UNITED STATES
Form S-1
Adeza Biomedical Corporation
| Delaware | 2835 | 77-0054952 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
1240 ELKO DRIVE
SUNNYVALE, CAElko Drive
Emory V. Anderson
Copies to:
| Sarah A. O’Dowd Heller Ehrman White & McAuliffe LLP 275 Middlefield Road Menlo Park, California 94025 (650) 324-7000 | Frederick W. Kanner Dewey Ballantine LLP 1301 Avenue of the Americas New York, NY 10019 (212) 259-8000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. [_]
o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [_]
o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [_]
o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. [_]
o
CALCULATION OF REGISTRATION FEE
- -------------------------------------------------------------------------------
- -------------------------------------------------------------------------------
| Title of Each Class of | Proposed Maximum Aggregate | Amount of | ||
| Securities to be Registered | Offering Price(1) | Registration Fee | ||
| Common Stock, par value $0.001 per share | $69,000,000 | $8,742.30 | ||
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the 2,500,000 sharesRegistrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of common stock, par value $0.001 per share (the
"Common Stock"), offered hereby are being sold by Adeza Biomedical Corporation
("Adeza" or the "Company").
Prior to this offering there has been no public market for the Common Stock of
the Company. It is currently anticipated that the initial public offering price
will be between $11.00 and $13.00 per share. See "Underwriting" for a
discussion of the factors to be considered in determining the initial public
offering price.
Application has been made for inclusion of the Company's Common Stock in The
Nasdaq Stock Market's National Market (the "Nasdaq National Market") under the
symbol "ADZA."
THE COMMON STOCK OFFERED HEREBY INVOLVES A HIGH DEGREE OF RISK. SEE "RISK
FACTORS" ON PAGES 6 TO 16 FOR A DISCUSSION OF CERTAIN MATERIAL FACTORS THAT
SHOULD BE CONSIDERED IN CONNECTION WITH AN INVESTMENT IN THE COMMON STOCK
OFFERED HEREBY.
- --------------------------------------------------------------------------------
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES AND
EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION NOR HAS THE
SECURITIES AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION
PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY
REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
- -----------------------------------------------------------------------------
- -----------------------------------------------------------------------------
Underwriting
Price to Discounts and Proceeds to
Public Commissions(1) Company(2)
- -----------------------------------------------------------------------------
Per Share........................... $ $ $
- -----------------------------------------------------------------------------
Total(3)............................ $ $ $
- -----------------------------------------------------------------------------
- -----------------------------------------------------------------------------
(1) The Company and certain stockholders of the Company (the "Selling
Stockholders") have agreed to indemnify the several Underwriters against
certain liabilities, including liabilities under the Securities Act of 1933 or until the Registration Statement shall become effective on such date as amended. See "Underwriting."
(2) Before deducting expenses payable by the Company estimatedCommission, acting pursuant to be $750,000.
(3) The Selling Stockholders have granted the several Underwriters 30-day over-
allotment options to purchase up to 375,000 additional shares of Section 8(a), may determine.
| The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted. |
| PRELIMINARY PROSPECTUS | Subject to Completion | August 6, 2004 |
Shares

| Adeza Biomedical Corporation |
This is the same terms and conditions as set forth above. Ifinitial public offering of our common stock. No public market currently exists for our common stock. We are offering all such
additional shares are purchased by the Underwriters, the total Price to
Public will be $ , the total Underwriting Discounts and Commissions will
be $ , the total Proceeds to Company will be $ , and the total Proceeds
to Selling Stockholders will be $ . See "Principal and Selling
Stockholders" and "Underwriting."
- --------------------------------------------------------------------------------
The shares of Common Stock are offered by the several Underwriters subject to
delivery by the Company and the Selling Stockholders and acceptance by the
Underwriters, to prior sale and to withdrawal, cancellation or modification of
the offer without notice. Delivery of the shares toof common stock offered by this prospectus. We expect the Underwriters is
expectedpublic offering price to be made atbetween $ and $ per share.
We have applied to have our common stock approved for quotation on The Nasdaq National Market under the office of Prudential Securities Incorporated, One
New York Plaza, New York, New York, on or about , 1996.
PRUDENTIAL SECURITIES INCORPORATED
NEEDHAM & COMPANY, INC.
TUCKER ANTHONY INCORPORATED
, 1996
DIAGNOSING PROBLEMS IN WOMEN'S HEALTH CARE
PREMATURE BIRTH
Premature birth is a life-threatening
problem affecting 10% of all birthssymbol “ADZA.”
Investing in the United States. More than 20,000
infants die each year in the U.S. from
premature delivery. Surviving infants
often suffer lifelong mental and physical
handicaps,and the high cost of neonatal [DRAWING OF CHEMICAL STRUCTURE]
intensive care imposes a significant
financial burden.
Adeza received an expedited PMA from
the FDA for a fetal fibronection fFN
ELISA (Enzyme-Linked Immunosorbent Assay)
test to aid in the diagnosis of premature
birth in symptomatic women. Adeza is
preparing PMA supplements for the use of
the assay in asymptomatic women and for
a point-of-care dipstick test. fFN: A Biochemical Indicator
For Premature Birth
[PHOTOS] [DIAGRAMMED DRAWING]
PREECLAMPSIA ENDOMETRIOSIS/INFERTILITY
Preeclampsia, severe hypertension during Endometriosis is a serious condition
pregnancy, is a leading cause of afflicting 10% of all women of
maternal death and affects 7% of all reproductive age in the United
pregnancies in the United States. If States.
If the condition progresses to eclampsia,
the lives of both mother and baby are at Adeza is developing analytical
risk. software and less-invasive
biochemical assays to identify
Adeza has developed an ELISA-based women with endometriosis and
diagnostic test to confirm severe endometriosis-related infertility.
preeclampsia in symptomatic women.
Preclinical evaluations are in
progress in the United States, Europe
and Australia.
Adeza(Registered Trademark) is a
registered trademark of the Company,
and the Adeza logo is a trademark of [LOGO OF
the Company. This Prospectus also ADEZA BIOMEDICAL
contains trademarks and trade names of CORPORATION]
other companies.
- --------------------------------------------------------------------------------
IN CONNECTION WITH THIS OFFERING, THE UNDERWRITERS MAY OVER-ALLOT OR EFFECT
TRANSACTIONS WHICH STABILIZE OR MAINTAIN THE MARKET PRICE OF THE COMMON STOCK
OFFERED HEREBY AT A LEVEL ABOVE THAT WHICH MIGHT OTHERWISE PREVAIL IN THE OPEN
MARKET. SUCH STABILIZING, IF COMMENCED, MAY BE DISCOUNTED AT ANY TIME.
2
PROSPECTUS SUMMARY
The following summary is qualified in its entirety by the more detailed
information and the Consolidated Financial Statements and Notes thereto
appearing elsewhere in this Prospectus, including information under "Risk
Factors." The Common Stock offered herebyour common stock involves a high degree of risk. Unless otherwise indicated,Before buying any shares, you should carefully read the discussion of material risks of investing in our common stock in “Risk factors” beginning on page 7 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ | ||||||
The underwriters may also purchase up to an additional shares of our common stock at the public offering price, less the underwriting discounts and commissions payable by us, to cover over-allotments, if any, within 30 days from the date of this prospectus. If the underwriters exercise this option in full, the total underwriting discounts and commissions will be $ and our total proceeds, before expenses, will be $ .
The underwriters are offering the common stock as set forth under “Underwriting.” Delivery of the shares will be made on or about , 2004.
UBS Investment Bank
SG Cowen & Co.
| Thomas Weisel Partners LLC |
| William Blair & Company |
You should rely only on the information contained in this Prospectus (i) assumesprospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional information or information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the Underwriters' over-allotment options will not be exercised, (ii) reflects
the one-for-2.4 reverse stock split effected in connection with the Company's
reincorporation in Delaware prior to the effectivenessdate of this offering and
(iii) gives effect toprospectus, regardless of the automatic conversiontime of all outstanding sharesdelivery of Series 1 Preferred Stock and Series 2 Preferred Stock into an equal numberthis prospectus or of any sale of shares of Common Stock uponour common stock.
Through and including , 2004 (the 25th day after the completiondate of this offering. Certain termsprospectus), federal securities laws may require all dealers that effect transactions in our common stock, whether or not participating in this offering, to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
TABLE OF CONTENTS
Adeza Biomedical Corporation Trademarks and Registered Trademarks are definedtrademarks of Adeza. Our trademarks and trade names include the stylized A, Adeza®, E-tegrity® Test, FULL TERM® and TLiIQ® System. Other service marks, trademarks and trade names referred to in this prospectus are the Glossaryproperty of Termstheir respective owners.
Prospectus summary
This summary highlights selected information appearing elsewhere in this prospectus. While this summary highlights what we consider to be the most important information about us, you should carefully read this prospectus and the registration statement of which this prospectus is a part in their entirety before investing in our common stock, especially the risks of investing in our common stock, which we discuss later in “Risk factors,” and our financial statements and related notes beginning on page 65 of this Prospectus.
THE COMPANYF-1. Unless the context requires otherwise, the words “Adeza,” “we,” “Company,” “us” and “our” refer to Adeza Biomedical Corporation ("Adeza" or the "Company") developsCorporation.
OUR BUSINESS
We design, develop, manufacture and markets
diagnosticmarket innovative products and services for women's reproductive health care. The
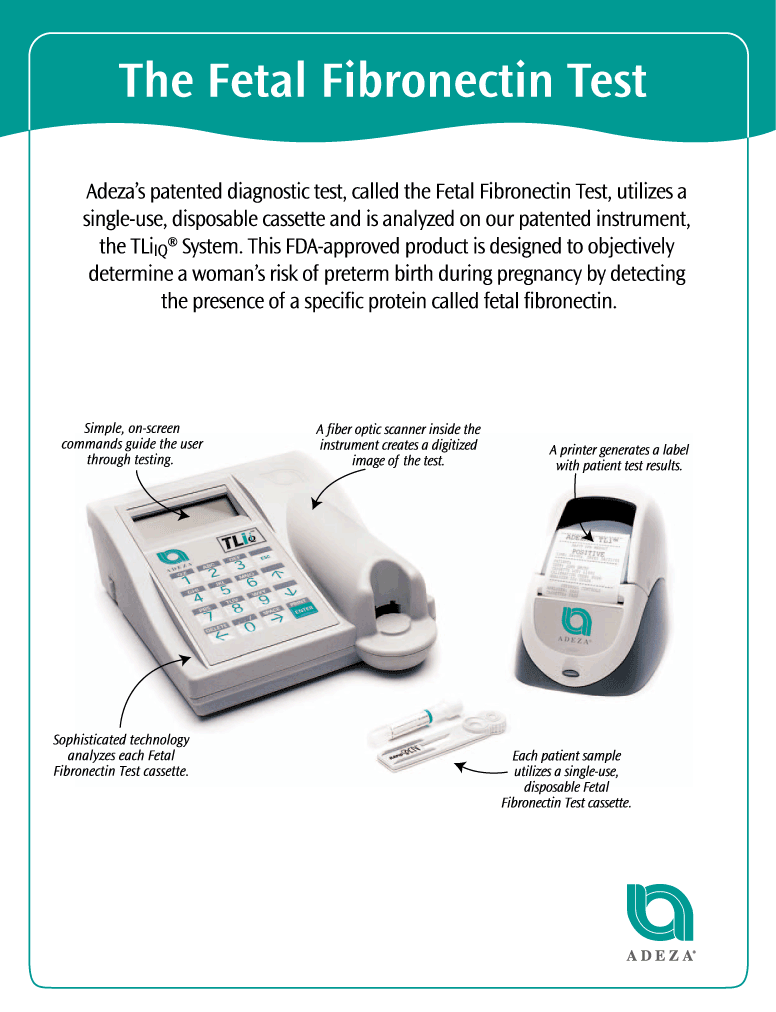
Company's primarywomen’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the developmentFetal Fibronectin Test, that utilizes a single-use, disposable cassette and marketing of proprietary tests
foris analyzed on our patented instrument, the diagnosis of pregnancy-related and female reproductive disorders,
including premature and late birth, preeclampsia, endometriosis and
infertility. The Company believes its products and services will result in
improved patient management, with a consequent reduction in both patient risk
and overall costs.
The Company has received an expedited premarket approval ("PMA") fromTLiIQ System. This product is approved by the Food and Drug Administration, ("FDA") to market its proprietary enzyme-linked
immunosorbent assay ("ELISA") diagnostic (the "fFN ELISA Test")or FDA, for broad use in women with symptomsassessing the risk of prematurepreterm birth. The fFN ELISA
Our Fetal Fibronectin Test is the only FDA-
approved immunodiagnostic test for this disorder and representsdesigned to objectively determine a significant
advance over currently used evaluation techniques. This test measureswoman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, ("fFN") in the vaginal fluidsecretions during pregnancy. Testing for fetal fibronectin during pregnancy provides a more accurate assessment of pregnant women in
order to assess the likelihood of premature birth. The Company believes that
its fFN-based productsa preterm birth than traditional methods. Preterm births have the potentialhistorically accounted for up to become an element85% of standard
prenatal care. The Company will distribute the fFN ELISA Testall pregnancy-related complications and deaths in the United States, through an exclusive strategic distribution arrangementand over $13 billion in costs were associated with Matria
Healthcare, Inc. ("Matria"),the care of preterm or low birth weight infants in 2001. By correctly identifying women at risk for preterm birth, we believe our Fetal Fibronectin Test leads to improved patient care and significant cost savings and has the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women.
The patient population for which our Fetal Fibronectin Test is approved can be divided into three patient categories. The first category consists of women who present with signs and symptoms of preterm labor and are typically directed to the hospital. The second and third categories include women designated as either “high-risk” or “low-risk” for preterm birth by their healthcare providers, and who currently exhibit no signs and symptoms of preterm labor. We believe that by using the Fetal Fibronectin Test periodically during a leading women'spregnancy, healthcare providers can more accurately assess the likelihood that women in all three categories will not deliver preterm.
We have shipped more than 1,200 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes for use in hospital and clinical laboratories. As of August 3, 2004, our direct sales force consisted of 66 representatives who sell to hospital and clinical laboratories, health care company. Matria
began shipment of fFN ELISA Tests toplans and healthcare providers. Our Fetal Fibronectin Test has a select number of initial customers in
the United States in April 1996specific reimbursement code, and is expected to commence full-scale
marketing efforts in the second half of 1996. The Companywe believe that reimbursement for our Fetal Fibronectin Test has been selling the
fFN ELISAregularly available through health plan organizations and most state Medicaid programs.
We also market and sell our E-tegrity Test, in Japan since 1995 through an exclusive arrangement with
Daiichi Pure Chemicals Co. Ltd. ("Daiichi") and in Europe throughinfertility-related test based on a limited
number of distributors.
The women's reproductive health care market represents a large and
increasingly important partproprietary analyte specific reagent, to assess receptivity of the health care economy,uterus to embryo implantation in women with an estimated $20
billion being spent annuallyunexplained infertility. The E-tegrity Test can be particularly useful for women who are considering assisted reproductive technologies, including in vitro fertilization, or IVF. We are also seeking to expand the United States on obstetricalindications for use of our Fetal Fibronectin Test for predicting successful induction of labor, for predicting delivery at term and gynecological ("Ob/Gyn") care. Pregnancy-related disorders being addressed byfor diagnostic applications in oncology, including bladder cancer.
We were profitable for both the Company, including prematureyear ended December 31, 2003 and for the six months ended June 30, 2004. For the year ended December 31, 2003, we had product sales of approximately $26.5 million and net income of $3.2 million. For the six months ended June 30, 2004, we had product sales of approximately $15.6 million and net income of $2.0 million.
OUR PRIMARY MARKET
Preterm birth and preeclampsia, represent significant
opportunities within the women's reproductive health care market. The American
College of Obstetrics and Gynecology estimates that of the annual
There are approximately four million births in the United States approximately 400,000annually. Births occurring before 37 weeks of pregnancy are prematuredefined as preterm, and approximately 300,000 births are affected by preeclampsia, which accounts for
10% to 25%in recent years, they have occurred on average at a rate of premature births. CIGNA Corporation estimates that the costs
associated with premature births are greater than $4.7 billionover 1,300 per day, or 480,000 per year. The
Company also targetsAccording to the reproductive disorderCenters for Disease Control and Prevention, the percentage of endometriosis, whichpreterm births in the United States is estimatedgrew to afflict six million women12% of reproductive age.
Endometriosis is also closely associated with anotherall births in 2002, an increase of the Company's other
targeted markets, infertility. Annual expenditures29% since 1981. This increase in the United Statespreterm birth rate is a growing public health concern. In January 2003, the March of Dimes launched a five-year, $75 million campaign to reduce the number of preterm births.
Historically, healthcare providers have had difficulty accurately predicting the likelihood of preterm birth, which has contributed to increased costs and complications. According to a 1994 publication cited by the March of Dimes, the cost of medical care for a complicated birth ranged between $20,000 and $400,000 from delivery to hospital discharge, while the diagnosisaverage cost of a normal birth was $6,400.
Women that are evaluated and potentially treated for preterm birth fall into three categories:
| 4 | Women with signs and symptoms of preterm labor— We believe that there are approximately 1 million episodes each year in the United States where women seek urgent medical care for signs and symptoms of preterm labor. Some of these signs and symptoms include uterine contractions, cervical dilation, vaginal infection, backache, pelvic pain, abdominal fullness or discomfort, change in vaginal discharge and vaginal bleeding. However, as these signs and symptoms are common throughout pregnancy, they do not provide a sufficient basis for making an accurate diagnosis of preterm labor and impending birth. |
| Prior to an episode, these women were designated as either “high-risk” or “low-risk” for preterm birth. |
| 4 | Women designated as “high-risk” for preterm birth— We believe that up to 1.2 million women in the United States annually may be designated as “high-risk” for preterm birth during their pregnancy. Risk factors include previous preterm birth, multiple gestation, uterine anomalies, cervical length, gestational diabetes, hypertension, low pre-pregnancy weight, use of illicit drugs, sexually transmitted diseases, vaginal infections, smoking, consumption of alcohol and demographic factors such as low socioeconomic status, certain age brackets and races. However, healthcare providers have had limited success in accurately determining the risk of preterm birth based on these risk factors and evaluations. |
| 4 | Women designated as “low-risk” for preterm birth— We believe that up to 2.8 million women in the United States annually with no known risk factors are designated as “low-risk.” However, “low-risk” women account for approximately 50% of all preterm births. We believe that the ability to accurately diagnose which of these women are truly at high risk for preterm birth is currently beyond the scope of traditional evaluation methods. Women who are inaccurately identified as “low-risk” are excluded from the potential benefits of existing interventions. |
OUR SOLUTION
We believe that our proprietary, FDA-approved diagnostic test and instrument, the single-use, disposable Fetal Fibronectin Test and the TLiIQ System, have the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women and to become a standard of infertilitycare for use in pregnancy. The clinical efficacy of our Fetal Fibronectin Test for preterm birth has been demonstrated in numerous large, multi-center peer-reviewed clinical publications.
The Fetal Fibronectin Test and the TLiIQ System have the following key characteristics:
| 4 | Objective result—Instrument provides a positive or negative result; |
| 4 | Low-cost instrument—Minimal cost is incurred to acquire the instrument; |
| 4 | Rapid turnaround—Produces a result in less than 25 minutes; |
| 4 | Easy to use—Simple and convenient test procedure and instrument user interface; |
| 4 | Established reimbursement—Reimbursement provided by large US health plans; and |
| 4 | Significant cost savings opportunity—Reduces hospital admissions and eliminates unnecessary transports and costly interventions. |
We believe women who present with signs and symptoms of preterm labor are estimatedcandidates for one Fetal Fibronectin Test per episode, and women designated as “high-risk” may be tested multiple times during their pregnancy. We estimate that our product has a potential market size in these two patient populations of over $400 million annually. If we are able to expand the use of our Fetal Fibronectin Test for women designated as “low-risk” and for other uses, we estimate that the potential annual market size can be approximately $2.0greater than $1 billion.
Adeza's
OUR STRATEGY
Our goal is to becomebe a global leader in the diagnosisdevelopment and treatmentcommercialization of
pregnancy-related and female reproductive disorders by designing, developing
and marketing proprietary diagnostic testsproducts for reproductive healthcare. We believe that our products can assist healthcare providers in making more timely and servicesaccurate diagnoses, leading to improved quality of care and significant cost savings for the women's
reproductive health care market.healthcare system. In additionorder to the fFN ELISA Test, the Company
is developing several other products foreffectively execute this market. The Company is preparing
supplementsstrategy, we plan to:
| 4 | leverage existing sales and marketing infrastructure to increase market penetration; |
| 4 | increase marketing for women designated as “high-risk”; |
| 4 | expand marketing to “low-risk” patient population; |
| 4 | expand international sales; |
| 4 | seek additional indications for our Fetal Fibronectin Test in pregnancy-related applications; |
| 4 | develop products for applications in oncology; and |
| 4 | strategically acquire or in-license complementary businesses, products and technologies. |
OUR CORPORATE INFORMATION
Our business was incorporated in California in 1985 as Aspen Diagnostics Corporation. We changed our name to its PMA ("PMA Supplements") for the use of the fFN ELISA TestAdeza Biomedical Corporation in assessing the likelihood of premature birth1989, and in asymptomatic
3
women, and for the use of a point-of-care rapid assay (the "fFN Dipstick Test")
for assessing the likelihood of premature birth in symptomatic women. Clinical
trials have been completed by the National Institutes of Health (the "NIH")
which support the use of the fFN ELISA Test for asymptomatic women. The Company
is currently conducting clinical trials to support the use of the fFN Dipstick
in symptomatic women. Additionally, the Company is1996 we re-incorporated in the processState of designing
clinical trials for the use of the fFN Dipstick Test in assessing the
likelihood of successful induction of labor at term, which may be useful in
avoiding complications related to late birth. The Company's proprietary fFN
vertical flow membrane test (the "fFN Vertical Flow Test") for the assessment
of premature rupture of amniotic membranes ("ROM") has been introduced for sale
in Japan. Finally, the Company has developed a proprietary test based on
cellular fibronectin (the "cFN Test") for the diagnosis of preeclampsia, a
leading cause of maternal death and fetal complications in the United States.
The cFN Test is currently undergoing preclinical evaluations in the United
States, Europe and Australia. The Company also maintains a significant product
development program with the goal of introducing additional proprietary
diagnostics and therapeutics for the women's reproductive health care market.
The Company intends to increase acceptance and create long-term demand by
marketing the cost effectiveness and improved patient care that its products
and services provide to physicians, hospitals, other health care providers and
third-party payors. The Company, together with strategic partners and
distributors, will educate this target market through a variety of means,
including marketing evaluations, seminars, workshops, publications and
professional and trade meetings. The Company believes that the trend toward
management of health care costs will lead to increased awareness of and
emphasis on disease prevention and early patient management, which will
increase demand for its cost-effective diagnostic tests and services.
The Company owns or has licenses to 23 United States patents, and the Company
owns, has licenses to or options to license technology covered by another 16
pending United States patent applications, each in the area of pregnancy-
related and reproductive disorder diagnostics.
The Company'sDelaware. Our principal executive offices are located at 1240 Elko Drive, Sunnyvale, CaliforniaCA 94089, and itsour telephone number is (408) 745-0975. This Prospectus contains forward-looking statements which involve risks Our websites are located athttp://www.adeza.comand
uncertainties. http://www.ffntest.com. We do not intend for the information contained on our websites to be incorporated by reference into, or to form any part of, this prospectus.
The Company's actual results may differ significantly from the
results discussed in the forward-looking statements. Factors that might cause
such a difference include, but are not limited to, those discussed in "Risk
Factors."
4
THE OFFERING
offering
| Common | shares | |
| Common | shares | |
| Use of | We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming an initial public offering price of $ per share. We expect to use the net proceeds from this offering to expand our sales and marketing efforts in the United States and internationally, for research and development activities, to expand | |
| Proposed Nasdaq National Market | ADZA |
The number of shares of our common stock outstanding immediately after this offering excludes:
| 4 | 1,981,744 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2004 under our 1995 Stock Option and Restricted Stock Plan at a weighted average exercise price of $1.75 per share; |
| 4 | 262,556 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2004 at an exercise price of $2.628 per share; and |
| 4 | 176,177 shares of our common stock available for future grant under our 1995 Stock Option and Restricted Stock Plan (based on options outstanding as of June 30, 2004). |
In July 2004, we granted options to purchase 31,000 shares of common stock under the Company'sour 1995 Stock Option and Restricted Stock Plan (iv) 250,000at an exercise price of $7.50 per share. In August 2004, we increased the number of shares reservedof common stock available for future issuance as
of May 9, 1996grant under the Company's 1996 Employee Stock Purchase Plan and
(v) 200,000 shares reserved for future issuance as of May 9, 1996 under the
Company's 1996 Directors'our 1995 Stock Option Plan. See "Management--Stock Option
and Incentive Plans"Restricted Stock Plan by 750,000 shares and "Descriptionwe granted options to purchase 880,000 shares of Capital Stock."
(2) See Note 1common stock at an exercise price of Notes to Consolidated Financial Statements$7.50 per share. Our stockholders approved this increase in August 2004.
Unless otherwise indicated, all information in this prospectus:
| 4 | assumes that the underwriters do not exercise their option to purchase up to additional shares of our common stock to cover over-allotments, if any; |
| 4 | gives effect to the amendment and restatement of our certificate of incorporation and bylaws, which will become effective at the completion of this offering; and |
| 4 | gives effect to the adoption of our 2004 Equity Incentive Plan, which will become effective upon the completion of this offering. |
Summary financial data
The following summary financial data for information
concerning calculationthe years ended December 31, 2001, 2002 and 2003 have been derived from our financial statements audited by Ernst & Young LLP, which appear elsewhere in this prospectus. The summary financial data for the six months ended June 30, 2003 and 2004 have been derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited summary financial data include, in the opinion of management, all adjustments, consisting of only normal recurring adjustments, that are necessary for a fair presentation of the pro forma net loss per share.
(3) As adjusted to reflectfinancial position and results for the saleinterim unaudited periods. Operating results for the six months ended June 30, 2004 are not necessarily indicative of the 2,500,000 shares of Common Stock
offered hereby at an assumed initial public offering price of $12.00 per
share after deducting underwriting discounts and commissions and estimated
offering expenses and the receipt of the estimated net proceeds therefrom.
Does not reflect application of a portion of the net proceeds for repayment
of indebtednessresults that may be incurred after Marchexpected for the year ending December 31, 1996 under2004. The summary financial data set forth below should be read together with the Company's $2.0 million linefinancial statements and the related notes to those statements, as well as “Management’s discussion and analysis of credit from certain existing investors. See
"Usefinancial condition and results of Proceeds" and Notes 5 and 9 to Notes to Consolidated Financial
Statements.
5
RISK FACTORS
An investmentoperations,” appearing elsewhere in the sharesthis prospectus.
| Six months ended | |||||||||||||||||||||
| Years ended December 31, | June 30, | ||||||||||||||||||||
| Statement of | |||||||||||||||||||||
| operations data: | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||||||||
| Product sales | $6,742 | $14,277 | $26,499 | $12,519 | $15,638 | ||||||||||||||||
| Cost of product sales | 2,521 | 3,715 | 6,087 | 3,059 | 3,343 | ||||||||||||||||
| Gross profit | 4,221 | 10,562 | 20,412 | 9,460 | 12,295 | ||||||||||||||||
| Contract revenues | 811 | 1,059 | — | — | — | ||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||||||
| Selling and marketing | 6,437 | 7,819 | 12,259 | 5,872 | 7,486 | ||||||||||||||||
| General and administrative | 2,033 | 2,069 | 2,730 | 1,113 | 1,552 | ||||||||||||||||
| Research and development | 2,145 | 2,047 | 2,001 | 980 | 1,196 | ||||||||||||||||
| Total operating costs and expenses | 10,615 | 11,935 | 16,990 | 7,965 | 10,234 | ||||||||||||||||
| Income (loss) from operations | (5,583 | ) | (314 | ) | 3,422 | 1,495 | 2,061 | ||||||||||||||
| Other expenses, net | (21 | ) | (8 | ) | (33 | ) | (9 | ) | — | ||||||||||||
| Interest income (expense), net | (85 | ) | (7 | ) | (19 | ) | (44 | ) | 56 | ||||||||||||
| Income (loss) before income taxes | (5,689 | ) | (329 | ) | 3,370 | 1,442 | 2,117 | ||||||||||||||
| Provision for income taxes | — | — | 135 | 85 | 80 | ||||||||||||||||
| Net income (loss) | $(5,689 | ) | $(329 | ) | $3,235 | $1,357 | $2,037 | ||||||||||||||
| Net income (loss) per share: | |||||||||||||||||||||
| Basic | $(27.20 | ) | $(1.36 | ) | $13.33 | $5.60 | $8.38 | ||||||||||||||
| Diluted | $(27.20 | ) | $(1.36 | ) | $0.19 | $0.08 | $0.11 | ||||||||||||||
| Shares used in computing net income (loss) per share: | |||||||||||||||||||||
| Basic | 209,150 | 241,596 | 242,720 | 242,460 | 242,980 | ||||||||||||||||
| �� | |||||||||||||||||||||
| Diluted | 209,150 | 241,596 | 16,692,907 | 16,573,159 | 17,760,275 | ||||||||||||||||
The following table contains a summary of Common Stock offered herebyour balance sheet as of June 30, 2004:
| 4 | on an actual basis; |
| 4 | on a pro forma basis to give effect to the conversion of all 15,409,062 shares of our preferred stock outstanding as of June 30, 2004 into 15,943,176 shares of our common stock, which will become effective at the closing of this offering; and |
| 4 | on a pro forma as adjusted basis to give further effect to the sale of the shares of our common stock we are offering at an assumed initial public offering price of $ per share, after deducting underwriting discounts and commissions and estimated offering expenses to be paid by us. |
| As of June 30, 2004 | ||||||||||||
| Pro forma | ||||||||||||
| Balance sheet data: | Actual | Pro forma | as adjusted | |||||||||
| (unaudited, in thousands) | ||||||||||||
| Cash and cash equivalents | $14,495 | $14,495 | $ | |||||||||
| Working capital | 11,735 | 11,735 | ||||||||||
| Total assets | 21,566 | 21,566 | ||||||||||
| Convertible preferred stock | 61,484 | — | — | |||||||||
| Accumulated deficit | (51,600 | ) | (51,600 | ) | ||||||||
| Total stockholders’ equity (deficit) | (49,219 | ) | 12,265 | |||||||||
Risk factors
Investing in our common stock involves a high degree of risk. Prospective investorsYou should carefully consider the following
risk factors, in addition torisks described below with all of the other information set forthincluded in this Prospectus,prospectus before deciding to invest in connectionour common stock. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In this event, the market price of our common stock could decline and you could lose part or all of your investment.
RISKS RELATING TO OUR BUSINESS
Because our revenues and financial results depend significantly on a limited product line, if we are unable to manufacture or sell our products in sufficient quantities and in a timely manner, our business will suffer.
To date, substantially all of our revenue has resulted from sales of our principal product line, our Fetal Fibronectin Test, the TLiIQ System (and its predecessor, the TLi System) and related consumables. Although we have introduced new products such as the E-tegrity Test, and intend to introduce additional products, we expect sales of the Fetal Fibronectin Test to account for substantially all of our near-term revenue. Because our business is highly dependent on our Fetal Fibronectin Tests, the TLiIQ System and the related consumables, factors adversely affecting the pricing of or demand for these products could have a material and adverse effect on our business and cause the value of our securities to decline substantially. We will lose revenue if alternative diagnostic products or technologies gain commercial acceptance, if our marketing activities are restricted or if reimbursement is limited. We cannot assure you that we will be able to continue to manufacture these products in commercial quantities at acceptable costs or that we will continue to market these products successfully. Our inability to do so would adversely affect our operating results and cause our business to suffer.
If our products do not achieve and sustain market acceptance, we may fail to generate sufficient revenue to maintain our business.
Our commercial success depends in large part on our ability to achieve and sustain market acceptance of our principal product line, the TLiIQ System and the Fetal Fibronectin Test. A key element of our business plan calls for us to expand sales of our TLiIQ System in hospitals and clinical laboratories and increase the related sales of the Fetal Fibronectin Test and other consumables used in conjunction with the TLiIQ System. To accomplish this, we will need to convince healthcare providers of the benefits of our products through various means, including through published papers, presentations at scientific conferences and additional clinical trials. If existing users of our products determine that these products do not satisfy their requirements, or if our competitors develop a product perceived to better satisfy their requirements, our sales of Fetal Fibronectin Tests and other consumables may decline, and our revenues may correspondingly decline.
In addition, our commercial success may depend on our ability to gain market acceptance for our other products and product candidates. Market acceptance of our product portfolio will depend on our ability to develop additional applications of our existing products and to introduce new products to additional markets, including the oncology diagnostic market, the reproductive endocrinology market and infertility market and other women’s health markets.
Other factors that might influence market acceptance of our products include the following:
| 4 | evidence of clinical utility; |
| 4 | ability to obtain sufficient third-party coverage or reimbursement; |
| 4 | convenience and ease of use; |
| 4 | availability of alternative and competing diagnostic products; |
| 4 | cost-effectiveness; |
| 4 | effectiveness of marketing, distribution and pricing strategy; and |
| 4 | publicity concerning these products or competitive products. |
In addition, our marketing and development efforts could require us to expend significant time and resources, and we cannot assure you that we will succeed in these efforts. If our products are unable to achieve or maintain broad market acceptance, our revenues and operating results may be negatively impacted and our business would suffer.
Our quarterly revenues and operating results are subject to significant fluctuations, and our stock price may decline if we do not meet the expectations of investors and analysts.
As of June 30, 2004, we had an investmentaccumulated deficit of $51.6 million. For the six months ended June 30, 2004, we had net income of $2.0 million. However, we cannot assure you that we will sustain profitability or that losses will not occur in the sharesfuture. Our quarterly revenues and operating results are difficult to predict and have in the past and may in the future fluctuate significantly from quarter to quarter due to a number of Common Stock
offered hereby.factors, many of which are outside our control. These factors include, but are not limited to:
| 4 | our ability to increase market acceptance of women’s health diagnostics generally and of our products in particular; |
| 4 | the seasonal nature of our business and quarterly variations in demand for our products based on procurement cycles of our customers; |
| 4 | our need and ability to generate and manage growth; |
| 4 | acceptance and continued reimbursement by third-party payors for our diagnostic tests; |
| 4 | changes in the manner in which our operations are regulated; |
| 4 | increases in the length of our sales cycle; |
| 4 | our reliance on international sales and growth, particularly the performance of our independent foreign distributors and fluctuations in foreign currency; |
| 4 | fluctuations in gross margins; |
| 4 | reductions in the efficiency of our manufacturing processes; |
| 4 | delays in, or failure of, delivery of components by our suppliers; and |
| 4 | continued difficult political and economic conditions. |
These and other factors make it difficult for us to predict sales for subsequent periods and future performance. If our quarterly operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly. We believe quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
In addition, we expect to incur additional expenses to execute our business plan, and these expenses will increase as we expand our marketing efforts, research and development activities, clinical testing and manufacturing capacity. These expenses, among other things, may cause our net income and working capital to decrease. If sales do not continue to grow, we may not be able to maintain profitability. Our expansion efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues sufficiently to offset these higher expenses. If we fail to do so, the market price for our common stock will likely decline.
If third-party payors do not adequately reimburse our customers, market acceptance of our products may be impaired, which may adversely affect our revenues and our operating results.
Market acceptance of our products and the majority of our sales depend, in large part, on the availability of adequate reimbursement for the use of our products from government insurance plans, including Medicare and Medicaid, managed care organizations, private insurance plans and other third-party payors in the United States and abroad. Though we believe that reimbursement for our Fetal Fibronectin Test has been regularly available through health plan organizations and most state Medicaid programs, third-party payors are often reluctant to reimburse healthcare providers for the use of medical diagnostic products incorporating new technology.
Because each third-party payor individually approves reimbursement, obtaining these approvals can be a time-consuming and costly process that requires us to provide scientific and clinical support for the use of each of these products to each third-party payor separately with no assurance that approval will be obtained. For example, while the policies of some third-party payors limit reimbursement for the use of our Fetal Fibronectin Test to women with signs and symptoms of preterm labor, other third-party payors provide reimbursement for broader use of our Fetal Fibronectin Test. This Prospectus contains forward-looking statementsindividualized process can delay the market acceptance of new products and may have a negative effect on our revenues and operating results.
Market acceptance of our products internationally may depend in part upon the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country and include both government sponsored healthcare and private insurance. We may not obtain international reimbursement approvals in a timely manner, if at all. Our failure to receive international reimbursement approvals may negatively impact market acceptance of our products in the international markets in which involve risksthose approvals are sought.
We believe third-party payors are increasingly limiting coverage for medical diagnostic products in the United States and uncertainties. The Company's actualinternationally, and in many instances are exerting pressure on medical products suppliers to reduce their prices. Consequently, third-party reimbursement may not be consistently available or adequate to cover the cost of our products. Additionally, third-party payors who have previously approved a specific level of reimbursement may reduce that level. We cannot assure you that under prospective payment systems, in which healthcare providers may be reimbursed a set amount based on the type of diagnostic procedure performed, such as those utilized by Medicare and in many privately managed care systems, the cost of our products will be justified and reimbursed. This could limit our ability to commercialize and sell new products and continue to sell our existing products, or may cause the prices of our existing products to be reduced, which may adversely affect our revenues and operating results.
If we fail to properly manage our anticipated growth in the United States or abroad, we may incur significant additional costs and expenses and our operating results may differ
significantly fromsuffer.
Rapid growth of our business is likely to place a significant strain on our managerial, operational and financial resources and systems. In the results discussedUnited States, while we anticipate hiring additional personnel to assist in the forward-looking statements.
Factorsplanned expansion of sales efforts for our current products and the development of future products, we cannot assure you that mightwe will be able to successfully increase sales of current products or introduce new products and meet our growth goals. To manage our anticipated growth, we must attract and retain qualified personnel and manage and train them effectively. We will depend on our personnel and third parties to effectively market our products to an increasing number of hospitals, physicians and other healthcare providers. We will also depend on our personnel to develop next generation technologies. Further, our anticipated growth will place additional strain on our suppliers
Our plans to significantly expand our presence in international markets will cause us to incur various costs and expenses and may strain our operating and financial systems and resources in a manner that could materially and adversely affect our operating results. We will be subject to the regulatory oversight of additional authorities as we expand internationally. These authorities may impose regulations and restrictions on the sales and marketing of our products that are different and potentially more restrictive than those placed on us by regulators in the United States. We may be required to expend considerable resources to comply with these requirements. We cannot assure you that we will ultimately be able to comply with such regulations in a differencetimely manner, if at all. If we are unable to satisfy these requirements on commercially reasonable terms, our ability to commercialize our products would be hampered and our revenues may be adversely affected.
We will need to devote considerable resources to comply with federal, state and foreign regulations and, if we are unable to fully comply, we could face substantial penalties.
We are directly or indirectly through our customers subject to extensive regulation by both the federal government and the states and foreign countries in which we conduct our business. The laws that directly or indirectly affect our ability to operate our business include, but are not limited to, those discussedthe following:
| 4 | the Federal Food, Drug and Cosmetic Act, which regulates the design, testing, manufacture, labeling, marketing, distribution and sale of medical devices; |
| 4 | the federal Anti-Kickback Law, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid Programs; |
| 4 | Medicare laws and regulations that prescribe the requirements for coverage and payment, including the amount of such payment, and laws prohibiting false claims for reimbursement under Medicare and Medicaid; |
| 4 | the federal physician self-referral prohibition, commonly known as the Stark Law, which, in the absence of a statutory or regulatory exception, prohibits the referral of Medicare patients by a physician to an entity for the provision of certain designated healthcare services, if the physician or a member of the physician’s immediate family has a direct or indirect financial relationship, including an ownership interest in, or a compensation arrangement with, the entity and also prohibits that entity from submitting a bill to a federal payor for services rendered pursuant to a prohibited referral; |
| 4 | state laws that prohibit the practice of medicine by non-physicians and fee-splitting arrangements between physicians and non-physicians, as well as state law equivalents to the Anti-Kickback Law and the Stark Law, which may not be limited to government reimbursed items; and |
| 4 | the Federal Trade Commission Act and similar laws regulating advertising and consumer protection. |
If our past or present operations are found to be in this section below.
UNCERTAINTY OF MARKET ACCEPTANCE. Salesviolation of any of the Company'slaws described above or the other governmental regulations to which we or our customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines,
If we are unable to maintain our existing regulatory approvals and clearances for our existing products, or obtain new regulatory approvals and clearances for our product candidates, our ability to commercially distribute our products and our business may be significantly harmed.
The US Food and Drug Administration, or FDA, and comparable agencies of other countries generally regulate our products as medical devices. In the United States, FDA regulations govern, among other things, the activities that we perform, including product development, product testing, product labeling, product storage, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution. Most of the new products that we plan to develop and commercialize in the United States will require either pre-market notification, also known as 510(k) clearance, or pre-market approval, from the FDA prior to marketing. The 510(k) clearance process requires us to notify the FDA of our intent to market a medical device. The overall 510(k) clearance process usually takes from three to twelve months from the time of submission to being able to sell a product in the market, but can take significantly longer. The pre-market approval process, often referred to as the PMA process, is much more costly, lengthy, uncertain and generally takes between one and three years from submission to PMA approval, but may take significantly longer.
All of the products that we have generated limited revenuessubmitted and may submit in the future for FDA clearance or approval are or will be subject to date. The Company's fFN ELISA Testsubstantial restrictions, including, among other things, restrictions on the indications for which we may market our products, which could result in reductions in or an inability to grow our revenues. Even if regulatory approval of a product is granted, the only
Companyapproval may be subject to limitations on the indicated uses for which the product may be marketed or certain requirements for costly post-marketing testing and surveillance to monitor the performance and clinical utility of the product. For example, any of our products that hashave received FDA approval, for salesuch as our TLiIQ System or Fetal Fibronectin Test, remain subject to ongoing post-marketing regulation and oversight by the FDA. The marketing claims that we are permitted to make in labeling our diagnostic products, if cleared or approved by the FDA, are limited to those specified in any clearance or approval. Our intention to expand the use of our products into new areas such as the prediction of successful induction of labor and oncology will require us to make new submissions to the FDA.
In addition, we are subject to review, periodic inspection and marketing surveillance by the FDA to determine our compliance with regulatory requirements for any product for which we obtain marketing approval. Following approval, our manufacturing processes, subsequent clinical data and promotional activities are subject to ongoing regulatory obligations. If the FDA finds that we have failed to comply with these requirements or later discovers previously unknown problems with our products, including unanticipated adverse events of unanticipated severity or frequency, manufacturer or manufacturing processes or failure to comply with regulatory requirements, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, including:
| 4 | fines, injunctions and civil penalties; |
| 4 | recall or seizure of our products; |
| 4 | restrictions on our products or manufacturing processes, including operating restrictions, partial suspension or total shutdown of production; |
| 4 | denial of requests for 510(k) clearances or PMAs of product candidates; |
| 4 | withdrawal of 510(k) clearances or PMAs already granted; |
| 4 | disgorgement of profits; and |
| 4 | criminal prosecution. |
Any of these enforcement actions could affect our ability to commercially distribute our products in the United States and may also harm our ability to conduct the clinical trials necessary to support the marketing, clearance or approval of these products and could materially and adversely affect our business.
Our PMA supplement seeking approval for use of our Fetal Fibronectin Test in predicting successful induction of labor has generated only limited sales in international markets.
There can be no assurancebeen submitted to the FDA. The FDA’s review of the application is currently on hold while a third party we have engaged conducts an audit of all of the clinical study sites for protocol deviations and accuracy of the data. Upon completion of the third-party audit, we will need to submit new analyses of the data and a corrective action plan to the FDA before it will resume its review of the application. We cannot assure you that the fFN ELISAnew analyses of the data or the corrective action plan will be acceptable to us or to the FDA or that we will continue to pursue or obtain FDA approval for this application.
We rely on our CLIA-certified laboratory located at our facility in Sunnyvale, California to process E-tegrity Tests. The Centers for Medicare and Medicaid Services, or the CMS, requires that operators of CLIA-certified laboratories submit to surveillance and follow-up inspections. If we are unable to meet the CMS’s requirements for continued operation pursuant to CLIA, our laboratory may lose its CLIA certification, and we may be unable to continue to process E-tegrity Tests. As a result, our business may be harmed.
If we modify our marketed products, we may be required to obtain new 510(k) clearances or PMAs, or we may be required to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)-cleared or pre-market approved diagnostic device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or PMA, such as the development of our Fetal Fibronectin Test as a diagnostic test for the induction of labor. The FDA requires every manufacturer to make the determination of whether new clearance or approval is required for 510(k)-cleared devices. The FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or PMA for any modification to a previously cleared or approved product, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Any recall or FDA requirement that we seek additional approvals or clearances could result in delays, fines, costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
If we or any of the Company's
other existing or future products,our third-party manufacturers do not operate in accordance with Quality System Regulations, we could be subject to FDA enforcement actions, including the fFN Dipstick Test,seizure of our products and the halt of our production.
We and any third-party manufacturers that we currently rely on or will gainrely on in the future, including those we rely on to produce components of our products, must continuously adhere to the current good manufacturing practices, or cGMP, set forth in the FDA’s Quality System Regulations, or QSR, and enforced by the FDA through its facilities inspection program. In complying with QSR, we and
We have received regulatory approvals for some of the operations located at our Sunnyvale, California headquarters, including our CLIA-certified laboratory. We currently lease our facility in Sunnyvale on a month-to-month basis while we negotiate a longer term renewal of the lease. Should we choose to relocate, or if for some reason we are obtained. required to relocate some or all of our facilities from this location, we may be required to apply for regulatory approvals for the new location. It may be difficult or impossible for us to obtain the necessary approvals to continue our business in its present form at any such new location, and our business may be harmed as a result.
If we experience delays in the development of new products or delays in planned improvements to our products, our commercial opportunities will be reduced and our future competitive position may be adversely affected.
To improve our competitive position, we believe that we will need to develop new products as well as improve our existing instruments, reagents and ancillary products. Improvements in automation and throughput, or the number of tests that can be performed in a specified period of time, of our products will be important to the competitive position of our products as we market to a broader, perhaps less technically proficient, group of customers. Our ability to develop new products and make improvements in our products may face difficult technological challenges leading to delays in development. If we are unable to successfully complete development of new products or if we are unable to successfully complete the planned enhancements to our products, in each case without significant delays, our future competitive position may be adversely affected.
If other companies develop and market technologies or diagnostic products faster than we do, or if those products are more cost effective or useful than our products, our commercial opportunities will be reduced or eliminated.
The Company believes that the commercial
successextent to which any of itsour technologies and products achieve and sustain market acceptance will depend on numerous competitive factors, many of which are beyond our control. Competition in the acceptancemedical devices and diagnostic products industries, is intense and has been accentuated by the rapid pace of technological development. While no company directly competes with us in our core markets, there are other diagnostic techniques currently in use to diagnose the likelihood of preterm birth, such as ultrasound. In addition, other companies may develop new diagnostic products or technologies that could compete with or entirely displace our products and technologies. For example, other biomarkers, including cytokines and other proteins indicative of infection, and proteomics are the subject of research that may yield new products or technologies. The effectiveness of these alternative techniques may improve with time and additional research by clinicians or manufacturers. The medical devices and diagnostic products industries include large diagnostics and life sciences companies. Most of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing, sales and service resources than we do. Some of them also have more experience than we do in research and development, clinical trials, regulatory matters, manufacturing, marketing and sales. These organizations also compete with us to:
| 4 | pursue acquisitions, joint ventures or other collaborations; |
| 4 | license proprietary technologies that are competitive with our technologies; |
| 4 | attract funding; and |
| 4 | attract and hire scientific and other talent. |
If we cannot successfully compete with new products or technologies, sales of our products and our competitive position will suffer. Because of their greater experience with commercializing technologies and larger research and development capabilities, other companies might succeed in developing and commercializing technologies or products earlier and obtaining regulatory approvals and clearances from the FDA more rapidly than we do. Other companies also might develop more effective technologies or products that are more predictive, more highly automated or more cost-effective, which may render our technologies or products obsolete or non-competitive.
We rely on a limited number of suppliers, including some single source suppliers, and if these suppliers fail or are unable to perform in a timely and satisfactory manner, we may be unable to manufacture our products or satisfy product demand in a timely manner, which could delay the production or sale of these products.
We rely on a limited number of suppliers for both raw materials and components necessary for the manufacture of our products, including our TLiIQSystem and Fetal Fibronectin Test. We also rely on single source suppliers for several components, assemblies and raw materials used to manufacture our products. We acquire all of these components, assemblies and raw materials on a purchase-order basis, which means that the supplier is not required to supply us with specified quantities over a certain period of time or to set aside part of its inventory for our forecasted requirements. If we need alternative sources for key components, assemblies or raw materials for any reason, such components, assemblies or raw materials may not be immediately available. If alternative suppliers are not immediately available, we will have to identify and qualify alternative suppliers, and delivery of such components, assemblies or raw materials may be delayed. Consequently, if we do not forecast properly, or if our suppliers are unable or unwilling to supply us in sufficient quantities or on commercially acceptable terms, we may not have access to sufficient quantities of these components, assemblies and raw materials on a timely basis and may not be able to satisfy product demand. We also rely upon a fulfillment provider to process orders for our products, by its
strategic partnerscoordinate invoicing and distributors and by physicians, hospitals, other health
care providers and third-party payorscollections, as clinically useful and cost effective,
and there can be no assurance that any such acceptance will be achieved.
Acceptance will also depend upon the ability of the Company and its strategic
partners and distributors to train physicians, hospitals and other health care
providers to use the fFN ELISA Test and the Company's other products, and the
willingness of such individuals to learn to use these products. Failure of the
Company'swell as ship our products to achieve significant market acceptancecustomers in the United States. We may not be able to find an adequate alternative supplier or fulfillment provider in a reasonable time period, or on commercially acceptable terms, if at all. Our inability to obtain a supplier for the manufacture of our products may force us to curtail or cease operations, which would have a material adverse effect on the Company's business, financial conditionour product sales and resultsprofitability.
In addition, if any of operations. See "Business--The Adeza Solution for Premature and
Late Birth/Successful Induction of Labor," "Business--Sales & Marketing;
Strategic Corporate Alliances" and "Business--Third-Party Reimbursement."
DEPENDENCE ON FFN ELISA TEST. The fFN ELISA Test is the primary product
being marketed by the Company and will remain so for the near term boththese components, assemblies or raw materials are no longer available in the United States and internationally. The Company has been granted an expedited
PMA to market the fFN ELISA Test for the assessment of the likelihood of
premature birth in symptomatic women. In order to market the fFN ELISA Test
for additional indications in the potentially larger market, the Companymarketplace, we will be requiredforced to further develop our technologies to incorporate alternate components, assemblies and raw materials and to do so in compliance with QSR. If we incorporate new components, assemblies or raw materials into our products, we may need to seek and obtain additional approvals or clearances from the FDA or foreign regulatory approvals. The Company plans to
useagencies, which could delay the resultscommercialization of a clinical trial performed by the NIH to file a supplement
to the Company's PMA (a "PMA Supplement") for use of the fFN ELISA Test in
asymptomatic women. There can be no assurance that a PMA Supplement will be
submitted for this indication or that further clinical trials will not be
required in addition to the NIH trials. The Company will also be required to
obtain additional regulatory approvals for the use of the fFN Dipstick Test in
assessing the likelihood of premature birth in symptomatic women, for which
clinical trials are in progress, and for the use of the fFN Dipstick Test in
assessing the likelihood of successful induction of labor in women at term for
which the Company is in the process of designing clinical trials. To date, the
Company has had only limited commercial sales of the fFN ELISA Test in
international markets and has only recently begun shipping to a limited number
of customers in the United States. There can be no assurance that the fFN
ELISA Test or any other product developed by the Company will be safe or
effective, capable of being manufactured in commercial quantities at
acceptable costs, approved by appropriate regulatory or reimbursement
authorities or successfully marketed. Furthermore, because the fFN ELISA Test
represents the Company's principal near-term focus, the Company could be
required to cease operations if the fFN ELISA Test is not successfully
commercialized, and even limited failure of the fFN ELISA Test to gain market
acceptance would have a material adverse effectthese products.
We depend on the Company's business,
financial condition and results of operations. See "Business--The Adeza
Solution for Premature and Late Birth/Successful Induction of Labor."
DEPENDENCE ON STRATEGIC PARTNERS AND DISTRIBUTORS. The Company depends on
strategic partners and distributors for substantially all currently
anticipated sales. Matria has exclusive rights to market and distribute the
fFN ELISA Test and the fFN Dipstick Test, as well as the right to participate
in the development of and distribute immunodiagnostic products for premature
birth that are developed by or acquired for development by the Company in the
United States, Canada and Puerto Rico. The Company currently expects that it
will derive a
6
substantial portion of its United States revenues for the foreseeable future
from sales of its fFN ELISA Test and fFN Dipstick Test through Matria. The
loss of Matria as a strategic partner or the failure of Matria to effectively
market and distribute the Company's fFN ELISA Test and the fFN Dipstick Test
would have a material adverse effect on the Company's business, financial
condition and results of operations. Because the Company's products are sold
to physicians, hospitals and other health care providers, to be effective,
strategic partners and distributors must possess sufficient technical,
marketing and sales resources and must devote these resources to education of
its target market, physician training and continuing product support. The
amount, timing and effectiveness of the resources that Matria will devote to
promoting the fFN ELISA Test or any other of the Company's products is not
within the control of the Company, and the Company does not have the
contractual right to terminate its marketing agreement with Matria for failure
to sell more than a minimum number of products. In addition, Matria may
terminate this agreement in the event a product competitive with either the
fFN ELISA Test or the fFN Dipstick Test captures a specified percentage of the
market for such product or in the event Matria determines it is unable to
legally market either the fFN ELISA Test or the fFN Dipstick Test due to the
patent position of any third party. Furthermore, Matria was recently formed by
the merger of Tokos Medical Corporation and HealthDyne, Inc., and the failure
of such merged companies to be successfully integrated or a change in
strategic focus of the merged entity could have a material adverse effect on
Matria's ability to market and sell the Company'sour products including the fFN
ELISA Test. There can also be no assurance that Matria will continuein overseas markets, and if our foreign distributors fail in their efforts or are unwilling or unable to devote sufficient resources to market and support the Company's existing or futuresell our products, our ability to effectively or at all,
or that Matria will not be adversely affected by economic conditions or
industry demand. Matria's failure to successfully market our products and support the
Company's products in the United States would have a material adverse effect
on the Company'sour business financial condition and results of operations.
Additionally, the Company expects it will be dependent on entering into
agreements with additional strategic partners and distributors for sales and
marketing of any future products in the United States, if and when such
products are developed. See "--No Assurance of Successful Product Development"
and "Business--Sales & Marketing; Strategic Corporate Alliances."
The Company anticipates thatharmed.
Our international sales will continue to represent
a significant portion of its total revenues in the future. Daiichi has
exclusive rights to market and distribute the fFN ELISA Test and the fFN
Vertical Flow Test in Japan. See "Business--Sales & Marketing; Strategic
Corporate Alliances." Additionally, the Company currently has a limited
network of other distributors that market the Company's products in Taiwan,
portions of Europe, Scandinavia, Japan and South Korea. The Company's
international sales are dependentdepend upon the marketing efforts of and sales by these distributors. The Companycertain distributors in Europe, Australia, the Pacific Rim region and South America. In most instances, our distribution arrangements are governed by short-term purchase orders. In Japan, Daiichi Pure Chemicals Co., Ltd. has exclusive rights to market and distribute certain of our products. We also relies onrely upon certain of these distributors to assist it in obtaining product registration and reimbursement approvals in certain international markets, and we may not be able to engage qualified distributors in our targeted markets.
The regulatory approval process outside the United States varies depending on foreign regulatory requirements and may limit our ability to develop, manufacture and sell our products internationally.
To market any of our products outside of the United States, we and our collaborative partners, including certain of our distributors, are subject to numerous and varying foreign regulatory requirements, implemented by foreign health authorities, governing the design and conduct of human clinical trials and marketing approval for diagnostic products. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval. The foreign regulatory approval process includes all of the risks associated with obtaining FDA approval set forth above, and approval by the FDA does not ensure approval by the health authorities of any other country, nor does the approval by foreign health authorities ensure approval by the FDA.
If our products do not perform as expected, we may experience reduced revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high quality medical diagnostic devices. Our customers are particularly sensitive to product defects and errors because of the use of our products in medical practice. Our reputation and the public image of our products may be impaired for any of the following reasons:
| 4 | failure of our products to perform as expected; |
| 4 | a perception that our products are difficult to use; and |
| 4 | litigation concerning the performance of our products. |
Even after any underlying problems are resolved, any manufacturing defects or performance errors in our products could result in lost revenue, delay in market acceptance, damage to our reputation, increased service and warranty costs and claims against us.
If product liability suits or other claims and product field actions are initiated against us, we may be required to engage in expensive and time-consuming litigation, pay substantial damages, face increased insurance rates and sustain damage to our reputation, which would significantly impair our financial condition.
Our business exposes us to potential product liability claims and field action risks that are inherent in the testing, manufacturing, marketing and sale of diagnostic products. We may be unable to avoid product liability claims or field actions, including those based on claims that the use or failure of our products resulted in a misdiagnosis or harm to a patient. While we intend to expand our product liability insurance coverage to any products for which we obtain marketing approval, insurance may be unavailable, prohibitively expensive or may not fully cover our potential liabilities. If we are unable
We depend on the Company'sservices of key personnel to implement our strategy, and if we lose key management or scientific personnel, scientific collaborators or other advisors or are unable to attract and retain other qualified personnel, we may be unable to execute our business financial conditionplan and our operations and business would suffer.
Our success depends, in large part, on the efforts and abilities of Emory Anderson, who is our President and Chief Executive Officer, Dr. Durlin Hickok, who is our Vice President, Medical Affairs, Dr. Robert Hussa, our Vice President, Research and Development, Mark Fischer-Colbrie, who is our Vice President of Finance and Administration and Chief Financial Officer, and Marian Sacco, our Vice President, Sales and Marketing, as well as the other members of our senior management and our scientific and technical personnel. We do not currently have employment agreements with any of these individuals. We do not currently carry key person insurance on the lives of any of these executives. Many of these people have been members of our executive team for several years, and their knowledge of our business would be difficult or time-consuming to replace. We also depend on our scientific collaborators and other advisors, particularly with respect to our research and development efforts. If we lose the services of one or more of our key officers, employees or consultants, or are unable to retain or attract the services of existing or new scientific collaborators and other advisors, our research and development and product development efforts could be delayed or curtailed and our ability to execute our business strategy would be impaired.
Most of our operations are currently conducted at a single location that may be at risk from earthquakes and other natural or unforeseen disasters.
We currently conduct all of our manufacturing, development and management activities at a single location in Sunnyvale, California near known fault zones. In addition, our E-tegrity Tests are currently processed solely through our CLIA-certified laboratory located at our Sunnyvale facility. Despite precautions taken by us, any future natural or man-made disaster, such as a fire, earthquake or terrorist activity, could cause substantial delays in our operations, damage or destroy our equipment or inventory, and reduce our sales or cause us to incur additional expenses. In addition, the facility and some pieces of manufacturing equipment would be difficult to replace and could require substantial replacement lead-time. A disaster could seriously harm our business and results of operations. See
"Business--Sales & Marketing; Strategic Corporate Alliances."
While we carry insurance for natural disasters and business interruption, the occurrence of such events could result in losses that exceed the amount of our insurance coverage, which would impair our operating results and financial condition.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our research and development activities sometimes involve the controlled use of potentially harmful biological materials, hazardous materials and chemicals. We cannot completely eliminate the risk of accidental contamination or injury to third parties from the use, storage, handling or disposal of these
Potential business combinations could require significant management attention and prove difficult to integrate with our business, which could distract our management, disrupt our business, dilute stockholder value and adversely affect our operating results.
If we become aware of potential business combination candidates that are complementary to our business, we may decide to combine with such businesses or their assets in the future. Business combinations involve a number of additional difficulties and risks to our business, including:
| 4 | failure to integrate management information systems, personnel, research and development and marketing, operations, sales and support; |
| 4 | potential loss of key current employees or employees of the other company; |
| 4 | disruption of our ongoing business and diversion of management’s attention from other business concerns; |
| 4 | potential loss of the other company’s customers; |
| 4 | failure to develop further the other company’s technology successfully; |
| 4 | unanticipated costs and liabilities; and |
| 4 | other accounting consequences. |
In addition, certainwe may not realize benefits from any business combination we may undertake in the future. If we fail to successfully integrate such businesses, or the technologies associated with such business combinations into our company, the revenue and operating results of the arrangements thatcombined company could be adversely affected. Any integration process would require significant time and resources, and we may not be able to manage the Companyprocess successfully. If our customers are uncertain about our ability to operate on a combined basis, they could delay or cancel orders for our products. We may enter intonot successfully evaluate or utilize the acquired technology or accurately forecast the financial impact of a combination, including accounting charges. If we fail to successfully integrate other companies with which we may combine in the future, with strategic partners may place responsibility onour business and your investment could be harmed.
If we fail to obtain necessary funds for our operations, we will be unable to continue to develop and commercialize new products and technologies and we would need to downsize or halt our operations.
We expect capital outlays and operating expenditures to increase over the Company's
partners for preclinical testing and humannext several years as we expand our infrastructure, commercialization, manufacturing, clinical trials and forresearch and development activities. We believe the preparationnet proceeds of this offering, together with our cash and submission of applications for regulatory approval of
potential diagnostic or therapeutic products. Should any strategic partner
fail to perform its obligations under such arrangements, the Company's
business, financial condition and results of operations could be materially
adversely affected.
UNCERTAINTY RELATING TO THIRD-PARTY REIMBURSEMENT. In the United States,
physicians, hospitals and other health care providers that perform medical
services generally rely on third-party payors, such as private
7
health insurance plans, to reimburse all or part of the cost associated with
the treatment of patients. Although reimbursement for diagnosing premature
birth has generally been available in the United States, because of the
uncertainty relating to health care reform, there can be no assurance that
this will continue to be the case. See "--Uncertainty Related to Health Care
Reform." Furthermore, there can be no assurance, even if the Company's fFN
ELISA Test and other future products are cleared by the FDA for new clinical
applications, that reimbursement at acceptable levels, or at all,cash equivalents, will be availablesufficient to meet our operating and capital requirements for such procedures. The Company could also be adversely affected by
changes in reimbursement policies of government or private third-party payors,
particularly toat least the extent that any such changes affect reimbursement for
diagnostic procedures in which the Company's products are used. Failure by
physicians, hospitalsnext two years. However, our present and future funding requirements will depend on many factors, including, among other health care providers to obtain sufficient
reimbursement from third-party payors for tests in which the Company's
products are used, or adverse changes in government and private third-party
payors' policies toward reimbursement for such tests, could have a material
adverse effect on the Company's business, financial condition and results of
operations. See "Business--Third-Party Reimbursement."
Market acceptance of the Company's products in international markets may be
dependent in part upon the availability of reimbursement within prevailing
health care payment systems. Reimbursement and health care payment systems in
international markets vary significantly by country, and can include both
government sponsored and private health care insurance. The Company's fFN
ELISA Test and fFN Vertical Flow Test have been approved for reimbursement in
Japan. Although the Company will seek additional international reimbursement
approvals, obtaining such approvals can require 12 to 18 months or longer, and
there can be no assurance that any such approvals will be obtained in a timely
manner, if at all. Failure to receive additional international reimbursement
approvals could have a material adverse effect on market acceptance of the
Company's products in the international markets in which the Company is
seeking approvals and could have a material adverse effect on the Company's
business, financial condition and results of operations. See "Business--Third-
Party Reimbursement."
LIMITED MANUFACTURING EXPERIENCE; MANAGEMENT OF EXPANDING OPERATIONS. The
Company has only limited experience in manufacturing its products in
commercial quantities. The Company currently manufactures its products for
research, United States clinical trials, international clinical trials and
limited commercial sales. things:
| 4 | the level of research and development investment required to maintain and improve our technology position; |
| 4 | costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| 4 | the success of our product sales and related collections; |
| 4 | our need or decision to acquire or license complementary businesses, products or technologies or acquire complementary businesses; |
| 4 | maintaining or expanding our manufacturing or commercialization capacity; |
| 4 | competing technological and market developments; and |
| 4 | costs relating to changes in regulatory policies or laws that affect our operations. |
As a result of these factors, we may need to raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms when needed, if at all. If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution and the receiptnew equity or debt securities may have rights, preferences and privileges senior to those of a PMA for the fFN
ELISA Test, the Company intendsour existing stockholders. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to expand its operations generally, including
its manufacturing and laboratory capabilities. Companies often encounter
difficulties associated with scaling up production of newrelinquish potentially valuable rights to our future products and
expanding operations, including problems involving production yields, quality
control and assurance, component supply and shortages of qualified personnel.
There can be no assuranceor proprietary technologies, or grant licenses on terms that the Company willare not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends significantly on our ability to protect our proprietary rights to the necessary manufacturing capability, buildtechnologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and train the necessary
manufacturing, salestrademark laws, and marketing teams, enter into the necessary
distributionnondisclosure, confidentiality and other contractual restrictions, to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or collaborative relationships, attract, retainpermit us to gain or keep any competitive advantage. For example, our pending US and integrate the
required key personnel,foreign patent applications may not issue as patents at all, or implement the financialif they do, they may not issue as patents in a form that will be advantageous to us or may issue and management systems as
required to meet any increased demand for its products. Failure of the Company
tobe subsequently successfully expand its operations in response to any increased demand for
its currentchallenged by others and future products, if any, could have a material adverse effect
on the Company's business, financial condition and results of operations.invalidated. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. Both the Company's manufacturing facilities are subjectpatent application process and the process of managing patent disputes can be time-consuming and expensive. Competitors may be able to applicable FDA
regulations regarding Good Manufacturing Practices ("GMP"), international
quality standardsdesign around our patents or develop products that provide outcomes comparable to ours. Although we have taken steps to protect our intellectual property and other regulatory requirements. Failure by the Company to
maintain its facilities in accordanceproprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with GMP regulations, international
quality standardsour employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other regulatory requirements may entail a delay or
termination of production, which could have a material adverse effect on the
Company's business, financial condition and results of operations. See
"Business--Laboratory Services; Manufacturing" and "Business--Government
Regulation."
LIMITED LABORATORY OPERATING EXPERIENCE. The Company has limited experience
in providing laboratory services to provide results of its diagnostic tests.
The Company intends to provide laboratory results to physicians and other
users of the Company's diagnostic test as a means of ensuring that the users
of such products will have ready access to results. The Company believes that
such laboratory services could generate a significant portion of the Company's
revenues in the near term. The Company expects that over time other commercial
laboratories will also provide results of its diagnostic tests. However, no
commercial laboratory is currently providing results with respect to the
Company's tests, and there can be no assurance that any laboratory
8
other than that of the Company will ultimately provide such results. Failure
by the Company to provide accurate and cost-efficient laboratory services and
to generate expected revenues through such services would have a material
adverse effect on the Company's business, financial condition and results of
operations and could expose the Company to significant liabilityproprietary information in the event of errors in test results. There can be no assurance that there will be demandunauthorized use or disclosure or other breaches of the agreements. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States.
Although we may initiate litigation to stop the infringement of our patent claims or to attempt to force an unauthorized user of our patented inventions or trade secrets to compensate us for the Company's laboratory servicesinfringement or that such services will generate any
additional revenues forunauthorized use, patent and trade secret litigation is complex and often difficult and expensive, and would consume the Company. See "Business--Laboratory Services;
Manufacturing."
LIMITED SALES, MARKETING AND DISTRIBUTION EXPERIENCE. The Company has
limited experience in sales, marketingtime of our management and distributionother significant resources. If the outcome of itslitigation is adverse to us, third parties may be able to use our technologies without payments to us. Moreover, other companies against whom we might initiate litigation may be better able to sustain the costs of litigation because they have substantially greater resources. Because of these factors relating to litigation, we may be effectively unable to prevent misappropriation of our patent and other proprietary rights.
Our rights to use technologies and patents licensed to us by third parties are not within our control, and we may not be able to commercialize our products and
currently relies on strategic partners and distributors for the sales,
marketing and distribution. The Company currently has agreementswithout these technologies.
We have licensed a number of patents, including patents related to sell its
fFN ELISAour Fetal Fibronectin Test and fFN Dipstick Test inour E-tegrity Test. Our business may significantly suffer if one or more of these licenses terminate, if we or our licensors fail to abide by the United States, Canada and Puerto
Rico through Matria and the fFN ELISA Test and the fFN Vertical Flow Test in
Japan through Daiichi. In connection with the commercializationterms of the fFN
ELISA Test,licenses or fail to prevent infringement by third parties or if the Company, in conjunction with Matria, is substantially
increasing its United States saleslicensed patents are found to be invalid.
If we violate the terms of our licenses, or otherwise lose our rights to these patents, we may be unable to continue developing and marketing efforts.selling our products. Our licensors or others may dispute the scope of our rights under any of these licenses. The Company intendslicensors under these licenses may breach the terms of their respective agreements or fail to sell its future products primarily through distributors orprevent infringement of the licensed patents by meansthird parties. Loss of strategic relationships, although the Company may also establish a direct
sales force to sell certain products. There can be no assurance that the
Company's sales and marketing efforts or direct sales force, if established,
will be successful. Failure to establish effective distribution or strategic
partner relationships or to achieve an effective sales and marketing
organization with respect to the Company's future productsany of these licenses for any reason could have a
material adverse effect on the Company's business,materially harm our financial condition and resultsoperating results.
In addition, if we determine that our products do not incorporate the patented technology that we have licensed from third parties, or that one or more of operations. See "Business--Laboratory Services; Manufacturing"the patents that we have licensed is not valid, we may dispute our obligation to pay royalties to our licensors. Any such dispute would be complex, expensive and "Business--Sales & Marketing; Strategic Corporate Alliances."
NO ASSURANCE OF SUCCESSFUL PRODUCT DEVELOPMENT. The Company'stime-consuming and an outcome adverse to us could materially harm our business and impair our ability to successfully developcommercialize our products.
If the use of our technologies conflicts with the intellectual property rights of third parties, we may incur substantial liabilities, and we may be unable to commercialize products based on these technologies in a profitable manner, if at all.
Other companies may have or acquire patent rights that they could enforce against us. If they do so, we may be required to alter our technologies, pay licensing fees or cease activities. If our technologies conflict with patent rights of others, third parties could bring legal action against us or our licensees, suppliers, customers or collaborators, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any additional products is uncertain. The Company's
research and development programs with respectpotential liability for damages, we might have to certain of its potential
products are at an early stage. The Company's goal isobtain a license in order to develop,continue to manufacture andor market diagnostic tests and, to a lesser extent, therapeutic products
concentrating on its targeted pregnancy-related and female reproductive
disorders. Except for the Company's fFN ELISA Test andaffected products. A required license under the related specimen
collection kit, none of the Company's products have been approved for
commercial sale in the United States. Potential new products will require
significant additional research, development, preclinical and clinical
testing, regulatory approval and additional investment prior to their
commercialization, whichpatent may not be successful. Thereavailable on acceptable terms, if at all.
Because patent applications can take many years to issue, there may be no assurancecurrently pending applications, unknown to us, that the Company's approach willmay later result in the developmentissued patents upon which our technologies may infringe. There could also be existing patents of commercially
successful products. Additionally, the Company has very limited experience in
the development of therapeutic products. See "Business--The Adeza Solution for
Premature and Late Birth/Successful Induction of Labor" and "Business--The
Adeza Solution for Reproductive Disorders."
DEPENDENCE ON LICENSES; POTENTIAL NEED FOR ADDITIONAL COLLABORATORS. The
Company's strategy for the research and development of certain of its products
contemplateswhich we are unaware that it will enter into arrangements with collaborators,
licensors, licensees and others. The Companyour technologies may therefore, be dependent upon
the subsequent success of theseinfringe. In addition, if third parties in performing their
responsibilities, including any research activities contemplated under such
arrangements. The Company has obtained, and intends to obtain in the future,
licensed rights to certain proprietary technologies from individuals,
universities and research institutions to which it is, or will be, obligated
to pay royalties and milestone payments if it develops products based upon the
licensed technology. The Company has a worldwide, sublicensable, exclusive
license from the Fred Hutchinson Cancer Research Center to certain patent
rights related to fFN, which constitutes the Company's core technology for its
fFN ELISA Test and fFN Dipstick Test. There can be no assurance that the
Company will be able to enter into additional collaborative, license or other
arrangements that the Company deems necessary or appropriate to develop,
commercialize and market its products, or that any or all of the contemplated
benefits from such collaborative, license or other arrangements will be
realized. There can be no assurance that partners or collaborators will not
pursue alternative technologies or products either on their own or in
collaboration with others, including the Company's competitors, as a means for
developing diagnostic or therapeutic products for the Company's targeted
pregnancy-related and female reproductive disorders. See "Business--Certain
License Agreements; Patents and Proprietary Technology" and "Business--
Advisors and Collaborators."
9
OPERATING LOSSES; FLUCTUATIONS IN OPERATING RESULTS. Adeza has generated
limited revenues from product sales which have not been sufficient to cover its
operating expenses. Adeza is substantially dependent upon research and
development contracts, external financing such as that being sought in this
offering and interest income to pursue its intended business activities.
Approximately $15.3 million of the Company's aggregate total revenues to date
have consisted of contract revenues received from its strategic partners,
primarily Matria and Daiichi, for research and product development. The Company
does not expect to generate further material research and development revenues
from these strategic agreements. The Company has not been profitable since
inception and has incurred a cumulative net loss of $23.7 million through March
31, 1996. Losses have resulted principally from costs incurred in research and
development activities, clinical trials, marketing and product introduction
expenses and from general and administrative costs. The Company expects to
incur additional operating losses at least through 1997. The Company's ability
to achieve profitability is dependent on its ability to successfully market and
sell its products, to develop and obtain patent protection and regulatory
approval for its products, to enter into agreements for product development and
commercialization with corporate sponsors and to manufacture its products in a
cost-effective manner. There can be no assurance that the Company will
successfully develop, commercialize, patent, manufacture or market its
products, obtain required regulatory approvals or achieve profitability. Future
revenues and results of operations may fluctuate significantly from quarter to
quarter and will depend upon, among other factors, actions relating to
regulatory and reimbursement matters, the extent to which Matria is successful
in achieving market acceptance of the Company's products, the rate at which the
Company expands its international distribution network, the progress of
clinical trials and the introduction of competitive products for diagnosis of
the Company's targeted pregnancy-related and female reproductive disorders. See
"--Uncertainty of Market Acceptance," "--Dependence on fFN ELISA Test" and
"Management's Discussion and Analysis of Financial Condition and Results of
Operations."
RELIANCE ON PATENTS AND PROTECTION OF PROPRIETARY TECHNOLOGY. The Company's
ability to compete effectively will depend substantially on its ability to
develop and maintain proprietary aspects of its technology. Although the
Company has been granted or has exclusive rights to various patents, the
Company's success will depend in large part on its ability to obtain United
States and foreign patent protection for its products, preserve its trade
secrets and operate without infringing on the proprietary rights of third
parties. There can be no assurance that the Company's issued patents, any
future patents that may be issued as a result of the Company's United States or
internationalfile patent applications or theobtain patents that the Company has
licensed, will offer any degree of protectionclaiming technology also claimed by us in pending applications, we may have to the Company's products against
competitive products. There can also be no assurance that any additional
patents will be issued from any of the patent applications owned by or licensed
to the Company, or that any patents that currently are or may be issued or
licensed to the Company or any of the Company's patent applications will not be
challenged, invalidated or circumventedparticipate in interference proceedings in the future, or that any patents
issued to or licensed by the Company will not be infringed upon or designed
around by others. In addition, there can be no assurance that competitors, many
of whom have substantial resources and have made substantial investments in
competing technologies, will not seek to apply for and obtain patents that will
prevent, limit or interfere with the Company's ability to make, use or sell its
products either in the United States or in international markets. Moreover,
patent law relating to certain of the Company's fields of interest,
particularly as to the scope of claims in issued patents, is still developing
and it is unclear how this uncertainty will affect the Company's patent rights.
The medical device and biotechnology industries have been characterized by
extensive litigation regarding patents and other intellectual property rights,
and companies in these industries have employed intellectual property
litigation as a strategy to gain a competitive advantage. There can be no
assurance that the Company will not in the future become subject to patent
infringement claims and litigation or interference proceedings declared by the
United StatesUS Patent and Trademark Office ("USPTO")to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of the filed foreign patent applications. We may have to participate in interference proceedings involving our issued patents or our pending applications.
If a third party claims that we infringe upon its proprietary rights, it could cause our business to suffer in a number of ways, including:
| 4 | we may become involved in time-consuming and expensive litigation, even if the claim is without merit; |
| 4 | we may become liable for substantial damages for past infringement if a court decides that our technologies infringe upon a competitor’s patent; |
| 4 | a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross-licenses to our patents; and |
| 4 | we may have to redesign our product so that it does not infringe upon others’ patent rights, which may not be possible or could require substantial funds or time. |
If any of these events occur, our business will suffer and the market price of our common stock may decline.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
In order to protect or enforce our patent rights, we may initiate patent litigation. In addition, others may initiate patent litigation against us. We may become subject to interference proceedings conducted in patent and trademark offices to determine the priority of inventions. There are numerous issued and pending patents in the medical device field. The defensevalidity and prosecutionbreadth of intellectual property suits,
USPTO interference proceedings and relatedmedical technology patents may involve complex legal and administrative proceedings
are both costly and time consuming. factual questions for which important legal principles may remain unresolved.
Litigation may be necessary to assert or defend against infringement claims, enforce our issued and licensed patents, issued to or licensed to the Company, to protect the Company'sour trade secrets or know-how or to determine the enforceability, scope and validity of the proprietary rights of others. 10
As is typicalOur involvement in its industry, the Company has received noticesintellectual property claims and litigation could:
| 4 | divert existing management, scientific and financial resources; |
| 4 | subject us to significant liabilities; |
| 4 | allow our competitors to market competitive products without obtaining a license from us; |
| 4 | cause product shipment delays and lost sales; |
| 4 | require us to enter into royalty or licensing agreements, which may not be available on terms acceptable to us, if at all; or |
| 4 | force us to discontinue selling or modify our products, or to develop new products. |
We may be subject to damages resulting from third
parties alleging infringement claims.claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other diagnostic companies, including our potential competitors. Although thereno claims against us are currently no pending, we may be subject to claims that these employees or lawsuitswe have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against the Company regarding any possible infringementthese claims. Even if we are successful in defending against these claims, there canlitigation could result in substantial costs and be no assurance that infringementa distraction to management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business.
If we cannot obtain additional licenses to intellectual property owned by third parties or
claims for indemnification resulting from infringement claims willthat we desire to incorporate into new products we plan to develop, we may not be assertedable to develop or commercialize these future products.
We are developing diagnostic products designed to expand the utility of fetal fibronectin in multiple applications. The technology that we ultimately may use in the development and commercialization of
RISKS RELATING TO THIS OFFERING
You will suffer immediate and substantial dilution.
We expect the initial public offering price of our shares to be substantially higher than the book value per share of our outstanding common stock. Accordingly, investors purchasing shares of common stock in this offering will:
| 4 | pay a price per share that substantially exceeds the value of our tangible assets after subtracting liabilities; and |
| 4 | contribute approximately % of the total amount invested to date to fund us but own only approximately % of the shares of common stock outstanding after this offering. |
To the extent outstanding stock options, warrants or the underwriters’ over-allotment option are exercised after this offering, there will be further dilution to new investors. See “Dilution.”
If our principal stockholders, executive officers and directors choose to act together, they may be able to control our management and operations, which may prevent us from taking actions that may be favorable to you.
Our executive officers, directors and principal stockholders, and entities affiliated with them, will beneficially own in the aggregate approximately % of our common stock following this offering. This significant concentration of share ownership may adversely affect the trading price of our common stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders. These stockholders, acting together, will have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets. In addition, they could dictate the management of our business and affairs. This concentration of ownership could have the effect of delaying, deferring or preventing a change in control of us or impeding a merger or consolidation, takeover or other business combination that could be favorable to you.
The future sale of our common stock could dilute your investment and negatively affect our stock price.
After this offering, we will have approximately million shares of common stock outstanding, or million shares if the underwriters exercise their over-allotment option in full. The shares sold in this offering, or shares if the underwriters exercise their over-allotment option in full, will be freely tradable without restriction under the federal securities laws unless purchased by our affiliates. The remaining shares of common stock outstanding after this offering will be available for public sale subject in some cases to volume and other limitations. See “Shares eligible for future sale.” Substantially all of our shares outstanding after this offering (excluding the shares sold in this offering) will be subject to the lock-up agreements with the underwriters described under “Underwriting.”
If our common stockholders sell substantial amounts of common stock in the public market, or the market perceives that such sales may occur, the market price of our common stock could fall. After this offering, the holders of approximately 15,943,176 shares of our common stock and the holders of warrants to purchase 262,556 shares of our common stock will have rights, subject to some
In addition, we may need to raise additional capital in the future to fund our operations. If we raise additional funds by issuing equity securities, our stock price may decline and our existing stockholders may experience significant dilution. Furthermore, we may enter into financing transactions at prices that represent a substantial discount to market price. Raising funds through the issuance of equity securities will dilute the ownership of our existing stockholders. A negative reaction by investors and securities analysts to any sale of our equity securities could result in a decline in the trading price of our common stock.
If an active, liquid trading market for our common stock does not develop, you may be unable to sell your shares quickly or at the market price.
Prior to this offering, there was no public market for our common stock. An active trading market for our common stock may not develop following this offering. You may not be able to sell your shares quickly or at the market price if trading in our stock is not active. The initial public offering price may not be indicative of prices that such assertions, if provenwill prevail in the trading market. See “Underwriting” for more information regarding the factors considered in determining the initial public offering price.
If the price and volume of our common stock experience extreme fluctuations, this could lead to costly litigation for us.
Because we operate within the medical devices and diagnostic products industries, our stock price is likely to be true,volatile. The market price of our common stock may fluctuate substantially due to a variety of factors, including:
| 4 | changes in reimbursement policies concerning the diagnostic products that we or other companies offer; |
| 4 | media reports and publications and announcements about women’s health and cancer diagnostic products or new cancer treatments or innovations that could compete with our products; |
| 4 | announcements concerning our competitors or the medical devices and diagnostic products industries in general; |
| 4 | new regulatory pronouncements, changes in regulatory guidelines, such as adverse changes in reimbursement for women’s health and cancer diagnostic products, and timing of regulatory approvals concerning the products in our pipeline; |
| 4 | market conditions or trends related to the medical devices and diagnostic products industries or the market in general; |
| 4 | additions to or departures of our key personnel; |
| 4 | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| 4 | changes in financial estimates or recommendations by securities analysts; |
| 4 | the seasonal nature of our revenues and expenses; |
| 4 | variations in our quarterly operating results; and |
| 4 | changes in accounting principles. |
The market prices of the securities of medical devices and diagnostic products companies, particularly companies like ours without consistent product sales and earnings, have been highly volatile and are likely to remain highly volatile in the future. This volatility has often been unrelated to the operating performance of particular companies. Moreover, market prices for stocks of biotechnology-related companies, particularly following an initial public offering, frequently reach levels that bear no relationship to the operating performance of these companies. These market prices generally are not sustainable and are highly volatile. In the past, companies that experience volatility in the market price of their securities have often faced securities class action litigation. Whether or not meritorious, litigation brought against us could result in substantial costs, divert our management’s attention and resources and harm our ability to grow our business.
We have reserved discretion in how we allocate our use of the net proceeds of this offering and if we do not use these proceeds effectively, we may fail to achieve our objectives and our stock price could decline.
We will have flexibility in applying the net proceeds of this offering among the categories of identified uses described in the “Use of proceeds” section of this prospectus. Although we expect to use the net proceeds in the approximate allocations described elsewhere in this prospectus, if we use the net proceeds for corporate purposes that do not yield a significant return or any return at all for our stockholders, our stock price could decline, and you may also not agree with how we allocate the net proceeds of this offering.
Anti-takeover provisions in our certificate of incorporation and bylaws and under Delaware law may inhibit a change in control or a change in management that you consider favorable.
Provisions in our certificate of incorporation and bylaws could delay or prevent a change of control or change in management that would provide you with a premium to the market price of your common stock. These provisions include those:
| 4 | authorizing the issuance without further approval of “blank check” preferred stock that could be issued by our board of directors to increase the number of outstanding shares and thwart a takeover attempt; |
| 4 | prohibiting cumulative voting in the election of directors, which would otherwise allow less than a majority of stockholders to elect director candidates; |
| 4 | limiting the ability to remove directors; |
| 4 | limiting the ability of stockholders to call special meetings of stockholders; |
| 4 | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of stockholders; and |
| 4 | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon by stockholders at stockholder meetings. |
In addition, Section 203 of the Delaware General Corporation Law limits business combination transactions with 15% stockholders that have not been approved by our board of directors. These provisions and others could make it difficult for a material adverse effectthird party to acquire us, or for members of our board of directors to be replaced, even if doing so would be beneficial to our stockholders. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt to replace the current management team. If a change of
We do not expect to pay dividends in the foreseeable future. As a result, you must rely on stock appreciation for any return on your investment.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. Accordingly, you will have to rely on capital appreciation, if any, to earn a return on your investment in our common stock. Furthermore, we may in the future become subject to contractual restrictions on, or prohibitions against, the payment of dividends.
Special note regarding forward-looking statements
This prospectus, including the sections titled “Prospectus summary,” “Risk factors,” “Management’s discussion and analysis of financial condition and results of operations” and “Business,” contains forward-looking statements. Forward-looking statements convey our current expectations or forecasts of future events. All statements contained in this prospectus other than statements of historical fact are forward-looking statements. Forward-looking statements include statements regarding our future financial position, business strategy, budgets, projected costs, plans and objectives of management for future operations. The words “may,” “continue,” “estimate,” “intend,” “plan,” “will,” “believe,” “project,” “expect,” “could,” “would,” “anticipate” and similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. These forward-looking statements include, among other things, statements about:
| 4 | the unpredictability of our quarterly revenues and results of operations; |
| 4 | our estimates regarding market size, future revenues, expenses and capital requirements and needs for additional financing; |
| 4 | the rate and degree of market acceptance of our products; |
| 4 | our marketing and manufacturing capacity and strategy; |
| 4 | our ability to develop and market new and enhanced products; |
| 4 | the timing of and our ability to obtain and maintain regulatory clearances for our products; |
| 4 | the timing of and ability to obtain reimbursement for our diagnostic products; and |
| 4 | our competitors. |
Any or all of our forward-looking statements in this prospectus may turn out to be inaccurate. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. They may be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including the risks, uncertainties and assumptions described in “Risk factors.” In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur as contemplated, and actual results could differ materially from those anticipated or implied by the forward-looking statements.
You should not unduly rely on these forward-looking statements, which speak only as of the date of this prospectus. Unless required by law, we undertake no obligation to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this prospectus. See “Where you can find additional information.”
Use of proceeds
We estimate that the net proceeds to us from the sale of the shares of common stock we are offering will be approximately $ million, assuming an initial public offering price of $ per share, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate the net proceeds to us from this offering will be approximately $ million.
We intend to use the net proceeds of this offering as follows:
| 4 | approximately $ million to expand our sales and marketing efforts in the United States and internationally; |
| 4 | approximately $ million for research and development activities; |
| 4 | approximately $ million for the relocation of our facility in Sunnyvale, California or expansion by the lease of additional property; and |
| 4 | the balance for other general corporate purposes. |
We may also use a portion of the net proceeds to acquire or invest in complementary businesses, products or technologies. Although we have no specific arrangements with respect to acquisitions, we evaluate acquisition opportunities and engage in related discussions from time to time. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds from this offering. We reserve the right to change the allocation of the net proceeds based on the changing needs of our business, including the progress and results of our research and development activities, the results of our sales and marketing efforts, acquisition opportunities and competitive developments.
Pending use of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments.
Dividend policy
We have never declared or paid any cash dividends on our capital stock and we do not currently anticipate declaring or paying cash dividends on our capital stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance operations. Any litigation or interference proceedings involvingfuture determination relating to our dividend policy will be made at the Companydiscretion of our board of directors and will resultdepend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and covenants and other factors that our board of directors may deem relevant.
Capitalization
The following table summarizes our capitalization as of June 30, 2004:
| 4 | on an actual basis; |
| 4 | on a pro forma basis to give effect to: |
| 4 | the conversion of all 15,409,062 shares of our preferred stock outstanding as of June 30, 2004 into 15,943,176 shares of our common stock, which will become effective at the closing of this offering; and | |
| 4 | the filing of an amended and restated certificate of incorporation to provide for an authorized capital stock of 5,000,000 shares of preferred stock and 100,000,000 shares of common stock; and |
| 4 | on a pro forma as adjusted basis to further reflect the sale of the shares of our common stock we are offering at an assumed public offering price of $ per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read the following table in substantial expense to the Companyconjunction with “Management’s discussion and significant
diversionanalysis of effort by the Company's technical and management personnel. An
adverse determination in litigation or interference proceedings to which the
Company may become a party could subject the Company to significant liabilities
to third parties or require the Company to seek licenses from third parties.
Although patent and intellectual property disputes in the medical device and
biotechnology industries have often been settled through licensing or similar
arrangements, costs associated with such arrangements may be substantial and
could include ongoing royalties. Furthermore, there can be no assurance that
necessary licenses would be available to the Company on satisfactory terms, if
at all. Adverse determinations in a judicial or administrative proceeding or
failure to obtain necessary licenses could prevent the Company from
manufacturing and selling its products, which would have a material adverse
effect on the Company's business, financial condition and results of operations.
operations” and our financial statements and related notes appearing elsewhere in this prospectus.
| As of June 30, 2004 | ||||||||||||||
| Pro forma | ||||||||||||||
| Actual | Pro forma | as adjusted | ||||||||||||
| (in thousands, except share and | ||||||||||||||
| per share data) | ||||||||||||||
| Cash and cash equivalents | $ | 14,495 | $ | 14,495 | $ | |||||||||
| Convertible preferred stock, $0.001 par value, 16,516,335 shares authorized, actual; 5,000,000 shares authorized, pro forma and pro forma as adjusted; 15,409,062 shares issued and outstanding, actual; no shares issued and outstanding, pro forma and pro forma as adjusted | $ | 61,484 | $ | — | $ | — | ||||||||
| Stockholders’ equity (deficit): | ||||||||||||||
| Common stock, $0.001 par value, 25,000,000 shares | ||||||||||||||
| authorized, actual; 100,000,000 shares authorized, pro forma and pro forma as adjusted; 242,980 shares issued and outstanding, actual; 16,186,156 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted | — | 16 | ||||||||||||
| Additional paid-in capital | 2,476 | 63,944 | ||||||||||||
| Deferred compensation | (95 | ) | (95 | ) | (95 | ) | ||||||||
| Accumulated deficit | (51,600 | ) | (51,600 | ) | ||||||||||
| Total stockholders’ equity (deficit) | (49,219 | ) | 12,265 | |||||||||||
| Total capitalization | $ | 12,265 | $ | 12,265 | $ | |||||||||
The number of shares of our common stock to be outstanding immediately after this offering is based on 16,186,156 shares of common stock outstanding as of June 30, 2004 after giving effect to the conversion of all 15,409,062 shares of preferred stock outstanding as of June 30, 2004 into 15,943,176 shares of common stock, which will become effective at the closing of this offering.
The number of shares of our common stock outstanding immediately after this offering excludes:
| 4 | 1,981,744 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2004 under our 1995 Stock Option and Restricted Stock Plan, at a weighted average exercise price of $1.75 per share; |
| 4 | 262,556 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2004 at an exercise price of $2.628 per share; and |
| 4 | 176,177 shares of our common stock available for future grant under our 1995 Stock Option and Restricted Stock Plan (based on options outstanding as of June 30, 2004). |
In July 2004, we granted option to purchase 31,000 shares of common stock under our 1995 Stock Option and Restricted Stock Plan at an exercise price of $7.50 per share. In August 2004, we increased the number of shares of common stock available for future grant under our 1995 Stock Option and Restricted Stock Plan by 750,000 shares, and we granted options to purchase 880,000 additional shares of common stock at an exercise price of $7.50 per share. Our stockholders approved this increase in August 2004.
Dilution
If you invest in our common stock, your interest will be diluted immediately to the extent of the difference between the public offering price per share you will pay in this offering and the pro forma net tangible book value per share of our common stock immediately after this offering.
Our net tangible book value as of June 30, 2004 was approximately $(49.4) million, or $(203.39) per share of common stock. Net tangible book value per share is equal to our total tangible assets minus total liabilities, all divided by 242,980 shares of common stock outstanding as of June 30, 2004.
Our pro forma net tangible book value per share as of June 30, 2004 was approximately $0.75 per share. Pro forma net tangible book value per share gives effect to the conversion of all outstanding shares of our preferred stock as of June 30, 2004 into 15,943,176 shares of our common stock, which will become effective at the closing of this offering.
After giving effect to the sale of the shares of common stock we are offering at an assumed initial public offering price of $ per share, and after deducting underwriting discounts and commissions and our estimated offering expenses, our pro forma as adjusted net tangible book value as of June 30, 2004 would have been approximately $ million, or $ per share.
This represents an immediate increase in pro forma net tangible book value of $ per share and an immediate dilution of $ per share to new investors. The following table illustrates this calculation on a per share basis:
| Assumed initial public offering price per share | $ | |||||||
| Net tangible book value per share as of June 30, 2004 | $ | (203.39 | ) | |||||
| Pro forma increase in net tangible book value per share attributable to conversion of preferred stock outstanding at June 30, 2004 | 204.14 | |||||||
| Pro forma net tangible book value per share of common stock as of June 30, 2004 | 0.75 | |||||||
| Pro forma increase per share attributable to the offering | ||||||||
| Pro forma as adjusted net tangible book value per share of common stock after this offering | ||||||||
| Pro forma dilution per share to new investors | $ | |||||||
If the underwriters exercise their over-allotment option in full, our pro forma as adjusted book value will increase to $ per share, representing an increase to existing holders of $ per share, and there will be an immediate dilution of $ per share to new investors.
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2004, after giving effect to this offering, and the pro forma adjustments referred to above, the total number of shares of our common stock purchased from us and the total consideration and average price per share by existing stockholders and by new investors:
| Total shares | Total consideration | ||||||||||||||||||||
| Average price | |||||||||||||||||||||
| Number | Percent | Amount | Percent | per share | |||||||||||||||||
| Existing stockholders | % | $ | % | $ | |||||||||||||||||
| New investors | |||||||||||||||||||||
| Total | 100.0 | % | $ | $ | 100.0 | % | |||||||||||||||
If the underwriters exercise their over-allotment option in full, the following will occur:
| 4 | the pro forma as adjusted percentage of shares of our common stock held by existing stockholders will decrease to approximately % of the total number of pro forma as adjusted shares of our common stock outstanding after this offering; and |
| 4 | the pro forma as adjusted number of shares of our common stock held by new public investors will increase to , or approximately % of the total pro forma as adjusted number of shares of our common stock outstanding after this offering. |
The tables and calculations above are based on 242,980 shares of our common stock outstanding as of June 30, 2004 and excludes:
| 4 | 1,981,744 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2004 under our 1995 Stock Option and Restricted Stock Plan at a weighted average exercise price of $1.75 per share; |
| 4 | 262,556 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2004 at an exercise price of $2.628 per share; and |
| 4 | 176,177 shares of our common stock available for future grant under our 1995 Stock Option and Restricted Stock Plan (based on options outstanding as of June 30, 2004). |
In July 2004, we granted options to purchase 31,000 shares of common stock under our 1995 Stock Option and Restricted Stock Plan at an exercise price of $7.50 per share. In August 2004, we increased the number of shares of common stock available for future grant under our 1995 Stock Option and Restricted Stock Plan by 750,000 shares, and we granted options to purchase 880,000 additional shares of common stock at an exercise price of $7.50 per share. Our stockholders approved this increase in August 2004.
If all of our outstanding options and warrants as of June 30, 2004 were exercised, the pro forma as adjusted net tangible book value per share after this offering would be $ per share, representing an increase to existing holders of $ per share, and there will be an immediate dilution of $ per share to new investors.
Selected financial data
We have derived the following statements of operations data for each of the three fiscal years ended December 31, 2001, 2002 and 2003 and the balance sheet data at December 31, 2002 and 2003 from our financial statements which have been audited by Ernst & Young LLP, independent registered public accounting firm, and which financial statements and the report thereon we include elsewhere in this prospectus. We have derived our statements of operations data for the fiscal years ended December 31, 1999 and 2000 and the balance sheet data at December 31, 1999, 2000 and 2001 from our audited financial statements that we do not include in this prospectus. The selected financial data at June 30, 2004 and for the six months ended June 30, 2003 and 2004 have been derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited selected financial data include, in the opinion of management, all adjustments, consisting of only normal recurring adjustments, that are necessary for a fair presentation of the financial position and the results of operations for the interim unaudited periods. Operating results for the six months ended June 30, 2004 are not necessarily indicative of the results that may be expected for the year ending December 31, 2004. You should read the selected financial data in conjunction with our financial statements and the related notes and “Management’s discussion and analysis of financial condition and results of operations” appearing elsewhere in this prospectus.
| Six months ended | |||||||||||||||||||||||||||||
| Years ended December 31, | June 30, | ||||||||||||||||||||||||||||
| Statement of | |||||||||||||||||||||||||||||
| operations data: | 1999 | 2000 | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||||||||||||||||
| (unaudited) | |||||||||||||||||||||||||||||
| Product sales | $3,776 | $3,404 | $6,742 | $14,277 | $26,499 | $12,519 | $15,638 | ||||||||||||||||||||||
| Cost of product sales | 1,880 | 2,023 | 2,521 | 3,715 | 6,087 | 3,059 | 3,343 | ||||||||||||||||||||||
| Gross profit | 1,896 | 1,381 | 4,221 | 10,562 | 20,412 | 9,460 | 12,295 | ||||||||||||||||||||||
| Contract revenues | 444 | 515 | 811 | 1,059 | — | — | — | ||||||||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||||||||||||||
| Selling and marketing | 3,630 | 5,337 | 6,437 | 7,819 | 12,259 | 5,872 | 7,486 | ||||||||||||||||||||||
| General and administrative | 1,481 | 1,641 | 2,033 | 2,069 | 2,730 | 1,113 | 1,552 | ||||||||||||||||||||||
| Research and development | 2,014 | 1,893 | 2,145 | 2,047 | 2,001 | 980 | 1,196 | ||||||||||||||||||||||
| Total operating costs and expenses | 7,125 | 8,871 | 10,615 | 11,935 | 16,990 | 7,965 | 10,234 | ||||||||||||||||||||||
| Income (loss) from operations | (4,785 | ) | (6,975 | ) | (5,583 | ) | (314 | ) | 3,422 | 1,495 | 2,061 | ||||||||||||||||||
| Other expenses, net | (11 | ) | (17 | ) | (21 | ) | (8 | ) | (33 | ) | (9 | ) | — | ||||||||||||||||
| Interest income (expense), net | (779 | ) | (646 | ) | (85 | ) | (7 | ) | (19 | ) | (44 | ) | 56 | ||||||||||||||||
| Income (loss) before income taxes | (5,575 | ) | (7,638 | ) | (5,689 | ) | (329 | ) | 3,370 | 1,442 | 2,117 | ||||||||||||||||||
| Provision for income taxes | — | — | — | — | 135 | 85 | 80 | ||||||||||||||||||||||
| Net income (loss) | $(5,575 | ) | $(7,638 | ) | $(5,689 | ) | $(329 | ) | $3,235 | $1,357 | $2,037 | ||||||||||||||||||
| Net income (loss) per share: | |||||||||||||||||||||||||||||
| Basic | $(21.08 | ) | $34.31 | $(27.20 | ) | $(1.36 | ) | $13.33 | $5.60 | $8.38 | |||||||||||||||||||
| Diluted | $(21.08 | ) | $34.31 | $(27.20 | ) | $(1.36 | ) | $0.19 | $0.08 | $0.11 | |||||||||||||||||||
| Shares used in computing net income (loss) per share: | |||||||||||||||||||||||||||||
| Basic | 264,478 | 222,590 | 209,150 | 241,596 | 242,720 | 242,460 | 242,980 | ||||||||||||||||||||||
| Diluted | 264,478 | 222,590 | 209,150 | 241,596 | 16,692,907 | 16,573,159 | 17,760,275 | ||||||||||||||||||||||
| As of December 31, | ||||||||||||||||||||||||
| As of June 30, | ||||||||||||||||||||||||
| Balance sheet data: | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | ||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Cash and cash equivalents | $3,071 | $6,515 | $16,674 | $10,751 | $12,092 | $14,495 | ||||||||||||||||||
| Working capital | (2,586 | ) | (1,888 | ) | 7,094 | 6,195 | 9,653 | 11,735 | ||||||||||||||||
| Total assets | 6,137 | 8,234 | 17,714 | 15,731 | 18,716 | 21,566 | ||||||||||||||||||
| Convertible preferred stock | 36,577 | 45,637 | 60,984 | 60,984 | 61,484 | 61,484 | ||||||||||||||||||
| Accumulated deficit | (43,216 | ) | (50,854 | ) | (56,543 | ) | (56,872 | ) | (53,637 | ) | (51,600 | ) | ||||||||||||
| Total stockholders’ equity (deficit) | (42,188 | ) | (48,540 | ) | (54,217 | ) | (54,537 | ) | (51,279 | ) | (49,219 | ) | ||||||||||||
Management’s discussion and analysis of financial condition and results of operations
The following discussion of our financial conditions and results of operations should be read in conjunction with our financial statements and the notes to those financial statements appearing elsewhere in this prospectus. This discussion contains forward-looking statements that involve significant risks and uncertainties. As a result of many factors, such as those set forth under “Risk factors” and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
OVERVIEW
We design, develop, manufacture and market innovative products for women’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the Fetal Fibronectin Test, that utilizes a single-use, disposable cassette and is analyzed on our patented instrument, the TLiIQ System. This FDA-approved product is designed to objectively determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. We began selling our single-use, disposable Fetal Fibronectin Test in 1999 and launched our second-generation system, the TLiIQ System, in 2001. Sales of TLiIQ Systems to hospital and clinical laboratories allow healthcare providers access to the Fetal Fibronectin Test, resulting in the potential for better patient care and for significant cost savings by avoiding unnecessary medical treatment. To date, we have shipped over 1,200 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes.
We believe the key factors underlying our growth since 1999 include greater healthcare provider acceptance, demonstrated cost savings, expanded reimbursement coverage by insurance companies, expansion of our sales force and increased marketing efforts. Continued growth in test volume and revenue will depend on a number of factors, including placing additional TLiIQ Systems in hospitals and clinical laboratories, increasing utilization of existing TLiIQ Systems and developing additional applications or products.
Product sales
Our product sales are derived primarily from the sale of our disposable Fetal Fibronectin Test cassettes. In addition, we derive a small portion of our revenues from the sale of TLiIQ Systems and other consumables. Sales in the United States accounted for 97% of our product sales in both the six months ended June 30, 2004 and the year ended December 31, 2003. International sales accounted for 3% of our product sales in both the six months ended June 30, 2004 and the year ended December 31, 2003. We currently use distributors for sales outside of the United States and Canada. Our business has been in the past and may continue to be seasonal and is affected by customer ordering patterns, which may involve quarterly or semi-annual orders, as well as other factors which may cause quarterly variances in our revenue. As such, revenue may not increase in sequential quarters and our net income may fluctuate significantly.
Cost of product sales
Our cost of product sales represents the cost of materials, overhead associated with the manufacture of our products, direct labor, delivery charges, lab services, royalties and product warranty obligations.
Selling and marketing expenses
Selling and marketing expenses consist primarily of sales and marketing personnel compensation, sales force incentive compensation, travel, trade-shows, promotional materials and programs, advertising and healthcare provider education materials and events.
General and administrative expenses
Our general and administrative expenses consist primarily of personnel expenses for accounting, human resources, information technology and corporate administration functions. Other costs include facility costs and professional fees for legal and accounting services.
Research and development expenses
Our research and development expenses consist of costs incurred for company-sponsored and collaborative research and development activities. These expenses consist primarily of direct and research-related allocated overhead expenses such as facilities costs, salaries and benefits, and material and supply costs.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
We prepare our financial statements in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. Our critical accounting policies are as follows:
Revenue recognition
Our revenue from product sales is recognized when there is persuasive evidence an arrangement exists, the price is fixed or determinable, delivery to the customer has occurred and collectibility is reasonably assured. We use contracts and customer purchase orders to determine the existence of an arrangement. We assess whether the fee is fixed or determinable based on the terms of the agreement associated with the transaction. We use shipping documents and, if necessary, third-party proof of delivery to verify delivery. In order to determine whether collection is probable, we assess a number of factors, including past transaction history with the customer and the credit-worthiness of the customer. Revenue from our laboratory services is recognized as tests are performed. Contract revenues are recorded over the life of the contract or as performance occurs and the related earnings process is completed based on the performance requirements of the contract.
With respect to sales to distributors, revenue is generally recognized upon shipment, as the title, risks and rewards of ownership of the products pass to the distributors and the selling price of our product is fixed and determinable at that point. The selling prices on sales to a certain distributor through June 30, 2002 were not fixed and determinable until the distributor shipped the products to the end user. Consequently, for this distributor, we recognized revenue only after the shipment of product to the end user. Additionally, on July 1, 2002, we entered into a services agreement with a laboratory and fulfillment company that performs diagnostic tests. Under the terms of the agreement, this company provides certain domestic product distribution and testing services for us. We recognize revenue upon the shipment of products from this company to the end user as the title, risks and rewards of ownership of the products pass from us to the end user at that time. Any advance payments received in excess of revenue recognized are classified as deferred revenue.
Valuation of inventory
Inventories are stated at the lower of standard cost (which approximates actual cost) or market. We calculate an inventory reserve for estimated obsolescence or excess inventory based upon historical demand and assumptions about future demand for our products and market conditions. If our current assumptions about future demand change and if actual market conditions are less favorable than anticipated, additional inventory reserves may be required which would negatively impact our gross profit.
Allowance for doubtful accounts
We maintain an allowance for doubtful accounts related to the estimated losses that may result from the inability of our customers to make required payments. This allowance is determined based upon historical experience and any specific customer collection issues that have been identified. Historically, we have not experienced significant credit losses related to an individual customer or groups of customers in any particular industry or geographic area. However, deterioration in our ability to collect our receivables could result in an increase in our allowance for doubtful accounts and increase our general and administrative expenses.
Stock-based compensation expense
In connection with the grant of stock options to employees and directors, we record deferred stock compensation as a component of stockholders’ equity. Deferred stock compensation for options granted to employees and directors is recorded if the estimated fair value of our common stock on the date the options are granted is greater than their exercise prices. Deferred stock compensation is amortized as a charge to operations over the vesting periods of the options using the straight-line method. Additionally, for options granted to non-employees, the fair value of the options, estimated using the Black-Scholes valuation model, is periodically re-measured with the resulting value charged to expense over the period of the related services being rendered. We recorded stock-based compensation expense related to all of our options of $0, $5,000, and $23,000 for the years ended December 31, 2001, 2002 and 2003, respectively, and $23,000 for the six months ended June 30, 2004. As of June 30, 2004, we had $95,000 of deferred stock compensation that will be expensed over the next four years. The amount of stock-based compensation expense to be recorded in future periods may decrease if unvested options, for which deferred stock compensation has been recorded, are subsequently canceled.
RESULTS OF OPERATIONS
Six months ended June 30, 2004 as compared to six months ended June 30, 2003
Product sales
Product sales for the six months ended June 30, 2004 were $15.6 million, an increase of 24.9%, or $3.1 million, from $12.5 million for the six months ended June 30, 2003. The growth in sales was primarily due to a $3.0 million increase in unit sales of Fetal Fibronectin Test cassettes.
Cost of product sales
Cost of product sales for the six months ended June 30, 2004 was $3.3 million, an increase of 9.3% from $3.1 million for the six months ended June 30, 2003 as the result of increased unit sales. As a percentage of product sales, the cost of product sales for the six months ended June 30, 2004 was 21.4%, a decrease from 24.4% for the six months ended June 30, 2003. The change in cost of product sales as a percentage of product sales was primarily attributable to an increase in units sold and a decrease in manufacturing variances.
Gross profit
Our gross profit for the six months ended June 30, 2004 was $12.3 million, an increase of $2.8 million from $9.5 million in the six months ended June 30, 2003. Gross margin was 78.6% for the six months ended June 30, 2004 as compared to 75.6% for the same period in 2003.
Selling and marketing expenses
Selling and marketing expenses for the six months ended June 30, 2004 were $7.5 million, an increase of 27.5% from $5.9 million for the six months ended June 30, 2003. Selling and marketing expenses as a percentage of product sales increased to 47.9% in the six months ended June 30, 2004 from 46.9% in the six months ended June 30, 2003. The increase was largely attributable to $0.9 million
General and administrative expenses
General and administrative expenses for the six months ended June 30, 2004 were $1.6 million, an increase of 39.4% from $1.1 million for the six months ended June 30, 2003. General and administrative expenses as a percentage of product sales were 9.9% and 8.9% for the six months ended June 30, 2004 and 2003, respectively. The increase was primarily attributable to legal expenses of $0.2 million and personnel related expenses of $0.1 million.
Research and development expenses
Research and development expenses for the six months ended June 30, 2004 were $1.2 million, an increase of 22% from $1.0 million for the same period in 2003. The absolute dollar increase was attributable to increased clinical trial expenses and personnel and related costs. Research and development expenses as a percentage of revenue decreased to 7.6% for the six months ended June 30, 2004 from 7.8% for the same period in 2003.
Interest income (expense)
We recognized interest income of $56,000 for the six months ended June 30, 2004, a decrease from $64,000 for the same period in 2003, primarily due to lower interest rates. We incurred no interest expense for the six months ended June 30, 2004 compared to $108,000 for the same period in 2003. The interest expense of $108,000 in 2003 was primarily related to notes payable in conjunction with the conclusion of a co-promotion and distribution agreement with a major US distributor. These notes were repaid by June 30, 2003.
Provision for income taxes
We recorded a provision for income taxes of $80,000 for the six months ended June 30, 2004 related to federal alternative minimum taxes and state taxes compared to $85,000 for the same period in 2003.
Our effective tax rate for the six months ended June 30, 2004 was 3.8% compared to 5.9% for the six months ended June 30, 2003. The effective tax rate in both periods is lower than the statutory rate due primarily to tax benefits arising from the utilization of net operating losses to the extent allowable under current tax law.
Year ended December 31, 2003 as compared to year ended December 31, 2002
Product sales
Product sales for the year ended December 31, 2003 were $26.5 million, an increase of 85.6%, or $12.2 million from $14.3 million in 2002. The increase was primarily attributable to the reduction in revenue sharing relating to the conclusion of our co-promotion and distribution agreement with a major US distributor. The increase was also attributable to growth in unit sales and an increase in average selling prices. Under the terms of our co-promotion and distribution agreement, we recognized only a portion of US product sales. After June 30, 2002, we began to recognize 100% of US product sales, leading to significantly increased revenue during the second half of 2002.
Cost of product sales
Cost of product sales for the year ended December 31, 2003 was $6.1 million, an increase of 63.8% from $3.7 million in 2002. The increase was related primarily to the conclusion of the co-promotion agreement and the resulting increase in our product sales of Fetal Fibronectin Test cassettes. As a percentage of product sales, cost of product sales in 2003 decreased to 23.0% in the year ended December 31, 2003 from 26.0% in the year ended December 31, 2002.
Gross profit
Gross profit was $20.4 million in the year ended December 31, 2003 and $10.6 million in the year ended December 31, 2002. Gross margin for the year ended December 31, 2003 was 77.0%, as compared to 73.9% in 2002. The increase in gross profit was primarily due to the conclusion of the co-promotion and distribution agreement and the resulting increase in our product sales.
Contract revenue
We had no contract revenue in the year ended December 31, 2003. Contract revenue of $1.1 million was recognized in the year ended December 31, 2002. In 1999, we entered into a co-promotion and distribution agreement with a major US distributor. Under the agreement, the distributor paid us a nonrefundable one-time payment of $2.0 million in 1999. This one-time payment was being recognized as revenue over the estimated five-year life of the agreement. At the termination of the agreement in 2002, we recognized the balance of this deferred contract revenue. The distributor was also to pay us a total of up to $2.0 million during 2001 and 2002 to fund certain portions of the research and development efforts related to the products and services covered by this agreement. During the year ended December 31, 2002, we recognized $176,000 of such research and development revenue.
Selling and marketing expenses
Selling and marketing expenses for the year ended December 31, 2003 were $12.3 million, an increase of 56.8% from $7.8 million for the same period in 2002. The increase was primarily due to $3.0 million in costs associated with the expansion of our direct sales force and $0.9 million related to marketing expenditures.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2003 were $2.7 million, an increase of 31.9% from $2.1 million for the same period in 2002. The increase was primarily attributable to increased patent and other legal fees of approximately $0.4 million and increased insurance expense of $0.2 million.
Research and development expenses
Research and development expenses for the year ended December 31, 2003 were $2.0 million, unchanged from the same period in 2002. Lower clinical trial expenses in 2003 were offset by higher usage of supplies and service charges relating to FDA filing and user fees.
Interest income (expense)
We recognized interest income of $112,000 in 2003, a decrease of 47.2% from $212,000 in 2002. The decrease was primarily due to a higher average cash balance in 2002 and lower interest rates in 2003. We recognized interest expense of $131,000 in 2003, a decrease of 40.2% from $219,000 in 2002. The decrease is attributable to interest on capital lease obligations and notes payable which were fully paid in 2002, and interest from the note payable related to the terminated co-promotion and distribution agreement issued in June 2002.
Provision for income taxes
We recorded an income tax provision of $135,000 for the year ended December 31, 2003 related to federal alternative minimum taxes and state taxes. There was no tax provision recorded for the year ended December 31, 2002 due to an operating loss.
Because of our lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance.
As of December 31, 2003, we had federal and state net operating loss carryforwards of approximately $40.3 million and $22.8 million, respectively. We also had federal and state research and development tax credit carryforwards of approximately $1.1 million and $0.9 million, respectively. The federal net
Utilization of our net operating losses may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses before utilization.
Year ended December 31, 2002 as compared to year ended December 31, 2001
Product sales
Product sales for the year ended December 31, 2002 were $14.3 million, an increase of 111.8% from $6.7 million for 2001. The increase was mainly attributable to the favorable impact from the conclusion of the co-promotion and distribution agreement with a major US distributor on June 30, 2002, as well as an increase in unit sales of Fetal Fibronectin Test cassettes. During the term of the distributor agreement, we recognized only a portion of the total US revenue generated by both parties. After the termination of the agreement on June 30, 2002, we began to recognize 100% of US product sales.
Cost of product sales
Cost of product sales for the year ended December 31, 2002 was $3.7 million, an increase of 47.4% from $2.5 million in 2001. The absolute dollar increase was primarily due to the favorable impact of the conclusion of the co-promotion and distribution agreement with a major US distributor. As a percentage of total revenue, cost of product sales in 2002 was 26.0%, significantly lower than the 37.4% in 2001.
Gross profit
Gross profit was $10.6 million in the year ended December 31, 2002 and $4.2 million in the year ended December 31, 2001. Gross margin was 73.9% in 2002 and 62.6% in 2001. The increase was primarily attributable to the conclusion of the co-promotion and distribution agreement with a major US distributor and the resulting increase in our sales.
Contract revenue
Contract revenue for the year ended December 31, 2002 was $1.1 million, an increase of 30.6% from $0.8 million for 2001. In the years ended December 31, 2002 and 2001, respectively, we recognized $176,000 and $244,000 of research and development revenue from a co-promotion and distribution agreement with a major US distributor. Additionally, the Company received a nonrefundable, one-time $2.0 million payment from the distributor in 1999 that was being recognized as revenue over the estimated five-year life of the agreement. The amount of revenue recognized for the years ended December 31, 2002 and 2001 from this one-time payment was $574,000 and $400,000, respectively. Also included in contract revenue are reimbursements for research and development expenses from National Institutes of Health, or NIH, grants. Reimbursements recognized as revenue in 2002 and 2001 were $35,000 and $167,000, respectively.
Selling and marketing expenses
Selling and marketing expenses for the year ended December 31, 2002 were $7.8 million, an increase of 21.5% from $6.4 million in 2001. The increase was primarily due to $0.9 million of expenses related to the expansion of our direct sales force.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2002 were $2.1 million, relatively consistent with $2.0 million in 2001. Increases in personnel related expenses and legal fees were offset by reduced bad debt expenses.
Research and development expenses
Research and development expenses for the year ended December 31, 2002 were $2.0 million, a decrease of 4.6% from $2.1 million in 2001. The decrease was primarily due to lower clinical trial expenses partially offset by increased personnel expenses.
Interest income (expense)
We recognized interest income of $212,000 in the year ended December 31, 2002, a decrease of 22.1% from $272,000 in 2001. The decrease was primarily due to lower interest rates.
We recognized interest expense of $219,000 in the year ended December 31, 2002, a decrease of 38.7% from $357,000 in 2001. The decrease was attributable to declining interest expense related to capital lease obligations and notes payable.
Provision for income taxes
We recorded no income tax provision for 2002 or 2001 due to operating losses.
Because of our lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance.
LIQUIDITY AND CAPITAL RESOURCES
Since our inception, our operations have been primarily financed through private equity investments, with the addition of capital leases, and research and development contracts. As of June 30, 2004 our cash and cash equivalents were $14.5 million. All of our cash equivalents have original maturities of three months or less.
During the six months ended June 30, 2004, our operating activities provided cash of approximately $2.5 million, compared to approximately $2.1 million during the six months ended June 30, 2003. The increase was due primarily to an increase in net income.
Our investing activities used cash of approximately $78,000 during the six months ended June 30, 2004 compared to $62,000 for the six months ended June 30, 2003. Investing activities were primarily related to investments in equipment.
No cash was used for financing activities during the six months ended June 30, 2004 compared to $4.3 million used during the six months ended June 30, 2003. The $4.3 million used during the six months ended June 30, 2003 was primarily related to repayments of notes payable.
For the year ended December 31, 2003 our operating activities provided cash of approximately $5.5 million. This was an increase of $9.4 million from the cash used in operating activities of $3.9 million for the year ended December 31, 2002. This change was primarily due to a loss of $0.3 million in the year ended December 31, 2002 in comparison to net income of $3.2 million in 2003. Additionally, accounts receivable increased by $1.3 million in the year ended December 31, 2003, in comparison to an increase of $3.9 million in 2002, and deferred revenue increased by $0.2 million in 2003, in comparison to a decrease of $1.9 million in 2002. These changes in accounts receivable and deferred revenue were due primarily to the conclusion of the co-promotion and distribution agreement with a major US distributor.
For the year ended December 31, 2003 our investing activities used cash of approximately $0.4 million. This was an increase of $0.2 million from cash used in investing activities of $0.1 million for the year ended December 31, 2002. The increase was due to the purchase of intangible assets and equipment.
For the year ended December 31, 2003 our financing activities used $3.8 million. This was an increase of $1.8 million from cash used in financing activities of approximately $1.9 million for the year ended December 31, 2002. The increase was primarily attributable to payments on notes payable.
As of June 30, 2004, we had no long-term debt, capital lease obligations or long-term purchase agreements or commitments. We are currently operating under a month-to-month lease, and are in negotiations for a new facility lease.
In 2004, we entered into an operating lease for a new telephone system for approximately $53,000 with the following schedule of future operating lease payments:
| Payments due in | ||||||||||||||||||||||||||||
| Contractual obligations | Total | 2004 | 2005 | 2006 | 2007 | 2008 | After 2008 | |||||||||||||||||||||
| Operating lease | $ | 53,305 | $ | 9,995 | $ | 13,326 | $ | 13,326 | $ | 13,326 | $ | 3,332 | — | |||||||||||||||
In addition to patents,cash generated from product sales, we believe our existing cash and cash equivalents, together with the Company relies on trade secretsestimated net proceeds from this offering, will be sufficient to meet our anticipated cash requirements for at least the next two years. However, future research and proprietary
know-how, which it seeks to protect, in part,development, clinical trials and sales and marketing expenses, as well as administration support, may require additional capital resources. We may raise additional funds through appropriate
confidentiality and proprietary information agreements. These agreements
generally provide that all confidential information developedpublic or made knownprivate equity offerings, debt financings, capital lease transactions, corporate collaborations or other means. Due to the individualuncertainty of financial markets, financing may not be available to us on acceptable terms or at all. Therefore, we may raise additional capital from time to time due to favorable market conditions or strategic considerations even if we have sufficient funds for planned operations.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
| 4 | success of our product sales and related collections; |
| 4 | future expenses to expand and support our sales and marketing activities; |
| 4 | costs relating to changes in regulatory policies or laws that affect our operations; |
| 4 | maintaining and expanding our manufacturing capacity; |
| 4 | the level of investment in research and development and clinical trials required to maintain and improve our technology position; |
| 4 | costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and |
| 4 | our need or decision to acquire or license complementary businesses, products or technologies. |
If at any time sufficient capital is not available, either through existing capital resources or through raising additional funds, we may be required to delay, reduce the scope of, eliminate or divest one or more of our research, clinical, sales and marketing programs or our entire business.
RECENT ACCOUNTING PRONOUNCEMENTS
In January 2003, the Financial Accounting Standards Board, or FASB, issued Interpretation No. 46,Consolidation of Variable Interest Entities(FIN 46). FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity’s activities or entitled to receive a majority of the entity’s residual returns or both. A variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. Our adoption of the requirements of FIN 46 did not have an impact on our financial position or results of operations.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
To date, all of our sales have been denominated in US dollars. Accordingly, we believe that there is no material exposure to risk from changes in foreign currency exchange rates.
We hold no derivative financial instruments and do not currently engage in hedging activities.
Our exposure to interest rate risk at December 31, 2003 and June 30, 2004 is related to the investment of our excess cash into highly liquid financial investments with original maturities of three months or less. We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments. Due to the short term nature of our investments, we have assessed that there is no material exposure to interest rate risk arising from them.
Business
OVERVIEW
We design, develop, manufacture and market innovative products for women’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the Fetal Fibronectin Test, that utilizes a single-use, disposable cassette, and is analyzed on our patented instrument, the TLiIQ System. This test is approved by the CompanyFDA for broad use in assessing the risk of preterm birth.
Our Fetal Fibronectin Test is designed to objectively determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. Testing for fetal fibronectin during pregnancy provides a more accurate assessment of the likelihood of a preterm birth than traditional methods. Preterm births have historically accounted for up to 85% of all pregnancy-related complications and deaths in the United States, and over $13 billion in costs were associated with the care of preterm or low birth weight infants in 2001. By correctly identifying women at risk for preterm birth, we believe our Fetal Fibronectin Test leads to improved patient care and significant cost savings and has the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women.
Healthcare providers have historically had difficulty with accurately predicting when a woman is likely to give birth. Data from numerous clinical studies have demonstrated that our Fetal Fibronectin Test has a greater predictive value than traditional risk assessment methods for identifying women at risk of preterm birth. For example, a negative Fetal Fibronectin Test for a woman presenting with signs and symptoms of preterm labor indicates a 99.5% probability that she will not deliver in the individuals relationshipnext 7 days. A negative test result enables the healthcare provider to avoid unnecessary and costly hospitalization and drug treatment. Although a positive Fetal Fibronectin Test result does not have the same predictive value as a negative test result, if the Fetal Fibronectin Test result is positive, the healthcare provider may proactively prescribe various treatments to delay or manage preterm labor and birth.
The patient population for which our Fetal Fibronectin Test is approved can be divided into three patient categories. The first category consists of women who present with signs and symptoms of preterm labor and are typically directed to the hospital. The second and third categories include women designated as either “high-risk” or “low-risk” for preterm birth by their healthcare providers, and who currently exhibit no signs and symptoms of preterm labor. We believe that by using the Fetal Fibronectin Test periodically during a pregnancy, healthcare providers can more accurately assess the likelihood that women in all three categories will not deliver preterm.
We have shipped more than 1,200 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes for use in hospital and clinical laboratories. As of August 3, 2004, our direct sales force consisted of 66 representatives who sell to hospital and clinical laboratories, health plans and healthcare providers. Our Fetal Fibronectin Test has a specific reimbursement code, and we believe that reimbursement for our Fetal Fibronectin Test has been regularly available through health plan organizations and most state Medicaid programs.
We also market and sell the E-tegrity Test, an infertility-related test based on a proprietary analyte specific reagent, to assess receptivity of the uterus to embryo implantation in women with unexplained infertility. The E-tegrity Test can be particularly useful for women who are considering assisted reproductive technologies, including in vitro fertilization, or IVF. We are also seeking to expand the indications for use of our Fetal Fibronectin Test for predicting successful induction of labor, for predicting delivery at term and for diagnostic applications in oncology, including bladder cancer.
OVERVIEW OF TARGETED MARKETS
Preterm birth
There are approximately four million births in the United States annually. Births occurring before 37 weeks of pregnancy are defined as preterm, and in recent years they have occurred on average at a rate of over 1,300 per day, or 480,000 per year. According to the Centers for Disease Control and Prevention, the percentage of preterm births in the United States grew to 12% of all births in 2002, an increase of 29% since 1981. This increase in the preterm birth rate is a growing public health concern. In January 2003, the March of Dimes launched a five-year, $75 million campaign to reduce the number of preterm births.
According to a 1994 publication cited by the March of Dimes, the cost of medical care for a complicated birth ranged between $20,000 and $400,000 from delivery to hospital discharge, while the average cost of a normal birth was $6,400. Infants born preterm often receive specialized care in a neonatal intensive care unit, or NICU, with charges ranging from approximately $800 to $2,700 per day in 1998. In addition, medical costs following discharge for preterm births can be substantial as a result of ongoing physical and mental developmental complications. We believe medical costs can be reduced if women at risk of preterm birth could be identified earlier and appropriately treated.
Preterm birth market segments
Women that are evaluated and potentially treated for preterm birth fall into three categories:
| 4 | Women with signs and symptoms of preterm labor— We believe that there are approximately 1 million episodes each year in the United States where women seek urgent medical care for signs and symptoms of preterm labor. Prior to an episode, these women were designated as either “high-risk” or “low-risk” for preterm births. Some of these signs and symptoms include uterine contractions, cervical dilation, vaginal infection, backache, pelvic pain, abdominal fullness or discomfort, change in vaginal discharge and vaginal bleeding. However, as these signs and symptoms are common throughout pregnancy, they do not provide a sufficient basis for making an accurate diagnosis of preterm labor and impending birth. Without a reliable method to assess the risk of preterm birth, the healthcare provider may not be able to make appropriate treatment decisions, such as whether to hospitalize the woman, prescribe medications to delay the onset of labor or accelerate fetal lung development, request expensive transport to an advanced NICU facility, or instruct women to remain home on bed rest and discontinue employment. If appropriate, these interventions can significantly increase the chance of infant survival. If healthcare providers could accurately identify women at risk, they could avoid many unnecessary interventions and their associated costs. |
| 4 | Women designated as “high-risk” for preterm birth— We believe that up to 1.2 million women in the United States annually may be designated as “high-risk” for preterm birth during their pregnancy. Risk factors include previous preterm birth, multiple gestation, uterine anomalies, gestational diabetes, hypertension, low pre-pregnancy weight, use of illicit drugs, sexually transmitted diseases, vaginal infections, smoking, consumption of alcohol and demographic factors such as low socioeconomic status, certain ages and races. In addition, some women may also be designated as “high-risk” later in pregnancy when evaluated using a vaginally inserted ultrasound probe to assess cervical length. This method requires specially trained medical personnel, and its accuracy is highly dependent on specific user technique. However, healthcare providers have had limited success in accurately determining the risk of preterm birth based on these risk factors and evaluations. As a result, there is a need for an easy-to-use, objective method to identify women at multiple times during pregnancy who are truly at high risk for preterm birth. |
| 4 | Women designated as “low-risk” for preterm birth— We believe that up to 2.8 million women annually in the United States with no known risk factors are designated as “low-risk.” However, “low-risk” women account for approximately 50% of all preterm births. We believe the ability to |
| accurately diagnose which of these women are truly at high risk for preterm birth is currently beyond the scope of traditional evaluation methods. Women who are inaccurately identified as “low-risk” are excluded from the potential benefits of existing interventions. If these women could be accurately identified as “high-risk” by periodic testing, their pregnancies potentially could be prolonged with appropriate medical treatment and lifestyle changes. These treatments could result in substantial medical benefits to the mother and infant, as well as significant cost savings. |
Current treatments and interventions for preterm birth
Identification of women at risk for preterm birth can be critical because the use of certain interventions to delay preterm birth may improve infant survival rates and reduce the severity of complications. Interventions may include hospitalization, consultation with a highly-skilled specialist, transport to a more advanced NICU facility, drug treatments such as tocolytics, which are used to delay the onset of birth by reducing contractions, corticosteroids for acceleration of fetal lung development, administration of progesterone to reduce the likelihood of preterm birth, antibiotics, and lifestyle modifications, including bed rest and discontinuing work.
There is ongoing research into the development of medications to further slow or prevent preterm births. For example, a multi-center, randomized, controlled, clinical trial was conducted by the National Institutes of Health and published in the New England Journal of Medicine in 2003 that used a progesterone formulation to prevent preterm birth in a group of “high-risk” women with a prior preterm birth. The study showed that in women with a prior preterm birth, there was a reduction in preterm birth by more than 33% in the treatment group as compared to a group treated with a placebo.
Infertility
According to the CDC National Center for Health Statistics, approximately 6.1 million women in the United States are affected by some form of infertility. It was estimated that approximately 1.2 million women had an infertility appointment in 1995 in the United States. We believe approximately 10% to 15% of infertile women are classified as suffering from unexplained infertility where extensive tests for known factors have failed to reveal a cause. Infertile women are candidates for assisted reproductive technologies, including IVF.
According to the American Society of Reproductive Medicine, the average cost of an IVF cycle is $12,400. Receptivity of the uterus to undergo implantation varies by patient, and we are unaware of any current methods by which to evaluate women on this basis. The ability to predict good candidates for IVF procedures would help prevent unnecessary and costly IVF cycles.
Induction of labor
Induction of labor is the process by which medications and other treatments are used to initiate labor and delivery. Of the estimated four million births in the United States in 2002, approximately 820,000 were induced. The percentage of induced labor more than doubled from approximately 9% in 1989 to approximately 21% in 2002. Induction of labor may be required for certain maternal or fetal conditions. In addition, we believe a number of inductions are elective in nature and performed for the convenience of the patient or healthcare provider. Healthcare providers have traditionally assessed women as candidates for successful induction of labor through the presence of certain clinical characteristics such as softness, dilation and thickness of the woman’s cervix. These clinical characteristics are not always reliable predictors of which women will be successfully induced. As a result, many women who are not good candidates for labor induction may endure prolonged dysfunctional induced labor with exposure to drugs such as cervical ripening agents or oxytocin and an elevated risk of cesarean section.
We believe the current use of subjective evaluation techniques to predict the successful induction of labor contributes to the annual cesarean section rate of 26% of all births in the United States in 2002. This is the highest rate ever reported in the United States and has risen 26% since 1996. Delivery by cesarean section typically results in costs of approximately $3,000 more than vaginal delivery based on our estimates. In addition, unsuccessful induction of labor may be associated with medical complications for both the mother and infant, as well as increased hospital stays and neonatal costs. In addition, women who undergo a cesarean section are often encouraged to continue to have cesarean sections in subsequent pregnancies. An objective diagnostic test to assist healthcare providers in predicting the successful induction of labor would help improve labor success rates and reduce unnecessary cesarean sections.
Oncology — bladder cancer
Bladder cancer is the fifth most common cancer in the United States. There are more than 55,000 new cases diagnosed annually and an existing patient population of approximately 500,000 cases. Following treatment, even patients initially diagnosed with superficial tumors must be monitored closely as two-thirds of these patients will experience a recurrence within 5 years and almost 90% will have a recurrence within 15 years. Current diagnostic tools and techniques include visual observation through cystoscopy, evaluation of potential cancer cells through cytology and assessment of tissue biopsies. There are several FDA-cleared tests for monitoring patients and a limited number of tests that have been approved by the FDA for use in screening for bladder cancer. However, these tests detect bladder cancer with varying degrees of success and are generally more successful in detecting more advanced cancers. We believe there is a market opportunity for a more accurate and reliable test to monitor and screen for bladder cancer at an early stage.
FETAL FIBRONECTIN OVERVIEW
Fetal fibronectin is a protein expressed in the fetal membranes and placenta at the interface between the mother and fetus. Fetal fibronectin is believed to play a role in the adhesion of the fetal membranes to the wall of the uterus. In a normal pregnancy, the level of fetal fibronectin in vaginal secretions is typically elevated through weeks 16 to 20 of gestation as the fetal membranes adhere to the uterine wall. From week 20 through week 35 of gestation, fetal fibronectin levels are typically low. As the pregnancy reaches term, the fetal fibronectin level rises significantly. Therefore, low levels of fetal fibronectin in vaginal secretions between week 20 and week 35 of gestation are highly correlated with a low risk of preterm birth, while high levels of fetal fibronectin during this time period indicate a greater risk of preterm birth. Testing for the presence or absence of fetal fibronectin enables healthcare providers to identify women at risk for preterm birth, and may also be useful in predicting the successful induction of labor.
Fetal fibronectin has also been shown to be present in certain forms of cancer. In oncology, fetal fibronectin is referred to as oncofetal fibronectin. The protein is expressed in several forms of cancer where its adhesive properties may help the cancer to attach to tissue and grow. Oncofetal fibronectin can potentially be detected in cancerous tissues or in fluids that come into contact with those tissues.
THE ADEZA SOLUTION
We believe that our proprietary, FDA-approved diagnostic test and instrument, the single-use, disposable Fetal Fibronectin Test and the TLiIQ System have the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women and to become a standard of care for use in pregnancy. The clinical efficacy of our fetal fibronectin test for preterm birth has been demonstrated in numerous large, multi-center peer-reviewed clinical publications. In addition, cost savings resulting from the use of our test have also been confirmed in peer-reviewed publications.
The Fetal Fibronectin Test and the TLiIQ System have the following key characteristics:
| 4 | Objective result—Instrument provides a positive or negative result; |
| 4 | Low-cost instrument—Minimal cost is incurred to acquire the instrument; |
| 4 | Rapid turnaround— Produces a result in less than 25 minutes; |
| 4 | Easy to use— Simple and convenient test procedure and instrument user interface; |
| 4 | Established reimbursement— Reimbursement provided by large US health plans; and |
| 4 | Significant cost savings opportunity—Reduces hospital admissions, eliminates unnecessary transports and avoids costly interventions. |
We market the Fetal Fibronectin Test to healthcare providers for women who are seeking urgent medical care for signs and symptoms of preterm labor in the hospital. While a woman is being evaluated, the Fetal Fibronectin Test is run in the hospital laboratory with the Companyresult generated in less than 25 minutes. A negative Fetal Fibronectin Test for women with signs and symptoms of preterm labor effectively rules out the chance of preterm birth in the next seven days, with a 99.5% probability, avoiding unnecessary hospitalization, medications, hospital transport, or bed rest. Although a positive Fetal Fibronectin Test result does not have the same predictive value as a negative test result, if the test result is positive, the healthcare provider may proactively prescribe various treatments to delay or manage preterm labor and birth.
We also market the Fetal Fibronectin Test to healthcare providers for women who have been designated as “high-risk.” These women should be carefully monitored throughout their pregnancy, and a Fetal Fibronectin Test can be used multiple times in their pregnancy during their frequent visits to their healthcare provider’s office. In those cases, fetal fibronectin samples are collected from women in healthcare providers’ offices and picked up for testing by a clinical laboratory. Results are typically returned to the healthcare provider within 24 hours. The use of the Fetal Fibronectin Test for this patient population helps to identify women who are not at risk of delivering preterm.
In addition, we believe that our product has the potential to be used in routine patient visits for women who have been designated as “low-risk” by their healthcare providers. Over 50% of all preterm births occur when no known risk factors are present. Our Fetal Fibronectin Test may provide an objective test for identifying additional women who are at risk of preterm birth and, when used in conjunction with traditional diagnostics such as ultrasound, could potentially enable early intervention.
In these “high-risk” and “low-risk” patient categories, a large, multi-center, peer reviewed clinical study comparing the most common predictors of preterm birth demonstrated that the Fetal Fibronectin Test was the single strongest predictor of preterm birth at less than 35 weeks of pregnancy.
A peer-reviewed, cost-benefit study published in 1999 addressed the potential impact of the Fetal Fibronectin Test in a hospital system. Data obtained from a year when the Fetal Fibronectin Test was utilized was compared to the prior year when the test was not available. Use of the Fetal Fibronectin Test resulted in a significant cost savings for the hospital system by reducing preterm labor hospital admissions, length of stay, and prescriptions for tocolytic agents. Preterm labor hospital admissions alone were decreased by approximately 40%.
We believe our potential aggregate market size is defined by the number of pregnant women who are potential candidates for the Fetal Fibronectin Test and the number of tests they may receive during their pregnancies. We believe women with signs and symptoms of preterm labor are candidates for one Fetal Fibronectin Test per episode, and women designated as “high-risk” may be tested multiple times during their pregnancy. We estimate that our product has a potential market size in these two patient populations of over $400 million annually. If we are able to expand the use of the Fetal Fibronectin Test for women designated as “low-risk” and for other uses, we estimate that the potential annual market size can be greater than $1 billion.
PRODUCTS
The following table summarizes information related to our principal products and certain products under development.
| Product | Use | Status | ||||
| Marketed products | ||||||
| Preterm birth | Fetal Fibronectin Test for the TLiIQSystem | Prediction of preterm birth 4 Women with signs and symptoms 4 Women at “high-risk” 4 Women at “low-risk” | Commercially available | |||
| Infertility | E-tegrity Test | Uterine receptivity | Commercially available | |||
| Products under development | ||||||
| Other pregnancy products | ||||||
| Induction of labor | Fetal Fibronectin Test for the TLiIQSystem | Successful induction of labor | FDA review pending | |||
| Preterm birth | SalEst Test | Prediction of preterm birth | In commercial development | |||
| Delivery date | Fetal Fibronectin Test SalEst Test | Prediction of delivery date | In development | |||
| Oncology products | ||||||
| Bladder cancer | oncofetal fibronectin test for the TLiIQSystem | Monitoring and screening | In development | |||
Marketed products
Preterm birth products
Our Fetal Fibronectin Test has been approved by the FDA for assessing the risk of preterm birth. We manufacture and market the patented single-use, disposable Fetal Fibronectin Test, which is performed on our patented instrument, the TLiIQ System. The Fetal Fibronectin Test cassette is sold directly to hospital and clinical laboratories that perform the test and provide results to healthcare providers.
TLiIQ System.The TLiIQ System consists of the TLiIQ instrument and printer. The TLiIQ instrument is small and compact, approximately eight inches long by seven inches wide by three inches high, and is composed of:
| 4 | A keypad which is used to enter patient and user identification information; |
| 4 | A display which provides simple on-screen commands to guide the user through the testing sequence; |
| 4 | A fiber-optic scanner that is contained inside the instrument, which creates a digitized image of the test result; and |
| 4 | Sophisticated technology that analyzes each Fetal Fibronectin Test cassette. |
The printer for the TLiIQ System generates a label with the patient test result.
Fetal Fibronectin Test cassette.The Fetal Fibronectin Test cassette is a single-use, dry chemistry cassette. All the necessary reagents for one test are contained within the cassette.
Test procedure.A healthcare provider collects a sample of the patient’s vaginal secretions using our Fetal Fibronectin Specimen Collection Kit. The Fetal Fibronectin Specimen Collection Kit contains a
Infertility products
E-tegrity Test.The E-tegrity Test is a patented diagnostic test designed to determine receptivity of the uterus to embryo implantation. The E-tegrity Test identifies the presence or absence of a unique protein, alpha-v beta-3 integrin, important for implantation to occur. This unique protein is the basis of our proprietary analyte specific reagent, or ASR, on which the E-tegrity Test is based. If a woman is missing the alpha-v beta-3 integrin protein between days 20 to 24 of her menstrual cycle, the fertilized egg may not attach properly to the epithelial lining of the uterus. As a result, a woman’s chances of a successful pregnancy are significantly decreased.
The E-tegrity Test provides healthcare providers with a new method for potentially explaining the cause of a woman’s infertility. Women who may be helped by the E-tegrity Test include women that are having difficulty getting pregnant by natural means, women who are considering assistive reproductive technologies, and women who have already tried IVF without success. A negative test provides a potential reason for the woman’s infertility, and the healthcare provider can then initiate appropriate treatments to potentially increase the chance for successful embryo implantation. We believe the E-tegrity Test can provide significant cost savings by potentially reducing the number of failed IVF cycles. Peer-reviewed publications have shown that women missing alpha-v beta-3 integrin can benefit from drug therapy that improves uterine receptivity for embryo implantation. We perform the E-tegrity Test exclusively in our CLIA-certified laboratory, Adeza Diagnostic Services.
Products under development
Pregnancy products
Induction of labor
Preterm birth
Delivery date
Oncology products
We are exploring applications for oncofetal fibronectin in oncology and have initially focused our efforts in the area of bladder cancer. In addition, we are developing and evaluating protocols for cervical and ovarian cancer detection studies.
Bladder cancer
OUR STRATEGY
Our goal is to be kept confidentiala leader in the development and not disclosedcommercialization of proprietary diagnostic products for reproductive healthcare. In addition, we intend to commercialize products for diagnostic applications in oncology. We believe that our products can assist healthcare providers in making more timely and accurate diagnoses, leading to improved quality of care and significant cost savings for the healthcare system. The key elements of our strategy are to maximize the value of our commercialized products and build for future growth by continuing to develop our product pipeline. In order to effectively execute this strategy we plan to:
Leverage existing sales and marketing infrastructure to increase market penetration
As of August 3, 2004, our existing sales force consisted of 66 direct sales representatives. We intend to leverage these representatives to increase market penetration of our TLiIQ System in hospital and clinical laboratories as well as to increase the number of Fetal Fibronectin Tests run on each TLIIQ System within our current customer base. Our direct sales representatives call on healthcare providers in the hospital and office setting, as well as on clinical laboratories and health plans, to broaden the overall awareness of our Fetal Fibronectin Test and its associated benefits. Our sales representatives use peer-reviewed publications, cost-benefit data, case studies and other marketing materials to promote the benefits of our Fetal Fibronectin Test and drive increased utilization. In addition, our marketing organization has implemented a national speakers program where healthcare providers, such as maternal fetal medicine specialists, obstetricians, nurses and midwives conduct educational presentations at hospitals, professional meetings and conferences to increase awareness of our Fetal Fibronectin Test.
Increase marketing for women designated as “high-risk”
We have expanded our target market for the Fetal Fibronectin Test to include women designated as “high-risk” for preterm birth by their healthcare provider. Of our 66 direct sales representatives, 47 are primarily focused on healthcare providers in the office setting to drive utilization of our Fetal Fibronectin Test for “high-risk” women. As we further penetrate the “high-risk” market, we plan to increase the number of direct sales representatives focused on healthcare providers serving this patient population. In addition to targeting healthcare providers, we are increasing our direct marketing efforts to women and health plans for this “high-risk” patient population. We provide specific patient-focused material for placement in healthcare provider offices. We also conduct presentations to medical directors and case managers of health plan organizations, exhibiting the benefits of utilizing our Fetal Fibronectin Test in this “high-risk” patient population.
Expand marketing to “low-risk” patient population
As we further penetrate the market for women with signs and symptoms of preterm birth and women designated as “high-risk,” we plan to expand our focus to market our Fetal Fibronectin Test for routine patient visits in “low-risk” women. By increasing market penetration and continuing to educate healthcare providers on the benefits of our Fetal Fibronectin Test, we believe we have the potential to penetrate the “low-risk” patient population for the assessment of the risk of preterm birth. Because approximately 50% of all preterm births occur when no known risk factors are present, there is a need to more accurately assess risk at multiple times during pregnancy. Our Fetal Fibronectin Test could be used to predict the likelihood of preterm birth during a woman’s routine visits to her healthcare provider. Our sales and marketing efforts will ultimately be focused on penetrating this “low-risk” patient population for routine patient visits.
Expand international sales
We are currently focused on the US market, but we intend to expand into additional geographic regions. We currently sell directly into Canada and have relationships with approximately 15 international distributors in areas such as Mexico, Europe, the Pacific Rim and South America. In Japan and South Korea, we have a strategic partnership with Daiichi Pure Chemicals Co. Ltd. We intend to increase our international distribution network through strategic partners and distributors that have significant presence and experience in their local markets.
Seek additional indications for our Fetal Fibronectin Test in pregnancy-related applications
In addition to the current indications for our Fetal Fibronectin Test, we have submitted a PMA supplement for FDA review and approval to expand the labeling of the Fetal Fibronectin Test to be used as a predictor of the successful induction of labor. Of the more than 820,000 births in the United States annually that are induced, we believe a number are elective in nature due to patient or healthcare provider convenience. Many women endure prolonged dysfunctional induced labor, increased hospital stays, elevated risk of cesarean section and increased neonatal costs. Our Fetal Fibronectin Test may provide an objective diagnostic test to assist healthcare providers in predicting the successful induction of labor. In addition, we are developing a product to more accurately predict delivery date in full-term pregnancies.
Develop products for applications in oncology
We are developing an oncofetal fibronectin test for the detection of bladder cancer. The results from two preliminary clinical studies in the United States and Germany suggest that bladder cancer can be detected in the urine of bladder cancer patients with our oncofetal fibronectin test. We recently initiated another study in Germany to expand on the earlier studies and to obtain further clinical data from a larger patient population. In addition, we are developing and evaluating protocols for cervical and ovarian cancer detection studies.
Strategically acquire or in-license complementary businesses, products or technologies
In addition to our own product development efforts, we intend to expand our product portfolio by selectively acquiring or in-licensing complementary businesses, products or technologies that could be sold by our existing direct sales force. For example, we acquired the exclusive rights to the SalEst Test in late 2003. We are continually identifying and evaluating new opportunities that would meet these criteria and further strengthen or expand our current programs.
SALES AND MARKETING
We focus our sales and marketing efforts on increasing awareness of our products and services among healthcare providers, hospitals, laboratories, health plans, and patients. Our strategy is to sell and market our products through our direct sales force in the United States and Canada, and distributors worldwide. We choose our partners and distributors on a country-by-country basis. As of August 3, 2004, we had 66 direct sales representatives in North America, including 15 who have a primary focus on hospital and laboratory sales, 47 who have a primary focus on healthcare providers in the office setting, three health plan sales representatives and one sales representative covering Canada. Our direct sales force includes some full-time sales representatives provided to us by a third parties,
exceptparty, who are directly managed by us, sell our products exclusively and are an integral part of our sales team.
US sales and marketing
Our hospital and clinical laboratory sales representatives focus primarily on selling the TLiIQ System and Fetal Fibronectin Test to hospital and clinical laboratories. Our healthcare provider sales representatives focus on the estimated 1,300 maternal fetal medicine specialists, who focus on high-risk pregnancies, 25,000 Ob/ Gyn physicians, as well as nurses and midwives. Our health plan sales representatives focus on chief medical officers, medical directors, and case managers of health plans.
We are focused on increasing healthcare provider, hospital, laboratory, health plan, and patient awareness of the Fetal Fibronectin Test and its associated benefits through our direct sales and marketing efforts. In our selling process, we use peer-reviewed publications, cost-benefit data and case studies. Peer-to-peer selling is also a critical element of our strategy. Our marketing organization has implemented a national speakers program where healthcare providers such as maternal fetal medicine specialists, obstetricians, nurses, and midwives make educational presentations at hospitals, professional meetings, and conferences to increase awareness of our Fetal Fibronectin Test. We also conduct presentations at health plan organizations to medical directors and case managers. Our marketing professionals support sales of our Fetal Fibronectin Test with product literature and training materials for healthcare providers, laboratories, and health plans. We also create awareness through specific patient-focused material for placement in healthcare provider offices and hospital labor and delivery units, as well as consumer publications.
We also sell our products through some of the leading national laboratories in the United States, including LabCorp, Quest Diagnostics and Specialty Laboratories.
International sales and marketing
Our international sales and marketing efforts address the particular healthcare systems of individual countries through a network of approximately 15 international distributors with expertise in their markets. Our internal marketing professionals support these distributors. We intend to expand our international marketing efforts by increasing our international direct sales force, as well as broadening our international distribution network through strategic partners and distributors that have significant presence and experience in their local markets. In Japan and South Korea, we have a strategic partnership with Daiichi Pure Chemicals Co. Ltd.
We also manufacture and market other Fetal Fibronectin Test formats that are sold in international markets and are designed to meet certain criteria specific circumstances.to these markets.
MANUFACTURING
We conduct a majority of the manufacturing for our TLiIQ System and Fetal Fibronectin Test cassettes at our 17,600 square foot manufacturing facility, located in Sunnyvale, California. This facility is subject to the current good manufacturing practices, or cGMP, enforced by the FDA. The agreements also generally providemanufacturing process for our products includes assembly, testing, packaging, labeling, component inspection and final inspection of products that have been manufactured by us or to our specifications
We are licensed by the individualState of California, Department of Health Services Food and Drug Branch and are also registered with the FDA as a device manufacturer.
We purchase components for our Fetal Fibronectin Test and TLiIQ System products from various suppliers. The components we purchase are generally available from more than one supplier. For those components for which there are relatively few alternate sources of supply, we believe that we could establish additional or replacement sources of supply in a timely manner to meet the requirements of our business.
Our quality assurance systems are required to be in conformance with the Quality System Regulations, or QSR, as mandated by the FDA. We have ISO 13485 certification for our quality system which is required in Canada. Our products are CE marked in accordance with the European In Vitro Diagnostic Directive.
RESEARCH AND DEVELOPMENT
Our research and development efforts are conducted internally and through collaborations with academic investigators and clinicians. Our research and development is focused on enhancements to existing products and developing additional products within women’s health, including pregnancy and infertility, and in oncology. As of August 3, 2004, we had 10 employees conducting research and development.
GOVERNMENT REGULATION
United States
The research, development, manufacture, labeling, distribution and marketing of our products are subject to extensive regulation by the Food and Drug Administration and other regulatory bodies. The FDA regulates our currently marketed products as medical devices and we are required to obtain review and clearance or approval from the FDA prior to commercializing our devices.
FDA’s premarket clearance and approval requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the courseUnited States will require either prior 510(k) clearance or prior premarket approval, or PMA, from the FDA. The FDA classifies medical devices into one of rendering servicesthree classes. Devices deemed to pose lower risk are placed in either Class I or II, which requires the manufacturer to submit to the Company shallFDA a premarket notification requesting permission for commercial distribution of a device that is substantially equivalent to a predicate device that has already received 510(k) clearance or was commercialized prior to May 28, 1976. This process is known as 510(k) clearance and was used for authorization of our Fetal Fibronectin Specimen Collection Kit. Some low-risk Class I devices are exempt from the 510(k) requirement altogether. Devices deemed by the FDA to pose greater risk, or devices deemed not substantially equivalent to a previously cleared 510(k) device are placed in Class III, most of which require premarket approval, such as our TLiIQ System. Both premarket clearance and premarket approval applications are subject to the payment of user fees, paid at the time of submission for FDA review.
510(k) clearance pathway
To obtain 510(k) clearance, a premarket notification must be submitted, demonstrating that the exclusive propertyproposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications. The FDA’s 510(k) clearance pathway usually takes
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new 510(k) clearance or could require premarket approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If the FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
Premarket approval pathway
A PMA application must be submitted if the device cannot be cleared through the 510(k) process. A PMA application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the Company. There can be no
assurance that proprietarydevice for its intended use.
After a PMA approval application is complete, the FDA begins an in-depth review of the submitted information, which generally takes between one and three years, but may take significantly longer. During this review period, the FDA may request additional information or confidentiality agreementsclarification of information already provided. For example, the FDA’s review of our PMA supplement seeking approval for use of our Fetal Fibronectin Test in predicting successful induction of labor is currently on hold while a third party we have engaged conducts an audit of all of our clinical study sites for protocol deviations and accuracy of the data. Upon completion of the third-party audit, we will need to submit new analyses of the data and a corrective action plan to the FDA before it will resume its review of the application.
Also during the PMA review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with employees, consultants and others will not be breached,quality system regulations. New PMA applications or PMA supplements are required for significant modifications to the manufacturing process, labeling or design of an approved device. PMA supplements often require submission of the same type of information as a PMA, except that the Company will
have adequate remediessupplement is limited to information needed to support any changes from the device covered by the original PMA, and may not require as extensive clinical data or the convening of an advisory panel, which makes recommendations to the FDA concerning the approval of a PMA.
In vitro diagnostic medical devices
In addition to our FDA-approved and cleared medical devices in distribution, we provide the E-tegrity Test as a test run in our own CLIA-certified laboratory, Adeza Diagnostic Services, for the detection of defects in uterine receptivity. This in-house test is permitted to utilize an analyte specific reagent, an ASR or active ingredient, the use of which has been validated in our clinical laboratory. While we receive specimens in our lab for the E-tegrity Test, the active ingredient of the test itself has not been validated outside of, and is not marketed outside of, our lab. Under the FDA’s requirements, E-tegrity Test results are provided with a disclaimer concerning the absence of an FDA requirement for clearance or approval of ASRs. We do not intend to seek FDA clearance or approval for broader use of the E-tegrity Test.
Continuing FDA regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
| 4 | quality system regulation, or QSR, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process, otherwise known as Good Manufacturing Practices or GMPs; |
| 4 | labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and |
| 4 | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any breach, or thatof the Company's trade secrets will
not otherwise become knownfollowing sanctions:
| 4 | fines, injunctions, and civil penalties; |
| 4 | recall or seizure of our products; |
| 4 | operating restrictions, partial suspension or total shutdown of production; |
| 4 | refusing our request for 510(k) clearance or premarket approval of new products; |
| 4 | withdrawing 510(k) clearance or premarket approvals that are already granted; and |
| 4 | criminal prosecution. |
We are also subject to or independently developedunannounced inspections by competitors. See
"Business--Certain License Agreements; Patentsthe FDA and Proprietary Technology."
GOVERNMENT REGULATION. The manufacturethe Food and saleDrug Branch of the California Department of Health Services.
International
International sales of medical devices are subject to extensive regulation by numerousforeign government authorities, both in the
United States and internationally. In the United States, the principal
regulatory authorities are the FDA and corresponding state agencies, such as
the California Department of Health Services ("CDHS"). The process of obtaining
and maintaining required regulatory clearances is lengthy, expensive and
uncertain. The FDA requires companies that desire to market a new medical
device or an existing medical device for use for a new indication to obtain
either a premarket notification clearance under Section 510(k) of the Federal
Food, Drug, and Cosmetic Act ("510(k)") or a PMA prior to the introduction of
such product into the market. In addition, material changes to medical devices
are also subject to FDA review and clearance or approval prior to marketing and
sale in the United States. Though generally believed to be a shorter, less
costly regulatory path than a PMA, the process of obtaining a 510(k) clearance
generally requires the submission of supporting data,regulations, which can be extensive
and extend the regulatory process for a considerable length of time. In
addition, the FDA may require review by an advisory panel as a condition for
510(k) clearances, which can further lengthen the regulatory process. The PMA
process can take several years from initial filing and requires the submission
of extensive supporting data and clinical information. No assurance can be
given that any future products or applications developed by the Company will
not require approval under the more lengthy and expensive PMA process or that
such approval will be received. If the Company is required to obtain approval
for any products pursuant to the PMA procedure or, if the 510(k) process with
respect to any products is extended for a considerable length of time, the
commencement of commercial sales of the Company's products will be delayedvary substantially or indefinitely.
Sales of medical devices outside of the United States are subject to
international regulatory requirements that vary from country to country. The time required to obtain approval for sale internationallyby a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ.
In Europe,
INTELLECTUAL PROPERTY
Protection of our intellectual property is a strategic priority for our business, and we rely on a combination of patent, trademark, copyright and trade secret laws to protect our interests. Our ability to protect and use our intellectual property rights in the Company will be requiredcontinued development and commercialization of our technologies and products, operate without infringing the proprietary rights of others, and prevent others from infringing our proprietary rights, is crucial to obtain the certifications necessary
to enable the CE mark, an international symbol of adherence to quality
assurance standards and compliance with applicable European Union Medical
Device Directives, to be
11
affixed to the Company's products in order to continue sales in member
countries of the European Union. The Company has not obtained such
certifications, and there can be no assurance that itour continued success. We will be able to do so in
a timely manner, if at all. Many countries in which the Company currently
operates or intends to operate either do not currently regulate medical devices
or have minimal registration requirements; however, these countries may develop
more extensive regulations in the future that could adversely affect the
Company's ability to market its products. In addition, significant costs and
requests by regulators for additional information may be encountered by the
Company in its efforts to obtain regulatory approvals. Any such events could
substantially delay or preclude the Company from marketing its products in the
United States or internationally.
Regulatory approvals, if granted, may include significant limitations on the
indicated uses for which the Company's products may be marketed. In addition,
in order for companies to obtain such approvals, the FDA and certain foreign
regulatory authorities impose numerous additional requirements with which
medical device manufacturers must comply. FDA enforcement policy strictly
prohibits the promotion of approved medical devices for uses other than those
specifically cleared for marketing by the FDA. The Company is required to
adhere to GMP regulations and similar regulations in other countries, which
include testing, control and documentation requirements. Ongoing compliance
with GMP and other applicable regulatory requirements will be monitored through
periodic inspections by federal and state agencies, including the FDA and the
CDHS, and by comparable agencies in other countries. Failure to comply with
applicable regulatory requirements could result in, among other things, warning
letters, fines, injunctions, civil penalties, recall or seizure of products,
total or partial suspension of production, refusal of the government to grant
premarket clearance or premarket approval for devices, withdrawal of previously
granted approvals and criminal prosecution. Changes in existing regulations or
adoption of new government regulations or policies could prevent or delay
regulatory approval of the Company's products.
While the Company has received a PMA after an expedited review for the use of
its fFN ELISA Test in assessing the likelihood of premature birth in
symptomatic women, there can be no assurance that the Company will be able to
obtain additional PMA approvals, that the Company will not be required to seek
510(k) clearances for certain products or indications or that necessary
clearances or approvals will be obtained. The Company is preparing a PMA
Supplement for the use of its fFN ELISA Test in screening asymptomatic women,
but there can be no assurance that this PMA Supplement will be filed or, if
filed, will be granted FDA approval. Moreover, regulatory clearances, if
granted, may include significant limitations on the indicated uses for which a
product may be marketed. Delays in receipt of or failure to receive such
approvals or clearances, the loss of previously obtained approvals or
clearances, or failure to comply with existing or future regulatory
requirements would have a material adverse effect on the Company's business,
financial condition and results of operations. See "Business--The Adeza
Solution for Premature and Late Birth/Successful Induction of Labor" and
"Business--Government Regulation."
As a provider of health care related services, the Company is subject to
extensive and frequently changing federal, state and local regulations
governing licensure, billing, financial relationships, conduct of operations,
cost-containment and other aspects of the Company's business relationships.
Federal and state certification and licensure programs establish standards for
the day-to-day operation of laboratories such as the Company's. Compliance with
such standards is verified by periodic inspections and requires participation
in proficiency testing programs. There can be no assurance that the Company's
laboratory facilities will pass all future inspections conducted to ensure
compliance with federal or any other applicable licensure or certification
laws. See "Business--Laboratory Services; Manufacturing."
COMPETITION. There is currently significant competition in the women's
reproductive health care market, which the Company expects to increase over
time. Currently, the fFN ELISA Test is the only FDA-approved immunodiagnostic
test for the assessment of the likelihood of premature birth for symptomatic
women. However, there can be no assurance that other, more effective diagnostic
tests for the assessment of the likelihood of premature birth will not receive
FDA approval in the near future. Other companies and institutions with
substantially greater financial, manufacturing, marketing, distribution and
technical resources than the Company are engaged in the research and
development of products similar to those currently being developed or
12
commercialized by the Company. Some or all of these products may compete
directly with the Company'sprotect our products and other companies and institutions may
choose to enter this market at a later date. Although none of these companies
currently concentrates exclusively on products for the women's reproductive
health care market, there can be no assurance that these or other companies or
institutions will not succeed in developing products or procedures that are
more effective than the Company's or that would render the Company's
technology or products obsolete or uncompetitive. The Company believes that
important competitive factors with respect to the development and
commercialization of its products include the relative speed with which it can
develop products, establish clinical utility, complete the clinical testing
and regulatory approval process, obtain reimbursement and supply commercial
quantities of the product to the market. The Company's inability to compete
favorably with respect to any of these factors could have a material adverse
effect on its business, financial condition and results of operations.
Additionally, Matria and the Company's other strategic partners compete with
other companies that distribute products related to the women's reproductive
health care market to physicians, hospitals and other health care providers,
and there can be no assurance that Matria or such other strategic partners
will be able to compete favorably with these companies. The Company also
competes with other companies for clinical sites to conduct trials. There can
be no assurance that the Company will be able to compete successfully or that
competition will not have a material adverse effect on the Company's business,
financial condition and results of operations. See "Business--Competition."
RISK OF LIABILITY; ADEQUACY OF INSURANCE COVERAGE. The medical device and
biotechnology industries have historically been litigious, and the Company
faces an inherent business risk of financial exposure to product liability
claims. Inherent in the manufacturing and distribution of the Company's
products is the risk of financial exposure to product liability claims in the
event that thetechnologies from unauthorized use of its products results in personal injury. Although the
Company has not experienced any claims to date, there can be no assurance that
the Company will not experience losses due to product liability claims in the
future. The marketing and sale of health care services through the Company's
laboratory could expose the Company to the risk of certain types of
litigation. Damages assessed in connection with, and the costs of defending,
any legal action could be substantial. Although the Company is presently
covered by general liability insurance in the amount of $5.0 million per
occurrence and $5.0 million in the aggregate, there can be no assurance that
insurance coverage will provide sufficient funds to satisfy judgments which,
in the future, may be entered against the Company or that liability insurance
in such amounts will be available or affordable in the future. In addition,
there can be no assurance that all of the activities encompassed within the
Company's business are covered under the Company's policies. The Company may
require increased product liability coverage as its products are
commercialized and as it provides increased laboratory services. Such
insurance is expensive, difficult to obtain and may not be available in the
future on acceptable terms, or at all. Furthermore, there can be no assurance
that the Company will have sufficient resources to satisfy any liability or
litigation expenses that may result from any uninsured or underinsured claims.
Any claims or series of claims against the Company, regardless of their merit
or eventual outcome, could have a material adverse effect on the Company's
business, financial condition and results of operations. See "Business--
Laboratory Services; Manufacturing."
UNCERTAINTY RELATED TO HEALTH CARE REFORM. Political, economic and
regulatory influences are subjecting the health care industry in the United
States to fundamental change. The Company anticipates that Congress, state
legislatures and the private sector will continue to review and assess
alternative health care delivery and payment systems. Potential approaches
that have been considered include mandated basic health care benefits,
controls on health care spending through limitations on the growth of private
health insurance premiums and Medicare and Medicaid spending, the creation of
large insurance purchasing groups, price controls and other fundamental
changes to the health care delivery system. Legislative debate is expected to
continue in the future, and market forces are expected to demand reduced
costs. The Company cannot predict what impact the adoption of any federal or
state health care reform measures, future private sector reform or market
forces may have on its business, financial condition or results of operations.
See "Business--Third-Party Reimbursement."
RISKS ASSOCIATED WITH INTERNATIONAL SALES. In fiscal 1993, 1994 and 1995 and
for the three months ended March 31, 1996, international sales accounted for
approximately 100%, 45%, 31% and 100%, respectively, of the Company's total
revenues. A number of risks are inherent in international operations and
transactions.
13
International sales and operations may be limited or disrupted by the
imposition of government controls, export license requirements, political
instability, trade restrictions, changes in tariffs and difficulties in
staffing, coordinating and managing international operations. Additionally,
the Company's business, financial condition and results of operations may be
adversely affected by fluctuations in international currency exchange rates as
well as constraints on the Company's ability to maintain or increase prices.
The international nature of the Company's business subjects it and its
representatives, agents and distributors to laws and regulations of the
foreign jurisdictions in which they operate or the Company's products are
sold. The regulation of medical devices in a number of such jurisdictions,
particularly in the European Union, continues to develop and there can be no
assurance that new laws or regulations, or new interpretations of existing
laws and regulations, will not have a material adverse effect on the Company's
business, financial condition and results of operations. In addition, the laws
of certain foreign countries do not protect the Company's intellectual
property rights to the same extent as do the laws of the United States. See
"Business--Government Regulation" and "Business--Certain License Agreements;
Patents and Proprietary Technology." There can be no assurance that the
Company will be able to successfully further commercialize its current
products or successfully commercialize any future products in any
international market. See "Business--Sales & Marketing; Strategic Corporate
Alliances."
POSSIBLE FUTURE CAPITAL REQUIREMENTS. The Company's capital requirements
depend upon numerous factors, including market acceptance of the fFN ELISA
Test and other products, the progress of the Company's clinical research and
product development programs, the receipt of and time required to obtain
regulatory clearances and approvals, the resources the Company devotes to
developing, manufacturing and marketing its products and other factors. The
timing and amount of such capital requirements cannot accurately be predicted.
There can be no assurance that the Company will not require additional
funding, or that such additional funding, if needed, will be available on
terms acceptable to the Company, or at all. Insufficient capital may require
the Company to delay, scaleback or eliminate certain of its research and
development programs or to attempt to license to third parties the rights to
commercialize products or technologies that the Company itself would otherwise
undertake. See "Use of Proceeds" and "Management's Discussion and Analysis of
Financial Condition and Results of Operations--Liquidity and Capital
Resources."
DEPENDENCE ON QUALIFIED PERSONNEL. The Company is highly dependent upon the
efforts of its senior management and scientific team. The loss of the services
of one or more of these individuals could impede the achievement of its
development objectives. Because of the specialized scientific nature of the
Company's business, Adeza is also highly dependent upon its ability to
continue to attract and retain qualified scientific and technical personnel.
There is intense competition for qualified personnel in the areas of the
Company's activities, and there can be no assurance that Adeza will be able to
continue to attract and retain the qualified personnel necessary for the
development of its business. Loss of the services of, or failure to recruit,
key scientific and technical personnel would be significantly detrimental to
the Company's product development programs. See "Management--Executive
Officers and Directors."
NO PRIOR PUBLIC MARKET; POSSIBLE VOLATILITY OF STOCK PRICE. Prior to this
offering, there has been no public market for the Common Stock. Accordingly,
there can be no assurance that an active trading market for the Common Stock
will develop or be sustained upon completion of this offering. The initial
public offering price of the Common Stock will be determined by negotiations
between the Company and the representatives of the Underwriters. The factors
to be considered in making such determination will include the prevailing
market conditions, the Company's financial and operating history and
condition, its prospects and the prospects for its industry in general, the
management of the Company and the market price of securities for companies in
businesses similar to that of the Company. The securities markets have, from
time to time, experienced significant price and volume fluctuations that may
be unrelated to the operating performance of particular companies. These
fluctuations often substantially affect the market price of a company's common
stock. The market prices for securities of medical device companies have in
the past been, and can in the future be expected to be, especially volatile.
The market price of the Common Stock may be subject to volatility from quarter
to quarter depending upon announcements regarding the results of regulatory
approval filings, clinical studies or other testing, technological innovations
or new commercial products by the Company or its competitors,
14
government regulations, developments or disputes concerning proprietary
rights, changes in reimbursement levels, public concern as to the safety of
products developed by the Company or others, changes in health care policy in
the United States and internationally, the issuance of new or changed stock
market analyst reports and recommendations and economic and other external
factors, as well as continued operating losses by the Company and fluctuations
in the Company's financial results. These factors could have a material
adverse effect on the Company's business, financial condition and results of
operations and may not be indicative of the prices that may prevail in the
public market. See "Underwriting" and "Management's Discussion and Analysis of
Financial Condition and Results of Operations."
CONTROL BY EXISTING STOCKHOLDERS. Following the completion of this offering,
officers and directors of the Company, together with entities affiliated with
them, will beneficially own approximately 38.5% of the Common Stock of the
Company (approximately 35.5% if the Underwriters' over-allotment options are
exercised in full). These stockholders, acting as a group, will continue to be
able to control the election of all members of the Company's Board of
Directors and to determine all corporate actions after the sale of the shares
offered hereby. The voting power of these stockholders could also have the
effect of delaying or preventing a change in control of the Company. See
"Principal and Selling Stockholders" and "Description of Capital Stock."
SHARES ELIGIBLE FOR FUTURE SALE. Upon completion of this offering and based
on the shares outstanding as of April 30, 1996, the Company will have a total
of 7,863,669 shares of Common Stock outstanding, assuming no exercise of
outstanding warrants for 404,468 shares of Common Stock immediately prior to
the closing of this offering and no exercise of options after April 30, 1996.
Of these shares, the 2,500,000 shares offered hereby (2,875,000 shares if the
Underwriters' over-allotment options are exercised in full) will be freely
tradable without restriction or registration under the Securities Act by
persons other than "affiliates" of the Company, as defined under the
Securities Act. The remaining 5,363,669 shares of Common Stock outstanding are
"restricted shares" as that term is defined by Rule 144 as promulgated under
the Securities Act ("Restricted Shares"). Sales of Restricted Shares in the
public market, or the availability of such shares for sale, could adversely
affect the market price of the Common Stock. All directors and executive
officers and certain other stockholders of the Company, holding in the
aggregate substantially all of the shares of Common Stock outstanding prior to
this offering, have agreed with the Underwriters not to sell or otherwise
dispose of any shares of Common Stock for a period of 180 days from the date
of this Prospectus (the "Lock-up Period"), or as earlier determined by
Prudential Securities Incorporated, on behalf of the Underwriters, without the
prior written consent of Prudential Securities Incorporated, on behalf of the
Underwriters. The Company is continuing to seek 180 day lock-up agreements
from certain of its current stockholders and option holders who have not yet
entered into such agreements. The number of shares of Common Stock available
for sale in the public market is further limited by restrictions under the
Securities Act.
Because of the restrictions noted above, beginning 180 days after the
effective date of this offering, 5,334,300 Restricted Shares will be eligible
for sale in the public market subject to Rule 144 and Rule 701 of the
Securities Act. On December 21, 1996, 16,626 shares held by stockholders will
become eligible for sale in the public market pursuant to Rule 144 upon
expiration of a two-year holding period from the date such shares were fully
paid. An additional 12,743 shares held by stockholders will become eligible
for sale in the public market 90 days after the effective date of this
offering pursuant to Rule 701.
In addition, holders of 5,203,465 shares of Common Stock and the holders of
warrants to purchase 136,274 shares of Common Stock may require the Company to
register their shares of Common Stock under the Securities Act, which would
permit such holders to resell a certain amount of their shares without
complying with Rule 144. Registration and sale of such shares could have an
adverse effect on the trading price of the Common Stock. See "Description of
Capital Stock--Registration Rights of Certain Holders."
As of May 9, 1996, options to purchase a total of 579,270 shares of Common
Stock pursuant to the Option Plan were outstanding with a weighted average
exercise price of $1.21 per share, all of which were exercisable as of April
30, 1996 and 237,855 of which were fully vested as of such date. An additional
662,025 shares of
15
Common Stock were available for future option grants under the 1995 Stock
Option and Restricted Stock Plan. 579,270 shares subject to options held by
officers, directors, certain other employees and former employees are subject
to lock-up agreements. See "Management--Stock Option and Incentive Plans,"
"Shares Eligible for Future Sale," "Underwriting" and Notes 5 and 9 to
Consolidated Financial Statements.
Rule 701 under the Securities Act provides that, beginning 90 days after the
date of this Prospectus, shares of Common Stock acquired on the exercise of
outstanding options may be resold by persons other than affiliates subject only to the manner of sale provisions of Rule 144,extent that they are covered by valid and by affiliates subject
to all provisions of Rule 144 except its two-year minimum holding period. The
Company intends to file oneenforceable patents, trademarks or more registration statements on Form S-8 under
the Securities Act to register shares of Common Stock subject to stock
options.
Prior to this offering, there has been no public marketcopyrights, or are effectively maintained as trade secrets, know-how or other proprietary information.
We seek US and international patent protection for the Common Stock
of the Company, and no predictions can be made of the effect, if any, that the
sale or availability for shares of additional Common Stock will have on the
trading price of the Common Stock. Nevertheless, sales of substantial amounts
of such shares in the public market, or the perception that such sales could
occur, could adversely affect the trading price of the Common Stock and could
impair the Company's future ability to raise capital through an offering of
its equity securities. See "Shares Eligible for Future Sale" and "Description
of Capital Stock."
EFFECT OF CERTAIN CHARTER AND BYLAW PROVISIONS. Certain provisions of the
Company's Certificate of Incorporation and Bylaws may have the effect of
making it more difficult for a third party to acquire, or of discouraging a
third party from attempting to acquire, control of the Company. Such
provisions could limit the price that certain investors might be willing to
pay in the future for shares of the Common Stock. Certain of these provisions
allow the Company to issue preferred stock without any vote or further action
by the stockholders, provide for a classified board of directors, eliminate
the right of stockholders to act by written consent without a meeting and
eliminate cumulative voting in the election of directors. These provisions may
make it more difficult for stockholders to take certain corporate actions and
could have the effect of delaying or preventing a change in control of the
Company. Certain provisions of Delaware law applicable to the Company could
also delay or make more difficult a merger, tender offer or proxy contest
involving the Company, including Section 203 of the Delaware General
Corporation Law, which prohibits a Delaware corporation from engaging in any
business combination with any interested stockholder for a period of three
years unless certain conditions are met. See "Management" and "Description of
Capital Stock."
ABSENCE OF DIVIDENDS. The Company has not declared or paid dividends on its
Common Stock since its inception and does not anticipate declaring or paying
cash dividends to its stockholders in the foreseeable future. See "Dividend
Policy."
DILUTION. Purchasers of the Common Stock offered hereby will experience
immediate and substantial dilution in the net tangible book value per share of
Common Stock from the initial offering price set forth on the cover of this
Prospectus. See "Dilution." A substantial dilution will occur upon exercise of
outstanding options to purchase Common Stock.
16
THE COMPANY
The Company was founded and incorporated in California in 1985 as "Aspen
Diagnostics Corporation." The Company has operated as "Adeza Biomedical
Corporation" since November 1989 and will reincorporate in Delaware prior to
the effective date of this offering. In December 1989, the Company merged with
Yellowstone Diagnostics Corporation, a health care diagnostics company. The
Company's principal executive offices are located at 1240 Elko Drive,
Sunnyvale, California, 94089 and its telephone number is (408) 745-0975. As
used in this Prospectus, and unless the context requires otherwise, the terms
"Adeza" and the "Company" refer to Adeza Biomedical Corporation, a Delaware
corporation, its California predecessor and its subsidiary.
USE OF PROCEEDS
The net proceeds to the Company from the sale of the 2,500,000 shares of
Common Stock offered hereby assuming an initial public offering price of
$12.00 per share, the mid-point of the price range set forth on the cover of
this Prospectus (after deducting underwriting discounts and commissions and
estimated offering expenses), are estimated to be approximately $27,150,000.
The Company currently intends to use approximately $6.0 million of the net
proceeds from this offering to fund research and development, approximately
$5.0 million to expand the Company's marketing and sales activities,
approximately $4.0 million to fund clinical trials, approximately $500,000 to
repay outstanding indebtedness to certain of the Company's stockholders that
may be incurred under the Company's $2.0 million line of credit from certain
existing investors and the balance for working capitalour reagents, collection kits, diagnostic systems and other general
corporate purposes. These amounts are estimates,components of our products, and the amountall other commercially important technologies we develop.
We devote significant resources to obtaining, enforcing and timing of
the expenditures of the net proceeds for these purposes will depend on
numerous factors, including market acceptance of the Company's fFN ELISA Testdefending patents and other products, the status of the Company's product development efforts,
the results of clinical trials, the regulatory approval process, relationships
with strategic corporate partnersintellectual property and distributors, competition and
manufacturing activities. The Company may also use a portion of the net
proceeds to acquireprotecting our other proprietary information. We have already obtained patents or invest in businesses, products and technologies that
are complementary to those of the Company, although no such acquisitions or
investments are planned or being negotiated as of the date of this Prospectus,
and no portion of the net proceeds has been allocated for any specific
acquisition or investment. Pending such uses, the Company intends to invest
the net proceeds from this offering in short-term, government securities and
other investment-grade, interest-bearing securities.
If the Underwriters' over-allotment options are exercised, the Company will
not receive any of the proceeds from the sale of shares of Common Stock by the
Selling Stockholders. See "Principal and Selling Stockholders."
DIVIDEND POLICY
The Company has never paid cash dividends on its capital stock and does not
anticipate paying cash dividends in the foreseeable future. Any future
determination to pay cash dividends will be at the discretion of the Board of
Directors and will be dependent upon the Company's financial condition,
results of operations, capital requirements and such other factors as the
Board of Directors deems relevant.
17
CAPITALIZATION
The following table sets forth the capitalization of the Company as of March
31, 1996 (i) on a pro forma basis to reflect the automatic conversion of all
outstanding shares of the Company's Series 1 and Series 2 Preferred Stock into
Common Stock and the filing of the Company's Restated Certificate of
Incorporation to authorize 22,462,220 shares of Common Stock at a par value of
$0.001 per share and 10,000,000 shares of preferred stock at a par value of
$0.001 per share prior to the effectiveness of this offering and (ii) on a pro
forma as adjusted basis to reflect the sale of the 2,500,000 shares of Common
Stock offered hereby at an assumed initial public offering price of $12.00 per
share after deducting underwriting discounts and commissions and estimated
offering expenses and the application of the estimated net proceeds therefrom.
This table should be read in conjunction with "Management's Discussion and
Analysis of Financial Condition and Results of Operations" and the
Consolidated Financial Statements and related Notes thereto included elsewhere
in this Prospectus.
MARCH 31, 1996
-------------------------
PRO FORMA AS ADJUSTED(1)
--------- --------------
(IN THOUSANDS)
Capital lease obligations, net of current portion..... $ 36 $ 36
Stockholders' equity:
Preferred stock, $0.001 par value; issuable in
series; 10,000,000 shares authorized, no shares
issued and outstanding, pro forma and as adjusted.. -- --
Common Stock, $0.001 par value; 22,462,220 shares
authorized, 5,363,669 and 7,863,669 shares issued
and outstanding pro forma and as adjusted,
respectively....................................... 5 8
Additional paid-in capital.......................... 24,455 51,602
Deferred compensation............................... (64) (64)
Deficit accumulated during development stage........ (23,727) (23,727)
-------- --------
Total stockholders' equity........................ 669 27,819
-------- --------
Total capitalization.............................. $ 705 $ 27,855
======== ========
- --------
(1) Excludes (i) 579,270 shares of Common Stock issuable upon exercise of
stock options outstanding as of May 9, 1996 with a weighted average
exercise price of $1.21 per share, (ii) 404,468 shares of Common Stock
issuable upon exercise of warrants outstanding at May 9, 1996 with a
weighted average exercise price of $2.74 per share, (iii) 662,025 shares
reserved for future issuance as of May 9, 1996 under the Company's 1995
Stock Option and Restricted Stock Plan, (iv) 250,000 shares reserved for
future issuance as of May 9, 1996 under the Company's 1996 Employee Stock
Purchase Plan and (v) 200,000 shares reserved for future issuance as of
May 9, 1996 under the Company's 1996 Directors' Stock Option Plan. See
"Management--Stock Option and Incentive Plans" and "Description of Capital
Stock."
18
DILUTION
Purchasers of the Common Stock offered hereby will experience an immediate
and substantial dilution in the net tangible book value of their Common Stock
from the assumed initial public offering price. The pro forma net tangible
book value of the Company at March 31, 1996 was approximately $669,000, or
$0.12 per share. "Net tangible book value" per share is equal to net tangible
assets (tangible assets of the Company less total liabilities) divided by the
number of shares of Common Stock outstanding. Net tangible book value dilution
per share represents the difference between the amount per share paid by
purchasers of Common Stock in this offering and the pro forma net tangible
book value per share of Common Stock immediately after the completion of this
offering. After giving effect to the sale of the 2,500,000 shares of Common
Stock offered hereby at an assumed initial public offering price of $12.00 per
share, after deducting underwriting discounts and commissions and estimated
offering expenses and the application of net proceeds therefrom, the pro forma
net tangible book value of the Company as of March 31, 1996 would have been
$27,819,000 or $3.54 per share. This represents an immediate increase in pro
forma net tangible book value of $3.42 per share to existing stockholders and
an immediate dilution in net tangible book value of $8.46 per share to new
investors purchasing shares of Common Stock in this offering. The following
table illustrates this per share dilution as of March 31, 1996:
Assumed initial public offering price.......................... $12.00
Pro forma net tangible book value before this offering....... $0.12
Increase per share attributable to new investors............. 3.42
-----
Adjusted pro forma net tangible book value after this offer-
ing........................................................... 3.54
------
Dilution in net tangible book value to new investors........... $ 8.46
======
The following table sets forth, on a pro forma basis as of March 31, 1996
after giving effect to the conversion of all outstanding shares of Series 1
and Series 2 Preferred Stock into Common Stock, the difference between the
existing stockholders and the purchasers of shares in this offering at an
assumed initial public offering price of $12.00 per share (before deducting
underwriting discounts and commissions and estimated offering expenses) with
respect to the number of shares purchased from the Company, the total cash
consideration paid and the average price per share paid:
SHARES PURCHASED TOTAL CASH CONSIDERATION
----------------- ----------------------------AVERAGE PRICE
NUMBER PERCENT AMOUNT PERCENT PER SHARE
--------- ------- --------------- -------------------------
Existing stockholders
(1).................... 5,363,669 68.2% $ 24,855,000 45.3% $ 4.63
New stockholders (1).... 2,500,000 31.8 30,000,000 54.7 12.00
--------- ----- --------------- ---------
Total................. 7,863,669 100.0% $54,855,000 100.0%
========= ===== =============== =========
- --------
(1) In the event the Underwriters' over-allotment options are exercised in
full, the number of shares held by existing stockholders will be reduced
to 4,988,669 shares, or 63.4% of the total number of shares outstanding
after this offering, and the number of shares held by new investors will
increase to 2,875,000 shares, or 36.6% of the total number of shares
outstanding after this offering. See "Principal and Selling Stockholders."
The foregoing tables assume no exercise of outstanding options or warrants.
As of May 9, 1996, there were 579,270 shares of Common Stock issuable upon
exercise of stock options outstanding under the Company's 1995 Stock Option
and Restricted Stock Plan with a weighted average exercise price of $1.21 per
share and 404,468 shares of Common Stock issuable upon exercise of warrants
outstanding with a weighted average exercise price of $2.74 per share. In
addition, as of May 9, 1996, 662,025 shares of Common Stock reserved for
future issuance under the Company's 1995 Stock Option and Restricted Stock
Plan, 250,000 shares of Common Stock reserved for future issuance under the
Company's 1996 Employee Stock Purchase Plan and 200,000 shares of Common Stock
reserved for future issuance under the Company's 1996 Directors' Stock Option
Plan. If all of the outstanding options and warrants were exercised for Common
Stock, the dilution per share to new investors would be $8.65 per share. See
"Management--Stock Option and Incentive Plans" and Notes 5 and 9 of Notes to
Consolidated Financial Statements.
19
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data is qualified by reference
to and should be read in conjunction with "Management's Discussion and
Analysis of Financial Condition and Results of Operations" and the
Consolidated Financial Statements and Notes thereto included elsewhere in this
Prospectus. The consolidated statements of operations data set forth below
with respect to each of the three years in the period ended December 31, 1995
and the consolidated balance sheet data at December 31, 1994 and 1995 are
derived from, and are qualified by reference to, the Consolidated Financial
Statements which have been audited by Ernst & Young LLP, independent auditors,
included elsewhere in this Prospectus. The consolidated statements of
operations data for the years ended December 31, 1991 and 1992 and the
consolidated balance sheet data at December 31, 1991, 1992 and 1993 are
derived from the Company's audited consolidated financial statements not
included in this Prospectus. The consolidated statements of operations data
for the three months ended March 31, 1995 and 1996 and the consolidated
balance sheet data at March 31, 1996 have been derived from the unaudited
consolidated financial statements also appearing elsewhere in this Prospectus
which have been prepared on the same basis as the audited consolidated
financial statements and, in the opinion of management, contain all
adjustments, consisting only of normal recurring adjustments, necessary for a
fair presentation of the results of operations for such periods. The results
of operations for the three months ended March 31, 1996 are not necessarily
indicative of results to be expected for the full year or for any subsequent
period.
PERIOD FROM
INCEPTION THREE MONTHS
(JANUARY 1985) ENDED
YEAR ENDED DECEMBER 31, TO MARCH 31,
------------------------------------------ DECEMBER 31, -----------------
1991 1992 1993 1994 1995 1995 1995 1996
------- ------- ------- ------- ------- -------------- -------- -------
CONSOLIDATED STATEMENTS
OF OPERATIONS DATA: (IN THOUSANDS, EXCEPT PER SHARE DATA)
Revenues:
Contract revenues...... $ 5,299 $ 3,486 $ 967 $ 1,977 $ 3,416 $ 15,300 $ 167 $ --
Product sales.......... 155 432 504 316 541 1,948 99 152
------- ------- ------- ------- ------- -------- -------- -------
Total revenues.......... 5,454 3,918 1,471 2,293 3,957 17,248 266 152
Operating costs and ex-
penses:
Research and develop-
ment, including
manufacturing start-up
costs and costs of
product sales (1)..... 3,311 5,161 4,320 3,635 3,460 25,550 785 830
Selling, general and
administrative........ 2,011 2,819 3,128 1,928 1,725 14,682 371 418
------- ------- ------- ------- ------- -------- -------- -------
Total operating costs
and expenses.......... 5,322 7,980 7,448 5,563 5,185 40,232 1,156 1,248
------- ------- ------- ------- ------- -------- -------- -------
Income (loss) from oper-
ations................. 132 (4,062) (5,977) (3,270) (1,228) (22,984) (890) (1,096)
Interest income (ex-
pense) and other, net.. 67 57 (132) (390) 84 334 16 19
------- ------- ------- ------- ------- -------- -------- -------
Net income (loss)....... $ 199 $(4,005) $(6,109) $(3,660) $(1,144) $(22,650) $ (874) $(1,077)
======= ======= ======= ======= ======= ======== ======== =======
Pro forma net loss per
share(2)............... $ (0.21) $ (0.16) $ (0.20)
======= ======== =======
Shares used in computing
pro forma net loss per
share (2).............. 5,446 5,446 5,448
DECEMBER 31, MARCH 31,
------------------------------------------------ ---------
1991 1992 1993 1994 1995 1996
-------- -------- -------- -------- -------- ---------
(IN THOUSANDS)
CONSOLIDATED BALANCE
SHEET DATA:
Working capital (defi-
cit)................... $ 4,838 $ 1,387 $ (1,638) $ 2,318 $ 1,273 $ 235
Total assets............ 6,603 3,370 2,666 5,175 2,923 1,860
Convertible notes pay-
able to related par-
ties, including accrued
interest............... -- -- 2,262 -- -- --
Capital lease obliga-
tions, net of current
portion................ 398 372 344 144 49 36
Deferred liability-
noncurrent............. 750 750 750 -- -- --
Deficit accumulated dur-
ing the development
stage.................. (7,732) (11,737) (17,846) (21,506) (22,650) (23,727)
Total stockholders' eq-
uity (deficit)......... 4,575 1,408 (1,755) 2,873 1,740 669
- --------
(1) Included in research and development expenses for the period from
inception (January 1985) to December 31, 1995 is approximately $553,000
related to the purchase of in-process research and development.
(2) See Note 1 of Notes to Consolidated Financial Statements for information
regarding the computation of pro forma net loss per share.
20
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion and analysis contains certain forward-looking statements
relating to future events or the future financial performance of the Company.
Such statements are only predictions and the actual events or results may
differ materially from the results discussed in the forward-looking
statements. Factors that could cause or contribute to such differences
include, but are not limited to, those discussed in "Risk Factors" as well as
those discussed elsewhere in this Prospectus. The historical results set forth
in this discussion and analysis are not necessarily indicative of trends with
respect to any actual or projected future financial performance of the
Company. This discussion and analysis should be read in conjunction with the
Consolidated Financial Statements and the related Notes thereto included
elsewhere in this Prospectus.
OVERVIEW
Adeza was founded in January 1985 and, since its inception, has been a
development stage company primarily involved in research and development
activities for the women's reproductive health care market. From inception
through 1992, the Company focused its efforts on developing an alternative to
amniocentesis and diagnostic devices for pregnancy-related and female
reproductive disorders. Since current senior management joined the Company in
the second half of 1992, Adeza has primarily focused on the development and
marketing of proprietary tests for the diagnosis of its targeted pregnancy-
related and female reproductive disorders, including premature and late birth,
preeclampsia, endometriosis and infertility. Adeza currently relies on its
strategic partners and distributors for the sale, marketing and distribution
of its products to physicians, hospitals, other health care providers and
third-party payors. The Company, together with its strategic partners and
distributors, intends to educate its target market as to the clinical
usefulness and cost effectiveness of its products through a variety of means,
including marketing evaluations, seminars, workshops, publications and
professional and trade meetings.
In September 1995, the Company received an expedited PMA from the FDA to
market its fFN ELISA Test in the United States as an aid in assessing the
likelihood of premature birth in symptomatic women. Prior to receiving its
PMA, the Company's sales and marketing efforts were focused on selected
international markets for the purpose of gathering relevant market data and
increasing product awareness. The fFN ELISA Test will be marketed in the
United States, Canada and Puerto Rico through Matria, the Company's strategic
partner. The Company anticipates that Matria will introduce the fFN ELISA Test
in the United States through full-scale marketing efforts commencing in the
second half of 1996. The Company currently expects that it will derive a
substantial amount of its United States revenues for the forseeable future
from sales of its fFN ELISA Test and fFN Dipstick Test through Matria. The
Company anticipates that international sales will continue to represent a
significant portion of its total revenues in the future and intends to expand
its network of international distributors. In general, the Company intends to
recognize revenues upon the shipment of products to Matria or upon the
completion of the laboratory services conducted by the Company. Adeza may
receive additional revenues upon collection of revenues by Matria from its
paying customers. The Company recognizes revenues from international sales
upon shipment of the product to a strategic partner or distributor.
Approximately $15.3 million of the Company's aggregate total revenues to
date have consisted of contract revenues received from its strategic partners,
primarily Matria and Daiichi, for research and product development. In the
future, the Company does not expect to generate further material research and
development revenues from these agreements with its strategic partners. The
Company has also generated approximately $2.1 million since inception through
March 31, 1996 from international product sales. Future revenues and results
of operations may fluctuate significantly from quarter to quarter and will
depend upon, among other factors, actions relating to regulatory and
reimbursement matters, the extent to which Matria is successful in achieving
market acceptance of the Company's products, the rate at which the Company
expands its international distribution network, the progress of clinical
trials and the introduction of competitive products for diagnosis of the
Company's targeted pregnancy-related and female reproductive disorders.
21
The Company has incurred cumulative net losses of $23.7 million through
March 31, 1996 in the course of its development activities. Losses have
resulted principally from research and development activities, clinical
trials, marketing and product introduction expenses and from general and
administrative costs. The Company expects to incur additional operating losses
at least through 1997. The Company's ability to achieve profitability is
dependentfiled patent applications on a number of factors,our technologies, including acceptanceimportant patents and patent applications relating to fetal fibronectin, our TLiIQ System, and the Fetal Fibronectin Test. If valid and enforceable, these patents may give us a means of blocking competitors from using similar or alternative technology to compete directly with our products. We also have certain proprietary trade secrets that are not patentable or for which we have chosen to maintain secrecy rather than file for
We believe that our portfolio of issued patents and patent applications, together with our exclusively licensed patents described under “License Agreements”, provides patent coverage for our proprietary technologies and products. As of August 3, 2004, our intellectual property estate consisted of 159 issued patents or patent applications, as follows:
| 4 | 28 issued US patents; |
| 4 | 8 US non-provisional patent applications; |
| 4 | 6 US provisional patent applications; |
| 4 | 102 issued foreign patents; |
| 4 | 14 foreign patent applications that are in various national stages of prosecution; and |
| 4 | 1 foreign patent application not at the national stage. |
We solely own all of the Company'spatents and patent applications set forth above.
LICENSE AGREEMENTS
While we own much of our intellectual property, including patents, patent applications, trademarks, copyrights, trade secrets, know-how and proprietary information, we also license related technology of importance to commercialization of our products. To continue developing and commercializing our current and future products, by physicians, hospitals, other health care providers and third-party
payors, its abilitywe may license intellectual property from commercial or academic entities to successfully commercialize the fFN ELISA Test, the
ability of the Company's strategic partners to successfully market and
distribute its products, its ability to obtain reimbursement from third-party
payors for the use of its products and its ability to develop and obtain
patent protection and regulatory approval for its products.
RESULTS OF OPERATIONS
REVENUES
Contract Revenues. Contract revenues increased from $967,000 in 1993 to $2.0
million in 1994 and $3.4 million in 1995, and were $167,000 for the three
months ended March 31, 1995. The Company received no contract revenues in the
three months ended March 31, 1996. Contract revenues were primarily earned as
research and development milestone payments under the Company's strategic
agreements with Matria and Daiichi. The Company does not expect additional
material contract revenues under these strategic agreements, as the research
and development phase of these agreements has been completed.
Product Sales. Product sales decreased from $504,000 in 1993 to $316,000 in
1994 and increased to $541,000 in 1995, and also increased from $99,000 in the
three months ended March 31, 1995 to $152,000 in the comparable 1996 period.
The year-to-year fluctuations were primarily the result of the timing of
inventory orders from international distributors. The increase from three-
month period to three-month period was primarily the result of increased
product sales through Daiichi. The Company expects that Matria will launch
full-scale marketing efforts for the fFN ELISA Test in the United States in
the second half of 1996 and also expects to expand its international
distribution network. There can be no assurance, however, that the Company and
Matria will be successful in their efforts to introduce the fFN ELISA Test in
the United States or that the Company's efforts to expand its international
distribution network will be successful. Additionally, there can be no
assurance that the fFN ELISA Test will be accepted as clinically useful or
cost effective by physicians, hospitals, other health care providers and
third-party payors.
COSTS AND EXPENSES
Research and Development. Research and development expenses consist of costs
related to product development, clinical trials, start-up manufacturing and
product sales. Future manufacturing start-up costs and costs of product sales
will be accounted for separately as costs of goods sold following the full-
scale commercial introduction of the fFN ELISA Test. The Company's research
and development expenses decreased from $4.3 million in 1993 to $3.6 million
in 1994 and $3.5 million in 1995 and increased from $785,000 in the three
months ended March 31, 1995 to $830,000 in the comparable 1996 three-month
period. The decreases from year-to-year were primarily the result of the
Company's cost containment efforts, which were achieved through reduced
personnel costs, partially offset by increased costs of products sold, outside
clinical trial costs and support of third-party research efforts. The increase
from three-month period to three-month period was primarily attributable to an
increase in the cost of products sold and clinical and regulatory expenses,
partially offset by a decrease in research and development expenses. However,
the Company expects that research and development expenses will increase in
future periods, as the sponsorship by the Company of third-party research and
development efforts increase and the Company pursues its research and
development of complementary diagnostic products. In addition, the Company
expects to report a non-cash charge of approximately $250,000 in the second
quarter of 1996 in connection with the issuance of a warrant to the Hutchinson
Center. See Note 9 to Notes to Consolidated Financial Statements.
Selling, General and Administrative. Selling, general and administrative
expenses decreased from $3.1 million in 1993 to $1.9 million in 1994 and $1.7
million in 1995, and increased from $371,000 for the three
22
months ended March 31, 1995 to $418,000 for the comparable 1996 period. The
decreases from year-to-year were primarily the result of the Company's efforts
to reduce overall operating expenses by reducing management and staff
personnel. The increase from three-month period to three-month period was
primarily attributable to an increase in patent and general corporate legal
expenses. The Company expects that selling, general and administrative
expenses will increase in future periods as the Company increases its sales
and marketing efforts in connection with the commercial introduction of the
fFN ELISA Test in the United States, expansion of its international
distribution network and increased responsibilities as a public company.
Interest Income (Expense) and Other, Net. Interest income (expense) and
other, net changed from $(132,000) in 1993 to $(390,000) in 1994, primarily
reflecting higher outstanding debt balances, and was $84,000 in 1995,
primarily reflecting lower outstanding debt balances. Interest income
(expense) and other, net increased from $16,000 for the three months ended
March 31, 1995 to $19,000 for the comparable 1996 three-month period,
primarily reflecting lower outstanding debt balances.
Income Taxes. At December 31, 1995, the Company had available net operating
loss carryforwards for federal and state tax purposes of approximately $18.8
million and $5.1 million, respectively. The federal net operating loss
carryforwards will expire at various dates beginning in 2000 through 2010, if
not utilized, and the state net operating loss carryforwards will expire in
1996 through 2000, if not utilized. Utilization of the net operating losses
and credits will be subject to a substantial annual limitation due to the
ownership change provisions of the Internal Revenue Code of 1986, as amended,
and similar state provisions. Under its current operating plan, the Company
expects that such limitations may result in the expiration of combined federal
and state net operating loss carryforwards and credits of approximately $18.7
million prior to their utilization.
LIQUIDITY AND CAPITAL RESOURCES
The Company has financed its operations to date primarily through issuances
of equity securities, from payments under agreements with strategic partners
and, to a lesser extent, cash receipts from product sales. Since inception,
the Company raised $23.9 million in aggregate net proceeds from issuances of
equity securities and received an additional $15.3 million in payments from
strategic partners. Further, in April 1996 the Company entered into a $2.0
million line of credit with certain of its existing investors. Amounts
borrowed by the Company under this line of credit will bear simple interest at
an annual rate equal to the applicable Internal Revenue Service imputed rate
in effect at the time such amount is borrowed and will become due and payable
upon the earlier of 30 days following the completion of this offering or May
1997.
The Company's net loss from inception through March 31, 1996 of $23.7
million exceeds the net cash used in operations of $21.1 million for the same
period primarily due to non-cash charges of $553,000 for the purchase of in-
process research and development and $1.5 million for depreciation and
amortization, as well as, to a lesser extent, increases in accounts payable
and accrued liabilities. The Company has also invested approximately $1.2
million in capital equipment, facilities improvements and other long-term
assets. The level of future capital expenditures will be dependent upon
available capital, and the Company currently has no commitments for material
capital expenditures.
At March 31, 1996, the Company's cash and cash equivalents were
approximately $1.0 million. The Company anticipates that its existing capital
resources, including the $2.0 million credit line obtained from certain
existing investors in April 1996 and the anticipated net proceeds from this
offering, will be adequate to fund its planned operations through 1997.
However, the Company expects to incur substantial additional operating costs
related to research and clinical trials, manufacturing start-up, development
of reference laboratory facilities and the expansion of its sales and
marketing activities. The Company's capital requirements will also depend upon
numerous factors, including market acceptance of the fFN ELISA Test and other
products, the progress of the Company's clinical research and product
development programs, the receipt of and time required to obtain regulatory
clearances and approvals, the resources the Company devotes to developing,
manufacturing and marketing its products and other factors. The timing and
amount of such capital requirements cannot be accurately predicted. There can
be no assurance that the Company will not require additional funding, or that
such additional funding, if needed, will be available on terms attractive to
the Company, or at all. Insufficient capital may require the Company to delay,
scaleback or eliminate certain of its research and product development
programs or to attempt to license to third parties the rights to commercialize
productstechnology that may be complementary to, or technologies that the Company itself would otherwise undertake.
23
BUSINESS
THE COMPANY
Adeza Biomedical Corporation ("Adeza" or the "Company") develops and markets
diagnostic products and servicesrequired for, women's reproductive health care. The
Company's primary focus is theour research, development and marketing of proprietary tests
for the diagnosis of pregnancy-related and female reproductive disorders,
including premature and late birth, preeclampsia, endometriosis and
infertility. The Company believes that its products and services will result
in improved patient management,commercialization activities.
For example, under an agreement with a consequent reduction in both patient
risk and overall costs. The Company, in conjunction with its strategic
partners and distributors, intends to market its products to obstetrical and
gynecological ("Ob/Gyn") physicians and third-party payors, who represent a
large and increasingly important part of the health care economy.
The Company has received an expedited premarket approval ("PMA") from the
Food and Drug Administration ("FDA") to begin marketing its enzyme-linked
immunosorbent assay ("ELISA") diagnostic (the "fFN ELISA Test") for use in
women with symptoms of premature birth. The fFN ELISA Test is the only FDA-
approved immunodiagnostic test for this disorder and represents a significant
advance over currently used evaluation techniques. This test measures the
presence of fetal fibronectin ("fFN") in the vaginal fluid of pregnant women
in order to assess the likelihood of premature birth. The Company believes
that its fFN-based products have the potential to become an element of
standard prenatal care. The Company will distribute the fFN ELISA Test in the
United States through an exclusive strategic distribution arrangement with
Matria Healthcare, Inc. ("Matria"), a leading women's health care company.
Matria began shipment of fFN ELISA Tests to a select number of initial
customers in the United States in April 1996 and is expected to commence full-
scale marketing efforts in the second half of 1996. The Company has been
selling the fFN ELISA Test in Japan since 1995 through an exclusive
arrangement with Daiichi Pure Chemicals Co. Ltd. ("Daiichi") and in Europe
through a limited number of distributors.
In addition to the fFN ELISA Test, the Company is developing several other
products for the women's reproductive health care market. The Company is
preparing supplements to its PMA ("PMA Supplements") for the use of the fFN
ELISA Test in assessing the likelihood of premature birth in asymptomatic
women, and for the use of a point-of-care rapid assay (the "fFN Dipstick
Test") for assessing the likelihood of premature birth in symptomatic women.
Clinical trials have been completed by the National Institutes of Health (the
"NIH") which support the use of the fFN ELISA Test for asymptomatic women, and
the Company is conductingmajor clinical trials for the use of the fFN Dipstick Test
in symptomatic women. Additionally, the Company is in the process of designing
clinical trials for the use of the fFN Dipstick Test in assessing the
likelihood of successful induction of labor at term. The Company has also
developed a proprietary fFN vertical flow membrane test (the "fFN Vertical
Flow Test") for the assessment of premature rupture of amniotic membranes
("ROM"), which has been introduced for sale in Japan. Accurate diagnosis of
suspected premature ROM is related to the diagnosis of premature birth and is
important for the prevention of potentially serious neonatal infection. In
addition, the Company has developed a proprietary diagnostic test based on
cellular fibronectin (the "cFN Test") for the pregnancy-related disorder of
preeclampsia, a leading causes of maternal death and serious fetal
complications in the United States. The cFN Test is currently undergoing
preclinical evaluation in the United States, Europe and Australia. The Company
also maintains a product development program with the goal of introducing
additional proprietary diagnostics and therapeutics for the women's
reproductive health care market.
24
THE ADEZA STRATEGY
Adeza's goal is to become a global leader in the diagnosis and treatment of
pregnancy-related and female reproductive disorders by designing, developing
and marketing proprietary diagnostic tests and services for the women's
reproductive health care market. Adeza's strategy is to:
. Penetrate the Market for Premature Birth Diagnostics. The Company's
strategy is to have its fFN-based products become an element of standard
prenatal care. The Company is focusing its initial marketing efforts on
sales of the fFN ELISA Test for symptomatic women through Matria to
physicians, hospitals, other health care providers and third-party
payors. The Company believes this target market will recognize the
significant benefits in patient health and reduced costs arising from
use of the fFN ELISA Test as a diagnostic for premature birth. In order
to further facilitate market penetration, the Company will also provide
a laboratory service to supply fFN ELISA Test results. The Company
believes that other commercial laboratories will provide the same
service as market penetration of the fFN ELISA Test increases.
. Leverage Expertise in fFN-based Diagnostics. The Company intends to
expand the market for its fFN-based diagnostics to include testing of
asymptomatic women for the likelihood of premature birth and testing for
the likelihood of successful induction at term. In addition, the Company
plans to introduce faster and more convenient formats for its fFN-based
products, as represented by the fFN Dipstick Test.
. Market to Health Care Providers and Third-Party Payors. The Company
intends to increase acceptance and create long-term demand by marketing
the cost effectiveness and improved patient care that its products and
services provide to physicians, hospitals, other health care providers
and third-party payors. The Company, together with its strategic
partners and distributors, intends to educate this target market through
a variety of means, including marketing evaluations, seminars,
workshops, publications and professional and trade meetings.
. Penetrate International Market. The Company may introduce new products
into selected international markets for the purpose of gathering
relevant market data and increasing market awareness. The Company
intends to expand its international distribution network through
agreements with strategic partners and distributors that have
significant presence and experience in the international women's health
care market.
. Continue Product Innovation. Through a focused research and development
program, including joint efforts with various collaborators, the Company
is developing additional products for the diagnosis of premature and
late birth, preeclampsia, endometriosis and infertility. With the goal
of becoming a comprehensive provider, the Company and its collaborators
are also researching a variety of complementary therapeutic approaches
to these targeted disorders. See "--Research and Development."
PREGNANCY-RELATED DISORDERS
The women's reproductive health care market represents a large and
increasingly important part of the health care economy, with an estimated $20
billion being spent annually in the United States on Ob/Gyn care. Obstetrical
care accounts for one in every eight hospital admissions in the United States
at an annual cost of over $10 billion. Obstetrical and neonatal tests result
in annual expenditures on laboratory services in the United States of
approximately $480 million. More than half of all women in the United States
of reproductive age visit their Ob/Gyn for primary health care. The Company
believes that the social and economic cost of inadequate prenatal care and
problem pregnancies has created a significant market opportunity for the
Company's products, and accordingly has targeted the pregnancy-related
disorders of premature and late birth and preeclampsia.
25
PREMATURE BIRTH AND LATE BIRTH/SUCCESSFUL INDUCTION AT TERM
Premature Birth
Premature birth, defined as delivery between 20 and 37 weeks of gestation,
is a major public health problem in the United States and the leading cause of
death among newly born babies. Of the estimated four million births in the
United States annually, approximately 10% occur prematurely. However, babies
born prematurely often have potentially fatal complications affecting the
cardiovascular, respiratory, digestive and central nervous systems that are
directly related to the premature birth. Even if not fatal, these problems are
often associated with long-term physical and developmental disabilities and
mental retardation.
Approximately $4.7 billion is spent annually in the United States for the
initial medical care of premature babies and their mothers. Compared to the
$10,000 average cost of a term birth, initial medical care for a premature
baby averages $20,000 and frequently exceeds $30,000. Over 20,000 premature
babies die annually during the neonatal period, and another 300,000 babies,
most of which are premature, require intensive care in one of the
approximately 600 neonatal intensive care units in the United States. For
babies born prior to 28 weeks of gestation or weighing less than two pounds
(1,000 grams), initial medical care averages $77,000. In addition, continuing
medical costs for significantly premature babies can be substantial as a
result of the physical and mental developmental complications that often arise
from premature birth.
With respect to the likelihood of premature birth, pregnant women can
generally be divided into two groups: symptomatic, defined as the presence of
symptoms indicating the possibility of premature birth and asymptomatic,
defined as the absence of symptoms indicating the possibility of premature
birth. The challenge for physicians and other health care providers is to be
able to accurately and reliably distinguish women who are actually undergoing
preterm labor from those with "false" labor symptoms. The Company estimates
that approximately 47% of symptomatic women who receive treatment for
premature birth may not need such treatment and that 50% of all asymptomatic
women who deliver prematurely do not otherwise fall into definable risk
categories. Objective assessment of the risk of premature birth in women with
symptoms would allow timely and effective patient management. In addition,
accurate diagnosis of suspected premature ROM is related to the diagnosis of
premature birth, and is important for the prevention of potentially serious
infections of the fetus. At least 20% of all women who experience premature
birth have also had premature ROM.
Symptomatic Women. In the United States, approximately 25% of all pregnant
women seek unscheduled medical care for symptoms of premature birth, resulting
in an estimated one million patient visits each year. Such symptoms may
include uterine contractions, backache, pelvic pain, abdominal fullness or
discomfort, change in vaginal discharge and/or vaginal bleeding. However,
these symptoms are not reliable predictors of premature birth because they
commonly occur in both premature and term births, and the Company believes
that other evaluation techniques that physicians currently use are not
sufficiently reliable to make an accurate diagnosis. Lacking an accurate
diagnostic test, physicians currently evaluate the amniotic membranes for
rupture, the level of uterine activity and the state of the cervix. However,
once the amniotic membranes have ruptured, premature birth is virtually
assured. Similarly, once detectable cervical change occurs, the onset of labor
and imminent delivery of the baby are virtually unavoidable, regardless of the
therapeutic intervention employed. Uterine contractions may be early
indicators of premature birth, but are often actually harmless contractions
that commonly occur in the last half of term pregnancies.
Asymptomatic Women. Diagnosis of the likelihood of premature delivery among
asymptomatic pregnant women currently depends on the evaluation of known risk
factors, such as multiple fetus pregnancies (e.g. twins), poor prenatal care
or previous premature birth. However, these risk factors have limited
usefulness because approximately 50% of women who deliver prematurely are
pregnant for the first time or have no identifiable risk factors. Even when
accurately identified, these risk factors (with the exception of multiple
fetus pregnancies) are not reliable predictors of premature birth.
26
Late Birth/Successful Induction at Term
Late births are those delivered after 42 weeks of gestation. Approximately
5% of the four million babies born in the United States annually are delivered
late. Late birth significantly increases the medical risk for both the mother
and baby and, if intervention is required, the overall cost of pregnancy and
delivery. The primary risk for late babies is asphyxiation. As a fetus grows
in the relatively confined space of the uterus, there is increased likelihood
of blood flow disruption resulting from umbilical cord compression. When blood
flow through the umbilical cord is compromised, the fetus may lose its source
of oxygen and face death or significant disability if not immediately
delivered, usually by emergency cesarean section. In addition, women with
post-term pregnancies are more likely to develop preeclampsia and therefore be
required to deliver their babies via cesarean section, which increases risk to
the mother and results in costs of approximately $3,000 more than a term
vaginal delivery. In order to limit the risks and costs associated with late
birth, physicians typically seek to induce labor at term. The significant
risks and costs associated with late birth and reasons of social convenience
have led to an increasing reliance on induced labor, which may account for as
many as 25% of all term deliveries.
Physicians induce labor through the application of preparations containing
prostaglandin E2 to the vagina to prepare the cervix for delivery and use
intravenous administration of pitocin to increase the number and force of
uterine contractions. Induction is often accompanied by protracted labor
resulting from the uterus and cervix not being prepared to deliver the baby.
There currently exists no reliable diagnostic to test whether the
physiological preparatory changes necessary for a successful induction have
occurred. Physicians currently assess subjective characteristics of the cervix
to determine preparedness. The Company believes that this subjective
determination does not allow practitioners reliably to determine whether or
not induction of labor will be successful and may contribute to the high
annual cesarean rate (24% of all births) in the United States.
27
THE ADEZA SOLUTION FOR PREMATURE BIRTH AND LATE BIRTH/SUCCESSFUL INDUCTION OF
LABOR
The Company believes that its current products and its near- and long-term
product candidates will address the need for objective diagnostic tests for
premature birth and late birth. The following table sets forth the Company's
current and near-term products for these disorders.
CURRENT AND NEAR-TERM FFN-BASED PRODUCTS FOR PREMATURE AND LATE BIRTH(/1/)
DISORDER/PRODUCT INDICATION STATUS CURRENT APPROACH
- -----------------------------------------------------------------------------------------------------------------------------------
PREMATURE BIRTH
fFN ELISA Test Laboratory assay to Expedited PMA for use Symptomatic women are
assess likelihood of of test in symptomatic typically held for
premature birth to be women received in observation and are
used in symptomatic 1995. Commercial in- often medicated and
or asymptomatic wom- troduction in Europe monitored for
en. in 1992, Japan in cervical change and
1995 and for symptom- contractions. No
atic women antici- immunodiagnostic test
pated in the United to assess likelihood
States in the second of premature birth
half of 1996. PMA has received FDA
Supplement for use of approval.
test in asymptomatic
woman being prepared
for planned submis-
sion in late 1996.
fFN Dipstick Test Rapid point-of-care FDA concordance trial Symptomatic women are
dipstick analyzed by in progress and PMA typically held for
practitioner on-site Supplement being observa-
to assess likelihood prepared for use of tion and are often
of premature birth. test in symptomatic medicated and moni-
women. tored for cervical
change and contrac-
tions. No
immunodiagnostic test
to assess likelihood of
premature birth has
received FDA approval.
Premature Rupture of
Amniotic Membranes
fFN Vertical Flow Multi-step membrane Commercial Pregnant women with
Test(/2/) test analyzed by introduction in Japan suspected ruptured
practitioner on-site in 1995. No current membranes are
to assess premature plans to introduce in assessed for excess
rupture of amniotic the United States. fluid in the vagina,
membranes. a vaginal secretion
slide test is
performed and vaginal
fluid acidity is
tested.
LATE BIRTH
fFN Dipstick Test Rapid point-of-care Clinical trials Pregnant women beyond
dipstick analyzed by currently being expected delivery
practitioner on-site designed to support a date have labor
to assess likelihood PMA Supplement. induced and/or
of successful cesarean sections. No
induction at or after immunodiagnostic test
term. to assess likelihood
of successful
induction at term has
received FDA
approval.
(1) The preceding table and other portions of this Business section, including
without limitation "The Adeza Solution for Premature and Late
Birth/Successful Induction of Labor," "The Adeza Solution for
Preeclampsia" and "The Adeza Solution for Reproductive Disorders," contain
forward-looking statements which involve risks and uncertainties with
respect to the products the Company currently intends to develop. Actual
results may differ significantly from those discussed in the forward-
looking statements. Factors that might cause such a difference include,
but are not limited to, those discussed in "Risk Factors."
(2) The fFN Vertical Flow Test for the premature rupture of amniotic membranes
has been specifically developed for sale and marketing in Japan. The
Company is in the early stages of developing a product for detection of
suspected ROM in a more cost-effective, rapid point-of-care format which
it intends to introduce in the United States.
28
The Company believes that its fFN-based products provide the first objective
and convenient immunodiagnostic tests for assessing the likelihood of
premature birth and late birth/successful induction of labor at term. As
indicated in the diagram below, fFN is a placental protein that is a component
of the placental membranes at the maternal/fetal interface.
NORMAL PREGNANCY
[DRAWING OF FETUS IN UTERUS]
The level of fFN in vaginal secretions is closely correlated to the onset of
labor and subsequent delivery. Although fFN is not the cause of labor and
delivery, its presence in vaginal secretions signals the weakening or
compromise of placental membranes and the probable onset of labor. Results of
the Company's clinical trials indicate that fFN testing will enable physicians
to objectively identify women at risk for preterm labor within the next seven
to 14 days. The Company believes that this will allow appropriate patient
care, resulting in reduced risk to the mother and baby and significantly lower
overall costs to the health care system.
The following chart demonstrates the levels of fFN normally present in
vaginal secretions of pregnant women at different gestational ages. High
levels of fFN between gestational ages of 24 weeks and 34 weeks are associated
with an increased risk of premature birth. By measuring the levels of fFN in
vaginal secretions during such period, physicians are able to better assess
the likelihood of premature birth.
VAGINAL fFN LEVEL AT EACH GESTATIONAL AGE
[CHART OF FFN LEVELS]
29
The Company has developed, or is developing, a number of tests based on the
detection of fFN as an indicator for premature birth and late birth/successful
induction of labor at term.
fFN ELISA Test
The Company's initial fFN diagnostic product is the fFN ELISA Test that
measures the presence of fFN in vaginal fluid. The fFN ELISA Test consists of
a specimen collection kit and a two-hour microtitre plate kit that will be
used by laboratories and hospitals to provide results on the specimens
collected. The Company believes that the fFN ELISA Test is the first
immunodiagnostic test that will allow accurate predictive assessment of the
risk of premature birth among both symptomatic and asymptomatic pregnant
women. The Company believes that the fFN ELISA Test's predictive ability will
enable physicians to more accurately select the appropriate course of
treatment. If the test is negative, the physician may decide to treat less
aggressively and, for example, not admit a symptomatic woman into a health
care facility for observation. If the test is positive, the physician may
choose to take steps to delay the onset of labor and perhaps initiate
treatment to aid the baby's lung development. The Company believes that early
recognition of women at increased risk leads to improved patient management
via more intense surveillance and selection of appropriate treatments, thereby
improving patient outcome and reducing costs. The fFN ELISA Test has received
an expedited PMA for marketing in the United States as an aid in assessing the
risk of premature birth in symptomatic women. This test is currently being
sold in portions of Europe through a limited number of distributors and in the
Pacific Rim and Japan through Daiichi, and has been approved and assigned
reimbursement points by the Japanese Ministry of Health and Welfare for
assessing the likelihood of premature birth. The Company's strategic partner,
Matria, will market the fFN ELISA Test for use by hospitals, other health care
providers, and eventually commercial reference laboratories, in the United
States, Canada and Puerto Rico. Each fFN ELISA Test will yield up to 44
results. Matria will also market the Company's laboratory service to hospitals
and physicians not equipped to use the fFN ELISA Test to provide results on
the specimen samples they have taken. See "Business--Laboratory Services;
Manufacturing."
Symptomatic Women. The fFN ELISA Test has received a PMA from the FDA for
use in assessing the likelihood of premature birth in symptomatic women
between 24 and 34 weeks of gestation. In clinical trials, symptomatic women
with a negative fFN ELISA Test result had less than a 1% (1-in-150) chance of
delivering within the 14-day period following sample collection. The Company
believes that this high negative correlation in symptomatic women will
significantly reduce costs by allowing physicians to treat some symptomatic
women more appropriately, such as on an outpatient basis, rather than through
observation and hospitalization, as is now often the case. In addition,
symptomatic women who test positive for the presence of fFN between 24 and 34
weeks of gestationcenter, we have a one in six chance of giving birth within a 14-day
period following administration of the test. The Company believes that this
will result in the proper diagnosis of women who will benefit from proper
patient management. For example, an NIH consensus panel indicates that proper
treatment, such as administration of corticosteroids will result in reduced
risk of respiratory complications for the baby as well as initial cost savings
estimated at more than $3,000 per treated baby born prematurely.
Asymptomatic Women. Based on results from the recently concluded Prematurity
Prediction Study, a multi-center clinical trial conducted by the Maternal
Fetal Medicine Unit of the NIH involving approximately 3,000 patients at 10
sites, the Company is in the process of preparing a PMA Supplement for use of
the fFN ELISA Test in the assessment of the likelihood of premature birth in
asymptomatic women. The results of this trial are expected to be submitted to
the FDA in late 1996, although there can be no assurance that a PMA Supplement
will be submitted at that time or at all. Results of this study indicate that
fFN is a meaningful predictor of premature birth of those asymptomatic women
evaluated between 24 and 30 weeks gestation. The study found that asymptomatic
women at 24 weeks gestation with a negative fFN ELISA Test result had less
than 1% (1-in-200) chance of delivering within the 28-day period following
sample collection. The study concluded that women who tested positive for the
presence of fFN at 24 weeks gestation were 59 times more likely to give birth
before 28 weeks of gestation, when infant morbidity and mortality is most
significant, than women with negative test results at such gestational age.
30
fFN Dipstick Test
Premature Birth. The fFN Dipstick Test uses the same core technology as the
fFN ELISA Test, but is a convenient, rapid assay designed to allow a physician
or other health care practitioner to test the pregnant woman on-site and have
a result in approximately 10 minutes, without the need for further processing.
The fFN Dipstick Test is currently being evaluated in an FDA concordance trial
that compares the fFN Dipstick Test to the fFN ELISA Test in symptomatic women
in the United States. The Company believes that use of its fFN Dipstick Test
will allow physicians to make more timely treatment decisions for symptomatic
women, particularly those women with an increased risk of premature birth. In
addition, the Company believes that the ease of use of the dipstick format and
the immediate availability of results will broaden the use of the fFN Dipstick
Test to include the physicians who do not have access to laboratories that are
equipped to analyze the fFN ELISA Test.
Late Birth/Successful Induction of Labor. The Company believes that the fFN
Dipstick Test may provide the first objective and convenient test to identify
women who are candidates for successful induction of labor at term. Based on
the results of clinical trials in three countries involving over 250 patients,
the Company believes that vaginal expression of fFN is associated with
successful induction of labor at term among women with cervices that are not
prepared for the process of labor as assessed using the current subjective
evaluation techniques. These studies indicated that women with a positive fFN
test have a shorter duration of induced labor, require less drug
administration and have a significantly lower cesarean section rate than
similar women with a negative fFN test result. The Company believes that more
accurate selection of patients for induction will decrease maternal and fetal
complications associated with failed induction and decrease the cesarean
section rate which, as a result, will decrease costs. The Company is in the
process of designing a multi-center clinical trial to expand the use of the
fFN Dipstick to include the assessment of the likelihood of successful
induction of labor at term.
fFN Vertical Flow Test
Premature Rupture of Amniotic Membranes. In addition to the fFN ELISA Test
and the fFN Dipstick Test, the Company has introduced in Japan its fFN
Vertical Flow Test for assessing premature ROM. Accurate diagnosis of
suspected premature ROM is essential to facilitate prevention of potentially
serious neonatal infection. In addition, premature ROM can be indicative of
the likelihood of premature birth. Prior to delivery of a baby, the mother's
amniotic membranes rupture, releasing amniotic fluid into the vagina. Current
procedures to diagnose premature ROM include pooling (visual evidence of
pooling of amniotic fluid in the vagina), ferning (microscopic evidence of a
fern-like pattern produced by dried vaginal secretions) and nitrazine paper (a
form of pH paper). These procedures are commonly used, but because each has
significant weaknesses, two of the three procedures must be positive to
establish the diagnosis of premature ROM under current standards of care.
Detection of fFN in vaginal secretions indicates the presence of amniotic
fluid verifying that the amniotic membranes have ruptured. The fFN Vertical
Flow Test has been approved and assigned reimbursement points by the Japanese
Ministry of Health and Welfare for identification of premature ROM. The fFN
Vertical Flow Test is currently being sold in Japan by Daiichi.
PREECLAMPSIA
The Company also focuses on the pregnancy-related disorder of preeclampsia.
Preeclampsia is a syndrome characterized by the presence of at least one of
the following symptoms: elevated blood pressure (hypertension), protein in the
urine (proteinuria) and swelling of the face and extremities (edema).
Preeclampsia typically occurs in the later stages of pregnancy, and although
its origin is not currently well understood, it is believed to start at the
time of implantation of the fertilized egg and may have immunological basis.
In the United States, preeclampsia occurs in approximately 7% of all
pregnancies per year and is one of the leading causes of maternal mortality.
Most women develop symptoms of preeclampsia late in pregnancy, although some
women become symptomatic as early as 24 weeks of gestation. The current
standard of care for identifying this disorder includes frequent blood
pressure checks of the mother between 24 and 36 weeks of gestation.
About 5% of all women with preeclampsia develop severe life-threatening
forms of this disorder that may lead to stroke, eclamptic seizures, liver or
kidney failure, severe blood clotting disorders and even death.
31
Preeclampsia can also have significant harmful effects on fetal well-being.
The only cure for preeclampsia is delivery of the fetus. Thus, many
preeclamptic women have a cesarean section before the onset of significant
complications. Consequently, preeclampsia accounts for approximately 10% to
25% of preterm deliveries, and, as a result, many of these babies suffer from
prematurity-related disorders.
Current diagnosis techniques for preeclampsia are not reliable. Although
diagnosed on the basis of the presence of hypertension, proteinuria and edema,
preeclampsia occasionally occurs in the complete absence of these symptoms. To
further complicate diagnosis of this disorder, many women experience
relatively harmless, transient blood pressure elevation during pregnancy which
may be incorrectly diagnosed as preeclampsia. At the present time, there are
no widely used immunodiagnostic markers for preeclampsia, and those tests that
do exist do not typically provide sufficient warning to prevent potentially
serious complications. Thus physicians must determine whether to perform a
cesarean section on patients that are believed to be preeclamptic regardless
of the gestational age of the fetus. This leads in some cases to unnecessary
cesarean sections, as well as the complications associated with premature
births.
THE ADEZA SOLUTION FOR PREECLAMPSIA
cFN ELISA Test
The Company has determined that cellular fibronectin ("cFN"), a protein
produced by the endothelial cells that line the vascular system that is
related to but distinct from fFN, is elevated in the plasma of women with
preeclampsia but not in the plasma of women with hypertension unrelated to
preeclampsia. Preclinical studies involving approximately 270 patients in
three sites indicate that cFN may be useful as a confirmatory marker for
preeclampsia and that its concentration seems to be proportional to the
severity of disease. Adeza has developed an ELISA test for the detection of
preeclampsia (the "cFN ELISA Test"), which is currently undergoing preclinical
evaluation in the United States, Europe and Australia.
HLA-G
Research conducted with Adeza's university collaborators has indicated that
preeclampsia may be a disease of abnormal trophoblast invasion. Trophoblasts
allow the placenta to implant on the uterus; adverse effects to trophoblasts
may prevent the placenta from gaining proper access to the maternal blood
supply. The Company's collaborations have identified new proteins which may
play roles in controlling trophoblast invasion and influencing the development
of preeclampsia. The first of these proteins, called Human Lymphocyte Antigen
G, or HLA-G, is present on the trophoblasts of normally invading, non-
preeclamptic placenta but markedly lower in concentration on the same types of
cells in preeclamptic placenta. The Company is conducting research on these
proteins with the goal of developing diagnostics for preeclampsia.
REPRODUCTIVE DISORDERS
In addition to the pregnancy-related disorders of premature birth and late
birth/successful induction of labor and preeclampsia, the Company has also
targeted the reproductive disorders of endometriosis and infertility.
ENDOMETRIOSIS
Endometriosis is a disorder manifested by the misplaced growth of the
endometrial tissue that normally lines the uterus and/or the development of
related lesions in the pelvic cavity. In the United States, the disease is
estimated to afflict 10% of women of reproductive age and is a leading cause
of infertility. Symptoms of endometriosis include pelvic pain, abnormal
vaginal bleeding and infertility. The symptoms of pain can range from minor
distress to excruciating pain that leads to possible bed rest and an inability
to function normally. The extent and location of the disease do not directly
correlate with the severity of the symptoms. In the United States, it is
estimated that approximately six million women of reproductive age have some
form of endometriosis. Approximately 30% to 45% of women who have
endometriosis are infertile. Endometriosis is responsible for 20% to 30% of
all gynecological operations and is the leading non-obstetric cause of
hospitalizations for women of reproductive age.
32
Physicians currently diagnose endometriosis by performing laparoscopic
surgery to view endometriosis lesions in the pelvic cavity. Laparoscopy is an
invasive surgical procedure usually performed under general anesthesia,
involving inflation of the abdomen and two punctures in order to inspect the
pelvic cavity and diagnose the disease. Laparoscopy is not consistently
accurate in detecting early stage, microscopic disease. Current treatments
include invasive laparoscopic procedures to remove tissue and hormonal therapy
to temporarily suppress the growth of the lesions. These laparoscopic
procedures cost $2,000 to $4,000 per patient. The Company believes that the
development of an accurate diagnostic test would significantly reduce the
costs of treating endometriosis by providing an effective alternative to
laparoscopic surgery in certain cases. Enhanced ability to diagnose
endometriosis would reduce the risk to the patient and allow the physician to
not only detect the disease, but also to determine and monitor the
effectiveness of treatments.
INFERTILITY
It is estimated that about 800,000 new female infertility patients are
diagnosed in the United States each year and that diagnosis and treatment of
such patients costs approximately $2.0 billion per year. While at least 50% of
female infertility cases involve diagnoses which are well understood, much of
the management of female infertility centers around the treatment of poorly
understood factors. At least 25% of infertile women fall into the category of
"unexplained infertility" where extensive tests for known factors have failed
to reveal the cause of their infertility. These women may face open-ended and
undefined therapy that elevates costs and frustrates patients and physicians.
Women with "unexplained infertility" are candidates for assisted reproductive
technologies ("ART"), which are not always successful. Each attempted ART
treatment costs between $8,000 and $10,000. The Company believes that the
ability to predict successful candidates for ART treatments would help prevent
unnecessary lengthy and costly procedures in failed treatments.
THE ADEZA SOLUTION FOR REPRODUCTIVE DISORDERS
ENDOMETRIOSIS
Neural Networks. A neural network is a computer-based form of artificial
intelligence that is capable of identifying and "learning" direct and indirect
relationships between complex sets of data and a given outcome. The strength
of neural network analysis lies in its ability to examine complex and
nonlinear systems, such as human physiologic processes. The Company is
developing a proprietary neural network software, using its endometriosis
database, that the Company believes may be useful in assessing the likelihood
of the existence of endometriosis in a particular patient. The Company may
also apply this technology to its other targeted pregnancy-related and female
reproductive disorders.
Endometriosis-Specific Chemotactic Factor Test. A chemotactic factor has the
property of attracting macrophages and neutrophils in a process called
chemotaxis. The endometriosis-specific chemotactic factor that has been
isolated by Adeza and its collaborators shows the highest level of activity in
peritoneal fluid of patients with minimal to mild endometriosis, as compared
to patients with no endometriosis or moderate to severe endometriosis. A
diagnostic test based on this endometriosis-specific chemotactic factor would,
as a result, allow physicians to detect endometriosis at an early stage. The
Company is developing a proprietary diagnostic test based on this
endometriosis-specific chemotactic factor.
Anti-Endometrial Antibody Assay. The Company has detected an elevated
antibody response against endometrial proteins in the blood of patients with
endometriosis. Adeza's scientists have identified certain antigens that they
believe elicit this antibody response. Adeza is developing an assay test based
on these antigens that would allow physicians to diagnose endometriosis
through the detection of endometriosis-specific antibodies in the blood of
patients with the disease.
33
INFERTILITY
Uterine Receptivity Test. The Company believes that the lack of expression
of the Beta-3 integrin correlates with defects in uterine receptivity to
embryo implantation. The Company is in the process of obtaining an exclusive
worldwide, option to a proprietary test for defects in uterine receptivity
based on Beta-3 integrin. Such a test would be of significant value by
allowing the triage of women for therapy to correct uterine receptivity
defects prior to attempting ART. Because patients with defined defects in
uterine receptivity often fail to conceive either normally or in response to
in vitro fertilization, the Company believes that such a test could also allow
significant cost savings by potentially reducing the number of unnecessary
failed in vitro fertilization ("IVF") cycles. After signing of the option, the
Company intends to conduct a multi-center trial with major IVF centers to
further evaluate the potential of this test.
Anti-Ovarian Antibody Assay. In order for in vitro fertilization
technologies to be successful, patients must produce mature ova, which is the
purpose of fertility drug treatment prior to the use of in vitro
fertilization. The Company believes that high levels of a certain anti-ovarian
antibody correlate to poor response to fertility drug treatments. The Company
is developing an assay to detect anti-ovarian antibodies in the blood of women
who are candidates to receive drug treatment for ovarian stimulation. The
Company believes this test has the potential to be a useful screen for poor
response to drug-induced ovarian stimulation. If the Company is able to
successfully develop such a test, it believes that the anti-ovarian antibody
assay could lead to significant savings by reducing the number of ovulation
induction treatment cycles that fail due to lack of response.
SALES & MARKETING; STRATEGIC CORPORATE ALLIANCES
DOMESTIC OPERATIONS
The Company's strategy is to sell and market its products primarily through
strategic partners and distributors. The Company, together with its strategic
partners and distributors, markets primarily to physicians, hospitals, other
health care providers and third-party payors. The Company believes that the
trend toward management of health care costs in the United States will lead to
increased awareness of and emphasis on disease prevention and early patient
management, which will increase demand from physicians and third-party payors
for cost-effective diagnostic tests.
The Company, together with its strategic partner, Matria, is currently
preparing for market launch of the fFN ELISA Test, which has exclusive rights
to market the Company's fFN ELISA Test and its fFN Dipstick Test in the United
States, Canada and Puerto Rico. Matria is the surviving entity of the merger
of Tokos Medical Corporation ("Tokos") and HealthDyne, Inc., two leaders in
providing health care services for pregnant women. As of April 30, 1996,
Matria had a field organization of approximately 114 sales and sales support
personnel and 400 nurses. It has over 30 specialists focused on third-party
payors and 100 reimbursement customer service claims management personnel.
Adeza and Matria are currently working to structure reimbursement rates with
major third-party payors in anticipation of the full-scale commercial launch
of the fFN ELISA Test in the United States in the second half of 1996. The
current end-user list price in the United States for the fFN ELISA Test is
$212 for a processed laboratory result from the Company and $2,400 for a
diagnostic kit that is analyzed by the end-user's laboratory, which may be
utilized for eight to 44 tests.
The Company's relationship with Matria is governed by an exclusive marketing
agreement (the "Matria Marketing Agreement") for the Company's fFN ELISA Test
and the fFN Dipstick Test. In addition, Matria has the right to participate in
the development of and obtain rights to distribute immunodiagnostic products
for premature birth that are developed by or acquired for development by the
Company, in the United States, Canada and Puerto Rico. Under the Matria
Marketing Agreement, initially executed in December 1991 with Tokos and
amended in May 1996, the Company received an initial $3.0 million in research
and development funding in 1991. In addition, the Company received another
$1.0 million in milestone payments in 1992 and $4.0 million in 1995 in
connection with the completion of other milestones. Additionally, in
connection with the Matria Marketing Agreement, Matria invested in $2.0
million of the Company's capital stock.
34
INTERNATIONAL OPERATIONS
Until the Company's receipt of FDA approval of its fFN ELISA Test in the
United States, Adeza's marketing and sales efforts were focused on selected
international markets for the purpose of gathering relevant market data and
increasing market awareness. These markets include Taiwan, portions of Europe,
Scandinavia, Japan and South Korea. Currently, the Company's international
sales and marketing efforts address the particular health care systems of
individual countries through a network of 11 international distributors with
expertise in their markets. These distributors are supported by the Company's
internal sales and marketing professionals. The Company intends to expand its
international distribution network through agreements with strategic partners
and distributors that have significant presence and experience in the
international women's health care market.
The fFN ELISA Test and fFN Vertical Flow Test are marketed in Japan and
South Korea by the Company's marketing partner, Daiichi. In December 1990, the
Company and Daiichi entered into agreements to co-develop and market Adeza's
in vitro diagnostic products in Japan. Under these agreements, the Company
recognized contract revenue for research and development of $2.0 million in
1991, $2.0 million in 1992, $667,000 in 1993, $667,000 in 1994 and $667,000 in
1995. Additionally, in connection with these agreements, Daiichi purchased an
aggregate of $1.0 million of the Company's capital stock.
The Company expects in the future to enter into additional strategic
partnerships to develop, commercialize and sell its current and future
products. However, there can be no assurance that the Company will be able to
negotiate acceptable agreements in the future, or that such new agreements or
existing agreements will be successful. The Company may also establish a
direct sales force to sell certain products.
Adeza's internal sales and marketing efforts provide technical and clinical
support for the Company's products to the Company's strategic partners and
distributors, including their journal advertising, seminars, congresses and
trade show participation. A primary goal of the Company's direct marketing is
to increase public awareness of the problems and high costs associated with
pregnancy-related and female reproductive disorders it targets. Adeza
collaborates with Ob/Gyn physicians, practitioners and academics to support
the use of the Company's technology. The Company and its strategic partners
have produced an in-service video to facilitate consistent and regular use of
its products by physicians and laboratory technicians, cost/benefit
information and informative product literature in five languages.
RESEARCH AND DEVELOPMENT
The Company's research and development efforts are conducted internally and
through collaborations with academic investigators and clinicians, with a
primary focus on the research and development of additional diagnostics and on
therapeutic products that will complement the Company's diagnostics in
addressing the needs of women with pregnancy-related and reproductive
disorders. Adeza's therapeutic research strategy emphasizes the selection of
naturally occurring compounds which have clear roles in the biochemical
processes frequently associated with premature birth. Total research and
development expenses for the years ended December 31, 1994 and December 31,
1995 and the three month period ended March 31, 1996 were $3.6 million, $3.5
million and $830,000, respectively.
LABORATORY SERVICES; MANUFACTURING
Laboratory Services. The Company offers laboratory services that will
provide results from the fFN ELISA Test. The Company expects that over time
other laboratory service providers will also provide results from this test.
The Company's strategy is to use its laboratory services to help it penetrate
the market by facilitating adoption of the Company's products by physicians,
clinicians and health care providers and to generate additional revenue
through fees for such laboratory services. The Company has limited experience
in providing laboratory services to provide results of its diagnostic tests.
The Company intends to provide laboratory results to physicians and other
users of the Company's diagnostic test as a means of ensuring that the users
of such products will have ready access to results. No commercial laboratory
service provider other than
35
the Company is currently providing results with respect to the Company's
tests, and there can be no assurance that any laboratory other than that of
the Company will ultimately provide such results. Failure by the Company to
provide accurate and cost-efficient laboratory services and to generate
expected revenues through such services could have a material adverse effect
on the Company's business, financial condition and results of operations and
could expose the Company to significant liability in the event of errors in
test results. There can be no assurance that there will be demand for the
Company's laboratory services or that such services will generate any
additional revenues for the Company. See "Risk Factors--Limited Laboratory
Operating Experience."
The ability to provide quality laboratory information for the physician
begins with obtaining a high-quality specimen. To maintain the specimen's
integrity during shipment, all specimens are sent following prescribed
shipping instructions. In the case of the fFN samples, a container with a
frozen ice pack is being used to help protect the samples from excess heat. An
overnight courier is used to ensure that the samples are transported from the
point of collection to the laboratory within 24 hours. Validation studies to
ensure the specimens have been transported properly have been performed and
will be an ongoing part of Adeza's laboratory quality assurance program.
As of April 30, 1996, Adeza employed five California Licensed Clinical
Laboratory Scientists and retained the services of a Laboratory Director and a
Clinical Consultant. The laboratory is staffed and equipped to process 240 to
480 fFN samples in the course of each eight hour shift, six days a week. The
Company believes that this laboratory capacity will be sufficient through
1997. In addition to the fFN testing, the Company intends to use its
laboratory to offer clinical testing services for additional products if and
as such products are introduced and expansion of the Company's existing
laboratory facilities may be required to perform such additional testing.
Adeza's laboratory is certified under the Clinical Laboratory Improvement
Amendments of 1988 ("CLIA") for its laboratory services. It is also certified
by the State of California Department of Health Services. CLIA is intended to
ensure the quality and reliability of all medical testing in laboratories in
the United States by requiring that any facility in which testing is performed
meet specified standards in areas such as personnel qualification,
administration, participation in proficiency testing, patient test management,
quality control, quality assurance and inspections. The regulations
promulgated under CLIA have established three levels of regulatory control
based on test complexity--"waived," "moderately" and "highly" complex. The fFN
ELISA Test is currently catagorized as highly complex, and Adeza's laboratory
has been certified to perform tests at the highly complex level.
The federal and state certification and licensure programs establish
standards for the day-to-day operation of a medical laboratory, including, but
not limited to, personnel and quality control. Compliance with such standards
is verified by periodic inspections by inspectors employed by federal or state
regulatory agencies. In addition, federal regulatory authorities require
participation in a proficiency testing program approved by the Department of
Health and Human Services ("DHHS") for each of the specialties and
subspecialties for which a laboratory seeks approval from Medicare or Medicaid
and licensure under CLIA. Proficiency testing programs involve actual testing
of specimens that have been prepared by an entity running an approved program
for testing by the laboratory. On March 14, 1990, the DHHS published final
regulations setting forth the standards for the day-to-day operation of all
laboratories that participate in Medicare and Medicaid, as well as those
laboratories which engage in interstate commerce and, therefore, are regulated
under CLIA. These regulations generally became effective on September 1, 1992
and apply to the Company's laboratory. The Company believes it is in
compliance with these regulations.
Existing federal laws governing Medicare and Medicaid, as well as some state
laws, also regulate certain aspects of the relationship between health care
providers, including laboratories, and their referral sources, including
physicians, hospitals and other laboratories. One provision of these laws,
known as the "anti-kickback law," contains extremely broad proscriptions, and
relatively little regulatory guidance or judicial precedent exists concerning
its application. Violation of this provision may result in exclusion from
Medicare and Medicaid or
36
criminal penalties. The Company seeks to structure its arrangements with
physicians and other providers to be in compliance with this law, and to keep
up-to-date on developments concerning the law's application by means including
consultation with legal counsel. However, the Company is unable to predict how
the law will be applied in the future, and no assurances can be given that its
arrangements will not be subject to scrutiny under this law.
Any exclusion or suspension from participation in the Medicare and Medicaid
programs, any loss of licensure or accreditation, or any inability to obtain
any required license or permit, whether arising from any action by DHHS, any
state, or any other regulatory authority, would have a material adverse effect
on the Company's business. Any significant civil or criminal penalty resulting
from such proceedings could have a material adverse effect on the Company.
The FDA does not currently regulate laboratory testing services. Certain
federal and state laws govern the handling and disposal of medical specimens,
infectious and hazardous wastes and radioactive materials. Failure to comply
could subject an entity covered by these laws to fines, criminal penalties
and/or other enforcement actions.
Pursuant to the Occupational Safety and Health Act, laboratories have a
general duty to provide a workplace to their employees that is safe from
hazard. Over the past few years, the Occupational Safety and Health
Administration ("OSHA") has issued rules relevant to certain hazards that are
found in the laboratory. Failure to comply with any applicable OSHA rules or
with the general duty to provide a safe workplace could subject an employer,
including a laboratory employer, to substantial fines and penalties.
Manufacturing. As of April 30, 1996, Adeza employed six full-time employees
in manufacturing and four full-time employees in its quality assurance
department. The manufacturing facility is located at the Company's
headquarters in Sunnyvale, California and consists of approximately 4,400
square feet of dedicated manufacturing and quality assurance space.
The Company currently manufactures the fFN ELISA Test, the fFN Dipstick Test
and the fFN Vertical Flow Test at its facilities in Sunnyvale. The Company
believes that it has the capacity to meet its estimated production
requirements for 1997. Adeza's manufacturing facility has a current license
from the State of California, Department of Health Services Food and Drug
Branch Manufacturing and is also registered with the FDA as a device
manufacturer.
The Company has no therapeutic manufacturing facilities at the present time
and plans to rely upon outside manufacturers to produce any therapeutic
products. The Company believes that there is substantial worldwide production
capacity for therapeutics and that it will be able to establish manufacturing
arrangements on acceptable terms. However, there can be no assurance that
appropriate production capacity for therapeutics will be available if and when
needed by the Company or that the Company will be able to establish
manufacturing arrangements on acceptable terms, if at all.
CERTAIN LICENSE AGREEMENTS; PATENTS AND PROPRIETARY TECHNOLOGY
Certain License Agreements. The Company seeks to obtain technologies that
complement and expand its existing technology base. Where consistent with its
business strategy, the Company intends to license product and marketing rights
from selected research and educational institutions to capitalize on the
research and development capabilities and technology bases of these entities.
The Company's material license agreement covering the technology related to
the Company's fFN-based products is with the Fred Hutchinson Cancer Research
Center (the "Hutchinson Center"). In August 1992, Adeza entered into an
amended license agreement (the "Hutchinson Agreement") which superseded
certain previous license agreements between the two entities. Under the
Hutchinson Agreement, the Company was granted a worldwide, sublicensable, exclusive license (subject to the rights of certain United StatesUS governmental agencies and a grant-back to the Hutchinson Centerresearch center for non-commercial research purposes) to certaina US patent rightsand corresponding foreign patents related to fFNfetal fibronectin and antibodies made against fFN,
37
which constitutes Adeza's core technology for its fFN-based products,
including the fFN ELISA Test and the fFN Dipstick Test. The Company paid up-
front license fees upon execution of the Hutchinson Agreement, in January 1993
and upon receipt of a PMA for the fFN ELISA Test. The Company is also
obligated tofetal fibronectin. We pay royalties on net sales of our Fetal Fibronectin Test under this agreement. We also have a non-exclusive license under two US patents and corresponding foreign patents under which we also pay royalties on net sales of our Fetal Fibronectin Test.
Under an agreement with a major university, we have a worldwide, sublicensable, exclusive license to the Hutchinson Center on actual product sales in
the United States by the Company during the remainder of the term of a
licensed patent, subjectthree US patents and corresponding foreign patents relating to an annual minimum royalty. In addition, in
connection with Matria's market launch of the fFN ELISA Test, the Company
granted the Hutchinson Center a warrant to purchase 25,000 shares of Common
Stock.
In addition to the Hutchinson Agreement, the Company is a party to numerous
option and license agreements with research and educational institutions for
the in-licensing of a variety of key technologies, including the University of
California at San Francisco for the use of HLA-G for diagnosis and treatmenta specific protein found in the lining of preeclampsia, the Universityuterus as a predictor of Pennsylvania for the potential diagnostic
and therapeutic applications of a chemotactic factor associated with endometriosis and for certain antibodies made againstthe determination of uterine receptivity toward embryo implantation. Under the agreement, we pay royalties on net sales of our E-tegrity Test.
We also have agreements with other third parties pursuant to which we have royalty-bearing, non-exclusive licenses to patents held by those third parties.
COMPETITION
Rapid product development, technological advances, intense competition and a strong emphasis on proprietary products characterize the medical devices and diagnostic products industries. Our products could be rendered obsolete or uneconomical by the introduction and market acceptance of competing products, by technological advances of potential competitors or by other approaches.
We are currently the only provider of a fetal fibronectin test for predicting preterm birth. However, we could experience competition for our preterm birth diagnostic products from companies that manufacture and the University of Alabama at Birmingham for a therapeutic agent for treatment
of women at risk for preterm delivery. Certain of these agreements require the
Company or the other parties to meet certain performance obligations in order
to retain their rights under such agreements or require the Company to make
certain payments in order to obtain or maintain rights to the subject
technology.
Patents and Proprietary Technology. The Company's success will depend in
part on its ability to obtain patent protection for its products and
processes, to preserve its trade secrets and to operate without infringing the
proprietary rights of third parties. The Company's policy is to protect its
technology base by filing patent applications on internal proprietary
technology as well as on in-licensed technology. The Company owns or has
licenses to 23 United States patents and the Company owns, has licenses to or
options to license technology covered by another 16 pending United States
patent applications, each in the area ofmarket pregnancy-related and reproductive
disorder diagnostics.
The Company's ability to protect its proprietary position is in part
dependent on the issuance of patents on current and future applications. The
Company currently has applications pending in the United States, Japan, Canada
and certain countries in Europe. The validity and breadth of claims in medical
technology patents involve complex legal and factual questions and therefore
are highly uncertain. No assurance can be given that any pending patent
applications or any future patent applications will be issued, that the scope
of any patent protection will exclude competitors or provide competitive
advantages to the Company, that any of the Company's patents will be held
valid if subsequently challenged or that others will not claim rights in or
ownership to the patents and other proprietary rights held by the Company.
Furthermore, there can be no assurance that others have not developed or will
not develop similar products, duplicate any of the Company's products or
design around the Company's patents. In addition, others may hold or receive
patents or file patent applications that contain claims having a scope that
covers products developed by the Company. In the event that any relevant
claims of third-party patents are upheld as valid and enforceable, the Company
could be prevented from practicing the subject matter claimed in such patents
or could be required to obtain licenses from the patent owners of each of such
patents or to redesign its products or processes to avoid infringement. There
can be no assurance that such licenses would be available or, if available,
would be on terms acceptable to the Company or that the Company would be
successful in any attempt to redesign its products or processes to avoid
infringement. The Company also relies upon unpatented trade secrets to protect
its proprietary technology, and no assurance can be given that others will not
independently develop or otherwise acquire substantially equivalent techniques
or otherwise gain access to the Company's proprietary technology or that the
Company can ultimately protect meaningful rights to such unpatented
proprietary technology.
Litigation regarding patent and other intellectual property rights which
would result in substantial cost to and diversion of effort by the Company may
be necessary to enforce patents issued to the Company, to protect trade
secrets or know-how owned by the Company, to defend the Company against
claimed infringement of the rights of others or to determine the ownership,
scope or validity of the proprietary rights of the Company and others. An
adverse determination in any such litigation could subject the Company to
significant liability to third parties, could require the Company to seek
licenses from third parties, which licenses may not be available or, if
38
available, may not be on terms acceptable to the Company, and ultimately could
prevent the Company from manufacturing, selling or using its products, any of
which could have a material adverse effect on the Company's business,
financial condition and results of operations.
Adeza also relies on trade secrets and proprietary know-how. The Company's
policy is to require each of its employees, consultants and advisors to
execute a confidentiality agreement upon the commencement of any employment,
consulting or advisory relationship with the Company. Each agreement provides
that all confidential information developed or made known to the individual
during the course of the relationship will be kept confidential and not
disclosed to third parties except in specified circumstances. In the case of
employees, the agreements provide that all inventions conceived of by an
individual shall be the exclusive property of the Company, other than
inventions unrelated to the Company and developed entirely on the employee's
own time. There can be no assurance, however, that these agreements will
provide meaningful protection or adequate remedies for misappropriation of the
Company's trade secrets in the event of unauthorized use or disclosure of such
information.
THIRD-PARTY REIMBURSEMENT
The Company's strategy is to increase acceptance of its products and create
long-term demand by marketing the cost effectiveness and improved patient care
that its products provide to third-party payors. The Company, together with
its strategic partners and distributors, intends to educate such third-party
payors through a variety of means, including marketing evaluations, seminars,
workshops, publications and professional and trade meetings. Adeza and Matria
are currently working to structure reimbursement rates with major third-party
payors in anticipation of the full-scale commercial launch of the fFN ELISA
Test in the United States in the second half of 1996.
The Company's ability to successfully commercialize its products depends in
part on the availability of, and the Company's ability to obtain, adequate
levels of third-party reimbursement for use of its diagnostic tests. In the
United States, hospitals, physicians and other health care providers that
purchase diagnostic tests such as the fFN ELISA Test generally rely on third-
party payors, principally federal Medicare, state Medicaid and private and
corporate health insurance plans, to reimburse all or part of the cost
associated with the use of such tests. Although reimbursement for diagnosing
premature birth has generally been available in the United States, there can
be no assurance that this will continue to be the case. Third-party payors may
deny reimbursement if they determine that a prescribed diagnostic test has not
received appropriate FDA or other governmental regulatory clearances, is not
used in accordance with cost-effective treatment methods as determined by the
payor, or is experimental, unnecessary or inappropriate. The Company's ability
to commercialize its products successfully will depend in part on the extent
to which appropriate reimbursement levels for the cost of such products and
related treatment are obtained from government authorities, private health
insurers and other organizations, such as health maintenance organizations
("HMOs"). Third-party payors are increasingly challenging the prices charged
for medical products and services. The Company is unableThese companies may be significantly larger and have access to predict what
changes will be made insubstantially more capital for new product development and sales and marketing. These companies may develop new diagnostic products or technologies that could
In addition, healthcare providers use diagnostic techniques such as HMOs, which could control
or significantly influence the purchase of health care servicesclinical examination and products,
as well as legislative proposalsultrasound to reform health care or reduce government
insurance programs, may all result in lower prices for the Company's products.
The Company is unable to forecast what additional legislation or regulation,
if any, relating to the health care industry or third-party coverage and
reimbursement may be enacted in the future or what effect such legislation or
regulation would have on the Company's business. The cost containment measures
that health care providers are instituting and the impact of any health care
reform could have an adverse effect on the Company's ability to sell its
products and may have a material adverse effect on the Company's business,
financial condition and results of operations.
Reimbursement systems in international markets vary significantly by
country, and by region within some countries, and reimbursement approvals must
be obtained on a country-by-country basis. Many international markets have
government managed health care systems that control reimbursement for new
diagnostic tests and procedures. In most markets, there are private insurance
systems as well as government managed systems.
39
Market acceptance of the Company's products will depend on the availability
and level of reimbursement in international markets targeted by the Company.
Currently, the Company's fFN ELISA Test and fFN Vertical Flow Test have been
approved for reimbursement in Japan. However, there can be no assurance that
the Company will obtain reimbursement in any other countries for these
products or in any country for its future products within a particular time,
for a particular amount, or at all.
Regardless of the type of reimbursement system, the Company believes that
physician and clinician acceptance and advocacy of the use of the Company's
products will be required to obtain reimbursement. Availability of
reimbursement will depend on the clinical efficacy and cost of the Company's
products. There can be no assurance that reimbursement for any of the
Company's products in the United States or foreign countries under either
government or private reimbursement systems will be available, or if
available, will not be decreased in the future, or that physicians and
clinicians will support and advocate reimbursement for the use of the
Company's products. Failure by physicians, clinicians and other users of the
Company's products to obtain sufficient reimbursement from third-party health
care payors for the use of the Company's products could have a material
adverse effect on the Company's business, financial condition and results of
operations.
GOVERNMENT REGULATION
The manufacturing, testing, labeling, distribution, marketing, promotion and
sale of the Company's products are subject to extensive and rigorous
regulation by the FDA and, to varying degrees of regulation, by other Federal,
state and foreign regulatory agencies. The Company's products are regulated by
the FDA under the Federal Food, Drug and Cosmetic Act (the "FDC Act"), as
amended by the Medical Device Amendments of 1976 and the Safe Medical Devices
Act of 1990, among other laws. Under the FDC Act and the regulations
promulgated thereunder, the FDA regulates, among other things, the clinical
testing, manufacturing, labeling, distribution, sale and promotion of medical
devices in the United States. In addition, various foreign countries in which
the Company's products are or may be sold, including Germany, France, Japan
and Canada, impose local regulatory requirements. The testing for, preparation
of and subsequent FDA and foreign regulatory review of required applications
is expensive, lengthy and uncertain. Failure to comply with FDA and similar
foreign requirements could result in civil penalties or criminal prosecutions,
restrictions on or injunction against marketing of the Company's products,
seizure or recall of the Company's products, total or partial suspension of
products, refusal by the government to grant premarket clearance or approval
of the Company's products, withdrawal of market approval or other regulatory
action. There can be no assurance that the Company will be able to obtain
necessary regulatory approvals or clearances on a timely basis or at all, and
delays in receipt of or failure to receive such approvals or clearances, the
loss of previously received approvals or clearances, or failure to comply with
existing or future regulatory requirements would have a material adverse
effect on the Company's business, financial condition and results of
operations.
The FDC Act, among other things, classifies medical devices into three
categories over which the FDA maintains increasing levels of regulation: Class
I (general controls--e.g., labeling, premarket notification and GMPs), II
(general and special controls--e.g., performance standards, postmarket
surveillance and FDA guidelines) and III (premarket approval). Before a new
device can be introduced into the market, the manufacturer typically must
obtain FDA clearance or approval through either a 510(k) premarket
notification or a PMA. Because the Company believes that the majority of
Adeza's products are or will ultimately be classified as Class III devices,
they are or will be subject to the requirement of premarket approval by the
FDA. However, a 510(k) premarket notification will be sufficient for a
proposed device that is "substantially equivalent" to a legally marketed Class
I or Class II medical device or a Class III medical device for which the FDA
has not required submission of PMAs. Prior to marketing any of these devices,
the Company is required to submit a 510(k) premarket notification to the FDA
and await the FDA's determination that the product may be marketed. In 510(k)
premarket notification the Company must, among other things, demonstrate that
the product to be marketed is substantially equivalent to the predicate
device. Test data from clinical trials is often required to demonstrate
substantial equivalence and that the products are safe and effective, which
may delay the 510(k) premarket notification review period or which may result
in a finding that the product is not substantially equivalent and that a full
PMA is required.
40
Following submission of a 510(k) premarket notification, a company may not
market the device for clinical use until the FDA finds that product is
substantially equivalent. It generally takes four to 12 months from the date
of submission of a 510(k) to obtain premarket clearance, but it may take
longer. The FDA may agree that the product is substantially equivalent to a
predicate device and allow the product to be marketed in the United States.
The FDA, however, may (i) determine that the new device is not substantially
equivalent and require a PMA or (ii) require further information, such as
additional test data, including data from clinical studies, before it is able
to make a determination regarding substantial equivalence. By requesting
additional information, the FDA can further delay market introduction of a
company's products. Further, for any devices cleared through the 510(k)
process, modifications or enhancements that could significantly affect safety
or effectiveness, or constitute a major change in intended use, require new
510(k) submissions.
In February 1990, Adeza received FDA clearance of its 510(k) premarket
notification for its specimen collection kit and received FDA clearance of
revisions to this 510(k) in September 1995. However there can be no assurance
that the FDA will act favorably or quickly in its review of the Company's
future 510(k) submissions, if any, and significant difficulties and costs may
be encountered by the Company in its efforts to obtain FDA clearance that
could delay or preclude the Company from selling its products in the United
States. Furthermore, there can be no assurance that the FDA will not request
additional data, require that the Company conduct further clinical studies or
require a PMA, causing the Company to incur further cost and delay.
If a device does not qualify for the premarket notification procedure, a
company must file a PMA. The PMA requires more extensive pre-filing testing
than required for a 510(k) premarket notification and usually involves a
significantly longer review process. The PMA generally must contain results of
laboratory and human clinical studies establishing the safety and
effectiveness of the device for its intended use. The PMA must also give a
detailed description of the proposed labeling as well as the methods,
facilities and controls used for manufacturing the device. After a preliminary
review, the FDA makes an initial determination regarding whether a PMA is
sufficiently complete to permit substantial review and fileable. If a PMA is
accepted for filing, the FDA begins a more in-depth review process, which
likely will include review by a scientific advisory panel. During the PMA
review process, the FDA will conduct an inspection of the manufacturer's
facilities to ensure compliance with the applicable Good Manufacturing
Practices ("GMP") requirements. If the FDA evaluation of both the PMA and the
manufacturing facilities is favorable, the FDA may issue an "approvable"
letter, which usually contains a number of conditions that must be met in
order to secure final approval of the PMA. When those conditions have been
fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval
letter, authorizing commercial distribution of the device for those
indications for which the FDA deems the data to be adequate. The FDA will also
indicate the intended uses or labeling claims, which the FDA will endorse, and
the product may only be marketed for these uses. In addition, the FDA can
revoke its approval after it has initially been given, and once approved, a
product is subject to various other regulatory controls that may be imposed by
the FDA. There can be no assurance that the existing FDA approval for the fFN
ELISA Test will not be revoked or that the fFN ELISA Test will meet other
regulatory controls that may be imposed. If the FDA's evaluation of the PMA or
manufacturing facilities is not favorable, the FDA will deny the application
or issue a "non-approvable" letter. The FDA may determine that additional
clinical data is necessary and delay the PMA to
allow conduction of clinical trials and submission of data to the FDA.
In October 1992, the Company submitted a PMA for the use of its fFN ELISA
Test for use in assessingdiagnose the likelihood of premature birthpreterm birth. Healthcare providers may choose to continue using these techniques to assess their patients, rather than use our Fetal Fibronectin Test. They may also choose to use these techniques in symptomatic
women. In January 1993, the FDA notified the Company of its initial
determination notconjunction with our Fetal Fibronectin Test to accept the PMA for filing pending the submission of
further clinical information by the Company. In response to subsequent
discussions with the FDA, Adeza expanded its clinical studiespredict preterm birth.
We believe that in light of the fFN ELISA
Testincreased rate of preterm birth, cesarean section delivery and submitted these results toassisted reproductive procedures, the FDA in October 1994. In December 1994,
the FDA notified the Company of its decision to file the PMAmarket for our products is growing. As a result, we expect additional competition from companies with expedited
review, and in April 1995, an FDA advisory panel determined that the fFN ELISA
Test was safe and effective for the symptomatic indication and recommended
approval of the PMA by the FDA. In September 1995, the Company was notified by
the FDA that it is permissible to commercially distribute the fFN ELISA Test
in the United States for its intended use.
41
Once an original PMA is reviewed and approved by the FDA, a Company may seek
authorization to market the approved product in a different format or for
additional clinical uses not described in the original PMA. Permission to
market the device for a claim different from that specified in the original
PMA is typically submitted to the FDA by a Company in the form of a PMA
Supplement. The FDA typically requires that the Company provide new clinical
data to facilitate its review of the new product format or clinical use. Adeza
is currently planning to submit two PMA Supplements to its original approved
PMA covering the fFN ELISA Test. In the first PMA Supplement, the Company
currently intends to request permission to expand the intended use of the fFN
ELISA Test to include assessment of the likelihood of premature birth for
asymptomatic women. In the second PMA Supplement, the Company currently
intends to request permission to market the fFN Dipstick Test for assessment
of the likelihood of premature birth in symptomatic women.
There can be no assurance that the Company will be able to meet the FDA's
PMA requirements or that any necessary regulatory approvals will be obtained
by the Company in the United States or any other country. Adeza's failure to
obtain any required regulatory approvals, as well as any delay which may be
experienced in obtaining such approvals, could materially and adversely affect
the marketing of the Company's products and its ability to generate revenues.
Adeza's failure to obtain any necessary regulatory approvals, any delay on
receiving such approvals, the restriction, suspension or revocation of
regulatory approvals, if obtained, or the failure to comply with regulatory
requirements would have a material adverse effect on the Company's business,
financial condition and results of operations.
Distribution of the Company's products outside the United States is subject
to FDA export and extensive foreign government regulation. These regulations,
including the requirements for approvals or clearance to market, the time
required for regulatory review and the sanctions imposed for violations, vary
from country to country. Since the fFN ELISA Test has been approved for
marketing in the United States by the FDA, there are currently no significant
restrictions limiting exportation of the device. Exportation of devices which
have not yet been cleared for domestic commercial distribution requires
permission from the country in which the device will be marketed and the
receipt of an 801(e) authorization, which is the approval of the FDA to export
a device. Adeza has obtained 801(e) clearances for export of the fFN Vertical
Flow Test to 22 countries, including the United Kingdom, Australia, Germany,
France and Spain. Additionally, Adeza has obtained approval and reimbursement
for the fFN ELISA Test and the from the Ministry of Health and Welfare in
Japan. On April 26, 1996, the export provisions in the FDC Act were amended in
Chapter 1A of Title II, Supplemental Appropriations For the Fiscal Year Ending
September 30, 1996, in the FDA Export Reform and Enhancement Act of 1996 to
revise the terms and conditions under which biologies may be exported under
Sections 801 and 801 of the FDC Act. This new legislation was designed to
facilitate the export of drugs by revising and in some circumstances
eliminating the requirement for FDA approval as a condition of export. Because
the legislation has just been enacted and there are no FDA regulations or
existing policies with respect to its implementation, the Company is unable to
reliably predict the extent of benefit that its export opportunities may
enjoy, if any. However, despite such new legislation, there can be no
assurance that the Company will obtain additional regulatory approvals in
other countries or that it will not be required to incur significant costs in
obtaining or maintaining its foreign regulatory approvals. Failure to obtain
necessary regulatory approvals, the restriction, suspension or revocation of
existing approvals or any other failure to comply with regulatory requirements
outside the United States could have a material adverse effect on the
Company's business, financial condition and results of operations.
The FDC Act and California laws also require the Company to be licensed and
to manufacture its products in compliance with current GMP regulations. These
regulations require that the Company manufacture its products and maintain
related documentation in a prescribed manner with respect to manufacturing,
testing and control activities. The Company is also required to comply with
various FDA requirements for labeling and the FDA prohibits a device, even if
approved, approved under a PMA, from being marketed for unapproved clinical
uses. If the FDA believes that a company is not in compliance with the
regulations, it can, among other things, institute proceedings to detain or
seize a product, issue a recall, prohibit marketing and sales of the company's
products and assess civil and criminal penalties against the company, its
officers or its employees. There can be
42
no assurance that its manufacturing facility will satisfy GMP or California
manufacturing requirements. The Company's facilities and manufacturing
processes have been periodically inspected by the State of California and
other agencies, but remain subject to audit from time to time. The Company
believes that it is in substantial compliance with all applicable federal and
state regulations. Nevertheless, there can be no assurance that the FDA or a
state agency will agree with the Company's position, or that its GMP
compliance will not be challenged at some subsequent point in time.
Enforcement of the GMP regulations has increased significantly in the last
several years and the FDA has publicly stated that compliance will be more
strictly scrutinized. In the event that the Company is determined to be in
noncompliance with FDA regulations, the FDA or state agency has the power to
assert penalties or remedies, including, among other things warning letters,
fines, injunctions, civil penalties, recall or seizure of products, total or
partial suspension of production, refusal of the government to grant premarket
clearance or premarket approval for devices, withdrawal of previously granted
approvals and criminal prosecution. Such penalties or remedies could have a
materially adverse effect on the Company's business, financial condition and
results of operations.
In addition, the manufacture, sale or use of the Company's products are also
subject to regulation by other federal entities, such as the OSHA and the
Environmental Protection Agency, and by various state agencies, including the
California Environmental Protection Agency. Federal and state regulations
regarding the manufacture, sale or use of the Company's products are subject
to future change, which changes could have a material adverse effect on the
Company's business, financial condition and results of operations.
COMPETITION
Although Adeza believes it is well positioned to take advantage of the
opportunities of the women's reproductive health care market, the Company
expects competition is this market to increase. The fFN ELISA Test is the only
FDA-approved immunodiagnostic test for the assessment of the likelihood of
premature birth for symptomatic women and represents a significant advance
over currently used subjective evaluation techniques. However, there can be no
assurance that other, more effective diagnostic tests for the assessment of
the likelihood of premature birth will not receive FDA approval in the near
future. Other companies and institutions with substantially greater financial, manufacturing, marketing, distributionmanagerial, and technical resources than the
Company are engagedwe have.
EMPLOYEES
As of August 3, 2004, we had 83 employees, including 46 in thesales, marketing and business development, 13 in manufacturing and laboratory services, 10 in research and development, of9 in administration and 5 in quality assurance. In addition, our direct sales force includes some full-time sales representatives provided to us by a third party, who are directly managed by us, sell our products similar to
those currently being developed or commercialized by the Company. For example,
companies such as Abbott Laboratories, American Home Products Corp., Bayer AG,
Boehringer Mannheim GmbH, Bristol Myers Squibb Co., Eli Lilly & Co., G.D.
Searle and Co. (a subsidiary of Monsanto Co.), Johnson & Johnson, TAP
Pharmaceuticals Inc. and Zeneca Group PLC. are engaged in the development,
manufacture and marketing of diagnostic and therapeutic products relating to
women's reproductive health careexclusively, and are therefore potential competitorsan integral part of the Company. Although noneour sales team. None of these companies currently concentrates
exclusively on products for the women's reproductive health care market, there
can be no assurance that theseour employees is represented by a labor union or other companies or institutions will not
succeed in developing products or procedures that are more effective than the
Company's or that would render the Company's technology or products obsolete
or uncompetitive.
The Company's fFN ELISA Test will be distributed in the United States
through an exclusive strategic distribution arrangement with Matria. The
Company believes Matria competes with other companies that distribute products
related to the women's reproductive health care market to physicians,
clinicians, hospitals and other health care providers. These companies include
Apria Healthcare, Caremark International and Coram Healthcare. The Company
believes that Matria's extensive sales and marketing network competes
favorably with these and other companies, although there can be no assurance
that it will continue to do so.
The medical industry is characterized by rapid and significant technological
change. Consequently, the Company's success will depend in part on its ability
to respond quickly to medical and technological changes through the
development and commercialization of new products. The Company believes that
for all its products, important competitive factors include the relative speed
with which companies can develop products, establish clinical utility,
complete the clinical testing and regulatory approval process, obtain
reimbursement and supply commercial quantities of the product to the market.
The Company's inability to compete favorably with respect
43
to any of these factors could have a material adverse effect on its business,
financial condition and results of operations. Product development involves a
high degree of risk and there can be no assurance that the Company's current
products will become commercially successful or that the Company's research
and development efforts will result in commercially successful future
products.
EMPLOYEES
As of April 30, 1996, the Company employs 40 full and part time personnel,
including 21 in research, development, clinical and regulatory affairs, six in
manufacturing, four in quality assurance, two in marketing and sales, and
seven in administration. Each of the Company's employees has signed a
confidentiality agreement and none is covered by a collective bargaining agreement. The Company hasWe have never experienced any employment-related work stoppages and considers itsconsider our employee relations to be good. PROPERTIES
Adeza maintains itsWe also employ independent contractors to support our development, regulatory, sales, marketing and administrative activities.
FACILITIES
We maintain our headquarters in Sunnyvale, California in one leased facility aggregatingof approximately 17,600 square feet, which contains laboratory, research and development, production, manufacturing, marketingsales and salesmarketing, and general administration, and finance. Thewhich is currently leased on a month-to-month basis while we negotiate a longer term lease for this facility
expires in August 1997. Adeza has a right of first refusalrenewal. We believe that if exercised,
would extend the term of the lease for an additional five year period, to
2002. The Company currently pays monthly rent of approximately $20,000. The
Company believes that itsour existing facilities are adequate to meet itsour immediate needs and that suitable additional space will be available in the future on commercially reasonablereasonably terms as needed.
LEGAL PROCEEDINGS
As of the date of this Prospectus, the Company is
We are not acurrently party to any material legal proceedings.
ADVISORS
Management
EXECUTIVE OFFICERS, SIGNIFICANT EMPLOYEES, AND COLLABORATORS
The Company has establishedDIRECTORS
Set forth below is the name, age, position and a network of medical, clinical and scientific
advisors and collaborators to consult with the Company's scientists and
clinical research staff and to advise the Company on its research and
development program, the design of its products and on other medical and
scientific matters relating to the Company's business. The Company's advisors
and collaborators include the following individuals:
Gary S. David, Ph.D., Independent Consultant, formerly conducted
immunogenetics and immunochemistry research at the City of Hope, cancer
immunobiology at the Salk Institute, and immunochemistry and tumor
immunobiology at the Scripps Clinic and Research Foundation.
Susan Fischer, Ph.D., Chairman and Professorbrief account of the Departmentbusiness experience of Oral
Biology, Professoreach of Obstetrics, Gynecology & Reproductive Sciences,
Professor of Pharmaceutical Chemistry and Associate Director of UCSF Mass
Spectrometry Facility, University of California at San Francisco, San
Francisco, California.
Thomas Garite, M.D., Chairman and Professor of the Department of Obstetrics
& Gynecology, University of California at Irvine, Irvine California; Diplomat
and Examiner, American Board of Obstetrics and Gynecology; Member, American
Board of Obstetrics and Gynecology Maternal Fetal Medicine Division Committee;
Editor, the American Journal of Obstetrics and Gynecology.
Linda Giudice, M.D., Ph.D., Associate Professor of Obstetrics & Gynecology;
Chief, Division of Reproductive Endocrinology and Infertility, Chief,
Gynecology, Lucille Packard Children's Hospital at Stanford University,
Stanford, California.
Jay Iams, M.D., Professor, Obstetrics & Gynecology, Director of Maternal
Medicine Division of the Department of Obstetrics & Gynecology, Ohio State
University, Columbus, Ohio.
Charles Lockwood, M.D., Chairman and Professor of Obstetrics & Gynecology,
New York University School of Medicine, New York, New York.
44
Judith Luborsky, Ph.D., Associate Professor of Obstetrics & Gynecology, Rush
Presbyterian St. Luke's Medical Center, Chicago, Illinois.
James Roberts, M.D., Professor of Obstetrics & Gynecology, University of
Pittsburgh, Pittsburgh, Pennsylvania; Chairman, Maternal-Fetal Medicine Unit
of the National Institutes of Health.
Robert Taylor, M.D., Ph.D., Associate Professor of Obstetrics & Gynecology,
University of California at San Francisco, San Francisco, California.
The Company has entered into consulting agreements with most of the listed
advisors and collaborators. Each of the Company's advisors and collaborators
has entered into a confidentiality and non-disclosure agreement with the
Company. These advisors and collaborators are generally employed by employers
other than the Company and may have commitments to or consulting or advisory
contracts with other entities that may limit their availability to the
Company. Although generally each advisor and collaborator agrees not to
perform services for another person or entity which would create a conflict of
interest with the individual's services for the Company, there can be no
assurance that such conflict will not arise.
45
MANAGEMENT
EXECUTIVE OFFICERS AND DIRECTORS
The following table sets forth certain information with respect to theour executive officers and directors of the Company and their ages and positions
as of April 30, 1996:
August 1, 2004.
| Name | Age | Position(s) | ||||
| Emory V. Anderson | 50 | President, Chief Executive Officer and Director | ||||
| Mark D. Fischer-Colbrie | 48 | Vice President, Finance and Administration and Chief Financial Officer | ||||
| Durlin E. Hickok, MD, MPH | 56 | Vice President, Medical Affairs | ||||
| Robert O. Hussa, | 63 | Vice President, Research and | ||||
| Marian E. Sacco | 50 | Vice President, | ||||
Andrew E. Senyei, | 54 | Chairman of the Board | ||||
Nancy D. | 50 | Director | ||||
Craig C. | 54 | Director | ||||
Kathleen D. LaPorte(3) | 42 | Director | ||||
| (1) | Member of audit committee |
| (2) | Member of compensation committee |
| (3) | Member of nominating and corporate governance committee |
Emory V. Andersonhas been theour President and Chief Executive Officer of Adeza
since July 1992. PriorFebruary 1997. From October 1992 to joining Adeza,February 1997, Mr. WildsAnderson was employed by Baxter
Healthcare Corporation ("Baxter"). From 1968 until June 1992, Mr. Wilds was
President of Baxter's Chemotherapy Service division, President and Chief
Operating Officer of its diagnostic joint venture with Genentech and President
and Chief Executive Officer of Medisense, Inc. (a fully integrated diagnostic
company in which Baxter owns a minority interest). Mr. Wilds was also General
Manager of Baxter's Mexico City operations, General Manager of its Container
Development Business Center, Director of Strategy Development and Vice
President of its Corporate Alliances function.
Emory V. Anderson has been theour Vice President and Chief Financial Officer of
Adeza since October 1992.Officer. Prior to joining Adeza,us, Mr. Anderson served as Executive Vice President Chief Operating Officer and Chief FinancialOperating Officer of Indesys, Inc., which he co-founded in 1984. Previously, Mr. Andersonhe held the position of Director of Finance for Atari, Inc. He serves on the board of directors of Curon Medical, Inc., a publicly held company.
Mark D. Fischer-Colbriehas been our Vice President of Finance and Administration and Chief Financial Officer since February 2001. From March 1992 to January 2001, Mr. Fischer-Colbrie served as Vice President, Finance and Administration and Chief Financial Officer for KeraVision, Inc., a company that filed for bankruptcy under federal bankruptcy laws in March 2001. He also held several financial positions at Maxtor Corporation from April 1986 through February 1992, including Vice President of Finance and Corporate Controller.
Durlin E. Hickok, MD, MPH,has been our Vice President of Medical Affairs since November 1998. From 1996 to 1998, Dr. Hickok was Vice President and Medical Director of Omnia, Inc., a women’s healthcare management company. He was also Chief of Obstetrics and Gynecology at the Virginia Mason Medical Center from 1993 to 1996, and Associate Director of Perinatal Medicine at Swedish Hospital Medical Center in Seattle, Washington from 1982 to 1993. Previously, he was an Assistant Professor at the University of Washington.
Robert O. Hussa, PhD,has been theour Vice President of Research and Development and
the Chief Technical Officer of Adeza since May 1993. From January 1990 to May 1993, Dr. Hussa was Vice President of Imaging and Therapeutics Research and Development at Hybritech, Inc. ("Hybritech"), where he developed in vitro
diagnostic tests in the areas of reproductive endocrinology, cardiovascular
disease and cancer. From June 1986 to December 1989, he was Director of Assay Development at Hybritech. Prior to joining Hybritech, Dr. Hussa was a Professor of Gynecology & Obstetrics and of Biochemistry at the Medical College of Wisconsin where he worked for 18 years.
David C. Casal
Marian E. Saccohas been our Vice President of Sales and Marketing since September 1997. From 1996 to 1997, Ms. Sacco was the Vice President Clinical and Regulatory Affairs
of Adeza since November 1994,Marketing at Behring Diagnostics. Previously, Ms. Sacco was Adeza'sthe Director of Scientific AffairsWorldwide Oncology Business and Worldwide Marketing Manager for CBA Corning/ Chiron Diagnostics from
October 1991 to November 19941996, and was Adeza's Directorthe US Sales and Marketing Manager for Centocor Diagnostics from 1987 to 1991.
Andrew E. Senyei, MD, Chairman of Clinical and
Regulatory Affairs from January 1990 to October 1991.the Board, has served as one of our directors since 1987. Dr. Casal was previously
the Manager of Clinical Programs at Ligand Pharmaceutical, Inc., formerly
Progenex Incorporated, and was also a Senior Research Scientist at Hybritech,
where he was responsible for the clinical evaluation and approval of numerous
in vitro diagnostic assays.
46
Andrew E. Senyei has been a director of Adeza since 1987Managing Director and has been
involved in Adeza's development since joining the Company as a consultant in
1986. Dr. Senyei has been a General Partner of Enterprise Partners, a venture capital firm, since 1987. Dr. Senyei was a founder of Molecular Biosystems and, prior to joining Enterprise Partners, was a practicing clinician and Adjunct Associate Professor of Obstetrics, Gynecology and Pediatrics at the University of California at Irvine. Dr. Senyei currentlyHe serves on the Boardboards of Directorsdirectors of Ligand Pharmaceutical, Inc.
Nelson N.H. Teng co-founded Adeza and has served on the Company's Board of
Directors since 1985. Dr. Teng was the Medical Director and Vice President of
Research at Adeza from 1988 to 1989. Dr. Teng is currently an Associate
Professor in Obstetrics and Gynecology and Director of Gynecological Oncology
at the Stanford University School of Medicine, where he has been on the
faculty since 1981. Dr. Teng currently serves on the Board of Directors of
Univax Biologics.
numerous private healthcare companies.
Nancy S. Amer D. Burrushas served as a directorone of Adezaour directors since May 1992. Ms. Amer has
been a Managing DirectorDecember of Harvard Private Capital Group, Inc., Boston,
Massachusetts since 1990. Prior to joining Harvard Private Capital Group,
Inc., Ms. Amer was a senior consultant with the Boston Consulting Group, Inc.
Ms. Amer currently serves on the Board of Directors of Cambridge NeuroScience,
Inc., Playtex Products, Inc. and Procept, Inc. and several privately held
companies.
Nancy D. Burrus has been a director of Adeza since December 1994. Ms. Burrus has been a General Partner withgeneral partner of Indosuez Ventures, a venture capital firm, since 1991 where she has
been active in early stage health care company investing.1990. Indosuez Ventures manages STF II, L.P., one of our institutional shareholders. Prior to joining Indosuez Ventures, Ms. Burrus was a Vice President ofwith Morgan Stanley Ventures. She serves on the boards of directors of several private companies.
Craig C. Taylorco-founded Adezaour company and has served on the Company's Boardas one of Directorsour directors since 1986. Mr. Taylor heads the life science investments at Alloy Ventures and has been active in venture capital since 1977 when he joined Asset Management Company. Alloy Ventures is the institutional fund management successor to Asset Management Company, a venture management firm founded in 1965. He serves on the boards of directors of Pharmacyclics and Lynx Pharmaceuticals, both publicly held companies, and several private companies.
Kathleen D. LaPortehas served as one of our directors since April of 2002. Ms. LaPorte has been a General Partner with Asset
Management Associates, an early stagegeneral partner of Sprout Group, a venture capital firm, since 1977 where
he1994. Prior to joining the Sprout Group, Ms. LaPorte was a principal at Asset Management Company, a venture capital firm focused on early stage investments. She has participated inalso worked as a financial analyst with The First Boston Corporation. She is the establishmentpast President, and growtha member of high technology and
health care companies including Amgen, Hybritech and Applied Biosystems. Mr.
Taylor currentlythe Board of the Western Association of Venture Capitalists. She serves on the Boardboard of Directorsdirectors of Telor Ophthalmic
Pharmaceutical, Metra Biosystems, Pharmacyclics and Lynx TherapeuticsISTA Pharmaceuticals, Inc., a publicly held company, and several privately heldprivate companies.
BOARD OF DIRECTORS COMMITTEES, COMPENSATION OF DIRECTORS AND OTHER INFORMATION
AllCOMPOSITION
Our board of directors hold office untilhas five members. Upon the nextcompletion of this offering and in accordance with the terms of our amended and restated certificate of incorporation, our board of directors will be divided into three classes:
| 4 | the class I directors are Ms. Burrus and Mr. Taylor, and their term will expire at the annual meeting of stockholders to be held in 2005; |
| 4 | the class II director is Dr. Senyei and his term will expire at the annual meeting of stockholders to be held in 2006; and |
| 4 | the class III directors are Mr. Anderson and Ms. LaPorte, and their term will expire at the annual meeting of stockholders to be held in 2007. |
At each annual meeting of stockholders, or special meeting in lieu thereof, after the initial classification of the Companyboard of directors, the successors to directors whose terms will then expire will be elected to serve from the time of election and qualification until their successors have been duly elected and qualified.the third annual meeting following the election or special meeting in lieu thereof. The officersauthorized number of directors may be changed only by resolution adopted by a majority of the Company are appointed annually and serve at the discretionboard of directors. This classification of the Boardboard of Directors.
Directorsdirectors may have the effect of delaying or preventing changes of control or management.
BOARD COMMITTEES
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Pursuant to our bylaws, our board of directors may from time to time establish other committees to facilitate the management of our business and operations.
Audit committee
Our audit committee currently receive no cash feesconsists of Ms. Burrus, Mr. Taylor and Dr. Senyei. Our audit committee is responsible for services provided in their
capacity as directors but may be reimbursed for out-of-pocket expenses
incurred in connectionassuring the integrity of our financial control, audit and reporting functions and reviews with services performed forour management and our independent auditors the benefiteffectiveness of our financial controls and accounting and reporting practices and procedures. In addition, this committee reviews the Company.
The Company has adopted the 1996 Directors' Stock Option Plan under which
current and future nonemployee directors will be eligible to receive stock
options in considerationqualifications of our independent auditors, is responsible for their services. See "Management--Stock Optionappointment, compensation, retention and Incentive Plans."
The Board of Directors designated a Compensation Committee (the
"Compensation Committee") in 1995 to reviewoversight and approvereviews the compensation and
benefits for the Company's executive officers, administer the Company's 1995
Stock Option and Restricted Stock Plan and make recommendations to the Board
of Directors regarding such matters. The Compensation Committee is currently
composed of Andrew E. Senyei and Nancy D. Burrus.
The Board of Directors designated an Audit Committee in 1995 to review the
scope, fees and results of financial auditsactivities related to audit and other services performed bynon-audit services. The composition of the Company's independent accountantsaudit committee will satisfy the independence requirements of The Nasdaq National Market and the SEC.
Compensation committee
Our compensation committee consists of Ms. Burrus and Dr. Senyei. The compensation committee’s principal responsibility is to administer our stock plans and to makeset the salary and incentive compensation, including stock option grants, for our Chief Executive Officer and senior staff members. The composition of the compensation committee will satisfy the independence requirements of The Nasdaq National Market.
Nominating and corporate governance committee
Our nominating and governance committee consists of Ms. LaPorte and Mr. Taylor. The nominating and governance committee is responsible for reviewing and making recommendations on the composition of our board and selection of directors, periodically assessing the functioning of our board of directors and its committees, and making recommendations to our board of directors regarding corporate governance matters and practices. The composition of the Boardnominating and governance committee will satisfy the independence requirements of DirectorsThe Nasdaq National Market.
We strive to operate within a comprehensive plan of corporate governance for the purpose of defining responsibilities, setting high standards of professional and personal conduct and assuring compliance with these responsibilities and standards. We have implemented changes to our corporate governance structure and procedures in response to the Sarbanes-Oxley Act of 2002 and the adopted changes in The Nasdaq National Market’s listing standards regarding such matters. The committee is currently composedcorporate governance. We believe that our current corporate governance structure and procedures comply with existing corporate governance requirements. We will strive to maintain our board of Nancy
S. Amerdirectors and Craig C. Taylor. committees in full compliance with these corporate governance requirements on an ongoing basis. We will also continue to regularly monitor developments in the area of corporate governance.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
No interlocking relationship existsis expected to exist between the
Company's Boardour board of Directors, the
47
Compensation Committeedirectors or Audit Committeecompensation committee and the board of directors compensation committee or auditcompensation committee of any other company,entity, nor has any
such interlocking relationship existed in the past. See "Certain
Transactions."
DIRECTOR COMPENSATION
Our 2004 Equity Incentive Plan, or the 2004 Plan, provides for automatic grants of options to purchase shares of our common stock to our non-employee directors. The 2004 Plan provides for the automatic grant to a non-employee director who is on our board of directors upon the completion of this offering, or who is appointed or elected to our board of directors at a later date, of an option to purchase 40,000 shares of our common stock for our chairman, and 30,000 shares of our common stock to each remaining non-employee director. Such grant is referred to as the Initial Option. The 2004 Plan provides for an automatic grant of an option to purchase 15,000 shares to our chairman and 10,000 shares to each remaining non-employee director on the date of each annual meeting of the stockholders that occurs on or after the date that the 2004 Plan first becomes effective, provided that
Each Initial Option and Annual Option will have an exercise price equal to the fair market value of a share of our common stock on the date of grant and will have a term of ten years. Each Initial Option will vest and become exercisable in 48 equal installments on each monthly anniversary of the date of grant so long as the non-employee director continuously remains a director or consultant to our company. Each Annual Option will vest and become exercisable in 12 equal installments on each monthly anniversary of the date of grant of the option for so long as the non-employee director remains a director or a consultant to, our company. All automatic non-employee director options granted under the 2004 Plan will be non-statutory stock options. Options must be exercised, if at all, within three months after a non-employee director’s termination of service, except in the case of death, in which event the director’s estate shall have one year from the date of death to exercise the option. In no event, however, shall any option granted to a director be exercisable later than the expiration date of the option’s term. In the event of our merger with another corporation or another change of control, the non-employee director options will become fully vested and exercisable. For a description of the 2004 Plan see “Equity Compensation Plan Information.”
We currently do not pay cash compensation to our chairman or other non-employee directors for their service as directors.
LIMITATION OFON LIABILITY AND INDEMNIFICATION MATTERS
The Company's CertificateOF OFFICERS AND DIRECTORS
Our amended and restated certificate of Incorporationincorporation limits the liability of our directors to the maximum extent permitted by Delaware law. Delaware law provides that a directorcorporation may eliminate the personal liability of a corporation will not be personally liableits directors for monetary damages for breach of such individual'stheir fiduciary duties as a directordirectors, except for
liability (i) for any breach of such director's duty of loyalty to the
corporation, (ii) for acts or omissions not in good faith or that involve
intentional misconduct or a knowing violation of law, (iii) for unlawful
payments of dividends or unlawful stock repurchases or redemptions as provided
in Section 174 of the Delaware General Corporation Law or (iv) for any
transaction from which a director derives an improper personal benefit.
The Company's Bylawsfollowing acts:
| 4 | breach of their duty of loyalty to us or our stockholders; |
| 4 | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| 4 | unlawful payments of dividends or unlawful stock repurchases or redemptions; and |
| 4 | any transaction from which the director derived an improper personal benefit. |
Our bylaws provide that the Companywe will indemnify itsour directors,
and may indemnify its officers, employees and other agents to the fullest extent permitted by law. The Company believes that indemnification under its
Bylaws covers at least negligence and gross negligencethe Delaware General Corporation Law. Our bylaws also permit us to secure insurance on the part of an
indemnified party and permits the Company to advance expenses incurred by an
indemnified party in connection with the defensebehalf of any action or proceeding
arising out of such party's status or service as aofficer, director, officer, employee or other agent of the Company upon an undertaking by such party to repay such
advances if it is ultimately determined that such party is not entitled to
indemnification.
The Company has entered into separate indemnification agreements with each
of its directors and officers. These agreements require the Company, among
other things, to indemnify such director or officer against expenses
(including attorney's fees), judgments, fines and settlements (collectively,
"Liabilities") paid by such individual in connection withours for any action, suit or
proceedingliability arising out of his or her actions in such individual's status or service ascapacity, regardless of whether the Delaware General Corporation Law would permit a director or
officercorporation to indemnify for such liability.
We have obtained directors’ and officers’ insurance providing indemnification for all of the Company (other than Liabilities arising from willful misconduct
or conductour directors and officers for certain liabilities. We believe that is knowingly fraudulent or deliberately dishonest) and to
advance expenses incurred by such individual in connection with any proceeding
against such individual with respect to which such individual may be entitled
to indemnification by the Company. The Company believes that its Certificate
of Incorporation and Bylawthese provisions and indemnification agreementsthis insurance are necessary to attract and retain qualified persons as directors and officers. At present, the Companythere is not aware of anyno pending litigation or proceeding involving any director, officer, employeeof our directors, officers, employees or agent of the Companyagents where indemnification willwould be required or permitted. The Company isWe are not aware of any pending or threatened litigation or proceeding that might result in a claim for such indemnification.
48
EXECUTIVE COMPENSATION
Summary compensation table
The following table sets forth certainsummarizes the compensation paid by the Companyto, awarded to or earned during the fiscal year ended December 31, 1995 to the Company's2003 by our Chief Executive Officer and each of the Company's otherfour most highly
| Long-term | |||||||||||||||||||||
| compensation | |||||||||||||||||||||
| Annual compensation | |||||||||||||||||||||
| Securities | |||||||||||||||||||||
| Other annual | underlying | All other | |||||||||||||||||||
| Name and principal position(s) | Salary | Bonus | compensation(1) | options | compensation | ||||||||||||||||
Emory V. Anderson(2) | $311,782 | $109,124 | — | — | — | ||||||||||||||||
| President and Chief Executive Officer | |||||||||||||||||||||
Mark D. Fischer-Colbrie(3) | 217,342 | 43,468 | — | — | — | ||||||||||||||||
| Vice President, Finance and Administration and Chief Financial Officer | |||||||||||||||||||||
Durlin E. Hickok(4) | 219,533 | 43,907 | — | — | — | ||||||||||||||||
| Vice President, Medical Affairs | |||||||||||||||||||||
Robert O. Hussa(5) | 199,510 | 19,951 | — | — | — | ||||||||||||||||
| Vice President, Research and Development | |||||||||||||||||||||
Marian E. Sacco(6) | 210,223 | 42,045 | — | — | — | ||||||||||||||||
| Vice President, Sales and Marketing | |||||||||||||||||||||
| (1) | In accordance with the rules of the SEC, the other annual compensation disclosed in this table does not include various perquisites and |
| (2) | Does not include 225,000 shares underlying options granted by the board of directors on August 4, 2004 that are exercisable within 60 days of June 30, 2004. |
| (3) | Does not include 50,000 shares underlying options granted by the board of directors on August 4, 2004 that are exercisable within 60 days of June 30, 2004. |
| (4) | Does not include 50,000 shares underlying options granted by the board of directors on August 4, 2004 that are exercisable within 60 days of June 30, 2004. |
| (5) | Does not include 25,000 shares underlying options granted by the board of directors on August 4, 2004 that are exercisable within 60 days of June 30, 2004. |
| (6) | Does not include 50,000 shares underlying options granted by the board of directors on August 4, 2004 that are exercisable within 60 days of June 30, 2004. |
Option grants in fiscal year 2003
We did not grant any options granted to the Named Executive Officersany of our named executive officers during theour fiscal year ended December 31, 1995. No stock appreciation rights2003.
Aggregated option exercises in last fiscal year and fiscal year-end option values
There were granted to these individuals
during the year.
OPTION GRANTS IN LAST FISCAL YEAR
POTENTIAL REALIZABLE VALUE
AT ASSUMED ANNUAL RATES OF
NUMBER OF SHARES PERCENTAGE OF STOCK PRICE APPRECIATION
UNDERLYING TOTAL OPTIONS EXERCISE FOR OPTION TERM(2)
OPTIONS GRANTED TO PRICE PER EXPIRATION ---------------------------
NAME GRANTED(#)(1) EMPLOYEES SHARE DATE 5% 10%
---- ---------------- ------------- --------- ---------- ------------- -------------
Daniel O. Wilds......... 248,463 53.43% $0.24 5/05 $ 37,502 $ 95,038
Emory V. Anderson....... 46,885 10.08 0.24 5/05 7,077 17,934
Robert O. Hussa......... 58,607 12.60 0.24 5/05 8,846 22,418
David C. Casal.......... 30,603 6.58 0.24 5/05 4,620 11,707
- --------
(1) These stock options, which were granted under the 1995 Stock Option and
Restricted Stock Plan, represent both vested and unvested options. The
maximum term of eachno option grant is ten years from the date of grant. The
exercise price is equal to the fair market value of the stock on the grant
date.
(2) The 5% and 10% assumed annual rates of compounded stock price appreciation
are mandatedexercises by the Securities and Exchange Commission. There is no
assurance provided to anynamed executive officer or any other holder of the
Company's securities that the actual stock price appreciation over the 10-
year option term will be at the assumed 5% and 10% levels or at any other
defined level. Unless the market price of the Common Stock appreciates
over the option term, no value will be realized from the option grants
made to the executive officers.
49
The following table sets forth information for the Named Executive Officers
with respect to exercises of options to purchase common stock in theofficers during our fiscal year ended December 31, 1995.
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR
AND FISCAL YEAR-END OPTION VALUES
NUMBER OF SHARES VALUE OF UNEXERCISED
UNDERLYING UNEXERCISED IN-THE-MONEY OPTIONS
OPTIONS AT FISCAL YEAR END AT FISCAL YEAR END(1)
---------------------------- -------------------------
NAME EXERCISABLE(2) UNEXERCISABLE EXERCISABLE UNEXERCISABLE
- ---- -------------- ------------- ----------- -------------
Daniel O. Wilds.......... 248,463 0 $1,788,934 0
Emory V. Anderson........ 46,885 0 337,572 0
Robert O. Hussa.......... 58,607 0 421,970 0
David C. Casal........... 30,603 0 220,342 0
- --------
(1) Based on2003. The following table summarizes the fair market value of the Common Stockoptions held by them as of December 31, 1995,2003. There was no public trading market for our common stock as determined byof December 31, 2003. Accordingly, the Boardvalue of Directors ($7.44 per share), minus unexercised in-the-money options listed below has been calculated on
| Number of securities | Value of unexercised | |||||||||||||||||||||||
| underlying unexercised options | in-the-money options | |||||||||||||||||||||||
| at December 31, 2003 | at December 31, 2003 | |||||||||||||||||||||||
| Shares acquired | Value | |||||||||||||||||||||||
| Name | upon exercise | realized | Exercisable(1) | Unexercisable | Exercisable | Unexercisable | ||||||||||||||||||
| Emory V. Anderson | — | $ | — | 812,718 | — | $ | $ | |||||||||||||||||
| Mark D. Fischer-Colbrie | — | — | 175,928 | — | ||||||||||||||||||||
| Durlin E. Hickok | — | — | 175,949 | — | ||||||||||||||||||||
| Robert O. Hussa | — | — | 72,621 | — | ||||||||||||||||||||
| Marian E. Sacco | — | — | 181,583 | — | ||||||||||||||||||||
| (1) | Each of the outstanding options listed may be exercised at any time, whether vested or unvested. Upon the exercise of an unvested option or the unvested portion of an option, the holder will receive shares of restricted stock that are subject to our repurchase right at the original purchase price of the shares, which repurchase right lapses in accordance with the vesting schedule previously applicable to the option. |
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
In December 2000, in connection with his offer of April 30, 1996,employment, we agreed to loan Mark Fischer-Colbrie up to $183,000 in connection with his offer letter. On February 1, 2003, we entered into a promissory note with Mr. Fischer-Colbrie in the amount of $109,800. The terms of the 248,463, 46,885, 58,607note provided that the principal amount would be due on January 31, 2006, and 30,603 option
shares heldinterest on the unpaid principal balance would accrue at an annual rate of 1.65%. The note also provides that the loan amount is forgiven by Daniel O. Wilds, Emory V. Anderson, Robert O. Hussa$36,600 in principal along with accumulated interest at the end of each 12 months of employment. The full amount of the note, including interest, would be due and David C. Casal, respectively, 127,687, 25,542, 33,095payable within 30 days of Mr. Fischer-Colbrie’s resignation from our company or his termination by us for cause. In the event of a change of control transaction, a merger with another company or his termination without cause by us, the loan and 16,122 option
shares, respectively, wereany accumulated interest would be forgiven. In August 2004, the $76,000 unpaid balance and interest of Mr. Fischer-Colbrie’s loan was forgiven.
Other agreements
All of our current employees and consultants have entered into agreements with us relating to the protection of our confidential information and the assignment of inventions.
None of our employees are employed for a specified term and each employee’s employment with us is subject to repurchasetermination at any time by either party for any reason, with or without cause, without further liability or obligation.
EQUITY COMPENSATION PLAN INFORMATION
1995 Stock Option and Restricted Stock Plan
In 1995, our board of directors and stockholders approved the Company.
STOCK OPTION AND INCENTIVE PLANSAdeza Biomedical Corporation 1995 Stock Option and Restricted Stock Plan, or the 1995 Plan. The Company's 1995 Stock Option and Restricted Stock Plan (the "Plan") was
adopted by the Board of Directors and approved by the stockholders in July
1995. The Plan was adopted as a successor to the Company'sour 1988 Employee Stock Plan and 1992 Key Executive Stock Plan. The 1995 Plan (collectively, the "Predecessor
Plans"). A total of 1,244,083 shares have been authorized for issuance under
the Plan, plus an automatic increase on the first trading dayis designed to provide employees, non-employee members of the 1997,
1998, 1999, 2000board of directors or non-employee members of the board of directors of any parent or subsidiary and 2001 calendar yearconsultants who provide services to us or any parent or subsidiary with the opportunity to receive grants of incentive stock options, non-statutory stock options and restricted stock issuances. We believe that the 1995 Plan will promote our interests
Under the 1995 Plan, we are authorized to grant shares equaland stock options for the purchase of up to 3.5%a maximum of 2,293,484 shares of our common stock. As of June 30, 2004:
| 4 | 1,981,744 shares were issuable upon the exercise of outstanding options granted under the 1995 plan at a weighted average exercise price of $1.75 per share; |
| 4 | 135,563 shares of common stock were issued upon the exercise of options and pursuant to restricted stock grants at purchase prices ranging between $0.24 and $8.88; and |
| 4 | 176,177 shares of common stock were available for future grant under the 1995 Plan at the completion of this offering (based on options outstanding as of June 30, 2004). |
In July 2004, we granted options to purchase 31,000 shares of common stock under our 1995 Stock Option and Restricted Stock Plan at an exercise price of $7.50 per share. In August 2004, we increased the number of shares of Common Stockcommon stock available for future grant under the 1995 Plan by 750,000 shares, and we granted options to purchase 880,000 additional shares of common stock under the 1995 Plan at an exercise price of $7.50 per share. Our stockholders approved this increase in August 2004.
Upon the effectiveness of this offering, we will no longer issue any additional options under the 1995 Plan. However, upon the effectiveness of this offering, any shares that are available for future grant under the 1995 Plan, and any shares issuable upon exercise of outstanding on December
31options that are subsequently forfeited, expire or are canceled, will be allocated to the 2004 Equity Incentive Plan.
Administration of the immediately preceding calendar year, with each such annual increase
not to exceed 500,000 shares. However, in no event may any one participant in
the1995 Plan receive option grants or direct stock issuances in any calendar year
for more than 1,000,000 shares.
The Plan provides for the grant to employees
of the Company (including officers and employee directors) of "incentive stock
options" within the meaning of Section 422 of the Code ("ISOs") and for the
grant of nonstatutory stock options ("NSOs") and stock purchase rights to
employees, employee directors and consultants of the Company. The1995 Plan is administered by the Boardour board of Directorsdirectors, or a committee thereof, (the plan administrator), which determines all terms of the Board of
Directors (the "Administrator") and is divided into two separate components:
the option grant program and the stock issuance program.
Option Grant Program. The Administrator has the authority to grant ISOs to
the employees of the Company and to grant NSOs to the employees, employee
directors and consultants of the Company. The Administrator determines the
individuals to be granted optionsgrants under the 1995 Plan. The plan administrator also determines who will receive grants under the 1995 Plan and the terms of such
options, including the exercise price, the number of shares of common stock subject to suchthe grant.
Eligibility
All employees, non-employee members of the board of directors or non-employee members of the board of directors of any parent or subsidiary, and consultants who provide services to us are eligible to participate in the 1995 Plan.
Options
The 1995 Plan authorizes the plan administrator to grant options the maximum term for which such options arein an amount and at an exercise price to remain outstanding,
the date upon which such options are to become exercisable and the vesting
schedule, if any, applicable to such options.be determined by it. The exercise price of all ISOs
granted under the Plan mustan incentive stock option cannot be at least equal toless than 100% of the fair market value of the
Common Stock of the Companyour common stock on the date of grant.option grant date. The exercise price of all
NSOs granted under the Plannon-statutory stock options must equalgenerally be at least 85% of the fair market value of the Common Stockcommon stock on the date of grant. Theoption grant date. With respect to options granted to a 10% shareholder, the exercise price of any ISO
granted to an optionee who owns stock representing moreshall not be less than 10% of the voting
power of the Company's outstanding capital stock (a "10% Stockholder") must
equal at least 110% of the fair market value of the Common Stockcommon stock on the date
of grant. Paymentoption grant date. The exercise price shall be immediately due upon the exercise of the exercise price may be made in cash, by check or
promissory note, in shares of properly registered Common Stockoption, and is generally payable:
| 4 | in cash or check; |
| 4 | by shares of common stock that meet certain holding period requirements (valued at the fair market value of our common stock on the exercise date); or |
| 4 | through a special sale and remittance procedure though a brokerage firm if the option is exercised for vested shares. |
The 1995 Plan also authorizes the fair market value as ofplan administrator to determine the exercise date of the option) that meet certain
holding requirements or,vesting schedule (if any) applicable to the extentoption shares. Generally, options granted under the 1995 plan vest in 48 successive equal monthly installments over four years. The plan administrator also determines the maximum term for which the option is exercised for vested
shares, through a special sale and remittance procedure conducted by a
Company-designated brokerage firm whereby the Company is paid sufficient funds
to cover the aggregate
50
exercise price of the purchased shares along with all taxes that the Company
would be required to withhold as a result of such exercise.remain outstanding. The term of a stockan option granted undercannot exceed ten years from the Plan may not exceed 10 years;
provided, however, thatoption grant date, and the term of an ISOincentive option granted to a 10% Stockholder may
notstockholder cannot exceed five years. No option may be transferred by the optionee other than
by will or the lawsAs of descent or distribution, except that an NSO may be
assigned in accordance with the terms of a domestic relations or order
(substantially complying with the requirements of Section 414(p)June 30, 2004, approximately 81.2% of the Code)
conveying marital property rights to any spouse or former spouse of the
optionee pursuant to applicable state domestic relations laws. Except as set
forth in the foregoing sentence, each option may be exercised during the
lifetime of the optionee only by such optionee. To the extent an optionee
would have the right in any calendar year to exercise for the first time one
or more ISOs for shares having an aggregate fair market value (under all plans
of the Company and determined for each share as of the date the option to
purchase the share was granted) in excess of $100,000, any such excessissuable under outstanding options shall be treated as NSOs. In the event of certain changes in control of the
Company, the Plan requires that each outstanding option accelerate so that
each such option shall immediately prior to the effective date of the change
in control, become fully exercisable for all the phases of Common Stock
subject to the option, except to the extent assumed by the successor
corporation (or parent thereof) replaced with a comparable option to purchase
shares of stock of the successor corporation (or parent thereof), replaced
with a cash incentive program of the successor corporation or in subject to
other limitation imposed by the Administration in connection with the change
in control. had vested.
Upon the termination of an optionee'soptionee’s employment or other relationship with the Company,us, such optionee will have a limited time within which to exercise any outstanding options, which time period will vary
depending onoptions.
The 1995 Plan also authorizes the length of the optionee's relationship with the Company and
the reason for termination. The Administrator has the discretionplan administrator to grant options that are exercisable for unvested shares of Common Stock and, tocommon stock. To the extent that an optionee holds options for such unvested shares of Common Stockcommon stock upon termination, from the Company, the Companywe will have the right to repurchase any or all of such unvested shares at the per-share exercise price paid by the optionee for such unvested shares. The plan administrator will determine the terms of such options, but the vesting schedule may not be more restrictive than 20% per year vesting, beginning one year after the grant of the option. Such minimum vesting requirement shall not apply to options granted to a highly compensated employee.
Until our common stock is first registered under Section 12(g) of the Securities Exchange Act, we retain a right of first refusal with respect to any disposition by an optionee of any shares of common stock issued under the 1995 Plan. Options issued under the 1995 Plan may not be assigned or transferred other than by will or the laws of descent and distribution following the optionee’s death, except that non-statutory stock options may be assigned in accordance with the terms of a qualified domestic relations order.
The aggregate fair market value of the shares of common stock for which one or more incentive stock options granted to any employee under the 1995 Plan may for the first time become exercisable during any one calendar year shall not exceed the sum of $100,000.
Change in control
Prior to its amendment in August 2004, the 1995 Plan provided that in the event of a corporate transaction where the acquiror does not assume or replace options granted under the 1995 Plan, each outstanding option will terminate on the effective date of the corporate transaction. Options assumed or replaced in connection with a corporate transaction shall be appropriately adjusted.
Following the amendment of the 1995 Plan, the 1995 Plan provides with regard to options granted after that date, that in the event of a corporate transaction where the acquiror does not assume or replace options granted under the 1995 Plan, such outstanding options will become fully vested and exercisable immediately prior to the consummation of the corporate transaction. Additionally the amendment 1995 Plan provides that in the event of a corporate transaction where the acquiror assumes or substitutes options granted under the 1995 Plan, options issued under the 1995 Plan will become fully vested only if the optionee is involuntarily terminated without cause within 12 months of the corporate transaction or if the optionee voluntarily resigns following a relocation of the optionee’s worksite to a location more than 50 miles from the original location within 12 months of the corporate transaction. Options assumed or replaced by an acquiror in connection with a corporate transaction shall be appropriately adjusted.
A “corporate transaction” is defined as either of the following shareholder approved transactions to which we are a party: (i) a merger or consolidation in which securities possessing more than 50% of the total combined voting power of our outstanding securities are transferred to a person or persons different from the persons holding those securities immediately prior to such transaction, or (ii) a sale, transfer or other disposition of all or substantially all of our assets in complete liquidation or dissolution of our company.
All outstanding repurchase rights shall automatically terminate upon a corporate transaction, except to the extent the repurchase rights are assigned to the successor corporation in connection with such corporate transaction. As of December 31,August 1, 2004, the 1995 Plan provides that for options to purchasegranted after that date in the event of a total of 986 shares of Common
Stock had been exercised, options to purchase a total of 516,284 shares at a
weighted average exercise price of $0.24 per share werecorporate transaction where outstanding and an
aggregate of 166,063 shares remained available for future option grants under
the Plan. Additionally, options to purchase a total of 32,888 shares of Common
Stock had been exercised under the Predecessor Plans.
Stock Issuance Program. In additionrepurchase rights are assigned to the Option Grant Program,successor corporation, all outstanding repurchase rights shall automatically terminate if the Administratorstockholder is involuntarily terminated without cause within 12 months of the corporate transaction or if the stockholder voluntarily resigns following a relocation of the stockholder’s worksite to a location more than 50 miles from the original location within 12 months of the corporate transaction.
Stock issuances
The plan administrator may grant direct and immediate issuances of shares of Common
Stock, without any intervening option grants,common stock pursuant to the Company's Stock
Issuance Program.1995 Plan’s stock issuance program. The purchase price per share of all Common Stockcommon stock issued under the Planstock issuance program must equalbe at least 85% of the fair market value of the Common
Stockcommon stock on the datestock issuance date. With respect to issuances to a 10% stockholder, the exercise price shall not be less than 110% of issuance.the fair market value of common stock on the stock issuance date. Payment offor the exercise price may be made in cash, by check or promissory note, or in past services rendered to the Companyus (or any parent or subsidiary of the Company)subsidiary).
Shares of Common Stockcommon stock issued under the Plan's stock issuance program may, in the discretion of the Administrator,plan administrator, be fully and immediately vested upon issuance or may vest in one or more installments over the term of the recipient'srecipient’s service for the Company or upon attainment of specified performance objectives. The plan administrator may not impose a vesting schedule that is more restrictive than 20% per year vesting, beginning one year after the stock issuance date. Such minimum vesting requirement shall not apply to stock issued to a highly compensated employee. An individual who is issued shares of Common Stockcommon stock under the Plan's stock issuance program will, upon issuance, have full stockholder rights with respect to such shares whether or not such shares are vested as of the date of issuance. To the extent that an individual who was issued Common Stockcommon stock pursuant to the Plan's stock issuance program either terminates his or her employment (or other relationship) with the Companyus while holding unvested shares of Common Stockcommon stock or fails to attain the specified performance objectives upon which thesuch vesting of such Common Stockcommon stock was contingent, the Company may cause such individualshares shall be immediately surrendered to immediately surrender the
unvested shares to the Companyus for cancellation in exchange for a refund of any cash or cash-equivalent consideration paid for such unvested shares. The Administratorplan administrator may waive the surrender and cancellation of unvested shares of common stock.
Until our common stock is first registered under Section 12(g) of the Securities Exchange Act, we retain a right of first refusal with respect to any disposition by the participant of any shares of common stock issued under the stock issuance program.
Prior to amendment in August 2004, the 1995 Plan provided that in the event of a corporate transaction where the outstanding repurchase rights under the stock issuance program are not assigned to the successor corporation in connection with such corporate transaction, all of the outstanding repurchase rights under the stock issuance program shall terminate automatically upon a corporate transaction. Following amendment of the 1995 Plan, the 1995 Plan provides that, with regard to shares of common stock issued under the stock issuance program after the date of amendment in the event of a corporate transaction where the repurchase rights are assigned to the successor corporation, the outstanding purchase rights will terminate if the stockholder is involuntarily terminated without cause within 12 months of the corporate transaction or if the stockholder voluntarily resigns following a relocation of the stockholder’s worksite to a location more than 50 miles from the original location within 12 months of the corporate transaction.
Amendment and termination
The board of directors has the complete and exclusive power and authority to amend or modify the 1995 Plan as long asin any or all respects. No such action
does notamendment or modification shall adversely affect the rights and obligations with respect to options or unvested stock issuances thenat the time outstanding under the 1995 Plan, and provided
further that stockholder approval shall be required for anunless the optionee or participant consents to such amendment toor modification. To increase
The 1995 Plan shall terminate upon the earliest of the expiration of the ten-year period of the 1995 Plan, the date on which all shares available for issuance have been issued, or the termination of all outstanding options in connection with a corporate transaction. Upon termination of the 1995 Plan, all outstanding options and unvested stock issuances shall continue to have full force and effect.
2004 Equity Incentive Plan
In August 2004, our board of directors and stockholders approved the 2004 Equity Incentive Plan, or the 2004 Plan, which will become effective upon the completion of this offering.
Stock options, stock appreciation rights, or SARs, stock awards and cash awards may be granted under the Plan; or2004 Plan. Each is referred to increaseas an award in the annual
limitation on grants to2004 Plan. Options granted under the 2004 Plan participants. If not terminated earlier, the Plan
will terminate in 2005.
1996 Employee Stock Purchase Plan
The Company's 1996 Employee Stock Purchase Plan (the "Purchase Plan") was
adopted by the Board of Directors in May 1996. The Company is currently
seeking stockholder approvalmay be either “incentive stock options,” as defined under Section 422 of the Purchase Plan and expects toInternal Revenue Code of 1986, as amended, or nonstatutory stock options.
Share reserve
We have such
approval prior to the commencement of this offering. Areserved a total of 250,0002,500,000 shares of Common Stock has been reservedour common stock, subject to adjustment, for issuance under the Purchase Plan.2004 Plan, all of which are available for future grant. The Purchasenumber of shares initially reserved under the 2004 Plan which is intendedwill be increased by up to qualify15,177 shares, represented by shares available for grant under Section 423the 1995 Plan as of the Code,completion of the offering. In addition, options outstanding under the 1995 Plan that are forfeited, expire or are cancelled after the date of this prospectus will be implemented by a seriesavailable for future grant under the 2004 Plan after the completion of offering periodsthis offering.
Automatic annual increase of 12 months duration,
with new offering periods (other thanshare reserve
The 2004 Plan provides that the first offering period) commencingshare reserve will be cumulatively increased on or about January 1 and July 1 of each year. Eachyear, beginning January 1, 2005 and for nine years thereafter, by a number of shares that is equal to the lesser of (a) 3% of the number of our company’s shares issued and outstanding as of the preceding December 31, (b) 700,000 shares and (c) a number of shares set by our board.
Automatic grants to non-employee directors
The 2004 Plan provides that non-employee directors will be automatically granted options under the 2004 Plan in the following amounts: (a) an option to purchase 30,000 shares of our common stock to non-employee directors upon the completion of this offering period will consist
of two consecutive purchase periods ofor upon their initial appointment or election, as applicable, to our board, and (b) commencing in 2005 and provided that such individual has served as a non-employee director for at least six months, duration, with the last day
of such period being designated aan option to purchase date. The initial offering period
will begin10,000 shares annually thereafter on the date of each stockholder meeting following which the non-employee director continues to serve on our board. In the case of a non-employee director who serves as the chairman of the board, such director will receive (a) an option to purchase 40,000 shares of our common stock upon the completion of this offering, or upon initial appointment or election as the chairman, as applicable, and will continue through June 30,
1997, with the first purchase date occurring on December 31, 1996 and
subsequent purchase dates to occur every six months thereafter. The Purchase
Plan permits eligible employees(b) an option to purchase Common Stock through payroll
deductions,15,000 shares annually thereafter on the date of each stockholder meeting following which the non-employee director continues to serve as chairman of our board.
Administration
Our board of directors or a committee of the board will administer the 2004 Plan. The board or its committee is referred to in the 2004 Plan as the administrator.
Eligibility
Awards under the 2004 Plan may be granted to our employees, directors and consultants. Incentive stock options may be granted only to our employees. The administrator, in its discretion, approves awards granted under the 2004 Plan.
Change in control
In the event of a corporate transaction, any or all outstanding awards may be assumed, converted or replaced. Awards assumed, converted or replaced in connection with a corporate transaction shall be appropriately adjusted. In the event of a corporate transaction where the acquiror does not assume or substitute awards granted under the 2004 Plan, the vesting with respect to such awards shall fully and immediately accelerate or the repurchase rights shall fully and immediately terminate, as the case may be, immediately prior to the consummation of the corporate transaction. In the event of a corporate transaction where the acquiror assumes or substitutes awards granted under the 2004 Plan, the vesting with respect to such awards shall fully and immediately accelerate or the repurchase rights shall fully and immediately terminate, as the case may be, if an awardee voluntarily resigns following relocation of the awardee’s workplace to a location more than 50 miles from the original location within 12 months following the corporate transaction.
Termination of awards
Generally, if an awardee’s service to us terminates other than by reason of death or disability, vested options and SARs will remain exercisable for a period of three months following the termination of the awardee’s service, or if earlier, the expiration of the term of such award. Unless otherwise provided for by the administrator in the award agreement, if an awardee’s termination is due to death or disability, the awardee’s vested options and SARs will be exercisable for one year following the awardee’s death or disability, or if earlier, the expiration of the term of such award.
Nontransferability of awards
Unless otherwise determined by the administrator, awards granted under the 2004 Plan are not transferable other than by will, a domestic relations order, or the laws of descent and distribution and may be exercised during the awardee’s lifetime only by the awardee.
Exercise price of stock options
The administrator determines the exercise price of options at the time the options are granted. The exercise price of an incentive stock option may not exceed 15%be less than 100% of an employee's compensation, atthe fair market value of our common stock on the date of grant. With respect to incentive stock options granted to a 10% stockholder, the exercise price equal toshall not be less than 110% of the lowerfair market value of our common stock on the date of grant. Unless otherwise determined by the administrator, the exercise price of a nonstatutory stock option will generally not be less than 85% of the fair market value of our common stock on the Common Stock atdate of grant. The fair market value of our common stock will generally be the beginningclosing sales price as quoted on The Nasdaq National Market on the date of the offeringgrant.
Exercise of option; form of payment
The administrator determines when options become exercisable. The means of payment for shares issued on exercise of an option are specified in each award agreement. The 2004 Plan permits payment to be made by cash, check, wire transfer, other shares of our common stock that meet certain holding period or the purchase date. Ifrequirements (valued at the fair market value of the Common Stock on a purchase date is less than the fair market
value at the beginning of the offering period, a new 12 month offering period
will automatically beginour common stock on the first business day followingexercise date), a broker-assisted same-day sale transaction or cancellation of any debt owed by us or any of our affiliates to the purchaseoptionholder.
Term of option
The term of an option may be no more than ten years from the date of grant. No option may be exercised after the expiration of its term. Any incentive stock option granted to a 10% stockholder may not have a term of more than five years.
Stock appreciation rights
The administrator may grant SARs alone, in addition to, or in tandem with, a new fair market value. The maximum number of shares an employee may
purchase during each offering period shall be determined onany other awards under this plan. A SAR entitles the offering dateparticipant to receive the amount by dividing $25,000 bywhich the fair market value of a sharespecified number of shares on the exercise date exceeds an exercise price established by the administrator. The excess amount will be payable in ordinary shares, in cash or in a combination thereof, as determined by the administrator. The terms and conditions of a SAR will be contained in an award agreement. The grant of a SAR may be made contingent upon the achievement of objective performance conditions.
Stock awards
The administrator may grant stock awards of restricted shares as payment of a bonus, as payment of any other compensation obligation, upon the occurrence of a special event or as otherwise determined by the administrator. The terms and conditions of a stock award will be found in an award agreement. Vesting and restrictions on the ability to exercise such stock awards may be conditioned upon the achievement of one or more goals, as determined by the administrator in its discretion. Recipients of restricted shares may have voting rights and may receive dividends on the granted shares prior to the time the restrictions lapse.
Cash awards
The administrator may grant cash awards, which entitle the recipient to a cash payment on the satisfaction of performance goals described in the award. The administrator determines the terms, conditions and restrictions related to cash awards.
Amendment and termination of the Company's
Common Stock on2004 Plan
Our board of directors may amend, alter, suspend or terminate the offering date (subject to certain limitations imposed by
the Code). Employees may end their participation in an offering2004 Plan, or any part thereof, at any time during this offering period priorand for any reason. However, we will solicit stockholder approval for any amendment to the purchase date,2004 Plan to the extent necessary and participation ends
automatically on terminationdesirable to comply with applicable laws. Generally, no such action by our board of employment withdirectors or stockholders may alter or impair any award previously granted under the Company. The Purchase2004 Plan provides that inwithout the event of a mergerwritten consent of the Company with or into another
corporation or a sale of substantially all of the Company's assets, each right
to purchase stock under the Purchaseawardee. The 2004 Plan will be assumed or an equivalent
right substituted by the successor corporation unless the Board of Directors
shortens this offering period so that employees' rights to purchase stock
under the plan are exercised prior to the merger or sale of assets. The Board
of Directors has the power to amend or terminate the Purchase Plan as long as
such action does not adversely affect any outstanding rights to purchase stock
thereunder. If not terminated earlier, the Purchase Plan will have a term of 20 years.
1996 Directors' Stock Option Plan
The 1996 Directors' Stock Option Plan (the "Directors' Plan") was adopted10 years, but it may be terminated by the Boardour board of Directors in May 1996. The Company is currently seeking
stockholder approvaldirectors at any time.
401(k) PLAN
We have established and maintain a retirement savings plan under section 401(k) of the Directors' Plan and expectsInternal Revenue Code, or the Code, to have such approval
priorcover our eligible employees. The Code allows eligible employees to defer a portion of their compensation, within prescribed limits, on a tax-deferred basis through contributions to the commencement of this offering. A total of 200,000 shares of
Common Stock has been reserved for issuance401(k) plan. Our 401(k) plan is qualified under the Directors' Plan. The
Directors' Plan provides for the grant of NSOs to non-employee directorsSection 401(a) of the Company. The Directors' PlanCode and its associated trust is designedexempt from federal income taxation under Section 501(a) of the Code. Our 401(k) permits us to work automatically without
administration;make matching contributions on behalf of eligible employees; however, to the extent administration is necessary, it will be
performed by the Board of Directors. The Directors' Plan provides that each
person who is a non-employee director onwe currently do not make any matching contributions.
Certain relationships and related party transactions
From January 1, 2003 until the date of this offeringprospectus, there has not been any transaction or series of similar transactions, nor is there currently proposed any transaction or series of similar transactions, to which we were, are, or would be a party, and each
person who first becomes a non-employee directorin which the amount involved exceeded or would exceed $60,000 and in which any of our directors or executive officers, any holder of more than 5% of any class of our voting securities or any member of the Company after the dateimmediate family of this offering shall be granted a NSO to purchase 10,000 sharesany of Common
Stock (the "First Option"). Thereafter, on the date of each Annual Meeting of
the Company's stockholders, commencing in 1997, each non-employee director
shall be automatically granted an additional NSO to purchase 5,000 shares of
Common Stock (a "Subsequent Option") if, on such date, hethese persons had or she shall have
served on the Company's Board of Directors for at least six months. The
Directors' Plan provides that the First Option shall become exercisable in
installments as to 25% of the total number of shares subject to the First
Option on each anniversary of the date of grant of the First Option; each
Subsequent Option shall become exercisable in full on the fourth anniversary
of the date of grant of that Subsequent Option. The exercise price of all
stock options granted under the Directors' Plan shall be equal to
52
the fair market value of a share of the Company's Common Stock on the date of
grant of the option. Options granted under the Directors' Planwill have a term of
10 years. Indirect or indirect material interest, other than the event ofcompensation arrangements (including with respect to equity compensation) described in “Management,” and the dissolution or liquidation of the Company, a sale
of all or substantiallytransactions described below.
We believe that we have executed all of the assetstransactions described below on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors and principal stockholders and their affiliates are approved by a majority of our board of directors, including a majority of the Company,independent and disinterested members of our board of directors, and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
ISSUANCES OF PREFERRED STOCK AND WARRANTS
We have engaged in transactions regarding sales of our preferred stock with one of our directors and with the mergerbeneficial owners of the
Company with or into another corporation in which the Company is not the
surviving corporation or any other capital reorganization in which more than
50%at least 5% of the shares of the Company entitled to vote are exchanged, each non-
employee director shall have either (i) a reasonable time within which to
exercise the option, including any part of the option that would not otherwise
be exercisable, prior to the effectiveness of such liquidation, dissolution,
sale, merger, consolidation or reorganization, at the end of which time the
option shall terminate or (ii) the right to exercise the option, including any
part of the option that would not otherwise be exercisable, or receive a
substitute option with comparable terms, as to an equivalent number of shares
of stock of the corporation succeeding the Company or acquiring its business by
reason of such liquidation, dissolution, sale, merger, consolidation or
reorganization. The Board of Directors may amend or terminate the Directors'
Plan; provided, however, that no such action may adversely affect any
outstanding option, and the provisions regarding the grant of options under the
plan may be amended only once in any six-month period, other than to comport
with changes in the Employee Retirement Income Security Act of 1974, as amended
or the Code. If not terminated earlier, the Directors' Plan will have a term of
10 years.
401(k) Plan
The Company has a tax-qualified employee savings and retirement plan (the
"401(k) Plan") covering all of the Company's employees. Pursuant to the 401(k)
Plan, employees may elect to reduce their current compensation by up to the
statutorily prescribed annual limit ($9,500 in 1996) and have the amount of
such reduction contributed to the 401(k) Plan. The 401(k) Plan permits, but
does not require, additional matching contributions to the 401(k) Plan by the
Company on behalf of all participants in the 401(k) Plan. To date, the Company
has not made matching contributions under the 401(k) Plan. The 401(k) Plan is
intended to qualify under Section 401 of the Code so that contributions by
employees or by the Company to the 401(k) Plan, and income earned on 401(k)
Plan contributions, are not taxable to employees until withdrawn from the
401(k) Plan, and so that contributions by the Company, if any, will be
deductible by the Company when made. The Trustee under the 401(k) Plan, at the
direction of each participant, invests the assets of the 401(k) Plan in any of
six investment options.
EMPLOYMENT AGREEMENTS
The Company does not presently have any employment contracts in effect with
its Chief Executive Officer or any of the other Named Executive Officers.
53
CERTAIN TRANSACTIONS
Prior to December 1994, the Companyour voting securities. On September 19, 2001, we sold to certain investors an aggregate of 525,6923,247,408 shares of itsour Series A5 Preferred Stock 3,199,436at a purchase price of $4.63 per share. On September 29, 2000, we sold an aggregate of 2,203,108 shares of itsour Series B4 Preferred Stock 4,992,955at a purchase price of $4.63 per share. On November 18, 1996, we sold an aggregate of 4,463,100 shares of itsour Series C3 Preferred Stock 362,318 shares of its Series D Preferred Stock, 654,761 shares of its Series E
Preferred Stock and 1,201,922 shares of its Series F Preferred Stock. In
December 1994, 64,635 shares of Series A Preferred Stock and 517,390 shares of
Series B Preferred Stock were converted to Common Stock. Subsequently in
December 1994, the Company effected a recapitalization, whereby all
outstanding shares of its Preferred Stock were converted into and exchanged
for shares of a newly created Series 1 Preferred Stock based upon the
outstanding series' respective liquidation preferences, and all outstanding
shares of Common Stock were subject to a reverse stock split (the "1994
Recapitalization"). Upon the consummation of the 1994 Recapitalization, each
outstanding share of Series A Preferred Stock was converted and reconstituted
as .081305 shares of Series 1 Preferred Stock; each outstanding share of
Series B Preferred Stock was converted and reconstituted as .081305 shares of
Series 1 Preferred Stock; each outstanding share of Series C Preferred Stock
was converted and reconstituted as .155837 shares of Series 1 Preferred Stock;
each outstanding share of Series D Preferred Stock was converted and
reconstituted as .311654 shares of Series 1 Preferred Stock; each outstanding
share of Series E Preferred Stock was converted and reconstituted as .474293
shares of Series 1 Preferred Stock; and each outstanding share of Series F
Preferred Stock was converted and reconstituted as .352341 shares of Series 1
Preferred Stock.
In December 1994, the Company sold shares of its Series 2 Preferred Stock to
certain investors, including Tokos, Hambrecht & Quist, Aeneas Venture
Corporation, Aspen Venture Partners, Asset Management Associates, Charter
Ventures, and Enterprise Partners (the "Series 2 Holders"), at a purchase price of $2.40$2.92 per share. The Series 2 Holders paid for the Series 2 Preferred Stock in
cashOn December 21, 1994, we sold an aggregate of 3,496,750 shares and by the surrendering for cancellation of certain outstanding
promissory notes issued by the Company. In addition, the Company concurrently
issued warrants to purchase shares of Series 2 Preferred Stock to certain of
the Series 2 Holders at ana purchase and exercise price of $2.40$2.42 per share. AllOn December 21, 1994, we effectuated a reverse stock split and converted and reconstituted:
| 4 | each share of the then existing Series A Preferred Stock as .08131 shares of Series 1 Preferred Stock; |
| 4 | each share of the then existing Series B Preferred Stock as .08131 shares of Series 1 Preferred Stock; |
| 4 | each share of the then existing Series C Preferred Stock as .15584 shares of Series 1 Preferred Stock; |
| 4 | each share of the then existing Series D Preferred Stock as .31165 shares of Series 1 Preferred Stock; |
| 4 | each share of the then existing Series E Preferred Stock as .47429 shares of Series 1 Preferred Stock; and |
| 4 | each share of the then existing Series F Preferred Stock into .35234 shares of Series 1 Preferred Stock. |
The following table summarizes the shares of our preferred stock purchased in these transactions by our directors, by our 5% stockholders and by the persons and entities associated with them in these private placement transactions. Each share of Series 1 andPreferred Stock, Series 2 Preferred Stock, issued by the
Company
| Series 1 | Series 2 | Series 3 | Series 4 | Series 5 | ||||||||||||||||
| Preferred | Preferred | Preferred | Preferred | Preferred | ||||||||||||||||
| Investor | Stock | Stock | Stock(1) | Stock | Stock | |||||||||||||||
Directors | ||||||||||||||||||||
Craig Taylor(2) | — | — | — | — | 21,598 | |||||||||||||||
5% Stockholders(3) | ||||||||||||||||||||
Entities affiliated with Sprout Capital(4) | — | — | 3,805,172 | — | — | |||||||||||||||
Enterprise Partners V, L.P.(5) | — | — | — | 1,079,914 | 1,295,896 | |||||||||||||||
Entities affiliated with Charter Ventures(6) | 180,305 | 590,904 | 295,487 | 204,242 | 291,577 | |||||||||||||||
| Entities affiliated with Aeneas Venture Corporation | 338,775 | 773,890 | 96,684 | — | — | |||||||||||||||
Entities affiliated with Alliance Technology Ventures(7) | 130,851 | 239,230 | 45,882 | 647,949 | 55,076 | |||||||||||||||
Entities affiliated with Asset Management(8) | 136,735 | 472,360 | 230,331 | 162,399 | 86,393 | |||||||||||||||
| Pantheon Global PCC Limited | 136,735 | 422,802 | 230,324 | 54,349 | 197,300 | |||||||||||||||
STF II, L.P.(9) | — | 416,666 | 349,817 | 54,255 | — | |||||||||||||||
| (1) | Represents shares of common stock into which Series 3 Preferred Stock will convert, rounded down to the nearest share. | |
| (2) | Craig C. Taylor is a member of our board of directors and is a general partner of Asset Management Associates 1984, L.P. and Asset Management Associates 1989, L.P. | |
| (3) | For additional detail as to the identities of the affiliated entities and additional information related to beneficial ownership of shares, see “Principal stockholders.” | |
| (4) | Includes 76,103 shares of Series 3 Preferred Stock issued to DLJ Capital Corp., 380,517 shares of Series 3 Preferred Stock issued to DLJ First ESC L.P., 1,830,658 shares of Series 3 Preferred Stock issued to Sprout Capital VII, L.P., 21,265 shares of Series 3 Preferred Stock issued to Sprout CEO Fund, L.P., and 1,496,627 shares of Series 3 Preferred Stock issued to Sprout Growth II, L.P. Kathleen LaPorte, a member of our board of directors, is a Managing Director of DLJ Capital Corp. DLJ Capital Corp. is the managing general partner of Sprout Capital VII, L.P., and the general partner of Sprout CEO Fund, L.P. and DLJ LBO Plans Management Corp. and DLJ LBO Plans Management Corp. II, are the general partners of DLJ First ESC, L.P. | |
| (5) | Andrew Senyei, a member of our board of directors, is a general partner of the general partner of Enterprise Partners V, L.P. | |
| (6) | Includes 180,305 shares of Series 1 Preferred Stock, 590,904 shares of Series 2 Preferred Stock, 295,487 shares of Series 3 Preferred Stock, 204,242 shares of Series 4 Preferred Stock and 277,685 shares of Series 5 Preferred Stock issued to CLS-I-IV, LLC, 3,331 shares of Series 5 Preferred Stock issued to Charter Advisors Fund IV, L.P., and 10,561 shares of Series 5 Preferred Stock issued to Charter Entrepreneurs Fund IV, L.P. | |
| (7) | Includes 128,286 shares of Series 1 Preferred Stock, 234,541 shares of Series 2 Preferred Stock and 45,005 shares of Series 3 Preferred Stock, 647,949 shares of Series 4 Preferred Stock and 53,996 shares of Series 5 Preferred Stock issued to Alliance Technology Ventures III, L.P., 2,565 shares of Series 1 Preferred Stock, 4,689 shares of Series 2 Preferred Stock, 877 shares of Series 3 Preferred Stock and 1,080 shares of Series 5 Preferred Stock issued to ATV III Affiliates Fund, L.P. | |
| (8) | Includes 136,735 shares of Series 1 Preferred Stock, 353,223 shares of Series 2 Preferred Stock, 31,876 shares of Series 3 Preferred Stock, 89,110 shares of Series 4 Preferred Stock issued to Asset Management Associates 1984, L.P., 119,137 shares of Series 2 Preferred Stock, 198,454 shares of Series 3 Preferred Stock, 73,289 shares of Series 4 Preferred Stock, and 86,393 shares of Series 5 Preferred Stock issued to Asset Management Associates 1989, L.P. Craig C. Taylor, a member of our board of directors, is a general partner of AMC Partners 84, L.P. and AMC Partners 89, L.P., and the general partner of Asset Management Associates 1984, L.P. and Asset Management Associates 1989, L.P. | |
| (9) | Includes 416,666 shares of Series 2 Preferred Stock, 349,817 shares of Series 3 Preferred Stock and 54,255 shares of Series 4 Preferred Stock issued to STF II, L.P. Nancy Burris, a member of our board of directors, is an affiliate of Indosuez Ventures, which is affiliated with STF II, L.P. |
INDEMNIFICATION AGREEMENTS
We have entered into indemnification agreements with our directors and executive officers for the closingindemnification of the saleand advancement of the shares of Common Stock offered hereby.
In April 1996, the Company obtained a $2.0 million aggregate committed line
of credit from certain of the Company's existing investors, pursuantexpenses to which
Aeneas Venture Corporation agreed to lend the Company up to $250,000, Asset
Management Associates agreed to lend the Company up to $356,118, Enterprise
Partners agreed to lend the Company up to $356,118, a fund affiliated with
Indosuez Partners agreed to lend the Company up to $265,134, and certain other
investors of the Company agreed to lend the Company an aggregate of up to
$772,630 in exchange for convertible secured promissory notes (the "Notes")
that will become due and payable upon the earlier of 30 days following the
completion of this offering or May 1997 (the "Line of Credit"). The Company
intends to use a portion of the proceeds obtained from this offering to repay
any indebtedness incurred pursuantthese persons to the Line of Credit. In connectionfullest extent permitted by law. We also intend to enter into these agreements with the Line of Credit, the Company issued warrants to purchase Common Stock,
exercisable at 85% of the offering price in an amount equal to 10% of their
respective commitments under the Line of Credit plus an additional 5% of the
outstanding principal amount of their respective Notes issued under the Line
of Credit for each full or partial calendar month that such principal amount
remains outstanding. The aggregate warrant coverage that the Company is
entitled to provide to each investor pursuant to the Line of Credit is capped
at 50% of each investor's contribution to the aggregate amount of the Notes
issued under the Line of Credit. The warrants issued in connection with the
Line of Credit are not exercisable for a period of one year following the
closing of this offering and expire on April 30, 2001, or earlier upon the
sale of all or substantially all of the assets of the Company or upon the
acquisition of the Company by another entity pursuant to which the
stockholders of the Company immediately prior to such acquisition possess a
minority of the voting power of the acquiring entity immediately following
such acquisition.
54
In December 1992, Adeza entered into a promissory note agreement with Daniel
O. Wilds, the Company's President and Chief Executive Officer, which allowed
Mr. Wilds to borrow up to $140,000. Interest on the note accrues at a rate of
6.15% per annum. The note and accrued interest are due upon the earlier of (i)
voluntary termination, (ii) 12 months after involuntary termination, (iii)
closing of an initial underwritten public offering of the Common Stock, (iv)
merger of the Company, (v) sale of all the assets of the Company or (vi) July
19, 1997. As of April 30, 1996, the outstanding principal balance under this
note was $100,000.
The Company believes that all of the transactions set forth above were made
on terms no less favorable to the Company than could have been obtained from
unaffiliated third parties. Allour future transactions, including loans, between
the Company and its officers, directors, principal stockholders and their
affiliates will be approved by a majority of the Board of Directors, including
a majority of the independent and disinterested directors and will continue to
be on terms no less favorable toexecutive officers.
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
See “Management—Employment, Severance and Change of Control Agreements.”
LOANS TO EXECUTIVE OFFICERS
We loaned Mark Fischer-Colbrie $109,800 in February 2003. In August 2004, the Company than could be obtained from
unaffiliated third parties.
55
PRINCIPAL AND SELLING STOCKHOLDERS
$76,000 unpaid balance and interest of Mr. Fischer-Colbrie’s loan was forgiven. See “Management—Employment, Severance and Change of Control Agreements.”
Principal stockholders
The following table sets forth certainshows information with respect to the beneficial ownership of the Company's Common Stockour common stock as of AprilJune 30, 1996 (after
giving effect to the conversion of the Company's Series 1 and Series 2
Preferred Stock into Common Stock at a one-to-one ratio),2004 and as adjusted to reflect the sale of sharesthe common stock being offered hereby, as to (i) each person (or group of
affiliated persons) known by the Company to own beneficially more than 5% of
the Company's outstanding Common Stock, (ii) each of the Company's directors,
(iii) each of the Named Executive Officers and (iv) all currentin this offering by:
| 4 | each of our directors; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | each of our named executive officers; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | all of our directors and executive
Beneficial ownership and percentage ownership The following table reflects the conversion of all shares of our preferred stock outstanding as of June 30, 2004 into an aggregate of 15,943,176 shares of our common stock that will become effective at the closing of this offering. This table is based on 16,186,156 shares of our common stock (reflecting the conversion of our preferred stock into our common stock) outstanding as of June 30, 2004 and shares outstanding immediately after this offering. Unless otherwise indicated, the address of each stockholder is c/o Adeza Biomedical Corporation, 1240 Elko Drive, Sunnyvale, California 94089. 72 Principal stockholders
73 Principal stockholders
74 Description of capital stock The description below of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to GENERAL Our amended and restated certificate of
COMMON STOCK Each holder of Subject to preferences that may be applicable to any then outstanding Holders of 75 Description of capital stock PREFERRED STOCK Upon the closing of this offering, our board of directors will be authorized, subject to any limitations prescribed by law, without stockholder approval, to issue up to an aggregate of 5,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions granted to or imposed upon the preferred stock, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences. The rights of the holders of common stock will be subject to, and may be adversely affected by, the rights of holders of any preferred stock that may be issued in the future. WARRANTS In March 1999, we issued two warrants to purchase a total of 219,178 shares of Series 3 Preferred Stock STOCK OPTIONS We intend to file a registration statement under the Securities Act covering 5,375,033 shares of As of June 30, 2004, options to purchase 1,981,744 shares of common stock were issued and outstanding at REGISTRATION RIGHTS We and the holders of our preferred stock entered into an amended and restated investor rights agreement, dated September 19, 2001. This agreement provides these holders with customary demand and piggyback registration rights with respect to the shares of common stock to be issued upon conversion of their preferred stock or exercise of their warrants. Demand registration According to the terms of the amended and restated investor rights agreement, holders of 40% of common stock issued or issuable upon conversion of the outstanding preferred stock and upon the exercise of stock purchase warrants of the company have the right to require us to register their shares with the SEC for resale to the public. To demand such a registration, holders who hold together an aggregate of at least 40% of the shares having registration rights must request a registration that is reasonably expected to have an 76 Description of capital stock Piggyback registration If we file a registration statement for a public offering of any of our securities (other than a registration relating solely to employee stock option or purchase plans, or a registration relating solely to an SEC Rule 145 transaction, or a registration on any form other than Form S-1, S-2 or S-3, or any successor to such form which does not include substantially the same information as would be required to be included in a registration statement), the holders of common stock issued or issuable upon conversion of the outstanding preferred stock will have the right to include their shares in the registration statement. Form S-3 registration At any time after we become eligible to file a registration statement on Form S-3, the holders of common stock issued or issuable upon conversion of the outstanding preferred stock may require us to file a Form S-3 registration statement, provided that the aggregate disposition price of These registration rights are subject to certain conditions and limitations, including the right of the underwriters of an ANTI-TAKEOVER EFFECTS OF PROVISIONS OF OUR AMENDED AND RESTATED CERTIFICATE OF INCORPORATION AND BYLAWS AND DELAWARE LAW Amended and restated certificate of incorporation and bylaws Some provisions of Delaware law and our amended and restated certificate of incorporation and bylaws contain provisions that could make the following transactions more difficult:
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids and to promote stability in our management.
77 Description of capital stock
These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. Delaware anti-takeover statute We are subject to Section 203 of the Delaware General Corporation Law. This law prohibits a publicly held Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless:
Section 203 defines “business combination” to include:
78 Description of capital stock
In general, Section 203 defines an “interested stockholder” as an entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person. LIMITATION OF LIABILITY Our amended and restated certificate of incorporation provides that no director shall be personally liable to us or to our stockholders for monetary damages for breach of fiduciary duty as a director, except that the limitation shall not eliminate or limit liability to the extent that the elimination or limitation of such THE NASDAQ NATIONAL MARKET We intend to apply for the quotation of TRANSFER AGENT AND REGISTRAR Upon the closing of this offering, the transfer agent and registrar for our common stock will be . 79 Shares eligible for future sale Prior to this offering, there was no public market for our common stock. Future sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur, could adversely affect the price of our common stock. Based on the number of shares outstanding as The holders of shares of outstanding common stock as of After the offering, the holders of In general, under Rule 144, as currently in effect, an affiliate of ours who beneficially owns shares of our common stock that are not restricted securities, or a person
Sales under Rule 144 are also subject to Generally, an employee, officer, director or consultant who purchased shares of our common stock before the effective date of the registration statement of which this 80 Shares eligible for future sale are our affiliates may generally sell their eligible securities under Rule 701, commencing 90 days after the effective date of the registration statement of which this prospectus is a part, without having to comply with Rule 144’s one-year holding period restriction. Neither Rule 144 nor Rule 701 supersedes the contractual obligations of our security holders The 16,186,156 pro forma shares of
Additionally, of the 2,244,300 shares issuable upon exercise of options or warrants to purchase our common stock outstanding as of June 30, 2004, approximately shares will be vested and eligible for sale 180 days after the date of this prospectus. EQUITY COMPENSATION We have reserved an aggregate of 5,375,033 shares of 81 Underwriting We are offering the shares of our common stock described in this prospectus through the underwriters named below. UBS Securities LLC, SG Cowen & Co., LLC, Thomas Weisel Partners LLC, and William Blair & Company, L.L.C. are the representatives of the underwriters. UBS Securities LLC is the sole book-running manager of this offering. We have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of common stock listed next to its name in the following table:
The underwriting agreement provides that the underwriters must buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below. Our common stock is offered subject to a number of conditions, including:
The representatives have advised us that the underwriters intend to make a market in our common stock In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically. Sales of shares made outside of the United States may be made by affiliates of the underwriters. OVER-ALLOTMENT OPTION We have granted the underwriters an option to buy up to additional shares of our common stock. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above. COMMISSIONS AND DISCOUNTS Shares sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from the initial public offering price. If all the shares are not sold at the initial public offering price, the representatives may change the offering price and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to 82 Underwriting purchase the shares at the prices and upon the terms stated therein and, as a result, will thereafter bear any risk associated with changing the offering price to the public or other selling terms. The underwriters have informed us that they do not expect discretionary sales to exceed 5% of the shares of common stock to be offered. The following table shows the per share and total underwriting discounts and commissions we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional shares.
We estimate that the total expenses of the offering payable by us, excluding underwriting discounts and commissions, will be approximately $ . NO SALES OF SIMILAR SECURITIES We, our executive officers and directors and our existing stockholders have entered into lock-up agreements with the underwriters. Under these agreements, subject to certain exceptions, we and each of these persons may not, without the prior written approval of UBS Securities LLC, offer, sell, contract to sell or otherwise dispose of, directly or indirectly, or hedge our common stock or securities convertible into or exchangeable or exercisable for our common stock. These restrictions will be in effect for a period of 180 days after the date of this prospectus. The 180-day lock-up period may be extended under certain circumstances where we release, or pre-announce a release of, our earnings or material news or a material event shortly before or after the termination of the 180-day period. At any time and without public notice, UBS Securities LLC may in its sole discretion release all or some of the securities from these lock-up agreements. INDEMNIFICATION We have agreed to indemnify the underwriters against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities. NASDAQ NATIONAL MARKET QUOTATION We have applied to have our common stock approved for quotation on The Nasdaq National Market under the trading symbol “ADZA.” PRICE STABILIZATION, SHORT POSITIONS In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including:
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. These transactions may also include making short sales of our common stock, which involve the sale by the underwriters 83 Underwriting of a greater number of shares of common stock than they are required to purchase in this offering and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked short sales,” which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. Naked short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchased in this offering. The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions. As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on The Nasdaq National Market, in the over-the-counter market or otherwise. DETERMINATION OF OFFERING PRICE Prior to this offering, there has been no public market for
DIRECTED SHARE PROGRAM At our request, certain of the 84 Underwriting Certain employees and other persons purchasing these reserved shares will be prohibited from disposing of or hedging the AFFILIATIONS Certain of the underwriters and their affiliates have in the past provided and may from time to time provide certain commercial banking, financial advisory, investment banking and other 85 Legal matters The validity of the Experts Ernst & 86 Where you can find more information We have filed with the You may read and copy the registration statement of which this prospectus is We maintain Internet websites at This prospectus includes statistical data obtained from industry publications. These industry publications generally indicate that the 87 Adeza Biomedical Corporation INDEX TO
F- 1 Report of Ernst & The Board of Directors and Stockholders Adeza Biomedical Corporation We have audited the accompanying We conducted our audits in accordance with In our opinion, the financial statements referred to above present fairly, in all material respects, the
Palo Alto, California March 8, 2004, except as to which the date is F- 2 Adeza Biomedical Corporation BALANCE SHEETS
See accompanying notes. F- 3 Adeza Biomedical Corporation STATEMENTS OF (in thousands, except share and per share
See accompanying notes. F- 4 Adeza Biomedical Corporation STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) (in thousands, except share and per share information)
See accompanying notes. F- 5 Adeza Biomedical Corporation STATEMENTS OF CASH FLOWS (in thousands)
See accompanying notes. F- 6 Adeza Biomedical Corporation NOTES TO December 31,
Organization and business Adeza Biomedical Corporation The Company’s primary products consist of:
Basis of Presentation The Company operates in one business segment, women’s health products. Certain reclassifications of prior year amounts have been Use of The preparation of financial statements in conformity with US generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates. Unaudited interim results The Unaudited pro forma convertible preferred stock and In F- 7 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the Revenue recognition Revenue from product sales is recognized when there is persuasive evidence an arrangement exists, delivery to the customer has occurred, the price is fixed or determinable and collectibility is reasonably assured. Contract revenues are recorded as performance occurs and the related earnings process is completed based on the performance requirements of the contract. In June 1999, Adeza entered into a Co-Promotion and Exclusive Distribution Agreement with a major US distributor (the “Distributor”). The Co-Promotion and Exclusive Distribution Agreement with the Distributor was terminated as of June 30, 2002. Under the terms of the agreement through June 30, 2002, the revenue earned for the sale of products to the Distributor was not fixed and determinable until the time the products were sold by the Distributor to the end user. Consequently, the Company Effective July 1, 2002, Adeza entered into a services agreement with a national laboratory that performs diagnostic tests. Under the terms of the agreement, the laboratory provides certain domestic product distribution and testing services for Adeza. The Company recognizes revenue upon the shipment of products to the end user as the title, risks and rewards of ownership of the products pass from the Company to the end user at that time. Revenue from the Company’s laboratory services is recognized as tests are performed. Revenue on all other product sales is recognized upon shipment to distributors as the title, risks, and rewards of ownership of the products pass to the distributors and the selling price of Adeza products is fixed and determinable at that point. Any advance payments received in excess of revenue recognized are classified as deferred revenue on the accompanying balance sheets. Customers have the right to return products that are defective. There are no other return rights. During the years ended December 31, 2001, 2002 and 2003, 87%, 94%, and 97% and the periods ended June 30, 2003 and 2004, 96% and 97%, respectively, of the Company’s product revenues were derived from customers located in the United States. Warranty policy The Company records a liability for product warranty obligations at the time of sale based upon historical warranty experience. The term of the warranty is generally twelve months. The Company also records any additional liability required for specific warranty matters when they become known F- 8 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) and are reasonably estimable. The Company’s product warranty obligations are included in other accrued liabilities as follows:
Research and development Research and development expenses consist of costs incurred for Company-sponsored and collaborative research and development activities. These costs include direct and research-related allocated overhead expenses such as facilities costs, salaries and benefits, and material and supply costs. Research and development expenses under collaborative agreements and other contracts approximate the revenue recognized under such agreements. The Company expenses research and development costs as such costs are incurred. Concentrations of risk Cash and cash equivalents, and accounts receivable are financial instruments which potentially subject Adeza to concentrations of credit risk. Adeza primarily invests in money market funds, and, by policy, limits the amount in any one type of investment, other than securities issued or guaranteed by the U.S. government. Adeza has not experienced any material credit losses and does not generally require collateral on receivables. For the year ended December 31, 2003 and the six-months ended June 30, 2003 and 2004, no single customer represented greater than 5% of total revenues. For the year ended December 31, 2001 and 2002, sales through the Distributor accounted for 86% and 24%, respectively, of Adeza’s total product sales. Cash and cash equivalents Cash equivalents consist of highly liquid financial instruments with original maturities of three months or less at the time of acquisition. Cash equivalents consist of money market funds held by a high-credit quality financial institution. Cash and cash equivalents are stated at cost which approximates fair value at December 31, 2002 and 2003 and June 30, 2004 based on available market information. Derivative financial instruments The Company holds no derivative financial instruments and does not currently engage in hedging activities. Property and equipment Property and equipment are stated at cost. Depreciation is calculated using the straight-line method, and the cost is amortized over the estimated useful lives of the assets, generally three to five years. F- 9 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter. Intangible assets Intangible assets consist of purchased patents. Accumulated amortization at December 31, 2003 and June 30, 2004, was $16,000 and $40,000, respectively. Intangible assets are amortized over their estimated useful lives of 5 years. Amortization expense is expected to be $48,000 per year in 2004 through 2007 and $32,000 for the year ended December 31, 2008. Long-lived assets The Company reviews long-lived assets, including property and equipment, and intangible assets for impairment whenever events or changes in business circumstances indicate that the carrying amounts of the assets may not be fully recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition are less than its carrying amount. Impairment, if any, is assessed using discounted cash flows. Through June 30, 2004, there have been no such impairment losses. Income taxes Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance to the amount that is more likely than not, in the opinion of management, to be realized. Stock-based compensation As permitted by Statement of Financial Accounting Standards No. 123,Accounting for Stock-Based Compensation(SFAS 123) as amended by Statement of Financial Accounting Standards No. 148,Accounting for Stock-Based Compensation—Transition and Disclosure(SFAS 148), the Company has elected to account for stock options granted to employees and directors using the intrinsic value method and, accordingly, does not recognize compensation expense for stock options granted to employees and directors with exercise prices equal to the fair value of the underlying common shares. Options granted to nonemployees have been accounted for in accordance with SFAS 123 and Emerging Issues Task Force Consensus No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, and are periodically remeasured with the resulting value charged to expense over the period of the related services being rendered. Pro forma information regarding net loss is required by SFAS 123, as if Adeza had accounted for its employee and director stock options granted under the fair value method of SFAS 123. The fair value F- 10 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) for these options was estimated at the date of grant using the Black-Scholes option pricing model and the following weighted-average assumptions:
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, the model requires the input of highly subjective assumptions, including the expected life of the option. Because Adeza’s employee and director stock options have characteristics significantly different from those of traded options and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee and director stock options. For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The pro forma information is as follows:
Allowance for doubtful accounts The Company maintains an allowance for doubtful accounts related to the estimated losses that may result from the inability of its customers to make required payments. This allowance is determined based upon historical experience and any specific customer collection issues that have been identified. F- 11 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) Historically, the Company has not experienced significant credit losses related to an individual customer or groups of customers in any particular industry or geographic area. The Company’s allowance for bad debt is included in accounts receivable as follows:
In addition to the bad debt reserve, the Company’s allowance for sales returns is included in accounts receivable as follows:
Inventories Inventories are stated at the lower of standard cost (which approximates actual cost) or market. Advertising expense The cost of advertising is expensed as incurred. Advertising expense for the years ended December 31, 2001, 2002, and 2003 was approximately $124,000, $334,000, and $941,000, respectively, and $454,000 and $193,000 for the six months ended June 30, 2003 and 2004, respectively. The cost of advertising was included in selling and marketing expenses in the statements of operations. Shipping and handling costs Shipping and handling costs incurred for inventory purchases and product shipments are included within cost of product sales in the statements of operations. Recent accounting pronouncements In January 2003, the Financial Accounting Standards Board (FASB), issued Interpretation No. 46,Consolidation of Variable Interest Entities(FIN 46). FIN 46 requires a variable interest entity to be F- 12 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity’s activities or entitled to receive a majority of the entity’s residual returns or both. A variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. The Company’s adoption of the requirements of FIN 46 did not have an impact on its financial position or results of operations. Net income (loss) per share Basic net income (loss) per share is calculated by dividing the net income (loss) by the weighted-average number of common shares outstanding for the period. Diluted net loss per share is computed by dividing the income (loss) by the weighted-average number of common shares outstanding for the period and dilutive potential common shares. For purposes of this calculation, common stock subject to repurchase by the Company, preferred stock, options, and warrants are considered to be potential common shares and are only included in the calculation of diluted net income (loss) per share when their effect is dilutive.
F- 13 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited)
On September 9, 2003, the Company completed the acquisition of substantially all of the assets of Biex, Inc. to expand its diagnostic product pipeline. Biex’s assets primarily consisted of several patents relating to FDA-approved preterm labor diagnostic testing products. The Biex acquisition has been accounted for as an acquisition of assets rather than as a business combination in accordance with the criteria outlined in Emerging Issues Task Force 98-3, because, at the date of acquisition, Biex lacked all of the elements of a business because it did not have any employees or processes. The aggregate purchase price of the Biex assets was $250,000 in cash. The following table summarizes the purchase price allocation (in thousands):
The purchased patents relate to methods of predicting premature labor. The Company believes that the patents may allow for complementary products to its existing product lines. The Company allocated the purchase price to the tangible and intangible assets acquired based on their estimated fair values. Such valuations require management to make significant estimates and assumptions, especially with respect to intangible assets. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable.
Adeza has entered into license, clinical trial, supply, and sponsored research and development agreements with universities, research organizations, and commercial companies. Certain of these agreements require payments of royalties on future sales of products resulting from such agreements and may subject Adeza to minimum annual royalty payments to such contract partners. During the years ended December 31, 2001, 2002, and 2003, the total of such royalty costs recorded were approximately $98,000, $253,000 and $3,330,000, respectively, and $1,575,000 and $1,921,000 for the six months ended June 30, 2003 and 2004, respectively. The royalty costs are included in cost of product sales. F- 14 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited)
In June 1999, Adeza entered into a Co-Promotion and Exclusive Distribution Agreement with the Distributor. This agreement was mutually terminated by both parties as of June 30, 2002. Under the terms of this agreement, the Distributor had the right to co-market and distribute, within the United States of America and its territories and possessions excluding the Commonwealth of Puerto Rico, certain existing products and next-generation improvements to those existing products related to their use for certain clinical indications owned by Adeza. In consideration of the co-promotion and exclusive distribution rights, and other terms and conditions, the Distributor paid Adeza a nonrefundable one-time payment of $2,000,000 in 1999. The Distributor was also to pay Adeza a total of up to $2,000,000 during 2001 and 2002 to fund certain portions of the research and development efforts related to the products and services covered by this agreement. During the years ended December 31, 2001, 2002 and 2003, the Company received $244,000, $176,000, and $0, respectively, of such research and development funding. No amounts were received in the six months ended June 30, 2003 and 2004. This funding is included within contract revenue in the statements of operations. The 1999 nonrefundable, one-time $2,000,000 payment received from the Distributor was deferred and was being recognized as revenue over the estimated five-year life of the agreement. At the termination of the agreement, Adeza recognized the balance of the deferred contract revenue in 2002, net of costs related to the contract termination. The amount of revenue recognized for the years ended December 31, 2001, 2002 and 2003 was $400,000, $574,000, and $0, respectively. As a result of the mutual termination of the contract, Adeza agreed to pay the Distributor $5,771,000. This amount, primarily related to deferred product revenue received by Adeza from sales to the Distributor, was reclassified from deferred revenue to notes payable excluding $273,000 of imputed interest to be recorded over the repayment period, in 2002 (see Note 7).
Inventories consist of the following (in thousands):
F- 15 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited)
Property and equipment consists of the following (in thousands):
Included in laboratory and other equipment at December 31, 2002 are assets with a cost of $70,000 acquired pursuant to capital lease obligations. Related accumulated amortization for these leased assets was $51,000 as of December 31, 2002. During 2003, the capital lease reached its term. As such, the assets underlying this lease were purchased and are no longer carried under capital lease. The assets continue to be included within laboratory and other equipment.
Leases Future minimum obligations under noncancelable operating leases at December 31, 2003 are as follows (in thousands):
Rent expense was approximately $300,000, $297,000, $195,000, $97,000, and $103,000 for the years ended December 31, 2001, 2002, 2003, and the six-month periods ended June 30, 2003 and 2004 (unaudited), respectively. Notes payable In March 1999, Adeza entered into a loan and security agreement with MMC/ GATX Partnership No. 1 and Transamerica Business Credit Corporation. Under the terms of the agreement, Adeza had $4,000,000 available as a loan for a period of 36 months, against which it borrowed $3,000,000 in March 1999 and an additional $1,000,000 in July 1999. Monthly payments under the notes were interest only through May 2000 and then reverted to combined principal and interest payments. The interest rate applicable to the borrowings was 13.66%. Adeza’s assets secured this borrowing. The final principal and interest payments were made as of March 31, 2002. In June 2002, Adeza entered into an agreement to terminate the Co-Promotion and Distribution Agreement between Adeza and the Distributor (see Note 4). Under the terms of the agreement, Adeza agreed to pay the Distributor $5,771,000 under a note payable due through June 30, 2003. In December 2002, $1,443,000 was paid to the Distributor. The remaining payments were paid in March and June 2003. The note was secured by Adeza’s assets. In the years ended December 31, 2002 and 2003, Adeza recorded $167,000 and $106,000, respectively, of interest expense related to this note. $106,000 was recorded in the six-month period ended June 30, 2003. No interest expense was recorded in the six-month period ended June 30, 2004. F- 16 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited)
Convertible preferred stock Convertible preferred stock consists of the following (in thousands, except share information):
Upon completion of, and subordinate to, the distribution of any Series 5 convertible preferred stock dividends, each share of Series 4 convertible preferred stock entitles the holder to receive, if and as declared, cumulative dividends of $0.463. Upon completion of and subordinate to the distribution to holders of Series 4 convertible preferred stock dividends, each share of Series 1, 2, and 3 convertible preferred stock entitles the holder to receive, if and as declared, noncumulative dividends of $0.24, $0.24, and $0.292 per share, respectively. If dividends are declared on Series 1, 2, 3, or 4 convertible preferred stock, all such series of convertible preferred stock must receive dividends. Series 5 convertible preferred stock entitles the holder to receive, if and as declared, cumulative dividends of $0.463 per share. No dividends have been declared to date. Each share of Series 1, 2, 4, and 5 convertible preferred stock is convertible, at the option of the holder, into one share of common stock. Each share of Series 3 convertible preferred stock is convertible, at the option of the holder, into such number of shares of common stock as determined by dividing $2.92 by the Series 3 conversion price of $2.628. The Series 1, 2, 3, 4, and 5 convertible preferred stock is convertible at the option of the holder, automatically upon a public offering with a price per share of at least $6.95 and aggregate proceeds greater than $15,000,000 or on the affirmative vote or written consent of the holders of at least 66 2/3% of Series 1, 2, 3, 4, or 5 convertible preferred stockholders consenting as a single class, respectively. Upon liquidation of Adeza, the holders of Series 5 convertible preferred stock shall have a liquidation preference, prior and in preference to any distribution to the holders of Series 1, 2, 3, and 4 convertible preferred stock of $9.26 per share plus any declared but unpaid dividends. Following the liquidation payments to the holders of Series 5 preferred stock, the holders of Series 4 convertible preferred stock shall have a liquidation preference, prior and in preference to any distribution to the holders of Series 1, 2, or 3 convertible preferred stock of $9.26 per share plus any declared but unpaid dividends. Following the liquidation payments to the holders of Series 4 and 5 preferred stock, the holders of Series 1, 2, and 3 convertible preferred stock shall be entitled to $2.40, $2.40, and $2.92 per share, respectively, plus any declared but unpaid dividends. A merger or consolidation of the Company in which the F- 17 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) company approval. Therefore, such a payment to the preferred stockholders is not solely within the Company’s control. The carrying value of the convertible preferred stock has not been adjusted to the liquidation value of such shares as it is not probable that such a payment will occur due to the potential for conversion of the preferred stock prior to any such change-in-control. After the The Series 1, 2, 3, 4, and 5 preferred stockholders have voting rights equal to the common stockholders on an as-if-converted basis. The Company has reserved 236,301 shares of convertible preferred stock for issuance upon the exercise of warrants at December 31, 2003 and June 30, 2004. Warrants In conjunction with a convertible note financing, Adeza issued warrants to purchase 190,259 shares of the In conjunction with the loan and security agreement with MMC/ GATX Partnership No. 1 and Transamerica Business Credit Corporation, Adeza issued warrants to purchase 236,301 shares of Series 3 convertible preferred stock at an exercise price of $2.92 per share. The warrants are scheduled to expire on the later of ten years from the date of the grant or five years after the closing of a All preferred stock underlying the warrants is convertible into common stock at a conversion ratio of 1:1.11111. F- 18 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) Common stock The Company has reserved the following shares of common stock for the issuance of options and rights granted under the Company’s stock option plans, the conversion of preferred stock and
Stock The Company Option activity under the 1995 Plan is as follows:
F- 19 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) The following summarizes options outstanding and exercisable as of December 31, 2003:
The following summarizes options outstanding and exercisable as of June 30, 2004:
The weighted-average fair value of options granted during 2001, 2002 and 2003 and the six months ended June 30, 2004, was $1.64, $1.36, $1.96, and $1.86, respectively. During the six months ended June 30, 2004, the Company recorded deferred stock compensation for the excess of the estimated fair value of its common stock over option exercise prices at the date of grant of approximately $106,000 related to options granted to employees and directors. Stock-based compensation expense is being recognized over the option vesting period of four years using the straight-line method. For options granted to nonemployees, the Company determined the estimated fair value of the options using the Black-Scholes option pricing model. Compensation expense is generally being recognized over the option vesting period. For the years ended December 31, 2002 and 2003 and the six months ended June 30, 2004, the Company recorded stock-based compensation expense of approximately $5,000, $23,000 and 11,000, respectively, in connection with options granted to nonemployees. No stock-based compensation expense was recorded for the F- 20 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited)
The Company’s accumulated deficit differs from the federal net operating loss carryforwards due to the losses of Adeza’s foreign subsidiary and temporary differences, which consist primarily of certain expenses not currently deductible for tax reporting purposes. The provision for income taxes consists of the
The Company’s income tax provision differs from the amounts computed by applying the federal statutory income tax rate of 34% to pretax income (loss) as follows:
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows:
F- 21 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) Because of Adeza’s lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance (increased) decreased by $(3,200,000), $1,800,000 and $1,860,000 during the years ended December 31, 2001, 2002 and 2003, respectively. As of December 31, 2003, the Company had federal and state net operating loss carryforwards of approximately $40,300,000 and $22,800,000, respectively. The Company also had federal and state research and development tax credit carryforwards of approximately $1,100,000 and $900,000, respectively. The federal net operating loss and tax credit carryforwards will expire at various dates beginning in 2004 through 2022, if not utilized. The state net operating loss carryforwards will expire at various dates beginning in 2004 through 2013, if not utilized. The state research and development tax credits carry forward indefinitely. Utilization of the Company’s net operating losses and research and development tax credits may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses and research and development tax credits before utilization.
The Company’s 401(k) Plan allows eligible employees to make contributions of their qualified compensation subject to IRS limits. The Company has the discretion to make matching contributions each year. The Company has not made any matching contributions to date.
In 2000, a loan offer was made to an officer of the Company. The agreement to the loan was ratified by the Board of Directors on April 21, 2001, for an amount of $183,000, with the minimum interest rate allowed by the internal revenue service. According to the terms agreed upon, 20% of the loan would be forgiven in principal and accrued interest at the end of each twelve months of employment. The loan would be due and payable within 30 days following termination by Adeza for cause. In the event of a change of control or merger with another company or of termination without cause, the loan and accumulated interest would be forgiven. The loan contemplated was executed on February 28, 2003 for $109,800 which was paid to the officer at that time. All other terms were in accordance with the original loan offer. Subject to the officer’s continued employment, the loan and related interest will be forgiven in 2003 to 2006. In the year ended December 31, 2003 and the six months ended to June 30, 2004, $33,550 and $18,300, respectively, of the principal was forgiven and recorded to general and administrative expenses.
Note receivable On August 4, 2004 the Company upon approval of its board of directors forgave the remaining balance of the note receivable from an officer. At this time, the remaining unamortized balance and interest totaling approximately $58,000 at June 30, 2004 was recorded as a general and administrative expense. F- 22 Adeza Biomedical Corporation NOTES TO FINANCIAL STATEMENTS (continued) December 31, 2003 (Information pertaining to June 30, 2004 and the six months ended June 30, 2004 and 2003 is unaudited) Employee option plans 2004 Equity Incentive Plan In August 2004, the Company’s board of directors and stockholders approved the 2004 Equity Incentive Plan (the 2004 Plan), which will become effective upon the completion of its initial public offering. The Company has reserved a total of 2,500,000 shares of its common stock for issuance under the 2004 Plan, all of which are available for future grant. 1995 Stock Option and Restricted Stock Plan In August 2004, the Company’s board of directors and stockholders approved amendments to the 1995 Plan to increase the number of shares reserved for issuance thereunder by 750,000 shares and to amend the provisions of the 1995 Plan governing the treatment of certain awards under the 1995 Plan in certain corporate transactions. Upon completion of the Company’s initial public offering, any shares that are available for future grant under the 1995 Plan, and any shares that are issuable upon exercise of options outstanding under the 1995 Plan that are forfeited after the completion of the Company’s initial public offering, will be allocated to the 2004 Plan. Option Grants In July 2004, we granted F- 23
Part II INFORMATION NOT REQUIRED IN PROSPECTUS Item 13. The following table sets forth the costs and expenses, other than underwriting discounts and commissions,
Item 14. Section 145 of the Delaware General Corporation Law authorizes a court to award, or a As permitted by the Delaware General Corporation Law, the Registrant’s amended restated certificate of incorporation includes a provision that eliminates the personal liability of its directors for monetary damages for breach of fiduciary duty as a director. As permitted by the Delaware General Corporation Law, the bylaws of the The Registrant has entered into II- 1 Part II The Underwriting Agreement provides for indemnification by the The indemnification provisions in the Registrant’s restated certificate of incorporation, restated bylaws and the indemnification agreements entered into between the Registrant and each of its directors and executive officers may be sufficiently broad to permit indemnification of the The Registrant has obtained liability insurance for its officers and Reference is made to the following documents filed as exhibits to this Registration Statement regarding relevant indemnification provisions described above and elsewhere in this prospectus:
Item 15. Recent sales of unregistered securities In the 1. We have issued options to 2. In 3. In November 2001, we issued 25,000 shares of our common stock to an investor for an aggregate cash consideration of $250. 4. In December 2003, we issued 190,259 shares of our Series 3 preferred stock upon exercise of warrants for an aggregate cash consideration of approximately $500,000. II- 2 Part II Item
Item 17. The undersigned Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the II- 3 Part II person of the The undersigned registrant hereby undertakes that:
II- 4 Signatures Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement
Power of attorney We, the undersigned directors and/or Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities
II- 5 Exhibit Index
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
