UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________________________________
FORM 20-F
| |
| ¨ | Registration Statement Pursuant to Section 12(b) or 12(g) of The Securities Exchange Act of 1934 |
OR
| |
| ý | Annual Report Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 for the fiscal year ended December 31, 20152018 |
OR
| |
| ¨ | Transition Report Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 |
OR
| |
| ¨ | Shell Company Report Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 |
Commission file number 0-30752
AETERNA ZENTARIS INC.
(Exact Name of Registrant as Specified in its Charter)
Not Applicable
(Translation of Registrant's Name into English)
Canada
(Jurisdiction of Incorporation)
c/o Norton Rose Fulbright Canada LLP315 Sigma Drive
1 Place Ville Marie, Suite 2500Summerville, South Carolina, USA
Montréal, Quebec
Canada H3B 1R129486
(Address of Principal Executive Offices)
Philip TheodoreMichael V. Ward
Telephone: 843-900-3211843-900-3201
E-mail: ptheodore@aezsinc.commward@aezsinc.com
315 Sigma Drive Suite 302D
Summerville, South Carolina
2948329486
(Name, Telephone, E-mail and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
| | |
| Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Shares | | NASDAQ Capital Market Toronto Stock Exchange |
Securities registered or to be registered pursuant to Section 12(g) of the Act: NONE
Securities for which there is a reporting obligation pursuant to Section 15(d) of the ACT: NONE
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as at the close of the period covered by the annual report: 9,928,697 16,440,760 Common Shares as at December 31, 2015.2018.
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ý
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ¨ No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ý No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or, or a non-accelerated filer.filer, or an emerging growth company. See definitions of "accelerated filer" andfiler," "large accelerated filer"filer," and "emerging growth company" in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨Accelerated filer ¨Non-accelerated filer ýEmerging growth company ¨
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act.
† The term "new or revised financial accounting standard" refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
US GAAP ¨ International Financial Reporting Standards as issued by the Other ¨
International Accounting Standards Board ý
If "other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ¨ Item 18 ¨
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No ý
Basis of Presentation
General
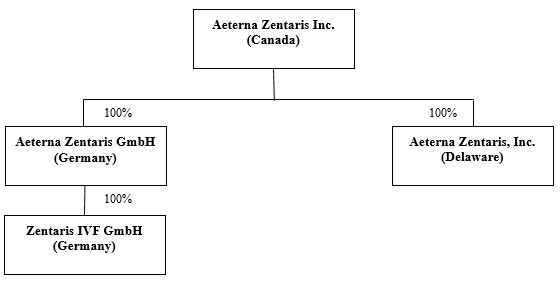
Except where the context otherwise requires, all references in this Annual Report on Form 20-F to the "Company", "Aeterna Zentaris Inc."Zentaris", "we", "us", "our" or similar words or phrases are to Aeterna Zentaris Inc. and its subsidiaries, taken together. In this Annual Report on Form 20-F, references to "$" and "US$"U.S.$" are to United States ("U.S.") dollars, references to "CAN$" are to Canadian dollars and references to "EUR" are to euros. Unless otherwise indicated, the statistical and financial data contained in this Annual Report on Form 20-F are presented as at December 31, 2015.2018.
All share, option and share purchase warrant as well as per share, option and share purchase warrant information presented in this Annual Report on Form 20-F hashave been adjusted, including proportionate adjustments being made to each option and share purchase warrant exercise price, to reflect and to give effect to a share consolidation (or reverse stock split), on November 17, 2015, of our issued and outstanding common shares on a 100-to-1 basis (the "Share Consolidation"). The Share Consolidation affected all shareholders, optionholders and warrantholders uniformly and thus did not materially affect any securityholder's percentage of ownership interest.
This Annual Report on Form 20-F also contains certain information regarding products or product candidates that may potentially compete with our products and product candidates, and such information has been primarily derived from information made publicly available by the companies developing such potentially competing products and product candidates and has not been independently verified by Aeterna Zentaris Inc.
Forward-Looking Statements
This Annual Report on Form 20-F contains forward-looking statements made pursuant to the safe harbor provisionssafe-harbor provision of the U.S. Securities Litigation Reform Act of 1995.1995, which reflect our current expectations regarding future events. Forward-looking statements can be identified by words such as: "intend," "believe," "designed to," "vision," "aimed at," "expect," "may," "should," "would," "will" and similar references. Such statementsmay include, but are not limited to statements aboutpreceded by, followed by, or that include the progress ofwords "will," "expects," "believes," "intends," "would," "could," "may," "anticipates," and similar terms that relate to future events, performance, or our research, development and clinical trials and the timing of, and prospects for, regulatory approval and commercialization of our product candidates, the timing of expected results of our studies and the anticipated results of these studies, statements about the status of our efforts to establish a commercial operation and to obtain the right to promote or sell products that we did not develop, and estimates regarding our capital requirements and our needs for, and our ability to obtain, additional financing.results. Forward-looking statements involve known risks and uncertainties, including those discussed in this Annual Report on Form 20-F, under the caption "Key Information - Risk Factors" filed with the relevant Canadian securities regulatory authorities in lieu of an annual information form and with the U.S. Securities and Exchange Commission ("SEC"). Known and unknown risks and uncertainties which could cause the Company'sour actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, our now heavy dependence on the success of Macrilen™ (macimorelin) and related out-licensing arrangements and the continued availability of funds and resources to pursue our researchsuccessfully launch the product, the ability of Aeterna Zentaris to enter into out-licensing, development, manufacturing and development ("R&D") projects,marketing and distribution agreements with other pharmaceutical companies and keep such agreements in effect, reliance on third parties for the successfulmanufacturing and timely completion of clinical studies, the risk that safety and efficacy data from anycommercialization of our Phase 3 trials may not coincideproduct candidates, potential disputes with third parties, leading to delays in or termination of the data analysis from previously reported Phase 1 and/manufacturing, development, out-licensing or Phase 2 clinical trials, the rejectioncommercialization of our product candidates, or non-acceptance of any new drug application by oneresulting in significant litigation or more regulatory authoritiesarbitration, and, more generally, uncertainties related to the regulatory process, the ability of the Company to efficiently commercialize one or more of our products or product candidates,out-license Macrilen™ (macimorelin), the degree of market acceptance onceof Macrilen™ (macimorelin), our ability to obtain necessary approvals from the relevant regulatory authorities to enable us to use the desired brand names for our products, are approved for commercialization, the abilityimpact of securities class action litigation, on our cash flow, results of operations and financial position; any evaluation of potential strategic alternatives to maximize potential future growth and stakeholder value may not result in any such alternative being pursued, and even if pursued, may not result in the Companyanticipated benefits, our ability to take advantage of business opportunities in the pharmaceutical industry, theour ability of the Company to protect itsour intellectual property, the potential of liability arising from shareholder lawsuits and general changes in economic conditions. Investors should consult the Company's quarterly and annual filings with the Canadian and United States ("U.S.") securities commissions for additional information on risks and uncertainties. Given these uncertainties relating to the forward-looking statements. Investorsand risk factors, readers are cautioned not to place undue reliance on these forward-looking statements. The Company does not undertake to update these forward-looking statements and disclaimsWe disclaim any obligation to update any such factors or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except ifunless required to do so by a governmental authority or applicable law.
TABLE OF CONTENTS
GENERAL INFORMATION
Page
|
| | |
| | Page |
| Item 1. | | |
| | | |
| | | |
| | | |
| Item 2. | | |
| | | |
| | | |
| Item 3. | | |
| | | |
| | | |
| | | |
| | | |
| Item 4. | | |
| | | |
| | | |
| | | |
| | | |
| Item 4A. | | |
| Item 5. | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Item 6. | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Item 7. | | |
| | | |
| | | |
| | | |
| Item 8. | | |
| | | |
| | | |
| Item 9. | | |
| | | |
| | | |
| | | |
| | | |
| | | |
|
| | |
| | | |
| Item 10. | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Item 11. | | |
| Item 12. | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Item 13. | | |
| Item 14. | | |
| Item 15. | | |
| Item 16A. | | |
| Item 16B. | | |
| Item 16C. | | |
| Item 16D. | | |
| Item 16E. | | |
| Item 16F. | | |
| Item 16G. | | |
| Item 16H. | | |
| | | |
| | |
| Item 17. | | |
| Item 18. | | |
| Item 19. | | |
PART I
| |
| Item 1. | Identity of Directors, Senior Management and Advisers |
| |
| A. | Directors and senior management |
Not applicable.
Not applicable.
Not applicable.
| |
| Item 2. | Offer Statistics and Expected Timetable |
Not applicable.
| |
| B. | Method and expected timetable |
Not applicable.
| |
| A. | Selected financial data |
The consolidated statement of comprehensive income (loss) income datainformation set forth in this Item 3.A3.A. with respect to the years ended December 31, 2015, 20142018, 2017 and 20132016 and the consolidated statement of financial position datainformation as at December 31, 20152018 and 20142017 have been derived from the audited consolidated financial statements set forth in Item 18, which have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards Board ("IASB"). The consolidated statement of comprehensive income (loss) income information with respect to the years ended December 31, 20122015 and 20112014 and the consolidated statement of financial position information as at December 31, 2013, 20122016, 2015 and 20112014 set forth in this Item 3.A. have been derived from our previous consolidated financial statements not included herein, and have also been prepared in accordance with IFRS, as issued by the IASB. The selected financial data should be read in conjunction with our audited consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 20-F, as well as "Item 5. – Operating and Financial Review and Prospects" of this Annual Report on Form 20-F.
1The Company has not declared or paid any dividends per share during the periods covered by the selected financial data.
Consolidated Statements of Comprehensive Income (Loss) Income Information
(in thousands of USU.S. dollars, except share and per share data)
Derived from consolidated audited financial statements prepared in accordance with IFRS, as issued by the IASB
| | | | | Years ended December 31, | December 31, |
| | | 2015 | | 2014 | | 2013 | | 2012 | | 2011 | 2018 | | 2017 | | 2016 | | 2015 | | 2014 |
| | | $ | | $ | | $ | | $ | | $ | $ | | $ | | $ | | $ | | $ |
| Revenues | | | | | | | | | | | | | | | | | | | |
| License fees | | 24,325 |
| | 458 |
| | 497 |
| | 248 |
| | 11 |
|
| Product sales | | 2,167 |
| | — |
| | — |
| | — |
| | — |
|
| Royalty income | | 184 |
| | — |
| | — |
| | — |
| | — |
|
| Sales commission and other | | 297 |
| | — |
| | 96 |
| | 834 |
| | 250 |
| 205 |
| | 465 |
| | 414 |
| | 297 |
| | — |
|
| License fees | | 248 |
| | 11 |
| | 6,079 |
| | 1,219 |
| | 4,455 |
| |
| | | 545 |
| | 11 |
| | 6,175 |
| | 2,053 |
| | 4,705 |
| 26,881 |
| | 923 |
| | 911 |
| | 545 |
| | 11 |
|
| Operating expenses | | | | | | | | | | | |
| Cost of sales | | — |
| | — |
| | 51 |
| | 591 |
| | 212 |
| 2,104 |
| | — |
| | — |
| | — |
| | — |
|
| Research and development costs | | 17,234 |
| | 23,716 |
| | 21,284 |
| | 20,592 |
| | 24,245 |
| 2,932 |
| | 10,704 |
| | 16,495 |
| | 17,234 |
| | 23,716 |
|
| General and administrative expenses | | 11,308 |
| | 9,840 |
| | 11,091 |
| | 9,226 |
| | 10,046 |
| 8,894 |
| | 8,198 |
| | 7,147 |
| | 11,308 |
| | 9,840 |
|
| Selling expenses | | 6,887 |
| | 3,850 |
| | 1,225 |
| | 1,380 |
| | 1,909 |
| 3,109 |
| | 5,095 |
| | 6,745 |
| | 6,887 |
| | 3,850 |
|
| | | 35,429 |
| | 37,406 |
| | 33,651 |
| | 31,789 |
| | 36,412 |
| 17,039 |
| | 23,997 |
| | 30,387 |
| | 35,429 |
| | 37,406 |
|
| Loss from operations | | (34,884 | ) | | (37,395 | ) | | (27,476 | ) | | (29,736 | ) | | (31,707 | ) | |
| Finance income | | 305 |
| | 20,319 |
| | 1,748 |
| | 6,974 |
| | 6,239 |
| |
| Finance costs | | (15,649 | ) | | — |
| | (1,512 | ) | | (382 | ) | | (8 | ) | |
| Net finance (costs) income | | (15,344 | ) | | 20,319 |
| | 236 |
| | 6,592 |
| | 6,231 |
| |
| Loss before income taxes | | (50,228 | ) | | (17,076 | ) | | (27,240 | ) | | (23,144 | ) | | (25,476 | ) | |
| Income tax expense | | — |
| | (111 | ) | | — |
| | — |
| | (1,104 | ) | |
| Net loss from continuing operations | | (50,228 | ) | | (17,187 | ) | | (27,240 | ) | | (23,144 | ) | | (26,580 | ) | |
| Income (loss) from operations | | 9,842 |
| | (23,074 | ) | | (29,476 | ) | | (34,884 | ) | | (37,395 | ) |
| Settlements | | (1,400 | ) | | — |
| | — |
| | — |
| | — |
|
| Gain (loss) due to changes in foreign currency exchange rates | | 656 |
| | 502 |
| | (70 | ) | | (1,767 | ) | | 1,879 |
|
| Change in fair value of warrant liability | | 263 |
| | 2,222 |
| | 4,437 |
| | (10,956 | ) | | 18,272 |
|
| Warrant exercise inducement fee | | — |
| | — |
| | — |
| | (2,926 | ) | | — |
|
| Other finance income | | 278 |
| | 75 |
| | 150 |
| | 305 |
| | 168 |
|
| Net finance income (costs) | | 1,197 |
| | 2,799 |
| | 4,517 |
| | (15,344 | ) | | 20,319 |
|
| Income (loss) before income taxes | | 9,639 |
| | (20,275 | ) | | (24,959 | ) | | (50,228 | ) | | (17,076 | ) |
| Income tax recovery (expense) | | (5,452 | ) | | 3,479 |
| | — |
| | — |
| | (111 | ) |
| Net income (loss) from operations | | 4,187 |
| | (16,796 | ) | | (24,959 | ) | | (50,228 | ) | | (17,187 | ) |
| Net income from discontinued operations | | 85 |
| | 623 |
| | 34,055 |
| | 2,732 |
| | (487 | ) | — |
| | — |
| | — |
| | 85 |
| | 623 |
|
| Net (loss) income | | (50,143 | ) | | (16,564 | ) | | 6,815 |
| | (20,412 | ) | | (27,067 | ) | 4,187 |
| | (16,796 | ) | | (24,959 | ) | | (50,143 | ) | | (16,564 | ) |
| Other comprehensive (loss) income: | | | | | | | |
| |
| |
| Other comprehensive income (loss): | | | | | | | | | | |
| Items that may be reclassified subsequently to profit or loss: | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustments | | 1,509 |
| | (1,158 | ) | | 1,073 |
| | (504 | ) | | (789 | ) | (260 | ) | | (1,430 | ) | | 569 |
| | 1,509 |
| | (1,158 | ) |
| Items that will not be reclassified to profit or loss: | | | | | | | | | | | | | | | | | | | |
| Actuarial gain (loss) on defined benefit plans | | 844 |
| | (1,833 | ) | | 2,346 |
| | (3,705 | ) | | (1,335 | ) | 193 |
| | 694 |
| | (1,479 | ) | | 844 |
| | (1,833 | ) |
| Comprehensive (loss) income | | (47,790 | ) | | (19,555 | ) | | 10,234 |
| | (24,621 | ) | | (29,191 | ) | 4,120 |
| | (17,532 | ) | | (25,869 | ) | | (47,790 | ) | | (19,555 | ) |
Net loss per share (basic and diluted) from continuing operations1 | | (18.17 | ) | | (29.12 | ) | | (92.41 | ) | | (117.04 | ) | | (168.75 | ) | |
Basic Net income (loss) per share from continuing operations(1) | | 0.25 |
| | (1.12 | ) | | (2.41 | ) | | (18.17 | ) | | (29.12 | ) |
Diluted Net income (loss) per share from continuing operations(1) | | 0.24 |
| | (1.12 | ) | | (2.41 | ) | | (18.17 | ) | | (29.12 | ) |
Net income per share (basic and diluted) from discontinued operations1 | | 0.03 |
| | 1.06 |
| | 115.53 |
| | 13.79 |
| | (3.09 | ) | — |
| | — |
| | — |
| | 0.03 |
| | 1.06 |
|
Net (loss) income per share (basic and diluted)1 | | (18.14 | ) | | (28.06 | ) | | 23.12 |
| | (103.22 | ) | | (171.84 | ) | |
Weighted average number of shares outstanding:1 | | | | | | | |
|
| |
|
| |
Net (loss) income per share (basic)1 | | 0.25 |
| | (1.12 | ) | | (2.41 | ) | | (18.14 | ) | | (28.06 | ) |
Net (loss) income per share (diluted)1 | | 0.24 |
| | (1.12 | ) | | (2.41 | ) | | (18.14 | ) | | (28.06 | ) |
| Weighted average number of shares outstanding: | | | | | | | | | | |
| Basic | | 2,763,603 |
| | 590,247 |
| | 294,765 |
| | 197,751 |
| | 157,513 |
| 16,440,760 |
| | 14,958,704 |
| | 10,348,879 |
| | 2,763,603 |
| | 590,247 |
|
| Diluted | | 3,424,336 |
| | 590,247 |
| | 294,765 |
| | 198,067 |
| | 157,513 |
| 17,034,812 |
| | 14,958,704 |
| | 10,348,879 |
| | 2,763,603 |
| | 590,247 |
|
| |
1 | Adjusted to reflect the November 17, 2015 100-to-1 Share Consolidation |
Consolidated Statement of Financial Position Information
(in thousands of USU.S. dollars)
Derived from consolidated financial statements prepared in accordance with IFRS, as issued by the IASB
| | | | | As at December 31, | | As at December 31, |
| | | 2015 | | 2014 | | 2013 | | 2012 | | 2011 | | 2018 | | 2017 | | 2016 | | 2015 | | 2014 |
| | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ |
| Cash and cash equivalents | | 41,450 |
| | 34,931 |
| | 43,202 |
| | 39,521 |
| | 46,881 |
| | 14,512 |
| | 7,780 |
| | 21,999 |
| | 41,450 |
| | 34,931 |
|
| Restricted cash equivalents | | 255 |
| | 760 |
| | 865 |
| | 826 |
| | 806 |
| | 418 |
| | 381 |
| | 496 |
| | 255 |
| | 760 |
|
| Total assets | | 51,498 |
| | 47,435 |
| | 59,196 |
| | 67,665 |
| | 75,369 |
| | 25,011 |
| | 22,195 |
| | 31,659 |
| | 51,498 |
| | 47,435 |
|
| Warrant liability (current and non-current portion) | | 10,891 |
| | 8,225 |
| | 18,010 |
| | 6,176 |
| | 9,162 |
| | 3,634 |
| | 3,897 |
| | 6,854 |
| | 10,891 |
| | 8,225 |
|
| Share capital | | 204,596 |
| | 150,544 |
| | 134,101 |
| | 122,791 |
| | 101,884 |
| | 222,335 |
| | 222,335 |
| | 213,980 |
| | 204,596 |
| | 150,544 |
|
| Shareholders' equity (deficiency) | | 21,615 |
| | 14,484 |
| | 17,064 |
| | (6,695 | ) | | (4,546 | ) | |
| Shareholders' (deficiency) equity | | | 1,907 |
| | (2,783 | ) | | 6,212 |
| | 21,615 |
| | 14,484 |
|
| |
| B. | Capitalization and indebtedness |
Not applicable.
| |
| C. | Reasons for the offer and use of proceeds |
Not applicable.
D. Risk factorsAn investment in our securities involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this Annual Report, before making an investment decision. If any of the following risks actually occurs, our business, prospects, financial condition or results of operations could suffer. In that case, the trading price, if any, of our securities could decline, and you may lose all or part of your investment.
Risks Relating to Us and Our Business
Investments in biopharmaceutical companies are generally considered to be speculative.speculative in nature.
The prospects for companies operating in the biopharmaceutical industry are uncertain, given the very nature of the industry, and, accordingly, investments in biopharmaceutical companies should be considered to be speculative assets.
We have a history of operating losses and we may never achieve or maintain operating profitability. If we are unsuccessful in generating new revenue, increasing our revenues and/or raising additional funding, we may not be able to continue as a going concern.
We have incurred, and expect to continue to incur, substantial expenses in our efforts to develop and marketcommercialize products. Consequently, we have incurred operating losses historically and in each of the last several years. As at December 31, 2015,2018, we had an accumulated deficit of approximately $271.6$310 million. Our operating losses have adversely impacted, and will continue to adversely impact, our working capital, total assets, operating cash flow and shareholders’shareholders' equity. We do not expect to reach operating profitability in the immediate future, and our operating expenses are likely to continue to represent a significant component of our overall cost profile as we continue our R&D and clinical study programs, seek regulatory approval for our product candidates and carry out commercial activities. Even if we succeed infocus on the commercialization of Macrilen™ (macimorelin). In developing, acquiring, or in-licensing new commercial products,out-licensing Macrilen™ (macimorelin), we could incur additional operating losses for at least the next several years. If we do not ultimately generate sufficient revenue from a commercialized products toproduct and achieve or maintain operating profitability, an investment in our Common Shares or other securities could result in a significant or total loss.
Our revenues and expenses may fluctuate significantly, and any failure to meet financial expectations may disappoint securities analysts or investors and result in a decline in the price or the value of our Common Shares or other securities.
We have a history of operating losses. Our revenues and expenses have fluctuated in the past and may continue to do so in the future. These fluctuations could cause our share price or the value of our other securities to decline. Some of the factors that could cause our revenues and expenses to fluctuate include but are not limited to:
the inability to complete product development in a timely manner that results in a failure or delay in receiving the required regulatory approvals to commercialize our product candidates;
the timing of regulatory submissions and approvals;
the timing and willingness of any current or future collaborators to invest the resources necessary to commercialize our product candidates;
the nature and timing of licensing fee revenues;
the outcome of litigation, including the securities class action litigation pending against us that is described elsewhere in this Annual Report on Form 20-F;
foreign currency fluctuations;
the timing of the achievement and the receipt of milestone payments from current or future collaborators; and
failure to enter into new or the expiration or termination of current agreements with collaborators.
Due to fluctuations in our revenues and expenses, we believe that period-to-period comparisons of our results of operations are not necessarily indicative of our future performance. It is possible that in some future quarters or years, our revenues and expenses will be above or below the expectations of securities analysts or investors. In this case, the price of our Common Shares and/or the value of our other securities could fluctuate significantly or decline.
Our clinical trials may not yield results that will enable us to obtain regulatory approval for our products, and a setback in any of our clinical trials would likely cause a drop in the price of our Common Shares or a decline in the value of our other securities.
We will only receive regulatory approval for a product candidate if we can demonstrate, in carefully designed and conducted clinical trials, that the product candidate is both safe and effective. We do not know whether our pending or any future clinical trials will demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals or will result in marketable products. Unfavorable data from those studies could result in the withdrawal of marketing approval for approved products or an extension of the review period for developmental products. Preclinical testing and clinical development are inherently lengthy, complex, expensive and uncertain processes and have a high risk of failure. It typically takes many years to complete testing, and failure can occur at any stage of testing. Results attained in preclinical testing and early clinical studies, or trials, may not be indicative of results that are obtained in later studies. In addition, we have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approval and, accordingly, may encounter unforeseen problems and delays in the approval process. Furthermore, errors in the conduct, monitoring and/or auditing of a clinical trial, whether made by us or by a contract research organization (a “CRO”) that we retain could invalidate the results from a regulatory perspective.
None of our current product candidates has to date received regulatory approval for their intended commercial sale. We cannot market a pharmaceutical product in any jurisdiction until it has completed rigorous preclinical testing and clinical trials and passed such jurisdiction’s extensive regulatory approval process. In general, significant R&D and clinical studies are required to demonstrate the safety and efficacy of our product candidates before we can submit regulatory applications. Even if a product candidate is approved by the applicable regulatory authority, we may not obtain approval for an indication whose market is large enough to recover our investment in that product candidate. In addition, there can be no assurance that we will ever obtain all or any required regulatory approvals for any of our product candidates.
We are currently developing our product candidates based on R&D activities, preclinical testing and clinical trials conducted to date, and we may not be successful in developing or introducing to the market these or any other new products or technology. If we fail to develop and deploy new products successfully and on a timely basis, we may become non-competitive and unable to recover the R&D and other expenses we incur to develop and test new products.
Interim results of preclinical or clinical studies do not necessarily predict their final results, and acceptable results in early studies might not be obtained in later studies. Safety signals detected during clinical studies and preclinical animal studies may require us to perform additional studies, which could delay the development of the drug or lead to a decision to discontinue development of the drug. Product candidates in the later stages of clinical development may fail to show the desired safety and efficacy traits despite positive results in initial clinical testing. Results from earlier studies may not be indicative of results from future clinical trials and the risk remains that a pivotal program may generate efficacy data that will be insufficient for the approval of the drug, or may raise safety concerns that may prevent approval of the drug. Interpretation of the prior preclinical and clinical safety and efficacy data of our product candidates may be flawed and there can be no assurance that safety and/or efficacy concerns from the prior data were overlooked or misinterpreted, which in subsequent, larger studies appear and prevent approval of such product candidates.
Furthermore, we may suffer significant setbacks in advanced clinical trials, even after promising results in earlier studies. Based on results at any stage of clinical trials, we may decide to repeat or redesign a trial or discontinue development of one or more of our product candidates. Further, actual results may vary once the final and quality‑controlled verification of data and analyses has been completed. If we fail to adequately demonstrate the safety and efficacy of our products under development, we will not be able to obtain the required regulatory approvals to commercialize our product candidates.
A failure in the development of any one of our programs or product candidates could have a negative impact on the development of the others. Setbacks in any phase of the clinical development of our product candidates would have an adverse financial impact (including with respect to any agreements and partnerships that may exist between us and other entities), could jeopardize regulatory approval and would likely cause a drop in the price of our Common Shares and/or a decline in the value of our other securities.
If we are unable to successfully complete our clinical trial programs, or if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected.
Whether or not and how quickly we complete clinical trials is dependent in part upon the rate at which we are able to engage clinical trial sites and, thereafter, the rate of enrollment of patients, and the rate at which we collect, clean, lock and analyze the clinical trial database. Patient enrollment is a function of many factors, including the design of the protocol, the size of the patient population, the proximity of patients to and availability of clinical sites, the eligibility criteria for the study, the perceived risks and benefits of the drug under study and of the control drug, if any, the efforts to facilitate timely enrollment in clinical trials, the patient referral practices of physicians, the existence of competitive clinical trials, and whether existing or new drugs are approved for the indication we are studying. Certain clinical trials are designed to continue until a pre-determined number of events have occurred to the patients enrolled. Such trials are subject to delays stemming from patient withdrawal and from lower than expected event rates and may also incur increased costs, if enrollment is increased in order to achieve the desired number of events. If we experience delays in identifying and contracting with sites and/or in patient enrollment in our clinical trial programs, we may incur additional costs and delays in our development programs, and may not be able to complete our clinical trials on a cost-effective or timely basis. In addition, conducting multi-national studies adds another level of complexity and risk as we are subject to events affecting countries other than the US and Canada. Moreover, negative or inconclusive results from the clinical trials we conduct or adverse medical events could cause us to have to repeat or terminate the clinical trials. Accordingly, we may not be able to complete the clinical trials within an acceptable time-frame, if at all. If we or any third party have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing clinical trials.
Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards and must (i) meet the requirements of these authorities; (ii) meet the requirements for informed consent; and (iii) meet the requirements for good clinical practices. We may not be able to comply with these requirements in respect of one or more of our product candidates.
Additionally, we have limited experience in filing an NDA or similar application for approval in the US or in any other country for our current product candidates, which may result in a delay in, or the rejection of, our filing of an NDA or similar application. During the drug development process, regulatory agencies will typically ask questions of drug sponsors. While we endeavor to answer all such questions in a timely fashion, some questions may not be answered in time to prevent the delay of acceptance of an NDA or the rejection of an NDA.
We have incurred, and expect to continue to incur, substantial expenses, and we have made, and expect to continue to make, substantial financial commitments to establish a commercial operation. There can be no assurance how quickly, if ever, we will realize a profit from our commercial operation.
Our business strategy is to become a specialty biopharmaceutical company with commercial operations to market and sell products that we may develop, acquire or in‑license. To that end, our commercial operations consist of 21 full-time sales representatives, who provide services pursuant to our agreement with a contract sales organization, and our sales-management employees. We have to date incurred, and expect to continue to incur, substantial expenses, and we have made, and expect to continue to make, substantial financial commitments to build out our commercial operations. Establishing a commercial operation is expensive and time-consuming, and there can be no assurance how quickly, if ever, we will realize a profit from our commercial operations. Factors that may inhibit our efforts to realize a profit from our commercial operations include:
our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel and representatives;
the inability of our sales personnel to obtain access to or to persuade adequate numbers of physicians to prescribe our products or the products that we in-license or co-promote;
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
unforeseen costs and expenses associated with creating an independent sales and marketing organization.
Our financial viability depends, in part, on our ability to acquire, in-license or otherwise obtain the right to sell other products. If we are unable to do so, our business, financial condition and results of operations may be materially adversely affected.
In connection with our strategy to further transform the Company into a commercially operating specialty biopharmaceutical organization, we may enter into commercial arrangements with third parties, including but not limited to promotion, co-promotion, acquisition or in-licensing agreements, in efforts to establish and expand our commercial revenue base. These business activities entail numerous operational and financial risks, including:
the difficulty or inability to secure financing to acquire or in-license products;
the incurrence of substantial debt or dilutive issuances of securities to pay for the acquisition or in-licensing of new products;
the disruption of our business and diversion of our management’s time and attention;
higher than expected development, acquisition or in-license and integration costs;
exposure to unknown liabilities; and
the difficulty in locating products that are in our targeted therapeutic areas and that are compatible with other products in our portfolio.
We can provide no assurance that we will be able to identify potential product candidates or strategic commercial partners or, if we identify such product candidates or partners, that any related commercial arrangements will be consummated on terms that are favorable to us. To the extent that we are successful in entering into any strategic commercial arrangements, including promotional, co-promotional or marketing agreements, or acquisition or in-licensing agreements with third parties, we cannot provide any assurance that any resulting initiatives or activities will be successful. To the extent that any related investments in such arrangements do not yield the expected benefits, our business, financial condition and results of operations may be materially adversely affected.
We have limited resources to identify and execute the procurement of additional products and to integrate them into our current commercial operations. The failure to successfully integrate the personnel and operations of businesses that we may acquire or of products that we may in-license in the future with our existing operations, business and products could have a material adverse effect on our operations and results. We compete with larger pharmaceutical companies and other competitors in our efforts to acquire, in-license, and/or obtain the right to market and/or detail new products. Our competitors likely will have access to greater financial resources than us and may have greater expertise in identifying and evaluating new opportunities. Moreover, we may devote resources to potential acquisition, in-licensing, promotion or co-promotion opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts.
We will require significant additional financing, and we may not have access to sufficient capital.
We will require significant additional capital to fund our commercial operations and may require additional capital to pursue planned clinical trials and regulatory approvals, as well as further R&D and marketing efforts for our product candidates and potential products. We do not anticipate generating significant revenues from operations in the near future, and we currently have no committed sources of capital.
We may attempt to raise additional funds through public or private financings, collaborations with other pharmaceutical companies or CROs or from other sources, including, without limitation, through at-the-market offerings and issuances of Common Shares. Additional funding may not be available on terms that are acceptable to us. If adequate funding is not available to us on reasonable terms, we may need to delay, reduce or eliminate one or more of our product development programs or obtain funds on terms less favorable than we would otherwise accept. To the extent that additional capital is raised through the sale of equity securities or securities convertible into or exchangeable or exercisable for equity securities (collectively, “Convertible Securities”), the issuance of those securities would result in dilution to our shareholders. Moreover, the incurrence of debt financing or the issuance of dividend-paying preferred shares, could result in a substantial portion of our future operating cash flow, if any, being dedicated to the payment of principal and interest on such indebtedness or the payment of dividends on such preferred shares and could impose restrictions on our operations and on our ability to make certain expenditures and/or to incur additional indebtedness, which could render us more vulnerable to competitive pressures and economic downturns.
We anticipate that our cash and cash equivalents as at December 31, 2015 will be sufficient to fund our commercial operations, development programs, clinical trials and other operating expenses at least through December 31, 2016. However, our future capital requirements are substantial and may increase beyond our current expectations depending on many factors, including:
the duration of, changes to and results of our clinical trials for our various product candidates going forward;
unexpected delays or developments in seeking regulatory approvals;
the time and cost involved in preparing, filing, prosecuting, maintaining and enforcing patent claims;
unexpected developments encountered in implementing our business development and commercialization strategies;
the potential addition of commercialized products to our portfolio;
lower sales commission than expected;
the outcome of litigation, including the securities class action litigation pending against us that is described elsewhere in this Annual Report on Form 20-F; and
further arrangements, if any, with collaborators.
In addition, global economic and market conditions as well as future developments in the credit and capital markets may make it even more difficult for us to raise additional financing in the future.
If we are unsuccessful in generating new revenues, increasing our revenues and/or raising additional funding, we may possibly cease to continue operating as we currently do.
We have incurred sustained operating losses, deficits and negative cash flows from operating activities over the past several years, and we expect that we will continue to do so for an extended period.
Our ability to continue as a going concern is dependent on the successful execution of our business plan, which will require an increase in revenue and/or additional funding to be provided by potential investors and/or non-traditional sources of financing. In 2018, our primary source of liquidity was the $24.0 million licensing payment received from Strongbridge Biopharma plc in January 2018.
We stated in our management’s discussion and analysis of financial condition and results of operations for the year ended 2018 that we expect existing cash balances and operating cash flows will provide us with adequate funds to support our current operating plan for at least twelve months. There can be no assurance, however, that weunplanned capital requirements or other future events, will achieve profitabilitynot require us to seek debt or positive cash flows or be able to obtain additional funding orequity financing and, if so required, that
if obtained, they it will be sufficient, or whether any other initiatives will be successful such that we may continue as a going concern. There could also be material uncertainties relatedavailable on terms acceptable to certain adverse conditions and events that could impact our ability to remain a going concern. If the going concern assumptions were deemed no longer appropriate for our consolidated financial statements, adjustments to the carrying value of assets and liabilities, reported expenses and consolidated statement of financial position classifications would be necessary. Such adjustments could be material.us, if at all.
Additional funding may be in the form of debt or equity or a hybrid instrument depending on our needs, the demands of investors and market conditions. Depending on the prevailing global economic and credit market conditions, we may not be able to raise additional liquiditycash through these traditional sources of financing. Although we may also pursue non-traditional sources of financing with third parties, the global equity and credit markets may adversely affect the ability of potential third parties to pursue such transactions with us. Accordingly, as a result of the foregoing, we continue to review traditional sources of financing, such as private and public debt or various equity financing alternatives, as well as other alternatives to enhance shareholder value, including, but not limited to, non-traditional sources of financing, such as strategic alliances with third parties, the sale of assets or licensing of our technology or intellectual property, a combination of operating and related initiatives or a substantial reorganization of our business.
There can be no assurance that we will achieve profitability or positive cash flows or be able to obtain additional funding or that, if obtained, the additional funding will be sufficient, or whether any other initiatives will be successful such that we may continue as a going concern. If we do not ultimately achieve operating profitability, an investment in our Common Shares or other securities could result in a significant or total loss. There could also be material uncertainties related to certain adverse conditions and events that could impact our ability to remain a going concern. If the going concern assumptions were deemed no longer appropriate for our consolidated financial statements, adjustments to the carrying value of assets and liabilities, reported expenses and consolidated statement of financial position classifications would be necessary. Such adjustments could be material.
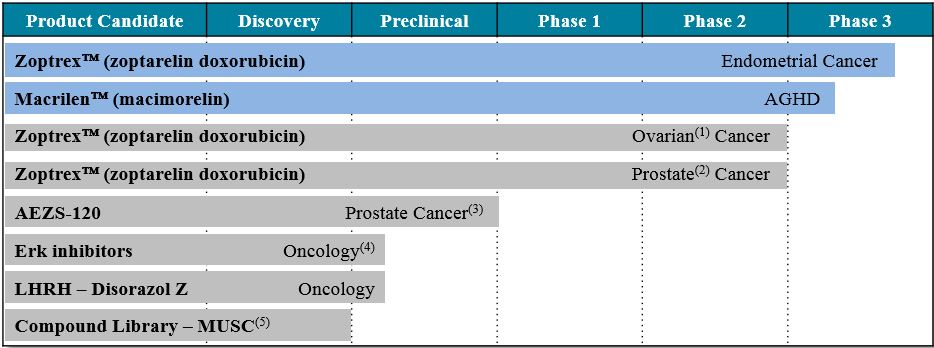
If we are unable to successfully commercialize or out-license Macrilen™ (macimorelin), or if we experience significant delays in doing so, our business would be materially harmed, and the future and viability of our Company could be imperiled.
Our principal focus is on the licensing and development of Macrilen™ (macimorelin) and we currently do not have any other product. The Company is a party to a license and assignment agreement with a subsidiary of Novo Nordisk A/S (“Novo”) to carry out development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) in the U.S. and Canada (the “License and Assignment Agreement”). The Company continues to explore licensing opportunities worldwide.
The commercial success of Macrilen™ (macimorelin) depends on several factors, including the following:
receipt of approvals from foreign regulatory authorities;
successfully contracting with qualified third-party suppliers to manufacture Macrilen™ (macimorelin);
developing appropriate distribution and marketing infrastructure and arrangements for our product;
launching and growing commercial sales of the product;
out-licensing Macrilen™ (macimorelin) to third parties; and
acceptance of the product in the medical community, among patients and with third party payers.
If we are unable to successfully achieve any of these factors, our business, financial condition and results of operations may be materially adversely affected.
Our revenues and expenses may fluctuate significantly, and any failure to meet financial expectations may disappoint securities analysts or investors and result in a decline in the price or the value of our Common Shares or other securities.
We have a history of operating losses. Our revenues and expenses have fluctuated in the past and may continue to do so in the future. These fluctuations could cause our share price or the value of our other securities to decline. Some of the factors that could cause our revenues and expenses to fluctuate include but are not limited to:
the timing and willingness of any current or future collaborators to invest the resources necessary to commercialize Macrilen™ (macimorelin);
not obtaining necessary regulatory approvals from the U.S. Food and Drug Administration ("FDA"), European Medicines Agency ("EMA") and other agencies that may delay or prevent us from obtaining approval of a pediatric indication for Macrilen™ (macimorelin), which may affect the price of our securities;
the timing of regulatory submissions and approvals;
the nature and timing of licensing fee revenues;
the outcome of litigation, including the securities class action litigation pending against us that is described elsewhere in this Annual Report on Form 20-F;
foreign currency fluctuations;
the timing of the achievement and the receipt of milestone payments from current or future licensing partners; and
failure to enter into new or the expiration or termination of current agreements with suppliers who manufacture Macrilen™ (macimorelin).
Due to fluctuations in our revenues and expenses, we believe that period-to-period comparisons of our results of operations are not necessarily indicative of our future performance. It is possible that in some future periods, our revenues and expenses will be above or below the expectations of securities analysts or investors. In this case, the price of our Common Shares and the value of our other securities could fluctuate significantly or decline.
If we are unable to successfully complete the pediatric clinical trial program for Macrilen™ (macimorelin), or if such clinical trial takes longer to complete than we project, our ability to execute any related business strategy will be adversely affected.
If we experience delays in identifying and contracting with sites and/or in-patient enrollment in our pediatric clinical trial program for Macrilen™ (macimorelin), we may incur additional costs and delays in our development programs, and may not be able to complete our clinical trials on a cost-effective or timely basis. In addition, conducting multi-national studies adds another level of complexity and risk as we are subject to events affecting countries other than the U.S. and Canada. Moreover, negative or inconclusive results from the clinical trials we conduct or adverse medical events could cause us to have to repeat or terminate the clinical trials. Accordingly, we may not be able to complete the pediatric clinical trial within an acceptable time-frame, if at all. If we or our contract resource organization (a "CRO") have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing clinical trials.
Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards and must, among other requirements:
meet the requirements of these authorities from multiple countries and jurisdictions and their related statutes, regulations, and guidances;
meet the requirements for informed consent;
meet the requirements for institutional review boards; and
meet the requirements for good clinical practices
We are currently dependent on certain strategic relationships with third parties for the development, manufacturing and licensing of Macrilen™ (macimorelin) and we may enter into future collaborations for the development, manufacturing and licensing of Macrilen™ (macimorelin).
We are currently dependent on certain strategic relationships with third parties for the development, manufacturing and licensing of Macrilen™ (macimorelin), and may enter into future collaborations for the development, manufacturing and licensing of Macrilen™ (macimorelin). Our arrangements with these third parties may not provide us with the benefits we expect and may expose us to a number of risks.
Currently, we are dependent on Novo to commercialize Macrilen™ (macimorelin) in the U.S and Canada. Most of our potential revenue consists of contingent payments, including regulatory milestones and royalties on the sale of Macrilen™ (macimorelin). The milestone and royalty revenue that we may receive under this collaboration will depend upon Novo’s ability to successfully introduce, market and sell Macrilen™ (macimorelin) in the United States. If Novo does not devote sufficient time and resources to its collaboration arrangement with us, we may not realize the potential commercial benefits of the arrangement, and our results of operations may be materially adversely affected.
Our reliance on relationships with Novo and other potential third parties poses a number of risks. We may not realize the contemplated benefits of such agreements nor can we be certain that any of these parties will fulfill their obligations in a manner which maximizes our revenue. These arrangements may also require us to transfer certain material rights to third parties. These agreements create certain additional risks. The occurrence of any of the following or other events may delay or impair commercialization of Macrilen™ (macimorelin):
in certain circumstances, third parties may assign their rights and obligations under these agreements to other third parties without our consent or approval;
the third parties may cease to conduct business for financial or other reasons;
we may not be able to renew such agreements;
the third parties may not properly maintain or defend certain intellectual property rights that may be important to the commercialization of Macrilen™ (macimorelin);
the third parties may encounter conflicts of interest, changes in business strategy or other issues which could adversely affect their willingness or ability to fulfill their obligations to us (for example, pharmaceutical companies historically have re-evaluated their priorities following mergers and consolidations, which have been common in this industry);
delays in, or failures to achieve, scale-up to commercial quantities, or changes to current raw material suppliers or product manufacturers (whether the change is attributable to us or the supplier or manufacturer) could delay clinical studies, regulatory submissions and commercialization of Macrilen™ (macimorelin); and
disputes may arise between us and the third parties that could result in the delay or termination of the manufacturing or commercialization of Macrilen™ (macimorelin), resulting in litigation or arbitration that could be time-consuming and expensive, or causing the third parties to act in their own self-interest and not in our interest or those of our shareholders.
| |
| i. | In addition, the third parties can terminate our agreements with them for a number of reasons based on the terms of the individual agreements that we have entered into with them. If one or more of these agreements were to be terminated, we would be required to devote additional resources to manufacturing and commercializing Macrilen™ (macimorelin), which would likely cause a drop in the price of our Common Shares. |
We may be unsuccessful in consummating further out-licensing arrangements for MacrilenTM (macimorelin) on favorable terms and conditions, or we may be significantly delayed in doing so.
As part of our product development and commercialization strategy, we are evaluating out-licensing opportunities for MacrilenTM (macimorelin) in addition to the License and Assignment Agreement. If we elect to collaborate with third parties in respect of MacrilenTM (macimorelin), we may not be able to negotiate a collaborative arrangement for MacrilenTM (macimorelin) on favorable terms and conditions, if at all. Should any partner fail to successfully commercialize MacrilenTM (macimorelin), our business, financial condition and results of operations may be adversely affected.
We may require significant additional financing, and we may not have access to sufficient capital.
We may require significant additional capital to fund our commercial operations and may require additional capital to pursue planned clinical trials and regulatory approvals. Although we have capital from the License and Assignment Agreement, we do not anticipate generating significant revenues from operations in the near future other than from the License and Assignment Agreement, and we currently have no committed sources of capital.
We may attempt to raise additional funds through public or private financings, collaborations with other pharmaceutical companies or from other sources, including, without limitation, through at-the-market offerings and issuances of Common Shares. Additional funding may not be available on terms that are acceptable to us. If adequate funding is not available to us on reasonable terms, we may need to delay, reduce or eliminate one or more of our product development programs or obtain funds on terms less favorable than we would otherwise accept. To the extent that additional capital is raised through the sale of equity securities or securities convertible into or exchangeable or exercisable for equity securities, the issuance of those securities would result in dilution to our shareholders. Moreover, the incurrence of debt financing or the issuance of dividend-paying preferred shares, could result in a substantial portion of our future operating cash flow, if any, being dedicated to the payment of principal and interest on such indebtedness or the payment of dividends on such preferred shares and could impose restrictions on our operations and on our ability to make certain expenditures and/or to incur additional indebtedness, which could render us more vulnerable to competitive pressures and economic downturns.
Our future capital requirements are substantial and may increase beyond our current expectations depending on many factors, including:
the duration of changes to and results of our clinical trials for any future products going forward;
unexpected delays or developments in seeking regulatory approvals;
the time and cost involved in preparing, filing, prosecuting, maintaining and enforcing patent claims;
unexpected developments encountered in implementing our business development and commercialization strategies;
the potential addition of commercialized products to our portfolio;
the outcome of current and future litigation, including the securities class action litigation pending against us that is described elsewhere in this Annual Report on Form 20-F; and
further arrangements, if any, with collaborators.
In addition, global economic and market conditions as well as future developments in the credit and capital markets may make it even more difficult for us to raise additional financing in the future.
We are and will be subject to stringent ongoing government regulation for our products and our product candidates, even if we obtain regulatory approvals for the latter.
The manufacture, marketing and sale of our products and product candidatesMacrilen™ (macimorelin) are and will be subject to strict and ongoing regulation, even if regulatory authorities approve any ofwith marketing approval by the latter.FDA and EMA for Macrilen™ (macimorelin). Compliance with such regulation will be expensive and consume substantial financial and management resources. For example, anthe EMA approval for a product may bemacimorelin was conditioned on our agreement to conduct costly post-marketing follow-up studies to monitor the safety or efficacy of the products.product. In addition, as clinical experience with a drug expands after approval because the drug is used by a greater number and more diverse group of patients than during clinical trials, side effects or other problems may be observed after approval that were not observed or anticipated during pre-approval clinical trials. In such a case, a regulatory authority could restrict the indications for which the product may be sold or revoke the product’sproduct's regulatory approval.
We and our contract manufacturers will be required to comply with applicable currentCurrent Good Manufacturing Practice regulations for the manufacture of our products.current or future products and other regulations. These regulations include requirements relating to quality assurance, as well as the corresponding maintenance of rigorous records and documentation. Manufacturing facilities must be approved before we can use them in the commercial manufacturing of our productsa product and are subject to subsequent periodic inspection by regulatory authorities. In addition, material changes in the methods of manufacturing or changes in the suppliers of raw materials are subject to further regulatory review and approval.
If we, or if any future marketing collaborators or contract manufacturers, fail to comply with applicable regulatory requirements, we may be subject to sanctions including fines, product recalls or seizures and related publicity requirements, injunctions, total or partial suspension of production, civil penalties, suspension or withdrawals of previously granted regulatory approvals, warning or untitled letters, refusal to approve pending applications for marketing approval of new products or of supplements to approved applications, complete withdrawal of a marketing application, exclusion from government healthcare programs, import or export bans or restrictions, andand/or criminal prosecution and penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products and product candidates.a product.
Even if we receivewith marketing approval for our product candidates, MacrilenTM (macimorelin),such product approvalsapproval could be subject to restrictions or withdrawals. Regulatory requirements are subject to change.
On December 20, 2017, the FDA granted marketing approval in the United States for Macrilen™ (macimorelin) to be used in the diagnosis of patients with adult growth hormone deficiency ("AGHD") and on January 16, 2019, the EMA granted marketing approval in Europe for macimorelin for the diagnosis of AGHD. Regulatory authorities generally approve products for particularspecified indications. If an approval is for a limited indication, this limitation reduces the size of the potential market for that product. Product approvals, once granted, are subject to continual review and periodic inspections by regulatory authorities. Our operations and practices are subject to regulation and scrutiny by the USU.S. government, as well as governments of any other countries in which we do business or conduct activities. Later discovery of previously unknown problems or safety issues and/or failure to comply with domestic or foreign laws, knowingly or unknowingly, can result in various adverse consequences, including, among other things, a possible delay in the approval or refusal to approve a product, warning or untitled letters, fines, injunctions, civil penalties, recalls or seizures of products and related publicity requirements, total or partial suspension of production, import or export bans or restrictions, refusal of the government to renew marketing applications, complete withdrawal of a marketing
application, criminal prosecution and penalties, suspension or withdrawals of previously granted regulatory approvals, withdrawal of an approved product from the market and/or exclusion from government healthcare programs. Such regulatory enforcement could have a direct and negative impact on the product for which approval is granted, but also could have a negative impact on the approval of any pending applications for marketing approval of new drugs or supplements to approved applications.
Because we operate in a highly regulated industry, regulatory authorities could take enforcement action against us in connection with our licensees' or our licensees’ or collaborators’collaborators', business and marketing activities for various reasons.
From time to time, new legislation is passed into law that could significantly change the statutory provisions governing the approval, manufacturing, and marketing of products regulated by the U.S. Food and Drug Administration ("FDA")FDA, EMA and other health authorities. Additionally, regulations and guidance are often revised or reinterpreted by health agencies in ways that may significantly affect
our business and our products.Macrilen™ (macimorelin). It is impossible to predict whether further legislative changes will be enacted, or whether regulations, guidance, or interpretations will change, and what the impact of such changes, if any, may be.
Healthcare reform measures could hinder or prevent the commercial success of oura product candidates and adversely affect our business.
The business prospects and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payers to contain or reduce the costs of healthcare. The U.S. government and other governments have shown significant interest in pursuing healthcare reform and reducing healthcare costs. Any government-adopted reform measures could cause significant pressure on the pricing of healthcare products and services, including Macrilen™ (macimorelin), both in the U.S. and internationally, as well as the amount of reimbursement available from governmental agencies and other third-party payers. If reimbursement for Macrilen™ (macimorelin) is substantially less than we expect, our revenue prospects could be materially and adversely impacted.
In the USU.S. and in other jurisdictions there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the healthcare system, such as proposals relating to the pricing of healthcare products and services in the USU.S. or internationally, the reimportation of drugs into the USU.S. from other countries (where they are then sold at a lower price), and the amount of reimbursement available from governmental agencies or other third party payers. For example,Furthermore, the pricing of pharmaceutical products, in general, and specialty drugs, in particular, has been a topic of concern in the U.S. Congress, where hearings on the topic have been held, and has been a topic of speeches given by political figures, including President Donald Trump. Additionally, in the U.S., states have also passed legislation and proposed bills that are aimed at drug manufacturers are requiredpricing transparency, which will likely impact drug pricing. There can be no assurance as to have a national rebate agreement with the Departmenthow this scrutiny on pricing of Health and Human Services in order to obtain state Medicaid coverage, which requires manufacturers to pay a rebate on drugs dispensed to Medicaid patients.pharmaceutical products will impact future pricing of Macrilen™ (macimorelin).
The Patient Protection and Affordable Care Act and the Healthcare and Education Affordability Reconciliation Act of 2010 (collectively, the “ACA”"ACA") may havehas had far-reaching consequences for most healthcare companies, including specialty biopharmaceutical companies like us. For example, if reimbursement for our product candidates is substantially less than we expect, our revenue prospects could be materially and adversely impacted.
Regardless of the impactThe future of the ACA is, however, uncertain. Since January 2017, the U.S. Congress has proposed various bills to revise the ACA. Additionally, President Donald Trump has suggested similar action and enacted Executive Orders to curtail the ACA and its impacts on us,healthcare in the US government and other governments have shown significant interest in pursuingU.S. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation, or potential legislation, regulation, and reducing healthcare costs. Any government-adopted reform measures could cause significant pressureorders or their impact on the pricing of healthcare products and services, including our product candidates, in the US and internationally, as well as the amount of reimbursement available from governmental agencies and other third-party payers.us.
In addition, on September 27, 2007, the Food and Drug Administration Amendments Act of 2007 was enacted, givinggives the FDA enhanced post-market authority, including the authority to require post-marketing studies and clinical trials, labeling changes based on new safety information, and compliance with risk evaluations and mitigation strategies approved by the FDA. The FDA’sFDA's exercise of this authority may result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, which may also increase costs related to complying with new post-approval regulatory requirements, and increase potential FDA restrictions on the sale or distribution of approved products.
If we or our licensees market products or interact with health care practitioners in a manner that violates healthcare fraud and abuse laws, we or our licensees may be subject to civil or criminal penalties, including exclusion from participation in government healthcare programs.
As a pharmaceutical company, even though we do not provide healthcare services or receive payments directly from or bill directly to Medicare, Medicaid or other third-party payers for our products,current product, certain federal and state healthcare laws and regulations pertaining to fraud and abuse are and will be applicable to our business. We and our licensees are subject to healthcare fraud and abuse regulation by both the federal government and the states in which we conduct our business.
The laws that may affect our and our licensee's ability to operate include the federal healthcare program anti-kickback statute, which prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce, or in return for, the purchase, lease, order, or arrangement for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute applies to arrangements between pharmaceutical manufacturers and prescribers, purchasers and formulary managers. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Pharmaceutical companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as providing free product to customers with the expectation that the customers would bill federal programs for the product; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered off-label uses; and submitting inflated best price information to the Medicaid Drug Rebate Program.
The Health Insurance Portability and Accountability Act of 1996 also created prohibitions against healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payers. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. The ACA, through the Physician Payment Sunshine Act, imposed new requirements on manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’sChildren's Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare and Medicaid Services (“CMS”("CMS") information related to payments or other “transfers"transfers of value”value" made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians (as defined above) and their immediate family members and payments or other “transfers"transfers of value”value" to such physician owners and their immediate family members. Manufacturers are required to report such data to the government by the 90th calendar day of each year.
The majority of states also have statutes or regulations similar to these federal laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer. In addition, some states have laws that require pharmaceutical companies to adopt comprehensive compliance programs. For example, under California law, pharmaceutical companies must comply with both the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and the PhRMA Code on Interactions with Healthcare Professionals, as amended. Certain states also mandate the tracking and reporting of gifts, compensation, and other remuneration paid by us to physicians and other healthcare providers.
Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us or our licensees for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, cause reputational harm and divert our management’smanagement's attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state laws may prove costly.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. The ACA also made several important changes to the federal Anti-Kickback Statute,anti-kickback statute, false claims laws, and healthcare fraud statute by weakening the intent requirement under the anti-kickback and healthcare fraud statutes that may make it easier for the government or whistleblowers to charge such fraud and abuse violations. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. In addition, the ACA increases penalties for fraud and abuse violations. If our past, present or future operations are found to be in violation of any of the laws described above or other similar governmental regulations to which we are subject, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and negatively impact our financial results.
If our products doMacrilen™ (macimorelin) does not gain market acceptance, we may be unable to generate significant revenues.
Even if our products are approved for commercialization, they may not be successful in the marketplace.
Market acceptance of any of our products will dependMacrilen™ (macimorelin) depends on a number of factors, including, but not limited to:
demonstration of clinical efficacy and safety;
the prevalence and severity of any adverse side effects;
limitations or warnings contained in the product’sproduct's approved labeling;
availability of alternative treatments for the indications we target;
the advantages and disadvantages of our productsMacrilen™ (macimorelin) relative to current or alternative treatments;
the availability of acceptable pricing and adequate third-party reimbursement; and
the effectiveness of marketing and distribution methods for the products.Macrilen™ (macimorelin).
If our products doMacrilen™ (macimorelin) does not gain market acceptance among physicians, patients, healthcare payers and others in the medical community, who may not accept or utilize our products,Macrilen™ (macimorelin), our ability to generate significant revenues from our productsMacrilen™ (macimorelin) would be limited, and our financial condition could be materially adversely affected. In addition, if we fail to further penetrate our core markets and existing geographic markets or to successfully expand our business into new markets, the growth in sales of our products,Macrilen™ (macimorelin), along with our operating results, could be negatively impacted.
Our ability to further penetrate our core markets and existing geographic markets in which we compete or to successfully expand our business into additional countries in Europe, Asia or elsewhere is subject to numerous factors, many of which are beyond our control. Our products,Macrilen™ (macimorelin), if successfully developed,commercialized, may compete with a number of drugs, therapies, products and tests currently manufactured and marketed by major pharmaceutical and other biotechnology companies. Our productsMacrilen™ (macimorelin) may also compete with new products currently under development by others or with products which may be less expensive than our products.Macrilen™ (macimorelin). There can be no assurance that our efforts to increase market penetration in our core markets and existing geographic markets will be successful. Our failure to do so could have an adverse effect on our operating results and would likely cause a drop in the price of our Common Shares and/or a decline in the value of our other securities.Shares.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidatesother products or indications for which there may be a greater likelihood of success.
Because we have limited financial and managerial resources, we are currently focusing our efforts on our lead, clinical-stage development compounds, Zoptrex™ (zoptarelin doxorubicin) and Macrilen™ (macimorelin), and we are doing so for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other potential indications for Macrilen™ (macimorelin) which there may be a greater likelihood of success or may prove to have greater commercial potential. Notwithstanding our investment to date and anticipated future expenditures on Zoptrex™, Macrilen™ and any earlier-stage programs, we have not yet developed, and may never successfully develop, any marketed treatments using these products. Research programs to identify new product candidates or pursue alternative indications for current product candidatesMacrilen™ (macimorelin) require substantial technical, financial and human resources. These activities may initially show promise in identifying potential product candidates or indications, yet fail to yield product candidates or indications for further clinical development.
We may not achieve our projected development goals in the time-frames we announce and expect.
We may set goals and make public statements regarding the timing of the accomplishment of objectives material to our success, such as the commencement, enrollment and anticipated completion of clinical trials, anticipated regulatory submission and approval dates and time of product launch. The actual timing of these events can vary dramatically due to factors such as delays or failures in ourany clinical trials, the uncertainties inherent in the regulatory approval process and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products.Macrilen™ (macimorelin). There can be no assurance that our clinical trials will be completed, that we will make regulatory submissions or receive regulatory approvals as planned or that we will be able to adhere to our current schedule for launching of Macrilen™ (macimorelin) outside of the launch of any of our products.U.S. If we fail to achieve one or more of these milestones as planned, the price of our Common Shares and/or the value of our other securities would likely decline.
If we fail to obtain acceptable prices or adequate reimbursement for our products,Macrilen™ (macimorelin), our ability to generate revenues will be diminished.
Our ability or that of our licensee(s) to successfully commercialize our productsMacrilen™ (macimorelin) will depend significantly on our or their ability to obtain acceptable prices and the availability of reimbursement to the patient from third-party payers, such as governmental and private insurance plans. These third-party payers frequently require companies to provide predetermined discounts from list prices, and they are increasingly challenging the prices charged for pharmaceuticals and other medical products. Our productsMacrilen™ (macimorelin) may not be considered cost-effective, and reimbursement to the patient may not be available or sufficient to allow us or our licensee(s) to sell our products on a competitive basis. It may not be possible to negotiate favorable reimbursement rates for our products.Macrilen™ (macimorelin). Adverse pricing and reimbursement conditions would also likely diminish our ability to induce third parties to co-promote our products.in-license Macrilen™ (macimorelin).
In addition, the continuing efforts of third-party payers to contain or reduce the costs of healthcare through various means may limit our commercial opportunity and reduce any associated revenue and profits. We expect that proposals to implement similar government controls will continue. The pricing of pharmaceutical products, in general, and specialty drugs, in particular, has been a topic of concern in the U.S. Congress, where hearings on the topic have been held, and has been a topic of speeches given by political figures, including President Donald Trump. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, continue.among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Additionally, there is drug pricing reform taking place at the state level in the U.S., in the form of laws and bills, that will impact how pharmaceutical companies can market and sell drug products and at what price. Further, third-party payers are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. There can be no assurance as to how this scrutiny on pricing of pharmaceutical products will impact future pricing of a product or orphan drugs or pharmaceutical products generally. In addition, increasing emphasis on managed care will continue to put pressure on the pricing of pharmaceutical and biopharmaceutical products. Cost control initiatives could decrease the price that we or any current or potential collaborators could receive for any of our productsa product and could adversely affect our profitability. In addition, in the US, inU.S., Canada and in many other countries, pricing and/or profitability of some or all prescription pharmaceuticals and biopharmaceuticals are subject to government control.
If we or our licensee(s) fail to obtain acceptable prices or an adequate level of reimbursement for our products,Macrilen™ (macimorelin), the sales of our productsMacrilen™ (macimorelin) would be adversely affected or there may be no commercially viable market for our products.Macrilen™ (macimorelin).
Competition in our targeted markets is intense, and development by other companies could render our products or technologiesMacrilen™ (macimorelin) non-competitive.
The biopharmaceutical field is highly competitive. New products developed by other companies in the industry could render our products or technologies non-competitive.Macrilen™ (macimorelin) uncompetitive. Competitors are developing and testing products and technologies that would compete with the products that we are developing.Macrilen™ (macimorelin). Some of these products may be more effective or have an entirely different approach or means of accomplishing the desired effect than our products.Macrilen™ (macimorelin). We expect competition from pharmaceutical and biopharmaceutical companies and academic research institutions to continue to increase over time. Many of our competitors and potential competitors have substantially greater product development capabilities and financial, scientific, marketing and human resources than we do. Our competitors may succeed in developing products earlier and in obtaining regulatory approvals and patent protection for such products more rapidly than we can or at a lower price.
We may not obtain adequate protection for our productsMacrilen™ (macimorelin) through our intellectual property.
We rely heavily on our proprietary information in developing and manufacturing our product candidates.Macrilen™ (macimorelin). Our success depends, in large part, on our ability to protect our competitive position through patents, trade secrets, trademarks and other intellectual property rights. The patent positions of pharmaceutical and biopharmaceutical firms, including us, are uncertain and involve complex questions of law and fact for which important legal issues remain unresolved. We have filed and are pursuing applications
for patents and trademarks in the US, in Canada and in other territories.many countries. Pending patent applications may not result in the issuance of patents and we may not be able to obtain additional issued patents relating to our technology or products.Macrilen™ (macimorelin).
The laws of some countries do not protect intellectual property rights to the same extent as the laws of the USU.S. and Canada. Many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. Compulsory licensing of life-saving drugs is also becoming increasingly popular in developing countries either through direct legislation or international initiatives. Such compulsory licenses could be extended to include some of our product candidates, which could limit our potential revenue opportunities. Moreover, the legal systems of certain countries, particularly certain developing countries, do not favor the aggressive enforcement of patent and other intellectual property protection, which makes it difficult to stop and prevent infringement.
Our patents and/or the patents that we license from others may be challenged, narrowed, invalidated, held to be unenforceable or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products.Macrilen™ (macimorelin). Changes in either patent laws or in interpretations of patent laws in the USU.S. and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.protection for Macrilen™ (macimorelin). The patents issued
or to be issued to us for Macrilen™ (macimorelin) may not provide us with any competitive advantage or protect us against competitors with similar technology. In addition, it is possible that third parties with products that are very similar to ours will circumvent our patents by means of alternate designs or processes. We may have to rely on method-of-use, methods of manufacture and/or new-formulation protection for our compounds in development, and any resulting products, which may not confer the same protection as claims to compounds per se.
In addition, our patents may be challenged by third parties in patent litigation, which is becoming widespread in the biopharmaceutical industry. There may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim. There may also be prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. No assurance can be given that our patents would, if challenged, be held by a court to be valid or enforceable or that a competitor’scompetitor's technology or product would be found by a court to infringe our patents. Our granted patents could also be challenged and revoked in USU.S. post-grant proceedings as well as in opposition or nullity proceedings in certain countries outside the US.U.S. In addition, we may be required to disclaim part of the term of certain patents.
Patent applications relating to or affecting our business have been filed by a number of pharmaceutical and biopharmaceutical companies and academic institutions. A number of the technologies in these applications or patents may conflict with our technologies, patents or patent applications, and any such conflict could reduce the scope of patent protection which we could otherwise obtain. Because patent applications in the US and many other jurisdictions are typically not published until eighteen months after their first effective filing date, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, we cannot be certain that we were the first to make the inventions claimed in issued patents or pending patent applications, or that we were the first to file for protection of the inventions set forth in the patent applications. If a third party has also filed a patent application in the US covering our product candidates or a similar invention, we may have to participate in adversarial proceedings, such as interferences and deviation proceedings, before the United States Patent and Trademark Office to determine which party is entitled to a US patent claiming the disputed invention. The costs of these proceedings could be substantial, and it is possible that our efforts could be unsuccessful, resulting in a loss of our USU.S. patent position.
We also rely on trade secrets and proprietary know-how to protect our intellectual property. If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected. We seek to protect our unpatented proprietary information in part by requiring our employees, consultants, outside scientific collaborators and sponsored researchers and other advisors to enter into confidentiality agreements. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’sindividual's relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of our employees, the agreements provide that all of the technology whichthat is conceived by the individual during the course of employment is our exclusive property. These agreements may not provide meaningful protection or adequate remedies in the event of unauthorized use or disclosure of our proprietary information. In addition, it is possible that third parties could independently develop proprietary information and techniques substantially similar to ours or otherwise gain access to our trade secrets. If we are unable to protect the confidentiality of our proprietary information and know-how, competitors may be able to use this information to develop products that compete with our products and technologies, which could adversely impact our business.
We currently have the right to use certain patents and technologies under license agreements with third parties. Our failure to comply with the requirements of one or more of our license agreements could result in the termination of such agreements, which could cause us to terminate the related development program and cause a complete loss of our investment in that program. Inventions
claimed in certain in-licensed patents may have been made with funding from the USU.S. government and may be subject to the rights of the USU.S. government and we may be subject to additional requirements in the event we seek to commercialize or manufacture product candidates incorporating such in-licensed technology.
As a result of the foregoing factors, we may not be able to rely on our intellectual property to protect our productsMacrilen™ (macimorelin) in the marketplace.
Some of our patents have expired or will be expiring in 2016.
The product development timeline for our products is lengthy and it is possible that our issued patents covering our product candidates in the US and other jurisdictions may expire prior to commercial launch of the products. The patent that covers Zoptrex™ and other related targeted cytotoxic anthracycline analogues, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of cancer expired in the US in November 2015 and will expire in the European Union, Japan, China and Hong Kong in November 2016. We did not apply for patent term extension for this US patent. As a result, our ability to protect this compound from competition will be based on the protections provided in the US for new chemical entities and similar protections, if any, provided in other countries. We cannot assure you that Zoptrex™ or any of our other drug candidates will obtain new chemical entity exclusivity or any other market exclusivity in the US, the European Union or any other territory, or that we will be the first to receive the respective regulatory approval for such drugs so as to be eligible for any market exclusivity protection.
We may infringe the intellectual property rights of others.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. There could be issued patents of which we are not aware that our products or methods may be found to infringe, or patents of which we are aware and believe we do not infringe but which we may ultimately be found to infringe. Moreover, patent applications and their underlying discoveries are in some cases maintained in secrecy until patents are issued. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products or technologies are found to infringe. Moreover, there may be published pending applications that do not currently include a claim covering our products or technologies but which nonetheless provide support for a later drafted claim that, if issued, our products or technologies could be found to infringe.
If we infringe or are alleged to infringe intellectual property rights of third parties, it will adversely affect our business. Our research, development and commercialization activities, as well as any product candidates or products resulting from these activities, may infringe or be accused of infringing one or more claims of an issued patent or may fall within the scope of one or more claims in a published patent application that may subsequently be issued and to which we do not hold a license or other rights. Third parties may own or control these patents or patent applications in the USU.S. and abroad. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
The biopharmaceutical industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. In the event of infringement or violation of another party’sparty's patent or other intellectual property
rights, we may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us or our partners and collaborators.
Patent litigation is costly and time consuming and may subject us to liabilities.
If we become involved in any patent litigation, interference, opposition, re-examination or other administrative proceedings we will likely incur substantial expenses in connection therewith, and the efforts of our technical and management personnel will be significantly diverted. In addition, an adverse determination in litigation could subject us to significant liabilities.
We may not obtain trademark registrations for our product candidates.current or future products.
We have filed applications for trademark registrations, in connection with Zoptrex™ andincluding Macrilen™ (macimorelin), in various jurisdictions, including the US.U.S. We may file applications for other possible trademarks for our product candidates in the future.Macrilen™ (macimorelin). No assurance can be given that any of our trademarks will be registered in the US or elsewhere, or that the use of any registered or unregistered trademarks will confer a competitive advantage in the marketplace. Furthermore, even if we are successful in our trademark registrations, the FDA and regulatory authorities in other countries have their own process for drug nomenclature and their own views concerning appropriate proprietary names. The FDA and other regulatory authorities also have the power, even after granting market approval, to request a company to reconsider the name for a product because of evidence of confusion in the marketplace. No assurance can be given that the FDA or any other regulatory authority will approve of any of our trademarks or will not request
reconsideration of one of our trademarks at some time in the future. The loss, abandonment, or cancellation of any of our trademarks or trademark applications could negatively affect the success of the product candidates to which they relate.
We are currently dependent on certain strategic relationships with third parties and we may enter into future collaborations for the R&D of our product candidates.
We are currently dependent on certain strategic relationships with third parties and may enter into future collaborations for the R&D of our product candidates. Our arrangements with these third parties may not provide us with the benefits we expect and may expose us to a number of risks.
We are dependent on, and rely upon, third parties to perform various functions related to our business, including, but not limited to, R&D with respect to some of our product candidates. Our reliance on these relationships poses a number of risks.
We may not realize the contemplated benefits of such agreements nor can we be certain that any of these parties will fulfill their obligations in a manner which maximizes our revenue. These arrangements may also require us to transfer certain material rights or to issue our equity, voting or other securities to third parties. Any license or sublicense of our commercial rights may reduce our product revenue.
These agreements create certain additional risks. The occurrence of any of the following or other events may delay product development or impair commercialization of our products:
not all of the third parties are contractually prohibited from developing or commercializing, either alone or with others, products and services that are similar to or competitive with our product candidates and, with respect to our contracts that do contain such contractual prohibitions or restrictions, prohibitions or restrictions do not always apply to the affiliates of the third parties and they may elect to pursue the development of any additional product candidates and pursue technologies or products either on their own or in collaboration with other parties, including our competitors, whose technologies or products may be competitive with ours;
the third parties may under-fund or fail to commit sufficient resources to marketing, distribution or other development of our products;
the third parties may cease to conduct business for financial or other reasons;
we may not be able to renew such agreements;
the third parties may not properly maintain or defend certain intellectual property rights that may be important to the commercialization of our products;
the third parties may encounter conflicts of interest, changes in business strategy or other issues which could adversely affect their willingness or ability to fulfill their obligations to us (for example, pharmaceutical companies historically have re-evaluated their priorities following mergers and consolidations, which have been common in recent years in this industry);
delays in, or failures to achieve, scale-up to commercial quantities, or changes to current raw material suppliers or product manufacturers (whether the change is attributable to us or the supplier or manufacturer) could delay clinical studies, regulatory submissions and commercialization of our product candidates; and
disputes may arise between us and the third parties that could result in the delay or termination of the development or commercialization of our product candidates, resulting in litigation or arbitration that could be time-consuming and expensive, or causing the third parties to act in their own self-interest and not in our interest or those of our shareholders or other stakeholders.
In addition, the third parties can terminate our agreements with them for a number of reasons based on the terms of the individual agreements that we have entered into with them. If one or more of these agreements were to be terminated, we would be required to devote additional resources to developing and commercializing our product candidates, seek a new third party with which to contract or abandon the product candidate, which would likely cause a drop in the price of our Common Shares and/or a decline in the value of our other securities.
We rely on third parties to conduct, supervise and monitor our clinical trials, and those third parties may not perform satisfactorily.
We rely on third parties such as CROs,contract resource organizations, medical institutions and clinical investigators to enroll qualified patients and to conduct, supervise and monitor our clinical trials. Our reliance on these third parties for clinical development activities reduces our control over these activities. Our reliance on these third parties, however, does not relieve us of our regulatory responsibilities, including ensuring that our clinical trials are conducted in accordance with Good Clinical Practice guidelines and the investigational plan and protocols contained in an Investigational New Drug application to the FDA, or a comparable foreign regulatory submission. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. In addition, they may not complete activities on schedule, or may not conduct our preclinical studies or clinical trials in accordance with regulatory requirements or our trial design. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, our efforts to obtain regulatory approvals for, and to commercialize, our product candidatesproducts may be delayed or prevented.
In carrying out our operations, we are dependent on a stable and consistent supply of ingredients and raw materials.
There can be no assurance that we, our contract manufacturers or our licensees, will be able, in the future, to continue to purchase products from our current suppliers or any other supplier on terms that are favorable or similar to current terms or at all. An interruption in the availability of certain raw materials or ingredients, or significant increases in the prices we pay for them, could have a material adverse effect on our business, financial condition, liquidity and operating results.
The failure to perform satisfactorily by third parties upon which we expect to rely to manufacture and supply products may lead to supply shortfalls.
We expect to rely on third parties to manufacture and supply marketed products.Macrilen™ (macimorelin). We also have or may have certain supply obligations vis-à-vis our existing and potential licensees, who are or will be responsible for the marketing of the products.Macrilen™ (macimorelin). To be successful, our products haveMacrilen™ (macimorelin) has to be manufactured in commercial quantities in compliance with quality controls and regulatory requirements. Even though it is our objective to minimize such risk by introducing alternative suppliers to ensure a constant supply at all times, there are a limited number of contract manufacturers or suppliers that are capable of manufacturing our product candidatesMacrilen™ (macimorelin) or the materials used in theirits manufacture. If we are unable to do so ourselves or to arrange for third-party manufacturing or supply of these product candidatesMacrilen™ (macimorelin) or materials, or to do so on commercially reasonable terms, we may not be able to complete development of these product candidates or commercialize them ourselves orMacrilen™ (macimorelin) through our licensees. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third party for regulatory compliance, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control, and the possibility of termination or non-renewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us.
We are subject to intense competition for our skilled personnel, and the loss of key personnel or the inability to attract additional personnel could impair our ability to conduct our operations.
We are highly dependent on our management and our clinical, regulatory and scientific staff, the loss of whose services might adversely impact our ability to achieve our objectives. Recruiting and retaining qualified management and clinical, scientific and regulatory personnel is critical to our success. Reductions in our staffing levels have eliminated redundancies in key capabilities and skill sets among our full-time staff and required us to rely more heavily on outside consultants and third parties. We have been unable to increase the compensation of our associates to the extent required to remain fully competitive for their services, which
increased our employee retention risk. The competition for qualified personnel in the biopharmaceutical field is intense, and if we are not able to continue to attract and retain qualified personnel and/or maintain positive relationships with our outside consultants, we may not be able to achieve our strategic and operational objectives.
We are currently subject to a securities class actionclass-action litigation matter and we may be subject to similar or other litigation in the future.
WeThe Company and certain of our current and former officers are defendants in a purported class-action lawsuit pending in the USU.S. District Court for the District of New Jersey, (the “Court”), brought on behalf of shareholders of the Company. The lawsuit alleges violations of the Securities Exchange Act of 1934 (the “Exchange Act”) in connection with allegedly false and misleading statements made by the defendants between April 2, 2012August 30, 2011 and November 6, 2014 or the Class Period,(the "Class Period"), regarding the safety and efficacy of Macrilen™ (macimorelin), a product we developed for use in the diagnosis of AGHD, and the prospects for the approval of the Company’s NDACompany's New Drug Application for the product by the FDA. The plaintiffs seek to represent a class comprised of purchasers of our Common Sharesthe Company's common shares during the Class Period and seek damages, costs and expenses and such other relief as determined by the Court. On September 14, 2015,The Company considers the Court dismissedclaims that have been asserted in the lawsuit stating thatto be without merit and is vigorously defending against them. The Company cannot, however, predict at this time the plaintiffs failedoutcome or potential losses, if any, with respect to state a claim, but granted the plaintiffs leave to amend. On October 14, 2015, the plaintiffs filed a Second Amended Complaint against us. We filed a motion to dismiss the Second Amended Complaint on November 11, 2015, because we believe that the Second Amended Complaint also fails to state a claim. The hearing of the motion to dismiss the Second Amended Complaint occurred on January 19, 2016.this lawsuit.
On March 2, 2016, the Court issued an order granting our motion to dismiss the complaint in part and denying it in part. The Court dismissed certain of our current and former officers from the lawsuit. The Court allowed the claim that we omitted material facts from our public statements during the Class Period to proceed against us and our former CEO who departed in 2013, while dismissing such claims against other current and former officers. The Court also allowed a claim for “controlling person” liability to proceed against certain current and former officers. We disagree with the Court's decision and we filed a motion for reconsideration on March 16, 2016.
While we believe we have meritorious defenses and intend to continue to defend this lawsuit vigorously, we cannot predict the outcome. Furthermore, we may, from time to time, be partiesa party to other litigation in the normal course of business. Monitoring and defending against legal actions, whether or not meritorious, is time-consuming for our management and detracts from our ability to fully focus our internal resources on our business activities. In addition, legal fees and costs incurred in connection with such activities may be significant and we could, in the future, be subject to judgments or enter into settlements of claims for significant monetary damages. A decision adverse to our interests could result in the payment of substantial damages and could have a material adverse effect on our cash flow, results of operations and financial position.
With respect to any litigation, our insurance may not reimburse us or may not be sufficient to reimburse us for the expenses or losses we may suffer in contesting and concluding such lawsuit. Substantial litigation costs, including the substantial self-insured retention that we are required to satisfy before any insurance applies to a claim, unreimbursed legal fees or an adverse result in any litigation may adversely impact our business, operating results or financial condition. We believe that our directors’directors' and officers’officers' liability insurance will cover our potential liability with respect to the securities class-action lawsuit described above;lawsuit; however, the insurer has reserved its rights to contest the applicability of the insurance to such claim,claims and the limits of the insurance may be insufficient to cover our eventual liability, and we will be required to satisfy a substantial self-insured retention before any insurance coverage applies to the claim.liability.
We are subject to the risk of product liability claims, for which we may not have or may not be able to obtain adequate insurance coverage.
The use of Zoptrex™ and Macrilen™ on human participants in our clinical trials subjects us to the risk of liability to such participants, who may suffer unintended consequences. If Zoptrex™ and/or Macrilen™ are approved for commercialization or if we acquire a marketed product from a third party, the sale and use of such productsMacrilen™ (macimorelin) will involve the risk of product liability claims and associated adverse publicity. Product liability claims might be made against us directly by patients, healthcare providers or pharmaceutical companies or others selling, buying or using our products. We attempt to manage our liability risks by means of insurance. We maintain insurance covering our liability for our preclinical and clinical studies.studies as well as products liability insurance. However, we may not have or be able to obtain or maintain sufficient and affordable insurance coverage, including coverage for potentially very significant legal expenses, and without sufficient coverage any claim brought against us could have a materially adverse effect on our business, financial condition or results of operations. We do not currently maintain product liability insurance because we do not currently market, sell, distribute or handle any products. We may not be able to obtain product liability insurance on reasonable terms, if at all, when we begin to market, sell, distribute or handle products.
Our business involves the use of hazardous materials. We are required to comply with environmental and occupational safety laws regulating the use of such materials. If we violate these laws, we could be subject to significant fines, liabilities or other adverse consequences.
Our discovery and development processes involve the controlled use of hazardous materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. The risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of an accident or a failure to comply with environmental or occupational safety laws, we could be held liable for any damages that result, and any such liability could exceed our resources. We may not be adequately insured against this type of liability. We may be required to incur significant costs to comply with environmental laws and regulations in the future, and our operations, business or assets may be materially adversely affected by current or future environmental laws or regulations.
We are a holding company, and claims of creditors of our subsidiaries will generally have priority as to the assets of such subsidiaries over our claims and those of our creditors and shareholders. In addition, our principal operating subsidiary, AEZS Germany, may become subject to insolvency proceedings if it is illiquid or “over-indebted” in accordance with German law.
Aeterna Zentaris Inc. is a holding company and a substantial portion of our non-cash assets is the share capital of our subsidiaries. AEZS Germany, our principal operating subsidiary, based in Frankfurt, Germany, holds most of our intellectual property rights, which represent the principal non-cash assets of our business.
rights. Because Aeterna Zentaris Inc. is a holding company, our obligations to our creditors are structurally subordinated to all existing and future liabilities of our subsidiaries.subsidiaries, which may incur additional or other liabilities and/or obligations. Therefore, our rights and the rights of our creditors to participate in any distribution of the assets of any subsidiary in the event that such subsidiary were to be liquidated or reorganized or in the event of any bankruptcy or insolvency proceeding relating to or involving such subsidiary, and therefore the rights of the holders of our Common Shares to participate in those assets, are subject to the prior claims of such subsidiary’ssubsidiary's creditors. To the extent that we may be a creditor with recognized claims against any such subsidiary, our claims would still be subject to the prior claims of our subsidiary’ssubsidiary's creditors to the extent that they are secured or senior to those held by us.
Holders of our Common Shares are not creditors of our subsidiaries. Claims to the assets of our subsidiaries will derive from our own ownership interest in those operating subsidiaries. Claims of our subsidiaries’subsidiaries' creditors will generally have priority as to the assets of such subsidiaries over our own ownership interest claims and will therefore have priority over the holders of our Common
Shares. Our subsidiaries’subsidiaries' creditors may from time to time include general creditors, trade creditors, employees, secured creditors, taxing authorities, and creditors holding guarantees. Accordingly, in the event of any foreclosure, dissolution, winding-up, liquidation or reorganization, or a bankruptcy, insolvency or insolvencycreditor protection proceeding relating to us or our property, or any subsidiary, there can be no assurance as to the value, if any, that would be available to holders of our Common Shares.
In addition, any distributions to us by our subsidiaries could be subject to monetary transfer restrictions in the jurisdictions in which our subsidiaries operate.
Our subsidiaries may incur additional indebtedness and other liabilities.German law, which governs our principal operating subsidiary, AEZS Germany imposes an obligation on the managing director of AEZS Germany to institute insolvency proceedings of that subsidiary if the managing director concludes that AEZS Germany is insolvent because it is either illiquid or "over-indebted" in accordance with the provisions of German law.
It may be difficult for USU.S. investors to obtain and enforce judgments against us because of our Canadian incorporation and German presence.
We are a company existing under the laws of Canada. A number of our directors and officers and certain of the experts named herein, are residents of Canada or otherwise reside outside the US,U.S., and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the US.U.S. Consequently, although we have appointed an agent for service of process in the US,U.S., it may be difficult for investors in the USU.S. to bring an action against such directors officers or expertsofficers or to enforce against those persons or us a judgment obtained in a USU.S. court predicated upon the civil liability provisions of federal securities laws or other laws of the US.U.S. Investors should not assume that foreign courts (1)(i) would enforce judgments of USU.S. courts obtained in actions against us or such directors, officers or experts predicated upon the civil liability provisions of the USU.S. federal securities laws or the securities or “blue sky”"blue sky" laws of any state within the USU.S. or (2)(ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the USU.S. federal securities laws or any such state securities or “blue sky”"blue sky" laws. In addition, we have been advised by our Canadian counsel that in normal circumstances, only civil judgments and not other rights arising from US securities legislation (for example, penal or similar awards made by a court in a regulatory prosecution or proceeding) are enforceable in Canada and that the protections afforded by Canadian securities laws may not be available to investors in the US.
We are subject to various internal control reporting requirements under applicable Canadian securities laws and the Sarbanes-Oxley Act in the US.U.S. We can provide no assurance that we will at all times in the future be able to report that our internal controls over financial reporting are effective.
As a public company, we are required to comply with Section 404 of the USU.S. Sarbanes-Oxley Act (“("Section 404”404") and National Instrument 52-109 - Certification of Disclosure in Issuers’Issuers' Annual and Interim Filings.of the Canadian securities administrators. In any given year, we cannot be certain as to the time of completion of our internal control evaluation, testing and remediation actions or of their impact on our operations. Upon completion of this process, we may identify control deficiencies of varying degrees of severity under applicable SEC and Public Company Accounting Oversight Board (US)(U.S.) rules and regulations. As a public company, we are required to report, among other things, control deficiencies that constitute material weaknesses or changes in internal controls that, or that are reasonably likely to, materially affect internal controls over financial reporting. A “material weakness”"material weakness" is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’sour annual consolidated financial statements will not be prevented or detected on a timely basis. If we fail to comply with the requirements of Section 404 or similar Canadian requirements or if we report a material weakness, we might be subject to regulatory sanction and investors may lose confidence in our consolidated financial statements, which may be inaccurate if we fail to remedy such material weakness.
We believe we were a passive foreign investment company for the 2015 taxable year andIt is possible that we may be a passive foreign investment company, for the 2016 taxable year, which could result in adverse tax consequences to USU.S. investors.
Adverse USU.S. federal income tax rules apply to “US Holders”"U.S. Holders" (as defined in “Item"Item 10.E - Taxation - Certain Material USU.S. Federal Income Tax Considerations”Considerations" in this Annual Report on Form 20-F) who directly or indirectly hold common sharesCommon Shares of a passive foreign investment company (“PFIC”("PFIC"). We will be classified as a PFIC for USU.S. federal income tax purposes for a taxable year if (i) at least 75% of our gross income is “passive income”"passive income" or (ii) at least 50% of the average value of our assets, including goodwill (based on annual quarterly average), is attributable to assets which produce passive income or are held for the production of passive income.
We believe that we were a PFIC for the 2015 taxable year. Theyear, but were not a PFIC for the 2016, 2017 and 2018 taxable years. However, the PFIC determination depends on the application of complex USU.S. federal income tax rules concerning the classification of our assets and income for this purpose, and these rules are uncertain in some respects. In addition, the fair market value of our assets may be determined in large part by the market price of our Common Shares, which is likely to fluctuate, and the composition of our income and assets will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction. No assurance can be provided that we will not be classified as a PFIC for the 2018 taxable year and for any future taxable year.
Since
If we believe we wereare a PFIC for any taxable year during which a U.S. Holder holds Common Shares, we generally would continue to be treated as a PFIC with respect to that U.S. Holder for all succeeding years during which the U.S. Holder holds such Common Shares, even if we ceased to meet the threshold requirements for PFIC status. PFIC characterization could result in 2015, US Holders may suffer adverse USU.S. federal income tax consequences.consequences to U.S. Holders. In particular, absent certain elections, a USU.S. Holder would generally be subject to USU.S. federal income tax at ordinary income tax rates, plus a possible interest charge, in respect of a gain derived from a disposition of our Common Shares, as well as certain distributions by us. A USIf we are treated as a PFIC for any taxable year, a U.S. Holder may be able to minimize the adverse tax consequences by makingmake an election to "mark to market" Common Shares each taxable year and recognize ordinary income pursuant to such election based upon increases in the value of the Common Shares. In addition, USU.S. Holders may mitigate the adverse tax consequences of the PFIC rules by making a "qualified electing fund" ("QEF") election. We will endeavor to satisfy the record keeping requirements that apply to a QEF and to supply requesting US Holders with the information that such US Holders are required to report under the QEF rules. However,election; however, there can be no assurance that wethe Company will satisfy the record keeping requirements applicable to a QEF or that it will provide the information requiredregarding its income that would be necessary for a U.S. Holder to be reported by US Holders.make a QEF election.
In addition, if we areIf the Company is a PFIC, USU.S. Holders will generally be required to file an annual information return with the Internal Revenue Service (the “IRS”"IRS") (on IRS Form 8621, which PFIC shareholders will be required to file with their USU.S. federal income tax or information returns) relating to their ownership of Common Shares. This filing requirement is in addition to any pre-existing reporting requirements that apply to a U.S. Holder's interest in a PFIC (which this requirement does not affect).
For a more detailed discussion of the potential tax impact of us being a PFIC, see “Item"Item 10.E - Taxation - Certain Material USU.S. Federal Income Tax Considerations”Considerations" in this Annual Report on Form 20-F. The PFIC rules are complex. USU.S. Holders should consult their tax advisors regarding the potential application of the PFIC regime and any reporting obligations to which they may be subject under that regime.
Our net operating losses may be limited for U.S. federal income tax purposes under Section 382 of the Internal Revenue Code.
If a corporation with net operating losses ("NOLs") undergoes an "ownership change" within the meaning of Section 382 of the United States Internal Revenue Code of 1986, as amended, then such corporation's use of such "pre-change" NOLs to offset income incurred following such ownership change may be limited. Such limitation also may apply to certain losses or deductions that are "built-in" (i.e., attributable to periods prior to the ownership change but not yet taken into account for tax purposes) as of the date of the ownership change that are subsequently recognized. An ownership change generally occurs when there is either (i) a shift in ownership involving one or more "5% shareholders"; or (ii) an "equity structure shift" and, as a result, the percentage of stock of the corporation owned by one or more 5% shareholders (based on value) has increased by more than 50 percentage points over the lowest percentage of stock of the corporation owned by such shareholders during the "testing period" (generally the 3 years preceding the testing date). In general, if such change occurs, the corporation's ability to utilize its net operating loss carry-forwards and certain other tax attributes would be subject to an annual limitation, as described below. The unused portion of any such net operating loss carry-forwards or tax attributes each year is carried forward, subject to the same limitation in future years. The impact of an ownership change on state NOL carryforwards may vary from state to state. Recent legislation added several limitations to the ability to claim deductions for NOLs, including a deduction limit equal to 80% of taxable income and a restriction on NOL carryback deductions.
We may incur losses associated with foreign currency fluctuations.
Our operations are in many instances conducted in currencies other than our functional currency or the functional currencies of our subsidiaries. Fluctuations in the value of currencies could cause us to incur currency exchange losses. We do not currently employ a hedging strategy against exchange rate risk. We cannot assert with any assurance that we will not suffer losses as a result of unfavorable fluctuations in the exchange rates between the USU.S. dollar, the euro, the Canadian dollar and other currencies.
Legislative actions, new accounting pronouncements and higher insurance costs may adversely impact our future financial position or results of operations.
Changes in financial accounting standards or implementation of accounting standards may cause adverse, unexpected revenue or expense fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with greater frequency and are expected to occur in the future, and we may make or be required to make changes in our accounting policies in the future. Compliance with changing regulations of corporate governance and public disclosure, notably with respect to internal controls over financial reporting, may result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for companies such as ours, and insurance costs are increasing as a result of this uncertainty.
SecurityData security breaches may disrupt our operations and adversely affect our operating results.
Our network security and data recovery measures and those of third parties with which we contract, may not be adequate to protect against computer viruses, cyber-attacks, break-ins,breaches, and similar disruptions from unauthorized tampering with our computer systems. The misappropriation, theft, sabotage or any other type of security breach with respect to any of our proprietary and confidential information that is electronically stored, including research or clinical data, could cause interruptions in our operations, could result in a material disruption of our clinical activities and business operations and could expose us to third-party legal claims. Furthermore, we could be required to make substantial expenditures of resources to remedy the cause of cyber attackscyber-attacks or break-ins. This disruption could have a material adverse impact on our business, operating results and financial condition. Additionally, any break-in or trespass of our facilities that results in the misappropriation, theft, sabotage or any other type of security breach with respect to our proprietary and confidential information, including research or clinical data, or that results in damage to our R&D equipment and assets could have a material adverse impact on our business, operating results, and financial condition.
Our business processes personal information, both in connection with clinical activities and our employees. The use of this information is critical to our operations and innovation, including the development of our products, as well as management of our employees. New and evolving regulations, such as the European Union General Data Protection Regulation, could bring increased scrutiny of our data management in the future. Any cyber-attacks or other failure to protect critical and sensitive systems and information could damage our reputation, prompt litigation or lead to regulatory sanctions, all of which could materially affect our financial condition and results of operation.
Risks Relating to our Common Shares
Our Common Shares may be delisted from NASDAQ or TSX, which could affect their market price and liquidity. If our Common Shares were to be delisted, investors may have difficulty in disposing of their shares.
Our Common Shares are currently listed on both NASDAQ under the symbol “AEZS” and on TSX under the symbol “AEZ”"AEZS". We must meet continuing listing requirements to maintain the listing of our Common Shares on NASDAQ and TSX. For continued listing, NASDAQ requires, among other things, that listed securities maintain a minimum closing bid price of not less than $1.00 per share. There can be no assurance that the market price of our Common Shares will not fall below $1.00 in the future or that, if it does, we will regain compliance with the minimum bid price requirement.
In addition to the minimum bid price requirement, the continued listing rules of NASDAQ require us to meet at least one of the following listing standards: (i) stockholders’stockholders' equity of at least $2.5 million, (ii) market value of listed securities (calculated by multiplying the daily closing bid price of our Common Shares by our total outstanding Common Shares) of at least $35 million or (iii) net income from continuing operations (in the latest fiscal year or in two of the last three fiscal years) of at least $500,000 (collectively, the “Additional"Additional Listing Standards”Standards"). If we fail to meet at least one of the Additional Listing Standards, our securitiesCommon Shares may be subject to delisting after the expiration of the period of time, if any, that we are allowed for regaining compliance.
There can be no assurance that our Common Shares will remain listed on NASDAQ or TSX. If we fail to meet any of NASDAQ’sNASDAQ's or TSX’sTSX's continued listing requirements, our Common Shares may be delisted. Any delisting of our Common Shares may adversely affect a shareholder’sshareholder's ability to dispose, or obtain quotations as to the market value, of such shares.
Our share price is volatile, which may result from factors outside of our control.
Our valuation and share price since the beginning of trading after our initial listings, first in Canada and then in the US,U.S., have had no meaningful relationship to current or historical financial results, asset values, book value or many other criteria based on conventional measures of the value of shares.
As adjusted for and giving effect to the Share Consolidation, betweenBetween January 1, 20152018 and December 31, 2015,2018, the closing price of our Common Shares ranged from $4.00$1.19 to $84.20$3.87 per share on NASDAQ and from C$ 5.391.53 to C$ 104.00 5.10per share on TSX. Our share price may be affected by developments directly affecting our business and by developments out of our control or unrelated to us. The stock market generally, and the biopharmaceutical sector in particular, are vulnerable to abrupt changes in investor sentiment. Prices of shares and trading volume of companies in the biopharmaceutical industry can swing dramatically in ways unrelated to, or that bear a disproportionate relationship to, operating performance. Our share price and trading volume may fluctuate based on a number of factors including, but not limited to:
clinical and regulatory developments regarding our product candidates;
delays in our anticipated development or commercialization timelines;
developments regarding current or future third-party collaborators;suppliers and licensee(s);
clinical and regulatory developments regarding Macrilen™ (macimorelin);
delays in our anticipated clinical development or commercialization timelines;
announcements by us regarding technological, product developmentregulatory or other matters;
arrivals or departures of key personnel;
governmental or regulatory action affecting our product candidates and our competitors’competitors' products in the US,U.S., Canada and other countries;
developments or disputes concerning patent or proprietary rights;
actual or anticipated fluctuations in our revenues or expenses;
general market conditions and fluctuations for the emerging growth and biopharmaceutical market sectors; and
economic conditions in the US, CanadaU.S. or abroad.
Our listing on both NASDAQ and TSX may increase price volatility due to various factors, including different ability to buy or sell our Common Shares, different market conditions in different capital markets and different trading volumes. In addition, low trading volume may increase the price volatility of our Common Shares. A thin trading market could cause the price of our Common Shares to fluctuate significantly more than the stock market as a whole.
We do not intend to pay dividends in the near future.
To date, we have not declared or paid any dividends on our Common Shares. We currently intend to retain our future earnings, if any, to finance further research and the overall commercial expansion of our business. As a result, the return on an investment in our Common Shares, or any of our other securities, will depend upon any future appreciation in value. There is no guarantee that our Common Shares or any of our other securities will appreciate in value or even maintain the price at which shareholders have purchased them.
Future issuances of securities and hedging activities may depress the trading price of our Common Shares.
Any additional or future issuance of Common Shares or Convertible Securities, including the issuance of Common Shares upon the exercise of stock options and upon the exercise of warrants or other Convertible Securities, could dilute the interests of our existing shareholders, and could substantially decrease the trading price of our Common Shares.
We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy, to satisfy our obligations upon the exercise of options or warrants or for other reasons. Our Stock Option Planstock option plan generally permits us to have outstanding, at any given time, stock options that are exercisable for a maximum number of Common Shares equal to 11.4% of all then issued and outstanding Common Shares. As at March 29, 2016,December 31, 2018, there were:
9,928,69716,440,760 Common Shares issued and outstanding;
no issued and outstanding Preferred Shares;
2,842,309115,844 Common Shares issuable upon exercise of warrants that we previously issued in March 2015, which had a weighted average exercise price as of December 31, 2018 of $1.07 per Common Share, 2,331,000 Common Shares issuable upon exercise of warrants that we previously issued in December 2015, which had a weighted average exercise price as of December 31, 2018 of $7.10 per Common Share, and 945,000 Common Shares issuable upon exercise of warrants that we previously issued in November 2016, which had a weighted average exercise price as of December 31, 2018 of $4.70 per Common Share;
888,816 Common Shares that underlie outstanding warrants (excluding any exercisesstock options and deferred share units granted under our Plans, having a weighted average exercise price of Series B Warrants$3.66 per Common Share;
869 Common Shares that underlie outstanding stock options and deferred share units granted under our Plans, having a weighted average exercise price of C$743.56 per Common Share; and
246,619 additional Common Shares available for future grants under our Stock Option Plan, and 737,942 additional Common Shares available for future grants under our Long Term Incentive Plan. The maximum number of Common Shares issuable under the alternate cashless exercise featurePlans may equal 11.4% of such warrants);the issued and
275,041 stock options outstanding. outstanding Common Shares at any given time.
In addition, the price of our Common Shares could also be affected by possible sales of Common Shares by investors who view other investment vehicles as more attractive means of equity participation in us and by hedging or arbitrage trading activity that may develop involving our Common Shares. This hedging or arbitrage could, in turn, affect the trading price of our Common Shares.
We believe that there is a reasonable likelihood thatIn the event we maywere to lose our foreign private issuer status as of June 30 2016, whichof a given financial year, we would require usbe required to comply with the Exchange Act’sAct's domestic reporting regime, andwhich could cause us to incur additional legal, accounting and other expenses.
In order to maintain our current status as a foreign private issuer, either (1) a majority of our common sharesCommon Shares must not be either directly or indirectly owned of record by residents of the USU.S. or (2) (a) a majority of our executive officers and of our directors must not be USU.S. citizens or residents, (b) more than 50 percent of our assets cannot be located in the USU.S. and (c) our business must
be administered principally outside the US. We believeU.S.
In 2018, our management conducted its annual assessment of the various facts and circumstances underlying the determination of our status as a foreign private issuer and, based on the foregoing, our management has determined that, there isas of the date of such determination and as of June 30, 2018, we continued to be a reasonable likelihoodforeign private issuer.
There can be no assurance, however, that we maywill remain a foreign private issuer either in 2019 or in future financial years.
If we were to lose our foreign private issuer status when it is next reassessed as of June 30 2016. If we lose this status,of any given financial year, we would be required to comply with the Exchange Act reporting and other requirements applicable to USU.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC rules and NASDAQ listing standards. The regulatory and compliance costs to us of complying with the reporting requirements applicable to a USU.S. domestic issuer under USU.S. securities laws may be higher than the cost we have historically incurred as a foreign private issuer.In addition, if we were to lose our foreign private issuer status, we would no longer qualify under the Canada-USCanada-U.S. multijurisdictional disclosure system to benefit from being able to file registration statements on Form F-10 (even if we satisfy the other conditions to eligibility), which could make it longer and more difficult to register our securities and raise funds by way of public, registered offerings in the US,U.S., and we would become subject to “baby shelf”"baby shelf" rules that place limitations on our ability to issue an amount of securities above a certain threshold depending on our market capitalization and public float at a given point in time. As a result, we would expect that a potential loss of foreign private issuer status mayat some future point in time could increase our legal, financial reporting and financialaccounting compliance costs, and it is difficult at this time to estimate by how much our legal, financial reporting and financialaccounting compliance costs may increase.increase in such eventuality.
Our articles of incorporation contain “blank check”"blank check" preferred share provisions, which could delay or impede an acquisition of our company.
Our articles of incorporation, as amended, authorize the issuance of an unlimited number of “blank check” Preferred Shares,"blank check" preferred shares, which could be issued by our boardBoard of directorsDirectors without shareholder approval and which may contain liquidation, dividend and other rights equivalent or superior to our Common Shares. In addition, we have implemented in our constating documents an advance notice procedure for shareholder approvals to be brought before an annual meeting of our shareholders, including proposed nominations of persons for election to our boardBoard of directors.Directors. These provisions, among others, whether alone or together, could delay or impede hostile takeovers and changes in control or changes in our management. Any provision of our constating documents that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their Common Shares and could also affect the price that some investors are willing to pay for our Common Shares.
Our business could be negatively affected as a result of the actions of activist shareholders.
Proxy contests have been waged against many companies in the biopharmaceutical industry over the last few years. If faced with a proxy contest, we may not be able to successfully respond to the contest, which would be disruptive to our business. Even if we are successful, our business could be adversely affected by a proxy contest because:
responding to proxy contests and other actions by activist shareholders may be costly and time‑consuming, and may disrupt our operations and divert the attention of management and our employees;
perceived uncertainties as to the potential outcome of any proxy contest may result in our inability to consummate potential acquisitions, collaborations or in‑licensing opportunities and may make it more difficult to attract and retain qualified personnel and business partners; and
if individuals that have a specific agenda different from that of our management or other members of our boardBoard of directorsDirectors are elected to our board as a result of any proxy contest, such an election may adversely affect our ability to effectively and timely implement our strategic plan and to create value for our shareholders.
| |
| Item 4. | Information on the Company |
| |
| A. | History and development of the Company |
We are a specialty biopharmaceutical company engaged in developing and commercializing novel treatments in oncology, endocrinologypharmaceutical therapies, currently focused on the development and women’s health.commercialization of Macrilen™ (macimorelin), including through out-licensing arrangements and pursuing in-licensing opportunities.
We were incorporated on September 12, 1990 under the Canada Business Corporations Act (the "CBCA") and continue to be governed by the CBCA. Our registered address is located at 1 Place Ville Marie, Suite 2500,1155 René-Lévesque Blvd, West 41st Floor, Montréal, Quebec, Canada H3B 1R1,3V2 c/o Norton Rose Fulbright CanadaStikeman Elliott, LLP. Our principal executive offices are located at 315 Sigma Drive, Suite 302D, Summerville, South Carolina 29483;29486; our telephone number is (843) 900-3223 and our website is www.aezsinc.com.www.zentaris.com. None of the documents or information found on our website shall be deemed to be included in or incorporated by reference into this Annual Report on Form 20-F, unless such document is specifically incorporated herein by reference.The SEC also maintains a website at www.sec.gov that contains reports, proxy statements and other information regarding registrants that file electronically with the SEC.
On December 30, 2002, we acquired Zentaris AG, a biopharmaceutical company based in Frankfurt, Germany. Zentaris was a spin-off of Asta Medica GmbH, a former pharmaceutical company affiliated with Degussa AG.
In May 2004, we changed our name to Aeterna Zentaris Inc. and on May 11, 2007, Zentaris GmbH was renamed Aeterna Zentaris GmbH. Aeterna Zentaris GmbH ("AEZS Germany"). AEZS Germany conducts our drug development efforts. In September 2007, we incorporated Aeterna Zentaris, Inc. under the laws of Delaware. This wholly-owned subsidiary, which is based in the Charleston, South Carolina area, conducts certain of our administrative and commercial operations.
On October 1, 2013, we announced the completion of our previously announced agreements with various partners and licensees
with respect to the manufacturing rights and obligations for our Cetrotide® product. The principal outcome of such agreements was the transfer of all manufacturing rights and the grant of a license to a subsidiary of Merck KGaA of Darmstadt, Germany for the manufacture, testing, assembling, packaging, storage and release of Cetrotide® in all territories (the "Cetrotide® Business"). Following this transfer, the Cetrotide® Business has been presented in our consolidated financial statements as a discontinued operation. Except for this discontinued operation, we have not made any material divestitures or capital expenditures from 2013 to present.
On November 17, 2015, we effected a 100-to-1 Share Consolidation (reverse stock split). Our Common Shares commenced trading on a consolidated and adjusted basis on both NASDAQ and TSX on November 20, 2015.
We currently have three wholly-owned direct and indirect subsidiaries, AEZS GmbH,Germany, based in Frankfurt, Germany; Zentaris IVF GmbH, a direct wholly-owned subsidiary of AEZS Germany based in Frankfurt, Germany; and Aeterna Zentaris, Inc., an entity incorporated in the State of Delaware with an office in the Charleston, South Carolina area in the United States.
|
| | | | | |
| | | |
| | Aeterna Zentaris Inc. (Canada) | |
| | | |
| | | | |
| | | | | | |
| | | | | | |
| | 100% | | | 100% | |
| | | |
Aeterna Zentaris GmbH (Germany) | | Aeterna Zentaris, Inc. (Delaware) |
| | | |
| | | | |
| | 100% | | |
| | | |
Zentaris IVF GmbH (Germany) | | | | |
| | | | | |
Our Common Shares are listed for trading on theboth NASDAQ and TSX under the trading symbol "AEZ" and on NASDAQ under the trading symbol "AEZS".
Our agent for service of process and SEC matters in the United States is our wholly-owned subsidiary, Aeterna Zentaris, Inc., located at 315 Sigma Drive, Suite 302D, Summerville, South Carolina 29483.29486.
There have been no public takeover offers by third parties with respect to us or by us in respect of other companies' shares during the last or current fiscalfinancial year.
Recent Developments
For a complete description of our recent corporate and pipeline developments, refer to "Item 5. - Operating and Financial Review and Prospects - Key Developments".
We are engaged in drug development activities and in the promotion of products for others. We are now conducting Phase 3 studies of two internally developed compounds. The focus of our business development efforts is the acquisition or license of products that are relevant to our therapeutic areas of focus. We also intend to license out certain commercial rights of internally developed products to licensees in territories where such out-licensing would enable us to ensure development, registration and launch of our product candidates. Our goal is to become a growth-oriented specialty biopharmaceutical company by pursuing successful development and commercialization of our product portfolio and by achieving successful commercial presence and growth, while consistently delivering value to our shareholders, employees and the medical providers and patients who will benefit from our products.
Our Business Strategy
Our primary business strategy is to pursuefinalize the development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) through the License and Assignment Agreement in the United States and Canada. We continue to explore various alternatives to monetize our principal product candidates -- Zoptrex™ (zoptarelin doxorubicin) andrights to Macrilen™ (macimorelin) in oncology and endocrinology, respectively -- andother countries around the globe, including whether to find other license partners in these jurisdictions or to use our internal resources to commercialize oncology, endocrinology and women's health products that we may acquire, in-licenseMacrilen™ (macimorelin) in one or promote.more of these countries. Our vision is to become a growth-oriented specialty biopharmaceutical company.
Overview of our Drug Development Efforts
Our product pipeline
| |
(1) | Phase 2 in ovarian cancer completed. |
| |
(2) | Investigator-driven and sponsored Phase 2 trial in castration and taxane resistant prostate cancer completed. |
| |
(3) | Potential oral prostate cancer vaccine available for co-development/out-licensing, subject to an option granted to a third party. |
| |
(4) | Available for co-development/out-licensing. |
| |
(5) | Compound library transferred to Medical University of South Carolina. Aeterna Zentaris has access to future potential development candidates. |
Our drug development efforts are focused currently on two compounds, Zoptrex™ and Macrilen™, which are in Phase 3 clinical development, and on a LHRH-disorazol Z conjugate (AEZS-138), which is in pre-clinical development in oncology and is available for partnering. We made the decision to focus our efforts in pre-clinical development on one compound following a review of our portfolio, during which we concluded that we lack the resources to pursue other earlier-stage opportunities. As a result of this decision, we discontinued drug discovery efforts, including basic research activities in medicinal chemistry and biology and our high-throughput-screening operations, which resulted in a reduction of our research and development staff by approximately 29 personnel during 2014.
Zoptrex™
Overview
Zoptrex™ represents a new targeting concept in oncology using a hybrid molecule composed of a synthetic peptide carrier, zoptarelin, and a well-known chemotherapy agent, doxorubicin, resulting in a cytotoxic conjugate. Most chemotherapeutic agents, including doxorubicin, are toxic to normally growing, healthy cells as well as to tumor cells that grow uncontrolled. Therefore, a method for targeting such drugs specifically to cancerous tissue offers a potential benefit for patients with tumors, and particularly with advanced or metastatic tumors.
The illustration above depicts the believed mode of action of our hybrid cytotoxic compound Zoptrex™. The LHRH receptor targeting part of the hybrid is believed to transport doxorubicin to a cancer cell presenting the LHRH receptor, which leads to the death of this cancer cell.
Zoptrex™ is the first intravenous drug in advanced clinical development that is considered to direct the chemotherapy agent specifically to LHRH-receptor expressing tumors, which then could result in a more targeted treatment with less damage to healthy tissue. This design is believed to allow for the specific binding and selective uptake of the cytotoxic conjugate by LHRH receptor-positive tumors. Potential benefits of this targeted approach include better efficacy and a more favorable safety profile with lower incidence and severity of side effects as compared to doxorubicin. In addition, the targeted approach may enable treatment of LHRH receptor-positive cancers that have become resistant to doxorubicin.
We are conducting a pivotal Phase 3 clinical study of Zoptrex™ in women with locally advanced, recurrent or metastatic endometrial cancer who have progressed and who have received one chemotherapeutic regimen with platinum and taxane (either as adjuvant or first-line treatment). The clinical study is known as the “ZoptEC” study (zoptarelin doxorubicin in endometrial cancer). ZoptEC is a fully-recruited (over 500 patients), open-label, randomized-controlled study, comparing the efficacy and safety of Zoptrex™ to doxorubicin alone. Patients are centrally randomized in a 1:1 ratio and receive either Zoptrex™ (267 mg/m2) or doxorubicin (60 mg/m2) intravenously, every three weeks and for up to nine cycles. Response is being evaluated every three cycles during treatment and thereafter every 12 weeks until progression.
We are conducting ZoptEC under a Special Protocol Assessment (“SPA”) with the FDA. The SPA agreement states that the proposed trial protocol design, clinical endpoints and planned analyzes are acceptable to the FDA to support a regulatory submission. Final marketing approval depends on the results of efficacy, the adverse event profile and an evaluation of the benefit/risk of treatment demonstrated in ZoptEC. The primary efficacy endpoint of the ZoptEC trial is improvement in median Overall Survival (“OS”). Secondary endpoints include progression-free survival, objective response rate and clinical benefit rate.
On October 13, 2015, we announced that the independent Data and Safety Monitoring Board (“DSMB”) appointed to monitor ZoptEC recommended that ZoptEC continue as planned to completion. The DSMB's decision followed completion of its pre- specified second interim analysis on efficacy and safety for ZoptEC at approximately 192 events. In April 2015, the DSMB made the same recommendation following its first pre-specified analysis on safety and futility at approximately 124 events. A final analysis of the data is expected at approximately 384 events.
ZoptEC is being conducted by Ergomed plc, a contract clinical development organization with which we have entered into a co-development and profit-sharing agreement. Under the terms of the agreement, Ergomed has agreed to assume 30% (up to $10 million) of the clinical and regulatory costs for ZoptEC, which are estimated at approximately $32.5 million. Ergomed will receive
its return on investment based on an agreed single-digit percentage of any net income or net proceeds from licensing activity we receive for Zoptrex™ in this indication, up to a specified maximum amount.
We are attempting to commercialize Zoptrex™ as a treatment for endometrial cancer because, according to the American Cancer Society, endometrial cancer is the most common invasive gynecologic cancer in women in the United States, with approximately 50,000 new cases annually. This disease primarily affects postmenopausal women at an average age of 60 years at diagnosis. In the United States, it is estimated that approximately 8,000 women will die of endometrial cancer annually. To the best of our knowledge, there is also no systemic therapy approved in either the United States or Europe (except Germany, where doxorubicin is approved for this indication) for treating advanced or recurrent endometrial cancer.
We expect to complete the ZoptEC trial in the third quarter of 2016 and, if the results of the trial warrant doing so, to file a new drug application ("NDA") in the United States for Zoptrex™ in 2017. We are now moving forward with our planning to commercialize Zoptrex™, looking toward commercial launch of the product in 2018, assuming positive Phase 3 results and that the NDA is granted.
Development History
The following is a summary of the history of our development of Zoptrex™ in ovarian and endometrial cancer:
| |
• | In 2007, a Phase 2 open-label, non-comparative, multicenter two-indication trial stratified with two stages Simon Design was prepared. The study was planned to involve up to 82 patients, with up to 41 patients each with a diagnosis of platinum-resistant ovarian cancer (stratum A) or disseminated endometrial cancer (stratum B). Under coordination by Prof. Günter Emons, M.D., Chairman of the Department of Obstetrics & Gynecology at the University of Göttingen, Germany, this open-label, multicenter and multinational Phase 2 study “AGO-GYN 5” was conducted by the German AGO Study Group (Arbeitsgemeinschaft Gynäkologische Onkologie / Gynaecological Oncology Working Group), in cooperation with clinical sites in Europe. An intravenous infusion of Zoptrex™ (267 mg/m2) was administered on every first day of a 21-day (three-week) cycle. The proposed duration of the study treatment was six cycles. The study was performed with 14 centers of the German Gynaecological Oncology Working Group, in cooperation with three clinical sites in Europe. The primary efficacy endpoint was a response rate with a success criterion at the end of Stage II defined as five or more patients with partial or complete tumor responses according to Response Evaluation Criteria in Solid Tumors (“RECIST”) and/or Gynaecologic Cancer Intergroup (“GCIG”) guidelines. Secondary endpoints included time to progression (“TTP”), survival and toxicity, as well as adverse effects. In October 2008, we announced that we had entered the second stage of patient recruitment for the Phase 2 trial in the platinum-resistant ovarian cancer indication. This decision was taken following the report of two partial responses (“PR”) among patients with ovarian cancer. The second stage of patient recruitment for the endometrial cancer indication was reached in November 2008 and was based on the report of one complete response (“CR”) and two PR among 14 patients with endometrial cancer.
|
| |
• | On June 7, 2010, Prof. Emons initially presented positive efficacy and safety data for Zoptrex™ in ovarian cancer at the American Society of Clinical Oncology’s (“ASCO”) Annual Meeting, now published in an article entitled "Phase 2 study of AEZS-108, a targeted cytotoxic LHRH analog, in patients with LHRH receptor-positive platinum resistant ovarian cancer" in the journal Gynecologic Oncology (Gynecol.Oncol. (2014) 133:427). Efficacy included PR in six patients (14.3%) and stable disease for more than twelve weeks in 16 patients (38%). Based on those data, a clinical benefit rate (“CBR”) of 52% was estimated. Median TTP and OS were evaluated at 2.8 months (12 weeks) and 12.2 months (53 weeks), respectively. Prof. Emons concluded that (i) Zoptrex™ was efficacious and well tolerated in patients with heavily pre-treated platinum- and taxane-resistant ovarian cancer; (ii) the safety profile confirmed the dose of 267 mg/m2; (iii) hematological toxicity was rapidly reversible; (iv) non-hematological toxicities were usually limited to lower severity; (v) tolerability and CBR compared with topotecan and liposomal doxorubicin; (vi) no cardiotoxic events were observed; and (vii) overall survival was encouraging as all patients treated with Zoptrex™ had platinum-resistant disease.
|
| |
• | On September 14, 2011, Prof. Emons presented positive final Phase 2 efficacy and safety data for zoptarelin doxorubicin in advanced endometrial cancer at the European Society of Gynecological Oncology in Milan, Italy. The results of the study were published in an article by Prof. Emons, et al. in the journal Gynecologic Oncology (Gynecol.Oncol. (2014) 24:260). The study involved 43 patients with LHRH positive advanced or recurrent endometrial cancer. Patients received Zoptrex™ at a dose of 267 mg/m2 by intravenous infusion, with retreatment every three weeks, for up to six courses. Response rate per RECIST was defined as the primary endpoint. Secondary endpoints were safety, TTP and OS. The responses, as confirmed by independent review, included two patients with complete response (5%), eight patients with PR (18%) and 20 patients with stable disease (“SD”) (47%). Based on such data, the estimated overall response rate (“ORR”) (ORR=CR+PR) was 23% and the CBR was 70%. Responses were also achieved in patients with prior chemotherapy - two PR and three SD in eight of the patients pre-treated with platinum/taxane regimens. Median TTP and OS were seven months (30 weeks) and 14.9 months (62 weeks), respectively. Prof. Emons concluded as follows: (i) Zoptrex™ was efficacious and well tolerated in patients with advanced endometrial cancer; (ii) the safety profile confirmed the dose of 267 mg/m2; (iii) hematological toxicity was rapidly reversible; (iv) non-hematological toxicities were usually not severe, causing few deviations from scheduled treatment; (v) no cardiotoxic events were observed; (vi)
|
the ORR of 23% compares well with those of single-agent platinum or taxane treatment; (vii) responders included patients pre-treated with platinum/taxane combination; (viii) in addition, the rate of SD was 47%, resulting in a CBR of 70%; and (ix) the OS after single agent Zoptrex™ was similar to that reported for modern triple combination chemotherapy, but was achieved with lower toxicity.
Competition
The following products are among some of the many products currently in clinical trial in endometrial cancer:
|
| | | | | | | |
Drug | Co-administered drugs & comparator arm | Target | Indication | Clinical Trial/ Approval Status | Innovator | Primary Endpoint | Comments/
Clinical History/
Commercial History
|
Ixabepilone (Ixempra; BMS-247550)
| Doxorubicin, paclitaxel | Tubulin-micro- tubules; epothilone B analog | Second-line endometrial cancer | IXAMPLE2; Phase III (not in Bristol-Myers Squibb's pipe-line, did not meet OS primary endpoint) | BMS | Overall survival
(OS)
| 500-patient trial; did not improve OS at interim analysis in Q4/13 |
Ixabepilone (Ixempra; BMS- 247550) | Paclitaxel, carboplatin, temsirolimus, bevacizumab (Avastin) | As above | Stage III/IV, recurrent endometrial cancer | Phase III | US NCI | PFS out to five years (RECIST) | 330-patient trial, PFS data expected in 2016 |
Lenvatinib
(E7080)
| Monotherapy | Tyrosine kinase
VEGFR2 inhibitor,
multi-targeted
| Second-line
endometrial
cancer
| Phase II, open-label
single-arm (still active, but not recruiting patients)
| Eisai | Objective
response rate to six months
| 167-patient trial, tumor response data was expected in H2/12 |
MK-2206 | Monotherapy | Serine/
threonine kinase Akt inhibitor
| Recurrent, advanced endometrial cancer | Phase II, two-arm, only patients with PIK3CA mutation | US NCI (Astra--Zeneca-Merck partnered drug) | Objective response, PFS | 90-patient trial, PFS/tumor response data in 2016 |
Buparlisib (BKM120) | Monotherapy | Phosphatidyl inositol-3-kinase (PI3K)-Akt-mTOR
pathway inhibitor
| Second-line endometrial cancer | Phase II (ENDOPIK) | Novartis | ORR/PFS out to six months | 56-patient trial, PFS/tumor response data in H2/16 |
BMN 673
(BioMarin)
| Monotherapy | Poly-ADP ribose polymerase inhibitor | Inoperable, advanced endometrial cancer | Phase II (PANDA trial) | BioMarin, University College London | PFS at six months, time-to-recurrence | 100-patient trial, started in June 2015, data probably by H1/18 |
GSK
2141795
| Mekinist (trametinib, MEK inhibitor) | Akt inhibitor | Recurrent, persistent endometrial cancer | Phase II, control arm is Mekinist alone | US NCI (is GSK drug, but GSK not identified as sponsor) | PFS, up to five years, impact of Kras status on response | 148-patients, interim PFS data by H1/17 |
|
| | | | | | | |
Virexxa
(Cridanimod
sodium)
| Progesterone | Carboxymethyl
-acridinone;
elevates PrR
expression
| Recurrent,
persistent endometrial cancer
(PrR-negative)
| Phase II | Pharmsynthez
(Estonia), AS
Kevelt
| ORR at one year, PFS at two years | 58-patients, first enrolled in Jan/15; data in H2/18 |
Cabozantinib s-malate (Exelixis' Comitriq) | Monotherapy | Multi-kinase inhibitor, already approved in thyroid cancer | Recurrent, metastatic endometrial cancer | Phase II | US NCI (Exelixis not identified as
partner)
| ORR/PFS out to three months | 72-patient, PFS data expected by Q3/16 |
LY3023414 | Monotherapy | PI3K-mTOR dual inhibitor | Recurrent endometrial cancer | Phase II (multiple cancer forms) | MSKC, Eli Lilly | Three-month CBR, one-year O/S | 25-patient, single-arm, clinical benefit rate data by Q4/16 |
IMMU-132 | Monotherapy | TROP-2-targeted mAb linked to SN38 (metabolite of irinotecan) | Endometrial cancer | Phase I/II (multiple epithelial cancers being tested simultane-ously) | Immuno medics | Safety, tumor response | 250-patient, three- month response rate data in H2/16 |
KPT-330
(Selinexor)
| Monotherapy | XPO1 (nuclear export protein) antagonist | Advanced gynecologic cancers | Phase II | Karyopharm Therapeutics | Safety, survival, QoL | 105-patient, two-year survival data in H2/17 |
HuMax-TF- ADC | Monotherapy | Tissue factor- targeted mAb lined to auristatin | Solid tumors, including endometrial cancer | Phase I/II | Genmab | Safety, PK, response rate | 80-patient, adverse event rate & response rate data in H2/17 |
Source: D. Loe, “Aeterna Zentaris: Recalibrating Valuation Following Capital Structure De-construction, But Still Positive on Zoptarelin Prospects” (Euro Pacific Canada) December 11, 2015 (quoting NIH data)
Additional Indications
We believe that Zoptrex™ may be useful in treating other cancers, including breast cancer, bladder cancer and prostate cancer. We terminated early clinical trials of the compound as a treatment for triple-negative breast cancer and bladder cancer as part of our ongoing review of our development activities to ensure the most effective use of our resources.
We assisted Dr. Jacek Pinski, Associate Professor of Medicine at the Norris Comprehensive Cancer Center of the University of Southern California, to conduct a Phase 1/2 study in refractory prostate cancer with Zoptrex™. Dr. Pinski received a $1.6 million grant from The National Institutes of Health (“NIH”) to conduct the study. The study, entitled “A Phase I/II Trial of AN-152 [AEZS-108] in Castration-and Taxane-Resistant Prostate Cancer”, was conducted in two portions: an abbreviated dose-escalation study followed by a single arm, Simon Optimum two-stage design Phase 2 study, using the dose selected in the Phase 1 portion.
The following is a summary of Dr. Pinski's study:
On December 14, 2010, we announced the initiation of the Phase 1/2 trial.
| |
• | On February 3, 2012, we reported updated results for the Phase 1 portion of the study. The results were based on 13 patients who had been previously treated with androgen-deprivation therapy (LHRH agonist) and at least one taxane-based chemotherapy regimen, who were treated on three dose levels of Zoptrex™: three at 160 mg/m2, three at 210 mg/m2, and seven at 267 mg/m2. Overall, Zoptrex™ was well tolerated among this group of heavily pretreated older patients. There were two dose-limiting toxicities, each of which having been a case of asymptomatic Grade 4 neutropenia at the 267 mg/m2 dose level and both patients fully recovered. The Grade 3 and 4 toxicities were primarily hematologic. There was minimal non-hematologic toxicity, most frequently fatigue and alopecia. Despite the low doses of Zoptrex™ in the first cohorts, there was some evidence of antitumor activity. One patient received eight cycles (at 210 mg/m2) due to continued benefit. Among the five evaluable patients with measurable disease, four achieved stable disease. At the time of submission of the abstract, a decrease in PSA was noted in six patients. Six of 13 (46%) treated patients received at least five cycles of therapy with no evidence of disease progression at twelve weeks. Correlative studies on CTC demonstrated the uptake of zoptarelin doxorubicin into the targeted tumor.
|
| |
• | On November 12, 2012, we announced the initiation of the Phase 2 portion of Dr. Pinski’s Phase 1/2 study of Zoptrex™ in prostate cancer. This was a single-arm Simon Optimum design Phase 2 study of Zoptrex™ in 25 patients with CRPC. Patients received Zoptrex™ (210 mg/m2) intravenously over two hours, every three weeks. The primary endpoint was CB, defined as remaining progression-free by RECIST and PSA after treatment for 12+ weeks. Secondary endpoints were progression free survival (“PFS”), best overall response, toxicity, pain and OS.
|
| |
• | On June 3, 2013, we announced that final data for the Phase 1 portion of Dr. Pinski’s Phase 1/2 trial with Zoptrex™ in prostate cancer demonstrated the compound's promising anti-tumor activity. Results were presented by Dr. Pinski during a poster session at the ASCO Annual Meeting in Chicago. The results of the study were published in an article by Liu et al in the journal Clinical Cancer Research (Clin. Cancer Res. (2014) 20:6277). Eighteen men were treated at three dose levels: (160 mg/m2; (ii) 210 mg/m2; and (iii) 267 mg/m2). Overall Zoptrex™ was well tolerated among this group of heavily pretreated patients. There were two dose-limiting toxicities (grade four neutropenia and grade three febrile neutropenia), prompting de-escalation to 210 mg/m2 and establishing it as the Maximum Tolerated Dose. Among the 15 evaluable patients with measurable disease, ten achieved SD, and a drop in PAS was noted in three patients.
|
On September 28, 2015, Dr. Pinski announced during a poster session at the 18th ECCO - 40th ESMO European Cancer Congress in Vienna, Austria, that among the 25 patients in the Phase 2 portion of the trial, 11 patients experienced clinical benefit as the primary endpoint and 13 patients achieved SD. Maximal PSA response was stable in 20 patients. Pain assessment improved for 11 patients. Zoptrex™ was well tolerated in this heavily pretreated patient population with hematological toxicities, usually limited to grade three, as the most common adverse events. Dr. Pinski concluded that Zoptrex™ was well tolerated and met the primary efficacy endpoint in castration- and taxane-resistant prostate cancer patients.
We believe that immuno-modulatory and targeted therapies have been key areas of innovation in oncology over the last few years. Zoptrex™ is a targeted cytotoxic therapy using a peptide as the targeting agent and is therefore part of the ongoing innovation in the treatment of cancer. Furthermore, we believe that Zoptrex™ is ahead of many of the immuno-oncology products that are in development. Due to our lack of resources, we intend to pursue the development of Zoptrex™ for indications other than endometrial cancer by seeking development partners to assist with the effort. In this regard, on December 1, 2014, we entered into an exclusive license and technology transfer agreement with Sinopharm A-Think Pharmaceuticals Co., Ltd. for the compound, for the initial indication of endometrial cancer and for all other human indications, in the territories of China, Hong Kong and Macau. We are currently seeking to sublicense the compound to others for development in additional markets. (macimorelin)
Macrilen™
Macrilen™ (macimorelin) is a novel orally available peptidomimetic ghrelin receptor agonist that stimulates the secretion of growth hormone by binding to the ghrelin receptor (GHSR-1a) and that has potential uses in both endocrinology and oncology indications. Macrilen™ has been(macimorelin) was granted orphan-drug designation by the FDA for use in evaluating growth hormone deficiency (“GHD”("GHD"). If approved by the FDA, Macrilen™ would be the first orally administered drug indicated for the evaluation of adult growth hormone deficiency (“AGHD”).
Competitors for Macrilen™ (macimorelin) as a product for the evaluation of AGHD are principally the diagnostic tests currently performed by endocrinologists, although none of these tests are approved by the FDA for this purpose. The most commonly used diagnostic tests for GHD are:
Measurement of blood levels of Insulin Growth Factor (“IGF”("IGF")-1, which is typically used as the first test when GHD is suspected. However, this test is not used to definitively diagnose GHD because many growth hormone deficient patients show normal IGF-1 levels.
The insulin tolerance test (“ITT”Insulin Tolerance Test ("ITT"), which ishas historically been considered the historical gold standard for the evaluation of AGHD because of its high sensitivity and specificity. However, the ITT is inconvenient to both patients and physicians, administered intravenously (IV), and contra-indicated in certain patients, such as patients with coronary heart disease or seizure disorder, because it requires the patient
to experience hypoglycemia to obtain aan accurate result. Some physicians will not induce full hypoglycemia, intentionally compromising accuracy to increase safety and comfort for the patient. Furthermore, administration of the ITT is expensive becauseincludes additional costs associated with the patient must be constantlybeing closely monitored by a physician for the two- to four-hour duration of the test and the test must be administered in a setting where emergency equipment is available and where the patient may be quickly hospitalized. The ITT is not used for patients with co-morbidities, such as cardiovascular disease, seizure disorder or a history of brain cancer or for patients who are elderly and frail, due to safety concerns.
The Glucagon test, which is simple to perform andStimulation Test ("GST") is considered relatively safe by endocrinologists. The mechanism of action for this test is unclear. Also, this test takes up to three to four hours. It produces side effects in up to one-third of the patients.patients with the most common being nausea during and after the test. This test is administered intramuscularly.intramuscularly (IM).
The GHRH + ARG test (growth hormone releasing hormone-arginine stimulation) which is an easier test to perform in an office setting and has a good safety profile but is considered to be costly to administer compared to the ITT and Glucagon.the GST. GHRH + ARG is approved in the EU and has been proposed to be the best alternative to ITT, but GHRH is no longer available in the United States. This test is administered intravenously.intravenously (IV).
Oral administration of Macrilen™ (macimorelin) offers more convenience and simplicity over the current GHD tests used, all of which require either intravenous or intramuscular administration. Additionally, Macrilen™ (macimorelin) may demonstrate a more favorable safety profile than existing diagnostic tests, some of which may be inappropriate for certain patient populations, e.g. diabetes mellitus or coronary heart disease, and have demonstrated a variety of side effects, which Macrilen™ (macimorelin) has not thus far. These factors may be limiting the use of GHD testing and may potentially enable Macrilen™ (macimorelin) to become the product of choice in evaluating AGHD. We believe that Macrilen™, if it is approved, (macimorelin) is likely to rapidly displace the ITT as the preferred means of evaluating AGHD for the following reasons:
•it is safer and more convenient than the ITT because it does not require the patient to become hypoglycemic;
•Macrilen™ (macimorelin) is administered orally, while the ITT requires an intravenous injection of insulin;
Macrilen™ (macimorelin) is a more robust test than the ITT leading to evaluable test results;
Macrilen™ (macimorelin) results are highly reproducible;
the evaluation of AGHD using Macrilen™ (macimorelin) is much less time consumingtime-consuming and labor intensivelabor-intensive than the ITTITT; and therefore, it is less expensive to conduct; and
the evaluation can be conducted in the physician's office rather than in a hospital-like setting.
There areWe believe that approximately 36,00060,000 AGHD tests performedwill be conducted annually, in the U.S. BasedU.S, after the introduction of Macrilen™ (macimorelin). In addition, based on published information from the U.S. Centers for Disease Control and Prevention, different scientific publications and by Navigant Research, we estimate that the total potential U.S. market for AGHD evaluation is approximately 158,000150,000 tests per year, including the evaluation of patients who have suffered traumatic brain injury (“TBI”("TBI"). In patients with TBI, GHD is frequent and may contribute to cognitive sequelsequelae and reduction in quality of life. GHD developsmay develop in approximately 19% of both severe and moderate hospitalized TBI victims.
Development History
The following is a summary of the history of our development of Macrilen™ (macimorelin):
We out-licensed the development compound macimorelin acetate to Ardana Bioscience in 2004. Ardana Bioscience subsequently initiated the clinical development program of macimorelin acetate as an orally active compound intended to be used in the diagnosis of adult growth hormone deficiency. Following agreement with the FDA on the study design, Ardana Bioscience initiated a pivotal Phase 3 study in 2007, which tested the compound compared to a test of growth hormone-releasing hormone (“GHRH”) + L-Arginine (“ARG”), using a competitor's compound. The study was discontinued in 2008 due to Ardana Bioscience's bankruptcy. We terminated Ardana Bioscience's license to the compound due to its bankruptcy.2004 - 2014
| |
| • | We out-licensed the development compound macimorelin acetate to Ardana Bioscience in 2004. Ardana Bioscience subsequently initiated the clinical development program of macimorelin acetate as an orally active compound intended to be used in the diagnosis of AGHD, however in 2008 Ardana Bioscience filed for bankruptcy so we terminated the license and regained rights to the compound. On October 19th, 2009, we announced that we would continue the macimorelin clinical development program for use in evaluating the AGHD and assumed the sponsorship of the Investigational New Drug Application (IND). On December 20, 2010, we announced we had reached agreement with the FDA on a Special Protocol Assessment ("SPA") for Macrilen™ (macimorelin), enabling us to complete the ongoing registration study required to gain approval for use in evaluating AGHD. On July 26, 2011, we announced the completion of the Phase 3 study of Macrilen™ (macimorelin) as a first oral product for use in evaluating AGHD and the decision to meet with the FDA for the future filing of an NDA for the registration of Macrilen™ (macimorelin) in the United States. On June 26, 2012, we announced that the final results from a Phase 3 trial for Macrilen™ (macimorelin) showed that the drug is safe and effective in evaluating AGHD. In November 2013, we filed an NDA for Macrilen™ (macimorelin) for the evaluation of AGHD by evaluating the pituitary gland secretion of growth hormone in response to an oral dose of the product. The FDA accepted the NDA for substantive review in January 2014. On November 6, 2014, the FDA informed us, by issuing a Complete Response Letter ("CRL"), that it had determined that our NDA could not be approved in its then present form. The CRL stated that the planned analysis of our pivotal trial did not meet its stated primary efficacy objective as agreed to in the SPA. The CRL further mentioned issues related to the lack of complete and verifiable source data for determining whether patients were accurately diagnosed with AGHD. The FDA concluded that, "in light of the failed primary analysis and data deficiencies noted, the clinical trial does not by itself support the indication." To address the deficiencies identified above, the CRL stated that we needed to demonstrate the efficacy of Macrilen™ (macimorelin) as a diagnostic test for GHD in a new, confirmatory clinical study. The CRL also stated that a serious event of electrocardiogram QT interval prolongation occurred for which attribution to drug could not be excluded. Therefore, a dedicated thorough QT study to evaluate the effect of macimorelin on the QT interval would be necessary for FDA clearance and approval. |
On October 19, 2009, we announced that we had initiated activities intended to complete the clinical development of Macrilen™ for use in evaluating AGHD. We had already assumed the sponsorship of the IND from Ardana Bioscience and discussed with the FDA the best way to complete the ongoing Phase 3 clinical trial and subsequently to file an NDA for approval of Macrilen™ for use in evaluating AGHD. The pivotal Phase 3 trial was designed to investigate the safety and efficacy of the oral administration of Macrilen™ as a growth hormone stimulator for use in evaluating AGHD. It was accepted by the FDA that for the ongoing part of the study, Macrilen™ would not be compared to the GHRH + ARG test because the competitor's compound had been removed from the market.
On December 20, 2010, we announced we had reached agreement with the FDA on a SPA for Macrilen™, enabling us to complete the ongoing registration study required to gain approval for use in evaluating AGHD. The first part of the study, conducted by our former licensee, Ardana, was a two-way cross-over study and included 42 patients with confirmed AGHD or multiple pituitary hormone deficiencies and a low IGF-1. A control group of ten subjects without AGHD was matched to patients for age, gender, body mass index and (for females) estrogen status.
On July 26, 2011, we announced the completion of the Phase 3 study of Macrilen™ as a first oral product for use in evaluating AGHD and the decision to meet with the FDA for the future filing of an NDA for the registration of Macrilen™ in the United States.
On June 26, 2012, we announced that the final results from a Phase 3 trial for Macrilen™ showed that the drug is safe and effective in evaluating AGHD. Jose M. Garcia, MD, PhD, then of the Baylor College of Medicine and the Michael E. DeBakey VA Medical Center, disclosed these data during an oral presentation at the 94th ENDO Annual Meeting and
Expo in Houston, Texas. The study had originally been designed as a cross-over trial of Macrilen™ compared to the GHRH + ARG test in AGHD patients and in controls matched for body mass index (“BMI”), estrogen status, gender and age. After 43 AGHD patients and ten controls had been tested, the GHRH + ARG test became unavailable because the competitor's compound was withdrawn from the market. The study was completed by testing ten more AGHD patients and 38 controls with Macrilen™ alone. Of the 53 AGHD subjects enrolled, 52 received Macrilen™, and 50 who had confirmed AGHD prior to study entry were included in this analysis, along with 48 controls. Two AGHD subjects could not be matched due to the combination of young age, high BMI and estrogen use. The objective of this clinical trial was to determine the efficacy and safety of Macrilen™ in the evaluation of AGHD. Mean peak growth hormone ("GH") levels in AGHD patients and controls following Macrilen™ administration were 2.36ng/mL (range 0.03-33) and 17.71ng/mL (range 10.5-94), respectively. The ROC plot analysis yielded an optimal GH cut-point of 2.7ng/mL, with 82% sensitivity, 92% specificity and a 13% misclassification rate. Obesity (BMI>30) was present in 58% of cases and controls, and peak GH levels were inversely associated with BMI in controls. Adverse events ("AE") were seen in 37% of AGHD patients and in 21% of controls following Macrilen™. In contrast, 61% of AGHD subjects and 30% of controls experienced AEs with L ARG+GHRH. The most common AEs after Macrilen™ were unpleasant taste (19.2%) and diarrhea (3.8%) for the AGHD patients and unpleasant taste (4.2%) and diarrhea (4.2%) for the matched controls. No clinically meaningful changes from baseline in ECG results during the study for AGHD patients were observed; however, one control subject had an ECG change (T wave abnormality and QTc interval prolongation) one hour after treatment with Macrilen™ that was considered a serious treatment-related adverse event and resolved spontaneously within 24 hours. The subject had been pre-treated with citalopram, a drug that was later reported by the FDA to be associated with QT prolongation, although the patient had stopped this medication seven days prior to dosing. In an expert statement of January 9, 2015 Prof. Dr. W. Haverkamp, Centrum Herz-, Kreislauf- und Gefäßmedizin, Charité, Berlin, considered the observed QT prolongation to be not related to Macrilen™. Overall, this study demonstrated that Macrilen™ is safe and effective for use in evaluating AGHD.
In November 2013, we filed an NDA for Macrilen™ for the evaluation of AGHD by evaluating the pituitary gland secretion of growth hormone in response to an oral dose of the product. The FDA accepted the NDA for substantive review in January 2014. On November 6, 2014, the FDA informed us, by issuing a Complete Response Letter (“CRL”), that it had determined that our NDA could not be approved in its then- present form. The CRL stated that the planned analysis of our pivotal trial did not meet its stated primary efficacy objective as agreed to in the SPA. The CRL further mentioned issues related to the lack of complete and verifiable source data for determining whether patients were accurately diagnosed with AGHD. The FDA concluded that, “in light of the failed primary analysis and data deficiencies noted, the clinical trial does not by itself support the indication.” To address the deficiencies identified above, the CRL stated that we needed to demonstrate the efficacy of Macrilen™ as a diagnostic test for GHD in a new, confirmatory clinical study. The CRL also stated that a serious event of electrocardiogram QT interval prolongation occurred for which attribution to drug could not be excluded. Therefore, a dedicated thorough QT study to evaluate the effect of macimorelin on the QT interval would be necessary.
Following receipt of the CRL, we assembled a panel of experts in the field of growth-hormone deficiency, including experts in the field from both the United States of America and the EU. The panel met on January 8, 2015, during which we discussed our conclusions from the CRL, as well as the potential design of a new pivotal study. The panel advised us to continue to seek approval for Macrilen™ (macimorelin) because of their confidence in its efficacy and because there currently is no FDA-approved diagnostic test for AGHD. In parallel, we collected information on timelines and costs for such a study.
During an end-of-review meeting with the FDA on March 6, 2015, we agreed with the FDA on the general design of the confirmatory study Phase 3 study of Macrilen™ (macimorelin) for the evaluation of AGHD, as well as evaluation criteria. We agreed with the FDA that the confirmatory study will be conducted as a two-way crossover with the ITT as the benchmark comparator.
On April 13, 2015, we announced plans to conduct a new, confirmatory Phase 3 clinical study to demonstrate the efficacy of Macrilen™ (macimorelin) for the evaluation of AGHD, as well as a dedicated thorough QT study to evaluate the effect of Macrilen™ (macimorelin) on myocardial repolarization. The confirmatory Phase 3 clinical study of Macrilen™ (macimorelin), entitled “Confirmatory"Confirmatory validation of oral macimorelin as a growth hormone (GH) stimulation test (ST) for the diagnosis of adult growth hormone deficiency (AGHD)AGHD in comparison with the insulin tolerance test (ITT)”", iswas designed as a two-way crossover study with the ITT as the benchmark comparator and will involve some 30involved 31 sites in the United States and Europe. The study population will consist of approximatelywas planned to include at least 110 subjects (at least 55 ITT-positive and 55 ITT-negative) with a medical history documenting risk factors for AGHD, and willwas planned to include a spectrum of subjects from those with a low risk of having AGHD to those with a high risk of having the condition. The primary endpoint is validation of a single oral dose of Macrilen™ for the diagnosis of AGHD, using the ITT as a comparator.
On May 26, 2015, we announced that we had received written scientific advice from the European Medicines Agency (“EMA”)EMA regarding the further development plan, including the study design, for the new confirmatory Phase 3 clinical study of Macrilen™ (macimorelin) for use in evaluating AGHD. As a result of the advice, we believe that the confirmatory Phase 3
study that was agreed with the FDA meets the EMA's study-design expectations as well, allowing for USU.S. and European approval, if the study is successful.
On November 19, 2015, we announced the enrollment of the first patient in the confirmatory Phase 3 clinical study of Macrilen™ (macimorelin). Based on the current rate
On October 26, 2016, we announced completion of enrollment, we expectpatient recruitment for the confirmatory Phase 3 clinical studytrial of Macrilen™ (macimorelin) as a growth hormone stimulation test for the evaluation of AGHD. In addition, we completed the dedicated QT study as requested by the FDA in the CRL to evaluate the effect of Macrilen™ (macimorelin) on the QT interval.
On January 4, 2017, we announced that, based on an analysis of top-line data, the confirmatory Phase 3 clinical trial of Macrilen™ (macimorelin) failed to achieve one of its co-primary endpoints. Under the study protocol, the evaluation of AGHD with Macrilen™ (macimorelin) would be considered successful, if the lower bound of the two-sided 95% confidence interval for the primary efficacy variables was 75% or higher for "percent negative agreement" with the ITT, and 70% or higher for the "percent positive agreement" with the ITT. While the estimated percent negative agreement met the success criteria, the estimated percent positive agreement did not reach the criteria for a successful outcome. Therefore, the results did not meet the pre-defined equivalence criteria which required success for both the percent negative agreement and the percent positive agreement.
On February 13,2017, we announced that, after reviewing the raw data on which the top-line data were based, we had concluded that Macrilen™ (macimorelin) had demonstrated performance supportive of achieving FDA registration and that we intended to pursue registration. The announcement set forth the facts on which our conclusion was based. The Company met with the FDA at the end of March 2017 to discuss this position.
On March 7, 2017, we announced that the Pediatric Committee ("PDCO") EMA agreed to the Company's Pediatric Investigation Plan ("PIP") for Macrilen™ (macimorelin) and agreed that the Company may defer conducting the PIP until after it files a Marketing Authorization Application ("MAA") seeking marketing authorization for the use of Macrilen™ (macimorelin) for the evaluation of AGHD.
On July 18, 2017, we were provided a PDUFA date of December 30, 2017 by the FDA.
On November 27, 2017, the EMA accepted our MMA submission for Macrilen™ (macimorelin).
On December 20, 2017, the FDA approved the market authorization for Macrilen™ (macimorelin), to be concludedused in the third quarterdiagnosis of 2016. Furthermore,patients with adult growth hormone deficiency (AGHD).
On January 16, 2018, the Company, through AEZS Germany, entered into the License and Assignment Agreement to carry out development, manufacturing, registration, regulatory and supply chain services for the commercialization of Macrilen™ (macimorelin) in the U.S. and Canada as further described below.
In the August 2018, Volume 103, Issue 8 edition of The Journal of Clinical Endocrinology and Metabolism, the pivotal Phase 3 data from the macimorelin confirmatory trial was published by Jose M. Garcia, MD, PhD, et al., titled ‘Macimorelin as a Diagnostic Test for Adult GH Deficiency’.
On November 19, 2018, we expectannounced the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion recommending a marketing authorization for macimorelin.
On January 16, 2019, the Company announced that the EMA has granted marketing authorization for macimorelin.
Macrilen™ (macimorelin) License and Assignment Agreement
On January 16, 2018, the Company, through AEZS Germany, entered into the License and Assignment Agreement with Strongbridge Ireland Limited ("Strongbridge") to carry out development, manufacturing, registration, regulatory and supply chain services for the commercialization of Macrilen™ (macimorelin) in the U.S. and Canada. This agreement provides (i) for the "right to use" license relating to the Adult Indication; (ii) for the right to acquire a license for the Pediatric Indication if and when the FDA approves a pediatric indication; (iii) that the licensee is to fund 70% of the costs of a pediatric clinical trial submitted for approval to the EMA and FDA (the "PIP") to be ablerun by the Company with customary oversight from a joint steering committee (the "JSC"); and (iv) the Interim Supply Arrangement.
Effective December 19, 2018, Strongbridge sold the United States and Canadian rights to submitMacrilen™ (macimorelin) under the License and Assignment Agreement to Novo for a payment plus tiered royalties on net sales and Novo will fund Strongbridge's Macrilen™(macimorelin) field organization as a contract field force to promote the product in the United States for up to three years.
(i) Adult Indication
Under the terms of the License and Assignment Agreement, and for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on annual net sales up to $75.0 million and an NDA18% royalty on annual net sales above $75.0 million. Following the end of patent protection in United States or Canada for Macrilen™ (macimorelin), the Company is entitled to a 5% royalty on net sales in that country. In addition, the Company will receive one-time payments ranging from $4.0 million to $100.0 million upon the achievement of commercial milestones going from $25.0 million annual net sales up to $500.0 million annual net sales.
In January 2018, the Company received a cash payment of $24.0 million from Strongbridge and on July 23, 2018, Strongbridge launched product sales of Macrilen™ (macimorelin) in the United States.
(ii) Pediatric Indication
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment from Strongbridge of $5.0 million.
(iii) PIP study
We have initiated an open label, single dose trial to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of macimorelin in pediatric patients from two to less than 18 years of age with suspected growth hormone deficiency ("GHD"). Under the terms of the License and Assignment Agreement, the licensee will pay 70% and the Company will pay the remaining 30% of the research and development costs associate with the PIP. During 2018, the Company invoiced Strongbridge $358,000 as its share of the costs incurred by mid-year the Company under the PIP; such amounts have been collected in full.
(iv) Interim supply arrangement
The Company has agreed to supply ingredients for the manufacture of Macrilen™ (macimorelin) during an interim period at a price that is set ‘at cost’, without any profit margin. During 2018, the Company invoiced $2,108,000 and has received payment in full of these invoices under an interim supply agreement.
Rest of world commercialization of macimorelin
On January 16, 2019, we announced that the EMA had granted marketing authorization for macimorelin for the diagnosis of AGHD. AGHD may occur in an adult patient who has a history of childhood onset GHD or may occur during adulthood as an acquired condition. Considering a population of 510 million for the European Union, research based on incidence prevalence suggests that at least 35,000 adults could be afflicted with GHD. This milestone marks a key development in our European commercialization strategy and we are in discussions with a variety of companies regarding licensing and/or distribution opportunities in the rest of the world.
Monetization of non-strategic assets
Other pipeline prospects for the Company include preclinical work done on AEZS-120, a prostate cancer vaccine, discovery research for ERK-inhibitors for Oncology indications; and discovery research conducted at the Medical University of South Carolina on Compound Library as well as other research and clinical development projects that have been undertaken by our German subsidiary.
2017 and if the study is successful in meeting its primary endpoint, to obtain approval of the drug by year-end 2017.
LHRH-Disorazol Z (AEZS-138)earlier - Zoptrex™
In search of new antitumor agents, we found that disorazol Z, a compound that was isolated from the myxobacterium ZoptrexSorangium cellulosumTM , possesses cytotoxic activity in the picomolar range in a panel of different tumor cell lines. Inhibition of tubulin polymerization, cell cycle arrest and efficient induction of apoptosis have been identified as modes of action. AEZS-138 is a cytotoxic conjugate of disorazol Z andcomplex molecule that combines a synthetic peptide carrier that targetswith doxorubicin, a well-known chemotherapy agent. The synthetic peptide carrier is a luteinizing hormone-releasing hormone ("LHRH") agonist, a modified natural hormone with affinity for the LHRH receptor. It is, therefore, an outgrowthThe design of our research that lead to our formulationthe compound allows for the specific binding and selective uptake of Zoptrex™. The following is a summary of our development efforts with respect to AEZS-138:the cytotoxic conjugate by LHRH receptor-positive tumors.
On March 24, 2011, we were awarded a $1.5 million grant from the German Ministry of Education and Research to develop, up to the clinical stage, cytotoxic conjugates of the proprietary cytotoxic compound disorazol Z and peptides targeting G-protein coupled receptors, including the LHRH receptors. The compounds combine the targeting principle being studied in Phase 3 with zoptarelin doxorubicin with the novel cytotoxic disorazol Z. The grant was payable as a partial reimbursement of qualifying expenditures over a three-year period, until January 31, 2014. The qualified project was performed with Morphisto GmbH and the Helmholtz Institute in Saarbrücken, Germany, which received additional funding of approximately US$0.7 million. Researchers from the departments of Gynecology and Obstetrics at both the University of Göttingen and the University of Würzburg, Germany, were also part of the collaboration.
On November 16, 2011,30, 2017, we announced the presentationcompletion of the clinical phase of the pivotal Phase 3 ZoptEC (Zoptarelin Doxorubicin in Endometrial Cancer) study with the occurrence of the 384th death.
On May 1, 2017, we announced that the ZoptEC pivotal Phase 3 clinical study of Zoptrex™ (zoptarelin doxorubicin) in women with locally advanced, recurrent or metastatic endometrial cancer did not achieve its primary endpoint of demonstrating a poster atstatistically significant increase in the AACR-NCI-EORTC International Conference on Molecular Targetsmedian period of overall survival of patients treated with Zoptrex™ (zoptarelin doxorubicin) as compared to patients treated with doxorubicin and Cancer Therapeutics on encouraging preclinical data for disorazol Z. The data showed that disorazol Z possesses cytotoxicity in a highly diverse panelwe discontinued its development. Similarly, we discontinued the development of 60 different tumor cell lines, and also underlined the identification of important aspects of this novel natural compound's mechanism of action. AEZS-138/Disorazol Z, has been identified as a tubulin binding agent with highly potent antitumor properties. Cell cycle analysis revealed that disorazol Z arrested cellsit was based on the same concept as Zoptrex™ (zoptarelin doxorubicin).
We have licensed the development, commercialization and certain other rights to Zoptrex™ to Sinopharm A-Think for China, Hong Kong and Macau; to an affiliate of Orient EuroPharma Co., Ltd. for Taiwan and southeast Asia; to Rafa Laboratories, Ltd for Israel and the Palestinian territories and to Specialised Therapeutics Asia Pte Ltd for Australia and New Zealand.
We do not anticipate significant revenues from the Sinopharm License Agreement in the G2/M cell cycle phase and subsequently induced apoptosis with remarkable potency, as shown by sub-nanomolar EC50 values. To expand our zoptarelin doxorubicin technology platform, we aim to evaluatefuture other than the utility of disorazol Z as a cytotoxic component in a drug-targeting approach utilizing GPCR ligands as the targeting moieties for the treatment of GPCR over-expressing cancers.
On April 10, 2013, we announced at the American Association for Cancer Research's ("AACR") annual meeting encouraging updated proof-of-concept results for disorazol Z cytotoxic conjugates, such as AEZS-138, in human ovarian and endometrial cancer xenograft models. Data demonstrated that conjugates of D-Lys6-LHRH and disorazol Z retained strong binding to the LHRH receptor and showed potent inhibition of tubulin polymerization. Cellular cytotoxicityamortization of the conjugates was in the low nanomolar EC50 range. Increased cytotoxicity in cells over-expressing the LHRH receptor, support receptor targeting as a mechanism of action. The LHRH receptor-dependent efficacies of disorazol Z - D-Lys6-LHRH conjugates in vitro and in mouse xenograft models that were presented support the principle of tumor targeting by the LHRH receptor as considered to be employed by zoptarelin doxorubicin.
remaining deferred revenue.On February 11, 2014, at the 11th International Symposium on GnRH in Salzburg, Austria, we presented further data on the mechanism of action and proof of concept of the disorazol Z cytotoxic conjugate, AEZS-138, which had led to the initiation of its preclinical development during the second quarter of 2013.Other
Overview of our Commercial Operations
Our commercial operations consist of a full-time sales force and a sales-management staff. We currently have 21 sales representativeswere significantly reduced in the United States, who provide services solely for us pursuant tofourth quarter of 2017. We eliminated our contract sales team in its entirety, as well as remaining sales management in November 2017, in accordance with the terms of our agreement with inVentiv Commercial Services, LLC, an affiliate of inVentiv Health, Inc. (“inVentiv”("inVentiv"), a contract-sales organization. Our sales force is managed by two Regional Sales Managers, a National Sales Director and led by our Senior Vice President and Chief Commercial Officer. Our sales force currently promotes EstroGel®, Saizen® and APIFINY®.
Our agreement with inVentiv provides that the inVentiv personnel who provide services to us are independent contractors and not our employees. Furthermore, inVentiv is solely responsible for the human-resource and performance-management functions of all such personnel. It is also responsible for paying the compensation, benefits, payroll-related or withholding taxes and any governmental charges or benefits, including unemployment and disability insurance contributions or benefits and workers compensation contributions with respect to such personnel and for reimbursing them for their expenses. We pay a fixed monthly fee to inVentiv for the services of the sales representatives it provides for us, which is subject to adjustment if the assumptions
regarding the annual salaries paid to the sales representatives prove to be too high or too low, and we also reimburse inVentiv for certain expenses that it incurs as a result of providing sales representatives to us.
Our agreement with inVentiv has a two-year term that started in November 2014. The term may be extended for additional periods of one year, if we reach a written agreement with inVentiv regarding the terms of the extension not less than 60 days before the end of the expiring term. The agreement is subject to customary termination provisions for non-payment of amounts due, material breach and bankruptcy or insolvency. In addition, we may terminate the agreement without cause by giving inVentiv at least 90 days' prior written notice.
We promote EstroGel®, a leading non-patch transdermal hormone replacement therapy product, pursuant to a co-promotion agreement (the “Ascend Agreement”) with ASCEND Therapeutics US LLC (“ASCEND”), which we entered into in August 2014. The Ascend Agreement provides that we will promote EstroGel® in specific agreed-upon U.S. territories in exchange for a sales commission that is based upon incremental sales volumes of the product that are generated over pre-established baselines.
The Ascend Agreement has a two-year term that commenced in November 2014. It is subject to extension for successive periods of two years each upon our agreement with ASCEND. The Ascend Agreement has customary termination provisions and, in addition, is subject
Pursuant to termination for convenience by either party upon the provision of not less than six months' written notice to the other party. During the term of the Ascend Agreement, either party may offer other products that it acquires toinVentiv agreement, we ended our co-promotion with EMD Serono, Inc. ("EMD Serono") and Armune BioScience, Inc. ("Armune").
Until September 1, 2016, we co-promoted a product, EstroGel®, and until termination of our sales team in November 2017, the other party for inclusion in the co-promotion arrangement established by the Ascend Agreement.inVentiv sales force promoted two products:
Saizen®Saizen® [somatropin (rDNA origin) for injection] is a prescription medicine indicated for the treatment of growth hormone deficiency in children and adults. We promote Saizen®promoted Saizen® pursuant to our co-promotionpromotional services agreement (the “EMD Serono Agreement”) with EMD Serono Inc. (“EMD Serono”), which we entered into in May 2015.2015 and amended as of December 31, 2016. The EMD Serono Agreement, providesas amended, provided that we willwere to promote Saizen® in specific agreed-upon U.S. territories to adult and pediatric endocrinologists in exchange for a sales commission that iswas based upon incremental new patient starts of the product that are generated over pre-established baselines.product. The agreement was terminated in accordance with its terms in December 2017.
The EMD Serono Agreement has a five-year term that began in May 2015, which is not subject to an agreed extension period, and is subject to customary termination provisions. EMD Serono has the right to terminate the EMD Serono Agreement for convenience at any time by giving us three months' advance written notice. EMD Serono has certain payment obligations to us that will arise if it terminates the EMD Serono Agreement for convenience, the type and amount of which will depend on the date that EMD exercises its right to terminate. We may terminate the EMD Serono Agreement for convenience at any time after the second anniversary of its date by giving EMD Serono three month’s advance written notice.
APIFINY®, is the only cancer-specific, non-PSA blood test for the evaluation of the risk of prostate cancer. The test was developed by Armune, BioScience, Inc. (“Armune”), a medical diagnostics company that develops and commercializes unique proprietary technology exclusively licensed from the University of Michigan for diagnostic and prognostic tests for cancer. We entered into a co-marketing agreement with Armune in November 2015 (the “Armune Agreement”), pursuantwhich was amended effective as of June 1, 2016, which allowed us to which we haveexclusively promote APIFINY® throughout the right to promote APIFINY® to designated medical professionals in our 21 U.S. territories.entire United States. We receivereceived a commission for each test performed resulting from our targeted promotion without regard to aany established baseline. The Armune Agreement, hasas amended, had a one-yearthree-year term that renewsrenewed automatically for successive one-year periods, unless either party terminates it by giving not less than 60 days' advance written notice toperiods. The parties agreed in January 2018 that the other, which either party may do at any time with or without cause.Armune Agreement was terminated.
Geographic Areas
A description of the principal geographic areas in which we compete, including a geographical and categorical breakdown of our revenues in the past three years is presented in note 2725 (Segment information) to our consolidated financial statements included in this Annual Report on Form 20-F at Item 18.
Seasonality
As a specialty biopharmaceutical company, the Company does not consider any of its products or services to be seasonal.
Raw Materials
Raw materials and supplies are generally available in quantities adequate to meet the needs of our business. We will be dependent on third-party manufacturers for the pharmaceutical products that we or our licensees will market. An interruption in the availability of certain raw materials or ingredients, or significant increases in the prices paid by us for them, could have a material adverse effect on our business, financial condition, liquidity and operating results.
Regulation of Drug Development
Generally. Governmental authorities in the United States, Canada, Europe and other countries extensively regulate the preclinical and clinical testing, manufacturing, labeling, storage, record keeping, advertising, promotion, export, marketing and distribution, among other things, of pharmaceuticals. Under the laws of the United States, the countries of the EU, and other countries, we and the institutions at which we sponsor research are subject to obligations to ensure that our clinical trials are conducted in accordance with Good Clinical Practices ("GCP") guidelines and the investigational plan and protocols contained in an INDInvestigational New Drug ("IND") application, or comparable foreign regulatory submission. The Japanese regulatory process for approval of new drugs is similar to the FDA approval process described below except that Japanese regulatory authorities request bridging studies to verify that foreign clinical data are applicable to Japanese patients and also require the tests to determine appropriate dosages for Japanese patients to be
conducted on Japanese patient volunteers. Due to these requirements, delays of two to three years in introducing a drug developed outside of Japan to the Japanese market are customary. Set forth below is a brief summary of the material governmental regulations affecting us in the major markets in which we intend to market our products and/or promote products that we acquire or in-license or to which we obtain promotional rights.
The United States. In the United States, the FDAFDA's Center for Drug Evaluation and Research (CDER) under the United StatesFederal Food, Drug and Cosmetic Act of 1938, as amended (the “FDA Act”"FDCA"), the Public Health Service Act and other federal statutes and regulations, subjects pharmaceutical products to rigorous review. In order to obtain approval ofmarket and sell a new drug product fromin the FDA,United States, we must among other requirements, submit proof of safetyfirst test it and efficacy as well as detailed information onsend CDER evidence from these tests to prove that the manufacturedrug is safe and composition of the product.effective for its intended use. In most cases, this proof entailsthese tests include extensive preclinical, clinical, and laboratory tests. A team of CDER physicians, statisticians, chemists, pharmacologists, and other scientists reviews the company's data and proposed labeling. If this independent and unbiased review establishes that a drug's health benefits outweigh its known risks, the drug is approved for sale. CDER does not test the drug itself but it does conduct limited research in the areas of drug quality, safety, and effectiveness standards. Before approving a new drug or marketing application, the FDA also typically conductsmay conduct pre-approval inspections of the company,developer of the drug (the "sponsor"), its CROs and/or its clinical trial sites to ensure that clinical, safety, quality control, and other regulated activities are compliant with GCP, or Good Laboratory Practices ("GLP"), for specific non-clinical toxicology studies. Manufacturing facilities used to produce a product are also subject to ongoing inspection by the FDA. The FDA may also require confirmatory trials, post-marketing testing, andand/or extra surveillance to monitor the effects of approved products, or place conditions on any approvals that could restrict the commercial applications of these products.a product. Once approved, the labeling, advertising, promotion, marketing, and distribution of a drug or biologic product must be in compliance with FDA regulatory requirements.
The first stage required for ultimate FDA approval of a new biologic or drug involves completion of preclinical studies whereby a sponsor must test new drugs on animals for toxicity. Multiple species are used to gather basic information on the safety and the submissionefficacy of the results of these studies to the FDA. This, together with proposed clinical protocols, manufacturing information, analytical data, and other information in an IND, must become effective before human clinical trials may commence. Preclinical studies involve laboratory evaluation of product characteristics and animal studies to assess the efficacy and safety of the product.compound being investigated and/or researched. The FDA regulates preclinical studies under a series of regulations called the current GLP regulations.regulations as well as regulatory requirements found in Part 21 subchapter D of the Code of Federal Regulations. If the sponsor violates these regulations, the FDA may require that the sponsor replicate those studies.studies or can subject the sponsor to enforcement actions or penalties as described further below. The sponsor then submits to the FDA an IND application based on the results from initial testing that include the drug's composition and manufacturing, along with a plan for testing the drug on humans. The FDA reviews the IND to ensure that the proposed studies (clinical trials) do not place human subjects at unreasonable risk of harm. FDA also verifies that there are adequate informed consent and human subject protections in place.
After a sponsor submits an IND application, it must wait 30 days before starting a clinical trial to allow FDA time to review the prospective study. If FDA finds a problem, it can order a clinical hold to delay an investigation, or interrupt a clinical trial if problems occur during the study. After the IND becomes effective,application is in effect, a sponsor may commence human clinical trials. The sponsor typically conducts human clinical trials in three sequential phases, but the phases may overlap. In Phase 1 trials, the sponsor tests the product in a small number of patients or healthy volunteers (typically 20-80 healthy volunteers), primarily for safety at one or more doses. The goal in this phase is to determine what the drug's most frequent side effects are and, often, how the drug is metabolized and excreted. Phase 2 studies begin if Phase 1 trials in cancer are often conducted with patients who have end-stage or metastatic cancer.studies do not reveal unacceptable toxicity. In Phase 2, in addition to safety, the sponsor evaluates the efficacy of the product in a patient population somewhat larger than Phase 1 trials. The number of subjects in Phase 2 studies typically ranges from a few dozen to about 300. This phase aims to obtain preliminary data on whether a drug works in people who have a certain disease or condition. At the end of Phase 2, the FDA and sponsor try to come to an agreement on how large-scale studies in Phase 3 should be done.
Phase 3 studies begin if evidence of effectiveness is shown in Phase 2. Phase 3 trials typically involve additional testing for safety and clinical efficacy in an expanded population at geographically dispersed test sites. The sponsor must submit to the FDA a clinical plan, or "protocol", accompanied by the approval of the institutions participating in the trials, prior to commencement of each clinical trial. The FDA may order the temporary or permanent discontinuation of a clinical trial at any time. In the case of product candidates for cancer, the initial human testing may be done in patients with the disease rather than in healthy volunteers. Because these patients are already afflicted with the target disease, such studies may provide results traditionally obtained in Phase 2 studies. Accordingly, these studies are often referred to as “Phase"Phase 1/2” studies.2" studies as they combine two phases. Even if patients participate in initial human testing and a Phase 1/2 study is carried out, the sponsor is still responsible for obtaining all the data usually obtained in both Phase 1 and Phase 2 studies.
The sponsor must submit to the FDA the results of the preclinical and clinical testing, together with, among other things, detailed information on the manufacture and composition of the product, in the form of an NDAa New Drug Application ("NDA") or, in the case of a biologic, a Biologics License Applications (“BLA”("BLA"). In a process that can take a year or more, the FDA reviews this application and, when and if it decides that adequate data are available to show that the new compound is both safe and effective for a particular indication and that other applicable requirements have been met, approves the drug or biologic for marketing. The amount of time taken for this approval process is a function of a number of variables, including the quality of the submission and studies presented and the potential contribution that the compound will make in improving the treatment of the disease in question.
Orphan-drug designation is granted by the FDA Office of Orphan Drug Products to novel drugs or biologics that treat aare intended for the safe and effective treatment, diagnosis or prevention of rare diseasediseases or condition affectingdisorders that affect fewer than 200,000 patientspeople in the U.S., or that affect more than 200,000 people but are not expected to recover the costs of developing and marketing a treatment drug. The designation provides the drug developersponsor with a seven-year period of U.S. marketing exclusivity if the drug is the first of its type approved for the specified indication or if it demonstrates superior safety, efficacy or a major contribution to patient care versus another drug of its type previously granted the designation for the same indication. We have been granted orphan drug designations for Zoptrex™ for the treatment of advanced ovarian cancer and for Macrilen™ (macimorelin) for the evaluation of growth hormone deficiency.
Under the Drug Price Competition and Patent Term Restoration Act of 1984 (the "Hatch-Waxman Act"), newly-approved drugs and indications may benefit from a statutory period of non-patent data exclusivity. The Hatch-Waxman Act provides five-year data exclusivity to the first applicant to gain approval of an NDA for a new chemical entity, or NCE, meaning that the FDA has not previously approved any other drug containing the same active pharmaceutical ingredient, or active moiety. Although protection under the Hatch-Waxman Act will not prevent the submission or approval of another full NDA, such an NDA applicant would be required to conduct its own preclinical and adequate, well-controlled clinical trials to demonstrate safety and effectiveness.
The Hatch-Waxman Act also provides three years of data exclusivity for the approval of new and supplemental NDAs, including Section 505(b)(2) applications, for, among other things, new indications, dosage forms, routes of administration, or strengths of an existing drug, or for a new use, if new clinical investigations that were conducted or sponsored by the applicantsponsor are determined by the FDA to be essential to the approval of the application. This exclusivity, which is sometimes referred to as clinical investigation exclusivity, would not prevent the approval of another application if the applicantsponsor has conducted its own adequate, well-controlled clinical trials demonstrating safety and efficacy, nor would it prevent approval of a generic product that did not incorporate the exclusivity-protected changes of the approved drug product.
The labeling, advertising, promotion, marketing, and distribution of a drug or biologic product must be in compliance with FDA regulatory requirements. Failure to comply with applicable requirements can lead to the FDA demanding that production and shipment cease and, in some cases, that the manufacturer recall products, or to enforcement actions that can include seizures, injunctions, and criminal prosecution. These failures can also lead to FDA withdrawal of approval to market a product.
Canada. In Canada, the Therapeutic Products Directorate of Health Canada is the Canadian federal authority that regulates pharmaceutical drugs and medical devices for human use. Prior to being given market authorization, a manufacturersponsor must present substantive scientific evidence of a product's safety, efficacy and quality as required by the Food and Drugs Act and other legislation and regulations. The requirements for the development and sale of pharmaceutical drugs in Canada are substantially similar to those in the United States, which are described above.
The European Union. Medicines can be authorized in the EU by using either the centralized authorization procedure or national authorization procedures. The EU has implemented a centralized procedure coordinated by the EMA for the approval of human medicines, which results in a single marketing authorization issued by the European Commission that is valid across the EU, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human medicines that are derived from biotechnology processes, such as genetic engineering, that contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions, and designated orphan medicines. For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health.
There are also two other possible routes to authorize medicinal products in several EU countries, which are available for investigational drug products that fall outside the scope of the centralized procedure:
•Decentralized procedure.Using the decentralized procedure, an applicanta sponsor may apply for simultaneous authorization in more than one EU country of medicinal products that have not yet been authorized in any EU country and that do not fall within the mandatory scope of the centralized procedure. The application will be reviewed by a selected Reference Member State ("RMS"). The Marketing Authorization granted by the RMS will then be recognized by the other Member States involved in this procedure.
•Mutual recognition procedure. In the mutual recognition procedure, a medicine is first authorized in one EU Member State, in accordance with the national procedures of that country. Following this, further marketing authorizations can be sought from other EU countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization.
Regulation of Commercial Operations
The marketing, promotional, and pricing practices of human pharmaceutical manufacturers, as well as the manner in which manufacturers interact with purchasers and prescribers, are subject to various U.S. federal and state laws, including the federal anti-kickback statute and the False Claims Act and state laws governing kickbacks, false claims, unfair trade practices, and consumer protection, and to similar laws in other countries. In the United States,U.S., these laws are administered by, among others, the Department of Justice (DOJ)("DOJ"), the Office of Inspector General of the Department of Health and Human Services, the Federal Trade Commission, the Office of Personnel Management, and state attorneys general. Over the past several years, the FDA, the DOJ, and many other agencies have increased their enforcement activities with respect to pharmaceutical companies and increased the inter-agency coordination of enforcement activities.
In the United States,U. S., biopharmaceutical and medical device manufacturers are required to record any transfers of value made to licensed physicians and teaching hospitals and to disclose such data to the Department of Health and Human Services (“HHS”("HHS"). In addition to civil penalties for failure to report transfers of value to physicians or teaching hospitals, there will be criminal penalties if a manufacturer intentionally makes false statements or excludes information in such reports. The payment data across biopharmaceutical and medical device companies is posted by HHS on a publicly available website. Increased access to such data by fraud and abuse investigators, industry critics and media will draw attention to our collaborations with reported entities and will importantly provide opportunities to underscore the critical nature of our collaborations for developing new medicines and exchanging scientific information. This national payment transparency effort coupled with industry commitment to uphold voluntary codes of conduct (such as the PhRMA Code on Interactions with Healthcare Professionals and PhRMA Guiding
Principles Direct to Consumer Advertisements About Prescription Medicines) and rigorous internal training and compliance efforts will complement existing laws and regulations to help ensure ethical collaboration and truthful product communications.
The Canadian association of Research-Based Pharmaceutical Companies (“("Rx & D”D") has adopted “Guidelines"Guidelines for Transparency in Stakeholder Funding”Funding" that require member companies to regularly disclose, by means of the web sites and annual reports, a list of all stakeholders to which they provide direct funding. The term “stakeholder”"stakeholder" is defined in Rx & D’sD's Code of Ethical Practices to include “Health"Health Care Professionals”Professionals". In the EU, the disclosure code of transfers of value to healthcare professionals and organizations adopted by the European Federation of Pharmaceutical Industries and Associations (“EFPIA”("EFPIA") requires all members of EFPIA to disclose transfers of value to healthcare professionals and healthcare organizations beginning in 2016, covering the relevant transfers in 2015. Each member company will be required to document and disclose: (i) the names of healthcare professionals and associations that have received payments or other transfers of value and (ii) the amounts or value transferred, and the type of relationship.
For more information about the regulatory risks associated with our business operations, see “Item 3. - Key Information -"Item 3D. Risk Factors”Factors".
Intellectual Property - Patents
We seek to protect our compounds, manufacturing processes, compositions and methods of medical use for our lead drugs and drug candidates through a combination of patents, trade secrets and know-how. Our patent portfolio consists of approximately 134 owned and in-licensed patent families (issued, granted or pending in the United States, Europe and other jurisdictions). The patent positions of companies in the biotechnology and pharmaceutical industries are highly uncertain and involve complex legal and factual questions. Therefore, we cannot predict the breadth of claims, if any, that may be allowed under any of our patent applications, or the enforceability of any of our allowed patents. See "Item 3D.3.D. Risk Factors - We may not obtain adequate protection for our products through our intellectual property."
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country. In the United States, the patent term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent, in which the patentee may file an application for yearly interim extensions within five years if the patent will expire and the FDA has not yet approved the NDA. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended.
Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In these jurisdictions, however, no interim extensions exist and the marketing approval must be granted before the patent expires. In the future, if and when our pharmaceutical products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. While we anticipate that any such applications for patent term extensions will likely be granted, we cannot predict the precise length of time for which such patent terms would be extended in the United States, Europe or other jurisdictions. If we are not able to secure patent term extensions on patents covering our products for meaningful periods of additional time, we may not achieve or sustain profitability, which would adversely affect our business.
In addition to patent protection, our products may benefit from the market-exclusivity provisions contained in the orphan-drug regulations or the pediatric-exclusivity provisions or other provisions of the FDA Act, such as new chemical entity exclusivity or new formulation exclusivity. Orphan drug regulations provide incentives to pharmaceutical and biotechnology companies to develop and manufacture drugs for the treatment of rare diseases, currently defined as diseases that exist in fewer than 200,000 individuals in the U.S., or diseases that affect more than 200,000 individuals in the U.S. but that the sponsor does not realistically anticipate will generate a net profit. Under these provisions, a manufacturer of a designated orphan drug can seek tax benefits, and the holder of the first FDA approval of a designated orphan product will be granted a seven-year period of marketing exclusivity for such FDA-approved orphan product. In the U.S., the FDA has the authority to grant additional data protection for approved drugs where the sponsor conducts specified testing in pediatric or adolescent populations. If granted, this pediatric exclusivity provides an additional six months which are added to the term of data protection as well as to the term of any relevant patents, to the extent these protections have not already expired. We may also seek to utilize market exclusivities in other territories, such as in the EU. We cannot assure youThere can be no assurance that any of our drug candidates will obtain such orphan drug designation, pediatric exclusivity, new chemical entity exclusivity or any other market exclusivity in the U.S., the EU or any other territory, or that we will be the first to receive the respective regulatory approval in a given country or territory for such drugs so as to be eligible for any market exclusivity protection.
Our drug development efforts are currently focused on two compounds, zoptarelin doxorubicin (Zoptrex™) and macimorelin (Macrilen™), which are in clinical development, and on an LHRH-disorazol Z conjugate (AEZS-138), which is in pre-clinical development. The following is a description of our intellectual property rights with respect to these compounds.
Zoptrex™:
We license intellectual property and associated rights relating to LHRH agonists and LH-RH antagonists carrying various cytotoxic radicals (including zoptarelin doxorubicin) from the Administrators of the Tulane Educational Fund ("Tulane") pursuant to a License Agreement dated September 17, 2002 between Tulane, as licensor, and AEZS Gmbh, as licensee (the "Tulane Agreement"). The Tulane Agreement grants to us an exclusive worldwide license for all therapeutic uses of LH-RH agonists and LH-RH antagonists carrying various cytotoxic radicals, to the extent covered by one of the patents listed below. The term of the Tulane Agreement continues for ten years after the first commercial sale of a product based on the licensed intellectual property (a "Licensed Product") or until the expiration of the last to expire of the patents listed below, whichever is longer, on a country-by-country basis.
Pursuant to the Tulane Agreement, we are required to pay Tulane the following amounts: (i) US$400,000 upon the first grant of regulatory approval for a Licensed Product in the United States, Canada, the European Union or Japan; (ii) 10% of all consideration received by us from a sublicensee for authorization to use the licensed intellectual property to develop, manufacture, market, distribute and sell a Licensed Product; (iii) 5% of our net sales of Licensed Products; and (iv) 50% of any royalties that we receive from a sublicensee with respect to its net sales of Licensed Products; provided, however, that the payment with respect to royalties received from a sublicensee shall not be less than 3.5% nor more than 5% of the sublicensee's net sales of the Licensed Product.
The following patents are covered by the Tulane Agreement:
US patent 5,843,903 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2015.
European patent 0 863 917 B1 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expires in November 2016.
Japanese patent 3 987 575 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expires in November 2016.
Chinese patent ZL96198605.0 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expires in November 2016.
Hong Kong patent 1017363 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expires in November 2016.
In early 2015, we filed a European patent application directed to a novel method of manufacturing Zoptrex™. Within the 12 months priority period, we also filed an international patent application for the manufacturing process, as well as national patent applications in selected countries, including the US, China, and Taiwan, Japan and India. We decided to file patent applications in additional territories after the European Patent Office issued a search report for the European patent application that we consider to be favorable. The claimed manufacturing process is expected to result in a significant reduction in our cost of manufacturing Zoptrex™, providing us with what should be a stronger competitive position and discouraging competition from generic manufacturers after our five-year period of data exclusivity expires.
Macrilen™ (macimorelin):
We hold the worldwide rights to macimorelin pursuant to an exclusive license agreement with Thethe French Centre National de la Recherche Scientifique, as licensor, and AEZS GmbH,Germany, as licensee. Macrilen™ is the approved marketing name for macimorelin as licensed under the License and Assignment Agreement for commercialization in the U.S. and Canada, only.
The following patents and patent applications relate to Macrilen™:macimorelin:
U.S. patent 6,861,409 covers Macrilen™macimorelin and U.S. patent 7,297,681 covers other related growth hormone secretagogue compounds, each also covering pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. U.S. patent 6,861,409 and U.S. patent 7,297,681 both expire in August 2022.
European patent 1 289 951 covers Macrilen™macimorelin and European patent 1 344 773 covers other related growth hormone secretagogue compounds, pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. EP patent 1 289 951 and EP patent 1 344 773 both expire in June 2021.
Japanese patent 3 522 265 covers Macrilen™macimorelin and pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. This patent expires in June 2021.
Canadian patent 2,407,659 covers Macrilen™macimorelin and pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. This patent expires in June 2021.
U.S. patent 8,192,719 covers a method of assessing pituitary-related growth hormone deficiency in a human or animal subject comprising an oral administration of the compound Macrilen™macimorelin and determination of the level of growth hormone in the sample and assessing whether the level of growth hormone in the sample is indicative of growth hormone deficiency. This patent expires in October 2027.
European patent 1 984 744 covers a method of assessing pituitary-related growth hormone deficiency by oral administration of Macrilen™.macimorelin. This patent expires in February 2027.
Japanese patent 4 852 728 covers a method of assessing pituitary-related growth hormone deficiency by oral administration of Macrilen™.macimorelin. This patent expires in February 2027.
U.S. provisional patent applications Serial No. 62/607,866 was filed on December 19, 2017 and Serial No. 62/609,059 was filed on December 21, 2017. Both are identical and are directed to a method of assessing growth hormone deficiency comprising oral administration of a macimorelin containing composition and collecting one or two post-administration samples.
A non-provisional U.S. application was filed on May 30, 2018 drawing the priority of both provisional applications. The US-PTO issued a Notice of Allowance on January 09, 2019. If granted, a patent would presumably expire December 19, 2037.
A PCT application was filed December 18, 2018 drawing the priority of both provisional U.S. applications. In addition to the method of assessing growth hormone deficiency comprising oral administration of a macimorelin containing composition and collecting one or two post-administration samples, the PCT application also covers a similar method of assessing growth hormone deficiency using 3 post-administration samples.
Zoptrex™
We have licensed the intellectual property and associated rights relating to LHRH agonists and LH-RH antagonists carrying various cytotoxic radicals (including zoptarelin doxorubicin) from the Administrators of the Tulane Educational Fund ("Tulane") pursuant to a license agreement dated September 17, 2002 between Tulane, as licensor, and AEZS Germany, as licensee (the "Tulane Agreement"). The Tulane Agreement grants to us an exclusive worldwide license for all therapeutic uses of LH-RH agonists and LH-RH antagonists carrying various cytotoxic radicals, to the extent covered by one of the licensed patents. The term of the Tulane Agreement continues for ten years after the first commercial sale of a product based on the licensed intellectual property (a "Licensed Product") or until the expiration of the last to expire of the licensed patents, whichever is longer, on a country-by- country basis.
Pursuant to the Tulane Agreement, we are required to pay Tulane the following amounts: (i) $400,000 upon the first grant of regulatory approval for a Licensed Product in the U.S., Canada, the EU or Japan; (ii) 10% of all consideration received by us from a sublicensee for authorization to use the licensed intellectual property to develop, manufacture, market, distribute and sell a Licensed Product; (iii) 2.5% of our net sales of Licensed Products; and (iv) 50% of any royalties that we receive from a sublicensee with respect to its net sales of Licensed Products; provided, however, that the payment with respect to royalties received from a sublicensee shall not be less than 1.75% nor more than 2.5% of the sublicensee's net sales of the Licensed Product.
All patents covered by the Tulane Agreement expired by November 2016. In early 2015, we filed a European patent application directed to a novel method of manufacturing Zoptrex™. Within the 12 months priority period, we also filed an international patent application for the manufacturing process, as well as national patent applications in selected countries, including the U.S., China, and Taiwan, Japan and India. As a consequence of the negative Phase 3 ZoptEC study received in April 2017, we ceased further Zoptrex™ development and intellectual property filings.
Disorazol Z - LHRH conjugates (AEZS-138):
We own a number of patents that relate to our Disorazol Z - LHRH conjugates, as follows:
U.S. patent 7,741,277 covers AEZS-138 (disorazolconjugates. As a consequence of the negative Phase III ZoptEC study received in April 2017, we ceased further Disorazol Z - LHRH conjugate). This patent will expire in January 2028 (including PTA).conjugate development and intellectual property filings.
U.S. patent 8,470,776 covers methods of treatment for compound AEZS-138 (disorazol Z - LHRH conjugate). This patent will expire in February 2029 (including PTA).
European patent application 2,066,679 covers AEZS-138 (disorazol Z - LHRH conjugate) as well as methods of treatment for this compound. If granted, this patent will expire in September 2027.
Japanese patent 5,340,155 covers AEZS-138 (disorazol Z - LHRH conjugate) as well as methods of treatment for this compound. This patent will expire in September 2027.
| |
| C. | Organizational structure |
Our corporate structure, the jurisdiction of incorporation of our direct and indirect subsidiaries and the percentage of shares that we held in those subsidiaries as at December 31, 20152018 is depicted in the chart set forth under the caption "Item 4-A.4.A. History and development of the Company".
| |
| D. | Property, plants and equipment |
Our registered address is located in Montreal, Canada. Our corporate head office is located in Summerville, South Carolina, which is a suburb of Charleston, South Carolina.Carolina and our largest office is located in Frankfurt, Germany. We do not own any real property. The following table sets forth information with respect to our main facilities as at December 31, 2015.
|
| | | | | | | |
| Location | | Use of space | | Square Footage | | Type of interest |
315 Sigma Drive, Suite 302D, Summerville SC 2948329486 | | Partially occupiedOccupied for management, administration, commercial operations and business development | | 4,623300 |
| | Leasehold |
Weismüllerstr. 50 D-60314 Frankfurt-am-Main, Germany | | Occupied for management, R&D, business development and administration | | 36,168 |
| | Leasehold |
We believe that our current facilities are adequate to meet our ongoing needs and that, if we require additional space, we will be able to obtain additional facilities on commercially reasonable terms.
Item 4A | |
| Item 4A | Unresolved Staff Comments |
Not required.
None.
| |
| Item 5. | Operating and Financial Review and Prospects |
Key Developments
Zoptrex™Macrilen™ (macimorelin) license agreement
ZoptrexTM Macrilen™ (macimorelin), a ghrelin receptor agonist, is a complexnovel orally-active small molecule that combines a synthetic peptide carrier with doxorubicin, a well-known chemotherapy agent.stimulates the secretion of growth hormone. The synthetic peptide carrier is a luteinizing hormone-releasing hormone ("LHRH") agonist, a modified natural hormone with affinityFDA has granted marketing approval for Macrilen™ (macimorelin) to be used in the diagnosis of AGHD.
On January 16, 2018, the Company, through AEZS Germany, entered into the License and Assignment Agreement to carry out development, manufacturing, registration, regulatory and supply chain services for the LHRH receptor. The designcommercialization of Macrilen™ (macimorelin) in the United States and Canada. This agreement provides (i) for the "right to use" license relating to the Adult Indication; (ii) for the right to acquire a license for the Pediatric Indication if and when the FDA approves a pediatric indication; (iii) that the licensee is to fund 70% of the compound allowscosts of a pediatric clinical trial submitted for approval to the EMA and FDA (the "PIP") to be run by the Company with customary oversight from a joint steering committee (the "JSC"); and (iv) for the specific binding and selective uptakeInterim Supply Arrangement.
(i) Adult Indication
Under the terms of the cytotoxic conjugate by LHRH receptor-positive tumors. Potential benefitsLicense and Assignment Agreement, and for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on annual net sales up to $75.0 million and an 18% royalty on annual net sales above $75.0 million. Following the end of this targeted approach includepatent protection in United States or Canada for Macrilen™ (macimorelin), the Company is entitled to a better efficacy5% royalty on net sales in that country. In addition, the Company will receive one-time payments ranging from $4.0 million to $100.0 million upon the achievement of commercial milestones going from $25.0 million annual net sales up to $500.0 million annual net sales.
In January 2018, the Company received a cash payment of $24.0 million from Strongbridge and a more favorable safety profile with lower incidence and severityon July 23, 2018, Strongbridge launched product sales of side effects as compared to doxorubicin alone.
We believe that ZoptrexTM has the potential to become the first FDA-approved medical therapy for advanced, recurrent endometrial cancer, potentially resultingMacrilen™ (macimorelin) in the compound's rapid adoptionUnited States.
(ii) Pediatric Indication
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment from Strongbridge of $5.0 million.
(iii) PIP study
We have initiated an open label, single dose trial to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of macimorelin in pediatric patients from two to less than 18 years of age with suspected GHD. Under the terms of the License and Assignment Agreement, the licensee will pay 70% and the Company will pay the remaining 30% of the research and
development costs associate with the PIP. During 2018, the Company invoiced Strongbridge $358,000 as its share of the costs incurred by the Company under the PIP; such amounts have been collected in full.
(iv) Interim supply arrangement
The Company has agreed to supply ingredients for the manufacture of Macrilen™ (macimorelin) during an interim period at a price that is set ‘at cost’, without any profit margin. During 2018, the Company invoiced $2,108,000 and has received payment in full of these invoices under an interim supply agreement.
Novo Nordisk purchase of Strongbridge License Agreement
Effective December 19, 2018, Strongbridge sold the United States and Canadian rights to Macrilen™ under the License and Assignment Agreement to Novo and Novo will fund Strongbridge's Macrilen™ (macimorelin) field organization as a novel core therapycontract field force to promote the product in the United States for up to three years.
Rest of world commercialization of macimorelin
On January 16, 2019, we announced that the European Medicines Agency ("EMA") had granted marketing authorization for macimorelin for the diagnosis of AGHD. AGHD may occur in an adult patient treatmentwho has a history of childhood onset GHD or may occur during adulthood as an acquired condition. Considering a population of 510 million for the European Community, research based on incidence prevalence suggests that at least 35,000 adults could be afflicted with GHD. This milestone marks a key development in our European commercialization strategy and management, representingwe are in discussions with a significant potential market opportunity for us. Moving forward,variety of companies regarding licensing and/or distribution opportunities in the rest of world (“ROW”).
Monetization of non-strategic assets
On May 1, 2017, we will continue to develop our commercialization plans regarding ZoptrexTM in this indication. In addition, contingent on the success ofannounced that the ZoptEC (Zoptarelin Doxorubicin in Endometrial Cancer) pivotal Phase 3 clinical trialstudy of Zoptrex™ (zoptarelin doxorubicin) in women with locally advanced, recurrent or metastatic endometrial cancer did not achieve its primary endpoint of demonstrating a statistically significant increase in the median period of overall survival of patients treated with Zoptrex™ (zoptarelin doxorubicin) as compared to patients treated with doxorubicin, and we have additional areasdiscontinued its development. Similarly, we discontinued the development of interest for further therapeutic development for zoptarelin doxorubicin, including ovarian, prostate, breast cancer and potentially bladder cancer.
On April 16, 2015, we announced that we had filed an application for a European patent on a novel method of manufacturing ZoptrexTM. Because this compound is a complex molecule,AEZS-138/Disorazol Z, as it is expensive to synthesize, and the requested patent, if granted, may make it difficult for generic manufacturers to produce ZoptrexTM on a financially feasible basis once our composition of matter patentwas based on the compound expires. Further, the claimed manufacturing process is expected to result in a significant reduction in cost of goods sold, which should place us in a stronger competitive position.same concept as Zoptrex™ (zoptarelin doxorubicin).
On April 27, 2015, we announced that an independent Data and Safety Monitoring Board ("DSMB")Other pipeline prospects for the pivotal Phase 3 ZoptrexTM clinical trial with zoptarelin doxorubicin in women with advanced, recurrent or metastatic endometrial cancer had completedCompany include preclinical work done on AEZS-120, a pre-specified first interim futility analysis at approximately 128 events, and on June 30, 2015, we announced that we had reached our goal of completing enrollment of 500 patients for this clinical trial.
On September 28, 2015, we announced that ZoptrexTM had met the primary end point of the investigator-driven and sponsored Phase 2 clinical trial in castration and taxane resistant prostate cancer ("CRPC")vaccine, discovery research for ERK-inhibitors for Oncology indications; and demonstrated good tolerability. This was a single-arm Simon Optimum design Phase 2 study in 25 patients with CRPC.
On October 13, 2015, we announced thatdiscovery research conducted at the independent DSMB had recommended that the pivotal Phase 3 ZoptEC study continue as planned. The DSMB's decision followed completion of its pre-specified second interim analysis on efficacy and safety at approximately 192 events. A final analysis of the data is expected at approximately 384 events.
Macrilen™
On April 13, 2015, we announced plans to conduct a new confirmatory Phase 3 clinical trial to demonstrate the efficacy of Macrilen™ for the evaluation of AGHD, as well as a dedicated thorough QT study to evaluate the effect of Macrilen™ on myocardial repolarization. During an end-of-review meeting with the FDA on March 6, 2015, we and the FDA agreed on the general design of the confirmatory Phase 3 clinical trial of Macrilen™, as well as on evaluation criteria.
On May 26, 2015, we announced that we had received written scientific advice from the European Medicines Agency (the "EMA") regarding the further development plan, including the study design, for the new confirmatory Phase 3 clinical trial of Macrilen™ for use in evaluating AGHD, following a Scientific Advice Meeting that had been held earlier that month. As a result of the advice, we believe that the confirmatory Phase 3 clinical trial that was agreed with the FDA meets the EMA's study-design expectations allowing for US and European approval if the study is successful.
On June 25, 2015, we announced that we had entered into an agreement with Ergomed PLC (formerly Ergomed Clinical Research Limited, hereafter referred to as "Ergomed"), pursuant to which Ergomed will manage the new confirmatory Phase 3 clinical trial of Macrilen™. Ergomed is already the clinical research organization supporting our pivotal Phase 3 ZoptEC clinical trial.
On November 19, 2015, we announced the first patient enrolled for confirmatory Phase 3 trial of Macrilen™ for the evaluation of AGHD. The confirmatory Phase 3 clinical study of Macrilen™ is designed as a two-way crossover study with the insulin tolerance test ("ITT") as the benchmark comparator and will involve some 30 sites in the US and Europe. The study population will consist of approximately 110 subjects (at least 55 ITT-positive and 55 ITT-negative) with a medical history documenting risk factors for AGHD, and will include a spectrum of subjects from those with a low risk of having AGHD to those with a high risk
of having the condition. The primary endpoint is validation of a single oral dose of macimorelin for the diagnosis of AGHD, using the ITT as a comparator.
Pre-clinical developments
On March 31, 2015, we announced the transfer of our discovery library of roughly 100,000 unique compounds to the South Carolina Center for Therapeutic Discovery and Development (the "Center") which is part of The Medical University of South Carolina ("MUSC"). Our material transfer agreement with the Center will result in the continued use of the library for the discovery of drugon Compound Library as well as other research and clinical development candidates for the Company in the areas of oncology, neurology, endocrinology and women's health. The Center may make the library available to all investigators in the University of South Carolina system without restriction on its use and will own any therapeutic compounds discovered outsideprojects which have been undertaken by our areas of therapeutic interest.
The Center has agreed to conduct screening and pre-clinical activities with respect to the library with a view toward submitting to us at least one development candidate per year in our areas of therapeutic interest over a ten-year period beginning in 2018. We also have a right of first refusal to in-license any submitted development candidates. Should we decide to further develop a development candidate submitted by the Center, MUSC will license the compound candidate to us and be entitled to a royalty on the net sales of all commercialized products developed from the development candidate. However, should we decide not to further develop the development candidate submitted by the Center, MUSC is required to pay us a royalty on net sales of all commercialized products developed from the development candidate.
On July 28, 2015, we announced that we had granted to German life sciences entrepreneurs with a proven track-record of funding the development and commercialization of biotechnology (the "Optionee"), an option to license our live recombinant allogenic oral cancer vaccine technology (the "Technology"), including AEZS-120, the most advanced product candidate for prostate cancer which is ready to enter into a Phase 1 clinical trial. This option was granted to the Optionee worldwide, for a period of twelve months, in exchange for an upfront fee. Pursuant to the option agreement, the Optionee has the right to obtain a worldwide exclusive license to develop, use and sell products relating to the Technology and AEZS-120, in exchange for milestone payments and royalties on net sales of any product developed from the Technology and an equity interest in the company formed to develop the Technology. At the present time, we hold worldwide rights to the Technology, including AEZS-120.
On July 29, 2015, we announced that we had selected an optimized Erk inhibitor molecule, AEZS-140 and back-up candidates, for development. We have since decided to suspend our efforts on internally developing this class of potential cancer therapies to conserve our resources for other projects. Therefore, we are seeking proposals from parties who are interested in either co-developing or licensing the compounds.
On January 13, 2016, during our participation in the annual J.P. Morgan Healthcare conference, we announced that, in addition to our focus on Zoptrex™, we are also focusing on Disorazol Z, because it is an ideal compound for the formation of cytotoxic conjugates with peptides, proteins and antibodies to selectively target cancer cells. We have one cytotoxic conjugate, AEZS-138, in preclinical development. It is a conjugate based on Disorazol Z and the LHRH receptor agonist that is utilized in Zoptrex™. We believe that the peptide directs the compound specifically to LHRH receptor expressing tumor cells, and mediates binding and uptake via endocytosis. Within the cancer cell, the conjugates are cleaved and Disorazol Z can deploy its potent anti-proliferative activity. We have patented the cytotoxic agent Disorazol Z in 35 countries, including the US, Japan, Europe, China, Russia, Korea and Taiwan. This patent protection expires in 2026. The conjugate of Disorazol Z and the LHRH receptor agonist as a targeted cytotoxic agent is patented in 15 countries, including the US, Japan, China, Russia, Korea and Taiwan. This patent protection expires in 2027. We expect the European patent to be granted in the near future.
subsidiary.
Commercial Operations
Our commercial operations consistwere significantly reduced in the fourth quarter of 21 full-time2017. We eliminated our contract sales representatives and a sales-management staff. Theteam in its entirety, as well as remaining sales representatives provide services pursuant tomanagement in November 2017, in accordance with the terms of our agreement with inVentiv Commercial Services, LLC, an affiliate of inVentiv Health, Inc. ("inVentiv"), a contract salescontract-sales organization. The structuring
Pursuant to termination of the inVentiv agreement, we ended our co-promotion with EMD Serono, Inc. and implementationArmune BioScience, Inc.
Until termination of our commercial operations organization is felt to provide direct value through our existing co-promotion commercial activities, discussed below, as well assales team in support of our efforts to in-license and/or acquire products into our portfolio.
EstroGel®
During 2015, we ramped up selling efforts related to our co-promotion agreement with Ascend, which we entered into in August 2014, for EstroGel®, a leading non-patch transdermal hormone replacement therapy product, in specific agreed-upon US territories in exchange for a sales commission that is based upon incremental sales volumes ofNovember 2017, the product that are generated over pre-established baselines.
Detailing efforts associated with EstroGel® commenced in earnest early in the first quarter of 2015, following the completion ofinVentiv sales force training and other knowledge-transfer activities that had been underway since late 2014. During 2015, we began exceeding pre-established unit sales baseline thresholds on a total nation basis.
promoted two products during 2017:
Saizen®
On May 8, 2015, we announced that we had entered into a promotional services agreement with EMD Serono, allowing us to promote Saizen® [somatropin[somatropin (rDNA origin) for injection] to designated medical professionals in specified US territories. Saizen®is a recombinant human growth hormone registered in the USprescription medicine indicated for the treatment of growth hormone deficiency in children and adults. Under this agreement, we are detailingWe promoted Saizen®pursuant to designated medical professionals, representing an important incremental field promotion activityour promotional services agreement (the "EMD Serono Agreement") with EMD Serono, which we entered into in supportMay 2015 and amended as of December 31, 2016. The EMD Serono's product. PaymentSerono Agreement, as amended, provided that we were to Aeterna Zentaris is based on new, eligible patient starts onpromote Saizen® above anin specific agreed-upon baseline.
We are currently promoting Saizen®U.S. territories to adult and pediatric endocrinologists in 21 US territories,exchange for a sales commission that was based upon new patient starts of the product. The agreement was terminated in accordance with efforts having commenced during the third quarter of 2015.its terms on December, 13 2017.
APIFINY®
On December 1, 2015, we announced the finalization of a co-marketing agreement that allows us to promote Armune's APIFINY®,is the only cancer specific,cancer-specific, non-PSA blood test for the detectionevaluation of the risk of prostate cancer. Pursuant to thisThe test was developed by Armune BioScience, Inc. ("Armune"), a medical diagnostics company that develops and commercializes unique proprietary technology exclusively licensed from the University of Michigan for diagnostic and prognostic tests for cancer. We entered into a co-marketing agreement wewith Armune in November 2015 (the "Armune Agreement"), which was amended effective as of June 1, 2016, which allowed us to exclusively promote APIFINY to designated medical professionals in 20 US territories and are entitled to receive®throughout the entire United States. We received a commission for each test performed resulting from our targeted promotion.promotion without regard to any established baseline. The Armune Agreement, as amended, had a three-year term that renewed automatically for successive one-year periods. The parties agreed in January 2018 that the Armune Agreement was terminated.
Corporate Activities
Share consolidation
Leadership
On November 18, 2015,May 8, 2018, the following individuals were elected to the Board of Directors: Carolyn Egbert, Michael Cardiff, Juergen Ernst, Gerard Limoges, Dr. Brent Norton, Jonathan Pollack, and Robin Smith Hoke. On September 25, 2018, we announced the details and implementationappointment of Leslie Auld as Senior Vice President, Chief Financial Officer. On March 26, 2019, the consolidation of our issued and outstanding common shares approved by shareholders at a special meeting held on November 16, 2015, which occurred at a consolidation ratio of 100-to-1 and became legally effective on November 17 2015. Our common shares began trading on a consolidated basis on each of the NASDAQ and the TSX at the opening of markets on November 20, 2015 under our current NASDAQ and TSX trading symbols, “AEZS” and “AEZ”, respectively. All common share and warrant data presented in the MD&A and pertaining to pre-share consolidation events or transactions have been retroactively adjusted to reflect this share consolidation.
On December 8, 2015, weCompany announced that Mr. Cardiff has resigned from the NASDAQ had notified the Company that we regained compliance with Rule 5450(a)(1), which requires a minimum bid priceboard of $1.00directors for continued listing on the NASDAQ.personal reasons.
Public offerings and related eventsSpecial Committee
On March 11, 2015, we completed12, 2019, the Company announced that its board of directors has formed a public offeringspecial committee of 596,775 unitsindependent directors (the "Units""Special Committee"), generating net proceeds of approximately $34.4 million, with each Unit consisting of either one common share or one pre-funded warrant to purchase one common share ("Series C Warrant"), 0.75review strategic options available to the Company. The Special Committee has approved the engagement by the Company of a warrantfinancial advisor that is working with management to purchase one common share ("Series A Warrant") and 0.50 of a warrant to purchase one common share ("Series B Warrant"), at a purchase price of $62.00 per Unit (the "March 2015 Offering"). The Series A Warrants are exercisable during a five-year term at an initial exercise price of $81.00 per share,assist the Special Committee and the Series B Warrants are exercisable during an 18-month term at an initial exercise priceboard of $81.00 per share. Bothdirectors in considering a wide range of transactions (including opportunities for the Series A and Series B warrants are subject to certain anti-dilution provisions. The Series C Warrants were exercisable for a periodlicense of five years at an exercise price of $62.00 per share. Total gross proceeds payable to us in connection with the exerciseMacrilen outside of the Series C Warrants were pre-paid by investors at the closingUnited States and Canada, or other monetization transactions relating to Macrilen. Management has evaluated whether material uncertainties exist relating to events or conditions as described in Note 4 of the March 2015 Offering and therefore areour Financial Statements included in this Form 20-F, and has considered the aforementioned proceeds. Between March 23, 2015following in making that critical judgment.
The Company’s current operating budget and June 16, 2015, allcash flows from operating activities in 2019 are expected to decline compared with 2018, however, the Company believes it will continue to generate growth in its royalty income, which, when combined with its forecasted cash and cash equivalents, the Company believes will provide liquidity that is in excess of the pre-funded Series C Warrants were exercised, resulting in the issuance of a total of 346,294 common shares.
Both the Series A and Series B Warrants mayits costs for at any time be exercised on a standard cashless basis. In addition,least, but not limited to, twelve months from the Series B Warrants may be exercised on an alternate net cashless basis. The exercise of Series B Warrants performed on an alternate net cashless basis results in the issuance of a substantially larger number of the Company's common shares than otherwise would be issued following a standard cash or cashless exercise. Specifically, between May 26, 2015 and December 31, 2015, 290,318 Series B Warrants were exercised on an alternate net cashless basis, resulting in the issuance of approximately 5.7 million common shares. The remaining 8,064 Series B Warrants expire on September 12, 2016.
In connection with the March 2015 Offering, the holders of 211,230 of the 219,000 outstanding warrants issued in connection with previous public offerings completed in November 2013 and January 2014 each entered into an amendment agreement that caused such previously issued warrants to expire and terminate in consideration for a cash payment made by us in the aggregate amount of approximately $5.7 million out of the proceeds of the March 2015 Offering.
On November 2, 2015, we announced that the holders (the "Participating Holders") of substantially all of the remaining outstanding Series B Warrants at that time had agreed to exercise all of the Series B Warrants held by them, as promptly as practicable, at a maximum exercise ratio of approximately 33.23 common shares per warrant in accordance with the alternate cashless exercise feature in such Series B Warrants. Following the exercise of Series B Warrants by the Participating Holders in accordance with the terms of the agreements, 8,064 Series B Warrants, with an expiry date of September 12, 2016, remain outstanding, representing approximately 2.7%approval of the originally issued number of Series B Warrants. A total of $2.9 million in cash was paid to the Participating Holders pursuant to the aforementioned agreements.these financial statements.
On December 14, 2015, we completed an underwritten public offering (the “December 2015 Offering”) of 3.0 million common shares and warrants to acquire 2.1 million common shares with a combined purchase price of $5.55 for one common share together with a warrant to purchase 0.7 of a common share, generating net proceeds of approximately $15.0 million. In addition, the Company granted the underwriter a 45-day option to purchase up to an additional 330,000 common shares and/or warrants to purchase up to an additional 231,000 common shares, to cover over-allotments, if any. Prior to closing, the underwriter exercised its over-allotment option with respect to the warrants to acquire an additional 231,000 common shares, resulting in an issuance of warrants to acquire an aggregate of approximately 2.3 million common shares at closing.Contingencies
The warrants are exercisable immediately and expire five years following issuance at an exercise price of $7.10 per share. The warrants do not contain any price or other adjustment provision, except for customary adjustment provisions that apply in the event of certain corporate events or transactions that affect all outstanding common shares. The warrants may at any time be exercised on a standard cashless basis in accordance with a customary formula but do not contain an alternate cashless exercise feature contained in our previously issued Series B common shares purchase warrants. The warrants are not listed on any stock exchange.
On December 30, 2015, we announced that we had filed a preliminary short form base shelf prospectus (the “Shelf Prospectus”) with the securities regulatory authorities in each of the provinces of Canada, and a corresponding shelf registration statement on Form F-10 with the SEC under the US/Canada Multijurisdictional Disclosure System. The Shelf Prospectus and corresponding shelf registration statement, which became effective subsequent to year-end on January 13, 2016, will allow us to offer up to $150 million of common shares, preferred shares, debt securities, subscription receipts, warrants or units comprised of one or more of such securities during the 25-month period that the Shelf Prospectus is effective.
ClassSecurities class action lawsuitlitigation
The Company and certain of its current and former officers are defendants in a putative class-action lawsuit pending in the U.S. District Court for the District of New Jersey, brought on behalf of shareholders of the Company. The pending lawsuit is the result of the consolidation of several lawsuits, the first of which was filed on November 11, 2014. The plaintiffs filed their amended consolidated complaint on April 10, 2015. The amended complaint allegedalleges violations of the Securities Exchange Act of 1934 in connection with allegedly false and misleading statements made by the defendants between August 30, 2011 and November 6, 2014 (the "Class Period"), regarding the safety and efficacy of Macrilen™ (macimorelin) and the prospects for the approval of the Company's new drug applicationNew Drug Application for the product by the FDA. The plaintiffs seek to represent a class comprised of purchasers of the Company's common shares during the Class Period and seek unspecified damages, costs and expenses and such other relief as determined by the court.
On September 14, 2015,Court. The Company considers the Court dismissedclaims that have been asserted in the lawsuit but grantedto be without merit and is vigorously defending against them. The Company cannot, however, predict at this time the plaintiffs leaveoutcome or potential losses, if any, with respect to amend. this lawsuit.
Other litigation
In dismissinglate July 2017, the Company terminated for cause the employment agreement of Mr. David A. Dodd, the former President and Chief Executive Officer and it also terminated the employment of Mr. Philip A. Theodore, the former Senior Vice President, Chief Administrative Officer, General Counsel and Corporate Secretary. On August 3, 2017, the Company filed a lawsuit against both Messrs. Dodd and Theodore for damages suffered by the Company for breach of confidence and/or breach of fiduciary duty in an amount to be determined prior to trial. On December 21, 2017, Messrs. Dodd and Theodore brought a counterclaim against the Company and its Chair, Carolyn Egbert, in the amount of CAN$6.0 million alleging, among other things, that defamatory statements were made against Messrs. Dodd and Theodore. On December 21, 2018, the matter was amicably resolved with the Company making a payment to Mr. Dodd in the amount of $775,000. The parties consider their contractual relationship as having been terminated.
Cogas Consulting, LLC ("Cogas") filed a lawsuit against the Company in state court in Fulton County, Georgia on February 2, 2018. The lawsuit was removed to federal court in Georgia. In the lawsuit, Cogas alleged that its employee (and sole shareholder) John Sharkey was entitled to a "success fee" commission on the court affirmed thatStrongbridge License Agreement. Cogas was claiming damages in the plaintiffs had failed to stateform of a claim. On Octoberlost commission on the transaction. Cogas claims its commission is 5% on payments the Company receives within the first three years after January 14, 2015, the plaintiffs filed a second amended complaint. We subsequently filed a motion to dismiss, because we believe that the second amended complaint also fails to state a claim. The hearing2018 including 5% of the motion to dismiss$24.0 million Strongbridge already paid the Second Amended Complaint occurred onCompany, plus 5% of any royalty Strongbridge pays the Company through January 19, 2016.17, 2021. On March 2, 2016,November 5, 2018, the Court issued an order granting our motion to dismiss the complaint in part and denying it in part. The Court dismissed certain of our current and former officers from the lawsuit. The Court allowed the claim that we omitted material facts from our public statements during the Class Period to proceed against us and our former CEO who departed in 2013, while dismissing such claims against other current and former officers. The Court also allowed a claim for “controlling person” liability to proceed against certain current and former officers. We disagreematter was amicably resolved with the Court's decision and we filedCompany making a motion for reconsideration on March 16, 2016.
Restructuring
On October 12, 2015, we announced that our Boardpayment to Cogas in the amount of Directors had approved a plan to restructure our finance and accounting operations and to close our Quebec City office (the “Corporate Restructuring”). We transferred all functions performed by the five employees in our Quebec City office to other personnel and we intend to add new finance and accounting personnel, including a new Chief Financial Officer, in our Summerville, South Carolina, office. We estimate that the Corporate Restructuring will be completed by September 2016.$625,000. The parties now consider their contractual relationship as having been terminated.
Consolidated Statements of Comprehensive (Loss) Income Information
|
| | | | | | | | | | | | | | | |
| | | Three-month periods ended December 31, | | Years ended December 31, |
| (in thousands, except share and per share data) | | 2015 | | 2014 | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ | | $ | | $ |
| Revenues | | | | | | | | | | |
| Sales commission and other | | 41 |
| | — |
| | 297 |
| | — |
| | 96 |
|
| License fees | | 61 |
| | 11 |
| | 248 |
| | 11 |
| | 6,079 |
|
| | | 102 |
| | 11 |
| | 545 |
| | 11 |
| | 6,175 |
|
| Operating expenses | | | | | | | | | | |
| Cost of sales | | — |
| | — |
| | — |
| | — |
| | 51 |
|
| R&D costs | | 4,243 |
| | 6,282 |
| | 17,234 |
| | 23,716 |
| | 21,284 |
|
| General and administrative expenses | | 3,953 |
| | 2,633 |
| | 11,308 |
| | 9,840 |
| | 11,091 |
|
| Selling expenses | | 1,764 |
| | 2,043 |
| | 6,887 |
| | 3,850 |
| | 1,225 |
|
| | | 9,960 |
| | 10,958 |
| | 35,429 |
| | 37,406 |
| | 33,651 |
|
| Loss from operations | | (9,858 | ) | | (10,947 | ) | | (34,884 | ) | | (37,395 | ) | | (27,476 | ) |
| Finance income | | 26 |
| | 15,053 |
| | 305 |
| | 20,319 |
| | 1,748 |
|
| Finance costs | | (211 | ) | | — |
| | (15,649 | ) | | — |
| | (1,512 | ) |
| Net finance (costs) income | | (185 | ) | | 15,053 |
| | (15,344 | ) | | 20,319 |
| | 236 |
|
| (Loss) income before income taxes | | (10,043 | ) | | 4,106 |
| | (50,228 | ) | | (17,076 | ) | | (27,240 | ) |
| Income tax expense | | — |
| | (111 | ) | | — |
| | (111 | ) | | — |
|
| Net (loss) income from continuing operations | | (10,043 | ) | | 3,995 |
| | (50,228 | ) | | (17,187 | ) | | (27,240 | ) |
| Net income from discontinued operations | | 25 |
| | 158 |
| | 85 |
| | 623 |
| | 34,055 |
|
| Net (loss) income | | (10,018 | ) | | 4,153 |
| | (50,143 | ) | | (16,564 | ) | | 6,815 |
|
| Other comprehensive (loss) income: | | | | | | | | | | |
| Items that may be reclassified subsequently to profit or loss: | | | | | | | | | | |
| Foreign currency translation adjustments | | 249 |
| | (677 | ) | | 1,509 |
| | (1,158 | ) | | 1,073 |
|
| Items that will not be reclassified to profit or loss: | | | | | | | | | | |
| Actuarial (loss) gain on defined benefit plans | | (116 | ) | | 1,336 |
| | 844 |
| | (1,833 | ) | | 2,346 |
|
| Comprehensive (loss) income | | (9,885 | ) | | 4,812 |
| | (47,790 | ) | | (19,555 | ) | | 10,234 |
|
Net (loss) income per share (basic and diluted) from continuing operations1 | | (1.46 | ) | | 6.11 |
| | (18.17 | ) | | (29.12 | ) | | (92.41 | ) |
Net income per share (basic and diluted) from discontinued operations1 | | — |
| | 0.24 |
| | 0.03 |
| | 1.06 |
| | 115.53 |
|
Net (loss) income per share (basic and diluted)1 | | (1.46 | ) | | 6.35 |
| | (18.14 | ) | | (28.06 | ) | | 23.12 |
|
Weighted average number of shares outstanding:1 | | | | | | | | | | |
| Basic | | 6,874,460 |
| | 653,833 |
| | 2,763,603 |
| | 590,247 |
| | 294,765 |
|
| Diluted | | 7,302,816 |
| | 653,833 |
| | 3,424,336 |
| | 590,247 |
| | 294,765 |
|
| |
1
| Adjusted to reflect the November 17, 2015 100-to-1 Share Consolidation |
Consolidated Statements of Comprehensive Loss Information
|
| | | | | | | | | | | | | | | |
| | | Three months ended December 31, | | Years ended December 31, |
| (in thousands, except share and per share data) | | 2018 | | 2017 | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ | | $ | | $ |
| Revenues | | | | | | | | | | |
| License fees | | (332 | ) | | 119 |
| | 24,325 |
| | 458 |
| | 497 |
|
| Product sales | | 1,446 |
| | — |
| | 2,167 |
| | — |
| | — |
|
| Royalty income | | 184 |
| | — |
| | 184 |
| | — |
| | — |
|
| Sales commission and other | | 94 |
| | 59 |
| | 205 |
| | 465 |
| | 414 |
|
| | | 1,392 |
| | 178 |
| | 26,881 |
| | 923 |
| | 911 |
|
| Cost of sales | | 1,413 |
| | — |
| | 2,104 |
| | — |
| | — |
|
| Gross income | | (21 | ) | | 178 |
| | 24,777 |
| | 923 |
| | 911 |
|
| | | | | | | | | | | |
| Operating expenses | | | | | | | | | | |
| Research and development costs | | 767 |
| | 526 |
| | 2,932 |
| | 10,704 |
| | 16,495 |
|
| General and administrative expenses | | 1,665 |
| | 2,778 |
| | 8,894 |
| | 8,198 |
| | 7,147 |
|
| Selling expenses | | 588 |
| | 452 |
| | 3,109 |
| | 5,095 |
| | 6,745 |
|
| | | 3,020 |
| | 3,756 |
| | 14,935 |
| | 23,997 |
| | 30,387 |
|
| Income (loss) from operations | | (3,041 | ) | | (3,578 | ) | | 9,842 |
| | (23,074 | ) | | (29,476 | ) |
| Settlements | | (1,400 | ) | | — |
| | (1,400 | ) | | — |
| | — |
|
| Gain (loss) due to changes in foreign currency exchange rates | | 64 |
| | 72 |
| | 656 |
| | 502 |
| | (70 | ) |
| Change in fair value of warrant liability | | (1,489 | ) | | (478 | ) | | 263 |
| | 2,222 |
| | 4,437 |
|
| Other finance income | | 104 |
| | 21 |
| | 278 |
| | 75 |
| | 150 |
|
| Net finance income (costs) | | (1,321 | ) | | (385 | ) | | 1,197 |
| | 2,799 |
| | 4,517 |
|
| Income (loss) before income taxes | | (5,762 | ) | | (3,963 | ) | | 9,639 |
| | (20,275 | ) | | (24,959 | ) |
| Income tax recovery (expense) | | 636 |
| | 3,479 |
| | (5,452 | ) | | 3,479 |
| | — |
|
| Net income (loss) | | (5,126 | ) | | (484 | ) | | 4,187 |
| | (16,796 | ) | | (24,959 | ) |
| Other comprehensive income (loss): | | | | | | | | | | |
| Items that may be reclassified subsequently to profit or loss: | | | | | | | | | | |
| Foreign currency translation adjustments | | (13 | ) | | (238 | ) | | (260 | ) | | (1,430 | ) | | 569 |
|
| Items that will not be reclassified to profit or loss: | | | | | | | | | | |
| Actuarial gain (loss) on defined benefit plans | | (418 | ) | | 59 |
| | 193 |
| | 694 |
| | (1,479 | ) |
| Comprehensive income (loss) | | (5,557 | ) | | (663 | ) | | 4,120 |
| | (17,532 | ) | | (25,869 | ) |
| Basic Net income (loss) per share | | (0.31 | ) | | (0.03 | ) | | 0.25 |
| | (1.12 | ) | | (2.41 | ) |
| Diluted Net income (loss) per share | | (0.31 | ) | | (0.03 | ) | | 0.24 |
| | (1.12 | ) | | (2.41 | ) |
Our operating and financial review and prospects should be read in conjunction with our consolidated financial statements, accompanying notes and other information appearing in this Annual Report.
2015
2018 compared with 2017
Fourth Quarter
Revenues
Our total revenue for the three-month period ended December 31, 2018 was $1.4 million as compared with $0.2 million for the same period in 2017, representing an increase of $1.2 million. The 2018 revenue comprised the net impact of $1.4 million in product sales less the $0.2 million reclassification of the $24.0 million license revenue associated with the Pediatric Indication, to the consolidated statements of financial position. For the same period in 2017, total revenue was comprised of $0.1 million in license fees and $0.1 million in sales commission and other. The increase in product sales in the fourth quarter of 2018 arises from the sale of Macrilen™ (macimorelin) inventory to our licensee for sale in the United States.
Cost of sales
Our total cost of goods sold for the three-month period ended December 31, 2018 was $1.4 million as compared with nil for the same period in 2017. The 2018 balance reflects the cost of Macrilen™ (macimorelin) inventory sold under an interim supply agreement to our licensee for future sales in the United States.
Operating expenses
Our total operating expenses for the three-month period ended December 31, 2018 was $3.0 million as compared with $3.8 million for the same period in 2017, representing a decrease of $0.8 million. This net decline arises primarily from a $1.1 million decrease in general and administration expenses, offset by a $0.2 million increase in research and development costs and a $0.1 million increase in selling expenses. These increases are in-line with the expected impact of the roll-out of our PIP study beginning in the third quarter of 2018.
Settlements
In the three-month period ended December 31, 2018, $1.4 million was classified as settlements as compared with nil in the same period in 2017. These were costs to settle a lawsuit against the Company from two of its former executives and former sales agent.
Net finance costs
Our net finance loss for the three-month period ended December 31, 2018 was $1.3 million, as compared to 2014$0.4 million for the same period in 2017, representing an increase of $0.9 million. The increase in net finance costs is primarily due to the change in fair value of warrant liability. Such change in fair value results from the periodic "mark-to-market" revaluation, via the application of pricing models, of outstanding share purchase warrants. The closing price of our common shares, which, on the NASDAQ, fluctuated from $1.19 to $3.87 during the twelve-month period ended December 31, 2018, compared to $2.67 to $2.70 during the same period in 2017, also had a direct impact on the change in fair value of warrant liability.
Net loss
For the three-month period ended December 31, 2018, we reported a consolidated net loss of $5.1 million, or $0.31 loss per common share, as compared with a consolidated net loss of $0.5 million, or $0.03 loss per common share, for the three-month period ended December 31, 2017. The $4.6 million increase in net loss, as compared with 2017, results primarily from a $2.8 million in tax expense, $1.4 million increase in cost of goods, $0.9 million increase in finance costs and $1.4 million increase in settlements, offset by $1.2 million increase in total revenues. In the fourth quarter of 2018, unlike in 2017, we earned $0.2 million in royalty income from our licensee, expensed $1.4 million in settlement costs and had actively begun the EMA and FDA pediatric study for Macrilen™ (macimorelin).
Fiscal Year-End
Revenues
Our total revenue for the year ended December 31, 2018 was $26.9 million as compared with $0.9 million for the same period in 2017, representing an increase of $26.0 million. The 2018 revenue comprised $24.3 million in license revenue, $2.2 million in product sales and $0.2 million in royalty income and $0.2 million in sales commissions as compared with $0.4 million in license fee and $0.5 million in sales commission in 2017. The increase in total revenue in 2018 relates to license fees and product sales associated with executing the License and Assignment Agreement signed for Macrilen™ (macimorelin) in January 2018.
Cost of sales
Our total cost of goods sold for the year ended December 31, 2018 was $2.1 million as compared with nil for the same period in 2017, reflecting the sale of Macrilen™ (macimorelin) inventory pursuant to an interim supply agreement under the License and Assignment Agreement.
Operating expenses
Our total operating expenses for the year ended December 31, 2018 was $14.9 million as compared with $24.0 million for the same period in 2017, representing a decline of $9.1 million. This was primarily due to a $7.8 million decrease in research and development costs and a $2.0 million decrease in selling expenses, offset by $0.7 million increase in general and administration expenses.
Research and development
In 2018, our focus was on our pediatric clinical trial submitted for approval to the EMA and FDA (the "PIP study") for Macrilen™ (macimorelin), for which we received $0.4 million from our licensee for their share of such costs. This study was initiated in the third quarter of 2018 with active screening of patients beginning in early 2019.
In 2017, we spent $2.5 million on third-party costs associated with the ZoptEC pivotal Phase 3 clinical study of Zoptrex™ (zoptarelin doxorubicin) and $1.2 million on Macrilen™ (macimorelin) third-party costs. In addition, we recorded $2.6 million in severance accruals and other directly related costs and an onerous lease provision related to the 2017 German Restructuring. This restructuring resulted from the May 2017 announcement that the ZoptEC pivotal Phase 3 clinical study of Zoptrex™ (zoptarelin doxorubicin) did not achieve its primary endpoint of demonstrating a statistically significant increase in the median period of overall survival of patients treated with Zoptrex™ (zoptarelin doxorubicin) as compared to patients treated with doxorubicin.
General and administrative expenses
These costs were higher in 2018 than expected as we incurred significant legal costs in the course of reaching settlement agreements for $1.4 million, as previously discussed in ContingenciesOther litigation.
Selling expenses
These costs are in-line with expectations and lower in 2018 than in 2017 due to the Q1 2018 termination of our North American sales team and their co-promotion activities as we shifted our focus to the commercialization of Macrilen™ (macimorelin) in markets in the rest of the world.
Settlements
In 2018, $1.4 million was expensed for settlements as compared with nil in the same period in 2017. These were costs to settle a two lawsuits against the Company from two of its former executives and from its former sales agent.
Net finance income
Our net finance income for the year ended December 31, 2018 was $1.2 million, as compared to $2.8 million for the same period in 2017, representing a decrease of $1.6 million. The decline in net finance income is primarily due to the change in fair value of our warrant liability. Such change in fair value results from the periodic "mark-to-market" revaluation via the application of pricing models to our outstanding share purchase warrants. The closing price of our common shares, which, on the NASDAQ, fluctuated from $1.19 to $3.87 during the twelve-month period ended December 31, 2018, compared to $2.67 to $2.70 during the same period in 2017, also had a direct impact on the change in fair value of warrant liability.
Net Income
For the year ended December 31, 2018, we reported a consolidated net income of $4.2 million, or $0.25 per common share, as compared with a consolidated net loss of $16.8 million, or $1.12 loss per common share, for the year ended December 31, 2017. The $21.0 million improvement in results, as compared with 2017, arose primarily from a $23.9 million increase in gross profit and $9.1 million reduction in operating expenses, offset by $8.9 million movement in income taxes from recovery to (expense) and $1.6 million decrease in net finance income.
2017 compared to 2016
Revenues
Sales commission and other were $0.1 million and $0.5 million for the three-month periodthree and the yeartwelve months ended December 31, 2015, respectively, compared to $0.02017 and $0.1 million and $6.2$0.4 million for the same periods in 2014.
The2016, and thus increased in 2017 as compared to 2016. In 2017, those revenues recorded during the year ended December 31, 2015mainly resulted primarily from the amortization of a one-time, non-refundable payment madeour sales team exceeding pre-established unit sales baseline thresholds under our co-promotion agreement to us in December 2014 in connection with a master collaboration agreement, a technology transfer and technical assistance agreement and a license agreement that we entered into with Sinopharm A-Think Pharmaceuticals Co., Ltd. ("Sinopharm") related to Zoptrexsell SaizenTM®. We deferred this non-refundable payment and we amortize it on a straightline basis over a four-year period. In addition, we started to generatealso generated sales commission in connection with our co-promotion effortspromotion of APIFINY®. In the corresponding periods in 2016, sales commission and other revenues were mainly related to EstroGel®, pursuant to the co-promotion services agreement entered into with Ascend..
We expect revenues duringLicense fees were $0.1 million and $0.5 million for the yearthree and twelve months ended December 31, 20162017, as compared to be higher than those recorded during the year ended December 31, 2015 due to the recording of expected higher sales commissions associated with our promotional efforts related to EstroGel® andaswe begin to generate sales commissions related to Saizen®,provided that we are able to begin to exceed the pre-established baselines outlined in the related co-marketing agreement, as well as sales commissions related to APIFINY®.
Operating Expenses
R&D costs were $4.2$0.2 million and $17.2 million for the three-month period and the year ended December 31, 2015, respectively, compared to $6.3 million and $23.7$0.5 million for the same periods in 2014.2016. The Company has deferred revenues at December 31, 2017 of $541,000 relating to non-refundable upfront payments it previously received for licensing and technology transfer arrangements that it entered into with respect to the development of Zoptrex™ in various territories. Due to events that occurred in 2018, the Company does not anticipate development of Zoptrex™ under the licensing agreements, therefore the Company's remaining carrying amount of deferred revenues was recognized in the first quarter of 2018 as income.
The decrease
Operating Expenses
R&D costs were $0.5 million and $10.7 million for the three and twelve months ended December 31, 2017, compared to $4.6 million and $16.5 million for the same periods in 2016. R&D costs decreased for the three-month periodand twelve-month periods ended December 31, 2015,2017 as compared to the same period in 2014,2016. The decrease in R&D costs is mainly attributable to lower comparative third-party costs, as described below, partially offset by the recording, in the third quarter of 2017, of a provision in connection with the 2017 German Restructuring.
Additionally, the decrease in our R&D costs for the twelve months ended December 31, 2017, as compared to the same period in 2016, is attributable to lower employee compensation and benefits costs, and lower facilities rent and maintenance costs as well as lower other costs. A substantial portion of this decrease is due to the realization of cost savings in connection with our effortongoing efforts to streamline our R&D activities and to increase our commercial operations and flexibility by reducing our R&D staff, which was started in 2014 (the "Resource Optimization Program"), for which a provision had been recorded in the third quarter of 2014. In addition, the decrease is also due to the weakening, in 2015, of the EUR against the US dollar, which has appreciated quarter-over-quarter on average by approximately 12.0% from the quarter ended December 31, 2014 to the same period in 2015.
. The decreaseR&D costs for the year ended December 31, 2015, as compared2017 were lower than anticipated mainly because we were able to the same period in 2014, is attributablenegotiate reductions to lower comparative employee compensation and benefits costs, facilities rent and maintenance costs as well as other costs. a change order received from our principal R&D third-party service provider.
A substantial portion of this decrease is due to the realization of cost savings in connection with our Resource Optimization Program rolled out in the third quarter of 2014, as well as to the weakening, in 2015, of the EUR against the US dollar, which has appreciated on average by approximately 16.5% from the year ended December 31, 2014 to the same period in 2015. The decrease for the year ended December 31, 2015 was partly offset by higher third-party costs, as described below.
The following table summarizes our net R&D costs by nature of expense:
|
| | | | | | | | | | | | | | | |
| | | Three-month periods ended December 31, | | Years ended December 31, |
| (in thousands) | | 2015 | | 2014 | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ | | $ | | $ |
| Third-party costs | | 2,899 |
| | 3,967 |
| | 11,891 |
| | 11,356 |
| | 10,049 |
|
| Employee compensation and benefits | | 905 |
| | 1,231 |
| | 3,699 |
| | 8,430 |
| * | 7,864 |
|
| Facilities rent and maintenance | | 224 |
| | 887 |
| | 940 |
| | 2,160 |
| | 1,758 |
|
| Other costs** | | 231 |
| | 197 |
| | 727 |
| | 1,901 |
| | 2,130 |
|
| R&D tax credits and grants | | (16 | ) | | — |
| | (23 | ) | | (131 | ) | | (517 | ) |
| | | 4,243 |
| | 6,282 |
| | 17,234 |
| | 23,716 |
| | 21,284 |
|
_________________________
* Includes a provision for restructuring in the amount of $2.2 million.
** Includes depreciation, amortization, impairment charges, loss (gain) on disposal of property, plant and equipment and onerous lease provision recognized.
The following table summarizes primary third-party R&D costs, by product candidate, incurred by the Company during the three-month periods ended December 31, 2015 and 2014.
|
| | | | | | | | | | | | |
| (in thousands, except percentages) | | Three-month periods ended December 31, |
| Product Candidate | | 2015 | | 2014 |
| | | $ | | % | | $ | | % |
| Zoptrex™ (zoptarelin doxorubicin) | | 1,488 |
| | 51.3 |
| | 3,609 |
| | 91.0 |
|
| Macrilen™ (macimorelin) | | 977 |
| | 33.7 |
| | 192 |
| | 4.8 |
|
| Erk inhibitors | | 71 |
| | 2.5 |
| | 112 |
| | 2.8 |
|
| LHRH - Disorazol Z | | 73 |
| | 2.5 |
| | 54 |
| | 1.4 |
|
| Other | | 290 |
| | 10.0 |
| | — |
| | — |
|
| | | 2,899 |
| | 100.0 |
| | 3,967 |
| | 100.0 |
|
The following table summarizes primary third-party R&D costs, by product candidate, incurred by the Company during the years ended December 31, 2015, 2014 and 2013.
|
| | | | | | | | | | | | | | | | | | |
| (in thousands, except percentages) | | Years ended December 31, |
| Product Candidate | | 2015 | | 2014 | | 2013 |
| | | $ | | % | | $ | | % | | $ | | % |
| Zoptrex™ (zoptarelin doxorubicin) | | 8,635 |
| | 72.6 |
| | 9,668 |
| | 85.1 |
| | 4,934 |
| | 49.1 |
|
| Macrilen™ (macimorelin) | | 1,555 |
| | 13.1 |
| | 404 |
| | 3.6 |
| | 1,238 |
| | 12.3 |
|
| Erk inhibitors | | 1,081 |
| | 9.1 |
| | 488 |
| | 4.3 |
| | 1,128 |
| | 11.2 |
|
| LHRH - Disorazol Z | | 212 |
| | 1.8 |
| | 257 |
| | 2.3 |
| | 659 |
| | 6.6 |
|
| Perifosine | | 29 |
| | 0.2 |
| | 196 |
| | 1.7 |
| | 1,134 |
| | 11.3 |
|
| Other | | 379 |
| | 3.2 |
| | 343 |
| | 3.0 |
| | 956 |
| | 9.5 |
|
| | | 11,891 |
| | 100.0 |
| | 11,356 |
| | 100.0 |
| | 10,049 |
| | 100.0 |
|
As shown above, a substantial portion of the quarter-to-date and year-to-date third-party R&D costs relates to development initiatives associated with ZoptrexTMZoptrex™, and in particular with our pivotal Phase 3 ZoptEC clinical trial initiated in 2013 with Ergomed. Excluding the impact of the foreign exchange rate fluctuations, third-partyThird-party costs attributable to ZoptrexTM increased slightlyZoptrex™ decreased considerably during the yeartwelve months ended December 31, 2015,2017, as compared to the same period in 2014,2016, mainly duesince we completed the clinical portion of the ZoptEC trial during the first quarter of 2017 which was partially offset by the additional liability recognized following the negative ZoptrexTM top-line results.
Third-party costs attributable to a higher comparative number of patients enrolledZoptrex™ decreased during the three and twelve months ended December 31, 2017, as compared
to the same period in 2016, mainly since we closed out the study and related activities in the clinical trial, which is now fully enrolled. However,second quarter following the quarter-over-quarter decrease is negative
ZoptrexTM top-line results on May 1, 2017. The negative costs for the three-month period ended December 31, 2017 are mainly
explained by lower close out costs as compared to the fact that the number of patients in active treatmentaccrual made in the clinical trial was lower in 2015second quarter.
Third-party costs attributable to Macrilen™ (macimorelin) decreased during the three and twelve months ended December 31, 2017, as compared to the same period in 2014.
During2016. This is mainly since we completed the year ended December 31, 2015, ongoing services provided by Ergomed included the conducting of monitoring visits at various clinical sites, screening and enrollment initiatives, investigation-related management and analysis as well as regulatory and quality assurance support. ZoptEC-related efforts are progressing in accordance with pre-established timelines. As we continue to closely monitor all initiatives supported by Ergomed, we may decide to revise some of the trial's parameters or expand the scope of work performed by Ergomed and, consequently, total estimated costs in connection with the co-development and revenue sharing agreement may be adjusted. To date, our arrangement with Ergomed has been revised following our decision to open additional clinical sites and to perform additional sub-studies, resulting in overall, cumulative cost increases of approximately $2.4 million, as compared to our original expectations. We currently estimate that we will incur approximately $6 million pursuant to our agreement with Ergomed over the next 12 months as we proceed with and complete our ZoptEC trial.
In addition, during the year 2015, we started the new confirmatory Phase 3 clinical trial at the end of Macrilen™,2016. The costs incurred in 2017 related to the detailed analysis of the top-line results as well as the preparation of the NDA filing which explains
was submitted on June 30, 2017. The costs reversal in the increase in costs for this product candidate.fourth quarter of 2017 are explained mainly by the reductions to close
out costs.
ExcludingG&A expenses were $2.8 million and $8.2 million for both the impact of foreign exchange rate fluctuations, we expect R&D costs for 2016 to increase,three and twelve-month periods ended December 31, 2017, as compared to 2015, with the recent initiation of our confirmatory Phase 3 clinical trial for MacrilenTM. Based on currently available information and taking into account our more detailed forecasts for the MacrilenTM trial, and excluding the impact of foreign exchange rate fluctuations, we expect that we will incur overall R&D costs of between $19$1.8 million and $20 million for the year ended December 31, 2016.
General and administrative ("G&A") expenses were $4.0 million and $11.3 million for the three-month period and the year ended December 31, 2015, respectively, as compared to $2.6 million and $9.8$7.1 million for the same periods in 2014.2016. The increase is mainly attributable toin our G&A costs for the recording of a provision related to our Corporate Restructuring in the fourth quarter of 2015, as well as to the recording of certain transaction costs associated with the completion of the March 2015 Offeringthree and thetwelve months ended December 2015 Offering, discussed above.
During 2016, excluding the impact of foreign exchange rate fluctuations and the recording of transaction costs related to potential financing activities (not currently known or estimable), we expect G&A expenses to be lower31, 2017, as compared to 2015, ranging between $6 million and $7 million, because we do not expect to record any restructuring chargesthe same period in 2016, as we had in 2015.is mainly due to outside legal costs.
Selling expenseswere $1.8$0.5 million and $6.9$5.1 million for the three months and the yeartwelve months ended December 31, 2015, respectively,2017, as compared to $2.0$1.5 million and $3.9$6.7 million for the same periods in 2014.
The decrease in selling2016. Selling expenses for the three-month periodthree and twelve months ended December 31, 2015 is explained by2017 and 2016 represent mainly the start-up costs related to the deployment of our contracted sales force related to the co-promotion activities which were launched duringas well as our sales management
team. The decrease in selling expenses is explained by the elimination of sales representatives. In the fourth quarter, we eliminated
all sales representatives as part of 2014.the restructuring efforts.
The increase in selling expensesNet finance income (costs) was $(0.4) million and $2.8 million for the yearthree and twelve months ended December 31, 20152017, as compared to $(0.6) million and $4.5 million, for the same periodperiods in 20142016. The decrease in finance income is mainly attributable to the fact that 2014 was not a full year of sales activity. During the third quarter of 2015, we also expanded the size of our contracted sales force from 19 to 21 sales representatives in order to support our promotional efforts associated with Saizen®. This sales force expense will also cover the recently initiated selling in support of APIFINY®.
During 2016, we expect selling expenses to increase slightly to reach a range of between $7 million and $8 million.
Net finance (costs) income arecomprised predominantly of
to the change in fair value of warrant liability and of gains and losses recorded due to changes in foreign currency exchange rates, as presented below.
|
| | | | | | | | | | | | | | | |
| | | Three-month periods ended December 31, | | Years ended December 31, |
| | | 2015 | | 2014 | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ | | $ | | $ |
| Finance income | | | | | | | | | | |
| Change in fair value of warrant liability | | 3,030 |
| | 14,079 |
| | — |
| | 18,272 |
| | 1,563 |
|
| Gain associated with the extinguishment of warrant liability | | — |
| | — |
| | 162 |
| | — |
| | — |
|
| Gains due to changes in foreign currency exchange rates | | — |
| | 924 |
| | — |
| | 1,879 |
| | — |
|
| Interest income | | 26 |
| | 50 |
| | 143 |
| | 168 |
| | 185 |
|
| | | 3,056 |
| | 15,053 |
| | 305 |
| | 20,319 |
| | 1,748 |
|
| Finance costs | | | | | | | | | | |
| Change in fair value of warrant liability | | — |
| | — |
| | (10,956 | ) | | — |
| | — |
|
| Warrant exercise inducement fee * | | (2,926 | ) | | — |
| | (2,926 | ) | | — |
| | — |
|
| Losses due to changes in foreign currency exchange rates | | (315 | ) | | — |
| | (1,767 | ) | | — |
| | (1,512 | ) |
| | | (3,241 | ) | | — |
| | (15,649 | ) | | — |
| | (1,512 | ) |
| | | (185 | ) | | 15,053 |
| | (15,344 | ) | | 20,319 |
| | 236 |
|
_________________________
| |
* | Recorded in connection with the agreement with the Participating Holders, as discussed above. |
Theliability. Such change in fair value of our warrant liability results from the periodic "mark-to-market" revaluation, via the application of the intrinsic valuation and the Black-Scholes option pricing model,models, of currently outstanding share purchase warrants. The "mark-to-market" warrant valuation has been most notably impacted by the issuance of 3.1 million additional share purchase warrants and by the closing price of our common shares, which, on the NASDAQ, has fluctuated from $4.00 to $84.20 during the year ended December 31, 2015, from $52.00 to $150.00 for the same period in 2014 and from $103.00 to $323.00 for the same period in 2013.
With specific reference to 2014, we recorded substantial fair value gains on our warrant liability, resulting from the significant reduction in our share price following our announcement, in November, that the FDA had issued a complete response letter ("CRL") in connection with our new drug application ("NDA") for Macrilen™. The lower closing price of our shares following our announcement of the CRL has resulted in a lower Black-Scholes valuation of our outstanding share purchase warrants during the fourth quarter of 2014.
In 2015, the change in fair value of warrant liability was significantly impacted by the issuance of the Series B Warrants. More than 97% of the Series B Warrants were exercised before the end of the year.
Net (loss) income for the three-month period and the year ended December 31, 2015was ($10.0) million and ($50.1) million, or ($1.46) and ($(18.14) per basic and diluted share, respectively, compared to $4.2 million and ($16.6) million, or $6.35 and ($28.06) per basic and diluted share for the same periods in 2014.
The increase in our net loss from continuing operations for the three-month period and for the year ended December 31, 2015, as compared to the same period in 2014, is due to the higher comparative G&A and selling expenses and net finance costs, partly offset by lower comparative R&D costs, as presented above.
2014 compared to 2013
Revenues
Revenues recorded during the year ended December 31, 2013 resulted predominantly from the non-recurring, accelerated recognition of remaining unamortized deferred revenue associated with an upfront payment received from a licensee following the termination of related R&D activities.
Operating Expenses
R&D costs were $23.7 million for the year ended December 31, 2014, compared to $21.3 million for the same period in 2013.
The increase for the year ended December 31, 2014, as compared to the same period in 2013, is attributable to higher comparative employee compensation and benefits costs, which in turn are mainly due to the recording of R&D restructuring costs. Following the approval of our aforementioned Resource Optimization Program, we recorded a provision for restructuring costs, amounting to approximately $2.5 million, for severance payments, onerous lease provisions and other directly related costs associated with the Resource Optimization Program. This increase was partly offset by lower comparative salaries and short-term employee benefits and share-based compensation costs.
A substantial portion of the increase in 2013-to-2014 third-party R&D costs relates to development initiatives associated with Zoptrex™, and in particular with our Phase 3 ZoptEC trial initiated in 2013 with Ergomed. This increase was partially offset by the lower comparative development costs associated with most of our other product candidates.
General and administrative ("G&A") expenses were $9.8 million for the year ended December 31, 2014, compared to $11.1 million for the same period in 2013.
For the year ended December 31, 2014, the decrease in G&A expenses, as compared to the same period in 2013, is mainly related to recognition in the second quarter of 2013 of non-recurring termination benefits paid to our former Chief Executive Officer and to the recording of related non-cash based compensation costs, partially offset by the recording of restructuring costs related to administrative staff redundancies resulting from the Resource Optimization Program.
Selling Expenses were $3.9 million for the year ended December 31, 2014 compared to $1.2 million for the same period in 2013.
For the year ended December 31, 2014, the increase in selling expenses, as compared to the same period in 2013, mainly relates to the ramping up of our pre-commercialization activities and the deployment of our contracted sales force related to our co-promotion activities.
Net finance income (costs) arecomprised predominantly of the change in fair value of warrant liability and of gains and losses recorded due to changes in foreign currency exchange rates.
The change in fair value of our warrant liability results from the periodic "mark-to-market" revaluation, via the application of the Black-Scholes option pricing model, of currently outstanding share purchase warrants. The Black-Scholes "mark-to-market" warrant valuation most notably has been impacted by the issuance of 8.8 million additional share purchase warrants and by the closing price of our common shares, which, on the NASDAQ, fluctuated from $52.00$0.84 to $150.00 during the year ended December 31, 2014 and from $103.00 to $323.00 for the same period in 2013.
With specific reference to 2014, we recorded substantial fair value gains on our warrant liability, resulting from the significant reduction in our share price following our announcement, in November, that the FDA had issued a CRL in connection with our NDA for Macrilen™. The lower closing price of our shares following our announcement of the CRL has resulted in a lower Black-Scholes valuation of our outstanding share purchase warrants during the fourth quarter of 2014.
Gains or losses due to changes in foreign currency exchange rates are mainly related to the US dollar, which strengthened against the EUR by approximately 12.2%,$3.65 during the twelve-month period ended December 31, 2014. During the twelve-month period ended December 31, 2013, however, the US dollar weakened against the EUR by approximately 4.5%.
Net loss from continuing operations for the year ended December 31, 2014was $(17.2) million, or $(29.12) per basic and diluted share,2017, compared to $(27.2) million, or $(92.41) per basic and diluted share for$2.67 to $4.94 during the same period in 2013.2016, also had a direct impact on the change in fair value of warrant liability.
Net loss for the three and twelve months ended December 31, 2017 was $0.5 million and $16.8 million (or $0.03 and $1.12 per share), as compared to a net loss of $8.2 million and $25.0 million (or $0.71 and $2.41 per share) for the same periods in 2016. The decrease in net loss from continuing operations for the yearthree-month period ended December 31, 2014, as compared to the same period in 2013,2017 is due largely to higher comparative net finance income, partly offset by lower comparative license fee revenues and by higher comparative net R&D costs and G&A and selling expenses, as presented above.
Discontinued Operations
Following a strategic review of our risks and prospects with respect to the manufacturing of Cetrotide® and related activities (collectively, the "Cetrotide® Business") and, in particular, having taken into account, as discussed below, the previous monetization of the corresponding royalty stream, we decided to transfer all manufacturing rights of Cetrotide® and to discontinue our involvement with the Cetrotide® Business. On April 3, 2013 (the "Cetrotide® Effective Date"), we entered into a transfer and service agreement ("TSA") and concurrent agreements with various partners and licensees with respect to our manufacturing rights for Cetrotide®, marketed for therapeutic use as part of in vitro fertilization programs. The principal effect of these agreements was to transfer, effective October 1, 2013 (the "Cetrotide®Closing Date"), our manufacturing rights for Cetrotide® to Merck Serono in all territories. Also per the TSA, we agreed to provide certain transition services to Merck Serono over a period of 36 months from the Cetrotide® Effective Date in order to assist Merck Serono in managing overall responsibility for the Cetrotide® Business.
Under the TSA, during the period commencing on the Cetrotide® Effective Date and ending on the Cetrotide® Closing Date (the "Cetrotide® Interim Period"), we were obligated to continue to conduct the Cetrotide® Business in the ordinary course in a manner consistent with past practices, subject to certain conditions. Per the TSA, we received a non-refundable, one-time payment of €2.5 million (approximately $3.3 million) in consideration for the transfer of our manufacturing rights referred to above, as well as other payments in exchange for the transfer, also on the Cetrotide® Closing Date, of certain assets, such as inventory and equipment used solely for the manufacture of Cetrotide®. We recognized the non-refundable, one-time payment on the Cetrotide® Closing Date, as we no longer had managerial involvement or effective control over the manufacturing of goods sold through the Cetrotide® Business. We provide the aforementioned transition services to Merck Serono in exchange for a monthly service fee. As a result of the transfer of substantially allreduction in third party R&D costs. The reduction is attributed to closing out the Zoptrex study and successful completion in the U.S. of the risks and rewards associated with the CetrotideMacrilen™ (macimorelin) filing.
® Business on the Cetrotide® Closing Date, the Cetrotide® Business has been classified as a discontinued operation in the consolidatedSelected quarterly financial statements. As such, relevant amounts in our consolidated statements of comprehensive (loss) income have been retroactively reclassified to reflect the Cetrotidedata® Business as a discontinued operation. |
| | | | | | | | | | | | |
| (in thousands, except for per share data) | | Three months ended |
| | | December 31, 2018 | | September 30, 2018 | | June 30, 2018 | | March 31, 2018 |
| | | $ | | $ | | $ | | $ |
| Revenues | | 1,392 |
| | 663 |
| | 168 |
| | 24,658 |
|
| Net income (loss) | | (5,126 | ) | | (2,509 | ) | | (2,602 | ) | | 14,424 |
|
| Net income (loss) per share [basic] | | (0.31 | ) | | (0.15 | ) | | (0.16 | ) | | 0.88 |
|
| Net income (loss) per share [diluted] | | (0.31 | ) | | (0.15 | ) | | (0.16 | ) | | 0.87 |
|
|
| | | | | | | | | | | | | | | |
| | | Three-month periods ended December 31, | | Years ended December 31, |
| (in thousands) | | 2015 | | 2014 | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ | | $ | | $ |
| Revenues | | | | | | | | | | |
| Sales and royalties | | — |
| | — |
| | — |
| | — |
| | 63,755 |
|
| License fees and other* | | 59 |
| | 118 |
| | 331 |
| | 1,037 |
| | 4,589 |
|
| | | 59 |
| | 118 |
| | 331 |
| | 1,037 |
| | 68,344 |
|
| Operating expenses | | | | | | | | | | |
| Cost of sales | | — |
| | — |
| | — |
| | — |
| | 30,002 |
|
| Research and development costs | | 2 |
| | 8 |
| | 31 |
| | 25 |
| | 8 |
|
| General and administrative expenses | | — |
| | — |
| | — |
| | 1 |
| | 15 |
|
| Selling Expenses | | 32 |
| | (48 | ) | | 215 |
| | 388 |
| | 4,264 |
|
| | | 34 |
| | (40 | ) | | 246 |
| | 414 |
| | 34,289 |
|
| Net income from discontinued operations | | 25 |
| | 158 |
| | 85 |
| | 623 |
| | 34,055 |
|
|
| | | | | | | | | | | | |
| (in thousands, except for per share data) | | Three months ended |
| | | December 31, 2017 | | September 30, 2017 | | June 30, 2017 | | March 31, 2017 |
| | | $ | | $ | | $ | | $ |
| Revenues | | 178 |
| | 241 |
| | 243 |
| | 261 |
|
| Net loss | | (484 | ) | | (9,631 | ) | | (2,550 | ) | | (4,131 | ) |
| Net loss per share [basic and diluted] | | (0.03 | ) | | (0.61 | ) | | (0.18 | ) | | (0.31 | ) |
_________________________
| |
* | Includes the non-refundable, one-time payment made by Merck Serono in exchange for the manufacturing rights for Cetrotide®and revenues from certain transition services provided pursuant to the aforementioned agreement.
|
The decrease in sales and royalties from discontinued operations, in cost* Net loss per share is based on the weighted average number of sales from discontinued operations and in selling expenses from discontinued operationsshares outstanding during each reporting period, which may differ on a quarter-to-quarter basis. As such, the year ended December 31, 2014, as compared to the same period in 2013, reflects the fact that we recorded no sales of Cetrotide® and royalties during the year ended December 31, 2014, as compared to the corresponding period of 2013, given that the transfersum of the Cetrotidequarterly net loss per share amounts may not equal full-year net loss per share.® Business was effective on October 1, 2013.
Net (loss) income
Net (loss) income for the year ended December 31, 2014 was $(16.6) million or $(28.06) per basic and diluted share compared to $6.8 million, or $23.12 per basic and diluted share, for the same period in 2013. The decrease in net income for the year ended December 31, 2014, as compared to the same period in 2013, is due largely to higher loss from operations and to lower net income from discontinued operations, partially offset by higher comparative net finance income.
Quarterly Consolidated Results of Operations Information
|
| | | | | | | | | | | | |
| (in thousands, except for per share data) | | Three-month periods ended |
| | | December 31, 2015 | | September 30, 2015 | | June 30, 2015 | | March 31, 2015 |
| | | $ | | $ | | $ | | $ |
| Revenues | | 102 |
| | 173 |
| | 197 |
| | 73 |
|
| Loss from operations | | (9,858 | ) | | (7,501 | ) | | (7,989 | ) | | (9,536 | ) |
| Net loss from continuing operations | | (10,043 | ) | | (15,401 | ) | | (15,148 | ) | | (9,636 | ) |
| Net loss | | (10,018 | ) | | (15,290 | ) | | (15,099 | ) | | (9,736 | ) |
| Net loss per share from continuing operations (basic and diluted)* | | (1.46 | ) | | (6.71 | ) | | (13.69 | ) | | (13.45 | ) |
| Net loss per share (basic and diluted)* | | (1.46 | ) | | (6.66 | ) | | (13.65 | ) | | (13.59 | ) |
|
| | | | | | | | | | | | |
| (in thousands, except for per share data) | | Three-month periods ended |
| | | December 31, 2014 | | September 30, 2014 | | June 30, 2014 | | March 31, 2014 |
| | | | | $ | | $ | | $ |
| Revenues | | 11 |
| | — |
| | — |
| | — |
|
| Loss from operations | | (10,947 | ) | | (9,843 | ) | | (8,410 | ) | | (8,195 | ) |
| Net income (loss) from continuing operations | | 3,995 |
| | (11,629 | ) | | (5,249 | ) | | (4,304 | ) |
| Net income (loss) | | 4,153 |
| | (11,337 | ) | | (5,024 | ) | | (4,356 | ) |
| Net income (loss) per share from continuing operations (basic and diluted)* | | 6.11 |
| | (19.66 | ) | | (9.29 | ) | | (7.84 | ) |
| Net income (loss) per share (basic and diluted)* | | 6.35 |
| | (19.16 | ) | | (8.89 | ) | | (7.93 | ) |
_________________________
| |
* | Net income (loss) per share is based on the weighted average number of shares outstanding during each reporting period, which may differ on a quarter-to-quarter basis. As such, the sum of the quarterly net income (loss) per share amounts may not equal year-to-date net (loss) income per share. |
Historical quarterly results of operations and net income (loss) from continuing operationsloss cannot be taken as reflective of recurring revenue or expenditure patterns or of predictable trends, largely given the non-recurring nature of certain components of our historical revenues, due most notably to the accelerated recognition of upfront payments and to unpredictable quarterly variations attributable to our net finance income, (costs), which in turn are comprised mainly of the impact of the periodic "mark-to-market" revaluation of our warrant liability and of foreign exchange gains and losses. Additionally, our net R&D costs have historically have varied on a quarter-over-quarter basis due to the ramping up or winding down of potential product candidate activities, which in turn are dependent upon a number ofmany factors that often do not occur on a linear or predictable basis.
Our selling expenses have increasedbeen consistent but can also vary on a quarter-over-quarter basis due to the ramping up of pre-commercialization activities associated with Macrilen™ (prior to the receipt in November 2014 of the CRL from the FDA) and to the deployment of our contracted sales force and managerial staff related to our co-promotion and other commercial activities.
In addition to the items referred to above, our net income (loss) also has been impacted by net variations attributable to the Cetrotide® Business, which, as discussed above, has been presented on a retrospective basis within discontinued operations.(macimorelin).
Condensed Consolidated Statement of Financial Position Information
| | | | | As at December 31, | | December 31, |
| (in thousands) | | 2015 | | 2014 | | 2018 | | 2017 |
| | | $ | | $ | | $ | | $ |
Cash and cash equivalents1 | | 41,450 |
| | 34,931 |
| |
| Cash and cash equivalents | | | 14,512 |
| | 7,780 |
|
| Trade and other receivables and other current assets | | 944 |
| | 1,286 |
| | 1,504 |
| | 1,047 |
|
| Restricted cash equivalents | | 255 |
| | 760 |
| | 418 |
| | 381 |
|
| Inventory | | | 240 |
| | 554 |
|
| Property, plant and equipment | | 256 |
| | 797 |
| | 65 |
| | 101 |
|
| Deferred tax assets | | | — |
| | 3,479 |
|
| Other non-current assets | | 8,593 |
| | 9,661 |
| | 8,272 |
| | 8,853 |
|
| Total assets | | 51,498 |
| | 47,435 |
| | 25,011 |
| | 22,195 |
|
Payables and other current liabilities2 | | 4,770 |
| | 7,304 |
| |
| Payables and accrued liabilities and income taxes payable | | | 4,635 |
| | 2,814 |
|
| Current portion of provision for restructuring and other costs | | | 887 |
| | 2,469 |
|
| Current portion of deferred revenues | | 244 |
| | 270 |
| | 74 |
| | 486 |
|
| Warrant liability (current and non-current portions) | | 10,891 |
| | 8,225 |
| |
Non-financial non-current liabilities3 | | 13,978 |
| | 17,152 |
| |
| Warrant liability | | | 3,634 |
| | 3,897 |
|
Non-financial non-current liabilities (1) | | | 13,874 |
| | 15,312 |
|
| Total liabilities | | 29,883 |
| | 32,951 |
| | 23,104 |
| | 24,978 |
|
| Shareholders' equity | | 21,615 |
| | 14,484 |
| |
| Shareholders' equity (deficiency) | | | 1,907 |
| | (2,783 | ) |
| Total liabilities and shareholders' equity | | 51,498 |
| | 47,435 |
| | 25,011 |
| | 22,195 |
|
_________________________
1 Of which approximately $1.5 million was denominated in EUR as of December 31, 2015 ($3.6 million as of December 31, 2014).
| |
2
1. | Of which approximately $0.6 million is related to a provisionComprised mainly of employee future benefits, provisions for restructuring and other costs asand non-current portion of December 31, 2015 ($1.5 million as of December 31, 2014).deferred revenues. |
3 Comprised mainly of employee future benefits, provisions for onerous contracts and non-current portion of deferred revenues.
The increase in cash and cash equivalents as at December 31, 2015,2018, as compared to December 31, 2014,2017, is due to the receipt of aggregate net proceeds of $49.4$24.0 million in connection withas the March 2015 Offering andpayment from the December 2015 Offering, as well asexecution of the proceeds from the disposal of property, plantLicense and equipment and the decrease in restricted cash equivalents, both of which were related to our Resource Optimization Program. ThisAssignment Agreement.
The increase was partially offset by the variations in components of our working capital and to net cash used in operating activities, as well as to the effect of exchange rate fluctuations. We also paid $8.6 million in connection with warrant amendment agreements and a warrant exercise inducement fee, as discussed above.
The decrease in trade and other receivables and other current assets as at December 31, 2015, as compared to December 31, 2014, is mainly due to lower accounts receivable related to Canadian sites for our ZoptEC trial.
The decrease in other non-current assets, which consist mainly of goodwill, as at December 31, 2015, as compared to December 31, 2014, is primarily due to the lower comparative exchange rate of the EUR against the US dollar, which weakened from December 31, 2014 to December 31, 2015.
The decrease in payables and other current liabilities is mainly attributable to taxes payable owing for the payments received from our MacrilenTM(macemorelin) licensee in 2018.
The decrease in non-financial non-current liabilities from December 31, 2017 to December 31, 2018 is primarily due to the decline in future obligations with employee future benefits and our restructuring and other costs.
The improvement in shareholders' equity (deficiency) as at December 31, 2015, 2018,as compared to December 31, 2014,2017, is due to the recording of a provision for restructuring costs related to the Resource Optimization Program in Q3-2014, discussed above.
Our warrant liability increased from December 31, 2014 to December 31, 2015. The increase is due to net fair value revaluation losses of $11.0 million, which were recorded pursuant to our periodic "mark-to-market" revaluation of the underlying outstanding share purchase warrants, as discussed above and by the issuance of 3.1 million additional share purchase warrants in connection with the March and December 2015 Offerings, which initially had increased our warrant liability by $28.7 million. Those increases were partly offset by the derecognition of part of the warrant liability due to early expiry as well as to the exercise of warrants for a total of $37.0 million.
Non-financial non-current liabilities decreased largely as a result of a change in discount rate underlying the calculation of the employee future benefit obligation.
The decrease in shareholders' equity as at December 31, 2015, as compared to December 31, 2014, is mainly attributable to the increasenet income of $4.2 million earned in our deficit due to2018 as compared with the recording of net loss partly offset by the increaseof $(16.8) million in our share capital following the issuance of common shares and warrants discussed above.2017.
Outstanding Share Data
As atMarch 29, 2016,2019 we had9,928,697 common shares 16,440,760,Common Shares issued and outstanding, as well as 275,041888,816 US dollar-denominated awards (including deferred share units and stock options) and 869 Canadian dollar-denominated stock options, outstanding. WarrantsShare purchase warrants outstanding as at March 29, 2016 2019represented a total of 2,842,3093,391,844 equivalent common shares (excluding any exercises of Series B Warrants under the alternate cashless exercise feature of such warrants).shares.
Recent Accounting Pronouncements
Not yet adopted
Annual improvementsThe IASB continues to IFRS (2012-2014) cycle: On September 25, 2014issue new and revised IFRS. A listing of the IASB issued narrow-scope amendments to a total of four standards as part of its annual improvements process. The amendments will apply for annual periods beginning on or after January 1, 2016. Amendments were made to clarify the following in their respective standards:
Changes in method for disposal under IFRS 5, Non-current Assets Held for Sale and Discontinued Operations ("IFRS 5");
Continuing involvement for servicing contracts and offsetting disclosures in condensed interim financial statements under IFRS 7, Financial Instruments: Disclosures (“IFRS 7”);
Discount rate in a regional market sharing the same currency under International Accounting Standard ("IAS") 19, Employee Benefits;
Disclosure of information "elsewhere in the interim financial reports" under IAS 34, Interim Financial Reporting;
We are currently assessing the impact that these amendments may have on our consolidated financial statements.
The final version of IFRS 9, Financial Instruments ("IFRS 9"), was issuedrecent accounting pronouncements promulgated by the IASB in July 2014 and will replace IAS 39, Financial Instruments: Recognition and Measurement ("IAS 39"). IFRS 9 introduces a model for classification and measurement, a single, forward-looking expected loss impairment model and a substantially reformed approach to hedge accounting. The new single, principle-based approach for determining the classification of financial assets is driven by cash flow characteristics and the business model in which an asset is held. The new model also results in a single impairment model being applied to all financial instruments, which will require more timely recognition of expected credit losses. It also includes changes in respect of an entity's own credit risk in measuring liabilities elected to be measured at fair value, so that gains causednot yet adopted by the deterioration of an entity's own credit risk on such liabilities are no longer recognizedCompany is included in profit or loss. IFRS 9, which isnote 5 to be applied retrospectively, is effective for annual periods beginning on or after January 1,the Company's December 31, 2018 and is available for early adoption. In addition, an entity's own credit risk changes can be applied early in isolation without otherwise changing the accounting for financial instruments. In addition, there are amendments to IFRS 7 which require additional disclosures on transition from IAS 39 to IFRS 9. These amendments are effective upon adoption of IFRS 9. We are currently assessing the impact, if any, that these new standards will have on our consolidated financial statements.
In May 2014, the IASB issued IFRS 15, Revenue from Contracts with Customers ("IFRS 15"). The objectivestatements which are included in Item 18 of this new standard is to provide a single, comprehensive revenue recognition framework for all contracts with customers to improve comparability of financial statements of companies globally. This new standard contains principles that an entity will apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity will recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to receive in exchange for those goods or services. This new standard is effective for annual periods beginningAnnual Report on or after January 1, 2018, with early adoption permitted. We are currently assessing the impact that this new standard may have on our consolidated financial statements.Form 20-F.
In January 2016, the IASB issued IFRS 16, Leases ("IFRS 16"), which supersedes IAS 17, Leases, and the related interpretations on leases: IFRIC 4, Determining Whether an Arrangement Contains a Lease; Standard Interpretations Committee ("SIC") 15, Operating Leases - Incentives; and SIC 27, Evaluating the Substance of Transactions in the Legal Form of a Lease. IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with earlier adoption permitted for companies that also apply IFRS 15. We are currently assessing the impact that this new standard may have on our consolidated financial statements.
| |
| B. | Liquidity, Cash Flows and Capital Resources |
B. Liquidity and capital resources
Our operations and capital expenditures have been generally been financed through certain transactions impacting our cash flows from operating activities, public equity offerings as well as from the drawdownsand issuances under various ATM programs. In 2018, we are investing the $24.0 million up front payment received from Strongbridge to fund operations and capital expenditures.
Based on our assessment, which took into account current cash levels, as well as our strategic plan and corresponding budgets and forecasts, we believe thatSince inception, we have incurred significant expenses in its efforts to develop and commercialize products. Consequently, we have incurred operating losses and negative cash flow from operations historically and in each of the last several years except for the year ended December 31, 2018 when the Company earned revenue from the sale of a license for the adult indication of MacrilenTM (macimorelin) in the United States and Canada. As at December 31, 2018, we had an accumulated deficit of $310 million.
We have $14,512 of cash and cash equivalents as at December 31, 2018, and management believes it has sufficient liquidity to meet its current obligations and financial resources to fundcontinue its planned expenditures and other working
capital needslevel of expenses for at least, but not limited to the 12-month period followingnext twelve months after the statement of financial position date of December 31, 2015.
We may endeavor to secure additional financing, as required, through strategic alliance arrangements or through other activities, as well as via the issuance of this Annual Report on Form 20-F. We are focused on managing our operating expenses, and have the discretion to limit research and development costs, administrative expenses and capital expenditures in order to maintain our liquidity, until such time that additional sources of funding can be obtained. Our principal focus is on the licensing and development of MacrilenTM (macimorelin) and we currently do not have any other approved product.
As described above under “Special Committee,” our current operating budget and cash flows from operating activities in 2019 are expected to decline compared with 2018, however, we believe we will experience an increase in our royalty income, which, when combined with our forecasted cash flows, will provide sufficient liquidity to finance operations and meet our commitments for at least, but not limited to, twelve months from the date of issuance of this Annual Report on Form 20-F.
License and Assignment Agreement
On January 16, 2018, the Company entered into the License and Assignment Arrangement.
(i) Adult Indication
Under the terms of the license agreement, and for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on annual net sales up to $75.0 million and an 18% royalty on annual net sales above $75.0 million. Following the end of patent protection in United States or Canada for Macrilen™ (macimorelin), the Company will be entitled to a 5% royalty on net sales in that country. In addition, the Company will also receive one-time payments ranging from $4.0 million to $100.0 million upon the achievement of commercial milestones going from $25.0 million annual net sales up to $500.0 million annual net sales.
(ii) Pediatric Indication
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment of $5.0 million. This amount will be recognized once it is probable that it will be received.
(iii) PIP study
We have initiated an open label, single dose trial to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of macimorelin in pediatric patients from two to less than 18 years of age with suspected growth hormone deficiency ("GHD"). Under the terms of the License and Assignment Agreement, the licensee will pay 70% and the Company will pay the remaining 30% of the research and development costs associate with the PIP. During 2018, the Company invoiced Strongbridge $358,000 as its share of the costs incurred by the Company under the PIP; such amounts have been collected in full.
(iv) Interim Supply Arrangement
The Company has agreed under the contract to supply ingredients for the manufacture of Macrilen™ (macimorelin) during an interim period at a price that is set ‘at cost’, without any profit margin. The Company believes the stand-alone selling price of the manufacturing ingredients to be their cost, as that approximates the amount at which Strongbridge would be able to procure those same goods with other suppliers. During 2018, the Company invoiced $2,108,000 and has received payment in full of these invoices under the Interim Supply Arrangement.
Novo purchase of Strongbridge License Agreement
Effective December 19, 2018, Strongbridge sold the United States and Canadian rights to Macrilen™ (macimorelin) to Novo and Novo will fund Strongbridge's Macrilen™ (macimorelin) contract field force to promote the product in the United States for up to three years.
Public Offerings
On April 1, 2016, we entered into an ATM sales agreement under which we are able, at our discretion and from time to time, to sell up to 3 million of our common shares through ATM issuances on the NASDAQ for aggregate gross proceeds of up to approximately $10 million (the "April 2016 ATM Program"). The ATM program provides that common shares are to be sold at market prices prevailing at the time of sale and, as a result, prices may vary. During the year ended December 31, 2017, the Company issued an additional 555,068 common shares under the April 2016 ATM Program at an average price of approximately $3.20 per share for gross proceeds of $1.8 million. The shelf registration statement pursuant to which this program was established expired on March 28, 2017.
On March 28, 2017, we commenced a new ATM offering pursuant to its existing ATM Sales Agreement, dated April 1, 2016, under which we were able, at our discretion, from time to time, to sell up to a maximum of 3 million common shares through ATM issuances on the NASDAQ, up to an aggregate amount of $9.0 million (the "March 2017 ATM Program"). The common shares were to be sold at market prices prevailing at the time of the sale of the common shares and, as a result, sale prices varied.
Between March 28, 2017 and April 18, 2017, we issued a total of 597,994 common shares under the March 2017 ATM Program at an average issuance price of $2.97 per share capitalfor aggregate gross proceeds of $1.8 million less cash transaction costs of $55,000 and previously deferred financing costs of $65,000.
On April 27, 2017, we entered into a new ATM Sales Agreement (the "New ATM Sales Agreement"), and filed with the SEC a prospectus supplement (the "Prospectus Supplement") related to sales and distributions of up to a maximum of 2,240,000 common shares through ATM issuances on the NASDAQ, up to an aggregate amount of $6.9 million under the New ATM Sales Agreement. The common shares will be sold at market prices prevailing at the time of the sale of the common shares and, as a result, prices may vary. The New ATM Sales Agreement and the Prospectus Supplement superseded and replaced the March 2017 ATM Program, which itself had superseded and replaced the April 2016 ATM Program. The Prospectus Supplement supplements the base prospectus included in our Shelf Registration Statement on Form F-3, as amended (the "2017 Shelf Registration Statement"), which was declared effective by the SEC on April 27, 2017. The 2017 Shelf Registration Statement allows us to offer up to $50 million of common shares and is effective for a three-year period. Between May 30, 2017 and December 31, 2017, we issued 1.8 million common shares at an average issuance price of $1.71 per share under the New ATM Sales Agreement.
On November 1, 2016, we completed a registered direct offering of 2,100,000 units (the "Units"), with each Unit consisting of one common share or other securities.one pre-funded warrant to purchase one common share and 0.45 of a warrant to purchase one common share (the "November 2016 Offering"). Total gross cash proceeds raised through the November 2016 Offering amounted to $7.6 million, less cash transaction costs of $1.0 million, including the placement agent's fee and expenses. The warrants are exercisable six months after their date of issuance and for a period of three years thereafter at an exercise price of $4.70 per share. The warrants contain a call provision which provides that, in the event our common shares trade at or above $10.00 on the principal trading market of our common shares during a specified measurement period and subject to a minimum volume of trading during such measurement period, then, subject to certain conditions, we have the right to call for cancellation all or any portion of the warrants which are not exercised by holders within 10 trading days following receipt of a call notice from us. Upon complete exercise for cash, these warrants would result in the issuance of an aggregate of 945,000 common shares that would generate additional proceeds of approximately $4.4 million, although these warrants may be exercised on a "net" or "cashless" basis.
The variations in our cash and cash equivalentsliquidity by activity are explained below.
| | | (in thousands) | | Three-month periods ended December 31, | | Years ended December 31, | | Three months ended December 31, | | Years ended December 31, |
| | | 2015 | | 2014 | | 2015 | | 2014 | | 2013 | | 2018 | | 2017 | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ |
| Cash and cash equivalents - Beginning of period | | 38,345 |
| | 41,952 |
| | 34,931 |
| | 43,202 |
| | 39,521 |
| | 16,800 |
| | 12,173 |
| | 7,780 |
| | 21,999 |
| | 41,450 |
|
| Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | |
| Cash used in operating activities from continuing operations | | (8,419 | ) | | (8,676 | ) | | (33,929 | ) | | (30,787 | ) | | (30,131 | ) | |
| Cash provided by (used in) operating activities from discontinued operations | | 25 |
| | 93 |
| | 85 |
| | (295 | ) | | 10,147 |
| |
| Net cash used in operating activities | | | (2,679 | ) | | (4,527 | ) | | 6,825 |
| | (22,913 | ) | | (29,010 | ) |
| | | (8,394 | ) | | (8,583 | ) | | (33,844 | ) | | (31,082 | ) | | (19,984 | ) | | (2,679 | ) | | (4,527 | ) | | 6,825 |
| | (22,913 | ) | | (29,010 | ) |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | |
| Net proceeds from issuance of common shares and warrants | | 14,987 |
| | 2,075 |
| | 49,427 |
| | 24,358 |
| | 23,708 |
| |
| Payment pursuant to warrant amendment agreements and Series B Warrant exercise inducement fee | | (2,926 | ) | | — |
| | (8,629 | ) | | — |
| | — |
| |
| | | 12,061 |
| | 2,075 |
| | 40,798 |
| | 24,358 |
| | 23,708 |
| |
| Net proceeds from issuance of common shares | | | — |
| | — |
| | — |
| | 7,788 |
| | 9,924 |
|
| Proceeds from warrants exercised (note 19) | | | — |
| | — |
| | — |
| | 242 |
| | — |
|
| | | | | | | | | | | | | — |
| | — |
| | — |
| | 8,030 |
| | 9,924 |
|
| Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | |
| Net cash (used in) provided by investing activities from continuing operations | | (6 | ) | | (4 | ) | | 913 |
| | (61 | ) | | (85 | ) | |
| Net cash provided by investing activities from discontinued operations | | — |
| | — |
| | — |
| | — |
| | 113 |
| |
| Net cash provided by (used in) investing activities | | | 4 |
| | 140 |
| | (35 | ) | | 307 |
| | (314 | ) |
| | | (6 | ) | | (4 | ) | | 913 |
| | (61 | ) | | 28 |
| | 4 |
| | 140 |
| | (35 | ) | | 307 |
| | (314 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | (556 | ) | | (509 | ) | | (1,348 | ) | | (1,486 | ) | | (71 | ) | | 387 |
| | (6 | ) | | (58 | ) | | 357 |
| | (51 | ) |
| Cash and cash equivalents - End of period | | 41,450 |
| | 34,931 |
| | 41,450 |
| | 34,931 |
| | 43,202 |
| | 14,512 |
| | 7,780 |
| | 14,512 |
| | 7,780 |
| | 21,999 |
|
Operating Activities
20152018 compared to 20142017
Cash flowsprovided by operating activities totaled $6.8 million for year ended December 31, 2018, as compared to $22.9 million used by operating activities in the same period in 2017, which is a net provision of cash from operating activities of $29.7 million. This increase is primarily due to the $24.0 million license payment received from Strongbridge in January 2018.
2017 compared to 2016
Cash used in operating activities were $8.4 totaled $4.5 million and $33.8$22.9 million for the three-month periodthree and the yeartwelve months ended December 31, 2015, respectively,2017 as compared to $8.6$8.1 million and $31.1$29.0 million for the same periods in 2014.2016. The increasedecrease in cash used in operating activities for the yeartwelve months ended December 31, 2015,2017, as compared to the same periodperiods in 2014,2016, is mainly due to higher trade accounts payable settlements and higher payments in connection with the aforementioned restructuring programs.lower operating expenses.
Financing Activities
We expect net cash used in operating2018 compared to 2017
Cash flows from financing activities to range from $30 million to $32 millionwere nil for the year ended December 31, 2016, mainly2018, as we continue to invest in our Zoptrex™ and Macrilen™ Phase 3 programs and related sub-studies and as we generate higher revenues in connection with the promotion of Estrogel®, Saizen® and APIFINY®. This guidance may vary significantly in future periods, most notably as we monitor our progress with regard to our co-promotion activities and in light of ongoing business development initiatives, as discussed further below.
2014 compared to 2013
Cash flows used in operating activities were $31.1$8.0 million and $20.0 million for the years ended December 31, 2014 and 2013, respectively. The significant increase in cash used in operating activities for the year ended December 31, 2014 as compared to the same period in 2013 is mainly due to2017. During 2018, we have focused on commercializing Macrilen™ (macimorelin) though the variations associated with our discontinued operations, following the transferapplication of the Cetrotide® Business$24.0 milestone payment from Strongbridge to our operating costs and working capital needs. This is a change from the same period in the fourth quarter of 2013, as discussed above.
Financing Activities2017 when we raised capital from certain At-The-Market programs.
20152017 compared to 20142016
Cash flows provided byfrom financing activities were $12.1totaled $0.0 million and $40.8$8.0 million for the three-month periodthree and the yeartwelve months ended December 31, 2015, respectively,2017, as compared to $2.1$9.4 million and $24.449.9 million for the same periods in 2014.2016. The increase for the three-month period and year ended December 31, 2015, as compared to the same period in 2014decrease is mainly due to higher net proceeds received from the issuanceNovember 2016 Offering.
Investing Activities
2018 compared to 2017
Cash flows from investing activities totaled $0.0 million for the year ended December 31, 2018, as compared with $0.3 million for the same period in 2017. We have reduced our investment in non-current assets over the last number of common sharesyears.
2017 compared to 2016
Cash (used in) provided by investing activities totaled $0.1 million and warrants.$0.3 million for the three and twelve months ended December 31, 2017, as compared to $0.0 million and ($0.3) million for the same periods in 2016.
Critical Accounting Policies, Estimates and Judgments
Our consolidated financial statements as at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 have been prepared in accordance with IFRS as issued by the IASB.
The preparation of consolidated financial statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of our assets, liabilities, revenues, expenses and related disclosures. Judgments, estimates and assumptions are based on historical experience, expectations, current trends and other factors that management believes to be relevant at the time at whichwhen our consolidated financial statements are prepared.
Management reviews, on a regular basis, the Company's accounting policies, assumptions, estimates and judgments in order to ensure that the consolidated financial statements are presented fairly and in accordance with IFRS. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
A summary of those criticalCritical accounting estimates and assumptions, as well as critical judgments used in applying accounting policies in the preparation of our interim condensed consolidated financial statements can be found in note 3were the same as those that applied to our annual consolidated financial statements as atof December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 2013.2016.
Capital Disclosures
Our objective in managing capital, consisting of shareholders' equity, with cash and cash equivalents and restricted cash equivalents being its primary components, is to ensure sufficient liquidity to fund R&D costs, selling expenses, general and administrativeG&A expenses, working capital and capital expenditures.
Over the past several years, we have increasingly raised capital via public equity offerings and drawdowns and issuances under various ATM sales programs as our primary source of liquidity. In 2018, we invested the $24.0 million up front payment received from Strongbridge to fund operations and capital expenditures.
Our capital management objective remains the same as that in previous periods. The policy on dividends is to retain cash to keep funds available to finance the activities required to advance our product development portfolio and to pursue appropriate commercial opportunities as they may arise. We are not subject to any capital requirements imposed by any regulators or by any other external source.
| |
| C. | Research and development, patents and licenses, etc. |
C. Research and development, patents and licenses, etc.
For a description of our R&D policies for the last three years, see "Item 4B.4.B. Business Overview" and "Recent"Key Developments" at the beginning of this Item 5.
D. Trend Information
Outlook for Over the past three years, our research and development activities have encompassed a 2016
Clinical Activities
Zoptrex confirmatory Phase 3 clinical trial of MacrilenTM
With the recent DSMB recommendation that the pivotal(macimorelin), a 2017 unsuccessful Phase 3 ZoptECclinical study in women with advanced, recurrent, or metastatic endometrial cancer continue as planned, we are expanding our commercialization planning forof ZoptrexTM. Our commercialization efforts will focus on the development of a scientific platform, the identification of key opinion leaders (zoptarelin doxorubicin) and the expansion2018 initiation of market research initiatives. We expect to complete the ZoptEC trial during the third quarter of 2016 and, if the resultspediatric indication study for MacrilenTM(macimorelin) for which our licensee is paying 70% of the trial warrant doing so,costs. You can also find relevant information in our consolidated financial statements in Item 18.
Outlook for 2019
By the end of December 2018, the License and Assignment Agreement for the U.S. and Canadian rights to fileMacrilen™ (macimorelin) had been purchased by Novo Nordisk from Strongbridge. In January 2019, the NDAJSC met to discuss Novo's commercialization plan for Zoptrex™ in 2017, looking toward commercial launchthe United States, their supply chain needs and the status of the product in 2018, assuming positive Phase 3 resultsPIP study. Quarterly meetings will continue as forecasts for sales, inventory build and purchases as well as clinical trial needs continue to occur.
The January 2019 announcement of marketing authorization for macimorelin for the diagnosis of AGHD by the EMA has further validated the clinical profile and commercial value of macimorelin.
On March 12, 2019, we announced that our NDAboard of directors formed the Special Committee to review our strategic options. The Special Committee has approved the engagement of Torreya, a global investment bank specializing in life sciences, as its financial advisor. Torreya is granted.
Macrilen™
We will focus on patient recruitmentworking with management to assist the Special Committee and the board of directors in considering a wide range of transactions (including opportunities for the confirmatory Phase 3 trial in AGHD. We also initiatedlicense of macimorelin outside of the QT study. We currently estimate thatUnited States and Canada, other monetization transactions relating to macimorelin or the trials will be completed in Q3potential sale of 2016, with a combined expected expenditurethe Company).
Our priority is the commercialization of approximately $5 million over the remaining trial period. This would permit us to submit a NDA by mid-year 2017. If the study is successful in meeting its primary endpoint,macimorelin; however, we anticipate FDA approval of Macrilen™ by year-end 2017.
Commercial Operations
EstroGel®
Our promotional efforts in support of EstroGel® continue to demonstrate positive promotional response, and ongoing activities bypursue out-licensing opportunities of our contract sales force are expected to continue to result in exceeding pre-established baseline thresholds for unit sales in our US territories. We expect steady incremental growth of EstroGel prescriptions by competitively targeting high-volume transdermal prescriber's, expanding our total prescriber base and increasing usage with our current high prescriber's. For the remainder of 2016, we anticipate continued year-over-year growth of new and total prescriptions.
non-strategic assets, as they arise.
Saizen®
During the third quarter of 2015, we initiated promotional efforts in support of Saizen®. We recognize the value of direct promotion in the category of growth hormone treatments, and our objective is to exceed pre-established baselines on a total nation basis by significantly increasing the share-of-voice in support of this product in territories not previously covered by EMD Serono. There are now 21 representatives actively promoting Saizen® in conjunction with our promotion of EstroGel®.
APIFINY®
During the fourth quarter of 2015, we signed a co-marketing agreement with Armune. We already started promotional efforts using our existing contracted sales force, and we expect to commence generating commission revenues in the first quarter of 2016.
Summary of key expectations for revenues and operating expenditures and cash flows
As noted above, we expect to continue to record commissions revenue in connection with our co-promotion agreement for EstroGel® and to begin to record commissions revenues in relation to our promotional services agreement for Saizen® and with our co-marketing agreement with Armune. As for license fee revenues, we will continue to recognize the amortization of deferred revenues related to the agreements we entered into with Sinopharm in 2014, as mentioned above.
As noted above, our main focusThe following represents forward-looking information and users are cautioned that actual results may vary.
The January 2018 licensing of Macrilen™ (macimorelin) for R&D efforts will be on ZoptrexTM, withits commercialization in the ongoing pivotal Phase 3 ZoptEC clinical trial, as well as on Macrilen™ withUnited States and Canada was a significant turning point for the initiated confirmatory Phase 3 clinical trialCompany and the QT study, where we continue to anticipate substantial investment to fund ongoingfurther development initiatives. More specifically, we currently estimateand commercialization of Macrilen™ (macimorelin) in 2019 is the Company's primary focus.
To that we will incur approximately $11 million pursuant to our agreements with Ergomed over the next 12 months as we complete our QT study and our confirmatory Phase 3 clinical trial for Macrilen™ and as we proceed with and complete our ZoptEC trial.
As discussed above, excluding the impact of foreign exchange rate fluctuations,end, we expect that weresearch and development costs will incur R&D costs of between $19 million and $20be up to $2.0 million for the year endedending December 31, 2016.2019 and will comprise commercial service, consultant, employee and patent costs related to the PIP study and to follow-up studies agreed with the EMA. In the third quarter of 2018, we began invoicing our licensee for its 70% share of the PIP study costs. In 2019, we will continue this collaboration and will work with Novo to optimize this trial.
WeIn addition, we expect that sellingour general and administrative expenses will slightly increaseto range between $6.5 million and $7.5 million for the year endedending December 31, 2016, as compared to the year ended December 31, 2015, mainly due to our increased promotional activities associated with Saizen® 2019 and APIFINY®.
Excluding the impactwill consist primarily of foreign exchange rate fluctuations, we expect that our G&A expenses will be lower for the year ended December 31, 2016, as compared to the year ended December 31, 2015, mainly due to the aforementioned recording of transaction costs in connection with our public offerings completed in March and December 2015 and to the recording of a provision for restructuring in connection to the closure of our Quebec City office during the fourth quarter of 2015.
Excluding any foreign exchange impacts,employee, insurance, rent, as well as income from newlegal and public company costs.
We are working with Torreya on European and the rest of the world business development initiatives, we expect that our overall useactivities to support the commercialization of cash for operations in 2016 will range from $30 million to $32 million as we continue to fund ongoing operating activitiesmacimorelin outside of Canada and working capital requirements.the United States.
The preceding summary with regard to our revenue, operating expenditure and cash flow expectations excludes any consideration of any potential strategic commercial initiatives that may be consummated in connection with our efforts to expand our commercial operations in the US or elsewhere. In addition, these expectations may be materially impacted by our expected growth in sales
commission. As such, the guidance presented in this MD&A is subject to revision based on new information that is not currently known or available.
Financial Risk Factors and Other Instruments
Fair value risk
As noted above, the change in our warrant liability, which is measured at fair value through profit or loss, results from the periodic "mark-to-market" revaluation, via the application of the intrinsic valuationThe nature and the Black-Scholes option pricing model, of currently outstanding share purchase warrants. These valuation models are impacted, among other inputs, by the market priceextent of our common shares. As a result,exposure to risks arising from financial instruments, including credit risk, liquidity risk and market risk (share price risk) and how we manage those risks are described in note 21 to the change in fair value ofCompany's annual audited consolidated financial statements as at December 31, 2018 and 2017 and for the warrant liability, which is reported as finance income (cost) in our consolidated statements of comprehensive income (loss), has been and may continue in future periods to be materially affected by changes in our common share closing price, which has ranged from $4.00 to $84.20 on the NASDAQ during the yearyears ended December 31, 2015.2018, 2017 and 2016.
If variations in the market price of our common shares of -10% and +10% were to occur, the impact on our net loss for the warrant liability held at December 31, 2015 would beThe consolidated financial statements filed as follows:
|
| | | | | | | | | |
| (in thousands) | | Carrying
amount | | -10% | | +10% |
| | | $ | | $ | | $ |
| Warrant liability | | 10,891 |
| | 1,059 |
| | (1,067 | ) |
| Total impact on net loss – decrease / (increase) | | | | 1,059 |
| | (1,067 | ) |
Liquidity risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they become due. We manage this risk through the management of our capital structure and by continuously monitoring actual and projected cash flows. Our Board of Directors reviews and approves our operating and capital budgets, as well as any material transactions out of the ordinary course of business. We have adopted an investment policy in respect of the safety and preservation of our capital to ensure our liquidity needs are met. The instruments are selected with regard to the expected timing of expenditures and prevailing interest rates.
We believe that we have sufficient funds to pay our ongoing general and administrative expenses, to pursue our R&D activities and to meet our obligations and existing commitments as they fall due at least through December 31, 2016. In making this assessment, we took into account all available information about the future, which is at least, but not limited to, twelve months from the end of the most recent reporting period. We expect to continue to incur operating losses and may require significant capital to fulfill our future obligations. Our ability to continue future operations beyond December 31, 2016 and to fund our activities is dependent on our ability to secure additional funding, which may be completed in a number of ways, including but not limited to licensing arrangements, partnerships, share and other security issuances and other financing activities. We will pursue such additional sources of financing when required, and while we have been successful in securing financing in the past, there can be no assurance we will be able to do so in the future or that these sources of funding or initiatives will be available for the Company or that they will be available on terms which are acceptable to us.
Credit risk
Credit risk is the risk of an unexpected loss if a customer or counterparty to a financial instrument fails to meet its contractual obligations. We regularly monitor credit risk exposure and take steps to mitigate the likelihoodpart of this exposure resulting in losses. Our exposure to credit risk currently relates to cash and cash equivalents, to trade and other receivables and to restricted cash equivalents. We hold our available cash in amounts thatAnnual Report on Form 20-F are readily convertible to known amounts of cash and deposit our cash balances with financial institutions that have an investment grade credit rating of at least "A" or the equivalent. This information is supplied by independent rating agencies where available and, if not available, we use publicly available financial information to ensure that we invest our cash in creditworthy and reputable financial institutions.presented under "Item 18. – Financial Statements".
| |
| E. | Off-Balance Sheet Arrangements |
As at December 31, 2015, trade accounts receivable for an amount of approximately $122,000 were with two counterparties and no trade accounts receivable were past due or impaired.
Generally, we do not require collateral or other security from customers for trade accounts receivable; however, credit is extended following an evaluation of creditworthiness. In addition, we perform ongoing credit reviews of all our customers and establish an allowance for doubtful accounts when accounts are determined to be uncollectible.
The maximum exposure to credit risk approximates the amount recognized on our condensed interim consolidated statement of financial position.
E. Off-Balance sheet arrangements
As at December 31, 2015,2018, we did not have any interests in special purpose entities or any other off-balance sheet arrangements.
| |
| F. | Tabular disclosure of contractual obligations |
F. Tabular disclosure of contractual obligations
Financial Liabilities, Obligations and Commitments
We have certain contractual lease obligation commitments as well as other long-term obligations related to unfunded pension plan benefits and unfunded post-employment benefit plans. The following tables summarize future cash requirements with respect to these obligations.
Expected future minimum lease payments, which also include future payments in connection with utility service agreements and future minimum sublease receipts under non-cancellable operating leases (subleases), as well as future payments in connection with service and manufacturing agreements, as at December 31, 20152018 are as follows:
| | | (in thousands) | | Minimum lease payments | | Minimum sublease receipts | | Service and manufacturing | | Minimum lease payments | | Minimum sublease receipts | | Service and manufacturing |
| | | $ | | $ | | $ | | $ | | $ | | $ |
| Less than 1 year | | 1,367 |
| | (385 | ) | | 639 |
| | 408 |
| | (117 | ) | | 2,180 |
|
| 1 - 3 years | | 2,394 |
| | (487 | ) | | 370 |
| | 533 |
| | (24 | ) | | — |
|
| 4 - 5 years | | 1,837 |
| | (23 | ) | | — |
| | 60 |
| | — |
| | — |
|
| More than 5 years | | 286 |
| | — |
| | — |
| | 5 |
| | — |
| | — |
|
| Total | | 5,884 |
| | (895 | ) | | 1,009 |
| | 1,006 |
| | (141 | ) | | 2,180 |
|
During the third quarter of 2015, our lease agreement in Germany for laboratory, office, and storage space was terminated, and we entered into a new lease agreement for the rental of less space on the same premises as compared to our former arrangement. The new lease expires on April 30, 2021 and is subject to renewal upon notice by us for two additional four-year periods. Under the terms of the arrangement, the minimum lease payment may be increased or decreased in accordance with the fluctuations in the German consumer price index up to 5% on a cumulative basis.
In accordance with the assumptions used in our employee future benefit obligation calculation as at December 31, 2015,2018, undiscounted benefits expected to be paid in Euros are as follows:
|
| | | |
| (in thousands) | | $Euros |
| Less than 1 year | | 453 |
|
| 1 – 3 years | | 944921 |
|
| 4 – 5 years | | 1,016944 |
|
| More than 5 years | | 17,43913,658 |
|
| Total | | 19,85215,976 |
|
| |
| Item 6. | Directors, Senior Management and Employees |
| |
| A. | Directors and senior management |
The following table sets forth information about our directors and our senior corporate officers as at March 29, 2016:2019:
|
| | |
| Name and Place of Residence | | Position with Aeterna Zentaris |
| | | |
Cardiff, MichaelAmmer, Nicola | | DirectorChief Medical Officer, Vice President Clinical Development |
Ontario, Canada | Frankfurt, Germany | |
| | | |
Dinges, JudeAuld, Leslie | | Senior Vice President, and Chief CommercialFinancial Officer |
Georgia, United States | | |
| | |
Dodd, David A. | | Chairman, President and Chief Executive Officer |
South Carolina, United States | Ontario, Canada | |
| | | |
| Egbert, Carolyn | | DirectorChair of the Board of Directors |
| Texas, United States | | |
| | | |
| Ernst, Juergen | | Lead Independent Director |
| North Rhine-Westphalia, Germany | |
| | |
| Garrison, Brian | | Senior Vice President, Global Commercial Operations |
| Pennsylvania, United States | |
| | |
| Grau, Günther | | Vice President, Finance |
| Frankfurt, Germany | |
| | | |
| Guenther, Eckhard | | Vice President, Business DevelopmentAlliance Management |
| Hessen, Germany | | |
| | |
Lapalme, Pierre | | Director |
Quebec, Canada | | |
| | |
Lemaire, Geneviève | | Vice President, Finance and Chief Accounting Officer |
Quebec, Canada | | |
| | | |
| Limoges, Gérard | | Director |
| Quebec, Canada | | |
| | | |
Newport, KenNorton, Brent | | Director |
| Ontario, Canada | | |
| | | |
Sachse, RichardPollack, Jonathan | | Senior Vice President, Chief Scientific Officer/Chief Medical OfficerDirector |
Baden-Württemberg, GermanyOntario, Canada | |
| | |
| Smith Hoke, Robin | | Director |
| Ohio, United States | |
| | | |
| Teifel, Michael | | Vice President, Pre-Clinical DevelopmentNon-Clinical Sciences |
| Hessen, Germany | | |
| | | |
Theodore, Philip A.Ward, Michael | | Senior Vice President and Chief AdministrativeExecutive Officer General Counsel and Corporate Secretary |
South Carolina,Illinois, United States | |
| | |
There are no family relationships among any of our directors or executive officers. The following is a brief biography of each of our directors and executive officers.
Michael CardiffNicola Ammer was appointed toas our Board of Directors on January 29, 2016. He was most recently Global Senior Vice President, forClinical Development and as Chief Medical Officer in January 2018. She serves as one of our executive officers. Dr. Ammer, who is based in the OfficeFrankfurt, Germany, office of our German subsidiary, began her career in the pharmaceutical medicine environment in the CRO business in 2002 and gained profound knowledge of all aspects of clinical research & development in various positions with increasing responsibility, including a Director of Clinical Operations. She joined Aeterna Zentaris GmbH in March 2015 as Clinical Program Director and took over the role of the CFO Business UnitHead of Clinical Development in January 2016. She possesses numerous skills in the area of pharmaceutical medicine and contributed significantly to the successful completion of the macimorelin clinical development program in the adult indication. Dr. Ammer obtained the license to practice medicine in 1995 after completion of her academic studies at INFOR,the University of Essen. She was awarded a $3 billion revenue software company. His business unit included software for financials, payroll, human resources, performance management, business improvement, planningdoctorate diploma in medicine by the University of Münster in 2004 and forecasting, compliance and risk management. Prior to holding that position, Mr. Cardiff held numerous senior positionsa Master of Science in a number of technology companies, including large multinationals such as EDS, SAP and IBM, as well as startup companies such as Fincentric, Convergent Technologies, Tandem, and Stratus Computer. Mr. Cardiff is currently a director of Hydrogenics Corporation (NASDAQ: HYGS; TSX: HYG), and Startech.Com. Mr. Cardiff has also served as a director of other publicly traded companies, including Husky Injection Molding, Descartes Systems Group, Visible Genetics and Burntsand Inc. He has also been a director of private companies, including Solcorp, Spectra Security Software and Visible Decisions and not-for-profot organizations such as The Toronto Film Festival, Roy Thomson Hall and Medic Alert Foundation. Mr. Cardiff is a member of, and holdsPharmaceutical Medicine by the ICD.D designation from, the Institute of Corporate Directors.University Duisburg-Essen in 2009.
Jude DingesLeslie Auld was appointed as our Senior Vice President, and Chief CommercialFinancial Officer in November 2013. He began his career nearly 30September 2018. She has over twenty-five years ago as a professional sales representativeof accounting, finance and pharmaceutical industry experience, with increasingly senior roles at Bristol LaboratoriesPricewaterhouseCoopers,
Helix BioPharma Corp., Luminex Diagnostics (formerly TM BioScience Corp.), Attwell Capital Inc. (formerly Fralex Therapeutics) and later at MerckGeneNews Limited. A Chartered Professional Accountant, Ms. Auld graduated with an Honors Bachelor of Science degree in Pharmacology & Co., where he was promoted to positions with increased responsibilities in training, sales, management, marketingToxicology from the University of Western Ontario and market development. While at Merck, Mr. Dinges won multiple awards, including the President's Achievement Award in 2001, awarded to one of 32 Business Directors each year. He received the Change Agent Award for his market development prelaunch business planning and contributions to sales force execution, while launching the blockbuster brands Cozaar®, Fosamax®, Singulair®, Maxalt®, Vioxx®, and Vytorin®. He was recognized with a Career Achievement Award for his consistent top performance as a Senior/Executive Business Director. Mr. Dinges joined Novartis Pharmaceuticals in 2006 and led his region to top performance in the launch of Tekturna® while balancing a broad antihypertensive portfolio across several Novartis divisions. His region also led the nation in market share for Exelon® and Exelon Patch®. In 2008, Mr. Dinges became the Respiratory & Infectious Disease Specialty Medicines Director. In 2009, Mr. Dinges joined Amgen Inc. as Executive Director of Region Sales, Bone Health Business Unit. Mr. Dinges led his region team to a highly successful launch of monoclonal antibody, Prolia®, across the southeastern United States and Puerto Rico.
David A. Dodd was appointed our President and Chief Executive Officer in April 2013 and then assumed the position of Chairman of the Board in May 2014. Mr. Dodd's executive management experience in the pharmaceutical and biotechnology industries spans more than 35 years. Prior to joining Aeterna Zentaris, Mr. Dodd was President and CEO of Solvay Pharmaceuticals, Inc. During his six-year tenure as President, CEO and director of Serologicals Corporation, the market value of the company increased from $85 million in June 2000 to an all-cash sale to Millipore Corporation in July 2006 for $1.5 billion. He was also President, CEO and Chairman of BioReliance Corporation, a leading provider of biological safety and related testing services. Prior to that, Mr. Dodd held various senior management positions at Wyeth-Ayerst Laboratories, the Mead Johnson Laboratories Division at Bristol-Myers Squibb, and Abbott Laboratories. Mr. Dodd holdshas a Master of ScienceBusiness Administration degree from Georgia Statethe University and he has completed the Harvard Business School Advanced Management Program.of Toronto.
Carolyn Egbert has served as a director on our Board since August 2012.2012 and as Chair of our Board since May 2016. After enjoying the private practice of law as a defense litigator in Michigan and Washington, D.C., she joined Solvay America, Inc. ("Solvay") (a chemical and pharmaceutical company) in Houston, Texas. Over the course of a twenty-year career with Solvay, she held the positions of Vice President, Human Resources, President of Solvay Management Services, Global Head of Human Resources and Senior Executive Vice President of Global Ethics and Compliance. During her tenure with Solvay, she served as a director on the Board of Directors of seven subsidiary companies and as Chair of one subsidiary company.board. After retiring in 2010, she established a consulting business providing expertise in corporate governance, ethics and compliance, organizational development, executive compensation and strategic human resources. She holds a Bachelor of Sciences degree in Biological Sciences from George Washington University, Washington D.C. and a Juris Doctor degree from Seattle University, Seattle, Washington. She also was a Ph.D. candidate in Pharmacology at both Georgetown University Medical School at Washington, D.C. and Northwestern University Medical School at Chicago, Illinois. She remains an active member of both the Michigan State Bar and the District of Columbia Bar, Washington, D.C.
Juergen Ernst has served as a director on our Board since 2005. As the former General Manager of the Pharmaceutical Sector of Solvay S.A. (international chemical and pharmaceutical group), Mr. Ernst has had extensive senior management experience, where, among other functions, he oversaw the human resources department. Mr. Ernst is also a member of the Board of Directors of Pharming Group N.V., a publicly traded biotechnology company based in the Netherlands.
Brian Garrison became our Senior Vice President, Global Commercial Operations in December 2017. For the last three years he has held the roles of National Sales Director, managing the co-promotion efforts for two endocrinology products and a urology diagnostic and as the Marketing Director for Macrilen™(macimorelin). Mr. Garrison worked at Amgen, Inc. where he held the role of Oncology Reimbursement Marketing Director. In this position, he was in charge of the Field Reimbursement Team and the Oncology Call Center for all of Amgen's oncology brands. Mr. Garrison also worked on the access strategy for several of the key oncology brands, such as Neulasta®, Neupogen®, Vectibix® and Imlygic®. Also, while at Amgen, Mr. Garrison served as a Marketing Manager in the Inflammatory Business Unit working on key access programs for Enbrel®. Prior to his work on Enbrel®, Mr. Garrison was a Sales Manager for the Bone Health Business Unit, launching the first-in-class biologic therapy for osteoporosis, Prolia®. Mr. Garrison began his career at Merck & Co. where he held various positions of increasing responsibility in sales and marketing, winning top national sales honors, both as a representative and sales manager. Mr. Garrison is a combat veteran, leading an infantry platoon with the 10th Mountain Division through combat operations in the Horn of Africa. Mr. Garrison is a graduate of the U.S. Military Academy, West Point, where he was commissioned as an Infantry officer, serving ten years active duty in the U.S. Army.
GüntherGrau was appointed as our Vice President, Finance in February 2018. Mr. Grau, has been part of the Company since 2000. He began his career in the pharmaceutical industry at ASTA Medica AG, a predecessor of our Company, in 1995, assuming roles of increasing responsibility in areas of internal and external accounting during his career. Mr. Grau obtained a diploma in Business Administration from the Philipps-University, Marburg, in 1991.
Eckhard Günther was appointed as our Vice President, Business Development in October 2014.2014 and as Vice President, Alliance Management in June 2016. He serves as one of our executive officers. From 2008 through 2014, he was our Vice President, Alliance Management and Intellectual Property and from 2006 through 2008, he was our Vice President, Head of Drug Discovery and Preclinical Development. Dr. Günther, who is based in the Frankfurt, Germany, office of our German subsidiary, began his career in the pharmaceutical industry in 1985. He joined ASTA Medica AG, a predecessor of our Company, in 1990, assuming roles of increasing responsibility in areas of medicinal
chemistry and drug discovery during his career. He possesses numerous scientific and business skills and has a long record of successful innovation and alliance building and management. Dr. Günther obtained a diploma in Chemistry from the Martin-Luther-University of Halle-Wittenberg in 1979 and was awarded his doctorate diploma in synthetic organic chemistry by the University of Halle-Wittenberg in 1985.
Pierre Lapalme has served as a director on our Board since December 2009. Mr. Lapalme has, over the course of his career, held numerous senior management positions in various global life sciences companies. He is former Senior Vice President, Sales and Marketing for Ciba-Geigy (which subsequently became Novartis) and former Chief Executive Officer and Chairman of the Board of Rhone-Poulenc Pharmaceuticals Inc. in Canada and in North America, as well as Executive Vice President and Chief Executive Officer of Rhone-Poulenc-Rorer Inc. North America (now sanofi-aventis), where he supervised the development, manufacturing and sales of prescription products in North and Central America. Mr. Lapalme served on the Board of Directors of the National Pharmaceutical Council USA and was a member of the Board of Directors of the Pharmaceutical Manufacturers Association of Canada, where he played a leading role in reinstituting patent protection for pharmaceuticals. Until recently, he was a member of the Board and Chairman of the Board of Sciele Pharma Inc., which was acquired by Shionogi and Co. Ltd. Mr. Lapalme is currently Chairman of the Board of Biomarin Pharmaceutical Inc., Chairman of the Board of Pediapharm Inc., Chairman of the Board of GlyPharma Therapeutics and a member of the Board of Directors of Algorithme Pharma Inc. and of Insy's Therapeutics Inc., a Phoenix-Arizona based specialty pharma company. He studied at the University of Western Ontario and at INSEAD, France.
Geneviève Lemaire was appointed our interim Corporate Controller in August 2015 and subsequently our Vice President, Finance and Chief Accountant Officer in February 2016. Ms. Lemaire, who is based in Quebec City, Canada is serving us on a contract basis. She has worked in various accounting and audit functions for Ernst & Young in Canada and Switzerland from 1997 until 2012 and in senior finance and accounting functions at Atrium Innovations from 2012 until 2014. Since then, Ms. Lemaire serves as an independent consultant. Ms. Lemaire is a chartered professional accountant in Canada and Certified Public Accountant, registered in the State of Illinois, and holds a Bachelor's degree in Accountancy from the University of Sherbrooke.
Gérard Limoges, C.M., FCPA, FCA has served as a director on our Board since 2004. Mr. Limoges served as the Deputy Chairman of Ernst & Young LLP Canada until his retirement in September 1999. After a career of 37 years with Ernst & Young, Mr. Limoges has been devoting his time as a director of a number of companies. Mr. Limoges began his career with Ernst & Young in Montreal in 1962. After graduating from the Management Faculty of the Université de Montréal (HEC Montréal) in 1966, he wrote the CICA exams the same year (Honors: Governor General's Gold Medal for the highest marks in Canada and Gold Medal of the Ordre des Comptables Agréés du Québec). He became a chartered accountant in 1967 and partner of Ernst & Young in 1971. After practicing as auditor since 1962 and partner since 1971, he was appointed Managing Partner of the Montreal Office in 1979 and Chairman for Quebec in 1984 when he also joined the National Executive Committee. In 1992, he was appointed
Vice Chairman of Ernst & Young Canada and the following year, Deputy Chairman of the Canadian firm. After retirement from practice at the end of September 1999, he was appointed Trustee of the School boardBoard of Greater Montreal (1999), member of the Quebec Commission on Health Care and Social Services (2000-2001) and special advisor to the Rector of the Université de Montréal and affiliate schools (2000-2003). Mr. Limoges, at the request of the Board of Directors of the Université de Montréal, participated in the selection of the Dean of the Faculty of Medicine in 2011. Mr. Limoges is also a trustee and chairman of the Audit Committee of PROREIT (TSX). He is also a board member of various private companies and charities. Mr. Limoges became an FCPA, FCA (Fellow) in 1984 and received the Order of Canada in 2002.
Dr. Brent Norton has served as a director on our Board since 2018. Dr. Norton is a business leader in the life science industry with operational and director experience across several successful enterprises which have achieved significant product sales and returns for investors. He uses his cross functional knowledge to develop strategy, raise capital and build important relationships in the academic and business community. Dr. Norton founded PreMD, completing IPO’s and listings on both the Toronto Stock Exchange and the American Stock Exchange. Operationally, he has research and development and commercial operations, led transactions with AstraZeneca, Eli Lilly, L’Oreal, Parke Davis/Pfizer, etc., and taken products through the FDA to global out-licensing with Johnson & Johnson. He is a founding Director of Novadaq Technologies (TSX:NDQ, NASDAQ:NVDQ) and was recently sold to Stryker Corporation. Dr. Norton has been an active member of several boards in Canada and the United States. He is a Venture Partner at Lumira Capital, Executive Chairman & CEO of Ortho RTI, a member of the Research Committee for CAMH, an Advisory BOD member for the Ivey International Centre for Health Innovation, a Director of Alpine Ontario and Past-President and Director of the Osler Bluff Ski Club.
Ken NewportJonathan Pollack has served as a director on our Board since 2018. Mr. Pollack is the President of The JMP Group, a private investment and consulting firm. He is also a director of several public and private companies including CECO Environmental Corp. (NASDAQ:CECE) and is an officer of AcuityAds Holdings, Inc. (TSX-V:AT). Mr. Pollack also served as a director of API Technologies Corp. (NASDAQ: ATNY), Pinetree Capital Ltd. (TSX:PNP), Hanfeng Evergreen Inc. (TSX:HF) and Lifebank Corp. (TSX-V:LBK). Previously, he served as Executive Vice President of API Technologies Corp. (NASDAQ:ATNY), a leading provider of RF/microwave, microelectronics and security technologies for critical and high-reliability applications from 2009 and as a director from 2007 until January 2011 when it was appointed tosold. From March 2005 through its sale in 2009, he served as the Chief Financial Officer and Corporate Secretary of Kaboose Inc. Prior thereto, he worked in investment banking in New York. Mr. Pollack received a Master of Science in Finance from the London School of Economics and a Bachelor of Commerce from McGill University. He sits on the boards of several philanthropic organizations including the Mt. Sinai Hospital Foundation, the Crescent School Foundation, and the Sterling Hall School Foundation.
Robin Smith Hoke has served as a director on our Board since 2018. Ms. Hoke is a business and legal executive with over 25 years of healthcare and pharmaceutical experience in various legal and business roles where she focused on operations, strategy, business development, acquisitions, strategic relationships, and commercialization. Ms. Hoke currently serves as President & CEO of Leiters, a 503B FDA registered outsourcing service provider with manufacturing facilities in Denver, Colorado and San Jose, California. She also serves as a member of the Board of Directors of Camargo Pharmaceutical Services, LLC., a privately held 505(b)(2) global drug development and regulatory services company in Cincinnati, Ohio. She previously served as a member of the board of Oncobiologics, Inc., a publicly held clinical stage biopharmaceutical company focused on January 29, 2016. He isidentifying, developing, manufacturing and commercializing complex biosimilar therapeutics. She previously served as chair of the Board of Directors and interim chief executive officer at Ricerca Biosciences, LLC, a chartered accountant, entrepreneurpre-clinical CRO. Prior to Ricerca, Ms. Hoke served as the president of GeneraMedix, Inc., a specialty generic injectable company and life-sciencesheld senior legal and business executiveroles at Cardinal Health, Inc. She also spent time with Abbott Laboratories, Inc., and served as Senior Vice-President and Executive Committee member at PRA International Inc. for three years until his retirement in 2005. He was co-founder and President of CroMedica Inc., a clinical trials contract research organization, which was sold to PRA International in 2002. Mr. Newport was also a founding member of Global Biomedical Capital Corporation, Zelos Therapeutics Inc., Prime Trials Inc. and other life sciences organizations. He has served or serves on the corporate Boards of Nordion Inc., Opmedic Group Inc., Jennerex Inc. and Medgenesis Therapeutics Inc. He sits on several non-profit boards, including his role as Chair of the BioCanRx, the National Centre of Excellence for Biotherapeutics cancer research.
Richard Sachse was appointed our Senior Vice President and Chief Scientific Officer in January 2014. In March 2014, he was also appointed Chief Medical Officer. Dr. Sachse holds a degree in medicine from the Friedrich-Alexander-University Erlangen, in Germany, and a board certification in Clinical Pharmacology. With more than 20 years’ experience as a physician and scientist, he has extensive expertise in a variety of different therapeutic areas, including endocrinology and oncology. In addition to registration studies, he is especially experiencedpartner in the design and implementationbusiness law firm of translational programs to bridge research programs to the clinic, as well as in the design and implementation of clinical pharmacology programs, including all required profiling studies and activities, enabling successful registration of products at the international level. From 1996 to 2000, he was International Project Leader at the Bayer AG Institute for Clinical Pharmacology, and Principal Investigator at the Bayer Clinical Pharmacology Unit, implementing innovative exploratory development tools, including biomarkers to demonstrate early Proof of Concept. From 2001 to 2006, Dr. Sachse held a variety of different management positions within early and late phase clinicalKegler, Brown, Hill & Ritter, Co., L.P.A.
development programs, including responsibilities for completed Phase 3 programs leading to successful NDA/MAA submissions. In 2007, after a merger, he became Senior Director, Head of Experimental Medicine, at UCB in Belgium, where he managed the implementation of novel biomarkers in clinical development to provide data supporting identification of appropriate target indication and target population. In 2010, Dr. Sachse became Vice President, Head of Global Translational Medicine at Boehringer Ingelheim.
Michael Teifel became our Vice President, Non-Clinical Sciences in October 2014. He joined our German subsidiary, which is based in Frankfurt, in 2004, where he has been involved in a number of roles focused on the design and implementation of non-clinical development programs for small molecule drugs, targeted therapies and biologics. He serves as one of our executive officers. Prior to joining us, Dr. Teifel co-founded Munich Biotech AG, which developed anti-tumor diagnostics and therapeutics, from 1998 through August 2004. Prior to founding Munich Biotech AG, Dr. Teifel was employed by Boehringer Mannheim GmbH/Roche Diagnostics GmbH where his focus was on gene therapy. He received his diploma in biology from the Technical University Darmstadt in 1992 and his doctorate from the same institution in 1996.
Philip A. TheodoreMichael Ward was appointedbecame our Senior Vice President and Chief AdministrativeExecutive Officer in July 2017. He has over thirty years of executive and General Counsellegal experience in the healthcare, pharmaceutical and technology industries. Most recently, Mr. Ward served as Chief Compliance & Legal Officer and Corporate Secretary in October 2014.for Sagent Pharmaceuticals, a global specialty generic pharmaceutical company, and led its sale to Nichi-Iko Pharmaceutical Co., Ltd. for $736 million. Mr. Ward has served as Strategic Advisor to Benevolent Capital Partners for the last five years and is an inactive Partner with Outside GC LLC. Prior to joining us, heSagent Pharmaceuticals, Mr. Ward was the Vice President, General Counsel and Corporate Secretary of Zep Inc., a consumable chemical packaged goods company based in Atlanta, Georgia, from July 2010 through September 2014; the Vice President of Corporate Development, Compliance, and Legal for BioReliance, Inc., a provider of biologics-safety-testing services based in Rockville, Maryland, from September 2008 to April 2009; the Senior Vice President andAssistant General Counsel of John H. Harland Company, a financial services company basedGlobal Compliance, Ethics & Litigation and Chief Privacy Officer at CDK Global. Mr. Ward has served in Atlanta, Georgia,several executive roles and was responsible for business development, compliance, legal and operational
matters in the healthcare, pharmaceutical and technology industries during his career. Mr. Ward graduated from September 2006Albion College and Case Western Reserve University Law School.
There are no family relationships between any of the persons named above and no arrangement with any customers, major shareholders, suppliers or others pursuant to September 2007; and the Vice President, General Counsel and Corporate Secretary of Serologicals Corporation, a life-sciences tools company based in Atlanta, Georgia, from 2004 through August 2006. Mr. Theodore also servedwhich any person above was selected as a partner in the corporate practice of King & Spalding, LLP, an Atlanta-based law firm, from 1986 through 2003.director or executive officer.
Our directors and executive officers are generally paid in their home country'scountry currency. Unless otherwise indicated, all directors' and executive's compensation information included in this document is presented in USU.S. dollars and, to the extent a director or officer has been paid in a currency other than USU.S. dollars, (i.e. Canadian dollars or euros), the amounts have been converted from such person's home country currency to USU.S. dollars based on the following annual average exchange rates: for the financial year ended December 31, 2015:2018: €1.000 = US$1.110U.S.$1.181 and CAN$1.000 = US$0.783;U.S.$0.772; for the financial year ended December 31, 2014:2017: €1.000 = US$1.329U.S.$1.198 and CAN$1.000 = US$0.905; andU.S.$0.797; for the financial year ended December 31, 2013:2016: €1.000 = US$1.329U.S.$1.110 and CAN$1.000 = US$0.971.U.S.$0.754.
1.Compensation of Outside Directors
The compensation paid to members of our Board of Directors who are not our employees (our "Outside Directors") is designed to (i) attract and retain the most qualified people to serve on the Board and its committees, (ii) align the interests of the Outside Directors with those of our shareholders, and (iii) provide appropriate compensation for the risks and responsibilities related to being an effective Outside Director. This compensation is recommended to the Board by the Nominating, Governance and Compensation Committee (the "Compensation Committee""NGCC") of the Board.. The Compensation CommitteeNGCC is composed of three Outside Directors, each of whom is independent, namely Ms. Carolyn Egbert (Chair), Mr. Gérard LimogesJuergen Ernst and Mr. Pierre Lapalme.Ms. Robin Smith Hoke.
Annual Retainers and Attendance Fees
Our Outside Directors are paid an annual retainer, the amount of which depends on the position held on the Board, and attendance fees.Board. Annual retainers and attendance fees are paid on a quarterly basis to our Outside Directors onDirectors. Each Outside Director is paid the following basis:equivalent value of the payment in his or her home currency, net of any withholdings or deductions required by applicable law. Members of the Strategic Review Committee (the "SRC") were granted a monthly retainer in the amount of U.S. $7,500 from July 2017 up to and including January 2018, Ms. Egbert and Mr. Ernst deferred payment of their SRC retainers to 2018.
|
| | | |
| Type of Compensation | | Annual CompensationRetainer for the year 2015 (in units of home country currency)
2018 | Monthly Retainer for January 2018 |
Lead DirectorChair of the Board Retainer | | 65,00080,000 | - |
| Board Member Retainer | | 15,000 |
Board Meeting Attendance Fees40,000 | | 1,000 per meeting- |
| Audit Committee Chair Retainer | | 15,00020,000 | - |
| Audit Committee Member Retainer | | 4,0005,000 | - |
Audit Committee Meeting Attendance Fees | | 1,000 per meeting |
Compensation CommitteeNGCC Chair Retainer | | 12,00015,000 | - |
Compensation CommitteeNGCC Member Retainer | | 2,0003,000 | - |
Compensation Committee Meeting Attendance FeesSRC Chair Retainer | | 1,000 per meeting- | 7,500 |
| SRC Member Retainer | | - | 7,500 |
The Chairman, President and Chief Executive Officer is the only member of the Board who is not an Outside Director and, as such, is not compensated in his capacity as a director. OutsideAll Directors are reimbursed for travel and other out-of-pocket expenses incurred in attending Board or committee meetings.
Outstanding Option-Based Awards and Share-Based Awards
The following table shows all awards outstanding to each Outside Director up to the end of the financial year ending and as at December 31, 2015:2018:
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Option-based Awards | | Share-based Awards |
| Name | | Issuance Date | | Number of Securities Underlying Unexercised Options(1) | | Option Exercise Price | | Option Expiration Date | | Value of Unexercised In-the-money Options(2) | | Issuance Date | | Number of Shares or Units of Shares that have Not Vested | | Market or Payout Value of Share-based Awards that have Not Vested |
| | | (mm-dd-yyyy) | | (#) | | (CAN$ or $) | | (mm-dd-yyyy) | | (CAN$ or $) | | (mm-dd-yyyy) | | (#) | | ($) |
| Egbert, Carolyn | | 12/06/2012 | | 75 | | | $217.00 | | 12/05/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2013 | | 50 | | | $186.00 | | 05/07/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 11/27/2013 | | 250 | | | $112.00 | | 11/26/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2014 | | 600 | | | $107.00 | | 05/08/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2015 | | 600 | | | $52.50 | | 05/07/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| Ernst, Juergen | | 01/04/2007 | | 8 | | | CAN$2,790.00 | | 01/03/2017 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/11/2007 | | 41 | | | CAN$1,092.00 | | 12/10/2017 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 11/14/2008 | | 166 | | | CAN$390.00 | | 11/13/2018 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/08/2008 | | 25 | | | CAN$330.00 | | 12/08/2018 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/09/2009 | | 33 | | | CAN$570.00 | | 12/08/2019 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/08/2010 | | 50 | | | CAN$912.00 | | 12/07/2020 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/07/2011 | | 83 | | | $1,044.00 | | 12/06/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2012 | | 100 | | | $354.00 | | 05/08/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2013 | | 50 | | | $186.00 | | 05/07/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 11/27/2013 | | 250 | | | $112.00 | | 11/26/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2014 | | 600 | | | $107.00 | | 05/08/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2015 | | 600 | | | $52.50 | | 05/07/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| Lapalme, Pierre | | 12/09/2009 | | 33 | | | CAN$570.00 | | 12/08/2019 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/08/2010 | | 50 | | | CAN$912.00 | | 12/07/2020 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/07/2011 | | 83 | | | $1,044.00 | | 12/06/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2012 | | 100 | | | $354.00 | | 05/08/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2013 | | 50 | | | $186.00 | | 05/07/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 11/27/2013 | | 250 | | | $112.00 | | 11/26/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2014 | | 600 | | | $107.00 | | 05/08/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2015 | | 600 | | | $52.50 | | 05/07/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| Limoges, Gérard | | 01/04/2007 | | 8 | | | CAN$2,790.00 | | 01/03/2017 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/11/2007 | | 41 | | | CAN$1,092.00 | | 12/10/2017 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/08/2008 | | 25 | | | CAN$330.00 | | 12/08/2018 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/09/2009 | | 33 | | | CAN$570.00 | | 12/08/2019 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/08/2010 | | 50 | | | CAN$912.00 | | 12/07/2020 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 12/07/2011 | | 83 | | | $1,044.00 | | 12/06/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2012 | | 100 | | | $354.00 | | 05/08/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2013 | | 50 | | | $186.00 | | 05/07/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 11/27/2013 | | 250 | | | $112.00 | | 11/26/2023 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/09/2014 | | 600 | | | $107.00 | | 05/08/2021 | | — |
| | | — |
| | | — |
| | | — |
| |
| | 05/08/2015 | | 600 | | | $52.50 | | 05/07/2022 | | — |
| | | — |
| | | — |
| | | — |
| |
_________________________
|
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Option-based Awards | | Share-based Awards |
| Name | | Issuance Date
| | Number of Securities Underlying Unexercised Options
| | Option Exercise Price
| | Option Expiration Date | | Value of Unexercised In-the-money Options(1)
| | Issuance Date | | Number of Shares or Units of Shares that have Not Vested
| | Market or Payout Value of Share-based Awards that have Not Vested(2)
|
| | | (mm-dd-yyyy) | | (#) | | ($) | | (mm-dd-yyyy) | | ($) | | (mm-dd-yyyy) | | (#) | | ($) |
| Cardiff, Michael | | 05-10-2016 |
| | 20,000 |
| | 3.48 |
| | 05-09-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 12-06-2016 |
| | 7,850 |
| | 3.45 |
| | 12-06-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 08-15-2017 |
| | 60,000 |
| | 2.05 |
| | 08-15-2024 |
| | 53,400 |
| | — |
| | — |
| | — |
|
| | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Egbert, Carolyn | | 05-10-2016 |
| | 10,000 |
| | 3.48 |
| | 05-09-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 12-06-2016 |
| | 7,850 |
| | 3.45 |
| | 12-06-2023 |
| | — |
| | — |
| | — |
| | ��� |
|
| | 08-15-2017 |
| | 60,000 |
| | 2.05 |
| | 08-15-2024 |
| | 53,400 |
| | — |
| | — |
| | — |
|
| | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Ernst, Juergen | | 05-10-2016 |
| | 10,000 |
| | 3.48 |
| | 05-09-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 12-06-2016 |
| | 7,850 |
| | 3.45 |
| | 12-06-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 08-15-2017 |
| | 60,000 |
| | 2.05 |
| | 08-15-2024 |
| | 53,400 |
| | — |
| | — |
| | — |
|
| | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Smith Hoke, Robin | | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Limoges, Gérard | | 05-10-2016 |
| | 10,000 |
| | 3.48 |
| | 05-09-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 12-06-2016 |
| | 7,850 |
| | 3.45 |
| | 12-06-2023 |
| | — |
| | — |
| | — |
| | — |
|
| | 08-15-2017 |
| | 60,000 |
| | 2.05 |
| | 08-15-2024 |
| | 53,400 |
| | — |
| | — |
| | — |
|
| | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Norton, Brent | | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| Pollack, Jonathan | | — |
| | — |
| | — |
| | — |
| | — |
| | 05-08-2018 |
| | 23,000 |
| | 67,620 |
|
| |
| (1) | The number of securities underlying unexercised options represents all awards outstanding as at December 31, 2015. Awards that were issued before November 17, 2015 have been adjusted to reflect and give effect to the 100-for-1 reverse stock split (or share consolidation) that occurred on that date. |
| |
(2) | "Value of unexercised in-the-money options" at financial year-end is calculated based on the difference between the closing prices of the Common Shares on the NASDAQ or the TSX, as applicable, on the last trading day of the fiscal year (December 31, 2015)2018) of $4.48 and CAN$6.19, respectively,$2.94 and the exercise price of the options, multiplied by the number of unexercised options. |
| |
| (2) | The Company used the closing price of its Common Shares on the NASDAQ as at the last trading day of the fiscal year (December 31, 2018) of $2.94 |
See "Summary of the Stock Option Plan" for more details on the Company's second amended and restated stock option plan adopted by the Board on March 29, 2016 and ratified by the shareholders on May 10, 2016 ("Stock Option Plan (as defined below)Plan") and see "Summary of Long-Term Incentive Plan" for more details on the Company's long-term incentive plan adopted by the Board of Directors on March 27, 2018, and ratified by the shareholders on May 8, 2018 ("Long-Term Incentive Plan").
Total Compensation of Outside Directors
The table below summarizes the total compensation paid to our Outside Directors during the financial year ended December 31, 20152018 (all amounts are in USU.S. dollars). Our Outside Directors are generally paid in their home currency, which is the Canadian dollar for all Outside Directors other than Ms. Egbert, who isMessrs. Cardiff, Limoges, Norton and Pollack were paid in USCanadian dollars. Mses. Egbert and Smith Hoke were paid in U.S. dollars and Mr. Ernst who iswas paid in euros.
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Fees earned ($) | | Share-based Awards | | Option-based Awards(1) | | Non-Equity Incentive Plan Compensation | | Pension Value | | All Other Compensation(2) | | Total |
| | Retainer | | Attendance | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) |
Aubut, Marcel(3) | | 8,809 |
| | 3,915 |
| | — |
| | | 25,200 |
| | | — |
| | | — |
| | | — |
| | | 37,924 |
| |
Dorais, José(4) | | 5,827 |
| | 4,307 |
| | — |
| | | — |
| | | — |
| | | — |
| | | — |
| | | 10,134 |
| |
| Egbert, Carolyn | | 29,593 |
| | 14,500 |
| | — |
| | | 25,200 |
| | | — |
| | | — |
| | | 1,000 |
| | | 70,293 |
| |
| Ernst, Juergen | | 63,473 |
| | 12,205 |
| | — |
| | | 25,200 |
| | | — |
| | | — |
| | | 51,862 |
| | | 152,740 |
| |
| Lapalme, Pierre | | 15,892 |
| | 12,528 |
| | — |
| | | 25,200 |
| | | — |
| | | — |
| | | — |
| | | 53,620 |
| |
| Limoges, Gérard | | 24,505 |
| | 11,354 |
| | — |
| | | 25,200 |
| | | — |
| | | — |
| | | — |
| | | 61,059 |
| |
|
| | | | | | | | | | | | | | |
| Name | | Fees earned(1) | | Share-based
Awards(2) | | Option-based
Awards | | Non-Equity
Incentive Plan
Compensation | | Pension
Value | | All Other
Compensation | | Total |
| | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) |
| Cardiff, Michael | | 53,555 | | 41,170 | | — | | — | | — | | — | | 94,725 |
Egbert, Carolyn(3) | | 177,500 | | 41,170 | | — | | — | | — | | — | | 218,670 |
| Ernst, Juergen | | 51,065 | | 41,170 | | — | | — | | — | | — | | 92,235 |
| Smith Hoke, Robin | | 27,879 | | 41,170 | | — | | — | | — | | — | | 69,049 |
| Limoges, Gérard | | 67,500 | | 41,170 | | — | | — | | — | | — | | 108,670 |
| Norton, Brent | | 29,176 | | 41,170 | | — | | — | | — | | — | | 70,346 |
| Pollack, Jonathan | | 29,176 | | 41,170 | | — | | — | | — | | — | | 70,346 |
_________________________
| |
| (1) | In respect of our financial year ended December 31, 2018, we paid an aggregate amount of $450,577to all of our Outside Directors for services rendered in their capacity as directors, excluding reimbursement of out-of-pocket expenses and the value of share-based awards and option-based awards granted in 2018. |
| |
| (2) | Amounts shown represent the value of the DSUs on the grant date ($1.79). The value of stock options representsone DSU on the grant date is the closing price of theone Common SharesShare on the NASDAQ on the last trading day preceding the date of grant ($52.50 for options granted on May 8, 2015) multiplied by the Black-Scholes factor as at such date (80.00% for options granted on May 8, 2015) and the number of stock options granted on such date. The number of shares subject to the options granted in 2015 and the corresponding exercise price have been adjusted to reflect and give effect to the 100-for-1 reverse stock split (or share consolidation) that occurred on November 17, 2015. |
| |
(2) | The amounts paid to Ms. Egbert was for special tasks she performed for us. The amount paid to Mr. Ernst was a recognition payment for his past service as both Chairman and interim President/CEO between April and August 2008.
|
| |
(3) | Mr. Aubut ceased to be a director effective November 16, 2015. |
| |
(4) | Mr. Dorais did not stand for election at the Company’s annual and special meeting of shareholders held on May 8, 2015.grant. |
During(3) Ms. Egbert was awarded a cash bonus in the financial year ended December 31, 2015, we paid an aggregate amount of $259,770 to all of our Outside Directors for services rendered in their capacity as directors, excluding reimbursement of out-of-pocket expenses and the value of stock options issued in 2015.$75,000.
2.Compensation of Executive Officers
The following is disclosure of information related to the compensation that we paid to our “Named Executive Officers” during 2015. Our “Named2018. For the 2018 year, our "Named Executive Officers”Officers" were as follows:
Mr. David A. Dodd,Michael V. Ward, who currently serves as President and Chief Executive Officer as an employee;
Mr. James Clavijo, who served as our Chief Executive Officer during all of 2015;
Mr. Dennis Turpin, who served as our Chief Financial Officer as an employee from January 1, 2015 through October 9, 2015;March 5, 2018 to September 24, 2018;
Mr. Keith Santorelli,Ms. Leslie Auld, who servedcurrently serves as our Vice President, Finance throughout 2015 as well as Chief Accounting Officer and as our interim principal financial officer from October 9, 2015 up to and including December 31, 2015; and
Messrs. Philip A. Theodore, our Senior Vice President, Chief AdministrativeFinancial Officer as an independent contractor from September 24, 2018; and General Counsel and Jude Dinges, our Senior Vice President and Chief Commercial Officer; and
Dr. Richard Sachse, ourwho served as Senior Vice President and Chief Scientific and Chief Medical Officer until June 14, 2018, Mr. Brian Garrison, who currently serves as Senior Vice President, Global Commercial Operations, and Eckhard Guenther, who currently serves as Vice President, Alliance Management, who were our three most highly compensated executive officers (other than our Chief Executive Officer and our current and former Chief Financial Officer and our former Chief Accounting Officer and interim principal financial officer)Officer) during 2015.2018.
Compensation Discussion & Analysis
Compensation Philosophy and Objectives
Our Board, of Directors, through the Compensation Committee,NGCC, establishes our executive compensation program that is market-based and at a competitive percentile grouping for both total cash and total direct compensation. The Compensation CommitteeNGCC has established a compensation program that is designed to attract, motivate and retain high-performing senior executives, encourage and reward superior performance and align the executives' interests with those of our shareholders by:
providing the opportunity for an executive to earn compensation that is competitive with the compensation received by executives serving in the same or measurably similar positions within comparable North American companies;
providing the opportunity for executives to participate in an equity-based incentive plan, namely a stock option plan;compensation plans;
aligning executive compensation with companyour corporate objectives; and
attracting and retaining highly qualified individuals in key positions.
Compensation Elements
Our executive compensation is targeted at the 50th percentile for small cap biopharmabiopharmaceutical companies within both the local and national marketmarkets and is comprised of both fixed and variable components. The variable components include equity and non-equity incentive plans. Each compensation component is intended to serve a different function, but all elements are intended to work in concert to maximize both corporate and individual performance by establishing specific, competitive operational and corporate goals and by providing financial incentives to employees based on their level of attainment of these goals.
Our current executive compensation program is comprised of the following four basic components: (i) base salary; (ii) an annual bonus linked to both individual and corporate performance; (iii) equity incentives, consisting solely ofincluding stock options, previously granted under our second amended and restated stock option plan adopted by the Board on March 29, 2016 and ratified by the shareholders of Aeterna on May 10, 2016 (the "Stock Option Plan"), and presently granted under the Company's long-term incentive plan adopted by the Board on March 27, 2018 and ratified by the shareholders of Aeterna on May 8, 2018 (the "Long-Term Incentive Plan"), established for the benefit of our directors, certain executive officers and other participants as may be designated from time to time by either the Board or the Compensation Committee (the "Stock Option Plan");NGCC; and (iv) other elements of compensation, consisting of benefits, perquisites and retirement benefits.
Base Salary.Base salaries are intended to provide a steady income to theour executive officers regardless of share price. In determining individual base salaries, the Compensation CommitteeNGCC takes into consideration individual circumstances that may include the scope of an executive's position, the executive's relevant competencies or experience and retention risk. The Compensation CommitteeNGCC also takes into consideration the fulfillment of our corporate objectives, as well as the individual performance of the executive.
Short-Term, Non-Equity Incentive Compensation.Our short-term, non-equity incentive compensation plan sets a target cash bonus for each executive officer, expressed as a percentage of the executive officer’sofficer's base salary. The amount of cash bonus paid to an executive officer depends on the extent to which he or she contributed to the achievement of the annual performance objectives established by the Board for the year. The annual performance objectives are specific operational, clinical, regulatory, financial, commercial and corporate goals that are intended to advance our product pipeline, to promote the success of our commercial efforts and to enhance our financial position. The annual performance objectives are set at the end of each financial year as part of the annual review of corporate strategies. The performance objectives are not established for individual executive officers but rather by functional area(s), many of which are carried out by or fall within the responsibility of our President and Chief Executive Officer, Chief Financial Officer (or principal financial officer) and our other executive officers, including our Named Executive Officers. The award of a cash bonus requires the approval of both the Compensation CommitteeNGCC and the Board and is based upon an assessment of each individual's performance, as well as our overall performance at a corporate level. The determination of individual performance does not involve quantitative measures using a mathematical calculation in which each individual performance objective is given a numerical weight. Instead, the Compensation Committee'sNGCC's determination of individual performance is a subjective determination as to whether a particular executive officer substantially achieved the stated objectives or over-performed or under-performed with respect to corporate objectives that were deemed to be important to our success.
Long-Term Equity Compensation Plan of Executive Officers.The long-term component of the compensation of our executive officers is based exclusively on the Stock OptionLong Term Incentive Plan, which permits the awardissuance of a number of optionsequity-based awards based on the contribution of the officers and their responsibilities. The Board adopted a policy regarding stock option grants in December 2014, which provides that each Named Executive Officer is eligible to receive options to acquire our Common Shares having a value, based on the Black-Scholes option pricing model, equal to a specified multiple of his or her salary. The specified multiple for the President and Chief Executive Officer is 1.5. The specified multiple for each other Named Executive Officer is 0.75. To encourage retention and focus management on developing and successfully implementing our continuing growth strategy, stock options vest over a period of three years, with the first third vesting on the first anniversary of the date of grant. StockSince the adoption of the Long-Term Incentive Plan in 2018, we have broadened the types of equity-based awards which we may issue beyond stock options are usually granted to executive officers in December of each year.(to include, among other types, restricted stock units, deferred share units and others).
Other Forms of Compensation. Our executive employee benefits program also includes life, medical, dental and disability insurance to the same extent and in the same manner as all other employees. Several of our executive officers also receive a car allowance as a perquisite. These benefits and perquisites are designed to be competitive overall with equivalent positions in comparable
North American organizations in the life sciences industry. We also contribute to our North American employees' retirement plans to the extent of 50% of the employee's contribution up to an annual maximum amount of $9,000$11,200 for employees in the United States, and up to a maximum of $12,000 for employees and executive officers over 50 years old in the United States. The contribution amounts for our United States employees are subject to limitations imposed by the United States Internal Revenue Service on contributions to our most highly compensated employees. Employees based in Frankfurt, Germany also benefit from certain employer contributions into the employees' pension funds. Our executive officers, including the Named Executive Officers, are eligible to participate in such employer-contribution plans to the same extent and in the same manner as all other employees.
Positioning
The Compensation CommitteeNGCC is authorized to engage its own independent consultant to advise it with respect to executive compensation matters. While the Compensation CommitteeNGCC may rely on external information and advice, all of the decisions with respect to executive compensation are made by the Board upon the recommendation of the Compensation CommitteeNGCC and may reflect factors and considerations other than, or that may differ from, the information and recommendations provided by any external compensation consultants that may be retained from time to time.
In 2013, the Compensation CommitteeNGCC retained a compensation consultant to benchmark our executive compensation plan in an effort to determine whether we were achieving our objective of providing market competitive compensation opportunities. The compensation consultant gathered compensation data from companies that it concluded were of comparable size and/or stage of
development as us and from other companies with which we compete for executive talent and advised the Compensation CommitteeNGCC that our executive compensation should be generally aligned with the 50th percentile, or the mid-point, of the companies surveyed by the consultant. Furthermore, the consultant advised the Compensation CommitteeNGCC that the total cash target payment (base salary and, if applicable or awarded in cash, annual bonus) for our executive officers in 2013 generally fell around the 50th percentile of the companies surveyed. The base salaries of our Chairman, President and Chief Executive Officer and our Senior Vice President and their target bonuses were not increased in 2014 or 2015. Therefore, the Compensation CommitteeNGCC did not repeat or update the benchmarking process in 2014 or 2015- 2018 because it concluded that doing so would not provide additional meaningful data, considering the expense of the process. However, the Compensation Committee,NGCC, as a matter of good governance, will reviewannually reviews and assessassesses the Company's current compensation program and makemakes appropriate adjustments, if any, during 2016.any.
In June 2018, the NGCC retained Bowers Consulting LLC (“Bowers”), an independent compensation consulting firm, to assist the NGCC in analyzing the Corporation’s director and executive compensation. The Corporation paid fees to Bowers in the amount of $5,400.
Risk Assessment of Executive Compensation Program
The Board, through the Compensation Committee,NGCC, oversees the implementation of compensation methods that tie a portion of executive compensation to our short-term and longer-termlong-term performance and that of each executive officer and that take into account the advantages and risks associated with such compensation methods. In addition, the Board oversees the creation of compensation policies that are intended to reward the creation of shareholder value while reflecting a balance between our short-term and longer-termlong-term performance and that of each executive officer. The Compensation CommitteeNGCC has considered in general terms the concept of risk as it relates to our executive compensation program.
Base salaries are fixed in amount to provide a steady income to the executive officers regardless of share price and thus do not encourage or reward risk-taking to the detriment of other important business, operational, commercial or clinical metrics or milestones. The variable compensation elements (annual bonuses and stock options)equity-based awards) are designed to reward each of short-term, mid-term and long-term performance. For short-term performance, a discretionary annual bonus may be awarded based on the timing and level of attainment of specific operational and corporate goals that the Compensation CommitteeNGCC believes to be challenging, yet does not encourage unnecessary or excessive risk-taking. While our bonus payments are generally based on annual performance, a maximum bonus payment is pre-fixed for each senior executive officer and represents only a portion of each individual's overall total compensation opportunities. In exceptional circumstances, a particular executive officer may be awarded a bonus that exceeds his or her maximum pre-fixed or target bonus amount. Finally, a significant portion of executive compensation is provided in the form of stock options,equity-based awards, which is intended to further align the interests of executives with those of shareholders. The Compensation CommitteeNGCC believes that these awards do not encourage unnecessary or excessive risk-taking since the ultimate value of the awards is tied to our share price, and in the case of grants under the long-term incentive compensation plan, are generally subject to mid-term and long-term vesting schedules to help ensure that executives generally have significant value tied to long-term share price performance.
The Compensation CommitteeNGCC believes that the variable compensation elements (annual bonuses and stock options)equity-based awards) represent a percentage of overall compensation that is sufficient to motivate our executive officers to produce superior short-term, mid-term and long-term corporate results, while the fixed compensation element (base salary) is also sufficient to discourage executive officers from taking unnecessary or excessive risks. The Compensation CommitteeNGCC and the Board also generally have the discretion to adjust annual bonuses and stock option grantsequity-based awards based on individual performance and any other factors they may determine to be appropriate in the circumstances. Such factors may include, where necessary or appropriate, the level of risk-taking a particular executive officer may have engaged in during the preceding year.
Based on the foregoing, the Compensation CommitteeNGCC has not identified any specific risks associated with our executive compensation program that are reasonably likely to have a material adverse effect on us. The Compensation CommitteeNGCC believes that our executive compensation program does not encourage or reward any unnecessary or excessive risk-taking behaviour.behavior.
While we have not formally adopted a policy prohibiting or restricting ourOur directors, executive officers and directorsemployees are prohibited from purchasing, selling or otherwise trading in derivative securities relating to our Common Shares. Derivative securities are securities whose value varies in relation to the price of our securities. Examples of derivative securities include warrants to purchase our Common Shares, and put or call options written on our Common Shares, as well as individually arranged derivative transactions, such as financial instruments, including, for greater certainty, pre-paid variable forward contracts, equity swaps, collars, or units of exchange funds, which are designed to hedge or offset a decrease in market value of our equity securities granted as executive compensation or directors' remuneration,remuneration. Options to acquire Common Shares and other equity-based awards issued pursuant to our executive officers and directors haveStock Option Plan or Long-Term Incentive Plan are not historically engaged in such financial instruments or transactions. In addition, our disclosure and trading policy requires that all "reporting insiders", including executive officers and directors, pre-clear with our Corporate Secretary each trade in ourderivative securities which would include the entering into of any such financial instrument or transaction, hedge, swap or forward contract.for this purpose.
20152018 Compensation
Base Salary. The primary element of our compensation program is base salary. Our view is that a competitive base salary is a necessary element for retaining qualified executive officers. In determining individual base salaries, the NGCC takes into consideration individual circumstances that may include the scope of an executive's position, the executive's relevant competencies or experience and retention risk. The NGCC also takes into consideration the fulfillment of our Chairman, President and Chief Executive Officer and our Senior Vice Presidents were not increased in 2015 becausecorporate objectives, as well as the Compensation Committee determined that the financial positionindividual performance of the Company did not justify an increase in base salaries.executive.
Short-Term, Non-Equity Incentive Compensation. The Board, of Directors, based on the Compensation Committee'sNGCC's recommendation, adopted the following performance objectives for 2015:2018:
|
| | |
Objectives for 2015Goal | | Result |
FinancingCommercialization of Macrilen™ (macimorelin) in Europe and ROW | SecureAssuming EMA approval, develop strategy and implementation plan for commercialization through the out-licensing of Macrilen™ (macimorelin) for Europe and ROW
| The Board developed and approved a minimumstrategy to out-license macimorelin for the ROW, but the Corporation did not secure acceptable ROW agreements in 2018. The Corporation subsequently (in 2019) engaged Torreya to assist in identifying and executing upon such opportunities. |
| Successfully execute the board-approved strategy and implementation plan. | Not completed. The Board approved a strategy and implementation plan to pursue commercialization opportunities for macimorelin for the ROW and to implement non-macimorelin related opportunities. The Corporation explored several potential opportunities, but none resulted in a transaction that was acceptable to the Corporation. |
Deploy all effective resources to ensure timely EMA approval of $10 millionMacrilen™ (macimorelin). | Completed. EMA approval of macimorelin was obtained in January 2019 based on the work of the Corporation during the first half2018. |
Commercialization of 2015End 2015 with a minimum of two years of cash
Macrilen™ (macimorelin) in United States and Canada | $37 million raisedProvide effective support to Strongbridge in March 2015 financing,its commercialization efforts to ensure Macrilen™ (macimorelin) is timely marketed in 2018. | Not completed. The Corporation provided support, but issuance of highly dilutive Series B Warrants precluded additional fund raising until December 2015. Ended year with $41.45 million of cash. Unable to build cash reserve to two yearsefforts were slowed due to impactStrongbridge’s sale of Series B Warrants. Inabilityits license rights to achieve funding goal was offsetNovo Nordisk A/S in December 2018. |
| Ensure effective clinical studies are in place to obtain approval of pediatric indication of Macrilen™ (macimorelin). | In progress. The Corporation is collaborating with Novo Nordisk (and previously with Strongbridge) and is providing appropriate activities with respect to the ongoing clinical studies that are required to obtain approval for the pediatric indication of Macrilen™. |
| Improve operations | Manage costs and control expenses to maximize cash conservation. | In progress. The Corporation is focused on cost-savings and cash conservation. To this end, the Corporation reduced operating costs in both Germany and the United States in 2018. This continues to be an important objective in 2019. |
Provide cash forecast by achievementmonth on a 24-month projection. | The Corporation remains focused on aligning essential personnel, both in Germany and the United States, with the Corporation’s strategy and improving cost-effectiveness. In 2018, this included the termination of a meaningful reduction inemployment of certain employees. |
Ensure effective and efficient use of cash for operating activities duringresources and personnel. | The Corporation remains focused on aligning essential personnel, both in Germany and the year.United States, with the Corporation’s strategy and improving cost-effectiveness. In 2018, this included the termination of employment of certain employees. |
EstroGel®
Ensure that performance milestones for key managers align with and support CEO milestones. | Achieve minimum of $5 million in annual revenue in AEZS territories | Growth in market share of total prescriptions from 31.2% in Q1 to 36.8% in Q4, resulting in a 17.4% increase in total prescriptions in our territories, but revenues far below target. |
ZoptEC Phase 3 trial | Issue first interim results
If trial continues, issue second interim results
Conduct clinical quality assessment of trial
| The first interim results were successful and were issued on April 27, 2015. The second interim results were successful and were issued on October 9, 2015. The quality assessment was conducted. |
Macrilen™ | Decide whether to continue with clinical development
If the decision is to continue, clarify protocol issues with the FDA
If the decision is to continue, initiate the clinical program
| We decided to continue with clinical development in the first quarter of 2015. We clarified the protocol issues with the FDA in the second quarter of 2015 and initiated the clinical program ahead of schedule, also in the second quarter of 2015. |
Erk Inhibitors | Determine a development candidate | During the second quarter of 2015, we selected AEZS-140 as the lead development candidate. Two back-up candidates were also identified. |
Business Development | Complete in-license, acquisition or promotion agreements with a minimum annual revenue or commission potential of $10 million | The Saizen® co-promotion agreement was signed on May 7 and selling was launched on July 27. Apifiny® co-marketing agreement was signed on November 30 and selling was launched on December 1.
Completed. |
The Chief Executive Officer recommended to the Compensation Committee that we award cash bonuses to two of our Named Executive Officers with respect to 2015. The Compensation Committee concurred with the Chief Executive Officer’s recommendation as did the full Board of Directors. Mr. Philip A. Theodore, our Senior Vice President, Chief Administrative Officer, General Counsel and Corporate Secretary, was awarded a cash bonus with respect to 2015 in the amount of $35,000, which represented approximately 25% of his target bonus. Dr. Richard Sachse, our Senior Vice President, Chief Medical Officer and Chief Scientific Officer, was awarded a cash bonus with respect to 2015 in the amount of €100,000, which represented 100% of his target bonus. Both bonuses were recommended by the Chief Executive Officer based on performance he deemed significant.
Long-Term Equity Compensation
The Compensation CommitteeBoard approved significant option awards to our Named Executive Officers in 2015 because, as a resultan award of the Share Consolidation and the significant decrease in our stock price following the dilution attributable to the issuance of Common Shares upon the exercise of the Series B Warrants, all previous option awards became extremely out-of-the-money and, therefore, ceased to provide a long-term equity incentive. Mr. Dodd was awarded 85,00050,000 stock options and Messrs. Dinges, Sachse and Theodore were each awarded 40,000 stock options. The stock options haveat an exercise price of $4.58 and vest$1.46, to Mr. Ward on April 2, 2018 in three annual installments, commencingaccordance with the Stock Option Plan. The Board approved an award of 100,000 stock options at an exercise price of $2.11, to Mr. Ward on December 21, 2016.June 22, 2018, in accordance with the Long-Term Incentive Plan.
Summary of the Stock Option Plan
We established the Stock Option Plan in order to attract and retain directors, officers, employees and suppliers of ongoing services, who will be motivated to work towards ensuring our success. The Board has full and complete authority to interpret the Stock Option Plan, to establish applicable rules and regulations and to make all other determinations it deems necessary or useful for the administration of the Stock Option Plan, provided that such interpretations, rules, regulations and determinations are consistent with the rules of all stock exchanges and quotation systems on which our securities are then traded and with all relevant securities legislation.
The Stock Option Plan provides that the sole persons eligible to receive grantsThere were 628,685 options outstanding under the Stock Option Plan (each, a "Participant") shall be: (i) our most senior executive officers, including the persons occupying the positionsrepresenting approximately 3.82% of Chief Executive Officer, Chief Financial Officer, Chief Scientific Officer, Chief Commercial Officer, Chief Administrative Officerall issued and Chief Compliance Officer; (ii) such other of our executive officers or executive officers of our subsidiaries that may, from time to time, report directly to the Chief Executive Officer; (iii) the non-employee, independent members of the Board; and (iv) such other of our officers or employees or the officers or employees of any of our subsidiaries, as the case may be, or suppliers of ongoing services, as may be expressly designated by resolution of the Board or the Compensation Committee.
outstanding Common Shares on March 29, 2019. The maximumproposed number of Common Shares issuable underpursuant to the Stock OptionLong-Term
Incentive Plan is fixed at 11.4% of the issued and outstanding Common Shares at any given time which, asless the number of March 29, 2016, represented 1,131,871 Common Shares. There were 275,041Shares issuable pursuant to stock options outstandinggranted at such time under the Stock Option Plan. See below for a complete description of the Long-Term Incentive Plan. The Company does not intend on issuing any new stock options under the Stock Option Plan, representing approximately 2.8% of all issued and outstanding Common Shares, on March 29, 2016.instead will issue any future stock options under the Long-Term Incentive Plan.
Under the Stock Option Plan, (i) the number of securities issuable to insiders, at any time, or issued within any one-year period, under all of our security-based compensation arrangements, cannot exceed 10% of our issued and outstanding securities and (ii) no single Participantperson eligible to receive grants under the Stock Option Plan (each a "Participant") may hold options to purchase, from time to time, more than 5% of our issued and outstanding Common Shares. In addition: (i) the aggregate fair value of options granted under all of our security-based compensation arrangements to any one of our Outside Directors entitled to receive a benefit under the Stock Option Plan, within any one-year period, cannot exceed $100,000 valued on a Black-Scholes basis and as determined by the Compensation Committee;NGCC; and (ii) the aggregate number of securities issuable to all of our Outside Directors entitled to receive a benefit under the Stock Option Plan, within any one-year period, under all of our security-based compensation arrangements, cannot exceed 1% of its issued and outstanding securities.
Options granted under the Stock Option Plan may be exercised at any time within a maximum period of seven or ten years following the date of their grant (the "Outside Expiry Date"), depending on the date of grant. The Board or the Compensation Committee,NGCC, as the case may be, designates, at its discretion, the specific Participants to whom stock options are granted under the Stock Option Plan and determines the number of Common Shares covered by each of such option grants, the grant date, the exercise price of each option, the Outside Expiry Date and any other matter relating thereto, in each case in accordance with the applicable rules and regulations of the regulatory authorities. The price at which the Common Shares may be purchased may not be lower than the greater of the closing prices of the Common Shares on the NASDAQ or the TSX, as applicable, on the last trading day preceding the date of grant of the option. Options granted under the Stock Option Plan shall vest in equal tranches over a three-year period (one-third each year, starting on the first anniversary of the grant date) or as otherwise determined by the Board or the Compensation Committee,NGCC, as the case may be. Participants may not assign their options (nor any interest therein) other than by will or in accordance with the applicable laws of estates and succession.
Unless the Board or the Compensation CommitteeNGCC decides otherwise, Participants cease to be entitled to exercise their options under the Stock Option Plan: (i) immediately, in the event a Participant who is an officer or employee resigns or voluntarily leaves his or her employment or his or her employment is terminated with cause and, in the case of a Participant who is a non-employee director of us or one of our subsidiaries, the date on which such Participant ceases to be a member of the relevant Board of Directors; (ii) six months following the date on which employment is terminated as a result of the death of a Participant who is an officer or employee and, in the case of a Participant who is an Outside Director, six months following the date on which such Participant ceases to be a member of the Board of Directors by reason of death; (iii) 90 days following the date on which a Participant's employment is terminated for a reason other than those mentioned in (i) or (ii) above including, without limitation, upon the disability, long-term illness, retirement or early retirement of the Participant; and (iv) where the Participant is a service supplier, 30 days following the date on which such Participant ceases to act as such, for any cause or reason (each, an "Early Expiry Date").
The Stock Option Plan also provides that, if the expiry date of one or more options (whether an Early Expiry Date or an Outside Expiry Date) occurs during a "blackout period" or within the seven business days immediately after a blackout period imposed by us, the expiry date will be automatically extended to the date that is seven business days after the last day of the blackout period. For the purposes of the foregoing, "blackout period" means the period during which trading in our securities is restricted in accordance with our corporate policies.
Participants may not assign their options (nor any interest therein) other than by will or in accordance with the applicable laws of estates and succession.
If (i) we accept an offer to amalgamate, merge or consolidate with any other entity (other than one of our wholly-owned subsidiaries) or to sell or license all or substantially all of our assets to any other entity (other than one of our wholly-owned subsidiaries); (ii) we sign a support agreement in customary form pursuant to which the Board agrees to support a takeover bid and recommends that our shareholders tender their Common Shares to such takeover bid; or (iii) holders of greatermore than 50% of our then outstanding Common Shares tender all of their Common Shares to a takeover bid made to all of the holders of the Common Shares to purchase all of the then issued and outstanding Common Shares, then, in each case, all of the outstanding options shall, without any further action required to be taken by us, immediately vest. Each Participant shall thereafter be entitled to exercise all of such options at any time up to and including, but not after the close of business on that date which is ten days following the Closing Date (as defined below). Upon the expiration of such ten-day period, all rights of the Participant to such options or to the exercise of same (to the extent not already exercised) shall automatically terminate and have no further force or effect whatsoever. "Closing Date" is defined to mean (x) the closing date of the amalgamation, merger, consolidation, sale or license transaction in the case of clause (i) above; (y) the first expiry date of the takeover bid on which each of the offeror's conditions are either satisfied or waived in the case of clause (ii) above; or (z) the date on which it is publicly announced that holders of greater than 50% of our then outstanding Common Shares have tendered their Common Shares to a takeover bid in the case of clause (iii) above.
The Stock Option Plan provides that the following amendments may be made to the plan only upon approval of each of the Board and our shareholders as well as receipt of all required regulatory approvals:
any amendment to Section 3.2 of the Stock Option Plan (which sets forth the limit on the number of options that may be granted to insiders) that would have the effect of permitting, without having to obtain shareholder approval on a "disinterested vote" at a duly convened shareholders' meeting, the grant of any option(s) under the Stock Option Plan otherwise prohibited by Section 3.2;
any amendment to the number of securities issuable under the Stock Option Plan (except for certain permitted adjustments, such as in the case of stock splits, consolidations or reclassifications);
any amendment whichthat would permit any option granted under the Stock Option Plan to be transferable or assignable other than by will or in accordance with the applicable laws of estates and succession;
the addition of a cashless exercise feature, payable in cash or securities, which does not provide for a full deduction of the number of underlying securities from the Stock Option Plan reserve;
the addition of a deferred or restricted share unit component or any other provision whichthat results in employees receiving securities while no cash consideration is received by us;
with respect to any Participant, whether or not such Participant is an "insider" and except in respect of certain permitted adjustments, such as in the case of stock splits, consolidations or reclassifications:
| |
◦ | any reduction in the exercise price of any option after the option has been granted; |
| |
◦ | any cancellation of an option and the re-grant of that option under different terms; |
| |
◦ | any extension to the term of an option beyond its Outside Expiry Date to a Participant who is an "insider" (except for extensions made in the context of a "blackout period"); |
any reduction in the exercise price of any option after the option has been granted, or
any cancellation of an option and the re-grant of that option under different terms, or
any extension to the term of an option beyond its Outside Expiry Date to a Participant who is an "insider" (except for extensions made in the context of a "blackout period");
any amendment to the method of determining the exercise price of an option granted pursuant to the Stock Option Plan;
the addition of any form of financial assistance or any amendment to a financial assistance provision which is more favorable to employees; and
any amendment to the foregoing amending provisions requiring Board, shareholder and regulatory approvals.
The Stock Option Plan further provides that the following amendments may be made to the Stock Option Plan upon approval of the Board and upon receipt of all required regulatory approvals, but without shareholder approval:
amendments of a "housekeeping" or clerical nature or to clarify the provisions of the Stock Option Plan;
amendments regarding any vesting period of an option;
amendments regarding the extension of an option beyond an Early Expiry Date in respect of any Participant, or the extension of an option beyond the Outside Expiry Date in respect of any Participant who is a "non-insider";
adjustments to the number of issuable Common Shares underlying, or the exercise price of, outstanding options resulting from a split or a consolidation of the Common Shares, a reclassification, the payment of a stock dividend, the payment of a special cash or non-cash distribution to our shareholders on a pro rata basis provided such distribution is approved by
our shareholders in accordance with applicable law, a recapitalization, a reorganization or any other event which necessitates an equitable adjustment to the outstanding options in proportion with corresponding adjustments made to all outstanding Common Shares;
discontinuing or terminating the Stock Option Plan; and
any other amendment which does not require shareholder approval under the terms of the Stock Option Plan.
Summary of the Long-Term Incentive Plan
The purpose of the Long-Term Incentive Plan is to (i) promote our long-term financial interests and growth by attracting and retaining management and other personnel and key service providers with the training, experience and ability to enable them to make a substantial contribution to the success of our business; (ii) motivate management personnel by means of growth-related incentives to achieve long-range goals; and (iii) further the alignment of interests of participants with those of our shareholders through opportunities for increased share ownership in the Company.
The NGCC is the administrator of the Long-Term Incentive Plan (the “Administrator”). At any time, the Board may serve as the Administrator of the Long-Term Incentive Plan, in lieu of, or in addition, to the NGCC. Except as provided otherwise under the Long-Term Incentive Plan, the Administrator has plenary authority to grant awards pursuant to the terms of the Long-Term Incentive Plan to eligible individuals, determine the types of awards and the number of shares to be covered by the awards, establish the terms and conditions for awards and take all other actions necessary or desirable to carry out the purpose and intent of the Long-Term Incentive Plan.
Participation in the Long-Term Incentive Plan is generally open to all officers, employees and other individuals, including Outside Directors. However, any individual whose services to the Company or any of its subsidiaries are limited to capital-raising transactions, or the promotion and maintenance of a market for the Company securities, are ineligible to participate in the Long-Term Incentive Plan. Prospective officers, employees and other service providers who have accepted offers to provide services to the Company may also participate in the Long-Term Incentive Plan.
The Long-Term Incentive Plan enables the grant of stock options, stock appreciation rights, stock awards, stock unit awards, performance shares, cash-based performance units and other stock-based awards, each of which may be granted separately or in tandem with other awards.
The maximum number of Common Shares issuable under the Long-Term Incentive Plan is fixed at 11.4% of the issued and outstanding Common Shares at any given time, less the number of Common Shares issuable pursuant to stock options granted at such time under the Stock Option Plan. There were 261,000 awards outstanding under the Long-Term Incentive Plan representing approximately 1.59% of all issued and outstanding Common Shares on March 26, 2019. See above for a complete description of the Stock Option Plan.
The number of securities issuable to insiders, at any time, or issued within any one-year period, under all of our security-based compensation arrangements, cannot exceed 10% of our issued and outstanding securities and no single participant may hold options to purchase, from time to time, more than 5% of our issued and outstanding Common Shares.
The aggregate fair value of options granted under all of our security-based compensation arrangements to any one of our Outside Directors entitled to receive a benefit under the Long-Term Incentive Plan, within any one-year period, cannot exceed $100,000 valued on a Black-Scholes basis and as determined by the NGCC; and the aggregate number of securities issuable to all of our Outside Directors entitled to receive a benefit under the Long-Term Incentive Plan, within any one-year period, under all of our security-based compensation arrangements, cannot exceed 1% of its issued and outstanding securities.
Except as provided below or within an award agreement, each award granted under the Long-Term Incentive Plan (other than a performance unit that cannot be paid in shares) will be subject to a minimum vesting period or minimum restriction period as follows: (i) each stock option or SAR will be subject to a minimum vesting period of 12 months from the date of grant, (ii) each award of stock, stock units, performance shares, performance units payable in shares and other stock- based awards (“Full Value Awards”) granted to non-employee directors will be subject to a minimum restriction period of 12 months from the date of grant, and (iii) each Full Value Award granted to a participant other than a non-employee director will be subject to a minimum restriction period of 12 months from the date of grant if vesting of or lapse of restrictions on such award is based on the satisfaction of performance goals and a minimum restriction period of 36 months from the date of grant, applied in either pro rata installments or a single installment, if vesting of or lapse of restrictions on such award is based solely on the participant’s satisfaction of specified service requirements with us (provided that no such Full Value Awards will vest or have its restrictions lapse during the first 12 months following the date of grant). If the grant of a performance award is conditioned on satisfaction of performance goals, the performance period must not be less than 12 months’ duration, but no additional minimum restriction period need apply to such award. The minimum vesting period or minimum restriction period will not apply in the case of death or disability of a participant or in the event of a change in control. Awards that result in the issuance of an aggregate of up to 5% of the share pool under the Long-Term Incentive Plan may be granted without regard to such minimum vesting period or minimum restriction period.
Awards granted under the Long-Term Incentive Plan shall not be subject in any manner to alienation, anticipation, sale, transfer, assignment, pledge, or encumbrance, except as otherwise determined by the Administrator; provided, however, that this restriction shall not apply to the Common Shares received in connection with an award after the date that the restrictions on transferability of such shares set forth in the applicable award agreement have lapsed.
Except as provided in the applicable award agreement or otherwise determined by the Administrator, and subject to the minimum vesting period or minimum restriction period described above, upon termination of service (as defined in the Long-Term Incentive Plan):
O•utstandingStock options or stock appreciation rights shall be forfeited, to the extent stock options or stock appreciation rights are not vested and exercisable;
•During the applicable restriction period, restricted stock and any accrued but unpaid dividends that are at that time subject to restrictions shall be forfeited; and
•During the applicable deferral period or portion thereof to which forfeiture conditions apply, or upon failure to satisfy any other conditions precedent to the delivery of common shares or cash to which RSUs, performance shares or performance units relate, all performance shares, performance units and RSUs and any other accrued but unpaid dividend equivalents with respect to such RSUs that are then subject to deferral or restriction shall be forfeited.
In the event of a change in control (as defined in the Long-Term Incentive Plan) of the Company, outstanding awards will terminate upon the effective time of the change in control unless provision is made for the continuation, assumption or substitution of awards by the surviving or successor entity or its parent. Unless an award agreement says otherwise, the following will occur with respect to awards that terminate in connection with a change in control of the Company:
•stock options and SARs, whether vested or unvested, will become fully exercisable and holders of these awards will be permitted immediately before the change in control to exercise them;
•restricted stock and RSUs with time-based vesting (i.e., not subject to achievement of performance goals) will become fully vested immediately before the change in control, and RSUs will be settled as promptly as is practicable in accordance with applicable law; and
•restricted stock, RSUs, performance shares, and performance units that vest based on the achievement of performance goals will become fully vested and earned based on the target performance level as to the performance goals, such that 100% of the target award is earned as of the date of the change of control; and the RSUs and performance units will be settled as promptly as is practicable in accordance with applicable law.
The Long-Term Incentive Plan will terminate on the earlier of (i) the earliest date as of which all awards granted under the Long-Term Incentive Plan have been satisfied in full or terminated and no shares approved for issuance under the Long-Term Incentive Plan remain available to be granted under new awards, or (ii) the tenth anniversary of date the Long-Term Incentive Plan, as amended and restated, is approved by our shareholders.
The Administrator may amend, alter or discontinue the Long-Term Incentive Plan, but no amendment, alteration or discontinuation will be made that would materially impair the rights of a participant with respect to a previously granted award without his or her consent, except such an amendment made to comply with applicable law or rule of any securities exchange or market on which our Common Shares are listed or admitted for trading or to prevent adverse tax or accounting consequences to the Company or the participant. In no event, however, will an amendment be made without the approval of our shareholders to the extent such amendment would (i) materially increase the benefits accruing to participants under the Long-Term Incentive Plan, (ii) increase the number of shares that may be issued under the Long-Term Incentive Plan or to a participant, (iii) materially expand the eligibility for participation in the Long-Term Incentive Plan, (iv) eliminate or modify the prohibition on repricing of stock options and SARs, (v) lengthen the maximum term or lower the minimum exercise price or base price permitted for stock options and SARs, (vi) modify the prohibition on the issuance of reload or replenishment options, (vii) amend the amendment provisions in the Long-Term Incentive Plan, or (viii) amend the Long-Term Incentive Plan to remove or exceed the 10% insider participation limit.
Outstanding Option-Based Awards and Share-Based Awards
The following table shows all awards outstanding to our Named Executive Officers as of December 31, 2015. The number of shares subject to the stock options and the corresponding exercise prices have been adjusted to reflect and give effect to the 100-for-1 reverse stock split (or share consolidation) that occurred on November 17, 2015.2018:
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option-based Awards | | Share-based Awards |
| Name | Issuance Date | | Number of Securities Underlying Unexercised Options(1) | | Option Exercise Price | | Option Expiration Date | | Value of Unexercised In-the-money Options(2) | | Issuance Date | | Number of Shares or Units of shares that have Not Vested
| | Market or Payout Value of Share-based Awards that have Not Vested (3) |
| | (mm-dd-yyyy) | | (#) | | (CAN$ or $) | | (mm-dd-yyyy) | | (CAN$ or $) | | | | (#) | | |
| Dodd, David A. | 04/15/2013 | | 3,000 |
| | (3) | | $198.00 | | 04/14/2023 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/04/2014 | | 4,750 |
| | | | $76.00 | | 12/04/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/21/2015 | | 85,000 |
| | | | $4.58 | | 12/20/2022 | | — |
| | | — |
| | | — |
| | | |
| Santorelli, Keith | 05/09/2014 | | 750 |
| | | | $107.00 | | 05/08/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/04/2014 | | 300 |
| | | | $76.00 | | 12/04/2021 | | — |
| | | — |
| | | — |
| | | — |
|
Turpin, Dennis(4) | 01/04/2007 | | 83 |
| | | | CAN$2,790.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/11/2007 | | 83 |
| | | | CAN$1,092.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/09/2009 | | 191 |
| | | | CAN$570.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/08/2010 | | 94 |
| | | | CAN$912.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/07/2011 | | 172 |
| | | | $1,044.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/06/2012 | | 840 |
| | | | $217.00 | | 01/08/2016 | | — |
| | | — |
| | | — |
| | | — |
|
| Sachse, Richard | 01/16/2014 | | 1,500 |
| | (5) | | $129.00 | | 01/15/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/04/2014 | | 1,300 |
| | | | $76.00 | | 12/04/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/21/2015 | | 40,000 |
| | | | $4.58 | | 12/20/2022 | | — |
| | | — |
| | | — |
| | | — |
|
| Dinges, Jude | 11/27/2013 | | 1,500 |
| | (6) | | $112.00 | | 11/26/2023 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/04/2014 | | 1,660 |
| | | | $76.00 | | 12/04/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/21/2015 | | 40,000 |
| | | | $4.58 | | 12/20/2022 | | — |
| | | — |
| | | — |
| | | — |
|
| Theodore, Philip A | 10/06/2014 | | 1,500 |
| | (7) | | $134.00 | | 10/05/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/04/2014 | | 500 |
| | | | $76.00 | | 12/04/2021 | | — |
| | | — |
| | | — |
| | | — |
|
| | 12/21/2015 | | 40,000 |
| | | | $4.58 | | 12/20/2022 | | — |
| | | — |
| | | — |
| | | — |
|
|
| | | | | | | | | | | | | | | | | |
| | | Option-based Awards | | Share-based Awards |
| Name | | Issuance Date | | Number of
Securities
Underlying
Unexercised
Options(1) | | Option
Exercise Price | | Option
Expiration Date | | Value of
Unexercised In-the-money
Options(2) | | Issuance Date | | Number of
Shares or
Units of shares
that have Not
Vested | | Market or Payout
Value of Share-based
Awards that have Not Vested |
| | | (mm-dd-yyyy) | | (#) | | ($) | | (mm-dd-yyyy) | | ($) | | | | (#) | | ($) |
| Auld, Leslie | | — | | — | | | — | | — | | — | | — | | — | | — |
Clavijo, James(3) | | — | | — | | | — | | — | | — | | — | | — | | — |
| Garrison, Brian | | 11/17/2015 | | 500 | | | 116.00 | | 11/17/2022 | | — | | — | | — | | — |
| | 12/21/2015 | | 3,000 | | | 4.58 | | 12/21/2022 | | — | | — | | — | | — |
| | 12/06/2016 | | 2,500 | | | 3.45 | | 12/06/2023 | | — | | — | | — | | — |
| Guenther, Eckhard | | 12/21/2015 | | 5,000 | | | 4.58 | | 12/21/2022 | | — | | — | | — | | — |
| | 11/08/2016 | | 398 | | | 3.50 | | 11/08/2023 | | — | | — | | — | | — |
| | 12/06/2016 | | 10,000 | | | 3.45 | | 12/06/2023 | | — | | — | | — | | — |
Sachse, Richard(4) | | — | | — | | | — | | — | | — | | — | | — | | — |
| Ward, Michael V. | | 08/15/2017 | | 150,000 | | | 2.05 | | 08/15/2024 | | 133,500 | | — | | — | | — |
| | 04/02/2018 | | 50,000 | | | 1.46 | | 04/02/2025 | | 74,000 | | — | | — | | — |
| | 06/22/2018 | | 100,000 | | | 2.11 | | 06/22/2025 | | 83,000 | | — | | — | | — |
_________________________
| |
| (1) | The number of securities underlying unexercised options represents all awards outstanding at December 31, 2015.2018. |
| |
| (2) | "Value of unexercised in-the-money options" at financial year-end is calculated based on the difference between the closing pricesprice of the Common Shares on the NASDAQ or the TSX, as applicable, on the last trading day of the fiscal year (December 31, 2015)2018) of $4.48 and CAN$6.19, respectively,$2.94 and the exercise price of the options, multiplied by the number of unexercised options. |
| |
| (3) | David A. Dodd was appointed President andMr. Clavijo ceased to be Chief ExecutiveFinancial Officer effective April 15, 2013 and was granted 3,000on September 24, 2018. All outstanding stock options in connection with such appointment. |
| |
(4) | The vested stock options issued toheld by Mr. Turpin expired without being exercised 90 days following the terminationClavijo were cancelled effective as of his employment on October 9, 2015termination date in accordance with the termsprovisions of the Stock Option Plan. |
| |
(5)(4) | Richard SachseDr. Sachse's employment was appointed Senior Vice President and Chief Scientific Officerterminated effective January 1, 2014 and was granted 1,500June 14, 2018. All outstanding stock options held by Dr. Sachse were cancelled in connectionaccordance with such appointment. |
| |
(6) | Jude Dinges was appointed Senior Vice President and Chief Commercial Officer effective November 1, 2013 and was granted 1,500 stock options in connection with such appointment. |
| |
(7) | Philip A. Theodore was appointed Senior Vice President, Chief Administrative Officer and General Counsel effective October 6, 2014 and was granted 1,500 stock options in connection with such appointment.the provisions of the Stock Option Plan. |
There arewere no vested share-based awards that have not yet been paid out or distributed.outstanding to our Named Executive Officers at December 31, 2018.
Incentive Plan Awards - Value Vested or Earned During the Year
The following table shows the incentive plan awards value vested or earned for each Named Executive Officer for the financial year ended December 31, 2015.2018:
|
| | | | | | |
Name | | Option-based awards - Value
vested during the year(1)
| | Share-based awards -
Value
vested during the year
| | Non-equity incentive
plan
compensation - Value
earned
during the year
($)
|
| | ($) | | ($) | |
Dodd, David A. | | — | | — | | — |
Santorelli, Keith | | — | | — | | — |
Turpin, Dennis | | — | | — | | — |
Sachse, Richard | | — | | — | | 111,000 |
Dinges, Jude | | — | | — | | — |
Theodore, Philip A. | | — | | — | | 35,000 |
_________________________ |
| | | | | | |
| Name | | Option-based awards — Value
vested during the year(1) | | Share-based awards —
Value
vested during the year | | Non-equity incentive plan compensation — Value earned during the year |
| | | ($) | | ($) | | ($) |
| Auld, Leslie | | — | | — | | — |
| Clavijo, James | | — | | — | | — |
| Garrison, Brian | | 3,074 | | — | | 35,000 |
| Guenther, Eckhard | | 12,299 | | — | | — |
| Sachse, Richard | | — | | — | | 120,000 |
| Ward, Michael V. | | — | | — | | 35,000 |
| |
| (1) | Represents the aggregate dollar value that would have been realized if the options had been exercised on the vesting date, based on the difference between the closing price of the Common Shares on the NASDAQ and the exercise price on such vesting date. |
Summary Compensation Table
The Summary Compensation Table set forth below shows compensation information for each of the Named Executive Officers for services rendered in all capacities during each of the financial years ended December 31, 2015, 20142018, 2017 and 2013.2016. All amounts in the table below are in USU.S. dollars. All cash amounts paid to Messrs. Dodd, Santorelli, DingesWard, Clavijo and TheodoreGarrison were paid in USU.S. dollars, while Mr. Turpin’sMs. Auld’s cash payments were made in Canadian dollars and Dr. Sachse’sSachse and Mr. Guenther’s cash payments were made in euros.
SUMMARY COMPENSATION TABLE
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and principal position | Years | Salary | Share based awards | Option based awards(1) | Non-equity incentive plan compensation | Pension Value | All other compensation(2) | Total compensation |
Annual incentive plan | Long-term incentive plans |
| | ($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) |
Dodd, David A. Chairman, President and Chief Executive Officer | 2015 | 475,000 |
| | — |
| | 358,690 |
| | — |
| | — |
| | — |
| | — |
| | 833,690 |
| |
| 2014 | 475,000 |
| | — |
| | 291,914 |
| | 100,000 |
| | — |
| | — |
| | — |
| | 866,914 |
| |
| 2013 | 328,846 |
| (3) | 414,048 |
| (4) | 474,606 |
| | 50,000 |
| | — |
| | — |
| | — |
| | 1,267,500 |
| |
Santorelli, Keith Former Vice President, Finance and Chief Accounting Officer and Interim Principal Financial Officer | 2015 | 244,800 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 244,800 |
| |
| 2014 | 240,000 |
| | — |
| | 82,554 |
| | — |
| | — |
| | | | — |
| | 322,554 |
| |
| 2013 | 27,692 |
| (5) | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 27,692 |
| |
Turpin, Dennis Former Senior Vice President and Chief Financial Officer | 2015 | 206,590 |
| (6) | — |
| | — |
| | — |
| | — |
| | — |
| | 481,569 |
| (7) | 688,159 |
| |
| 2014 | 309,299 |
| | — |
| | 107,547 |
| | 22,013 |
| | — |
| | — |
| | — |
| | 438,859 |
| |
| 2013 | 331,652 |
| | — |
| | — |
| | 66,677 |
| | — |
| | — |
| | — |
| | 398,329 |
| |
Sachse, Richard Senior Vice President, Chief Scientific Officer and Chief Medical Officer | 2015 | 221,900 |
| | — |
| | 168,795 |
| | 111,000 |
| | — |
| | 47,349 |
| (8) | — |
| | 549,044 |
| |
| 2014 | 265,752 |
| | — |
| | 235,017 |
| | 62,463 |
| | — |
| | 27,239 |
| | — |
| | 590,471 |
| |
| 2013 | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| |
Dinges, Jude Senior Vice President and Chief Commercial Officer | 2015 | 320,000 |
| | | | 168,795 |
| | — |
| | — |
| | — |
| | — |
| | 488,795 |
| |
| 2014 | 320,000 |
| | — |
| | 102,016 |
| | 25,000 |
| | — |
| | — |
| | — |
| | 447,016 |
| |
| 2013 | 121,988 |
| (9) | — |
| | 135,542 |
| | — |
| | — |
| | — |
| | — |
| | 257,530 |
| |
| Theodore, Philip A Senior Vice President, Chief Administrative Officer and General Counsel | 2015 | 320,000 |
| | — |
| | 168,795 |
| | 35,000 |
| | — |
| | — |
| | — |
| | 523,795 |
| |
| 2014 | 67, 692 |
| (10) | — |
| | 189,433 |
| | — |
| | — |
| | — |
| | — |
| | 257,125 |
| |
| 2013 | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| |
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | Non-equity incentive plan compensation | |
| Name and principal position | Years | | Salary | | Share
based
awards | | Option based awards (1) | | Annual
incentive
plan | | Long-term
incentive
plans | Pension
Value | | All other compensation | | Total
compensation |
| | | | ($) | | ($) | | ($) | | ($) | | ($) | ($) | | ($) | | ($) |
Ward, Michael V. President and Chief Executive Officer | 2018 | | 325,000 | | — |
| | 227,241 |
| | 35,000 |
| | — |
| — |
| | — |
| | 587,241 |
|
| 2017 | | 121,461 | | — |
| | 242,495 |
| | — |
| | — |
| — |
| | — |
| | 363,956 |
|
| 2016 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
Clavijo, James(2) Former Chief Financial Officer | 2018 | | 190,574 | | — |
| | 130,240 |
| | — |
| | — |
| — |
| | 137,500 |
| | 458,314 |
|
| 2017 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
| 2016 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
Auld, Leslie
Senior Vice President, Chief Financial Officer | 2018 | | 62,385 |
| — |
| | — |
| | — |
| | — |
| — |
| | — |
| | 62,385 |
|
| 2017 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
| 2016 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
Sachse, Richard(4)
Former Senior Vice President, Chief Scientific Officer and Chief Medical Officer | 2018 | | 403,297 | | — |
| | — |
| | — |
|
| — |
| — |
| | — |
|
| 403,297 |
|
| 2017 | | 222,000 | | — |
| | — |
| | 120,000 |
|
| — |
| 37,067 |
| | — |
| | 379,067 |
|
| 2016 | | 222,000 | | — |
| | 257,000 |
| | 55,500 |
|
| — |
| 37,067 |
| | — |
| | 571,567 |
|
Garrison, Brian
Senior Vice President, Global Commercial Operations | 2018 | | 235,015 | | — |
| | 3,550 |
| | 35,000 |
| | — |
| — |
| | — |
| | 273,565 |
|
| 2017 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
| 2016 | | — | | — |
| | — |
| | — |
| | — |
| — |
| | — |
| | — |
|
Guenther, Eckhard
Vice President, Alliance Management | 2018 | | 191,242 | | — |
| | — |
| | 13,154 |
| | — |
| 3,298 |
| | — |
| | 207,694 |
|
| 2017 | | 155,318 | | — |
| | — |
| | — |
| | — |
| 2,970 |
| | — |
| | 158,288 |
|
| 2016 | | 152,510 |
| — |
| | 27,797 |
| | — |
| | — |
| 2,851 |
| | — |
| | 183,158 |
|
_________________________
| |
| (1) | The value of option-based awards represents the closing price of the Common Shares on the NASDAQ on the last trading day preceding the date of grant ($198.00for options granted on April 15, 2013, $112.00for options granted on November 27, 2013,$129.00 for options granted on January 16, 2014, $107.00 for options granted on May 9, 2014,$134.00 for options granted on October 6, 2014, $76.00 for options granted on December 4, 2014 and $4.58 for options granted on December 21, 2015) multiplied by the Black-Scholes factor as at such date (79.96%for options granted on April 15, 2013, 80.68%for options granted on November 27, 2013, 80.17% for options granted on January 16, 2014, 79.90% for options granted on May 9, 2014, 78.96% for options granted on October 6, 2014, 80.86% for options granted on December 4, 2014 and92.14% for options granted on December 21, 2015) and the number of stock options granted on such date. The following table sets forth the value of the option-based awards and the corresponding Black-Scholes factor: |
|
| | |
| Date of Grant | Value of Grant | Black-Scholes Factor |
| November 9, 2016 | $3.50 | 80.35% |
| December 6, 2016 | $3.45 | 80.57% |
| December 16, 2016 | $3.80 | 80.68% |
| August 15, 2017 | $2.05 | 78.86% |
| April 2, 2018 | $1.46 | 77.57% |
| June 22, 2018 | $2.11 | 80.86% |
| |
| (2) | “Mr. Clavijo received a severance payment of $137,500 following the date that he ceased to be the Chief Financial Officer on September 24, 2018. All Other Compensation” represents perquisites and other personal benefits which,outstanding stock options held by Mr. Clavijo were cancelled in accordance with the aggregate, amount to $50,000 or more, or are equivalent to 10% or more of a Named Executive Officer's total salary for the financial year ended December 31, 2015. The type and amount of each perquisite, the value of which exceeds 25%provisions of the total value of perquisites, is separately disclosed for each Named Executive Officer, if applicable.Stock Option Plan. |
| |
| (3) | Represents the salary earned by and paid to Mr. Dodd following his appointment as President and Chief Executive Officer on April 15, 2013. |
| |
(4) | The value of Mr. Dodd's share-based awards represents the closing price of the Common Shares on the NASDAQ on the last trading day preceding the date of grant ($1.98 for share appreciation rights ("SARs") granted on April 15, 2013) multiplied by the Black-Scholes factor as at such date (175,000 SARs at a factor of 54% and 200,000 SARs at a factor of 58%) and the number of SARs granted on such date. The SARs expired on December 31, 2015 without being exercised. |
| |
(5) | Represents the salary earned by and paid to Mr. Santorelli following his appointment as Vice President, Finance on November 11, 2013. |
| |
(6) | Mr. Turpin served as Chief Financial Officer through October 9, 2015. The indicated salary amount represents salary earned and paid to Mr. Turpin up until the date of his departure. |
| |
(7) | Represents severance payment, perquisites and other personal benefits paid to Mr. Turpin in 2015, of which $468,736 was paid in the form of a termination payment. |
| |
(8) | We maintainmaintained a reinsured benevolent fund (Rückgedeckte Unterstützungskasse), which is a type of private defined contribution pension plan, for Dr. Sachse. We contributecontributed to a private pension provider an amount equal to 2.4% of Dr. Sachse’s salary, up to a monthly salary limit of €6,050, plus an additional contribution of 18% of the amount of Dr. Sachse’s salary that exceeds the monthly limit. Dr. Sachse also contributescontributed a percentage of his salary to the plan. We are liable to Dr. Sachse for the pension benefits that have been promised, if the private pension provider does not, or cannot, pay the promised pension payments. We obtained reinsurance against the insolvency or liquidation of the private pension provider. The table below sets forth additional information regarding Dr. Sachse’s pension plan. The difference between (i) the sum of the Accumulated Value at Start of Year column plus the Compensatory column and (ii) the Accumulated Value at End of Year column is attributable to Dr. Sachse’s contributions to the pension plan during the year ended December 31, 2015, as well as changes in foreign exchange rate, his contributions being made in euros. |
regarding Dr. Sachse’s pension plan. The difference between (i) the sum of the Accumulated Value at Start of Year column plus the Compensatory column and (ii) the Accumulated Value at End of Year column is attributable to Dr. Sachse’s contributions to the pension plan during the year ended December 31, 2018, as well as changes in the foreign exchange rate, his contributions being made in euros.
| | | Accumulated value at start of year | Compensatory | Accumulated value at year end | Compensatory | Accumulated value at year end |
| $28,187 | $47,349 | $73,729 | |
| $133,639 | | $12,258 | $145,897 |
| |
(9) | Represents consultant fees paid to Mr. Dinges between May 12, 2013 and October 31, 2013 combined with the salary paid to him following his appointment as Senior Vice President and Chief Commercial Officer on November 1, 2013. |
| |
(10) | Represents the salary earned by and paid to Mr. Theodore following his appointment as Senior Vice President, Chief Administrative Officer, General Counsel and Corporate Secretary on October 6, 2014. |
Compensation of the Chief Executive Officer
The compensation of our Chairman, President and Chief Executive Officer is governed by our executive compensation policy described in the section titled "Compensation of Executive Officers", and the Chairman, President and Chief Executive Officer participates, together with the other Named Executive Officers, in all of our incentive plans.
Mr. Dodd'sWard's total earned salaryearnings during the financial year ended December 31, 20152018 was $475,000. Mr. Dodd was not awarded$360,000 including an annual incentive bonus with respect to 2015.in the amount of $35,000.
For the financial year ended December 31, 2015,2018, the Compensation Committee recommended that 85,000Board approved an award of 50,000 stock options be grantedat an exercise price of $1.46, to Mr. Dodd underWard on April 2, 2018, in accordance with the long-term equity compensation plan.Stock Option Plan. The grantBoard approved an award of 100,000 stock options at an exercise price of $2.11, to Mr. Dodd is includedWard on June 22, 2018, in accordance with the table above captioned "Grants of Plan Based Awards".Long-Term Incentive Plan.
See "Long-Term Equity Compensation Plan of Executive Officers - Summary of the Stock Option Plan", for a complete description of the Stock Option Plan. See "Long-Term Equity Compensation Plan of Executive Officers - Summary of the Long-Term Incentive Plan", for a complete description of the Long-Term Incentive Plan.
Pension, retirement or similar benefits
As at December 31, 2018, the Company and its subsidiaries had accrued pension, retirement or similar benefits obligations amounting to $13.1 million. See note 18 - Employee future benefits, to the audited consolidated financial statements included in Item 18 of this Annual Report on Form 20-F.
| |
| C. | Board Practicespractices |
Our Articles provide that our Board shall be composed of a minimum of five and a maximum of 15 directors. Directors are elected annually by our shareholders, but the directors may from time to time appoint one or more directors, provided that the total number of directors so appointed does not exceed one-third of the number of directors elected at the last annual meeting of shareholders. Each elected director will remain in office until termination of the next annual meeting of the shareholders or until his or her successor is duly elected or appointed, unless his or her post is vacated earlier. We do not have service agreements with our independent directors.
See Item 6A. for information about the period of service of each of our directors and senior corporate officers.
Standing Committees of the Board of Directors
Our Board has established an Audit Committee and a Compensation Committee.NGCC.
Audit Committee
The Audit Committee assists the Board in fulfilling its oversight responsibilities. The Audit Committee reviews the financial reporting process, the system of internal control, the audit process, and our process for monitoring compliance with laws and regulations and with our Code of Ethical Conduct. In performing its duties, the Audit Committee will maintain effective working relationships with the Board, management, and the external auditors. To effectively perform his or her role, each committee member will obtain an understanding of the detailed responsibilities of committee membership as well as our business, operations and risks.
The function of the Audit Committee is oversight and while it has the responsibilities and powers set forth in its charter (incorporated by reference to Exhibit 11.2)11.3 to this Annual Report on Form 20-F), it is neither the duty of the committee to plan or to conduct audits or to determine that our financial statements are complete, accurate and in accordance with generally accepted accounting principles, nor to maintain internal controls and procedures.
The current members of the Audit Committee are Carolyn Egbert, Pierre Lapalme and Gérard Limoges (Chair)., Brent Norton, and Jonathan Pollack.
Compensation Committee
NGCC
The Compensation CommitteeNGCC is responsible for, among other matters, (i) assisting the Board in developing our approach to corporate governance issues, (ii) proposing new Board nominees, (iii) overseeing the assessment of the effectiveness of the Board and its committees, their respective chairs and individual directors and (iv) making recommendations to the Board with respect to board member nominees and directors' compensation, and generally playingas well as serving in a leadership role infor our corporate governance practices. It is also responsible for taking all reasonable measuresactions to ensure that appropriate human resources policies, procedures and systems, e.g., recruitment and procedures, such as hiringretention policies, competency profiles,and performance metrics and measurements, training policies and development programs, and market-based, competitive compensation and benefits structures, are in place so that we can attract, motivate and retain the quality of personnel required to meetachieve our business objectives. The Compensation CommitteeNGCC also assists the Board in discharging its responsibilities relating to executive and other human resources hiring,the recruitment, retention, development, assessment, compensation and succession planning matters.for our executive and senior management members.
Thus, the Compensation CommitteeNGCC recommends the appointment of senior officers, including the terms and conditions of their appointment and termination, and reviews the evaluation of the performance of our senior officers, including recommending their compensation and overseeing risk identification and management in relation to executive compensation policies and practices. The Board, which includes the members of the Compensation Committee,NGCC, reviews the Chief Executive Officer's corporate strategy, goals and performance objectives and evaluates and measures his or her performance and compensation in lightagainst the achievement of such goals and objectives.
The Compensation CommitteeNGCC recognizes that the industry, regulatory and competitive environment in which we operate requires a balanced level of risk-taking to promote and achieve the performance expectations of executives of a specialty biopharmaceutical company that is also seeking to acquire or in-license new commercial products.company. The Compensation CommitteeNGCC is of the view that our executive compensation program should not encourage senior executives to take excessiveinappropriate or unreasonable risk. In this regard, the Compensation CommitteeNGCC recommends the implementation of compensation methods that tieappropriately connect a portion of senior executive compensation towith our short-term and longer-term performance, as well as that of each individual executive officer and that take into account the advantages and risks associated with such compensation methods. The Compensation CommitteeNGCC is also responsible for creatingestablishing compensation policies that are intended to reward the creation of shareholder value while reflecting a balance between our short-term and longer-term performance and that of each executive officer.
The current members of the Compensation Committee are Carolyn Egbert (Chair), Pierre LapalmeJuergen Ernst and Gérard Limoges.Robin Smith Hoke.
As at December 31, 2015,2018, we had a total of 4622 active employees, of which 3618 are based in Frankfurt, Germany. The remaining 10four employees are based in the United States.States and our CFO is based in Toronto, Canada. Our employees are engaged in the following activities: (i) 2912 are engaged in research and development, regulatory affairs and quality assurance; (ii) eightfour are involved in commercial operations and business development; and (iii) nine6 are involved in various administrative functions, including finance and accounting. We do not employ any sales representatives. Under the German Restructuring Plan started in 2017 , 14 employees left our German subsidiary in 2018 (22 were terminated in 2017, three of them left in 2017, 14 of them left in 2018. Five of the employees who were terminated in 2017 were re-employed in 2018). The Managing Director of the German site was replaced during 2018.
We have agreements with our employees covering confidentiality, loyalty, non-competition and assignment of all intellectual property rights developed during the employment period. From August 17, 2014 to December 31, 2015, we conducted a resource optimization program that resulted in the termination of 28 employees.
The information in the table below issets forth information as of March 29, 2019 provided as at December 31, 2015:to us by our directors and executive officers concerning their ownership of Common Shares and stock options of the Company:
|
| | | | | | | | | | | |
| Name | No. of Common Shares owned or held | Percent(1) | No. of stock options held(2) | No. of currently exercisable options |
| Auld, Leslie | — |
| | — | | — |
| | — |
|
Cardiff, Michael(3) | — |
| | — | | 87,850 |
| | 87,850 |
|
Clavijo, James(4) | — |
| | — | | — |
| | — |
|
| Egbert, Carolyn | 1,920 |
| | * | | 77,850 |
| | 5,951 |
|
| Ernst, Juergen | 1,348 |
| | * | | 77,850 |
| | 5,951 |
|
| Garrison, Brian | — |
| | — | | 6,000 |
| | — |
|
| Guenther, Eckhard | — |
| | — | | 15,398 |
| | 6,801 |
|
| Hoke Smith, Robin | — |
| | — | | — |
| | — |
|
| Limoges, Gérard | 1,200 |
| | * | | 77,850 |
| | 5,951 |
|
| Norton, Brent | — |
| | * | | — |
| | — |
|
| Pollack, Jonathan | — |
| | * | | — |
| | — |
|
Sachse, Richard(5) | — |
| | — | | — |
| | — |
|
| Teifel, Michael | — |
| | — | | 30,350 |
| | 13,451 |
|
| Ward, Michael V. | — |
| | — | | — |
| | — |
|
| Total | 4,468 |
| | * | | 373,148 |
| | 125,955 |
|
|
| | | | | | | | | | | |
| Name | No. of Common Shares owned or held | Percent(1) | No. of stock options held(2) | No. of currently exercisable options |
| Dinges, Jude | 6,533 |
| | * | | 43,160 |
| | 1,554 | |
| Dodd, David A. | 19,003 |
| | * | | 92,750 |
| | 3,584 | |
| Egbert, Carolyn | 1,920 |
| | * | | 1,575 |
| | 476 | |
| Ernst, Juergen | 1,348 |
| | * | | 2,006 |
| | 907 | |
| Guenther, Eckhard | — |
| | — | | 5,597 |
| | 464 | |
| Lapalme, Pierre | — |
| | — | | 1,766 |
| | 667 | |
| Limoges, Gérard | 14 |
| | * | | 1,840 |
| | 741 | |
| Sachse, Richard | — |
| | — | | 42,800 |
| | 934 | |
| Santorelli, Keith | — |
| | — | | 1,050 |
| | 350 | |
| Teifel, Michael | — |
| | — | | 10,526 |
| | 393 | |
| Theodore, Philip A. | 10,894 |
| | * | | 42,000 |
| | 667 | |
| Total | 39,712 |
| | | 245,070 |
| | 10,737 | |
_________________________
* Less than 1%________________________
| |
| (1) | Based on 9,928,697 16,440,760Common Shares outstanding as at December 31, 2015.2018. |
| |
| (2) | For information regarding option expiration dates and exercise price refer to the tables included under the caption "Outstanding Option-Based Awards and Share-Based Awards". |
| |
| (3) | Mr. Cardiff resigned from the Board for personal reasons in March 2019. At such time, the Board amended the Stock Option Plan to accelerate vesting of Mr. Cardiff’s stock options. His stock options will remain exercisable until the seventh business day following the end of the current blackout period, following which time any unexercised options of Mr. Cardiff will be forfeited and cancelled. |
| |
| (4) | Mr. Clavijo ceased to be the Company's Chief Financial Officer on September 24, 2018. All outstanding stock options held by Mr. Clavijo were cancelled effective as of his termination date in accordance with the provisions of the Stock Option Plan. |
| |
| (5) | Dr. Sachse employment was terminated effective June 14, 2018. All outstanding stock options held by Dr. Sachse were cancelled in accordance with the provisions of the Stock Option Plan. |
| |
| Item 7. | Major Shareholders and Related Party Transactions |
We are not directly or indirectly owned or controlled by another corporation or by any foreign government. Based on filings with the SEC and the Canadian securities regulatory authorities, as at March 29, 2016, the only2019, no individual or entity, thatother than as set out below, beneficially owned, directly or indirectly, or exercised control or direction over our Common Shares carrying more than 5% of the voting rights attached to all our Common Shares were Sabby Healthcare Master Fund, Ltd., Sabby Volatility Warrant Master Fund, Ltd., Sabby Management, LLC and Hal Mintz, who together beneficially owned 534,145(to whom we refer as our major shareholders). The ownership percentages reflected below are based on 16,440,760 Common Shares outstanding as of March 26, 2019. The shareholders listed below do not have any different voting rights from any of our Common shares, representing approximately 5.38%other shareholders. We know of no arrangements that would, at a subsequent date, result in a change of control of the Company.
|
| | |
| Beneficial Owner | No. of Common Shares | Percentage |
J. Goldman & Co., L.P. J. Goldman Capital Management, Inc. Jay G. Goldman1 | 997,494 | 6.067201% |
|
| |
1 Based solely on a Schedule 13G, dated February 11, 2019, filed by J. Goldman & Co., L.P. (“JGC”), J. Goldman Capital Management, Inc. (“JGCM”) and Jay G. Goldman (“JGG”) with the SEC. As indicated in that statement, JGC, JGCM, and JGG possess shared voting and dispositive power with respect to all of such Common Shares, all of which are beneficially owned by J. Goldman Master Fund, L.P. | |
Changes in Percentage Ownership by Major Shareholders
We had no major shareholders in 2016 or 2017. During 2018, the above listed major shareholders became major shareholders due to the acquisition of over 5% of our outstanding Common Shares as further described in their Schedule 13G/A filed with the SEC on January 14, 2016..
United States Shareholders
AsBased on a review of the information provide to us by our transfer agent, as at February 29, 2016,March 7, 2019, there were 10thirteen holders of record of our Common Shares, of which one wastwo were registered with an address in the United States holding in the aggregate approximately 99.69%99.8% of our outstanding Common Shares. We believe that the number of beneficial owners of our Common Shares is substantially greater than the number of record holders, because the overwhelming majority of our Common Shares are held in broker "street names".names."
| |
| B. | Related party transactions |
In addition to recurring payments made to members ofOther than employment agreements and indemnification agreements with our key management, team, during the years ended December 31, 2015 and 2014, we incurred nil and $38,000, respectively, in professional fees for services rendered by one of the members of the Company's Board of Directors in connection with special tasks mandated by our Compensation Committee.there are no related party transactions.
| |
| C. | Interests of experts and counsel |
Not applicable.required.
| |
| Item 8. | Financial Information |
| |
| A. | Consolidated statements and other financial information |
The consolidated financial statements filed as part of this Annual Report on Form 20-F are presented under "Item 18. – Financial Statements".
No significant changes occurred since the date of our annual consolidated financial statements included elsewhere in this Annual Report on Form 20-F.
| |
| Item 9. | The OfferingOffer and Listing |
| |
| A. | Offer and listing details |
Not Applicable, except for Item 9A(4).
Our Common Shares are listed on both NASDAQ and TSX under the symbol "AEZS" and on the TSX under the symbol "AEZ". The following table indicates, for the relevant periods, the high and low closing prices of our Common Shares on NASDAQ and on the TSX:
|
| | | | | | | | |
| | NASDAQ (US$) | TSX (CAN$) |
| | High | Low | High | Low |
| 2018 | 3.87 |
| 1.19 |
| 5.10 |
| 1.53 |
|
| 2017 | 3.65 |
| 0.84 |
| 4.81 |
| 1.13 |
|
| 2019 | | | | |
First quarter 1 | 4.27 |
| 3.03 |
| 5.70 |
| 4.12 |
|
| 2018 | | | | |
| Fourth quarter | 3.87 |
| 1.30 |
| 5.10 |
| 1.69 |
|
| Third quarter | 2.03 |
| 1.60 |
| 2.69 |
| 2.10 |
|
| Second quarter | 2.62 |
| 1.19 |
| 3.34 |
| 1.53 |
|
| First quarter | 2.41 |
| 1.46 |
| 3.01 |
| 1.89 |
|
| 2017 | | | | |
| Fourth quarter | 2.70 |
| 1.87 |
| 3.48 |
| 2.38 |
|
| Third quarter | 2.87 |
| 0.98 |
| 3.57 |
| 1.28 |
|
| Second quarter | 3.35 |
| 0.84 |
| 4.50 |
| 1.13 |
|
| First quarter | 3.65 |
| 2.45 |
| 4.81 |
| 3.24 |
|
| | | | | |
|
| | | | | | | | |
| | NASDAQ (US$) | TSX (CAN$) |
| | High | Low | High | Low |
| 2015 | 84.20 |
| 4.00 |
| 104.00 |
| 5.39 |
|
| 2014 | 150.00 |
| 52.00 |
| 166.00 |
| 57.00 |
|
| 2013 | 323.00 |
| 103.00 |
| 327.00 |
| 108.00 |
|
| 2012 | 1,290.00 |
| 187.00 |
| 1,284.00 |
| 187.00 |
|
| 2011 | 1,548.00 |
| 858.00 |
| 1,506.00 |
| 846.00 |
|
| | | | | |
| 2016 | | | | |
First quarter(1) | 4.40 |
| 2.67 |
| 6.08 |
| 3.85 |
|
| 2015 | | | | |
| Fourth quarter | 11.43 |
| 4.00 |
| 15.41 |
| 5.39 |
|
| Third quarter | 27.50 |
| 5.02 |
| 35.00 |
| 7.00 |
|
| Second quarter | 64.10 |
| 27.00 |
| 78.00 |
| 32.50 |
|
| First quarter | 84.20 |
| 51.00 |
| 104.00 |
| 64.00 |
|
| 2014 | | | | |
| Fourth quarter | 134.00 |
| 52.00 |
| 151.00 |
| 57.00 |
|
| Third quarter | 150.00 |
| 114.00 |
| 164.00 |
| 123.00 |
|
| Second quarter | 123.00 |
| 105.00 |
| 135.00 |
| 113.00 |
|
| First quarter | 149.00 |
| 117.00 |
| 166.00 |
| 129.00 |
|
| | | | | |
| Most recent 6 months | | | | |
| February 2016 | 3.18 |
| 2.81 |
| 4.37 |
| 3.92 |
|
| January 2016 | 4.40 |
| 2.67 |
| 6.08 |
| 3.85 |
|
| December 2015 | 9.95 |
| 4.42 |
| 13.27 |
| 6.06 |
|
| November 2015 | 11.43 |
| 4.00 |
| 15.41 |
| 5.39 |
|
| October 2015 | 9.30 |
| 4.25 |
| 12.50 |
| 5.50 |
|
| September 2015 | 11.85 |
| 5.02 |
| 16.00 |
| 7.00 |
|
_________________________
(1) Up to and including March 28, 20162019.
Not applicable.
Our Common Shares are listed and posted for trading on both NASDAQ under the symbol "AEZS" and on the TSX under the symbol "AEZ""AEZS".
Not applicable.
Not applicable.
Not applicable.
| |
| Item 10. | Additional Information |
Item 10.Additional Information
Not applicable.required.
| |
| B. | Memorandum and articles of association |
We are governed by our restated articles of incorporation (the "Restated Articles of Incorporation") under the CBCA and by articles of amendment dated October 2, 2012 and November 16,17, 2015 (together with the Restated Articles of Incorporation, the "Articles") and by our bylaws, as amended and restated on March 21, 2013 (the "bylaws"). Our Articles are on file with the Corporations Directorate of Industry Canada under Corporation Number 264271-9. The Articles do not include a stated purpose and do not place any restrictions on the business that we may carry on.
Inspection Rights of Shareholders
Under the CBCA, shareholders are entitled to be provided with a copy of the list of our registered shareholders. In order to obtain the shareholder list, a shareholder must provide to us an affidavit including, among other things, a statement that the list will only be used for the purposes permitted by the CBCA. These permitted purposes include an effort to influence the voting of our shareholders, an offer to acquire our securities and any other matter relating to our affairs. We are entitled to charge a reasonable fee for the provision of the shareholder list and must deliver that list no more than ten days after receipt of the affidavit described above.
Under the CBCA, shareholders have the right to inspect certain corporate records, including the Corporation'sour Articles and bylaws and minutes of meetings and resolutions of the shareholders. Shareholders have no statutory right to inspect minutes of meetings and resolutions of our directors. Our shareholders have the right to certain financial information respecting us. In addition to the annual and quarterly financial statements required to be filed under applicable securities laws, under the CBCA, we are required by the CBCA to place before every annual meeting of shareholders our audited comparative annual financial statements. In addition, shareholders have the right to examine the financial statements of each of our subsidiaries and any other corporate entity whose accounts are consolidated in our financial statements.
Directors
The minimum number of directors we must have is five and the maximum number is 15. In accordance with the CBCA, at least 25% of our directors must be residents of Canada. In order to serve as a director, a person must be a natural person at least 18 years of age, of sound mind, not bankrupt, and must not be prohibited by any court from holding the office of director. None of the Articles, the bylaws and the CBCA imposesimpose any mandatory retirement requirements for directors.
The directors are elected by a majority of the votes cast at the annual meeting at which an election of directors is required, to hold office until the election of their successors, except in the case of resignations or if their offices become vacant by death or otherwise. Subject to the provisions of our bylaws, all directors may, if still qualified to serve as directors, stand for re-election. The Board is not replaced at staggered intervals but is elected annually.
There is no provision in our bylaws or Articles that requires that a director must be a shareholder.
The directors are entitled to remuneration as shall from time to time be determined by the Board or by a committee to which the Board may delegate the power to do so. Under the mandate of our Compensation Committee,the NGCC, such committee, comprised of at least a majority of independent directors, is tasked with making recommendations to the Board concerning director remuneration.
The CBCA provides that a director who is a party to, or who is a director or officer of, or has a material interest in, any person who is a party to a material contract or transaction or proposed material contract or transaction with us must disclose to us the nature and extent of his or her interest at the time and in the manner provided by the CBCA, or request that same be entered in the minutes of the meetings of the Board, even if such contract, in connection with our normal business activity, does not require the approval of either the directors or the shareholders. At the request of the president or any director, the director placed in a situation of conflict of interest must leave the meeting while the Board discusses the matter. The CBCA prohibits such a director from voting on any resolution to approve the contract or transaction unless the contract or transaction:
relates primarily to his or her remuneration as our director, officer, employee or agent or as a director, officer, employee or agent of an affiliate;affiliate of us;
is for indemnity or insurance for director's liability as permitted by the CBCA; or
is with our affiliate.
The CBCA provides that the Board may, on our behalf and without authorization of our shareholders:
borrow money upon our credit;
issue, reissue, sell or pledge our debt obligations;
give a guarantee on our behalf to secure performance of an obligation of any person; and
mortgage, hypothecate, pledge or otherwise create a security interest in all or any of our property, owned or subsequently acquired, to secure any of our obligations.
The shareholders have the ability to restrict such powers through our Articles or bylaws (or through a unanimous shareholder agreement), but no such restrictions are in place.
The CBCA prohibits the giving of a guarantee to any of our shareholders, directors, officers or employees or of an affiliated corporation or to an associate of any such person for any purpose or to any person for the purpose of or in connection with a purchase of a share issued or to be issued by us or our affiliates, where there are reasonable grounds for believing that we are or, after giving the guarantee, would be unable to pay our liabilities as they become due, or the realizable value of our assets in the form of assets pledged or encumbered to secure a guarantee, after giving the guarantee, would be less than the aggregate of our liabilities and stated capital of all classes. These borrowing powers may be varied by our bylaws or Articles. However, our bylaws and Articles do not contain any restrictions on or variations of these borrowing powers.
Pursuant to the CBCA, our directors manage and administer our business and affairs and exercise all such powers and authority as we are authorized to exercise pursuant to the CBCA, the Articles and the bylaws. The general duties of our directors and officers under the CBCA are to act honestly and in good faith with a view to our best interests and to exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances. Any breach of these duties may lead to liability to us and our shareholders for breach of fiduciary duty. In addition, a breach of certain provisions of the CBCA, including the improper payment of dividends or the improper purchase or redemption of shares, will render the directors who authorized such action liable to account to us for any amounts improperly paid or distributed.
Our bylaws provide that the Board may, from time to time, appoint from amongst their number committees of the Board, and delegate to any such committee any of the powers of the Board except those which pursuant to the CBCA a committee of the Board has no authority to exercise. As such, the Board has two standing committees: the Audit Committee and the Nominating, Governance and Compensation Committee, or the Compensation Committee.NGCC.
Subject to the limitations provided by the CBCA, our bylaws provide that we shall, to the full extent provided by law, indemnify a director or an officer, a former director or officer or a person who acts or acted at our request as a director or officer of a body corporate of which we are or were a shareholder or creditor, and his or her heirs and legal representatives, against all costs, losses, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by him or her in respect of any civil, criminal or administrative action or proceeding to which he or she is made a party by reason of having been our director or officer or such body corporate, provided: (a) he or she acted in good faith in our best interests and (b) in the case of a criminal or an administrative action or proceeding that is enforced by a monetary penalty, he or she had reasonable grounds to believe that his or her conduct was lawful.
| |
(a) | he or she acted in good faith in our best interests; and |
| |
(b) | in the case of a criminal or an administrative action or proceeding that is enforced by a monetary penalty, he or she had reasonable grounds to believe that his or her conduct was lawful. |
Our directors are authorized to indemnify from time to time any director or other person who has assumed or is about to assume in the normal course of business any liability for us or for any corporation controlled by us and to secure such director or other person against any loss by the pledge of all or part of our movable or immovable property through the creation of a hypothec or any other real right in all or part of such property or in any other manner.
We have also agreed to indemnify and save harmless our directors and senior corporate officers as well as the managing directors of our German subsidiary pursuant to various Director and Officer Indemnification Agreements against certain charges, damages, awards, settlements, liabilities, interest, judgments, fines, penalties, statutory obligations, professional fees and retainers and other expenses of whatever nature or kind, provided that any such costs, charges, professional fees and other expenses are reasonable (collectively, "Expenses") and from and against all Expenses sustained or incurred by the indemnified party as a result of serving as a director, officer or employee of the Company (or its subsidiary) in respect of any act, matter, deed or thing whatsoever made, done, committed, permitted, omitted or acquiesced in by the indemnified party as a director, officer or employee of the Company (or its subsidiary). The form of Director and Officer Indemnification Agreement has been furnished to the SEC as Exhibit 99.1 to our Report on Form 6-K dated October 21, 2016.
Share Capitalization
Our authorized share capital structure consists of an unlimited number of shares of the following classes (all classes are without nominal or par value): Common Shares; and first preferred shares (the "First Preferred Shares") and second preferred shares (the "Second Preferred Shares" and, together with the First Preferred Shares, the "Preferred Shares"), both issuable in series. As at March 29, 2016,2019, there were 9,928,697 approximately 16.4 millionCommon Shares outstanding. No Preferred Shares have been issued to date. We have also issued warrants to acquire Common Shares in connection with certain equity financings.
Common Shares
The holders of the Common Shares are entitled to one vote for each common shareCommon Share held by them at all meetings of shareholders, except meetings at which only shareholders of a specified class of shares are entitled to vote. In addition, the holders are entitled to receive dividends if, as and when declared by our Board of Directors on the Common Shares. Finally, the holders of the Common Shares are entitled to receive our remaining property upon any liquidation, dissolution or winding-up of our affairs, whether voluntary or involuntary. Shareholders have no liability to further capital calls as all shares issued and outstanding are fully paid and non-assessable.
Preferred Shares
The First and Second Preferred Shares are issuable in series with rights and privileges specific to each class. The holders of Preferred Shares are generally not entitled to receive notice of or to attend or vote at meetings of shareholders. The holders of First Preferred Shares are entitled to preference and priority to any participation of holders of Second Preferred Shares, Common Shares or shares of any other class of shares of our share capital ranking junior to the First Preferred Shares with respect to dividends and, in the event of our liquidation, the distribution of our property upon our dissolution or winding-up, or the distribution of all or part of our assets among the shareholders, to an amount equal to the value of the consideration paid in respect of such shares outstanding, as credited to our issued and paid-up share capital, on an equal basis, in proportion to the amount of their respective claims in regard to such shares held by them. The holders of Second Preferred Shares are entitled to preference and priority to any participation of holders of Common Shares or shares of any other class of shares of our share capital ranking junior to the Second Preferred Shares with respect to dividends and, in the event of our liquidation, the distribution of our property upon our dissolution or winding-up, or the distribution of all or part of our assets among the shareholders, to an amount equal to the value of the consideration paid in respect of such shares outstanding, as credited to our issued and paid-up share capital, on an equal basis, in proportion to the amount of their respective claims in regard to such shares held by them.
Our Board of Directors may, from time to time, provide for additional series of Preferred Shares to be created and issued, but the issuance of any Preferred Shares is subject to the general duties of the directors under the CBCA to act honestly and in good faith with a view to our best interests and to exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances.
Warrants
For a description of our Warrants, see note 17 - warrant liability, to the audited consolidated financial statements included in Item 18 of this Annual Report on Form 20-F.
Shareholder Actions
The CBCA provides that our shareholders may, with leave of a court, bring an action in our name and on our behalf for the purpose of prosecuting, defending or discontinuing an action on our behalf. In order to grant leave to permit such an action, the CBCA provides that the court must be satisfied that our directors were given adequate notice of the application, the shareholder is acting in good faith and that it appears to be in our best interests that the action be brought.
Amended and Restated Shareholder Rights Plan
OurThe Board of Directors adoptedof the Corporation approved a shareholder rights plan of the Corporation on March 29, 2016, which was approved, ratified and confirmed by the shareholders at the annual and special meeting of shareholders of the Corporation on May 10, 2016 (the “Existing Rights Plan”). The Existing Rights Plan was implemented to ensure, to the extent possible, that all shareholders of the Corporation are treated fairly in connection with any take-over offer or other acquisition of control of the Corporation.
Pursuant to the terms of the Existing Rights Plan, the Existing Rights Plan will expire upon the termination of the Meeting unless shareholders ratify its continued existence. The Board of Directors reviewed the terms of the Existing Rights Plan for conformity
with current Canadian securities laws, as well as the evolving practices of public corporations in Canada, with respect to shareholder rights plan design and has made some minor amendments thereto as a result.
The Board of Directors determined it appropriate and in the best interests of the shareholders to continue the Existing Rights Plan and approved the amended and restated shareholder rights plan (the "Rights Plan"). Under the rules on March 26, 2019. The Rights Plan will take effect immediately upon receipt of approval of the TSX, shareholder rights plans must be ratified by shareholders of a listed company within six monthsthe Corporation at the annual and special meeting of their adoption. Our shareholders will be askedscheduled to confirm and ratify the Rights Plan at our 2016 Annual Meeting, which will be held on May 10, 2016. 8, 2019.
If our shareholders do not confirm and ratify the Rights Plan at such meeting,is approved by the shareholders, the Existing Rights Plan will be amended as set forth below:
the provisions in which future shareholder approval is required to ratify the continued existence of the Rights Plan will be revised to specify that such events will occur at every third annual meeting of the shareholders subsequent to the annual meeting of shareholders whereby the Rights Plan is initially approved, as well as the addition of certain provisions in respect of the effective date of the plan to give effect to the fact that the Rights Plan is in effect a continuation of the Existing Rights Plan;
the definition of “Acquiring Person” will exclude Convertible Security Acquisitions (as defined below);
the definition of “Beneficial Owner”, “Beneficial Ownership” and “Beneficially Own” will:
exclude securities that may be acquired pursuant to any agreement related to an amalgamation, merger, arrangement, business combination or other similar transaction (statutory or otherwise, but for greater certainty not including a Take-over Bid) that is conditional upon shareholder approval prior to such Person acquiring such securities; and
include securities which are subject to a lock-up or similar agreement to tender or deposit them into any Take-over Bid made by such Person or made by any Affiliate or Associate of such Person or made by any other person acting jointly or in concert with such Person, other than Permitted Lock-up Agreements;
“Convertible Security Acquisitions” will be defined to mean an acquisition of Voting Shares by a Person upon the purchase, exercise, conversion or exchange of Convertible Securities, where such Convertible Securities are acquired or received by such Person pursuant to a Permitted Bid Acquisition, an Exempt Acquisition or a Pro Rata Acquisition;
“Market Price” will be defined to mean the average of the daily closing price per security on the 20 consecutive trading days (i.e. days on which the TSX or another stock exchange or national securities quotation system on which the Common Shares are traded (including for greater certainty, each of the Nasdaq Global Select Market, the Nasdaq Global Market and the rights issued thereunderNasdaq Capital Market) is open for the transaction of business, subject to certain exceptions), through and including the trading day immediately preceding such date of determination, subject to certain exceptions;
the definition of “Permitted Lock-Up Agreement” will terminate atbe added (as described below); and
certain other amendments of a non-substantive, “housekeeping” nature have been made to provide for greater clarity and consistency.
Other than the close ofamendments as described above, the Annual Meeting. The Rights Plan replaces ais substantially similar plan that was adopted by our Board of Directors and confirmed and ratified by our shareholders in 2010 and reconfirmed by our shareholders in 2013.to the Existing Rights Plan.
Objectives and Background of the Shareholder Rights Plan
The fundamental objectives of the Rights Plan are to provide adequate time for our Board and shareholders to assess an unsolicited
take-over bid for us, to provide the Board with sufficient time to explore and develop alternatives for maximizing shareholder value if a take-over bid is made, and to provide shareholders with an equal opportunity to participate in a take-over bid.
The Rights Plan encourages a potential acquiror who makes a take-over bid to proceed either by way of a "Permitted Bid", as described below, which requires a take-over bid to satisfy certain minimum standards designed to promote fairness, or with the concurrence of our Board. If a take-over bid fails to meet these minimum standards and the Rights Plan is not waived by the Board, the Rights Plan provides that holders of Common Shares,, other than the acquiror, will be able to purchase additional Common Shares at a significant discount to market, thus exposing the person acquiring Common Shares to substantial dilution of its holdings.
Summary of the Rights Plan
The following is a summary of the principal terms of the Rights Plan, which summary is qualified in its entirety by reference to the terms thereof. Capitalized terms not otherwise defined in this summary shall have the meaning ascribed to such terms in the Shareholder Rights Plan Agreement which sets forth the Rights Plan. TheA draft of the Rights Plan is filed as an exhibit to this Annual Report on Form 20-F. In preparing this summary we reviewedavailable at the amendments to the regulatory framework governing take-over bids published by the Canadian Securities Administrators that are scheduled to generally come into effect on May 9, 2016 (the “Amendments”).
In particular, the Amendments will require that all “non-exempt” take-over bids remain open for a minimum of 105 days, subject to the ability of a target issuer’s board of directors to shorten, in a non-discriminatory manner with respect to any potential other bids, the minimum period to a period of no less than 35 days by issuing a news release to such effect. We will continue to monitor the regulatoryfollowing websites: www.zenataris.com, www.sedar.com and governance landscape in Canada regarding the interaction of the Amendments and shareholder rights plans generally.www.sec.gov.
For the purposes of this summary and as set out in the Rights Plan, the term “MI 62-104”"NI 62-104" refers to MultilateralNational Instrument 62-104-Take-Over Bids and Issuer Bids adopted by certain of the Canadian securities regulatory authorities, as now in effect or as the same may from time to time be amended, re-enacted or replaced and including for greater certainty any successor instrument thereto (including, without limitation, National Instrument 62-104-Take-Over Bids and Issuer Bids of the Canadian Securities Administrators proposed to come into force on or about May 9, 2016).thereto.
Operation of the Rights Plan
Pursuant to the terms of the Rights Plan, we issued one right was issued in respect of each common share outstanding at 5:01 p.m. on March 29,2016 (the "Record Time"). In addition, we will issue one right for each additional Common Share issued after the Record Time andprior to the earlier of the Separation Time (as defined below) and the Expiration Time (as defined below). The rights have an initial exercise price equal to the Market Price (as defined below) of the Common Shares as determined at the Separation Time, multiplied by five, subject to certain anti-dilution adjustments (the "Exercise Price"), and they are not exercisable until the Separation Time. Upon the occurrence of a Flip-in Event (as defined below), each right will entitle the holder thereof, other than an Acquiring Person or any other person whose rights are or become void pursuant to the provisions of the Rights Plan, to purchase from us, effective at the close of business on the eighth trading day after the Stock Acquisition Date (as defined below), upon payment to us of the Exercise Price, Common Shares having an aggregate Market Price equal to twice the Exercise Price on the date of consummation or occurrence of such Flip-in Event, subject to certain anti-dilution adjustments.
Definition of Market Price
Market Price is generally defined in the Rights Plan, on any given day on which a determination must be made, as the volume weighted average trading price of the Common Shares for the five20 consecutive trading days (i.e. days on which the TSX or another stock exchange or national securities quotation system on which the Common Shares are traded (including for greater certainty, each of the Nasdaq Global Select Market, the Nasdaq Global Market and the Nasdaq Capital Market) is open for the transaction of business, subject to certain exceptions), through and including the trading day immediately preceding such date of determination, subject to certain exceptions.
Trading of Rights
Until the Separation Time (or the earlier termination or expiration of the rights), the rights trade together with the Common Shares and are represented by the same share certificates as the Common Shares or an entry in our securities register in respect of any outstanding Common Shares.Shares. From and after the Separation Time and prior to the Expiration Time, the rights are evidenced by rights certificates and trade separately from the Common Shares.Shares. The rights do not carry any of the rights attaching to the Common Shares such as voting or dividend rights.
Separation Time
The rights will separate from the Common Shares to which they are attached and become exercisable at the time (the "Separation Time") of the close of business on the eighth business day after the earliest to occur of:
| |
| 1. | the first date (the "Stock Acquisition Date") of a public announcement of facts indicating that a person has become an Acquiring Person; and |
| |
| 2. | the date of the commencement of, or first public announcement of the intention of any person (other than us or any of our subsidiaries) to commence a take-over bid or a share exchange bid for more than 20% of our outstanding Common Shares other than a Permitted Bid or a Competing Permitted Bid (as defined below), so long as such take-over bid continues to satisfy the requirements of a Permitted Bid or a Competing Permitted Bid, as the case may be. |
The Separation Time can also be such later time as may from time to time be determined by the Board, provided that if any such take-over bid expires, or is canceled, terminated or otherwise withdrawn prior to the Separation Time, without securities deposited thereunder being taken up and paid for, it shall be deemed never to have been made and if the Board determines to waive the application of the Rights Plan to a particular Flip-in Event, the Separation Time in respect of such Flip-in Event shall be deemed never tohave occurred.
From and after the Separation Time and prior to the Expiration Time, each right entitles the holder thereof to purchase one Common Share upon payment of the Exercise Price to us.
Flip-in Event
The acquisition by a person (an "Acquiring Person"), including others acting jointly or in concert with such person, of more than 20% of the outstanding Common Shares,, other than by way of a Permitted Bid, a Competing Permitted Bid or in certain other limited circumstances described in the Rights Plan, is referred to as a "Flip-in Event".
In the event that, prior to the Expiration Time, a Flip-in Event that has not been waived occurs (see "Waiver and Redemption" below), each right (other than those held by or deemed to be held by the Acquiring Person) will thereafter entitle the holder thereof, effective as at the close of business on the eighth trading day after the Stock Acquisition Date, to purchase from us, upon payment of the Exercise Price and otherwise exercising such right in accordance with the terms of the Rights Plan, that number of Common Shares having an aggregate Market Price on the date of consummation or occurrence of the Flip-in Event equal to twice the Exercise Price, for an amount in cash equal to the Exercise Price (subject to certain anti-dilution adjustments described in theRights Plan).
A bidder may enter into Permitted Lock-up Agreements with our shareholders ("Locked-up Persons") who are not affiliates or associates of the bidder and who are not, other than by virtue of entering into such agreement, acting jointly or in concert with the bidder, whereby such shareholders agree to tender their Common Shares to the take-over bid (the "Lock-up Bid") without the bidder being deemed to beneficially own the Common Shares deposited pursuant to the Lock-up Bid. Any such agreement must include a provision that permits the Locked-up Person to withdraw the Common Shares to tender to another take-over bid or to support another transaction that will either provide greater consideration to the shareholder than the Lock-up Bid or provide for a right to sell a greater number of shares than the Lock-up Bid contemplates (provided that the Permitted Lock-up Agreement may require that such greater number exceed the number of shares under the Locked-up Bid by a specified percentage not to exceed 7%).
TheA Permitted Lock-up Agreement may require that the consideration under the other transaction exceed the consideration under the Lock-up Bid by a specified amount. The specified amount may not be greater than 7%. For greater certainty, a Permitted Lock-up Agreement may contain a right of first refusal or require a period of delay (or other similar limitation) to give a bidder an opportunity to match a higher price in another transaction as long as the limitation does not preclude the exercise by the Locked-up Person of the right to withdraw the Common Shares during the period of the other take-over bid or transaction.
The Rights Plan requires that any Permitted Lock-up Agreement be made available to us and the public. The definition of Permitted Lock-up Agreement also provides that under a Permitted Lock-up Agreement, no "break up" fees, "topping" fees, penalties, expenses or other amounts that exceed in aggregate the greater of (i) 2.5% of the price or value of the aggregate consideration payable under the Lock-up Bid, and (ii) 50% of the amount by which the price or value of the consideration received by a Locked-up Person under another take-over bid or transaction exceeds what such Locked-up Person would have received under the Lock-up Bid, can be payable by such Locked-up Person if the Locked-up Person fails to deposit or tender Common Shares to the Lock-up Bid or withdraws Common Shares previously tendered thereto in order to deposit such Common Shares to another take-over bid or support another transaction.
Permitted Bid Requirements
The requirements of a Permitted Bid include the following:
| |
| 1. | the take-over bid must be made by means of a take-over bid circular; |
| |
| 2. | the take-over bid must be made to all holders of Common Shares wherever resident, on identical terms and conditions, other than the bidder; |
| |
| 3. | the take-over bid must not permit Common Shares tendered pursuant to the bid to be taken up or paid for: |
| |
| a) | prior to the close of business on a date that is not less than 105 days following the date of the relevant take-over bid or such shorter minimum period that a take-over bid (that is not exempt from any of the requirements of Division 5 (Bid Mechanics of MINI 62-104)) must remain open for deposits of securities thereunder, in the applicable circumstances at such time, pursuant to MINI 62-104; |
| |
| b) | then only if at the close of business on the date Common Shares (and/or “Convertible Securities”"Convertible Securities", as defined in the Rights Plan) are first taken up or paid for under such take-over bid, outstanding Common Shares and Convertible Securities held by shareholders other than any other Acquiring Person, the bidder, the bidder’s affiliates or associates, persons acting jointly or in concert with the bidder and any employee benefit plan, deferred profit-sharing plan, stock participation plan or trust for the benefit of our employees or the employees of any of our subsidiaries, unless the beneficiaries of such plan or trust direct the manner in which the Common Shares are to be voted or direct whether the Common Shares are to be tendered to a take-over bid (collectively, “Independent Shareholders”) that represent more than 50% of the aggregate of (I) then outstanding Common Shares and (II) Common Shares issuable upon the exercise of Convertible Securities, have been deposited or tendered pursuant to the take-over bid and not withdrawn; |
of any of our subsidiaries, unless the beneficiaries of such plan or trust direct the manner in which the Common Shares are to be voted or direct whether the Common Shares are to be tendered to a take-over bid (collectively, "Independent Shareholders") that represent more than 50% of the aggregate of (I) then outstanding Common Shares and (II) Common Shares issuable upon the exercise of Convertible Securities, have been deposited or tendered pursuant to the take-over bid and not withdrawn;
| |
| 4. | the take-over bid must allow Common Shares and/or Convertible Securities to be deposited or tendered pursuant to such take-over bid, unless such take-over bid is withdrawn, at any time prior to the close of business on the date Common Shares and/or Convertible Securities are first taken up or paid for under the take-over bid; |
| |
| 5. | the take-over bid must allow Common Shares and/or Convertible Securities to be withdrawn until taken up and paid for; and |
| |
| 6. | in the event the requirement set forth in clause 3.b.3.b) above is satisfied, the bidder must make a public announcement of that fact and the take-over bid must remain open for deposits and tenders of Common Shares for not less than ten days from the date of such public announcement. |
A Permitted Bid need not be a bid for all outstanding Common Shares not held by the bidder, i.e., a Permitted Bid may be a partial bid. The Rights Plan also allows a competing Permitted Bid (a “Competing"Competing Permitted Bid”Bid") to be made while a Permitted Bid is in existence. A Competing Permitted Bid must satisfy all the requirements of a Permitted Bid other than the requirement set out in clause 3.a3.a) above and must not permit Common Shares tendered or deposited pursuant to the bid to be taken up or paid for prior to the close of business on the last day of the minimum initial deposit period that such take-over bid must remain open for deposits of securities thereunder pursuant to MINI 62-104 after the date of the take-over bid constituting the Competing Permitted Bid; provided, however, that a take-over bid that has qualified as a Competing Permitted Bid shall cease to be a Competing Permitted Bid at any time and as soon as such time as when such take-over bid ceases to meet any or all of the foregoing provisions of the definition of “Competing"Competing Permitted Bid”Bid" and any acquisition of Common Shares and/or Convertible Securities made pursuant to such take-over bid that qualified as a Competing Permitted Bid, including any acquisition of Common Shares and/or Convertible Securities made before such take-over bid ceased to be a Competing Permitted Bid, will not be a “Permitted"Permitted Bid Acquisition”Acquisition" (as defined in the Rights Plan).
Waiver and Redemption
The Board may, prior to the occurrence of a Flip-in Event, waive the dilutive effects of the Rights Plan in respect of, among other things, a particular Flip-in Event resulting from a take-over bid made by way of a take-over bid circular to all holders of our Common Shares.Shares. In such an event, such waiver shall also be deemed to be a waiver in respect of any other Flip-in Event occurring under a take-over bid made by way of a take-over bid circular to all holders of Common Shares prior to the expiry of the first mentioned take-over bid.
The Board may, with the approval of a majority of Independent Shareholders (or, after the Separation Time has occurred, holders of rights, other than rights which are void pursuant to the provisions of the Rights Plan or which, prior to the Separation Time, are held otherwise than by Independent Shareholders), at any time prior to the occurrence of a Flip-in Event which has not been waived, elect to redeem all, but not less than all, of the then outstanding rights at a price of CAN$0.00001 each, appropriately adjusted as provided in the Rights Plan (the "Redemption Price").
Where a take-over bid that is not a Permitted Bid or Competing Permitted Bid is withdrawn or otherwise terminated after the Separation Time has occurred and prior to the occurrence of a Flip-in Event, the Board may elect to redeem all the outstanding rights at the Redemption Price without the consent of the holders of the Common Shares or the rights and reissue rights under the Rights Plan to holders of record of Common Shares immediately following such redemption. Upon the rights being so redeemed and reissued, all the provisions of the Rights Plan will continue to apply as if the Separation Time had not occurred, and the Separation Time will be deemed not to have occurred and we shall be deemed to have issued replacement rights to the holders of its thenoutstanding Common Shares.Shares.
Amendment to the Rights Plan
The Rights Plan may be amended to correct any clerical or typographical error or to make such changes as are required to maintain the validity of the Rights Plan as a result of any change in any applicable legislation, regulations or rules thereunder, without the approval of the holders of the Common Shares or rights. Prior to the Separation Time, we may, with the prior consent of the holders of Common Shares,, amend, vary or delete any of the provisions of the Rights Plan in order to effect any changes which the Board, acting in good faith, considers necessary or desirable. We may, with the prior consent of the holders of rights, atany time after the Separation Time and before the Expiration Time, amend, vary or delete any of the provisions of the Rights Plan.
Protection Against Dilution
The Exercise Price, the number and nature of securities which may be purchased upon the exercise of rights and the number of rights outstanding are subject to adjustment from time to time to prevent dilution in the event of stock dividends, subdivisions, consolidations, reclassifications or other changes in the outstanding Common Shares,, pro rata distributions to holders of Common Shares and other circumstances where adjustments are required to appropriately protect the interests of the holders of rights.
Fiduciary Duty of Board
The Rights Plan will not detract from or lessen the duty of the Board to act honestly and in good faith with a view to our best interests and the best interests of our shareholders. The Board will continue to have the duty and power to take such actions and make such recommendations to our shareholders as are considered appropriate.
Exemptions for Investment Advisors
Fund managers, investment advisors (for fully-managed accounts), trust companies (acting in their capacities as trustees and administrators), statutory bodies whose business includes the management of funds, and administrators of registered pension plans are exempt from triggering a Flip-in Event, provided that they are not making, or are not part of a group making, a take-over bid.
Term
The Rights Plan will expire (the "Expiration Time") at on the closeearlier of business(i) the Termination Time; and (ii) the Close of Business on the date on which the first annual meeting of our shareholders following March 29, 2019 (being the Corporation to be held in 2022 and at every third anniversaryannual meeting of the Record Time) is held; provided, however, that if our Independent Shareholders approveCorporation thereafter (each such annual meeting being a resolution confirming“Reconfirmation Meeting”) occurs and at which the Rights Plan atis not reconfirmed or prior topresented for reconfirmation as contemplated in the 2019 annual meeting of our shareholders, Expiration Time shall mean the close of business on the date on which the first annual meeting of our shareholders following March 29, 2022 (being the sixth anniversary of the Record Time) is held.Rights Plan (the “Expiration Time”).
Action Necessary to Change Rights of Shareholders
In order to change the rights of our shareholders, we would need to amend our Articles to effect the change. Such an amendment would require the approval of holders of two-thirds of the issued and outstanding shares cast at a duly called special meeting. For certain amendments, a shareholder is entitled under the CBCA to dissent in respect of such a resolution amending the Articles and, if the resolution is adopted and we implement such changes, demand payment of the fair value of its shares.
Disclosure of Share Ownership
In general, under applicable securities regulation in Canada, a person or company who beneficially owns, or who directly or indirectly exercises control or direction over voting securities of a reporting issuer, voting securities of an issuer or a combination of both, carrying more than ten percent of the voting rights attached to all the issuer's outstanding voting securities is an insider and must, within ten days of becoming an insider, file a report in the required form effective the date on which the person became an insider, disclosing any direct or indirect beneficial ownership of, or control or direction over, securities of the reporting issuer.
Additionally, securities regulation in Canada provides for the filing of a report by an insider of a reporting issuer whose holdings change, which report must be filed within five days from the day on which the change takes place.
Section 13 of the United States Securities Exchange Act of 1934 (the "Exchange Act") imposes reporting requirements on persons
who acquire beneficial ownership (as such term is defined in the Rule 13d-3 under the Exchange Act) of more than five percent of a class of an equity security registered under Section 12 of the Exchange Act. Our Common Shares are so registered. In general, such persons must file, within ten days after such acquisition, a report of beneficial ownership with the SEC containing the information prescribed by the regulations under Section 13 of the Exchange Act. This information is also required to be sent to the issuer of the securities and to each exchange where the securities are traded.
Meeting of Shareholders
An annual meeting of shareholders is held each year for the purpose of considering the financial statements and reports, electing directors, appointing auditors and fixing or authorizing the Board to fix their remuneration and for the transaction of other business as may properly come before a meeting of shareholders. Any annual meeting may also constitute a special meeting to take cognizance and dispose of any matter of which a special meeting may take cognizance and dispose. Under the bylaws, our Chief Executive Officer or our President has the power to call a meeting of shareholders.
The CBCA provides that the holders of not less than 5% of our outstanding voting shares may requisition our directors to call a meeting of shareholders for the purpose stated in the requisition. Except in limited circumstances, including where a meeting of
shareholders has already been called and a notice of meeting already given or where it is clear that the primary purpose of the requisition is to redress a personal grievance against us or our directors, officers or shareholders, our directors, on receipt of such requisition, must call a meeting of shareholders. If the directors fail to call a meeting of shareholders within twenty-one days after receiving the requisition, any shareholder who signed the requisition may call the meeting of shareholders and, unless the shareholders resolve otherwise at the meeting, we shall reimburse the shareholders for the expenses reasonably incurred by them in requisitioning, calling and holding the meeting of shareholders.
The CBCA also provides that, except in limited circumstances, a resolution in writing signed by all of the shareholders entitled to vote on that resolution at a meeting of shareholders is as valid as if it had been passed at a meeting of shareholders.
A quorum of shareholders is present at an annual or special meeting of shareholders, regardless of the number of persons present in person at the meeting, if the holder(s) of shares representing at least 10% of the outstanding voting shares at such meeting are present in person or represented in accordance with our bylaws. In the case where the CBCA, our Articles or our bylaws require or permit the vote by class of holders of a given class of shares of our share capital, the quorum at any meeting will be one or more persons representing 10% of the outstanding shares of such class.
Notice of the time and place of each annual or special meeting of shareholders must be given not less than 21 days, nor more than 50 days, before the date of each meeting to each director, to the auditor and to each shareholder entitled to vote thereat. If the address of any shareholder, director or auditor does not appear in our books, the notice may be sent to such address as the person sending the notice may consider to be most likely to reach such shareholder, director or auditor promptly. Every person who, by operation of the CBCA, transfers or by any other means whatsoever, becomes entitled to any share, shall be bound by every notice given in respect of such share which, prior to the entry of his or her name and address on our register, is given to the person whose name appears on the register at the time such notice is sent. Notice of meeting of shareholders called for any other purpose other than consideration of the financial statements and auditor's report, election of directors and reappointment of the incumbent auditor, must state the nature of the business in sufficient detail to permit the shareholder to form a reasoned judgment on and must state the text of any special resolution or bylaw to be submitted to the meeting.
On March 21, 2013, the Board of Directors approved an amendment to ourOur bylaws in order to include an advance notice provision (the "Advance Notice Requirement") and concurrently approved an amendment to and restatement of our bylaws giving effect to the Advance Notice Requirement (the "Amended and Restated Bylaws"). The Amended and Restated Bylaws giving effect to the Advance Notice Requirement were subsequently ratified and approved by our shareholders on May 8, 2013. The Advance Notice Requirement applies in certain circumstances where nominations of persons for election to the Board of Directors are made by our shareholders other than pursuant to: (a) a requisition of a meeting made pursuant to the provisions of the CBCA; or (b) a shareholder proposal made pursuant to the provisions of the CBCA.
Among other things, the Advance Notice Requirement fixes a deadline by which shareholders must submit a notice of director nominations to us prior to any annual or special meeting of shareholders where directors are to be elected and sets forth the information that a shareholder must include in the notice for it to be valid. In the case of an annual meeting of shareholders, we must be given not less than 30 nor more than 65 days' notice prior to the date of the annual meeting; provided, however, that in the event that the annual meeting is to be held on a date that is less than 50 days after the date on which the first public announcement of the date of the annual meeting was made, notice may be made not later than the close of business on the 10th day following such public announcement. In the case of a special meeting of shareholders (which is not also an annual meeting), we must be given notice not later than the close of business on the 15th day following the day on which the first public announcement of the date of the special meeting was made.
The Board of Directors may, in its sole discretion, waive any requirement of the Advance Notice Requirement.
Limitations on Right to Own Securities
Neither Canadian law nor our Restated Articles of Incorporation, our articles of amendment or bylaws limit the right of a non-resident to hold or vote our Common Shares, other than as provided in the Investment Canada Act (the "Investment Act").
The Investment Act requires any person that is a “non-Canadian”"non-Canadian" (as defined in the Investment Act) who acquires “control”"control" (as defined in the Investment Act) of an existing Canadian business to file either a pre-closing application for review or a post-closing notification with IndustryInnovation, Science and Economic Development Canada.
On March 25, 2015, the Canadian government announced new Investment Act regulations that changed the thresholds for determining when an acquisition of control of a Canadian business is a reviewable transaction (from an asset value-based test to an enterprise value-based test, in most cases). As of April 24, 2015, when amendments to the Investment Act and the regulations come into force,February 2, 2019, the threshold for review of a direct acquisition of control of a non-cultural Canadian business by a World Trade Organization member country investor that is not a state-owned enterprise is an enterprise value of assets that exceeds CAN$600 million.1.045 billion. For “trade agreement investors” that are not state-owned enterprises (as defined in the Investment Act), which as of March 2019 include investors ultimately controlled by nationals of Australia, Chile, Colombia, EU member states, Honduras, Japan, Korea, Mexico, New Zealand, Panama, Peru, Singapore, the United States or Vietnam, the threshold for review of a direct acquisition of control of a non-cultural Canadian business is an enterprise value of assets that exceeds C$1.568 billion. The enterprise value review threshold will remain at CAN$600 millionthresholds for two years, before increasingboth World Trade Organization member countries and trade agreement investors are indexed to CAN$800 million for theannual GDP growth and are adjusted accordingly each year. following two years, and then to CAN$1 billion. For purposes of a publicly traded company, the “enterprise value” "enterprise value"
of the assets of the Canadian business is equal to the market capitalization of the entity, plus its liabilities (excluding its operating liabilities), minus its cash and cash equivalents.
As such, under the Investment Act, the acquisition of control of us (either through the acquisition of our Common Shares or all or substantially all our assets) by a non-Canadian who is a World Trade Organization member country investor or a trade agreement investor, including a U.S. investor, would be reviewable only if the enterprise value of our assets exceeds the specified threshold for review.
Where the acquisition of control is a reviewable transaction, the Investment Act generally prohibits the implementation of the reviewable transaction unless, after review, the relevant Minister is satisfied or deemed to be satisfied that the acquisition is likely to be of net benefit to Canada.
The acquisition of a majority of the voting interests of an entity is deemed to be acquisition of “control”"control" of that entity. The acquisition of less than a majority but one-third or more of the total number of votes attached to all of the voting shares of a corporation or of an equivalent undivided ownership interest in the total number of votes attached to all of the voting shares of the corporation is presumed to be an acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquiror through the ownership of voting shares. The acquisition of less than one-third of the total number of votes attached to all of the voting shares of a corporation is deemed not to be acquisition of control of that corporation subject to certain discretionary rights relative to investments involving state ownedstate-owned enterprises. Other than in connection with a "national security" review, discussed below, certain transactions in relation to our Common Shares would be exempt from the Investment Act including:
the acquisition of our Common Shares by a person in the ordinary course of that person's business as a trader or dealer in securities;
the acquisition or control of us in connection with the realization of security granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Act,if the acquisition is subject to approval under the Bank Act, the Cooperative Credit Associations Act, the Insurance Companies Act or the Trust and Loan Companies Act; and
the acquisition or control of us by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of us, through the ownership of our voting interests, remains unchanged.
Under the national security regime in the Investment Act, review on a discretionary basis may also be undertaken by the federal government in respect of a much broader range of investments by a non-Canadian to "acquire, in whole or in part, or to establish an entity carrying on all or any part of its operations in Canada". The relevant test is whether such an investment by a non-Canadian could be "injurious to national security". The Minister of Innovation, Science and Economic Development has broad discretion to determine whether an investor is a non-Canadian and therefore may be subject to national security review. Review on national security grounds is at the discretion of the federal government and may occur on a pre or post-closing basis.
There is no law, governmental decree or regulation in Canada that restricts the export or import of capital, or which would affect the remittance of dividends or other payments by us to non-resident holders of our Common Shares, other than withholding tax requirements.
Other than as disclosed herein under "Shareholder"Amended and Restated Shareholder Rights Plan" and below, and except for contracts entered into in the ordinary course of business, there are no material contracts to which we or any of our subsidiaries is a party.
License and Assignment Agreement
79On January 16, 2018, the Company, through AEZS Germany, entered into a license and assignment agreement (the "License and Assignment Agreement") with Strongbridge, to carry out development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) in the United States and Canada.
The Company received a cash payment of $24,000,000 from Strongbridge, and, for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on net sales up to $75,000,000 and an 18% royalty on net sales above $75,000,000. Following the end of patent protection in U.S. or Canada for Macrilen™ (macimorelin), the Company will be entitled to a 5% royalty on net sales in that country. In addition, the Company will also receive one-time payments from Strongbridge following the first achievement of the following commercial milestone events:
$4,000,000 on achieving $25,000,000 annual net sales,
$10,000,000 on achieving $50,000,000 annual net sales,
$20,000,000 on achieving $100,000,000 annual net sales,
$40,000,000 on achieving $200,000,000 annual net sales, and
$100,000,000 on achieving $500,000,000 annual net sales.
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment of $5,000,000 from the licensee.
The licensee will fund 70% of the costs of a pediatric clinical submitted for approval to the EMA and FDA to be run by the Company with customary oversight from a joint steering committee. The joint steering committee will be comprised of four persons, two of whom will be appointed by each of Strongbridge and the Company.
The License and Assignment Agreement will expire at the end of a defined royalty period in each of the U.S. and Canada (the "Territory"), at which time the license that the Company granted will become irrevocable, fully paid-up, perpetual and royalty-free in such country. The licensee has the right to terminate the License and Assignment Agreement if there is a safety concern related to Macrilen™ (macimorelin), withdrawal of regulatory approval for Macrilen™ (macimorelin) in the U.S. believed to be permanent, two hundred and seventy (270) days' prior written notice, or if the Company commits a material breach of any term of the License and Assignment Agreement that it fails to cure within 90 days after receiving written notice of the breach. The Company has the right to terminate the License and Assignment Agreement if the licensee commits a material breach of any term of the License and Assignment Agreement that it fails to cure within 90 days after receiving written notice of the breach. If the breach relates to Canada then the Company shall only have the right to terminate the License and Assignment Agreement in relation to Canada. If the breach relates to the United States, then the Company shall have the right to terminate the License and Assignment Agreement in its entirety.
The License and Assignment Agreement contains customary provisions related to, among other things, confidentiality and non-disclosure, representations and warranties, indemnity and dispute resolution. The License and Assignment Agreement is governed by the laws of the State of New York, United States.
The License and Assignment Agreement is incorporated by reference as Exhibit 4.3 to this Annual Report on Form 20-F.
Effective December 19, 2018, Strongbridge sold its rights to Macrilen™ (macimorelin) in Canada and the United States to Novo and Novo will fund Strongbridge’s Macrilen™ (macimorelin) field organization as a contract field force to promote the product in the United States for up to three years.
Sinopharm Agreements
On December 1, 2014, we entered into an exclusive master collaboration agreement ("Master Collaboration Agreement,Agreement"), a Technology Transfertechnology transfer and Technical Assistance Agreementtechnical assistance agreement ("Tech Transfer Agreement") and a License Agreementlicense agreement ("Sinopharm License Agreement") with Sinopharm A-Think Pharmaceuticals Co., Ltd. ("Sinopharm") for the development, manufacture and commercialization of Zoptrex™ in all human uses, in the People's Republic of China, including Hong Kong and Macau (collectively, the “Territory""Sinopharm Territory"). Under the terms of the Tech Transfer Agreement, Sinopharm made a one-time, non-refundable payment of $1,101,000 ("Transfer Fee") to us for the transfer of technical documentation and materials, know-how and technical assistance services. We will be entitled to receive additional consideration upon achieving certain milestones, including the occurrence of certain regulatory and commercial events in the Sinopharm Territory. Furthermore, we will be entitled to royalties on future net sales of Zoptrex™ in the Sinopharm Territory. Sinopharm will be responsible for the development, production, registration and commercialization of Zoptrex™ in the Sinopharm Territory.
Sinopharm is required to use commercially reasonable efforts to develop, manufacture and commercialize Zoptrex™ in the Sinopharm Territory, in order to maximize the net sales derived from Zoptrex™ during the royalty term of the Sinopharm License Agreement. In particular, Sinopharm is required to use commercially reasonable efforts to: (i) develop Zoptrex™ for the indication of endometrial cancer in the Sinopharm Territory in accordance with an agreed development plan and not to terminate, suspend, halt or delay development, unless there are substantial safety, efficacy, commercial or regulatory reasons for doing so; (ii) apply for and obtain all required regulatory approvals in the Sinopharm Territory following successful completion of all appropriate clinical studies; (iii) make the first commercial sale of Zoptrex™ in the Sinopharm Territory within a specified period of time following the approval of Zoptrex™ for endometrial cancer; (iv) maintain an adequate sales force and provide for relevant staff to manage the pre- and post-launch activities required to commercialize Zoptrex™ in the Sinopharm Territory; and (v) seek to maximize sales of Zoptrex™ in the Sinopharm Territory. Sinopharm’s failure to use commercially reasonable efforts to develop, manufacture and commercialize Zoptrex™ would be a material breach of the Sinopharm License Agreement.
The Sinopharm License Agreement imposes on Sinopharm the responsibility for marketing, promoting and selling Zoptrex™ in the Sinopharm Territory after all regulatory approvals for commercial sale have been obtained, including pre-launch and post-launch marketing, promoting, conducting market research, distributing, offering to commercially sell and commercially selling Zoptrex™, importing, exporting or transporting Zoptrex™ for commercial sale, conducting medical education activities, conducting clinical studies that are not required to obtain or maintain regulatory approval of Zoptrex™ for an indication, which may include epidemiological studies, modeling and pharmacoeconomic studies, conducting post-marketing surveillance studies, conducting investigator sponsored studies and health economics studies and regulatory affairs.
The Sinopharm License Agreement will expire at the end of a defined royalty period, at which time the license that we granted to Sinopharm will become a fully paid-up, perpetual license. Sinopharm has the right to terminate the Sinopharm License Agreement if there are material safety, efficacy, commercial or regulatory reasons for doing so; if we commit a material breach of any term of the Sinopharm License Agreement that we fail to cure within 90 days after receiving written notice of the breach; if we file or institute bankruptcy, reorganization, liquidation or receivership proceedings; or if we assign a substantial portion of our assets for the benefit of our creditors. If Sinopharm has the right to terminate because a third party institutes involuntary bankruptcy proceedings against us, we will have 90 days to obtain the dismissal of the proceedings, during which time, Sinopharm may not terminate the Agreement.
We have the right to terminate the Sinopharm License Agreement if Sinopharm commits a material breach of any term of the Sinopharm License Agreement that it fails to cure within 90 days after receiving written notice of the breach; if it files or institutes bankruptcy, reorganization, liquidation or receivership proceedings, or if it assigns a substantial portion of its assets for the benefit of its creditors. If we have the right to terminate because a third-party institutes involuntary bankruptcy proceedings against Sinopharm, it will have 90 days to obtain the dismissal of the proceedings, during which time, we may not terminate the Agreement.
The Sinopharm License Agreement contains customary provisions related to, among other things, our oversight of Sinopharm’sSinopharm's commercialization efforts, intellectual property, pharmacovigilance, confidentiality and non-disclosure, representations and warranties, indemnity and dispute resolution. The Sinopharm License Agreement is governed by the laws of Hong Kong.
We do not anticipate significant revenues from the Sinopharm License Agreement in the future other than the amoritzation of the remaining deferred revenue.
The Master Collaboration Agreement, the Sinopharm License Agreement and the Tech Transfer Agreement are incorporated by reference as Exhibits 4.11, 4.124.9, 4.10 and 4.134.11 to this Annual Report on Form 20-F.
Employment and Service Agreements
We have,had, or one of our subsidiaries has,had, entered into an employment agreement orand, in some cases, a service contract (collectively, the "Employment Agreements")change of control agreement with each of theour Named Executive Officers who remain in ourOfficers. We terminated Dr. Sachse’s employment except foron January 17, 2018, effective June 14, 2018, and terminated Mr. Philip A. Theodore, our Senior Vice President,Clavijo’s employment on September 24, 2018.
The employment and change of control agreements of Mr. Ward, the employment and change of control agreements of Mr. Clavijo. and the consulting agreement of Ms. Auld described below are filed as Exhibits 4.4, 4.5, 4.6, 4.7 and 4.8 to this Annual Report on Form 20-F.
Michael Ward
We entered into an employment agreement and a change of control agreement with Michael V. Ward, Chief AdministrativeExecutive Officer, General Counsel and Secretary.effective as of October 1, 2017 (the "Employment Agreement"). The Employment Agreements provideAgreement provides that we will pay the executive aMr. Ward (the "Executive") an initial base salary of $250,000 and an annual cash bonus, if our economicfinancial results and position justify payment of a bonus and subject to the determination and approval of the Governance CommitteeNGCC and our Board, and that such executivesBoard. Additionally, the Executive will be eligible to receive long-term incentive grants in the form of stock options, which will be reviewed annually in accordance with our policies. Under the terms of the Employment Agreement, Mr. Ward's base salary increased to $325,000, upon approval of Macrilen™ (macimorelin) by the FDA, effective as of December 11, 2017.
The Employment Agreements have an indefinite term; provided, however,Agreement provides that Dr. Sachse's Employment Agreement will end without the need to give notice not later than the expiry of the month during which Dr. Sachse attains the minimum age of legal retirement in Germany.
The Employment Agreements of Messrs. Dodd, Dinges and Santorelli provide that (i) if we terminate their employment without “Cause”, (ii) in the case of Mr. Dinges, there is a “separation from service”"separation form service" within the meaning of Section 409A of the U.S. Internal Revenue Code of 1986, as amended, (a “Separation from Service”)as a result of (i) termination of the Executive's employment by us without "Cause" or (iii) if they resign(ii) the Executive resigns for “Good Reason”,"Good Reason," then the executiveExecutive will be entitled to receive severance payments in the case of Mr. Dodd, a lump-sum payment (less applicable tax withholdings) in an amount equal to twice the sumat least eighteen (18) months of his then base salary his then annual bonus,paid in equal installments over one (1) year, and conditional upon the amount of his then car allowance, plus any earned retention bonusExecutive executing a full and eighteen months of the value of the other benefits to which he is entitled (through the purchase by us of eighteen months of the coverage required under the Consolidated Omnibus Budget Reconciliation Act of 1986 ("COBRA")). In the case of Messrs. Dingesgeneral Release and Santorelli, the executive is entitled to receive a lump-sum payment (less applicable tax withholdings) in an amount equal to one times the sumof his then base salary, his then annual bonus, pro-rated as applicable, any earned retention bonus, if applicable, the amount of his then car allowance, if applicable,complying with certain non-compete and eighteen months of the value of the other benefits to which he is entitled (through our purchase of eighteen months of the coverage required under COBRA). In addition, in the case of Messrs. Dodd, Dinges and Santorelli, if the executive has a Separation of Service, then the executive's right to exercise all then outstanding stock options granted to him shall fully and immediately vest on the effective date of the Separation from Service.
In connection with the closure of our Quebec City office and the restructuring of our finance and accounting staff, on October 9, 2015, we entered into a transition agreement with Mr. Santorelli pursuant to which we agreed to pay him the sum of $336,600 and to maintain coverage under our health insurance plan until August 31, 2016, and to permit all stock options issued to him to immediately vest in exchange for his provision of certain transition services to us. We also agreed to waive the non-competition covenant contained in his employment agreement. Mr. Santorelli’s employment with us terminated on February 18, 2016 after he fulfilled his obligations to us pursuant to the transition agreement.
Dr. Sachse's Employment Agreement provides that we are entitled to terminate his agreement without cause by giving him six months' prior notice effective to the end of any calendar month. During the six-month notice period, Dr. Sachse is entitled only to his salary and heconfidentiality agreements. The Executive has no right to receive a cash bonus or any other form of remuneration.
Furthermore, Messrs. Dodd and DingesThe Executive shall not, for a period equal to one year following such executive'shis termination of employment with us, directly or indirectly,
compete with us;us in a business in the development and commercialization of substantially similar endocrine therapies and oncology treatments; solicit any of our clients or do anything whatsoever to induce or to lead any person to end, in whole or in part, its business relations with us; induce, attempt to induce or otherwise interfere in the relations whichthat we have with our distributors, suppliers, representatives, agents and other parties with whom we deal; or induce, attempt to induce or otherwise solicit our personnel to leave their employment with us or hire our personnel for any enterprise in which the executiveExecutive has an interest. The foregoing agreement applies in each territorythose geographic areas in the United States, Canada and Europe in which we had “actively exploited” (as defined in each executive's employment agreement) a product during the two years preceding the date of such executive's termination of employment.
Dr. Sachse's Employment Agreement also contains a non-competition provision. Dr. Sachse is prohibited from competing with us,same or any of our subsidiaries, during the term of his Employment Agreement and for a period of one year following the date of termination of his Employment Agreement. The non-competition provision prohibits Dr. Sachse from participating in any capacity whatsoever, and from having any interest whatsoever, in a business that would directly or indirectly compete with us, or with any of our subsidiaries, including a business involved in the development and commercialization of the specificsubstantially similar endocrine therapies and oncology treatments that we, or any of our subsidiaries,treatment are actively developing. The territory covereddeveloped and commercialized by Dr. Sachse's non-competition provision is the geographical areas in which a specific product had been actively exploited by us or one of our subsidiaries during the two years preceding the date of termination of his employment. The non-competition provision prohibits Dr. Sachse from performing duties for the competing business that are identical or substantially similar to those duties he performed or carried on for us during the 24 months preceding the termination of his Employment Agreement. If Dr. Sachse is unable to find a new employment because of the existence of the non-competition provision, we will pay him his base salary during a period ending on the first to occur of (i) the date on which he starts a new employment and (ii) the date on which the non-competition provision expires.us.
Pursuant to histhe Employment Agreement, Mr. Doddthe Executive is also entitled to receive certain payments in lieu of and not in addition to any severance payments provided under the Employment Agreement (the "Change of Control Payments") in the event (i) a "Change of Control" occurs, and (ii) during the twelve-month period following the Change of Control, either we terminate his employment without "Cause", or he terminates his employment for "Good Reason" during such period. The Change of Control Payment will equal the sum of the following amounts: (i) the equivalent of thirty-sixeighteen (18) months of histhe Executive’s then annual base salary, (ii) an amount equivalent to twiceeighteen (18) months of the Executive’s annual bonus, if any, which he would have been entitled to receivereceived in the year during whichimmediately prior to the year the Change of Control occurred, and (iii) an amount equivalent to eighteen (18) months of the then monthly premium to provide the group medical benefits to the any earned retention bonus, and (iv) an amount equivalent to 12eighteen (18) months of the then annual cost monthly premium to provide the other benefits to which he is entitled, or our cost to purchase coverage under COBRA for such benefits, whichever is applicable. group medical benefits Executive, his spouse and dependents determined by utilizing the applicable COBRA premium rates for the month the Executive’s employment terminates. The Change of Control Payment is subject to applicable statutory withholdings. Any outstanding stock options held by Mr. Doddto acquire our stock shall, in such circumstances, become fully exercisable, vested and immediately vestnon-forfeitable on the date the Executive’s employment terminates following a Change of his Separation from Service.Contract during the term of the agreement. The payments are conditional on the Executive executing a full and general Release.
For the purposes of the applicable Employment Agreements (including the annexes and schedules thereto):Agreement:
a "Change of Control" shall be deemed to have occurred in any of the following circumstances: (i) subject to certain exceptions, upon the acquisition by a person (or one or more persons who are affiliates of one another or who are acting jointly or in concert) of a beneficial interest in our securities representing in any circumstance 50% or more of the voting rights attaching to our then outstanding securities; (ii) upon a sale or other disposition of all or substantially all of our assets; (iii) upon a plan of liquidation or dissolution of us; or (iv) if, for any reason, including our amalgamation, merger or consolidation with or into another company, the individuals who, as atduring the dateterm of the relevant Employment Agreement,change of control agreement, constituted the Board (and any new directors whose appointment by the Board or whose nomination for election by our shareholders was approved by a vote of at least two-thirds of the directors then still in office who either were directors as atduring the dateterm of the relevant Employment Agreementchange of control agreement or whose appointment or nomination for election was previously so approved) cease to constitute a majority of the members of the Board;
termination of employment for "Cause" includes (but is not limited to) (i) if the executiveExecutive commits any fraud, theft, embezzlement or other criminal act of a similar nature, andor (ii) if the executive is guiltyExecutive commits an act of serious misconduct or willful or gross negligence in the performance of his duties; andduties.
terminationTermination of employment by the executive officerExecutive for "Good Reason" means the occurrence, without the Executive’s express written consent, of any of the following acts: (i) a material reduction of the Executive’s base salary as in effect on the date of his Employment Agreement or as same may be increased from time to time, and (ii) any material and sustained reduction in the Executive’s duties and responsibilities as Chief Executive Officer and the Board has been provided with notice and fails to cure the situation within thirty (30) days following receipt of notice.
James Clavijo
We entered into an employment agreement and a change of control agreement with Mr. James Clavijo, Chief Financial Officer. Mr. Clavijo left the Company in September 2018, at which time, he received a severance payment in accordance with his employment agreement.
Leslie Auld
We entered into a consulting agreement with Leslie Auld, Senior Vice President, Chief Financial Officer, effective as of September 24, 2018 (the "Consulting Agreement"). The Consulting Agreement provides that Ms. Auld (the "Consultant") will perform specified services for us for up to 120 hours per month. The Consultant will be paid $150 per hour (plus HST) (the “base fees”) for these services. Additionally, the Consultant will be paid for up to eight (8) hours of travel time per round trip, at a rate of $150 per hour.
The Consulting Agreement may be terminated by either party for convenience, upon thirty (30) days written notice. The Consulting Agreement may also be terminated by us upon the material breach or default of any provision of the Consulting Agreement by the Consultant, immediately upon the Consultants death or upon the parties’ mutual agreement. In the event of termination, the Consultant will be entitled to receive any outstanding base fees and reimbursement for incurred expenses to the effective date of termination.
The Consulting Agreement provides the Consultant indemnifies us from and against any and all claims, costs, liabilities, damages, charges and expenses arising out of the Consulting Agreement or the services, including in respect of misclassification.
The table below shows estimated incremental payments triggered pursuant to termination of employment of our Named Executive Officers who remained employed on December 31, 2018. The amounts shown are in U.S. dollars.
|
| |
| Name | Termination Provisions
Value ($)(1) (2) |
| Auld, Leslie | 0 |
| Garrison, Brian | 0 |
| Guenther, Eckhard | 94,800 |
| Ward, Michael V. | 487,500 |
| |
◦(1) | inThe termination values assume that the case of Mr. Dodd, the occurrence, without his express written consent, of any of the following acts: (i) a material reduction of his total compensation (including annual base salary plus annual bonus, benefits and number of stock options) as in effecttriggering event took place on the datelast business day of his Employment Agreement or as same may be increased from time to time, provided such reduction is not warranted and due to our performance; (ii) any change in his direct reporting relationship to the Board; (iii) any reduction in his duties and responsibilities as our President and Chief Executive Officer; or (iv) a physical change of one hundred miles of more in his principal place of business; andfinancial year-end (December 31, 2018). |
| |
◦(2) | Value of earned/unused vacation, if applicable, and amounts owing for expense reimbursement are not included as they are not considered as “incremental” payments made in the caseconnection with termination of Mr. Dinges, the occurrence, without his express written consent, of any of the following acts: (i) a more than 25% reduction of his base annual salary as in effect on the date of his Employment Agreement or as the same may be increased from time to time, provided such reduction is not warranted and due to either our performance or failure of Mr. Dinges to achieve performance standards or objectives as determined by our President in his sole and absolute discretion and judgment; or (ii) a material reduction in his duties and responsibilities as our Chief Commercial Officer.employment. |
Canada has no system of exchange controls. There are no exchange restrictions on borrowing from foreign countries or on the remittance of dividends, interest, royalties and similar payments, management fees, loan repayments, settlement of trade debts or the repatriation of capital.
E. Taxation
THE FOLLOWING SUMMARY IS OF A GENERAL NATURE ONLY AND IS NOT INTENDED TO BE, NOR SHOULD IT BE CONSTRUED TO BE, LEGAL OR TAX ADVICE TO ANY PARTICULAR HOLDER. CONSEQUENTLY, HOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS FOR ADVICE AS TO THE TAX CONSEQUENCES OF AN INVESTMENT IN THE COMMON SHARES HAVING REGARD TO THEIR PARTICULAR CIRCUMSTANCES.
Material Canadian Income Tax Considerations
The following summary describes the principal Canadian federal income tax considerations applicable to a holder who acquiresof Common Shares (a "holder") and who, for the purposes of the Canadian federal Income Tax Act, R.S.C. 1985, as amended (the "Tax Act"), and at all relevant times, deals at arm's length with, and is not affiliated with, the Company and holds their Common Shares as capital property.property (a "holder"). Common Shares will generally be considered to be capital property to a holder for purposes of the Tax Act unless either the holder holds such Common Shares in the course of carrying on a business of trading or dealing in securities, or the holder has held or acquired such Common Shares in a transaction or transactions considered to be an adventure in the nature of trade.
This summary is not applicable to a holder (i) that is a "financial institution", as defined in the Tax Act for purposes of the mark-to- market rules, (ii) that is a "specified financial institution", as defined in the Tax Act, (iii) an interest in which would be a "tax shelter investment" as defined in the Tax Act, (iv) that has made a functional currency reporting election for purposes of the Tax Act, or (v) that has entered or will enter into a "derivative forward agreement", as defined in the Tax Act, in respect of Common Shares.Shares, or (vi) that receives dividends on Common Shares under or as part of a dividend rental arrangement as defined in the Tax Act. Such holders should consult their own tax advisors.
Additional considerations, not discussed herein, may be applicable to a holder that is a corporation resident in Canada, and is, or becomes, or does not deal at arm's length for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or series of transactions or events that includes the acquisition of the Common Shares, controlled by a non-residentnon-
resident corporation for the purposes of the "foreign affiliate dumping" rules in section 212.3 of the Tax Act. Such holders should consult their tax advisors with respect to the consequences of acquiring Common Shares.
This summary is based upon the current provisions of the Tax Act and the regulations promulgated thereunder (the "Regulations") and the Company's understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency ("CRA"). It also takes into account all proposed amendments to the Tax Act and the Regulations publicly released by the Minister of Finance (Canada) prior to the date hereof ("Tax Proposals"), and assumes that all such Tax Proposals will be enacted as currently proposed. No assurance can be given that the Tax Proposals will be enacted in the form proposed or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative or assessing practice or policy of the CRA, whether by legislative, regulatory, judicial or administrative action or interpretation, nor does it address any provincial, local, territorial or foreign tax considerations.
For purposes of the Tax Act, all amounts, including dividends, adjusted cost base and proceeds of disposition, must generally be determined in Canadian dollars. Amounts denominated in US dollarsa foreign currency must be converted to Canadian currency using exchange rates determined in accordance with the Bank of Canada noon rate on the day on which the amount arose or such other rate of exchange that is acceptable to the Minister of National Revenue (Canada).Tax Act. The amount of any capital gain or any capital loss to a holder with respect to the Common Shares may be affected by fluctuations in Canadian dollar exchange rates.
Holders Not Resident in Canada
The following discussion applies to a holder of Common Shares who, at all relevant times, for purposes of the Tax Act, is neither resident nor deemed to be resident in Canada and does not, and is not deemed to, use or hold Common Shares in carrying on a business or part of a business in Canada (a "Non-Resident holder"). In addition, this discussion does not apply to an insurer who carries or is deemed to carry on, an insurance business in Canada and elsewhere or to an "authorized foreign bank" (as defined in the Tax Act).
Disposition of Common Shares
A Non-Resident holder generally will not be subject to tax under the Tax Act in respect of any capital gain realized by such Non- Resident holder on a disposition or deemed disposition of Common Shares unless such shares constitute "taxable Canadian property" (as defined in the Tax Act) of the Non-Resident holder at the time of disposition and the gain is not exempt from tax pursuant to the terms of an applicable income tax treaty or convention. As long as the Common Shares are listed on a designated stock exchange (which currently includes NASDAQ and the TSX) at the time of their disposition, the Common Shares generally will not constitute taxable Canadian property of a Non-Resident holder, unless (a) at any time during the 60-month period immediately preceding the disposition (i) one or any combination of (A) the Non-Resident holder, (B) persons with whom the Non-Resident holder did not deal at arm's length, and (C) partnerships in which the Non-Resident holder or a person described in (B) holds a membership interest directly or indirectly through one or more partnerships, owned 25% or more of the issued shares of any class or series of shares of the Company; and (ii) more than 50% of the fair market value of the shares of the Company was derived directly or indirectly from one or any combination of real or immovable property situated in Canada, "Canadian resource properties" (as defined in the Tax Act), "timber resource properties" (as defined in the Tax Act) or options in respect of, or interests in, or for civil law rights in, any such property whether or not such property exists or (b) ourthe Common Shares are otherwise deemed to be taxable Canadian property to the Non-Resident holder.
A Non-Resident holder's capital gain (or capital loss) in respect of Common Shares that constitute or are deemed to constitute taxable Canadian property (and are not "treaty-protected property" as defined in the Tax Act) will generally be computed in the manner described below under the heading "Holders"Holders Resident in Canada - Disposition of Common Shares". If the Common Shares were to cease being listed on NASDAQ, the TSX or another "recognized stock exchange" (as defined in the Tax Act), a Non-Resident holder who disposes of Common Shares that are taxable Canadian property may be required to fulfill the requirements of section 116 of the Tax Act, unless the Common Shares are "treaty-protected property" (as defined in the Tax Act) of the disposing Non-Resident holder.
Non-Resident holders whose Common Shares are taxable Canadian property should consult their own tax advisors.
Taxation of Dividends on Common Shares
Dividends paid or credited or deemed to be paid or credited to a Non-Resident holder by the Company are subject to Canadian withholding tax at the rate of 25% unless reduced by the terms of an applicable tax treaty or convention. Under the Canada - United States Tax Convention (1980) (the "Convention") as amended, the rate of withholding tax on dividends paid or credited to a Non-
ResidentNon-Resident holder who is the beneficial owner of the dividends, is resident in the USU.S. for purposes of the Convention and entitled to the benefits of the Convention (a "US"U.S. holder") is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a USU.S. holder that is a company beneficially owning at least 10% of the Company's voting shares). Non-Resident holders should consult their own tax advisors.
Holders Resident in Canada
The following discussion applies to a holder of Common Shares who, at all relevant times, for purposes of the Tax Act, is or is deemed to be resident in Canada (a "Canadian holder"). Certain Canadian holders whose Common Shares might not otherwise qualify as capital property may, in certain circumstances, treat the Common Shares and every other "Canadian security" (as defined in the Tax Act) owned by the Canadian holder as capital property by making an irrevocable election provided by subsection 39(4) of the Tax Act.
Canadian holders should consult their own tax advisors for advice as to whether an election under subsection 39(4) of the Tax Act is available and/or advisable in their particular circumstances.
Taxation of Dividends on Common Shares
Dividends received or deemed to have been received on the Common Shares will be included in a Canadian holder's income for purposes of the Tax Act. Such dividends received or deemed to have been received by a Canadian holder that is an individual (other than certain trusts) will be subject to the gross-up and dividend tax credit rules generally applicable under the Tax Act in respect of dividends received on shares of taxable Canadian corporations. Generally, a dividend will be eligible for the enhanced gross-up and dividend tax credit if the Company designates the dividend as an "eligible dividend" (within the meaning of the Tax Act) in accordance with the provisions of the Tax Act. There may be limitations on the ability of the Company to designate dividends as eligible dividends. A Canadian holder that is a corporation will be required to include such dividends in computing its income and will generally be entitled to deduct the amount of such dividends in computing its taxable income. In certain circumstances, subsection 55(2) of the Tax Act may treat a taxable dividend received by a Canadian holder that is a corporation as proceeds of disposition or a capital gain. A Canadian holder that is a "private corporation" or a "subject corporation" (as such terms are defined in the Tax Act), may be liable under Part IV of the Tax Act to pay a refundable tax of 38 1/3% on dividends received or deemed to have been received on the Common Shares to the extent such dividends are deductible in computing the holder's taxable income.
Disposition of Common Shares
A disposition, or a deemed disposition, of a Common Share by a Canadian holder will generally give rise to a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of the share, net of any reasonable costs of disposition, exceed (or are less than) the adjusted cost base of the share to the holder. Such capital gain (or capital loss) will be subject to the treatment described below under "Taxation of Capital Gains and Capital Losses".
Additional Refundable Tax
A Canadian holder that is a "Canadian-controlled private corporation" (as such term is defined in the Tax Act) may be liable to pay an additional refundable tax of 10 2/3% on certain investment income including amounts in respect of "Taxable Capital Gains", as defined below.
Taxation of Capital Gains and Capital Losses
In general, one half of any capital gain (a "Taxable Capital Gain") realized by a Canadian holder in a taxation year will be included in the holder's income in the year. Subject to and in accordance with the provisions of the Tax Act, one half of any capital loss (an "Allowable Capital Loss") realized by a Canadian holder in a taxation year must be deducted from Taxable Capital Gains realized by the holder in the year and Allowable Capital Losses in excess of Taxable Capital Gains may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net Taxable Capital Gains realized in such years. The amount of any capital loss realized by a Canadian holder that is a corporation on the disposition or deemed disposition of a Common Share may be reduced by the amount of dividends received or deemed to have been received by it on such Common Share (or on a share for which the Common Share has been substituted) to the extent and under the circumstances prescribed by the Tax Act. Similar rules may apply where a corporation is a member of a partnership or a beneficiary of a trust that owns Common Shares, directly or indirectly, through a partnership or a trust.
Alternative Minimum Tax
A Taxable Capital Gain realized and taxable dividends received or deemed to have been received by a Canadian holder who is an individual (including a trust, other than certain specified trusts) may give rise to liability for alternative minimum tax.
Certain Material USU.S. Federal Income Tax Considerations
The following discussion is a summary of certainthe material USU.S. federal income tax consequences applicable to the ownership and disposition of Common Shares by a USU.S. Holder (as defined below), but does not purport to be a complete analysis of all potential USU.S. federal income tax effects. This summary is based on the Internal Revenue Code of 1986, as amended (the "Code"), USU.S. Treasury regulations promulgated thereunder, IRS rulings and judicial decisions in effect on the date hereof. All of these are subject to change, possibly with retroactive effect, or different interpretations. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive basis. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary.
This summary does not address all aspects of USU.S. federal income taxation that may be relevant to particular USU.S. Holders in light of their specific circumstances (for example, USU.S. Holders subject to the alternative minimum tax or the Medicare contribution tax on net investment income under the Code) or to holders that may be subject to special rules under USU.S. federal income tax law, including:
dealers in stocks, securities or currencies;
securities traders that use a mark-to-market accounting method;
banks and financial institutions;
insurance companies;
regulated investment companies;
real estate investment trusts;
tax-exempt organizations;
retirement plans, individual plans, individual retirement accounts and tax-deferred accounts;
partnerships or other pass-through entities for USU.S. federal income tax purposes and their partners or members;
persons holding Common Shares as part of a hedging or conversion transaction straddle or other integrated or risk reduction transaction;
persons who or that are, or may become, subject to the expatriation provisions of the Code;
persons whose functional currency is not the USU.S. dollar; and
direct, indirect or constructive owners of 10% or more of the total combined voting power of all classes of our voting stock or 10% or more of the total value of shares of all classes of our stock.
This summary also does not address the tax consequences of holding, exercising or disposing of warrants in the Company. If the Company is a PFIC, as described below, USU.S. Holders of its warrants will be subject to adverse tax rules and will not be able to make the mark-to-market or the QEF election described below with respect to such warrants. We believe that we were a PFIC for the 2015 taxable year. USU.S. Holders of warrants should consult their tax advisors with regard to the USU.S. federal income tax consequences of holding, exercising or disposing of warrants in the Company, including in the situation in which the Company is classified as a PFIC.
This summary also does not discuss any aspect of state, local or foreign law, or estate or gift tax law as applicable to USU.S. Holders. In addition, this discussion is limited to USU.S. Holders holding Common Shares as capital assets. For purposes of this summary, "US"U.S. Holder" means a beneficial holder of Common Shares who or that for USU.S. federal income tax purposes is:
an individual citizen or resident of the United States;
a corporation or other entity classified as a corporation for USU.S. federal income tax purposes created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
an estate, the income of which is subject to USU.S. federal income taxation regardless of its source; or
a trust, if (a) a court within the United States is able to exercise primary supervision over the administration of such trust and one or more "US"U.S. persons" (within the meaning of the Code) have the authority to control all substantial decisions of the trust, or (b) a valid election is in effect to be treated as a USU.S. person for USU.S. federal income tax purposes.
If a partnership or other entity or arrangement classified as a partnership for USU.S. federal income tax purposes holds Common Shares,, the USU.S. federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. This summary does not address the tax consequences to any such partner. Such a partner should consult its own tax advisor as to the tax consequences of the partnership owning and disposing of Common Shares.Shares.
USU.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH REGARD TO THE APPLICATION OF THE TAX CONSEQUENCES DESCRIBED BELOW TO THEIR PARTICULAR SITUATIONS AS WELL AS THE APPLICATION OF ANY STATE, LOCAL, FOREIGN OR OTHER TAX LAWS, INCLUDING GIFT AND ESTATE TAX LAWS.
Tax Consequences if we are a Passive Foreign Investment Company ("PFIC")
A foreign corporation will be classified as a PFIC for any taxable year in which, after taking into account the income and assets of the corporation and certain subsidiaries pursuant to applicable "look-through rules", either (i) at least 75% of its gross income is "passive income" or (ii) at least 50% of the average quarterly value of its assets is attributable to assets which produce passive income or are held for the production of passive income. Passive income generally includes dividends, interest, rents and royalties (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce passive income. If a non-USnon-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-USnon-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation's income.
The Company believes it was a PFIC for the 2015 taxable year.year but not for the 2016, 2017 and 2018 taxable years. However, the fair market value of the Company's assets may be determined in large part by the market price of the Common Shares,, which is likely to fluctuate, and the composition of the Company's income and assets will be affected by how, and how quickly, the Company spends any cash that is raised in any financing transaction. Thus, no assurance can be provided that the Company will not be classified as a PFIC for 2018 or any future taxable year. USU.S. Holders should consult their tax advisors regarding the Company's PFIC status.
If the Company is classified as a PFIC for any taxable year during which a USU.S. Holder owns Common Shares, the USU.S. Holder, absent certain elections (including the mark-to-market and QEF elections described below), will generally be subject to adverse rules (regardless of whether the Company continues to be classified as a PFIC) with respect to (i) any "excess distributions" (generally, any distributions received by the USU.S. Holder on the Common Shares in a taxable year that are greater than 125% of the average annual distributions received by the USU.S. Holder in the three preceding taxable years or, if shorter, the USU.S. Holder's holding period for the Common Shares) and (ii) any gain realized on the sale or other disposition of the Common Shares.
Under these adverse rules (a) the excess distribution or gain will be allocated ratably over the USU.S. Holder's holding period, (b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which the Company is classified as a PFIC will be taxed as ordinary income, and (c) the amount allocated to each of the other taxable years during which the Company was classified as a PFIC will be subject to tax at the highest rate of tax in effect for the applicable category of taxpayer for that year and an interest charge will be imposed with respect to the resulting tax attributable to each such other taxable year. A USU.S. Holder that is not a corporation will be required to treat any such interest paid as "personal interest", which is not deductible.
USU.S. Holders can avoid the adverse rules described above in part by making a mark-to-market election with respect to the Common Shares,, provided that the Common Shares are "marketable". The Common Shares will be marketable if they are "regularly traded" on a "qualified exchange" or other market within the meaning of applicable USU.S. Treasury regulations. For this purpose, the Common Shares generally will be considered to be regularly traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. The Common Shares are currently listed on NASDAQ, which constitutes a qualified exchange; however, there can be no assurance that the Common Shares will be treated as regularly traded for purposes of the mark-to-market election on a qualified exchange. If the Common Shares were not regularly traded on NASDAQ or were delisted from NASDAQ and were not traded on another qualified exchange for the requisite time period described above, the mark-to-market election would not be available.
A USU.S. Holder that makes a mark-to-market election must include in gross income, as ordinary income, for each taxable year an amount equal to the excess, if any, of the fair market value of the USU.S. Holder's Common Shares at the close of the taxable year
over the USU.S. Holder's adjusted tax basis in the Common Shares.Shares. An electing USU.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the USU.S. Holder's adjusted tax basis in the Common Shares over the fair market value of the Common Shares at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains previously included in income. A USU.S. Holder that makes a mark-to-market election generally will adjust such USU.S. Holder's tax basis in the Common Shares to reflect the amount included in gross income or allowed as a deduction because of such mark-to-market election. Gains from an actual sale or other disposition of the Common Shares will be treated as ordinary income, and any losses incurred on a sale or other disposition of the Common Shares will be treated as ordinary losses to the extent of any net mark-to-market gains previously included in income.
If the Company is classified as a PFIC for any taxable year in which a USU.S. Holder owns Common Shares but before a mark-to-market election is made, the adverse PFIC rules described above will apply to any mark-to-market gain recognized in the year the election is made. Otherwise, a mark-to-market election will be effective for the taxable year for which the election is made and all subsequent taxable years. The election cannot be revoked without the consent of the IRS unless the Common Shares cease to be marketable, in which case the election is automatically terminated.
If the Company is classified as a PFIC, a USU.S. Holder of Common Shares will generally be treated as owning stock owned by the Company in any direct or indirect subsidiaries that are also PFICs and will be subject to similar adverse rules with respect to distributions to the Company by, and dispositions by the Company of, the stock of such subsidiaries. A mark-to-market election is not permitted for the shares of any subsidiary of the Company that is also classified as a PFIC. USU.S. Holders should consult their tax advisors regarding the availability of, and procedure for making, a mark-to-market election.
In some cases, a shareholder of a PFIC can avoid the interest charge and the other adverse PFIC consequences described above by making a QEF election to be taxed currently on its share of the PFIC's undistributed income. WeWe will endeavor to satisfy the record keeping requirements that apply to a QEF and to supply requesting USU.S. Holders with the information that such USU.S. Holders are required to report under the QEF rules. However, there can be no assurance that the Company will satisfy the record keeping requirements or provide the information required to be reported by USU.S. Holders.
A USU.S. Holder that makes a timely and effective QEF election for the first tax year in which its holding period of its Common Shares begins generally will not be subject to the adverse PFIC consequences described above with respect to its Common Shares. Rather, a USU.S. Holder that makes a timely and effective QEF election will be subject to USU.S. federal income tax on such USU.S. Holder's pro rata share of (a) the Company's net capital gain, which will be taxed as long-term capital gain to such USU.S. Holder, and (b) the Company's ordinary earnings, which will be taxed as ordinary income to such USU.S. Holder, in each case regardless of which such amounts are actually distributed to the USU.S. Holder by the Company. Generally, "net capital gain" is the excess of (a) net long-term capital gain over (b) net short-term capital loss, and "ordinary earnings" are the excess of (a) "earnings and profits" over (b) net capital gain.
A USU.S. Holder that makes a timely and effective QEF election with respect to the Company generally (a) may receive a tax-free distribution from us to the extent that such distribution represents "earnings and profits" that were previously included in income by the USU.S. Holder because of such QEF election and (b) will adjust such USU.S. Holder's tax basis in the Common Shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF election. In addition, a USU.S. Holder that makes a QEF election generally will recognize capital gain or loss on the sale or other taxable disposition of Common Shares.
The QEF election is made on a shareholder-by-shareholder basis. Once made, a QEF election will apply to the tax year for which the QEF election is made and to all subsequent tax years, unless the QEF election is invalidated or terminated or the IRS consents to revocation of the QEF election. In addition, if a USU.S. Holder makes a QEF election, the QEF election will remain in effect (although it will not be applicable) during those tax years in which the Company is not a PFIC.
If the Company is classified as a PFIC and then ceases to be so classified, a USU.S. Holder may make an election (a "deemed sale election") to be treated for USU.S. federal income tax purposes as having sold such USU.S. Holder's Common Shares on the last day of the taxable year of the Company during which it was a PFIC. A USU.S. Holder that made a deemed sale election would then cease to be treated as owning stock in a PFIC by reason of ownership of Common Shares in the Company. However, gain recognized as a result of making the deemed sale election would be subject to the adverse rules described above and loss would not be recognized.
If the Company is a PFIC in any year with respect to a USU.S. Holder, the USU.S. Holder will be required to file an annual information return on IRS Form 8621 regarding distributions received on Common Shares and any gain realized on the disposition of Common Shares.
Shares.
In addition, if the Company is a PFIC, USU.S. Holders will generally be required to file an annual information return with the IRS (also on IRS Form 8621, which PFIC shareholders are required to file with their USU.S. federal income tax or information returns)
relating to their ownership of Common Shares. This new filing requirement is in addition to the preexisting reporting requirements described above that apply to a US Holder's interest in a PFIC (which this requirement does not affect).Shares.
USU.S. Holders should consult their tax advisors regarding the potential application of the PFIC regime and any reporting obligations to which they may be subject under that regime.
Dividends
Subject to the PFIC rules discussed above, any distributions paid by the Company out of current or accumulated earnings and profits (as determined for USU.S. federal income tax purposes), before reduction for any Canadian withholding tax paid with respect thereto, will generally be taxable to a USU.S. Holder as foreign source dividend income, and generally will not be eligible for the dividends received deduction generally allowed to corporations.
Distributions in excess of current and accumulated earnings and profits will be treated as a non-taxable return of capital to the extent of the USU.S. Holder's adjusted tax basis in the Common Shares and thereafter as capital gain. The Company does not, however, intend to calculate its earnings and profits under USU.S. federal income tax principles. Therefore, USU.S. Holders should expect that any distribution from the Company generally will be treated for USU.S. federal income tax purposes as a dividend. USU.S. Holders
should consult their own tax advisors with respect to the appropriate USU.S. federal income tax treatment of any distribution received from the Company.
Dividends paid to non-corporate USU.S. Holders by the Company in a taxable year in which it is treated as a PFIC, or in the immediately following taxable year, will not be eligible for the special reduced rates normally applicable to long-term capital gains. In all other taxable years, dividends paid by the Company should be taxable to a non-corporate USU.S. Holder at the special reduced rates normally applicable to long-term capital gains, provided that certain conditions are satisfied. (including a minimum holding period requirement). The Company believes it was not a PFIC for the 20152018 taxable yearyear. However, no assurance can be provided that the Company will not be classified as a PFIC for 2019 and, therefore, no assurance can be provided that a USU.S. Holder will not be able to claim a reduced rate for dividends paid in 20162019 or 2020 (if any).See "Passive Foreign Investment Company Considerations" above.
Under current law, payments of dividends by the Company to non-Canadian investors are generally subject to a 25% Canadian withholding tax. The rate of withholding tax applicable to USU.S. Holders that are eligible for benefits under the Canada-United States Tax Convention (the "Convention") is reduced to a maximum of 15%. This reduced rate of withholding will not apply if the dividends received by a USU.S. Holder are effectively connected with a permanent establishment of the USU.S. Holder in Canada. For USU.S. federal income tax purposes, USU.S. Holders will be treated as having received the amount of Canadian taxes withheld by the Company, and as then having paid over the withheld taxes to the Canadian taxing authorities. As a result of this rule, the amount of dividend income included in gross income for USU.S. federal income tax purposes by a USU.S. Holder with respect to a payment of dividends may be greater than the amount of cash actually received (or receivable) by the USU.S. Holder from the Company with respect to the payment.
Subject to certain limitations, a USU.S. Holder will generally be entitled, at the election of the USU.S. Holder, to a credit against its USU.S. federal income tax liability, or a deduction in computing its USU.S. federal taxable income, for Canadian income taxes withheld by the Company. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a USU.S. Holder during a year. For purposes of the foreign tax credit limitation, dividends paid by the Company generally will constitute foreign source income in the "passive category income" basket. The foreign tax credit rules are complex and USU.S. Holders should consult their tax advisors concerning the availability of the foreign tax credit in their particular circumstances.
Dividends paid in Canadian dollars will be included in the gross income of a USU.S. Holder in a USU.S. dollar amount calculated by reference to the exchange rate in effect on the date the USU.S. Holder (actually or constructively) receives the dividend, regardless of whether such Canadian dollars are actually converted into USU.S. dollars at that time. If the Canadian dollars received are not converted into USU.S. dollars on the date of receipt, a USU.S. Holder will have a tax basis in the Canadian dollars equal to their USU.S. dollar value on the date of receipt. Gain or loss, if any, realized on a sale or other disposition of the Canadian dollars will generally be USU.S. source ordinary income or loss to a USU.S. Holder.
The Company generally does not pay any dividends and does not anticipate paying any dividends in the foreseeable future.
Sale, Exchange or Other Taxable Disposition of Common Shares
Subject to the PFIC rules discussed above, upon a sale, exchange or other taxable disposition of Common Shares,, a USU.S. Holder generally will recognize capital gain or loss for USU.S. federal income tax purposes equal to the difference, if any, between the amount realized on the sale, exchange or other taxable disposition and the USU.S. Holder's adjusted tax basis in the Common Shares.Shares.
This capital gain or loss will be long-term capital gain or loss if the USU.S. Holder's holding period in the Common Shares exceeds one year. The deductibility of capital losses is subject to limitations. Any gain or loss will generally be USU.S. source for USU.S. foreign tax credit purposes.
Information Reporting and Backup Withholding
Payments made within the United States,U.S., or by a USU.S. payor or USU.S. middleman, of dividends on, and proceeds arising from sales or other dispositions of Common Shares,, generally will be reported to the IRS and to the USU.S. Holder as required under applicable regulations. Backup withholding tax may apply to these payments if the USU.S. Holder fails to timely provide in the appropriate manner an accurate taxpayer identification number or otherwise fails to comply with, or establish an exemption from, such backup withholding tax requirements. Certain USU.S. Holders are not subject to the information reporting or backup withholding tax requirements described herein. USU.S. Holders should consult their tax advisors as to their qualification for exemption from backup withholding tax and the procedure for establishing an exemption.
Backup withholding tax is not an additional tax. USU.S. Holders generally will be allowed a refund or credit against their USU.S. federal income tax liability for amounts withheld, provided the required information is timely furnished to the IRS.
Subject to certain exceptions and future guidance, USU.S. tax legislation generally requires a USU.S. Holder that is a specified individual or to the extent provided in future guidance, a domestic entity, to report annually to the IRS on IRS Form 8938 such USU.S. Holder's interests in stock or securities issued by a non-USnon-U.S. person (such as the Company). USU.S. Holders should consult their tax advisors regarding the information reporting obligations that may arise from their acquisition, ownership or disposition of Common Shares.Shares.
| |
| F. | Dividends and paying agents |
Not applicable.required.
Not applicable.required.
In addition to placing our audited consolidated annual financial statements before every annual meeting of shareholders as described above, we are subject to the information requirements of the Securities Exchange Act of 1934, as amended. In accordance with these requirements, we file and furnish reports and other information with the SEC. These materials, including this Annual Report on Form 20-F and the exhibits hereto, may be inspected and copied at the SEC's Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. The public may obtain information on the operation of the SEC's Public Reference Room by calling the SEC in the United States at 1-800-SEC-0330. The SEC also maintains a website at www.sec.gov that contains reports, proxy statements and other information regarding registrants that file electronically with the SEC. Our annual reports and some of the other information we submitted to the SEC may be accessed through this website. In addition, material we filed can be inspected on the Canadian Securities Administrators' electronic filing system, SEDAR, accessible at the website www.sedar.com. This material includes our Management Information Circular for our annual meeting of shareholders to be held on May 10, 20168, 2019 to be furnished to the SEC on Form 6-K, which provides information including directors' and officers' remuneration and indebtedness and principal holders of securities. Additional financial information is provided in our audited annual financial statements for the year ended December 31, 20182015 and our MD&A relating to these statements included elsewhere in this Annual Report on Form 20-F. These documents are also accessible on SEDAR (www.sedar.com) and on EDGAR (www.sec.gov).
Our subsidiaries are set forth under "Item 4C. – Organizational Structure".Not required.
| |
| Item 11. | Quantitative and Qualitative Disclosures About Market Risk |
Fair value
The Company classifies its financial instruments in the following categories: "Financial assets at fair value through profit or loss ("FVTPL")"; "Loans and receivables"; "Financial liabilities at FVTPL"; and "Other financial liabilities".
The Company's loans and receivables are comprised of cash and cash equivalents, trade and other receivables and restricted cash equivalents.
Financial liabilities at FVTPL are currently comprised of the Company's warrant liability.
Other financial liabilities include trade accounts payable andpayables, accrued liabilities, and provision for restructuring costs and other non-current liabilities.costs.
The carrying values of all of the aforementioned financial instruments, excluding warrant liability which is stated at fair value, approximate their fair values due to their short-term maturity or to the prevailing interest rates of these instruments, which are comparable to those of the market.
Financial risk factors
The following provides disclosures relating to the nature and extent of the Company's exposure to risks arising from financial instruments, including credit risk, liquidity risk, and market risk (share price riskrisk) and currency risk),foreign exchange risk and how the Company manages those risks.
(a) Credit risk
Credit risk is the risk of an unexpected loss if a customer or counterparty to a financial instrument fails to meet its contractual obligations. The Company regularly monitors credit risk exposure and takes steps to mitigate the likelihood of this exposure resulting in losses. The Company's exposure to credit risk currently relates to cash and cash equivalents, trade and other receivables and restricted cash equivalents.the financial assets at amortized cost in the table
above. The Company holds its available cash in amounts that are readily convertible to known amounts of cash and deposits its cash balances with financial institutions that have an investment grade credit rating of at least "A""P-2" or the equivalent. This information is supplied by independent rating agencies where available and, if not available, the Company uses publicly available financial information to ensure that it invests its cash in creditworthy and reputable financial institutions.
As at December 31, 2015,2018 trade accounts receivable for an amount of approximately $122,000$197 were with twofour counterparties and no trade accounts receivable wereof whichl $55 was past due or impaired.and impaired andimpaired amounts were fully reserved and not a part of the balance .
Generally, the Company does not require collateral or other security from customers for trade accounts receivable; however, credit is extended following an evaluation of creditworthiness. In addition, the Company performs ongoing credit reviews of all of its customers and establishes an allowance for doubtful accounts when accounts are determined to be uncollectible. On this basis, as at December 31, 2018, the Company has provided for all outstanding and unpaid amounts relating to its operations before its licensing of MacrilenTM(macemorelin). The licensee has paid all amounts owing within 90 days of invoicing.
The maximum exposure to credit risk approximates the amount recognized onin the Company's consolidated statement of financial position.
(b) Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due. As indicated in the capitalnote 23 - Capital disclosures section (see "Item 5 – Operating and Financial Review and Prospects")of our financial statements included in this Form 20-F, the Company manages this risk through the management of its capital structure. It also manages liquidity risk by continuously monitoring actual and projected cash flows.flows as further discussed in note 2 - Assessment of liquidity and management's plans. The Board of Directors reviews and approves the Company's operating and capital budgets, as well as any material transactions outoccurring outside of the ordinary course of business. The Company has adopted an investment policy in respect of the safety and preservation of its capital to ensure the Company's liquidity needs are met. The instruments are selected with regard to the expected timing of expenditures and prevailing interest rates.
The Company expectsOn December 20, 2017, the FDA granted marketing approval for Macrilen™ (macimorelin) to continue to incur operating expenses and may require significant capital to fulfill its future obligationsbe used in absencethe diagnosis of sufficient corresponding revenues. The Company's ability to continue future operations beyond December 31, 2016 and to fund its activities is dependent on its ability to secure additional financings, which may be completed in a number of ways, including but not limited to licensing arrangements, partnerships, promotional arrangements, the issuance of securities and other financing activities. Management will pursue such additional sources of financing when required, and whilepatients with AGHD. On January 16, 2018, the Company, has been successful in securing financing inthrough AEZS Germany entered into the past, there can be no assurance itLicense and Assignment Agreement. The License and Assignment Agreement will be ablecontribute to do so infulfilling the Company's future or that these sources of funding or initiatives will be available or on terms acceptable to the Company.obligations.
(c) Market risk
90
Share price risk
The change in fair value of the Company's warrant liability, which is measured at FVTPL, results from the periodic "mark-to-market" revaluation, via the applicationas further described in note 17 of the intrinsic valuation and the Black-Scholes option pricing model. Theseour financial statements included in this Form 20-F as it applies to its outstanding share purchase warrants. The valuation models are impacted, among other inputs, by the market price of the Company's common shares. As a result, the change in fair value of the warrant liability, which is reported as finance income (costs) in the accompanying consolidated statements of comprehensive income (loss) income,, has been and may continue in future periods to be materially affected most notably by changes in the Company's common share closing price, which on the NASDAQ has ranged from $4.00$1.19 to $84.20 $3.87during the year ended December 31, 2015.2018.
If variations in the market price of our Common Sharescommon shares of -10%-30% and +10%+30% were to occur, the impact on the Company's net (loss) income forrelated to the warrant liability held at December 31, 20152018 would be as follows:
| | | (in thousands) | | Carrying
amount | | -10% | | +10% | | Carrying
amount | | -30% | | +30% |
| | | $ | | $ | | $ | | $ | | $ | | $ |
| Warrant liability | | 10,891 |
| | 1,059 |
| | (1,067 | ) | | 3,634 |
| | 1,792 |
| | (1,504 | ) |
| Total impact on net income – decrease / (increase) | | | | 1,059 |
| | (1,067 | ) | |
| Total impact on net income – (decrease) / increase | | | | | 1,792 |
| | (1,504 | ) |
Foreign currency risk
We have not entered into any forward currency contracts or other financial derivatives to hedge foreign exchange risk. We are therefore subject to foreign currency transaction and translation gains and losses.
| |
| Item 12. | Description of Securities Other than Equity Securities |
Not applicable.required.
Not applicable.required.
Not applicable.required.
| |
| D. | American depositary shares |
Not applicable.
PART II
| |
| Item 13. | Defaults, Dividend Arrearages and Delinquencies |
None.
| |
| Item 14. | Material ModificationModifications to the Rights of Security Holders and Use of Proceeds |
None.
| |
| Item 15. | Controls and Procedures |
Under the supervision of and with the participation of the Registrant'sour management, including the Chief Executive Officer and the PrincipalChief Financial Officer, we have conducted an evaluation pursuant to Rule 13a-15, promulgated under the Securities Exchange Act of 1934, as amended, ofevaluated the effectiveness of our disclosure controls and procedures as at December 31, 2015.2018. Based on that evaluation, the Chief Executive Officer and the PrincipalChief Financial Officer have concluded that these disclosure controls and procedures were effective as at December 31, 2015.2018.
Management's Annual Report on Internal Control over Financial Reporting
The Registrant'sOur management is responsible for establishing and maintaining adequate internal control over financial reporting. The Registrant'sOur internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as issued by the IASB.
The Registrant'sOur internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the Registrant's assets;assets of Aeterna Zentaris; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that receipts and expenditures of the RegistrantCompany are being made only in accordance with authorizations of the Registrant'sCompany management; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Registrant'sCompany assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management conducted an evaluation of the effectiveness of the Registrant'sour internal control over financial reporting based on the criteria established in Internal Control -– Integrated Framework: 2013 issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management has concluded that the Registrant'sour internal control over financial reporting was effective as at December 31, 2015.2018.
Changes in Internal ControlControls over Financial Reporting
There have beenwere no changes in our internal control over financial reporting during the year ended December 31, 20152018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The design of any system of controls and procedures is based in part upon certain assumptions about the likelihood of certain events. There can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, including conditions that are remote.
In accordance with Securities and Exchange Commission’s rules regarding non-accelerated filers, this Annual Report on Form 20-F does not include an attestation report of the Company's independent registered public accounting firm regarding the Company's internal control over financial reporting.
| |
| Item 16A. | Audit Committee Financial Expert |
Our Board has determined that we have at least one audit committee financial expert (as defined in paragraph (b) of Item 16A to Form 20-F). The name of the audit committee financial expert is Mr. Gérard Limoges, FCPA, FCA, the Audit Committee's Chairman. In accordance with Item 6A,16A, paragraph (d) of Form 20-F, the designation of Mr. Limoges as our audit committee financial expert does not: (i) make Mr. Limoges an "expert" for any purpose, including without limitation for purposes of Section 11 of the Securities Act of 1933, as amended, as a result of this designation; (ii) impose any duties, obligations or liability on Mr. Limoges that are greater than those imposed on him as a member of the Audit Committee and the Board in the absence of such designation; or (iii) affect the duties, obligations or liability of any other member of the Audit Committee or the Board. The other current members of the Audit Committee are Mr. Pierre LapalmeMessrs. Brent Norton and Ms. Carolyn Egbert,Jonathan Pollack, each of whom, along with Mr. Limoges, is independent, as that term is defined in the NASDAQ listing standards. For a description of their respective education and experience, please refer to "Item 6. – Directors, Senior Management and Employees".
On March 29, 2004,December 16, 2017, the Board adopted a "Code of Ethical Conduct"Conduct and Business Ethics", which replaced the then existing Code of Ethical Conduct as of January 1, 2018. The Code of Conduct and Business Ethics expanded on the previous Code of Ethical Conduct to provide additional details of expected conduct of all employees and directors of the Company, including specific obligations the Company and its employees has been amended byas a member of the Board on November 3, 2004, December 13, 2005, March 2, 2007 and March 10, 2009. The December 13, 2005 amendment incorporates changes to the duty to report violations consistent with applicable laws.healthcare industry. We selected an independent third party supplier to provide a confidential and anonymous communication channel for reporting concerns about possible violations to the our Code of Ethical Conduct as well as financial and/or accounting irregularities or fraud. A copy of the Code of Ethical Conduct, as amended, is includedincorporated by reference as Exhibit 11.1 to this Annual Report on Form 20-F and is also available on our Web site at www.aezsinc.comwww.zentaris.com under the Investors - Corporate Governance tab. The Code of Ethical Conduct is a "code of ethics" as defined in paragraph (b) of Item 16B to Form 20- F. The Code of Ethical Conduct applies to all of our employees, directors and officers, including our principal executive officer, principal financial officer, and principal accounting officer or controller, or persons performing similar functions, and includes specific
provisions dealing with integrity in accounting matters, conflicts of interest and compliance with applicable laws and regulations. On December 4, 2014, our Board of Directors adopted a "Code of Business Conduct and Ethics for Members of the Board of Directors", which is includedincorporated by reference as Exhibit 11.2 to this Annual Report on Form 20-F. We will provide these documents without charge to any person or company upon request to our Corporate Secretary, at our head office at 315 Sigma Drive, Suite 302D, Summerville, South Carolina 29483.29486.
| |
| Item 16C. | Principal Accountant Fees and Services |
(All amounts are in USU.S. dollars)
(a) Audit Fees
During thethe financial years ended December 31, 20152018 and 2014,2017, the Registrant'sCompany's principal accountant, PricewaterhouseCoopers LLP, billed $473,515$563,558 and $458,248,$506,309, respectively, for the audit of the Registrant's Company's annual consolidated financial statements and for services rendered in connection with the Registrant's statutory and regulatory filings.
(b) Audit-related Fees
During the financial years ended December 31, 20152018 and 2014,2017, the Registrant'sCompany's principal accountant, PricewaterhouseCoopers LLP, billed $57,524$37,663 and $92,241,$113,430, respectively, for audit or attest services not required by statute or regulation, for accounting consultations on proposed transactions, for the review of prospectuses and prospectus supplements, including the delivery of customary consent and comfort letters in connection therewith.
(c) Tax Fees
During the financial years ended December 31, 20152018 and 2014,2017, the Registrant'sCompany's principal accountant,accountants, PricewaterhouseCoopers LLP billed $24,269$36,224 and $27,661,$5,426, respectively, for services related to tax compliance, tax planning and tax advice.
(d) All Other Fees
During the financial years ended December 31, 20152018 and 2014,2017, the Registrant'sCompany's principal accountant, PricewaterhouseCoopers LLP, did not bill us for services not included in audit fees, audit-related fees and tax fees.
(e) Audit Committee Pre-Approval Policies and Procedures
| |
| (e) | Audit Committee Pre-Approval Policies and Procedures |
Under applicable Canadian securities regulations, the Registrant iswe are required to disclose whether itsour Audit Committee has adopted specific policies and procedures for the engagement of non-audit services and to prepare a summary of these policies and procedures. The Audit Committee Charter (included(incorporated by reference as Exhibit 11.3 to this Annual Report on Form 20-F, incorporated by reference to Exhibit 11.3 of the Registrant's Annual Report on Form 20-F for the financial year ended December 31, 2014 filed with the Commission on March 17, 2015)20-F) provides that it is such committee's responsibility to approve all audit engagement fees and terms as well as reviewing policies for the provision of non-audit services by the external auditors and, when required, the framework for pre-approval of such services. The Audit Committee delegates to its Chairman the pre-approval of such non-audit fees. The pre-approval by the Chairman is then presented to the Audit Committee at its first scheduled meeting following such pre-approval.
For each of the years ended December 31, 20152018 and 2014,2017, there were no non-audit services provided by the Registrant'sour external auditor that required the approval from the Audit Committee pursuant to the "de minimis exception" to the pre-approval requirement for non-audit services.
| |
| (f) | Work performed by Full-time, Permanent Employees of Principal Accountant |
(f) Work performed by Full-time, Permanent Employees of Principal Accountant
During the financial year ended on December 31, 2015,2018, no person other than the full-time, permanent employees of the Registrant'sour principal accountant, PricewaterhouseCoopers LLP, performed more than 50% of the audit work on the Registrant'sour financial statements.
| |
| Item 16D. | Exemptions from the Listing Standards for Audit Committees |
None.
| |
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
None.
| |
| Item 16F. | Change in Registrant's Certifying Accountant |
None.
| |
| Item 16G. | Corporate Governance |
We are generally in compliance with the corporate governance requirements of NASDAQ except as described below. We are not in compliance with the NASDAQ requirement that a quorum for a meeting of the holders of our Common Shares be no less than 33 1/3% of such outstanding shares. Our bylaws provide that a quorum for purposes of any meeting of our shareholders consists of at least 10% of the outstanding voting shares. We benefit from an exemption from NASDAQ from this quorum requirement because the quorum provided for in our bylaws complies with the requirements of the CBCA, our governing corporate statute, and with the rules of TSX, the home country exchange on which our voting shares are traded. In accordance with applicable current
NASDAQ requirements, we have in the past, and upon request, provided to NASDAQ letters from outside counsel certifying that these practices are not prohibited by our home country law.
| |
| Item 16H. | Mine Safety Disclosure |
None.
PART III
| |
| Item 17 | Financial Statements |
We have elected to provide financial statements pursuant to Item 18.
Item 18. Financial Statements
| |
| Item 18. | Financial Statements |
The financial statements appear on pages 95 to 144.137.
Aeterna Zentaris Inc.
Consolidated Financial Statements
As at December 31, 20152018 and December 31, 20142017 and for the years ended
December 31, 2015, 20142018, 2017 and 20132016
(presented in thousands of USU.S. dollars)
Independent Auditor's Report
To the Shareholders of
Aeterna Zentaris Inc.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Aeterna Zentaris Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial statementsposition of Aeterna Zentaris Inc. and its subsidiaries which comprise the consolidated statements(the “Company”) as of financial position as at December 31, 20152018 and December 31, 20142017, and the related consolidated statements of changes in shareholders'shareholders’ (deficiency) equity, comprehensive income (loss) income and cash flows for each of the three years in the period ended December 31, 2015, and2018, including the related notes which comprise a summary of significant accounting policies and other explanatory information.
Management's responsibility for(collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements
Management is responsible present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the preparation and fair presentation of these consolidated financial statementsthree years in accordancethe period ended December 31, 2018 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards BoardBoard.
andBasis for such internal control as management determines is necessary to enable the preparation ofOpinion
These consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditor'sthe responsibility
of the Company’s management. Our responsibility is to express an opinion on thesethe Company’s consolidated financial statements based on our audits. We conducted our audits in accordanceare a public accounting firm registered with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement. Canadian generally accepted auditing standards also require that we comply with ethical requirements.
An audit involves performing procedures to obtain audit evidence, on a test basis, about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor's judgment, including the assessment of the risks of material misstatement, of the consolidated financial statements, whether due to frauderror or error. Wefraud. The Company is not required to have, nor were notwe engaged to perform, an audit of the company'sits internal control over financial reporting. OurAs part of our audits included considerationwe are required to obtain an understanding of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company'sCompany's internal control over financial reporting. Accordingly, we express no such opinion. An audit
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also includesincluded evaluating the appropriateness of accounting principles and policies used and the reasonableness of accountingsignificant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a reasonable basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Aeterna Zentaris Inc. and its subsidiaries as at December 31, 2015 and December 31, 2014 and their financial performance and their cash flows for each of the three years in the period ended December 31, 2015 in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Quebec, Quebec,
Toronto, Ontario, Canada
March 29, 201628, 2019
We have served as the Company’s auditor since 1993.
1 CPA auditor, CA, public accountancy permit No. A121191
|
|
| Aeterna Zentaris Inc. |
| Consolidated Statements of Financial Position |
| (in thousands of US dollars) |
|
| | | | | | |
| | | December 31, 2015 | | December 31, 2014 |
| | | $ | | $ |
| ASSETS | | | | |
| Current assets | | | | |
| Cash and cash equivalents (note 7) | | 41,450 |
| | 34,931 |
|
| Trade and other receivables (note 8) | | 598 |
| | 867 |
|
| Prepaid expenses and other current assets | | 346 |
| | 419 |
|
| | | 42,394 |
| | 36,217 |
|
| Restricted cash equivalents (note 9) | | 255 |
| | 760 |
|
| Property, plant and equipment (note 10) | | 256 |
| | 797 |
|
| Identifiable intangible assets (note 11) | | 237 |
| | 352 |
|
| Other non-current assets | | 520 |
| | 622 |
|
| Goodwill (note 12) | | 7,836 |
| | 8,687 |
|
| | | 51,498 |
| | 47,435 |
|
| LIABILITIES | | | | |
| Current liabilities | | | | |
| Payables and accrued liabilities (note 13) | | 4,172 |
| | 5,799 |
|
| Provision for restructuring costs (note 14) | | 598 |
| | 1,505 |
|
| Current portion of deferred revenues (note 5) | | 244 |
| | 270 |
|
| Current portion of warrant liability (note 15) | | 1,411 |
| | — |
|
| | | 6,425 |
| | 7,574 |
|
| Deferred revenues (note 5) | | 487 |
| | 809 |
|
| Warrant liability (note 15) | | 9,480 |
| | 8,225 |
|
| Employee future benefits (note 19) | | 12,656 |
| | 15,053 |
|
| Provisions and other non-current liabilities (note 16) | | 835 |
| | 1,290 |
|
| | | 29,883 |
| | 32,951 |
|
| SHAREHOLDERS' EQUITY | | | | |
| Share capital (note 17) | | 204,596 |
| | 150,544 |
|
| Other capital | | 87,508 |
| | 86,639 |
|
| Deficit | | (271,621 | ) | | (222,322 | ) |
| Accumulated other comprehensive income (loss) | | 1,132 |
| | (377 | ) |
| | | 21,615 |
| | 14,484 |
|
| | | 51,498 |
| | 47,435 |
|
|
| | | | | | |
| | | December 31, 2018 | | December 31, 2017 |
| | | $ | | $ |
| ASSETS | | | | |
| Current assets | | | | |
| Cash and cash equivalents (note 7) | | 14,512 |
| | 7,780 |
|
| Trade and other receivables (note 8) | | 294 |
| | 221 |
|
| Inventory (note 9) | | 240 |
| | 554 |
|
| Prepaid expenses and other current assets (note 10) | | 1,210 |
| | 826 |
|
| Total current assets | | 16,256 |
| | 9,381 |
|
| Restricted cash equivalents (note 11) | | 418 |
| | 381 |
|
| Property, plant and equipment (note 12) | | 65 |
| | 101 |
|
| Deferred tax assets (note 20) | | — |
| | 3,479 |
|
| Identifiable intangible assets (note 13) | | 62 |
| | 90 |
|
| Other non-current assets | | — |
| | 150 |
|
| Goodwill (note 14) | | 8,210 |
| | 8,613 |
|
| Total Assets | | 25,011 |
| | 22,195 |
|
| LIABILITIES | | | | |
| Current liabilities | | | | |
| Payables and accrued liabilities (note 15) | | 2,966 |
| | 2,814 |
|
| Provision for restructuring and other costs (note 16) | | 887 |
| | 2,469 |
|
| Income taxes (note 22) | | 1,669 |
| | — |
|
| Current portion of deferred revenues (note 6) | | 74 |
| | 486 |
|
| Total current liabilities | | 5,596 |
| | 5,769 |
|
| Deferred revenues (note 6) | | 258 |
| | 55 |
|
| Warrant liability (note 17) | | 3,634 |
| | 3,897 |
|
| Employee future benefits (note 18) | | 13,205 |
| | 14,229 |
|
| Non-current portion of provision for restructuring and other costs (note 16) | | 411 |
| | 1,028 |
|
| Total liabilities | | 23,104 |
| | 24,978 |
|
| SHAREHOLDERS' EQUITY (DEFICIENCY) | | | | |
| Share capital (note 19) | | 222,335 |
| | 222,335 |
|
| Other capital (note 19) | | 89,342 |
| | 88,772 |
|
| Deficit | | (309,781 | ) | | (314,161 | ) |
| Accumulated other comprehensive income | | 11 |
| | 271 |
|
| Total shareholders' equity (deficiency) | | 1,907 |
| | (2,783 | ) |
| Total liabilities and shareholders' equity | | 25,011 |
| | 22,195 |
|
Commitments and contingencies (note 25)
Subsequent event (note 28)27)
The accompanying notes are an integral part of these consolidated financial statements.
Approved by the Board of Directors 
|
| | |
| /s/ Carolyn Egbert | | /s/ Gérard Limoges |
Carolyn Egbert Chair of the Board | | Gérard Limoges Director |
|
|
| Aeterna Zentaris Inc. |
| Consolidated Statements of Changes in Shareholders' (Deficiency) Equity |
For the years ended December 31, 2015, 20142018, 2017 and 20132016 |
| (in thousands of US dollars, except share data) |
|
| | | | | | | | | | | | | | | | | | | | | |
| | | Common shares (number of)1, 2 | | Share capital | | Pre-funded warrants | | Other capital | | Deficit | | Accumulated other comprehensive income (loss) | | Total |
| | | | | $ | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2015 | | 655,091 |
| | 150,544 |
| | — |
| | 86,639 |
| | (222,322 | ) | | (377 | ) | | 14,484 |
|
| Net loss | | — |
| | — |
| | — |
| | — |
| | (50,143 | ) | | — |
| | (50,143 | ) |
| Other comprehensive loss: | | | | | | | | | | | | | | — |
|
| Foreign currency translation adjustments | | — |
| | — |
| | — |
| | — |
| | — |
| | 1,509 |
| | 1,509 |
|
| Actuarial gain on defined benefit plans (note 19) | | — |
| | — |
| | — |
| | — |
| | 844 |
| | — |
| | 844 |
|
| Comprehensive loss | | — |
| | — |
| | — |
| | — |
| | (49,299 | ) | | 1,509 |
| | (47,790 | ) |
| Share issuances in connection with public offerings (note 17) | | 3,250,481 |
| | 14,322 |
| | — |
| | — |
| | — |
| | — |
| | 14,322 |
|
| Pre-funded warrant issuances in connection with a public offering (note 17) | | — |
| | — |
| | 8,653 |
| | — |
| | — |
| | — |
| | 8,653 |
|
| Share issuances pursuant to the exercise of pre-funded warrants (note 17) | | 346,294 |
| | 8,653 |
| | (8,653 | ) | | — |
| | — |
| | — |
| | — |
|
| Share issuances pursuant to the exercise of warrants (other than pre-funded warrants) (notes 15 and 17) | | 5,676,831 |
| | 31,077 |
| | — |
| | — |
| | — |
| | — |
| | 31,077 |
|
| Share-based compensation costs | | — |
| | — |
| | — |
| | 869 |
| | — |
| | — |
| | 869 |
|
| Balance - December 31, 2015 | | 9,928,697 |
| | 204,596 |
| | — |
| | 87,508 |
| | (271,621 | ) | | 1,132 |
| | 21,615 |
|
|
| | | | | | | | | | | | | | | | | | |
| | | Common shares (number of) 1 | | Share capital | | Other capital | | Deficit | | Accumulated other comprehensive income | | Total |
| | | | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2018 | | 16,440,760 |
| | 222,335 |
| | 88,772 |
| | (314,161 | ) | | 271 |
| | (2,783 | ) |
| Net income | | — |
| | — |
| | — |
| | 4,187 |
| | — |
| | 4,187 |
|
| Other comprehensive income (loss): | | | | | | | | | | | | |
| Foreign currency translation adjustments | | — |
| | — |
| | — |
| | — |
| | (260 | ) | | (260 | ) |
| Actuarial gain on defined benefit plans (note 18) | | — |
| | — |
| | — |
| | 193 |
| | — |
| | 193 |
|
| Comprehensive loss | | — |
| | — |
| | — |
| | 4,380 |
| | (260 | ) | | 4,120 |
|
| Share-based compensation costs | | — |
| | — |
| | 570 |
| | — |
| | — |
| | 570 |
|
| Balance - December 31, 2018 | | 16,440,760 |
| | 222,335 |
| | 89,342 |
| | (309,781 | ) | | 11 |
| | 1,907 |
|
|
| | | | | | | | | | | | | | | | | | |
| | | Common shares (number of)1, 2 | | Share capital | | Other capital | | Deficit | | Accumulated other comprehensive (loss) income | | Total |
| | | | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2014 | | 453,120 |
| | 134,101 |
| | 86,107 |
| | (203,925 | ) | | 781 |
| | 17,064 |
|
| Net loss | | — |
| | — |
| | — |
| | (16,564 | ) | | — |
| | (16,564 | ) |
| Other comprehensive loss: | | | | | | | | | | | | |
| Foreign currency translation adjustments | | — |
| | — |
| | — |
| | — |
| | (1,158 | ) | | (1,158 | ) |
| Actuarial loss on defined benefit plans (note 19) | | — |
| | — |
| | — |
| | (1,833 | ) | | — |
| | (1,833 | ) |
| Comprehensive loss | | — |
| | — |
| | — |
| | (18,397 | ) | | (1,158 | ) | | (19,555 | ) |
| Share issuances in connection with a public offering (note 17) | | 110,000 |
| | 4,340 |
| | — |
| | — |
| | — |
| | 4,340 |
|
| Share issuances in connection with "At-the-Market" drawdowns (note 17) | | 91,971 |
| | 12,103 |
| | — |
| | — |
| | — |
| | 12,103 |
|
| Share-based compensation costs | | | | — |
| | 532 |
| | — |
| | — |
| | 532 |
|
| Balance - December 31, 2014 | | 655,091 |
| | 150,544 |
| | 86,639 |
| | (222,322 | ) | | (377 | ) | | 14,484 |
|
_________________________
1 Issued and paid in full.________________________
| |
21
| Adjusted to reflect the November 17, 2015 100-to-1 Share Consolidation (see note 1 – Summary of business, liquidity risk, reporting entity, share consolidationIssued and basis of preparation;paid in full. |
|
| | | | | | | | | | | | | | | | | | | | | |
| | | Common shares (number of) 1 | | Share capital | | Pre-funded warrants | | Other capital | | Deficit | | Accumulated other comprehensive income (loss) | | Total |
| | | | | $ | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2017 | | 12,917,995 |
| | 213,980 |
| | — |
| | 88,590 |
| | (298,059 | ) | | 1,701 |
| | 6,212 |
|
| Net loss | | — |
| | — |
| | — |
| | — |
| | (16,796 | ) | | — |
| | (16,796 | ) |
| Other comprehensive income (loss): | | | | | | | | | | | | | | |
| Foreign currency translation adjustments | | — |
| | — |
| | — |
| | — |
| | — |
| | (1,430 | ) | | (1,430 | ) |
| Actuarial gain on defined benefit plans (note 18) | | — |
| | — |
| | — |
| | — |
| | 694 |
| | — |
| | 694 |
|
| Comprehensive loss | | — |
| | — |
| | — |
| | — |
| | (16,102 | ) | | (1,430 | ) | | (17,532 | ) |
| Share issuances pursuant to the exercise of pre-funded warrants (note 19) | | 301,343 |
| | 977 |
| | — |
| | — |
| | — |
| | — |
| | 977 |
|
| Share issuances in connection with "at-the-market" drawdowns (note 19) | | 3,221,422 |
| | 7,378 |
| | — |
| | — |
| | — |
| | — |
| | 7,378 |
|
| Share-based compensation costs | | | | — |
| | — |
| | 182 |
| | — |
| | — |
| | 182 |
|
| Balance - December 31, 2017 | | 16,440,760 |
| | 222,335 |
| | — |
| | 88,772 |
| | (314,161 | ) | | 271 |
| | (2,783 | ) |
________________________
| |
1 | Issued and note 17 – Share capital).paid in full. |
The accompanying notes are an integral part of these consolidated financial statements.
|
|
| Aeterna Zentaris Inc. |
| Consolidated Statements of Changes in Shareholders' (Deficiency) Equity |
For the years ended December 31, 2015, 20142018, 2017 and 20132016 |
| (in thousands of US dollars, except share data) |
|
| | | | | | | | | | | | | | | | | | |
| | | Common shares (number of)1, 2 | | Share capital | | Other capital | | Deficit | | Accumulated other comprehensive income (loss) | | Total |
| | | | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2013 | | 253,293 |
| | 122,791 |
| | 83,892 |
| | (213,086 | ) | | (292 | ) | | (6,695 | ) |
| Net income | | — |
| | — |
| | — |
| | 6,815 |
| | — |
| | 6,815 |
|
| Other comprehensive income: | | | | | | | | | | | | |
| Foreign currency translation adjustments | | — |
| | — |
| | — |
| | — |
| | 1,073 |
| | 1,073 |
|
| Actuarial gain on defined benefit plans (note 19) | | — |
| | — |
| | — |
| | 2,346 |
| | — |
| | 2,346 |
|
| Comprehensive income | | — |
| | — |
| | — |
| | 9,161 |
| | 1,073 |
| | 10,234 |
|
| Share issuances in connection with registered direct and public offerings | | 183,000 |
| | 8,573 |
| | — |
| | — |
| | — |
| | 8,573 |
|
| Share issuances in connection with "At-the-Market" drawdowns | | 16,827 |
| | 2,737 |
| | — |
| | — |
| | — |
| | 2,737 |
|
| Share-based compensation costs | | — |
| | — |
| | 2,215 |
| | — |
| | — |
| | 2,215 |
|
| Balance - December 31, 2013 | | 453,120 |
| | 134,101 |
| | 86,107 |
| | (203,925 | ) | | 781 |
| | 17,064 |
|
_________________________
| |
1
| Issued and paid in full. |
| |
2
| Adjusted to reflect the November 17, 2015 100-to-1 Share Consolidation (see note 1 – Summary of business, liquidity risk, reporting entity, share consolidation and basis of preparations; and note 17 – Share capital). |
|
| | | | | | | | | | | | | | | | | | | | |
| | Common shares (number of)1 | | Share capital | | Pre-funded warrants | | Other capital | | Deficit | | Accumulated other comprehensive income (loss) | | Total |
| | | | $ | | $ | | $ | | $ | | $ | | $ |
| Balance - January 1, 2016 | 9,928,697 |
| | 204,596 |
| | — |
| | 87,508 |
| | (271,621 | ) | | 1,132 |
| | 21,615 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | (24,959 | ) | | — |
| | (24,959 | ) |
| Other comprehensive income (loss): | | | | | | | | | | | | | |
| Foreign currency translation adjustments | — |
| | — |
| | — |
| | — |
| | — |
| | 569 |
| | 569 |
|
| Actuarial loss on defined benefit plan (note 18) | — |
| | — |
| | — |
| | — |
| | (1,479 | ) | | — |
| | (1,479 | ) |
| Comprehensive loss | — |
| | — |
| | — |
| | — |
| | (26,438 | ) | | 569 |
| | (25,869 | ) |
| Share issuances in connection with a public offering (note 19) | 1,150,000 |
| | 3,377 |
| | — |
| | — |
| | — |
| | — |
| | 3,377 |
|
| Pre-funded warrant issuances in connection with a public offering (note 19) | — |
| | — |
| | 2,789 |
| | — |
| | — |
| | — |
| | 2,789 |
|
| Share issuances pursuant to the exercise of pre-funded warrants (note 19) | 950,000 |
| | 2,789 |
| | (2,789 | ) | | — |
| | — |
| | — |
| | — |
|
| Share issuances in connection with "at-the-market" drawdowns (note 19) | 889,298 |
| | 3,218 |
| | — |
| | — |
| | — |
| | — |
| | 3,218 |
|
| Share-based compensation costs | | | | | | | 1,082 |
| | — |
| | — |
| | 1,082 |
|
| Balance - December 31, 2016 | 12,917,995 |
| | 213,980 |
| | — |
| | 88,590 |
| | (298,059 | ) | | 1,701 |
| | 6,212 |
|
1 Issued and paid in full.
The accompanying notes are an integral part of these consolidated financial statements.
|
|
| Aeterna Zentaris Inc. |
Consolidated Statements of Comprehensive Income (Loss) Income |
For the years ended December 31, 2015, 20142018, 2017 and 20132016 |
| (in thousands of US dollars, except share and per share data) |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Revenues | | | | | | |
| Sales commission and other | | 297 |
| | — |
| | 96 |
|
| License fees (note 5) | | 248 |
| | 11 |
| | 6,079 |
|
| | | 545 |
| | 11 |
| | 6,175 |
|
| Operating expenses (note 18) | | | | | | |
| Cost of sales | | — |
| | — |
| | 51 |
|
| Research and development costs | | 17,234 |
| | 23,716 |
| | 21,284 |
|
| General and administrative expenses | | 11,308 |
| | 9,840 |
| | 11,091 |
|
| Selling expenses | | 6,887 |
| | 3,850 |
| | 1,225 |
|
| | | 35,429 |
| | 37,406 |
| | 33,651 |
|
| Loss from operations | | (34,884 | ) | | (37,395 | ) | | (27,476 | ) |
| | | | | | | |
| Finance income (note 20) | | 305 |
| | 20,319 |
| | 1,748 |
|
| Finance costs (note 20) | | (15,649 | ) | | — |
| | (1,512 | ) |
| Net finance (costs) income | | (15,344 | ) | | 20,319 |
| | 236 |
|
| Loss before income taxes | | (50,228 | ) | | (17,076 | ) | | (27,240 | ) |
| Income tax expense (note 22) | | — |
| | (111 | ) | | — |
|
| Net loss from continuing operations | | (50,228 | ) | | (17,187 | ) | | (27,240 | ) |
| Net income from discontinued operations (note 6) | | 85 |
| | 623 |
| | 34,055 |
|
| Net (loss) income | | (50,143 | ) | | (16,564 | ) | | 6,815 |
|
| Other comprehensive (loss) income: | | | | | | |
| Items that may be reclassified subsequently to profit or loss: | | | | | | |
| Foreign currency translation adjustments | | 1,509 |
| | (1,158 | ) | | 1,073 |
|
| Items that will not be reclassified to profit or loss: | | | | | | |
| Actuarial gain (loss) on defined benefit plans (note 19) | | 844 |
| | (1,833 | ) | | 2,346 |
|
| Comprehensive (loss) income | | (47,790 | ) | | (19,555 | ) | | 10,234 |
|
Net loss per share (basic and diluted) from continuing operations (note 26)1 | | (18.17 | ) | | (29.12 | ) | | (92.41 | ) |
Net income per share (basic and diluted) from discontinued operations (notes 6 and 26)1 | | 0.03 |
| | 1.06 |
| | 115.53 |
|
Net (loss) income per share (basic and diluted) (note 26)1 | | (18.14 | ) | | (28.06 | ) | | 23.12 |
|
Weighted average number of shares outstanding
(notes 17 and 26):1 | | | | | | |
| Basic | | 2,763,603 |
| | 590,247 |
| | 294,765 |
|
| Diluted | | 3,424,336 |
| | 590,247 |
| | 294,765 |
|
|
| | | | | | | | | |
| | | Years Ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Revenues | | | | | | |
| License fees (note 6) | | 24,325 |
| | 458 |
| | 497 |
|
| Product sales (note 6) | | 2,167 |
| | — |
| | — |
|
| Royalty income (note 6) | | 184 |
| | — |
| | — |
|
| Sales commission and other | | 205 |
| | 465 |
| | 414 |
|
| Total revenues | | 26,881 |
| | 923 |
| | 911 |
|
| Cost of sales | | 2,104 |
| | — |
| | — |
|
| Gross income | | 24,777 |
| | 923 |
| | 911 |
|
| Operating expenses (note 20) | | | | | | |
| Research and development costs | | 2,932 |
| | 10,704 |
| | 16,495 |
|
| General and administrative expenses | | 8,894 |
| | 8,198 |
| | 7,147 |
|
| Selling expenses | | 3,109 |
| | 5,095 |
| | 6,745 |
|
| Total operating expenses | | 14,935 |
| | 23,997 |
| | 30,387 |
|
| Income (loss) from operations | | 9,842 |
| | (23,074 | ) | | (29,476 | ) |
| Settlements (note 27) | | (1,400 | ) | | — |
| | — |
|
| Gain (loss) due to changes in foreign currency exchange rates | | 656 |
| | 502 |
| | (70 | ) |
| Change in fair value of warrant liability (note 17) | | 263 |
| | 2,222 |
| | 4,437 |
|
| Other finance income | | 278 |
| | 75 |
| | 150 |
|
| Net finance income (costs) | | 1,197 |
| | 2,799 |
| | 4,517 |
|
| Income (loss) before income taxes | | 9,639 |
| | (20,275 | ) | | (24,959 | ) |
| Income tax (expense) recovery (note 22) | | (5,452 | ) | | 3,479 |
| | — |
|
| Net income (loss) | | 4,187 |
| | (16,796 | ) | | (24,959 | ) |
| Other comprehensive income (loss): | | | | | | |
| Items that may be reclassified subsequently to profit or loss: | | | | | | |
| Foreign currency translation adjustments | | (260 | ) | | (1,430 | ) | | 569 |
|
| Items that will not be reclassified to profit or loss: | | | | | | |
| Actuarial gain (loss) on defined benefit plans | | 193 |
| | 694 |
| | (1,479 | ) |
| Comprehensive income (loss) | | 4,120 |
| | (17,532 | ) | | (25,869 | ) |
| Net income (loss) per share (basic) (note 26) | | 0.25 |
| | (1.12 | ) | | (2.41 | ) |
| Net income (loss) per share (diluted) (note 26) | | 0.24 |
| | (1.12 | ) | | (2.41 | ) |
| Weighted average number of shares outstanding (note 26) | | | | | | |
| Basic | | 16,440,760 |
| | 14,958,704 |
| | 10,348,879 |
|
| Diluted | | 17,034,812 |
| | 14,958,704 |
| | 10,348,879 |
|
| |
| Adjusted to reflect the November 17, 2015 100-to-1 Share Consolidation (see note 1 – Summary of business, liquidity risk, reporting entity, share consolidation and basis of preparation; and note 17 – Share capital). |
The accompanying notes are an integral part of these consolidated financial statements.
|
|
| Aeterna Zentaris Inc. |
| Consolidated Statements of Cash Flows |
For the years ended December 31, 2015, 20142018, 2017 and 20132016 |
| (in thousands of US dollars) |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Cash flows from operating activities | | | | | | |
| Net loss from continuing operations | | (50,228 | ) | | (17,187 | ) | | (27,240 | ) |
| Items not affecting cash and cash equivalents: | | | | | | |
| Change in fair value of warrant liability (note 15) | | 10,956 |
| | (18,272 | ) | | (1,563 | ) |
| Provision for restructuring costs (note 14) | | 932 |
| | 2,489 |
| | — |
|
| Depreciation, amortization and impairment (notes 10 and 11) | | 341 |
| | 878 |
| | 949 |
|
| Share-based compensation costs (note 17) | | 919 |
| | 497 |
| | 2,215 |
|
| Employee future benefits (note 19) | | 351 |
| | 605 |
| | 470 |
|
| Amortization of deferred revenues (note 5) | | (248 | ) | | — |
| | (6,046 | ) |
| Foreign exchange loss (gain) on items denominated in foreign currencies | | 1,581 |
| | (1,164 | ) | | 1,078 |
|
| Gain on disposal of property, plant and equipment | | (264 | ) | | (66 | ) | | — |
|
| Amortization of prepaid expenses and other non-cash items | | 154 |
| | 2,640 |
| | 6,831 |
|
| Gain associated with the extinguishment of warrant liability (note 17) | | (162 | ) | | — |
| | — |
|
| Transaction costs allocated to warrants issued (note 17) | | 2,208 |
| | 666 |
| | 1,165 |
|
| Series B Warrant exercise inducement fee (note 15) | | 2,926 |
| | — |
| | — |
|
| Changes in operating assets and liabilities (note 21) | | (3,395 | ) | | (1,873 | ) | | (7,990 | ) |
| Net cash provided by (used in) operating activities of discontinued operations (note 6) | | 85 |
| | (295 | ) | | 10,147 |
|
Net cash used in operating activities | | (33,844 | ) | | (31,082 | ) | | (19,984 | ) |
| Cash flows from financing activities | | | | | | |
| Proceeds from issuances of common shares and warrants (including pre-funded warrants), net of cash transaction costs of $4,223 in 2015, $1,348 in 2014 and $2,119 in 2013 (note 17) | | 49,427 |
| | 24,358 |
| | 23,708 |
|
| Series B Warrrant exercise inducement fee (note 17) | | (2,926 | ) | | — |
| | — |
|
| Payment pursuant to warrant amendment agreements (note 15) | | (5,703 | ) | | — |
| | — |
|
| Net cash provided by financing activities | | 40,798 |
| | 24,358 |
| | 23,708 |
|
| Cash flows from investing activities | | | | | | |
| Purchase of property, plant and equipment (note 10) | | (26 | ) | | (127 | ) | | (85 | ) |
| Disposals of property, plant and equipment (note 10) | | 505 |
| | 66 |
| | — |
|
| Decrease in restricted cash equivalents | | 434 |
| | — |
| | — |
|
Net cash provided by investing activities of discontinued operations | | — |
| | — |
| | 113 |
|
Net cash provided by (used in) investing activities | | 913 |
| | (61 | ) | | 28 |
|
| Effect of exchange rate changes on cash and cash equivalents | | (1,348 | ) | | (1,486 | ) | | (71 | ) |
| Net change in cash and cash equivalents | | 6,519 |
| | (8,271 | ) | | 3,681 |
|
| Cash and cash equivalents – Beginning of the year | | 34,931 |
| | 43,202 |
| | 39,521 |
|
| Cash and cash equivalents – End of the year | | 41,450 |
| | 34,931 |
| | 43,202 |
|
|
| | | | | | | | |
| | Years ended December 31, |
| | 2018 | | 2017 | | 2016 |
| | $ | | $ | | $ |
| Cash flows from operating activities | | | | | |
| Net income (loss) for the year | 4,187 |
| | (16,796 | ) | | (24,959 | ) |
| Items not affecting cash and cash equivalents: |
|
| | | | |
| Change in fair value of warrant liability (note 17) | (263 | ) | | (2,222 | ) | | (4,437 | ) |
| Provision for restructuring and other costs (note 16) | (136 | ) | | 3,083 |
| | (8 | ) |
| Recapture of inventory previously written off | — |
| | (643 | ) | | — |
|
| Depreciation, amortization and impairment (notes 12 and 13) | 58 |
| | 94 |
| | 280 |
|
| Deferred income taxes (note 22) | 3,479 |
| | (3,479 | ) | | — |
|
| Share-based compensation costs | 570 |
| | 182 |
| | 1,082 |
|
| Employee future benefits (note 18) | 316 |
| | 246 |
| | 382 |
|
| Amortization of deferred revenues (note 6) | (609 | ) | | (458 | ) | | (345 | ) |
| Foreign exchange (gain) loss on items denominated in foreign currencies | (652 | ) | | (553 | ) | | 87 |
|
| Gain on disposal of property, plant and equipment | (9 | ) | | (136 | ) | | (1 | ) |
| Other non-cash items | 35 |
| | (19 | ) | | (83 | ) |
| Transaction cost allocated to warrants issued (note 19) | — |
| | — |
| | 56 |
|
| Changes in operating assets and liabilities (note 21) | (151 | ) | | (2,212 | ) | | (1,064 | ) |
| Net cash provided by/(used in) operating activities | 6,825 |
| | (22,913 | ) | | (29,010 | ) |
| Cash flows from financing activities |
| | | | |
| Proceeds from issuances of common shares, warrants (including pre-funded warrants), net of cash transaction costs of $nil, $250 and $1,107 in 2018, 2017, and 2016, respectively (note 19) | — |
| | 7,788 |
| | 9,924 |
|
| Proceeds from warrants exercised (note 19) | — |
| | 242 |
| | — |
|
| Net cash provided by financing activities | — |
| | 8,030 |
| | 9,924 |
|
| Cash flows from investing activities |
| | | | |
| Purchase of property, plant and equipment (note 12) | (9 | ) | | (4 | ) | | (66 | ) |
| Proceeds for disposals of property, plant and equipment (note 12) | 24 |
| | 161 |
| | 2 |
|
| Change in restricted cash equivalents | (50 | ) | | 150 |
| | (250 | ) |
| Net cash provided by (used in) investing activities | (35 | ) | | 307 |
| | (314 | ) |
| Effect of exchange rate changes on cash and cash equivalents | (58 | ) | | 357 |
| | (51 | ) |
| Net change in cash and cash equivalents | 6,732 |
| | (14,219 | ) | | (19,451 | ) |
| Cash and cash equivalents – beginning of year (note 6) | 7,780 |
| | 21,999 |
| | 41,450 |
|
| Cash and cash equivalents – end of year (note 6) | 14,512 |
| | 7,780 |
| | 21,999 |
|
The accompanying notes are an integral part of these consolidated financial statements.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
| |
1 | Summary of business, liquidity risk, reporting entity, share consolidation and basis of preparation |
1 Business overview
Summary of business
Aeterna Zentaris Inc. (the("Aeterna Zentaris" or the "Company") is a specialty biopharmaceutical company engaged in developing andwhich is commercializing novel treatmentspharmaceutical therapies. On December 20, 2017, the United States Food and Drug Administration ("FDA") granted marketing approval for Macrilen™ (macimorelin) to be used in oncology, endocrinologythe diagnosis of patients with adult growth hormone deficiency ("AGHD"). On January 16, 2018, the Company, through Aeterna Zentaris GmbH, entered into a license and women's health.
Liquidity risk
The Company has a historyassignment agreement with Strongbridge Ireland Limited ("Strongbridge") to carry out development, manufacturing, registration, regulatory and supply chain services for the commercialization of operating losses, due largely to significant researchMacrilen™ (macimorelin) in the United States and development ("R&D") investment, as well as to the incurrence of substantial selling expensesCanada (the "License and general and administrative expenses ("G&A"Assignment Agreement"). The Company has financed its operations more recently through different sources, includingEffective December 19, 2018, Strongbridge sold the issuance of common sharesUnited States and warrants and the conclusion of strategic alliances with licensee partners and other collaborators. The Company expectsCanadian rights to continueMacrilen™ to incur operating expenses and may require significant capital to fulfill its future obligations in absence of sufficient corresponding revenues. See note 23 – Capital disclosures and note 24(b) – Financial instruments and financial risk management – Liquidity risk.Novo Nordisk ("Novo").
Reporting entity
The accompanying consolidated financial statements include the accounts of Aeterna Zentaris Inc., an entity incorporated under the Canada Business Corporations Act, and its wholly ownedwholly-owned subsidiaries (collectively referred to as the "Group"). Aeterna Zentaris Inc. is the ultimate parent company of the Group.
The Company currently has three wholly ownedwholly-owned direct and indirect subsidiaries, Aeterna Zentaris GmbH ("AEZS Germany"), based in Frankfurt, Germany, Zentaris IVF GmbH, a wholly ownedwholly-owned subsidiary of AEZS Germany, based in Frankfurt, Germany, and Aeterna Zentaris, Inc., an entity incorporated in the state of Delaware and with offices in Summerville, South Carolina, in the United States.
The registered office of the Company is located at 1 Place Ville Marie, Suite 2500,1155 Rene-Levesque Blvd. West, 41st Floor, Montreal, Quebec H3B 1R1, Canada.3V2, Canada and its principal place of business is 315 Sigma Drive, Summerville, South Carolina 29486.
The Company's common shares are listed on both on the Toronto Stock Exchange (the "TSX") and on the NASDAQ Capital Market (the "NASDAQ").
Share consolidation (reverse stock split)
On November 17, 2015, the Company effected a consolidation of its issued and outstanding common shares on a 100-to-1 basis (the "Share Consolidation"). The Share Consolidation affected all shareholders, option holders and warrant holders uniformly and thus did not materially affect any security holder's percentage of ownership interest. All references in these consolidated financial statements to common shares, options and share purchase warrants have been retroactively adjusted to reflect the Share Consolidation.
Basis of preparationpresentation
| |
(a) | Statement of compliance |
The(a) Statement of compliance
These consolidated financial statements as at December 31, 2015 2018and December 31, 20142017 and for the years ended December 31, 2015, 2014 2018, 2017and 20132016 have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB").
The accounting policies in these consolidated financial statements are consistent with those of the previous financial year except for the adoption of those standards in 2018 (note 4) and are consistent with the previous quarter.
These consolidated financial statements were approved by the Company's Board of Directors on March 29, 2016.2019.
The accompanying consolidated financial statements were prepared on a going concern basis, under the historical cost convention, except for the warrant liability, which is measured at fair value.
The preparation of financial statements in accordance with IFRS requires the use of certain critical accounting estimates and the exercise of management's judgment in applying the Company's accounting policies. Areas involving a high
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
degree of judgment or complexity and areas where assumptions and estimates are significant to the Company's consolidated financial statements are discussed in note 3 –4 - Critical accounting estimates and judgments.
| |
(b) | Principles of consolidation |
(b) Principles of consolidation
These consolidated financial statements include any entity in which the Company directly or indirectly holds more than 50% of the voting rights or over which the Company exercises control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. An entity is included in the consolidation from the date that control is transferred to the Company, while any entities that are sold are excluded from the consolidation from the date that control ceases. All inter-company balances and transactions are eliminated on consolidation.
| |
(c) |
Foreign currencyAeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
(c) Foreign currency
Items included in the financial statements of the Group's entities are measured using the currency of the primary economic environment in which the entities operate (the "functional currency"). On January 1, 2015, which is U.S. dollars for the Company and its USU.S. subsidiary, Aeterna Zentaris, Inc., changed their functional currency from the and Euro ("EUR") to the US dollar, given that changes to underlying transactions, events and conditions indicated that the US dollar more appropriately reflects the primary economic environment in which these entities operate. This change in functional currency was accounted for prospectively. The functional currency of theits German subsidiaries remains the EUR.subsidiaries.
Assets and liabilities of the German subsidiaries are translated from EUR balances at the period-end exchange rates, and the results of operations are translated from EUR amounts at average rates of exchange for the period. The resulting translation adjustments are included in accumulated other comprehensive (loss) income within shareholders' equity.equity (deficiency).
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the underlying transaction. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of monetary assets and liabilities not denominated in the functional currency are recognized in the consolidated statement of comprehensive income (loss) income..
2 Assessment of liquidity and management's plans
Since inception, the Company has incurred significant expenses in its efforts to develop and commercialize products. Consequently, the Company has incurred operating losses that relate toand negative cash flow from operations historically and in each of the last several years except for the year ended December 31, 2018 when the Company earned revenue from the sale of a license for the adult indication of MacrilenTM (macimorelin) in the United States and Canada (note 6). As at December 31, 2018, the Company had an accumulated deficit of $310 million.
The Company has $14,512 of cash and cash equivalents are presented within finance income or finance costs in the consolidated statementas at December 31, 2018, and management believes it has sufficient liquidity to meet its current obligations of comprehensive (loss) income. All other foreign exchange gains$5,596 and losses are presented in the consolidated statement of comprehensive (loss) income within operating expenses.
In light of the increasedcontinue its planned level of commercial initiatives, management determined thatexpenses for at least, but not limited to the next twelve months from the date of issuance of these consolidated financial statements. The Company is focused on managing its operating expenses, and has the discretion to limit research and development costs, administrative expenses and capital expenditures in order to appropriately reflectmaintain its liquidity, until such time that additional sources of funding can be obtained. The Company’s principal focus is on the current naturelicensing and development of MacrilenTM (macimorelin) and it currently does not have any other approved product. In January 2018, the Company signed a license and assignment agreement with Strongbridge Ireland Ltd., which as of December 19, 2018 is a wholly-owned subsidiary of Novo Nordisk A/S (“Novo”), to carry out development, manufacturing, registration and commercialization of MacrilenTM (macimorelin) in the U.S. and Canada (the “License and Assignment Agreement”) (see note 6). Consistent with Strongbridge, Novo is funding 70% of the pediatric clinical trial submitted to the EMA and FDA, the Company's sole development priority.
On March 12, 2019, the Company announced that its board of directors has formed a special committee of independent directors (the "Special Committee") to review strategic options available to the Company. The Special Committee has approved the engagement by the Company of a financial advisor that is working with management to assist the Special Committee and the board of directors in considering a wide range of transactions (including opportunities for the license of MacrilenTM (macimorelin) outside of the United States and Canada, or other monetization transactions relating to MacrilenTM (macimorelin). Management has evaluated whether material uncertainties exist relating to events or conditions as described in Note 4 and has considered the following in making that critical judgment.
The Company’s current operating budget and cash flows from operating activities in 2019 are expected to decline compared with 2018, however, the Company believes it will experience an increase in its royalty income, which, when combined with its forecasted cash flows, the Company believes will provide sufficient liquidity to finance operations and meet its commitments for at least, but not limited to, twelve months from the former consolidated statementdate of comprehensive loss line item "Selling, general and administrative expenses" should be disaggregated into two separate line items: "General and administrative expenses" and "Selling expenses". Comparative amounts have been disaggregated consistently.approval of these financial statements.
| |
2 | Summary of significant accounting policies |
3 Summary of significant accounting policies
The accounting policies set out below have been applied consistently to all years presented in these consolidated financial statements except for the adoption of those standards in 2018 (note 4) and have been applied consistently by all Group entities.
Cash and cash equivalents
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
Cash and cash equivalents consist of unrestricted cash on hand and balances with banks, as well as short-term interest-bearing deposits, such as money market accounts, that are readily convertible to known amounts of cash and are subject to an insignificant risk of changes in value, with a maturity of three months or less from the date of acquisition.
Inventories
Inventories are valued at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method for all inventories. The Company's policy is to write down inventory that has become obsolete and inventory that has a cost basis in excess of its expected net realizable value. Increases in the reserve are recorded as charges in cost of product sales. For product candidates that have not been approved by the FDA, inventory used in clinical trials is written down at the time of production and recorded as research and development ("R&D") costs. For products that have been approved by the FDA, inventory used in clinical trials is expensed at the time the inventory is packaged for the clinical trial. All direct manufacturing costs incurred after approval are capitalized into inventory.
Restricted cash equivalents
Restricted cash equivalents are comprised of a bank deposit,deposits, related to a guarantee for a long-term operating lease obligation and for a corporate credit card program that cannot be used for current purposes.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Property, plant and equipment and depreciation
Items of property, plant and equipment are recorded at cost, net of related government grants and accumulated depreciation and impairment charges. Depreciation is calculated using the following methods, annual rates and period:
|
| | | | |
| | | Methods | | Annual rates and period |
| Equipment | | Declining balance and straight-line | | 20% |
| Furniture and fixtures | | Declining balance and straight-line | | 10% and 20% |
| Computer equipment | | Straight-line | | 25% and 331/3%3% |
| Leasehold improvements | | Straight-line | | Remaining lease term |
Depreciation expense, which is recorded in the consolidated statement of comprehensive income (loss) income,, is allocated to the appropriate functional expense categories to which the underlying items of property, plant and equipment relate.
Identifiable intangible assets and amortization
Identifiable intangible assets with finite useful lives consist of in-process R&D acquired in business combinations, patents and trademarks. In-process R&D acquired in business combinations is recognized at fair value at the acquisition date. Patents and trademarks are comprised of costs, including professional fees incurred in connection with the filing of patents and the registration of trademarks for product marketing and manufacturing purposes net of related government grants, impairment losses, where applicable, and accumulated amortization. Identifiable intangible assets with finite useful lives are amortized, from the time at which the assets are available for use, on a straight-line basis over their estimated useful lives of eight to fifteen years for in-process R&D and patents and ten years for trademarks. Amortization expense, which is recorded in the consolidated statement of comprehensive income (loss) income,, is allocated to the appropriate functional expense categories to which the underlying identifiable intangible assets relate.
Goodwill
Goodwill representsis recognized as the excessfair value of the purchase price overconsideration transferred including the recognized amount of any non-controlling interest in the acquiree, less the fair valuesvalue of the net identifiable assets acquired and liabilities assumed, as of entities acquired at their respective dates of acquisition. Goodwillthe acquisition date. Subsequent to initial recognition, goodwill is carriedmeasured at cost less accumulated impairment losses. Goodwill acquired in business combinations is allocated to each cash-generating unitgroups of cash generating units ("CGU") or group of CGUs that are expected to benefit from the related businesssynergies of the combination.
Impairment of assets
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
Items of property, plant and equipment and identifiable intangible assets with finite lives subject to depreciation or amortization, respectively, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be recoverable. Management is required to assess at each reporting date whether there is any indication that an asset may be impaired. Where such an indication exists, the asset's recoverable amount is compared to its carrying value, and an impairment loss is recognized for the amount by which the asset's carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset's fair value less costs to sell and value in use. For the purpose of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows, or CGU. In determining value in use of a given asset or CGU, estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. Impairment losses are allocated to the appropriate functional expense categories to which the underlying identifiable intangible assets relate, and are recorded in the consolidated statement of comprehensive income (loss) income..
Items of property, plant and equipment and amortizable identifiable intangible assets with finite lives that suffered impairment are reviewed for possible reversal of the impairment if there has been a change, since the date of the most recent impairment test, in the estimates used to determine the impaired asset's recoverable amount. However, an asset's carrying amount, increased due to the reversal of a prior impairment loss, must not exceed the carrying amount that would have been determined, net of depreciation or amortization, had the original impairment not occurred.
Goodwill is not subject to amortization and instead is tested for impairment annually or more often if there is an indication that the CGU to which the goodwill has been allocated may be impaired. Impairment is determined for goodwill by assessing
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
whether the carrying value of a CGU, including the allocated goodwill, exceeds its recoverable amount, which is the higher of fair value less costs to sell and value in use. In the event that the carrying amount of goodwill exceeds its recoverable amount, an impairment loss is recognized in an amount equal to the excess. Impairment losses related to goodwill are not subsequently reversed.
Share purchase warrants
Share purchase warrants are classified as liabilities when the Company does not have the unconditional right to avoid delivering cash to the holders in the future. Each of the Company's share purchase warrants contains a written put option, arising upon the occurrence of a Fundamental Transaction,fundamental transaction, as that term is defined in the share purchase warrants, including a change of control. As a result of the existence of these put options, and despite the fact that the repurchase feature is conditional on a defined contingency, the share purchase warrants are required to be classified as a financial liability, since such contingency could ultimately result in the transfer of assets by the Company.
The warrant liability is initially measured at fair value, and any subsequent changes in fair value are recognized as gains or losses through profit or loss. Any transaction costs related to the share purchase warrants are expensed as incurred.
The warrant liability is classified as non-current, unless the underlying share purchase warrants are about towill expire or be settled within 12months from the end of a given reporting period.
Employee benefits
Salaries and other short-term benefits
Salaries and other short-term benefit obligations are measured on an undiscounted basis and are recognized in the consolidated statement of comprehensive income (loss) income over the related service period or when the Company has a present legal or constructive obligation to make payments as a result of past events and when the amount payable can be estimated reliably.
Post-employment benefits
The Company's subsidiary in Germany maintains defined contribution and unfunded defined benefit plans, as well as other benefit plans for its employees. For defined benefit pension plans and other post-employment benefits, net periodic pension expense is actuarially determined on a quarterly basis using the projected unit credit method. The cost of pension and other benefits earned by employees is determined by applying certain assumptions, including discount rates, the projected age of employees upon retirement, the expected rate of future compensation and employee turnover.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
The employee future benefits liability is recognized at its present value, which is determined by discounting the estimated future cash outflows using interest rates of high-quality corporate bonds that are denominated in the currency in which the benefits will be paid and that have terms to maturity approximating the terms of the related future benefit liability. Actuarial gains and losses that arise in calculating the present value of the defined benefit obligation are recognized in other comprehensive income (loss) income,, net of tax, and simultaneously reclassified in the deficit in the consolidated statement of financial position in the year in which the actuarial gains and losses arise and without recycling to the consolidated statement of comprehensive income (loss) income in subsequent periods.
For defined contribution plans, expenses are recorded in the consolidated statement of comprehensive income (loss) income as incurred–namely, over the period that the related employee service is rendered.
Termination benefits
Termination benefits are recognized in the consolidated statement of comprehensive income (loss) income when the Company is demonstrably committed, without the realistic possibility of withdrawal, to a formal detailed plan to terminate employment earlier than originally expected. Termination benefit liabilities expected to be settled after 12 months from the end of a given reporting period are discounted to their present value, where material.
Financial instruments
105The Company classifies its financial instruments in the following categories: "Financial assets at fair value through profit or loss ("FVTPL"); "Financial assets at amortized cost"; "Financial liabilities at "FVTPL"; and "Financial liabilities at amortized cost".
Financial assets at FVTPL: Financial assets carried at FVTPL are initially recorded at fair value and transaction costs are expensed in the statement of comprehensive income (loss). Realized and unrealized gains and losses arising from changes in the fair value of the financial assets held at FVTPL are included in the statement of comprehensive income (loss) in the period in which they arise.
Financial liabilities at FVTPL: These financial liabilities are initially recognized at fair value, and transaction costs directly attributable to issuing the warrants are expensed in the statement of comprehensive income (loss). Financial liabilities that are required to be measured at FVTPL have all fair value movements, excluding those related to changes in the credit risk of the liability which are recorded in other comprehensive income (loss), recognized in the statement of comprehensive income (loss).
Financial assets at fair value through other comprehensive income (FVTOCI): Investments in equity instruments at FVTOCI are initially recognized at fair value plus transaction costs. Subsequently they are measured at fair value, with gains and losses arising from changes in fair value recognized in other comprehensive income (loss) in the period in which they arise.
Financial assets at amortized cost: A financial asset is measured at amortized cost if the objective of the business model is to hold the financial asset for the collection of contractual cash flows, and the asset's contractual cash flows are comprised solely of payments of principal and interest. They are classified as current assets or non-current assets based on their maturity date, and are initially recognized at fair value and subsequently carried at amortized cost less any impairment.
Impairment of financial assets at amortized cost: The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
The following table shows the classification of the Company’s financial assets/liabilities under IFRS 9 Financial instruments
The Company classifies its financial instruments inInstruments ("IFRS 9") and the following categories: "Financial assets at fair value through profit or loss ("FVTPL"); "Loans and receivables"; "Financial liabilities at "FVTPL"; and "Other financial liabilities".previous classifications under IAS 39:
Financial assetsasset/liability IFRS 9 Classification IAS 39 Classification
Cash and liabilities are offset, and the net amount is reported in the consolidated statement of financial position, when there is a legally enforceable right to offset the recognized amounts and there is an intention to settle on a net basis or realize the asset and settle the liability simultaneously.
Financial assets at fair value through profit or loss
Financial assets at FVTPL are financial assets held for trading. Fair value is defined as the amount at which the financial assets could be exchanged in a current transaction between willing parties, other than in a forced or liquidation sale. A financial asset is classified as at FVTPL if the instrument is acquired or received as consideration principally for the purpose of selling in the short-term. Financial assets at FVTPL are classified as current assets if expected to be settled within 12 months from the end of a given reporting period; otherwise, the assets are classified as non-current.
As at December 31, 2015 and 2014, the Company held no assets classified as financial assets at FVTPL.
cash equivalents Amortized cost Loans and receivables
Trade and other receivables Amortized cost Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Loans and receivables are included in current assets, except for instruments with maturities greater than 12 months after the end of a given reporting period or where restrictions apply that limit the Company from using the instrument for current purposes, which are classified as non-current assets.
The Company's loans and receivables are comprised ofRestricted cash and cash equivalents tradeAmortized cost Loans and other receivables
Warrant liability (derivative) FVTPL FVTPL
Payable and restricted cash equivalents.
Financialaccrued liabilities at fair value through profit or loss
Financial liabilities at FVTPL are financial liabilities held for trading. A financial liability is classified as at FVTPL if the instrument is acquired or incurred principally for the purpose of selling or repurchasing in the short-term or where the Company does not have the unconditional right to avoid delivering cash or another financial asset to the holders in certain circumstances. Financial liabilities at FVTPL are classified as current liabilities if required to be settled within 12 months from the end of a given reporting period; otherwise, the liabilities are classified as non-current.
Financial liabilities at FVTPL are currently comprised of the Company's warrant liability.
Amortized cost Other financial liabilities
Other financial liabilities include trade accounts payable and accrued liabilities, provision for restructuring and other non-current liabilities.
| |
(b) | Recognition and measurement |
Financial assets at fair value through profit or loss
Financial assets at FVTPL are recognized on the settlement date, which is the date on which the asset is delivered to the Company. Financial assets at FVTPL are initially recognized at fair value, and transaction costs are expensed immediately in the consolidated statement of comprehensive (loss) income. Financial assets at FVTPL are derecognized when the right to receive cash flows from the underlying investment have expired or have been transferred and when the Group has transferred substantially all risks and rewards of ownership. Gains and losses arising from changes in the fair value of financial assets at FVTPL are presented in the consolidated statement of comprehensive (loss) income within finance income or finance costs in the period in which they arise.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Loans and receivables
Loans and receivables are recognized on the settlement date and are measured initially at fair value and subsequently at amortized cost using the effective interest rate method.
Financial liabilities at fair value through profit or loss
Financial liabilities at FVTPL are recognized on the settlement date. Financial liabilities at FVTPL are initially recognized at fair value, and transaction costs are expensed immediately in the consolidated statement of comprehensive (loss) income. Gains and losses arising from changes in the fair value of financial liabilities at FVTPL are presented in the consolidated statement of comprehensive (loss) income within finance income or finance costs in the period in which they arise.
Other financial liabilities
Financial instruments classified as "Other financial liabilities" are measured initially at fair value and subsequently at amortized cost using the effective interest rate method.
Financial assets measured at amortized cost are reviewed for impairment at each reporting date. Where there is objective evidence that impairment exists for a financial asset measured at amortized cost, an impairment charge equivalent to the difference between the asset's carrying amount and the present value of estimated future cash flows is recorded in the consolidated statement of comprehensive (loss) income. The expected cash flows exclude future credit losses that have not been incurred and are discounted at the financial asset's original effective interest rate.
Impairment charges related to financial assets carried at amortized cost are reversed if, in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after the impairment was recognized. However, the reversal cannot result in a carrying amount of the financial asset that exceeds what the amortized cost would have been had the impairment not been recognized at the date the impairment is reversed.
Share capital
Common shares are classified as equity. Incremental costs that are directly attributable to the issueissuance of common shares and stock options are recognized as a deduction from equity, net of any tax effects.
Where offerings result in the issuance of units (where each unit is comprised of a common share of the Company and a share purchase warrant, exercisable in order to purchase a common share or fraction thereof), proceeds received in connection with those offerings are allocated between Shareshare capital and Shareshare purchase warrants based on the residual method. Proceeds are allocated to warrant liability based on the fair value of the share purchase warrants, fair value, and the residual amount of proceeds is allocated to Shareshare capital. Transaction costs in connection with such offerings are allocated to the liability and equity unitsunit components in proportion to the allocation of proceeds.
Provisions
Provisions represent liabilities to the Company for which the amount or timing is uncertain. Provisions are recognized when the Company has a present legal or constructive obligation as a result of past events, such as organizational restructuring, when it is probable that an outflow of resources will be required to settle the obligation and where the amount can be reliably estimated. Provisions are not recognized for future operating losses.
Provisions are made for any contracts which are deemed onerous. A contract is onerous if the unavoidable costs of meeting the obligations under the contract exceed the economic benefits expected to be received under it. Provisions for onerous contracts are measured at the present value of the lower of the expected cost of terminating the contract and the expected net cost of continuing with the contract. Present value is determined based on expected future cash flows that are discounted at a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the liability. The unwinding of the discount is recognized in finance costs.
Revenue recognition
107License fees
License fees representing non-refundable payments received at the time of executing the license agreements. The Company’s promise to grant a license provides its customer with either a right to access the Company’s intellectual property ("IP") or a right to use the Company’s IP. Revenue from a license that provides a customer the right to use the Company’s IP is recognized at a point in time when the transfers to the licensee is completed and the license period begins. Revenue from a license that provides access to the Company’s IP over a license term is considered to be a performance obligation satisfied over time and, therefore, revenue is recognized over the term of the license arrangement.
Royalty and milestone income
Royalty income earned through a license is recognized when the underlying sales have occurred. Milestone income is recognized at the point in time when it is highly probable that the respective milestone event criteria are met, and the risk of reversal of revenue recognition is remote. Other revenue also includes revenue from activities such as manufacturing or
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
Revenue recognition
Sales of products
Revenues fromother services rendered, to the sale of goods areextent such revenue is not recorded under net sales, and is recognized when the Company has transferredcontrol transfers to the buyer the significant risks and rewards of ownership of the goods (which is at the time the goods are shipped), when the Company retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold, when the amount of revenues can be measured reliably, when it is probable that the economic benefits associated with the transaction will flow to the Company and when the costs incurred or to be incurred in respect of the transaction can be measured reliably.
Royalty revenues
The Company had deferred recognition of proceeds received in December 2008 from Healthcare Royalty Partners L.P. (formerly Cowen Healthcare Royalty Partners L.P. ) ("HRP") relating to the Company's rights to royalties on future sales of Cetrotide® covered by a license agreement with ARES Trading S.A. ("Merck Serono") in which the latter had been granted worldwide marketing, distribution and selling rights, except in Japan, for Cetrotide®, a compound used for in vitro fertilization.
The Company recognized the proceeds received from HRP as royalty revenues over the life of the underlying royalty sale arrangement, pursuant to the "units-of-revenue" method. Under that method, periodic royalty revenues are calculated as the ratio of the remaining deferred revenue amount to the total estimated remaining royalties that Merck Serono expected to pay to HRP over the term of the underlying arrangement multiplied by the royalty payments due to HRP for the period.
As mentioned in note 6 – Discontinued operations, from April 3, 2013 to October 1, 2013, the Company accelerated the amortization of the remaining deferred revenues.
Licensing revenues and multiple element arrangements
The Company is currently in a phase in which certain potential products are being further developed or marketed jointly with partners and licensees. Existing licensing agreements usually foresee one-time payments (upfront payments), payments for R&D services in the form of cost reimbursements, milestone payments and royalty receipts for licensing and marketing product candidates. Revenues associated with those multiple-element arrangements are allocated to the various elements based on their relative fair value.
Agreements containing multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered obligation(s). The consideration received is allocated among the separate units based on each unit's fair value,third party and the applicable revenue recognition criteria are applied to each of the separate units.
License fees representing non-refundable payments received at the time of signature of license agreements are recognized as revenue upon signature of the license agreements when the Company has no significant futurerelated performance obligations and collectibility of the fees is probable. Upfront payments received at the beginning of licensing agreements are deferred and recognized as revenue on a systematic basis over the period during which the related services are rendered and all obligations are performed.satisfied.
Milestone payments
Milestone payments, which are generally based on developmental or regulatory events, are recognized as revenue when the milestones are achieved, collectibility is assured, and when the Company has no significant future performance obligations in connection with the milestones.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Share-based compensation costs
The Company operates an equity-settled share-based compensation plan under which the Company receives services from directors, senior executives, employees and other collaborators as consideration for equity instruments of the Company.
The Company accounts for all forms of share-based compensation using the fair value-based method. Fair value of stock options is determined at the date of grant using the Black-Scholes option pricing model, which includes estimates of the number of awards that are expected to vest over the vesting period. Where granted share options vest in installments over the vesting period (defined as graded vesting), the Company treats each installment as a separate share option grant. Share-based compensation expense is recognized over the vesting period, or as specified vesting conditions are satisfied, and credited to Other Capital.other capital.
Any consideration received by the Company in connection with the exercise of stock options is credited to Share Capital.share capital. Any Other Capitalother capital component of the share-based compensation is transferred to Share Capitalshare capital upon the issuance of shares.
Current and deferred income tax
Income tax on profit or loss comprises current and deferred tax. Tax is recognized in profit or loss, except that a change attributable to an item of income or expense recognized as other comprehensive income (loss) income or directly in equity is also recognized directly in other comprehensive income (loss) income or directly in equity. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation and establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
The current income tax charge is calculated in accordance with tax rates and laws that have been enacted or substantively enacted by the reporting date in the countries where the Company's subsidiaries operate and generate taxable income.
Deferred income tax is recognized on temporary differences (other than, where applicable, temporary differences associated with unremitted earnings from foreign subsidiaries and associates to the extent that the investment is essentially permanent in duration, and temporary differences associated with the initial recognition of goodwill) arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements and on unused tax losses or R&D non-refundable tax credits in the Group. Deferred income tax is determined using tax rates and laws that have been enacted or substantively enacted by the reporting date.
Deferred income tax assets are recognized only to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized.
Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income taxes assets and liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities where there is an intention to settle the balances on a net basis.
Research and development costs
Research costs are expensed as incurred. Development costs are expensed as incurred, except for those that meet generally acceptedthe criteria for deferral, in which case the costs are capitalized and amortized to operations over the estimated period of benefit. No development costs have been capitalized during any of the periods presented.
Discontinued operations
A discontinued operation is a component of the Company that has been disposed of, or is classified as held for sale, and represents a separate major line of business or geographical area of operations and/or is part of a single co-ordinated plan to dispose of a separate major line of business or geographical area of operations. Classification as a discontinued operation occurs upon the earlier of the disposal of the operation (or disposal group) or the date at which the operation meets the criteria for classification as held for sale. When an operation is classified as discontinued, comparative statements of comprehensiveNet income (loss) income and cash flows are presented as if the operations had been discontinued at the beginning of the earliest comparative period presented.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Net (loss) income per share
Basic net income (loss) income per share is calculated using the weighted average number of common shares outstanding during the year.
Diluted net income (loss) income per share is calculated based on the weighted average number of common shares outstanding during the year, plus the effects of dilutive common share equivalents, such as stock options and share purchase warrants.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
This method requires that diluted net income (loss) income per share be calculated using the treasury stock method, as if all common share equivalents had been exercised at the beginning of the reporting period, or period of issuance, as the case may be, and that the funds obtained thereby were used to purchase common shares of the Company at the average trading price of the common shares during the period.
| |
3 | Critical accounting estimates and judgments |
4 Critical accounting estimates and judgments
The preparation of consolidated financial statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of the Company's assets, liabilities, revenues, expenses and related disclosures. Judgments, estimates and assumptions are based on historical experience, expectations, current trends and other factors that management believes to be relevant at the time at which the Company's consolidated financial statements are prepared.
Management reviews, on a regular basis, the Company's accounting policies, assumptions, estimates and judgments in order to ensure that the consolidated financial statements are presented fairly and in accordance with IFRS. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
| |
(a) | Critical accounting estimates and assumptions |
(a) Critical accounting estimates and assumptions
Critical accounting estimates and assumptions are those that have a significant risk of causing material adjustment and are often applied to matters or outcomes that are inherently uncertain and subject to change. As such, management cautions that future events often vary from forecasts and expectations and that estimates routinely require adjustment.
The following discusses the most significant accounting estimates and assumptions that the Company has made in the preparation of the consolidated financial statements.
Accounting for the Macrilen License and Assignment Agreement
See the performance obligations further described in note 6 - Licensing arrangements.
Fair value of the warrant liability and stock options
Determining the fair value of the warrant liability and stock options requires judgment related to the choiceselection of athe most appropriate pricing model, the estimation of stock price volatility and the expected term of the underlying instruments. Any changes in the estimates or inputs utilized to determine fair value could result in a significant impact on the Company's future operating results, liabilities or other components of shareholders' equity. Fair value assumptions used are described in notes 15 –note 17 - Warrant liability and 17 –19 - Share and other capital.
Goodwill impairmentImpairment of goodwill and identifiable intangible assets
The annual impairment assessment related to goodwill requires to estimate the recoverable amount, which has been determined using fair value less costs of disposal. This evaluation is based on estimates that are derived from current market capitalization and on other factors, including assumptions related to relevant industry-specific market analyses and potential costs to dispose.in use model. The Company also concluded that there was only one CGU as management monitors goodwill and identifiable intangible assets on an overall entity basis. Future events including a significant reduction in the Company's share price, could cause the assumptions utilized in the impairment tests to change, resulting in a potentially adverse effect on the Company's future results due to increased impairment charges.
Employee future benefits
The determination of expenses and obligations associated with employee future benefits requires the use of assumptions, such as the discount rate to measure obligations, the projected age of employees upon retirement, the expected rate of future compensation and estimated employee turnover. Because the determination of the cost and obligations associated with employee future benefits requires the use of various assumptions, there is measurement uncertainty inherent in
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
the actuarial valuation process. Actual results will differ from results that are estimated based on the aforementioned assumptions. Additional information is included in note 19 –18 - Employee future benefits.
Income taxes
The estimation of income taxes includes evaluating the recoverability of deferred tax assets based on an assessment of Group entities' ability to utilize the underlying future tax deductions against future taxable income prior to expiry of
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
those deductions. Management assesses whether it is probable that some or all of the deferred income tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income, which in turn is dependent upon the successful commercialization of the Company's products. To the extent that management's assessment of any Group entity's ability to utilize future tax deductions changes, the Company would be required to recognize more or fewer deferred tax assets, and future income tax provisions or recoveries could be affected. Additionalaffected.Additional information is included in note 22 –- Income taxes.
| |
(b) | Critical judgments in applying the Company's accounting policies |
Revenue recognition .
Management's assessments related to the recognition of revenues related to arrangements containing multiple elements are based on judgment. Judgment is necessary to identify separate units of5 Recent accounting and to allocate related consideration to each separate unit of accounting. Where deferral of upfront payments or license fees is deemed appropriate, subsequent revenue recognition is often determined based upon the assessment of the Company's continuing involvementpronouncements
Accounting standards adopted in the arrangement, the benefits expected to be derived by the customer and, where applicable, expected patent lives. Additional information is included in note 5 – Development, commercialization and licensing arrangements.
| |
4 | Recent accounting pronouncements |
Not yet adopted
Annual improvements to IFRS (2012-2014) cycle: On September 25, 2014 the IASB issued narrow-scope amendments to a total of four standards as part of its annual improvements process. The amendments will apply for annual periods beginning on or after January 1, 2016. Amendments were made to clarify the following in their respective standards:
Changes in method for disposal under IFRS 5, Non-current Assets Held for Sale and Discontinued Operations
("IFRS 5");
Continuing involvement for servicing contracts and offsetting disclosures in condensed interim financial statements under IFRS 7, Financial Instruments: Disclosures (“IFRS 7”);
Discount rate in a regional market sharing the same currency under International Accounting Standard ("IAS") 19, Employee Benefits;
2018•Disclosure of information "elsewhere in the interim financial reports" under IAS 34, Interim Financial Reporting;
The Company is currently assessing the impact that these amendments may have on the Company’s consolidated financial statements.
The final version of IFRS 9 Financial Instruments instruments
("IFRS 9"), was issued by9 replaces the IASB in July 2014 and will replaceprovisions of IAS 39 Financial Instruments: Recognition and Measurement ("IAS 39"). IFRS 9 introduces a model for that relate to the recognition, classification and measurement a single, forward-looking expected loss impairment model and a substantially reformed approach to hedge accounting. The new single, principle-based approach for determining the classification of financial assets is driven byand financial liabilities, de-recognition of financial instruments, impairment of financial assets and hedge accounting.
The Company's financial assets are mainly comprised of cash flow characteristicsand cash equivalents, trade and other receivables, and restricted cash equivalents, which are classified and accounted for under IFRS 9 at amortized cost. Financial liabilities are mainly comprised of payables and accrued liabilities, which are accounted for at amortized cost, and the business model inwarrant liability, which an asset is held. The new model also results in a single impairment model being applied to all financial instruments, which will require more timely recognition of expected credit losses. It also includes changes in respect of an entity's own credit risk in measuring liabilities elected to be measuredderivative that is accounted for at fair value so that gains caused bythrough profit and loss (FVTPL).
The impairment of financial assets, including trade and other receivables, is now assessed using the deteriorationsimplified method of an entity's ownthe expected credit riskloss model: previously, the incurred loss model was used. Applying the expected credit loss model has not had a significant impact on such liabilities are no longer recognized in profit or loss.the value of the financial assets.
The Company applied the modified retrospective method upon adoption of IFRS 9 which ison January 1, 2018. This method requires the recognition of the cumulative effect of initially applying IFRS 9 to be applied retrospectively, is effective for annual periods beginningretained earnings (deficit) and not to restate prior years. The application of this new standard resulted in changes in accounting policies but has no impact on or afteropening deficit.
IFRS 15 Revenue from contracts with customers
Effective January 1, 2018, the Company has adopted IFRS 15 Revenue from Contracts with Customers (“IFRS 15”). This new standard was applied using a modified retrospective approach. The adoption of IFRS 15 did not have a significant impact on the timing or measurement of the Company’s revenue and no adjustment to the opening balance of deficit as at January 1, 2018 has been recorded as result of adopting IFRS 15.
The impacts of adoption of the new standard are summarized below:
The Company's revenue consists of licensing fees representing non-refundable payments received at the time of executing the license agreement, which are recognized as revenue upon execution of the license agreements when the Company has no significant future performance obligation and collectability of the fees is available for early adoption. In addition, an entity's own credit risk changes canprobable. Under IFRS 15, the Company determines whether the Company's promise to grant a license provides its customer with either a right to access the Company’s IP or a right to use the Company’s IP. Revenue from a license that provides a customer the right to use the Company’s IP is recognized at a point in time when the transfer to the licensee is completed and the license period begins. Revenue from a license that provides access to the Company's IP over a license term is considered to be applied early in isolation without otherwise changinga performance obligation satisfied over time and, therefore, revenue is recognized over the term of the license arrangement.
Revenue consists also of royalty income from the out-licensing of IP, which is recognized as earned and from manufacturing and other services, where revenue is recognized when control transfers to the third party and the Company’s performance obligations are satisfied. The adoption of IFRS 15 did not significantly change the timing or amount of revenue recognized from these manufacturing and other services arrangements, nor did it change accounting for financial instruments. In addition, there are amendments to IFRS 7 which requirethese royalty arrangements, as the standard's royalty exception is applied for IP licenses.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
additional disclosures on transition fromFurthermore, the Company receives milestone payments related to the out-licensing of IP. IFRS 15 resulted in the following changes in timing and amount of revenue recognized under these arrangements.In January 2018, the Company received $24.0 million of which $23.6 million was recognized in the consolidated statements of comprehensive income (loss) and $0.4 million was deferred to the consolidated statements of financial position and is being amortized until June 2023 when we expect to commence product sales for the pediatric indication. Under IAS 3918, the full $24.0 million would have been deferred to IFRS 9. These amendments are effectivethe consolidated statements of financial position and would have been amortized to the consolidated statements of comprehensive income (loss) evenly until October 2027, representing the expiry date of the underlying patents.
The Company applied the modified retrospective method upon adoption of IFRS 9. The Company is currently assessing15 on January 1, 2018. This method requires the impact, if any, that these new standards will have onrecognition of the Company's consolidated financial statements.
In May 2014, the IASB issuedcumulative effect of initially applying IFRS 15 Revenue from Contracts with Customers ("IFRS 15").to deficit and not to restate prior years. The objectiveapplication of this new standard is to provide a single, comprehensive revenue recognition framework for all contracts with customers to improve comparability of financial statements of companies globally. This new standard contains principles that an entity will apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity will recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to receive in exchange for those goods or services. This new standard is effective for annual periods beginning on or after January 1, 2018 with early adoption permitted. The Company is currently assessing thehad no impact that this new standard may have on the Company's consolidated financial statements.opening deficit.
Accounting standards not yet adopted
In January 2016, the IASB issued IFRS 16, Leases ("IFRS 16"), which supersedes IAS 17, Leases, and the related interpretations on leases: IFRIC 4, Determining Whether an Arrangement Contains a Lease; Standard Interpretations Committee ("SIC") 15, Operating Leases - Incentives; and SIC 27, Evaluating the Substance of Transactions in the Legal Form of a Lease. IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with earlier adoption permitted for companies that also apply IFRS 15. The Company is currently assessing the impact that this new standard may have on the Company's consolidated financial statements.
In June 2017, IFRIC 23, "Uncertainty over Income Tax Treatment" ("IFRIC 23"), was issued. IFRIC 23 provides guidance on how to value uncertain income tax positions based on the probability of whether the relevant tax authorities will accept the company's tax treatments. A company is to assume that a taxation authority with the right to examine any amounts reported to it will examine those amounts and will have full knowledge of all relevant information when doing so. IFRIC 23 is effective for annual periods beginning on or after January 1, 2019. The adoption of this interpretation is not expected to have a significant impact on the Company's consolidated financial statements.
In June 2015, the IASB published ED/2015/5 Remeasurement on a Plan Amendment, Curtailment or Settlement/Availability of a Refund from a Defined Benefit Plan (Proposed amendments to IAS 19 and IFRIC 14) combining two issues submitted separately to the IFRS Interpretations Committee into a single package of narrow-scope amendments to IAS 19 Employee Benefits and IFRIC 14 IAS 19 - The Limit on a Defined Benefit Asset, Minimum Funding Requirements and their Interaction. However, in April 2017 the IASB decided to pursue the amendments to IAS 19 and in September 2017 confirmed it would do so despite putting off the amendments to IFRIC 14. The amendments in Plan Amendment, Curtailment or Settlement (Amendments to IAS 19) are: (i) if a plan amendment, curtailment or settlement occurs, it is now mandatory that the current service cost and the net interest for the period after the remeasurement are determined using the assumptions used for the remeasurement and (ii) amendments have been included to clarify the effect of a plan amendment, curtailment or settlement on the requirements regarding the asset ceiling. An entity applies the amendments to plan amendments, curtailments or settlements occurring on or after the beginning of the first annual reporting period that begins on or after 1 January 2019. The adoption of these amendments is not expected to have a significant impact on the Company's consolidated financial statements.
6 Licensing arrangements
Macrilen License and Assignment Agreement
On January 16, 2018, the Company through Aeterna Zentaris GmbH entered into a license and assignment agreement (the "License and Assignment Agreement") with Strongbridge to carry out development, manufacturing, registration, regulatory and supply chain services for the commercialization of Macrilen™ (macimorelin) in the United States and Canada, which provides for (i) the "right to use" license relating to the Adult Indication; (ii) the sale of the right to acquire a license of a future FDA-approved Pediatric Indication; (iii) Strongbridge has agreed to fund 70% of the costs of a pediatric clinical trial submitted for approval to the EMA and FDA to be run by the Company with customary oversight from a joint steering committee; and (iv) for an Interim Supply Arrangement.
(i) Adult Indication
| |
5 |
Development, commercializationAeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 2018 and licensing arrangementsDecember 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
Sinopharm
Under the terms of the License and Assignment Agreement, and for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on annual net sales up to $75.0 million and an 18% royalty on annual net sales above $75.0 million. Following the end of patent protection in United States or Canada for Macrilen™ (macimorelin), the Company will be entitled to a 5% royalty on net sales in that country. In addition, the Company will also receive one-time payments ranging from $4.0 million to $100.0 million upon the achievement of commercial milestones going from $25.0 million annual net sales up to $500.0 million annual net sales.
In January 2018, the Company received a cash payment of $24.0 million from Strongbridge and on July 23, 2018, Strongbridge launched product sales of Macrilen™ (macimorelin) in the United States.
(ii) Pediatric Indication
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment of $5.0 million. This amount will be recognized once it is probable that it will be received.
Transaction price
Analysis of the total discounted cash flows of both the $24.0 million payment and the $5.0 million payment upon FDA approval of the Pediatric Instance demonstrates that 84% of the future revenue streams would be derived from the Adult Indication and 16% from the Pediatric Indication. On a relative fair value basis, the Company has allocated the transaction price to the performance obligations resulting in $23.6 million being allocated to the Adult Indication and being recognized as license fee revenue in the consolidated statements of comprehensive income (loss) effective January 2018, and $400 being allocated to the right to a future Pediatric Indication, which is recognized as deferred revenue on the consolidated statements of financial position and amortized monthly beginning January 2018 into the consolidated statements of comprehensive income (loss).
(iii) PIP study
During 2018, the Company invoiced Strongbridge $358 as its share of the costs incurred by the Company under the PIP. The Company considers the funding arrangement under the PIP to be a collaboration arrangement under IFRS 11 and has accounted for the invoicing as a reduction of costs incurred during the period. This amount is presented in the consolidated statement of financial position as trade and other receivables and has been fully collected.
(iv) Interim Supply Arrangement
The Company has agreed under the License and Assignment Agreement to supply ingredients for the manufacture of Macrilen™ (macimorelin) during an interim period at a price that is set ‘at cost’, without any profit margin. The Company believes the stand-alone selling price of the manufacturing ingredients to be their cost, as that approximates the amount at which Strongbridge would be able to procure those same goods with other suppliers. During 2018, the Company invoiced $2,167 and has received payment in full of these invoices. These items are presented in the consolidated statements of comprehensive income (loss) as product sales and cost of goods sold.
Novo purchase of Strongbridge License Agreement
Effective December 19, 2018, Strongbridge sold the entity which owned the License and Assignment Agreement for the United States and Canadian rights to Macrilen™ to Novo .
Zoptrex™ License Agreements
On July 1, 2016, the Company entered into a license agreement (the "Cyntec License Agreement") with Cyntec Co., Ltd. ("Cyntec"), an affiliate of Orient EuroPharma Co., Ltd. ("OEP") for Zoptrex™ (zoptarelin doxorubicin) for the initial indication of endometrial cancer. Under the terms of the Cyntec License Agreement, the Company was paid a nonrefundable
upfront cash payment (the "License Fee") of EUR 0.5 million in consideration for the license to Cyntec of the Company's intellectual property related to Zoptrex™ and the grant to Cyntec of the right to commercialize Zoptrex™ in a territory
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
consisting of Taiwan and nine countries in southeast Asia (the "OEP Territory"). Cyntec has also agreed to make additional payments to the Company upon achieving certain pre-established regulatory and commercial milestones.
Furthermore, the Company will receive royalties based on future net sales of Zoptrex™ in the OEP Territory. Cyntec will be responsible for the development, registration, reimbursement and commercialization of the product in the OEP Territory. The Company also entered into related Technology Transfer and Supply Agreements with another affiliate of OEP, pursuant to which the Company will transfer to such affiliate the technology necessary to permit the affiliate to manufacture finished Zoptrex™ using quantities of the active pharmaceutical agreement purchased from the Company pursuant to the Supply Agreement.
On December 1, 2014, the Company entered into a an exclusive master collaboration agreement ("Master Collaboration Agreement,Agreement"), a Technologytechnology transfer and technical assistance agreement ("Tech Transfer and Technical Assistance Agreement ("TTA"Agreement") and a license agreement ("Sinopharm License Agreement ("LA"Agreement") with Sinopharm A-Think Pharmaceuticals Co., Ltd. ("Sinopharm") for the development, manufacture and commercialization of Zoptrex™ (zoptarelin doxorubicin) in all human uses, in the People's Republic of China, including Hong Kong and Macau (collectively, "thethe "Sinopharm Territory"). Under the terms of the TTA, Sinopharm made a one-time, non-refundable payment of $1,101,000 (the "Transfer Fee") of $1,000 to the Company in consideration for the transfer of technical documentation and materials, know-how and technical assistance services. Additionally, perpursuant to the LA,Sinopharm License Agreement, the Company will beis entitled to receive additional consideration upon achieving certain milestones, including the occurrence of certain regulatory and commercial events in the Sinopharm Territory.
Furthermore, the Company will beis entitled to royalties on future net sales of Zoptrex™ in the Sinopharm Territory.
The Company has substantial continuing involvement in the aforementioned arrangements, including the transfer of documentation, know-how and materials, as well as the provision of technical assistance, such as quality systems implementation, analytical and stability testing, territory-specific development initiatives, and other services.
The Company has applied the provisions of IAS 18, Revenue ("IAS 18"), and has determined that all deliverables and performance obligations contemplated by the agreements with Sinopharm should be accounted for as a single unit of accounting, limited to amounts that are not contingent upon the delivery of additional items or the meeting of other specified performance conditions which are not known, probable or estimable at the time at which the agreements with Sinopharm were entered into.
The Company has deferred the non-refundable License and Transfer FeeFees and is amortizing the related payment as revenue on a straight-line basis over the period during which the aforementioned services are rendered and obligations are performed.
In determining the period over which Transfer Fee revenues are to be recognized,At December 31, 2017, the Company concludedhad deferred revenues net of amortization of $541 relating to non-refundable upfront payments and, due to events that its significant continuing involvementoccurred in 2017, the Company does not anticipate development of Zoptrex™ under the licensing agreements. In the first quarter of 2018, the Company recognized this amount as revenue.
7 Cash and cash equivalents
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Cash on hand and balances with banks | | 3,501 |
| | 7,099 |
|
| Interest-bearing deposits with maturities of three months or less | | 11,011 |
| | 681 |
|
| | | 14,512 |
| | 7,780 |
|
8 Trade and other receivables
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Trade accounts receivable (net of allowance for doubtful accounts of $55 (2017 - $5)) | | 142 |
| | 20 |
|
| Value added tax | | 49 |
| | 186 |
|
| Other receivables | | 103 |
| | 15 |
|
| | | 294 |
| | 221 |
|
See note 24 - Financial instruments and financial risk management for discussion of credit losses.
9 Inventory
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Finished goods | | — |
| | 554 |
|
| Work in process | | 240 |
| | — |
|
| | | 240 |
| | 554 |
|
The Company recognized $2,087 of inventory costs as cost of sales in the aforementioned agreements will span approximately four years, commencing in lateconsolidated statement of comprehensive income (loss) for the year ended December 2014. However, the31, 2018 (2017 - nil).
10 Prepaid expenses and other current assets
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Prepaid insurance | | 832 |
| | 410 |
|
| Prepaid inventory | | 175 |
| | 87 |
|
| Other | | 203 |
| | 329 |
|
| | | 1,210 |
| | 826 |
|
| | | | | |
11 Restricted cash equivalents
The Company may adjust the amortization period based on appropriate factshad restricted cash equivalents amounting to $418 at December 31, 2018 and circumstances not yet known,$381 at December 31, 2017. These balances consist of certificates of deposit that would significantly change the duration of the Company's continuing involvementare used as collateral for corporate credit cards and performance obligations or benefits expected to be derived by Sinopharm.leases.
Future milestones will be recognized as revenue individually and in full upon the actual achievement of the related milestone, given the substantive nature of each milestone. Lastly, upon initial commercialization and sale of the developed product,
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
the Company will recognize royalty revenues as earned, based on the contractual percentage applied to the actual net sales achieved by Sinopharm, as per the LA.12 Property, plant and equipment
Pursuant to the aforementioned agreements, the Company was required to remit to the Chinese tax authorities $111,000Components of the gross proceeds received from Sinopharm. This amount, which was withheld at source, was recognized as income tax expenseCompany's property, plant and equipment are summarized below. |
| | | | | | | | | | | | | | | |
| | | Cost |
| | | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | | $ | | $ | | $ | | $ | | $ |
| At January 1, 2017 | | 3,919 |
| | 19 |
| | 737 |
| | 37 |
| | 4,712 |
|
| Additions | | 2 |
| | — |
| | 2 |
| | — |
| | 4 |
|
| Disposals / Retirements | | (2,160 | ) | | — |
| | (43 | ) | | — |
| | (2,203 | ) |
| Impact of foreign exchange rate changes | | 507 |
| | — |
| | 94 |
| | 5 |
| | 606 |
|
| At December 31, 2017 | | 2,268 |
| | 19 |
| | 790 |
| | 42 |
| | 3,119 |
|
| Additions | | 1 |
| | — |
| | 8 |
| | — |
| | 9 |
|
| Disposals / Retirements | | (758 | ) | | — |
| | (137 | ) | | — |
| | (895 | ) |
| Reclassifications | | 11 |
| | (11 | ) | | — |
| | — |
| | — |
|
| Impact of foreign exchange rate changes | | (64 | ) | | (1 | ) | | (24 | ) | | (2 | ) | | (91 | ) |
| At December 31, 2018 | | 1,458 |
| | 7 |
| | 637 |
| | 40 |
| | 2,142 |
|
|
| | | | | | | | | | | | | | |
| | Accumulated depreciation |
| | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | $ | | $ | | $ | | $ | | $ |
| At January 1, 2017 | 3,799 |
| | 2 |
| | 692 |
| | 15 |
| | 4,508 |
|
| Disposals / Retirements | (2,135 | ) | | — |
| | (43 | ) | | — |
| | (2,178 | ) |
| Depreciation expense | 50 |
| | 2 |
| | 30 |
| | 18 |
| | 100 |
|
| Impact of foreign exchange rate changes | 496 |
| | — |
| | 90 |
| | 2 |
| | 588 |
|
| At December 31, 2017 | 2,210 |
| | 4 |
| | 769 |
| | 35 |
| | 3,018 |
|
| Disposals / Retirements | (752 | ) | | — |
| | (137 | ) | | — |
| | (889 | ) |
| Depreciation expense | 19 |
| | 1 |
| | 14 |
| | 1 |
| | 35 |
|
| Impact of foreign exchange rate changes | (63 | ) | | — |
| | (22 | ) | | (2 | ) | | (87 | ) |
| At December 31, 2018 | 1,414 |
| | 5 |
| | 624 |
| | 34 |
| | 2,077 |
|
|
| | | | | | | | | | | | | | |
| | Carrying amount |
| | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | $ | | $ | | $ | | $ | | $ |
| At December 31, 2017 | 58 |
| | 15 |
| | 21 |
| | 7 |
| | 101 |
|
| At December 31, 2018 | 44 |
| | 2 |
| | 13 |
| | 6 |
| | 65 |
|
Depreciation of $35 ($100 in 2017 and $112 in 2016) is presented in the consolidated statement of comprehensive income (loss) income,as follows: $20 ($69 in 2014,2017 and $80 in accordance with the provision of IAS 12, Income Taxes.
Ergomed agreement
On April 10, 2013, the Company entered into a co-development and revenue-sharing agreement ("CDRSA") with Ergomed Clinical Research Limited ("Ergomed"), pursuant to which Ergomed has agreed to assist the Company2016) in the clinical development program for Zoptrex™ for the purpose of maximizing the commercialization potential of Zoptrex™ with the ultimate aim of selling or licensing Zoptrex™. Concurrently with the execution of the CDRSA, the Company entered into a master services agreement ("MSA") with Ergomed for a clinical Phase 3 trial of Zoptrex™ in endometrial cancer, pursuant to which Ergomed will provide clinical development services with respect to the co-development initiative referred to above.
Under the CDRSA, Ergomed will not charge the Company for 30% of the total costs up to a maximum of $10,000,000 for which an amount of $2,330,000 remains to be claimed as of December 31, 2015. While Ergomed will not directly contribute any cash proceeds towards the completion of the activities contemplated by the CDRSA, Ergomed, as primary supplier of a substantial portion of Zoptrex™ related clinical and regulatory activities, will contribute to the overall funding of the initiative via the application of a 30% discount from the costs set forth in the MSA until the cumulative total of such reductions reaches a maximum of $10,000,000. Ergomed will be entitled to receive an agreed upon single-digit percentage of any future net income (as defined in the CDRSA) or other proceeds related to the licensing of received zoptarelin doxorubicin in endometrial cancer indication, up to a specified maximum amount.
The Company recognizes R&D costs, associated with the CDRSA$10 ($10 in 2017 and MSA net of the 30% discount, as services are rendered by Ergomed$11 in the consolidated statement of comprehensive (loss) income. During the years ended December 31, 2015, 20142016) in general and 2013, the Company expensed a total of $7,140,000, $7,195,000administrative ("G&A") expenses and $3,560,000, respectively, pursuant to the CDRSA$5 ($21 in 2017 and MSA.
Yakult agreement
On March 8, 2011, the Company entered into an agreement with Yakult Honsha Co., Ltd. ("Yakult") for the development, manufacture and commercialization of perifosine$21 in all human uses, excluding leishmaniasis,2016) in Japan. Under the terms of this agreement, Yakult had made an initial, non-refundable gross upfront payment to the Company of €6,000,000 (approximately $8,412,000). The Company applied the provisions of IAS 18 and recognized deferred revenue, which was being amortized on a straight-line basis through the estimated end of the estimated life cycle of perifosine in colorectal cancer ("CRC") and multiple myeloma ("MM").selling expenses.
On April 1, 2012, following disclosure of the results of the Phase 3 study of perifosine in CRC, the Company discontinued the perifosine program in that indication. Furthermore, in March 2013, following an analysis of interim results of the Phase 3 study of perifosine in MM, the Company also discontinued the development of perifosine in the MM indication.
Based on these events, the Company determined that it no longer had significant obligations under the agreement with Yakult to continue with the development of perifosine. Accordingly, the Company recognized, in March 2013, the remaining amount of deferred revenue of $5,860,000 related to the above licensing agreement within License fees in the consolidated statement of comprehensive (loss) income.
On April 3, 2013 (the "Cetrotide® Effective Date"), the Company entered into a transfer and service agreement ("TSA") and concurrent agreements with various partners and licensees with respect to the manufacturing rights for Cetrotide®, marketed for therapeutic use as part of in vitro fertilization programs. The principal effect of these agreements was to transfer, effective October 1, 2013 (the "Cetrotide® Closing Date"), the manufacturing rights for Cetrotide® and to grant a license to Merck Serono for the manufacture, testing, assembling, packaging, storage and release of Cetrotide® in all territories. Also
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
per the TSA, the Company agreed to provide certain transition services to Merck Serono over a period of 36 months from the Cetrotide® Effective Date in order to assist Merck Serono in managing overall responsibility for the manufacturing of Cetrotide® and related activities (collectively, the "Cetrotide® Business").13 Identifiable intangible assets
Under the TSA, during the period commencing on the Cetrotide® Effective DateIdentifiable intangible assets with finite useful lives consist entirely of in-process R&D costs, patents and ending on the Cetrotide® Closing Date (the "Interim Period"), the Company was obligatedtrademarks with such assets expected to continue to conduct the Cetrotide® Businessbe fully amortized by 2021. Changes in the ordinary course in a manner consistent with past practices, subject to certain conditions.
Per the TSA, the Company received a non-refundable, one-time payment of €2,500,000 (approximately $3,300,000) in consideration for the transfercarrying value of the manufacturing rights referred to above,Company's identifiable intangible assets with finite useful lives are summarized below. |
| | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, 2018 | | Year ended December 31, 2017 |
| | | Cost | | Accumulated amortization | | Carrying value | | Cost | | Accumulated amortization | | Carrying value |
| | | $ | | $ | | $ | | $ | | $ | | $ |
| Balances – Beginning of the year | | 34,246 |
| | (34,156 | ) | | 90 |
| | 30,032 |
| | (29,962 | ) | | 70 |
|
| Additions | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Impairment (loss) reversal* | | — |
| | — |
| | — |
| | — |
| | 44 |
| | 44 |
|
| Recurring amortization expense* | | — |
| | (23 | ) | | (23 | ) | | — |
| | (38 | ) | | (38 | ) |
| Impact of foreign exchange rate changes | | (1,603 | ) | | 1,598 |
| | (5 | ) | | 4,214 |
| | (4,200 | ) | | 14 |
|
| Balances – End of the year | | 32,643 |
| | (32,581 | ) | | 62 |
| | 34,246 |
| | (34,156 | ) | | 90 |
|
* Recorded as well as other payments in exchange for the transfer, also on the Cetrotide® Closing Date, of certain assets and equipment (see note 10 – Property, plant and equipment) used solely for the manufacture of Cetrotide®.
The Company has agreed to provide the aforementioned transition services in exchange for a monthly service fee, which is payable by Merck Serono. The related transition services revenues are recognized as License fees and other within net income from discontinued operations in the Company's consolidated statement of comprehensive (loss) income as the transition services are provided over the corresponding term of the transition services contract.
Impact of the TSA on previously deferred revenues
In 2008, the Company had monetized its royalty stream related to Cetrotide® via a transaction with HRP, which resulted, among other things, in the payment by HRP to the Company of $52,500,000, less certain transactionR&D costs in exchange for the Company's rights to royalties on future net sales of Cetrotide® generated by Merck Serono. The Company initially recorded the proceeds received from HRP as deferred revenue due to the Company's significant continuing involvement with the Cetrotide® Business. Since then, the Company amortized the deferred revenue into income (as Sales and royalties within net income from discontinued operations in the Company's consolidated statement of comprehensive (loss) income) over the life of the underlying license agreement, based on the "units-of-revenue" method. Under that method, periodic royalty revenues were calculated by multiplying the ratio of the unamortized deferred revenue amount to the total estimated remaining royalties that Merck Serono expected to pay to HRP over the term of the underlying arrangement by the royalty payments due to HRP for the period.
Management has determined that, as of the Cetrotide® Closing Date, there is no basis to continue amortizing the deferred revenue associated with HRP, primarily due to the fact that the Company no longer has significant continuing involvement in the Cetrotide® Business, as discussed above. As such, commencing on the Cetrotide® Effective Date, the Company accelerated the amortization of the remaining deferred revenues of approximately $31,875,000 over the Interim Period, by continuing to apply the units-of-revenue method, which is consistent with past practice. The remaining deferred revenues were fully amortized in 2013 and were recorded as Sales and royalties within net income from discontinued operations in the Company's consolidated statement of comprehensive (loss) income.
Presentation of Cetrotide® Business subsequent to the Cetrotide®Closing Date
In accordance with the provisions of IFRS 5, upon the transfer of substantially all of the risks and rewards associated with the Cetrotide® Business on the Cetrotide® Closing Date, the Cetrotide® Business was classified as a discontinued operation. As such, relevant amounts in the consolidated statements of comprehensive (loss) income (loss).
14 Goodwill
The change in carrying value is as follows:
|
| | | | | | | | | |
| | | Cost | | Accumulated impairment loss | | Carrying amount |
| | | $ | | $ | | $ |
| At January 1, 2017 | | 7,553 |
| | — |
| | 7,553 |
|
| Impact of foreign exchange rate changes | | 1,060 |
| | — |
| | 1,060 |
|
| At December 31, 2017 | | 8,613 |
| | — |
| | 8,613 |
|
| Impact of foreign exchange rate changes | | (403 | ) | | — |
| | (403 | ) |
| At December 31, 2018 | | 8,210 |
| | — |
| | 8,210 |
|
Management's evaluation of impairment in goodwill is based on estimates that are derived from our licensee's projected sales of Macrilen for 2019 (both units and cash flows have been retroactively reclassified to reflectselling price), annual revenue growth rate, growth in operating expenses, the Cetrotide® Business as a discontinued operation.effect of future costs of the pediatric development program (the "PIP") and discount rate for generating the Company's net present value. There was no impairment assessed at December 31, 2018.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
Components of the Company's net income from discontinued operations are summarized below.15 Payables and accrued liabilities
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Revenues* | | | | | | |
| Sales and royalties | | — |
| | — |
| | 63,755 |
|
| License fees and other | | 331 |
| | 1,037 |
| | 4,589 |
|
| | | 331 |
| | 1,037 |
| | 68,344 |
|
| Operating expenses | | | | | | |
| Cost of sales | | — |
| | — |
| | 30,002 |
|
| Research and development costs | | 31 |
| | 25 |
| | 8 |
|
| General and administrative expenses | | — |
| | 1 |
| | 15 |
|
| Selling expenses | | 215 |
| | 388 |
| | 4,264 |
|
| | | 246 |
| | 414 |
| | 34,289 |
|
| Net income from discontinued operations | | 85 |
| | 623 |
| | 34,055 |
|
| Components of operating expenses presented as discontinued include the following: | | | | | | |
| Subcontractor fees | | — |
| | — |
| | 24,930 |
|
| Raw material purchases | | — |
| | — |
| | 579 |
|
| Change in inventory | | — |
| | — |
| | 4,173 |
|
| Impairment of equipment | | — |
| | — |
| | 268 |
|
| Depreciation of equipment | | — |
| | — |
| | 52 |
|
| Cost of sales | | — |
| | — |
| | 30,002 |
|
| Goods and services** | | 32 |
| | 191 |
| | 2,987 |
|
| Royalty and patent expenses related to onerous contracts | | 214 |
| | 223 |
| | 1,300 |
|
| | | 246 |
| | 414 |
| | 34,289 |
|
_________________________ |
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Trade accounts payable | | 1,282 |
| | 1,222 |
|
| Accrued research and development costs | | 26 |
| | 127 |
|
| Salaries, employment taxes and benefits | | 183 |
| | 390 |
|
| Financing of insurance premiums (a) | | 738 |
| | — |
|
| Other accrued liabilities | | 737 |
| | 1,075 |
|
| | | 2,966 |
| | 2,814 |
|
| |
*(a) | In addition to recurring salesRepresents financing of Cetrotide®, the revenues presented above include the aforementioned non-refundable, one-time payment of €2,500,000 (approximately $3,300,000), as well as royalty revenues of $33,631,000 in 2013, which represent the amortization of proceeds received in connection with the Company's transaction with HRP.
|
| |
** | Goods2019 insurance premiums, carrying interest at 6.5% and services include professional fees, marketing services, insurance, travel and representation costs.repayable in eight equal monthly installments commencing January 31, 2019. |
16 Provision for restructuring and other costs
In the third quarter of 2017, Aeterna Zentaris GmbH, and its Works Council approved a restructuring program (the "2017 German Restructuring"), which was rolled out as a consequence of the negative Phase 3 clinical trial results of Zoptrex™ and the related impact on the product pipeline. This was also part of the continued strategy to transition into a commercially operating specialty biopharmaceutical organization focused on the development and commercialization of Macrilen™ (macimorelin), including through out-licensing arrangements and pursuing in-licensing opportunities.
The general and administrative expenses presented above forchanges in the year ended December 31, 2013 also include $1,300,000 associated with the initial recognition of aCompany's provision for certain non-cancellable contracts related to the Cetrotide® Business that were deemed onerous due to the fact that management expected no economic benefits to flow to the Company following the transfer of the Cetrotide® Business on the Cetrotide® Closing Date. The provisions for onerous contracts represent the present value of estimated unavoidable future royalty and patent costs associated with the intellectual property underlying Cetrotide®. The estimate may vary as a result of changes in estimated future royalty and patent costs. The unexpired term of these contracts is seven years as at December 31, 2015. See also note 16 – Provisionsrestructuring and other non-current liabilities.costs can be summarized as follows:
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
Components of the Company's net cash (used in) provided by operating activities of discontinued operations are summarized below.
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Cash flows from operating activities | | | | | | |
| Net income from discontinued operations | | 85 |
| | 623 |
| | 34,055 |
|
| Items not affecting cash and cash equivalents: | | | | | | |
| Provision for onerous contracts | | 214 |
| | 223 |
| | 1,300 |
|
| Depreciation, amortization and impairment | | — |
| | — |
| | 320 |
|
| Amortization of deferred revenues | | — |
| | — |
| | (33,631 | ) |
| Other non-cash items | | — |
| | 96 |
| | — |
|
| Changes in operating assets and liabilities: | | | | | | |
| Trade and other receivables | | 15 |
| | 1,460 |
| | 6,212 |
|
| Inventory | | — |
| | — |
| | 4,061 |
|
| Prepaid expenses and other current assets | | — |
| | — |
| | 882 |
|
| Payables and accrued liabilities | | (78 | ) | | (2,300 | ) | | (2,996 | ) |
| Provisions and other non-current liabilities | | (151 | ) | | (397 | ) | | (56 | ) |
| Net cash provided by (used in) operating activities of discontinued operations | | 85 |
| | (295 | ) | | 10,147 |
|
|
| | | | | | | | | | | | | | | |
| | | Other provision | | Cetrotide(R) onerous contracts | | 2017 German Restructuring: onerous lease | | 2017 German Restructuring: severance | | Total |
| | | | | $ | | $ | | $ | | $ |
| January 1, 2017 | | 158 |
| | 574 |
| | — |
| | — |
| | 732 |
|
| Provision recognized | | — |
| | — |
| | 1,113 |
| | 2,002 |
| | 3,115 |
|
| Utilization of provision | | (152 | ) | | (145 | ) | | (19 | ) | | (138 | ) | | (454 | ) |
| Change in the provision | | — |
| | (20 | ) | | 10 |
| | (41 | ) | | (51 | ) |
| Unwinding of discount and impact of foreign exchange rate changes | | 3 |
| | 64 |
| | 104 |
| | (16 | ) | | 155 |
|
| December 31, 2017 | | 9 |
| | 473 |
| | 1,208 |
| | 1,807 |
| | 3,497 |
|
| Provision recognized | | — |
| | 317 |
| | — |
| | — |
| | 317 |
|
| Utilization of provision | | (9 | ) | | (222 | ) | | (467 | ) | | (1,202 | ) | | (1,900 | ) |
| Change in the provision | | — |
| | — |
| | (21 | ) | | (432 | ) | | (453 | ) |
| Unwinding of discount and impact of foreign exchange rate changes | | — |
| | (21 | ) | | (57 | ) |
| (85 | ) | | (163 | ) |
| December 31, 2018 | | — |
| | 547 |
| | 663 |
| | 88 |
| | 1,298 |
|
| Less: current portion | | — |
| | (136 | ) | | (663 | ) | | (88 | ) | | (887 | ) |
| Non-current portion | | — |
| | 411 |
| | — |
| | — |
| | 411 |
|
| |
7 | Cash and cash equivalents |
17 Warrant liability
The change in the Company's warrant liability can be summarized as follows: |
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Cash on hand and balances with banks | | 11,233 |
| | 10,803 |
|
| Interest-bearing deposits with maturities of three months or less | | 30,217 |
| | 24,128 |
|
| | | 41,450 |
| | 34,931 |
|
| |
8 | Trade and other receivables |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Balance – Beginning of the year | | 3,897 |
| | 6,854 |
| | 10,891 |
|
| Share purchase warrants issued during the year (note 19) | | — |
| | — |
| | 400 |
|
| Share purchase warrants exercised during the year | | — |
| | (735 | ) | | — |
|
| Change in fair value of share purchase warrants | | (263 | ) | | (2,222 | ) | | (4,437 | ) |
| Balance - End of the year | | 3,634 |
| | 3,897 |
| | 6,854 |
|
|
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Trade accounts receivable | | 180 |
| | 583 |
|
| Value added tax | | 291 |
| | 47 |
|
| Other | | 127 |
| | 237 |
|
| | | 598 |
| | 867 |
|
| |
9 | Restricted cash equivalents |
In supportA summary of the activity related to the Company's long-term operating lease obligation in Germany and in replacement of a related bank guarantee, the Company transferred funds to a restricted cash account. These funds, including any interest earned thereon, are restricted for as long as the underlying lease arrangement (note 25 – Commitments and contingencies) has not expired and therefore cannot be utilized for current purposes as at December 31, 2015.share purchase warrants is provided below.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
| |
10 | Property, plant and equipment |
Components of the Company's property, plant and equipment are summarized below.
|
| | | | | | | | | | | | | | | |
| | | Cost |
| | | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | | $ | | $ | | $ | | $ | | $ |
| At January 1, 2014 | | 9,054 |
| | 1,237 |
| | 1,852 |
| | 1,196 |
| | 13,339 |
|
| Additions | | 16 |
| | 20 |
| | 86 |
| | 5 |
| | 127 |
|
| Disposals / Retirements | | (1,212 | ) | | — |
| | (182 | ) | | — |
| | (1,394 | ) |
| Impact of foreign exchange rate changes | | (1,046 | ) | | (151 | ) | | (222 | ) | | (146 | ) | | (1,565 | ) |
| At December 31, 2014 | | 6,812 |
| | 1,106 |
| | 1,534 |
| | 1,055 |
| | 10,507 |
|
| Additions | | 2 |
| | 8 |
| | 16 |
| | — |
| | 26 |
|
| Disposals / Retirements | | (2,108 | ) | | (1,021 | ) | | (719 | ) | | (962 | ) | | (4,810 | ) |
| Impact of foreign exchange rate changes | | (667 | ) | | (74 | ) | | (85 | ) | | (74 | ) | | (900 | ) |
| At December 31, 2015 | | 4,039 |
| | 19 |
| | 746 |
| | 19 |
| | 4,823 |
|
|
| | | | | | | | | | | | | | | |
| | | Accumulated depreciation |
| | | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | | $ | | $ | | $ | | $ | | $ |
| At January 1, 2014 | | 8,016 |
| | 1,222 |
| | 1,821 |
| | 929 |
| | 11,988 |
|
| Disposals / Retirements | | (1,212 | ) | | — |
| | (182 | ) | | — |
| | (1,394 | ) |
| Impairment loss* | | 206 |
| | — |
| | — |
| | — |
| | 206 |
|
| Recurring depreciation expense | | 282 |
| | 17 |
| | 21 |
| | 51 |
| | 371 |
|
| Impact of foreign exchange rate changes | | (979 | ) | | (152 | ) | | (212 | ) | | (118 | ) | | (1,461 | ) |
| At December 31, 2014 | | 6,313 |
| | 1,087 |
| | 1,448 |
| | 862 |
| | 9,710 |
|
| Disposals / Retirements | | (1,957 | ) | | (1,015 | ) | | (719 | ) | | (882 | ) | | (4,573 | ) |
| Impairment loss* | | — |
| | — |
| | — |
| | 70 |
| | 70 |
|
| Recurring depreciation expense | | 138 |
| | 1 |
| | 36 |
| | 15 |
| | 190 |
|
| Impact of foreign exchange rate changes | | (621 | ) | | (73 | ) | | (82 | ) | | (54 | ) | | (830 | ) |
| At December 31, 2015 | | 3,873 |
| | — |
| | 683 |
| | 11 |
| | 4,567 |
|
|
| | | | | | | | | | | | | | | | | | |
| | Years ended December 31, | |
| | 2018 | | 2017 | | 2016 | |
| | Number | | Weighted average exercise price ($) | | Number | | Weighted average exercise price ($) | | Number | | Weighted average exercise price ($) | |
| Balance – Beginning of the year | 3,417,840 |
| | 7.59 |
| | 3,779,245 |
| | 9.66 |
| | 2,842,309 |
| | 11.30 |
| |
| Issued (note 19) | — |
| | — |
| | — |
| | — |
| | 945,000 |
| | 4.70 |
| |
| Exercised | — |
| | — |
| | (331,730 | ) | * | 1.07 |
| | — |
| | — |
| |
| Expired (note 19) | (25,996 | ) | | 185.00 |
| | (29,675 | ) | | 345.00 |
| | (8,064 | ) | | 4.23 |
| |
| Balance – End of the year | 3,391,844 |
| | 6.23 |
| | 3,417,840 |
| | 7.59 |
| | 3,779,245 |
| | 9.66 |
| |
_________________________
*Related to R&D equipment impaired A portion of the Series A warrants was exercised using the cashless feature. Therefore, the total number of equivalent shares issued was 301,343.
The following table summarizes the share purchase warrants outstanding and exercisable as a result of a restructuring (note 14 – Restructuring).
at December 31, 2018:
|
| | | | | |
| | | |
| Exercise price ($) | | Number | | Weighted average remaining contractual life (years) |
| 1.07 | | 115,844 |
| | 1.19 |
| 4.70 | | 945,000 |
| | 1.34 |
| 7.10 | | 2,331,000 |
| | 1.96 |
| | | 3,391,844 |
| | 1.76 |
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
|
| | | | | | | | | | | | | | | |
| | | Carrying amount |
| | | Equipment | | Furniture and fixtures | | Computer equipment | | Leasehold improvements | | Total |
| | | $ | | $ | | $ | | $ | | $ |
| At December 31, 2014 | | 499 |
| | 19 |
| | 86 |
| | 193 |
| | 797 |
|
| At December 31, 2015 | | 166 |
| | 19 |
| | 63 |
| | 8 |
| | 256 |
|
Depreciation of $260,000 ($577,000 in 2014 and $495,000 in 2013) is presented in the consolidated statement of comprehensive (loss) income as follows: $231,000 ($530,000 in 2014 and $480,000 in 2013) in R&D costs, $13,000 ($47,000 in 2014 and $15,000 in 2013) in general and administrative ("G&A") expenses and $16,000 (nil in 2014 and 2013) in selling expenses. See also note 6 – Discontinued operations for depreciation expense related to discontinued operations.
| |
11 | Identifiable intangible assets |
Identifiable intangible assets with finite useful lives consist entirely of in-process R&D costs, patents and trademarks. Changes in the carrying value of the Company's identifiable intangible assets with finite useful lives are summarized below.
|
| | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, 2015 | | Year ended December 31, 2014 |
| | | Cost | | Accumulated amortization | | Carrying value | | Cost | | Accumulated amortization | | Carrying value |
| | | $ | | $ | | $ | | $ | | $ | | $ |
| Balances – Beginning of the year | | 35,032 |
| | (34,680 | ) | | 352 |
| | 39,890 |
| | (39,182 | ) | | 708 |
|
| Disposal/Retirements | | (538 | ) | | 538 |
| | — |
| | — |
| | — |
| | — |
|
| Impairment loss* | | — |
| | — |
| | — |
| | — |
| | (184 | ) | | (184 | ) |
| Recurring amortization expense* | | — |
| | (81 | ) | | (81 | ) | | — |
| | (117 | ) | | (117 | ) |
| Impact of foreign exchange rate changes | | (3,343 | ) | | 3,309 |
| | (34 | ) | | (4,858 | ) | | 4,803 |
| | (55 | ) |
| Balances – End of the year | | 31,151 |
| | (30,914 | ) | | 237 |
| | 35,032 |
| | (34,680 | ) | | 352 |
|
_________________________
* Recorded as R&D costs in the consolidated statements of comprehensive (loss) income.
The change in carrying value is as follows:
|
| | | | | | | | | |
| | | Cost | | Accumulated impairment loss | | Carrying amount |
| | | $ | | $ | | $ |
| At January 1, 2014 | | 9,892 |
| | — |
| | 9,892 |
|
| Impact of foreign exchange rate changes | | (1,205 | ) | | — |
| | (1,205 | ) |
| At December 31, 2014 | | 8,687 |
| | — |
| | 8,687 |
|
| Impact of foreign exchange rate changes | | (851 | ) | | — |
| | (851 | ) |
| At December 31, 2015 | | 7,836 |
| | — |
| | 7,836 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
13 Payables and accrued liabilities
|
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Trade accounts payable | | 2,488 |
| | 3,153 |
|
| Accrued research and development costs | | 312 |
| | 1,073 |
|
| Salaries, employment taxes and benefits | | 256 |
| | 560 |
|
| Current portion of onerous contract provisions (note 16) | | 334 |
| | 322 |
|
| Other accrued liabilities | | 782 |
| | 691 |
|
| | | 4,172 |
| | 5,799 |
|
On August 7, 2014, the Company's Nominating, Governance and Compensation Committee and Board of Directors approved the Company's global resources optimization program (the "Resource Optimization Program"), which was rolled out as part of a strategy to transition Aeterna Zentaris into a commercially operating specialty biopharmaceutical organization. The Resource Optimization Program, the goal of which was to streamline R&D activities and to increase commercial operations and flexibility, resulted in the termination of 28 employees at the Company. As at December 31, 2015, the Resource Optimization Program was substantially complete.
Upon approval of the Resource Optimization Program, a provision for restructuring costs was recorded. Total restructuring costs associated with the Resource Optimization Program included severance payments, onerous lease provision and other directly related costs, and were recorded as follows in the accompanying consolidated statement of comprehensive (loss) income: $2,201,000 in R&D costs, and $288,000 in G&A expenses. All the changes in the provision recorded in 2015 for the Resource optimization Program was recorded in R&D costs.
On October 9, 2015, the Company's Board of Directors approved a plan to restructure the finance and accounting operations and to close the Company's Quebec City office (the "Corporate Restructuring"). The Company transferred all functions performed by the five employees in its Quebec City office to other personnel and will be adding new finance and accounting personnel, including a new Chief Financial Officer, in the Company's Summerville, South Carolina, office. As of December 31, 2015, management estimates that the Corporate Restructuring will be completed by the end of September 2016.
Upon approval of the Corporate Restructuring, a provision for severance payments, lease cancellation fees and other directly related costs was recorded in G&A expenses in the accompanying consolidated statement of comprehensive (loss) income. This estimate may vary as a result of changes in the underlying assumptions applied thereto.
Restructuring costs are recognized in the consolidated statement of comprehensive (loss) income when the Company has a detailed formal plan for the restructuring and has raised a valid expectation in those affected that it will carry out the restructuring by starting to implement that plan or announcing the plan's main features to those affected by it.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
The change in the Company's provision for restructuring costs can be summarized as follows:
|
| | | | | | | | | |
| | | Resource Optimization Program | | Corporate Restructuring | | Total |
| | | $ | | $ | | $ |
| At January 1, 2014 | | — |
| | — |
| | — |
|
| Provision recognized | | 2,489 |
| | — |
| | 2,489 |
|
| Utilization of provision | | (687 | ) | | — |
| | (687 | ) |
| Impact of foreign exchange rate changes | | (151 | ) | | — |
| | (151 | ) |
| At December 31, 2014 | | 1,651 |
| | — |
| | 1,651 |
|
| Less: non current portion (note 16) | | (146 | ) | | — |
| | (146 | ) |
| | | 1,505 |
| | — |
| | 1,505 |
|
| | | | | | | |
| At December 31, 2014 | | 1,651 |
| | — |
| | 1,651 |
|
| Provision Recognized | | — |
| | 1,244 |
| | 1,244 |
|
| Utilization of provision | | (1,154 | ) | | (636 | ) | | (1,790 | ) |
| Change in the provision | | (265 | ) | | (47 | ) | | (312 | ) |
| Impact of foreign exchange rate changes | | (157 | ) | | (4 | ) | | (161 | ) |
| At December 31, 2015 | | 75 |
| | 557 |
| | 632 |
|
| Less: non-current portion (note 16) | | (34 | ) | | — |
| | (34 | ) |
| | | 41 |
| | 557 |
| | 598 |
|
The change in the Company's warrant liability can be summarized as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Balance – Beginning of the year | | 8,225 |
| | 18,010 |
| | 6,176 |
|
| Share purchase warrants issued during the year (note 17) | | 28,678 |
| | 8,487 |
| | 13,397 |
|
| Derecognition due to early expiry (note 17) | | (5,865 | ) | | — |
| | — |
|
| Share purchase warrants exercised during the year | | (31,103 | ) | | — |
| | — |
|
| Change in fair value of share purchase warrants (note 20) | | 10,956 |
| | (18,272 | ) | | (1,563 | ) |
| Balance - End of the year | | 10,891 |
| | 8,225 |
| | 18,010 |
|
| Less: current portion | | (1,411 | ) | | — |
| | — |
|
| | | 9,480 |
| | 8,225 |
| | 18,010 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
A summary of the activity related to the Company's share purchase warrants is provided below. |
| | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | Number | | Weighted average exercise price (US$) | | Number | | Weighted average exercise price (US$) | | Number | | Weighted average exercise price (US$) |
| Balance – Beginning of the year | | 287,852 |
| | 187.00 |
| | 201,074 |
| | 234.00 |
| | 44,074 |
| | 514.00 |
|
| Issued (note 17) | | 3,076,956 |
| ** | 6.58 |
| * | 88,000 |
| | 125.00 |
| * | 157,000 |
| | 155.00 |
|
| Exercised | | (298,088 | ) | | 4.94 |
| | — |
| | — |
| | — |
| | — |
|
| Expired (note 17) | | (224.411 | ) | | 66.90 |
| | (1,222 | ) | | 75.00 |
| | — |
| | — |
|
| Balance – End of the year | | 2,842,309 |
| | 11.91 |
| | 287,852 |
| | 187.00 |
| | 201,074 |
| | 234.00 |
|
_________________________
* As adjusted (note 17 – Share capital)
** 298,382 of which represent the Series B Warrants (see note 17 - Share Capital), which may be exercised on an alternate cashless basis, as discussed below.
The following table summarizes the share purchase warrants outstanding and exercisable as at December 31, 2015: |
| | | | | | |
| | | |
| Exercise price ($) | | Number | | Weighted average remaining contractual life (years) |
| 4.95 | | 455,638 |
| | 4.14 |
|
| 7.10 | | 2,331,000 |
| | 4.95 |
|
| 185.00 | | 25,996 |
| | 2.58 |
|
| 345.00 | | 29,675 |
| | 1.80 |
|
| | | 2,842,309 |
| | 4.77 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
The table presented below shows the inputs and assumptions applied to the Black-Scholes option pricing model in order to determine the fair value of all warrants outstanding as at December 31, 2015 in order to determine the fair value of all outstanding warrants, with the exception of the Series B Warrants, as defined and discussed below.2018. The Black-Scholes option pricing model uses "Level 2" inputs, as defined by IFRS 13, Fair value measurement ("IFRS 13") and as discussed in note 24 - Financial instruments and financial risk management.
|
| | | | | | | | | | | | | | | | | | | |
| | Number of equivalent shares | | Market-value per share price | | Weighted average exercise price | | Risk-free annual interest rate | | Expected volatility | | Expected life (years) | | Expected dividend yield |
| | | | ($) | | ($) | | (a) | | (b) | | (c) | | (d) |
| March 2015 Series A Warrants (e) | 115,844 |
| | 2.94 |
| | 1.07 |
| | 2.58 | % | | 81.81 | % | | 1.19 | | 0.00 | % |
| December 2015 Warrants | 2,331,000 |
| | 2.94 |
| | 7.10 |
| | 2.47 | % | | 122.00 | % | | 1.96 | | 0.00 | % |
| November 2016 Warrants (f) | 945,000 |
| | 2.94 |
| | 4.70 |
| | 2.56 | % | | 78.95 | % | | 1.34 | | 0.00 | % |
|
| | | | | | | | | | | | | | | | | | | | |
| | | Number of equivalent shares | | Market-value per share price ($) | | Weighted average exercise price ($) | | Risk-free annual interest rate (a) | | Expected volatility (b) | | Expected life (years) (c) | | Expected dividend yield (d) |
| October 2012 Investor Warrants | | 29,675 |
| | 4.48 |
| | 345.00 |
| | 0.97 | % | | 141.94 | % | | 1.80 |
| | 0.00% |
| July 2013 Warrants | | 25,996 |
| | 4.48 |
| | 185.00 |
| | 1.20 | % | | 125.35 | % | | 2.58 |
| | 0.00% |
| March 2015 Series A Warrants (e) | | 447,574 |
| | 4.48 |
| | 4.95 |
|
| 1.57 | % | | 121.27 | % | | 4.19 |
| | 0.00% |
| December 2015 Warrants | | 2,331,000 |
| | 4.48 |
| | 7.10 |
|
| 1.74 | % | | 113.75 | % | | 4.95 |
| | 0.00% |
_________________________________________________
| |
| (a) | Based on United States Treasury Government Bond interest rates with a term that is consistent with the expected life of the warrants. |
| |
| (b) | Based on the historical volatility of the Company's stock price over the most recent period consistent with the expected life of the warrants, as well as on future expectations. |
| |
| (c) | Based upon time to expiry from the reporting period date. |
| |
| (d) | The Company has not paid dividends and it does not intend to pay dividends in the foreseeable future. |
| |
| (e) | For the March 2015 Series A Warrants, the inputs and assumptions applied to the Black-Scholes option pricing model have been further adjusted to take into consideration the value attributed to certain anti-dilution provisions. Specifically, the weighted average exercise price is subject to adjustment (see note 17 –19 - Share capital). |
Series B Warrants
In addition to the availability of standard cashless exercise provisions, the Series B Warrants (defined and discussed in note 17 – Share capital) were entitled to be exercised on an alternate cashless basis in accordance with their terms. Such an exercise permitted the holder to obtain a number of common shares equal to: 200% of (i) the total number of common shares with respect to which the Series B Warrant is then being exercised multiplied by (ii) 81.00 divided by (iii) 85% of the quotient of (A) the sum of the per share volume weighted average price ("VWAP") of the common share for each of the five lowest trading days during the fifteen trading day period ending on and including the trading day immediately prior to the applicable Exercise Date, divided by (B) five, less (iv) the total number of common shares with respect to which the Series B Warrant is then being exercised.
Exercises of Series B Warrants that are performed on an alternate cashless basis results in the issuance of a substantially larger number of the Company's common shares than otherwise would be issued following a standard cash or cashless exercise of the Series B Warrants.
Management has determined that, in light of the alternate cashless exercise feature and of actual Series B Warrant exercises since original issuance, application of the Black-Scholes option pricing model does not appropriately reflect the fair value of the Series B Warrants outstanding at a given statement of financial position date. Instead, management has determined that the application of an intrinsic valuation method is more representative of the market value of the Series B Warrants.
On November 2, 2015, the Company announced that the holders (the "Participating Holders") of substantially all of the remaining outstanding Series B Warrants at that time had agreed to exercise all Series B Warrants held by them, at a maximum exercise ratio of approximately 33.23 common shares per warrant in accordance with the alternate cashless exercise feature in such Series B Warrants. Following the exercise of Series B Warrants by the Participating Holders in accordance with the terms of the agreements, 8,064 Series B Warrants, expiring in September 2016, remain outstanding, representing approximately 2.7% of the originally issued number of Series B Warrants. A total of $2,925,653 was paid to the Participating Holders pursuant to the aforementioned agreements. (see note 20 - Finance income and finance costs)
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
As such, the Company has attributed a value to the remaining Series B Warrants via the application of the aforementioned alternate cashless exercise formula, reflecting relevant market data as at December 31, 2015, summarized as follows:
|
| | | | |
Number of Series B Warrants outstanding | | 8,064 |
| |
Estimated potential number of equivalent shares | (a) | 325,254 |
| |
Applicable VWAP, as calculated per above | | $4.502 | |
Market value per share price | | $4.48 | |
Estimated intrinsic value per Series B Warrant | | $175 | |
Fair value of Series B Warrants outstanding | | $1,411 | |
(a) The number of common shares that would be issued pursuant to an alternative cashless exercise if the exercise of all of the
Series B Warrants had occurred on December 31, 2015.
The intrinsic valuation model described above applies "Level 2" inputs, as defined by IFRS 13.
| |
16 | Provisions and other non-current liabilities |
|
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Onerous contract provisions (detailed below) | | 703 |
| | 1,014 |
|
| Non-current portion of provision for restructuring costs (note 14) | | 34 |
| | 146 |
|
| Other | | 98 |
| | 130 |
|
| | | 835 |
| | 1,290 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Onerous contract provisions
|
| | | | | | | | | |
| | | Cetrotide® onerous contracts* | | Onerous lease** | | Total |
| | | $ | | $ | | $ |
| At January 1, 2014 | | 1,296 |
| | 436 |
| | 1,732 |
|
| Additional provision recognized | | 223 |
| | — |
| | 223 |
|
| Utilization of provision | | (397 | ) | | (102 | ) | | (499 | ) |
| Unwinding of discount and effect of change in the discount rate | | (124 | ) | | 4 |
| | (120 | ) |
| At December 31, 2014 | | 998 |
| | 338 |
| | 1,336 |
|
| Less: current portion (note 13) | | (218 | ) | | (104 | ) | | (322 | ) |
| | | 780 |
| | 234 |
| | 1,014 |
|
| | | | | | | |
| At January 1, 2015 | | 998 |
| | 338 |
| | 1,336 |
|
| Additional provision recognized | | 170 |
| | — |
| | 170 |
|
| Utilization of provision | | (278 | ) | | (108 | ) | | (386 | ) |
| Unwinding of discount and effect of change in the discount rate | | (87 | ) | | 4 |
| | (83 | ) |
| At December 31, 2015 | | 803 |
| | 234 |
| | 1,037 |
|
| Less: current portion (note 13) | | (225 | ) | | (109 | ) | | (334 | ) |
| | | 578 |
| | 125 |
| | 703 |
|
_________________________
| |
* | Recorded following the transfer of the Cetrotidecapital® Business, as discussed in note 6 – Discontinued operations.).
|
| |
**(f) | RepresentsFor the presentNovember 2016 Warrants, the Company reduced the fair value of these warrants to take into consideration the fair value of the future lease payments that$10 call option, which was also calculated using the Company is obligated to make pursuant to a non-cancellable operating lease in the United States, net of estimated future sublease income. The estimate may vary as a result of changes in the utilization of the leased premisesBlack-Scholes pricing model. (see note 19 - Share and of the sublease arrangement. The remaining term of the lease is two years as at December 31, 2015. |
The Company has an unlimited number of authorized common shares (being voting and participating shares) with no par value, as well as an unlimited number of preferred, first and second ranking shares, issuable in series, with rights and privileges specific to each class, with no par value.
Share consolidation
The 655,984,512 common shares issued and outstanding immediately prior to the Share Consolidation, which became legally effective on November 17, 2015, were consolidated into 6,559,846 common shares (the "Post-Consolidation Shares"). The Post-Consolidation Shares began trading on each of the TSX and NASDAQ at the opening of markets on November 20, 2015. The number of outstanding stock options and share purchase warrants were adjusted on the same basis with proportionate adjustments being made to each stock option and share purchase warrant exercise price.
All share, option and share purchase warrant and per share, option and share purchase warrant data have been retroactively adjusted in these consolidated financial statements to reflect and give effect to the Share Consolidation as if it occurred at the beginning of the earliest period presented.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Common shares issued in connection with "At-the-Market" ("ATM") drawdowns
May 2013 ATM Program
On May 22, 2013, the Company entered into an ATM sales agreement (the "May 2013 ATM Program"), under which the Company was able, at its discretion and from time to time, to sell up to 25,000 of its common shares through ATM issuances on the NASDAQ for aggregate gross proceeds not to exceed $4,600,000. The May 2013 ATM Program provided that common shares were to be sold at market prices prevailing at the time of sale and, as a result, prices may have varied.
Between January 1, 2014 and March 31, 2014, the Company issued a total of 2,020 common shares under the May 2013 ATM Program at an average price of approximately $143.00 per share, resulting in aggregate gross proceeds of approximately $288,000, less cash transaction costs of $8,600 and previously deferred transaction costs of $17,000. The May 2013 ATM Program was subsequently discontinued in connection with the implementation of the May 2014 ATM Program described below.
May 2014 ATM Program
On May 9, 2014, the Company entered into an ATM sales agreement (the "May 2014 ATM Program"), under which the Company is able, at its discretion and from time to time, to sell up to 140,187 of its common shares through ATM issuances on the NASDAQ for aggregate gross proceeds not to exceed $15,000,000. The May 2014 ATM Program provides that common shares are to be sold at market prices prevailing at the time of sale and, as a result, prices may vary.
Between July 1, 2014 and December 31, 2014, the Company issued a total of 89,951common shares under the May 2014 ATM Program at an average price of approximately $136.00 per share for aggregate gross proceeds of approximately $12,200,000 less cash transaction costs of $305,430 and previously deferred transaction costs of $71,575.
Public offerings
January 2014 Offering
On January 14, 2014, the Company completed a public offering (the "January 2014 Offering") of 110,000 units, at a purchase price of $120.00 per unit, with each unit consisting of one common share and 0.8 of a warrant to purchase a common share. The related warrants (the "January 2014 Warrants") represent the right to acquire an aggregate of 88,000 common shares, as discussed below.
Total gross cash proceeds raised through the January 2014 Offering amounted to $13,200,000, less cash transaction costs of approximately $1,034,000 and previously deferred transaction costs of $5,000.
The Company issued the January 2014 Warrants to the investors who participated in the January 2014 Offering at an exercise price of $125.00 per share, with the January 2014 Warrants containing certain anti-dilution provisions. These warrants were exercisable at any time during their five-year term and, upon complete exercise, would have resulted in the issuance of an aggregate of 88,000 common shares that would generate additional proceeds for an amount that would be determined based on the then adjusted exercise price.
The Company estimated the fair value attributable to the January 2014 Warrants as of the date of grant by applying the Black-Scholes pricing model, to which the following additional assumptions were applied: a risk-free annual interest rate of 1.64%, an expected volatility of 102.31%, an expected life of 5 years and a dividend yield of 0.0%. As a result, the fair value of the share purchase warrants was estimated at $8,487,000.
Total gross proceeds of the January 2014 Offering were allocated as follows: $8,487,000 was allocated to Warrant liability, and the balance of $4,713,000 was allocated to Share capital. Transaction costs were allocated to the liability and equity components in proportion to the allocation of proceeds. As such, an amount of $666,000 was allocated to the share purchase warrants and immediately recognized in general and administrative expenses in the consolidated statement of comprehensive (loss) income, and an amount of $373,000 was allocated to Share capital.
In connection with the January 2014 Offering, the holders of warrants issued as part of a financing in November 2013 (the "November 2013 Warrants"), who participated in the January 2014 Offering agreed to waive certain anti-dilution provisions
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
of such warrants solely in connection with the January 2014 Offering, and agreed to an adjustment of the exercise price of such warrants following the closing of the January 2014 Offering from their original exercise price of $160.00 per share to an exercise price equal to $125.00 per share. The exercise price of the November 2013 Warrants held by the sole holder who did not participate in the January 2014 Offering, was further reduced by $5.00 per share.
March 2015 Offering
On March 11, 2015, the Company completed a public offering of 596,775 units (the "Units"), with each Unit consisting of either one common share or one pre-funded warrant to purchase one common share ("Series C Warrant"), 0.75 of a warrant to purchase one common share ("Series A Warrant") and 0.50 of a warrant to purchase one common share ("Series B Warrant"), at a purchase price of $62.00 per Unit (the "March 2015 Offering").
Total gross cash proceeds raised through the March 2015 Offering amounted to $37,000,000, less cash transaction costs of approximately $2,560,000 and previously deferred transaction costs of $7,000.
The Series A Warrants are exercisable during a five-year term at an initial exercise price of $81.00 per share, and the Series B Warrants are exercisable during an 18-month term at an initial exercise price of $81.00 per share. Both the Series A and Series B Warrants are subject to certain anti-dilution provision and may at any time be exercised on a standard cashless basis and, in addition, the Series B Warrants may be exercised on an alternate net cashless basis. The exercise of Series B Warrants performed on an alternate net cashless basis results in the issuance of a substantially larger number of the Company's common shares than otherwise would be issued following a standard cash or cashless exercise. See also note 15 – Warrant liability. The remaining 8,064 Series B Warrants expire in September 2016.
Between May 26, 2015 and December 31, 2015, 290,318 of the Series B Warrants were exercised on an alternate cashless basis, resulting in the issuance of 5,670,118 common shares.
The Company estimated the fair value attributable to the Series A and Series B warrants as of the date of grant by applying the Black-Scholes pricing model, to which the following additional assumptions were applied: Series A warrants: a risk-free annual interest rate of 1.59%, an expected volatility of 95.11%, an expected life of 5 years and a dividend yield of 0.0%; Series B warrants: a risk-free annual interest rate of 0.47%, an expected volatility of 97.34%, an expected life of 18 months and a dividend yield of 0.0%. As a result, on March 11, 2015, the total fair value of the share purchase warrants was estimated at $20,980,000.
The Series C Warrants were offered in the March 2015 Offering to investors whose purchase of Units would have resulted in their beneficially owning more than an "initial beneficial ownership limitation" of either 4.9% or 9.9% of our common shares following the offering. The Series C Warrants, which were exercisable immediately upon issuance and for a period of five years at an exercise price of $62.00 per share, were fully exercised between March 23, 2015 and June 15, 2015. Total gross proceeds payable to the Company in connection with the exercise of the Series C Warrants were pre-funded by investors and therefore were included in the proceeds of the offering. No additional consideration was required to be paid to the Company upon exercise of the Series C Warrants.
Total gross proceeds of the March 2015 Offering were allocated as follows: $20,980,000 was allocated to the warrant liability, $9,296,000 was allocated to pre-funded warrants, and the balance of $6,724,000 was allocated to Share capital. Transaction costs were allocated to the liability and equity components in proportion to the allocation of proceeds. As such, an amount of $1,451,000 was allocated to the warrant liability and immediately recognized in general and administrative expenses in the consolidated statement of comprehensive loss, an amount of $473,000 was allocated to Share capital and an amount of $643,000 was allocated to pre-funded warrants. Upon exercise of the Series C Warrants, the net proceeds initially allocated to the pre-funded warrants were re-allocated to Share capital.
In connection with the March 2015 Offering, the holders of 211,230 of the 219,000 then outstanding warrants issued by the Company in connection with public offerings completed in November 2013 and January 2014 entered into an amendment agreement that caused such previously issued warrants to expire and terminate. The Company made a cash payment in the aggregate amount of $5,703,000 out of the proceeds of the March 2015 Offering as consideration to the relevant warrantholders in exchange for the latter agreeing to the aforementioned amendment. Upon expiry of the warrants in question, the Company recognized a gain of $5,865,000 and derecognized the expired warrants. The gain on derecognition was
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
recorded, net of the aforementioned amendment fee, within finance income in the accompanying condensed interim consolidated statement of comprehensive loss (see note 20 – Finance income and finance costs). For holders of the remaining 7,770 outstanding warrants issued by the Company in connection with the November 2013 and the January 2014 offerings who did not enter into a warrant amendment agreement, the exercise price of the corresponding warrants was reduced to $14.00 per share in accordance with the terms thereof.
December 2015 Offering
On December 14, 2015, the Company completed a public offering of 3,000,000 common shares at a purchase price of $5.54 per share and 2,100,000 warrants to purchase one common share at a purchase price of $0.01 per warrant (the "December 2015 Offering").
In connection with the December 2015 Offering, the Company granted the underwriter a 45-day over-allotment option to separately acquire up to an additional 330,000 common shares at the same purchase price of $5.54 per share and/or up to an additional 231,000 warrants at the same purchase price of $0.01 per warrants. The underwriter exercised its option in full with respect to the 231,000 warrants for market stabilization purposes but did not exercise any of its option in respect of common shares.
Total gross cash proceeds raised through the December 2015 Offering amounted to approximately $16,650,000, less cash transaction costs of approximately $1,638,000.
The warrants are exercisable for a period of five years at an exercise price of $7.10 per share. Upon complete exercise for cash, these warrants would result in the issuance of an aggregate of 2,331,000 common shares that would generate additional proceeds for an amount of $16,550,100. However, those warrants may at any time be exercised on a "net" or "cashless" basis.
The Company estimated the fair value attributable to the warrants as of the date of grant by applying the Black-Scholes pricing model, to which the following additional assumptions were applied: a risk-free annual interest rate of 1.68%, an expected volatility of 107.57%, an expected life of 5 years and a dividend yield of 0.0%. As a result, on December 14, 2015, the total fair value of the share purchase warrants was estimated at $7,698,000.
Total gross proceeds of the December 2015 Offering were allocated as follows: $7,698,000 was allocated to the warrant liability and $8,952,000 was allocated to Share capital. Transaction costs were allocated to the liability and equity components in proportion to the allocation of proceeds. As such, an amount of $757,000 was allocated to the warrant liability and immediately recognized in general and administrative expenses in the consolidated statement of comprehensive loss, an amount of $881,000 was allocated to Share capital.
In connection with the December 2015 Offering and in accordance with the anti-dilution provisions, the exercise prices of the January 2014 and March 2015 Series A and Series B warrants were adjusted at $0.00 and $4.95, respectively. The remaining January 2014 Warrants were exercised on December 30, 2015 and no longer remain outstanding.
Shareholder rights plan
The Company has a shareholder rights plan (the "Rights Plan") that provides the Board of Directors and the Company's shareholders with additional time to assess any unsolicited take-over bid for the Company and, where appropriate, to pursue other alternatives for maximizing shareholder value. Under the Rights Plan, one right has been issued for each currently issued common share, and one right will be issued with each additional common share to be issued. The Rights Plan was most recently re-confirmed and approved by the Company's shareholders at its annual meeting of shareholders held on May 8, 2013.
Stock options
In December 1995, the Company's Board of Directors adopted a stock option plan (the "Stock Option Plan") for its directors, senior executives, employees and other collaborators who provide services to the Company. The total number of common shares that may be issued under the Stock Option Plan cannot exceed 11.4% of the total number of issued and outstanding common shares at any given time. The Company's Board of Directors amended the Stock Option Plan on March 20, 2014.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Options granted under the Stock Option Plan prior to the 2014 amendment expire after a maximum period of ten years following the date of grant. Options granted after the 2014 amendment expire after a maximum period of seven years following the date of grant.
The following tables summarize the activity under the Stock Option Plan. |
| | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| US dollar-denominated options | | Number | | Weighted
average
exercise
price
(US$) | | Number | | Weighted
average
exercise
price
(US$) | | Number | | Weighted
average
exercise
price
(US$) |
| Balance – Beginning of the year | | 33,956 |
| | 187.36 |
| | 17,575 |
| | 339.61 |
| | 13,260 |
| | 426.22 |
|
| Granted | | 243,000 |
| | 5.17 |
| | 19,515 |
| | 93.03 |
| | 6,300 |
| | 156.48 |
|
| Forfeited | | (4,082 | ) | | 136.17 |
| | (3,134 | ) | | 453.77 |
| | (1,985 | ) | | 336.97 |
|
| Balance – End of the year | | 272,874 |
| | 25.88 |
| | 33,956 |
| | 187.36 |
| | 17,575 |
| | 339.61 |
|
|
| | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Canadian dollar-denominated options | | Number | | Weighted
average
exercise
price
(CAN$) | | Number | | Weighted
average
exercise
price
(CAN$) | | Number | | Weighted
average
exercise
price
(CAN$) |
| Balance – Beginning of the year | | 4,909 |
| | 1,010.4 |
| | 6,484 |
| | 1,290.50 |
| | 7,232 |
| | 1,270.57 |
|
| Exercised | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Forfeited | | (271 | ) | | 923.20 |
| | (810 | ) | | 748.53 |
| | (97 | ) | | 1,254.56 |
|
| Expired | | (851 | ) | | 1,772.17 |
| | (765 | ) | | 3,661.77 |
| | (651 | ) | | 1,074.38 |
|
| Balance – End of the year | | 3,787 |
| | 845.46 |
| | 4,909 |
| | 1,010.40 |
| | 6,484 |
| | 1,290.50 |
|
|
| | | | | | | | | |
| | | US$ options outstanding as at December 31, 2015 |
Exercise price (US$) | | Number | | Weighted average remaining
contractual life (years) | | Weighted average exercise price (US$) |
| 4.58 to 28.54 | | 240,000 |
| | 6.97 |
| | 4.58 |
|
| 28.55 to 91.50 | | 11,852 |
| | 6.01 |
| | 71.24 |
|
| 91.51 to 122.50 | | 7,300 |
| | 6.13 |
| | 109.73 |
|
| 122.51 to 207.50 | | 6,250 |
| | 7.08 |
| | 165.60 |
|
| 207.51 to 2,178.00 | | 7,472 |
| | 6.05 |
| | 439.41 |
|
| | | 272,874 |
| | 6.89 |
| | 25.88 |
|
|
| | | | | | | | | |
| | | US$ options exercisable as at December 31, 2015 |
Exercise price (US$) | | Number | | Weighted average remaining
contractual life (years) | | Weighted average exercise price (US$) |
| 28.55 to 91.50 | | 3,199 |
| | 5.93 |
| | 76.00 |
|
| 91.51 to 122.50 | | 3,352 |
| | 6.53 |
| | 110.35 |
|
| 122.51 to 207.50 | | 3,170 |
| | 7.14 |
| | 176.38 |
|
| 207.51 to 2,178.00 | | 7,472 |
| | 6.05 |
| | 439.41 |
|
| | | 17,193 |
| | 6.32 |
| | 259.14 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
|
| | | | | | | | | |
| | | CAN$ options both outstanding and exercisable as at December 31, 2015 |
Exercise price (CAN$) | | Number | | Weighted average remaining
contractual life (years) | | Weighted average exercise price (CAN$) |
| 330.00 to 480.00 | | 862 |
| | 2.89 |
| | 364.73 |
|
| 480.01 to 741.00 | | 1,192 |
| | 3.94 |
| | 570.00 |
|
| 741.01 to 1,002.00 | | 983 |
| | 4.91 |
| | 912.00 |
|
| 1,002.01 to 1,941.00 | | 460 |
| | 1.94 |
| | 1,092.00 |
|
| 1,941.01 to 2,790.00 | | 290 |
| | 1.01 |
| | 2,790.00 |
|
| | | 3,787 |
| | 3.48 |
| | 845.46 |
|
As at December 31, 2015, the total compensation cost related to unvested US Dollar stock options not yet recognized amounted to $1,221,998 ($1,126,261 in 2014). This amount is expected to be recognized over a weighted average period of 1.70 years (1.70 years in 2014).
The Company settles stock options exercised through the issuance of new common shares as opposed to purchasing common shares on the market to settle stock option exercises.
Fair value input assumptions for US dollar-denominated options granted
The table below shows the assumptions, or weighted average parameters, applied to the Black-Scholes option pricing model in order to determine share-based compensation costs over the life of the awards.
|
| | | | | | | |
| | | | | Years ended December 31, |
| | | | | 2015 | | 2014 |
| Expected dividend yield | | (a) | | 0.0% |
| | 0.0% |
| Expected volatility | | (b) | | 110.5 | % | | 101.6% |
| Risk-free annual interest rate | | (c) | | 1.79 | % | | 1.87% |
| Expected life (years) | | (d) | | 5.77 |
| | 6.16 |
| Weighted average share price | | | | $5.65 | | $93.00 |
| Weighted average exercise price | | | | $5.17 | | $93.00 |
| Weighted average grant date fair value | | | | $4.69 | | $75.00 |
_________________________
| |
(a) | The Company has not paid dividends and it does not intend to pay dividends in the foreseeable future. |
| |
(b) | Based on the historical volatility of the Company's stock price over the most recent period consistent with the expected life of the stock options, as well as on future expectations. |
| |
(c) | Based on United States Treasury Government Bond interest rates with a term that is consistent with the expected life of the stock options. |
| |
(d) | Based upon historical data related to the exercise of stock options, on post-vesting employment terminations and on future expectations related to exercise behavior. |
The Black-Scholes pricing models referred above use "Level 2" inputs in calculating fair value, as defined by IFRS 7, and as discussed in note 24 – Financial instruments and financial risk management.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Components of the Company's operating expenses from continuing operations include the following:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Subcontractor fees | | — |
| | — |
| | 51 |
|
| Cost of sales | | — |
| | — |
| | 51 |
|
Key management personnel compensation(1) | | | | | | |
| Salaries and short-term employee benefits | | 2,957 |
| | 2,405 |
| | 2,280 |
|
| Termination benefits | | 843 |
| | 439 |
| | 1,438 |
|
| Post-employment benefits | | 119 |
| | 77 |
| | 58 |
|
| Share-based compensation costs | | 828 |
| | 392 |
| | 1,795 |
|
| | | 4,747 |
| | 3,313 |
| | 5,571 |
|
| Other employees compensation: | | | | | | |
| Salaries and short-term employee benefits | | 4,431 |
| | 7,663 |
| | 7,955 |
|
| Termination benefits (note 14) | | 245 |
| | 1,984 |
| | 7 |
|
| Post-employment benefits | | 511 |
| | 832 |
| | 626 |
|
| Share-based compensation costs | | 91 |
| | 105 |
| | 572 |
|
| | | 5,278 |
| | 10,584 |
| | 9,160 |
|
Goods and services(2) | | 21,429 |
| | 19,016 |
| | 15,954 |
|
Leasing costs, net of sublease receipts of $155,000 in 2015, $344,000 in 2014 and $226,000 in 2013(3) | | 1,452 |
| | 1,802 |
| | 1,879 |
|
| Refundable tax credits and grants | | (23 | ) | | (131 | ) | | (517 | ) |
| Onerous contract expenses resulting from the Resource Optimization Program and from the Corporate Restructuring (note 14) | | (202 | ) | | 563 |
| | — |
|
| Share-based compensation costs related to collaborators | | — |
| | — |
| | (148 | ) |
| Transaction costs related to share purchase warrants | | 2,208 |
| | 666 |
| | 1,165 |
|
| Depreciation and amortization | | 271 |
| | 488 |
| | 949 |
|
| Impairment losses | | 70 |
| | 390 |
| | — |
|
| Operating foreign exchange losses (gains) | | 199 |
| | 715 |
| | (413 | ) |
| | | 25,404 |
| | 23,509 |
| | 18,869 |
|
| | | 35,429 |
| | 37,406 |
| | 33,651 |
|
_________________________
| |
(1)
| Key management includes the Company's directors and members of the executive management team. |
| |
(2)
| Goods and services include third-party R&D costs, laboratory supplies, professional fees, contracted sales force costs, marketing services, insurance and travel expenses. |
| |
(3)
| Leasing costs also include changes in the onerous lease provision (note 16 – Provisions and other non-current liabilities), other than attributable to the unwinding of the discount. |
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
On April 15, 2013, the Company appointed a new President and Chief Executive Officer (the "CEO"), who also was appointed and subsequently elected to the Company's Board of Directors. In accordance with his employment agreement, the Company's new President and CEO was granted, as a retention bonus, 375,000 share appreciation rights ("SARs"), pursuant to which he would have been entitled to receive a18 Employee future cash payment if he remained employed through a certain date. The retention bonus was to be based on the increase, if any, in the company's share price from $198.00 over a specified period of time. 175,000 SARs vested (and expired) on December 31, 2014, and 200,000 SARs vested (and expired) on December 31, 2015. As a result of the share price, the CEO did not receive any payment under the SARs prior to their expiry.benefits
The Company's former President and CEO received, upon termination of his employment, benefits of approximately $1,438,000. Additionally, the Company's former President and CEO was permitted to retain all of his stock options, which, pursuant to IFRS 2, Share-based Payment, constitutes a modification to the terms of the existing stock options granted in a share-based payment transaction, by allowing such stock options to expire at the original expiry date, based on the original date of grant, despite the termination of employment. As a result of this modification, an amount of $682,000, which corresponds to the compensation cost related to unvested stock options not yet recognized immediately before the modification and to the incremental fair value of the stock options, measured by comparing the stock options immediately before and immediately after the modification date, was recognized during the year ended December 31, 2013 within G&A expenses in the consolidated statement of comprehensive (loss) income.
Most of the employment agreements entered into between the Company and its executive officers include termination provisions, whereby the executive officers would be entitled to receive benefits that would be payable if the Company were to terminate the executive officers' employment without cause or if their employment is terminated following a change of control. Separation benefits generally are calculated based on an agreed-upon multiple of applicable base salary and incentive compensation and, in certain cases, other benefit amounts.
In addition to payments made to members of the Company's key management team, during the years ended December 31, 2013, 2014 and 2015 the Company paid $76,800; $38,000; and nil respectively, in professional fees to one of the members of the Company's Board of Directors for special tasks mandated by the Company's Nominating, Corporate Governance and Compensation Committee.
| |
19 | Employee future benefits |
The Company's subsidiary in Germany provides unfunded defined benefit pension plans and unfunded post-employment benefit plans for certain groups of employees. Provisions for pension obligations are established for benefits payable in the form of retirement, disability and surviving dependent pensions.
The unfunded defined benefit pension plans are final salary pension plans, which provide benefits to members (or to their surviving dependents) in the form of a guaranteed level of pension payable for life. The level of benefits provided depends on the member’smember's length of service and on his or her base salary in the final years leading up to retirement. Current pensions vary in accordance with applicable statutory requirements, which foresee an adjustment every three years on an individual basis that is based on inflationary increases or in relation to salaries of comparable groups of active employees in the Company. An adjustment may be denied by the Company if the Company's financial situation does not allow for an increase in pensions. These plans are unfunded, and the Company meets benefit payment obligations as they fall due.
The change in the Company's accrued benefit obligations is summarized as follows:
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
The following table presents the changes in the aforementioned plans' accrued benefit obligations:
| | | | | Pension benefit plans Years ended December 31, | | Other benefit plans Years ended December 31, | | Pension benefit plans
Years ended December 31, | | Other benefit plans
Years ended December 31, |
| | | 2015 | | 2014 | | 2013 | | 2015 | | 2014 | | 2013 | | 2018 | | 2017 | | 2016 | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ |
| Balance – Beginning of year | | 14,619 |
| | 14,646 |
| | 16,062 |
| | 433 |
| | 762 |
| | 1,169 |
| |
| Balances – Beginning of the year | | | 14,145 |
| | 13,197 |
| | 12,375 |
| | 84 |
| | 217 |
| | 281 |
|
| Current service cost | | 103 |
| | 176 |
| | 219 |
| | 14 |
| | 24 |
| | 57 |
| | 66 |
| | 107 |
| | 87 |
| | 6 |
| | 14 |
| | 13 |
|
| Interest cost | | 260 |
| | 476 |
| | 421 |
| | 8 |
| | 25 |
| | 31 |
| | 224 |
| | 237 |
| | 282 |
| | 1 |
| | 3 |
| | — |
|
| Actuarial loss (gain) arising from changes in financial assumptions | | (844 | ) | | 1,833 |
| | (2,346 | ) | | (34 | ) | | (96 | ) | | (258 | ) | |
| Actuarial (gain) loss arising from changes in financial assumptions | | | (193 | ) | | (694 | ) | | 1,479 |
| | 19 |
| | (115 | ) | | — |
|
| Benefits paid | | (410 | ) | | (411 | ) | | (357 | ) | | (97 | ) | | (210 | ) | | (274 | ) | | (492 | ) | | (485 | ) | | (399 | ) | | (2 | ) | | (66 | ) | | (60 | ) |
| Impact of foreign exchange rate changes | | (1,353 | ) | | (2,101 | ) | | 647 |
| | (43 | ) | | (72 | ) | | 36 |
| | (650 | ) | | 1,783 |
| | (627 | ) | | (3 | ) | | 31 |
| | (17 | ) |
| Balance – End of year | | 12,375 |
| | 14,619 |
| | 14,646 |
| | 281 |
| | 433 |
| | 761 |
| |
| Balances – End of the year | | | 13,100 |
| | 14,145 |
| | 13,197 |
| | 105 |
| | 84 |
| | 217 |
|
| Amounts recognized: | | | | | | | | | | | | | | | | | | | | | | | | |
| In comprehensive (loss) income | | (363 | ) | | (652 | ) | | (640 | ) | | 12 |
| | 47 |
| | 170 |
| |
| In other comprehensive income(loss) | | 2,197 |
| | 268 |
| | 1,699 |
| | 43 |
| | 72 |
| | (36 | ) | |
| In net loss | | | (290 | ) | | (344 | ) | | (369 | ) | | (26 | ) | | 98 |
| | (13 | ) |
| In other comprehensive income (loss) | | | 843 |
| | (1,089 | ) | | (852 | ) | | 3 |
| | (31 | ) | | 17 |
|
The cumulative amount of actuarial net losses recognized in other comprehensive income (loss) income as at December 31, 20152018 is approximately $3,492,000 (approximately $4,336,000$4,084($4,277 as at December 31, 20142017 and approximately $2,503,000$4,971 as at December 31, 2013)2016).
The significant actuarial assumptions applied to determine the Company's accrued benefit obligations are as follows:
| | | | | Pension benefit plans | | Other benefit plans | | Pension benefit plans | | Other benefit plans |
| | | Years ended December 31, | | Years ended December 31, | | Years ended December 31, | | Years ended December 31, |
| Actuarial assumptions | | 2015 | | 2014 | | 2013 | | 2015 | | 2014 | | 2013 | | 2018 | | 2017 | | 2016 | | 2018 | | 2017 | | 2016 |
| | | % | | % | | % | | % | | % | | % | | % | | % | | % | | % | | % | | % |
| Discount rate | | 2.40 | | 2.00 | | 3.37 | | 2.40 | | 2.00 | | 3.37 | | 1.90 | | 1.70 | | 1.60 | | 1.90 | | 1.70 | | 1.60 |
| Pension benefits increase | | 1.80 | | 1.80 | | 2.00 | | 2.40 | | 1.80 | | 2.00 | | 1.80 | | 1.80 | | 1.80 | | 1.80 | | 1.80 | | 1.80 |
| Rate of compensation increase | | 2.00 | | 2.00 | | 2.75 to 3.75 | | 2.00 | | 2.00 | | 2.75 | | 2.00 | | 2.00 | | 2.00 | | 2.00 | | 2.00 | | 2.00 |
The calculation of the pension benefit obligation is sensitive to the discount rate assumption. SinceEffective January 1, 2015,2018, management determined that the discount rate assumption should be adjusted from 2.0%1.7% to 2.4%1.9% as a result of changes in the European economic environment.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Assumptions regarding future mortality are set based on actuarial advice in accordance with published statistics and experience in Germany. These assumptions translate into an average remaining life expectancy in years for a pensioner retiring at age 65:
| | | | | 2015 | | 2014 | | 2013 | | 2018 | | 2017 | | 2016 |
| Retiring at the end of the reporting period: | | | | | | | | | | | | |
| Male | | 20 |
| | 19 |
| | 19 |
| | 20 | | 20 | | 20 |
| Female | | 24 |
| | 23 |
| | 23 |
| | 24 | | 24 | | 24 |
| Retiring 20 years after the end of the reporting period: | | | | | | | |
| Male | | 22 |
| | 22 |
| | 22 |
| | 28 | | 22 | | 22 |
| Female | | 26 |
| | 26 |
| | 26 |
| | 31 | | 26 | | 26 |
The most recent actuarial reports give effect to the pension and post-employment benefit obligations as at December 31, 2015. The next actuarial reports are planned for December 31, 2016.
In accordance with the assumptions used as at December 31, 2015, undiscounted defined pension benefits expected to be paid are as follows:
|
| | | |
| | | $ |
| 2016 | | 453 |
|
| 2017 | | 463 |
|
| 2018 | | 481 |
|
| 2019 | | 502 |
|
| 2020 | | 514 |
|
| Thereafter | | 17,439 |
|
| | | 19,852 |
|
The weighted average duration of the defined benefit obligation is 16.3 years.
Total expenses for the Company's defined contribution plan in its German subsidiary amounted to approximately $159,000 for the year ended December 31, 2015 ($232,954 for 2014 and $228,771 for 2013).
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
| |
20 | Finance income and finance costs |
ComponentsThe most recent actuarial reports give effect to the pension and post-employment benefit obligations as at December 31, 2018. The next actuarial reports are planned for December 31, 2019.
In accordance with the assumptions used as at December 31, 2018, undiscounted defined pension benefits expected to be paid, in Euro, are as follows:
|
| | | |
| | | $ |
| 2019 | | 453 |
|
| 2020 | | 458 |
|
| 2021 | | 463 |
|
| 2022 | | 468 |
|
| 2023 | | 476 |
|
| Thereafter | | 13,658 |
|
| | | 15,976 |
|
The weighted average duration of the defined benefit obligation is 15.3 years.
Total expenses for the Company's finance incomedefined contribution plan in its German subsidiary amounted to approximately $75 for the year ended December 31, 2018 ($119 for 2017 and finance costs can be summarized$129 for 2016).
If variations in the following assumptions had occurred during 2018, the impact on the Company's pension benefit obligation of $13,100 as at December 31, 2018 would have been as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Finance income | | | | | | |
| Change in fair value of warrant liability | | — |
| | 18,272 |
| | 1,563 |
|
| Gain associated with the extinguishment of warrant liability (note 17) | | 162 |
| | — |
| | — |
|
| Gains due to changes in foreign currency exchange rates | | — |
| | 1,879 |
| | — |
|
| Interest income | | 143 |
| | 168 |
| | 185 |
|
| | | 305 |
| | 20,319 |
| | 1,748 |
|
| Finance costs | | | | | | |
| Change in fair value of warrant liability | | (10,956 | ) | | — |
| | — |
|
| Warrant exercise inducement fee (note 15) | | (2,926 | ) | | — |
| | — |
|
| Losses due to changes in foreign currency exchange rates | | (1,767 | ) | | — |
| | (1,512 | ) |
| | | (15,649 | ) | | — |
| | (1,512 | ) |
| | | (15,344 | ) | | 20,319 |
| | 236 |
|
| |
21 | Supplemental disclosure of cash flow information |
|
| | | | |
| Assumption | Increase | Decrease |
| | | |
| Change interest rate by 0.25% | (467 | ) | 498 |
|
| Change salary rate by 0.25% | 19 |
| (17 | ) |
| Change pension by 0.25% | 372 |
| (355 | ) |
| Change mortality by 1 year | 464 |
| (463 | ) |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Changes in operating assets and liabilities: | | | | | | |
| Trade and other receivables | | 270 |
| | (578 | ) | | (3 | ) |
| Inventory | | — |
| | — |
| | 112 |
|
| Prepaid expenses and other current assets | | (111 | ) | | (2,453 | ) | | (6,454 | ) |
| Other non-current assets | | 58 |
| | (204 | ) | | (124 | ) |
| Payables and accrued liabilities | | (1,013 | ) | | 1,732 |
| | (900 | ) |
| Deferred revenues | | — |
| | 1,101 |
| | — |
|
| Provision for restructuring costs (note 14) | | (1,840 | ) | | (687 | ) | | — |
|
| Employee future benefits (note 19) | | (507 | ) | | (621 | ) | | (631 | ) |
| Provisions and other non-current liabilities | | (252 | ) | | (163 | ) | | 10 |
|
| | | (3,395 | ) | | (1,873 | ) | | (7,990 | ) |
19 Share and other capital
During the year ended December 31, 2014,The Company has an unlimited number of authorized common shares (being voting and participating shares) with no par value, as well as an unlimited number of preferred, first and second ranking shares, issuable in series, with rights and privileges specific to each class, with no par value.
Common shares issued in connection with "At-the-Market" ("ATM") drawdowns
April 2016 ATM Program
On April 1, 2016, the Company paid approximately $111,000 in income taxes in the form of foreign jurisdiction withholding tax on payments received pursuant to the agreements entered into with Sinopharm,an ATM sales agreement (the "April 1, 2016 ATM Program"), under which the Company was able, at its discretion and from time to time, to sell up to 3 million common shares through ATM issuances on the NASDAQ for aggregate gross proceeds of up to approximately $10 million. The April 2016 ATM Program provides that common shares were to be sold at market prices prevailing at the time of sale and, as discussed in note 5 – Development, commercializationa result, prices varied.
Between April 1, 2016 and license arrangements.March 24, 2017, the Company issued a total of 1,706,968 common shares under the April 2016 ATM Program at an average issuance price of $3.52 per share for aggregate gross proceeds of $6.0 million less cash transaction costs of $190 and previously deferred financing costs of $225.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
March 2017 ATM Program
Significant componentsOn March 28, 2017, the Company commenced a new ATM offering pursuant to its existing ATM Sales Agreement, dated April 1, 2016, under which the Company was able, at its discretion, from time to time, to sell up to a maximum of current3 million common shares through ATM issuances on the NASDAQ, up to an aggregate amount of $9.0 million (the "March 2017 ATM Program"). The common shares were to be sold at market prices prevailing at the time of the sale of the common shares and, as a result, sale prices varied.
Between March 28, 2017 and April 18, 2017, the Company issued a total of 597,994 common shares under the March 2017 ATM Program at an average issuance price of $2.97 per share for aggregate gross proceeds of $1,780,000 less cash transaction costs of $55 and previously deferred income tax expensefinancing costs of $65.
April 2017 ATM Program
On April 27, 2017, the Company entered into a New ATM Sales Agreement and filed with the Securities and Exchange Commission (the "SEC") a prospectus supplement (the "April 2017 ATM Prospectus Supplement" or "April 2017 ATM Program") related to sales and distributions of up to a maximum of 2.24 million common shares through ATM issuances on the NASDAQ, up to an aggregate amount of $6.9 million under the New ATM Sales Agreement. The common shares will be sold at market prices prevailing at the time of the sale of the common shares and, as a result, prices may vary. The New ATM Sales Agreement and the April 2017 ATM Program superseded and replaced the March 2017 ATM Program, which itself superseded and replaced the April 2016 ATM Program. The April 2017 ATM Prospectus Supplement supplements the base prospectus included in the Company's Shelf Registration Statement on Form F-3, as amended (the "2017 Shelf Registration Statement"), which was declared effective by the SEC on April 27, 2017. The 2017 Shelf Registration Statement allowed the Company to offer up to $50 million of common shares and is effective for a three-year period.
Between May 30, 2017 and December 31, 2017, the Company issued a total of 1,805,758 common shares under the April 2017 ATM Program at an average issuance price of $2.08 per share for aggregate gross proceeds of $3,761,000 less cash transaction costs of $115 and previously deferred financing costs of $285. Because of these issuances, the exercise price of the Series A warrants issued in March 2015 was adjusted to $1.07 pursuant to the anti-dilution provisions contained in such warrants.
Public offerings
November 2016 Offering
On November 1, 2016, the Company completed a registered direct offering of 2.1 million units (the "Units"), with each Unit consisting of one common share or one pre-funded warrant to purchase one common share and 0.45 of a warrant to purchase one common share (the "November 2016 Offering").
Total gross cash proceeds raised through the November 2016 Offering amounted to $7.6 million, less cash transaction costs of $1.0 million, and previously deferred transactions costs of $27. The warrants are as follows:exercisable six months after their date of issuance and for a period of three years thereafter at an exercise price of $4.70 per share.
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Current tax expense | | — |
| | 111 |
| | — |
|
| Deferred tax: | | | | | | |
| Origination and reversal of temporary differences | | 8,920 |
| | 10,246 |
| | (4,253 | ) |
| Adjustments in respect of prior years | | — |
| | 5 |
| | 418 |
|
| Change in unrecognized tax assets | | (8,920 | ) | | (10,251 | ) | | 3,835 |
|
| Income tax expense | | — |
| | 111 |
| | — |
|
The warrants contain a call provision which provides that, in the event the Company's common shares trade at or above $10 on the market during a specified measurement period and subject to a minimum volume of trading during such measurement period, then, subject to certain conditions, the Company has the right to call for cancellation all or any portion of the warrants which are not exercised by holders within 10 trading days following receipt of a call notice from the Company. Upon complete exercise for cash, these warrants would result in the issuance of an aggregate of 945,000 common shares that would generate additional proceeds of approximately $4.4 million, although these warrants may be exercised on a "net" or "cashless" basis. See also note 17 - Warrant liability.
The reconciliationCompany estimated the fair value attributable to the warrants as of the combined Canadian federaldate of grant by applying probability to multiple Black-Scholes pricing models, to which the following weighed average assumptions were applied: a risk-free annual interest rate of 0.63%, an expected volatility of 112.48%, an expected life of 1.63 years and provincial income tax ratea dividend yield of 0.0%. In addition, the Company reduced the fair value of these warrants to take into consideration the income tax expense is provided below:fair value of the $10.00 call option,
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| Combined Canadian federal and provincial statutory income tax rate | | 26.9 | % | | 26.9 | % | | 26.9 | % |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Income tax recovery (expense) based on combined statutory income tax rate | | 13,511 |
| | 4,426 |
| | (1,833 | ) |
| Change in unrecognized tax assets | | (8,581 | ) | | (10,251 | ) | | 3,835 |
|
| Permanent difference attributable to the use of local currency for tax reporting | | (1,297 | ) | | 145 |
| | (892 | ) |
| Permanent difference attributable to net change in fair value of warrant liability | | (3,754 | ) | | 4,408 |
| | (217 | ) |
| Share-based compensation costs | | (248 | ) | | (133 | ) | | (596 | ) |
| Difference in statutory income tax rate of foreign subsidiaries | | 1,135 |
| | 1,398 |
| | (809 | ) |
| Permanent difference attributable to expiring loss carry forward | | (563 | ) | | | | |
| Permanent difference attributable to unrealized foreign exchange gain/loss | | — |
| | 18 |
| | 131 |
|
| Foreign withholding tax | | — |
| | (111 | ) | | — |
|
| Adjustments in respect of prior years | | — |
| | 5 |
| | 418 |
|
| Other | | (203 | ) | | (16 | ) | | (37 | ) |
| | | — |
| | (111 | ) | | — |
|
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
which was also calculated using the Black-Scholes pricing model with similar assumptions as described above. As a result, on November 1, 2016, being the date of issuance, the total fair value of the share purchase warrants was estimated at $400.
The pre-funded warrants were offered in the November 2016 Offering to the investor because the purchase of Units would have resulted in the investor beneficially owning more than an "initial beneficial ownership limitation" of 4.9% of the Company's common shares following the offering. The pre-funded warrants, which were exercisable immediately upon issuance and for a period of five years at an exercise price of $3.60 per share, were fully exercised between November 10, 2016 and December 19, 2016. Total gross proceeds payable to the Company in connection with the exercise of the pre-funded warrants were pre-funded by the investor and therefore were included in the proceeds of the offering. No additional consideration was required to be paid to the Company upon exercise of the pre-funded warrants.
Total gross proceeds of the November 2016 Offering were allocated as follows: $400 was allocated to the warrant liability, $3,239 was allocated to the pre-funded warrants, and the balance of $3,921 was allocated to Share capital. Transaction costs were allocated to the liability and equity components in proportion to the allocation of proceeds. As such, an amount of $56 was allocated to the warrant liability and immediately recognized in general and administrative expenses in the consolidated statement of comprehensive income (loss), an amount of $544 was allocated to share capital and an amount of $450 was allocated to pre-funded warrants. Upon exercise of the pre-funded warrants, the net proceeds initially allocated to the pre-funded warrants were re-allocated to share capital.
Shareholder rights plan
The Company has a shareholder rights plan (the "Rights Plan") that provides the Board of Directors and the Company's shareholders with additional time to assess any unsolicited take-over bid for the Company and, where appropriate, to pursue other alternatives for maximizing shareholder value. Under the Rights Plan, one right has been issued for each currently issued common share, and one right will be issued with each additional common share that may be issued from time to time. The Rights Plan was approved, ratified and confirmed by the Company's shareholders at its annual meeting of shareholders held on May 10, 2016.
The Board of Directors reviewed the terms of the Existing Rights Plan for conformity with current Canadian securities laws, as well as the evolving practices of public corporations in Canada, with respect to shareholder rights plan design and has made some minor amendments thereto as a result. The Board of Directors determined it appropriate and in the best interests of the shareholders to continue the Rights Plan and approved the amended and restated rights plan (the "Rights Plan") on March 26, 2019. The Rights Plan will take effect immediately upon receipt of approval of the shareholders of the Corporation at the annual and special meeting of shareholders scheduled to be held on May 8, 2019.
Other capital
The Company accounts for costs associated with share-based compensation from security grants under its long-term incentive plan and stock option plans as other capital in its consolidated statements of changes in shareholders' equity (deficiency) and as general and administrative expenses in its consolidated statements of comprehensive income (loss).
Long-term incentive plan
At the 2018 annual and special meeting of shareholders, the Company's shareholders approved the adoption of the 2018 long-term incentive plan (the "LTIP"), which allows the Board of Directors to issue up to 11.4% of the total issued and outstanding common shares at any given time to eligible individuals at an exercise price to be determined by the Board of Directors at the time of the grant, subject to a ceiling, as stock options, stock appreciation rights, stock awards, stock units, performance shares, performance units, and other stock-based awards. This LTIP replaces the stock option plan (the "Stock Option Plan") for its directors, senior executives, employees and other collaborators who provide services to the Company. The Company's Board of Directors amended the Stock Option Plan on March 20, 2014 and the Company's Shareholders approved, ratified and confirmed the Stock Option Plan on May 10, 2016.
Options granted under the Stock Option Plan prior to the 2014 amendment expire after a maximum period of10 years following the date of grant. Options granted after the 2014 amendment expire after a maximum period of seven years following the date of grant.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
During 2018, the Company granted Deferred Share Units (DSU) and stock options.The following tables summarizes the activity under the LTIP and, previously, the Stock Option Plan:
|
| | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| US dollar-denominated options | | Number | | Weighted average exercise price (US$) | | Number | | Weighted average exercise price (US$) | | Number | | Weighted average exercise price (US$) |
| Balance – Beginning of the year | | 712,415 |
| | 4.66 |
| | 966,539 |
| | 7.23 |
| | 272,874 |
| | 25.88 |
|
| Granted | | 426,000 |
| | 1.74 |
| | 390,000 |
| | 2.05 |
| | 713,573 |
| | 3.47 |
|
| Forfeited | | (249,599 | ) | | 3.23 |
| | (643,271 | ) | | 6.02 |
| | (10,034 | ) | | 99.22 |
|
| Cancelled | | — |
| | — |
| | — |
| | — |
| | (9,874 | ) | | 157.11 |
|
| Expired | | — |
| | — |
| | (853 | ) | | 704.88 |
| | — |
| | — |
|
| Balance – End of period | | 888,816 |
| | 3.66 |
| | 712,415 |
| | 4.66 |
| | 966,539 |
| | 7.23 |
|
|
| | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| Canadian dollar-denominated stock options | | Number | | Weighted average exercise price (CAN$) | | Number | | Weighted average exercise price (CAN$) | | Number | | Weighted average exercise price (CAN$) |
| Balance – Beginning of the year | | 1,503 |
| | 605.84 |
| | 1,858 |
| | 820.27 |
| | 3,787 |
| | 845.46 |
|
| Forfeited | | (104 | ) | | 668.65 |
| | — |
| | — |
| | (1,028 | ) | | 967.63 |
|
| Cancelled | | — |
| | — |
| | — |
| | — |
| | (901 | ) | | 758 |
|
| Expired | | (530 | ) | | 367.70 |
| | (355 | ) | | 1,728.15 |
| | — |
| | — |
|
| Balance – End of the year | | 869 |
| | 743.56 |
| | 1,503 |
| | 605.84 |
| | 1,858 |
| | 820.27 |
|
|
| | | | | | | | | |
| | | Total US$ share-based awards as at December 31, 2018 |
Exercise price
(US$) | | Number | | Weighted average remaining
contractual life
(years) | | Weighted average exercise price
(US$) |
| 1.46 to 1.79 | | 211,000 |
| | 8.62 |
| | 1.71 |
|
| 1.80 to 2.11 | | 490,000 |
| | 6.41 |
| | 2.06 |
|
| 2.12 to 3.50 | | 157,148 |
| | 4.75 |
| | 3.46 |
|
| 3.51 to 4.58 | | 26,000 |
| | 3.97 |
| | 4.58 |
|
| 4.59 to 1,044.00 | | 4,668 |
| | 2.77 |
| | 260.87 |
|
| | | 888,816 |
| | 6.55 |
| | 3.66 |
|
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
|
| | | | | | | | | |
| | | Total exercisable US$ share-based awards as at December 31, 2018 |
Exercise price
(US$) | | Number | | Weighted average remaining
contractual life
(years) | | Weighted average exercise price
(US$) |
| 1.46 to 1.79 | | 161,000 |
| | 9.35 |
| | 1.79 |
|
| 1.80 to 2.11 | | 130,000 |
| | 5.62 |
| | 2.05 |
|
| 2.12 to 3.50 | | 104,774 |
| | 4.75 |
| | 3.46 |
|
| 3.51 to 4.58 | | 26,000 |
| | 3.97 |
| | 4.58 |
|
| 4.59 to 1,044.00 | | 4,668 |
| | 2.77 |
| | 260.87 |
|
| | | 426,442 |
| | 6.68 |
| | 5.29 |
|
|
| | | | | | | | | |
| | | CAN$ options outstanding and exercisable as at December 31, 2018 |
Exercise price
(CAN$) | | Number | | Weighted average remaining
contractual life (years) | | Weighted average exercise price
(CAN$) |
| 570.00 to 741.00 | | 428 |
| | 0.94 |
| | 570.00 |
|
| 741.01 to 912.00 | | 441 |
| | 1.87 |
| | 912.00 |
|
| | | 869 |
| | 1.41 |
| | 743.56 |
|
As at December 31, 2018, the total compensation cost related to unvested US Dollar stock options not yet recognized amounted to $198 ($444 in 2017). This amount is expected to be recognized over a weighted average period of 1.15 years (1.38 years in 2017).
The Company settles stock options exercised through the issuance of new common shares as opposed to purchasing common shares on the market to settle stock option exercises.
Fair value input assumptions for US dollar-denominated grants
The table below shows the assumptions, or weighted average parameters, applied to the Black-Scholes option pricing model in order to determine share-based compensation costs over the life of the awards. |
| | | | | | |
| | | Years ended December 31, |
| | | 2018 | | | 2017 | |
| Expected dividend yield | (a) | 0.00 | % | | 0.00 | % |
| Expected volatility | (b) | 129.23 | % | | 137.60 | % |
| Risk-free annual interest rate | (c) | 2.51 | % | | 1.53 | % |
| Expected life (years) | (d) | 3.60 | | | 3.26 | |
| Weighted average share price | | $1.74 | | | $2.05 | |
| Weighted average exercise price | | $1.74 | | | $2.05 | |
| Weighted average grant date fair value | | $1.39 | | | $1.62 | |
| |
| (a) | The Company has not paid dividends and it does not intend to pay dividends in the foreseeable future. |
| |
| (b) | Based on the historical volatility of the Company's stock price over the most recent period consistent with the expected life of the stock options, as well as on future expectations. |
| |
| (c) | Based on United States Treasury Government Bond interest rates with a term that is consistent with the expected life of the stock options. |
| |
| (d) | Based upon historical data related to the exercise of stock options, on post-vesting employment terminations and on future expectations related to exercise behavior. |
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
The Black-Scholes pricing models referred above use "Level 2" inputs in calculating fair value, as defined by IFRS 13, and as discussed in note 24 - Financial instruments and financial risk management.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
20 Operating expenses
The nature of the Company's operating expenses from continuing operations include the following: |
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
Key management personnel compensation(1) | | | | | | |
| Salaries and short-term employee benefits | | 2,388 |
| | 2,081 |
| | 2,430 |
|
| Consultants fees | | 62 |
| | — |
| | — |
|
| Termination benefits | | 356 |
| | — |
| | — |
|
| Post-employment benefits | | 147 |
| | 59 |
| | 78 |
|
| Share-based compensation costs | | 462 |
| | 87 |
| | 1,051 |
|
| | | 3,415 |
| | 2,227 |
| | 3,559 |
|
| Other employees compensation: | | | | | | |
| Salaries and short-term employee benefits | | 1,325 |
| | 3,584 |
| | 3,574 |
|
| Termination benefits (note 16) | | — |
| | 1,806 |
| | — |
|
| Post-employment benefits | | 275 |
| | 441 |
| | 500 |
|
| Share-based compensation costs | | 108 |
| | 95 |
| | 31 |
|
| | | 1,708 |
| | 5,926 |
| | 4,105 |
|
| Professional fees | | 6,421 |
| | 7,153 |
| | 7,157 |
|
| Insurance | | 1,303 |
| | 949 |
| | 870 |
|
| Third-party R&D | | 498 |
| | 3,758 |
| | 11,796 |
|
| Contracted sales force | | 256 |
| | 22 |
| | 14 |
|
| Travel | | 256 |
| | 831 |
| | 1,185 |
|
| Marketing services | | 176 |
| | 698 |
| | 5 |
|
| Laboratory supplies | | 139 |
| | 2 |
| | 30 |
|
| Other goods and services | | 342 |
| | 162 |
| | 160 |
|
Leasing costs, net of sublease receipts of $121 in 2018, $359 in 2017 and $345 in 2016(2) | | 344 |
| | 2,247 |
| | 1,131 |
|
| Transaction costs related to share purchase warrants | | — |
| | — |
| | 56 |
|
| Depreciation and amortization | | 60 |
| | 138 |
| | 195 |
|
| Impairment (reversal) losses | | — |
| | (44 | ) | | 85 |
|
| Operating foreign exchange (gains) losses | | 17 |
| | (72 | ) | | 39 |
|
| | | 9,812 |
| | 15,844 |
| | 22,723 |
|
| | | 14,935 |
| | 23,997 |
| | 30,387 |
|
| |
(1) | Key management includes the Company's executive management team and directors. |
| |
(2) | Leasing costs also include changes in the onerous lease provision (note 16 - provisions for restructuring and other costs), other than attributable to the unwinding of the discount. |
Most of the employment agreements entered into between the Company and its executive officers include termination provisions, whereby the executive officers would be entitled to receive benefits that would be payable if the Company were
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
to terminate the executive officers' employment without cause or if their employment is terminated following a change of control. Separation benefits generally are calculated based on an agreed-upon multiple of applicable base salary and incentive compensation and, in certain cases, other benefit amounts.
21 Supplemental disclosure of cash flow information
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Changes in operating assets and liabilities: | | | | | | |
| Trade and other receivables | | (95 | ) | | 158 |
| | 228 |
|
| Inventory | | 314 |
| | — |
| | — |
|
| Prepaid expenses and other current assets | | 448 |
| | (343 | ) | | (45 | ) |
| Other non-current assets | | 150 |
| | 39 |
| | (233 | ) |
| Payables and accrued liabilities | | (586 | ) | | (1,080 | ) | | (199 | ) |
| Taxes payable | | 1,669 |
| | — |
| | — |
|
| Deferred revenues | | 400 |
| | — |
| | 555 |
|
| Provision for restructuring and other costs (note 16) | | (1,957 | ) | | (435 | ) | | (911 | ) |
| Employee future benefits (note 18) | | (494 | ) | | (551 | ) | | (459 | ) |
| | | (151 | ) | | (2,212 | ) | | (1,064 | ) |
22 Income taxes
Significant components of current and deferred income tax expense (recovery) are as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Current tax (expense) recovery | | — |
| | — |
| | — |
|
| Deferred tax: | | | | | | |
| Origination and reversal of temporary differences | | (4,003 | ) | | 6,395 |
| | 9,199 |
|
| Adjustments in respect of prior years | | 742 |
| | (149 | ) | | 36 |
|
| Change in unrecognized tax assets | | (2,191 | ) | | (2,767 | ) | | (9,235 | ) |
| Income tax (expense) recovery | | (5,452 | ) | | 3,479 |
| | — |
|
The reconciliation of $111,000 for the year ended December 31, 2014 represents current taxation in the form of foreign jurisdictioncombined Canadian federal and provincial income tax withholdings on payments pursuantrate to the licensing agreement entered into with Sinopharm (note 5 – Development, commercialization and licensing arrangements).income tax expense is provided below:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| Combined Canadian federal and provincial statutory income tax rate | | 26.7 | % | | 26.8 | % | | 26.9 | % |
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Income tax (expense) recovery based on combined statutory income tax rate | | (2,574 | ) | | 5,434 |
| | 6,714 |
|
| Change in unrecognized tax assets | | (1,963 | ) | | (2,701 | ) | | (9,235 | ) |
| Change in unrecognized tax assets related to OCI | | (188 | ) | | (228 | ) | | 436 |
|
| Share issuance costs | | (40 | ) | | 164 |
| | 224 |
|
| Permanent difference attributable to the use of local currency for tax reporting | | 792 |
| | (71 | ) | | (30 | ) |
| Change in enacted rates used | | (58 | ) | | (358 | ) | | (16 | ) |
| Permanent difference attributable to net change in fair value of warrant liability | | 70 |
| | 595 |
| | 1,194 |
|
| Share-based compensation costs | | (152 | ) | | (49 | ) | | (291 | ) |
| Difference in statutory income tax rate of foreign subsidiaries | | (917 | ) | | 768 |
| | 972 |
|
| Adjustments in respect of prior years | | (372 | ) | | (149 | ) | | 36 |
|
| Other | | (50 | ) | | 74 |
| | (4 | ) |
| | | (5,452 | ) | | 3,479 |
| | — |
|
Deferred income tax assets are recognized to the extent that the realization of the related tax benefit through reversal of temporary differences and future taxable profits is probable.
LossIncome (loss) before income taxes
LossIncome (loss) before income taxes is attributable to the Company's tax jurisdictions as follows:
| | | | | Years ended December 31, | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ | | $ | | $ | | $ |
| Germany | | (20,500 | ) | | (29,672 | ) | | (19,784 | ) | | 16,297 |
| | (13,950 | ) | | (19,179 | ) |
| Canada | | (29,496 | ) | | 12,867 |
| | (7,639 | ) | | (5,504 | ) | | (5,592 | ) | | (5,659 | ) |
| United States | | (232 | ) | | (271 | ) | | 183 |
| | (1,154 | ) | | (733 | ) | | (121 | ) |
| | | (50,228 | ) | | (17,076 | ) | | (27,240 | ) | | 9,639 |
| | (20,275 | ) | | (24,959 | ) |
Significant components of deferred tax assets and liabilities are as follows:
|
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Deferred tax assets | | | | |
| Non-current: | | | | |
| Operating losses carried forward | | 1,355 |
| | 2,139 |
|
| Intangible assets | | 6,242 |
| | 7,918 |
|
| | | 7,597 |
| | 10,057 |
|
| Deferred tax liabilities | | | | |
| Current: | | | | |
| Deferred revenues | | 327 |
| | 941 |
|
| | | 327 |
| | 941 |
|
| Non-current: | | | | |
| Property, plant and equipment | | 9 |
| | 17 |
|
| Deferred revenues | | 6,868 |
| | 7,979 |
|
| Warrant liability | | 390 |
| | 1,116 |
|
| Other | | 3 |
| | 4 |
|
| | | 7,270 |
| | 9,116 |
|
| | | 7,597 |
| | 10,057 |
|
| Deferred tax assets (liabilities), net | | — |
| | — |
|
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Deferred tax assets | | | | |
| Current: | | | | |
| Operating losses carried forward | | — |
| | 3,479 |
|
| Non-current: | | | | |
| Operating losses carried forward | | 764 |
| | 696 |
|
| Intangible assets | | 3,646 |
| | 4,812 |
|
| | | 4,410 |
| | 8,987 |
|
| Deferred tax liabilities | | | | |
| Current: | | | | |
| Deferred revenues | | 38 |
| | — |
|
| Restricted cash | | 153 |
| | — |
|
| Payables and accrued liabilities | | 95 |
| | — |
|
| | | 286 |
| | — |
|
| Non-current: | | | | |
| Property, plant and equipment | | 3 |
| | 5 |
|
| Deferred revenues | | 4,074 |
| | 5,316 |
|
| Other | | 47 |
| | 187 |
|
| | | 4,124 |
| | 5,508 |
|
| | | 4,410 |
| | 5,508 |
|
| Deferred tax assets (liabilities), net | | — |
| | 3,479 |
|
Significant components of unrecognized deferred tax assets are as follows: | | | | | As at December 31, | | December 31, |
| | | 2015 | | 2014 | | 2018 | | 2017 |
| | | $ | | $ | | $ | | $ |
| Deferred tax assets | | | | | | | | |
| Current: | | | | | | | | |
| Onerous contract and other provisions | | 167 |
| | 102 |
| |
| Deferred revenues and other provisions | | | 649 |
| | 584 |
|
| | | 167 |
| | 102 |
| | 649 |
| | 584 |
|
| Non-current: | | | | | | | | |
| Deferred Revenues | | 155 |
| | — |
| |
| Deferred revenues | | | — |
| | — |
|
| Operating losses carried forward | | 64,471 |
| | 62,094 |
| | 81,731 |
| | 82,421 |
|
| Research and development costs | | 9,207 |
| | 10,987 |
| |
| SR&ED Pool | | | 9,148 |
| | 9,167 |
|
| Unused tax credits | | 7,977 |
| | 9,517 |
| | 5,894 |
| | 8,019 |
|
| Employee future benefits | | 1,919 |
| | 2,455 |
| | 2,048 |
| | 2,296 |
|
| Property, plant and equipment | | 219 |
| | 1,175 |
| | 448 |
| | 407 |
|
| Share issue expenses | | 1,226 |
| | 817 |
| |
| Onerous contract provisions | | 96 |
| | 198 |
| |
| Intangible assets | | 190 |
| | 227 |
| |
| Share issuance expenses | | | 467 |
| | 841 |
|
| Other | | 197 |
| | 296 |
| | 241 |
| | 335 |
|
| | | 85,657 |
| | 87,766 |
| | 99,977 |
| | 103,486 |
|
| Unrecognized deferred tax assets | | 85,824 |
| | 87,868 |
| | 100,626 |
| | 104,070 |
|
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
As at December 31, 2015,2018, amounts and expiry dates of tax attributes to be deferred for which no deferred tax asset was recognized were as follows:
| | | | | Canada | | Canada |
| | | Federal | | Provincial | | Federal | | Provincial |
| | | $ | | $ | | $ | | $ |
| 2028 | | 6,592 |
| | 5,206 |
| | 7,880 |
| | 6,494 |
|
| 2029 | | 4,791 |
| | 4,773 |
| | 4,791 |
| | 4,773 |
|
| 2030 | | 4,105 |
| | 4,089 |
| | 4,104 |
| | 4,089 |
|
| 2031 | | 1,753 |
| | 1,738 |
| | 1,753 |
| | 1,737 |
|
| 2032 | | 4,250 |
| | 4,250 |
| | 4,250 |
| | 4,250 |
|
| 2033 | | 3,721 |
| | 3,721 |
| | 3,721 |
| | 3,721 |
|
| 2034 | | 4,154 |
| | 4,154 |
| | 4,153 |
| | 4,153 |
|
| 2035 | | 9,587 |
| | 9,625 |
| | 10,418 |
| | 10,452 |
|
| 2036 | | | 10,592 |
| | 10,592 |
|
| 2037 | | | 7,343 |
| | 7,343 |
|
| 2038 | | | 6,557 |
| | 6,557 |
|
| | | 38,953 |
| | 37,556 |
| | 65,562 |
| | 64,161 |
|
The Company has estimated non-refundable R&D investment tax credits of approximately $7,977,000$5,894 which can be carried forward to reduce Canadian federal income taxes payable and which expire at dates ranging from 20182019 to 2034.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
2038. Furthermore, the Company has unrecognized tax assets in respect of operating losses to be carried forward in Germany and in the United States. The federal tax losses amount to approximately $171,064,000$205,343 in Germany (EUR 173,733) for which there is no expiry date, and to $1,145,000$3,322 in the United States, which expire as follows:
| | | | | United States | | United States |
| | | $ | | $ |
| 2028 | | 369 |
| | 369 |
|
| 2029 | | 178 |
| | 178 |
|
| 2034 | | 151 |
| | 151 |
|
| 2035 | | 447 |
| | 447 |
|
| 2036 | | | 195 |
|
| 2037 | | | 709 |
|
| 2038 | | | 1,273 |
|
| | | 1,145 |
| | 3,322 |
|
The operating loss carryforwards and the tax credits claimed are subject to review, and potential adjustment, by tax authorities.
Other deductible temporary differences for which tax assets have not been booked are not subject to a time limit, except for share issueissuance expenses which are amortizable over five years.
23 Capital disclosures
The Company's objective in managing capital, consisting of shareholders' equity, with cash and cash equivalents and restricted cash equivalents being its primary components, is to ensure sufficient liquidity to fund R&D activities,costs, selling andexpenses, G&A expenses and working capital and capital expenditures.requirements.
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
Over the past several years, the Company has increasingly raised capital via public equity offerings and drawdownsissuances under various ATM sales programs as its primary source of liquidity, as discussed in note 17 –19 - Share and other capital.
The capital management objective of the Company remains the same as that in previous periods. The policy on dividends is to retain cash to keep funds available to finance the activities required to advance the Company's product development portfolio and to pursue appropriate commercial opportunities as they may arise.
The Company is not subject to any capital requirements imposed by any regulators or by any other external source.
| |
24 | Financial instruments and financial risk management |
24 Financial instruments and financial risk management
Financial assets (liabilities) as at December 31, 20152018 and December 31, 20142017 are presented below.
|
| | | | | | | | | | | | |
| December 31, 2015 | | Loans and
receivables | | Financial
liabilities at
FVTPL | | Other
financial
liabilities | | Total |
| | | $ | | $ | | $ | | $ |
| Cash and cash equivalents (note 7) | | 41,450 |
| | — |
| | — |
| | 41,450 |
|
| Trade and other receivables (note 8) | | 297 |
| | — |
| | — |
| | 297 |
|
| Restricted cash equivalents (note 9) | | 255 |
| | — |
| | — |
| | 255 |
|
| Payables and accrued liabilities (note 13) | | — |
| | — |
| | (3,837 | ) | | (3,837 | ) |
| Provision for restructuring costs (note 14) | | — |
| | — |
| | (625 | ) | | (625 | ) |
| Warrant liability including current portion (note 15) | | — |
| | (10,891 | ) | | — |
| | (10,891 | ) |
| Other non-current liabilities (note 16) | | — |
| | — |
| | (98 | ) | | (98 | ) |
| | | 42,002 |
| | (10,891 | ) | | (4,560 | ) | | 26,551 |
|
|
| | | | | | | | | | | |
| December 31, 2018 | Financial assets at amortized cost | | Financial liabilities at FVTPL | | Financial liabilities at amortized cost | | Total |
| | $ | | $ | | $ | | $ |
| Cash and cash equivalents (note 7) | 14,512 |
| | — |
| | — |
| | 14,512 |
|
| Trade and other receivables (note 8) | 245 |
| | — |
| | — |
| | 245 |
|
| Restricted cash equivalents (note 11) | 418 |
| | — |
| | — |
| | 418 |
|
| Payables and accrued liabilities (note 15) | — |
| | — |
| | (2,940 | ) | | (2,940 | ) |
| Provision for restructuring and other costs (note 16) | — |
| | — |
| | (1,298 | ) | | (1,298 | ) |
| Warrant liability (note 17) | — |
| | (3,634 | ) | | — |
| | (3,634 | ) |
| | 15,175 |
| | (3,634 | ) | | (4,238 | ) | | 7,303 |
|
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
|
| | | | | | | | | | | | |
| December 31, 2014 | | Loans and receivables | | Financial liabilities at FVTPL | | Other financial liabilities | | Total |
| | | $ | | $ | | $ | | $ |
| Cash and cash equivalents (note 7) | | 34,931 |
| | — |
| | — |
| | 34,931 |
|
| Trade and other receivables (note 8) | | 796 |
| | — |
| | — |
| | 796 |
|
| Restricted cash equivalents (note 9) | | 760 |
| | — |
| | — |
| | 760 |
|
| Payables and accrued liabilities (note 13) | | — |
| | — |
| | (5,256 | ) | | (5,256 | ) |
| Provision for restructuring costs (note 14) | | — |
| | — |
| | (1,105 | ) | | (1,105 | ) |
| Warrant liability (note 15) | | — |
| | (8,225 | ) | | — |
| | (8,225 | ) |
| Other non-current liabilities (note 16) | | — |
| | — |
| | (130 | ) | | (130 | ) |
| | | 36,487 |
| | (8,225 | ) | | (6,491 | ) | | 21,771 |
|
|
| | | | | | | | | | | | |
| December 31, 2017 | | Financial assets at amortized cost | | Financial liabilities at FVTPL | | Financial liabilities at amortized cost | | Total |
| | | $ | | $ | | $ | | $ |
| Cash and cash equivalents (note 7) | | 7,780 |
| | — |
| | — |
| | 7,780 |
|
| Trade and other receivables (note 8) | | 35 |
| | — |
| | — |
| | 35 |
|
| Restricted cash equivalents (note 11) | | 381 |
| | — |
| | — |
| | 381 |
|
| Payables and accrued liabilities (note 15) | | — |
| | — |
| | (2,687 | ) | | (2,687 | ) |
| Provision for restructuring and other costs (note 16) | | — |
| | — |
| | (3,497 | ) | | (3,497 | ) |
| Warrant liability (note 17) | | — |
| | (3,897 | ) | | — |
| | (3,897 | ) |
| | | 8,196 |
| | (3,897 | ) | | (6,184 | ) | | (1,885 | ) |
Fair value
TheAs discussed in note 17 - Warrant liability, the Black-Scholes valuation methodology uses "Level 2" inputs in calculating fair value, as defined in IFRS 7,13, which establishes a hierarchy that prioritizes the inputs used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement). The input levels discussed in IFRS 713 are:
Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities.
| |
Level 1 – | Unadjusted quoted prices in active markets for identical assets or liabilities. |
| |
Level 2 – | Inputs other than quoted prices included within Level 1 that are observable for an asset or liability, either directly (i.e. prices) or indirectly (i.e. derived from prices). |
| Aeterna Zentaris Inc. |
Level 3 –Notes to Consolidated Financial Statements |
InputsAs at December 31, 2018 and December 31, 2017 and for an asset or liability that are not based on observable marketthe years ended December 31, 2018, 2017 and 2016 |
(tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data (unobservable inputs).and as otherwise noted) |
Level 2 – Inputs other than quoted prices included within Level 1 that are observable for an asset or liability, either directly (i.e. prices) or indirectly (i.e. derived from prices).
Level 3 – Inputs for an asset or liability that are not based on observable market data (unobservable inputs).
The carrying values of the Company's cash and cash equivalents, trade and other receivables, restricted cash equivalents, payables and accrued liabilities and provision for restructuring costs and other non-current liabilitiescosts approximate their fair values due to their short-term maturities or to the prevailing interest rates of the related instruments, which are comparable to those of the market.
Financial risk factors
The following provides disclosures relating to the nature and extent of the Company's exposure to risks arising from financial instruments, including credit risk, liquidity risk, and market risk (share price risk), and foreign exchange risk and how the Company manages those risks.
(a) Credit risk
Credit risk is the risk of an unexpected loss if a customer or counterparty to a financial instrument fails to meet its contractual obligations. The Company regularly monitors credit risk exposure and takes steps to mitigate the likelihood of this exposure resulting in losses. The Company's exposure to credit risk currently relates to cash and cash equivalents, trade and other receivables and restricted cash equivalents.the financial assets at amortized cost in the table above. The Company holds its available cash in amounts that are readily convertible to known amounts of cash and deposits its cash balances with financial institutions that have an investment grade credit rating of at least "A""P-2" or the equivalent. This information is supplied by independent rating agencies where available and, if not available, the Company uses publicly available financial information to ensure that it invests its cash in creditworthy and reputable financial institutions. Once there are indicators that there is no reasonable expectation of recovery, such financial assets are written off but are still subject to enforcement activity.
As at December 31, 2015,2018, trade accounts receivable for an amount of approximately $122,000$197 were with twofour counterparties and no trade accounts receivable wereof which $55 was past due or impaired.
impaired and fully provided for (2017 - $25 with three counterparties and $5 past due or impaired and fully provided for).The licensee is obligated to pay its quarterly royalties, 60 days after quarter-end.
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Generally, the Company does not require collateral or other security from customers for trade accounts receivable; however, credit is extended following an evaluation of creditworthiness. In addition, the Company performs ongoing credit reviews of all of its customers and establishes an allowance for doubtful accounts when accounts are determined to be uncollectible. On this basis, as at December 31, 2018, the Company has provided for all outstanding and unpaid amounts relating to its operations before its licensing of MacrilenTM(macemorelin). The licensee has paid all amounts owing within 90 days of invoicing.
The maximum exposure to credit risk approximates the amount recognized in the Company's consolidated statement of financial position.
(b) Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due. As indicated in note 23 –- Capital disclosures, the Company manages this risk through the management of its capital structure. It also manages liquidity risk by continuously monitoring actual and projected cash flows.flows as further discussed in note 2 - Assessment of liquidity and management's plans. The Board of Directors reviews and approves the Company's operating and capital budgets, as well as any material transactions occurring outside of the ordinary course of business. The Company has adopted an investment policy in respect of the safety and preservation of its capital to ensure the Company's liquidity needs are met. The instruments are selected with regard to the expected timing of expenditures and prevailing interest rates.
The Company expects to continue to incur operating expenses and may require significant capital to fulfill its future obligations in absence of sufficient corresponding revenues. The Company's ability to continue future operations beyond December 31, 2016 and to fund its activities is dependent on its ability to secure additional financings, which may be completed in a number of ways, including but not limited to licensing arrangements, partnerships, promotional arrangements, the issuance of securities and other financing activities. Management will pursue such additional sources of financing when required, and while the Company has been successful in securing financing in the past, there can be no assurance it will be able to do so in the future or that these sources of funding or initiatives will be available or on terms acceptable to the Company.(c) Market risk
| |
(c) |
Market riskAeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
Share price risk
The change in fair value of the Company's warrant liability, which is measured at FVTPL, results from the periodic "mark-to-market" revaluation via the application of the intrinsic valuation and the Black-Scholes option pricing model. Theseas further described in note 15 as it applies to its outstanding share purchase warrants. The valuation models are impacted, among other inputs, by the market price of the Company's common shares. As a result, the change in fair value of the warrant liability, which is reported as finance income (costs) in the accompanying consolidated statements of comprehensive income (loss) income,, has been and may continue in future periods to be materially affected most notably by changes in the Company's common share closing price, which on the NASDAQ has ranged from $4.00$1.19 to $84.20 $3.87during the year ended December 31, 2015.2018.
If variations in the market price of our common shares of -10%-30% and +10%+30% were to occur, the impact on the Company's net (loss)income related to the warrant liability held at December 31, 20152018 would be as follows:
|
| | | | | | | | | |
| | | Carrying
amount | | -10% | | +10% |
| | | $ | | $ | | $ |
| Warrant liability, including current portion | | 10,891 |
| | 1,059 |
| | (1,067 | ) |
| Total impact on net loss – decrease / (increase) | | | | 1,059 |
| | (1,067 | ) |
|
| | | | | | | | | |
| | | Carrying
amount | | -30% | | +30% |
| | | $ | | $ | | $ |
| Warrant liability | | 3,634 |
| | 1,792 |
| | (1,504 | ) |
| Total impact on net income – (decrease) / increase | | | | 1,792 |
| | (1,504 | ) |
(d) Foreign exchange risk
Entities using the Euro as their functional currency
The Company is exposed to foreign exchange risk due to its investments in foreign operations whose functional currency is the Euro. As at December 31, 2018, if the US dollar had increased or decreased by 10% against the Euro, with all variables held constant, net income for the year ended December 31, 2018 would have been lower or higher by approximately $1,134 (2017 - $1,087).
25 Segment information
The Company operates in a single operating segment, being the biopharmaceutical segment.
Geographical information
Revenues by geographical area are detailed as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Ireland | | 24,910 |
| | — |
| | — |
|
| United States | | 1,416 |
| | 452 |
| | 410 |
|
| China | | 275 |
| | 262 |
| | 249 |
|
| Singapore | | — |
| | — |
| | 101 |
|
| British Virgin Islands | | 280 |
| | 206 |
| | 100 |
|
| Other | | — |
| | 3 |
| | 51 |
|
| | | 26,881 |
| | 923 |
| | 911 |
|
Revenues have been allocated to geographic regions based on the country of residence of the Company's external customers or licensees.
Non-current assets* by geographical area are detailed as follows:
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
|
| | | | | | |
| | | December 31, |
| | | 2018 | | 2017 |
| | | $ | | $ |
| Germany | | 8,599 |
| | 12,552 |
|
| United States | | 153 |
| | 102 |
|
| Canada | | 3 |
| | 160 |
|
| | | 8,755 |
| | 12,814 |
|
| |
25* | CommitmentsNon-current assets include property, plant and contingenciesequipment, identifiable intangible assets and goodwill. |
Major customers representing 10% or more of the Company's revenues in each of the last three years are as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Company 1 | | 26,127 |
| | — |
| | — |
|
| Company 2 | | — |
| | — |
| | 20 |
|
| Company 3 | | 275 |
| | 262 |
| | 249 |
|
| Company 4 | | — |
| | 323 |
| | 222 |
|
| Company 5 | | — |
| | 129 |
| | 167 |
|
| Company 6 | | — |
| | — |
| | 101 |
|
| Company 7 | | 280 |
| | 206 |
| | 100 |
|
26 Net income (loss) per share
The following table sets forth pertinent data relating to the computation of basic and diluted net income (loss) per share attributable to common shareholders.
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2018 | | 2017 | | 2016 |
| | | $ | | $ | | $ |
| Net income (loss) | | 4,187 |
| | (16,796 | ) | | (24,959 | ) |
| Basic weighted average number of shares outstanding | | 16,440,760 |
| | 14,958,704 |
| | 10,348,879 |
|
| Diluted weighted average number of shares outstanding | | 17,034,812 |
| | 14,958,704 |
| | 10,348,879 |
|
| Items excluded from the calculation of diluted net income (loss) per share because the exercise price was greater than the average market price of the common shares or due to their anti-dilutive effect | |
| |
| |
|
| Stock options | | 889,685 |
| | 713,918 |
| | 968,397 |
|
| Share purchase warrants | | 3,391,844 |
| | 3,417,840 |
| | 3,779,245 |
|
Net income (loss) per share is calculated by dividing net income (loss) by the weighted average number of shares outstanding during the relevant period. Diluted weighted average number of shares reflects the dilutive effect of equity instruments, such as any "in the money" stock options and share purchase warrants. In periods with reported net losses, all stock options and
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
| As at December 31, 2018 and December 31, 2017 and for the years ended December 31, 2018, 2017 and 2016 |
| (tabular amounts in thousands of US dollars, except share/option/warrant/DSU and per share/option/warrant/DSU data and as otherwise noted) |
share purchase warrants are deemed anti-dilutive such that basic net loss per share and diluted net loss per share are equal, and thus "in the money" stock options and share purchase warrants have not been included in the computation of net loss per share because to do so would be anti-dilutive.
27 Commitments and contingencies
The Company is committed to various operating leases for its premises. Expected future minimum lease payments, which also include future payments in connection with utility service agreements and future minimum sublease receipts under non-cancellable operating leases (subleases), as well as future payments in connection with service and manufacturing agreements, as at December 31, 20152018 are as follows:
|
| | | | | | | | | |
| | | Minimum lease payments | | Minimum sublease receipts | | Service and manufacturing |
| | | $ | | $ | | $ |
| Less than 1 year | | 1,367 |
| | (385 | ) | | 639 |
|
| 1 - 3 years | | 2,394 |
| | (487 | ) | | 370 |
|
| 4 - 5 years | | 1,837 |
| | (23 | ) | | — |
|
| More than 5 years | | 286 |
| | — |
| | — |
|
| Total | | 5,884 |
| | (895 | ) | | 1,009 |
|
During the quarter, the Company's lease agreement in Germany for laboratory, office, and storage space was terminated, and the Company entered into a new lease agreement for the rental of less space on the same premises as compared to the Company's former arrangement. The new lease expires on April 30, 2021 and is subject to renewal upon notice by the Company for two additional four-year periods. Under the terms of the arrangement, the minimum lease payment may be increased or decreased in accordance with the fluctuations in the German consumer price index up to 5% on a cumulative basis. |
| | | | | | | | | | | | |
| | | Minimum lease payments | | Minimum sublease receipts | | Service and manufacturing | | Total |
| | | $ | | $ | | $ | | $ |
| Less than 1 year | | 408 |
| | (117 | ) | | 2,180 |
| | 2,471 |
|
| 1 - 3 years | | 533 |
| | (24 | ) | | — |
| | 509 |
|
| 4 - 5 years | | 60 |
| | — |
| | — |
| | 60 |
|
| More than 5 years | | 5 |
| | — |
| | — |
| | 5 |
|
| Total | | 1,006 |
| | (141 | ) | | 2,180 |
| | 3,045 |
|
Contingencies
In the normal course of operations, the Company may become involved in various claims and legal proceedings related to, for example, contract terminations and employee-related and other matters. No contingent liabilities have been accrued as at December 31, 2015 or 2014.
Class Action LawsuitSecurities class action lawsuit
The Company and certain of its current and former officers are defendants in a class-action lawsuit pending in the United StatesU.S. District Court for the District of New Jersey, (the "Court"), brought on behalf of shareholders of the Company. The pending lawsuit is the result of the consolidation of several lawsuits, the first of which was filed on November 11, 2014. The plaintiffs filed their amended consolidated complaint on April 10, 2015. The amended complaint allegedalleges violations of the Securities Exchange Act of 1934 in connection with allegedly false and misleading statements made by the defendants between August 30, 2011 and November 6, 2014 (the "Class Period"), regarding the safety and efficacy of Macrilen™, a product that the Company developed for use in the diagnosis of adult growth hormone deficiency, (macimorelin) and the prospects for the approval of the Company's new drug applicationNew Drug Application for the product by the US Food and Drug Administration.FDA. The plaintiffs seek to represent a class comprised of purchasers of the Company's common shares during the Class Period and seek unspecified damages, costs and expenses and such other relief as determined by the court.
On September 14, 2015,Court. The Company considers the Court dismissedclaims that have been asserted in the lawsuit but grantedto be without merit and is vigorously defending against them. The Company cannot, however, predict at this time the plaintiffs leaveoutcome or potential losses, if any, with respect to amend. this lawsuit.
Other lawsuits
In dismissinglate July 2017, the lawsuit,Company terminated for cause the Court stated that "takingemployment agreement of Mr. David A. Dodd, the complaint as a whole, plaintiffs have failed to state a claim" underformer President and Chief Executive Officer and it also terminated the Private Securities Litigation Reform Actemployment of 1995 or Rule 9 ofMr. Philip A. Theodore, the Federal Rules of Civil Procedure.former Senior Vice President, Chief Administrative Officer, General Counsel and Corporate Secretary. On October 14, 2015,August 3, 2017, the plaintiffs filed a Second Amended Complaint against the Company. The Company filed a motionlawsuit against both Messrs. Dodd and Theodore for damages suffered by the Company for breach of confidence and/or breach of fiduciary duty in an amount to dismissbe determined prior to trial. On December 21, 2017, Messrs. Dodd and Theodore brought a counterclaim against the Second Amended Complaint on November 11, 2015, because management believesCompany and its Chair, Carolyn Egbert, in the amount of CAN$6.0 million alleging, among other things, that defamatory statements were made against Messrs. Dodd and Theodore. On December 21, 2018, the Second Amended Complaint also fails to state a claim. See note 28 – Subsequent event.
The Company's directors' and officers' insurance policies ("D&O Insurance") provide for reimbursement of certain costs and expenses incurred in connectionmatter was amicably resolved with the defenseCompany agreeing to make a payment to Mr. Dodd in the amount of this$775. The parties consider their contractual relationship as having been terminated.
Cogas Consulting, LLC ("Cogas") filed a lawsuit including legal and professional fees, as well as other loss (damages, settlements, and judgments), if any, subjectagainst the Company in state court in Fulton County, Georgia on February 2, 2018. The lawsuit was removed to certain policy exclusions, restrictions, limits, deductibles and other terms. The Company believesfederal court in Georgia. In the lawsuit, Cogas alleged that its employee (and sole shareholder) John Sharkey was entitled to a "success fee" commission on the D&O Insurance applies to the purported lawsuit; however, the insurers haveStrongbridge License Agreement. Cogas was
|
|
| Aeterna Zentaris Inc. |
| Notes to Consolidated Financial Statements |
As at December 31, 20152018 and December 31, 20142017 and for the years ended December 31, 2015, 20142018, 2017 and 20132016 |
(tabular amounts in thousands of US dollars, except share/option/warrantwarrant/DSU and per share/option/warrantwarrant/DSU data and as otherwise noted) |
issued standard reservationsclaiming damages in the form of rights letters reserving all rights undera lost commission on the D&O Insurance. Legal and professional fees are expensed as incurred, and no reservetransaction. Cogas claims its commission is established for them.
While5% on payments the Company believes that it has meritorious defenses and intends to defend this purported lawsuit vigorously, management cannot currently predictreceives within the outcomefirst three years after January 14, 2018 including 5% of this suit or reasonably estimate any potential loss that may result from this suit. Accordingly,the $24.0 million Strongbridge already paid the Company, has not recordedplus 5% of any liability related to the lawsuit. No assurance can be given with respect to the ultimate outcome of such proceedings, androyalty Strongbridge pays the Company could incur substantial unreimbursed legal fees, damages, settlements, judgments,through January 17, 2021. On November 5, 2018, the matter was amicably resolved with the Company agreeing to make a payment to Cogas in the amount of $625. The parties now consider their contractual relationship as having been terminated.
28 Reclassification on comparative figures
To consolidate the presentation of similar items, during 2018, the Company reclassified certain of its prior year comparative balance sheet items as follows:
Prepaid expenses and other expenses in connection with these proceedingscurrent assets
The semi-finished goods inventory of $87 that may not qualify for coverage under, or may exceed the limits of, its applicable D&O Insurance and could have a material adverse impact on the Company's financial condition, results of operations, liquidity, and cash flows.
| |
26 | Net (loss) income per share |
The following table sets forth pertinent data relating to the computation of basic and diluted net (loss) income per share attributable to common shareholders.
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Net loss from continuing operations | | (50,228 | ) | | (17,187 | ) | | (27,240 | ) |
| Net income from discontinued operations | | 85 |
| | 623 |
| | 34,055 |
|
| Net (loss) income | | (50,143 | ) | | (16,564 | ) | | 6,815 |
|
| Basic weighted average number of shares outstanding | | 2,763,603 |
| | 590,247 |
| | 294,765 |
|
| Dilutive effect of stock options | | 5,094 |
| | — |
| | — |
|
| Dilutive effect of share purchase warrants | | 655,639 |
| | — |
| | — |
|
| Diluted weighted average number of shares outstanding | | 3,424,336 |
| | 590,247 |
| | 294,765 |
|
| Items excluded from the calculation of diluted net (loss) income per share because the exercise price was greater than the average market price of the common shares or due to their anti-dilutive effect. | |
| |
| |
|
| Stock options | | 36,661 |
| | 23,242 |
| | 21,155 |
|
| Warrants (number of equivalent shares) | | 55,671 |
| | 287,852 |
| | 71,419 |
|
For the year endedwas classified as inventory as at December 31, 2015, the diluted net loss per share2017 has been reclassified to prepaid expenses and other current assets as at December 31, 2018.
Provision for restructuring and other costs
The current portion of onerous contract provisions of $173 that was the sameclassified as the basic net loss per share, since the effectpayables and accrued liabilities as at December 31, 2017 has been reclassified to provision for restructuring and other costs as at December 31, 2018.
The full balance of the assumed exerciseprovisions, comprising $310 of stock optionsonerous contract provisions and warrants$718 of non-current portion of provision for restructuring costs, as at December 31, 2017 has been reclassified to purchase common shares is anti-dilutive. Accordingly, the diluted net loss per shareprovision for this period was calculated using the basic weighted average number of shares outstanding.restructuring and other costs at December 31, 2018.
The weighted average number of shares is influenced most notably by share issuances made in connection with financing activities, such as registered direct and public offerings and ATM drawdowns, which resulted in the issuance of a total of 3,250,481 common shares during the year (201,971 and 199,827 common shares during the years ended December 31, 2014 and 2013, respectively). Additionally, during the year ended December 31, 2015, 6,023,125 common shares were issued in connection with the exercise of warrants (see note 15 - Warrant liability and note 17 - Share capital).
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
The Company operates in a single operating segment, being the biopharmaceutical segment.
Geographical information
Revenues by geographical area are detailed as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| United States | | 217 |
| | 6 |
| | 33,640 |
|
| Switzerland | | 312 |
| | 956 |
| | 34,081 |
|
| Japan | | 18 |
| | 61 |
| | 6,586 |
|
| China | | 302 |
| | — |
| | — |
|
| Other | | 27 |
| | 25 |
| | 212 |
|
| | | 876 |
| | 1,048 |
| | 74,519 |
|
| Amounts presented: | | | | | | |
| Within discontinued operations | | 331 |
| | 1,037 |
| | 68,344 |
|
| Within continuing operations | | 545 |
| | 11 |
| | 6,175 |
|
| | | 876 |
| | 1,048 |
| | 74,519 |
|
Revenues have been allocated to geographic regions based on the country of residence of the Company's external customers or licensees.
Non-current assets* by geographical area are detailed as follows:
|
| | | | | | |
| | | As at December 31, |
| | | 2015 | | 2014 |
| | | $ | | $ |
| Germany | | 8,280 |
| | 9,778 |
|
| Canada | | 49 |
| | 58 |
|
| | | 8,329 |
| | 9,836 |
|
_______________________________
* Non-current assets include property, plant and equipment, identifiable intangible assets and goodwill.
Major customers representing 10% or more of the Company's revenues in each of the last three years are as follows:
|
| | | | | | | | | |
| | | Years ended December 31, |
| | | 2015 | | 2014 | | 2013 |
| | | $ | | $ | | $ |
| Company 1* | | 312 |
| | 956 |
| | 34,081 |
|
| Company 2* | | — |
| | — |
| | 33,640 |
|
| Company 3 | | — |
| | — |
| | 5,952 |
|
| Company 4 | | 217 |
| | — |
| | — |
|
| Company 5 | | 302 |
| | — |
| | — |
|
*Related to the Cetrotide® Business (see note 6 – Discontinued operations).
|
|
Aeterna Zentaris Inc. |
Notes to Consolidated Financial Statements |
As at December 31, 2015 and December 31, 2014 and for the years ended December 31, 2015, 2014 and 2013 |
(tabular amounts in thousands of US dollars, except share/option/warrant and per share/option/warrant data and as otherwise noted) |
Class Action Lawsuit
The hearing of the motion to dismiss the Second Amended Complaint occurred on January 19, 2016. On March 2, 2016, the Court issued an order granting the Company's motion to dismiss the complaint in part and denying it in part. The Court dismissed certain of the Company's' current and former officers from the lawsuit. The Court allowed the claim that the Company omitted material facts from public statements during the Class Period to proceed against the Company and the former CEO who departed in 2013, while dismissing such claims against other current and former officers. The Court also allowed a claim for “controlling person” liability to proceed against certain current and former officers. The Company disagrees with the Court's decision and filed a motion for reconsideration on March 16, 2016.
|
| | |
| 1.1 | | |
| 1.2 | | |
| 1.3 | | |
| 1.4 | | |
| 2.1 | | |
| 2.2 | | |
| 4.1 | | |
| 4.2 | | |
| 4.3 | | |
| 4.4 | | |
| 4.5 | | |
4.34.6 | | |
4.44.7 | | Service Contract dated January 1, 2014 between Richard Sachse, MD and Aeterna Zentaris GmbH, a subsidiary |
4.5 | | EmploymentControl Agreement dated November 11, 2013March 5, 2018 between Keith SantorelliJames Clavijo and a subsidiary of the Registrant (incorporated by reference to Exhibit 4.5 of the Registrant's Annual Report on Form 20-F for the financial year ended December 31, 2014 filed with the Commission on March 17, 2015) |
4.6 | | Amendment #1 to Employment Agreement dated May 29, 2014 between a subsidiary of the Registrant and Keith Santorelli (incorporated by reference to Exhibit 4.6 of the Registrant's Annual Report on Form 20-F for the financial year ended December 31, 2014 filed with the Commission on March 17, 2015) |
4.7 | | Amendment #2 to Employment Agreement, dated October 9, 2015, between a subsidiary of the Registrant and Keith Santorelli |
4.8 | | Transition letter agreement, dated October 9, 2015, between a subsidiary of the Registrant and Keith Santorelli |
4.9 | | Amendment to Amended Employment Agreement dated as at June 20, 2007 among the Registrant, Aeterna Zentaris, Inc. and Dennis Turpin (incorporated by reference to Exhibit 4.8 of the Registrant's Annual Report on Form 20-F for the financial year ended December 31, 20072017 filed with the Commission on March 28, 2008)2018) |
4.104.8 | | Termination of |
4.114.9 | | |
4.124.10 | | |
4.134.11 | | |
| 4.12 | | |
| 4.13 | | |
| 8.1 | | |
| 11.1 | | |
| 11.2 | | |
| 11.3 | | Audit Committee Charter of the Registrant (incorporated(incorporated by reference to Exhibit 11.3 of the Registrant's Annual Report on Form 20F for the financial year ended December 31, 2014 filed with the Commission on March 17, 2015) |
| 12.1 | | |
| 12.2 | | |
| 13.1 | | |
| 13.2 | | |
| 15.1 | | |
101. INS XBRL Instance Document
145101. SCH XBRL Taxonomy Extension Schema
101. CAL XBRL Taxonomy Extension Schema Calculation Linkbase
101. DEF XBRL Taxonomy Extension Schema Definition Linkbase
101. LAB XBRL Taxonomy Extension Schema Label Linkbase
101. PRE XBRL Taxonomy Extension Schema Presentation Linkbase
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
|
| AETERNA ZENTARIS INC. |
| |
/s/ David A. DoddMichael V. Ward |
| |
David A. DoddMichael V. Ward |
Chairman, President and Chief Executive Officer |
Date: March 29, 20162019