and 10%, depending on each individual scheme’s mortality experience, to make allowance for future improvements in life expectancy. In the USA, mortality rates are calculated using the RP2000 fully generational table, projected using scale AA, with the white collar adjustment. This builds in a full allowance for future improvements in life expectancy. During 2005, the Group made special funding contributions to the UK and US pension schemes totalling £366 million. GSK has agreed with the trustees of the UK and US defined benefit pension schemes that the Group would make additional contributions of approximately £370 million per year over a five-year period ending 31st December 2009 in order to eliminate the deficits on an IAS 19 basis, by that point. In the UK the defined benefit pension schemes operated for the benefit of former Glaxo Wellcome employees and former SmithKline Beecham employees remain separate. These schemes were closed to new entrants in 2001 and subsequent UK employees are entitled to join a defined contribution scheme. In the USA the former Glaxo Wellcome and SmithKline Beecham defined benefit schemes were merged during 2001. In addition, the Group operates a number of post-retirement healthcare schemes, the principal one of which is in the USA. The following information relates to the Group’s defined benefit pension and post-retirement healthcare schemes.
The amounts recorded in the income statement and statement of recognised income and expense for the three years ended 31st December 2005 in relation to the defined benefit pension and post-retirement healthcare schemes were as follows:
The total actuarial losses recorded in the statement of recognised income and expense since 1st January 2003 amount to £1,118 million.
The fair values of the assets and liabilities of the UK and US defined benefit schemes, together with aggregated data for other defined benefit schemes in the Group are as follows:
26Pensions and other post-employment benefitscontinued
The liability for the US post-retirement healthcare scheme has been assessed using the same assumptions as for the US pension scheme, together with the assumption for future medical inflation of 10%, reducing by 0.75% per year to 5% in 2013 and thereafter. On this basis the liability for the US scheme has been assessed at £1,133 million (2004 – £895 million; 2003 – £851 million). The defined benefit pension obligation is analysed as follows:
Post-retirement benefits are unfunded.
26Pensions and other post-employment benefitscontinued
The UK defined benefit schemes include defined contribution sections with account balances totalling £515 million at 31st December 2005 (2004 – £404 million, 2003 – £327 million). Information on scheme assets under US GAAP is given in Note 38. Employer contributions for 2006 are estimated to be approximately £700 million in respect of deferred benefit pension schemes and £50 million in respect of post-retirement benefits. The transition date for conversion to IFRS for GSK was 1st January 2003 and therefore the following historical data has been presented from that date. This will be built up to a rolling five year record over the next two years.
26Pensions and other post-employment benefitscontinued
Sensitivity analysis Changes in the assumptions used may have a material impact on the annual defined benefit pension and post-retirement costs or the benefit obligations.
27 Other provisions
The Group has recognised costs in The Group has recognised costs in GlaxoSmithKline is involved in a number of legal and other disputes, including notification of possible claims. Provisions for legal and other disputes
It is in the nature of the Group’s business that a number of these matters, including those provided using the IBNR actuarial technique, may be the subject of negotiation and litigation over several years. The largest individual amounts provided are expected to be settled within At 31st December 2005, it is expected that £115 million (2004 – £236 million) of the provision made for legal and other disputes will be reimbursed. This amount is included within non-current assets. For a discussion of
29 Contingent liabilities
30Net debt
Current assets The effective interest rate on cash and cash equivalents at 31st December 2005 was approximately 4.0%.
The weighted average interest rate on commercial paper borrowings at 31st December The weighted average interest rate on current bank loans and overdrafts at 31st December 2005 was 4.0% (2004 – 3.0%).
The loans repayable after five years carry interest at effective rates between The average effective interest rate of all Notes at 31st December 2005 was approximately 4.5%. Secured loans
At 31st December 2005, of
A total of £6.5 billion has been spent by the company since 2001 on
The table below sets out the monthly purchases under the share buy-back programme:
All shares purchased in 2005 are held as Treasury shares. For details of substantial shareholdings refer
Retained earnings and other reserves
32Movements in equitycontinued
Other reserves include the merger reserve created on the merger of Glaxo Wellcome and SmithKline Beecham amounting to £1,561 million at 31st December
GlaxoSmithKline held an 18.4% interest in Quest Diagnostics Inc. at 31st December In 2005, both the Group and Shionogi & Co. Ltd. entered into transactions with their 50/50 US joint venture company in support of the research and development activities conducted by that joint venture company. During 2005, GlaxoSmithKline provided services to the joint venture of £1 million (2004 – £1 million). At 31st December 2005 the balance due to GlaxoSmithKline from the joint venture was £1 million (2004 – £2 million). Dr Shapiro, a Non-Executive Director of GlaxoSmithKline plc, received fees of $85,000 (2004 – $85,000) of which Dr Barzach, a former Non-Executive Director of GlaxoSmithKline plc, received fees of €84,244 (2004 –€83,005) from a subsidiary of the company for healthcare consultancy provided. These are included within ‘Annual remuneration’ in the Remuneration Report. The aggregate compensation of the Directors, CET and
34Acquisitions and disposals Details of the acquisition and disposal of subsidiary and associated undertakings, joint ventures and 2005
The total consideration included directly attributable costs of £3 million. On 12th July 2005, the Group acquired 92% of the issued share capital of Corixa Corporation, a biotechnology company specialising in developing vaccine adjuvants and immunology based products, for a cash consideration of £150 million. This investment increased the Group’s holding in Corixa to 100%. The Group had a number of business relationships with Corixa prior to the acquisition date, principally in relation to an adjuvant developed by Corixa and used in some of the Group’s vaccines. This transaction has been accounted for by the purchase method of accounting. The existing 8% investment in Corixa, with a book value of £12 million, was
34Acquisitions and disposalscontinued Euclid SR Partners, LP GlaxoSmithKline Consumer Healthcare Limited GlaxoSmithKline Pharmaceuticals Limited GlaxoSmithKline Biologicals (Shanghai) Limited Disposals Aseptic Technologies S.A.
34 Acquisitions and disposals continued 2004
Euclid SR Partners, LP Disposals Quest Diagnostics Inc. GlaxoSmithKline Vehicle Finance Ltd GlaxoSmithKline Pharmaceuticals (Chongqing) Ltd Beeyar Investments (Pty) Ltd OptiLead S.r.l.
34 Acquisitions and disposalscontinued 2003 Acquisitions
Iterfi – Disposals
35 Commitments
A number of commitments were made in 2005 under licensing and other agreements, principally with Vertex Pharmaceuticals Inc. The commitments related to intangible assets include milestone payments, which are dependent on successful clinical development and which represent the maximum that would be paid if all milestones are achieved. GSK has agreed with the trustees of the UK and US pension schemes to make additional contributions of approximately £370 million The Group also has other commitments relating to revenue payments to be made under licences and other alliances, principally to Exelixis Inc. Commitments in respect of future interest payable on loans are disclosed after taking into account the effect of interest rate swaps.
36Financial instruments and related disclosures Financial risk management GSK maintains treasury control systems and procedures to monitor foreign exchange, interest rate, liquidity, credit and other financial risks. GSK uses a variety of financial instruments, including derivatives, to finance its operation and to manage market risks from these operations. Financial instruments include cash and liquid resources, borrowings and spot foreign exchange contracts. A number of derivative financial instruments are used to manage the market risks from Treasury operations. Derivative instruments, principally comprising forward foreign currency contracts and interest rate and currency swaps, are used to swap borrowings and liquid assets into the currencies required for Group purposes and to manage exposure to funding risks from changes in foreign exchange rates and interest rates. GSK balances the use of borrowings and liquid assets having regard to the cash flow from operating activities and the currencies in which it is earned; the tax cost of intra-Group distributions; the currencies in which business assets are denominated; and the post-tax cost of borrowings compared to the post-tax return on liquid assets. Liquid assets surplus to the immediate operating requirements of Group companies are generally invested and managed centrally by Corporate Treasury. Requirements of Group companies for operating finance are met whenever possible from central resources. External borrowings, mainly managed centrally by Corporate Treasury, comprise a portfolio of long and medium-term instruments and short-term finance. GSK does not hold or issue derivative financial instruments for trading purposes and the Group’s Treasury policies specifically prohibit such activity. All transactions in financial instruments are undertaken to manage the risks arising from underlying business activities, not for speculation. Foreign exchange risk management A significant proportion of Group borrowings, including the commercial paper programme, is in US dollars, to benefit from the liquidity of US dollar denominated capital markets. Certain of these and other borrowings are swapped into other currencies as required for Group purposes. The Group seeks to denominate borrowings in the currencies of its principal assets and cash flows. Borrowings denominated in, or swapped into, foreign currencies that match investments in overseas Group assets are treated as a hedge against the relevant net assets. At 31st December 2005, the Group had outstanding contracts to sell or purchase foreign currency having a total gross notional principal amount of £15,974 million (2004 – Based on the composition of net debt at 31st December 2005, a 10% appreciation in sterling against major currencies would result in a reduction in the Group’s net debt of approximately £61 million. A 10% weakening in sterling against major currencies would result in an increase in the Group’s net debt of approximately £75 million. Interest rate risk management The Group uses a limited number of interest rate swaps to redenominate external borrowings into the interest rate coupon required for Group purposes. The duration of these swaps matches the duration of the principal instruments. Interest rate derivative instruments are accounted for as fair value or cash flow hedges of the relevant assets or liabilities. The Group manages centrally the short-term cash surpluses or borrowing requirements of subsidiary companies and uses forward contracts to hedge future repayments back into originating currency. Sensitivity analysis considers the sensitivity of the Group’s net debt to hypothetical changes in market rates and assumes that all other variables remain constant. Based on the composition of net debt and financing arrangements at 31st December 2005, and taking into consideration all fixed rate borrowings in place, a one percentage point (100 basis points) decrease in average interest rates would result in an increase in the Group’s annual net interest charge of approximately £19 million. Market risk of financial assets Equity investments are classified as available-for-sale investments and the Group manages disposals to meet overall business requirements as they arise. The Group regularly monitors the value of its equity investments and only enters into hedges selectively with the approval of the Board. Credit risk
36 Financial instruments and related disclosurescontinued The Group is exposed to a concentration of credit risk in respect of these wholesalers such that, if one or more of them is affected by financial difficulty, it could materially and adversely affect the Group’s financial results. The Group does not believe it is exposed to major concentrations of credit risk on other classes of financial instruments. The Group is exposed to credit-related losses in the event of non-performance by counterparties to financial instruments, but does not expect any counterparties to fail to meet their obligations. Where the Group has significant investments with a single counterparty, collateral is obtained in order to reduce risk. The Group applies Board-approved limits to the amount of credit exposure to any one counterparty and employs strict minimum credit worthiness criteria as to the choice of counterparty. Liquidity Operating cash flow is used to fund investment in the research and development of new products as well as routine outflows of capital expenditure, tax, dividends and repayment of maturing debt. The Group may, from time to time, have additional demands for finance, such as for share purchases and acquisitions. GSK operates with a high level of interest cover and at low levels of net debt relative to its market capitalisation. In addition to the strong positive cash flow from normal trading activities, additional liquidity is readily available via its commercial paper programme and short-term investments. The Group also has a European Medium Term Note programme of £5 billion, of which £3.5 billion was in issue at 31st December 2005. In March 2004, the Group established a US Shelf Registration of $5 billion; at 31st December 2005 $2.4 billion (£1.4 billion) was in issue. Fair value of financial assets and liabilities The fair values of the financial assets and liabilities are included at the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a forced or liquidation sale. The following methods and assumptions were used to estimate the fair values:
In the year ended 31st December 2005, the total amount of the change in fair values estimated using valuation techniques referred to above resulted in a credit to the income statement of £1 million. Fair value of investments in GSK shares Committed facilities
36Financial instruments and related disclosurescontinued 2005 – IFRS disclosures Classification and fair values of financial assets and liabilities
36Financial instruments and related disclosurescontinued Interest rate profiles of financial assets and liabilities
Maturity analysis of interest earning financial assets
36Financial instruments and related disclosurescontinued Currency profiles of financial assets and liabilities
Derivative financial instruments
In 2002, GSK hedged part of the equity value of its holdings in its largest equity investment Quest Diagnostics Inc. through a series of variable sale forward contracts. The contracts (‘the equity collar’) are structured in five series, each over one million Quest shares, and mature between 2006 and 2008. The Group has entered into a put option agreement whereby Theravance’s shareholders can sell up to half of their Theravance shares to GSK at a pre-determined price ($19.375). Given the maximum number of shares subject to the put option, the Group’s obligation is capped at $525 million. At 31st December 2005, this option is recorded as a liability of $81 million (2004 – $132 million). As at 31st December 2005, the maximum potential exposure to GSK from fair value movements of these options is therefore approximately $444 million. The expiry date is August 2007. The Group has entered into a call option agreement whereby it can purchase half of the outstanding Theravance shares in issue at a predetermined price ($54.25). At 31st December 2005, this option is recorded as an asset of $28 million (2004 – $31 million). As at 31st December 2005, the maximum potential exposure to GSK from fair value movements of this option is therefore $28 million. The expiry date is July 2007.
Cash flow hedges Fair value hedges The Group has designated interest rate swaps and the interest element of cross currency swaps as fair value hedges. The risk being hedged is the variability of the fair value of the bonds arising from interest rate fluctuations. Net investment hedges 2004 – UK GAAP disclosures UK GAAP accounting policy for derivative financial instruments Derivative financial instruments are used to manage exposure to market risks from treasury operations. The principal derivative instruments are currency swaps, forward foreign exchange contracts and interest rate swaps. The derivative contracts are treated from inception as an economic hedge of the underlying financial instrument, with matching accounting treatment and cash flows. The derivative contracts have a high correlation with the specific financial instrument being hedged both at inception and throughout the hedge period. Derivative instruments no longer designated as hedges are restated at market value and any future changes in value are taken directly to the income statement. Currency swaps and forward foreign exchange contracts used to fix the value of the related asset or liability in the contract currency and at the contract rate are accrued to the income statement over the life of the contract. Gains and losses on foreign forward exchange contracts designated as hedges of forecast foreign exchange transactions are deferred and included in the measurement of the related foreign currency transactions in the period they occur. Gains and losses on balance sheet hedges are accrued and are taken directly to reserves, except that forward premiums/discounts are recognised as interest over the life of the contracts. Interest differentials under interest swap agreements are recognised in the income statement by adjustment of interest expense over the life of the agreement.
36Financial instruments and related disclosures continued Classification and fair values of financial assets and liabilities
36Financial instruments and related disclosures continued Currency and interest rate risk profile of financial liabilities Total financial liabilities comprise total borrowings of £5,963 million, other long-term payables of £244 million and provisions of £256 million but exclude short-term payables and foreign exchange derivatives of £67 million. The benchmark rate for determining interest payments for all floating rate financial liabilities in the tables below is LIBOR.
Currency and interest rate risk profile of financial assets
36Financial instruments and related disclosurescontinued Currency exposure of net monetary assets/(liabilities)
The unrecognised gains and losses above represent the difference between the carrying amount and the fair value of the currency swaps, interest rate swaps, equity collar and other foreign exchange derivatives. Impact of IAS 32 and IAS 39 adoption on comparative information
37Employee share schemes The Group operates share option schemes, whereby options are granted to employees to acquire shares or ADSs in GlaxoSmithKline plc at the grant price, and share award schemes, whereby awards are granted to employees to acquire shares or ADSs in GlaxoSmithKline plc at no cost, subject to the achievement by the Group of specified performance targets. In 2004, the Group introduced a new share award scheme, the Restricted Share Plan, whereby awards are granted to employees to acquire shares or ADSs in GlaxoSmithKline plc at no cost after a three year vesting period. The granting of restricted share awards has replaced the granting of options to certain employees as the cost of the scheme more readily equates to the potential gain to be made by the employee. The Group operates share option schemes and savings-related share option schemes. Grants under share option schemes are normally exercisable between three and ten years from the date of grant. Grants of restricted shares and share awards are normally exercisable at the end of the three year vesting/performance period. Grants under savings-related share option schemes are normally exercisable after three years’ saving. Options under the share option schemes are normally granted at the market price ruling at the date of grant. In accordance with UK practice, the majority of options under the savings-related share option schemes are granted at a price 20% below the market price ruling at the date of grant. Share options awarded to the Directors and, with effect from the 2004 grant, the CET are subject to performance criteria as laid out in the Remuneration Report. Option pricing
Volatility was determined based on the three year share price history. The fair value of performance share plan grants take into account market conditions. Expected lives of options were determined based on weighted average historic exercises of options.
The average share price in 2005 was £13.42 and $48.88
37Employee share schemescontinued In order to encourage employees to convert options, excluding savings-related share options, held over Glaxo Wellcome or SmithKline Beecham shares or ADSs, into those over GlaxoSmithKline shares or ADSs, a programme was established to give an additional cash benefit of 10% of the exercise price of the original option provided that the employee did not voluntarily leave the Group for two years from the date of the merger and did not exercise the option before the earlier of six months from the expiry date of the original option and two years from the date of the merger. The cash benefit will also be paid if the options expire unexercised if the market price is below the exercise price on the date of expiry.
All of the above options are exercisable, except all options over shares and ADSs granted in 2003, 2004 and 2005 and the savings-related share options granted in 2003, 2004 and 2005. There has been no change in the effective exercise price of any outstanding options during the year.
GlaxoSmithKline share award schemes
Restricted Share Plan
Employee Share Ownership Plan Trusts
The Trusts also acquire and hold shares to meet notional dividends re-invested on deferred awards under the SmithKline Beecham Mid-Term Incentive Plan. The trustees have waived their rights to dividends on the shares held by the ESOP Trusts.
38Reconciliation to US accounting principles The analyses and reconciliations presented in this Note represent the financial information prepared on the basis of US Generally Accepted Accounting Principles (US GAAP) rather than IFRS. Summary of material differences between IFRS and US GAAP Accordingly the net assets of SmithKline Beecham were recognised at fair value as at the date of acquisition. As a result of the fair value exercise, increases in the values of SmithKline Beecham’s inventory, property, plant and equipment, intangible assets, investments and pension obligations were recognised and fair market values attributed to its internally-generated intangible assets, mainly product rights (inclusive of patents and trademarks) and in-process research and development, together with appropriate deferred taxation effects. The difference between the cost of acquisition and the fair value of the assets and liabilities of SmithKline Beecham is recorded as goodwill. Capitalised interest Goodwill Under US GAAP, goodwill arising on acquisitions prior to 30th June 2001 was capitalised and amortised over a period not exceeding 40 years. In July 2001, the Financial Accounting Standards Board (FASB) issued Statement of Financial Accounting Standard (SFAS) 142, ‘Goodwill and Other Intangible Assets’. Like IFRS 3, SFAS 142 requires that goodwill must not be amortised and that annual impairment tests of goodwill must be undertaken. The implementation of SFAS 142 in 2002, a year earlier than the Group’s transition to IFRS, results in goodwill balances acquired between 1998 and 2003 reflecting one year less of amortisation under US GAAP than under IFRS. Under IFRS, costs to be incurred in integrating and restructuring the Wellcome, SmithKline Beecham and Block Drug businesses following the acquisitions in 1995, 2000 and 2001 respectively were charged to the income statement post acquisition. Similarly, integration and restructuring costs arising in respect of the acquisitions of Corixa and ID Biomedical in 2005 have been charged to the income statement under IFRS. Under US GAAP, certain of these costs are considered in the allocation of purchase consideration thereby affecting the goodwill arising on acquisition. In-process research & development (IPR&D) IPR&D acquired in transactions other than business combinations is discussed under Intangible assets below. Intangible assets Under IFRS, intangible assets are amortised over their estimated useful economic life except in the case of certain acquired brands where the end of the useful economic life of the brand cannot be foreseen. Under US GAAP, until the implementation of SFAS 142 ‘Goodwill and
Back to
38 Reconciliation to US accounting principles continued Restructuring costs Other restructuring costs are recorded as a provision under IFRS when a restructuring plan has been announced. Under US GAAP, a provision may only be recognised when further criteria are met or the liability is incurred. Therefore adjustments have been made to eliminate provisions for restructuring costs that do not meet US GAAP requirements. Marketable securities The accounting treatment for marketable securities under US GAAP and IFRS is similar. However, differences do arise, principally as a result of the category of marketable securities as defined by SFAS 115 being smaller than the category of available-for-sale financial assets as defined by IAS 39. Investments which are not marketable securities under the SFAS 115 definition are accounted for at cost less impairments under US GAAP rather than at fair value. The Group did not adopt IAS 39 until 1st January 2005, and, in accordance with the exemption available under IFRS 1, has presented financial instruments in the comparative periods in accordance with UK GAAP. Therefore in 2004 these securities are stated at the lower of cost and net realisable value. Marketable securities are reviewed at least every six months for other than temporary impairment. For equity securities, the factors considered include:
Gross unrealised gains and losses on marketable securities were £36 million and £4 million, respectively, at 31st December The proceeds from sale of marketable securities under US GAAP were £19,416 million in the year ended 31st December 2005. The proceeds include the roll-over of liquid funds on short-term deposit. The gross gains and losses reflected in the consolidated income statement in respect of marketable securities were £7 million and £nil, respectively. Pensions and other post-retirement benefits Stock-based compensation Derivative instruments The Group also evaluates contracts for ‘embedded’ derivatives. In accordance with SFAS 133 requirements, if embedded derivatives are not clearly and closely related to the host contract, they are accounted for separately from the host contract as derivatives. The key differences between IFRS under which the Group’s financial statements are prepared and US GAAP, and in the Group’s application of their respective requirements, are: certain derivatives which are designated by the Group as hedging instruments under IAS 39 are not designated as hedging instruments under SFAS 133. Accordingly, hedge accounting is not applied under US GAAP in respect of these arrangements
38 Reconciliation to US accounting principles
The Group has exercised the exemption available under IFRS 1 to present financial instruments in the comparative periods in accordance with UK GAAP. Under UK GAAP, some derivative instruments used for hedges were not recognised on the balance sheet and the matching principle was used to match the gain or loss under these hedging contracts to the foreign currency transaction or profits to which they related. Gains and losses related to the fair value adjustments on these derivative instruments are therefore reconciling items. As in 2005, the Group did not designate any of its derivatives as qualifying hedge instruments under SFAS 133. The fair value and book value of derivative instruments as at 31st December 2004 is disclosed in the ‘Classification and fair value of financial assets and liabilities’ table in Note 36. Valuation of derivative instruments Foreign exchange contracts are valued using forward rates observed from quoted prices in the relevant markets when possible. The Group assumes parties to long-term contracts are economically viable but reserves the right to exercise early termination rights if economically beneficial when such rights exist in the contract. Dividends Other
Consolidated summary statement of cash flows Comprehensive income statement
38 Reconciliation to US accounting principles Recent Financial Accounting Standards Board (FASB) EITF 05-06 EITF 05-6 is effective immediately. The adoption of EITF 05-6 has not had a material impact on the Group’s consolidated financial position, results of operations or cash flows under US GAAP. SFAS 123R and related FSPs During 2005 the FASB issued FSP 123R-1, FSP 123R-2 and FSP 123R-3. These FSPs detail with various aspects of the implementation of SFAS 123R. GSK is in the process of assessing the impact of the adoption of SFAS 123R on the Group’s consolidated financial position, results of operations and cash flows under US GAAP. Other recent FASB pronouncements In December 2004, the FASB issued SFAS 153, ‘Exchanges of Non-monetary Assets - an amendment of APB Opinion 29’, which amends APB Opinion 29, ‘Accounting for Non-monetary Transactions’ to eliminate the exception for non-monetary exchanges of similar productive assets and replaces it with a general exception for exchanges of non-monetary assets that do not have commercial substance. SFAS 153 is effective for non-monetary asset exchanges occurring in fiscal years beginning after 15th June 2005. In March 2005, the FASB published FASB Staff Position (FSP) FIN 47, ‘Accounting for Conditional Asset Retirement Obligations – an interpretation of FASB Statement No. 143’ which clarifies the application of SFAS 143 ‘Accounting for Obligations Associated with the Retirement of Long-Lived Assets’ in respect of conditional asset retirement obligations. The FSP is effective in the first period beginning after 15th December 2005. In November 2005, the FASB issued FSP 115-1 and FSP 124-1, ‘The Meaning of Other-Than-Temporary Impairment and Its Application to Certain Investments’ which nullify certain requirements of EITF 03-1 and supersede EITF D-44. The FSPs provide guidance for identifying impaired investments and new disclosure requirements for investments that are deemed to be temporarily impaired. The FSPs are effective for fiscal years beginning after 15th December 2005. In November 2005, the FASB issued FSP FIN 45-3 to provide clarification with respect to the application of FIN 45, ‘Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others’. FSP FIN 45-3 includes within its scope and provides guidance concerning the application of FIN 45 to a guarantee granted to a business (or to its owners) that the entity’s revenue (or the revenue of a specified portion of the entity) will meet a minimum amount (referred to as a minimum revenue guarantee). The Group does not expect the adoption of the above pronouncements to have a material impact on its consolidated financial position, results of operations or cash flows under US GAAP. In May 2005, SFAS 154, ‘Accounting Changes and Error Corrections – replacement of APB Opinion 20 and SFAS 3,’ was issued. SFAS 154 changes the accounting for and reporting of a change in accounting principle by requiring retrospective application to prior periods’ financial statements of changes in accounting principle unless impracticable. SFAS 154 is effective for accounting changes made in fiscal years beginning after 15th December 2005. The Group cannot determine the impact of SFAS 154 as it depends in part upon future changes to US accounting principles.
38Reconciliation to US accounting principlescontinued The following is a summary of the material adjustments to profit and shareholders’ funds which would be required if US GAAP had been applied instead of IFRS.
38Reconciliation to US accounting principlescontinued
Notes to the Profit and Equity shareholders’ funds reconciliations (a) Goodwill
Of the £18,672 million (2004 – £18,121 million) US GAAP goodwill balance at 31st December 2005, £15,875 million (2004 – £15,875 million) is in respect of the goodwill arising on the acquisition of SmithKline Beecham by Glaxo Wellcome in 2000. The following tables present the changes in goodwill allocated to the Group’s reportable segments:
(b) Intangible assets
In addition to the above adjustments for amortisation and impairments, further IFRS to US GAAP adjustments arose during the year of £98 million (2004 – £173 million; 2003 – £105 million) in respect of the acquisition and disposal of in-process R&D, licences, patents etc. which are capitalised under IFRS but charged directly to research and development expense under US GAAP, and £nil million (2004 – £37 million; 2003 – £nil) in respect of disposals of product rights which have a higher carrying value under US GAAP than under IFRS.
38Reconciliation to US accounting principlescontinued
Product rights intangible assets under US GAAP are analysed as follows:
The acquired products are pharmaceutical products, principally arising from the acquisition of SmithKline Beecham plc, with book values net of accumulated amortisation and impairment as follows:
The indefinite lived brands relate to a large number of Consumer Healthcare products, principally arising from the acquisitions of SmithKline Beecham plc (including products previously acquired by SmithKline Beecham from Sterling Winthrop Inc.) and the Block Drug Company, with book values as follows:
38Reconciliation to US accounting principlescontinued Each of these brands is considered to have an indefinite life, given the strength and durability of the brand and the level of marketing support. The brands are in relatively stable and profitable market sectors, and their size, diversification and market shares mean that the risk of market-related factors causing a shortening of the brands’ lives is considered to be relatively low. The Group is not aware of any material legal, regulatory, contractual, competitive, economic or other factor which could limit their useful lives. Accordingly, they are not amortised. Each brand is tested annually for impairment applying a fair value less costs to sell methodology and using five year post-tax cash flow forecasts with a terminal value calculation and applying a discount rate of the Group post-tax weighted average cost of capital of 8%. This approximates to applying a pre-tax discount rate to pre-tax cash flows. The carrying values of certain intangibles subject to amortisation were reviewed and an impairment of £68 million (2004 – £26 million) has been recorded. Of this, £46 million (2004 – £nil) relates to pharmaceutical products and £22 million (2004 – £26 million) to Consumer Healthcare products. An impairment charge in respect of Consumer Healthcare intangible assets not subject to amortisation of £50 million was recognised during 2005 (2004 – £nil). As discussed in Note 41 ‘Legal proceedings’, a number of distributors of generic drugs have filed applications to market generic versions of a number of the Group’s products prior to the expiration of the Group’s patents. If generic versions of products are launched in future periods at earlier dates than the Group currently expects, impairments of the carrying value of the products may arise. The estimated future amortisation expense for the next five years for intangible assets subject to amortisation as of 31st December 2005 is as follows:
In-process R&D of £26 million (2004 – £nil; 2003 – £nil) arising on the acquisitions of ID Biomedical and Corixa Corporation has been written-off. This has been valued on the same basis as the other intangible assets acquired and relates to various development projects in the pre-approval stage where the technological feasibility of the projects had not been established at the point of acquisition. (c) Theravance Additionally, the Group has accounted for the Theravance put option discussed above in accordance with SFAS 150, ‘Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity’, which requires the Group to record the fair value of the put option as a liability. The fair value of the Theravance put option at 31st December 2005 is £47 million (2004 – £69 million). In accordance with SFAS 133 ‘Accounting for Derivative Instruments and Hedging Activities’ the call option is not recognised in the financial statements as it is not readily convertible into cash.
38Reconciliation to US accounting principlescontinued
The IFRS to US GAAP adjustment in respect of current tax expense includes £37 million (2004 – £40 million; 2003 – £40 million) for the Group’s share of the tax expense of associates. This is recognised in the Taxation charge in the income statement under US GAAP but recorded in Share of after tax profits of associates in the income statement presented in accordance with IFRS. (e) Deferred taxation under US GAAP Classification of GSK’s deferred taxation liabilities and assets under US GAAP is as follows:
38Reconciliation to US accounting principlescontinued
The disclosures below include the additional information required by SFAS 132R. The pension costs of the UK, US and major overseas defined benefit pension plans have been restated in the following tables in accordance with US GAAP. Minor retirement plans with pension costs in 2005 of £8 million (2004 – £5 million; 2003 – £9 million), have not been recalculated in accordance with the requirements of SFAS 87, and have been excluded.
38Reconciliation to US accounting principlescontinued
Plan assets consist primarily of investments in UK and overseas equities, fixed interest securities, index-linked securities and property. At 31st December 2005 UK equities included 1.9 million GSK shares (2004 – 0.3 million shares) with a market value of £28 million (2004 – £4 million). An analysis of the percentage of total plan assets for each major category is disclosed in Note 26. This analysis includes assets valued at £101 million in minor retirement plans, which have been excluded from these tables.
38Reconciliation to US accounting principlescontinued Post-retirement healthcare under US GAAP
The rate of future healthcare inflation reflects the fact that the benefits of certain groups of participants are capped.
39Principal Group companies The following represent the principal subsidiary and associated undertakings of the GlaxoSmithKline Group at 31st December 2005. Details are given of the principal country of operation, the location of the headquarters, the business segment and the business activities. The equity share capital of these undertakings is wholly owned by the Group except where its percentage interest is shown otherwise. All companies are incorporated in their principal country of operation except where stated.
39Principal Group companiescontinued
39 Principal Group companiescontinued
Background The IFRS project The GlaxoSmithKline Annual Report for the year ending 31st December 2005 is the first Annual Report prepared under IFRS. As 2003 is the earliest year for which full IFRS financial statements are presented in the Annual Report 2005, the transition date to IFRS for GlaxoSmithKline is 1st January 2003. Normally, accounting changes of this nature would require full retrospective application, but GSK has taken advantage of exemptions available under the IFRS transitional rules to apply certain requirements only with effect from the transition date of 1st January 2003 or, in the case of financial instruments, from 1st January 2005. Financial instruments On 1st January 2005 there was an adjustment of £12 million to the opening balance sheet to reflect the movements from the UK GAAP carrying values to the IAS 39 values, which for many financial instruments will be fair value. The financial instruments concerned are:
If the IAS 39 valuation rules had been applied in 2004 there would have been a charge to profit before tax, the largest elements of which arise from the Quest collar (£42 million; 2003 – £42 million) and the Theravance put and call options (£53 million; 2003 – nil). Valuations are inherently unpredictable and changes in the fair values of financial instruments could have a material impact on the future results and financial position of GSK. IFRS adjustments A summary of the principal differences between UK GAAP and IFRS as they apply to GSK is set out below and the financial effect is shown on pages 153 to 156. Customer allowances GSK believes that this reflects best practice in revenue recognition and hence, in the absence of detailed guidance under IFRS, has decided to adopt a revenue recognition policy under IFRS in line with EITF 01-09. Therefore there is not expected to be any difference between turnover reported under IFRS and turnover reported under US GAAP. This adjustment has no impact on profit before tax or EPS. Share-based payments IFRS 2, ‘Share-based Payment’, and its UK GAAP equivalent FRS 20, ‘Share-based Payment’, both of which came into force in 2005, require the fair value of the equity instruments issued to be charged to the income statement. The Group has chosen to recognise all unvested options and awards retrospectively. GSK receives a tax credit, as appropriate, which relates to share options and awards when exercised, based on the gains the holders make and dependent on the tax rules in the country in which the deduction is claimed. The deferred tax asset represents an estimate of future tax relief for this gain and is based on the potential gains available to the option or award holders at the balance sheet date. The movement in deferred tax asset from one balance sheet to the next may result in either a tax credit or a tax charge recorded in the income statement. The amount of any tax credit recognised in the income statement is capped at the cumulative amount of the tax effect of the share-based payment charge. Any excess credit is taken to equity. This adjustment reduced profit before tax in 2004 by £309 million (2003 – £368 million), earnings by £314 million (2003 – £344 million) and EPS by 5.5 pence (2003 – 5.9 pence).
40Transition to IFRScontinued IFRS adjustments Coreg capitalisation and amortisation Other intangible assets amortisation Goodwill amortisation Pensions and other post-employment benefits IAS 19, ‘Employee Benefits’, recognises surpluses and deficits in the accounts, and in accordance with the transitional provisions of IFRS 1, the surpluses and deficits have been recognised in full on the balance sheet at the transition date of 1st January 2003. In addition, following an amendment to IAS 19 issued by the IASB in December 2004, it is permitted to recognise any movements in the surpluses or deficits immediately in the balance sheet, but outside the income statement, in the Statement of recognised income and expense. This means that, in most cases, the balance sheet reflects the full surplus or deficit positions of the funds. The Group’s policy is to charge out to the operating businesses the service cost element of the pension charge, which then gets reported within cost of sales, selling, general and administrative expenditure or research and development as appropriate, but not to charge out the element related to the funding deficit, which is all reported in selling, general and administrative expenditure. Under IAS 19, the service cost element of the total charge is considerably higher than under SSAP 24 and the funding deficit element lower. This has led to an additional reclassification adjustment between the income statement expense headings. The overall impact of the adjustments to pensions and OPEBs in 2004 was a decrease in profit before tax of £36 million (2003 – increase of £11 million) and a decrease in EPS of 0.4 pence (2003 – nil). Share of profits of associates Deferred tax on intercompany profit
40Transition to IFRS continued Other adjustments
Cash flow statement IFRS 1 exemptions and elections
40Transition to IFRScontinued IFRS Consolidated income statement
IFRS Consolidated statement of recognised income and expense
40Transition to IFRScontinued IFRS Consolidated balance sheet
40Transition to IFRScontinued Analysis of IFRS adjustments to the Income Statement Year ended 31st December 2004
Reconciliation of opening equity by component of equity At 1st January 2003
40Transition to IFRScontinued Analysis of IFRS balance sheet adjustments
41Legal proceedings The Group is involved in Intellectual property claims include challenges to the validity of the Group’s patents on various products or processes and assertions of non-infringement of those patents. A loss in any of these cases could result in loss of patent protection for the product at issue. The consequences of any such loss could be a significant decrease in sales of that product and could materially affect future results of operations for the Group. Legal expenses incurred and provisions related to legal claims are charged to selling, general and administration costs. Provisions are made, after taking appropriate legal advice, when a reasonable estimate can be made of the likely outcome of the dispute. In 2004 the Group established an actuarially determined provision for product liability claims incurred but not yet reported as described in Note 27. At 31st December 2005 the Group’s aggregate provision for legal and other disputes (not including tax matters described under ‘Taxation’ in Note 12) was over £1.1 billion. The ultimate liability for legal claims may vary from the amounts provided and is dependent upon the outcome of litigation proceedings, investigations and possible settlement negotiations. The most significant of Intellectual property Advair The Group holds other US patents relating toAdvairwhich are not affected by the re-issue application, including the compound patent related to the active ingredient salmeterol which affords protection through August 2008 (after giving effect to an expected grant of paediatric exclusivity by the FDA) and various patents relating to theDiskusdevice which expire over a period from 2011 to 2016. Avandia and Avandamet Teva subsequently filed an additional certification challenging the validity of the Group’s basic compound patent for rosiglitazone, and in January 2004 the Group commenced an action against Teva in the same court for infringement of that patent. The basic compound patent currently expires in 2012 after giving effect to patent term restoration and paediatric exclusivity. In January 2005, the Group filed an action in the US District Court for the District of New Jersey against Teva for infringement of the same two patents – the basic compound and maleate salt patents for rosiglitazone. Teva had filed an ANDA with the FDA for a generic version ofAvandametwith a certification that those patents are invalid or not infringed. FDA approval of that ANDA is stayed until the earlier of June 2007 or resolution of the patent infringement action. SinceAvandametis protected by the same patents asAvandia, any earlier holding of invalidity in theAvandiacases would be dispositive forAvandametas well. Imitrex In March 2004, the Group commenced an infringement action against Cobalt Pharmaceuticals which was transferred to the US District Court for the Southern District of New York. The defendant had filed an ANDA for sumatripan oral tablets with a certification of invalidity or non-infringement of the same compound patent at issue in the Dr Reddy’s case. Final pre-trial conference in the consolidated Dr Reddy’s and Cobalt case is scheduled for May 2006.
41Legal proceedingscontinued In February 2005, the Group commenced an infringement action in the US District Court for the District of Delaware against Spectrum Pharmaceuticals. The defendant had filed an ANDA for injectable sumatriptan with a certification of invalidity or non-infringement of the same compound patent at issue in the Dr Reddy’s and Cobalt cases. Trial date in this case is set at November 2006. Lamictal Paxil/Seroxat In July 1998,
In February
The Group’s US patent litigation with Synthon BV was settled in December 2003 enabling US marketing of Synthon’s paroxetine mesylate product. This was followed with settlement in August 2004 of most of the Group’s
Paxil CR
41Legal proceedingscontinued Requip Valtrex
Wellbutrin XL
In December 2005, Andrx Pharmaceuticals filed an action against the Group in the US District Court for the
In August 2001, the Group commenced an action in the US District Court for the District of New Jersey against Reddy-Cheminor and Prior to the trial both Reddy-Cheminor and West-ward withdrew their challenge to the compound patent. The trial over infringement and validity of the In March 2002, the Group filed a similar action against Teva In January 2003, In Additional actions remain pending against generic distributors which are asserting that their products do not infringe the Group’s patent for a reduced crystal size of ondansetron, which expires in March 2012 taking into account the extension for paediatric exclusivity, but which are not asserting invalidity or non-infringement of the Group’s compound
41Legal proceedingscontinued Product Liability Paxil The Group has received lawsuits filed in state and federal courts in the USA and Canada on behalf of thousands of plaintiffs, including purported class actions, alleging that paroxetine (the active ingredient in The Group has received numerous claims and lawsuits alleging that treatment withPaxil Avandia
The federal cases have been consolidated in a multidistrict litigation proceeding in the US District Court for the District of
Baycol Following the withdrawal, Bayer and GSK have been named as defendants in thousands of lawsuits filed in state and federal courts in the USA on behalf of both individuals and putative classes of formerBaycolusers. A number of the suits allege that the plaintiffs have suffered personal injuries, including rhabdomyolosis, from the use ofBaycol. Others claim that persons who tookBaycol, although not injured, may be at risk of future injury or may have suffered economic damages from purchasing and usingBaycol. Plaintiffs seek remedies including compensatory, punitive and statutory damages and creation of funds for medical monitoring. GSK and Bayer Corporation, the principal US subsidiary of Bayer AG, have signed an allocation agreement under which Bayer Corporation has agreed to pay 95% of all settlements and compensatory damages judgments with each party retaining responsibility for its own attorneys’ fees and any punitive damages. The federal cases have been consolidated in a multidistrict litigation proceeding in the US District Court for the District of Minnesota. Numerous cases are scheduled for trial in state and federal courts during 2006. To date two statewide class actions have been certified – a medical monitoring case in Pennsylvania and a Consumer Fraud and Deceptive Business Practices Act case in Illinois. The medical monitoring action was dismissed by the court on summary judgment. Another class action, in which GSK was not named as a defendant, has been certified in Oklahoma. A substantial number of claims for death or serious injury have been settled and many others alleging muscle aches and pains have been voluntarily or involuntarily dismissed.
41
Most of the lawsuits seek relief including some combination of compensatory and punitive damages, medical monitoring and refunds for purchases of drugs. In 1997, the Judicial Panel on Multidistrict Litigation issued an order consolidating and transferring all federal actions to the District Court for the Eastern District of Pennsylvania. That court approved a global settlement proposed by defendant Wyeth, which sold fenfluramine and dexfenfluramine. The settlement, subsequently
Lotronex Sales and Marketing and Regulation
On 22nd February 2006, the FDA approved an ANDA filed by Roxane Laboratories for a generic form ofFlonasenasal spray and denied two citizens petitions that had been filed by the Group concerning regulatory criteria that should be applied in determining whether proposed generic products are bioequivalent to, and have the same quality control standards as,Flonase. On 23rd February the US District Court for the District of Maryland granted a temporary restraining order suspending the FDA’s approval of the Roxane ANDA for ten days. The Group will file a motion for a preliminary injunction to continue the interim relief granted in the temporary restraining order and will request a ruling on such motion before the temporary restraining order (as it may be extended for up to an additional ten days) expires. In February 2003, the Verona Public Prosecutor commenced a criminal investigation into GSK’s sales and marketing practices in Italy. Specific areas of investigation include medical education programmes, clinical studies and congresses as well as the interaction between GSK representatives and physicians. Similar issues are being investigated by the Bari public prosecutor. The US Securities and Exchange Commission (SEC) staff has In February 2006, the Group received a subpoena from the SEC in respect of the Group’s participation in the United Nations Oil for Food Programme. The Group is co-operating with the SEC and providing documents responsive to the subpoena.
41Legal proceedingscontinued Average wholesale price Subsequent to the initial subpoenas, several states through their respective attorneys general
Nominal pricing Paxil/Seroxat In the
Cidra, Puerto Rico manufacturing site The Cidra site is engaged in tableting and packaging for a range of GSK products – primarily for the US market – includingPaxil,Paxil CR,Coreg,AvandiaandAvandamet. In April 2005, the Group reached agreement with the FDA on a Consent Decree. The Consent Decree provides for an independent expert to review manufacturing processes at the site for compliance with FDA Good Manufacturing Practice (GMP) requirements. As provided in the Consent Decree, the Group provided a report to the FDA on the deficiencies identified in this review, setting out a corrective plan and timetable for completion. FDA inspectors recently conducted a general GMP inspection and follow-up to the Group’s report. In January 2006, the FDA issued a Form 483, listing five observations that were made during the inspection to which the Group responded in February. Those observations were consistent with the findings of the
The Group was also required to post a bond to ensure that product previously seized by the FDA was appropriately destroyed or reconditioned. The Group has met all the requirements of the bond, which expires in March 2006. In April 2005, the Group received a subpoena from the US Attorney’s Office in Boston requesting production of records regarding manufacturing at the Cidra site covering the same type of information as that collected by the US government in Puerto Rico in 2003.
41Legal proceedingscontinued Anti-trust Paxil/Seroxat
Following public reference to the FTC investigation regardingPaxil, purported class actions In October 2005, the Competition Directorate of the European Commission initiated an inspection concerning allegations that Relafen Following the District Court decision, Canadian importation
In relation to the same matter, the Minnesota state attorney general has filed a civil investigative demand and, subsequently, a complaint alleging that the Group has violated state anti-trust and commercial laws. The Group has filed The Group has also been named as a defendant, along with thirteen other drug companies, in a state court action in California, in which Wellbutrin SR
41Legal proceedingscontinued Commercial and corporate Relenza
Environmental matters GSK has been advised that it may be a responsible party at approximately These proceedings seek to require the operators of hazardous waste facilities, transporters of waste to the sites and generators of hazardous waste disposed of at the sites to clean up the sites or to reimburse the government for cleanup costs. In most instances,
Tax matters
To provide a link between IFRS and UK GAAP, 2003 information is presented also under UK GAAP. The accounting
The
An unaudited analysis is provided by quarter of the Group results in sterling for the financial year
Pharmaceutical turnover – total Group
Pharmaceutical turnover includes co-promotion income.
Pharmaceutical turnover includes co-promotion income.
Pharmaceutical turnover – Europe
Pharmaceutical turnover includes co-promotion income.
Pharmaceutical turnover – International
Pharmaceutical turnover includes co-promotion income.
Five year record A record of financial performance is provided analysed in accordance with current reporting practice. The transition date to IFRS for GlaxoSmithKline is 1st January 2003. Therefore, the 2005, 2004 and 2003 information included in the Five year record is in accordance with IFRS as adopted for use in the European Union. For GSK there are no differences between IFRS as adopted for use in the European Union and full IFRS as published by the International Accounting Standards Board. The 2002 and 2001 information is in accordance with UK GAAP.
To provide a link between IFRS and UK GAAP, 2003 information is also presented under UK GAAP. The accounting policies used in the preparation of the UK GAAP information are disclosed in the 2004 Annual Report. Information prepared under IFRS is not directly comparable with information prepared under UK GAAP. The Five year record also presents information in accordance with US GAAP.
The information presented in accordance with US GAAP is derived from financial information prepared under IFRS, as adopted for use in the European Union, for 2003-2005 and from UK GAAP for 2001-2002. The information below presents US GAAP net income/(loss) and net income/(loss) per share as if the results for the year ended 31st December 2001 were adjusted to reverse the amortisation expense for goodwill and indefinite-lived intangible assets, that is, as if SFAS 142 had also applied in those years.
Exchange rates
The average rate for the year is calculated as the average of the noon buying rates on the last day of each month during the year.
The number of employees is the number of permanent employed staff at the end of the financial period. It excludes those employees who are employed and managed by GlaxoSmithKline on a contract basis.
Share price
The table above sets out the middle market closing prices derived from the London Stock Exchange Daily Official List. The company’s share price Market capitalisation SmithKline Beecham plc Floating Rate Unsecured Loan Stock 1990/2010 The loan stock is not listed on any exchange but holders may require SmithKline Beecham plc to redeem their loan stock at par, i.e. £1 for every £1 of loan stock held, on the first business day of March, June, September and December. Holders wishing to redeem all or part of their loan stock should complete the notice on the back of their loan stock certificate and return it to the registrar, to arrive at least 30 days before the relevant redemption date. Taxation General information concerning the UK and US tax effects of share ownership is set out in 'Taxation information for shareholders' on page 180. Dividends GlaxoSmithKline pays dividends quarterly.
Dividends
Dividends per ADS
Information made available on the website does not constitute part of this Annual Report.
Registrar Lloyds TSB Registrars The registrars also provide the following services:
The Bank of New York’s holding held through BNY (Nominees) Limited represents the company’s ADR programme, whereby each ADS represents two Ordinary Shares of 25p nominal value. At 24th February 2006, the number of holders of record of shares in the USA was 1,190 with holdings of 1,543,844 shares, and the number of registered holders of the ADRs was 41,589 with holdings of 415,217,646 ADRs. Certain of these shares and ADRs were held by brokers or other nominees. As a result the number of holders of record or registered holders in the USA is not representative of the number of beneficial holders or of the residence of beneficial holders. Control of company Major shareholders have the same voting rights per share as all other shareholders. Substantial shareholdings
As far as is known to the company, no other person was the owner of 3% or more of the shares in issue, excluding Treasury Shares of the company. Directors and Officers
<<<
The The following tables set out, for the periods indicated, the high and low middle market closing quotations in pence for the shares on the London Stock Exchange, and the high and low last reported sales prices in US dollars for the ADSs on the NYSE.
Share dealing service Glaxo Wellcome and SmithKline Beecham corporate PEPs The provision of the details above is not intended to be an invitation or inducement to engage in an investment activity. Advice on share dealing should be obtained from a stockbroker or independent financial adviser. AMERICAN DEPOSITARY SHARES In general, the NYSE’s rules permit the company to follow UK corporate governance practices instead of those that apply in the USA, provided that the company explains any significant variations. This explanation is provided on the company’s website. ADR programme administrator The Bank of New York Customer Response Center The administrators also provide
INVESTOR RELATIONS
USA Annual General Meeting
The Annual General Meeting is the Investors holding shares in the company through a nominee service should arrange with that nominee service to be appointed as a corporate representative or proxy in respect of their shareholding in order to attend and vote at the meeting. ADR holders wishing to attend the meeting must obtain a proxy from The Bank of New York which will enable them to attend the meeting and vote on the business to be transacted. ADR holders may instruct The Bank of New York as to
Results Announcements Financial reports The Annual Review is sent to all shareholders on the date of publication. Shareholders may also elect to receive Copies of previous financial reports are available on the
Information for shareholders A summary of the
This statement is based upon UK and US tax laws and practices at the date of this report. The new UK/US Income Tax Convention US holders of ADRs generally will be treated as the owners of the underlying shares for the purposes of the current The following analysis deals with dividends paid after 6th April UK shareholders Taxation of dividends Taxation of capital gains Inheritance tax Such a gift or other disposal is subject to both UK inheritance tax and US estate or gift tax. The Estate and Gift Tax Convention would generally provide for tax paid in the Stamp duty US shareholders The following is a summary of certain Taxation of dividends
Taxation of capital gains Estate and gift taxes Stamp duty No SDRT would be payable on the transfer of an ADR. No UK stamp duty should be payable on the transfer of an ADR provided that the instrument of transfer is executed and remains at all times outside the UK. Any stamp duty on the transfer of an ADR would be payable at a rate of
This table has been provided as a cross reference from the information included in this Annual Report to the requirements of Form 20-F.
Item 19 Exhibits Exhibit Index
Signature The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||