UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20212023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report
For the transition period from __________ to __________
Commission file number 001-40173
Steakholder Foods Ltd.
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
Israel
(Jurisdiction of incorporation or organization)
5 David Fikes St., P.O. Box 4061
Rehovot 7638205 Israel
(Address of principal executive offices)
+
info@meatech3d.com
info@steakholderfoods.com
5 David Fikes St., Rehovot 7638205 Israel
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||
American Depositary Shares, each representing | STKH | The Nasdaq Stock Market LLC | ||||
Ordinary shares, no par value per share | ⸺ | The Nasdaq Stock Market LLC* |
| * | Listed not for trading or quotation purposes, but only in connection with the registration of American Depositary Shares representing such ordinary shares pursuant to the requirements of the Securities and Exchange Commission. The American Depositary Shares are registered under the Securities Act of 1933, as amended, pursuant to a separate registration statement on Form F-6 (File No. 333-253915). |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report. As of December 31, 2021,2023, the registrant had outstanding 125,770,107251,756,047 ordinary shares, no par value.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Note — Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ | Non-accelerated filer | ☒ |
| Emerging growth company | ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP | International Financial Reporting Standards as issued by the International Accounting | ☐ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
TABLE OF CONTENTS
INTRODUCTION
| 1 | ||
| PART II | 74 | |
| ITEM 16J | Insider Trading Policies | 77 |
| ITEM 16K | Cybersecurity | 77 |
| PART III | 79 | |
i
INTRODUCTION
Certain Definitions
In this Annual Report on Form 20-F, unless the context otherwise requires:
| ● | references to “Steakholder Foods,” the “Company,” “us,” “we” and “our” refer to Steakholder Innovation Ltd. (formerly MeaTech MT Ltd. and MeaTech Ltd.) from its inception until the consummation of the January 2020 merger described herein, and Steakholder Foods Ltd. (formerly MeaTech 3D Ltd.) (the “Registrant”), an Israeli company, thereafter, unless otherwise required by the context; |
| ● | references to “ordinary shares,” “our shares” and similar expressions refer to the Registrant’s ordinary shares, no nominal (par) value per share; |
| ● | references to “ADS” refer to the American Depositary Shares listed on the Nasdaq Capital Market (“Nasdaq”) under the symbol “STKH,” each representing ten ordinary shares of the Registrant; |
| ● | references to “dollars,” “U.S. dollars,” “USD” and “$” are to United States Dollars; |
| ● | references to “NIS” are to New Israeli Shekels, the currency of the State of Israel; |
| ● | references to the “Companies Law” are to Israel’s Companies Law, 5759-1999, as amended; and |
| ● | references to the “SEC” are to the United States Securities and Exchange Commission. |
Forward-Looking Statements
This Annual Report on Form 20-F contains forward-looking statements concerning our business, operations and financial performance and condition as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements that are not historical facts may be deemed to be forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms including “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” and similar expressions intended to identify forward-looking statements, but these are not the only ways these statements are identified. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. You should not put undue reliance on any forward-looking statements. Unless we are required to do so under U.S. federal securities laws or other applicable laws, we do not intend to update or revise any forward-looking statements. Readers are encouraged to consult the Company’s filings made on Form 6-K, which are periodically filed with or furnished to the SEC.
Forward-looking statements contained in this prospectus include, but are not limited to:
| ● | our estimates regarding our expenses, future revenue, capital requirements and needs for additional financing; |
● |
● |
| ● | our expectations regarding the timing for the potential commercial launch of our cultured meat technologies; |
| ● | our ability to successfully manage our planned growth, and any future acquisitions, joint ventures, collaborations or similar transactions; |
| ● | the competitiveness of the market for our cultured meat technologies; |
| ● | our ability to enforce our intellectual property rights and to operate our business without infringing, misappropriating, or otherwise violating the intellectual property rights and proprietary technology of third parties; |
| ● | our ability to predict and timely respond to preferences for alternative proteins and cultured meats and new trends; |
| ● | our ability to attract, hire and retain qualified employees and key personnel; |
| ● | security, political and economic instability in the Middle East that could harm our business, including due to the current war between Israel and Hamas; and |
| ● | other risks and uncertainties, including those listed in “Item 3. —Key Information—Risk Factors.” |
ii
PART I
| ITEM 1. | Identity Of Directors, Senior Management And Advisers |
Not applicable.
| ITEM 2. | Offer Statistics And Expected Timetable |
Not applicable.
| ITEM 3. | Key Information |
A. Selected Financial Data
[Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Our business is subject to various risks, including those described below. You should carefully consider the risks and uncertainties described below and in our future filings with the SEC. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. Additionally, risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, results of operations and/or prospects.
Summary of Risks Associated with our Business
Our business is subject to a number of risks of which you should be aware before a decision to invest in the ADSs.aware. You should carefully consider all the information set forth in this prospectusAnnual Report on Form 20-F and, in particular, should evaluate the specific factors set forth in the sections titled “Risk Factors” before deciding whether to invest in the ADSs.. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. Among these important risks are, but not limited to, the following:
RISKS RELATED TO OUR FINANCIAL CONDITION AND LIQUIDITY REQUIREMENTS
| ● | We expect to continue incurring significant losses for the foreseeable future. |
| ● | We may require substantial additional funds to complete our research and development activities. |
| ● | There is substantial doubt as to whether we can continue as a going concern. |
| ● | Raising additional capital may cause dilution to our existing shareholders or restrict our operations. |
RISKS RELATED TO OUR BUSINESS AND STRATEGY
| ● | We have a limited operating history to date. |
| ● | The research and development associated with alternative protein manufacturing is a lengthy process. |
| ● | We intend to engage in future acquisitions, joint ventures or collaborations, which may not be successful. |
| ● | We may not be able to successfully manage our planned growth, and if the market does not grow as we expect, we may not achieve sustainable revenues. |
| ● | Business or economic disruptions may have an adverse impact on our business. |
RISKS RELATED TO COMPETITION AND COMMERCIALIZATION OF OUR TECHNOLOGIES
| ● | We are an early-stage company with an unproven business model. |
| ● | We may suffer reputational harm due to issues with products manufactured by our licensees. |
| ● | Failure to improve our technologies may adversely affect our ability to continue to grow. |
| ● | We may face difficulties if we expand our operations into new geographic regions. |
| ● | Consumer preferences for alternative proteins in general are difficult to predict and may change. |
| ● | We have no manufacturing experience or resources, and we may have issues in obtaining raw materials. |
| ● | Litigation or legal proceedings, government investigations or other regulatory enforcement actions could subject us to civil and criminal penalties or otherwise expose us to significant liabilities. |
RISKS RELATED TO OUR OPERATIONS
| ● | We expect that a small number of customers will account for a significant portion of our revenues, and we may be exposed to the credit risks of our customers. |
| ● | If we are unable to attract and retain qualified employees, our ability to implement our business plan may be adversely affected, and we may not be able to enforce covenants not to compete under applicable employment laws. |
| ● | Insurance policies may not fully cover the risk of loss to which we are exposed. |
| ● | Our business, reputation and operations could suffer in the event of information technology system failures or a cybersecurity incident. |
| ● | Food safety and food-borne illness incidents may materially adversely affect our business. |
RISKS RELATED TO GOVERNMENT REGULATION
| ● | Products utilizing our technologies will be subject to regulations that could adversely affect our business and results of operations. |
| ● | Any changes in, or failure by our supplier to comply with, applicable laws, regulations or policies could adversely affect our business. |
RISKS RELATED TO OUR INTELLECTUAL PROPERTY AND POTENTIAL LITIGATION
| ● | If we are unable to obtain and maintain our intellectual property rights, we may not be able to compete effectively in our markets. |
| ● | Intellectual property rights of third parties could adversely affect our ability to successfully commercialize our products and may prevent or delay our development and commercialization. |
| ● | Patent policy and rule changes could increase uncertainties and costs. |
| ● | We may be involved in lawsuits to protect or enforce our or third party intellectual property rights. |
| ● | Our articles of association provide that unless we consent to an alternate forum, the federal district courts of the United States shall be the exclusive forum of resolution of any claims arising under the Securities Act. |
RISKS RELATED TO OUR OPERATIONS IN ISRAEL
| ● | Political, economic and military conditions in Israel could have an adverse impact on our business. |
| ● | We are exposed to fluctuations in currency exchange rates. |
| ● | Enforcing a U.S. judgment against us and our executive officers and directors, or asserting U.S. securities law claims in Israel, may be difficult. |
| ● | Our articles of association provide that unless we consent otherwise, the competent courts of Tel Aviv, Israel shall be the sole and exclusive forum for substantially all disputes between the Company and its shareholders under the Companies Law and the Israeli Securities Law. |
| ● | Your rights and responsibilities as our shareholder will be governed by Israeli law, which may differ in some respects from the rights and responsibilities of shareholders of U.S. corporations. |
| ● | Our articles of association and Israeli law could prevent a takeover that shareholders consider favorable and could also reduce the market price of our ADSs. |
RISKS RELATED TO OWNERSHIP OF THE ADSs
| ● | The ADS price may be volatile, and you may lose all or part of your investment. |
| ● | We have never paid dividends on our share capital, nor do we intend to pay dividends for the foreseeable future. |
| ● | ADS holders may not receive the same distributions or dividends as those we make to the holders of our ordinary shares. |
| ● | ADS holders do not have the same rights as our shareholders. |
| ● | ADS holders may be subject to limitations on transfer of their ADSs. |
| ● | We follow certain home country corporate governance practices instead of certain Nasdaq and Exchange Act requirements. |
| ● | If we are a “passive foreign investment company” for U.S. income tax purposes, there may be adverse tax consequences to U.S. investors. |
| ● | If we are a controlled foreign corporation, there could be adverse U.S. income tax consequences to certain U.S. holders. |
RISKS RELATED TO OUR FINANCIAL CONDITION AND LIQUIDITY REQUIREMENTS
We expect to continue incurring significant losses for the foreseeable future and may never become profitable.
We have experienced net losses in every period since the inception of MeaTech.our predecessor entity, Steakholder Innovation Ltd., or Steakholder Innovation. We anticipate that we will continue to incur significant losses for the foreseeable future as our operating expenses and capital expenditures increase substantially due to our continued investment in our research and development activities and as we hire additional employees over the coming years. These activities may prove more expensive than we anticipate. We incur significant expenses in developing our technologies. Accordingly, we may not be able to achieve or sustain profitability, and we expect to incur significant losses for the foreseeable future.
Steakholder Innovation commenced cultured meatfood technology development operations in September 2019, and we continue to be in the early stages of development of our technologies. As a result, we have not generated any revenues since inception of our cultured meatfood technology operations, and we do not expect to generate anycannot guarantee whether or when we will commence generating revenue from operations in the near term.operations. We may not be able to develop the technology for manufacturing cultured meat at all, or meet the additional technological challenges to scaling such technology up to an industrial scale from our research and development effortsfood production machines, or successfully market and licensecommercialize our technologies, once approved.technologies. In addition, there is no certainty that there will be sufficient demand to justify the production and marketing of cultured meat products.our food production machines. The market for alternative proteins in general, and cultured meats specifically, may be small or may not develop.
If cultured meats produced using our industrial-scale cultured meat manufacturing processesfood production machines do not gain wide market acceptance, we will not be able to achieve our anticipated growth, revenues or profitability and we may not be able to continue our business operations.
We willmay require substantial additional funds to complete our research and development activities and, if additional funds are not available on acceptable terms or at all, we may need to significantly scale back or cease our operations.
A significant portion of our research and development activities has been financed by the issuance of equity securities. We believe that we will continue to expend substantial resources for the foreseeable future as we work to develop our technologies. These expenditures are expected to include costs associated with research and development, and manufacturing and supply, as well as general operating expenses. In addition, other unanticipated costs may arise.
There is no certainty that we will be able to obtain funding for our research and development activities when we need it, on acceptable terms or at all. A lack of adequate funding may force us to reduce or cease all or part of our research and development activities and business operations. Our operating plan may change because of factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to shareholders, imposition of debt covenants and repayment obligations, or other restrictions that may adversely affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements depend on many factors, including:
| ● | our progress with current research and development activities; |
| ● | the number and characteristics of any products or manufacturing processes we develop or acquire; |
| ● | the expenses associated with our marketing initiatives; |
| ● | the timing, receipt and amount of milestone, royalty and other payments from future customers and collaborators, if any; |
| ● | the scope, progress, results and costs of researching and developing future products or improvements to existing products or manufacturing processes; |
| ● | any lawsuits related to our products or commenced against us; |
| ● | the expenses needed to attract, hire and retain skilled personnel; |
| ● | the costs associated with being a public company in the United States; and |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing intellectual property claims, including litigation costs and the outcome of such litigation. |
If our estimates and predictions relating to any of these factors are incorrect, we may need to modify our operating plan. Additional funds may not be available to us when needed on acceptable terms, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate our manufacturing, research and development activities or other activities that may be necessary to generate revenue and achieve profitability.
There is substantial doubt as to whether we can continue as a going concern.
Our consolidated financial statements as of December 31, 20212023 contain an explanatory paragraph that states that our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. Our financial statements do not include any measurement or presentation adjustment for assets or liabilities that might result if we would be unable to continue as a going concern. We have incurred operating losses since inception, have not generated any revenues and have not achieved profitable operations. Our net loss, accumulated during the development stage through December 31, 2021,2023, totaled approximately $37USD 69.8 million, and we expect to continue to incur substantial losses in future periods while we continue our research and development activities.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through equity offerings, debt financings, government contracts, government and/or other third-party grants or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We will require substantial funding to fund our anticipated commercialization efforts and fund our operating expenses and other activities. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail our research or development program, or a part thereof, which would adversely impact our potential revenues, results of operations and financial condition.
RISKS RELATED TO OUR BUSINESS AND STRATEGY
We have a limited operating history to date and our business prospects will be dependent on our ability to meet a number of challenges.
Our business prospects are difficult to predict due to a lack of operational history, and our success will be dependent on our ability to meet a number of challenges. We are focused on developing commercial technologies to manufacture alternative foods without the need for animal butchery. If we are unable to successfully develop our alternative protein manufacturing technologies, we may not be able to achieve our anticipated growth, revenues or profitability and we may not be able to continue our business operations.
Because we have a limited operating history and we are in the early stages of development, you may not be able to evaluate our future prospects accurately. Our prospects will be primarily dependent on our ability to successfully develop industrial-scale cultured meat manufacturing technologies and processes,food production machines, and market these to our customers. If we are not able to successfully meet these challenges, our prospects, business, financial condition and results of operations could be adversely impacted.
We are wholly dependent on the success of our cultured meatfood manufacturing technologies, including our cultured steak technologies, and we have limited data on the performance of our technologies to date.
We do not currently have any products or technologies approved for sale and we are still in the early stages of development. To date, we have limited data on the ability of our technologies to successfully manufacture cultured meat,alternative proteins, towards which we have devoted substantial resources to date.resources. We may not be successful in developing our technologies in a manner sufficient to support our expected scale-ups and future growth, or at all. We expect that a substantial portion of our efforts and expenditures over the next few years will be devoted to the development of technologies designed to enable us to market industrial-scale cultured meat manufacturing processes.food production machines. We cannot guarantee that we will be successful in developing these technologies on the timeline we expect or at all. If we are able to successfully develop our cultured meat technologies, we cannot ensure that we will obtain regulatory approval or that, following approval, upon commercialization our technologies will achieve market acceptance. Any such delay or failure would materially and adversely affect our financial condition, results of operations and prospects.
To develop our cultured meat steak technology, we are developing cellular agriculture technology, such as cell lines and approaches to working with plant-based cell-growth media in a scalable process.
We are currently planning to scale up the printing process to provide us with industrial-scale capabilities. If we are unable to successfully develop our cultured meat manufacturing technologies, we may not be able to achieve our anticipated growth, revenues or profitability and we may not be able to continue our business operations.
We may evaluate various acquisitions and collaborations, including licensing or acquiring complementary technologies, intellectual property rights, or businesses. Any potential acquisition, joint venture or collaboration will entail numerous potential risks, including:
| ● | increased operating expenses and cash requirements; |
| ● | the assumption of additional indebtedness or contingent liabilities; |
| ● | assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel; |
| ● | the diversion of our management’s attention from our existing programs and initiatives in pursuing such a strategic merger or acquisition; |
| ● | retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships; |
| ● | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing technologies; and |
| ● | our inability to generate revenue from acquired technologies or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
In addition, if we undertake acquisitions, we may utilize our cash, issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense.
Moreover, we may not be able to locate suitable acquisition or collaboration opportunities and this inability could impair our ability to grow or obtain access to technologies that may be important to the development of our business.
Our existing potential collaborations, or any future collaboration arrangements that we may enter into, may not be successful, which could significantly limit the likelihood of receiving the potential economic benefits of the collaboration and adversely affect our ability to develop and commercialize our product candidates.
We have entered into a potential collaborationcollaborations with Tiv Ta’am, which is reflected in a non-binding letter of intent. Tiv Ta’am has no obligation to collaborate with uspartner organizations, and may withdraw from the proposed collaboration at any time without any liability.
| ● | collaborators may not perform or prioritize their obligations as expected; |
| ● | collaborators may not pursue development and commercialization of any of our alternative protein manufacturing technologies or may elect not to continue or renew development or commercialization, changes in the collaborators’ focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities; |
| ● | collaborators may provide insufficient funding for the successful development or commercialization of our alternative protein manufacturing technologies; |
| ● | collaborators could independently develop, or develop with third parties, products or technologies that compete directly or indirectly with our products or alternative protein manufacturing technologies if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| ● | alternative protein manufacturing technologies developed in collaborations with us may be viewed by our collaborators as competitive with their own products or technologies, which may cause collaborators to cease to devote resources to the development or commercialization of our products; |
| ● | a collaborator with marketing and distribution rights to one or more of our products or technologies that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of any such product; |
| ● | disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development of alternative protein manufacturing technologies, may cause delays or termination of the research, development or commercialization of such technologies, may lead to additional responsibilities for us with respect to such technologies, or may result in litigation or arbitration, any of which would be time-consuming and expensive; |
| ● | collaborators may not properly maintain, protect, defend or enforce our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
| ● | disputes may arise with respect to the ownership of intellectual property developed pursuant to our collaborations; |
| ● | collaborators may infringe, misappropriate or otherwise violate the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | collaborations may be terminated for the convenience of the collaborator and, if terminated, the development of our alternative protein manufacturing technologies may be delayed, and we could be required to raise additional capital to pursue further development or commercialization ; |
| ● | future relationships may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing shareholders, or disrupt our management and business; and |
| ● | we could face significant competition in seeking appropriate collaborators, and the negotiation process is time-consuming and complex. |
If our collaborations do not result in the successful development of our products, or if one of our collaborators terminates its agreement with us, we may not receive any future research funding or milestone, earn-out, royalty or other contingent payments under the collaborations. If we do not receive the funding we expect under these agreements, our development of our cultured meatalternative protein manufacturing technologies could be delayed and we may need additional resources to develop our technologies. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and the perception of us in the business and financial communities could be adversely affected. All of the risks relating to product development, regulatory approval and commercialization described in this report apply to the activities of our collaborators.
We may not be able to successfully manage our planned growth.
We expect to continue to make investments in our cultured meatalternative protein manufacturing technologies. We expect that our annual operating expenses will continue to increase as we invest in further research and development activities and, ultimately, sales and marketing efforts and customer service and support resources for future customers. Our failure to expand operational and financial systems in a timely or efficient manner could result in operating inefficiencies, which could increase our costs and expenses more than we had planned and prevent us from successfully executing our business plan. We may not be able to offset the costs of operation expansion by leveraging the economies of scale from our growth in negotiations with our suppliers and contract manufacturers. Additionally, if we increase our operating expenses in anticipation of the growth of our business and this growth does not meet our expectations, our financial results will be negatively impacted.
If our business grows, we will have to manage additional product design projects, materials procurement processes, and sales efforts and marketing for an increasing number of products, as well as expand the number and scope of our relationships with suppliers, distributors and end customers. If we fail to manage these additional responsibilities and relationships successfully, we may incur significant costs, which may negatively impact our operating results. Additionally, in our efforts to be first to market with new products with innovative functionality and features, we may devote significant research and development resources to products and product features for which a market does not develop quickly, or at all. If we are not able to predict market trends accurately, we may not benefit from such research and development activities, and our results of operations may suffer.
As our future development and commercialization plans and strategies develop, we expect to need additional managerial, operational, sales, marketing, financial and legal personnel. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, failure to deliver and timely deliver our products to customers, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional new products. If our management is unable to manage our growth effectively, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced, and we may not be able to implement our business strategy.
If the market does not grow as we expect, we may not achieve sustainable revenues.
The marketplace for alternative protein manufacturing plants, which we expect to be our primary market, is dominated by methods that do not involve three-dimensional printing technology. If the market does not broadly accept three-dimensional printing of cultured meatsalternative protein as an alternative for conventional meat harvesting, or if it adopts three-dimensional printing based on a technology other than our proprietary bio-ink technology, we may not be able to achieve a sustainable level of revenues, and our results of operations would be adversely affected as a result. Additionally, cultivated meat is significantlyalternative proteins may be more expensive than conventional meat. If the price of cultivated meatalternative proteins remains high, this may limit the consumer demand for, and market acceptance of, products manufactured using our technologies, and we may never be able to compete successfully or generate sufficient revenue or sustained profitability.
Business or economic disruptions or global health concerns including COVID-19, may have an adverse impact on our business and results of operations.
The COVID-19 pandemic has negatively impacted the global economy, disrupted consumer spending and global supply chains, and created significant volatility and disruption of financial markets. Many countries around the world, including in Israel, haveimplemented significant governmental measures being implemented to control the spread of the virus, including temporary closure of businesses, severe restrictions on travel and the movement of people, and other material limitations on the conduct of business. To date, the impact of the pandemic on our operations has been mainly limited to a temporary closure of our facility earlier in the year, in the context of a government-mandated general lockdown, which temporary delayed certain of our development activities, while we implemented remote working and workplace protocols for our employees in accordance with government requirements. While government restrictions have recently been eased in Israel following a successful vaccination campaign, theThe extent of the impact of the COVID-19future disruptions, such as another pandemic, on our business and financial performance, including our ability to execute our near-term and long-term business strategies and initiatives in the expected time frame, will depend on future developments, including the duration and severity of the pandemic and the impacts of reopening, including possible additional waves, which are uncertain and cannot be predicted.
RISKS RELATED TO COMPETITION AND COMMERCIALIZATION OF OUR TECHNOLOGIES
We are an early-stage company with an unproven business model, which makes it difficult to evaluate our current business and future prospects.
We have no established basis to assure investors that our business strategies will be successful. We are dependent on unproven technologies and we have no basis to predict acceptance of our technologies by potential licensees and their customers. The market for cultured meatprinted alternative proteins is new and as yet untested. As a result, the revenue and income potential of our business and our market are unproven. Further, because of our limited operating history and early stage of development, and because the market for cultured meatalternative proteins is relatively new and rapidly evolving, we have limited insight into trends that may emerge and affect our business. We may make errors in predicting and reacting to relevant business trends, which could harm our business.
We may not be able to compete successfully in our highly competitive market.
The alternative protein market is expected to be highly competitive, with numerous brands and products competing for limited retailer shelf space, foodservice and restaurant customers and consumers. For us to compete successfully, we expect that the cultured meatsalternative proteins printed using our technologies will need to be competitive in taste, ingredients, texture, ease of integration into the consumer diet, nutritional claims, convenience, brand recognition and loyalty, product variety, product packaging and package design, shelf space, reputation, price, advertising and access to restaurant and foodservice customers.
Generally, the food industry is dominated by multinational corporations with substantially greater resources and operations than us.we. We cannot be certain that we will successfully compete with larger competitors that have greater financial, marketing, sales, manufacturing, distributing and technical resources than we do. Conventional food companies may acquire our competitors or launch their own competing products, and they may be able to use their resources and scale to respond to competitive pressures and changes in consumer preferences by introducing new products, reducing prices or increasing promotional activities, among other things. Competitive pressures or other factors could prevent us from acquiring market share or cause us to lose market share, which may require us to lower prices, or increase marketing and advertising expenditures, either of which would adversely affect our margins and could result in a decrease in our operating results and profitability. We cannot assure you that we will be able to maintain a competitive position or compete successfully against such sources of competition.
We may suffer reputational harm due to real or perceived quality or health issues with products manufactured by our licenseescustomers using our technology.
Any real or perceived quality or food safety concerns or failures to comply with applicable food regulations and requirements, whether or not ultimately based on fact and whether or not involving us, or even merely involving unrelated manufacturers, could cause negative publicity and reduced confidence in our company, or the industry as a whole, which could in turn harm our reputation and sales, and could adversely impact our business, financial condition and operating results. There can be no assurance that products manufactured by our licenseescustomers will always comply with regulatory standards. Although we expect that our licenseescustomers will strive to manufacture products free of pathogenic organisms, these may not be easily detected and cross-contamination can occur. We cannot assure you that this health risk will always be preempted by quality control processes.
We will have no control over the products manufactured by our licensees,customers, especially once they are purchased by consumers, who may prepare these products in a manner that is inconsistent with directions or store them for excessive periods of time, which may adversely affect their quality and safety. If the products manufactured by our licensees are not perceived as safe or of high quality, then our business, results of operations and financial condition could be adversely affected.
The growing use of social and digital media increases the speed and extent that information or misinformation and opinions can be shared. Negative publicity about cultured meats produced using our technologies could seriously damage our reputation.
Failure to improve our technologies may adversely affect our ability to continue to grow.
In order to continue to grow, we expect we will need to continue to innovate by developing new technologies or improving existing ones, in ways that meet our standards for quality and will enable our eventual licenseescustomers to manufacture products that appeal to consumer preferences. Such innovation will depend on the technical capability of our staff in developing and testing product prototypes, including complying with applicable governmental regulations, and the success of our management and sales and marketing teams in introducing and marketing new technologies. Failure to develop and market new technologies may cause a negative impact on our business and results of operations.
Additionally, the development and introduction of new technologies requires substantial research, development and marketing expenditures, which we may be unable to recoup if the new technologies do not lead to products that gain widespread market acceptance. If we are unsuccessful in meeting our objectives with respect to new or improved technologies, our business could be harmed.
We may face difficulties if we expand our operations into new geographic regions, in which we have no prior operating experience.
We intend to licensecommercialize our technologies in numerous geographical markets. International operations involve a number of risks, including foreign regulatory compliance, tariffs, taxes and exchange controls, economic downturns, inflation, foreign currency fluctuations and political and social instability in the countries in which we will operate. Expansion may involve expanding into less developed countries, which may have less political, social or economic stability and less developed infrastructure and legal systems. It is costly to establish, develop and maintain international operations and develop and promote our brands in international markets. As we expand our business into other countries, we may encounter regulatory, legal, personnel, technological and other difficulties that increase our expenses and/or delay our ability to become profitable in such countries, which may have an adverse impact on our business and brand.
Consumer preferences for alternative proteins in general, and more specifically cultured meats, are difficult to predict and may change, and, if we are unable to respond quickly to new trends, our business may be adversely affected.
Our business is focused on the development and marketing of licensable cultured meatalternative protein manufacturing technologies. Consumer demand for the cultured meatsalternative proteins manufactured using these technologies could change based on a number of possible factors, including dietary habits and nutritional values, concerns regarding the health effects of ingredients and shifts in preference for various product attributes. If consumer demand for our products decreases, our business and financial condition wouldmay suffer. Consumer trends that we believe favor sales of products manufactured using our licensed technologies could change based on a number of possible factors, including a shift in preference from animal-based protein products, economic factors and social trends. A significant shift in consumer demand away from products manufactured using our technologies could reduce our sales or our market share and the prestige of our brand, which would harm our business and financial condition.
We have nolimited manufacturing experience orand resources and we expect we will incur significant costs to develop this expertise or need to rely on third parties for manufacturing.
We have nolimited manufacturing experience. In order to develop and licensecommercialize our technologies, we will need to develop, contract for or otherwise arrange for the necessary manufacturing capabilities. We may experience difficulty in obtaining adequate and timely manufacturing capacity for our proprietary cultured meatalternative protein printers and bio-inks. We do not own or lease facilities currently that could be used to manufacture any products that we might develop on an industrial scale, nor do we have the resources at this time to acquire or lease suitable facilities. If we are unable to build the necessary internal manufacturing capability or obtain this capability through third parties we will not be able to commercialize our technologies.blends. Even if we develop or obtain the necessary manufacturing capacity, if we fail to comply with regulations, to obtain the necessary licenses and knowhow or to obtain the requisite financing in order to comply with all applicable regulations and to own or lease the required facilities in order to manufacture products, we could be forced to cease operations, which would cause you to lose all of your investment.operations.
Our limited manufacturing capabilities are nascent, and if we fail to effectively develop manufacturing and production capabilities, our business and operating results and our brand reputation could be harmed.
If we are unable to develop manufacturing capacity to commence cultured chicken fatcultivated meat production, we may not be able to expedite our market entry or develop an industrial process for cultivating and producing real meat cuts. Additionally, there is risk in our ability to effectively scale production processes, if developed, to optimize manufacturing capacity for specific products and effectively manage our supply chain requirements, which involves accurately forecasting demand for each of our products and inventory needs in order to ensure we have adequate available manufacturing capacity for each such product and to ensure we are effectively managing our inventory. Our forecasts may be based on multiple assumptions that may cause our estimates to be inaccurate and affect our ability to obtain adequate manufacturing capacity (whether our own manufacturing capacity or co-manufacturing capacity) and adequate inventory supply in order to meet the demand for our products, which could prevent us from developing our industrial process and harm our business and prospects.
However, if we overestimate our demand and overbuild our capacity or inventory, we may have significantly underutilized assets, experience reduced margins, and have excess inventory which we may be required to write-down or write-off. If we do not accurately align our manufacturing capabilities and inventory supply with demand, if we experience disruptions or delays in our supply chain, or if we cannot obtain raw materials of sufficient quantity and quality at reasonable prices and in a timely manner, our business, financial condition and results of operations may be adversely affected.
If developed, our manufacturing and production operations will be subject to additional risks and uncertainties.
The interruption in, or the loss of operations at, our pilot facility, which may be caused by work stoppages, labor shortages, strikes or other labor unrest, production disruptions, product quality issues, local economic and political conditions, restrictive governmental actions, border closures, disease outbreaks or pandemics (such as COVID-19), the outbreak of hostilities, acts of war, terrorism, fire, earthquakes, severe weather, flooding or other natural disasters at one or more of these facilities, could delay, postpone or reduce production of some of our products, could impede our ability to develop an industrial process for cultivating and producing real meat cuts or could otherwise have an adverse effect on our business, results of operations and financial condition until such time as such interruption is resolved or an alternate source of production is secured.
Because we rely on a limited number of third-party suppliers, we may not be able to obtain raw materials on a timely basis or in sufficient quantities at competitive prices to produce our products or meet the demand for our products.
We rely on a limited number of vendors, a portion of which are located internationally, to supply us with raw materials. Our financial performance depends in large part on our ability to arrange for the purchase of raw materials in sufficient quantities at competitive prices. We are not assured a continued supply or pricing of raw materials. Any of our other suppliers could discontinue or seek to alter their relationship with us. We could experience similar delays in the future from any of our suppliers. Any disruption in the supply of embryonic stem cells or other raw materials would have a material adverse effect on our business if we cannot replace these suppliers in a timely manner, on commercially reasonable terms, or at all.
In addition, our suppliers manufacture their products at a limited number of facilities. A natural disaster, severe weather, fire, power interruption, work stoppage or other calamity affecting any of these facilities, or any interruption in their operations, could negatively impact our ability to obtain required quantities of raw materials in a timely manner, or at all, which could materially increase our cost and delay our timeline, and have a material effect on our business and financial condition.
Events that adversely affect our suppliers of raw materials could impair our ability to obtain raw material inventory in the quantities at competitive prices that we desire. Such events include problems with our suppliers’ businesses, finances, labor relations and/or shortages, strikes or other labor unrest, ability to import raw materials, product quality issues, costs, production, insurance and reputation, as well as local economic and political conditions, restrictive U.S. and foreign governmental actions, such as restrictions on transfers of funds and trade protection measures, including export/import duties and quotas and customs duties and tariffs, adverse fluctuations in foreign currency exchange rates, changes in legal or regulatory requirements, border closures, disease outbreaks or pandemics (such as COVID-19), acts of war, terrorism, natural disasters, fires, earthquakes, flooding, severe weather, agricultural diseases or other catastrophic occurrences. We continuously seek alternative sources of raw materials to use in our products, but we may not be successful in diversifying the raw materials we use in our products.
If we need to replace an existing supplier, there can be no assurance that supplies of raw materials will be available when required on acceptable terms, or at all, or that a new supplier would allocate sufficient capacity to us in order to meet our requirements, fill our orders in a timely manner or meet our strict quality standards.
Litigation or legal proceedings, government investigations or other regulatory enforcement actions could subject us to civil and criminal penalties or otherwise expose us to significant liabilities and have a negative impact on our reputation or business.
We operate in a constantly evolving legal and regulatory framework. Consequently, we are subject to heightened risk of legal claims, government investigations or other regulatory enforcement actions. Although we have implemented policies and procedures designed to ensure compliance with existing laws and regulations, we cannot assure you that our employees, temporary workers, contractors or agents will not violate our policies and procedures. Moreover, a failure to maintain effective control processes could lead to violations, unintentional or otherwise, of laws and regulations. Legal claims, government investigations or regulatory enforcement actions arising out of our failure or alleged failure to comply with applicable laws and regulations could subject us to civil and criminal penalties that could materially and adversely affect our product sales, reputation, financial condition and operating results. For example, in November 2020, the Israel Securities Authority, or ISA, initiated an administrative proceeding against us claiming negligent misstatement regarding certain immediate and periodic reports published by our predecessor (Ophectra) during the years 2017 and 2018, prior to the merger with MeaTech. These reports relate to Ophectra’s activities prior to establishment of the settlement fund in connection with the merger. In April 2021, following negotiations with the ISA, we agreed to settle the matter for NIS 0.7 million ($0.220.2 million). The settlement is subject to approval of the ISA’s Enforcement Committee.
RISKS RELATED TO OUR OPERATIONS
We expect that a small number of customers will account for a significant portion of our revenues, and the loss of one or more of these customers could adversely affect our financial condition and results of operations.
We do not expect to generate revenue in the short or medium term. If we are able to generate revenue, we believe that we will do so through three primary streams: (i) licensing our proprietary intellectual property to customers for the purpose of commercializing our technologies,selling alternative protein manufacturing machines, including by way of setting up and operating cultured meat production factories;three-dimensional printers; (ii) brokering the supply of materials needed in the manufacturing process; and (iii) providing consulting and implementation services to customers. These streams may arise in the context of product co-development. Under this model, we initially expect to derive a significant portion of our revenues from a few customers. Our financial condition and results of operations could be adversely impacted if any one of these customers interrupt or curtail their activities, fail to pay for the services that have been performed, terminate their cultured meat operations, or if we are unable to enter into agreements with new customers on favorable terms. The loss of customers could adversely affect our financial condition and results of operations.
We may be exposed to the credit risks of our customers, and nonpayment by these customers and other parties could adversely affect our financial position, results of operations and cash flows.
We may be subject to risks of loss resulting from nonpayment by our customers. Any material nonpayment by these entities could adversely affect our financial position, results of operations and cash flows. If customers default on their obligations to us, our financial results and condition could be adversely affected. Some of these customers may be highly leveraged and subject to their own operating and regulatory risks.
If we are unable to attract and retain qualified employees, our ability to implement our business plan may be adversely affected.
The loss of the service of our employees, such as Mr. Arik Kaufman, our Chief Executive Officer, would likely delay our achievement of product development and other business objectives, as we may not be able to find suitable individuals to replace them on a timely basis, if at all. In addition, any such departure could be viewed in a negative light by investors and analysts, which may cause the price of our ordinary sharessecurities to decline. Although we have employment agreements with our key employees, these employees could terminate their employment with us at any time on relatively short notice. We do not carry key man life insurance on any of our executive officers.
Recruiting and retaining qualified scientific, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among high technology and life sciences companies for similar personnel. We also experience competition from universities and research institutions in attracting and retaining scientific personnel. In addition, we rely on consultants and advisors, including scientific advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
Under applicable employment laws, we may not be able to enforce covenants not to compete.
Our employment agreements generally include covenants not to compete. These agreements prohibit our employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work. For example, Israeli courts have required employers seeking to enforce covenants not to compete to demonstrate that the competitive activities of a former employee will harm one of a limited number of material interests of the employer, such as the secrecy of a company’s confidential commercial information or the protection of its intellectual property. If we cannot demonstrate that such an interest will be harmed, we may be unable to prevent our competitors from benefiting from the expertise of our former employees or consultants and our competitiveness may be diminished.
We are exposed to a risk of substantial loss due to claims that may be filed against us in the future because our insurance policies may not fully cover the risk of loss to which we are exposed.
We are exposed to the risk of having claims seeking monetary damages being filed against us, for example with regard to securities-related claims. In the event that we are required to pay damages for any such claim, we may be forced to seek bankruptcy or to liquidate because our asset base and revenue base may be insufficient to satisfy the payment of damages and any insurance that we have obtained may not provide sufficient coverage against potential liabilities.
Our business and operations would suffer in the event of information technology system failures, including security breaches.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, causing our business to suffer. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product could be delayed.
A cybersecurity incident, other technology disruptions or failure to comply with laws and regulations relating to privacy and the protection of data relating to individuals could negatively impact our business, our reputation and our relationships with customers.
We use computers in substantially all aspects of our business operations. We also use mobile devices, social networking and other online activities to connect with our employees, suppliers and co-manufacturers. Such uses give rise to cybersecurity risks, including security breaches, espionage, system disruption, theft and inadvertent release of information. Our business involves the storage and transmission of numerous classes of sensitive and/or confidential information and intellectual property, including suppliers’ information, private information about employees and financial and strategic information about us and our business partners. Further, as we pursue new initiatives that improve our operations and cost structure, potentially including acquisitions, we may also be expand and improve our information technologies, resulting in a larger technological presence and corresponding exposure to cybersecurity risk. If we fail to assess and identify cybersecurity risks associated with new initiatives or acquisitions, we may become increasingly vulnerable to such risks. Additionally, while we have implemented measures to prevent security breaches and cyber incidents, our preventative measures and incident response efforts may not be entirely effective. The theft, destruction, loss, misappropriation, or release of sensitive and/or confidential information or intellectual property, or interference with our information technology systems or the technology systems of third parties on which we rely, could result in business disruption, negative publicity, brand damage, violation of privacy laws, loss of customers, potential liability and competitive disadvantage all of which could have a material adverse effect on our business, financial condition or results of operations.
In addition, we are subject to laws, rules and regulations in the United States, the European Union and other jurisdictions relating to the collection, use and security of personal information and data. Such data privacy laws, regulations and other obligations may require us to change our business practices and may negatively impact our ability to expand our business and pursue business opportunities. We may incur significant expenses to comply with the laws, regulations and other obligations that apply to us. Additionally, the privacy- and data protection-related laws, rules and regulations applicable to us are subject to significant change. Several jurisdictions have passed new laws and regulations in this area, and other jurisdictions are considering imposing additional restrictions. Privacy- and data protection-related laws and regulations also may be interpreted and enforced inconsistently over time and from jurisdiction to jurisdiction. Any actual or perceived inability to comply with applicable privacy or data protection laws, regulations, or other obligations could result in significant cost and liability, litigation or governmental investigations, damage our reputation, and adversely affect our business.
Disruptions in the worldwide economy may adversely affect our business, results of operations and financial condition.
The global economy can be negatively impacted by a variety of factors such as the spread or fear of spread of contagious diseases (such as the COVID-19 pandemic), man-made or natural disasters, actual or threatened war, terrorist activity, political unrest, civil strife and other geopolitical uncertainty. Such adverse and uncertain economic conditions may impact consumer demand for alternative proteins, in general, and clean meats specifically, which may in turn impact manufacturer and retailer demand for our technologies. In addition, our ability to manage normal commercial relationships with suppliers may suffer. Consumers may shift purchases to lower-priced or other perceived value offerings during economic downturns as a result of various factors, including job losses, inflation, higher taxes, reduced access to credit, change in government economic policy and international trade disputes. In particular, consumers may reduce the amount of cultured meatalternative proteins that they purchase in favor of conventional meat, or other alternative proteins, which may have lower retail prices, which could indirectly affect our results of operations. Manufacturer and retailers may become more conservative in response to these conditions and seek to delay commencing cultured marketalternative protein manufacturing operations or reduce existing operations. Our results of operations will depend upon, among other things, the financial condition of our business customers and our ability to supply them with the means to manufacture products that appeal to consumers at the right price. Decreases in demand for the products manufactured by our customers would put downward pressure on margins and would negatively impact our financial results. Prolonged unfavorable economic conditions or uncertainty may result in end consumers making long-lasting changes to their discretionary spending behavior on a more permanent basis, which may likewise have an indirect adverse effect on our sales and profitability.
Food safety and food-borne illness incidents may materially adversely affect our business by exposing us to lawsuits, product recalls or regulatory enforcement actions, increasing our operating costs and reducing demand for our product offerings.
Selling food for human consumption involves inherent legal and other risks, and there is increasing governmental scrutiny of and public awareness regarding food safety. Unexpected side effects, illness, injury or death related to allergens, food-borne illnesses or other food safety incidents caused by products we sell, or involving our suppliers or co-manufacturers, could result in the discontinuance of sales of these products or our relationships with such suppliers or co-manufacturers, or otherwise result in increased operating costs, regulatory enforcement actions or harm to our reputation. Shipment of adulterated or misbranded products, even if inadvertent, can result in criminal or civil liability. Such incidents could also expose us to product liability, negligence or other lawsuits, including consumer class action lawsuits. Any claims brought against us may exceed or be outside the scope of our existing or future insurance policy coverage or limits. Any judgment against us that is more than our policy limits or not covered by our policies or not subject to insurance would have to be paid from our cash reserves, which would reduce our capital resources.
The occurrence of food-borne illnesses or other food safety incidents could also adversely affect the price and availability of affected ingredients, resulting in higher costs, disruptions in supply and a reduction in our sales. Furthermore, any instances of food contamination or regulatory noncompliance, whether or not caused by our actions, could compel us, our suppliers, our distributors or our customers, depending on the circumstances, to conduct a recall in accordance with FDA regulations, comparable state laws or foreign laws such as those of the European Union and the United Kingdom. Food recalls could result in significant losses due to their costs, the destruction of product inventory, lost sales due to the unavailability of the product for a period of time and potential loss of existing distributors or customers and a potential negative impact on our ability to attract new customers due to negative consumer experiences or because of an adverse impact on our brand and reputation. The costs of a recall could exceed or be outside the scope of our existing or future insurance policy coverage or limits.
In addition, food companies have been subject to targeted, large-scale tampering as well as to opportunistic, individual product tampering, and we, like any food company, could be a target for product tampering. Forms of tampering could include the introduction of foreign material, chemical contaminants and pathological organisms into consumer products as well as product substitution. FDA regulations require companies like us to analyze, prepare and implement mitigation strategies specifically to address tampering (i.e., intentional adulteration) designed to inflict widespread public health harm. If we do not adequately address the possibility, or any actual instance, of intentional adulteration, we could face possible seizure or recall of our products and the imposition of civil or criminal sanctions, which could materially adversely affect our business, financial condition and operating results.
Non-compliance with environmental, social, and governance, or ESG, practices could harm our reputation, or otherwise adversely impact our business, while increased attention to ESG initiatives could increase our costs.
Companies across industries are facing increasing scrutiny from a variety of stakeholders related to their ESG and sustainability practices. Certain market participants, including institutional investors and capital providers, are increasingly placing importance on the impact of their investments and are thus focusing on corporate ESG practices, including the use of third-party benchmarks and scores to assess companies’ ESG profiles in making investment or voting decisions, and engaging with companies to encourage changes to their practices. Unfavorable ESG ratings could lead to increased negative investor sentiment towards us or our industry. If we do not comply with investor or shareholder expectations and standards in connection with our ESG initiatives or are perceived to have not addressed ESG issues within our company, our business and reputation could be negatively impacted and our share price could be materially and adversely affected, as well as our access to and cost of capital.
While we may, at times, engage in voluntary initiatives (such as voluntary disclosures, certifications, or goals, among others) or commitments to improve the ESG profile of our company and/or products, such initiatives or achievements of such commitments may not have the desired effect and may be costly.
In addition, we may commit to certain initiatives or goals but not ultimately achieve such commitments or goals due to factors that are both within or outside of our control. Moreover, actions or statements that we may take based on expectations, assumptions, or third-party information that we currently believe to be reasonable may subsequently be determined to be erroneous or be subject to misinterpretation. Even if this is not the case, our current actions may subsequently be determined to be insufficient by various stakeholders, and we may be subject to investor or regulator engagement on our ESG initiatives and disclosures, even if such initiatives are currently voluntary. In addition, increasing ESG-related regulation, such as the SEC’s climate disclosure proposal, may also result in increased compliance costs or scrutiny.
Expectations around a company’s management of ESG matters continues to evolve rapidly, in many instances due to factors that are out of our control. To the extent ESG matters negatively impact our reputation, it may also impede our ability to compete as effectively to attract and retain employees or customers, which may adversely impact our operations.
RISKS RELATED TO GOVERNMENT REGULATION
We expect that products utilizing our technologies will be subject to regulations that could adversely affect our business and results of operations.
The manufacture and marketing of food products is highly regulated. We, our suppliers and licensees,customers, may be subject to a variety of laws and regulations. These laws and regulations apply, directly or indirectly, to many aspects of our business, including the manufacture, composition and ingredients, packaging, labeling, distribution, advertising, sale, quality and safety of food products, as well as the health and safety of our employees and the protection of the environment.
We are focused on developing a novel, proprietary three-dimensional bioprinterprinters to deposit layers of cells (including stem cells and differentiated stem cells), scaffolding, and cell nutrients in a three-dimensional form ofprint structured cultured meat. The cultured meat, in turn, will be produced by our customers. Peace of Meat intends to produce cultured avian fat that is anticipated to be used as an ingredient, inter alia, in the production of finished cultured poultry. Neither we nor Peace of Meatfood. We do not intend to manufacture, distribute and sell branded cultured-meat end products for consumer consumption.
For the reasons discussed below, we ourselves do not expect to be directly regulated by the FDA for United States compliance purposes but will apply FDA’s food contact substance standards or analogous foreign regulations when developing our three-dimensional bioprinter.printers. Specifically, we intend to licensesell our production technology,machines, as well as provide associated products and services to food processing and food retail companies through a B2B model. From a regulatory perspective, in the United States, we expect companies manufacturing finishedproducts that include cultured meat products to be subject to regulation by various government agencies, including the FDA, U.S. Department of Agriculture,the USDA, the U.S. Federal Trade Commission, or FTC, Occupational Safety and Health Administration and the Environmental Protection Agency, as well as the requirements of various state and local agencies and laws, such as the California Safe Drinking Water and Toxic Enforcement Act of 1986. We likewise expect these products to be regulated by equivalent agencies outside the United States by various international regulatory bodies.
As the manufacturer of technologymachines used to produce food, including cultured meat, and consistent with the Federal Food, Drug and Cosmetic Act, Federal Meat Inspection Act, and Poultry Products Inspection Act, we believe we will not be directly be regulated by the FDA or USDA. Rather, we believe the regulatory obligation falls on our customers — cultured meat producers — to ensure that all food produced using our technologyproducts is wholesome and not adulterated. Consistent with food industry norms, we expect that our customers will therefore request assurances from us that our products are suitable for their intended use from an FDA regulatory perspective. Therefore, we plan to apply FDA food safety standards when developing our three-dimensional bioprinterprinters as a means of assuring our customers that our bioprinter isprinters are safe for itstheir intended use and will not result in the production of adulterated food. In particular, we plan to apply applicable food contact substance requirements, such as those of the FDA, when developing itsour three-dimensional bioprinterprinters as a means of assuring customers using the Company'sour technology that our bioprinter isprinters are safe for itstheir intended use and will not result in the production of adulterated food. If we are unable to provide regulatory compliance assurance to our customers, we expect that our ability to licensecommercialize our production technology would be adversely impacted.
The manufacturing of cultured meat, for which we are developing pipeline technologies, is expected to be subject to extensive regulations internationally, with products subject to numerous food safety and other laws and regulations relating to the sourcing, manufacturing, composition and ingredients, storing, labeling, marketing, advertising and distribution of these products. In addition, enforcement of existing laws and regulations, changes in legal requirements and/or evolving interpretations of existing regulatory requirements may result in increased compliance costs and create other obligations, financial or otherwise, that could adversely affect our business, financial condition or operating results. In addition, we could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act, or FCPA, and similar worldwide anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to officials or other third parties for the purpose of obtaining or retaining business. While our policies mandate compliance with anti-bribery laws, our internal control policies and procedures may not protect us from reckless or criminal acts committed by our employees, contractors or agents. Violations of these laws, or allegations of such violations, could disrupt our business and adversely impact our results of operations, cash flows and financial condition.
Any changes in, or changes in the interpretation of, applicable laws, regulations or policies of the USDA, state regulators or similar foreign regulatory authorities that relate to the use of the word “meat” or other similar words in connection with cultured meatalternative protein products could adversely affect our business, prospects, results of operations or financial condition.
The USDA, state regulators or similar foreign regulatory authorities, such as Health Canada or the Canadian Food Inspection Agency, or CFIA, or authorities of the European Union (EU)EU or the EU member states (e.g., European Food Safety Authority, or EFSA), could take action to impact our ability to use the term “meat” or similar words, such as “beef”, to describe the productproducts our bioprintersprinters will produce. In addition, a food may be deemed misbranded if its labeling is false or misleading in any particular way, and the USDA, CFIA, EFSA or other regulators could interpret the use of the term “meat” or any similar phrase(s) to describe our cultured meatalternative protein products as false or misleading or likely to create an erroneous impression regarding their composition. In the U.S., USDA will be developingdevelop new labeling requirements for foods under its jurisdiction produced through cell culture technology as noted in an Advance Notice of Proposed Rulemaking, (ANPR)or ANPR, published in September 2021.
Failure by our raw materials suppliers to comply with food safety, environmental or other laws and regulations, or with the specifications and requirements of our products, may disrupt our supply of products and adversely affect our business.
If our suppliers fail to comply with food safety, environmental or other laws and regulations, or face allegations of non-compliance, their operations may be disrupted. In the event of actual or even alleged non-compliance, we might be forced to find an alternative supplier and we may be subject to lawsuits related to such non-compliance by our suppliers. As a result, our supply of raw materials could be disrupted or our costs could increase, which would adversely affect our business, results of operations and financial condition. The failure of any supplier to produce products that conform to our standards could adversely affect our reputation in the marketplace and result in economic loss. Additionally, actions we may take to mitigate the impact of any disruption or potential disruption in our supply of raw materials, including increasing inventory in anticipation of a potential supply or production interruption, may adversely affect our business, results of operations and financial condition.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY AND POTENTIAL LITIGATION
If we are unable to obtain and maintain effective patent rights for our products, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us.
Since September 2019, we have sought patent protection for certain of our products, systems, processes, designs and applications. Our success depends in large part on our ability to obtain, maintain, monitor and enforce patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and new products.
We seek to protect our proprietary position and sustain our competitive advantage by filing patent applications in the United States and in other countries. Patent prosecution in the United States and the rest of the world is uncertain, expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner in all the necessary locations. It is also possible that we will fail to identify Patentablepatentable aspects of our research and development output before it is too late to obtain patent protection.
We have a growing portfolio of 1416 patents and provisional and non-provisional pending patent applications, with a robust pipeline. These are filed with the U.S. Patent and Trademark Office, or USPTO, the World Intellectual Property Organization, or WIPO, and when the time comes, in various patent offices around the world, such as Israel, China, Japan, Europe, Canada, and South Korea. Three of the pending patent applications were filed through the Paris Convention Treaty, or PCT. We cannot offer any assurances about which, if any of the pending patent applications will issue, the scope of protection of any such patent or whether any issued patents will be found invalid and/or unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any new products that we may develop.
Further, there is no assurance that all potentially relevant prior art relating to our patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents doare successfully issue,issued, and even if such patents cover our products, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patent applications and any future patents may not adequately protect our intellectual property, provide exclusivity for our new products, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
If we cannot obtain and maintain effective patent rights for our products, we may not be able to compete effectively, and our business and results of operations would potentially be harmed.
If we are unable to maintain effective proprietary rights for our products, we may not be able to compete effectively in our markets.
In addition to the protection afforded by any patents currently owned and that may be granted, historically, we have relied on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes and helpful devices that are not easily known, knowable or easily ascertainable, and for which patent infringement is difficult to monitor and enforce and any other elements of our product development processes, that involve proprietary know-how, as well as information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data, trade secrets and intellectual property by maintaining physical security of our premises and physical and electronic security of our information technology systems, as well as implementing various standard operating procedures designed to maintain that integrity. Agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors.
We cannot provide any assurances that our trade secrets and other confidential proprietary information will not be disclosed in violation of our confidentiality agreements or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Also, misappropriation or unauthorized and unavoidable disclosure of our trade secrets and intellectual property could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secret.
Intellectual property rights of third parties could adversely affect our ability to successfully commercialize our products, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
At this stage, and in the future it is inherently difficult to conclusively assess our freedom to operate without infringing on third party rights. Our competitive position may be adversely affected if existing patents or patents resulting from patent applications issued to third parties or other third-party intellectual property rights are held to cover our products, systems and processes or elements thereof, or our manufacturing or uses relevant to our development plans. In such cases, we may be limited, or not be in a position to develop or commercialize products or our product candidates unless we successfully pursue litigation to nullify or invalidate the third party intellectual property right concerned, or enter into a license agreement with the intellectual property rights’ holder, if available on commercially reasonable terms. There may also be pending patent applications that should they result in issued patents, could be alleged to be infringed by our new products. If such an infringement claim should be brought and be successful, we may be required to pay substantial damages, royalties, be forced to abandon our new products or seek a license from any patent holders. No assurances can be given that a license will be available on commercially reasonable terms, if at all.
It is also possible that we have failed to identify relevant third party patents or applications. For example, U.S. patent applications filed before July 8, 2019 and certain U.S. patent applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our new products or platform technology could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our new products or the use of our new products. Third party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in pursuing the development of and/or marketing our new products. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing our new products that are held to be infringing. We might, if possible, also be forced to redesign our new products so that we no longer infringe the third party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing new products. As our industries expand and more patents are issued, the risk increases that our products may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, designs or methods of manufacture related to the use or manufacture of our products. There may be currently pending patent applications that may later result in issued patents that our products may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
If any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for designs, or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our products. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of any patents that may issue from our patent applications, or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we were the first to file the invention claimed in our owned and licensed patent or pending applications, or that we or our licensor were the first to file for patent protection of such inventions. Assuming all other requirements for patentability are met, in the United States prior to March 15, 2013, the first to make the claimed invention without undue delay in filing, is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, enacted on September 16, 2011, the United States has moved to a first to file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents, all of which could have a material adverse effect on our business and financial condition.
We may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our intellectual property. If we were to initiate legal proceedings against a third party to enforce a patent covering one of our new products, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Under the Leahy-Smith Act, the validity of U.S. patents may also be challenged in post-grant proceedings before the USPTO. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Derivation proceedings initiated by third parties or brought by us may be necessary to determine the priority of inventions and/or their scope with respect to our patent or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our new products to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our traded securities.
We may, in the future, be subject to claims that our employees, consultants, or independent contractors have wrongfully or unavoidably used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We continue to employ individuals who were previously employed at our competitors or potential competitors. We have established standard operating procedures to try and ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, but we may nevertheless be subject to claims that we or our employees, consultants, or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employees’ former employers or other third parties. Litigation may result and be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship of our intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in, or right to compensation, with respect to our current patent and patent applications, future patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our products. Litigation may be necessary to defend against these and other claims challenging inventorship or claiming the right to compensation. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on products, as well as monitoring their infringement in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products. Future patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, which could make it difficult for us to stop the marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our future patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to monitor and enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Our articles of association provide that unless we consent to an alternate forum, the federal district courts of the United States shall be the exclusive forum of resolution of any claims arising under the Securities Act.
Our articles of association provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the sole and exclusive forum for any claim asserting a cause of action arising under the Securities Act (for the avoidance of any doubt, such provision does not apply to any claim asserting a cause of action arising under the Exchange Act). Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both U.S. state and federal courts have jurisdiction to entertain such claims. This choice of forum provision may limit a shareholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees and may increase the costs associated with such lawsuits, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find these provisions of the articles of association inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition. Any person or entity purchasing or otherwise acquiring any interest in our share capital shall be deemed to have notice of and to have consented to the choice of forum provisions of the articles of association described above. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction.
RISKS RELATED TO OUR OPERATIONS IN ISRAEL
Conditions in Israel, including the ongoing war between Israel and Hamas and other conflicts in the region, as well as political and economic instability, may adversely impact our business operations.
Our headquarters, all members of our board of directors and management, all of our research and development activities, and other significant operations are located in Israel and may be impacted by regional instability and extreme security tension. Political, economic and militarysecurity conditions in Israel itand the surrounding region could have an adverse impact on our operations.
In recent years,October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on Israeli population and industrial centers. These attacks resulted in extensive deaths, injuries and kidnapping of civilians and soldiers, as well as evacuations of tens of thousands of civilians from their homes. Following the attack, Israel’s security cabinet declared war against Hamas and commenced a military campaign.
Since the commencement of these events, there have includedbeen additional active hostilities, fromincluding with Hezbollah in Lebanon, the Houthi movement which controls parts of Yemen and betweenmost recently with Iran. It is possible that these hostilities will escalate in the future into a greater regional conflict, and that additional terrorist organizations and countries, will actively join the hostilities.
Currently, the war has impacted the availability of a limited number of our workforce in Israel in various ways – a small part of our workforce has been called to active duty, and others have been supporting friends or family members engaged in the war. While many military reservists have been released, some remain obligated to return in the coming months. If the situation escalates, they may be called up for additional reserve duty sooner than expected, additional employees may be called for service, and such persons may be absent for an extended period of time. This may materially and adversely affect our business operations, including product development, and our ability to meet our customers’ expectations, and could impact our competitive position and cause our sales to decrease.
The scope, intensity and duration of the current war are difficult to predict, as are the economic implications on our business and operations and on Israel’s economy in general. For example, these events may be intertwined with wider macroeconomic factors relating to a deterioration of Israel’s economic standing, that may involve, for instance, a downgrade in Israel’s credit rating by rating agencies (such as the recent downgrade by Moody’s of its credit rating of Israel from A1 to A2, as well as the downgrade of its outlook rating from “stable” to “negative”). Any of these implications on Israel’s economy or financial conditions may have an adverse effect on our ability to effectively conduct our operations.
Moreover, the perception of Israel and Israeli companies by the global community (including, for example, in light of the interim ruling rendered by the International Court of Justice (ICJ) in a case filed by South Africa against Israel in January 2024) may cause an increase in sanctions and other adverse measures against Israel, Israeli companies and their products and services. Additionally, there have been increased efforts by countries, activists and organizations to cause companies and consumers to boycott Israeli goods and services or otherwise restrict business with Israel and with Israeli companies, which may impact our ability to do business with our existing and potential customers. Such efforts, particularly if they become widespread, as well as the ICJ ruling and possible future rulings and orders of other tribunals against Israel, could materially and adversely impact our business operations.
The current hostilities with Hamas, Hezbollah, Iran and other organizations and countries have included and may include various methods of armed attacks that have already caused and may cause further damage to private and public facilities, infrastructure, utilities, and telecommunication networks. This may require the temporary closure of our offices or facilities or affect our employees’ ability to work, negatively impacting our operational capacity and disrupting supply chains that impact our ability to conduct business efficiently, thereby leading to increased costs associated with alternative solutions or contingency measures. Such attacks may also pose risks to the safety and effectiveness of our workforce and impair our ability to maintain business continuity, which would likely result in the Gaza Strip, which resulted in rockets being fired into Israel, causing casualtiessubstantial direct and disruption of economic activities. In addition, Israel faces threats from the civil war in Syria and from Iran. Our commercial insurance does not cover lossesindirect costs that may occur as a result of an event associated with the security situation in the Middle East.not be recoverable from our commercial insurance. Although we expect that the Israeli government will covercurrently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure yoube assured that such government coverage will be maintained or if maintained,that it will be sufficient to compensate us fully for damages incurred. Several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies, whether as a result of hostilities in the region or otherwise.sufficiently cover our potential damages. Any such matters could adversely affect our operations and results of operations, and any losses or damages incurred by us could have a material adverse effect on our business.
Further, Israel has held five general elections between 2019 and 2022, and prior to the Hamas attack in October 2023, the Israeli government had been pursuing legislative changes, which, if adopted, may alter the current state of separation of powers among the three branches of government and, as a result, have sparked a considerable political debate. Many individuals, organizations, and institutions, within and outside of Israel, voiced concerns over the resultpotential negative impacts of such a conflictchanges and the controversy surrounding them on the business and financial environment in Israel. Such negative impacts may include, among others, increased interest rates, currency fluctuations, inflation, civil unrest and volatility in securities markets, which could adversely affect the conditions in which we operate and potentially deter foreign investors and organizations from investing or transacting business with Israeli-based companies. To date, these initiatives have been substantially put on hold, but if such changes to Israel’s judicial system are again pursued by the government and approved by the parliament, or if any of the foregoing negative impacts were to materialize, it may have an adverse impacteffect on our business.
Because a substantial portion of our revenues is expected to be generated in currencies other than our functional currency, we will be exposed to fluctuations in currency exchange rates, which could negatively affect our financial condition.
In the future, we expect that a substantial portion of our revenues will be generated in currencies other than our functional currency. Our functional and presentation currency, in which we currently maintain our financial records is NIS, and our presentation currency, in which we report our financial results, is USD. The functional currency of Peace of Meat, in which it currently maintains its financial records, is Euros. As a result, our revenues for financial statement purposes might be negatively affected by fluctuations in the exchange rates of currencies in theother countries in which our technologiesproducts may be licensed,sold, and supplementary services provided and products sold.provided.
Currency exchange controls may restrict our ability to utilize our cash flows.
We expect to receive proceeds from sales of any product we may develop, and also to pay a portion of our operational costs and expenses, in U.S. dollars, Euros and other foreign currencies. However, we may be subject to existing or future rules and regulations on currency conversion. In 1998, the Israeli currency control regulations were liberalized significantly, and there are currently no currency controls in place. Legislation remains in effect, however, pursuant to which such currency controls could be imposed in Israel by administrative action at any time. We cannot assure that such controls will not be reinstated, or if reinstated, that they would not have an adverse effect on our operations.
Enforcing a U.S. judgment against us and our executive officers and directors, or asserting U.S. securities law claims in Israel, may be difficult.
We are incorporated in Israel. Most of ourOur executive officers and directors reside in Israel and most of our assets and the assets of these persons are located outside of the United States. Therefore,We have been informed by our legal counsel in Israel that it may be difficult to assert claims under U.S. securities laws in original actions instituted in Israel or obtain, a judgment obtained against us or any of these persons in the United States, including one based on the civil liability provisions of the U.S. federal securities laws. Israeli courts may refuse hear a claim based on a violation of U.S. securities laws against us or any of our non-U.S. executive officers and directors because Israel may not be collectible in the United States and may not be enforced by an Israeli court. It may also be difficultmost appropriate forum to affect service of process on these persons in the United States or to assert U.S. securities laws claims in original actions instituted in Israel.bring such a claim.
In addition, even if an Israeli court agrees to hear such a claim, it may determine that Israeli, and not U.S., law is applicable to the claim. Under Israeli law, if U.S. law is found to be applicable to such a claim, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would be governed by Israeli law. There is little binding case law in Israel addressing these matters.
Moreover, an Israeli court will not enforce a final U.S.non-Israeli judgment if (among other things) it was given in a civil matter, including judgments based upon the civil liability provisions of the U.S. securitiesstate whose laws and including a monetary or compensatory judgment in a non-civil matter, provided that:
As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index, or Israeli CPI, plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rate fluctuations.
Our articles of association provide that unless we consent otherwise, the competent courts of Tel Aviv, Israel shall be the sole and exclusive forum for substantially all disputes between the Company and its shareholders under the Companies Law and the Israeli Securities Law.
The competent courts of Tel Aviv, Israel shall, unless we consent otherwise in writing, be the exclusive forum for (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s shareholders, or (iii) any action asserting a claim arising pursuant to any provision of the Companies Law or the Israeli Securities Law, 1968, or the Israeli Securities Law. This exclusive forum provision is intended to apply to claims arising under Israeli law and would not apply to claims brought pursuant to the Securities Act or the Exchange Act or any other claim for which federal courts would have exclusive jurisdiction. Such exclusive forum provision in the articles of association will not relieve us of our duties to comply with federal securities laws and the rules and regulations thereunder, and our shareholders will not be deemed to have waived our compliance with these laws, rules and regulations. This exclusive forum provision may limit a shareholder’s ability to bring a claim in a judicial forum of its choosing for disputes us Company or our directors or other employees which may discourage lawsuits against us, our directors, officers and employees.
Your rights and responsibilities as our shareholder will be governed by Israeli law, which may differ in some respects from the rights and responsibilities of shareholders of U.S. corporations.
Since we are incorporated under Israeli law, the rights and responsibilities of our shareholders are governed by our articles of association and Israeli law. These rights and responsibilities differ in some respects from the rights and responsibilities of shareholders of U.S. corporations. In particular, each of our shareholders has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations toward us and other shareholders and to refrain from abusing its power in, among other things, voting at shareholder meetings on certain matters, such as an amendment to our articles of association, an increase of our authorized share capital, a merger and approval of related party transactions that require shareholder approval. A shareholder also has a general duty to refrain from discriminating against other shareholders. In addition, a controlling shareholder or a shareholder who knows that it possesses the power to determine the outcome of a shareholders vote or to appoint or prevent the appointment of an office holder has a duty to act in fairness toward us. Israeli law does not clearly define the substance of these duties, butduties. There is limited case law available to assist in understanding the implications of these provisions may be interpreted to impose additional obligations and liabilities on our shareholders that are not typically imposed on shareholders of U.S. corporations.govern shareholder behavior.
Provisions of Israeli corporate and tax law may deter acquisition transactions.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date on which a merger proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days have passed from the date on which the shareholders of both merging companies have approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, the Companies law requires that an acquirer in a full tender offer, which as a result would result in the acquirer to hold over 90% of the target company’s voting rights or the target company’s issued and outstanding share capital (or of a class thereof), to make a tender offer forto all of a company'sthe company’s shareholders for the purchase of all of the issued and outstanding shares of the company (or the applicable class). The full tender offer can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion ofcapital (or the tender offer also requires approval ofapplicable class) and if a majority of the offerees that do not have a personal interest in the tender offer,offer; unless, following consummation ofthe shareholders who did not accept the tender offer hold less than 2% of the issued and outstanding share capital of the company (or of the applicable class), all of the shares that the acquirer would hold at least 98%offered to purchase will be transferred to the acquirer by operation of the company's outstanding shares. Furthermore,law. the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completiondate of acceptance of the tender offer, claim that the consideration for the acquisition of the shares does not reflect their fair market value, and petition an Israeli court to alterdetermine whether the considerationtender offer was for less than fair value and whether the acquisition accordingly, unlessfair value should be paid as determined by the acquirer stipulatedcourt. However, an offeror may provide in its tenderthe offer that a shareholder that acceptswho accepted the offer will not be entitled to petition the court for appraisal rights as described in the preceding sentence, as long as the offeror and the company disclosed the information required by law in connection with the full tender offer. If the full tender offer was not accepted in accordance with any of the above alternatives, the acquirer may not seek such appraisalacquire shares of the company that will increase its holdings to more than 90% of the company’s voting rights or the company’s issued and outstanding share capital (or of the applicable class) from shareholders who accepted the tender offer. Shares purchased in contradiction to the full tender offer rules under the Companies Law will have no rights and the acquirer or the company published all required information with respect to the tender offer prior to the tender offer's response date.will become dormant shares.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred. These provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders.
Our articles of association and Israeli law could prevent a takeover that shareholders consider favorable and could also reduce the market price of our ADSs.
Certain provisions of Israeli law and our articles of association could have the effect of delaying or preventing a change in control and may make it more difficult for a third party to acquire us or for our shareholders to elect different individuals to its board of directors, even if doing so would be beneficial to its shareholders, and may limit the price that investors may be willing to pay in the future for the our ADSs. Among other things:
| ● | Israeli corporate law regulates mergers and requires that a tender offer be effected when more than a specified percentage of shares in a company are purchased; |
| ● | Israeli corporate law requires special approvals for certain transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to these types of transactions; |
| ● | Israeli corporate law does not provide for shareholder action by written consent for public companies, thereby requiring all shareholder actions to be taken at a general meeting of shareholders; |
| ● | our articles of association divide our directors into three classes, each of which is elected once every three years; |
| ● | our articles of association generally require a vote of the holders of a majority of our outstanding ordinary shares entitled to vote present and voting on the matter at a general meeting of shareholders (referred to as simple majority), and solely the amendment of the provision relating to the removal of members of our board of directors, require a vote of the holders of 65% of our outstanding ordinary shares entitled to vote at a general meeting; |
| ● | our articles of association provide that director vacancies may be filled by our board of directors. |
RISKS RELATED TO OWNERSHIP OF THE ADSs
If we are unable to maintain compliance with Nasdaq listing standards, our ADSs may be delisted from the Nasdaq Stock Market and you may face significant restrictions on the resale of your shares.
There can be no assurances that we will be able to maintain our Nasdaq listing in the future. On March 27, 2023, we received a notification from Nasdaq that the trading price of our ADSs did not meet the minimum bid price requirement of $1.00 per share for a period of 30 consecutive trading days. We were granted a 180-day compliance period, or September 18, 2023, to regain compliance by achieving a closing bid price of at least $1.00 per share for a minimum of 10 consecutive trading days. The Nasdaq confirmed that we had regained compliance on August 7, 2023. On October 31, 2023, we received another notification from Nasdaq that we were granted an additional 180-day period, or April 29, 2024, to regain compliance by achieving a closing bid price of at least $1.00 per share for a minimum of 10 consecutive trading days. The Nasdaq confirmed that we had regained compliance on April 18, 2024. In the event we are unable to maintain compliance with the Nasdaq Capital Market listing standards and our ADSs are delisted from the exchange, it would, among other things, likely lead to a number of negative implications, including an adverse effect on the price of our ADSs, reduced liquidity in our ADSs and greater difficulty in obtaining financing. In the event of a de-listing, we would take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our ADSs to become listed again, stabilize the market price or improve the liquidity of our ADSs, prevent our ADSs from dropping below the Nasdaq minimum bid price requirement in the future, or prevent future non-compliance with Nasdaq’s listing requirements. If we cannot maintain our compliance with Nasdaq’s listing requirements, we would pursue eligibility for trading of these securities on other markets or exchanges, such as the OTCQB or OTCQX, which are unorganized, inter-dealer, over-the-counter markets which provides significantly less liquidity than the Nasdaq Capital Market or other national securities exchanges.
The ADS price may be volatile, and you may lose all or part of your investment.
The market price of the ADSs could be highly volatile and may fluctuate substantially, including downward, as a result of many factors, including:
| ● | changes in the prices of our raw materials or the products manufactured in factories using our technologies; |
| ● | the trading volume of the ADSs; |
| ● | general economic, market and political conditions, including negative effects on consumer confidence and spending levels that could indirectly affect our results of operations; |
| ● | actual or anticipated fluctuations in our financial condition and operating results, including fluctuations in our quarterly and annual results; |
| ● | announcements by us or our competitors of innovations, other significant business developments, changes in distributor relationships, acquisitions or expansion plans; |
| ● | announcement by competitors or new market entrants of their entry into or exit from the alternative protein market; |
| ● | overall conditions in our industry and the markets in which we intend to operate; |
| ● | market conditions or trends in the packaged food sales industry that could indirectly affect our results of operations; |
| ● | addition or loss of significant customers or other developments with respect to significant customers; |
| ● | adverse developments concerning our manufacturers and suppliers; |
| ● | changes in laws or regulations applicable to our products or business; |
| ● | our ability to effectively manage our growth and market expectations with respect to our growth, including relative to our competitors; |
| ● | changes in the estimation of the future size and growth rate of our markets; |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| ● | additions or departures of key personnel; |
| ● | competition from existing products or new products that may emerge; |
| ● | issuance of new or updated research or reports about us or our industry, or positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| ● | variance in our financial performance from the expectations of market analysts; |
| ● | our failure to meet or exceed the estimates and projections of the investment community or that we may otherwise provide to the public; |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| ● | disputes or other developments related to proprietary rights, including patents, and our ability to obtain intellectual property protection for our products; |
| ● | litigation or regulatory matters; |
| ● | announcement or expectation of additional financing efforts; |
| ● | our cash position; |
| ● | sales and short-selling of the ADSs; |
| ● | our issuance of equity or debt; |
| ● | changes in accounting practices; |
| ● | ineffectiveness of our internal controls; |
| ● | negative media or marketing campaigns undertaken by our competitors or lobbyists supporting the conventional meat industry; |
| ● | the public’s response to publicity relating to the health aspects or nutritional value of products to be manufactured in factories using our technologies; and |
| ● | other events or factors, many of which are beyond our control. |
In addition, the stock markets have experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of the ADSs, regardless of our operating performance. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes, tariffs, international currency fluctuations, or the effects of disease outbreaks or pandemics (such as the COVID-19 pandemic), may negatively impact the market price of the ADSs. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
We have never paid dividends on our share capital and we do not intend to pay dividends for the foreseeable future.
We have never declared or paid any dividends on our share capital and do not intend to pay any dividends in the foreseeable future. We anticipate that we will retain all of our future earnings for use in the development and growth of our business and for general corporate purposes. Accordingly, any gains from an investment in the ADSs will depend on price appreciation of the ADSs, which may never occur. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to certain Israeli withholding taxes.
ADS holders may not receive the same distributions or dividends as those we make to the holders of our ordinary shares, and, in some limited circumstances, they may not receive dividends or other distributions on our ordinary shares and may not receive any value for them, if it is illegal or impractical to make them available.
The depositary for the ADSs has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities underlying the ADSs, after deducting its fees and expenses. ADS holders will receive these distributions in proportion to the number of ordinary shares their ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act of 1933, as amended, or the Securities Act, but that are not properly registered or distributed under an applicable exemption from registration. In addition, conversion into U.S. dollars from foreign currency that was part of a dividend made in respect of deposited ordinary shares may require the approval or license of, or a filing with, any government or agency thereof, which may be unobtainable. In these cases, the depositary may determine not to distribute such property and hold it as “deposited securities” or may seek to affect a substitute dividend or distribution, including net cash proceeds from the sale of the dividends that the depositary deems an equitable and practicable substitute. We have no obligation to register under U.S. securities laws any ADSs, ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. In addition, the depositary may deduct from such dividends or distributions its fees and may withhold an amount on account of taxes or other governmental charges to the extent the depositary believes it is required to make such withholding. These restrictions may cause a material decline in the value of the ADSs.
ADS holders do not have the same rights as our shareholders.
ADS holders do not have the same rights as our shareholders. For example, ADS holders may not attend shareholders’ meetings or directly exercise the voting rights attaching to the ordinary shares underlying their ADSs. ADS holders may vote only by instructing the depositary to vote on their behalf. If we request the depositary to solicit voting instructions from ADS holders (which we are not required to do), the depositary will notify ADS holders of a shareholders’ meeting and send or make voting materials available to them. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject to the laws of Israel and the provisions of our articles of association or similar documents, to vote or to have its agents vote the deposited ordinary shares as instructed by ADS holders. If we do not request the depositary to solicit voting instructions from ADS holders, they can still send voting instructions, and, in that case, the depositary may try to vote as they instruct, but it is not required to do so. Except by instructing the depositary as described above, ADS holders won’t be able to exercise voting rights unless they surrender their ADSs and withdraw the ordinary shares. However, they may not know about the meeting enough in advance to withdraw the ordinary shares. We cannot assure ADS holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ordinary shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may not be able to exercise voting rights and there may be nothing they can do if their ordinary shares are not voted as they requested. In addition, ADS holders have no right to call a shareholders’ meeting.
ADS holders may be subject to limitations on transfer of their ADSs.
ADSs will be transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, under any provision of the deposit agreement, or for any other reason in accordance with the terms of the deposit agreement.
As a foreign private issuer whose ADSs are listed on Nasdaq, we follow certain home country corporate governance practices instead of certain Nasdaq requirements, we are not subject to U.S. proxy rules and are exempt from certain Exchange Act reporting requirements. If we were to lose our foreign private issuer status, our costs to modify our practices and maintain compliance under U.S. securities laws and Nasdaq rules would be significantly higher.
We are a foreign private issuer and are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. We are permitted to follow certain home country corporate governance practices instead of certain requirements of the rules of Nasdaq. As permitted under the Israeli Companies Law 1999, or Companies Law, pursuant to our articles of association, the quorum for an ordinary meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a voting instrument, who hold at least 25% of the voting power of our shares (and in an adjourned meeting, with some exceptions, a minimum of one shareholder) instead of 33 1⁄3% of our issued share capital as otherwise required under the Nasdaq corporate governance rules. We may also adopt and approve material changes to equity incentive plans in accordance with the Companies Law, which does not impose a requirement of shareholder approval for such actions. In addition, we follow Israeli corporate governance practice instead of the Nasdaq requirements to obtain shareholder approval for certain dilutive events (such as issuances that will result in a change of control, certain transactions other than a public offering involving issuances of a 20% or greater interest in us and certain acquisitions of the stock or assets of another company). Additionally, we follow Israeli corporate governance practices instead of Nasdaq requirements with regard to, among other things, the composition of our board of directors. For example, our board of directors currently comprises four directors, three of whom we have determined are independent, however during 2021, the majority of our board of directors was not deemed to be independent, in compliance with our home-country requirements. Accordingly, our shareholders may be afforded less protection that what is provided under the Nasdaq corporate governance rules to investors in U.S. domestic issuers. See “Item 16G. —Corporate Governance—Nasdaq Listing Rules and Home Country Practices.”
Additionally, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Furthermore, although under regulations promulgated under the Companies Law, as an Israeli public company listed on Nasdaq, we are required to disclose the compensation of our five most highly compensated officers on an individual basis, this disclosure may not be as extensive as that required of U.S. domestic reporting companies. In addition, we are not required under the Exchange Act to file current reports and quarterly reports, including financial statements, with the SEC as frequently or as promptly as U.S. domestic reporting companies whose securities are registered under the Exchange Act. Moreover, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information. These exemptions and leniencies reduce the frequency and scope of information and protections available to ADS holders in comparison to those applicable to U.S. domestic reporting companies.
If we cease to qualify as a foreign private issuer, we would be required to comply fully with the reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We would lose our foreign private issuer status if a majority of our shares are owned by U.S. residents and a majority of our directors or executive officers are U.S. citizens or residents or we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers and we would lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers. Such modifications and subsequent compliance would cause us to incur significant legal, accounting and other expenses that we would not incur as a foreign private issuer.
If we are a “passive foreign investment company” for U.S. federal income tax purposes, there may be adverse U.S. federal income tax consequences to U.S. investorsinvestors.
Based on our income and assets, we believe that we shouldmay be treated as a PFIC for the preceding taxable year. However, the determination of our PFIC status is made annually based on the factual tests described below. Consequently, while we may be a PFIC in future years, we cannot estimate with certainty at this stage whether or not we are likely to be treated as a PFIC in the current taxable year or any future taxable years. Generally, if, for any taxable year, at least 75 percent of our gross income is “passive income” or at least 50 percent of the average percentage of our gross assets during the taxable year (based on the average of the fair market values of the assets determined at the end of each quarterly period) are assets that produce or are held for the production of passive income, we will be characterized as a PFIC for U.S. federal income tax purposes. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions, and gains from assets that produce passive income. However, rents and royalties received from unrelated parties in connection with the active conduct of a trade or business should not be considered passive income for purposes of the PFIC test. For example, if we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. Holder (as defined in “Item 10.—Additional Information—Taxation — Material United States federal income tax considerations”) holds ordinary shares or ADSs, such U.S. Holder could be subject to additional taxes and interest charges upon certain distributions by us and any gain recognized on a sale, exchange or other disposition of our shares, whether or not we continue to be characterized as a PFIC. Certain adverse consequences of PFIC status can be mitigated if a U.S. Holder makes a “mark to market” election or an election to treat us as a qualified electing fund, or QEF. Upon request, we expect to provide the information necessary for U.S. Holders to make “qualified electing fund elections” if we are classified as a PFIC. See “Item 10.—Additional Information—Taxation—Passive foreign investment company considerations.”
Whether we are a PFIC for any taxable year will depend on the composition of our income and the composition and value of our assets from time to time. Each U.S. Holder is strongly urged to consult its tax advisor regarding these issues and any available elections to mitigate such tax consequences.
If we are a controlled foreign corporation, there could be adverse U.S. federal income tax consequences to certain U.S. Holders.
Each “Ten Percent Shareholder” (as defined below) in a non-U.S. corporation that is classified as a “controlled foreign corporation,” or a CFC, for U.S. federal income tax purposes generally is required to include in income for U.S. federal tax purposes such Ten Percent Shareholder’s pro rata share of the CFC’s “Subpart F income,” “tested income,” “global intangible low-taxed income” and investment of earnings in U.S. property, even if the CFC has made no distributions to its shareholders. Subpart F income generally includes dividends, interest, rents, royalties, gains from the sale of securities and income from certain transactions with related parties. In addition, a Ten Percent Shareholder that realizes gain from the sale or exchange of shares in a CFC may be required to classify a portion of such gain as dividend income rather than capital gain. A non-U.S. corporation generally will be classified as a CFC for U.S. federal income tax purposes if Ten Percent Shareholders own, directly or indirectly, more than 50% of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock of such corporation. A “Ten Percent Shareholder” is a United States person (as defined by the Internal Revenue Code of 1986, as amended, or the Code) who owns or is considered to own, directly, indirectly, or constructively, 10% or more of the value or total combined voting power of all classes of stock entitled to vote of such corporation.
The determination of CFC status is complex and includes complex attribution rules. A non-corporate Ten Percent Shareholder with respect to a CFC generally will not be allowed certain tax deductions or foreign tax credits generally available to a corporate Ten Percent Shareholder. Failure to comply with CFC reporting obligations may subject a Ten Percent Shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any Ten Percent Shareholder information that may be necessary to comply with the reporting and tax paying obligations applicable under the CFC rules of the Code. U.S. Holders should consult their own tax advisors with respect to the potential adverse U.S. tax consequences of becoming a Ten Percent Shareholder in a CFC.
ITEM 4. |
A. History and Development of the Company
We were incorporated in May 2018 in Israel as DocoMed Ltd., and originally provided digital health services. In July 2019, we changed our name to MeaTech Ltd. and later Steakholder Innovation Ltd., or MeaTech,Steakholder Innovation, and commenced our cultured meat technology development operations. In January 2020, MeaTechSteakholder Innovation completed a merger with Ophectra Real Estate and Investment Ltd., or Ophectra, a company incorporated in Israel whose shares were traded on the TASE, whereupon the name of Ophectra was changed to Meat-Tech 3D Ltd., and later further changed tothen MeaTech 3D Ltd. and finally Steakholder Foods Ltd., or MeaTech 3D.Steakholder Foods.
According to the terms of the merger, MeaTech 3DSteakholder Foods acquired all outstanding shares of MeaTechSteakholder Innovation from MeaTech’sthe shareholders of Steakholder Innovation, in return for the issuance of ordinary shares to the shareholders of MeaTech,Steakholder Innovation, as well as non-tradable merger warrants to receive ordinary shares upon the achievement of pre-defined milestones, which were achieved in 2020 and 2021. MeaTechSteakholder Innovation become MeaTech 3D’sSteakholder Foods’ wholly-owned subsidiary, and later changed its name to Chicken Meat-Tech Ltd. and then to MeaTech MT Ltd.subsidiary.
In connection with the merger, the Tel Aviv District Court for Economic Affairs approved an arrangement whereby all of Ophectra’s assets and liabilities, whether certain or contingent, at the time of the merger were irrevocably assigned to a settlement fund, or Settlement Fund, for the purpose of settling Ophectra’s pre-merger liabilities (except for Ophectra’s ownership of 14.74% of the outstanding shares of Therapin Ltd., as described below). This includes all future liabilities arising from Ophectra’s activities prior to the merger (including tax liabilities, if any), and any commitments made by Ophectra prior to the merger. We also provided the Settlement Fund approximately NIS 1.3 million (approximately $0.4 million), which we included in our public listing expenses, for the purpose of settling any of Ophectra’s debts, and bear no additional liabilities to the Settlement Fund. Anyone who believed they had a claim to Ophectra’s assets were invited to lodge their claims to the trustees. Due to the fact that more than two years have passed since the merger, and the fact that the Settlement Fund no longer contains any assets, its trustees are expected to instigate proceedings to wind up the Settlement Fund.
Although MeaTech 3DSteakholder Foods was the legal acquirer of MeaTech’sSteakholder Innovation’s shares as described above, because (i) the shareholders of MeaTech received the majority of the voting rights in MeaTech 3D and the ability to determine its financial and operational policy, (ii) the management of MeaTech continues to serve as the management of MeaTech 3D and (iii) at the time of completion of the merger MeaTech 3D was a company without significant business operations, the merger is not considered a business acquisition as defined in IFRS 3.ASC 805. As a result, it was determined that MeaTechSteakholder Innovation is the acquirer of the business for accounting purposes and the transaction was treated as a reverse acquisition that does not constitute a business combination.
In March 2021, we completed an initial public offering on the consolidated financial statementsNasdaq Capital Market, listing American Depositary Shares (ADSs) for trade under the ticker STKH, and financial data included herein for all periods through and including December 31, 2019 were adjusted retroactively to reflectlater voluntarily de-listed our ordinary shares from the financial statements of MeaTech (now called MeaTech MTTASE.
On April 3, 2023, we announced our participation in an investment round in Wilk Technologies Ltd.), other than the information concerning earnings per share, which is presented according to the equity information of MeaTech 3D (then called Ophectra Real Estate and Investments Ltd.), and our consolidated financial statements and financial data included herein from January 1, 2020 onward relate to MeaTech 3D.
B. Business Overview
Overview
We are an international deep-tech food company that initiated activities in 2019 and isare listed on the Nasdaq Capital Market under the ticker “MITC”“STKH”. We maintain facilities in Rehovot, Israelare focusing on alternative protein machinery production, initially for three-dimensional printing of meat and Antwerp, Belgiumseafood analogs, followed by hybrid meats that combine cultivated and are in the process of expanding activities to California, USA.plant-based elements. We believe that our alternative protein and cultivated meat technologies hold significant potential to reduce the environmental impact of food production (including reducing carbon footprint and promoting biodiversity), improve meat production, simplify the meat supply chain, and offer consumers a range of new product offerings.
We aim to provide anproduction technology and associated supplies needed to commercially produce structured alternative to industrialized animal farmingprotein products. To that reduces carbon footprint, minimizes water and land usage, and prevents the slaughtering of animals. By adopting a modular factory design,end, we expect to be able to offer a sustainable solution for producing a variety of beef, chicken and pork products, both as raw materials and whole cuts.
During 2023, we focused our efforts on commercializing our three-dimensional printers and their ingredient blend supplies for plant-based foods initially. As a result, our first commercial offering is intended to affordably generate revenues for our partners and customers by manufacturing plant-based meat and fish analogs, which are not expected to require the lengthy regulatory processes associated with cultivated meats and other novel foods.
In addition, we are developing hybrid cultivated meat technologies including three-dimensional printing technology, together with biotechnologyto be integrated into our production processes and customizable manufacturing processes in order to manufacture cultivated meat that does not require animal slaughter. We are developing a novel, proprietary three-dimensional bioprinter to deposit layers of differentiated stem cells, scaffolding, and cell nutrients in a three-dimensional form of structured cultured meat.at high throughput, once these technologies become commercially viable. We believe that the culturedcultivated meat production processes we are developing, which are designed to offer our eventualpartners and customers an alternative to industrial slaughter, will have the potential to improve the quality of the environment, shorten global food supply chains, and reduce the likelihood of health hazards such as zoonotic diseases transferred from animals to humans (including viruses, such as virulent avian influenza and COVID-19, and drug-resistant bacterial pathogens, such as some strains of salmonella).
In December 2021,May 2022, we joined the United Nations, or UN, Global Compact initiative, committing to ten universally accepted principles in the areas of human rights, labor, environment, and anti-corruption and to act in support of the issues embodied in the UN’s Sustainable Development Goals.
In April 2023, we announced that we had successfully three-dimensionally printed the world’s first hybrid fish fillet, by customizing our plant-based white fish ingredient blend, combined with cultivated grouper cells.
In July 2023, we announced that we had entered into a 3.67 oz cultivated steak, primarily composedMemorandum of cultivated real fat and muscle tissues. While cultivated meat companies have made some progress developing unstructured alternative meat products, suchAgreement for Strategic Cooperation, or MOA, with an accredited GCC-based governmental body as minced meat and sausage,our strategic partner, to advance food security efforts through the bestapplication of our knowledge,3D printing technology. Commencing with an investment by the industry has struggledstrategic partner in developing high-margin, high-value structuredthe construction of a pilot plant to produce printed products, the MOA eventually aims to create a first-of-its-kind large-scale production facility in the Persian Gulf region. The agreement foresees a material initial down payment to us for the procurement of at least one three-dimensional food printer, followed by a milestone-based sales and cultured meat products such as steak. Unlike minced meat, a cultured meat steak product has to grow in fibers and contain connective tissues and fat. To be adopted by diners,procurement plan for industrial-scale output.
In February 2024, we believe cultured steaks will need to be meticulously engineered to look and smell like conventional meat, both before and after cooking, and to taste and feel like meat to the diner. We believeannounced that we are the first company to be developing both a proprietary bioprinterhad entered into an inaugural private-sector Memorandum of Understanding, or MoU, for strategic cooperation with Wyler Farm, one of Israel’s leading alternative protein producers and the related processes for growing cultivated meat to focus on what we believe is a high value sectorits premier tofu producer. The terms of the alternative protein market.non-binding MoU contemplate Wyler Farm acquiring one of our proprietary meat printers, along with a subscription to our software and plant-based meat ingredient blend, by early 2025.
We are led by our Chief Executive Officer, Arik Kaufman, who has founded various Nasdaq- and TASE-traded foodtech companies, and currently serves as director of Wilk Technologies Ltd.companies. He is also a founding partner of theBlueOcean Sustainability Fund, LLC, or BlueSoundWaves, collective, led by Ashton Kutcher, Guy Oseary and Effie Epstein, which recentlyhas partnered with MeaTechSteakholder to assist in attempting to accelerate the Company’sour growth. Mr. Kaufman holds extensive personal experience in the fields of food-tech and bio-tech law, and has led and managed numerous complex commercial negotiations, as part of local and international fundraising, mergers and acquisitions, or M&A, transactions and licensing agreements. We have carefully selected personnel for the rest of our executive management team who possess substantial industry experience and share our core values, from fields as diverse as tissue engineering, industrial stem cell growth, and printer and print materials development.
Alternative Protein Market Opportunity
Protein is a necessary staple for healthy nutrition. The growth in recent years of both the human population and global wealth is driving a decades-long trend of accelerating demand for meat. The demand for protein products has consistently risen in recent decades and is expected to continue to do so. Theso, however the rising growth of demand for farm animals for the food industry has created significant environmental, health, financial and ethical challenges.
Alternative proteins, with an emphasis on meat substitutes, have emerged as a leading method for dealing with these challenges. Globally, more than 670 million kilograms of meat substitutes are consumed annually, at a value of the global meat sector was estimated at $838exceeding USD 11 billion in 2020, and was forecast to increase to $1,157 billion by 2025. According to market research firm Fortune Business Insights,2024. In the global meat substitute market was estimated at $5.4 billion in 2021 andnear term alone, this is expected to growrise to $10.8over 980 million kilograms by 2028, valued at over USD 16 billion.
Meat substitutes are the second most valuable category of plant-based food products in the United States, where sales of plant-based analogs were estimated to be worth USD 1.8 billion in 2023, exceeded only by 2028. According to Factsthe People’s Republic of China (USD 2.1 billion in 2023 and Factors Market Research,2.4 billion in 2024). Other countries in the cultivatedworld’s most populous continent led global rates of consumer spending for meat substitutes, chiefly Vietnam (24%) and Hong Kong (21%).
Meat substitutes are likewise the second-most valuable category alone is expected to reach $248 millionof plant-based food products in Europe, with sales reaching EUR 2 billion in 2022, led by 2026, with an annual growth ratethe United Kingdom (21%) and the Netherlands (19%) in rates of approximately 16%. With regard to the longer term, Barclays predicted in November 2021 that by 2040, 20%consumer spending.
Among those taking note of the demand for meat globally will be provided by cultivated meat – a $450 billion market opportunity.
Limitations of Conventional Meat Production
In addition to questions about whether conventional meat production can adequately provide for the growing global population, conventional meat production raises serious environmental issues. According to the United Nations, 8% of the world'sworld’s freshwater is used for raising livestock for meat and leather. At least 18% of the greenhouse gases entering the atmosphere are from the livestock industry. 26% of the planet'splanet’s ice-free land is used for livestock grazing and 33% of croplands are used for animal feed. With regard to treatment of animals in conventional meat production, approximately Morethe Humane Society of the United States estimated in 2023 that more than 7092 billion animals are slaughtered annually, with steady increases to be expected in line with increased demand for meat.
Another common consumer concern with industrial-scale animal rearing is the reliance on the intensive use of antibiotics. Antibiotics are used in livestock, especially pigs and poultry, to manage animal health, and to treat or prophylactically prevent diseases such as avian flu and swine flu. Their effects on human health have not been fully resolved, with concerns including the potential growth of antibiotic-resistant diseases in meat for human consumption.
Existing Alternative Proteins and their Limitations
Negative consumer sentiment towards the perceived ethical, health and environmental effects of the global meat industry help explain the strong focus that has developed on creating methods of protein production that are more sustainable, nutritious and conscious of animal welfare. Recent years have seen a combination of increasing consumer awareness and advanced technological development that has led to substantially increased demand for proteins that do not involve animal slaughter beyondbesides traditional plant-based proteins, such as soy, peas and chickpeas. Some of the alternative proteins being developed for human consumption for this purpose include:
Mycoproteins:Some of the most commercially successful novel alternative proteinPlant-Based Meat: These products are currentlydesigned to look, cook, and taste like traditional meat, with the texture of traditional meat while using plant ingredients. Innovations in this field have led to plant-based burgers, sausages and nuggets that mimic their animal-based counterparts in an approach known as biomimicry.
Cultivated Meat: Meat grown through cellular agriculture, which aims to produce cultivated animal proteins without the need for slaughter. Cultivated meat is grown in cell culture rather than inside animals and applies tissue engineering practices for fat and muscle production for the purpose of human consumption. Stem cells are isolated from animal tissue, such as from an umbilical cord (following birth), an adipose or a muscle tissue, and then cultivated in vitro to form protein biomass, muscle fibers and fat cells.
Fermentation-Derived Proteins: Created through microbial fermentation. Examples include precision fermentation products, and mycoproteins, which are derived from fungi. They are high in protein high inand fiber, low in saturated fat, and contain no cholesterol. However, they have been associated with allergic and gastrointestinal reactions. They are fermented to become a dough, which can develop a texture similar to that of meat.
Insects: Insect-Based Proteins: Insects are an environmentally-friendly source of protein requiringthat requires significantly less land and water, and emittingemits significantly less greenhouse gases than large mammals raised for slaughter. In addition, they can be fed food unsuitable for livestock that would otherwise be wasted. While crickets are the most common source of edible insects, research is currently taking place on new insect species of value for food production, as well as methods to produce them economically at scale. Insects can be consumed in their natural state; however many cultures consider insect consumption to be taboo and many people are disgusted by the idea. As a result, research is taking place into developing insect-based products in different forms not easily discernable as insect-based, including flour.
Three-Dimensional Printing Solution
Our three-dimensional food printers are designed as specialized, large-scale production machines, tailored to meet the distinct requirements for producing plant-based meat and seafood alternatives. We are developing two distinct types of industrial-grade printers, engineered to handle the specific textures and production requirements of meat or seafood alternatives, respectively, within high-volume food manufacturing environments.
Our Three-Dimensional Printer Types
Meat-Specific Three-Dimensional Printers
Our meat-specific printers are equipped with advanced Fused Paste Layering, or FPL, technology. This technology is ideal for creating the dense, fibrous textures associated with various types of meat, from beef to poultry. The FPL process meticulously layers plant-based materials to mimic the natural structure of meats, including intricate marbling and texture variations that are critical for authentic taste and mouthfeel. These printers are designed to integrate seamlessly into existing meat production lines within food factories, complementing traditional meat processing equipment with minimal adjustment required.
Seafood-Specific Three-Dimensional Printers
Our seafood-specific printers utilize Drop Location in Space, or DLS, technology, which specializes in replicating the delicate, flaky textures of Peacevarious seafoods, from white fish to shellfish. DLS technology is designed to precisely position plant-based materials to emulate the unique physical properties of Meatseafood, including layering and tender flakiness, essential for authentic experiences in alternative-protein seafood dishes. These machines are also built to integrate with standard seafood processing lines in food factories, ensuring that they can operate alongside other familiar equipment without disrupting existing workflows.
Key Features of Both Meat- and Seafood-Specific Printers
| ● | Industrial Scale Production: Both types of printers are designed for high output, capable of continuous operation in demanding factory settings. They support large-scale production needs, ensuring that food manufacturers can meet substantial market demands efficiently. |
| ● | Seamless Factory Integration: Each printer model is crafted to fit into existing production environments easily. They interface smoothly with other factory equipment such as automated feed systems, conveyors, and packaging lines, ensuring a cohesive and streamlined production process. |
| ● | Compliance with Regulatory Standards: We are committed to maintaining strict standards of food safety and hygiene in all of our manufacturing processes. In line with this commitment, all of our three-dimensional food printers – whether meat-specific or seafood-specific – are designed in accordance with regulatory requirements and guidelines, such as the European Hygienic Engineering & Design Group guidelines. This ensures that our printers meet rigorous standards for safe food production, which we believe is essential for maintaining consumer health and trust. |
| ● | Customization and Flexibility: Despite their specialized functions, both printer types offer customizable settings to adjust textures, shapes, and sizes, allowing for a high degree of product personalization and innovation within the plant-based food industry. |
By offering these two distinct types of three-dimensional food printers, we finalizedaim to provide food manufacturers with the precise tools needed to expand into or enhance their plant-based product offerings. Whether producing plant-based meats with realistic textures or crafting delicate seafood alternatives, our acquisition of Peace of Meat, a Belgian producer of cultured avian products, for uptechnology is designed to $19.9 millionempower manufacturers to be leaders in cash and equity, depending on milestone achievements. We intend to leverage Peace of Meat’s cultured avian technologies to diversify our own bovine-oriented technologies and expedite our entry into the rapidly growing market for plant-based meat alternativessustainable and cultured products.innovative food solutions.
Premix Blends
At the core of our product catalog are the SHMeat™ and voluntary delisting from the Tel Aviv Stock Exchange
We are developing SHMeat™ Premix Blends in an incubatorarray of premix blends to mature, allowingbe available in both a printed format, for structured end products such as steaks, and a minced format, for unstructured end products, crafted to enhance culinary creativity and versatility with a range that extends to include pork, lamb, and exotic meat options. Each blend is being developed to mimic the stem cellsexact taste and texture profile of its traditional meat counterpart, in order to differentiate into fat and muscle cells and develop into fat and muscle tissueprovide an authentic culinary experience. The variety to form our steak.
We are fused into significant muscle fibers that better resemble those in whole cuts of meat. Bovine stem cells were isolated, proliferated in the lab, and differentiated into matured muscle cells with improved muscle fiber density, thickness and length. Based on these improvements, we have filed a provisional patent applicationalso developing SHFish™ Premix Blends, with the USPTO.
By choosing Steakholder Foods’ innovative solutions, our customers can provide consumers with traditional flavors in a few dozen companiesmore sustainable and institutions actively workingethical way, helping to develop technologiestransform the global food landscape and other products to meet this demand, some of whompaving the way for a more sustainable culinary future.
Advantages
We are focused on producing red meats, while othersdeveloping a process that will allow our food technology customers to operate a high-throughput manufacturing process for high-quality, healthy meat. Our cellular agriculture and bioprinting processes are focused on fish and crustaceans. Some of these companies are working on culturing various types of cells, such as chicken, pork, kangaroo and foie gras.being designed to be modular, meaning that they can work using different factory sizes. We believe this push on scaling-upwe could license our technology to customers with industrial plants close to urban areas seeking to provide “just in time”, logistically-efficient, local and premium cellular agriculture hasagriculture. In addition, we believe a licensee of our technology could build a plant in a locality that does not have the potentialresources needed for industrial animal husbandry, which would allow places like the United Arab Emirates, Hong Kong or Singapore to offer a solution to the scale and environmental challenges confronting conventional meat production. Other alternative protein competitors are already selling plant-based meat substitutes, but topotentially become more agriculturally independent by increasing food security. As costs decrease, we believe that our knowledge, these companies are not focused on thecustomers could also build production of real meat products produced with animal cells with no pea or soy ingredients.
We believe that cultivated meatplant-based meats could have several potential advantages over conventionally-harvested meat:
| ● |
| ● |
| ● | Animal |
| ● |
Alternate Use of Natural |
| ● | Food |
Our Strategy
Our vision is to be a global leader in making realthe alternative protein machinery field, offering printers based on our three-dimensional printing technology, as well as ingredient blends for use in the printers. In the future, we also aim to provide ingredient blends that include cultivated cells, for the printing of hybrid meat through advanced biotechnology and engineering solutions for a more sustainable world.seafood. We are committed to making the right choice of meatprotein simple for end consumers, simple by developing high-quality realcommercial production machines that are capable of mimicking meat and seafood texture, flavor and nutritional values, all at an affordable cost so that is slaughter-free, delicious, nutritious,we can offer our customers food printers to create products that end consumers will want to buy. Operators of our printers will have wide-ranging digital control over parameters of the products they print, including with respect to meat type, three-dimensional modeling, texture, flavor and safer than farm-raisednutritional values. In this way, end products can be adjusted and customized based on market demand. Our commercial-scale production machines are being designed to be modular, scalable starting from minimal production capacity of up to a few hundred kilograms per hour.
We are in the process of establishing a demonstration center at our headquarters, in order to demonstrate the full commercial production process, including all pre- and post-production stages, such as ingredients blend preparation, pasteurization and packaging, culminating in edible meat by adoptinganalogs produced from a factory design intendedcommercial-scale printer. Once we successfully commercialize our first printers, we expect to offer a sustainable solution for producing a variety of beef, chicken, and pork products, whether as raw materials or final consumer products.also be able to demonstrate our printers at work in our customers’ food production sites.
Our technologies and processes have the potential to be sustainable. We are developing a meat production processprocesses that isare designed to provide sustainability in an industry that, due to inefficiencies inherent in conventional meat farming, is not otherwise expected to be able to meet the growing demand for protein caused by rising population numbers and global affluence, due to inefficiencies inherent in conventional meat farming.affluence. These include the large amountamounts of land and water use that are needed for raising livestock, causingwhich causes precious natural resources to be squandered.
We have carefully selected personnel for our management team who possess substantial industry experience, from diverse fields including the food industry, bioprinting,business development, three-dimensional printing, food technologies, tissue engineering, industrial stem cell growth, software engineering, electronic and bioprintermechanical engineering and print materials development. We believe that this blend of talent and experience in managers who share our core values gives us the requisite insights and capabilities to execute our plan to develop technologies designed to meet demand in a scalable, profitable and sustainable way.
Pipeline Application – Hybrid Product Inks
Perfect the development of ourThe cultivated meat manufacturing technology and processes. We intend to continue developing and refining our processes, procedures and equipment until we are in a position to commercialize our technologies, whether by manufacturing final products for consumers (B2C and B2B2C models) or ingredientsindustry aims for industrial use, as well asproduction at a competitive price compared to conventional meat. However, challenges in outlicensing (B2B models). We are continuingscalability and cost have slowed progress. While the vision of cultivated meats steak remains a goal and the industry is actively working to tackleovercome the technological challenges involved in scaling up both our biological and printing processes to industrial-scale levels.
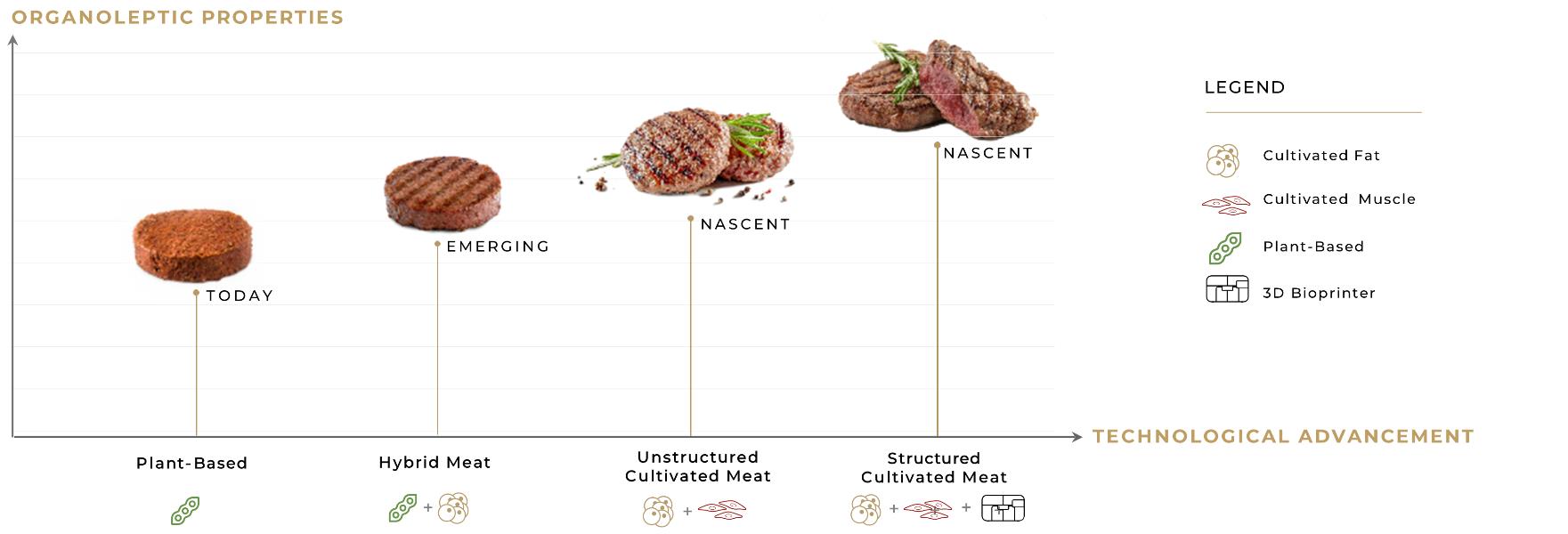
The process of cultivating bovine cells is developed in-house. Integrating these cells into the hybrid product could involve either undifferentiated stem cell biomass or cells differentiated into specialized cell types. Traditional steak is naturally composed of muscle, fat, and connective tissue, so cells forming any of these parts could potentially enhance the final product. We are developing cellular agriculture technology, including cell lines and methods for working with growth media and develop differentiation media, to support the production of cells such as fat and muscle cells as well as undifferentiated biomass. The processes we are developing are designed to allow cells of interest, following humane tissue extraction from the umbilical cord or biopsy, to be isolated, replicated, grown and maintained in vitro under controlled, laboratory conditions.
We are engaged in exploring and evaluating the contributions of fat cells, muscle cells, and undifferentiated stem cells to the hybrid product, while also considering the unique obstacles each of these cell types faces in scaling up to industrial production. In February 2023, we announced that we had analyzed our muscle cells and found that they offer the same amino acid profile as that of the native tissue. The amino acid profile has two important roles in our cultivated beef products – taste and nutritional value. Our biology team tested 17 amino acids and compared them to native tissue, with the results showing that the team was able to create the same amino acid profile in the lab as in animals, For this reason, we believe that hybrid meats printed using our three-dimensional printers have the potential to provide similar nutritional value as that of conventional meats.
Meat is a rich source of amino acids, the building blocks of proteins. which play a crucial role in human nutrition. Essential amino acids, which the human body cannot produce on its own, include leucine, isoleucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, and histidine. These amino acids are important for a variety of bodily functions, including muscle growth and repair, immune function, and hormone production. The specific amino acid profile of meat, as well as its taste, varies depending on the animal species and how it is raised, as well as the cut of meat.
Integrating fat cells into the final hybrid product also appears to be a promising direction of development. Our cultivated fat biomass is engineered to be antibiotic-free and can be customized to offer personalized nutritional profiles and precise flavors, similar to natural beef fat. To this end, we have conducted a number of taste tests where we demonstrated the potential that our cultivated fat biomass has to enhance the taste of plant-based protein products. We believe that a product comprised of as little as 10-25% of our cultivated fat biomass combined with plant-based proteiningredients has the potential to enhance meatiness.mouthfeel and overall experience.
Cell lines development for cultivated meat products
The process of industrial scale meat printing necessitates the isolation and development of cells which have the potential to produce animal muscle or fat tissues. Our cultivated fat biomass is designed to be free of antibiotics, can provide enhanced fatty acid profiles, andproprietary cell lines are isolated from various sources that harbor these properties. For example, adult stem cells can be tailoredisolated from various adult tissues and give rise to provide personalized nutritional profiles.more cells of the same type, such as fat or muscle tissues, while stem cells isolated from the umbilical cord immediately following birth can give rise to multiple types of cells, including both fat and muscle cells. Each of these cells has advantages and disadvantages and their adaptation to our robust meat production process is currently being evaluated.
Once isolated and plated, stem cells derived from proprietaryadult tissues or umbilical cord exhibit the intrinsic ability to continue dividing and propagating in vitro; however, their proliferative capacity is limited, rendering them unable to yield a sufficient number of cells for large-scale production. To facilitate industrial-scale production, we have developed an immortalization process. Through this process, our immortalized cell lines can continue proliferating for up to one year, maintaining a steady division rate. It’s noteworthy that this division rate is significantly lower than the primary source cells, ensuring a consistent and prolonged expansion for enhanced scalability.
In order to facilitate scale-up processes, it is imperative for cells to grow in suspension, as monolayer growth on traditional laboratory plates proves both cost-ineffective and incompatible with scaling up operations. To address this challenge, we have successfully developed an adaptation to a suspension growth process for our cell lines. These cells grow naturallyNotably, this innovative approach has been successfully applied to various cell lines derived from adult tissues and umbilical cord origins. This achievement positions us strategically for the next crucial step in suspension,upscaling our production capabilities.
Media development
We are in high densities. They also proliferate continuously, are relatively large and tend to easily accumulate lipid. This quality of the cells makes them an excellent candidate for producing cultured fat, so we have used them to build a robust cell line, free of genetic modifications, which we are now attempting to upscale towards industrial production volumes. Our most advanced cell line is being built with non-GMO pluripotent stem cells that can differentiate into muscle cells and fat cells, and form connective tissue, which need fewer high-cost media components, such as growth factors, for their development, which is why these cells may have higher growth potential with lower costs than alternative technologies. We have likewise developed the process for isolating, growing and differentiating bovine stem cells into muscle fibers, fat biomass and connective tissue.

Some of the steps that we are developing are designedtaking in order to allow cells of interest, following humane tissue extraction fromkeep the umbilical cord or biopsy, to be isolated, replicated, grown and maintained in vitro under controlled, laboratory conditions.

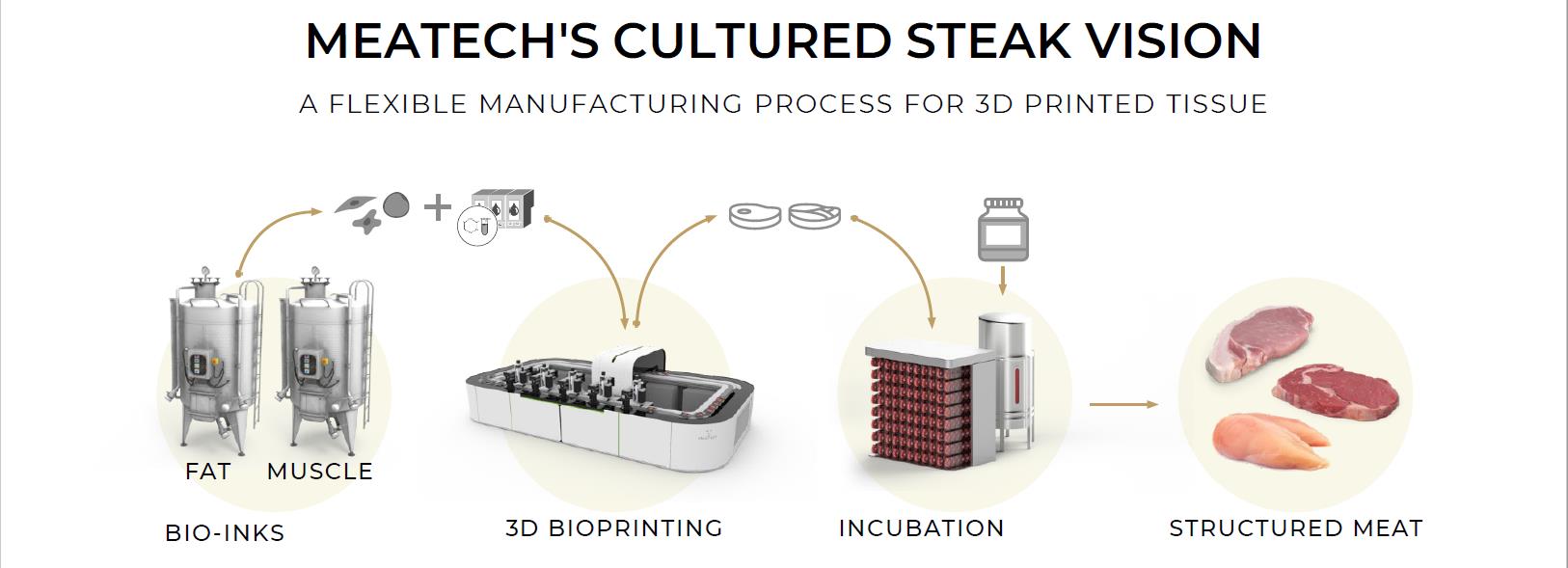
| ● | Developing our own in-house medium tailored specifically for our cell types. By including only the essential components necessary for the growth of each particular cell line, our medium costs significantly less than commercial alternatives; |
| ● | Replacing expensive, animal-derived components in cell growth media with lab-developed compounds or proteins developed through fermentation; |
| ● | Cell line screening and optimizations for efficient growth in low-cost media; and |
| ● | Bioprocess optimization and media recycling. |
Bioreactors
We are using software-controlled bioreactors to foster cell proliferation.proliferation for hybrid meats, and eventually cultivated meats. The initial growth phase leverages exponential growth of stem cells to achieve sufficient cell volumes for food production. These stem cells undergoinitiate differentiation processes into multiple cell types, such as muscle and fat, as well as cell maturation, where cells continue to proliferate, spread and, for printed cells, produce the extra-cellular matrix which transforms the dispersed printed cells into a whole tissue.fat.
We are in the process of developing cell-culturingcell-cultivation processes and protocols unique to each cell line for use in bioreactors, by regulating the system parameters while monitoring the growth, viability and metabolism of our cells. We are continuously exploring diverse bioreactor systems. Such bioreactor systemsinfrastructures and refining cell harvesting techniques to optimize the yield of biomass production. To date, we have successfully advanced our yield and achieved high cell density in a short period of time, and we expect that our cell cultivation records in small-scale bioreactors will enable monitoring and control of growth parameters, as well as testing andus to develop the proprietary information needed for the development of efficient and economical cell-growth processes in industrial breeding containers. Separate fromindustrial-scale bioreactors.