

As filed with the Securities and Exchange Commission on September 26, 2003February 3, 2006
Registration No. 333- 333-128827
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 6 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ACORDA THERAPEUTICS, INC.
(Exact nameName of registrantRegistrant as specifiedSpecified in its charter)Charter)
| Delaware | 2836 | 13-3831168 | ||
| (State or Other Jurisdiction of | (Primary Standard Industrial Classification Code Number) | ( |
15 Skyline Drive
Hawthorne, New York 10532
(914) 347-4300
(Address, Including Zip Code, and Telephone Number,
Including Area Code, of Registrant's Principal Executive Offices)
Ron Cohen
Chief Executive Officer
15 Skyline Drive
Hawthorne, New York 10532
(914) 347-4300
(Name, address, including zip code,Address, Including Zip Code, and telephone number, includingTelephone Number,area code,Including Area Code, of agent for service)Agent For Service)
Copy To:
| Ellen B. Corenswet | ||
New York, New York (212) | Danielle Carbone Shearman & Sterling LLP 599 Lexington Avenue New York, New York 10022 (212) 848-4000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this form are to bebeing offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the "Securities Act") check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434 under the Securities Act, please check the following box. o
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Amount to be Registered(1) | Proposed Maximum Offering Price Per Share | Proposed Maximum Aggregate Offering Price(2) | Amount of Registration Fee | ||||
|---|---|---|---|---|---|---|---|---|
| Common Stock, $0.001 par value per share | $ | $75,000,000 | $6,067.50 | |||||
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until thethis registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we areis not soliciting an offeroffers to buy these securities in any jurisdictionstate where the offer or sale is not permitted.
Prospectus
SUBJECT TO COMPLETION, DATED SEPTEMBER 26, 2003FEBRUARY 3, 2006
Prospectus
5,500,000 Shares


Common Stock
Acorda Therapeutics, Inc. is offering 5,500,000 shares of common stock. This is our initial public offering, and no public market currently exists for our shares. We anticipate that the initial public offering price will be between $$11.00 and $$13.00 per share. After the offering, the market price for our shares may be outside this range.
We will applyhave applied to list our common stock on Thethe Nasdaq National Market under the symbol "ACRD."ACOR."
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 8.9.
| | Per Share | Total | ||||
|---|---|---|---|---|---|---|
| Offering price | $ | $ | ||||
| Discounts and commissions to underwriters | $ | $ | ||||
Offering proceeds to Acorda Therapeutics, Inc., before expenses | $ | $ | ||||
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this prospectus is accurate or complete. Any representation to the contrary is a criminal offense.
We have granted the underwriters the right to purchase up to 825,000 additional shares of common stock to cover any over-allotments. The underwriters can exercise this right at any time within 30 days after the offering. The underwriters expect to deliver the shares of common stock to investors on or about , 2003.2006.
Banc of America Securities LLC
Lazard
U.S. Bancorp Piper Jaffray
RBC Capital Markets
| Lazard Capital Markets |
| Piper Jaffray |
| SG Cowen & Co. |
, 20032006
![[INSIDE FRONT COVER]](https://capedge.com/proxy/S-1/0001047469-03-031904/g148063.jpg)
You should rely only on the information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with different information. We are not making offers to sell or seeking offers to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this prospectus is accurate as of the date on the front of this prospectus only. Our business, financial condition, results of operations and prospects may have changed since that date.
We have registered "Acorda Therapeutics" and our logo as trademarks in the United States. Other trademarks mentioned in this prospectus are the property of their respective owners.
| | Page | |
|---|---|---|
| Summary | ||
Risk Factors | ||
Forward-Looking Statements | ||
Use of Proceeds | ||
Dividend Policy | ||
Capitalization | ||
Dilution | ||
Selected Consolidated Financial | ||
Management's Discussion and Analysis of Financial Condition and Results of Operations | ||
Business | ||
Management | ||
Summary Compensation Table | 99 | |
| Certain Relationships and Related Transactions | ||
Principal Stockholders | ||
Description of Capital Stock | ||
Shares Eligible | ||
Underwriting | 120 | |
| Legal Matters | ||
Experts | ||
Where You Can Find | ||
2i
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information you should consider before investing in our common stock. You should read the entire prospectus carefully including the "Risk Factors" section and our consolidated financial statements and the related notes included in this prospectus before making an investment decision. References in this prospectus to "Acorda," "the company," "we," "us" and "our" refer to Acorda Therapeutics, Inc.
OUR BUSINESS
Acorda Therapeutics isWe are a late-stagecommercial-stage biopharmaceutical company dedicated to the identification, development and commercialization of novel therapies that improve neurological function in people with multiple sclerosis, or MS, spinal cord injury, multiple sclerosisor SCI, and relatedother disorders of the central nervous system, or CNS. Our currentmarketed product, candidatesZanaflex Capsules, is FDA-approved for the management of spasticity. Our lead product candidate, Fampridine-SR, is in a Phase 3 clinical trial for the improvement of walking ability in people with MS. Our preclinical programs also target the treatment of a wide range ofMS and SCI, as well as other CNS disorders, affecting individuals with chronic spinal cord injury, referred to as SCI,including stroke and multiple sclerosis, referred to as MS, including spasticity, muscle weakness, loss of bowel and bladder control and sexual dysfunction.traumatic brain injury.
Approximately 500,000650,000 people in the United States suffer from MS or SCI and MS and we believe that the combined annual cost of treatment for these conditions exceeds $9$13 billion. It is estimated that a total of approximately 10 million people live with the long-term consequences of traumatic brain injury and stroke.
Our goal is to becomecontinue to grow as a fully integratedfully-integrated biopharmaceutical company by commercializing multiple therapeuticpharmaceutical products, developing our product candidates and advancing our preclinical programs for these large and underserved markets while continuingmarkets. We plan to augmentaccomplish this through our product pipelinesales and to identify new applications formarketing infrastructure, our core technologies.extensive scientific and medical network, our partnerships and our clinical and management experience.
Our Product CandidatesPipeline
Zanaflex
Our products, Zanaflex Capsules and Zanaflex tablets, are FDA-approved for the management of spasticity, a symptom of conditions such as MS and SCI that is commonly characterized by stiffness and rigidity, restriction of movement and painful muscle spasms. Zanaflex Capsules and Zanaflex tablets contain tizanidine hydrochloride, or tizanidine, one of the two leading treatments currently used for the management of spasticity. We acquired Zanaflex Capsules and Zanaflex tablets from a wholly-owned subsidiary of Elan Corporation, plc, or Elan, in July 2004. This strategic acquisition provided us with the opportunity to build a commercial infrastructure, develop sales and marketing expertise and create a foundation for future product launches, in addition to generating product revenue.
In April 2005, we launched Zanaflex Capsules, a new capsule formulation of tizanidine. This product is protected by an issued U.S. patent. Zanaflex tablets lost compound patent protection in 2002 and both products now compete with 11 generic versions of tizanidine tablets.
We believe that Zanaflex Capsules offer important benefits over Zanaflex tablets and generic tizanidine tablets. When taken with food, Zanaflex Capsules have a different blood absorption profile, referred to as pharmacokinetic profile, than Zanaflex tablets and generic tizanidine tablets, generally resulting in a lower level and more gradual rise of peak levels of tizanidine in a patient's blood. As a result of this different pharmacokinetic profile, Zanaflex tablets and generic tizanidine tablets are not therapeutically equivalent, or AB-rated, with Zanaflex Capsules. Therefore, under state pharmacy laws, prescriptions written for Zanaflex Capsules may not properly be filled by the pharmacist with Zanaflex tablets or generic tizanidine tablets. Zanaflex Capsules are also available in a higher dose, which gives patients and prescribers an additional choice in dosing and an opportunity to reduce the number of pills a person must take daily. In addition, people who have difficulty swallowing may find Zanaflex Capsules easier to take.
To support our commercialization of Zanaflex Capsules, we have established a sales and marketing infrastructure consisting of our internal specialty sales force, a contract sales force and a pharmaceutical telesales group. Our internal specialty sales force currently consists of 14 sales professionals who call on neurologists and other prescribers specializing in treating patients with conditions that involve spasticity. Members of this sales force also call on managed care organizations, pharmacists and wholesale drug distribution customers. We plan to expand our specialty sales force to approximately 30 sales professionals in the first quarter of 2006. Our contract sales force is provided by Cardinal Health PTS, LLC, or Cardinal Health, and consists of approximately 160 sales representatives who market Zanaflex Capsules to primary care physicians, on a non-exclusive basis. We also have a contract with Access Worldwide Communications to provide a small, dedicated sales force of pharmaceutical telesales professionals to contact primary care physicians, specialty physicians and pharmacists. Our current sales and marketing infrastructure enables us to reach virtually all high-volume prescribers of Zanaflex tablets and generic tizanidine. We believe that these prescribers are also potential high-volume prescribers for our lead product candidate, Fampridine-SR, if approved.
Fampridine-SR
Fampridine-SR is an oral,currently in a Phase 3 clinical trial for the improvement of walking ability in people with MS. The trial is being conducted pursuant to a Special Protocol Assessment, or SPA, with the FDA. The FDA has agreed that, if successful, this trial could qualify as one of the pivotal efficacy studies required for drug approval. Fampridine-SR is a small molecule drug contained in a sustained release oral tablet form. Laboratory studies have shown that fampridine, the active molecule ofin Fampridine-SR, improves impulse conduction in nerve fibers in which the insulating outer layer, ofcalled the spinal cord, called myelin sheath, has been damaged. This damage may be caused by physical trauma, in the case of SCI, or by the body's own immune system, in the case of MS. We are developing Fampridine-SR for useMS, or by people with SCI or MS.physical trauma, in the case of SCI.
Clinical trials of Fampridine-SR are the first, to our knowledge, that have demonstrated improved neurological function in people with chronic SCI or MS. In cooperation with Elan Corporation plc, or Elan, we have conducted a series of clinical trials during the past six years evaluating Fampridine-SR. Approximately 550More than 800 people have been treated with Fampridine-SR in 14over 25 clinical trials, including eightnine clinical trials for SCIin MS and six11 clinical trials for MS.in SCI. In six Phase 2 clinical trials, treatment with Fampridine-SR has been associated with a variety of neurological benefits in people with SCIMS or MS.
We are currently conducting two Phase 3 clinical trials in people with SCI for the reduction of muscle stiffness, referred to as spasticity, and one lateSCI. In our most recently completed Phase 2 clinical trial, there was a trend toward improvement in the primary endpoint of walking speed and, when analyzed using the same methodology that the FDA has now agreed to in the SPA for our Phase 3 clinical trial, these results are statistically significant. We expect the recruitment period for the current Phase 3 clinical trial, which began in June 2005, to end in February 2006. The treatment period is 14 weeks and the subjects are involved in trial procedures for approximately five months. We expect to be able to evaluate data from this clinical trial in the third quarter of 2006.
We believe Fampridine-SR is the first potential therapy in late-stage clinical development for MS that seeks to improve the function of damaged nerve fibers, rather than only treating the symptoms of MS or slowing the progression of disease. To our knowledge, there are no current drug therapies that improve walking ability in people with MS for the improvement of walking speed. Our goals are to submit a New Drug Application, or NDA, to the United States Food and Drug Administration, or the FDA, for Fampridine-SR for the treatment of spasticity in SCI in 2004 and for the treatment of lower extremity motor dysfunction in people with MS in 2005.MS. We plan to commercialize Fampridine-SR, if approved, ourselves in the United States, and possibly Canada, and with partners in various other markets throughout the rest of the world. We have received Orphan Drug designation from the FDA for Fampridine-SR for the treatment of both SCI and MS.
Our second most advanced product candidate is valrocemide, which is currently in Phase 2 clinical trials for the treatment of epilepsy. Valrocemide is a small molecule drug with early Phase 2 clinical evidence of safety and efficacy as an add-on therapy for partial seizures, a type of epilepsy, and pre-clinical evidence of activity in animal models of epilepsy and neuropathic pain. We plan to move valrocemide into late Phase 2 clinical trials for epilepsy and early Phase 2 clinical trials for bipolar disorder in 2004. We may also pursue
3
clinical development of valrocemide for the treatment of neuropathic pain. Valrocemide is being co-developed and co-promoted with Teva Pharmaceutical Industries Ltd., or Teva, and its affiliates in the United States.Preclinical programs
We have three preclinical programs focused on novel approaches to repair damaged components of the CNS:
We believe that all of our preclinical therapies have also developed a chondroitinase program based on the concept of breaking down scar tissue that forms as a result of injury,potential to address conditions for which is believed to limit the regeneration of nerve fibers in the CNS.no effective treatment currently exists. In addition to applicability in MS, SCI and various other CNS disorders, we believe that our preclinical programs also may have initiated a regenerative antibody program to identify novel approaches to stimulate nerve fiber regenerationapplicability in CNS. To support our researchsuch fields as orthopedics, cardiology, oncology and development efforts, we have substantial laboratory capabilities employing both tissue culture methods and predictive animal models of SCI repair. These capabilities allow us to rapidly screen and validate potentially useful therapeutic approaches to SCI.
Our product development programs include a patent portfolio comprised of 24 U.S. patents and 40 U.S. patent applications and numerous foreign counterparts, of which we are the assignee or have in-licensed.ophthalmology.
Our core initial focus on the development of treatments for SCI has led, and we believe will continue to lead, to the identification and development of therapies applicable to other CNS disorders. Since many of the mechanisms of tissue damage and repair in SCI are shared by other conditions, such as MS, stroke and traumatic brain injury, our core technologies have potentially broad applicability for these and other CNS indications.
Our strategy is to focuscontinue to grow as a fully-integrated biopharmaceutical company focused on the identification, development and marketingcommercialization of a broad range of CNS therapeutics,nervous system therapeutics. We are using our scientific and clinical expertise in MS and SCI as a strategic pointpoints of access. In orderaccess to implement thisadditional CNS markets, including stroke and traumatic brain injury. Key aspects of our strategy we plan to pursue the following initiatives:are to:
To keep us apprised of the latest technological advances and help us identify and evaluate business development opportunities, weWe have established an advisory team and network of well-recognized scientists, clinicians and opinion leaders in the fields of SCIMS and MS.SCI. Depending on their expertise, these advisors provide assistance in trial design, conduct clinical trials, keep us apprised of the latest scientific advances and help us identify and evaluate business development opportunities. In addition, we have recruited 80 SCI rehabilitationover 35 MS centers and 24 MS80 SCI rehabilitation centers in the United States and Canada to conduct our clinical trials. Our clinical management team has extensive experience in the areas of SCIMS and MSSCI and works closely with this network.
4
Our business is subject to numerous risks, as more fully described in the section entitled "Risk Factors" immediately following this prospectus summary. We may be unable, for many reasons, including those that are beyond our control, to implement our current business strategy. Those reasons
could include failure to successfully promote Zanaflex Capsules and any other future marketed products; delays in obtaining, or a failure to obtain, regulatory approval for our product candidates; and failure to maintain and to protect our proprietary intellectual property assets, among others. The information about our preclinical and clinical trials may be useful to you in evaluating our company's current stage of development and our near-term and long-term prospects; however, you should note that of the large number of drugs in development only a small percentage successfully complete the FDA regulatory approval process and are commercialized.
We have a limited operating history and, as of September 30, 2005, had an accumulated deficit of approximately $198.5 million. We expect to incur losses for at least the next several years. We had net losses of $26.0 million and $44.7 million for the nine months ended September 30, 2005 and for the year ended December 31, 2004, respectively. We are unable to predict the extent of future losses or when we will become profitable, if at all. Even if we succeed in promoting Zanaflex Capsules and developing and commercializing one or more of our product candidates, we may never generate sufficient sales revenue to achieve and sustain profitability.
We were incorporated in 1995 as a Delaware corporation. Our principal executive offices are located at 15 Skyline Drive, Hawthorne, New York 10532. Our telephone number is (914) 347-4300. Our website iswww.acorda.com. The information on our website is not part of this prospectus.
"Acorda Therapeutics" is a registered trademark that we own and "Zanaflex" is a registered trademark that we exclusively license. We have pending U.S. trademark applications for our logo and "Zanaflex Capsules." Other trademarks, trade names and service marks used in this prospectus are the property of their respective owners.
| Common stock offered | 5,500,000 shares | |
Common stock outstanding after this offering | 19,047,022 shares | |
Use of proceeds | We intend to use the net proceeds of this offering for | |
Proposed Nasdaq National Market symbol | ACOR | |
Risk factors | See "Risk Factors" and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. |
The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of JuneSeptember 30, 20032005 and assumesexcludes the following:
In the table above, the number of shares of common stock outstanding after this offering excludes:
5 Unless we specifically state otherwise, all information in this prospectus, including the number of shares of common stock to be outstanding after this offering:
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table presents a summary of our historical financial information. You should read this information in conjunction with our consolidated financial statements and related notes and the information under "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this prospectus.
| | Year Ended June 30, | Period from March 17, 1995 (inception) to June 30, 2003 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||||||||
| | ($ in thousands, except per share data) | ||||||||||||||||||||
| Statement of Operations Data: | |||||||||||||||||||||
| Grant revenue | $ | 1,036 | $ | 756 | $ | 462 | $ | 132 | $ | 474 | $ | 3,637 | |||||||||
| Operating expenses incurred in the development stage: | |||||||||||||||||||||
| Research and development | 3,083 | 4,777 | 6,142 | 11,146 | 17,527 | 46,175 | |||||||||||||||
| Research and development—Related party | 1,152 | 2,024 | 2,223 | 4,687 | 2,265 | 32,351 | |||||||||||||||
| General and administrative | 1,342 | 1,406 | 3,489 | 6,636 | 6,388 | 22,855 | |||||||||||||||
| Total operating expenses | 5,577 | 8,207 | 11,854 | 22,469 | 26,180 | 101,381 | |||||||||||||||
| Operating loss | (4,541 | ) | (7,451 | ) | (11,392 | ) | (22,337 | ) | (25,706 | ) | (97,744 | ) | |||||||||
| Other income (expense): | |||||||||||||||||||||
| Interest expense | — | — | — | — | (78 | ) | (78 | ) | |||||||||||||
| Interest expense—Related party | (425 | ) | (448 | ) | (444 | ) | (408 | ) | (369 | ) | (2,580 | ) | |||||||||
| Interest income | 611 | 1,001 | 1,824 | 984 | 393 | 5,038 | |||||||||||||||
| Other income | — | — | — | — | 26 | 26 | |||||||||||||||
| Total other income (expense) | 186 | 553 | 1,380 | 576 | (28 | ) | 2,406 | ||||||||||||||
| Minority interest—Related party | — | — | 699 | 580 | — | 4,279 | |||||||||||||||
| Net loss | (4,355 | ) | (6,898 | ) | (9,313 | ) | (21,181 | ) | (25,734 | ) | (91,059 | ) | |||||||||
| Beneficial conversion feature, accretion of issuance costs, preferred dividends and fair value of warrants issued to convertible preferred stockholders | (18 | ) | (27 | ) | (36 | ) | (55 | ) | (23,793 | ) | (24,426 | ) | |||||||||
| Net loss allocable to common stockholders | $ | (4,373 | ) | $ | (6,925 | ) | $ | (9,349 | ) | $ | (21,236 | ) | $ | (49,527 | ) | $ | (115,485 | ) | |||
| Net loss per share allocable to common stockholders—basic and diluted | $ | (18.38 | ) | $ | (29.34 | ) | $ | (39.08 | ) | $ | (86.05 | ) | $ | (198.91 | ) | ||||||
| Pro forma net loss per share allocable to common stockholders—basic and diluted (unaudited)(1) | ($ | 15.38 | ) | ||||||||||||||||||
| Weighted average shares of common stock outstanding used in computing net loss per share allocable to common stockholders—basic and diluted | 232 | 236 | 239 | 247 | 249 | ||||||||||||||||
| Weighted average shares of common stock outstanding used in computing pro forma net loss per share allocable to common stock-holders—basic and diluted (unaudited)(1)(2) | 8,321 | ||||||||||||||||||||
Pro forma amounts in the following table reflect the conversion of our outstanding convertible preferred stock and mandatorily redeemable convertible preferred stock were converted into common stock as of the beginning of the year ended June 30, 2003 or from their respective dates of issuance,
6
if issued after the beginning of the year. The pro forma net loss per share allocable to common stockholders has been computed assuming the offering was completed at the beginning of the fiscal year presented and has been adjusted to give effect to the following: (a) recognition of the unamortized portion of a beneficial conversion charge of $78.6 million; (b) recognition of the unamortized portion of issuance costs relating to Series E, Series I and Series J preferred stock of $479,000; and (c) reversal of accrued preferred dividends on Series J preferred stock of $630,000 (see Note 2 to the consolidated financial statements).
| | | | | | | Nine Months Ended September 30, | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | Six Months Ended December 31, | | |||||||||||||||||||
| | Year Ended June 30, | Year Ended December 31, | ||||||||||||||||||||||
| | 2004 | 2005 | ||||||||||||||||||||||
| | 2001 | 2002 | 2003 | 2003 | 2004 | (unaudited) | ||||||||||||||||||
| | ||||||||||||||||||||||||
| | (in thousands, except per share data) | |||||||||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||||||
| Gross sales—Zanaflex | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 3,239 | ||||||||||
| Less: discounts and allowances | — | — | — | — | (4,417 | ) | (144 | ) | (992 | ) | ||||||||||||||
| Net sales | — | — | — | — | (4,417 | ) | (144 | ) | 2,247 | |||||||||||||||
| Grant revenue | 462 | 132 | 474 | 382 | 479 | 445 | 184 | |||||||||||||||||
| Total net revenue | 462 | 132 | 474 | 382 | (3,938 | ) | (301 | ) | 2,431 | |||||||||||||||
| Less: cost of sales | — | — | — | — | (885 | ) | (363 | ) | (2,274 | ) | ||||||||||||||
| Gross profit | 462 | 132 | 474 | 382 | (4,823 | ) | (62 | ) | 157 | |||||||||||||||
Operating expenses: | ||||||||||||||||||||||||
| Research and development | 6,142 | 11,147 | 17,527 | 16,743 | 21,999 | 18,621 | 9,652 | |||||||||||||||||
| Research and development—related party | 2,223 | 4,687 | 2,265 | 3,343 | — | — | — | |||||||||||||||||
| Sales and marketing | — | — | — | — | 4,662 | 2,793 | 9,657 | |||||||||||||||||
| General and administrative | 3,489 | 6,636 | 6,388 | 17,069 | 13,283 | 11,034 | 6,339 | |||||||||||||||||
| Total operating expenses | 11,854 | 22,470 | 26,180 | 37,155 | 39,944 | 32,448 | 25,648 | |||||||||||||||||
| Operating loss | (11,392 | ) | (22,338 | ) | (25,706 | ) | (36,773 | ) | (44,767 | ) | (32,510 | ) | (25,491 | ) | ||||||||||
Other income (expense): | ||||||||||||||||||||||||
| Interest and amortization of debt discount expense | — | — | (78 | ) | (38 | ) | (385 | ) | (297 | ) | (824 | ) | ||||||||||||
| Interest and amortization of debt discount expense—related party | (443 | ) | (408 | ) | (369 | ) | (184 | ) | — | — | — | |||||||||||||
| Interest income | 1,824 | 984 | 393 | 276 | 409 | 329 | 347 | |||||||||||||||||
| Other income | — | — | 26 | 7 | 2 | 2 | 1 | |||||||||||||||||
| Total other income (expense) | 1,381 | 576 | (28 | ) | 61 | 26 | 34 | (476 | ) | |||||||||||||||
| Minority interest—related party | 699 | 580 | — | — | — | — | — | |||||||||||||||||
| Cumulative effect of change in accounting principle | — | — | — | — | — | — | 3 | |||||||||||||||||
| Net loss | (9,313 | ) | (21,181 | ) | (25,734 | ) | (36,712 | ) | (44,741 | ) | (32,476 | ) | (25,964 | ) | ||||||||||
Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders | (36 | ) | (55 | ) | (24,320 | ) | (11,985 | ) | (24,746 | ) | (18,496 | ) | (18,636 | ) | ||||||||||
| Net loss allocable to common stockholders | $ | (9,349 | ) | $ | (21,236 | ) | $ | (50,054 | ) | $ | (48,697 | ) | $ | (69,487 | ) | $ | (50,972 | ) | $ | (44,600 | ) | |||
| Net loss per share allocable to common stockholders—basic & diluted | $ | (50.81 | ) | $ | (111.90 | ) | $ | (261.38 | ) | $ | (252.87 | ) | $ | (351.76 | ) | $ | (259.22 | ) | $ | (221.17 | ) | |||
| | | | | | | Nine Months Ended September 30, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | Six Months Ended December 31, | | ||||||||||||
| | Year Ended June 30, | Year Ended December 31, | |||||||||||||||
| | 2004 | 2005 | |||||||||||||||
| | 2001 | 2002 | 2003 | 2003 | 2004 | (unaudited) | |||||||||||
| | |||||||||||||||||
| Pro forma net loss per share allocable to common stockholders—basic & diluted (unaudited) | $ | (9.63 | ) | $ | (1.92 | ) | |||||||||||
| Weighted average shares of common stock outstanding used in computing net loss per share allocable to common stockholders—basic & diluted | 184 | 190 | 191 | 193 | 198 | 197 | 202 | ||||||||||
| Weighted average shares of common stock outstanding used in computing pro forma net loss per share allocable to common stockholders—basic & diluted (unaudited) | 13,536 | 13,547 | |||||||||||||||
The following table sets forth our cash, cash equivalents and short-term investments and capitalization as of September 30, 2005:
The pro forma consolidated balance sheet data below reflects the assumed conversionall of our outstanding convertible preferred stock and mandatorily redeemable convertible preferred stock into 15,806,61713,338,279 shares of our common stock upon completionon the closing of this offering. Theoffering; and
| | June 30, 2003 | |||||||
|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma (unaudited) | Pro Forma as Adjusted (unaudited) | |||||
| | ($ in thousands) | |||||||
| Consolidated Balance Sheet Data: | ||||||||
| Cash and cash equivalents | $ | 48,319 | $ | 48,319 | ||||
| Restricted cash | 253 | 253 | ||||||
| Short-term investments | 12,250 | 12,250 | ||||||
| Working capital | 58,975 | 58,975 | ||||||
| Total assets | 64,807 | 64,807 | ||||||
| Deferred revenue | 95 | 95 | ||||||
| Current portion of notes payable | 310 | 310 | ||||||
| Non-current portion of notes payable | 612 | 612 | ||||||
| Long-term convertible notes payable—principal amount plus accrued interest, less unamortized debt discount—Related party | 7,907 | 7,907 | ||||||
| Mandatorily redeemable preferred stock | 36,712 | |||||||
| Total stockholders' equity | $ | 16,803 | $ | 53,515 | ||||
7
| | As of September 30, 2005 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| | Actual (unaudited) | Pro Forma (unaudited) | Pro Forma As Adjusted (unaudited) | ||||||
| | (in thousands) | ||||||||
| Balance Sheet Data: | |||||||||
| Cash and cash equivalents | $ | 3,581 | $ | 14,879 | $ | 74,296 | |||
| Restricted cash | 261 | 261 | 261 | ||||||
| Short-term investments | 5,160 | 5,160 | 5,160 | ||||||
| Working capital | (12,203 | ) | (14,207 | ) | 45,473 | ||||
| Capitalized transaction costs—PRF transaction | — | 500 | |||||||
| Total assets | 25,543 | 37,842 | 96,622 | ||||||
| Deferred product revenue—Zanaflex Capsules | 4,960 | 4,960 | 4,960 | ||||||
| Deferred product revenue—Zanaflex tablets | 10,686 | 10,686 | 10,686 | ||||||
| Current portion of notes payable | 2,347 | 1,150 | 1,150 | ||||||
| Revenue interest liability—PRF transaction | — | 14,600 | 14,600 | ||||||
| Put/call option liability—PRF transaction | — | 400 | 400 | ||||||
| Long-term portion of notes payable | 3,534 | 1,731 | 1,731 | ||||||
| Long-term convertible notes payable—principal amount plus accrued interest, less unamortized debt discount—related party | 8,695 | 8,695 | 8,695 | ||||||
| Mandatorily redeemable preferred stock | 85,000 | — | |||||||
| Total stockholders' (deficit) | (101,669 | ) | (16,869 | ) | 42,410 | ||||
An investment in our common stock involves a high degree of risk. You should consider carefully readthe following risk factors and consider the risks described belowother information contained in this prospectus before decidingyou decide to invest inpurchase our common stock. If any of the followingAdditional risks actually occur, our business, financial condition, results of operationthat are not currently known or cash flows could be materially harmed. In any such case, theforeseeable to us may materialize at a future date. The trading price of our common stock could decline if any of these risks or uncertainties occur and you couldmight lose all or part of your investment. When determining whether to buy our common stock, you should also refer to the other information in this prospectus, including our consolidated financial statements and the related notes.
Risks Related To Our Business
We have a history of operating losses and we expect to continue to incur losses and may never be profitableprofitable.
As of JuneSeptember 30, 2003,2005, we had an accumulated deficit of approximately $91.1$198.5 million. AsWe had net losses of $26.0 million and $44.7 million for the nine months ended September 30, 2005, and the year ended December 31, 2004, respectively. We have had operating losses since inception as a result of our significant clinical development, research and development, clinical development, general and administrative, sales and marketing and business development expenses and the lack of any products to generate revenue, we have generated operating losses since our inception.expenses. We expect to continue to incur losses for at least the next several years and expect that our losses will increase as we expand our sales and marketing capabilities and continue our clinical trials and research and development activities and incur significant clinical testing costs. To date, our working capital has primarily been generated through financing activities consisting of the sale of shares of our preferred stock and the issuance of convertible debt securities.activities.
Our prospects for achieving profitability will depend primarily on how successful we are in executing our business plan to:
We cannot assure you when or if If we will beare not successful in executing our business plan. If we are not successful,plan, we may never generate revenuesachieve or achievemay not sustain profitability.
The resultsWe will be substantially dependent on sales of our late stage clinical trials may be insufficientone product, Zanaflex Capsules, to obtain FDA approvalgenerate revenue for the foreseeable future.
Positive resultsWe currently derive substantially all of our revenue from preclinical studiesthe sale of Zanaflex Capsules and early clinical trialsZanaflex tablets, which are our only FDA-approved products. Although we currently distribute Zanaflex tablets, our marketing efforts are focused on Zanaflex Capsules and we do not, ensure positive results in more advanced clinical trials.and do not intend to, actively promote Zanaflex tablets. As a result, prescriptions for Zanaflex tablets have declined and we expect that they will continue to decline. Our goal is to convert sales of Zanaflex tablets and generic tizanidine tablets to sales of Zanaflex Capsules. We believe that sales of Zanaflex Capsules will constitute a significant portion of our total revenue for the foreseeable future. If we are unable to demonstrateconvert tablet sales to capsule sales or are otherwise unable to increase our revenue from the sale of this product, our business, financial condition and results of operations could be adversely affected.
If we are unable to successfully differentiate Zanaflex Capsules from both Zanaflex tablets and generic tizanidine tablets we may not be able to increase sales of Zanaflex Capsules.
There are currently 11 generic versions of tizanidine tablets on the market and they are significantly cheaper than either Zanaflex Capsules or Zanaflex tablets. In 2004, these generic versions of tizanidine tablets constituted 95% of tizanidine sales in the United States. Although Zanaflex Capsules have a different pharmacokinetic profile when taken with food and are available in a higher dose than Zanaflex tablets and their generic equivalents, we may be unsuccessful in convincing prescribers, patients and third-party payors that athese differences justify the higher price of Zanaflex Capsules. Prescribers may prescribe generic tizanidine tablets instead of Zanaflex Capsules, and third-party payors may establish unfavorable reimbursement policies for Zanaflex Capsules or otherwise seek to encourage patients and prescribers to use generic tizanidine tablets instead of Zanaflex Capsules. In
addition, although the FDA has determined that neither Zanaflex tablets nor generic tizanidine tablets are therapeutically equivalent, or "AB-rated," to Zanaflex Capsules, it is possible that pharmacists may improperly fill prescriptions with generic tizanidine tablets or may seek to influence patients or physicians to change prescriptions from Zanaflex Capsules to generic tizanidine tablets. If we are unable to successfully differentiate Zanaflex Capsules from Zanaflex tablets and generic tizanidine tablets in the minds of prescribers, pharmacists, patients and third-party payors, our ability to generate meaningful revenue from this product candidate will be safeadversely affected.
Our company has limited sales and marketing experience and we may not be successful in building an effective sales and marketing organization to market Zanaflex Capsules to specialty physicians.
As a company, we have limited sales and marketing experience, having only launched Zanaflex Capsules in advanced clinical trials involving larger numbers of patients,April 2005. In order to successfully commercialize Zanaflex Capsules or any other products that we may bring to market, we will be unable to submit the NDA, necessary to receive approval from the FDA to commercialize that product.
Fampridine-SR is currently in Phase 3 clinical trials for the treatment of spasticity in SCI. We expectneed to have results from the Phase 3 clinical trials by the endadequate sales, marketing and distribution capabilities. Although we plan to expand our internal specialty sales force of 14 persons to approximately 30 persons in the first quarter of 2006, we may need to further expand that sales force in the future. We may not be able to attract and train skilled sales and marketing personnel, in a timely manner or at all, or integrate and manage a growing sales and marketing organization.
Returns of Zanaflex tablets may adversely affect our results of operations.
Prior to the launch of generic tizanidine tablets in June 2002, wholesalers established larger than normal inventories of Zanaflex tablets. These inventories had expiration dates that extended to June 2005. Our return policy is to accept returns for six months before and 12 months after the product's expiration date. According to our Zanaflex asset purchase agreement with Elan, we are responsible for all returns of Zanaflex tablets after January 17, 2005. Zanaflex tablets sold by Elan can be returned to us through June 2006. In the year ended December 31, 2004, and expectwe took a $4.1 million charge to file our NDA shortly thereafter.establish a reserve for expected returns of Zanaflex tablets sold by Elan. This charge is an estimate. If returns for products not sold by us are higher than we fail to achieve the primary endpoints in our Phase 3 clinical trials or the results are ambiguous,have estimated, we will have to determine whether to redesignrecord additional charges, which will adversely affect our Fampridine-SR in SCI development program and protocols and continue with additional testing, or cease activities in this area. Redesigning the program could be extremely costly and time-consuming. A substantial delay in obtaining FDA approval or terminationresults of the Fampridine-SR SCI program could result in a delay in our ability to generate revenue. We face the same risk of failure to meet our primary endpoints with respect to our Fampridine-SR in MS and valrocemide clinical trial programs.operations.
8
Our product candidates must undergo rigorous clinical testing, the results of which are in various stages of developmentuncertain and may not be successfully developedcould substantially delay or commercializedprevent us from bringing them to market.
We currently do not sell any products. We are subjectBefore we can obtain regulatory approval for a product candidate, we must undertake extensive clinical testing in humans to demonstrate safety and efficacy to the risk that some or allsatisfaction of our product candidates:
We cannot predict whether we will successfully develop and commercialize any of ournew product candidates sufficient to obtain regulatory marketing approval are expensive and take years to complete, and the failure to do so would result in our inability to generate revenue.
If the FDA does not accept the measure we are using in our clinicaloutcome of such trials for Fampridine-SR in MS, FDA approval for treatment of patients with MS will be significantly delayedis uncertain.
We are using the Timed 25 Foot Walk to measure improvement in walking speed in people taking Fampridine-SR for MS in our Phase 2 clinical trials. To our knowledge, the FDA has not approved a drug based on this measure to date. Although the resultsClinical development of our Phase 2 clinical testing may demonstrate a statistically significant, clinically meaningful benefit to patients when using Fampridine-SR in MS, the FDA may decide that the Timed 25 Foot Walk is an insufficient measure to determine whether this product should receive FDA approval, and may require us to re-design our clinical trials using different measures. If we are required to identify new measures to test our primary endpoints, we will face substantial delays in our current timeline to commercialize and launch Fampridine-SR in MS and will incur additional costs associated with these activities. Any delays in regulatory approval will delay commercialization of Fampridine-SR in MS, which would harm our business prospects.
Our other product candidates are in early stages of development and may never be commercialized
Research, development and pre-clinical testing are long, expensive and uncertain processes. Other than Fampridine-SR and valrocemide, none of our other product candidates have reached clinical trial testing. Our GGF-2any product candidate and our remyelinating antibodies are in pre-clinical testing. Our chondroitinase and regenerative antibodies programs are in the research stage. Our future success depends, in part, on our abilitythat we determine to complete pre-clinical development of our other product candidates and advance them to thetake into clinical trials.
Our product development programstrials may be curtailed, redirected, delayed or eliminated at any time for some or all of the following reasons:
9
A delay in or termination of any of our clinical development programs could have an adverse effect on our business.
If our Phase 3 clinical trials of Fampridine-SR are unsuccessful, or if we are unable to obtain regulatory approval for this product candidate or any approval is unduly limited in scope, our business prospects will be adversely affected.
In June 2005, we initiated a Phase 3 clinical trial for Fampridine-SR for the improvement of walking ability in patients with MS. In April 2004, we released results from a Phase 2 clinical trial designed to assess the relative safety and efficacy of varying doses of Fampridine-SR in MS. Our results did not reach statistical significance for the primary endpoint in this trial. Although we have designed the current Phase 3 clinical trial to address the difficulties we encountered in interpreting the patient data from the earlier trial, we cannot be sure that the results from our current clinical trial will be statistically significant.
To achieve the primary endpoint in our current Phase 3 clinical trial for MS, we need to show statistical improvement in the walking speed of the patients in the trial and that this improvement is both sustained and clinically meaningful to these patients. If we fail to achieve the primary endpoint in this clinical trial or the results are ambiguous, we will have to determine whether to re-design our MS trial and protocols and continue with additional testing, or cease development activities in this area. Redesigning the program could be extremely costly and time-consuming. Even if we are able to achieve the primary endpoint, we will need positive results from at least one other clinical trial to support the filing of a new drug application, or NDA, with the FDA. We cannot predict how long the second trial, or any additional trial that might be required by the FDA, will take or what the cost will be.
Our Phase 3 clinical trial for Fampridine-SR in MS is being conducted pursuant to a special protocol assessment, or SPA, with the FDA and the FDA has agreed that, if successful, this trial could qualify as one of the pivotal trials needed to support regulatory approval. This SPA may not be changed by either us or the FDA. However, if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of Fampridine-SR is identified after the trial began, the FDA may alter its conclusion on the adequacy of the protocol. In addition, even if the SPA remains in place and the trial meets its primary endpoint, the FDA could determine that the overall balance of risks and benefits for Fampridine-SR is not adequate to support approval, or only justifies approval for a narrow set of uses or approval with restricted distribution or other burdensome post-approval requirements and limitations. If the FDA denies approval of Fampridine-SR in MS, FDA approval is substantially delayed, approval is granted on a narrow basis or with restricted distribution or other burdensome post-approval requirements, or if the Fampridine-SR program is terminated, our business prospects will be adversely affected.
In March 2004, we completed two Phase 3 clinical trials of Fampridine-SR in SCI in which our results failed to reach their primary endpoints. We expect to resume development of Fampridine-SR for SCI after we have completed further development of the drug for MS. We cannot predict whether future clinical trials of Fampridine-SR in SCI will achieve their primary endpoints, how long these clinical trials will take or how much they will cost.
Our other drug development programs are in early stages of development and may never be commercialized.
All of our development programs other than Fampridine-SR are in the preclinical phase. Our future success depends, in part, on our ability to select promising product candidates, complete preclinical development of these product candidates and advance them to clinical trials. These product candidates will require significant development, preclinical studies and clinical trials, regulatory clearances and substantial additional investment before they can be commercialized.
Our preclinical programs may not lead to commercially viable products for several reasons. For example, we may fail to identify promising product candidates, our product candidates may fail to be safe and effective in preclinical tests or clinical trials, or we may have inadequate financial or other resources to pursue discovery and development efforts for new product candidates. In addition, because we have limited resources, we are focusing on product candidates that we believe are the most promising. As a result, we may delay or forego pursuit of opportunities with other product candidates. From time to time, we may establish and announce certain development goals for our product candidates and programs; however, given the complex nature of the drug discovery and development process, it is difficult to predict accurately if and when we will achieve these goals. If we are unsuccessful in advancing our early stage product candidatespreclinical programs into clinical testing for any reason,or in obtaining regulatory approval, our long-term business prospects will be harmed.
The pharmaceutical industry is subject to stringent regulation and failure to obtain regulatory approval will prevent commercialization of our product candidates.
Our research, development, preclinical and clinical trial activities, as well as the manufacture and marketing of any products that we may successfully develop, are subject to an extensive regulatory approval process by the FDA and other regulatory agencies abroad. The process of obtaining required regulatory approvals for drugs is lengthy, expensive and uncertain, and any regulatory approvals may contain limitations on the indicated usage of a drug, distribution restrictions or may be conditioned on burdensome post-approval study or other requirements, including the requirement that we institute and follow a special risk management plan to monitor and manage potential safety issues, all of which may eliminate or reduce the drug's market potential. Post-market evaluation of a product could result in marketing restrictions or withdrawal from the market.
The results of preclinical and Phase 1 and Phase 2 clinical studies are not necessarily indicative of whether a product will demonstrate safety and efficacy in larger patient populations, as evaluated in Phase 3 clinical trials. Additional adverse events that could impact commercial success, or even continued regulatory approval, might emerge with more extensive post-approval patient use. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are approved for commercialization.
In order to conduct clinical trials to obtain FDA approval to commercialize any product candidate, an IND application must first be submitted to the FDA and must become effective before clinical trials may begin. Subsequently, an NDA must be submitted to the FDA, including the results of adequate and well-controlled clinical trials demonstrating, among other things, that the product candidate is safe and effective for use in humans for each target indication. In addition, the manufacturing facilities used to produce the products must comply with current good manufacturing practices and must pass a pre-approval FDA inspection. Extensive submissions of preclinical and clinical trial data are required to demonstrate the safety, efficacy, potency and purity for each intended use. The FDA may refuse to accept our regulatory submissions for filing if they are incomplete.
Clinical trials are subject to oversight by institutional review boards and the FDA to ensure compliance with the FDA's good clinical practice requirements, as well as other requirements for the protection of clinical trial participants. We depend, in part, on third-party laboratories and medical institutions to conduct preclinical studies and clinical trials for our products and other third-party organizations to perform data collection and analysis, all of which must maintain both good laboratory and good clinical practices required by regulators. If any such standards are not complied with in our clinical trials, the resulting data from the clinical trial may not be usable or we, an institutional review board or the FDA may suspend or terminate such trial, which would severely delay our development and possibly end the development of such product candidate. We also depend upon third party manufacturers of our products to qualify for FDA approval and to comply with good manufacturing practices required by regulators. We cannot be certain that our present or future manufacturers and suppliers will comply with current good manufacturing practices. The failure to comply with good
manufacturing practices may result in the termination of clinical studies, restrictions in the sale of, or withdrawal of the products from the market. Compliance by third parties with these standards and practices is outside of our direct control.
In addition, we are subject to regulation under other state and federal laws, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other local, state, federal and foreign regulations. We cannot predict the impact of such regulations on us, although it could impose significant restrictions on our business and additional expenses to comply with these regulations.
Our products and product candidates may not gain market acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenue.
Even if we obtain regulatory approval forMarket acceptance of our products market acceptanceand product candidates will depend on our ability to demonstrate to physicians and patients the benefits of our products in terms of safety, efficacy, convenience, ease of administration and cost effectiveness. In addition, weeffectiveness and our ability to demonstrate these benefits to physicians and patients. We believe market acceptance also depends on the effectiveness of our marketing strategy, the pricing of our products and the reimbursement policies of government and third-party payors.payors, as well as on the effectiveness of our sales and marketing activities. Physicians may not prescribe our products, and patients may determine, for any reason, that our product isproducts are not useful to them. IfFor example, physicians may not believe that the benefits of Zanaflex Capsules outweigh their higher cost in relation to Zanaflex tablets or generic tizanidine tablets. The failure of any of our products or product candidates, failsonce approved, to achieve market acceptance would limit our ability to generate revenue will be limited.and would adversely affect our results of operations.
If we fail to meet our obligations under our license agreements, or our agreements are terminated for any other reasons, weOur potential products may lose our rights to in-licensed technologies
We have licensed the rights for most of our products. We could lose the rights to Fampridine-SR, for example, in certain countriesnot be commercially viable if we fail to file regulatory approvalsobtain an adequate level of reimbursement for these products by Medicaid, Medicare or launch a productother third-party payors.
Our commercial success will depend in part on third-party payors, such countries within specified periods, or if we failas government health administrative authorities, including Medicaid and Medicare, private health insurers and other such organizations, agreeing to fulfillreimburse patients for the cost of our payment obligations underproducts. Significant uncertainty exists as to the license agreement. Furthermore, if Elan were to file for bankruptcy in Ireland, there is the possibility that our licensed rights could be transferred, altered or terminated, or we could incur substantial expenses to keep our license effective. If we lose our rights to Fampridine-SR, ourreimbursement status of newly-approved healthcare products. Our business and prospects would be materially harmed.adversely affected if the Medicaid program, Medicare program or other third-party payors were to deny reimbursement for our products or provide reimbursement only on unfavorable terms. Our business could also be adversely affected if the Medicaid program, Medicare program or other reimbursing bodies or payors limit the indications for which our products will be reimbursed to a smaller set of indications than we believe is appropriate.
Third-party payors frequently require that drug companies negotiate agreements with them that provide discounts or rebates from list prices. At present we do not have any such agreements with private third-party payors and only a small number of such agreements with government payors. If sales of Zanaflex Capsules increase we may need to offer larger discounts or discounts to a greater number of third-party payors to maintain acceptable reimbursement levels. If we were required to negotiate such agreements, there is no guarantee that we would be able to negotiate them at price levels that are profitable to us, or at all. If we are unsuccessful in maintaining reimbursement for our products at acceptable levels, our business will be adversely affected. In addition, if our competitors reduce the prices of their products, or otherwise demonstrate that they are better or more cost effective than our products, this may result in a greater level of reimbursement for their products relative to our products, which would reduce our sales and adversely affect our results of operations.
We may experience pressure to lower prices on our approved products due to new and/or proposed federal legislation.
Federal legislation enacted in December 2003 added an outpatient prescription drug benefit to Medicare, effective January 2006. In the interim, Congress has established a discount drug card program for Medicare beneficiaries. Both benefits will be provided primarily through private entities,
which will attempt to negotiate price concessions from pharmaceutical manufacturers. These negotiations may increase pressure to lower prescription drug prices. While the new law specifically prohibits the U.S. government from interfering in price negotiations between manufacturers and Medicare drug plan sponsors, some members of Congress are pursuing legislation that would permit the U.S. government to use its enormous purchasing power to demand discounts from pharmaceutical companies, thereby creating de facto price controls on prescription drugs. In addition, the new law contains triggers for Congressional consideration of cost containment measures for Medicare in the event Medicare cost increases exceed a certain level. These cost containment measures could include limitations on prescription drug prices. This Medicare prescription drug coverage legislation, as well as additional healthcare legislation that may be enacted at a future date, could reduce our sales and adversely affect our results of operations.
If our competitors develop and market products that are more effective, safer or more convenient than our approved products, or obtain marketing approval before we obtain approval of future products, our commercial opportunity will be reduced or eliminated.
Competition in the pharmaceutical and biotechnology industries is intense and is expected to increase. Composition of matter patents on tizanidine, the active ingredient in Zanaflex Capsules and Zanaflex tablets, expired in 2002. There are currently 11 generic versions of tizanidine tablets on the market. To the extent that we are not able to differentiate Zanaflex Capsules from Zanaflex tablets and generic tizanidine tablets and/or pharmacists improperly substitute generic tizanidine tablets when filling prescriptions for Zanaflex Capsules, we may be unable to convert a meaningful amount of sales of Zanaflex tablets and generic tizanidine tablets to Zanaflex Capsules and our ability to generate revenue from this product will be adversely affected. Although no other FDA-approved capsule formulation of tizanidine exists, another company could develop a capsule or other formulation of tizanidine that competes with Zanaflex Capsules.
Many biotechnology and pharmaceutical companies, as well as academic laboratories, are involved in research and/or product development for various neurological diseases, including MS and SCI. We are aware of a company developing a sodium/potassium channel blocker and a second company developing an immediate release form of fampridine, both of which may compete with Fampridine-SR, if approved. In certain circumstances, pharmacists are not prohibited from formulating certain drug compounds to fill prescriptions on an individual patient basis. We are aware that at present compounded fampridine is used by some people with MS or SCI and it is possible that some people will want to continue to use compounded formulations even if Fampridine-SR is approved. Several companies are engaged in developing products that include novel immune system approaches and cell transplant approaches to remyelination for the treatment of people with MS. These programs are in early stages of development and may compete in the future with Fampridine-SR or our preclinical candidates.
Our rights tocompetitors may succeed in developing products that are more effective, safer or more convenient than our products or the ones we have under development use and marketing of all ofor that render our otherapproved or proposed products or technologies noncompetitive or obsolete. In addition, our competitors may achieve product candidates are also governed by license agreements thatcommercialization before we entered into with licensors of these technologies. Our failure to achieve milestones, or meetdo. If any of our financialcompetitors develops a product that is more effective, safer or more convenient for patients, or is able to obtain FDA approval for commercialization before we do, we may not be able to achieve market acceptance for our products, which would adversely affect our ability to generate revenues and recover the substantial development costs we have incurred and will continue to incur.
Our products may be subject to competition from lower-priced versions of such products and competing products imported into the United States from Canada, Mexico and other countries where there are government price controls or other obligations under these license agreementsmarket dynamics that make the products lower priced.
Our operations could result in the loss ofbe curtailed if we are unable to obtain any necessary additional financing on favorable terms or at all.
On September 30, 2005, on a pro forma as-adjusted basis after giving effect to this offering and our rights to these technologies. If we loseentry into our rights under any of these license agreements,revenue interest assignment arrangement with PRF, we would have had approximately $79.5 million in cash, cash equivalents and short-term investments. Although we anticipate this will be unablesufficient to fund our operations for approximately the next 24 months, we have several product candidates in various stages of development, and all will require significant further investment to develop, test and obtain regulatory approval prior to commercialization. We will likely need to seek additional equity or debt financing or strategic collaborations to continue our product development programs, whichactivities, and could require substantial funding to commercialize any products that we successfully develop. We may not be able to raise additional capital on favorable terms or at all.
To the extent that we are able to raise additional capital through the sale of equity securities, the issuance of those securities would result in lost revenuedilution to our stockholders. Holders of such new equity securities may also have rights, preference or privileges that are senior to yours. If additional capital is raised through the incurrence of indebtedness, we may become subject to various restrictions and would harmcovenants that could limit our ability to respond to market conditions, provide for unanticipated capital investments or take advantage of business prospects.
We depend upon Elan foropportunities. To the manufactureextent funding is raised through collaborations or intellectual property-based financings, we may be required to give up some or all of Fampridine-SR,the rights and other manufacturers for the manufacturerelated intellectual property to one or more of our otherproducts, product candidates
Our supply agreement with Elan obligates us to purchase at least 75% of our yearly supply of Fampridine-SR from Elan. We are in the process of qualifying a second manufacturing source in the event that Elan is unable or unwilling, due to financial difficulties or otherwise, to fulfill our manufacturing and supply needs.preclinical programs. If we are unable to qualify a second manufacturing source,obtain sufficient financing on favorable terms when and Elan ceasesif needed, we may be required to manufacture the product for us, we could experience substantial delays before we are able to qualify another supplier. Any significant delays in product shipments could slow the current progressreduce, defer or discontinue one or more of our clinical trials and, ifproduct development programs or devote fewer resources to marketing Zanaflex Capsules.
Under our financing arrangement with PRF, upon the occurrence of certain events, PRF may require us to repurchase the right to receive revenues that we receive approvalassigned to commercialize Fampridine-SR, would materiallyit or may foreclose on certain assets that secure our obligations to PRF. Any exercise by PRF of its right to cause us to repurchase the assigned right or any foreclosure by PRF could adversely affect our ability to commercialize Fampridine-SR. In addition, if we do not purchase at least 100%results of operations and our requirements from Elan under the supply agreement we are required to make certain compensatory payments to Elan which could increase our total manufacturing costs.
We are also substantially dependent upon Elan to complete the chemistry, manufacturing and controls section of the NDA for Fampridine-SR in SCI. If Elan fails to provide this section in a complete and timely manner we could incur delays in filing our NDA for Fampridine-SR in SCI.
10
We are also wholly dependent on third parties to manufacture our other product candidates, including valrocemide. If we lose and are unable to replace these manufacturers, we will be unable to continue developing and testing our other product candidates.
If we must obtain the active pharmaceutical ingredient in Fampridine-SR from new suppliers, we may face serious delays in manufacturing Fampridine-SRfinancial condition.
We do not have direct contractual relationshipsOn December 23, 2005, we entered into a revenue interests assignment agreement with the suppliers of fampridine, the active pharmaceutical ingredient in Fampridine-SR,PRF pursuant to which we referassigned to as API. Currently,PRF the right to receive a portion of our net revenues from Zanaflex Capsules, Zanaflex tablets and any future Zanaflex products. To secure our obligations to PRF, we rely on Elan's contractsalso granted PRF a security interest in substantially all of our assets related to Zanaflex.
Under our arrangement with third parties to supply API. If Elan or an alternative manufacturer is unable to obtain API supplies from these suppliers for any reason, a new supplier would have to be identified. Although other suppliersPRF, upon the occurrence of API are readily available,certain events, including if we experience a change of control, undergo certain bankruptcy events, transfer any of our interests in Zanaflex (other than pursuant to a supplierlicense agreement, development, commercialization, co-promotion, collaboration, partnering or similar agreement), transfer all or substantially all of our assets, or breach certain of the covenants, representations or warranties under the revenue interests assignment agreement, PRF may (i) require us to repurchase the rights we assigned to it at the "put/call price" in effect on the date such right is exercised or (ii) foreclose on the Zanaflex assets that wassecure our obligations to PRF. Except in the case of certain bankruptcy events, if PRF exercises its right to cause us to repurchase the rights we assigned to it, PRF may not previously approved in our NDA may require formal approvalforeclose unless we fail to pay the put/call price as required. The put/call price on a given date is the greater of (i) 150% of all payments made by the FDA before we could use their API in our product. Any delays in obtaining APIPRF to manufacture Fampridine-SRus as of such date, less all payments received by PRF from us as of such date, and (ii) an amount that would delay the commercialization of Fampridine-SR.
We do not havegenerate an internal sales force,rate of return to PRF of 25% on all payments made by PRF to us as of such date, taking into account the amount and timing of all payments received by PRF from us as of such date.
If PRF were to exercise its right to cause us to repurchase the right we will rely on a third party providerassigned to assist us in commercializing Fampridine-SR
We do not currently have our own internal sales force and will rely on third parties to commercialize Fampridine-SR. We have agreements with Cardinal Health and inChord Communications to use their RxPedite program to commercialize Fampridine-SR in SCI. The RxPedite program involves the development and implementation of a marketing plan to launch Fampridine-SR and provides for a sales force to market the product. Weit, we cannot assure you that we would have sufficient funds available to pay the commercializationput/call price in effect at that time. Even if we have sufficient funds available, we may have to use funds that we planned to use for other purposes and marketingour results of operations and financial condition could be adversely affected. If PRF were to foreclose on the Zanaflex assets that secure our obligations to PRF, our results of operations and
financial condition could also be adversely affected. Because PRF's right to cause us to repurchase the rights we assigned to it is triggered by, among other things, a change in control, transfer of any of our product through the RxPedite program will be successful. If our agreements with Cardinal and inChord are terminated for any reason, it could be time-consuming to identify another party to assist us, and we would be subjectinterests in Zanaflex (other than pursuant to a material disruption in ourlicense agreement, development, commercialization, and marketing process. Without an active sales force, there could be serious delays in marketing Fampridine-SR. Disruptionco-promotion, collaboration, partnering or similar agreement) or transfer of the commercializationall or marketing of Fampridine-SR would have a material adverse effect on our ability to generate revenues.
Our success in developing our product candidates depends upon the performancesubstantially all of our licensees and collaborative partners
Our efforts to develop, obtain regulatory approval for and commercialize our existing and any future product candidates depend in part uponassets, the performanceexistence of our licensees and collaborative partners. Currently, we have license and collaborative agreements with Elan, Rush-Presbyterian St. Luke's Medical Center, Teva, Canadian Spinal Research Organization, Cornell Research Foundation, Inc., Mayo Clinic Foundation and CeNeS Pharmaceuticals plc. We do not have day-to-day control over the activities of our licenseesthat right could discourage us or collaborative partners and cannot assure youa potential acquirer from entering into a business transaction that they will fulfill their obligations to us. Further, our licensees and collaborators may encounter conflicts of interest, changes in business strategy or other business issues, or they may acquire or develop rights to competing products, all of which could limit our ability to commercialize our product candidates.
Any failure on the part of our licensees or collaborators to perform or satisfy their obligations to us could lead to delayswould result in the development or commercializationoccurrence of our product candidates and affect our ability to realize product revenues. Disagreements with our licensees or collaborators could require or result in litigation or arbitration, which could be time-consuming and expensive. If we fail to maintain our existing agreements or establish new agreements as necessary, we could be required to undertake development, manufacturing and commercialization activities solely at our own expense. This would significantly increase our capital requirements and may also delay the commercializationany of our product candidates.
11
If we fail to manage our growth, our business could be harmed
If we are unable to manage our growth effectively, our business could be materially adversely affected. For example, pursuant to our agreement with Cardinal, we have an option to acquire the Fampridine-SR dedicated sales force provided by the RxPedite program in the future. Assimilation of this sales force into our operations would require us to assume the administrative burden of managing our own sales operations. Our inability to successfully assimilate this sales force, or manage rapid growth in other areas, could materially adversely affect our business.those events.
The loss of our key management and scientific personnel may hinder our ability to execute our business planplan.
As a small company with 68 employees, ourOur success depends on the continuing contributions of our management team and scientific personnel, and maintaining relationships with the members of our Scientific Advisory Boardscientific and medical network and the network of centers in the United States and Canada that conducts our clinical trials. We are highly dependent on the services of Dr. Ron Cohen, our Chairman, President and Chief Executive Officer, as well as the other principal members of our management and scientific staff. Our success depends in large part upon our ability to attract and retain highly qualified personnel. We face intense competition in our hiring efforts with other pharmaceutical and biotechnology companies, as well as universities and nonprofit research organizations, and we may have to pay higher salaries to attract and retain qualified personnel. With the exception of Dr. Ron Cohen, we do not maintain "key man" life insurance policies on the lives of our officers, directors or employees. The loss of one or more of such individuals,our key employees, or our inability to attract additional qualified personnel, could substantially impair our ability to implement our business plan.
We face an inherent risk of liability in the event that the use or misuse of our products results in personal injury or death.
If the use or misuse of Zanaflex Capsules or any other FDA-approved products we may sell in the future harms people, we may be subject to costly and damaging product liability claims brought against us by consumers, healthcare providers, pharmaceutical companies, third-party payors or others. The use of our product candidates in clinical trials could also expose us to product liability claims. We currently maintain a product liability insurance policy that includes coverage of our clinical trials. This insurance policy has a $10 million per claim limit and the aggregate amount of claims under the policy is also capped at $10 million. We cannot predict all of the possible harms or side effects that may result from the use of our products or the testing of product candidates and, therefore, the amount of insurance coverage we currently have may not be adequate to cover all liabilities or defense costs we might incur. A product liability claim or series of claims brought against us could give rise to a substantial liability that could exceed our resources. Even if claims are not successful, the costs of defending such claims and potential adverse publicity could be harmful to our business.
We are subject to various federal and state laws regulating the marketing of Zanaflex Capsules and, if we do not comply with these regulations, we could face substantial penalties.
Our sales, promotion and other activities related to Zanaflex Capsules, or any of our other products under development following their regulatory approval, are subject to regulatory and law enforcement authorities in addition to the FDA, including the Federal Trade Commission, the Department of Justice, and state and local governments. We are subject to various federal and state laws pertaining to health care "fraud and abuse," including both federal and state anti-kickback laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive or pay any remuneration as an inducement for the referral of business, including the use, recommendation, purchase or prescription of a particular drug. The federal government has published regulations that identify "safe harbors" or exemptions for certain payment arrangements that do not violate the anti-kickback statutes. Although we seek to comply with these statutes, it is possible that our practices, or those of our contract sales force, might be challenged under anti-kickback or similar laws. Violations of fraud and abuse laws may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, and future exclusion from participation in government healthcare programs.
We may be subject to penalties if we fail to comply with post-approval legal and regulatory requirements and our products could be subject to restrictions or withdrawal from the market.
Any product for which we currently have or may obtain marketing approval, along with the associated manufacturing processes, any post-approval clinical data that we might be required to collect and the advertising and promotional activities for the product, are subject to continual recordkeeping and reporting requirements, review and periodic inspections by the FDA and other regulatory bodies. Regulatory approval of a product may be subject to limitations on the indicated uses for which the product may be marketed or to other restrictive conditions of approval that limit our ability to promote, sell or distribute a product. Furthermore, any approval may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product.
We have an outstanding commitment with the FDA, inherited from Elan, to evaluate Zanaflex Capsules for pediatric use. Although the commitment was orginally set by the FDA to be completed by December 2005, we believe that it was extended to February 2007, by the Pediatric Research Equity Act, or PREA. We are seeking from the FDA either confirmation that the commitment is due February 2007 or, if not confirmed, we will seek a deferral until that date. The NDA for Zanaflex Capsules was approved with a plan to address the requirements of the PREA through a pediatric pharmacokinetic study. We have submitted a proposed design for this pharmacokinetic study to the FDA. Depending on the FDA's response to our submission or the outcome of this study, we may be required to conduct additional studies. These studies could be more extensive and more costly than the currently-planned study.
Our advertising and promotion are subject to stringent FDA rules and oversight. In particular, the claims in our promotional materials and activities must be consistent with the FDA approvals for our products, and must be appropriately substantiated and fairly balanced with information on the safety risks and limitations of the products. Any free samples we distribute to physicians must be carefully monitored and controlled, and must otherwise comply with the requirements of the Prescription Drug Marketing Act, as amended, and FDA regulations. We must continually review adverse event information that we receive concerning our drugs and make expedited and periodic adverse event reports to the FDA and other regulatory authorities.
In addition, the research, manufacturing, distribution, sale and promotion of drug and biological products are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-kickback and fraud and abuse provisions of the Social Security Act, as amended, the False Claims Act, as amended, the privacy provisions of the Health Insurance Portability and Accountability Act and similar state laws. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
We may be slow to adapt, or we may not be able to adapt, to changes in existing regulatory requirements or adoption of new legal or regulatory requirements or policies. Later discovery of previously unknown problems with our products, manufacturing processes, or failure to comply with regulatory requirements, may result in:
In addition, the FDA or another regulatory agency may conduct periodic unannounced inspections. If they determine that we are not in compliance with applicable requirements, they may issue a notice of inspectional observations. If the observations are significant, we may have to devote significant resources to respond and undertake appropriate corrective and preventive actions, which could adversely affect our business prospects. For example, the FDA recently completed an inspection relating to our adverse event and product complaint handling and reporting for Zanaflex. The FDA has issued to us a Form 483, Inspectional Observations, with five observations. We have completed or expect to complete shortly all necessary corrective actions. The cost of the corrective actions is not expected to be material.
State pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
In recent years, several states, including California, Vermont, Maine, Minnesota, New Mexico and West Virginia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports with the state on sales, marketing, pricing and other activities. For example, California has enacted a statute requiring pharmaceutical companies to adopt a comprehensive compliance program that is in accordance with the Office of Inspector General of the Department of Health and Human ServicesCompliance Program Guidance for Pharmaceutical Manufacturers. This compliance program must include policies for compliance with the Pharmaceutical Research and Manufacturers of AmericaCode on Interactions with Healthcare Professionals, as well as a specific annual dollar limit on gifts or other items given to individual healthcare professionals in California. The law requires posting policies on a company's public web site along with an annual declaration of compliance.
Vermont, Maine, Minnesota, New Mexico, and West Virginia have also enacted statutes of varying scope that impose reporting and disclosure requirements upon pharmaceutical companies pertaining to drug pricing and payments and costs associated with pharmaceutical marketing, advertising and promotional activities, as well as restrictions upon the types of gifts that may be provided to healthcare practitioners. Similar legislation is being considered in other states. Many of these requirements are new and uncertain and the penalties for failure to comply with these requirements are unclear. We are not aware of any companies against which fines or penalties have been assessed under these state reporting and disclosure laws to date. We are currently in the process of developing a formal compliance infrastructure and standard operating procedures to comply with such laws. Unless we are in full compliance with these laws, we could face enforcement action and fines and other penalties, and could receive adverse publicity.
If we seek to market our products in foreign jurisdictions, we will need to obtain regulatory approval in these jurisdictions.
In order to market our products in the European Union and many other foreign jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. Approval procedures vary among countries and can involve additional clinical testing. The time required to obtain approval may differ from that required to obtain FDA approval. Should we decide to market our products abroad, we may fail to obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. We may not be able to file for, and may not receive, necessary regulatory approvals to commercialize our products in any foreign market, which could adversely affect our business prospects.
If we use biological and hazardous materials in a manner that causes injury, we may be liable for damages.
Our research and development activities involve the controlled use of potentially harmful biological materials, hazardous materials and chemicals that are subject to federal, state and local laws and regulations governing their use, storage, handling and disposal. These materials include ketamine, buprenophine, sodium pentobarbital, ether, acetonitrile, hexanes, chloroform, xylene, dehydrated alcohol, methanol, ethyl alcohol, isopropanol and formaldehyde. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. If we fail to comply with environmental regulations, we could be subject to criminal sanctions and/or substantial liability for any damages that result, and any substantial liability could exceed our resources. We currently maintain a general liability insurance policy that has a $2 million per claim limit and also caps aggregate claims at $2 million. In addition, we have an umbrella insurance policy that covers up to $9 million of liability in excess of the general liability policy's $2 million limit. This amount of insurance coverage may not be adequate to cover all liabilities or defense costs we might incur. In addition, the cost of compliance with environmental and health and safety regulations may be substantial.
Risks Related to Our Dependence on Third Parties
We currently have no manufacturing capabilities and are substantially dependent upon Elan, Novartis and other third party suppliers to manufacture Zanaflex Capsules, Zanaflex tablets and Fampridine-SR.
We do not own or operate, and currently do not plan to own or operate, manufacturing facilities for production of Zanaflex Capsules, Zanaflex tablets or Fampridine-SR. We rely and expect to continue to rely on third parties for the production of our products and clinical trial materials.
We rely on a single manufacturer, Elan, for the supply of Zanaflex Capsules. Zanaflex Capsules are manufactured using Elan's proprietary SODAS (spheroidal oral drug absorption system) multiparticulate drug delivery technology. Elan is obligated, in the event of a failure to supply Zanaflex Capsules, to use commercially reasonable efforts to assist us in either producing Zanaflex Capsules ourselves or in transferring production of Zanaflex Capsules to a third-party manufacturer, provided that such third-party manufacturer is not a technological competitor of Elan. In the event production is transferred to a third party, the FDA may require us to demonstrate through bioequivalence studies and laboratory testing that the product made by the new supplier is equivalent to the current Zanaflex Capsules before we could distribute products from that supplier. The process of transferring the technology and qualifying the new supplier could take a year or more.
Under our supply agreement with Elan, we provide Elan with monthly written 18-month forecasts and with annual written two-year forecasts of our supply requirements for Zanaflex Capsules. In each of the five months following the submission of our written 18-month forecast we are obligated to purchase the quantity specified in the forecast, even if our actual requirements are greater or less. Elan is not obligated to supply us with quantities in excess of our forecasted amounts, although it has agreed to use commercially reasonable efforts to do so. Because we have a limited history of selling Zanaflex Capsules, our forecasts of our supply requirements may be inaccurate. As a result, we may have an excess or insufficient supply of Zanaflex Capsules.
The Elan facility located in Gainesville, Georgia, which is responsible for bottling Zanaflex Capsules, has been operating under a court-ordered consent decree and injunction since 2001, which were imposed following adverse FDA inspections and FDA allegations that the facility was failing to comply with current good manufacturing requirements. These prior issues were not related to the manufacture of our products. If, however, Elan fails to comply with the requirements of the consent decree and injunction, it could be held in contempt and the facility could be shut down and the manufacturing of our products halted or interrupted.
We currently rely on Novartis for our supply of Zanaflex tablets and tizanidine, the active pharmaceutical ingredient, or API, in both Zanaflex Capsules and Zanaflex tablets. Under a supply agreement we assumed from Elan, Novartis is responsible for manufacturing Zanaflex tablets and
tizanidine for us through February 2007. This includes the tizanidine that Elan uses to manufacture Zanaflex Capsules for us. Novartis currently produces tizanidine, but has arranged with another party to formulate Zanaflex tablets. We have arranged for another company, Sharp Corporation, to package and bottle Zanaflex tablets. Novartis has informed us that it intends to discontinue production of tizanidine by the end of the first quarter of 2006. It is our understanding that Novartis is currently in the process of transferring the methods of manufacturing tizanidine to Rohner, a manufacturer in Pratteln, Switzerland. We have also identified an alternate source for tizanidine in collaboration with Elan but do not have an agreement with that alternative source or any other alternate manufacturer. By the expiration of our contract with Novartis in 2007, we will need to have established a direct relationship with an alternative supplier of tizanidine for Zanaflex tablets if we want them to continue to be manufactured.
We also rely exclusively on Elan to supply us with our requirements for Fampridine-SR. Elan relies on a third-party manufacturer to supply fampridine, the API in Fampridine-SR. Under our supply agreement with Elan, we are obligated to purchase at least 75% of our yearly supply of Fampridine-SR from Elan, and we are required to make compensatory payments if we do not purchase 100% of our requirements from Elan, subject to certain exceptions. We and Elan have agreed that we may purchase up to 25% of our annual requirements from Patheon, Inc., a mutually agreed-upon and qualified second manufacturing source, without compensatory payment.
Our dependence on others to manufacture our marketed products and clinical trial materials may adversely affect our ability to develop and commercialize our products on a timely and competitive basis.
If third-party contract research organizations do not perform in an acceptable and timely manner, our preclinical testing or clinical trials could be delayed or unsuccessful.
We do not have the ability to conduct all aspects of our preclinical testing or clinical trials ourselves. We rely and will continue to rely on clinical investigators, third-party contract research organizations and consultants to perform some or all of the functions associated with preclinical testing or clinical trials. The failure of any of these vendors to perform in an acceptable and timely manner in the future, including in accordance with any applicable regulatory requirements, such as good clinical and laboratory practices, or preclinical testing or clinical trial protocols, could cause a delay or otherwise adversely affect on our preclinical testing or clinical trials and ultimately on the timely advancement of our development programs.
We rely on a third party to provide the sales representatives to market Zanaflex Capsules to primary care physicians.
We recently entered into a contract with Cardinal Health pursuant to which it provides us with approximately 160 sales representatives who market Zanaflex Capsules to primary care physicians. These sales representatives are not our employees and we do not have control over their performance or compliance with applicable laws. Their failure to increase prescriptions for Zanaflex Capsules from the targeted primary care physicians would negatively impact our sales growth, and their failure to comply with applicable laws could subject us to liability.
Risks Related to Our Intellectual Property
If we cannot protect our intellectual property, our ability to develop and commercialize our products will be severely limitedlimited.
Our success will depend in part on our and our licensors' ability to obtain, maintain and enforce patent protection for each party'sthe technologies, compounds and products, if any, resulting from these technologies.our licenses and development programs. Without protection for the intellectual property we use, other companies could offer substantially identical products for sale without incurring the sizable discovery, development and
licensing costs that we have incurred. Our ability to recover these expenditures and realize profits upon the sale of products could be diminished.
We have in-licensed or are the assignee of more than 25 U.S. patents, more than 60 foreign patents and over 65 patent applications filed and pending in the United States andor abroad for our own technologies and for technologies that we have developed from our in-licensed programs. The process of obtaining patents can be time consuming and expensive with no certainty of success. Even if we spend the necessary time and money, a patent may not issue or it may insufficientlynot have sufficient scope or strength to protect the technology it was intended to protect.protect or to provide us with any commercial advantage. We canmay never be certain that we were the first to develop the technology or that we were the first to file a patent application for the particular technology because many U.S. patent applications are confidential until a patent issues,they are published, and publications in the scientific or patent literature lag behind actual discoveries. The degree of future protection for our proprietary rights will remain uncertain if our pending patent applications are not approved for any reason or if we are unable to develop additional proprietary technologies that are patentable. Furthermore, third parties may independently develop similar or alternative technologies, duplicate some or all of our technologies, design around our patented technologies or challenge our issued patents or the patents of our licensors.
InWe may initiate actions to protect our intellectual property and in any litigation wherein which our patents or our licensors' patents are asserted, a court may determine that the patents are invalid or unenforceable. Even if the validity or enforceability of these patents is upheld by a court, a court may not prevent alleged infringement on the grounds that such activity is not covered by the patent claims. In addition, effective intellectual property enforcement may be unavailable or limited in some foreign countries. Any litigation, whether to enforce our rights to use our or our licensors' patents or to defend against allegations that we infringe third party rights, willwould be costly, time consuming, and may distract management from other important tasks.
12
As is commonplace in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. To the extent our employees are involved in research areas whichthat are similar to those areas in which they were involved at their former employers, we may be subject to claims that such employees and/or we have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of the former employers. Litigation may be necessary to defend against such claims, which could result in substantial costs and be a distraction to management and which maycould have a materialan adverse effect on us, even if we are successful in defending such claims.
We also rely in our business on trade secrets, know-how and other proprietary information. We seek to protect this information, in part, through the use of confidentiality agreements with employees, consultants, advisors and others. Nonetheless, we cannot assure you that those agreements willmay not provide adequate protection for our trade secrets, know-how or other proprietary information and prevent their unauthorized use or disclosure. To the extent that consultants, key employees or other third parties apply technological information independently developed by them or by others to our proposed products, disputes may arise as to the proprietary rights to such information which may not be resolved in our favor. The risk that other parties may breach confidentiality agreements or that our trade secrets become known or independently discovered by competitors, could adversely affect us by enabling our competitors, who may have greater experience and financial resources, to copy or use our trade secrets and other proprietary information in the advancement of their products, methods or technologies. Policing unauthorized use of our or our licensors' intellectual property is difficult, expensive and time-consuming, and we may be unable to determine the extent of any unauthorized use. Adequate remedies may not exist in the event of unauthorized use or disclosure.
Our success also depends on our ability to operate and commercialize products without infringing on theIf third parties successfully claim that we infringed their patents or proprietary rights, of othersour ability to continue to develop and successfully commercialize our product candidates could be delayed.
Third parties may claim that we or our licensors or suppliers are infringing their patents or are misappropriating their proprietary information. In the event of a successful claim against us or our licensors or suppliers for infringement of the patents or proprietary rights of others relating to any of our marketed products or product candidates, we may be required to:
In addition, from time to time, we become aware of third parties who have, or claim to have, intellectual property rights covering matters such as methods for doing business, conducting research, diagnosing diseases or prescribing medications that are alleged to be broadly applicable across sectors of the industry, and we may receive assertions that these rights apply to us. The existence of such intellectual property rights could present a risk to our business.
A license required under any such patents or proprietary rights held by a third party may not be available to us, or may not be available on acceptable terms. If we or our licensors or suppliers are sued for infringement we could encounter substantial delays in, or be prohibited from developing, manufacturing and commercializing our product candidates.
Our operations could be curtailed if we are unable to obtain additional financing on favorable terms, if at all
On June 30, 2003, after giving effect to this offering on a pro forma as adjusted basis, we would have had approximately $ million in cash, cash equivalentscandidates and short-term investments. We anticipate this will be sufficient to fundadvancing our operations for at least the next 18 months. Our product candidates are in various stages of development, and all will require significant further investment to develop, test and obtain regulatory approval prior to commercialization. We may need to seek additional financing to continue our product development activities, and could require substantial funding to commercialize any of the products that we successfully develop. We do not currently have any funding commitments or arrangements with third parties to provide funding. We may not be able to raise additional capital on favorable terms, if at all.
13
To the extent that we are able to raise additional capital through the sale of equity securities, the issuance of those securities could result in dilution to our stockholders. In addition, if we incur debt financing, we will be required to make cash payments to the principal and interest on such indebtedness, which could substantially reduce our cash balance. If we are unable to successfully commercialize Fampridine-SR, introduce other product candidates, or otherwise obtain sufficient financing on favorable terms when and if needed, we may be required to reduce, defer or discontinue one or more of our product developmentpreclinical programs. Our inability to continue development of any one or more of our product candidates may result in reduced revenue, which could harm our business prospects.
The pharmaceutical industry is subject to stringent regulation, and failure to obtain regulatory approval will prevent commercialization of our products
Our research, development, preclinical and clinical trial activities and the manufacture and marketing of any products that we may successfully develop are subject to an extensive regulatory approval process by the FDA and other regulatory agencies abroad. The process of obtaining required regulatory approvals for drugs is lengthy, expensive and uncertain, and any such regulatory approvals may entail limitations on the indicated usage of a drug, which may reduce the drug's market potential.
In order to obtain FDA approval to commercialize any product candidate, an NDA must be submitted to the FDA demonstrating, among other things, that the product candidate is safe and effective for use in humans for each target indication. Our regulatory submissions may be delayed, or we may cancel plans to make submissions for product candidates for a number of reasons, including:
Consequently, we cannot assure you that we will make our submissions to the FDA in the timeframe that we have planned, or at all, or that our submissions will be approved by the FDA. Even if regulatory clearance is obtained, post-market evaluation of our products, if required, could result in restrictions on our product's marketing or withdrawal of our product from the market as well as possible civil and criminal sanctions.
Clinical trials are subject to oversight by institutional review boards and the FDA to ensure compliance with the FDA's good clinical practice regulations, as well as other requirements for good clinical practices. We depend, in part, on third-party laboratories and medical institutions to conduct preclinical studies and clinical trials for our products and other third-party organizations to perform data collection and analysis, all of which must maintain both good laboratory and good clinical practices. If any such standards are not complied with in our clinical trials, the FDA may suspend or terminate such trial, which would severely delay our development and possibly end the development of such product candidate. We also currently and in the future will depend upon third party manufacturers of our products to comply with Good Manufacturing Practices. We cannot be certain that our present or future manufacturers and suppliers will comply with current Good Manufacturing Practices. The failure to comply with Good Manufacturing Practices may result in restrictions in the sale of, or withdrawal of the products from the market. Compliance by third parties with these standards and practices are outside of our direct control.
In addition, we are subject to regulation under state and federal laws, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other local, state, federal and foreign regulation. We cannot predict the impact of such regulation on us, although it could impose significant restrictions on our business and additional expenses to comply.
14
If our competitors develop and market products that are more effective than ours, or obtain marketing approval before we do, our commercial opportunity will be reduced or eliminated
Competition in the pharmaceutical and biotechnology industries is intense and is expected to increase. Several biotechnology and pharmaceutical companies, as well as academic laboratories, universities and other research institutions, are involved in research and/or product development for various treatments for SCI, MS, epilepsy and bipolar disorder. For example, we are aware that Aventis is developing a sodium/potassium channel blocker, HP 184, with a potential indication in SCI. We believe that HP 184 is now in clinical trials and any resulting product could compete with Fampridine-SR. Many of our competitors have significantly greater research and development capabilities, experience in obtaining regulatory approvals and manufacturing, marketing, financial and managerial resources than we have.
Our competitors may succeed in developing products that are more effective than the ones we have under development or that render our proposed products or technologies noncompetitive or obsolete. In addition, certain of such competitors may achieve product commercialization before we do. If any of our competitors develop a product that is more effective, or are able to obtain FDA approval for commercialization before we do, we may not be able to achieve significant market acceptance, which would have a material adverse effect on our business.
Our products will also compete with numerous drugs used to treat symptoms related to SCI and MS. Although the mechanism by which Fampridine-SR works to treat patients is different than current treatments, these treatments are well-known and widely prescribed by health care providers who may be reluctant to prescribe a new product to their patients.
Valrocemide is a new chemical entity derived from valproic acid, which is a commonly used anti-epileptic drug for the treatment of most seizure types. If valrocemide is not shown to have similar or better efficacy than valproic acid, and a more favorable side effect profile, the commercialization of the product may not be successful.
We face an inherent risk of liability in the event that the useare dependent on our license agreements and if we fail to meet our obligations under these license agreements, or misuse of our products result in personal injury or deathagreements are terminated for any reason, we may lose our rights to our in-licensed patents and technologies.
The useWe are dependent on licenses for intellectual property related to Zanaflex, Fampridine-SR and all of our product candidates in clinical trials, and the salepreclinical programs. Our failure to meet any of any approved products, may expose us to product liability claims, whichour obligations under these license agreements could result in financial losses. Our clinical liability insurance coverage may not be sufficientthe loss of our rights to cover claims that may be made against us. In addition, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts or scope to protect us against losses. Any claims against us, regardless of their merit, could severely harm our financial condition, strain our management and other resources and adversely impact or destroy the prospects for commercialization of the product which is the subject of any such claim.
this intellectual property. If we use biological and hazardous materials in a manner that causes injury,lose our rights under any of these license agreements, we may be liable for damagesunable to commercialize a product that uses licensed intellectual property.
Our research and development activities involveWe could lose our rights to Fampridine-SR under our license agreement with Elan in countries in which we have a license, including the controlled use of potentially harmful biological materials, hazardous materials and chemicals, and are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. IfUnited States, if we fail to comply with environmental regulations, we could be subject to criminal sanctions and/or substantial liability for any damages that result,file regulatory approvals within a commercially reasonable time after completion and any liability could exceed our resources.
Healthcare reformreceipt of positive data from all preclinical and restrictions on reimbursements may limit our financial returns
Our ability to successfully commercialize our products may depend in part on the extent to which government health administration authorities, private health insurers and other third party payors will reimburse consumersclinical studies required for the costrelated NDA, or any NDA-equivalent. We could also lose our rights under our license agreement with Elan if we fail to launch a product in such countries, within 180 days of these products. Third party payors are increasingly challenging bothNDA or equivalent approval. Elan could also terminate our license agreement if we fail to make payments due under the needlicense agreement. If we lose our rights to Fampridine-SR our prospects for generating revenue and recovering our substantial investment in the pricedevelopment of novel therapeutic drugs and uncertainty exists as to the reimbursement status of newly approved therapeutics. Adequate third party reimbursement may notthis product would be available for our drug products to enable us to maintain price levels sufficient to realize an appropriate return on our investments in research and product development, which could restrict our ability to commercialize that particular drug.materially harmed.
15
Risks Relating To The Offering
Our stock priceThere is no existing market for our common stock. An active trading market may be volatile,not develop and you may not be able to resell your shares at or above the initial offering priceprice.
Prior to this offering, there has been no public market for our common stock. We cannot predict the extent to which investor trading will lead to the development of an active and liquid trading market in our common stock. The initial public offering price of our common stock was determined by negotiations between the representatives of the underwriters and us and may not be indicative of future market
prices. The market price for our common stock may decline below the initial offering price. Our stock price may experience substantial fluctuations and could fluctuate significantly due to a number of factors, including:
Many of these factors are beyond our control. In addition, the stock markets in general, and the Nasdaq National Market and the market for biotechnologicalbiopharmaceutical companies in particular, have experienced extreme price and volume fluctuations recently. These fluctuations often have been unrelated or disproportionate to the operating performance of these companies. These broad market and industry factors may adversely affect the market price of our common stock, regardless of our actual operating performance.
As a new investor, you will experience immediate and substantial dilution in the net tangible book value of your investment and may experience further dilution in the future.
The initial public offering price for this offering is substantially higher than the pro forma net tangible book value per share of our outstanding common stock. Investors purchasing shares of our common stock in this offering will pay more for their shares than the amount paid by existing stockholders who acquired shares prior to this offering. Accordingly, if you purchase common stock in this offering, you will incur immediate dilution in pro forma net tangible book value of approximately $$10.10 per share. If the holders of outstanding options or warrants exercise these options or warrants, you will incur further dilution. See "Dilution."Investors purchasing shares of our common stock in this offering will contribute approximately 30.8% of the total amount we have raised since our inception, but will own only approximately 28.9% of our total common stock immediately following the completion of this offering.
Future sales of our common stock or the perception that these sales may occur, could adversely impactcause our stock price to decline.
Sales of substantial amounts of our common stock in the public market after this offering, includingor the possibility of those sales of our common stock by existing stockholders pursuant to Rule 144 underor other distributions, could put downward pressure on the Securities Act and sales of common stock issued upon the exercise of outstanding stock options and warrants, could adversely affect themarket price of our common stock. After the consummation of this offering, our current stockholders will be subject to a 180-day lock up on the sale of their shares. After the lock-up expires, at least 5,649,379based on the number of shares outstanding as of our common stock will become freely tradable, 10,406,232December 31, 2005, 19,698,104 shares of common stock will be tradableeligible for sale subject to Rule 144, Rule 144(k) or Rule 701. The remaining 913,155 shares held by existing stockholders will be eligible for sale from time to time in the future under Rule 144, Rule 144(k) or Rule 701 and holders of 15,806,61613,338,279 shares of our common stock will have rights to cause us to file a registration statement on their behalf and to include their shares in registration statements that we may file on
16
our behalf or on behalf of other stockholders. By exercising their registration rights and selling a large number of shares, these holders could cause the price of our common stock to decline.
We will have broad discretion regardingIf our officers, directors and largest stockholders choose to act together, they may be able to control the useoutcome of proceeds from this offeringa stockholder vote.
Our management will have broad discretion in utilizing the proceeds fromAfter this offering, our officers, directors and holders of 5% or more of our outstanding common stock will beneficially own approximately 37.5% of our common stock. Moreover, a majority of our directors are principals or representatives of entities that own substantial amounts of our common stock. As a result, these stockholders, acting together, will be able to significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval or mergers or other business combination transactions. The interests of this group of stockholders may usenot always coincide with the proceedsinterests of other stockholders, and they may act in ways witha manner that advances their best interests and not necessarily those of other stockholders.
Certain provisions of Delaware law, our certificate of incorporation and our by-laws may delay or prevent an acquisition of us that stockholders may consider favorable or may prevent efforts by our stockholders to change our directors or our management, which you may disagree. We may use the proceeds for corporate purposes that do not immediately enhance our prospects for the future or increasecould decrease the value of your investment.
Provisions in Delaware law and our charter may prevent or delay a change of control, even if that change of control may be beneficial to our stockholdersshares.
We are subject to the Delaware anti-takeover laws regulating corporate takeovers. These anti-takeover laws prevent Delaware corporations from engaging in business combinations with any stockholder, including all affiliates and associates of the stockholder, who owns 15% or more of the corporations' outstanding voting stock, for three years following the date that the stockholder acquired 15% or more of the corporation's voting stock unless specified conditions are met, as further described in "Description of Capital Stock".
Following this offering, our chartercertificate of incorporation and by-laws will providecontain provisions that could make it more difficult for a classified board of directors, whichthird party to acquire us, and may have the effect of preventing or hindering any attempt by our stockholders to replace our current directors. In addition, our charter will authorize usdirectors or officers. These provisions include:
As a Delaware corporation, we are also subject to certain anti-takeover provisions of Delaware law. Under Delaware law, a corporation may not engage in a business combination with any holder of 15% or more of its capital stock unless the holders has held the stock for three years or, among other things, the board of directors has approved the transaction. Our board of directors could rely on Delaware law to prevent or delay an acquisition of us, which could have the effect of reducing your ability to receive a premium on your common stock.
Because we do not intend to pay dividends, you will benefit from an investment in our common stock only if it appreciates in value.
We have not paid cash dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. The success of your investment in our common stock will depend entirely upon any future appreciation. There is no guarantee that could be used to hinder a takeover attempt by a third party.our common stock will appreciate in value after the offering or even maintain the price at which you purchased your shares.
17
This prospectus, including the sections entitled "Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations," and "Business," contains forward-looking statements. These statements relate to future events or to our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. In some cases, you can identify forward-looking statements by the use of words such as "may," "could," "expect," "intend," "plan," "seek," "anticipate," "believe," "estimate," "predict," "potential," "continue," or the negative of these terms or other comparable terminology. You should not place undue reliance on forward-looking statements, since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our control and which could materially affect actual results, levels of activity, performance or achievements. Factors that may cause actual results to differ materially from current expectations, which we describe in more detail elsewhere in this prospectus under the heading "Risk Factors",Factors," include, but are not limited to:
If one or more of these or other risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect, actual results may vary significantly from what we projected. Any forward-looking statement you read in this prospectus reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, growth strategy and liquidity. We assume no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
The safe harbor for forward-looking statements contained in the Securities Litigation Reform Act of 1995 protects companies from liability for their forward looking statements if they comply with the requirements of the Act. The Act does not provide this protection for initial public offerings.
18
We estimate that we will receive approximately $$59.3 million in net proceeds from the sale of our common stock in this offering, or approximately $$68.5 million if the underwriters'underwriters exercise their over-allotment option is exercised in full, based on an assumed initial public offering price of $$12.00 per share (the midpoint of the estimated initial public offering price range)range shown on the cover of this prospectus) after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the proceeds of this offering:offering as follows:
Under our revenue interest assignment arrangement with PRF, if this offering results in our having a total market capitalization in excess of $150.0 million, we will have the option, for 180 days, to repurchase PRF's rights at the "put/call price" in effect on the date such right is exercised. The put/call price on a given date is the greater of (i) 150% of the payments made by PRF to us as of such date, less all payments received by PRF from us as of such date, and (ii) an amount that would generate an internal rate of return to PRF of 25% of all payments made by PRF to us as of such date, taking into account the amount and timing of all payments received by PRF from us as of such date. We do not currently intend to exercise this option if it becomes exercisable, but we may reevaluate whether we would exercise the option during the 180-day period. If we do exercise any such option, we would use a portion of the proceeds from this offering to make the repayment. Unless earlier terminated, the revenue interest assignment arrangement will expire on December 31, 2015. We entered into our revenue interest assignment arrangement with PRF in order to provide additional immediate funding to support the commercialization of Zanaflex Capsules. All funds from the PRF transaction must be used for the commercialization and other activities and obligations related specifically to our Zanaflex operations. We currently intend to use $3 million of the PRF proceeds to pay a Zanaflex Capsules sales-based milestone due to Elan on March 31, 2006.
We expect that the proceeds of this offering will allow us to complete our current Fampridine-SR Phase 3 clinical trial. The amount and timing of our actual expenditures on sales and marketing and our research and development programs will depend on numerous factors, including the progress of our research and development activities, the progress of our clinical trials and regulatory approval process, the number and breadth of our product development programs, our ability to maintain our manufacturing andsuccess in marketing collaborations and other arrangements,Zanaflex Capsules, and any in-licensing and acquisition activities. Our research programs are in an early stage of development and it is difficult to predict what advances, if any, we will make in our research activities using the proceeds of this offering. Accordingly, we will retain broad discretion in the allocation and use of the proceeds of this offering. Currently we have no specific plans or commitments related to any acquisitions or licenses.
Pending application of the net proceeds, we intend to invest them in short-term, investment-grade, interest-bearing instruments.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not intend to pay cash dividends for the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments and other factors our board of directors deems relevant.
19
The following table sets forth our cash, cash equivalents and short-term investments and capitalization as of JuneSeptember 30, 2003:2005:
| | As of June 30, 2003 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma | Pro Forma As Adjusted | ||||||||
| | ($ in thousands, except per share amounts) | ||||||||||
| Cash, cash equivalents and short-term investments | $ | 60,570 | $ | 60,570 | $ | ||||||
| Long-term portion of notes payable | 612 | 612 | $ | ||||||||
Long-term convertible notes payable—principal amount plus accrued interest, less unamortized debt discount-Related party | $ | 7,907 | $ | 7,907 | $ | ||||||
| Mandatorily Redeemable Convertible Preferred Stock, $.001 par value: 7,472,612 shares of Series E convertible preferred stock authorized, issued and outstanding at June 30, 2003; 10,204,047 shares of Series I convertible preferred stock authorized, issued and outstanding at June 30, 2003; 112,790,246 shares of Series J convertible preferred stock authorized, issued and outstanding at June 30, 2003; 0 shares authorized, issued and outstanding on a pro forma and pro forma as adjusted basis | 36,712 | — | |||||||||
| Stockholders' equity (deficit): | |||||||||||
| Non-redeemable Convertible Preferred Stock, $.001 par value: 1,306,068 shares of Series A convertible preferred stock; 900,000 shares of Series B convertible preferred stock; 333,333 shares of Series C convertible preferred stock; 0 shares of Series D preferred stock; 2,300,000 shares of Series F convertible preferred stock; 0 shares of Series G preferred stock; 1,575,229 shares of Series H convertible preferred stock; 0 shares issued and outstanding on a pro forma and pro forma as adjusted basis | 6 | — | |||||||||
| Common stock, $.001 par value; 260,000,000 shares authorized at June 30, 2003 and 75,000,000 shares authorized on a pro forma and on a pro forma as adjusted basis; 248,994 shares issued and outstanding at June 30, 2003, 16,055,611 on a pro forma basis and on a pro forma as adjusted basis, respectively | — | 16 | |||||||||
| Additional paid-in capital | 107,862 | 144,564 | |||||||||
| Deficit accumulated during the development stage | (91,059 | ) | (91,059 | ) | |||||||
| Other comprehensive loss | (6 | ) | (6 | ) | |||||||
| Total stockholders' equity | 16,803 | 53,515 | |||||||||
| Total capitalization | $ | 62,034 | $ | 62,034 | $ | ||||||
| | As of September 30, 2005 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | Actual (unaudited) | Pro Forma (unaudited) | Pro Forma As Adjusted (unaudited) | ||||||||
| | (in thousands) | ||||||||||
| Cash, cash equivalents and short-term investments | $ | 8,741 | $ | 20,039 | $ | 79,456 | |||||
| Current portion of notes payable | 2,347 | 1,150 | 1,150 | ||||||||
| Revenue interest liability—PRF transaction | — | 14,600 | 14,600 | ||||||||
| Put/call option liability—PRF transaction | — | 400 | 400 | ||||||||
| Long-term portion of notes payable | 3,534 | 1,731 | 1,731 | ||||||||
| Long-term convertible notes payable—principal amount plus accrued interest, less unamortized debt discount—Related party | 8,695 | 8,695 | 8,695 | ||||||||
| Mandatorily Redeemable Convertible Preferred Stock, $.001 par value: 7,472,612 shares of Series E convertible preferred stock authorized, issued and outstanding at September 30, 2005; 10,204,047 shares of Series I convertible preferred stock authorized, issued and outstanding at September 30, 2005; 112,790,246 shares of Series J convertible preferred stock authorized, 112,790,233 shares issued and outstanding at September 30, 2005; 1,573,330 shares of Series K convertible preferred stock authorized, 1,533,327 shares issued and outstanding at September 30, 2005; 0 shares issued and outstanding on a pro forma and pro forma as adjusted basis | 85,000 | — | — | ||||||||
| Stockholders' equity (deficit): | |||||||||||
| Non-redeemable Convertible Preferred Stock, $.001 par value: 1,306,068 shares of Series A convertible preferred stock; 900,000 shares of Series B convertible preferred stock; 333,333 shares of Series C convertible preferred stock; 0 shares of Series D preferred stock; 2,300,000 shares of Series F convertible preferred stock; 0 shares of Series G preferred stock; 1,575,229 shares of Series H convertible preferred stock; 0 shares issued and outstanding on a pro forma and pro forma as adjusted basis | 6 | — | — | ||||||||
| Common stock, $.001 par value; 200,000,000 shares authorized at September 30, 2005 and 80,000,000 shares authorized on a pro forma and on a pro forma as adjusted basis; 208,766 shares issued and outstanding at September 30, 2005, issued and outstanding on a pro forma basis and on a pro forma as adjusted basis, respectively | — | 13 | 19 | ||||||||
| Additional paid-in capital | 96,806 | 181,801 | 241,074 | ||||||||
| Accumulated deficit | (198,475 | ) | (198,677 | ) | (198,677 | ) | |||||
| Other comprehensive loss | (6 | ) | (6 | ) | (6 | ) | |||||
| Total stockholders' (deficit) | (101,669 | ) | (16,869 | ) | 42,410 | ||||||
| Total capitalization | $ | (2,093 | ) | $ | 9,707 | $ | 68,986 | ||||
The table above does not include the following amountsexcludes, as of JuneSeptember 30, 2003:2005:
20
Our pro forma net tangible book value as of JuneSeptember 30, 20032005 was approximately $$(23.1) million, or approximately $$(1.71) per share based on 16,055,61113,547,022 shares of common stock outstanding as of JuneSeptember 30, 2003, calculated2005, after giving effect to to:
Net tangible book value per share represents our total tangible assets reduced by our total liabilities, mandatorily redeemable convertible preferred stock, deferred offering costs and the liquidation value of our convertible preferred stock and divided by the number of shares of common stock outstanding. Dilution in pro forma net tangible book value per share to new investors represents the difference between the amount per share that you pay for our common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
Our pro forma as adjusted net tangible book value per share as of JuneSeptember 30, 20032005, would have been approximately $$36.3 million, or approximately $$1.90 per share, after giving further effect to the receiptour sale of 5,500,000 shares in this offering, assuming an initial public offering price of $12.00 per share (the midpoint of the estimated net proceeds fromprice range shown on the sale by uscover of shares.this prospectus), after deducting underwriting discounts and commissions and estimated offering expenses. This represents an immediate increase in net tangible book value of $$3.61 per share to existing stockholders and an immediate decrease in net tangible book value per share of $$10.10 to you. The following table illustrates the dilution.
| Assumed initial public offering price per share | $ | 12.00 | |||||
Pro forma net tangible book value per share as of September 30, 2005 | $ | (1.71 | ) | ||||
Pro forma as adjusted increase in net tangible book value per share attributable to this offering | $ | 3.61 | |||||
Pro forma as adjusted net tangible book value per share after this offering | 1.90 | ||||||
| Dilution per share to new investors | $ | 10.10 | |||||
If the underwriters exercise their over-allotment option in full, the pro forma net tangible book value per share after the offering would be $2.30 per share, the increase in net tangible book value per share to existing stockholders would be $.40 per share and the dilution to new investors would be $9.71 per share.
The following table sets forth, as of JuneSeptember 30, 2003,2005, on a pro forma as adjusted basis, (i) the difference between the holders set forth below with respect to thetotal number of shares of common stock purchased from us, after giving effect to this offering and the reverse stock split and automatic conversion of our outstanding preferred stock described above,
(ii) the total consideration paid to us, and (iii) the average price per share paid.paid by the existing stockholders and by new investors purchasing shares in this offering.
| | Shares Purchased | Total Consideration | | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Average Price Per Share | |||||||||||
| | Number | % | Amount | % | ||||||||
| Existing stockholders | 13,547,022 | 71.1 | % | $ | 147,863,000 | 69.1 | % | $ | 1.04 | |||
| New investors(1) | 5,500,000 | 28.9 | 66,000,000 | 30.8 | 12.00 | |||||||
| Total | 19,047,022 | 100.0 | % | $ | 213,863,000 | 100.0 | % | |||||
The table above assumes no exercise of stock options or warrants outstanding as of September 30, 2005. At September 30, 2005, there were 1,816,518 shares of our common stock issuable upon exercise of outstanding stock options expectedand warrants at a weighted average exercise price of $5.13 per share. To the extent that outstanding options or warrants are exercised in the future, there will be further dilution to be granted to our officers, directorsnew investors. To the extent all of such outstanding options and employees in connection withwarrants had been exercised as of September 30, 2005, pro forma as adjusted net tangible book value per share after this offering would be $2.54 and shares of our common stock issuable upon the exercise of options previously grantedtotal dilution per share to our officers and directors.
The foregoing discussion and tables are based upon the number of shares issued and outstanding as of June 30, 2003 and excludes:new investors would be $9.47.
The issuance of additional common stock will result in further dilution to new investors.
If the underwriters' over-allotment option is exercised in full, the number of shares of our common stock held by existing stockholders will be reduced to , %68.2% of the aggregate number of shares of our common stock outstanding after this offering, and the number of shares of common stock held by new investors will be increased to 6,325,000 or %31.8% of the aggregate number of shares of common stock outstanding after this offering.
21
SELECTED CONSOLIDATED FINANCIAL AND OPERATING DATA
The selected consolidated statement of operations data for the fiscal years ended June 30, 2001, 2002 and 2003, six month period ended December 31, 2003, and the year ended December 31, 2004 and the selected consolidated balance sheet data presented below as of June 30, 2001, 2002 and 2003, have beenand December 31, 2003 and 2004, set forth below are derived from, and are qualified by reference to, our consolidated financial statements included in this prospectus other than the pro forma financial information, which consolidated financial statements have been audited by KPMG LLP, independent auditors.our Independent Registered Public Accounting Firm, and that are included elsewhere in this prospectus for the years ended June 30, 2002 and 2003, six months ended December 31, 2003 and year ended 2004.
We changed our fiscal year end from June 30 to December 31, effective for the six months ended December 31, 2003. The selected consolidated statement of operations data presented below for the yearsnine months ended JuneSeptember 30, 19992004 and 20002005, and selected consolidated balance sheet data presented below as of JuneSeptember 30, 1999, 2000 and 20012005, have been derived from our audited Consolidated Financial Statementsunaudited consolidated financial statements included elsewhere in this prospectus. The unaudited consolidated financial information include, in the opinion of management, all adjustments, consisting of normal and recurring adjustments, that management considers necessary for a fair presentation, in all material respects, of its consolidated results for those periods. Our historical results are not included herein.necessarily indicative of the results to be expected in the future periods and the results for the nine months ended September 30, 2005, should not be considered indicative of results expected for the full year.
This data should be read in conjunction with our "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our Consolidated Financial Statements and the related notes included elsewhere in this prospectus.
| | | | | | | Period from March 17, 1995 (inception) to June 30, 2003 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Year Ended June 30, | ||||||||||||||||||||
| Statement of Operations Data: | 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||||||||
| | ($ in thousands, except per share data) | ||||||||||||||||||||
| Grant revenue | $ | 1,036 | $ | 756 | $ | 462 | $ | 132 | $ | 474 | $ | 3,637 | |||||||||
| Operating expenses incurred in the development stage: | |||||||||||||||||||||
| Research and development | 3,083 | 4,777 | 6,142 | 11,146 | 17,527 | 46,175 | |||||||||||||||
| Research and development—Related party | 1,152 | 2,024 | 2,223 | 4,687 | 2,265 | 32,351 | |||||||||||||||
| General and administrative | 1,342 | 1,406 | 3,489 | 6,636 | 6,388 | 22,855 | |||||||||||||||
| Total operating expenses | 5,577 | 8,207 | 11,854 | 22,469 | 26,180 | 101,381 | |||||||||||||||
| Operating loss | (4,541 | ) | (7,451 | ) | (11,392 | ) | (22,337 | ) | (25,706 | ) | (97,744 | ) | |||||||||
| Other income (expense): | |||||||||||||||||||||
| Interest expense | — | — | — | — | (78 | ) | (78 | ) | |||||||||||||
| Interest expense—Related party | (425 | ) | (448 | ) | (444 | ) | (408 | ) | (369 | ) | (2,580 | ) | |||||||||
| Interest income | 611 | 1,001 | 1,824 | 984 | 393 | 5,038 | |||||||||||||||
| Other income | — | — | — | — | 26 | 26 | |||||||||||||||
| Total other income (expense) | 186 | 553 | 1,380 | 576 | (28 | ) | 2,406 | ||||||||||||||
| Minority interest—Related party | — | — | 699 | 580 | — | 4,279 | |||||||||||||||
| Net loss | (4,355 | ) | (6,898 | ) | (9,313 | ) | (21,181 | ) | (25,734 | ) | (91,059 | ) | |||||||||
| Beneficial conversion feature, accretion of issuance costs preferred dividends and fair value of warrants issued to convertible preferred stockholders | (18 | ) | (27 | ) | (36 | ) | (55 | ) | (23,793 | ) | (24,426 | ) | |||||||||
| Net loss allocable to common stockholders | $ | (4,373 | ) | $ | (6,925 | ) | $ | (9,349 | ) | $ | (21,236 | ) | $ | (49,527 | ) | $ | (115,485 | ) | |||
| Net loss per share allocable to common stockholders—basic and diluted | $ | (18.83 | ) | $ | (29.34 | ) | $ | (39.08 | ) | $ | (86.05 | ) | $ | (198.91 | ) | ||||||
| Pro forma net loss per share allocable to common stockholders—basic and diluted (unaudited)(1) | $ | (15.38 | ) | ||||||||||||||||||
| Weighted average shares of common stock outstanding used in computing net loss per share allocable to common stockholders—basic and diluted | 232 | 236 | 239 | 247 | 249 | ||||||||||||||||
| Weighted average shares of common stock outstanding used in computing pro forma net loss per share allocable to common stockholders—basic and diluted (unaudited)(1)(2) | 8,321 | ||||||||||||||||||||
Footnotes Pro forma per share amounts in the following table reflect the conversion of our outstanding convertible and mandatorily redeemable convertible preferred stock into 13,338,279 shares of common stock on next pagethe closing of this offering, assuming that shares of our preferred stock were outstanding for the entire periods presented. Pro forma balance sheet data amounts in the following table reflect the conversion of our outstanding convertible and mandatorily redeemable convertible preferred stock, as well as our entry into a revenue interest assignment arrangement with PRF on December 23, 2005, including (i) our receipt at signing of a payment in the amount of $15.0 million, (ii) our use of approximately $3.0 million of that payment to repay a portion of the amount we owe to GE Capital and approximately $700,000 of that payment to pay fees and expenses related to the transaction, including expenses incurred by PRF, (iii) our recognition of a revenue interest liability of approximately $14.6 million, (iv) our recognition of a put/call option liability of approximately $400,000, and (v) our capitalization of approximately $500,000 in fees and expenses related to the transaction.
22
| | | | | | | Nine Months Ended September 30, | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | Six Months Ended December 31, | | |||||||||||||||||||
| | Year Ended June 30, | Year Ended December 31, | ||||||||||||||||||||||
| | 2004 | 2005 | ||||||||||||||||||||||
| | 2001 | 2002 | 2003 | 2003 | 2004 | (unaudited) | ||||||||||||||||||
| | ||||||||||||||||||||||||
| | (in thousands, except per share data) | |||||||||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||||||
| Gross sales—Zanaflex | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 3,239 | ||||||||||
| Less: discounts and allowances | — | — | — | — | (4,417 | ) | (144 | ) | (992 | ) | ||||||||||||||
| Net sales | — | — | — | — | (4,417 | ) | (144 | ) | 2,247 | |||||||||||||||
| Grant revenue | 462 | 132 | 474 | 382 | 479 | 445 | 184 | |||||||||||||||||
| Total net revenue | 462 | 132 | 474 | 382 | (3,938 | ) | (301 | ) | 2,431 | |||||||||||||||
| Less: cost of sales | — | — | — | — | (885 | ) | (363 | ) | (2,274 | ) | ||||||||||||||
| Gross profit | 462 | 132 | 474 | 382 | (4,823 | ) | (62 | ) | 157 | |||||||||||||||
Operating expenses: | ||||||||||||||||||||||||
| Research and development | 6,142 | 11,147 | 17,527 | 16,743 | 21,999 | 18,621 | 9,652 | |||||||||||||||||
| Research and development—related party | 2,223 | 4,687 | 2,265 | 3,343 | — | — | — | |||||||||||||||||
| Sales and marketing | — | — | — | — | 4,662 | 2,793 | 9,657 | |||||||||||||||||
| General and administrative | 3,489 | 6,636 | 6,388 | 17,069 | 13,283 | 11,034 | 6,339 | |||||||||||||||||
| Total operating expenses | 11,854 | 22,470 | 26,180 | 37,155 | 39,944 | 32,448 | 25,648 | |||||||||||||||||
| Operating loss | (11,392 | ) | (22,338 | ) | (25,706 | ) | (36,773 | ) | (44,767 | ) | (32,510 | ) | (25,491 | ) | ||||||||||
Other income (expense): | ||||||||||||||||||||||||
| Interest and amortization of debt discount expense | — | — | (78 | ) | (38 | ) | (385 | ) | (297 | ) | (824 | ) | ||||||||||||
| Interest and amortization of debt discount expense—related party | (443 | ) | (408 | ) | (369 | ) | (184 | ) | — | — | — | |||||||||||||
| Interest income | 1,824 | 984 | 393 | 276 | 409 | 329 | 347 | |||||||||||||||||
| Other income | — | — | 26 | 7 | 2 | 2 | 1 | |||||||||||||||||
| Total other income (expense) | 1,381 | 576 | (28 | ) | 61 | 26 | 34 | (476 | ) | |||||||||||||||
| Minority interest—related party | 699 | 580 | — | — | — | — | — | |||||||||||||||||
| Cumulative effect of change in accounting principle | — | — | — | — | — | — | 3 | |||||||||||||||||
| Net loss | (9,313 | ) | (21,181 | ) | (25,734 | ) | (36,712 | ) | (44,741 | ) | (32,476 | ) | (25,964 | ) | ||||||||||
Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders | (36 | ) | (55 | ) | (24,320 | ) | (11,985 | ) | (24,746 | ) | (18,496 | ) | (18,636 | ) | ||||||||||
| Net loss allocable to common stockholders | $ | (9,349 | ) | $ | (21,236 | ) | $ | (50,054 | ) | $ | (48,697 | ) | $ | (69,487 | ) | $ | (50,972 | ) | $ | (44,600 | ) | |||
| Net loss per share allocable to common stockholders—basic & diluted | $ | (50.81 | ) | $ | (111.90 | ) | $ | (261.38 | ) | $ | (252.87 | ) | $ | (351.76 | ) | $ | (259.22 | ) | $ | (221.17 | ) | |||
| | | | | | | Nine Months Ended September 30, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | Six Months Ended December 31, | | ||||||||||||
| | Year Ended June 30, | Year Ended December 31, | |||||||||||||||
| | 2004 | 2005 | |||||||||||||||
| | 2001 | 2002 | 2003 | 2003 | 2004 | (unaudited) | |||||||||||
| | |||||||||||||||||
| Pro forma net loss per share allocable to common stockholders—basic & diluted (unaudited)(1) | $ | (9.63 | ) | $ | (1.92 | ) | |||||||||||
| Weighted average shares of common stock outstanding used in computing net loss per share allocable to common stockholders—basic & diluted | 184 | 190 | 191 | 193 | 198 | 197 | 202 | ||||||||||
| Weighted average shares of common stock outstanding used in computing pro forma net loss per share allocable to common stockholders—basic & diluted (unaudited)(1)(2) | 13,536 | 13,547 | |||||||||||||||
| | As of June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consolidated Balance Sheet Data: | 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||
| | ($ in thousands) | ||||||||||||||
| Cash and cash equivalents | $ | 16,862 | $ | 17,193 | $ | 48,083 | $ | 27,012 | $ | 48,319 | |||||
| Restricted Cash | — | 232 | 243 | 250 | 253 | ||||||||||
| Short-term investments | — | — | — | 2,836 | 12,250 | ||||||||||
| Working capital | 16,873 | 15,894 | 46,115 | 27,097 | 58,975 | ||||||||||
| Total assets | 17,487 | 18,260 | 50,349 | 33,597 | 64,807 | ||||||||||
| Deferred revenue | — | — | — | — | 95 | ||||||||||
| Current portion of notes payable | — | — | — | — | 310 | ||||||||||
| Non-current portion of notes payable | — | — | — | — | 612 | ||||||||||
| Long-term convertible notes payable—principal amount plus accrued interest, less unamortized debt discount—Related party | 6,239 | 6,687 | 7,131 | 7,538 | 7,907 | ||||||||||
| Mandatorily redeemable preferred stock | 19,985 | 20,012 | 59,604 | 59,659 | 36,712 | ||||||||||
| Total stockholders' equity (deficit) | (9,045 | ) | (10,438 | ) | (19,041 | ) | (36,910 | ) | 16,803 | ||||||
23
| | As of June 30, | As of December 31, | As of September 30, | Pro Forma As of September 30, | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | 2003 | 2004 | 2005 | 2005 | |||||||||||||||
| | (in thousands) | (unaudited) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: | ||||||||||||||||||||||
| Cash and cash equivalents | $ | 48,083 | $ | 27,012 | $ | 48,319 | $ | 8,965 | $ | 11,729 | $ | 3,581 | $ | 14,879 | ||||||||
| Restricted cash | 243 | 250 | 253 | 254 | 257 | 261 | 261 | |||||||||||||||
| Short-term investments | — | 2,836 | 12,250 | 32,250 | 9,397 | 5,160 | 5,160 | |||||||||||||||
| Capitalized transaction costs—PRF transaction | — | — | — | — | — | — | 500 | |||||||||||||||
| Working capital | 46,115 | 27,097 | 58,975 | 35,375 | 9,067 | (12,203 | ) | (14,207 | ) | |||||||||||||
| Total assets | 50,349 | 33,597 | 64,807 | 45,960 | 30,982 | 25,543 | 37,842 | |||||||||||||||
| Deferred grant revenue | — | — | 95 | 48 | — | — | — | |||||||||||||||
| Deferred product revenue—Zanaflex Capsules | — | — | — | — | — | 4,960 | 4,960 | |||||||||||||||
| Deferred product revenue—Zanaflex tablets | — | — | — | — | 6,668 | 10,686 | 10,686 | |||||||||||||||
| Current portion of notes payable | — | — | 310 | 324 | 302 | 2,347 | 1,150 | |||||||||||||||
| Non-current portion of notes payable | — | — | 612 | 447 | 145 | 3,534 | 1,731 | |||||||||||||||
| Revenue interest liability—PRF transaction | — | — | — | — | — | — | 14,600 | |||||||||||||||
| Put/call option liability—PRF transaction | — | — | — | — | — | — | 400 | |||||||||||||||
| Long-term convertible notes payable—related party | 7,131 | 7,538 | 7,907 | 8,091 | 8,422 | 8,695 | 8,695 | |||||||||||||||
| Mandatorily redeemable preferred stock | 59,604 | 59,659 | 18,187 | 30,171 | 66,364 | 85,000 | — | |||||||||||||||
| Total stockholders' (deficit) | (19,041 | ) | (36,910 | ) | 35,328 | (130 | ) | (60,571 | ) | (101,669 | ) | (16,869 | ) | |||||||||
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our consolidated financial condition and results of operations should be read in conjunction with our audited consolidated financial statements and related notes included in this prospectus. This discussion and analysis contains forward-looking statements that are subject to risks, uncertainties and other factors, including, but not limited to, those discussed under "Risk Factors" and elsewhere in this prospectus, that could cause our actual results, performance, prospects or opportunities to differ materially from those expressed in, or implied by, these forward-looking statements. See "Forward-Looking Statements".Statements."
Background
Since we commenced operations in 1995, we have devoted substantially all of our resources to the identification, development and commercialization of novel therapies that improve neurological function in people with MS, SCI MS and relatedother disorders of the CNS. Our currentmarketed drug, Zanaflex Capsules, is FDA-approved for the management of spasticity. Our lead product candidate, targetsFampridine-SR, is in a Phase 3 clinical trial for the improvement of walking ability in people with MS. Our preclinical programs also target MS and SCI, as well as other CNS disorders, including stroke and traumatic brain injury.
From 1995 until mid-2004, we were engaged almost exclusively in the in-licensing of compounds and the preclinical and clinical development of these compounds. We licensed the rights to Fampridine-SR from Elan for the treatment of SCI in 1997. In 1998, we formed a wide rangejoint venture, MS Research & Development Corporation, or MSRD, with Elan International Services, Ltd., or EIS, a subsidiary of disorders affecting individualsElan, to develop Fampridine-SR for the treatment of MS under an exclusive worldwide license from Elan.
In September 2003, we entered into a termination and assignment agreement with SCIElan, EIS and MS,MSRD, pursuant to which MSRD assigned to us its assets, including spasticity, muscle weakness, lossthe license from Elan for Fampridine-SR for MS. We paid MSRD approximately $11.5 million for all of bowelthe assets and bladder controlassumed all of the liabilities of MSRD, and sexual dysfunction. Our pipeline currently includes oneMSRD distributed to us approximately $9.5 million as our pro rata portion of the purchase price. From the time of establishment of MSRD until the sale of MSRD's assets to us, Elan was considered to be a related party under generally accepted accounting principles. In conjunction with the termination and assignment, we entered into an amended license agreement with Elan that granted us exclusive worldwide rights to Fampridine-SR in return for the payment of royalties and milestones. In addition, we entered into a supply agreement under which Elan provides Fampridine-SR based upon an agreed upon price schedule.
In September 2003, we entered into a collaboration agreement with Teva Pharmaceutical Industries Ltd., or Teva, to jointly develop and promote in the United States products containing valrocemide, pursuant to which we made an initial payment to Teva of $2.1 million. We and Teva amicably terminated this collaboration agreement in June 2005 and in connection with the termination we paid Teva approximately $3.1 million. We and Teva have no further obligations to each other under this collaboration agreement.
We have expended a significant portion of our funds on a number of clinical trials for Fampridine-SR, our most advanced product incandidate, including two Phase 3 clinical trials two productsof Fampridine-SR in SCI and a Phase 2 clinical trial in MS, the results of which were announced in March 2004. An earlier Phase 2 clinical trial in MS was completed in 2001. In mid-2004, we decided to put our clinical trials of Fampridine-SR in SCI on hold, and multiple pre-clinicalrefocused our efforts on our ongoing Fampridine-SR in MS program, leading to our current Phase 3 clinical trial of Fampridine-SR for improvement of walking ability in people with MS. We may resume our clinical development of Fampridine-SR for SCI following completion of our MS clinical program, or sooner.
In July 2004, we acquired all of Elan's research, development, distribution, sales and marketing rights to Zanaflex Capsules and Zanaflex tablets in the United States. These products are FDA-approved for the management of spasticity. We made an upfront payment to Elan of $2.0 million and are obligated to pay royalties on sales and to make milestone payments upon achievement of specified sales levels. To date, we have achieved two milestones, the first triggering a payment of $1.5 million, 50% of which was paid in the first quarter of 2005 and 50% of which is due in the first quarter of 2006. The second milestone of $3.0 million is due on March 31, 2006. As part of our Zanaflex acquisition, we entered into a long-term supply agreement with Elan under which Elan provides us with Zanaflex Capsules. Elan also assigned us its rights under an agreement with Novartis for the supply of tizanidine and Zanaflex tablets.
Our marketing efforts are focused on Zanaflex Capsules, which we launched in April 2005. Zanaflex tablets lost compound patent protection in 2002 and both Zanaflex Capsules and Zanaflex tablets compete with 11 generic tizanidine products. Although we currently distribute Zanaflex tablets, we do not, and do not intend to, actively promote Zanaflex tablets. As a result, prescriptions for Zanaflex tablets have declined and we expect that they will continue to decline. Our goal is to convert as many sales of Zanaflex tablets and generic tizanidine tablets to sales of Zanaflex Capsules as possible. We believe that sales of Zanaflex Capsules will constitute a significant portion of our total revenue for the foreseeable future.
In late 2004, we began establishing our own specialty sales force in the United States, which consisted of 14 sales professionals as of September 30, 2005. This sales force targets neurologists and other prescribers who specialize in treating people with conditions that involve spasticity. Members of this sales force also call on managed care organizations, pharmacists and distribution customers. We plan to expand our specialty sales force to approximately 30 sales professionals in the first quarter of 2006. We have also entered into an agreement with Cardinal Health, under which, since August 2005, they have provided approximately 160 sales representatives to market Zanaflex Capsules, on a non-exclusive basis, to primary care physicians in the United States. We have retained Access Worldwide Communications to provide a small, dedicated sales force of pharmaceutical telesales professionals to contact primary care, specialty physicians and pharmacists. We expect to expand this sales and marketing infrastructure in the future, as appropriate.
In February 2004, we changed our fiscal year end from June 30 to December 31, effective for the six months ended December 31, 2003.
In December 2005, we entered into a revenue interests assignment agreement with PRF pursuant to which we assigned PRF the right to receive a portion of our net revenues (as defined in the agreement) from Zanaflex Capsules, Zanaflex tablets and any future Zanaflex products. The agreement covers all Zanaflex net revenues generated from October 1, 2005 through and including December 31, 2015, unless the agreement is terminated earlier. In consideration for the assignment, PRF paid us $15.0 million at signing. We used approximately $3.0 million of that payment to repay a portion of the amount we owe to GE Capital, $200,000 of that payment for expenses associated with such repayment and $500,000 of that payment to reimburse PRF for expenses it incurred in the transaction. Under our agreement with PRF, we are required to use the remainder of the amount we received at signing and any other amounts we receive under the agreement to support commercialization, sales, marketing, clinical and regulatory activities and other financial obligations related specifically and solely to our Zanaflex operations. At our election, PRF is also required to pay us (i) an additional $5.0 million if our Zanaflex net revenues in 2005 equal or exceed $11.0 million and our Zanaflex net revenues in the first six months of 2006 equal or exceed $16.0 million, and (ii) an additional $5.0 million if our Zanaflex net revenues in 2006 equal or exceed $33.5 million. If we meet these milestones and decide to borrow these additional funds, we would be required to pay PRF $5.0 million on December 1, 2009 in the case of the first additional payment and $5.0 million on December 1, 2010 in the case of the second additional payment. For more information regarding our agreement with PRF, see "—Liquidity and Capital Resources—Financing Arrangements."
Product Revenue and Returns
Ongoing Zanaflex Capsule and Tablet Sales
Product revenue consists of sales of Zanaflex Capsules and Zanaflex tablets. Under SFAS 48,Revenue Recognition When the Right of Return Exists, we are not permitted to recognize revenue until we can reasonably estimate the likely return rate for our products. Since we have only limited sales history with Zanaflex Capsules and due to generic competition and customer conversion from Zanaflex tablets to Zanaflex Capsules, we cannot reasonably determine a return rate. As a result, we account for sales of these products using a deferred revenue recognition model. At a future point in time, which could be in a number of years, when we are able to reasonably estimate product returns we will begin to recognize revenue based on shipments of product to our wholesale drug distributors.
Under our deferred revenue model, we do not recognize revenue upon shipment of product to our wholesale drug distributors. Instead, we record deferred revenue at gross invoice sales price, and classify the cost basis of the inventory held by the wholesaler as a component of inventory. We recognize revenue when prescriptions are filled to end-users because once prescriptions are filled the product cannot be returned. We use monthly prescription data that we purchase from NDC Health, a leading provider of healthcare data, to determine the amount of revenue to be recognized. When we receive the prescription data, we use the number of units of product prescribed to record gross sales. We then reduce deferred revenue and record cost of goods sold. We began receiving end-user prescription data in March 2005 which enabled us to begin recognizing revenue from Zanaflex tablet sales. We began marketing Zanaflex Capsules in April 2005 and began receiving prescription data and recognizing revenue in the same month. Through September 30, 2005, we have recognized $2.1 million in revenue from Zanaflex tablets and $1.1 million from Zanaflex Capsules.
Under our revenue interests assignment agreement with PRF, PRF is entitled to a portion of our net revenues from Zanaflex Capsules, Zanaflex tablets and any future Zanaflex products. The agreement covers all Zanaflex net revenues generated from October 1, 2005 through and including December 31, 2015, unless the agreement terminates earlier. Under the agreement, PRF is entitled to the following portion of Zanaflex net revenues:
Notwithstanding the foregoing, once PRF has received and retained payments under the agreement that are at least twice the aggregate amount PRF has paid us under the agreement, PRF will only be entitled to 1% of Zanaflex net revenues.
We accept returns of products for six months prior to and 12 months after their expiration date. Returns of products sold by us are charged directly against deferred revenue, reducing the amount of deferred revenue that we may recognize.
Sale of Zanaflex Tablet Inventory Acquired From Elan
When we acquired Zanaflex from Elan, we also acquired Elan's inventory of Zanaflex tablets. This inventory included partial lots with expiration dating of less than 12 months and full lots with expiration dating greater than 12 months. We have not generateddeferred recognition of any revenue from product sales since our inception. If our development efforts result in clinical success, regulatory approval and successful commercialization of our products, we would expect to generate revenue from sales of the partial lot inventory until the return period for the product expires in June 2006, and will recognize revenue
then only to the extent that deferred revenues exceed returns. We cannot use prescription data to recognize revenue associated with the partial lot inventory acquired from Elan because we cannot determine whether the prescription was filled with product that Elan sold prior to our acquisition of Zanaflex or with product we sold.
All Zanaflex tablet partial lot inventory that we acquired from Elan has either been sold or is no longer being sold by us. As a result, after the return period expires in June 2006, there will no longer be deferred revenue associated with the Zanaflex tablet partial lot inventory acquired from Elan.
In July 2005 we began to recognize revenue from the full lots based on prescriptions filled for Zanaflex tablets. All of the Zanaflex tablet inventory sold by Elan prior to our acquisition reached expiration in June 2005, therefore any prescriptions filled for Zanaflex tablets subsequent to June 2005 must be from the full inventory lots acquired by and sold by us.
We are uncertain about the amount of returns that we may receive on these products, in-licensed productsfor a number of reasons including our limited historical returns experience. Returns of Zanaflex tablet inventory acquired from Elan and from receiptsold by us are charged against deferred revenue, reducing the amount of royalties on salesdeferred revenue that we may recognize.
Returns of out-licensed products. Since our inception,Zanaflex Tablets sold by Elan
As part of the acquisition of Zanaflex, we agreed to accept returns of Zanaflex tablets that were returned subsequent to January 17, 2005, including returns of product that was originally sold by Elan. Product returns prior to January 17, 2005, were the responsibility of Elan. We have recognized $3.6recorded a charge of $4.1 million in revenuethe year ended December 31, 2004, for the estimated returns of Zanaflex tablets sold by Elan. To the extent that returns exceed the estimated charge, we will be required to record further charges. The return period for Zanaflex tablets sold by Elan ends in June 2006, after which time we do not anticipate any further charges resulting from government grants.Zanaflex tablets sold by Elan.
Discounts and Allowances
Discounts and allowances consist of estimated reserves for cash discounts, rebates and chargebacks. At the time product is shipped to wholesalers an allowance is recorded for these discounts and allowances. Allowances are established on a product-by-product basis. These allowances are established by management as its best estimate based on each product's historical experience adjusted to reflect known changes in the factors that impact such reserves. Reserves for chargebacks, rebates and discounts are established based on the contractual terms with customers, analysis of historical levels of discounts, chargebacks and rebates, communications with customers and the levels of inventory remaining in the distribution channel as well as expectations about the market for each product and anticipated introduction of competitive products.
Grant Revenue
Grant revenue is recognized when the related research expenses are incurred and our performance obligations under the terms of the respective contract are satisfied. To the extent expended, grant revenue related to purchase of equipment is deferred and amortized over the shorter of its useful life or the life of the related contract.
Cost of Sales
Cost of sales consists of cost of inventory, royalty expense and milestone amortization of intangible assets associated with the Zanaflex acquisition, packaging costs, freight and required inventory stability testing costs. Our inventory costs, royalty obligations and milestone obligations are set forth in the agreements entered into in connection with our Zanaflex acquisition. The Company does not expect
any payments it makes to PRF in connection with the revenue interests assignment transaction entered into in December 2005 to constitute royalty expense or otherwise affect the Company's cost of sales or other operating expenses. See "—Liquidity and Capital Resources—Financing Arrangements."
Research and Development Expenses
Research and development expenses consist primarily of salaries and related expenses for personnel, fees paid to professional service providers in conjunction with independently monitoring our clinical trials and acquiring and evaluating data in conjunction withfrom our clinical trials, costs of contract manufacturing services, costs of materials used in clinical trials and research and development, depreciation of capital resources used to develop our products, costs of facilities and the legal costs of pursuing patent protection of our intellectual property. We expense research and development costs as incurred. We expect our research and development expenses to increase as we continue to develop our product candidates. From inception throughcandidates and preclinical programs.
The following table summarizes our research and development expenses for the fiscal years ended June 30, 2001, 2002, 2003, we spent an aggregatethe six months ended December 31, 2003, the year ended December 31, 2004 and the nine months ended September 30, 2005. Included in this table are our external research and development costs, consisting largely of $78.5 million,clinical trial and research services provided by outside laboratories and vendors recognized in connection with each product candidate currently in clinical development and all preclinical programs as a group. Many of our internal research and development costs, including personnel costs, related benefits and stock-based compensation, expenses of $3.1 million, onare not attributable to any individual project because we use these resources across several development projects. Compensation expense for option grants is classified between clinical development and preclinical research and development.development based on employee job function.
| | | | | | | Nine Months Ended September 30, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Year Ended June 30, | Six Months Ended December 31, 2003 | | ||||||||||||||||||
| | Year Ended December 31, 2004 | ||||||||||||||||||||
| | 2001 | 2002 | 2003 | 2004 | 2005 | ||||||||||||||||
| | | | | | | (unaudited) | |||||||||||||||
| | (in thousands) | ||||||||||||||||||||
| Clinical development: | |||||||||||||||||||||
| Contract expense—SCI | $ | 1,557 | $ | 3,359 | $ | 5,777 | $ | 4,266 | $ | 5,853 | $ | 5,630 | $ | 30 | |||||||
| Contract expense—MS | 649 | 908 | 1,613 | 2,116 | 2,850 | 2,205 | 2,296 | ||||||||||||||
| Other contract expense | — | — | 1,015 | 1,388 | 4,945 | 4,292 | 3,729 | ||||||||||||||
| Operating expense | 695 | 1,518 | 2,356 | 1,789 | 2,652 | 2,108 | 951 | ||||||||||||||
| Licensing expense—Teva | — | — | — | 2,000 | — | — | — | ||||||||||||||
| Total clinical development | 2,901 | 5,785 | 10,761 | 11,559 | 16,300 | 14,235 | 7,006 | ||||||||||||||
| Preclinical research & development: | |||||||||||||||||||||
| Research contracts | 586 | 617 | 271 | 412 | 628 | 469 | 68 | ||||||||||||||
| Contract expense | — | 213 | 1,441 | 216 | 113 | 47 | 62 | ||||||||||||||
| Operating expense | 2,655 | 4,531 | 5,054 | 4,556 | 4,958 | 3,870 | 2,516 | ||||||||||||||
| Total preclinical research & development | 3,241 | 5,361 | 6,766 | 5,184 | 5,699 | 4,386 | 2,646 | ||||||||||||||
| Total research & development | 6,142 | 11,146 | 17,527 | 16,743 | 21,999 | 18,621 | 9,652 | ||||||||||||||
| Research & development—related party expense | 2,223 | 4,687 | 2,265 | 3,343 | — | — | — | ||||||||||||||
| Total | $ | 8,365 | $ | 15,833 | $ | 19,792 | $ | 20,086 | $ | 21,999 | $ | 18,621 | $ | 9,652 | |||||||
Research and development—Development—Related partyParty
In cooperation with Elan, we have conducted a series of clinical trials during the past sixeight years evaluating Fampridine-SR. Elan is an Acorda stockholder and iswas considered to be a related party.party during the period from April, 1998 when MSRD, our jointly-owned venture with Elan to develop Fampridine-SR in MS, was formed until September 2003, when Elan's interest in MSRD was sold to us (see Note 11 to our consolidated financial statements included in this prospectus). Related party research and development or sales and marketing expenses have been included as a separate line item in our financial statements.statements for this period and in the table above. These expenses consistconsisted of the contracted development and supply of our lead product candidate, Fampridine-SR.Fampridine-SR, license fees and expenses associated with our acquisition of Elan's interest in MSRD.
Sales and Marketing Expenses
Sales and marketing expenses includes the costs of salaries for our sales and marketing personnel and the cost of our advertising, promotion and education programs. Sales and marketing expenses include the cost of our contract sales force provided by Cardinal Health and our contract pharmaceutical telesales services provided by Access Worldwide.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs for personnel serving executive, finance, sales and marketing, business development, legal, information technology and human resource functions. Other costs include facility costs not otherwise included in research and development or sales and marketing expense and professional fees for legal and accounting services. We expect that our general and administrative expenses will increase as we add personnel and become subject to the reporting obligations applicable to public companies. From inception through June 30, 2003, we spent an aggregate of $22.9 million, including stock-based compensation expenses of $2.6 million, on general and administrative expenses.
24
Stock-Based Compensation
We have accounted for options and restricted stock granted to employees and directors in accordance with SFAS No. 123,Accounting for Stock-Based Compensation, and related interpretations. As such, compensation expense is recorded on stock option and restricted stock grants based on the fair value of the restricted stock and options granted, which is estimated on the date of grant using an option-pricing model and it is recognized on a straight-line basis over the vesting period. Compensation expense for options and restricted stock granted to employees amounted to $643,000, $1.3 million, $1.6 million, $13.2 million, $9.0 million, and $3.8$3.5 million for the years ended June 30, 2001, 2002 and 2003, the six months ended December 31, 2003, the year ended December 31, 2004 and for the period from March 17, 1995, the date of our inception, to Junenine months ended September 30, 2003, respectively.2005. Compensation expense for options and restricted stock granted to employees are classified between research and development and general and administrative expense based on employee job function.
We have accounted for stock options granted to non-employees on a fair-value basis in accordance with SFAS No. 123,Accounting for Stock-Based Compensation, Emerging Issues Task Force ("EITF") Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, and FASB Interpretations No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans an Interpretation of APB Opinion No. 15 and 25. As a result, the non-cash charge to operations for non-employee options with vesting or other performance criteria is affected each reporting period by changes in the fair value of our common stock. Compensation expense for options granted to non-employees amounted to $94,000, $75,000, ($6,000)7,000), $8,000, $15,000 and $1.9 million$47,000 for the years ended June 30, 2001, 2002 and 2003, the six months ended December 31, 2003, the year ended December 31, 2004 and for the period from March 17, 1995, the date of our inception, to Junenine months ended September 30, 2003,2005, respectively. The amount of compensation expense to be recorded in the future for options granted to non-employees is subject to change each reporting period based upon changes in the fair value of our common stock, estimated volatility and risk free interest rate until the non-employee completes performance under the option agreement.
We may record additional deferred stock-based compensation if we grant additional options or change the terms of the options granted to our employees. On September 11, 2003, we granted 1,374,997 stock options, that had been authorized for issuance under the Plan in May 2003, to employees under the stock option plan at an exercise price of $5.88 per share which is below the fair value of our common stock at the date of grant. We will immediately recognize a compensation expense of approximately $8.3 million in the first quarter of fiscal 2004, as a certain number of the options issued will vest immediately, and the balance of $5.2 million will be recognized over the remaining respective vesting periods of the options. In addition, we reduced the exercise price per share of 150,251 stock options issued to employees to $5.88 per share. As a result of this repricing, the Company will recognize an additional compensation charge estimated at approximately $520,000, of which $449,000 will be recognized during the first fiscal quarter of 2004, with the balance to be recognized over the remaining respective vesting periods of the options.
Beneficial Conversion Feature
In May 2003, we completed a private placement of 112,790,233 shares of Series J convertible preferred stock for an aggregate purchase price of approximately $55.3 million. As a result of this financing, our Series A through Series I preferred stockholders' original conversion price wasprices were reduced as a result ofdue to anti-dilution adjustments, which resulted in a beneficial conversion of $80.8$80.7 million in accordance with EITF No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios and EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments. The beneficial conversion of $20.9 million was recorded as an immediate charge to additional paid-in capital, relatedrelating to our Series A, Series B, Series C, Series F and Series H convertible preferred stock, which are not mandatorily redeemable and may be converted to common stock at any time at the option of the holders. The remaining beneficial conversion of $59.9 million, relatedrelating to our Series E and Series I convertible preferred stock, which isare mandatorily redeemable at any time on or after June 30, 2008, is being accreted ratably over the mandatory redemption period. Such accretion amounted to $1.7 million, $5.8 million, $11.6 million and $8.7 million for the year ended June 30, 2003, amounted to $1.7 millionthe six months ended December 31, 2003, the year ended December 31, 2004, and the nine months ended September 30, 2005, respectively, and is charged to additional paid-in capital.
25
In addition, the The issuance of our Series J mandatorily redeemable convertible preferred stock resulted in a beneficial conversion of $20.9amounting to $40.0 million in accordance with EITF No. 98-5. The beneficial conversion is calculated using an estimate ofbased on the estimated fair value of our common stock onprice per share at the date of issuance of Series J preferred stock of approximately $8.16$10.14 per share of common stock, which iswas calculated based on the estimated projected midpoint of the range of the Company'sour initial public offering price per common share, which was planned in the fourth calendar quarter of 2003, and the stock price appreciation in comparable public companies from May 2003 to August 2003. The beneficial conversion feature is being accreted ratably over the mandatory redemption period. Theperiod, with a charge to additional paid-in capital of $1.1 million, $3.9 million, $7.8 million and $5.8 million for the year ended June 30, 2003, amounted to $580,000.the six months ended December 31, 2003, the year ended December 31, 2004, and the nine months ended September 30, 2005, respectively.
The unamortized portion of the beneficial conversion at JuneSeptember 30, 20032005 was $78.6$53.3 million. Upon the closing of this offering, we will recognize a one time non-cash charge to additional paid in capital, reflecting the unamortized portion of the beneficial conversion feature as a result of the automatic conversion of theall outstanding convertible preferred stock and mandatorily redeemable convertible preferred stock to common stock upon completion of this offering.
Other Income (Expense)
Interest income consists of interest earned on our cash, cash equivalents and short-term investments. Interest expense consists of interest expense on our GE Capital notes payable.notes. Interest expense—Relatedexpense-related party consists of amortization of debt discount and accrued interest on our $7.5 million aggregate principal amount of EIS convertible notes, payable to Elan International Services, Ltd., or EIS, an affiliateoutstanding as of Elan.September 30, 2005. Other income consists primarily consists of unrealized gains from our investment securities. In future periods, we expect to recognize higher levels of interest expense in connection with the revenue interests assignment arrangement we entered into with PRF in December 2005. In addition, we expect to recognize a net liability associated with the fair value of PRF's put option and our call option under the revenue interests assignment arrangement. This liability will be revalued on a quarterly basis to reflect any changes in the fair value and we will recognize a gain or loss based on an option transaction.that revaluation. See "—Liquidity and Capital Resources—Financing Arrangements."
Results of Operations
Nine Months Ended September 30, 2005 Compared to Nine Months Ended September 30, 2004
Product Sales
We have a limited historyrecognized revenue from the sale of operations. We anticipate that our quarterly resultsZanaflex Capsules and Zanaflex tablets of operations will fluctuate$3.2 million for the foreseeablenine months ended September 30, 2005, as compared to $0 for the nine months ended September 30, 2004. We recognize product sales using a deferred revenue recognition model meaning that product sales are recorded as deferred revenue when shipped to the wholesaler and only recognized as revenue when end-user prescriptions of the product are filled. Product sales in the nine months ended September 30, 2005, consist of Zanaflex tablet sales beginning in March 2005, which is when we began receiving prescription data for tablets containing a code clearly identifying these prescriptions as having been filled with product we sold, and Zanaflex Capsules prescription data beginning after our launch of the product in April 2005.
Deferred revenue from Zanaflex Capsules was $5.0 million as of September 30, 2005, as compared to $0 as of September 30, 2004. The increase in deferred revenue of Zanaflex Capsules was a result of our launch of the product in April 2005. We expect deferred revenue from Zanaflex Capsules to increase in the future dueas our sales and marketing efforts ramp up, and prescription data continues to several factors, includinglag wholesaler orders made in anticipation of demand.
Deferred revenue from Zanaflex tablets was $10.7 million as of September 30, 2005, an increase of $4.0 million since December 31, 2004, as compared to $2.8 million as of September 30, 2004. The increase in deferred revenue of Zanaflex tablets was a result of continued sales of the timingproduct and amountthe fact that we are not recognizing any of payments madethe deferred revenue from Zanaflex tablet inventory acquired from Elan that had an expiration date of less than twelve months at the date of acquisition until after the return period expires in June 2006. With respect to the $10.7 million of deferred revenue at September 30, 2005, approximately $2.5 million relates to product that we acquired from Elan that had an expiration date of less than 12 months at the time we sold it during 2004. We believe there is a high likelihood that this product will be returned, which would result in our inability to recognize revenue related to these sales. We expect deferred revenue from Zanaflex tablets to decline over time as we attempt to convert Zanaflex tablet sales to Zanaflex Capsules sales.
Discounts and Allowances
We recorded discounts and allowances of $992,000 for the nine months ended September 30, 2005 as compared to $144,000 for the nine months ended September 30, 2004. Discounts and allowances are recorded when Zanaflex Capsules and Zanaflex tablets are shipped to wholesalers. We began shipping Zanaflex tablets after our acquisition from Elan in July 2004 and Zanaflex Capsules in April 2005. Discounts and allowances for the nine months ended September 30, 2005 consisted of $683,000 in cash discounts and $308,000 in reserves for chargebacks and rebates. Discounts and allowances for the nine months ended September 30, 2004, consisted of $55,000 in cash discounts and $89,000 for chargebacks and rebates. As part of our April 2005 launch of Zanaflex Capsules, in April, May and September 2005 we extended a 6% promotional cash discount over and above the standard 2% discount provided to drug wholesalers and a 4% rebate on products resold by the wholesalers to pharmacies, hospitals and other third parties. We expect cash discounts to decrease in future periods as a percentage of sales.
Grant Revenue
Grant revenue for the nine months ended September 30, 2005 was $184,000 compared to $445,000 for the nine months ended September 30, 2004. Grant revenue is recognized when the related research expenses are incurred and our performance obligations under the terms of the respective contract are satisfied.
Cost of Sales
We recorded cost of sales of $2.3 million for the nine months ended September 30, 2005 as compared to $363,000 for the nine months ended September 30, 2004. Cost of sales for the nine
months ended September 30, 2005, consisted of $1.2 million in royalty fees, $249,000 in milestone amortization of intangible assets, $561,000 in inventory costs and $275,000 in costs related to packaging, freight, and stability testing. Cost of sales for the nine months ended September 30, 2004, consisted of $261,000 in royalty fees, $37,000 in milestone amortization of intangible assets and $65,000 in inventory costs. We began incurring cost of sales upon the acquisition of Zanaflex in July 2004.
Research and Development
Research and development expenses for the nine months ended September 30, 2005, were $9.7 million as compared to $18.6 million for the nine months ended September 30, 2004, a decrease of approximately $8.9 million, or received pursuant to licensing or collaboration agreements, progress of our47.8%. The decrease in research and development efforts,expenses was primarily attributable to completion of two Phase 3 clinical trials of Fampridine-SR in SCI, and one Phase 2 clinical trial of Fampridine-SR in MS, during the first quarter of 2004. The SCI clinical development program expense decreased from $5.6 million for the nine months ended September 30, 2004 to $30,000 for the nine months ended September 30, 2005, due to our decision to put the program on hold. The MS clinical development program expense increased from $2.2 million for the nine months ended September 30, 2004 to $2.3 million for the nine months ended September 30, 2005, an increase of 4.5%. We expect that expenses associated with our MS clinical development program will increase as we continue our Phase 3 clinical trial.
Other contract expenses decreased to $3.8 million in the nine months ended September 30, 2005, from $4.3 million in the nine months ended September 30, 2004, a decrease of 11.6%. This decrease is primarily due to a $3.1 million decrease in expenses for the manufacture of clinical supplies from the period ended September 30, 2004, offset by a $2.2 million increase in expenses related to the valrocemide collaboration, primarily due to expenses of $3.1 million related to the termination of that collaboration in the nine months ended September 30, 2005.
Operating expenses for clinical development and preclinical research and development decreased to $3.3 million in the nine months ended September 30, 2005, from $6.0 million in the nine months ended September 30, 2004, a decrease of $2.5 million, or 41.7%. This decrease was a result of a $1.1 million decrease in preclinical salaries and benefits due to a staff reduction in early 2005. These expenses also include non-cash stock-based compensation expense of $465,000 for the nine months ended September 30, 2005, and $1.4 million for the nine months ended September 30, 2004.
Sales and Marketing
Sales and marketing expenses for the nine months ended September 30, 2005, were $9.7 million compared to $2.8 million for the nine months ended September 30, 2004, an increase of approximately $6.9 million or 246.4%. This increase was primarily attributable to $3.3 million for marketing and distribution and sales administration expense related to the launch of Zanaflex Capsules and the timingdistribution of Zanaflex tablets and outcome$3.0 million in salaries and benefits related to our Zanaflex Capsules specialty sales force.
General and Administrative
General and administrative expenses for the nine months ended September 30, 2005, were $6.3 million compared to $11.0 million for the nine months ended September 30, 2004, a decrease of regulatory approvals.approximately $4.7 million, or 42.7%. Total general and administrative expenses include non-cash stock based compensation expense of $2.0 million for the nine months ended September 30, 2005, as compared to $5.3 million for the nine months ended September 30, 2004, primarily attributable to the repricing in the first quarter of 2004 of options granted prior to 2004. In addition, the nine months ended September 30, 2004, included approximately $1.2 million in outside NDA preparation services related to our Phase 3 trials of Fampridine-SR in SCI.
Other Income (Expense)
Other income (expense) decreased to a loss of $476,000 for the nine months ended September 30, 2005, from a gain of $34,000 in the nine months ended September 30, 2004, a decrease of $510,000.
Interest expense increased by $527,000 primarily related to a $6.0 million secured term loan with GE Capital entered into in January 2005. The increase in interest expense was offset by an increase in interest income of $18,000 during the nine months ended September 30, 2005.
Beneficial Conversion Feature, Accretion of Issuance Costs, Preferred Dividends and Fair Value of Warrants Issued to Convertible Preferred Stockholders
Charges related to preferred stock remained relatively flat at $18.6 million for the nine months ended September 30, 2005, and $18.5 million for the nine months ended September 30, 2004. These charges primarily comprised accretion of issuance costs on Series E, Series I and Series J mandatorily redeemable convertible preferred stock, accrual of preferred dividend of on Series J and Series K mandatorily redeemable convertible preferred stock, accretion of beneficial conversion feature on Series A through Series I preferred stock for reset in conversion price and accretion of beneficial conversion feature on Series J preferred stock (see Notes 3, 8 and 11 to our consolidated financial statements included in this prospectus).
Year Ended December 31, 2004 Compared to Twelve Months Ended December 31, 2003(1)
| | Twelve Months Ended December 31, 2003 | Year Ended December 31, 2004 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| | (unaudited) | | |||||||
| | (in thousands) | ||||||||
| Gross sales—Zanaflex | $ | — | $ | — | |||||
| Less: discounts and allowances | — | (4,417 | ) | ||||||
| Net sales | — | (4,417 | ) | ||||||
| Grant revenue | 764 | 479 | |||||||
| Total net revenue | 764 | (3,938 | ) | ||||||
| Less: cost of sales | — | (885 | ) | ||||||
| Gross profit | 764 | (4,823 | ) | ||||||
| Operating expenses: | |||||||||
| Research and development | 26,228 | 21,999 | |||||||
| Research and development—related party | 4,016 | — | |||||||
| Sales and marketing | — | 4,662 | |||||||
| General and administrative | 21,220 | 13,283 | |||||||
| Total operating expenses | 51,464 | 39,944 | |||||||
| Operating loss | (50,700 | ) | (44,767 | ) | |||||
| Other income (expense): | |||||||||
| Interest and amortization of debt discount expense | (82 | ) | (385 | ) | |||||
| Interest and amortization of debt discount expense—related party | (445 | ) | — | ||||||
| Interest income | 417 | 409 | |||||||
| Other income | 30 | 2 | |||||||
| Total other income (expense) | (80 | ) | 26 | ||||||
| Net loss | (50,780 | ) | (44,741 | ) | |||||
| Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders | (36,277 | ) | (24,746 | ) | |||||
| Net loss allocable to common stockholders | $ | (87,057 | ) | $ | (69,487 | ) | |||
Product Sales
We did not record product sales from the sale of either Zanaflex Capsules or Zanaflex tablets in the year ended December 31, 2004, or the twelve months ended December 31, 2003.
We did not record deferred revenue from Zanaflex Capsules in either period, as the product was not launched until April 2005. Deferred revenue from Zanaflex tablets was $6.7 million as of December 31, 2004, as compared to $0 as of December 31, 2003. With respect to the $6.7 million of deferred revenue at December 31, 2004, approximately $3.6 million related to product that we acquired from Elan that had an expiration date of less than 12 months at the time we sold it during 2004. We believe there is a high likelihood that this product will be returned, which would result in our inability to recognize revenue related to these sales.
Discounts and Allowances
We recorded discounts and allowances of $4.4 million for the year ended December 31, 2004, as compared to $0 for the twelve months ended December 31, 2003. Discounts and allowances for the year ended December 31, 2004, consisted of $128,000 in cash discounts and $207,000 for chargebacks and rebates. Additionally, in the year ended December 31, 2004, we took a $4.1 million charge to establish a reserve for expected returns of Zanaflex tablets sold by Elan prior to our acquisition of Zanaflex. As part of the acquisition of Zanaflex, we agreed to accept any returns of Zanaflex tablets that were returned subsequent to January 17, 2005, including returns of product that was originally sold by Elan.
Grant Revenue
Grant revenue for the year ended December 31, 2004, was $479,000 compared to $764,000 for the twelve months ended December 31, 2003. Grant revenue is recognized when the related research expenses are incurred and our performance obligations under the terms of the respective contract are satisfied.
Cost of Sales
We recorded cost of sales of $885,000 for the year ended December 31, 2004 as compared with $0 for the twelve months ended December 31, 2003. Cost of sales for the year ended December 31, 2004, consisted of $519,000 in royalty fees, $114,000 in milestone amortization of intangible assets and $252,000 in inventory costs related to the sale of Zanaflex tablets. For the twelve months ended December 31, 2003, we had no product sales and, as a result, no cost of sales.
Research and Development
Research and development expense for the year ended December 31, 2004, was $22.0 million, as compared to $26.2 million for the twelve months ended December 31, 2003, a decrease of approximately $4.2 million, or 16.0%. Contributing to this decrease was completion of two Phase 3 clinical trials of Fampridine-SR in SCI, and one Phase 2 clinical trial of Fampridine-SR in MS, during the first quarter of 2004. The SCI clinical development program expense decreased to $5.9 million for the year ended December 31, 2004, as compared to $7.2 million for the twelve months ended December 31, 2003, a decrease of $1.3 million, or 18.1%. The MS clinical development program expense decreased to $2.9 million for the year ended December 31, 2004, as compared to $3.3 million for the twelve months ended December 31, 2003, a decrease of $400,000, or 12.1%. We expect that expenses associated with our MS clinical development program will increase as we continue our Phase 3 clinical trial. Our limited operating history makes predictionslicensing expense decreased to $0 for the year ended December 31, 2004, as compared to $2.0 million for the twelve months ended December 31, 2003. This expense was attributable to an initial payment to Teva for our collaboration agreement for valrocemide.
Other contract expenses increased to $5.0 million for the year ended December 31, 2004, as compared to $1.9 million for the twelve months ended December 31, 2003, an increase of future operations difficult$3.1 million, or impossible. Since163.2%. This increase is primarily the result of the inclusion of costs related to the drug development and supply of Fampridine-SR in other contract expenses for the year ended December 31, 2004. Prior to the termination of the joint venture with Elan in September 2003, this cost was included in Research and development—related party expense. Also contributing to this increase was a cost of $914,000 relating to a terminated development program.
Operating expense for clinical development and preclinical research and development decreased to $7.6 million for the year ended December 31, 2004, as compared to $11.2 million for the twelve months ended December 31, 2003, a decrease of $3.6 million, or 32.1%. This decrease was partly attributable to a decline in non-cash stock-based compensation expense to $1.8 million for the year ended December 31, 2004, as compared to $3.0 million for the twelve months ended December 31, 2003. The decrease was also attributable to other expenses in the twelve months ended December 31, 2003, which included $508,000 of NDA expense and a $452,000 bonus accrual. In addition, research and development lab expense for the year ended December 31, 2004 was $277,000, as compared to $557,000 for the twelve months ended December 31, 2003, a decrease of $280,000.
Research and development—related party expenses for the year ended December 31, 2004, were $0, as compared to $4.0 million for the twelve months ended December 31, 2003. This decrease was attributable to the termination of our inception, we have incurred significant losses. AsMSRD joint venture with Elan in September 2003, after which all MSRD-related research and development expenses were included in clinical development expenses. Research and development—related party expenses for the twelve months ended December 31, 2003 also included $2.0 million related to termination of June 30,the joint venture and $2.0 million in drug development and supply cost.
Sales and Marketing
Sales and marketing expense was $4.7 million for the year ended December 31, 2004, as compared to $0 for the twelve months ended December 31, 2003. This increase was attributable to the beginning of our commercial efforts after our acquisition of the Zanaflex products in July 2004 and included $2.1 million in expense for marketing, distribution, and sales administration, $1.2 million in salaries and benefits, approximately $765,000 in non-cash stock-based compensation expense, and approximately $600,000 in additional sales and marketing overhead expenses.
General and Administrative
General and administrative expense decreased to $13.3 million for the year ended December 31, 2004, from $21.2 million for the twelve months ended December 31, 2003, a decrease of approximately $7.9 million, or 37.3%. This decrease was partly attributable to a decrease in non-cash stock based compensation expense to $6.5 million for the year ended December 31, 2004, as compared to $11.8 million for the twelve months ended December 31, 2003, a decrease of approximately $5.3 million, or 44.9%. In addition, for the twelve months ended December 31, 2003, we had an accumulated deficitadditional $1.4 million in financing-related expenses as compared to the year ended December 31, 2004.
Other Income (Expense)
Other income (expense) increased to a gain of approximately $91.1 million. We anticipate incurring additional losses, which we expect will increase,$26,000 for the foreseeable future.year ended December 31, 2004, compared to a loss of $80,000 for the twelve months ended December 31, 2003, an increase of $106,000. Interest expense decreased by $142,000 due to a decrease in interest expense on our EIS convertible promissory notes, offset by an increase in interest expense from our GE Capital notes, and a decrease of $8,000 in interest income for the year ended December 31, 2004, as compared to the twelve months ended December 31, 2003.
Beneficial Conversion Feature Accretion of Issuance Costs, Preferred Dividends and Fair Value of Warrants Issued to Convertible Preferred Stockholders
Charges related to preferred stock decreased to $24.8 million for the year ended December 31, 2004, from $36.3 million for the twelve months ended December 31, 2003. For the year ended December 31, 2004, charges were primarily comprised of beneficial conversion charges of $19.5 million on Series E, Series I and Series J convertible preferred stock, accretion of issuance costs of $106,000, and preferred dividends of $5.2 million (see Notes 3 and 8 to our consolidated financial statements in this prospectus). For the twelve months ended December 31, 2003, charges were primarily comprised of beneficial conversion charges of $33.9 million on Series A, B, C, F and H convertible preferred stock, and Series E, I and J mandatorily redeemable convertible preferred stock, accretion of issuance costs of $86,000, and preferred dividends of $2.8 million (see Notes 3, 8 and 11 to our consolidated financial statements in this prospectus).
Year Ended June 30, 2003 Compared to Year Ended June 30, 2002
Grant Revenue
Grant revenue for the year ended June 30, 2003, was $474,000 compared to $132,000 for the year ended June 30, 2002. Grant revenue is recognized when the related research expenses are incurred and our performance obligations under the terms of the respective contract are satisfied. To the extent expended, grant revenue related to purchase of equipment is deferred and amortized over the shorter of its useful life or the life of the related contract. For the year ended June 30, 2002,2003, we deferred approximately $95,000 in grant revenue assince it relatesrelated to funding for the purchase of equipment. Since inception, we have recognized grant revenue of approximately $3.6 million of which approximately $3.4 million has been received by us as of June 30, 2003.
Operating Expenses Incurred in the Development Stage
Research and Development
Research and development expensesexpense for the year ended June 30, 2003, werewas $17.5 million, as compared to $11.1 million for the year ended June 30, 2002, an increase of approximately $6.4 million, or 57.2%57.7%. TotalThe increase was primarily attributable to acceleration in patient enrollment for both the Phase 2 clinical trial of Fampridine-SR in MS, as well as two Phase 3 clinical trials of Fampridine-SR in SCI. The SCI study expenses increased to $5.8 million for the year ended June 30, 2003, as compared to $3.4 million for the year ended June 30, 2002, an increase of $2.4 million, or 70.6%. The MS study expense increased to $1.6 million for the year ended June 30, 2003, as compared to $900,000 for the year ended June 30, 2002.
Operating and other contract expense for clinical development and preclinical research and development increased to $8.4 million for the year ended June 30, 2003, as compared to $6.0 million for the year ended June 30, 2002, an increase of $2.4 million, or 40.0%. These expenses include a non-cash stock-based compensation expense of $478,000 for the year ended June 30, 2003, as compared to $455,000 for the year ended June 30, 2002, an2002. This increase of approximately $23,000 or 5.0%. The increase in researchis also attributable to increased staffing and development expensessupport required for the year ended June 30, 2003 was primarily attributable to acceleration in patient enrollment in both the Phase 2new clinical trial in MStrials.
Research and two Phase 3 clinical trials in SCI. The SCI studydevelopment-related party expenses increased from $3.4 million for the
26
year ended June 30, 2002 to $5.7were $2.3 million for the year ended June 30, 2003, an increase of $2.3 million. The MS study expenses increased from $900,000 for the year ended June 30, 2002 to $2.0 million for the year ended June 30, 2003, an increase of $1.1 million. Expenses related to the clinical study and outside contract work for drug stability testing increased by approximately $1.0 million from the year ended June 30, 2002. Expenses incurred in the development of our remyelinating agents and the associated costs increased by approximately $800,000 from the year ended June 30, 2002. Salaries and benefit costs relating to research and development activities increased by approximately $1.0 million from the year ended June 30, 2002 primarily due to an increase in headcount.
Research and Development—Related party
The cost of the drug development and supply of Fampridine-SR decreased by $2.4 million, or 51.7%, to $2.3 million for the year ended June 30, 2003as compared to $4.7 million for the year ended June 30, 2002.2002, a decrease of $2.4 million, or 51.1%. This decrease in expense iswas due to reduced development activities by Elan related to Fampridine-SR during the year ended June 30, 2003.
General and Administrative
General and administrative expensesexpense of $6.4 million remained relatively flat for the year ended June 30, 2003, were $6.4 millionas compared to $6.6 million for the year ended June 30, 2002, a2002. The decrease of approximately $248,000, or 3.7%. Totalin general and administrative expense was primarily due to management's decision to defer spending for market research and medical communications during the year ended June 30, 2003. General and administrative expenses also include non-cash stock based compensation expense of $1.1 million for the year ended June 30, 2003, as compared to $951,000$950,000 for the year ended June 30, 2002, an increase of approximately $144,000,$150,000, or 15.2%15.8%. The decrease in general and administrative expenses was primarily due to management's decision to defer spending for market research and medical communications until we had reached a later stage of clinical development of Fampridine-SR.
Other Income (Expense)
Interest Income (Expense) Net
Interest expense—others Other income (expense) decreased to a loss of $28,000 for the year ended June 30, 2003, consistscompared to a gain of approximately $78,000 on our GE Capital notes payable. Interest expense—Related party decreased $39,000, or 9.5%, to $369,000$576,000 for the year ended June 30, 2003 from $408,000 for the year ended June 30, 2002. Interest expense—Related party for the year ended June 30, 20032002, a decrease of $604,000. This decrease was primarily consists of accrued interest of $150,000 and approximately $219,000 on amortization of debt discount relatingattributable to the EIS convertible promissory notes. Interest income decreased $591,000, or 60.1%, to $393,000 for the year ended June 30, 2003 from $984,000 for the year ended June 30, 2002. Thea decrease in interest income was attributableof $591,000 due to lower average cash balances and lower interest earned on cash balances induring the year ended June 30, 2003.
Other Income
We recorded other income of $26,000 for the year ended June 30, 2003 as compared with $0 for the year ended June 30, 2002. During the first quarter of 2003, we entered into a foreign currency option transaction to sell U.S. dollars to British Pounds amounting to approximately $295,000, with a strike price of $1.4280. The option expiration date was January 31, 2003. The Company's primary purpose for entering into this transaction was to cover an exchange gain or loss on a British Pound denominated contract to be entered into with a foreign company. The gain of $26,000 relating to this option transaction is classified as other income.
Minority Interest
Minority interest decreased to $0 for the year ended June 30, 2003, as compared withto $580,000 for the year ended June 30, 2002. Elan's previous ownership interest in MS Research and Development Corporation, or MSRD, a joint venture that was owned approximately 83% by Acorda and approximately 17% by Elan and another minority stockholder, iswas reflected as minority interest in our consolidated financial statements. In the year ended June 30, 2003, Elan ceased funding its share of the joint venture's expenses, and therefore there is no minority interest for the year ended June 30, 2003. The assets of this joint venture have beenwere transferred to us. See "R&D and Product Collaborations, Alliances and License Agreements".us as of September 2003.
27
Beneficial conversion feature, accretion of issuance costs, preferred dividends and fair value of warrants
issued to convertible preferred stockholders
Charges related to preferred stock increased fromto $24.3 million for the year ended June 30, 2003, as compared to $55,000 for the year ended June 30, 2002 to $23.8 million for the year ended June 30, 2003. For the year ended June 30, 2002 charges were primarily comprised of accretion of issuance costs on Series E and Series I mandatorily redeemable convertible preferred stock (see Note 7 to the consolidated financial statements).2002. For the year ended June 30, 2003, charges were primarily comprised of accretion of issuance costs of $66,000 on Series E, Series I and Series J mandatorily redeemable convertible preferred stock, accrual of preferred dividend of $630,000 on Series J mandatorily redeemable convertible preferred stock, accretion of beneficial conversion feature of $22.5$23.6 million on Series A through Series IJ preferred stock for reset in conversion price and accretion of beneficial conversion feature of $580,000$1.1 million on Series J preferred stock (see Notes 3, 8 and 711 to theour consolidated financial statements)statements included in this prospectus).
Year Ended June 30, 2002 Compared to Year Ended June 30, 2001
Grant Revenue
Grant revenue for For the year ended June 30, 2002, was $132,000 compared to $462,000 for the year ended June 30, 2001, a decreasecharges were primarily comprised of approximately $331,000, or 71.5%. The decrease is primarily due to lower grants receivedaccretion of issuance costs on Series E and recognized for the year ended June 30, 2002.
Operating Expenses Incurred in the Development Stage
Research and Development
Research and development expenses for the year ended June 30, 2002 were $11.1 million compared to $6.1 million for the year ended June 30, 2001, an increase of approximately $5.0 million, or 81.5%. The research and development expenses include non-cash stock based compensation expense of $455,000 for the year ended June 30, 2002, as compared to $286,000 for the year ended June 30, 2001, an increase of approximately $169,000, or 59.4%, primarily due to options granted to employees. SCI clinical study expenses increased from $1.5 million in fiscal 2001 to $3.5 million for the year ended June 30, 2002, an increase of approximately $2.0 million. This increase was due to the increased clinical trial activity for the year ended June 30, 2002. Expenses incurred for salaries and benefits totaled approximately $2.7 million for the year ended June 30, 2002 as compared with $1.8 million for the year ended June 30, 2001, an increase of approximately $900,000 due to increases in research and development headcount. Other research and development expenses associated with our remyelinating agents and other internal costs totaled $1.0 million for the year ended June 30, 2002 compared with $500,000 for the year ended June 30, 2001, an increase of approximately $500,000. We recognized a non-cash charge of $618,000 in research and development expense for the year ended June 30, 2002 on the estimated fair value of a five-year warrant to purchase 100,000 shares of Series B preferred stock of $321,000 and $296,666 as a beneficial conversion charge on issuance of Series CI mandatorily redeemable convertible preferred stock (see Note 103 and 8 to theour consolidated financial statements)statements included in this prospectus).
Research and Development—Related party
The cost of the drug development and supply of Fampridine-SR increased by $2.4 million, or 110.8%, to $4.7 million for the year ended June 30, 2002 compared to $2.2 million for the year ended June 30, 2001. This increase in expense was due to increased development activities by Elan related to Fampridine-SR for the year ended June 30, 2002.
General and Administrative
General and administrative expenses for the year ended June 30, 2002 were $6.6 million compared to $3.5 million for the year ended June 30, 2001, an increase of approximately $3.1 million, or 90.2%. Total general and administrative expenses include non-cash stock based compensation expense of $951,000 for the year ended June 30, 2002, as compared to $452,000 for the year ended June 30, 2001, an increase of approximately $499,000, or 110.5%. Expenses incurred for public relations, marketing research, medical
28
communications, salaries, benefits and the other related sales and marketing operating costs totaled $1.9 million in the year ended June 30, 2002 as compared with $325,000 for the year ended June 30, 2001, an increase of approximately $1.6 million, or 492.3%, was primarily due to the creation of the sales and marketing department in the fourth quarter of the year ended June 30, 2001 and the business development department in the year ended June 30, 2002. Expenses incurred for salaries, benefits and other business development operating costs totalled $700,000 for the year ended June 30, 2002.
Other Income (Expense)
Interest Income (Expense) Net
Interest expense—Related party decreased $36,000, or 8.1%, to $408,000 for the year ended June 30, 2002 from $444,000 for the year ended June 30, 2001. Interest expense—Related party for the year ended June 30, 2002 primarily related to amortization of debt discount of approximately $258,000 and accrued interest of $150,000 relating to the EIS convertible promissory notes. Interest income decreased $840,000, or 46.1%, to $984,000 for the year ended June 30, 2002 from $1.8 million for the year ended June 30, 2001. The decrease in interest income was attributable to lower average cash balances and lower interest earned on cash balances for the year ended June 30, 2002.
Minority Interest
Minority interest decreased $118,000, or 16.9%, to $580,000 for the year ended June 30, 2002 as compared with $699,000 for the year ended June 30, 2001. The minority interest for the year ended June 30, 2002 represents the 19.9% interest in MSRD owned by Elan and another party.
Liquidity and Capital Resources
We have incurred annual operating losses since inception, and, as of JuneSeptember 30, 2003,2005, we have incurredhad an accumulated deficit of approximately $91.1 million, including non-cash charges of $6.7 million related to grants of stock options, warrants, and common stock. Since our inception, we$198.5 million. We have financed our operations primarily through the proceeds of private placements of our securities, and, to a lesser extent, from loans, government grants.grants and, more recently, our financing arrangement with PRF.
Financing Arrangements
From our inception through JuneSeptember 30, 2003,2005, we raised aggregate net proceeds of $136$147.9 million through the proceeds of private placements of equity securities. In addition, in fiscalJanuary 1997, EIS loaned us an aggregate of $7.5 million pursuant to two convertible promissory notes to partly fund our research and development activities.activities, all of which was outstanding as of June 30, 2005. In fiscal 2003,August and September 2002, we financed certain of our fixed assets through two financing agreements with General Electric Capital Corporation, or GE Capital, in the aggregate amount of approximately $1.2 million.million, of which $194,000 was outstanding as of September 30, 2005. In January 2005, we entered into a $6.0 million senior secured term loan, which is collateralized by all of our personal property and fixtures, other than the property that secures our revenue interests assignment arrangement with PRF.
On December 23, 2005, we entered into a revenue interests assignment agreement with PRF, a dedicated healthcare investment fund, pursuant to which we assigned to PRF the right to a portion of our net revenues (as defined in the agreement) from Zanaflex Capsules, Zanaflex tablets and any
future Zanaflex products. To secure our obligations to PRF, we also granted PRF a security interest in substantially all of our assets related to Zanaflex. Our agreement with PRF covers all Zanaflex net revenues generated from October 1, 2005 through and including December 31, 2015, unless the agreement terminates earlier. Under the agreement, PRF is entitled to the following portion of Zanaflex net revenues:
Notwithstanding the foregoing, once PRF has received and retained payments under the agreement that are at least twice the aggregate amount PRF has paid us under the agreement, PRF will only be entitled to 1% of Zanaflex net revenues. In connection with the transaction, we expect to record a liability, referred to as the revenue interest liability, of approximately $14.6 million in accordance with EITF 88-18,Sales of Future Revenues. We will also impute interest expense associated with this liability using the effective interest rate method and will record a corresponding accrued interest liability. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the life of the arrangement. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of Zanaflex sales. We currently estimate that the imputed interest rate associated with this liability will be approximately 8.9%. Payments made to PRF as a result of Zanaflex sales levels will reduce the accrued interest liability and the principal amount of the revenue interest liability.
In consideration for our assignment of the right to receive a portion of Zanaflex net revenues, PRF paid us $15.0 million at signing of the agreement. We used approximately $3.0 million of the signing payment to repay a portion of the amount we owe to GE Capital, approximately $200,000 of the signing payment for fees and expenses associated with such repayment and $500,000 of the signing payment to reimburse PRF for expenses it estimated it incurred in the transaction. If $500,000 exceeds the actual amount of expenses PRF incurred in the transaction, PRF is required to pay us the excess within 90 days of the signing date. Under our agreement with PRF, we are required to use the remainder of the amount we received at signing and any other amounts we receive under the agreement to support commercialization, sales, marketing, clinical and regulatory activities and other financial obligations related specifically and solely to our Zanaflex operations. We may not use any proceeds from our agreement with PRF to support any of our other products unless such use is ancillary to the support of commercialization of Zanaflex.
At Juneour election, PRF is also required to pay us (i) an additional $5.0 million if our Zanaflex net revenues in 2005 equal or exceed $11.0 million and our Zanaflex net revenues in the first six months of 2006 equal or exceed $16.0 million, and (ii) an additional $5.0 million if our Zanaflex net revenues in 2006 equal or exceed $33.5 million. If we meet these milestones and decide to borrow these additional funds, we would be required to pay PRF $5.0 million on December 1, 2009 in the case of the first additional payment and $5.0 million on December 1, 2010 in the case of the second additional payment.
Upon the occurrence of certain events, including if we experience a change of control, undergo certain bankruptcy events, transfer any of our interests in Zanaflex (other than pursuant to a license agreement, development, commercialization, co-promotion, collaboration, partnering or similar agreement), transfer all or substantially all of our assets, or breach certain of the covenants, representations or warranties we make under the agreement, PRF may (i) require us to repurchase the rights we sold them at the "put/call price" in effect on the date such right is exercised or (ii) foreclose on the Zanaflex assets that secure our
obligations to PRF. Except in the case of certain bankruptcy events, if PRF exercises its right, which we refer to as PRF's put option, to cause us to repurchase the rights we assigned to it, PRF may not foreclose unless we fail to pay the put/call price as required. If we experience a change of control or complete an initial public offering of shares of our common stock that results in our having a total market capitalization in excess of $150.0 million, we have the right, which we refer to as our call option, to repurchase the rights we sold to PRF at the "put/call price" in effect on the date such right is exercised. If our call option becomes exercisable as a result of this offering, we will have a period of 180 days during which to exercise the option. We do not currently intend to exercise our call option if it becomes exercisable as a result of this offering but we may reevaluate whether we would exercise the option during the 180-day period. If we do exercise our call option, we would use a portion of the proceeds from this offering to make the repayment. See "Use of Proceeds." The put/call price on a given date is the greater of (i) 150% of all payments made by PRF to us as of such date, less all payments received by PRF from us as of such date, and (ii) an amount that would generate an internal rate of return to PRF of 25% on all payments made by PRF to us as of such date, taking into account the amount and timing of all payments received by PRF from us as of such date. We have determined that PRF's put option and our call option meet the criteria to be considered an embedded derivative and should be accounted for as such. Therefore, we expect to record a net liability of approximately $400,000 related to the put/call option to reflect its estimated fair value as of the date of the agreement, in accordance with SFAS No. 133,Accounting for Derivatives Instruments and Hedging Activities. This liability will be revalued on a quarterly basis to reflect any changes in the fair value and any gain or loss resulting from the revaluation will be recorded in earnings.
During any period during which PRF has the right to receive 15% of Zanaflex net revenues, then 8% of the first $30.0 million in gross product revenues (as defined in the agreement) for Zanaflex we receive in any fiscal year will be distributed to PRF on a daily basis. Following the end of each fiscal quarter, if the aggregate amount actually received by PRF during such quarter exceeds the amount of net revenues PRF was entitled to receive, PRF will remit such excess to us. If the amount of net revenues PRF was entitled to receive during such quarter under the first paragraph above exceeds the aggregate amount actually received by PRF during such quarter, we will remit such excess to PRF.
PRF also has the right to appoint a representative to receive all notices and materials provided to our board of directors and to attend as an observer all meetings of our board of directors, subject to certain exceptions. This right will terminate on the earlier to occur of the fourth anniversary of the completion of an initial public offering of shares of our common stock or termination of the revenue interests assignment agreement.
Investment Activities
At September 30, 2003,2005, cash and cash equivalents and short-term investments were approximately $60.6$8.7 million, as compared to $29.8$23.6 million at JuneSeptember 30, 2002.2004. Our cash and cash equivalents consist of highly liquid investments with original maturitymaturities of three months or less at date of purchase and consist of time deposits and investments in money market funds with commercial banks and financial institutions and high-quality government and investment grade corporate bonds. Also, we maintain cash balances with financial institutions in excess of insured limits. We do not anticipate any losses with respect to such cash balances. Our short-term investments consist of corporate debt securities with original maturities greater than three months.
Our future cash requirements include, but are not limited to, supporting our clinical trial effortsmonths and continuing our other research and development programs. Since inception we have entered into various agreements with institutions and contract research organizations to conduct and monitor our current clinical trials. Underless than one year. The balance of these agreements, at June 30, 2003 we have estimated our payments to be approximately $2.6investments was $5.2 million over the remaining term of the clinical trials. Through June 30, 2003, approximately $78.6 million has been expensed as research and development expenses in the accompanying consolidated statements of operations, $32.3 million has been paid to Elan and $19.2 million has been paid related to these clinical trials. The timing of our expense recognition and future payments related to these agreements are subject to the enrollment of patients and performance by the applicable institution of certain services. As we expand our clinical trials, we will enter into additional agreements and significant additional expenditures will
29
be required as we complete our clinical trials, apply for regulatory approvals, continue development of our product candidates and expand our operations and bring our products to market. In addition, we have entered into various other research and license agreements which, as of JuneSeptember 30, 2003, upon accomplishment2005, as compared to $18.7 million as of certain milestones, will require paymentsSeptember 30, 2004. As of September 30, 2005, our cash and cash equivalents were $3.6 million, and our short-term investments were $5.2 million, as compared to $4.9 million and $18.7 million respectively, as of September 30, 2004.
Net Cash Used by us aggregating up to $15.8 million. Approximately $375,000 of these milestone payments can be taken as credits against earned royalties. Upon regulatory approval, these agreements also require us to make royalty payments as a percentage of product sales.Operations
Net cash used inby operations was $8.1 million, $18.1 million, $24.3 million, and $24.5$26.9 million for the years ended June 30, 2001, 2002 and 2003 and the year ended December 31, 2004, respectively, and $25.8 million and $16.9 million for the nine months ended September 30, 2004 and 2005, respectively. Cash used by
operations for the nine months ended September 30, 2005 was primarily attributable to a net loss of $26.0 million, an increase in prepaid expenses and other current assets of $1.5 million, a decrease in return-related liabilities of $2.1 million and an increase in inventory of $4.5 million attributable to the launch of Zanaflex Capsules. Cash used in operations for the nine months ended September 30, 2005, was offset by stock compensation expense of $3.5 million, an increase in deferred revenue of $9.0 million from Zanaflex sales, and a $2.7 million increase in accounts payable, accrued expenses and other current liabilities. Amounts classified as royalty payable as of December 31, 2004, are included in accounts payable, accrued expenses and other current liabilities as of September 30, 2005, due to their reclassification as a current liability.
Cash used by operations for the nine months ended September 30, 2004, of $25.8 million was primarily due to a net loss of approximately $32.5 million, an increase in accounts receivable of $1.6 million due to the acquisition of Zanaflex and a decrease in accounts payable of $2.4 million due to timing of our payments. The cash used in operations for the nine months ended September 30, 2004, was offset by stock compensation expense of $7.3 million and an increase in deferred revenue of $2.8 million due to Zanaflex sales.
Cash used by operations for the year ended December 31, 2004, of $26.9 million was due to a net loss of $44.7 million, a $1.9 million increase in accounts receivable from Zanaflex sales and a $3.4 million decrease in accounts payable; accrued expenses and other current liabilities, primarily due to a $1.1 million decrease in bonus accruals. Cash used by operations for the year ended December 31, 2004, was offset by stock compensation expense of $9.1 million, depreciation and amortization expense of $1.2 million; an increase in deferred product revenue of $6.7 million; an increase in returns liability of $4.1 million; amortization of discount on short-term investments of $1.7 million; and an increase in royalty payable of $750,000 for Zanaflex sales.
Cash used by operations for the year ended June 30, 2003, of $24.3 million was due to a net loss of $25.7 million; a decreasereduction in amounts due to Elan of $593,000, primarily due to lower drug development charges from Elan for the year ended June 30, 2003;charges; an increase in prepaid expenses and other current assets of $402,000 primarily due to an $118,000 increase in prepaid insurance premiums as a result of increases in general liability insurance and product liability insurance associated with the clinical trial programs,$402,000; a $154,000 increase in interest receivable on our short term investments and an increase in other receivables of $78,000; and an increase in grant receivable of $214,000 primarily due to an increase in grant revenue during the current fiscal year by approximately $437,000.$214,000. The cash used in operations for the year ended June 30, 2003 was offset by stock compensation expense of $1.6 million;million, depreciation and amortization expense of $740,000 and amortization of debt discount relating to our $7.5 million aggregate principal amount convertible notes payable to EIS of $219,000.
Cash was used by operations for the year ended June 30, 2002, of $18.1 million was due to a net loss of approximately $21.2 million and minority interest of $580,000. The cash used in operations for the year ended June 30, 20032002, was partially offset by stock compensation expenses of $1.4 million; expensing of warrants and beneficial conversion charge of $618,000 on Series C preferred stock issued to Elan, on completion of Phase 2 clinical trials of $618,000; an increase in due to Elan of $580,000 primarily due to increased drug development charges from Elan, as the program progressed in the year ended June 30, 2002; depreciation and amortization expense of $417,000;$417,000, amortization of debt discount relating to our $7.5 million aggregate principal amount of convertible promissory notes payable to EIS of $258,000;$258,000, increase in accounts payable and accrued expenses and other current liabilities of $224,000 due to higher expenses incurred as research and development projects progress.
Net Cash Used in/Provided by Investing
Net cash provided by investing activities for the nine months ended September 30, 2005, was $3.2 million, primarily due to $4.1 million in net proceeds received from maturities of short-term investments. In addition, we purchased property and equipment of $142,000 in the nine months ended September 30, 2005. Net cash provided by investing activities for the nine months ended September 30, 2005 was offset by $750,000 in purchases of intangible assets relating to the Zanaflex milestone accrual. Net cash provided by investing activities for the nine months ended September 30, 2004, was $10.5 million, primarily due to $12.9 million in net proceeds received from maturities of short-term investments. Net cash provided by investing activities for the nine months ended September 30, 2004,
was offset by $2 million in purchases of intangible assets related to the acquisition of Zanaflex and $463,000 in purchases of property and equipment. We had no material commitments to purchase property and equipment as of September 30, 2005.
Net cash provided by investing activities for the year ended December 31, 2004, was $18.6 million, primarily due to $21.1 million in net proceeds received from maturities of short-term investments. Net cash provided by investing activities for the year ended December 31, 2004, was offset by $2.0 million in purchases of intangible assets related to the acquisition of Zanaflex and $532,000 in purchases of property and equipment.
Net cash used in investing activities for the year ended June 30, 2003 was $10.2$10.4 million, and wasprimarily due primarily to the net reinvestment of $9.4$9.7 million of surplus cash into marketable securities. In addition, we purchasedsecurities and purchase of property and equipment of $748,000 in fiscal 2003.$748,000. Net cash used in investing activities for the year ended June 30, 2002 was $5.1 million and was primarily due to purchase of short-term investment of $2.8 million and purchase of purchased property and equipment of $2.2 million in the year ended June 30, 2002. We incurred significant expenses in acquisition ofacquiring property and equipment in the year ended June 30, 2002 as a result of the expansion of our office and laboratory facilities. We had no material commitments
Net Cash Used in/Provided by Financing
Net cash provided by financing activities for the nine months ended September 30, 2005, was $5.6 million, primarily due to $5.8 million in proceeds received from the GE Capital senior secured loan and $215,000 in proceeds received from issuance of warrants to GE Capital in conjunction with the issuance of the GE Capital senior secured loan, offset by approximately $429,000 in repayments of notes payable.
Net cash provided by financing activities for the nine months ended September 30, 2004, was $11.2 million, primarily due to proceeds from issuance of preferred stock. In March 2004, we completed a private placement of 1,533,327 shares of Series K mandatorily redeemable convertible preferred stock at $7.50 per share for an aggregate purchase property and equipment at Juneprice of approximately $11.5 million. Issuance costs of $55,000 related to this financing were netted against proceeds received. Net cash provided by financing activities for the nine months ended September 30, 2003.2004, was offset by $240,000 in repayments of notes payable to GE Capital.
Net cash provided by financing activities for the year ended December 31, 2004, was $11.1 million, primarily due to proceeds from issuance of the Series K preferred stock. Net cash provided by financing activities for the year ended December 31, 2004, was offset by $324,000 in repayments of notes payable to GE Capital.
Net cash provided by financing activities in the years ended June 30, 2003, and 2002 was $55.9 million and 2003 was $2.1 million, and $55.9 million, respectively, andrespectively. The cash provided in the year ended June 30, 2003, was primarily due to proceeds of $55.3 million from the issuance of preferred stock. In May 2003, we completed a private placement of 112,790,233 shares of Series J mandatorily redeemable convertible preferred stock at $0.49 per share for an aggregate purchase price of approximately $55.3 million.stock. Issuance costs of $334,000 related to this financing were netted against proceeds received. In the year ended June 30, 2003, also we entered into two financing agreements with GE Capital and received aggregate proceeds in the amount of $1.2 million, $241,000 of such proceeds have been repaid in fiscal 2003.million. In the year ended June 30, 2002, we received proceeds from the issuance of preferred stock of approximately $1.3 million. Proceeds from the issuance of preferred stock primarily consisted of the issuance of 150,000 Series B preferred stock for an aggregate purchase price of $300,000 and 333,333 Series C preferred stock for an aggregate purchase price of $1.0 million to Elan as part of our January 1997 License and Supply Agreement.
We expect to incur losses from operations for the foreseeable future. We expect to incur increasing research and development expenses, including expenses related to additions to personnel and clinical trials. We expect that our general and administrative expenses will increase in the future as we expand our businessFuture Capital Needs
30
development, legal and accounting staff, add infrastructure and incur additional costs related to being a public company, including directors' and officers' insurance, investor relations programs and increased professional fees. Our future capital requirements will depend on a number of factors, including the amount of revenue generated from sales of Zanaflex Capsules, the continued progress of our research and development of product candidates,activities, the timing and outcome of regulatory approvals, the amount and timing of milestone or other payments received or made under collaborative agreements, the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims and other intellectual property
rights and the acquisition of licenses to new products or compounds,compounds. We expect to incur losses from operations for at least the statusnext several years as we continue to expand our sales and marketing infrastructure and increase our marketing efforts to support the commercialization of competitive products, the availabilityZanaflex Capsules, continue our clinical development of financingFampridine-SR and advance our success in developing markets for our product candidates.preclinical programs.
We believe our existing cash and cash equivalents and short-term investment, together with the net proceeds of the Series J preferred stockfrom our financing arrangement with PRF and this offering, will be sufficient to fund our operating expenses, debt repayments and capital equipment requirements for approximately the next 18 months from the date of this prospectus.
We have no credit facility or other committed sources of capital.24 months. To the extent our capital resources are insufficient to meet future operating requirements, we will need to raise additional capital or incur indebtedness to fund our operations. We cannot assure you thatmay be unable to obtain additional debt or equity financing will be available on acceptable terms, if at all. If adequate funds are not available, we may be required to curtail our sales and marketing efforts, delay, reduce the scope of or eliminate some of our research and development programs reduce our commercialization efforts or obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to certain product candidates that we might otherwise seek to develop or commercialize independently. Any future funding may dilute the ownership of our equity investors.
Contractual Obligations and Commitments
In September 2003,January 2005, we entered into several agreementsa $6.0 million senior secured term loan with Elan, including an agreementGE Capital. In December 2005, we used a portion of the initial payment we received under our revenue interest assignment arrangement with PRF to license Elan's sustained release formulationrepay approximately $3.0 million of aminopyridines, including Fampridine-SR.this loan. We are obligated to make milestone payments to Elan, which includes a payment upon approval of an NDA for Fampridine-SR, and royalty payments as a percentage of product sales.
We entered into various other research, license and collaboration agreements which will also require milestone payments upon the achievement of certain milestones and royalty payments as a percentage of product sales.
Under the terms of the employment agreement with our Chief Executive Officer and Chief Financial Officer, we are obligatedrequired to pay severance under certain circumstances. See "Management—Employment Contracts, Terminationmonthly installments until February 2008, with interest-only payments for the first six months followed by principal and interest payments for the remaining 29 months. Interest is fixed at the rate of Employment9.93% per annum. The loan is secured by all of our personal property and Change-in-Control Arrangements."fixtures, other than the property that secures our arrangement with PRF.
In August and September 2002, we entered into two financing agreements with GE Capital in thefor an aggregate amount of approximately $1.1$1.2 million, bearing annual fixed interest rates of 8.57% and 8.88% to finance the purchase of certain property and equipment. BorrowingsOne note is for $766,781 and bears an annual fixed interest rate of 8.88%. The second note is for $386,731 and bears an annual fixed interest rate of 8.57%. These financing arrangements are secured by security interest in certain of our property and equipment.equipment and do not include any debt covenants. We are required to pay monthly installments until October 2006. Future payment obligations for each of the four yearsThe aggregate principal payments required subsequent to June 30, 2003 are: $379,0002005 are $129,115 in each of 20042005, and 2005, $246,000$144,654 in 2006 and $42,000 in 2007.2006.
In January 1997, EIS loaned us an aggregate of $7.5 million pursuant to two convertible promissory notes. One promissory note in the principal amount of $5$5.0 million bears interest at a rate of 3% beginningwhich began on the first anniversary of the note. The other promissory note in the amount of $2.5 million is non-interest bearing. The unpaid principal of $5.0 million note is convertible into shares of our Series D preferred stock at a conversion price of $12.50 per share. Principal and interest are repayable, if not converted, ratably over a seven-year period beginning one year after we receive certain regulatory approval for the products to be developed, subject to limitations related to gross margin on product sales. If it is determined by Elan and us that regulatory approval will not likely occur, all principal and interest will be forgiven. If our license and supply agreements with Elan are terminated for any other reason, the principal and interest is repayable ratably over 15 years.
The other promissory note in the amount of $2.5 million is non-interest bearing. This promissory note is convertible after January 22, 1999, into either shares of Series B preferred stock at a conversion price of $2.00 per share or into an undesignated series of preferred stock at a conversion price equal to 80% of the most recently completed equity financing, as defined, whichever conversion price is greater. This promissory noteIf our preferred stock is no longer outstanding, these notes will be convertible into shares of our common stock. Principal and interest are repayable, by us, if not converted, by EIS, ratably over a seven-year period, beginning one year after we receive certain regulatory approval for thecertain products to be developed.developed, subject to limitations related to gross margin on product sales. If it is determined by uswe and Elan determine that regulatory approval will not likely occur, or ifthe $5.0 million promissory note will automatically convert into the underlying common stock. If our license and supply agreements with Elan are terminated for any other reason, the noteprincipal and interest is repayable ratably over 15 years. Both promissory notes restrict our ability to incur indebtedness that is senior to the notes, subject to certain exceptions, including for our revenue interests assignment arrangement with PRF.
31 Under our Zanaflex purchase agreement with Elan, we are obligated to make milestone payments to Elan of up to $19.5 million based on cumulative gross sales of Zanaflex tablets and Zanaflex Capsules. As of September 30, 2005, we have made or accrued $4.5 million of these milestone
payments in the consolidated financial statements. Under our Zanaflex supply agreement with Elan, we are required to provide to Elan an 18-month rolling forecast at the beginning of each month and a two-year forecast not later than July 1 of each year. We are bound to order one hundred percent of the forecast required quantities for each five month period immediately following each monthly forecast report. At September 30, 2005, the forecast requirement for the five month period following September 30, 2005 amounted to approximately $4.9 million. Under our Fampridine-SR license agreement with Elan, we are obligated to make milestone payments to Elan of up to $15.0 million over the life of the contract and royalty payments as a percentage of product sales. In addition, under our various other research, license and collaboration agreements we are obligated to make milestone payments of up to an aggregate of approximately $16.8 million over the life of the contracts. The following table summarizes our minimum contractual obligations as of June 30, 2003.December 31, 2004. This table does not reflect contingent milestone or royalty payments that may result in future periods from our collaborations, alliances and/or license agreements. This table should be read in conjunction with the accompanying notes to our consolidated financial statements:
| Fiscal Year Ending June 30, | Notes Payable(1) | Operating Leases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Twelve Month Period Ending December 31, | Notes Payable(1) | Operating Leases | ||||||||
| | ($ in thousands) | (in thousands) | ||||||||
2004 | $ 379 | $ 642 | ||||||||
2005 | 379 | 642 | $ | 1,202 | $ | 642 | ||||
2006 | 246 | 642 | 2,462 | 642 | ||||||
2007 | 42 | 642 | 2,558 | 642 | ||||||
2008 | — | 374 | 225 | 53 | ||||||
Total | $1,046 | $2,942 | $ | 6,447 | $ | 1,979 | ||||
Under the terms of the employment agreement with our chief executive officer, Ron Cohen, we are obligated to pay severance under certain circumstances. If the employment agreement is terminated by us or by our chief executive officer for reasons other than for cause, we must pay (i) an amount equal to the base salary the chief executive officer would have received during the fifteen month period immediately following the date of termination, plus (ii) bonus equal to last annual bonus received by chief executive officer multiplied by a fraction, the numerator of which shall be the number of days in the calendar year elapsed as of the termination date and the denominator of which shall be 365.
Under the terms of the employment agreements with our chief scientific officer, Andrew Blight, our chief operating officer, Mary Fisher, our chief financial officer, David Lawrence and our general counsel, Jane Wasman, we are obligated to pay severance under certain circumstances. In the event we terminate our employment agreement with Dr. Blight, Ms. Fisher, Mr. Lawrence or Ms. Wasman without cause, or if one of them voluntarily terminates his or her agreements with good reason, we are obligated to make severance payments equal to nine months base annual salary, in the case of Dr. Blight and Ms. Fisher, and seven months base annual salary, in the case of Mr. Lawrence and Ms. Wasman, as well as COBRA premium payments for the severance period. In such event, all options, stock appreciation rights awards and restricted stock awards that have vested as of the termination date shall remain exercisable for 90 days following such date. All unvested options, stock
appreciation rights awards and stock awards will be cancelled on the date of termination. If Dr. Blight, Ms. Fisher, Mr. Lawrence or Ms. Wasman voluntarily terminates his or her employment without good reason or if we terminate his or her employment without cause within 18 months after a change in control, we are obligated to make severance payments equal to one year's base annual salary, in the case of Dr. Blight and Ms. Fisher, and nine months base annual salary, in the case of Mr. Lawrence and Ms. Wasman, in each case paid in a lump sum within 30 days after termination, as well as COBRA premium payments for the severance period plus a bonus equal to the prior year's bonus pro rated for the number of days worked prior to termination. We are also obligated to pay salary earned but not paid, vacation and sick leave days that have accrued, and reimbursable business expenses incurred through the date of termination. In such event, not less than 50% of the unvested options, stock appreciation rights and restricted or other stock awards shall become immediately and full vested and shall remain exercisable for 18 months following such date. All options that have vested as of the termination date shall remain exercisable for 90 days following such date. All unvested options, stock appreciation rights awards and stock awards will be cancelled on the date of termination.
Subsequent Events
For a discussion of material events that have taken place subsequent to JuneSeptember 30, 2003,2005, please refer to Note 1317 to our consolidated financial statements included in this prospectus.
Quantitative and Qualitative Disclosures about Market Risk
Our financial instruments consist of cash and cash equivalents, short-term investments, grant receivable, notes payable, convertible notes payable and accounts payable. The estimated fair values of all of our financial instruments, excluding convertible notes payable to EIS, approximate their carrying amounts at JuneSeptember 30, 2003.2005. The terms of these notes are disclosed at Note 1011 to the consolidated financial statements.
We have cash equivalents and marketable securitiesshort-term investments at JuneSeptember 30, 2003,2005, which are exposed to the impact of interest rate changes and our interest income fluctuates as our interest rates change. Due to the short-term nature of our investments in money market funds and corporate debt securities, the carrying value of our cash equivalents and marketable securitiesshort-term investments approximate their fair value at JuneSeptember 30, 2003.2005.
We maintain an investment portfolio in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Although our investments are subject to credit risk, our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure from any single issue, issuer or type of investment. Our investments are also subject to interest rate risk and will decrease in value if market interest rates increase. However, due to the conservative nature of our investments and relatively short duration, interest rate risk is mitigated. We do not own derivative financial instruments. Accordingly, we do not believe that there is any material market risk exposure with respect to derivative or other financial instruments.
Effects of Inflation
Our most liquid assets are cash, cash equivalents and short-term investments. Because of their liquidity, these assets are not directly affected by inflation. Because we intend to retain and continue to use our equipment, furniture and fixtures and leasehold improvements, we believe that the incremental inflation
32
related to replacement costs of such items will not materially affect our operations. However, the rate of inflation affects our expenses, such as those forprimarily employee compensation and contract services, which could increase our level of expenses and the rate at which we use our resources.expenses.
Critical Accounting Policies and Estimates
The following discussion of critical accounting policies identifies the accounting policies that require application of management's most difficult, subjective or complex judgments, often as a result
of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. It is not intended to be a comprehensive list of all of our significant accounting policies, which are more fully described in Note 2 of the notes to the consolidated financial statements included in this prospectus. In many cases, the accounting treatment of a particular transaction is specifically dictated by generally accepted accounting principles, with no need for management's judgment in their application. There are also areas in which the selection of an available alternative policy would not produce a materially different result. We have identified the following as our areas of critical accounting policies: sales revenue recognition, research and development, income taxes, and stock warrantsstock-based compensation.
Revenue Recognition
We apply the revenue recognition guidance in SFAS No. 48,Revenue Recognition When the Right of Return Exists, which among other criteria requires that future returns can be reasonably estimated in order to recognize revenue. Under SFAS 48 we are not permitted to recognize revenue until we can reasonably estimate the likely return rate for our products. Since we have only limited sales history with Zanaflex Capsules and option accounting.due to generic competition and customer conversion from Zanaflex tablets to Zanaflex Capsules, we do not believe we can reasonably determine a return rate. As a result, we account for sales of these products using a deferred revenue recognition model. At a future point in time, which could be in a number of years, when we are able to reasonably estimate product returns we will begin to recognize revenue based on shipments of product to our wholesale drug distributors.
Under our deferred revenue model, we do not recognize revenue upon shipment of product to our wholesale drug distributors. Instead, we record deferred revenue at gross invoice sales price, and classify the cost basis of the inventory shipped as a component of inventory. We recognize revenue when prescriptions are filled to an end-user because once a prescription is filled the product cannot be returned. We use monthly prescription data that we purchase from NDC Health, a leading provider of healthcare data, to determine the amount of revenue to be recognized. We receive this data approximately 45 days after the end of a given month. We estimate prescription sales until the NDC data becomes available, at which time adjustments are made to revenue and cost of sales to account for any differences between our estimates and the actual data. To date such differences have been immaterial. The estimated prescription sales are based on assumed monthly prescription growth rates of 0% for Zanaflex tablets and an amount equal to the prior month's prescription growth rate for Zanaflex Capsules. The method for estimating prescription growth for Zanaflex Capsules will be reevaluated as more prescription data becomes available. When we receive the prescription data, we use the number of units of product prescribed to record gross sales. We then reduce deferred revenue and record cost of goods sold. We began receiving end-user prescription data in March 2005 which enabled us to begin recognizing revenue from Zanaflex tablet sales. We began marketing Zanaflex Capsules in April 2005 and began receiving prescription data and recognizing revenue in the same month.
In addition to the prescription data we receive from NDC Health, we also receive data that we use to monitor trends in sales from wholesalers to their customers. We receive this data from an outside vendor on a monthly basis. This data includes bottles shipped from certain wholesalers to their customers. We also compare our shipments to wholesalers to prescription reports to further assess inventory in the distribution channel on a monthly basis. We use the wholesaler sales trend data and the wholesaler vs. prescription comparison to better understand market conditions, but not as a basis for recognizing revenue.
We accept returns of products for six months prior to and 12 months after their expiration date. Returns of products sold by us are charged directly against deferred revenue, reducing the amount of deferred revenue that we may recognize.
Sale of Zanaflex Tablet Inventory Acquired From Elan
When we acquired Zanaflex from Elan, we also acquired Elan's inventory of Zanaflex tablets. This inventory included partial lots with expiration dating of less than 12 months and full lots with expiration dating greater than 12 months. We have deferred recognition of any revenue from sales of the partial lot inventory until the return period for the product expires in June 2006, and will recognize revenue then only to the extent that deferred revenues exceed returns. We cannot use prescription data to recognize revenue associated with the partial lot inventory acquired from Elan because we cannot determine whether the prescription was filled with product that Elan sold prior to our acquisition of Zanaflex or with product we sold.
All Zanaflex tablet partial lot inventory that we acquired from Elan has either been sold or is no longer being sold by us. As a result, after the return period expires in June 2006, there will no longer be deferred revenue associated with the Zanaflex tablet partial lot inventory acquired from Elan.
In July 2005 we began to recognize revenue from the full lots based on prescriptions filled for Zanaflex tablets. All of the Zanaflex tablet inventory sold by Elan prior to our acquisition reached expiration in June 2005, therefore any prescriptions filled for Zanaflex tablets subsequent to June 2005 must be from the full inventory lots acquired by and sold by us.
We are uncertain about the amount of returns that we may receive on these products, for a number of reasons including our limited historical returns experience. Returns of Zanaflex tablet inventory acquired from Elan and sold by us are charged against deferred revenue, reducing the amount of deferred revenue that we may recognize.
At December 31, 2004, and September 30, 2005, we had deferred revenue from Zanaflex tablets of $6.6 million and $10.7 million (unaudited), respectively, of which $3.6 million and $2.5 million (unaudited), respectively, was related to product acquired from Elan that had an expiration date of less than 12 months at the time it was sold during 2004. We believe there is a high likelihood that this product will be returned, which would result in our inability to recognize related revenue.
Returns of Zanaflex Tablets sold by Elan
As part of the acquisition of Zanaflex, we agreed to accept any returns of Zanaflex tablets that were returned subsequent to January 17, 2005, including returns of product that was originally sold by Elan. Product returns prior to January 17, 2005, were the responsibility of Elan. We have recorded a charge of $4.1 million in the year ended December 31, 2004, for the estimated returns of Zanaflex tablets sold by Elan. To the extent that returns exceed the estimated charge, we will be required to record further charges. The return period for Zanaflex tablets sold by Elan ends in June 2006, after which time we do not anticipate any further charges resulting from Zanaflex tablets sold by Elan.
Research and Development
Research and development expenses include the costs associated with our internal research and development activities including, salaries and benefits, occupancy costs, and research and development conducted for us by third parties, such as sponsored university-based research, and clinical trial vendors. In addition, research and development expenses include expenses related to grant revenue and the cost of clinical trial drug supply shipped to our clinical study vendors. We account for our clinical study costs by estimating the total cost to treat a patient in each clinical trial and recognizing this cost as we estimate when the patient receives treatment, beginning when the patient enrolls in the trial. This estimated cost includes payments to the trial site and patient-related costs, including laboratory costs related to the conduct of the trial. Cost per patient varies based on the type of clinical trial, the site of the clinical trial, and the length of the treatment period for each patient. As actual costs become known to us, we adjust our accrual; such changes in estimate may be a material change in our clinical study accrual, which could also materially affect our results of operations.
Income Taxes
As part of the process of preparing our financial statements we are required to estimate our income taxes in each of the jurisdictions in which we operate. We account for income taxes by the liability method. Under this method, deferred income taxes are recognized for tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end, based on enacted laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
We have not recorded any tax provision or benefit for the years ended June 30, 2002 and 2003.2003 and December 31, 2004 and for the nine months ended September 30, 2005. We have provided a valuation allowance for the full amount of our net deferred tax assets since realization of any future benefit from deductible temporary differences and net operating loss carry-forwards cannot be sufficiently assured at JuneSeptember 30, 2003.2005.
As of JuneSeptember 30, 2003,2005, we had available net operating loss carry-forwards of approximately $75.6$63.5 million for federal and state income tax purposes, which are available to offset future federal and state taxable income, if any, and expire between 2009 and 2023. We also have2024 and research and development tax credit carry-forwards of approximately $704,000$1.5 million for federal income tax reporting purposes which are available to reduce federal income taxes, if any, through 2017.2018. Since our inception, we have incurred substantial losses and expectsexpect to incur substantial and recurring losses in future periods. The Tax Reform ActInternal Revenue Code of 1986, as amended, the Act,Code, provides for a limitation of the annual use of NOLnet operating loss and research and development tax credit carry forwards (following certain ownership changes, as defined by the Act)Code) that could
33
significantly limit our ability to utilize these carry-forwards. We have experienced various ownership changes, as defined by the Act,Code, as a result of past financings. Accordingly, our ability to utilize the aforementioned carry-forwards may be limited. Additionally, because U.S. tax laws limit the time during which these carry forwards may be applied against future taxes we may not be able to take full advantage of these attributes for federal income tax purposes.
Stock Warrants and Options AccountingStock-Based Compensation
We account for options and restricted stock granted to employees and directors in accordance with Statement of Financial Accounting Standards ("SFAS") No. 123,Accounting for Stock-Based Compensation, and related interpretations. As such, compensation expense is recorded on stock option grants based on the fair value of the options granted, which is estimated on the date of grant using the Black-Scholes option-pricing model and it is recognized on a straight-line basis over the vesting period. Compensation expense for restricted stock granted is based on the fair value of the restricted stock granted and is recognized on a straight-line basis over the vesting period. We account for stock options granted to non-employees on a fair-value basis in accordance with SFAS No. 123, Emerging Issues Task Force Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, and FASB Interpretations No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans an Interpretation of APB Opinion No. 15 and 25. As a result, the non-cash charge to operations for non-employee options with vesting or other performance criteria is affected each reporting period by changes in the estimated fair value of our common stock. The two factors which most affect charges or credits to operations related to stock-based compensation are the fair value of the common stock underlying stock options for which stock-based compensation is recorded and the volatility of such fair value. If our estimates of the fair value of these equity instruments change, it would have the effect of changing compensation expenses. Because shares of our common stock have not been publicly traded, we estimate the fair value of our common stock considering, among other factors, the most recent previous sale of convertible preferred stock (convertible on a one-for-one basis, or one-for-twelve post(pro forma for the 1-for-1.3 reverse split into common stock)on January 11, 2006). We do not discount the issuance price of our preferred stock in estimating the fair value of our common stock.
Recent Accounting Pronouncements
In June 2002, the FASB issued SFAS No. 146,Accounting for Costs Associated with Exit or Disposal Activities ("SFAS No. 146"). SFAS No. 146 addresses financial accounting and reporting for costs associated with exit or disposal activities and nullifies Emerging Issues Task Force ("EITF") Issue No. 94-3,Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring). SFAS No. 146 became effective January 1, 2003. The adoption of SFAS No. 146 did not impact our consolidated financial statements for the fiscal year ended June 30, 2003.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity ("SFAS No. 150"). SFAS No. 150 revises the accounting for certain financial instruments that, under previous guidance, issuers could account for as equity. SFAS No. 150 requires that those instruments be classified as liabilities in statements of financial position. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective for interim periods beginning after June 15, 2003. The adoption of SFAS No. 150 did not impact our consolidated financial statements for the fiscal year ended June 30, 2003.
34
BUSINESS
Overview
Acorda Therapeutics is a late-stagecommercial-stage biopharmaceutical company dedicated to the identification, development and commercialization of novel therapies that improve neurological function in people with multiple sclerosis, or MS, spinal cord injury, or SCI, MS and relatedother disorders of the central nervous system, or CNS. Our marketed drug, Zanaflex Capsules, is FDA-approved for the management of spasticity. Our lead product candidate, Fampridine-SR, which is in a Phase 3 clinical trialstrial for SCI and Phase 2 clinical trials for MS, targets the treatmentimprovement of a wide range of disorders affecting individualswalking ability in people with chronic SCI and MS, including spasticity, muscle weakness, loss of bowel and bladder control and sexual dysfunction.MS. Our other product candidatespreclinical programs also target SCIMS and MSSCI as well as other CNS disorders.disorders, including stroke and traumatic brain injury.
We estimate that approximately 500,000Approximately 650,000 people in the United States suffer from MS or SCI and MS and that the combined annual cost of treatment for these conditions exceeds $9$13 billion. In addition, it is estimated that a total of approximately 10 million people live with the long-term consequences of traumatic brain injury and stroke in the United States. Our goal is to becomecontinue to grow as a fully integratedfully-integrated biopharmaceutical company by commercializing multiple therapeutic products, developing our product candidates and advancing our preclinical programs for these large and underserved markets, while continuing to augment our product pipeline and identify new applications for our core technologies.markets.
Company Highlights
pivotal efficacy studies required for drug approval. We believe Fampridine-SR is the first potential therapy in late-stage clinical development for MS that seeks to improve the function of damaged nerve fibers and would be complementary to existing drugs used to treat MS. To our knowledge, there are no current therapies approved or in development that improve walking ability in people with MS.
Fampridine-SR
Our lead product candidate, Fampridine-SR, is an oral, small molecule drug, contained in a sustained release tablet form. Laboratory studies have shown that fampridine, the active molecule of Fampridine-SR, improves impulse conduction in nerve fibers in which the insulating layer, called myelin, has been damaged. This damage may be caused by physical trauma, in the case of SCI, or by the body's own immune system, in the case of MS. We are developing Fampridine-SR for the treatment of both SCI and MS. We plan to
35
commercialize Fampridine-SR ourselves in the United States and Canada and with partners in various markets throughout the rest of the world.
Key attributes of our Fampridine-SR program include:
Other Products in Our Pipeline
Our other research and development programs are also focused on novel therapeutics for CNS disorders. Our product candidates include small molecules, antibodies, and other protein therapeutics.
Valrocemide
We have entered into a collaboration agreement with Teva to co-develop and co-promote valrocemide in the United States. Valrocemide is a small molecule drug with early Phase 2 clinical evidence of safety and efficacy as an add-on therapy for partial seizures, a type of epilepsy, and preclinical evidence of activity in animal models of epilepsy and neuropathic pain. We plan to move valrocemide into late Phase 2 clinical trials for epilepsy and early Phase 2 clinical trials for bipolar disorder in 2004. We may also pursue clinical development of valrocemide for the treatment of neuropathic pain.
Remyelinating Agents
Our pre-clinical programs include two distinct myelin restoring therapies, Glial Growth Factor 2, which we refer to as GGF-2, and remyelinating antibodies. GGF-2 has been shown in various published studies to stimulate remyelination in animal models of MS and to have a variety of other effects in neural protection and repair. We plan to develop GGF-2 initially for treatment of MS, pending successful completion of preclinical toxicology testing. Our remyelinating antibody program involves monoclonal antibodies that have demonstrated the ability to stimulate repair of myelin in three different animal models of MS. We are currently in the process of final preclinical validation and selection of the lead candidate molecule.
Chondroitinase Program for Scar Matrix Modification
This novel therapeutic approach to repair the injured brain and spinal cord targets the scar tissue that develops as a result of injury, which is believed to impede nerve fiber regeneration and limit functional recovery. We have initiated a collaborative program to develop this promising technology and we are in the process of building our intellectual property position with respect to this technology.
Regenerative Antibody Program
Our pre-clinical programs have identified novel approaches to stimulate axon regeneration in CNS. Our primary focus involves antibodies that react with CNS antigens. Our second strategy employs the use of neuronally restricted precursor cells.
36
Background and Market Opportunity
Our Approach to the Market for CNS
We are focused on identifying, developing and commercializing novel pharmaceutical products that address large and underserved CNS markets. We view SCI as a strategic point of access to a broad range of additional neurological conditions, particularly those resulting from focused cellular damage in the CNS, for the following reasons:
MS is a second major focus for the company because, like SCI, it involves critical damage to nerve fibers and their myelin within the CNS. Three of our product candidates, Fampridine-SR, GGF-2, and the remyelinating antibodies, address demyelination. Epilepsy is another rational development focus because it is also often caused by damage to neurons that produces a disturbance of normal control over excitability and communication between nerve cells.
The Challenge of Central Nervous System Disorders
The spinal cord and brain together comprise the CNS. The billions of nerve cells that make up the CNS, in conjunction with the nerve bundles that run through all parts of the body, which is calledmake up the peripheral nervous system, transmit the electrical impulses necessary to sustain, regulate and monitor every aspect of human life. The spinal cord serves as the master link between the brain and the body.body and carries information that regulates movement, sensation and involuntary functions, such as breathing, blood pressure, temperature control, and bladder, bowel and sexual functions.
Nerve impulses travel within and between the brain and spinal cord via long, thin fibers, or axons, that transmit information. The spinal cord also acts as a conduit for information to other nerve cells through microscopic junctions called synapses. When axons are damaged or lost, they do not normally regenerate, and there is only very limited adaptability, or plasticity, of the surviving axons that regulates involuntary functions, such as breathing, blood pressure, temperature control, and bladder, bowel and sexual functions.allow them to take over the role of damaged or lost axons. The myelin sheath that surrounds nerve fibersaxons in the brain and spinal cord provides insulation that facilitates the effective transmission of nerve impulses. ItWe refer to the axon and its surrounding myelin sheath as a nerve fiber. The myelin sheath is composed of multiple layers of tightly packed cell membrane and is vulnerable to damage in conditions like SCIMS and MS.SCI. Once damaged, it is often not effectively repaired. Although nerve fibers can survive in a demyelinated state, their ability to conduct nerve impulses may be completely lost or severely compromised.
Our Approach to the Market for CNS Disorders
We are focused on identifying, developing and commercializing novel pharmaceutical products that address large and underserved CNS markets. We view MS and SCI as the primary markets for our
products as well as strategic points of access to a broad range of additional neurological conditions for the following reasons:
For people with MS, SCI and similar chronic neurological conditions, even relatively small and incremental improvements in CNS function can produce meaningful benefits in their quality of life.
Spasticity
Spasticity refers to the often painful involuntary tensing, stiffening or contracting of muscles. Spasticity is not a disease but a symptom of other conditions, such as MS, SCI, stroke, traumatic brain injury and cerebral palsy, where portions of the nervous system that control voluntary movement have been damaged. This damage results in the nerve cells in the spinal cord becoming disconnected from controlling centers in the brain and, as a result, transmitting unregulated impulses to the muscles. People who have spasticity may not experience it all the time—it may be triggered by a stimulus, such as pain, pressure sores, cold weather or a urinary tract infection. Up to 75% of people with chronic SCI, and the majority of people with MS, experience some form of spasticity. We Move, a non-profit organization dedicated to movement disorders, estimates that spasticity affects approximately 500,000 people in the United States and over 12 million worldwide.
Current treatments for spasticity are focused on reducing spasm frequency, pain or irritating stimuli that can provoke spasticity. Treatment of spasticity often involves a combination of physical therapy and oral medications. Baclofen and tizanidine, the active ingredient in the Zanaflex products, are the two most frequently prescribed oral medications for spasticity. For more intractable spasticity, treatments sometimes include surgical or chemical destruction of nerve roots in the affected area.
Multiple Sclerosis
The National Multiple Sclerosis Society, or NMSS, currently estimates that 400,000 people in the United States have multiple sclerosis. The NMSS estimates that the medical costs associated with treating MS in the United States were approximately $6.2 billion in 2004. Medications accounted for approximately $3.5 billion of these costs. MS is more prevalent in Caucasians and women and is generally diagnosed between the ages of 20 and 50.
MS is a degenerative CNS disorder in which the immune system attacks and damages the insulating myelin sheath. This damage, which can occur at multiple sites in the CNS, blocks or diminishes conduction of electrical impulses. People with MS may suffer impairments in any number of neurological functions. These impairments vary from individual to individual and over the course of time, depending on which parts of the brain and spinal cord are affected, and often include difficulty walking, spasticity, fatigue, lack of stamina and loss or disturbance of vision. They may also include loss of sensation, loss of bowel and bladder control, sexual dysfunction, depression, neuropathic pain,
muscle paralysis, dizziness, tremors and cognitive difficulties. Individuals vary in the severity of the impairments they suffer on a day-to-day basis, with impairments becoming better or worse depending on the activity of the disease on a given day. An individual with MS may function normally one day and experience one or more symptoms of MS the next.
MS is generally classified by how the disease progresses. The most common classification is relapsing-remitting MS, in which people go through periods during which their disease is relatively stable or in remission, only to experience a recurrence of their disease, known as a relapse, which creates additional damage and loss of function. Approximately 10% of MS cases in the United States. are diagnosed as primary progressive MS, which does not involve distinct attacks but rather a steady worsening of symptoms. Secondary progressive MS involves an initial period of relapsing-remitting disease followed by a steady worsening that is punctuated by more severe flare-ups and partial remissions. Most people with relapsing-remitting disease will eventually convert to secondary progressive disease, though this may not occur for many years.
There are no current treatments that address the weakness and loss of mobility that is a major aspect of the progressive disability experienced by people with MS. Existing treatments are classified as relapse management, disease course management and symptom relief.
Relapse Management. The majority of neurologists treating people with MS use intravenous high-dose corticosteroids for the treatment of sudden and severe relapses. Generally, people experiencing a severe relapse receive a four-day course of steroids on either an in-patient or out-patient basis. This treatment may shorten the time required for recovery from such a relapse.
Disease Course Management. Drugs that modify the immune reactions associated with nerve damage in MS include Avonex, Betaseron, Copaxone and Rebif. These drugs are approved only for the relapsing-remitting form of the disease. Other drugs that suppress the immune system include drugs initially approved to treat cancer, such as Novantrone, which is approved for the treatment of relapsing or secondary progressive MS, and methotrexate. These medications produce a reduction in relapse rate, rather than a halting or reversal of the disease process. They do not restore lost neurological function.
Symptom Relief. Doctors also prescribe a number of drugs to address the secondary disabilities, or symptoms, associated with MS. These include treatments for spasticity, fatigue, bladder and bowel control, depression and pain. Baclofen and tizanidine are the most frequently prescribed drugs for spasticity. Commonly prescribed drugs for other symptoms include Ditropan or Detrol for bladder dysfunction, Provigil for fatigue, fluoxetine for depression, and amitriptyline for pain.
Spinal Cord Injury
ApproximatelyAccording to the National Spinal Cord Injury Statistical Center, approximately 250,000 people in the United States live with the long-term consequences of SCI and approximately 10,000 to 12,00011,000 new spinal cord injuries occur each year, typically in young men. The majority of people with SCI are injured under the age of 30 and live with permanent disability and multiple related medical conditions for more than 40 years. It is estimated by the Center for Disease Control that the annual direct cost to the U.S. healthcare system for people with SCI exceeds $9 billion.years after their injury. The National Spinal Cord Injury Database at the University of Alabama estimates that the average lifetime costs directly attributable to SCI for an individual injured at age 25 would varyvaries from $700,000approximately $600,000 to $2.8 million depending on the severity of injury. We believe that the number of patients with SCI outside of the United States is approximately equal to the number in the United States.injury.
The spinal cord can be injured by physical trauma that bends the neck or body violently, such as vehicular or diving accidents, or by objects that penetrate or impact the spinal cord, such as a bullet or a
37
knife. The spinal cord can also be injured by loss of blood flow due to damage to major blood vessels or during surgical procedures. When an area of the spinal cord is damaged, motor and sensory function are impaired throughout those parts of the body that are below the level of the injury.
Until recently, SCI was considered an untreatable and incurable condition. Within the last two decades, researchers have shown that the spinal cord is not severed in most people with SCI. Rather, stretching or compression of the cord causes nerve fibers and blood vessels to tear and unleashes a secondary process of bleeding, loss of blood flow and inflammation that causes more tissue damage. The majority of people with spinal cord injury have some axons that survive within or around the site of injury. Because of these surviving axons, approximately 50% of people with SCI have some motor and/or sensory function remaining below the level of the injury and are said to have incomplete SCI. Those with no detectable function below the injury level are said to have complete SCI. Researchers have also shown that many axons that survive trauma are damaged and permanently lose part of their myelin the insulating sheath that permits electrical impulses to be conducted rapidly down the axon. Loss of myelin insulation in surviving axons can cause nerve impulses to be delayed or lost entirely, resulting in impaired neurological function.sheath.
In addition to the more obvious impact of paralysis on mobility and independence, chronic SCI is often associated with several life-altering conditions that vary depending on the individual and the extent of injury. These include spasticity, as well as persistent pain, loss of control of bowel and bladder functions, loss of sexual function, compromised breathing, loss of sensation, and unstable control of blood pressure, heart rate and body temperature. We believe that novel therapies that offer even an incremental improvement of these conditions would have a meaningful impact on the quality of life for people with SCI.
Current Approaches to the Treatment of SCI
There is no cure for SCI and no treatment available that is capable of improving neurological function. Currently, there are only treatments for some of the symptoms and conditions associated with SCI. In the early 1990s clinical trials showed thatMethylprednisolone, a very high dose of a commonhigh-dose steroid, methylprednisolone, MP, resulted in some protection of function after acute SCI. The effect was small, but statistically significant. MP is believed to act as an antioxidant and thus prevents secondary damage to the tissue during the first few hours after injury. Treatment immediately after the injury with MP iscurrently the standard of care in the United States, thoughStates. Methylprednisolone is a one-time treatment administered to the benefits have been debated in recent years.
Treatmentspatient immediately following an injury to prevent secondary tissue damage. There are several treatments for the symptoms of SCI, are limited. Spasticity is most often treated with muscle relaxants such as baclofen, tizanidine, diazepam and dantrolene sodium. Although these medications appear to help in symptom relief in some people, they are often only partially effective and generally require dosing every two to four hours. In addition, these medications are often associated with unwanted side effects such as sedation, weakness and loss of normal muscle tone, or flaccidity. Treatments focused on maintaining the health of the bladder and kidneys include Ditropan for the relief of bladder spasticity and antibiotics for the treatment of urinary tract infections. Loss of control over bowel function is treated with a number of prescription and non-prescription medications, often combined with a regular regimen of physical manipulation. Male sexual dysfunction may be treated with Viagra, which is useful in improving erectile function in some people. Loss of mobility is almost always accompanied by a loss of sensation leading to a high risk for skin breakdown and ulceration at pressure points. These conditions are treated with physical care and anti-infective drugs. Chronic pain is treated with a range of prescription and non-prescription drugs; however, neuropathic pain, which is common in SCI patients, is usually not relieved by normal pain medications because it is generated by disordered activity in the nervous system rather than by some physical source of tissue injury.
Multiple Sclerosis
According to The National Institutes of Health, it is believed that approximately 250,000 to 350,000 people in the United States have been diagnosed with MS, and approximately 10,000 people are newly diagnosed annually. MS is more prevalent in Caucasians and women and is generally diagnosed between the ages of 20 and 50. The NIH estimates that the annual economic, social and medical cost of treating MS in the United States exceeds $2.5 billion.
38
MS is a degenerative CNS disorder, the causemany of which is unknown,are the same treatments used to address the symptoms of MS. We believe that novel therapies that offer even an incremental improvement in whichthese conditions would have a meaningful impact on the immune system attacks and damages the insulating myelin sheath around nerve fibers in the brain and spinal cord. As in SCI, this myelin sheath damage blocks or diminishes conductionquality of electrical impulses. However, the loss of conduction caused by MS can occur at multiple sites in the CNS rather than at a specific point of injury as it does in SCI.
People with MS may suffer impairments in any number of neurological functions. These impairments vary from individual to individual, and over the course of time, depending on which parts of the brain and spinal cord are affected. Some of these impairments are also common in SCI including loss of sensation, loss of bowel and bladder control, sexual dysfunction, spasticity, neuropathic pain, and muscle paralysis. However, other aspects of MS are not characteristic of SCI, including severe fatigue or lack of stamina, dizziness, tremors, loss or disturbance of vision, and cognitive difficulties. The great majority oflife for people with MS experience general weakness and difficulty walking.SCI.
Current Approaches to the Treatment of MS
Current therapies for MS are focused on the control of symptoms associated with exacerbations and progression of the disease. Many of the symptoms that result from MS are also common in SCI, including spasticity, and issues with bladder, bowel and sexual function. Similar approaches to treating symptoms in SCI are used in treating people with MS, often resulting in the occurrence of the same unwanted side effects. Treatments are defined as relapse management and disease course management.
Relapse Management. The majority of neurologists treating people with MS utilize intravenous high-dose corticosteroids for the treatment of a sudden and severe relapses. Generally, people experiencing a severe relapse receive a four-day course of steroids on either an in-patient or out-patient basis. This treatment may shorten the time required for recovery from an acute relapse.
Disease Course Management. Drugs that modify the immune reactions associated with nerve damage in MS include Avonex, Betaseron, Copaxone, and Rebif. Other drugs that suppress the immune system include drugs initially approved to treat cancer such as Novantrone and methotrexate. However, these medications produce a slowing of deterioration, rather than a halting or reversal of the disease process. They do not restore lost neurological function.
Epilepsy
In 1998, the Centers for Disease Control estimated that approximately 180,000 new cases of epilepsy are diagnosed annually in the United States. In 1995, the Epilepsy Foundation Report to the Nation stated that epilepsy and seizures affect approximately 2.3 million Americans of all ages. Both sources estimate annual costs of medical care as approximately $12.5 billion.
Epilepsy is a condition defined by the presence of recurrent seizures that are not provoked by any immediate clinical cause, such as fever or trauma. Seizures are a manifestation of abnormal and excessive excitation of neurons in an area of the brain. There may be many different underlying causes of epilepsy, including genetic predisposition and localized chemical or mechanical damage to the brain. The cause is often not identified, particularly in children. Epilepsy may develop at any stage of life, but is most likely to develop in childhood, adolescence or in old age.
Current Approaches to the Treatment of Epilepsy
The diagnosis of a particular seizure type, and of a specific type of epilepsy, or epilepsy syndrome, determines the initial therapy. The decision to treat with anti-epileptic drugs, or AEDs, after an initial seizure is still controversial, although there is growing evidence that repeated seizures in themselves can further damage the affected area of the brain. This makes it important to limit seizure recurrence to the extent possible. The decision to treat is based on a consideration of the individual's risk of relapse and potential consequences of further seizures and of drug treatment, including the potential adverse effects of the available AEDs.
39
Most cases of epilepsy are managed with a single AED, and the choice of drug and dosage is typically adjusted individually based on the degree of seizure control and the type and degree of adverse responses. For partial onset seizures with secondary generalization, cabamazepine, phenytoin, valproic acid, phenobarbital and primidone have been used for many years. More recently approved drugs include felbamate, gabapentin, lamotrigine, topiramate and tiagabine. The more recent drugs were approved as adjunctive treatment, based on studies in which they were tested as add-on therapy with older compounds. This clinical trial design is required by the ethical consideration of the need to maintain subjects on an established drug, even if it is only partially effective.
For people with more severe, generalized onset seizures, particularly tonic-clonic, myoclonic, absence, or photosensitive forms, valproic acid is often the drug of choice. The rare but potentially fatal risk of liver toxicity with valproic acid means that ethosuxamide is considered slightly safer, particularly in very young children, who are most at risk for this complication.
Many AEDs are associated with birth defects, which may be offset with folic acid administration. Also, AEDs tend to affect metabolism of other drugs in the liver and most are metabolized in the liver themselves, which complicates the use of drug combinations, particularly with other AEDs. Some drugs, such as phenytoin and valproic acid are significantly bound to proteins in the blood, which reduces their activity. This means that the effective dose can influence or be influenced by other drugs that affect protein binding, such as common aspirin.
Other Disorders of the Central Nervous System
Neurological injuries and degenerative diseases of the CNS, including stroke, traumatic brain injury, Parkinson's Diseasedisease and Alzheimer's Disease,disease, are among the most devastating and costly of human ailments. These conditions are most often chronic and historically have been extremely difficult to treat.
These disorders, like SCIMS and MS,SCI, involve damage to nerve cells and nerve fibers and would likely benefit from similar approaches to tissue protection and repair. For example, the inflammation process that occurs naturally after many types of tissue injury may damage both injured and healthy CNS cells. Several of these conditions could benefit from treatments that inhibit aspects of this inflammatory process. As with MS and SCI, these conditions could be treated with interventions that replace nerve cells, stimulate new nerve fiber growth, or increase the adaptability of connections within the nervous system.
Our Strategy
Our strategy is to become an integratedcontinue to grow as a fully-integrated biopharmaceutical company focused on the identification, development and marketingcommercialization of a broad range of CNS therapeutics,nervous system therapeutics. We are using our scientific, clinical and clinicalcommercial expertise in MS and SCI as a strategic pointpoints of access. In implementing thisaccess to additional CNS markets, including stroke and traumatic brain injury. Key aspects of our strategy we have the following initiatives in place:are:
40
trials necessary for regulatory approval. We may also pursue subsequent approvals of Fampridine-SR in additional CNS disorders, including SCI.
Our Product Pipeline
| Status | Marketing Rights | |||||
|---|---|---|---|---|---|---|
U.S. | ||||||
MS | Worldwide | |||||
Chondroitinase Program | SCI | Preclinical | Worldwide | |||
Neuregulin Program | MS | Preclinical | Worldwide | |||
Remyelinating | MS | |||||
Worldwide |
Fampridine-SR for SCI and MSZanaflex Products
Zanaflex Capsules and Zanaflex tablets are short-acting drugs approved by the FDA for the management of spasticity. We acquired all of Elan's U.S. sales, marketing and distribution rights to Zanaflex Capsules and Zanaflex tablets in July 2004. These products contain tizanidine, one of the two leading treatments for the management of spasticity. Zanaflex tablets were approved by the FDA in 1996 and lost compound patent protection in 2002. There are currently 11 generic versions of tizanidine tablets on the market. However, substantial brand loyalty remains in the prescriber community for the Zanaflex brand. Approximately 90% of all prescriptions for tizanidine are written as "Zanaflex," although most are switched automatically at the pharmacy for a generic tizanidine tablet. Zanaflex Capsules were approved by the FDA in 2002, but were never marketed by Elan. We began marketing Zanaflex Capsules in April 2005.
Clinical trials conducted by Elan demonstrated that Zanaflex Capsules, when taken with food, produce average peak levels of tizanidine in a person's blood that are lower and rise more gradually compared to the peak levels following a similar dose of the tablet form. The FDA recognizes these differences and has determined that Zanaflex tablets and generic tizanidine tablets are not
therapeutically equivalent and are not AB-rated to Zanaflex Capsules. As a result, under state pharmacy laws, prescriptions written for Zanaflex Capsules may not be filled by the pharmacist with Zanaflex tablets or generic tizanidine tablets, although some substitution does take place in practice. Zanaflex Capsules are available in 2 mg, 4 mg and 6 mg doses, while tablet formulations are only available in 2 mg and 4 mg doses. The 6 mg capsule gives patients and physicians an additional dosing choice and an opportunity to reduce the number of pills a patient must take daily. In addition, many patients may find capsules easier to swallow than tablets. In addition, people who have difficulty swallowing may open the capsule and sprinkle it on food. The pharmacokinetic effect of sprinkling contents of the capsule on food, however, is different from when the intact capsule is taken with food.
In 2004, retail sales of Zanaflex tablets and generic equivalents of Zanaflex tablets totaled approximately $300 million in the United States, with Zanaflex tablets accounting for about $15 million of that amount. The vast majority of prescriptions for these products are written by a relatively small group of prescribers. In 2004, over 117,000 physicians wrote one or more prescriptions for generic tizanidine or Zanaflex tablets. However, 78% of all such prescriptions were generated by approximately 9,200 prescribers. We believe that our internal specialty sales force, contract sales force and contract telesales group, will be able to reach virtually all of these high-volume prescribers.
Sales and promotional support for Zanaflex Capsules
To support our commercialization of Zanaflex Capsules, we have established a sales and marketing infrastructure consisting of an internal specialty sales force, a contract sales force and a pharmaceutical telesales group. Our internal specialty sales force currently consists of 14 sales professionals who call on neurologists and other prescribers specializing in treating patients with conditions that involve spasticity. Members of our internal sales force also call on managed care organizations, pharmacists and distribution customers. We plan to expand our specialty sales force to approximately 30 sales professionals in the first quarter of 2006. In addition, Cardinal Health provides us with a contract sales force of approximately 160 sales representatives to market Zanaflex Capsules, on a non-exclusive basis, to primary care physicians who currently prescribe Zanaflex tablets or generic tizanidine tablets. We use a pharmaceutical telesales group to contact primary care physicians, specialty physicians and pharmacists to provide information regarding Zanaflex Capsules or determine their interest in receiving samples of Zanaflex Capsules or a visit from a sales representative.
After the introduction of generic tizanidine tablets in June 2002, Elan discontinued promotional and educational support for Zanaflex tablets. To our knowledge, none of the distributors of generic tizanidine or baclofen, the other leading spasticity treatment, which is also generic, has engaged in any educational programs on the treatment of spasticity. Concurrent with our launch of Zanaflex Capsules in April 2005, we initiated a sampling program as well as a number of educational, promotional and drug safety monitoring programs for prescribers and patients. In addition to our programs for prescribers and patients, we also have a number of programs in place to educate pharmacists about Zanaflex Capsules and the pharmacokinetic differences between tizanidine tablets, including generic tizanidine tablets and Zanaflex tablets, and Zanaflex Capsules.
Since April 2005, we have seen continued growth in monthly prescriptions of Zanaflex Capsules. We believe that this trend will continue as we extend our reach into the population of high-volume prescribers of tizanidine. We are seeking FDA approval of improvements in labeling and we will also explore the potential for new indications.
Pharmacokinetic differences between Zanaflex Capsules and tizanidine tablets
Although tizanidine, the active ingredient in Zanaflex Capsules, Zanaflex tablets and generic tizanidine tablets, is the same, there are some important differences between the capsule and tablet formulations. To establish the differences between Zanaflex Capsules and Zanaflex tablets, Elan conducted a single dose clinical trial with 96 healthy volunteers. That trial demonstrated that Zanaflex Capsules, when taken with food, resulted, on average, in a more gradual rise in tizanidine levels in the blood and a lower peak concentration. By contrast, the trial demonstrated that Zanaflex Capsules taken without food resulted in essentially the same pharmacokinetic effect as the tablet formulation of tizanidine. The results of the trial are illustrated in Figure 1 below.
Figure 1. Average Blood Concentration Over Time
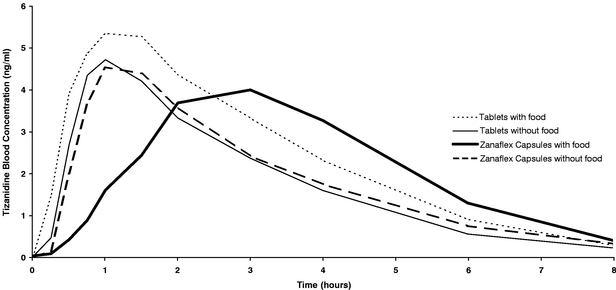
Average blood concentrations of tizanidine in subjects following a single dose of 4 mg Zanaflex tablet or a 4 mg dose of Zanaflex Capsules, taken either with or without food.
As a result of this difference in absorption rate and blood level when taken with food, the FDA has determined that neither Zanaflex tablets nor generic tizanidine tablets are therapeutically equivalent or AB-rated, to Zanaflex Capsules. Therefore, under state pharmacy laws, pharmacists cannot fill prescriptions written for Zanaflex Capsules with Zanaflex tablets or generic tizanidine tablets. The FDA-approved package insert for Zanaflex Capsules contains the following language regarding the differences between the products: "Food has complex effects on tizanidine pharmacokinetics, which differ with different formulations. These pharmacokinetic differences may result in clinically significant differences when (1) switching administration of the tablet between the fed or fasted state, (2) switching administration of the capsule between the fed or fasted state, (3) switching between the tablet and capsule in the fed state, or (4) switching between the intact capsule and sprinkling the contents of the capsule on applesauce. These changes may result in increased adverse events or delayed/more rapid onset of activity, depending on the nature of the switch. For this reason, the prescriber should be thoroughly familiar with the changes in kinetics associated with these different conditions."
The most frequent adverse events associated with the use of tizanidine include dry mouth, drowsiness, fatigue and dizziness. These events are generally mild to moderate and are believed to be dose-related. In one single-dose study where patients were not titrated, two-thirds of patients experienced hypotension. Zanaflex Capsules have a short-acting effect, and patients are advised to take it at the times during the day when they most need relief from spasticity.
Fampridine-SR
Fampridine-SR, our lead product candidate, is currently in a Phase 3 clinical trial for the improvement of walking ability in people with MS pursuant to an SPA issued by the FDA. The FDA has agreed that this trial, if successful, could qualify as one of the pivotal efficacy studies required for drug approval. Fampridine-SR is a sustained release, oral tablet formulation of fampridine, suitable for twice daily dosing. It is based on a small molecule drug contained in a sustained-release tablet form. Laboratory studies have shown that fampridine, which acts to block potassium ion channelsthe active ingredient in Fampridine-SR, improves impulse conduction in nerve cell membranes.fibers in which the myelin sheath has been damaged. Fampridine is not currently FDA-approved for use in MS or any other indications. We believe that Fampridine-SR representscould represent a fundamental shift in the treatment of both SCI andpeople with MS because it may improve neurological function rather than only treating the symptoms or slowing the progression of these diseases.disease, as current treatments do. We are currently conducting two Phase 3 clinical trials ofhave obtained Orphan Drug designations from the FDA for Fampridine-SR for chronic SCI,in both MS and one Phase 2 clinical trial for MS.incomplete SCI.
Recent clinical research using imaging and post-mortem studies has shown that the majority of people with SCI do not have severed spinal cords and maintain some nerve fibers, or axons, that cross the site of injury. However, these surviving axons are often damaged and lose their myelin. In MS, the myelin sheath is damaged by the body's own immune system, rather than by physical trauma.causing areas of myelin sheath loss, also known as demyelination. When an axona nerve fiber is demyelinated after injury, large numbers of the specialized potassium channels on the surface of axonsthe axon that are normally hidden or covered by the myelin sheath are exposed and leak potassium ions, causing the axonnerve fiber to short circuit
41
its electrical impulses. Fampridine is able to blockblocks these exposed channels, thereby permitting the axonnerve fiber to transmit nerve impulses again, even in a demyelinated state. Fampridine may also serve to amplify electrical signals at sites of contact or synapses between nerve cells by blocking the same channels in the tips of the nerve fiber, thereby improving the function of surviving tissue in the injured nervous system. Fampridine does not repair damaged nerve fibers or axons, but worksFampridine-SR is a sustained release formulation of fampridine that we believe enables safer and more effective blood levels to improve impulse conduction and must be given continuously to maintain its effects.maintained throughout the day, which cannot be easily accomplished with an immediate-release formulation.
Fampridine-SR was developed by and is manufactured for us by Elan. We have a worldwide, exclusive license from Elan tofor all of its patent for the sustained release formulation of aminopyridines, which includes fampridine, for the rights to, among other things, develop, promote, distribute, use and sell Fampridine-SR in all human clinical indications, and to develop, promote, distribute, use and sell other patented sustained-release formulations of the drug. We hold an exclusive license from the Canadian Spinal Research Organization to a patentElan also manufactures Fampridine-SR for the use of fampridine in the treatment of neuropathic pain and spasticity in SCI, as well as an exclusive license from Cornell University to a patent for the use of fampridine in the treatment of anterior horn cell diseases, which include amyotrophic lateral sclerosis, known as Lou Gehrig's disease. We have obtained Orphan Drug designations from the FDA for Fampridine-SR in both incomplete SCI and MS.us.
We have performed a series of clinical trials of Fampridine-SR in chronic SCI and MS to establish the pharmacokinetics, safety, and optimal dosing of the drug, as well as to assess its efficacy. Our previous clinical trials have indicated that people with chronic and incomplete SCI experience benefits with Fampridine-SR on a range of measures including improvements in spasticity, bowel and bladder function, and sexual function. We are currently sponsoring two independent Phase 3 clinical trials of Fampridine-SR in SCI, each designed to establish a significant clinical benefit of the drug on spasticity, which is marked by muscle stiffness, and we are preparing a regulatory filing based on this specific indication. These clinical trials also include secondary endpoints related to bowel, bladder and sexual function.
We believe there are several compelling reasons for the development of Fampridine-SR with a primary indication of spasticity:
We believe there are compelling reasons for the development ofto develop Fampridine-SR as a new therapy for MS,improving walking ability in people with a primary indication of lower extremity motor function:MS:
Clinical Trials of Fampridine-SR
In cooperation with Elan weWe have conducted a series of clinical trials duringto establish the past six years evaluating Fampridine-SR. Approximately 550safety, pharmacokinetics and optimal dosing of Fampridine-SR in MS and SCI, as well as to assess its efficacy. More than 800 people have been treated with Fampridine-SR in 14over 25 clinical trials, including eightnine clinical trials for SCIin MS and six11 clinical trials in SCI.
Clinical Trials in Multiple Sclerosis
Current Phase 3 Trial. Our current Phase 3 clinical trial, MS-F203, was initiated in June 2005, after we reached agreement with the FDA on the protocol design and received a Special Protocol Assessment from the FDA Division of Neuropharmacological Drug Products. The FDA has agreed that this trial, if successful, could qualify as one of the pivotal efficacy studies required for MS.drug approval.
MS-F203 is a double-blind clinical trial designed to enroll 240 people at up to 35 MS centers in the United States and Canada. Subjects will complete a Timed 25-Foot Walking Test at each visit during the clinical trial. This test involves timing the subject's completion of a 25-foot walk as fast as he or she can do so safely. Such a test is relevant as a measure of the subject's ability to perform tasks that are required in daily life, such as crossing the street in the time period allotted by a traffic light. In alladdition, subjects will also be asked to fill out a 12-item questionnaire known as the MS Walking Scale or MSWS-12. The MSWS-12 is a subjective measure of the degree to which walking disability impacts the subject's daily life.
Trial results will be analyzed using our proprietary responder analysis, for which we have applied for a patent. A subject will be deemed to be a responder if his or her score on the 25-foot walk was better during the majority of his or her visits in the treatment phase of the trial, than the best visit during the non-treatment phase. The primary endpoint of the trial will be the comparison of the percentage of responders in the Fampridine-SR group to the percentage of responders in the placebo group. To validate the clinical importance of improvements in the timed walk measurements, the MSWS-12 scores of the responders will be compared against those of non-responders. This analysis is designed to ensure that being deemed a responder is clinically meaningful to the subject. In addition, the trial will also test for significant improvement in walking ability in the Fampridine-SR-treated responder group at the last treatment visit versus the placebo group. This analysis is designed to ensure that the improvements seen by responders are maintained over the duration of the trial. As a secondary endpoint, the trial will also measure lower extremity muscle strength, as assessed by the modified British Medical Research Council manual muscle testing procedures, referred to as the Lower Extremity Manual Muscle Test or LEMMT.
The design of our Phase 3 clinical trial was closely modeled on the design of the preceding Phase 2 clinical trials, treatment with Fampridine-SR has been associated with a variety oftrial, MS-F202, and builds on our clinical trial experience in measuring improvements in neurological benefitsfunction against the variability in function that is inherent in people with SCIMS. Individuals who suffer from MS vary in the severity of the impairments they experience on a day-to-day basis, depending on the activity of the disease on a given day. As a result, from one clinical trial visit to the next, a subject's walking ability can vary significantly. This variability makes it difficult to distinguish treatment-related changes in walking ability from disease-related changes in walking ability. Our review of MS-F202 data demonstrated that a responder form of analysis helps overcome the effect of the inherent variability of disease activity that people with MS experience.
We expect the recruitment period for the current trial, which began in June 2005, to end in February 2006. The treatment period is 14 weeks and MS.each subject is involved in trial procedures for approximately five months overall. We currently expect to be able to evaluate data from this clinical trial in the third quarter of 2006, if patient recruitment proceeds as planned.
42 Phase 2 Clinical Trials. Our most recently completed Phase 2 clinical trial, MS-F202, was designed to compare 10 mg, 15 mg and 20 mg doses of Fampridine-SR taken twice per day and to assess their relative safety and efficacy over a stable treatment period of 12 weeks. The pre-specified primary endpoint of the clinical trial was an improvement in average walking speed using the Timed 25-Foot Walk. The clinical trial was initiated in early 2003 and completed enrollment of 211 subjects in 24 major MS centers in August 2003. The clinical trial was designed to give us a clear indication of optimal dose and the number of subjects that we would need to establish efficacy in a subsequent Phase 3 trial. The overall design of our MS-F202 Phase 2 clinical trial is illustrated in Figure 2 below.
Figure 2. Design of Fampridine-SR MS-F202 Phase 2 clinical trial.

The efficacy results, based on the prospective analysis plan of MS-F202, indicated a trend for improvement from baseline in walking ability (using the Timed 25-Foot Walk test) in the Fampridine-SR-treated subjects, relative to the placebo-treated subjects. Statistical significance was not reached on the primary efficacy analysis, which was defined as the percentage change from baseline in average walking speed during the 12 weeks of stable double-blind treatment. Statistical significance was obtained for the secondary outcome measure of lower extremity muscle strength, as assessed by LEMMT. All three Fampridine-SR dose groups showed greater mean increases from baseline in LEMMT scores relative to the placebo group and the differences were statistically significant for the 10 mg and 15 mg Fampridine-SR groups (p< 0.05). A p-value is a statistical term that indicates the probability that a difference between treatment groups is random. The smaller the p-value, the lower the likelihood that the difference was random. Generally a p-value of less than 0.05 is considered to represent a statistically significant difference.
Our analysis of the data led us to believe that part of the reason that statistical significance was not achieved on the primary endpoint was related to the disease-related variability of walking ability for a subject from visit to visit, together with the fact that not all subjects are expected to respond to the treatment. We believe this variability in walking ability, much of which is contributed by subjects who do not respond, made it difficult to establish the significance of treatment-related improvements using the average walking speed measure that had been prospectively defined as the endpoint of the trial. In order to try to reduce the effect of this variability, we developed an analysis designed to classify subjects as responders only if they demonstrated consistent improvement during the treatment period, when subjects were taking either Fampridine-SR or placebo. Subjects were deemed to be responders if their Timed 25-Foot Walk test results were better during at least three of the four treatment visits than their best score during the non-treatment period. When examined using this form of analysis, all three of the groups receiving Fampridine-SR had a statistically significant increase in the number of responders compared to placebo, as shown in Figure 3.
Figure 3. Responder rates for treatment groups in MS-F202.
Since the differences in responder rates among the three doses examined were small, more detailed analyses were performed comparing the pooled Fampridine-SR-treated groups against the placebo-treated group. The difference in responder rate between the pooled Fampridine-SR-treated subjects and the placebo-treated subjects was statistically significant (p-value<0.001), as shown below.
| Status | Placebo | Fampridine-SR Pooled | ||
|---|---|---|---|---|
| | (N=47) | (N=158) | ||
Responders | 8.5% | 36.7% | ||
Non-responders | 91.5% | 63.3% |
The responder analysis allows characteristics of the response to be appreciated in more detail. The improvement in walking in responders appeared to be substantial and sustained. The average increase in walking speed of responders was more than 25%, as compared to approximately 2% for non-responders. This was consistent over the 3-month period of treatment and was statistically significant at every visit, as shown in Figure 4.
Figure 4. The average percent change from baseline in walking speed.
The graph depicts the average change in walking speed during the treatment period for study MS-F202, comparing Fampridine-SR-treated responders to the placebo-treated group. Differences between the groups were statistically significant (p<0.001) at all visits.
In MS-F202, subjects were required to fill out the MSWS-12 questionnaire. When the results of this questionnaire were analyzed for all evaluable subjects, the average improvement, or reduction in score, during the treatment period was greater for responders than for non-responders, in each case including those subjects on placebo, and the difference was statistically significant. We believe this result demonstrates that being a timed-walk responder is clinically meaningful to patients. These results are shown in Figure 5.
Figure 5. Average change in MS Walking Scale Score.
Histogram to show the average change in score for the MS Walking Scale for responders and non-responders between the baseline and stable treatment periods. A reduction in score represents a subject's perception that there has been improvement in the effect of walking disability on activities of daily life.
This analysis of the MS-F202 clinical trial served as the basis for the design of the Phase 3 MS-F203 clinical trial. The results of MS-F202 using this analysis showed that there was a statistically significant increase in the number of people being treated who experienced a consistent increase in walking ability, compared to placebo, and that this improvement was sustained and clinically meaningful to patients. These data also show that the benefit was maintained for the full 14 weeks of treatment. These results are similar whether the pooled Fampridine-SR-treated subjects or just those subjects receiving the current target dose of 10 mg twice a day are compared with the placebo-treated group.
In 2001, we completed a smaller double-blind Phase 2 clinical trial of Fampridine-SR, MS-F201. This clinical trial was designed to determine the optimal dose range of Fampridine-SR and to evaluate possible ways in which to measure the effect of the drug on symptoms of the disease, including motor strength, timed walking and self-reported fatigue. The clinical trial involved a total of 36 MS subjects in four major academic MS research centers. A total of 25 subjects received Fampridine-SR in doses increasing from 10 mg to 40 mg twice per day during seven weeks of treatment and 11 subjects were given placebo during the same period. This treatment period was preceded by a series of baseline evaluations during the course of four weeks to allow the subjects to become adjusted to the clinic visits and allow the various measurements to stabilize. A one-week blinded treatment with placebo tablets preceded the first drug administration to look for potential placebo effects on the various outcome measures.
The clinical trial demonstrated that doses up to 25 mg twice a day were well tolerated and were associated with statistically significant improvements in walking ability and leg muscle strength. All the improvement in strength and walking ability was apparent within these first four weeks of the
treatment, at doses from 10 mg to 25 mg twice a day. The placebo-treated subjects showed some tendency to improve or worsen in walking ability, mostly within 20% of their baseline average. However, the Fampridine-SR-treated group showed a marked tendency for improvement in walking speed, with 9 of 25 subjects improving more than 20% from baseline and two with greater than 50% improvement. These findings were consistent with the results of an earlier, small, crossover study sponsored by Elan, using doses of 17.5 mg twice a day for one week, which was published in the journalNeurology in 1997.
We re-examined the data from the MS-F201 clinical trial using an equivalent responder analysis in which we defined a responder as a subject who showed walking ability on the 25-Foot Walk that was faster in a majority of treatment visits than the fastest speed recorded during the non-treatment period. In MS-F201, this meant that four or more of the seven treatment visits had to show faster walking than the visits during the non-treatment period. We found that the responder rates in this trial were 40% (10 of 25) for the Fampridine-SR-treated subjects and 9.1% (1 of 11) for the placebo-treated subjects. Hence, the response rate by this measurement was similar to that seen in the MS-F202 clinical trial. We did not incorporate the MSWS-12 measure in the MS-F201 clinical trial.
Fampridine-SRClinical Trials in Spinal Cord Injury
Recent clinical research using imaging and post-mortem studies has shown that the majority of people with SCI do not have severed spinal cords and maintain some nerve fibers that cross the site of injury. However, these surviving nerve fibers are often damaged and lose their myelin sheath. A series of preclinical studies and clinical trials have indicated that fampridine can potentially improve conduction in nerve fibers injured by spinal cord injury and improve function in people with spinal cord injury.
Phase 3 Clinical Trials. We are currently conductingIn March 2004, we released results from two parallel Phase 3 double-blind clinical trials of Fampridine-SR in SCI, designatedpeople with SCI. The trials did not reach statistical significance in their primary endpoints, which were reduction of spasticity, as SCI-F301 and SCI-F302. The protocols for these two clinical trials are identical. They are designed to provide evidence of efficacy of Fampridine-SR sufficient to allow submission of an NDA to the FDA in 2004. Each double-blinded clinical trial enrolled approximately 200 subjects with chronic and incomplete SCI at approximately 40 clinical centers in North America. Subjects entering the clinical trials were randomly assigned to receive a stable dose of either 25 mg of Fampridine-SR or placebo twice a day for 12 weeks. During the 12-week study period, subjects visit the clinic for evaluation once every four weeks. We anticipate that the data produced by these clinical trials should be unblinded and analyzedmeasured by the end of March 2004.
The primary endpoints for these two clinical trials are improvement in spasticity in the legs of the subjects, which is measured by a reduction in the Ashworth score,scale, and improvement in the subject's rating of the effect of treatment, which is measured by a test called the Subject Global Impression. The Ashworth score is assigned by a clinician who measures the stiffness of the affected leg muscles by attempting to move the subject's relaxed leg around the knee joint. The stiffness is rated on a scale of 1, indicating no resistance and no spasticity, to 5, indicating stiffness so complete that the leg cannot be moved around the joint. Spasticity associated with an Ashworth score of 3 or greater is considered to have significantly negative effects on a person's quality of life. Our current Phase 3 clinical trials have required subjects to have average Ashworth scores greater than 2 both at their initial screening visit and after two weeks of placebo treatment. Thepatients' Subject Global Impression, or SGI. The Ashworth scale is a validated, 5-point clinician assessment of an individual's spasticity. The SGI is a standard seven-point scale onin which subjectstrial participants rate how they feel about the overall effect of the studytrial drug. In two previousone of the SCI trials, the data showed a positive trend (p=0.069) toward improvement on the Ashworth scale when analyzed across all observations during the double-blind trial treatment period, which was the trial's pre-specified plan of analysis. When analyzed based on the subjects' last observation carried forward, a commonly used method of analysis, the improvement in, or reduction of, Ashworth score in that trial was statistically significant (p=0.006). The drug groups in both trials showed a progressive mean improvement on the Ashworth score during the double-blind treatment period. However, the placebo group in one of the trials showed a more pronounced reduction in Ashworth Score than expected.
The design of these Phase 3 clinical trials was based on a series of earlier Phase 2 clinical trials of Fampridine-SR in which the most consistent finding was a greater reduction in spasticity in Fampridine-SR-treated subjects with chronic SCI, the improvement registeredrelative to placebo-treated subjects, as measured by the SGI was statistically significant, even with relatively small study groups of 30 people or less.
We discussedAshworth Score. Other benefits observed in the primary endpoints and the other aspects of the design of these clinical trials with the FDA during development of the protocols in order to determine the expected requirements for submission of an NDA. The FDA is familiar with the Ashworth score as a measure of spasticity and has approved two other drugs using it as a primary outcome measure. We have also agreed to use SGI, in addition to the Ashworth score, to avoid potential uncertainty about the degree of change in score that represents a clinically meaningful effect.
Phase 2 Clinical Trials. We conducted two double-blind Phase 2 clinical trials SCI-200 and SCI-F201, and Elan sponsored a double-blind Phase 2 clinical trial, ELN 0295-001US, which was published in theJournal of Neurotrauma in October 1998. These clinical trials involved a total of 177 subjects with SCI. In these three clinical trials there was evidence of benefit across a broad spectrum of neurological functions in subjects treated with Fampridine-SR at doses ranging from 17.5 mg to 25 mg twice per day, compared to placebo. These benefits included improvements in sensory,were improved motor, bowel, bladder and sexual function. The most consistent finding across the clinical trials was a reduction in spasticity in Fampridine-SR treated subjects.
Unlike the design of our Phase 3 clinical trials, our Phase 2 clinical trials did not require a minimum spasticity level for enrollment. The average Ashworth score at baselineenrollment and the treatment period was approximately 2,from one to four weeks rather than 14 weeks. These changes were made in the mildest levelPhase 3 trials because the FDA required minimum twelve week duration of treatment for approval of a long-term therapy of this kind and because adequate measurement of benefit required a certain degree of spasticity at baseline.
Based on the scale. Thereforeentire body of data in study SCI-F201,clinical trials of fampridine in people with SCI and the new approaches to evaluating response to the drug that we have learned in MS trials, we expect to resume
development of Fampridine-SR for example, althoughSCI after we saw improvementhave completed further development of the drug for MS.
Safety Profile of Fampridine-SR
To date, Fampridine-SR has been tested in over 800 subjects. The adverse events most commonly experienced in all double blind, placebo-controlled Phase 2 and Phase 3 studies were insomnia, numbness or tingling in the entire group treatedextremities, dizziness and nausea. These events were generally mild to moderate and are believed to have been dose-related. Seizures have also been observed in some prior trials of Fampridine-SR with 25 mg twice a day, the difference was not quite statistically significant, as shown in Figure 1. A much clearer assessment of effects on spasticity was produced by separately analyzing the measurements for those subjects who had more than minimal spasticity before treatment, with average Ashworth scores greater than 2 at their initial baseline examination. The average improvement for those treated with Fampridine-SR, compared to those treated with placebo, was then both statistically significant and clinically meaningful, at more than half a point improvement to the average Ashworth score. Moreover, subjects whose spasticity at the beginninghigher doses of the study registered as greater than 3 on the Ashworth scale, showed even larger mean improvements, at more than a full point on the scale, although statistical significance was reduced by the smaller number of subjects that could be included, as showndrug. No seizures have been reported to date in Figure 1.
43
Figure 1: SCI-F201 Phase 2 Ashworth ScoresPost-Treatment with Placebo or Fampridine-SR Treatment vs. Baseline
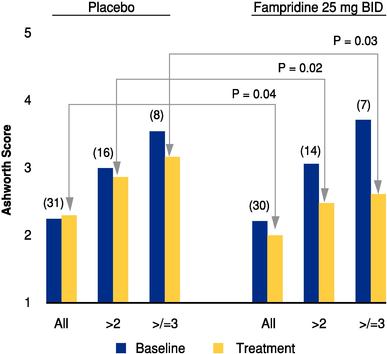
The illustration depicts Ashworth scores for the placebo group, shown on left, as compared to the Fampridine-SR treatment group, shown on right. The arrows highlight the differences in Ashworth scores between the placebo and Fampridine-SR treatment groups. The Ashworth scores were averaged over four muscle groups in each subject and compared against baseline for the four weekly clinic visits during the stable treatment period. Statistical significance for this clinical trial was established at p<0.025.
Similar results were derived from our earlier clinical trial, SCI-200, which examined a dose of 20 mg twice a day for one week, compared to placebo. A statistically significant improvement in Ashworth score was also seen in the published Elan-sponsored clinical trial for subjects receiving 17.5 mg of Fampridine-SR twice a day for one week.
Subjects treatedpatients with the 25 mg dose twice daily experiencedthat we have selected for our Phase 3 clinical trial. We are carefully monitoring the potential for seizure as a side effectseffect, including the possibility of interaction with other drugs that were not significantly differentare known to lower the threshold for seizure in susceptible subjects. We are also aware that people with MS are reported to have a higher incidence of seizures than the unaffected population. We have excluded from those experienced bythese trials subjects receiving the placebo. The number of subjects who discontinued the clinical trialat known risk for seizures because of side effects was similar between the 25 mg twice a day and placebo groups at approximately 10% and 8%, respectively. Side effects included dizziness, tingling, nervousness, insomnia and pain. Thisprevious experience was similar to the earlier clinical trials that used lower dosesor abnormal electroencephalogram indicative of Fampridine-SR. Side effects were more severe and more frequent in the group treated with 40 mg of Fampridine-SR twice a day, including nausea, tremor, and abnormal thinking. These side effects led 11 of the 30 subjects in this group to discontinue the study, most during the stage of increasing doses at the beginning of the clinical trial. One subject in the 40 mg group experienced a seizure near the end of the designated treatment period. This subject had a prior history of traumatic brain injury and amnesia, combined with spinal cord injury two years earlier, and had been treated with Dilantin for three weeks following possible post-traumatic seizure activity that could not be thoroughly investigated at the time.such risk.
Phase 1 Clinical Trials. A series of earlier clinical trials, called SCI-101, SCI-102 and SCI-103, were designed to measure the concentration of drug achieved in the blood with a wide range of doses, and to relate those levels to side effects and possible clinical benefits. These clinical trials showed that a dose of 25 mg every 12 hours produced peak concentrations of Fampridine-SR in the subjects' blood that were usually in the target effective range of 50 to 100 ng/ml.
Overall, subject and clinician reports and clinical measures in these non-blinded clinical trials indicated that there was evidence of increasing dose-response through the range of 10 to 25 mg twice a day, but that evidence of increasing efficacy at doses higher than 25 mg twice a day was limited, possibly being offset by increased side effects.
44
Safety Studies. As part of our continuing evaluation of safety, we have established extension studies that allow subjects in earlier clinical trials to receive Fampridine-SR on an unblinded, or open-label basis, with their progress followed for at least a year but withand the potential for continuing treatment until the drug is approved. By their open-label design, these studies will allow us to gain some additional knowledge of the longer term efficacy and safety of the drug, albeit limited by the lack of a placebo control group. These studies are intended primarily to gain sufficient subject experience to satisfy the regulatory guidelines for long-term and overall safety assessments. As of September 2003,2005, approximately 90176 subjects from Phase 1 and Phase 2 SCI clinical trials have beenMS-F202 were enrolled in ongoingan extension trials.trial and 137 remained active in the trial, with approximately 42 subjects who had taken the drug for over a year. A separatenew extension study for subjects of the current Phase 3 clinical trialstrial is expected to enroll a totalmajority of approximately 350the MS-F203 trial subjects, beginning in the thirdfourth quarter of 2003.2005.
Only limited data are yet available from these ongoing safety studies, since no interim analysis of the data is planned, but there have been threetwo incidences of seizures in subjects enrolled in the SCI-F201MS-F202 extension. Two of theseThese seizures occurred in subjects who had been taking more than 25 mg twice per day (70 mg/day for 9 months and 80 mg/day for 2 months, respectively). Overall, including the subject in study SCI-F201, this represents 3 seizures in the 115 subjects (2.6%) exposed to doses greater than 50 mg daily, and 1 seizure in the 308 subjects (0.3%) exposed to doses less than or equal to 50 mg daily, the proposed commercial dose for Fampridine-SR in SCI applications. Some currently marketed drugs cite incidence of seizures in clinical trials of 3% or greater. We are carefully monitoring the potential for seizure as a side effect, including the possibility of interaction with other drugs that are known to lower the threshold for seizure in susceptible subjects.
Fampridine-SR in Multiple Sclerosis
Phase 2 Clinical Trial. The current late Phase 2 clinical trial, MS-F202, was designed, after extensive consultation with a panel of expert MS neurologists and with the FDA, to provide pivotal data for support of an NDA for the use of Fampridine-SR in MS. The clinical trial is also designed to compare three doses of 10, 15 and 20 mg, twice per day, and to assess their relative safety and efficacy over a treatment period of 12 weeks. The primary endpoint of the study is an improvement in average walking speed using the Timed 25 Foot Walk. The Timed 25 Foot Walk is part of a standardized set of neurological tests, called the Multiple Sclerosis Functional Composite Score, MSFC, and involves timing the subject completing a 25 foot walk. We plan to use these measurements to support an indication for the treatment of lower extremity motor dysfunction, characterized by weakness and walking impairment.
The clinical trial was initiated early in 2003 and completed enrollment of approximately 200 subjects in 24 major MS centers in July 2003. We expect the data from this clinical trial to be available by the end of March 2004. If the clinical trial is successful, we plan to sponsor a second clinical trial as soon as possible to provide the necessary confirmation. It is also possible that the clinical trial may not provide statistical significance on the primary endpoint but give us a clear indication of dose and group size to inform the design of two subsequent Phase 3 clinical trials that should provide sufficient pivotal data for submission of the MS NDA.
In 2001, we completed a double-blind Phase 2 clinical trial of Fampridine-SR in Multiple Sclerosis, MS-F201. The clinical trial was designed to determine the optimal dose level of Fampridine-SR and to evaluate possible ways in which to measure the effect of the drug on symptomsat a dose of the disease, including motor strength, timed walking, and self-reported fatigue. The clinical trial involved a total of 36 MS subjects in four major academic MS research centers. A total of 25 subjects received Fampridine-SR in doses increasing from 10 mg to 40 mg twice per day over eight weeks of treatment, and 11 subjects were given placebo over the same period. This treatment period was preceded by a series of baseline evaluations over the course of four weeks to allow the subjects to become adjusted to the clinic visits and allow the various measurements to stabilize. A one week blinded treatment with placebo preceded the first drug administration to look for potential placebo effects on the various outcome measures.
45
The clinical trial demonstrated that doses up to 2515 mg twice a day were well tolerated,for six months and were associated with statistically significant improvements in walking speed and leg muscle strength. Most offive months respectively, before the improvement in strength and walking speed was apparent within the first three weeks of the Fampridine-SR treatment, atadverse event. The protocol has now been amended to restrict doses fromto 10 to 25 mg twice a day.
When we examined the measurements from individual subjects, and looked at the improvement in walking speed between the baseline period and the average over the first four treatment weeks, we found clear differences in the pattern of response between Fampridine-SR and placebo-treated subjects, as shown in Figure 2. The placebo-treated subjects showed some tendency to improve or worsen slightly in walking speed, mostly within 20% of their baseline average. However, the Fampridine-SR treated group showed a marked tendency for improvement in speed, with 9 of 25 subjects improving more than 20% from baseline, and 2 with greater than 50% improvement. These findings were consistent with the results of a small crossover study sponsored by Elan, using doses of 17.5 mg. twice a day for one week, which was published in the journalNeurologyin 1997.
Figure 2: Change in Walking Speed During the First Four Weeks of Treatment
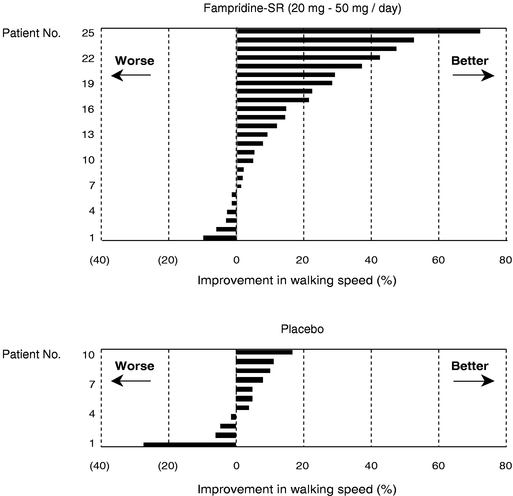
The subjects (25 Fampridine-treated and 11 placebo-treated) were placed in order of degree of improvement, from top to bottom. The Fampridine-SR treated group showed a significantly greater improvement in walking speed during the treatment period.
The side effects experienced by subjects in MS-F201 given Fampridine-SR up to 25 mg twice a day were similarin order to thosegather more safety data at the dose that we are examining in our SCI clinical trials. Two subjects experienced seizures while on dosesthe current Phase 3 trial and for which we intend to seek approval. To date, we have had no report of 30 to 35seizure at the 10 mg twice a day respectively. This was consistent with previous experience and the expected greater
46
susceptibility of people with MS to seizure, which is thought to be related to the presence of demyelinating damage in the brain.
Phase 1 Clinical Trials. Two earlier Phase 1 clinical trials, AN751-101 and AN751-102, sponsored by Elan, addressed the pharmacokinetics of Fampridine-SR in subjects with MS in a manner equivalent to our SCI-101 and SCI-102 clinical trials. The pharmacokinetic characteristics of Fampridine-SR measured in these clinical trials were similar to those established in normal subjects and in people with SCI.dose.
Valrocemide for Epilepsy
Valrocemide is a new chemical entity derived from valproic acid which is a commonly used AED. Valrocemide was discovered as part of a program to identify a compound with similar or better efficacy than valproic acid, but with a more favorable side effect profile. Valrocemide has been studied by Teva in both animal models and in clinical trials in Europe.
Based on responses in pre-clinical models of epilepsy and neuropathic pain and on the close relation between valrocemide and valproic acid, we believe this drug may be effective in epilepsy and bipolar disorder, and potentially in neuropathic pain, for which valproic acid is frequently prescribed.
In pre-clinical studies and clinical trials carried out by Teva, valrocemide appears to have minimal interactions with other drugs, unlike valproic acid which significantly affects liver enzymes that are used to metabolize many compounds. Valrocemide also shows less affinity for binding to proteins in the blood compared to valproic acid, which should simplify dosing. Based on animal toxicology studies the side effects of valrocemide appear to be less significant than those of valproic acid.
Studies in human volunteers have indicated that the maximum tolerated dose is in the range of 4 grams per day. Teva has completed a 13-week, early Phase 2 clinical trial in Europe involving 22 people with refractory epilepsy, using valrocemide as an add-on therapy with doses increasing up to four grams per day. Side effects were considered mild to moderate. However, one patient reported severe loss of strength, which may have been related to the treatment. All but one subject completed the study, with one leaving the study because of the requirement for multiple blood samples. This was an open-label study and was not designed to demonstrate safety or efficacy, although there were indications of clinical benefit that were consistent with the expectations for the drug. Larger scale clinical trials have yet to be conducted to demonstrate safety and efficacy.
Together with Teva, we plan to develop valrocemide initially for the treatment of epilepsy, for a number of reasons:
We also plan to initiate a clinical program to explore the potential for efficacy in bipolar disorder in 2004. We may also pursue clinical development of valrocemide for neuropathic pain in the future.
Other Research and Development Programs
Remyelination Programs
We believe that if the myelin sheath can be repaired, we can expect greater improvements in neurological function than those achieved with Fampridine-SR, which addresses a single, although crucial, consequence of demyelination. We licensed two separate groups of patents, one group relating to the neuregulin growth factors and another group relating to a family of remyelinating antibodies, that may provide access to remyelinating therapies via different and potentially complementary routes.
47
Neuregulins/GGF-2
We are conducting a research and development program directed towards applying a neuregulin growth factor, GGF-2, to stimulate the cells that normally form the myelin sheath. GGF-2 was the subject of a comprehensive discovery program by CeNeS Pharmaceuticals, PLC, previously Cambridge Neuroscience, Inc. In 2002, we obtained an exclusive worldwide license to the neuregulin technology, including GGF-2, from CeNeS. In addition, we licensed cell lines for the production of the molecule from both CeNeS and Bayer AG, which had earlier partnered with CeNeS on the GGF-2 program. We have entered into an agreement with Lonza Biologics, plc for the manufacturing and process development for GGF-2. If our pre-clinical testing is successful, we intend to advance GGF-2 to clinical development by early 2005.
Remyelinating Antibody Program
Our remyelinating antibody program is based on an exclusive license to patents derived from more than 15 years of research performed at the Mayo Clinic. Our remyelinating antibody program is designed to promote remyelination of affected areas in the brain and spinal cord. In particular, these remyelinating antibodies were found to react with molecules on the surface of the cells that make myelin, and stimulate them in a number of ways, leading to increased activity and remyelination.
In addition, we are continuing to support pre-clinical studies at the Mayo Clinic to learn more about the ways the antibodies act to stimulate the myelin-forming cells. The development costs of this program will be partially offset by a small business grant of $1.0 million awarded to us in September of 2002 by the NIH.
Regeneration and Plasticity Programs
Chondroitinase Program for Scar Matrix Modification
We have developed a chondroitinase program aroundbased on the concept of breaking down the matrix of scar tissuestissue that developdevelops as a result of an injury. We believeinjury to the CNS. Published research has demonstrated that this scar tissuematrix is partly responsible for limiting the regeneration of nerve fibers in the CNS and restricting their ability to modify existing neural connections, which is the process known as plasticity. This scar matrix may also theinhibit repair of the myelin sheath because it restrictsby restricting the movements of the myelinating cells into the area of damage. We believe that some of the molecular
Major components of the scarring reaction are particularly inhibitory to nerve fiber growth.
Chondroitin sulfatescar matrix, known as proteoglycans, CSPGs, are a group of large molecules that form a major component of the scar matrix. Proteoglycans are composedconsist of a combination of protein and sugar molecules, producing essentially a sugar-coated protein.molecules. Chondroitin sulfate proteoglycans, or CSPGs, are the specific types of proteoglycans found in the scar matrix. Cell culture studies and a number of animal studies have shown that these CSPGs inhibit the growth of nerve fibers and are likely to be key factors in the failure of the spinal cord or brain to regenerate and repair. Cell culture studiesStudies also have shown that it is possible to reduce the inhibitory activity of CSPG molecules by breaking them down with bacterial enzymes called chondroitinases. These enzymes clip off somechondroitinases break down the CSPG molecules, thereby reducing their inhibitory activity.
Four independent laboratories have published animal studies showing that application of chondroitinase results in recovery of function following injuries to various areas of the surface sugarbrain or spinal cord. These functions have included walking, forelimb grasping, sensation, and visual and bladder function. We have produced a recombinant version of naturally-occurring Chondroitinase ABC-I and successfully tested its ability to improve function in an animal model of spinal cord injury. These studies were recently published in the Journal of Neurotrauma. In these studies, rats that sustained a spinal cord injury were treated with either chondroitinase or an ineffective enzyme control and evaluated over 10 weeks of recovery. Animals treated with chondroitinase showed significant improvements both in motor function of the limbs and in bladder function, compared to those treated with the control enzyme.
We are conducting a research program, which has been funded in part by federal and state grants, to develop second generation approaches to overcoming the proteoglycan matrix. These include novel enzyme molecules and alternative approaches to blocking matrix formation. We are now exploring research grants from the protein coreNIH and potential partnerships with other companies for completing our preclinical program in chondroitinase. In 2003, we obtained an exclusive worldwide license to certain patents and technology from Cambridge University Technical Services Limited and King's College London related to our chondroitinase program. We are also building our intellectual property position with respect to this technology with patent applications around uses of the CSPG, which are responsible for muchknown compound and new chemical structures.
Remyelination Programs
Our remyelination programs include two distinct therapeutic approaches to stimulate repair of the inhibitory action.damaged myelin sheath in MS, Glial Growth Factor 2, or GGF-2, and remyelinating antibodies. These two approaches address remyelination by different and potentially complementary routes. Both programs require finalizing production of clinical-grade material and completion of preclinical toxicology tests before moving into clinical development. We believe a therapy that could permanently repair myelin sheaths has the potential to restore substantial neurological function to those affected by demyelinating conditions.
Regenerative AntibodiesNeuregulins/GGF-2
DuringGGF-2 is a member of the course of our remyelinating antibody program, for which we evaluated a range of antibodies that react with CNS tissues, we identified anotherneuregulin family of antibodies that appeargrowth factors related to epidermal growth factor. The neuregulins bind to erbB receptors, which translate the growth factor signal to the cell and cause changes in cell growth, protein production and gene expression. The molecule was shown in published studies to stimulate growth of nerve fibers. Studies are under way to determine whether these antibodies are effective in promoting regenerationremyelination in animal models of SCI.MS and to have a range of other effects in neural protection and repair. In 2002, we obtained from CeNeS an exclusive worldwide license to its neuregulin patents and related technology, including GGF-2. We initially plan to develop GGF-2 for the treatment of MS.
Neuregulins covered in the portfolio from CeNeS have additional potential applications in treatment of heart disease and cancer. Neuregulins and their erbB receptors are essential for cardiac development and have been shown to protect cardiac muscle cells from stressors that model congestive heart failure and myocardial infarction. Additionally, GGF-2 has been shown to protect the heart and brain from the toxicity of commonly used chemotherapeutic agents, such as cisplatin. The neuregulins may also have the potential, when coupled with toxins, to target erbB receptor positive tumors such as those found in certain types of breast cancers.
Remyelinating Antibodies Program
Our remyelinating antibodies program is based on more than 15 years of research performed at Mayo Clinic. Under a license agreement entered into with Mayo Clinic in September 2000, we have exclusive worldwide rights to patents and other intellectual property for these antibodies related to use and treatment of CNS disorders. Studies have demonstrated the ability of this family of antibodies to stimulate repair of the myelin sheath in three different animal models of MS. In particular, these antibodies were found to react with molecules on the surface of the cells that make the myelin sheath and stimulate them in a number of ways, leading to increased remyelination activity. First identified in mice, similar antibodies were subsequently identified in human blood samples by the Mayo team and we have been able to produce a recombinant human antibody that may be suitable for clinical development.
We have also supported preclinical studies at Mayo Clinic to learn more about the ways the antibodies act to stimulate the myelin sheath-forming cells. In 2004, Mayo Clinic received a $2 million grant to develop and manufacture clinical-grade material and progress the program towards clinical development.
R&DSales and Product Marketing
We have established three sales channels for marketing Zanaflex Capsules: an internal specialty sales force, a contract sales force and a telemarketing group.
We focus our sales and marketing efforts on physicians and other prescribers who treat spasticity in the United States. Approximately 9,200 physicians generated roughly 78% of the prescriptions for Zanaflex and generic tizanidine tablets in the United States in 2004. Most of these physicians are located at major medical centers. We have existing relationships with the majority of these centers through our Fampridine-SR clinical trial process.
We believe that, in general, people with MS and SCI are knowledgeable about their conditions, actively seek new treatments, and directly influence their prescriber's evaluation of treatment options. We have existing relationships with the major advocacy groups that focus on MS and SCI. We provide regular updates regarding our development programs and we sponsor or support several educational initiatives. We have implemented a comprehensive series of educational and promotional programs to support Zanaflex Capsules. These include educational materials, a peer-to-peer speakers' program, samples, medical information and drug safety monitoring services, as well as a patient assistance program. At the request of the FDA, we have also implemented an educational program to inform
pharmacists, prescribers and patients that Zanaflex tablets or generic tizanidine tablets are not therapeutically equivalent to Zanaflex Capsules and that, as a result, a prescription for Zanaflex Capsules should not be substituted with any tablet formulations at the pharmacy.
We believe that the expertise we are developing through commercializing Zanaflex Capsules will provide a strong foundation for our marketing of Fampridine-SR, if approved, as well as for additional potential treatments in CNS conditions. As a result, we plan to market Fampridine-SR ourselves in the United States and possibly in Canada, if it is approved in both countries. We expect that the sales force for Zanaflex Capsules would also promote Fampridine-SR in the United States since both products would have many of the same prescribers. We do not currently intend to build commercial capabilities outside North America but intend to secure those capabilities through one or more partners.
Similar to other phamaceutical companies, our principal customers are wholesale pharmaceutical distributors. We currently depend on three key customers. For the nine months ended September 30, 2005, Cardinal Health, McKesson Corporation and AmerisourceBergen Corporation accounted for approximately 51.4%, 27.3% and 13.2% of our shipments, respectively.
Scientific and Medical Network
We have an established advisory team and network of well-recognized scientists, clinicians and opinion leaders in the fields of MS and SCI. Depending on their expertise, these advisors provide assistance in trial design, conduct clinical trials, keep us apprised of the latest scientific advances and help us identify and evaluate business development opportunities. A number of the members of this network form our Scientific Advisory Board. The members of our Scientific Advisory Board are highlighted below:
| Name | Affilation | |
|---|---|---|
| Michael S. Beattie, Ph.D. | Brumbaugh Professor and Chair of the Department of Neuroscience, Ohio State University. | |
Jacqueline C. Breshnahan, Ph.D. | Professor of Neuroscience, Ohio State University. | |
Mary B. Bunge, Ph.D. | Professer of Cell Biology and Anatomy, Neurological Surgery and Neurology, University of Miami School of Medicine. | |
Carl W. Cotman, Ph.D. | Professor of Psychobiology and Neurology, University of California, Irvine. | |
James W. Fawcett, Ph.D. | Merck Company Professor of Experimental Neurology, Cambridge University, and Chairman of the MRC Cambridge Centre for Brain Repair. | |
Martin Grumet, Ph.D. | Professor of Cell Biology and Neuroscience, Rutgers University Director, W. M. Keck Center for Collaborative Neuroscience. | |
Eugene Johnson, Jr., Ph.D. | Norman J. Stupp Professor of Neurology, and Professor of Molecular Biology and Pharmacology at Washington University School of Medicine, St. Louis. | |
Mark D. Noble, Ph.D. | Professor of Genetics at the Center for Cancer Biology, University of Rochester Medical Center. | |
Melitta Schachner, Ph.D. | Professor and Director of the Institute for Synthesis of Neural Structures, University of Hamburg, Germany. | |
Jerry Silver, Ph.D. | Professor of Neurosciences, Case Western Reserve University. | |
Patrick A. Tresco, Ph.D. | Professor of Bioengineering, Director Keck Center for Bioengineering, University of Utah. | |
Mark H. Tuszynski, M.D., Ph.D. | Professor of Neurosciences, Director of the Center for Neural Repair, and Attending Neurologist at the University of California, San Diego. | |
Stephen G. Waxman, M.D., Ph.D. | Chairman of the Department of Neurology, Yale University School of Medicine. | |
Wise Young, Ph.D., M.D. | Professor II and Founding Director of the W. M. Keck Center for Collaborative Neuroscience, Rutgers University. |
In addition, we have recruited over 35 MS centers and 80 SCI rehabilitation centers in the United States and Canada to conduct our clinical trials. Our clinical management team has extensive experience in the areas of MS and SCI and works closely with this network.
Collaborations, Alliances and License Agreements
Elan Corporation plc
Zanaflex
In July 2004, we entered into an Asset Purchase Agreement with Elan pursuant to which we acquired all of Elan's research, development, distribution, sales and marketing rights to Zanaflex Capsules and Zanaflex tablets in the United States. The assets acquired include the products' FDA registrations and FDA dossiers, proprietary product know-how, a patent and two related patent applications, certain inventory of Zanaflex tablets and certain product books and records. Elan has granted us a license that allows us to use the Zanaflex trademarks in the United States and has given us the right to buy the Zanaflex trademark for a nominal sum once specified milestone and royalty payments have been made. Elan also granted us an exclusive, perpetual and royalty-free license to certain intellectual property relating to technology contained in Zanaflex Capsules and Zanaflex tablets or used in the manufacture of Zanaflex Capsules, for use in connection with the sale and marketing of Zanaflex Capsules and Zanaflex tablets in the United States. We also acquired the right to develop new indications, formulations, dosage forms, delivery systems and process improvements of Zanaflex. Under the agreement, Elan agreed not to directly or indirectly market, distribute or sell any products containing tizanidine as an active pharmaceutical ingredient in the United States until the later of the end of our obligation to pay royalties to Elan or valid termination of our supply agreement with Elan. In addition, we agreed not to directly or indirectly market, distribute or sell any products containing tizanidine as its active pharmaceutical ingredient in the United Kingdom or Ireland until July 2007.
Our agreement with Elan obligates us to pay a combination of sales-based milestone payments of up to $19.5 million and royalties on future sales of Zanaflex Capsules and Zanaflex tablets. We have made or accrued an aggregate of $3.5 million in payments under this agreement through September 30, 2005. Our obligation to pay royalties to Elan for Zanaflex tablets and Zanaflex Capsules ends on the later of July 2014 or when the last patent included in the acquisition expires. We also agreed to use commercially reasonable efforts to commercialize Zanaflex Capsules.
As part of the acquisition, we assumed certain of Elan's rights and obligations relating to Zanaflex under a license agreement with Novartis, to the extent that these rights and obligations arise subsequent to our acquisition of Zanaflex. Under this agreement we obtained certain rights to market and sell tizanidine products and rights to product improvements developed by Novartis. We are obligated to pay Novartis royalties based on net sales of Zanaflex Capsules and Zanaflex tablets until
the agreement expires in February 2007, after which time we will have a fully paid-up license from Novartis to these rights.
Elan and Novartis manufacture Zanaflex Capsules and tablets for us, respectively. See "—Manufacturing." In December 2005, we entered into a financing arrangement with PRF pursuant to which we assigned PRF the right to receive a portion of our net revenues from Zanaflex Capsules, Zanaflex tablets and any future Zanaflex products. The arrangement covers all Zanaflex net revenues generated from October 1, 2005 through and including December 31, 2015, unless the arrangement is terminated earlier. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Financing Activities."
Fampridine-SR
In January 1997, we licensed from Elan exclusive worldwide rights to Elan's sustained release formulation of fampridine, Fampridine-SR, for the treatment of SCI. In April 1998, we formed MS Research & Development Corporation, or MSRD, with Elan's subsidiary, Elan International Services, Ltd., or
48
EIS, to develop Fampridine-SR for treatment of MS. At that time, MSRD licensed from Elan exclusive worldwide rights to Fampridine-SR for the treatment of MS.
Termination and Assignment Agreement
In September 2003, we entered into a termination and assignment agreement with Elan, EIS and MSRD pursuant to which MSRD assigned to us its assets, including the license from Elan for Fampridine-SR for MS. We paid MSRD approximately $11.5 million for all the assets and assumed liabilities of MSRD. MSRD will distributedistributed the purchase price to its shareholders according to their equity ownership interest. We will receivereceived a distribution of approximately $9.5 million as a result of this distribution. We also purchased EIS's shares at par value, and own approximately 88% of MSRD, which now has no assets or liabilities and is inactive.
Amended and Restated License
In September 2003, we entered into an amended and restated license with Elan, which replaced the two prior licenses for Fampridine-SR in oral sustained release dosage form. Under this agreement, Elan granted us exclusive worldwide rights to Fampridine-SR for all indications, including SCI, MS and all other indications. We agreed to pay Elan milestone payments of up to $15.0 million and royalties based on net sales of the product.product, if approved. We have not made any payments under this agreement through September 30, 2005.
Elan is responsible for completing the chemistry, manufacturing and controls section of our NDA for Fampridine-SR and equivalent regulatory applications outside the United States. Elan is also supplying us with product for our clinical trials under this agreement.
Elan may terminate our license in countries in which we have a license, including the US, the major European markets or JapanUnited States, if we do notfail to file regulatory approvals within a commercially reasonable time after completion and receipt of positive data from all preclinical and clinical studies required for the related NDA or any NDA equivalent. We could also lose our rights under the license agreement if we fail to obtain regulatorylaunch a product in such countries within 180 days of NDA or equivalent approval or launchif we fail to fulfill our payment obligations under the product after regulatory approval in the applicable country within specified periods.license agreement. If Elan terminates our license in any applicable country, Elan is entitled to license from us our patent rights and know-how relating to the product and to market the product in the applicable country, subject to royalty payments to us.
We have the right to terminate the Elan is responsible for completinglicense at any time by written notice. In addition, the chemistry, manufacturingElan license may be immediately terminated by either party following an incurable breach of any term or provision by the other party. The Elan license may also be terminated by either party following notice and controls section of our NDA and equivalent regulatory applications outside the US. Elan is also supplying usa cure period with product for our clinical trials under this agreement.respect to an uncured breach by either party.
Subject to the early termination provisions, the Elan license terminates on a country by country basis on the last to occur of fifteen years from the date of the agreement, the expiration of the last to expire Elan patent or the existence of competition in that country.
Supply AgreementCardinal Health PTS, LLC
In September 2003,August 2005, we entered into a supplysales force agreement with ElanCardinal Health. Under this agreement, approximately 160 of Cardinal Health's sales representatives market Zanaflex Capsules to approximately 4,000 high prescribing primary care physicians identified by us throughout the United States. Although these sales representatives do not exclusively represent Acorda, our agreement with Cardinal Health provides that they will not market any other products during their sales calls related to Zanaflex Capsules. We are responsible for providing training to the Cardinal Health sales representatives regarding the medical and technical aspects of Zanaflex Capsules and on our specific sales strategies and policies. We also provide all samples and promotional materials for use by these sales representatives. Cardinal Health is responsible for general supervision and management of the sales force, including ensuring legal and regulatory compliance, including maintaining procedures relating to the manufacturehandling of drugs by their sales representatives in compliance with applicable laws and supply of Fampridine-SR by Elan.prudent management practices.
We have agreed to purchase at least 75% of our annual requirements of product from Elan, unless Elan is unable or unwilling to meet our requirements, for a purchase pricepay Cardinal Health service fees based on a specified percentagethe achievement of net sales. In those circumstances where we electtargeted sales levels and to purchase less than 100% of our requirements from Elan, we agreed to makereimburse Cardinal Health for certain compensatory payments to Elan. Elan agreed to assist us in qualifying a second manufacturer to manufacture and supply us with Fampridine-SR subject to our obligations to Elan.
Teva Pharmaceutical Industries Ltd.
In September 2003, we entered into a collaboration agreement with Teva under which we were granted a co-exclusive license with Teva to jointly develop and promote in the United States products containing valrocemide as an active ingredient in any formulation and dosage form for any indication for human use, except MS. However, in the event that Teva seeks to develop and promote products containing valrocemide for MS it must provide us with notice and negotiate with us an amendment to the agreement.costs. The agreement provides that Teva will own all right, title and interest in and to all intellectual property jointly developed by the parties while Teva has the sole right and obligation to defend against any infringement claims.
The agreement further provides that Teva is responsible for seeking and maintaining regulatory approval from the FDA upon the completion of any co-developed product and that Teva will consult with us in
49
preparing the filings to obtain regulatory approval. Teva is also solely responsible for commercializing, manufacturing and supplying all co-developed products.
We made an initial payment to Teva upon execution of the collaboration agreement and are obligated to make payments to Teva upon achieving certain milestones. We are also responsible for the cost and conduct of the next clinical trial in epilepsy and a toxicology study for valrocemide. We will use commercially reasonable efforts to complete the next clinical trial by the first quarter of 2006 and, if further clinical trials are required after the completion of the next clinical trial, we will share the costs of such trials with Teva. Following the completion of the next clinical trial in epilepsy, we will share in 50% of co-development expenses and co-development profits. We are entitled to receive a royalty, on a country by country basis, on net sales by Teva of valrocemide outside of the United States if the clinical data used to obtain regulatory approval for sale of the product in such country was jointly developed, or independently developed by us, under this Agreement.
Unless earlier terminated under provisions of the Teva agreement, the agreement will expire on the earlier to occur of (i) September 23, 2009, if the parties have not achieved a statistically significant primary endpoint that is accepted by the FDA for the first pivotal trial in connection with any product, (ii) six months after the first generic version of any product is launched in the United States, or (iii) September 23, 2012, if the parties have not commenced the promotion and/or commercialization of any product under the Teva agreement.
In addition, if we seek a co-promotion partner for our products containing Fampridine-SR, Teva has a rightterm of first negotiation for the co-developmenttwo years and co-promotion of these products in the United States. Teva's right of first negotiation terminates 60 days after we deliver to Teva the summary results of the completed SCI-F301, SCI-F302 and MS-F202 clinical trials for Fampridine-SR.can be terminated without cause.
Rush-Presbyterian St. Luke's Medical Center
In 1990, Elan licensed from Rush-Presbyterian St. Luke's Medical Center, or Rush, know-how relating to fampridine for the treatment of MS. We subsequently licensed this know-how from Elan. In September 2003, we entered into an agreement with Rush and Elan terminating the Rush license to Elan and providing for mutual releases. We also entered into a license agreement with Rush in which Rush granted us an exclusive worldwide license to theirits know-how relating to fampridine for the treatment of MS. Rush has also assigned to us theirits Orphan Drug Designation for fampridine for the relief of symptoms of MS.
We agreed to pay Rush a license fee, milestone payments of up to $1.15 million and royalties based on net sales of the product for neurological indications. We have made an aggregate of $200,000 in payments under this agreement through September 30, 2005.
The Rush license may be terminated by either party following an uncured material breach by the other party and notice. The Rush license may also be terminated upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party. We also entered into an agreement with Elan relating to the allocation of payments between us and Elan of certain payments to Rush under the Rush license.
Subject to the early termination provisions, the Rush license terminates upon expiration of the royalty obligations, which expire fifteen years from the date of the agreement.
Canadian Spinal Research Organization
In August 2003, we entered into an Amended and Restated License Agreement with the Canadian Spinal Research Organization, CSRO. Under this agreement we were granted an exclusive and worldwide license under certain patent assets and know-how of CSRO relating to the use of fampridine in the reduction of chronic pain and spasticity in a spinal cord injured patient.subject.
We are required to pay to CSRO royalties based on a percentage of net sales of any product incorporating the licensed rights, including royalties on the sale of Fampridine-SR for any indication.
We have the right to terminate the CSRO agreement at any time by written notice. In addition, the CSRO agreement may be terminated by either party following an uncured material breach by the other party. The CSRO agreement may also be terminated by either party upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of assets, by the other party. Subject to the early termination provisions, the CSRO agreement will expire upon the termination of all royalty or other payment obligations on a country-by-country basis, which will be
no longer than the earlier of the expiration of the last to expire licensed patent in such country or ten years from the date of the first commercial sale of the product in such country.
50
Cornell Research Foundation, Inc.
In February 2003, we entered into a license agreement with Cornell Research Foundation, Inc., pursuant to which we were granted an exclusive license under a patent for the use of fampridine in the treatment of anterior horn cell diseases. In consideration for the license, we paid Cornell an upfront license fee and are required to make payments of up to $150,000 to Cornell upon the achievement of certain milestones relating to the successful reissuance or reexamination of the patents licensed to us and, the completion of a clinical trial testing the use of Fampridine-SR in amyotrophic lateral sclerosis. We have made an aggregate of $50,000 in payments under this agreement through September 30, 2005. We are also obligated to pay Cornell royaltiesan annual royalty on net sales of Fampridine-SR in any and all indications.indications, subject to a minimum annual royalty requirement of $25,000.
Under the Cornell agreement, Cornell is responsible for all patent prosecution and maintenance activities relating to the licensed patent, and we are responsible for paying all fees incurred by Cornell in connection therewith. We have the right under this agreement to enforce any patent rights within the licensed patents against infringement by third parties at our own expense.
We have the right to terminate the Cornell Agreement at any time by written notice. In addition, the Cornell agreement may be terminated by either party following an uncured material breach by the other party. Subject to the early termination by either of us,provisions, the term of the Cornell agreement will continue until the expiration of the last to expire valid claim under the licensed patent.
Cambridge University Technical Services Limited and King's College London
In December 2003, we entered into a license agreement with Cambridge University Technical Services Limited and King's College London, pursuant to which we were granted an exclusive worldwide license, including the right to sublicense, under a U.S. patent application and its foreign counterpart to develop and commercialize products related to enzymatic methods, including chondroitinase, of treating CNS disorders. We were also granted a non-exclusive worldwide license, including the right to sublicense, under the same U.S. and foreign patent applications to develop and commercialize products related to small molecule inhibitors for use in treating CNS disorders.
In consideration for these licenses, we paid an upfront license fee and are required to make payments of up to $2.15 million upon the achievement of certain milestones. We have made an aggregate of $45,000 in payments under this agreement through September 30, 2005. We are also obligated to pay royalties on net sales and on any sublicense royalties that we receive.
The King's College license may be terminated by any party following an uncured material breach by any other party. The King's College license may also be terminated by any party if any other party ceases to carry on business, is declared by a court of competent jurisdiction to be bankrupt or upon the appointment of a liquidator of that party. Subject to the early termination provisions, the King's College license agreement will continue until the expiration of the last to expire valid claim under the licensed patent applications, at which time the licenses granted under the license agreement will automatically become non-exclusive, worldwide, fully paid-up and irrevocable.
Mayo Clinic Foundation for Medical Education and Research
In September 2000, we entered into a license agreement with The Mayo Foundation for Education and Research, or the Mayo Clinic, pursuant to which we were granted an exclusive worldwide license to its patents and other intellectual property on remyelinating antibodies. Under this agreement, we have the right to develop, make, use and sell the remyelinating antibody products for the prevention, mitigation and treatment of CNS disorders. We have worked closely with theone of Mayo ClinicClinic's research groupgroups on developing and patenting this emerging technology in connection with the therapeutic use of these
antibodies, specifically myelination and re-myelinationremyelination in SCIMS and MS. TheSCI. Mayo Clinic has the right to continue researching the antibodies and, in the event it develops other applications related to the licensed patent, which are outside of the scope of our current license, but are for the treatment of CNS disorders. Mayo Clinic is required to offer rights in these new applications to us before it offers such rights to a third party.
Under the Mayo Clinic agreement, we are obligated to make milestone payments andof up to $1.875 million. We also pay royalties based on net sales. ThisWe have not made any milestone or royalty payments under this agreement through December 31, 2005. The Mayo Clinic agreement may be terminated by either party following an uncured material breach by the other party. We may terminate the Mayo Clinic agreement at will upon prior written notice to Mayo. In addition, either party also has the right to terminate upon the insolvency of the other party, the filing of bankruptcy by or against the other party, or the assignment of assets to the benefit of creditors by the other party. Unless otherwise terminated, this license agreement will terminate upon the expiration of the last licensed patent in any such licensed product.
We have also supported preclinical studies at Mayo Clinic to learn more about the ways the antibodies act to stimulate the myelin sheath-forming cells. In 2004, Mayo Clinic received a $2 million grant to develop and manufacture clinical-grade material and progress the program towards clinical development. A subsequent letter agreement between Mayo Clinic and us acknowledges that the work under this grant is being performed subject to and pursuant to the Mayo Clinic agreement.
CeNeS Pharmaceuticals plc
In November 2002, we entered into two license agreements with CeNeS Pharmaceuticals plc. The first agreement relates to an exclusive worldwide sublicense under certain patents, patent applications and know-how to make, have made, use, import, offer for sale and sell protein products composed of GGF-2 and non-protein products developed through the use of material covered by a valid claim in the patents. The license to these patents and the right to sub-license these patents were granted to CeNeS by the Ludwig Institute for Cancer Research.
Our payment obligations to CeNeS include payment of an upfront license fee, royalties based on annual net sales of the product, if any, as well as payments of up to $8.5 million upon achieving certain milestones in connection with the development, testing and regulatory approval of any protein products. We have not made any payments under this agreement through December 31, 2005. We are obligated to make minimum royalty payments commencing on the third calendar year following the first commercial sale of any licensed product. If we fail to pay any minimum royalty, CeNeS will have the option to convert our license or any sublicense to a non-exclusive license.
This agreement with CeNeS is effective until the later of November 12, 2017 or the expiration of the last-to-expire valid claim in the licensed patents. We may terminate this agreement at will upon prior written notice to CeNeS. In addition, this first agreement may be terminated by either party following an uncured material breach by the other party and if this agreement is terminated under that provision, we may retain the exclusive worldwide sublicense granted to us under this agreement, provided that we continue to pay royalties.
The second agreement relates to an exclusive worldwide sublicense to us under certain patents, patent applications and know-how to make and have made, use and have used, sell, offer for sale, have sold and import protein products composed of one or more proteins encoded by the growth factor genenrg-2 nrg-2 and non-protein products developed through the use of material covered by a valid claim of the patents. The license to this patent and the right to sub-license this patent was granted to CeNeS by the President and Fellows of Harvard College.
51
We have agreed to a timeline to achieve certain milestones relating to the research and development and the clinical testing and filing of regulatory approvals for the products. We are also required to make milestone payments.payments of up to $5.93 million. If we are unable to meet a milestone, CeNeS has agreed to negotiate in good faith with us to agree for a reasonable extension of the time to
achieve the milestone up to one year. We are obligated to pay CeNeS a license fee and royalties based on a percentage of net sales of protein products and non-protein products covered under the agreement. We have not made any payments under this agreement through December 31, 2005.
This second agreement may be terminated by either party following an unremedied default of a material obligation by the other party. CeNeS may terminate this agreement upon our failure to cure a default in our obligations relating to maintenance of insurance liability or our failure meet certain milestones. Harvard may terminate the underlying Harvard license if CeNeS becomes insolvent, makes an assignment of assets for the benefit of creditors, or has a petition bankruptcy filed for or against it. In that case, Harvard is required, upon our written request, to enter into a direct license with us under the same terms as those set forth in the agreement. We have the right to terminate this agreement upon written notice to CeNeS. The license granted to us pursuant to this agreement continues after the expiration of this agreement and may continue after the termination of this agreement, depending upon the circumstances under which this agreement is terminated.
Subject to early termination provisions, this agreement remains effective until the last patent, patent application or claim included in the licensed patents has expired, been abandoned or been held finally rejected or invalid.
Aeres Biomedical LimitedTeva Pharmaceuticals Industries Ltd.
In February 2002,September 2003, we entered into a research collaboration and commercialization agreement with Aeres Biomedical Limited, pursuantTeva Pharmaceuticals Industries Ltd., or Teva, under which we were granted a co-exclusive license with Teva to which Aeres will modify our Lym22jointly develop and Lym46 antibodies to switch their class from IgM to IgG and create a stable cell line expressing each antibody. We will have all right, title and interest in the DNA and RNA sequences expressing our antibodies, any and all cell lines, and all inventions and all patents and patent applications arising out of the research under this agreement.
We will pay Aeres for the costs of its research and will make payments to Aeres upon completion of each research milestone. We will also pay Aeres a royalty on our net sales of any product incorporating or derived from an antibody that is produced by Aeres' stable cell line under this agreement for a period of 12 years after commercial introduction of the product in a particular country.
Unless earlier terminated, the Aeres agreement will expire on a country by country basis until no further payments are due by us to Aeres in such country.
Sales and Marketing
We plan to market Fampridine-SR for both SCI and MSpromote in the United States and Canada. We believe marketing synergies exist between the planned initial indication of SCI and the follow-on indication of MS. SCI and MS have several symptoms in common, and these symptoms are treated with similar medications prescribed by similar physicians. This concentrated group of physicians may be targeted with a relatively small sales force. We do not intend to build commercial capabilities outside North America at this time, but intend to secure those capabilities through a partner or partners.
Our sales and marketing efforts in North America will focus on SCI specialists, physiatrists, neurologists, physical therapists and nurse practitioners involved in the treatment of SCI and MS. These health care professionals form a small group, and are located at major medical centers in the U.S. and Canada. We have existing relationships with a majority of these centers already, as a result of extensive interaction throughout the Fampridine-SR clinical testing process.products containing valrocemide.
We believemade an initial payment to Teva of $2 million that in general, people with SCIwas charged as research and MS are knowledgeable about their condition, actively seek new treatments, and directly influence their prescriber's evaluation of treatment options. We have existing relationships with the major advocacy groups that focus on SCI and MS. We provide regular updates regarding our development programs, and sponsor or support several SCI and MS educational initiatives.
We have developed the following three-part strategyexpenses for the developmentyear ended December 31, 2003, upon execution of the collaboration agreement, and commercialization of our product candidates:
52
leverage the introduction of Fampridine-SR for SCI into additional indications in MS. In addition, we plan to build on the unusually large role that people with SCI and MS play in the development of their own treatment. Thisvalrocemide.
We and Teva amicably terminated the collaboration agreement as of June 27, 2005 and in connection with the termination we paid Teva approximately $3.1 million. We and Teva have no further obligations to each other under the collaboration agreement.
Manufacturing
Zanaflex
We currently rely on Elan, Novartis and other third parties to supply us with Zanaflex Capsules and Zanaflex tablets. Zanaflex Capsules are manufactured using Elan's proprietary SODAS (spheroidal oral drug absorption system) multiparticulate drug delivery technology. We agreed to provide Elan with monthly written 18-month forecasts, and with annual written two-year forecasts, of our supply requirements for Zanaflex Capsules. In each of the five months following the submission of our 18-month forecast we are obligated to purchase the quantity specified in the forecast, even if our actual requirements are greater or less. Elan is not obligated to supply products in excess of our forecast requirements, but will involveuse commercially reasonable efforts to fulfill any such orders. The initial term of the agreement expires in 2009, with two automatic two-year renewal terms. Either party may terminate the agreement by notifying the other party at least 12 months prior to the expiration of the initial term or any renewal term. In addition, either party may terminate the agreement if the other party commits a varietymaterial breach that remains uncured. If a failure to supply occurs under the agreement, other than a force majeure event, or if we terminate the supply agreement for cause, Elan must use commercially reasonable efforts to assist us in transferring production of advocacy initiatives,Zanaflex Capsules to us or a third-party manufacturer, provided that such third party is not a technological competitor of Elan. If we need to transfer production, Elan has agreed to grant us a royalty-free, fully paid-up license of its manufacturing know-how and other information and rights related to the production of Zanaflex Capsules, including consumer education, professional medical seminars, continuing medical education programs, advisory boards and publications. To date, we have presented clinical results at most major medical conferences for physical medicine and rehabilitation as well as neurology.
technological competitor of Elan. In the event of termination of the supply agreement due to a force majeure event that continues for more than three months, Elan has agreed to enter into negotiations with us to preserve the continuity of supply of products, including the possibility of transferring manufacturing of Zanaflex Capsules to us or a third party manufacturer. Elan obtains tizanidine, the active ingredient in Zanaflex Capsules, from Novartis.
Under a supply agreement we assumed from Elan, Novartis is responsible for manufacturing Zanaflex tablets and tizanidine, the API in both Zanaflex Capsules and Zanaflex tablets, for us through February 2007. Although Novartis currently produces tizanidine, it has informed us that it intends to discontinue production by the end of the first quarter of 2006. It is our understanding that Novartis is currently in the process of transferring the methods of manufacturing tizanidine to Rohner, an API manufacturer in Pratteln, Switzerland. We have verified this transfer and plan to audit Rohner's manufacturing site toward the end of the first quarter of 2006, following the commencement of Rohner's manufacture of tizanidine. We have also identified an alternate source for tizanidine in collaboration with Elan. However, we do not have an agreement with an alternative tizanidine manufacturer, and we do not anticipate entering into a supply agreement for API with Elan or Rohner. It is the responsibility of each of Novartis and Elan to procure the API required to meet their contractual obligations under their respective supply agreements with us. We do not anticipate an interruption in API supply, and any cost associated with validating API suppliers would be incurred by Novartis or Elan. The costs of our audit of Rohner or any selected member of Cardinal Health's sales force in the RxPedite program. Cardinal will help us transition those members of their sales force who accept our offer to work for us asother supplier are not material and are considered part of our own direct sales force.
ManufacturingFampridine-SR
Under our supplyIn September 2003, we entered into an agreement with Elan Elan will manufacture andfor the supply to us,of Fampridine-SR. We agreedUnder that agreement, we are required to purchase at least 75% of our annual requirements of productFampridine-SR from Elan unless Elan is unable or unwilling to meet our requirements, for a purchase price based on a specified percentage of net sales. Except in those circumstances,requirements. In addition, the agreement also obligates us to make compensatory payments if we elect todo not purchase less than 100% of our requirements from Elan.
As permitted by our agreement with Elan, we have designated Patheon, Inc. as a qualified second manufacturing source of Fampridine-SR. In connection with that designation, Elan assisted us in transferring manufacturing technology to Patheon. We and Elan have agreed that we may purchase up to make certain additional25% of our annual requirements from Patheon without making compensatory payments to Elan. Elan agreed to assist us in qualifying a second manufacturer andIn addition, Patheon may supply us with Fampridine-SR subject to our obligations to Elan.
We have entered into a technical transfer program agreement with Patheon, Inc., pursuant to which Patheon will perform certain manufacturing and analytical services on Fampridine-SR in connection with the contemplated transfer of Elan's Fampridine-SR technology to Patheon.if Elan is supporting the technology transferunable or unwilling to Patheon, and if it is successful, we intend to pursue qualifying Patheon as an alternate manufacturer of Fampridine-SR inmeet our NDA filing.requirements.
Fampridine Active Pharmaceutical Ingredient, API, is supplied by third parties directly under contract to Elan. Acorda may or may not have future direct contracts with these API suppliers. We currently participate in quality audits of these API suppliers, and have met with the FDA regarding our plans to submit two alternate suppliers of API.
53
Teva and its affiliates have the exclusive right to manufacture valrocemide. Commercially reasonable efforts, consistent with good pharmaceutical industry practices, shall be used to manufacture and supply valrocemide.Preclinical Products
We have established the internal capability to manufacture research quantities of antibody and protein drug candidates. In April 2003, we entered into an agreement with Lonza Biologics, pursuant to which it will performproduct candidates and have contracted for testing and manufacturing development activities for GGF-2. We have also retained the services of Aeres Biomedical for antibody engineering support. As NDA holder we planGGF-2 to have quality assurance agreements with all manufacturing parties to ensure compliance with regulatory requirements.be performed by an outside contractor.
Intellectual Property
We have in-licensed, or are the assignee of, 24over 25 U.S. patents, 40 U.S.over 60 foreign patents and over 65 patent applications and their numerous foreign counterparts.pending in the United States or abroad. There are fourfive major families of patents in our portfolio, corresponding to our four current major programs of research and development, i.e., fampridine, remyelinating antibodies, Neuregulins, including GGF-2, and the chondroitinase program.portfolio.
FampridineZanaflex
As part of our purchase from Elan of the Zanaflex assets, we acquired one issued U.S. patent and two pending U.S. patent applications. Our issued patent is generally directed to certain methods of reducing somnolence and reducing peak plasma concentrations in patients receiving tizanidine therapy. This issued patent expires in 2021. Our two pending U.S. patent applications are directed to
multiparticulate formulations of tizanidine and certain other methods of using tizanidine. The process of seeking patent protection can be time consuming and we cannot assure you that patents will be issued from these pending applications or that, if patents are issued, they will be of sufficient scope to provide meaningful protection of our products.
In addition, we entered into a Supply Agreement with Elan as part of the acquisition, whereby Zanaflex Capsules are manufactured for us by Elan using Elan's proprietary SODAS technology and proprietary information. This proprietary technology is owned by Elan and, in the event Elan ceases to manufacture Zanaflex Capsules, Elan has agreed to grant us a royalty-free, fully paid-up license of its manufacturing know-how and other information and rights related to the production of Zanaflex Capsules, including a license to use its SODAS technology for specified purposes. We have the right to sublicense this know-how to a third party manufacturer, so long as this third party is not a technological competitor of Elan.
Elan has granted us a license that allows us to use the Zanaflex trademark in the United States and gave us the right to buy the Zanaflex trademark for a nominal sum once specified milestone and royalty payments have been made.
Fampridine-SR
We hold an exclusive, worldwide license from CSRO for a CanadianU.S. patent application and its foreign counterparts for the use of fampridine in the treatment of spasticity and neuropathic pain in chronic SCI. Foreign counterpart patents have been granted in the U.S. and in a number of other countries worldwide. The U.S. patent expires in 2013.
We hold an exclusive, worldwide license from Elan to three issued U.S. patents, from Elanwith corresponding issued patents and pending applications in a number of foreign countries, relating to timed delivery formulations of a family of aminopyridine compounds, including fampridine, and which also claim methods of administration and treatment for relevant neurological conditions. Foreign counterparts of these patents are also covered by the license. One of the three U.S. patents expires in 2011 and the other two U.S. patents expire in 2013.
We also hold an exclusive license from Cornell University for an issued U.S. patent that relates to the use of aminopyridine compositions, including fampridine, for the treatment of diseases of anterior horn cells, including amyotrophic lateral sclerosis, which is also known as Lou Gehrig's disease. This patent expires in 2016.
Valrocemide
Teva owns, has in-licensed or is the assignee of patents related to valrocemide. The We also have a pending U.S. patent for valrocemide expires in 2013.application and its foreign equivalent directed to methods of using aminopyridines and a pending U.S. patent directed to aminopyridine formulations.
Remyelinating AntibodiesChondroitinase
We are the exclusive licenseehave a license to a U.S. application and its foreign counterpart from King's College, University of Cambridge directed to treatment of CNS damage. We have recently filed a portfolionumber of patents andU.S. patent applications relatedand their foreign counterparts directed to a serieschondroitinase enzymes and methods of remyelinating monoclonal antibodies discovered in the laboratoryuse and preparation. In particular, we have filed eight U.S. applications, with foreign equivalents to five of Dr. Moses Rodriguez at the Mayo Clinic in Minnesota. One U.S. patent has issued. Foreign counterpartsthem, and an additional international application directed to fusion proteins of this patent are also issued in Australia, Mexico,chondroitinase, chimeric proteins including chondroitinase, deletion mutants, and New Zealand, and applications are pending elsewhere, including Europe, Canada, Japan and Korea.certain methods relating to chondroitinase.
Neuregulins
We are the exclusive licensee under a license agreement with CeNeS Pharmaceuticals, plc, of a worldwide portfolio of patents and patent applications related to products of neuregulin genes, including glial growth factors.GGF-2. These patents claim the use of particular neuregulins to treat various pathophysiological conditions, particularly stimulating myelinating cells in order to treat demyelinating conditions of the central and peripheral nervous system. These patents also claim a number of additional potential applications of neuregulins, including stimulation of growth in mammalian muscle cells and treating cardiac failure, peripheral neuropathy and nerve injury.
54
Chondroitinase / Matrix ModificationRemyelinating Antibodies
We have filedare the exclusive licensee of a portfolio of patents and patent applications related to our own researcha series of remyelinating antibodies discovered in the laboratory of Dr. Moses Rodriguez at the Mayo Clinic in Rochester, Minnesota for the treatment of CNS disorders. One U.S. patent has been issued and development programforeign counterparts of this patent have also issued in matrix modification.Australia, Mexico, New Zealand and South Korea, as well as in Europe, where patents have been validated in Germany, Spain, France, Great Britain and Italy. Applications are pending elsewhere, including Canada and Japan.
Competition
A numberThe market for developing and marketing pharmaceutical products is highly competitive. We are aware of many biotechnology and pharmaceutical companies that are engaged in development and/or marketing of therapeutics for a broad range of CNS conditions, though none of them have focused on the spinal cord as a point of access to this field. The current therapies for people with SCI and MS focus on treating symptoms associated with these diseases. Current approaches to symptom management include the use of compounds such as baclofen for spasticity, tricyclic antidepressants for neurological pain, fluoxetine for depression, amantadine or modafinil for fatigue, and oxybutinin for bladder contraction. Although our approach to the treatment of SCI and MS does not focus on treating symptoms, but rather on improving neurological function, our products will compete for market acceptance with these current treatments because they have been accepted and regularly prescribed to people with SCI and MS by health care providers.
Several biotechnology and pharmaceutical companies, as well as academic laboratories, are involved in research and/or product development for various neurological diseases, including SCI. However, to our knowledge, none has a comprehensive, multi-disciplinary approach for SCI therapeutic product development that is comparable to ours. We are aware that Aventis is developing a sodium/potassium channel blocker, HP 184, with a potential indication in SCI. We believe that HP 184 is now in clinical trials and any resulting product could compete with Fampridine-SR.conditions. Many of our competitors have substantially greater financial, research and development, human and other resources than we do. Furthermore, large pharmaceuticalmany of these companies have significantly more experience than we do in preclinical testing, human clinical trials, and regulatory approval procedures.procedures and sales and marketing.
Spasticity
CommerciallyTizanidine, the active pharmaceutical ingredient in Zanaflex Capsules, Zanaflex tablets and generic tizanidine tablets, is one of the two leading FDA-approved treatments for spasticity, a symptom suffered by both MS and SCI patients. Zanaflex tablets were approved by the FDA in 1996 and lost compound patent protection in 2002. Eleven generic manufacturers of tizanidine are distributing their own tablet formulations. Baclofen, which is also available therapiesgenerically, is the other leading drug for the treatment of spasticity. The mechanism of action and associated effects of baclofen are different from those of tizanidine. Due to the different pharmacokinetic profile of Zanaflex Capsules, Zanaflex tablets and generic tizanidine tablets are not AB-rated with Zanaflex Capsules. To our knowledge there are currently no other treatments for spasticity in clinical development.
MS and SCI
Current disease management approaches to MS are centered on immunomodulatory compounds.classified either as relapse management or disease course management approaches. For relapse management, the majority of neurologists treat sudden and severe relapses with a four-day course of intravenous high-dose corticosteroids. Many of these corticosteroids are available generically. For disease course management, there are a number of FDA-approved MS therapies that seek to modify the immune system. These treatments attempt to reduce the frequency and severity of exacerbations or slow the accumulation of physical disability for people with certain types of MS, though their precise mechanisms of action are not known. These products include Avonex from Biogen-IDEC, Betaseron from Schering AG, Copaxone from Teva and Rebif from Serono.
Several biotechnology and pharmaceutical companies, as well as academic laboratories, are involved in research and/or product development for various neurological diseases, including MS and SCI. We are aware that Aventis is developing a sodium/potassium channel blocker, HP 184, with a potential indication in SCI, MS and other conditions. We believe that HP 184 is in clinical trials for SCI and any resulting product could compete with Fampridine-SR. Neurorecovery Inc. has publicly disclosed that it has an immediate release form of fampridine for peripheral nervous system conditions in Phase 2 trials and any resulting product might compete with Fampridine-SR. In certain circumstances, pharmacists are not prohibited from formulating certain drug compounds to fill prescriptions on an individual patient basis. We are aware that at present compounded fampridine is used by some people with MS or SCI. Although we expect this use to decrease substantially if Fampridine-SR is approved, it is possible that some people will continue to use this formulation of fampridine. Several companies are engaged in developing products that include novel immunomodulatorimmune system approaches and cell transplant approaches to remyelination for the treatment of people with MS. These
programs are in early stages of development and may compete with Fampridine-SR or our preclinical candidates in the future.
ThereOur lead product candidate, Fampridine-SR, is the first product to our knowledge that acts to improve neurological function in subjects with MS. We are also numerous drugs usednot aware of other companies in clinical development with products that specifically address walking ability in subjects with MS. As a result of its focus on improving function, we believe that Fampridine-SR may be complementary to treat epilepsyboth the relapse management and bipolar disorderdisease course management therapies that are commercially available. Nonetheless, Fampridine-SR will compete for market acceptance with which our valrocemide product as well as other products we develop in collaborationthese current treatments because they have been accepted and regularly prescribed to people with Teva may compete.MS by physicians.
Government Regulation
FDA Regulation and Product Approval
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the preclinical testing, clinical development, manufacture, distribution and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising, sale, promotion, import and promotionexport of our products and product candidates.
In the United States, Zanaflex tablets, Zanaflex Capsules, and some of our product candidates.candidates are regulated by the FDA as drugs. Other of our product candidates are potentially regulated both as drugs and as biological products. Drugs are subject to the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations of the FDA, as well as to other federal, state, and local statutes and regulations. Biologics are also regulated under the Public Health Service Act, as amended. Violations of regulatory requirements at any stage may result in various adverse consequences, including FDA's and other health authorities' delay in approving or refusal to approve a product. Violations of regulatory requirements also may result in enforcement actions, including withdrawal of approval, labeling restrictions, seizure of products, fines, injunctions and/or civil or criminal penalties.
The process required by the FDA under these laws before our product candidates may be marketed in the United States generally involves the following:
55
The testingresearch, development and approval process requires substantial time, effort, and financial resources and we cannot be certain that any approval will be granted on a timely or commercially viable basis, if at all.
Preclinical studies include laboratory evaluation of the product candidate, its chemistry, formulation and stability, as well as animal studies to assess its potential safety and efficacy. We then submit the results of the preclinical studies, together with manufacturing information, and analytical data and any available clinical data or literature to the FDA as part of an IND application, which must
become effective before we may begin human clinical trials. The IND automatically becomes effective 30 days after the FDA acknowledges that the filing is complete, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the preclinical trials or the design of the proposed clinical trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. Further, an independent Institutional Review Board charged with protecting the welfare of human subjects involved in research at each medical center proposing to conduct the clinical trials must review and approve any clinical study.trial and study subjects must provide informed consent for their participation in the research.
Human clinical trials are typically conducted in three sequential phases which may overlap:
In the case of product candidates for severe or life-threatening diseases such as MS, the initial human testing is often conducted in affected subjects rather than in healthy volunteers. Since these subjects already have the target condition, these clinical trials may provide initial evidence of efficacy traditionally obtained in Phase 2 clinical trials and thus these clinical trials are frequently referred to as Phase 1b clinical trials.
The orphan designation processBefore proceeding with a study, sponsors may seek a written agreement from the FDA regarding the design, size, and conduct of a clinical trial. This is known as an SPA. Three types of studies are eligible for SPAs: (1) animal carcinogenicity studies, (2) final product stability studies, and (3) clinical studies for pivotal trials whose data will form the mechanismprimary basis to establish a product's efficacy. Where the FDA agrees to an SPA, the agreement may not be changed by which sponsorseither the sponsor or the FDA except if the sponsor and the FDA agree to a change, or an appropriately senior FDA official determines that a substantial scientific issue essential to determining the safety or effectiveness of drugsthe product was identified after the testing began. SPAs thus help establish up front agreement with the FDA about the adequacy of the design of a clinical trial to support a regulatory approval, but the agreement is not binding if new circumstances arise. In addition, even if an SPA remains in place and biologicsthe trial meets its endpoints with statistical significance, the FDA could determine that the overall balance of risks and benefits for rare diseases qualifythe product candidate is not adequate to support approval, or only justifies approval for a tax creditnarrow set of uses or approval with restricted distribution or other burdensome post-approval requirements or limitations. There is thus no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA.
U.S. law requires that studies conducted to support approval for product marketing be "adequate and seven-year marketing exclusivity incentives of the Orphan Drug Act. Orphan Drug designations are specific towell controlled." In general, this means that either a placebo or a product and its FDA-approved indication. Market exclusivity begins at the time of FDA approval of a productalready approved for its designated use. We have received Orphan Drug designation for Fampridine-SR for both the treatment of incomplete SCI and MS.the disease or condition under study must be used as a reference control. Studies must also be conducted in compliance with good clinical practice, or GCP, requirements.
We cannot be certain that we will successfully complete Phase 1, Phase 2 or Phase 3 testing of our product candidates within any specific time period, if at all. Furthermore, the FDA or the Institutional Review Boards or the sponsor may suspendprevent clinical trials from beginning or may place clinical trials on hold or terminate them at any time on various grounds, including a findingpoint in this process if, among other reasons, they conclude that thestudy subjects are being exposed to an unacceptable health risk.
TheIn the U.S., the results of product development, preclinical studies and clinical trials aremust be submitted to the FDA as part of a new drug application for review and approval of theprior to marketing and commercial shipment of the product candidate. If the product is regulated as a drug, a New Drug Application, or NDA, must be submitted and approved before commercial marketing may begin. If the product, such as an antibody, is regulated as a biologic, a Biologic License Application, or BLA, must be submitted and approved before commercial marketing may begin. The NDA or BLA must include a substantial amount of data and other information concerning the safety and effectiveness (and, in the case of a biologic, purity and potency) of the compound from laboratory, animal and clinical testing, as well as data and information on manufacturing, product stability, and proposed product labeling.
Each domestic and foreign manufacturing establishment, including any contract manufacturers we may decide to use, must be listed in the NDA or BLA and must be registered with the FDA. The application will generally not be approved until the FDA conducts a manufacturing inspection, approves the applicable manufacturing process for the drug or biological product, and determines that the facility is in compliance with current good manufacturing practice, or cGMP, requirements. If the manufacturing facilities and processes fail to pass the FDA inspection, we will not receive approval to market these products.
Under the Prescription Drug User Fee Act, as amended, the FDA receives fees for reviewing a BLA or NDA and supplements thereto, as well as annual fees for commercial manufacturing establishments and for approved products. These fees can be significant. The NDA or BLA review fee alone can exceed $700,000, although certain limited deferrals, waivers and reductions may be available.
Under applicable laws and FDA regulations, each NDA or BLA submitted for FDA approval is usually reviewed for administrative completeness and reviewability within 45 to 60 days following submission of the application. If deemed complete, the FDA will "file" the NDA or BLA, thereby triggering substantive review of the application. The FDA can refuse to file any NDA or BLA that it deems incomplete or not properly reviewable. If the FDA refuses to file an application, the FDA will retain 25% of the user fee as a penalty. The FDA has established performance goals for the review of NDAs and BLAs—six months for priority applications and 10 months for regular applications. However, the FDA is not legally required to complete its review within these periods and these performance goals may change over time. Moreover, the outcome of the review, even if generally favorable, typically is not an actual approval but an "action letter" that describes additional work that must be done before the application can be approved. The FDA's review of an application may involve review and recommendations by an independent FDA advisory committee.
The FDA may deny a new drug applicationan NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical data. Even if such data is submitted, the FDA may ultimately decide that the new drug applicationNDA or BLA does not satisfy the criteria for approval. If the FDA approves a product, it may limit the approved therapeutic uses for the product as described in the product labeling, require that contraindications, warning statements or precautions be included in the product labeling, require that additional studies be conducted following approval as a condition of the approval, impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval or post-approval, or limit labeling. Once issued, the FDA may withdraw product approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the effect
56
of approved products which have been commercialized, and the agency has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
Satisfaction of the above FDA requirements or similar requirements of state, local and foreign regulatory agencies typically takes several years or more and the actual time required may vary substantially, based upon the type, complexity and novelty of the pharmaceutical product candidate. Government regulation may delay or prevent marketing of potential products for a considerable period
of time or permanently and to impose costly procedures upon our activities. We cannot be certain that the FDA or any other regulatory agency will grant approval for any of our product candidates on a timely basis, or on a commercially viable basis, if at all. Success in preclinical or early stage clinical trials does not assure success in later stage clinical trials. Data obtained from preclinical and clinical activities is not always conclusive and may be susceptible to varying interpretations which could delay, limit or prevent regulatory approval. Even if a product candidate receives regulatory approval, the approval may be significantly limited to specific indications. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain regulatory approvals would have a material adverse effect on our business. Marketing our product candidates abroad will require similar regulatory approvals and is subject to similar risks. In addition, we cannot predict what adverse governmental regulations may arise from future United StatesU.S. or foreign governmental action.
Any products manufactured or distributed by us pursuant to FDA clearances or approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements, and reporting of adverse experiences with the drug.drug, other reporting, advertising and promotion restrictions. The FDA's rules for advertising and promotion require in particular that we not promote our products for unapproved uses, and that our promotion be fairly balanced and adequately substantiated. We must also submit appropriate new and supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with current Good Manufacturing Practices, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. We cannot be certain that we or our present or future suppliers will be able to comply with the current Good Manufacturing Practices and other FDA regulatory requirements.
The FDA regulates drug labelingalso enforces the requirements of the Prescription Drug Marketing Act, or PDMA, which, among other things, imposes various requirements in connection with the distribution of product samples to physicians.
In addition to inspections related to manufacturing, we are subject to periodic unannounced inspections by the FDA and promotion activities. Theother regulatory bodies related to the other regulatory requirements that apply to drugs manufactured or distributed by us. For example, the FDA may conduct periodic inspections regarding our reporting of adverse events, and the FDA has actively enforced regulations prohibitingindicated to the marketingindustry that it may be conducting increased inspections related to compliance with the requirements of products for unapproved uses. Under the Modernization ActPDMA concerning the handling of 1997,drug samples. When the FDA conducts an inspection, it will permitidentify any deficiencies it believes exist in the promotionform of a drug for an unapproved usenotice of inspectional observations, or Form FDA 483. The observations may be more or less significant. If we receive a notice of inspectional observations, we likely will be required to respond in certain circumstances, but subjectwriting, and may be required to very stringent requirements.undertake corrective and preventive actions in order to address the FDA's concerns.
We and our product candidates are also subject to a variety of state laws and regulations in those states or localities where they are or will be marketed. Any applicable state or local regulations may hinder our ability to market our product candidates in those states or localities.
The FDA's policies may change and additional government regulations may be enacted which could prevent or delay regulatory approval of our potential products. Moreover, increased attention to the containment of health care costs in the United States and in foreign markets could result in new government regulations which could have a material adverse effect on our business. We cannot predict the likelihood, nature or extent of adverse governmental regulation which might arise from future legislative or administrative action, either in the United States or abroad.
Orphan Drugs
Under the Orphan Drug Act, special incentives exist for sponsors to develop products for rare diseases or conditions, which are defined to include those diseases or conditions that affect fewer than 200,000 people in the U.S. Sponsors may request that FDA grant a drug orphan designation prior to approval. We have received Orphan Drug designation for Fampridine-SR for the treatment of both MS and incomplete SCI.
Products designated as orphan drugs are eligible for special grant funding for research and development, FDA assistance with the review of clinical trial protocols, potential tax credits for research, reduced filing fees for marketing applications, and a special seven-year period of market exclusivity after marketing approval. Orphan drug exclusivity prevents FDA approval of applications by others for the same drug and the designated orphan disease or condition. FDA may approve a subsequent application from another person if FDA determines that the application is for a different drug or different use, or if FDA determines that the subsequent product is clinically superior, or that the holder of the initial orphan drug approval cannot assure the availability of sufficient quantities of the drug to meet the public's need. In addition, even when a drug has orphan exclusivity, the FDA may approve a competing drug for the same orphan use. The FDA may also approve someone else's application for the same drug that has orphan exclusivity, but for a different use, in which case the competing drug could be prescribed by physicians outside its FDA approval for the orphan use, notwithstanding the existence of orphan exclusivity. A grant of an orphan designation is not a guarantee that a product will be approved. If a sponsor receives orphan drug exclusivity upon approval, there can be no assurance that the exclusivity will prevent another person from receiving approval for the same or a similar drug for the same or other uses.
Generic Drugs, AB Ratings and Pharmacy Substitution
Generic drugs are approved through an abbreviated process, which differs in important ways from the process followed for innovative products. Generally an abbreviated new drug application, or ANDA, is filed with the FDA. The ANDA must seek approval of a drug product that has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use (labeling) as a so-called "reference listed drug" approved under an NDA with full supporting data to establish safety and effectiveness. Only limited exceptions exist to this ANDA sameness requirement, including certain limited variations approved by the FDA through a special suitability petition process. The ANDA also generally contains clinical data to demonstrate that the product covered by the ANDA is absorbed in the body at the same rate and to the same extent as the reference listed drug. This is known as bioequivalence. In addition, the ANDA must contain information regarding the manufacturing processes and facilities that will be used to ensure product quality, and must contain certifications to patents listed with the FDA for the reference listed drug.
Every state has a law permitting or requiring pharmacists to substitute generic equivalents for brand-name prescriptions unless the physician has prohibited substitution. Managed care organizations often urge physicians to prescribe drugs with generic equivalents, and to authorize substitution, as a means of controlling costs. They also may require lower copayments as an incentive to patients to ask for and accept generics.
While the question of substitutability is one of state law, most states look to the FDA to determine whether a generic is substitutable. FDA lists therapeutic equivalence ratings in a publication often referred to as the Orange Book. In general, a generic drug that is listed in the Orange Book as therapeutically equivalent to the branded product will be substitutable under state law and, conversely, a generic drug that is not so listed will not be substitutable. To be considered therapeutically equivalent, a generic drug must first be a pharmaceutical equivalent of the branded drug. This means that the generic has the same active ingredient, dosage form, strength or concentration and route of
administration as the brand-name drug. Tablets and capsules are presently considered different dosage forms that are pharmaceutical alternatives and not substitutable pharmaceutical equivalents.
In addition to being pharmaceutical equivalents, therapeutic equivalents must be bioequivalent to their branded counterparts. Bioequivalence for this purpose is defined in the same manner as for ANDA approvals, and usually requires a showing of comparable rate and extent of absorption in a small human study.
Solid oral dosage form drug products generally are rated AB in the Orange Book if they are considered therapeutic equivalents. If bioequivalence has been adequately demonstrated, the products will be rated "AB."
Foreign Regulation and Product Approval
Outside the United States, our ability to market a product candidate is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Community, or EC, registration procedures are available to companies wishing to market a product in more than one EC member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. This foreign regulatory approval process involves all of the risks associated with FDA clearance discussed above.
57
Other Regulations
In the U.S., the research, manufacturing, distribution, sale, and promotion of drug and biological products are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration), other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-kickback and fraud and abuse provisions of the Social Security Act, as amended, the False Claims Act, also as amended, the privacy provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Reimbursement and Pricing Controls
In many of the markets where we or our collaborative partners would commercialize a product following regulatory approval, the prices of pharmaceutical products are subject to direct price controls (by law) and to drug reimbursement programs with varying price control mechanisms.
In the United States, there has been an increased focus on drug pricing in recent years. Although there are currently no direct government price controls over private sector purchases in the United States, federal legislation requires pharmaceutical manufacturers to pay prescribed rebates on certain drugs to enable them to be eligible for reimbursement under certain public health care programs such
as Medicaid. Various states have adopted further mechanisms under Medicaid and otherwise that seek to control drug prices, including by disfavoring certain higher priced drugs and by seeking supplemental rebates from manufacturers. Managed care has also become a potent force in the market place that increases downward pressure on the prices of pharmaceutical products. Federal legislation, enacted in December 2003, has altered the way in which physician-administered drugs covered by Medicare are reimbursed. Under the new reimbursement methodology, physicians are reimbursed based on a product's "average sales price," or ASP. This new reimbursement methodology has generally led to lower reimbursement levels. The new federal legislation also has added an outpatient prescription drug benefit to Medicare, effective January 2006. In the interim, Congress has established a discount drug card program for Medicare beneficiaries. Both benefits will be provided primarily through private entities, which will attempt to negotiate price concessions from pharmaceutical manufacturers.
Public and private health care payors control costs and influence drug pricing through a variety of mechanisms, including through negotiating discounts with the manufacturers and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Payors also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. In particular, many public and private health care payors limit reimbursement and coverage to the uses of a drug that are either approved by the FDA or that are supported by other appropriate evidence (for example, published medical literature) and appear in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence, whether or not such uses have been approved by the FDA. For example, in the case of Medicare coverage for physician-administered oncology drugs, the Omnibus Budget Reconciliation Act of 1993, or OBRA '93, with certain exceptions, prohibits Medicare carriers from refusing to cover unapproved uses of an FDA-approved drug if the unapproved use is supported by one or more citations in the American Hospital Formulary Service Drug Information, the American Medical Association Drug Evaluations, or the U.S. Pharmacopoeia Drug Information. Another commonly cited compendium, for example under Medicaid, is the DRUGDEX Information System.
Different pricing and reimbursement schemes exist in other countries. For example, in the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from member state to member state. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products, as exemplified by the National Institute for Clinical Excellence in the UK which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert a commercial pressure on pricing within a country.
Employees
As of July 31, 2003,January 3, 2006, we had 6867 employees. Of the 6867 employees, 2724 perform scientificresearch and researchdevelopment activities, including both preclinical programs and 15 hold advanced degrees.clinical trials, 29 work in sales, marketing, business development and communications and 14 perform general and administrative tasks.
Facilities
Our principal executive offices are located in an approximately 30,000 square foot facility in Hawthorne, NY, which houses offices and laboratory space. The current annual rent for this facility is $642,000. We believe that our facility is currently adequate for our purposes and that it will continue to be so for the foreseeable future. The lease for this facility expires in January 2008.
Legal Proceedings
We are not currently a party to any material legal proceedings.
58
Executive Officers and Directors
The following table sets forth, as of January 11, 2006, information about our directorsexecutive officers and executive officers.directors.
| Name | Age | |||
|---|---|---|---|---|
| Ron Cohen, M.D. | President, Chief Executive Officer and Director | |||
| Andrew R. Blight, Ph.D. | ||||
| Mary Fisher | ||||
| David Lawrence, M.B.A. | ||||
| 49 | ||||
| John | Director | |||
| Sandra Panem, Ph.D.(1) | Director | |||
| Barclay A. Phillips(2) | 43 | Director | ||
| Mark R.E. Pinney, M.B.A., C.F.A., M.S. | 51 | Director | ||
| Lorin J. Randall(2) | 62 | Director | ||
| Steven M. Rauscher(1) | 52 | Director | ||
| Michael Steinmetz, Ph.D.(2) | Director | |||
| Wise Young, Ph.D., M.D.(1) | Director |
Ron Cohen, M.D.,has served as our President and Chief Executive Officer and a director since he founded Acorda in 1995. Dr. Cohen previously was a principal in the startup of Advanced Tissue Sciences, Inc., a biotechnology company engaged in the growth of human organ tissues for transplantation uses. Dr. Cohen serves as the Chairman of the Board of the New York Biotechnology Association and on the Board of Directors of Ceregene, Inc. He also serves on the Advisory Editorial Board of BioPeople magazine, and as a member of the Columbia-Presbyterian Health Sciences Council. Dr. Cohen received his B.A. degree with honors in Psychology from Princeton University, and his M.D. from the Columbia College of Physicians & Surgeons. He completed a residency in Internal Medicine at the University of Virginia Medical Center, and is Board Certified in Internal Medicine. Dr. Cohen serves on the Board of Directors of Zymenex A/S, a Danish pharmaceutical company, and on the Emerging Company Section of the Board of the Biotechnology Industry Organization (BIO). He is Chairman Emeritus and a Director of the Board of the New York Biotechnology Association and also serves as on the Scientific Advisory Board of the Daniel Heumann Fund and as a member of the Columbia-Presbyterian Health Sciences Advisory Council.
Andrew R. Blight, Ph.D., has been our Chief Scientific Officer since January 2004 and previously served as our Executive Vice President, Research and Development sincefrom 2000 to 2004, and was Vice President, Research and Development, from 1998 to 2000. Prior to joining Acorda, Dr. Blight spent approximately 6six years as Professor and Director of the Neurosurgery Research Laboratory at the University of North Carolina at Chapel Hill. Dr. Blight held prior academic positions at Purdue University and New York University. Dr. Blight is a leader in SCI pathophysiology research and has made several important contributions to the field, particularly on the role of demyelination in SCI. He also pioneered the therapeutic application of 4-AP in SCI animal models and in human clinical trials. Dr. Blight is a member of the editorial board of the Journal of Neurotrauma and has served as a member of the NIH NSDA review committee. He was previously Secretary, Treasurer and Vice President of the National Neurotrauma Society. Dr. Blight received his B.S. in Zoology and his Ph.D. in Zoology/Neurobiology from the University of Bristol, U.K.
Mary Fisher has been our Chief Operating Officer since January 2005 and previously served as our Vice President, Commercial Operations since Septemberfrom 2003 through 2004 and was Vice President, Marketing and Strategic Planning sincefrom 2000 to 2003. From 1999 to 2000, Ms. Fisher was an independent consultant to various pharmaceutical companies. From 1994 to 1999, MsMs. Fisher was Vice President, Strategic Healthcare and Commercial Operations for Cephalon, Inc. In that capacity she had responsibilitywas responsible for the company's corporate sales, managed care marketing, pricing, reimbursement, health economics, patient support programs, product planning, commercial manufacturing, distribution and customer service. From 1990 until joining Cephalon, Ms. Fisher was Corporate Communications Manager for Immunex
59
Corporation. Previously, she spent nine years in a variety of line and staff positions, including production planning, purchasing, accounting, and public affairs at Boehringer Ingelheim Pharmaceuticals, Inc. Ms. Fisher currently serves as a director of PharmaMetrics GmbH.
Elliott A. Gruskin, Ph.D. has served as our Vice President, Research & Development since 2001. From November 1990 until joining Acorda, Dr. Gruskin served as Senior Director of the Life Sciences Division of United States Surgical Corporation where he was responsible for all operations including strategic planning, budgets, corporate development, contracts, external research agreements, intellectual property, research, development and transfer of products to manufacturing. Dr. Gruskin was responsible for two approved wound healing products, has been issued 8 U.S. patents and has published scientific papers in DNA repair enzymology biomaterials, wound healing and tissue engineering. Dr. Gruskin received his B.S. Degree in Biochemistry from the University of Rochester and his Ph.D. in Biochemistry from Vanderbilt University as a Harold Sterling Vanderbilt Scholar and completed his postdoctoral training at MIT in Biophysics.
Mitchell Katz, Ph.D. has served as our Vice President, Clinical Programs since 2000. Prior to joining Acorda, he served as the Director of Clinical Operations at SCP Communications from 1998 to 2000. He also supported clinical and preclinical operations at several start-up biotechnology companies, and held management positions at R.W. Johnson Pharmaceutical Research Institute, and Schering-Plough. In these prior positions, he participated in four successful NDA applications. He received a B.A. in Biology from CUNY Brooklyn and a Ph.D. in microbiology from Rutgers University Graduate School and served as a postdoctoral research follow in the Department of Microbiology and Immunology at Downstate Medical Center, Brooklyn, New York. Dr. Katz has also published scientific papers in numerous peer-review journals.
David Lawrence, M.B.A. joined us, has been our Chief Financial Officer since January 2005. He previously served as our Vice President, Finance in 1999.from January 2001 through 2004, and Director, Finance from 1999 to 2001. From 1991 to 1999, Mr. Lawrence held several positions for Tel-Air Communications, Inc. including Vice President and Controller. Prior to Tel-Air, he held financial management positions of Controller and Finance Manager for Southwestern Bell and Metromedia Telecommunications respectively. Mr. Lawrence received his undergraduate degree in Accounting from Roger Williams College, in Rhode Island, and an M.B.A in Finance from Iona College in New York.College. Mr. Lawrence is a founding member and currently serves on the Board of Directors as Treasurer of The Brian Ahearn Children's Fund.
King Lee, Ph.D. R.A.C.Jane Wasman, J.D. joined us in August 2003 as, has been our Executive Vice President, of Regulatory Affairs.General Counsel and Corporate Secretary since May 2004. From 20011995 to 2003, Dr. Lee was Executive Director of Regulatory Affairs/Quality Assurance/Quality Control at ATON Pharma, Inc. From 1996 to 2001, Dr. Lee was with VION Pharmaceuticals, Inc., where he had2004, Ms. Wasman held several management positions, most recently the Senior Director in Regulatory Affairs/Quality Assurance. Prior to VION, Dr. Lee heldvarious leadership positions at IBEX, Sterling-Winthrop Research Institute,Schering-Plough Corporation, including Staff Vice President and Wyeth Pharmaceuticals. Dr. Lee is a member ofAssociate General Counsel responsible for legal support for U.S. Pharmaceuticals operations, including sales, marketing and compliance; FDA regulatory matters; global research and development; and, corporate licensing and business development. She served as Staff Vice President, International in 2001 and as Staff Vice President, European Operations—Legal from 1998 to 2000. Previously, Ms. Wasman specialized in litigation at Fried, Frank, Harris, Shriver & Jacobson. She also served as Associate General Counsel to the Regulatory Affairs Professional SocietyU.S. Senate Committee on Veteran's Affairs. Ms. Wasman graduated Magna Cum Laude from Princeton University and has authored over 40 publications. Dr. Lee received his Ph.D. in Pharmacologyearned her J.D. from the College of Medicine of the University of Kentucky and was certified by the Regulatory Affairs Professionals Board in 1994.Harvard Law School.
Mark R. E. Pinney, M.B.A., C.F.A., M.S.Standish M. Fleming has served as our Chief Financial Officer since 2001 and has been a member of theour Board of Directors since 2004. He is a 19-year veteran of life sciences venture capital investing. Mr. Fleming co-founded Forward Ventures in 1993. Before establishing Forward Ventures II in 1993, Mr. Fleming served as start-up chairman, president and CEO of GeneSys Therapeutics (now part of Cell Genesys). He has served as founding director and acting president of Triangle Pharmaceuticals (now part of Gilead Sciences, Inc.), CombiChem (now part of Bristol-Myers Squibb) and Corixa and GenQuest (now both part of GlaxoSmithKline). Mr. Fleming was a founding board member of directors since the foundingCiphergen Biosystems and Gryphon Sciences. He is a former president of the companyBiotechnology Venture Investors Group. Mr. Fleming began his venture career with Ventana Growth Funds in 1995. Prior to joining Acorda,San Diego in 1986. Mr. Fleming earned his B.A. from 2000 to 2001,Amherst College and his M.B.A. from the UCLA Graduate School of Management. Mr. Pinney was ChairmanFleming has served on the boards of CanDo, Inc., an Internet company that offered product19 venture-backed companies and service solutions to people with disabilities. In 1998, he co-founded and was Chief Executive Officer of LifeWire, Inc., a company developing community-based destination web sites for the disability population. LifeWire merged with CanDo in 2000. Mr. Pinneyis also co-founded Real Media, Inc., an Internet advertising software and services firm, in 1996, and participated in the start-up of Acorda. From 1984 to 1988, he was Vice President, Corporate Finance for Merrill Lynch Capital Markets and from 1988 to 1992, he was Vice President, Private Transactions at Dillon Read & Co., Inc. Mr. Pinney serves as a Trustee of the Christopher Reeve Paralysis Foundation and currently serves as a director of Healthlink Systems, Inc. He received an undergraduate degree in engineering at the University of Exeter, England, an M.B.A. from the University of Chicago Graduate School of Business,Ambit and Sanarus Medical, and a masters degree in engineering from Columbia University. He is a Chartered Financial Analyst.
60
Harold Safferstein, Ph.D., J.D. has served as our Vice President, Business Development since 2001. From 1997 to 2001, Dr. Safferstein spent approximately 5 years at Bristol-Myers Squibb, most recently as Director, Lifecycle Management, Business Developmentfounding director of Arizeke Pharmaceuticals and Strategic Planning for the Diabetes Franchise. Prior to joining Bristol-Myers Squibb, Dr. Safferstein was Director, Technology Transfer at the National Heart, Lung and Blood Institute, Cooperative Venture Manager for the National Institute of Allergy and Infectious Diseases and Chair, PHS Technology Development Coordinators Committee at the National Institutes of Health. Dr. Safferstein was a NIH Post-Doctoral Fellow and Fellow of the Multiple Sclerosis Society. Dr. Safferstein received a B.A. in biochemistry from Rutgers University, a Ph.D. in neurobiology from the University of Louisville, a J.D. from The American University, Washington College of Law and an M.B.A. from Columbia University.Nereus Pharmaceuticals.
John Friedman has served asbeen a directormember of Acordaour Board of Directors since May 2003. Mr. Friedman is the founder and principalManaging Partner of Easton Hunt Capital Partners, whichL.P., a private investment firm that he founded in 1998. Prior to founding Easton Hunt Capital Partners he was managing director1999. Since 1991, Mr. Friedman has also been the President of Easton Capital Corp., a private investment firm. He also helped manage Atrium Capital Corporation, an investment firm, from 19931991 to 1998, which he also founded.1993. From 1989 to 1991, Mr. Friedman was also the founder and Managing General Partner of Security Pacific Capital Investors, a $200-million private equity fund geared towards expansion financings and recapitalizations.investment firm. Prior to joining Security Pacific, he spent more than eight yearsMr. Friedman was a Managing Director and Partner at E.M. Warburg, Pincus & Co., Inc., serving last as a Managing Director and Partner. Before joining Warburg,where he was employed from 1981 to 1989. From 1978 to 1980, Mr. Friedman was an attorney with Sullivan & Cromwell LLP and Cromwell LLP. He holdsduring 1980 he was employed at Shearson Loeb Rhoades. Mr. Friedman received a B.A. in History from Yale
College and a J.D. degree from Yale Law School andSchool. Mr. Friedman is a B.A. degree from Yale College. He currently serves onmember of the boardsboard of directors of Transave, Inc., Renovis, Inc., Conor Med Systems, Comverse Technology, Inc., Trellis Bioscience, Assistive Technology,a telecommunications equipment company, YM BioSciences, Inc., and ModelWire,a biotechnology company, Renovis, a biotechnology company, Conor Medsystems, Inc., anda drug delivery technology company, as well as several private companies. Mr. Friedman is onalso co-chairman of the President's Council atof the Cold Spring Harbor Laboratory.
Sandra Panem, Ph.D., has served asbeen a directormember of Acordaour Board of Directors since 1998. She is currently a partner at Cross Atlantic Partners, which she joined in 2000. From 1994 to 1999, Dr. Panem was President of Vector Fund Management, the then asset management affiliate of Vector Securities International. Prior thereto, Dr. Panem served as Vice President and Portfolio Manager for the Oppenheimer Global BioTech Fund, a mutual fund that invested in public and private biotechnology companies. Previously, she was Vice President at Salomon Brothers Venture Capital, a fund focused on early and later-stage life sciences and technology investments. Dr. Panem was also a Science and Public Policy Fellow in economic studies at the Brookings Institution, and an Assistant Professor of Pathology at the University of Chicago. She received a B.S. in biochemistry and Ph.D. in microbiology from the University of Chicago. Dr. Panem currently serves on the boards of directors of Martek Biosciences Corp., Bioject Medical Technologies, Inc., AirLogix,Labcyte, Inc. and Confluent Surgical, Inc.
Michael Steinmetz, Ph.D.Barclay A. Phillips joinedhas been a member of our boardBoard of directorsDirectors since September 2004. Mr. Phillips has been a Managing Director of Vector Fund Management, a venture capital firm focused on investments in 1998. Dr. Michael Steinmetzthe life sciences and healthcare industry, since 1999. From 1991 to 1999, Mr. Phillips served in various roles including Director of Private Placements and Biotechnology Analyst for INVESCO Funds Group, Inc. From 1985 to 1990, Mr. Phillips held positions in sales and trading with Paine Webber, Inc. and Shearson Lehman Hutton, Inc. Over the last twelve years, Mr. Phillips has served on the boards of a number of private companies and currently serves as a Director of CancerVax Corp. Mr. Phillips received a B.A. in economics from the University of Colorado.
Mark R. E. Pinney, M.B.A., C.F.A., M.S., joined our Board of Directors at our founding. He was also our Chief Financial Officer from 2001 to 2004. Since 2004, he has served as Chief Financial Officer and Chief Privacy Officer of Tacoda Systems, Inc. From 2000 to 2001, Mr. Pinney was Chairman of CanDo, Inc., an Internet company that offered product and service solutions to people with disabilities. In 1998, he co-founded and was Chief Executive Officer of LifeWire, Inc., a company developing community-based destination web sites for the disability population. LifeWire merged with CanDo in 2000. Mr. Pinney also co-founded Real Media, Inc., an Internet advertising software and services firm, in 1996. From 1984 to 1988, he was Vice President, Corporate Finance for Merrill Lynch Capital Markets and from 1988 to 1992, he was Vice President, Private Transactions at Dillon Read & Co., Inc. Mr. Pinney also serves on the Advisory Board of United Spinal Association. He received an undergraduate degree in engineering at the University of Exeter, England, an M.B.A. from the University of Chicago Graduate School of Business and a masters degree in engineering from Columbia University. He is a Chartered Financial Analyst.
Lorin J. Randallhas been a member of our Board of Directors since January 2006. Mr. Randall has been Senior Vice President and Chief Financial Officer of Eximias Pharmaceutical Corporation, a development-stage drug development company since 2004. From 2002 to 2004, Mr. Randall served as Senior Vice President and Chief Financial Officer of i-STAT Corporation, a publicly-traded manufacturer of medical diagnostic devices which was acquired by Abbott Laboratories in 2004. From 1995 to 2001, Mr. Randall was Vice President and Chief Financial Officer of CFM Technologies, Inc. a publicly-traded manufacturer of semiconductor manufacturing equipment. Mr. Randall previously served on the board of Quad Systems Corporation, a publicly-traded manufacturer of electronics manufacturing equipment where he served as Chairman of the Audit Committee. Mr. Randall received a B.S. in accounting from The Pennsylvania State University and an M.B.A. from Northeastern University.
Steven M. Rauscher has served on our Board of Directors since 2005. He is President and CEO of Oscient Pharmaceuticals Corporation, a commercial stage biopharmaceutical company. He joined Oscient in 2000 having served as a member of the Board of Directors since 1993. Previously, Mr. Rauscher was CEO of AmericasDoctor, a company providing clinical research services to the pharmaceutical industry. Prior to AmericasDoctor, he held a number of leadership positions at Abbott Laboratories, including Vice President of Corporate Licensing, Vice President of Business Development, International Division and Vice President of Sales, U.S. Pharmaceuticals. Mr. Rauscher received a B.S. from Indiana University and an M.B.A. from the University of Chicago.
Michael Steinmetz, Ph.D., has been a member of our Board of Directors since 1999, when MPM invested in the company. Dr. Steinmetz is a Managing Director at Clarus Ventures LLC, a company he co-founded in 2005. Since 1999, Dr. Steinmetz has been a General Partner of MPM Capital, which he joined in 1997.MPM's BioVentures' I-III funds. Prior to MPM, he held positions at various academic institutions, including the California Institute of Technology and the Basel Institute for Immunology where he was a permanent member. In 1986, he joined Hoffmann-La Roche and held various leadership positions in R&D, initially in Switzerland and subsequently in the U.S.A.United States where, as Vice President of Preclinical Research and Development, he was responsible for Roche's drug discovery activities in the U.S.A.United States and Roche's global biotechnology efforts. Dr. Steinmetz was trained asreceived a chemistdegree in chemistry from the University of Hamburg, Germany and holds a Ph.D. from the University of Munich.Munich, Germany. He didhas done academic research in the areas of Biochemistry, Molecular Biology and Immunology and has published over 130 manuscripts in leading scientific journals. He is currently Chairman of the Board at BioXell SpA and the ISB Accelerator Corporation and a director of Amphora Discovery Corporation, atugen AG, Biovitrum AB, Cellular Genomics, Inc., Epigenomics AG, Intracel Resources, LLC, MacroGenics Inc., and TaiGen Bioscience Corporation.
Wise Young, Ph.D., M.D., has been a member of the board of directors and of our scientific advisory board since the founding of the company in 1995. Dr. Young has been at Rutger'sRutgers University since 1997, where he serves as Professor and Chair of the Department of Cell Biology and Neuroscience, Professor II and Director of the Neuroscience Center and founderFounding Director of the W.M. Keck Center for Neuroscience. Dr. Young is one of the preeminent scientists in the fields of spinal cord injury and neurotrauma, SCI animal models, and the pharmacological therapy of SCI. He was the Principal Investigator for the Multicenter Animal Spinal
61
Cord Injury Study, funded by the National Institutes of Health; is editor-in-chief ofCurrent Concepts in Critical Care and Trauma; and serves on numerous editorial boards, including those ofExperimental Neurology, Journal of Neurotrauma, Brain Research andStroke. Dr. Young has received the Wakeman Award for Research in Neurosciences, and a Jacob Javits Neuroscience Award from the National Institute of Neurological Disorder and Stroke. He is also a member of the Scientific Advisory Council of the American Paralysis Association and of the National Acute Spinal Cord Injury Study executive committee. Dr. Young received a B.A. in biology and chemistry from Reed College, a Ph.D. in physiology and biophysics from the University of Iowa and an M.D. from Stanford University.
Scientific Advisory Board
Our Scientific Advisory Board is comprised of the following individuals who are well recognized scientists and academic leaders in the SCI and MS fields, representing multiple areas of expertise, including neuropharmacology, cellular and molecular neurobiology, clinical neurology, SCI animal models, and spinal cord pathophysiology. They provide us with advance access to information and technology within the SCI and MS fields, as well as advise us on particular project needs, perform experiments and write grants that help to support in-house research.
Michael S. Beattie, Ph.D. holds the Brumbaugh Chair in Brain Research and Teaching, and is Professor of and Chair of the Department of Neuroscience at Ohio State University. In collaboration with Dr. Jacqueline Bresnahan, Dr. Beattie has contributed seminal work in the areas of SCI mechanisms and regeneration, most recently in elucidating the role of programmed cell death in SCI, and he is co-developer of the Beattie-Bresnahan-Basso, or BBB, scale that is now a standard for measurement of behavioral recovery in animals after SCI. He has served on the editorial board ofJournal of Neurotrauma, NIH study sections, and has chaired international symposia on neural transplantation and neurotrauma. Dr. Beattie received his B.S. in psychology from the University of California, and his M.A. and Ph.D. in neuropsychology from Ohio State University.
Jacqueline C. Bresnahan, Ph.D. is Professor of Neuroscience at Ohio State University. In collaboration with Dr. Michael Beattie, Dr. Bresnahan has contributed seminal work in the areas of SCI mechanisms and regeneration, most recently in elucidating the role of apoptosis in SCI, and she is co-developer of the BBB scale that is now a standard for measurement of behavioral recovery in animals after SCI. She is a past President of the Neurotrauma Society, serves on the editorial boards of theJournal of Neurotrauma and theNeurotrauma Society Newsletter, and is a member of the Scientific Advisory Council of the American Paralysis Association. Dr. Bresnahan received her B.A. in psychology and biology from Kent State University, and her M.A. and Ph.D. in physiological psychology from Ohio State University.
Mary B. Bunge, Ph.D. is Professor of Cell Biology and Anatomy, Neurological Surgery and Neurology at the University of Miami School of Medicine. Dr. Bunge's research focuses on the development and repair of neural tissue, particularly by the application of cultured Schwann cell grafts and various nerve growth factors. She has served on the editorial boards of theJournal of Cell Biology andJournal of Neurocytology. She is the first winner of the Mika Salpeter Women in Neuroscience Lifetime Achievement Award and the recipient of the 1996 Wakeman Award for her seminal contributions to the field of spinal cord injury repair. Dr. Bunge received her B.S. in biology from Simmons College, her M.S. in medical physiology and Ph.D. in zoology/cytology from the University of Wisconsin Medical School, and her Post-Doctoral Fellowship in developmental neurobiology at the Columbia College of Physicians and Surgeons.
Carl W. Cotman, Ph.D. is Professor of Psychobiology and Neurology at the University of California, Irvine. His research focus is on programmed cell death in the CNS, and on beta amyloid-associated neurotoxicity. Among other honors, Dr. Cotman has received the Bristol Myers Neuroscience Research Award, the Pattison Prize in Neuroscience, and the Camhi Research Award of the American Paralysis Association, or APA. He has published nearly 500 papers in the field of neuroscience and serves on several editorial boards, including theJournal of Neurochemical Research, Journal of Biological Chemistry andCentral Nervous
62
System Trauma. Dr. Cotman also is past Chairman of the Scientific Advisory Council of the APA, and continues to serve as a Scientific Advisor. He received his B.A. in chemistry from Wooster College, and his Ph.D. in biochemistry from Indiana University.
James W. Fawcett, Ph.D. is Merck Company Professor of Experimental Neurology, Cambridge University, and Chairman of the MRC Cambridge Centre for Brain Repair. He is a world authority on regeneration in the nervous system, has published extensively in the field of neural development and repair. He is also edited of a number of books, including the recentBrain Damage and Brain Repair.
Martin Grumet, Ph.D. is Professor of Cell Biology and Neuroscience in the Division of Life Sciences at Rutgers University and he is Director of the W.M. Keck Center for Neuroscience. He is the discoverer of the Ng-CAM protein, a chick homologue of L1. He is a leading researcher of cell adhesion molecules in the nervous system and their roles in CNS development and regeneration. Dr. Grumet received his B.Sc. in physics and biology from, respectively, The Cooper Union and New York University, his Ph.D. in biophysics at Johns Hopkins University, and did his Post-Doctoral Fellowship in developmental and molecular biology at The Rockefeller University.
Eugene Johnson, Jr., Ph.D. is Norman J. Stupp Professor of Neurology, and Professor of Molecular Biology and Pharmacology at Washington University School of Medicine, St. Louis. He is preeminent in the field of pharmacologic regulation of nerve growth factors, and in the mechanisms and prevention of programmed nerve cell death. Dr. Johnson has received both a Jacob Javits Neurosciences Investigator Award and a MERIT Award from the National Institutes of Health, and a Decade of the Brain Medal from the American Association of Neurological Surgeons. He serves as the Co-Director of the Washington University Alzheimer's Disease Research Center. His editorial board service includesNeuron, Journal of Neuroscience, Synapse andJournal of Neurotrauma. Dr. Johnson received his B.Sc. in pharmacy, and his Ph.D. in medicinal chemistry from the University of Maryland.
Mark D. Noble, Ph.D. is Professor of Genetics at the Center for Cancer Biology, University of Rochester Medical Center, Rochester, NY. Dr. Noble is a world leader in the areas of stem cell biology, CNS myelin repair, glial progenitor cells, and CNS regeneration. He is a recipient of the Jean Monnet Prize of the European Neurological Society, and serves on the editorial boards of, among others,Developmental Neuroscience, International Journal of Developmental Neuroscience andGlia. Dr. Noble received his B.A. in Biology and Philosophy from Franklin and Marshall College, and his Ph.D. in Genetics from Stanford University
Melitta Schachner, Ph.D. is Professor and Director of the Institute for Synthesis of Neural Structures at the University of Hamburg, in Germany. Dr. Schachner is the discoverer of the L1 protein, a promoter of axonal outgrowth, and the L2/HNK-1 carbohydrate, a critical motor neuron guidance factor. Her research focus is on cell adhesion molecules in the nervous system, and their role in nerve cell regeneration. She serves on the editorial boards of numerous scientific journals, including theJournal of Neurobiology, Brain Research, Molecular Brain Research, Journal of Neuroscience and theJournal of Neuroimmunology. Dr. Schachner received her undergraduate degree in biochemistry at the University of Tubingen, and her Ph.D. in biochemistry from the Max-Planck Institute in Munich.
Jerry Silver, Ph.D. is Professor of Neurosciences at Case Western Reserve University. Dr. Silver is a world authority on neuroglial cells, extracellular matrix and nerve regeneration, particularly in relation to spinal cord injury. He is associate editor ofExperimental Neurology, and serves on the editorial boards ofGlia, The Journal of Neurobiology, and Restorative Neurobiology andNeuroscience. He is a member of the Scientific Committee of the International Spinal Research Trust and the Daniel Heumann Fund. He has served as Chairman of the Workshop on New Developments in Spinal Cord Injury: Acute Interventions and Neural Grafts, sponsored by the U.S. Congress. Dr. Silver received his B.S. in biology from Cleveland State University, and his Ph.D. in anatomy from Case Western Reserve University.
63
Patrick A. Tresco, Ph.D. is Associate Professor of Bioengineering at the University of Utah. He has performed groundbreaking research in the development of cell encapsulation systems as sustained delivery devices for treatment for CNS disorders. Dr. Tresco holds 11 issued and pending patents relating to this and other bioengineering areas. Dr. Tresco is a regular peer reviewer forExperimental Neurology andBioengineering and Biotechnology. He received his B.A. in biology from Susquehanna University, his M.S. in pharmacology and toxicology from the University of Rhode Island, and his Ph.D. in medical sciences from Brown University.
Mark H. Tuszynski, M.D., Ph.D. is Professor of Neurosciences, Director of the Center for Neural Repair, and Attending Neurologist at the University of California, San Diego, or UCSD. Dr. Tuszynski has performed pioneering research in the regeneration of CNS axons by use of genetically modified cell grafts, notably in the areas of SCI and Alzheimer's disease. He holds a Physician Scientist Award from the University of Minnesota, and in 1995 was the first recipient of the Silvio O. Conte Neuroscience Research Award from the American Academy of Neurology. He serves as a reviewer for, among others, theJournal of Neuroscience, Experimental Neurology, Cell Transplantation, and theJournal of Neuroscience Methods. Dr. Tuszynski received his B.A. in biology and his M.D. from the University of Minnesota, and his Ph.D. in neurosciences from UCSD. He completed his neurology residency at Cornell University Medical Center.
Stephen G. Waxman, M.D., Ph.D. is Chairman of the Department of Neurology, Yale University School of Medicine, and Neurologist-in-Chief, Yale-New Haven Hospital. He also is founder and Director of the PVA/EPVA Neuroscience Research Center. Dr. Waxman is internationally recognized for elucidating the molecular architecture of nerve fibers and glial cells, and mechanisms of injury to nerve fibers in the spinal cord and brain. He has published over 400 scientific papers and has authored three books on neuroscience. He is editor ofThe Neuroscientist, associate editor of theJournal of Neurological Sciences andMuscle and Nerve, and serves on the editorial boards of numerous other journals. Dr. Waxman is a member of the Institute of Medicine of the National Academy of Sciences; and has served on the Advisory Boards of the American Paralysis Association and the Spinal Cord Research Foundation. A graduate of Harvard College, his honors include the Wartenburg Award of the American Academy of Neurology and the Distinguished Alumnus Award of the Albert Einstein College of Medicine, where he received his MD and Ph.D. degrees.
Wise Young, Ph.D., M.D. See "Executive Officers and Directors" above.
Board Composition
FollowingOur board of directors currently has ten members. Upon completion of this offering, our board of directors will consist of sixnine directors divided into three classes, with each class serving for a term of three years.years:
At each annual meeting of stockholders, the successors to directors whose terms will then expire will be elected for three-year terms. This classification of the board of directors may have the effect of delaying or preventing changes in control or management. See "Risk Factors—Certain provisions of Delaware law, our certificate of incorporation and our by-laws may delay or prevent an acquisition of us that stockholders may consider favorable or may prevent efforts by our stockholders to change our directors or our management, which could decrease the value of your shares."
We believe that a three-year term to succeedmajority of the directors whose terms are expiring. Mr. Pinney and Dr. Youngmembers of our Board of Directors will be Class Iindependent under the current independence requirements of the Nasdaq National Market and the Securities and Exchange Commission, or the SEC. The authorized number of directors whose termsmay be changed by resolution adopted by a majority of the board of directors.
Board Observation Rights
In connection with our revenue interests assignment agreement with PRF, we granted PRF the right to appoint a representative to receive all notices and materials provided to our board of directors and to attend as an observer all meetings of our board of directors, subject to certain exceptions. Mr. Gregory B. Brown, M.D. is the initial representative designated by PRF for such purpose. This right will expire in 2004; Drs. Panem and Steinmetz will be Class II directors whose terms will expire in 2005 and Dr. Cohen and Mr. Friedman will be Class III directors whose terms will expire in 2006.terminate on the earlier to occur of the fourth anniversary of the completion of an initial public offering of shares of our common stock or termination of our agreement with PRF.
Director Compensation
CommencingOur outside directors compensation policy provides that new outside directors on our board receive an initial grant of stock options in the amount of 0.2% of the fully diluted shares of our common stock, or a comparable adjusted number of stock appreciation rights or shares of restricted stock, with a fair market value exercise price and a three-year quarterly vesting schedule commencing on the date of this prospectus, eachthe award, unless they hold at least an equivalent amount of common stock through prior ownership. On an annual basis, at the discretion of the board of directors upon the recommendation of the compensation committee, outside directors can receive stock options in the amount of up to 0.02% of the fully diluted shares of our non-employee directors will receive non-qualifiedcommon stock, options to purchase 20,000or a comparable adjusted number of stock appreciation rights or shares at the initial public offeringof restricted stock, with a fair market value exercise price per share, which options will vest in 12 equaland a one-year quarterly installments commencing 180 days after the date of grant.
Commencing on the datevesting schedule. Upon consummation of this prospectus, eachoffering, this compensation policy will be extended to all of the outside directors on our directors will receive an annual option grant to purchase 10,000 shares on the dateboard of our annual stockholder meeting. In addition, directors will receive an annual grant of 5,000 options exercisable to purchase shares for each committee on which they serve.directors. Directors willare also be reimbursed for reasonable expenses incurred in attending meetings.related to their service on our board of directors.
64
Board Committees
TheOur board of directors has an audit committee, a compensation committee and a governancenominations committee.
Audit Committee
Our audit committee consists of Mr. Phillips, Mr. Fleming, Mr. Randall and Dr. Steinmetz. Mr. Phillips serves as chair of our audit committee. Our board of directors has determined that Mr. Fleming and Mr. Randall each qualify as an "audit committee financial expert" as that term is defined in Item 401(h) of Regulation S-K of the Securities Act. We believe that the composition of our audit committee meets, and the functioning of our audit committee will comply with, the applicable requirements of the Sarbanes-Oxley Act of 2002, the Nasdaq National Market and SEC rules and regulations.
Our audit committee is responsible for the following functions:for:
We have adopted a written audit committee charter that we will make available on our website.
Compensation Committee
Our compensation committee consists of Dr. Panem, Mr. Rauscher and Dr. Young. Dr. Panem serves as chair of our compensation committee. We believe that the composition of our compensation committee meets, and the functioning of our compensation committee will comply with, the applicable requirements of the Nasdaq National Market and SEC rules and regulations. Our compensation committee is responsible for the following functions:for:
We have adopted a written compensation committee charter that we will make available on our website.
Nominations Committee
Our nominations committee will consist of Mr. Friedman, Dr. Panem and Dr. Steinmetz. Mr. Friedman will serve as chair of the committee. The governancenominations committee iswill be responsible for identifying potential candidates to serve on our board. We have approved a written nominations committee charter that sets forth procedures for the consideration of director nominees and other related matters.
Code of Ethics
Our board of directors has adopted a code of ethics for all directors, officers and employees. We will make this code available on our website upon completion of this offering.
Compensation Committee Interlocks and Insider Participation
Our Compensation Committee is comprised of three members, Mr. John Friedman and Drs. Sandra Panem and Michael Steinmetz. No memberThe compensation of our Compensation Committee has at any time been an officer or employee of ours, orexecutive officers is currently determined by our subsidiary. No interlocking relationship exists between our board of directors or compensation committee, andas described above. None of our executive officers has served as a member of the board of directors or compensation committee of any other company, norentity that has any interlocking relationship existed in the past.one or more executive officers serving as a member of our board of directors or compensation committee.
Mr. Friedman, Dr. Panem and Dr. Steinmetz areis affiliated with Easton Hunt Capital Partners, Cross Atlantic Partners, and MPM/BB Bioventure group, respectively, each of which participated in the sale of our Series J preferred stock in a private placement consummated in May 2003, and MPM/BB Bioventure group also participated in our Series I preferred stock a private placement consummated in March 2001.2003. Pursuant to an amended and
restated registration rights agreement among us and certain of our stockholders, including entities affiliated with Mr. Friedman and Drs.Dr. Panem, and Steinmetz, the parties to the registration rights agreement have demand and piggy-back registration rights. See "Certain Relationships and Related Transactions."
65
Executive Compensation
The following summary compensation table sets forth the aggregate compensation awarded to, earned by or paid to the Chief Executive Officerfollowing individuals during the fiscal years ended December 31, 2005 and to 2004:
We refer to these individuals as our "named executive officers."
Summary Compensation TableSUMMARY COMPENSATION TABLE
| | Annual Compensation | Long-Term Compensation | | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name and Principal Position | Year | Salary | Bonus | Other Annual Compensation | Securities Underlying Options | All Other Compensation | ||||||||
| Ron Cohen, M.D. President and Chief Executive Officer | 2003 | $ | 285,000 | $ | 60,000 | — | — | — | ||||||
Andrew R. Blight, Ph.D. Executive Vice President, Research and Development | 2003 | $ | 179,500 | $ | 28,915 | — | — | — | ||||||
Mary Fisher Vice President, Marketing | 2003 | $ | 176,000 | $ | 28,263 | — | — | — | ||||||
Mitchell Katz, Ph.D. Vice President, Clinical Programs | 2003 | $ | 187,292 | $ | 20,161 | — | — | — | ||||||
Mark Pinney, M.B.A., C.F.A.(1) Chief Financial Officer | 2003 | $ | 191,250 | $ | 33,306 | — | — | — | ||||||
| Long-Term Compensation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual Compensation | Securities Underlying | ||||||||||||||
| Name and Principal Position | Year | Salary | Bonus(1) | Restricted Stock Award(2) | Options(3) | All Other Compensation | |||||||||
| Ron Cohen, M.D. President and Chief Executive Officer | 2005 2004 | $ | 305,000 305,000 | $ | — 120,000 | 260,385 | 51,265 — | — — | |||||||
Andrew R. Blight, Ph.D. Chief Scientific Officer | 2005 2004 | 215,000 210,000 | — 65,450 | 97,385 | 52,338 — | — — | |||||||||
Mary Fisher Chief Operating Officer(4) | 2005 2004 | 225,000 210,000 | — 74,000 | 157,231 | 132,323 — | $ | — 109,087 | ||||||||
Jane Wasman Executive VP & General Counsel(5) | 2005 2004 | 225,000 225,000 | — — | 77,615 | 44,769 — | $ | — 25,000 | ||||||||
David Lawrence M.B.A. Chief Financial Officer(6) | 2005 2004 | 180,000 164,800 | — 64,000 | 64,231 | 70,109 — | — — | |||||||||
Stock Options
Aggregate Exercise of Stock Options and Year-end Option Values
The following table contains information regarding the amountnumber of $91,250, which was paid in September 2003, was deferredshares of common stock subject to both exercisable and unexercisable stock options, as well as the value of unexercisable in-the-money options as of June 30, 2003.
Option Grants in Last Fiscal Year
During the fiscal year ended June 30, 2003 no stock option grants were made toDecember 31, 2005 for the named executive officers. NoThere was no public market for our common stock appreciation rights were granted to these individuals duringas of December 31, 2005. Accordingly, the value of unexercised in-the-money options as of such year.date has been calculated by determining the difference between the exercise price per share
66
Aggregate Option Exercises in Last Fiscal Year and Fiscal Year-End Option Valuesan assumed offering price of $12.00 per share, which is the midpoint of the estimated price range shown on the cover of this prospectus.
| | | | Number of Securities Underlying Unexercised Options at December 31, 2005 | Value of Unexercised In-the-Money Options at December 31, 2005 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Shares Acquired on Exercise | | ||||||||||||
| Name | Value Realized ($) | |||||||||||||
| Exercisable | Unexercisable | Exercisable | Unexercisable | |||||||||||
| Ron Cohen | 0 | 0 | 665,721 | 38,452 | $ | 6,186,793 | $ | 148,425 | ||||||
| Andrew Blight | 0 | 0 | 108,205 | 39,255 | 950,313 | 151,524 | ||||||||
| Mary Fisher | 0 | 0 | 69,965 | 99,245 | 474,419 | 383,086 | ||||||||
| Jane Wasman | 0 | 0 | 11,193 | 33,576 | 43,205 | 129,603 | ||||||||
| David Lawrence | 0 | 0 | 46,415 | 52,584 | 339,213 | 202,974 | ||||||||
The following table sets forth information concerning option exercises and option holdings for the fiscal year ended June 30, 2003 with respectnumber of shares underlying options that have been issued to each of the named executive officers.officers in calendar year 2005. No stock appreciation rights werehave been granted to these individuals. The potential realizable value set forth in the last column of the table is calculated based on the term of the option at the time of grant, which is ten years. This value is based on assumed rates of stock price appreciation of 0%, 5% and 10% compounded annually from the date of grant until their expiration date, assuming a fair market value equal to the mid-point of the estimated price range of an offering, minus the applicable exercise price. These numbers are calculated based on the requirements of the SEC and do not reflect our estimate of future stock price growth. Actual gains, if any, on stock option exercises will depend on future performance of the common stock on the date on which the options are exercised.
| | | | | | | Potential Realizable Value at Assumed Annual Rates of Stock Price Appreciation for Option Term(2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Number of Shares Underlying Stock Options Granted in Calendar Year 2005(1) | | | | | |||||||||||||
| | Percent of Total Options Granted to Employees in Fiscal Year | | Market Price on Date of Grant ($/share) | | ||||||||||||||
| Name | Exercise Price ($/share) | Expiration Date | ||||||||||||||||
| 5% | 10% | |||||||||||||||||
| Ron Cohen | 51,265 | 8.8% | $ | 8.14 | $ | 8.14 | 1/1/2015 | $ | 548,761 | $ | 1,068,244 | |||||||
| Andrew Blight | 52,338 | 9.0% | $ | 8.14 | $ | 8.14 | 1/1/2015 | 560,247 | 1,090,602 | |||||||||
| Mary Fisher | 132,323 | 22.7% | $ | 8.14 | $ | 8.14 | 1/1/2015 | 1,416,438 | 2,757,304 | |||||||||
| Jane Wasman | 44,769 | 7.7% | $ | 8.14 | $ | 8.14 | 1/1/2015 | 479,225 | 932,882 | |||||||||
| David Lawrence | 70,109 | 12.0% | $ | 8.14 | $ | 8.14 | 1/1/2015 | 750,475 | 1,460,909 | |||||||||
| | | | Number of Securities Underlying Unexercised Options at June 30, 2003 | Value of Unexercised In-the-Money Options at June 30, 2003 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Shares Acquired on Exercise | Value Realized ($) | ||||||||||||
| Name | Exercisable | Unexercisable | Exercisable | Unexercisable | ||||||||||
| Ron Cohen | 0 | 0 | 39,063 | 19,618 | $ | 19,922 | $ | 10,005 | ||||||
| Andrew Blight | 0 | 0 | 18,438 | 2,813 | 13,332 | 1,433 | ||||||||
| Mary Fisher | 0 | 0 | 4,896 | 3,437 | 2,847 | 1,870 | ||||||||
| Mitchell Katz | 0 | 0 | 4,531 | 2,552 | 2,690 | 1,389 | ||||||||
| Mark Pinney | 0 | 0 | 16,111 | 12,854 | 8,542 | 6,556 | ||||||||
Stock Incentive Plans
Our board of directors has adopted two equity incentive plans: our 2006 Employee Incentive Plan (the 2006 Plan) and our 1999 Employee Stock Option Plan (the 1999 Plan and, together with the 2006 Plan, the Plans). As of January 15, 2006, a total of 5,481,334 shares of our common stock had been reserved for issuance under the Plans, including 3,000,000 shares reserved for the grant of awards under the 2006 Plan. No additional awards will be made under the 1999 Plan.
In JuneThe 2006 Plan was adopted on January 3, 2006 and became effective, following approval by our stockholders, on January 11, 2006. The 1999 Plan was adopted by our board of directors adopted the 1999 Employee Stock Option Plan. We obtained stockholder approval of the plan in AugustJune 1999. The plan allowsPlans allow us to issue awards of incentive orand nonstatutory stock options, restricted stock awards and stock
appreciation rights for shares of our common stock and stock appreciation rights. Our compensation committee administersstock. The 2006 Plan will terminate ten years after the plan, selects those persons who are to be granted awards undereffective date of the plan and determines the terms and conditions of those awards. Our directors, key employees, officers, independent contractors, agents and consultants are eligible to receive awards under our plan, but only employees and officers may receive incentive stock options. As of June 30, 2003 we reserved a total of 1,658,333plan.
The 3,000,000 shares of common stock reserved for issuance and have granted options to purchase 188,291 shares under the plan. In September 2003,2006 Plan will be increased by the number of shares of our board of directors adopted an amendmentcommon stock, if any, that are subject to outstanding awards under the 1999 Plan at January 11, 2006, to the plan,extent that such shares are surrendered before exercise, lapse, are terminated without being exercised or are forfeited after such date.
In addition, the 2006 Plan contains an "evergreen" provision which is subject to approval by our stockholders. The amendment to the plan, which will become effective upon consummation of this offering, provides for automatic annual increases to the share reserve under the 2006 Plan on the first day of each fiscal year by a number of shares equal to the lesser of:
Although a similar evergreen provision is contained in our 1999 Plan, this provision has been amended, effective upon the adoption of the 2006 Plan by our board of directors, so as not to increase the reserved shares under the 1999 Plan.
Our compensation committee administers the Plans, selects those persons who are to be granted awards under the 2006 Plan and determines the terms and conditions of those awards. Our directors, key employees, independent contractors, agents and consultants are eligible to receive awards under our Plans, but only employees and officers may receive incentive stock options.
The exercise price per share of the incentive stock options awarded under the planPlans must be at least equal to the fair market value of a share of our common stock on the date of grant. The exercise price per share of nonstatutory stock options awarded under the planPlans must be equal to the fair market value of a share of our common stock on the date of grant, or such other price thanthat the compensation committee may determine is appropriate. The compensation committee determines the exercise period of the stock options, but in no event will the stock options expire later than ten years from the date of grant. Except as the compensation committee may otherwise determine, upon the voluntary termination or involuntary termination without cause of the option holder, the stock options may be exercised for a period of three months after such termination. In the case of termination of the option holder by reason of retirement or due to disability, the stock options may be exercised at any time to the extent that such stock option was vested, but only within one year of termination in the case of incentive stock options. In the case of termination by death, the option holder's
67
estate, or any person who acquires the stock option by reason of the option holder's death, may exercise the stock option within a period of three years after the option holder's death.
TheAn award under our Plans will become vested only if the vesting conditions set forth in the award agreement, as determined by the compensation committee, hasare satisfied. The vesting conditions may include performance of services for a specified period, achievement of performance objectives or a combination of the authoritytwo types of criteria. Performance objectives may be based on financial or operating measures. In granting performance-based awards under the 2006 Plan, which are regulated by Section 162(m) of the Internal Revenue Code, the compensation committee is bound to includefollow the criteria established under the 2006 Plan.
Under the 2006 Plan, upon a reorganization event, as defined, each outstanding award under the 2006 Plan, with any stock optioncertain exceptions, must either be assumed or an equivalent exercisable or unrestricted award substituted by the successor entity in the reorganization. If an award is assumed and, within 18 months after the reorganization event the recipient's employment is terminated without cause or he or she terminates employment for good reason, the award will become exercisable in full. If the successor entity does not assume outstanding awards at the time of a reorganization event, the compensation committee must provide that either (i) all or some portion of outstanding awards will be accelerated immediately prior to the reorganization event, or (ii) all outstanding awards will terminate upon consummation of the reorganization event and each recipient of an award will receive, in
exchange for the award, a progressive stock option, which allows an option holder to exercise their stock option by surrendering shares of common stock and entitles them to receive additional shares of common stockcash payment equal to the numbervalue of shares surrendered. The compensation committee also has the authority to grant stock appreciation rights in connection with any stock option award, which may be paid in shares ofor (iii) if our common stock cash or both, atremains publicly traded, the discretion ofawards will remain in place unchanged.
Under the compensation committee and subject to the requirements of the plan.
In1999 Plan, in the event of a tender offer by a person or persons other than us, for all or any part of the outstanding stock which, if uponfollowing consummation of the tender offer would result in the offerorofferor's or offerors would, own,offerors' owning, beneficially or of record, an aggregate of more than 25% of our outstanding common stock, or in the event of a change of control theas defined, stock options under the 1999 Plan will become immediately exercisable to the extent of the total number of shares subject to the stock options. The compensation committee may authorize payment of cash upon exercise of a stock appreciation right in the event of a tender offer as described above, or a change of control.
At June 30, 2003, options to purchase an aggregate of 188,291 shares at prices ranging from $4.20 to $24.00 were outstanding under the 1999 option plan. In September 2003, we unilaterally reduced therepriced 115,578 stock options issued to employees, which had an exercise price per option of more than $7.64, with a new exercise price of 150,251 of such options to $5.88, the effective common$7.64. In March 2004, we repriced 1,227,648 stock price per share at which we sold our Series J preferred stock. Also in September 2003, we granted stock options to purchase an aggregate of 1,374,997 shares of our common stock at $5.88 per share to our officers and employees. These options had been authorized for issuance under the Plan by the board of directors at the closing of the Series J financing in May 2003. The following table sets forth the number of shares underlying such options issued to eachemployees, which had an exercise price per option of the named executive officers and directors and the numbermore than $2.60, with a new exercise price per option of stock options repriced:$2.60. We recognized additional compensation charges for these repricings (see Note 9 to our consolidated financial statements included in this prospectus).
| Name | Number of Shares Underlying Stock Options Granted | Number of Stock Options Repriced | ||
|---|---|---|---|---|
| Ron Cohen | 740,100 | 58,681 | ||
| Andrew Blight | 102,411 | 21,250 | ||
| Mary Fisher | 39,621 | 8,333 | ||
| Mitchell Katz | 32,570 | 7,083 | ||
| Mark Pinney | 204,508 | 28,965 |
401(k) Plan
Effective September 1, 1999, we adopted a defined contribution 401(k) savings plan covering all of our employees. Participants may elect to defer a percentage of their annual pre-tax compensation to the 401(k) plan, subject to defined limitations. Our board of directors has discretion to match contributions made by our employees. We did not make any matching contributions to the plan in fiscal years 2000, 2001, 2002 or in calendar years 2003 and 2003.2004.
Employment Contracts, Termination of Employment and Change-in-Control Arrangements
We are a party to an employment agreement with Dr. Cohen whichthat governs the terms and conditions of his employment as our President and Chief Executive Officer. The employment agreement provides for a base annual salary of $280,000, subject to annual increases and bonuses at the discretion of the board of directors. His current salary is $305,000. Dr. Cohen is eligible to receive annual performance-based stock options to purchase common stock in an amount determined by the board of directors based on Dr. Cohen's individual performance and the achievement of our goals and objectives. Dr. Cohen's employment agreement expireswould have expired in January 2004, but is subject to automatic successive one-year renewal periods unless either Dr. Cohen or we give the other written notice at least 60 days prior to the expiration date that Dr. Cohen or we do not intend to renew the contract. Dr. Cohen's employment agreement has been renewed effective January 2006 for a one-year period. In the event we terminate the agreement with Dr. Cohen without cause, or if Dr. Cohen voluntarily terminates
68
the agreement with good reason, we are obligated to make severance payments equal to one year's base annual salary and COBRA premium payments for the severance period plus a bonus equal to his prior year's bonus pro rated for the number of days worked prior to termination. In such event, all of Dr. Cohen's options will become immediately exercisable and will remain exercisable for 48 months following termination. If Mr.Dr. Cohen's employment terminates for death or disability, we are obligated to pay his base salary for three months and COBRA premiums for the COBRA coverage period and 65% of his outstanding options will become immediately vested and remain exercisable for 48 months following such termination. In the event of a change in control, the vesting of Dr. Cohen's options will be governed by the terms of our stock option plan and his stock option agreement, but in no event will less than 65% of Dr. Cohen's then unvested stock options become immediately vested and exercisable. If Dr. Cohen voluntarily terminates his employment without good reason following a change in control, he is entitled to receive the same severance and bonus package described above, however, only 65% of his outstanding options will become immediately vested and remain exercisable for 48 months following termination. Following his termination of employment, Mr.Dr. Cohen will remain subject to confidentiality, non-competition and non-solicitation covenants for one year in the case of non-competition and non-solicitation and five years in the case of confidentiality.
On September 26, 2004, we entered into an amendment to Dr. Cohen's employment agreement to increase the amount of severance to which he would be entitled in the event of a termination of his employment by us without cause or by Dr. Cohen with good reason from one year to 15 months and to make such severance, together with his prorated bonus, payable in one lump sum within 30 days after such termination.
We are party to an employment agreement with Dr. Blight that governs the terms and conditions of his employment as our Chief Scientific Officer. The employment agreement provides for a base annual salary of $215,000, subject to annual review by Dr. Cohen and by the compensation committee of the Board of Directors. His current salary is $215,000.
We are party to an employment agreement with Ms. Fisher that governs the terms and conditions of her employment as our Chief Operating Officer. The employment agreement provides for a base annual salary of $225,000, subject to annual review by Dr. Cohen and by the compensation committee of the Board of Directors. Her current salary is $225,000.
We are party to an employment agreement with Mr. Pinney whichLawrence that governs the terms and conditions of his employment as our Chief Financial Officer. The employment agreement provides for a base annual salary of $197,500.$180,000, subject to annual review by Dr. Cohen and by the compensation committee of the Board of Directors. His current salary is $180,000.
We are party to an employment agreement with Ms. Wasman that governs the terms and conditions of her employment as our Executive Vice President, General Counsel and Corporate Secretary. The employment agreement provides for a base annual salary of $225,000, subject to annual review by Dr. Cohen and by the compensation committee of the Board of Directors. Her current salary is $225,000.
Pursuant to their employment agreements, Dr. Blight, Ms. Fisher, Mr. Pinney isLawrence and Ms. Wasman are eligible to receive an annual bonus and to receive annual performance-based stock options to purchase common stock, stock appreciation rights awards and/or restricted stock awards of common stock in an amount to be determinedrecommended by the compensation committee and approved by the board of directors based on Mr. Pinney's performancetheir respective performances and in the case of the stock options, upon the achievement of our goals and objectives. Mr. Pinney'sEach of their employment agreementagreements expires in September 2004,on December 19, 2006 but is subject to automatic successive one-year renewal periods unless either Mr. Pinney or we give written notice toextension by the other at least 60 days prior to the expiration date that either Mr. Pinney or we do not intend to renew the agreement.mutual agreement of both parties.
In the event we terminate theour employment agreement with Dr. Blight, Ms. Fisher, Mr. PinneyLawrence or Ms. Wasman without cause, or if Mr. Pinneyone of them voluntarily terminates the agreementhis or her agreements with good reason, we are obligated to make severance payments equal to sixnine months base annual salary, in the case of Dr. Blight and Ms. Fisher, and seven months base annual salary, in the case of Mr. Lawrence and Ms. Wasman, as well as COBRA premium payments for the severance period. In such event, all options, stock appreciation rights awards and restricted stock awards that have vested as of the termination date shall remain exercisable for 90 days following such date. All unvested options, stock appreciation rights awards and stock awards will be cancelled on the date of termination. If Dr. Blight, Ms. Fisher, Mr. Lawrence or Ms. Wasman voluntarily terminates his or her employment without good reason or if we terminate his or her employment without cause within 18 months after a change in control, we are obligated to make severance payments equal to one year's base annual salary, in the case of Dr. Blight and Ms. Fisher, and nine months base annual salary, in the case of Mr. Lawrence and Ms. Wasman, in each case paid in a lump sum within 30 days after termination, as well as COBRA premium payments for the severance period plus a bonus equal to hisa prior year's bonus pro rated for the number of days worked prior to termination. We are also obligated to pay salary earned but not paid, vacation and sick leave days that have accrued, and reimbursable business expenses incurred through the date of termination. In such event, allnot less than 50% of Mr. Pinney'sthe unvested options, willstock appreciation rights and restricted or other stock awards shall become immediately exercisable and willfull vested and shall remain exercisable for 4818 months following termination. If Mr. Pinney's employment terminates for death or disability, we are obligated to pay his base salary for three months and COBRA premiums forsuch date. All options that have vested
as of the COBRA coverage period and 33% of his outstanding options will become immediately vested andtermination date shall remain exercisable for 48 months90 days following such termination. In the event of a change in control, the vesting of Mr. Pinney'sdate. All unvested options, stock appreciation rights awards and stock awards will be governed bycancelled on the termsdate of our stock option plan and his stock option agreements, but in no event will less than 33% of Mr. Pinney's then unvested stock options become immediately vested and exercisable. If Mr. Pinney voluntarily terminates his employment without good reason following a change in control, he is entitled to receive the same severance and bonus package described above. However, only 33% of his outstanding options will become immediately vested and remain exercisable for 48 months following termination. Following his termination of employment, Mr. Pinney will remain subject to confidentiality, non-competition and non-solicitation covenants for one year in the case of non-competition and non-solicitation and five years in the case of confidentiality.
Indemnification of Directors and Executive Officers and Limitation on Liability
Our bylawscertificate of incorporation currently provideprovides and, upon the closing of this offering, our amended and restated bylawscertificate of incorporation will provide, that we shall indemnify our directors and officers to the fullest extent permitted by Delaware law, providedlaw. Upon the closing of this offering, our amended and restated certificate of incorporation will also provide that, with respect to proceedings initiated by our officers and directors, we are only required to indemnify these persons if the proceeding was authorized by our board of directors. Our amended bylaws permit us, by action of our board of directors, to indemnify our other employees and agents to the same extent as we are required to indemnify our officers and directors. We are also empowered under our bylaws to enter into indemnification agreements with our directors, officers, employees or agents and to purchase insurance on behalf of any of our director, officer, employee or agent whether or not we are required or permitted to indemnify such persons under Delaware law.
69
We have entered into indemnification agreements with certain of our directors and executive officers and intend to enter into indemnification agreements with all of our other directors and executive officers prior to the consummation of this offering. Under these agreements, we will indemnify our directors and executive officers against amounts actually and reasonably incurred in connection with actual or threatened proceedings if any of them may be made a party because of their role as one of our directors or officers. We are obligated to pay these amounts only if the officer or director acted in good faith and in a manner that he or she reasonably believed to be in or not opposed to our best interests. For any criminal proceedings, we are obligated to pay these amounts only if the officer or director had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth procedures that will apply in the event of a claim for indemnification thereunder.
In addition, our bylawscertificate of incorporation provides, and upon the closing of this offering our amended and restated certificate of incorporation will provide, that our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, except for liability:
There is no pending litigation or proceeding involving any of our directors or officers for which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
70
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Sale of Securities
In March 2001, we consummated a private placement of 10,204,047 shares of Series I preferred stock for an aggregate purchase price of approximately $39,694,000. Except for Michael Steinmetz, Standish Fleming and Barclay Phillips, who isare affiliated with MPM/BB Bioventure,MPM BioVentures I LLC, Forward Ventures and Vector Fund Management, respectively, none of our executive officers or directors purchased any shares of the Series I preferred stock.
The following table sets forth, with respect to the Series I preferred stock transaction, the purchase price per share, the aggregate shares purchased and the total investment for MPM/BB Bioventure group.Group, Forward Ventures and Vector Fund Management:
| Investor | Purchase Price per Share of Series I Preferred | Aggregate Shares of Series I Preferred Purchased | Total Investment in Series I Preferred | Purchase Price per Share of Series I Preferred | Aggregate Shares of Series I Preferred Purchased | Total Investment in Series I Preferred | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPM/BB Bioventure group | $ | 3.89 | 639,359 | $ | 2,487,107 | |||||||||
| MPM BioVentures I LLC | $3.89 | 639,359 | $2,487,107 | |||||||||||
| Forward Ventures | $3.89 | 1,542,417 | $6,000,002 | |||||||||||
| Vector Fund Management | $3.89 | 398,547 | $1,550,348 | |||||||||||
In May 2003, we consummated a private placement of 112,790,233 shares of Series J preferred stock for an aggregate purchase price of approximately $55,267,000. Except for Michael Steinmetz, John Friedman, and Sandra Panem, Standish Fleming and Barclay Phillips, who are affiliated with MPM/BB Bioventure group,MPM BioVentures I LLC, Easton Hunt Capital Partners, and Cross Atlantic Partners, Forward Ventures and Vector Fund Management, respectively, none of our executive officers or directors purchased any shares of the Series J preferred stock.
The following table sets forth, with respect to the Series J preferred stock transaction, the purchase price per share, the aggregate shares purchased and the total investment for each of MPM/BB Bioventure group,MPM BioVentures I LLC, Easton Hunt Capital Partners, Cross Atlantic Partners, Forward Ventures and Vector Fund Management:
| Investor | Purchase Price per Share of Series J Preferred | Aggregate Shares of Series J Preferred Purchased | Total Investment in Series J Preferred | |||||
|---|---|---|---|---|---|---|---|---|
| MPM BioVentures I LLC | $ | 0.49 | 15,306,121 | $ | 7,500,000 | |||
| Easton Hunt Capital Partners | $ | 0.49 | 11,224,490 | $ | 5,500,000 | |||
| Cross Atlantic Partners | $ | 0.49 | 8,506,256 | $ | 4,168,065 | |||
| Forward Ventures | $ | 0.49 | 8,163,264 | $ | 4,000,000 | |||
| Vector Fund Management | $ | 0.49 | 2,040,816 | $ | 1,000,000 | |||
In March 2004, we consummated a private placement of 1,533,330 shares of Series K preferred stock for an aggregate purchase price of approximately $11,499,958. Except for John Friedman and Sandra Panem, who are affiliated with Easton Hunt Capital Partners and Cross Atlantic Partners, respectively, none of our executive officers or directors purchased any shares of the Series K preferred stock.
The following table sets forth, with respect to the Series K preferred stock transaction, the purchase price per share, the aggregate shares purchased and the total investment for each of Easton Hunt Capital Partners, Easton Hunt New York and Cross Atlantic Partners:
| Investor | Purchase Price per Share of Series J Preferred | Aggregate Shares of Series J Preferred Purchased | Total Investment in Series J Preferred | Purchase Price per Share of Series K Preferred | Aggregate Shares of Series K Preferred Purchased | Total Investment in Series K Preferred | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPM/BB Bioventure group | $ | 0.49 | 15,306,121 | $ | 7,500,000 | |||||||||||
| Easton Hunt Capital Partners | $ | 0.49 | 11,224,490 | $ | 5,500,000 | $ | 7.50 | 100,000 | $ | 750,000 | ||||||
| Easton Hunt New York | $ | 7.50 | 100,000 | $ | 750,000 | |||||||||||
| Cross Atlantic Partners | $ | 0.49 | 8,506,256 | $ | 4,168,065 | $ | 7.50 | 55,574 | $ | 416,805 | ||||||
Board Representation and Registration Rights
Pursuant to an amended and restated registration rights agreement dated as of March 3, 2004, the above partiesholders of our Series I Preferred, Series J Preferred and Series K preferred stock have demand and piggy-back registration rights; provided thatrights. Pursuant to the boardterms of directors hasthis agreement, holders of at least 30% of outstanding "registrable securities" have the right to postponeinitiate a demand registration, insubject to our ability to delay registration under certain circumstances. However, under the amended and restated registration rights agreement, we are not obligated to register the registrable securities prior to the date that is six months after the effective date of our registration statement for this offering. We have agreed to pay for all expenses in connection with the registration. See "Description of Capital Stock—Registration Rights".
In addition, if we propose to register any of our securities under the Securities Act, including in this offering, certain of our other stockholders are entitled to notice of the registration and to include their registrable shares in the offering. If the managing underwriter determines that marketing factors require a limitation on the number of shares to be underwritten, the managing underwriters may limit or exclude from such underwriting the registrable securities and other securities of the holders to be so distributed.these stockholders. If we are so advised by the managing underwriter, then all securities other than registrable securities shall first be excluded from the registration. In no event, however, will the amount of stockholders' securities to be included in the offering be reduced below 30% of the total securities in the offering. We are required to bear substantially all costs incurred in these registrations, other than underwriting discounts and commissions.
71 Pursuant to the lock-up agreements with the underwriters, holders of greater than 70% of the "registrable securities" under our registration rights agreement have waived their rights to demand registration and participation in this offering under the registration rights agreement until the later of October 30, 2006 or expiration of the lock-up agreements.
Indemnification agreementsAgreement with Director
ForIn November, 2004, we entered into an agreement with Mark Pinney, under which we agreed to extend the last date by which Mr. Pinney is entitled to exercise vested stock options previously granted to him to 90 days after he is no longer a descriptiondirector or consultant to us. In addition, he will be entitled to retain certain shares of restricted stock if the vesting requirements for these shares are met within the extended time period. On September 26, 2005, Mr. Pinney was issued 5,000 shares of restricted stock for services rendered as a member of our indemnification arrangements with ourboard of directors and executive officers, see "Management—Indemnificationfrom November 1, 2004 through December 31, 2005. Mr. Pinney's shares of Directors and Executive Officers and Limitationrestricted stock are otherwise subject to the vesting in the manner described in footnote 3 to the Summary Compensation Table found on Liability".page 92.
Agreements with Elan
In September 2003, we entered into the following agreements with Elan, which holds more than 5% of our outstanding common stock:Elan:
In July 2004, we entered into the following agreements with Elan:
For a more detailed description of these agreements with Elan see "Business—R&D and Product Collaborations Alliances and License Agreements".
72 Until December 23, 2005, Elan held more than 5% of our outstanding common stock. On December 23, 2005, Elan transferred to funds affiliated with Saints Capital 900,000 shares of our Series B convertible preferred stock, 333,333 shares of our Series C convertible preferred stock, 2,300,000 shares of our Series F convertible preferred stock, a warrant to purchase 100,000 shares of our Series B convertible preferred stock, a $2,500,000 convertible promissory note convertible into shares of our Series B convertible stock or an undesignated series and a $5,000,000 convertible promissory note convertible into shares of our Series D convertible preferred stock.
The following table contains information as of December 31, 2005 about the beneficial ownership of our common stock before and after the consummation of this offering for:
Unless otherwise indicated, the address for each person or entity named below is c/o Acorda Therapeutics, Inc., 15 Skyline Drive, Hawthorne, New York 10532.
Beneficial ownership is determined in accordance withon the basis of the rules and regulations of the Securities and Exchange Commission. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options held by that person that are currently exercisable or exercisable within 60 days of the date hereof are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. For the purpose of calculating the amounts set forth in the following table, all outstanding shares of preferred stock have been deemed to have been converted into shares of common stock, which conversion will occur upon the closing of this offering. Except as indicated in the footnotes to the following table or pursuant to applicable community property laws, each stockholder named in the table has sole voting and investment power with respect to the shares set forth opposite such stockholder's name. The percentage of beneficial ownership is based on 16,055,61113,547,022 shares of common stock outstanding, on the date of this prospectus.an as converted basis, on December 31, 2005.
| | | | Percentage of Common Stock Outstanding | | | Percentage of Common Stock Outstanding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beneficial Owner | Beneficial Owner | Number of Shares(1) | Before Offering | After Offering(2) | Beneficial Owner | Number of Shares(1) | Before Offering | After Offering(2) | |||||||
| Five Percent Stockholders | |||||||||||||||
| MPM/BB Bioventure group(3) | 2,132,189 | 13.3 | % | ||||||||||||
| Elan group(4) | 1,297,115 | 7.9 | % | ||||||||||||
| Five Percent Stockholders: | Five Percent Stockholders: | ||||||||||||||
| Forward Ventures group(5) | 1,136,061 | 7.1 | % | MPM BioVentures I LLC(3) | 1,640,137 | 12.1 | % | 8.6 | % | ||||||
| Easton Hunt Capital Partners, LP(6) | 935,374 | 5.8 | % | Saints Capital(4) | 965,170 | 7.1 | 5.2 | ||||||||
| MDS/Neuroscience Partners Healthcare(7) | 876,597 | 5.5 | % | Forward Ventures group(5) | 873,890 | 6.5 | 4.6 | ||||||||
| TVM Life Sciences(8) | 850,340 | 5.3 | % | Easton Hunt(6) | 873,364 | 6.4 | 4.6 | ||||||||
| ABN AMRO(9) | 850,340 | 5.3 | % | Cross Atlantic Partners(7) | 705,388 | 5.2 | 3.7 | ||||||||
| J.P. Morgan(10) | 850,340 | 5.3 | % | TVM Life Sciences(8) | 705,388 | 5.2 | 3.7 | ||||||||
| Cross Atlantic Partners(11) | 850,339 | 5.3 | % | MDS/Neuroscience Partners Healthcare(9) | 674,295 | 5.0 | 3.5 | ||||||||
Directors and Executive Officers: | Directors and Executive Officers: | Directors and Executive Officers: | |||||||||||||
| Ron Cohen, M.D.(12) | 670,240 | 4.0 | % | Ron Cohen, M.D.(10) | 887,229 | 6.5 | 4.7 | ||||||||
| Andrew R. Blight, Ph.D.(13) | 109,985 | * | Andrew R. Blight, Ph.D.(11) | 152,411 | 1.1 | 0.8 | |||||||||
| Mary Fisher(14) | 28,903 | * | Mary Fisher(12) | 138,749 | 1.0 | * | |||||||||
| Elliott A. Gruskin, Ph.D.(15) | 19,796 | * | David Lawrence, M.B.A.(13) | 74,514 | * | * | |||||||||
| Mitchell Katz, Ph.D.(16) | 25,964 | * | Jane Wasman, J.D.(14) | 45,147 | * | * | |||||||||
| David Lawrence, M.B.A.(17) | 22,821 | * | John Friedman(15) | 873,364 | 6.4 | 4.6 | |||||||||
| King Lee, Ph.D. | 0 | * | Sandra Panem, Ph.D.(16) | 711,102 | 5.2 | 3.7 | |||||||||
| Mark R.E. Pinney, M.B.A., C.F.A.(18) | 147,842 | * | Michael Steinmetz, Ph.D.(17) | 1,640,137 | 12.1 | 8.6 | |||||||||
| Harold Safferstein, Ph.D., J.D.(19) | 15,293 | * | Wise Young, Ph.D., M.D.(18) | 22,434 | * | * | |||||||||
| John Friedman(20) | 935,374 | 5.8 | % | Standish Fleming(19) | 873,890 | 6.5 | 4.6 | ||||||||
| Sandra Panem, Ph.D.(21) | 854,556 | 4.4 | % | Mark Pinney, M.B.A., C.F.A.(20) | 174,497 | 1.3 | * | ||||||||
| Michael Steinmetz, Ph.D.(22) | 2,132,189 | 13.3 | % | Barclay Phillips(21) | 543,803 | 4.0 | 2.9 | ||||||||
| Wise Young, Ph.D., M.D.(23) | 24,167 | * | Steven Rauscher(22) | 8,173 | * | * | |||||||||
| All directors and executive officers as a group (13 persons)(24) | 4,837,003 | 28.5 | % | ||||||||||||
| Lorin J. Randall | — | — | — | ||||||||||||
| All directors and executive officers as a group (14 persons)(23) | All directors and executive officers as a group (14 persons)(23) | 6,145,450 | 45.4 | % | 32.3 | % | |||||||||
73
Investors 1998 LLC. BB Bio Ventures L.P., MPM Bio Ventures Parallel Fund, L.P. and MPM Asset Management Investors 1998 LLC are affiliates of MPM Bio Ventures I LLC. The address of MPM/BB Bioventures groupMPM Bio Ventures I LLC and its affiliates is c/o MPM Capital Asset Management, 111 Huntington Avenue, 31st200 Clarendon St., 54th Floor, Boston, Massachusetts 02199.
Dr. Panem is a partner of Cross Atlantic Partners and exercises investment and voting power over these shares. Dr. Panem disclaims beneficial ownership of these shares.
74
PriorThe following is a description of the material terms of our amended and restated certificate of incorporation and bylaws as each is anticipated to be in effect immediately following the consummationclosing of this offering and the filing of our amended and restated certificate of incorporation. We refer you to our amended and restated certificate of incorporation and bylaws, copies of which have been filed as exhibits to the registration statement of which this prospectus forms a part.
Authorized Capitalization
On September 18, 2005, our Board of Directors approved a 1-for-1.3 reverse stock split, which we effected on January 11, 2006. All references to common stock, common shares outstanding, average number of common shares outstanding, per share amounts, options and warrants and Elan notes payable in this registration statement have been restated to reflect the 1-for-1.3 common stock reverse split on a retroactive basis.
As of September 30, 2005, our authorized capital stock consistsconsisted of (i) 260,000,000 shares of common stock, $0.001with a par value of $0.001 per share, of which 208,743 shares were issued and 140,221,535outstanding, and (ii) 141,754,865 shares of preferred stock, $0.001with a par value of $0.001 per share.share, of which 106,472,984 shares are issued and outstanding. Immediately following the consummationclosing of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of 75,000,00080,000,000 shares of common stock, $0.001with a par value of $0.001 per share and 1,000,00020,000,000 shares of preferred stock, $0.001with a par value of $0.001 per share. As of September 19, 2003, there were outstanding:
As of the consummation of this offering, all of the outstanding shares of preferred stock will automatically convert into 13,338,279 shares of common stock. After giving effect to thethis conversion, we will have outstanding 16,055,611expect there to be 19,047,022 shares of common stock issued and outstanding (or 19,872,022 shares of common stock if the underwriter exercises its over-allotment option in full), and no shares of preferred stock.stock issued and outstanding.
Common stockStock
Voting Rights
Under our amended and restated certificate of incorporation, the holdersHolders of common stock are entitled to one vote per share on all matters to be voted onsubmitted for action by the stockholders. After paymentThe holders of any dividends due and owing tocommon stock do not have cumulative voting rights in the election of directors. Accordingly, the holders of preferredmore than 50% of the shares of common stock can, if they choose to do so, elect all the directors. In such event, the holders of the remaining shares of common stock will not be able to elect any directors.
Dividend Rights
Holders of common stock are entitled to receive ratably dividends if, as and when dividends are declared from time to time by theour board of directors out of funds legally available for dividends. In the eventthat purpose, after payment of dividends required to be paid on outstanding preferred stock, if any. Our secured term loan imposes restrictions on our ability to declare dividends on our common stock.
Liquidation Rights
Upon our liquidation, dissolution or winding up, any business combination or a sale or disposition of all or substantially all of our assets, the holders of common stock are entitled to share in allreceive ratably the assets remainingavailable for distribution to the stockholders after payment of liabilities and accumulated and unpaid dividends and liquidation preferences ofon outstanding shares of preferred stock.stock, if any.
Other Matters
Holders of common stock have no preemptive conversion, subscriptionrights and are not subject to further calls or other rights.assessment by us. There are no redemption or sinking fund provisions applicable to theour common stock. All outstanding shares of our common stock, are, and allincluding the shares of common stock to be outstanding upon completion ofoffered in this offering, will be,are fully paid and nonassessable.non-assessable.
Preferred stockStock
In accordance with ourOur amended and restated certificate of incorporation authorizes our board of directors hasto establish one or more series of up to 20,000,000 shares of preferred stock. Unless required by law or by any stock exchange on which our common stock is listed, the authority,authorized shares of preferred stock will be available for issuance without further action by the stockholders, to issue up to 1,000,000 shares of preferred stock.our stockholders. Our board of directors may issueis able to determine, with respect to any series of preferred stock, in one or morethe terms and rights of that series andincluding:
Restricted Stock
As of preferred stock could also have the effect of decreasing the market price of the common stock and could delay, deter or prevent a change in control of our company. We have no present plans to issue anyDecember 31, 2005, we had 755,083 shares of preferred stock.restricted stock outstanding.
Warrants
We haveAs of December 31, 2005, we had outstanding warrants to purchase 38,42450,200 shares of common stock at a weighted average exercise price of $1.47$16.54 per share.
Stock optionsOptions
1,623,705As of December 31, 2005, 1,767,904 shares of common stock are issuable upon the exercise of outstanding stock options to purchase our common stock. AfterOn the effective date of the registration statement, of which this offering,prospectus is a part, we intend to file a registration statement on Form S-8 to register the shares of common stock reserved for issuance upon exercise of outstanding options. The registration statement is expected to be filedoptions under both our 1999 Plan and become effective approximately six months after the closing
75
of this offering.our 2006 Plan. Accordingly, shares registered under the registration statement
will be available for sale in the open market without restriction, except with respect to Rule 144 volume limitations that apply to our affiliates.
Convertible Promissory Notes
In January 1997, EIS loaned us an aggregate of $7.5 million pursuant to two promissory notes that are convertible into 361,842278,339 shares of our common stock.
Classified Board of Directors
Our amended and restated certificate of incorporation provides for our board of directors On December 23, 2005, Elan transferred these promissory notes to be divided into three classes,funds affiliated with each class serving for a term of three years. At each annual meeting of stockholders, directors will be elected for a three-year term to succeed the directors whose terms are expiring. Mr. Pinney and Dr. Young will be class I directors whose terms will expire in 2004; Drs. Panem and Steinmetz will be class II directors whose terms will expire in 2005 and Dr. Cohen and Mr. Friedman will be class III directors whose terms will expire in 2006. There will be no cumulative voting with respect to the election of directors.Saints Capital.
Registration rightsRights
Pursuant to an amended and restated registration rights agreement between us and certain of our stockholders commencing six months after the effective datedated as of our registration statement for this offering,March 3, 2004, holders of an aggregate of 13,338,279 shares of our common stock have demand and piggy-back registration rights. The demand rights may be exercised by holders of 30% of the registrable securities.securities at any time after completion of this offering. Additionally, if at any time we propose to register our common stock under the Securities Act for our own account or the account of any of our stockholders or both, the stockholders party to the registration rights agreement are entitled to notice of the registration and to include registrable shares in the offering, provided that the underwriters of that offering do not limit the number of shares included in the registration. In no event, however, will the amount of stockholders' securities to be included in the offering be reduced below 30% of the total securities in the offering. We are required to bear substantially all costs incurred in these registrations, other than underwriting discounts and commissions. The registration rights described above could result in substantial future expenses for us and adversely affect any future equity or debt offerings.offering. Pursuant to the lock-up agreements with the underwriters, holders of greater than 70% of the "registrable securities" under our registration rights agreement have waived their rights to demand registration and participation in this offering under the registration rights agreement until the later of October 30, 2006 and expiration of the lock-up agreements. In addition, holders of the requisite amount of registrable securities have waived their rights to registration through the completion of this offering.
Anti-takeover provisionsAuthorized but Unissued Capital Stock
The Delaware General Corporation Law does not require stockholder approval for any issuance of authorized shares. These additional shares may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive the stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Delaware Anti-Takeover Statute
We are governed by the provisions ofsubject to Section 203 of the Delaware General Corporation Law. In general,Subject to specific exceptions, Section 203 prohibits a publicpublicly held Delaware corporation from engaging in a business combination"business combination" with an interested stockholder"interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder, unless unless:
"Business combinations" include mergers, asset sales orand other transactions resulting in a financial benefit to the interested"interested stockholder. An interested stockholder" Subject to various exceptions, an "interested stockholder" is a person who, together with his or her affiliates and associates, owns, or within three years did own, 15.0%15% or more of the company'scorporation's outstanding voting stock. The statuteThese restrictions could prohibit or delay deferthe accomplishment of mergers or prevent aother takeover or change in control ofattempts with respect to us and, therefore, may discourage attempts to acquire us.
Transfer Agent and Registrar
Registrar and Transfer Company is the transfer agent and registrar for our company.common stock.
Listing
We will applyhave applied to list our common stock on The Nasdaq Stock Market's National Market, subject to official notice of issuance, under the trading symbol "ACRD."ACOR."
Transfer agent and registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company.
76
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common stock. Upon completion of this offering, we will have outstanding an aggregate of 19,047,022 million shares of common stock, and if the underwriters exercise their over-allotment option in full, we will have outstanding an aggregate of 19,872,022 million shares of our common stock. Of these shares,All of the shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except that any shares purchased in the offering by our affiliates, as that term is defined in Rule 144 of the Securities Act, may generally only be sold in compliance with the limitations of Rule 144 described below. Persons whoAfter the offering, all 20,611,259 shares of our outstanding common stock will be "restricted securities," as that term is defined under Rule 144 and Rule 701 under the Securities Act. Restricted securities may be deemed to be affiliates generally include individualssold in the public market only if registered or entities that control,if they qualify for an exemption from registration under Rule 144 or 144(k) under the Securities Act, which are controlled by, or are under common control with, us and may include our directors and officers.summarized below.
Sales of substantial amounts of our common stock in the public market could adversely affectput downward pressure on the market price of our common stock. We cannot estimate the number of shares of common stock that may be sold by third parties in the future because such sales will depend on market prices, the circumstances of sellers and other factors.
Sales of restricted shares
16,055,611 shares of our common stock held by existing stockholders as of the date of this prospectus are restricted securities under Rule 144. The number of these shares of common stock available for sale in the public market is limited by restrictions under the Securities Act.144
In general, under Rule 144 as currently in effect, beginning 90 days after the date of this prospectus, a person (oror persons whose shares are aggregated), including any of our affiliates,aggregated, who has beneficially owned restricted shares for at least one year, (including the holding period of any prior owner, except if the prior owner was an affiliate) willincluding persons who may be deemed to be our "affiliates," would be entitled to sell within any three-month period a number of shares that does not exceed the greater of: (a) one percent
Sales of restricted securities pursuant tounder Rule 144 are also subject to requirements relating to manner of sale provisions and notice requirements and to the availability of current public information about us. Under
Rule 144(k)
In addition, under Rule 144(k), a person who is not deemed to haveand has not been anour affiliate of ours at any time during the three months90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least two years (includinghave elapsed since the holding periodshares were acquired from us or any affiliate of any prior owners except a prior owner who was an affiliate),ours, is entitled to sell itsthose shares immediately after the consummation of this offering without complying with the volume limitation orregard to the manner of sale, notice or current public information, volume limitation or notice requirements of Rule 144.
Rule 701
Generally, an employee, officer, director or consultant who purchased shares of our common stock before the effective date of the registration statement of which this prospectus is a part, or who holds options as of that date, pursuant to a written compensatory plan or contract, may rely on the resale provisions of Rule 701 under the Securities Act. Under Rule 701, these persons who are not our affiliates may generally sell their eligible securities, commencing 90 days after the effective date of the registration statement of which this prospectus is a part, without having to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Therefore, unless otherwise restricted, 144(k)
Sale of Restricted Shares
Based on the shares could be sold immediatelyoutstanding as of December 31, 2005, 20,611,259 shares of our common stock will become eligible for sale pursuant to Rule 144 or Rule 701 without registration approximately as follows, assuming conversion of our preferred stock upon the completionconsummation of this offering. Asoffering and no exercise of outstanding options and warrants, and assuming no shares are released from the lock-up agreements described below prior to 180 days after the date of this prospectus, an aggregateprospectus:
Rules 144, 144(k) shares which areand Rule 701 do not otherwise restricted.supersede the contractual obligations of our security holders set forth in the lock-up agreements described below.
Lock-up Agreements
We, and our directors and executive officers and substantially all of our existing stockholders and option holders have entered into lock-up agreements with the underwriters pursuantunderwriters. Under these agreements, subject to whichexceptions, we may not issue any new shares of common stock, and those holders of stock and options have agreedmay not, to, directly or indirectly, sell, offer, contract or grant any option to sell, pledge, transfer or otherwise dispose of or hedge any shares of our common stock or securities convertible into or exchangeable for shares of our common stock, or publicly announce the intention to do any of the foregoing, without the prior written consent of Banc of America Securities LLC, for a period of 180 days afterfrom the date of this prospectus.prospectus related to this offering, subject to a potential extension of up to an additional 34 days under certain circumstances. This consent may be given at any time without public notice. In addition, during this 180 day period, we have also agreed not to file any registration statement for and eachany shares of our officers hascommon stock without the prior written consent of Banc of America Securities LLC. Pursuant to the lock-up agreements holders of greater than 70% of the "registrable securities" under our registration rights agreement have also agreed not to make any demand for, or exercise any right of, theto registration of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, without the prior written consent of Banc of America Securities LLC.
77
Registration rightsRights
Commencing six months afterFollowing the effective datecompletion of our registration statement for this offering, holders of 15,806,616an aggregate of 13,338,279 shares of our common stock will be entitled to certain rights with respect to the registration of their shares under the Securities Act.
See "Description of Capital Stock—Registration Rights." Except for shares purchased by affiliates, registrationRegistration of their shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. These stockholders
CERTAIN UNITED STATES FEDERAL INCOME AND ESTATE TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of certain U.S. federal income and estate tax consequences of the purchase, ownership and disposition of our common stock as of the date hereof. Except where noted, this summary deals only with common stock that is held as a capital asset by a non-U.S. holder.
A "non-U.S. holder" means a beneficial owner of our common stock (other than a partnership) that is not, for U.S. federal income tax purposes, any of the following:
This summary is based upon provisions of the Code and regulations, rulings and judicial decisions as of the date hereof. Those authorities may be changed, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those summarized below. This summary does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state, local or other tax considerations that may be relevant to non-U.S. holders in light of their personal circumstances. In addition, it does not represent a description of the U.S. federal income and estate tax consequences applicable to you if you are subject to special treatment under the U.S. federal income tax laws (including if you are a U.S. expatriate, "controlled foreign corporation," "passive foreign investment company," corporation that accumulates earnings to avoid U.S. federal income tax, a tax-exempt organization, a bank, an insurance company, a dealer in securities, a person that holds our common stock as part of a "straddle," "hedge," "conversion transaction," or other integrated transaction, a pass-through entity or an investor in a pass-through entity). We cannot assure you that a change in law will not alter significantly the tax considerations that we describe in this summary.
If a partnership holds our common stock, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership holding our common stock, you should consult your tax advisors. In this summary, "partnership" includes any entity treated as a partnership and "partner" includes any person treated as a partner for U.S. federal income tax purposes.
If you are considering the purchase of our common stock, you should consult your own tax advisors concerning the particular U.S. federal income and estate tax consequences to you of the ownership of our common stock, as well as the consequences to you arising under the laws of any other taxing jurisdiction.
Dividends
We do not currently anticipate paying dividends on our common stock. See "Dividend Policy" above. If we were to pay dividends in the future, dividends paid to a non-U.S. holder of our common stock generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However, dividends that are effectively connected with the conduct of a trade or business by the non-U.S. holder within the United States (and, where a tax treaty applies, are attributable to a U.S. permanent establishment of the non-U.S.
holder) are not permittedsubject to exercise their registration rightsthe withholding tax, provided certain certification and disclosure requirements are satisfied. Instead, such dividends are subject to U.S. federal income tax on a net income basis in the same manner as if the non-U.S. holder were a U.S. person as defined under the Code. Any such effectively connected dividends received by a foreign corporation may be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A non-U.S. holder of our common stock who wishes to claim the benefit of an applicable treaty rate for dividends will be required to complete Internal Revenue Service Form W-8BEN (or other applicable form) and certify under penalty of perjury that such holder is eligible for benefits under the applicable treaty. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals. In addition, Treasury regulations provide special procedures for payments of dividends through certain intermediaries.
A non-U.S. holder of our common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by filing an appropriate claim for refund with the Internal Revenue Service.
Gain on Disposition of Common Stock
Any gain realized on the disposition of our common stock generally will not be subject to U.S. federal income tax unless:
An individual non-U.S. holder described in the first bullet point immediately above will be subject to tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates. An individual non-U.S. holder described in the second bullet point immediately above will be subject to a flat 30% tax on the gain derived from the sale, which may be offset by U.S. source capital losses, even though the individual is not considered a resident of the United States. If a non-U.S. holder that is a foreign corporation falls under the first bullet point immediately above, it will be subject to tax on its net gain in the same manner as if it were a U.S. person as defined under the Code and, in addition, may be subject to the branch profits tax equal to 30% of its effectively connected earnings and profits or at least six months following this offering.such lower rate as may be specified by an applicable income tax treaty.
78 We believe we are not and do not anticipate becoming a USRPHC for U.S. federal income tax purposes, however no assurances can be provided that we will not be a USRPHC in the future.
U.S. Federal Estate Tax
Common stock owned or treated as owned by an individual who is not a citizen or resident of the United States, as specifically defined for U.S. estate tax purposes, at the time of death will be included in such holder's gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
We must report annually to the Internal Revenue Service and to each non-U.S. holder the amount of dividends paid to such holder and the tax withheld with respect to such dividends, regardless of whether withholding was required. Copies of the information returns reporting such dividends and withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
A non-U.S. holder will be subject to backup withholding for dividends paid to such holder unless such holder certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that such holder is a U.S. person as defined under the Code), or such holder otherwise establishes an exemption.
Information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale of our common stock within the United States or conducted through certain U.S.-related financial intermediaries, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that the beneficial owner is a U.S. person as defined under the Code) or such owner otherwise establishes an exemption. Certain shareholders, including all corporations, are exempt from the backup withholding rules.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder's U.S. federal income tax liability provided the required information is furnished to the Internal Revenue Service.
We are offering the shares of common stock described in this prospectus through a number of underwriters. Banc of America Securities LLC, Lazard FrèresCapital Markets LLC, Piper Jaffray & Co. LLC, U.S. Bancorp Piper Jaffray Inc. and RBC Dain Rauscher, Inc.SG Cowen & Co., LLC, are the representatives of the underwriters. We have entered into a firm commitment underwriting agreement with the underwriters.representatives. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has agreed to purchase, the number of shares of common stock listed next to its name in the following table:
| Underwriter | Number of Shares | |
|---|---|---|
| Banc of America Securities LLC | ||
| Lazard | ||
Total | 5,500,000 | |
The underwriting agreement is subject to a number of terms and conditions and provides that the underwriters must buy all of the shares if they buy any of them. The underwriters will sell the shares to the public when and if the underwriters buy the shares from us.
The underwriters initially will offer the shares to the public at the price specified on the cover page of this prospectus. The underwriters may allow to selected dealers a concession of not more than $ per share.share to selected dealers. The underwriters may also allow, and anythose dealers may reallow, a concession of not more than $ per share to some other dealers. If all the shares of common stock are not sold at the public offering price, the underwriters may change the public offering price and the other selling terms. Our common stock is offered subject to a number of conditions, including:
Over-Allotment Options. We have granted the underwriters an over-allotment option to purchasebuy up to 825,000 additional shares of our common stock at the public offeringsame price lessper share as they are paying for the underwriting discounts and commissions.shares shown in the table below. These additional shares would cover sales of shares by the underwriters that exceed the total number of shares shown in the table above. The underwriters may exercise this option solely for the purpose of coveringat any over-allotments made in connection with this offering. The underwriters havetime within 30 days fromafter the date of this prospectus to exercise this option. Ifprospectus. To the extent that the underwriters exercise this option, theyeach underwriter will each purchase additional shares from us in approximately inthe same proportion toas it purchased the amounts specifiedshares shown in the table above. If purchased, the additional shares will be sold by the underwriters on the same terms as those on which the other shares are sold.
Discount and Commissions. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters by us. These amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
We estimate that the expenses of the offering to be paid by us, not including the underwriting discounts and commissions, will be approximately $2.1 million.
| | Paid by Us | |||||
|---|---|---|---|---|---|---|
| | No Exercise | Full Exercise | ||||
| Per share | $ | $ | ||||
| Total | $ | $ | ||||
We estimate that the total expenses of this offering to be paid by us, not including the underwriting discounts and commissions, will be approximately $ million.
We, our executive officers and directors and our existing stockholders have entered into lock-up agreements with the underwriters. Under these agreements, we and each of these persons may not, without
79
the prior written approval of Banc of America Securities LLC, offer, sell, contact to sell or otherwise dispose of or hedge our common stock or securities convertible into or exchangeable for our common stock. These restrictions will be in effect for a period of 180 days after the date of this prospectus. At any time and without notice, Banc of America Securities LLC may, in its sole discretion, release all or some of the securities from these lock-up agreements.
We will indemnify the underwriters against various liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments the underwriters may be required to make in respect of those liabilities.
Listing. We have applied to have our common stock included for quotation on the Nasdaq National Market under the symbol "ACRD."ACOR."
Stabilization. In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including:
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. Stabilizing transactions may include making short sales of our common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock from us or in the open market to cover positions created by short sales. Short sales may be "covered" shorts, which are short positions in an amount not greater than the underwriters' over-allotment option referred to above, or may be "naked" shorts, which are short positions in excess of that amount. Syndicate covering transactions involve purchases of our common stock in the open market after the distribution has been completed in order to cover syndicate short positions.
The underwriters may close out any covered short position either by exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares pursuant to the over-allotment option.
A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchased in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The representatives also may impose a penalty bid on underwriters and selling group members.dealers participating in the offering. This means that if the representatives purchase sharesmay reclaim from any syndicate members or other dealers participating in the open marketoffering the underwriting discounts on shares sold by them and purchased by the representatives in stabilizing transactions or to cover short sales,covering transactions.
These activities may have the representatives can requireeffect of raising or maintaining the underwritersmarket price of our common stock or selling group members that sold those shares as partpreventing or retarding a decline in the market price of this offering to repay the selling concession received by them.
our common stock. As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any
time. The underwriters may carry out these transactions on the Nasdaq National Market, in the over-the-counter market or otherwise.
The underwriters have informed us that they do not expect to make sales to accounts over which they exercise discretionary authority to exceedin excess of 5% of the total number of shares of common stock offered by this prospectus.being offered.
80
IPO Pricing. Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiations amongnegotiated between us and the representatives of the underwriters. The primaryAmong the factors to be considered in determining the initial public offering price include:these negotiations are:
At Lock-up Agreement. We, our request,executive officers and directors and substantially all of our stockholders have entered into or will, prior to the underwriters have reserved upcompletion of this offering, enter into lock-up agreements with the underwriters. Under these agreements, subject to 5%exceptions, we may not issue any new shares of common stock, and those holders of stock and options may not, directly or indirectly, sell, offer, contract or grant any option to sell, pledge, transfer or otherwise dispose of or hedge any common securities convertible into or exchangeable for shares of common stock, or publicly announce the intention to do any of the common stock being offered by this prospectus for sale to our directors, employees, business associates and related persons atforegoing, without the public offering price. The sales will be made byprior written consent of Banc of America Securities LLC throughfor a directed share program. We do not know if these persons will choose to purchase all or any portionperiod of these reserved shares, but any purchases they do make will reduce the number of shares available to the general public. These persons must commit to purchase no later than the close of business on the day following180 days from the date of this prospectus. Any directors, employeesprospectus related to this offering, subject to a potential extension of up to an additional 34 days under certain circumstances. This consent may be given at any time without public notice. In addition, during this period, we have also agreed not to file any registration statement for any shares of our common stock, other than a registration statement covering shares issued under our incentive compensation plans, without the prior written consent of Banc of America Securities LLC. Pursuant to the lock-up agreements holders of greater than 70% of the "registrable securities" under our registration rights agreement have also agreed not to make any demand for, or other persons purchasing such reservedexercise any right to registration of any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock, without the prior written consent of Banc of America Securities LLC.
Indemnification. We will indemnify the underwriters against some liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments the underwriters may be prohibited from disposingrequired to make in respect of or hedging such shares for a period of at least 180 days after the date of this prospectus.those liabilities.
The underwriters and their affiliates have provided, and may in the future provide, various investment banking, commercial banking and other financial services for us.
Compliance with Non-U.S. Laws and Regulations
Each underwriter intends to comply with all applicable laws and regulations in each jurisdiction in which it acquires, offers, sells or delivers shares of our common stock or has in its possession or distributes the prospectus.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), with effect from timeand including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) an offer of shares to timethe public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of shares to the public in that Relevant Member State at any time:
For the purposes of this provision, the expression an "offer of shares to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression Prospectus Directive means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
France
No prospectus (including any amendment, supplement or replacement thereto) has been prepared in connection with the offering of the shares that has been approved by theAutorité des marchés financiers or by the competent authority of another State that is a contracting party to the Agreement on the European Economic Area and notified to theAutorité des marchés financiers; no shares have been offered or sold and will be offered or sold, directly or indirectly, to the public in France except to permitted investors ("Permitted Investors") consisting of persons licensed to provide the investment service of portfolio management for the account of third parties, qualified investors (investisseurs qualifiés) acting for their own account and/or corporate investors meeting one of the four criteria provided in Article 1 of Decree No 2004-1019 of September 28, 2004 and belonging to a limited circle of investors (cercle restreint d'investisseurs) acting for their own account, with "qualified investors" and "limited circle of investors" having the meaning ascribed to them in Article L. 411-2 of the FrenchCode Monétaire et Financier and applicable regulations thereunder; none of the prospectus supplement, the accompanying prospectus, or any other materials related to the offering or information contained therein relating to the shares has been released, issued or distributed to the public in France except to Permitted Investors; and the direct or indirect resale to the public in France of any shares acquired by
any Permitted Investors may be made only as provided by articles L. 412-1 and L. 621-8 of the FrenchCode Monétaire et Financier and applicable regulations thereunder.
United Kingdom
Each underwriter acknowledges and agrees that:
This document is only being distributed to and our affiliatesis only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons"). The shares are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such shares will be engaged in the ordinary courseonly with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of their business.its contents.
Italy
Each underwriter acknowledges and agrees that the offering of the shares has not been cleared by the Italian Securities Exchange Commission (Commissione Nazionale per le Società e la Borsa, the "CONSOB") pursuant to Italian securities legislation and, accordingly, acknowledges and agrees that the shares may not and will not be offered, sold or delivered, nor may or will copies of the prospectus or any other documents relating to the shares or the prospectus be distributed in Italy other than to professional investors (investitori professionali), as defined in Article 31, paragraph 2 of CONSOB Regulation No. 11522 of July 1, 1998, as amended ("Regulation No. 11522") or pursuant to another exemption from the requirements of Articles 94 and seq. of Legislative Decree No. 58 of February 24, 1998 (the "Italian Finance Law") and CONSOB Regulation No. 11971 of May 14, 1999 ("Regulation No. 11971").
Each underwriter acknowledges and agrees that any offer, sale or delivery of the shares or distribution of copies of the prospectus or any other document relating to the shares or the prospectus in Italy may and will be effected in accordance with all Italian securities, tax, exchange control and other applicable laws and regulations, and, in particular, will be:
(the "Italian Banking Law"), Legislative Decree No. 58 of February 24, 1998, as amended, CONSOB Regulation No. 11522 of July 1, 1998, and any other applicable laws and regulations;
Any investor purchasing the shares in this offering is solely responsible for ensuring that any offer or resale of the shares it purchased in this offering occurs in compliance with applicable laws and regulations.
This prospectus and the information contained herein are intended only for the use of its recipient and are not to be distributed to any third party resident or located in Italy for any reason. No person resident or located in Italy other than the original recipients of this document may rely on it or its content.
In addition to the above (which shall continue to apply to the extent not inconsistent with the implementing measures of the Prospectus Directive in Italy), after the implementation of the Prospectus Directive in Italy, the restrictions, acknowledgments and agreements set out under the heading "European Economic Area" above shall apply to Italy.
The validity of the issuance of the shares of common stock offered in this prospectushereby will be passed upon for us by LoebCovington & Loeb LLP,Burling, New York, New York. Shearman & Sterling LLP, New York, New York, will pass upon certain legal matters in connection with this offering for the underwriters.
TheOur consolidated financial statements of Acorda Therapeutics, Inc. and subsidiary as of June 30, 2002December 31, 2004 and 2003 and for the related consolidated statements of operations, stockholders' equityyear ended December 31, 2004, the six month period ended December 31, 2003, and cash flows for each of the years in the three-year period ended June 30, 2003 and for the period from March 17, 1995 (inception) to June 30, 2003,2002 have been included herein and in the registration statement in reliance upon the report of KPMG LLP, independent accountants,registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
The statements of net revenues and direct expenses for the period from January 1, 2004 through July 21, 2004 and the year ended December 31, 2003 of the Zanaflex Product Line of Elan Corporation, plc have been included herein in reliance upon the report of KPMG, independent public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MOREADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. As permitted by the rules and regulations of the SEC, thisThis prospectus, which is a part of the registration statement, omits variousdoes not include all of the information exhibits, schedules and undertakings included in the registration statement. For further information pertainingwith respect to us and theour common stock, offered under this prospectus, reference is made to the registration statement and the attached exhibits and schedules. Although required material information has been presented in this prospectus, statements contained in this prospectus asstatement.
We are not currently subject to the contentsinformational requirements of the Securities Exchange Act of 1934, or provisionsthe Exchange Act. As a result of any contract or other document referredthis offering, we will become subject to in this prospectus may be summary in nature,the informational requirements of the Exchange Act, and, in each instance reference is made toaccordance therewith, will file reports and other information with the copy of this contract or other document filed as an exhibit to theSEC. The registration statement, such reports and each statement is qualified in all respects by this reference.
81
A copy of the registration statement mayother information can be inspected without charge at the public reference facilities maintained by the SECand copied at the Public Reference Room 450 Fifthof the SEC located at 100 F Street, N.W.N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any partportion of the registration statement, maycan be obtained from the SEC's offices upon the paymentPublic Reference Room of the feesSEC at prescribed by the SEC. Pleaserates. You can call the SEC at 1-800-SEC-0330 for furtherto obtain information on the operation of the Public Reference facilities. In addition, registration statements and certain other filings made with the commission through its Electronic Data Gathering, Analysis and Retrieval system, including our registration statement and all exhibits and amendments to our registration statement, are publicly available throughRoom. Such materials may also be accessed electronically by means of the SEC's website at www.sec.gov.
After this offering, we will have to providehome page on the information and reports required by the Securities Exchange Act of 1934, as amended, and we will file periodic reports, proxy statements and other information with the Securities and Exchange Commission. Our SEC filings are also available at the office of The Nasdaq National Market. For further information on obtaining copies of our public filings at The Nasdaq National Market you should call (212) 656-5060.Internet (www.sec.gov).
82
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | PAGE | ||
|---|---|---|---|
| Consolidated Financial | |||
Report of Independent | F-2 | ||
Consolidated Balance Sheets | F-3 | ||
Consolidated Statements of Operations | |||
Consolidated Statements of Stockholders' | |||
Consolidated Statements of Cash Flows | |||
Notes to Consolidated Financial Statements | F-12 | ||
| Statements of Net Revenues and Direct Expenses of Zanaflex Product Line of Elan | |||
| Report of Independent Public Accounting Firm | F-47 | ||
| Statements of Net Revenues and Direct Expenses | F-48 | ||
| Notes to Financial Statements | F-49 | ||
| Unaudited Pro Forma Combined Statement of Operations: | |||
| Introduction to Unaudited Pro Forma Combined Statement of Operations | F-52 | ||
| Unaudited Pro Forma Combined Statement of Operations | F-53 | ||
| Notes to Unaudited Pro Forma Combined Statement of Operations | F-54 | ||
F-1
When the transaction referred to in note 13(i) of the notes to the consolidated financial statements has been consummated, we will be in a position to render the following report.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
KPMG LLP
The Board of Directors and Stockholders
Acorda Therapeutics, Inc.:
We have audited the accompanying consolidated balance sheets of Acorda Therapeutics, Inc. and subsidiary (a development stage enterprise)(the Company) as of June 30, 2002December 31, 2004 and 2003, and the related consolidated statements of operations, stockholders' equity (deficit), and cash flows for each of the years inyear ended December 31, 2004, the three-yearsix-month period ended December 31, 2003, and years ended June 30, 2003 and for the period from March 17, 1995 (inception) to June 30, 2003.2002. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with auditingthe standards generally accepted inof the United States of America.Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Acorda Therapeutics, Inc. and subsidiary (a development stage enterprise)the Company as of June 30, 2002December 31, 2004 and 2003, and the results of their operations and their cash flows for each of the years inyear ended December 31, 2004, the three-yearsix-month period ended December 31, 2003, and years ended June 30, 2003 and for the period from March 17, 1995 (inception) to June 30, 2003,2002, in conformity with accounting principlesU.S. generally accepted in the United States of America.accounting principles.
| /s/ KPMG LLP |
September 26, 2003,Short Hills, New Jersey
October 3, 2005, except for note 16
(as to Note 13(i)the effects of a reverse stock split)
which is as of January 11, 2006
F-2
See accompanying Notes to Consolidated Financial Statements See accompanying Notes to Consolidated Financial Statements ACORDA THERAPEUTICS, INC. AND SUBSIDIARY Consolidated Statements of Changes in Stockholders' (Deficit) See accompanying Notes to Consolidated Financial Statements. ACORDA THERAPEUTICS, INC. AND SUBSIDIARY Consolidated Statements of Changes in Stockholders' (Deficit) (continued) See accompanying Notes to Consolidated Financial Statements. ACORDA THERAPEUTICS, INC. AND SUBSIDIARY Consolidated Statements of Changes in Stockholders' (Deficit) (continued) See accompanying Notes to Consolidated Financial Statements. ACORDA THERAPEUTICS, INC. AND SUBSIDIARY Consolidated Statements of Changes in Stockholders' (Deficit) (continued) See accompanying Notes to Consolidated Financial Statements. ACORDA THERAPEUTICS, INC. AND SUBSIDIARY Consolidated Statements of Changes in Stockholders' (Deficit) (continued) See accompanying Notes to Consolidated Financial Statements. See accompanying Notes to Consolidated Financial Statements. (1) Organization and Business Activities Acorda Therapeutics, Inc. On February 24, 2004, the Board of Directors of Acorda adopted a resolution to change the Company's fiscal year end from June 30 to December 31, effective for the six-month period ended December 31, 2003. For the six-month period ended December 31, 2002 (unaudited) grant revenue and gross profit were $25,494, net loss was $7,215,511, beneficial conversion feature accretion of issuance costs was $27,487 and the net loss applicable to common stockholders was $7,188,024. The Company acquired all of Elan Corporation plc's ("Elan") U.S. sales, marketing and distribution rights to Zanaflex Capsules and Zanaflex tablets in July 2004. These products are approved for the management of spasticity. Zanaflex tablets were approved by the FDA in 1996 and lost patent protection in 2002. There are currently 11 generic versions of Zanaflex tablets on the market. Zanaflex Capsules were approved by the FDA in 2002, but were never marketed by Elan. The Company began marketing Zanaflex Capsules in April 2005. The Company made an initial payment to Elan of $2 million and is obligated to make royalty payments as well as additional contingent payments upon achieving certain cumulative sales milestones. The Company is devoting substantially all of its efforts engaging in preclinical development. The Company has The Company plans to finance its operations (2) Summary of Significant Accounting Policies Unaudited Interim Financial Information The accompanying consolidated balance sheet as of September 30, 2005, the consolidated statements of operations and cash flow for the nine months ended September 30, 2004 and 2005, and the statement of Stockholders' (deficit) for the nine months ended September 30, 2005 are unaudited. The unaudited interim financial statements have been prepared in accordance with U.S. generally accepted accounting principles. In the opinion of the Company's management, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments consisting of normal recurring adjustments and accruals necessary for the fair presentation of the Company's financial position, results of operations and its cash flows for the nine months ended September 30, 2004 and 2005. The results for the nine months ended September 30, 2005 are not necessarily indicative of the results to be expected for the year ended December 31, 2005. Principles of Consolidation The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America and include the results of operations of the Company and its Use of Estimates The preparation of the consolidated financial statements requires management of the Company to make a number of estimates and assumptions relating to the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include research and development Cash and Cash Equivalents The Company considers all highly liquid debt instruments with original maturities of three months or less to be cash equivalents. All cash and cash equivalents are held in United States financial institutions and money market funds, which are unrestricted as to withdrawal or use. To date, the Company has not experienced any losses on its cash and cash equivalents. The carrying amount of cash and cash equivalents approximates its fair value due to its short-term and liquid nature. Restricted Cash Restricted cash represents a certificate of deposit placed by the Company with a bank for issuance of a letter of credit Short-Term Investments Short-term investments consist of corporate debt securities with original maturities greater than three months. In accordance with Statement of Financial Accounting Standards ("SFAS") No. 115 ("SFAS 115"),Accounting for Certain Investments in Debt and Equity Securities, the Company classifies its short-term investments as available-for-sale. Available-for-sale securities are recorded at fair value of the investments based on quoted market Unrealized holding gains and losses on available-for-sale securities, which are determined to be temporary, Premiums and discounts on investments are amortized over the life of the related available-for-sale security as an adjustment to yield using the Inventory Inventory is stated at the lower of cost or market value and includes amounts for both Zanaflex tablet and Zanaflex capsule inventories. All inventories consist of finished goods. Cost is determined using the first-in, first-out method (FIFO) for all inventories. The Company adjusts its inventory value based on an estimate of inventory that may expire. Property and Equipment Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed on the straight-line basis over the estimated useful lives of the Intangible Assets The Company has recorded intangible assets related to its Zanaflex acquisition. These intangible assets are amortized on a straight line basis over the period in which the Company expects to receive economic benefit and are reviewed for impairment when facts and circumstances indicate that the carrying value of the asset may not be recoverable. The determination of the expected life will be dependent upon the use and underlying characteristics of the intangible asset. In the Company's evaluation of the intangible assets, it considers the term of the underlying patent life and the expected life of the product line. If the carrying value is not recoverable, impairment is measured as the amount by which the carrying value exceeds its estimated fair value. Fair value is generally estimated based on either appraised value or other valuation techniques. Impairment of Long-Lived Assets In accordance with the Financial Accounting Standards Board ("FASB") SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, the Company continually evaluates whether events or circumstances have occurred that indicate that the estimated remaining useful life of its long-lived assets may warrant revision or that the carrying value of these assets may be impaired. The Company evaluates the realizability of its long-lived assets based on profitability and cash flow expectations for the related asset. Any write-downs are treated as permanent reductions in the carrying amount of the assets. Based on this evaluation, the Company believes that, as of each of the balance sheet dates presented, none of the Company's long-lived assets was impaired. Warrants In January 2005, the Company issued warrants that provide the holder with the right to purchase $300,000 (unaudited) worth of shares of preferred stock in the Company's next qualifying equity round or 40,000 shares of Series K mandatorily redeemable preferred stock if no such round is completed prior to December 31, 2005. Beginning July 1, 2005, these warrants are subject to FASB Staff Position No. 150-5 ("FSP 150-5"), which addresses whether freestanding warrants and other similar instruments on shares that are either puttable or mandatorily redeemable would be subject to the requirements of FASB Statement No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, regardless of the timing of the redemption feature or the redemption price. Upon adoption of FSP 150-5 on July 1, 2005, the Company reclassified the warrants from additional paid in capital to a liability based on its fair value on July 1, 2005. The warrant will be marked to market each reporting period thereafter with the change in fair value recorded to earnings. The adoption of this statement resulted in gain from a net effect of change in accounting principle of $2,805, as a result of the change in fair value of the warrant from January 2005 to July 1, 2005. In November 2005, the Company modified the terms of this warrant to provide the holder with the right to purchase $300,000 (unaudited) worth of (i) shares of preferred stock in the Company's next Qualifying Equity Round or, (ii) to the extent the Company has consummated an IPO on or before February 28, 2006, shares of Common Stock at the lower of (A) the per share price of the Common Stock sold in the IPO and (B) $7.50 per share, or (iii) to the extent the Company has not consummated either a qualifying equity round or an IPO on or before February 28, 2006, then Series K mandatorily redeemable preferred stock at $7.50 per share. Patent Costs Patent application and maintenance costs are expensed as incurred. Research and Development Research and development expenses include the clinical development costs associated with the Company's product candidates and research and development costs associated with the Company's preclinical programs. These expenses include internal research and Accounting for Income Taxes Income taxes are accounted for under the asset and liability method with deferred tax assets and liabilities recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be reversed or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance for the amounts of any tax benefits which, more likely than not, will not be realized. Revenue Recognition The Company applies the revenue recognition guidance in SFAS No. 48,Revenue Recognition When the Right of Return Exists, which amongst other criteria requires that future returns can be reasonably estimated in order to recognize revenue. The amount of future tablet returns is uncertain due to generic competition and customer conversion to Zanaflex Capsules. Zanaflex Capsules are a new product with no historical return data. Due to the uncertainty of returns for both products, the Company is accounting for these product shipments using a deferred revenue recognition model. Under the deferred revenue model, the Company does not recognize revenue upon product shipment. For these product shipments, the Company invoices the wholesaler, records deferred revenue at gross invoice sales price, and classifies the cost basis of the product held by the wholesaler as a component of inventory. The Company recognizes revenue when prescribed to the end-user, on a first-in first-out (FIFO) basis. The Company's revenue to be recognized is based on (1) the estimated prescription demand-based on pharmacy sales for its products, and (2) the Company's analysis of third-party information, including third-party market research data. The Company's estimates are subject to the inherent limitations of estimates that rely on third-party data, as certain third-party information was itself in the form of estimates, and reflect other limitations. The Company's sales and revenue recognition reflects the Company's estimates of actual product prescribed to the end-user. When the Company acquired Zanaflex from Elan, it also acquired Elan's inventory of Zanaflex tablets, which included both partial lots with expiration dating of less than 12 months and full lots with expiration dating greater than 12 months. The Zanaflex tablet inventory the Company acquired from Elan was labeled with a code identifying the inventory as Elan's. For partial tablet lots acquired, the Company has deferred recognition of revenue until the product return period expires in June 2006, since it is unable to determine whether the prescriptions filled for Zanaflex tablet with Elan's code relate to product sold by Elan or sold by the Company. After the product return period expires in June 2006, the Company will recognize revenue only to the extent of unreturned product. With regards to the full tablet lots acquired, the Company began recognizing revenue in July 2005 for prescriptions filled for Zanaflex tablets with Elan's code. All of the Zanaflex tablet inventory sold by Elan prior to the acquisition reached its expiration date in June 2005, therefore any prescriptions filled for Zanaflex tablets subsequent to June 2005 can only be from the full inventory lots acquired by the Company and sold by the Company. Inventory manufactured after the Company's acquisition of Zanaflex is labeled with a code that enables it to identify the inventory as the Company's. These codes are contained on end-user prescription data that the Company uses to recognize revenue. The Company began receiving end-user prescription data containing its code, which enabled it to begin recognizing revenue from Zanaflex tablet sales in March 2005. The Company began marketing Zanaflex Capsules in April 2005 and began recognizing revenue in the same month. At December 31, 2004 and September 30, 2005 the Company had deferred revenue from Zanaflex tablets of $6.7 million and $10.7 million (unaudited), respectively, of which $3.6 million and $2.5 million (unaudited), respectively, was related to product acquired from Elan that had an expiration date of less than 12 months at the time the Company sold it during 2004. The Company believes there is a high likelihood that this product will be returned which would result in its inability to recognize related revenue. If such product is returned the deferred revenue liability upon a return would offset the associated receivable or any credit we may issue if the wholesaler previously paid the invoice. The Company's net revenues represent total revenues less allowances for customer credits, including estimated discounts, rebates, and chargebacks. Product shipping and handling costs are included in cost of sales. These reserves are recorded in accordance with Emerging Issues Task Force (EITF) Issue No. 01-9,Accounting for Consideration Given by a Vendor to a Customer, which states that cash consideration given by a vendor to a customer is presumed to be a reduction of the selling prices of the vendor's products or services and, therefore, should be characterized as a reduction of revenue when recognized in the vendor's income statement. At the time product is shipped to wholesalers, an adjustment is recorded for estimated chargebacks, rebates, and discounts. These reserves are established by management as its best estimate based on available information and is adjusted to reflect known changes in the factors that impact such reserves. Reserves for chargebacks, rebates and discounts are established based on the contractual terms with customers, analysis of historical levels of discounts, chargebacks and rebates, communications with customers and the levels of inventory remaining in the distribution channel, as well as expectations about the market for each product and anticipated introduction of competitive products. In addition, the Company records a charge to cost of goods sold for the cost basis of the estimated product returns the Company believes may ultimately be realized at the time of product shipment to wholesalers. The Company has recognized this charge at the date of shipment since it is probable that it will receive a level of returned products; upon the return of such product it will be unable to resell the product considering its expiration dating; and it can reasonably estimate a range of returns. This charge represents the cost basis for the low end of the range of the Company's estimated returns. As part of the acquisition of Zanaflex, the Company agreed to accept any returns of Zanaflex tablets that were returned subsequent to January 17, 2005, including returns of product that was originally sold by Elan. Product returns prior to that date were the responsibility of Elan. The Company has recorded a charge to discounts and allowances of $4.1 million in 2004 for the estimated liability for product originally sold by Elan that will ultimately be returned to the Company for a refund under its returned product policy of Zanaflex tablets sold by Elan. This obligation to accept returns for product sold by Elan expires in June 2006. Revenue Recognition—Grants Revenue related to research and development grants is recognized when the related research expenses are incurred and the Company's specific performance obligations under the terms of the respective contract are satisfied. To the extent expended, grant funding related to purchases of equipment is deferred and amortized over the shorter of the equipment's useful life or the life of the related contract. Revenue recognized in the accompanying consolidated financial statements is not subject to repayment. Payments, if any, received in advance of performance under the contract are deferred and recognized as revenue when earned. Planned Initial Public Offering Costs The Company originally deferred the planned initial public offering costs incurred in 2003 in accordance with SEC Staff Accounting Bulletin ("SAB") Topic 5A, Expenses of Offering. In December 2003, the Company Concentration of Risk Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of investments in cash and cash equivalents, restricted cash, accounts receivable and debt securities. The Company maintains The Company is substantially dependent upon Elan The Company relies The Company has agreed to purchase at least 75% of its Fampridine-SR product with third parties to supply API. If Elan or an alternative manufacturer is unable to obtain API Similar to other pharmaceutical companies, the Company's principal customers are wholesale pharmaceutical distributors. The Company periodically assesses the financial strength of these customers and establishes allowances for anticipated losses, if necessary. To date, such losses have been minimal. Allowance for Doubtful Accounts A portion of the Company's accounts receivable may not be collected due principally to customer disputes and sales returns. The Company provides reserves for these situations based on the evaluation of the aging of its trade receivable portfolio and an analysis of high-risk customers. The Company has not recognized an allowance as of December 31, 2004 or September 30, 2005 (unaudited) as management believes all outstanding accounts receivable are fully collectible. Fair Value of Financial Instruments The fair value of a financial instrument represents the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a forced sale or liquidation. Significant differences can arise between the fair value and carrying amounts of financial instruments that are recognized at historical cost amounts. The following methods are used to estimate the Company's financial instruments: It is not practical for the Company to estimate the fair value of the convertible notes payable to Elan due to the specific provisions of these notes including the uncertainty of the timing of repayment which is Net loss per share is computed in accordance with SFAS No. 128,Earnings Per Share, by dividing the net loss allocable to common stockholders by the weighted average number of shares of common stock outstanding. The Company has certain options, warrants, convertible preferred stock and mandatorily redeemable convertible preferred stock (see Notes 3 and ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)Consolidated Balance Sheets June 30, Pro Forma
June 30, 2003 2002 2003
(Unaudited) Assets Current assets: Cash and cash equivalents $ 27,012,412 $ 48,319,175 $ 48,319,175 Restricted cash 249,502 252,997 252,997 Short-term investments 2,835,526 12,250,449 12,250,449 Grant receivable 147,721 361,607 361,607 Prepaid expenses 74,128 244,982 244,982 Other current assets 87,324 318,176 318,176 Total current assets 30,406,613 61,747,386 61,747,386 Property and equipment, net of accumulated depreciation 2,939,968 2,947,747 2,947,747 Minority interest—Related party 110,374 — — Other assets 139,816 111,516 111,516 Total assets $ 33,596,771 $ 64,806,649 $ 64,806,649 Liabilities, Mandatorily Redeemable
Convertible Preferred Stock and Stockholders' Equity (Deficit) Current liabilities: Accounts payable $ 1,454,636 $ 787,980 $ 787,980 Accounts payable to Related party 965,435 173,668 173,668 Accrued expenses and other current liabilities 825,192 1,291,775 1,291,775 Due to Related party 64,394 113,260 113,260 Deferred revenue — 95,462 95,462 Current portion of notes payable — 310,233 310,233 Total current liabilities 3,309,657 2,772,378 2,772,378 Long-term portion of notes payable — 612,087 612,087 Long-term convertible notes payable—principal amount, plus accrued interest less unamortized debt discount of $636,749 and $417,814 as of June 30, 2002 and 2003, respectively-Related party 7,538,251 7,907,186 7,907,186 Mandatorily Redeemable Convertible Preferred Stock: Series E convertible preferred stock—$0.001 par value. Authorized, issued, and outstanding 7,472,612 shares in 2002 and 2003 (Redemption and liquidation value of $20,176,052 at June 30, 2003) 20,066,835 476,478 — Series I convertible preferred stock—$0.001 par value. Authorized 10,282,777 and 10,204,047 shares in 2002 and 2003, respectively; issued and outstanding 10,204,047 shares in 2002 and 2003 (Redemption and liquidation value of $39,693,743 at June 30, 2003) 39,591,760 1,024,149 — Series J convertible preferred stock—$0.001 par value. Authorized, issued, and outstanding 112,790,246 shares in 2003 (Redemption and liquidation value of $56,003,896 at June 30, 2003) — 35,211,047 — Commitments and contingencies Stockholders' equity (deficit): Series A convertible preferred stock, $0.001 par value. Authorized 1,656,000 and 1,646,068 shares in 2002 and 2003, respectively; issued and outstanding 1,306,068 shares in 2002 and 2003 (liquidation value of $1,306,068 at June 30, 2003) 1,306 1,306 — Series B convertible preferred stock, $0.001 par value. Authorized 2,250,000 shares in 2002 and 2003; issued and outstanding 900,000 shares in 2002 and 2003 (liquidation value of $1,800,000 at June 30, 2003) 900 900 — Series C convertible preferred stock, $0.001. Authorized, issued, and outstanding 333,333 shares in 2002 and 2003 (liquidation value of $999,999 at June 30, 2003) 333 333 — Series D convertible preferred stock, $0.001. Authorized 400,000 shares in 2002 and 2003; issued, and outstanding—none — — — Series F convertible preferred stock, $0.001 par value. Authorized, issued, and outstanding 2,300,000 shares in 2002 and 2003 (liquidation value of $11,999,100 at June 30, 2003) 2,300 2,300 — Series G convertible preferred stock, $0.001. Authorized 1,250,000 shares in 2002 and 2003; issued, and outstanding—none — — — Series H convertible preferred stock, $0.001 par value. Authorized, issued, and outstanding 1,575,229 shares in 2002 and 2003 (liquidation value of $5,119,494 at June 30, 2003) 1,575 1,575 — Common stock, $0.001 par value. Authorized 33,670,451 and 260,000,000 shares in 2002 and 2003, respectively; issued and outstanding 248,994 shares in 2002 and 2003 249 249 16,056 Additional paid-in capital 28,408,252 107,861,689 144,563,969 Deficit accumulated during the development stage (65,324,647 ) (91,058,949 ) (91,058,949 ) Other comprehensive loss — (6,078 ) (6,078 ) Total stockholders' equity (deficit) (36,909,732 ) 16,803,325 53,514,998 Total liabilities, mandatorily redeemable convertible preferred stock and stockholders' equity (deficit) $ 33,596,771 $ 64,806,649 $ 64,806,649 See accompanying Notes to Consolidated Financial Statements.F-3ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)Consolidated Statements of Operations Period From
March 17,
1995
(inception)
to June 30,
2003 Year ended June 30 2001 2002 2003 Grant revenue $ 462,407 $ 131,592 $ 473,588 $ 3,637,117 Operating expenses incurred in the development stage: Research and development 6,141,705 11,146,415 17,526,656 46,175,443 Research and development—Related party 2,223,407 4,686,671 2,265,233 32,350,958 General and administrative 3,489,509 6,636,306 6,387,999 22,854,793 Total operating expenses 11,854,621 22,469,392 26,179,888 101,381,194 Operating loss (11,392,214 ) (22,337,800 ) (25,706,300 ) (97,744,077 ) Other income (expense): Interest expense — — (77,712 ) (77,712 ) Interest expense—Related party (443,400 ) (407,686 ) (368,935 ) (2,580,413 ) Interest income 1,824,050 983,876 392,742 5,037,989 Other income — — 25,903 25,903 Total other income (expense) 1,380,650 576,190 (28,002 ) 2,405,767 Minority interest—Related party 698,894 580,467 — 4,279,361 Net loss (9,312,670 ) (21,181,143 ) (25,734,302 ) (91,058,949 ) Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders (35,897 ) (54,973 ) (23,792,769 ) (24,426,130 ) Net loss allocable to common stockholders $ (9,348,567 ) $ (21,236,116 ) $ (49,527,071 ) $ (115,485,079 ) Net loss per share allocable to common stockholders—basic and diluted $ (39.08 ) $ (86.05 ) $ (198.91 ) Weighted average common shares outstanding used in computing net loss per share allocable to common stockholders—basic and diluted 239,207 246,778 248,994 See accompanying Notes to Consolidated Financial Statements.F-4ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)Consolidated Statements of Changes in Stockholders' Equity (Deficit) Stockholders' Equity (Deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Deficit
accumulated
during the
development
stage Accumulated
other
Comprehensive
Income
(Loss) Total
stockholders'
equity
(deficit) Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Additional
paid-in
capital Stock
subscriptions
receivable Issuance of common stock in March 1995, $0.12 per share — $ — — $ — — $ — — $ — — $ — 216,875 $ 2,603 $ 23,422 $ (24,750 ) $ — $ — $ 1,275 One-for twelve reverse stock split — — — — — — — — — — — (2,386 ) 2,386 — — — — Issuance of Series A convertible preferred stock in May 1995, $1.00 per share 610,000 610 — — — — — — — — — — 609,390 — — — 610,000 Contribution of services — — — — — — — — — ��� — — 125,000 — — — 125,000 Issuance of detachable warrants with Series A convertible preferred stock — — — — — — — — — — — — 44,971 — — — 44,971 Amortization of Series A warrants — — — — — — — — — — — — (44,971 ) — — — (44,971 ) Net loss — — — — — — — — — — — — — — (276,998 ) — (276,998 ) Balance at June 30, 1995 610,000 610 — — — — — — — — 216,875 217 760,198 (24,750 ) (276,998 ) — 459,277 Issuance of common stock in February through March and June 1996, $1.20 per share — — — — — — — — — — 4,333 52 5,148 (4,800 ) — — 400 Exercise of stock warrants — — — — — — — — — — 7,500 90 810 — — — 900 One-for twelve reverse stock split — — — — — — — — — — — (130 ) 130 — — — — Issuance of Series A convertible preferred stock in December 1995 and April 1996, $1.00 per share 450,000 450 — — — — — — — — — — 449,550 — — — 450,000 Contribution of services — — — — — — — — — — — — 125,000 — — — 125,000 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 155,189 155,189 Net loss — — — — — — — — — — — — — — (1,116,738 ) — (1,116,738 ) Balance at June 30, 1996 1,060,000 1,060 — — — — — — — — 228,708 229 1,496,025 (29,550 ) (1,393,736 ) — 74,028 Issuance of Series A convertible preferred stock in January 1997, $1.00 per share 195,000 195 — — — — — — — — — — 194,805 — — — 195,000 Issuance of Series B convertible preferred stock, net in January 1997, $2.00 per share — — 750,000 750 — — — — — — — — 1,499,250 — — — 1,500,000 Discount on below-market interest rate convertible notes — — — — — — — — — — — — 2,173,127 — — — 2,173,127 Contribution of services — — — — — — — — — — — 70,000 — — — 70,000 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 653,214 — — — 653,214 Issuance of detachable warrants with preferred stock — — — — — — — — — — — — 452,141 — — — 452,141 Amortization of warrants — — — — — — — — — — — — (452,141 ) — — — (452,141 ) Net loss — — — — — — — — — — — — — — (6,798,970 ) — (6,798,970 ) Balance at June 30, 1997 1,255,000 1,255 750,000 750 — — — — — — 228,708 229 6,086,421 (29,550 ) (8,192,706 ) — (2,133,601 ) Issuance of common stock in September 1997 and June 1998, $1.44 per share — — — — — — — — — — 2,208 26 3,024 — — — 3,050 One-for twelve reverse stock split — — — — — — — — — — — (24 ) 24 — — — — Issuance of Series F convertible preferred stock in April 1998, $5.22 per share — — — — — — 2,300,000 2,300 — — — — 11,997,700 — — — 12,000,000 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 342,658 — — — 342,658 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,059 — — — 1,059 Net loss — — — — — — — — — — — — — — (15,385,329 ) — (15,385,329 ) Balance at June 30, 1998 1,255,000 1,255 750,000 750 — — 2,300,000 2,300 — — 230,916 231 18,430,886 (29,550 ) (23,578,035 ) — (5,172,163 ) Issuance of common stock in July 1998, $1.44 per share — — — — — — — — — — 1,334 16 1,834 — — — 1,850 One-for twelve reverse stock split — — — — — — — — — — — (15 ) 15 — — — — Payments received on notes due from shareholders — — — — — — — — — — — — — 22,350 — — 22,350 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 378,814 — — — 378,814 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 97,349 — — — 97,349 Accretion of issuance costs related to Series E mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (18,042 ) — — — (18,042 ) Net loss — — — — — — — — — — — — — — (4,354,718 ) — (4,354,718 ) Balance at June 30, 1999 1,255,000 1,255 750,000 750 — — 2,300,000 2,300 — — 232,250 232 18,890,856 (7,200 ) (27,932,753 ) — (9,044,560 ) Issuance of common stock in November and December 1999, $0.36 per share — — — — — — — — — — 6,041 73 2,227 — — — 2,300 One-for twelve reverse stock split — — — — — — — — — — — (67 ) 67 — — — — Issuance of Series H convertible preferred stock in August 1999, $3.25 per share — — — — — — — — 1,575,229 1,575 — — 5,117,919 — — — 5,119,494 Payment received on notes due from shareholders — — — — — — — — — — — — — 4,200 — — 4,200 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 227,318 — — — 227,318 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 178,383 — — — 178,383 Accretion of issuance costs related to Series E mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — — (27,337 ) Net loss — — — — — — — — — — — — — — (6,898,081 ) — (6,898,081 ) Balance at June 30, 2000 1,255,000 1,255 750,000 750 — — 2,300,000 2,300 1,575,229 1,575 238,291 238 24,389,433 (3,000 ) (34,830,834 ) — (10,438,283 ) F-5ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)Consolidated Statements of Changes in Stockholders' Equity (Deficit) (Continued) Stockholders' Equity (Deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Deficit
accumulated
during the
development
stage Accumulated
other
Comprehensive
Income
(Loss) Stock
sub-
scriptions
receivable Total
stockholders'
equity
(deficit) Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Number
of shares Par
value Additional
paid-in
capital Issuance of common stock in January and June 2001, $0.84 per share — $ — — $ — — $ — — $ — — $ — 6,307 $ 75 $ 5,115 $ — $ — $ — $ 5,190 Payment received on notes due from shareholders — — — — — — — — — — — (68 ) 68 3,000 — — 3,000 Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 94,397 — — — 94,397 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 643,028 — — — 643,028 Accretion of issuance costs related to Series E mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — — (27,337 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (8,560 ) — — — (8,560 ) Net loss — — — — — — — — — — — — — — (9,312,670 ) — (9,312,670 ) Balance at June 30, 2001 1,255,000 1,255 750,000 750 — — 2,300,000 2,300 1,575,229 1,575 244,598 245 25,096,144 — (44,143,504 ) — (19,041,235 ) Issuance of Series A convertible preferred stock in May 2002, $1.00 per share 51,068 51 — — — — — — — — — — 22,749 — — — 22,800 Issuance of Series B convertible preferred stock in January 2002, $2.00 per share — — 150,000 150 — — — — — — — — 299,850 — — — 300,000 Issuance of Series C convertible preferred stock in February 2002, $3.00 per share — — — — 333,333 333 — — — — — — 999,666 — — — 999,999 Issuance of common stock in September and October 2001 and February 2002, $4.68 per share — — — — — — — — — — 4,396 53 20,566 — — — 20,619 One-for-twelve reverse stock split — — — — — — — — — — — (49 ) 49 — — — — Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — 74,624 — — — 74,624 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,331,911 — — — 1,331,911 Accretion of issuance costs related to Series E mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — — (27,337 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,636 ) — — — (27,636 ) Research and development expense for issuance of warrants and Series C preferred stock on obtaining Phase II clinical trial approval — — — — — — — — — — — — 617,666 — — — 617,666 Net loss — — — — — — — — — — — — — — (21,181,143 ) — (21,181,143 ) Balance at June 30, 2002 1,306,068 1,306 900,000 900 333,333 333 2,300,000 2,300 1,575,229 1,575 248,994 249 28,408,252 — (65,324,647 ) — (36,909,732 ) Research and development expense for issuance of stock options to non-employees — — — — — — — — — — — — (6,539 ) — — — (6,539 ) Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,580,054 — — — 1,580,054 Accretion of issuance costs related to Series E mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — — (27,337 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,636 ) — — — (27,636 ) Accretion of issuance costs related to Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (10,990 ) — — — (10,990 ) Accrual of preferred dividend on Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (629,895 ) — — — (629,895 ) Beneficial conversion feature for reduction in conversion price — — — — — — — — — — — — 80,730,286 — — — 80,730,286 Beneficial conversion feature for reduction in conversion price — — — — — — — — — — — — (20,860,491 ) — — — (20,860,491 ) Deemed dividends on preferred stock for reduction in conversion price — — — — — — — — — — — — (1,656,854 ) — — — (1,656,854 ) Issuance of preferred stock with beneficial conversion feature — — — — — — — — — — — — 20,942,405 — — — 20,942,405 Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature — — — — — — — — — — — — (579,566 ) — — — (579,566 ) Comprehensive loss Unrealized loss on investment securities — — — — — — — — — — — — — — — (6,078 ) (6,078 ) Net loss — — — — — — — — — — — — — — (25,734,302 ) — (25,734,302 ) Total Comprehensive loss (25,740,380 ) Balance at June 30, 2003 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 248,994 $ 249 $ 107,861,689 $ — (91,058,949 ) $ (6,078 ) $ 16,803,325 See accompanying Notes to Consolidated Financial Statements.F-6ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)Consolidated Statements of Cash Flows Period From
March 17,
1995
(inception)
to June 30,
2003 Year ended June 30, 2001 2002 2003 Cash flows from operating activities: Net loss $ (9,312,670 ) $ (21,181,143 ) $ (25,734,302 ) $ (91,058,949 ) Adjustments to reconcile net loss to net cash used in operating activities: Stock compensation expense 737,425 1,406,535 1,573,514 6,071,458 Expensing of warrants and beneficial conversion feature on completing Phase II clinical trial — 617,666 — 617,666 Amortization of note discount 293,400 257,686 218,935 1,755,313 Depreciation and amortization expense 123,449 417,479 740,201 1,378,346 Minority interest—Related party (698,894 ) (580,467 ) — (4,279,361 ) Changes in assets and liabilities: Decrease (increase) in grant receivables 143,263 50,993 (213,886 ) (361,607 ) (Increase) decrease in prepaid expenses and other current assets (119,353 ) 84,917 (401,706 ) (563,158 ) (Increase) decrease in other assets (110,200 ) 23,639 28,300 (111,516 ) Increase (decrease) in accounts payable and accrued expenses and other current liabilities 56,720 223,984 (200,072 ) 2,079,756 Increase (decrease) in amounts due to Related party 749,846 579,983 (592,901 ) 1,111,928 Deferred revenue — — 95,462 95,462 Restricted cash (11,901 ) (6,015 ) (3,495 ) (252,997 ) Net cash used in operating activities (8,148,915 ) (18,104,743 ) (24,489,950 ) (83,517,659 ) Cash flows from investing activities: Purchases of property and equipment (936,510 ) (2,230,916 ) (747,981 ) (4,326,094 ) Purchases of short-term investments — (2,835,526 ) (18,669,923 ) (21,505,449 ) Proceeds from maturities of short-term investments — — 9,248,922 9,248,922 Net cash used in investing activities (936,510 ) (5,066,442 ) (10,168,982 ) (16,582,621 ) Cash flows from financing activities: Proceeds from issuance of preferred stock, net of issuance costs 39,555,564 1,322,799 54,933,001 135,652,640 Proceeds from sale of interest in subsidiary — — — 3,000,000 Funding received from minority owner 411,421 757,566 110,374 1,279,361 Proceeds received on notes due from shareholders 3,000 — — 29,550 Proceeds from issuance of common stock 5,190 20,619 — 35,584 Proceeds from issuance of notes payable — — 1,163,511 1,163,511 Repayments of notes payable — — (241,191 ) (241,191 ) Proceeds from issuance of long-term convertible notes payable — — — 7,500,000 Net cash provided by financing activities 39,975,175 2,100,984 55,965,695 148,419,455 Net increase (decrease) in cash and cash equivalents 30,889,750 (21,070,201 ) 21,306,763 48,319,175 Cash and cash equivalents at beginning of period 17,192,863 48,082,613 27,012,412 — Cash and cash equivalents at end of period $ 48,082,613 $ 27,012,412 $ 48,319,175 $ 48,319,175 Supplemental disclosure: Issuance of common stock for notes receivable $ — $ — $ — $ 29,550 Discount on below-market interest rate convertible notes $ — $ — $ — $ 2,173,127 Cash paid for interest $ — $ — $ 77,712 $ 77,712 See accompanying Notes to Consolidated Financial Statements.F-7
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY(A Development Stage Enterprise)
Consolidated Balance Sheets December 31, September 30,
2005 2003 2004 (unaudited) Assets Current assets: Cash and cash equivalents $ 8,965,173 $ 11,729,112 $ 3,580,613 Restricted cash 254,078 256,568 261,037 Short-term investments 32,250,263 9,396,677 5,160,275 Trade accounts receivable, net — 1,922,838 729,811 Grant receivable 171,181 141,815 90,530 Prepaid expenses 920,084 827,891 1,460,959 Finished goods inventory held by the Company — 192,452 3,852,435 Finished goods inventory held by others — 230,748 1,101,183 Other current assets 194,962 241,251 1,154,966 Total current assets 42,755,741 24,939,352 17,391,809 Property and equipment, net of accumulated depreciation 3,093,154 2,547,014 1,905,063 Intangible Assets, net of accumulated amortization — 3,386,050 6,137,023 Other assets 111,516 109,234 109,235 Total assets $ 45,960,411 $ 30,981,650 $ 25,543,130 Liabilities, Mandatorily Redeemable Convertible Preferred Stock and Stockholders' (Deficit) Current liabilities: Accounts payable $ 2,354,131 $ 1,929,394 1,810,123 Accounts payable to related party 305,088 — — Accrued expenses and other current liabilities 4,349,828 2,890,218 7,825,869 Accrued product returns — 4,081,910 1,966,343 Deferred grant revenue 48,043 — — Deferred product revenue—Zanaflex tablets — 6,668,491 10,685,860 Deferred product revenue—Zanaflex Capsules — — 4,959,993 Current portion of notes payable 323,971 301,938 2,346,985 Total current liabilities 7,381,061 15,871,951 29,595,173 Long-term portion of notes payable 446,592 144,654 3,533,601 Other long-term liabilities — 750,000 — Warrant liability — — 388,135 Long-term convertible notes payable—principal amount, plus accrued interest less unamortized debt discount of $329,374, $175,312 and $82,818 as of December 31, 2003 and 2004 and September 30, 2005 (unaudited) respectively 8,091,412 8,421,996 8,694,877 Mandatorily Redeemable Convertible Preferred Stock: Series E convertible preferred stock—$0.001 par value. Authorized, issued, and outstanding 7,472,612 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (Redemption and liquidation value of $20,176,052 as of December 31, 2004) 2,449,656 6,396,021 9,347,741 Series I convertible preferred stock—$0.001 par value. Authorized, issued and outstanding, 10,204,047 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (Redemption and liquidation value of $39,693,743 as of December 31, 2004) 4,897,447 12,644,040 18,438,141 Series J convertible preferred stock—$0.001 par value. Authorized, 112,790,233 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued, and outstanding 112,790,233 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (Redemption and liquidation value of $64,109,973 as of December 31, 2004) 22,824,094 35,100,482 44,291,812 Series K convertible preferred stock—$0.001 par value. Authorized, 1,573,330 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding 1,533,327 shares at December 31, 2004 and September 30, 2005 (unaudited) (Redemption and liquidation value of $12,720,513 at December 31, 2004) — 12,223,211 12,922,497 Commitments and contingencies Stockholders' (deficit): Series A convertible preferred stock, $0.001 par value. Authorized 1,646,068 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding 1,306,068 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (liquidation value of $1,306,068 as of December 31, 2004) 1,306 1,306 1,306 Series B convertible preferred stock, $0.001 par value. Authorized 2,250,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding 900,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (liquidation value of $1,800,000 as of December 31, 2004) 900 900 900 Series C convertible preferred stock, $0.001 par value. Authorized, issued, and outstanding 333,333 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (liquidation value of $999,999 as of December 31, 2004) 333 333 333 Series D convertible preferred stock, $0.001 par value. Authorized 400,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding—none — — — Series F convertible preferred stock, $0.001 par value. Authorized, issued, and outstanding 2,300,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (liquidation value of $11,999,100 as of December 31, 2004) 2,300 2,300 2,300 Series G convertible preferred stock, $0.001 par value. Authorized 1,250,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding—none — — — Series H convertible preferred stock, $0.001 par value. Authorized, issued, and outstanding 1,575,229 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited) (liquidation value of $5,119,494 as of December 31, 2004) 1,575 1,575 1,575 Common stock, $0.001 par value. Authorized 200,000,000 shares at December 31, 2003 and 2004 and September 30, 2005 (unaudited); issued and outstanding 195,209, 197,569 and 208,743 shares as of December 31, 2003 and 2004 and September 30, 2005 (unaudited), respectively 195 198 209 Additional paid-in capital 127,631,942 111,957,403 96,806,331 Accumulated deficit (127,770,920 ) (172,511,684 ) (198,475,470 ) Other comprehensive income (loss) 2,518 (23,036 ) (6,331 ) Total stockholders' (deficit) (129,851 ) (60,570,705 ) (101,668,847 ) Total liabilities, mandatorily redeemable convertible preferred stock and stockholders' (deficit) $ 45,960,411 $ 30,981,650 $ 25,543,130
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Consolidated Statements of Operations Nine-month period ended September 30, Year ended June 30, Six-month period ended
December 31,
2003 Year ended December 31,
2004 2002 2003 2004 2005 (unaudited) (unaudited) Gross sales—Zanaflex $ — $ — $ — $ — $ — $ 3,239,091 Less: discounts and allowances — — — (4,416,691 ) (144,338 ) (991,560 ) Net sales — — — (4,416,691 ) (144,338 ) 2,247,531 Grant revenue 131,592 473,588 382,094 479,495 444,920 184,195 Total net revenue 131,592 473,588 382,094 (3,937,196 ) 300,582 2,431,726 Less: cost of sales — — — (885,450 ) (362,695 ) (2,273,970 ) Gross profit 131,592 473,588 382,094 (4,822,646 ) (62,113 ) 157,756
Operating expenses:
Research and development 11,146,415 17,526,656 16,743,098 21,999,091 18,620,938 9,652,543 Research and development—related party 4,686,671 2,265,233 3,343,681 — — — Sales and marketing — — — 4,661,643 2,792,962 9,657,233 General and administrative 6,636,306 6,387,999 17,068,746 13,283,506 11,033,820 6,338,716 Total operating expenses 22,469,392 26,179,888 37,155,525 39,944,240 32,447,720 25,648,492 Operating loss (22,337,800 ) (25,706,300 ) (36,773,431 ) (44,766,886 ) (32,509,833 ) (25,490,736 ) Other income (expense): Interest and amortization of debt discount expense — (77,712 ) (37,646 ) (385,419 ) (297,419 ) (824,196 ) Interest and amortization of debt discount expense—related party (407,686 ) (368,935 ) (184,226 ) — — — Interest income 983,876 392,742 276,334 409,118 329,263 347,352 Other income — 25,903 6,998 2,423 2,423 989 Total other income (expense) 576,190 (28,002 ) 61,460 26,122 34,267 (475,855 ) Minority interest—related party 580,467 — — — — — Cumulative effect of change in accounting principle — — — — — 2,805 Net loss (21,181,143 ) (25,734,302 ) (36,711,971 ) (44,740,764 ) (32,475,566 ) (25,963,786 ) Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders (54,973 ) (24,320,031 ) (11,984,669 ) (24,746,337 ) (18,496,128 ) (18,636,443 ) Net loss allocable to common stockholders $ (21,236,116 ) $ (50,054,333 ) $ (48,696,640 ) $ (69,487,101 ) $ (50,971,694 ) $ (44,600,229 ) Net loss per share allocable to common stockholders—basic and diluted $(111.90 ) $(261.38 ) $(252.87 ) $(351.76 ) $(259.22 ) $(221.17 ) Weighted average common shares outstanding used in computing net loss per share allocable to common stockholders—basic and diluted 189,786 191,497 192,573 197,541 196,636 201,656 Stockholders' (deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Accumulated
Comprehensive
Income
(Loss) Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Additional
paid-in
capital Accumulated
Deficit Total
stockholders'
(deficit) Balance at June 30, 2001 1,255,000 $ 1,255 750,000 $ 750 — $ — 2,300,000 $ 2,300 1,575,229 $ 1,575 188,120 $ 188 $ 25,096,201 $ (44,143,504 ) $ — $ (19,041,235 ) Issuance of Series A convertible preferred stock in May 2002, $1.00 per share 51,068 51 — — — — — — — — — — 22,749 — — 22,800 Issuance of Series B convertible preferred stock in January 2002, $2.00 per share — — 150,000 150 — — — — — — — — 299,850 — — 300,000 Issuance of Series C convertible preferred stock in February 2002, $3.00 per share — — — — 333,333 333 — — — — — — 999,666 — — 999,999 Issuance of common stock in September and October 2001 and February 2002, $4.68 per share — — — — — — — — — — 3,381 4 20,615 — — 20,619 Research and development expense for issuance of stock options to nonemployees — — — — — — — — — — — — 74,624 — — 74,624 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,331,911 — — 1,331,911 Accretion of issuance costs related to Series F mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — (27,337 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,636 ) — — (27,636 ) Research and development expense for issuance of warrants and Series C preferred stock on obtaining Phase II clinical trial approval — — — — — — — — — — — — 617,666 — — 617,666 Net loss — — — — — — — — — — — — — (21,181,143 ) — (21,181,143 ) Balance at June 30, 2002 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 191,501 $ 192 $ 28,408,309 $ (65,324,647 ) $ - $ (36,909,732 ) Stockholders' (deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Accumulated
Comprehensive
Income
(Loss) Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Additional
paid-in
capital Accumulated
Deficit Total
stockholders'
(deficit) Research and development expense for issuance of stock options to nonemployees — — — — — — — — — — — — (6,539 ) — — (6,539 ) Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,580,054 — — 1,580,054 Accretion of issuance costs related to Series E, I and J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,337 ) — — (27,337 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (27,636 ) — — (27,636 ) Accretion of issuance costs related to Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (10,990 ) — — (10,990 ) Accrual of preferred dividends of Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (629,895 ) — — (629,895 ) Beneficial conversion feature for reduction in conversion price — — — — — — — — — — — — 80,730,286 — — 80,730,286 Deemed dividends on preferred stock for reduction in conversion price, Series A, B, C, F and H — — — — — — — — — — — — (20,860,491 ) — — (20,860,491 ) Deemed dividends on preferred stock for reduction in conversion price, Series E and I — — — — — — — — — — — — (1,656,854 ) — — (1,656,854 ) Issuance of preferred stock with beneficial conversion feature, Series J — — — — — — — — — — — — 39,994,812 — — 39,994,812 Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature, Series J — — — — — — — — — — — — (1,106,828 ) — — (1,106,828 ) Comprehensive loss -Unrealized loss on investment securities — — — — — — — — — — — — — — (6,078 ) (6,078 ) Net loss — — — — — — — — — — — — — (25,734,302 ) — (25,734,302 ) Total Comprehensive loss (25,740,380 ) Balance at June 30, 2003 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 191,501 $ 192 $ 126,386,891 $ (91,058,949 ) $ (6,078 ) $ 35,328,470 Stockholders' (deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Accumulated
Comprehensive
Income
(Loss) Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Additional
paid-in
capital Accumulated
Deficit Total
stockholders'
(deficit) Research and development expense for issuance of stock options to nonemployees — — — — — — — — — — — — 8,488 — — 8,488 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 13,198,080 — — 13,198,080 Exercise of stock options — — — — — — — — — — 3,687 3 23,232 — — 23,235 Accretion of issuance costs related to Series E, I and mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (8,188 ) — — (8,188 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (7,434 ) — — (7,434 ) Accretion of issuance costs related to Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (32,323 ) — — (32,323 ) Deemed dividends on preferred stock for reduction in conversion price, Series E and I — — — — — — — — — — — — (5,830,852 ) — — (5,830,852 ) Accrual of preferred dividends on Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (2,210,688 ) — — (2,210,688 ) Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature, Series J — — — — — — — — — — — — (3,895,184 ) — — (3,895,184 ) Fractional share reimbursement liability due to reverse stock split — — — — — — — — — — — — (80 ) — — (80 ) Comprehensive loss Unrealized gain on investment securities — — — — — — — — — — — — — — 8,596 8,596 Net loss — — — — — — — — — — — — — (36,711,971 ) — (36,711,971 ) Total Comprehensive loss (36,703,375 ) Balance at December 31, 2003 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 195,188 $ 195 $ 127,631,942 $ (127,770,920 ) $ 2,518 $(129,851 ) Stockholders' (deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Accumulated
Comprehensive
Income
(Loss) Total
stockholders'
equity
(deficit) Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Additional
paid-in
capital Accumulated
Deficit Research and development expense for issuance of stock options to nonemployees — — — — — — — — — — — — 15,458 — — 15,458 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 6,812,795 — — 6,812,795 Compensation expense for issuance of restricted stock to employees — — — — — �� — — — — — — — 2,235,263 — — 2,235,263 Exercise of stock options — — — — — — — — — — 2,360 3 8,282 — — 8,285 Accretion of issuance costs related to Series E, I, J and K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (16,376 ) — — (16,376 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (14,869 ) — — (14,869 ) Accretion of issuance costs related to Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (64,646 ) — — (64,646 ) Accretion of issuance costs related to Series K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (10,332 ) — — (10,332 ) Accrual of preferred dividends of Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (4,421,377 ) — — (4,421,377 ) Accrual of preferred dividends of Series K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (766,664 ) — — (766,664 ) Deemed dividends on preferred stock for reduction in conversion price, Series E and I — — — — — — — — — — — — (11,661,705 ) — — (11,661,705 ) Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature, Series J — — — — — — — — — — — — (7,790,368 ) — — (7,790,368 ) Comprehensive loss Unrealized loss on investment securities — — — — — — — — — — — — — — (25,554 ) (25,554 ) Net loss — — — — — — — — — — — — — (44,740,764 ) — (44,740,764 ) Total Comprehensive loss (44,766,318 ) Balance at December 31, 2004 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 197,548 $ 198 $ 111,957,403 $ (172,511,684 ) $ (23,036 ) $ (60,570,705 ) Stockholders' (deficit) Series A convertible
preferred stock Series B convertible
preferred stock Series C convertible
preferred stock Series F convertible
preferred stock Series H convertible
preferred stock Common Stock Accumulated
Comprehensive
Income
(Loss) (Unaudited) Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Number
of
shares Par
value Additional
paid-in
capital Accumulated
Deficit Total
stockholders'
(deficit) Research and development expense for issuance of stock options to nonemployees — — — — — — — — — — — — 47,246 — — 47,246 Compensation expense for issuance of stock options to employees — — — — — — — — — — — — 1,991,827 — — 1,991,827 Compensation expense for issuance of restricted stock to employees — — — — — — — — — — — — 1,425,861 — — 1,425,861 Exercise of stock options — — — — — 11,195 15 20,433 — — 20,448 One for one point three reverse stock split — — — — — — — — — — — (4 ) 4 — — — Accretion of issuance costs related to Series E, I, J and K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (12,282 ) — — (12,282 ) Accretion of issuance costs related to Series I mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (11,152 ) — — (11,152 ) Accretion of issuance costs related to Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (48,485 ) — — (48,485 ) Accretion of issuance costs related to Series K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (9,300 ) — — (9,300 ) Accrual of preferred dividends of Series J mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (3,316,033 ) — — (3,316,033 ) Accrual of preferred dividends of Series K mandatorily redeemable convertible preferred stock — — — — — — — — — — — — (689,997 ) — — (689,997 ) Deemed dividends on preferred stock for reduction in conversion price, Series E and I — — — — — — — — — — — — (8,722,382 ) — — (8,722,382 ) Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature, Series J — — — — — — — — — — — — (5,826,812 ) — — (5,826,812 ) Comprehensive loss Unrealized gain on investment securities — — — — — — — — — — — — — — 16,705 16,705 Net loss — — — — — — — — — — — — — (25,963,786 ) — (25,963,786 ) Total Comprehensive loss (25,947,081 ) Balance at September 30, 2005 (unaudited) 1,306,068 $ 1,306 900,000 $ 900 333,333 $ 333 2,300,000 $ 2,300 1,575,229 $ 1,575 208,743 $ 209 $ 96,806,331 $ (198,475,470 ) $ (6,331 ) $ (101,668,847 )
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Consolidated Statements of Cash Flows Year ended June 30, Six months
ended
December 31, Year ended December 31, Nine months ended September 30, 2002 2003 2003 2004 2004 2005 (unaudited) (unaudited) Cash flows from operating activities: Net loss $ (21,181,143 ) $ (25,734,302 ) $ (36,711,971 ) $ (44,740,764 ) $ (32,475,566 ) $ (25,963,786 ) Adjustments to reconcile net loss to net cash used in operating activities: Stock compensation expense 1,406,535 1,573,514 13,206,567 9,063,517 7,307,146 3,464,928 Expensing of warrants and beneficial conversion 617,666 — — — — — Amortization of note discount 257,686 218,935 88,440 154,062 116,244 92,495 Amortization of discount on short-term investments — 264,519 160,735 1,723,827 563,108 193,930 Accretion of note payable — — — — — 251,373 Depreciation and amortization expense 417,479 740,201 445,260 1,191,860 886,862 1,033,130 Minority interest—Related party (580,467 ) — — — — — Changes in assets and liabilities: Increase in accounts receivable — — — (1,922,838 ) (1,576,380 ) 1,193,027 Decrease (increase) in grant receivables 50,993 (213,886 ) 190,426 29,366 (11,493 ) 51,285 Decrease (increase) in prepaid expenses and other current assets 84,917 (401,706 ) (551,888 ) 45,904 1,313 (1,546,783 ) Increase in inventory held by the Company — — — (192,452 ) (610,672 ) (3,659,983 ) Increase in inventory held by others — — — (230,748 ) — (870,435 ) Decrease in other assets 23,639 28,300 — 2,282 2,281 — Increase (decrease) in accounts payable, accrued expenses, other current liabilities 223,984 (200,072 ) 4,624,205 (3,384,347 ) (2,425,393 ) 2,746,766 Increase (decrease) in returns liability — — — 4,081,910 — (2,115,567 ) Increase (decrease) in amounts due to related party 579,983 (592,901 ) 113,946 (128,566 ) (305,088 ) — Increase (decrease) in deferred grant revenue — 95,462 (47,419 ) (48,043 ) (38,502 ) — Increase in deferred product revenue—tablets — — — 6,668,491 2,764,881 4,959,993 Increase in deferred product revenue—capsules — — — — — 4,017,369 Increase (decrease) in royalty payable — — — 750,000 — (750,000 ) Restricted cash (6,015 ) (3,495 ) (1,081 ) (2,490 ) (1,754 ) (4,470 ) Net cash used in operating activities (18,104,743 ) (24,225,431 ) (18,482,780 ) (26,939,029 ) (25,803,013 ) (16,906,728 ) Cash flows from investing activities: Purchases of property and equipment (2,230,916 ) (747,981 ) (590,666 ) (531,770 ) (462,822 ) (142,154 ) Purchases of intangible assets — — — (2,000,000 ) (2,000,000 ) (750,000 ) Purchases of short-term investments (2,835,526 ) (18,940,520 ) (39,763,681 ) (19,179,583 ) (19,179,583 ) (11,520,820 ) Proceeds from maturities of short-term investments — 9,255,000 19,611,727 40,283,788 32,118,787 15,580,000 Net cash (used in) provided by investing activities (5,066,442 ) (10,433,501 ) (20,742,620 ) 18,572,435 10,476,382 3,167,026 Cash flows from financing activities: Proceeds from issuance of preferred stock, net of issuance costs 1,322,799 54,933,001 — 11,446,219 11,446,219 — Funding received from minority owner 757,566 110,374 — — — — Proceeds from issuance of common stock 20,619 — 23,235 8,285 8,285 20,448 Proceeds from issuance of notes payable — 1,163,511 — — — 5,785,215 Proceeds from issuance of warrants — — — — — 214,785 Repayments of notes payable — (241,191 ) (151,757 ) (323,971 ) (240,327 ) (429,245 ) Reverse stock split fractional share liability — — (80 ) — — — Net cash provided by (used in) financing activities 2,100,984 55,965,695 (128,602 ) 11,130,533 11,214,177 5,591,203 Net increase (decrease) in cash and cash equivalents (21,070,201 ) 21,306,763 (39,354,002 ) 2,763,939 (4,112,454 ) (8,148,499 )
Cash and cash equivalents at beginning of period
48,082,613
27,012,412
48,319,175
8,965,173
8,965,173
11,729,112
Cash and cash equivalents at end of period $ 27,012,412 $ 48,319,175 $ 8,965,173 $ 11,729,112 $ 4,852,719 $ 3,580,613
Supplemental disclosure:
Cash paid for interest $ — $ 77,293 $ 37,646 $ 54,835 $ 43,777 $ 374,535
Non-cash charges related to convertible preferred stock:
Beneficial conversion feature — 23,624,173 9,726,036 19,452,073 14,564,279 14,549,194 Accretion of issuance costs 54,973 65,963 47,945 106,223 79,151 81,219 Preferred dividend — 629,895 2,210,688 5,188,041 3,852,697 4,006,030
Non-cash investing activities:
Accrued Zanaflex milestone payments — — — 1,500,000 — 2,250,000
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Notes to Consolidated Financial Statements(Acorda("Acorda" or the Company)"Company") was incorporated in Delaware on March 17, 1995. The Company is a developmentcommercial stage biopharmaceutical company engaged indedicated to the identification, development and commercialization of novel therapies that improve neurological function in people with multiple sclerosis, spinal cord injury multiple sclerosis and relatedother disorders of the central nervous system. Prior to the fiscal year ended December 31, 2004, the Company was a development stage enterprise.toward conducting pharmaceutical development,to promoting sales of Zanaflex Capsules, conducting clinical trials, pursuing regulatory approval for products under development, and raising needed capital. In the course of its activities the Company has sustained operating losses and expects such losses to continue for the foreseeable future.not generatedbegun to generate product revenues andbut has not achieved profitable operations or positive cash flows from operations. There is no assurance that profitable operations, if ever achieved, could be sustained on a continuing basis. The Company's accumulated deficit accumulated duringsince inception through September 30, 2005 was $198.5 million (unaudited) and the development stage aggregated $91,058,949, through June 30, 2003, and itCompany expects to continue to incur substantial and increasing losses in future periods.for the foreseeable future. Further, the Company's future operations are dependent on the success of the Company's researchCompany in commercializing Zanaflex Capsules, completing the clinical development of Fampridine-SR in MS and commercialization efforts and, ultimately, uponobtaining regulatory approval and market acceptance of the Company's products.this product candidate and advancing its preclinical programs.withthrough a combination of public and/or private placementissuance of equity securities, grants, payments from strategic research and development arrangements, and revenues from future product sales.Zanaflex Capsules, loans and, to a lesser extent, grants. There are no assurances that the Company will be successful in obtaining an adequate level of financing needed for the long-termto fund its development and commercialization of its planned products.efforts. The Company believes that its current financial resources and sources of liquidity should be adequate to fund operations at least through December 31, 2004,January 1, 2006 based on the Company's current projected spending levels.approximately 83%majority owned subsidiary (see Note 7)Notes 7 and 11). All intercompany accounts and transactions have been eliminated in consolidation.income taxes,(clinical trial accrual), beneficial conversion charge andcharges, stock warrants and option accounting.accounting, which are all dependent on the fair value of the Company's equity security. In addition, the Company recognizes revenue based on estimated prescriptions filled. The Company adjusts its inventory value based on an estimate of inventory that may expire. Actual results could differ from those estimates.F-8 in the amount of $226,825 to the Company's lessor for office space.prices at June 30, 2003.prices. The Company consideredconsiders all of these investments to be available-for-sale. on available-for-sale securities are excluded from earnings and are reported as a separate component of other comprehensive loss.income (loss).effective-interesteffective- interest method. Dividend and interest income are recognized when earned. Realized gains and losses are determined on the average cost method. Amortized premiums and discounts, dividend and interest income and realized gains and losses are included in interest income. various assets, which range from three to seven years. Leasehold improvements are recorded at cost, less accumulated amortization, which is computed on the straight-line basis over the shorter of the useful lives of the asset or the remaining lease term. Expenditures for maintenance and repairs are charged to expense as incurred.development bydevelopments costs and the Company andcosts of research and development conducted on behalf of the Company by outside advisors,third parties, including sponsored university-based research agreements, and clinical study vendors. All research and development costs are expensed as incurred. Costs incurred in obtaining technology licenses are charged immediately to research and development expense if the technology licensed has not reached technological feasibility and has no alternative future uses.F-9Long-Lived Assets In accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, which the Company adopted on July 1, 2002, the Company monitors events or changes in circumstances that may indicate carrying amounts of its long-lived assets may not be recoverable. When such events or changes in circumstances are present, the Company assesses the recoverability of its assets by determining whether the carrying amount of its assets will be recovered through undiscounted, expected future cash flows. Should the Company determine that the carrying values of specific long-lived assets are not recoverable, the Company would record a charge to operations to reduce the carrying value of such assets to their fair values. The Company considers various valuation factors, principally discounted cash flows, to assess the fair values of long-lived assets. Prior to the adoption of SFAS No. 144, the Company accounted for long-lived assets in accordance with SFAS No. 121,Accounting for Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of. Since inception,has recognized $3,637,117deferred its plan for an initial public offering. As a result, the related costs of approximately $1.3 million were expensed and included in grant revenue,the Company's consolidated statements of which $3,370,972 has been received byoperations for the Company.six month period ended December 31, 2003.such financial instrumentscash and cash equivalents, restricted cash and debt securities with approved financial institutions. The Company is exposed to credit risks in the event of default by the financial institutions or issuers of investments in excess of FDIC insured limits. The Company performs periodic evaluations of the relative credit standing of these financial institutions and limits the amount of credit exposure with any institution.F-10 Corporation plc ("Elan") for several activities related to the development and commercialization of Fampridine-SR. The Company will rely on Elan to complete the chemistry, manufacturing and controls section of the New Drug Application ("NDA") for Fampridine-SR in SCI.multiple sclerosis. If Elan fails to provide itthese parts of the NDA in a complete and timely manner the Company could incur delays in filing of its NDA for Fampridine-SR in SCI.multiple sclerosis.uponon a single manufacturer, Elan, for the supply of Zanaflex Capsules and on another single manufacturer, Novartis, for the supply of tizanadine, the active pharmaceutical ingredient, or API, in Zanaflex Capsules. If either Elan or Novartis experiences any disruption in their operations, a delay or interruption in the supply of the Company's products could result until the affected supplier cures the problem or the Company locates an alternative source of supply. The Company may not be able to enter into alternative supply arrangements on terms that are commercially favorable, if at all. Any new supplier would also be required to qualify under applicable regulatory requirements. The Company could experience substantial delays before it is able to qualify any new supplier and transfer the required manufacturing technology to that supplier. Novartis has informed the Company that it intends to discontinue tizanidine production by the end of the first quarter of 2006. The Company has established relationships with the company that currently formulates the tablets and the company that bottles and package Zanaflex tablets; however, the Company does not have an agreement with an alternative manufacturer of tizanidine. It is the responsibility of each of Novartis and Elan to procure the API required to meet its contractual obligations to supply the Company with product. The Company does not anticipate an interruption in API supply. Novartis is currently transferring the methods of manufacturing tizanidine to Rohner, an API manufacturer in Pratteln, Switzerland. The Company has verified this transfer and plans to audit Rohner's manufacturing site towards the end of first quarter 2006, following the commencement of Rohner's manufacture of tizanidine. The Company has also identified an alternate source for tizanidine in collaboration with Elan. The Company does not anticipate entering into a supply agreement for API with either party. Any cost associated with validating API suppliers would be incurred by Novartis or Elan. The costs of the Company's audit of Rohner or any other supplier are not material and are considered part of its normal course of business.requirements.requirements from Elan, and must make compensatory payments if it does not purchase 100% of its requirements from Elan. The Company and Elan have agreed that the Company may purchase up to 25% of its annual Fampridine-SR requirements from Patheon, Inc., a qualified manufacturing source of Fampridine-SR, without making compensatory payments to Elan. In addition, the Company does not have direct contractual relationships with the suppliers of fampridine, the active pharmaceutical ingredient in Fampridine-SR, referred to as API. Currently, the Company is relying on Elan's contracts supplies from these suppliers for any reason, a new supplier would have to be identified by the Company. Although there are other supplierspotential sources of API are readily available, a changeany new supplier would be required to a supplier, that was not previously approved in the Company's NDA may require formal approval by the Food and Drug Administration ("FDA") before the Company could use the new suppliers' API product.qualify under applicable regulatory requirements. Any delays in obtaining API to manufacture Fampridine-SR wouldcould delay the commercializationclinical trials of Fampridine-SR. % of total trade accounts receivable As of December 31, 2004 As of September 30, 2005 (unaudited) Major customers: Cardinal 27 % 11 % McKesson 52 60 Amerisource 13 11 Total 92 % 82 % tied todependent upon regulatory approval of certain products. The terms of these notes are disclosed at Note 10. In September 2003, the notes were restructured, see Note 13.11.Net Loss PerEarnings per Share7)8), which have not been used in the calculation of diluted net loss per share because to do so would be anti-dilutive. Anti- dilutive shares totaled 24,091,289 as of June 30, 2002, 136,881,522 as of June 30, 2003 and December 31, 2003, and 138,414,849 as of December 31, 2004 and September 30, 2005 (unaudited). As such, the numerator and the denominator used in computing both basic and diluted net loss per share allocable to common stockholders for each year are equal. The Company has reflected the beneficial conversion feature of $23,096,911,for Series E, Series I and Series J, accretion of issuance costs of $65,963,for Series E, Series I, Series J and Series K, and preferred dividend of $629,895 in the 2003 net loss allocable toF-11common stockholders (See Notes 3for Series J and 7). The 2001 and 2002 net loss allocable to common stockholders reflect accretion of issuance costs and the beneficial conversion feature on issuance of Series C convertible preferred stockK in the net loss allocable to common stockholders (See Note 11).as set forth below.
| | Beneficial conversion feature | Accretion of issuance costs | Preferred dividend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| For the year ended December 31, 2004 | $ | 19,452,073 | $ | 106,223 | $ | 5,188,041 | |||
| For the six month period ended December 31, 2003 | 9,726,036 | 47,945 | 2,210,688 | ||||||
| For the year ended June 30, 2003 | 23,624,173 | 65,963 | 629,895 | ||||||
| For the year ended June 30, 2002 | — | 54,973 | — | ||||||
For the nine month period ended September 30, 2005 (unaudited) | 14,549,194 | 81,219 | 4,006,030 | ||||||
| For the nine month period ended September 30, 2004 (unaudited) | 14,564,279 | 79,151 | 3,852,697 | ||||||
Stock-Based Compensation
At June 30, 2003, theThe Company hadhas various stock-based employee and non-employee compensation plans, which are described more fully in Note 8.9. The Company accounts for options and restricted stock granted to employees and directors in accordance with the fair value method of SFAS No. 123,Accounting for Stock-Based Compensation ("SFAS No. 123"), as amended by SFAS No. 148,Accounting for Stock-Based Compensation—Compensation Transition and Disclosure an amendment of FASB Statement No. 123 and related interpretations. As such, compensation expense is recorded on stock option and restricted stock grants based on the fair value of the options or restricted stock granted, which is estimated on the date of grant using the Black-Scholes option-pricing model for stock options granted, and it is recognized on a straight-line basis over the vesting period. The Company accounts for stock options granted to non-employees on a fair-value basis in accordance with SFAS No. 123, Emerging Issues Task Force Issue ("EITF")EITF No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, and FASB Interpretation No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans an Interpretation of APB Opinion No. 15 and 25. As a result, the non-cash charge to operations for non-employee options with vesting or other performance criteria is affected each reporting period by changes in the estimated fair value of the Company's common stock. All the stock option grants made by the Company since fiscal 1996 are in the money. The two factors which most affect charges or credits to operations related to stock-based compensation are the fair value of the common stock underlying stock options for which stock-based compensation is
recorded and the volatility of such fair value. If the Company's estimates of the fair value of these equity instruments changes, it would have the effect of changing compensation expense. Because shares of the Company's common stock have not been publicly traded, the Company generally estimates the fair value of its common stock based on the most recent previous sale of convertible preferred stock (convertible on a one-for-one basis (one-for-twelve post reverse split) into common stock). The Company does not discount the issuance price of its preferred stock in estimating for financial reporting purposes the fair value of its common stock.
Segment Information
The Company is managed and operated as one business. The entire business is managed by a single management team that reports to the chief executive officer. The Company does not operate separate lines of business with respect to any of its product candidates. Accordingly, the Company does not prepare discrete financial information with respect to separate product candidates or by location and does not have separately reportable segments as defined by SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information.
Derivatives
On July 1, 2001, the Company adopted SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities. This Statement requires the recognition of all derivative instruments as either assets or liabilities in the consolidated balance sheet, and the periodic adjustment of those instruments to fair value. The classification of gains and losses resulting from changes in the fair values of derivatives is dependent on the intended use of the derivative and its resultant designation.
F-12
During the first quarter of fiscal 2003, the Company entered into a foreign currency option transaction to sell (put option) U.S. dollars to British Pounds amounting to $294,739, with a strike price of $1.4280. The option expiration date was January 31, 2003. The Company's primary purpose for entering into this transaction was to cover an exchange gain or loss on a British Pound denominated contract to be entered into with a foreign company. This contract was not entered into by the Company. During fiscal 2003, the Company recorded a gain of $25,903 related to this option transaction, which is classified as other income in the consolidated statement of operations.
Comprehensive income (loss)Income (Loss)
SFAS No. 130,Reporting Comprehensive Income ("SFAS No. 130") establishes standards for the reporting and display of comprehensive income (loss) and its components in a full set of financial statements. SFAS No. 130 requires that unrealized lossesgains (losses) from the Company's investment securities be included in other comprehensive income (loss).
Pro Forma Consolidated Balance Sheet (Unaudited)
Upon the consummation of the initial public offering contemplated herein, all of the outstanding shares of convertible preferred stock and mandatorily redeemable convertible preferred stock at June 30, 2003 automatically convert into 15,806,617 shares of common stock (see Note 7). The June 30, 2003 unaudited pro forma consolidated balance sheet have been prepared assuming the conversion of the convertible preferred stock and the mandatorily redeemable convertible preferred stock outstanding as of June 30, 2003, into common stock as of June 30, 2003.
Pro Forma Net Loss Per Share (Unaudited)
The following pro forma basic and diluted net loss per share allocable to common stockholders and shares used in computing pro forma basic and diluted net loss per share allocable to common stockholders have been presented reflecting the automatic conversion into shares of common stock of the convertible preferred stock and mandatorily redeemable convertible preferred stock upon completion of the initial public offering contemplated herein (see Note 7), using the as converted method from their respective dates of issuance. Pro forma adjustments related to the pro forma consolidated statement of operations have been computed assuming the initial public offering was consummated at the beginning of the fiscal year ended June 30, 2003 and include adjustments giving effect to the following: (a) recognition of unamortized portion of beneficial conversion charge of $78,575,780, which is calculated as follows: (x) beneficial conversion feature of $80,730,286 for reset in conversion price (see Note 2), plus (y) beneficial conversion feature of $20,942,405 on issuance of Series J mandatorily redeemable convertible preferred stock (see Note 2), less (z) beneficial conversion feature charged to additional paid-in capital for the year ended June 30, 2003 of $23,096,911 (b) recognition of unamortized portion of issuance costs relating to Series E, Series I and Series J preferred stock charge of $479,457, which is calculated as follows: (x) total issuance costs relating to Series E, Series I and Series J of $681,668 (see Note 7), less (y) accumulated amortization of issuance costs as of June 30, 2003 of $202,211, and (c) reversal of accrued preferred dividend on Series J preferred stock in the amount of $629,895 (see Note 7). Weighted average common shares outstanding used in computing pro forma net less per share allocable to common stockholder is calculated based on (a) Series A through Series I equivalent common shares from the beginning of the fiscal year; (b) additional equivalent common shares
F-13
issuable for Series A through Series I, based on reset to conversion price from the date of reset; and (c) Series J equivalent common shares issuable from the date of issuance of Series J preferred stock.
| | Pro Forma Year ended June 30, 2003 | |||
|---|---|---|---|---|
| | (unaudited) | |||
| Pro forma net loss per share allocable to common stockholders—basic and diluted | $ | (15.38 | ) | |
| Weighted average common shares outstanding used in computing pro forma net loss per share allocable to common stockholders—basic and diluted | 8,320,796 | |||
Recent Accounting Pronouncements
In June 2002,December 2004, the FASB issued SFAS No. 146,123R. This statement is a revision of FASB Statement No. 123,Accounting for Costs Associated with ExitStock-Based Compensation. This statement supersedes APB Opinion No. 25,Accounting for Stock Issued to Employees, and its related implementation guidance. This statement establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or Disposal Activities ("SFAS No. 146"). SFAS No. 146services. It also addresses financial accounting and reportingtransactions in which an entity incurs liabilities in exchange for costs associated with exitgoods or disposal activities and nullifies EITF Issue No. 94-3,Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring).services that are based on the fair value of the entity's equity instruments or that may be settled by the issuance of those equity instruments. The full impact of adoption of SFAS No. 146 did not123R cannot be predicted at this time because it will depend on levels of share-based payments granted in the future. However, had the Company adopted SFAS 123R in prior periods, management believes the impact of that standard would have approximated the Company's consolidated financial statements for the fiscal year ended June 30, 2003.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity ("SFAS No. 150"). SFAS No. 150 revises the accounting for certain financial instruments that, under previous guidance, issuers could account for as equity. SFAS No. 150 requires that those instruments be classified as liabilities in statements of financial position. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective for interim periods beginning after June 15, 2003. The adoptionimpact of SFAS No. 150 did not impact the Company's consolidated financial statements for the fiscal year ended June 30, 2003.123.
(3) Beneficial Conversion Feature
In May 2003, the Company completed a private placement of 112,790,246112,790,233 shares of Series J mandatorily reedeemableredeemable convertible preferred stock at $0.49 per share for an aggregate purchase price of approximately $55,267,000. The terms of the preferred stock are more fully described in Note 7.8.
As part of this financing, the original conversion price onof the Series A through Series I preferred stock was reduced as a result of anti-dilution adjustments, which resulted in a beneficial conversion amounting to $80,730,286 in accordance with EITF No. 98-5,Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios and EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments. The beneficial conversion charge of $20,860,491 relatedrelating to Series A, Series B, Series C, Series F and Series H convertible preferred stock, which are not mandatorily redeemable and may be converted at any time at the option of the holders to common stock, has been recorded as an immediate charge to additional paid-in capital. The remaining beneficial conversion amount of $59,869,795 related to Series E and Series I convertible preferred stock, which are mandatorily redeemable at any time on or after June 30, 2008, is being accreted ratably over the mandatory redemption period. Such
F-14
accretion amounted to $1,656,854, $5,830,852, $11,661,705 and $8,722,382 (unaudited) for the year ended June 30, 2003, amounted to $1,656,854the six month period ended December 31, 2003, the year ended December 31, 2004 and the nine-month period ended September 30, 2005, respectively, and is charged to additional paid-in capital.
In addition, the issuance of Series J mandatorily redeemable convertible preferred stock resulted in a beneficial conversion amounting to $20,942,405$39,994,812 in accordance with EITF No. 98-5. The beneficial conversion is calculated based on the estimated fair value of the Company's common stock price per share at the date of issuance of Series J preferred stock of approximately $8.16$10.14 per share of common stock, which iswas calculated based on the estimated projected midpoint of the range of the estimated Company's initial public offering price per common share, which was planned in the fourth calendar quarter of 2003, and the stock price appreciation in comparable public companies from May 2003 to August 2003. The beneficial conversion feature is being accreted ratably over the mandatory redemption period, with a charge to additional paid-in capital of $579,566$1,106,828, $3,895,184, $7,790,368 and $5,826,812 (unaudited) for the year ended June 30, 2003.2003, the six month period ended December 31, 2003, the year ended December 31, 2004 and the nine-month period ended September 30, 2005 (unaudited), respectively.
(4) Short-Term Investments
The Company has accounted for its investments in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities, and determined that all of its short-term investments are classified as available-for-sale. Available-for-sale securities are carried at fair value with interest on these securities included in interest income. Available-for-sale securities consisted of the following:
| | Amortized Cost | Gross unrealized gains | Gross unrealized losses | Estimated fair value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Corporate debt securities | ||||||||||
| As of June 30, 2002 | $ | 2,835,526 | — | — | 2,835,526 | |||||
| As of June 30, 2003 | $ | 12,256,527 | 518,122 | (524,200 | ) | 12,250,449 | ||||
| | Amortized Cost | Gross unrealized gains | Gross unrealized losses | Estimated fair value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corporate debt securities | |||||||||||||
| As of December 31, 2003 | $ | 32,247,745 | $ | 25,690 | $ | (23,172 | ) | $ | 32,250,263 | ||||
| As of December 31, 2004 | 9,419,713 | — | (23,036 | ) | 9,396,677 | ||||||||
| As of September 30, 2005 (unaudited) | 5,166,606 | — | (6,331 | ) | 5,160,275 | ||||||||
The contractual maturities of available-for-sale debt securities at JuneDecember 31, 2004 and September 30, 20032005 are within one year.
Proceeds from sales Investments are considered impaired when a decline in fair value is determined to be other-than-temporary. The Company employs a systematic methodology that considers available evidence in evaluating potential impairment of its investments in accordance with EITF Issue No. 03-1,The
Meaning of Other-Than-Temporary Impairment and maturitiesIts Application to Certain Investments ("EITF 03-01"). In the event that the cost of an investment securities available-for-sale during fiscal 2001, 2002exceeds its fair value, the Company evaluates, among other factors, the duration and 2003extent to which the fair value is less than cost; the financial health of and business outlook for the period from March 17, 1995 (inception)investment or investee; and the Company's intent and ability to June 30, 2003,hold the investment. The Company has determined that there were $0, $0, $9,255,000no other-than-temporary declines in the fair values of its short term investments as of December 31, 2004.
The following table shows the gross unrealized losses and $9,255,000, respectively. No gains/(losses) were realized on those sales during anyfair value of the periods presented. PurchasesCompany's available-for-sale securities with unrealized losses that are not deemed to be other-than-temporarily impaired, aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position, at December 31, 2004 (in thousands):
| | Less than 12 months | 12 Months or Greater | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description of Securities | Fair value | Unrealized loss | Fair value | Unrealized loss | |||||||||
| Corporate debt securities(1) | $ | 9,397 | $ | (23 | ) | $ | — | $ | — | ||||
| Total | $ | 9,397 | $ | (23 | ) | $ | — | $ | — | ||||
Short-term investments with original maturity of three months or less have been classified as cash and cash equivalents, and amounted to $24,902,027$7,551,356, $7,878,024 and $46,638,611$2,277,746 (unaudited) as of JuneDecember 31, 2003, December 31, 2004 and September 30, 2002 and June 30, 2003,2005, respectively.
F-15
(5) Property and Equipment
Property and equipment consisted of the following as of June 30, 2003 and 2002:following:
| | December 31, 2003 | December 31, 2004 | September 30, 2005 | Estimated useful lives | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | (unaudited) | | |||||||
| Laboratory equipment | $ | 2,068,502 | $ | 2,113,093 | $ | 2,099,396 | 5 years | ||||
| Furniture and fixtures | 535,200 | 537,473 | 514,181 | 5 years | |||||||
| Computer equipment | 585,244 | 759,949 | 680,008 | 3 years | |||||||
| Leasehold improvements | 1,727,813 | 2,023,033 | 2,052,309 | 5 to 7 years | |||||||
| 4,916,759 | 5,433,548 | 5,345,894 | |||||||||
| Less accumulated depreciation | 1,823,605 | 2,886,534 | 3,440,831 | ||||||||
| $ | 3,093,154 | $ | 2,547,014 | $ | 1,905,063 | ||||||
| | 2002 | 2003 | Estimated useful lives | ||||
|---|---|---|---|---|---|---|---|
| Laboratory equipment | $ | 1,399,729 | 1,641,423 | 5 years | |||
| Furniture and fixtures | 518,772 | 499,743 | 5 years | ||||
| Computer equipement | 315,197 | 474,239 | 3 years | ||||
| Leasehold improvements | 1,344,415 | 1,710,688 | 5 to 7 years | ||||
| 3,578,113 | 4,326,093 | ||||||
| Less accumulated depreciation | 638,145 | 1,378,346 | |||||
| $ | 2,939,968 | 2,947,747 | |||||
Depreciation and amortization expense on property and equipment was $123,449, $417,479 $740,201 and $1,378,346$740,201 for the yearsfiscal year ended June 30, 2001, 2002 and 2003, and$445,260 for the six-month period from March 17, 1995 (inception) to Juneended December 31, 2003, $1,077,910 for the year ended December 31, 2004 and $788,572 (unaudited) for the nine-month period ended September 30, 2003, respectively.2005.
(6) Accrued Expenses and Other Current Liabilities
Accrued expense and other current liabilities consisted of the following:
| | December 31, 2003 | December 31, 2004 | September 30, 2005 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| | | | (unaudited) | ||||||
| Bonus payable | $ | 1,106,698 | $ | — | $ | 514,002 | |||
| Milestone payable to Elan | — | 750,000 | 3,750,000 | ||||||
| Royalties payable | — | 519,531 | 223,493 | ||||||
| Accrued research and development expenses | 2,649,368 | 825,166 | 859,812 | ||||||
| Return credits payable to customers | — | — | 1,278,801 | ||||||
| Other accrued expenses | 593,762 | 795,521 | 1,199,761 | ||||||
| $ | 4,349,828 | $ | 2,890,218 | $ | 7,825,869 | ||||
Accrued research and development expenses include amounts relating to the clinical trials as well as preclinical operating costs. Other accrued expenses include legal and business development accruals, payroll liabilities, vacation and commission accruals and other operating expense accruals.
(7) Notes Payable
In 2003, the Company entered into two financing agreements with General Electric Capital Corporation in the aggregate amount of $1,163,511,$1,153,511, bearing annual fixed interest rates of 8.57% and 8.88%, to finance the purchase of certain property and equipment. Borrowings are secured by a security interest in certain property and equipment of the Company.Company and the agreements do not include any debt covenants. The Company is required to pay monthly installments until October 2006. The aggregate maturitiesprincipal payments required subsequent to December 31, 2004 are: $301,938 in 2005, and $144,654 in 2006. The related interest payments required subsequent to December 31, 2004 are: $26,001 in 2005, and $5,109 in 2006.
In 2005 (unaudited), the Company entered into a $6 million senior secured term loan with General Electric Capital Corporation ("GE"), that bears an annual fixed interest rate of 9.93%. The Company is required to pay monthly installments until February 2008, with interest-only payments for eachthe first six months followed by principal and interest payments for the remaining 29 months. The loan is secured by all of the four yearsCompany's personal property and fixtures owned at closing or subsequently acquired. The aggregate principal payments required subsequent to June 30, 2003December 31, 2004 are: $310,233 in 2004, $338,317$899,887 in 2005, $231,937$2,317,217 in 2006, $2,558,084 in 2007 and $41,833$224,813 in 2007.2008. The related interest payments required subsequent to December 31, 2004 are: $283,130 in 2005, $402,862 in 2006, $161,995 in 2007 and $1,860 in 2008. See Note 8.
For Long-termlong-term convertible notes payable to related party see Note 11.
(7)(8) Mandatorily Redeemable Convertible Preferred Stock and Convertible Preferred Stock
The board of directors of the Company has authorized 140,221,535141,754,865 shares of convertible preferred stock, designated as Series A, B, C, D, E, F, G, H, I, J and JK preferred stock (Series A, Series B, Series C, Series D, Series E, Series F, Series G, Series H, Series I, Series J and Series J;K; collectively, the Preferred Stock). Series E, Series I, Series J and Series JK are mandatorily redeemable convertible preferred stock (Redeemable Preferred Stock). Upon an initial public offering, the preferred stock will automatically convert into common stock.
The terms of the Preferred Stock are as follows:
(a)
The Preferred Stock (except Series J and Series K) is entitled to noncumulative dividends prior to and in preference to dividends declared or paid on the common stock, at the rate of $0.10 per share per annum for Series A through Series H and at the rate of $0.39 per share per annum for Series I when and if declared by the board of directors. Dividends on Series J and Series K are cumulative and accrue on each share of Series J Preferred Stock and Series K Preferred Stock commencing on the date of issuance, whether or not earned or declared at the rate of $0.0392 per share per annum for Series J and at the rate of $0.60 per share per annum for Series K, based on the original issue price of Series J Preferred Stock and Series K Preferred Stock, prior and in preference to any declaration or payment of any dividend on any other Series of Preferred Stock holders (Series A through Series I). Series J and Series K dividends are payable when declared by the Board of Directors or upon liquidation, as defined or upon redemption.redemption, provided, however, that the amount of any dividend payable shall not exceed the original issue price of such series of preferred stock. Accrued dividends for Series J and K were $6.6 million and $761,000 as of December 31, 2004 and $9.9 million and $1.5 million (unaudited) as of September 30, 2005, respectively.
F-16
The preferred stockholders have liquidation preferences over common stockholders based on the series of Preferred Stock held. In the event of liquidation, dissolution, or winding up of the Company, each holder of shares of Series J Preferred Stock and Series K Preferred Stock is entitled to be paid in preference to common stockholders and any other Series of Preferred Stock holders (Series A through Series I) an amount equal to the original issue price per share of $0.49 for Series J and $7.50 for Series K, plus all accrued or declared but unpaid dividend, as defined.dividends. After payment has been made to Series J and Series K Preferred Stock, the Series I, Series E,E-1, Series E-2, Series F and Series H shall be entitled to receive out of the available assets, on a pro rata basis, an amount per share of $3.89, $1.31, $1.07, $1.09 and $1.36, respectively, plus all declared but unpaid dividends on each such share issued. After payment of the above mentioned preferential amounts have been made by the Company, the holders of Series E, Series F and Series H Preferred Stock shall be entitled to be paid out of the remaining available assets an amount per share equal to $0.26, $0.21 and $0.27, respectively, plus all declared but unpaid dividends. After payment of the above mentioned preferential amounts, the holders of Series A through Series H Preferred Stock shall be entitled to be paid out of remaining available assets an amount per share up to and including such amounts paid in accordance with as mentioned above, equal to $1.00, $2.00, $3.00, $12.50, $3.13, $5.22, the greater of $2.00 and 80% of the closing price per share of the Institutional Financing, as defined, most recently completed by the Company prior to the issuance of the Series G Preferred Stock and $3.25, respectively.
(c)
The Preferred Stock is convertible into common stock at the option of the stockholder. The per share conversion price on preferred stock are Series A—$6.97, Series B—$9.12, Series C—$11.26, Series D—$9.12, Series E—$10.62, Series F—$10.62, Series G—the product of (x) the number of Series G Preferred Stock surrendered and (y) the number determined by dividing (i) the greater of $24.0 or 80% of the closing price per share of the most recently completed bona fide equity financing of the Company prior to the issuance of Series G Preferred Stock by (ii) the Series G conversion price in effect, Series H—$11.80, Series I—$13.16 and Series J—$5.88. The Preferred Stock will be automatically converted into common stock upon an initial public offering of the Company's common stock or upon either the approval by written consent of the holders of a majority of the then outstanding shares of Series A, Series B, Series C, Series D, Series E, Series F, Series G, Series H and Series I voting together as a single class and upon approval by written consent of the holders of a majority of the then outstanding shares of Series J or upon an Initial Public Offering, provided thatand Series K.
| Preferred stock | Shares outstanding at December 31, 2004 | Conversion Price | Shares of Common Stock | ||||
|---|---|---|---|---|---|---|---|
| Series A | 1,306,068 | $ | 9.06 | 144,074 | |||
| Series B | 900,000 | 11.86 | 151,820 | ||||
| Series C | 333,333 | 14.64 | 68,339 | ||||
| Series D | — | 11.86 | — | ||||
| Series E | 7,472,612 | 13.81 | 1,461,363 | ||||
| Series F | 2,300,000 | 13.81 | 449,803 | ||||
| Series G | — | (1 | ) | — | |||
| Series H | 1,575,229 | 15.34 | 333,827 | ||||
| Series I | 10,204,047 | 17.11 | 2,319,457 | ||||
| Series J | 112,790,233 | 7.64 | 7,230,118 | ||||
| Series K | 1,533,327 | 9.75 | 1,179,478 | ||||
In the event the convertible promissory notes payable to Elan are converted into common stock, the per share conversion price on the Series I and Series J preferred stock would be adjusted to $12.59$16.37 and $5.48,$7.12, respectively. InOther than in an initial public offering or certain other specified instances, in the event the Company issues common stock (or securities convertible into common stock) at an effective common stock issuance price of less than $5.88 per share,a specified amount the conversion price on all convertible preferred stock will be reduced based on anti-dilution provisions.
F-17
Holders of Series E, Series I, Series J and Series JK Preferred Stock may at any time on or after June 30, 2008, require the Company to redeem all or any portion of such Holders' Redeemable Preferred Stockholders' redeemable preferred stock at a redemption price, as specified below, provided, however, that no holder of Redeemable Preferred Stockredeemable preferred stock may so require such redemption unless and until (i) the holders of not less than a majority of the Redeemable Preferred Stockredeemable preferred stock then issued and outstanding make such election and (ii) the holders of a majority of the Series J Preferred Stockand Series K preferred stock then issued and outstanding make such election prior to September 30, 2008.2008 (these terms collectively, the Redemption Date). The redemption price for each share of Redeemable Preferred Stockredeemable preferred stock shall be the original issue price plus accrued but unpaid dividends. One half of such aggregate redemption price for all Redeemable Preferred Stockredeemable preferred stock shall be payable in cash on the Redemption Date, as defined and the second half of such aggregate redemption price shall be payable in cash on the first anniversary of the Redemption Date, as defined. For the fiscal year ended June 30, 2003, the Company has accrued preferred dividends in the amount of $629,895 for Series J Preferred Stockholders.
(e)
Each holder of outstanding Preferred Stock (other than Series F) shall be entitled to the number of votes equal to the number of shares of common stock into which the shares of Preferred Stock so held could be converted. The holders of Series F Preferred Stock shall have no voting rights except as required by Delaware General Corporation Law. The board of directors consistconsists of sevennine directors: (i) two directors elected by the holders of Series A, Series E and Series H Preferred Stock, voting as a single class; (ii) one director elected by Series I Preferred Stock; (iii) two directors elected by Series J Preferred Stock; (iv) one director elected by holders of common stock; and (v) one directorthree directors elected by the holders of common stock and Preferred Stock, voting as a single class. The Company's certificate of incorporation includes provisions which restrict the Company from certain actions without the approval of a defined percentage of the preferred stockholders.
Convertible Preferred Stock
Series A
In May 1995, the Company issued 610,000 shares of Series A, at a per share price of $1.00, for aggregate proceeds of $610,000, and granted each purchaser a warrant to purchase one additional share of Series A for every ten Series A shares purchased, at an exercise price of $1$1.00 per share. 51,068 of these warrants were exercised in fiscal 2002. The Company estimated the fair value of warrants at approximately $44,971. SuchThe fair value was determined by the Black-Scholes valuation method, using a risk free interest rate of 6.5%, itsthe warrants' contractual life of seven years, an annual volatility of 73% and no expected dividends. Such amount was credited to additional paid-in capital and charged immediately to additional paid-in capital, as the warrants were exercisable at any time at the option of the holder. Each warrant was exercised for one share of Series A. Through June 30, 2003,During fiscal 2002, 22,800 of these warrants were exercised on a cash basis and 28,268 were exercised in a cashless exercise resulting in total proceeds of $22,800. The remaining 9,932 of these warrants were not exercised and have expired.
In fiscal 1996 and 1997, the Company issued 450,000 and 195,000 shares of Series A, at a per share price of $1.00, for aggregate proceeds of $450,000 and $195,000, respectively. In August 1996 and
F-18
January 1997, the Company granted 340,000 warrants to purchase shares of Series A at an exercise price of $1.00. These warrants expireexpired in August 2003 and January 2004, respectively, and the numberrespectively. None of Series A shares to be received upon exercise is one share for one warrant.these warrants were exercised. The Company estimated the fair value of warrants at approximately $254,110. Such value was determined by the Black-Scholes valuation method, using a risk free interest rate of 6.5%, the warrant's contractual life of seven years, an annual volatility of 75% and no expected dividends. Such amount was credited to additional paid-in capital and charged immediately to additional paid-in capital as the warrants were exercisable at any time at the option of the holder.
Series B
In January 1997, the Company issued 750,000 shares of Series B, at a per share price of $2.00, for aggregate proceeds of $1,500,000. In January 2002, the Company issued 150,000 shares of Series B, at a per share price of $2.00, for aggregate proceeds of $300,000 (see Note 10)11).
As of September 30, 2005 (unaudited), 100,000 Series B warrants were outstanding with an exercise price per share of $2.00. The warrants to acquire Series B Preferred Stock enable the holder to acquire 21,929 shares of common stock.
Series C
In February 2002, the Company issued to Elan and affiliates 333,333 shares of Series C, at a per share price of $3.00, for aggregate proceeds of $999,999.
Series F
In April 1998, the Company issued to Elan 2,300,000 shares of Series F, at a per share price of approximately $5.22, for aggregate proceeds of approximately $12 million. Also, in April 1998, the Company entered into a joint venture agreement with Elan. The $12 million proceeds from the sale of the shares of Series F was then transferred to MS Research and Development Corp. (MSRD)("MSRD"), a joint venture company of which the Company owned approximately 80% and Elan owned 20%, approximately.. To purchase its approximate 20% interest, Elan invested an additional $3 million into MSRD. The combined $15 million was subsequently used to license research and development technology from Elan to develop Elan's proprietary oral sustained release formulation of fampridine for the treatment of multiple sclerosis. This purchase is recorded as a license payment expense in the consolidated financial statements for the fiscal year ended June 30, 1998. For the years ended June 30, 2001, 2002 and 2003 and thefor six-month period from March 17, 1995 (inception) to June 30,ended December 31, 2003, MSRD incurred approximately $2.2 million, $2.9 million, $3.2 million and $24.6$3.3 million, respectively, in research and development expenses, which is included as research and development expense in the accompanying statements of operations, of which Acordathe Company funded 80% and Elan funded 20% until June 30, 2002, in accordance with the terms of the original development agreement. Elan's ownership interest in MSRD is reflected as minority interest in the accompanying statement of operations. The minority interest share of the MSRD losses were being funded by Elan, and through June 30, 2002 the Company received $1,279,361 as a reimbursement of this funding. In fiscal 2003, Elan ceased funding its approximately 20% share of its minority interest inthe operating expenses of MSRD and the Company ceased recognizing the related minority interest benefit resulting in an increase in the Company's ownership interest to 83% pursuant to the original agreement (see Note 11 for discussion on license and research agreement.) In September 2003, the Company entered into a termination and assignment agreement with Elan, EIS and MSRD, pursuant to which MSRD assigned to the Company its assets, including the license from Elan for Fampridine-SR for MS. The Company paid MSRD approximately $11.5 million for all of the assets and assumed all of the liabilities of MSRD, and MSRD distributed to the Company approximately $9.5 million as pro rata portion of the purchase price. From the time of establishment of MSRD until the sale of MSRD's assets to the Company, Elan was considered to be a related party under generally accepted accounting principles.
Series H
In August 1999, the Company completed a private placement of 1,575,229 shares of Series H at $3.25 per share, resulting in net proceeds to the Company of $5,119,494 after payment of legal and certain other fees.
As of June 30, 2003, 340,000 Series A and 100,000 Series B warrants were outstanding with a weighted average exercise price per share of $1.23. The warrants to acquire Series A and Series B Preferred Stock enable the holder to acquire 70,695 shares of common stock.
F-19
Mandatorily Redeemable Convertible Preferred Stock
The following convertible preferred stock are classified as free standing instruments based on the redemption rights and conversion option as discussed above under terms of the Preferred Stock.
Series E
In July and November 1998, the Company issued 7,472,612 shares of Series E, whichthat are mandatorily redeemable at $2.70 per share for an aggregate purchase price of approximately $20,176,000.
$20,176,000. The Company incurred issuance costs of $209,270. Such costs are netted against the proceeds of the Series E, and are being amortized over the mandatory redemption period.
Series I
In March 2001, the Company issued 10,204,047 shares of Series I whichthat are mandatorily redeemable at $3.89 per share for an aggregate purchase price of approximately $39,694,000. The Company incurred issuance costs of $138,179. Such costs are netted against the proceeds of the Series I, and are being amortized over the mandatory redemption period.
Series J
In May 2003, the Company issued 112,790,246112,790,233 shares of Series J whichthat are mandatorily redeemable at $0.49 per share for an aggregate purchase price of approximately $55,267,000. The Company incurred issuance costs of $334,219. Such costs are netted against the proceeds of the Series J, and are being amortized over the mandatory redemption period.
F-20 In September 2003, the Company obtained approval by the written consent from the holders of Series J preferred stock voting together as a single class and the holders of the Preferred Stock, voting separately as a single class on an as if converted basis for a reduction in the price per share of common stock offered to the public in an initial public offering which would trigger automatic conversion of the preferred stock into common stock from an offering price of not less than $14.76 per share to an offering price of not less than $12.00 per share.
Series K
In March 2004, the Company issued 1,533,327 shares of Series K which are mandatorily redeemable at $7.50 per share for an aggregate purchase price of $11,499,943. The Company incurred issuance costs of $53,728. Such costs are netted against the proceeds of the Series K, and will be amortized over the mandatory redemption period.
In January 2005, the Company granted warrants to purchase $300,000 (unaudited) worth of shares of Preferred Stock in the Company's next qualifying equity round, or Series K if no such round is issued prior to December 31, 2005. The number of Series K shares to be received upon exercise is 40,000 at the Series K issue price of $7.50 per share, which converts to 30,769 common shares. The Company estimated the fair value of warrants at approximately $214,785. Such value was determined by the Black-Scholes valuation method, using a risk free interest rate of 3.5%, the warrant's contractual life of ten years, an annual volatility of 90% and no expected dividends. These warrants were issued to GE in conjunction with the $6 million senior secured term loan (see Note 7). The discount of the note related to the warrants is being accreted over the life of the notes and resulted in a $78,023 (unaudited) charge to interest expense for the nine-month period ended September 30, 2005.
The changes in mandatorily redeemable convertible preferred stock since inception are as follows:
| | Mandatorily Redeemable Convertible Preferred Stock | Mandatorily Redeemable Convertible Preferred Stock (in thousands) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Series E mandatorily redeemable convertible preferred stock | Series I mandatorily redeemable convertible preferred stock | Series J mandatorily redeemable convertible preferred stock | Series E | Series I | Series J | Series K | |||||||||||||||||||||||||||
| | Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Amount | ||||||||||||||||||||||||||||
| Balance at June 30, 1998 | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||||||||||||
| Issuance of Series E mandatorily redeemable convertible preferred stock | 7,472,612 | 19,966,782 | — | — | — | — | ||||||||||||||||||||||||||||
| Accretion of issuance costs | — | 18,042 | — | — | — | — | ||||||||||||||||||||||||||||
| Balance at June 30, 1999 | 7,472,612 | 19,984,824 | — | — | — | — | ||||||||||||||||||||||||||||
| Accretion of issuance costs | — | 27,337 | — | — | — | — | ||||||||||||||||||||||||||||
| Balance at June 30, 2000 | 7,472,612 | 20,012,161 | — | — | — | — | ||||||||||||||||||||||||||||
| Issuance of Series I mandatorily redeemable convertible preferred stock | — | — | 10,204,047 | 39,555,564 | — | — | ||||||||||||||||||||||||||||
| Accretion of issuance costs | — | 27,337 | — | 8,560 | — | — | ||||||||||||||||||||||||||||
| Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Amount | |||||||||||||||||||||||||||
| Balance at June 30, 2001 | 7,472,612 | 20,039,498 | 10,204,047 | 39,564,124 | — | — | 7,473 | $ | 20,040 | 10,204 | $ | 39,564 | — | — | — | — | ||||||||||||||||||
| Accretion of issuance costs | — | 27,337 | — | 27,636 | — | — | — | 27 | — | 28 | — | — | — | — | ||||||||||||||||||||
| Balance at June 30, 2002 | 7,472,612 | 20,066,835 | 10,204,047 | 39,591,760 | — | — | 7,473 | 20,067 | 10,204 | 39,592 | — | — | — | — | ||||||||||||||||||||
| Issuance of Series J mandatorily redeemable convertible preferred stock | — | — | — | — | 112,790,246 | 54,933,001 | — | — | — | — | 112,790 | 54,933 | — | — | ||||||||||||||||||||
| Accretion of issuance costs | — | 27,337 | — | 27,636 | — | 10,990 | — | 27 | — | 28 | — | 11 | — | — | ||||||||||||||||||||
| Accrual of preferred dividend on Series J mandatorily redeemable convertible preferred stock | — | — | — | — | — | 629,895 | — | — | — | — | — | 630 | — | — | ||||||||||||||||||||
| Beneficial conversion feature for reduction in conversion price | — | — | — | (39,693,743 | ) | — | (20,942,405 | ) | — | (20,176 | ) | — | (39,694 | ) | — | — | — | — | ||||||||||||||||
| Beneficial conversion feature on issuance | — | (20,176,052 | ) | — | — | — | — | — | — | — | — | — | (39,995 | ) | — | — | ||||||||||||||||||
| Deemed dividends on preferred stock for reduction in conversion price | — | 558,358 | — | 1,098,496 | — | — | — | 558 | — | 1,098 | — | — | — | — | ||||||||||||||||||||
| Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature | — | — | — | — | — | 579,566 | — | — | — | — | — | 1,107 | — | — | ||||||||||||||||||||
| Balance at June 30, 2003 | 7,472,612 | $ | 476,478 | 10,204,047 | $ | 1,024,149 | 112,790,246 | $ | 35,211,047 | |||||||||||||||||||||||||
| | Mandatorily Redeemable Convertible Preferred Stock (in thousands) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Series E | Series I | Series J | Series K | ||||||||||||||||
| | Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Amount | Number of Shares | Amount | ||||||||||||
| Balance at June 30, 2003 | 7,473 | $ | 476 | 10,204 | $ | 1,024 | 112,790 | $ | 16,686 | — | — | |||||||||
| Accretion of issuance costs | — | 8 | — | 7 | — | 32 | — | — | ||||||||||||
| Accrual of preferred dividend on Series J mandatorily redeemable convertible preferred stock | — | — | — | — | — | 2,211 | — | — | ||||||||||||
| Deemed dividends on preferred stock for reduction in conversion price | — | 1,965 | — | 3,866 | — | — | — | — | ||||||||||||
| Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature | — | — | — | — | — | 3,895 | — | — | ||||||||||||
| Balance at December 31, 2003 | 7,473 | $ | 2,450 | 10,204 | $ | 4,897 | 112,790 | $ | 22,824 | — | — | |||||||||
| Issuance of Series K mandatorily redeemable convertible preferred stock | 1,533 | $ | 11,446 | |||||||||||||||||
| Accretion of issuance costs | — | 16 | — | 15 | — | 65 | — | 10 | ||||||||||||
| Accrual of preferred dividend on Series K mandatorily redeemable convertible preferred stock | — | — | — | — | — | — | — | 767 | ||||||||||||
| Accrual of preferred dividend on Series J mandatorily redeemable convertible preferred stock | — | — | — | — | — | 4,421 | — | — | ||||||||||||
| Deemed dividends on preferred stock for reduction in conversion price | — | 3,930 | — | 7,732 | — | — | — | — | ||||||||||||
| Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature | — | — | — | — | — | 7,790 | — | — | ||||||||||||
| Balance at December 31, 2004 | 7,473 | $ | 6,396 | 10,204 | $ | 12,644 | 112,790 | $ | 35,100 | 1,533 | $ | 12,223 | ||||||||
| Accretion of issuance costs | — | 12 | — | 11 | — | 48 | — | 9 | ||||||||||||
| Accrual of preferred dividend on Series K mandatorily redeemable convertible preferred stock | — | — | — | — | — | — | — | 690 | ||||||||||||
| Accrual of preferred dividend on Series J mandatorily redeemable convertible preferred stock | — | — | — | — | — | 3,316 | — | — | ||||||||||||
| Deemed dividends on preferred stock for reduction in conversion price | — | 2,939 | — | 5,783 | — | — | — | — | ||||||||||||
| Deemed dividends on preferred stock for issuance of preferred stock with beneficial conversion feature | — | — | — | — | — | 5,827 | — | — | ||||||||||||
| Balance at September 30, 2005 (unaudited) | 7,473 | $ | 9,347 | 10,204 | $ | 18,438 | 112,790 | $ | 44,291 | 1,533 | $ | 12,922 | ||||||||
(8) Common Stock and(9) Common Stock Options, Warrants and WarrantsRestricted Stock
Upon inception of the Company in March 1995, the founders, directors, and certain employees purchased 216,875166,827 shares of restricted common stock at a per share price of $0.12. Full recourse promissory notes with interest rates of 7.75% were issued for the purchase price and have been received by the Company for 206,250 of these shares. The remaining amounts due were paid during 2001.
The Company's president began devoting substantial time to the formation and then-operation of the Company in November 1993. Compensation expense and additional paid in capital for the estimated value of his services has been recorded in the amount of $125,000 in both fiscal 1995 and 1996 and $70,000 in fiscal 1997, as his salary was not paid and has been forgiven. The amount recorded in fiscal 1995 includes an amount related to the period prior to incorporation. The Company's president has received a salary since 1997.
F-21
In fiscal 1996, the Company issued 4,000 shares of common stock at a per share price of $1.20. Promissory notes due and received in fiscal 2001 were for 1,500 of these shares. The remaining promissory notes for 2,500 of these shares were paid in August 2000.$0.16.
On June 18, 1999, the Company's board of directors approved the adoption of the Acorda Therapeutics, Inc. 1999 Employee Stock Option Plan (the "Plan"). The Plan reserves 1.7 million shares for issuance through option grants (see Note 13 for changes to stock option plan). All employees of the Company are eligible to participate in the Plan, including executive officers, as well as directors, independent contractors, and agents of the Company. The Plan is administered by the Compensation Committee of the board of directors, which selects the individuals to be granted options and stock appreciation rights, determines the time or times at which options and stock appreciation rights shall be granted under the Plan, determines the number of shares to be granted subject to any option or stock appreciation right under the Plan and the duration of each option and stock appreciation right, and makes any other determinations necessary, advisable, and/or appropriate to administer the Plan. Under the Plan, each option granted expires no later than the tenth anniversary of the date of its grant. Options vest over a four year period on a quarterly basis commencing with the date of award. Compensation expense is calculated using a Black-Scholes calculation with the expense being recognized over the vesting period. No option may be granted pursuant to the Plan more than ten years after the date on which the Plan was adopted by the board of directors and any option granted under the Plan shall, by its terms, not be exercisable more than ten years after the date of grant. In March 2004, the number of shares authorized for issuance under the Plan was increased from 1,275,641 shares to 2,451,088 shares. In
September 2005, the number of shares authorized for issuance under the Plan was increased from 2,451,088 to 3,186,856.
The effects of applying SFAS No. 123 in a particular year, may not be representative of the effects on reported net income or loss for future years. The fair value of each option granted is estimated on the date of grant using an option-pricing model with the following weighted average assumptions:
| | 2001 | 2002 | 2003 | |||||
|---|---|---|---|---|---|---|---|---|
| Employees and directors | ||||||||
| Estimated volatility | 104.4 | % | 97.7 | % | 94.0 | % | ||
| Expected life in years | 5 | 5 | 5 | |||||
| Risk free interest rate | 4.79 | % | 4.41 | % | 3.04 | % | ||
| Dividend yield | — | — | — | |||||
The weighted average fair value per share of options granted to employees for the years ended June 30, 2001, 2002 and 2003 amounted to approximately $33.96, $39.96 and $40.56, respectively. The weighted average fair value per share of options granted to non-employees for the year ended June 30, 2001 amounted to approximately $28.68. No options were granted to non-employees for the years ended June 30, 2002 and 2003. The fair value of each option granted in 2001 to non-employees was calculated at the date of grant using an option-pricing model with the following weighted average assumptions (a) estimated volatility—104.5%; (b) expected life in years—10 years; (c) risk free interest rate—5.11%; and (d) dividend yield—0%.
| | Year ended June 30, | | | | | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Six-month period ended December 31, 2003 | | Nine-month period ended September 30, 2004 | Nine-month period ended September 30, 2005 | ||||||||||
| | Year ended December 31, 2004 | |||||||||||||
| | 2002 | 2003 | ||||||||||||
| | | | | | (unaudited) | (unaudited) | ||||||||
| Employees and directors: | ||||||||||||||
| Estimated volatility | 97.7 | % | 94.0 | % | 89.8 | % | 90.0 | % | 88.42 | % | 78.4 | % | ||
| Expected life in years | 5 | 5 | 5 | 5 | 5 | 5 | ||||||||
| Risk free interest rate | 4.41 | % | 3.04 | % | 3.28 | % | 3.41 | % | 2.78 | % | 4.1 | % | ||
| Dividend yield | — | — | — | — | — | — | ||||||||
The Company estimated volatility for purposes of computing compensation expense on its employee and non-employee options using the volatility of public companies that the Company considered comparable. The expected life used to estimate the fair value of non-employee options is equal to the contractual life of the option granted, which is 10 years.
F-22 The weighted average fair value per share of options granted to employees for the years ended June 30, 2002 and 2003, the six month period ended December 31, 2003, the year ended December 31, 2004 and the nine month period ended September 30, 2005 amounted to approximately $51.95, $52.73, $16.22, $6.95, and $5.41 (unaudited) respectively. The weighted average fair value per share of options granted to non-employees for the six month period ended December 31, 2003 amounted to approximately $7.64. No options were granted to non-employees for the years ended June 30, 2002, and 2003, and December 31, 2004, and the nine month period ended September 30, 2005 (unaudited).
Common stock option and warrant activity from March 17, 1995 (inception) to June 30, 20032001 to September 30, 2005 is as follows (this table does not include Warrantswarrants to acquire Series AB and Series BK Preferred stock, which are discussed in Note 7.)8):
| | Shares | Exercise Price per share | |||||||
|---|---|---|---|---|---|---|---|---|---|
| March 17, 1995 (inception) | — | $ | — | ||||||
| Granted | 5,000 | 0.12 | |||||||
| Exercised | — | — | |||||||
| Balance at June 30, 1995 | 5,000 | ||||||||
| Granted | 29,583 | 0.12–1.20 | |||||||
| Exercised | (7,500 | ) | 0.12 | ||||||
| Balance at June 30, 1996 | 27,083 | ||||||||
| Granted | 45,417 | 1.20–2.40 | |||||||
| Exercised | — | — | |||||||
| Balance at June 30, 1997 | 72,500 | ||||||||
| Granted | 3,500 | 2.40–4.20 | |||||||
| Forfeited | (500 | ) | 2.40 | ||||||
| Exercised | (2,209 | ) | 1.20–2.40 | ||||||
| Balance at June 30, 1998 | 73,291 | ||||||||
| Granted | 24,833 | 4.20 | |||||||
| Exercised | (1,333 | ) | 1.20–4.20 | ||||||
| Balance at June 30, 1999 | 96,791 | ||||||||
| Granted | 13,625 | 4.20 | |||||||
| Exercised | (6,041 | ) | 0.12–1.20 | ||||||
| Balance at June 30, 2000 | 104,375 | ||||||||
| Granted | 95,709 | 4.20–18.0 | |||||||
| Forfeited | (1,984 | ) | 4.20–18.0 | ||||||
| Exercised | (6,308 | ) | 0.12–4.20 | ||||||
| Shares | Exercise Price per share | ||||||||
| Balance at June 30, 2001 | 191,792 | 147,532 | |||||||
| Granted | 56,720 | 18.0–24.0 | 43,631 | $23.40 - $31.20 | |||||
| Forfeited | (3,965 | ) | 4.20–18.0 | (3,050 | ) | 5.46 - 23.40 | |||
| Exercised | (4,395 | ) | 4.20–18.0 | (3,381 | ) | 5.46 - 23.40 | |||
| Balance at June 30, 2002 | 240,152 | 184,732 | |||||||
| Granted | 14,521 | 9.60–24.0 | 11,170 | 12.48 - 31.20 | |||||
| Forfeited | (5,965 | ) | 4.20–24.0 | (4,588 | ) | 5.46 - 31.20 | |||
| Balance at June 30, 2003 | 248,708 | 191,314 | |||||||
| Granted (original price $7.64, repriced to $2.60) | 1,112,082 | 2.60 | |||||||
| Forfeited | (14,114 | ) | 7.64 - 31.20 | ||||||
| Exercised | (3,687 | ) | 1.56 - 12.48 | ||||||
| Balance at December 31, 2003 | 1,285,595 | ||||||||
| Granted | 67,488 | 2.60 - 9.75 | |||||||
| Forfeited | (61,588 | ) | 2.60 - 7.64 | ||||||
| Exercised | (2,360 | ) | 1.56 - 7.64 | ||||||
| Balance at December 31, 2004 | 1,289,135 | ||||||||
| Granted | 615,798 | 8.14 | |||||||
| Forfeited | (124,737 | ) | 2.60 | ||||||
| Exercised | (11,195 | ) | 2.60 | ||||||
| Balance at September 30, 2005 (unaudited) | 1,769,001 | ||||||||
F-23 Options available to grant at December 31, 2004 were 70,570.
Options and Warrants outstanding | Options and Warrants exercisable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of exercise prices | Outstanding as of December 31, 2004 | Weighted average remaining contractual life | Weighted average exercise price | Exercisable as of December 31, 2004 | Weighted average exercise price | |||||||
| $.16 - $3.12 | 42,628 | 1.67 | $ | 1.52 | 42,628 | $ | 1.52 | |||||
| $2.60 | 1,177,815 | 8.40 | 2.60 | 1,102,774 | 2.60 | |||||||
| $9.75 - $12.48 | 64,619 | 9.69 | 9.80 | 5,072 | 10.44 | |||||||
| $23.40 | 4,071 | 6.69 | 23.40 | 3,451 | 23.40 | |||||||
| 1,289,135 | 8.24 | 2.99 | 1,153,926 | 2.66 | ||||||||
This table represents the equity instruments issued since January 1, 2004 through September 30, 2005.
| Transaction Date | Options Granted | Restricted Shares Granted | Fair Market Value at Grant Date | Exercise Price Per Share | Intrinsic Value | Recipient | Compensation Expense Recognized Thru September 2005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan-04(1) | 1,912 | — | $ | 7.64 | $ | 2.60 | $ | 9,636 | Employees | $ | 5,796 | |||||||
| Mar-04 | 17,192 | — | 9.75 | 9.75 | — | Employees | 40,618 | |||||||||||
| Mar-04 | — | 1,134,423 | 9.75 | n/a | — | Employees | 3,581,830 | |||||||||||
| Aug-04 | 3,769 | — | 9.75 | 9.75 | — | Employees | 3,129 | |||||||||||
| Aug-04 | — | 5,077 | 9.75 | n/a | — | Employees | 2,149 | |||||||||||
| Nov-04 | 44,615 | — | 9.75 | 9.75 | — | Employees | 67,462 | |||||||||||
| Total 2004 | 67,488 | 1,139,500 | ||||||||||||||||
| Jan-05 (unaudited) | 34,615 | — | 9.75 | Employees | 41,021 | |||||||||||||
| Aug-05 (unaudited) | 548,484 | — | 8.14 | Employees | 720,092 | |||||||||||||
| Aug-05 (unaudited) | 32,699 | — | 8.14 | Director | 46,458 | |||||||||||||
| Sep-05 (unaudited) | — | 7,692 | n/a | Directors | 77,140 | |||||||||||||
| �� | ||||||||||||||||||
| Total 2005 (unaudited) | 615,798 | 7,692 | ||||||||||||||||
In January 2005, the Company issued warrants that provide the holder with the right to purchase $300,000 (unaudited) worth of shares of preferred stock in the Company's next qualifying equity round or 40,000 shares of Series K mandatorily redeemable preferred stock if no such round is issued prior to December 31, 2005. In November 2005, the Company modified the terms of this warrant to provide the holder with the right to purchase $300,000 worth of shares of preferred stock in the Company's next qualifying equity round or; to the extent the Company has consummated an IPO on or before February 28, 2006, shares of Common Stock at the lower of (A) the per share price of the Common Stock sold in the IPO and (B) $9.75 per share or; to the extent the Company has not consummated either a qualifying equity round or an IPO on or before February 28, 2006, then $9.75 per share.
In September 2003, the Company re-priced 118,142 stock options issued to employees, which had an exercise price per option of more than $7.64, with a new exercise price per share of $7.64. As a result of this repricing, the Company has recognized an additional compensation charge based on the estimated fair value of the repriced options of $575,563, of which $449,585, $92,054 and $15,737 (unaudited) was recognized during the six-month period ended December 31, 2003, the year ended December 31, 2004 and the nine-month period ended September 30, 2005, respectively, with the balance to be recognized over the remaining respective vesting periods of the repriced options. Such compensation expense was calculated based on the estimated fair value based upon the Black-Scholes model of the repriced options compared to the value of the options immediately prior to the date of the repricing based on the original terms.
In September 2003, the Company granted 1,062,081 stock options, that had been authorized for issuance under the Plan in May 2003, to employees under the Plan at an exercise price of $7.64 per share, which was below the estimated fair value of the Company's common stock at the date of grant. Compensation expense of approximately $11.0 million, attributable to the fair value of the options granted, was recognized for the six-month period ended December 31, 2003 as certain of the options issued to employees vested immediately and the balance of $6.1 million will be recognized over the remaining respective vesting periods of the options. Such compensation expense was calculated based on the estimated projected midpoint of the range of the Company's initial public offering price per common share, which
| Options and Warrants outstanding | Options and Warrants exercisable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of exercise prices | Outstanding as of June 30, 2003 | Weighted average remaining contractual life | Weighted average exercise price | Exercisable as of June 30, 2003 | Weighted average exercise price | |||||||
| $0.12–2.40 | 58,333 | 3.19 | $ | 1.20 | 58,333 | $ | 1.20 | |||||
| 4.20 and 9.60 | 41,579 | 6.67 | 5.40 | 30,423 | 4.32 | |||||||
| 18.0 and 24.0 | 148,796 | 7.93 | 18.36 | 79,846 | 18.12 | |||||||
| 248,708 | 6.61 | $ | 12.12 | 168,602 | $ | 9.72 | ||||||
was planned in the fourth calendar quarter of 2003, and the stock price appreciation in comparable public companies from May 2003 to August 2003 (the estimated fair value of the Company's common stock on the date of grant.) In December 2003, the Company deferred its plan for an initial public offering.
In October 2003, the Company granted 38,462 stock options to its chief executive officer and 9,615 stock options to its executive director-marketing and commercialization at exercise prices of $7.64 per share, which was below the estimated fair value of the Company's common stock at the date of grant. Compensation expense of approximately $425,000 attributable to the fair value of the options granted was recognized for the six-month period ended December 31, 2003, as certain of the options issued to employees vested immediately and the balance of $355,000 will be recognized over the remaining respective vesting periods of the options.
In March 2004, the Company repriced 1,250,853 stock options issued to employees, which had an exercise price per share of more than $2.60, with a new exercise price per share of $2.60. Most of these options were originally issued in September 2003. As a result of this repricing, the Company has recognized an additional compensation charge of $2,200,330, of which $1,869,872 and $89,198 was recognized during the year ended December 31, 2004 and the nine-month period ended September 30, 2005 (unaudited), respectively, with the balance to be recognized over the remaining respective vesting periods of the repriced options. Such compensation expense was calculated based on the estimated fair value of the repriced options compared to the value of the options immediately prior to the date of the repricing based on the original terms.
In March 2004, the Company granted 1,134,423 restricted shares and 17,192 stock options to employees under the Plan. The stock options were issued with an exercise price of $9.75 per share which was the fair value of the Company's common stock at the date of grant. The restricted shares were granted for no cash consideration. The option grants are exercisable based on a four-year quarterly vesting schedule, commencing with the date of award March 9, 2004. The restricted stock awards are subject to vesting over a four-year period as follows: the first installment will vest on the last to occur of (a) the expiration of the lock-up period following our initial public offering, and (b) the third day after public announcement of data regarding either the primary outcome measure of our Fampridine-SR Phase 3 trial in MS or suspension or termination of the trial, whichever comes first, and (c) in the case of Ron Cohen, June 30, 2007; except that if the vesting date under (a) or (b) or (c) would occur during a "blackout" period under our insider trading policy, the vesting date will be the first day following termination of the blackout period. The first vested installment under each restricted stock award will be calculated as the total number of shares covered by the award multiplied by a fraction, the numerator of which is the number of months from the vesting commencement date to the date on which the first installment of restricted shares vest, or the "initial vesting date," and the denominator is 48. All remaining restricted shares will vest in equal quarterly installments, measured from the vesting commencement date, except that for any partial quarter in which the initial vesting date occurs, the unvested portion of shares remaining for that quarter will vest at the end of such quarter. As a result of these grants, the total compensation charge is approximately $11,177,540, of which compensation expense of $2,256,103 and $1,366,345 (unaudited) was recognized during the year ended December 31, 2004 and the nine months ended September 30, 2005, with the balance to be recognized over the remaining respective vesting periods of the options and restricted shares. The Company hasrecognized compensation expense ratably over four years.
In August and November 2004, the Company granted certain common48,384 options to employees under the plan. The stock options were issued with an exercise price of $9.75 per share. The options will vest over a four-year vesting schedule. As a result of these grants the total compensation charge is $325,109 of which $14,053 and warrants. These$56,538 (unaudited) was recognized during the year ended December 31, 2004 and the nine months ended September 30, 2005.
In January 2005, the Company granted 34,615 options to employees under the plan. The stock options were issued with an exercise price of $9.75 per share. The options will vest over a four-year vesting schedule. As a result of these grants the total compensation charge is $228,495 (unaudited) of which $41,021 (unaudited) was recognized during the nine months ended September 30, 2005.
In August 2005, the Company granted 548,484 and warrants are exercisable32,699 stock options to employees and non-employees, respectively, under the Plan. The stock options were issued with an exercise price of $8.14 per share. 87,624 and 6,122 of the employee and non-employee grants, respectively, vested immediately upon the grant date of the award. The balance will be vested based on a four-year quarterly vesting schedule for employee grants and a periodthree-year quarterly vesting schedule for non-employee grants. $4,538,220 (unaudited), of ten yearswhich compensation expense of $766,550 (unaudited) was recognized during the nine months ended September 30, 2005, with the balance to be recognized over the remaining respective vesting periods of the options.
In August 2005, the Company granted 7,692 restricted shares to non-employees. 5,769 of these grants vested immediately upon the date of award of August 3, 2005, and vest upthe balance will be vested based on a one-year quarterly vesting schedule, contingent upon certain restrictions defined in the restricted stock agreement. As a result of these grants, the total compensation charge is approximately $84,612 (unaudited), of which compensation expense of $77,140 (unaudited) was recognized through September 30, 2005, with the balance to four years.be recognized over the remaining respective vesting periods of the restricted shares. As of December 31, 2004 and September 30, 2005 1,127,808 and 756,620 (unaudited) restricted shares remain outstanding.
Compensation expense for options and restricted stock granted to employees amounted to $643,028, $1,331,911, $1,580,054, $13,198,079, $9,049,858 and $3,831,784$3,417,684 (unaudited) for the years ended June 30, 2001, 2002 and 2003, the six-month period ended December 31, 2003, the year ended December 31, 2004 and for the nine-month period from March 17, 1995 (inception) to Juneended September 30, 2003,2005, respectively. Compensation expense for options and restricted stock granted to employees are classified between research and development and general and administrative expense based on employee job function.
Options granted to non-employees vest immediately or over a one to four year period based upon future service requirements. Compensation expense for options granted to non-employees amounted to $94,397, $74,624, ($6,539), $8,488, $15,458 and $1,919,675$47,246 (unaudited) for the years ended June 30, 2001, 2002 and 2003, the six-month period ended December 31, 2003, the year ended December 31, 2004 and for the nine-month period from March 17, 1995 (inception) to Juneended September 30, 2003,2005, respectively. The amount of compensation expense to be recorded in the future for options granted to non-employees is subject to change each reporting period based upon changes in the estimated fair value of the Company's common stock, estimated volatility and risk free interest rate until the non-employee completescompleted performance under the option agreement. 1,770As of December 31, 2004 and September 30, 2005, respectively, 1,756 and 27,619 (unaudited) options subject to this treatment remain unvestedunvested.
With the exception of options to purchase 1,912 shares of common stock made in January 2004, the Company granted all of its 2004 common stock options and warrants at June 30, 2003.an exercise price of $9.75, which was equal to the price of its March 2004 financing transaction of Series K Preferred Stock. Although the preferred shares have certain preferential rights such as liquidation preferences, convertibility features, dividend rights and anti-dilution protections that would result in differences between the fair value of its preferred and common stock, the Company estimated the fair value of its common stock at $9.75 per share during 2004 and recorded compensation expense based on the $9.75 fair market value.
(9)(10) Income Taxes
As of June 30, 2003, theThe Company had available net operating loss carry-forwards ("NOL") of approximately $75,591,000$131,843,113 and $154,641,684 as of December 31, 2004 and September 30, 2005 (unaudited), for federal and state income tax purposes, which are available to offset future federal and state taxable income, if any, and expire between 20092010 and 2023.2025. The Company also has research and development tax credit carryforwards of approximately $704,000$1,254,000 and $1,515,641 as of December 31, 2004 and September 30, 2005 (unaudited), for federal income tax reporting purposes whichthat are available to reduce federal income taxes, if any, and expire in future years through 2017.beginning in 2018.
F-24
The tax effect of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities as of JuneDecember 31, 2003 and 2004 and September 30, 2002 and 2003,2005, are presented below:
| | December 31, 2003 | December 31, 2004 | September 30, 2005 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | | | (unaudited) | |||||||||||
| Net operating loss carryforwards | $ | 16,320,233 | 32,504,088 | $ | 39,167,783 | $ | 54,055,676 | $ | 63,403,090 | |||||||
| Research and development tax credit | 503,000 | 703,500 | 783,500 | 1,254,426 | 1,515,641 | |||||||||||
| Property and equipment | 6,000 | (270,300 | ) | (298,728 | ) | 110,266 | 219,207 | |||||||||
| Intellectual property | 6,521,667 | 5,948,334 | 5,398,333 | 5,310,070 | 4,970,111 | |||||||||||
| Stock options and warrants | 5,413,596 | 9,130,376 | 11,360,515 | |||||||||||||
| Other temporary differences | 35,482 | 42,061 | 43,302 | 124,141 | 136,465 | |||||||||||
| 23,386,382 | 38,927,683 | 59,507,786 | 69,984,955 | 81,605,030 | ||||||||||||
| Less valuation allowance | (23,386,382 | ) | (38,927,683 | ) | (59,507,786 | ) | (69,984,955 | ) | (81,605,030 | ) | ||||||
| Net deferred tax assets | $ | — | $ | — | $ | — | ||||||||||
| $ | — | — | ||||||||||||||
Changes in the valuation allowance for the yearssix-month period ended JuneDecember 31, 2003, for the year ended December 31, 2004, and for the nine-month period ended September 30, 2001, 2002 and 20032005 (unaudited) amounted to approximately $5,300,000, $3,853,000$11,580,075, $19,477,170 and $15,541,000$11,620,075, respectively. Since inception, the Company has incurred substantial losses and expects to incur substantial losses in future periods. The Tax Reform Act of 1986 (the "Act") provides for a limitation of the annual use of NOL and research and development tax credit carryforwards (following certain ownership changes, as defined by the Act) that could significantly limit the Company's ability to utilize these carryforwards. The Company has experienced various ownership changes, as defined by the Act, as a result of past financings. Accordingly, the Company's
ability to utilize the aforementioned carryforwards may be limited. Additionally, because U.S. tax laws limit the time during which these carryforwards may be applied against future taxes, the Company may not be able to take full advantage of these attributes for federal income tax purposes. Because of the above mentioned factors, and the development stage nature of its operations (see Note 1), the Company has not recognized its net deferred tax assets as of and for all periods presented. Accordingly, the Company has provided a full valuation allowance against its net deferred tax assets and no tax benefit has been recognized relative to its pretax losses.
(10)(11) License and Research Agreements
Elan
In January 1997, the Company entered into several agreements with Elan, including a License and Supply Agreement to develop Elan's, proprietary oral, sustained-release formulation of Fampridine-SR for treatment of SCI.spinal cord injury. In return for this exclusive license granted by Elan, the Company paid a license fee of $5 million which was expensed in fiscal 1997. The term of the agreement is equal to the greater of 20 years or the duration of relevant fampridineFampridine-SR patent rights. Any mutually agreed to research conducted by Elan will be paid by the Company at cost plus 45%. The Company will be responsible for all clinical trials and regulatory approvals. Elan will have the right to manufacture, subject to certain exceptions, products for the Company upon regulatory approval at specified prices as a percentage of net selling price. In the event Elan does not manufacture the products, it is entitled to a royalty as a stated percentage of the products' net selling price. The Company may recover up to $2.5 million of the license fee by reducing the royalty payable to Elan by stated percentages of the products' net selling price.
Series B and C Preferred Stock Purchase
Concurrent with the License and Supply Agreement, the Company entered into a Preferred Stock, Convertible Note and Warrant Purchase Agreement (the "Agreement") with Elan. Under this Agreement, Elan purchased 750,000 shares of the Company's Series B at a per share price of $2.00 and also agreed to purchase 333,333 shares of the Company's Series C at a per share price of $3.00 within 30 days of the
F-25
completion of Phase II2 clinical trials relating to products to be developed under the License and Supply Agreement. Concurrent with the purchase of Series B, the Company issued to Elan a warrant to purchase an additional 150,000 shares of Series B at a per share exercise price of $2.00 for a period of five years. The Company estimated the fair value of warrants at approximately $198,031. Such value was determined by the Black-Scholes valuation method, using a risk free interest rate of 6.4%, the warrant's contractual life of five years, an annual volatility of 75% and no expected dividends. Such amount was credited to additional paid-in capital and charged immediately to additional paid-in capital as the warrants were exercisableSeries B was convertible at any time at the option of the holder. Concurrent with the purchase of Series C, described below the Company issued to Elan a warrant to purchase 100,000 shares of Series B at a per share exercise price of $2.00 for a period of five years from the date of issuance.
Phase II2 clinical trials relating to products to be developed under the License and Supply Agreement were completed in February 2002 and Elan purchased 333,333 shares of Series C at a per share price of $3.00 resulting in total proceeds of $999,999. Elan also exercised its Series B warrant and the Company issued 150,000 shares of Series B at a per share price of $2.00 resulting in total proceeds of $300,000. The Company also issued an additional five-year warrant to purchase 100,000 shares of Series B at a per share exercise price of $2.00 for a period of five years from the date of issuance on January 4, 2002. The Company estimated the fair value of the five-year warrant to purchase 100,000 shares of Series B at approximately $321,000. Such value was determined by the Black-Scholes valuation method, using a risk free interest rate of 4.3%, the warrant's contractual life of five years, an annual volatility of 102% and no expected dividends. Such amount was credited to additional paid-in
capital and charged immediately to research and development expenses as these warrants were issued in connection with the Company completing Phase II2 clinical trials. In addition, the Company recognized $296,666 as a beneficial conversion feature on issuance of Series C convertible preferred stock and charged this amount to research and development expenses as these shares were issued upon the Company completing Phase II clinical trials pursuant to a previous arrangement.
Convertible Note
Under the Agreement, Elan also loaned to the Company an aggregate of $7.5 million pursuant to two convertible promissory notes. One promissory note in the amount of $5$5.0 million bears interest at a rate of 3% beginning on the first anniversary of the issuance of the note. The unpaid principal is convertible into shares of the Company's Series D at a conversion price of $12.50 per share. Principal and interest are repayable, if not converted, ratably over a seven-year period beginning one year after the Company receives certain regulatory approval for the products to be developed, subject to limitations related to gross margin on product sales. If it is determined by both parties that regulatory approval will not likely occur, all principal and interest shallwill not be repayable and the note will be cancellable after a defined notice period, if not earlier converted. If the License and Supply Agreement is otherwise terminated, the principal and interest is repayable ratably over 15 years. Both promissory notes restrict our ability to incur indebtedness that is senior to the notes, subject to certain exceptions, including for our revenue interests assignment arrangement with PRF (See Note 17).
The second promissory note in the amount of $2.5 million is non-interest bearing. This promissory note is convertible after January 22, 1999 into either shares of Series B at a conversion price of $2$2.00 per share or into an undesignated series (currently authorized as Series D) of Preferred Stock at a conversion price equal to 80% of the-then most recently completed equity financing, as defined, whichever conversion price is greater. This promissory note is repayable by the Company, if not converted by Elan, ratably over a seven-year period beginning one year after the Company receives certain regulatory approval for the products to be developed. If it is determined by both parties that regulatory approval will not likely occur or if the License and Supply Agreement is otherwise terminated, the note is repayable ratably over 15 years from the date of determination. Interest on these
F-26
convertible promissory notes has been imputed using 9% on 50% of the $5 million note and 8% on the $2.5 million note. In case of the $5 million note, the Company hasdid not imputedimpute interest on 50% of the $5 million note based on the provision in the License and Supply Agreement which providesthat provided for a recovery of up to $2.5 million of the license fee paid, which iswas dependent upon regulatory approval of the product. If regulatory approval of the product is received, the convertible note would be repayable and the Company would behave been entitled to recovery of up to $2.5 million based on the aforementioned provision. If the parties determine that regulatory approval will not likely occur, the note shallwill not be repayable and the Company would not receive recovery of up to $2.5 million of the license fee. The $2,173,127 difference between the $7.5 million principal amount of the notes and the discounted balance is being accreted to interest expense over the estimated term of the notes. Elan iswas considered to be a related party based on its ownership interest in the Company, significant license agreements entered into and involvement with research and development activities of the Company. In addition, Elan had a right to appoint a representative on the board of directors from January 22, 1997 through May 8, 2003. Elan ceased to be a related party in September 2003 upon termination of the jointly owned corporation as described below. The aggregate amount of the $7.5 million convertible notes payable are convertible into 361,842278,339 shares of common stock.
In April 1998, the Company entered into an agreement with Elan to develop Elan's proprietary oral sustained release formulation of fampridine for the treatment of multiple sclerosis (see note 7).sclerosis. Upon approval of aan NDA for the product by the FDA in the United States, the Company is obligated to pay Elan $2.5 million. In addition, the Company is obligated to pay an additional amount of $2.5 million to Elan, upon the earlier occurrence of the following: (a) first anniversary from the date of approval of NDA approval in the United States, or (b) upon approval of the product by a regulatory authority in Japan, the United Kingdom, Germany, France or Italy.
The Company has entered into various other research and license agreements which, as of June 30, 2003, upon accomplishment of certain milestones, will require payments by the Company aggregating up to $15.8 million. Approximately $375,000 of these milestone payments can be taken as credits against earned royalties. Upon regulatory approval, these agreements also require the Company to make royalty payments as a percentage of product sales.
(11) Employee Benefit Plan
Effective September 1, 1999, the Company adopted a defined contribution 401(k) savings plan (the "401(k) plan") covering all employees of the Company. Participants may elect to defer a percentage of their annual pretax compensation to the 401(k) plan, subject to defined limitations. No contributions were made by the Company for the years ended June 30, 2001, 2002 and 2003, respectively.
(12) Commitments and Contingencies
During 1998, the Company entered into a lease agreement for its facility. During November, 2000 and May, 2001, the Company entered into amendments of the lease for its facility. Under the amendments, the Company increased the total leased space and extended the lease term for its original leased space. Future minimum commitments under the facility leases are as follows:
| 2004 | $ | 641,808 | |
| 2005 | 641,808 | ||
| 2006 | 641,808 | ||
| 2007 | 641,808 | ||
| 2008 | 374,388 | ||
| $ | 2,941,620 | ||
F-27
Rent expense under these operating leases during the years ended June 30, 2001, 2002, and 2003 was $309,442, $468,309, and $652,339, respectively.
Under the terms of the employment agreement with the Company's chief executive officer, the Company is obligated to pay severance under certain circumstances. If the employment agreement is terminated by the Company or by the Company's chief executive officer for reasons other than for cause, the Company must pay (i) an amount equal to the base salary for a period of one year following the date of termination, plus (ii) bonus equal to last annual bonus received by chief executive officer multiplied by a fraction, the numerator of which shall be the number of days Also, in the calendar year elapsed as of the termination date and the denominator of which shall be 365.
The Company has entered into various clinical trial agreements which, as of June 30, 2003, upon accomplishment of certain milestones, will require payments by the Company aggregating up to $5.1 million. Approximately $2.5 million of these milestone payments have been made or accrued in the consolidated financial statements.
The Company is not a party to any material legal proceedings. It is the Company's policy to accrue for amounts related to legal matters if it is probable that a liability has been incurred and the amount is reasonably estimable.
(13) Subsequent events
On September 24, 2003, the Company entered into an employment agreement with the Company's chief financial officer, which was effective September 1, 2003. Under the terms of the agreement the Company is obligated to pay severance under certain circumstances. If the employment agreement is terminated by the Company or by the Company's chief financial officer for reasons other than for cause, the Company must pay (i) an amount equal to the base salary for a period of six months following the date of termination, plus (ii) bonus equal to last annual bonus received by chief financial officer multiplied by a fraction, the numerator of which shall be the number of days in the calendar year elapsed as of the termination date and the denominator of which shall be 365.
In September 2003, the Company granted 1,374,997 stock options, that had been authorized for issuance under the Plan in May 2003, to employees under the Plan at exercise prices of $5.88 per share, which is below the estimated fair value of the Company's common stock at the date of grant. Compensation expense of approximately $8.3 million will be recognized immediately in the first quarter of fiscal 2004, as a certain number of options issued to employees vested immediately and the balance of $5.2 million will be recognized over the remaining respective vesting periods of the options.
F-28
In September 2003, the Company re-priced 150,251 stock options issued to employees, which had an exercise price per option of more than $5.88, with a new exercise price per share of $5.88. As a result of this re-pricing, the Company will recognize an additional compensation charge estimated at approximately $520,000, of which $449,000 will be recognized during the first quarter of fiscal 2004, with the balance to be recognized over the remaining respective vesting periods of the repriced options.
Elan
In January 1997, the Company licensed from Elan exclusive worldwide rights to Elan's sustained release formulation of fampridine, Fampridine-SR, for the treatment of SCI. In April 1998, the Company formed MS Research & Development Corporation or ("MSRD,") with Elan and one of its affiliates to develop Fampridine-SR for treatment of MS.multiple sclerosis. At that time, MSR&DMSRD licensed from Elan exclusive worldwide rights to Fampridine-SR for the treatment of MS.multiple sclerosis.
Termination and Assignment Agreement. In September 2003, the Company entered into a termination and assignment agreement with Elan, Elan's affiliate, and MSRD pursuant to which MSRD (83% owned by Acorda immediately prior to entering into the agreement) assigned to the Company its assets, including the license from Elan for Fampridine-SR for treatment of MS.multiple sclerosis. The Company paid MSRD approximately $11.5 million for all the assets and liabilities of MSRD. MSRD will distributedistributed the purchase price to its shareholders according to their equity ownership interest. The Company will receivehas received a distribution of approximately $9.5 million as a result of this distribution and the remaining distribution of $2 million will bewas expensed in the first quarter of fiscal 2004September 30, 2003 as acquired in-process research and development.development and classified under Research and Development-related party. The Company also purchased Elan's affiliate shares at par value and now owns approximately 88% of MSRD, which now has no assets or liabilities and is inactive.
Amended and Restated License. In September 2003, the Company entered into an amended and restated license with Elan, which replaced the two prior licenses for Fampridine-SR. Under this agreement, Elan granted the Company exclusive worldwide rights to Fampridine-SR, as well as Elan's formulation for any other mono- or di-aminopyridines, for all indications, including SCIspinal cord injury and MS.multiple sclerosis. The Company agreed to pay Elan milestone payments and royalties based on net sales of the product.product if and when approved.
Elan may terminate the Company's license in the United States, the major European markets or Japan if the Company does not file to obtain regulatory approval or launch the product after regulatory approval in the applicable country within specified periods. If Elan terminates the Company's license in any applicable country, Elan is entitled to license from the Company patents rights and know-how relating to the product and to market the product in the applicable country, subject to royalty payments.
F-29
Elan is responsible for completing the chemistry, manufacturing and controls section of the NDA for Fampridine-SR and equivalent regulatory applications outside the United States. Elan is also responsible for supplying the product for clinical trials under this agreement.
Subject to early termination provisions, the Elan license terminates on a country by country basis on the latter to occur of fifteen years from the date of the agreement, the expiration of the last to expire Elan patent or the existence of competition in that country.
Supply agreement.Agreement. In September 2003, the Company entered into a supply agreement with Elan relating to the manufacture and supply of Fampridine-SR by Elan. The Company agreed to purchase at least 75% of its annual requirements of product from Elan, unless Elan is unable or unwilling to meet
its requirements, for a purchase price based on a specified percentage of net sales. In those circumstances, where the Company elects to purchase less than 100% of its requirements from Elan, the Company agreed to make certain compensatory payments to Elan. Elan agreed to assist the Company in qualifying a second manufacturer to manufacture and supply the Company with Fampridine-SR subject to its obligations to Elan.
Securities Amendment Agreement. In September 2003, the Company entered into a securities amendment agreement with Elan to modify certain provisions in some existing agreements between Elan and the Company. These included:
restrictions.
Teva Pharmaceuticals Industries Ltd.Collaboration Agreement.
In September 2003, the Company entered into a collaboration agreement with Teva Pharmaceuticals Industries Ltd. (Teva)("Teva") under which the Company was granted a co-exclusive license with Teva to jointly develop and promote in the United States products containing valrocemide as an active ingredient in any formulation and dosage form for any indication, except MS. However, in the event that Teva seeks to develop and promote products containing valrocemide for MS it must provide the Company with notice and negotiate an amendment to the agreement. The agreement provides that Teva will own all right, title and interest in and to all intellectual property jointly developed by the parties while Teva has the sole right and obligation to defend against any infringement claims.valrocemide.
The agreement further provides that Teva is responsible for seeking and maintaining regulatory approval from the FDA upon the completion of any co-developed product and that Teva will consult in preparing the filings to obtain regulatory approval. Teva is also solely responsible for the
F-30
commercializing, manufacture and supply of the product, and has the sole responsibility to commercialize the products in the territory.
The Company made an initial payment to Teva of $2.0 million that was charged as research and development expenses for the year ended December 31, 2003, upon execution of the collaboration agreement, and iswas obligated to make payments to Teva upon achievingrelating to the development of valrocemide.
The Company and Teva amicably terminated the Collaboration Agreement as of June 27, 2005, and in connection with the termination the Company paid Teva approximately $3.1 million. The Company and Teva have no further obligations to each other under the Collaboration Agreement.
(12) Employee Benefit Plan
Effective September 1, 1999, the Company adopted a defined contribution 401(k) savings plan (the "401(k) plan") covering all employees of the Company. Participants may elect to defer a percentage of their annual pretax compensation to the 401(k) plan, subject to defined limitations. No contributions were made by the Company for the years ended June 30, 2002 and 2003, the six-month period ended December 31, 2003, the year ended December 31, 2004 and the nine-month period ended September 30, 2005, respectively.
(13) Commitments and Contingencies
During 1998, the Company entered into a lease agreement for its facility. During November 2000 and May 2001, the Company entered into amendments of the lease for its facility. Under the amendments, the Company increased the total leased space and extended the lease term for its original
leased space. Future minimum commitments under all non-cancelable leases required subsequent to December 31, 2004 are as follows:
| 2005 | $ | 641,808 | |
| 2006 | 641,808 | ||
| 2007 | 641,808 | ||
| 2008 | 53,484 | ||
| $ | 1,978,908 | ||
Rent expense under these operating leases during the years ended June 30, 2002 and 2003, the six-month period ended December 31, 2003, the year ended December 31, 2004 and the nine-month period ended September 30, 2005 was $468,309, $652,339, $334,348, $670,413 and $501,395 (unaudited) respectively.
Under the terms of the employment agreement with the Company's chief executive officer, the Company is obligated to pay severance under certain milestones.circumstances. If the employment agreement is terminated by the Company or by the Company's chief executive officer for reasons other than for cause, the Company must pay (i) an amount equal to the base salary the chief executive officer would have received during the fifteen month period immediately following the date of termination, plus (ii) bonus equal to last annual bonus received by chief executive officer multiplied by a fraction, the numerator of which shall be the number of days in the calendar year elapsed as of the termination date and the denominator of which shall be 365.
The Company is not a party to any material legal proceedings. It is the Company's policy to accrue for amounts related to legal matters if it is probable that a liability has been incurred and the amount is reasonably estimable.
(14) Product Returns
As part of the terms of the Zanaflex asset purchase agreement, any product returned within six months of acquisition date was the obligation of Elan. Beginning in January 2005, such returns became a liability of the Company. Through September 30, 2005, the Company has accepted $4.0 million (unaudited) in total product returns, of which $2.1 million (unaudited) was for product not sold by the Company. As the Company will accept product returned up to twelve months subsequent to its expiration date, the Company expects to continue to receive returns of Zanaflex tablets sold by Elan through June 2006. The Company has recorded a charge to discounts and allowances of $4.1 million in the year ending December 31, 2004 to record an estimated liability for these returns, of which $1,966,343 (unaudited) remains as of September 30, 2005.
As part of the Zanaflex acquisition, the Company purchased certain tablet from Elan that expires within one year. The majority of this product was sold by the Company during July 2004 though March 2005. The Company has deferred revenue for this product due to the uncertainty of future returns. Included in deferred product revenue-tablets is $3.7 million and $2.5 million (unaudited) as of December 31, 2004 and September 30, 2005, respectively related to the sale of short dated product. The Company recorded a charge of $177,000 during 2004 to write-off the cost of the short dated product.
(15) Zanaflex Asset Purchase Agreement
The Company acquired all of Elan's U.S. sales, marketing and distribution rights to Zanaflex Capsules and Zanaflex tablets in July 2004 for $2.0 million plus $675,000 for finished goods inventory. The Company is also responsible for the paymentup to $19.5 million in future contingent milestone payments based on cumulative gross sales of costs as well as conducting the next clinical trial of a productZanaflex tablets and Zanaflex Capsules. These products are approved for the treatmentmanagement of therapy. The Company must make the best reasonable efforts to complete the next clinical trial by the first quarter of 2006; if further clinical trials are required after the completion of the next clinical trial, then the costs of such trials will be shared. Such costs will be shared equally with Teva during any calendar quarter based on the net sales of any co-developed product. However, in the event the costs incurred by both parties under the agreement exceed net sales, the Company is obligated to pay Teva a fee equal to 50% of the amount by which the aggregate costs have exceeded net sales. Under the agreement, the Company is entitled to receive a fee on a country by country basis, based on sales by Teva of co-developed products outside the United States, if clinical data used to obtain regulatory approval of sale of the product in such country was jointly developed, or independently by the Company, under this Agreement.
Unless earlier terminated under provisions of the Teva agreement, the agreement will expire on the earlier to occur of (i) September 2009, if parties have not achieved a statistically significant primary endpoint that is acceptedspasticity. Zanaflex tablets were approved by the FDA in 1996 and lost patent protection in 2002. There are currently 11 generic versions of Zanaflex tablets on the market. Zanaflex Capsules were approved by the FDA in 2002, but were never marketed by Elan. The Company began marketing Zanaflex Capsules in April 2005.
The Company is responsible for royalty payments to Elan and Novartis, based upon Net Sales of Zanaflex Capsules and tablets beginning on the closing date.
In connection with this transaction, the Company acquired the rights to the tradename "Zanaflex®", one issued U.S. patent and two patent applications related to Zanaflex Capsules, and the remaining tablet inventory on hand with Elan. Additionally, the Company assumed Elan's existing contract with Novartis to manufacture Zanaflex tablets and entered into a separate contract with Elan to manufacture Zanaflex Capsules. The Company separately launched Zanaflex Capsules in April 2005. The Company did not acquire any receivables, employees, facilities or fixed assets. The Company has allocated, on a relative fair value basis, the initial consideration paid to Elan to the assets acquired, principally the Zanaflex tradename $200,000 and the capsulation patent $1.8 million. The Company has allocated $150,000 and $1,350,000 of the first pivotal trialmilestone payment owed to the tradename and patent, respectively, upon achievement of that milestone's criteria in connection with any product, (ii) six months afterOctober 2004, and has allocated $300,000 (unaudited) and $2,700,000 (unaudited) of the first generic versionsecond milestone payment owed to the tradename and patent, respectively, upon achievement of any productthat milestone's criteria in September 2005. There is launchedno expected residual value of these intangible assets. As future milestone payments are made to Elan, such amounts will be allocated, on a relative fair value basis, to the assets acquired. The Company will amortize the allocated fair value of the tradename and patent over their estimated economic benefit to be achieved of approximately 2.5 years and 17 years, respectively.
Intangible Assets consisted of the following:
| | December 31, 2004 | September 30, 2005 | Estimated useful lives | |||||
|---|---|---|---|---|---|---|---|---|
| | | (unaudited) | | |||||
| Zanaflex patent | $ | 3,150,000 | $ | 5,850,000 | 17 years | |||
| Zanaflex tradename | 350,000 | 650,000 | 2.5 years | |||||
| 3,500,000 | 6,500,000 | |||||||
| Less accumulated amortization | 113,950 | 362,977 | ||||||
| $ | 3,386,050 | $ | 6,137,023 | |||||
The Company recorded $114,000 and $249,000 (unaudited) in amortization expense related to these intangible assets in the United States, or (iii)year ending December 31, 2004 and the nine-month period ending September 2012, if30, 2005, respectively.
Estimated future amortization expense for these intangible assets subsequent to December 31, 2004 is as follows:
| 2005 | $ | 433,789 | |
| 2006 | 739,047 | ||
| 2007 | 349,489 | ||
| 2008 | 349,489 | ||
| $ | 1,871,814 | ||
(16) Reverse Stock Split
On September 18, 2005, the partiesCompany's Board of Directors approved a 1-for-1.3 reverse stock split, which became effective on January 11, 2006. All references to common stock, common shares outstanding, average number of common shares outstanding, per share amounts, options and warrants and Elan notes payable in these financial statements and notes to financial statements have not commencedbeen restated to reflect the promotion and/or commercialization of any product under the Teva agreement.
In addition, we have granted Tevaone-for-one point three common stock reverse split on a right of first negotiation for the co-development and co-promotion of Fampridine-SR in North America.retroactive basis.
Rush-Presbyterian St. Luke's Medical Center(17) Subsequent Events (unaudited)
In 1990, Elan licensed from Rush know-how relating to fampridine for the treatment of MS. The Company subsequently licensed this know-how from Elan. In September 2003, On December 23, 2005, the Company entered into an agreement with Rushan affiliate of Paul Royalty Fund, or PRF, under which the Company received $15.0 million in cash. In exchange the Company has assigned PRF revenue interests in Zanaflex Capsules, Zanaflex tablets and Elan which terminatedany future Zanaflex products. The agreement covers all Zanaflex net revenues (as defined in the Rush licenseagreement) generated from October 1, 2005 through and including December 31, 2015, unless the agreement terminates earlier. Under the agreement, PRF is entitled to the following portion of Zanaflex net revenues:
Notwithstanding the foregoing, once PRF has received and retained payments under the agreement that are at least twice the aggregate amount PRF has paid the Company under the agreement, PRF will only be entitled to 1% of Zanaflex net revenues. If PRF is entitled to 15% of net revenues as described above, the Company will remit 8% of cash payments received from wholesalers to PRF on a daily basis, with a quarterly reconciliation and settlement.
Under the terms of the agreement the Company used $3.0 million of the $15.0 million in proceeds to partially repay its $6 million senior secured term loan with GE (see Note 7). The Company also entered intoincurred approximately $200,000 of expenses associated with the repayment of the GE term loan and approximately $500,000 of other transaction-related expenses that will be capitalized and amortized over the life of the arrangement. Under the terms of the prepayment GE relinquished its rights to the Company's personal property owned at closing or subsequently acquired that relates to the interests in Zanaflex acquired by PRF. This payment changed the aggregate principal payments to GE required subsequent to December 31, 2004 to: $3,858,654 in 2005; $890,521 in 2006; $1,062,180 in 2007; and
$187,645 in 2008. The related interest payments required subsequent to December 31, 2004 are: $524,687 in 2005; $163,196 in 2006; $76,683 in 2007; and $2,332 in 2008.
In connection with the transaction, the Company expects to record a liability, referred to as the revenue interest liability, of approximately $14.6 million in accordance with EITF 88-18,Sales of Future Revenues.The Company will impute interest expense associated with this liability using the effective interest rate method and will record a corresponding accrued interest liability. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the life of the arrangement. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level of Zanaflex sales. The Company currently estimates that the imputed interest rate associated with this liability will be approximately 8.9%. Payments made to PRF as a result of Zanaflex sales levels will reduce the accrued interest liability and the principal amount of the revenue interest liability.
The Company also has the option to receive two additional payments from PRF under the revenue interest assignment agreement, which essentially take the form of additional borrowings:
If the Company meets these milestones and decides to borrow these additional funds, it would be required to repay PRF $5.0 million on December 1, 2009 in the case of the first additional payment and $5.0 million on December 1, 2010 in the case of the second additional payment.
The agreement also contains put and call options whereby the Company may repurchase the revenue interest at its option or can be required by PRF to repurchase the revenue interest, contingent upon certain events. If the Company experiences a change of control, undergoes certain bankruptcy events, transfers any of their interests in Zanaflex (other than pursuant to a license agreement, with Rush indevelopment, commercialization, co-promotion, collaboration, partnering or similar agreement), transfers all or substantially all of its assets, or breaches certain of the covenants, representations or warranties made under the agreement, PRF has the right, which Rush grantedwe refer to as PRF's put option, to require the Company to repurchase the rights sold to PRF at the "put/call price" in effect on the date such right is exercised. If the Company experiences a change of control or completes an exclusive worldwide licenseinitial public offering of shares of its common stock that results in the Company having a total market capitalization in excess of $150.0 million, it has the right, which we refer to their know-how relatingas the Company's call option, to fampridinerepurchase the rights sold to PRF at the "put/call price" in effect on the date such right is exercised. If the Company's call option becomes exercisable as a result of this offering, the Company will have a period of 180 days during which to exercise the option. The Company does not currently intend to exercise its call option if it becomes exercisable as a result of this offering but may reevaluate whether it would exercise the option during the 180-day period. The put/call price on a given date is the greater of (i) 150% of all payments made by PRF as of such date, less all payments received by PRF as of such date, and (ii) an amount that would generate an internal rate of return to PRF of 25% on all payments made by PRF as of such date, taking into account the amount and timing of all payments received by PRF as of such date. The Company has determined that PRF's put option and the Company's call option meet the criteria to be considered an embedded derivative and should be accounted for as such. Therefore, the treatmentCompany expects to record a net liability of MS. Rush has also assignedapproximately $400,000 related to the Company their Orphan Drug Designation for fampridine for the reliefput/call option to reflect its estimated fair value as of symptoms of MS.
The Company agreed to pay Rush a license fee, milestone payments and royalties based on net sales of the product for neurological indications. The Company also entered into an agreement with Elan relating to the allocation of payments between the Company and Elan of certain payments to Rush under the Rush license.
Subject to early termination provisions, the Rush license terminates upon expiration of the royalty obligations, which expire fifteen years from the date of the agreement.
F-31
In September 2003, the Company also entered into an agreement, in accordance with RushSFAS No. 133,Accounting for Derivatives Instruments and Elan, pursuant to which the Company agreed that the payment of license fees, milestones and royalties to Rush under the license agreement with Rush,Hedging Activities. This liability will be allocated between Elan and the Company.
Upon entering into the above mentioned R&D and Product Collaborations, Alliances and License agreements, the Company expended approximately $2.1 million. Upon accomplishment of certain milestones, the Company will be required to make payments aggregating up to approximately $30 million.
In September 2003, the Company entered into two marketing service agreements with inChord Communication, Inc. and Cardinal Health PTC, Inc. for contract sales and marketing services associated with the commercialization of Fampridine-SR in the United States. RxPedite is a program developed by inChord Communications, Inc. and Cardinal Health PTC, Inc. pursuant to which they provide pharmaceutical drug manufacturers and biotech companies with comprehensive outsourced marketing communications, selling and distribution/manufacturing capabilities. The Company will receive the RxPedite services at a discounted market rate in exchange for full repayment of such discounts, if the product achieves 100% of its revenue targets (based on gross sales) through the twelve month period following launch, plus a risk premium in the event that the product exceeds such target revenues. The repayment terms range between no repayment if the product fails to achieve more than 50% of its revenue target in any quarter and full repayment of all discounts plus 200% of such amounts if the product achieves over 130% of its revenue target. The discount from the market rate is to be determined periodically according to a target product profile, which is in the range of 10-28%. The discounts and premiums will be repaidrevalued on a quarterly basis beginning six months afterto reflect any changes in the fair value and any gain or loss resulting from the revaluation will be recorded in earnings.
Report of Independent Public Accounting Firm
The Board of Directors
Elan Corporation, plc:
We have audited the accompanying statements of net revenues and direct expenses for the period from January 1, 2004 through July 21, 2004, and the year ended December 31, 2003 of the Zanaflex Product Line of Elan Corporation, plc. These financial statements are the responsibility of Elan Corporation, plc's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of Elan Corporation, plc's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying statements were prepared for the purpose of complying with the rules and regulations of the Securities and Exchange Commission and for inclusion in the Form S-1 of Acorda Therapeutics, Inc., as described in Note 2. The presentation is not intended to be a complete presentation of the Zanaflex results of operations.
In our opinion, the financial statements referred to above present fairly, in all material respects, the Zanaflex Product Line's net revenues and direct expenses for the period from January 1, 2004 through July 21, 2004 and the year ended December 31, 2003, in conformity with U.S. generally accepted accounting principles.
/s/ KPMG
KPMG
Dublin, Ireland
January 18, 2006
ZANAFLEX PRODUCT LINE
(A Product of the Elan Group)
Statements of Net Revenues and Direct Expenses
For the period from January 1, 2004 through July 21, 2004
and the year ended December 31, 2003
(In US $000)
| | Period from January 1, 2004 through July 21, 2004 | Year Ended December 31, 2003 | |||||
|---|---|---|---|---|---|---|---|
| Net product revenue (note 4) | $ | 5,656 | $ | 4,121 | |||
| Cost of product sold (note 2) | 1,265 | 891 | |||||
| Gross profit | 4,391 | 3,230 | |||||
| Research and development expenses | 572 | 609 | |||||
| Excess of net product revenue over direct expenses | $ | 3,819 | $ | 2,621 | |||
See accompanying notes to financial statements.
ZANAFLEX PRODUCT LINE
(A Product of the Elan Group)
Notes to Financial Statements
(1) Description of Business
Elan licensed Zanaflex (tizanidine hydrochloride) Product Line (Zanaflex or the Product) for the U.S. market from Novartis Pharma A.G. and subsequently launched the tablet formulation in 1997. It was approved by the FDA as a short-acting drug for the management of spasticity. In June 2002, Eon Laboratories received FDA approval to market a generic version of tizanidine tablets. A number of other generic applications for tizanidine tablets have since received approval from the FDA. Elan has experienced a significant decline in the sales and profitability of the Zanaflex brand, which it believes is attributable to the availability of generic alternatives to Zanaflex. The Zanaflex brand is sold primarily to drug wholesalers in the U.S.
In the third quarter of 2002, Elan received FDA approval of an alternative capsule formulation of tizanidine which was not marketed until the product launchwas acquired by Acorda Therapeutics.
Pursuant to an Asset Purchase Agreement, dated as of July 21, 2004 (the Asset Purchase Agreement), Elan Pharmaceuticals, Inc., and ending eighteen months post launch,certain wholly owned subsidiaries of Elan (collectively, the Elan Group) agreed to sell to Acorda Therapeutics their rights to the Zanaflex product, along with certain related assets and agreements.
(2) Basis of Presentation
The statements of net revenues and direct expenses of the Zanaflex Product Line have been prepared in accordance with accounting principles generally accepted in the United States of America. These statements were prepared for the purpose of complying with the rules and regulations of the Securities and Exchange Commission. The net revenues and direct expenses have been derived from the historical accounting records of the Company. Historically, financial statements have not been prepared for the Zanaflex Product Line as the Company did not maintain the Zanaflex Product Line as a final reconciliation afterseparate business unit. The statements of net revenues and direct expenses set forth the first twelve monthsrevenues, net and direct expenses attributable to the Zanaflex Product Line and do not purport to reflect all the costs, expenses, and resultant operating earnings and cash flows that would have been incurred had the Zanaflex Product Line been operated as a stand-alone, separate company. Net revenues include gross sales less product-specific sales returns, cash discounts, government and commercial rebates, and customer chargebacks. Direct expenses consist principally of cost of sales and research and development expenses. The statements do not include general administrative expenses or general corporate overhead such as corporate management, legal services, administration of insurance, treasury, payroll administration, corporate income tax, administration and benefit management, nor does it include any interest income and expense, as such items are not directly associated with Zanaflex Product Line.
Costs of product sold include the purchase price of the inventories, distribution costs for the respective product, and royalty payments to third-parties which are based on percentage or unit basis of gross product sales in accordance with the eventrespective royalty agreements. The amounts of over/under payment.royalties were $581,409 and $445,431 for the period from January 1, 2004 through July 21, 2004 and for the year ended December 31, 2003, respectively.
A joint commercialization committee, composed Since the product was not promoted in 2003 and 2004, there were minimal selling and marketing expenses associated with the Zanaflex Product Line. Certain research and development expenses are specifically identifiable and others, although direct expenses, require allocation to the Zanaflex Product Line since certain individuals performed research services on multiple product lines. These allocations
are made based on the percentage of time worked on the Zanaflex Product Line by individuals in research and development, and include salaries and professional fees.
(3) Summary of Significant Accounting Policies
(a) Revenue Recognition
Revenues from the sale of the CompanyProduct are recognized when title passes, net of applicable discounts and RxPedite representatives, will coordinateallowances.
(b) Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the overallamounts reported in the financial statements and accompanying disclosures. Actual results could differ from these estimates. As discussed in note 2, the financial statements include allocations and estimates that are not necessarily indicative of the costs and expenses that would have resulted if the Zanaflex Product Line had been operated as a separate entity.
(c) Rebates and Chargebacks, Cash Discounts, and Sales Returns
Revenue is recorded net of provision, made at the time of sale for rebates, chargebacks and cash discounts, as described below. The Elan Group entered into contracts with certain managed care organizations to provide access to the Zanaflex Product through formularies. Based on the managed care organization's market share performance and utilization of the Zanaflex Product, the organization receives managed care rebates from the Elan Group. In addition, the Elan Group is bound under certain laws and regulations to provide product at a discounted rate to Medicaid recipients. Medicaid rebates are paid to each state in the United States based on claims filed by pharmacies that provide the Zanaflex Product to Medicaid recipients at the reduced rate. Managed care and Medicaid rebates are charged to the Zanaflex Product, determined by estimating the actual usage of the Zanaflex Product by Medicaid recipients and managed care participants covered by the contracted prices.
Chargebacks are amounts credited to wholesalers to reimburse the wholesaler for sales to third parties at reduced prices based on contracts the Elan Group negotiates. Chargebacks are recorded based on the historical experience.
The Elan Group offers a cash discount to customers if invoices are paid within a certain time period.
In addition, sales returns are charged to the Zanaflex Product based upon estimates derived from the Elan Group's historical experience updated for changes in fact and circumstances, as appropriate.
(4) Net Product Revenues
Returns, chargebacks, and discounts and rebates charged to gross product revenues are as follows (in US $000):
| | Period from January 1, 2004 through July 21, 2004 | Year Ended December 31, 2003 | ||||||
|---|---|---|---|---|---|---|---|---|
| Zanaflex: | ||||||||
| Gross product revenues | $ | 8,979 | $ | 6,879 | ||||
| Returns | 1,777 | 1,500 | ||||||
| Customer chargebacks | 1,153 | 719 | ||||||
| Discounts and rebates | 393 | 539 | ||||||
| Net product revenues | $ | 5,656 | $ | 4,121 | ||||
(5) Corporate Services
The Elan Group performed certain corporate functions for the Zanaflex Product Line for the period from January 1, 2004 through July 21, 2004 and the year ended December 31, 2003, including, but not limited to, corporate management, certain legal services, administration of insurance, treasury, payroll administration, corporate income tax administration, employee compensation and benefit management, and administration. The costs of these corporate services are not included in costs of goods sold, as the Elan Group does not allocate such costs by product.
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Introduction to Unaudited Pro Forma Combined Statement of Operations
On July 21, 2004, Acorda Therapeutics, Inc (the "Company") acquired the U.S. sales, marketing and distribution rights to Zanaflex Capsules and Zanaflex tablets (the "Zanaflex Product Line") from Elan Pharmaceuticals, Inc., a wholly owned subsidiary of Elan Corporation, plc (with its consolidated subsidiaries, "Elan") pursuant to an Asset Purchase Agreement. The assets acquired included the U.S. Food and Drug Administration registration for each product, a patent and two related patent applications and existing Zanaflex tablet inventory. The acquisition did not include physical facilities, an employee base, working capital accounts, customer base, production techniques or a market distribution system. The Company made an upfront payment to Elan of $2.0 million plus $675,000 for finished goods inventory. The Company is also responsible for up to $19.5 million in future contingent milestone payments based on cumulative gross sales strategiesof Zanaflex tablets and Zanaflex Capsules. These products are approved for the productmanagement of spasticity. Zanaflex tablets were approved by the FDA in 1996 and associated workplans and budgets; however,lost patent protection in 2002. There are currently 11 generic versions of Zanaflex tablets on the market. Zanaflex Capsules were approved by the FDA in 2002, but were never marketed by Elan. The Company retains ultimate approval authority over all relevant decisions.began marketing Zanaflex Capsules in April 2005. The Company is responsible for all regulatory affairs including: filingsroyalty payments to Elan and approvals, complianceNovartis, based upon Net Sales of promotional materials, adverse event reporting, product recalls,Zanaflex Capsules and communicationZanaflex tablets beginning on the closing date.
In connection with regulatory authorities. Acorda owns all work product developed pursuantthis transaction, the Company acquired the rights to the marketing service agreements unless otherwise agreedtradename "Zanaflex", one issued U.S. patent and two patent applications related to Zanaflex Capsules, and the remaining Zanaflex tablet inventory on hand with Elan. Additionally, the Company assumed Elan's existing contract with Novartis to manufacture Zanaflex tablets and entered into a separate contract with Elan to manufacture Zanaflex Capsules. The Company separately launched Zanaflex Capsules in a specific work plan.April 2005.
The marketing service agreements designate RxPediteaccompanying unaudited pro forma statement of operations for the year ended December 31, 2004 combines the historical consolidated statement of operations of the Company for the year ended December 31, 2004 with the statements of net revenues and direct expenses of the Zanaflex Product Line of Elan for the period January 1, 2004 through July 21, 2004 included elsewhere in this registration statement to give effect to our acquisition of the Zanaflex Product Line as if the "agencyacquisition had occurred on January 1, 2004. The Company made pro forma adjustments to its historical consolidated consolidated statement of record" with respectoperations information to give effect to events that are (i) directly attributable to the services providedacquisition, (ii) expected to have a continuing impact on the combined results, and contain buy-out provisions(iii) factually supportable.
It is impracticable to prepare complete financial statements in accordance with Rules 3-01 and 3-02 of Regulation S-X relating to the assets acquired, as Elan did not account for the Zanaflex Product Line as a separate entity and cannot objectively allocate certain expenses to the assets. The statements of net revenues and direct expenses for the Zanaflex Product Line of Elan included elsewhere in this registration statement include certain allocations that Elan's management believes are reasonable; however, these allocations are not necessarily indicative of costs that would have been incurred related to the Zanaflex Product Line on a stand-alone basis. For instance, tax expense and interest income have not been included in the event thataccompanying statements of net revenues and direct expenses, as they are not specifically identifiable to the Company enters into a co-promote agreement with a third party and can no longer allocate a substantial amountZanaflex Product Line.
The unaudited pro forma combined statement of operations is not necessarily indicative of the servicesresults that would have occurred had our acquisition of the Zanaflex Product Line occurred on January 1, 2004 and should not be construed as being representative of future operating results. The unaudited financial information should be read in conjunction with:
F-32
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Unaudited Pro Forma Combined Statement of Operations
For the Year Ended December 31, 2004
| | Acorda | Zanaflex Product Line | Pro forma adjustments | Notes | Pro forma Acorda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gross sales—Zanaflex | $ | — | $ | 8,979 | $ | (25 | ) | A | $ | 8,954 | |||||||
| Less: Discounts and allowances | (4,417 | ) | (3,323 | ) | (7,740 | ) | |||||||||||
| Net sales | (4,417 | ) | 5,656 | (25 | ) | 1,214 | |||||||||||
| Grant revenue | 479 | — | 479 | ||||||||||||||
| Total net revenue | (3,938 | ) | 5,656 | (25 | ) | 1,693 | |||||||||||
| Less: Cost of sales | (885 | ) | (1,265 | ) | (324 | ) | A, B, C | (2,474 | ) | ||||||||
| Gross profit | (4,823 | ) | 4,391 | (349 | ) | (781 | ) | ||||||||||
Operating expenses: | |||||||||||||||||
| Research and development | 21,999 | 572 | 22,571 | ||||||||||||||
| Sales and Marketing | 4,662 | 4,662 | |||||||||||||||
| General and administrative | 13,283 | 13,283 | |||||||||||||||
| Total operating expenses | 39,944 | 572 | — | 40,516 | |||||||||||||
| Operating loss | (44,767 | ) | 3,819 | (349 | ) | (41,297 | ) | ||||||||||
| Other income (expense): | |||||||||||||||||
Interest and amortization of debt discount expense | (385 | ) | (385 | ) | |||||||||||||
| Interest income | 409 | (55 | ) | D | 354 | ||||||||||||
| Other income | 2 | 2 | |||||||||||||||
| Total other income (expense) | 26 | — | (55 | ) | (29 | ) | |||||||||||
| Net loss | (44,741 | ) | 3,819 | (404 | ) | (41,326 | ) | ||||||||||
| Beneficial conversion feature, accretion of issuance costs, preferred dividends, and fair value of warrants issued to convertible preferred stockholders | (24,746 | ) | (24,746 | ) | |||||||||||||
| Net loss allocable to common stockholders | $ | (69,487 | ) | $ | 3,819 | $ | (404 | ) | $ | (66,072 | ) | ||||||
Subject
ACORDA THERAPEUTICS, INC. AND SUBSIDIARY
Notes to early termination provisions, the agreements expire on the laterUnaudited Pro Forma Combined Statement of five years from the effective dateOperations
(1) Adjustments to Unaudited Pro Forma Combined Statement of the agreements or twenty-four months following the program launch in the case of the sales services, or eighteen months following product launch for spinal chord indication in the case of the marketing services.Operations
In September 2003, the Company obtained approval by the written consentA To eliminate sales and profit recognition on inter-company sales from the holders of Series J preferred stock voting together as a single class and the holders of the Preferred Stock (as defined in note 7), voting separately as a single class on an as if converted basis for a reduction in the price per share of common stock offeredElan to the public in an initial public offering which would trigger automatic conversion of the preferred stock into common stock from an offering price ofAcorda that has not less than $14.76 per sharebeen sold to an offering price of not less than $12.00 per share.
On September 25, 2003, the Company amended the Plan, which will become effective upon consummation of the Company's initial public offering contemplated herein, providing for automatic annual increases to the share reserve under the Plan on the first day of each fiscal year by a number of shares equal to the lessor of: (a) the number that will bring the total reserve to 2.5% of then outstanding shares of common stock; (b) 647,151 shares; (c) or a number determined by the board of directors.
On September 25, 2003, the Board of Directors authorized the filing of a registration statement with the SECB To record amortization expense for the sale of shares of common stock. Ifperiod January 1 to July 21 for the offering is consummated under the terms presently anticipated, all shares of Series A, B, C, E, F, H, I and J preferred stock outstanding as of the Consummation of the Offering will automatically convert into shares of common stock. No dividends will be payable on any of the Series A, B, C, E, F, H, I and J preferred stock.
On September 25, 2003, the Company stockholders approved a one-for-twelve reverse stock split to be effective immediately prior to the effectiveness of the registration statement filedacquired intangible assets that were recognized in connection with the acquisition of Zanaflex Product Line.
(2) Intangible Assets
The Company has allocated, on a relative fair value basis, the initial public offering contemplated herein.consideration paid to Elan to the assets acquired, principally the Zanaflex tradename $200,000 and the capsulation patent $1.8 million. The reverse stock splitCompany has been retroactively reflectedallocated $150,000 and $1,350,000 of the first milestone payment owed to the tradename and patent, respectively, upon achievement of that milestone's criteria in October 2004. There is no expected residual value of these intangible assets. As future milestone payments are made to Elan, such amounts will be allocated, on a relative fair value basis, to the accompanying consolidated financial statements.assets acquired. The Company will amortize the allocated fair value of the tradename and patent over their estimated period of economic benefit, of approximately 2.5 years and 17 years, respectively.
The pro forma amounts below give effect to the acquisition of the Zanaflex Product Line as if the transaction had occurred on January 1, 2004. The pro forma effects below reflect the achievement of the sales milestones based on cumulative sales from January 1, 2004. This results in additional pro forma expense of approximately $158,000. The pro forma effects include:
Upon consummation of an initial public offering, eachIntangible Assets consisted of the Company non-employee directors will receive non-qualified stock options to purchase 20,000 shares at the initial public offering price per share, which options will vest in 12 equal quarterly installments commencing 180 days after the date of grant.following:
Upon consummation of an initial public offering, each of the Company directors will receive an annual option grant to purchase 10,000 shares on the date of the Company annual stockholder meeting. In addition, the Company's directors will receive an annual grant of 5,000 options exercisable to purchase shares for each
F-33
| | Historical December 31, 2004 | Useful lives | Pro forma December 31, 2004 | Useful lives | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zanaflex Capsules patent | $ | 3,150,000 | 17 years | $ | 5,850,000 | 18 years | ||||
| Zanaflex tradename | 350,000 | 2.5 years | 650,000 | 3 years | ||||||
| 3,600,000 | 6,500,000 | |||||||||
Less accumulated amortization | 113,950 | 271,835 | ||||||||
| $ | 3,386,050 | $ | 6,228,165 | |||||||
committee on which they serve. Directors will also be reimbursed for expenses incurred in attending meetings.
In accordance with the Company amended and restated certificate of incorporation which we plan to file upon consummation of an initial public offering, the Company board of directors has the authority, without further action by the stockholders, to issue up to 1,000,000 shares of preferred stock. The Company board of directors may issue preferred stock in one or more series and may determine the rights, preferences, privileges, qualifications and restrictions granted to or imposed upon the preferred stock, including dividend rights, conversion rights, voting rights, rights and terms of redemption, liquidation preferences and sinking fund terms, any or all of which may be greater than the rights of the common stock. The issuance of preferred stock could adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation. The issuance of preferred stock could also have the effect of decreasing the market price of the common stock and could delay, deter or prevent a change in control of the company. We have no present plans to issue any shares of preferred stock.
F-34
5,500,000 Shares


Common Stock
Prospectus
, 20032006
Banc of America Securities LLC
LazardU.S. Bancorp Capital Markets
Piper JaffrayRBC Capital Markets
SG Cowen & Co.
Until , 200 ,2006, all dealers that buy, sell or trade the common stock may be required to deliver a prospectus, regardless of whether they are participating in this offering. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PARTPart II
INFORMATION NOT REQUIRED IN PROSPECTUS
ItemITEM 13. Other expenses of issuance and distributionOTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth allour estimated costs and expenses (other than underwriting discounts) payable byin connection with this offering.
| SEC Registration Fee | $ | 10,152.00 | ||
| NASD Filing Fee | 9,125.00 | |||
| Nasdaq National Market Listing Fee | 5,000.00 | |||
| Printing and Engraving Expenses | 375,000.00 | |||
| Legal Fees and Expenses | 900,000.00 | |||
| Accounting Fees and Expenses | 750,000.00 | |||
| NASD-related Legal Fees and Expenses | 20,000.00 | |||
| Transfer Agent and Registrar Fees and Expenses | 20,000.00 | |||
| Miscellaneous | 10,000.00 | |||
| Total | $ | 2,099,277.00 | ||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Acorda Therapeutics, Inc. (the "Registrant") in connection with, or the sale of the common stock being registered, other than underwriting commissions and discounts. All amounts are estimates, except for the SEC registration fee, the NASD filing fee and the Nasdaq National Market listing application fee.
Item 14. Indemnification of officers and directors
UnderRegistrant, is a Delaware corporation. Section 145 of the Delaware General Corporation Law, or the Registrant has broad powers to indemnify its directors and officers against liabilities they may incur in such capacities, including liabilities under the Securities Act of 1933, as amended (the "Securities Act").
The Registrant's Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws include provisions to (i) eliminate the personal liability of its directors and officers for monetary damages resulting from breaches of their fiduciary duty to the extent permitted by Section 102(b)(7) of the General Corporation Law of Delaware (the "Delaware Law") and (ii) require the Registrant to indemnify its directors and officers to the fullest extent permitted by Section 145 of the Delaware Law, including circumstances in which indemnification is otherwise discretionary. Pursuant to Section 145 of the Delaware Law, aDGCL, grants each corporation generally hasorganized thereunder the power to indemnify its present and former directors, officers, employees and agents"indemnify any person who is or was a director, officer, employee or agent of a corporation or enterprise, against expenses, attorneys' fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by themhim in connection with any threatened, pending or completed action, suit to which they are or are threatened to be made, a partyproceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of their servingbeing or having been in any such positions so long as theycapacity if he acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action theyor proceeding, had no reasonable cause to believe theirhis conduct was unlawful. The Registrant believes that these provisions are necessary"
Section 102(b)(7) of the DGCL enables a corporation in its certificate of incorporation or an amendment thereto to attract and retain qualified persons as directors and officers. These provisions do not eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations or the directors' fiduciary duty of care, and, in appropriate circumstances, equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Delaware Law. In addition, each director will continue to be subject to liabilityexcept (i) for any breach of the director's duty of loyalty to the Registrant,corporation or its stockholders, (ii) for acts or omissions not in good faith or involvingwhich involve intentional misconduct foror a knowing violationsviolation of law, for acts or omissions that the director believes(iii) pursuant to be contrary to the best interestsSection l74 of the RegistrantDGCL (providing for liability of directors for unlawful payment of dividends or its stockholders,unlawful stock purchases or redemptions) or (iv) for any transaction from which thea director derived an improper personal benefit, for acts or omissions involving a reckless disregard forbenefit.
Article Six of the director's duty toRegistrant's Amended and Restated Certificate of Incorporation (filed as Exhibit 3.1) provides that except as otherwise provided by the DGCL, no director of the Registrant or its stockholders when the director was aware or should have been aware of a risk of serious injuryshall be personally liable to the Registrant or its stockholders for acts or omissions that constitute an unexcused patternmonetary damages for breach of inattention that amounts to an abdicationfiduciary duty as a director.
Article Six of the director's dutyRegistrant's Amended and Restated Certificate of Incorporation and Article Six of the Registrant's Amended Bylaws provide that, to the fullest extent permitted by the DGCL, the Registrant shall indemnify any current or its stockholders, for improper transactions between theformer director andor officer of the Registrant and for improper distributions to stockholders and loans to directors and officers. The provision
II-1
also does not affect a director's responsibilities under any other law, such asmay, at the federal securities law or state or federal environmental laws.
The Registrant has entered into indemnification agreements with certain directors and executive officers and intends to enter into indemnification agreements with all of its other directors and executive officers prior to the consummationdiscretion of the offering. Under these agreements,Board of Directors, indemnify any current or former employee or agent of the Registrant will indemnify its directorsagainst all expenses (including attorneys' fees) judgments, fines and executive officers against amounts paid in settlement actually and reasonably incurred in connection with actualany threatened, pending or threatened proceedings if anycompleted action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of them may be made a party because of their role as a director or officer. The Registrant is obligated to pay these amounts only if the officer or director acted in good faith and in a manner fact
II-1
that he or she reasonably believed to be inis or not opposed to the Registrant's best interests. For any criminal proceedings, the Registrant is obligated to pay these amounts only if the officer or director had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth procedures that will apply in the event of a claim for indemnification thereunder.
At present, there is no pending litigation or proceeding involving a director or officer of the Registrant, or is or was serving as to which indemnification is being sought nor isa director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise.
Article Six of the Registrant's Amended and Restated Certificate of Incorporation also provides that the Registrant aware of any threatened litigation that may result in claims for indemnificationshall advance expenses incurred by anya director or officer or director.
The Registrant intends to apply for an insurance policy covering the officers and directors of the Registrant with respectin defending any civil, criminal, administrative or investigative such action, suit or proceeding in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such director or officer to certain liabilities, including liabilities arisingrepay such advances if it shall ultimately be determined that he is not entitled to be indemnified by the Registrant as authorized by the Registrant's By-laws. In addition, upon the closing of this offering, our amended and restated certificate of incorporation (filed as exhibit 3.2) will provide that if a claim under the Registrant's By-laws is not paid in full by the Registrant within thirty days after a written claim has been received by the Registrant, the claimant may at any time thereafter bring suit against the Registrant to recover the unpaid amount of the claim, and if successful in whole or in part on the merits or otherwise in establishing his or her right to indemnification or to the advancement of expenses, the claimant shall be paid also the expense of prosecuting such claim.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
Within the past three years, the Registrant has issued securities in the following transactions, each of which was exempt from the registration requirements of the Securities Act or otherwise.
Reference is made to the indemnification and contribution provisionsof 1933, as amended, as transactions by an issuer not involving any public offering thereunder. All of the Underwriting Agreement filed as an exhibit to this Registration Statement.
Item 15. Recent sales of unregisteredbelow-referenced securities
The following sets forth information regarding sales are deemed restricted securities for the purpose of the Registrant's unregistered securities during the last three years (giving effect to a one-for-12 reverse stock split to be effected prior to the completion of the offering):
In March 2001, we consummated a private placement of 10,204,047 shares of our Series I Convertible Preferred Stock to a group of accredited investors at a purchase price of $3.89 per share for aggregate consideration of $39,693,743.Securities Act.
In May 2003, we consummated a private placement of 112,790,296112,790,233 shares of our Series J Convertible Preferred Stock to a group of accredited investors at a purchase price of $0.49 per share for aggregate consideration of $55,000,000.approximately $55,267,000.
AllIn March 2004, we consummated a private placement of 1,533,330 shares of our Series K Convertible Preferred Stock to a group of accredited investors at a purchase price of $7.50 per share for aggregate consideration of approximately $11,499,958.
Stock Options
In the above-described issuances were exempt from registration pursuantfourth quarter of 2002, we issued options to Section 4(2)purchase 2,218 shares of our common stock with a fair market value price of $2.60 to a number of our employees.
In the Securities Act, or Regulation D or Rule 144A promulgated thereunder, as transactions not involvingfirst quarter of 2003, we issued options to purchase 5,465 shares of our common stock with a public offering. With respectfair market value price of $2.60 to each transaction listed above, no general solicitation was made by either us or any person acting ona number of our behalf;employees. We also issued 1,282 options to purchase our common stock with a fair market value price of $2.60 to a non-employee director.
In the securities sold are subjectsecond quarter of 2003, we issued options to transfer restrictions;purchase 288 shares of our common stock with a fair market value price of $2.60 to a number of our employees.
In the third quarter of 2003, we issued options to purchase 1,062,081 shares of our common stock with a fair market value price of $2.60 to a number of our employees.
In the fourth quarter of 2003, we issued options to purchase 48,077 shares of our common stock with a fair market value price of $2.60 to a number of our employees. We also issued 1,924 options to purchase our common stock with a fair market value price of $2.60 to a number of non-employees.
In the first quarter of 2004, we issued options to purchase 17,192 shares of our common stock with a fair market value price of $9.75 to a number of our employees. We also issued 1,912 options to purchase our common stock with a fair market value price of $7.64 to a number of employees.
II-2
In the third quarter of 2004, we issued options to purchase 3,769 shares of our common stock with a fair market value price of $9.75 to a number of our employees.
In the fourth quarter of 2004, we issued options to purchase 44,615 shares of our common stock with a fair market value price of $9.75 to a number of our employees.
In the first quarter of 2005, we issued options to purchase 34,615 shares of our common stock with a exercise price of $8.14 to a number of our employees.
In the third quarter of 2005, we issued options to purchase 548,484 shares of our common stock with a exercise price of $8.14 to a number of our employees. We also issued 32,699 options to purchase our common stock with a exercise price of $8.14 to a non-employee director.
In the fourth quarter of 2005, we issued options to purchase 3,461 shares of our common stock with an exercise price of $8.14 to a number of our employees.
Restricted Shares
On March 9, 2004, and the certificates for theAugust 6, 2004, we issued 1,134,393 and 5,077 restricted shares, contained an appropriate legend stating such securities have not been registered under the Securities Act and may not be offered or sold absent registration or pursuantrespectively, to an exemption therefrom. No underwriters were involveda number of our employees.
On August 3, 2005, we issued 7,692 restricted shares to two of our non-employee directors.
Warrants
On January 28, 2005, in connection with entering into our senior secured term loan with GE Capital, we issued to GE Capital a warrant to purchase up to $300,000 worth of shares of our preferred stock (or, if we have consummated our initial public offering, shares of our common stock) in an amount and at a price to be determined pursuant to the salesterms thereof.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Exhibit Index
A list of securities referred toexhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated in this Item 15.16(a) by reference.
Item 16. Exhibits and financial statement schedule
(a) Exhibits.
II-2
II-3
(b) Financial statement schedules.Statement Schedules
All schedules are omitted because they are not required, are not applicable or the information is included in our financial statements or notes thereto.None
ItemITEM 17. UndertakingsUNDERTAKINGS
The Registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant hasregistrants have been advised that in the opinion of the Securities and Exchange CommissionSEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrantregistrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
(1) The undersigned Registrantregistrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(2) The undersigned registrant hereby undertakes that:
(1)(a) For purposes of determining any liability under the Securities Act, of 1933, the information omitted from the form of prospectus filed as part of this Registration Statementregistration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrantregistrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statementregistration statement as of the time it was declared effective.
(2)(b) For the purposepurposes of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offeredoffering therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Amendment to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on September 26, 2003.February 3, 2006
| By: | /s/ RON COHEN Ron Cohen, President and Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Ron Cohen and Mark Pinney, and each of them (with full power of each to act alone), severally, as his or her true and lawful attorneys-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto and other documents in connection therewith) to this Registration Statement and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agent, or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment to Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
Signature | Title | Date | ||||
|---|---|---|---|---|---|---|
/s/ RON COHEN Ron Cohen, M.D. | President, Chief Executive Officer and Director | |||||
/s/ | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | February 3, 2006 | ||||
* Standish M. Fleming, M.B.A. | Director | |||||
Director | February 3, 2006 | |||||
* Sandra Panem, Ph.D. | Director | |||||
Director | February 3, 2006 | |||||
* Mark R.E. Pinney, M.B.A., C.F.A., M.Sc. | Director | February 3, 2006 | ||||
* Lorin J. Randall | Director | February 3, 2006 | ||||
II-5
* Steven M. Rauscher, M.B.A. | Director | February 3, 2006 | ||||
* Michael Steinmetz, Ph.D. | Director | |||||
Wise Young, Ph.D., M.D. | Director | |||||
*By: | /s/ Attorney-in-fact |
II-5II-6
| Exhibit No. | Description | |
|---|---|---|
1.1 | Form of Underwriting Agreement | |
3.1 | * | Amended and Restated Certificate of Incorporation |
3.2 | * | Amended Bylaws |
3.3 | * | Form of Post-IPO Amended and Restated Certificate of Incorporation |
3.4 | * | Form of Post-IPO Amended Bylaws |
3.5 | * | Amendment No. 1 to Amended and Restated Certificate of Incorporation |
3.6 | * | Amendment No. 2 to Amended and Restated Certificate of Incorporation |
4.1 | * | Specimen Stock Certificate |
4.2 | * | Warrant to purchase 100,000 shares of Series B Preferred Stock, $2.00 par value per share, dated February 4, 2002, issued by the Registrant to Elan International Services, Ltd. |
4.3 | * | Warrant to purchase 40,000 shares of common stock, $0.10 par value per share, dated May 1, 1996, issued by the Registrant to Mark Noble and |
4.4 | * | Warrant to purchase $300,000 worth of Warrant Shares, dated January 28, 2005, issued by the Registrant to General Electric Capital Corporation |
5.1 | ** | Opinion of Covington & Burling |
10.1 | * | Acorda Therapeutics 1999 Employee Stock Option Plan |
10.2 | * | Amendment to 1999 Employee Stock Option Plan |
10.3 | * | Amendment No. 2 to 1999 Employee Stock Option Plan |
10.4 | * | Acorda Therapeutics 2006 Employee Incentive Plan |
10.5 | * | Acorda Therapeutics 2006 Employee Incentive Plan, as amended as of January 13, 2005 |
10.6 | * | Sixth Amended and Restated Registration Rights Agreement, dated |
10.7 | * | Employment Agreement, dated August 11, 2002, by and between the Registrant and Ron Cohen |
10.8 | * | Amendment to August 11, 2002 Employment Agreement, dated September 26, 2005, by and between the Registrant and Ron Cohen |
10.9 | * | Letter Agreement, dated November 30, 2004, by and between the Registrant and Mark Pinney |
10.10 | * | Employment Agreement, dated as of December 19, 2005, by and between the Registrant and Andrew R. Blight |
10.11 | * | Employment Agreement, dated as of December 19, 2005, by and between the Registrant and Mary Fisher |
10.12 | * | Employment Agreement, dated as of December 19, 2005, by and between the Registrant and David Lawrence |
10.13 | * | Employment Agreement, dated as of December 19, 2005, by and between the Registrant and Jane Wasman |
10.14 | †* | Amended and Restated License Agreement, dated September 26, 2003, by and between the Registrant and Elan Corporation, | |
10.15 | †* | Supply Agreement, dated September 26, 2003, by and between the Registrant and Elan Corporation, | |
10.16 | †* | License Agreement, dated September 26, 2003, by and between the Registrant and Rush-Presbyterian-St. Luke's Medical Center | |
10.17 | * | Side Agreement, dated September 26, 2003, by and among the Registrant, Rush-Presbyterian-St. Luke's Medical Center, and Elan Corporation, | |
10.18 | †* | Payment Agreement, dated September 26, 2003, by and among the Registrant, Rush-Presbyterian-St. Luke's Medical Center, and Elan Corporation, | |
10.19 | †* | Amendment No. 1 to the Payment Agreement, dated as of October 27, 2003, by and between the Registrant and Elan Corporation, plc. | |
10.20 | †* | Amended and Restated License Agreement, dated August 1, 2003, by and between the Registrant and Canadian Spinal Research Organization | |
10.21 | †* | License Agreement, dated February 3, 2003, by and between the Registrant and Cornell Research Foundation, Inc. | |
10.22 | †* | License Agreement, dated November 12, 2002, by and between the Registrant and CeNeS Pharmaceuticals, plc | |
10.23 | †* | License Agreement, dated November 12, 2002, by and between the Registrant and CeNeS Pharmaceuticals, plc | |
10.24 | †* | ||
License Agreement, dated September 8, 2000, by and between the Registrant and | |||
10.25 | †* | Side Letter Agreement, dated | |
10.26 | †* | Asset Purchase Agreement, dated | |
10.27 | †* | Zanaflex Supply Agreement, dated | |
10.28 | †* | Assignment and Assumption Agreement, dated as of July 21, 2004, by and among the Registrant, Elan Pharmaceuticals, Inc., and Novartis Pharma AG | |
10.29 | †* | License Agreement, dated April 17, 1991, by and between Sandoz Pharma, now Novartis Pharma AG and Athena Neurosciences, Inc., now Elan Pharmaceuticals, Inc. | |
10.30 | * | Patent Assignment Agreement, dated as of July 21, 2004, by and between the Registrant and Elan Pharmaceuticals, Inc. | |
10.31 | * | Trademark License Agreement, dated as of July 21, 2004, by and between the Registrant and Elan Pharmaceuticals, Inc. | |
10.32 | * | Agreement Relating to Additional Trademark, dated as of July 2005, by and between the Registrant and Elan Pharmaceuticals, Inc. | |
10.33 | * | Domain Name Assignment Agreement, dated as of July 21, 2004, by and between the Registrant and Elan Pharmaceuticals, Inc. | |
10.34 | * | Bill of Sale and Assignment and Assumption Agreement, dated as of July 21, 2004, by and between the Registrant and Elan Pharmaceuticals, Inc. | |
10.35 | * | Limited Recourse Convertible Promissory Note issued to Elan International Services, Ltd. | |
10.36 | * | Full Recourse Convertible Promissory Note issued to Elan International Services, Ltd. | |
10.37 | * | Note Modification and Amendment, dated as of December 23, 2005, by and between the Registrant and Elan Pharma International Limited | |
10.38 | †* | Fampridine Tablet Technical Transfer Program Proposal for Commercial Registration, dated February 26, 2003, by and between the Registrant and Patheon, Inc. | |
10.39 | * | Securities Amendment Agreement, dated September 26, 2003, by and among the Registrant, Elan | |
10.40 | †* | Syndicated Sales Force Agreement, dated | |
10.41 |
†* | License Agreement, dated | |
10.42 | * | Promissory Note issued to General Electric Capital Corporation |
10.43 | * | Revenue Interests Assignment Agreement, dated as of December 23, 2005, between the Registrant and |
21.1 | * | List of Subsidiaries of the |
23.1 | * | Consent of KPMG LLP, Independent |
23.2 | * | Consent of |
23.3 | ** | Consent of Covington & Burling (included in Exhibit 5.1) |
24.1 | * | Power of |
24.2 | * | Power of Attorney of Lorin J. Randall |