United StatesSecurities and Exchange CommissionWashington,UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
☐REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 20192022
OR
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report
For the transition period from ____ to ____
Commission file number 001-36578
Enlivex Therapeutics Ltd.
(Exact name of Registrant as specified in its charter)
State of Israel
(Jurisdiction of incorporation or organization)
14 Einstein Street, NesNess Ziona, Israel 7403618
(Address of principal executive offices)
Mr. Oren Hershkovitz
Tel: +972.2.6208072
Email: Oren@enlivexpharm.com
Facsimile: +972.2.6208070
14 Einstein Street, NesNess Ziona, Israel 7403618
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class | Trading symbol | Name of each exchange on which registered | ||
| Ordinary Shares, par value of NIS 0.40 | ENLV | Nasdaq Capital Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
10,334,12618,421,852 Ordinary Shares, par value NIS 0.40 per share as of December 31, 2019
2022
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☒ |
| Non-Accelerated filer | ☐ | Emerging growth company | ☐ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are being registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statement. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.1D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP☒ | International Financial Reporting Standards as issued by the International Accounting Standards Board☐ | Other☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒
Yes ☐ No ☒
TABLE OF CONTENTS
i
INTRODUCTION
Overview of Corporate Structure
On March 26, 2019 (the “Closing Date”), Bioblast Pharma Ltd., a company organized under the laws of the State of Israel (“Bioblast”), and Enlivex Therapeutics Ltd., a company organized under the laws of the State of Israel (n/k/a Enlivex Therapeutics R&D Ltd., “Enlivex R&D”), consummated a merger transaction whereby Enlivex R&D merged with a mergerwholly owned subsidiary of Bioblast, with Enlivex R&D as the surviving entity in the merger (the “Merger”). As a result of the Merger, Enlivex R&D became a wholly owned subsidiary of Bioblast. ConcurrentlyIn connection with the consummation of the Merger, on the Closing Date, Bioblast changedamended its Articles of Association (the “Amended and Restated Articles of Association”), in order to change its name from “Bioblast Pharma Ltd.” to Enlivex“Enlivex Therapeutics Ltd.” and to change its registered capital to NIS 18,000,000 divided into 45,000,000 ordinary shares with a nominal value of NIS 0.40 each. In this Annual Report on Form 20-F, when we refer to the registrant as a combination of Bioblast and Enlivex R&D after giving effect to the Merger, we use the terms “Enlivex,” the “Company,” “we,” “us,” and “ours”. When we refer to the historic business, operations and corporate status of the parent in the Merger we use the term “Bioblast” and when we refer to the historic business, operations and corporate status of the subsidiary in the Merger, we use the term “Enlivex R&D”.“ours.”
Overview of the Merger Transaction
The Merger
On the Closing Date, pursuant to an Agreement and Plan of Merger dated as of November 19, 2018 among Bioblast, Enlivex R&D and a merger subsidiary of Bioblast (the “Merger Agreement”), the Merger was consummated and Enlivex R&D became a wholly-owned subsidiary of Bioblast. The Merger was structured as a statutory merger pursuant to Sections 314-327 of the Companies Law, 5759-1999 of the State of Israel. On the Closing Date, the Company was admitted for continued listing on the Nasdaq Capital Market under the new symbol “ENLV”.
Pursuant to the Merger Agreement, upon consummation of the Merger (the “Effective Time”), each outstanding ordinary share of Enlivex R&D was converted into approximately 0.04841 ordinary shares of the Company (the “Exchange Ratio”).
In addition, all outstanding Enlivex R&D options that were unexercised immediately prior to the Effective Time, whether or not vested, were assumed by the Company, and contain the same terms, conditions, vesting and other provisions, except that each such option is now exercisable for such number of ordinary shares of the Company, as adjusted in accordance with the Exchange Ratio and otherwise in accordance with the Merger Agreement.
Following the Merger and after giving effect to a private placement consummated in connection with the Merger, the former equity holders of Enlivex R&D owned approximately 89.1% of the Company’s issued and outstanding equity, Bioblast shareholders immediately prior to the Merger owned approximately 4.016% of the Company’s issued and outstanding equity, and the Investors (as defined below) owned approximately 6.74% of the Company’s issued and outstanding equity.
Pursuant to a Contingent Value Rights Agreement (the “CVR Agreement”), Bioblast shareholders received one contingent value right (“CVR”) for each ordinary share of Bioblast held as of the record date, March 25, 2019, which CVRs entitle the holders to potential payments that the Company receives in connection with a Trehalose transaction.
The foregoing descriptions of the Merger Agreement and CVR Agreement are only summaries, do not purport to be complete and are qualified in their entirety by reference to the full text of the Merger Agreement and the CVR Agreement, copies of which are filed as exhibits to this Annual Report on Form 20-F and are incorporated by reference herein.
All references to share amounts in this Annual Report on Form 20-F have been retroactively adjusted to reflect the impact of the Exchange Ratio.
ii
Accounting Treatment
The Merger has been treated as a reverse recapitalization of Bioblast for financial accounting and reporting purposes. As such, Enlivex R&D is treated as the acquirer for accounting and financial reporting purposes while Bioblast is treated as the acquired entity for accounting and financial reporting purposes. Further, as a result, the assets and liabilities and the historical operations that will be reflected in the Company’s future financial statements filed with the SEC will be those of Enlivex R&D, and the Company’s assets, liabilities and results of operations will be consolidated with the assets, liabilities and results of operations of Enlivex R&D.
Amendment of Articles of Association
In connection with the consummation of the Merger, on the Closing Date, the Company amended its Articles of Association (the “Amended and Restated Articles of Association”), in order to change its name from “Bioblast Pharma Ltd.” to “Enlivex Therapeutics Ltd.” and to change the registered capital of the Company to NIS 18,000,000 divided into 45,000,000 ordinary shares with a nominal value of NIS 0.40 each.
Business Description of Enlivex
The Company is a clinical stageclinical-stage macrophage reprogramming immunotherapy company, developing an allogeneic drug pipelineAllocetraTM, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing. Immune system rebalancing isand resolution of life-threatening conditions. These non-homeostatic macrophages contribute significantly to the severity of the respective diseases. By restoring macrophage homeostasis, Allocetra™ has the potential to provide a novel immunotherapeutic mechanism of action for life-threatening clinical indications that are defined as “unmet medical needs”, as a stand-alone therapy or in combination with leading therapeutic agents.
Macrophages are tissue-resident or infiltrating immune cells critical for innate immunity, normal tissue development, and repair of damaged tissue. Macrophages’ function is a result of their original designation, their local micro-environment, and the treatmenttype of life-threatening immunemetabolites, substances or pathogens to which they are exposed. Reprogrammed out of their homeostatic state, macrophages contribute to the pathophysiology of multiple diseases, including cancer, sepsis, and various inflammatory conditions, which involve the hyper-expression of cytokines (“Cytokine Release Syndrome”) and for which there are no U.S. Food and Drug Administration (“FDA”)-approved treatments, as well as treating solid tumors by modulating such tumors’ microenvironment.disorders.
TheWe believe the Company’s primary innovative immunotherapy, candidates are novel candidates based on the same unique mechanismAllocetraTM, represents a paradigm shift in macrophage reprogramming, moving from targeting a specific subset of action, which inducesmacrophages or a specific pathway effecting macrophages activity, to a fundamental view of macrophage homeostasis. Restoring macrophage homeostasis may induce the immune system to rebalance itself to normal levels of operation. These candidates targetoperation, thereby promoting disease resolution.
The Company is currently focused on two main clinical indications that are defined as “unmet medical needs,” such as acute organ failure associated withverticals: sepsis and preventing or treating complications associated with bone marrow transplants (“BMT”) and/or hematopoietic stem cell transplants (“HSCTsolid tumors (the “Indications”). The Company developed a product candidate from matched-donor cells,believes that negatively-reprogrammed macrophages may be key contributors to disease severity across these two Indications, and thus effective reprogramming of these negative-reprogrammed macrophages into their respective homeostatic states may facilitate disease resolution to these Indications, some of which has historically been referred to simply as Allocetra™. This product candidate has beenare considered “unmet medical needs.” All planned and expected timelines for execution of the subject of clinical trials for preventing or treating complications associated with bone marrow transplants (“BMT”) and/or hematopoietic stem cell transplants (“HSCT”). in the Indications are subject to certain risks and uncertainties. For further discussion of risks and uncertainties related to our clinical trial in the Indications please see Item 3.D. “Risk Factors” below.
Recent Developments
Frozen Formulation Allocetra™
We now refer to this product candidate as “Allocetra™ from matched-donor cells.” The Company developed a related product candidate,completed development of frozen formulation Allocetra™ in the first quarter of 2022, which has historically been referred to as off-the-shelf Allocetra™ or “Allocetra-OTS”, whichwas twelve months ahead of schedule. While the liquid formulation of Allocetra™ has a shelf life of 96 hours, the frozen formulation is expected to have a shelf life spanning multiple years. We expect that this will substantially identical mechanismimprove the product candidate’s scalability and shipping logistics, lower its production costs, and increase profitability upon potential commercialization. Due to the early completion of action to Allocetra™ from matched-donor cells, but it does not require matched-donor cells as the source of therapeutic cells.The Company’s timeline for the continued development of frozen formulation Allocetra™, we currently utilize it in our ongoing Phase II sepsis trial going forward, rather than introducing it only at the start of a Phase III study, as previously planned. In addition, we initiated the utilization of frozen formulation Allocetra™ from matched donor cells forin our ongoing solid tumor Phase I/II trials, and plan to solely use the prevention of GvHDfrozen formulation Allocetra™ in allogeneic HSCT patients has been modified to reflect the Company’s re-prioritization of product development in light of the encouraging results of theall subsequent clinical studies.
ii
Sepsis: Integrating Frozen Formulation Allocetra™ into Phase Ib of Allocetra-OTSII Trial, Expanding Study Population
In 2021, we initiated a placebo-controlled, randomized, dose-finding, multi-center, Phase II trial evaluating liquid Allocetra™ in patients with severepneumonia-associated sepsis. During 2022, we amended the protocol of this clinical trial to treat newly recruited patients with frozen formulation Allocetra™ and expand the study population to include patients whose septic condition stems from biliary, urinary tract, or peritoneal infections. We are planning to file another protocol amendment and submit the proposed changes to regulators in the second quarter of 2023. The amendment will include an increase in the patients’ SOFA score range, effectively allowing recruitment of patients with higher levels of sepsis severity, and a change to two cohorts (treatment and placebo) in lieu of the current four-cohort structure. The Company does not expect a material timeline delay for top-line data readouts, which are expected in Q1 2024.
Our Phase II sepsis trial is supported by previously reported positive results from a Phase Ib trial that demonstrated Allocetra’s favorable safety profile and showed vastly improved clinical outcomes, including SOFA (sequential organ failure assessment) scores, duration of hospitalization, and mortality in Allocetra-treated sepsis patients compared to a group of matched historical controls who received standard-of-care therapy. Sepsis is a life-threatening disease with no therapies approved by the U.S. Food and Drug Administration (the “FDA”) and a high unmet need. Each year, more than 1.7 million adults in the United States develop sepsis, with more than 270,000 dying of the disease.
Solid Tumors: Ongoing Clinical Trials Evaluating Allocetra™ in Patients with Solid Tumors
A major shortcoming of immunotherapeutic cancer treatments is limited efficacy against solid tumors, which represent approximately 90% of all cancer cases. We believe that this is due in large part to the negative reprogramming of macrophages in the tumor microenvironment, which results in the proliferation of pro-tumor macrophages that contribute to drug resistance, prevent disease resolution, and promote disease severity. Previously reported preclinical data from solid tumor models suggest that Allocetra™ has the potential to reprogram pro-tumor macrophages back to their homeostatic state and thereby may promote disease resolution and provide patients that do not respond well to existing FDA-approved immunotherapies with an effective treatment option. Based on these and other data, we initiated a Phase I/II trial evaluating Allocetra™ in combination with chemotherapy in patients with peritoneal metastases arising from solid cancers in the third quarter of 2022 and a Phase I/II trial evaluating Allocetra™ in combination with an immune checkpoint inhibitor in advanced-stage solid tumors in the fourth quarter of 2022.
We received a positive recommendation from the Data and Safety Monitoring Board decided(the “DSMB”) and clearance from the Israeli Ministry of Health (“IMOH”) to prioritizecontinue our Phase I/II clinical trial of Allocetra™ combined with chemotherapy in patients with peritoneal metastases arising from solid cancers. Recruitment of patients for this clinical trial has been on-track, and the Company’s cash reservescurrently expects to complete enrollment and resources on Allocetra-OTS in orderannounce data readouts by Q2 2024.
We also received a positive DSMB recommendation and IMOH clearance to generate additional clinical data, first and foremost, in a randomized, controlled sepsiscontinue our second Phase I/II clinical trial, which is evaluating Allocetra™ as monotherapy and to consider the initiationin combination with anti-PD1 checkpoint inhibitors in patients with advanced-stage solid tumors. Recruitment of smallerpatients for this clinical trials in high-value indications with an assumed pathophysiology markedly similar to that of sepsis.Because the product candidate historically referred as Allocetra-OTS is substantially identical to the product candidate formerly referred to as Allocetra™ (now“Allocetra™ from matched-donor cells”),study has been on-track, and the Company now referscurrently expects to Allocetra-OTScomplete enrollment in the intravenous-infusion monotherapy and low-dose combination cohorts by the end of Q2 2023. This study also received the U.S. Food & Drug Administration (“FDA”) clearance for an Investigational New Drug (“IND”) application at the end of 2022, as “Allocetra™”. Aswell as clearance in February 2023 from the Spanish Agency of Medicines and Medical Devices to open the study in Spain.
We also announced a resultClinical collaboration with BeiGene to evaluate the safety and efficacy of Allocetra™ in combination with tislelizumab, an anti-PD-1 immune checkpoint inhibitor, for the treatment of patients with advanced-stage solid tumors, a part of the Company’s decision, the planned continuation of additionalongoing Phase I/II clinical trials of Allocetra™ from matched-donor cells for the prevention GvHDtrial in allogeneic HSCT patients have currently been rescheduled to 2022, pending the expected results of clinical trials in sepsis and potentially other related indications during 2020-2022.with advanced-stage solid tumors.
For more information on each of Allocetra™ and Allocetra™ from matched-donor cells and a description of the respective clinical trials of Allocetra™ and Allocetra™ from matched-donor cells,Indications, see Item 4. “Information on the Company — Business—Business overview.”
BecauseManufacturing Plant Update
In the fourth quarter of 2022, we completed construction of our previously announced cGMP Allocetra™ manufacturing plant on schedule. As part of a Company-wide cost cutting program designed to extend our cash runway potentially into 2025, our planned move into the new manufacturing plant has been deferred for an approximate period of three years, and Allocetra™ from matched-donor cells are based onwe have determined to offer lease and supporting services of the same unique mechanism of action and are otherwise substantially identical, , the term Allocetra™ as used inleased space to potential clients during this Annual Report on Form 20-F refers to both Allocetra™ and Allocetra™ from matched-donor cells unless otherwise indicated.three-year period.
iii
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 20-F contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. These forward-looking statements include, but are not limited to:
| ● | our expectations regarding the timing of clinical trials with respect to Allocetra™ |
| ● | the continued listing of our ordinary shares on Nasdaq; |
| ● | our expectations regarding the progress of our clinical trials, including the duration, cost and whether such trials will be conducted at all; |
| ● | our intention to successfully complete clinical trials in order to be in a position to submit applications for accelerated regulatory paths in the EU and the United States; |
| ● | the possibility that we will apply in the future for regulatory approval for our current and any future product candidates we may develop, and the costs and timing of such regulatory approvals; |
| ● | the likelihood of regulatory approvals for any product candidate we may develop; |
| ● | the timing, cost or other aspects of the commercial launch of any product candidate we may develop, including the possibility that we will build a commercial infrastructure to support commercialization of our current and any future product candidates we may develop; |
| ● | future sales of our product candidates or any other future products or product candidates; |
| ● | our ability to achieve favorable pricing for our product candidates; |
| ● | the potential for our product candidates to receive orphan drug designations; |
| ● | that any product candidate we develop potentially offers effective solutions for various diseases; |
| ● | whether we will develop any future product candidates internally or through strategic partnerships; |
| ● | our expectations regarding the manufacturing and supply of any product candidate for use in our clinical trials, and the commercial supply of those product candidates; |
| ● | third-party payer reimbursement for our current or any future product candidates; |
| ● | our estimates regarding anticipated expenses, capital requirements and our needs for substantial additional financing; |
| ● | patient market sizes and market adoption of our current or any future product candidates by physicians and patients; |
| ● | completion and receiving favorable results of clinical trials for our product candidates; |
| ● | protection of our intellectual property, including issuance of patents to us by the United States Patent and Trademark Office (the “USPTO”), and other governmental patent agencies; |
iv
| ● | our intention to pursue marketing and orphan drug exclusivity periods that are available to us under regulatory provisions in certain countries; |
iv
| ● | the development and approval of the use of our current or any future product candidates for |
| ● | our expectations regarding commercial and pre-commercial activities; |
| ● | our expectations regarding licensing, acquisitions, and strategic operations; and |
| ● | our liquidity. |
In some cases, forward-looking statements are identified by terminology such as “may,” “will,” “could,” “should,” “expects,” “plans,” “anticipates,” “believes,” “intends,” “estimates,” “predicts,” “hope,” “targets,” “potential,” “goal” or “continue” or the negative of these terms or other comparable terminology. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results or performance to differ materially from those suggested in such forward-looking statements. These statements are current only as of the date of this Annual Report on Form 20-F and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our actual results, levels of activity, performance or achievements to be materially different from those suggested in the forward-looking statements. In addition, historic results of scientific research and clinical and preclinical trials do not guarantee that the conclusions of future research or trials would not suggest different conclusions or that historic results referred to in this Annual Report on Form 20-F would not be interpreted differently in light of additional research, clinical and preclinical trials results. The forward-looking statements contained in this Annual Report on Form 20-F are subject to risks and uncertainties, including those discussed underin Item 3.D. - “Risk Factors” and in our other filings with the Securities and Exchange Commission (the “SEC”). Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 20-F. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we do not intend to (and expressly disclaim any such obligation to) update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this Annual Report on Form 20-F.
RISK FACTOR SUMMARY
Our business is subject to numerous risks and uncertainties, including those described in Item 3.D. “Risk Factors.” These risks include, but are not limited to the following:
| ● | We are a clinical-stage macrophage reprogramming immunotherapy company with a history of operating losses; we expect to incur additional losses in the future and may never be profitable; |
| ● | We have not generated any revenue from Allocetra™ or any other product candidate, and we may never be profitable; |
| ● | We will need substantial additional capital in the future; if additional capital is not available, we will have to delay, reduce or cease operations; |
| ● | We are unable to estimate our long-term capital requirements due to uncertainties associated with the development and commercialization of our product candidates; if we fail to obtain necessary funds for our operations, we will be unable to develop and commercialize any of our product candidates; |
| ● | Our business, operating results and growth may be adversely affected by current or future unfavorable economic and market conditions due to geopolitical tensions and political, economic and military instability; |
v
| ● | We have focused substantially all of our efforts and resources on Allocetra™, and we may not obtain regulatory approval of Allocetra™; |
| ● | None of our product candidates may achieve commercial success in a timely and cost-effective manner, or ever; |
| ● | Results from our clinical trials may be negative or may not replicate the results of our preclinical trials or earlier clinical trials, which could require that we abandon development of Allocetra™, our other product candidates or any future product candidates, which will significantly impair our ability to generate revenues; |
| ● | The clinical trial process is complex and expensive, and commencement and completion of clinical trials can be delayed or prevented for a number of reasons; |
| ● | We cannot be certain that the results of our potential clinical trials, even if all endpoints are met, will support regulatory approval in any territory, of any of our product candidates for any indication; |
| ● | Our manufacturing processes are complex, delicate and susceptible to contamination, and involve biological intermediates that are subject to stringent regulations; |
| ● | If we or any potential CMOs we retain in the future fail to comply with manufacturing regulations, our financial results and financial condition could be adversely affected; |
| ● | Our product candidates may produce undesirable side effects that we may not detect in our clinical trials, which could prevent us from achieving or maintaining market acceptance of any such product candidate and could substantially increase commercialization costs or even force us to cease operations; |
| ● | Even if Allocetra™ or any other product candidate that we are developing or may develop receives marketing approval in any territory, we will continue to face extensive regulatory requirements, and any such product may still face future development and regulatory difficulties; |
| ● | We expect the healthcare industry to face increased limitations on reimbursement, rebates and other payments as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products; | |
| ● | Significant disruptions of information technology systems, cyberattacks and other security breaches could compromise our proprietary and confidential information, which could harm our business and reputation; |
| ● | If product liability lawsuits are successfully brought against us, our insurance may be inadequate; |
| ● | We manage our business through a small number of senior executive officers; | |
| ● | We intend to rely primarily on third parties to market and sell Allocetra™ and any other product candidate; |
| ● | Any collaboration arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our current and any future product candidates; |
| ● | We depend on third parties to conduct our clinical trials; |
| ● | The failure to obtain or maintain patents, licensing agreements and other intellectual property could impact our ability to compete effectively; and |
| ● | We cannot predict the scope and extent of patent protection for our product candidates because the patent positions of pharmaceutical products are complex and uncertain. |
vi
PART ONE
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
3.A. Selected financial data[Reserved]
Our historical financial statements are prepared in accordance with generally accepted accounting principles in the United States and are presented in U.S. dollars. The following summary financial data for the years ended December 31, 2019, 2018, 2017 and 2016 and as of December 31, 2019, 2018, 2017 and 2016 are derived from, and should be read in conjunction with, the audited financial statements, and notes thereto, appearing elsewhere in this Annual Report on Form 20-F. Selected financial data for the year ended December 31, 2015 is unaudited, and the Company could not provide audited data for such years without unreasonable effort or expense.
The information presented below is qualified by the more detailed historical financial statements set forth in this Annual Report on Form 20-F, and should be read in conjunction with those financial statements, the notes thereto and the discussion under Item 5 - “Operating and Financial Review and Prospects.”
Merger
For a description of the Merger, please see the “Introduction” appearing before Part I, Item 1 of this Annual Report on Form 20-F. The Merger was accounted for as an issuance of shares by the Company for the net assets of Bioblast Pharma Ltd., accompanied by a recapitalization. Accordingly, Enlivex R&D is reflected as the predecessor and acquirer and therefore the accompanying financial statements reflect the historical financial statements of Enlivex R&D for all periods presented and do not include the historical financial statements of pre-merger Bioblast. All historical information presented herein has been retroactively restated to reflect the effect of the merger shares exchange ratio, reverse stock split and change to the authorized number of ordinary shares in accordance with Accounting Standards Codification Topic 260, “Earnings Per Share”.
Statement of Operations Data - Year Ended December 31,
U.S. dollars in thousands, except share and per share data
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Revenues | $ | $ | $ | $ | $ | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development expenses | 5,724 | 4,255 | 1,691 | 2,029 | 1,625 | |||||||||||||||
| General and administrative expenses | 2,895 | 1,044 | 480 | 793 | 710 | |||||||||||||||
| Operating (loss) | (8,619 | ) | (5,299 | ) | (2,171 | ) | (2,822 | ) | (2,335 | ) | ||||||||||
| Financial income | 238 | 1,060 | 37 | 30 | 20 | |||||||||||||||
| Financial expenses | 1,003 | 3 | 370 | 86 | 120 | |||||||||||||||
| Net Income (loss) | (9,384 | ) | $ | (4,242 | ) | $ | (2,504 | ) | $ | (2,878 | ) | $ | (2,435 | ) | ||||||
| Other comprehensive gain (loss) | ||||||||||||||||||||
| Interest on convertible notes | - | (67 | ) | (617 | ) | |||||||||||||||
| Exchange differences arising from translating financial statements from functional to presentations currency | (951 | ) | (748 | ) | 336 | 93 | 15 | |||||||||||||
| Total other comprehensive gain (loss) | (951 | ) | (748 | ) | 336 | 26 | (602 | ) | ||||||||||||
| Total comprehensive (loss) | (8,433 | ) | (4,990 | ) | (2,168 | ) | (2,852 | ) | (3,037 | ) | ||||||||||
| Basic (loss) earnings per share | (1.12 | ) | $ | (1.4 | ) | (0.94 | ) | (1.03 | ) | (0.99 | ) | |||||||||
| Weighted average number of shares outstanding | 8,649,486 | 3,509,346 | 3,425,925 | 3,357,647 | 3,059,508 | |||||||||||||||
| Diluted (loss) per share | (1.11 | ) | $ | (1.4 | ) | (0.94 | ) | (1.03 | ) | (0.99 | ) | |||||||||
| Weighted average number of shares outstanding | 8,649,486 | 3,509,346 | 3,425,925 | 3,357,647 | 3,059,508 | |||||||||||||||
Balance Sheet Data - December 31,
U.S. dollars in thousands
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Assets | ||||||||||||||||||||
| Current Assets: | ||||||||||||||||||||
| Cash and cash equivalents | 3,948 | $ | 9,736 | $ | 9,005 | $ | 3,020 | $ | 262 | |||||||||||
| Short Term Deposits | 8,060 | 40 | - | - | 5,009 | |||||||||||||||
| Prepaid expenses | 510 | 288 | 14 | 46 | 154 | |||||||||||||||
| Other receivables | 403 | 213 | 95 | 47 | 510 | |||||||||||||||
| Cash held for CVR holders | 1,500 | - | - | - | - | |||||||||||||||
| Receivables for the sale of Trehalose | 2,000 | - | - | - | - | |||||||||||||||
| Total Current Assets | 16,421 | 10,277 | 9,114 | 3,113 | 5,935 | |||||||||||||||
| Non-Current Assets | ||||||||||||||||||||
| Restricted cash | 76 | 56 | 27 | 25 | 19 | |||||||||||||||
| Long-term prepaid expenses | 5 | 16 | 11 | 7 | 5 | |||||||||||||||
| Property and equipment, net | 648 | 685 | 388 | 314 | 188 | |||||||||||||||
| Other assets | 410 | |||||||||||||||||||
| Total Non-Current Assets | 1,139 | 757 | 426 | 346 | 212 | |||||||||||||||
| Total Assets | 17,560 | $ | 11,034 | $ | 9,540 | $ | 3,459 | $ | 6,147 | |||||||||||
| Liabilities | ||||||||||||||||||||
| Current liabilities | ||||||||||||||||||||
| Accounts payable trade | 316 | $ | 173 | $ | 37 | $ | 32 | $ | 71 | |||||||||||
| Accrued expenses and other liabilities | 1,897 | 944 | 634 | 583 | 577 | |||||||||||||||
| Payables to related parties | 367 | 13 | 25 | 28 | 28 | |||||||||||||||
| CVR holders | 3,400 | |||||||||||||||||||
| Total Current Liabilities | 5,980 | 1,130 | 696 | 643 | 676 | |||||||||||||||
| Non-Current Liabilities | ||||||||||||||||||||
| Retirement benefit obligations | - | 6 | 7 | 6 | 6 | |||||||||||||||
| Other long-term Liabilities | 298 | |||||||||||||||||||
| Warrants | - | 192 | 344 | - | ||||||||||||||||
| Total Non-Current Liabilities | 298 | 198 | 351 | 6 | 6 | |||||||||||||||
| Commitments and Contingent Liabilities | - | |||||||||||||||||||
| Total Liabilities | 6,278 | 1,328 | 1,047 | 649 | 682 | |||||||||||||||
3.B. Capitalization and indebtednessIndebtedness
Not applicable.
3.C. Reasons for the offerOffer and useUse of proceedsProceeds
Not applicable.
3.D. Risk factorsFactors
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the risks described below before investing in our ordinary shares.
There are a number of risks and uncertainties that could affect our business and cause our actual results to differ from past performance or expected results. We consider the following risks and uncertainties to be those material to our business. If any of these risks actually occur, our business, financial condition and results of operations could suffer, and the trading price of our ordinary shares could decline. We urge investors to consider carefully the risk factors described below, in evaluating any investment in our ordinary shares andtogether with the other information contained in this Annual Report on Form 20-F.20-F, in evaluating any investment in our ordinary shares.
Risks Related to Our Financial Position and Capital Requirements
We are a clinical-stage cellmacrophage reprogramming immunotherapy company with a history of operating losses. We expect to incur additional losses in the future and may never be profitable.
We are a clinical-stage cellmacrophage reprogramming immunotherapy company with a limited operating history and no currently approved products. To date, we have focused almost exclusively on developing our related product candidates, Allocetra™ and Allocetra™ from matched-donor cells., a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic stage, focusing in particular on treatment of the Indications. We have funded our operations to date primarily through proceeds from private placements of ordinary shares and convertible debt. We have no saleable products and have not generated any revenue from product sales. We have incurred losses in each year since our inception in 2005. Our loss attributable to holders of our ordinary shares for the years ended December 31, 20192022 and 20182021 was approximately $9.3$31 million and $4.2$14.4 million, respectively. As of December 31, 2019,2022, we had an accumulated deficit of approximately $25.6$83 million. Substantially all of our operating losses resulted from costs incurred in connection with our development program and from general and administrative costs.
We expect our research and development expenses to increase in connection with our planned pre-clinical studies and clinical trials. We may also incur expenses in connection with third-party studies and trials involving our product candidates or other intellectual property. In addition, if we obtain marketing approval for any of our product candidates, we will likely initially incur significant outsourced sales, marketing and manufacturing expenses, as well as continued research and development expenses. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing cell immunotherapy products, we are unable to predict the extent of any future losses or when we will become profitable, if at all.
We have not generated any revenue from Allocetra™ or any other product candidate, and we may never be profitable.
Our ability to become profitable depends upon our ability to generate revenue in excess of our expenses. We have not generated any revenue from our development of Allocetra™, or any other product candidate. We do not know when, or if, we will generate any revenue. We do not expect to generate revenue unless and until we obtain regulatory and marketing approval of, and commercialize, Allocetra™ or Allocetra™ from matched-donor cells or any other product candidate. We will continue to incur research and development and general and administrative expenses related to our operations. We expect to continue to incur losses for the foreseeable future, and such losses will likely increase as we:
| ● | initiate and manage preclinical development and clinical trials for our current and any new product candidates; |
| ● | seek regulatory approvals for our product candidates, or future product candidates, if any; |
| ● | implement internal systems and infrastructure, including, without limitation, hiring of additional personnel as needed and to develop sales and marketing functions if and when our product candidate receives applicable regulatory approval; |
| ● | seek to in-license additional technologies for development, such as cell delivery, processing and testing technologies; |
| ● | hire additional management and other personnel; and |
| ● | move towards commercialization of our product candidates and future product candidates, if any. |
We may out-license our ability to generate revenue from our product candidates, depending on a number of factors, including our ability to:
| ● | obtain favorable results from and progress the clinical development of our product candidates, particularly Allocetra™; |
| ● | develop and obtain regulatory approvals in various countries and for the uses we intend to pursue for our product candidates; |
| ● | subject to successful completion of registration, clinical trials and perhaps additional clinical trials of any product candidate, apply for and obtain marketing approval in the countries we intend to pursue for such product candidate; |
| ● | contract for the manufacture of commercial quantities of our product candidates at acceptable cost levels, subject to the receipt of marketing approval; and |
| ● | establish external, and potentially, internal, sales and marketing capabilities to effectively market and sell our product candidates in the United States and other countries. |
Even if either Allocetra™, our leading product candidate, which has beenis being developed for treating organ dysfunctionsepsis and failure in sepsis patients, or Allocetra™ from matched-donor cells, which has been developed for prevention of GvHD in allogeneic HSCT patients,solid tumors, is approved for commercial sale for any indication, either product candidate may not gain market acceptance or achieve commercial success. In addition, we anticipate incurring significant costs associated with commercialization. We may not achieve profitability soon after generating product revenue, if ever. If we are unable to generate product revenue, we will not become profitable and would be unable to continue operations without additional funding.
We have not yet commercialized any products and we may never become profitable.
We have not yet commercialized any products, and we may never do so. We do not know when or if we will complete any of our product development efforts, obtain regulatory approval for any product candidates or successfully commercialize any approved products. Even if we are successful in developing products that are approved for marketing, we will not be successful unless these products gain market acceptance for appropriate indications. The degree of market acceptance of any of our planned future products will depend on a number of factors, including, but not limited to:
| ● | the timing of regulatory approvals in the countries, and for the uses, we intend to pursue with respect to the commercialization of our product candidates; |
| ● | the competitive environment; |
| ● | the acceptance by the medical community of the safety and clinical efficacy of our products and their potential advantages over other therapeutic products; |
| ● | the adequacy and success of distribution, sales and marketing efforts, including through strategic agreements with pharmaceutical and biotechnology companies; and |
| ● | the pricing and reimbursement policies of government and third-party payors, such as insurance companies, health maintenance organizations and other plan administrators. |
Physicians, patients, third-party payors or the medical community in general may be unwilling to accept, utilize or recommend coverage of, and in the case of third-party payors, cover any of our planned future products. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products, we may not become profitable.
Our limited operating history makes it difficult to evaluate our business and prospects.
Although we have been in existence since 2005, we have a limited operating history, and our operations to date have been limited primarily to research and development, raising capital and recruiting scientific and management personnel. Therefore, it is difficult to evaluate our business and prospects. We have not yet demonstrated an ability to commercialize or obtain regulatory approval for any product candidate. Consequently, any predictions about our future performance may not be accurate, and you may not be able to fully assess our ability to complete development or commercialize our product candidates, or any future product candidates, obtain regulatory approvals or achieve market acceptance or favorable pricing for our product candidates or any future product candidates.
We have not yet commercialized any products and we may never become profitable.
We have not yet commercialized any products, and we may never do so. We do not know when or if we will complete any of our product development efforts, obtain regulatory approval for any product candidates or successfully commercialize any approved products. Even if we are successful in developing products that are approved for marketing, we will not be successful unless these products gain market acceptance for appropriate indications. The degree of market acceptance of any of our planned future products will depend on a number of factors, including, but not limited to:
Physicians, patients, third-party payors or the medical community in general may be unwilling to accept, utilize or recommend coverage of, and in the case of third-party payors, cover any of our planned future products. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products, we may not become profitable.
We will need substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
We will need to raise substantial additional capital to fund our operations and to develop and commercialize our product candidates, particularly Allocetra™. Our future capital requirements may be substantial and will depend on many factors, including, but not limited to:
| ● | our clinical trial results; |
| ● | the cost, timing and outcomes of seeking marketing approval of our product candidates; |
| ● | the cost of filing and prosecuting patent applications and the cost of defending our patents; |
| ● | the cost of prosecuting infringement actions against third parties; |
| ● | exploration and possible label expansion of our product candidates for the treatment of other conditions or indications; |
| ● | the costs associated with commercializing our product candidates if we receive marketing approval, including the cost and timing of establishing external, and potentially in the future, internal, sales and marketing capabilities to market and sell such product candidates; |
| ● | subject to receipt of marketing approval, revenue received from sales of approved products, if any, in the future; |
| ● | any product liability or other lawsuits related to our future product candidates or products, if any; |
| ● | the demand for our products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; and |
| ● | the costs associated with being a public company. |
Based on our current operating plan, we anticipate our existing resources will be sufficient to enable us to maintain our currently planned operations, including our continued product development, through the fourth quarter of 2022.2024; however, we have a contingency plan that may potentially extend our cash runway into 2025. We will require significant additional funds to initiate and complete the FDA and the European Medicines Agency (“EMA”) approval process. However, changing circumstances may cause us to consume capital significantly faster than we currently anticipate, including, without limitation, regulatory requests by the FDA or EMA, changes in our development strategy, delays in or an inability to execute our development plans, unsuccessful preclinical or clinical studies and losing our “Small and Medium Enterprise” status at the EMA, which entitles us to significant fee reductions. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amount of increased capital and operating expenditures associated with our anticipated clinical trials and general operations. We have no committed external sources of funds. Additional financing may not be available when we need it or on terms that are favorable to us. If adequate funds are not available to us on a timely basis, or at all, we may be required to terminate or delay planned clinical trials or other development activities for our product candidates, which would materially and adversely affect our liquidity and results of operations.
Raising additional financing may be costly or difficult to obtain, may dilute current shareholders’ ownership interests and may require that we relinquish our rights to certain of our technologies, products or marketing territories.
Any debt or equity financing that we may need may not be available on terms favorable to us, or at all. If we obtain funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to certain of our technologies, products or marketing territories. If we are unable to obtain required additional capital, we may have to curtail our growth plans or cut back on existing business, and we may not be able to continue operating.
We may incur substantial costs in pursuing future financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition and results of operations.
Any additional capital raised through the sale of equity or equity-linked securities may dilute our current shareholders’ ownership in us and could also result in a decrease in the market price of our ordinary shares. The terms of the securities issued by us in future capital transactions may be more favorable to new investors and may include the issuance of warrants or other derivative securities, which may have a further dilutive effect.
We are unable to estimate our long-term capital requirements due to uncertainties associated with the development and commercialization of our product candidates. If we fail to obtain necessary funds for our operations, we will be unable to develop and commercialize any of our product candidates.
We expect our long-term capital requirements to depend on many potential factors, including, among others:
| ● | the number of product candidates in development; |
| ● | the duration and cost of discovery and preclinical development; |
| ● | the regulatory path of product candidates, including our lead product candidate, Allocetra™, which has been developed for treating |
| ● | the results of preclinical and clinical testing, which can be unpredictable in product candidate development; |
| ● | our ability to successfully commercialize our product candidates, including securing commercialization and out-licensing agreements with third parties and favorable pricing and market share; |
| ● | the progress, success and costs of our clinical trials and research and development programs, including those associated with milestones and royalties; |
| ● | the costs, timing and outcome of regulatory review and obtaining regulatory approval of our lead product candidate and addressing regulatory and other issues that may arise post-approval; |
| ● | the breadth of the labeling, assuming that any of our product candidates are approved for commercialization by the relevant regulatory authority; |
| ● | our need, or decision, to acquire or in-license complementary technologies or new platform technologies or product candidate targets; |
| ● | the costs of enforcing our issued patents and defending intellectual property-related claims; |
| ● | the costs of investigating patents that might block us from developing potential product candidates; |
| ● | the costs of recruiting and retaining qualified personnel; |
| ● | our revenue, if any; and |
| ● | our consumption of available resources more rapidly than currently anticipated, resulting in the need for additional funding sooner than anticipated. |
If we are unable to obtain the funds necessary for our operations, we will be unable to develop and commercialize any of our product candidates, or any future product candidates, which would materially and adversely affect our business, liquidity and results of operations.
Due to our recurring operating losses, our ability to continue to operate as a going concern is dependent on additional financial support.
We devote substantially all of our efforts toward research and development activities. In the course of such activities, we have sustained operating losses and expect such losses to continue for the foreseeable future. We have no current source of revenue to sustain our present activities, and we do not expect to generate revenue until, and unless, the FDA or other regulatory authorities approve one of our product candidates and we successfully commercialize (including out-licensing) such product candidate. Accordingly, our ability to continue operating will require us to obtain additional financing to fund our operations. According to our estimates, if we are not successful in obtaining additional capital resources, there is a substantial doubt that we will be able to continue our activities beyond the fourth quarter of 2022.2024. The perception of our inability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
Our business, operating results and growth may be adversely affected by current or future unfavorable economic and market conditions due to geopolitical tensions and political, economic and military instability.
Our business depends on the economic health of the global economies. U.S. and global markets have recently experienced volatility and disruption, including as a result of the escalation of geopolitical tensions following the start of the war between Russia and Ukraine in February 2022. Furthermore, the Israeli government is currently pursuing extensive changes to Israel’s judicial system, which has sparked extensive political debate. In response to the foregoing developments, a series of civil unrests and demonstrations throughout Israel took place. Additionally, individuals, organizations and institutions, both within and outside of Israel, have voiced concerns that the proposed changes may negatively impact the business environment in Israel including due to reluctance of foreign investors to invest or conduct business in Israel, as well as to increased currency fluctuations, downgrades in credit rating, increased interest rates, increased volatility in securities markets, and other changes in macroeconomic conditions. Such proposed changes may also adversely affect the labor market in Israel or lead to political instability or civil unrest, where we are headquartered, and our ability to raise additional funds.
If the conditions in the global economies remain uncertain or continue to be volatile, or if they deteriorate, including as a result of the impact of military conflict, such as the war between Russia and Ukraine, terrorism or other geopolitical events, such as political instability in Israel, our business, operating results and financial condition may be materially adversely affected. Economic weakness, inflation and increases in interest rates, limited availability of credit, liquidity shortages and constrained capital spending have at times in the past resulted, and may in the future result, in a challenging capital raising environment, slower adoption of new technologies and increased competition, and any such disruptions may also magnify the impact of other risks described in this Annual Report on Form 20-F.
Risks Related to Our Business, Industry and Regulatory Requirements
We have focused substantially all of our efforts and resources on Allocetra™, and we may not obtain regulatory approval of Allocetra™.
We have invested almost all of our efforts and financial resources in the research and development of Allocetra™. As a result, our business is primarily dependent on our ability to complete the development of, obtain regulatory approval for and successfully commercialize Allocetra™. The process to develop, obtain regulatory approval for and commercialize Allocetra™ is long, complex and costly, and its outcome is uncertain.
The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drugs and pharmaceutical products, including biologics, are subject to extensive regulation by the FDA, the EMA and regulatory agencies in other countries. These regulations differ from jurisdiction to jurisdiction. We are not permitted to market Allocetra™, or any other product candidate, in the United States until we receive approval of a biologics license application (“BLA”) from the FDA, or in the European Union until we receive a marketing authorization application (“MAA”) from the EMA, or in any foreign countries until we receive the requisite approval from the respective regulatory agencies in such countries. We have not received regulatory clearance to conduct the clinical trials that are necessary to file a BLA with the FDA or comparable applications to other regulatory authorities in other countries or received marketing approval for Allocetra™. The results of clinical trials may be unsatisfactory, even if we believe those clinical trials to be successful, the FDA, or other regulatory authorities, may not approve our BLA should we be in a position to file one.
Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The marketing approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. In particular, in many countries outside the United States, it is required that a product receives pricing and reimbursement approval before it can be commercialized. This can result in substantial delays in such countries. In other countries, product approval depends on showing superiority to an approved alternative therapy. This can result in significant expense for conducting complex clinical trials. If we fail to comply with regulatory requirements in the United States or international markets or to obtain and maintain required approvals or if regulatory approvals in the United States or international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products will be harmed.
Marketing approval in one jurisdiction does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one jurisdiction may have a negative effect on the regulatory process in others. Failure to obtain marketing approval in other countries or any delay or setback in obtaining such approval would impair our ability to develop foreign markets for Allocetra™ or any other product candidate. This would reduce our target market and limit the full commercial potential of Allocetra™ or any other product candidate.
None of our product candidates may achieve commercial success in a timely and cost-effective manner, or ever.
Even if regulatory authorities approve any of our product candidates, they may not be commercially successful. Our product candidates may not be commercially successful because, among other things, government agencies or other third-party payors may not provide reimbursement for the costs of the product or the reimbursement may be too low to be commercially successful. Also, physicians and others may not use or recommend our product candidates, even following regulatory approval. In addition, a product approval, even if issued, may limit the uses for which such product may be distributed, which could adversely affect the commercial viability of the product. Moreover, third parties may develop superior products or have proprietary rights that preclude us from marketing our product. Physician and patient acceptance of, and demand for, our products, if we obtain regulatory approval, will depend largely on many factors, including, but not limited to, the extent, if any, of reimbursement of costs by government agencies and other third-party payors, pricing, the effectiveness of our marketing and distribution efforts, the safety and effectiveness of alternative products, and the prevalence and severity of side effects associated with such products. If physicians, government agencies and other third-party payors do not accept the use or efficacy of our products, we will not be able to generate significant revenue, if any.
Results from our clinical trials may be negative or may not replicate the results of our preclinical trials or earlier clinical trials, which could require that we abandon development of Allocetra™, our other product candidates or any future product candidates, which will significantly impair our ability to generate revenues.
Upon the completion of any clinical trial, the results might not support the outcomes sought by us. Further, success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and the results of later clinical trials may not replicate the results of prior clinical trials and preclinical testing. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage development. Accordingly, the results from the completed preclinical studies and clinical trials for Allocetra™ may not be predictive of the results we may obtain in later stage trials of Allocetra™ or clinical trials of any of our other product candidates. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain FDA or EMA, or other regulatory agency, approval for their products.
In addition, the clinical trial process may fail to demonstrate that Allocetra™ is safe and effective for its indicated uses. Any such failure may cause us to abandon Allocetra™ and may delay development of other product candidates. Any delay in, or termination or suspension of, our clinical trials will delay the requisite filings with the FDA or other regulatory agencies and, ultimately, our ability to commercialize our product candidates and generate revenues. If the clinical trials do not support our product claims, the completion of development of such product candidate may be significantly delayed or abandoned, which will significantly impair our ability to generate revenues and will materially adversely affect our results of operations.
The clinical trial process is complex and expensive, and commencement and completion of clinical trials can be delayed or prevented for a number of reasons.
We may not be able to commence or complete the clinical trials required to support our submission of a BLA to the FDA or a MAA to the EMA or any similar submission to regulatory authorities in other countries. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of any of our clinical trials. The fact that the FDA, EMA or other regulatory authorities permit a company to conduct human clinical trials is no guarantee that the trial will be successful. To the contrary, most product candidates that enter clinical trials do not prove to be successful and do not result in the filing of a BLA, MAA or similar filing. Drug candidates that prove successful at one clinical trial phase may prove unsuccessful at a subsequent phase. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements and in part because the results of clinical trials are inherently uncertain and unpredictable. Regulatory authorities, such as the FDA, may preclude clinical trials from proceeding. Additionally, the clinical trial process is time-consuming, and failure can occur at any stage of the trials. We may encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be delayed by several factors, including:
| ● | difficulties obtaining regulatory clearance or approval to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; |
| ● | delays in reaching or failing to reach agreement on acceptable terms with prospective contract research organizations (“CROs”), contract manufacturing organizations (“CMOs”), pharmaceutical shipping companies and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs, CMOs, shipping companies and trial sites; |
| ● | insufficient or inadequate supply or quality of a product candidate or other materials necessary to conduct our clinical trials; |
| ● | difficulties in obtaining institutional review board (“IRB”) approval to conduct a clinical trial at a prospective site; |
| ● | delays resulting from a decision of the FDA or EMA not to review a BLA or MAA for Allocetra™, respectively, or any of our other product candidates, under the FDA’s Fast Track Development Program or as a Breakthrough Therapy; and |
| ● | challenges in recruiting and enrolling patients or donors to participate in clinical trials for a variety of reasons, including size and nature of patient population, proximity of patients to clinical sites, eligibility criteria for the trial, nature of trial protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications. |
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA or other regulatory authorities, the IRBs at the sites where such boards are overseeing a trial, or a data safety monitoring board overseeing the clinical trial at issue or other regulatory authorities due to a number of factors, including:
| ● | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| ● | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
| ● | unforeseen safety issues or lack of effectiveness; and |
| ● | lack of adequate funding to continue the clinical trials. |
In addition, we or regulatory authorities may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks, or if others report that similar products pose an unacceptable risk to patients, or if the regulatory authorities find deficiencies in our regulatory submissions or the conduct of such trials. Any suspension of clinical trials will delay possible regulatory approval, if any, and adversely affect our ability to develop products and generate revenue.
We cannot be certain that the results of our potential Phase III clinical trials, even if all endpoints are met, will support regulatory approval of any of our product candidates for any indication.
Currently, the FDA, EMA and other regulatory agencies do not have any clear guidance on which endpoints of a Phase III clinical trial would be sufficient for approval of a drug for the treatment of any indication. Therefore, the development pathway for Allocetra™ and our other product candidates is not completely clear. For example, even if the FDA approves a certain primary endpoint for a pivotal clinical trial, and the trial meets that primary endpoint, the FDA may still deny approval of a BLA for various reasons. It is possible that even if the results of a potential Phase III clinical trial meet the primary endpoints for a particular product candidate, the FDA may still require longer-term studies of that product candidate prior to granting marketing approval.
Obtaining approval of a BLA, MAA or other regulatory approval, even after clinical trials that are believed to be successful is an uncertain process.
Even if we complete our planned clinical trials and believe the results to be successful, all of which are uncertain, obtaining approval of a BLA, or similar regulatory application, is an extensive, lengthy, expensive and uncertain process, and the EMA, IMOH, the FDA and other regulatory agencies may delay, limit or deny approval of our product candidates for many reasons, including:
| ● | we may not be able to demonstrate to the satisfaction of the applicable regulatory agencies that our product candidates are safe and effective for any indication; |
| ● | the results of our clinical trials may not meet the level of statistical significance or clinical significance required by the applicable regulatory agencies for approval; |
| ● | the applicable regulatory agencies may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| ● | the applicable regulatory agencies may not find the data from preclinical studies and clinical trials sufficient to demonstrate that our product candidates’ clinical and other benefits outweigh their respective safety risks; |
| ● | the applicable regulatory agencies may disagree with our interpretation of data from preclinical studies or clinical trials; |
| ● | the applicable regulatory agencies may not accept data generated at our clinical trial sites; |
| ● | the data collected from preclinical studies and clinical trials of our product candidates may not be sufficient to support the submission of a BLA or similar regulatory application; |
| ● | the applicable regulatory agencies may not schedule an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the applicable regulatory agencies require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| ● | the applicable regulatory agencies may require development of a risk evaluation and mitigation strategy as a condition of approval; |
| ● | the applicable regulatory agencies may require simultaneous approval for both adults and children which would delay needed approvals, or we may have successful clinical trial results for adults, but not children, or vice versa; |
| ● | the applicable regulatory agencies may change their approval policies or adopt new regulations that may impede consideration or approval of our BLA, or similar regulatory application; |
| ● | the applicable regulatory agencies may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers, or suppliers of blood and cell samples or providers of cell collection, freezing and transportation services, with which we enter into agreements for clinical and commercial supplies; and |
| ● | the applicable regulatory agencies may demand post-marketing approval studies, such as Phase IV clinical trials, in connection with our product candidates. |
With respect to Allocetra™ for
For example, in the treatment of organ dysfunction and failure associated with sepsis clinical vertical, before we can submit a BLA,marketing application, or similar regulatory application, to the FDA,EMA, or other regulatory authorities, as applicable, we will first be required to conduct acomplete our ongoing Phase II clinical trial and then must conduct pivotal Phase III clinical trials. With respect to Allocetra™ for the prevention of GvHD, before we can submit a BLA, or similar regulatory application, to the FDA, or other regulatory authorities, as applicable, we may first be required to conduct additional Phase II clinical trials and then must conduct pivotal Phase III clinical trials that will be substantially broader than our previously conducted Phase IIa trial. However, certain regulations in Europe may allow us to obtain conditional marketing authorization in Europe for some of our products for certain indications that are classified as unmet medical need and fall under certain guidelines. We will also need to agree on a protocol with the FDA for the clinical trials before commencing those trials in the United States. Phase III clinical trials frequently produce unsatisfactory results even though prior clinical trials were successful. Therefore, the results of the additional trials that we conduct may or may not be successful. The applicable regulatory agencies may suspend all clinical trials or require that we conduct additional clinical, nonclinical, manufacturing, validation or drug product quality studies and submit those data before considering or reconsidering the BLA,marketing application, or similar regulatory application. Depending on the extent of these, or any other studies, approval of any applications that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the applicable regulatory agencies to provide regulatory approval. If any of these outcomes occur, we likely would not receive approval for Allocetra™, or any of our other product candidates, and may be forced to cease operations.
Even if we obtain regulatory approval for Allocetra™, or any of our other product candidates, the approval might contain significant limitations related to the intended uses for which the product is approved, including, without limitation, restrictions related to certain labeled populations, age groups, warnings, precautions or contraindications, or an approval may be subject to significant post-marketing studies or risk mitigation requirements. If we are unable to successfully commercialize Allocetra™, or any of our other product candidates, we may be forced to cease operations.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, which could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of our clinical trials.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, and as a result, we may need to amend our clinical trial protocols. Amendments may require us to resubmit our clinical trial protocols to IRBs for review and approval, which may adversely affect the cost, timing and successful completion of a clinical trial. If we experience delays in the completion of, or if we terminate, any of our clinical trials, the commercial prospects for our affected product candidates would be harmed and our ability to generate product revenue would be delayed, possibly materially.
Our manufacturing processes are complex, delicate and susceptible to contamination, and involve biological intermediates that are subject to stringent regulations.
Blood is a raw material that is susceptible to damage and contamination and may contain human pathogens, any of which would render the blood unsuitable as raw material for further manufacturing. For instance, improper storage of blood, by us or third-party suppliers, may require us to destroy some of our raw material. If unsuitable blood is not identified and discarded prior to the release of the blood to the manufacturing process, it may be necessary to discard intermediate or finished product made from that blood or to recall any finished product released to the market or individual patients, resulting in a charge to cost of goods sold.
The manufacture of Allocetra™ is a complex and delicate process of cell collection, separation, freezing, storing, incubation, harvesting, formulating and testing, each under aseptic conditions. First, cells are collected by separation from the blood donations at collection centers and medical centers. The manufacture of AllocetraTM and AllocetraTM from matched donor cells differ only in the source of the blood donations. Donations for AllocetraTM from matched donor cells come from the blood of either (i) a donor matched by his or her human leukocyte antigen (i.e., a protein found on white blood cells that is the standard genetic marker for the regulation of the immune system and is used to match donors and recipients in transplantations), (ii) the patient, or (iii) an allogeneic, unmatched donor that has undergone ex-vivo (i.e., prior to infusion) manipulation to stabilize the “early apoptosis” status of the cells for a prolonged period of time. Donations for AllocetraTM are collected from healthy donors through apheresis. The cells sourced for either AllocetraTM or AllocetraTM from matched donor cells are then shipped to a manufacturing site for cryopreservation by trained personnel pursuant to current Good Manufacturing Practices (“cGMP”) requirements, FDA guidelines and our manufacturing protocol, as detailed in our Chemistry Manufacturing and Controls (“CMC”) protocols. Second, the cells are thawed, processed, prepared in an intravenous bag and tested according to our quality assurance and quality control assays and cGMP requirements. The final product is then shipped to the clinical site where it is injectedinfused into the patient within the predetermined expiration period. All shipping and handling isare pursuant to carefully controlled conditions, including controlled temperatures, as required by applicable regulations. The manufacturing sites must be certifiedregistered manufacturing facilities operating under cGMP requirements and all manufacturing activities, including cell collection, processing, testing, freezing, shipping, final product preparations, packaging and labeling, must be conducted by properly and adequately trained personnel in accordance with detailed protocols, batch records and our CMC and based on cGMP requirements and FDA, or other applicable regulatory, guidelines.
Allocetra™, and our other potential drug candidates, if any, may fail to meet our stringent specifications through a failure in one or more of these process steps. Such failure would prohibit us from releasing the drug at issue for human use until the failure is properly and sufficiently corrected and resolved. We may detect instances in which an unreleased product was produced, either internally (as is the case for small scale preclinical or early stage clinical production) or by a CMO (as would be the case for large scale production for which we would provide appropriate technology training and require EMA or FDA approval), without adherence to our manufacturing procedures or blood used in our production process was not collected, shipped, processed or stored in a compliant manner consistent with our current cGMP, or other regulations or regulatory requests, including those by the FDA.EMA. Such an event of non-compliance would likely result in our determination that the implicated product candidates should not be released and therefore should be destroyed. Even if handled properly, biologics may form or contain particulates or have other issues or problems after storage which may require destruction or recalls. The impact of such non-compliance or issues or problems would be exacerbated if our manufacturing efforts are scaled to conduct a Phase II or Phase III clinical trial in Europe, Israel or the United States, or Europe, where there may be numerous collection sites and where shipments may be made to multiple locations with large numbers of patients across a large geographical area. There can be no assurance that we can scale such a manufacturing process, including in Europe, Israel and the United States, in a cost-effective or efficient manner, or in a manner that will meet all regulatory requirements, including EMA, IMOH or FDA requirements, if at all.
While we expect to write-off small amounts of work-in-progress in the ordinary course of business due to the complex nature of blood, our processes and our product candidates, unanticipated events may lead to write-offs and other costs materially in excess of our expectations and the reserves we have established for these purposes. Such write-offs and other costs could cause material fluctuations in our liquidity and results of operations. Furthermore, contamination of our product candidates could cause consumers or other third parties with whom we conduct business to lose confidence in the reliability of our manufacturing procedures, which could adversely affect our liquidity and results of operations. In addition, faulty or contaminated product candidates that are unknowingly distributed could result in patient harm, threaten the reputation of our products and expose us to product liability damages and claims from companies for whom we do contract manufacturing.
If we or ourany potential CMOs we retain in the future fail to comply with manufacturing regulations, our financial results and financial condition could be adversely affected.
Before a BLAmarketing application is approved, or before we begin the commercial manufacture of any of our products, CMOs and other outsourced manufacturing service providers we may engage must obtain regulatory approval of their manufacturing facilities, processes and quality systems. In addition, pharmaceutical manufacturing facilities are continuously subject to inspection by the FDAEMA and foreign regulatory authorities before and after product approval. Due to the complexity of the processes used to manufacture pharmaceutical products and product candidates, any potential third-party manufacturer may be unable to continue to pass or initially pass federal, state or international regulatory inspections in a cost-effective manner.
The FDAEMA and foreign regulators require manufacturers to register manufacturing facilities. The FDAEMA and foreign regulators also inspect these facilities to confirm compliance with requirements that the FDAEMA or foreign regulators establish. We, to the extent we may manufacture our products in the future, or our materials suppliers may face manufacturing or quality control problems causing product production and shipment delays or a situation where we or the supplier may not be able to maintain compliance with the FDA’sEMA’s or foreign regulators’ requirements necessary to continue manufacturing our product candidate. Any failure to comply with FDAEMA or foreign regulatory requirements could adversely affect our clinical research activities and our ability to develop and market our product candidate and any future product candidates.
If a third-party manufacturer with whom we contract is unable to comply with manufacturing regulations, we may be subject to fines, unanticipated compliance expenses, recall or seizure of our products, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or civil prosecution. These possible sanctions would adversely affect our financial results and financial condition.
In January 2019September 2021 we completedinitiated the construction of a new facility in Israel to support the production of the Allocetra™ drug product, for any clinical trial that will be conductedwhich we completed in the EU or Israel.fourth quarter of 2022. We do not have experience in manufacturing products on a commercial scale. If, due to our lack of manufacturing experience and resources, we cannot manufacture our products on a commercial scale successfully or manufacture sufficient product to meet our expected commercial requirements, our business may be materially harmed.
In January 2019 we completed construction of a new facility in Israel for the manufacture of Allocetra™ for the planned clinical trials that will be conducted in the EU or Israel. We do not have experience in manufacturing products on a commercial scale or using automated processes and we have limited personnel. In addition, because we are not aware of any company that has manufactured Allocetra™ for clinical use, there are limited precedents from which we can learn. If we do not receive regulatory approval for Allocetra™, our costs for the construction and maintenance of the manufacturing facility may exceed revenue derived from the sale of products manufactured at such facility. If we do not have sufficient revenues to cover the costs of the manufacturing facility, we may need to shut down the facility at a loss or borrow or raise funds to maintain the facility until sufficient revenues can be generated. As part of a Company-wide cost cutting program designed to extend our cash runway potentially into 2025, our planned move into the new manufacturing plant has been deferred for an approximate period of three years, and we have determined to offer lease and supporting services of the leased space to potential clients during this three-year period, and we will not have access to the manufacturing facility during such time. We may encounter difficulties in the manufacture of our products due to our limited manufacturing experience and resources. These difficulties could delay the build-out and equipping of a commercial manufacturing facility and regulatory approval of the manufacture of our products, increase our costs or cause production delays or result in us not manufacturing sufficient product to meet our expected commercial requirements, any of which could damage our reputation and hurt our profitability. If we are unable to successfully increase our manufacturing capacity to commercial scale, our business may be materially adversely affected.
Our ability to produce safe and effective products depends on the safety of our blood supply against transmittable diseases.
Despite overlapping safeguards, including the screening of donors and GMP aseptic manufacturing under a quality controlled governing system, the risk of transmissible disease through blood products cannot be entirely eliminated. For example, because blood-derived therapeutics involve the use and purification of human blood, there has been concern raised about the risk of transmitting coronavirus (COVID-19),COVID-19, human immunodeficiency virus (“HIV”), West Nile virus, H1N1 virus or “swine flu” and other blood-borne pathogens and infectious agents through blood-derived products. There are also concerns about the future transmission of H5N1 virus, or “bird flu.” In the 1980s, thousands of hemophiliacs worldwide were infected with HIV through the use of contaminated Factor VIII.
New infectious diseases emerge in the human population from time to time. If a new infectious disease has a period during which time the causative agent is present in the bloodstream but symptoms are not present, it is possible that blood donations could be contaminated by that infectious agent. Typically, early in an outbreak of a new disease, tests for the causative agent do not exist. During this early phase, we must rely on screening of donors and patients (e.g., for behavioral risk factors or physical symptoms) to reduce the risk of blood contamination. Screening methods are generally less sensitive and specific than a direct test as a means of identifying potentially contaminated blood units.
During the early phase of an outbreak of a new infectious disease, our ability to manufacture safe products would depend on the manufacturing process’ capacity to inactivate or remove the infectious agent. To the extent that a product’s manufacturing process is inadequate to inactivate or remove an infectious agent, our ability to manufacture and distribute that product would be impaired.
If a new infectious disease were to emerge in the human population, the regulatory and public health authorities could impose precautions to limit the transmission of the disease that would impair our ability to procure blood, manufacture our product candidates or both. Such precautionary measures could be taken before there is conclusive medical or scientific evidence that a disease poses a risk for blood-derived products.
In recent years, new testing and viral inactivation methods have been developed that more effectively detect and inactivate infectious viruses in collected blood. There can be no assurance, however, that such new testing and inactivation methods will adequately screen for, and inactivate, infectious agents in the blood used in the production of our product candidates.
Our product candidates may produce undesirable side effects that we may not detect in our clinical trials, which could prevent us from achieving or maintaining market acceptance of any such product candidate and could substantially increase commercialization costs or even force us to cease operations.
Even if Allocetra™, or any of our other product candidates, receives marketing approval, we or others may later identify undesirable side effects caused by the product, and, in that event, a number of potentially significant negative consequences could result, including, without limitation:
| ● | regulatory authorities may suspend or withdraw their approval of the product; |
| ● | regulatory authorities may require the addition of labeling statements, such as warnings or contraindications or distribution and use restrictions, or they may require that these statements be placed in a black box on the product’s labeling; |
| ● | regulatory authorities may require us to issue specific communications to healthcare professionals, such as “Dear Doctor” letters; |
| ● | regulatory authorities may issue negative publicity regarding the affected product, including safety communications; |
| ● | we may be required to change the way the product is administered, conduct additional preclinical studies or clinical trials or restrict or cease the distribution or use of the product; and |
| ● | we could be sued and held liable for harm caused to patients, and in certain cases, certain relatives. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could substantially increase commercialization costs or even force us to cease operations.
Even if Allocetra™ or any other product candidate that we are developing or may develop receives marketing approval, we will continue to face extensive regulatory requirements, and any such product may still face future development and regulatory difficulties. In addition, we are subject to government regulations and we may experience delays in obtaining required regulatory approvals to market our proposed product candidates.
Even if we receive regulatory approval to market a particular product candidate, any such product will remain subject to extensive regulatory requirements, including requirements relating to manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution and recordkeeping. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the uses for which the product may be marketed or the conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product, which could adversely affect us by reducing revenues or increasing expenses, and cause the approved product candidate not to be commercially viable. In addition, as clinical experience with a drug expands after approval, typically because it is used by a greater number and more diverse group of patients after approval than during clinical trials, side effects and other problems may be observed over time after approval that were not seen or anticipated during pre-approval clinical trials or other studies. Any adverse effects observed after the approval and marketing of a product candidate could result in limitations on the use of or withdrawal of any approved products from the marketplace. Absence of long-term safety data may also limit the approved uses of our products, if any. If we fail to comply with the regulatory requirements of the FDA,EMA, and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other setbacks, including, without limitation, the following:
| ● | suspend or impose restrictions on operations, including costly new manufacturing requirements; |
| ● | refuse to approve pending applications or supplements to applications; |
| ● | suspend any ongoing clinical trials; |
| ● | suspend or withdraw marketing approval; |
| ● | seek an injunction or impose civil or criminal penalties or monetary fines; |
| ● | seize or detain products; |
| ● | ban or restrict imports and exports; |
| ● | issue warning letters or untitled letters; or |
| ● | refuse to approve pending applications or supplements to applications. |
In addition, various aspects of our operations are subject to federal, state or local laws, rules and regulations, any of which may change from time to time. Costs arising out of any regulatory developments could be time-consuming and expensive and could divert management resources and attention and, consequently, could adversely affect our business operations and financial performance.
Delays in regulatory approval, limitations in regulatory approval and withdrawals of regulatory approval may have a material adverse effect on the Company.
If we receive marketing approval for any of our product candidates, sales will be limited unless the product achieves broad market acceptance.
The commercial success of our product candidates and any future product candidate for which we obtain marketing approval from the FDA,EMA, or other regulatory authorities, will depend on the breadth of its approved labeling and upon the acceptance of the product by the medical community, including physicians, patients and third-party payors. The degree of market acceptance of any approved product will depend on a number of factors, including, without limitation:
| ● | demonstration of clinical safety and efficacy compared to other products; |
| ● | ability of physicians to accurately diagnose the targeted indications; |
| ● | the relative convenience and ease of administration; |
| ● | the prevalence and severity of any adverse side effects; |
| ● | limitations or warnings contained in the product’s approved labeling; |
| ● | distribution and use restrictions imposed by the |
| ● | availability of alternative treatments, including a number of competitive products already approved or expected to be commercially launched in the near future; |
| ● | pricing and cost effectiveness; |
| ● | the effectiveness of our, or any future collaborators’, sales and marketing strategies; |
| ● | our ability to obtain sufficient third-party coverage or reimbursement; and |
| ● | the willingness of patients to pay for drugs out of pocket in the absence of third-party coverage. |
If any of our product candidates is approved, but does not achieve an adequate level of acceptance by physicians, third-party payors and patients, we may not generate sufficient revenue from the product, and we may not become profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of the product may require significant resources and may never be successful.
If we acquire or in-license additional technologies or product candidates, we may incur additional costs, have integration difficulties and experience other risks that could harm our business and results of operations.
We may acquire and in-license additional product candidates and technologies. Any product candidate or technologies we in-license or acquire will likely require additional development efforts prior to commercial sale, including extensive preclinical or clinical testing, or both, and approval by the FDAEMA and applicable foreign regulatory authorities, if any. All product candidates are prone to risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate, or products developed based on in-licensed technology, will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any product candidate that we develop based on acquired or in-licensed technology that is granted regulatory approval will be manufactured or produced economically, successfully commercialized or widely accepted or competitive in the marketplace. Moreover, integrating any newly acquired or in-licensed product candidates could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may not succeed.
The FDAEMA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. If we are found to have improperly promoted off-label uses, we may become subject to significant liability.
The FDAEMA and other regulatory agencies strictly regulate promotional claims about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDAEMA or such other regulatory agencies as reflected in the product’s approved labeling. In particular, any labeling approved by such regulatory agencies for our products, if any, may also include restrictions on use. Such regulatory agencies may impose further requirements or restrictions on the distribution or use of our products as part of a mandatory plan, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. If we receive marketing approval for our product candidates, physicians may nevertheless prescribe our products to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such “off-label” uses, we may become subject to significant liability. In particular, the U.S. federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDAEMA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
We may be subject to extensive environmental, health and safety, and other laws and regulations in multiple jurisdictions.
Our business involves the controlled use, including through our service providers, of hazardous materials, various biological compounds and chemicals; therefore, we, our agents and our service providers may be subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, the use, management and disposal of hazardous, radioactive and biological materials and wastes and the cleanup of contaminated sites. The risk of accidental contamination or injury from these materials cannot be eliminated. If an accident, spill or release of any regulated chemicals or substances occurs, we could be held liable for resulting damages, including for investigation, remediation and monitoring of the contamination, including natural resource damages, the costs of which could be substantial. We may incur substantial capital costs and operating expenses and may be required to obtain consents to comply with any environmental and health laws or regulations and the terms and conditions of any permits required pursuant to such laws and regulations, including costs incurred by us to install new or updated pollution control equipment for our service providers, modify our operations or perform other corrective actions at our facilities or the facilities of our service providers. In addition, fines and penalties may be imposed on us, our agents and/or our service providers for non-compliance with environmental, health and safety and other laws and regulations or for the failure to have, or comply with the terms and conditions of, required environmental or other permits or consents.
We expect the healthcare industry to face increased limitations on reimbursement, rebates and other payments as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products.
In both the United StatesEurope and in other countries, sales of our products, if any, will depend in part upon the availability of reimbursement from third-party payors, which include governmental authorities, managed care organizations and other private health insurers. Third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services.
Increasing expenditures for healthcare have been the subject of considerable public attention in Europe and the United States. Both private and government entities are seeking ways to reduce or contain healthcare costs. Numerous proposals that would effect changes in the European and U.S. healthcare systemsystems have been introduced or proposed, in Congress and in some state legislatures, including reducing reimbursement for prescription products and reducing the levels at which consumers and healthcare providers are reimbursed for purchases of pharmaceutical products.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, (the “Medicare Modernization Act”), changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs under Medicare Part B. In recent years, Congress has considered further reductions in Medicare reimbursement for drugs administered by physicians. The Centers for Medicare & Medicaid Services (“CMS”), has issued and will continue to issue regulations to implement the new law which will affect Medicare, Medicaid and other third-party payors. Medicare, which is the single largest third-party payment program and which is administered by CMS, covers prescription drugs in one of two ways. Medicare Part B covers outpatient prescription drugs that are administered by physicians and Medicare Part D covers other outpatient prescription drugs, but through private insurers. Medicare Part A covers hospitalizations and pays hospitals a fixed fee, subject to certain limited exceptions, for treating a given ailment. Drug or biologics administered during a patient’s hospitalization are not separately reimbursed by CMS, but are paid by the hospital out of its fixed fee payment from CMS. Hospitals, therefore, may be reluctant to purchase new costly products unless those products can significantly reduce a patient’s length of stay. Medicaid, a health insurance program for the poor, is funded jointly by CMS and the states, but is administered by the states; states are authorized to cover outpatient prescription drugs, but that coverage is subject to caps and to substantial rebates. The CMS also has the authority to revise reimbursement rates and to implement coverage restrictions for some drugs. Cost reduction initiatives and changes in coverage implemented through legislation or regulation could decrease utilization of and reimbursement for any approved products, which in turn would affect the price we can receive for those products. While the Medicare Modernization Act and Medicare regulations apply only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from federal legislation or regulation may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 (the “Affordable Care Act”), a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. The Affordable Care Act expanded manufacturers’ rebate liability to include covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, increased the minimum rebate due for innovator drugs from 15.1% of average manufacturer price (“AMP”), to 23.1% of AMP. The rebate on innovator drugs is the greater of 23.1 % of the AMP per unit or the difference between the AMP and the best price per unit and adjusted by the Consumer Price Index-Urban (“CPI-U”) based on launch date and current quarter AMP. The total rebate amount for innovator drugs is capped at 100.0% of AMP. The Affordable Care Act and subsequent legislation also narrowed the definition of AMP. Furthermore, the Affordable Care Act imposes a significant annual, nondeductible fee on companies that manufacture or import certain branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with healthcare practitioners, and a significant number of provisions are not yet, or have only recently become, effective. Although it is too early to determine the effect of the Affordable Care Act, it appears likely to continue to put pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
The Food and Drug Administration Reauthorization Act (FDARA) was signed into law on August 18, 2017. The law comprises the reauthorization of the Prescription Drug User Fee Act (PDUFA), which is intended to give the FDA the resources to sustain a predictable and efficient review process for human drugs and biologics. The current PDUFA (PDUFA VI), which includes the Fiscal Years 2018-2022, generates a new user fee program with some changes including removal of the prescription drug establishment fees and added a human prescription drug program fee, Although the establishment fee has been eliminated, the previous establishment registration and drug listing requirements are still in effect. PDUFA VI created a structure whereby human drug application fees will account for 20% and prescription drug program fees will account for 80% of the total revenue amount for that fiscal year. Previously, FDA collected human drug application and supplement fees, prescription drug establishment fees and prescription drug product fees. PDUFA VI eliminates fees for supplements as well as for establishments, though applicants will be assessed annual prescription drug program fees for prescription drug products, rather than the prescription drug product fee assessed under the previous iteration of PDUFA. In addition, PDUFA VI eliminates a provision under which applicants could apply for a waiver or refund of user fees on the basis that the fees to be paid by such person will exceed the anticipated present and future costs incurred by the Secretary in conducting the process for the review of human drug applications for such person, also known as the ‘the fees-exceed-costs waiver.
A significant component of the Affordable Care Act (ACA) is the individual mandate that obligates individuals to purchase health insurance. The 2017 Tax Cuts and Jobs Act eliminated the penalty for not doing so. A December 2018 Federal District Court in Texas has ruled that the mandate is now unconstitutional and since that provision is not severable from the ACA, this unconstitutionality applies to the entire ACT. This decision is under appeal. The U.S. Department of Health and Human Services issued a statement indicating that the decision “is not an injunction that halts the enforcement of the law and not a final judgment. Therefore, HHS will continue administering and enforcing all aspects of the ACA as it had before the court issued its decision.” The Centers for Medicare and Medicaid Services (CMS) also indicated on its healthcare.gov website that the decision does not impact enrollment or coverage on the health care exchanges for 2019. While it is business as usual for now, this decision may be far-reaching and eventually could significantly impact employer health plans, individuals, health care providers, and federal- and state-administered health programs and their coverage of and reimbursement for our product.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. If we ever obtain regulatory approval and commercialization of any of our product candidates, these new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates may be.
Although we cannot predict the full effect on our business of the implementation of existing legislation or the enactment of additional legislation pursuant to healthcare and other legislative reform, we believe that legislation or regulations that would reduce reimbursement for, or restrict coverage of, our products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could materially and adversely affect our business by reducing our ability to generate revenue, raise capital, obtain additional collaborators and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact product sales.
It will be difficult for us to profitably sell our future products, if any, if reimbursement for any such product is limited by government authorities and third-party payor policies.
In addition to any healthcare reform measures that may affect reimbursement, market acceptance and sales of our future products, if any, will depend on the reimbursement policies of government authorities and third-party payors. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the European and U.S. healthcare industry andindustries, as well as elsewhere, is cost containment. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that reimbursement will be available for our future products, if any, and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. In addition, third-party payors are likely to impose strict requirements for reimbursement in order to limit off-label use of a higher priced drug or treatment. Reimbursement by a third-party payor may depend upon a number of factors including the third-party payor’s determination that use of a product is:
| ● | a covered benefit under its health plan; |
| ● | safe, effective and medically necessary; |
| ● | appropriate for the specific patient; |
| ● | cost-effective; and |
| ● | neither experimental nor investigational. |
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide supporting scientific, clinical and cost effectiveness data for the use of our products to the payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage or adequate reimbursement will be available for our future products. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our future products. If reimbursement is not available, or is available only to limited levels, we may not be able to commercialize our product candidate, or any future product candidates, profitably, or at all, even if approved.
Governments outside the United States tend to impose strict price controls, which may adversely affect our future revenues, if any.
In some countries, particularly the countries comprising the EU, the pricing of pharmaceuticals and certain other therapeutics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
We are subject to anti-kickback laws and regulations. Our failure to comply with these laws and regulations could have adverse consequences to us.
There are extensive international and U.S. federal and state laws and regulations prohibiting fraud and abuse in the healthcare industry that can result in significant criminal and civil penalties. Such U.S. federal laws include: the anti-kickback statute, which prohibits certain business practices and relationships, including the payment or receipt of compensation for the referralgeneration of patients whose carebusiness that will be paid by Medicare or other federal healthcare programs; the physician self-referral prohibition, commonly referred to as the Stark Law; the anti-inducement law, which prohibits providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services covered by either program; the False Claims Act, which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment by the federal government, including the Medicare and Medicaid programs; and the Civil Monetary Penalties Law, which authorizes the U.S. Department of Health and Human Services to impose civil penalties administratively for fraudulent or abusive acts. In addition, the Affordable Care Act requires drug manufacturers to report to the government any payments to physicians for consulting services and the like. Many jurisdictions outside the United States have similar anti-kickback, fraud and abuse, and healthcare laws and regulations, and we could be subject to these laws and regulations to the extent that we operate in such jurisdictions.
Sanctions for violating these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, monetary penalties, imprisonment, denial of Medicare and Medicaid payments or exclusion from the Medicare and Medicaid programs, or both, and debarment. As federal and state budget pressures continue, federal and state administrative agencies may also continue to escalate investigation and enforcement efforts to reduce or eliminate waste and to control fraud and abuse in governmental healthcare programs. Private enforcement of healthcare fraud has also increased, due in large part to amendments to the Civil False Claims Act in 1986 that were designed to encourage private persons to sue on behalf of the government. Efforts to ensure compliance with any of these federal, state and other fraud and abuse laws and regulations may involve substantial costs, and a violation of the same could have a material adverse effect on our liquidity and financial condition. An investigation into the use by physicians of any of our products, if ever commercialized, may dissuade physicians from either purchasing or using them, and could have a material adverse effect on our ability to commercialize those products.
Our market is subject to intense competition. If we are unable to compete effectively, Allocetra™ or any other product candidate that we are developing or may develop may be rendered uncompetitive or obsolete.
There are a number of products in development for the treatment or prevention of sepsis acute GvHDand solid tumors and the other autoimmune and inflammatory disorders that we are targeting or intend to target in the future, most of which are being developed by companies that are far larger than us, with significantly greater resources and more experience. Further, our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large, fully-integrated pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. All of these competitors currently engage in, have engaged in or may engage in the future in the development, manufacturing, marketing and commercialization of new pharmaceuticals, some of which may compete with our product candidates. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. These companies may have products in development that are superior to Allocetra™ or any other product candidate that we are developing or may develop. Key competitive factors affecting the commercial success of Allocetra™ and any other product candidates that we are developing or may develop are likely to be efficacy, time of onset, safety and tolerability profile, reliability, convenience of dosing, price and reimbursement.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drug candidates, obtaining FDAEMA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than us in obtaining FDAEMA and other marketing approvals for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize, which may render Allocetra™ or any other product candidates that we are developing or may develop obsolete or uncompetitive before we can recover the expenses of developing and commercializing the product. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases and disorders we are targeting could render Allocetra™ or any other product candidates that we are developing or may develop, uncompetitive or obsolete. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may never be profitable.
Our competitors currently include companies with marketed products and/or an advanced research and development pipeline. Moreover, several companies have reported the commencement of research projects related to the treatment or prevention of GvHDsepsis and solid tumors. With respect to our solid tumor Indication, the development and commercialization of cancer immunotherapy products is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary rights. We face competition with respect to our current product candidate, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major biopharmaceutical companies, specialty biopharmaceutical companies, and biotechnology companies worldwide. There are a number of large biopharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of solid tumors.
Significant disruptions of information technology systems, cyberattacks and other autoimmunesecurity breaches could compromise our proprietary and inflammatory conditions, such as Crohn’s diseaseconfidential information, which could harm our business and reputation.
In the ordinary course of our business, we generate, collect and store proprietary information, including clinical trial subject, intellectual property and business information. We rely on sophisticated information technology systems, including software, cloud services and network-connected control systems, some of which are managed, hosted, provided or serviced by third parties. The secure storage, maintenance, and transmission of and access to this information is important to our operations, including our research and development efforts, and reputation. The future operation, success and growth of our business depends on streamlined processes made available through our uninhibited access to information systems, global communications, internet activity and other inflammatory bowel disorders, RA, gout, MSnetwork processes. Further, because certain employees are working remotely, our reliance on our and solid organ transplant rejection, as well asthird-party information technology systems has increased, which could increase our cybersecurity risk, create data accessibility concerns and make us more susceptible to communication disruptions, any of which could adversely impact our business operations.
Like most other companies, despite our current security measures and process controls, our information technology systems, and those of our third-party service providers, may be vulnerable to information security breaches, ransomware or extortion, mishandled data, acts of vandalism, computer viruses and interruption or loss of valuable business data. Stored data might be improperly accessed due to a variety of events beyond our control, including, but not limited to, damage and interruption from power loss or natural disasters, computer system and network failures, loss of telecommunications services, physical and electronic loss of access to data and information, terrorist attacks, hackers, security breaches or other security incidents, and computer viruses or attacks. A failure at any third parties who maintain our information technology systems to provide adequate and timely support could adversely affect the treatmentoperation of our information technology systems.
Hackers and data thieves are increasingly sophisticated and operate large-scale and complex attacks which may remain undetected until after they occur. Computer hackers may attempt to penetrate our computer systems or preventionthose of sepsis.our third party vendors and, if successful, misappropriate our proprietary and confidential information including e-mails and other electronic communications. In addition, an employee, contractor, or other third party with whom we do business may attempt to obtain such information, and may purposefully or inadvertently cause a breach involving such information. Any such compromise of our data security and access to, or public disclosure or loss of, confidential business or proprietary information could disrupt our operations, damage our reputation, provide our competitors with valuable information and subject us to additional costs, which could adversely affect our business.
The costs of mitigating cybersecurity risks are significant and are likely to increase in the future. These costs include, but are not limited to, retaining the services of cybersecurity providers; compliance costs arising out of existing and future cybersecurity, data protection and privacy laws and regulations; costs related to maintaining redundant networks, data backups and other damage-mitigation measures; and extra administrative costs to mitigate risk and deal with any system breaches.
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
Our products and product candidates could cause adverse effects. These adverse effects may not be observed in clinical trials, but may nonetheless occur in the future. If any of these adverse effects occur, they may render our product candidates ineffective or harmful in some patients, and our sales would suffer, materially adversely affecting our business, financial conditions and results of operations.
In addition, potential adverse effects caused by our product candidates, or products, could lead to product liability claims. Product liability claims might be brought against us by consumers, healthcare providers or others coming into contact with our products. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | decreased demand for our product candidates for which we obtain marketing approval; |
| ● | impairment of our business reputation and exposure to adverse publicity; |
| ● | increased warnings on product labels; |
| ● | withdrawal of clinical trial participants; |
| ● | costs of related litigation; |
| ● | distraction of management’s attention from our primary business; |
| ● | substantial monetary awards to patients or other claimants; |
| ● | loss of revenue; and |
| ● | the inability to successfully commercialize our product candidates for which we obtain marketing approval. |
If product liability lawsuits are successfully brought against us, our insurance may be inadequate.
We have obtained liability insurance coverage for our clinical trials with limits that are customary for such trials. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for any of our product candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain this product liability insurance on commercially reasonable terms. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A successful product liability claim, or series of claims, brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
The product liability insurance we will need to obtain in connection with the commercial sales of our product candidates, if and when they receive regulatory approval, may be unavailable in meaningful amounts or at a reasonable cost. If we are the subject of a successful product liability claim that exceeds the limits of any insurance coverage we obtain, we would incur substantial charges that would adversely affect our earnings and require the commitment of capital resources that might otherwise be available for the development and commercial launch of our product programs.
If we are unable to obtain adequate insurance to protect our business and property against damage, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance.
We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. To the extent our business or property suffers any damages, losses or claims by third parties, which are not covered, or adequately covered, by insurance, our financial condition may be materially adversely affected. If we are unable to obtain appropriate insurance, medical centers may be unwilling or unable to enter into site agreements to clinically test our candidate products.
We may be unable to maintain sufficient insurance as a public company to cover liability claims made against our officers and directors. If we are unable to adequately insure our officers and directors, we may not be able to retain or recruit qualified officers and directors to manage the Company.
We manage our business through a small number of senior executive officers. We depend on them even more than similarly-situated companies.
Our future growth and success depends on our ability to recruit, retain, manage and motivate our senior executive officers. The loss of the services of our management personnel, including without limitation, our Executive Chairman, Shai Novik, our Chief Executive Officer, Dr. Oren Hershkovitz, our VP Medical, Dr. Einat Galamidi, or our founder and Chief Scientific & Medical Officer, Prof. Dror Mevorach, or the inability to hire or retain experienced management personnel could adversely affect our ability to execute our business plan and harm our operating results.
Because of the specialized scientific nature of our business, we rely heavily on our ability to attract and retain qualified senior executive officers with scientific and technical experience. In particular, the loss of one or more of our senior executive officers could be detrimental to us if we cannot recruit suitable replacements in a timely manner. We do not currently carry “key person” insurance on the lives of members of senior management. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense competition, we may be unable to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
We incur significant costs as a result of operating as a public company, and our management devotes substantial time to compliance initiatives. We may fail to comply with the rules that apply to public companies, including Section 404 of the Sarbanes-Oxley Act of 2002, which could result in sanctions or other penalties that would harm our business.
We incur significant legal, accounting and other expenses as a public company, including costs relating to public company reporting obligations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). and regulations regarding corporate governance practices. Our management and other personnel devote a substantial amount of time to ensure that we comply with all of these requirements. Moreover, the reporting requirements, rules and regulations result in considerable legal and financial compliance costs. Any changes we make to comply with these obligations may not be sufficient to allow us to satisfy our obligations as a public company on a timely basis, or at all. These reporting requirements, rules and regulations, coupled with the increase in potential litigation exposure associated with being a public company, could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors (the “Board,”, or the “Board of Directors”) or board committees or to serve as executive officers, or to obtain certain types of insurance, including directors’ and officers’ liability insurance, on acceptable terms.
We are subject to Section 404 of The Sarbanes-Oxley Act of 2002, or Section 404, and the related rules of the SEC, which generally require our management and independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting. Section 404 requires an annual management assessment of the effectiveness of our internal control over financial reporting. Prior to December 31, 2019, we were an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and we took advantage of certain exemptions from various reporting requirements that were applicable to public companies that are emerging growth companies, including, but not limited to, the auditor attestation requirements of Section 404. We ceased to be an emerging growth company as of December 31, 2019 and are required to include an opinion from our independent registered public accounting firm on the effectiveness of our internal controls over financial reporting as part of this Annual Report on Form 20-F for the fiscal year ended December 31, 2019.
During the course of our review and testing of our internal controls, we may identify deficiencies and be unable to remediate them before we must provide the required reports. Furthermore, if we have a material weakness in our internal controls over financial reporting, we may not detect errors on a timely basis and our financial statements may be materially misstated. We or our independent registered public accounting firm may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting, which could harm our operating results, cause investors to lose confidence in our reported financial information and cause the trading price of our shares to fall.
If our internal controls over financial reporting are not effective, the reliability of our financial statements may be questioned, and our share price may suffer.
The continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. As our business continues to grow both domestically and internationally, our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of its testing, our management may identify material weaknesses or significant deficiencies. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our independent registered public accounting firm identifies material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our ordinary shares may suffer.
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
We may require rapid and substantial growth in order to achieve our operating plans, which will place a strain on our human and capital resources. Successful implementation of our business plan will require management of growth, which will increase management’s responsibilities. We currently have a limited number of employees, and, in order to continue the development and the commercialization of product candidates and future products, if any, we will need to substantially increase our operations, including expanding our employee base of managerial, operational and financial personnel. We currently intend to establish our manufacturing capabilities in Israel, Europe and the United States through CMOs, or through our own additional manufacturing factories as well as through clinical study management and monitoring service providers and CROs, which we may require additional funds. Any future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. To that end, we must be able to:
| ● | manage our clinical trials and the regulatory process effectively and efficiently; |
| ● | develop our administrative, accounting and management information systems and controls; |
| ● | hire and train additional qualified personnel; and |
| ● | integrate current and additional management, administrative, financial and sales and marketing personnel. |
If we are unable to establish, scale-up and implement improvements to our control systems in an efficient or timely manner, or if we encounter deficiencies in existing systems and controls, investors may choose not to invest in us, which could cause our share price to decline and negatively impact our ability to successfully commercialize our product candidates and future product candidates.
Failure to attract and retain sufficient numbers of talented employees will further strain our human resources and could impede our growth or result in ineffective growth. If we are unable to manage our growth effectively, our losses could materially increase and it will have a material adverse effect on our business, results of operations and financial condition.
Environmental, social and corporate governance (“ESG”) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. If our ESG practices fail to meet regulatory requirements or investor, customer, consumer, employee or other stakeholders’ evolving expectations and standards for responsible corporate citizenship in areas including environmental stewardship, support for local communities, Board of Director and employee diversity, human capital management, employee health and safety practices, product quality, supply chain management, corporate governance and transparency, our reputation, brand and employee retention may be negatively impacted, and our customers and suppliers may be unwilling to continue to do business with us.
Customers, consumers, investors and other stakeholders are increasingly focusing on environmental issues, including climate change, energy and water use, plastic waste and other sustainability concerns. Concern over climate change may result in new or increased legal and regulatory requirements to reduce or mitigate impacts to the environment. Changing customer and consumer preferences or increased regulatory requirements may result in increased demands or requirements regarding plastics and packaging materials, including single-use and non-recyclable plastic products and packaging, other components of our products and their environmental impact on sustainability, or increased customer and consumer concerns or perceptions (whether accurate or inaccurate) regarding the effects of substances present in certain of our products. Complying with these demands or requirements could cause us to incur additional manufacturing, operating or product development costs.
If we do not adapt to or comply with new regulations, including the SEC’s published proposed rules that would require companies to provide significantly expanded climate-related disclosures in their periodic reporting, which may require us to incur significant additional costs to comply and impose increased oversight obligations on our management and board of directors, or fail to meet evolving investor, industry or stakeholder expectations and concerns regarding ESG issues, investors may reconsider their capital investment in our Company, we may become subject to penalties, and customers and consumers may choose to stop purchasing our products, if approved for commercialization, which could have a material adverse effect on our reputation, business or financial condition.
Risks Related to Our Reliance on Third Parties
In January 2019 we completedWe expect to rely on third party manufacturers to manufacture our product candidates for purposes of clinical trials and commercial quantities of our product candidates, if and when approved for marketing by the construction of a new facility in Israelapplicable regulatory authorities.
With respect to support the production of the Allocetra™ drug product for any clinical trial that will be conducted in the EU or Israel and anticipate relying on third-party manufacturers and service providers to produce our products.
In January 2019 we completed the construction of a new facility in Israel to support the production of the Allocetra™ drug product for any clinical trial that will be conducted in the EU or Israel. For the starting material production required for future clinical trials, we expect to rely either our own manufacturing capabilities or on third-parties. We currently and in the future will rely upon blood banks and collection service facilities for the collection of starting material for the production of Allocetra™. We plan to initially rely upon hospitals, other health care providers, contract manufacturers and, potentially, collaboration partners, to manufacture commercial quantities of our product candidates, if and when approved for marketing by the applicable regulatory authorities. Although we have not yet engaged any contract manufacturers or other service providers, if and when we do, our contract manufacturers and service providers must complete technology transfer, process validation for the manufacturing process and demonstrate successful manufacturing of comparable product. If our contract manufacturers and service providers, and their respective facilities, as applicable, are not approved by the FDA,EMA, or other applicable regulatory authorities, our commercial supply of the product candidate will be significantly delayed and may result in significant additional costs. If we need to identify additional finished product manufacturers, we would not be able to do so without significant delay and likely significant additional cost.
Our and our contract manufacturers’ and other service providers’ failure to achieve and maintain high manufacturing standards, in accordance with applicable regulatory requirements, or the incidence of manufacturing errors, could result in patient injury or death, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business. Contract manufacturers and service providers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. Our future contract manufacturers and service providers may not perform as agreed or may not remain in the contract manufacturing business. In the event of a natural disaster, business failure, strike or other difficulty, we may be unable to replace our manufacturing capacity or a third-party manufacturer or provider in a timely manner and the production of our product candidates would be interrupted, resulting in delays and additional costs. See also “Risk Factors—Risks Related to our Business, Industry and Regulatory Requirements—Our manufacturing processes are complex, delicate and susceptible to contamination, and involve biological intermediates that are subject to stringent regulations.”
We intend to rely primarily on third parties to market and sell Allocetra™ and any other product candidate.
We have no sales or distribution capabilities. To the extent we rely on third parties to commercialize our products, if marketing approval is obtained, we may receive less revenue than if we commercialize such products ourselves. In addition, we would have less control over the sales efforts of any third parties involved in our commercialization efforts. In the event we are unable to collaborate with a third-party marketing and sales organization to commercialize our products, particularly for broader patient populations, our ability to generate revenue will be limited.
Although we may ultimately develop a marketing and sales force with technical expertise and supporting distribution capabilities in the longer term, we do not currently intend to do so; therefore, we will be unable to directly market our products, if any, in the near future. To promote any of our potential products through third parties, we will have to locate acceptable third parties for these functions and enter into agreements with them on acceptable terms, and we may not be able to do so. Any third-party arrangements we are able to enter into may result in lower revenues than we could achieve by directly marketing and selling our potential products. In addition, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, as well as the terms of our agreements with such third parties, which cannot be predicted in most cases at this time. As a result, we might not be able to market and sell our products in the United States or overseas, which would have a material adverse effect on us.
Any collaboration arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our current and any future product candidates.
We have collaboration arrangements with certain medical institutions and we may determine to seek collaboration arrangements with pharmaceutical or biotechnology companies for the development and commercialization of our current and any future product candidates. We will face, to the extent that we decide to enter into future collaboration agreements, significant competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements. Additionally, the terms of any collaborations or other arrangements that we may establish may not be favorable to us.
Any future collaborations that we enter into may not be successful. The success of our collaboration arrangements, if any, would depend heavily on the efforts and activities of our collaborators. Collaborators generally have significant discretion in determining the efforts and resources that they will apply to these collaborations.
Disagreements between parties to a collaboration arrangement regarding clinical development and commercialization matters can lead to delays in the development process or commercializing the applicable product candidate and, in some cases, termination of the collaboration arrangement. These disagreements can be difficult to resolve.
Collaborations with pharmaceutical or biotechnology companies and other third parties are often terminated or allowed to expire by the other party. Any such termination or expiration could adversely affect us financially and could harm our business reputation.
We depend on third parties to conduct our clinical trials.
We currently rely, and for the foreseeable future, will continue to rely, on third parties, such as CROs, medical institutions, clinical investigators and contract laboratories to oversee most of the operations of our clinical trials and to perform data collection and analysis. As a result, we may face additional delays outside of our control if these parties do not perform their obligations in a timely fashion or in accordance with regulatory requirements. If these third parties do not successfully carry out their contractual duties or obligations and meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our financial results and the commercial prospects for our product candidates or any other potential product candidates could be harmed, our costs could increase and our ability to obtain regulatory approval and commence product sales could be delayed.
The recent coronavirus outbreak may adversely affect our business, results of operations and financial condition.
In December 2019, a novel strain of coronavirus, COVID-19 (“Coronavirus”) was reported to have surfaced in Wuhan, China, and has reached multiple other countries, resulting in government-imposed quarantines, travel restrictions and other public health safety measures in China, the United States, Israel, Italy, Spain, Australia and other affected countries. The various precautionary measures taken by many governmental authorities around the world in order to limit the spread of the Coronavirus, which has had, and may continue to have, an adverse effect on the global markets and economy, including on the availability and pricing of employees, resources, materials, manufacturing and delivery efforts and other aspects of the global economy. Therefore, the Coronavirus could disrupt production and cause delays in the supply and delivery of products used in our operations, may affect our operations, including the conduct of clinical studies, or the ability of regulatory bodies to grant approvals or supervise our candidates and products, may further divert the attention and efforts of the medical community to coping with the Coronavirus and disrupt the marketplace in which we operate and may have a material adverse effects on our operations. The extent to which the Coronavirus impacts our business and financial condition will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the Coronavirus and the actions to contain the Coronavirus or treat its impact, among others. The development of the Coronavirus outbreak could materially disrupt our business and operations, slow down the overall economy, interrupt our sources of supply and make it hard to adequately staff our operations, any of which could adversely affect our business, results of operations and financial condition.
Cyberattacks and other security breaches could compromise our proprietary and confidential information, which could harm our business and reputation.
In the ordinary course of our business, we generate, collect and store proprietary information, including clinical trial subject, intellectual property and business information. The secure storage, maintenance, and transmission of and access to this information is important to our operations and reputation. Computer hackers may attempt to penetrate our computer systems and, if successful, misappropriate our proprietary and confidential information including e-mails and other electronic communications. In addition, an employee, contractor, or other third party with whom we do business may attempt to obtain such information, and may purposefully or inadvertently cause a breach involving such information. While we have certain safeguards in place to reduce the risk of and detect cyberattacks, our information technology networks and infrastructure may be vulnerable to unpermitted access by hackers or other breaches, or employee error or malfeasance. Any such compromise of our data security and access to, or public disclosure or loss of, confidential business or proprietary information could disrupt our operations, damage our reputation, provide our competitors with valuable information and subject us to additional costs, which could adversely affect our business.
Risks Related to Our Intellectual Property
The failure to obtain or maintain patents, licensing agreements and other intellectual property could impact our ability to compete effectively.
To compete effectively, we need to develop and maintain a proprietary position with regard to our own technologies, intellectual property, licensing agreements, product candidates and business. Legal standards relating to the validity and scope of claims in the biotechnology and biopharmaceutical fields are still evolving. Therefore, the degree of future protection for our proprietary rights in our core technologies and any product candidates or products that might be developed using these technologies is also uncertain. The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| ● | while the patents we own have been issued, pending patent applications we have filed may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | we may be subject to interference or reexamination proceedings; |
| ● | we may be subject to opposition proceedings in foreign countries; |
| ● | any patents that are issued may not provide meaningful protection for any significant period of time, if at all; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge and invalidate patents licensed or issued to us or our customers; |
| ● | other companies may independently develop similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design around technologies we have licensed or developed; and |
| ● | enforcement of patents is complex, uncertain and expensive, and our patents may be found invalid or enforceable. |
We cannot be certain that patents will be issued as a result of any of our pending applications, and we cannot be certain that any of our issued patents, whether issued pursuant to our pending applications or licensed from third parties, will give us adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope, and changes in the law may affect the utility of a pending patent application or issued patent. In addition, because publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions. If any of our composition of matter patents, or pending applications, was subject to a successful challenge or failed to issue, our business and competitive advantage could be significantly affected. Our current patents will expire or they may otherwise cease to provide meaningful competitive advantage, and we may be unable to adequately develop new technologies and obtain future patent protection to preserve our competitive advantage or avoid adverse effects on our business.
Although we expect to do so, we may not be able to submit a BLAmarketing application seeking approval of Allocetra™ prior to the applicable patents’ expiration date, assuming all necessary patents are in fact issued. Moreover, we cannot be certain that we will be the first applicant to obtain an FDAEMA approval for any indication of our product candidates and we cannot be certain that we will be entitled to any other exclusivity with respect to the same. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
Others may obtain issued patents that could prevent us from commercializing our product candidates or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon trade secrets and proprietary know-how to protect our proprietary technology. We require our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the disclosure of confidential information to any other parties. We also require our employees and consultants to disclose and assign to us their ideas, developments, discoveries and inventions. These agreements may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
We cannot predict the scope and extent of patent protection for our product candidates because the patent positions of pharmaceutical products are complex and uncertain.
Any patents issued to us will not ensure the protection of our intellectual property for a number of reasons, including, without limitation, the following:
| ● | any issued patents may not be broad or strong enough to prevent competition from other products including identical or similar products; |
| ● | if we are not awarded patents or if issued patents expire or are declared invalid or not infringed, there may be no protections against competitors attempting to make |
| ● | there may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim; |
| ● | there may be other patents or pending patent applications existing in the patent landscape for our product candidates that will affect our freedom to operate; |
| ● | if our patents are challenged, a court could determine that they are not valid or enforceable; |
| ● | a court could determine that a competitor’s technology or product does not infringe our patents; |
| ● | our patents could irretrievably lapse due to failure to pay fees or otherwise comply with regulations, or could be subject to compulsory licensing; and |
| ● | if we encounter delays in our development or clinical trials, the period of time during which we could market our products under patent protection would be reduced. |
We may not be able to enforce our intellectual property rights throughout the world. This risk is exacerbated because we expect that our product candidates will be manufactured and used in a number of countries.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This risk is exacerbated for us because we expect our product candidates will be manufactured and used in a number of countries.
The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to life sciences. This could make it difficult for us to stop the infringement of our other intellectual property rights. For example, several foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, some countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit.
Although most jurisdictions in which we have applied for, intend to apply for, or have been issued patents have patent protection laws similar to those of the United States, some of them do not. For example, in the future, we may consider doing business in South America, Eurasia, China and Indochina, and the countries in these regions may not provide the same or similar protection as that provided in the United States. Additionally, due to uncertainty in patent protection law, we have not filed applications in many countries where significant markets exist, including, without limitation, South American countries, Eurasian countries, African countries and Taiwan.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other pharmaceutical and biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical and biotechnology industries involve both technological and legal complexity. Therefore, obtaining and enforcing related patents is costly, time-consuming and inherently uncertain. In particular, the United States has recently enacted, and is currently implementing changes in the law, including wide-ranging patent reform legislation. The U.S. Supreme Court and the U.S. Court of Appeals for the Federal Circuit have ruled on several patent cases in recent years, and could do so again in the future, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition, the U.S. Patent and Trademark Office, or the USPTO, has implemented patentability guidelines that may render the subject matter of a patent as non-patentable based on a lack of utility. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by applicable courts and legislatures in the countries in which we may pursue patent protection, including those of the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents and the interpretations of such laws could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may be unable to protect the intellectual property rights of the third parties from whom we license or may license certain of our intellectual property or with whom we have entered into other strategic relationships, which could have a material adverse effect on our business, results of operations and financial condition.
Certain of our intellectual property rights are and may in the future continue to be licensed from third parties, including universities and/or strategic partners. Such third parties may not protect the intellectual property rights that we license from them and we may be unable to defend such intellectual property rights on our own (even if we contractually agree to manage, maintain and defend such rights) or we may have to undertake costly litigation to defend the intellectual property rights of such third parties. There can be no assurances that we will continue to have proprietary rights to any of the intellectual property that we license from such third parties or otherwise have the right to use through similar strategic relationships. Any loss or limitations on use with respect to such intellectual property licensed from third parties or otherwise obtained from third parties with whom we have entered into strategic relationships could have a material adverse effect on our business, results of operations and financial condition.
We may infringe on the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing, or increase the costs of commercializing, our products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. For example, there could be issued patents of which we are not aware that our products infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes.
Third parties may assert that we are employing their proprietary technology without authorization. If a court held that any third-party patents are valid, enforceable and cover our products or their use, the holders of any of these patents may be able to block our ability to commercialize our product candidates or products unless we obtained a license under the applicable patents, or until the patents expire. In addition to litigation proceedings which may be filed against us, we may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost or on reasonable terms. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us.
We may be unable to adequately prevent disclosure and unauthorized use of trade secrets and other proprietary information by third parties.
Our ability to obtain and maintain patent protection and trade secret protection for our intellectual property and proprietary technologies, our products and their uses is important to our commercial success. We rely on a combination of patent, copyright, trademark and trade secret laws, non-disclosure and confidentiality agreements, licenses, assignment of inventions agreements and other restrictions on disclosure and use to protect our intellectual property rights.
We also rely on trade secrets to protect our proprietary know-how and technological advances, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements, to the extent they are in place and in effect, may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. Failure to obtain or maintain trade secret protection could enable competitors to use our proprietary information to develop products that compete with our product candidates or products or cause additional material adverse effects upon our competitive business position.
We cannot be certain that the steps that we have taken will prevent the misappropriation or other violation of our confidential information and other intellectual property, particularly in foreign countries in which laws may not protect our proprietary rights as fully as in the United States and other developed economies. Moreover, if we lose any key personnel, we may not be able to prevent these persons from impermissibly disclosing or using our technical knowledge or other trade secrets. If we are unable to maintain the security of our proprietary technology, this could materially adversely affect our competitive advantage, business and results of operations.
Under applicable U.S. and Israeli law, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We generally enter into non-competition agreements with our employees and certain key consultants, or our employment and consulting agreements contain non-competition provisions. These agreements, to the extent they are in place and in effect, prohibit our employees and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period of time. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us. For example, Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or the protection of its intellectual property. If we cannot demonstrate that such interests will be harmed, we may be unable to prevent our competitors from benefiting from the expertise of our former employees or consultants and our ability to remain competitive may be diminished.
Our employment agreements include employees’ undertakings with respect to confidentiality and the assignment to us of intellectual property rights developed in the course of employment, as well as a waiver of royalties related to intellectual property developed by the employee during his or her employment. However, in view of recent Israeli case law, these waivers may be deemed insufficient, and our employees could be able to assert claims for compensation with respect to our future revenue. As a result, we may receive less revenue from future products if such claims are successful, which in turn could impact our future profitability.
Any lawsuits relating to infringement of intellectual property rights necessary to defend ourselves or enforce our rights will be costly and time consuming.
We may be required to initiate litigation to enforce our rights or defend our activities in response to alleged infringement of a third-party. In addition, we may be sued by others who hold intellectual property rights and who claim that their rights are infringed by our product candidates or any of our future products or product candidates. These lawsuits can be very time consuming and costly. There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries generally.
A third-party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume time and other resources. There is a risk that such court will decide that we are infringing the third-party’s patents and will order us to stop the activities claimed by the patents, redesign our products or processes to avoid infringement or obtain licenses, which may not be available on commercially reasonable terms. In addition, there is a risk that a court will order us to pay the other party damages for infringement.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license, if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our product candidates, technologies or other matters.
In addition, our patents and patent applications could face other challenges, such as interference proceedings, opposition proceedings and re-examination proceedings. Any of these challenges, if successful, could result in the invalidation of, or in a narrowing of the scope of, any of our patents and patent applications subject to challenge. Any of these challenges, regardless of their success, would likely be time consuming and expensive to defend and resolve and would divert our management’s time and attention.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which non-compliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
Risks Related to the Ownership of Our Ordinary Shares
We do not know whether a market for our ordinary shares will be sustained or what the market price of our ordinary shares will be and as a result it may be difficult for you to sell your shares.
The trading price of our ordinary shares is likely to be volatile. The following factors, some of which are beyond our control, in addition to other risk factors described in this section, may have a significant impact on the market price of our ordinary shares:
| ● | inability to obtain the approvals necessary to commence further clinical trials; |
| ● | unsatisfactory results of clinical trials; |
| ● | announcements of regulatory approval or the failure to obtain it, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| ● | announcements of therapeutic innovations or new products by us or our competitors; |
| ● | adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| ● | changes or developments in laws or regulations applicable to our product candidates; |
| ● | any adverse changes to our relationship with manufacturers or suppliers; |
| ● | any product liability actions or intellectual property infringement actions in which we may become involved; |
| ● | announcements concerning our competitors or the pharmaceutical or biotechnology industries in general; |
| ● | achievement of expected product sales and profitability or our failure to meet expectations; |
| ● | our commencement of, or involvement in, litigation; |
| ● | any major changes in our Board, management or other key personnel; |
| ● | legislation in the United States, Europe and other foreign countries relating to the sale or pricing of pharmaceuticals; |
| ● | announcements by us of significant strategic partnerships, out-licensing, in-licensing, joint ventures, acquisitions or capital commitments; |
| ● | expiration or terminations of licenses, research contracts or other collaboration agreements; |
| ● | public concern as to the safety of therapeutics we, our licensees or others develop; |
| ● | success of research and development projects; |
| ● | variations in our and our competitors’ results of operations; |
| ● | changes in earnings estimates or recommendations by securities analysts, if our ordinary shares are covered by analysts; |
| ● | developments by our licensees, if any; |
| ● | future issuances of ordinary shares or other |
| ● | general political, economic and market conditions. |
These factors may materially and adversely affect the market price of our ordinary shares, which could result in substantial losses by our investors.
In addition, the stock market in general, and the Nasdaq Capital Market and the market for biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies like ours. Broad market and industry factors may negatively affect the market price of our ordinary shares, regardless of our actual operating performance. Further, a systemic decline in the financial markets and related factors beyond our control may cause our share price to decline rapidly and unexpectedly. Price volatility of our ordinary shares might be worse if the trading volume of our ordinary shares is low.
Moreover, the liquidity of our ordinary shares is limited. Among other factors, the number of ordinary shares that can be bought and sold at a given price, potential delays in the timing of executing transactions in our ordinary shares and a reduction in security analyst and media coverage of our company, if any, may result in lower prices for our ordinary shares and a larger spread between the bid and ask prices therefor. In addition, without a large float, our ordinary shares are less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our ordinary shares may be more volatile. In the absence of an active public trading market, an investor may be unable to liquidate its investment in our ordinary shares. Trading of a relatively small volume of our ordinary shares may have a greater impact on the trading price of our shares than would be the case if our public float were larger. We cannot predict the prices at which our ordinary shares will trade in the future.
We may be subject to securities litigation, which may be expensive and could divert management attention.
Companies that have experienced volatility and other negative fluctuations in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources from our business, which could materially harm our business, even if we were to successfully defend against such litigation. Any adverse determination in litigation could also subject us to significant liabilities.
Our principal shareholders, directors and officers currently own approximately 46%19.47% of our outstanding ordinary shares. They will therefore be able to exert significant controlinfluence over matters submitted to our shareholders for approval.
Our principal shareholders, directors and officers beneficially own approximately 46%19.47% of our outstanding ordinary shares. As a result, these shareholders, if they acted together, could significantly influence or even unilaterally approve matters requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these shareholders may not always coincide with our interests or the interests of other shareholders. This significant concentration of share ownership may adversely affect the trading price for our ordinary shares because investors often perceive disadvantages in owning stock in companies with controlling shareholders.
Raising additional capital would result in dilution to our existing shareholders and may restrict our operations or require us to relinquish rights.
We may seek additional capital through a combination of private and public equity offerings, debt financings, collaborations and licensing arrangements. To the extent that we raise additional capital through the sale of equity, convertible debt or other equity-linked securities, your ownership interest will be diluted, and the terms of the equity or equity-linked securities that we issue may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, would result in increased payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Our U.S. shareholders may suffer adverse tax consequences if we were to be characterized as a passive foreign investment company, or PFIC.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of our assets are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. There can be no assurance that we will not be classified as a PFIC in any year. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. Holder, as defined in “Taxation — United States Federal Income Tax Consequences”,Consequences,” owns ordinary shares, such U.S. Holder could face adverse U.S. federal income tax consequences, including having gains realized on the sale of our ordinary shares classified as ordinary income, rather than as capital gains, a loss of the preferential rate applicable to dividends received on our ordinary shares by individuals who are U.S. Holders and having interest charges apply to distributions by us and the proceeds of share sales. Certain elections exist that may alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment (such as mark-to-market treatment) of our ordinary shares; however, we do not intend to provide the information necessary for U.S. holders to make “qualified electing fund elections”,elections,” or QEF elections, if we are classified as a PFIC, and, accordingly, such elections would not be available to U.S. Holders. See “Taxation — “Taxation—United States Federal Income Tax Consequences”.Consequences.”
If our internal controls over financial reporting are not effective, the reliability of our financial statements may be questioned, and our share price may suffer.
We are subject to Section 404 of The Sarbanes-Oxley Act of 2002, or Section 404, and the related rules of the SEC, which generally require our management and independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting. Section 404 requires an annual management assessment of the effectiveness of our internal control over financial reporting. Prior to December 31, 2019, we were an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and we took advantage of certain exemptions from various reporting requirements that were applicable to public companies that are emerging growth companies, including, but not limited to, the auditor attestation requirements of Section 404. We ceased to be an emerging growth company as of December 31, 2019, and we are required to include an opinion from our independent registered public accounting firm on the effectiveness of our internal controls over financial reporting as part of this Annual Report on Form 20-F for the fiscal year ended December 31, 2019.
The continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. As our business continues to grow both domestically and internationally, our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of its testing, our management may identify material weaknesses or significant deficiencies. For example, as described in Amendment No. 2 to our Annual Report on Form 20-F for the year ended December 31, 2018, management concluded that the Company’s internal control over financial reporting and disclosure controls and procedures were not effective at December 31, 2018 due to a material weakness in internal control over financial reporting, resulting in the filing of Enlivex R&D’s historical audited financial statements for the year ended December 31, 2018 in our Annual Report on Form 20-F for the year ended December 31, 2018, filed on April 30, 2019, rather than those of Bioblast. This material weakness has been fully remediated, but any material weaknesses or significant deficiencies identified by our management in the future may not be remedied in a timely manner to meet the deadline imposed by the Sarbanes-Oxley Act. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our independent registered public accounting firm identifies material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our ordinary shares may suffer.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our shares, our share price and trading volume could be negatively impacted.
The trading market for our ordinary shares may be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts, and we cannot provide any assurance that analysts will cover us or, if they do, provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our shares, or provide more favorable relative recommendations about our competitors, our share price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could negatively impact our share price or trading volume.
Because we do not intend to declare cash dividends on our ordinary shares in the foreseeable future, shareholders must rely on appreciation of the value of our ordinary shares for any return on their investment.
We have never declared or paid cash dividends on our ordinary shares. We currently anticipate that we will retain future earnings, if any, for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Moreover, the Israeli Companies Law, 1999 as amended (the “Companies Law”), imposes certain restrictions on our ability to declare and pay dividends.Payment of dividends may also be subject to Israeli withholding taxes. See Item 10.E “Taxation—Israeli Taxation Considerations” for more information. As a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future. Consequently, in the foreseeable future, you will likely only experience a gain from your investment in our ordinary shares if the price of our ordinary shares increases beyond the price in which you originally acquired the ordinary shares.
We are a “foreign private issuer” under the Exchange Act, and our disclosure and reporting requirements are different than those of a U.S. domestic reporting company.
We are a “foreign private issuer” under the Exchange Act and the rules of the SEC promulgated thereunder. As a result, we are subject to the reporting requirements under the Exchange Act applicable to foreign private issuers, meaning that, among other things, we are required to file our Annual Report on Form 20-F with the SEC within four months of our fiscal year end. In addition, we are not subject to quarterly financial reporting, as would be the case for a U.S. domestic reporting company; therefore, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. We are additionally not required to comply with Regulation FD, which addresses certain restrictions on the selective disclosure of material non-public information. Also, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. If we lose our status as a foreign private issuer, we will no longer be exempt from such rules and, among other things, will be required to file periodic reports and financial statements as if it were a company incorporated in the United States.
As a “foreign private issuer”,issuer,” we are permitted to follow certain home country corporate governance practices instead of otherwise applicable SEC and Nasdaq Capital Market requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the listing rules of the Nasdaq Capital Market (the “Nasdaq Listing Rules”) for U.S. issuers. For instance, we follow home country practice in Israel with regard to, among other things, director nomination procedures, quorum requirements, and approval of compensation of officers.officers and distribution of periodic reports. In addition, we follow our home country law instead of the Nasdaq Listing Rules that require that we obtain shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity basedequity-based compensation plans, an issuance that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or greater interest in the company, and certain acquisitions of the stock or assets of another company. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on the Nasdaq Capital Market may provide less protection to you than what is accorded to investors under the Nasdaq Listing Rules applicable to domestic U.S. issuers.
Our ordinary shares are traded on more than one market and this may result in price variations.
Our ordinary shares are traded on the Nasdaq Capital Market and the Tel Aviv Stock Exchange (“TASE”). Trading in our ordinary shares on these markets takes place in different currencies (U.S. dollars on Nasdaq and New Israeli Shekels (“NIS”) on the TASE) and at different times, as a result of different time zones, trading days and public holidays in the United States and Israel. The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on the TASE could contribute to a decrease in the trading price of our ordinary shares on Nasdaq, and a decrease in the price of our ordinary shares on Nasdaq could likewise contribute to a decrease in the trading price of our ordinary shares on the TASE.
Risks Related to Israeli Law and Our Operations in Israel
Our headquarters and other significant operations are located in Israel and, therefore, our results may be adversely affected by political, economic and military instability in Israel.
Our executive offices are located in NesNess Ziona, Israel. In addition, the majority of our officers and several of our directors are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations. Furthermore, in recent years, Israel is currentlyhas been engaged in ansporadic armed conflictconflicts with Hamas, a militiaan Islamist terrorist group and political party whothat controls the Gaza Strip, and during the summer of 2006, Israel was engaged in an armed conflict with Hezbollah, a Lebanesean Islamist Shiite militiaterrorist group that controls large portions of southern Lebanon, and political party.with Iranian-backed military forces in Syria. In July 2014, there was an escalation in violence amongaddition, Iran has threatened to attack Israel, Hamas, the Palestinian Authoritymay be developing nuclear weapons and other groups, as well as extensivehas targeted cyber attacks against Israeli entities. Some of these hostilities along Israel’s border with the Gaza Strip, which resulted inwere accompanied by missiles being fired from the Gaza Strip into Southern Israel, as well as areas more centrally located near Tel Aviv and areas surrounding Jerusalem. This conflict, as well as a previous round of escalation which took place in November 2012, involved missile strikes against civilian targets in various parts of Israel, including areas in which our employees and some of our consultants are located. The continuation of such strikes may negatively affectaffected business conditions in Israel. Since February 2011, Egypt has experienced political turbulence and an increase in terrorist activity in the Sinai Peninsula following the resignation of Hosni Mubarak as president. This included protests throughout Egypt, and the appointment of a military regime in his stead, followed by the elections to parliament which brought groups affiliated with the Muslim Brotherhood (which had been previously outlawed by Egypt), and the subsequent overthrow of this elected government by a military regime instead. Such political turbulence and violence may damage peaceful and diplomatic relations between Israel and Egypt, and could affect the region as a whole. Similar civil unrest and political turbulence has occurred in other countries in the region, including Syria which shares a common border with Israel, and is affecting the political stability of those countries. Since April 2011, internal conflict in Syria has escalated, and evidence indicates that chemical weapons have been used in the region. Intervention may be contemplated by outside parties in order to prevent further chemical weapon use. This instability and any intervention may lead to deterioration of the political and economic relationships that exist between the State of Israel and some of these countries, and may have the potential for additional conflicts in the region. In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza, Hezbollah in Lebanon, and various rebel militia groups in Syria. These situations may potentially escalate in the future to more violent events which may affect Israel and us. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions and could harm our results of operations and could make it more difficult for us to raise capital. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, and airline companies may cancel or delay scheduled air travel to Israel, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions generally and could harm our results of operations.
Further, in the past, the State of Israel and Israeli companies have been subjects of economic boycotts. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
For additional information on risks relating to restrictions in A campaign of boycotts, divestment, and sanctions has been undertaken against Israel, in connection with Coronavirus, See –– “The recent coronavirus outbreak maywhich could also adversely affect our business.
Furthermore, the Israeli government is currently pursuing extensive changes to Israel’s judicial system, which has sparked widespread protests across Israel and, if adopted, may negatively impact the political and business environment in Israel. Actual or perceived instability with respect to the current public dispute over changes to the Israeli legal systems or the impact thereof, may individually or in the aggregate adversely affect the Israeli economy and our ability to do business, financial condition, results of operations, growth prospects, and financial condition”.share price as well as our ability to raise additional funds.
Our operations may be disrupted as a result of the obligation of Israeli citizens to perform military service.
Many Israeli citizens are obligated to perform up to one month, and in some cases more, of annual military reserve duty until they reach the age of 40 (or older, for reservists who are officers or who have certain occupations) and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups, which may include the call-up of our employees, including members of our senior management, or the employees or management of our Israeli business partners. Such disruption could materially adversely affect our business, financial condition and results of operations.
Exchange rate fluctuations between the U.S. dollar, Euro and the New Israeli Shekel currenciesNIS may negatively affect our earnings.
Our functional currency is the NIS. We incur expenses in U.S. dollars, Euros and NIS. As a result, we are exposed to the risks that the Euro anddollar because the U.S. dollar is the currency of the primary economic environment in which we operate and expect to continue to operate for the foreseeable future. The functional currency of Enlivex R&D had been the NIS; however, we reassessed Enlivex R&D’s functional currency and determined that, as of September 30, 2021, the U.S. dollar became the functional currency of Enlivex R&D rather than the NIS. Monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the applicable balance sheet date. Because we incur expenses denominated in currencies other than the U.S. dollar, predominantly the NIS and Euro, fluctuations in exchange rates among these currencies may appreciate relative tonegatively impact our earnings, particularly if the NIS or if either the Euro andappreciates against the U.S. dollar devalue relative to the NIS, that the inflation rate in the EU and in the United States may exceed such rate of devaluation of the Euro and the U.S. dollar, or that the timing of such devaluation may lag behind inflation in the EU and in the United States. In any such event, the NIS cost of our operations in the EU and in the United States would increase and our NIS-denominated results of operations would be adversely affected.dollar. The average exchange rate for the year ended December 31, 20192022 was $1.00 = Euro 0.890.94 and $1.00 = NIS 3.456. We cannot predict any future trends in the rate of inflation in the EU and in the United States or the rate of devaluation, if any, of either the Euro or the U.S. dollar against the NIS.3.519.
We received Israeli government grants for certain of our research and development activities. The terms of those grants may require us, in addition to payment of royalties, to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. We may be required to pay penalties in addition to repayment of the grants.
Our research and development efforts, during the period between 2007 and 2016 were financed in part through royalty-bearing grants, in anthe aggregate amount of approximately $3.2 million, from the Israel Innovation Authority (the “IIA”), pursuant to the Encouragement of Research, Development and Technological Innovation in the Industry Law 5744-1984 (formerly known as the Encouragement of Industrial Research and Development Law, 5744-1984), (the “Innovation Law”). During 20192019-2022, our research and development efforts were also financed in part through royalty-bearing grants from the IIA, in anthe aggregate amount of approximately $1.11$4.56 million. With respectAs of December 31, 2022, we have not paid any royalties to such grants,the IIA and had a contingent obligation to the IIA, including interest of $9.2 million.
Under the Innovation Law as currently in effect, we are committed to pay royalties at a rate of 3% to 5% on our sales proceeds from any product that is a treatment, deviceproducts or medical kit designed for therapeutic treatments using apoptotic cellsservices based on know-how funded by the IIA research and development grants we have received (which rates may be increased under certain circumstances) up to the total amount of grants received (which may be increased under certain circumstances), linked to the U.S. dollar plus interest equal to LIBOR as it would apply to U.S. dollar deposits.12-month LIBOR.
Regardless of any royalty payment, we are further required to comply with the requirements of the Israeli Encouragement of Research, Development and Technological Innovation in the Industry Law 1984 (formerly known as the Israeli Encouragement of Industrial Research and Development Law, 1984, and related regulations, (the “Research Law”), with respect to those past grants. When a company develops know-how, technology or products using IIA grants, or is otherwise IIA-supported, theThe terms of such IIA grants and the ResearchInnovation Law restrict the transfer of such IIA-supported know-how and rights related thereto, technology and products to a third party or the transfer of manufacturing or manufacturing rights of the same outside of Israel (except for the transfer of less than 10% of the manufacturing capacity in the aggregate which requires only a notice), without the prior IIA approval. Therefore, if deemed IIA-supported, the discretionary approval of an IIA committee would be required for any transfer to third parties, which could, if we receive such approvals, result in the payment of increased royalties (both increased royalty rates and increased royalties ceilings), in cases of transfer of manufacturing outside of Israel (up to three times the amount of the IIA grants received, depending on the manufacturing volume performed outside Israel (plus accrued interest)) and/or payment of additional amounts to the IIA in cases of transfer of IIA-supported know-how outside of Israel. Furthermore, the IIA may impose certain conditions on any arrangement under which we may transfer technology or development outside of Israel (including for the purpose of manufacturing). Currently,(calculated according to a formula under the ResearchInnovation Law, there is no mechanism for the approval of licensing transactions of IIA-supported technologies to third parties outside of Israel, other than the Licensing Rules described below; however, licensing IIA-supported technologieswhich may under certain circumstances be considered a transfer of know-how and therefore require IIA approval, as described above. On May 7, 2017, the IIA published the Rules for Granting Authorization for Use of Know-How Outside of Israel (the “Licensing Rules”). The Licensing Rules enable the approval of out-licensing arrangements and other arrangements for granting of an authorization to an entity outside of Israel to use know-how developed under research and development programs funded by the IIA and any derivatives thereof. Subject to payment of a “License Fee” to the IIA, at a rate that will be determined by the IIA in accordance with the Licensing Rules, the IIA may now approve arrangements for the license of know-how outside of Israel. This allows companies that have received IIA support to commercialize know-how in a manner which was not previously available.
The transfer of IIA-supported know-how, technology or products outside of Israel may involve the payment of additional amounts depending upon the value of the transferred know-how, technology or products, the amount of IIA support, the time of completion of the development of IIA-supported know-how, technology or products, and other factors up to a maximum of six times the amount of the grants received (less paid royalties, if any, and depreciation, but no less than the total grants received), plus LIBOR and minus any royalties paid. accrued interest).
These restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of IIA-supported know-how, technology or products (such as a merger or similar transaction) may be reduced by any amounts that we may be required to pay to the IIA.
Our obligations and limitations pursuant to the ResearchInnovation Law are not limited in time and may not be terminated by us at will and the obligations pursuant to the Research Law remain in force even after we have paid all required royalties, which may require us to obtain IIA approval prior to consummating certain transactions, including licensing IIA-supported know-how, technology and products outside of Israel. Although as of the date of this Annual Report on Form 20-F we have not been required to pay any royalties or additional payments with respect to any IIA grant, there can be no assurance that we will not be required to do so in the future.royalties. Such restrictions and payments could materially restrict or limit our ability to perform or outsource manufacturing outside of Israel or otherwise transfer or license our IIA-supported know-how, technology or products, which could materially affect our business, results of operations and financial position.
Provisions of Israeli law and our Amended and Restated Articles of Association may delay, prevent or otherwise impede a merger with, or an acquisition of, our company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Provisions of Israeli law and our amended and restated articles of association could have the effect of delaying or preventing a change in control and may make it more difficult for a third-party to acquire us or our shareholders to elect different individuals to our board of directors, even if doing so would be considered to be beneficial by some of our shareholders, and may limit the price that investors may be willing to pay in the future for our ordinary shares. Among other things, Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date on which a merger proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days have passed from the date on which the shareholders of both merging companies have approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless, following consummation of the tender offer, the acquirer would hold at least 98% of the Company’s outstanding shares. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition, unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek such appraisal rights.
Furthermore, under the ResearchInnovation Law, a recipient of IIA grants, such as us, must report to the IIA regarding any change of control or any change in the holding of its means of control of our Company which transforms any non-Israeli citizen or resident into an “interested party”,party,” as defined in the Israeli Securities Law, 1968, and in the latter event, the non-Israeli citizen or resident shallis required to execute an undertaking in favor of IIA, in a form prescribed by the IIA.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. See “Taxation — Item 10.E. “Taxation—Israeli Taxation Considerations” for additional information.
Our Amended and Restated Articles of Association also contain provisions that could delay or prevent changes in control or changes in our management without the consent of our Board. These provisions will include the following:
| ● | no cumulative voting in the election of directors, which limits the ability of minority shareholders to elect director candidates; and |
| ● | the |
It may be difficult to enforce a judgment of a United States court against us, our officers and directors in Israel or the United States, to assert United States securities laws claims in Israel or to serve process on our officers directors and these experts.directors.
We were and continue to beare organized in Israel. Substantially all of our executive officers and directors reside outside of the United States, and all of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not necessarily be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Your rights, liabilities and responsibilities as a shareholder will be governed by Israeli law and will differ in some material respects from those under U.S. law.
Because we are an Israeli company, the rights and responsibilities of our shareholders are governed by our Amended and Restated Articles of Association and Israeli law, including the Companies Law. These rights, liabilities and responsibilities differ in some material respects from the rights, liabilities and responsibilities of shareholders in a U.S. corporation. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders and to refrain from abusing his, her or its power in the company, including, among other things, in voting at the general meeting of shareholders on certain matters. Israeli law provides that these duties are applicable to shareholder votes on, among other things, amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and interested party transactions requiring shareholder approval.approval under Israeli law. In addition, a controlling shareholder of an Israeli company or a shareholder who knowsis aware that it possesses the power to determine the outcome of a shareholders’ vote or to appoint or prevent the appointment of a director or executive officer in the company or has other powers towards the company, has a duty of fairness towards the company. However, Israeli law does not define the substance of this duty of fairness. There is limited case law available to assist us in understanding the implications of these provisions that govern shareholders’ actions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. corporations.
ITEM 4. INFORMATION ON THE COMPANY
4.A. History and developmentDevelopment
We were originally incorporated on January 22, 2012 under the laws of the State of Israel as Bioblast Pharma Ltd. Upon consummation of the Merger, we changed our name to Enlivex Therapeutics Ltd. Our primary operating subsidiary, Enlivex Therapeutics R&D Ltd. was originally incorporated in September 2005 under the laws of the State of Israel as an Israeli privately held company under the name Tolarex Ltd. In February 2010, Enlivex R&D changed its to Enlivex Therapeutics Ltd., and, upon consummation of the Merger, to Enlivex Therapeutics R&D Ltd. In June 2021 Enlivex Therapeutics RDO Ltd. was established in Israel as a wholly owned subsidiary. Our principal executive offices are located at 14 Einstein Street, NesNess Ziona, Israel 7403618 and our telephone number is: +972 26208072. Our wholly owned U.S. subsidiary, Bio Blast Pharma,Enlivex Therapeutics Inc., incorporated in Delaware, has been appointed our agent in the United States, and its registered address is 1811 Silverside Road, Wilmington, Delaware 19810. Bio Blast Pharma, Inc. existed as a subsidiary of Bioblast prior to the Merger. Our website address is https://www.enlivex.com/.www.enlivex.com. The information contained on, or that can be accessed through, our website is not part of this Annual Report.Report on Form 20-F. We have included our website address herein solely as an inactive textual reference. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of this website is http://www.sec.gov. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
On March 26, 2019, we consummated the Merger. See “Introduction”.
Prior to December 31, 2019, we were an “emerging growth company” for a period of five years following the completion of our initial public offering in 2014.
Our capital expenditures for the three years ended 2019, 2018December 31, 2022, 2021 and 20172020 were insignificant. See “Operating and Financial Review and Prospects -LiquidityProspects—Liquidity and Capital Resources”.Resources.”
4.B. Business overviewOverview
The Company is a clinical stageclinical-stage macrophage reprogramming immunotherapy company, developing an allogeneic drug pipelineAllocetraTM, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing. Immune system rebalancing is critical for the treatmentand resolution of life-threatening immune and inflammatory conditions, which involveconditions. These non-homeostatic macrophages contribute significantly to the hyper-expressionseverity of cytokines (“Cytokine Release Syndrome”) and for which there are no U.S. Food and Drug Administration (“FDA”)-approved treatments, as well as treating solid tumors by modulating such tumors’ microenvironment. The Company’s innovative immunotherapy candidates,the respective diseases. By restoring macrophage homeostasis, Allocetra™ and Allocetra™ from matched-donor cells, arehas the potential to provide a novel immunotherapy candidates based on a uniqueimmunotherapeutic mechanism of action that targetsfor life-threatening clinical indications that are defined as “unmet medical needs.” Allocetra™ has been developedneeds”, as a stand-alone therapy or in combination with leading therapeutic agents.
Macrophages are tissue-resident or infiltrating immune cells critical for innate immunity, normal tissue development, and repair of damaged tissue. Macrophages’ function is a result of their original designation, their local micro-environment, and the type of metabolites, substances or pathogens to treat acute organ failure associated with sepsis.which they are exposed. Reprogrammed out of their homeostatic state, macrophages contribute to the pathophysiology of multiple diseases, including cancer, sepsis, and various inflammatory disorders.
We believe the Company’s primary innovative immunotherapy, Allocetra™TM, represents a paradigm shift in macrophage reprogramming, moving from matched-donor cells has been developedtargeting a specific subset of macrophages or a specific pathway effecting macrophages activity, to prevent or treat complications associated with bone marrow transplants (“BMT”) and/or hematopoietic stem cell transplants (“HSCTa fundamental view of macrophage homeostasis. Restoring macrophage homeostasis may induce the immune system to rebalance itself to normal levels of operation, thereby promoting disease resolution.
The Company is currently focused on two main clinical verticals: sepsis and solid tumors (the “Indications”). BecauseThe Company believes that negatively-reprogrammed macrophages may be key contributors to disease severity across these two Indications, and thus effective reprogramming of these negative-reprogrammed macrophages into their respective homeostatic states may facilitate disease resolution to these Indications, some of which are considered “unmet medical needs.” All planned and expected timelines for execution of the candidatesclinical trials in the Indications are basedsubject to certain risks and uncertainties. For further discussion of risks and uncertainties related to our clinical trial in the Indications please see Item 3.D. “Risk Factors” below.
The Company had previously included treatment of COVID-19 as a clinical vertical and conducted certain trials in respect of the treatment of COVID-19; however, the Company has determined to currently focus its resources on the same unique mechanismIndications. See “—COVID-19 De-Prioritization” below.
Macrophage Reprogramming
When macrophages are negatively reprogrammed by certain diseases, they may become highly aggressive or highly passive, depending on the disease and the environment in which they reside. In their aggressive modality, macrophages can cause a systemic inflammatory response in which cytokine release composition and amplitude spirals out of action and are otherwise substantially identical, we refer to both as Allocetra™, unless indicated otherwise.control.
Cytokines are a broad and loose category of small proteins (~5–20 kDa) produced by a broad range of cells, including immune cells, and are especially important in the immune system as they promote, modulate and balance immune responses. They are released by cells and affect the behavior of other cells, and include chemokines, interferons, interleukins, lymphokines, tumor necrosis factors and others, but generally not hormones or growth factors. Cytokines are important in health and disease, specifically in host responses to infection, immune responses, inflammation, trauma, sepsis, cancer and other conditions. Cytokine Release Syndrome is a systemic inflammatory response in which cytokine release composition and amplitude spirals out of control. It is considered difficult to treat with traditional small molecules or biologics because the condition involves dozens of cytokines that induce multiple biological paths of hyper immune activity. Such hyper immune activity may result in an attack of immune killer cells (e.g., T-Cells, B-Cells and Natural Killer Cells) on healthy organs of the patient, including the heart, brain, lungs, liver, kidney and others, which may lead to organ damage, multiple organ failure and mortality.
In their passive mode, macrophages, who in their homeostatic state orchestrate proper immune reaction against certain diseases, become reprogrammed into a passive state, do not orchestrate immune response targeted at disease resolution, and in some diseases interfere with the attempts to treat patients with therapeutic agents.
The Company believes that the only approach to handling such a multi-factorial complex life-threatening situation in diseases in which one key aspect of disease severity is the negative reprogramming of macrophages is via an integrated cell-based immunotherapy that inducespositively reprograms macrophages back into their respective homeostatic state, thereby inducing the immune system to rebalance itself to normal levels of operation utilizing a mechanism of action used regularly by themacrophage-modulated proper immune system and developed through evolution.responses to disease challenges. The Company’s unique therapeutic approach is based on inducing immunotolerance and the specific normal rebalancing of the immune systemmacrophage homeostasis by infusing early and stable apoptotic cells (dying cells)AllocetraTM into the patient. Once infused, such apoptotic the AllocetraTM cells interact with macrophages and dendritic cells via well-defined mechanisms causing rebalancinga reprogramming of an over-agitated immune response.these negatively reprogrammed macrophages back to their homeostatic state. Using this inherent immune pathway, the Company believes that it can use Allocetra™ to shape a patient’s innate immune response to a disease, leadingdisease.
Frozen Formulation of Allocetra™
We completed development of frozen formulation Allocetra™, in the first quarter of 2022, which was twelve months ahead of schedule. While the liquid formulation of Allocetra™ has a shelf life of 96 hours, the frozen formulation is expected to have a decrease in unwanted immune response.
There are many clinical conditions in which a patient hasshelf life spanning multiple years. We expect that this will substantially improve the product candidate’s scalability and shipping logistics, lower its production costs, and increase profitability upon potential commercialization. Due to develop Cytokine Release Syndrome. Those clinical conditions include complications associated with sepsis, HSCT and several autoimmune and inflammatory conditions, such as Crohn’s disease, rheumatoid arthritis, gout and multiple sclerosis.
Immune System Triggering and Relaxation
The immune system constantly handles multiple challenges of bacterial, viral, fungal and other infections via a sophisticated elevation of immune activity utilizing enhanced cytokine releases from macrophages and dendritic cells, resulting in recruitment of antibodies and immune cells (e.g., T-Cells, B-Cells and Natural Killer Cells). Once the threat has been eliminated, the immune system rebalances itself into a normal state. Such rebalancing occurs naturally through antigen presenting cells, macrophages and dendritic cells that engulf and clear infected cells that have been instigated into apoptosis and cells from the immune system that have gone through programmed cell death, causing a decrease to normal levels of cytokines and immune activity.
Apoptosis is a natural and critical process in tissue and organ maintenance that occurs when a cell is damaged beyond repair, infected with a virus or undergoing other stressful conditions. Apoptosis involves a series of biochemical events leading to changes in cell morphology and, ultimately, cell death. Immediate removalearly completion of the dying cell is performed by antigen presenting cells, macrophages and dendritic cells. The primary functiondevelopment of dendritic cells is phagocytosis, orfrozen formulation Allocetra™, we currently utilize it in our ongoing Phase II sepsis trial going forward], rather than introducing it only at the capturing and transportation of antigens to draining lymphoid tissues. Immature dendritic cells are capable of large-scale phagocytosis of apoptotic cells.
As many as 3x108 cells undergo apoptosis every hour in the human body. One of the primary “eat me” signals expressed by apoptotic cells is phosphatidylserine (PtdSer). Apoptotic cells themselves serve as major contributors to the “non-inflammatory” nature of the engulfment process, some by secreting thrombospondin-1 (TSP-1) or adenosine monophosphate and possibly other immune modulating “calm-down” signals that interact with antigen presenting cells, macrophages and dendritic cells. Apoptotic cells also produce “find me” and “tolerate me” signals to attract and immune-modulate antigen presenting cells, macrophages and dendritic cells that express specific receptors for some of these signals (Trahtemberg and Mevorach; 2017).
Injectionstart of a high volumePhase III study, as previously planned. We initiated the utilization of densely concentrated early apoptotic cells activates dendritic cells, causing themfrozen formulation Allocetra™ in our ongoing solid tumor Phase I/II trials, and plan to migrate tosolely use the lymphoid tissues, such as the spleen, where they interact with T-cells and B-cells, which are lymphocytes involvedfrozen formulation Allocetra™ in the regulation of the immune system, remove the apoptotic cells and suppress inflammation. The foregoing process induces immunotolerance, as opposed to general immunosuppression, which would otherwise make the patient more susceptible to infection and other immunological challenges.all subsequent clinical studies.
The Company’s unique therapeutic approach is based on inducing immunotolerance and the specific normal rebalancing of the immune system by infusing early and stable apoptotic cells (dying cells) into the patient. Once infused, such apoptotic cells interact with macrophages and dendritic cells via well-defined mechanisms causing rebalancing of an over-agitated immune response.Sepsis
Using this inherent immune pathway, the Company believes that it can use Allocetra™ to shape a patient’s innate immune response to a disease, leading to a decrease in unwanted immune response. During the apoptotic cell removal process, several therapeutic responses are induced, such as inflammation suppression, modulation of macrophage-directed deletion of invading pathogens and regulation of immune responses. These responses are the target of Allocetra™. The Company believes that Allocetra™ can specifically target the immune response without simultaneously diminishing the general immune capabilities of the patient or compromising the patient’s ability to respond to immunological challenges.
The Company’s current clinical development programs focus on preventing or treating complications associated with sepsis, HSCT and solid tumors. The Company’s leading product candidate, Allocetra™, has been developed for treating organ dysfunction and failure in sepsis patients. and potentially for prevention of in allogeneic HSCT patients. Allocetra™ from matched-donor cells has been developed to prevent or treat complications associated with BMT and/or HSCT. Additionally, the Company is currently examining the potential for clinical studies to evaluate the efficacy of existing immunotherapy treatments in combination with Allocetra™ for treatment of solid tumors.
Sepsis
The Company is developing Allocetra™ as an adjunctive immunomodulating cell therapy for avoiding organ failure caused by sepsis. The drug would be administered intravenously to the patient following the diagnosis of sepsis in addition to standard of care treatment.
Sepsis is a highly heterogeneous syndrome that is caused by an unbalanced immune host response to an infection. Sepsis was not clinically defined until the early 1990s when a group of key opinion leaders released the first consensus definition of sepsis. Sepsis has been defined as a systemic inflammatory response syndrome (“SIRS”) caused by an infection; and increasing severities have been designated as ’severe‘severe sepsis’ (referring to sepsis and organ dysfunction) and ’septic‘septic shock’ (referring to sepsis and refractory hypotension). In the most recent ’Sepsis-3’‘Sepsis-3’ consensus definition, sepsis is defined as a life-threatening organ dysfunction that is caused by a dysregulated host response to infection, and the term “severe sepsis” has been removed. Of note, although infection is the triggering event in this definition of sepsis, the aberrant immune response often remains after successful treatment of the infection. Sepsis imposes a substantial global burden in terms of morbidity and mortality. Nearly all patients with severe sepsis require treatment in an intensive care unit. Sepsis, which has been identified by the World Health Organization as a global health priority, has no proven pharmacologic treatment other than appropriate antibiotic agents, fluids, and vasopressors. Sepsis affects approximately 1.7 million adults in the United States each year and potentially contributes to more than 250,000 deaths. Various studies estimate that sepsis is present in 30% to 50% of hospitalizations that culminate in death (Rhee et al; 2019) Previous attempts to find a therapy for sepsis failed partially due to the parallel and complex course of biological activities that occur within a sepsis patient. For many years, a disproportionate inflammatory response to invasive infection was considered to be central to the pathogenesis of sepsis, but it is now clear that the host response is disturbed in a much more complex way, involving both sustained excessive inflammation and immune suppression, and a failure to return to normal homeostasis.
This outcome may lead to organ damage, multiple organ failure and mortality. If the immune system could be rebalanced, we believe that many of the outcomes, specifically organ damage and failure, could be prevented and significantly increase a patient’s chance of survival with reduced morbidity.
Preclinical Data, Sepsis
In its preclinical study, the Company utilized a murine cecal ligation puncture (“CLP”) sepsis model. The CLP model has been proposed to more closely replicate the nature and course of clinical sepsis, as compared to other models.
We evaluated the effect of Allocetra™ in mice, given 4 hours after the end of a CLP procedure, in combination with Ertapenem© a highly effective antibiotic commonly used for the treatment of severe or high-risk bacterial infections. Mice were monitored for clinical signs and determination of the murine sepsis score. The endpoint was defined as survival (either death or sacrifice when a total clinical score of 15 or maximum score in one of the categories was reached).
As shown in Figure 2A,1A, antibiotic treatment showed a non-significant tendency to ameliorate mortality of the mice (Ertapenem + vehicle, n=15) compared to the control group (CLP only, n=16). Treating CLP mice with the combination of antibiotics and Allocetra™ significantly delayed and prevented mortality in 60% of the animals (Ertapenem + Allocetra™, n=20, p<0.001). In comparison to the control group, the Company’s study reflected an approximately 10-fold improvement in the survival rate (p<0.001 in a log-rank analysis). As shown in Figure 2B,1B, Allocetra™ treated mice had significantly lower murine sepsis clinical scores indicating superior clinical condition. Finally, the Company correlated the clinical score to serum cytokines/chemokines in vivo measurements and as shown in Figure 2C.1C. Allocetra™ downregulated pro-inflammatory cytokines/chemokines. In the preclinical study, Allocetra™ delayed and prevented mortality in animal models with sepsis by rebalancing the immune system.
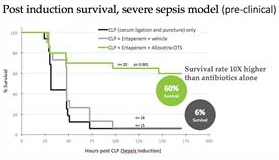

Figure 2A1A

Figure 1B

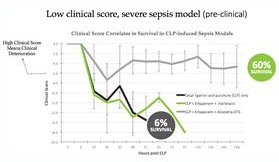
Figure 2B1C

Figure 2C
Allocetra™for the treatmentTreatment of Organ Dysfunction and Failure Associated with Sepsis
In late 2019, the Company completed its Phase Ib clinical trial of Allocetra™ (historically referred to as Allocetra OTS) in patients with severe sepsis. The Company has designed Allocetra™to eliminate the need to find matched donors as the source of the therapeutic cells. Following regulatory approval, if obtained, Allocetra™would allow the Company to manufacture therapeutic product inventory that could be used for additional clinical indications that require swift infusion of the product to patients. The first such indication Enlivex is targeting is prevention of cytokine storms and organ dysfunction associated with sepsis.
The aim of the Phase Ib clinical trial was to determine the safety and efficacy profile and tolerability of Allocetra™, in subjects admitted to the emergency room with sepsis. Allocetra™ (140x106 cells/kg) was administered in either a single dose to 6 patients at day 1 or in two doses to 4 additional patients at days 1 and 3, to patients admitted to the emergency room with sepsis. Patients were followed for 28 days. The study subjects were also compared to historical controls hospitalized in the ICU, matched by age, gender, Sequential Organ Failure Assessment (SOFA) score, and infection source.
On March 18, 2020, the Company announced the final safety and efficacy data from the Company’s completed Phase Ib. The final analysis compared the clinical data of 10 patients admitted to the intensive care unit with sepsis who were administered Allocetra™upon their admission, with 37 patients who were matched controls (matched by age, gender, Sequential Organ Failure Assessment (SOFA) score, and infection source) who received only the standard of care treatment at the same hospital during 2014-2019 but did not receive Allocetra™. The clinical trial was conducted at Hadassah Medical Center, which is one of the largest and most prestigious hospitals in Israel (“Haddasah”).Israel. The Acute Physiology and Chronic Health Evaluation (APACHEII) score of the Allocetra™-treated group was 12.3,12.9, and the corresponding probability of mortality of at least one patient in that group was predicted at 85% based on the hospital’s ICU staff’s clinical assessment of each patient’s overall condition at admission. However, none (0%) of the Allocetra™-treated patients died during the 28-day study period, as compared to 27% 28-day mortality in the matched controls group. Each of the 10 Allocetra™-treated patients had between 2 to 5 dysfunctional organ systems upon admission to the ICU. All (100%) of the Allocetra™-treated patients had rapid and complete recovery from their septic conditions and of any organ dysfunction that was present upon admission to the ICU. Despite the similarity of organ-failure state (SOFA) at entry between the Allocetra™-treated patients and the matched controls group (average of 3.4 versus 3.47), not a single patient treated with Allocetra™ had any increase in organ-failure state post administration of Allocetra™, while the majority of the patients in the matched controls group had an increase in organ-failure state. The average worsening in organ-failure state of patients in the matched controls group was approximately 100% compared with their ICU hospitalization state vs zero (0%) percent worsening in organ-failure state of Allocetra™-treated patients post administration of Allocetra™ (p< <0.0001). The ICU length-of-stay for all Allocetra™-treated patients was significantly shorter than those patients who received only the standard of care, with an average of 44.7 days compared to 11.11 in the matched controls group, a 64% reduction (p<0.0001). The slowest ICU discharge of a patient treated with Allocetra™ was after 8 days, while approximately 50% of the matched controls group were still at the ICU after 28 days. Allocetra™ was shown to be safe and tolerable, with no serious unexpected severe adverse reactions and no serious adverse events.
Summary of Planned Clinical Trials of Allocetra™for the treatment of Organ Dysfunction and Failure Associated with Sepsis
In light of the encouraging results of the Phase Ib in patients with severe sepsis, the Company plans, subject to regulatory approvals, to initiate a Phase IIb or Phase II/III clinical trial with Allocetra™ for the treatment of organ dysfunction and failure associated with sepsis during 2020. The Company currently intends to enroll up to 100 patients in such a clinical trial. The Company intends, subject to the clinical trial results, to request a conditional marketing authorization from the EMA for this indication.
43
Complications Associated with Bone Marrow Transplants
Allocetra™ from matched-donor cells for preventing or treating complications associated with HSCT is an immunomodulation cell-therapy drug in development that involves injection of early-apoptotic cells that have been retrieved from the blood of either (i)In 2021, Enlivex initiated a donor matched by his or her human leukocyte antigen (i.e., a protein found on white blood cells that is the standard genetic marker for the regulation of the immune system and is used to match donors and recipients in transplantations), (ii) the patient, or (iii) an allogeneic, unmatched donor that has undergone ex-vivo (i.e., prior to infusion) manipulation to stabilize the “early apoptosis” status of the cells for a prolonged period of time. Allocetra™ from matched-donor cells’ specific clinical indications include (i) preventing complications associated with HSCT through an injection prior to and two weeks following the bone marrow transplantation procedure, and (ii) treatment of steroid refractory patients upon occurrence of GvHD post HSC transplantation. Systemic corticosteroids are the standard of care for the initial treatment of grade 2–4 GvHD. However, many patients with acute GvHD (“aGvHD”) do not experience sustained responses to corticosteroids which may lead to multiple organ failure and potential death, and for which 6-month survival rates among steroid-refractory patients are approximately 49% with long-term survival rates of only 5–30%.
Graft Versus Host Disease (GvHD)
Allogeneic HSCT has revolutionized the treatment of hematopoietic malignancies, inherited hematopoietic disorders, aplastic anemia, and other severe diseases (Copelan 2006). The HSCT clinical benefit is in part a result of the graft-versus-leukemia (“GVL”) effect, in which a donor immune response is targeted against recipient malignant cells. Although alloreactive donor T-cells play an important role in GVL by targeting tumor cells for elimination, the serious complication of GvHD develops when alloreactive donor cells attack healthy host tissues. Despite promising advances in HSCT methodology, including prophylactic immunosuppressive therapies, approximately 50% of HSCT recipients develop GvHD. GvHD can present as an acute disease, aGvHD, or a chronic (“cGvHD”) disease. Both aGvHD and cGvHD are inflammatory disorders initiated by the infiltration of alloreactive T cells into target organs, followed by activation of proinflammatory signaling cascades, tissue damage and organ failure. Previously, the distinction between aGvHD and cGvHD was based solely on the time of onset (i.e., during or after 100 days post-transplant). However, important pathophysiological distinctions have since been identified, requiring evaluation of clinical presentation to make an accurate disease diagnosis. The skin is the organ most typically affected at the onset of aGvHD, followed by the gastrointestinal tract and liver. Several organ systems, including the skin and gastrointestinal tract, are also affected in cGvHD, but clinical distinctions can be made to differentiate cGvHD from aGvHD in these organ systems. Additional diagnostic symptoms of cGvHD manifest in the mouth, genitalia, lungs and muscles. The target organ damage observed in aGvHD is primarily characterized by apoptosis, whereas cGvHD is associated with fibrosis and many autoimmune features, indicative of an expanded role for macrophages and B cells compared with aGvHD (Jagasia et al; 2018). The Allocetra™ from matched-donor cells clinical development program is aimed to prevent, and in some cases treat, post transplantation complications such as aGvHD.
The standard of care for treatment of complications associated with HSCT, including GvHD, often involves immune-suppressants, such as corticosteroids. Some patients do not respond to corticosteroids, and lack of any other treatment alternative leave these patients with a bleak survival prognosis. The subset of patients who do respond to corticosteroids faces the risk that the immune system may become so suppressed that the ability of the immune system to fight pathogens severely deteriorates and becomes unable to fight severe infections, which are abundant in a typical hospital setting.
The Company conducted a Phase IIa clinical study, which evaluated the safety, tolerability and preliminary efficacy profile of Allocetra™ from matched-donor cells for the prevention of complications post-HSCT. The study demonstrated that Allocetra™ from matched-donor cells has the potential to induce immune-tolerance and immune system rebalancing to normal activity levels in a patient post-HSCT, thus preventing Cytokine Release Syndrome and complications associated with HSCT, without undermining the ability of the transplanted bone marrow to attack the remainder of the cancer disease in the patient. Specifically, patients who received effective doses of Allocetra™ from matched-donor cells experienced no Cytokine Release Syndrome and no GvHD grade II-IV and were discharged from the hospital after an average duration of 21 days of hospitalization compared to an historical data expected duration of 41-45 days. In trials to date, Allocetra™ from matched-donor cells has been well-tolerated, and there has been no observable, significant adverse side effects.
Summary of Allocetra™ from matched-donor cells Clinical Trials
Phase IIa Trial: Allocetra™ from matched-donor cells for the prevention of aGvHD
After completing all pre-clinical safety and efficacy testing in animals, the Company began aplacebo-controlled, randomized, dose-finding, multi-center, Phase IIa clinicalII trial of Allocetra™ from matched-donor cells to evaluate the safety, tolerability and preliminary efficacy profile of the drug for the prevention of aGvHD in allogeneic HSCT patients at Hadassah Medical Center, Rambam and Sheba Medical Centers in Israel. The study protocol included 13 patients who were intravenously infused with ranging doses of Allocetra™ from matched-donor cells 24 hours prior to an allogeneic HSCT procedure and then monitored for 180 days following transplantation. The Company published a summary of the results from such trial in the peer-reviewed journal of the American Society for Blood and Marrow Transplantation, the Biology of Blood and Marrow Transplantation, titled “Single Infusion of Donor Mononuclear Early Apoptotic Cells as Prophylaxis for Graft-versus-Host Disease in Myeloablative HLA-Match Allogeneic Bone Marrow Transplantation: A Phase I/IIa Clinical Trial.”
The primary objective of the Phase IIa clinical trial in Israel was to determine the safety profile and tolerability, or dose limiting toxicity, of ascending doses of Allocetra™ from matched-donor cells within 180 days post-transplantation in subjects undergoing allogeneic HSCT from matched-related donors (i.e., donors’ whose tissues were immunologically compatible with the recipient). The secondary objectives of the trial were to determine (i) the success rate of allogeneic HSCT and the time to successful engraftment, (ii) the rates and severity of aGvHD following Allocetra™ from matched-donor cells infusion and (iii) the immunological function of the patient following the HSCT procedure and Allocetra™ from matched-donor cells infusion.
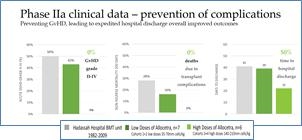
The Company’s clinical data from its Phase IIa trial indicate that Allocetra™ from matched-donor cells was well-tolerated at all doses administered for up to six months post-transplantation, which was the observed duration of the trial. The Company did not observe or receive reports of any definite or probable adverse effects related to Allocetra™ from matched-donor cells. Although historical data shows that approximately 50% of patients with aGvHD are expected to advance to the most severe grades of GvHD (i.e., Grades II-IV), none of the six patients treated with the two highest doses of Allocetra™ from matched-donor cells (defined as the effective doses) in the study advanced to such grades. In fact, the number of overall adverse effects decreased with Allocetra™ from matched-donor cells dose escalation, Grade I aGvHD was 50% in the same cohorts, and mild chronic GvHD was present in a number of patients. This finding might suggest that Allocetra™ from matched-donor cells treatment, as a physiological modality, reduces high grade GvHD rather than abolishing it, supporting a favorable GvL response. In this trial, Allocetra™ from matched-donor cells injections were not associated with prolongation of time to engraftment, chimerism delay (i.e., an increase in the time it takes for donor immune cells to become immunologically effective in the patient’s body), increased mortality rate or serious infections when compared with similar patients described in scientific literature. Patients who received effective doses of Allocetra™ from matched-donor cells experienced no Cytokine Release Syndrome symptoms and were discharged from the hospital after an average duration of 21 days of hospitalization compared to an expected duration of 41 days as per historical controls, the charts above summarize certain of these findings.
The FDA granted the Company’s orphan drug designation request for the active moiety, or the part of the drug responsible for the physiological or pharmacological action of the drug substance, for the prevention of aGvHD. Orphan designation qualifies the sponsor of the drug or biologic for various development incentives, including tax credits for qualified clinical testing and 7-year marketing exclusivity post commercialization. In addition, Allocetra™ from matched-donor cells received from the European Medicinal Authority (the “EMA”) an (i) Advanced Therapy Medicinal Product (“ATMP”) certification for the prevention of aGvHD, and (ii) Orphan medicinal product designation for the indication: Prevention of GvHD. This designation may provide Allocetra™ from matched-donor cells with a 10-year market exclusivity incentive upon commercialization.
Clinical trials of Allocetra™ for the prevention of aGvHD in Allogeneic HSCT Patients
The Company’s timeline for the continued development of Allocetra™ for the prevention of GvHD in allogeneic HSCT patients has been modified to reflect the Company’s re-prioritization of product development in light of the encouraging results of the Phase Ib ofevaluating liquid Allocetra™ in patients with severepneumonia-associated sepsis. The Board decided to utilizeDuring 2022, we amended the Company’s cash reserves in order to generate additional clinical data, first and foremost, in a randomized, controlled sepsisprotocol of this clinical trial to treat newly recruited patients with frozen formulation Allocetra™ and expand the study population to considerinclude patients whose septic condition stems from biliary, urinary tract, or peritoneal infections. We are planning to file another protocol amendment and submit the initiationproposed changes to regulators in the second quarter of smaller clinical trials2023. The amendment will include an increase in high-value indicationsthe patients’ SOFA score range, effectively allowing recruitment of patients with an assumed pathophysiology markedly similarhigher levels of sepsis severity, and a change to thattwo cohorts (treatment and placebo) in lieu of sepsis. Asthe current four-cohort structure. The Company does not expect a result, clinical trials of Allocetra™material timeline delay for the prevention of GvHDtop-line data readouts, which are expected in allogeneic HSCT patients have currently been rescheduled to 2022, pending the expected results of clinical trials in sepsis and potentially other related indications during 2020-2022.Q1 2024.
Solid Tumors, Macrophage Programming and Solid Cancer Treatments
The Company is also developing Allocetra™ as a next-generation solid cancer immunotherapy. While first-generation immuno-oncology therapies, such as checkpoint inhibitors, are a significant therapeutic advancement, most patients do not achieve durable clinical benefit. Companies such as Novartis, Juno and Kite have made significant advances in treatment of recurring blood cancers via Chimeric Antigen Receptor T-Cell (“CAR-T”) therapies, immunological treatments that use the body’s own immune system to treat cancerous cells. CAR-T therapies have not proven highly successful against solid tumors. Other approved compounds for solid tumors such as anti- programmedanti-programmed cell death protein 1 (“anti-PD1”) treatments have demonstrated superior efficacy but only in a small subset of the patient population.
Solid tumors are harder to treat primarily due to the complex and interconnected tumor microenvironment. The Company believes that Allocetra™ presents a significant opportunity to engage the body’s immune system, enabling anti-cancer therapies such as CAR-T, T-Cell Receptor Therapy, anti-PD1 and others to effectively treat solid tumors thus improving cure rates for patients with a variety of solid cancers.
The data from the Company’sour extensive preclinical studies showshows that the Allocetra™ cells, which have demonstrated a strongrobust safety profile in sepsis and COVID-19 clinical trials, have reprogrammed macrophage populations in the tumor microenvironment and surrounding tissues, resulting in a previous clinical trial, have not only caused a significantmajor increase in duration of survival compared with stand-alone CAR-T treatment, but also have demonstrated an ability for complete remission for some preclinical subjects.subjects and survival duration.
In the Company’s preclinical study, SCID-Bg mice were injected intra-peritoneally with 2 consecutive doses of 0.25x106human HeLa-CD19-luciferase cells (HeLa cancer cells expressing CD19), on days 1 and 2 of the experiment. Mice also received 10x106 Allocetra™ or vehicle, on day 9;9, 16 and 23; and 10x106 CD19-CAR-T (third generation) cells or mock T cells on day 10. Mice were weighed twice a week and monitored daily for clinical signs and peritoneal fluid accumulations. Pre-scheduled sacrifices were performed to characterize the cell and macrophage sub-population profile. The rest of the mice were kept for survival analysis. The survival endpoint was defined by a score based on severe peritoneal fluid accumulation manifested as an enlarged and tense abdomen, and reduced mobility or increased respiratory effort. These preclinical findings correlated to large accumulation of HeLa cells in the peritoneum. Survival analysis was performed according to the Kaplan-Meier Log rank statistical test. The CompanyAllocetra-OTS co-administered with CAR-T significantly ameliorated survival over CAR-T alone, resulting in complete recovery of 20-40% of the treated mice as presented in Figure 2 and the table below.

Figure 2

Based on these and other data, we have initiated a Phase I/II trial evaluating Allocetra™ in combination with chemotherapy in solid peritoneal tumors in the third quarter of 2022 and a Phase I/II trial evaluating Allocetra™ in combination with an immune checkpoint inhibitor in the fourth quarter of 2022. We received a positive DSMB recommendation and IMOH clearance to continue our Phase I/II clinical trial of Allocetra™ combined with chemotherapy in patients with peritoneal metastases arising from solid cancers. Recruitment of patients has been on-track, and we currently expect to complete enrollment and announce data readouts by Q2 2024.
We also received a positive DSMB recommendation and IMOH clearance to continue our second Phase I/II clinical trial, which is evaluating Allocetra™ as monotherapy and in combination with anti-PD1 checkpoint inhibitors in patients with advanced-stage solid tumors. Recruitment of patients has been on-track, and we currently examiningexpect to complete enrollment in the potentialintravenous-infusion monotherapy and low-dose combination cohorts by the end of Q2 2023. This study also received FDA clearance for collaboratingan IND application at the end of 2022, as well as clearance in February 2023 from the Spanish Agency of Medicines and Medical Devices to open the study in Spain . We recently announced a clinical collaboration with companies developing leading potential immune therapiesBeiGene to evaluate the safety and efficacy of immune therapy treatmentsAllocetra™ in combination with Allocetra™tislelizumab, an anti-PD-1 immune checkpoint inhibitor, for the treatment of patients with advanced-stage solid tumors, a part of the Company’s ongoing Phase I/II clinical trial in patients with advanced-stage solid tumors.
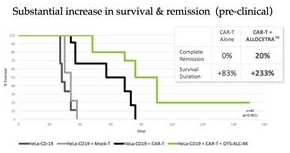
COVID-19, Severe and Critical Patient Population
The Company had previously included treatment of COVID-19 as a clinical vertical and conducted certain trials in respect of the treatment of COVID-19; however, the Company has determined to currently focus its resources on the Indications. See “—COVID-19 De-Prioritization” below.
On February 3, 2021, the Company reported positive top-line results from Phase II clinical trial that evaluated Allocetra™ in severe and critical COVID-19 patients and provided a program update. The top line results of the combined analysis of a previously reported Phase Ib and the Phase II were detailed as follows:
| ● | Phase II + Ib (21 patients treated, 11/21 (52%) with severe illness, 10/21 (48%) with critical illness); |
| ● | 0/21 (0%) mortality on day-28; |
| ● | 19/21 (90.5%) patients recovered and were discharged from the hospital by day-28; |
| ● | Average duration of hospitalization post administration of AllocetraTM for discharged patients was 5.6 days; and |
| ● | 2/21 (9.5%) patients, both of whom had critical illness at the time of AllocetraTM treatment, were hospitalized in the ICU on a respirator on day-28. |
| Clinical Outcome | Hospitalization Discharged patients, post Administration of AllocetraTM | |||||||||||
| Clinical Trial | # Patients enrolled | Disease Severity | Recovered Day 28 | Recovered Day 28 | Discharged Day 28 | Duration (days, avg.) | ||||||
| Phase Ib | 5 | 2 Severe 3 Critical | 5/5 (100%) | 5/5 (100%) | 5/5 (100%) | 6.6 | ||||||
| Phase II | 16 | 9 Severe 7 Critical | 14/16 (87.5%) | 14/16 (87.5%) | 14/16 (87.5%) | 5.3 | ||||||
| Total | 21 | 11 Severe 10 Critical | 19/21 (90.5%) | 19/21 (90.5%) | 19/21 (90.5%) | 5.6 | ||||||
In light of the encouraging results of the Phase Ib and Phase II clinical trials of AllocetraTM in severe and critical COVID-19 patients, the Company initiated during 2021 a randomized, controlled Phase IIb clinical trial of AllocetraTM in severe and critical COVID-19 patients hospitalized in medical centers in Israel and certain European countries.
COVID-19 De-Prioritization:
Due to (a) the emergence of variants that may be associated with less severe disease, (b) a more stringent regulatory environment, and (c) increased volatility of the potential business segment, we de-prioritized Allocetra’s clinical development for treatment of COVID-19 and re-allocated the program’s cash resources to our primary development priorities, sepsis and oncology. Due to slow recruitment, the total number of patients enrolled in our Phase IIb COVID-19 is insufficient to make any meaningful assessments of Allocetra’s efficacy. Patients already enrolled in this trial will continued to be followed for assessments of safety.
Accelerated Regulatory Approval Processes for Life Saving Therapies
The Company anticipates that its therapeutic drugs and their respective indications could qualify under specific accelerated regulatory paths in both the EU and the United States. Specifically for the EU, an accelerated path allowing conditional marketing approval is available for certain therapeutic drugs following a Phase II study. There is no assurance that the Company will qualify for such accelerated regulatory paths.
If the Company’s products continue to indicate that they may increase long-term survival for patients in life-threatening indications, defined as “unmet medical needs,” such as sepsis and complications following bone marrow transplantation, the Company could be eligible to initiate marketing of these drugs in the EU if it receives conditional approval, following submission of a marketing application after completion of a Phase II study to the EMA.
In general, therapeutic products are eligible for conditional marketing approval if they meet at least one of the following categories:
a. Aimed at treating, preventing or diagnosing seriously debilitating or life-threatening diseases ;diseases;
b. Intended for use in emergency situations; or
c. Designated as orphan medicines.
For a product to be granted a conditional marketing authorization following submission of a marketing application, it must fulfil all the following criteria:
a. The risk–benefit balance of the medicinal product is positive;
b. It is likely that the applicant will be able to provide the comprehensive clinical data in future studies post initiation of commercialization;
c. Unmet medical needs will be fulfilled; and
d. The benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required.
Clinical Trials and Commercial Manufacturing of Allocetra™
To prepareIn the fourth quarter of 2022, we completed construction of our new cGMP Allocetra™ manufacturing plant in Israel. The facility is currently approximately 36,400 square feet, and has the ability to be expanded to approximately 45,600 square feet. As part of a Company-wide cost cutting program designed to extend our cash runway potentially into 2025, our planned move into the new manufacturing plant has been deferred for an approximate period of three years, and we have determined to offer lease and supporting services of the planned initiation of itsleased space to potential clients during this three-year period. Although currently deferred, we intend to use the additional manufacturing capacity provided by the facility to support ongoing clinical trials, the Company has constructed a good manufacturing process (“GMP”) manufacturing facility in Israel to support thefuture clinical trials, and initial commercial production of the Allocetra™ drug product for any clinical trial that will be conducted in the EU or Israel.may occur if we receive applicable regulatory approvals.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly evolving technology, intense competition and a highly uncertain, costly and lengthy research and development process. Adequate protection of intellectual property, successful product development, adequate funding and retention of skilled, experienced and professional personnel are among the many factors critical to success in these industries.
We believe that our product candidates offercandidate offers key potential advantages over other drugs and therapies currently in use or in development that could enable our product candidates,candidate, if approved for the intended indications, to capture meaningful market share. In particular, we believe that, based on our studies to date, Allocetra’s ™ ability to induce immunotolerance and reduce inflammation without observable, significant adverse side effects and without general immunosuppression make Allocetra™ a potentially valuable therapy for the treatment of autoimmune and inflammatory disorders.
See “Risk Factors — Factors—Risks Related to Our Business, Industry and Regulatory Requirements — Requirements—We might be unable to develop any of our product candidates to achieve commercial success in a timely and cost-effective manner, or ever” and “Risk Factors — Factors—Risks Related to Our Business, Industry and Regulatory Requirements — Requirements—Our market is subject to intense competition. If we are unable to compete effectively, Allocetra™ or any other product candidate that we are developing or may develop may be rendered uncompetitive or obsolete”.obsolete.”
License Agreements
Tolaren Ltd.
In April 2008, Tolaren Ltd., which we refer to as Tolaren, granted to us an exclusive, irrevocable, worldwide, royalty free and sublicensable license to research, develop, commercialize, manufacture, market, sell, distribute and otherwise use and exploit a certain patent, patent rights and pending patent applications relating to the method for using apoptotic cells as a treatment for various autoimmune and inflammatory disorders and the production processes with respect to the same. The license further stipulates that all intellectual property rights, including, any inventions, developments, discoveries, results, products data, information and know-how developed by the Company based on the licensed intellectual property rights, belong solely and exclusively to the Company and, to the extent such intellectual property rights are registrable, they may be registered in the name of the Company. We have used and continue to use such licensed technology to develop and produce Allocetra™. Pursuant to the license, we have agreed to manage, maintain and defend the licensed patents, including managing the registration of such patents in different countries. The license expires upon the expiration of the licensed patent; however, upon such expiration, we will have a fully paid-up, nonexclusive, unlimited, worldwide, sublicensable license to the technology developed on the basis of the patent and related patent rights and all inventions, know-how and other intellectual property owned or licensed by us and covered by the agreement or related thereto. The license is terminable by the Company upon 30-days prior written notice or by Tolaren if the Company ceases operations for a period of more than 360 days. Otherwise, the license for each of the patents endures until the expiration of such patent, and the license for any other licensed technology survives indefinitely.
Approximately 97% of the issued and outstanding share capital of Toleran is held by Hadasit Bio-Holdings Ltd., which currently holds approximately 10%5.97% % of our issued and outstanding share capital.
Hadasit Medical Research Services and Development Ltd. and Yissum Research and Development Company Ltd.
In March 2006, the institutes jointly granted us an exclusive, worldwide, royalty free and sublicensable license to research, develop, commercialize, manufacture, market, sell, distribute and otherwise use and exploit a certain patent and patent rights relating to the therapeutic use of dead or dying cells, including apoptotic or necrotic cells, as well as any associated materials, methods or technology, as well as a method of using the heparin-binding domain of TSP thrombospondin-1, or TSP-1, which we may develop in the future as a molecular-based therapy for the treatment of inflammatory bowel disease. The license further stipulates that all intellectual property rights, including, any inventions, developments, discoveries, results, products data, information and know-how developed by the Company based on the licensed intellectual rights, belong solely and exclusively to the Company and, to the extent such intellectual property rights are registrable, they may be registered in the name of the Company. Pursuant to the license, we agreed to manage, maintain and defend the licensed patents, including managing the registration of such patents in different countries. The license expires upon the expiration of the licensed patent; however, upon such expiration, we will have a fully paid-up, nonexclusive, unlimited, worldwide, sublicensable license to the technology developed on the basis of the patent and related patent rights and all inventions, know-how and other intellectual property owned or licensed by us and covered by the agreement or related thereto. In addition to certain standard termination provisions relating to the financial condition of each party, we may terminate the license upon 30-days’ prior written notice, and the institutes may terminate the license if we cease our operations for more than 120 days or if the institutes determine, in their reasonable discretion, that we have ceased making reasonable efforts to commercialize the licensed technology.
Hadasit Medical Research Services and Development Ltd. is the technology transfer office of Hadassah Hospital in Jerusalem, where Prof. Dror Mevorach, one of our directors,Chief Science & Medical Office, is currently the Director of the Rheumatology Research Centre.
Intellectual Property and Patents and Proprietary Rights
The proprietary nature of, and protection for, our product candidates and our discovery programs, processes and know-how are important to our business. We own and in-license issued patents and pending patent applications in various jurisdictions worldwide, including three issued patents and several pending patent application in the United States, one issued patent in Israel, two issued patents and several pending patent application in the EU and several international patent application filed with the World Intellectual Property Organization under the PCT. We have sought patent protection for certain methods of producing and using autologous and allogeneic Allocetra. We also intend to seek patent protection for our discovery programs, and any other inventions to which we have rights, where available and when appropriate.
Our policy is to pursue, maintain and defend patent rights, whether developed internally or licensed from third parties, and to protect the technology, inventions and improvements that are commercially important to the development of our business. We also rely on trade secrets that may be important to the development of our business.
Our commercial success will substantially depend on obtaining and maintaining patent protection and trade secret protection for our current and future product candidates and the methods used to develop and manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing our products depends on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities. We believe that our patents do, and filed patent applications will, provide broad and comprehensive coverage for the use of Allocetra™ as a treatment for the treatment of certain autoimmune and inflammatory disorders.our key target clinical indications. However, the patent positions of biotechnology companies, such as ourselves, are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position, if any, for the technology will depend on our success in obtaining effective claims and enforcing those claims once granted.
There is no certainty that any of our pending patent applications will result in the issuance of any patents. Our issued patents and those that may be issued in the future, could be challenged, narrowed, circumvented or found to be invalid or unenforceable, which could limit our ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products. In addition, our competitors may independently develop similar technologies or duplicate any technology developed by us, and the rights granted under any issued or future patents may not provide us with any meaningful competitive advantages against these competitors. Furthermore, because of the extensive time required for development, testing and regulatory review of a potential product, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of such patent.
The Company has filed severalmultiple patent families in which certain patents have been granted and there are other pending patent applications covering products under development.development and the uses thereof. The first patent family, titled “Disease Therapy Using Dying Oror Dead Cells” washas IP granted by the U.S. patent office (patent numberPatent and Trademark Office (USPTO; Patent No. 9,567,568), the EU (patent number 187, 9601)European Patent Office (EPO; Patent No. EP1879601), and Israel (patent number 187,122)Patent Office (IPO; Patent No. IL187122) with a term expiring in 2025-2026 in the United Statespatent terms of 04-May-2025 (US) and Israel, EU (DE,04-May-2026 (Israel and Europe – DE, FR, IE, GB), respectively. The second patent family, titled “Therapeutic Apoptotic Cell Preparations, Method for Producing Same and Uses Thereof” washas patents granted byin the U.S. patent office (patent number(Patent Nos. 10,077,426 B2) on September 18, 2018and 10,927,343) with term dates in 2033 and 2034, respectively. Patents in this family have also been granted in Australia, Canada, China, Europe, Israel, and Japan, which have a term expiring in 2033date of December 5, 2033. A third patent family, titled “THERAPEUTIC POOLED BLOOD APOPTOTIC CELL PREPARATIONS AND USES THEREOF” has granted patents in the United States, Japan,U.S. (Patent No. 10,857,181), Australia, China, Europe, Israel, and Japan, having a term date of April 21, 2036 (July 15, 2036 for the U.S. patent). Pending applications in this patent family include those filed in Australia, Canada, China, Europe, Hong Kong, Israel, Japan, South Korea, and is currently underthe U.S., many of which have entered prosecution. A fourth patent family, titled “COMBINATION IMMUNE THERAPY AND CYTOKINE CONTROL THERAPY FOR CANCER TREATMENT,” for combination therapies with CAR T-cells, has granted patents in the U.S. (Patent Nos. 11,000,548 and 11,000,548), Australia, Europe, Israel, and Japan, having a term date of February 18, 2036. Pending applications in prosecution in this family include those filed in the U.S., Australia, Canada, China, Europe, Israel, Japan, and South Korea. A fifth patent family, titled “EARLY APOPTOTIC CELLS FOR USE TREATING SEPSIS” has granted patents in the U. S. (Patent Nos. 11,304,976; 11,497,767; 11,318,163) having a term date of February 18, 2036. Pending application sin this patent family include those filed in the U.S. (allowed), Australia, Canada, China, Europe, Israel, Japan, and South Korea. A sixth patent family, titled “THERAPEUTIC APOPTOTIC CELLS FOR CANCER THERAPY” has pending applications in the U.S., Australia, Canada, China, Europe, Israel, Japan, and South Korea. A seventh patent family, titled “THERAPEUTIC APOPTOTIC CELLS FOR TREATMENT OF OSTEOARTHRITIS” has pending applications in the U.S., Australia, Canada, China, Europe, Israel, Japan, and South Korea. An eighth patent family, titled “USE OF EARLY APOPTOTIC CELLS FOR TREATING COVID-19” has pending applications in the U.S., Australia, Canada, China, Europe, Israel, Japan, and South Korea. Various additional patent families with pending applications in the U.S. and in other countries are under prosecution or are in the preliminary stages and have been filed and are under prosecution.as provisional U.S. patents or International (PCT) applications.
Trade Secrets
In addition to owned and licensed patents, we rely on trade secrets and know-how to develop and maintain our competitive position. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary processes, in part, by confidentiality and intellectual property ownership and assignment agreements or provisions with certain of our employees, consultants, scientific advisors, contractors and commercial partners involved in research and development activities or who may otherwise have access to our confidential or proprietary information. These agreements are designed to protect our proprietary information. We also seek to preserve the integrity and confidentiality of our data, trade secrets and know-how by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, such agreements or security measures may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors or others, which would significantly affect our competitive advantage and have a material adverse effect on our business, results of operation and financial condition. See also, “Risk Factors—Risks Related to Our Intellectual Property—Under applicable U.S. and Israeli law, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees”.employees.”
Raw Materials, Suppliers and Manufacturing
In order to produce Allocetra™, blood donations are collected from healthy donors through apheresis and then shipped to a manufacturing site for cryopreservation by trained personnel pursuant to cGMP requirements and otherwise in accordance with applicable FDA guidelines and our CMC protocols. The manufacture of AllocetraTM and AllocetraTM from matched donor cells differ only in the source of the blood donations. Donations for AllocetraTM from matched donor cells come from the blood of either (i) a donor matched by his or her human leukocyte antigen (i.e., a protein found on white blood cells that is the standard genetic marker for the regulation of the immune system and is used to match donors and recipients in transplantations), (ii) the patient, or (iii) an allogeneic, unmatched donor that has undergone ex-vivo (i.e., prior to infusion) manipulation to stabilize the “early apoptosis” status of the cells for a prolonged period of time. Donations for AllocetraTM are collected from healthy donors through apheresis. The cells sourced for either AllocetraTM or AllocetraTM from matched donor cells then undergo quality control testing and are thawed and manipulatedex vivo by inducing apoptosis to retrieve and harvest stable early apoptotic cells. The agents used in theex vivo manipulation for Allocetra™ are then washed and removed before the apoptotic cells are combined with a saline solution for delivery and injection in patients. We use standard collection equipment and procedures to collect blood for Allocetra™ production and anticipate entering into long-term agreements with various collection and medical centers to properly train their personnel pursuant to cGMP requirements, FDA guidelines and our CMC protocols and thereafter collect blood at their facilities upon receipt of patient or donor consent. Other than the blood collections, we believe that the raw materials required to manufacture our product candidates are readily available commodities commonly used in the pharmaceutical and biotechnology industries and are generally widely available from numerous suppliers at market prices. However, biologically sourced raw materials are subject to unique contamination risks and their use may be restricted in certain countries. See also “Risk Factors—Risks Related to our Business, Industry and Regulatory Requirements—Our manufacturing processes are complex, delicate and susceptible to contamination, and involve biological intermediates that are subject to stringent regulations”,regulations,” “Risk Factors—Risks Related to our Business, Industry and Regulatory Requirements—Our ability to produce safe and effective products depends on the safety of our blood supply against transmittable diseases.”
In January 2019 we completed construction of a new facility in Israel for the manufacture of Allocetra™ for the planned clinical trials that will be conducted in the EU or Israel. The new facility received QP declaration confirming compliance with EU GMP regulations. However, we do not have experience in manufacturing products on a commercial scale or using automated processes and we have limited personnel. We do not have any current contractual relationships for the manufacture of commercial supplies of Allocetra™ or any other product candidate. If any of our product candidates or future product candidates are approved by any regulatory authority, we intend to enter into agreements with one or more third-party contract manufacturers for the commercial production of those products. Development and production of commercial quantities of any products that we develop will need to be manufactured in facilities, and by processes, that comply with the requirements of the FDA and the regulatory agencies of other jurisdictions in which we are seeking approval, as well as with our CMC. When selecting a CMO and other third-party service providers and suppliers to produce Allocetra™, we and certain hired quality assurance consultants verify that such CMOs and third party service providers and suppliers are compliant with both cGMP requirements.
There can be no assurance that our product candidates, if approved, can be manufactured in sufficient commercial quantities, in compliance with regulatory requirements and at an acceptable cost. We and our any potential future contract manufacturers are, and will be, subject to extensive governmental regulation in connection with the manufacture of any pharmaceutical products. We and our future contract manufacturers must ensure that all of the processes, methods and equipment are compliant with our CMC and cGMP for drugs and biologics on an ongoing basis, as mandated by the FDAEMA and other applicable regulatory authorities, and conduct extensive audits of vendors, contract laboratories and suppliers.
Contract Research Organizations
We intend to outsource certain future clinical trial activities, including the administration of treatments, to CROs. Such clinical CROs must comply with guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which attempt to harmonize the FDA and the EMA regulations and guidelines. We intend to create and implement the development plans and manage the CROs according to the specific requirements of the product candidate under development. To the extent clinical research is conducted by the CROs (or us in the future), compliance with certain federal regulations, including but not limited to 21 C.F.R. parts 50, 54, 56, 58 and 312, which pertain to, among other things, informed consent, financial conflicts of interest by investigators, IRBs, good laboratory practices, GCP and submitting IND applications, may be required.
Marketing, Sales and Commercialization
Given our stage of development, we do not have any internal sales, marketing or distribution infrastructure or capabilities. If we receive regulatory approval for any of our product candidates, we intend, as appropriate, to pursue commercialization relationships, including strategic alliances and licensing, with biotechnology companies and other strategic partners, which are equipped to market and sell our products, if any, through their sales, marketing and distribution organizations. In addition, we may out-license some or all of our worldwide patent rights to more than one party to achieve the fullest development, marketing and distribution of any products we develop. Over the longer term, we may consider building an internal marketing, sales and commercial infrastructure.
Environmental Matters
We, our agents and our service providers, including our manufacturers, are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous and biological materials and wastes and the cleanup of contaminated sites. We believe that our business, operations and facilities, including, to our knowledge, those of our agents and service providers, are currently operated in compliance in all material respects with applicable environmental and health and safety laws and regulations. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. However, significant expenditures could be required in the future if we, our agents or our service providers are required to comply with new or more stringent environmental or health and safety laws, regulations or requirements.
Government Regulation
Clinical trials, the drug approval process and the marketing of drugs are intensively regulated in the United States and in all other major foreign countries. Governmental authorities in the United States (including federal, state and local authorities) and in other countries, extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, storage, distribution, post-approval monitoring and reporting, advertising and promotion, pricing and export and import of pharmaceutical products, such as those we are developing. The process for obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (the “FDCA”), and related regulations, and the Public Health Service Act (the “PHSA”) and its implementing regulations. In addition, drug innovation, prescribing and reimbursement are influenced by Titles XVIII and XIX of the Social Security Act (commonly referred to as Medicare and Medicaid) and the Patient Protection and Affordable Care Act, 42 U.S.C. § 18001, as amended, and their implementing regulations. FDA approval is required before any new drug candidate or dosage form, including a new use of a previously approved drug, can be marketed in the United States. We intend to submit an NDA in the United States. Failure to comply with the applicable United States regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, other corrective action, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and foreign regulatory authorities impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising and promotion of our products.
The FDA’s policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our platforms and candidate products or any future product candidates or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Approval
The process required by the FDA before a product candidate may be marketed in the United States generally involves the following:
The testing and approval process requires substantial time and financial resources and we cannot be certain that any approvals for our candidate products will be granted on a timely basis, if at all.
An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of in vitro and in vivo studies and animal testing results assessing the toxicology, pharmacokinetics and pharmacodynamic characteristics of the product; chemistry, manufacturing and controls information; and any available human data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence.
We will need to successfully complete clinical trials in order to be in a position to submit an NDA to the FDA. Our planned future clinical trials for our candidate products may not begin or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including:
We must reach an agreement with the FDA on the proposed protocols for our future clinical trials in the United States. A separate submission apart from an IND application must be made for each clinical trial to be conducted during product development. Further, an independent IRB for each site proposed to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each trial subject. Regulatory authorities, an IRB or the sponsor, may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk.
Clinical trials
Clinical trials involve the administration of the product candidate to human subjects under the supervision of qualified investigators in accordance with current good clinical practices (“cGCP”), which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site’s IRB before the studies may be initiated and the IRB must monitor the trial until completed. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
Our objective is to conduct clinical trials for our candidate products and, if those trials are successful, seek marketing approval from the FDA and other worldwide regulatory bodies.
For purposes of NDA approval, human clinical trials are typically conducted in phases that may overlap.
A pivotal trial is a clinical trial that adequately meets regulatory agency requirements for the evaluation of a drug candidate’s efficacy and safety such that it can be used to justify the approval of the product. Generally, pivotal trials are Phase 3 trials, but the FDA may accept results from Phase 2 trials if the trial design provides a well-controlled and reliable assessment of clinical benefit, particularly in situations where there is an unmet medical need and the results are sufficiently robust.
The FDA, the IRB, or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk.
Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a Data Safety Monitoring Board or Committee. This group provides oversight and assessment of designated milestones based on access to certain data during the conduct of the trial. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
All of these trials must be conducted in accordance with GCP requirements in order for the data to be considered reliable for regulatory purposes.
The clinical trial process can take three to ten years or more to complete and there can be no assurance that the data collected will support FDA approval or licensure of the product. Government regulation may delay or prevent marketing of a product candidate or new drugs for a considerable period of time and impose costly procedures upon our activities. We cannot be certain that the FDA or any other regulatory agency will grant approvals for a product candidate on a timely basis, if at all. Success in early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
The NDA Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational new drug product information is submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. Under federal law, the submission of most NDAs is subject to an application user fee. For the FDA’s fiscal year 2018, the application user fee with clinical data was $2,421,495 and for 2019 the fee is $2,588,478 and the sponsor of an approved NDA is also subject to annual product and program user fees. For the FDA’s fiscal year 2018, these program fees were set at $304,162 per product and in 2019 they are $309,915 per product. These fees are typically increased annually. Applications for orphan drug products are exempted from the NDA user fees and may be exempted from product and establishment user fees, unless the application includes an indication for other than a rare disease or condition.
An NDA must include all relevant data available from pertinent nonclinical and clinical trials, regardless of the results or findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data is generated from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or in certain instances, from other sources, including trials initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational new drug product to the satisfaction of the FDA.
The FDA will initially review the NDA for completeness before it accepts it for filing. The FDA has 60 days from receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an Advisory Committee, typically a panel that includes independent clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an Advisory Committee, but it considers such recommendations carefully when making decisions.
Upon the request of an applicant, the FDA may grant a Priority Review designation to a product, which sets the target date for FDA action on the application at six months, rather than the standard ten months. Priority review is given where preliminary assessments indicates that a product, if approved, has the potential to provide a significant improvement compared to marketed products or offers a therapy where no satisfactory alternative therapy exists. Priority Review designation does not alter the scientific/medical standard for approval or the quality of evidence necessary to support approval.
The FDA is required to complete its review in a certain amount of time, for which the user fees are paid to help with the costs of the evaluation. However, FDA and the sponsor can agree to extend this review time. After the FDA completes its review of an NDA, it will communicate to the sponsor that the drug will either be approved, or it will issue a Complete Response Letter to communicate that the NDA will not be approved in its current form and inform the sponsor of changes that must be made or additional clinical, nonclinical or manufacturing data that must be received before the application can be approved, with no implication regarding the ultimate approvability of the application.
Before approving an NDA, the FDA will typically inspect the facilities at which the drug substance or drug product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
Additionally, before approving an NDA, the FDA may inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the NDA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 trials may be made a condition to be satisfied for continuing drug approval. The results of Phase 4 trials can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA now has express statutory authority to require sponsors to conduct post-market trials to specifically address safety issues identified by the agency.
Any approvals that we may ultimately receive could be withdrawn if required post-marketing trials or analyses do not meet the FDA requirements, which could materially harm the commercial prospects for our candidate products.
The FDA also has authority to require a Risk Evaluation and Mitigation Strategy (“REMS”), from sponsors to ensure that the benefits of a drug or biological product outweigh its risks. A sponsor may also propose a REMS as part of the NDA submission. The need for a REMS is determined as part of the review of the NDA. Based on statutory standards, elements of a REMS may include “Dear Doctor” letters, a Medication Guide, more elaborate targeted educational programs and in some cases restrictions on distribution. These elements are negotiated as part of the NDA approval, and in some cases if consensus is not obtained until after the Prescription Drug User Fee Act review cycle, the approval date may be delayed. Once adopted, REMS are subject to periodic assessment and modification.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, including Black Box Warnings, or in the form of risk management plans, restrictions on distribution, or post-marketing trial requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or complete withdrawal of the product from the market. Delay in obtaining, or failure to obtain, regulatory approval for our candidate products, or obtaining approval but for significantly limited use, would harm our business. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
FDA Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, changes to the approved product or the addition of new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
Drug sponsors and their manufacturers are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We rely, and expect to continue to rely, on third parties for the production of clinical quantities of our current product candidate, and expect to rely in the future on third parties for the production of commercial quantities. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of a requirement to conduct post-market trials or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, but not limited to the following:
The FDA strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant enforcement and product liability exposure.
Orphan Drug Designation and Exclusivity
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, or if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making the drug for this type of disease or condition will be recovered from sales in the United States.
Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user-fee waivers. In addition, if a product receives FDA approval for the indication for which it has orphan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity. Orphan drug designation does not affect the regulatory review standards or shorten the review period. Designation does not imply FDA approval, and it is possible a company may, in certain cases, lose designation before a product’s approval and, thus, may not obtain orphan drug exclusivity.
European Union/Rest of World Government Regulation
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a clinical trial application “(“CTA”), must be submitted for each clinical protocol to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is accepted in accordance with a country’s requirements, the clinical trial may proceed.
The requirements and process governing the conduct of clinical trials vary from country to country. In all cases, the clinical trials are conducted in accordance with cGCP,GCP, the applicable regulatory requirements, and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational medicinal product under European Union regulatory systems, we must submit a marketing authorization application. The content of the NDA or BLA filed in the United States is similar to that required in the European Union, with the exception of, among other things, country and EU-specific document requirements.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing product licensing, pricing, and reimbursement vary from country to country.
Countries that are part of the European Union, as well as countries outside of the European Union, have their own governing bodies, requirements, and processes with respect to the approval of pharmaceutical products. If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Authorization Procedures in the European Union
Medicines can be authorized in the European Union by using either the centralized authorization procedure or national authorization procedures.
Centralized procedure. The European Commission implemented the centralized procedure for the approval of human medicines to facilitate marketing authorizations that are valid throughout the EEA which is comprised of the 27 member states of the European Union plus Norway, Iceland, and Lichtenstein. This procedure results in a single marketing authorization issued by the European Commission that is valid across the EEA. The centralized procedure is compulsory for human medicines that are: derived from biotechnology processes, such as genetic engineering; contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions; and officially designated orphan medicines.
For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA following a favorable eligibility request by the EMA, as long as the medicine concerned is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health.
National authorization procedures. There are also two other possible routes to authorize medicinal products in several European Union countries, which are available for investigational medicinal products that fall outside the scope of the centralized procedure:
Decentralized procedure. Using the decentralized procedure, an applicant may apply for simultaneous authorization in more than one European Union country of medicinal products that have not yet been authorized in any European Union country and that do not fall within the mandatory scope of the centralized procedure.
Mutual recognition procedure.procedure. In the mutual recognition procedure, a medicine is first authorized in one European Union Member State, in accordance with the procedure laid down in the EU directive 2001/83 as amended and implemented into national legislation. Following this, further marketing authorizations can be sought from other European Union countries in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization.
In some cases, a Pediatric Investigation Plan, or PIP, and/or a request for waiver or deferral, is required for submission prior to submitting a marketing authorization application. A PIP describes, among other things, proposed pediatric trials and their timing relative to clinical trials in adults.
New Chemical Entity Exclusivity
In the European Union, new chemical entities, sometimes referred to as new active substances or new molecular entities, as well as submissions following Article 8.3 of Directive 2001/83 as amended, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, the product may be approved but must not be launched prior to the end of the 10 years data exclusivity period. The overall ten-year period will be extended by one year if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, is held to bring a significant clinical benefit, in comparison with existing therapies, or by six months if there is a pediatric development in accordance with a PIP has been performed.
Orphan Drug Designation and Exclusivity
In the European Union, the EMA’s COMP grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the European Union and for which no satisfactory method of diagnosis, prevention, or treatment has been authorized (or the product would be a significant benefit to those affected, i.e. where a prior approval was granted). Additionally, designation is granted for products intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the medicinal product.
In the European Union, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity is granted following medicinal product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. This period can be prolonged to 12 years in case a pediatric development has been performed following an agreed PIP.
Orphan drug designation must be requested and granted before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Exceptional Circumstances/Conditional Approval
Orphan drugs or drugs with unmet medical needs may be eligible for European Union approval under exceptional circumstances or with conditional approval. Approval under exceptional circumstances is applicable to all applications including orphan products and is used when an applicant is unable to provide comprehensive data on the efficacy and safety under normal conditions of use because the indication for which the product is intended is encountered so rarely that the applicant cannot reasonably be expected to provide comprehensive evidence, when the present state of scientific knowledge does not allow comprehensive information to be provided, or when it is medically unethical to collect such information. Conditional marketing authorization is applicable to orphan medicinal products, medicinal products for seriously debilitating or life-threatening diseases, or medicinal products to be used in emergency situations in response to recognized public threats. Conditional marketing authorization can be granted on the basis of less complete data than is normally required in order to meet unmet medical needs and in the interest of public health, provided the risk-benefit balance is positive, it is likely that the applicant will be able to provide the comprehensive clinical data after approval, and unmet medical needs will be fulfilled. Conditional marketing authorization is subject to certain specific obligations to be reviewed annually. The initial approval needs to be renewed annually. This renewal is controlled by the CHMP and, if not granted, may lead to cessation of the marketing authorization at the end of this particular year.
Accelerated Review
Under the Centralized Procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the EMA’s Committee for Medicinal Products for Human Use, or CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, particularly from the point of view of therapeutic innovation. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days, excluding clock stops.
U.S. Government Regulation
In the United States, the FDA regulates drugs and biologics under the Federal Food, Drug, and Cosmetic Act (the “FDCA”), and related regulations, and the Public Health Service Act (the “PHSA”) and its implementing regulations. In addition, drug innovation, prescribing and reimbursement are influenced by Titles XVIII and XIX of the Social Security Act (commonly referred to as Medicare and Medicaid, respectively) and the Patient Protection and Affordable Care Act, 42 U.S.C. § 18001, as amended, and their implementing regulations. FDA approval is required before any new drug or biologic candidate or dosage form, including a new use of a previously approved drug, can be marketed in the United States. We intend to submit a BLA in the United States. Failure to comply with the applicable United States regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, other corrective action, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and foreign regulatory authorities impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising and promotion of our products.
The FDA’s policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our platforms and candidate products or any future product candidates or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Approval
The process required by the FDA before a product candidate may be marketed in the United States generally involves the following:
| ● | completion of extensive nonclinical laboratory tests and nonclinical animal studies, all performed in accordance with cGMP and current Good Laboratory Practices, guidance and regulations; |
| ● | submission to the FDA of an investigational new drug (“IND”), application which must become effective before human clinical trials may begin and must be updated annually; |
| ● | approval by an IRB or ethics committee representing each clinical site before each clinical trial may be initiated; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; |
| ● | preparation of and submission to the FDA an NDA or BLA after completion of all clinical trials; |
| ● | potential review of the product application by an FDA Advisory Committee, where appropriate and if applicable; |
| ● | a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review; |
| ● | satisfactory completion of FDA pre-approval inspection of the manufacturing facilities where the proposed product is produced to assess compliance with cGMP; and |
| ● | FDA review and approval of an NDA or BLA prior to any commercial marketing or sale of the drug in the United States. |
The testing and approval processes require substantial time and financial resources, and we cannot be certain that any approvals for our candidate products will be granted on a timely basis, if at all.
An IND is a request for authorization from the FDA to administer an investigational new drug or biologic product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of in vitro and in vivo studies and animal testing results assessing the toxicology, pharmacokinetics and pharmacodynamic characteristics of the product; chemistry, manufacturing and controls information; and any available human data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence.
We will need to successfully complete clinical trials in order to be in a position to submit a BLA to the FDA. Our planned future clinical trials for our candidate products may not begin or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including:
| ● | not obtaining regulatory approval to commence a trial; |
| ● | not reaching agreement with third-party clinical trial sites and their subsequent performance in conducting accurate and reliable studies on a timely basis; |
| ● | not obtaining IRB approval to conduct a trial at a prospective site; |
| ● | recruiting an insufficient number of patients to participate in a trial; |
| ● | inadequate supply of the drug; and |
| ● | clinical adverse finding(s) during the trial itself. |
We must reach an agreement with the FDA on the proposed protocols for our future clinical trials in the United States. A separate submission apart from an IND application must be made for each clinical trial to be conducted during product development. Further, an independent IRB for each site proposed to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each trial subject. Regulatory authorities, an IRB or the sponsor, may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk.
Clinical trials
Clinical trials involve the administration of the product candidate to human subjects under the supervision of qualified investigators in accordance with current clinical practices (“GCP”), which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site’s IRB before the studies may be initiated and the IRB must monitor the trial until completed. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
Our objective is to conduct clinical trials for our candidate products and, if those trials are successful, seek marketing approval from the FDA and other worldwide regulatory bodies.
For purposes of NDA approval, human clinical trials are typically conducted in phases that may overlap.
| ● | Phase 1. The drug or biologic is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients; |
| ● | Phase 2. This phase involves trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage; |
| ● | Phase 3. This phase involves trials undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population, often at geographically dispersed clinical trial sites. These trials are intended to establish the overall benefit/risk profile of the product and provide an adequate basis for product labeling; and |
| ● | Phase 4. In some cases, the FDA may condition approval of an NDA or BLA for a product candidate on the sponsor’s agreement to conduct additional clinical trials after approval. In other cases, a sponsor may voluntarily conduct additional clinical trials after approval to gain more information about the drug. Such post-approval studies are typically referred to as Phase 4 clinical trials. |
A pivotal trial is a clinical trial that adequately meets regulatory agency requirements for the evaluation of a drug candidate’s efficacy and safety such that it can be used to justify the approval of the product. Generally, pivotal trials are Phase 3 trials, but the FDA may accept results from Phase 2 trials if the trial design provides a well-controlled and reliable assessment of clinical benefit, particularly in situations where there is an unmet medical need and the results are sufficiently robust.
The FDA, the IRB, or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk.
Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a Data Safety Monitoring Board or Committee. This group provides oversight and assessment of designated milestones based on access to certain data during the conduct of the trial. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
All of these trials must be conducted in accordance with GCP requirements in order for the data to be considered reliable for regulatory purposes.
The clinical trial process can take three to ten years or more to complete and there can be no assurance that the data collected will support FDA approval or licensure of the product. Government regulation may delay or prevent marketing of a product candidate or new drugs for a considerable period of time and impose costly procedures upon our activities. We cannot be certain that the FDA or any other regulatory agency will grant approvals for a product candidate on a timely basis, if at all. Success in early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
The NDA and BLA Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational new drug product information is submitted to the FDA in the form of an NDA, and a BLA for new biologics, requesting approval to market the product for one or more indications. Under federal law, the submission of most NDAs and BLAs is subject to an application user fee. For the FDA’s fiscal year 2022, the application user fee with clinical data was $3,117,218 and for 2023 the fee is $3,242,026 and the sponsor of an approved NDA or BLA is also subject to annual product and program user fees. For the FDA’s fiscal year 2022, these program fees were set at $369,413 per product and in 2023 they are $393,933 per product. These fees are typically increased annually. Applications for orphan drug products are exempted from these user fees and may be exempted from product and establishment user fees, unless the application includes an indication for other than a rare disease or condition.
An NDA and BLA must include all relevant data available from pertinent nonclinical and clinical trials, regardless of the results or findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data is generated from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or in certain instances, from other sources, including trials initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational new drug product to the satisfaction of the FDA.
The FDA will initially review the NDA or BLA for completeness before it accepts it for filing. The FDA has 60 days from receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the NDA or BLA submission is accepted for filing, the FDA reviews the NDA or BLA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an Advisory Committee, typically a panel that includes independent clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an Advisory Committee, but it considers such recommendations carefully when making decisions.
Upon the request of an applicant, the FDA may grant a Priority Review designation to a product, which sets the target date for FDA action on the application at six months, rather than the standard ten months. Priority review is given where preliminary assessments indicates that a product, if approved, has the potential to provide a significant improvement compared to marketed products or offers a therapy where no satisfactory alternative therapy exists. Priority Review designation does not alter the scientific/medical standard for approval or the quality of evidence necessary to support approval.
The FDA is required to complete its review in a certain amount of time, for which the user fees are paid to help with the costs of the evaluation. However, FDA and the sponsor can agree to extend this review time. After the FDA completes its review of an NDA or BLA, it will communicate to the sponsor that the drug or biologic will either be approved, or it will issue a Complete Response Letter to communicate that the NDA will not be approved in its current form and inform the sponsor of changes that must be made or additional clinical, nonclinical or manufacturing data that must be received before the application can be approved, with no implication regarding the ultimate approvability of the application.
Before approving an NDA or BLA, the FDA will typically inspect the facilities at which the drug substance or drug product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
Additionally, before approving an NDA or BLA, the FDA may inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the NDA or BLA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 trials may be made a condition to be satisfied for continuing drug approval. The results of Phase 4 trials can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA now has express statutory authority to require sponsors to conduct post-market trials to specifically address safety issues identified by the agency.
Any approvals that we may ultimately receive could be withdrawn if required post-marketing trials or analyses do not meet the FDA requirements, which could materially harm the commercial prospects for our candidate products.
The FDA also has authority to require a Risk Evaluation and Mitigation Strategy (“REMS”), from sponsors to ensure that the benefits of a drug or biological product outweigh its risks. A sponsor may also propose a REMS as part of the NDA submission. The need for a REMS is determined as part of the review of the NDA. Based on statutory standards, elements of a REMS may include “Dear Doctor” letters, a Medication Guide, more elaborate targeted educational programs and in some cases restrictions on distribution. These elements are negotiated as part of the NDA or BLA approval, and in some cases if consensus is not obtained until after the Prescription Drug User Fee Act review cycle, the approval date may be delayed. Once adopted, REMS are subject to periodic assessment and modification.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, including Black Box Warnings, or in the form of risk management plans, restrictions on distribution, or post-marketing trial requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or complete withdrawal of the product from the market. Delay in obtaining, or failure to obtain, regulatory approval for our candidate products, or obtaining approval but for significantly limited use, would harm our business. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
FDA Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, changes to the approved product or the addition of new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
Drug sponsors and their manufacturers are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We rely, and expect to continue to rely, on third parties for the production of clinical quantities of our current product candidate, and expect to rely in the future on third parties for the production of commercial quantities. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA or BLA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of a requirement to conduct post-market trials or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, but not limited to the following:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters or holds on post-approval clinical trials; |
| ● | refusal of the FDA to approve pending NDAs or BLAs or supplements to approved NDAs or BLAs, or suspension or revocation of product license approvals; |
| ● | injunctions or the imposition of civil or criminal penalties; or |
| ● | product seizure or detention, or refusal to permit the import or export of products. |
The FDA strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant enforcement and product liability exposure.
Orphan Drug Designation and Exclusivity
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, or if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making the drug for this type of disease or condition will be recovered from sales in the United States.
Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user-fee waivers. In addition, if a product receives FDA approval for the indication for which it has orphan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity. Orphan drug designation does not affect the regulatory review standards or shorten the review period. Designation does not imply FDA approval, and it is possible a company may, in certain cases, lose designation before a product’s approval and, thus, may not obtain orphan drug exclusivity.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payers. Third-party payers include government authorities, managed care providers, private health insurers and other organizations. If we obtain regulatory approval for our products, third-party payers may not provide coverage for our products, or may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Moreover, a payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third-party payers are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. To obtain coverage and reimbursement for any product that receives regulatory approval for commercial sale, we may need to provide supporting scientific, clinical and cost-effectiveness data, which may be difficult and costly to obtain. Our current or any future product candidates may not be considered medically necessary or cost-effective. If third-party payers do not consider a product to be cost-effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of health care costs, including price controls, reporting requirements, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. By way of example, the ACA contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Adoption of additional government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals.
In the U.S., judicial challenges as well as legislative initiatives to modify, limit, or repeal the ACA have been initiated and continue, including a recent Executive Order signed by the U.S. President directing executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of provisions of the ACA that would impose a fiscal or regulatory burden on individuals and certain entities to the maximum extent permitted by law.continue. The extent to which any repeal or replacement of elements of the ACA, or other legislation, would affect our ability to obtain regulatory approval for the sale of Allocetra™, or the prices and net revenues from its sale is unknown at the time of this filing and represent an additional uncertainty.
In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules, legislation and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed to by the government. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
In Canada, the federal government, provinces and territories provide coverage to about one third of residents through publicly financed programs. Both the federal and provincial governments play a role in regulating drug prices and reimbursement. The prices of patented drugs are regulated at the federal level by the Patented Medicine Prices Review Board, which ensures that prices are not excessive. Also, drugs must be approved at the provincial level in order to be covered under provincial health insurance systems. Once Health Canada has approved a drug for use, the country’s public drug plans must decide if the drug will be eligible for public reimbursement. The Canadian Agency for Drugs and Technologies in Health (“CADTH”), an independent non-profit agency has a mandate to provide advice and evidence-based information about the effectiveness of drugs and other health technologies to Canadian health care decision makers. CADTH implements a Common Drug Review (“CDR”) process to provide formulary recommendations for all provinces except Quebec. Through the CDR process, CADTH conducts evaluations of the clinical, economic, and patient evidence on drugs, and uses this evaluation to provide reimbursement recommendations and advice to Canada’s federal, provincial, and territorial public drug plans, with the exception of Quebec. About two-thirds of Canada’s residents are covered for prescription drugs by private insurance. Private plans establish their own lists of covered drugs.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if governmental and other third-party payers fail to provide adequate coverage and reimbursement. In addition, there is an increasing emphasis on cost containment measures in the United States and other countries, which we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
If we obtain regulatory approval for our current or any future product candidates, we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce or reward, or in return for, the referral of an individual, or the purchase, order or recommendation of any good, item or service reimbursable under a federal healthcare program, such as Medicare and Medicaid; |
| ● | the federal physician self-referral prohibition law, or Stark Law, which prohibits, among other things, a physician (defined to include a doctor of medicine or osteopathy, a doctor of dental surgery or dental medicine, a doctor of podiatric medicine, a doctor of optometry, or a chiropractor) from referring Medicare and Medicaid patients to certain types of entities with which the physician or any of the physician’s immediate family members have a financial relationship; | |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, including the False Claims Act, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from the federal government, including Medicare, Medicaid, or other third-party payers, that are false or fraudulent; |
| ● |
| ● | the federal transparency laws, including the physician sunshine provisions of the Affordable Care Act, that requires certain drug manufacturers to disclose certain payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and their family members; |
| ● | the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and its implementing regulations, which imposes certain requirements relating to the privacy and security of individually identifiable health information; |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; and |
| ● | the FCPA, which prohibits companies from making improper payments to foreign government officials and other persons for the purpose of obtaining or retaining business. |
The ACA broadened the reach of the fraud and abuse laws by, among other things, amending the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes contained within 42 U.S.C. §1320a-7b. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act (discussed below) or the civil monetary penalties statute. Many states have adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only federal healthcare programs such as the Medicare and Medicaid programs.
Safeguards we implement to prohibit improper payments or offers of payments by our employees, consultants, and others may be ineffective, and violations of the fraud and abuse laws, the FCPA and similar laws may result in severe criminal or civil sanctions, or other liabilities or proceedings against us, any of which would likely harm our reputation, business, financial condition and result of operations.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, damages, fines, disgorgement, contractual remedies, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Labeling, Marketing and Promotion
The FDA closely regulates the labeling, marketing and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a drug that are consistent with FDA approval, and the company is allowed to actively market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, enforcement letters, such as publicly-posted warning letters, corrective advertising, injunctions and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of drugs. These federal enforcement actions can also potentially lead to state actions and product liability claims, as well as competitor challenges of deceptive advertising.
Anti-Kickback Statute, False Claims Act, and Other Laws
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other federal, state and local government agencies. For example, sales, marketing and scientific/educational grant programs must comply with, among others, the federal Anti-Kickback Statute, the Stark Law, the federal False Claims Act, privacy and security regulations promulgated under HIPAA, and similar state laws, as applicable. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The federal Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce or reward referrals, or the purchase, order, or prescription of a particular drug or other item or service, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
The federal Stark Law prohibits a physician (defined to include a doctor of medicine or osteopathy, a doctor of dental surgery or dental medicine, a doctor of podiatric medicine, a doctor of optometry, or a chiropractor) from referring Medicare and Medicaid patients to certain types of entities with which the physician or any of the physician’s immediate family members have a financial relationship, unless an exception to the law’s prohibition is met.
The federal False Claims Act prohibits anyone from knowingly presenting, or causing to be presented, for payment to the government, claims for items or services, including drugs that are false or fraudulent, such as claims for items or services not provided as claimed, or claims for medically unnecessary items or services.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, a similar federal requirement requires manufacturers to track and report to the federal government certain payments made to physicians and certain teaching hospitals made in the previous calendar year. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us, and additional laws and regulations may be enacted in the future that expand our compliance obligations even further. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and federal authorities.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Israel
Clinical Testing in Israel
In order to conduct clinical testing on humans in the State of Israel, special authorization must first be obtained from the ethics committee and general manager of the institution in which the clinical trials are scheduled to be conducted, as required under the Guidelines for Clinical Trials in Human Subjects implemented pursuant to the Israeli Public Health Regulations (Clinical Trials in Human Subjects), as amended from time to time, and other applicable legislation. These regulations require authorization by the institutional ethics committee and general manager as well as from the Israeli Ministry of Health, except in certain circumstances, and in the case of genetic trials, special fertility trials and complex clinical trials, an additional authorization of the Ministry of Health’s overseeing ethics committee. The institutional ethics committee must, among other things, evaluate the anticipated benefits that are likely to be derived from the project to determine if it justifies the risks and inconvenience to be inflicted on the human subjects, and the committee must ensure that adequate protection exists for the rights and safety of the participants as well as the accuracy of the information gathered in the course of the clinical testing. Since we perform a portion of the clinical trials on certain of our therapeutic candidates in Israel, we are required to obtain authorization from the ethics committee and general manager of each institution in which we intend to conduct our clinical trials, and in most cases, from the Israeli Ministry of Health.
4.C. Organizational structureStructure
Our soleWe have the following wholly-owned subsidiaries aresubsidiaries: (i) Enlivex Therapeutics R&D Ltd., a company formedorganized under the laws of the State of Israel, and Bio Blast Pharma,(ii) Enlivex Therapeutics Inc., a Delaware corporation.corporation, and (iii) Enlivex Therapeutics RDO Ltd., a company organized under the laws of the State of Israel.
4.D. Property, plantsPlants and equipmentEquipment
The Company’sOur corporate headquarters are located at 14 Einstein Street, NesNess Ziona, Israel 7403618, where it leases and occupieswe lease approximately 420 square meters of space. The facility includes office space and current good manufacturing practice (“cGMP”) clean rooms, which are designed to enable the manufacturing of clinical batches to support theour planned clinical trials in Israel and the EU and commercial products for these regions. The lease for this space expires on August 31, 2023 at which time we are planning extend the Companylease for an additional 60-month period. In October 2020, we entered into an additional lease agreement for approximately 421 square meters of space. The lease for this space expires on September 30, 2025, at which time we may extend the lease for an additional 60 months’33-month period, and we currently plan to effect such extension. In July 2021, we entered into an additional lease agreement for approximately 455 square meters of office space. The lease for this office space expires on October 31, 2026, at which time we may extend the lease for an additional 22-month period.
In September 2021 we entered into another lease agreement for a 2,500 square meter property in Yavne, Israel to construct a new 1,600 square meter facility for the manufacture of AllocetraTM. This lease agreement expires on September 30, 2026 at which time we are planning to extend the lease for an additional 60-month period. In September 2022, our management decided to postpone the use of the manufacturing plant for an approximate period of three years and to offer lease and supporting services of the leased space to potential clients during this three-year period.
In addition, the Company leaseswe lease and occupiesoccupy approximately 55 square meters of office and research labs space at the BioPark Building, Hadassah Ein-Kerem Campus in Jerusalem, Israel. The lease for BioPark space expires on SepSeptember 1, 20212024 at which time the Companywe may extend the lease for an additional 48 months. The CompanyWe also hashave access to and utilizes,utilize, on an as-needed basis, additional research and development facilities and services located at the Hadassah Medical Center, including, without limitation, testing equipment, cell collection equipment and services and blood bank services. The Company believesWe believe that itsour facilities are suitable and adequate for itsour current needs.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
Overview
The Company is a clinical stage immunotherapy company, developing an allogeneic drug pipeline for immune system rebalancing. Immune system rebalancing is critical forYou should read the treatmentfollowing discussion and analysis of life-threatening immuneour financial condition and inflammatory conditions, which involve the Cytokine Release Syndromeresults of operations together with our audited consolidated financial statements and for which there are no FDA-approved treatments, as well as treating solid tumors by modulating such tumors’ microenvironment. The Company’s innovative immunotherapy candidates, Allocetra™ and Allocetra™ from matched-donor cells, are novel immunotherapy candidates based on a unique mechanism of action that targets clinical indications that are defined as “unmet medical needs.” Allocetra™ has been developed to treat acute organ failure associated with sepsis. Allocetra™ from matched-donor cells has been developed to prevent or treat complications associated with BMT and/or HSCT.
The Merger
For a description of the Merger, please see the “Introduction” appearing before Part I, Item 1 ofrelated notes included elsewhere in this Annual Report on Form 20-F. The Merger was accounted forfollowing discussion and analysis contains forward-looking statements that involve risk and uncertainties, such as an issuance of shares by the Company for the net assets of Bioblast Pharma Ltd., accompanied by a recapitalization. Accordingly, Enlivex R&D is reflected as the predecessor and acquirer and therefore the accompanying financial statements reflect the historical financial statements of Enlivex R&Dour plans, objectives, expectations, and intentions. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factor Summary” and Item 3.D. “Risk Factors” contained in this Annual Report on Form 20-F.
Overview
The Company is a clinical-stage macrophage reprogramming immunotherapy company, developing AllocetraTM, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for all periods presentedimmune system rebalancing and do not includeresolution of life-threatening conditions. Non-homeostatic macrophages contribute significantly to the historical financial statements of pre-merger Bioblast. All historical information presented herein has been retroactively restated to reflect the effectseverity of the merger shares exchange ratio, reverse stock splitrespective diseases, which include solid tumors, sepsis and change to the authorized number of ordinary shares in accordance with Accounting Standards Codification Topic 260, “Earnings Per Share”.others.
Financing During First Quarter 20205.A. Operating Results
On February 26, 2020, we consummated a registered direct offering to certain institutional investors of 1,000,000 ordinary shares, at a purchase price of $8.00 per share, for aggregate gross proceeds of $8.0 million (the “February 2020 offering”). Additionally, we issued to the placement agent in the February 2020 offering warrants to purchase up to 70,000 ordinary shares pursuant, which are exercisable at a price of $10.00 per ordinary share at any time during a period of five years from the issuance date.
On March 1, 2020, we consummated a second registered direct offering to certain institutional investors of 2,093,750 ordinary shares, together with warrants to purchase up to 2,093,750 of ordinary shares, at a combined purchase price of $8.00 per share and associated warrant to purchase one ordinary share, for aggregate gross proceeds of $16.75 million (the “March 2020 offering”). The investor warrants are exercisable at a price of $9.00 per ordinary share at any time during a period of two years from the issuance date. Additionally, we issued to the placement agent in the March 2020 offering warrants to purchase up to 146,563 ordinary shares, which are exercisable at a price of $10.00 per ordinary share at any time during a period of two years from the issuance date.
Financial Overview
Since inception, we have incurred significant losses in connection with our research and development and have not generated any revenue. We have funded our operations primarily through grants from the Israel Innovation AuthorityIIA and the sale of equity and equity linked securities in public and private offerings. As of December 31, 2019,2022, we had approximately $12$50.2 million in cash and cash equivalents and short-term bank deposits and had an accumulated deficit of approximately $26 million. As described above, during February$83 million, see “—Liquidity and March 2020 we consummated two registered-direct equity offerings for aggregate gross proceeds of $24.75 million.Capital Resources” below.
Although we provide no assurance, weWe currently believe that suchour existing funds and the proceeds of our recently consummated registered direct offerings will be sufficient to continue our business and operations as currently conducted through the fourth quarter of 2022.2024. During 2022, we introduced a Company-wide cost cutting program designed to extend our cash runway potentially into 2025. We expect that we will continue to incur operating losses, which may be substantial over the next several years, and we may need to obtain additional funds to further develop our research and development programs.
Revenue
We have not generated any revenue since our inception. To date, we have funded our operations primarily through grants from the Israel Innovation AuthorityIIA and the sale of equity and equity linked securities in public and private offerings. Our ability to generate revenue and become profitable depends upon the clinical success of our product candidates, regulatory approvals and our ability to successfully commercialize products.
Costs and Operating Expenses
Our current costs and operating expenses consist of two components: (i) research and development expenses; and (ii) general and administrative expenses.
Research and Development Expenses
Our research and development expenses consist primarily of research and development activities at our laboratory in Israel, including drug and laboratory supplies and costs for facilities and equipment, outsourced development expenses, including the costs of regulatory consultants and certain other service providers, salaries and related personnel expenses (including stock based compensation) and fees paid to external service providers patent-related legal fees and the costs of preclinical studies and clinical trials. We charge all research and development expenses to operations as they are incurred. We expect our research and development expenses to remain our primary expenses in the near future as we continue to develop our product candidates. Increases or decreases in research and development expenditures are attributable to the number and duration of our preclinical and clinical studies.
We expect that a large percentage of our research and development expenses in the future will be incurred in support of our current and future preclinical and clinical development projects. Due to the inherently unpredictable nature of preclinical and clinical development processes, we are unable to estimate with any certainty the costs we will incur in the continued development of our product candidates in our pipeline for potential commercialization. Furthermore, although we expect to obtainapply for additional grants from the Israel Innovation Authority,IIA, we cannot be certain that we will do so.obtain such grants. Clinical development timelines, the probability of success and development costs can differ materially from expectations. We expect to continue to test our product candidates in preclinical studies for toxicology, safety and efficacy and to conduct additional clinical trials for our product candidates.
While we are currently focused on advancing our product development, our future research and development expenses will depend on the clinical success of our product candidates, as well as ongoing assessments of each candidate’s commercial potential. As we obtain results from clinical trials, we may elect to discontinue or delay clinical trials for our product candidates in certain indications in order to focus our resources on more promising indications for any such product candidate. Completion of clinical trials may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate.
We expect our research and development expenses to increase in the future as we continue the advancement of our clinical product development for our current indications and as we potentially pursue additional indications. The lengthy process of completing clinical trials and seeking regulatory approval for our product candidates requires the expenditure of substantial resources. Any failure or delay in completing clinical trials, or in obtaining regulatory approvals, could cause a delay in generating product revenue and cause our research and development expenses to increase and, in turn, have a material adverse effect on our operations.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation (including stock-based compensation) for employees in executive and operational roles, including accounting, finance, investor relations, information technology and human resources. Our other significant general and administrative expenses include facilities costs, professional fees for outside accounting and legal services, including legal work in connection with patent applications, travel costs and insurance premiums.
We expect that our general and administrative expenses suchwill increase over time, as accounting and legal fees, to increase having completed the Merger, and we currently expect increases in the number of our executive, accounting and administrative personnel due to our anticipated growth.
Financial Expenses
Other income (expenses), net
Our financial expenses consist
Other income (expenses), net consists of bank fees, exchange rate differences and gains and losses resulting from our investments in marketable securities.
Other Comprehensive Income (Loss)
Our functional currency is the U.S. dollar because the U.S. dollar is the currency of the primary economic environment in which we operate and expect to continue to operate for the foreseeable future. Monetary assets and liabilities denominated in foreign currencies, including in NIS, were translated into U.S. dollars using exchange rates in effect at the balance sheet date. Balances related to non-monetary assets and liabilities are based on translated amounts as of the date of the change, and non-monetary assets acquired, and non-monetary liabilities incurred after September 30, 2021 (the date on which Enlivex R&D’s functional currency changed from NIS to U.S. dollars) have been translated at the approximate exchange rate prevailing at the date of the particular transaction. Transactions included in the statement of operations and comprehensive loss until September 30, 2021 were translated at average exchange rates during the applicable period, and transactions after September 30, 2021 were translated at the approximate exchange rate in effect at the time of the particular transaction. Gains or losses resulting from translation adjustments until September 30, 2021 are reported in other comprehensive gain.
Results of Operations
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
The table below provides our results of operations for the years ended December 31, 2022 and 2021:
| Year Ended December 31 | ||||||||
| 2022 | 2021 | |||||||
| (In thousands, except per share data) | ||||||||
| Research and development expenses, net | $ | 18,693 | $ | 12,881 | ||||
| General and administrative expenses | 7,104 | 6,407 | ||||||
| Operating loss | (25,797 | ) | (19,288 | ) | ||||
| Other income (expenses), net | (5,263 | ) | 4,820 | |||||
| Operating loss post- other income (expenses), net | (31,060 | ) | (14,468 | ) | ||||
| Taxes on income | ||||||||
| Net loss | (31,060 | ) | (14,468 | ) | ||||
| Other comprehensive income | - | 124 | ||||||
| Total comprehensive loss | $ | (31,060 | ) | $ | (14,344 | ) | ||
| Basic loss per share | $ | (1.69 | ) | $ | (0.81 | ) | ||
| Diluted loss per share | $ | (1.69 | ) | $ | (0.81 | ) | ||
Research and Development Expenses
For the years ended December 31, 2022 and 2021, we incurred research and development expenses in the aggregate of $18,693,000 and $12,881,000, respectively. The increase of $5,812,000, or 45%, in research and development expenses for 2022 as compared to 2021 was primarily due to a $1,317,000 increase in salaries, as a result of hiring additional R&D personnel, and certain salary increases for existing personnel, a $3,708,000 increase in expenses for pre-clinical studies and clinical studies, a $539,000 increase in consumption of materials due to an increase in the number of AllocetraTM doses that were manufactured and inventoried, and a $576,000 increase in lease payments and overhead expenses with respect to the lease of our plant space used for the manufacture of AllocetraTM to support ongoing clinical trials (as described above, our planned move into this plant space has been deferred for an approximate period of three years), offset by a $334,000 decrease in stock-based compensation expense with respect to equity granted to employees and consultants and a $187,000 increase of grants from the IIA for an approved clinical development program for the prevention of cytokine storm and organ dysfunction associated with financial derivative liabilities.sepsis.
FinancialGeneral and Administrative Expenses
For the years ended December 31, 2022 and 2021, we incurred general and administrative expenses in the aggregate of $7,104,000 and $6,407,000, respectively. The increase of $697,000, or 11%, in general and administrative expenses for 2022 as compared to 2021 was primarily due to a $151,000 increase in salaries, as a result of hiring additional personnel, and certain pay raises for existing personnel, a $132,000 increase in stock-based compensation expense with respect to equity granted to employees and directors, a $214,000 increase in professional fees, a $112,000 increase in insurance expenses, and a $163,000 increase resulting from intellectual property regulatory expenses, offset by a $487,000 decrease in compensation to directors.
Operating Loss
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenue since our inception, for the year ended December 31, 2022, our operating loss was $25,797,000, as compared to $19,288,000 for the year ended December 31, 2021, representing an increase of $6,509,000, or 34%.
Other Income (Expenses), Net
Our financialOther income (expenses), net consists of interestthe following:
| ● | Interest earned on our cash and cash equivalents; |
| ● | Expenses or income resulting from fluctuations of the NIS and Euro, in which a portion of our assets and liabilities are denominated, against the U.S. dollar; and | |
● | Realized and unrealized gains and losses from marketable equity securities. | |
For the years ended December 31, 2022 and 2021, we recorded other income on deposits, exchange rate differences(expenses), net of $(5,263,000) and income on$4,820,000, respectively. The increase in other expenses, net for the year ended December 31, 2022 as compared to the year ended December 31, 2021 was mainly due to a $1,983,000 loss from changes in the fair value of financial derivative liabilities.marketable equity securities compared to income of $5,590,000 from changes in the fair value of marketable equity securities for the year ended December 31, 2021, as well as $4,101,000 exchange differences expenses resulting from currency fluctuations mainly on cash and cash equivalents and deposits denominated in NIS compared to $562,000 of expenses for the year ended December 31, 2021.
Net Loss
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenue since our inception, for the year ended December 31, 2022, our net loss was $31,060,000, as compared to our net loss of $14,468,000 for the year ended December 31, 2021, representing an increase of $16,592,000, or 115%. This increase in loss resulted primarily from a $6,084,000 non-operating expense associated with a change in fair value of marketable securities and currency fluctuations on cash and cash equivalents and deposits denominated in NIS for 2022 as compared to a non-operating gain of $5,028,000 for 2021, together representing $11,112,000, or 67%, of the increase in net loss.
Other Comprehensive Income (Loss)
Our functional currency is the U.S. dollar because the U.S. dollar is the currency of the primary economic environment in which we operate and expect to continue to operate for the foreseeable future.
As of October 1, 2021, the U.S. dollar became the functional currency of Enlivex R&D; therefore, for 2022, we did not record any other comprehensive income (loss) from exchange rate differences arising from the translation of our subsidiary’s financial statements from its functional to its presentation currency.
Year Ended December 31, 2021 Compared to Year Ended December 31, 2020
The table below provides our results of operations for the years ended December 31, 2021 and 2020:
| Year Ended December 31 | ||||||||
| 2021 | 2020 | |||||||
| (In thousands, except per share data) | ||||||||
| Research and development expenses | $ | 12,881 | $ | 6,086 | ||||
| General and administrative expenses | 6,407 | 3,699 | ||||||
| Operating loss | (19,288 | ) | (9,785 | ) | ||||
| Financial income (expenses), net | 4,820 | (2,039 | ) | |||||
| Operating income (loss) post-finance expense & other income, net | (14,468 | ) | (11,824 | ) | ||||
| Taxes on income | - | |||||||
| Net income (loss) | (14,468 | ) | (11,824 | ) | ||||
| Other comprehensive income (loss) | 124 | 2,277 | ||||||
| Total comprehensive income (loss) | $ | (14,344 | ) | $ | (9,547 | ) | ||
| Basic income (loss) per share | $ | (0.81 | ) | $ | (0.90 | ) | ||
| Diluted income (loss) per share | $ | (0.81 | ) | $ | (0.90 | ) | ||
Research and Development Expenses
For the years ended December 31, 2021 and 2020, we incurred research and development expenses in the aggregate of $12,881,000 and $6,086,000, respectively. The increase of $6,795,000, or 112%, in research and development expenses for 2021 as compared to 2020 was primarily due to a $1,871,000 increase in salaries as a result of hiring additional R&D personnel and certain salary increases for existing personnel, an $809,000 increase in stock-based compensation expense with respect to equity granted to employees and consultants, a $3,012,000 increase in expenses for pre-clinical studies, clinical studies and consumption of materials, and a $195,000 decrease of grants from the IIA for an approved clinical development program for the prevention of cytokine storm and organ dysfunction associated with sepsis and COVID-19.
General and Administrative Expenses
For the years ended December 31, 2021 and 2020, we incurred general and administrative expenses in the aggregate of $6,407,000 and $3,699,000, respectively. The increase of $2,708,000, or 73%, in general and administrative expenses for 2021 as compared to 2020 was primarily due to a $401,000 increase in salaries, a $306,000 increase in compensation to directors, a $1,435,000 increase in stock-based compensation expense with respect to equity granted to employees and directors and a $297,000 increase in insurance expenses.
Operating Loss
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenue since our inception, for the year ended December 31, 2021, our operating loss was $19,288,000, as compared to $9,785,000 for the year ended December 31, 2020, representing an increase of $9,503,000, or 97%.
Other Income (Expenses), Net
Other income (expenses), net consists of the following:
| ● | Interest earned on our cash and cash equivalents; |
| ● | Expenses or income resulting from fluctuations of the U.S. dollar and Euro, in which a portion of our assets and liabilities are denominated, against the NIS for the period from January 1, 2021 through September 30, 2021; and |
| ● | Expenses or income resulting from fluctuations of the NIS and Euro, in which a portion of our assets and liabilities are denominated, against the U.S. dollar for the period from October 1, 2021 through December 31, 2021; and |
| ● | Realized and unrealized gains from marketable securities. |
For the years ended December 31, 2021 and 2020, we recorded net other income (expenses) of $4,820,000 and $(2,039,000), respectively. The increase in net other income for the year ended December 31, 2021 as compared to net other expenses for the year ended December 31, 2020 was mainly due to unrealized gains from an increase in the fair value of marketable securities of $4,599,000 and realized gains on equity securities of $991,000 for the year ended December 31, 2021 and decrease in currency fluctuations expenses on cash and cash equivalents and deposits denominated in NIS of $1,696,000.
Net Loss
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenue since our inception, for the year ended December 31, 2021, our net loss was $14,468,000, as compared to our net loss of $11,824,000 for the year ended December 31, 2020, representing an increase of $2,644,000, or 22%.
Other Comprehensive Income (Loss)
Our functional currency is the U.S. dollar because the U.S. dollar is the currency of the primary economic environment in which we operate and expect to continue to operate for the foreseeable future.
The functional currency of Enlivex R&D was the NIS. We reassessed Enlivex R&D’s functional currency and determined that, from September 30, 2021, the U.S. dollar to be the functional currency of Enlivex R&D. Gain resulting from translation adjustments until September 30, 2021 is reported in other comprehensive gain.
As a result of 0.44% increase in the U.S. dollar against the NIS for the nine months ended September 30, 2021, as compared to a decrease of 7% in the twelve-month period ended December 31, 2020, we recorded income of $124,000 from exchange rate differences arising from translating financial statements from functional to presentation currency, as compared to income of $2,277,000 for the twelve-month period ended December 31, 2020.
Cash Flows
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
For the years ended December 31, 2022 and 2021, net cash used in operations was$23,953,000 and $17,542,000, respectively. The increase in net cash used in operations for 2022 was primarily due to an increase in research and development expenses and general and administrative expenses as a result of increases in salaries, expenses for clinical studies, pre-clinical studies and consumption of materials, lease payments, professional services fees and insurance expenses.
For the years ended December 31, 2022 and 2021, net cash provided by (used in) investing activities was $62,524,000 and $(38,408,000), respectively. The increase in net cash provided by investing activities for 2022 as compared to 2021 resulted primarily from net cash proceeds on sales of marketable securities of $60,941,000 in 2022, as compared to net cash investments on purchases of marketable securities of $56,813,000, in 2021. In addition, we incurred a non-recurring expense of $6,960,000 related to the construction of our new manufacturing facility.
For the years ended December 31, 2022 and 2021, net cash provided by financing activities was $150,000 and $60,982,000, respectively. This decrease in cash provided by financing activities for 2022 as compared to 2021 resulted primarily from net proceeds of $53,174,000 from our issuance of ordinary shares and warrants in an offering in February 2021 and proceeds of $7,702,000 from the exercise of warrants in 2021.
5.B. Liquidity and Capital Resources
We have incurred substantial losses since our inception. As of December 31, 2022, we had an accumulated deficit of approximately $83 million and working capital (current assets less current liabilities) of approximately $45.7 million. We expect to incur losses from operations for the foreseeable future, and we expect to incur increasing research and development expenses, including expenses related to the hiring of personnel, conducting preclinical studies and clinical trials and outsourcing of certain development activities. We expect that general and administrative expenses will also increase as we expand our finance and administrative staff and add infrastructure.
Developing product candidates, conducting clinical trials and commercializing products are expensive, and we will need to raise substantial additional funds to achieve our strategic objectives. We believe that our existing cash resources will be sufficient to fund our projected cash requirements approximately through the fourth quarter of 2024. During 2022, we introduced a Company-wide cost cutting program designed to extend our cash runway potentially into 2025. Nevertheless, we will require significant additional financing in the future to fund our operations, including if and when we progress into additional clinical trials, obtain regulatory approval for any of our product candidates and commercialize the same. We believe that we will need to raise significant additional funds before we have any cash flow from operations, if at all. Our future capital requirements will depend on many factors, including:
| ● | the progress and costs of our preclinical studies, clinical trials and other research and development activities; |
| ● | the scope, prioritization and number of our clinical trials and other research and development programs; |
| ● | the amount of revenues and contributions we receive under future licensing, development and commercialization arrangements with respect to our product candidates; |
| ● | the costs of the development and expansion of our operational infrastructure; |
| ● | the costs and timing of obtaining regulatory approval for our product candidates; |
| ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; |
| ● | the costs and timing of securing manufacturing arrangements for clinical or commercial production; |
| ● | the costs of contracting with third parties to provide sales and marketing capabilities for us; |
| ● | the costs of acquiring or undertaking development and commercialization efforts for any future products, product candidates or platforms; |
| ● | receipt of additional government grants; |
| ● | the magnitude of our general and administrative expenses; and |
| ● | any cost that we may incur under future in- and out-licensing arrangements relating to our product candidates. |
Other than under our ATM Agreement (as defined and described below), we currently do not have any agreements for future external funding. In the future, we will need to raise additional funds, and we may decide to raise additional funds even before we need such funds if the conditions for raising capital are favorable. Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through debt or equity financings, credit facilities or by out-licensing applications of our product candidates. The sale of equity, including under our ATM Agreement, or convertible debt securities may result in dilution to our existing shareholders. The incurrence of indebtedness would result in increased fixed obligations and could also subject us to covenants that restrict our operations. We cannot be certain that additional funding, whether through grants from the IIA, financings, credit facilities or out-licensing arrangements, will be available to us on acceptable terms, if at all. If sufficient funds are not available, we may be required to delay, reduce the scope of or eliminate research or development plans for, or commercialization efforts with respect to, one or more applications of our product candidates, or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain potential products that we might otherwise seek to develop or commercialize independently.
Financing During 2022
On December 30, 2022 we entered into an agreement (the “ATM Agreement”) with Cantor Fitzgerald & Co. and JMP Securities LLC (each referred to as an “Agent”, and together, the “Agents”), as sales agents, pursuant to which we may elect to sell, but are not obligated to sell, ordinary shares having an aggregate offering price of up to $100,000,000 from time to time through the Agents. Our offer and sale of ordinary shares under the ATM Agreement may be made in transactions deemed to be “at-the-market” offerings as defined in Rule 415 under the Securities Act, including sales made directly on or through the Nasdaq Capital Market, or any other existing trading market in the United States for the ordinary shares, sales made to or through a market maker other than on an exchange or otherwise, directly to an Agent as principal, in negotiated transactions, or in any other method permitted by law, which may include block trades. We have agreed to pay the Agents an aggregate commission of 3.0% of the gross sales price from each sale of ordinary shares under the ATM Agreement. Any sale of ordinary shares under the ATM Agreement will be made pursuant to our effective shelf registration statement on Form F-3, including the prospectus contained therein (File No. 333-264561).
Financing During 2020 and 2021
On October 22, 2020 we and H.C. Wainwright & Co., LLC (“Wainwright”) entered into a sales agreement (the “Former ATM Agreement”), pursuant to which we could elect to sell, from time to time, through Wainwright as sales agent, up to an aggregate of $25 million of our ordinary shares in “at-the-market” offerings under our prior registration statement on Form F-3. We received aggregate gross proceeds of approximately $8,557,000 from the sale of 476,983 ordinary shares pursuant to the Former ATM Agreement prior to terminating such agreement on December 19, 2022.
On February 9, 2021, we entered into an amended and restated underwriting agreement with Wainwright with respect to our offer, issuance and sale (the “February 2021 offering”) of an aggregate of 2,296,107 ordinary shares, together with an option granted to Wainwright to purchase up to 344,416 additional ordinary shares in a registered offering under our prior registration statement on Form F-3. The ordinary shares were offered to the public at a price of $20.00 per share.
We issued an aggregate of 2,564,312 ordinary shares in the February 2021 offering (including 268,205 ordinary shares issued pursuant the partial exercise by Wainwright of its option to purchase additional ordinary shares). We paid Wainwright underwriting discounts and commissions equal to 7% of the gross proceeds from the sale of ordinary shares to the public in the February 2021 offering, as well as a management fee equal to 1% of the gross proceeds from the sale of the ordinary shares in the February 2021 offering. In addition, we issued to Wainwright or its designees 179,501 warrants to purchase ordinary shares. Such warrants are exercisable for five years from the commencement of the February 2021 offering and have an exercise price of $25 per ordinary share, subject to customary adjustments. We also reimbursed Wainwright approximately $126,000 for various expenses.
During February 2021, 855,813 of our previously issued and outstanding warrants were exercised for an aggregate of 855,813 ordinary shares, at a price of $9.00 per share, providing us with aggregate gross proceeds of $7,702,000.
Contractual Obligations
The following table summarizes our significant contractual obligations at December 31, 2022.
| Total | Less than 1 year | 1 – 3 years | 3 – 5 years | More than 5 Years | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating Lease Obligations | $ | 5,531 | 788 | 1,384 | 1,186 | 2,173 | ||||||||||||||
| Other Long-Term Contingent Liabilities | 9,200 | - | - | - | 9,200 | |||||||||||||||
| Total | $ | 14,731 | 788 | 1,384 | 1,186 | 11,373 | ||||||||||||||
Quantitative and Qualitative Disclosure About Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position, results of operations or cash flows due to adverse changes in financial market prices and rates, including interest rates and foreign exchange rates, of financial instruments. Our market risk exposure is primarily a result of foreign currency exchange rates. As of December 31, 2022 $25,763,000 of our net assets (i.e., total assets net of total liabilities) were denominated in NIS while our presentationfunctional currency is the U.S. dollar. GainsChanges of 5% in the U.S. dollar against the NIS exchange rate will increase/decrease our expenses by $1,288,000.
Interest Rate Risk
We are currently not exposed to significant interest rate risk. Our only interest-bearing financial assets are principally of cash and cash equivalents and restricted cash, which are invested in major banks in Israel. Given the short-term nature of these investments, we do not believe our sensitivity with respect to interest rate fluctuations is significant. Therefore, the effect of an increase or losses resultingdecrease in interest rates would only have an immaterial effect on our financial results.
Foreign Currency Exchange Risk
Our foreign currency exposures give rise to market risk associated with exchange rate movements of the NIS mainly against the U.S. dollar, and vice versa, because a considerable portion of our expenses are denominated in NIS. Our NIS expenses consist principally of payments made to employees, subcontractors and consultants for preclinical studies, clinical trials and other research and development activities. We anticipate that a sizable portion of our expenses will continue to be denominated in NIS. Our financial position, results of operations and cash flow are subject to fluctuations due to changes in foreign currency exchange rates. Our results of operations and cash flow are, therefore, subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates.
As of December 31, 2022, we were not engaged in foreign currency hedging transactions. In the future, we may enter into formal currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currency. These measures, however, may not adequately protect us from the translation frommaterial adverse effects of such fluctuations.
5.C. Research and Development, Patents and Licenses, Etc.
For our functional currencyresearch and development efforts, see Item 4.B. “Business Overview.” For information regarding our patents and proprietary rights, see Item 4.B. “Business Overview––Intellectual Property and Patents and Proprietary Rights.” For information regarding our license agreements, see Item 4.B. “Business Overview––License Agreements.”
5.D. Trend Information
We are a clinical-stage macrophage reprogramming immunotherapy company, and it is not possible for us to predict with any degree of accuracy the outcome of our presentation currencyresearch, development or commercialization efforts. As such, it is not possible for us to predict with any degree of accuracy any known trends, uncertainties, demands, commitments or events that are recognizedreasonably likely to have a material effect on our financial condition, including our liquidity and capital resources, or that would cause reported financial information to not necessarily be indicative of future operating results or financial conditions. Our results of operations and financial condition may be affected by various trends and factors discussed in other comprehensive income (loss).Item 3.D. “Risk Factors,” Item 4 “Information on The Company” and elsewhere in this Item 5 “Operating and Financial Review and Prospects.”
5.E. Critical Accounting Policies and EstimateEstimates
The preparation of financial statements in accordance with United States generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Management bases its estimates on historical experience, market and other conditions, and various other assumptions it believes to be reasonable. Although these estimates are based on management’s best knowledge of current events and actions that may impact us in the future, the estimation process is, by its nature, uncertain given that estimates depend on events over which we may not have control. If market and other conditions change from those that we anticipate, our financial statements may be materially affected. In addition, if our assumptions change, we may need to revise our estimates, or take other corrective actions, either of which may also have a material effect in our financial statements. We review our estimates, judgments, and assumptions used in our accounting practices periodically and reflect the effects of revisions in the period in which they are deemed to be necessary. We believe that these estimates are reasonable; however, our actual results may differ from these estimates.
While our significant accounting policies are described in more detail in the notes to our financial statements appearingcontained elsewhere in this Annual Report on Form 20-F, we believe the following accounting policies to be the most critical to the judgments and estimates used in the preparation of our financial statements.
Share-BasedStock-Based Compensation
We have issued restricted stock units and Fair Value of Ordinary Shares
ASC 718 - “Compensation-stock Compensation”- (“ASC 718”) requires companiesoptions to estimate the fair value of equity-based payment awards on the date of grant using an option pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods.
We estimate the fair value of our share-based awards to employees and non-employees using Black-Scholes, which requires the input of assumptions, some of which are highly subjective, including:
Until the closing of the Merger, there was no active external or internal market forpurchase our ordinary shares duringshares. Stock-based compensation cost is measured at the periods presented in our audited financial statements contained elsewhere in this Annual Reportgrant date based on Form 20-F. Thus, it was not possible to estimate the expected volatility of our share price in estimating fair value of options granted. Accordingly, as a substitute for such volatility, we used the historical volatility of comparable companies in the industry. The expected term of options granted represents the period of time that options granted are expected to be outstanding, we use management’s estimates for the expected term of options due to insufficient readily available historical exercise data.
Compensation expense for options granted to non-employees is determined in accordance with the standard as the fair value of the consideration received oraward and is recognized as expense over the fair value of the equity instruments issued, whichever is more reliably measured. Compensation expense for awards granted to non-employees is re-measured eachrequisite service/vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based payment awards require the stock-based compensation requires the inputuse of highly subjective assumptions, including the expected life of the stock-based payment awards and stock price volatility andvolatility.
We estimate the grant date fair value of stock options and the shares upon measurement date.
Valuationrelated compensation expense, using the Black-Scholes option valuation model. This option valuation model requires the input of Warrant Liability
Our outstanding warrants issued in September and October 2017 are classified as derivative liabilities as it permits net settlement. The warrants are valued at each financial reportingsubjective assumptions including: (1) expected life (estimated period using option pricing models which incorporateof time outstanding) of the Company’s stock price,options granted, (2) volatility, U.S.(3) risk-free rate dividend rate, and estimated life, with changes(4) dividends. In general, the assumptions used in fair value being recognized as profit of loss.
Results of Operations
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
The table below provides our results of operations for the years ended December 31, 2019 and December 31, 2018:
| Year Ended December 31 | ||||||||
| 2019 | 2018 | |||||||
| (In thousands, except per share data) | ||||||||
| Research and development expenses | $ | 5,724 | $ | 4,255 | ||||
| General and administrative expenses | 2,895 | 1,044 | ||||||
| Operating loss | (8,619 | ) | (5,299 | ) | ||||
| Financial income (expenses), net | (765 | ) | 1,057 | |||||
| Operating income (loss) post-finance expense & other income, net | (9,384 | ) | (4,242 | ) | ||||
| Taxes on income | - | - | ||||||
| Net income (loss) | (9,384 | ) | (4,242 | ) | ||||
| Other comprehensive income (loss) | 951 | (748 | ) | |||||
| Total comprehensive income (loss) | $ | (8,433 | ) | $ | (4,990 | ) | ||
| Basic income (loss) per share | $ | (1.11 | ) | $ | (1.4 | ) | ||
| Diluted income (loss) per share | $ | (1.11 | ) | $ | (1.4 | ) | ||
Research and Development Expenses
For the years ended December 31, 2019 and 2018, we incurred research and development expenses in the aggregate of $5,724,000 and $4,255,000, respectively. The increase of $1,469,000, or 34%, in research and development expenses for 2019 as compared to 2018 was primarily due to a $942,000 increase in R&D salaries and a $1,808,000 increase in preclinical and clinical studies and consumption of materials, offset by a $255,000 decrease in stock-based compensation to employees and to consultants, and a $1,082,000 increase in Israel Innovation Authority grants relating to a 50% participation of an approved clinical development program of the Company for the prevention of cytokine storm and organ dysfunction associated with sepsis.
General and Administrative Expenses
For the years ended December 31, 2019 and 2018, we incurred general and administrative expenses in the aggregate of $2,895,000 and $1,044,000, respectively. The increase of $1,851,000, or 177%, in general and administrative expenses for 2019 as compared to 2018 was primarily due to a $382,000 increase in withholding tax provision on preferred share dividends (all such preferred shares were converted to ordinary shares in connection with the Merger), a $226,000 increase in salaries, a $344,000 increase in compensation to directors, a $517,000 increase in professional fees, a $232,000 increase in insurance expenses and a $56,000 increase in stock-based compensation to employees and directors.
Operating Loss
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenues since our inception, for year ended December 31, 2019, our operating loss was $8,619,000, representing an increase of $3,320,000, or 63%, as compared to our operating loss for the year ended December 31, 2018.
Financial (Expenses) Income, Net
Financial expenses, net and income, net consist of the following:
For the years ended December 31, 2019 and 2018, we recorded net financial (expenses) income of $(765,000) and $1,057,000, respectively. The increase in financial expenses for the year ended December 31, 2019 as compared to the year ended December 31, 2018 was primarily due to currency fluctuations on cash and cash equivalents and deposits denominated in currencies other than the New Israeli Shekels, changes incalculating the fair value of stock-based payment awards represent management’s best estimates, but the warrants we granted to HBL (See Item 7.B. – “Related party transactions” – “Certain Relationshipsestimates involve inherent uncertainties and Related Party Transactions” – “Agreement with HBL – Hadasit Bio-Holdings Ltd.”) and from interest earned on our cash and cash equivalents.the application of management judgment.
Net Loss
Leases
We determine if an arrangement includes a lease at inception. Right-of-use assets represent our right to use an underlying asset for the lease term; and lease liabilities represent our obligation to make lease payments arising from the lease. Right-of-use assets and lease liabilities are recognized at the commencement date of the lease, renewal date of the lease or significant remodeling of the lease space based on the present value of the remaining future minimum lease payments. Leases with a term greater than one year are recognized on the balance sheet as right-of-use assets and short-term and long-term lease liabilities, as applicable.
Operating lease liabilities and their corresponding right-of-use assets are initially recorded based on the present value of lease payments over the expected remaining lease term. The interest rate implicit in lease contracts is typically not readily determinable. As a result, we utilize our incremental borrowing rate to discount lease payments, which reflects the fixed rate at which we could borrow on a collateralized basis the amount of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenues since our inception, for the year ended December 31, 2019, our net loss was $9,384,000, representing an increase of $5,142,000 as compared to our net loss for the comparable prior year period.
Other Comprehensive Income (Loss)
As a result of a decrease of 7.8%lease payments in the U.S. dollar againstsame currency, for a similar term, in a similar economic environment.
Our leases include options to extend or terminate the NIS in twelve months ended December 31, 2019, as comparedlease. In determining the lease term, management uses its judgement to determine whether or not an increase of 8.1%option would be reasonably certain to be exercised. Management considers all facts and circumstances including their past practice and any cost that will be incurred to change the asset if an option to extend is not taken to help determine the lease term. Extension options are only included in the comparable prior year period, we recorded income of $951,000 from exchange rate differences arising from translating financial statements from functional to presentation currency, as compared to losses of $748,000 for the comparable prior year period.
Year Ended December 31, 2018 Compared to Year Ended December 31, 2017
The table below provides our results of operations for the year ended December 31, 2018 as compared to the year ended December 31, 2017.
| Year ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| (In thousands, except per share data) | ||||||||
| Research and development expenses | $ | 4,255 | $ | 1,691 | ||||
| General and administrative expenses | 1,044 | 480 | ||||||
| Operating loss | (5,299 | ) | (2,171 | ) | ||||
| Financial income (expenses), net | 1,057 | (333 | ) | |||||
| Operating income (loss) post-finance expense & other income, net | (4,242 | ) | (2,504 | ) | ||||
| Taxes on income | - | - | ||||||
| Net income (loss) | (4,242 | ) | (2,504 | ) | ||||
| Other comprehensive income (loss) | (748 | ) | 336 | |||||
| Total comprehensive income (loss) | $ | (4,990 | ) | $ | (2,168 | ) | ||
| Basic loss per share | $ | (1.4 | ) | $ | (0.94 | ) | ||
| Diluted loss per share | $ | (1.4 | ) | $ | (0.94 | ) | ||
Research and Development Expenses
For the years ended December 31, 2018 and 2017, we incurred research and development expenses in the aggregate of $4,255,000 and $1,691,000, respectively. The increase of $2,564,000, or 152%, in research and development expenses for 2018 as compared to 2017 was primarily due to a $724,000 increase in R&D salaries, a $739,000 increase in stock-based compensation to employees and to consultants, and a $744,000 increase in preclinical studies and consumption of materials.
General and Administrative Expenses
For the years ended December 31, 2018 and 2017, we incurred general and administrative expenses in the aggregate of $1,044,000 and $480,000, respectively. The increase of $564,000, or 118%, in general and administrative expenses for 2018 as compared to 2017 was primarily due to a $160,000 increase in salaries, $138,000 increase in professional fees and a $149,000 increase in stock-based compensation to employees and directors.
Operating Loss
As a result of the foregoing research and development and general and administrative expenses as well as our failure to generate revenues since our inception, for year ended December 31, 2018, our operating loss was $5,299,000, representing an increase of $3,128,000, or 144%, as compared to our operating loss for the year ended December 31, 2017. This increase primarily resulted from an increase in research and development salaries and stock-based compensation, the costs of preclinical studies and material consumption and an increase in accounting and legal expenses.
Financial Expenses, Net
For the years ended December 31, 2018 and 2017, we recorded net financial (expenses) income of $1,057,000 and $(333,000), respectively. The increase in financial income for the year ended December 31, 2018 as compared to the year ended December 31, 2017 was primarily due to currency fluctuations on cash and cash equivalents and deposits denominated in currencies other than the New Israeli Shekels, changes in the fair value of our warrants and from interest earned on our cash and cash equivalents.
Net Income (Loss)
As a result of the foregoing research and development and general and administrative expenses, as well as our failure to have generated revenues since our inception, for the year ended December 31, 2018, our net loss was $4,242,000, representing an increase of $1,738,000 as compared to our net loss for the comparable prior year period. The increase was primarily a result of increase in research and development activities.
Other Comprehensive Income (Loss)
As a result of an increase of 8% in the U.S. dollar against the NIS in twelve months ended December 31, 2018, as compared to a decrease of 10% in the comparable prior year period, we recorded losses of $748,000 from exchange rate differences arising from translating financial statements from functional to presentation currency, as compared to income of $336,000 for the comparable prior year period.
Cash Flows
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
For the years ended December 31, 2019 and 2018, net cash used in operations was $7,041,000 and $3,161,000, respectively. The increase in net cash used in operations for 2019, as compared to the comparable prior year period, was primarily due to an increase in research and development expenses as a result of increases in salaries, preclinical study expenses, consultants’ fees, as well as the commencement of clinical trials in 2019.
For the years ended December 31, 2019 and 2018, net cash used in investing activities was $6,359,000 and $501,000, respectively. The increase in net cash used in investing activities for 2019 as compared to 2018 resulted primarily from our investment in short-term bank deposits and from the purchase of property and equipment for our new GMP manufacturing facility in Israel offset by cash, cash equivalents and restricted cash received in the issuance of shares for the net assets of Bioblast Pharma Ltd..
For the years ended December 31, 2019 and 2018, net cash provided by financing activities was $8,306,000 and $5,194,000 respectively. This increase in cash provided by financing activities for 2019 as compared to 2018 resulted primarily from net proceeds of $7,707,000 from the issuance of ordinary shares in the private placement that closed concurrently with the closing of the Merger, as well as $599,000 of proceeds from the exercise of options.
Year Ended December 31, 2018 Compared to Year Ended December 31, 2017
For the years ended December 31, 2018 and 2017 net cash used in operations was $3,161,000 and $2,304,000, respectively. The increase in net cash used in operations for 2018 as compared to the comparable prior year period was primarily due to an increase in research and development expenses as a result of increases in salaries, preclinical study expenses, consultants’ fees, as well as preparation for commencement of clinical trials in 2019.
For the years ended December 31, 2018 and 2017, net cash used in investing activities was $501,000 and $130,000, respectively. The increase in net cash used in investing activities for 2018 as compared to 2017 resulted primarily from purchase of property and equipment for the new GMP manufacturing facility.
For the years ended December 31, 2018 and 2017, net cash provided by financing activities was $5,194,000 and $8,055,000 respectively. This decrease in cash provided by financing activities for 2018 as compared to 2017 resulted primarily from a capital raising transaction of Series C Preferred Shares in the net amount of $5,194,000 in 2018 as compared to a capital raising transaction of Series B Preferred Shares in the net amount of $8,055,000 in 2017. There are no preferred shares outstanding as of the date of this Annual Report on Form 20-F.
Liquidity and Capital Resources
We have incurred substantial losses since our inception. As of December 31, 2019, we had an accumulated deficit of approximately $25.6 million and working capital (current assets less current liabilities) of approximately $10.4 million. We expect to incur losses from operations for the foreseeable future, and we expect to incur increasing research and development expenses, including expenses related to the hiring of personnel, conducting preclinical studies and clinical trials and outsourcing of certain development activities. We expect that general and administrative expenses will also increase as we expand our finance and administrative staff and add infrastructure.
Developing product candidates, conducting clinical trials and commercializing products are expensive, and we will need to raise substantial additional funds to achieve our strategic objectives. We believe that our existing cash resources, due to the consummation of two registered direct equity offerings during the first quarter of 2020, will be sufficient to fund our projected cash requirements approximately through the fourth quarter of 2022. Nevertheless, we will require significant additional financing in the future to fund our operations, including if and when we progress into additional clinical trials, obtain regulatory approval for any of our product candidates and commercialize the same. We believe that we will need to raise significant additional funds before we have any cash flow from operations, if at all. Our future capital requirements will depend on many factors, including:
We currently do not have any commitments for future external funding. In the future, we will need to raise additional funds, and we may decide to raise additional funds even before we need such fundslease term if the conditions for raising capital are favorable. Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through debt or equity financings, credit facilities or by out-licensing applications of our product candidates. The sale of equity or convertible debt securities may result in dilution to our existing shareholders. The incurrence of indebtedness would result in increased fixed obligations and could also subject us to covenants that restrict our operations. We cannot belease is reasonably certain that additional funding, whether through grants from the Israel Innovation Authority, financings, credit facilities or out-licensing arrangements, will be available to us on acceptable terms, if at all. If sufficient funds are not available, we may be required to delay, reduce the scope of or eliminate research or development plans for, or commercialization efforts with respect to, one or more applications of our product candidates, or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain potential products that we might otherwise seek to develop or commercialize independently.
72
Contractual Obligations
The following table summarizes our significant contractual obligations at December 31, 2019.
| Total | Less than 1 year | 1 – 3 years | 3 - 5 years | More than 5 Years | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating Lease Obligations | $ | 525 | 172 | 353 | - | - | ||||||||||||||
| Purchase Obligations | $ | 575 | 575 | |||||||||||||||||
| Other Long-Term Contingent Liabilities | 5,197 | - | - | - | 5,197 | |||||||||||||||
| Total | $ | 6,297 | 747- | 353- | - | 5,197- | ||||||||||||||
Off-Balance Sheet Arrangements
We currently do not have any off-balance sheet arrangements that have had, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Quantitative and Qualitative Disclosure About Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position, results of operations or cash flows due to adverse changes in financial market prices and rates, including interest rates and foreign exchange rates, of financial instruments. Our market risk exposure is primarily a result of foreign currency exchange rates. As of December 31, 2019 and December 31, 2018, $12,131,896 and $9,374,000, respectively, of our net assets (i.e., total assets net of total liabilities) were denominated and presented in U.S. dollars while our functional currency is the NIS. Changes of 5% in the U.S. dollar against the NIS exchange rate will increase/decrease our expenses by $606,595 and $469,000, respectively.
Interest Rate Risk
We are currently not exposed to significant interest rate risk. Our only interest-bearing financial assets are principally of cash and cash equivalents and restricted cash, which are invested in major banks in Israel. Given the short-term nature of these investments, we do not believe our sensitivity with respect to interest rate fluctuations is significant. Therefore, the effect of an increase or decrease in interest rates would only have an immaterial effect on our financial results.
Foreign Currency Exchange Risk
Our foreign currency exposures give rise to market risk associated with exchange rate movements of the NIS mainly against the U.S. dollar, and vice versa, because most of our expenses are denominated in NIS and the U.S. dollar. Our NIS and U.S. dollar expenses consist principally of payments made to employees, sub-contractors and consultants for preclinical studies, clinical trials and other research and development activities. We anticipate that a sizable portion of our expenses will continue to be denominated in the NIS and U.S. dollar. Our financial position, results of operations and cash flow are subject to fluctuations due to changes in foreign currency exchange rates. Our results of operations and cash flow are, therefore, subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates.extended.
To date, we have not engaged in hedging our foreign currency exchange risk. In the future, we may enter into formal currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
6.A. Directors and executive officersExecutive Officers
The following table lists the names and positions of the current executive officers and directors of the Company. The business address for each of our directors, senior management and executive officers is c/o Enlivex Therapeutics Ltd., 14 Einstein Street, NesNess Ziona, Israel 7403618.
| Name | Age | Position | ||
| Shai Novik, MBA | Executive Chairman of the Board | |||
| Roger Pomerantz, M.D. | 66 | Director and Vice Chairman of the Board | ||
| Oren | Chief Executive Officer | |||
| Prof. Dror Mevorach, M.D. | Chief Scientific & Medical Officer | |||
| Shachar Shlosberger, CPA | Chief Financial Officer | |||
| Abraham (Avri) Havron, Ph.D. | Director | |||
| Gili Hart, Ph.D. | Director | |||
| Director | ||||
| Director | ||||
Backgrounds of Current Executive Officers and Directors
Shai Novik ishas served as the Company’s Executive Chairman of the Board and has been such since 2014. Mr. Novik founded PROLOR Biotech, Inc. in 2005, and served as its President until 2014. PROLOR Biotech was listed on the NYSE MKT (N/K/A NYSE American) in 2010 and was sold in 2013 to OPKO Health, Inc. (“OPKO”) in one of the second largest biotech exitexits ($560 million) in the history of Israeli biotech. Mr. Novik has also served asbeen a member of the Chairmanboard of Innovsion LabsCortex Therapeutics, Inc., a neuroscience technology company, since 2007.2020 and of Innovision Labs Inc. since 2013. Mr. Novik previously served as Chief Operating Officer and Head of Strategic Planning of THCG, Inc., a technology and life sciences investment company. THCG was a portfolio company of Greenwich Street Partners, one of the largest U.S. private equity funds. THCG’s portfolio included several life sciences and medical devices companies. Mr. Novik received his M.B.A., with distinction, from Cornell University.
Roger Pomerantz, M.D. has served as a director and Vice Chairman of the Board of Directors since May 2022. Dr. Pomerantz has served as Chairman of the board of directors and Chief Executive Officer of ContraFect Corporation (Nasdaq: CFRX), a clinical-stage biotechnology company, since April 2019. Prior to that, Dr. Pomerantz served as Vice Chairman of the board of directors of ContraFect Corporation since May 2014. From November 2013 to December 2019, Dr. Pomerantz served as Chairman of the board of directors of Seres Therapeutics, Inc. (Nasdaq: MCRB), a biotechnology company, and as its President and Chief Executive Officer from June 2014 to January 2019. From 2011 to 2013, Dr. Pomerantz was formerly Worldwide Head of Licensing & Acquisitions, Senior Vice President at Merck & Co., Inc. where he oversaw all licensing and acquisitions at Merck Research Laboratories. Previously, Dr. Pomerantz served as Senior Vice President and Global Franchise Head of Infectious Diseases at Merck. Prior to joining Merck, Dr. Pomerantz was Global Head of Infectious Diseases for Johnson & Johnson Pharmaceuticals. Dr. Pomerantz joined Johnson & Johnson in 2005 as President of Tibotec Pharmaceuticals, Inc. Dr. Pomerantz serves as Chairman of the board of directors of the following public companies: Collplant Biotechnologies, Ltd. (Nasdaq: CLGN) since 2021, Indaptus Therapeutics, Inc. (Nasdaq: INDP) since 2021 and Viracta Therapeutics Inc (Nasdaq: VIRX) since 2020. Dr. Pomerantz also serves as Chairman of the board of directors of the private company Silicon Therapeutics Inc. since 2019, and a member of the board of the following private companies: X-VAX Technology, Inc. since 2019 and VerImmune since 2020. Previously, Dr. Pomerantz served on the board of directors of public companies Rubius Therapeutics from 2014 to 2019 and Evelo Therapeutics from 2015 to 2016. Dr. Pomerantz received his B.A. in Biochemistry at the Johns Hopkins University and his M.D. at the Johns Hopkins School of Medicine. Dr. Pomerantz received post-graduate training at the Massachusetts General Hospital, Harvard Medical School and M.I.T. Dr. Pomerantz is Board Certified in both Internal Medicine and Infectious Diseases. Dr. Pomerantz was Professor of Medicine, Biochemistry and Molecular Pharmacology, Chief of Infectious Diseases, and the Founding Director and Chair of the Institute for Human Virology and Biodefense at the Thomas Jefferson University and Medical School. Dr. Pomerantz has developed nine drugs approved world-wide in important diseases, including HIV, HCV, and tuberculosis.
Oren Hershkovitz Ph.D, has beenserved as the Company’s Chief Executive Officer since November 2019. Prior to that, Dr. Hershkovitz served for nearly a decade in managerial and executive roles at PROLOR Biotech, Inc., including following its acquisition by OPKO and change in name to OPKO Biologics, Ltd.Inc., acting as General Manager for more than 4.5 years. Dr. Hershkovitz successfully managed more than 70 employees and led manufacturing, non-clinical and clinical development for various Phase I, II and III programs, including for obesity, hemophilia and growth hormone deficiency. Dr. Hershkovitz earned his Ph.D. in immunology from the BGU of the Negev with distinction.
Prof. Dror Mevorach, M.D.,the Company’s founder, has beenserved as the Company’s Chief Scientific & Medical Officer since 2009. Prof. Mevorach is a leading scientist on the removal of apoptotic cells and the Co-Chair of the 2015 Apoptotic Cell Recognition and Clearance Gordon Research Conference at the University of New England in Maine. Prof. Mevorach is currently the Director of the Rheumatology Research Centre of Hadassah Hospital and a Senior Lecturer in Medicine at the Hebrew University of Jerusalem, Hadassah School of Medicine. Since 2009, Prof. Mevorach has managed the internal medicine department at Hadassah Hospital in Jerusalem. Prof. Mevorach published more than 112 scientific papers and frequently lectures frequently at international conferences. Prof. Mevorach earned his M.D. from The Technion – Israel Institute of Technology in Haifa, Israel.
74
Shachar Shlosberger, CPA., has served as the Company’s Chief Financial Officer of the Company since 2016, bringing with her more than 11 years of financial experience in the hi-tech and biotechnology industries. Prior to her position at the Company, Mrs. Shlosberger worked for 4four years at PROLOR Biotech, Ltd (NYSE-American: PBTH)Inc. as Finance Director where she was responsible for the overall financial operations in Israel and the US. Mrs.United States. Ms. Shlosberger is a Certified Public Accountant and holds a M.B.A. in Accounting and Business Administration from the College of Management in Israel.
Bernhard Kirschbaum, Ph.D., has been a Director of the Company since 2018. Dr. Kirschbaum served as Executive Vice President and a member of the board of directors at Merck Serono, and Head of Global Research & Early Development reporting to the Chief Executive Officer of Merck Serono from 2011 to 2013. He led a global team of more than 1,200 employees, with a 400 million Euro annual budget. Since then, he has served as a member of the board of directors of several biotechnology companies, including Redx Pharma Plc, Protagen Diagnostics, Omeicos Therapeutics GmbH, BioMedx, KAHR Medical, Ltd. and FutuRx. Dr. Kirschbaum has significant expertise in a broad range of disease areas, including rheumatology/immunology, thrombosis, cardiometabolic diseases, oncology and neurology. He has successfully participated in the profiling of several drugs in their course to the market or during market expansion, including Arava, Velcade, Lovenox, Erbitux and Avelumab. Dr. Kirschbaum led drug portfolio re-allocation with focus on the therapeutic areas: oncology, neurodegenerative diseases (MS, Alzheimers, Parkinsons), autoimmune and inflammatory diseases. Dr. Kirschbaum has also been involved in research activities with respect to fertility, mainly focusing on embryo technologies. He implemented the new Merck Serono research organization, including an exploratory medicine department and all non-clinical development functions (toxicology, general & safety pharmacology, Chemistry, Manufacturing and Control (CMC) development and Drug Metabolism and Pharmacokinetics (DMPK)). Previously, Dr. Kirschbaum was Vice President Discovery Research, Global Head of Thrombosis and Angiogenesis at Sanofi-Aventis; and Vice President, Drug Innovation and Approval at Sanofi-Aventis. Dr. Kirschbaum earned his Ph.D. in biochemistry, summa cum laude, from the University of Konstanz, Germany, was a postdoctoral fellow with Dr. R.G. Roeder, at the Rockefeller University in New York, and a Research Associate with Dr. M. Buckingham at Institut Pasteur in Paris.
Abraham (Avri) Havron, Ph.D.,has been a Directorserved as one of the CompanyCompany’s directors since 2014. Dr. Havron served as the Chief Executive Officer of PROLOR Biotech, Inc. from 2005 through 2013. Dr. Havron is a 35-year43-year veteran of the biotechnology industry and was a member of the founding team and Director of Research and Development of Interpharm Laboratories (then, a subsidiary of Serono, later acquired by Merck) from 1980 to 1987, and headed the development of the multiple sclerosis drug REBIF, with current sales of more than $1.5 billion annually. Dr. Havron served as Vice-President Manufacturing and Process-Development of BioTechnology General Ltd., from 1987 to 1999; and Vice President and Chief Technology Officer of Clal Biotechnology Industries Ltd. from 1999 to 2003. Dr. Havron’s managerial responsibilities included the co-development of several therapeutic proteins and other bio-pharmaceuticals currently in the market, including recombinant human growth hormone (BioTropin), recombinant Hepatitis B Vaccine (Bio-Hep-B), recombinant Beta Interferon (REBIF), recombinant human insulin and hyaluronic acid for ophthalmic and orthopedic applications. Dr. Havron earned his Ph.D. in Bio-Organic Chemistry from the Weizmann Institute of Science, and served as a Research Fellow in theat Harvard Medical School, Department of Radiology. Dr. Havron served as a director of Kamada Ltd. (KMDA) from 2010 to 2018.2018, and PamBio Ltd., a private biotech company, from 2016 to 2019. Dr. Havron also currently serves on the board of directors of Collplant HoldingsCollPlant Biotechnologies Ltd. (CLGN), which position he has held since 2016, and PamBio, a private biotech company.2016.
Gili Hart, Ph.D.,has been a Directorserved as one of the CompanyCompany’s directors since 2014. Dr. Hart previously held various positions at OPKO Biologics, (f.k.a.Inc. (f/k/a PROLOR Biotech)Biotech, Inc.) and led the pre-clinical, clinical and pharmacological activities there from 2008 until her move in 2018 to Mitoconix Bio Ltd., a biopharmaceutical company developing disease modifying therapies addressing unmet medical needs by improving mitochondrial health, where she currently serves as Chief Executive Officer. Dr. Hart was a research fellow in the Immunology Department of Yale University from 2005 to 2007 and a research fellow at the Immunology Department of the Weizmann Institute of Science in Israel. Dr. Hart currently serves as a member of the board of directors of Collplant Holdings Ltd. (CLGN), which position she has held since 2017. Dr. Hart received her Ph.D. with distinction from the Immunology Department of the Weizmann Institute of Science in Immunology, and a M.S. degree in Biotechnology Engineering, summa cum laude, from the Technion Institute in Israel. Dr. Hart has published numerous papers and patents, in each case focusing on autoimmunity disease and immune system activation.
75
Sangwoo Lee has been a Directorserved as one of the CompanyCompany’s directors since 2017. Mr. Lee has served as an Executive Director of the Investment Department at Korea Investment Partners Co. Ltd., the largest capital venture fund in Korea, since 2014 and headPresident of its U.S. branch since 2017. Korea Investment Partners Co. Ltd. is an affiliate of KIP Global Pharma Private Equity Fund, one of the Company’s major shareholders. HeMr. Lee is responsible for sourcing and evaluation of start-up companies, investment and participation in business development and growth expansion of the fund’s investments in the United States and Europe. Previously, from 2013 to 2014, Mr. Lee was General Manager of the MSC Department at Samsung Electronics, responsible for strategic and business planning; and from 2004 to 2013, Vice President, CTO & Foreign Marketing Group Leader at Polidigm Co. Ltd. Mr. Lee received his B.Sc. and M.Sc. from Seoul National University, Department of Control and Instrumentation.
Hyun Gyu Lee M.D, Ph.D has beencurrently serves as a Directormember of the Companyboard of directors of Celleron Therapeutics since 2017.2016, Pavilion Data Systems Inc. since 2018, Prokarium Ltd. since 2018, Atrogi AB since 2019, PDC*Line Pharma since 2019, and Geneos Therapeutics since 2020. Additionally, Mr Lee served as a member of the board of Directors of Elastagen Pty, Ltd. from 2015 to 2017, KAHR Medical Ltd. from 2015 to 2019 (observer since 2020), and SecuLetter from 2019 to 2021. Mr. Lee currently serves as a board observer of Global Kinetics Pty, Ltd since 2018, and Elicio Therapeutics since 2020, and served as a board observer of Lyncean Technologies from 2018 to 2022, Vaccitech Limited from 2018 to 2021, and Tapas Media from 2019 to 2021.
Brian Schwartz, M.D.has served as an Executive Director, Investment Division, of Korea Investment Partners Co. Ltd., the largest venture capital fund in Korea, since 2016. Korea Investment Partners Co. Ltd. is an affiliate of KIP Global Pharma Private Equity Fund, one of the Company’s major shareholders. Hedirectors since December 2020. Dr. Schwartz has wide-ranging experience as a drug development expert in pharmaceutical and biotechnology industries, spanning several therapeutic areas including oncology, hematology, dermatology, neurology and rare diseases. During the past decade he has served as Senior Vice President, Head of Research & Development and Chief Medical Officer of ArQule Inc., which was from 2011acquired for $2.7 billion by Merck & Co. in 2020. Prior to 2016 Research Assistant Professor withArQule, Dr. Schwartz was CMO at ZIOPHARM Oncology Inc. (Nasdaq: ZIO), and previously held several senior leadership roles at Bayer AG and LEO Pharma. The majority of Dr. Schwartz’s achievements have been in oncology, encompassing the Departmentdevelopment of Microbiologytargeted, cytotoxic agents and Immunology, Institute for Immunology and Immunological Diseases, Yonsei University, College of Medicine in Seoul, Korea. He received his Ph.D. in Immuno-Pathology from the Seoul National University, College of Medicine in Seoul, Korea, and his M.D. from Seoul National University, College of Medicine in Seoul, Korea.
Baruch Halpert has been a Director of the Company since 2017. With more than 20 years of experience in venture capital and private equity as an entrepreneur, corporate finance advisor, senior executive and an investor, Mr. Halpert has developed a large network of contacts across the globe. Since 2010, Mr. Halpertimmunotherapy. Dr. Schwartz has been involved with a number of drug approvals, including sorafenib at Bayer, which has been used as a foundation for a number of initiatives in turn-arounds through active managementU.S. based cancer/rare disease focused biotechnology companies. At ArQule and Ziopharm, Dr. Schwartz was a key member of and private equity investments in high yield opportunities. In this capacity, Mr. Halpert is active in investing in companies with annual revenues of at least $100 million in special situations and took part in the successful turnarounds of, among others, Hemaclear (www.hemaclear.com), Apnano (www.nisusacorp.com) and HBL (www.hbl.co.il). Mr. Halpert currently serves as Executive Chairman of Terragenic International Limited, which position he has held since 2018. Early in his career, Mr. Halpert was active in oil and gas exploration in Israel. In that capacity he obtained, developed and sold the rights to an Israeli oil and gas exploration license, the Megiddo Prospect, to Ultra Petroleum Corp. (Nasdaq: UPL). In 1997, Mr. Halpert founded E*TRADE Israel (www.etrade.com). After obtaining a license from E*TRADE, Mr. Halpert put together a core management team and headed severalmanaged diverse interdisciplinary teams to prepare multiple successful roundsNew Drug Applications (NDA), numerous Investigational New Drug (IND) applications, preclinical and clinical drug development programs. Dr. Schwartz has acquired knowledge and experience of financing. Following E*TRADE, Mr. Halpert was Headmedical affairs, cooperative oncology group, investors relations, partnering and capital raising. Dr. Schwartz is currently a Board Member of Corporate Finance at Fantine Capital. Mr. Halpert holdsCyclacel Pharmaceutical Inc (Nasdaq: CYCC) and Infinity Pharmaceuticals Inc. (Nasdaq: INFI). In addition, Dr. Schwartz serves as an LLB Degree (Hons.) from Reading University, United Kingdom.
Michel Habib has been a Director of the Company since 2017. Mr. Habib is the Chief Executiveadvisor, SAB member, Medical Officer of Hadasit Bio-Holdings Ltd., which position he has held since 2018. Hadasit Bio-Holdings currently beneficially owns 18.23% of the outstanding shares of the Company. Mr. Habib was the co-founder and managed Agate Medical Investmentsindependent consultant for numerous private biotech and Agate MaC VC funds from 2007-2016 with over $100 million under management. His portfolio companies have attracted investments from leading global and Chinese companies, including Boston Scientific, Johnson & Johnson, Medtronic, Haisco, Longtech, and Xio. Currently, Mr. Habib serves on the board of several investment companies and startups, including Xenia Ventures, Kahr Medical (Chairman), Cellcure, Bioprotect and Ornim Medical. Prior to that he managed Matar Capital Advisors, a venture boutique. Mr. Habib served for nearly four years as Business Development Director of Elron (TASE: ELRN), focusing on thecompanies. Dr. Schwartz received his medical devices sector. Prior to Elron, he established and managed the investment banking activity of ING Barings in Israel. Formerly, he served as Vice President Investment Banking at Cukierman & Co. where he led private placements and IPOs in Europe. During the 1990s, Mr. Habib served as a diplomat in Israel’s foreign service, where he served as Economic Consul in Boston, and earlier as the first Commercial Attaché to Seoul, South Korea. As Navy Officer (Captain Res.) in the Israel Defense Forces, he was involved in the development of advanced Naval warfare systems for the Navy’s elite unit. Mr. Habib holds an Aeronautical Engineering degree from the Technion-Israel InstituteUniversity of Technology, and is a graduate from Harvard Law School Executive Program On Negotiation. He is a graduate from the foreign service cadet school, and member of the Technion Alumni “100 Club.” Mr. Habib was born in Paris, France, and immigrated to Israel in 1973.Pretoria, South Africa.
There are no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our directors or members of senior management were selected as such. In addition, there are no family relationships among our executive officers and directors.
6.B. Compensation of Board Members and Executives
The table below reflects theaggregate compensation granted to our five most highly compensated directorsof all board members and executive officers, including share-based compensation, for the year ended December 31, 2019. All2022, was $3,536,310. This amount includes $220,000 set aside or accrued to provide pension, severance, education fund, retirement, annual leave and recuperation or similar benefits or expenses, commonly provided by Israeli companies to their employees. This amount does not include any business travel and expenses reimbursed to office holders.
As of December 31, 2022, aggregate options to purchase 2,094,650 ordinary shares outstanding under our Global Share Incentive Plan (2014) and Global Share Incentive Plan (2019) and 123,626 restricted share units (“RSUs”) outstanding under our Global Share Incentive Plan (2019) were granted to directors and executive officers. The options have a weighted average exercise price of $5.85 per option.
The aggregate compensation of our five most highly compensated executive directors and officers for the year ended December 31, 2022 (the “Covered Executives”), included cash-based salaries of $1,179,000 and $304,000 in amounts reportedset aside or accrued to provide pension, severance, education fund, retirement, annual leave and recuperation or similar benefits or expenses, commonly provided by Israeli companies to their employees (the “Benefits”). These aggregated amounts were allocated to the Covered Executives as follows: of the aggregate cash salaries, 35%, 21%, 20%, 13% and 12% was allocated to our Executive Chairman, Chief Executive Officer, Chief Scientific Officer, Head of Clinical Operations and Chief Financial Officer, respectively, and of the aggregate Benefits, 42%, 27% and 31% was allocated to our Chief Executive Officer, Head of Clinical Operations and Chief Financial Officer, respectively. Our Executive Chairman and Chief Scientific Officer did not receive Benefits in 2022. In addition, the Covered Executives received cash bonuses in 2022 in the table reflect the costaggregate amount of $217,000 which was allocated to the Company, in U.S. Dollars,Covered Executives as recognizedfollows: 69%, 12%, 9%, 6% and 3% to our Executive Chairman, Chief Executive Officer, Chief Scientific Officer, Head of Clinical Operations and Chief Financial Officer, respectively. We recorded a non-cash equity-based compensation expense in our financial statements for the year ended December 31, 2019. Amounts paid in NIS are translated into U.S. dollars at the rate of NIS 3.5645 = U.S. $1.00, based on the average representative rate of exchange between the NIS2022, for options and the U.S. dollar as reported by the Bank of Israel for the year ended December 31, 2019.
| Name and Position | Salary/Fees(1) | Share-Based Compensation (2) | Bonus/Severance | Total | ||||||||||||
| Shai Novik, Chairman | $ | 286,000 | $ | 8,000 | $ | 242,000 | $ | 536,000 | ||||||||
| Shmulik Hess, Former CEO | $ | 307,000 | $ | 217,000 | $ | $ | 524,000 | |||||||||
| Oren Hershkovits CEO | $ | 38,000 | $ | 22,000 | $ | $ | 60,000 | |||||||||
| Prof. Dror Mevorach, CSO | $ | 180,000 | $ | 365,000 | $ | $ | 545,000 | |||||||||
| Shlosberger Shachar, CFO | $ | 126,000 | $ | 6,000 | $ | $ | 132,000 | |||||||||
The aggregate amount of compensation paid or accrued to all of our directors and executive officers as a group with respect to the year ended December 31, 2019 was approximately $1,888,920. Such amount is inclusive of the grant date fair value of option awards granted or modified during the year ended December 31, 2019Covered Executives, in the amountaggregate, of $709,000. The amount does not include business travel, relocation, professional$1,658,000 which was allocated as follows: 76%, 15%, 5%, 2% and business association due2% to our Executive Chairman, Chief Executive Officer, Chief Scientific & Medical Officer, Head of Clinical Operations and expenses.Chief Financial Officer, respectively. For information regarding our accounting for stock-based compensation arrangements, see Note 2 to our audited consolidated financial statements contained in this Annual Report on Form 20-F.
Employment and Consulting Agreements with Executive Officers and Directors
We have entered into a written consulting and employment agreementagreements with our Executive Chairman, our Chief Executive Officer, and a written consulting agreement with our Chief Scientific & Medical Officer, and our Chief Financial Officer. TheseAll such agreements as well as other agreements with our other officers, employees and consultants, contain provisions standard for a company in our industry and customary for the respective positions regarding non-competition, confidentiality of information and assignment of inventions. However,The non-competition provisions apply for a period of twelve months following termination of the respective officer’s employment. In addition, we are required to provide notice of between one and twelve months prior to terminating the employment of such executive officers other than in the case of a termination for cause.
Other than with respect to the Executive Chairman’s consulting agreement, these agreements do not provide for benefits upon the termination of these executives’ respective employment with us, other than payment of salary and benefits during the required notice period for termination of these agreements, which varies under current applicable employment laws,these individual agreements.
With respect to the Executive Chairman’s consulting agreement, if we may notterminate the Executive Chairman’s Board service other than for cause, the Executive Chairman is entitled to the base retainer for the twelve-month period following the effective date of termination. The Executive Chairman is also entitled to certain other stock option payments upon such termination, and to certain benefits in the case the termination is due to long-term disability. On November 4, 2021, the Company and the Executive Chairman entered into an amendment to the agreement, granting the Executive Chairman 3.33% of future gross proceeds actually received by the Company during the first five (5) years from consummation of a commercial transaction or sale, as defined in the amendment. Such commissions will be ablepaid once the aggregate consideration actually received by the Company in respect of such transactions will equal or exceed $20 million.
As approved by our shareholders at the 2021 annual general meeting of shareholders, for the year ended December 31, 2022, the non-executive directors (namely, all directors other than Mr. Novik) were paid an annual fee of NIS 61,725 (approximately $17,600) and a per meeting fee of NIS 1,594 (approximately $455) until June 30, 2022, which thereafter increased to enforce covenants notan annual fee of NIS 66,828 (approximately $18,900) and a per meeting fee of NIS 1,993 (approximately $560) until June 30, 2023, and thereafter will increase annually by 25% up to competethe maximum fixed amount payable from time to time by us under the Companies Regulations (Rules Regarding Compensation and therefore may be unable to prevent our competitors from benefiting from the expertiseExpense Reimbursement of some of our former employees. External Directors), 2000.
Please see “Risk Factors — Factors—Risks Related to Our Business, Industry and Regulatory Requirements” for a further description of the enforceability of non-competition clauses. See “Related Party Transactions” below for additional information.
Directors’ Service Contracts
We have executed a services agreement with our Chairman.
6.C. Board practicesPractices
Board of Directors
AccordingPursuant to the Companies Law, the management of our business is vested in our Board. Our Board may exercise all powers and may take all actions that are not specifically granted to our shareholders. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our Board. Executive officers are appointed by, and serve at the discretion of, our Board, subject to any applicable employment agreements we have entered into with the executive officers.
Under our Amended and Restated Articles of Association, the Board must consist of at least five and not more than eleven directors. The Board of the Company is currently composed of eightsix members, and includes Mr. Shai Novik, Dr. Bernhard Kirschbaum, Dr. Abraham (Avri) Havron, Dr. Gili Hart, Mr. Sangwoo Lee, Mr. Hyun Gyu Lee, Mr. Baruch HalpertDr. Roger Pomerantz and Mr. Michel Habib.Brian Schwartz. These directors were nominated immediately after the closing of the Merger, except for Mr. Brian Schwartz, who was originally appointed in November 2020, and Dr. Roger Pomerantz, who was originally appointed in May 2022. All of our directors were reelected at our annual general meeting of shareholders held on November 17, 2022, and will serve until theour next annual general meeting of shareholders (a “General Meeting”) of the Company orand until their respective successors are duly elected and qualified. Each of our directors shall be elected at an Annualannual General Meeting and shall serve in theirhis or her office until the next annual General Meeting, or until they cease to serve in their office in accordance with the provisions of our Amended and Restated Articles of Association or any law, whichever is the earlier.
Under the Israeli Companies Law, 5759-1999 (the “Companies Law”), the Board must determine the minimum number of directors who are required to have accounting and financial expertise. Under applicable regulations, a director with accounting and financial expertise is a director who, by reason of his or her education, professional experience and skill, has a high level of proficiency in and understanding of business accounting matters and financial statements, sufficient to be able to thoroughly comprehend the financial statements of the company and initiate debate regarding the manner in which financial information is presented. In determining the number of directors required to have such expertise, the Board must consider, among other things, the type and size of the company and the scope and complexity of its operations. The existing Board of the Company has determined that the Company requires one director with such expertise, and that both Mr. Shai Novik hasand Mr. Sangwoo Lee have such accounting and financial expertise.
External Directors
Under the Companies Law, except as provided below (“Exempted Companies”Companies”), companies incorporated under the laws of the State of Israel that are publicly traded, including Israeli companies with shares listed on the Nasdaq such as the Company, are required to appoint at least two external directors, who meet the qualifications requirements set forth in the Companies Law.
Pursuant to the Israeli Companies Regulations (Relief for Companies Whose Shares are Registered for Trading Outside of Israel), 2000 (the “Relief Regulations”), a company will be an Exempted Company, if: (i) the company’s shares are listed on a foreign securities exchange which is referenced in Section 5A(c) of the Regulations, which includes, among others, the NASDAQ Capital Market; (ii) the company does not have a controlling shareholder (as such term is defined in the Companies Law); (iii) a majority of the directors serving on the board of directors are “independent,” as defined under Nasdaq Listing Rule 5605(a)(2); and (iv) the company complies with the U.S. laws (including applicable Nasdaq Listing RulesRules) as to the required composition of the audit and compensation committees of the Board as applicable to U.S. domestic issuers (which require that such committees consist solely of independent directors (at least three and two members, respectively)), as described under the Nasdaq Listing Rules. Under the Relief Regulations, if a company is an Exempted Company, the board of directors of asuch company such as Enlivex, is not requiredmay elect to have“opt out” of the Companies Law requirement to appoint external directors if it is an Exempted Company. An external director who was elected to serve as such prior to the date on which the company opted to comply with the applicable foreign exchangeand related Companies Law rules governing the appointment of independent directors andconcerning the composition of the audit committee and compensation committees as set forth above may continue to serve out his/her term as a non-external director on the company’s boardcommittee of directors until the earlier of (i) the end of his/her three year term, or (ii) the second annual General Meeting following the company’s decision to comply with the said applicable foreign exchange rules, without any further action on the part of the Company or its shareholders. Such director may be elected to the board of directors by the Company’s shareholders, but he/she would now be elected as a regular director (not an external director) and his/her election would be no different(other than the electiongender diversification rule under the Companies Law, which requires the appointment of anya director from the other director. Thegender if at the time of appointment of a director all members of the board of directors are of the same gender),. . In accordance with the Relief Regulations, our Board determined that the Company meets all of thesethe requirements of an Exempted Company and does not have external directors followingelected to “opt out” from such requirements of the board’s determination to follow the exemption provided under the Relief Regulations,Companies Law, such that following the board’s determination, we do not have external directors and the Company wouldmust comply with the Nasdaq Listing Rules governing the appointment of independent directors and the composition of the audit committee and compensation committee applicable to domestic U.S. issuers, provided that the Company continues to meet the requisite requirements for said relief and unless the Company’s board of directors determines otherwise.
Leadership Structure of the Board
In accordance with the Companies Law and the Amended and Restated Articles of Association, the Board is required to appoint one of its members to serve as Chairman of the Board. The Board has appointed Mr. Shai Novik to serve as Executive Chairman of the Board. In addition, the Board has appointed Dr. Roger Pomerantz to serve as Vice Chairman of the Board.
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our Board of Directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the Board of Directors at regular board meetings, including a description of steps taken by management to mitigate or eliminate such risks.
Board Committees
Audit Committee
The Companies Law requires public companies to appoint an audit committee. In accordance with the Relief Regulations promulgated under the Companies Law described above, we elected to “opt out” from the Companies Law requirement to appoint external directors and related rules concerning the composition of the audit committee and compensation committee. Under such exemption, among other things, our audit committee is required to comply with the audit committee composition requirements under U.S. laws (including applicable Nasdaq Listing Rules and SEC rules) as applicable to U.S. domestic issuers.
Under the Nasdaq Listing Rules, the Company is required to maintain an audit committee consisting of at least three independent directors, all of whom are financially literate and one of whom has accounting or related financial management expertise.expertise, and none of whom has participated in the preparation of our or any of our subsidiaries’ financial statements at any time during the prior three years. In addition, each member of the Audit Committee is required to be “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act.
The audit committee of the Company (the “Audit Committee”) consists of three members, all of whom are independent under the listing standards of the Nasdaq Listing Rules.Rules and are “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act. The members of the Audit Committee are Mr. Shai Novik, Dr. Avri Havron and(as Chairman of the Audit Committee), Dr. Gili Hart.Hart and Mr. Sangwoo Lee. The Board of the Company has determined that Mr. NovikLee is an audit committee financial expert as defined by the SEC rules and has the requisite financial sophistication as defined by the Nasdaq Listing Rules. All of the members of the Audit Committee meet the requirements for financial literacy under the applicable Nasdaq Listing Rules.
Each member of the Audit Committee is required to be “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act.
In addition, the Companies Law requires public companies to appoint an audit committee. The responsibilities of the audit committee as set forth in the Companies Law include identifying irregularities in the management of our business and approving related party transactions as required by law, classifying company transactions as extraordinary transactions or non-extraordinary transactions and as material or non-material transactions in which an office holder has an interest (which will have the effect of determining the kind of corporate approvals required for such transaction), assessing the proper function of the company’s internal audit regime and determining whether its internal auditor has the requisite tools and resources required to perform his or her role and to regulate the company’s rules on employee complaints, reviewing the scope of work of the company’s independent accountants and their fees, and implementing a whistleblower protection plan with respect to employee complaints of business irregularities. In addition, the responsibilities of the audit committee under the Companies Law also include the following matters: (i) to establish procedures to be followed in respect of related party transactions with a “controlling shareholder” (where such are not extraordinary transactions), which may include, where applicable, the establishment of a competitive process for such transaction, under the supervision of the audit committee, or individual, or other committee or body selected by the audit committee, in accordance with criteria determined by the audit committee; and (ii) to determine procedures for approving certain related party transactions with a “controlling shareholder”,shareholder,” which were determined by the audit committee not to be extraordinary transactions, but which were also determined by the audit committee not to be negligible transactions.
Compensation Committee
Under the Companies Law, an audit committee must consistthe board of at least three directors, including all the external directors of Israeli publicly traded companies are required to appoint a compensation committee. In accordance with the company, and a majority of the members of the audit committee must be independent or external directors. The Companies Law defines independent directors as either external directors or directors who: (1) meet the requirements of an external director, other than the requirement to possess accounting and financial expertise or “professional qualifications”, with Audit Committee confirmation of such; (2) have been directors in the company for an uninterrupted duration of less than 9 years (and any interim period during which such person was not a director which is less than 2 years shall not be deemed to interrupt the duration); and, (3) were classified as such by the company.
The following persons may not be a member of the audit committee:
According to the Companies Law (1)described above, we elected to “opt out” from the chairman of the audit committee must be anCompanies Law requirement to appoint external director (unless it is an Exempted Company), (2) the required quorum for audit committee meetings and decisions is a majority of the committee members, of which the majority of members present must be independent directors and (3) any person who is not eligible to serve on the audit committee is further restricted from participating in its meetings and votes, unless the chairman of the audit committee determines that such person’s presence is necessary in order to present a certain matter, provided however, that company employees who are not controlling shareholders or relatives of such shareholders may be present in the meetings but not for the actual votes, and likewise, company counsel and company secretary who are not controlling shareholders or relatives of such shareholders may be present in the meetings and for the decisions if such presence is requested by the audit committee.
As stated above, pursuant to an exemption available in the Relief Regulations which we follow, companies whose shares are listed for trading on specified exchanges outside of Israel, including the NASDAQ Capital Market, and which satisfy the criteria detailed above, are exempt from the followingrelated rules regardingconcerning the composition of the audit committee and compensation committee. Under such exemption, among other things, our compensation committee is required to comply with the compensation committee composition requirements under the Companies Law: (i) the committee shall be comprised of at least 3 members, who shall include all of the external directors,U.S. laws (including applicable Nasdaq Listing Rules and the majority of the members shall be independent; (ii) the audit committee may not include the chairman of the board, or any director employed by the Company, by a controlling shareholder or by any entity controlled by a controlling shareholder, or any director providing services to us, to a controlling shareholder or to any entity controlled by a controlling shareholder on a regular basis, or any director whose income is primarily dependent on a controlling shareholder, and may not include a controlling shareholder or any relatives of a controlling shareholder; (iii) the controlling shareholder or his relatives shall not be members of the audit committee; (iv) the chairman of the audit committee shall be an external director; (v) a person who is prohibited from being a member of the audit committee shall not be present at the committee’s meetings; (vi) if the committee also servesSEC rules) as a financials committee, the rules applicable to the financials committee shall apply; (vii) the legal quorum shall be the majority of the committee members, provided that the majority of directors present are independent, at least one of whom is an external director.U.S. domestic issuers.
Compensation Committee
Under the Nasdaq Listing Rules, the Company is required to maintain a compensation committee consisting entirely of independent directors (or the determination of such compensation solely by the independent members of the board of directors of the company).
The compensation committee of the Company (the “Compensation Committee”) consists of three members, Dr. Roger Pomerantz (as Chairman of the Compensation Committee), Dr. Avri Havron and Dr. Gili Hart, all of whom are independent under the listing standards of the Nasdaq Listing Rules.
The responsibilities of the compensation committee as set forth in the Companies Law include the following:
| 1. | To recommend to the Board of Directors as to a compensation policy for office holders of the company, as well as to recommend, once every three years to extend the compensation policy subject to receipt of the required corporate approvals; |
| 2. | To recommend to the Board of Directors as to any updates to the compensation policy which may be required; |
| 3. | To review the implementation of the compensation policy by the company; |
| 4. | To approve transactions relating to terms of office and employment of certain company office holders, which require the approval of the compensation committee pursuant to the Companies Law; and |
| 5. | To exempt, under certain circumstances, a transaction relating to terms of office and employment from the requirement of approval of the shareholders meeting. |
Compensation Policy
Under the Companies Law, the board of directors of Israeli publicly traded companies are required to appoint a compensation committee comprised of at least three members, including all external directors, who must also comprise a majority of the members of the compensation committee. In addition, the chairman of the compensation committee must be an external director. Followingfollowing the compensation committee’s recommendations, the board of directors is required to establish a compensation policy, which includes a framework for establishing the terms of office and employment of the office holders and guidelines with respect to the structure of the variable pay of office holders. Such guidelines are the basis for adequate balance between the components of compensation, which exists when a linkage is maintained between compensation and performance and the creation of value for shareholders in the Company, while maintaining the Company’s ability to recruit and maintain talented officeholders and incentivizing them to pursue the Company’s objectives. In particular, an appropriate balance between the fixed component (base salary and additional benefits) and the variable component and capital compensation avoids placing an exaggerated emphasis on one component.
Any person who is not eligible to serve on the compensation committee is further restricted from participating in its meetings and votes, unless the chairman of the compensation committee determines that such person’s presence is necessary in order to present a certain matter, provided however, that employees who are not controlling shareholders, or relatives of such shareholders, may be present at such meetings, but not for the actual votes. Likewise, company counsel and the secretary if not controlling shareholders, or relatives of such shareholders, may be present at the compensation committee meetings and for the decisions if such presence is requested by the compensation committee.
Under the Companies Law, a company’s compensation policy shall be determined based on, and take into account, the following parameters:
| a. | Advancement of the goals of the company, its working plan and its long term policy; |
| b. | The creation of proper incentives for the office holders while taking into consideration, inter alia, the company’s risk management policies; |
| c. | The company’s size and nature of its operations; |
| d. | The contributions of the relevant office holders in achieving the goals of the company and profit in the long term in light of their positions; |
| e. | The education, skills, expertise and achievements of the relevant office holders; |
| f. | The role of the office holders, areas of their responsibilities and previous agreements with them; |
| g. | The correlation of the proposed compensation with the compensation of other employees of the company, and the effect of such differences in compensation on the employment relations in the company; and |
| h. | The long term performance of the office holder. |
In addition, the compensation policy should take into account that in the event the compensation paid to office holders shall include variable components – it should address the ability of the board of directors to reduce the value of the variable component from time to time or to set a cap on the exercise value of convertible securities components that are not paid out in cash. Additionally, in the event that the terms of office and employment include grants or payments made upon termination – such grants should take into consideration the length of the term of office or period of employment, the terms of employment of the office holder during such period, the company’s success during said period and the office holder’s contribution to obtaining the company’s goals and maximizing its profits as well as the circumstances and context of the termination.
In addition, the compensation policy must set forth standards and rules on the following issues: (a) with respect to variable components of compensation -– basing the compensation on long term performance and measurable criteria (though a non-material portion of the variable components can be discretionary awards taking into account the contribution of the office holder to the company. Pursuant to the provisions of the Companies Law, variable components in the amount of up to a three month salary of the relevant office holder, on an annual basis, shall be considered a non-material portion of the variable components); (b) establishing the appropriate ratio between variable components and fixed components and placing a cap on such variable components (including a cap on the grant date value of convertible securities components that are not paid out in cash); (c) setting forth a ruleclawback rules requiring, an office holder to return amounts paid, in the event that it is later revealed that such amounts were paid on the basis of data which prove to be erroneous and resulted in an amendment and restatement of the company’s financial statements; (d) determining minimum holding or vesting periods for equity based variable components of compensation, while taking into consideration appropriate long term incentives; and (e) setting a cap on grants or benefits paid upon termination.
The board of directors of a company is obligated to adopt a compensation policy after considering the recommendations of the compensation committee. The final adoption of the compensation committee is subject to the approval of the shareholders of the company, which such approval is subject to certain special majority requirements, as set forth in the Companies Law, pursuant to which one of the following must be met:
| (i) | the majority of the votes includes at least a majority of all the votes of shareholders who are not controlling shareholders of the company or who do not have a personal interest in the compensation policy and participating in the vote; abstentions shall not be included in the total of the votes of the aforesaid shareholders; or |
| (ii) | the total of opposing votes from among the shareholders described in subsection (i) above does not exceed 2% of all the voting rights in the company. |
For this purpose, under the Companies Law “personal interest” is defined as: (1) a shareholder’s personal interest in the approval of an act or a transaction of the company, including (i) the personal interest of his or her relative (which includes for these purposes any members of his/her (or his/her spouse’s) immediate family); and (ii) a personal interest of a corporate body in which a shareholder or any of his/her aforementioned relatives serves as a director or the chief executive officer, owns at least 5% of its issued share capital or its voting rights or has the right to appoint a director or chief executive officer, but (2) excluding a personal interest arising solely from the fact of holding shares in the company or in a corporate body.
Nonetheless, even if the shareholders of the company do not approve the compensation policy, the board of directors of a company may approve the compensation policy, provided that the compensation committee and, thereafter, the board of directors determined, based on detailed, documented, reasons and after a second review of the compensation policy, that the approval of the compensation policy is for the benefit of the company.
The following persons may not be a member of the compensation committee:
The term “controlling shareholder” is defined in the Companies Law as a shareholder with the ability to direct the activities of a company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in a company or has the right to appoint the majority of the directors of such company or its general manager.
The responsibilities of the compensation committee as set forth in the Companies Law include the following:
Pursuant to the provisions of the Companies Law, the audit committee may serve as the company’s compensation committee, provided that it meets the composition requirements of the compensation committee.
Pursuant to anNominating Committee
We rely on the exemption available to foreign private issuers under the Nasdaq Listing Rules and follow Israeli law and practice with regard to the process of nominating directors, in accordance with which our Board (or a committee thereof) is authorized to recommend to our shareholders director nominees for election.
In May 2022, our Board established a non-independent nominating committee (the “Nominating Committee”), whose role is (among other things) to identify and recommend director nominees for election by the Relief Regulations which we follow, companies whose shares are listed for trading on specified exchanges outside of Israel, includingshareholders, while considering the NASDAQ Capital Market,appropriate size and satisfying the criteria detailed above, are exempt from the following rules regarding the composition of the compensation committee underBoard of Directors, the Companies Law: (i) the board of a public company is requiredrequirements applicable to appoint a compensation committee; (ii) the compensation committee shall be comprised of at least 3all members (iii) all of the external directors shall beBoard of Directors and the criteria for the selection of new members and shall constitute the majority of its members and (iv) the rest of the members shall be members whose termsBoard of service are as required under the Companies Law.
Directors. The compensation committee of the Company (the “CompensationNominating Committee”) consists of three members, Mr. Shai Novik Dr. Avri Havron, and(as Chairman of the Nominating Committee), Dr. Gili Hart all of whom are independent under the listing standards of the Nasdaq Listing Rules.and Mr. Sangwoo Lee.
Nominating Committee
Our Board of Directors does not have an independent nominating committee. Board nominees are selected by a majority of the Board’s independent directors.
Internal auditor
Under the Companies Law, the Board of Directors of an Israeli public company must appoint an internal auditor recommended by the audit committee and nominated bycommittee. Under the Board of Directors. AnCompanies Law, an internal auditor may not be:
| ● | a person (or a relative of a person) who holds more than 5% of the Company’s outstanding shares or voting rights; |
| ● | a person (or a relative of a person) who has the power to appoint a director or the general manager of the company; |
| ● | an office holder (including a director) of the company (or a relative thereof); or |
| ● | a member of the company’s independent accounting firm, or anyone on his or her behalf. |
The role of the internal auditor is, to examine, among other things, to examine our compliance with applicable law and orderly business procedures. Alon Amit of Raveh Ravid Internal Audit Services Ltd. serves as our internal auditor.
6.D. Employees
As of December 31, 2019,2022, the Company had 3677 full time employees. TheIn addition, the Company’s Chief Scientific & Medical Officer provides services on a part-time basis pursuant to a consulting agreement. ThirtySixty-six of the Company’s employees are currently involved in product development, and six11 provide general and administrative services. All of these employees are located in Israel.
None of the Company’s employees are party to any collective bargaining agreements or represented by any labor unions. However, in Israel, the Company is subject to certain Israeli labor laws, regulations, rulings of Israeli labor courts and certain provisions of collective bargaining agreements that apply to its employees by virtue of extension orders issued by the Israel Ministry of Economy and which apply such agreement provisions to the Company’s employees even though they are not part of a union that has signed a collective bargaining agreement. These labor laws and regulations primarily govern the length of the workday, minimum daily wages for professional workers, pension fund benefits for all employees, insurance for work-related accidents, procedures for dismissing employees, determination of severance pay and other conditions of employment. Israeli law generally requires severance pay, which may be funded by managers’ insurance and/or a pension fund described below, upon the retirement or death of an employee or termination of employment without cause (as may be defined in the law)employment agreements). The payments to the managers’ insurance and/or pension fund in respect of severance pay amount to approximately 8.33% of an employee’s wages, in the aggregate. Furthermore, Israeli employees and employers are required to pay predetermined sums to the National Insurance Institute, which is similar to the United States Social Security Administration.Institute. Such amounts also include payments for national health insurance. The payments to the National Insurance Institute (including payments for healthcare insurance) are paid on a differential basis, such that with respect to the part of the employee’s wage which is equal to up to 60% of the average wage in Israel, the employer is required to pay an amount equal to 3.55% of such part of the employee’s wage and the employee is required to pay an amount equal to 3.50% of such part of the employee’s wage, and for the remainder of the employee’s wage up to the maximum wage amount on which payments to the National Insurance Institute are required to be paid, the employer is required to pay an amount equal to 7.60% of such part of the employee’s wage and the employee is required to pay an amount equal to 12% of such part of the employee’s wage. Such practice are further reinforcedIn addition, pursuant to the provisions of Section 14 to the Israeli Severance Pay Law, according to which1963 (the “Section 14 Arrangement”), the payment of monthly deposits by us into managers’ insurance and/or pension fund are in respect of severance obligation to such employees. These funds provide a combination of savings plan, insurance and severance pay benefits to the employee, giving the employee a lump sum payment upon retirement and securing the severance pay or part of it, if legally entitled, upon termination of employment. Each employee contributes an aggregate amount equal to 6% of his or her base salary to such funds, and the Company contributes, in the aggregate, an additional 14.83% to 15.83% of the employee’s base salary, with such amount including the 8.33% which is contributed as severance pay as noted above. The monthly contributions as mentioned above constitute the required payment for severance pay, and if the respective employment agreement includes the required provisions pursuant to the Section 14 Arrangement, the Company is not required to pay any additional sum upon termination of employment for the period during which the Sections 14 Arrangement applies. The Company generally provides its employees with benefits and working conditions above the required minimums. The Company has never experienced any employment-related work stoppages and believes its relationship with its employees is good.
All of the Company’s employment agreements include employees’ undertakings with respect to non-competition, confidentiality and the assignment to the Company of intellectual property rights developed in the course of employment. However, under current applicable Israeli labor laws, the Company may not be able to enforce (either in whole or in part) covenants not to compete and therefore may be unable to prevent its competitors from benefiting from the expertise of some of the Company’s former employees.
6.E. Share ownership
Equity Incentive Option Plans
Pursuant to the Merger Agreement, all outstanding Enlivex R&D options that were unexercised immediately prior to the Effective TimeMerger were assumed by the Company in the Merger and are administered under the 2013 Incentive Option Plan described below.
2013 and 2014 Incentive Option Plans
We maintain the pre-mergerpre-Merger Bioblast 2013 Incentive Option Plan (the “2013 Plan”). As of December 31, 2019,2022, there were a total of 83,649 options to purchase 500 ordinary shares outstanding under ourthe 2013 Plan, of which 15,500 options to purchase ordinary shares were issued and outstanding and 58,071 remained available for future issuance. A total of 15,500 options to purchase ordinary shares were vested as of that date, withare exercisable at a weighted average exercise price of $90.16 per share. No additional awards may be issued under the 2013 Plan.
We also maintain ourIn May 2014, we adopted the Global Share Incentive Plan (2014) (the “2014 Plan”). Following the adoption of the Global Share Incentive Plan (2019), as described below, no additional awards may be granted under the 2014 Plan.
In June 2019, the Companywe adopted itsthe Global Share Incentive Plan (2019) (the “2019 Plan”), under which all ordinary shares that remained available for future grant under all existing plans were reserved for issuance with respect to awards that may be granted under the 2019 Plan.
As of December 31, 2019,2022, there were a total of 2,350,704 options to purchase 2,939,434 ordinary shares under our 2019 Plan, of which 1,625,042 options to purchase ordinary shares were issued and outstanding under the 2014 Plan and 490,749the 2019 Plan and 157,560 RSUs issued and outstanding under the 2019 Plan, and 635,594 ordinary shares remained available for future issuance. Aissuance under the 2019 Plan. Of such outstanding options, a total of 1,098,6911,953,429 options to purchase ordinary shares were vested as of that date, with a weighted average exercise price of $3.88$5.55 per share.
Our 2013 Plan, which was adopted by the pre-merger Bioblast board of directors on November 13, 2013, and amended most recently on March 28, 2016, provides for the grant of options to our and our affiliates’ respective directors, employees, office holders, service providers and consultants.
OurThe 2019 Plan which was adopted by the Enlivex Board of Directors on June 3, 2019, was assumed by the Company pursuant to the Merger. A copy of the 2019 plan is filed as Exhibit 4.2 to this Annual Report on Form 20-F.
The 2013 and 2019 Plans are administered by our Board of Directors, which shall determine, subject to Israeli law, the grantees of awards and various terms of the grant. The 2013 and 2019 Plans providePlan provides for granting options in compliance with Section 102 of the Israeli Income Tax Ordinance, 1961 (the “Ordinance”).
Options and RSUs granted under the 2013 and 2019 PlansPlan to Israeli employees have been granted under the capital gains track of Section 102 of the Ordinance. Section 102 of the Ordinance allows employees, directors and officers, who are not controlling shareholders, to receive favorable tax treatment for compensation in the form of shares or options. Our Israeli non-employee service providers and controlling shareholders may only be granted options under Section 3(i) of the Ordinance, which does not provide for similar tax benefits. Section 102 of the Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees and also includes an additional alternative for the issuance of options or shares directly to the grantee (without a trustee). Section 102(b)(2) of the Ordinance, the most favorable tax treatment for grantees, permits the issuance to a trustee under the “capital gains track.” However, under this track we are not allowed to deduct an expense with respect to the issuance of the options or shares. In order to comply with the terms of the capital gains track, all options granted under the 2013 and 2019 Plans pursuant and subject to the provisions of Section 102 of the Ordinance, as well as the ordinary shares issued upon exercise of these options and other shares received subsequently following any realization of rights with respect to such options, such as share dividends and share splits, must be granted to a trustee for the benefit of the relevant employee, director or officer and should be held by the trustee for at least two years after the date of the grant.
Options and RSUs granted under the 2013 and 2019 PlansPlan will generally vest over four years commencing on the date of grant, such that 25% vest after one year and an additional 6.25%thereafter, the options or RSUs vest atin 3 equal annual installments (every 12 months), each equal to 25% of the end of each subsequent three-month period thereafter for 36 months.shares subject to the option or RSU granted, over three years. Options that are not exercised within ten years from the grant date expire, unless otherwise determined by the Board of Directors or its designated committee, as applicable. In case of termination for reasons of disability or death, the grantee or his legal successor may exercise options that have vested prior to termination within a period of six to twelve months from the date of disability or death. If we terminate a grantee’s employment or service for cause, all of the grantee’s vested and unvested options will expire on the date of termination. If a grantee’s employment or service is terminated for any other reason, the grantee may exercise his or her vested options within 90 days of the date of termination. Any expired or unvested options return to the pool for reissuance.
In the event of a merger or consolidation of our company subsequent to which we shall no longer exist as a legal entity, or a sale of all, or substantially all, of our shares or assets or other transaction having a similar effect on us, then any outstanding option shall be assumed, or an equivalent option shall be substituted, by such successor corporation or an affiliate thereof or, in case the successor corporation refuses to assume or substitute the option, our Board of Directors or its designated committee may (a) provide the grantee with the opportunity to exercise the option as to all or part of the shares, vested or otherwise, and (b) specify a period of time, no less than 7 days, following which all outstanding options shall terminate.
Copies of the 2013 Plan, the 2014 Plan and the 2019 Plan are filed as exhibits to this Annual Report on Form 20-F.
See also Item 7A7.A. “Major Shareholders” below.
6.F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
7.A. Major shareholdersShareholders
The following table and the related notes present information with respect the beneficial ownership of the Company’s ordinary shares as of March 30, 202031, 2023 by:
| ● | each shareholder known by us to beneficially own more than 5% of the Company’s outstanding ordinary |
| ● | each director of the Company; |
| ● | each executive officer of the Company; and |
| ● | all of the Company’s directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. The percentage of ordinary shares beneficially owned is based on 13,427,87618,566,262 ordinary shares issued and outstanding as of March 30, 2020.31, 2023.
Ordinary shares of the Company that may be acquired by an individual or group within 60 days of March 30, 2020,31, 2023, pursuant to the exercise of the Company’s outstanding options or warrants or the vesting of outstanding RSUs, are deemed outstanding for the purposes of computing the percentage of ordinary shares beneficially owned by such individual or group, but are not deemed outstanding for purposes of computing the percentage of ordinary shares beneficially owned by any other individual or group shown in the table.
| Beneficial Owner | Number of Ordinary Shares Beneficially Owned | Percentage of Ordinary Shares Beneficially Owned | ||||||
| The Company’s 5% or Greater Shareholders (other than Directors and Executive Officers) | ||||||||
| HBL-Hadasit Bio-Holdings Ltd | 1,728,411 | 12.87 | % | |||||
| Kretzmer and Associates PLLC | 830,973 | 6.19 | % | |||||
| KIP Global Pharma-Ecosystem Private Equity Fund | 1,417,950 | 10.56 | % | |||||
| Directors and Executive Officers | ||||||||
| Shai Novik (1) | 918,824 | 6.74 | % | |||||
| Avri Havron (2) | 488,356 | 3.62 | % | |||||
| Dror Mevorach (3) | 491,900 | 3.59 | % | |||||
| Gili Hart, Director (4) | 95,192 | * | ||||||
| Sangwoo Lee (5) | 26,596 | * | ||||||
| Hyun-Gyu Lee (5) | 26,596 | * | ||||||
| Michel Habib (5) | 26,596 | * | ||||||
| Baruch Halpert (6) | 107,099 | * | ||||||
| Bernhard Kirschbaum (7) | 13,298 | (* | ) | |||||
| Dr. Oren Hershkovitz | 6,481 | |||||||
| Shachar Shlosberger (8) | 4,115 | * | ||||||
| All directors and executive officers as a group | 2,205,052 | 15.56 | % | |||||
| Beneficial Owner | Number of Ordinary Shares Beneficially Owned | Percentage of Ordinary Shares Beneficially Owned | ||||||
| The Company’s 5% or Greater Shareholders (other than Directors and Executive Officers) | ||||||||
| KIP Global Pharma-Ecosystem Private Equity Fund | 1,417,950 | 7.64 | % | |||||
| HBL-Hadasit Bio-Holdings Ltd | 1,006,007 | 5.42 | % | |||||
| Directors and Executive Officers | ||||||||
| Shai Novik (1) | 1,531,981 | 7.92 | % | |||||
| Roger Pomerantz | -- | -- | ||||||
| Oren Hershkovitz (2) | 131,657 | * | ||||||
| Dror Mevorach (3) | 579,895 | 3.07 | % | |||||
| Shachar Shlosberger (4) | 13,794 | * | ||||||
| Avri Havron (5) | 231,044 | 1.24 | % | |||||
| Gili Hart (6) | 68,178 | * | ||||||
| Sangwoo Lee (7) | 54,880 | * | ||||||
| Brian Schwartz (8) | 21,164 | * | ||||||
| All directors and executive officers as a group (9 persons) | 2,632,593 | 13.16 | % | |||||
| * | Less than 1%. |
| (1) | Includes |
(2) | Includes |
| (3) | Includes |
| (4) | Includes |
| (5) | Includes 53,192 shares underlying options currently exercisable or exercisable within 60 days from March 31, 2023, which expire in January 2025 and have an exercise price of $2.69. | |
| (6) | Includes 66,490 shares underlying options currently exercisable or exercisable within 60 days from March 31, 2023, which expire in January 2025 and have an exercise price of $2.69. |
| (7) | Includes 53,192 shares underlying options currently exercisable or exercisable within 60 days from March 31, 2023, which expire in December 2027 and have an exercise price of $6.22. |
| (8) | Includes 20,250 shares underlying options currently exercisable or exercisable within 60 days from March 31, 2023, which expire in November 2030 and have an exercise price of $9.02. |
To our knowledge, other than as disclosed in the table above, our other filings with the SEC and this Annual Report, there has been no significant change in the percentage ownership held by any major shareholder since January 1, 2020.
According to our transfer agent, as of March 30, 2020,31, 2023, there were 7442 record holders of our ordinary shares, among whom are 5 U.S. holders (including Cede & Co., the nominee of the Depositary Trust Company, holding 19.89 %87.7% of our outstanding ordinary shares). The number of record holders in the United States is not representative of the number of beneficial holders nor is it representative of where such beneficial holders are resident since many of these ordinary shares are held by brokers or other nominees. None of our shareholders has different voting rights from other shareholders.
The Company is not directly or indirectly owned or controlled by another corporation, by any foreign government or by any natural or legal persons, severally or jointly.
7.B. Related party transactionsParty Transactions
Approval of Related Party Transactions under Israeli Law
Fiduciary duties of office holders
The Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company. The duty of care of an office holder is based on the duty of care set forth in connection with the tort of negligence under the Israeli Torts Ordinance (New Version) 5728-1968. This duty of care requires an office holder to act with the degree of proficiency with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means, in light of the circumstances, to obtain:
| ● | information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and |
| ● | all other important information pertaining to these actions. |
The duty of loyalty requires an office holder to act in good faith and for the benefit of a company, and includes the duty to:
| ● | refrain from any act involving a conflict of interest between the performance of his or her duties in a company and his or her other duties or personal affairs; |
| ● | refrain from any activity that is competitive with the business of a company; |
| ● | refrain from exploiting any business opportunity of the company for the purpose of gaining a personal advantage for himself or herself or others; and |
| ● | disclose to the company any information or documents relating to a company’s affairs which the office holder received as a result of his or her position as an office holder. |
We may approve an act performed in breach of the duty of loyalty of an office holder provided that the office holder acted in good faith, the act or its approval does not harm the Company, and the office holder discloses his or her personal interest, as described below.
Disclosure of personal interests of an office holder and approval of acts and transactions
The Companies Law requires that an office holder promptly disclose to the Company any personal interest that he or she may have and all related material information or documents relating to any existing or proposed transaction by the Company. An interested office holder’s disclosure must be made promptly and, in any event, no later than the first meeting of the board of directors at which the transaction is considered. An office holder is not obliged to disclose such information if the personal interest of the office holder derives solely from the personal interest of his or her relative in a transaction that is not considered as an extraordinary transaction.
The term “personal interest” is defined under the Companies Law to include the personal interest of a person in an action or in the business of a company, including the personal interest of such person’s relative or the interest of any corporation in which the person is an interested party, but excluding a personal interest stemming solely from the fact of holding shares in such company. A personal interest furthermore includes the personal interest of a person for whom the office holder holds a voting proxy or the interest of the office holder with respect to his or her vote on behalf of the shareholder for whom he or she holds a proxy even if such shareholder itself has no personal interest in the approval of the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction.
Under the Companies Law, an extraordinary transaction which requires approval is defined as any of the following:
| ● | a transaction other than in the ordinary course of business; |
| ● | a transaction that is not on market terms; or |
| ● | a transaction that may have a material impact on the company’s profitability, assets or liabilities. |
Under the Companies Law, once an office holder has complied with the disclosure requirement described above, a company may approve a transaction between the company and the office holder or a third party in which the office holder has a personal interest, or approve an action by the office holder that would otherwise be deemed a breach of duty of loyalty. However, a company may not approve a transaction or action that is adverse to the company’s interest or that is not performed by the office holder in good faith.
Under the Companies Law, unless the articles of association of a company provide otherwise, a transaction with an office holder, a transaction with a third party in which the office holder has a personal interest, and an action of an office holder that would otherwise be deemed a breach of duty of loyalty requires approval by the board of directors. Our Articles of Association do not provide otherwise. If the transaction or action considered is (i) an extraordinary transaction or (ii) an action of an office holder that would otherwise be deemed a breach of duty of loyalty and may have a material impact on a company’s profitability, assets or liabilities, then audit committee approval is required prior to approval by the board of directors.
Under the Companies Law, a transaction with an office holder in a public company regarding his or her terms of office and employment should be determined in accordance with the company’s compensation policy. Nonetheless, provisions were established that allow a company, under special circumstances, to approve terms of office and employment that are not in line with the approved compensation policy. The following are required for the approval of the terms of office or employment of the officers of a public company:
| ● | A transaction with an office holder in a public company that is neither a director nor the chief executive officer regarding his or her terms of office and employment requires approval by the (i) compensation committee; and (ii) the board of directors. Approval of terms of office and employment for such officers which do not comply with the compensation policy may nonetheless be approved subject to two cumulative conditions: (i) the compensation committee and thereafter the board of directors, approved the terms after having taken into account the various considerations and mandatory requirements set forth in the Companies Law with respect to office holder compensation, and (ii) the shareholders of the company have approved the terms by means of the following special majority requirements, (the “Special Majority Requirements”), as set forth in the Companies Law, pursuant to which the shareholder approval must either include at least one-half of the shares held by non-controlling and disinterested shareholders who actively participate in the voting process (without taking abstaining votes into account), or, alternatively, the total shareholdings of the non-controlling and disinterested shareholders who vote against the transaction must not represent more than 2% of the voting rights in the company. However, the transaction may still be approved despite shareholder rejection, provided that the company’s compensation committee and thereafter the board of directors have determined to approve the proposal, based on detailed reasoning, after having re-examined the terms of office and employment, and taken the shareholder rejection into consideration. |
| ● | A transaction with the chief executive officer in a public company regarding his or her terms of office and employment requires approval by (i) the compensation committee; (ii) the board of directors; and (iii) the shareholders of the company by the Special Majority Requirements. Approval of terms of office and employment for the chief executive officer which do not comply with the compensation policy may nonetheless be approved subject to two cumulative conditions: (i) the compensation committee and thereafter the board of directors, approved the terms after having taken into account the various considerations and mandatory requirements set forth in the Companies Law with respect to office holder compensation and (ii) the shareholders of the company have approved the terms by means of the Special Majority Requirements, as detailed above. However, a transaction with a chief executive officer that is not approved by shareholders may still be approved despite shareholder rejection, provided that the company’s compensation committee and thereafter the board of directors have determined to approve the proposal, based on detailed reasoning, after having re-examined the terms of office and employment, and taken the shareholder rejection into consideration. In addition, the compensation committee may exempt the transaction regarding terms of office and employment with a candidate for the office of chief executive officer where such officer has no relationship with the controlling shareholder or the company from shareholder approval if it has found, based on detailed reasons, that bringing the transaction to the approval of the shareholders meeting shall prevent the employment of such candidate by the company. Such approval may be given only in respect of terms of office and employment which are in accordance with the company’s compensation policy. |
| ● | A transaction with a director who is not an executive officer in a public company regarding his or her terms of office and engagement requires approval by the (i) compensation committee; (ii) the board of directors; and (iii) the shareholders of the company. Approval of terms of office and employment for directors of a company which do not comply with the compensation policy may nonetheless be approved subject to two conditions: (i) the compensation committee and thereafter the board of directors, approved the terms after having taken into account the various considerations and mandatory requirements set forth in the Companies Law with respect to office holder compensation and (ii) the shareholders of the company have approved the terms by means of the Special Majority Requirements, as detailed above. In addition, pursuant to a relief provided under the Companies Regulations (Relief in Interested Party Transactions), 2000, the compensation committee may exempt the transaction regarding terms of office and engagement with a non-executive director, if the compensation committee and board of directors determined that such terms of office are only for the benefit of the company, or if the compensation terms of the director do not exceed the maximum compensation paid to external directors pursuant to the applicable regulations. |
A director who has a personal interest in a matter that is considered at a meeting of the board of directors or the audit committee may generally not be present at the meeting or vote on the matter unless a majority of the directors or members of the audit committee have a personal interest in the matter, or, unless the chairman of the audit committee or board of directors (as applicable) determines that he or she should be in attendance to present the transaction that is subject to approval. If a majority of the directors have a personal interest in the matter, such matter also requires approval of the shareholders of the company by the Special Majority Requirements.
Disclosure of personal interests of a controlling shareholder and approval of transactions
Under the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. The definition of “controlling shareholder” in connection with matters governing: (i) extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, (ii) certain private placements in which the controlling shareholder has a personal interest, (iii) certain transactions with a controlling shareholder or relative with respect to services provided to or employment by the company, (iv) the terms of employment and compensation of the general manager, and (v) the terms of employment and compensation of office holders of the company when such terms deviate from the compensation policy previously approved by the company’s shareholders, also includes shareholders that hold 25% or more of the voting rights if no other shareholder owns more than 50% of the voting rights in the company (and the holdings of two or more shareholders which each have a personal interest in such matter will be aggregated for the purposes of determining such threshold).
Under the Companies Law, extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, require the approval of the audit committee, the board of directors and the shareholders, in that order. Transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the compensation committee, the board of directors and the shareholders by means of the Special Majority Requirements, as detailed above.
If such transaction concerns the terms of office and employment of such controlling shareholder, in his capacity as an office holder or an employee of the company, such terms of office and employment approved by the compensation committee and board of directors shall be in accordance with the compensation policy of the company. Nonetheless, in special circumstances, the compensation committee and the board of directors may approve terms of office and compensation of a controlling shareholder which do not comply with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by means of the Special Majority Requirements, as detailed above.
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval, in the same manner described above, is required once every three years, unless, with respect to extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
Certain Relationships and Related Party Transactions
The following is a summary description of the material terms of those transactions with related parties to which we are party, and which were in effect within the 20192022 fiscal year through March 30, 2020.
Agreement with HBL – Hadasit Bio-Holdings Ltd.
On May 22, 2017, Enlivex entered into an agreement (the “Hadasit Agreement”) with HBL – Hadasit Bio-Holdings Ltd. (“HBL”). Subject to the agreement, Enlivex agreed to pay HBL cash compensation in an aggregate amount of 5% of the proceeds received by Enlivex from investors introduced (“Introduced Investors”) by HBL to Enlivex, plus VAT, and grant to HBL a warrant to purchase securities of Enlivex of the same type issued to the Introduced Investors in a financing, in an amount equal to up to 5% of the total amount invested by the Introduced Investors in the financing, at an exercise price equal to the per share price paid to by the Introduced Investors to Enlivex in such financing. On September 18, 2017, Enlivex paid HBL a total cash amount of $351,000, and granted HBL a warrant to purchase 48,204 shares of Enlivex. The warrant was exercised in full immediately prior to the closing of the Merger. The Hadasit Agreement expired on May 21, 2018.
Employment Agreements and Arrangements with Directors and Related Parties
Chairman Services Agreement with A.S. Novik and Shai Novik
On September 7, 2018, we entered into an agreement with A.S. Novik Ltd., a company organized under the laws of Israel and family-owned entity of Shai Novik (“A.S. Novik”), pursuant to which we retained Shai Novik as our Executive Chairman of the Board for an initial term of two years, to be automatically extended for additional one-year periods, unless either party provides at least 180 days written notice prior to the expiration of the term. A.S. Novik is entitled to a base retainer of $150,000, payable in equal monthly installments, subject to review and adjustment upon certain specified events. Upon the closing of the Merger, A.S. Novik’s base retainer was increased to $250,000, which will increase to $350,000 upon the Company having a cash and cash equivalents balance of $20 million. A.S. Novik is eligible to receive an annual cash bonus up to 100% of the base retainer, as determined by the Board, which will be based upon performance criteria established by the Board. The minimum guaranteed annual bonus for the first two fiscal years after the closing of the Merger shall be 50% of the annual base retainer. If we terminate Mr. Novik’s Board service other than for cause, A.S. Novik is entitled to the base retainer for the twelve-month period following the effective date of termination. We have also agreed to reimburse A.S. Novik for up to $3,000 of monthly expenses in connection with Mr. Novik’s Board service as our Executive Chairman. Mr. Novik is also entitled to certain other stock option payments upon termination.
31, 2023.
Employment Agreement with Oren Hershkovitz
On November 16, 2019, we entered into an employment agreement (the “Hershkovitz Employment Agreement”) with Oren Hershkovitz, Ph.D., to serve as our Chief Executive Officer, for an undefined term, unless and until terminated by either party. Dr. Hershkovitz is entitled to a monthly salary of NIS 65,000. The Hershkovitz Employment Agreement provides for certain other benefits, including pension, expense reimbursement and use of a company car. The Hershkovitz Employment Agreement may be terminated by either party, at any time and for any reason, pursuant to 90-days prior written notice by the terminating party.
Employment Agreement with Shachar Shlosberger
On May 3, 2016, we entered into an employment agreement (the “Shlosberger Employment Agreement”) with Shachar Shlosberger, to serve as our Chief Financial Officer, for an undefined term, unless and until terminated by either party. The Shlosberger Employment Agreement may be terminated by either party, at any time and for any reason, pursuant to 30-days prior written notice by the terminating party. Ms. Shlosberger is entitled to a monthly salary of NIS 23,040 and an annual bonus of up to 15% of her annual salary, at the Company’s discretion. The Shlosberger Employment Agreement provides for certain other benefits, including pension benefits and use of a cellphone.
Consulting Agreement with Prof. Dror Mevorach and Hadasit Medical Research Services
Prof. Dror Mevorach, M.D., our founder, has also served as our Chief Scientific Officer and as a member of our Board since 2005. On January 1, 2017, we entered into a consulting agreement, as amended as of April 1, 2020 (the “Consulting Agreement”) with Hadasit Medical Research Services and Development (“Hadasit”) and Prof. Mevorach for the provision by Prof. Mevorach of services as our Chief Scientific Officer for an initial period of 12 months, which is automatically extended for additional twelve-month periods thereafter, unless earlier terminated by either party. The Consulting Agreement, which may be terminated upon certain breaches or actions, is also terminable by either party upon 30 days prior written notice. Prof. Mevorach is entitled, effective as of November 1, 2019, to an annual fee of $250,000, plus VAT, to be paid in equal monthly installments in NIS. We paid Hadasit $63,333.33 pursuant to the Consulting Agreement. We also granted options to purchase pre-merger Enlivex ordinary shares to Prof. Mevorach and Hadasit pursuant to the Consulting Agreement, which include an aggregate of 419,281 ordinary shares of the Company granted to Prof. Mevorach and 110,304 ordinary shares of the Company granted to Hadasit which are exercisable at a price of $2.68 per share and additional grants of 145,237 and 29,047 ordinary shares of the Company to Prof. Mevorach and Hadasit, respectively, which are exercisable at a price of $6.22 per share.
Research Agreement with Prof. Dror Mevorach and Cell Generation (C-G) Ltd.
On May 12, 2019, we entered into a research agreement with Cell Generation (C-G) Ltd. (“C-G”), pursuant to which C-G will carry out cell collections from Apheresis site at facilities operated by C-G, employing the services of Prof. Dror Mevorach as the principal investigator (the “C-G Research Agreement”). The C-G Research Agreement will expire on the earlier of: (i) the completion of the subject study according to the protocols provided therein and submission of all reports, case report forms and other documentation required thereunder, and (ii) the termination of the C-G Research Agreement by us or C-G pursuant to the terms of the C-G Research Agreement, which either party may do without cause. Upon entry into the C-G Research Agreement, we paid C-G a non-refundable advance in the amount of NIS 430,000, plus VAT, and we are obligated to pay C-G upon request certain collection, screening and recruitment remuneration. The C-G Research Agreement provides us with the option to acquire all of the outstanding shares of C-G, starting on the second anniversary of the C-G Research Agreement, at an exercise price that shall be defined in good faith by an appraiser acceptable to both parties.
Agreement with Baruch Halpert, Director
On January 2, 2018, Enlivex entered into an agreement (the “Halpert Agreement”) with Baruch Halpert, a member of its Board of Directors. Subject to the agreement, Enlivex agreed to pay Mr. Halpert cash compensation in an aggregate amount of 5% of the proceeds received by Enlivex from investors introduced (“Introduced Investors”) by Halpert to Enlivex, plus VAT,Indemnification and grant to Mr. Halpert a warrant to purchase securities of Enlivex of the same type issued to the Introduced Investors in a financing, in an amount equal to up to 5% of the total amount invested by the Introduced Investors in the financing, at an exercise price equal to the per share price paid to by the Introduced Investors to the Company in such financing. Subject to the Halpert Agreement, Enlivex paid Mr. Halpert in the aggregate, a total cash amount of $50,000 in relating to the closing of a financing on September 12, 2018 (the “Financing”). Halpert executed a waiver nullifying his right to receive a warrant relating to the closing of the Financing immediately prior to the closing of the Financing. The Halpert Agreement expired on January 1, 2019.Exculpation Agreements
Indemnification Agreements
Our Amended and Restated Articles of Association permit us to exculpate, indemnify and insure each of our directors and officers to the fullest extent permitted by the Companies Law. We have entered into agreements with each of our directors and Professor Mevorach,officers, exculpating them, to the fullest extent permitted by the Companies Law, from liability for monetary or other damages due to, or arising or resulting from, a breach of the duty of care to the Company and undertaking to indemnify them to the fullest extent permitted by Israeli law, including with respect to liabilities resulting from certain acts performed by such office holdersdirectors and officers in their capacity as an office holder of the Company, our subsidiaries or affiliates. The indemnification is limited both in terms of amount and coverage.
Insurance
In addition to the indemnification and exculpation agreements described above, we have previously obtained directors’ and officers’ liability insurance with maximum coverage of $3$5 million in the aggregate for the benefit of the Company and our office holders and directors, and purchased, post the closing of the Merger, a different policy with maximum coverage of $5 million in the aggregate.directors. Such directors’ and officers’ liability insurance contains certain standard exclusions.
We also maintain insurance for our offices in Nes Ziona and Jerusalem, Israel. Our insurance program covers approximately $925,000 of equipment and lease improvements against risk of loss and damage from inventory. In addition, we maintain the following insurance: employer liability with coverage of approximately $5,610,885; and third-party liability with coverage of approximately $1,318,558.
We also intend to purchase worldwide product and clinical trial liability insurance to cover each of our clinical trials studies with respect to our product candidates used in clinical trials in accordance with applicable local regulations in the territories in which the studies will take place. We also procure additional insurance for each specific clinical trial which covers a certain number of trial participants and which varies based on the particular clinical trial. Certain of such policies are based on the Declaration of Helsinki, which is a set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association, and certain protocols of the Israeli Ministry of Health.
We believe that our insurance policies are adequate and customary for a business of our kind. However, because of the nature of our business, we cannot assure you that we will be able to maintain insurance on a commercially reasonable basis or at all, or that any future claims will not exceed our insurance coverage.
7.C. Interests of expertsExperts and counselCounsel
Not applicable.
Not applicable.
ITEM 8. FINANCIAL INFORMATION
8.A. Consolidated Statements and Other Financial statements and other financial informationInformation
Financial Statements
See Item 18 - Financial“Financial Statements.”
Legal Proceedings
From time to time, we are involved in various routine legal proceedings incidental to the ordinary course of our business. We do not currently believe that the outcome of these legal proceedings havehas had in the recent past, or will have (with respect to any pending proceedings)proceeding), significant effects on our financial position or profitability. Any future litigation may result in substantial costs and be a distraction to management. No assurance can be given that future litigation will not have a material adverse effect on our financial position. For an additional discussion of certain risks associated with legal proceedings, see Item 3.D. “Risk Factors.”
Dividends
We have never paid any cash dividends on our ordinary shares and do not anticipate paying any cash dividends infor the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our Board of Directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our Board of Directors may deem relevant.
The Companies Law imposes further restrictions on our ability to declare and pay dividends. See Item 10.B. -“Articles“Articles of Association” Association–– “Rights,Rights, Preferences, Restrictions of Shares and Shareholder Meetings” Meetings–– “DividendDividend and Liquidation Rights” for additional information.
Payment of dividends may be subject to Israeli withholding taxes. See Item 10.E. - “Taxation” below for additional information.
8.B. Significant changes
Except as disclosed elsewhere in this annual report,Annual Report on Form 20-F, there have been no other significant changes since December 31, 2019,2022, until the date of the filing of this annual report.Annual Report on Form 20-F.
ITEM 9. THE OFFER AND LISTING
9.A. Offer and listing detailsListing Details
Not applicable.
9.B. Plan of distributionDistribution
Not applicable.
9.C. Market for Ordinary Shares
On the Closing Date, ourOur ordinary shares were admitted for continuedare listed on the Nasdaq Capital Market under the new symbol “ENLV”. From April 27, 2017 through the Closing Date, our ordinary shares were listed and on the Nasdaq Capital Market, and from July 31, 2014 until April 27, 2017, our ordinary shares were listed on the Nasdaq Global Market, in each caseTel Aviv Stock Exchange under the symbol “ORPN”.“ENLV.”
9.D. Selling shareholdersShareholders
Not applicable.
9.E. Dilution
Not applicable.
Not applicable.
9.E. Dilution
Not applicable.
9.F. Expenses of the issueIssue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
10.A. Share capitalCapital
Not applicable.
10.B. Articles of Association
Securities Register
We are registered with the Israeli Registrar of Companies. Our registration number is 51-471648-9. Our Amended and Restated Articles of Association provide that we may engage in any type of lawful business.
Board of Directors
The Companies Law requires that certain transactions, actions and arrangements be approved as provided for in a company’s articles of association and in certain circumstances by the Audit Committee or the Compensation Committee, by the Board of Directors itself and by the shareholders. The vote required by the Audit Committee, Compensation Committee and the Board of Directors for approval of such matters, in each case, is a majority of the disinterested directors participating in a duly convened meeting. If, however, a majority of the members participating in such meeting have a personal interest in the approval of such matter, then all directors may participate in the discussions and the voting on approval thereof and in such case the matter shall be subject to further shareholder approval.
Our Amended and Restated Articles of Association provide that, subject to the Companies Law, our Board of Directors may delegate its authority, in whole or in part, to such committees of the Board of Directors as it deems appropriate, and it may from time to time revoke such delegation.
Borrowing Powers
Pursuant to the Companies Law, our Board of Directors may exercise all powers and take all actions that are not required under law or under our Amended and Restated Articles of Association to be exercised or taken by our shareholders or other corporate bodies, including the power to borrow money for company purposes.
Rights, Preferences, Restrictions of Shares and Shareholders Meetings
| ● | General. Our share capital is NIS 18,000,000 divided into 45,000,000 ordinary shares with a nominal value of NIS 0.40 each. Our ordinary shares may be certificated or uncertificated, subject to the Companies Law. |
| ● | Voting. The ordinary shares do not have cumulative voting rights in the election of directors. As a result, the holders of ordinary shares that represent more than 50% of the voting power have the power to elect all the Directors. |
| ● | Dividend and liquidation rights. Our Board of Directors may declare a dividend to be paid to the holders of our ordinary shares according to their rights and interests in our profits and may fix the record date for eligibility and the time for payment, subject to the Companies Law. Our ordinary shares entitle each owner thereof to an equal right to participate in the distribution of the surplus assets of the company in the event of our liquidation in accordance with the proportionate nominal value of the shares held thereby. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future. |
| ● | Transfer of shares; record dates. Fully paid up ordinary shares may be freely transferred pursuant to our Amended and Restated Articles of Association unless such transfer is restricted or prohibited by another instrument or securities laws. Each shareholder who would be entitled to attend and vote at a General Meeting of shareholders is entitled to receive notice of any such meeting. For purposes of determining the shareholders entitled to notice and to vote at such meeting, the Board of Directors will fix a record date. |
| ● | Voting; annual general and extraordinary meetings. Subject to any rights or restrictions for the time being attached to any class or classes of shares, each shareholder shall have one vote for each share of which he or she is the holder, whether on a show of hands or on a poll. Our Amended and Restated Articles of Association do not permit cumulative voting and it is not mandated by Israeli law. Votes may be given either personally or by proxy. A proxy need not be a shareholder. If any shareholder is without legal capacity, he may vote by means of a trustee or a legal custodian, who may vote either personally or by proxy. If two or more persons are jointly entitled to a share then, in voting upon any question, the vote of the person whose name is registered first in the registry of shareholders as the owner of that share shall be accepted, whether in person or by proxy, and he, she or it is shall be entitled to vote such share. |
| ● | Quorum for General Meeting. The quorum required for our General Meetings of shareholders consists of at least two shareholders present in person, by proxy or written ballot who holds or represent between them at least one-third of the total outstanding voting rights. A meeting adjourned for lack of a quorum is generally adjourned to the same day in the following week at the same time and place or to a later time/date if so specified in the summons or notice of the meeting. At the reconvened meeting, any two or more shareholders present in person or by proxy shall constitute a lawful quorum. |
| ● | Notice of General Meeting. Under the Companies Law, shareholder meetings generally require prior notice of not less than 21 days or, with respect to certain matters, such as election of directors and affiliated party transactions, not less than 35 days. Only shareholders of record as reflected on our share register at the close of business on the date fixed by the Board of Directors as the record date determining the then shareholders who will be entitled to vote, shall be entitled to notice of, and to vote, in person or by proxy, at a General Meeting and any postponement or adjournment thereof. |
| ● | Annual; agenda; calling a General Meeting. General Meetings are required to be held at least once in every calendar year at such time (within a period of 15 months after the holding of the last preceding General Meeting), and at such time and place as may be determined by the Board of Directors. At a General Meeting, decisions shall be adopted only on matters that were specified on the agenda. The Board of Directors is obligated to call extraordinary general meeting of the shareholders upon a written request in accordance with the Companies Law. The Companies Law provides that an extraordinary general meeting of shareholder may be called by the Board of Directors or by a request of two directors or 25% of the directors in office, or by shareholders holding at least 5% of the issued share capital of the company and at least 1% of the voting rights, or of shareholders holding at least 5% of the voting rights of the company. |
| ● | Majority vote. Except as otherwise provided in the Companies Law or our Amended and Restated Articles of Association, any resolution at a General Meeting shall be deemed adopted if approved by the holders of a majority of our voting rights represented at the meeting in person or by proxy and voting thereon. Under the Israeli Companies Law, certain actions require a special majority, including (among others): (i) approval of a compensation policy, requiring the approval described in Item 6C “Directors, Senior Management and Employees— Board Practices— Compensation Committee — Compensation Policy”; and (ii) approval of executive officer compensation inconsistent with our compensation policy or the compensation of our chief executive officer (subject to limited exceptions), requiring the approval described in Item 7B “Related Party Transactions — Approval of Related Party Transactions under Israeli Law— Disclosure of personal interests of an office holder and approval of acts and transactions”; and (iii) approval of transactions with and compensation of controlling shareholders, requiring the approval described in Item 7B “Related Party Transactions — Approval of Related Party Transactions under Israeli Law— Disclosure of personal interests of a controlling shareholder and approval of transactions.” In the case of an equality of votes, the chairman of the meeting shall not be entitled to a further vote. |
| ● | Discrimination against shareholders. There are no discriminating provisions in our amended and restated articles of association, against any existing or prospective holders of our ordinary shares as a result of a shareholder holding a substantial number of ordinary shares. |
Modification of Class Rights
If, at any time, the share capital is divided into different classes of shares, the rights attached to any class (unless otherwise provided by the terms of issuance of the shares of that class) may be varied with the consent in writing of the holders of all the issued shares of that class, or with the sanction of a majority vote at a meeting of the shareholders passed at a separate meeting of the holders of the shares of that class. The provisions of our Amended and Restated Articles of Association relating to General Meetings shall apply, mutatis mutandis, to every such separate class meeting.
Unless otherwise provided by the conditions of issuance, the enlargement of an existing class of shares, or the issuance of additional shares thereof, shall not be deemed to modify or abrogate the rights attached to the previously issued shares of such class or of any other class. These conditions provide for the minimum shareholder approvals permitted by the Companies Law.
Restrictions on Shareholders Rights to Own Securities
Our Amended and Restated Articles of Association and the laws of the State of Israel do not restrict in any way the ownership or voting or our shares by non-residents of Israel, except with respect to subjects of countries which are in a state of war with Israel.
Acquisitions under Israeli Law
Full tender offerTender Offer
A person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the target company’s issued and outstanding share capital or of the issued and outstanding share capital of a certain class of shares is required by the Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company or of all of the issued and outstanding shares of the same class.
If the shareholders who do not respond to or accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class of the shares, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will be accepted if the shareholders who do not accept it hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of the shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition the Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company or of the applicable class or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special tender offerTender Offer
The Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of at least 25% of the voting rights in the company. This rule does not apply if there is already another holder of at least 25% of the voting rights in the company.
Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company.
These requirements do not apply if the acquisition (i) occurs in the context of a private offering, on the condition that the shareholders meeting approved the acquisition as a private offering whose purpose is to give the acquirer at least 25% of the voting rights in the company if there is no person who holds at least 25% of the voting rights in the company, or as a private offering whose purpose is to give the acquirer 45% of the voting rights in the company, if there is no person who holds 45% of the voting rights in the company; (ii) was from a shareholder holding at least 25% of the voting rights in the company and resulted in the acquirer becoming a holder of at least 25% of the voting rights in the company; or (iii) was from a holder of more than 45% of the voting rights in the company and resulted in the acquirer becoming a holder of more than 45% of the voting rights in the company.
The special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (ii) the special tender offer is accepted by a majority of the votes of those offerees who gave notice of their position in respect of the offer; in counting the votes of offerees, the votes of a holder of control in the offeror, a person who has personal interest in acceptance of the special tender offer, a holder of at least 25% of the voting rights in the company, or any person acting on their or on the offeror’s behalf, including their relatives or companies under their control, are not taken into account.
In the event that a special tender offer is made, a company’s board of directors is required to express its opinion on the advisability of the offer or shall abstain from expressing any opinion if it is unable to do so, provided that it gives the reasons for its abstention.
An office holder in a target company who, in his or her capacity as an office holder, performs an action the purpose of which is to cause the failure of an existing or foreseeable special tender offer or is to impair the chances of its acceptance, is liable to the potential purchaser and shareholders for damages resulting from his acts, unless such office holder acted in good faith and had reasonable grounds to believe he or she was acting for the benefit of the company. However, office holders of the target company may negotiate with the potential purchaser in order to improve the terms of the special tender offer, and may further negotiate with third parties in order to obtain a competing offer.
If a special tender offer was accepted by a majority of the shareholders who announced their stand on such offer, then shareholders who did not respond to the special offer or had objected to the special tender offer may accept the offer within four days of the last day set for the acceptance of the offer.
In the event that a special tender offer is accepted, then the purchaser or any person or entity controlling it and any corporation controlled by them shall refrain from making a subsequent tender offer for the purchase of shares of the target company and may not execute a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Companies Law permits merger transactions if approved by each party’s Board of Directors and, unless certain requirements described under the Companies Law are met, a majority of each party’s shareholders, by a majority of each party’s shares that are voted on the proposed merger at a shareholders’ meeting.
The Board of Directors of a merging company is required pursuant to the Companies Law to discuss and determine whether in its opinion there exists a reasonable concern that as a result of a proposed merger, the surviving company will not be able to satisfy its obligations towards its creditors, taking into account the financial condition of the merging companies. If the Board of Directors has determined that such a concern exists, it may not approve a proposed merger. Following the approval of the Board of Directors of each of the merging companies, the Boards of Directors must jointly prepare a merger proposal for submission to the Israeli Registrar of Companies.
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more of the directors of the other party, vote against the merger.
If the transaction would have been approved but for the separate approval of each class of shares or the exclusion of the votes of certain shareholders as provided above, a court may still rule that the company has approved the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the appraisal of the merging companies’ value and the consideration offered to the shareholders.
Under the Companies Law, each merging company must send a copy of the proposed merger plan to its secured creditors. Unsecured creditors are entitled to receive notice of the merger, as provided by the regulations promulgated under the Companies Law. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the target company. The court may also give instructions in order to secure the rights of creditors.
In addition, a merger may not be completed unless at least 50 days have passed from the date that a proposal for approval of the merger was filed with the Israeli Registrar of Companies and 30 days from the date that shareholder approval of both merging companies was obtained.
Potential Issues that Could Delay a Merger
Certain provisions of Israeli corporate and tax law may have the effect of delaying, preventing or making more difficult any merger or acquisition of us.
Requirement of Disclosure of Shareholder Ownership
There are no provisions of our Amended and Restated Articles of Association governing the ownership threshold above which shareholder ownership must be disclosed. We are subject, however, to U.S. securities rules that require beneficial owners of more than 5% of our ordinary shares to make certain filings with the SEC.
Changes in Capital
Our Amended and Restated Articles of Association do not impose any conditions governing changes in capital that are more stringent than required by the Companies Law.
10.C. Material contractsContracts
For a description of our license agreements, see Item 4.B. “Business Overview -LicenseOverview––License Agreements” and for a description of the agreements related to our directors and officers, see Item 7.B. “Related party transactions” – “Employment6.B. “Compensation of Board Members and Executives—Employment and Consulting Agreements with Executive Officers and Arrangements with Directors and Related Parties”.Directors.”
10.D. Exchange controlsControls
There are currently no Israeli currency control restrictions on payments of dividends or other distributions with respect to our ordinary shares or the proceeds from the sale of our ordinary shares, except for the obligation of Israeli residents to file reports with the Bank of Israel regarding certain transactions. However, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
Non-residents of Israel who purchase our securities with non-Israeli currency will be able to repatriate dividends (if any), liquidation distributions and the proceeds of any sale of such securities, into non-Israeli currencies at the rate of exchange prevailing at the time of repatriation, provided that any applicable Israeli taxes have been paid (or withheld) on such amounts.
Neither our Amended and Restated Articles of Association nor the laws of the State of Israel restrict in any way the ownership or voting of our ordinary shares by non-residents of Israel, except with respect to citizens of countries that are in a state of war with Israel.
10.E. Taxation
The following is a summary of the current tax structure, which is applicable to companies in Israel, with special reference to its effect on us. The following also contains a discussion of material Israeli and U.S. tax consequences to persons purchasing our ordinary shares and government programs from which we and some of our group companies benefit.shares. To the extent that the discussion is based on new tax legislation, which has yet to be subject to judicial or administrative interpretation, there can be no assurance that the views expressed in the discussion will accord with any such interpretation in the future. The discussion is not intended and should not be construed as legal or professional tax advice and is not exhaustive of all possible tax considerations. An Israeli company that is subject to Israeli taxes on the income of its non-Israeli subsidiaries will receive a credit for income taxes paid/withheld or that will be paid/withheld by the subsidiary in its country of residence, according to the terms and conditions determined in the Israeli Tax Ordinance.
The following summary is included herein as general information only and is not intended as a substitute for careful tax planning. Accordingly, each investor should consult his or her own tax advisor as to the particular tax consequences to such investor of the purchase, ownership or sale of an ordinary share, including the effect of applicable state, local, foreign or other tax laws and possible changes in tax laws.
Israeli Taxation Considerations
THE FOLLOWING IS A SUMMARY OF THE MATERIAL ISRAELI INCOME TAX LAWS APPLICABLE TO US. THIS SECTION ALSO CONTAINS A DISCUSSION OF MATERIAL ISRAELI INCOME TAX CONSEQUENCES CONCERNING THE OWNERSHIP AND DISPOSITION OF OUR ORDINARY SHARES. THIS SUMMARY DOES NOT DISCUSS ALL THE ASPECTS OF ISRAELI INCOME TAX LAW THAT MAY BE RELEVANT TO A PARTICULAR INVESTOR IN LIGHT OF HIS OR HER PERSONAL INVESTMENT CIRCUMSTANCES OR TO SOME TYPES OF INVESTORS SUBJECT TO SPECIAL TREATMENT UNDER ISRAELI LAW. EXAMPLES OF THIS KIND OF INVESTOR INCLUDE RESIDENTS OF ISRAEL OR TRADERS IN SECURITIES WHO ARE SUBJECT TO SPECIAL TAX REGIMES NOT COVERED IN THIS DISCUSSION. TO THE EXTENT THAT THE DISCUSSION IS BASED ON NEW TAX LEGISLATION THAT HAS NOT YET BEEN SUBJECT TO JUDICIAL OR ADMINISTRATIVE INTERPRETATION, WE CANNOT ASSURE YOU THAT THE APPROPRIATE TAX AUTHORITIES OR THE COURTS WILL ACCEPT THE VIEWS EXPRESSED IN THIS DISCUSSION. THIS SUMMARY IS BASED ON LAWS AND REGULATIONS IN EFFECT AS OF THE DATE OF THIS ANNUAL REPORT AND DOES NOT TAKE INTO ACCOUNT POSSIBLE FUTURE AMENDMENTS WHICH MAY BE UNDER CONSIDERATION.
General corporate tax structure in Israel
As of January 1, 2018, Israeli resident companies, such as us, were generally subject to the standard corporate tax at the rate of 23%.
Capital gains derived by an Israeli resident company are generally subject to tax at the same rate as the prevailing corporate tax rate. Under Israeli tax legislation, a corporation will be considered as an “Israeli Resident” if it meets one of the following: (a) it was incorporated in Israel; or (b) its business is managed and controlled from Israel.
Taxation of our Israeli individual shareholders on receipt of dividends
Israeli residents who are individuals are generally subject to Israeli income tax for dividends paid on our ordinary shares (other than bonus shares or share dividends) at a rate of 25%, or 30% if the recipient of such dividend is a “substantial shareholder” (as defined below) at the time of distribution or at any time during the preceding 12-month period.
AnIndividuals who are subject to tax in Israel (whether any such individual is an Israeli resident or non-Israeli resident) are also subject to an additional income tax at a rate of 3% will bewhich is imposed on high earners whose annual taxable income or gain exceeded NIS 649,560,663,240, in 2019.2022 and NIS 698,280 in 2023.
A “substantial shareholder” is generally a person who alone, or together with his relative or another person who collaborates with him on a regular basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote in a General Meeting of shareholders, the right to receive profits, the right to nominate a director or an officer, the right to receive assets upon liquidation (after settling the debts), or the right to instruct someone who holds any of the aforesaid rights regarding the manner in which he or she is to exercise such right(s), and whether by virtue of shares, rights to shares or other rights, or in any other manner, including by means of voting agreements or trusteeship agreements.
The term “Israeli Resident” for individuals is generally defined under Israeli Income Tax Ordinance [New Version], 1961, or the Israeli Tax Ordinance, as an individual whose center of life is in Israel. According to the Israeli Tax Ordinance, in order to determine the center of life of an individual, account will be taken of the individual’s family, economic and social connections, including: (a) the place of the individual’s permanent home; (b) the place of residence of the individual and his family; (c) the place of the individual’s regular or permanent place of business or the place of his permanent employment; (d) place of the individual’s active and substantial economic interests; (e) place of the individual’s activities in organizations, associations and other institutions. The center of life of an individual will be presumed to be in Israel if: (a) the individual was present in Israel for 183 days or more in the tax year; or (b) the individual was present in Israel for 30 days or more in the tax year, and the total period of the individual’s presence in Israel in that tax year and the two previous tax years is 425 days or more. The presumption in this paragraph may be rebutted either by the individual or by the assessing officer.
Taxation of Israeli Resident Corporations on Receipt of Dividends
Israeli resident corporations are generally exempt from Israeli corporate income tax with respect to dividends paid on our ordinary shares.
Capital Gains Taxes Applicable to Israeli Resident Shareholders
The income tax rate applicable to Real Capital Gain derived by an Israeli individual from the sale of shares which had been purchased after January 1, 2012, whether listed on a stock exchange or not, is 25%. However, if such shareholder is considered a “substantial shareholder” (as defined above) at the time of sale or at any time during the preceding 12-month period, such gain will be taxed at the rate of 30%. An additional tax at a rate of 3% will be imposed on high earners whose annual income or gains exceeded NIS 649,560,663,240, in 2019.2022 and NIS 698,280 in 2023.
Moreover, capital gains derived by a shareholder who is a dealer or trader in securities, or to whom such income is otherwise taxable as ordinary business income, are taxed in Israel at ordinary marginal income rates (currently, up to 50% for individuals and 23% for Israeli resident corporations).
Taxation of Non-Israeli Shareholders on Receipt of Dividends
Non-Israeli residents (corporations or individuals) are generally subject to Israeli income tax on the receipt of dividends paid on our Shares at the rate of 25% (or 30%, if such non-Israeli resident is a “substantial shareholder” at the time receiving the dividend or on any date in the 12 months preceding such date), which tax will be withheld at source, unless a tax certificate is obtained from the Israeli Tax Authority authorizing withholding-exempt remittances or a reduced rate of tax pursuant to an applicable tax treaty.
A non-Israeli resident who receives dividends from which tax was withheld is generally exempt from the duty to file tax returns in Israel in respect of such income.
For example, under the Convention Between the Government of the United States of America and the Government of Israel with Respect to Taxes on Income, as amended, Israeli withholding tax on dividends paid to a U.S. resident for treaty purposes may not, in general, exceed 25%, or 15% in the case of dividends paid out of the profits of a Benefitedan Approved Enterprise (within the meaning of the Law for the Encouragement of Capital Investments, 1959), subject to certain conditions. Where the recipient is a U.S. corporation owning 10% or more of the outstanding shares of the voting stock of the paying corporation during the part of the paying corporation’s taxable year which precedes the date of payment of the dividend and during the whole of its prior taxable year (if any) and not more than 25% of the gross income of the paying corporation for such prior taxable year (if any) consists certain interest or dividends, the Israeli tax withheld may not exceed 12.5%, subject to certain conditions.
Capital gains income taxes applicable to non-Israeli shareholders.shareholders
Non-Israeli resident shareholders are generally exempt from Israeli capital gains tax on any gains derived from the sale, exchange or disposition of our ordinary shares, provided that such gains were not derived from a permanent establishment or business activity of such shareholders in Israel and if additional conditions are met. However, non-Israeli corporations’ shareholders will not be entitled to the foregoing exemptions if an Israeli resident (i) has a controlling interest of more than 25% in such non-Israeli corporation or (ii) is the beneficiary of or is entitled to 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly.
Regardless of whether shareholders may be liable for Israeli income tax on the sale of our ordinary shares, the payment of the consideration may be subject to withholding of Israeli tax at the source. Accordingly, shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding tax at source at the time of sale.
Estate and gift tax
Currently, Israeli law does not impose estate or gift taxes.
United States Federal Income Tax Consequences
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF ORDINARY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
U.S. Federal Income Taxation
On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “TCJA”), was signed into law making significant changes to U.S. income tax law, including a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017, modification of the U.S. international taxation system, and a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings as of December 31, 2017. We dodid not see a material direct impact on our financials as of December 31, 2019.2022.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the ordinary shares. For this purpose, a “U.S. Holder” is a holder of ordinary shares that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury Regulations) created or organized in or under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is subject to U.S. federal income tax regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations; or (6) any person otherwise subject to U.S. federal income tax on a net income basis in respect of the ordinary shares, if such status as a U.S. Holder is not overridden pursuant to the provisions of an applicable tax treaty.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase or hold our ordinary shares. This summary generally considers only U.S. Holders that will own our ordinary shares as capital assets. Except as explicitly discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Code, final, temporary and proposed U.S. Treasury Regulations promulgated thereunder, administrative and judicial interpretations thereof, and the U.S./Israel Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the U.S. Internal Revenue Service, or the IRS, with regard to the U.S. federal income tax treatment of an investment in our ordinary shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular shareholder based on such shareholder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is subject to special tax rules, including any U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity”;entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our ordinary shares in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our ordinary shares as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, ordinary shares representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of persons who hold ordinary shares through a partnership or other pass-through entity are not considered.
You are encouraged to consult your own tax advisor with respect to the specific U.S. federal and state income tax consequences to you of purchasing, holding or disposing of our ordinary shares, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Distributions on Ordinary Shares
The entire discussion in this section is subject to the discussion under the heading “Passive“Passive Foreign Investment Companies”Companies” below.
A U.S. Holder, other than certain U.S. Holders that are U.S. corporations, will be required to include in gross income as ordinary income the amount of any distribution paid on ordinary shares (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution that exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the ordinary shares to the extent thereof, and then capital gain. Corporate holders generally will not be allowed a deduction for dividends received. For noncorporate U.S. Holders whose total adjusted income exceeds certain income thresholds, the maximum federal income tax rate for “qualified dividend income” and long-term capital gains is generally 20%, and for noncorporate U.S. Holders, whose total adjusted income does not exceed such thresholds, the maximum federal income tax rate for “qualified dividend income” and long-term capital gains is generally 15%. For this purpose, “qualified dividend income” includes, among other things, dividends received from a “qualified foreign corporation.” A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United States which includes an exchange of information program. The IRS has stated that the Israel/U.S. Tax Treaty satisfies this requirement and we believe we are eligible for the benefits of that treaty.
For U.S. Holders that are corporations, the TCJA provides a 100% deduction for the foreign-source portion of dividends received from “specified 10-percent owned foreign corporations” by U.S. corporate holders, subject to a one-year holding period. No foreign tax credit, including Israeli withholding tax (or deduction for foreign taxes paid with respect to qualifying dividends) would be permitted for foreign taxes paid or accrued with respect to a qualifying dividend. This deduction would be unavailable for “hybrid dividends.”
In addition, our dividends will be qualified dividend income if our ordinary shares are readily tradable on Nasdaq or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a PFIC. A U.S. Holder will not be entitled to the preferential rate: (1) if the U.S. Holder has not held our ordinary shares or ADRs for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished its risk of loss on our ordinary shares are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Code section 163(d)(4) will not be eligible for the preferential rate of taxation.
The amount of a distribution with respect to our ordinary shares will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the amount of any Israeli taxes withheld therefrom. (SeeSee discussion above under Item 10.E - “Israeli Tax Considerations - Considerations––Taxation of Our Shareholders - Shareholders––Dividends.”) Cash distributions paid by us in NIS will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the NIS, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Distributions paid by us will generally be foreign source income for U.S. foreign tax credit purposes. Subject to the limitations set forth in the Code, U.S. Holders, other than certain U.S. Holders that are corporations, may elect to claim a foreign tax credit against their U.S. income tax liability for Israeli income tax withheld from distributions received in respect of the ordinary shares. In general, these rules limit the amount allowable as a foreign tax credit in any year to the amount of regular U.S. tax for the year attributable to foreign source taxable income. This limitation on the use of foreign tax credits generally will not apply to an electing individual U.S. Holder whose creditable foreign taxes during the year do not exceed $300, or $600 for joint filers, if such individual’s gross income for the taxable year from non-U.S. sources consists solely of certain passive income. A U.S. Holder will be denied a foreign tax credit with respect to Israeli income tax withheld from dividends received with respect to the ordinary shares if such U.S. Holder has not held the ordinary shares for at least 16 days out of the 31-day period beginning on the date that is 15 days before the ex-dividend date or to the extent that such U.S. Holder is under an obligation to make certain related payments with respect to substantially similar or related property. Any day during which a U.S. Holder has substantially diminished his or her risk of loss with respect to the ordinary shares will not count toward meeting the 16-day holding period. A U.S. Holder will also be denied a foreign tax credit if the U.S. Holder holds the ordinary shares in an arrangement in which the U.S. Holder’s reasonably expected economic profit is insubstantial compared to the foreign taxes expected to be paid or accrued. The rules relating to the determination of the U.S. foreign tax credit are complex, and U.S. Holders should consult with their own tax advisors to determine whether, and to what extent, they are entitled to such credit. U.S. Holders that do not elect to claim a foreign tax credit may instead claim a deduction for Israeli income taxes withheld, provided such U.S. Holders itemize their deductions.
Disposition of Shares
The entire discussion in this section is subject to the discussion under the heading “Passive“Passive Foreign Investment Companies”Companies” below.
Except as provided under the PFIC rules described below, upon the sale, exchange or other disposition of our ordinary shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis in the sold ordinary shares and the amount realized on the disposition of such ordinary shares (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale or exchange or other disposition of ordinary shares will be long-term capital gain or loss if the United States Holder has a holding period of more than one year at the time of the disposition.
In general, gain realized by a U.S. Holder on a sale, exchange or other disposition of ordinary shares will generally be treated as U.S. source income for U.S. foreign tax credit purposes. A loss realized by a U.S. Holder on the sale, exchange or other disposition of ordinary shares is generally allocated to U.S. source income. However, U.S. Treasury Regulations require such loss to be allocated to foreign source income to the extent specified dividends were received by the taxpayer within the 24-month period preceding the date on which the taxpayer recognized the loss. The deductibility of a loss realized on the sale, exchange or other disposition of ordinary shares is subject to limitations.
Tax on Net Investment Income
U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% tax on their net investment income (including dividends on and gains from the sale or other disposition of our ordinary shares), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Passive Foreign Investment Companies.Companies
Special U.S. federal income tax laws apply to a U.S. Holder who owns shares of a corporation that was (at any time during the U.S. Holder’s holding period) a PFIC. We would be treated as a PFIC for U.S. federal income tax purposes for any tax year if, in such tax year, either:
| ● | 75% or more of our gross income (including our pro rata share of gross income for any company, U.S. or foreign, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| ● | At least 50% of our assets, averaged over the year and generally determined based upon value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value), in a taxable year are held for the production of, or produce, passive income |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income. If we are or become classified as a PFIC while a U.S. Holder holds shares of our stock, we generally will continue to be classified as a PFIC as to that U.S. Holder in later years even if we no longer satisfy the foregoing tests, unless the U.S. Holder makes a “deemed sale” election under the PFIC rules. If the “deemed sale” election is made, a U.S. Holder will be deemed to have sold the ordinary shares the U.S. Holder holds at their fair market value as of the date of such deemed sale and any gain from such deemed sale would be subject to the PFIC rules described below.
If we are or become a PFIC, each U.S. Holder who has not elected to treat us as a qualified electing fund by making a “QEF election”,election,” or who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our ordinary shares at a gain, be liable to pay U.S. federal income tax at the then prevailing highest tax rates on ordinary income plus interest on such tax, as if the distribution or gain had been recognized ratably over the taxpayer’s holding period for the ordinary shares. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to special U.S. federal income tax rules.
The PFIC taxation regime would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the ordinary shares while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS.InIRS. In general, a QEF election is effective only if we make available certain required information, and we do not intend to provide such information; accordingly, a QEF election would not be available to U.S. Holders.
A U.S. Holder of PFIC shares which are traded on qualifying public markets, including the Nasdaq, can elect to mark the shares to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the PFIC shares and the U.S. Holder’s adjusted tax basis in the PFIC shares. The PFIC interest charges do not apply to taxes arising from mark-to-market gains pursuant to such election. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years. As with a QEF election, a mark-to-market election is made on a shareholder-by-shareholder basis, applies to all ordinary shares held or subsequently acquired by an electing U.S. holder and can only be revoked with consent of the IRS (except to the extent the ordinary shares no longer constitute “marketable stock”).
Based on the nature of our business, the projected composition of our income and the projected composition and estimated fair market values of our assets, we likely will be classified as a PFIC. In addition, we may have been a PFIC in prior years and may be a PFIC in the future. U.S. Holders who hold ordinary shares during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC, subject to exceptions for U.S. Holders described above .above. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making applicable elections under the PFIC rules.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding (at a rate of 24% under current law) with respect to cash dividends and proceeds from a disposition of ordinary shares. In general, back-up withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting
Certain U.S. Holders who are individuals may be required to report information relating to an interest in the ordinary shares, subject to certain exceptions. U.S. Holders are urged to consult their tax advisors regarding the application of these and other reporting requirements that may apply to their ownership of ordinary shares.
Non-U.S. Holders of Ordinary Shares
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our ordinary shares.
A non-U.S. Holder may be subject to U.S. federal income or withholding tax on a dividend paid on our ordinary shares or the proceeds from the disposition of our ordinary shares if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States or, in the case of a non-U.S. Holder that is a resident of a country which has an income tax treaty with the United States, such item is attributable to a permanent establishment or, in the case of gain realized by an individual non-U.S. Holder, a fixed place of business in the United States; (2) in the case of a disposition of our ordinary shares, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the sale and other specified conditions are met; (3) the non-U.S. Holder is subject to U.S. federal income tax pursuant to the provisions of the U.S. tax law applicable to U.S. expatriates.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our ordinary shares if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides on an applicable Form W-8 (or a substantially similar form) a taxpayer identification number, certifies to its foreign status, or otherwise establishes an exemption. A U.S. related person for these purposes is a person with one or more current relationships with the United States.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
10.F. Dividends and paying agentsPaying Agents
Not applicable.
10.G. Statement by expertsExperts
Not applicable.
10.H. Documents on displayDisplay
We are subject to certain of the information reporting requirements of the Exchange Act. As a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions contained in Section 16 of the Exchange Act, with respect to their purchase and sale of our ordinary shares. In addition, we are not required to file reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we are required to file with the SEC, within four months after the end of each fiscal year, an annual report on Form 20-F containing financial statements audited by an independent accounting firm. We publish unaudited interim financial information after the end of each quarter. We furnish this quarterly financial information to the SEC under cover of a Form 6-K.
You may read and copy any document we file with the SEC at its public reference facilities at 100 F Street, NE, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, NE, Washington, D.C. 20549. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants, like us, that file electronically with the SEC. The address of this website is http://www.sec.gov. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
10.I. Subsidiary information
Not applicable.
10.J. Annual Report to Security Holders
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
For a discussion related to our market risk, see
The information set forth under the heading “Quantitative and Qualitative Disclosure About Market Risk” contained in Item 5 - “Operating and Financial Review and Prospects”. of this Annual Report on Form 20-F is incorporated by reference in this Item 11.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
We do not have any outstanding American Depositary Shares or American Depositary Receipts.
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
ITEM 15. CONTROLS AND PROCEDURES
(a) Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief ExecutiveFinancial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2019 (the “Evaluation Date”).2022. Based on such evaluation, those officers have concluded that, as of the Evaluation Date,December 31, 2022, our disclosure controls and procedures arewere effective in recording, processing, summarizing and reporting, on a timely basis, information required to be included in periodic filings under the Exchange Act and that such information is accumulated and communicated to management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
(b) Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based principally on the framework and criteria established in Internal Control -– Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission as of the end of the period covered by this report. Based on that evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 20192022 at providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
(c) Attestation Report of the Registered Public Accounting Firm
TheThe effectiveness of our internal control over financial reporting as of December 31, 20192022 has been audited by Yarel + Partners, our principal independent registered public accounting firm, as stated in their report that appears herein.
(d) Changes in Internal Control over Financial Reporting
As described in Amendment No. 2 to our Annual Report on Form 20-F forDuring the year ended December 31, 2018 (the “2018 20-F Amendment No. 2”), management concluded that the Company’s internal control over financial reporting and disclosure controls and procedures were not effective at December 31, 2018 due to a material weakness in internal control over financial reporting, resulting in the filing of Enlivex R&D’s historical audited financial statements for the year ended December 31, 2018 in our Annual Report on Form 20-F for the year ended December 31, 2018, filed on April 30, 2019, rather than those of Bioblast.
As discussed in greater detail in Item 15 of 2018 20-F Amendment No. 2, management identified that a material weakness existed as of December 31, 2018 due to a deficiency in the design of controls to appropriately perform effective and timely review of the accounting for, and disclosure of, non-routine business combinations. We believe the identified material weakness has been fully remediated after the Company’s management took steps to strengthen the Company’s controls relating to non-routine material business combination activity, including, enhancing policies and procedures and strengthening communication and information flows between legal and finance personnel. Management is committed to maintaining a strong internal control environment and believes the above noted efforts will represent significant improvements to the internal control environment.
Other than as described in the preceding paragraph, during the year ended December 31, 2019,2022, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our Board of Directors has determined that Shai Novik, our Executive Chairman andMr. Sangwoo Lee, a member of our Audit Committee, is an audit committee financial expert, as defined under the rules under the Exchange Act, and is independent in accordance with applicable Exchange Act rules and Nasdaq rules.
ITEM 16B. CODE OF ETHICS
We have adopted a written code of ethics that applies to our officers and employees, including our principal executive officer, principal financial officer, principal controller and persons performing similar functions as well as our directors. Our Code of Business Conduct and Ethics is posted on our website at https://www.enlivex.com. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report on Form 20-F and is not incorporated by reference herein. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the SEC including the instructions to Item 16B of Form 20-F. We have not granted any waivers under our Code of Business Conduct and Ethics.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table provides information regarding fees paid by us to Yarel + Partners, our principal independent registered public accounting firm, for all services, including audit services, for the years ended December 31, 20192022 and 2018:2021:
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Audit fees(1) | $ | 171,180 | $ | 77,500 | ||||
| Audit related fees(2) | 12,500 | |||||||
| Tax Fees(3) | 4,500 | 4,500 | ||||||
| All other fees | - | |||||||
| Total | $ | 175,680 | $ | 94,500 | ||||
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Audit fees (1) | $ | 129,000 | $ | 116,000 | ||||
| Audit related fees (2) | — | 9,000 | ||||||
| Tax Fees (3) | 21,000 | 16,000 | ||||||
| All other fees | — | — | ||||||
| Total | $ | 150,000 | $ | 141,000 | ||||
| (1) | Includes professional services rendered in connection with the audit of our annual financial statements and the review of our interim financial statements. |
| (2) | Audit-related fees relate to assurance and |
| (3) | Includes |
Pre-Approval of Auditors’ Compensation
Our Audit Committee has adopted a pre-approval policy for the engagementAll of our independent registered public accounting firm to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the Audit Committee pre-approves annually a catalog of specific audit and non-audit services in the categories of audit services, audit-related services and tax services that may be performed by our independent registered public accounting firm. If a type of service, that is to be provided by our auditors, has not received such general pre-approval, it will require specific pre-approval by our Audit Committee. The policy prohibits retention of the independent registered public accounting firm to perform the prohibited non-audit functions defined in applicable SEC rules. All of the feesdescribed in the table above were either pre-approved according to this policy, or otherwise pre-approvedapproved in advance by our Audit Committee orin accordance with paragraph (c)(7)(i)(A) of Rule 2-01 of Regulation S-X and thereafter approved by our Board of Directors. Prior to the Merger during 2018 andDirectors in 2017, Kost Forer Gabbay & Kasierer (“Kost Forer”) served as the independent registered public accounting firm for Bioblast, and all audit-related services, tax services and all other services provided by Bioblast to Kost Forer, were pre-approved by the Audit Committee.accordance with Israeli law.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
On March 17, 2019, Enlivex appointed Yarel + Partners as the Company’s principal independent registered public accountant to audit the Company’s consolidated financial statements for the fiscal year ended December 31, 2018. This action, taken at the General Meeting of shareholders, effectively dismissed Kost Forer as of March 17, 2019, as the Company’s principal independent registered public accountants.
Not applicable.
Kost Forer served as the independent auditor for Bioblast prior to the Merger and previously provided an Independent Auditor’s Report dated April 23, 2018, for Bioblast’s financial statements, which comprised the balance sheet as of December 31, 2017, and the related statements of operations, changes in shareholders’ equity, and cash flows for the year then ended, and the related notes to the financial statements.
ITEM 16G. CORPORATE GOVERNANCE
The audit report of Kost Forer
Our ordinary shares are listed on the financial statements of the Company, as ofNasdaq Capital Market and for the years ended December 31, 2017 and December 31, 2016, did not contain any adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles, except that the report for the year ended December 31, 2017 dated April 23, 2018 contained an explanatory paragraph stating that: “The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.”
During the years ended December 31, 2017 and 2016 and through the date of this Annual Report on Form 20-F, there were no disagreements with Kost Forer on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which if not resolved to Kost Forer’s satisfaction would have caused it to make reference thereto in connection with its reports on the financial statements for such years. During the years ended December 31, 2017 and 2016 and through March 17, 2019, there were no reportable events of the type described in Item 16F(a)(1)(v) of Form 20-F.
During the years ended December 31, 2017 and 2016 and through March 17, 2019, the Company did not consult with Yarel + Partners with respect to any of (i) the application of accounting principles to a specified transaction, either completed or proposed; (ii) the type of audit opinion that might be rendered on the Company’s financial statements; or (iii) any matter that was either the subject of a disagreement or an event of the type described in Item 16F(a)(1)(v) of Form 20-F.
The Company provided Kost Forer with a copy of the foregoing disclosure and requested Kost Forer to furnish the Company with a letter addressed to the Securities and Exchange Commission stating whether it agrees with the statements made therein. A copy of such letter, dated April 30, 2019, furnished by Kost Forer is filed as Exhibit 16.1 to this Annual Report on Form 20-F.
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, requires foreign private issuers, such as us, to comply with various corporate governance practices. In addition,accordingly, we are required to comply with Nasdaq Stock Market rules. Under those rules, as a “foreign private issuer” (as such term is defined in Rule 3b-4 under the Exchange Act), we may elect to follow certain corporate governance practices permitted under the Companies Law in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Nasdaq Stock Market rules for U.S. domestic issuers.
In accordance with Israeli law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Listing Rules, we have elected to follow the provisions of the Companies Law, rather than the Nasdaq Listing Rules, with respect to the following requirements:
| ● | Distribution of periodic reports to |
| ● | Quorum. |
| ● | Compensation of officers. We follow Israeli law and practice with respect to the approval of officer compensation. While our compensation committee currently complies with the provisions of the Nasdaq Listing Rules relating to composition requirements and Israeli law generally requires that the compensation of the chief executive officer and all other executive officers be approved, or recommended to the board for approval, by the compensation committee (and in certain instances, shareholder approval is required), Israeli law includes relief from compensation committee approval in certain instances. For details regarding the approvals required under the Israeli Companies Law and regulation promulgated thereunder for the approval of compensation of the chief executive officer, all other executive officers and directors, see Item 7B “Related Party Transactions — Approval of Related Party Transactions under Israeli Law— Disclosure of personal interests of an office holder and approval of acts and transactions.” For details regarding the approvals required under the Israeli Companies Law for the approval of compensation of controlling shareholders, see Item 7B “Related Party Transactions — Approval of Related Party Transactions under Israeli Law— Disclosure of personal interests of a controlling shareholder and approval of transactions.” |
| ● | Nomination of directors. Israeli law and our Amended and Restated Articles of Association do not require |
Shareholder approval is generally required in the event (i) approval by our Board of Directors and our Compensation Committee is not consistent with our office holders compensation policy, or (ii) compensation required to be approved is that of our Chief Executive Officer or an executive officer who is also the controlling shareholder of us (including an affiliate thereof). Such shareholder approval shall require a majority vote of the shares present and voting at a shareholders meeting, provided either (i) such majority includes a majority of the shares held by non-controlling shareholders who do not have a personal interest in the compensation arrangement that are voted at the meeting, excluding for such purpose any abstentions disinterested majority, or (ii) the total shares held by non-controlling disinterested shareholders voted against the arrangement does not exceed 2% of the voting rights in us.
Additionally, approval of the compensation of a director, including a director who is also an executive officer, shall require a simple majority vote of the shares present and voting at a shareholders meeting, if consistent with our office holders compensation policy or a special majority as set forth above if the proposed compensation for the director is not consistent with our compensation policy. Our Compensation Committee and Board of Directors may, in special circumstances, approve the compensation of an executive officer (other than a director or a controlling shareholder) despite shareholders’ objection, based on specified arguments and taking shareholders’ objection into account. Our Compensation Committee may exempt an engagement with a nominee for the position of Chief Executive Officer, who meets the non-affiliation requirements for an external director, as set forth in the Companies Law, from requiring shareholders’ approval, if such engagement is consistent with our office holders compensation policy and our Compensation Committee determines based on specified arguments that presentation of such engagement to shareholders’ approval is likely to prevent such engagement.
A director or executive officer may not be present when the Compensation Committee or Board of Directors of a company discusses or votes upon the terms of his or her compensation, unless the Chairman of the Compensation Committee or Board of Directors (as applicable) determines that he or she should be present to present the transaction that is subject to approval.
| ● | Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than pursuant to Nasdaq Listing Rule 5635. In particular, under this Nasdaq rule, shareholder approval would otherwise generally be required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of equity compensation arrangements; and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. |
DisclosureExcept as stated above, we currently intend to comply with the rules generally applicable to U.S. domestic companies listed on Nasdaq. We may in the future decide to use the foreign private issuer exemption with respect to some or all of personal interests of a controlling shareholder and approval of transactions
Under the Companies Law,other Nasdaq corporate governance rules. Following our home country governance practices, as opposed to the disclosure requirements that apply to an office holder alsowould otherwise apply to a controlling shareholdercompany listed on Nasdaq, may provide less protection than is accorded to investors under Nasdaq listing requirements applicable to domestic issuers. For more information, see “Item 3D “Key Information - Risks Related to the Ownership of Our Ordinary Shares - As a public company. The definition“foreign private issuer,” we are permitted to follow certain home country corporate governance practices instead of “controlling shareholder”otherwise applicable Nasdaq Capital Market requirements, which may result in connection with matters governing: (i) extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, (ii) certain private placements in which the controlling shareholder has a personal interest, (iii) certain transactions with a controlling shareholder or relative with respectless protection than is accorded to services providedinvestors under rules applicable to or employment by the company, (iv) the terms of employment and compensation of the general manager, and (v) the terms of employment and compensation of office holders of the company when such terms deviate from the compensation policy previously approved by the company’s shareholders, also includes shareholders that hold 25% or more of the voting rights if no other shareholder owns more than 50% of the voting rights in the company (and the holdings of two or more shareholders which each have a personal interest in such matter will be aggregated for the purposes of determining such threshold).domestic U.S. issuers.”
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
Under the Companies Law, extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, require the approval of the audit committee, the board of directors and the shareholders, in that order. Transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the compensation committee, the board of directors and the shareholders, in that order. In addition, the approval of such extraordinary transactions by the shareholders require at least a majority of the shares voted by the shareholders of the company participating and voting in a shareholders’ meeting, provided that one of the following requirements is fulfilled:
If such transaction concerns the terms of office and employment of such controlling shareholder, in his capacity as an office holder or an employee of the company, such terms of office and employment approved by the compensation committee and board of directors shall be in accordance with the compensation policy of the company. Nonetheless, the compensation committee and the board of directors may approve terms of office and compensation of a controlling shareholder which do not comply with the company’s compensation policy, provided that the compensation committee and, thereafter, the board of directors approve such terms, based on, among other things, the considerations listed under Section 267B(a) and Parts A and B of Annex 1A of the Companies Law, as those are described above. Following such approval by the compensation committee and board of directors, shareholder approval would be required.
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval, in the same manner described above, is required once every three years, unless, with respect to extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
Not applicable.
ITEM 17. FINANCIAL STATEMENTS
Not applicable.
The Registrant has responded to Item 18 in lieu of responding to this Item 17.
ITEM 18. FINANCIAL STATEMENTS
The following financial statements, and the related notes thereto, and the Report of Independent Public Accountants are filed as a part of this annual report.Annual Report on Form 20-F.
Audited Financial Statements
ITEM 19. EXHIBITS
EXHIBIT INDEX
| EXHIBIT NUMBER |
EXHIBIT INDEX
| * | Filed herewith. |
| † | Portions of the exhibit have been omitted because such information is both (i) not material and (ii) could be competitively harmful if publicly disclosed. |
SIGNATURES
SIGNATURES
Enlivex Therapeutics Ltd. hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Enlivex Therapeutics Ltd. | ||
| By: | /s/ Oren Hershkovitz | |
| Oren Hershkovitz | ||
| Chief Executive Officer | ||
| Date: April | ||
ENLIVEX THERAPEUTICS LTD.
FINANCIAL STATEMENTS
AS OF DECEMBER 31, 20192022
F-1
ENLIVEX THERAPEUTICS LTD.
FINANCIAL STATEMENTS
AS OF DECEMBER 31, 20192022
INDEX

 |  |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of ENLIVEX THERAPEUTICS LTD.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying balance sheets of Enlivex Therapeutics Ltd. and subsidiaries (the “Company”) as of December 31, 20192022 and 2018,2021, and the related statements of income,operations, comprehensive income,loss, shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2019,2022, and the related notes (collectively referred to as the financial statements).
We also have audited the Company’s internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 20192022 and 2018,2021, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2019,2022, in conformity with accounting principles generally accepted in the United States of America.
Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control—Integrated Framework (2013) issued by COSO.
Basis for Opinion
The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.

Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Yarel + Partners
Yarel + Partners
Certified Public Accountants (Isr.)
Tel-Aviv, Israel
April 2, 2020March 31, 2023
We have served as the Company’s auditor since 2013.
F-4
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands (except share data)
December 31, | ||||||||
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents (notes 2d, 3) | $ | 3,948 | $ | 9,736 | ||||
| Short term deposits | 8,060 | 40 | ||||||
| Prepaid expenses | 510 | 288 | ||||||
| Other receivables | 403 | 213 | ||||||
| Cash held with respect to CVR Agreement (notes 1a,3) | 1,500 | - | ||||||
| Receivables for the sale of Trehalose (note 1a) | 2,000 | - | ||||||
| Total Current Assets | 16,421 | 10,277 | ||||||
| Non-Current Assets | ||||||||
| Restricted cash (notes 2e, 3) | 76 | 56 | ||||||
| Long-term prepaid expenses | 5 | 16 | ||||||
| Property and equipment, net (notes 2f, 4) | 648 | 685 | ||||||
| Other assets (notes 2h, 8) | 410 | - | ||||||
| Total Non-Current Assets | 1,139 | 757 | ||||||
| TOTAL ASSETS | $ | 17,560 | $ | 11,034 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Accounts payable trade | $ | 316 | $ | 173 | ||||
| Accrued expenses and other liabilities (note 5) | 1,897 | 944 | ||||||
| Payables to related parties (note 13) | 367 | 13 | ||||||
| CVR holders (note 1a) | 3,400 | - | ||||||
| Total Current Liabilities | 5,980 | 1,130 | ||||||
| Non-Current Liabilities | ||||||||
| Retirement benefit obligations (note 2j) | - | 6 | ||||||
| Other long-term Liabilities (notes 2h, 8) | 298 | - | ||||||
| Warrants (note 6) | - | 192 | ||||||
| Total Non-Current Liabilities | 298 | 198 | ||||||
| Commitments and Contingent Liabilities(note 9) | - | |||||||
| TOTAL LIABILITIES | 6,278 | 1,328 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Common stock of NIS 0.40 ($0.11) par value: (note 10) Authorized: 45,000,000 and 11,861,073 shares as of December 31, 2019 and 2018; Issued and outstanding: 10,334,126 and 3,509,405 as of December 31, 2019 and 2018; | 1,151 | 396 | ||||||
| Series A Preferred Stock, NIS 0.4 ($0.11) par value: (note 10) Authorized and outstanding 3,146,815 and 3,059,730 shares as of December 31, 2018 | - | 309 | ||||||
| Series B Preferred Stock, NIS 0.4 ($0.11) par value: (note 10) Authorized and outstanding 3,485,703 and 1,373,804 shares as of December 31, 2018 | - | 156 | ||||||
| Series C Preferred Stock, NIS 0.4 ($0.11) par value: (note 10) Authorized and outstanding 3,146,815 and 525,171 shares as of December 31, 2018 | - | 59 | ||||||
| Additional paid in capital | 37,104 | 27,326 | ||||||
| Foreign currency translation adjustments (note 2c) | (1,300 | ) | (2,251 | ) | ||||
| Accumulated deficit | (25,673 | ) | (16,289 | ) | ||||
| TOTAL SHAREHOLDERS’ EQUITY | 11,282 | 9,706 | ||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | 17,560 | $ | 11,034 | ||||
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents (note 3) | $ | 49,945 | $ | 11,202 | ||||
| Short term deposits (note 4) | 299 | 10,004 | ||||||
| Marketable securities | - | 62,924 | ||||||
| Prepaid expenses and other receivables (note 5) | 2,086 | 2,312 | ||||||
| Total Current Assets | 52,330 | 86,442 | ||||||
| Non-Current Assets | ||||||||
| Property and equipment, net (note 6) | 9,875 | 2,530 | ||||||
| Other assets (notes 7 & 9) | 5,437 | 6,174 | ||||||
| Total Non-Current Assets | 15,312 | 8,704 | ||||||
| TOTAL ASSETS | $ | 67,642 | $ | 95,146 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Accounts payable trade (note 14) | $ | 1,948 | $ | 878 | ||||
| Accrued expenses and other liabilities (note 8) | 4,659 | 3,840 | ||||||
| Total Current Liabilities | 6,607 | 4,718 | ||||||
| Non-Current Liabilities | ||||||||
| Other long-term Liabilities (note 9) | 4,194 | 5,389 | ||||||
| Total Non-Current Liabilities | 4,194 | 5,389 | ||||||
| Commitments and Contingent Liabilities (note 10) | ||||||||
| TOTAL LIABILITIES | 10,801 | 10,107 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary shares of NIS 0.40 par value: (note 11) Authorized: 45,000,000 shares as of December 31, 2022 and 2021; Issued and outstanding: 18,421,852 and 18,331,507 as of December 31, 2022 and 2021, respectively; | 2,117 | 2,107 | ||||||
| Additional paid in capital | 136,648 | 133,796 | ||||||
| Foreign currency translation reserve | 1,101 | 1,101 | ||||||
| Accumulated deficit | (83,025 | ) | (51,965 | ) | ||||
| TOTAL SHAREHOLDERS’ EQUITY | 56,841 | 85,039 | ||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | 67,642 | $ | 95,146 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
ENLIVEX THERAPEUTICS LTD.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
U.S. dollars in thousands (except share and per share data)
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Revenues(note 2k) | $ | - | $ | - | $ | - | ||||||
| Operating expenses: | ||||||||||||
| Research and development expenses, net (notes 2l, 15a) | 5,724 | 4,255 | 1,691 | |||||||||
| General and administrative expenses (note 15b) | 2,895 | 1,044 | 480 | |||||||||
| 8,619 | 5,299 | 2,171 | ||||||||||
| Operating (loss) | (8,619 | ) | (5,299 | ) | (2,171 | ) | ||||||
| Financial income (note 15c) | 238 | 1,060 | 37 | |||||||||
| Financial expenses (note 15d) | (1,003 | ) | (3 | ) | (370 | ) | ||||||
| Net (loss) | $ | (9,384 | ) | $ | (4,242 | ) | $ | (2,504 | ) | |||
| Other comprehensive gain (loss) | ||||||||||||
| Interest on convertible notes | - | |||||||||||
| Exchange differences arising from translating financial statements from functional to presentation currency (note 2c, 2r) | 951 | (748 | ) | 336 | ||||||||
| Total other comprehensive gain (loss) | 951 | (748 | ) | 336 | ||||||||
| Total comprehensive (loss) | $ | (8,433 | ) | $ | (4,990 | ) | $ | (2,168 | ) | |||
| Basic & diluted (loss) per share (note 2o) | $ | (1.11 | ) | $ | (1.40 | ) | $ | (0.94 | ) | |||
| Weighted average number of shares outstanding | 8,649,486 | 3,509,346 | 3,425,925 | |||||||||
| Year ended December 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Revenues | $ | - | $ | - | $ | - | ||||||
| Operating expenses: | ||||||||||||
| Research and development expenses, net (note 16a) | 18,693 | 12,881 | 6,086 | |||||||||
| General and administrative expenses (note 16b) | 7,104 | 6,407 | 3,699 | |||||||||
| 25,797 | 19,288 | 9,785 | ||||||||||
| Operating (loss) | (25,797 | ) | (19,288 | ) | (9,785 | ) | ||||||
| Other income (expenses), net (note 16c) | (5,263 | ) | 4,820 | (2,039 | ) | |||||||
| Net (loss) | $ | (31,060 | ) | $ | (14,468 | ) | $ | (11,824 | ) | |||
| Other comprehensive gain | ||||||||||||
| Exchange differences arising from translating financial statements from functional to presentation currency | - | 124 | 2,277 | |||||||||
| Total other comprehensive gain | - | 124 | 2,277 | |||||||||
| Total comprehensive (loss) | $ | (31,060 | ) | $ | (14,344 | ) | $ | (9,547 | ) | |||
| Basic & diluted (loss) per share | $ | (1.69 | ) | $ | (0.81 | ) | $ | (0.90 | ) | |||
| Weighted average number of shares outstanding | 18,394,886 | 17,859,713 | 13,169,208 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
ENLIVEX THERAPEUTICS LTD.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
U.S. dollars in thousands (except share data)data)
| Common stock | Preferred Stock | Currency | ||||||||||||||||||||||||||||||
| Number of shares | Share capital | Number of shares | Share capital | Additional paid in capital | translation adjustments | Accumulated deficit | Total | |||||||||||||||||||||||||
| JANUARY 1, 2017 | 3,390,733 | $ | 383 | 3,059,730 | $ | 309 | $ | 13,500 | $ | (1,839 | ) | $ | (9,543 | ) | $ | 2,810 | ||||||||||||||||
| Issuance of Common Stock in connection with conversion of notes in 2016 | 118,611 | 13 | - | - | (13 | ) | - | - | - | |||||||||||||||||||||||
| Issuance of Preferred Stock Series B for cash consideration of $8,249 net of $519 issuance costs | - | - | 1,373,804 | 156 | 7,574 | - | - | 7,730 | ||||||||||||||||||||||||
| Stock based compensation | - | - | - | - | 121 | - | - | 121 | ||||||||||||||||||||||||
| Other comprehensive gain | - | - | - | - | - | 336 | - | 336 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (2,504 | ) | (2,504 | ) | ||||||||||||||||||||||
| DECEMBER 31, 2017 | 3,509,344 | 396 | 4,433,534 | 465 | 21,182 | (1,503 | ) | (12,047 | ) | 8,493 | ||||||||||||||||||||||
| Exercise of Options | 61 | * | - | - | * | - | - | * | ||||||||||||||||||||||||
| Issuance of Preferred Stock Series C for cash consideration of $5,350 net of $156 issuance costs | - | - | 525,171 | 59 | 5,135 | - | - | 5,194 | ||||||||||||||||||||||||
| Stock based compensation | - | - | - | - | 1,009 | - | - | 1,009 | ||||||||||||||||||||||||
| Other comprehensive loss | - | - | - | - | - | (748 | ) | - | (748 | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (4,242 | ) | (4,242 | ) | ||||||||||||||||||||||
| DECEMBER 31, 2018 | 3,509,405 | 396 | 4,958,705 | 524 | 27,326 | (2,251 | ) | (16,289 | ) | 9,706 | ||||||||||||||||||||||
| Conversion of Preferred Shares to Ordinary Shares | 5,479,547 | 605 | (4,958,705 | ) | (524 | ) | (81 | ) | - | - | - | |||||||||||||||||||||
| Issuance of shares upon exercise of warrants | 20,348 | 2 | 247 | 249 | ||||||||||||||||||||||||||||
| Issuance of shares for cash consideration of $8,362 net of $655 issuance costs | 682,631 | 76 | 7,631 | 7,707 | ||||||||||||||||||||||||||||
| Shares issued in conjunction with share option exercise | 221,641 | 25 | 574 | 599 | ||||||||||||||||||||||||||||
| Issuance of shares in connection with the merger | 420,554 | 47 | 597 | 644 | ||||||||||||||||||||||||||||
| Stock based compensation | 810 | 810 | ||||||||||||||||||||||||||||||
| Other comprehensive gain | - | 951 | 951 | |||||||||||||||||||||||||||||
| Net loss | - | (9,384 | ) | (9,384 | ) | |||||||||||||||||||||||||||
| DECEMBER 31, 2019 | 10,334,126 | $ | 1,151 | - | $ | - | $ | 37,104 | $ | (1,300 | ) | $ | (25,673 | ) | $ | 11,282 | ||||||||||||||||
| Ordinary Shares | ||||||||||||||||||||||||
| Number of shares | Share capital | Additional paid in capital | Currency translation reserve | Accumulated deficit | Total | |||||||||||||||||||
| JANUARY 1, 2020 | 10,334,126 | 1,151 | 37,104 | (1,300 | ) | (25,673 | ) | 11,282 | ||||||||||||||||
| Issuance of shares and warrants for cash consideration of $26,968 net of $2,501 issuance costs | 3,286,416 | 382 | 24,085 | 24,467 | ||||||||||||||||||||
| Exercise of options | 37,746 | 4 | 97 | 101 | ||||||||||||||||||||
| Exercise of warrants | 929,646 | 109 | 8,405 | 8,514 | ||||||||||||||||||||
| Stock based compensation | - | 670 | 670 | |||||||||||||||||||||
| Other comprehensive loss | - | 2,277 | 2,277 | |||||||||||||||||||||
| Net loss | - | - | (11,824 | ) | (11,824 | ) | ||||||||||||||||||
| DECEMBER 31, 2020 | 14,587,934 | 1,646 | 70,361 | 977 | (37,497 | ) | 35,487 | |||||||||||||||||
| Issuance of shares and warrants for cash consideration of $57,629 net of $4,455 issuance costs | 2,848,629 | 352 | 52,822 | 53,174 | ||||||||||||||||||||
| Exercise of options | 39,131 | 5 | 101 | 106 | ||||||||||||||||||||
| Exercise of warrants | 855,813 | 104 | 7,598 | 7,702 | ||||||||||||||||||||
| Stock based compensation | 2,914 | 2,914 | ||||||||||||||||||||||
| Other comprehensive loss | 124 | 124 | ||||||||||||||||||||||
| Net loss | - | (14,468 | ) | (14,468 | ) | |||||||||||||||||||
| DECEMBER 31, 2021 | 18,331,507 | 2,107 | 133,796 | 1,101 | (51,965 | ) | 85,039 | |||||||||||||||||
| Exercise of options | 22,875 | 3 | 147 | 150 | ||||||||||||||||||||
| Exercise of warrants | - | |||||||||||||||||||||||
| Restricted stock units vested | 67,470 | 7 | (7 | ) | - | |||||||||||||||||||
| Stock based compensation | - | 2,712 | 2,712 | |||||||||||||||||||||
| Net loss | - | (31,060 | ) | (31,060 | ) | |||||||||||||||||||
| DECEMBER 31, 2022 | 18,421,852 | $ | 2,117 | $ | 136,648 | $ | 1,101 | $ | (83,025 | ) | $ | 56,841 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
ENLIVEX THERAPEUTICS LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net (loss) | $ | (9,384 | ) | $ | (4,242 | ) | $ | (2,504 | ) | |||
| Adjustments required to reflect net cash used in operating activities: | ||||||||||||
| Income and expenses not involving cash flows: | ||||||||||||
| Depreciation | 206 | 121 | 83 | |||||||||
| Non-cash operating lease expense | 168 | - | - | |||||||||
| Decrease in retirement benefit obligations | (7 | ) | - | - | ||||||||
| Loss on sale of property and equipment | 75 | - | - | |||||||||
| Share-based compensation | 810 | 1,009 | 121 | |||||||||
| Issuance expenses related to warrants exercisable into shares | - | - | 19 | |||||||||
| Changes in fair value of warrants exercisable into shares | 51 | (132 | ) | - | ||||||||
| Changes in operating asset and liability items: | ||||||||||||
| (Increases) decrease in prepaid expenses | 277 | (293 | ) | 33 | ||||||||
| (Increase) decrease in other receivables | 41 | (130 | ) | (40 | ) | |||||||
| Increase (decrease) in accounts payable trade | 114 | 145 | 1 | |||||||||
| Increase (decrease) in accrued expenses and other liabilities | 433 | 372 | (11 | ) | ||||||||
| Operating lease liabilities | (167 | ) | - | - | ||||||||
| Increase (decrease) in related parties payable | 342 | (11 | ) | (6 | ) | |||||||
| Net cash (used in) operating activities | (7,041 | ) | (3,161 | ) | (2,304 | ) | ||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property and equipment | (193 | ) | (461 | ) | (130 | ) | ||||||
| Proceeds from sale of property and equipment | 4 | - | - | |||||||||
| Investment in short-term bank deposits | (7,714 | ) | (40 | ) | - | |||||||
| Net cash, cash equivalents and restricted cash received in the issuance of shares for the net assets of Bioblast Pharma Ltd. | 1,544 | |||||||||||
| Net cash provided by (used in) investing activities | (6,359 | ) | (501 | ) | (130 | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of ordinary shares, preferred shares and warrants, net | 7,707 | 5,194 | 8,055 | |||||||||
| Proceeds from exercise of options | 599 | * | - | |||||||||
| Net cash provided by financing activities | 8,306 | 5,194 | 8,055 | |||||||||
| Increase in cash, cash equivalents and restricted cash | (5,094 | ) | 1,532 | 5,621 | ||||||||
| Cash, cash equivalents and restricted cash - beginning of year | 9,792 | 9,032 | 3,045 | |||||||||
| Exchange rate differences on cash, cash equivalents and restricted cash | 826 | (772 | ) | 366 | ||||||||
| Cash, cash equivalents and restricted cash - end of year | $ | 5,524 | $ | 9,792 | $ | 9,032 | ||||||
| Non-cash transactions: | ||||||||||||
| Conversion of preferred stock to ordinary shares | $ | 525 | $ | - | $ | - | ||||||
| Conversion of 6% preference on preferred stock to ordinary shares | $ | 2,071 | $ | $ | ||||||||
| Issuance of ordinary shares upon exercise of warrants | $ | 249 | $ | $ | ||||||||
| Issuance of common and preferred shares in connection with conversion of convertible notes and accrued interest | $ | - | $ | - | $ | 7 | ||||||
| Issuance of shares to the former shareholders of Bioblast Pharma Ltd. | $ | 47 | $ | $ | ||||||||
| Net assets acquired in the issuance of shares for the net assets of Bioblast Pharma Ltd. excluding cash, cash equivalents and restricted cash: | ||||||||||||
| Assets acquired | (2,632 | ) | ||||||||||
| Less - liabilities assumed | 3,532 | |||||||||||
| $ | 900 | $ | $ | |||||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for taxes | $ | - | $ | - | $ | - | ||||||
| Cash received for interest, net | $ | 112 | $ | 138 | $ | 37 | ||||||
| Year ended December 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net loss | $ | (31,060 | ) | $ | (14,468 | ) | $ | (11,824 | ) | |||
| Adjustments required to reflect net cash used in operating activities: | ||||||||||||
| Income and expenses not involving cash flows: | ||||||||||||
| Depreciation | 777 | 546 | 286 | |||||||||
| Loss (income) on marketable securities and short-term bank deposits | 1,983 | (5,590 | ) | - | ||||||||
| Non-cash operating lease expenses | 831 | 413 | 167 | |||||||||
| Share-based compensation | 2,712 | 2,914 | 670 | |||||||||
| Changes in operating asset and liability items: | ||||||||||||
| (Increase) decrease in prepaid expenses and other receivables | 278 | (1,249 | ) | 1,823 | ||||||||
| Increase in accounts payable trade | 1,168 | 442 | 36 | |||||||||
| (Decrease) increase in accrued expenses and other liabilities | 684 | (466 | ) | (2,022 | ) | |||||||
| Operating lease liabilities | (1,326 | ) | (84 | ) | (143 | ) | ||||||
| Net cash used in operating activities | (23,953 | ) | (17,542 | ) | (11,007 | ) | ||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property and equipment | (8,122 | ) | (1,625 | ) | (1,019 | ) | ||||||
| (Release) investment in short-term bank deposits, net | 9,705 | 20,030 | (19,958 | ) | ||||||||
| Purchases of marketable securities | (1,608 | ) | (104,417 | ) | - | |||||||
| Proceeds from sale of marketable securities | 62,549 | 47,604 | - | |||||||||
| Net cash provided by (used in) investing activities | 62,524 | (38,408 | ) | (20,977 | ) | |||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of shares and warrants, net | - | 53,174 | 24,467 | |||||||||
| Proceeds from exercise of warrants | - | 7,702 | 8,514 | |||||||||
| Proceeds from exercise of options | 150 | 106 | 101 | |||||||||
| Net cash provided by financing activities | 150 | 60,982 | 33,082 | |||||||||
| Increase (decrease) in cash, cash equivalents and restricted cash | 38,721 | 5,032 | 1,098 | |||||||||
| Cash, cash equivalents and restricted cash - beginning of year | 11,636 | 7,012 | 5,524 | |||||||||
| Exchange rate differences on cash, cash equivalents and restricted cash | - | (408 | ) | 390 | ||||||||
| Cash, cash equivalents and restricted cash - end of year | $ | 50,357 | $ | 11,636 | $ | 7,012 | ||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for taxes | $ | - | $ | - | $ | - | ||||||
| Cash received for interest, net | $ | 666 | $ | 39 | $ | 160 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1 – GENERAL INFORMATION
| a. | General |
Enlivex Therapeutics Ltd. (the “Parent” and, including its consolidated subsidiaries, “we”, “us”, “our” or the “Company”“Company”) wasis a clinical-stage macrophage reprogramming immunotherapy company originally incorporated on January 22, 2012 under the laws of the State of Israel as Bioblast Pharma Ltd.Israel.
In January 2015, Bioblast Pharma Inc. was established in the State of Delaware as a wholly owned subsidiary of the Parent (the “Subsidiary”).
On March 26, 2019, upon consummation of a merger transaction between the Parent and Enlivex Therapeutics R&D Ltd., (“Enlivex R&D”, formerly known as Enlivex Therapeutics Ltd.), whereby Enlivex R&D merged with Treblast Ltd. (a subsidiary of the Parent) with Enlivex R&D remaining as the surviving entity in the merger (the “Merger”), the Parent changed its name to Enlivex Therapeutics Ltd.
The Merger has been treated as a reverse recapitalization of the Parent for financial accounting and reporting purposes, as such, Enlivex R&D is treated as the acquirer and the Parent is treated as the acquired entity.
Excess of consideration paid over net assets acquired and other merger-related costs were recorded as a charge to additional paid-in capital as discussed in Note 7.
As a result, the financial statements of the Company prior to the Merger are the historical financial statements of Enlivex R&D whereas the financial statements of the Company after the Merger reflect the results of the consolidated operations. All historical information presented herein has been retroactively restated to reflect the effect of the merger shares exchange ratio, reverse stock split and change to the authorized number of Ordinary Shares, in each case effected in connection with the Merger and in accordance with Accounting Standards Codification Topic 260, “Earnings Per Share”.
The Company is a clinical-stageclinical stage macrophage reprogramming immunotherapy company. Enlivex R&D was incorporated in September 2005 under the laws of the State of Israel and has been engaged since inception in the development of an allogeneic drug pipelinecompany, developing AllocetraTM, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing. Immune system rebalancing is critical for the treatmentand resolution of life-threatening immune and inflammatory conditions,conditions. Non-homeostatic macrophages contribute significantly to the severity of certain diseases, which involve the hyper-expression of cytokines (Cytokine Release Syndrome) and for which there are no U.S. Food and Drug Administration (“FDA”) approved treatments, as well as treatinginclude solid tumors, via modulating immune-checkpoint rebalancing. The Company’s innovative immunotherapy candidate, Allocetra™, is a novel immunotherapy candidate based on a unique mechanism of action that targets clinical indications that are defined as “unmet medical needs,” such as preventing or treating complications associated with bone marrow transplants and/or hematopoietic stem cell transplants, sepsis and acute multiple organ failure. The Company also intends to develop its cell-based therapy to be combined with currently effective treatments of solid tumors via immune checkpoint rebalancing to increase the efficacy of various anti-cancer therapies, including Chimeric Antigen Receptor T-Cell Therapy and therapies targeting T-Cell Receptor Therapy. The Company’s developmentothers.
AllocetraTM is based on the discoveries of Professor Dror Mevorach, an expert on immune activity, macrophage activation and clearance of dying (apoptotic) cells, in his laboratory in the Hadassah University Hospital located in the State of Israel.
At the closing of the Merger, the Parent, Enlivex R&D, the Parent’s pre-Merger CEO, as representative of the pre-Merger Parent’s shareholders, and a rights agent entered into a Contingent Value Rights Agreement (the “CVR Agreement”). Pursuant to the CVR Agreement, the Parent’s shareholders immediately prior to the Merger received one CVR for each of the Parent’s’ ordinary shares held of record immediately prior to the closing of the Merger. Each CVR represents the right to receive payments based on the Parent’s pre-Merger clinical development programs. CVR holders are entitled to receive 100% of any payments up to $20,000 received by the Company and 50% of any subsequent consideration in excess of such amount, in each case, net of all related transaction expenses.
On February 19, 2019, the Parent sold its pre-Merger clinical development programs for “Trehalose” to Seelos Therapeutics, Inc. (“Seelos”), a clinical-stage biopharmaceutical company. Under the terms of the agreement between the Parent and Seelos, Seelos paid $1,500 upon closing and paid an additional $2,000 upon the first anniversary of the closing. Seelos has agreed to pay additional milestone payments of up to $17,000 upon completion of the related clinical study for Trehalose and approval of a New Drug Application by the FDA, as well as royalties.
The Company’s ordinary shares, par value of NIS 0.40 per share (“Ordinary Shares” or “ordinary shares”Shares”), are traded under the symbol “ENLV” on both the Nasdaq Capital Market and on the on the Tel Aviv Stock Exchange.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| b. | Financial resources |
The Company devotes substantially all of its efforts toward research and development activities and raising capital.capital to support such activities. The Company’s activities are subject to significant risks and uncertainties, including failing to secure additional funding before the Company achieves sustainable revenues and profit from operations.
Research and development activities have required significant capital investment since the Company’s inception. The Company expects that its operations to continue towill require additional cash investment to pursue the Company’s research and development activities, including preclinical studies, formulation development, clinical trials and related drug manufacturing. The Company has not generated any revenues or product sales and has not achieved profitable operations or positive cash flow from operations. The Company’sCompany has experiencedincurred net losses since its inception, and, as of December 31, 2019,2022, had an accumulated deficit of $25,673.$83.2 million.
The Company raised $7,751 in cash in conjunction with the Merger and the issuance of shares immediately after the Merger, as well as additional cash of $22,629 subsequent to December 31, 2019 in conjunction with certain registered securities offerings. See note 16 to these audited consolidated financial statements. However, the Company expects to continue to incur additional losses for at least the next several years, and over that period the Company will need to raise additional debt or equity financing or enter into partnerships to fund its development. If the Company is not able to achieve its funding requirements, it may be required to reduce discretionary spending, may not be able to continue the development of its product candidates or may be required to delay part of its development programs, which could have a material adverse effect on the Company’s ability to achieve its intended business objectives. There can be no assurances that additional financing will be secured or, if secured, will be on favorable terms.
The ability of the Company to transition to profitability in the longer term is dependent on developing productsa combination of factors, including (i) successfully obtaining additional financing discussed above; (ii) the Company’s ability to successfully begin marketing of its product candidates or complete revenue-generating partnerships with other companies; (iii) the success of its research and development; (iv) the impact of competition from other biotechnology and pharmaceutical companies that may develop competitive therapies; and (v) regulatory approval and market acceptance of the Company’s product revenues to support its expenses.candidates.
The Company’s management and board of directors (the “Board”) are of the opinion that itsthe Company’s current financial resources will be sufficient to continue the development of the Company’s productsproduct candidates for at least twelve months from the filing of these financial statements with the U.S. Securities and Exchange Commission. The Company may determine, however, to raise additional capital during such period as the Board deems prudent. The Company’s management plans to finance its operations with issuances of the Company’s Annual Report on Form 20-Fequity securities and, in the longer term, revenues. There are no assurances, however, that the Company will be successful in obtaining the financing necessary for its long-term development. The Company’s ability to continue to operate in the year ended December 31, 2019, of which these audited consolidatedlong term is dependent upon additional financial support.
| c. | Approval of financial statements |
These financial statements form a part.were approved by the Board on March 31, 2023.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The Company’s audited consolidated financial statements as of December 31, 20192022 and 2018,2021 and for each of the years in the three-year period ended December 31, 2019,2022 have been prepared in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”).Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
The accompanying consolidated financial statements include the accounts of the Company and Enlivex Therapeutics R&D Ltd. and Enlivex Therapeutics RDO Ltd., its wholly-owned subsidiaries incorporated in Israel and Enlivex Theraputics Inc. its wholly-owned subsidiary incorporated in the State of Delaware.
All intercompany accounts and transactions have been eliminated in consolidation.
The significant accounting policies described below have been applied on a consistent basis for all years presented.
The financial statements have been prepared on the basis of historical cost, subject to adjustment of financial assets and liabilities to their fair value through profit or loss. The Company classifies its expenses on the statement of comprehensive loss based on the operating characteristics of such expenses.
Use of estimates
The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions, it also requires that management exercise its judgment in applying the Company’s accounting policies. The Company’s management believes that the estimates, judgments and assumptions used were reasonable based upon information available at the time they were made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts in the statements of revenue and expensesoperations during theeach reporting period. Actual results could differ materially from those estimates.
Functional currency and translation to the reporting currency
The functional currency of the Company is the U.S. dollar because the U.S. dollar is the currency of the primary economic environment in which the Company operates and expects to continue to operate in the foreseeable future.
The functional currency of Enlivex Therapeutics R&D Ltd. was the New Israeli Shekel (“NIS”), until September 30, 2021. The Company reassessed the functional currency and determined that the factors supported the U.S. dollar as the functional currency prospectively from September 30, 2021. Significant elements in the decision to effect the functional currency change involved (i) a change in the Company’s business strategy resulting from a planned initiation of oncology clinical trials (ii) the newly-developed frozen formulation of Allocetra, which isremoved the local currency in which the Company operates.
The financial statementsconstraints of short shelf life and storage of the Companyliquid formulation and enabled the conduct of clinical trials outside of Israel. Additionally, the Company’s sources of financing are the U.S. capital markets and U.S. dollar-denominated government grants from the State of Israel, and all of the Company’s budgeting and planning are conducted solely in U.S. dollars, all of which contributed to the decision to change the Company’s functional currency. Monetary assets and liabilities denominated in foreign currencies were translated into U.S. dollars using exchange rates in accordance with ASC 830, “Foreign Currency Matters”. Accordingly,effect at the balance sheet date. Balances related to non-monetary assets and liabilities wereare based on translated from local currencies to U.S. dollars using year end exchange rates, equity items were translated at the exchange ratesamounts as of the date of the equity transaction,change, and non-monetary assets acquired and liabilities incurred after September 30, 2021 were translated at the approximate exchange rate prevailing at the date of the transaction. Transactions included in the statement of income and expense itemsuntil the date of change were translated at average exchange rates during the year.
applicable period, and transactions after September 30, 2021 were translated at the approximate exchange rate in effect at the time of the transaction. Gains or losses resulting from translation adjustments (which result from translating an entity’s financial statements into U.S. dollars if its functional currency is different than the U.S. dollar)until September 30, 2021 are reported in other comprehensive income (loss).
The following table presents data regarding U.S. dollar exchange rates:
| 2022 | 2021 | 2020 | ||||||||||
| At December 31, 1 U.S. $ = | NIS 3.519 | NIS 3.110 | NIS 3.215 | |||||||||
| Increase (decrease) during the year | 13.15 | % | (3.27 | )% | (6.97 | )% | ||||||
F-10
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share
Cash and per share data)cash equivalents
Balances denominated in, or linked to foreign currency are stated on the basis of the exchange rates prevailing at the balance sheet date. For foreign currency transactions included in the statement of income, the exchange rates applicable on the relevant transaction dates are used. Transaction gains or losses arising from changes in the exchange rates used in the translation of such balances are carried to financing income or expenses as applicable.
The exchange rate for 1 U.S. $ was 3.456, 3.748 and 3.467 NIS as of December 31, 2019, 2018 and 2017, respectively. The increase (decrease) of the U.S. $ against the NIS for the years ended December 31, 2019, 2018 and 2017 was (7.8)%, 8.1% and (9.8)%, respectively.
Cash equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less at acquisition.
Restricted cash
Amounts included in restricted cash are held in interest bearing saving accounts and represent cash amounts required to be set aside by a contractual agreement for the rental of the Company’s premises and for credit cards.cards and cash amounts required to be set aside by other contractual agreements.
Marketable securities
The Company has an investment policy that includes guidelines on acceptable investment securities, minimum credit quality, maturity parameters, and concentration and diversification. The Company invests its excess cash primarily in mutual funds that are classified based on the nature of their underlying securities and their availability for use in current operations. The Company’s marketable equity securities are measured at fair value with gains and losses recognized in other income/(expense), net.
Net (loss) gain recognized on equity securities for the years ended December 31, 2022, 2021 and 2020 were $(1,983) thousand, $5,590 thousand and $0.
Property and equipment
Property and equipment are stated at historical cost less depreciation. Assets are depreciated using the straight-line method over the estimated useful lives of the assets. The annual depreciation rates are as follows:
| % | ||||
| Computers | ||||
| Office furniture and equipment | 7 | |||
| Leasehold improvements | ||||
| Laboratory equipment | ||||
Impairment of non-financial assets
The long-lived assets of the Company are reviewed for impairment in accordance with ASC 360, “Property, Plant, and Equipment”, whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. RecoverabilityASC 360 requires three steps to identify, recognize and measure the impairment of assetsa long-lived asset (asset group) to be held and used is measured byused:
Step 1 - consider whether indicators of impairment are present;
Step 2 - if indicators of impairment are present, perform a comparisonrecoverability test comparing the sum of the carrying amount of an asset with the futureestimated undiscounted cash flows expectedattributable to be generated by the assets. If such assets are consideredlong-lived asset or asset group in question to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceedslong-lived asset or asset group; and
Step 3 - if the undiscounted cash flows used in the recoverability test are less than the carrying amount of the long-lived asset (asset group), estimate the fair value of the assets.long-lived asset or asset group and recognize an impairment loss when the carrying amount of the long-lived asset or asset group exceeds the estimated fair value. During the years 2018, 2017,2022, 2021, and 2016,2020, no impairment losses were identified.
Leases
In accordance with ASU No. 2016-02, Leases (Topic 842), right-of-use (“ROU”) assets represent our right to use the underlying leased assets over the lease term, and lease liabilities represent our obligation to make lease payments arising from the related leases. ROU assets and lease liabilities are recognized at the lease commencement date based on the present value of lease payments over the lease term. Lease terms may include options to extend or terminate the lease when we believe it is reasonably certain that we will exercise such options. Operating lease ROU assets are reported in other assets, and operating lease liabilities are reported in accounts payable and accrued liabilities (current), and other long-term liabilities (non-current) in our condensed consolidated balance sheets.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Because most of the Company’s leases do not provide an implicit interest rate, the Company, with input from a third-party appraiser, uses its estimated incremental borrowing rate to determine the present value of lease payments. Lease expenses for operating lease payments are recognized on a straight-line basis over the lease term, and the related ROU assets and liabilities are reduced to the present value of the remaining lease payments at the end of each period. Short-term leases (with a term of 12 months or less) are not recorded as ROU assets or liabilities in the consolidated balance sheets. The Company’s lease agreements include rental payments that adjust periodically for inflation and do not contain any material residual value guarantees or material restrictive covenants.
F-11
ENLIVEX THERAPEUTICS LTD.Stock-based compensation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
The Company recognizes equity-basedaccounts for stock-based compensation expenses for awards of equity instruments toarrangements with employees and non-employees based on the grant dateusing a fair value method which requires the recognition of those awards in accordance with FASB ASC Topic 718, Stock Compensation (“ASC 718”). ASC 718 requirescompensation expense for costs related to all equity-based compensation awards to employees and non-employee directors,stock-based payments including grants of restricted shares and stock options, to be recognized as expense in the consolidated statements of operations based on their grant date fair values.options. The Company estimates the fair value of stock options usingmethod requires the Black-Scholes option pricing model. The option pricing model requires a number of assumptions. Until the closing of the Merger, was no market for the Ordinary Shares, consequently the Company utilized third-party valuations to estimate the fair value of its Ordinary Shares. For the estimation of the expected volatility of the Company’s share price, the Company used the historical volatility of comparable companies in the industry with characteristics similar to the Company, including stage of product development and focusstock-based payment awards on the life science industry.date of grant using an option-pricing model. The expected termCompany uses the Black-Scholes option-pricing model to estimate the fair value of options granted representsthat are expensed on a straight-line basis over the requisite service period, which is generally the vesting period. The Company accounts for forfeitures as they occur. Option valuation models, including the Black-Scholes option-pricing model, require the input of time that options granted areseveral assumptions. Changes in the assumptions used can materially affect the grant-date fair value of an award. These assumptions include the risk-free rate of interest, expected to be outstanding, the company uses management’s estimates fordividend yield, expected volatility and the expected termlife of options due to insufficient readily available historical exercise data. The risk-free interest rate is based on the yield rates of U.S. Government Treasury Bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.award.
The Company accounted for stock options issued to non-employees in accordance with ASC Topic 505-50 “Equity-Based Payment to Non-Employees”. Effective January 1, 2019, the Company adopted changes issued by the FASB related to Stock Compensation. In June 2018, the FASB issued ASU No. 2018-07, Compensation - Stock Compensation (Topic 718), Improvements to Nonemployee Share-Based Payment Accounting. This ASU simplifies aspects of share-based compensation issued to non-employees by making the guidance consistent with the accounting for employee share-based compensation.Employee benefits
The Company is required by Israeli law to make severance payments to Israeli employees upon their dismissal or termination of employment in certain other circumstances. The Company operates a number of post-employment defined contribution plans. A defined contribution plan is a program that benefits an employee after termination of employment, under which the Company regularly makes fixed payments to a fund administered by a separate and independent entity so that the Company has no legal or constructive obligation to pay additional contributions if such fund does not contain sufficient assets to pay all employees the benefits to which they may be entitled relating to employee service in the current and prior periods. The fund assets are not included in the Company’s consolidated balance sheets. The Company operates pension and severance compensation plans subject to Section 14 of the Israeli Severance Pay Law. The plans are funded through payments to insurance companies or pension funds administered by trustees. In accordance with its terms, the plans meet the definition of a defined contribution plan.plan.
Short term employee benefits - Labor laws in Israel entitle every employee to vacation days, paid sick leave and recreation pay, computed annually. The Company recognizes a liability and an expense in respect of vacation and recreation pay based on the individual entitlement of each employee.
Revenue recognition
The Company has not yet generated any revenue from product sales or otherwise.
Research and development expenses, net
Research and development expenses are chargedinclude costs directly attributable to the statementconduct of operationsresearch and comprehensive loss as incurred and consistdevelopment programs, including the cost of salaries, stock-basedshare-based compensation benefitsexpenses, payroll taxes and other personnel-related costs, fees paid to consultants,employee benefits, subcontractors and materials used for research and development activities, including clinical trials, patentmanufacturing costs, consulting fees and facilities and overhead costs. All costs associated with research and developments are expensed as incurred. As of December 31, 2019,2022, the Company had not yet capitalized development expenses. Costs incurred in purchasing technology assets and intellectual property are charged to research and development expense if the technology has not been conclusively proven to be feasible and has no alternative future use.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Grants received from Israel Innovation Authority, Ministry of Industry, Trade and Labor (the “IIA”), are recognized when the grant becomes receivable, provided there is reasonable assurance that the Company will comply with the conditions attached to the grant and there was reasonable assurance the grant will be received. The grant is deducted from the research and development expenses as the applicable costs are incurred. Clinical trial expenses are charged to research and development expense as incurred. The Company’s objective is to reflect the appropriate trial expense in the consolidated financial statements by matching the appropriate expenses with the period in which services and efforts are expended. In the event advance payments are made, the payments are recorded as assets, which are expensed as services are rendered.
Preclinical and clinical trial accruals
The Company makes estimates of its accrued expenses as of each balance sheet date in the financial statements based on the facts and circumstances known at that time. Accrued expenses for preclinical studies and clinical trials are based on estimates of costs incurred and fees that may be associated with services provided by contract research organizations, clinical trial sites and other clinical trial-related activities. Payments under certain contracts with such parties depend on factors such as successful enrollment of patients, site initiation and the completion of clinical trial milestones. If possible, the Company obtains information regarding unbilled services directly from these service providers. However, the Company may be required to estimate these services based on other available information. If the Company underestimates or overestimates the activities or fees associated with a study or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, estimated accrued liabilities have approximated actual expense incurred.
General and administrative
General and administrative expenses consist of compensation and related benefits, including stock-based compensation, for executive and corporate personnel; professional and consulting fees; and allocated overhead, such as facilities and equipment rent and maintenance, insurance costs allocated to general and administrative expenses, costs of patent applications, maintenance, depreciation expense, marketing costs, and other miscellaneous expenses which are allocated to general and administrative expense.
Patents
The Company expenses all costs associated with patents (including application fees, and the legal and consulting expenses related to making such applications) for product candidates under development as incurred. As a result of the Company’s research and development efforts, the Company is applyingregularly applies for a number of patents to protect proprietary technology and inventions. To date, the Company has not capitalized patent costs. The Company recorded a chargecharges to general and administrative expenses in the accompanying statements of operations and comprehensive loss of approximately $120, $242$536 thousand, $373 thousand and $219$374 thousand for the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively, related to patent costs.
Income taxes
The Company accounts for income taxes in accordance with ASC 740-10 “Accounting for Income Taxes”. This Statement, which requires the use of the liability method of accounting for income taxes, whereby deferred tax asset and liability account balances are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
As the Company is currently engaged primarily in development activities and is not expected to generate taxable income in the foreseeable future, the Company provides a valuation allowance to reduce deferred tax assets to their estimated realizable value.
ASC 740 contains a two-step approach to recognizing and measuring a liability for uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. As of December 31, 2019 and 2018,2022, the Company recorded a liability of $635 and $222, respectively,$705 thousand for uncertain tax positions. The Company does not expect that the amountsamount of uncertain tax positions will change significantly within the next year.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Loss per share
Basic loss per share is calculated based on the weighted average number of ordinary sharesOrdinary Shares outstanding during each year. Diluted net loss per share is calculated based on the weighted average number of ordinary sharesOrdinary Shares outstanding during each year, plus dilutive potential shares in accordance with ASC 260, “Earnings per Share.”
All outstanding preferredrestricted stock units, options and warrants forin the years ended December 31, 2019, 20182022, 2021 and 20172020 have been excluded from the calculation of the diluted net loss per share because all such securities are anti-dilutive for all years presented. For the years ended December 31, 2019, 20182022, 2021 and 2017,2020, the total weighted average number of shares related to outstanding potential shares excluded from the calculations of diluted net loss per share was 3,247,127, 8,638,7892,902,423, 2,910,491 and 4,926,621,3,526,214, respectively. The following data show the amounts used in computing income (loss) per share and the effect on income (loss):
| Year ended December 31, | ||||||||||||
| Basic and diluted (loss) per share: | 2019 | 2018 | 2017 | |||||||||
| (Loss) income from continuing operations | $ | (9,384 | ) | $ | (4,242 | ) | $ | (2,503 | ) | |||
| Interest of 6% to Cumulative Preferred Stock | (198 | ) | (659 | ) | (717 | ) | ||||||
| $ | (9,582 | ) | $ | (4,901 | ) | $ | (3,220 | ) | ||||
| Number of common shares at the beginning of the year | 3,509,405 | 3,509,344 | 3,390,733 | |||||||||
| Issuance of shares for cash | 518,295 | - | - | |||||||||
| Issuance of shares in connection with the merger | 322,617 | - | - | |||||||||
| Stock options exercised | 80,073 | 2 | - | |||||||||
| Warrants exercised | 15,609 | - | - | |||||||||
| Conversion of Preferred Shares | 4,203,487 | - | - | |||||||||
| Conversion of convertible notes | - | 35,192 | ||||||||||
| Number of shares used in per share computation | 8,649,486 | 3,509,346 | 3,425,925 | |||||||||
| Basic and diluted net income (loss) per share | $ | (1.11 | ) | $ | (1.40 | ) | $ | (0.94 | ) | |||
| (in thousands except share and per share data) | Year ended December 31, | |||||||||||
| Basic and diluted (loss) per share: | 2022 | 2021 | 2020 | |||||||||
| (Loss) from continuing operations | $ | (31,060 | ) | $ | (14,468 | ) | $ | (11,824 | ) | |||
| $ | (31,060 | ) | $ | (14,468 | ) | $ | (11,824 | ) | ||||
| Number of common shares at the beginning of the year | 18,331,507 | 14,587,934 | 10,334,126 | |||||||||
| Weighted average number of shares issued for cash | - | 2,485,245 | 2,603,763 | |||||||||
| Weighted average number of stock options exercised | 13,015 | 16,715 | 21,749 | |||||||||
| Weighted average number of restricted stock units vested | 50,364 | - | - | |||||||||
| Weighted average number of warrants exercised | - | 769,819 | 209,570 | |||||||||
| Number of shares used in per share computation | 18,394,886 | 17,859,713 | 13,169,208 | |||||||||
| Basic and diluted net (loss) per share | $ | (1.69 | ) | $ | (0.81 | ) | $ | (0.90 | ) | |||
Concentrations of credit risk:
FinancialThe Company’s financial instruments that potentially subject the Companyare exposed to concentrations of credit risk consist principallyprimarily of cash and cash equivalents, restricted cash and restricted cash.bank deposits.
Cash and cash equivalents, and restricted cash and deposits are invested in major banks in Israel. Such deposits in Israel are not insured. Management believes that the financial institutions that hold the Company’s investments are financially sound and, accordingly, minimal credit risk exists with respect to these investments.
The Company hashad no foreign exchange contracts or any other hedging arrangements.arrangements as of December 31, 2022.
Fair value of financial instruments
The Company applies ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”), pursuant to whichthe guidance defines fair value, is definedprovides guidance for measuring fair value and requires certain disclosures. The guidance does not apply to measurements related to share-based payments. The guidance discusses valuation techniques such as the price that would be receivedmarket approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to sellreplace the service capacity of an asset or paidreplacement cost). The guidance establishes a fair value hierarchy that prioritizes the inputs to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date.
ENLIVEX THERAPEUTICS LTD.valuation techniques used to measure fair value into three broad levels.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollarsThe Company’s financial instruments consist of cash and cash equivalents, restricted cash, deposits, marketable securities, accounts receivable, accounts payable, accrued liabilities, and lease liability. Fair value estimates of these instruments are made at each reporting period end based on relevant market information. These estimates may be subjective in thousands (except sharenature and per share data)involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. The carrying amount of cash and cash equivalents, restricted cash, accounts receivable, accounts payable, and accrued liabilities are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. The Company believes that the fair value of the lease liability approximates its carrying value.
ASC 820 establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The financial instruments presented on the balance sheet at fair value are grouped into classes with similar characteristics using the following fair value hierarchy which is determined based on the source of input used in measuring fair value:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 - inputs other than quoted prices included within level 1 that are observable either directly or indirectly.
Level 3 - inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data). The Company’s warrants exercisable for ordinary shares were classified as level 3 in the fair value hierarchy and measured at fair value on a recurring basis. All such warrants were exercised for ordinary shares in 2019.
Comprehensive income (loss)
Comprehensive loss is the change in shareholders’ equity from transactions and other events and circumstances other than those resulting from investments by shareholders and distributions to shareholders.
The Company accounts for comprehensive income (loss) in accordance with ASC 220, “Comprehensive Income”. This statement establishes standards for the reporting and display of comprehensive income (loss) and its components in a full set of general purposegeneral-purpose financial statements.
The Company’s other comprehensive income (loss) is currently comprisedfor the years ended December 31, 2021 and 2020 was composed of gains or losses resulting from translation adjustments which result from translating the Company’s financial statements into U.S. dollars when its functional currency is differentin currencies other than the U.S. dollar.dollar into U.S. dollars.
Reclassification
Certain amounts in the prior periods financial statementscomparative figures have been reclassified to conform to the presentation of the current period financial statements. Theseyear presentation. Such reclassifications had no effectdid not have any significant impact on the previously reportedCompany’s equity, net loss.income or cash flows.
Recently Adopted Accounting Standards
In August 2018,From time to time, the Financial Accounting Standards Board (“FASB”(the “FASB”) issued ASU 2018-13, “Fair Value Measurement (Topic 820), - Disclosure Framework - Changes toor other standard-setting bodies issue accounting standards that are adopted by the Disclosure Requirements for Fair Value Measurement,” which makes a numberCompany as of changes meant to add, modify or remove certain disclosure requirements associated with the movement amongst or hierarchy associated with Level 1, Level 2 and Level 3 fair value measurements. This guidance isspecified effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The Company adopted this ASU on January 1, 2020. The adoption of this ASU did not have a material impact on its consolidated financial statements. date.
In December 2019,August 2020, the FASB issued ASUAccounting Standards Update (“ASU”) No. 2019-12, “Income Taxes (Topic 740): Simplifying the2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40); Accounting for Income TaxesConvertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2019-12”2020-06”), which is intended to simplify various aspects related toaddresses issues identified as a result of the complexities associated with applying U.S. GAAP for certain financial instruments with characteristics of liabilities and equity. This update addresses, among other things, the number of accounting models for income taxes. ASU 2019-12 removes certain exceptionsconvertible debt instruments and convertible preferred stock, targeted improvements to the general principles in Topic 740disclosures for convertible instruments and also clarifiesearnings-per-share (“EPS”) guidance and amends existingamendments to the guidance to improve consistent application. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company is currently evaluating the impact of this standard on its consolidated financial statements and related disclosures, the adoption of this ASU is not expected to have a material impact on its consolidated financial statements.
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606)” (ASU 2014-09) as modified by ASU No. 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date,” ASU 2016-08, “Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net),” ASU No. 2016-10, “Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing,” and ASU No. 2016-12, “Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients.” The revenue recognition principle in ASU 2014-09 is that an entity should recognize revenue to depict the transfer of goods or services to customersderivatives scope exception for contracts in an amountentity’s own equity, as well as the related EPS guidance. This update applies to all entities that reflects the consideration to which the entity expects to be entitledissue convertible instruments and/or contracts in exchange for those goods or services. In addition, new and enhanced disclosures will be required. Companies may adopt the new standard either using the full retrospective approach, a modified retrospective approach with practical expedients, or a cumulative effect upon adoption approach. The Company adopted the new standard effective January 1, 2018. The adoption of this ASU did not have a material impact on its consolidated financial statements.
F-14
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
In January 2016, the FASB issued ASU No. 2016-01, Recognition and Measurement of Financial Assets and Financial Liabilities. ASU No. 2016-01 requires equity investments to be measured at fair value with changes in fair value recognized in net income; simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment; eliminates the requirement for public business entities to disclose the methods and significant assumptions used to estimate the fair value that is required to be disclosed for financial instruments measured at amortized cost on the balance sheet; requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes; requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments; requires separate presentation of financial assets and financial liabilities by measurement category and form of financial assets on the balance sheet or the accompanying notes to the financial statements; and clarifies that an entity should evaluate the need for a valuation allowance on a deferred tax asset related to available-for-sale securities in combination with the entity’s other deferred tax assets. ASU No. 2016-01own equity. This guidance is effective for financial statements issued for fiscal years beginning after December 15, 2017,2021, and interim periods within those fiscal years. The Company adoptedFASB specified that an entity should adopt the provisionsguidance as of ASU 2016-01 on January 1, 2018.the beginning of its annual fiscal year. The adoption of this update did not impact the Company’s consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842), which supersedes FASB ASC Topic 840, Leases (Topic 840) and provides principles for the recognition, measurement, presentation and disclosure of leases for both lessees and lessors. The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease, respectively. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than twelve months regardless of classification. Leases with a term of twelve months or less will be accounted for similar to existing guidance for operating leases. The standard is effective for annual and interim periods beginning after December 15, 2018, with early adoption permitted upon issuance. The Company adopted this standard on January 1, 2019. See Note 8 to these audited consolidated financial statements.
In May 2017, the Financial Accounting Standards Board (the FASB) issued ASU 2017-09, Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting, (ASU 2017-09). ASU 2017-09 provides clarity and reduces both (1) diversity in practice and (2) cost and complexity when applying the guidance in Topic 718, to a change to the terms or conditions of a share-based payment award. The amendments in ASU 2017-09 should be applied prospectively to an award modified on or after the adoption date. This ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2017. The Company adopted ASU 2017-09 on January 1, 2018.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features; II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception, (ASU 2017-11). Part I of this update addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments (such as warrants and convertible instruments) with down round features that require fair value measurement of the entire instrument or conversion option. Part II of this update addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification. This pending content is the result of the indefinite deferral of accounting requirements about mandatorily redeemable financial instruments of certain nonpublic entities and certain mandatorily redeemable noncontrolling interests. The amendments in Part II of this update do not have an accounting effect. This ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2018. The Company adopted ASU 2017-11 on January 1, 2019, and the adoption2020-06 did not have ana material impact on the Company’s consolidated financial statements.
ENLIVEX THERAPEUTICS LTD.statements and disclosures.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
In June 2018,May 2021, the FASB issued ASU 2018-07, Compensation-StockNo. 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment, and Derivatives and Hedging— Contracts in Entity’s Own Equity (Subtopic 815-40); Issuer’s Accounting (“ASU 2018-07”). ASU 2018-07 simplifies several aspectsfor Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options, a consensus of the FASB Emerging Issues Task Force (“ASU 2021-04”), which aims to clarify and reduce diversity in issuer’s accounting for nonemployee share-based payment transactions resulting from expanding the scopemodifications or exchanges of Topic 718, Compensation-Stock Compensation,freestanding equity-classified written call options that remain equity classified after modification or exchange. This update applies to include share-based payment transactions for acquiring goods and services from nonemployees. ASU 2018-07all entities that issue freestanding written call options that are classified in equity. This guidance is effective for public business entitiesfinancial statements issued for fiscal years beginning after December 15, 2018, including2021, and interim periods within those fiscal years. FASB specified that an entity should adopt the guidance as of the beginning of its annual fiscal year. The Company adoptedadoption of ASU 2018-072021-04 did not have a material impact on January 1, 2019.the Company’s consolidated financial statements and disclosures.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – CASH, CASH EQUIVALENTS AND RESTRICTED CASH
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the consolidated balance sheet, including location of amounts reported in the accompanying consolidated balance sheets, that sum to the total of the same such amounts shown in the statement of cash flows.
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Cash held in banks | $ | 937 | $ | 1,029 | ||||
| Bank deposits in U.S.$ (annual average interest rates 1.80% and 1.81%) | 3,011 | 8,707 | ||||||
| Total cash and cash equivalents | 3,948 | 9,736 | ||||||
| Cash held with respect to CVR Agreement | 1,500 | - | ||||||
| Long-term restricted bank deposits | 76 | 56 | ||||||
| Total cash, cash equivalents and restricted cash shown in the statement of cash flows | $ | 5,524 | $ | 9,792 | ||||
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Cash held in banks | $ | 2,123 | $ | 1,199 | ||||
| Bank deposits in U.S.$ with original maturities of three months or less (annual average interest rates 3.95% and 0.1%) | 47,822 | 10,003 | ||||||
| Total cash and cash equivalents | 49,945 | 11,202 | ||||||
| Restricted cash – current – Prepaid expenses and other receivables | 113 | 113 | ||||||
| Restricted cash – noncurrent – Other assets | 299 | 321 | ||||||
| Total cash, cash equivalents and restricted cash shown in the statement of cash flows | $ | 50,357 | $ | 11,636 | ||||
NOTE 4 – SHORT TERM DEPOSITS
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Bank deposits in U.S.$ (annual average interest rates 0.3% and 0.6%) | $ | 299 | $ | 10,004 | ||||
NOTE 5 – PREPAID EXPENSES AND OTHER RECEIVABLES
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Prepaid expenses | $ | 1,439 | $ | 1,112 | ||||
| Tax authorities | 522 | 1,073 | ||||||
| Others | 125 | 127 | ||||||
| $ | 2,086 | $ | 2,312 | |||||
NOTE 46 – PROPERTY AND EQUIPMENT
Property and equipment, net consists of the following:
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Cost: | ||||||||
| Laboratory equipment | $ | 2,851 | $ | 1,891 | ||||
| Computers | 276 | 256 | ||||||
| Office furniture & equipment | 249 | 192 | ||||||
| Leasehold improvements | 8,606 | 1,521 | ||||||
| Total cost | 11,982 | 3,860 | ||||||
| Accumulated depreciation: | ||||||||
| Laboratory equipment | 1,432 | 960 | ||||||
| Computers | 203 | 138 | ||||||
| Office furniture & equipment | 28 | 15 | ||||||
| Leasehold improvements | 444 | 217 | ||||||
| Total accumulated depreciation | 2,107 | 1,330 | ||||||
| Depreciated cost | $ | 9,875 | $ | 2,530 | ||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Cost: | ||||||||
| Laboratory equipment | $ | 782 | $ | 797 | ||||
| Computers | 94 | 86 | ||||||
| Office furniture & equipment | 36 | 58 | ||||||
| Leasehold improvements | 152 | 228 | ||||||
| Total cost | 1,064 | 1,169 | ||||||
| Accumulated depreciation: | ||||||||
| Laboratory equipment | 340 | 331 | ||||||
| Computers | 51 | 62 | ||||||
| Office furniture & equipment | 1 | 13 | ||||||
| Leasehold improvements | 24 | 78 | ||||||
| Totalaccumulated depreciation | 416 | 484 | ||||||
| Depreciated cost | $ | 648 | $ | 685 | ||||
For the years ended December 31, 2019, 2018 and 2017, depreciation expenses were $210, $121 and $83, respectively.ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – OTHER ASSETS
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Restricted cash | $ | 299 | $ | 321 | ||||
| Long Term Deposit | 8 | 3 | ||||||
| Long-term prepaid expenses | 105 | 161 | ||||||
| Right-of-Use assets, net | 5,025 | 5,689 | ||||||
| $ | 5,437 | $ | 6,174 | |||||
NOTE 58 – ACCRUED EXPENSES AND OTHER LIABILITIES
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Vacation, convalescence and bonus accruals | $ | 318 | $ | 211 | ||||
| Employees and payroll related | 261 | 158 | ||||||
| Short term operating lease liabilities | 123 | - | ||||||
| Accrued expenses and other | 1,195 | 575 | ||||||
| $ | 1,897 | $ | 944 | |||||
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Vacation, convalescence and employees’ bonus accruals | $ | 759 | $ | 1,068 | ||||
| Employees and payroll related | 539 | 519 | ||||||
| Short term operating lease liabilities | 653 | 617 | ||||||
| Accrued expenses and other | 2,708 | 1,636 | ||||||
| $ | 4,659 | $ | 3,840 | |||||
NOTE 9 – LEASES
The Company determines if a contract contains a lease at inception and recognizes operating lease right-of-use assets and operating lease liabilities based on the present value of the future minimum lease payments at the commencement date. As the Company’s leases do not provide an implicit rate, management develops incremental borrowing rates based on the information available at the commencement date in determining the present value of future payments. Lease expenses are recognized on a straight-line basis over the lease term.
The Company’s operating leases are as follows:
In September 2019, the Company entered into a 24 month lease agreement for approximately 55 square meters of office and research lab space at the BioPark Building, Hadassah Ein-Kerem Campus in Jerusalem, Israel. The Company exercised an extension option under this lease and extended the term of the lease by an additional 24 months, which commenced on September 1, 2021.
In July 2018, the Company entered into a lease agreement for offices, laboratory and parking spaces at the Science Park in Nes Ziona, Israel for approximately 420 square meters of space. The lease for this space expires on August 31, 2023 at which time the Company may extend the lease for an additional 60-month period.
In October 2020, the Company entered into an additional lease agreement for approximately 421 square meters of space in Nes Ziona, Israel. The lease for this space expires on September 30, 2025 at which time the Company may extend the lease for an additional 33-month period.
On July 29, 2021, the Company entered into an amendment to its lease agreement in Nes Ziona, which added an additional 455 square meters of office space to the existing leased space. The lease for the additional 455 square meters is for a period of 63 months, which commenced on August 1, 2021. The lease expires on October 31, 2026 with an option to extend the lease for an additional 22 months.
Upon entering into the Jerusalem and the Nes Ziona lease agreements, and the amendment to the Nes Ziona lease agreement, the Company was not reasonably certain that it would exercise the extension options contained therein and therefore did not include the options in the determination of the total lease terms of the leases for accounting purposes.
On September 19, 2021, the Company entered into a lease agreement for a manufacturing plant space for the purpose of manufacturing AllocetraTM to support ongoing clinical trials in Yavne Israel, for a period of 60 months that commenced on September 19, 2021. The Company completed construction on the manufacturing plant in the fourth quarter of 2022. The lease expires on September 18, 2026 with options for two successive 60-month extensions. Upon entering into the plant space lease agreement, the Company estimated that it would exercise the first extension option and therefore included one option term in the determination of the total lease term for accounting purposes. In September 2022, the Company’s management decided to postpone the use of the manufacturing plant for an approximate period of three years and to offer lease and supporting services of the leased space to potential clients during this three-year period. The Company’s initial estimation regarding the exercise of the first extension option has not changed.
F-16
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 6 – WARRANTS
On September 15, 2017,As of December 31, 2022 the Company issued 48,232 warrants in settlementwas a party to seven vehicle lease agreements. All vehicle lease agreements are for 36 months periods.
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| The components of lease expense were as follows: | ||||||||||||
| Operating leases expenses | $ | 1,037 | $ | 698 | $ | 243 | ||||||
| Cash flow information related to operating leases: | ||||||||||||
| Cash used in operating activities | $ | 875 | $ | 449 | $ | 223 | ||||||
| Non-cash activity - Right of use assets obtained in exchange for new operating lease liabilities | $ | 179 | $ | 5,446 | $ | 398 | ||||||
| December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Supplemental information related to operating leases, including location of amounts reported in the accompanying consolidated balance sheets, follows: | ||||||||
| Other assets - Right-of-Use assets | $ | 6,445 | $ | 6,329 | ||||
| Accumulated amortization | 1,420 | 640 | ||||||
| Operating lease Right-of-Use assets, net | $ | 5,025 | $ | 5,689 | ||||
| Lease liabilities – current - Accounts payable and accrued liabilities | $ | 653 | $ | 617 | ||||
| Lease liabilities – noncurrent | 4,194 | 5,389 | ||||||
| Total operating lease liabilities | $ | 4,847 | $ | 6,006 | ||||
| Weighted average remaining lease term in years | 7.6 | 8.6 | ||||||
| Weighted average annual discount rate | 3.4 | % | 3.6 | % | ||||
Maturities of a finder’s fee, which warrants were exercisable on a cashless basis for a period of seven years. All 48,232 warrants were exercised for 20,348 ordinary shares upon consummation of the Merger.
In accordance with ASC 815: “Derivatives and Hedging”, one of the characteristics of a derivative is that its terms require or permit net settlement. Accordingly, all warrants were classified as a non-current liability measured at fair value.
| Year ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Balance at January 1 | $ | 192 | $ | 344 | ||||
| Exchange differences arising from translation to presentation currency | 6 | (20 | ) | |||||
| Changes in values of warrants exercisable into shares liability | 51 | (132 | ) | |||||
| Fair value of warrants at exercise date | (249 | ) | - | |||||
| Balance at December 31 | $ | - | $ | 192 | ||||
The fair value of warrants prior to the Merger was valued by using the OPM pricing model. Fair valueundiscounted operating lease liabilities as of December 31, 2018 was estimated using the following assumptions for the warrants’ call option:2022, were as follows:
| (in thousands) | ||||
| 2023 | $ | 788 | ||
| 2024 | 681 | |||
| 2025 | 703 | |||
| 2026 | 607 | |||
| 2027 and onwards | 2,752 | |||
| Total undiscounted lease liability | 5,531 | |||
| Less: Imputed interest | (684 | ) | ||
| Present value of lease liabilities | $ | 4,847 | ||
NOTE 7 – REVERSE MERGER WITH BIOBLAST PHARMA LTD.
As described in Note 1, the Merger completed between Enlivex R&D and Bioblast Pharma Ltd. (the “Parent”) was accounted for as an issuance of shares by Enlivex R&D for the net assets of the Parent, accompanied by a recapitalization. Enlivex R&D was considered the acquirer for accounting and financial reporting purposes and acquired the assets and assumed the liabilities of the Parent, and Enlivex R&D gained control of the combined company after the merger. The annual consolidated financial statements of the Company reflect the operations of the acquirer for accounting purposes together with a deemed issuance of shares, equivalent to the shares held by the former shareholders of the legal acquirer and a recapitalization of the equity of the accounting acquirer. The annual consolidated financial statements include the accounts of the Company and its subsidiary since the effective date of the Merger and the accounts of Enlivex R&D since inception.
The estimated fair value of the total considerations was $5,152 based on the shares of the Parent outstanding on the Merger date as adjusted per the merger agreement of 420,554 multiplied by the Company’s share price of $12.25 on the date of the Merger. The excess of the fair value of the consideration paid over the fair value of the net assets acquired as detailed below was $4,508.
The following summarizes the estimated fair value of the assets and liabilities acquired at the date of the merger:
| Cash and cash equivalents | $ | 44 | ||
| Prepaid expenses and other receivables | 632 | |||
| Cash held with respect to CVR Agreement | 1,500 | |||
| Receivables for the sale of Trehalose | 2,000 | |||
| Trade payables | (10 | ) | ||
| Due to CVR Holders | (3,500 | ) | ||
| Other current liabilities | (22 | ) | ||
| $ | 644 |
F-17
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 8 – LEASES
The Company elected the transition provision provided by ASU no. 2018-11, Leases (Topic 842) that allows entities to continue to apply the legacy guidance in Topic 840, Leases, including its disclosure requirements, for the comparative periods presented in the year of adoption. Accordingly, the Topic 842 disclosures below are presented only as of and for the year ended December 31, 2019 and not for any prior period.
The Company is a party to operating leases for its corporate offices, laboratory space and vehicles. The Company’s operating leases have remaining lease terms of up to 3.66 years, some of which include options to extend the leases for up to five years. On January 1, 2019, the Company recognized $399 of ROU assets and of lease liabilities on the consolidated balance sheet for operating leases.
| December 31, | ||||
| 2019 | ||||
| The components of lease expense were as follows: | ||||
| Operating leases expenses | $ | 192 | ||
| Supplemental consolidated cash flow information related to operating leases follows: | ||||
| Cash used in operating activities | $ | 188 | ||
| Non-cash activity: Right of use assets obtained in exchange for new operating lease liabilities | $
| 141 | ||
| Supplemental information related to operating leases, including location of amounts reported in the accompanying consolidated balance sheets, follows: | ||||
| Other assets - Right-of-Use assets | $ | 501 | ||
| Accumulated amortization | 91 | |||
| Operating lease Right-of-Use assets, net | $ | 410 | ||
| Accounts payable and accrued liabilities | $ | 123 | ||
| Other long-term liabilities | 298 | |||
| Total operating lease liabilities | $ | 421 | ||
| Weighted average remaining lease term in years | 3.33 | |||
| Weighted average annual discount rate | 10.7 | % | ||
| Maturities of operating lease liabilities as of December 31, 2019, were as follows: | ||||
| 2020 | $ | 172 | ||
| 2021 | 157 | |||
| 2022 | 126 | |||
| 2023 | 70 | |||
| Total undiscounted lease liability | 525 | |||
| Less: Imputed interest | (104 | ) | ||
| Present value of lease liabilities | $ | 421 | ||
NOTE 910 – COMMITMENTS AND CONTINGENT LIABILITIES
| a) | Obligation to pay royalties to the State of Israel |
The Company is required to pay royalties to the State of Israel (represented by the Israel Innovation Authority)IIA), computed on the basis of proceeds from the sale or license of products whichfor development was supported by StateIIA grants.
In accordance with the terms of the financial participation, the State is entitled to royalties on the sale or license of any product which development was supported with State participation.
These royalties are generally 3% - 5% of sales until repayment of 100% of the grants (linked to the U.S. dollar) received by the Company plus annual interest at the LIBORa LIBOR-based rate.
The aggregate contingent obligation payable by the Company to the State of Israel as of December 31, 20192022 was approximately $5,197$9.2 million, which representsrepresented the gross amount of grants actually received by the Company from the Israel Innovation Authority,IIA, including accrued interest as of December 31, 2019.2022. As of December 31, 2022, the Company had not paid any royalties to the IIA.
F-18
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
In January 2019,2022, the Company submitted a new grant application to the Israel Innovation AuthorityIIA to approve an expenditure of $4.8 million for fundingits clinical development program for the prevention of cytokine storms and organ dysfunction associated with sepsis for a period that commenced January 1, 2022 and ended December 31, 2022 (the “2022 Sepsis Grant Application”). In May 2022 The IIA approved the 2022 Sepsis Grant Application of $1 million, for expenses associated with the ongoing sepsis clinical Phase II trial.
In January 2021, the Company submitted a new grant application to the IIA for reimbursement by the IIA of certain expenses associated with its clinical development program of prevention of cytokine storms and organ dysfunction associated with sepsis. On April 16, 2019, the Company’s application for grants at 50% participation of a NIS 7,668 ($2,219) plan to be executed in Israel was approved by the Israel Innovation Authority. The plan was approvedsepsis for a period commencing January 1, 20192021 and ending December 31, 2019.2021 (the “2021 Sepsis Grant Application”). In May 2021 the IIA approved the 2021 Sepsis Grant Application in the total amount of $1.2 million.
| b) | Executive Chairman Agreement |
On September 7, 2018 the Company entered into an agreement with its Executive Chairman. Pursuant to the agreement, upon termination of the Chairman’s board service, under certain conditions defined in the agreement, the Executive Chairman will be entitled to receive an amount of up to three times his then annual base retainer plus the value of accrued benefits. As of December 31, 2019,2022, no termination liability was accrued or paid.
On November 4, 2021 the Company had not paid any royaltiesand the Executive Chairman entered into an amendment to the Israel Innovation Authority.agreement, granting the Executive Chairman 3.33% of future gross proceeds actually received by the Company during the first five (5) years following consummation of a commercial transaction involving the Company or sale of the Company, in each case as defined in the amendment. In the case of a Commercial Transaction, the fee with respect to such commercial transaction would become payable only once the aggregate consideration actually received by the Company in respect of such commercial transaction is equal to or greater than $20 million.
From time to time, the Company may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business. Management believes that there were no claims or actions pending against the Company at December 31, 2022 which will have, individually or in the aggregate, a material adverse effect on the Company’s business, liquidity, financial position or results of operations. Litigation, however, is subject to inherent uncertainties, and an adverse result in such matters may arise from time to time that may harm the Company’s business.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1011 – EQUITY
| a) |
Shares of Preferred Stock conferred on their holders all rights accruing to holders of Common Stock, and, in addition, contained certain conversion and preference rights. Preferred A, Preferred B and Preferred C Stock also conferred upon their holders the right to receive an annual dividend amount equal to the original issue price of each Preferred Stock plus a cumulative preference of 6% of the original issue price per annum compounded annually until the lapse of two years from their issuance date.
Accumulated unpaid dividends to series A, series B and series C Convertible Preferred Stock holders as of the date of the Merger were $2,071.
| b) |
| c) | On February 9, 2021, the Company entered into an amended and restated underwriting agreement (the “February 2021 Underwriting Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”) with respect to the offer, issuance and sale (the “February 2021 Offering”) of an aggregate of 2,296,107 Ordinary Shares, together with an option granted to Wainwright to purchase up to 344,416 additional Ordinary Shares. The Ordinary Shares were offered to the public at a price of $20 per share. |
The February 2021 Offering closed on February 12, 2021, on which date the Company completed the issuance of 2,296,107 Ordinary Shares to Wainwright at a price, including the underwriting discount but before other associated fees, of $18.60 per ordinary share, as set forth in the February 2021 Underwriting Agreement.
On February 17, 2021, Wainwright exercised in part its option to purchase additional Ordinary Shares and purchased 268,205 Ordinary Shares at a price, including the underwriting discount but before other associated fees, of $18.60 per share, as set forth in the February 2021 Underwriting Agreement.
In accordance with the February 2021 Underwriting Agreement, the Company paid Wainwright underwriting discounts and commissions equal to 7% of the gross proceeds received by the Company from the sale of the Ordinary Shares in the February 2021 Offering, as well as a management fee equal to 1% of the gross proceeds received by the Company from the sale of the Ordinary Shares in the February 2021 Offering. In addition, the Company issued to Wainwright 179,501 warrants to purchase Ordinary Shares of the Company. The Company has also paid Wainwright approximately $126,000 for various expenses.
The net proceeds from the February 2021 Offering were $47,023 thousand after deducting Wainwright’s fees and other expenses relating to the February 2021 Offering.
| d) | On October 22, 2020, the Company entered into an at the market offering agreement (the “Former ATM Agreement”), with Wainwright pursuant to which the Company was able, from time to time and at the Company’s option, to issue and sell Ordinary Shares having an aggregate offering price of up to $25 million through Wainwright as sales agent, subject to certain terms and conditions. The Company terminated the Former ATM Agreement on December 19, 2022. Between February 5, 2021 and February 9, 2021, the Company received gross proceeds of $6,339,000 from the sale of 284,317 Ordinary Shares under the Former ATM Agreement at an average gross price per share of $22.29. Issuance expenses totaled $192 thousand. |
The Company sold an aggregate of 476,983 Ordinary Shares under the Former ATM Agreement, resulting in a gross aggregate offering price of $8,557,000 at a gross average price per share of $17.94.
| e) | On March |
Also prior to and in connection with the Merger, the Company effected a 1-for-8 reverse stock split and changed the total number of shares of all classes authorized to be issued to 45,000,000 with a par value of NIS 0.40 per share. All historical information presented herein has been retroactively restated to reflect the effect of the Merger exchange ratio and reverse stock split in accordance with Accounting Standards Codification Topic 260, “Earnings Per Share”.
| g) |
The
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
All Company warrants are classified as a component of shareholders’ equity because theysuch warrants are free standing financial instruments that are legally detachable, separately exercisable, do not embody an obligation for the Company to repurchase its own shares, and permit the holders to receive a fixed number of Ordinary Shares upon exercise. In addition, the warrants requireexercise, requires physical settlement and do not provide any guarantee of value or return.
ENLIVEX THERAPEUTICS LTD.The following table contains additional information concerning warrants activity for the years 2022 and 2021:
| For the year ended December 31, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
Number of warrants | Weighted average exercise | Number of warrants | Weighted average exercise price | |||||||||||||
| Outstanding at beginning of the year | 704,355 | $ | 13.18 | 1,407,683 | $ | 12.33 | ||||||||||
| Issued | - | $ | - | 179,501 | $ | 25.00 | ||||||||||
| Forfeited and expired | (502,104 | ) | $ | 9.09 | (27,016 | ) | $ | 180.00 | ||||||||
| Exercised | - | $ | - | (855,813 | ) | $ | 9.00 | |||||||||
| Outstanding and exercisable at year-end | 202,251 | $ | 23.31 | 704,355 | $ | 13.18 | ||||||||||
Set forth below is data regarding the range of exercise prices and remaining contractual life (in years) for warrants outstanding at December 31, 2022:
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Exercise Price | Number of | Remaining | ||||||
| $10 | 22,750 | 2.15 | ||||||
| $25 | 179,501 | 3.11 | ||||||
| 202,251 | ||||||||
U.S. dollars in thousands (except share and per share data)
NOTE 1112 – SHARE-BASED COMPENSATION
| a) |
Prior to the merger, Bioblast Pharma Ltd. adopted the 2013 Incentive Option Plan (the “2013 Plan”), which provided for the grant of incentive Ordinary Share options and nonqualified Ordinary Share options to employees, directors, and non-employees of the Company. As of December 31, 2019, there was a total of 15,500 options to purchase Ordinary Shares outstanding under the 2013 Plan. All outstanding options under the 2013 Plan were fully vested upon the closing of the Merger. The number of shares subject to and the exercise prices applicable to these outstanding options were adjusted in connection with the one-for-eight reverse stock-split. In June 2019, the 2013 Plan was assumed by the Company’s Global Share Incentive Plan (2019) the “2019 Equity Incentive Plan”), and the Company intends for the 2019 Equity Incentive Plan to be its primary stock compensation plan for future awards.
Prior to the Merger, Enlivex R&D granted Options under the 2007 Incentive Compensation Plan (the “2007 Equity Incentive Plan”) and the Global Share Incentive Plan (2014) (the “2014 Equity Incentive Plan”).
In June 2019, the Company adopted the 2019 EquityGlobal Share Incentive Plan under which all(2019) (the “2019 Plan”). On May 28, 2021, the Board approved an increase in the number of Ordinary Shares that remained availableauthorized for future grant under all existing plans were reserved for issuance with respect to awards that may be granted under the 2019 Equity Incentive Plan.
As of December 31, 2019, 2,350,704 Ordinary Shares were authorized for issuancePlan to employees, directors and consultants under the 2019 Equity Incentive Plan,from 2,350,704 to 4,150,704, of which 490,749635,594 shares were available for future grant.grant as of December 31, 2022.
Equity Incentive Plans and agreementsOptions granted under the 2019 Plan generally expire after ten years from the date of grant. Upon termination of the optionee’sholder’s employment or other relationship with the Company, restricted shares/options cease vesting, vested unvested shares/options forfeit. OrdinaryUnderlying shares underlying options that are canceled or not exercised within the option termor forfeited become available for future grant.
| b) |
The estimated fair value of stock option awards was determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions during the periods indicated:
| Year ended December 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Weighted Average Risk-free interest rate | 3.41 | % | 0.82 | % | 0.46 | % | ||||||
| Dividend yield | - | - | - | |||||||||
| Weighted Average Volatility factor | 91.62 | % | 91.57 | % | 76.32 | % | ||||||
| Weighted Average Expected life of the options | 5 | 5 | 6 | |||||||||
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Weighted Average Risk-free interest rate | 1.67 | % | 2.88 | % | 2.25 | % | ||||||
| Dividend yield | - | - | - | |||||||||
| Weighted Average Volatility factor | 73.71 | % | 74.91 | % | 73.31 | % | ||||||
| Weighted Average Expected life of the options | 5 | 6 | 6 | |||||||||
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table contains additional information concerning options granted to employees and directors under the existing stock-option plans:plan:
| For the year ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
Number of options | Weighted average exercise | Number of options | Weighted average exercise |
Number of options | Weighted average exercise | |||||||||||||||||||
| Outstanding at beginning of the year | 1,083,022 | $ | 4.79 | 974,271 | $ | 4.54 | 453,972 | $ | 2.69 | |||||||||||||||
| Granted | 158,021 | $ | 8.72 | 271,355 | $ | 5.78 | 590,839 | $ | 5.78 | |||||||||||||||
| Forfeited and expired | (128,256 | ) | $ | 5.24 | (162,543 | ) | $ | 4.96 | (70,540 | ) | $ | 2.69 | ||||||||||||
| Exercised | (2,360 | ) | $ | 3.77 | (61 | ) | $ | 2.69 | - | $ | - | |||||||||||||
| Pre-merger Bioblast options | 15,500 | $ | 90.17 | - | $ | - | - | $ | - | |||||||||||||||
| Outstanding at end of the year | 1,125,927 | $ | 6.47 | 1,083,022 | $ | 4.79 | 974,271 | $ | 4.54 | |||||||||||||||
| Exercisable at end of the year | 700,032 | $ | 3.92 | 457,661 | $ | 3.72 | 243,313 | $ | 2.69 | |||||||||||||||
| For the year ended December 31, | ||||||||||||||||||||||||
| 2022 | 2021 | 2020 | ||||||||||||||||||||||
Number of | Weighted average exercise price | Number of | Weighted exercise price | Number of | Weighted exercise price | |||||||||||||||||||
| Outstanding at beginning of the year | 2,142,547 | $ | 6.02 | 1,884,420 | $ | 5.52 | 1,625,042 | $ | 5.72 | |||||||||||||||
| Granted | 860,492 | $ | 5.49 | 345,500 | $ | 12.14 | 360,500 | $ | 4.46 | |||||||||||||||
| Forfeited and expired | (40,730 | ) | $ | 6.42 | (48,242 | ) | $ | 33.11 | (63,376 | ) | $ | 6.26 | ||||||||||||
| Exercised | (22,875 | ) | $ | 6.49 | (39,131 | ) | $ | 2.89 | (37,746 | ) | $ | 2.69 | ||||||||||||
| Outstanding at end of the year | 2,939,434 | $ | 5.85 | 2,142,547 | $ | 6.02 | 1,884,420 | $ | 5.52 | |||||||||||||||
| Exercisable at end of the year | 1,953,429 | $ | 5.55 | 1,613,465 | $ | 4.90 | 1,283,193 | $ | 5.15 | |||||||||||||||
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
Following is a summary of changes in nonvested shares granted to employees and directors:granted:
| For the year ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
Number of options | Weighted average exercise | Number of options | Weighted average exercise |
Number of options | Weighted average exercise | |||||||||||||||||||
| Balance at beginning of the year | 625,361 | $ | 5.58 | 730,958 | $ | 5.37 | 310,058 | $ | 2.69 | |||||||||||||||
| Granted | 158,021 | $ | 8.72 | 271,355 | $ | 5.78 | 590,839 | $ | 5.78 | |||||||||||||||
| Vested during the year | (229,231 | ) | $ | 5.01 | (217,733 | ) | $ | 4.75 | (102,724 | ) | $ | 2.69 | ||||||||||||
| Forfeited during the year | (128,256 | ) | $ | 5.24 | (159,219 | ) | $ | 5.16 | (67,215 | ) | $ | 2.69 | ||||||||||||
| Balance at end of the year | 425,895 | $ | 7.21 | 625,361 | $ | 5.58 | 730,958 | $ | 5.37 | |||||||||||||||
| For the year ended December 31, | ||||||||||||||||||||||||
| 2022 | 2021 | 2020 | ||||||||||||||||||||||
Number of options | Weighted average exercise price | Number of options | Weighted exercise price |
Number of options | Weighted exercise price | |||||||||||||||||||
| Balance at beginning of the year | 529,082 | $ | 8.69 | 601,227 | $ | 5.93 | 526,351 | $ | 7.03 | |||||||||||||||
| Granted | 860,492 | $ | 5.49 | 345,500 | $ | 12.14 | 360,500 | $ | 3.86 | |||||||||||||||
| Vested during the year | (370,839 | ) | $ | 7.14 | (385,798 | ) | $ | 7.39 | (249,965 | ) | $ | 6.07 | ||||||||||||
| Forfeited during the year | (32,730 | ) | $ | 6.43 | (31,847 | ) | $ | 6.75 | (35,659 | ) | $ | 6.24 | ||||||||||||
| Balance at end of the year | 986,005 | $ | 6.46 | 529,082 | $ | 8.69 | 601,227 | $ | 5.93 | |||||||||||||||
The weighted-average fair valuevalues at grant date of options granted 2019, 2018during the years ended December 31, 2022, 2021 and 20172020 were $5.29, $5.78$5.49, $6.23 and $0.207.$2.93 respectively.
The total unrecognized estimated compensation cost related to non-vested employees’ and directors’ stock options granted until December 31, 20192022 was $934,$2,746, which is expected to be recognized over a weighted average period of 1.951.61 years.
| c) |
The estimated fair value of stock option awards was determined using the Black-Scholes option valuation model with the following weighted-average assumptions during the periods indicated:
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Weighted Average Risk-free interest rate | - | 2.55 | % | 2.25 | % | |||||||
| Dividend yield | - | - | - | |||||||||
| Weighted Average Volatility factor | - | 75.23 | % | 73.31 | % | |||||||
| Weighted Average Expected life of the options | - | 6 | 6 | |||||||||
The following table contains additional information concerning options granted to consultants under the existing stock-option plans:
| For the year ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
Number of options | Weighted average exercise | Number of options | Weighted average exercise |
Number of options | Weighted average exercise | |||||||||||||||||||
| Outstanding at beginning of the year | 718,396 | $ | 3.63 | 684,507 | $ | 3.51 | 398,938 | $ | 2.69 | |||||||||||||||
| Granted | - | $ | - | 33,889 | $ | 6.40 | 285,569 | $ | 4.54 | |||||||||||||||
| Forfeited and expired | - | $ | - | - | $ | - | - | $ | - | |||||||||||||||
| Exercised | (219,281 | ) | $ | 2.69 | - | $ | - | - | $ | - | ||||||||||||||
| Outstanding at end of the year | 499,115 | $ | 4.04 | 718,396 | $ | 3.63 | 684,507 | $ | 3.51 | |||||||||||||||
| Exercisable at end of the year | 398,659 | $ | 3.35 | 568,921 | $ | 2.89 | 529,586 | $ | 2.69 | |||||||||||||||
F-21
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
Following is a summary of changes in nonvested shares granted to consultants:
| For the year ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
Number of options | Weighted average exercise | Number of options | Weighted average exercise |
Number of options | Weighted average exercise | |||||||||||||||||||
| Balance at beginning of the year | 149,475 | $ | 6.19 | 154,921 | $ | 6.20 | - | $ | - | |||||||||||||||
| Granted | - | $ | - | 33,889 | $ | 6.40 | 285,569 | $ | 4.54 | |||||||||||||||
| Vested during the year | (49,019 | ) | $ | 6.17 | (39,335 | ) | $ | 6.20 | (130,648 | ) | $ | 2.69 | ||||||||||||
| Forfeited during the year | - | $ | - | - | $ | - | - | $ | - | |||||||||||||||
| Balance at end of the year | 100,456 | $ | 6.27 | 149,475 | $ | 6.19 | 154,921 | $ | 6.20 | |||||||||||||||
The total unrecognized estimated compensation cost related to non-vested consultants’ stock options granted until December 31, 2019 was $315, which is expected to be recognized over a weighted average period of 1.94 years.
| Set forth below is data regarding the range of exercise prices and remaining contractual life (in years) for |
| Exercise price | Number of options outstanding | Remaining contractual Life (in years) | Intrinsic Value of Options Outstanding | No. of options exercisable | ||||||||||||||
| (in thousands) | ||||||||||||||||||
| $ | 2.69 | 649,883 | 2.42 | $ | 811 | 649,883 | ||||||||||||
| $ | 3.66 | 250,000 | 7.34 | 69 | 222,222 | |||||||||||||
| $ | 4.68 | 52,250 | 7.25 | - | 27,375 | |||||||||||||
| $ | 5.34 | 216,200 | 9.25 | - | - | |||||||||||||
| $ | 5.34 | 445,792 | 9.88 | - | 69,264 | |||||||||||||
| $ | 5.96 | 150,000 | 9.88 | - | ||||||||||||||
| $ | 6.22 | 634,177 | 4.5 | - | 634,177 | |||||||||||||
| $ | 8.19 | 150,000 | 6.88 | - | 112,500 | |||||||||||||
| $ | 8.23 | 20,000 | 8.88 | - | 5,000 | |||||||||||||
| $ | 8.30 | 3,750 | 8.6 | - | 3,750 | |||||||||||||
| $ | 9.02 | 40,500 | 7.88 | - | 20,250 | |||||||||||||
| $ | 10.12 | 12,126 | 5.93 | - | 12,126 | |||||||||||||
| $ | 12.21 | 1,816 | 6.24 | - | 1,816 | |||||||||||||
| $ | 12.23 | 250,000 | 8.41 | - | 152,778 | |||||||||||||
| $ | 14.00 | 60,500 | 8.32 | - | 40,333 | |||||||||||||
| $ | 21.40 | 1,940 | 6.57 | - | 1,455 | |||||||||||||
| $ | 90.16 | 500 | 1.92 | - | 500 | |||||||||||||
| 2,939,434 | $ | 880 | 1,953,429 | |||||||||||||||
| Exercise Price | Number of Options Outstanding | Average Remaining Contractual Life (in years) | Intrinsic Value of Options Outstanding | No. of options exercisable | ||||||||||||||
| $ | 2.69 | 723,922 | 5.27 | $ | 4,130 | 719,695 | ||||||||||||
| $ | 6.22 | 714,201 | 8.04 | $ | 1,547 | 359,135 | ||||||||||||
| $ | 8.19 | 150,000 | 9.57 | $ | 30 | - | ||||||||||||
| $ | 10.12 | 13,398 | 8.93 | $ | - | 4,091 | ||||||||||||
| $ | 12.21 | 2,421 | 9.25 | $ | - | - | ||||||||||||
| $ | 21.4 | 5,600 | 9.57 | $ | - | - | ||||||||||||
| $ | 90.16 | 15,500 | 1.69 | $ | - | 15,500 | ||||||||||||
| 1,625,042 | $ | 5,707 | 1,098,691 | |||||||||||||||
The total intrinsic value of options exercised during 20192022 was $1,262.$0.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| The following table contains information concerning restricted stock units granted under the existing equity incentive plans: |
| For the year ended December 31, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| Number of shares | Weighted average grant date fair value | Number of shares | Weighted average grant date fair value | |||||||||||||
| Nonvested at beginning of period | 229,331 | $ | 10.08 | - | $ | - | ||||||||||
| Granted | - | $ | - | 236,706 | $ | 10.22 | ||||||||||
| Vested | (67,458 | ) | $ | 9.91 | - | $ | - | |||||||||
| Forfeited | (4,313 | ) | $ | 14.67 | (7,375 | ) | $ | 14.67 | ||||||||
| Nonvested at end of period | 157,560 | $ | 10.02 | 229,331 | $ | 10.08 | ||||||||||
The Company estimates the fair value of restricted stock units based on the closing sales price of the Ordinary Shares on the date of grant (or the closing bid price, if no sales were reported). During the years ended December 31, 2022 and 2021, the Company recognized $744 thousand and $1,024, respectively, of share-based compensation expense related to restricted stock units. Total share-based compensation expense related to restricted stock units not yet recognized as of December 31, 2022 was $516 thousand, which is expected to be recognized over a weighted average period of 1.16 years.
| e) | The following table summarizes share-based compensation expenses related to grants under the |
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Research & development | $ | 607 | $ | 862 | $ | 123 | ||||||
| General & administrative | 203 | 147 | (2 | ) | ||||||||
| Total | $ | 810 | $ | 1,009 | $ | 121 | ||||||
F-22
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| Research & development | $ | 876 | $ | 1,210 | $ | 401 | ||||||
| General & administrative | 1,836 | 1,704 | 269 | |||||||||
| Total | $ | 2,712 | $ | 2,914 | $ | 670 | ||||||
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1213 – TAXES ON INCOME
| a. | The Israeli corporate tax rate | |
| b. | The Company has not | |
| c. | The Parent and its subsidiaries are taxed separately. |
The Company measures its results for tax purposes in nominal terms in NIS based on financial reporting under Israeli accounting principles, therefore, there are differences between the Company’s taxable income (loss) and income (loss) reflected in these financial statements.
As of December 31, 2019, the
The Parent and Enlivex R&D hadhas loss carry-forwards amounting to approximately $282$42 million deductible only against sale of assets and/or activities in connection with income generated from its clinical development programs prior to the Company’s merger transaction in March 2019, upon consummation of which Enlivex Therapeutics R&D Ltd. became a wholly owned subsidiary of the Company, and $20,343,the Company changed its name from Bioblast Pharma Ltd. to Enlivex Therapeutics Ltd. As of December 31, 2022, the Parent had additional losses carry-forward amounting to approximately $14.5 million, deductible from future taxable income.
As of December 31, 2022, Enlivex Therapeutics R&D Ltd. and Enlivex Therapeutics RDO Ltd. had losses carry-forward amounting to approximately $46 and $0.5 million, respectively, deductible from future taxable income.
These loss carry-forwardslosses carry-forward have no expiration date.
| d. | The components of the provision for income taxes are as follows: |
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| Current tax | $ | - | $ | - | $ | - | ||||||
| Deferred tax | - | - | - | |||||||||
| Provision for income taxes, net | $ | - | $ | - | $ | - | ||||||
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Current tax | $ | - | $ | - | $ | - | ||||||
| Deferred tax | - | - | - | |||||||||
| Provision for income taxes, net | $ | - | $ | - | $ | - | ||||||
| e. | A reconciliation between the |
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Loss before taxes | $ | 9,384 | $ | 4,242 | $ | 2,503 | ||||||
| Statutory tax rate | 23 | % | 23 | % | 24 | % | ||||||
| Tax benefit | 2,158 | 976 | 601 | |||||||||
| Permanent differences | (322 | ) | (237 | ) | (30 | ) | ||||||
| Valuation allowance | (1,836 | ) | (739 | ) | (535 | ) | ||||||
| Differences in tax rate | - | - | (36 | ) | ||||||||
| Tax expenses | $ | - | $ | - | $ | - | ||||||
| Year ended December 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Statutory tax rate | 23 | % | 23 | % | 23 | % | ||||||
| Permanent differences | (4 | )% | 7 | % | 5 | % | ||||||
| Change in valuation allowance | (19 | )% | (30 | )% | (28 | )% | ||||||
| Effective tax rate | - | - | - | |||||||||
| f. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax purposes.
As of December 31, 2019,2022, the Company had provided a full valuation allowance in respect of deferred tax assets. Management currently believes that because the Company has a history of losses, it is more likely than not that the deferred tax regarding the loss carry-forward and other temporary differences will not be realized for the foreseeable future.
Components of the Company’s deferred tax liabilities and assets are as follows:
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Tax assets in respect of: | $ | $ | $ | |||||||||
| Accrued employees’ and directors’ compensation | 136 | 31 | 18 | |||||||||
| Research and development expenses | 738 | 609 | 379 | |||||||||
| Net loss carry forward | 4,744 | 2,743 | 2,242 | |||||||||
| Total deferred tax assets | 5,595 | 3,383 | 2,639 | |||||||||
| Less - valuation allowance | (5,595 | ) | (3,383 | ) | (2,639 | ) | ||||||
| Deferred tax assets | $ | - | $ | - | $ | - | ||||||
| Year ended December 31, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Tax assets in respect of: | ||||||||
| Accrued employees’ and directors’ compensation | $ | 213 | $ | 308 | ||||
| Stock based compensation | 1,365 | 746 | ||||||
| Research and development expenses | 3,486 | 2,527 | ||||||
| Fixed assets | 185 | - | ||||||
| Net loss carryforward | 23,693 | 22,444 | ||||||
| Total deferred tax assets | 28,942 | 26,025 | ||||||
| Less - valuation allowance | (28,942 | ) | (26,025 | ) | ||||
| Deferred tax assets | $ | - | $ | - | ||||
F-23
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1314 – BALANCES AND TRANSACTIONS WITH RELATED PARTIES
Related party’s balances and transactions excluding compensation of members of management and the Board, were as follows:
| a) |
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Key management personnel and directors | $ | 367 | $ | 13 | ||||
| (in thousands) | December 31, | |||||||
| 2022 | 2021 | |||||||
| Current Liabilities - Accounts payable trade | $ | 113 | $ | 275 | ||||
| Current Liabilities - Accrued expenses | $ | 99 | $ | 115 | ||||
| b) | Related parties’ transactions |
On May 12, 2019, the Company entered into a research agreement with its Chief Scientific & Medical Officer under which the executive officer undertook to perform a patients’ study according to the terms defined in the agreement. The agreement will terminate upon the completion of the study according to the study protocol and the submission of all reports and other documentation, or if earlier, upon termination by any of the parties pursuant to the terms of the agreement. In the event of early termination for any reason, the Company shall pay the executive officer the following payments: (i) the agreed remuneration for work performed in accordance with the agreement until the date of termination; and (ii) in the event that the termination is not due to a breach of the agreement by the executive officer, the Company shall reimburse the executive officer for all documented expenses arising from non-cancellable commitments incurred prior to such termination, and (iii), the balance between the aggregate amount of the remuneration and the advance (as defined below) actually paid to the executive officer, and the amount of $816, in consideration for any expenses incurred by executive officer in preparation for the study.termination. In consideration for the executive officer’s fulfilment of his obligations, the Company has agreed to pay a fixed price for each of the activities performed as defined in the agreement. Upon entering the agreement, the Company paid the executive officer a non-refundable advance in the amount of $125. Total Research and development expenses related to this agreement and included in the Company’s consolidated statement of operations for the yearyears ended December 31, 20192022, 2021 and 2020 were $251.$1,322 thousand, $1,331 thousand and $522 thousand.
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1415 – FAIR VALUE MEASUREMENT
The Company’s financial assets measured at fair value on a recurring basis consisted of the following types of instruments as of December 31, 2019, 20182022 and 2017:2021:
| December 31, 2019 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash and cash equivalents | $ | 3,948 | $ | 3,948 | $ | - | $ | - | ||||||||
| Short term deposits | 8,060 | 8,060 | - | - | ||||||||||||
| Cash held with respect to CVR Agreement | 1,500 | 1,500 | - | - | ||||||||||||
| Restricted cash | 76 | 76 | - | - | ||||||||||||
| Total financial assets | $ | 13,584 | $ | 13,584 | $ | - | $ | - | ||||||||
| December 31, 2022 | ||||||||||||||||
| (in thousands) | Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Cash and cash equivalents | $ | 49,945 | $ | 49,945 | $ | - | $ | - | ||||||||
| Short term deposits | 299 | 299 | - | - | ||||||||||||
| Restricted cash | 412 | 412 | - | - | ||||||||||||
| Total financial assets | $ | 50,656 | $ | 50,656 | $ | - | $ | - | ||||||||
| December 31, 2018 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash and cash equivalents | $ | 9,736 | $ | 9,736 | $ | - | $ | - | ||||||||
| Short term deposits | 40 | 40 | - | - | ||||||||||||
| Restricted cash | 56 | 56 | - | - | ||||||||||||
| Total financial assets | $ | 9,832 | $ | 9,832 | $ | - | $ | - | ||||||||
| Warrants | $ | 192 | $ | - | $ | - | $ | 192 | ||||||||
| Total financial liabilities | $ | 192 | $ | - | $ | - | $ | 192 | ||||||||
| December 31, 2017 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash and cash equivalents | $ | 9,005 | $ | 9,005 | $ | - | $ | - | ||||||||
| Restricted cash | 27 | 27 | - | - | ||||||||||||
| Total financial assets | $ | 9,032 | $ | 9,032 | $ | - | $ | - | ||||||||
| Warrants | $ | 344 | $ | - | $ | - | $ | 344 | ||||||||
| Total financial liabilities | $ | 344 | $ | - | $ | - | $ | 344 | ||||||||
| December 31, 2021 | ||||||||||||||||
| (in thousands) | Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Cash and cash equivalents | $ | 11,202 | $ | 11,202 | $ | - | $ | - | ||||||||
| Short term deposits | 10,004 | 10,004 | - | - | ||||||||||||
| Marketable securities | 62,924 | 62,924 | - | - | ||||||||||||
| Restricted cash | 434 | 434 | - | - | ||||||||||||
| Total financial assets | $ | 84,564 | $ | 84,564 | $ | - | $ | - | ||||||||
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
NOTE 1516 – SUPPLEMENTARY FINANCIAL STATEMENT INFORMATION
| a. | Research and development expenses – net |
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| Payroll and related expenses | $ | 6,371 | $ | 5,054 | $ | 3,183 | ||||||
| Research and development services | 8,264 | 4,557 | 2,438 | |||||||||
| Materials | 2,044 | 1,505 | 612 | |||||||||
| Share Based Compensation | 876 | 1,210 | 401 | |||||||||
| Depreciation | 740 | 546 | 273 | |||||||||
| Other | 1,605 | 1,029 | 394 | |||||||||
| 19,900 | 13,901 | 7,301 | ||||||||||
| Israel Innovation Authority participation in research and development costs and royalties payable | (1,207 | ) | (1,020 | ) | (1,215 | ) | ||||||
| $ 18,693, | $ | 12,881 | $ | 6,086 | ||||||||
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Payroll and related expenses | $ | 2,259 | $ | 1,317 | $ | 593 | ||||||
| Research and development services | 2,682 | 874 | 298 | |||||||||
| Materials | 454 | 363 | 195 | |||||||||
| Patent-related expenses | 120 | 242 | 219 | |||||||||
| Share Based Compensation | 607 | 862 | 123 | |||||||||
| Depreciation | 198 | 121 | 83 | |||||||||
| Other | 486 | 476 | 180 | |||||||||
| 6,806 | 4,255 | 1,691 | ||||||||||
| Israel Innovation Authority participation in research and development costs and royalties payable | (1,082 | ) | - | - | ||||||||
| $ | 5,724 | $ | 4,255 | $ | 1,691 | |||||||
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Payroll expenses and management fees | $ | 569 | $ | 343 | $ | 189 | ||||||
| Compensation to directors | 526 | 182 | 176 | |||||||||
| Professional fees | 725 | 208 | 70 | |||||||||
| Office maintenance and office expenses | 145 | 96 | 16 | |||||||||
| Insurance | 243 | 11 | 7 | |||||||||
| Share Based Compensation | 203 | 147 | (2 | ) | ||||||||
| Other | 484 | 57 | 24 | |||||||||
| $ | 2,895 | $ | 1,044 | $ | 480 | |||||||
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Interest income | $ | 238 | $ | 138 | $ | 37 | ||||||
| Exchange differences, net | - | 790 | - | |||||||||
| Net change in fair value warrants | - | 132 | - | |||||||||
| $ | 238 | $ | 1,060 | $ | 37 | |||||||
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Issuance expenses related to warrants | $ | - | $ | - | $ | 19 | ||||||
| Exchange differences, net | 945 | - | 350 | |||||||||
| Net change in fair value warrants | 51 | - | - | |||||||||
| Bank commissions | 7 | 3 | 1 | |||||||||
| $ | 1,003 | $ | 3 | $ | 370 | |||||||
F-25
ENLIVEX THERAPEUTICS LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| b. | General and administrative expenses |
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| Payroll expenses | $ | 1,289 | $ | 1,138 | $ | 737 | ||||||
| Compensation to directors | 681 | 1,168 | 862 | |||||||||
| Professional fees | 1,294 | 1,080 | 995 | |||||||||
| Office maintenance and office expenses | 402 | 167 | 97 | |||||||||
| Insurance | 895 | 783 | 486 | |||||||||
| Share Based Compensation | 1,836 | 1,704 | 269 | |||||||||
| Other | 707 | 367 | 253 | |||||||||
| $ | 7,104 | $ | 6,407 | $ | 3,699 | |||||||
| c. | Other income (expenses), net |
| Year ended December 31, | ||||||||||||
| (in thousands) | 2022 | 2021 | 2020 | |||||||||
| Interest income | $ | 835 | $ | 120 | $ | 225 | ||||||
| (Loss) income related to marketable securities | (1,983 | ) | 5,590 | - | ||||||||
| Exchange differences, net | (4,101 | ) | (562 | ) | (2,258 | ) | ||||||
| Bank commissions and other expenses | (14 | ) | (328 | ) | (6 | ) | ||||||
| $ | (5,263 | ) | $ | 4,820 | $ | (2,039 | ) | |||||
NOTE 1617 – EVENTS SUBSEQUENT TO THE BALANCE SHEET DATE
The Company evaluated all events and transactions that occurred subsequent to the balance sheet date and prior to the date on which these financial statements were issued, and determined that the following events necessitated disclosure:disclosure:
| 1. | In February 2023, the Board approved the payment of bonuses for 2022 in the following manner: (i) up to an aggregate of $410,000, representing 5% of the aggregate 2022 base salaries, will be paid in cash, and (ii)and an aggregate of $616,000, representing 7.5% of the aggregate 2022 base salaries, will be paid in either cash or via a formula that converts the cash bonus to restricted stock units, to be determined by the Board 75 days prior to the annual general meeting of shareholders of the Company to be held in 2023, based on the Company’s cash balance and the market conditions at such time. The Company accrued for the $410,000 cash bonus as of December 31, 2022. |
On February 24, 2020 the Company entered into a securities purchase agreement with certain institutional investors in connection with the issuance and sale in a registered direct offering of 1,000,000 Ordinary Shares at a purchase price of $8.00 per share. In settlement of issuance costs to the placement agent, in addition to a cash fee equal to 7% of the gross proceeds and management fee of 1% of the gross proceeds and $113 for fees and expenses, the Company issued on February 26, 2020 warrants to purchase up to 70,000 Ordinary Shares at an exercise price of $10 per Ordinary Share. These warrants became exercisable immediately upon issuance and have a term of five years.
| 2. | In February 2023 the Board approved an increase in the number of shares reserved for issuance under the 2019 Plan by 878,000 Ordinary Shares. |
| 3. | During the first quarter of 2023 the Company issued 110,115 Ordinary Shares under the ATM Agreement, see Note 11(b) above for further information on the ATM Agreement. |
On March 1, 2020, the Company entered into a securities purchase agreement with certain institutional investors in connection with the issuance and sale in a registered direct offering of 2,093,750 Ordinary Shares, and warrants to purchase up to 2,093,750 Ordinary Shares, at a combined purchase price of $8 per share, together with the related warrant to purchase one Ordinary Share. The warrants became exercisable immediately upon issuance at a price of $9 per share and have a term of two years from the issuance date. In settlement of issuance costs to the placement agent, in addition to a cash fee equal to 7% and management fee of 1% of the gross proceeds and $113 for fees and expenses, the Company registered and issued warrants to purchase up to 146,563 Ordinary Shares, which became exercisable immediately upon issuance at a price of $10 per share and have a term of two years from the issuance date.
F-24
F-24