
As filed with the Securities and Exchange Commission on May 5, 2023
Registration No. 333-333-271413
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1 TO
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
| MODULAR MEDICAL, INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | 3841 | 87-0620495 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) | ||
16772 W. Bernardo Drive
10740 Thornmint Road
San Diego,, California92127
(858)800-3500
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
James E. Besser
Chief Executive Officer
16772 W. Bernardo Drive
10740 Thornmint Road
San Diego, California 92127
(858) 800-3500
(Name, address, including zip code, and telephone
number, including
area code, of agent for service)
With copies to:
Joseph M. Lucosky, Esq.
Lawrence Metelitsa, Esq.
Lucosky Brookman LLP
101 Wood Avenue South, 5th Floor
Woodbridge, New Jersey 08830
Tel. No.: (732) 395-4400
Fax No.: (732) 395-4401
Joseph Lucosky Lawrence Metelitsa Lucosky Brookman LLP 101 Wood Avenue South Woodbridge, NJ 08830 (732) 395-4400 | Stephen E. Older David S. Wolpa McGuireWoods LLP 1251 Avenue of the Americas, 20th Floor New York, NY 10020 (212) 548-2100 |
| As soon as practicable after the effective date of this registration statement |
| (Approximate date of commencement of proposed sale to the public) |
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o☐
If this Form is a post-effective amendment filed pursuant to ruleRule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer | ||
| Non-accelerated Filer ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by checkmarkcheck mark if the registrant has elected not elected to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. o☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuantpursuant to said Section 8(a), may determine.
PRELIMINARY PROSPECTUS SUBJECT TO COMPLETION DATED JUNE 6, 2022
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities andnor does it is not soliciting offersseek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.

This prospectus relatesPRELIMINARY PROSPECTUS

2,116,402 Units
Each Unit Consisting of Two Shares of Common Stock and
One Warrant to Purchase One Share of Common Stock
We are offering 2,116,402 units (each a “unit” and collectively, the resale“units”) of up to 1,438,202Modular Medical, Inc. (the “Company,” “Modular Medical,” “we,” “our” or “us”) with each unit consisting of two shares of our common stock, par value $0.001 per share, which we refer to as the “common stock,” and one warrant (a “warrant”) to purchase one share of Modular Medical, Inc. (“we,” “us,” “our,”our common stock. The public offering price is $ per unit. The units have no stand-alone rights and will not be certificated or issued as stand-alone securities. We do not intend to apply for listing of the “Company”), consistingwarrants on any national securities exchange. The shares of up to 1,438,202our common stock and the warrants comprising the units will be issued separately. The warrants included in the units are exercisable immediately, will expire five years from the date of issuance and have an exercise price of $ per share (120% of the public offering price per unit sold in this offering.) This offering also includes the shares of common stock issuable from time to time upon exercise of the warrants. The warrants (the “May Warrants”) to purchase shares of common stock at an exercise price of $6.60 per share originallywill be issued by us on May 5, 2022 in a private placement of warrants that occurred concurrently with a registered offering of shares of common stock (the “May Offering”).
This registration does not mean that the selling stockholder named herein will actually offer or sell any of these shares. We will not receive any proceeds from the resale of any of the shares of common stock being registered hereby sold by the selling stockholders. However, we may receive proceeds from the exercise of the May Warrants held by the selling stockholders exercised, other thanbook-entry form pursuant to any applicable cashless exercise provisions of such warrants.a warrant agency agreement between us and Colonial Stock Transfer Company, Inc. as warrant agent (the “Warrant Agent”).
Our common stock is listed on the Nasdaq Capital Market, or Nasdaq, under the symbol “MODD.” On JuneMay 2, 2022,2023, the last reported sale price of our common stock was $4.75$1.89 per share.
Following the effectiveness of the registration statement of which this prospectus forms a part, the sale and distribution of securities offered hereby may The public offering price per unit will be effected from time to time in one or more transactions that may take place on Nasdaq (or such other market or quotation system on which our common stock is then listed or quoted), including ordinary brokers’ transactions, privately negotiated transactions or through sales to one or more dealers for resale of such securities as principals, at market prices prevailingdetermined at the time of sale, at prices related to such prevailing market prices or at negotiated prices. Usualpricing and customary or specifically negotiated brokerage fees or commissions may be paid byat a discount to the selling stockholders.current market price. The selling stockholders and intermediaries through whom such securities are soldrecent market price used throughout this prospectus may not be deemed “underwriters” within the meaningindicative of the Securities Act of 1933, as amended (the “Securities Act”), with respectfinal offering price. There is no established trading market for the warrants, and we do not expect a market to the securities offered hereby, and any profits realized or commissions received may be deemed underwriting compensation.develop.
| Effective Price Per Share and Accompanying Warrant | Total | |||||||
| Public offering price(1) | $ | $ | ||||||
| Underwriting discounts and commissions(2) | $ | $ | ||||||
| Proceeds to us (before expenses) | $ | $ | ||||||
| (1) | Based on a price of $ per unit, with each unit consisting of two (2) shares of common stock and one warrant to purchase one share of common stock. |
| (2) | The underwriter will receive compensation in addition to the underwriting discount. See “Underwriting” beginning on page 71. |
| (3) | The amount of offering proceeds to us presented in this table does not give effect to any exercise of the: (i) over-allotment Option we have granted to the underwriter as described below and (ii) the Underwriter’s Warrants (as defined herein) being issued to the underwriter as described below. |
This prospectus describes the general manner in which shares of common stock may be offered and sold by any selling stockholders. When the selling stockholders sell shares of common stock under this prospectus, we may, if necessary and required by law, provide a prospectus supplement that will contain specific information about the terms of that offering. Any prospectus supplement may also add to, update, modify or replace information contained in this prospectus. We urge you to read carefully this prospectus, any accompanying prospectus supplement and any documents we incorporate by reference into this prospectus and any accompanying prospectus supplement before you make your investment decision.
We are an “emerging growth company” under applicable Securities and Exchange Commission rules and will be subject to reduced public company reporting requirements.
Investing in our common stock is highly speculative andsecurities involves a significanthigh degree of risk. See “Risk Factors,” beginning on page 14 of this prospectus for a discussion of information that should be considered before making a decision to purchase our common stock.10.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined ifpassed upon the adequacy or accuracy of this prospectus is truthful or complete.prospectus. Any representation to the contrary is a criminal offense.
The underwriter expects to deliver the units to investors on or about , 2023.
Sole Book-Running Manager
Newbridge Securities Corporation
The date of this prospectus is June 6, 2022., 2023
TABLE OF CONTENTS
Please read this prospectus carefully. It describes our business, our financial condition and our results of operations. We have prepared this prospectus so that you will have the information necessary to make an informed investment decision. You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with any information or to make any representations about us, the securities being offered pursuant toother than those contained in this prospectus or in any other matter discussed in this prospectus, other thanfree writing prospectuses we have prepared. We take no responsibility for, and can provide no assurance as to the information and representations contained in this prospectus. Ifreliability of, any other information or representationthat others may give you. This prospectus is given or made, such information or representation may not be relied upon as having been authorized by us.
an offer to sell only the units offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is accuratecurrent only as of its date.
For investors outside the dateUnited States: We have not, and the underwriter has not, done anything that would permit this offering or possession or distribution of this prospectus regardlessin any jurisdiction where action for that purpose is required, other than the United States. Persons outside of the time of deliveryUnited States who come into possession of this prospectus ormust inform themselves about, and observe any restrictions relating to, the offering of any sale of our common stock. Neither the deliveryunits and the distribution of this prospectus nor any distribution of securities in accordance with this prospectus shall, under any circumstances, imply that there has been no change in our affairs since the date of this prospectus. This prospectus will be updated and made available for delivery to the extent required by the federal securities laws.
We further note that the representations, warranties and covenants made by us in any document that is filed as an exhibit to the registration statement of which this prospectus is a part were made solely for the benefitoutside of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.United States.
Market and Other Industry Data
Unless otherwise indicated, market data and certain industry forecasts used throughout this prospectus were obtained from various sources, including from internal surveys, market research, consultant surveys, publicly available information and industry publications and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained fromWhile we believe these sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third-party sources nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s knowledge of the industry, have not been independently verified. The future performance of the industry and markets in which we operate and intend to operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the sections titled “Risk Factors”“Risk Factors” and “Special“Special Note Regarding Forward-looking Statements”Statements” and elsewhere in this prospectus. These and other factors could cause results to differ materially from those expressed in these publications and reports.
All brand names or trademarks appearing in this prospectus are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
i
This summary highlights selected information contained elsewhere in this prospectus and does not contain all the information that you should consider before making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, including the information set forth under the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our consolidated financial statements and the accompanying notes included in this prospectus. Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Modular Medical,” the “Company,” “we,” “us,” and “our” refer to Modular Medical, Inc. and its wholly-owned subsidiary, Quasuras, Inc.
Overview
Modular Medical is a development stagedevelopment-stage, medical device company focused on the design, development, and commercialization of an innovative insulin pump using modernized technology to increase pump adoption in the diabetes marketplace. Through the creation of a novel two-part patch pump, our MODD1 product candidate, or MODD1,“MODD1,” the Company seeks to fundamentally alter the trade-offs between cost and complexity and access to the higher standards of care that presently available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training, and day-to-day use, we seek to expand the wearable insulin delivery device market beyond the highly motivated “super users” and expand the category into the mass market. The product candidate seeks to serve both the type 1 and the rapidly growing, especially in terms of device adoption, type 2 diabetes markets.
Differentiation
We believe that there are a number of shortcomings and issues with currently available insulin pumps that prevent a substantial number of people who require insulin on a daily basis from choosing an insulin pump to treat their diabetes. We believe that by tailoring our insulin pump to address such factors, we can expand the scope and adoption rate of insulin pump usage. We believe that to achieve broader market acceptance, an insulin pump must improve the clinical experience of the user, be easier to learn toand use, less time consumingtime-consuming to operate, more intuitive to both patients and physicians, and meet the standards for coverage by insurance providers so that co-payments required from patients are affordable and the hurdles to insurance coverage are significantly reduced.
Among the more prominent issues are:
| Complexity: Many existing pumps are highly complex and require significant technical expertise to use effectively. We believe such pumps were designed for “super users,” who have high levels of motivation, technical competence and |
| ● | ||
| ● | ||
| Cost: Costs associated with insulin pump therapy are high and can be prohibitive, especially for those on fixed or limited incomes. These costs vary by pump, but multi-thousand-dollar upfront payments, often with substantial co-payments in addition to |
1
Our team has substantial knowledge of the diabetes industry and experience in developing, obtaining regulatory authorization for, and bringing insulin pumps to market. Based on this experience, we believe that our innovative insulin pump, using a new, low cost and proprietary method of pumping insulin, can address most or all of these shortcomings. It provides a state-of-the-art insulin pump capable of both basal (steady flow) and bolus (mealtime dosing) insulin disbursement. It also has been designed considering a natural migration path to multi-chamber/multi-liquid pumps, potentially offering an exciting array of new therapies to patients with diabetes and other conditions.conditions in the future.
Our goal is to become the leader in expanding access to insulin pump technology to a wider portion of diabetes sufferers and provide not just care for the super users, but “diabetes care for the rest of us.”
The MODD1 is a high-precision first-line pumpproduct that we believe represents the best choice for new pump patients because it is easy to afford, easy to learn, easy to use, and has a revolutionary design and technology that enable precision with low-cost manufacture and high reproducibility.
Key features include:
A proprietary survey of American healthcare payors representing 50 million covered lives (approximately 1/3 of U.S. covered lives) performed for us by industry leading survey firm ISA has demonstrated that a majority of payors are willing to grant equivalent or preferential coverage for a product with this feature set at launch in exchange for about a 20% rebate. These costs are built into all of our models.
Diabetes Classifications and Therapies
Diabetes is typically classified as either type 1, or T1D,“T1D,” or type 2, or T2D:“T2D”:
| T1D is an auto-immune condition characterized by the body’s nearly complete inability to produce insulin. It is frequently diagnosed during childhood or |
| T2D represents over 90% of all individuals diagnosed with diabetes and is characterized by the body’s inability to either properly utilize insulin or produce sufficient insulin. Initially, many people with T2D attempt to manage their condition with improvements in diet and exercise and/or the use of oral medications and/or injection of glucagon-like peptide-1 (GLP-1) drugs. However, as their diabetes advances, patients often progress to requiring insulin therapies such as once-daily long-acting insulin and ultimately to intensified mealtime rapid-acting insulin therapy. This represents an important portion of the diabetes market in the United States with an estimated 1.6 million T2D intensively treated (multiple daily injections) with |
Glucose, the primary source of energy for cells, must be maintained at certain levels in the blood in order to permit optimal cell function and health. In people with diabetes, blood glucose levels are not well controlled and frequently become very high, a condition known as hyperglycemia, and very low, a condition called hypoglycemia. Hyperglycemia can lead to serious long-term complications, including blindness, kidney disease, nervous system disorders, occlusive vascular diseases, lower-limb amputation, stroke, cardiovascular disease, and death. Hypoglycemia can lead to confusion or loss of consciousness, often requiring a visit to the emergency room or, in certain cases, result in seizures, coma, and/or death.
All people with T1D, which is our primary market, require daily insulin. According to the Seagrove 2021 Diabetes Blue Book, approximately 18% of people with T2D in the United States, or approximately 4.7 million people, also require insulin (basal (steady supply) alone representsinsulin. These 4.7 million people comprise 3.1 million who require multiple daily injections (“MDI”) and the remainder, 1.6 million, who require basal plus mealtime represent 1.6 million)delivery only to manage their diabetes. In this prospectus, we refer to people with T1D and people with T2D who require mealtime insulin as “insulin-requiring people with diabetes.”
2
Currently, there are two primary therapies available for insulin-requiring people with diabetes: multiple daily insulin injections directly into the body through syringes or insulin pens referred to as Multiple Daily Injection, or MDI therapy,(a type of syringe) or the use of an insulin pump to deliver mealtime insulin boluses (single doses) to help with glucose absorption after carbohydrate consumption andalong with a continuous subcutaneous insulin infusion, or CSII“CSII therapy,” into the body. Generally, CSII therapy is considered to provide a number of advantages over MDI therapy, primarily an improvement in glycemic control, as measured by certain diabetes management tests such as hemoglobin A1c (HbA1c) measure and more recently Time in Range (TIR) where a continuous glucose measuring device is used to calculate this test. Among other medicalclinical benefits, it has beena study conducted by Tandem Diabetes Care, Inc. in 2021 demonstrated that insulin pump use can decrease glucose variability, reduce the number of hypoglycemia decrease the daily doses of insulinevents, and reduce the fear of hypoglycemia.
Notwithstanding these advantages, the difficulty in use resulting from the complexity and cumbersome design of available insulin pumps, optimized for the well-motivated, educated and well-insured, as well as high and often prohibitive costs for both the patient and insurance provider has resulted not only in dissatisfaction among many existing pump users (fewer than half purchase a newreplacement pump after the warranty expires per a Seagrove Partners estimate)estimate in 2020), but also has severely limited the adoption rate of insulin pumps by a large segment of the MDI diabetes population, whowhom we refer to in this prospectus as “Almost Pumpers.”

We define Almost Pumpers as individuals who treat their diabetes with MDI. These individuals are aware of pumps, may have been on pumps and understand the potential benefits, however, for a variety of reasons, they choose to continue giving themselves shots. WeIn 2018, we undertook one-on-one interviews with over 200 of these individuals to understand their past experiences on or considering pumps, existing pump shortcomings, the cost and insurance hurtles challenges, complexity to learn and time and complexity to operate that drives them to remain on MDI. With this detailed understanding, we brought a series of prototype models to them to react to, so we could refine the design and include features that would motivate them to be able to use this technology to better care for their diabetes. To date, the MODD1 pump has been well received by these individuals.individuals and our clinical advisors.
It is estimated that 32%33% of Americans with T1D use insulin pump therapy. Clinicians were surveyed on potential pump users and identified that 28% of Americans with T1D, including 44% of those who currently utilize MDI, can be classified as i) having an interest in pump adoption and ii) meeting the American Diabetes Association guidelines for required glucose control. These individuals often do not want to closely manage their glucose levels and incur the associatedconsiderable time and effort involved with the existing offerings - they are the Almost Pumpers. We have developed what we believe to be the most technologically advanced delivery system to overcome their objections and provide motivation. We believe that there are four addressable hurdles to adoption:perform with the motivation level they can provide.
Our initial focus for our insulin pump is the almost pumperAlmost Pumper segment population located in the United States. We will then focus on a European introduction, and we believe our cost position and reuse of part of the product, which eliminates waste, will have significant environmental and financial appeal.
We believe this conversion process, engaging people to try and thereby receive the benefits of our technology will substantially increase adoption of insulin pumps among both those with T1D and T2D who remain reliant upon multiple daily injections. Diabetes is a disease that appears throughout the world. Therefore, we cannot segment the market by socioeconomics, education or level of care. We intend to create an insulin pump that appeals to all Almost Pumpers.
3
Our Insulin Pump
Risk Factor Summary
Instead of building complex, bespoke, and difficult to manufacture and maintain pumping and control systems, we began with the technology and the user in mind. Using proprietary and patented methods of insulin measurement, we were able to eschew the complex mechanisms used today and instead build a product candidate using only parts from high volume consumer electronics manufacturing lines, breaking the cost vs. functionality curve that has existed in the insulin pump space and representing the first truly modern insulin pump design.
The pre-production models of our low-cost insulin pump are now undergoing the testing required to submit to the Food and Drug Administration, or FDA, for clearance to market them in the United States. We expect to submit our product candidate to the FDA in March 2022 through a premarket notification (or 510(k)) process (see the section titled Government Regulation below for a discussion of the FDA submission process and requirements). After submission, we expect to receive two rounds of comments from the FDA, and we believe it will take approximately six months to obtain clearance from the FDA. After we obtain clearance from the FDA, we can commence our commercialization process, as discussed in the section titled Commercialization Strategy: Overcoming the Insurance Hurdles below. We continue to devote substantial time and resources to better understand the needs and preferences of Almost Pumpers and the specific patent/provider/payor requirements to motivate change from MDI patients.
MODD1 has several distinguishing features:

1 - The pump has a simple button to press to deliver insulin as the patient requires it. The electronic pump uses a simple motor and rotating cam to motivate the insulin into the patient along with low power Bluetooth (LPBT) and near field communication (NFC) chips to allow the patient to communicate with their smart phone, tablet, or other mobile computing platform, as appropriate.
2 - The pump snaps together with a three-day disposable cartridge that is patient filled with insulin for delivery. It includes the power source and a simple coin cell that allows it to run through the 80-hour life of the cartridge.
3 - There is a set (not shown) that contains a soft 6 mm cannula and an introducer for insertion into the skin and removal of the needle used to transfer insulin to the body.
4 - MODD1 comes with a variety of methods for the patient to wear the pump. Options include: a base plate with adhesive (shown) for attaching to the body that has features for holding the pump to the patient; overwraps to hold the product candidate to the patient; and a velcro strap with a base plate suitable for wrapping around the arm or leg of the patient. The system will deliver a small continuous rate, called a basal, that will provide approximately 50% of the total daily dose required and the user will use the on-pump button to administer boluses, typically before and after meals.
The objective is to make the product candidate simple to acquire and take home, simple to learn and most importantly, simple to use to expand the pump market, drive adoption and ultimately better clinical outcomes.
Technological Advantages
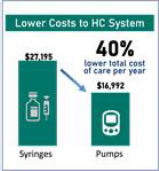
The adoption of new ultra-high volume technologies will result in far easier manufacturing scale up as parts sourcing and assembly processes are far easier. The MODD1 was designed from the beginning for mass manufacturing processes and fully automated production assembly lines. This advantage is compounded by the high availability and already optimized cost reduction in its components. This has resulted in a cost of goods, estimated on our competitors’ announced margins and sales, 50% lower than our closest patch pump competitor.
The adoption of modern, miniaturized technologies has led to numerous other advantages as well. The MODD1 pump is smaller in overall volume than Insulet’s popular Omnipod product and has a lower profile to the skin. Despite this, it holds a full 3mL (300 units) of insulin in line with full sized pumps such as Tandem and Medtronic, 50% more than the 2mL reservoir in the Omnipod. We believe that this volume advantage over other patch pumps will be significant as 24% of type 1 and over 50% of the rapidly growing type 2 market require more than 2 milliliters of insulin every 3 days (the expected wear time of patch pumps).
In addition, our new and patented pumping modality will provide what we believe is the most even (and thus closest to the function of a healthy pancreas) delivery of basal insulin in the industry. Basal rate can be delivered almost continuously while other pumps are delivering micro-boluses every five minutes for the Tandem, Omnipod and Medtronic pumps. We plan to demonstrate the impact of our system on glycemic control in a future clinical study.
The technology allows the patient to simply add insulin and operate. The battery is included in each cartridge and the device is operated without a controller. Nothing requires charging. MODD1 has been made push button simple to appeal to a wider audience of users.
This new technology has also made the MODD1 lighter than existing offerings. Compared to the Insulet Omnipod pump, MODD1 weighs 20 grams (vs. 26 grams) empty and 23 grams (vs. 28 grams) fully filled (despite carrying 50% more insulin), a reduction of 23% and 18% respectively. Also, unlike existing patch pumps, the MODD1 can be removed from the needle and taken off and replaced later if the user desires. This avoids loss of insulin in a pump due to accidental dislodging of the soft canula, an issue that users have expressed considerable dissatisfaction with on other patch pumps.
This technology is also uniquely suited to dual (or more) chamber pumps. We believe that such pumps will be integral to the realization of high time in range artificial pancreas solutions that require no human intervention, the next step forward from the cumbersome and awkward solutions today that require the user to announce meals, count and input carbs, and adjust delivery for exercise and sleep. The advantages of cost and miniaturization are multiplied in a multi-chamber setup and we expect to be able to reach price points, ease of use, and form factor unlike anything seen in the industry thus far. We believe that a prefilled, multi-hormone peel and stick-patch pump able to function in a fully autonomous closed loop system with CGM’s represents the next generation of diabetes care. We believe that we have demonstrated our technology and are securing the intellectual property on our approach.
We believe this technology, especially in dual chamber, will open up numerous applications outside of diabetes where medication compliance of complex therapy regimes is difficult, addressing such spaces as weight loss and fertility, and simplifying complex delivery of multi-drug cocktails, especially those with diverse and challenging dosing schedules.
Commercialization Strategy: Overcoming the Insurance Hurdles
Our goal is to establish MODD1 as the best option for new pump patients as we expand the market into the Almost Pumpers (Type 1 and Type 2) and the newly motivated CGM users. We seek to grow the market by providing first-line insulin pump therapy that is well suited to meet the needs of both diabetes patients requiring insulin and their clinicians.
Europe represents another large potential market for MODD1. Sixty million people in Europe live with diabetes, and approximately $161 billion is spent annually on diabetes healthcare costs in Europe. At present, cost containment is restricting pump uptake across Europe. Current pump usage across Europe hovers between 10% and 20% in many markets. Single payor healthcare systems across Europe traditionally attempt to contain costs in the short term and seek low price technologies with moderate medical benefits. MODD1 will offer a rebalance of this risk/reward strategy in that payors will incur only minor incremental short-term costs with the benefit of longer-term cost savings associated with reliable pump use. We intend to employ a partnership strategy across Europe following in-house managed regulatory and pricing activities in the major markets (e.g., the United Kingdom) and more cost receptive markets (e.g., Nordics). We are targeting European and United Kingdom approval towards early 2023.
Intellectual Property
Our success depends in part on our ability to obtain patents and trademarks, maintain trade secrets and know-how protection, enforce our proprietary rights against infringers, and operate without infringing on the proprietary rights of third parties. Because of the length of time and expense associated with developing new products and bringing them through the regulatory approval process, the health care industry places considerable emphasis on obtaining patent protection and maintaining trade secret protection for new technologies, products, processes, know-how, and methods.
As of May 31, 2022, we had one issued U.S. utility patent, five published U.S. utility patents, two pending foreign patent applications, and two pending international Patent Cooperation Treaty (PCT) patent applications covering various aspects of our technology, including our proprietary fluid movement technology. There can be no assurance that our pending patent applications will result in the issuance of patents, that patents issued to or licensed by us will not be challenged or circumvented by competitors, or that these patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage.
Recent Developments
On May 2, 2022, we entered into a securities purchase agreement (the “May 2022 Purchase Agreement”) with Sio Capital Management, LLC, an institutional investor (the “Investor”), pursuant to which we agreed to sell, in a registered direct offering (the “May 2022 Registered Offering”), an aggregate of 449,438 shares (the “Shares”) of our common stock, at a purchase price per Share of $4.45 and pre-funded warrants (the “Pre-Funded Warrants”) to purchase an aggregate of 1,348,314 shares of common stock at a purchase price per Pre-Funded Warrant of $4.44. The Pre-Funded Warrants will be exercisable immediately on the date of issuance at an exercise price of $0.01 per share and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
In a concurrent private placement (the “May 2022 Private Placement” and together with the Registered Offering, the “May 2022 Offerings”) under the May 2022 Purchase Agreement, we issued to the Investor warrants (the “Private Placement Warrants”) to purchase an aggregate of 1,438,202 shares of our common stock at an exercise price of $6.60 per share. The May 2022 Private Placement Warrants will be exercisable six months from date of issuance and have a five-year term.
Risks Related to Our Business
Our business is subject to many significant risks, as more fully described in the section entitled “Risk Factors”titled “Risk Factors” immediately following this prospectus summary. You should read and carefully consider these summary risks, together with the risks set forth under the section entitled “Risk Factors”titled “Risk Factors” and all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in our common stock.securities. If any of the risks discussed in this prospectus actually occur, our business, prospects, financial condition or operating results could be materially and adversely affected. In particular, our risks include, but are not limited to, the following:
Risk Factors
| We might not be able to continue as a going concern; |
| ● | We are a developmental-stage, medical-device company and have a history of significant operating losses; we expect to continue to incur operating losses for the foreseeable future, and we may never achieve or maintain profitability; |
| ● | We will need substantial additional funding to complete subsequent phases of our insulin pump product candidate and to operate our business, and such funding may not be available or, if it is available, is likely to substantially dilute our existing stockholders; |
| ● | We have a limited operating history and historical financial information upon which you may evaluate our performance; |
| ● | The amount of financing we require will depend on a number of factors, many of which are beyond our control. Our results of operations, financial condition and stock price are likely to be adversely affected if our funding requirements increase or are otherwise greater than we expect; |
| ● | We are subject to extensive regulation by the FDA, which could restrict the sales and marketing of our insulin pump product candidate and could cause us to incur significant costs; |
| ● | Even if we are able to obtain all regulatory approvals and have completed all other steps needed to be taken to commercialize our insulin pump, if we or any contract manufacturers we select fails to comply with the FDA’s quality system regulations, the manufacturing and distribution of our product candidate could be interrupted, and our product sales and operating results could |
| ● | ||
| We will need to outsource and rely on third parties for various aspects relating to the development, manufacture, sales and marketing of our insulin pump as well as in connection with assisting us in the preparation and filing of our FDA submission, and our future success will be dependent on the timeliness and effectiveness of the efforts of these third |
| ● | Our future cash requirements may differ significantly from our current estimates; |
| We may not receive the necessary regulatory clearance or approvals for our insulin pump, and failure to timely obtain necessary clearances and/or approvals could harm our then operations, including our ability to commercialize our product |
| Obtaining marketing authorization in the United States will not obviate the need to obtain marketing authorization in other jurisdictions We must obtain approval from foreign regulatory authorities before we can market and sell any of our product candidates in countries outside the United States. We will incur additional costs in seeking such approvals, may experience delays in obtaining such approvals and cannot be certain that such approvals will be |
| Although our product candidate does not presently require clinical trials to apply to the FDA for clearance, and even if a clinical trial is conducted, the results of our clinical testing may not demonstrate the safety and efficacy of the device or may be equivocal or otherwise not be sufficient for us to obtain approval of our product |
| We |
| Our competitors may develop products that are more effective, safer and less expensive than |
4
| Sales of a significant number of shares of our |
| We have limited internal research and development personnel, making us dependent on consulting |
| Technological breakthroughs in diabetes monitoring, treatment or prevention could render our insulin pump |
| We may not be able to identify, negotiate and maintain the strategic alliances necessary to develop and commercialize our products and technologies, and we will be dependent on our corporate partners if we |
Implications of Being an Emerging Growth Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act. We will remain an emerging growth company until the earlier of (1) December 31, 2024 (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.07 billion, (3) the last day of the fiscal year in which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which would occur on the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company, we may:
| ● | We may be subject to potential product liability and other claims that could materially impact our business and financial condition; |
| ● | If we are sued for infringing on third-party intellectual property rights, it will be costly and time-consuming, and an unfavorable outcome would have a significant adverse effect on our business; |
| ● | If we are unable to protect the confidentiality of |
| ● | Healthcare reform laws could adversely affect our product candidate and financial |
| ● | We may bring infringement claims or other legal proceedings against third parties, causing us to spend substantial resources on litigation and exposing our own intellectual property portfolio to challenge; |
| ● | Assuming our insulin pump receives FDA clearance or approval, our insulin pump will still be subject to recalls, which would harm our reputation, business operations and financial |
| ● | Because our current insulin pump is still in the pre clearance stage with the FDA, it does not have reimbursement and is not approved for insurance coverage. If in the future we are cleared for and are otherwise able to commercialize our insulin pump, but are unable to obtain adequate reimbursement or insurance coverage for such product candidate from third-party payors, we will be unable to generate significant revenue; |
| ● | If we fail to establish and maintain an effective system of internal controls, we may not be able to report our financial results accurately or prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely affect the trading price of our common stock; |
| ● | Our board of directors is able to adopt recapitalizations through forward or reverse splits of our outstanding shares of common stock without stockholder approval; |
| ● | We are a “smaller reporting company” and, as a result of the reduced disclosure and governance requirements applicable to smaller reporting companies, our common stock may be less attractive to investors; |
| ● | As we have broad discretion in how we use the proceeds from this offering, we may use the proceeds in ways with which you disagree; |
| ● | Holders of our warrants will have no rights as a common stockholder until they acquire our common stock; |
| ● | Provisions of the warrants offered by this prospectus could discourage an acquisition of us by a third party; |
| ● | The warrants offered by this prospectus are speculative in nature and may not have any |
| ● | There is no public market for the warrants being offered in this |
| ● | You may experience future dilution as a result of future equity offerings and other issuances of our |
In addition, under the JOBS Act, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period, and, as a result we will adopt new or revised accounting standards on relevant dates on which adoption of such standards is required for other public companies.
We are also a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act and have elected to take advantage of certain of the scaled disclosure available to smaller reporting companies.
Corporate Information
Our principal executive offices are located at 16772 West Bernardo Drive,10740 Thornmint Road, San Diego, CA 92127 and our telephone number is (858) 800-3500. We maintain a website at www.modular-medical.com to which we regularly post copies of our press releases, as well as additional information about us. Our filings with the Securities and Exchange Commission, or SEC, will be available free of charge through the website as soon as reasonably practicable after being electronically filed with or furnished to the SEC.www.modular-medical.com. Information contained on, or accessible through, our website does not constitute a part of this prospectus or our other filings with the SEC, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our securities.
5
All brand names or trademarks appearing in this prospectus are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.THE OFFERING
SUMMARY RISK FACTORS
Our business is subject to a number of risks, including risks that could prevent us from achieving our business objectives or financial goals or that otherwise could adversely affect our business, results of operations, financial condition and liquidity, that you should carefully consider before making a decision to invest in our common stock. These risks are discussed more fully in “Risk Factors.” These risks include the following:
Securities offered by us: |
THE OFFERING
| Common stock outstanding prior to this offering(1): | 10,949,389 shares of common stock outstanding as of May | |||
| Common stock to be outstanding after this offering(1): | 15,182,193 shares, or 15,817,113 shares, if the | |||
| Use of | We estimate that the net proceeds from this We intend to use the net proceeds of this offering for general corporate | |||
| Over-allotment option | We have granted a 30-day option to the underwriter, exercisable one or more times in whole or in part, to purchase up to an additional 634,920 shares of common stock at the effective public offering price per share of $ and additional warrants to purchase up to 317,460 additional shares of common stock at a price of $0.01 per warrant, in each case less underwriting discounts and commissions, to cover over-allotments, if any. | |||
| Risk factors: | Investing in our securities involves substantial risk. You should read the “Risk Factors | |||
| We and our directors and officers and holders of | ||||
| Nasdaq Capital Market | MODD |
The number of shares of our Common Stock to becommon stock outstanding before and after this offering is based on 10,911,68410,949,389 shares of our Common Stockcommon stock outstanding as of June 2, 2022,May 4, 2023 and excludes:
| 2,116,402 shares of our common stock issuable upon the exercise of the warrants to be issued as part of the units; |
| ● | 296,296 shares of common stock issuable upon exercise by the underwriter of the underwriter’s warrants; |
| ● | 767,796 shares of our |
| 1,348,314 |
6
The following tables summarize our financial data for the periods presented and should be read together with the sections of this prospectus titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our financial statements and related notes thereto appearing elsewhere in this prospectus. The following summary statements of operations data for the nine months ended December 31, 2022 and 2021 have been derived from our unaudited consolidated financial statements and footnotes included elsewhere in this prospectus and the summary statements of operations data for the years ended March 31, 2022 and 2021 have been derived from our audited consolidated financial statements and footnotes included elsewhere in this prospectus. The summary balance sheet information as of December 31 and March 31, 2022 has been derived from our unaudited condensed consolidated financial statements and footnotes included elsewhere in this prospectus. Our historical results are not necessarily indicative of our future results or of the results we expect in the future and results of interim periods are not necessarily indicative of results for the entire year.
| Twelve Months Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating expenses | ||||||||
| Research and development | $ | 7,729,240 | $ | 4,083,303 | ||||
| General and administrative | 7,197,162 | 3,253,412 | ||||||
| Total operating expenses | 14,926,402 | 7,336,715 | ||||||
| Loss from operations | (14,926,402 | ) | (7,336,715 | ) | ||||
| Other income | 368,920 | 130 | ||||||
| Interest expense | (2,752,229 | ) | (39,791 | ) | ||||
| Loss on debt extinguishment | (1,321,450 | ) | — | |||||
| Loss before income taxes | (18,631,161 | ) | (7,376,376 | ) | ||||
| Provision for income taxes | 1,600 | 1,600 | ||||||
| Net loss | $ | (18,632,761 | ) | $ | (7,377,976 | ) | ||
| Net loss per share | ||||||||
| Basic and diluted | $ | (2.74 | ) | $ | (1.20 | ) | ||
| Shares used in computing net loss per share | ||||||||
| Basic and diluted | 6,807,710 | 6,211,562 | ||||||
7
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
| Operating expenses | ||||||||||||||||
| Research and development | $ | 2,196,546 | $ | 1,849,399 | $ | 6,804,069 | $ | 5,742,911 | ||||||||
| General and administrative | 1,161,351 | 1,981,665 | 3,502,029 | 5,156,152 | ||||||||||||
| Total operating expenses | 3,357,897 | 3,831,064 | 10,306,098 | 10,899,063 | ||||||||||||
| Loss from operations | (3,357,897 | ) | (3,831,064 | ) | (10,306,098 | ) | (10,899,063 | ) | ||||||||
| Other income (expense) | (587 | ) | 4 | 16 | 368,876 | |||||||||||
| Interest expense | — | (1,010,247 | ) | — | (2,204,917 | ) | ||||||||||
| Loss on debt extinguishment | — | — | — | (1,321,450 | ) | |||||||||||
| Loss before income taxes | (3,358,484 | ) | (4,841,307 | ) | (10,306,082 | ) | (14,056,554 | ) | ||||||||
| Provision for income taxes | — | — | 1,600 | 1,600 | ||||||||||||
| Net loss | $ | (3,358,484 | ) | $ | (4,841,307 | ) | $ | (10,307,682 | ) | $ | (14,058,154 | ) | ||||
| Net loss per share | ||||||||||||||||
| Basic and diluted | $ | (0.31 | ) | $ | (0.76 | ) | $ | (0.95 | ) | $ | (2.22 | ) | ||||
| Shares used in computing net loss per share | ||||||||||||||||
| Basic and diluted | 10,925,862 | 6,354,145 | 10,863,082 | 6,331,982 | ||||||||||||
8
| December 31, 2022 (Unaudited) | March 31, 2022 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 7,690,957 | $ | 9,076,372 | ||||
| Prepaid expenses and other | 180,164 | 313,422 | ||||||
| Security deposit | 100,000 | — | ||||||
| TOTAL CURRENT ASSETS | 7,971,121 | 9,389,794 | ||||||
| Property and equipment, net | 716,409 | 235,959 | ||||||
| Right of use asset, net | 51,312 | 120,693 | ||||||
| Security deposit | — | 100,000 | ||||||
| TOTAL NON-CURRENT ASSETS | 767,721 | 456,652 | ||||||
| TOTAL ASSETS | $ | 8,738,842 | $ | 9,846,446 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | 382,080 | $ | 299,951 | ||||
| Accrued expenses | 255,545 | 524,891 | ||||||
| Short-term lease liability | 77,672 | 144,857 | ||||||
| TOTAL CURRENT LIABILITIES | 715,297 | 969,699 | ||||||
| Long-term lease liability | — | 39,957 | ||||||
| TOTAL LIABILITIES | 715,297 | 1,009,656 | ||||||
| Commitments and Contingencies (Note 8) | ||||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred Stock, $0.001 par value, 5,000,000 shares authorized, none issued and outstanding | — | — | ||||||
| Common Stock, $0.001 par value, 50,000,000 shares authorized; 10,932,098 and 10,461,898 shares issued and outstanding as of December 31, 2022 and March 31, 2022, respectively | 10,932 | 10,462 | ||||||
| Additional paid-in capital | 52,900,066 | 43,406,099 | ||||||
| Accumulated deficit | (44,887,453 | ) | (34,579,771 | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY | 8,023,545 | 8,836,790 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 8,738,842 | $ | 9,846,446 | ||||
9
Investing in our securities involves a high degree of risk. Before you invest in the units, the warrants and the common stock, involves a great dealyou should carefully consider the following risks, as well as general economic and business risks, and all of risk. Careful consideration should be madethe other information contained in this registration statement. Any of the following factors as well as other information included in this prospectus before deciding to purchase our common stock. There are many risks that affectcould harm our business, andoperating results of operations, some of which are beyond our control. Our business,and financial condition or operating results could be materially harmed by any of these risks. This couldand cause the trading price of our common stock to decline, andwhich would cause you mayto lose all or part of your investment. Additional risks that we do not yet know of or that we currently think are immaterial mayWhen determining whether to invest, you should also affectrefer to the other information contained in this prospectus, including our businessfinancial statements and results of operations.the related notes thereto.
Risks Related To Our Operations
We might not be able to continue as a going concern.
Our unaudited condensed consolidated financial statements as of December 31, 2022 have been prepared under the assumption that we will continue as a going concern for the next twelve months. At December 31, 2022, we had cash and cash equivalents of $7.7 million and an accumulated deficit of $44.9 million. Without the proceeds of this offering, we do not believe that our cash, cash equivalents and investments would be sufficient to fund our operations for the next 12 months, and we would need to raise additional capital. As a result of our expected operating losses and cash burn for the foreseeable future and recurring losses from operations, if we are unable to raise sufficient capital through this offering or additional debt or equity arrangements, there will be uncertainty regarding our ability to maintain liquidity sufficient to operate our business effectively, which raises substantial doubt as to our ability to continue as a going concern. If we cannot continue as a viable entity, our stockholders would likely lose most or all of their investment in us.
If we are unable to generate sustainable operating profit and sufficient cash flows, then our future success will depend on our ability to raise capital. In addition to this offering, we are seeking additional financing and evaluating financing alternatives in order to meet our cash requirements for the next 12 months. We cannot be certain that raising additional capital, whether through this offering, selling additional debt or equity securities or obtaining a line of credit or other loan, will be available to us or, if available, will be on terms acceptable to us. If we issue additional securities to raise funds, these securities may have rights, preferences, or privileges senior to those of our common stock, and our current stockholders may experience dilution. If we are unable to obtain funds when needed or on acceptable terms, we may be required to curtail our current product development programs, cut operating costs, forego future development and other opportunities or even terminate our operations.
We are a developmental stage medical device company and have a history of significant operating losses; we expect to continue to incur operating losses, and we may never achieve or maintain profitability.
As a development-stage enterprise, we do not currently have revenues to generate cash flows to cover operating expenses. Since our inception, we have incurred operating losses in each year due to costs incurred in connection with research and development activities and general and administrative expenses associated with our operations. For the yearsnine months ended December 31, 2022 and year ended March 31, 2021 and 2020,2022, we incurred net losses of approximately $7.4$10.3 million and $5.3$18.6 million, respectively. At March 31, 2021,As a result, we had an accumulated deficit of approximately $15.9 million. Forneed to raise additional capital in the nine months ended December 31, 2021, we incurred a net loss of approximately $4.8 million. At December 31, 2021, we had an accumulated deficit of approximately $30 million.future, which may or may not be available to us at all or only on unfavorable terms.
We expect to incur losses for the foreseeable future as we continue the development of, and seek regulatory clearance and approvals for, our insulin pump. As our prototypeMODD1 insulin pump is currently our only product, candidate, if it fails to gain regulatory approval and market acceptance, we will not be able to generate any revenue, or explore other opportunities to enhance shareholderstockholder value, such as through a sale. If we fail to generate revenue and eventually become profitable, or if we are unable to fund our continuing losses, our shareholdersstockholders could lose all or a substantial part of their investment.
We might not be able to continue as a going concern which would likely cause our stockholders to lose most or all of their investment.
Our audited financial statements for the year ended March 31, 2021 were prepared under the assumption that we would continue as a going concern. However, our independent registered public accounting firm included a “going concern” explanatory paragraph in its report on our financial statements for the year ended March 31, 2021, indicating that, without additional sources of funding, our cash at March 31, 2021 was not sufficient for us to operate as a going concern for a period of at least one year from the date of issuance of the financial statements. Management’s plans concerning these matters, including our need to raise additional capital, are described in Management’s Discussion and Analysis of Financial Conditions and Results of Operations included in this prospectus and in Note 1 to our audited consolidated financial statements included in this prospectus. However, we cannot assure you that our plans will be successful. In light of the foregoing, there is substantial doubt about our ability to continue as a going concern. If we cannot continue as a viable entity, our stockholders would likely lose most or all of their investment in us.
The full effects of COVID-19 and other potential future public health crises, epidemics, pandemics or similar events are uncertain and could have a material and adverse effect on our business, financial condition, operating results and cash flows.
The global outbreak of the coronavirus disease 2019, or COVID-19, was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the world economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The extent of the impact on our operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of our control and cannot be predicted.
We have been complying with county and state orders and, until May 2021, had implemented a teleworking policy for our employees and contractors and significantly minimized the number of employees who visit our office. However, a facility closure, work slowdowns or temporary stoppage at one of our suppliers could occur, which could have a longer-term impact and could delay our prototype production and ability to conduct business.
If our workforce is unable to work effectively, including because of illness, quarantines, absenteeism, government actions, facility closures, travel restrictions or other restrictions in connection with the COVID-19 pandemic, our operations will be negatively impacted. We may be unable to develop our product candidate, and our costs may increase as a result of the COVID-19 outbreak. The impacts could worsen if there is an extended duration of any COVID-19 outbreak or a resurgence of COVID-19 infection in affected regions after they have begun to experience improvement.
We rely on other companies to provide components and to perform services for us. An extended period of supply chain disruption caused by the response to COVID-19 could impact our ability to produce our initial product quantities and, if we are not able to implement alternatives or other mitigations, product deliveries would be adversely impacted and negatively impact our business, financial condition, operating results and cash flows. Limitations on government operations can also impact regulatory approvals that are necessary for us to operate our business.
The continued spread of COVID-19 has also led to disruption and volatility in the global capital markets. We were recently able to raise additional capital through equity offerings in February 2022 and May 2022, however, we will need to raise additional capital to support our operations in the future. We may be unable to access the capital markets, and additional capital may only be available to us on terms that could be significantly detrimental to our existing stockholders and to our business.
We will need substantial additional funding to complete subsequent phases of the development of our insulin pump product candidate and to operate our business and such funding may not be available or, if it is available, such financing is likely to substantially dilute our existing shareholders.stockholders.
The discovery, development, and commercialization of new medical devices, such as our insulin pump, entails significant costs. While we believe that we have generally completed the engineering and mechanical aspects of our insulin pump prototype,and cartridge along with production-level assembly equipment, we still must modify, refine and finalize our insulin pump to, among other things, meet the general needs and preferences of the almost pumperAlmost Pumper marketplace and the guidelines of third-party payors. To enable us to accomplish these and other related items and continue to operate our business, we will need to raise substantial additional capital and/or enter into strategic partnerships or joint ventures to enable us to:
| fund clinical studies and seek regulatory approvals; |
| build or access manufacturing and commercialization capabilities; |
| develop, test, and, if approved, market our product candidate; |
| acquire or license additional internal systems and other infrastructure; and |
| hire and support additional management, engineering and scientific personnel. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never achieve, we expect to finance our cash needs primarily through public or private equity offerings, debt financings or through the establishment of possible strategic alliances. WeThis offering is being conducted to obtain such funding, although there can be no guarantee that we will successfully raise all the funding we require in this offering. Depending on the amount of funding we receive in this offering, as well as other factors, we may in the future seek additional capital from public or private offerings of our capital stock or borrow additional amounts under new credit lines or from other sources. If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, we may incur significant financing costs, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaborations, licensing, joint ventures, strategic alliances, partnership arrangements or other similar arrangements, it may be necessary to relinquish valuable rights to the MODD1 pump or our potential future products or proprietary technologies or grant licenses on terms that are not favorable to us.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are not able to secure additional equity funding when needed, we may have to delay, reduce the scope of, or eliminate one or more of our clinical studies, development programs or future commercialization initiatives. In addition, any additional equity funding that we do obtain will dilute the ownership held by our existing equity holders. The amount of this dilution may be substantially increased if the trading price of our Common Stockcommon stock is lower at the time of any financing. Regardless, the economic dilution to shareholdersstockholders will be significant if our stock price does not increase significantly, or if the effective price of any sale is below the price paid by a particular shareholder.stockholder. Any debt financing that we obtain in the future could involve substantial restrictions on activities and creditors could seek a pledge of some or all of our assets. We have not identified potential sources for such financing that we will require, and we do not have commitments from any third parties to provide any future debt financing. If we fail to obtain funding as needed, we may be forced to cease or scale back operations, and our business, prospects, results of operations, financial condition and stock price would be adversely affected.
We have a limited operating history and historical financial information upon which you may evaluate our performance.
You should consider, among other factors, our prospects for success in light of the risks and uncertainties encountered by companies that, like us, are in their early stages of development. We may not successfully address these risks and uncertainties or successfully complete our studies and/or implement our existing and new products. If we fail to do so, it could materially harm our business and impair the value of our Common Stock.common stock. Unanticipated problems, expenses and delays are frequently encountered in establishing a new business, conducting research, and developing new products. These include, but are not limited to, inadequate funding, failure to obtain regulatory approval, unforeseen research issues, lack of consumer, physician or third-party payor acceptance, competition, sluggish product development, and inadequate sales and marketing. The failure by us to meet any of these conditions would have a materially adverse effect upon us and may force us to reduce or curtail operations. No assurance can be given that we can or will ever operate profitably.
We may not be able to meet our future capital needs.
To date, we have no revenue and we have limited cash liquidity and capital resources. We will need additional capital in the near future. Any equity financings will result in dilution and may contain other terms that are not favorable to our then-existing stockholders. We currently have debt financing, and any additional sources of debt financing that we may obtain in the future may result in a high interest expense. Any financing, if available, may be on unfavorable terms. If adequate funds are not obtained, we will be required to reduce or curtail operations.
The amount of financing we require will depend on a number of factors, many of which are beyond our control. Our results of operations, financial condition and stock price are likely to be adversely affected if our funding requirements increase or are otherwise greater than we expect.
Our future funding requirements will depend on many factors, including, but not limited to:
| the testing costs for our insulin pump product candidate and other development activities conducted by us directly, and our ability to successfully conclude the studies and activities and achieve favorable results; |
| our ability to attract future strategic partners to pay for or share costs related to our product development efforts; |
| the costs and timing of seeking and obtaining regulatory clearance and approvals for our product candidate; |
| the costs of filing, prosecuting, maintaining and enforcing any patents and other intellectual property rights that we may have and defending against potential claims of infringement; |
| decisions to hire additional scientific, engineering or administrative personnel or consultants; |
| our ability to manage administrative and other costs of our operations; and |
| the presence or absence of adverse developments in our research program. |
If any of these factors cause our funding needs to be greater than expected, our operations, financial condition, ability to continue operations and stock price may be adversely affected.
11
Our future cash requirements may differ significantly from our current estimates.
Our cash requirements may differ significantly from our estimates from time to time, depending on a number of factors, including:
| the costs and results of our clinical studies regarding our insulin pump product candidate; |
| the time and costs involved in obtaining regulatory clearance and approvals; |
| whether we are able to obtain funding under future licensing agreements, strategic partnerships, or other collaborative relationships, if any; |
| the costs of compliance with laws, regulations, or judicial decisions applicable to us; and |
| the costs of general and administrative infrastructure required to manage our business and protect corporate assets and |
If we fail to raise additional funds on a timely basis, even after the completion of this offering, we will need to scale back our business plans, which would adversely affect our business, prospects, results of operations, financial condition, and stock price, and we may even be forced to discontinue our operations and liquidate our assets.
Technological breakthroughs in diabetes monitoring, treatment or prevention could render our insulin pump obsolete.
The diabetes treatment market is subject to rapid technological change and product innovation. Our insulin pump is based on our proprietary technology, but a number of companies, medical researchers and existing pharmaceutical companies are pursuing new delivery devices, delivery technologies, sensing technologies, procedures, drugs and other therapeutics for the monitoring, treatment and/or prevention of insulin-dependent diabetes. Any technological breakthroughs in diabetes monitoring, treatment or prevention could render our insulin pump obsolete, which, since our insulin pump is our only product candidate, would have a material adverse effect on our business, financial condition andprospectus, results of operations and financial condition and could result in shareholdersstockholders losing their entire investment.
Any failure to attract and retain skilled directors, executives, employees and consultants could impair our product development and commercialization activities.
Our business depends on the skills, performance, and dedication of our directors, executive officers and key engineering, scientific and technical advisors. Many of our current engineering or scientific advisors are independent contractors and are either self-employed or employed by other organizations. As a result, they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, which may affect their ability to provide services to us in a timely manner. We will need to recruit additional directors, executive management employees, and advisers, particularly engineering, scientific and technical personnel, which will require additional financial resources. In addition, there is currently intense competition for skilled directors, executives and employees with relevant engineering, scientific and technical expertise, and this competition is likely to continue. If we are unable to attract and retain persons with sufficient engineering, scientific, technical and managerial experience, we may be forced to limit or delay our product development activities or may experience difficulties in successfully conducting our business, which would adversely affect our business, prospects, results of operations and financial condition.
Our operations are substantially dependent upon key personnel.
Our performance is substantially dependent on the continued services and performance of our senior management and certain other key personnel The loss of services of any of our executive officers or other key employees could have a material adverse effect on our business, financial condition and results of operations. In addition, any future expansion of our business will depend on our ability to identify, attract, hire, train, retain and motivate other highly skilled managerial, marketing, customer service and manufacturing personnel, and our inability to do so could have a material adverse effect on our business, financial condition and results of operations.
We are dependent on the performance and continued engagement of our Chairman, President and Principal Financial Officer.
We are dependent on the performance and continued engagement of Paul DiPerna, our Chairman, President and Principal Financial Officer. Although we believe we will be able to engage qualified personnel for such purposes, an inability to do so could materially adversely affect our ability to market, sell, and enhance our products. While Mr. DiPerna is currently devoting his full-time working efforts to us, other employees and consultants may only be available to us on a part-time basis. The loss of one or more of our key employees, especially Mr. DiPerna, or our inability to hire and retain other qualified employees, including but not limited to research and development, sales, manufacturing, and administrative support staff, could have a material adverse effect on our business, prospects, results of operations and financial condition.
We have limited internal research and development personnel, making us dependent on consulting relationships.
We consider research and development to be an important part of the process of designing, developing, obtaining regulatory required approvals and the eventual commercialization of our insulin pump. We continue to incur increased research and development expenditures, which are primarily attributable to effort and expenses incurred in designing and developing our innovative insulin pump. We expect to continue to incur substantial costs related to research and development.
12
We will need to outsource and rely on third parties for various aspects relating to the development, manufacture, sales and marketing of our insulin pump as well as in connection with assisting us in the preparation and filing of our FDA submission, and our future success will be dependent on the timeliness and effectiveness of the efforts of these third parties.
We are dependent on consultants for important aspects of our product development strategy. We do not have the required financial resources and personnel to carry out independently the development of our product candidate, and do not have the capability or resources to manufacture, market or sell our current product candidate. As a result, we contract with and rely on third parties for important functions, including in connection with the development and finalization of our insulin pump, the preparation and filing of our FDA submission and eventual manufacturing and commercialization of our product candidate. We have recently entered into several agreements with third parties for such services. If problems develop in our relationships with third parties, or if such parties fail to perform as expected, it could lead to delays or lack of progress in obtaining FDA clearance, significant cost increases, changes in our strategies, and even failure of our product initiatives.
We may not be able to identify, negotiate and maintain the strategic alliances necessary to develop and commercialize our products and technologies, and we will be dependent on our corporate partners if we do.
We may seek to enter into a strategic alliance with a diabetes relateddiabetes-related service providing company for the further development and approval of our insulin pump product candidate. At this time, we have not entered into any such strategic alliance. Strategic alliances, if entered into, could potentially provide us with additional funds, expertise, access, and other resources in exchange for exclusive or non-exclusive licenses or other rights to the product that we are currently developing or a product we may explore in the future. We cannot give any assurance that we will be able to enter into strategic relationships with a diabetes relateddiabetes-related service providing company or others in the near future or at all. In addition, we cannot assure you that any agreements that we do reach will allow us to achieve our goals or that such grants will be on terms that prove to be economically beneficial to us. When we do enter into strategic or contractual relationships, we become dependent on the successful performance of our partners or counter-parties. If they fail to perform as expected, such failure could adversely affect our financial condition, lead to increases in our capital needs, or hinder or delay our development efforts. See “Our Business -Employees” below.
We may not receive the necessary regulatory clearance or approvals for our insulin pump, and failure to timely obtain necessary clearances and/or approvals could harm our then operations, including our ability to commercialize our product candidate.
Before we can market a new medical device, such as our insulin pump, we must first receive clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA.“FDCA.” In the 510(k) clearance process, before a device may be marketed, the FDA must determine that such proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally on the U.S. market pursuant to a premarket approval (PMA) and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device.
Certain future modifications made to our product candidate, which we currently expect to be cleared through 510(k), may require a new 510(k) clearance. The 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last longer. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory authorizations could harm our business, including our ability to commercialize our product candidate and our shareholdersstockholders could lose their entire investment. Furthermore, even if we are granted the required regulatory authorizations, such authorizations may be subject to significant limitations on the indicated uses for the device, which may limit the market for our product candidate.
If the FDA requires us to go through a lengthier, more rigorous examination for our product candidate than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The FDA can delay, limit or deny clearance or approval for our insulin pump medical device for many reasons, including:including, for example:
| our inability to demonstrate to the satisfaction of the FDA that our product candidate is substantially equivalent to the proposed predicate device; |
| the disagreement of the FDA with the design or implementation of our performance testing protocols or the interpretation of data from our performance testing; |
| the data from performance testing may be insufficient to support a determination of substantial equivalence or that our device meets required special controls or applicable performance standards; |
| our inability to demonstrate that the benefits of our pump outweigh the risks; |
| the manufacturing process or facilities we intend to use may not meet applicable requirements; for example, we experienced issues maintaining insulin stability on an initial version of our pump product candidate, and we attributed this issue to the materials used in the production of our product; we believe we have made the necessary changes to our materials and process to address this issue and will be completing the required testing prior to our FDA submission; and |
| the potential for approval policies or regulations of the FDA to change significantly in a manner rendering our data or regulatory filings insufficient for clearance or approval. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our product candidate or impact our ability to modify our product candidate after clearance on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain clearance for our pump, increase the costs of compliance or restrict our ability to maintain our current approval.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of data supporting the safety and performance of the product candidates during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence.
Obtaining marketing authorization in the United States will not obviate the need to obtain marketing authorization in other jurisdictions We must obtain approval from foreign regulatory authorities before we can market and sell any of our product candidates in countries outside the United States. We will incur additional costs in seeking such approvals, may experience delays in obtaining such approvals and cannot be certain that such approvals will be granted.
The development, manufacture, and marketing of our product candidates outside the United States is subject to government regulation. In most foreign countries, we must complete rigorous pre-clinical testing and extensive human clinical trials that demonstrate the safety and efficacy of a product in order to apply for regulatory approval to market the product. If foreign regulatory authorities grant regulatory approval of a product, the approval may be limited to specific indications or limited with respect to its distribution. Expanded or additional indications for approved devices may not be approved, which could limit our potential revenues. Foreign regulatory authorities may refuse to grant any approval. Consequently, even if we believe that pre-clinical and clinical data are sufficient to support regulatory approval for our products, foreign regulatory authorities may not ultimately grant approval for commercial sale in any jurisdiction. If our product candidates are not approved in such jurisdictions, our ability to generate revenues will be limited and our business will be adversely affected.
Our competitors may develop products that are more effective, safer and less expensive than ours.
Existing insulin pumps are expensive, with the more popular models having purchase prices exceeding $4,000 for individuals without health insurance and often require significant patient copays. Others have daily use costs that exceed the reimbursement rates of many health insurance plans, forcing some users to spend thousands of dollars a year in copays. We believe this makes insurers hesitant to pay for any pumps and places pumps out of reach for many patients whomwho cannot afford such out of pocket expenses.
We are engaged in the diabetes treatment sector of the healthcare marketplace, which is intensely competitive. There are current products that are quite effective at addressing the effects of diabetes, and we expect that new developments by other companies and academic institutions in the areas of diabetes treatment will continue. If approved for marketing by the FDA, depending on the approved clinical indication, our product will be competing with existing and future products related to treatments for diabetes.
Our competitors may:
| develop product candidates and market products that increase the levels of safety or efficacy that our product candidates will need to show in order to obtain regulatory approval; |
| develop product candidates and market products that are less expensive or more effective than ours; |
| commercialize competing products before we can launch any products we are working to develop; |
| hold or obtain proprietary rights that could prevent us from commercializing our products; or |
| introduce therapies or market medical products that render our potential product candidates obsolete. |
We expect to compete against large medical device companies, such as Medtronic, Inc., Tandem Diabetes Care, Inc. and Insulet Corporation and smaller companies that are collaborating with larger medical device companies, new companies, academic institutions, government agencies and other public and private research organizations. These competitors, in nearly all cases, produce similar products relative to the treatment of diabetes thatand have substantially greater financial resources than we do. Our competitors also have significantly greater experience in:
| developing medical device and other product candidates; |
| undertaking testing and clinical studies; |
| building relationships with key customers and opinion-leading physicians; |
| obtaining and maintaining FDA and other regulatory approvals; |
| formulating and manufacturing medical devices; |
| launching, marketing and selling medical devices; |
| providing management oversight for all of the above-listed operational |
| ● | obtaining insurance coverage and reimbursement for our product. |
If we fail to achieve superiorityacceptance over other existing or newly developed products, we may be unable to obtain regulatory approval.approval or successfully commercialize our MODD1 insulin pump product candidate or any future products. If our competitors’ market medical devices that are less expensive, safer or more effective than our insulin pump, or that gain or maintain greater market acceptance, we may not be able to compete effectively.effectively, which would adversely affect our business, prospects, results of operations and financial condition. See “Business – Competition.“Business - Competition.”
We expect to rely on third-party manufacturers and will be dependent on their quality and effectiveness.
Our insulin pump requires precise, high-quality manufacturing. The failure to achieve and maintain high manufacturing standards, including failure to detect or control anticipated or unanticipated manufacturing errors or the frequent occurrence of such errors, could result in patient injury or death, discontinuance or delay of ongoing or planned clinical studies, delays or failures in product testing or delivery, cost overruns, product recalls or withdrawals and other problems that could seriously hurt our business. Contract medical device manufacturers often encounter difficulties involving production yields, quality control and quality assurance and shortages of qualified personnel. These manufacturers are subject to stringent regulatory requirements, including the FDA’s current good-manufacturing-practices regulations. If our contract manufacturers fail to maintain ongoing compliance at any time, the production of our product could be interrupted, resulting in delays or discontinuance of our clinical studies, additional costs and loss of potential revenues.
15
We may not be able to successfully scale-up manufacturing of our product candidate in sufficient quality and quantity, which would delay or prevent us from developing our product candidate and commercializing our product candidate.
In order to conduct larger-scale or late-stage clinical studies and for commercialization of our insulin pump, if 510(k) clearance is granted, we will need to manufacture it in larger quantities. We may not be able to successfully increase the manufacturing capacity for our product candidate in a timely or cost-effective manner, or at all. In addition, quality issues may arise during scale-up activities. If we are unable to successfully scale up the manufacture of our product candidate in sufficient quality and quantity, the development and testing of our product candidate and regulatory approval or commercial launch may be delayed, which could significantly harm our business.
We are dependent upon third-party suppliers to manufacture our product, and this makes us vulnerable to supply shortages and price increases; we may not be able to obtain an adequate supply of components on a timely basis or at all.
The future manufacture of our product will require the timely delivery of sufficient amounts of components from multiple suppliers in various countries. We intend to work closely with our suppliers to ensure continuity of supply, but we cannot guarantee these efforts will be successful. Due to the supply chain issues experienced by the semiconductor industry, at times, we have experienced delays obtaining integrated circuits from certain suppliers. We may need to enter into “take or pay” contracts with suppliers. We have also seen price increases for various components. We do not have supply agreements with any of our suppliers, and we make purchases based on individual purchase orders. An interruption, delay, or inability to obtain components from our third-party suppliers at acceptable prices in a timely manner, could hinder our ability to manufacture our products and have a material adverse effect on our business, prospects, financial condition and results of operations.
We may be subject to potential product liability and other claims that could materially impact our business and financial condition.
The development and sale of our insulin pump exposes us to the risk of significant damages from product liability and other claims, and the use of our product candidate in clinical studies may result in adverse effects.effects from liability claims. We cannot predict all the possible harms or adverse effects that may result. We maintain a modest amount ofintend to obtain product liability insurance to provide some protection from claims. Nonetheless, we may not have sufficient resources to pay for any liabilities resulting from a personal injury or other claim, even if it is partially covered by insurance. In addition to the possibility of direct claims, we may be required to indemnify third parties against damages and other liabilities arising out of our development, commercialization and other business activities, which would increase our liability exposure. If third parties that have agreed to indemnify us fail to do so, we may be held responsible for those damages and other liabilities as well.
Legislative, regulatory, or medical cost reimbursement changes may adversely impact our business.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, that relate to the health care system in the U.S. and in other jurisdictions may change the nature of and regulatory requirements relating to innovations in medical devices, testing and regulatory approvals, limit or eliminate payments for medical procedures and treatments, or subject the pricing of medical devices to government control. In addition, third-party payors in the U.S. are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new products. Consequently, significant uncertainty exists as to the reimbursement status of newly approved health care products. Significant changes in the health care system in the U.S. or elsewhere, including changes resulting from adverse trends in third-party reimbursement programs, could have a material adverse effect on our projected future operating results and our ability to raise capital, commercialize products, and remain in business.
We are subject to extensive regulation by the U.S. Food and Drug Administration, which could restrict the sales and marketing of our insulin pump and could cause us to incur significant costs.
Our insulin pump is subject to extensive regulation by the FDA. These regulations relate to manufacturing, labeling, sale, promotion, distribution and shipping. Before a new medical device, or a new intended use of a legally marketed device, can be marketed in the United States, it must be cleared or approved by FDA through the applicable premarket review process (510(k), PMA, or de novo classification), unless an exemption applies. If we receive 510(k) clearance for our insulin pump, we may be required to obtain new 510(k) clearances for significant post-market modifications to the pump. Each premarket submission and review process can be expensive and lengthy, and entail significant user fees, unless exempt.
Medical devices may be marketed only for the indications for which they are approved or cleared. Further, 510(k) clearances can be revoked if safety or effectiveness problems develop once the device is on the market.
The current regulatory requirements to which we are subject may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by the FDA, which may include any of the following sanctions:
| untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| customer notification, or orders for repair, replacement or refunds; |
| voluntary or mandatory recall or seizure of our current or future products; |
| administrative detention by the FDA of medical devices believed to be adulterated or misbranded; |
| imposing operating restrictions, suspension or shutdown of production; |
| refusing our requests for 510(k) clearance, PMA or de novo classification of any new products, new intended uses or modifications to our insulin pump; |
| rescinding 510(k) clearance that has already been granted; and |
| ● | criminal prosecution. |
The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in shareholdersstockholders losing their entire investment.
Although our systeminsulin pump product candidate does not presently require clinical trials to apply to the FDA for clearance and even if a clinical trial is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device or may be equivocal or otherwise not be sufficient for us to obtain approval of our product candidate.
Clinical trials are almost always required to support a PMA application and may also be required to support 510(k) submissions although at this time ours does not require a PMA. If the device presents a “significant risk” to human health as defined by the FDA, the FDA requires the study sponsor to submit an investigational device exemption (“IDE”) application and obtain IDE approval prior to commencing human clinical trials. The IDE must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. An IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. The FDA may also notify the sponsor that the study is approved as proposed. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the study, the FDA may permit a clinical trial to proceed under a conditional approval. Furthermore, the agency may withdraw approval of an IDE under certain circumstances. Clinical trials for a significant risk device may begin once an IDE is approved by the FDA and the appropriate Institutional Review Board (“IRB”) at each clinical trial site. If the product is deemed a “non-significant risk” device, IDE approval from the FDA would not be required, but the clinical trial would need to meet other requirements including IRB approval. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. A clinical trial may be suspended by the FDA or at a specific site by the relevant IRB at any time for various reasons, including a determination that the risks to the trial participants outweigh the benefits of participation in the clinical trial. Even if a clinical trial is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device or may be equivocal or otherwise not be sufficient for us to obtain approval of our product.
Our success depends substantially upon our ability to obtain and maintain intellectual property protection relating to our product candidate and research technologies.
We have applied to the U.S. Patent and Trademark Office (the USPTO) and various foreign patent agencies for patents on our proprietary fluid movement technology and the configuration of our insulin pump.delivery methodology. To date, the USPTO has granted three patents to us, and we have additional applications pending and in various stages of review by the USPTO and foreign patent agencies. There iscan be no assurance that these patentswe will be issued additional patents by the USPTO or foreign patent agencies and no assurance that theyany of our patents will prevent other companies from competing with us. We will continue to attempt to patent our innovations, as appropriate, to help ensure a sustainable competitive advantage.
Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering health care product inventions, our ability to enforce our existing patents and to obtain and enforce patents that may issue from any pending or future patent applications is uncertain and involves complex legal, scientific and factual questions. To date, no consistent policy has emerged regarding the breadth of claims allowed in medical device patents. Thus, we cannot be sure that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents do issue, we cannot be sure that the claims of these patents will be held valid or enforceable by a court of law, will provide us with any significant protection against competing products, or will afford us a commercial advantage over competitive products. If, at some point in the future, one or more products resulting from our product candidates is approved for sale by the FDA and we do not have adequate intellectual property protection for those products, competitors could duplicate them for approval and sale in the United States without repeating the extensive testing required of us to obtain FDA approval.
If we are sued for infringing on third-party intellectual property rights, it will be costly and time-consuming, and an unfavorable outcome would have a significant adverse effect on our business.
Our ability to commercialize our product candidate depends on our ability to use, manufacture and sell our product candidate without infringing the patents or other proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the diabetes medical device area. There may be existing patents, unknown to us, on which our activities with our insulin pump candidate could infringe.
If a third party claims that our actions infringe on its patents or other proprietary rights, we could face a number of issues that could seriouslymaterially harm our competitive position, including, but not limited to:
| infringement and other intellectual property claims that, even if meritless, can be costly and time-consuming, delay the regulatory approval process and divert management’s attention from our core business operations; |
| An order that we pay substantial damages for infringement, including consequential damages for lost of profits or market share, if a court determines that our products or technologies infringe on a third party’s patent or other proprietary rights; |
| a court prohibiting us from selling or licensing our products or technologies unless the holder licenses the patent or other proprietary rights to us, which it is not required to do; and |
| even if a license is available from a holder, we may have to pay substantial royalties or grant cross-licenses to our patents or other proprietary rights. |
If any of these events occur, it could significantly harm our operations and financial condition and negatively affect our stock price.
If we are unable to protect the confidentiality of our proprietary information, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely on our unpatented technology, trade secrets and know-how. We generally seek to protect this information by confidentiality, non-disclosure and assignment of invention agreements with our officers, employees, contractors and other service providers and with parties with which we do business. These agreements may be breached, which breach may result in the misappropriation of such information, and we may not have adequate remedies for any such breach. We cannot be certain that the steps we have taken will prevent unauthorized use or reverse engineering of our technology.
Moreover, our trade secrets may be disclosed to or otherwise become known or be independently developed by competitors. To the extent that our officers, employees, contractors, other service providers, or other third parties with whom we do business use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. If, for any of the above reasons, our intellectual property is disclosed or misappropriated, it would harm our ability to protect our rights and have a material adverse effect on our business, financial condition, and results of operations.
18
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to gain and maintain a competitive advantage. The following examples are illustrative:
| others may be able to make devices that are similar to our insulin pump but that are not covered by the claims of the patents that we own; |
| we or any collaborators might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own; |
| we might not have been the first to file patent applications covering certain of our inventions; |
| others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| it is possible that our pending patent applications will not lead to issued patents; |
| issued patents that we own may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges; |
| our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights, and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; and |
| we may not develop additional proprietary technologies that are patentable. |
Healthcare reform and drug-pricing reform laws could adversely affect our product candidate and financial condition.
In the United States, there have been, and continue to be, a number of legislative initiatives to contain healthcare costs. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (ACA), was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other ways in which it may affect our business, the ACA implemented payment system reforms, including a national pilot program on payment bundling to encourage hospitals, physicians, and other providers to improve the coordination, quality, and efficiency of certain healthcare services through bundled payment models and expanded the eligibility criteria for Medicaid programs.
Since its enactment, there have been judicial, executive, and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal, or replace the ACA will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, reduced Medicare payments to providers by 2% per fiscal year, effective on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through the end of 2021, unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, the Bipartisan Budget Act of 2018 among other things, amended the Medicare statute, effective January 1, 2019, to reduce the coverage gap in most Medicare drug plans, commonly known as the “donut hole,” by raising the manufacturer discount under the Medicare Part D coverage gap discount program to 70%. It is unclear how the ACA and its implementation, as well as efforts to repeal or replace, or invalidate, the ACA, or portions thereof, will affect our insulin pump or our business. Additional legislative changes, regulatory changes, and judicial challenges related to the ACA remain possible. It is possible that the ACA, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future, could have an adverse effect on our industry generally and on our ability to commercialize our insulin pump and achieve profitability. We have assumed in all of our financial projections that there is not an increase in the reimbursement for our product through the pharmacy or durable medical equipment routes.
President Biden intends, as his predecessor did, to take action against drug prices which are considered “high.” Drug pricing continues to be a subject of debate at the executive and legislative levels of U.S. government. The American Rescue Plan Act of 2021 includes a provision that will eliminate the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. With the elimination of the rebate cap, manufacturers may be required to compensate states in an amount greater than what the state Medicaid programs pay for the drug. Additionally, the Inflation Reduction Act of 2022 contains substantial drug pricing reforms, including the establishment of a drug price negotiation program within the U.S. Department of Health and Human Services that would require manufacturers to charge a negotiated “maximum fair price” for certain selected drugs or pay an excise tax for noncompliance, the establishment of rebate payment requirements on manufacturers of certain drugs payable under Medicare Parts B and D to penalize price increases that outpace inflation, and requires manufacturers to provide discounts on Part D drugs. Substantial penalties can be assessed for noncompliance with the drug pricing provisions in the Inflation Reduction Act of 2022. The Inflation Reduction Act of 2022 could have the effect of reducing the prices we can charge and reimbursement we receive for our products, if approved, thereby reducing our profitability, and could have a material adverse effect on our financial condition, results of operations and growth prospects. The effect of Inflation Reduction Act of 2022 on our business and the pharmaceutical industry in general is not yet known.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We expect that additional federal, state and foreign healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in limited coverage and reimbursement and reduced demand for our products, once approved, or additional pricing pressures.
These and other healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any current product or future product candidate. Any reduction in reimbursement from Medicare or other government healthcare programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of any current or future product candidates, if any, may be. In addition, increased Congressional scrutiny of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Even if we are able to obtain all regulatory approvals and have completed all other steps needed to be taken to commercialize our insulin pump, if we or any contract manufacturers we select fails to comply with the FDA’s quality system regulations, the manufacturing and distribution of our product candidate could be interrupted, and our product sales and operating results could suffer.
A material step in the process of the commercialization of our product candidate will involve selecting a manufacturer or manufacturers for our pump. We and any future contract manufacturers of our insulin pump will be required to comply with the FDA’s quality system regulations, which impose a complex regulatory framework that covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices. The FDA enforces its quality system regulations through periodic unannounced inspections. We cannot assure you that, in the future, any manufacturing facilities owned by us or any contract manufacturer will pass any quality system inspection. In the event that our or any contract manufacturer’s facilities fails a quality system inspection, the manufacturing or distribution of our product candidate could be interrupted and our operations disrupted. Failure to take adequate and timely corrective action in response to an adverse quality system inspection could force a suspension or shutdown of any packaging and labeling operations or then manufacturing operations of any contract manufacturers, or a recall of our insulin pump. If any of these events were to occur, we at such time would not be able to provide our customers with the quantity of insulin pumps that they require on a timely basis, our reputation could be harmed and we could lose any customers we then have, any or all of which could have a material adverse effect on our business, financial condition and results of operations.
We may undertakebring infringement claims or other legal proceedings against third parties, causing us to spend substantial resources on litigation and exposing our own intellectual property portfolio to challenge.
We may come to believe that third parties are infringing on our patents or other proprietary rights. To prevent infringement or unauthorized use, we may need to file infringement and/or misappropriation suits, which are very expensive and time-consuming, could result in meritorious counterclaims against us and would distract management’s attention. Also, in an infringement or misappropriation proceeding, a court may decide that one or more of our patents is invalid, unenforceable, or both, in which case third parties may be able to use our technology without paying license fees or royalties. Even if the validity of our patents is upheld, a court may refuse to stop the other party from using the technology at issue on the grounds that the other party’s activities are not covered by our patents. See “Business –- Patents,” below.
20
We may become involved in disputes with our present or future contract partners over intellectual property ownership or other matters, which would have a significant effect on our business.
Inventions discovered in the course of performance of contracts with third parties or contractors may become jointly owned by such third party contractors and us, in some cases, and the exclusive property of one of us, in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention or whether it is jointly owned, and disputes could arise regarding ownership or use of those inventions or jointly developed improvements thereto. Other disputes may also arise relating to the performance or alleged breach of our agreements with third parties. Any disputes could be costly and time-consuming, and an unfavorable outcome could have a significant adverse effect on our business.
Assuming our insulin pump receives FDA clearance or approval, our insulin pump will still be subject to recalls, which would harm our reputation, business operations and financial results.
Even assuming we obtain FDA approval or clearance with regard to our insulin pump, the FDA has the authority to require the recall of our pump if we commence manufacturing of our insulin pump and we or any contract manufacturers we retain fail to comply with relevant regulations pertaining to manufacturing practices, labeling, advertising or promotional activities, or if new information is obtained concerning the safety or efficacy of the device. A government-mandated recall could occur if the FDA finds that there is a reasonable probability that our device would cause serious, adverse health consequences or death. A voluntary recall by us could occur as a result of manufacturing defects, labeling deficiencies, packaging defects or other failures to comply with applicable regulations. Any recall would divert management’s attention and financial resources and harm our reputation with customers. A recall involving our insulin pump would be particularly harmful to our business, financial condition and results of operations because it is currently our only product candidate.
Any disruption and/or instability in economic conditions and capital markets could adversely affect our ability to access the capital markets, and thus adversely affect our business and liquidity.
Negative economic conditions and issues with regard toinstability or uncertainty in the financial markets could have a negative impact on our ability to access the capital markets, and thus have a negative impact on our then operations and liquidity. We face certain risks in the event of a sustained deterioration of financial market liquidity, as well as in the event of sustained deterioration in the liquidity, or failure, of our banking, cash management and custodial financial institutions. A general shortage of liquidity and credit combined with the substantial losses in worldwide equity markets could lead to an extended worldwide recession in the future. If such occurred, we would face significant challenges if conditions in the capital markets did not improve. Our ability to access the capital markets under such circumstances could be severely restricted at a time when we need to access such markets, which could have a negative impact on our business plans. Even if we are able to raise capital under such circumstances, it may not be at a price or on terms that are favorable to us. We cannot predict the occurrence of future disruptions or how long such negative conditions might continue.
Because our current insulin pump prototype is still in the developmentpre clearance stage with the FDA, it does not have reimbursement and is not approved for insurance coverage. If in the future we are approvedcleared for and are otherwise able to commercialize our insulin pump, but are unable to obtain adequate reimbursement or insurance coverage for such product candidate from third-party payors, we will be unable to generate significant revenue.
Because our current insulin pump prototype is still in the developmentpre clearance by the FDA stage, it does not have reimbursement and is not approved for insurance coverage. The future availability of insurance coverage and reimbursement for newly approved medical devices is highly uncertain. In the United States, patients using insulin pumps are generally reimbursed for all or part of the product cost by Medicare or other third-party payors. Any future commercial success of our insulin pump will be substantially dependent on whether third-party coverage and reimbursement is available for future customers. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new medical devices, and, as a result, they may not cover or provide adequate reimbursement for our insulin pump, assuming we are able to fully develop and obtain all regulatory approval to market it in the United States. In addition, in certain countries, no uniform policy of coverage and reimbursement for medical device products and services exists among third-party payors. Therefore, coverage and reimbursement for medical device products and services can differ significantly from payor to payor. In addition, payors continually review new technologies for possible coverage and can, without notice, deny coverage for these new products and procedures. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained, or maintained if obtained. Reimbursement systems in international markets vary significantly by country and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. In many international markets, a product must be approved for reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control reimbursement for new devices and procedures. Accordingly, unless government and other third-party payors provide coverage and reimbursement for our insulin pump, patients may not use it, which would cause investors to lose their entire investment.
21
We are subject to the oversight ofby the SEC and other regulatory agencies. Investigations by those agencies could divert management’s focus and could have a material adverse effect on our reputation and financial condition.
We are subject to the regulation and oversight of the SEC and state regulatory agencies, in addition to the FDA. As a result, we may face legal or administrative proceedings by these agencies. We are unable to predict the effect of any investigations on our business, financial condition or reputation. In addition, publicity surrounding any investigation, even if ultimately resolved in our favor, could have a material adverse effect on our business.
We are a “smaller reporting company” and, as a result of the reduced disclosure and governance requirements applicable to smaller reporting companies, our Common Stockcommon stock may be less attractive to investors.
We are a “smaller reporting company,” and are subject to lesser disclosure obligations in our SEC filings compared to other issuers. Specifically, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our operating results and financial prospects.
We do not expect any cash dividends to be paid on our shares of Common Stockcommon stock for the foreseeable future.
We have never declared or paid a cash dividend and we do not anticipate declaring or paying dividends on our Common Stockcommon stock for the foreseeable future. We expect to use future financing proceeds and earnings, if any, to fund operating expenses. Consequently, shareholders’stockholders’ only opportunity to achieve a return on their investment is if the price of our stock appreciates and they sell their shares at a profit. We cannot assure shareholdersstockholders of a positive return on their investment when they sell their shares or that shareholdersstockholders will not lose the entire amount of their investment.
If the beneficial ownership of our Common Stockcommon stock continues to be highly concentrated, it may prevent our shareholdersstockholders from influencing significant corporate decisions.
OurAs of March 24, 2023, our executive officers, directors and certain persons who may be deemed affiliates beneficially own in excess ofapproximately 50% of our issued and outstanding Common Stock.common stock. Specifically, James Besser, our chief executive officer, is the beneficial owner of approximately 29% of our outstanding capital stock. As a result, such persons may exercise substantial influence over the outcome of corporate actions requiring shareholderstockholder approval including, without limitation, the election of directors, certain mergers, consolidations and sales of all or substantially all of our assets or any other significant corporate transactions. Such persons may also vote against a change of control, even if such a change of control would benefit our other shareholders.
Salestockholders. Thus, investors in our common stock cannot reasonably expect to have any influence over the election of our Common Stockdirectors or other matters submitted to a vote of our stockholders. Instead, our existing significant stockholders may exert a substantial influence on the election of our directors and any actions requiring or otherwise put to a stockholder vote, potentially in a manner that you do not support. The concentrated amount of control over our affairs held by shareholdersa relatively few number of significant investors could encourage short sales by third parties,serve to reduce the attractiveness or liquidity of our common stock, and thereby depress its trading price. Additionally, conflicts of interest may arise between these executive officers, directors and other affiliates, on the one hand, and us and our other stockholders, on the other hand. In resolving these conflicts of interests, these investors may favor their own interests and the interests of their affiliates, over the interests of our other stockholders, which could contribute to the further declinecause a material adverse effect on our business, prospects, financial condition and results of our stock price.operations.
The significant downward pressure on the price of our Common Stock that would be caused by the sale of material amounts of our Common Stock could encourage short sales by third parties. Such an event could place further downward pressure on the price of our Common Stock.
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the JOBS Act). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We will remain an emerging growth company until the earlier of (i) the last day of the fiscal year (a) following the fifth anniversary of the completion of the first sale of shares covered by this prospectus, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a large accelerated filer, which requires the market value of our Common Stock that is held by non-affiliates to exceed $700.0 million as of the prior September 30th, and (ii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Future sales of our securities could adversely affect the market price of our Common Stockcommon stock and our future capital-raising activities could involve the issuance of equity securities, which would dilute your investment and could result in a decline in the trading price of our Common Stock.common stock.
We may sell securities in the public or private equity markets at prices per share below the current market price of our Common Stock,common stock, even if we do not have an immediate need for additional capital at that time. Sales of substantial amounts of shares of our Common Stock,common stock, or the perception that such sales could occur, could adversely affect the prevailing market price of our shares and our ability to raise capital. We may issue additional shares of Common Stockcommon stock in future financing transactions or as incentive compensation for our executive management and other key personnel, consultants and advisors. Issuing any equity securities would be dilutive to the equity interests represented by our then-outstanding shares of Common Stock.common stock. Moreover, sales of substantial amounts of shares in the public market, or the perception that such sales could occur, may adversely affect the prevailing market price of our Common Stockcommon stock and make it more difficult for us to raise additional capital. Such resulting significant downward pressure on the price of our common stock could also encourage short sales by third parties. Such an event could place further downward pressure on the price of our common stock.
Furthermore, we and our directors and officers and holders of more than 5% of the Company’s outstanding shares of common stock have agreed with the underwriter not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock or securities convertible into common stock for a period of three months after the date of this prospectus. The underwriter may, in its discretion, release the restrictions on any such shares at any time without notice. See “Underwriting.” We cannot predict the effect that future sales of our common stock would have on the market price of our common stock.
Our articles of incorporation allow for our board of directors to create new series of preferred stock without further approval by our shareholders,stockholders, which could adversely affect the rights of the holders of our Common Stock.common stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. Currently, our board of directors has the authority to designate and issue up to 5,000,000 shares of our preferred stock without further shareholderstockholder approval. In the future, our board of directors could authorize the issuance of one or more series of preferred stock that would grant to holders, among other rights, the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of Common Stockcommon stock and the right to the redemption of our preferred shares acquired by such persons, together with a premium, prior to the redemption of our Common Stock.common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our Common Stockcommon stock or that is convertible into our Common Stock,common stock, which could decrease the relative voting power of our Common Stockcommon stock or result in dilution to our existing shareholders.stockholders.
If we fail to establish and maintain an effective system of internal controls, we may not be able to report our financial results accurately or prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely affect the trading price of our Common Stock.common stock.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. If we are unable to maintain effective internal controls, we may not have adequate, accurate or timely financial information, and we may be unable to meet our reporting obligations as a public company, including the requirements of the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act). In addition, we may be unable to accurately report our financial results in future periods, or report them within the timeframes required by the requirements of the SEC or the Sarbanes-Oxley Act. Failure to comply with the Sarbanes-Oxley Act, when and as applicable, could also potentially subject us to sanctions or investigations by the SEC or other regulatory authorities. Any failure to maintain or implement required new or improved controls, or any difficulties we encounter in their implementation, could result in identification of additional material weaknesses or significant deficiencies, cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements.
Furthermore, Section 404 of the Sarbanes-Oxley Act and related regulations require our management to evaluate the effectiveness of our internal control over financial reporting as of the end of each fiscal year. Based on its evaluation, our management concluded that our internal controls over financial reporting were effective as of March 31, 2021.2022. We cannot provide assurance that, in the future, a material weakness or significant deficiency will not exist or otherwise be discovered. If that were to happen, it could harm our operating results and cause shareholdersstockholders to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our securities.
Our board of directors is able to adopt recapitalizations through forward or reverse splits of our outstanding shares of Common Stockcommon stock without shareholderstockholder approval.
Pursuant to our amended and restated articles of incorporation, our board of directors has the power, without obtaining shareholderstockholder approval, to effectuate recapitalizations of us through forward or reverse splits of our outstanding Common Stock.common stock. As a result of such provision, our board of directors can implement recapitalizations of us by effectuating a forward or reverse stock split of our outstanding Common Stock,common stock, which would increase or decrease each of our shareholder’sstockholder’s number of shares owned, and our shareholdersstockholders will have no right to approve or disapprove any such action even if such actions have a material adverse effect on them.
Risks Related to This Offering
CAUTIONARY
After this offering, we will need to raise additional capital in the future to execute our business plan.
We expect the net proceeds to us from this offering will approximate $7.0 million, which assumes that the over-allotment option is not exercised. Upon closing of this offering, we expect to have cash and cash equivalents of approximately $14.7 million. After the closing of this offering, we believe we will have adequate funds to operate our business for the at least the next nine months. During that timeframe, we intend to complete our 510(k) submission and obtain approval from the FDA, commence our initial commercialization efforts and complete our manufacturing capability. We will require additional funding to fully commercialize our pump product and bring it to market on a large scale.
We cannot be certain that additional capital, whether through selling additional debt or equity securities or obtaining a line of credit or other loan, will be available to us or, if available, will be on terms acceptable to us. If we issue additional securities to raise funds, these securities may have rights, preferences, or privileges senior to those of our common stock, and our current stockholders may experience dilution. If we are unable to obtain funds when needed or on acceptable terms, we may be required to curtail our current product development programs, cut operating costs, forego future development and other opportunities or even terminate our operations.
As we have broad discretion in how we use the proceeds from this offering, we may use the proceeds in ways with which you disagree.
We have not allocated the net proceeds from this offering for any specific purpose, except as generally set forth under “Use of Proceeds.” As set forth therein, our management will have significant flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used in ways you would agree with or ways which are likely to increase the value of your investment. Because of the number and variability of factors that will determine our use of our net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for our company or your investment. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
Holders of our warrants will have no rights as a common stockholder until they acquire our common stock.
The warrants included in the units in this offering do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire the common stock and pay the exercise price per share, prior to five years from the date of issuance, after which date any unexercised warrants will expire and have no further value. Until holders of the warrants acquire common stock upon exercise of the warrants, the holders will have no rights with respect to the common stock issuable upon exercise of the warrants. Upon exercise of the warrants, the holder will be entitled to exercise the rights of a stockholder as to the security exercised only as to matters for which the record date occurs after the exercise. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the warrants, and consequently, whether it will ever be profitable for holders of the warrants to exercise the warrants.
24
Provisions of the warrants offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the discussion of the provisions of our governing organizational documents, certain provisions of the warrants offered by this prospectus could make it more difficult or expensive for a third party to acquire us. The warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the warrants. These and other provisions of the warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
The warrants offered by this prospectus are speculative in nature and may not have any value.
The warrants offered by this prospectus will be exercisable for five years from the date of issuance. There can be no assurance that the market price of our common stock will ever exceed the exercise price of the warrants. In the event that our common stock price does not exceed the exercise price of the warrants during the term of the warrants, the warrants may not have any value.
There is no public market for the warrants being offered in this offering.
There is no public trading market for the warrants offered by this prospectus, and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants on a national securities exchange. Without an active market, the liquidity of the warrants will be limited.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
As of December 31, 2022, our net tangible book value (deficit) was approximately $8.0 million, or approximately $0.73 per share of common stock. As the effective price per share of our common stock being offered in this offering is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial dilution with respect to the net tangible book value of the common stock you purchase in this offering. Based on the assumed public offering price of $3.78 per unit being sold in this offering, and our net tangible book value per share as of December 31, 2022, if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of $0.91 per share with respect to the net tangible book value of the common stock (assuming no exercise of the underwriter’s option to purchase additional shares). See the section titled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
You may experience future dilution as a result of future equity offerings and other issuances of our common stock or other securities. In addition, this offering and future equity offerings and other issuances of our common stock or other securities may adversely affect the price of our common stock.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock (such as warrants, convertible debt or preferred stock) at prices that may not be the same as the price per share in this offering. We may not be able to sell shares of common stock or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares of common stock or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock or securities convertible into common stock in future transactions may be higher or lower than the price per share in this offering. In addition, the sale of shares of common stock in this offering and any future sales of a substantial number of shares of our common stock in the public market, or the perception that such sales may occur, could adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales of those shares of common stock or the availability of those shares of common stock for sale will have on the market price of our common stock.
25
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, which we refer to as the Securities“Securities Act,” and Section 21E of the Securities Exchange Act of 1934, as amended, which we refer to as the Exchange“Exchange Act,” that relate to future events or to our future operations or financial performance. Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statement.
Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “forecast,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “targets,” “likely,” “will,” “would,” “could,” “should,” “continue,” “scheduled” and similar expressions or phrases, or the negative of those expressions or phrases, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Although we believe that we have a reasonable basis for each forward-looking statement contained in this registration statement,prospectus, we caution you that these statements are based on our estimates or projections of the future that are subject to known and unknown risks and uncertainties and other important factors that may cause our actual results, level of activity, performance, experience or achievements to differ materially from those expressed or implied by any forward-looking statement. Actual results, level of activity, performance, experience or achievements may differ materially from those expressed or implied by any forward-looking statement as a result of various important factors, including our critical accounting policies and risks and uncertainties relating, to:
| our strategies, prospects, plans, expectations, forecasts or objectives; |
| our ability to achieve a marketable product (i.e., our insulin pump) and the costs and timing thereof; |
| acceptance of our product candidate by our target market and our ability to compete in such market; |
| our ability to raise additional financing when needed and the terms and timing thereof; |
| our ability to expand, protect and maintain our intellectual property rights; |
| our future operations, financial position, revenues, costs, expenses, uses of cash, capital requirements, our need for additional financing or the period for which our existing cash resources will be sufficient to meet our operating requirements; |
| our analysis of the target market for our insulin pump; |
| our ability to obtain all regulatory approvals and clearances relating to our insulin pump including those of the |
| regulatory developments in the United States and other countries; |
| the timing and costs of our obtaining all regulatory approvals and clearances identified immediately above; |
| our compliance with all applicable laws, rules and regulations, including those of the |
| our ability to compete in the diabetes marketplace with larger and more substantial medical device companies; |
| general economic, business, political and social conditions; |
| our reliance on and our ability to retain (and if necessary, timely recruit and replace) our officers, directors and key employees and their ability to timely and competently perform at levels expected of them; |
| our ability to generate significant revenues and achieve profitability; |
| our ability to manage the growth of our business; |
| our commercialization, marketing and manufacturing capabilities and strategies; |
| our ability to expand, protect and maintain our intellectual property position; |
| the success of competing third-party products; |
| our ability to fully remediate our identified internal control material weaknesses; |
| our ability to meet the initial or continuing listing requirement of the Nasdaq Capital Market; |
| ● | our ability to comply with regulatory requirements relating to our business, and the costs of compliance with those requirements, including those on data privacy and security; |
| the specific risk factors discussed under the heading |
| various other matters, many of which are beyond our control. |
26
We will not receive anyestimate that our net proceeds from this offering will be approximately $7,031,490, based on an assumed public offering price of $3.78 per unit which is based on the last reported sale price of our common stock on the Nasdaq Capital Market on May 2, 2023, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
As of December 31, 2022, we had cash and cash equivalents of approximately $7.7 million. We currently expect to use the net proceeds from this offering, together with the $7.7 million of cash and cash equivalents, primarily for the following purposes:
| ● | Approximately $10,900,000 to fund research and development for new products and improvements to our initial pump product candidate including, but not limited to, hiring of key personnel, and costs for continued research activities; |
| ● | Approximately $1,500,000 for the initial development of our sales, marketing and administrative capabilities and organization, including but not limited to adding additional staff, public relations and advertising; |
| ● | Approximately $2,000,000 for the continued development of our manufacturing and production capability, including personnel costs and capital expenditures; and |
| ● | The remainder for working capital, other capital expenditures and general corporate purposes. |
We believe that our existing cash and cash equivalents, along with the net proceeds from this offering, together with interest on cash balances, will be sufficient to fund our operating expenses and capital expenditure requirements through at least the next 12 months. The amount and timing of our actual expenditures and actual use of the common stock by the selling stockholders. We may receivenet proceeds upon the exercise of the May Warrants (tooffering will depend upon numerous factors, including the extenttiming of our submission to the registration statementFDA for 510(k) clearance of our product candidate, which is necessary to commence commercialization, the timing and results of our product launch, including all commercialization activities, the progress of our continuing product research and development activities, our ability to establish our outsourced manufacturing operations, and our ability to add the required staff to execute our business plan, any collaborations that we may enter into with third parties, and any unforeseen delays or cash needs.
Our expected use of the net proceeds from this prospectus isoffering represents our current intentions based upon our present plans and business conditions. As a part is then effectiveresult, our management will have broad discretion in the application of the net proceeds, and if applicable, the “cashless exercise” provision is not utilized by the holder). Any proceedsinvestors will be used for general corporate and working capital or for other purposes thatrelying on our board of directors, in its good faith, deems to be injudgment regarding the best interestapplication of the Company. No assurances can be given thatnet proceeds of this offering. In addition, we might decide to postpone or not pursue these certain of these activities if the net proceeds from this offering and the other sources of cash are less than, or do not last as long as, expected. We have no current understandings, agreements or commitments for any material acquisitions or licenses of such May Warrants will be exercised.any products, businesses or technologies.
Pending their use, we plan to invest the net proceeds from this offering in high-quality, short-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
We have never declared or paid any cash dividend on our capital stock. We do not anticipate paying any cash dividends in the foreseeable future and we intend to retain all of our earnings, if any, to finance our growth and operations and to fund the expansion of our business. Payment of any dividends will be made in the discretion of our board of directors, after its taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion. Any dividends that may be declared or paid on our common stock, must also be paid in the same consideration or manner, as the case may be, on our shares of preferred stock, if any.
28
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2022.
| ● | an actual basis; and |
DETERMINATION OF OFFERING PRICE
| ● | on a pro-forma basis to give effect to the issuance and sale of the units by us in this offering at the assumed public offering price of $3.78 per unit, after deducting underwriting discussions and commissions and estimated offering expenses payable by us for net proceeds of $7,031,490, which assumes that the over-allotment option is not exercised. |
The selling stockholdersas adjusted information below is illustrative only and our capitalization following the closing of this offering will offer common stock atbe adjusted based on the prevailing market prices or privately negotiated price.
Theactual public offering price and other terms of our common stock by the selling stockholders does not necessarily bear any relationship to our book value, assets, past operating results, financial condition or any other established criteria of value. The facts considered in determining thethis offering price were our financial condition and prospects, our limited operating history and the general condition of the securities market.
In addition, there is no assurance that our common stock will tradedetermined at market prices in excess of the offering price as prices for common stock in any public market will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock began trading on Nasdaq under the symbol “MODD” on February 10, 2022.
Holders of Record
On June 2, 2022, the closing price per share of our common stock was $4.75 as reported on The Nasdaq Capital Market, and we had approximately 80 stockholders of record. On June 2, 2022 there were 10,911,684 shares of our common stock issued and outstanding. In addition, we believe that a significant number of beneficial owners of our common stock hold their shares in street name.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis is based on, andpricing. You should be read in conjunctionthis information together with our financial statements which areand the related notes thereto included elsewhere in this prospectus. prospectus and the information set forth under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations contains statements that are forward-looking. These statements are based on current expectations and assumptions that are subject to risk, uncertainties and other factors. These statements are often identified by the use of words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” or “continue,” and similar expressions or variations. Actual results could differ materially because of the factors discussed in “Risk Factors”included elsewhere in this prospectus.
| As of December 31, 2022 | ||||||||
| Unaudited | ||||||||
| Actual | Pro Forma(1) | |||||||
| Cash and cash equivalents | $ | 7,690,957 | $ | 14,722,447 | ||||
| Stockholders’ equity: | ||||||||
| Preferred stock, par value $0.001; 5,000,000 shares authorized and undesignated, actual, pro forma; no shares issued and outstanding, actual or pro forma | — | |||||||
| Common Stock, $0.001 par value, 50,000,000 shares authorized; 10,932,098 shares issued and outstanding, actual; 14,979,718 shares issued and outstanding, pro forma | 10,932 | 15,165 | ||||||
| Additional paid-in capital | 52,900,066 | 59,927,323 | ||||||
| Accumulated deficit | (44,887,453 | ) | (44,887,453 | ) | ||||
| Total stockholders’ equity | $ | 8,023,545 | $ | 15,055,035 | ||||
| Total capitalization | $ | 8,738,842 | $ | 15,770,332 | ||||
| (1) | If the underwriter’s option to purchase up to an additional 634,920 shares of our common stock and warrants to purchase an additional 317,460 shares of our common stock is exercised in full, and assuming no exercise of the warrants, (i) we would receive approximately $1,116,000 in additional net proceeds, based on the assumed initial public offering price per unit of $3.78, which is based on the last reported sale price of our common stock on the Nasdaq Capital Market on May 2, 2023, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us; and (ii) cash and cash equivalents, total stockholders’ equity and total capitalization would each also increase by approximately $1,116,000. |
Each $1.00 increase (decrease) in the assumed public offering price of $3.78 per unit would increase (decrease) the as-adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $1,968,000, assuming that the number of units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of units we are offering. Each increase (decrease) of 100,000 units in the number of units we are offering would increase (decrease) the as adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $351,500, assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other factorsterms of this offering determined at pricing.
The number of shares of our common stock to be outstanding after this offering is based on 10,932,098 shares of our common stock outstanding as of December 31, 2022, and excludes:
| ● | 2,116,402 shares of our common stock issuable upon the exercise of the warrants to be issued as part of the units; |
| ● | 296,296 shares of common stock issuable upon exercise by the underwriter of the underwriter’s Warrants; |
| ● | 2,174,198 shares of our common stock issuable upon exercise of outstanding stock options with a weighted average exercise price of approximately $5.69 per share; |
| ● | 767,796 shares of our common stock issuable upon the exercise of warrants with an exercise price of $6.00 per share; |
| ● | 5,449,478 shares of our common stock issuable upon the exercise of warrants with an exercise price of $6.60 per share; |
| ● | 1,348,314 shares of our common stock issuable upon exercise of pre-funded warrants with an exercise price of $0.01 per share; and |
| ● | 445,559 shares of our common stock reserved for issuance pursuant to future awards under our Amended 2017 Equity Incentive Plan, or the 2017 Plan. |
29
Each unit, with an assumed public offering price of $3.78 per unit, which is based on the last reported sale price of our common stock on The Nasdaq Capital Market on May 2, 2023, consists of two shares of common stock and one warrant to purchase one share of common stock.
If you invest in our units, your interest will be diluted immediately to the extent of the difference between the offering price per share of our common stock that is part of the unit and the as adjusted net tangible book value per share of our common stock immediately after giving effect to this offering.
As of December 31, 2022, our historical net tangible book value, which represents our total tangible assets less total liabilities, was approximately $8.0 million, or $0.73 per share of common stock. Historical net tangible book value per share represents the amount of our total tangible assets reduced by total liabilities, divided by 10,932,098, the number of shares of common stock outstanding on December 31, 2022.
After giving effect to the sale of the units, at the assumed offering price of $3.78 per unit, which is based on the last reported sale price of our common stock on The Nasdaq Capital Market on May 2, 2023, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, but assuming no exercise of the warrants offered hereby or the underwriter’s warrant, our net tangible book value as of December 31, 2022 would have been $15,055,060 or $0.99 per share of common stock. This amount represents an immediate increase in net tangible book value of $0.26 per share to our existing stockholders. Investors purchasing our common stock in this offering will have paid $0.90 more than the as adjusted net tangible book value per share of common stock after this offering.
The following table illustrates this dilution on a per share basis:
| Assumed offering price per share | $ | 1.89 | ||||||
| Historical net tangible book value per share as of December 31, 2022 | $ | 0.73 | ||||||
| Increase in net tangible book value per share attributable to new investors | $ | 0.26 | ||||||
| Net tangible book value per share after the offering | 0.99 | |||||||
| Dilution per share to new investors | $ | 0.90 |
The dilution information discussed above is illustrative only and may change based on the actual initial public offering price and other terms of this offering.
Each $1.00 increase (decrease) in the assumed public offering price of $3.78 per unit would increase (decrease) our net tangible book value after this offering by approximately $1,968,000 per share, and increase (decrease) the dilution per share to new investors by approximately $0.13 per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us full.
The number of shares of our common stock to be outstanding after this offering is based on 10,932,098 shares of our common stock outstanding as of December 31, 2022, and excludes:
| ● | 2,116,402 shares of our common stock issuable upon the exercise of the warrants to be issued as part of the units; |
| ● | 296,296 shares of common stock issuable upon exercise by the underwriter of the underwriter’s warrants; |
| ● | 2,174,198 shares of our common stock issuable upon exercise of outstanding stock options with a weighted average exercise price of approximately $5.69 per share; |
| ● | 767,796 shares of our common stock issuable upon the exercise of warrants with an exercise price of $6.00 per share; |
| ● | 5,449,478 shares of our common stock issuable upon the exercise of warrants with an exercise price of $6.60 per share; |
| ● | 1,348,314 shares of our common stock issuable upon exercise of pre-funded warrants with an exercise price of $0.01 per share; and |
| ● | 445,559 shares of our common stock reserved for issuance pursuant to future awards under the 2017 Plan. |
If the shares described above that are reserved for issuance to the holders of our options and under our 2017 Plan are issued, or we otherwise issue additional shares of common stock in the future, there could be further dilution to investors participating in this offering. In addition, we anticipate needing to raise additional capital before generating positive cash flows and we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we may not know.have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
Our fiscal year endsIf the underwriter exercises its option to purchase additional shares of our common stock and warrants full, the pro forma as adjusted net tangible book value after this offering would be $1.02 per share, the increase in pro forma net tangible book value would be $0.29 per share and the dilution to new investors would be $0.87 per share, in each case assuming an public offering price of $3.78 per unit, which is based on Marchthe last reported sale price of our common stock on the Nasdaq Capital Market on May 2, 2023.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis in conjunction with our unaudited condensed consolidated financial statements and the notes to those financial statements for the three and nine months ended December 31, of each calendar year. Each reference2022 and December 31, 2021 and our audited consolidated financial statements and notes to a fiscal year in this Report, refers tothose financial statements for the fiscal yearyears ended March 31, of the calendar year indicated (for example, fiscal 2022 refers to the fiscal year endingand March 31, 2022). Unless the context requires otherwise, references to “we,2021, in each case, included elsewhere in this prospectus. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.” “us,” “our,” and the “Company” refer to Modular Medical, Inc. and its consolidated subsidiary.Our actual results may differ materially from those contained in or implied by any forward-looking statements.
Company Overview
We are a development stagedevelopment-stage medical device company focused on the design, development and commercialization of an innovative insulin pump using modernized technology to increase pump adoption in the diabetes marketplace. Through the creation of a novel two-part patch pump, our MODD1 product, the Company seekswe seek to fundamentally alter the trade-offs between cost and complexity and access to the higher standards of care that presently-available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training and day-to-day use, we seek to expand the wearable insulin delivery device market beyond the highly motivated “super users” and expand the category into the mass market. The product seeks to serve both the type 1 and the rapidly growing, especially in terms of device adoption, type 2 diabetes markets.
Historically, we have financed our operations principally through private placements and public offerings of our common stock and sales of convertible promissory notes. Based on our current operating plan, substantial doubt about our ability to continue as a going concernwe believe we have adequate cash for a period of at least one year from the date that the financial statements included in Item 1 of this Report are issued exists.next 12 months. Our long-term ability to continue as a going concern depends on our ability to raise additional capital, through the sale of equity or debt securities, to support our future operations. If we are unable to secure additional capital, we will be required to curtail our research and development initiatives and take additional measures to reduce costs. We have provided additional disclosure in Note 1 to the condensed consolidated financial statements in Item 1 of this ReportRegistration Statement and under Liquidity and Capital Resources below.
Impacts of COVID-19
Recent Economic Disruptions
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place”“shelter-in- place” and created significant disruption of the financial markets. The full extent ofmarkets during 2020, 2021 and into 2022. While the national emergency is set to expire in May 2023 and most closures and “shelter-in-place” orders have ended, there can be no assurance that the COVID-19 pandemic will not impact on our operational and financial performance will depend onin the future, developments, including, without limitation,as the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread all of which are uncertain, out of our control, and cannot be predicted.
In March 2020, Santa Diego County
The Russian invasion of Ukraine in California, where we are based, and the state of California issued “shelter-in-place” orders (the Orders). We complied with the Orders and minimized business activities at our San Diego facility from March 2020 until May 2021. During that time, we implemented a teleworking policy for our employees and contractors to reduce on-site activity at our facility. In May 2021, our employees and certain contractors returned to work in our office. We have and continue to experience longer lead times for certain components used to manufacture initial quantities of our products for our submission to the FDA. We remain diligent in continuing to identify and manage risks to our business given the changing uncertainties related to COVID-19. While we believe that our operations personnel are currently in a position to build an adequate supply of products for our FDA submission, we recognize that unpredictable events could create difficulties in the months ahead. We may not be able to address these difficulties in a timely manner, which could delay our submission to the FDA and negatively impact our business, results of operations, financial condition and cash flows.
The continued spread of COVID-19February 2022 has also led to disruptionfurther economic disruptions. Mounting inflationary costs pressures and volatility inrecessionary fears have negatively impacted the global capital markets.economy. During the third quarter of 2022, the U.S. Federal Reserve continued to aggressively address elevated inflation by increasing interest rates. The U.S. Federal reserve increased interest rates by 75 basis points in each of its meetings held in July, September and November 2022, 50 basis points in its meeting held in December 2022, and 25 basis points in its meeting held in each of February 2023 and March 2023, as inflation remains elevated. We were recently able to raise additional capital through equity offerings in a private placement of convertible promissory notes (see discussion below under Liquidity). However,February 2022 and May 2022, however, we will need to raise additional capital to commercialize our pump product candidate and support our operations in the future. We may be unable to access the capital markets, orand additional capital may only be available to us on terms that could be significantly detrimental to our existing stockholders and holders of the convertible promissory notes and to our business.
For additional information on risks that could impact our future results, please refer to “Risk Factors” on page 10.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based upon our condensed consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these condensed consolidated financial statements requires us to make certain estimates and judgments that affect the reported amounts of assets, liabilities, and expenses. On an ongoing basis, we make these estimates based on our historical experience and on assumptions that we consider reasonable under the circumstances. Actual results may differ from these estimates and reported results could differ under different assumptions or conditions. Our significant accounting policies and estimates are disclosed in Note 1 of the Notes to Consolidated Financial Statements in our Annual Report on Form 10-K for the year ended March 31, 2021.2022. As of December 31, 2021,2022, there have been no material changes to our significant accounting policies and estimates.
Results of Operations
Nine Months Ended December 31, 2022 and December 31, 2021
Research and Development
| December 31, | Change | |||||||||||||||
| 2021 | 2020 | 2020 to 2021 | ||||||||||||||
| Research and development – Three months ended | $ | 1,849,399 | $ | 1,086,669 | $ | 762,730 | 70.2 | % | ||||||||
| Research and development – Nine months ended | $ | 5,742,911 | $ | 3,150,149 | $ | 2,592,762 | 82.3 | % | ||||||||
| December 31, | Change | |||||||||||||||
| 2022 | 2021 | Fiscal 2022 to Fiscal 2023 | ||||||||||||||
| Research and development – Three months ended | $ | 2,196,546 | $ | 1,849,399 | $ | 347,147 | 18.8 | % | ||||||||
| Research and development – Nine months ended | $ | 6,804,069 | $ | 5,742,911 | $ | 1,061,158 | 18.5 | % | ||||||||
Our research and development expenses include personnel, consulting, materialsproduct prototyping and other costs associated with the development and initial production of our insulin pump product. We expense research and development costs as they are incurred.
Research and development, or R&D, expenses increased for the three and nine months ended December 31, 2021 as2022 compared with the priorsame period of fiscal 2021, primarily due to increased engineering and manufacturingoperations personnel costs, prototype and production component and material costs and higher stock-based compensation expenses. The increases in R&D expenses were partially offset by a decrease in consulting costs, as we havereduced our utilization of consultants, as we increased our employee headcount and the consultants completed development of aspects of our pump design and manufacturing activities.features. Our full-time R&D employee headcount increased to 32 at December 31, 2022 from 18 at December 31, 2021. R&D expenses increasedincluded stock-based compensation expenses of $356,752 and $204,962 for the three months ended December 31, 2022 and 2021, respectively, and $1,034,674 and $459,989 for the nine months ended December 31, 2021 as compared with the prior period of fiscal 2021 primarily due to increased engineering2022 and manufacturing personnel and consulting costs, protype and production component and material costs and stock-based compensation expenses. R&D expenses included non-cash, stock-based compensation expenses of $204,962 and $96,127 for the three months ended December 31, 2021, and 2020, respectively, and $459,989 and $301,767 for the nine months ended December 31, 2021 and 2020, respectively. We expect R&Dresearch and development expenses to remain flatcomparable for the remainder of fiscal 2022,2023, as we continue to advance the development of our pump product and develop an initial low-volumeour manufacturing process.
General and Administrative
| December 31, | Change | |||||||||||||||
| 2021 | 2020 | 2020 to 2021 | ||||||||||||||
| General and administrative – Three months ended | $ | 1,981,665 | $ | 783,898 | $ | 1,197,767 | 152.8 | % | ||||||||
| General and administrative – Nine months ended | $ | 5,156,152 | $ | 2,453,808 | $ | 2,702,344 | 110.1 | % | ||||||||
| December 31, | Change | |||||||||||||||
| 2022 | 2021 | Fiscal 2022 to Fiscal 2023 | ||||||||||||||
| General and administrative – Three months ended | $ | 1,161,351 | $ | 1,981,665 | $ | (820,314 | ) | (41.4 | )% | |||||||
| General and administrative – Nine months ended | $ | 3,502,029 | $ | 5,156,152 | $ | (1,654,123 | ) | (32.1 | )% | |||||||
General and administrative expenses consist primarily of personnel and related overhead costs for facilities,finance, human resources, legal, marketing and general management.
General and administrative, or G&A, expenses decreased for the three months ended December 31, 2022 compared with the same period of 2021, primarily as a result of decreased stock-based compensation, personnel and benefit costs and legal fees, which in fiscal 2022 related to our public offering and listing on the Nasdaq that was completed in February 2022. These decreases were partially offset by increased consulting and professional services fees.
G&A expenses decreased for the nine months ended December 31, 2022 compared with the same period of 2021, primarily as a result of decreased stock-based compensation, personnel and benefit costs, consulting and legal fees and marketing costs. These decreases were partially offset by increased accounting fees, travel costs and office-related expenses. Our full-time G&A employee headcount increased to 3 at December 31, 2022 from 2 at December 31, 2021. G&A expenses included stock-based compensation expenses of $282,753 and $1,016,774 for the three months ended December 31, 2022 and 2021, respectively and $1,085,839 and $2,280,098 for the nine months ended December 31, 2022 and 2021, respectively. We expect G&A expenses to remain flat for the remainder of fiscal 2023.
Years Ended March 31, 2022 and March 31, 2021
Research and Development
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| Research and development | $ | 7,729,240 | $ | 4,083,303 | $ | 3,645,937 | 89.3 | % | ||||||||
Our research and development expenses include personnel, materials and supplies and other costs associated with the development of our insulin pump product candidate. We expense research and development costs as they are incurred.
Research and development, or R&D, expenses increased in fiscal 2022 compared with fiscal 2021 primarily due to increased consulting costs, engineering and operations personnel, stock compensation expense and materials and supplies expenditures. Our R&D employee headcount increased to 23 at March 31, 2022, from 17 at March 31, 2021. R&D expenses included stock-based compensation expenses of $758,938 and $390,045 for fiscal 2022 and fiscal 2021, respectively. We expect R&D expenses to continue to increase in fiscal 2023, as we complete the development of our pump product candidate, engage third parties to test our product and develop a low-volume manufacturing process.
General and Administrative
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| General and administrative | $ | 7,197,162 | $ | 3,253,412 | $ | 3,943,750 | 121.2 | % | ||||||||
General and administrative expenses consist primarily of personnel and related overhead costs for marketing, finance, human resources and general management.
General and administrative expenses, or G&A, expenses, increased for the three and nine months ended December 31, 2021 asin fiscal 2022 compared with the prior periods of fiscal 2021 primarily as a result of increased personnel and consulting costs, stock-based compensation expenseexpenses and increased consulting and legal fees.professional services fees, primarily related to our financing activities, including our public offering that was completed in February 2022. G&A expenses included stock-based compensation expenses of $1,016,774$3,272,964 and $198,926$837,533 for the three months ended December 31,fiscal 2022 and fiscal 2021, and 2020, respectively, and $2,280,098 and $638,607 for the nine months ended December 31, 2021 and 2020, respectively. We expect G&A expenses to continue to increase in fiscal 2023, as we will increase headcount as we expand our organization to support our anticipated growth and prepare for the remainderexpected commencement of fiscal 2022, as we pursue a public offeringthe commercialization of our common stock.product in late fiscal 2023.
Interest Expense
| December 30, | Change | |||||||||||||||
| 2021 | 2020 | 2020 to 2021 | ||||||||||||||
| Interest expense – Three months ended | $ | 1,010,247 | $ | — | $ | (1,010,247 | ) | — | ||||||||
| Interest expense – Nine months ended | $ | 2,204,917 | $ | — | $ | (2,204,917 | ) | — | ||||||||
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| Interest expense | $ | 2,752,229 | $ | 39,791 | $ | 2,712,438 | 6,816.7 | % | ||||||||
Interest expense consisted of interest expense incurred from our convertible promissory notes, including amortization of debt issuance costs, and our promissory (bridge) note. We retired our outstanding debt in February 2022. See Notes 45 and 56 to the condensed consolidated financial statements included in Item 1 of this ReportRegistration Statement for additional disclosure.
Liquidity and Capital Resources
December 31, 2022
As a development-stage enterprise, we do not currently have revenues to generate cash flows to cover operating expenses. Since our inception, we have incurred operating losses and negative cash flows in each year due to costs incurred in connection with R&D activities and G&A expenses associated with our operations. For the nine months ended December 31, 2021,2022, we incurred a net loss of approximately $14.1$10.3 million. For the years ended March 31, 20212022 and 2020,2021, we incurred net losses of approximately $7.4$18.6 million and $5.3$7.4 million, respectively. At December 31, 2021,2022, we had a cash balance of approximately $0.2$7.7 million and an accumulated deficit of approximately $29.9$44.9 million. When considered with our current operating plan, and the requirement to repay the Notes (as defined below) and the draws under the Bridge Note (as defined below) by May 2022, these conditions raise substantial doubt about our ability to continue as a going concern for a period of at least one year from the date that of issuance of the consolidated financial statements included in Item 1 ofin this Report.prospectus. Our consolidated financial statements do not include adjustments to the amounts and classification of assets and liabilities that may be necessary should we be unable to continue as a going concern. Our ability to continue as a going concern depends on our ability to raise additional capital through the sale of equity or debt securities to support our future operations, and we are currently seeking such additional financing. As discussed in Note 3 to our condensed consolidated financial statements in Item 1 of this Report, we obtained forgiveness of the $368,000 principal balance and interest on the PPP Note we received from Silicon Valley Bank in April 2020 under the U.S. Small Business Administration Paycheck Protection Program. As discussed in Note 4 to our condensed consolidated financial statements in Item 1 of this Report, inIn May 2021,2022, we completed a private placementregistered direct offering of $6,610,500 aggregate principal amountsecurities for net proceeds of our convertible promissory notes (the Notes). The Notes are unsecured obligations of ours with each Note having a stated maturity date of 12 months from its issue date (the Issue Date). The Notes bear interest at a rate of 12% per annum, payable on maturity, provided that, if we fail to pay any amounts when due under a Note, the interest rate increases to the greater of 16% or the maximum amount permitted by law. Each Note may be prepaid at our option during the first 270 calendar days following its Issue Date (the 270th day, the Trigger Date), subject to a 110% prepayment penalty on all principal and accrued interest then outstanding. No Notes may be prepaid in whole or in part after the Trigger Date. As discussed in Note 9 to our condensed consolidated financial statements in Item 1 of this Report, on October 28, 2021, we sold $250,000 of shares of our common stock to officers, and we issued a secured promissory note (the Bridge Note) to an investor. The Bridge Note provides us with a $3,000,000 revolving credit facility with all amounts being drawn down by the Company thereunder being due and payable, subject to acceleration in the event of a default, on March 15, 2022. For the three months ended December 31, 2021, we drew down $1,500,000 under the Bridge Note.approximately $7.4 million.
Our operating needs include the planned costs to operate our business, including amounts required to fund research and development activities, including clinical studies, working capital and capital expenditures. During the nine months ended December 31, we made capital expenditures of approximately $574,000, as we have begun procuring equipment to develop a low-volume manufacturing production line to build our pump product to demonstrate and develop our manufacturing process. We expect to incur increased capital expenditures for the remainder of fiscal 2023. At December 31, 2022, we had outstanding, non-cancelable purchase orders for production equipment totaling $735,000, and we expect to receive and pay for this equipment over the following six months. Our future capital requirements and the adequacy of our available funds will depend on many factors, including, without limitation, our ability to successfully commercialize our product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement our product offering.offerings. If we are unable to secure additional capital timely, we will be required to curtail our research and development initiatives and take additional measures to reduce costs in order to conserve our cash.
For the nine months ended December 30,31, 2022, we used $8,184,696 in operating activities, which primarily resulted from our net loss of $10,307,682, as adjusted for stock-based compensation expenses of $2,120,513, $150,412 for issuances of shares of common stock in exchange for services and depreciation and amortization expenses of $92,616, and increased by net changes in operating lease assets and liabilities of $37,761 and operating assets and liabilities $202,794 and other immaterial adjustments. For the nine months ended December 31, 2021, we used $7,128,787 in operating activities, which primarily resulted from our net loss of $14,058,155$14,058,154, increased for a non-cash gain on the PPP Note extinguishment of $368,780 and net changes in operating lease assets and liabilities of $34,422, as adjusted for changes to operating assets and liabilities of $1,197,989,$1,197,988, a loss on debt extinguishment of $1,321,450 stock-based compensation expenses of $2,740,086, $388,021 for issuances of shares of common stock in exchange for services, $149,994 for issuable shares of common stock in exchange for services, depreciation and amortization expenses of $80,268 and interest expense of $1,454,762 for amortization of debt discount.
For the nine months ended December 31, 2020, we2022 and 2021, cash used $4,570,713 in operatinginvesting activities which primarily resulted from our net loss of $5,605,431$573,066 and changes to operating assets$22,779, respectively, was for the purchase of property and liabilitiesequipment.
Cash provided by financing activities of $107,758, as adjusted$7,372,347 for stock-based compensation expenses of $940,374, depreciation and amortization expenses of $82,016, net changes in lease assets and liabilities of $120,085.
For the nine months ended December 31, 20212022 was attributable to net proceeds from the issuance of common stock upon completion of an equity offering, net of underwriting fees and 2020, cash used in investing activities of $22,779 and $109,541, respectively was due to the purchase of property and equipment.
issuance costs. Cash provided by financing activities of $6,037,199$5,887,199 for the nine months ended December 31, 2021 was primarily attributable to $4,137,199 of net proceeds from the issuance of our Notes of $5,637,199 and net proceeds ofconvertible promissory notes, $250,000 from the sale of shares of common stock to officers of the Company. Cash provided by financing activitiesCompany and $1,500,000 from the issuance of $2,154,662a promissory bride note.
Recently Issued Accounting Pronouncements
Recently Issued Accounting Pronouncements are detailed in Note 1 in the Notes to the Condensed Consolidated Financial Statements for the three and nine months ended December 31, 2020 was attributable to net proceeds of $1,785,882 from the sale of shares of our common stock2022, included in a private placement that was initiated in March 2020 and $368,780 in proceeds from the PPP Note.this prospectus.
Contractual ObligationsBUSINESS
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, we are not required to provide the information requested by paragraph (a)(5) of this Item.Overview
BUSINESS
Overview
Modular Medical is a development stage-stage, medical device company focused on the design, development, and commercialization of an innovative insulin pump using modernized technology to increase pump adoption in the diabetes marketplace. Through the creation of a novel two-part patch pump, the Company seeks to fundamentally alter the trade-offs between cost and complexity and access to the higher standards of care that presently available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training and day-to-day use, we seek to expand the wearable insulin delivery device market beyond the highly motivated “super users” and expand the category into the mass market. The product candidate seeks to serve both the type 1 and the rapidly growing, especially in terms of device adoption, type 2 diabetes markets.
Differentiation
We believe that there are a number of shortcomings and issues with currently available insulin pumps that prevent a substantial number of people who require insulin on a daily basis from choosing an insulin pump to treat their diabetes. We believe, that by tailoring our insulin pump to address such factors, we can expand the scope and adoption rate of insulin pump usage. We believe that to achieve broader market acceptance, an insulin pump must be easier to learn to use, be less timetime- consuming to operate, more intuitive to both patients and physicians, and meet the standards for coverage by insurance providers so that co-payments required from patients are affordable and the hurdles to insurance coverage are significantly reduced.
Among the more prominent issues are:
| Complexity: Many existing pumps are highly complex and require significant technical expertise to use effectively. We believe such pumps were designed for “super users,” who have high levels of motivation and technical competence. The complexity of pumps |
| Cumbersome: We believe that a majority of existing pumps are bulky and difficult to manage, |
| Cost: Costs associated with insulin pump therapy | ||
| Outdated style: Consumer electronics devices have evolved in both form and function. Diabetes pumps have not experienced similar progress. We believe that consumers will be more receptive of products designed with the user experience in mind and that many have low tolerance for complex, difficult procedures for use and maintenance of products. | ||
| Pump mechanism limitations: Traditional pumps generally utilize a syringe and plunger mechanism to deliver insulin. We believe this design limits the ability to reduce the size of the pump, and also potentially exposes the user to the unintended delivery of the full volume of insulin within the pump, which can cause hypoglycemia or death. We believe that the fear of adverse health events due to technical malfunctions related to traditional pump mechanism limitations deters the adoption of insulin pump therapy. |
Our team has substantial knowledge of the diabetes industry and experience in developing, obtaining marketing authorization for, and bringing insulin pumps to market. Based on this experience, we believe that our innovative insulin pump, using a new and proprietary method of pumping insulin, can address most or all of these shortcomings. It provides a state-of-the-art insulin pump capable of both basal (steady flow) and bolus (mealtime dosing) insulin disbursement. It also has been designed considering a natural migration path to multi-chamber/multi-liquid pumps, potentially offering an exciting array of new therapies to patients with diabetes and other conditions.
Our goal is to become the leader in expanding access to insulin pump technology to a wider portion of diabetes sufferers and provide not just care for the super users, but “diabetes care for the rest of us.” WeWhile our initial target market is people with Type 1 diabetes, we believe there is a substantial opportunity to penetrate the type 2 MDI marketplace, whether through this new insulinour initial MODD1 pump or further simplification of pumps forour pump to address the type 2 marketplace.
The MODD1 is a high-precision first-line pump that we believe represents the best choice for new pump patients because it is easy to afford, easy to learn, easy to use, and has a revolutionary design and technology that enable precision with low-cost manufacture and high reproducibility.
Key features include:
| Two parts |
| One button interface, easy to learn and use; |
| 90-day reusable, 3-day disposable; |
| No external controller required, no charging, no battery replacement; and |
| Slim profile, lighter weight. |
A proprietary survey of American healthcare payors representing 50 million covered lives (approximately 1/3 of U.S. covered lives) performed for us by industry leading survey firm ISA in 2019 has demonstrated that payors are willing to grant equivalent or preferential coverage for a product with this feature set at launch in exchange for rebatesdiscounts of aboutapproximately 20%. These costs are built into all of our models.
Diabetes Classifications and Therapies
Diabetes is typically classified as either type 1 or type 2:
| T1D is an auto-immune condition characterized by the body’s nearly complete inability to produce insulin. It is frequently diagnosed during childhood or |
| T2D represents over 90% of all individuals diagnosed with diabetes and is characterized by the body’s inability to either properly utilize insulin or produce sufficient insulin. Initially, many people with T2D attempt to manage their condition with improvements in diet and exercise and/or the use of oral medications and/or injection of glucagon-like peptide-1 (GLP-1) drugs. However, as their diabetes advances, patients often progress to requiring insulin therapies such as once-daily long-acting insulin and ultimately to intensified mealtime rapid-acting insulin therapy. This represents an important portion of the diabetes market with an estimated 1.6 million T2D intensively treated with insulin currently in the United States. |
Glucose, the primary source of energy for cells, must be maintained at certain levels in the blood in order to permit optimal cell function and health. In people with diabetes, blood glucose levels are not well controlled and frequently become very high, a condition known as hyperglycemia, and very low, a condition called hypoglycemia. Hyperglycemia can lead to serious long-term complications, including blindness, kidney disease, nervous system disorders, occlusive vascular diseases, lower-limb amputation, stroke, cardiovascular disease, and death. Hypoglycemia can lead to confusion or loss of consciousness, often requiring a visit to the emergency room or, in certain cases, result in seizures, coma, and/or death.
All people with T1D, which is our primary market, require daily insulin. According to the Seagrove 2021 Diabetes Blue Book, approximately 18% of people with T2D in the United States, or 4.7 million people, require insulin (basal alone represent 3.1 million and basal plus mealtime represent 1.6 million) to manage their diabetes. In this prospectus, we refer to people with T1D and people with T2D who require mealtime insulin as “insulin-requiring people with diabetes.”
Currently, there are two primary therapies available for insulin-requiring people with diabetes: multiple daily insulin injections directly into the body through syringes or insulin pens, referred to as Multiple Daily Injection, or MDI therapy, or the use of an insulin pump to deliver mealtime insulin boluses to help with glucose absorption after carbohydrate consumption and a continuous subcutaneous insulin infusion, or CSII therapy, into the body. Generally, CSII therapy is considered to provide a number of advantages over MDI therapy, primarily an improvement in glycemic control, as measured by certain diabetes management tests such as hemoglobin A1c (HbA1c) measure and more recently Time in Range (TIR) where a continuous glucose measuring device is used to calculate this test. Among other medical benefits, it has beena study conducted by Tandem Diabetes Care, Inc. in 2021 demonstrated that insulin pump use can decrease glucose variability, reduce the number of hypoglycemia,hypoglycemic events, decrease the daily doses of insulin and reduce the fear of hypoglycemia.
Notwithstanding these advantages, we believe the difficulty in use resulting from the complexity and cumbersome design of available insulin pumps as well as high and often prohibitive costs for both the patient and insurance provider has resulted not only in dissatisfaction among many existing pump users (fewer than half purchase a new pump after the warranty expires, peras noted in a Seagrove Partners estimate)2021 study), but also has severely limited the adoption rate of insulin pumps by a large segment of the MDI diabetes population, whowhom we refer to in this prospectus as “Almost Pumpers.”
We define Almost Pumpers as insulin-requiring people with diabetes who are aware of pumps and their potential benefits but because of past experiences, pump shortcomings, cost, complexity, and time and learning required to adopt and utilize currently available insulin pumps, continue to receive their daily insulin through MDI therapy.
Our initial focus for our insulin pump is the almost pumper segment population located in the United States.


Our research, along with marketplace data provided by Seagrove Partners in 2023, estimates that 32%33% of Americans with T1D usehave an insulin pump therapy and 28% of Americans with T1D (44% of those who currently utilize MDI) can be classified as having an interest in pump adoption and meeting the American Diabetes Association guidelines of glucose control if their objections to the currently available suite of products can be overcome. They do not want to closely manage their glucose levels and incur the associated time and effort involved. They are the Almost Pumpers. We have developed what we believe to be the most technologically advanced delivery system overcome the objections and provided motivation for this market. We believe that there are four addressable hurdles to adoption:
| Usability: the device needs to be easy to learn and to operate; |
| Affordability: we will focus on overcoming copay and insurance hurdles rather than leaving the “insurance journey” to the clinician and patient; |
| Accessibility and Education: we will seek to engage patients to sample this new technology by supplying clinicians with free samples and simple training to allow people to see first-hand the typical barriers to adoption that have been overcome; and |
| Service and Support: where we will answer their questions and concerns during this diabetes experience. |
We believe this conversion process, engaging people to try and thereby receive the benefits of our technology will substantially increase adoption of insulin pumps among both those with T1D and T2D who remain reliant upon multiple daily injections. Diabetes is a disease that appears throughout the world. Therefore, we cannot segment the market by socioeconomics, education or level of care. We intend to create an insulin pump that appeals to all Almost Pumpers.
Market
Market
The International Diabetes Federation or IDF, estimatesestimated that, in 2019, approximately 460 million people were living with diabetes worldwide, and that by 2045, this number will increase to approximately 700 million peoplepeople.
An estimated 34 million people in the United States live with diabetes. Within this group, T1D accounts for approximately 1.8 million people (7% of total) with the remainder being T2D. All people with T1D require daily insulin. However, of the approximately 25.532.2 million people with T2D, about 1.6 million of them require intensivemultiple daily injections of insulin treatments to manage their diabetes. This represents a large and growing market with the effects of diabetes accounting for roughly 25% of all healthcare dollars spent annually in the United States.
According to the National Diabetes HCP Survey conducted by Seagrove Partners, LLC in 2021, approximately 25% of the 1.6 million highly insulin intensive T2D have considered going “on pump.”
Insulin pumps have been shown to provide a higher level of care for insulin dependent people with diabetes and result in better glycemic control, fewer comorbidities, fewer trips to the emergency room, and higher overall quality of life. They also result in lower overall costs to the healthcare system, reducing typical expense per patient year from $27,195 to $16,992.
Despite these benefits, only 1 in 3 (32%(33%) of the 1.8 million Americans with T1D and very few of the 1.6 million T2D intensively treated with insulin currently use an insulin pump, for a total of approximately 670,000 current users, with only a slow increase of insulin pump use. The remaining 68% of T1D’s and virtually all of the T2D’s rely on multiple daily injections (MDI) for glucose control. Decades of advances in technology advances have left these non-pumpers at a significant disadvantage from a control perspective versus their “pumping” counterparts.
We have identified a large segment of the market that we refer to as “Almost Pumpers.” Almost Pumpers are those insulin-requiring people with diabetes (T1D and T2D) who feel that they would adopt the pump if it were less expensive, less time consuming, less technically intimidating, and if there was no separate controller. TheyWe believe that they represent approximately 32% of the T1D market correlating to a $1.9 billion growth opportunity.
Insulin pumps on the market today require a substantial amount of time to manage the therapy, have high out of pocketout-of-pocket costs that place these technologies out of reach for a large part of the population, and are feature-heavy with complex systems that we believe have hampered adoption and intimidated many users. The most commonly used insulin pumps today require extensive training and hours of daily management. The average pump user must go through 42 steps of setup and refill process every 72 hours to “stay on track.” Our product only requires nine steps for setup and refill every 72 hours.
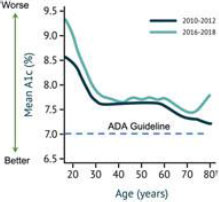
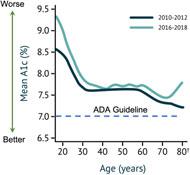
The current reluctance to adopt the insulin pump has had serious consequences on the healthcare system. In the United States, people living with T1D have struggled to attain glycemic targets. A 2019 analysis of the large T1D Exchange clinical registry found that only 21% of U.S. adults with T1D achieved the ADA A1c goal (<7.0%). Further, according to a study published in JAMA Internal Medicine, researchers found no significant improvements in diabetes care between 2005 and 2016, with persistent gaps in care related to socioeconomic status.
The recentAnother transition in the care of diabetes is the measuring of glucose from finger-stick tests to continuous glucose monitoring, or “CGM”, sensors, which are wearable devices. These sensors are placed under the skin and give a reading every five minutes of the user’s glucose level. While Dexcom has been a market leader in this field, the introduction and rapid adoption of the Freestyle Libre by Abbott Labs Freestyle Libre has made Continuous Glucose Monitoring (CGM)CGM easier and more affordable, expandingexpanded the product category, and doubling itsdoubled the market size. The Freestyle Libre product is a more affordable, easier to use and smaller version of the popular Dexcom, Inc. (Dexcom) CGM product. Now for the first time, there is an easy, less painful-nopainful, i.e., no more finger sticks–sticks, way for patients to have the data they need to understand more about their glucose levels and their insulin requirements. Access to such data has motivated patients to ask their diabetes clinician how they can achieve better glycemic control and made them more comfortable with using technology and wearables to treat their diabetes. Pumps offer a clear pathway to better control and better overall care. We believe that the insulin pump market is ready for a similar transition as that experienced in the CGM space. MODD1 pump represents a new and better offering to assist and induce a wide variety of patients to make the transition and bridge the void to superior control by becoming a “pumper.”
We believe the present pump marketplace is approximately a $1.9 billion market, comprising 32%33% of T1D pumpers and a small group of T2D pumpers. Seagrove Partners estimates in its 2021 report that 28% of T1D patients and 25% of T2D patients would adopt technology that was easier to use, access and pay for. We believe the total addressable market approximates $3 billion, assuming revenue of $4,128 per patient, per year. We expect to spend approximately 15% of our total revenue on discounts and free samples to encourage adoption of our pump product.
We are dedicated to helping all people with diabetes gain access to high quality care. We aim to help people with diabetes –- especially Almost Pumpers and the historically underserved communities –- gain access to insulin pump technology by making it affordable and easy to use.
Diabetes Care is at an Inflection Point
We believe that the insulin pump market stands at a crossroads as a confluence of events makes the timing for a new product introduction ideal.
2020 was a very difficult year in diabetes. Between COVID-19 and a loss of glycemic control during quarantines and isolation, deaths from diabetes rose by 17% in 2020 versus the prior year. This was sharpest among the young who saw deaths rise 29% in the 25-44 year old demographic. This has created a pain point and a desire to find new and better solutions and has raised awareness among patients, caregivers, payors, and policy makers.
COVID-19 also encouraged (and required) trial and adoption of telehealth models and a great many people have found them to their liking with a high proportion of patients and of health care providers (HCPs) that want to continue to use these technologies. We expect much of this shift and newfound comfort with distance care models to persist and believes that this can provide a patient acquisition and engagement model for insulin pumps and diabetes care, especially for pumps optimized for free trial and easy learning.
At the same time, reimbursement for patch pumps has been increasingly moving to a pharmacy benefits manager (PBM) model, which simplifies reimbursement which will further aid in a “frictionless launch.” This represents a fundamental shift in the insulin pump market, making onboarding rapid and simplifying a previously complex and time-consuming “insurance journey.”
The continuous glucose monitoring (CGM) space (wearable devices that monitor blood glucose levels) has been experiencing explosive growth largely driven by a new product introduction from Abbott Labs called the “libre.” This product was a more affordable, easier to use version of the popular Dexcom CGM. Not only is it now a larger (by revenues) product than Dexcom, but it accomplished this without seeming to slow Dexcom’s growth but rather by growing a new category with a new type of user.
TheseWe believe these CGM users are increasingly interested in adopting technology and wearables to manage their diabetes. We believe they are a natural market for a new type of pump if it can meet their needs and address their objections and that the conjunction of the above trends represents a unique opportunity in the insulin pump market’s history.
Diabetes technology companies understand that we are at a turning point with new markets (T2D, T1D that are currently not using technologies). This can be seen with increased discussion around this topic during recent national diabetes conferences, as well as but also an increase in marketing promotion. For example, Dexcom purchased a $5.5 million 30-second spot during the 2021 Super Bowl.
All these recent changes support the high proportion of T1D and T2D intensively treated with insulin that are considered as Almost Pumpers, a number that may grow in the next years and that may be more reachable with adequate marketing strategies.
Our Insulin Pump
Instead of building complex, bespoke, and difficult to manufacture and maintain pumping and control systems, we began with the technology and the user in mind. Using proprietary and patented methods of insulin measurement, we were able to eschew complex mechanisms and instead built a product candidate using only parts from high volume consumer electronics manufacturing lines, breaking the cost vs functionality curve that has existed in the insulin pump space and representing the first truly modern insulin pump design. This is a new kind of product for a new kind of patient.
The pre-productionproduction models of our low-cost insulin pump are now undergoing the testing required to submit to the FDA for 510(k) clearance to market them in the United States. We continue to devote, substantial time and resources to better understand the needs and preferences of Almost Pumpers and the specific patent/provider/payor requirements to motivate change from MDI.
MODD1 has several distinguishing features:

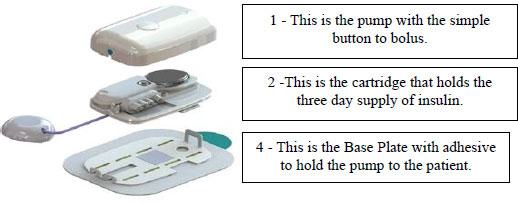
1 –- The pump has a simple button to press to deliver insulin as the patient requires it. The electronic pump uses a simple motor and rotating cam to motivate the insulin into the patient along with a low power Bluetooth (LPBT) and near fieldnear-field communication (NFC) chips to optionally allow the patient to communicate with their smart phone, tablet, or other mobile computing platform, as appropriate.platform. Our mobile device application will be included in our 510(k) submission and will be a part of our introductory product.
2 –- The pump snaps together with a three-day disposable cartridge that is patient filled with insulin for delivery. It includes the power source and a simple coin cell that allows it to run through the 80-hour life of the cartridge.
3 –- There is aan infusion set (not shown) that contains a soft 6 mm cannula and an introducer for insertion into the skin and removal of the needle used to transfer insulin to the body.
4 –- MODD1 comes with a variety of methods for the patient to wear the pump. Options include: a base plate with adhesive (shown) for attaching to the body that has features for holding the pump to the patient; overwraps to hold the product candidate to the patient; and a velcro strap with a base plate suitable for wrapping around the arm or leg of the patient.
The system will deliver a small continuous rate called a basal that will provide approximately 50% of the total daily dose required and the user will use the on-pump button to administer boluses, typically before and after meals.
The objective is to make the product candidate simple to acquire and take home, simple to learn and most importantly, simple to use to expand the pump market, drive adoption and ultimately better clinical outcomes.
Technological Advantages
The adoption of new ultra-high volume technologies will result in far easier manufacturing scale up as parts sourcing and assembly processes are far easier. The MODD1 was designed from the beginning for mass manufacturing processes and “lights out” or near lights out production assembly lines.lines whereby a minimal number of workers will be required in our production facility. This advantage is compounded by the high availability and already optimized cost reduction in its components. This has resulted in a cost of goods, estimated on the competitors’ announced margins and sales, 50% lower than our closest patch pump competitor.
The adoption of modern, miniaturized technologies has led to numerous other advantages as well. TheOur MODD1 pump is smaller in overall volume than Insulet’s popular Omnipod product and has a lower profile to the skin. Despite this, it holds a full 3mL (300 units) of insulin in line with full sized pumps such as Tandem and Medtronic, 50% more than the 2mL reservoir in the Omnipod. We believe that this volume advantage over other patch pumps will be significant as 24% of type 1 and over 50% of the rapidly growing type 2 market require more than 2mL of insulin every three days (the expected wear time of patch pumps).
In addition, our new and patented pumping modality will provide what we believe is the most even (and thus closest to the function of a healthy pancreas) delivery of basal insulin in the industry. Basal rate can be delivered almost continuously while other pumps are delivering micro-boluses every 5 minutes for the Omnipod, Tandem and Medtronic pumps. We plan to demonstrate the impact of our system on glycemic control in a future clinical study.
The technology allows the patient to simply add insulin and operate. The battery is included in each cartridge and the device is operated without a controller. Nothing needs charging. MODD1 has been made push button simple to appeal to a wider audience of users.
This new technology has also made the MODD1 lighter than existing offerings. Compared to the Insulet Omnipod, MODD1 weighs 20 grams (vs. 26 grams) empty and 23 grams (vs. 28 grams) fully filled (despite carrying 50% more insulin), a reduction of 23% and 18%, respectively. Also, unlike existing patch pumps, the MODD1 can be removed from the needle and taken off and replaced later if the user desires. This avoids loss of insulin in a pump due to accidental dislodging of the soft canula, an issue that users have expressed considerable dissatisfaction with on other patch pumps.
This
Our approach to the care of diabetes can be further enhanced by leveraging the MODD1 single-pumping chamber technology is also uniquely suitedand reusable pump approach to apply to dual (or more) chamber pumps.pumping solutions. We believe that such multi-chamber pumps will be integral to the realization of high time in rangetime-in-range artificial pancreas solutions that require no human intervention because of the application of, for instance, drugs to raise glucose levels coupled with drugs to lower glucose. They will be the next step forward from the cumbersome and awkward solutions today that require the user to announce meals, count and input carbs,carbohydrates, and adjust delivery for exercise and sleep. The advantagessleep to prevent overdosing of cost and miniaturization are multipliedinsulin. Instead, if a user overdosed insulin, the user would simply pump in a multi-chamber setup and we expectdrug to be ablerelease sugar stores to reach price points, ease of use, and form factor unlike anything seen in the industry thus far.raise it up. We believe that a prefilled, multi-hormonepre-filled peel and stick patch pump ablewith the ability to function in a fully autonomous closed loop system with CGM’sa CGM device, which is measuring and transmitting glucose-level information, represents the next generation of diabetes care. We believe that we have demonstrated our technology and are securing intellectual property protection on our approach.
We believe this technology, especially in dual chamber, will open up numerous applications outside of diabetes where medication compliance of complex therapy regimes is difficult addressing such spaces asdifficult. Example applications would include weight loss, fertility, and simplifying complexthe delivery of complex multi-drug cocktails, especially those with diverse and challenging dosing schedules.
Our Solution
Our proposed pump is being designed and developed to address the aforementioned shortcomings of the existing pump market and to appeal to: (i) the substantial group of ���Almost-Pumpers”“Almost-Pumpers” who are currentlymay be interested in using an insulin pump, but have not done so because of the complexity, cost or cumbersome nature of existing products, and (ii) people who are using one of the currently available insulin pumps but are dissatisfied with such products. We believe that, owing to our new proprietary technology, our proposed insulin pump will be the simplest and least expensive product on the market and the easiest for providers to prescribe.
Our current pump prototype of our proposed pump has been built to test what we believe to be our novel approach to insulin pumps. By providing a pump that we believe will establish industry standards in terms of technology, simplicity to understand, ease of use and price, we believe our proposed pump will offer the vast majority of benefits afforded by more expensive and complex pumps but remain accessible to a substantially greater percentage of diabetes sufferers requiring daily insulin therapy.
We believe people generally will not use technology that intimidates them andthem. In addition, we believe that physicians are hesitant to prescribe such technology.technology due to the level of training and support required with the present pump product offerings. We believe mass marketmass-market products, such as is intended for our proposed pump, must be “user friendly” and affordable. We believe this approach is fundamentally different from that applied to the existing pump market today, where most pumps are continuously adding complex features appealing to super users and are “user friendly” to onlyleaving the most technically astute.other people with diabetes further behind.
Our current goal is to successfully design, develop and obtain all required regulatory approvals for our proposed insulin pump, and, thereafter, commercialize the finished product. Our long-term goal is to become a leading provider of insulin pump therapy by focusing on both consumer and clinical needs.
To achieve our above stated immediate and current goals, we intend to pursue the following business strategies:
| Use of innovative proprietary technology. |
Based upon the substantial experience of Paul DiPerna, our President, Chief Financial Officer, Treasurer and Chairman of our Board of Directors, in engineering design and innovative technology in the medical device industry and, in particular, with the invention, market vision and technical development of insulin pumps, we have generated proprietary technology that has been incorporated into our proposed insulin pump. Generally, this technology is involved in the delivery of insulin to the user at the appropriate and necessary times. We believe this technology allowing for a two-part, yet small enough to wear, pump product, along with simplified mechanics for pumping, will greatly assist us in creating a simpler, user-friendly pump. We believe the proposed design, engineering and technology being incorporated into our proposed pump will make it substantially simpler and more affordable than those currently available. These features, together with the safety and reliability of our proposed pump, are designed to create the next generation of insulin pumps that will feature important and well-differentiated attributes compared to those currently available and make it available to consumers across mostly all socioeconomic groups in the United States and around the world.
| Keep costs low during our design and development process. |
To attempt to ensure that we have sufficient funds to design, develop, and obtain all required regulatory approvals for our proposed insulin pump without having to sacrifice quality and efficiency, we intend to maintain a tight budget and limit expenditures where possible. We believe this will be possible because of the extensive knowledge and experience of Mr. DiPerna, not only in the diabetes industry and more specifically in the insulin pump device market, but also his experience in designing and developing insulin pumps and other medical devices and his ability to manage a small, focused development team. We currently expect that various other expenses, such as product scale up, and sales and marketing costs, will not be incurred until such time as development work is completed and regulatory approvals obtained.
| Employ experienced engineers selected, supervised, and led by Mr. DiPerna, a highly experienced and respected engineer and executive in the insulin pump industry. |
To attempt to ensure our proposed insulin pump is “state of the art,” functional, and efficient, as well as to conserve funds, substantially all of our employees will initially be hand-picked engineers under the leadership of Mr. DiPerna. We believe that there is a strong pool of engineers with significant applicable experience and knowledge who we will be able to initially employ on a contract and/or outsource basis to help us design and develop our proposed insulin pump. We believe by hiring such persons on an out-source basis, we will save substantial resources and by having Mr. DiPerna lead and focus the team on technological and mechanical aspects of our proposed insulin pump, we believe our team will be well guided, focused, cost efficient, and able to efficiently design and develop our product candidate that we believe can eventually be a competitive and popular choice for people with insulin requiring diabetes.
Commercialization Strategy: Overcoming the Insurance Hurdles
Our goal is to establish MODD1 as the best option for new pump patients as we expand the market into the Almost Pumpers (Type 1 and Type 2) and the newly motivated CGM users. We seek to grow the market by providing first-line insulin pump therapy that is well suited to meet the needs of both diabetes patients requiring insulin and their clinicians.
| We believe that MODD1 is approximately 50% less expensive to manufacture than Omnipod. This low cost allows us to spend more on patients and sampling. |
| 20% discount vs Insulet (PODD) will drive preferred status; |
| Designed to use |
| No new code needed to be reimbursed at |
| Saves provider an estimated |
| The MODD1 will be sampled and given to patients by the doctor or diabetes nurse educator at the time of the patient visit. When a patient is motivated to make change, our starter kit will make it easy for the clinician to initiate the new therapy that same day. We seek to eliminate the currently challenging “insurance journey” and product acquisition timeline and significantly reduce training time for the busy clinician, which we believe are all major hurdles to pump adoption. We intend to add telehealth support to help the patient throughout adoption and use and to facilitate greater collaboration between patients and their physicians. |
Europe represents another large potential market for MODD1. ApproximatelyMODD1, as approximately 60 million people in Europe live with diabetes. ($161$161 billion is spent annually inon diabetes healthcare costs in the Europe).Europe based on data from a Seagrove Partners 2023 study. At present, cost containment is restricting pump uptake across Europe. Current pump usage hovers between 10% and 20% in many markets. Single payor healthcare systems across the Europe traditionally attempt to contain costs in the short term and seek low price technologies with moderate medical benefits. We anticipate MODD1 will offer a rebalance of this risk/reward strategy in that payors will incur only minor incremental short-term costs with the benefit of longer -term cost savings associated with reliable pump use. We intend to employ a partnership strategy across Europe following in-house managed regulatory and pricing activities in the major markets (e.g., UK) and more cost receptive markets (e.g., Nordics). We are targeting European and United Kingdom approval towards early 2023.mid 2024. Our initial target market for our insulin pump is the Almost Pumper population located in the United States followed quickly by an effort to obtain CE mark approval for distribution throughout Europe.
Marketing
MODD1 tackles the most significant barriers to pump use-access and affordability-and makes it easier for clinicians, caregivers and individuals to manage diabetes care. Our commercialization plan will drive adoption and is designed to expand the market and is intended to do the following:
| Maximize adoption with a comprehensive frictionless launch program. We will seek to decrease the level of reimbursement effort and cost to encourage HCPs to offer our pumps and encourage patient trials. Our product candidate reduces the technical hurdles to widen appeal, new starts and increase adherence. We will encourage MDI patients who want or need more control to make the switch to the pump earlier in their treatment-ideally right at diagnosis. |
| Leverage technology to support sales and new patient acquisition. We intend to set up |
| Facilitate patient trials. To facilitate patient trials, we intend to: |
| Provide a free sample pump, |
| Partner with connected care companies to provide superb support of patients from trial through the first |
We believe that MODD1 will be the only insulin pump that patients can take home immediately from the doctor’s office.
Leverage MODD1 300-unit chamber to increase adoption with Type 2 patients. We believe MODD1 has a major advantage over existing patch pumps in that the chamber carries enough insulin to meet the high doses many Type 2 patients need. We intend to promote this advantage and capture a significant share of the existing Type 2 pump users as well as new starts. |
| Work with key organizations and policy makers to pave the way for greater access to pumps. We will promote MODD1 technology among the underserved, who are typically low users of health technology. We will identify individuals, patient organizations, professional societies, and policy and DEI organizations that are critically important to the adoption of new technologies in the diabetes space and build relationships with these influential stakeholders. |
| Initiate a clinical study program (with key diabetes centers) to provide additional clinical support for MODD1 in special patient types and clinical setting. After obtaining 510(k) clearance, we intend to conduct a soft launch and clinical research program in major markets to pave the way for the full launch in late |
| Work with major health plans to establish MODD1 as the first line pump for Type 2 patients. We believe MODD1 will be payor preferred for both Type 1 and Type 2 patients. It was designed to attain preferential reimbursement and avoid the coverage pitfalls many other pumps have experienced. |
| Payors want |
| Designed to use existing PBM codes as a disposable |
| No new reimbursement code: Reimbursed at launch |
Tie-in with the massive movement to telehealth.
2020 saw personal telehealth go from beta test to mainstream. Customers and providers have become comfortable with it. There are only 4,000 patient-facing endocrinologists in the United States. The treatment of diabetes will be significantly enhanced with telehealth to drive more volume and clinical enhancements through their practices. Telemedicine is a force multiplier for a small group of doctors to better serve a large market. MODD1 was designed to be affordable enough for free sampling and trial, and simple enough for self-guided user training. We believe that by combining telehealth support with MODD1, we will decrease the burden of diabetes care and improve the lives of people with diabetes.
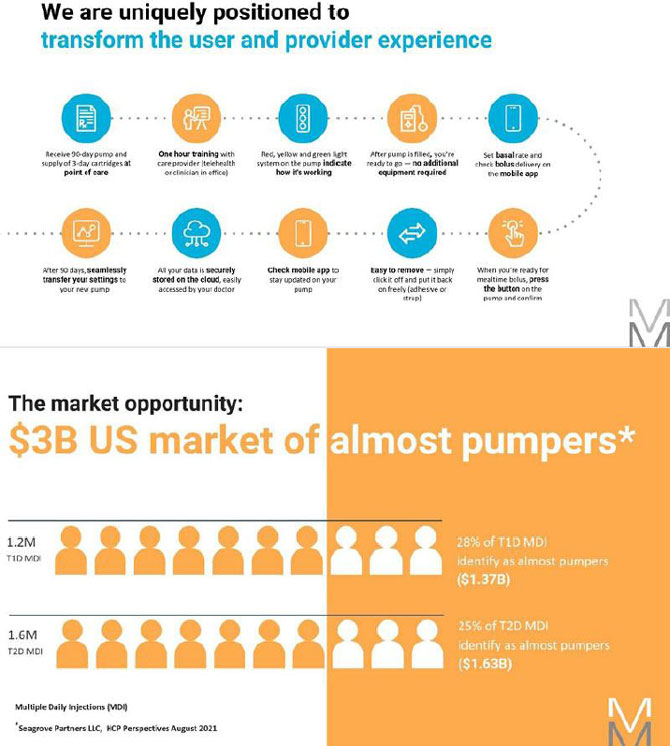
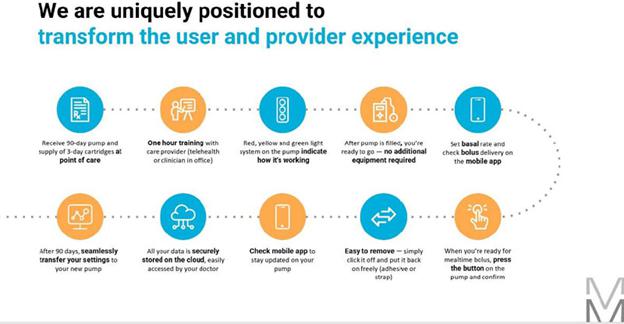
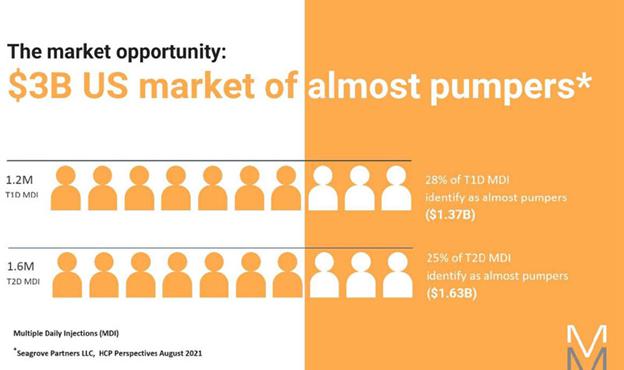
Pre-Launch/Trial
We intend to initiate a “soft launch” following FDA clearance of the MODD1 device. Our plan is to select a group of clinicians who are well trained, experienced and have the support infrastructure to take on initial patients and monitor them carefully to provide clinical feedback on our performance to further refine our product candidate and support infrastructure prior to full commercial launch. Many of these clinicians will have been those who assisted in the development of the MODD1 offering.
We intend to continue to modify, refine and finalize our system to best meet:
| The general needs and preferences of our |
| The general guidelines of third-party payors, private and public insurance companies, preferred provider organizations and other managed care providers with particular focus on the guidelines established by the Center for Medicare and Medicaid Services, or |
Manufacturing
Manufacturing requires
Our pump product comprises the production of pumps, cartridges,pump, a disposable cartridge that holds the insulin reservoir, a baseplate that affixes the pump product to the user’s body and baseplates as well as assemblythe infusion set, which includes a cannula to infuse the insulin into the body. We intend to manufacture the pump, the cartridge and the baseplate and purchase the infusion set from third parties. Prior to shipment, our pump product will be packaged with sets.an infusion set. In connection therewith:
| We |
| The infusion sets will be purchased |
The pumps will be built and testedWe have commenced working with a tier-one medical product manufacturer to develop the tooling equipment required to produce the components used in our San Diego facility whileproduct. As discussed above, we build volumeare in the process of implementing the cartridge assembly tooling equipment in our facility. Prior to product launch, we expect to transfer the cartridge automation equipment to this contract manufacturer in early 2024 to verify and expertise. When the production methodology has matured and the volumesvalidate into our manufacturing process. The medical product manufacturer would perform all manufacturing responsibilities to ensure compliance with FDA regulations. To date, we have risen,entered into a development agreement with this contract manufacturer for these development activities, but we will considerhave not yet entered into a transition to outside and offshorecontract manufacturing as appropriate.agreement.
FDA Clearance
The FDA requires us to meet all applicable regulations for insulin pumps, a subcategory of infusion pumps, which are generally considered Class 2 devices.II devices by the FDA. The design of the MODD1 pump has been completed, units have been built and testing is underway to verify that the design meets all FDA requirements.requirements prior to submission. There are 17 specific tests required to submit for 510(k) clearance. One of the more important tests addresses insulin stability, and we must demonstrate that our MODD1 pump does not damage the insulin molecules during infusion. We breakhave performed preliminary tests on insulin stability to increase the likelihood that we will pass these required tests into four testing categories: wetted surface, electricaltests. In an earlier version of our pump product candidate, we experienced issues demonstrating insulin stability. To address those issues, we began working with a medical product manufacturer to develop a full commercial version of our product utilizing alternative component parts. In addition, we must demonstrate: i) biocompatibility, meaning that we don’t use materials that compromise long-term patient safety, usabilityii) occlusion detection, meaning that our device will inform the patient of a lack of insulin delivery and internal.iii) cybersecurity controls to ensure that our product has adequate security protection to ensure it cannot be hacked by a third party. Appropriate design control and standard operating procedures have been implemented to allow us, when testing is completed, to submit for clearance under the premarket notification (or 510(k)) process. To achieve this, we will continue to work closely with our regulatory consultants to complete, finalize and file our submission to the FDA for 510(k) clearance and all other documentation necessary to obtain marketing authorization of our insulin pump.
| We have engaged the FDA in |
| We are currently preparing |
Commercialization Steps
While
To commercialize our product, we have substantially completed the general engineering and mechanical aspects of our insulin pump prototype, prior to commercializing, we still must successfully complete a number of material steps including:
| Continue to |
| the general needs and preferences of our |
| the general guidelines of third-party payors, private and public insurance companies, preferred provider organizations and other managed care providers with particular focus on the guidelines established by the Center for Medicare and Medicaid Services, or CMS which |
| Refine our manufacturing process during the submission process to |
| Take such actions, if any, as may be required by the FDA as a condition to granting approval and providing 510(k) clearance for our insulin pump; and |
Hire and retain appropriate sales and marketing personnel to develop, implement and launch a promotional campaign for our insulin pump substantially focused on our target market. |
As with any medical device attempting to enter and successfully compete with existing products in an established and competitive marketplace, we will face significant hurdles to accomplish the above steps to commercialization including:
| Obtaining FDA 510(k) clearance to market and sell our insulin pump to the public; |
| Obtaining any other FDA-required authorizations with regard to our product candidate, as required by the FDCA; |
| Educating endocrinologists, physician’s assistants, nurse practitioners and nurse educators, who typically prescribe pump usage, and certified diabetes educators and dieticians, who provide education and guidance to diabetes patients, as to what we believe to be the superior qualities of our product candidate; |
| Demonstrating to select general practitioners, who have historically been skeptical of the heightened support inherent in insulin pumps, our product candidate’s ease of use and convenience; |
| Ensuring that our final product does, in fact, meet the needs of Almost-Pumpers; |
| Overcoming the historic obstacles and reluctance of Almost-Pumpers to using insulin pumps to treat their diabetes; and |
| Ensuring that third party payors agree to cover all or a substantial portion of the purchase price and recurring costs of the use of our insulin pump. |
46
Looking Forward
Going forward, we expect to continue to evolve the MODD1 pumps and their capabilities and functionality both in response to patient needs and as part of our current platform roadmap.
| With our future MODD1+ |
| Additionally, adds AID control functionality via an “ACE” |
| Any approved algorithm controller can drive insulin delivery in “auto” mode |
| CGM integration allows the controller to potentially adjust basal insulin rate for meals and exercise with an approved algorithm. |
| With |
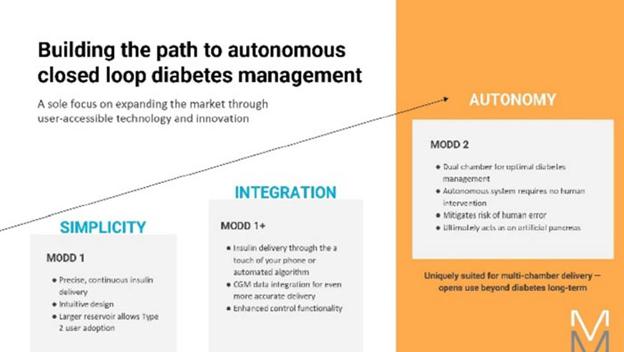

Employees
As of March 31, 2023, we had 38 employees all of whom are located in the United States and 37 of whom are full-time employees, consisting of 36 in research and development and manufacturing operations and 2 in general and administrative functions.
Competition
Today, in the United States, only three companies are commercializing insulin pumps to T1D patients and insulin treated T2D patients:
| ● | Medtronic - commercializes the durable Minimed 770G and also offers older durable pumps (670G, 630G etc.). In 2020, they held approximately 51% of the US insulin pump market. |
| ● | Tandem - commercializes the durable t:slim X2 pump (with or without algorithms - Basal-IQ and Control-IQ). In 2020, they held approximately 28% of the US insulin pump market. |
| ● | Insulet - commercializes the disposable Omnipod patch pump with about 19% of the US market in 2020. |
Older insulin pumps are also still being used by a minority of patients previously provided by Roche or Animas though these pumps are not commercialized any longer. To a lesser extent, the pumps described below are also used in small numbers.
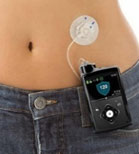 | 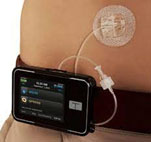 |
| Medtronic pump and infusion set | Tandem pump and infusion set |
These three insulin pump offerings are vying for the attention of the most motivated and well insured in hope of converting them away from their reliance on MDI. The t:slim X2 and Minimed 770G each have a ~$5,000 list price that is covered through Durable Medical Equipment (DME) reimbursement and daily consumables, which comprise cartridge, tubing and set for each three-day period, as well. These products have controllers integrated into the pump, making them cumbersome and bulky, along with long (> 20 inch) tubing between the pump and the cannular site. The Omnipod is the third offering, a patch pump that attaches to your body for 72 hours and uses a separate controller to manage the insulin delivery process. Insurance coverage for Omnipod can be provided via DME but also via Pharmacy Benefit (PB). The Omnipod patch pump is more expensive per day and less accurate than other insulin pumps, according to a Mende 2022 study. Around 33% of people living with T1D are currently using insulin pumps; of these, the vast majority are using one of these three offerings, a statistic that has not changed significantly over the last 5+ years.

All of these pump products require extensive training to initiate, two to four hours per day to use and manage on an ongoing basis. We believe this level of sophistication and effort along with the cost and awkwardness of these products contribute to the limited uptake.
Although there are purely mechanical pumps available to patients with a small percentage of T2D patients using the Mannkind V-Go patch pump, a fixed basal rate and a button to deliver small boluses. This pump is simple to use though gives little performance decision to the user (no possibility to change the basal rate, no possibility to stop bolus doses, small reservoir, pump that needs to be changed every day, etc.). The last available patch pump is provided by Cequr, called Simplicity, a bolus only delivery option without basal delivery that is yet to be commercially available.
In the future, Medtronic intends to launch a new version of their insulin pump, the Minimed 780G, already available in some European countries with an advanced algorithm, but no obvious change in hardware. Tandem is currently developing a patch pump called Mobi, coupled with an algorithm with potential launch expected in 2024. The Mobi is expected to have a small 2mL reservoir and would be controlled by a separate unit, similar to the current Omnipod product. Insulet has also launched the Omnipod 5, a similar patch pump to their offering today, that includes an AID algorithm.
Approximately 79% of the people who rely upon MDI choose to not administer a shot outside of their house, which creates a poorly controlled group. MODD1 is designed to focus upon a segment of these people and mobilize them via a simple, easy to use, affordable product.

Intellectual Property
Our success depends in part on our ability to obtain patents and trademarks, maintain trade secret and know-how protection, enforce our proprietary rights against infringers, and operate without infringing on the proprietary rights of third parties. Because of the length of time and expense associated with developing new products and bringing them through the regulatory approval process, the health care industry places considerable emphasis on obtaining patent protection and maintaining trade secret protection for new technologies, products, processes, know-how, and methods.
As of March 31, 2023, we held three U.S. utility and no foreign patents, and we also held 22 pending applications in the United States and abroad. The patents and patent applications cover various aspects of our technology, including our proprietary fluid movement technology and associated features of our insulin delivery methodology. There can be no assurance that the pending patent applications will result in the issuance of patents, that patents issued to or licensed by us will not be challenged or circumvented by competitors, or that these patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage.
Government Regulation
Our operations are subject to comprehensive federal, state, and local laws and regulations in the jurisdictions in which we or our research and development partners do business. The laws and regulations governing our business and interpretations of those laws and regulations and are subject to frequent change. Our ability to operate profitably will depend in part upon our ability, and that of our research and development partners and affiliates, to operate in compliance with applicable laws and regulations. The laws and regulations relating to medical products and healthcare services that apply to our business and that of our partners and affiliates continue to evolve, and we must, therefore, devote significant resources to monitoring developments in legislation, enforcement, and regulation in such areas. As the applicable laws and regulations change, we are likely to make conforming modifications in our business processes from time to time. We cannot provide assurance that a review of our business by courts or regulatory authorities will not result in determinations that could adversely affect our operations or that the regulatory environment will not change in a way that restricts our operations.
FDA Regulation
In the United States, medical devices are strictly regulated by the FDA. Under the FDCA, a medical device is defined as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component, part or accessory which is, among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.” This definition provides a clear distinction between a medical device and other FDA regulated products such as drugs. If the primary intended use of a medical product is achieved through chemical action or by being metabolized by the body, the product is usually a drug or biologic. If not, it is generally a medical device.
We are currently developing an insulin pump delivery system, which is regulated by the FDA as a medical device under the FDCA, as implemented and enforced by the FDA. The FDA regulates the development, testing, manufacturing, labeling, packaging, storage, installation, servicing, advertising, promotion, marketing, distribution, import, export, and market surveillance of our medical devices.
Device Premarket Regulatory Requirements
Before being introduced into the U.S. market, each medical device must obtain marketing clearance or approval from the FDA through the premarket notification (or 510(k)) process, the de novo classification process, or the premarket approval, or PMA,“PMA,” process, unless they are determined to be Class I devices or to otherwise qualify for an exemption from one of these available forms of premarket review and authorization by the FDA. Under the FDCA, medical devices are classified into one of three classes –- Class I, Class II or Class III –- depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurance of safety and effectiveness. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. Class I devices are those for which reasonable assurance of safety and effectiveness can be maintained through adherence to general controls which include compliance with the applicable portions of the FDA’s Quality System Regulation or the QSR,(the “QSR”), as well as regulations requiring facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. The Class I designation also applies to devices for which there is insufficient information to determine that general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device or to establish special controls to provide such assurance, but that are not life-supporting or life-sustaining or for a use which is of substantial importance in preventing impairment of human health, and that do not present a potential, unreasonable risk of illness or injury.
Class II devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish “special controls.” These special controls can include performance standards, post-market surveillance requirements, patient registries and FDA guidance documents describing device-specific special controls. While most Class I devices are exempt from the premarket notification requirement, most Class II devices require a premarket notification prior to commercialization in the United States; however, the FDA has the authority to exempt Class II devices from the premarket notification requirement under certain circumstances. As a result, manufacturers of most Class II devices must submit premarket notifications to the FDA under Section 510(k) of the FDCA (21 U.S.C. § 360(k)) in order to obtain the necessary clearance to market or commercially distribute such devices. To obtain 510(k) clearance, manufacturers must submit to the FDA adequate information demonstrating that the proposed device is “substantially equivalent” to a “predicate device” that is already on the market. A predicate device is a legally marketed device that is not subject to PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976 (“pre-amendments device”) and for which a PMA is not required, (ii) a device that has been reclassified from Class III to Class II or I or (iii) a device that was found substantially equivalent through the 510(k) process. If the FDA agrees that the device is substantially equivalent to the predicate device identified by the applicant in a premarket notification submission, the agency will grant 510(k) clearance for the new device, permitting the applicant to commercialize the device. Premarket notifications are subject to user fees, unless a specific exemption applies.
If there is no adequate predicate to which a manufacturer can compare its proposed device, the proposed device is automatically classified as a Class III device. In such cases, a device manufacturer must then fulfill the more rigorous PMA requirements or can request a risk-based classification determination for its device in accordance with the de novo classification process.
Devices that are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to a predicate device and for which safety and effectiveness cannot be assured solely by the general controls and special controls are placed in Class III. Such devices generally require FDA approval through the PMA process, unless the device is a pre-amendments device not yet subject to a regulation requiring premarket approval. The PMA process is more demanding than the 510(k) process. For a PMA, the manufacturer must demonstrate through extensive data, including data from preclinical studies and one or more clinical trials, that the device is safe and effective for its proposed indication. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review and determine whether the proposed device can be approved for commercialization, although in practice, PMA reviews often take significantly longer, and it can take up to several years for the FDA to issue a final decision. Before approving a PMA, the FDA generally also performs an on-site inspection of manufacturing facilities for the product to ensure compliance with the QSR.
The de novo classification process allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its device to Class I or Class II, on the basis that the device presents low or moderate risk, as an alternative to following the typical Class III device pathway requiring the submission and approval of a PMA application. Under the FoodWe expect our MODD1 insulin pump product candidate to be a Class II device, and Drug Administration Safety and Innovation Act of 2012, the FDA iswe do not expect to be required to classify a device within 120 days following receipt ofapply for down-classification under the de novo classification request from an applicant; however, the most recent FDA premarket review goals state that in fiscal year 2021, FDA will attempt to issue a decision within 150 days of receipt on 65% of all de novo classification requests received during the year and on 70% of de novo requests received during fiscal year 2022. If the manufacturer seeks reclassification into Class II, the classification request must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. The FDA may reject the classification request if it identifies a legally marketed predicate device that would be appropriate for a 510(k) notification or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.process.
Clinical trials are almost always required to support PMAs and are sometimes required to support 510(k) and de novo classification submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption or IDE,(“IDE”) regulations that govern investigational device labeling, prohibit promotion of investigational devices, and specify recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the study sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the study, the FDA may permit a clinical trial to proceed under a conditional approval. The FDA may also notify the sponsor that the study is approved as proposed or approved with specific requested modification. Furthermore, the agency may withdraw approval of an IDE under certain circumstances. In addition, the study must be approved by, and conducted under the oversight of, an institutional review board, or IRB, for each clinical site. If the device presents a non-significant risk to the patient according to criteria established by the FDA as part of the IDE regulations, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate authorization from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
Post-Marketing Restrictions and Enforcement
After a device is placed on the market, numerous regulatory requirements apply. These include, but are not limited to:
| submitting and updating establishment registration and device listings with the FDA; |
| compliance with the QSR, which requires manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process; |
| unannounced routine or for-cause device facility inspections by the FDA, which may include our suppliers’ facilities; and |
| labeling regulations, which prohibit the promotion of products for uncleared or unapproved (or “off-label”) uses and impose other restrictions relating to promotional activities; |
| corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FDCA that may present a risk to health; and |
| post-market surveillance regulations, which apply to certain Class II or III devices when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
In addition, under the FDA medical device reporting or MDR,(“MDR”) regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or a similar device of such manufacturer were to recur. The decision to file an MDR involves a judgment by the manufacturer. If the FDA disagrees with the manufacturer’s determination, the FDA can take enforcement action.
The MDR requirements also extend to health care facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both the FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to the FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
The FDA also has the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious adverse health consequences or death. Manufacturers may, under their own initiative, recall a product if any distributed devices fail to meet established specifications, are otherwise misbranded or adulterated under the FDCA, or if any other material deficiency is found. The FDA requires that certain classifications of recalls be reported to the FDA within ten working days after the recall is initiated.
The failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| warning letters, fines, injunctions or civil penalties; |
| recalls, detentions or seizures of products; |
| operating restrictions; |
| delays in the introduction of products into the market; |
| total or partial suspension of production; |
| delay or refusal of the FDA or other regulators to grant 510(k) clearance, PMA approvals, or other marketing authorization to new products; |
| withdrawals of marketing authorizations; or |
| in the most serious cases, criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of subcontractors.
Federal Trade Commission Regulatory Oversight
Our advertising for our products and services is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission or the FTC,(the “FTC”) as well as comparable state consumer protection laws. Under the Federal Trade Commission Act or FTC Act,(the “FTC Act”), the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Healthcare Law and Regulation
United States
If our MODD1 product candidate or our other future product candidates are approved in the United States, we will have to comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws, rules and regulations. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. These laws include the following:
| the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| the federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Department of Health and Human Services information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician health care practitioners and physician ownership and investment interests; and |
| analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. |
Some state laws require pharmaceutical or medical device companies to comply with the relevant industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug and device manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures.
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. We also may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base and thereby decrease our future revenues.
The European Union approves the use of medical devices in a very different way. They have similar regulations and requirements to adhere to, however a Notified Body, in the form of a private company, will represent their interests and is required to have sufficient expertise to review all applications and the company’s internal processes to ensure the safety of the product for which approval is being requested. We are in the process of identifying a Notified Body to represent us, and we will follow our FDA submission process with regard to preparing the materials and processes required to meet the regulations and gain clearance.
European Union
EEA
In the European Economic Area, (which is comprised of the 27 member states of the European Union plus Norway, Iceland and Liechtenstein), or EEA, manufacturers of medical devices need to comply with the Essential Requirements laid out in Annex I to the EU Medical Devices Directive (Council Directive 93/42/EEC) or with the General Safety and Performance Requirements (GSPR) of the new EU Medical Devices Regulation (EU 2017/745). Compliance with these requirements is a prerequisite to be able to affix the CE mark to medical devices, without which they cannot be marketed or sold in the EEA. To demonstrate compliance with the Essential Requirements and the GSPR and obtain the right to affix the CE Mark, manufacturers of medical devices must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements and the GSPR, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization designated by a competent authority of an EEA country to conduct conformity assessments. Depending on the relevant conformity assessment procedure, the Notified Body would audit and examine the Technical File and the quality system for the manufacture, design and final inspection of the devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements and GSPR. This Certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity. As a general rule, demonstration of conformity of medical devices and their manufacturers with the Essential Requirements and GSPR must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence.
All manufacturers placing medical devices into the market in the EEA must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the member states of the EEA, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices. Where appropriate, our products commercialized in Europe are CE marked and classified as either Class I or Class II.
In 2017, the European Parliament passed the Medical Devices Regulation, which repeals and replaces the EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable (i.e., without the need for adoption of EEA member State laws implementing them) in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and in vitro diagnostic devices and ensure a high level of safety and health while supporting innovation.
The Medical Device Regulation was meant to become applicable three years after publication (in May 2020). However, in April 2020, to allow EEA national authorities, notified bodies, manufacturers and other actors to focus fully on urgent priorities related to the COVID-19 pandemic, the European Council and Parliament adopted Regulation 2020/561, postponing the date of application of the Medical Device Regulation by one year. The Medical Device Regulation became applicable on May 26, 2021. Devices lawfully placed on the market pursuant to the EU Medical Devices Directive prior to May 26, 2021 may generally continue to be made available on the market or put into service until May 26, 2025. The Medical Devices Regulation, among other things:
| strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
| establishes explicit provisions on |
| improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and |
| strengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
Competition
Today, in the United States, only three companies are commercializing insulin pumps to T1D patients and insulin treated T2D patients:
Older insulin pumps are also still being used by a minority of patients previously provided by Roche or Animas though these pumps are not commercialized any longer. To a lesser extent, the pumps described below are also used in small numbers.
 | 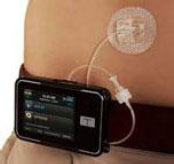 |
These three insulin pump offerings are vying for the attention of the most motivated and well insured in hope of converting them away from their reliance on multi-day insulin injections. The t:slim X2 and Minimed 770G each have a ~$5,000 list price that is covered through Durable Medical Equipment (DME) reimbursement; daily consumables and insulin are also required to complete these offerings. These products have controllers integrated into the pump, making them cumbersome and bulky, along with long (>20 inch) tubing between the pump and the cannular site. The Omnipod is the third offering, a patch pump that attaches to your body for 72 hours and uses a separate controller to manage the insulin delivery process. Insurance coverage can be provided via DME but also via Pharmacy Benefit (PB). The Omnipod patch pump is more expensive per day and less accurate than other insulin pumps. Around 32% of people living with T1D are currently using insulin pumps; of these, the vast majority are using one of these three offerings, a statistic that has not changed significantly over the last 5+ years.
 |
Although there are purely mechanical pumps available to patients with a small percentage of T2D patients are using the Zealand V-Go patch pump, a fixed basal rate and a button to deliver small boluses. This pump is simple to use though gives little performance decision to the user (no possibility to change the basal rate, no possibility to stop bolus doses, small reservoir, pump that needs to be changed every day, etc.). The last available patch pump is provided by Cequr, called Simplicity, a bolus only delivery option without basal delivery that is yet to be available.
In the future, Medtronic intends to launch a new version of their insulin pump, the Minimed 780G, already available in some European countries with an advanced algorithm, but no obvious change in hardware. Tandem is currently developing a patch pump called t:sport, coupled with an algorithm with potential launch expected in summer 2022. The t:sport should have a small 2mL reservoir and would be controlled by a separate unit as is the current Omnipod. Insulet should launch in the coming quarters the Omnipod 5, a similar patch pump to their offering today, that includes an algorithm.
Approximately 71% of the people who rely upon MDI choose to not administer a shot outside of their house, which creates a poorly controlled group. MOD is designed to focus upon a segment of these people and mobilize them via a simple, easy to use, affordable product.Available Information
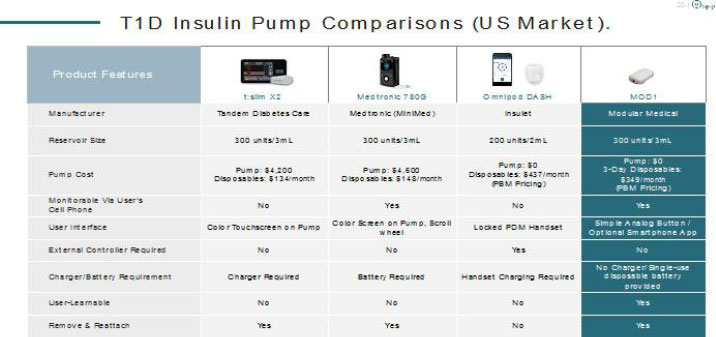
Intellectual Property
Our success depends in part on our ability to obtain patents and trademarks, maintain trade secret and know-how protection, enforce our proprietary rights against infringers, and operate without infringing on the proprietary rights of third parties. Because of the length of time and expense associated with developing new products and bringing them through the regulatory approval process, the health care industry places considerable emphasis on obtaining patent protection and maintaining trade secret protection for new technologies, products, processes, know-how, and methods.
As of December 31, 2021, we had one issued U.S. utility patent, five published U.S. utility patents, two pending foreign patent applications, and two pending international PCT patent applications covering various aspects of our technology, including our proprietary fluid movement technology. There can be no assurance that the pending patent applications will result in the issuance of patents, that patents issued to or licensed by us will not be challenged or circumvented by competitors, or that these patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage.
Available Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to such reports filed or furnished pursuant to section 13(a) or 15(d) of the Securities Exchange Act of 1934, as well as section 16 reports on Form 3, 4, or 5, are available free of charge on our website at www.modular-medical.com. as soon as it is reasonably practicable after they are filed or furnished with the SEC. Our Code of Business Conduct and Ethics and the charters for the Audit Committee, Compensation Committee and Nominating and Governance Committee are also available on our website. The Code of Business Conduct and charters are also available in print to any shareholderstockholder upon request without charge. Requests for such documents should be directed to James Sullivan, at Modular Medical, Inc., 16772 W. Bernardo Drive,10740 Thornmint Road, San Diego CA 92127. Our Internet website and the information contained on it or connected to it are not part of, or incorporated by, reference into this prospectus. Our filings with the SEC are also available on the SEC’s website at http://www.sec.gov.
Corporate History and Background
We were formed as a corporation under the laws of the State of Nevada in October 1998 under the name Bear Lake Recreation Inc. We had no material business operations from 2002 until July 2017, when we acquired Quasuras, Inc., a Delaware corporation (“Quasuras”), in the Control Block Acquisition (as defined below). Prior to the Control Block Acquisition, and, since at least 2002, we were a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the “Exchange Act”).
The Control Block Acquisition. On April 26, 2017, pursuant to a Common Stock Purchase Agreement, dated as of April 5, 2017, by and among Manchester Explorer, LP, a Delaware limited partnership, we and certain persons named therein, Manchester Explorer, LP purchased from us 966,667 shares of our Common Stockcommon stock, representing a number of shares in excess of a majority of our then issued and outstanding Common Stock,common stock, for a purchase price of $375,000 (the “Control Block Acquisition”), resulting in a change in control of the Company. In connection with the Control Block Acquisition, James E. Besser was appointed president and a director and Morgan C. Frank was appointed the chief executive officer, chief financial officer, secretary, treasurer and a director of ours and immediately following such appointments, our then officers and directors resigned. Mr. Besser is the managing member of and Mr. Frank is the portfolio manager and a consultant to Manchester Management Company, LLC, a Delaware limited liability company also referred to herein as MMC. MMC is the general partner of Manchester Explorer, LP and Jeb Partners, L.P. (Jeb Partners, and together with Manchester Explorer, LP, collectively, the Purchasing Funds).
The Acquisition. On July 24, 2017, pursuant to a Reorganization and Share Exchange Agreement, by and among us, Paul M. DiPerna, the sole officer, director and a controlling stockholder of Quasuras, Messrs. Besser and Frank (Messrs. Besser, Frank and DiPerna, collectively, the “3 Quasuras Shareholders”), and Quasuras Inc. (the “Share Exchange Agreement”), we acquired all of the issued and outstanding shares of Quasuras Inc. owned by the 3 Quasuras Shareholders, resulting in Quasuras Inc. becoming our wholly-owned subsidiary (the “Acquisition”). Simultaneously with the closing of the Acquisition, Manchester Explorer, LP cancelled the 2,900,000 shares of our Common Stockcommon stock purchased in the Control Block Acquisition, Mr. Besser resigned as our president and a director and Mr. Frank resigned as our chief executive officer, chief financial officer, secretary, and treasurer, but remained a director, and Mr. DiPerna was appointed our chairman of the board of directors, chief executive officer, chief financial officer, president, secretary and treasurer. Mr. DiPerna served as our chief executive officer until August 2021 and as our secretary until October 2021.
Subsidiaries
Quasuras, Inc., a Delaware corporation, is our only subsidiary.
EmployeesProperties
AsIn January 2023, we entered into a new lease agreement with a 48-month term, effective February 1, 2023, pursuant to which we leased approximately 24,000 square feet of May 31, 2022, we had 29 employees all of whom area building located in the United States, consisting of 26San Diego, California. We occupied this space in research and development and manufacturing operations and 3 in general and administrative functions.
Properties
In January 2020, we executed aMarch 2023, as the lease for aour former corporate facility located at 16772 West Bernardo Drive,in San Diego, CA 92127 and paid a $100,000 security deposit. The 39-month lease term commenced April 1, 2020, and provides for an initial monthly rent of approximately $12,400 with annual rent increases of approximately 3%.California expires June 30, 2023. In addition to the minimum lease payments under these leases, we are responsible for property taxes, insurance and certain other operating costs.costs under. We believe that our existing facility isfacilities are adequate to meet our current needs.
Corporate Information
We are a Nevada corporation. Our corporate headquarters and operating facilities are located at 16772 West Bernardo Drive,10740 Thornmint Road, San Diego, CA 92127 Our telephone number is (858) 800-3500. We maintain a website at www.modular-medical.com.
The following table sets forth information on our executive officers and directors as of June 2, 2022.May 4, 2023. The term for each of our directors is generally three years or until their successors are duly elected and qualified. We do not have any promoters or control persons.
| Name | Age | Position | ||
| James Besser | Chief Executive Officer | |||
| Paul DiPerna | Chief Financial Officer, President, Treasurer and Director (Chairman of the Board of Directors) | |||
| William J. Febbo(1) | 54 | Director | ||
| Steven Felsher(2)(3) | 74 | Director | ||
| Morgan C. Frank | 50 | Director | ||
| Philip Sheibley(2)(3) | 64 | Director | ||
| Carmen Volkart(1)(2) | 62 | Director | ||
| Ellen O’ Connor Vos | Director | |||
| (1) | Member of Compensation Committee |
| (2) | Member of Audit Committee |
| (3) | Member of Nominating and Governance Committee |
Family Relationships
There are no family relationships between or among any of our officers and members of our Board of Directors.
The principal occupations and positions for at least the past five years of our directors are described below. There are no family relationships among any of our directors or executive officers.
James “Jeb” Besser. Mr. Besser has served as our chief executive officer since February 23, 2022 and combines over 25 years of experience in alternative investments, strategic advisory, corporate strategy and corporate governance. Since 1999, he has been a Managing Member at Manchester Management Company, LLC (“Manchester”), an investment management firm. JamesMr. Besser is also currently a director of River Stone Biotech, a development stage specialty bioprocessing company. He holds a B.A. in history from Brown University. We believe that Mr. Besser is qualified to serve as member of our board of directors due to his extensive prior experience conducting financial analysis of public companies (certain of which were in the development stage), including such public companies’ management teams, products, including products in the development stage, the potential markets for such products and other factors that could affect the likelihood and timing of success and market penetration of such entities’ products as well as his capital raising activities. We believe this provides us with valuable insights into the financial markets and investment criteria of institutional and other investors as well as capital raising activities.
Paul DiPerna.Mr. DiPerna has been our chairman, chief financial officer, president and treasurer since we acquired Quasuras Inc. in July 2017. He also served as our chief executive officer from July 2017 until August 2021, and as our Secretary from July 2017 to October 2021. In 2015, he founded Quasuras, Inc., an early-stage medical device company developing an insulin pump product, and, until its acquisition by us, he served as its chief executive officer and chairman. Prior to that, Mr. DiPerna founded Fuel Source Partners, LLC to incubate early stage medical device products and accumulate technical talent. Our current pump product was one of such proposed products and was spun-out to Quasuras in 2015. From 2012 to 2015, he served as a co-inventor at a private company with property rights in a medical device used for blood borne infection control called the Curos Cap, which was acquired by 3M Corporation. In 2003, Mr. DiPerna founded Tandem Diabetes Care, Inc. (“Tandem”) and held various positions, including as director, chief executive officer and chief technology officer and was primarily responsible for the design concept and development of Tandem’s initial insulin pump. Prior to that, he held executive and management positions at Baxter Healthcare Corporation (“Baxter”) where he was tasked with identifying synergistic opportunities in the diabetes industry. As a result, Mr. DiPerna developed substantial expertise and knowledge in the diabetes industry and led attempts by Baxter to acquire three insulin pump manufacturers. Previously, he held mechanical design engineering positions in the automated test equipment and blood separation sciences industries. Mr. DiPerna holds approximately 70 patents in medical device and microfluidic technology and has achieved numerous product clearances fromwith the FDA. He has also achieved multiple successful exits with previous companies. Mr. DiPerna received a Masters in Engineering Management from Northeastern University and a B.S. in Mechanical Engineering from the University of Massachusetts and has spent over 35 years in the medical-device industry. We believe that Mr. DiPerna is qualified to serve as the chairman of our board of directors due to his extensive knowledge and experience in the medical devicemedical-device industry generally, and, in particular, with regard to insulin pumps and the diabetes industry, as well as his management and leadership experience from holding director and senior executive positions in other public and private companies and leading project development teams of medical device companies.
Ellen O’Connor Vos. Ms. Vos was appointed to our board of directors in May 2021 and served as our chief executive officer from August 2021 until February 23, 2022. Ms. Vos has served as a member of VosHealth LLC since November 2020. Prior to that, she served as the president and chief executive officer of the Muscular Dystrophy Association from October 2017 to November 2020. Previously, Ms. Vos had been chief executive officer of ghg | greyhealth group from 1996 to 2017, and she has been a champion of using digital capabilities to improve the public health. Ms. Vos also serves on the board of OptimizeRX Corporation, a publicly-traded digital health company, and the Jed Foundation, a leading nonprofit dedicated to protecting the emotional health of college students, and was a founding board member of MMRF, a pioneering cancer research foundation. Ms. Vos holds a B.S. in nursing from Alfred University. We believe that Ms. Vos is qualified to serve on our board of directors because of her executive experience and extensive executive skills in digital marketing, commercialization and communications in the healthcare industry.
William J. Febbo.Mr. Febbo was appointed to our board of directors in January 2020. He is currently the Chief Executive Officer and a director of OptimizeRx Corporation, a digital health company focused on bringing life sciences support to patients and providers, having joined usthe company in 2016. Since April 2022, he has served as member of the board of directors of Augmedix, Inc., a Nasdaq-listed provider of automated medical documentation and data services. Mr. Febbo founded Plexuus, LLC, a payment processing business for medical professionals in September 2015 and remained its Chairman from September 2015 to December 2020. From April 2007 to September 2015, he served as Chief Operating Officer of Merriman Holdings, Inc., an investment banking firm, where he assisted with capital raises in the technology, biotechnology, clean technology, consumer and resources industries. Mr. Febbo was a co-founder of, and from September 2013 to September 2015 served as Chief Executive Officer of, Digital Capital Network, Inc. a transaction platform for institutional and accredited investors. He was a co-founder of, and from January 1999 to September 2015 was Chief Executive Officer of, MedPanel, LLC, a provider of market intelligence and communications for the pharmaceutical, biomedical, and medical device industries. Since 2017, Mr. Febbo has been a faculty member of the Massachusetts Institute of Technology’s linQ program, which is a collaborative initiative focused on increasing the potential of innovative research to benefit society and the economy. Since 2004, he has been a board member of the United Nations Association of Greater Boston, a resource for the citizens of Greater Boston on the broad agenda of critical global issues addressed by the United Nations and its agencies. He holds a B.A. in international studies and Spanish from Dickinson College. We believe that Mr. Febbo is qualified to serve on our board of directors because of his wealth of experience in building and managing health services and financial businesses. Mr. Febbo brings more than 20 years of experience in building and managing health services and financial businesses.
On January 29, 2018, the Financial Industry Regulatory Authority (FINRA)(“FINRA”) accepted a Letter of Acceptance, Waiver and Consent (the Consent)“Consent”) submitted by Mr. Febbo. Without admitting or denying the findings, Mr. Febbo consented to the sanctions and to the entry of findings that he permitted Merriman Capital, Inc. to conduct a securities business while below its net capital requirement. From August 2012 to October 2015, he was the Financial and Operations Principal (FinOp)(“FinOp”) for a registered broker-dealer, Merriman Capital, Inc. (Merriman)(“Merriman”). During certain months, while Mr. Febbo was FinOp, FINRA found that certain of Merriman’s net capital filings with FINRA were inaccurate because of the method by which Merriman calculated net capital and that, when corrected, it was retroactively determined that Merriman had operated below its minimum net capital requirements. Mr. Febbo, as FinOp, signed certain of these reports and was thus held responsible. Based on the Consent, in settlement, Mr. Febbo, who was then no longer registered with any broker-dealer, accepted a fine of $5,000, a 10-business day suspension from acting as FinOp for any FINRA member and required to requalify by examination for the Series 27 license before again acting in a FinOp capacity.
Steven Felsher. Mr. Felsher was appointed to our board of directors in November 2021. Mr. Felsher is an experienced executive with respect to finance, administration, governance and other aspects of public and private company management. He has served as a member of the board of directors of Signal Hill Acquisition Corp., a special purpose acquisition company, since March 2021. From August 2018 to July 2020, he served as a member of the board of directors of Sito Mobile, Inc., a publicly-traded company that provided customized, data-driven solutions for brands spanning all forms of media. From January 2011 to June 2019, Mr. Felsher was a senior advisor at Quadrangle Group LLC, a private investment firm focused on the information and communications technology sectors. He spent a substantial portion of his career with Grey Global Group Inc., a global marketing services company, where he served as a senior executive from 1979 until 2007, most recently as vice chairman and chief financial officer. He holds a BA in classical Greek from Dickinson College and a J.D. from Yale University School of Law. We believe that Mr. FebboFelsher is qualified to serve on our board of directors because of his wealthextensive business experience with administration, governance, capital allocation and other aspects of experience in buildingpublic and managing health services and financial businesses. Mr. Febbo brings more than 20 years of experience in building and managing health services and financial businesses.private company management.
57
Morgan C. Frank.Mr. Frank was appointed to our board of directors in April 2017. In August 2022, he was appointed as chairman of the board of directors of SANUWAVE Health, Inc., a publicly-traded provider of wound-care products. Mr. Frank has worked with Manchester, Explorer, LP since May 2002, and, prior to such time, he was a founder and managing director at First Principles Group, a boutique consultancy and principal investor specializing in corporate restructuring, restarts, intellectual property assessment and salvage, and spin outs. Prior to such time, Mr. Frank spent approximately five years as an analyst and portfolio manager at Hollis Capital, a San Francisco based hedge fund and prior thereto, Mr. Frank worked for an independent private client group at Paine Webber specializing in primary research to develop investment ideas (particularly short sale ideas) for institutional clients. Prior to his employment at Paine Webber, Mr. Frank was a currency trader for Eastern Vanguard. Mr. Frank holds a BA in Economics and in Political Science from Brown University. We believe that Mr. Frank is qualified to serve as member of our board of directors due to his extensive prior experience conducting financial analysis of public companies (certain of which were in the development stage), including such public companies’ management teams, products, including products in the development stage, the potential markets for such products and other factors that could affect the likelihood and timing of success and market penetration of such entities’ products as well as his capital raising activities. We believe this provides us with valuable insights into the financial markets and investment criteria of institutional and other investors as well as capital raising activities.
Carmen Volkart. Ms. Volkartwas appointed to our board of directors in December 2019. She has served as chief financial officer of Natureworks LLC, an advanced materials company offering a portfolio of renewably-sourced polymers, since October 2018. From October 2012 to July 2018, Ms. Volkart served as chief financial officer and, for a portion of that time, as senior vice president of commercialization for NxThera, Inc., a medical device company pioneering the application of convective radiofrequency thermotherapy to treat endurological conditions. She served as global chief financial officer of Tornier N.V. from 2010 to 2012, and was chief operating and financial officer, corporate secretary, compliance officer and treasurer of Spine Wave, Inc. from 2006 to 2010. Prior to 2006, Ms. Volkart held various executive and financial positions at American Medical Systems, Inc., Medtronic, Inc. and Honeywell, Inc. She holds a B.S. in accounting from the University of North Dakota and an MBA with a concentration in strategic management from the University of Minnesota. We believe that Ms. Volkart is qualified to serve on our board of directors because of her substantial financial and public company experience, as she has served as chief financial officer at multiple medical device and other companies.
Steven Felsher. Mr. Felsher was appointed to our board of directors in November 2021. Mr. Felsher is an experienced executive with respect to finance, administration, governance and other aspects of public and private company management. He has served as a member of the board of directors of Signal Hill Acquisition Corp., a special purpose acquisition company, since March 2021. From August 2018 to July 2020, he served as a member of the board of directors of Sito Mobile, Inc., a publicly-traded company that provided customized, data-driven solutions for brands spanning all forms of media. From January 2011 to June 2019, Mr. Felsher was a senior advisor at Quadrangle Group LLC, a private investment firm focused on the information and communications technology sectors. He spent a substantial portion of his career with Grey Global Group Inc., a global marketing services company, where he served as a senior executive from 1979 until 2007, most recently as vice chairman and chief financial officer. He holds a BA in classical Greek from Dickinson College and a J.D. from Yale University School of Law. We believe that Mr. Felsher is qualified to serve on our board of directors because of his extensive business experience with administration, governance, capital allocation and other aspects of public and private company management.
Philip Sheibley.Mr. Sheibley was appointed to our board of directors in November 2021. Mr. Sheibley is an experienced executive and venture capitalist. Since 2011, he has served as a principal at Alumni Investment Partners, a private equity firm. From 1981 to 2010, Mr. Sheibley served as a management and technology consultant with Accenture, where he focused on the life sciences area, holding a variety of leadership positions, including North American industry director for life sciences and global lead for management consulting. Mr. Sheibley holds a B.S. in industrial and systems engineering with a business minor from Lehigh University. We believe that Mr. Sheibley is qualified to serve on our board of directors because of his extensive business experience in the life sciences area and experience with venture capital investment and consulting, including financing transactions for early-stage and scale-up stage companies, assisting with scale-up strategy/execution, and participating as a board member in the medical products industry.
Carmen Volkart. Ms. Volkart was appointed to our board of directors in December 2019. She has served as chief financial officer of Natureworks LLC, an advanced materials company offering a portfolio of renewably-sourced polymers, since October 2018. Ms. Volkart served as a member of the board of directors, including as a member of the audit committee of Antares Pharma, Inc., a Nasdaq-listed, specialty pharmaceutical company, from October 2021 to May 2022, when it was acquired by another Nasdaq-listed company. From October 2012 to July 2018, she served as chief financial officer and, for a portion of that time, as senior vice president of commercialization for NxThera, Inc., a medical device company pioneering the application of convective radiofrequency thermotherapy to treat endurological conditions. Ms. Volkart served as global chief financial officer of Tornier N.V. from 2010 to 2012, and was chief operating and financial officer, corporate secretary, compliance officer and treasurer of Spine Wave, Inc. from 2006 to 2010. Prior to 2006, she held various executive and financial positions at American Medical Systems, Inc., Medtronic, Inc. and Honeywell, Inc. Ms. Volkart holds a B.S. in accounting from the University of North Dakota and an MBA with a concentration in strategic management from the University of Minnesota. We believe that Ms. Volkart is qualified to serve on our board of directors because of her substantial financial and public-company experience, as she has served as chief financial officer at multiple medical device and other companies.
Ellen O’Connor Vos. Ms. Vos was appointed to our board of directors in May 2021 and served as our chief executive officer from August 2021 until February 23, 2022. Ms. Vos has served as a member of VosHealth LLC since November 2020. Prior to that, she served as the president and chief executive officer of the Muscular Dystrophy Association from October 2017 to November 2020. Previously, Ms. Vos had been chief executive officer of ghg | greyhealth group from 1996 to 2017, and she has been a champion of using digital capabilities to improve the public health. Ms. Vos also serves on the board of OptimizeRX Corporation, a publicly-traded digital health company, and the Jed Foundation, a leading nonprofit dedicated to protecting the emotional health of college students, and was a founding board member of MMRF, a pioneering cancer research foundation. Ms. Vos holds a B.S. in nursing from Alfred University. We believe that Ms. Vos is qualified to serve on our board of directors because of her executive experience and extensive executive skills in digital marketing, commercialization and communications in the healthcare industry.
58
Involvement in Legal Proceedings
Except as described above with regard to Mr. Febbo, to our knowledge, none of our executive officers or our directors has, during the last ten years:
| had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
To our knowledge, there are no material proceedings to which any director, officer or affiliate of ours, any owner of record or beneficially of more than 5% of any class of voting securities of us, or any associate of any such director, officer, affiliate of ours, or security holder is a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries.
Arrangements for Appointment of Directors and Officers
Pursuant to the Reorganization and Share Exchange Agreement, hereinafter referred to as the Share Agreement, dated as of July 24, 2017, by and among us, Quasuras, Inc., Mr. DiPerna and the other stockholders of Quasuras, Inc., until July 24, 2022, our board of directors shallwas required to consist of no more than five and no less than two directors of which (i) Manchester Explorer, LP has the right to appoint two directors, pursuant to which Manchester Explorer, LP appointed Mr. Frank and Ms. Volkart, and (ii) Mr. DiPerna, in addition to being our chairman of the board, had the right to appoint two additional directors, pursuant to which he appointed Liam Burns, who resigned from our board of directors in December 2021, and Mr. Febbo. In May 2021, the parties amended the Share Agreement and removed Manchester Explorer, LP’s and Mr. DiPerna’s rights to appoint directors. In addition, the parties agreed that Mr. DiPerna shall remain chairman of our board of directors until July 2022; provided, that in the event Mr. DiPerna resigns or is otherwise replaced as our chief executive officer, Mr. DiPerna shall remain as chairman of our board of directors for an additional period of three years. Following such amendment, our board of directors increased the size of the board to six members and, on May 18, 2021, appointed Ms. Vos as a director to our board.
The DiPerna Employment and Related Agreements
We entered into an employment agreement dated August 1, 2018, with Mr. DiPerna pursuant to which Mr. DiPerna is employed by us as our president for an initial two-year term with automatic one-year renewals. Pursuant to such agreement, we agreed to pay Mr. DiPerna: i)(i) an annual salary of $200,000 in cash, ii)(ii) $100,000 per year in fully-vested stock options granted monthly at an exercise price determined by our board of directors in its sole discretion and iii)(iii) an annual bonus of $300,000, payable at the discretion of our board of directors, either in shares or in cash. If the board chooses to pay the bonus in shares, such shares will be valued at a price determined by our board of directors. Pursuant to such employment agreement (i) if (a) we terminate Mr. DiPerna’s employment without cause or he resigns with good reason, we will pay Mr. DiPerna a lump sum of $200,000, and (b) we terminate Mr. DiPerna’s employment for cause, we are not obligated to make any severance payment and Mr. DiPerna will receive only his base compensation through the last day of his employment, (ii) upon Mr. DiPerna’s death or disability, he will receive his base compensation through the last day of his employment and will remain eligible for all applicable benefits relative to death or disability pursuant to any plans that we have in place at such time, and (iii) upon a change of control (as defined in the employment agreement), Mr. DiPerna will be paid a lump sum of $100,000 within sixty days of the time at which such change of control takes place.
In May 2020, we amended our employment agreement with Mr. DiPerna to provide that in the event of a change in control:
| within 60 days of the date the change in control occurs, Mr. DiPerna shall be paid by us or our successor in interest a lump sum cash payment equal to 12 months of Mr. DiPerna’s then annual Base Compensation (as defined in the employment agreement); and |
| immediately prior to such change of control, any unvested stock options or other unvested securities of ours issued to Mr. DiPerna shall automatically accelerate and immediately become fully vested and exercisable. |
In June 2020, our board of directors approved an amendment to the employment agreement to provide that Mr. DiPerna’s base salary would be paid entirely in cash commencing July 1, 2020. The payment of the additional cash component of Mr. DiPerna’s annual base salary ($8,333.33 per month) was initially deferred (the “Deferred Salary”) and accrue for Mr. DiPerna’s benefit until we have received $5,000,000 of cumulative gross proceeds of financing, at which time the Deferred Salary shall be paid to Mr. DiPerna and the salary deferrals will cease.The salary deferrals ceased and the Deferred Salary was paid to Mr. DiPerna in May 2021. In August 2021, Mr. DiPerna resigned as our chief executive officer, and he continues to serve as our president, chief financial officer, treasurer and chairman of our board of directors.
On April 15, 2021, our board of directors authorized a $50,000 bonus for Mr. DiPerna for fiscal 2021.
If a change of control occurred on March 31, 2021,2022, under his employment agreement, Mr. DiPerna would be entitled to the following:
| payment of a lump sum of $300,000 within 60 days of the time at which such change of control takes place; and |
| accelerated vesting of |
In connection with our acquisition of Quasuras, we entered into an Intellectual Property Transfer Agreement dated as of July 24, 2017, with Quasuras and Mr. DiPerna, pursuant to which Mr. DiPerna transferred to us all intellectual property rights owned directly and/or indirectly by him related to our business. Separately, we agreed to pay Mr. DiPerna, as part of his compensation for services to be performed for us, pursuant to a royalty agreement, certain fees based upon future sales, if any, of our potential product subject to a maximum $10,000,000 cap on the aggregate amount of fees that Mr. DiPerna could earn from such arrangement.
The Vos Employment Agreement
On August 11, 2021, we entered into a two-year employment agreement (the “Agreement”) with Ms. Vos for her service as our chief executive officer, and the Agreement renews for one-year terms, unless either party provides the other with 90-day prior written notice of termination. The Agreement provided that Ms. Vos was entitled to total base compensation of $300,000 annually, as follows: a cash salary of $250,000 per year (the “Cash Salary”), plus deferred salary of $50,000 per year (the “Deferred Salary” and, together with the Cash Salary, the “Base Compensation”).
On February 23, 2022, Ellen O’Connor Vos informed the board of directors the Company that she was resigning from her position as chief executive officer of the Company, effective immediately (the “Resignation”). In connection with the Resignation, the Company and Ms. Vos entered into a Severance and Release Agreement dated February 23, 2022 (the “Separation Agreement”). Pursuant to the Separation Agreement, Ms. Vos is entitled to receive separation payments in an aggregate gross amount of $375,000. Under the terms of the Separation Agreement, the vesting of an option to purchase 362,452 shares of the Company’s common stock, which was granted to Ms. Vos on August 11, 2021, will ceaseceased on May 24, 2022 and the remaining unvested shares will bewere forfeited.
James Besser
As compensation for his services as Chief Executive Officer, Mr. Besser is paid de minimis compensation of $1.00 per year.
Communications with our Board of Directors
Stockholders who desire to communicate with the board of directors, or a specific director, may do so by sending the communication addressed to either the board of directors or any individual director, c/o Modular Medical, Inc., 16772 West Bernardo Drive,10740 Thornmint Road, San Diego, California 92127. These communications will be delivered to the board of directors, or any individual director, as specified.
Corporate Governance
Director Independence
Our Board has undertaken a review of the independence of our directors and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. As a result of this review, our Boardboard of directors has determined that William Febbo, Steven Felsher, PhilpPhilip Sheibley and Carmen Volkart are “independent directors”directors,” as defined under the rules of Nasdaq.
Board Leadership Structure and Role in Risk Oversight
Due to ourthe small size and early stage of the Company, we have not adopted a formal policy on whether the chairman and chief executive officer positions should be separate or combined. Since August 11, 2021,2017, Mr. DiPerna has been serving as our chairman, and, since February 23, 2022, Mr. Besser has been serving as our chief executive officer. Our board of directors has oversight responsibility for our risk management processes. Our board of directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate, regarding our assessment of risks. Our board of directors will focus on the most significant risks facing us and our general risk management strategy, and also ensure that risks undertaken by us are consistent with our appetite for risk. While our board of directors oversees our risk management processes, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing us and that the leadership structure of our board of directors supports this approach.
We have established an audit committee, a compensation committee, and a nominating and governance committee. Each committee’s members and functions are described below.
Audit Committee
Our board of directors established the audit committee (the “Audit Committee”) for the purpose of overseeing the accounting and financial reporting processes and audits of our financial statements. The Audit Committee also is charged with reviewing any internal control violations under our whistleblower policy. The responsibilities of our audit committee are described in the Audit Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Ms. Volkart, Mr. Felsher and Mr. Sheibley are the current members of the Audit Committee. Mr. Felsher serves as the chairperson and has been designated by the board of directors as the “audit committee financial expert,” as defined by Item 407(d)(5) of Regulation S-K under the Securities Act of 1933, as amended, and the Exchange Act. That status does not impose duties, liabilities or obligations that are greater than the duties, liabilities or obligations otherwise imposed on her as a member of the audit committee and the board of directors, however. Our board of directors has determined that each of our Audit Committee members satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act.
Compensation Committee
Our board of directors established the compensation committee for the purpose of reviewing, recommending and approving our compensation policies and benefits, including the compensation of all of our executive officers and directors. Mr. Febbo and Ms. Volkart are the current members of the compensation committee, and Mr. Febbo serves as the chairperson. Each of our Compensation Committee members satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act.
Our compensation committee is responsible for reviewing, recommending and approving our compensation policies and benefits, including the compensation of all of our executive officers and directors, and it also has the principal responsibility for the administration of our equity incentive plan. The responsibilities of our compensation committee are more fully described in the Compensation Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Nominating and Corporate Governance Committee.
The Nominating and Governance Committee consists of Mr. Sheibley and Mr. Felsher, and Mr. Sheibley serves as the chairperson. Each of the members of our Nominating and Governance Committee satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act. The Nominating and Governance Committee will consider persons recommended by stockholders for inclusion as nominees for election to our board of directors if the information required by our bylaws is submitted in writing in a timely manner addressed and delivered to our secretary at the address of our executive offices.
The Nominating and Governance Committee will identify and evaluate nominees for our board of directors, including nominees recommended by stockholders, based on numerous factors it considers appropriate, some of which may include strength of character, mature judgment, career specialization, relevant technical skills, diversity, and the extent to which the nominee would fill a present need on our board of directors. The responsibilities of our Nominating and Governance committee are more fully described in the Nominating and Governance Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Code of Business Conduct and Ethics for Employees, Executive Officers and Directors
We have adopted a Code of Business Conduct and Ethics, or the Code of Conduct, applicable to all of our employees, executive officers and members of our board of directors. The Code of Conduct is available on our website at www.modular-medical.com. Our Nominating and Governance Committee is responsible for overseeing the Code of Conduct, and our board of directors must approve any waivers of the Code of Conduct. In addition, we intend to post on our website all disclosures that are required by law concerning any amendments to, or waivers from, any provision of the Code of Conduct.
Board Diversity
We seek diversity in experience, viewpoint, education, skill, and other individual qualities and attributes to be represented on our board of directors. We believe directors should have various qualifications, including individual character and integrity; business experience; leadership ability; strategic planning skills, ability, and experience; requisite knowledge of our industry and finance, accounting, and legal matters; communications and interpersonal skills; and the ability and willingness to devote time to our company. We also believe the skill sets, backgrounds, and qualifications of our directors, taken as a whole, should provide a significant mix of diversity in personal and professional experience, background, viewpoints, perspectives, knowledge, and abilities. Nominees are not to be discriminated against on the basis of race, religion, national origin, sex, sexual orientation, disability, or any other basis proscribed by law. The assessment of prospective directors is made in the context of the perceived needs of our board of directors from time to time.
All of our directors have held high-level positions in business or professional service firms and have experience in dealing with complex issues. We believe that all of our directors are individuals of high character and integrity, are able to work well with others, and have committed to devote sufficient time to the business and affairs of our company. In addition to these attributes, the description of each director’s background set forth above indicates the specific qualifications, skills, perspectives, and experience necessary to conclude that each individual should continue to serve as a director of ours.
63
Executive Compensation
SUMMARY COMPENSATION TABLESummary Compensation Table
The following table sets forth compensation information for the years ended March 31, 20212022 and March 31, 20202021 for each of our named executive officers.
| Name and Principal Position | Year | Salary ($) | Stock Awards ($) | Option Awards ($)(1) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||
| Paul DiPerna, CEO, CFO, Secretary, | 2021 | 200,000 | — | 25,000 | — | 50,000 | (3) | 275,000 | |||||||||||||||||||
| Treasurer and Director(2) | 2020 | 200,000 | — | 584,200 | — | 280,000 | (4) | 1,064,200 | |||||||||||||||||||
| Stephen Daly, Chief Commercial Officer(5) | 2021 | 234,000 | — | — | — | — | 234,000 | ||||||||||||||||||||
| 2020 | 20,833 | — | 355,240 | — | — | 376,073 | |||||||||||||||||||||
| Name and Principal Position | Year | Salary ($) | Stock Awards ($) | Option Awards ($)(1) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||
| Paul DiPerna, President, Chief Financial Officer, | 2022 | 370,833 | (3) | — | — | — | — | 370,833 | ||||||||||||||||||
| Treasurer and Chairman(2) | 2021 | 200,000 | — | 25,000 | — | 50,000 | (4) | 275,000 | ||||||||||||||||||
| James E. Besser, Chief Executive Officer(5) | 2022 | — | — | — | — | — | — | |||||||||||||||||||
| Ellen O’Connor Vos, Chief Executive Officer(6) | 2022 | 133,654 | — | 4,414,645 | — | 409,662 | (7) | 4,957,961 | ||||||||||||||||||
| Stephen Daly, Chief Commercial Officer(8) | 2022 | 59,395 | — | — | — | 6,046 | 65,441 | |||||||||||||||||||
| 2021 | 234,000 | — | — | — | — | 234,000 | ||||||||||||||||||||
| (1) | Award amounts reflect the aggregate grant date fair value with respect to awards granted, as determined pursuant to FASB ASC Topic 718. The assumptions used to calculate the aggregate grant date fair value of option awards are set forth in the notes to the consolidated financial statements included in |
| (2) | |
| (3) | Includes payment of |
| Earned as a bonus of which $22,000 was paid on April 30, 2021, and the remainder |
| (6) | Ms. Vos was appointed our chief executive officer in August 2021 at an annual cash salary of $250,000 per year plus deferred salary of $50,000 per year. She resigned as our chief executive officer in February 2022. The compensation amounts disclosed in the table above exclude amounts paid to Ms. Vos for her service as a |
| (7) | Represents payment during fiscal 2022 of i) accrued holiday and |
| Mr. Daly became our |
Outstanding Equity Awards at Fiscal Year-End
The following table shows certain information regarding outstanding equity awards held by our named executive officers as of March 31, 2021.2022.
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price($) | Option Expiration Date(1) | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price($) | Option Expiration Date(1) | ||||||||||||||||||||
| Paul DiPerna | 1,155 | (2) | — | 9.48 | 6/1/2030 | 1,155 | (2) | — | 9.48 | 6/1/2030 | ||||||||||||||||||
| 1,168 | (3) | — | 9.48 | 5/1/2030 | 1,169 | (3) | — | 9.48 | 5/1/2030 | |||||||||||||||||||
| 1,169 | (4) | — | 9.48 | 4/1/2030 | 1,170 | (4) | — | 9.48 | 4/1/2030 | |||||||||||||||||||
| 1,660 | (5) | — | 7.44 | 3/2/2030 | 1,660 | (5) | — | 7.44 | 3/2/2030 | |||||||||||||||||||
| 1,745 | (3) | — | 7.44 | 2/1/2030 | 1,745 | (6) | — | 7.44 | 2/1/2030 | |||||||||||||||||||
| 1,727 | (4) | — | 7.44 | 1/1/2030 | 1,727 | (7) | — | 7.44 | 1/1/2030 | |||||||||||||||||||
| 1,808 | (5) | — | 6.75 | 12/1/2029 | 1,809 | (8) | — | 6.75 | 12/1/2029 | |||||||||||||||||||
| 1,811 | (6) | — | 6.75 | 11/1/2029 | 1,811 | (9) | — | 6.75 | 11/1/2029 | |||||||||||||||||||
| 51,721 | (7) | — | 6.75 | 10/1/2029 | 1,721 | (10) | — | 6.75 | 10/1/2029 | |||||||||||||||||||
| 1,662 | (8) | — | 6.75 | 9/15/2029 | 1,662 | (11) | — | 6.75 | 9/15/2029 | |||||||||||||||||||
| 1,666 | (9) | — | 6.75 | 8/15/2029 | 1,666 | (12) | — | 6.75 | 8/15/2029 | |||||||||||||||||||
| 1,660 | (10) | — | 6.75 | 7/15/2029 | 1,660 | (13) | — | 6.75 | 7/15/2029 | |||||||||||||||||||
| 1,650 | (11) | — | 6.75 | 6/15/2029 | 1,650 | (14) | — | 6.75 | 6/15/2029 | |||||||||||||||||||
| 1,676 | (12) | — | 6.75 | 5/15/2029 | 1,677 | (15) | — | 6.75 | 5/15/2029 | |||||||||||||||||||
| 1,623 | (13) | — | 6.75 | 4/15/2029 | 1,624 | (16) | — | 6.75 | 4/15/2029 | |||||||||||||||||||
| 1,694 | (14) | — | 6.75 | 3/15/2029 | 1,694 | (17) | — | 6.75 | 3/15/2029 | |||||||||||||||||||
| 1,641 | (15) | — | 6.75 | 2/15/2029 | 1,641 | (18) | — | 6.75 | 2/15/2029 | |||||||||||||||||||
| 1,603 | (16) | — | 6.75 | 1/15/2029 | 1,603 | (19) | — | 6.75 | 1/15/2029 | |||||||||||||||||||
| 1.775 | (17) | — | 6.75 | 12/28/2028 | 1,775 | (20) | — | 6.75 | 12/15/2028 | |||||||||||||||||||
| 1.775 | (18) | — | 6.75 | 11/14/2028 | 1,775 | (21) | — | 6.75 | 11/15/2028 | |||||||||||||||||||
| 6,005 | (19) | — | 1.98 | 10/14/2028 | 6,005 | (22) | — | 1.98 | 10/15/2028 | |||||||||||||||||||
| 6,005 | (20) | — | 1.98 | 09/14/2028 | 6,005 | (23) | — | 1.98 | 09/15/2028 | |||||||||||||||||||
| 6,005 | (21) | — | 1.98 | 08/14/2028 | 6,005 | (24) | — | 1.98 | 08/15/2028 | |||||||||||||||||||
| 38,889 | (22) | 61,111 | 6.75 | 11/25/2029 | 2,222 | (25) | 27,778 | 6.75 | 11/25/2029 | |||||||||||||||||||
| Stephen Daly | 22,223 | (23) | 44,445 | 6.75 | 3/3/2030 | |||||||||||||||||||||||
| (1) | The standard option term is ten years, but all of the options expire automatically unless exercised within 90 days after the cessation of service as an employee, director or consultant. |
| (2) | The option was granted on June 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (3) | The option was granted on May 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (4) | The option was granted on April 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (5) | The option was granted on March 2, 2020, and the shares subject to this option were fully vested on the grant date. |
| (6) | The option was granted on February |
| (7) | The option was granted on January 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (8) | The option was granted on December 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (9) | The option was granted on November 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (10) | The option was granted on October 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (11) | The option was granted on September 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (12) | The option was granted on August 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (13) | The option was granted on July 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (14) | The option was granted on June 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (15) | The option was granted on May 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (16) | The option was granted on April 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (17) | The option was granted on March 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (18) | The option was granted on February 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (19) | The option was granted on January 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (20) | The option was granted on December 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (21) | The option was granted on November 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (22) | The option was granted on October 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (23) | The option was granted on September 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (24) | The option was granted on August 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (25) | The option was granted on November 25, 2019, and the shares subject to this option vest monthly over three years commencing January 1, 2020, subject to continued service as an employee, director or consultant. | |
Employment Agreements
We have entered into our standard form of employment, confidential information and invention assignment agreement with each of our named executive officers. We also have entered into agreements to indemnify our directors and certain executive officers, in addition to the indemnification provided for in our articles of incorporation and bylaws. These agreements, among other things, provide for indemnification of our directors and certain executive officers for many expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by or in the right of us, arising out of such person’s services as a director or executive officer of ours, any subsidiary of ours or any other company or enterprise to which such person provided services at our request.
Director Compensation
Effective April 1, 2021, our board of directors approved our outside (non-employee) director compensation plan (the “Director Plan”). Pursuant to the Director Plan, outside directors are paid the following annual retainers:
| ● | $25,000 for service as a member of the board of directors; |
| ● | $5,000 for service as chair of the audit committee; and |
| ● | $5,000 for service as chair of the compensation committee. |
The annual retainers will be paid in quarterly installments in either cash, options to purchase shares of our common stock or in shares of our common stock, as directed by each director based on an annual election. In addition, under the Director Plan, each director will also receive an annual service equity award of $100,000 paid in quarterly installments in either options to purchase shares of our common stock or shares of our common stock, as directed by each director based on an annual election.
In addition, upon appointment to our board of directors, we award our non-employee directors a stock option grant under our Amended 2017 Equity Incentive Plan (the “2017 Plan”). During fiscal 2022, we awarded each of the new non-employee directors a stock option to purchase 16,667 shares of our common stock. These options vest annually over three years from the date of appointment to our board of directors.
The following table summarizes the compensation we paid to our non-employee directors for the year ended March 31, 2021:2022:
| Fee | Restricted Stock | Option | ||||||||||||||||||||||||||||||||||||||
| Compensation | Awards | Awards | All Other | Total | ||||||||||||||||||||||||||||||||||||
| Name | ($) | ($) | ($)(1)(2) | Compensation | ($) | Fee Compensation ($) | Restricted Stock Awards ($) | Option Awards ($)(1)(2) | All Other Compensation (3) | Total ($) | ||||||||||||||||||||||||||||||
| Liam Burns(3) | 10,000 | — | — | — | 10,000 | |||||||||||||||||||||||||||||||||||
| Liam Burns(4) | 18,750 | — | 222,291 | — | 241,041 | |||||||||||||||||||||||||||||||||||
| William Febbo | 10,000 | — | — | — | 10,000 | 30,000 | — | — | 100,000 | 130,000 | ||||||||||||||||||||||||||||||
| Morgan Frank(4) | — | — | 375,105 | — | 375,105 | |||||||||||||||||||||||||||||||||||
| Steven Felsher(5) | — | — | 194,981 | 8,657 | 203,638 | |||||||||||||||||||||||||||||||||||
| Morgan Frank | — | — | 375,105 | — | 375,105 | |||||||||||||||||||||||||||||||||||
| Philip Sheibley(5) | 8,424 | — | 169,588 | 33,696 | 211,078 | |||||||||||||||||||||||||||||||||||
| Carmen Volkart | 10,000 | — | — | — | 10,000 | — | — | 296,423 | 29,671 | 326,094 | ||||||||||||||||||||||||||||||
| Ellen O’Connor Vos(6) | 2,953 | — | 281,339 | — | 284,292 | |||||||||||||||||||||||||||||||||||
| (1) | Award amounts reflect the aggregate grant date fair value with respect to awards granted, as determined pursuant to FASB ASC Topic 718. The assumptions used to calculate the aggregate grant date fair value of option awards are set forth in the notes to the consolidated financial statements included in |
| (2) | As of March 31, |
| (3) | Represents stock awards; we calculated the estimated fair value of the stock awards issued to our non-employee directors using the closing price per share of our common stock on the day prior to the grant date in accordance with the Director Plan. |
| (4) | Mr. Burns resigned as a |
| Messrs. Felsher and Sheibley were appointed to our board of directors on November 29, 2021. |
| (6) | Ms. Vos was |
During the year ended March 31, 2021, our board of directors had authorized an annual cash retainer fee of $10,000, payable in quarterly installments, for our non-employee directors, with the exception of Mr. Frank, as compensation for their service. Effective April 1, 2021, our board of directors approved our outside (non-employee) director compensation plan (the Director Plan). Pursuant to the Director Plan, outside directors will be paid the following annual retainers:
The retainers will be paid in quarterly installments in either cash or in shares of our Common Stock, as directed by each director based on an annual election. In addition, under the Director Plan, each director will also receive an annual service equity award of $100,000 paid in quarterly installments in either options to purchase shares our Common Stock or shares of our Common Stock, as directed by each director based on an annual election.
In addition, upon appointment to our board of directors, we award our non-employee directors a stock option grant under our Amended 2017 Equity Incentive Plan, hereinafter referred to as the 2017 Plan, ranging from 16,667 to 66,667 shares of our Common Stock. These options vest annually over three years from the date of appointment to our board of directors.
In connection with her May 2021 appointment and service as a non-employee director, Ms. Vos has entered into our standard form of indemnification agreement. We also awarded Ms. Vos an initial option to purchase 16,667 shares of our Common Stock vesting over three years, with one-third of the shares subject to the option vesting on each one-year anniversary of the date of grant.
Equity Compensation Plan Information
The following table shows the number of securities to be issued upon exercise or vesting of outstanding equity awards under the 2017 Plan as of March 31, 2021.2022.
| Plan Category | Number of securities to be issued upon exercise or vesting of outstanding equity awards (a) | Weighted- average exercise price of outstanding options (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column(a)) © | Number of securities to be issued upon exercise or vesting of outstanding equity awards (a) | Weighted- average exercise price of outstanding options (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column(a)) (c) | ||||||||||||||||||
| Equity compensation plans not approved by security holders | 1,197,252 | $5.25 | 136,082 | 1,650,705 | $ | 6.58 | 989,466 | |||||||||||||||||
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Related Party TransactionTransactions
Manchester Management Company LLC (“Manchester”) as the general partner of Manchester Explorer, L.P. (“Explorer”), combined with the holdings of its affiliates, JEB Partners LP, Mr. Besser and Mr. Frank, owns approximately 25% of the Company’s outstanding shares of common stock. Mr. Besser is our chief executive officer and a managing member of Manchester. Mr. Frank is one of our directors and serves as the portfolio manager of Explorer and as a managing member of Manchester.
Mr. DiPerna’s daughter is an employee of ours, and, during the year ended March 31, 2022, we paid her $169,589, which includes the aggregate grant date fair value, as determined pursuant to FASB ASC Topic 718, of a stock option granted in November 2021.
In October 2021, we issued a secured promissory note (the “Bridge Note”) to Explorer that provided the Company with a $3,000,000 revolving credit facility with all amounts being drawn down by us thereunder being due and payable, subject to acceleration in the event of a default, on March 15, 2022. Interest at the rate of 12% was payable on each amount drawn down without regard to the draw-down date or the date when interest is paid. During the period from October 2021 to February 2022, we made draws of $2,100,000 on the Bridge Note, and we repaid to Explorer the draws and accrued interest in the amount of $2,352,000 in February 2022.
In October 2021, we sold 12,346 shares of common stock to Mr. DiPerna and 18,519 shares to Ms. Vos at a price per share of $8.10 in a private placement.
In May 2021, we issued a convertible promissory note (the “Febbo Convertible Note”) in theMr. Febbo purchased $200,000 aggregate principal amount of $200,000our convertible notes and received a warrant to William Febbo, a memberpurchase 23,229 shares of our boardcommon stock (the “Director Warrant”). In connection with a public offering of directors. Onour equity securities in February 14, 2022, in accordance with the terms of the Manchester Convertible Note, principal and accrued interest of $273,507 for theconvertible note held by Mr. Febbo Convertible Note was converted into our equity securities. Upon conversion, Mr. Febbo received 45,586 shares of our common stock. In addition, in connection with the conversion, Mr. Febbo receivedstock and a five-year warrant to purchase 45,586 shares of our common stock at aan exercise price of $6.60 per share. In addition, the exercise price of the Director Warrant was reduced to $6.00 per share.
In AprilFebruary 2021, we issued a convertible promissory noteMr. DiPerna and Explorer (the “Manchester Convertible Note”“Related Party Holders”) in the, which is represented by Mr. Frank on our board of directors, purchased $100,000 and $1,000,000, aggregate principal amount of $1,026,630our convertible notes and received warrants to Explorer. On February 14, 2022, in accordancepurchase 119,237 and 11,924 shares of our common stock (the “Note Warrants”), respectively. Effective April 30, 2021, the Related Party Holders entered into revocation agreements with the termsCompany pursuant to which their collective $1,100,000 aggregate principal amount of the Manchester Convertible Note, principalconvertible notes and accrued interest of $1,124,511 for$50,091 were replaced with new convertible notes. In connection with a public offering of our equity securities in February 2022, the Manchester Convertible Note wasconvertible notes and accrued interest held by the Related Party Holders were converted into our equity securities and Mr. DiPerna received 23,429 shares of our common stock and a warrant to purchase 23,429 shares of our common stock at an exercise price of $6.60 per share and Explorer received 234,274 shares of our common stock. In addition, in connection with the conversion, Explorer receivedstock and a five-year warrant to purchase 234,274 shares of our common stock at aan exercise price of $6.60 per share.
In April 2021, we issued a convertible promissory note (the “DiPerna Convertible Note”) inaddition, the amount of $102,663 to Explorer. On February 14, 2022, in accordance with the termsexercise prices of the DiPerna Convertible Note principal and accrued interest of $112,451 for the DiPerna Convertible Note was converted into 23,429 shares of our common stock. In addition, in connection with the conversion, Mr. DiPerna received a five-year warrantWarrants were reduced to purchase 23,429 shares of our common stock at a price of $6.60$6.00 per share.
See “Management” above for other related party transactions involving our executive officers and directors.
67
SECURITY OWNERSHIP OF CERTAIN
The following table sets forth certain information as of May 31, 20224, 2023 concerning the ownership of our Common Stockcommon stock by:
| each |
| each of our directors; |
| each of our executive officers; and |
| all directors and executive officers as a group. |
Beneficial ownership is determined in accordance with Rule 13d-3 of the Exchange Act, and includes all shares over which the beneficial owner exercises voting or investment power. Shares that are issuable upon the exercise of options, warrants and other rights to acquire Common Stockcommon stock that are presently exercisable or exercisable within 60 days of May 31, 20224, 2023 are reflected in a separate column in the table below. These shares are taken into account in the calculation of the total number of shares beneficially owned by a particular holder and the total number of shares outstanding for the purpose of calculating percentage ownership of the particular holder. We have relied on information supplied by our officers, directors and certain stockholders and on information contained in filings with the SEC. Except as otherwise indicated, and subject to community property laws where applicable, we believe, based on information provided by these persons, that the persons named in the table have sole voting and investment power with respect to all shares of Common Stockcommon stock shown as beneficially owned by them. The percentage of beneficial ownership is based on 10,911,68410,949,389 shares of Common Stockcommon stock outstanding as of May 31, 2022.4, 2023.
Unless otherwise stated, the business address of each of our directors and executive officers listed in the table is 16772 West Bernardo Drive,10740 Thornmint Road, San Diego, California 92127.
| Name and principal position | Number of Shares Beneficially Owned (Excluding Outstanding Options)(1) | Number of Shares Issuable on Exercise of Outstanding Options and Warrants(2) | Percent of Class | |||||||||
| JEB Partners, L.P. | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Manchester Explorer, L.P. | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Manchester Management LLC | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
Sio Capital Management, LLC | 449,438 | (4) | 712,000 | (5) | 9.99 | |||||||
| Directors and Officers: | ||||||||||||
| James Besser | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Paul DiPerna | 2,553,586 | (6) | 186,862 | 24.69 | ||||||||
| Ellen O’Connor Vos | 18,519 | 95,206 | 1.03 | |||||||||
| William J. Febbo | 79,105 | 113,260 | 1.74 | |||||||||
| Morgan C. Frank | 2,720,577 | (3) | 443,836 | 27.87 | ||||||||
| Carmen Volkart | 3,999 | 73,892 | * | |||||||||
| Steven Felsher | 2,174 | 21,417 | * | |||||||||
| Philip Sheibley | 8,139 | — | * | |||||||||
| All current directors and executive officers as a group (8) persons | 5,386,099 | 934,473 | 56.34 | |||||||||
| Name and principal position | Number of Shares Beneficially Owned (Excluding Outstanding Options and warrants)(1) | Number of Shares Issuable on Exercise of Outstanding Options and Warrants(2) | Percent of Class | |||||||||
| JEB Partners, L.P. | 2,720,577 | (3) | 653,511 | 29.08 | ||||||||
| Manchester Explorer, L.P. | 2,720,577 | (3) | 653,511 | 29.08 | ||||||||
| Manchester Management LLC | 2,720,577 | (3) | 653,511 | 29.08 | ||||||||
| Sio Capital Management, LLC | 689,352 | (4) | 402,127 | (5) | 9.62 | |||||||
| Directors and officers: | ||||||||||||
| James Besser | 2,720,577 | (3) | 653,511 | 29.08 | ||||||||
| Paul DiPerna | 2,553,586 | (6) | 203,262 | 24.71 | ||||||||
| Kevin Schmid | — | — | * | |||||||||
| William J. Febbo | 89,105 | 135,482 | 2.03 | |||||||||
| Steven Felsher | 5,877 | 56,973 | * | |||||||||
| Morgan C. Frank | 2,720,577 | (3) | 793,469 | 29.92 | ||||||||
| Philip Sheibley | 21,139 | 5,556 | * | |||||||||
| Carmen Volkart | 7,085 | 120,558 | 1.15 | |||||||||
| Ellen O’Connor Vos | 18,519 | 124,910 | 1.30 | |||||||||
| All current directors and executive officers as a group (9 persons) | 5,415,888 | 1,440,209 | 59.93 | |||||||||
| * | Represents less than 1% |
| (1) | Excludes shares subject to outstanding options and warrants to acquire |
| (2) | Represents the number of shares subject to outstanding options and warrants to acquire |
| (3) | Includes (i) 124,750 shares directly held by Mr. Besser, of which: (a) 60,277 shares were received in exchange for Mr. Besser’s shares as a result of our acquisition of Quasuras; (b) 29,630 shares purchased in a private placement in 2018 (the “2018 Placement”) and (c) 34,843 shares were purchased in a private placement in 2020 (the “2020 |
| (4) |
| (5) | These shares are issuable upon exercise of outstanding pre-funded warrants to purchase shares of our |
| (6) | Includes (i) 2,000,000 shares directly held by the Paul DiPerna Irrevocable Trust, (ii) 333,334 shares directly held by Mr. DiPerna’s adult daughters, Kelsie DiPerna and Alaria DiPerna, which shares Mr. DiPerna has sole voting power over; (iii) 207,906 shares directly held by the Paul DiPerna Trust, of which 101,010 shares were purchased in the 2017 Placement and 23,429 shares were acquired upon the conversion off a convertible note in February 2022 and (iv) 12,346 shares held by Mr. DiPerna. The 2,000,000 shares held by the Paul DiPerna Irrevocable Trust, 333,334 shares held by Mr. DiPerna’s adult daughters and 73,480 shares held by the Paul DiPerna Trust that were issued in 2017 to Mr. DiPerna in the Control Block Acquisition and transferred to such persons in December 2020 by Mr. DiPerna. Mr. DiPerna is the chairman of our board of directors, and also serves as our president, chief financial officer and treasurer. Mr. DiPerna is the trustee of both the Paul DiPerna Irrevocable Trust and the Paul DiPerna Trust. |
DESCRIPTION OF SECURITIES TO BE REGISTEREDWE ARE OFFERING
General
We are authorized to issue up to 50,000,000offering 2,116,402 units, with each unit consisting of two shares of common stock and one warrant to purchase one share of our common stock. The units have no stand-alone rights and will not be certificated or issued as stand-alone securities. This offering also includes the offer and sale of the shares of common stock issuable from time to time upon exercise of the warrants.
Common Stock par value $0.001 per
As of December 31, 2022, we had 10,932,098 shares of common stock issued and outstanding, 7,565,588 shares of common Stock issuable upon exercise of outstanding warrants, and 2,174,198 shares of common stock issuable upon exercise of outstanding stock options.
Under the terms of our certificate of incorporation, holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, including the election of directors, and do not have cumulative voting rights. The holders of outstanding shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as our Board of Directors from time to time may determine. Our common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to 5,000,000stockholders are distributable ratably among the holders of our common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock par value $0.001 per share. As of June 2, 2022,that we had 10,911,684 shares of our Common Stock outstanding.may designate and issue in the future.
On November 29, 2021, we effected a 1-for-3 reverse stock split of our outstanding Common Stock, which caused our then outstanding Common Stock to decrease from 19,100,154 to 6,366,736 shares while keeping our authorized capital unchanged.
Warrants
The following descriptionsummary of certain terms and provisions of the warrants that are being offered hereby is a summary, does not purport to be complete and is subject to, and qualified in its entirety by, reference to our second amended and restated articlesthe provisions of incorporation, and our bylaws, as amended, eachthe warrants, the form of which is incorporated herein by reference and are exhibitsfiled as an exhibit to the registration statement of which this prospectus forms a part. We encourage you to read our articles of incorporation, our bylawsProspective investors should carefully review the terms and the applicable provisions of the Nevada Revised Statutes (the “NRS”)form of warrant for additional information.a complete description of the terms and conditions of the warrants.
Common Stock
Duration and Exercise Price.Each holderwarrant offered hereby will have an initial exercise price per share equal to $ (120% of the public offering price per unit sold in this offering. The warrants will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The warrants will be issued separately from the common stock and may be transferred separately immediately thereafter. One warrant to purchase one share of our Common Stockcommon stock will be issued for every two shares of common stock purchased in this offering.
Exercisability. The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is entitleddetermined in accordance with the terms of the warrants. No fractional shares of common stock will be issued in connection with the exercise of a warrant.
Cashless Exercise. If, at the time a holder exercises its warrants, a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a pro rata shareformula set forth in the warrants.
Transferability. Subject to applicable laws, a warrant in book entry form may be transferred at the option of the holder through the facilities of the Depository Trust Company and warrants in physical form may be transferred upon surrender of the warrant to the Warrant Agent together with the appropriate instruments of transfer. Pursuant to a warrant agency agreement between us and the Warrant Agent, the warrants initially will be issued in book-entry form and will be represented by one or more global certificates deposited with The Depository Trust Company (“DTC”) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Exchange Listing. There is no established public trading market for the warrants, and we do not expect a market to develop. In addition, we do not intend to list the warrants on any cash distributions made to shareholders, including any dividend payments. Thesecurities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the warrants will be limited.
Right as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our Common Stockcommon stock, including any voting rights, until they exercise their warrants.
Fundamental Transaction. In the event we consummate a merger or consolidation with or into another person or other reorganization event in which shares of our common stock are converted or exchanged for securities, cash or other property, or we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of our assets or we or another person acquire 50% or more of our outstanding common shares (each, a “Fundamental Transaction”), then following such Fundamental Transaction the holders of the warrants will be entitled to one vote for each sharereceive upon exercise of the warrants the same kind and amount of securities, cash or record on all mattersproperty which the holders would have received had they exercised the warrants immediately prior to be voted on by our shareholders. Theresuch Fundamental Transaction. Any successor to us or surviving entity will assume the obligations under the warrants.
Newbridge Securities Corporation (“Newbridge” or the “underwriter”) is no cumulative votingacting as sole underwriter of this offering. We entered into an underwriting agreement with Newbridge with respect to the electionsecurities subject to this offering. Subject to the terms and conditions stated in the underwriting agreement dated the date of our directorsthis prospectus, the underwriter has agreed to purchase, and we have agreed to sell to the underwriter, the number of units shown in the table below.
| Underwriter | Number of Units | |||
| Newbridge Securities Corporation | 2,116,402 | |||
| Total | 2,116,402 | |||
The underwriting agreement provides that the obligations of the underwriter to purchase the units included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriting agreement provides that the underwriter will purchase all of the units if any of them are purchased.
Units sold by the underwriter to the public will initially be offered at the public offering price set forth on the cover of this prospectus. In addition, the underwriter may offer some of the securities to other securities dealers at such price less a concession of $ per unit. After the initial offering of the units, the public offering price or any other matter. Therefore,term of the offering may be changed by the underwriter.
Underwriting discounts and commissions
We are offering 2,116,402 units pursuant to this prospectus. Each unit comprises two shares of common stock and one warrant to purchase one share. The following table shows the effective public offering price per share of common stock and accompanying warrant and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us.
| Effective Price Per Share and accompanying Warrant | Total Without Option | Total With Option | ||||||||||
| Public offering price(1) | $ | |||||||||||
| Underwriting discounts and commissions(2) | $ | |||||||||||
| Proceeds, before expenses, to us | $ | |||||||||||
| (1) | Based on a price of $ per unit comprising two shares of common stock and one warrant to purchase one share of common stock. |
| (2) | We have agreed to pay the underwriters a commission of 7% of the gross proceeds of this offering. |
Over-Allotment Option
In addition to the discount set forth in the above table, we have granted to the underwriters an option, exercisable not later than 30 days after the date of this prospectus, to purchase up to a number of additional shares of common stock equal to 15% of the shares of common stock included in the units sold in this offering and/or a number of additional warrants equal to 15% of the warrants included in the units sold in this offering, in any combination thereof, firmly committed in this offering at a price of $ per share or $0.01 per warrant to purchase one share of common stock, in each case, less the underwriting discounts and commissions. The underwriters may exercise the option solely to cover over-allotments, if any, made in connection with this offering. If any additional shares of our common stock and/or warrants are purchased pursuant to the over-allotment option, the underwriters will offer these additional shares of our common stock and/or warrants on the same terms as those on which the other shares of common stock and Warrants are being offered hereby.
Underwriter’s Warrants
Upon the closing of this offering, we have agreed to issue to Newbridge, or its designees, warrants (the “underwriter’s warrants”) to purchase a number of shares of common stock equal to an aggregate of 7% of the total shares sold in this public offering. The underwriter’s warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share of common stock sold in this offering. The underwriter’s warrants are exercisable at any time and from time to time, in whole or in part, during the three and one-half-year period commencing six months after the effective date of the registration statement related to this offering.
We are registering hereby the issuance of the underwriter’s warrants and the shares of common stock issuable upon exercise of such warrants. The underwriter’s warrants and the shares of common stock underlying the underwriter’s warrants have been deemed compensation by the Financial Industry Regulatory Authority, or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. Neither Newbridge or its permitted assignees under our charter documents,such rule, may sell, transfer, assign, pledge, or hypothecate the underwriter’s warrants or the securities underlying the underwriter’s warrants, nor will Newbridge engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the underwriter’s warrants or the underlying shares for a period of 180 days from the effective date of the registration statement.
Additionally, the underwriter’s warrants may not be sold, transferred, assigned, pledged or hypothecated for a 180-day period following the effective date of the registration statement except to any underwriter and selected dealer participating in this offering and their bona fide officers or partners. The underwriter’s warrants will provide for adjustment in the number and price of the underwriter’s warrants and the shares of common stock underlying such underwriter’s warrants in the event of recapitalization, merger, stock split or other structural transaction, or a future financing undertaken by us.
Lock-Ups
Our officers, directors and holders of more than 50%5.0% of the shares voted for the election of those directors can elect all of the directors. Our board of directors currently are elected as a single class. Our board of directors may from time to time declare dividends on our outstanding shares. Inshares of common stock have agreed that, for a period of 90 days from the eventdate of our liquidation, dissolutionthis prospectus, they will not, subject to certain exceptions, offer, pledge, sell, contract to sell, sell any option, right or winding up, the holders of our Common Stock are entitledwarrant to share ratably in all assets remaining available for distribution to them after payment of our liabilities and after provision has been made for each class of stock, ifpurchase, lend or otherwise transfer or dispose, directly or indirectly, any having any preference in relation to our Common Stock. Holders of shares of our Common Stockcapital stock or any securities convertible into or exercisable or exchangeable for shares of capital stock without the prior written consent of Newbridge. Additionally, we have no conversion, preemptivealso agreed that, for a period of 90 days from the date of this prospectus, we will not, subject to certain exceptions, offer, pledge, sell, contract to sell, sell any option, right or other subscription rights, and there are no redemption provisions applicablewarrant to purchase, lend or otherwise transfer or dispose, directly or indirectly, any shares of our Common Stock.capital stock or any securities convertible into or exercisable or exchangeable for shares of capital stock without the prior written consent of Newbridge. Newbridge, in its sole discretion, may release any of the securities subject to these lock-up agreements at any time without notice.
ExchangeNasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “MODD”.
Transfer Agent“MODD.” There is no established trading market for the warrants, and Registrar
The transfer agent and registrarwe do not intend to apply for our Common Stock is Colonial Stock Transfer Company, Inc., 7840 S 700 E, Sandy, UT 84070. Its telephone number is 801-355-5740.
SELLING STOCKHOLDERS
The shares of common stock being registered for resale hereby consist of shares that have been issued or are issuable upon exercise of outstanding warrants that were issued to the selling stockholders in a past private placementlisting of the Company. Warrants on The Nasdaq Capital Market or on any national securities exchange. Without a trading market, the liquidity of the warrants will be extremely limited.
Expenses and Reimbursements
We are registeringestimate that our portion of the total expenses of this offering will be approximately $400,000, which includes the fees and expenses for which we have agreed to reimburse the underwriter, including the fees and disbursements of counsel for the underwriter, in connection with the offering in an amount not to exceed $125,000.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of shares of common stock in orderthis offering is complete, SEC rules may limit the ability of the underwriter to permit the selling stockholders to offer thebid for and purchase shares for resale from time to time. Except as set forth in this prospectus and except for certain ownership of our securities,common stock. As an exception to these rules, underwriters are permitted to engage in certain transactions which stabilize the selling stockholders have not had any material relationship with us within the past three years.
The table below lists the selling stockholders and other information regarding the ownershipprice of the shares of common stock, (including shares of common stock issuable upon conversion or exercise of outstanding securities registered hereunder) by the selling stockholders. The second column lists the numberwhich may include short sales, covering transactions and stabilizing transactions. Short sales involve sales of shares of common stock (includingin excess of the number of shares to be purchased by the underwriter in the offering, which creates a short position. “Covered” short sales are sales made in an amount not greater than the underwriter’s option to purchase additional shares of common stock issuable upon conversion or exercise of outstanding securities registered hereunder) ownedfrom us in the offering. An underwriter may close out any covered short position by the selling stockholders prioreither exercising its option to this offering. The third column lists thepurchase additional shares of common stock (includingor purchasing shares of common stock issuable upon conversion or exercise of outstanding securities registered hereunder) being offered by this prospectus byin the selling stockholders. The fourth and fifth columns listopen market. In determining the number and percentage, respectively,source of shares of common stock owned byto close out the selling stockholders aftercovered short position, the closingunderwriter will consider, among other things, the price of the offering, based on their ownership as of the date of this prospectus, based on 10,911,684 shares of common stock outstanding, and assumingavailable for purchase in the saleopen market as compared to the share price at which the underwriter may purchase through its option to purchase additional shares. “Naked” short sales are any sales in excess of all of the shares offeredsuch option. The underwriter must close out any naked short position by the selling stockholders pursuant to this prospectus.
| Name of Selling Stockholder | Number of Shares Owned Prior to Offering(1) | Maximum Number of Shares to be Sold Pursuant to this Prospectus(1) | Number of Shares Owned After Offering(2) | Percentage of Shares Owned After Offering(3) | ||||||||||||
| Sio Capital Management, LLC | 2,599,640 | (4) | 1,438,202 | 1,161,438 | 9.99 | |||||||||||
PLAN OF DISTRIBUTION
We are registering thepurchasing shares of common stock in the open market. A naked short position is more likely to permitbe created if the resale of these shares of common stock (including shares of common stock issuable upon conversion or exercise of outstanding securities) by the holders thereof (and such holders’ successors and assigns) from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling stockholders of the shares of common stock. We will bear all fees and expenses incident to our obligation to register the shares of common stock.
The selling stockholders may sell all or a portion of the shares of common stock owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common stockunderwriter is concerned that there may be sold in one or more transactions at fixed prices, at prevailing market prices atdownward pressure on the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions,
If the selling stockholders effect such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the courseopen market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of hedging in positions they assume. The selling stockholders may also sell shares of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loanvarious bids for or pledge shares of common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may pledge or grant a security interest in some or allpurchases of the shares of common stock ownedmade by them and, if they defaultthe underwriters in the performanceopen market prior to the completion of their secured obligations, the pledgeesoffering.
The underwriter may also impose a penalty bid. This occurs when a particular underwriter repays to another underwriter a portion of the underwriting discount received by it because the representative has repurchased shares sold by or secured parties may offer and sellfor the account of such underwriter in stabilizing or short covering transactions.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above might have on our shares of common stock from time to time pursuant to this prospectusstock. Any of these activities may have the effect of preventing or any amendment to this prospectus under Rule 424(b)(3) or other applicable provisionretarding a decline in the market price of the Securities Act, amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate theour shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling owners for purposes of this prospectus.
The selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration statement, of which this prospectus forms a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. Regulation Mstock. They may also restrictcause the ability of any person engaged in the distributionprice of the shares of common stock to be higher than the price that would otherwise exist in the open market in the absence of these transactions. If an underwriter commences any of these transactions, it may discontinue them at any time without notice.
We expect that delivery of the shares will be made to investors on or about , 2023 (such settlement being referred to as “T+2”).
Electronic Distribution
In connection with the offering, the underwriter or any securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
The underwriter is a full service financial institution engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, principal investment, hedging, financing and brokerage activities. The underwriter and its affiliates may, from time to time, engage in market-makingtransactions with and perform services for us in the ordinary course of its business for which it may receive customary fees and reimbursement of expenses. In the ordinary course of its various business activities, withthe underwriter and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (which may include bank loans and/or credit default swaps) for its own account and for the accounts of its customers and may at any time hold long and short positions in such securities and instruments. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Sales Outside the United States
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of our common stock or accompanying warrants, or the possession, circulation or distribution of this prospectus or any other material relating to us or our common stock in any jurisdiction where action for that purpose is required. Accordingly, the shares of common stock. Allstock and accompanying warrants may not be offered or sold, directly or indirectly, and neither this prospectus nor any other offering material or advertisements in connection with our common stock and accompanying warrants may be distributed or published, in or from any country or jurisdiction, except in compliance with any applicable rules and regulations of any such country or jurisdiction.
The underwriter may arrange to sell the foregoing may affectcommon stock and accompanying warrants offered hereby in certain jurisdictions outside the marketabilityUnited States, either directly or through affiliates, where it is permitted to do so.
The validity of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
Once sold under the registration statement, of whichwarrants offered by this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
LEGAL MATTERS
Certain legal matters with respect to the shares of common stock offered hereby will behas been passed upon for us by Lucosky BrookmanBookman LLP, Woodbridge, New Jersey. McGuireWoods LLP is acting as counsel for the underwriters in connection with this offering.
The consolidated balance sheets of Modular Medical, Inc. as of March 31, 20212022 and March 31, 2020,2021, and the related consolidated statements of operations, changes in stockholders’ equity (deficit) and cash flows for the years then ended have been audited by Farber Hass Harley LLP, an independent registered public accounting firm, as stated in their report which is incorporated herein. Such consolidated financial statements have been incorporated herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1, including and schedules, under the Securities Act with respect tothat registers the shares of our common stock offered by this prospectus. This prospectus, which constitutes a partoffer and sale of the registration statement,securities to be sold in this offering. This prospectus does not contain all of the information set forthcontained in the registration statement someand the exhibits and schedules filed as part of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC.statement. For further information with respect to us and our common stock and warrants, we refer you to the registration statement includingand the exhibits and schedules filed as a part of the registration statement. Statements contained in this prospectus concerningas to the contents of any contract or any other document isare not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please seewe refer you to the copycopies of the contract or document that has been filed. Each statement isin this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The
You may read and copy all materials that we file with the SEC, maintains an Internetincluding the registration statement and its exhibits and schedules, on the website that containsmaintained by the SEC at www.sec.gov. Information contained on any website referenced in this prospectus does not and will not constitute a part of this prospectus or the registration statement on Form S-1 of which this prospectus is a part.
In addition, upon the closing of this offering, we will be subject to the information reporting requirements of the Exchange Act and we will file periodic reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and the website of the SEC referred to above. We also maintain a website at www.modular-medical.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The address ofinformation contained in, or that can be accessed through, our website is www.sec.gov.not a part of, and is not incorporated into, this prospectus. Additionally, you may request a copy of any of our filings with the SEC at no cost, by writing or telephoning us at the following address:
Attn.: CFO
Modular Medical, Inc.
10740 Thornmint Road
San Diego, California 92127
(858) 800-3500
You should rely only on the information contained in this prospectus or to which we have referred you. We have not and the underwriters have not authorized any person to provide you with different information or to make any representation not contained in this prospectus.
F-1
Modular Medical, Inc.
Condensed Consolidated Balance Sheets
| December 31, 2021 (Unaudited) | March 31, 2021 | December 31, 2022 (Unaudited) | March 31, 2022 | |||||||||||||
| ASSETS | ||||||||||||||||
| CURRENT ASSETS | ||||||||||||||||
| Cash and cash equivalents | $ | 204,098 | $ | 1,468,465 | $ | 7,690,957 | $ | 9,076,372 | ||||||||
| Prepaid expenses | 63,607 | 178,158 | ||||||||||||||
| Other current assets | 946 | 2,466 | ||||||||||||||
| Prepaid expenses and other | 180,164 | 313,422 | ||||||||||||||
| Security deposit | 100,000 | — | ||||||||||||||
| TOTAL CURRENT ASSETS | 268,651 | 1,649,089 | 7,971,121 | 9,389,794 | ||||||||||||
| Property and equipment, net | 241,468 | 298,958 | 716,409 | 235,959 | ||||||||||||
| Right of use asset, net | 141,720 | 200,124 | 51,312 | 120,693 | ||||||||||||
| Security deposit | 100,000 | 100,000 | — | 100,000 | ||||||||||||
| TOTAL NON-CURRENT ASSETS | 483,188 | 599,082 | 767,721 | 456,652 | ||||||||||||
| TOTAL ASSETS | $ | 751,839 | $ | 2,248,171 | $ | 8,738,842 | $ | 9,846,446 | ||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||||||
| CURRENT LIABILITIES | ||||||||||||||||
| Accounts payable | $ | 671,363 | $ | 169,284 | $ | 382,080 | $ | 299,951 | ||||||||
| Accrued expenses | 1,124,499 | 499,948 | 255,545 | 524,891 | ||||||||||||
| Short-term lease liability | 139,817 | 125,500 | 77,672 | 144,857 | ||||||||||||
| PPP note payable | — | 368,780 | ||||||||||||||
| Promissory note payable | 1,500,000 | — | ||||||||||||||
| Convertible notes payable | 5,485,583 | 2,133,453 | ||||||||||||||
| TOTAL CURRENT LIABILITIES | 8,921,262 | 3,296,965 | 715,297 | 969,699 | ||||||||||||
| LONG-TERM LIABILITIES | ||||||||||||||||
| Long-term lease liability | 77,212 | 184,355 | — | 39,957 | ||||||||||||
| Bonus payable | — | 42,000 | ||||||||||||||
| TOTAL LIABILITIES | 8,998,474 | 3,523,320 | 715,297 | 1,009,656 | ||||||||||||
| Commitments and Contingencies (Note 9) | ||||||||||||||||
| Commitments and Contingencies (Note 8) | ||||||||||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||||||||||
| Preferred Stock, $ par value, shares authorized, issued and outstanding | — | — | ||||||||||||||
| Common Stock, $ par value, shares authorized; and shares issued and outstanding as of December 31, 2021 and March 31, 2021, respectively | 6,374 | 6,302 | ||||||||||||||
| STOCKHOLDERS’ EQUITY | ||||||||||||||||
| Preferred Stock, $0.001 par value, 5,000,000 shares authorized, none issued and outstanding | — | — | ||||||||||||||
| Common Stock, $0.001 par value, 50,000,000 shares authorized; 10,932,098 and 10,461,898 shares issued and outstanding as of December 31, 2022 and March 31, 2022, respectively | 10,932 | 10,462 | ||||||||||||||
| Additional paid-in capital | 21,602,162 | 14,665,559 | 52,900,066 | 43,406,099 | ||||||||||||
| Common stock issuable | 149,994 | — | ||||||||||||||
| Accumulated deficit | (30,005,165 | ) | (15,947,010 | ) | (44,887,453 | ) | (34,579,771 | ) | ||||||||
| TOTAL STOCKHOLDERS’ DEFICIT | (8,246,635 | ) | (1,275,149 | ) | ||||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 751,839 | $ | 2,248,171 | ||||||||||||
| TOTAL STOCKHOLDERS’ EQUITY | 8,023,545 | 8,836,790 | ||||||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 8,738,842 | $ | 9,846,446 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-2
Condensed Consolidated Statements of Operations
(Unaudited)
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Operating expenses | ||||||||||||||||
| Research and development | $ | 1,849,399 | $ | 1,086,669 | $ | 5,742,911 | $ | 3,150,149 | ||||||||
| General and administrative | 1,981,665 | 783,898 | 5,156,152 | 2,453,808 | ||||||||||||
| Total operating expenses | 3,831,064 | 1,870,567 | 10,899,063 | 5,603,957 | ||||||||||||
| Loss from operations | (3,831,064 | ) | (1,870,567 | ) | (10,899,063 | ) | (5,603,957 | ) | ||||||||
| Other income | 4 | 22 | 368,876 | 126 | ||||||||||||
| Interest expense | (1,010,247 | ) | — | (2,204,917 | ) | — | ||||||||||
| Loss on debt extinguishment | — | — | (1,321,450 | ) | — | |||||||||||
| Loss before income taxes | (4,841,307 | ) | (1,870,545 | ) | (14,056,554 | ) | (5,603,831 | ) | ||||||||
| Provision for income taxes | — | — | 1,600 | 1,600 | ||||||||||||
| Net loss | $ | (4,841,307 | ) | $ | (1,870,545 | ) | $ | (14,058,155 | ) | $ | (5,605,431 | ) | ||||
| Net loss per share | ||||||||||||||||
| Basic and diluted | $ | (0.76 | ) | $ | (0.30 | ) | $ | (2.22 | ) | $ | (0.91 | ) | ||||
| Shares used in computing net loss per share | ||||||||||||||||
| Basic and diluted | 6,354,145 | 6,249,038 | 6,331,982 | 6,187,526 | ||||||||||||
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
| Operating expenses | ||||||||||||||||
| Research and development | $ | 2,196,546 | $ | 1,849,399 | $ | 6,804,069 | $ | 5,742,911 | ||||||||
| General and administrative | 1,161,351 | 1,981,665 | 3,502,029 | 5,156,152 | ||||||||||||
| Total operating expenses | 3,357,897 | 3,831,064 | 10,306,098 | 10,899,063 | ||||||||||||
| Loss from operations | (3,357,897 | ) | (3,831,064 | ) | (10,306,098 | ) | (10,899,063 | ) | ||||||||
| Other income (expense) | (587 | ) | 4 | 16 | 368,876 | |||||||||||
| Interest expense | — | (1,010,247 | ) | — | (2,204,917 | ) | ||||||||||
| Loss on debt extinguishment | — | — | — | (1,321,450 | ) | |||||||||||
| Loss before income taxes | (3,358,484 | ) | (4,841,307 | ) | (10,306,082 | ) | (14,056,554 | ) | ||||||||
| Provision for income taxes | — | — | 1,600 | 1,600 | ||||||||||||
| Net loss | $ | (3,358,484 | ) | $ | (4,841,307 | ) | $ | (10,307,682 | ) | $ | (14,058,154 | ) | ||||
| Net loss per share | ||||||||||||||||
| Basic and diluted | $ | (0.31 | ) | $ | (0.76 | ) | $ | (0.95 | ) | $ | (2.22 | ) | ||||
| Shares used in computing net loss per share | ||||||||||||||||
| Basic and diluted | 10,925,862 | 6,354,145 | 10,863,082 | 6,331,982 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-3
Condensed Consolidated Statements of Stockholders’ Equity (Deficit)
(Unaudited)
| Additional | Common | |||||||||||||||||||||||
| Common Stock | Paid-In | Stock | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Deficit | |||||||||||||||||||
| Balance as of March 31, 2021 | 6,302,050 | $ | 6,302 | $ | 14,665,559 | — | $ | (15,947,010 | ) | $ | (1,275,149 | ) | ||||||||||||
| Shares issued for services | 20,000 | 20 | 172,180 | — | — | 172,200 | ||||||||||||||||||
| Warrants issued with convertible notes | — | — | 3,700,632 | — | — | 3,700,632 | ||||||||||||||||||
| Stock-based compensation | 1,836 | 2 | 655,918 | — | — | 655,920 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,835,091 | ) | (4,835,091 | ) | ||||||||||||||||
| Balance as of June 30, 2021 | 6,323,886 | $ | 6,324 | $ | 19,194,289 | $ | — | $ | (20,782,101 | ) | $ | (1,581,488 | ) | |||||||||||
| Stock-based compensation | 3,635 | 4 | 862,427 | — | — | 862,431 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,381,757 | ) | (4,381,757 | ) | ||||||||||||||||
| Balance as of September 30, 2021 | 6,327,521 | $ | 6,328 | $ | 20,056,716 | $ | — | $ | (25,163,858 | ) | $ | (5,100,814 | ) | |||||||||||
| Private placement of common stock | 30,865 | 31 | 249,969 | — | — | 250,000 | ||||||||||||||||||
| Shares issued for services | 8,334 | 8 | 73,748 | — | — | 73,756 | ||||||||||||||||||
| Shares issuable for services | — | — | — | 149,994 | — | 149,994 | ||||||||||||||||||
| Shares issued for reverse stock split | 1,211 | 1 | — | — | — | 1 | ||||||||||||||||||
| Stock-based compensation | 5,775 | 6 | 1,221,729 | — | — | 1,221,735 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,841,307 | ) | (4,841,307 | ) | ||||||||||||||||
| Balance as of December 31, 2021 | 6,373,706 | $ | 6,374 | 21,602,162 | 149,994 | (30,005,165 | ) | (8,246,635 | ) | |||||||||||||||
| Additional | Common | Stockholders’ | ||||||||||||||||||||||
| Common Stock | Paid-In | Stock | Accumulated | Equity | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | (Deficit) | |||||||||||||||||||
| Balance as of March 31, 2020 | 5,956,754 | $ | 5,957 | $ | 10,517,505 | $ | 923,994 | $ | (8,569,034 | ) | $ | 2,878,422 | ||||||||||||
| Private placement of common stock | 243,299 | 243 | 2,042,385 | (923,994 | ) | — | 1,118,634 | |||||||||||||||||
| Stock-based compensation | — | — | 344,716 | — | — | 344,716 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,874,157 | ) | (1,874,157 | ) | ||||||||||||||||
| Balance as of June 30, 2020 | 6,200,053 | $ | 6,200 | $ | 12,904,606 | $ | — | $ | (10,443,191 | ) | $ | 2,467,615 | ||||||||||||
| Stock-based compensation | — | — | 300,604 | — | — | 300,604 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,860,729 | ) | (1,860,729 | ) | ||||||||||||||||
| Balance as of September 30, 2020 | 6,200,053 | $ | 6,200 | $ | 13,205,210 | $ | — | $ | (12,303,920 | ) | $ | 907,490 | ||||||||||||
| Private placement of common stock | 77,497 | 77 | 667,171 | — | — | 667,248 | ||||||||||||||||||
| Stock-based compensation | — | — | 295,054 | — | — | 295,054 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,870,545 | ) | (1,870,545 | ) | ||||||||||||||||
| Balance as of December 31, 2020 | 6,277,550 | $ | 6,277 | $ | 14,167,435 | $ | — | $ | (14,174,465 | ) | $ | (753 | ) | |||||||||||
| Common Stock | Additional Paid-In | Common Stock | Accumulated | Stockholders’ | |||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Equity | ||||||||||||||||||
| Balance as of March 31, 2022 | 10,461,898 | $ | 10,462 | $ | 43,406,099 | $ | — | $ | (34,579,771 | ) | $ | 8,836,790 | |||||||||||
| Shares issued for services | 348 | — | 1,576 | — | — | 1,576 | |||||||||||||||||
| Issuance of common stock and warrants in equity offering, net | 449,438 | 449 | 7,371,898 | — | — | 7,372,347 | |||||||||||||||||
| Issuance of common stock under equity incentive plan | 2,664 | 3 | 13,747 | — | — | 13,750 | |||||||||||||||||
| Stock-based compensation | — | — | 724,819 | — | — | 724,819 | |||||||||||||||||
| Net loss | — | — | — | — | (3,498,791 | ) | (3,498,791 | ) | |||||||||||||||
| Balance as of June 30, 2022 | 10,914,348 | $ | 10,914 | $ | 51,518,139 | $ | — | $ | (38,078,562 | ) | $ | 13,450,491 | |||||||||||
| Issuance of common stock under equity incentive plan | 11,375 | 12 | 50,368 | — | — | 50,380 | |||||||||||||||||
| Stock-based compensation | — | — | 692,060 | — | — | 692,060 | |||||||||||||||||
| Net loss | — | — | — | — | (3,450,407 | ) | (3,450,407 | ) | |||||||||||||||
| Balance as of September 30, 2022 | 10,925,723 | $ | 10,926 | $ | 52,260,567 | $ | — | $ | (41,528,969 | ) | $ | 10,742,524 | |||||||||||
| Issuance of common stock under equity incentive plan | 6,375 | 6 | 12,737 | — | — | 12,743 | |||||||||||||||||
| Stock-based compensation | — | — | 626,762 | — | — | 626,762 | |||||||||||||||||
| Net loss | — | — | — | — | (3,358,484 | ) | (3,358,484 | ) | |||||||||||||||
| Balance as of December 31, 2022 | 10,932,098 | $ | 10,932 | $ | 52,900,066 | $ | — | $ | (44,887,453 | ) | $ | 8,023,545 | |||||||||||
| Common Stock | Additional Paid-In | Common Stock | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Deficit | |||||||||||||||||||
| Balance as of March 31, 2021 | 6,302,050 | $ | 6,302 | $ | 14,665,559 | $ | — | $ | (15,947,010 | ) | $ | (1,275,149 | ) | |||||||||||
| Shares issued for services | 20,000 | 20 | 172,180 | — | — | 172,200 | ||||||||||||||||||
| Warrants issued with convertible notes | — | — | 3,700,632 | — | — | 3,700,632 | ||||||||||||||||||
| Issuance of common stock under equity incentive plan | 1,836 | 2 | 32,495 | — | — | 32,497 | ||||||||||||||||||
| Stock-based compensation | — | — | 623,423 | — | — | 623,423 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,835,091 | ) | (4,835,091 | ) | ||||||||||||||||
| Balance as of June 30, 2021 | 6,323,886 | $ | 6,324 | $ | 19,194,289 | $ | — | $ | (20,782,101 | ) | $ | (1,581,488 | ) | |||||||||||
| Stock-based compensation | 3,635 | 4 | 862,427 | — | — | 862,431 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,381,757 | ) | (4,381,757 | ) | ||||||||||||||||
| Balance as of September 30, 2021 | 6,327,521 | $ | 6,328 | $ | 20,056,716 | $ | — | $ | (25,163,858 | ) | $ | (5,100,814 | ) | |||||||||||
| Private placement of common stock | 30,865 | 31 | 249,969 | — | — | 250,000 | ||||||||||||||||||
| Shares issued for services | 8,334 | 8 | 73,748 | — | — | 73,756 | ||||||||||||||||||
| Shares issuable for services | — | — | — | 149,994 | — | 149,994 | ||||||||||||||||||
| Shares issued for reverse stock split | 1,211 | 1 | — | — | — | 1 | ||||||||||||||||||
| Stock-based compensation | 5,775 | 6 | 1,221,729 | — | — | 1,221,735 | ||||||||||||||||||
| Net loss | — | — | — | — | (4,841,307 | ) | (4,841,307 | ) | ||||||||||||||||
| Balance as of December 31, 2021 | 6,373,706 | $ | 6,374 | $ | 21,602,162 | $ | 149,994 | $ | (30,005,165 | ) | $ | (8,246,635 | ) | |||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-4
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| Nine Months Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Net loss | $ | (14,058,155 | ) | $ | (5,605,431 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain on PPP note forgiveness | (368,780 | ) | — | |||||
| Loss on debt extinguishment | 1,321,450 | — | ||||||
| Stock-based compensation expense | 2,740,086 | 940,374 | ||||||
| Depreciation and amortization | 80,268 | 82,016 | ||||||
| Shares issued for services | 388,021 | — | ||||||
| Shares issuable for services | 149,994 | — | ||||||
| Amortization of lease right-to-use asset | 58,404 | 52,994 | ||||||
| Change in lease liability | (92,826 | ) | 67,092 | |||||
| Amortization of debt discount | 1,454,762 | — | ||||||
| Changes in assets and liabilities: | ||||||||
| Prepaid expenses and other assets | (25,995 | ) | 15,964 | |||||
| Accounts payable and accrued expenses | 1,223,983 | (123,722 | ) | |||||
| Net cash used in operating activities | (7,128,787 | ) | (4,570,713 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (22,779 | ) | (109,541 | ) | ||||
| Net cash used in investing activities | (22,779 | ) | (109,541 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from private placement, net of issuance costs | 250,000 | 1,785,882 | ||||||
| Proceeds from issuance of convertible notes, net of placement fees | 4,137,199 | — | ||||||
| Proceeds from issuance of promissory note | 1,500,000 | — | ||||||
| Proceeds from issuance of PPP note payable | — | 368,780 | ||||||
| Net cash provided by financing activities | 5,887,199 | 2,154,662 | ||||||
| Net decrease in cash and cash equivalents | (1,264,367 | ) | (2,525,592 | ) | ||||
| Cash and cash equivalents at beginning of period | 1,468,465 | 3,122,134 | ||||||
| Cash and cash equivalents at end of period | $ | 204,098 | $ | 596,542 | ||||
| Supplemental disclosure: | ||||||||
| Noncash investing and financing activities: | ||||||||
| Fair value of detachable warrants issued with convertible notes | $ | 3,700,632 | $ | — | ||||
| Nine Months Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net loss | $ | (10,307,682 | ) | $ | (14,058,154 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain on PPP note forgiveness | — | (368,780 | ) | |||||
| Loss on debt extinguishment | — | 1,321,450 | ||||||
| Stock-based compensation expense | 2,120,513 | 2,740,086 | ||||||
| Depreciation and amortization | 92,616 | 80,268 | ||||||
| Shares issued for services | 150,412 | 388,021 | ||||||
| Shares issuable for services | — | 149,994 | ||||||
| Amortization of lease right-of-use asset | 69,381 | 58,404 | ||||||
| Change in lease liability | (107,142 | ) | (92,826 | ) | ||||
| Amortization of debt discount | — | 1,454,762 | ||||||
| Changes in assets and liabilities: | ||||||||
| Other assets and prepaid expenses | (15,577 | ) | (25,995 | ) | ||||
| Accounts payable and accrued expenses | (187,217 | ) | 1,223,983 | |||||
| Net cash used in operating activities | (8,184,696 | ) | (7,128,787 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (573,066 | ) | (22,779 | ) | ||||
| Net cash used in investing activities | (573,066 | ) | (22,779 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of common stock and warrants, net | 7,372,347 | — | ||||||
| Proceed from private placement, net of issuance costs | — | 250,000 | ||||||
| Proceeds from issuance of convertible notes, net | — | 4,137,199 | ||||||
| Proceeds from issuance of promissory note | — | 1,500,000 | ||||||
| Net cash provided by financing activities | 7,372,347 | 5,887,199 | ||||||
| Net decrease in cash and cash equivalents | (1,385,415 | ) | (1,264,367 | ) | ||||
| Cash and cash equivalents at beginning of period | 9,076,372 | 1,468,465 | ||||||
| Cash and cash equivalents at end of period | $ | 7,690,957 | $ | 204,098 | ||||
| Supplemental disclosure: | ||||||||
| Noncash investing and financing activities: | ||||||||
| Fair value of detachable warrants issued with convertible notes | $ | — | $ | 3,700,632 | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-5
F/K/A BEAR LAKE RECREATION, INC.
(UNAUDITED)
NOTE 1 – THE COMPANY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Modular Medical, Inc. (the Company) was incorporated in Nevada in October 1998 under the name Bear Lake Recreation, Inc. The Company had no material business operations from 2002 until approximately 2017 when it acquired all of the issued and outstanding shares of Quasuras, Inc., a Delaware corporation (Quasuras). As the major shareholderstockholder of Quasuras retained control of both the Company and Quasuras, the share exchange was accounted for as a reverse merger. As such, the Company recognized the assets and liabilities of Quasuras acquired in the merger at their historical carrying amounts. Prior to the acquisition of Quasuras and, since at least 2002, the Company was a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In June 2017, the Company changed its name from Bear Lake Recreation, Inc. to Modular Medical, Inc.
The Company is a development-stage medical-devicemedical device company focused on the design, development and eventual commercialization of an innovative insulin pump using modernizedto address shortcomings and problems represented by the relatively limited adoption of currently available pumps for insulin-dependent people with diabetes. The Company has developed a hardware technology allowing people with insulin-dependent diabetes to increase pump adoptionreceive their daily insulin in two ways, through a continuous “basal” delivery allowing a small amount of insulin to be in the diabetes marketplace. Throughblood at all times and a “bolus” delivery to address meal time glucose input and to address when the creation of a novel two-part, patch pump product,blood glucose level becomes excessively high. By addressing the time and effort required to effectively treat their condition, the Company seeks to fundamentally alterbelieves it can address the trade-offs between cost and complexity and access toless technically savvy, less motivated part of the higher standards of care that presently available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training and day-to-day use,market.
In February 2022, the Company seekscompleted a public offering of its equity securities, and its common stock was approved to expandlist on the wearable insulin delivery device market beyondNasdaq Capital Market under the highly motivated “super users”symbol “MODD” and expand the category into the mass market. The Company’s pump product seeks to serve both the type 1 and type 2 diabetes markets.began trading there on February 10, 2022.
Liquidity
Liquidity
Financial Accounting Standards Board (FASB) Accounting Standard Update (ASU) No. 2014-15 (ASU 2014-15), Going Concern, requires management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. If management identifies conditions or events that raise substantial doubt about an entity’s ability to continue as a going concern, management must consider if there are plans that are probable to be implemented, and whether it is probable that the plans will mitigate the conditions or events raising the substantial doubt about the entity’s ability to continue as a going concern. If the substantial doubt is not alleviated after consideration of management’s plans, the entity must include a statement in the notes to the financial statements indicating that there is substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued including: 1) the principal conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern, 2) management’s evaluation of the significance of those conditions or events in relation to the entity’s ability to meet its obligations, and 3) management’s plans to attempt to mitigate the conditions or events causing the substantial doubt about the entity’s ability to continue as a going concern.
The Company expects to continue to incur operating losses for the foreseeable future and incur cash outflows from operations as it continues to invest in the development and subsequent commercialization of its product. The Company expects that its research and development and general and administrative expenses will continue to increase, and, as a result, it will eventually need to generate significant product revenuesrevenue to achieve profitability. The Company’s expected operating losses and cash burn and the need to repay the convertible promissory notes and accrued interest in the first half of 2022 raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these financial statements are issued. Implementation of the Company’s plans and its ability to continue as a going concern will depend upon the Company’s ability to raise additional capital, through the sale of additional equity or debt securities, to support its future operations. There can be no assurance that such additional capital, whether in the form of debt or equity financing, will be sufficient or available and, if available, that such capital will be offered on terms and conditions acceptable to the Company. As disclosed in note 9, the Company recently sold shares of its common stock to two of its officers, obtained access to a credit facility and filed a registration statement to offer shares of its common stock.
F-6
The Company’s operating needs include the planned costs to operate its business, including amounts required to repay its convertible promissory notes (if not converted), fund working capital and capital expenditures. The Company’s future capital requirements and the adequacy of its available funds will depend on many factors, including the Company’s ability to successfully commercialize its product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement its product offering. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash. These condensed consolidated financial statements do not include any adjustments that might result from this uncertainty.
Basis of Presentation
The Company’s fiscal year ends on March 31 of each calendar year. Each reference to a fiscal year in these notes to the condensed consolidated financial statements refers to the fiscal year ended March 31 of the calendar year indicated (for example, fiscal 20222023 refers to the fiscal year ending March 31, 2022)2023). The condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Quasuras. All significant intercompany transactions and balances have been eliminated in consolidation.
The accompanying condensed consolidated financial statements of the Companyare unaudited and have been prepared without audit.in accordance with generally accepted accounting principles in the United States (GAAP) and with the rules and regulations of the United States Security and Exchange Commission (SEC) regarding interim financial reporting. The condensed consolidated balance sheet as of March 31, 20212022 has been derived from the audited consolidated financial statements at that date. Certain information and disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States (GAAP)GAAP have been condensed or omitted in accordance with these rules and regulations of the SecuritiesSEC. The information in this report should be read in conjunction with the Company’s consolidated financial statements and Exchange Commission (SEC).notes thereto included in its most recent annual report on Form 10-K filed with the SEC.
In the opinion of management, the accompanying unaudited condensed consolidated financial statements reflect all adjustments (consisting only of normal recurring adjustments) necessary to summarize fairly the Company’s financial position, results of operations and cash flows for the interim periods presented. The operating results for the three months ended December 31, 20212022 are not necessarily indicative of the results that may be expected for the year ending March 31, 20222023 or for any other future period.
Reverse Stock Split
On November 24, 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Nevada to effect a 1-for-3 reverse stock split of the Company’s shares of common stock. Such amendment and ratio were previously approved by a majority of the Company’s stockholders and the board of directors. As a result of the reverse stock split, which was effective November 29, 2021, every three shares of the Company’s pre-reverse split outstanding common stock were combined and reclassified into one share of common stock. Proportionate voting rights and other rights of common stock holders were not affected by the reverse stock split. Any fractional shares of common stock resulting from the reverse split were rounded up to the nearest whole share. All stock options outstanding and common stock reserved for issuance under the Company’s equity incentive plans and warrants outstanding immediately prior to the reverse stock split were adjusted by dividing the number of affected shares of common stock by three and, as applicable, multiplying the exercise price by three, as a result of the reverse stock split. All share numbers, share prices, exercise prices and per share amounts have been adjusted, on a retroactive basis to reflect this 1-for-3 reverse stock split.
Use of Estimates
The preparation of the accompanying condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Estimates may include those pertaining to accruals, stock-based compensation and income taxes. Actual results could differ from those estimates.
F-7
Reportable Segment
The Company operates in one business segment and uses one measurement of profitability for its business.
Research and Development
The Company expenses research and development expenditures as incurred.
General and Administrative
General and administrative expenses consist primarily of payroll and benefit costs, rent, stock-based compensation, legal and accounting fees, and office and other administrative expenses.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of cash. The Company maintains its cash at high-quality financial institutions within the United States, which are insured by the Federal Deposit Insurance Corporation up to limits of approximately $250,000. No reserve has been made in the financial statements for any possible loss due to financial institution failure.
Risks and Uncertainties
The Company is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history and the volatility of public markets.
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in- place” and created significant disruption of the financial markets. The full extent of the COVID-19 impact on the Company’s operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of the Company’s control, and cannot be predicted.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and cash in demand deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Property and Equipment
Property and equipment are recorded at historical cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally three to five years. Depreciation is recorded in operating expenses in the consolidated statements of operations. Leasehold improvements and assets acquired through capital leases are amortized over the shorter of their estimated useful life or the lease term, and amortization is recorded in operating expenses in the consolidated statements of operations. Construction-in-process includes machinery and equipment and is stated at cost and not depreciated. Depreciation on construction-in-process commences when the assets are ready for their intended use.
Fixed assets comprised:
| December 31, | March 31, | |||||||
| 2022 | 2022 | |||||||
| Machinery and equipment | $ | 487,855 | $ | 346,358 | ||||
| Construction-in-process | 427,670 | — | ||||||
| Leasehold improvements | 139,197 | 139,197 | ||||||
| Total property and equipment | 1,054,722 | 485,555 | ||||||
| Less: accumulated depreciation and amortization | (338,313 | ) | (249,596 | ) | ||||
| Total property and equipment, net | $ | 716,409 | $ | 235,959 | ||||
F-8
Fair Value of Financial Instruments
The Company measures the fair value of financial instruments using a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels:
| ● | Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets. |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
Due to their short-term nature, the carrying values of cash equivalents, accounts payable and accrued expenses, approximate fair value.
Right-of-Use Asset
The Company’s right-of-use assets consist of leased assets recognized in accordance with FASB Accounting Standards Codification (ASC) No. 842, Leases which requires lessees to recognize a lease liability and a corresponding lease asset for virtually all lease contracts. Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and the lease liability represents the Company’s obligation to make lease payments arising from the lease, both of which are recognized based on the present value of the future minimum lease payments over the lease term at the commencement date. Leases with a lease term of 12 months or less at inception are not recorded on the condensed consolidated balance sheets and are expensed on a straight-line basis over the lease term in the condensed consolidated statement of operations and comprehensive loss. The Company determines the lease term by agreement with the lessor. In cases where the lease does not provide an implicit interest rate, the Company uses the Company’s incremental borrowing rate based on the information available at commencement date in determining the present value of future payments.
Stock-Based Compensation
The Company recognizes stock-based compensation for stock options granted to employees and non-employees on a straight-line basis over the requisite service period, usually the vesting period, based on the grant-date fair value. The Company estimates the value of stock options on the date of grant using the Black-Scholes pricing model. The determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the option price, as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards, and projected stock option exercise behaviors.
Per-Share Amounts
Basic net loss per share is computed by dividing the net loss for the period by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share gives effect to all potentially dilutive common shares outstanding during the period. Potentially dilutive common shares consist of incremental shares of common stock issuable upon the exercise of stock options and warrants.
For the nine months ended December 31, 2022 and 2021, the following table sets forth securities outstanding which were excluded from the computation of diluted net loss per share as their inclusion would be anti-dilutive.
| Nine Months Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Options to purchase common stock | 2,174,198 | 1,967,188 | ||||||
| Common stock warrants | 7,565,588 | 767,796 | ||||||
| Total | 9,739,786 | 2,734,984 | ||||||
F-9
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations or cash flows.
Comprehensive Loss
Comprehensive loss represents the changes in equity of an enterprise, other than those resulting from stockholder transactions. Accordingly, comprehensive loss may include certain changes in equity that are excluded from net loss. For the three and nine months ended December 31, 2022 and 2021, the Company’s comprehensive loss was the same as its net loss.
Recently Issued Accounting Pronouncement
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses. This ASU added a new impairment model (known as the current expected credit loss (CECL) model) that is based on expected losses rather than incurred losses. Under the new guidance, an entity recognizes an allowance for its estimate of expected credit losses and applies to most debt instruments, trade receivables, lease receivables, financial guarantee contracts, and other loan commitments. The CECL model does not have a minimum threshold for recognition of impairment losses and entities will need to measure expected credit losses on assets that have a low risk of loss. This update is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years for smaller reporting companies. The adoption of this ASU is not expected to have a material impact on the Company’s results of operations and financial position.
NOTE 2 – LEASES
The Company accounts for the lease of its corporate facility in San Diego, California in accordance with ASC No. 842. The 39- month lease term commenced April 1, 2020, and the lease provides for an initial monthly rent of approximately $12,400 with annual rent increases of approximately 3%. In addition to the minimum lease payments, the Company is responsible for property taxes, insurance and certain other operating costs. The right-to-use asset and corresponding liability for the facility lease have been measured at the present value of the future minimum lease payments. A discount rate of 11%, which approximated the Company’s incremental borrowing rate, was used to measure the lease asset and liability. Lease expense is recognized on a straight-line basis over the lease term.
The Company obtained a right-of-use asset of $270,950 in exchange for its obligations under the operating lease. The landlord also provided a lease incentive of approximately $139,000, which was paid to the Company in June 2020, for the Company to make improvements to the leased space. In addition, the Company paid a $100,000 security deposit.
Future minimum payments under the facility operating lease, as of December 31, 2022, are listed in the table below.
| Annual Fiscal Years | Operating Lease | |||
| 2023 | 39,507 | |||
| 2024 | 40,692 | |||
| Less: | ||||
| Imputed interest | (2,527 | ) | ||
| Present value of lease liabilities | $ | 77,672 | ||
Cash paid for amounts included in the measurement of lease liabilities was $118,521 for the nine months ended December 31, 2022. Rent expense was $80,698 for each of the nine-month periods ended December 31, 2022 and 2021 and $26,930 for each of the three-month periods ended December 31, 2022 and 2021.
F-10
NOTE 3 – PPP NOTE
On April 24, 2020, the Company received a $368,780 unsecured loan (the PPP Note) under the Paycheck Protection Program (the PPP), which was established under the U.S. government’s Coronavirus Aid, Relief, and Economic Security Act (the CARES Act). The PPP Note to the Company was made through Silicon Valley Bank (the Lender), and the Company entered into a U.S. Small Business Administration Paycheck Protection Program Note (the Agreement) with the Lender evidencing the PPP Note. The full amount of the PPP Note was due in April 2022 and interest accrued on the outstanding principal balance of the PPP Note at a fixed rate of 1.0% per annum, which was deferred for 10 months after the covered period during which the Company used the proceeds.
In May 2021, the Lender and the U.S. Small Business Administration notified the Company that the outstanding principal and accrued interest for the PPP Note was forgiven in full. The Company accounted for the forgiveness of the PPP Note in accordance with ASC Topic 470: Debt (ASC 470), and the amount forgiven was recorded as a gain on extinguishment and recognized in the other income line of the consolidated statement of operations.
NOTE 4 – CONVERTIBLE PROMISSORY NOTES
From February through April 2021, the Company sold $2,310,000 of convertible promissory notes (each an Original Note and, collectively, the Original Notes), at par in a private placement transaction effected pursuant to an exemption from the registration requirements under the Securities Act of 1933, as amended. Effective April 30, 2021, pursuant to a revocation and replacement agreement between each holder of an Original Note and the Company, the $2,310,000 of Original Notes and accrued interest thereon as of April 30, 2021 were replaced with $2,360,550 aggregate principal amount of Notes and 2021 Warrants (as defined below). The Company accounted for the replacement of the Original Notes in accordance with ASC 470 and recorded a loss on extinguishment of $1,321,450 and interest expense of $70,647 for unamortized debt issuance costs as of April 30, 2021.
In April and May 2021, pursuant to a securities purchase agreement by and between the Company and each investor (the SPA), the Company sold to investors $4,250,000 aggregate principal amount of convertible promissory notes (the Notes) and warrants to purchase shares of its common stock (the 2021 Warrants). The Notes were unsecured obligations of the Company with each Note having a stated maturity date of 12 months from its issue date and accrued interest at a rate of 12% per annum, payable on maturity. If the Company completed an offering of its common stock or other securities in excess of $12,000,000 of gross proceeds (a Qualified Capital Raise, as defined in the Notes), each Note holder would be required to convert its Adjusted Note Amount (as defined below) into the securities of such Qualified Capital Raise. Adjusted Note Amount equals the product of (i) the sum of all outstanding principal plus accrued interest on a Note, multiplied by (ii) 1.25.
In connection with the issuance of the Notes, the Company issued the 2021 Warrants to purchase in the aggregate 767,796 shares of its common stock at an initial exercise price of $24.00 per share. The fair value of the 2021 Warrants was $3,700,632, of which $2,379,182 was recorded as a debt discount and amortized to interest expense, and $1,321,450 was recorded as a loss on debt extinguishment. The Company calculated the fair value of the Warrants utilizing the Black-Scholes valuation model with the following assumptions: volatility of 88.98%, risk-free interest rate of 0.86%, a term of 5.75 years and a dividend yield of zero.
Upon the closing of a public offering in February 2022, which was a Qualified Capital Raise, in accordance with their terms, the Notes converted into 1,511,276 shares of common stock and the holders of the Notes received an additional 1,511,276 common stock purchase warrants with an exercise price of $6.60 per share. In addition, as a result of the February 2022 equity offering, the exercise price of the 767,796 outstanding 2021 Warrants was reduced to $6.00 per share.
F-11
NOTE 5 – STOCKHOLDERS’ EQUITY (DEFICIT)
Placements of Common Stock and Warrants
On May 2, 2022, the Company entered into a securities purchase agreement (the Purchase Agreement) with an institutional investor, pursuant to which the Company sold, in a registered direct offering (the Registered Offering), which closed on May 5, 2022, an aggregate of 449,438 shares (the Shares) of the Company’s common stock, par value $0.001 per share, at a purchase price per Share of $4.45 and pre-funded warrants (the Pre-Funded Warrants) to purchase an aggregate of 1,348,314 shares of common stock at a purchase price per Pre-Funded Warrant of $4.44. The Pre-Funded Warrants will be exercisable immediately on the date of issuance at an exercise price of $0.01 per share and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
In a concurrent private placement under the Purchase Agreement, the Company issued to the Investor warrants (the Private Placement Warrants) to purchase an aggregate of 1,438,202 shares of common stock at an exercise price of $6.60 per share. The Private Placement Warrants will be exercisable beginning on the six-month anniversary of the date of issuance (the Initial Exercise Date) and will expire on the five-year anniversary of the Initial Exercise Date.
Warrants
As of December 31, 2022, the Company had the following warrants outstanding:
| Type | Number of Shares | Exercise Price | Expiration Date | ||||||||
| Common stock | 1,348,314 | $ | 0.01 | — | |||||||
| Common stock | 767,796 | $ | 6.00 | January - February 2027 | |||||||
| Common stock | 4,011,276 | $ | 6.60 | February 2027 | |||||||
| Common stock | 1,438,202 | $ | 6.60 | November 2027 | |||||||
| Total | 7,565,588 | ||||||||||
Other
During the nine months ended December 31, 2022 and 2021, the Company issued 348 and 28,334 shares of common stock, respectively, with a fair value of approximately $1,576 and $245,956, respectively, to service providers.
NOTE 6 – STOCK-BASED COMPENSATION
Amended 2017 Equity Incentive Plan
In October 2017, the Company’s board of directors (the Board) approved the 2017 Equity Incentive Plan (the Plan), as amended, with 1,000,000 shares of common stock reserved for issuance. In January 2020 and August 2021, the Board approved increases in the number of shares reserved for issuance under the Plan by 333,334 and 1,333,334 shares, respectively. Under the Plan, eligible employees, directors and consultants may be granted a broad range of awards, including stock options, stock appreciation rights, restricted stock, performance-based awards and restricted stock units. The Plan is administered by the Board or, in the alternative, a committee designated by the Board.
Stock-Based Compensation Expense
The expense relating to stock options is recognized on a straight-line basis over the requisite service period, usually the vesting period, based on the grant date fair value. As of December 31, 2022, the unamortized compensation cost was $3,443,902 related to stock options and is expected to be recognized as expense over a weighted-average period of approximately 2.07 years.
F-12
During the three and nine months ended December 31, 2022, the Company awarded 6,375 and 20,414 shares, respectively, to members of the Board in accordance with the compensation plan for non-employee directors. During the nine months ended December 31, 2022, the Company granted options with 10-year terms to purchase 677,199 shares of its common stock to employees, directors and consultants. The fair value of the options granted and shares awarded was $2,503,979. The following assumptions were used in the fair value calculations of the options granted:
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
| Risk-free interest rates | 3.93% - 3.99 | % | 1.26% - 1.36 | % | 2.82% - 4.06 | % | 0.8% - 1.36 | % | ||||||||
| Volatility | 149 | % | 197% - 253 | % | 149% - 223 | % | 89% - 370 | % | ||||||||
| Expected life (years) | 5.0 - 5.7 | 5.0 - 6.0 | 5.0 - 5.7 | 5.0 - 6.2 | ||||||||||||
The fair values of options at the grant date were estimated utilizing the Black-Scholes valuation model, which includes simplified methods to establish the fair term of options, as well as average volatility. The risk-free interest rate was derived from the Daily Treasury Yield Curve Rates, as published by the U.S. Department of the Treasury as of the grant date for terms equal to the expected terms of the options. A dividend yield of zero was applied because the Company has never paid dividends and has no intention to pay dividends in the foreseeable future. The Company accounts for forfeitures as they occur.
A summary of stock option activity under the Plan is presented below:
| Options Outstanding | ||||||||||||
| Shares Available for Grant | Number of Shares | Weighted Average Exercise Prices | ||||||||||
| Balance at March 31, 2022 | 989,466 | 1,650,705 | $ | 6.58 | ||||||||
| Options granted | (265,634 | ) | 265,634 | 4.35 | ||||||||
| Share awards | (2,664 | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | 96,668 | (96,668 | ) | 7.69 | ||||||||
| Balance at June 30, 2022 | 817,836 | 1,819,671 | 6.19 | |||||||||
| Options granted | (241,023 | ) | 241,023 | 4.35 | ||||||||
| Share awards | (11,375 | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | 30,444 | (30,444 | ) | 4.67 | ||||||||
| Balance at September 30, 2022 | 595,882 | 2,030,250 | 6.00 | |||||||||
| Options granted | (170,542 | ) | 170,542 | 2.00 | ||||||||
| Share awards | (6,375 | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | 26,594 | (26,594 | ) | 5.82 | ||||||||
| Balance at December 31, 2022 | 445,559 | 2,174,198 | $ | 5.69 | ||||||||
There were no stock options exercised during the nine months ended December 31, 2022 and 2021.
F-13
The following table summarizes the range of outstanding and exercisable options as of December 31, 2022:
| Options Outstanding | Options Exercisable | ||||||||||||||||||||
| Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (in Years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | Aggregate Intrinsic value | |||||||||||||||
| $1.98 - $17.70 | 2,174,198 | 7.94 | $ | 5.69 | 1,360,318 | $ | 5.87 | $ | 8,894 | ||||||||||||
The intrinsic value per share is calculated as the excess of the closing price of the common stock on the Company’s principal trading market over the exercise price of the option.
The Company is required to present the tax benefits resulting from tax deductions in excess of the compensation cost recognized from the exercise of stock options as financing cash flows in the consolidated statements of cash flows. For the nine months ended December 31, 2022 and 2021, there were no such tax benefits associated with the exercise of stock options, as no stock options were exercised.
NOTE 7 – INCOME TAXES
The Company determines deferred tax assets and liabilities based upon the differences between the financial statement and tax bases of the Company’s assets and liabilities using tax rates in effect for the year in which the Company expects the differences to affect taxable income. A valuation allowance is established for any deferred tax assets for which it is more likely than not that all or a portion of the deferred tax assets will not be realized. Based on the available information and other factors, management believes it is more likely than not that its federal and state net deferred tax assets will not be fully realized, and the Company has recorded a full valuation allowance.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations. All tax returns for fiscal 2016 to fiscal 2022 may be subject to examination by the U.S. federal and state tax authorities. As of December 31, 2022, the Company has not recorded any liability for unrecognized tax benefits related to uncertain tax positions.
NOTE 8 – COMMITMENTS & CONTINGENCIES
Litigations, Claims and Assessments
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. The Company records legal costs associated with loss contingencies as incurred and accrues for all probable and estimable settlements.
F-14
Indemnification
In the ordinary course of business, the Company enters into contractual arrangements under which it may agree to indemnify the counterparties from any losses incurred relating to breach of representations and warranties, failure to perform certain covenants, or claims and losses arising from certain events as outlined within the particular contract, which may include, for example, losses arising from litigation or claims relating to past performance. Such indemnification clauses may not be subject to maximum loss clauses. The Company has also entered into indemnification agreements with its officers and directors. No amounts were reflected in the Company’s consolidated financial statements for the nine months ended December 31, 2022 and 2021 related to these indemnifications. The Company has not estimated the maximum potential amount of indemnification liability under these agreements due to the limited history of prior claims and the unique facts and circumstances applicable to each particular agreement. To date, the Company has not made any payments related to these indemnification agreements, and no claims for payment have been made under such agreements.
Purchase Obligations
The Company’s primary purchase obligations include purchase orders for machinery and equipment. At December 31, 2022, the Company had outstanding purchase orders for machinery and equipment and related expenditures of approximately $735,000.
NOTE 9 – SUBSEQUENT EVENTS
New Lease Agreement
On January 5, 2023, the Company entered into a lease agreement (the Thornhill Lease) with Michael Summers (the Lessor) for a new headquarters facility pursuant to which the Company will lease approximately 24,000 square feet of a building located in San Diego, California, commencing on or about February 1, 2023. The monthly base rent is $36,000 for the first 12 months of the lease and will increase by 4% of the prior year’s base rent at the beginning of each 12-month period thereafter. The lease term is 48 months.
Under the Thornhill Lease, the Company will pay the Lessor a monthly fee for its pro-rated share of specified common area charges, including maintenance costs, property taxes and insurance, in addition to base rent. The monthly fee for the common area charges is approximately $10,700 and will be adjusted based on actual costs incurred by the Lessor.
Amended 2017 Equity Incentive Plan
In January 2023, the Company’s stockholders approved an increase in the number of shares reserved for issuance under the Plan by 2,000,000 shares.
F-15
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee and
Stockholders of Modular Medical, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Modular Medical, Inc. (the “Company”) as of March 31, 2022 and 2021, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
F-16
Going Concern
As described further in Note 1 to the financial statements, the Company has incurred losses since inception, and expects to continue to incur operating losses for the foreseeable future and incur cash outflows from operations as it continues to invest in the development and subsequent commercialization of its product. The Company expects that its research and development and general and administrative expenses will continue to increase, and, as a result, it will eventually need to generate significant product revenues to achieve profitability. As of March 31, 2022, the Company had cash balances of approximately $9,076,000, as a result of the capital raised in the public offering in February 2022. In addition, subsequent to March 31, 2022, the Company raised net proceeds from an equity offering of approximately $7,372,000. The Company has concluded that these plans alleviate the doubt related to its ability to continue as a going concern.
We identified management’s assessment of the Company’s ability to continue as a going concern as a critical audit matter due to inherent complexities and uncertainties related to the Company’s projections of operations. Auditing management’s going concern assessment involved a high degree of auditor judgment and audit effort due to the impact of these assumptions on the determination of the degree of doubt regarding the ability of the entity to continue as a going concern. The primary procedures we performed to address this critical audit matter included:
| ● | We evaluated the reasonableness of key assumptions underlying management’s conclusion. |
| ● | We evaluated that the disclosures included in the Form 10-K were complete and accurate and in accordance with accounting principles generally accepted in the United States of America. |
| ● | We evaluated the impact of the Company’s existing financing arrangements and future capital needs over the next 12 months on its ability to continue as a going concern. |
Stock Based Compensation
As discussed in Note 8, during the year ended March 31, 2022, the Company granted 827,427 options to purchase shares of its common stock with 10-year terms and a grant-date fair value of $8,507,311 to employees, directors and consultants. Management is required to analyze the fair value of each option granted and amortize it over its vesting period.
We identified the grant of stock options as a critical audit matter. Management’s estimates regarding fair value of options result in the application of a high degree of auditor judgment.
The primary procedures we performed to address this critical audit matter included the following:
| ● | We gained an understanding of Company’s processes and controls in place for determining the fair value of each granted option. |
| ● | We evaluated the option price model the management selected to determine the fair value, and analyzed the underlying data used in the calculations. |
| ● | We also recalculated the fair value of each option granted. |
/s/ Farber Hass Hurley LLP
Firm Id 223
We have served as the Company’s auditor since 2018.
Chatsworth, California
June 28, 2022
F-17
Consolidated Balance Sheets
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 9,076,372 | $ | 1,468,465 | ||||
| Prepaid expenses | 312,464 | 178,158 | ||||||
| Other current assets | 958 | 2,466 | ||||||
| TOTAL CURRENT ASSETS | 9,389,794 | 1,649,089 | ||||||
| Property and equipment, net | 235,959 | 298,958 | ||||||
| Right of use asset, net | 120,693 | 200,124 | ||||||
| Security deposit | 100,000 | 100,000 | ||||||
| TOTAL NON-CURRENT ASSETS | 456,652 | 599,082 | ||||||
| TOTAL ASSETS | $ | 9,846,446 | $ | 2,248,171 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | 299,951 | $ | 169,284 | ||||
| Accrued expenses | 524,891 | 499,948 | ||||||
| Short-term lease liability | 144,857 | 125,500 | ||||||
| PPP note payable | — | 368,780 | ||||||
| Convertible notes payable | — | 2,133,453 | ||||||
| TOTAL CURRENT LIABILITIES | 969,699 | 3,296,965 | ||||||
| Long-term lease liability | 39,957 | 184,355 | ||||||
| Bonus payable | — | 42,000 | ||||||
| TOTAL LIABILITIES | 1,009,656 | 3,523,320 | ||||||
| Commitments and Contingencies (Note 11) | ||||||||
| STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Preferred Stock, $0.001 par value, 5,000,000 shares authorized, none issued and outstanding | — | — | ||||||
| Common Stock, $0.001 par value, 50,000,000 shares authorized, 10,461,898 shares and 6,302,050 shares issued and outstanding as of March 31, 2022 and 2021, respectively | 10,462 | 6,302 | ||||||
| Additional paid-in capital | 43,406,099 | 14,665,559 | ||||||
| Accumulated deficit | (34,579,771 | ) | (15,947,010 | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | 8,836,790 | (1,275,149 | ) | |||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | 9,846,446 | $ | 2,248,171 | ||||
The accompanying notes are an integral part of these audited consolidated financial statements.
F-18
Consolidated Statements of Operations
| Twelve Months Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating expenses | ||||||||
| Research and development | $ | 7,729,240 | $ | 4,083,303 | ||||
| General and administrative | 7,197,162 | 3,253,412 | ||||||
| Total operating expenses | 14,926,402 | 7,336,715 | ||||||
| Loss from operations | (14,926,402 | ) | (7,336,715 | ) | ||||
| Other income | 368,920 | 130 | ||||||
| Interest expense | (2,752,229 | ) | (39,791 | ) | ||||
| Loss on debt extinguishment | (1,321,450 | ) | — | |||||
| Loss before income taxes | (18,631,161 | ) | (7,376,376 | ) | ||||
| Provision for income taxes | 1,600 | 1,600 | ||||||
| Net loss | $ | (18,632,761 | ) | $ | (7,377,976 | ) | ||
| Net loss per share | ||||||||
| Basic and diluted | $ | (2.74 | ) | $ | (1.20 | ) | ||
| Shares used in computing net loss per share | ||||||||
| Basic and diluted | 6,807,710 | 6,211,562 | ||||||
The accompanying notes are an integral part of these audited consolidated financial statements.
F-19
Consolidated Statements of Stockholders’ Equity (Deficit)
| Common Stock | Additional Paid-In | Common Stock | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Equity (Deficit) | |||||||||||||||||||
| Balance as of March 31, 2020 | 5,956,754 | $ | 5,957 | $ | 10,517,505 | $ | 923,994 | $ | (8,569,034 | ) | $ | 2,878,422 | ||||||||||||
| Placement of common stock | 320,796 | 321 | 2,709,555 | (923,994 | ) | — | 1,785,882 | |||||||||||||||||
| Shares issued for services | 24,500 | 24 | 210,921 | — | — | 210,945 | ||||||||||||||||||
| Stock-based compensation | — | — | 1,227,578 | — | — | 1,227,578 | ||||||||||||||||||
| Net loss | — | — | — | — | (7,377,976 | ) | (7,377,976 | ) | ||||||||||||||||
| Balance as of March 31, 2021 | 6,302,050 | $ | 6,302 | $ | 14,665,559 | $ | — | $ | (15,947,010 | ) | $ | (1,275,149 | ) | |||||||||||
| Issuance of common stock upon public offering, net of issuance costs | 2,500,000 | 2,500 | 13,657,500 | — | — | 13,660,000 | ||||||||||||||||||
| Issuance of common stock in settlement of convertible notes and accrued interest | 1,511,276 | 1,511 | 6,506,254 | — | — | 6,507,765 | ||||||||||||||||||
| Placement of common stock | 30,864 | 31 | 249,969 | — | — | 250,000 | ||||||||||||||||||
| Warrants issued with convertible notes | — | — | 3,700,632 | — | — | 3,700,632 | ||||||||||||||||||
| Shares issued for services | 90,000 | 90 | 594,310 | — | — | 594,400 | ||||||||||||||||||
| Shares issued for reverse stock split | 1,211 | 1 | (1 | ) | — | — | — | |||||||||||||||||
| Issuance of common stock under equity incentive plan | 26,497 | 27 | 172,091 | — | — | 172,118 | ||||||||||||||||||
| Stock-based compensation | — | — | 3,859,785 | — | — | 3,859,785 | ||||||||||||||||||
| Net loss | — | — | — | — | (18,632,761 | ) | (18,632,761 | ) | ||||||||||||||||
| Balance as of March 31, 2022 | 10,461,898 | $ | 10,462 | $ | 43,406,099 | $ | — | $ | (34,579,771 | ) | $ | 8,836,790 | ||||||||||||
The accompanying notes are an integral part of these audited consolidated financial statements.
F-20
Consolidated Statements of Cash Flows
| Year ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash Flows from operating activities | ||||||||
| Net loss | $ | (18,632,761 | ) | $ | (7,377,976 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain on PPP note forgiveness | (368,780 | ) | — | |||||
| Loss on debt extinguishment | 1,321,450 | — | ||||||
| Stock-based compensation expense | 4,031,902 | 1,227,578 | ||||||
| Depreciation and amortization | 117,490 | 111,015 | ||||||
| Accrued interest | 666,338 | — | ||||||
| Shares issued for services | 395,950 | 68,880 | ||||||
| Amortization of lease right-of-use asset | 79,431 | 70,826 | ||||||
| Change in lease liability | (125,040 | ) | 38,905 | |||||
| Amortization of debt issuance costs | 1,833,618 | 12,253 | ||||||
| Other | 274 | 1,004 | ||||||
| Changes in assets and liabilities: | ||||||||
| Other assets and prepaid expenses | 65,652 | 25,600 | ||||||
| Accounts payable and accrued expenses | 354,948 | (86,747 | ) | |||||
| Net cash used in operating activities | (10,259,528 | ) | (5,908,662 | ) | ||||
| Cash flows from investing activities | ||||||||
| Purchases of property and equipment | (54,764 | ) | (109,669 | ) | ||||
| Net cash used in investing activities | (54,764 | ) | (109,669 | ) | ||||
| Cash flows from financing activities | ||||||||
| Proceeds from private placements, net of issuance costs | 250,000 | 1,785,882 | ||||||
| Proceeds from issuance of convertible notes, net of placement fees | 4,137,199 | 2,210,000 | ||||||
| Proceeds from issuance of promissory note | 2,100,000 | — | ||||||
| Repayment of promissory note | (2,100,000 | ) | — | |||||
| Proceeds from issuance of PPP note payable | — | 368,780 | ||||||
| Proceeds from issuance of common stock upon public offering, net of issuance costs | 13,535,000 | — | ||||||
| Net cash provided by financing activities | 17,922,199 | 4,364,662 | ||||||
| Net increase (decrease) in cash and cash equivalents | 7,607,907 | (1,653,669 | ) | |||||
| Cash and cash equivalents, at beginning of year | 1,468,465 | 3,122,134 | ||||||
| Cash and cash equivalents, at end of year | $ | 9,076,372 | $ | 1,468,465 | ||||
| Supplemental disclosure: | ||||||||
| Noncash investing and financing activities: | ||||||||
| Fair value of detachable warrants issued with convertible notes | $ | 3,700,632 | — | |||||
| Conversion of convertible notes and accrued interest into common stock | $ | 7,253,876 | — | |||||
| Cash paid for: | ||||||||
| Income taxes | $ | 1,600 | $ | 1,600 | ||||
| Interest paid | $ | 252,000 | — | |||||
The accompanying notes are an integral part of these audited consolidated financial statements.
F-21
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – THE COMPANY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Modular Medical, Inc. (the Company) was incorporated in Nevada in October 1998 under the name Bear Lake Recreation, Inc. The Company had no material business operations from 2002 until approximately 2017 when it acquired all of the issued and outstanding shares of Quasuras, Inc., a Delaware corporation (Quasuras). As the major stockholder of Quasuras retained control of both the Company and Quasuras, the share exchange was accounted for as a reverse merger. As such, the Company recognized the assets and liabilities of Quasuras, acquired in the merger, at their historical carrying amounts. Prior to the acquisition of Quasuras and, since at least 2002, the Company was a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In June 2017, the Company changed its name from Bear Lake Recreation, Inc. to Modular Medical, Inc.
The Company is a development-stage medical device company focused on the design, development and eventual commercialization of an innovative insulin pump to address shortcomings and problems represented by the relatively limited adoption of currently available pumps for insulin-dependent people with diabetes. The Company has developed a hardware technology allowing people with insulin-dependent diabetes to receive their daily insulin in two ways, through a continuous “basal” delivery allowing a small amount of insulin to be in the blood at all times and a “bolus” delivery to address meal time glucose input and to address when the blood glucose level becomes excessively high. By addressing the time and effort required to effectively treat their condition, the Company believes it can address the less technically savvy, less motivated part of the market.
As discussed in Note 7, in February 2022, the Company completed a public offering of its equity securities, and its common stock was approved to list on the Nasdaq Capital Market under the symbol “MODD” and began trading there on February 10, 2022.
Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The realization of assets and the satisfaction of liabilities in the normal course of business are dependent on, among other things, the Company’s ability to operate profitably, to generate cash flows from operations, and to pursue financing arrangements to support its working capital requirements.
At issuance of the Company’s financial statements for the year ended March 31, 2021, management had determined that there was significant doubt as to the ability of the Company to meet its obligations and continue as a going concern. As a result of the Offering (see Note 7), which was completed in February 2022, and the Registered Offering (see Note 13), which was completed in May 2022, and resulting improved financial position, the Company believes it has sufficient liquidity to meet its obligations as they come due and conduct its business for a period of at least 12 months from the date of issuance of these financial statements.
The Company’s operating needs include the planned costs to operate its business, including amounts required to fund working capital and capital expenditures. The Company’s future capital requirements and the adequacy of its available funds will depend on many factors, including the Company’s ability to successfully commercialize its product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement its product offering. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash.
Basis of Presentation
The consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America. The Company’s fiscal year ends on March 31 of each calendar year.Each reference to a fiscal year in these notes to the consolidated financial statements refers to the fiscal year ended March 31 of the calendar year indicated (for example, fiscal 2022 refers to the fiscal year ending March 31, 2022). The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Quasuras. All significant intercompany transactions and balances have been eliminated in consolidation.
F-22
Reverse Stock Split
On November 24, 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Nevada to effect a 1-for-3 reverse stock split of the Company’s shares of common stock. Such amendment and ratio were previously approved by a majority of the Company’s stockholders and the board of directors. As a result of the reverse stock split, which was effective November 29, 2021, every three shares of the Company’s pre-reverse split outstanding common stock were combined and reclassified into one share of common stock. Proportionate voting rights and other rights of common stock holders were not affected by the reverse stock split. Any fractional shares of common stock resulting from the Reverse Split were rounded up to the nearest whole share. All stock options outstanding and common stock reserved for issuance under the Company’s equity incentive plans and warrants outstanding immediately prior to the reverse stock split were adjusted by dividing the number of affected shares of common stock by three (3) and, as applicable, multiplying the exercise price by three, (3), as a result of the reverse stock split. All share numbers, share prices, exercise prices and per share amounts have been adjusted, on a retroactive basis to reflect this 1-for-3 reverse stock split.
Use of Estimates
The preparation of the accompanying consolidated financial statements in conformity with GAAPU.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Estimates may include those pertaining to accruals, stock-based compensation and income taxes. Actual results could differ from those estimates.
Reportable Segment
The Company operates in 1one business segment and uses one measurement of profitability for its business.
Research and Development
The Company expenses research and development expenditures as incurred.
General and Administrative
General and administrative expenses consist primarily of payroll and benefit costs, rent, stock-based compensation, legal and accounting fees, and office and other administrative expenses.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of cash. The Company maintains its cash balances at high-qualityhigh quality financial institutions within the United States, which are insured by the Federal Deposit Insurance Corporation (FDIC) up to limits of approximately $250,000. No reserve has been made in the financial statements for any possible loss due to financial institution failure.
Risks and Uncertainties
The Company is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing technology and customer requirements, limited operating history and the volatility of public markets.
COVID-19
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The full extent of the COVID-19 impact on the Company’s operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of the Company’s control, and cannot be predicted.
F-23
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and cash in demand deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Property &and Equipment
Property and equipment are originally recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally three to five years. Depreciation is recorded in operating expenses in the condensed consolidated statements of operations. Leasehold improvements and assets acquired through capital leases are amortized over the shorter of their estimated useful life or the lease term, and amortization is recorded in operating expenses in the condensed consolidated statements of operations.
Fair Value of Financial Instruments
The Company measures the fair value of financial instruments using a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels:
Due to their short-term nature, the carrying values of cash equivalents, accounts payable and accrued expenses approximate fair value.
Basic net loss per share is computed by dividing loss for the period by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share gives effect to all potentially dilutive common shares outstanding during the period. For the nine months ended December 31, 2021 and 2020, outstanding options to purchase and shares of common stock, respectively, were excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive.
Reclassification
Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations or cash flows.
Comprehensive Loss
Comprehensive loss represents the changes in equity of an enterprise, other than those resulting from stockholder transactions. Accordingly, comprehensive loss may include certain changes in equity that are excluded from net loss. For the three and nine months ended December 31, 2021 and 2020, the Company’s comprehensive loss was the same as its net loss.
Recently Adopted Accounting Pronouncement
In August 2020, the FASB issued ASU No. 2020-06, Debtwith Conversion and Other Options(Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40)-Accounting For Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06). ASU 2020-06 simplifies the accounting for convertible instruments by removing major separation models required under current GAAP. Consequently, more convertible debt instruments will be reported as a single liability instrument with no separate accounting for embedded conversion features. ASU 2020-06 removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception, which will permit more equity contracts to qualify for it. ASU 2020-06 also simplifies the diluted net income per share calculation in certain areas. The new guidance is effective for annual and interim periods beginning after December 15, 2021, and early adoption is permitted for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years. The Company early adopted ASU 2020-06 effective April 1, 2021, and the impact of the adoption was not material to the Company’s consolidated financial statements.
NOTE 2 – LEASES
Effective April 1, 2019, the Company adopted ASU No. 2016-02, Leases (ASC 842),and related ASUs, as amended, using the alternative transition method, which allowed the Company to initially apply the new lease standard at the adoption date (the “effective date method”). In January 2020, the Company executed a lease for a new, larger corporate facility in San Diego, California and paid a $100,000 security deposit. The 39-month lease term commenced April 1, 2020, and the lease provides for an initial monthly rent of approximately $12,400 annual rent increases of approximately 3%. In addition to the minimum lease payments, the Company is responsible for property taxes, insurance and certain other operating costs. The right-to-use asset and corresponding liability for the facility lease have been measured at the present value of the future minimum lease payments. A discount rate of 11%, which approximated the Company’s incremental borrowing rate, was used to measure the lease asset and liability. Lease expense is recognized on a straight line basis over the lease term.
The Company obtained a right-of-use asset of $270,950 in exchange for its obligations under the operating lease. The landlord also provided a lease incentive of approximately $139,000, which was paid to the Company in June 2020, for the Company to make improvements to the leased space.
Future minimum payments under the facility operating lease, as of December 31, 2021, are listed in the table below.
| Annual Fiscal Years | Operating lease | |||
| 2022 | 38,358 | |||
| 2023 | 158,028 | |||
| 2024 | 40,692 | |||
| Less: | ||||
| Imputed interest | (20,049 | ) | ||
| Present value of lease liabilities | $ | 217,029 | ||
Cash paid for amounts included in the measurement of lease liabilities was $115,073 for the nine months ended December 31, 2021. Rent expense was $80,698 and $80,654 for the nine months ended December 31, 2021 and 2020, respectively, and $26,930 and $26,844 for the three months ended December 31, 2021 and 2020, respectively.
NOTE 3 – PPP NOTE
On April 24, 2020, the Company received a $368,780 unsecured loan (the PPP Note) under the Paycheck Protection Program (the PPP), which was established under the U.S. government’s Coronavirus Aid, Relief, and Economic Security Act (the CARES Act). The PPP Note to the Company was made through Silicon Valley Bank (the Lender), and the Company entered into a U.S. Small Business Administration Paycheck Protection Program Note with the Lender evidencing the PPP Note. The full amount of the PPP Note was due in April 2022 and interest accrued on the outstanding principal balance of the PPP Note at a fixed rate of 1.0% per annum, which was deferred for 10 months after the covered period during which the Company used the proceeds.
The Company applied to the Lender for forgiveness of the PPP Note in October 2020, and, in May 2021, the Company was notified by the Lender and the U.S. Small Business Administration that the outstanding principal and accrued interest for the PPP Note was forgiven in full. The Company accounted for the forgiveness of the PPP Note in accordance with Accounting Standards Codification Topic 470: Debt (ASC 470), and the amount forgiven was recorded as a gain on extinguishment and recognized in the other income line of the condensed consolidated statement of operations.
NOTE 4 – CONVERTIBLE PROMISSORY NOTES
From February through April 2021, the Company sold $2,310,000 of convertible promissory notes (each an Original Notes and, collectively, the Original Notes), at par in a private placement transaction effected pursuant to an exemption from the registration requirements under the Securities Act of 1933, as amended. Effective April 30, 2021, pursuant to a revocation and replacement agreement between each holder of an Original Note and the Company (the Revocation Agreement), the $2,310,000 of Original Notes and accrued interest thereon as of April 30, 2021 were replaced with $2,360,550 aggregate principal amount of new Notes (as defined below). The Company accounted for the replacement of the Original Notes in accordance with ASC 470 and recorded a loss on extinguishment of $1,321,450 and interest expense of $70,647 for unamortized debt issuance costs as of April 30, 2021.
In April and May 2021, pursuant to a Securities Purchase Agreement by and between the Company and each investor (the SPA), the Company sold to investors $4,250,000 aggregate principal amount of convertible promissory notes (the Notes) and warrants to purchase shares of its common stock (the Warrants). The Notes are unsecured obligations of the Company with each Note having a stated maturity date of 12 months from its issue date (the Issue Date). The Notes bear interest at a rate of 12% per annum, payable on maturity, provided that, if the Company fails to pay any amounts when due under a Note, the interest rate increases to the greater of 16% or the maximum amount permitted by law. Each Note may be prepaid at the Company’s option during the first 270 calendar days following its Issue Date (the 270th day, the Trigger Date), subject to a 110% prepayment penalty on outstanding principal and accrued interest then outstanding. No Note may be prepaid in whole or in part after the Trigger Date.
Notes outstanding after the Trigger Date may be converted into shares of the Company’s common stock at an initial conversion price of $8.61 per share; provided that a Note holder may not convert any portion of its Note that would cause it to beneficially own in excess of 4.99% of the Company’s outstanding common stock. The conversion price and number of shares of Company common stock issuable upon conversion of the Notes are subject to adjustment from time to time for subdivisions and consolidations of shares and other standard dilutive and corporate events, as provided in the Notes. Subject to certain Exempt Issuances (as defined in the Notes), if while a Note is outstanding, the Company sells, issues or grants any shares of its common stock or other securities to acquire shares of common stock at a price per share less than the then conversion price, such conversion price shall be reduced to such lesser price, and the number of conversion shares issuable upon conversion of the Notes shall be increased, as provided in the Notes.
If the Company completes an offering of its common stock or other securities in excess of $12,000,000 of gross proceeds (a Qualified Capital Raise, as defined in the Notes), each Note holder will be required to convert its Adjusted Note Amount (as defined below) into the securities of such Qualified Capital Raise. Adjusted Note Amount equals the product of (i) the sum of all outstanding principal plus accrued interest on a Note, multiplied by (ii) 1.25.
The Notes contain a number of Company events of default (Events of Default) including, without limitation (i) failure to pay any principal or interest thereon when due, (ii) failure to timely deliver shares upon conversions, (iii) failure to comply with SEC reporting requirements under the Exchange Act, (iv) certain breaches of the SPA, the Notes, the Warrants, and the Registration Rights Agreement, (v) material restatements of the Company’s consolidated financial statements filed with the SEC, (vi) a holder’s inability to rely on Rule 144 for sales of shares underlying the Notes, (vii) the Company’s common stock is suspended or halted from trading and/or fails to be quoted or listed (as applicable) on the OTCQB, OTCQX, any tier of the NASDAQ Stock Market, the New York Stock Exchange, or the NYSE American within 10 days thereafter, (viii) failure to file with the SEC a registration statement covering the resale of shares of common stock underlying the Notes and Warrants within 60 calendar days following the Issue Date, (ix) failure to cause such registration statement to become effective within 120 calendar days following the Issue Date, or (x) certain mergers consolidations, business combinations and sales of all or substantially all of the Company’s assets in the event the Company is not the survivor of such transaction.
Upon an Event of Default, a Note holder may declare all amounts under its Note(s) due and payable, in which event the Company will be required to pay such Note holder the sum of (i) the product of (a) all then outstanding principal amount and accrued interest thereon, multiplied by (b) 125%; and (ii) all collection costs including legal fees and expenses in connection therewith. At the option of a Note holder, in the event the Company receives cash proceeds as a result of certain events, including, but not limited to, payments from customers, issuances of debt or equity securities, exercise of warrants or asset sales, the Company will be required to use such proceeds to repay all or any lesser outstanding amounts due under such holder’s Note.
The Notes include covenants, representations, warranties, other payment obligations and agreements by the Company including, without limitation, most-favored nation rights, rights of participation and first refusal and exchange rights.
In connection with the issuance of the Notes, the Company issued Warrants to purchase in the aggregate 767,796 shares of its common stock at an initial exercise price of $24.00 per share. The Warrants may be exercised for a period of five years from the Trigger Date, provided that, if prior to the Trigger Date, the Company (i) completes a Qualified Capital Raise, the outstanding Warrants shall be cancelled or (ii) prepays a holder’s Note(s) in whole or in part, such holder’s pro-rata number of Warrants shall be cancelled. The fair value of the Warrants was $3,700,632, of which $2,379,182 was recorded as a debt discount, which is being amortized to interest expense over the term of the Warrants, and $1,321,450 was recorded as a loss on debt extinguishment. The Company calculated the fair value of the Warrants utilizing the Black-Scholes valuation model with the following assumptions: volatility of , risk-free interest rate of , a term of years and a dividend yield of .
In connection with the April and May 2021 sales of the $4,250,000 aggregate principal amount of the Notes, the Company incurred debt issuance costs of $116,000, which were recorded as a debt discount and are being amortized to interest expense over the term of the Notes using the effective interest rate method. The interest expense attributable to the debt discount, comprising the debt issuance costs and Warrants, during the three and nine months ended December 31, 2021 was $630,323 and $1,454,762, respectively.
The $6,610,550 aggregate principal amount of Notes are due and payable in full in the first quarter of fiscal 2023. Subsequent to the Trigger Date, the Notes can be converted into 767,783 shares of common stock at a conversion price of $8.61 per share.
NOTE 5 – PROMISSORY NOTE
On October 28, 2021, the Company issued a secured promissory note (the Bridge Note) to Manchester Explorer, L.P. (“Manchester”) that provides the Company with a $3,000,000 revolving credit facility with all amounts being drawn down by the Company thereunder being due and payable, subject to acceleration in the event of a default, on March 15, 2022 (the “Maturity Date”). Interest at the rate of 12% is payable on each drawn down without regard to the draw down date or the date when interest is paid.
The principal amount of the Bridge Note and interest due thereon is payable to Manchester no later than the earlier of: (i) the Maturity Date and (ii) the date on which the Company has received proceeds in excess of $12,000,000 from a transaction or series of related transactions occurring prior to the Maturity Date, which such transactions constitute equity financings or other issuances of the Company’s equity securities. Provided that no Event of Default (as such term is defined in the Bridge Note) has occurred, on any date prior to the Maturity Date, upon no less than three days written notice by the Company specifying the draw amount, Manchester will advance the draw amount to the Company. No draw amount can be in an amount less than $100,000 or exceed an amount equal to $3,000,000 minus the aggregate principal amount outstanding under the Bridge Note at the time of such draw request. If an Event of Default occurs and is continuing, Manchester may declare all of the Bridge Note, including any interest and other amounts due, to be due and payable immediately.
In connection with the issuance of the Note, on October 28, 2021, the Company entered into a Security Agreement with Manchester (the “Security Agreement”) under which the Company granted Manchester a continuing and unconditional first priority security interest in and to any and all of the Company’s property of any kind or description, tangible or intangible, wheresoever located and whether now existing or hereafter arising or acquired.
During the quarter ended December 31, 2021, the Company made draws on the Bridge Note of $1,500,000 and incurred interest charges of $180,000.
During the three months ended December 31, 2021, the Company sold 30,865 shares of common stock to its chief executive officer and its chairman of the board of directors, president, chief financial officer and treasurer, issued 8,334 shares of common stock to a service provider and issued 5,775 shares to its non-employee directors under the Company’s outside director compensation plan. At December 31, 2021, the Company had an obligation to issue 16,666 shares of common stock to service providers, and the value of these shares was recorded as common stock issuable in the condensed consolidated balance sheet.
Amended 2017 Equity Incentive Plan
In October 2017, the Company’s board of directors (the Board) approved the 2017 Equity Incentive Plan (the Plan) with shares of common stock reserved for issuance. In January 2020 and August 2021, the Board approved increases in the number of shares reserved for issuance under the Plan by and shares, respectively. Under the Plan, eligible employees, directors and consultants may be granted a broad range of awards, including stock options, stock appreciation rights, restricted stock, performance-based awards and restricted stock units. The Plan is administered by the Board or, in the alternative, a committee designated by the Board.
Stock-Based Compensation Expense
The expense relating to stock options is recognized on a straight-line basis over the requisite service period, usually the vesting period, based on the grant date fair value. The unamortized compensation cost, as of December 31, 2021, was $ related to stock options and is expected to be recognized as expense over a weighted-average period of approximately years.
During the nine months ended December 31, 2021, the Company granted options to purchase shares of its common stock to employees, directors and consultants. The options had 10-year terms, and options vested immediately when granted. The fair value of the options was determined to be $8,108,043 of which $ was recorded as stock-based compensation expense and included in the condensed consolidated statement of operations for the nine months ended December 31, 2021.
The following assumptions were used in the fair value method calculations:
Schedule of Fair Value Assumptions
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Risk-free interest rates | - | - | - | |||||||||||||
| Volatility | - | - | - | |||||||||||||
| Expected life (years) | – | – | - | - | ||||||||||||
| Dividend yield | ||||||||||||||||
The fair values of options at the grant date were estimated utilizing the Black-Scholes valuation model, which includes simplified methods to establish the fair term of options as well as average volatility of three comparable organizations. The risk-free interest rate was derived from the Daily Treasury Yield Curve Rates, as published by the U.S. Department of the Treasury as of the grant date for terms equal to the expected terms of the options. A dividend yield of zero was applied because the Company has never paid dividends and has no intention to pay dividends in the foreseeable future. In accordance with ASU No. 2016-09, the Company accounts for forfeitures as they occur.
Schedule of Stock Option activity
| Options Outstanding | ||||||||||||
| Shares Available for Grant | Number of Shares | Weighted Average Exercise Prices | ||||||||||
| Balance at March 31, 2021 | 136,082 | 1,197,252 | $ | 5.25 | ||||||||
| Options granted | (60,774 | ) | 60,774 | 12.69 | ||||||||
| Share awards | (1,836 | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | 7,547 | (7,547 | ) | 8.61 | ||||||||
| Balance at June 30, 2021 | 81,089 | 1,250,479 | 5.58 | |||||||||
| Additional shares authorized under the Plan | 1,333,334 | — | — | |||||||||
| Options granted | (396,384 | ) | 396,384 | 12.15 | ||||||||
| Share awards | (3,636 | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | 49,213 | (49,213 | ) | 7.02 | ||||||||
| Balance at September 30, 2021 | 1,063,546 | 1,597,650 | 7.17 | |||||||||
| Options granted | (266,112 | ) | 259,559 | 9.99 | ||||||||
| Share awards | (5,775 | ) | — | |||||||||
| Options cancelled and returned to the Plan | 29,073 | (11,256 | ) | 7.24 | ||||||||
| Balance at December 31, 2021 | 820,732 | 1,845,953 | 7.57 | |||||||||
There were no stock options exercised during the nine months ended December 31, 2021 and 2020.
Schedule of Outstanding and Exercisable Option, Range
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (in Years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | Aggregate Intrinsic value | ||||||||||||||||||
| $ - $ | 1,845,953 | 8.13 | $ | 7.57 | 1,011,586 | $ | 5.10 | $ | 3,564,268 | |||||||||||||||
The intrinsic value per share is calculated as the excess of the closing price of the common stock on the Company’s principal trading market over the exercise price of the option at December 31, 2021.
The Company is required to present the tax benefits resulting from tax deductions in excess of the compensation cost recognized from the exercise of stock options as financing cash flows in the consolidated statements of cash flows. For the nine months ended December 31, 2021 and 2020, there were no such tax benefits associated with the exercise of stock options.
NOTE 7 – INCOME TAXES
The Company determines deferred tax assets and liabilities based upon the differences between the financial statement and tax bases of the Company’s assets and liabilities using tax rates in effect for the year in which the Company expects the differences to affect taxable income. A valuation allowance is established for any deferred tax assets for which it is more likely than not that all or a portion of the deferred tax assets will not be realized. Based on the available information and other factors, management believes it is more likely than not that its federal and state net deferred tax assets will not be fully realized, and the Company has recorded a full valuation allowance.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations. All tax returns for fiscal 2016 to fiscal 2021 may be subject to examination by the U.S. federal and state tax authorities. As of December 31, 2021, the Company has not recorded any liability for unrecognized tax benefits related to uncertain tax positions.
NOTE 8 – RELATED PARTY TRANSACTIONS
In February 2021, the Company’s chairman of the board of directors and president and an existing investor, who is represented by a member of the Company’s board of directors, purchased $100,000 and $1,000,000, aggregate principal amount of the Original Notes, respectively. Effective April 30, 2021, the related party holders entered into revocation agreements with the Company pursuant to which their collective $1,100,000 aggregate principal amount of Original Notes and accrued interest of $50,091 were replaced with Notes. At December 31, 2021, the investor and executive officer held Notes in an aggregate principal amount of $1,026,630 and $102,663, respectively, with $82,693 and $8,269 of interest payable thereon. For the three months ended December 31, 2021, the Company incurred interest expense of approximately $31,052 and $3,105, respectively, and for the nine months ended December 31, 2021, the Company incurred interest expense of approximately $82,693 and $8,269, respectively, on the related party holder Notes.
In May 2021, a member of the Board purchased $200,000 aggregate principal amount of Notes (the Director Note). For the three and nine months ended December 31, 2021, the Company incurred expense of approximately $6,049 and $16,110, respectively, on the Director Note. At December 31, 2021, approximately $16,110 of interest was payable by the Company on the Director Note.
In October 2021, the Company entered into purchase agreements with Ellen O’Connor (Lynn) Vos, the Company’s chief executive officer, and Paul DiPerna, the chairman of the Company’s board of directors and its president, chief financial officer and treasurer, providing for the sale and issuance by the Company of shares of the Company’s common stock, par value $0.001 per share at the closing market price on October 28, 2021 of $8.10 per share. The Company received proceeds of approximately $250,000 from the sale of the shares, comprising $ from Ms. Vos and $ from Mr. DiPerna.
NOTE 9 – COMMITMENTS AND CONTINGENCIES
Litigations, Claims and Assessments
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. The Company records legal costs associated with loss contingencies as incurred and accrues for all probable and estimable settlements.
Indemnification
In the ordinary course of business, the Company enters into contractual arrangements under which it may agree to indemnify the counterparties from any losses incurred relating to breach of representations and warranties, failure to perform certain covenants, or claims and losses arising from certain events as outlined within the particular contract, which may include, for example, losses arising from litigation or claims relating to past performance. Such indemnification clauses may not be subject to maximum loss clauses. The Company has also entered into indemnification agreements with its officers and directors. No amounts were reflected in the Company’s consolidated financial statements for the nine months ended December 31, 2021 and 2020 related to these indemnifications. The Company has not estimated the maximum potential amount of indemnification liability under these agreements due to the limited history of prior claims and the unique facts and circumstances applicable to each particular agreement. To date, the Company has not made any payments related to these indemnification agreements, and no claims for payment have been made under such agreements.
NOTE 10 – SUBSEQUENT EVENTS
Bridge Note
Subsequent to December 31, 2021, the Company made additional draws totaling $600,000 under the Bridge Note.
Public Offering
On February 9, 2022, the Company entered into an underwriting agreement (the Underwriting Agreement), with Oppenheimer & Co. Inc., which acted as the representative of the several underwriters (the “Representative”), in a firm commitment underwritten public offering (the Offering) pursuant to which the Company agreed to sell to the Representative an aggregate of 2,500,000 shares of the Company’s common stock, par value $0.001 per share (the Common Stock), and 2,500,000 warrants (Warrants and, collectively with the Common Stock, the Units), each to purchase one share of Common Stock, at a public offering price of $6.00 per Unit. The Warrants included in the Units are exercisable immediately, have an exercise price of $6.60 per share and expire five years from the date of issuance. The Common Stock was approved to list on the Nasdaq Capital Market under the symbol “MODD” and began trading there on February 10, 2022. The gross proceeds from the Offering were $15 million, before deducting underwriting discounts and commissions and other offering expenses.
The Units were offered and sold to the public pursuant to the Company’s registration statement on Form S-1 (File No. 333-260682), initially filed by the Company with the Securities and Exchange Commission under the Securities Act of 1933, as amended (the Securities Act), on November 2, 2021, and declared effective on February 9, 2022.
The Underwriting Agreement contains customary representations, warranties and agreements by the Company, customary conditions to closing, indemnification obligations of the Company and the Representative, including for liabilities under the Securities Act of 1933, as amended, other obligations of the parties and termination provisions. In addition, pursuant to the terms of the Underwriting Agreement and related “lock-up” agreements, the Company, each director and executive officer of the Company, and certain stockholders have agreed with the Representative not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our Common Stock or securities convertible into Common Stock for a period of 180 days after February 9, 2022, the date of the final prospectus.
Modular Medical, Inc.
Consolidated Balance Sheets
| March 31, | ||||||||
| ASSETS | 2021 | 2020 | ||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 1,468,465 | $ | 3,122,134 | ||||
| Prepaid expenses | 178,158 | 63,853 | ||||||
| Other current assets | 2,466 | 306 | ||||||
| TOTAL CURRENT ASSETS | 1,649,089 | 3,186,293 | ||||||
| Property and equipment, net | 298,958 | 301,308 | ||||||
| Right of use asset, net | 200,124 | 270,950 | ||||||
| Security deposit | 100,000 | 100,000 | ||||||
| TOTAL NON-CURRENT ASSETS | 599,082 | 672,258 | ||||||
| TOTAL ASSETS | $ | 2,248,171 | $ | 3,858,551 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | 169,284 | $ | 367,019 | ||||
| Accrued expenses | 499,948 | 202,160 | ||||||
| Short-term lease liability | 125,500 | 92,214 | ||||||
| PPP note payable | 368,780 | — | ||||||
| Convertible notes payable | 2,133,453 | — | ||||||
| TOTAL CURRENT LIABILITIES | 3,296,965 | 661,393 | ||||||
| Long-term lease liability | 184,355 | 178,736 | ||||||
| Bonus payable | 42,000 | 140,000 | ||||||
| TOTAL LIABILITIES | 3,523,320 | 980,129 | ||||||
| Commitments and Contingencies (Note 10) | ||||||||
| STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Preferred Stock, $ par value, shares authorized, issued and outstanding | — | — | ||||||
| Common Stock, $ par value, shares authorized, shares and shares issued and outstanding as of March 31, 2021 and 2020, respectively | 6,302 | 5,957 | ||||||
| Additional paid-in capital | 14,665,559 | 10,517,505 | ||||||
| Common stock issuable | — | 923,994 | ||||||
| Accumulated deficit | (15,947,010 | ) | (8,569,034 | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | (1,275,149 | ) | 2,878,422 | |||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | 2,248,171 | $ | 3,858,551 | ||||
The accompanying notes are an integral part of these audited consolidated financial statements
Modular Medical, Inc.
Consolidated Statements of Operations
| Year ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Operating expenses | ||||||||
| Research and development | $ | 4,083,303 | $ | 3,034,152 | ||||
| General and administrative expenses | 3,253,412 | 2,313,870 | ||||||
| Total operating expenses | 7,336,715 | 5,348,022 | ||||||
| Loss from operations | (7,336,715 | ) | (5,348,022 | ) | ||||
| Other income | ||||||||
| Interest income | 130 | 28,749 | ||||||
| Interest expense | (39,791 | ) | — | |||||
| Loss before income taxes | (7,376,376 | ) | (5,319,273 | ) | ||||
| Provision for income taxes | 1,600 | 1,600 | ||||||
| Net loss | $ | (7,377,976 | ) | $ | (5,320,873 | ) | ||
| Net loss per share | ||||||||
| Basic and diluted | $ | (1.19 | ) | $ | (0.89 | ) | ||
| Shares used in computing net loss per share | ||||||||
| Basic and diluted | 6,211,562 | 5,954,923 | ||||||
The accompanying notes are an integral part of these audited consolidated financial statements
Modular Medical, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
| Common Stock | Additional Paid-In | Common Stock | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Equity (Deficit) | |||||||||||||||||||
| Balance as of March 31, 2019 | 5,946,754 | $ | 5,947 | $ | 9,696,471 | $ | 19,800 | $ | (3,248,161 | ) | $ | 6,474,057 | ||||||||||||
| Placement of common stock | — | — | — | 923,994 | — | 923,994 | ||||||||||||||||||
| Shares issued for services | 10,000 | 10 | 19,790 | (19,800 | ) | — | — | |||||||||||||||||
| Stock-based compensation | — | — | 801,244 | — | — | 801,244 | ||||||||||||||||||
| Net loss | — | — | — | — | (5,320,873 | ) | (5,320,873 | ) | ||||||||||||||||
| Balance as of March 31, 2020 | 5,956,754 | $ | 5,957 | $ | 10,517,505 | $ | 923,994 | $ | (8,569,034 | ) | $ | 2,878,422 | ||||||||||||
| Placement of common stock | 320,796 | 321 | 2,709,555 | (923,994 | ) | — | 1,785,882 | |||||||||||||||||
| Shares issued for services | 24,500 | 24 | 210,920 | — | — | 210,945 | ||||||||||||||||||
| Stock-based compensation | — | — | 1,227,578 | — | — | 1,227,578 | ||||||||||||||||||
| Net loss | — | — | �� | — | — | (7,377,976 | ) | (7,377,976 | ) | |||||||||||||||
| Balance as of March 31, 2021 | 6,302,050 | $ | 6,302 | $ | 14,665,559 | $ | — | $ | (15,947,010 | ) | $ | (1,275,149 | ) | |||||||||||
The accompanying notes are an integral part of these audited consolidated financial statements
Modular Medical, Inc.
Consolidated Statements of Cash Flows
| Year ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash Flows from operating activities | ||||||||
| Net loss | $ | (7,377,976 | ) | $ | (5,320,873 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation expense | 1,227,578 | 801,244 | ||||||
| Depreciation and amortization | 111,015 | 35,431 | ||||||
| Shares for services | 68,880 | — | ||||||
| Amortization of lease right-of-use asset | 70,826 | — | ||||||
| Change in lease liability | 38,905 | — | ||||||
| Amortization of debt issuance costs | 12,253 | — | ||||||
| Other | 1,004 | — | ||||||
| Changes in assets and liabilities: | ||||||||
| Other assets and prepaid expenses | 25,600 | (48,391 | ) | |||||
| Security deposits | — | (92,500 | ) | |||||
| Accounts payable and accrued expenses | (86,747 | ) | 530,250 | |||||
| Net cash used in operating activities | (5,908,662 | ) | (4,094,839 | ) | ||||
| Cash flows from investing activities | ||||||||
| Purchases of property and equipment | (109,669 | ) | (260,789 | ) | ||||
| Net cash used in investing activities | (109,669 | ) | (260,789 | ) | ||||
| Cash flows from financing activities | ||||||||
| Proceeds from private placement, net of issuance costs | 1,785,882 | 923,994 | ||||||
| Proceeds from issuance of convertible notes | 2,210,000 | — | ||||||
| Proceeds from issuance of PPP note payable | 368,780 | — | ||||||
| Net cash provided by financing activities | 4,364,662 | 923,994 | ||||||
| Net decrease in cash and cash equivalents | (1,653,669 | ) | (3,431,634 | ) | ||||
| Cash and cash equivalents, at beginning of year | 3,122,134 | 6,553,768 | ||||||
| Cash and cash equivalents, at end of year | $ | 1,468,465 | $ | 3,122,134 | ||||
| Supplemental disclosure: | ||||||||
| Cash paid for: | ||||||||
| Income taxes | $ | 1,600 | $ | 1,600 | ||||
The accompanying notes are an integral part of these audited consolidated financial statements
MODULAR MEDICAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – THE COMPANY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Modular Medical, Inc. (the Company) was incorporated in Nevada in October 1998 under the name Bear Lake Recreation, Inc. The Company had no material business operations from 2002 until approximately 2017 when it acquired all of the issued and outstanding shares of Quasuras, Inc., a Delaware corporation (Quasuras). As the major shareholder of Quasuras retained control of both the Company and Quasuras, the share exchange was accounted for as a reverse merger. As such, the Company recognized the assets and liabilities of Quasuras, acquired in the merger, at their historical carrying amounts. Prior to the acquisition of Quasuras and, since at least 2002, the Company was a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In June 2017, the Company changed its name from Bear Lake Recreation, Inc. to Modular Medical, Inc.
The Company is a development-stage medical device company focused on the design, development and eventual commercialization of an innovative insulin pump to address shortcomings and problems represented by the relatively limited adoption of currently available pumps for insulin-dependent people with diabetes. The Company has developed a hardware technology allowing people with insulin-dependent diabetes to receive their daily insulin in two ways, through a continuous “basal” delivery allowing a small amount of insulin to be in the blood at all times and a “bolus” delivery to address meal time glucose input and to address when the blood glucose level becomes excessively high. By addressing the time and effort required to effectively treat their condition, the Company believes it can address the less technically savvy, less motivated part of the market.
The consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America. The following summarizes the more significant of such policies:
Liquidity
Financial Accounting Standards Board (FASB) Accounting Standard Update (ASU) No. 2014-15 (ASU 2014-15), Going Concern, requires management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. If management identifies conditions or events that raise substantial doubt about an entity’s ability to continue as a going concern, management must consider if there are plans that are probable to be implemented, and whether it is probable that the plans will mitigate the conditions or events raising the substantial doubt about the entity’s ability to continue as a going concern. If the substantial doubt is not alleviated after consideration of management’s plans, the entity must include a statement in the notes to the financial statements indicating that there is substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued including: 1) the principal conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern, 2) management’s evaluation of the significance of those conditions or events in relation to the entity’s ability to meet its obligations, and 3) management’s plans to attempt to mitigate the conditions or events causing the substantial doubt about the entity’s ability to continue as a going concern.
The Company expects to continue to incur operating losses for the foreseeable future and incur cash outflows from operations as it continues to invest in the development and subsequent commercialization of its product. The Company expects that its research and development and general and administrative expenses will continue to increase, and, as a result, it will eventually need to generate significant product revenues to achieve profitability. These circumstances raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued. Implementation of the Company’s plans and its ability to continue as a going concern will depend upon the Company’s ability to raise additional capital, through the sale of additional equity or debt securities, to support its future operations. There can be no assurance that such additional capital, whether in the form of debt or equity financing, will be sufficient or available and, if available, that such capital will be offered on terms and conditions acceptable to the Company. As discussed in notes 3 and 11, in February 2021, the Company commenced a private placement of its convertible promissory notes to investors to fund its operations. In addition, during fiscal 2021, the Company obtained additional equity financing through a private placement of its common stock (see note 6), and the Company obtained a loan from Silicon Valley Bank in April 2020 (see notes 3 and 12).
The Company’s operating needs include the planned costs to operate its business, including amounts required to fund working capital and capital expenditures. The Company’s future capital requirements and the adequacy of its available funds will depend on many factors, including the Company’s ability to successfully commercialize its product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement its product offering. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash. These consolidated financial statements do not include any adjustments that might result from this uncertainty.
Basis of Presentation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Quasuras. All significant intercompany transactions and balances have been eliminated in consolidation. The Company’s fiscal year ends on March 31 of each calendar year.Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations or cash flows.
Use of Estimates
The preparation of the accompanying consolidated financial statements in conformity with U.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Estimates may include those pertaining to accruals, stock-based compensation and income taxes. Actual results could differ from those estimates.
Reportable Segment
The Company operates in one business segment and uses one measurement of profitability for its business.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are deposited with high credit-quality institutions within the United States, which are insured by the Federal Deposit Insurance Corporation (FDIC) up to limits of approximately $250,000.
Risks and Uncertainties
The Company is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history and the volatility of public markets.
COVID-19
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The full extent of the COVID-19 impact on the Company’s operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of the Company’s control, and cannot be predicted.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in demand deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Property and Equipment
Property and equipment are originally recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally three to five years. Depreciation is recorded in operating expenses in the consolidated statements of operations. Leasehold improvements and assets acquired through capital leases are amortized over the shorter of their estimated useful life or the lease term, and amortization is recorded in operating expenses in the consolidated statements of operations.
Fair Value of Financial Instruments
The Company measures the fair value of financial instruments using a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels:
| Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets. |
| Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
Due to their short-term nature, the carrying values of cash equivalents, accounts payable and accrued expenses, approximate fair value.
ResearchDebt Modifications and DevelopmentExtinguishments
When the Company modifies or extinguishes debt, it does so in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 470-50, Debt — Modifications and Extinguishments, which requires modification to debt instruments to be evaluated to assess whether the modifications are considered “substantial modifications.” A substantial modification of terms shall be accounted for like an extinguishment. Based on the guidance relied upon and the analysis performed, if the Company believes the embedded conversion feature has no fair value on the date of issuance (measurement date) and the embedded conversion feature has no beneficial conversion feature, the embedded conversion feature does not meet the criteria in ASC 470-50-40-10 or 470-20-25 and the issuance of the convertible note payable is considered a modification, and not an extinguishment that would require the recognition of a gain or loss. If the Company determines the change in terms meet the criteria for substantial modification under ASC 470 it will treat the modification as extinguishment and recognize a loss from debt extinguishment.
Leases
Effective April 1, 2019, the Company adopted ASC No. 842, Leases (ASC 842). ASC 842 requires an entity to recognize a right-of-use asset and a lease liability for all leases with terms longer than 12 months. The Company expenses research and development expenditures as incurred.adopted ASC 842 utilizing the modified retrospective transition method. The Company elected the practical expedient afforded in ASC 842 in which the Company did not reassess whether any contracts that existed prior to adoption have or contain leases or the classification of its existing leases.
General and Administrative
F-24
General and administrative expense consists primarily of payroll and benefit related costs, rent, office expenses, equipment supplies and meetings and travel.
Stock-Based Compensation
The Company recognizes stock-based compensation for stock options granted to employees and non-employees on a straight-line basis over the requisite service period, usually the vesting period, based on the grant-date fair value. The Company estimates the value of stock options on the date of grant using the Black-Scholes pricing model. The determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the option price, as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards, and projected stock option exercise behaviors.
Per-Share Amounts
Basic net loss per share is computed by dividing net loss for the period by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share gives effect to all potentially dilutive common shares outstanding during the period. ForPotentially dilutive common shares consist of incremental shares of common stock issuable upon the years ended March 31, 2021exercise of stock options and 2020, and exercise of warrants.
The following table sets forth securities outstanding options to purchase common stockwhich were excluded from the calculationcomputation of diluted net loss per share becauseas their effectinclusion would be anti-dilutive. anti-dilutive:
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| Options to purchase common stock | 1,650,705 | 1,197,252 | ||||||
| Warrants | 4,779,072 | — | ||||||
| Total | 6,429,777 | 1,197,252 | ||||||
Reclassification
Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations or cash flows.
Income Taxes
The Company determines deferred tax assets and liabilities based upon the differences between the financial statement and tax bases of the Company’s assets and liabilities using tax rates in effect for the year in which the Company expects the differences to affect taxable income. A valuation allowance is established for any deferred tax assets for which it is more likely than not that all or a portion of the deferred tax assets will not be realized. Based on the available information and other factors, management believes it is more likely than not that its federal and state net deferred tax assets will not be fully realized, and the Company has recorded a full valuation allowance.
The Company accounts for uncertain tax positions in accordance with FASB Accounting Standards Codification (ASC)ASC Topic 740, Income Taxes. When tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying consolidated balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative expenses in the consolidated statements of operations.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations. All tax returns from 2016 to 20202021 may be subject to examination by the U.S. federal and state tax authorities. As of March 31, 2022 and 2021, the Company hashad not recorded any liability for unrecognized tax benefits related to uncertain tax positions.
F-25
Comprehensive Loss
Comprehensive loss represents the changes in equity of an enterprise, other than those resulting from stockholder transactions. Accordingly, comprehensive loss may include certain changes in equity that are excluded from net loss. For the years ended March 31, 20212022 and 2020,2021, the Company’s comprehensive loss was the same as its net loss.
Recently Issued Accounting Pronouncement
In June 2016, the FASB issued Accounting Standards Update (ASU) No. 2016-13, Financial Instruments—Credit Losses. This ASU added a new impairment model (known as the current expected credit loss (CECL) model) that is based on expected losses rather than incurred losses. Under the new guidance, an entity recognizes an allowance for its estimate of expected credit losses and applies to most debt instruments, trade receivables, lease receivables, financial guarantee contracts, and other loan commitments. The CECL model does not have a minimum threshold for recognition of impairment losses and entities will need to measure expected credit losses on assets that have a low risk of loss. This update is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years for smaller reporting companies. The Company is still evaluating the impact of this accounting guidance on its results of operations and financial position.
NOTE 2 – CONSOLIDATED BALANCE SHEET DETAIL
Schedule of Property Plant And Equipment
| March 31, | ||||||||||||||||
| March 31, | 2022 | 2021 | ||||||||||||||
| Property and equipment, net: | 2021 | 2020 | ||||||||||||||
| Leasehold improvements | $ | 139,197 | $ | 139,197 | $ | 139,197 | $ | 139,197 | ||||||||
| Office equipment | 56,476 | 49,724 | 63,298 | 56,476 | ||||||||||||
| Computer equipment and software | 52,383 | 51,882 | 52,114 | 52,383 | ||||||||||||
| Machinery and equipment | 202,993 | 112,198 | 230,947 | 202,993 | ||||||||||||
| Property and equipment, gross | 451,049 | 353,001 | ||||||||||||||
| 485,556 | 451,049 | |||||||||||||||
| Less: accumulated depreciation and amortization | (152,091 | ) | (51,693 | ) | (249,597 | ) | (152,091 | ) | ||||||||
| Property and equipment, net | $ | 298,958 | $ | 301,308 | ||||||||||||
| $ | 235,959 | $ | 298,958 | |||||||||||||
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| Accrued expenses: | ||||||||
| Accrued wages and bonus | $ | 457,891 | $ | 372,563 | ||||
| Accrued placement fees | — | 88,800 | ||||||
| Accrued interest | — | 27,538 | ||||||
| Other | 67,000 | 11,047 | ||||||
| $ | 524,891 | $ | 499,948 | |||||
Schedule of Accrued Expenses
| March 31, | ||||||||
| Accrued expenses: | 2021 | 2020 | ||||||
| Accrued wages and bonus | $ | 372,563 | $ | 198,160 | ||||
| Accrued placement fees | 88,800 | — | ||||||
| Accrued interest | 27,538 | — | ||||||
| Other | 11,047 | 4,000 | ||||||
| Accrued expenses | $ | 499,948 | $ | 202,160 | ||||
NOTE 3 – NOTES PAYABLE
PPP Note
On April 24, 2020, the Company received a $368,780 unsecured loan (the PPP Note) under the Paycheck Protection Program (the PPP), which was established under the U.S. government’s Coronavirus Aid, Relief, and Economic Security Act (the CARES Act). The PPP Note to the Company was made through Silicon Valley Bank (the Lender), and the Company entered into a U.S. Small Business Administration Paycheck Protection Program Note (the Agreement) with the Lender evidencing the PPP Note.
The full amount of the PPP Note is due in April 2022. Interest will accrue on the outstanding principal balance of the PPP Note at a fixed rate of 1.0% per annum, which shall be deferred for 10 months after the covered period during which the Company used the proceeds. The Company may prepay principal of the PPP Note at any time in any amount without penalty. The Agreement contains customary events of default relating to, among other things, payment defaults, breach of representations and warranties or provisions of the PPP Note. The occurrence of an event of default may result in the repayment of all amounts outstanding, collection of all amounts owing from the Company, and/or filing suit and obtaining judgment against the Company.
The Company applied to the Lender for forgiveness of the PPP Note in October 2020, and the amount which may be forgiven will be equal to the sum of the payroll and benefit costs and covered rent and utility payments incurred by the Company, as calculated in accordance with the terms of the CARES Act.
Convertible Promissory NotesLEASES
In February and March 2021, the Company sold $2,210,000 of convertible promissory notes (the Notes), at par in a private placement transaction effected pursuant to an exemption from the registration requirements under the Securities Act of 1933, as amended (the 2021 Placement). The Notes bear interest at an annual rate of 12%, and interest is accrued or payable monthly in cash. The Notes mature on September 30, 2021 (the Maturity Date) and may be prepaid prior to the Maturity Date.
The aggregate principal amount of the Notes plus accrued but unpaid interest thereon shall automatically convert upon the closing of an offering of the Company’s equity securities to investors or a strategic corporate investor resulting in aggregate gross proceeds to the Company of at least $5,000,000 (excluding conversion of the Notes or other convertible securities issued for capital raising purposes) (a Qualified Financing). In the event of a Qualified Financing, all such outstanding principal and accrued interest shall convert into the same equity securities purchased by and on the same terms and conditions as the other investors in such Qualified Financing at a conversion price equal to 80% (a 20% discount) of the lowest price paid per unit or share by investors in the Qualified Financing. In the event that additional bridge financing is obtained by the Company, the Notes shall convert into the same securities and on the same terms and conditions as the other investors therein and all such purchases will be treated as one, single round of financing going forward. As of March 31, 2021, the Notes could be converted into 770,305 shares of common stock, excluding the effects of any payments of interest in kind.
At any time on or following the Maturity Date, the holders of the Notes may demand repayment of the Notes, and the Company shall repay the outstanding aggregate principal amount plus accrued but unpaid interest thereon. The holders of the Notes, however, retain the right for 30 days after the Maturity Date to convert all or part of the aggregate principal amount plus accrued but unpaid interest on the Notes into the Company’s common stock at the conversion price of $2.87 per share or at a 20% discount to any financing consummated during the 30-day period following the Maturity Date.
If a Qualified Financing has not occurred immediately prior to the consummation of a Change of Control (as defined below), the Note holders shall have the option of either (i) converting all or any portion of the aggregate principal amount of the Notes plus accrued but unpaid interest thereon into common stock of the Company at a conversion price equal to $2.87 per share or (ii) having the Company repay the aggregate principal amount of the Notes and accrued but unpaid interest. The term “Change of Control” means (i) a consolidation or merger of the Company with or into any other corporation or other entity or person, or any other corporate reorganization, other than any such consolidation, merger or reorganization in which the shares of capital stock of the Company immediately prior to such consolidation, merger or reorganization continue to represent a majority of the voting power of the surviving entity immediately after such consolidation, merger or reorganization; (ii) any transaction or series of related transactions to which the Company is a party in which in excess of 50% of the Company’s voting power is transferred; (iii) the sale or transfer of all or substantially all of the Company’s assets, or the exclusive license of all or substantially all of the Company’s material intellectual property; or (iv) the dissolution and winding up of the Company.
The Company incurred debt issuance costs of $88,800, which were recorded as a debt discount and are being amortized to interest expense overaccounts for the term of the Notes using the effective interest rate method. The interest expense related to the debt discount during the year ended March 31, 2021 was approximately $13,000.
NOTE 4 – LEASES
Effective April 1, 2019, the Company adopted ASC No. 842, as amended, using the alternative transition method, which allowed the Company to initially apply the new lease standard at the adoption date (the “effective date method”). In January 2020, the Company executed a lease for a new, largerits corporate facility in San Diego, California and paid a $100,000 security deposit.in accordance with ASC 842. The 39-month lease term commenced April 1, 2020, and the lease provides for an initial monthly rent of approximately $12,400 with$12,400 annual rent increases of approximately 3%. In addition to the minimum lease payments, the Company is responsible for property taxes, insurance and certain other operating costs. The right-to-use asset and corresponding liability for the facility lease have been measured at the present value of the future minimum lease payments. A discount rate of 11%, which approximated the Company’s incremental borrowing rate, was used to measure the lease asset and liability. Lease expense is recognized on a straight linestraight-line basis over the lease term.
F-26
The Company obtained a right-of-use asset of $270,950$270,950 in exchange for its obligations under the operating lease. The landlord also provided a lease incentive of approximately $139,000,$139,000, which was paid to the Company in June 2020, for the Company to make improvements to the leased space. In addition, the Company paid a $100,000 security deposit.
Future minimum payments under the facility operating lease, net of the lease incentive, as of March 31, 2021,2022, are listed in the table below.
| Operating | ||||||||
| Annual Fiscal Years | lease | Operating lease | ||||||
| 2022 | $ | 153,432 | ||||||
| 2023 | 158,028 | 158,028 | ||||||
| 2024 | 40,692 | 40,692 | ||||||
| Less: | ||||||||
| Imputed interest | (42,297 | ) | (13,906 | ) | ||||
| Present value of lease liabilities | $ | 309,855 | $ | 184,814 | ||||
Cash paid for amounts included in the measurement of lease liabilities was $153,432 for the year ended March 31, 2022. Rent expense was $107,540$107,820 and $35,766$107,540 for the years ended March 31, 2022 and 2021, respectively.
NOTE 4 – PPP NOTE
In April 2020, the Company received a $368,780 unsecured loan (the PPP Note) under the Paycheck Protection Program (the PPP), which was established under the U.S. government’s Coronavirus Aid, Relief, and 2020, respectively.Economic Security Act (the CARES Act). The PPP Note to the Company was made through Silicon Valley Bank (the Lender), and the Company entered into a U.S. Small Business Administration Paycheck Protection Program Note with the Lender evidencing the PPP Note. The full amount of the PPP Note was due in April 2022 and interest accrued on the outstanding principal balance of the PPP Note at a fixed rate of 1.0% per annum, which was deferred for 10 months after the covered period during which the Company used the proceeds.
In May 2021, the Lender and the U.S. Small Business Administration notified the Company that the outstanding principal and accrued interest for the PPP Note was forgiven in full. The Company accounted for the forgiveness of the PPP Note in accordance with ASC Topic 470: Debt (ASC 470), and the amount forgiven was recorded as a gain on extinguishment and recognized in the other income line of the consolidated statement of operations.
NOTE 5 – STOCK-BASED COMPENSATIONCONVERTIBLE PROMISSORY NOTES
Equity Compensation PlanFrom February through April 2021, the Company sold $2,310,000 of convertible promissory notes (each an Original Note and, collectively, the Original Notes), at par in a private placement transaction effected pursuant to an exemption from the registration requirements under the Securities Act of 1933, as amended. Effective April 30, 2021, pursuant to a revocation and replacement agreement between each holder of an Original Note and the Company (the Revocation Agreement), the $2,310,000 of Original Notes and accrued interest thereon as of April 30, 2021 were replaced with $2,360,550 aggregate principal amount of new Notes (as defined below). The Company accounted for the replacement of the Original Notes in accordance with ASC 470 and recorded a loss on extinguishment of $1,321,450 and interest expense of $70,647 for unamortized debt issuance costs as of April 30, 2021.
F-27
In April and May 2021, pursuant to a securities purchase agreement by and between the Company and each investor (the SPA), the Company sold to investors $4,250,000 aggregate principal amount of convertible promissory notes (the Notes) and warrants to purchase shares of its common stock (the Warrants). The Notes are unsecured obligations of the Company with each Note having a stated maturity date of 12 months from its issue date (the Issue Date). The Notes bear interest at a rate of 12% per annum, payable on maturity, provided that, if the Company fails to pay any amounts when due under a Note, the interest rate increases to the greater of 16% or the maximum amount permitted by law. Each Note may be prepaid at the Company’s option during the first 270 calendar days following its Issue Date (the 270th day, the Trigger Date), subject to a 110% prepayment penalty on outstanding principal and accrued interest then outstanding. No Note may be prepaid in whole or in part after the Trigger Date.
Notes outstanding after the Trigger Date may be converted into shares of the Company’s common stock at an initial conversion price of $8.61 per share; provided that a Note holder may not convert any portion of its Note that would cause it to beneficially own in excess of 4.99% of the Company’s outstanding common stock. The conversion price and number of shares of Company common stock issuable upon conversion of the Notes are subject to adjustment from time to time for subdivisions and consolidations of shares and other standard dilutive and corporate events, as provided in the Notes. Subject to certain Exempt Issuances (as defined in the Notes), if while a Note is outstanding, the Company sells, issues or grants any shares of its common stock or other securities to acquire shares of common stock at a price per share less than the then conversion price, such conversion price shall be reduced to such lesser price, and the number of conversion shares issuable upon conversion of the Notes shall be increased, as provided in the Notes.
If the Company completes an offering of its common stock or other securities in excess of $12,000,000 of gross proceeds (a Qualified Capital Raise, as defined in the Notes), each Note holder will be required to convert its Adjusted Note Amount (as defined below) into the securities of such Qualified Capital Raise. Adjusted Note Amount equals the product of (i) the sum of all outstanding principal plus accrued interest on a Note, multiplied by (ii) 1.25.
The Notes contained a number of Company events of default (Events of Default) including, without limitation (i) failure to pay any principal or interest thereon when due, (ii) failure to timely deliver shares upon conversions, (iii) failure to comply with SEC reporting requirements under the Exchange Act, (iv) certain breaches of the SPA, the Notes, the Warrants, and the Registration Rights Agreement, (v) material restatements of the Company’s consolidated financial statements filed with the SEC, (vi) a holder’s inability to rely on Rule 144 for sales of shares underlying the Notes, (vii) the Company’s common stock is suspended or halted from trading and/or fails to be quoted or listed (as applicable) on the OTCQB, OTCQX, any tier of the NASDAQ Stock Market, the New York Stock Exchange, or the NYSE American within 10 days thereafter, (viii) failure to file with the SEC a registration statement covering the resale of shares of common stock underlying the Notes and Warrants within 60 calendar days following the Issue Date, (ix) failure to cause such registration statement to become effective within 120 calendar days following the Issue Date, or (x) certain mergers consolidations, business combinations and sales of all or substantially all of the Company’s assets in the event the Company is not the survivor of such transaction.
Upon an Event of Default, a Note holder may declare all amounts under its Note(s) due and payable, in which event the Company will be required to pay such Note holder the sum of (i) the product of (a) all then outstanding principal amount and accrued interest thereon, multiplied by (b) 125%; and (ii) all collection costs including legal fees and expenses in connection therewith. At the option of a Note holder, in the event the Company receives cash proceeds as a result of certain events, including, but not limited to, payments from customers, issuances of debt or equity securities, exercise of warrants or asset sales, the Company will be required to use such proceeds to repay all or any lesser outstanding amounts due under such holder’s Note.
The Notes include covenants, representations, warranties, other payment obligations and agreements by the Company including, without limitation, most-favored nation rights, rights of participation and first refusal and exchange rights.
F-28
In connection with the issuance of the Notes, the Company issued Warrants to purchase in the aggregate 767,796 shares of its common stock at an initial exercise price of $24.00 per share. The Warrants may be exercised for a period of five years from the Trigger Date, provided that, if prior to the Trigger Date, the Company (i) completes a Qualified Capital Raise, the outstanding Warrants shall be cancelled or (ii) prepays a holder’s Note(s) in whole or in part, such holder’s pro-rata number of Warrants shall be cancelled. The fair value of the Warrants was $3,700,632, of which $2,379,182 was recorded as a debt discount, which is being amortized to interest expense over the term of the Warrants, and $1,321,450 was recorded as a loss on debt extinguishment. The Company calculated the fair value of the Warrants utilizing the Black-Scholes valuation model with the following assumptions: volatility of 88.98%, risk-free interest rate of 0.86%, a term of 5.75 years and a dividend yield of zero.
In connection with the April and May 2021 sales of the $4,250,000 aggregate principal amount of the Notes, the Company incurred debt issuance costs of $116,000, which were recorded as a debt discount and were amortized to interest expense over the term of the Notes using the effective interest rate method. The interest expense attributable to the debt discount, comprising the debt issuance costs and Warrants, during the year ended March 31, 2022 was $1,833,618.
Upon the closing of the Offering (see Note 7), which was a Qualified Capital Raise, in accordance with their terms, the Notes converted into 1,511,276 shares of common stock and the holders of the Notes received 1,511,276 Offering Warrants (as defined in Note 7). As a result of the Offering, the exercise price of the 767,796 outstanding Warrants was reduced to $6.00 per share.
NOTE 6 – PROMISSORY NOTE
In October 2017,2021, the Company issued a secured promissory note (the Bridge Note) to Manchester Explorer, L.P. (Manchester) that provided the Company with a $3,000,000 revolving credit facility with all amounts being drawn down by the Company thereunder being due and payable, subject to acceleration in the event of a default, on March 15, 2022 (the Maturity Date). Interest at the rate of 12% was payable on each drawn down without regard to the draw down date or the date when interest is paid.
The principal amount of the Bridge Note and interest due thereon is payable to Manchester no later than the earlier of: (i) the Maturity Date and (ii) the date on which the Company has received proceeds in excess of $12,000,000 from a transaction or series of related transactions occurring prior to the Maturity Date, which such transactions constitute equity financings or other issuances of the Company’s equity securities. Provided that no Event of Default (as such term is defined in the Bridge Note) has occurred, on any date prior to the Maturity Date, upon no less than three days written notice by the Company specifying the draw amount, Manchester will advance the draw amount to the Company. No draw amount can be in an amount less than $100,000 or exceed an amount equal to $3,000,000 minus the aggregate principal amount outstanding under the Bridge Note at the time of such draw request. If an Event of Default occurs and is continuing, Manchester may declare all of the Bridge Note, including any interest and other amounts due, to be due and payable immediately.
In connection with the issuance of the Note, on October 28, 2021, the Company entered into a security agreement with Manchester (the Security Agreement) under which the Company granted Manchester a continuing and unconditional first priority security interest in and to any and all of the Company’s property of any kind or description, tangible or intangible, wheresoever located and whether now existing or hereafter arising or acquired.
During fiscal 2022, the Company made draws on the Bridge Note of $2,100,000 and incurred interest charges of $252,000. In February 2022, subsequent to the completion of the Offering (see Note 7), the Bridge Note and accrued interest was paid in full, and the Security Agreement was terminated.
NOTE 7 – STOCKHOLDERS’ EQUITY (DEFICIT)
Public Offering
On February 9, 2022, the Company entered into an underwriting agreement (the Underwriting Agreement) with Oppenheimer & Co. Inc., who acted as the representative of the several underwriters (the Underwriters), in a firm commitment underwritten public offering (the Offering) pursuant to which, on February 14, 2022, the Company sold to the Underwriters an aggregate of 2,500,000 shares of the Company’s common stock and 2,500,000 warrants (the Offering Warrants and, collectively with the shares of common stock, the Units), each to purchase one share of common stock. The price to the public in the Offering was $6.00 per Unit, before underwriting discounts and commissions. The common stock and the Offering Warrants comprising the Units were immediately separable upon issuance and were issued separately. The Offering Warrants were exercisable immediately, have an exercise price of $6.60 per share and expire on February 14, 2027. The gross proceeds from the Offering were $15,000,000, before deducting underwriting discounts and commissions and other offering expenses.
F-29
Placements of Common Stock
Between March and December 2020, the Company completed a private placement of shares of its common stock (the 2020 Placement). The Company sold 962,387 shares of common stock, at a purchase price of $2.87 per share, for gross proceeds of $2,762,054. The Company paid placement agent fees on the 2020 Placement of $52,256 during fiscal 2021.
In October 2021, the Company sold 30,864 shares of common stock to two officers, its i) chief executive officer and ii) the chairman of the Company’s board of directors (the Board), president, chief financial officer and treasurer, at a purchase price of $8.10 per share, for gross proceeds of approximately $250,000.
During the year ended March 31, 2022, the Company issued to service providers 90,000 shares of common stock with a fair value of approximately $594,400.
NOTE 8 – STOCK-BASED COMPENSATION
Amended 2017 Equity Incentive Plan
In October 2017, the Company’s Board approved the 2017 Equity Incentive Plan (the 2017 Plan) with 3,000,0001,000,000 shares of common stock reserved for issuance. In January 2020 and August 2021, the Board approved an amendment to the 2017 Plan to increaseincreases in the number of shares reserved for issuance under the Plan by 1,000,000 shares.333,334 and 1,333,334 shares, respectively. Under the 2017 Plan, eligible employees, directors and consultants may be granted a broad range of awards, including stock options, stock appreciation rights, restricted stock, performance-based awards and restricted stock units. The 2017 Plan is administered by the Board or, in the alternative, a committee designated by the Board.
The exercise or purchase price of a stock option shall be calculated as follows:
The Board is responsible for determining the consideration to be paid for the shares of common stock to be issued upon exercise or purchase. The 2017 Plan generally does not allow for the transfer of awards, and the Board may amend, suspend or terminate the 2017 Plan at any time.
Stock-Based Compensation Expense
The expense relating to stock options is recognized on a straight-line basis over the requisite service period, usually the vesting period, based on the grant date fair value. The unamortized compensation cost, as of March 31, 20212022 was $2,242,352$3,286,370 related to stock options and is expected to be recognized as expense over a weighted-average period of approximately 2two years.
During the year ended March 31, 2021,2022, the Company granted options granted to purchase 827,427 shares of its common stock to employees, directors and consultantsconsultants. The options had 10-year terms and a137,292 options vested immediately when granted. The grant-date fair value was determined to be $8,507,311 of $1,101,737. Options to purchase 10,476 shares vested immediately onwhich $2,739,490 was recorded as stock-based compensation expense and included in the respective grant dates. consolidated statements of operations for the year ended March 31, 2022.
The following assumptions were used in the fair-value method calculations: calculations:
| Year ended March 31, | Year Ended March 31, | ||||||||||||||
| 2021 | 2020 | 2022 | 2021 | ||||||||||||
| Risk-free interest rates | - | - | 0.8% - 2.42 | % | 0.28% - 0.71 | % | |||||||||
| Volatility | - | - | 89% - 370 | % | 87% - 127 | % | |||||||||
| Expected life (years) | - | - | 5.0 - 6.2 | 5.0 - 6.0 | |||||||||||
| Dividend yield | — | — | |||||||||||||
The fair values of options at the grant date were estimated utilizing the Black-Scholes valuation model, which includes simplified methods to establish the fair term of options as well as average volatility of three comparable organizations. The risk-free interest rate was derived from the Daily Treasury Yield Curve Rates, as published by the U.S. Department of the Treasury as of the grant date for terms equal to the expected terms of the options. A dividend yield of zero was applied because the Company has never paid dividends and has no intention to pay dividends in the foreseeable future. In accordance with ASU No. 2016-09, the Company accounts for forfeitures as they occur.
F-30
A summary of stock option activity under the 2017 Plan is presented below:
| Shares | Options Outstanding | Options Outstanding | ||||||||||||||||||||||
| Available | Number of | Weighted Average | Shares Available | Number of | Weighted Average Exercise | |||||||||||||||||||
| for Grant | Shares | Exercise Price | for Grant | Shares | Price | |||||||||||||||||||
| Balance at March 31, 2019 | 490,031 | 509,969 | 2.56 | |||||||||||||||||||||
| Additional shares authorized under the Plan | 333,334 | — | — | |||||||||||||||||||||
| Options granted | (572,402 | ) | 572,402 | 6.75 | ||||||||||||||||||||
| Options cancelled and returned to the Plan | 23,056 | (23,056 | ) | 6.75 | ||||||||||||||||||||
| Balance at March 31, 2020 | 274,019 | 1,059,315 | 4.74 | 274,019 | 1,059,315 | $ | 4.74 | |||||||||||||||||
| Options granted | (163,492 | ) | 163,492 | 8.64 | (163,492 | ) | 163,492 | 8.64 | ||||||||||||||||
| Options cancelled and returned to the Plan | 25,555 | (25,555 | ) | 6.75 | 25,555 | (25,555 | ) | 6.75 | ||||||||||||||||
| Balance at March 31, 2021 | 136,082 | 1,197,252 | 5.25 | 136,082 | 1,197,252 | 5.25 | ||||||||||||||||||
| Additional shares authorized under the Plan | 1,333,334 | — | — | |||||||||||||||||||||
| Options granted | (827,427 | ) | 827,427 | 10.39 | ||||||||||||||||||||
| Share awards | (26,497 | ) | — | — | ||||||||||||||||||||
| Options cancelled and returned to the Plan | 373,974 | (373,974 | ) | 10.73 | ||||||||||||||||||||
| Balance at March 31, 2022 | 989,466 | 1,650,705 | $ | 6.58 | ||||||||||||||||||||
There were no stock options exercised during the years ended March 31, 20212022 and 2020.2021. The Company issued 26,497 shares to its non-employee directors under the Company’s outside director compensation plan and approximately $172,100 was recorded as stock-based compensation expense for these share awards during the year ended March 31, 2022.
The following table summarizes the range of outstanding and exercisable options as of March 31, 2021:2022:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (in Years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | Aggregate Intrinsic value | ||||||||||||||||||
| $ - $ | 1,197,252 | 8.25 | $ | 5.25 | 742,913 | $ | 3.96 | $ | 8,763,260 | |||||||||||||||
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (in Years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | Aggregate Intrinsic value | ||||||||||||||
| $1.98 - $17.70 | 1,650,705 | 8.01 | $ | 6.58 | 1,193,680 | $ | 5.69 | $ | 1,090,966 | |||||||||||
The intrinsic value per share is calculated as the excess of the closing price of the common stock on the Company’s principal trading market over the exercise price of the option.option at March 31, 2022.
The Company is required to present the tax benefits resulting from tax deductions in excess of the compensation cost recognized from the exercise of stock options as financing cash flows in the consolidated statements of cash flows. For the years ended March 31, 20212022 and 2020,2021, there were no such tax benefits associated with the exercise of stock options.
NOTE 6 – STOCKHOLDERS’ EQUITY
Private Placement
Between March and December 2020, the Company completed a private placement of shares of its common stock (the 2020 Placement). The Company sold shares of common stock, at a purchase price of $8.61 per share, for gross proceeds of $2,762,054. The Company paid placement agent fees on the 2020 Placement of $52,256 during fiscal 2021. Under the terms of the common stock purchase agreements between the Company and the investors, the Company must use commercially reasonable efforts to file a registration statement with the SEC to register for resale the shares of common stock sold.
NOTE 79 – INCOME TAXES
The income tax provision (benefit) consisted of the following:
| Year Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Current portion: | ||||||||
| Federal | $ | — | $ | — | ||||
| State | 1,600 | 1,600 | ||||||
| 1,600 | 1,600 | |||||||
| Deferred portion: | ||||||||
| Federal | (1,931,390 | ) | (1,180,434 | ) | ||||
| State | (576,868 | ) | (391,865 | ) | ||||
| (2,508,258 | ) | (1,572,299 | ) | |||||
| Change in valuation allowance | 2,508,258 | 1,572,299 | ||||||
| Provision for income taxes | $ | 1,600 | $ | 1,600 | ||||
As of
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Current portion: | ||||||||
| Federal | $ | — | $ | — | ||||
| State | 1,600 | 1,600 | ||||||
| 1,600 | 1,600 | |||||||
| Deferred portion: | ||||||||
| Federal | (4,109,000 | ) | (1,931,390 | ) | ||||
| State | (1,300,000 | ) | (576,868 | ) | ||||
| (5,409,000 | ) | (2,508,258 | ) | |||||
| Change in valuation allowance | 5,409,000 | 2,508,258 | ||||||
| Provision for income taxes | $ | 1,600 | $ | 1,600 | ||||
F-31
At March 31, 2021,2022, the Company had net operating loss carryforwards (NOLs) of approximately $13,954,000$27,600,000 for federal income tax purposes and $14,019,000$27,800,000 for state income tax purposes. These NOLs are available to reduce future taxable income and will expire at various times from 2037 through 2041,2042, except federal NOLs from fiscal 2018, 2019, 2020, 2021 and 20202022, which will never expire.
The Company also had federal research and development tax credit carryforwards of approximately $535,000,$800,000, which will begin expiring at various times from 2038 through 2040,2041, and state research and development credits of approximately $141,000,$200,000, which do not have an expiration date.
A reconciliation of income taxes provided at the federal statutory rate (21% for each of fiscal 20212022 and 2020)2021) to the actual income tax provision is as follows:
| Year Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Federal statutory rate | (21 | )% | (21 | )% | ||||
| State tax rate, net of federal benefit | (7 | )% | (7 | )% | ||||
| Permanent differences | — | % | — | % | ||||
| Research and development tax credits | (6 | )% | (3 | )% | ||||
| Section 179 assets | — | % | — | % | ||||
| Change in valuation allowance | 34 | % | 31 | % | ||||
| Effective income tax rate | — | % | — | % | ||||
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Federal statutory rate | (21 | )% | (21 | )% | ||||
| State tax rate, net of federal benefit | (7 | )% | (7 | )% | ||||
| Permanent differences | — | % | — | % | ||||
| Research and development tax credits | (2 | )% | (6 | )% | ||||
| Section 179 assets | — | % | — | % | ||||
| Change in valuation allowance | 30 | % | 34 | % | ||||
| Effective income tax rate | — | % | — | % | ||||
The losses before income tax provision for the years ended March 31, 2022 and 2021 were solely attributable to US operations.
Significant components of the Company’s deferred tax assets and liabilities were:
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| Net operating loss carryforwards | $ | 3,909,434 | $ | 1,965,118 | ||||
| Stock-based compensation expense | 554,892 | 364,989 | ||||||
| Property and equipment | (18,039 | ) | 6,842 | |||||
| Reserves, accruals & other | (79,878 | ) | (7,181 | ) | ||||
| Research and development tax credits | 646,296 | 237,716 | ||||||
| Total deferred tax assets | 5,012,705 | 2,567,484 | ||||||
| Less: valuation allowance | (5,012,705 | ) | (2,567,484 | ) | ||||
| Deferred tax assets, net | $ | — | $ | — | ||||
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| Net operating loss carryforwards | $ | 7,731,000 | $ | 3,909,434 | ||||
| Stock-based compensation expense | 1,824,000 | 554,892 | ||||||
| Property and equipment | 80,000 | (18,039 | ) | |||||
| Reserves, accruals & other | (104,000 | ) | (79,878 | ) | ||||
| Research and development tax credits | 988,000 | 646,296 | ||||||
| Total deferred tax assets | 10,519,000 | 5,012,705 | ||||||
| Section 179 assets | (97,000 | ) | — | |||||
| Total deferred tax liabilities | (97,000 | ) | — | |||||
| Less: valuation allowance | (10,422,000 | ) | (5,012,705 | ) | ||||
| Deferred tax assets, net | $ | — | $ | — | ||||
Based on the available information and other factors, management believes it is more likely than not that the net deferred tax assets at March 31, 20212022 and 2020,2021, will not be fully realizable. Accordingly, management has recorded a full valuation allowance against its net deferred tax assets at March 31, 20212022 and 2020.2021.
Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company’s consolidated financial statements at March 31, 20212022 and 2020.2021. The Company does not expect any significant changes in its unrecognized tax benefits within twelve months of the reporting date.
NOTE 810 – ROYALTY AGREEMENT
In July 2017, the Company entered into a royalty agreement with its founder, chiefthen-chief executive officer, president and major shareholderstockholder (the Founder). Pursuant to the agreement, the Founder assigned and transferred all of his rights in the intellectual property of Quasuras in return for future royalty payments on the Company’s product. The Company is obligated to make royalty payments under the agreement to the Founder on any sales of the royalty product sold or otherwise commercialized by the Company equal to (a) $0.75 on each sale of a royalty product or (b) 5% of the gross sale price of the royalty product, whichever is less. The royalty payments will cease, and the agreement will terminate, at such time as the total sum of royalty payments actually paid to the Founder, pursuant to the agreement, reaches $10,000,000. The Company has the option to terminate the agreement at any time upon payment, to the Founder, of the difference between total royalty payments actually made to him to date and the sum of $10,000,000. All payments of the royalties, if due, for the preceding quarter, will be made by the Company to the Founder within thirty days after the end of each calendar quarter.
F-32
NOTE 911 – RETIREMENT SAVINGS PLAN
Effective March 2020, the Company adopted the Modular Medical, Inc. 401(k) Plan (the Savings Plan), which qualifies as a thrift plan under Section 401(k) of the Internal Revenue Code. Full-time and part-time employees who are at least 21 years of age are eligible to participate in the Savings Plan at the time of hire. Participants may contribute up to 15% of their earnings to the Savings Plan. The Plan became effective and began accepting participant contributions in April 2020.
NOTE 10 – COMMITMENTS AND CONTINGENCIES
Litigations, Claims and Assessments
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. The Company records legal costs associated with loss contingencies as incurred and accrues for all probable and estimable settlements.
Indemnification
In the ordinary course of business, the Company enters into contractual arrangements under which it may agree to indemnify the counterparties from any losses incurred relating to breach of representations and warranties, failure to perform certain covenants, or claims and losses arising from certain events as outlined within the particular contract, which may include, for example, losses arising from litigation or claims relating to past performance. Such indemnification clauses may not be subject to maximum loss clauses. The Company has also entered into indemnification agreements with its officers and directors. No amounts were reflected in the Company’s consolidated financial statements for the years ended March 31, 20212022 and 20202021 related to these indemnifications. The Company has not estimated the maximum potential amount of indemnification liability under these agreements due to the limited history of prior claims and the unique facts and circumstances applicable to each particular agreement. To date, the Company has not made any payments related to these indemnification agreements.
NOTE 1112 – RELATED PARTY TRANSACTIONS
Consulting Services
DuringIn February 2021, the year ended March 31, 2020,Company’s chairman of the Company entered into consulting agreements with a member of its board of directors. Under the consulting agreements, during the year ended March 31, 2020, the Company paid the director consulting fees of $140,625in cash,Board and the director was granted stock options with a fair value of $76,875. The options were for a total of 15,687 shares of common stock, were fully vested on the grant datespresident and have terms of 10 years. The most recent consulting agreement, which was entered into between the Company and the director in September 2019, was terminated in March 2020. At March 31, 2020, the Company had an outstanding payable to the director of $5,585, which was included in accounts payable in the consolidated balance sheet. The Company paid the $5,585 to the director during fiscal 2021.
2021 Placement
The Company’s chief executive officer and an existing investor,Manchester, which is represented by a member of the Company’s board of directors, purchased $100,000 and $1,000,000, respectively, aggregate principal amount of the Original Notes, (the Related Party Notes)respectively. Effective April 30, 2021, the related party holders entered into revocation agreements with the Company pursuant to which their aggregate principal amount of Original Notes and accrued interest were replaced with Notes. On February 14, 2022, Manchester and the executive officer held Notes in the 2021 Placement. Asan aggregate principal amount of March 31, 2021, $1,677$1,026,630 and $16,767$102,663, respectively, with $97,881 and $9,788 of interest was payable bythereon. In connection with the Company onOffering, Manchester and the Related Party Notes to its chief executive officer received 234,274 and to the investor, respectively.
NOTE 12 – SUBSEQUENT EVENTS
Convertible Promissory Notes
Subsequent to March 31, 2021, the Company issued an additional $4,250,000 of the Notes in the 2021 Placement pursuant to a Securities Purchase Agreement between the Company and each investor (the SPA) and warrants to purchase shares of its common stock (the Warrants). The Notes are unsecured obligations of the Company with each Note having a stated maturity date of 12 months from its issue date (the Issue Date). The Notes bear interest at a rate of 12% per annum, payable on maturity, provided that, if the Company fails to pay any amounts when due under a Note, the interest rate increases to the greater of 16% or the maximum amount permitted by law. Each Note may be prepaid at the Company’s option during the first 270 calendar days following its Issue Date (the 270th day, the Trigger Date), subject to a 110% prepayment penalty on all principal and accrued but unpaid interest then outstanding. No Notes may be prepaid in whole or in part after the Trigger Date.
If the Notes remain outstanding after the Trigger Date, the Notes may be converted into shares of the Company’s common stock at an initial conversion price of $8.61 per share; provided, that a Note holder may not convert any portion of its Note that would cause it to beneficially own in excess of 4.99% of the Company’s outstanding common stock. The conversion price and number of shares of Company common stock issuable upon conversion of the Notes will be subject to adjustment from time to time for any subdivision or consolidation of shares and other standard dilutive and certain other corporate events, as provided in the Notes. Subject to certain Exempt Issuances (as defined in the Notes), if at any time while a Note is outstanding, the Company sells, issues or grants any shares of its common stock or other securities entitling the holder to acquire shares of the Company’s common stock at a price per share less than the then conversion price, such conversion price shall be reduced to such lesser price, and the number of shares of the Company’s common stock issuable upon conversion of the Notes shall be increased, as provided in the Notes.
If the Company completes an offering of its common stock or other securities in excess of $12,000,000 of gross proceeds (a Qualified Capital Raise), each Note holder will be required to convert its Adjusted Note Amount into the securities of such Qualified Capital Raise. For purposes hereof, Adjusted Note Amount equals the product of (i) the sum of all outstanding principal plus accrued but unpaid interest on a Note, multiplied by (ii) 1.25.
The Notes contain a number of Company events of default (Events of Default) including, without limitation (i) failure to pay any principal or interest thereon when due, (ii) failure to timely deliver shares upon conversions, (iii) failure to comply with SEC reporting requirements under the Exchange Act, (iv) certain breaches of the SPA, the Notes, the Warrants, and the Registration Rights Agreement, (v) material restatements of the Company’s consolidated financial statements filed with the SEC, (vi) a holder’s inability to rely on Rule 144 for sales of shares underlying the Notes, (vii) the Company’s common stock is suspended or halted from trading and/or fails to be quoted or listed (as applicable) on the OTCQB, OTCQX, any tier of the Nasdaq Stock Market, the New York Stock Exchange, or the NYSE American within 10 days thereafter, (viii) failure to file with the SEC a registration statement covering the resale of23,429 shares of common stock, underlying the Notesrespectively, and 234,274 and 23,429 Offering Warrants, within 60 calendar days following the Issue Date, (ix) failure to cause such registration statement to become effective within 120 calendar days following the Issue Date, or (x) certain merger consolidations, business combinations and sales of all or substantially all of the Company’s assets in the event the Company is not the survivor of such transaction.respectively.
Upon an Event of Default, a Note holder may declare all amounts under its Note(s) due and payable, in which event the Company will be required to pay such Noteholder the product of (i) all then outstanding principal amount and accrued but unpaid interest thereon, multiplied by (ii) 125%; and all collection costs including legal fees and expenses in connection therewith. At the option of a Note holder, in the event the Company receive cash proceeds as a result of certain events including, but not limited to, from customers, issuances of debt or equity securities, exercise of warrants or asset sales, the Company will be required to use such proceeds to repay all or any lesser outstanding amounts due under such holder’s Note.
The Notes also includes various covenants, including negative covenants, representations, warranties, other payment obligations and agreements by the Company including, without limitation, most-favored nation clauses, rights of participation and first refusal and exchange rights. In connection with the issuance of the Notes, the Company issued Warrants to purchase 761,912 shares of its common stock (Warrant Shares) at an initial exercise price of $24.00 per share. The Warrants may be exercised for a period of 5 years from the Trigger Date.
In the event that, prior to the Trigger Date, the Company (i) completes a Qualified Capital Raise, the outstanding Warrants shall be cancelled or (ii) prepays a holder’s Note(s) in whole or in part, such holder’s pro-rata number of its Warrants shall be cancelled.
Effective April 30, 2021, each of the holders of the $2,210,000 of Notes outstanding at March 31, 2021 entered into a revocation and replacement agreement with the Company (the Revocation Agreement). Under the terms of the Revocation Agreement, the $2,210,000 of Notes and accrued interest of $50,091 were replaced with new Notes consistent with the terms described above.
In May 2021, a member of the Board purchased $200,000 aggregate principal amount of Notes (the Director Note). On February 14, 2022, in connection with the Notes.
PPPOffering, the Director Note and $18,805 of accrued interest thereon were converted into 45,586 shares of common stock 45,586 Offering Warrants.
As a resultThe daughter of the Company’s request for loan forgiveness, on May 29, 2021,president, chief financial officer, treasurer and chairman of the Board is an employee of the Company. During fiscal 2022, the Company was notified thatpaid her $169,589, which includes the outstanding principalaggregate grant date fair value, as determined pursuant to FASB ASC Topic 718, of a stock option granted in November 2021.
NOTE 13 – SUBSEQUENT EVENT
On May 2, 2022, the Company entered into a securities purchase agreement (the Purchase Agreement) with an institutional investor (the Investor) pursuant to which the Company sold, in a registered direct offering (the Registered Offering), for gross proceeds of $8,000,000 an aggregate of 449,438 shares (the Shares) of the Company’s common stock, at a purchase price per Share of $4.45 and accrued interest forpre-funded warrants (the Pre-Funded Warrants) to purchase an aggregate of 1,348,314 shares of common stock at a purchase price per Pre-Funded Warrant of $4.44. The Pre-Funded Warrants were exercisable immediately on the PPP Note was forgivendate of issuance at an exercise price of $0.01 per share and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full byfull.
In a concurrent private placement under the U.S. Small Business Administration.Purchase Agreement, the Company issued warrants (the Private Placement Warrants) to the Investor to purchase an aggregate of 1,438,202 shares of common stock at an exercise price of $6.60 per share. The Private Placement Warrants will be exercisable commencing November 5, 2022 and have a five-year term.
F-33
Warrants to Purchase up to 1,438,202
2,116,402 Units
Each Unit Consisting of Two Shares of Common Stock and
One Warrant to Purchase One Share of Common Stock


MODULAR MEDICAL, Inc.
PROSPECTUS
June 6, 2022
PRELIMINARY PROSPECTUS
Sole Book-Running Manager
Newbridge Securities Corporation
, 2023
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The registrant estimates thatfollowing table sets forth all costs and expenses, other than underwriting discounts and commissions, paid or payable by the registrantRegistrant in connection with the offering described in this Registration Statement will be as follows:sale of the securities being registered. All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee:
| Securities and Exchange Commission registration fee | $ | 880 | ||
| Accounting fees and expenses * | $ | 2,500 | ||
| Legal fees and expenses | $ | 25,000 | ||
| Total | $ | 28,380* |
| Amount to be Paid | ||||
| SEC Registration Fee | $ | 1,711 | ||
| FINRA Filing Fee | 2,628 | |||
| Printing and Engraving Fees and Expenses | 25,000 | |||
| Legal Fees and Expenses | 300,000 | |||
| Accounting Fees and Expenses | 20,000 | |||
| Transfer Agent and Registrar Fees and Expenses | 10,000 | |||
| Miscellaneous Fees and Expenses | 50,000 | |||
| Total | $ | 409,339 | ||
Item 14.Indemnification of DirectorsOfficers and OfficersDirectors.
Our Second Amended and Restated Articles of Incorporation and our Amended and Restated Bylaws provide that each person who was or is made a party or is threatened to be made a party to or is otherwise involved (including, without limitation, as a witness) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he or she is or was one of our directors or officers or is or was serving at our request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, whether the basis of such action, suit or proceeding is alleged action in an official capacity as a director, officer or trustee or in any other capacity while serving as a director, officer or trustee, shall be indemnified and held harmless by us to the fullest extent authorized by the Nevada Revised Statutes, or NRS, against all expense, liability and loss (including attorneys’ fees and amounts paid in settlement) reasonably incurred or suffered by such.
NRS 78.7502 permits a corporation to indemnify any director or officer of the corporation against expenses (including attorneys’ fees) and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person (i) is not liable pursuant to NRS 78.138 and (ii) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the conduct was unlawful. In a derivative action (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or the suit if such person (i) is not liable pursuant to NRS 78.138 and (ii) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought or some other court of competent jurisdiction determines that such person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
II-1
Our Second Amended and Restated Articles of Incorporation provide that the liability of our directors and officers shall be eliminated or limited to the fullest extent permitted by the NRS. NRS 78.138(7) provides that, subject to limited statutory exceptions and unless the articles of incorporation or an amendment thereto (in each case filed on or after October 1, 2003) provide for greater individual liability, a director or officer is not individually liable to a corporation or its shareholdersstockholders or creditors for any damages as a result of any act or failure to act in his or her capacity as a director or officer unless it is proven that: (i) the act or failure to act constituted a breach of his or her fiduciary duties as a director or officer and (ii) the breach of those duties involved intentional misconduct, fraud or a knowing violation of law.
The foregoing discussion of our Second Amended and Restated Articles of Incorporation, Amended and Restated Bylaws and Nevada law is not intended to be exhaustive and is qualified in its entirety by such Second Amended and Restated Articles of Incorporation, Amended and Restated Bylaws, indemnification agreements, indemnity agreement, or law.
Nevada Revised Statutes provide that a corporation may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses.
We have been advised that in the opinion of the SEC, insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and other persons pursuant to the foregoing provisions, or otherwise, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event a claim for indemnification against such liabilities (other than payment of expenses incurred or paid by a director or officer in the successful defense of any action, suit or proceeding) is asserted by such director, officer or other person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act of 1933, as amended, against certain liabilities.
Item 15.Recent Sales of Unregistered Securities.Securities
Set forth below is information regarding shares of Common Stock,common stock , convertible notes and warrants issued, and options granted, by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such shares, convertible notes, warrants and options, and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
II-2
Director Compensation
On December 30, 2022, we issued 6,375 shares of our common stock to four of our non-employee directors in accordance with our Outside Director Compensation Plan (the “Director Plan”). On September 30, 2022, we issued 6,375 shares of our restricted common stock to four of our non-employee directors in accordance with the Director Plan. On August 8, 2022, we issued 5,000 shares of our restricted common stock to two of our non-employee directors in accordance with the Director Plan. On June 30, 2022, we issued 2,664 shares of our common stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On March 31, 2022, we issued 15,250 shares of our Common Stockcommon stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On December 31, 2021, we issued 5,775 shares of our Common Stockcommon stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On September 30, 2021, we issued 3,636 shares of our Common Stockcommon stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On June 30, 2021, we issued 1,836 shares of our Common Stockcommon stock to non-employee members of our board of directors for service as directors in accordance with our Outsidethe Director Compensation Plan (the Director Plan).Plan.
Service Providers
On March 23,20, 2023, we issued 10,000 shares of our common stock to a service provider. On March 13, 2023, we issued 478 shares of our common stock to a service provider. On February 13, 2023, we issued 438 shares of our common stock to a service provider. On May 9, 2022, we issued 45,000348 shares of our Common Stockcommon stock to a service provider. On January 5, 2022, we issued 16,666 shares of our Common Stockcommon stock to service providers.
2022 Placement
In May 2022, the Company issued warrants in a private placement to purchase an aggregate of 1,438,202 shares of common stock at an exercise price of $6.60 per share. The warrants were exercisable six months from the date of issuance and have a five-year term from the date the warrants become exercisable.
Officer Purchases of Common Stock
On October 28, 2021, we sold to two of our executive officers a total of 30,864 shares of our Common Stockcommon stock at a purchase price of $8.10 per share, which resulted in gross proceeds to us of $250,000.
2021 Placement
Between February and May 2021, we issued to accredited investors in a private placement (the “2021 Placement”) $6,610,550 aggregate principal amount of our 12% unsecured convertible promissory notes, due 12 months from each respective issuance date, at par and warrants to purchase in the aggregate 761,912 shares of our Common Stockcommon stock at an exercise price of $24.00 per share, exercisable for a 5-year period, as provided in such warrants.
2020 Placement
II-3
Item 16. Exhibits.
Between March and December 2020, we sold
The Index to accredited investors in a private placement (the 2020 Placement) a total of 320,796 shares of our Common Stock at a purchase price of $8.61 per share. The 2020 Placement resulted in gross proceeds to us of $2,762,054.
Other Transactions
In 2021, we issued a total of 52,836 shares of our Common Stock to five service providers in exchange for services rendered. In 2019, we issued 10,000 shares of our Common Stock for cash to a service provider.
2018 Placement
Between November 2018 and March 2019, we sold to accredited investors in a private placement (the 2018 Placement) a total of 618,996 shares of our Common Stock at a purchase price of $6.75 per share, resulting in gross proceeds to us of $4,142,666.
The above sales of our securities were made pursuant to exemptions from registration pursuant to Section 4(2) and/or Rule 506Exhibits listing the exhibits required by Item 601 of Regulation D ofS-K is located on the Securities Act. We made such determinations based upon representations bypage immediately following the purchasers of such securities including, without limitation, that such purchasers were “accredited investors” as defined in the Securities Act.signature page to this registration statement.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
* Filed herewith
+ Management contract, compensatory plan or arrangement.
(b) Financial statement schedules.
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of |
| (ii) | ||
| To reflect in the prospectus any facts or events arising after the effective date of the |
| (iii) | ||
| To include any material information with respect to the plan of distribution not previously disclosed in the |
| (2) | That, for the purpose of determining any liability under the Securities Act, |
| (3) | ||
| To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act |
| (i) | each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the Registration Statement as of the date the filed prospectus was deemed part of and included in the Registration Statement; and |
| (ii) | each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering |
II-4
| (5) | ||
| (6) | That, for the purpose of determining liability of the registrant under the Securities Act | |
| The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | ||
| Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | ||
| The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | ||
| Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. (a) Exhibits. See the Exhibit Index included immediately prior to the signature page to this registration statement, which is incorporated by reference herein. (b) Financial Statement Schedules. No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or notes. II-5 EXHIBIT INDEX (a) Exhibits
II-6
II-7
SIGNATURES Pursuant to the requirements of the Securities Act of 1933,
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
II-8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||