
As filed with the Securities and Exchange Commission on MarchMay 19, 20042020
Registration No. 333- 333-237928
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
DIGIRAD CORPORATION
(Exact Namename of Registrantregistrant as Specifiedspecified in its Charter)charter)
| Delaware | 3845 | 33-0145723 | ||
| (State or | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification Number) | ||
1048 Industrial Court
Suwanee, Georgia 30024
(858) 726-1600
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Matthew G. Molchan
President and Chief Executive Officer
Digirad Corporation
1048 Industrial Court
Suwanee, Georgia 30024
(858) 726-1600
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to: | ||||
| Adam W. Finerman, Esq. | ||||
| Olshan Frome Wolosky LLP | ||||
| 1325 Avenue of the Americas | ||||
| New York, New York 10019 | ||||
| Telephone: (212) 451-2300 | ||||
Angela Dowd, Esq. Loeb & |
|
(212) 407-4000 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement becomes effective.registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. o☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o☐
If delivery
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the prospectus is expectedExchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
| Non-accelerated filer ☒ | Smaller reporting company ☒ | |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to be madeuse the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Rule 434, checkSection 7(a)(2)(B) of the following box. oSecurities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee | ||
|---|---|---|---|---|
| Common Stock, $0.001 par value per share | $86,250,000 | $10,928 | ||
| Title of each Class of Security being registered | Proposed Maximum Price(2) | Amount of Fee(3) | ||||||
| Common stock, par value $0.0001 per share(1) (5) | $ | 5,750,000 | $ | 746.35 | ||||
| Pre-funded warrants, each warrant exercisable for one share of common stock(4) (5) | — | — | ||||||
| Shares of common stock issuable upon exercise of the pre-funded warrants to purchase shares of common stock(5) (7) | — | — | ||||||
| Common stock purchase warrants to purchase shares of common stock(8) | — | — | ||||||
| Common stock underlying common stock purchase warrants(7) (8) | 3,593,750 | 466.47 | ||||||
| Underwriter’s warrant to purchase shares of common stock(6) | — | — | ||||||
| Common stock issuable upon exercise of the underwriter’s warrant(7) | 158,125 | 20.53 | ||||||
| Total | $ | 9,501,875 | (7) | $ | 1,233.35 | (9) | ||
| (1) | Includes shares to cover the exercise of the over-allotment option granted to the underwriter. |
| (2) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). Includes the offering price of any additional securities that the underwriter has the option to purchase. In accordance with Rule 457(o) under the Securities Act, the number of securities being registered and the proposed maximum offering price per pre-funded warrant and per common stock purchase warrant are not included in this table. | |
| (3) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. | |
| (4) | No separate fee is required pursuant to Rule 457(i) of the Securities Act. | |
| (5) | The proposed maximum aggregate offering price of the common stock proposed to be sold in the offering will be reduced on a dollar-for-dollar basis based on the aggregate offering price of any pre-funded warrants offered and sold in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants to be sold in the offering will be reduced on a dollar-for-dollar basis based on the aggregate offering price of any shares of common stock sold in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock and the pre-funded warrants (including the common stock issuable upon exercise of the pre-funded warrants and the underwriter’s over-allotment option), if any, is $5,750,000. | |
| (6) | Represents a warrant issuable to the underwriter (the “Underwriter’s Warrant”) to purchase a number of shares of common stock equal to 2.5% of the number of shares of common stock (including the shares of common stock issued pursuant to the underwriter’s exercise of its over-allotment option and upon exercise of the pre-funded warrants) being offered at an exercise price equal to 110% of the public offering price of the common stock. See the “Underwriting” section of the prospectus included in this registration statement. In accordance with Rule 457(g) under the Securities Act, because the common stock of the registrant underlying the Underwriter’s Warrant is registered hereby, no separate registration fee is required with respect to the Underwriter’s Warrant registered hereby. | |
| (7) | Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. | |
| (8) | Each share of common stock and pre-funded warrant is being sold together with an accompanying warrant to purchase 0.5 of a share of our common stock Two common stock purchase warrants will be exercisable for one share of common at a per share exercise price of up to 125% of the public offering price of one share of common stock. The proposed maximum aggregate public offering price of the shares of common stock issuable upon exercise of the common warrants was calculated to be $3,593,750, which is equal to 125% of one-half of the proposed maximum aggregate public offering price of the shares of common stock in this offering. In accordance with Rule 457(g) under the Securities Act, because the common stock of the registrant underlying the common stock purchase warrants is registered hereby, no separate registration fee is required with respect to the common stock purchase warrants registered hereby. | |
| (9) | A filing fee of $766.88 was previously paid. |
The Registrantregistrant hereby amends this Registration Statementregistration statement on such date or dates as may be necessary to delay its effective date until the Registrantregistrant shall file a further amendment which specifically states that this Registration Statementregistration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statementregistration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting offersan offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 19, 2020
SubjectPRELIMINARY PROSPECTUS

Digirad Corporation
Up to CompletionPreliminary Prospectus dated , 2004
PROSPECTUS
[●] Shares

of Common Stock
Up to [●] Pre-funded Warrants (each Pre-funded Warrant to purchase one Share of Common Stock)
Up to [●] Shares of Common Stock underlying the Pre-funded Warrants
Common Warrants to Purchase up to [●] Shares of Common Stock and
Up to [●] Shares of Common Stock underlying the Common Warrants
This is our initial publican offering of [●] shares of our common stock. stock, par value of $0.0001 per share, which we refer to as the “common stock”, together with a number of common stock purchase warrants (the “warrants”) to purchase up to an aggregate of [●] shares of common stock (and the shares of common stock that are issuable from time to time upon exercise of the warrants).
We are also offering shares.to each purchaser whose purchase of shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants, in lieu of shares that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% of our outstanding shares of common stock. Each pre-funded warrant will be exercisable for one share of common stock. The purchase price of each pre-funded warrant will be equal to the price per share being sold to the public in this offering, minus $0.01, and the exercise price of each pre-funded warrant will be $0.01 per share. The pre-funded warrants will be certificated and will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. Any exercise of the pre-funded warrants which would result in a holder beneficially owning more than 4.99% of our outstanding shares of common stock will be subject to our consent. We expectmay, in our sole discretion, waive the initial4.99% ownership limitation in connection with this offering with respect to one or more potential purchasers.
For each pre-funded warrant we sell, the number of shares we are offering will be decreased on a one-for-one basis. Each share of common stock and pre-funded warrant is being sold together with an accompanying warrant to purchase 0.5 of a share of our common stock, at an exercise price of $[●] per whole share (at least 100%, and up to 125%, of the public offering price to be between $of one share of common stock). Because we will issue a warrant for each share of our common stock and $ per share.
Currently, no public market exists for each pre-funded warrant sold in this offering, the shares. After pricingnumber of warrants sold in this offering will not change as a result of a change in the mix of the offering, we expect that the shares of our common stock and pre-funded warrants sold.
The warrants will be quotedexercisable immediately, and will expire five years from the date of issuance. The warrants will only be exercisable for whole shares of common stock. The shares of common stock or pre-funded warrants can be purchased only with the accompanying warrants (other than the over-allotment option), but will be issued separately and will be immediately separable upon issuance.
Our common stock is listed on the Nasdaq NationalGlobal Market under the symbol "DRAD."“DRAD”. On May 15, 2020, the last reported sale price of our common stock on the Nasdaq Global Market was $2.37 per share. The actual public offering price per share of common stock or pre-funded warrant, as the case may be, will be determined between us and the investors in the offering and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price. There is no established public trading market for the warrants or the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply for a listing of the warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the warrants and the pre-funded warrants will be limited.
Investing in our securities involves a high degree of risk. See the common stock involves risks that are described in the "Risk Factors" section entitled “Risk Factors” beginning on page 724 of this prospectus.prospectus and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered in connection with an investment in our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY OTHER REGULATORY BODY HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
| Per | Per Pre-funded Warrant and Accompanying Warrant | Total(2) | |||||||||||||
| Public offering price(1) | $ | $ | |||||||||||||
| $ | |||||||||||||||
| Underwriting discounts and commissions (3) | $ | $ | $ | ||||||||||||
| Proceeds, before expenses, to us | $ | $ | $ | ||||||||||||
| (1) | The public offering price is $[●] per share of common stock and $0.01 per accompanying warrant and $[●] per pre-funded warrant and $0.01 per accompanying warrant. | |
| (2) | Assumes no exercise of the underwriters’ over-allotment option. | |
| (3) | See “Underwriting” for a description of the compensation payable to the underwriters; including reimbursable expenses. |
The underwriters may alsounderwriter has the option to purchase up to an additional [●] shares of common stock, or pre-funded warrants and/or warrants to purchase up to an additional [●] shares of common stock from us at the public offering price, less the underwriting discounts and commissions, within 3045 days fromafter the date of this prospectus to cover over-allotments.over-allotments, if any.
NeitherJeffrey E. Eberwein, the SecuritiesChairman of our Board of Directors, and Exchange Commission norMitchell Quain, one of our directors, have indicated to us their intention to purchase up to $100,000 of common stock and/or pre-funded warrants each (representing up to 42,194 shares based on an assumed offering price of $2.37 per share), along with accompanying warrants for the purchase of up to an additional 21,097 shares, in this offering. In addition, Matthew Molchan, our Chief Executive Officer, and David Noble, our Chief Financial Officer and Chief Operating Officer, have indicated to us their intention to purchase up to $5,000 and $50,000, respectively, of common stock and/or pre-funded warrants each (representing up to 2,109 and 21,097 shares, respectively, based on an assumed offering price of $2.37 per share), along with accompanying warrants for the purchase of up to an additional 1,054 and 10,548 shares, respectively). Certain other non-executive employees of our company may also purchase shares, warrants and/or pre-funded warrants in this offering. However, because indications of interest are not binding agreements or commitments to purchase, there can be no assurance that the underwriter will determine to sell shares of common stock, warrants and/or pre-funded warrants in this offering to any state securities commission has approved or disapproved of these persons or entities, or that any of these persons or entities will determine to purchase securities in this offering. Any securities sold to our employees, officers and directors will be at the same price and on the same terms as the securities sold to other investors in this offering. The underwriter will receive the same underwriting discount on any securities purchased by these persons or determined if this prospectus is truthful or complete. Any representationentities as they will on any other securities sold to the contrary is a criminal offense.public in this offering.
The underwriter expects to deliver the shares, will be ready for deliverywarrants and pre-funded warrants against payment in New York, New York on or about[●], 2004.2020.
JointSole Book-Running ManagersManager
Maxim Group LLC
|
The date of this prospectus is [●], 2004.
| Page | ||
| PROSPECTUS SUMMARY | 1 | |
| 22 | ||
| 24 | ||
| USE OF PROCEEDS | 46 | |
| 46 | ||
| 46 | ||
| 48 | ||
| 50 | ||
| 63 | ||
| 70 | ||
| 74 | ||
| 74 | ||
| 74 | ||
| 74 | ||
| DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES | 75 |
You should rely only on the information contained or incorporated into this prospectus. Neither we nor the underwriter has authorized anyone to provide any information or to make any representations other than those contained in this prospectus.prospectus or in any free writing prospectuses we have prepared. We have not,take no responsibility for, and can provide no assurance as to the underwriters have not, authorizedreliability of, any other person to provide you with different information. If anyone provides you with different or inconsistent information you should not rely on it. We are not, and the underwriters are not, makingthat others may give you. This prospectus is an offer to sell theseonly the securities offered hereby, but only under circumstances and in any jurisdictionjurisdictions where the offer or saleit is not permitted. You should assume that thelawful to do so. The information appearingcontained in this prospectus is accuratecurrent only as of its date regardless of the date on the front covertime of delivery of this prospectus.prospectus or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
i
PROSPECTUS SUMMARY
You should also read this prospectus together with the additional information described under “Where You Can Find More Information” and “Incorporation of Information by Reference”.
No action is being taken in any jurisdiction outside the U.S. to permit a public offering of our securities or possession or distribution of this prospectus in any such jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the U.S. are required to inform themselves about and to observe any restrictions about this offering and the distribution of this prospectus applicable to those jurisdictions.
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections entitled “Prospectus Summary,” “Risk Factors” and “Use of Proceeds,” as well as the information we incorporate herein by reference contain forward-looking statements within the meaning of the federal securities laws. Forward-looking statements include, but are not limited to, statements regarding expectations, intentions and strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “target,” “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would,” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this prospectus are based on current expectations and beliefs concerning future developments and their potential effects on our company and its subsidiaries. There can be no assurance that future developments will be those that have been anticipated. Factors that might cause such differences include, but are not limited to, those discussed in the section of this prospectus entitled “Risk Factors”. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all the risks and uncertainties that could have an impact on the forward-looking statements, including without limitation, risks and uncertainties relating to:
| ● | our financial performance, including our ability to generate revenue; |
| ● | our recent conversion into a diversified holding company; |
| ● | business interruptions resulting from health epidemics or pandemics or other contagious outbreaks, such as the recent coronavirus outbreak or geopolitical actions, including war and terrorism, natural disasters, including earthquakes, typhoons, floods and fires; |
| ● | ability of our products and services to achieve and/or maintain market success; |
| ● | success in retaining or recruiting, or changes required in, our officers, key employees or directors; |
| ● | potential ability to obtain additional financing when and if needed; |
| ● | our ability to protect our intellectual property; |
| ● | our ability to complete strategic acquisitions; |
| ● | our ability to complete strategic divestitures; |
| ● | our ability to manage growth and integrate acquired operations; |
| ● | our inability to pay dividends at the present time; |
| ● | our ability to maintain compliance with The Nasdaq Stock Market LLC’s listing maintenance standards; |
| ● | potential liquidity and trading of our securities; |
| ● | regulatory or operational risks; |
| ● | the effects of outbreaks of pandemic or contagious diseases, including the length and severity of the recent worldwide outbreak of Coronavirus, now named as COVID-19, including its impact on our business; |
| ● | downward revisions to, or withdrawals of, our credit ratings, if any, by third-party rating agencies; and |
ii
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. |
We cannot guarantee future results, levels of activity or performance. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this prospectus. These cautionary statements should be considered with any written or oral forward-looking statements that we may issue in the future. Except as required by applicable law, including the securities laws of the U.S., we do not intend to update any of the forward-looking statements to conform these statements to reflect actual results, later events or circumstances or to reflect the occurrence of unanticipated events. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or other investments or strategic transactions we may engage in.
iii
ThisThe following summary highlights selected information contained in other parts of this prospectus or incorporated by reference into this prospectus from our filings with the Securities and Exchange Commission, or SEC, listed in the section of the prospectus entitled “Incorporation of Certain Information by Reference”. Because it is only a summary, it does not contain all of the information that you should consider before buying shares ofpurchasing our common stock.securities in this offering and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere or incorporated by reference into this prospectus. You should read the entire prospectus, carefully, especially the "Risk Factors" sectionregistration statement of which this prospectus is a part, and the information incorporated by reference herein in their entirety, including our consolidated financial statements and the related notes appearing at the end ofincorporated by reference into this prospectus, before deciding to invest in sharesmaking an investment decision. Some of our common stock. Referencesthe statements in this prospectus to our certificate of incorporation and bylaws refer to the certificate of incorporationdocuments incorporated by reference herein constitute forward-looking statements that involve risks and bylaws that will be in effect upon completion of this offering.uncertainties. See information set forth under the section “Special Note Regarding Forward-Looking Statements.”
Unless the context otherwise requires or indicates, all references in this prospectus supplement and the accompanying prospectus to “we,” “our,” “us,” “Digirad” and the “Company” each mean Digirad Corporation,
a Delaware corporation, and its consolidated subsidiaries.
We are
Overview
Upon Digirad’s acquisition of ATRM Holdings, Inc. (“ATRM”) on September 10, 2019 (the “ATRM Merger” or the “ATRM Acquisition”), Digirad converted into a leader in the development, manufacturediversified holding company (the “HoldCo Conversion”). As a diversified holding company, Digirad has three divisions:
| ● | Healthcare (Digirad Health): designs, manufactures, and distributes diagnostic medical imaging products. Digirad Health operates in three businesses: Diagnostic Services, Mobile Healthcare, and Diagnostic Imaging. The Diagnostic Services business offers imaging and monitoring services to healthcare providers as an alternative to purchasing the equipment or outsourcing the job to another physician or imaging center. The Mobile Healthcare business provides contract diagnostic imaging, including computerized tomography (“CT”), magnetic resonance imaging (“MRI”), positron emission tomography (“PET”), PET/CT, and nuclear medicine and healthcare expertise through a convenient mobile service. The Diagnostic Imaging business develops, sells, and maintains solid-state gamma cameras. |
| ● | Building and Construction (ATRM): services residential and commercial construction projects by manufacturing modular housing units, structural wall panels, permanent wood foundation systems, and other engineered wood products, and supplies general contractors with building materials. |
| ● | Real Estate and Investments: manages real estate assets (currently three manufacturing plants in Maine) and investments. |
Healthcare (Digirad Health) delivers convenient, effective, and distributionefficient healthcare solutions on an as needed, when needed, and where needed basis. Digirad’s diverse portfolio of solid-state medicalmobile healthcare solutions and diagnostic imaging productsequipment and services for the detection of cardiovascular disease and other medical conditions. We designed and commercialized the first solid-state gamma camera. Our initial focus is on nuclear cardiology imaging procedures performed with gamma cameras, which we believe generate revenue of approximately $10.0 billion annually in the United States. Our target markets are primarilyprovides hospitals, physician practices, and outpatient clinics,imaging centers throughout the United States access to technology and services necessary to provide patient care in the rapidly changing healthcare environment. Digirad’s direct and indirect subsidiaries that are included in this division are referred to collectively herein as the “Healthcare Subsidiaries”.
Building and Construction (ATRM) manufactures modular housing units for commercial and residential applications. ATRM operates in two businesses: (i) modular building manufacturing and (ii) structural wall panel and wood foundation manufacturing, including building supply retail operations. The modular building manufacturing business is operated by KBS Builders, Inc. (“KBS”), and the structural wall panel and wood foundation manufacturing segment is operated by EdgeBuilder, Inc. (“EdgeBuilder”), and the retail building supplies are sold through Glenbrook Building Supply, Inc. (“Glenbrook” and together with EdgeBuilder, “EBGL”). KBS, EdgeBuilder and Glenbrook are wholly-owned subsidiaries of ATRM and are referred to collectively herein, and together with ATRM, as the “Construction Subsidiaries”.
1
Real Estate & Investments generates revenue from the lease of commercial properties and equipment through Star Real Estate Holdings USA, Inc. (“SRE”), a wholly-owned subsidiary of Digirad, and provides services that include investment advisory services and the servicing of pooled investment vehicles through Lone Star Value Management, LLC (“LSVM”), a Connecticut based exempt reporting advisor. LSVM, which we believe constitute approximately 25%was a wholly owned subsidiary of ATRM on the ATRM Acquisition Date (as defined below), was acquired by the Company in the ATRM Acquisition. In April 2019, as an initial transaction to create Digirad’s real estate division under SRE and launch that aspect of the total market,HoldCo Conversion, Digirad funded the initial purchase of three modular building manufacturing facilities in Maine and then leased those three properties to KBS. The funding of the assets acquisition was primarily through the revolver loan under our credit facility with Sterling National Bank (“Sterling” or $2.5 billion.“SNB”), a national banking association. LSVM, SRE and the subsidiaries of SRE that are included in this division are referred to collectively herein as the “Investments Subsidiaries”.
On September 10, 2019, Digirad completed its acquisition of ATRM pursuant to an Agreement and Plan of Merger, dated as of July 3, 2019 (the “ATRM Merger Agreement”), among Digirad, Digirad Acquisition Corporation, a Minnesota corporation and wholly-owned subsidiary of Digirad (“Merger Sub”), and ATRM. Under the terms of the ATRM Merger Agreement, Merger Sub merged with and into ATRM, with ATRM surviving as a wholly owned subsidiary of Digirad.
At the effective time of the ATRM Merger, (i) each share of ATRM common stock was converted into the right to receive three one-hundredths (0.03) of a share of 10.0% Series A Cumulative Perpetual Preferred Stock, par value $0.0001 per share, of the Company (“Series A Preferred Stock”) and (ii) each share of ATRM 10.00% Series B Cumulative Preferred Stock, par value $0.001 per share (“ATRM Preferred Stock”), converted into the right to receive two and one-half (2.5) shares of Series A Preferred Stock, for an approximate aggregate total of 1.6 million shares of Series A Preferred Stock. No fractional shares of Series A Preferred Stock were issued to any ATRM shareholder in the ATRM Merger. Each ATRM shareholder who would otherwise have been entitled to receive a fraction of a share of Company common stock in the ATRM Merger received one whole share of Series A Preferred Stock.
As a result of the ATRM Merger, ATRM’s operations have been included in our consolidated financial statements since the ATRM Acquisition Date. Digirad’s aim with this acquisition is to continue to grow its business into an integrated healthcare services company while simultaneously converting into a diversified holding company through the acquisition of businesses that meet Digirad’s internally developed financial screen for acquisitions. The Company expects to achieve significant synergies and cost reductions by eliminating redundant processes and facilities.
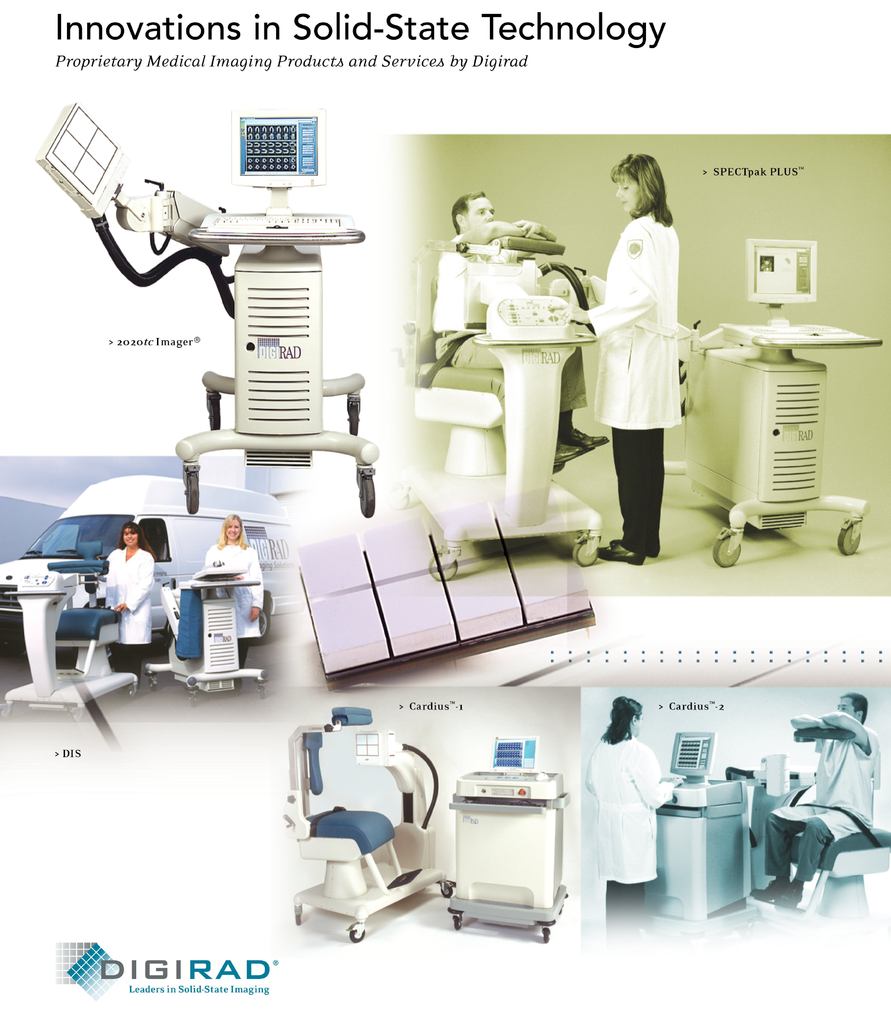
Our gamma cameras use small semiconductors to replace the bulky vacuum tubes used historically in gamma cameras. By utilizing solid-state technology,Competitive Strengths
Healthcare Services and Products
For Digirad Health, we believe that our competitive strengths are our streamlined and cost-efficient approach to providing healthcare solutions to our customers at the point of need, while providing an array of industry-leading, technologically-relevant healthcare imaging and monitoring services:
| ● | Broad Portfolio of Imaging Services.Approximately 77.9% of our revenues are derived from diagnostic imaging services to our customers. We have developed and continue to refine an industry-leading, customer-service focused approach to our customers. We have found our focus in this area is a key factor in acquiring and keeping our service-based customers. |
| ● | Unique Dual Sales and Service Offering. For the majority of our businesses, we offer a service-based model to our customers, allowing them to avoid making costly capital and logistical investments required to offer these services internally. Further, for a portion of our business, we have the ability to sell the underlying capital equipment directly to our customers should their needs change and they desire to provide services on their own with the underlying capital equipment. This ability to serve our customers in a variety of capacities from selling equipment directly, or providing more flexibility through a service-based model, allows us to serve our customers according to their exact needs, as well as the ability to capture both ends of the revenue spectrum. |
2
| ● | Utilization of Highly Trained Staff.We recruit and maintain highly trained staff for our clinical and repair services, which in turn allows us to provide superior and more efficient services. |
| ● | Leading Solid-State Technology. Our solid-state gamma cameras utilize proprietary photo detector modules that enable us to build smaller and lighter cameras that are portable with a degree of ruggedness that can withstand the vibration associated with transportation. Our dedicated cardiac imagers require a floor space of as little as seven feet by eight feet, can generally can be installed without facility renovations, and use standard power. Our portable cameras are ideal for mobile operators or practices desiring to service multiple office locations or imaging facilities. |
Construction Services and Products
Our competitive strengths at KBS include our ability to provide high quality products for both commercial and residential buildings with a focus on customization to suit the project requirements, provide value with our engineering and design expertise, and to meet the time frame needed by the customer:
| ● | Customization of high quality products for both commercial and residential buildings.KBS is able to adapt any floor plan and engineer it to modular design. KBS and its highly trained engineering and sales staff work with builders and designers to create a fully customized building plan, using the latest and most advanced materials and products available. KBS’ long-term experience across a broad range of market sectors, building types and geographies allows us to provide a compelling end-to-end modular solution for ground-up construction, including integrated design, engineering, materials selection and manufacturing. Our highly skilled staff utilizes technology and proven construction methods to achieve high quality results. |
| ● | Environmentally friendly building processes. We maintain precise dimensional tolerances throughout our building process, improving quality and speed of construction, reducing waste, and causing less disruption to the environment than traditional on-site construction methods. KBS is committed to offering homes and buildings that use recognized sustainable building techniques to meet the highest standards of energy efficiency, while creating high performance, healthy and architecturally distinctive designs. |
| ● | Utilization of experienced and highly trained engineering and design experts. KBS’ engineering department has over 30 years of combined work experience in modular design, giving us the ability to execute technically complex and challenging designs. Our in-house knowledge base spans across materials, construction practices and state building codes. Utilizing CADWORKS, an advanced three-dimensional drawing technology platform, our engineering and design team consistently produces timely, cost-effective residential and commercial designs. Our staff is able to export digital drawings and information directly into our state-of-the-art machinery. This allows for the precise milling of structural components for each module in any given project. New automation features have been developed and implemented in our CADWORKS software, which also allow us to create detailed bills of materials, which improve costing and inventory control. Our engineering and design team members are encouraged to work on all aspects of our projects, cultivating a team-centric culture, which supports mentorship and professional growth. The hallmark of KBS’ engineering department is communication and teamwork, with a strong focus on collaboration with the client. |
Our competitive strengths at Glenbrook include high quality building materials and unmatched service and attention to detail to building professionals and homeowners. In addition, we provide highly personalized service, knowledgeable salespeople and attention to detail that the larger, big-box chain home stores do not provide. In EdgeBuilder, we offer a superior product unique to the project’s requirements, provide value with our engineering and design expertise, and deliver product when required by the customer, while staying cost-competitive. Our production strategy is to utilize automation and the most efficient methods of manufacturing and high-quality materials in all EBGL projects.
3
| ● | High quality products are used in our building contracts.We provide brand name, high quality windows, such as Andersen, Marvin and Thermo-Tech. We also supply well-known composite decking, including Trex, TimberTech, Azek and Fiberon. All brands available through Glenbrook add profit margin to our larger commodities business, including premium brands like Selkirk, Cedar and Bessemer Plywood. |
| ● | Utilization of knowledgeable sales force.Our experienced sales force provides knowledgeable personal service to our customers to help bring our products to “life” in the form of a successful project through partnership with our customers. |
| ● | Efficiencies and reliability from our indoor manufacturing facility.We utilize efficiencies and reliability provided by our indoor manufacturing facility, which allows us to maximize labor productivity, reduce waste and foresee project specific design conflicts among trades before they arise on site. |
Real Estate and Investments
Our competitive strengths in real estate include a focus on acquisition opportunities that have underappreciated real estate value, which assets the Company anticipates placing into SRE. SRE expects to be largely self-funded over time by raising its own capital through commercial mortgages on its properties and other forms of external capital.
Our competitive strengths in investments include shareholder activism through the Lone Star Value brand name, which will be less confusing to investment targets and the investing public than pursuing investments through one of our operating companies. We also expect to make strategic acquisitions in the future. Investments and acquisitions will be made using our internally developed financially disciplined approach for acquisitions.
Strategy
We seek to grow our business by, among other things:
| ● | Organic growth from our core businesses.We believe that we operate in markets and geographies that will allow us to continue to grow our core businesses, allowing us to benefit from our scale and strengths. We plan to focus our efforts on markets in which we already have a presence in order to take advantage of personnel, infrastructure, and brand recognition we have in these areas. |
| ● | Introduction of new services.We plan to continue to focus on healthcare solutions related businesses that deliver necessary assets, services and logistics directly to the customer site. We believe that over time we can either purchase or develop new and complementary businesses and take advantage of our customer loyalty and distribution channels. In addition, as we transform into a multi-industry holding company, our largest near-term growth opportunity is to execute a successful turnaround of the KBS modular business that we acquired pursuant to the ATRM Merger. While we intend to continue to pursue and grow our residential modular building business, which provides us with positive margins and cash flow, we are also targeting large multi-family projects in the 25 to 100-building modules range. |
| ● | Acquisition of complementary businesses.The current economic environment offers significant opportunities for strategic acquisitions which can be either be bolt-on acquisitions for existing platform businesses, or new core businesses complementary to our new holding company structure. We will have a disciplined approach for making any potential acquisitions and will focus on faster growing and higher margin businesses. We believe there are many potential targets in the range of $3 million to $10 million in annual revenues that can be acquired over time and integrated into our businesses. We will also look at larger, more transformational acquisitions if we believe the appropriate mix of value, risk and return is present for our shareholders. The timing of these potential acquisitions will always depend on market conditions, available capital, and the value for each transaction. In general, we want to be “value” buyers, and will not pursue any transaction unless we believe the post-transaction potential value is high for shareholders. |
4
| ● | Divestiture of business units. From time to time we consider divestitures of business units and/or assets which are not consistent with the future strategy of our business, or if we believe the sale of such units and/or assets could be in the best interests of our business and its stockholders, subject to our ability to agree on acceptable terms with a prospective buyer. We currently are considering several possible transactions, including discussions regarding the sale of a business unit. Upon any such sale, a significant portion of the proceeds would be used to repay indebtedness, with the remainder being used for working capital purposes, and potentially the acquisition of complementary businesses as discussed above. There is no assurance that any such divestiture will occur, or if it does, if it would occur for a sales price currently contemplated. If it occurs, there is no assurance that we will be able to use the proceeds in the acquisition of a complementary business, or if we do if such acquisition will be successful. Because we have concluded, as of the date of this prospectus, that such divestiture is not “probable” under the applicable rules of the Securities Act of 1933, as amended, we are not including additional information about it in this prospectus and you will, therefore, not have the opportunity to evaluate such information as part of this offering. |
We continue to explore strategic alternatives to improve the market position and profitability of our product offerings in the marketplace, generate additional liquidity, and enhance our valuation. We may pursue our goals through organic growth and through strategic alternatives. Some of these alternatives have included, and could continue to include, selective acquisitions of business segments or entire businesses, divestitures of assets or divisions, or a restructuring of our company.
History of our Business
In January 2016 we acquired Project Rendezvous Holding Corporation (“PRHC”), the ultimate parent company of DMS Health Technologies, Inc. (collectively referred to hereinafter as “DMS Health Technologies” or “DMS Health”) for $32.3 million. DMS Health is a provider of mobile diagnostic imaging services and provides medical product sales and service. The acquisition resulted in two new reportable segments: Mobile Healthcare and Medical Device Sales and Services.
In February of 2018, we completed the sale of our customer contracts relating to our Medical Device Sales and Service (“MDSS”) post-warranty service business to Philips for $8.0 million. On October 31, 2018, we sold our Telerhythmics, LLC (“Telerhythmics”) business to G Medical Innovations USA, Inc., for $1.95 million cash.
On December 14, 2018, Digirad and ATRM entered into a joint venture and formed Star Procurement, LLC, with Digirad and ATRM each holding a 50% interest. The purpose of the joint venture is to provide the service of purchasing and selling building materials and related goods to KBS with which Star Procurement entered into a Services Agreement on January 2, 2019. In accordance with the terms of the Star Procurement Limited Liability Company Agreement, Digirad made a $1.0 million capital contribution to the joint venture, which was made in January 2019. This entity was subsequently consolidated within the consolidated financial statements upon completion of the ATRM Merger.
Digirad formed SRE in March 2019 in connection with establishing its Real Estate and Investments Division. In April 2019, as an initial transaction for the Real Estate and Investments Division under SRE, Digirad funded the initial purchase of three manufacturing facilities in Maine and leased those three properties.
On September 10, 2019 (the “ATRM Acquisition Date”), Digirad completed the ATRM Acquisition and thereby converted into a diversified holding company. As a result of the ATRM Acquisition, ATRM became a wholly owned subsidiary of Digirad and KBS, EdgeBuilder, Glenbrook and LSVM became wholly owned indirect subsidiaries of Digirad. As a result of internal restructuring, LSVM is now a direct wholly owned subsidiary of Digirad.
5
Business Segments
Prior to September 10, 2019, we were organized as four reportable segments: Diagnostic Services, Diagnostic Imaging, Mobile Healthcare, and Medical Device Sales and Service. On February 1, 2018, we sold our Medical Device Sales and Service (“MDSS”) business. As of March 31, 2020, our business is organized into five reportable segments: Diagnostic Services, Mobile Healthcare, Diagnostic Imaging, Building and Construction, and Real Estate and Investments. See Note 11.Segments, within the notes to our unaudited condensed consolidated financial statements included in our Quarterly Report on Form 10-Q for the three months ended March 31, 2020 and incorporated herein by reference for financial data relating to our segments. For discussion purposes, we categorized our Diagnostic Imaging, Diagnostic Services and Mobile Healthcare reportable segments as “Healthcare”. For the last periods indicated below, Healthcare had the following relative contribution to consolidated revenues:
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2020 | 2019 | 2019 | 2018 | |||||||||||||
| Healthcare Revenues: | ||||||||||||||||
| Diagnostic Services | 37.5 | % | 49.0 | % | 41.8 | % | 47.3 | % | ||||||||
| Mobile Healthcare | 33.5 | % | 40.4 | % | 36.1 | % | 41.2 | % | ||||||||
| Diagnostic Imaging | 9.9 | % | 10.6 | % | 12.1 | % | 11.5 | % | ||||||||
| Total Healthcare revenues | 80.9 | % | 100.0 | % | 90.0 | % | 100.0 | % | ||||||||
Building and Construction
Building and construction revenue is summarized as follows:
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2020 | 2019 | 2019 | 2018 | |||||||||||||
| Building and Construction | 19.0 | % | — | % | 9.9 | % | — | % | ||||||||
| Total Building and Construction Revenue | 19.0 | % | — | % | 9.9 | % | — | % | ||||||||
Real Estate and Investments
Real estate and investments revenue is summarized as follows:
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2020 | 2019 | 2019 | 2018 | |||||||||||||
| Real Estate and Investments | 0.1 | % | — | % | 0.1 | % | — | % | ||||||||
| Total Real Estate and Investments | 0.1 | % | — | % | 0.1 | % | — | % | ||||||||
Diagnostic Services
Through Diagnostic Services, we offer a convenient and economically efficient imaging and monitoring services program as an alternative to purchasing equipment or outsourcing the procedures to another physician or imaging center. For physicians who wish to perform nuclear imaging, echocardiography, vascular or general ultrasound tests, we provide imaging systems, qualified personnel, radiopharmaceuticals, licensing services, and the logistics required to perform imaging in their own offices, and thereby the ability to bill Medicare, Medicaid, or one of the third-party healthcare insurers directly for those services, which are primarily cardiac in nature. We provide imaging services primarily to cardiologists, internal medicine physicians, and family practice doctors who typically enter into annual contracts for a set number of days ranging from once per month to five times per week. Many of our physician customers are reliant on reimbursements from Medicare, Medicaid, and third-party insurers. Although reimbursement for procedures provided by our services have been stable during the last several years, any future changes to underlying reimbursements may require modifications to our current business model in order for us to maintain imagea viable economic model.
6
Our portable nuclear and ultrasound imaging operations utilize a “hub and spoke” model in which centrally located regional hubs anchor multiple van routes in the surrounding metropolitan areas. At these hubs, clinical personnel load the equipment, radiopharmaceuticals, and other supplies onto specially equipped vans for transport to customer locations, where they set up the equipment for the day. After quality while offering significant advantages over vacuum tube-basedassurance testing, a technologist under the physician’s supervision will gather patient information, inject the patient with a radiopharmaceutical, and then acquire images for interpretation by the physician. At the conclusion of the day of service, all equipment and supplies are removed from the customer location and transported back to the central hub location. Our model relies on density and customer concentration to allow for efficiencies and maximum profitability, and therefore we are only located in geographies where there is a high concentration of people, cardiac disease and associated likely customer locations.
For our nuclear imaging services, we have obtained Intersocietal Accreditation Commission (“IAC”) and Intersocietal Commission for Echocardiography Laboratories (“ICAEL”) accreditation for our services. Our licensing infrastructure provides radioactive materials licensing, radiation safety officer services, radiation safety training, monitoring and compliance policies and procedures, and quality assurance functions, to ensure adherence to applicable state and federal nuclear regulations.
Mobile Healthcare
Through Mobile Healthcare, we provide contract diagnostic imaging, including computerized tomography (“CT”), magnetic resonance imaging (“MRI”), positron emission tomography (“PET”), PET/CT, and nuclear medicine and healthcare expertise to hospitals, integrated delivery networks (“IDNs”), and federal institutions on a long-term contract basis, as well as provisional (short-term) services to institutions that are in transition. Rather than our customers owning the equipment directly and operating the related services, we provide this service when there is a cost, ease, and efficiency benefit.
Our Mobile Healthcare operations operate throughout the United States, with a heavier concentration in rural areas, particularly in the Upper Midwest region of the United States. We have a range of customer types, but our most typical customer is a small or regional hospital that does not have enough volume of activity to justify owning a piece of imaging equipment on a full-time basis. Our services typically offer the diagnostic imaging equipment, placed in a large patient friendly coach or tractor-trailer, coupled with either an owned or operator-owned tractor, which is then transported to each customer location. Our mobile routes are designed to provide for maximum utilization and efficiency by allowing our units to travel to the next customer location during non-working hours of a typical imaging clinic, meeting our technical staff at each location. Our customers commit to annual contracts ranging from service once every two weeks to up to two days of service per week, depending on modality type and their local demand for services.
Diagnostic Imaging
Through Diagnostic Imaging, we sell our internally developed solid-state gamma cameras, imaging systems including mobility through reduced size and weight,camera maintenance contracts. Our imaging systems include nuclear cardiac imaging systems, as well as general purpose nuclear imaging systems. We sell our imaging systems to physician offices and hospitals primarily in the United States, although we have sold a small number of imaging systems internationally. Our imaging systems are sold in both portable and fixed configurations, provide enhanced operability and reliability and improved patient comfort, and utilization. Our imaging systems, consisting of a gamma camera and accessories,fit easily fit into floor spaces as small as seven feet by eight feet. Due tofeet, and facilitate the size and other limitationsdelivery of vacuum tube cameras, nuclear imaging has traditionally been confined to dedicated and customized space within a hospital or imaging center. The mobility of our imaging systems enables us to deliver nuclear imagingmedicine procedures in a wide range of clinical settings—physician offices,physician’s office, an outpatient clinicshospital setting, or within multiple departments inof a hospital.hospital (e.g., emergency and operating rooms). Our Diagnostic Imaging segment revenues derive primarily from selling solid-state gamma cameras and post-warranty camera maintenance contracts.
We sell our imaging systems to physicians, outpatient clinics hospitals. In addition, through our wholly-owned subsidiary, Digirad Imaging Solutions, Inc., or DIS, we also offer
The central component of a comprehensive and mobile imaging leasing service, called FlexImaging®, for physicians who wish to perform nuclear cardiology imaging procedures in their offices but do not have the patient volume, capital or resources to justify purchasing a gamma camera. DIS provides our physician customers with an imaging system, certified personnel, required licensure and other support for the performance of nuclear imaging procedures under the supervision of our physician customers. Physicians enter into annual contracts for imaging services delivered on a per-day basis ranging from one day per month to several days per week. DIS currently operates 23 regional hubs and seven fixed sites in 17 states.
Our unique dual sales and leasing distribution model offers physicians, clinics and hospitals versatile delivery options that appeal to medical establishments of all sizes, capabilities and imaging expertise. The mobility of our imaging systems and the flexibility of our DIS service allow cardiologists to provide nuclear imaging procedures in their offices to patients that they historically had to refer to hospitals or imaging centers. As a result, we provide physicians with more control over the diagnosis and treatment of their patients and enable physicians to capture revenue from procedures that would otherwise be referred to these hospitals and imaging centers.
Nuclear imaging is a clinical diagnostic tool, with established reimbursement codes, that has been in use for over 40 years. According to industry sources, approximately 18.4 million nuclear imaging procedures were performed in the United States in 2002, of which approximately 9.9 million were cardiac procedures, a volume that is expected to grow by approximately 25% annually over the next three years. We believe the growth in nuclear cardiology imaging will be driven by an increase in coronary heart disease resulting from the aging of baby boomers and the record rate of obesity and diabetes in all age groups. We estimate that the growth rate in 2002 for nuclear imaging procedures performed in physician offices was approximately 44% and in hospitals was approximately 6%. We expect the mobility of our imaging systems
1
to continue to allow us to capitalize on this shift in the delivery of nuclear cardiology imaging services from hospitals to physician offices.
The target market for our productscamera is the approximately 30,000 cardiologists indetector, which ultimately determines the United States that performoverall clinical quality of images a camera produces. Our nuclear cardiology procedures. To date, wecameras feature detectors with advanced proprietary solid-state technology developed by us. Solid-state systems have sold or provided imaging services through DISa number of benefits over conventional photomultiplier tube-based camera designs typically offered by our competitors. Our solid-state technology systems are typically 2 to approximately 500 physicians. In 2003, DIS performed over 66,000 patient procedures.
5 times lighter and considerably more compact than most traditional nuclear systems, making them far easier and less costly to build, very reliable, and able to be utilized for mobile applications. We sold our first gamma camera in March 2000, and we established DIS in September 2000. We had consolidated revenues and net losses of $41.5 million and $12.8 million, respectively, in fiscal 2002 and $56.2 million and $1.7 million, respectively, in fiscal 2003. Revenues from DIS and from our camera sales constituted 62% and 38%, respectively, of our 2003 consolidated revenues. We believe DIS will continue to provide us with recurring annual contractual revenue and comprise the largest component of our consolidated revenues.
Our Competitive Strengths
We believe that our position asare a market leader in the mobile solid-state nuclear cardiac imaging market is a product of the following competitive strengths:
7
We believe our current imaging systems, with their state-of-the-art technology and underlying patents, will continue to be relevant for the foreseeable future. We will continue to enhance and adjust our existing systems for the changing nuclear imaging market, including software updates and smaller enhancements. However, to accomplish any significant changes and enhancements, we will utilize what we believe is a deep available pool of contract engineers on a flexible, as needed basis and do not maintain a high-quality image despitestaff research and development department, thereby eliminating the rigorsfixed costs of a mobile environment.
Building and Construction
ATRM through its wholly-owned subsidiaries KBS, Glenbrook and EdgeBuilder, services residential and commercial construction projects by manufacturing modular housing units, structural wall panels, permanent wood foundation systems, and other engineered wood products, and supplies general contractors with building materials. KBS is a Maine-based manufacturer that started business in 2001 as a manufacturer of modular homes. Our focus is to offer high quality products for both commercial and residential buildings with a focus on customization to suit the project requirements, provide value with our engineering and design expertise, and deliver product when required by the customer. Having operated at below 50% capacity at our South Paris, Maine facility throughout 2019, we have rebuilt our sales team and embarked on a new strategy that broadens our target market beyond single-family residential installations, which typically involve producing three to four building modules per project. While we intend to continue to pursue and grow our residential modular building business, which provides us with positive margins and cash flow, we are also targeting large multi-family projects in the 25 to 100 building modules range.
Having produced approximately 230 building modules in 2019, we are in advanced discussions for various large commercial construction projects in New England that together will account for nearly 250 building modules and over $10.0 million in potential revenue. The modular manufacturing industry brings different financial challenges than the conventional real estate construction industry, as factory production requires that materials be purchased up front and well ahead of final payment for a project. We recently received $2.0 million in financing from Gerber to add to our working capital in preparation for projects of our KBS subsidiary, which are anticipated to begin in May 2020 and be completed later in 2020.
Glenbrook is a retail supplier of lumber, windows, doors, cabinets, drywall, roofing, decking and other building materials and conducts its operations in Oakdale, Minnesota. EdgeBuilder is a manufacturer of structural wall panels, permanent wood foundation systems and other engineered wood products and conducts its operations in Prescott, Wisconsin. We provide high quality building materials and unmatched service and attention to detail to building professionals, as well as homeowners. In addition, we provide highly personalized service, knowledgeable salespeople and attention to detail that the larger, big-box chain home stores do not provide. We offer a superior products unique to each project’s requirements, provide value with our engineering and design expertise that meet the customer’s needs, while staying cost-competitive and on schedule. While EdgeBuilder supplies the wall panels, Glenbrook supplies “loose lumber” such as floor and roof sheathing, bracing, and hardware with a “tandem” approach to every project. Residential home projects require much less production time than larger projects. The framing on a single-family home can be manufactured in a few hours, while larger buildings take many weeks. Our production strategy is to utilize automation and the most efficient methods of manufacturing and high-quality materials in all of our projects.
Real Estate and Investments
As part of the HoldCo Conversion, Digirad formed a real estate division under a newly formed subsidiary named Star Real Estate Holdings USA, Inc. (“SRE”) for the purposes of holding significant real estate assets that Digirad acquires. As an initial transaction to create Digirad’s real estate division under SRE and launch that aspect of the HoldCo Conversion, in April 2019, Digirad funded the initial purchase of three manufacturing facilities in Maine that manufacture modular buildings and leased those three properties. The funding of the asset acquisition was primarily through the revolver loan under our credit facility with Sterling. Digirad expects SRE to be substantially self-funded over time by raising its own capital in the form of commercial mortgages on the properties it owns or by raising other forms of external capital. Lone Star Value Management, LLC (LSVM), which was a wholly owned subsidiary of ATRM on the ATRM Acquisition Date, is a Connecticut based exempt reporting advisor that was acquired by the Company in the ATRM Acquisition. LSVM provides services that include investment advisory services and the servicing of pooled investment vehicles. The Company expects to use LSVM to make strategic investments in future potential acquisition targets for the Company.
8
Market Opportunity
Healthcare Services and Products
Diagnostic imaging depictions of the internal anatomy or physiology are generated primarily through non-invasive means. Diagnostic imaging facilitates the early diagnosis of diseases and disorders, often minimizing the scope, cost, and amount of care required and reducing the need for more invasive procedures. Currently, the major types of non-invasive diagnostic imaging technologies available are: x-ray, MRI, CT, ultrasound, PET, and nuclear imaging. The most widely used imaging acquisition technology utilizing gamma cameras is single photon emission computed tomography, or SPECT. All our current internally-developed cardiac gamma cameras employ SPECT technology.
Diagnostic imaging is the standard of care in diagnosis of diseases and disorders. We offer, through our businesses, the majority of these diagnostic imaging modalities. All of the diagnostic imaging modalities that we offer (both from provision of services and product sales) have been consistently utilized in clinical applications for many years, and are stable in their use and need. By offering a wide array of these modalities, we believe that we have strategically diversified our operations in possible changing trends of utilization of one diagnostic imaging modality from another.
Construction Services and Products
In the building and construction business, KBS markets its modular homes products through a direct sales organization and through inside sales, outside sales, a network of independent dealers, builders, and contractors in the New England states (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont). KBS’s direct sales organization is responsible for all commercial building projects, and works with developers, architects, owners, and general contractors to establish the scope of work, terms of payment, and general requirements for each project. KBS’s sales people also work with independent dealers, builders, and contractors to accurately configure and place orders for residential homes for their end customers. KBS’s network of independent dealers and contractors do not work with it exclusively, although many have KBS model homes on display at their retail centers. KBS does not assign exclusive territories to its independent dealers and contractors, but they tend to sell in areas of New England where they will not be competing against another KBS dealer or contractor. KBS’s backlog and pipeline, along with its market initiatives to build more workforce housing, are expected to position KBS for growth in 2020.
EBGL markets its engineered structural wall panels and permanent wood foundation systems through direct sales people and a network of builders, contractors and developers in and around Minneapolis and St. Paul areas. EBGL’s direct sales organization is responsible for both residential and commercial projects and it works with general contractors, developers and builders to provide bids and quotes for specific projects. Our marketing efforts include participation in industry trade shows, production of product literature, and sales support tools. These efforts are designed to generate sales leads for our independent builders and dealers, and direct salespeople. EBGL’s backlog and pipeline are currently estimated to exceed $20.0 million, as EBGL also strives to build more workforce housing.
Competition
The market for diagnostic products and services is highly competitive. Our business, which is focused primarily on the private practice and hospital sectors, continues to face challenges of demand for diagnostic services and imaging equipment, which we believe is due in part to the impact of the Deficit Reduction Act on the reimbursement environment and the 2010 Healthcare Reform laws, as well as general uncertainty in overall healthcare and legislative changes in healthcare, such as the Affordable Care Act. These challenges have impacted, and will likely continue to impact, our operations. We believe that the principal competitive factors in our market include acceptance by hospitals and physicians, relationships that we develop with our customers, budget availability for our capital equipment, requirements for reimbursement, pricing, ease-of-use, reliability, and mobility.
9
Diagnostic Services.In providing diagnostic services, we compete against many smaller local and regional nuclear and/or ultrasound providers, often owner-operators that may have lower operating costs. The fixed-installation operators often utilize older, used equipment, and the mobile operators may use older Digirad single-head cameras or newer dual-head cameras. We are the only mobile provider with our own exclusive source of triple-head mobile systems. Some competing operators place new or used cameras into physician offices and then provide the staffing, supplies, and other support as an alternative to a Diagnostic Services service contract. In addition, we compete against imaging centers that install fixed nuclear gamma cameras and make them available to referring physicians in their geographic vicinity. In these cases, the physician sends their patients to the imaging center.
Diagnostic Imaging. In selling our imaging systems, providewe compete against several large medical device manufacturers who offer a full line of imaging cameras for each diagnostic imaging technology, including x-ray, MRI, CT, ultrasound, nuclear medicine, or SPECT/CT and PET/CT hybrid imagers. The existing nuclear imaging systems sold by these competitors have been in use for a longer period of time than internally developed nuclear gamma cameras, and are more convenient operation, better power efficiencywidely recognized and increased durabilityused by physicians and hospitals for nuclear imaging; however, they are generally not solid-state, lightweight, as compared to vacuum tube cameras.
Mobile Healthcare. The market for selling, servicing, and operating diagnostic imaging services, patient monitoring equipment, and imaging systems through DIS.
is highly competitive. In providing our Mobile Healthcare services, we compete against a few large national and regional providers. In addition to direct competition from other providers of services similar to those offered by us, we compete with freestanding imaging centers and healthcare providers that have their own diagnostic imaging systems, as well as with equipment manufacturers that sell imaging equipment directly to healthcare providers for permanent installation. Some of the direct competitors, which provide contract MRI and PET/CT services, have access to greater financial resources than we do. In addition, some of our customers are capable of providing the same services we provide to their patients directly, subject only to their decision to acquire a high-cost diagnostic imaging system, assume the financial and technology risk, and employ the necessary technologists, rather than obtain equipment and services from us. We may also experience greater competition in states that currently have certificate of need laws if such laws were repealed, thereby reducing barriers to entry and competition in those states. We also compete against other similar providers in quality of services, quality of imaging systems, relationships with healthcare providers, knowledge and service quality of technologists, price, availability, and reliability.
Building and Construction. The market for building and construction is highly competitive. KBS is a regional manufacturer of modular housing units with its primary market in the New England states. Several modular manufacturers are located in these New England states and in nearby Pennsylvania. Some competitors have manufacturing locations in Canada and ship their products to the United States. KBS’s competitors include Apex Homes, Commodore Corporation, Skyline Champion Homes, Custom Building Systems, Durabuilt, Excel Homes, Huntington Homes, Icon Legacy Homes, Kent Homes (Canada), Maple Leaf Homes (Canada), Muncy Homes, New England Homes, New Era, Pennwest, Premier Builders (PA), Professional Builders Systems, RCM (Canada), Redmond Homes, Ritz-Craft, Simplex Homes, and Westchester Modular. EBGL is a regional manufacturer of engineered structural wall panels and permanent wood foundation systems, and also has a local retail business. EBGL’s market is primarily the Upper Midwest states (Iowa, Minnesota, Missouri, North Dakota, South Dakota, and Wisconsin). EBGL’s competitors include Precision Wall Systems, Component Manufacturing Company, JL Schwieters Construction, Arrow Building Center, and Marshall Truss Systems Incorporated. EBGL’s professional building supply business competes on a local level against both small, local lumber yards, regional building supply companies and to a certain degree, the “big box” stores such as Home Depot, Lowe’s, and Menard’s.
10
Intellectual Property Portfolio
We rely on a combination of patent, trademark, copyright, trade secret, and other intellectual property laws, nondisclosure agreements, and other measures to protect our intellectual property. We require our employees, consultants, and advisors to execute confidentiality agreements and to agree to disclose and assign to us all inventions conceived during the workday, using our property, or which relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary. As discussed herein, Digirad Health intellectual property is currently subject to a security interest to Sterling. ATRM’s intellectual property, consisting of a registered copyright and its domain name, and EBGL’s intellectual property, consisting of registered trademarks, unregistered trademarks and domain names, are currently subject to a security interest to Gerber Finance Inc. (“Gerber”).
Patents.
We have developed an intellectual propertya patent portfolio that includes product, componentcovers our products, components, and processprocesses. We have 15 non-expired U.S. patents. The patents covering variouscover, among other things, aspects of solid-state radiation detectors that make it possible for Digirad to provide mobile imaging services, and our scan technology that provides for lower patient doses and more specific cardiac images. Our patents expire between 2021 (U.S. Patent 6,504,178) and 2030 (U.S. Patent 8,362,438). While each of our patents applies to nuclear medicine, many also apply to the construction of area detectors for other types of medical and non-medical imagers and imaging systems. Currently, we have 21 patents issuedmethods.
Trademarks and 10 pending patent applicationsCopyrights
Our registered trademark portfolio consists of registrations in the United States for Digirad® and CARDIUS®. Digirad has produced proprietary software for Digirad Imaging systems including: nSPEED™ 3D-OSEM Reconstruction, SEEQUANTA™ acquisition, and STASYS™ motion correction software. We also license certain software products, and their related copyrights, on a nonexclusive basis from Cedars-Sinai Health System. The license includes updates to the software. The license may be terminated at any time by either party upon notice if the other party materially breaches the agreement. Non-payment to licensor is considered a material breach. The license may also be automatically terminated by licensor if (i) an “event of default” occurs under indebtedness for borrowed money of licensee; (ii) licensee ceases business operations; (iii) licensee dissolves or (iv) licensee commences bankruptcy proceedings. On May 23, 2018, the parties entered into an amendment to the license agreement to, among other things, extend the term of license through July 1, 2023.
Raw Materials
Diagnostic Imaging.We and our contract manufacturers use a wide variety of materials, metals, and mechanical and electrical components for production of our nuclear imaging gamma cameras. These materials are primarily purchased from external suppliers, some of which are single-source suppliers. Materials are purchased from selected suppliers based on quality assurance, cost effectiveness, and constraints resulting from regulatory requirements, and we work closely with our suppliers to assure continuity of supply while maintaining high quality and reliability. Global commodity supply and demand can ultimately affect pricing of certain of these raw materials. Though we believe we have two patents issued and 21 pending patent applications internationally.
Our Business Strategyadequate available sources of raw materials, there can be no guarantee that we will be able to access the quantity of raw material needed to sustain operations, as well as at a cost-effective price.
Diagnostic Services and Mobile Healthcare.Our Diagnostic Services and Mobile Healthcare operations utilize radiopharmaceuticals for our nuclear services. The underlying raw material for creation of the array of doses utilized in nuclear medicine is produced from a total of five main production facilities throughout the world, typically from highly enriched uranium resources. These resources have been and are expected to continue to produce enough raw materials to address the global market, but there continues to be pressure to utilize low or non-enriched uranium resources to produce the underlying nuclear doses.
11
Building and Construction. KBS is a Maine-based manufacturer that started business in 2001 as a manufacturer of modular homes. The majority of underlying raw material for KBS are produced locally, with a small percentage coming from Canada. EdgeBuilder (EB) and Glenbrook (GL), referred to together as EBGL, maintain corporate offices in Oakdale, Minnesota. EdgeBuilder, manufactures wall panels, at its facility in Prescott, Wisconsin. Glenbrook, which is located in Oakdale, MN, is a retail supplier of lumber, windows, doors, cabinets, drywall, roofing, decking and other building materials. The underlying raw materials for EdgeBuilder and Glenbrook are dimensional lumber and structural panels (oriented strand board (OSB), plywood, and exterior gypsum sheathing). These resources have been and are expected to continue to produce high quality of wood panels to address global needs.
Manufacturing
Diagnostic Imaging.We manufacture our nuclear imaging gamma cameras by employing a strategy that combines using internal manufacturing resources for devices requiring specific expertise due to our proprietary design coupled with qualified contract manufacturers. Mechanical and electronic components of our systems are produced by contract manufacturers, whereas the most complex components, final assembly and final system performance tests are performed at our facility. All of our suppliers of critical materials, components, and subassemblies undergo supplier qualifications and ongoing quality audits in accordance with our supplier quality process.
We and our contract manufacturers are subject to FDA Quality System Regulations, state regulations, and standards set by the International Organization for Standardization, or ISO. We are currently certified to the EN ISO 13485:2016 quality standard. We have received U.S. Food and Drug Administration (“FDA”) 510(k) clearance for our complete nuclear imaging camera product line (Cardius® XPO, Cardius® X-ACT, and Ergo™ gamma cameras). In addition, the X-ACT camera utilizes an x-ray technology to provide attenuation correction information for the SPECT reconstruction. We also have received additional FDA clearance of our Ergo™ large-field-of-view General Purpose Imager for use in intraoperative and molecular breast imaging.
Building and Construction. KBS began manufacturing commercial modular multi-family housing units in 2008. In subsequent years, KBS expanded its product offerings to include a variety of commercial buildings including apartments, condominiums, townhouses, dormitories, hospitals, office buildings, and other structures. The structures are built inside our climate-controlled factories and are then transported to the site where they are set, assembled and secured on the foundation. Electrical, plumbing, and HVAC systems are inspected and tested in the factory, prior to transportation to the site, to ensure the modules meet all local building codes and quality requirements. Modular construction has gained increased acceptance and is a preferred method of building by many architects and general contractors. The advantages of modular construction include: modules are constructed in a climate-controlled environment; weather conditions usually do not interrupt or delay construction; the building is protected from weather, reducing the risk of mold due to materials absorbing moisture from rain or snow; reduced site work; improved safety and security; reduced vandalism and attrition, as the building is immediately secured; and a significant reduction in overall project time.
Having operated at below 50% capacity at our South Paris, Maine facility throughout 2019, we have rebuilt our sales team and embarked on a new strategy that broadens our target market beyond single-family residential installations, which typically involve producing three to four building modules per project. While we intend to continue to pursue and grow our residential modular building business, which provides us with positive margins and cash flow, we are also targeting large multi-family projects in the 25 to 100-building modules range.
EBGL consists of two separate companies (EdgeBuilder (EB) and Glenbrook (GL)) operating in tandem with a common management team. EdgeBuilder manufactures wall panels and permanent wood foundations (PWF) in a climate-controlled factory, then transports the panels to the construction site via flat-bed trucks. The panels are typically unloaded by crane and erected or assembled, on site, by professional framing contractors. Panelized construction, especially in large-scale, multi-unit projects, is becoming increasingly popular due to the heightened demand on construction labor. Additionally, because the wall panels are constructed in a controlled indoor environment, waste, weather related delays, and mistakes are minimized. This shaves weeks off large, multi-unit construction schedules, leaving room for more annual builds. Glenbrook fills in all the areas where EdgeBuilder leaves off, with Glenbrook’s vast offerings of professional building products. As International Building Code® continues to evolve, KBS and EBGL, along with our professional partners in the industry, meet code changes with innovative products and a dedicated staff for adherent builds.
12
Reimbursement for Digirad Health
All of Digirad Health customers typically rely primarily on the Medicare and Medicaid programs and private payors for reimbursement. As a result, demand for our products and services are dependent in part on the coverage and reimbursement policies of these payors. Third party coverage and reimbursement is subject to extensive federal, state, local, and foreign regulation, and private payor rules and policies. In many instances, the applicable regulations, policies, and rules have not been definitively interpreted by regulatory authorities or the courts, are open to a variety of interpretations, and are subject to change without notice.
The scope of coverage and payment policies vary among third-party private payors. For example, some payors will not reimburse a provider unless the provider has a contract with the payor, and in many instances such payors will not enter into such contracts without the approval of a third party “radiology benefit manager” that the payor compensates based on reducing the payor’s imaging expense. Other payors prohibit reimbursement unless physicians own or lease our cameras on a full-time basis, or meet certain accreditation or privileging standards. Such payor requirements and limitations can significantly restrict the types of business models we can successfully utilize.
Medicare reimbursement rules are subject to annual changes that may affect payment for services that our customers provide. In addition, Congress has passed healthcare reform proposals that are intended to expand the availability of healthcare coverage and reduce the growth in healthcare spending in the U.S. Many of these laws affect the services that our customers provide, and could change further over time.
Medicare reimbursement rules impose many standards and policies on the payment of services that our customers provide. For instance, physicians billing for the technical component of nuclear imaging tests must be accredited by a government-approved independent accreditation body and many private payors are adopting similar requirements. We offer our customers a service to assist them in obtaining and maintaining the required accreditation. We believe we have structured our contracts in a manner that allows our customers to seek reimbursement from third-party payors in compliance with Medicare reimbursement rules. Our physician customers typically bill for both the technical and professional components of the tests. Assuming they meet certain requirements including, but not limited to, performing and documenting bona fide interpretations and providing the requisite supervision of the non-physician personnel performing the tests, they may bill and be paid by Medicare. If the failure to comply is deemed to be “knowing” or “willful,” the government could seek to impose fines or penalties, and we may be required to restructure our agreements and/or respond to any resultant claims by such customers or the government. Our hospital customers typically seek reimbursement by Medicare for outpatient services under the Medicare Hospital Outpatient Prospective Payment System.
Sales
We maintain separate sales organizations that are aligned with each of our business improveunits, which operate independently but in cooperation with each other. Mobile Healthcare sales efforts are throughout the United States and Canada, though there typically is more effort expended in rural and smaller hospital areas, as these are the primary customers that we sell our market positionservices to and increaseprovide the most value. Diagnostic Services concentrates its efforts on twelve regional areas where the majority of our revenuebusiness is concentrated based on concentrations of people and profits by pursuingcardiac disease. Diagnostic Imaging sales efforts are conducted throughout the followingUnited States and certain foreign countries, and are not concentrated to any particular region or area within the United States as the customer profile for this business strategies:
In our building and construction division, KBS’s sales people also work with independent dealers, builders, and contractors to accurately configure and place orders for residential homes for their end customers. At KBS, we have two internal salespeople, two external salespeople and a Head of Business Development. KBS’s network of independent dealers and contractors do not work with it exclusively, although many have KBS model homes on display at their retail centers. KBS does not assign exclusive territories to its independent dealers and contractors, but they tend to sell in areas of New England where they will not be competing against another KBS dealer or contractor. EBGL markets its engineered structural wall panels and permanent wood foundation systems through direct sales people and a network of builders, contractors and developers in the Upper Midwest states. EBGL’s direct sales organization is responsible for both residential and commercial projects and it works with general contractors, developers and builders to provide bids and quotes for specific projects.
13
Employees.
As of March 31, 2020, we had a total of 595 full time employees in all our divisions, of which 347 were employed in clinical-related positions, 78 in manufacturing, 84 in operational roles, 61 in general and administrative functions, and 25 in marketing and sales. All positions are in the United States. We intendalso utilize varying amounts of temporary workers as necessary to maintainfulfill customer requirements. As described below, recent events have required us to temporarily reduce our leadership positionoperations and staffing significantly.
Recent Developments
COVID-19
On March 11, 2020, the World Health Organization declared the outbreak of a novel strain of coronavirus, COVID-19, a global pandemic, which continues to spread throughout the United States and around the world. Governmental authorities in solid-state imaging technology by continuing to invest resources in research and development.
During the three months ended March 31, 2020, we operated our business in the ordinary course. However, we experienced a $0.9 million decrease in revenue, as compared to the same period of the prior year, related to a decrease in our diagnostic services due to the COVID-19 pandemic. While the COVID-19 pandemic did not have a significant impact on the results of other segments of our business during the three months ended March 31, 2020, we have taken steps to contain the impact of the COVID-19 pandemic on our business.
On April 1, 2020, we announced that in response to the COVID-19 pandemic, Matthew G. Molchan, our President and Chief Executive Officer, David J. Noble, our Chief Financial Officer and Chief Operating Officer, and Martin B. Shirley, the president of our Diagnostic Imaging Solutions Inc. subsidiary, have each agreed to have their base salaries reduced by 20%. These reductions were effective as of April 6, 2020, and will remain in effect until May 15, 2020, subject to extension or adjustment based on developments surrounding the COVID-19 pandemic.
On April 1, 2020, we also announced that in response to the COVID-19 pandemic, we planned to furlough certain employees (without pay, but with our payment of health insurance) and that we would institute a 20% salary reduction for most of our salaried employees and reduce the number of working hours of most of our hourly employees by 20%. These reductions, which apply to our healthcare division, were effective as of April 6, 2020, and will remain in effect until May 15, 2020, subject to extension or adjustment developments surrounding the COVID-19 pandemic.
Throughout the COVID-19 pandemic, our building and construction division has furloughed employees or reduced employee hours based on fluctuations in demand for our products. Many of these employees are expected to return to work within the next few weeks.
This partial disruption, although temporary, may impact our operations and overall business. The impact of COVID-19 is evolving rapidly and its future effects are uncertain. Given the uncertainty of the situation, the duration of the disruption and related financial impact cannot be reasonably estimated at this time.
As a result of the evolving impact of COVID-19 on the economy, on April 7, 2020, we withdrew our 2020 full-year guidance.
On April 30, 2020, each of KBS, EdgeBuilder and Glenbrook executed a separate promissory note evidencing unsecured loans under the “Paycheck Protection Program” (the “PPP”). The promissory note executed by KBS is for $0.8 million (the “KBS Note”), the promissory note executed by EdgeBuilder is for $0.2 million (the “EdgeBuilder Note”) and the promissory note executed by Glenbrook is for $0.2 million (the “Glenbrook Note”). The KBS Note, the EdgeBuilder Note and the Glenbrook Note, each dated April 30, 2020, are referred to together as the “Construction Notes”.
On May 11, 2020, the Company and each of Digirad Imaging Solutions, Inc. (“DIS”), DMS Imaging, Inc. (“DMS Imaging”) and DMS Health Technologies, Inc. (“DMS Health”), each a direct or indirect wholly owned subsidiary of the Company, executed a separate promissory note evidencing unsecured loans under the PPP. The promissory note executed by the Company, dated May 7, 2020, is for $0.8 million (the “Company Note”); the promissory note executed by DIS, dated May 5, 2020, is for $3.0 million (the “DIS Note”); the promissory note executed by DMS Imaging, dated May 5, 2020, is for $1.6 million (the “DMS Imaging Note”) and the promissory note executed by DMS Health, dated May 7, 2020, is for $0.1 million (the “DMS Health Note”). Loans under the Company Note and the DIS Note were disbursed on May 12, 2020, and the loans under the DMS Health Note and DMS Imaging Note were disbursed on May 13, 2020. The Company Note, the DIS Note, the DMS Imaging Note, and the DMS Health Note are referred to together as the “Healthcare Notes”. The Construction Notes and the Healthcare Notes are referred to collectively as the “PPP Notes” and each promissory note individually as a “PPP Note”.
The PPP was established under the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) and is administered by the U.S. Small Business Administration (“SBA”). The loans evidenced by the Construction Notes are being made through Bremer Bank (“Bremer”) as lender, and the loans evidenced by the Healthcare Notes are being made through Sterling as lender.
The loans evidenced by the PPP Notes (the “PPP Loans”) have two-year terms and bear interest at a rate of 1.00% per annum. Monthly principal and interest payments under the PPP Loans are deferred for six months. Beginning seven months from the date of a PPP Note, unless fully forgiven prior thereto, the applicable borrower will pay to its lender thereunder a monthly principal and interest payments. The PPP Loans may be prepaid at any time prior to maturity with no prepayment penalties. The Construction Notes mature on April 30, 2022, and the Healthcare Notes mature two years from the date the loans under the Healthcare Notes are disbursed. The PPP Notes contain customary events of default relating to, among other things, payment defaults, making materially false and misleading representations to the SBA or lender, or breaching the terms of the applicable PPP Loan documents. Upon an event of default under a PPP Note, the lender thereunder may, among other things, require immediate payment of all amounts owing under the applicable PPP Note, collect all amounts owing from the applicable borrower, or file suit and obtain judgment.
Under the terms of the CARES Act, recipients of loans under the PPP can apply for and be granted forgiveness for all or a portion of loan granted under the PPP. Such forgiveness will be determined, subject to limitations, based on the use of loan proceeds for payment of payroll costs and certain other eligible costs. However, no assurance is provided that forgiveness for any portion of the PPP Loans will be obtained. In order to apply for the PPP Loan, we were required to certify, among other things, that the current economic uncertainty made the PPP Loan request necessary to support ongoing operations of the Company. This certification further required the Company to take into account its current business activity and its ability to access other sources of liquidity sufficient to support ongoing operations in a manner that is not significantly detrimental to the business. The Company is continuing to evaluate the criteria and new guidance put out by the SBA regarding qualification of loans under the PPP and the criteria for meeting loan conditions.
At Digirad, our highest priority remains the safety, health and well-being of our employees, their families and our communities and we remain committed to serving the needs of our customers. The COVID-19 pandemic is a highly fluid situation and it is not currently operatepossible for us to reasonably estimate the impact it may have on our financial and increase hub utilization by adding physician
2
customersoperating results. We will continue to evaluate the impact of the COVID-19 pandemic on our business as we learn more and routes.the impact of COVID-19 on our industry becomes clearer.
14
KBS Projects
As we transform Digirad into a multi-industry holding company, our largest near-term growth opportunity is to execute a successful turnaround of the KBS modular business that we acquired pursuant to the ATRM Merger. We alsoown three modular building manufacturing facilities within a short distance from each other in Maine. Currently, we are operating one of these facilities (our South Paris, Maine facility), leasing another to a third party and our remaining facility is idle but ready to bring on line when our production levels warrant additional capacity. Having operated at below 50% capacity at our South Paris facility throughout 2019, we have rebuilt our sales team and embarked on a new strategy that broadens our target market beyond single-family residential installations, which typically involve producing three to four building modules per project. While we intend to continue to pursue cardiology opportunitiesand grow our residential modular building business, which provides us with positive margins and cash flow, we are also targeting large multi-family projects in the 25 to 100-building modules range.
Having produced approximately 230 building modules in 2019, we are in advanced discussions for DISvarious large commercial construction projects for the New England market. Even if just a few of these projects are awarded, they will require a significantly higher utilization rate for KBS’s manufacturing plant in hospitalsSouth Paris, Maine and an increased investment in working capital. Three of these projects (the “KBS Projects”) are in the advanced stages of negotiation and are expected to start production in the coming months. Each of these projects involve construction of additional housing units in New England. These three projects alone are expected to generate revenue of over $10 million for KBS and result in the production of nearly 250 building modules. For comparison, in 2019, KBS generated approximately $12 million of revenue and produced approximately 230 building modules, entirely for the residential market. We believe KBS’s South Paris, Maine plant is capable of producing between 500 and 600 building modules per year at full capacity. KBS’ current potential sales pipeline totals more than $50 million, representing production of approximately 1,000 building modules. If KBS grows as expected in 2020, it will explore re-opening its Oxford, Maine plant, which we believe is capable of producing an additional 500 to 600 building modules per year at full capacity. KBS will also explore other add-on revenue streams, such as manufacturing structural wall panels for the New England market, as we are currently doing in the Minneapolis, Minnesota area. Our goal with these growth initiatives is to generate significantly higher revenue, cash flow, and earnings for our building and construction division, creating new clinical applicationsjobs and supporting local economic activity. While we anticipate securing the contracts for DISthe KBS Projects, we can provide no assurance that we will be awarded the KBS Projects, or that we will be able to successfully complete the KBS Projects or achieve the anticipated revenues from the KBS Projects if they are awarded to KBS.
Related Party Transactions Currently in neurology, oncologyEffect
Perma-Fix
Prior to his resignation on April 6, 2020, John Climaco served as one of our directors and surgery.
15
Eberwein Guarantees
On March 29, 2019, in connection with our entry into the SNB Loan Agreement, Jeffrey Eberwein, the Chairman of our board of directors, entered into a Limited Guaranty Agreement (the “SNB Eberwein Guaranty”) with SNB pursuant to which he guaranteed to SNB the prompt performance of all the obligations of the borrowers under our credit facility with SNB to SNB, including the full payment of all indebtedness owed by such borrowers to SNB under or in connection with the SNB credit facility. Mr. Eberwein’s obligations under the SNB Eberwein Guaranty are limited in the aggregate to the amount of (a) $1.5 million, plus (b) reasonable costs and expenses of SNB incurred in connection with the SNB Eberwein Guaranty. Mr. Eberwein’s obligations under the SNB Eberwein Guaranty terminate upon our and the SNB credit facility borrowers achieving certain milestones set forth therein.
On January 31, 2020, contemporaneously with our execution and delivery of a Loan and Security Agreement with certain of our subsidiaries and Gerber (the “Star Loan Agreement”), which provides for a credit facility with borrowing availability of up to $2.5 million and matures on January 1, 2025, Mr. Eberwein executed and delivered a guaranty (the “Gerber Eberwein Guaranty”) to Gerber pursuant to which he guaranteed the performance of all the borrowers’ obligations under the Star Loan Agreement to Gerber, including the full payment of all indebtedness owing by such borrowers to Gerber under or in connection with the Star Loan Agreement and related financing documents. Mr. Eberwein’s obligations under the Gerber Eberwein Guaranty are limited in the aggregate to the amount of (a) $2.5 million, plus (b) costs of Gerber incidental to the enforcement of the Gerber Eberwein Guaranty or any guaranteed obligations.
On March 5, 2020, contemporaneously with the execution and delivery of a First Amendment to Loan and Security Agreement with Gerber that amended a January 31, 2020 Loan Agreement (the “EBGL Loan Agreement”) between certain of our subsidiaries (the “EBGL Borrowers”) and Gerber, Mr. Eberwein executed and delivered a guaranty (the “EBGL Eberwein Guaranty”) to Gerber pursuant to which he guaranteed the performance of all the EBGL Borrowers’ obligations to Gerber under the EBGL Loan Agreement, including the full payment of all indebtedness owing by the EBGL Borrowers to Gerber under or in connection with the EBGL Loan Agreement and related financing documents. Mr. Eberwein’s obligations under the EBGL Eberwein Guaranty are limited in the aggregate to the amount of (a) $500 thousand, plus (b) costs of Gerber incidental to the enforcement of the EBGL Eberwein Guaranty or any guaranteed obligations.
As a condition to a loan made by Premier Bank (“Premier”) to Glenbrook and EdgeBuilder pursuant to that certain Revolving Credit Loan Agreement, dated June 30, 2017, by and among Glenbrook, EdgeBuilder and Premier (the “Premier Loan Agreement”), Mr. Eberwein entered into a guaranty in favor of Premier, absolutely and unconditionally guaranteeing all of the borrowers’ obligations thereunder.
Eberwein Premier Participation
Pursuant to a certain Participation Agreement by and between Mr. Eberwein and Premier, which was signed on March 31, 2020 and was effective as of March 26, 2020, Mr. Eberwein purchased a ratable participation in, and assumed a ratable part of, the aggregate maximum principal amount of the outstanding balance of the loan under the Premier Loan Agreement in the amount of $0.25 million.
Put Option Agreement
In addition, prior to the effective time of the ATRM Merger, we entered into a put option purchase agreement with Mr. Eberwein, pursuant to which we have the right to require Mr. Eberwein to acquire up to 0.1 million shares of our Series A Preferred Stock at a price of $10.00 per share for aggregate proceeds of up to $1.0 million at any time, in our discretion, during the 12 months following the effective time of the ATRM Merger (the “Issuance Option”). In March 2020, Mr. Eberwein extended the Issuance Option through June 30, 2021. See “Description of Our Capital Stock and Securities Offered” for additional information about our Series A Preferred Stock.
16
ATRM Notes Payable
ATRM, our wholly owned subsidiary as a result of the ATRM Merger, has the following related party promissory notes outstanding:
| (i) | Unsecured promissory note (principal amount of $0.6 million payable to Lone Star Value Co-Invest I, LP (“LSV Co-Invest I”)), with interest payable semi-annually at a rate of 10.0% per annum (LSV Co-Invest I may elect to receive interest in-kind at a rate of 12.0% per annum), with any unpaid principal and interest due on January 12, 2020 (the “January Note”). Mr. Eberwein is the sole manager of Lone Star Value Investors GP, LLC (“LSV GP”), the general partner of LSV Co-Invest I, and is the sole owner of LSV Co-Invest I. On November 13, 2019, LSV Co-Invest I extended the maturity date of the January Note from January 12, 2020, to the earlier of (i) October 1, 2020 and (ii) the date when the January Note is no longer subject to a certain Subordination Agreement dated January 12, 2018, as amended, in favor of Gerber. |
| (ii) | Unsecured promissory note (principal amount of $1.0 million payable to LSV Co-Invest I), with interest payable semi-annually at a rate of 10.0% per annum (LSV Co-Invest I may elect to receive interest in-kind at a rate of 12.0% per annum), with any unpaid principal and interest due on June 1, 2020 (the “June Note”). On November 13, 2019 LSV Co-Invest I also extended the maturity date of the June Note from June 1, 2020, to the earlier of (i) October 1, 2020 and (ii) the date when the January Note is no longer subject to a certain Subordinate Agreement dated June 1, 2018, as amended, in favor of Gerber Finance. |
| (iii) | Unsecured promissory note (principal amount of $0.3 million payable to Lone Star Value Management, LLC (“LSVM”)), with interest payable annually at a rate of 10.0% per annum (LSVM may elect to receive any interest payment entirely in-kind at a rate of 12.0% per annum), with any unpaid principal and interest due on November 30, 2020 (the “LSVM Note”). Mr. Eberwein is also the Chief Executive Officer of LSVM, which is the investment manager of Lone Star Value Investors, LP and LSV Co-Invest I. |
LSVM and LSV Co-Invest I on July 17, 2019, waived any right to accelerate payment with respect to the ATRM Merger under the LSVM Note, the January Note, and the June Note. In March 2020, Mr. Eberwein, sole manager of LSV Co-Invest I and LSVM, provided us with a Letter of Support for the LSVM Note, the January Note, and the June Note indicating that the applicable holder of such notes will take no adverse action against ATRM for failure to pay the principal due on the applicable note by the maturity date and intends to work with us and ATRM to assure our financial success.
Subordination Agreement
LSVM and LSV Co-Invest I are party to subordination agreements with ATRM and Gerber pursuant to which LSVM and LSV Co-Invest I agreed to subordinate the obligations of ATRM under their unsecured promissory notes to the obligations of the borrowers to Gerber.
Acquisition of LSVM
On April 1, 2019, ATRM entered into a Membership Interest Purchase Agreement (the “LSVM Purchase Agreement”) with LSVM and Mr. Eberwein. Pursuant to the terms of the LSVM Purchase Agreement, Mr. Eberwein sold all of the issued and outstanding membership interests of LSVM to ATRM (the “LSVM Acquisition”) for a purchase price of $100.00, subject to a working capital adjustment provision. The LSVM Acquisition closed simultaneously with the execution and delivery of the LSVM Purchase Agreement, and was deemed effective as of January 1, 2019 for accounting purposes, as a result of which LSVM became a wholly-owned subsidiary of ATRM. Pursuant to the LSVM Purchase Agreement, the current assets as well as the $0.3 million promissory note issued by ATRM and current liabilities existing prior to January 1, 2019 remain with Mr. Eberwein. Cash contributions made by Mr. Eberwein subsequent to the ATRM Acquisition also exist as a payment due to Mr. Eberwein by ATRM. The LSVM Purchase Agreement contains representations, warranties, covenants and indemnification provisions customary for transactions of this type. LSVM was acquired by us as part of the ATRM Acquisition.
17
Adoption of Stock Repurchase Program
On October 31, 2018, our board of directors approved a stock repurchase program that will enable us to repurchase up to 200,000 shares of our common stock from time to time in market or private transactions. We believe that the program will help offset the dilutive impact of employee stock option exercises, and maximize the value of our common stock, and that the program reflects our belief in our strategy and operations and our commitment to our stockholders.
Under the stock repurchase program, we can growmay purchase shares of our common stock through various means, including open market sharetransactions in compliance with Rule 10b-18 under the Exchange Act of 1934, as amended (the “Exchange Act”), privately negotiated transactions, tender offers or any combination thereof. The number of shares repurchased and the timing of repurchases will depend on a number of factors, including, but not limited to, stock price, trading volume and general market conditions, along with our working capital requirements, general business conditions and other factors. The stock repurchase program has no time limit and may be modified, suspended or terminated at any time by capitalizing on the recent trendboard of nuclear cardiology procedures shiftingdirectors. Repurchases under the stock repurchase program will be funded from our existing cash and cash equivalents or future cash flow and equity or debt financings. As of the hospital to the physician office.
Corporate Information
Our business was
We are a Delaware corporation, originally incorporated in California in November 1985, and we reincorporated in Delaware in January 1997. We have 19 wholly-owned subsidiaries. Our principal executive offices are located at 13950 Stowe Drive, Poway, California 920641048 Industrial Court, Suwanee, GA 30024, and our telephone number is (858) 726-1600. We maintain aOur website on the Internet atwww.digirad.com.is www.digirad.com. The information contained in,on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website is not aas part of this prospectus. Unless the context requires otherwise, as usedprospectus or in this prospectus the terms "Digirad," "we," "us" and "our" referdeciding whether to Digirad Corporation, a Delaware corporation, and its subsidiaries.
We have trademark registrations in the United States for 2020tc Imager®, CardiusSST®, Digirad®, Digirad Logo®, Digirad Imaging Solutions®, FlexImaging® and SPECTour®. We have trademark applications pending in the United States for the following marks: Cardius™, DigiServSM, DigiSpectSM, DigiTechSM and SolidiumSM. We have obtained and sought trademark protection for some of the above listed marks in the European Community and Japan.
3
The number of shares of common stock to be outstanding after this offering is based on the shares of common stock outstanding as of December 31, 2003. This number excludes as of December 31, 2003:
In addition, except where we state otherwise, the information we present in this prospectus reflects:
4
SUMMARY CONSOLIDATED FINANCIAL INFORMATION
DATA
The following table summarizes oursummary consolidated financial information for the periods presented which areset forth below is derived from our audited consolidated financial statements. Youstatements which are incorporated herein by reference from our Annual Report on Form 10-K for the year ended December 31, 2019 (“2019 10-K”) filed on March 9, 2020 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020 (“2020 Q1 10-Q”) filed on May 15, 2020. The summary consolidated financial information presented is only a summary of consolidated financial data and should be read this information togetherin conjunction with ourthe consolidated financial statements and the relatedaccompanying notes appearing elsewherethereto, included in this prospectusour 2019 10-K and 2020 Q1 10-Q. In addition, the "Management'ssummary consolidated financial information set forth below should be read in conjunction with the information presented under the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations"Operations” in our 2020 Q1 10-Q.
18
Condensed Consolidated Statements of Operations Data
Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| (in thousands, except per share data) | ||||||||
| Total revenues | $ | 114,185 | $ | 104,180 | ||||
| Total cost of revenues | 92,070 | 85,909 | ||||||
| Gross profit | 22,115 | 18,271 | ||||||
| Operating expenses | ||||||||
| Marketing, sales, general and administrative | 21,575 | 20,456 | ||||||
| Amortization of intangible assets | 1,794 | 1,377 | ||||||
| Merger and financing costs | 2,342 | — | ||||||
| Goodwill impairment | — | 476 | ||||||
| Loss on sale of buildings | 232 | 507 | ||||||
| Total operating expenses | 25,943 | 22,816 | ||||||
| Loss from operations | (3,828 | ) | (4,545 | ) | ||||
| Other expense, net | (133 | ) | (61 | ) | ||||
| Interest expense, net | (1,156 | ) | (751 | ) | ||||
| Loss on extinguishment of debt | (151 | ) | (43 | ) | ||||
| Total other expense | (1,440 | ) | (855 | ) | ||||
| Loss before income taxes | (5,268 | ) | (5,400 | ) | ||||
| Income tax benefit | 375 | 1,561 | ||||||
| Net loss from continuing operations | (4,893 | ) | (3,839 | ) | ||||
| Net income from discontinued operations | 266 | 4,575 | ||||||
| Net (loss) income | $ | (4,627 | ) | $ | 736 | |||
| Basic and diluted net (loss) income per common share attributable to common stockholders | $ | (2.56 | ) | $ | 0.37 | |||
Three Months Ended March 31, | ||||||||
| 2020 | 2019 | |||||||
| (in thousands, except per share data) | (unaudited) | |||||||
| Total revenues | $ | 28,857 | $ | 23,912 | ||||
| Total cost of revenues | 24,416 | 19,931 | ||||||
| Gross profit | 4,441 | 3,981 | ||||||
| Operating expenses | ||||||||
| Marketing, sales, general and administrative | 6,228 | 4,833 | ||||||
| Amortization of intangible assets | 817 | 283 | ||||||
| Total operating expenses | 7,045 | 5,116 | ||||||
| Loss from operations | (2,604 | ) | (1,135 | ) | ||||
| Other income (expense), net | 160 | (198 | ) | |||||
| Interest expense, net | (475 | ) | (181 | ) | ||||
| Loss on extinguishment of debt | ---- | (151 | ) | |||||
| Total other expense | (315 | ) | (530 | ) | ||||
| Loss before income taxes | (2,919 | ) | (1,665 | ) | ||||
| Income tax (expense) benefit | (34 | ) | 8 | |||||
| Net loss | $ | (2,953 | ) | $ | (1,657 | ) | ||
| Basic and diluted net loss per common share attributable to common stockholders | $ | (1.67 | ) | $ | (0.82 | ) | ||
Unaudited Condensed Consolidated Balance Sheet Data
| As of March 31, 2020 | |||||||||
| Pro Forma As | |||||||||
| Actual | Adjusted(1) | ||||||||
| (in thousands) | |||||||||
| Cash and cash equivalents | $ | 1,423 | $ | 5,867 | |||||
| Working capital (net)(2) | 2,203 | 2,203 | |||||||
| Total current assets | 24,576 | 29,020 | |||||||
| Total assets | 83,807 | 88,251 | |||||||
| Total current liabilities, including current portion of long term debt | 22,373 | 22,373 | |||||||
| Total long term debt | 23,581 | 23,581 | |||||||
| Total liabilities | 45,954 | 45,954 | |||||||
| Total mezzanine equity and stockholders’ equity | 37,853 | 42,297 | |||||||
| (1) | The pro forma as adjusted condensed consolidated balance sheet data (a) does not give effect to the PPP Loans and (b) gives effect to our issuance and sale of 2,109,704 shares of common stock in this offering based on an assumed offering price of $2.37 per share, assuming net proceeds of approximately $4.44 million, after deducting the underwriter’s fees and estimated offering expenses payable by us, and assuming no sale of any pre-funded warrants in this offering and no exercise of any accompanying warrants or the underwriter’s warrant. |
| (2) | Working capital is defined as current assets less current liabilities. |
19
Use of Non-GAAP Financial Measurements
The following non-GAAP (defined below) financial measures are intended to supplement the GAAP financial information by providing additional insight regarding results of operations of the Company. The non-GAAP EBITDA and adjusted EBITDA financial measures used by the Company are intended to provide an enhanced understanding of our underlying operational measures to manage the Company’s business, to evaluate performance compared to prior periods and the marketplace, and to establish operational goals. Certain items are excluded from these non-GAAP financial measures to provide additional comparability measures from period to period. Specifically, the table below presents the non-GAAP financial measure “EBITDA” (defined as earnings before interest, taxes, depreciation, amortization) and “Adjusted EBITDA” (defined as earnings before interest, taxes, depreciation, amortization adjusted for stock-based compensation and other one-time transaction costs such as mergers and acquisitions, financings and other extraordinary items), respectively. The most directly comparable measures for these non-GAAP financial measure are net income and diluted net income per share. EBITDA and Adjusted EBITDA are intended as supplemental measures of our performance that are not required by or presented in accordance with accounting principles generally accepted in the United States of America (“GAAP”). We believe that EBITDA and Adjusted EBITDA provides useful information to management and investors regarding certain financial and business trends relating to our financial condition and operating results.
We believe that the use of EBITDA and Adjusted EBITDA provide additional tools for investors to use in evaluating ongoing operating results and trends and in comparing our financial measures with other businesses which may present similar non-GAAP financial measures to investors. We believe that EBITDA and Adjusted EBITDA are useful measures because they normalize operating results by excluding non-recurring gains, losses and other items and help to demonstrate how much cash we are able to generate annually. In addition, you should be aware when evaluating EBITDA and Adjusted EBITDA that in the future we may incur expenses similar to those excluded when calculating these measures. Our presentation of these measures should not be construed as an inference that our future results will be unaffected by unusual or non-recurring items. Our computation of EBITDA and Adjusted EBITDA may not be comparable to other similarly titled measures computed by other companies, because all companies do not calculate IBITDA and Adjusted EBITDA in the same fashion.
Our management does not consider EBITDA and Adjusted EBITDA in isolation or as an alternative to financial measures determined in accordance with GAAP. The principal limitations of EBITDA and Adjusted EBITDA are that they exclude significant expenses and income that are required by GAAP to be recorded in our financial statements. Some of these limitations are:
| a. | EBITDA and Adjusted EBITDA do not reflect our cash expenditures, or future requirements, for capital expenditures or contractual commitments; |
| b. | EBITDA and Adjusted EBITDA do not reflect changes in, or cash requirements for, our working capital needs; |
| c. | EBITDA and Adjusted EBITDA do not reflect interest expense, or the cash requirements necessary to service interest or principal payments, on our debts; |
| d. | although depreciation and amortization are non-cash charges, the assets being depreciated and amortized will often have to be replaced in the future, and EBITDA and Adjusted EBITDA do not reflect any cash requirements for such replacements; |
| e. | EBITDA and Adjusted EBITDA do not reflect the impact of certain cash charges resulting from matters we consider not to be indicative of our ongoing operations; and |
| f. | other companies in our industry may calculate EBITDA and Adjusted EBITDA differently than we do, limiting its usefulness as a comparative measure. |
Because of these limitations, EBITDA and Adjusted EBITDA should not be considered in isolation or as a substitute for performance measures calculated in accordance with GAAP. We compensate for these limitations by relying primarily on our GAAP results and using EBITDA and Adjusted EBITDA only as supplements. You should review the reconciliation of net income to EBITDA and Adjusted EBITDA below and not rely on any single financial measure to evaluate our business.
20
Reconciliation of Non-GAAP Financial Measures
(unaudited)
(in thousands)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2020 | 2019 | |||||||
| Net income (loss) from continuing operations | $ | (2,953 | ) | $ | (1,657 | ) | ||
| Depreciation and amortization | 2,407 | 1,809 | ||||||
| Interest expense | 475 | 181 | ||||||
| Income tax benefit | 34 | (8 | ) | |||||
| EBITDA from continuing operations | (37) | 325 | ||||||
| Unrealized (gain) loss on equity securities (1) | 26 | (28) | ||||||
| Litigation costs (2) | 160 | --- | ||||||
| Loss on extinguishment of debt | --- | 151 | ||||||
| Stock-based compensation | 109 | 112 | ||||||
| Write-off of assets | 135 | --- | ||||||
| Transaction costs (3) | 115 | 230 | ||||||
| Adjusted EBITDA from continuing operations | $ | 508 | $ | 790 | ||||
| (1) | Reflects change in fair value of investments in equity securities. | |
| (2) | Reflects one-time litigation costs. | |
| (3) | Reflects legal and other costs related to the ATRM Merger and HoldCo Conversion. |
21
The following summary contains basic terms about this offering and the securities in this offering and is not intended to be complete. It may not contain all of the information that is important to you. You should read the more detailed information contained in this prospectus, including but not limited to, the Risk Factors beginning on page 24 of this prospectus. For a more complete description of the terms of the common stock, accompanying warrants and pre-funded warrants, see the section of this prospectus.
| | Years ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Statement of Operations Data: | 2001 | 2002 | 2003 | ||||||||
| | (In thousands, except per share amounts and operating data) | ||||||||||
| Revenues: | |||||||||||
| DIS | $ | 10,239 | $ | 23,005 | $ | 34,849 | |||||
| Product | 18,065 | 18,527 | 21,388 | ||||||||
| Total revenues | 28,304 | 41,532 | 56,237 | ||||||||
Cost of revenues: | |||||||||||
| DIS | 8,344 | 16,599 | 24,463 | ||||||||
| Product | 13,192 | 13,633 | 15,092 | ||||||||
| Total cost of revenues | 21,536 | 30,232 | 39,555 | ||||||||
Gross profit | 6,768 | 11,300 | 16,682 | ||||||||
Operating expenses: | |||||||||||
| Research and development | 3,009 | 2,967 | 2,191 | ||||||||
| Sales and marketing | 9,974 | 8,066 | 6,008 | ||||||||
| General and administrative | 8,161 | 9,497 | 8,097 | ||||||||
| Amortization and impairment of intangible assets | 991 | 1,011 | 444 | ||||||||
| Stock-based compensation(1) | 1,579 | 606 | 226 | ||||||||
| Total operating expenses | 23,714 | 22,147 | 16,966 | ||||||||
| Loss from operations | (16,946 | ) | (10,847 | ) | (284 | ) | |||||
| Other income (expense), net | (2,965 | ) | (1,925 | ) | (1,396 | ) | |||||
| Net loss | $ | (19,911 | ) | $ | (12,772 | ) | $ | (1,680 | ) | ||
| Net loss applicable to common stockholders | $ | (20,041 | ) | $ | (13,037 | ) | $ | (2,006 | ) | ||
| Basic and diluted net loss per share(2): | |||||||||||
| Historical | $ | (898.86 | ) | $ | (409.23 | ) | $ | (36.46 | ) | ||
| Pro forma | $ | (0.04 | ) | ||||||||
Shares used to compute basic and diluted net loss per share(2): | |||||||||||
| Historical | 22 | 32 | 55 | ||||||||
| Pro forma | 43,610 | ||||||||||
| | As of December 31, 2003 | ||||
|---|---|---|---|---|---|
| | Actual | As adjusted(3) | |||
| | (In thousands) | ||||
| Balance sheet data: | |||||
| Cash and cash equivalents | $ | 7,681 | |||
| Working capital | 2,578 | ||||
| Total assets | 35,159 | ||||
| Total debt | 16,441 | ||||
| Redeemable convertible preferred stock | 84,278 | ||||
| Total stockholders' equity (deficit) | (75,703 | ) | |||
5
| Issuer: | Digirad Corporation |
| Shares offered: | [●] shares of common stock. Jeffrey E. Eberwein, the Chairman of our Board of Directors, and Mitchell Quain, one of our directors, have indicated to us their intention to purchase up to $100,000 of common stock and/or pre-funded warrants each (representing up to 42,194 shares based on an assumed offering price of $2.37 per share), along with accompanying warrants for the purchase of up to an additional 21,097 shares, in this offering. In addition, Matthew Molchan, our Chief Executive Officer, and David Noble, our Chief Financial Officer and Chief Operating Officer, have indicated to us their intention to purchase up to $5,000 and $50,000, respectively, of common stock and/or pre-funded warrants each (representing up to 2,109 and 21,097 shares, respectively, based on an assumed offering price of $2.37 per share), along with accompanying warrants for the purchase of up to an additional 1,054 and 10,548 shares, respectively). Certain other non-executive employees of our company may also purchase shares, warrants and/or pre-funded warrants in this offering. However, because indications of interest are not binding agreements or commitments to purchase, there can be no assurance that the underwriter will determine to sell shares of common stock, warrants and/or pre-funded warrants in this offering to any of these persons or entities, or that any of these persons or entities will determine to purchase securities in this offering. Any securities sold to our employees, officers and directors will be at the same price and on the same terms as the securities sold to other investors in this offering. The underwriter will receive the same underwriting discount on any securities purchased by these persons or entities as they will on any other securities sold to the public in this offering. |
| Over-allotment option: | We have granted the underwriters an option to purchase up to [●] additional shares of common stock, warrants and/or pre-funded warrants and/or up to [●] additional warrants, in each case, solely to cover over-allotments, if any. |
| Offering price: | $[●] per share of common stock. |
| Pre-funded warrants offered by us: | We are also offering to each purchaser whose purchase of shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, pre-funded warrants, in lieu of shares of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% of our outstanding shares of common stock. Each pre-funded warrant will be exercisable for one share of common stock. The purchase price of each pre-funded warrant will equal the public offering price at which shares are being sold to the public in this offering, minus $0.01, and the exercise price of each pre-funded warrant included will be $0.01 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised in full. Any exercise of the pre-funded warrants which would result in a holder beneficially owning more than 4.99% of our outstanding shares of common stock will be subject to our consent. For each pre-funded warrant we sell, the number of shares we are offering will be decreased on a one-for-one basis. We may, in our sole discretion, waive the 4.99% ownership limitation in connection with this offering with respect to one or more potential purchasers. |
Warrants offered by us: | We are also offering warrants to purchase up to an aggregate of [●] shares of common stock. Each share of common stock and each pre-funded warrant (other than those sold pursuant to the over-allotment option) is being sold together with a warrant to purchase 0.5 of a share of common stock. The warrants will only be exercisable for whole shares of common stock, and will have an exercise price of $[●] per share (at least 100%, and up to 125%, of the public offering price per share of our common stock in this offering), and will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. This offering also relates to the shares of common stock issuable upon exercise of the warrants. |
| Common stock to be outstanding after this offering: | [●] shares, assuming all shares offered by this prospectus are sold and assuming no sale of pre-funded warrants and no exercise of the accompanying warrants or the underwriter’s warrant (or [●] shares if the over-allotment option is exercised in full and no sale of pre-funded warrants and no exercise of the accompanying warrants or the underwriter’s warrant). |
Use of proceeds: | We estimate that our net proceeds from the offering will be approximately $4.65 million (approximately $5.35 million if the underwriter’s over-allotment option is exercised in full) before deducting estimated offering expenses of approximately $0.2 million. We intend to use at least $3.0 million of the net proceeds from this offering to fund the KBS Projects (three modular housing projects to be constructed in New England), and the remainder (if any) for working capital and for other general corporate purposes. |
| Risk factors: | You should read the “Risk Factors” section of this prospectus as well as all other information included in this prospectus, including the information in the documents incorporated by reference into this prospectus, for a discussion of certain of the factors to consider carefully before deciding to purchase any securities in this offering. |
| Lock-up: | We and each of our officers and directors have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock for a period of 90 days after this offering is completed without the prior written consent of Maxim. |
22
| Transfer agent: | The registrar and transfer agent for the common stock, and the warrant agent for the warrants, is American Stock Transfer & Trust Company. |
| National securities exchange listing: | Our common stock is listed on the Nasdaq Global Market under the symbol “DRAD”. There is no established trading market for the pre-funded warrants or the warrants and we do not expect a market for such securities to develop. We do not intend to list the warrants or pre-funded warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants and the warrants will be limited. |
The number of shares of our common stock at an assumed initial publicto outstanding before and after this offering priceis based on 2,097,381 shares of $ per share, the mid-pointcommon stock outstanding as of the range on the coverdate of this prospectus after deducting the estimated underwriting discounts and commission and the estimated expenses payable by usexcludes, as of such date:
| ● | 26,517 shares of common stock reserved for issuance pursuant to grants outstanding under our 2011 Inducement Award Plan; |
| ● | 40,621 shares of common stock reserved for issuance pursuant to grants outstanding under our 2014 Equity Incentive Award Plan; |
| ● | 30,766 shares of common stock reserved for future issuance under our 2018 Equity Incentive Award Plan; and |
| ● | [●] shares of common stock issuable upon the exercise of the warrant granted to the underwriter in connection with this offering. |
Except as otherwise noted, all information in this prospectus reflects and assumes (i) no sale of pre-funded warrants in this offering, andwhich, if sold, would reduce the repaymentnumber of $9.4 million dueshares that we are offering on a one-for-one basis (and the shares of common stock issuable upon exercise of the pre-funded warrants would be at an exercise price of $0.01 per share), (ii) no exercise of outstanding options issued under our short-term linesequity incentive plans and (iii) no exercise of credit.the accompanying warrants or the underwriter’s warrant listed above.
6
23
An investmentInvesting in our common stocksecurities involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information includedcontained in this prospectus, including the consolidatedour financial statements and the related notes appearing elsewhere inat the end of this prospectus, before making an investment decision. Ifyour decision to invest in our securities. We cannot assure you that any of the followingevents discussed in the risk factors below will not occur. These risks actually occurs,could have a material and adverse impact on our business, financial condition, results of operations, financial condition and cash flows, and our future prospects would likely be materially and adversely affected. InIf that event,were to happen, the markettrading price of our common stock could decline, and you could lose all or part of your investment. In addition, you should also carefully consider the other risks described under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2019 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, which are incorporated herein by reference, as updated by annual, quarterly and other reports and documents we file with the Securities and Exchange Commission after the date of this prospectus and that are incorporated by reference herein.
Risks Related to Our Business and Industrythis Offering
If our imaging systems and DIS services are not accepted by physicians or hospitals, we may be unable to develop a sustainable, profitable business.
We expect that substantially all of our revenue in the foreseeable future will be derived from sales of our products in the nuclear imaging market and our leasing services offered through our wholly owned subsidiary, Digirad Imaging Solutions, Inc., or DIS. Our solid-state gamma cameras and DIS services represent a new approach in the nuclear imaging market, and we have sold our products only in limited quantities and leased our services for only a limited time. We began full commercial release of our imaging systems in March 2000 and established DIS in September 2000. Because of the recent commercial introduction of our nuclear imaging systems, we have limited product and brand recognition and our imaging systems have been used by a limited number of physicians and hospitals. Physicians and hospitals may generally be slow to adopt our products and leasing services for a number of reasons, including:
Our net proceeds from this offering are expected to be approximately $4.44 million, assuming no exercise of reimbursement from health care payors for procedures using our system;
Our successmanagement at this juncture will arise in the nuclear imaging market depends on whether physicians and hospitals view our imaging systems and DIS services as effective and economically beneficial. We believe that physicians and hospitals will not adopt our imaging systems or lease our DIS services unless they determine, based on experience and other factors, that our imaging systems and DIS services are an attractive alternative to vacuum tube imaging systems. We also believe that recommendations and support of our products and services by influential physicians and other health care providers are essential for market acceptance and adoption. We cannot assure you that physicians or hospitals will adopt or accept our imaging systems or DIS services. If physicians and hospitals do not adopt our imaging systems or DIS services, our operating results and business will be harmed.
We sell our imaging systems and provide our services in a highly competitive industry, and we often compete against large, well-established competitors that have significantly greater financial resources than we have.
The medical device industry, including the market for imaging systems and services, is highly competitive, subject to rapid change and significantly affected by new product introductions and market activities of other industry participants. Our primary competitors with respect to imaging systems include several large medical device manufacturers, including Philips Medical Systems, General Electric Healthcare, Siemens Medical Systems and Toshiba Medical Systems. All of these competitors offer a full line of imaging cameras for each diagnostic imaging technology, including x-ray, magnetic resonance
7
imaging, computerized tomography, ultrasound and nuclear medicine. The existing imaging systems sold by our competitors have been in use for a longer time than our products and are more widely recognized and used by physicians and hospitals for nuclear imaging. Many of our competitors and potential competitors enjoy significant competitive advantages over us, including:
In providing comprehensive mobile nuclear imaging solutions, we generally compete against small businesses employing traditional vacuum tube cameras that must be transported in large vehicles and cannot be moved in and out of physician offices.
We are aware of certain major medical device companies that are attempting to develop solid-state cameras and we believe these efforts will continue. In addition, we are aware of a privately-held company, Gamma Medica, which is currently marketing a solid-state gamma camera for breast imaging. We do not believe that this camerafuture. There can be used in a cardiac application. However, we cannot assure youno assurance that Gamma Medicamanagement’s use of proceeds generated through this offering will not attemptprove optimal or translate into revenue or profitability for the Company. Investors are urged to modify its existing camera for useconsult with their attorneys, accountants and personal investment advisors prior to making any decision to invest in the cardiac segmentCompany.
Even if this offering is successful, we may need to raise additional capital in the future to continue operations, which may not be available on acceptable terms, or develop another gamma camera for cardiac applications. Because of the size of the potential market, we anticipate that companies will dedicate significant resourcesat all. Failure to developing competing products and services. Current or future competitorsobtain this necessary capital if needed may develop technologies and products that demonstrate better image quality, ease of use or mobility than our imaging systems. Our ability to compete successfully will dependhave an adverse impact on our ability to develop proprietary products that reach the market in a timely manner, receive adequate reimbursementbusiness and are less expensive than alternatives availableoperations.
While we had net loss of approximately $1.7 million for the same purpose. Ifthree months ended March 31, 2019, we are unable to compete effectively against our existing and future competitors our sales will declinehad a net loss of approximately $3.0 million for the three months ended March 31, 2020, and our business will be harmed.
Changesnet loss attributable to common stockholders was approximately $1.67 per share for the three months ended March 31, 2020. We may incur additional net losses in domesticthe future, and international legislation, regulation, or coveragewe may experience quarter-to-quarter fluctuations in revenues, expenses, and reimbursement policieslosses, some of third-party payors may adversely impact our ability to market and sell our products and services.
Physicians and hospitals purchasing and using our products rely on adequate third-party payor coverage and reimbursement to maintain their operations. Changes in domestic and international legislation, regulation or coverage and reimbursement policies of third-party payors may adversely affect the demand for our existing and future products and services and may limit our ability to market and sell our products and services on a profitable basis. For example, on December 8, 2003, President Bush signed into law the Medicare Prescription Drug, Improvement and Modernization Act of 2003 (the "Medicare Modernization Act"), which contains a wide variety of changes that impact Medicare reimbursement to physicians and hospitals. We cannot predict what additional changes will be made to such legislation, regulation, or coverage and reimbursement policies, but we believe that future coverage and reimbursement may be subject to increased restrictions bothsignificant.
Assuming all shares offered by this prospectus are sold, we estimate that we will receive net proceeds of approximately $4.44 million from the sale of common stock offered by us in the United States and in international markets. Additionally, we cannot be certain that under prospective payment systems, or established fee schedule payment formulas, under which healthcare providers may be reimbursed a fixed amountthis offering, based on the patient's condition or the typeassumed public offering price of procedure performed, the costs$2.37 per share (the last reported sale price of our productscommon stock on the Nasdaq Global Market on May 15, 2020), and services will be justifiedafter deducting the estimated underwriting fees and incorporated intoestimated offering expenses payable by us. In the overall payment for the procedure. Third-party payors continue to act to contain or reduce healthcare costs through various means, including the movement to managed care systems where healthcare providers contract to provide comprehensive healthcare for a fixed fee per patient. These continued efforts to reduce healthcare costs may result in third-party payors refusing to reimburse patients or healthcare providers for our imaging services or allowing only specific providers to provide imaging services. As a result, salesevent of our gamma cameras would suffer and we may receive
8
pressure from our customers to terminate or otherwise modify the lease arrangements for our DIS services. Under such circumstances, our business, financial condition and results of operations could be materially adversely affected.
Because our imaging systems and DIS services are not widely diversified, a decrease in salesthe net proceeds to us from this offering as a result of a decrease in the assumed public offering price per share or the number of shares offered by us, we may need to scale back or eliminate certain of our products and leasing services could seriously harm our business.
Our current product and leasing service offerings consist primarily of our line of gamma cameras, including our Cardius-1, Cardius-2, 2020tc Imager and SPECTpak PLUS camera systems, each of which is used in the nuclear imaging market segment and all of which utilize the same solid-state technology. In addition, we offer a mobile imaging leasing service through DIS, which includes an imaging system, certified personnel, required licensure and other support for nuclear imaging procedures. As such, our line of products and services is not as diversified as those of some of our competitors. Consequently, if sales of our products or leasing services decline precipitously, our business would be seriously harmed, and it would likely be difficult for us to recover because we do not have the breadth of products or services that would enable us to sustain our business while seeking to develop new types of products or services or other markets for our existing products and services. In addition, because our technical know-how and intellectual property have limited applications, we may be unable to leverage our technical know-how and intellectual property to diversify our products and servicesplans or to develop other products or sources of revenue outside of the nuclear imaging market.
Our imaging systems and DIS services may become obsolete, andraise additional capital sooner than we anticipate. However, we may not be able to timely develop new products, product enhancementsraise additional funds on acceptable terms, or at all. Conditions in the capital markets and the financial services industry may make equity and debt financing more difficult to obtain, and may negatively impact our ability to complete financing transactions. Any debt financing, if available, may involve restrictive covenants, such as limitations on our ability to incur additional indebtedness and other operating restrictions that could adversely impact our ability to conduct our business.
We face risks related to health pandemics and other widespread outbreaks of contagious disease, including the novel coronavirus, COVID-19, which could significantly disrupt our operations and impact our financial results.
Our business has been disrupted and could be materially adversely affected by the recent outbreak of COVID-19. The outbreak and government measures taken in response have also had a significant impact, both direct and indirect, on businesses and commerce. Global health concerns, such as coronavirus, could also result in social, economic, and labor instability in the countries in which we or the third parties with whom we engage operate. The future progression of the outbreak and its effects on our business and operations are uncertain. We cannot presently predict the scope and severity of any potential business shutdowns or disruptions, including downturns in global economies and financial markets that could affect our future operating results. Any adverse impact on our results and financial condition could have a negative impact on our ability to comply with certain financial covenants in certain of our loan agreements (as described further below) and on your investment in our common stock.
An investment in our securities is speculative and there can be no assurance of any return on any such investment.
An investment in our securities is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be accepted bysubject to substantial risks involved in an investment in the market.Company, including the risk of losing their entire investment.
Our nuclear imaging system
You may experience dilution if we issue additional equity or equity-linked securities in the future.
If we issue additional shares of common stock, or securities convertible into or exchangeable or exercisable for shares of common stock, our stockholders, including investors who purchase shares of common stock and DIS servicesaccompanying warrants in this offering, will experience dilution if we issue shares in the future at a price higher than the net tangible book value per share of our common stock, and any such issuances may become obsolete or unmarketable if other products or services utilizing new technologies are introduced byresult in downward pressure on the price of our competitors or new industry standards emerge.common stock. We also cannot assure you that we will be able to successfully developsell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. Furthermore, investors may experience further dilution to the extent that shares of our common stock are issued upon the exercise of the warrants and pre-funded warrants issued in this offering. If we issue additional equity securities, employee stock grants vest, or there are any issuances and subsequent exercises of stock options issued in the future, you will experience additional dilution. See the section entitled “Dilution” for a more detailed discussion of the net tangible book value per share of our common stock as impacted by this offering.
Resales of our common stock in the public market by our stockholders as a result of this offering may cause the market price of our common stock to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. The issuance of new shares of our common stock could result in resales of our common stock by our current stockholders concerned about the potential ownership dilution of their holdings. In turn, these resales could have the effect of depressing the market price for our common stock.
There is no public market for the warrants or pre-funded warrants being offered in this offering.
There is no established public trading market for the warrants or pre-funded warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants or pre-funded warrants on any securities exchange or nationally recognized trading system, including the Nasdaq Global Market. Without an active market, the liquidity of the warrants and the pre-funded warrants will be limited.
Holders of warrants and/or pre-funded warrants purchased in this offering will have no rights as stockholders of shares of common stock until such holders exercise their warrants and/or pre-funded warrants and acquire our shares of common stock, except as set forth in the warrants and/or pre-funded warrants.
Except as set forth in the warrants and pre-funded warrants, until holders of warrants and/or pre-funded warrants acquire our shares of common stock upon exercise of the warrants and/or pre-funded warrants, holders of warrants and/or pre-funded warrants have no rights with respect to our shares of common stock underlying such warrants and pre-funded warrants. Upon exercise of the warrants and/or pre-funded warrants, the holders will be entitled to exercise the rights of a stockholder of shares of common stock only as to matters for which the record date occurs after the exercise date.
The warrants and pre-funded warrants are speculative in nature.
The warrants and pre-funded warrants offered hereby do not confer any rights of share of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically, commencing on the date of issuance, (i) holders of the pre-funded warrants may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of $0.01 per share of common stock and (ii) holders of the warrants may exercise their right to acquire the common stock and pay an exercise price of $[●] per share (at least 100%, and up to 125%, of the public offering price per share of our common stock). Moreover, following this offering, the market value of the warrants and/or pre-funded warrants is uncertain and there can be no assurance that the market value of the warrants and/or pre-funded warrants will equal or exceed their public offering price. There can be no assurance that the market price of the shares of common stock will ever equal or exceed the exercise price of the warrants and/or pre-funded warrants, and consequently, whether it will ever be profitable for holders of the warrants and/or pre-funded warrants to exercise the warrants and/or pre-funded warrants.
There may be future sales of our securities or other dilution of our equity, which may adversely affect the market price of our common stock.
We are generally not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable for, or that represent the right to receive, common stock. The market price of our common stock could decline as a result of a future sale of common stock or securities that are convertible into or exchangeable for, or that represent the right to receive, common stock after this offering or the perception that such sales could occur. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution.
As a smaller reporting company, we are subject to scaled disclosure requirements that may make it more challenging for investors to analyze our results of operations and financial prospects.
Currently, we are a “smaller reporting company,” as defined by Rule 12b-2 of the Exchange Act. As a “smaller reporting company,” we are able to provide simplified executive compensation disclosures in our filings and have certain other decreased disclosure obligations in our filings with the SEC, including being required to provide only two years of audited financial statements in annual reports. Consequently, it may be more challenging for investors to analyze our results of operations and financial prospects.
Furthermore, we are a non-accelerated filer as defined by Rule 12b-2 of the Exchange Act, and, as such, are not required to provide an auditor attestation of management’s assessment of internal control over financial reporting, which is generally required for SEC reporting companies under Section 404(b) of the Sarbanes-Oxley Act. Because we are not required to, and have not, had our auditor’s provide an attestation of our management’s assessment of internal control over financial reporting, a material weakness in internal controls may remain undetected for a longer period.
If we cannot continue to satisfy the Nasdaq Capital Market continued listing standards and other Nasdaq rules, our common stock could be delisted, which would harm our business, the trading price of our common stock, our ability to raise additional capital and the liquidity of the market for our common stock.
Our common stock is currently listed on the Nasdaq Global Market. To maintain the listing of our common stock on the Nasdaq Global Market, we are required to meet certain listing requirements, including, among others, either: (i) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $5 million and stockholders’ equity of at least $10 million; or (ii) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $15 million and total assets of at least $50 million and total revenue of at least $50 million (in the latest fiscal year or in two of the last three fiscal years).
There is no assurance that we will be able to maintain compliance with the minimum closing price requirement and other listing requirements. In the event that we fail to maintain compliance with Nasdaq listing requirements for 30 consecutive trading days, our common stock may be delisted from the Nasdaq Global Market. If our common stock were to be delisted from Nasdaq and was not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, the price and trading volume of our securities could decline.
The trading market for our securities will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our securities would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our clinical trials and operating results fail to meet the expectations of analysts, the price of our securities would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause the price or trading volume of our securities to decline.
We may be limited in our ability to utilize, or may not be able to utilize, net operating loss carryforwards to reduce our future tax liability as a result of this offering.
As disclosed in our most recent Annual Report on Form 10-K, under Section 382 of the Code (as defined below) if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss (“NOL”) carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. This offering may, alone or in conjunction with other changes in our stock ownership that we cannot control, result in an “ownership change.” We may also experience ownership changes in the future as a result of strategic transactions or partnerships, equity offerings and other shifts in our stock ownership. As a result, if we earn net taxable income, our ability to use our pre-change NOL carryforwards and other deferred tax assets to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, similar limitations may apply at the state level and there may be periods during which the use of NOL carryforwards is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
If we become a personal holding company, our results could be negatively impacted.
We could be subject to a second level of U.S. federal income tax on a portion of our income if we are determined to be a personal holding company (“PHC”) for U.S. federal income tax purposes. A U.S. corporation generally will be classified as a PHC for U.S. federal income tax purposes in a given taxable year if (i) at any time during the last half of such taxable year, five or fewer individuals (without regard to their citizenship or residency and including as individuals for this purpose certain entities such as certain tax-exempt organizations, pension funds and charitable trusts) own or are deemed to own (pursuant to certain constructive ownership rules) more than 50% of the stock of the corporation by value (by reference to all common, preferred and all other classes of stock) (the “Ownership Test”) and (ii) at least 60% of the corporation’s adjusted ordinary gross income, as determined for U.S. federal income tax purposes, for such taxable year consists of PHC income (which includes, among other things, dividends, interest, certain royalties, annuities and, under certain circumstances, rents) (the “Income Test”).
Based on our knowledge, we believe we meet the Ownership Test discussed above. However, based on our present operations and income sources, we believe that we will not meet the Income Test, and as a result we should not be treated as a PHC for the foreseeable future. If we are or were to become a PHC in a given taxable year, we would be subject to an additional PHC tax, currently 20%, on our undistributed PHC income, which generally includes our taxable income (without the benefit of any federal net operating loss carry forward), subject to certain adjustments. We can give no assurance that we will not become a PHC in the future.
Risks Related to Our Business and Industry
Our HoldCo Conversion and related acquisitions or investments could involve unknown risks that could harm our business and adversely affect our financial condition.
We are in the process of becoming a diversified holding company with interests in a variety of industries and market sectors. The real estate acquisitions that we have made under our SRE real estate division and the pending and future acquisitions that we consummate will involve unknown risks, some of which will be particular to the industry in which the acquisition target operates. Although we intend to conduct extensive business, financial, and legal due diligence in connection with the evaluation of all our acquisition and investment opportunities, there can be no assurance our due diligence investigations will identify every matter that could have a material adverse effect on us. We may be unable to adequately address the financial, legal, and operational risks raised by such acquisitions or investments, especially if we are unfamiliar with the industry in which we invest. The realization of any unknown risks could prevent or limit us from realizing the projected benefits of the acquisitions or investments, which could adversely affect our financial condition and liquidity. In addition, our financial condition, results of operations and the ability to service our debt will be subject to the specific risks applicable to any company we acquire or in which we invest.
We rely on information technology in our operations, and any material failure, inadequacy, interruption or security failure of that technology could materially harm our business.
We rely on information technology and systems, including the Internet, commercially available software, and other applications, to process, transmit, store, and safeguard information and to manage or support a variety of our business processes, including financial transactions and maintenance of records, which may include personal identifying information and other valuable or confidential information. If we experience material failures, inadequacies, or interruptions or security failures of our information technology, we could incur material costs and losses. Further, third-party vendors could experience similar events with respect to their information technology and systems that impact the products and services they provide to us or enhancements to our existing products,customers. We rely on commercially available systems, software, tools, and monitoring, as well as other applications and internal procedures and personnel, to provide security for processing, transmitting, storing, and safeguarding confidential information such as personally identifiable information related to our employees and others, information regarding financial accounts, and information regarding customers and vendors. We take various actions, and we incur significant costs, to maintain and protect the operation and security of our information technology and systems, including the data maintained in those systems. However, it is possible that these measures will not prevent the systems’ improper functioning or that our future products and enhancements will be accepted by our current or potential customersa compromise in security, such as in the event of a cyberattack or the third-party payors who financially support manyimproper disclosure of information. Security breaches, computer viruses, attacks by hackers, online fraud schemes, and similar breaches can create significant system disruptions, shutdowns, fraudulent transfer of assets, or unauthorized disclosure of confidential information. For example, in April 2019, we became aware that we had been a victim of criminal fraud commonly referred to as “business email compromise fraud.” The incident involved the impersonation of an officer of the procedures performed withCompany and improper access to his email, resulting in the transfer by the Company of funds to a third-party account.
Despite any defensive measures we take to manage threats to our products.business, our risk and exposure to these matters remain heightened because of, among other things, the evolving nature of such threats in light of advances in computer capabilities, new discoveries in the field of cryptography, new and sophisticated methods used by criminals including phishing, social engineering, or other illicit acts, or other events or developments that we may be unable to anticipate or fail to adequately mitigate. Any failure to maintain the security, proper function and availability of these circumstances mayour information technology and systems, or certain third-party vendors’ failure to similarly protect their information technology and systems that are relevant to our operations, or to safeguard our business processes, assets, and information could result in financial losses, interrupt our operations, damage our reputation, cause us to lose customers, disruptbe in default of material contracts, and subject us to liability claims or regulatory penalties, any of which could materially and adversely affect us.
We may not be able to achieve the anticipated synergies and benefits from business acquisitions.
Part of our business operationsstrategy is to acquire businesses that we believe can complement or expand our current business activities, both financially and harm our product salesstrategically. On September 10, 2019, Digirad acquired ATRM and services. Toits subsidiaries, including KBS, EdgeBuilder and Glenbrook with these synergistic benefits in mind. Previously, Digirad acquired PRHC and its subsidiaries, (including DMS Heath) on January 1, 2016; MD Office on March 5, 2015; and Telerhythmics on March 13, 2014, which Digirad subsequently sold on October 31, 2018. Acquisitions involve many complexities, including, but not limited to, risks associated with the acquired business’ past activities, loss of customers, regulatory changes that are not anticipated, difficulties in integrating personnel and human resource programs, integrating ERP systems and other infrastructures, general underperformance of the business under Digirad control versus the prior owners, unanticipated expenses and liabilities, and the impact on its internal controls of compliance with the regulatory requirements under the Sarbanes-Oxley Act of 2002. There is no guarantee that its acquisitions will increase the profitability and cash flow of Digirad, and its efforts could cause unforeseen complexities and additional cash outflows, including financial losses. As a result, the realization of anticipated synergies or benefits from acquisitions may be delayed or substantially reduced, and could potentially result in the impairment of its investment in these businesses.
There can be no assurances that we will be successful weas a diversified holding company.
Part of Digirad’s strategy is to become a diversified holding company through the acquisition of businesses that, Digirad believes, will needrealize a material benefit from being part of a larger holding company structure, both financially and strategically. There can be no assurances that Digirad will find suitable acquisition targets that will enable Digirad to enhance our productssuccessfully realize its conversion into a diversified holding company, and even if such targets are identified, there can be no assurances that Digirad can negotiate and complete such acquisitions on attractive terms.
If Digirad is unable to make successful acquisitions, its ability to grow its business could be adversely affected and its conversion to a diversified holding company structure may not succeeds. If Digirad succeed in making suitable acquisitions, Digirad may not be able to obtain the expected profitability or servicesother benefits in the short or long term from such acquisitions.
Acquisitions, including the ATRM Acquisition, involve many complexities, including, but not limited to, risks associated with the acquired business’ past activities, loss of customers, regulatory changes that are not anticipated, difficulties in integrating personnel and to design, develophuman resource programs, integrating ERP systems and market new productsother infrastructures under Company control, unanticipated expenses and liabilities, and the impact on its internal controls of compliance with the regulatory requirements under the Sarbanes- Oxley Act of 2002. There is no guarantee that successfully respond to competitive developments, allits acquisitions will increase the profitability and cash flow of whichDigirad, and its efforts could cause unforeseen complexities and additional cash outflows, including financial losses. As a result, the realization of anticipated benefits from acquisitions may be expensivedelayed or substantially reduced. In addition, its leadership team’s attention may also be diverted by any historical or potential acquisitions.
We are subject to particular risks associated with real estate ownership, which could result in unanticipated losses or expenses.
Following our recent acquisition of real estate, our business is subject to many risks that are associated with the ownership of real estate. For example, if our tenants do not renew their leases or default on their leases, we may be unable to re-lease the facilities at favorable rental rates. Other risks that are associated with real estate acquisition and time consuming.ownership include, without limitation, the following:
| ● | general liability, property and casualty losses, some of which may be uninsured; |
| ● | the inability to purchase or sell our assets rapidly to respond to changing economic conditions, due to the illiquid nature of real estate and the real estate market; |
| ● | leases which are not renewed or are renewed at lower rental amounts at expiration; |
| ● | the default by a tenant or guarantor under any lease; |
| ● | costs relating to maintenance and repair of our facilities and the need to make expenditures due to changes in governmental regulations, including the Americans with Disabilities Act; |
| ● | environmental hazards created by prior owners or occupants, existing tenants, mortgagors or other persons for which we may be liable; |
| ● | acts of God affecting our properties; and |
| ● | acts of terrorism affecting our properties. |
Our revenues may decline due to reductions in Medicare and Medicaid reimbursement rates.
The success of any new product offering or enhancement to an existing product will depend on several factors, including our ability to:
If we do not develop and obtain regulatory approvals or clearances for new products, services or product enhancements in time to meet market demand, or if there is insufficient demand for these products, services or enhancements, our business financial condition and resultsis largely dependent upon our medical professional customers’ ability to provide diagnostic care to their patients in an economically sustainable manner, either through the purchase of operations will likely suffer. In addition, even if our customers acquire new products, services or product enhancements we may
9
offer, the revenues from any such products, services or enhancements may not be sufficient to offset the significant costs associated with offering such products, services or enhancements to customers. In addition, any announcements of new products, services or enhancements may cause customers to decline or cancel their purchasing decisions in anticipation of such products, services or enhancements.
If we experience problems with the technologies used in our imaging systems or if deliveryusing our diagnostic services, or both. Our customers are directly impacted by changes (decreases and increases) in governmental and private payor reimbursements for diagnostic services. We are directly and indirectly impacted by changes in reimbursements. In our businesses, where we are indirectly affected by reimbursement changes, we make every effort to act as business partners with our physician customers. For example, in 2010, we proactively adjusted our diagnostic imaging services rates down due to the dramatic reimbursement declines that our customers experienced from the Centers for Medicare & Medicaid Services. Reimbursements remain a source of concern for our customers and downward pressure on reimbursements causes greater pricing pressure on our services and influences the buying decisions of our DIScustomers. Although the gap is closing, hospital reimbursements remain higher than in-office reimbursements. Our Diagnostic Imaging segment’s products are targeted to serve the hospital market. A smaller portion of our Diagnostic Services business segment operates in the hospital market.
Reductions in reimbursements could significantly impact the viability of in-office imaging performed by independent physicians, as well as the viability of our cardiac event monitoring services business. The historical decline in reimbursements in diagnostic imaging has resulted in cancellations of imaging days in our Diagnostic Services business and the delay of purchase and service decisions by our existing and prospective customers in our Diagnostic Imaging business.
Our Diagnostic Services revenues may decline due to changes in diagnostic imaging regulations and the use of third party benefit managers by states and private payors to drive down diagnostic imaging volumes.
Nuclear medicine is a “designated health service” under the federal physician self-referral prohibition law known as the “Stark Law,” which states that a physician may not refer designated health services to an entity with which the physician or an immediate family member has a financial relationship, unless a statutory exception applies. Our business model and service agreements are delayed, public perceptionstructured to enable our physician customers to meet the statutory in-office ancillary services (“IOAS”) exception to the Stark Law, allowing them to perform nuclear diagnostic imaging services on their patients in the convenience of their own office. From time-to-time, the Centers for Medicare and Medicaid Services and Congress have proposed to modify the IOAS to further limit or eliminate this exception. Various lobbying organizations, including the Medicare Payment Advisory Commission (“MedPAC”), in the past have pushed for, discussed, and recommended that Congress limit the availability of the IOAS exception in order to reduce federal healthcare costs. Legislation has been introduced in prior Congresses to modify or eliminate the exception, but has not been enacted. The outcome of these efforts is uncertain at this time; however, the limitation or elimination of the IOAS exception could significantly impact our Diagnostic Services business segment as currently structured.
Our customers who perform imaging services in their office also experience the continuing efforts by some private insurance companies to reduce healthcare expenditures by hiring radiology benefit managers to help them manage and limit imaging. The federal government has also set aside monies in the 2009 recession recovery acts to hire radiology benefit managers to provide image management services to Medicare/Medicaid and MedPAC has recommended and the Centers for Medicare & Medicaid Services has, in the past, proposed legislation requiring Medicare physicians who engage in a relatively high volume of medical imaging be required to obtain pre-authorization through a radiology benefit manager. A radiology benefit manager is an unregulated entity that performs various functions for private payors and managed care organizations. Radiology benefit manager activities can include pre-authorization for imaging procedures, setting and enforcing standards, approving which contracted physicians can perform the services, such as requiring even the most experienced and highly qualified cardiologists to obtain additional board certifications, or interfering with the financial decision of the private practitioner by requiring them to own their own imaging system and not allowing them to lease the system. The radiology benefit managers often do not provide written documentation of their decisions or an appeals process, leaving leasing physicians unable to challenge their decisions with the carrier or the state insurance department. Unregulated radiology benefit manager activities have and could continue to adversely affect our physician customers’ ability to receive reimbursement, therefore impacting our customers’ decision to utilize our Diagnostic Services imaging services.
Manufacturing and providing service for our nuclear imaging cameras is highly dependent upon the availability of certain suppliers, thereby making us vulnerable to supply problems that could harm our business.
Our manufacturing process within Diagnostic Imaging, and our warranty and post-warranty camera support business, rely on a limited number of third parties to supply certain key components and manufacture our products. Alternative sources of production and supply may not be readily available or may take several months to scale-up and develop effective production processes. If a disruption in the availability of parts or in the operations of our suppliers were to occur, our ability to have gamma cameras built as well as our ability to provide support could be harmedmaterially adversely affected. In certain cases, we have developed backup plans and cause us to lose customershave alternative procedures should we experience a disruption. However, if these plans are unsuccessful or if we have a single source, delays in the production and revenue.
Our gamma cameras have only recently been introduced into the marketplace. Most of our cameras currently in use are less than three years old. We have experienced some reliability issues with a prior version of our detector heads. In July 2003, we began selling mostsupport of our gamma cameras withfor an extended period of time could cause a new versionloss of revenue and/or higher production and support costs, which could significantly harm our business and results of operations.
Our Diagnostic Services and portions of our detector heads that we believe offers increased reliability. In addition, as the period of use of our cameras increases, other significant defects may occur. If significant defects do arise with our gamma cameras, our reputation among physicians and hospitals could be damaged.
Additionally, physicians rely on our DIS services to provide nuclear imaging procedures to their patients on the dates and at the times they have requested. Many factors could prevent us from delivering our DIS services on a timely basis, including weather and the availability of staffing, transportation and necessary supplies. If we are unable to provide physicians or hospitals our DIS services in a timely and effective manner, our reputation among physicians and hospitals could be damaged.
The performance and reliability of our products and services are critical to our reputation and to our ability to achieve market acceptance of those products and services. Widespread or other failures of our cameras and other products to consistently meet the expectations of purchasers or customers that use our DIS services could adversely affect our reputation, our ability to provide our DIS services, our relations with current customers and our business operations. Such failures could also reduce the attractiveness of our products and services to potential customers. Equipment failures could result from any number of causes, including equipment aging, ordinary wear and tear due to regular transportation and relocation, failure to perform routine maintenance and latent hardware or software defects of which we are unaware. Such failures, whether actual or perceived, could adversely affect our business even if we correct the underlying problems.
Our manufacturingMobile Healthcare operations are highly dependent upon third-party suppliers,the availability of certain radiopharmaceuticals, thereby making us vulnerable to supply problems and price fluctuations whichthat could harm our business.
We rely on
Both our Diagnostic Service business and portions of our Mobile Healthcare business involve the use of radiopharmaceuticals. There is a limited number of third partiesmajor nuclear reactors supplying medical radiopharmaceuticals worldwide and there is no guarantee that the reactors will remain in good repair or that our supplier will have continuing access to manufacture andample supply certain of the key components of our products. While many of the components used in our products are available from multiple sources, we obtain some components from single sources. For example, key components of the detector heads and the acquisition and control software utilized in our gamma cameras are manufactured or supplied by a single source. To be successful, our contract manufacturers and suppliers must provide us with the components of our systems in requisite quantities, in compliance with regulatory requirements, in accordance with agreed-upon specifications, at acceptable cost and on a timely basis. Segami Corporation, or Segami, has developed image acquisition and processing software for our camera under a non-exclusive license agreement. In the event that Segami attempts to terminate the license agreement, refuses to extend the term of the license or seeks to impose unreasonable pricing or terms, we would have to find an alternative software system to use in our gamma camera. Our reliance on these outside suppliers subjects us to a number of risks that could harm our business, including:
10
Any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competitive procedures. These events could harm our business and operating results.
We have limited marketing, sales and distribution capabilities, and our efforts in those areas are dependent in part on third parties.
We began commercial production and shipped our first imaging products in 2000, and therefore have limited experience in marketing, selling and distributing our products and services. Additionally, while we have a direct sales team focused on domestic marketing, sales and distribution, we also use four independent distributors in the United States and two independent, international sales distributors to market, sell and distribute our products and services. As a result, we are dependent in part upon the marketing, sales and distribution efforts of our third-party distributors. To date, one of our domestic third-party distributors is permitted to market, sell and distribute competing imaging services and products. Additionally, one of our domestic third-party distributors, as well as one of our international distributors, is generally permitted to market, sell and distribute competing imaging products that are used or refurbished and meet specified age requirements. Our other international distributor is prohibited from promoting or distributing any other gamma camera product, but is not prohibited from offering competing services.
Our future revenue growth will depend in large part on our success in maintaining and expanding our marketing, sales and distribution channels, which will likely be an expensive and time-consuming process. We are highly dependent upon the efforts of our sales force and third-party distributors to increase our revenue. We face intense competition for qualified sales employees and may be unable to hire, train, manage and retain such personnel, which could adversely affect our ability to maintain and expand our marketing, sales and distribution network, which would negatively affect our ability to compete effectively as a distributor of nuclear imaging devices. Additionally, even if we are able to expand our sales force and enter into agreements with additional third-party distributors on commercially reasonable terms, they may not commit the necessary resources to effectively market, sell and distribute our products and services domestically and internationally.radiopharmaceutical product. If we are unable to maintain and expand our direct and third-party marketing, sales and distribution networks,obtain an adequate supply of the necessary radiopharmaceuticals, we may be unable to sell enoughutilize our personnel and equipment through our in-office service operations, or the volume of our productsservices could decline and imaging services for our business tomay be profitable and our financial condition and results of operations will likely suffer accordingly.
If we fail to meet the demand for our imaging systems and DIS services as we transition manufacturing operations to a single facility, we may experience a decrease in sales.
We are currently in the process of transitioning our manufacturing operations from several separate facilities to a single facility. We must complete construction of and equip our new facility and obtain U.S.
11
Food and Drug Administration, or FDA, and California Food and Drug Branch approval prior to manufacturing our imaging systems at such facility. The completion of this process could be delayed by unforeseen circumstances, including:
As we scale-up operations at our new facility, we mayadversely affected. Shortages can also experience difficulties in producing sufficient quantities or quality of products or in achieving sufficient quality and manufacturing yield levels. If we are unable to commence manufacturing at our new facility on schedule or otherwise fail to meet our manufacturing needs, wecause price increases that may not be ableaccounted for in third party reimbursement rates, thereby causing us to providelose margin or require us to pass increases on to our customers with the quantity or quality of products they require, and we could lose customers and suffer reduced revenues.physician customers.
We are subject tocompete against businesses that have greater resources and different competitive strengths.
The market for mobile diagnostic services and diagnostic imaging systems is limited and has experienced some declines in the past. Some of our competitors have greater resources and a more diverse product offering than we do. Some of our competitors also enjoy significant advantages over us, including greater brand recognition, greater financial risks associatedand technical resources, established relationships with providing services through our DIS business.
There are numerous risks associated with any leasing arrangement, including the possibility that physicians may fail to make the required payments under the termshealthcare professionals, larger distribution networks, and provisions of their lease commitments. Our DIS business is also affected by the ability of physicians to pay us, which in turn may be affected by general economicgreater resources for product development and business conditionscapital expenditures, as well as more extensive marketing and the availability of reimbursement for the physicians. Such circumstances could adversely affect our business and financial condition.
sales resources. If we are unable to expand our DIS business,current market share, our business could be materially harmed.
We plan to grow our DIS business by expanding into several new states, adding new hub locations in states in which we currently operaterevenues and increasing hub utilization by adding physician customers and routes. As we undertake this expansion, we will need to hire, train and retain qualified personnel. We cannot assure you that physicians or hospitals in these new markets will accept our imaging products or services. Our expansion into additional domestic markets is subject to inherent risk, including the burden of complying with applicable state regulations, including but not limited to regulations concerning the use, storage, handling and disposal of radioactive materials, the difficulties in obtaining the necessary radioactive licensures and difficulties in staffing and managing operations. Further, certain states have regulations that will not allow the conduct of our DIS business as it is currently structured or at all.
A loss of key executives or failure to attract qualified managers, engineers and imaging technologists could limit our growth and adversely affect our business.
Our success is dependent on the efforts of our key technical, sales and managerial personnel and our ability to retain them, particularly David M. Sheehan, Paul J. Early, Herb Bellucci, Todd P. Clyde, Richard Conwell and Vera P. Pardee. The loss of any one or more of these individuals could place a significant strain on our remaining management team and we may have difficulty replacing any of these individuals. Furthermore, our future growth will depend in part upon our ability to identify, hire and retain additional key personnel, including nuclear imaging technologists, or CNMTs, paramedics, nurses, radiation safety officers, engineers, management, sales personnel and other highly skilled personnel. Hiring qualified management and technical personnel will be difficult due to the limited number of qualified candidates. Competition for these types of employees, particularly CNMTs and engineers, is intense in the medical imaging field. Given the competition for such qualified personnel, we cannot assure you that we will be able to continue to attract, hire and retain the personnel necessary to maintain and develop our business. Failure to attract, hire and retain key personnel could have an adverse effect on our business,related financial condition and results of operations. We do not have any employment agreements with, or key person insurance on, any of our employees.
12
If we choose to acquire new or complementary businesses, products or technologies instead of developing them ourselves, we may be unable to complete those acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner.could decline.
Our success depends on our ability to continually enhance and broaden our product and service offerings in response to changing customer demands, competitive pressures and technologies. While we have no current plans or commitments regarding any acquisitions of new or complementary businesses, products or technologies, we may in the future choose to pursue such acquisitions instead of developing those businesses, products or technologies ourselves. We cannot assure you, however, that we would be able to successfully complete any acquisition we choose to pursue, or that we would be able to successfully integrate any acquired business, product or technology into our company in a cost-effective and non-disruptive manner. Furthermore, there is no certainty that we would be able to attract, hire or retain key employees associated with any acquired businesses, products or technologies.
Integrating any acquired businesses, products or technologies could be expensive and time consuming, disrupt our ongoing business and divert the attention and resources of our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business will likely suffer. Additionally, any amortization of assets or charges resulting from the costs of acquisitions could harm our business and operating results.
We will face additional risks as we expand into international markets.
We have sales distributors for our imaging systems in Canada and Russia and are beginning to build an international sales organization. As we expand internationally, we will need to hire, train and retain qualified personnel in countries where language, cultural or regulatory impediments may exist. We cannot assure you that distributors, physicians or other involved parties in foreign markets will accept our nuclear imaging products, services and business practices. Our international operations will be subject to inherent risks, including:
Our manufacturing operations and executive offices will be located at a single facility that may be at risk from fire, earthquakes or other natural or man-made disasters or crises.
Our manufacturing operations and executive offices will soon be located at a single facility in Poway, California, near known fire areas and earthquake fault zones. This facility is located a short distance from the recent wildfires that destroyed many homes and businesses in San Diego County, California. We have taken precautions to safeguard our facilities, including insurance and health and safety protocols.
13
However, any future natural disaster, such as a fire or an earthquake, could cause substantial delays in our operations, damage to or destroy our manufacturing equipment or inventory, and cause us to incur additional expenses. A disaster could significantly harm our business and results of operations. The insurance we maintain against fires, earthquakes and other natural disasters may not be adequate to cover our losses in any particular case.
Additionally, electrical power is vital to our operations and we rely on a continuous power supply to conduct our business. California has experienced significant electrical power shortages and price volatility in recent years, and such shortages and price volatility may occur in the future. In the event of an acute power shortage, the California system operator has on some occasions implemented, and may in the future implement, rolling blackouts throughout California. If our energy costs substantially increase or blackouts interrupt our power supply frequently or for more than a few days, we may have to reduce or temporarily discontinue our normal operations. In addition, the cost of our research and development efforts may increase because of the disruption to our operations. Any such reduction or disruption of our operations at our facilities could harm our business.
We are exposed to risks relating to product liability, product recalls, property damage and personal injury for which insurance coverage is expensive, limited and potentially inadequate, and our business may be impacted by increased insurance costs.
Our operations entail a number of risks, including risks relating to product liability claims, product recalls, property damage and personal injury. We currently maintain insurance that we believe is adequate with respect to the nature of the risks insured against, including product liability insurance, professional liability insurance, automobile insurance, property insurance, workers compensation insurance and general liability insurance. In many cases such insurance is expensive and difficult to obtain, and no assurance can be given that we will be able to maintain our current insurance or that we will be able to obtain or maintain comparable or additional insurance in the future on reasonable terms, if at all. Additionally, we may be negatively affected by increased costs of insurance, including workers compensation insurance. For example, in October 2003, the Governor of California signed a bill which, if it takes effect, will require California businesses with 50 or more employees either to pay at least 80% of the premiums for a basic individual health insurance package for each of its employees and their families, or to pay a fee into a state pool for the purchase of health insurance for uninsured, low income workers.
Risks Related to Our Financial Results and Need for Financing
We have incurred significant and recurring operating losses since our inception in 1985 and we expect to incur increased operating expenses in the near term.
We have incurred significant net losses since our inception in November 1985, including losses of approximately $19.9 million in 2001, $12.8 million in 2002 and $1.7 million in 2003. As of December 31, 2003 we had an accumulated deficit of $80.2 million. We expect to incur increased operating expenses in the near term as we, among other things:
As a result of these activities, we may not be able to achieve profitability. If our revenue grows more slowly than anticipated, or if our operating expenses exceed our expectations, our ability to achieve our development and expansion goals would be adversely affected.
14
Our quarterly and annual financial results are difficult to predict and are likely to fluctuate significantly from period to period becauseperiod.
We have historically experienced seasonality in all of our business prospects are uncertain andbusinesses, volatility due to the seasonalitychanging healthcare environment, the variable supply of our DIS leasing services business.
Our revenueradiopharmaceuticals, and results of operations at any given time will be primarilydownturns based on the following factors, many of which we cannot control:
Additionally, we have experienced seasonality in the leasing services offered by DIS.changing U.S. economy. While our physicianscustomers are typically obligated to pay us for all leaseimaging days to which they have committed, our contracts permit some flexibility in scheduling when services are to be performed. This accounts for some of the seasonality of our DIS revenues. For example, our daily services have typically declined from our second fiscal quarter to our third fiscal quarter due to summer holidays and vacation schedules. We have also experienced declining daily services in December due to holidays and in our first quarter due to weather conditions in certain parts of the United States. We cannot predict with certainty the degree to which seasonal circumstances such as the summer slowdown, winter holiday variationsvacations, and weather conditions may makeaffect the results of our revenue unpredictable or lead tooperations. We have also experienced fluctuations in demand of our diagnostic imaging product sales due to economic conditions, capital budget availability, and other financial or business reasons. In addition, due to the way that customers in our target markets acquire our products, a large percentage of our products are booked during the last month of each quarterly operating resultsaccounting period, and often there can be a large amount in the future.
For these reasons, we believe that quarterly sales and operating resultslast month of the year. As such, a delivery delay of only a few days may vary significantly in the future and that period-to-periodimpact quarter-to-quarter comparisons of our results of operations are not necessarily meaningful and should not be relied upon as indicators of future performance. We cannot assure you that ouroperations. Moreover, the sales will increase or be sustained in future periods. Accordingly, we may experience significant, unanticipated quarterly losses. Because of these and other factors, our operating results in one or more future quarters may fail to meet the expectations of securities analysts or investors, which could cause our stock price to decline significantly.
Our reliance on a limited number of customers may cause our sales to be volatile.
We currently have a small number of customers, whom we typically bill after the delivery of our products and imaging services. If orders for our gamma cameras were to be cancelled, or our leasing service customers stopped using us or do not renew their lease agreements with us, our business would be adversely affected. Furthermore, in view of our small customer base, our failure to gain additional customers, the loss of any current customers or a significant reduction in the level of leasing services
15
provided to any one customer could disrupt our business, harm our reputation and adversely affect our sales.
The sales cycle for our gamma cameras is typically lengthy, which may result in significant fluctuations in our revenue.
Our sales efforts for our gamma cameras are dependent on the capital expenditures budgets of the physicians and hospitals to which we market. Often physicians and hospitals require a significant amount of lead time to plan for a major acquisition such as the purchase of our imaging systems. We may spend substantial time, effort and expense long before we actually consummate a sale of our cameras and with no assurance that we will ultimately be successful in achieving any such sales. As a result, we may experience significant fluctuations in our revenues. Furthermore, evaluating and predicting our future sales and operating performance is difficult and may not be as accurate as it could be if we had shorter sales cycles.
Our future capital needs are uncertain and we may need to raise additional funds in the future, and such funds may not be available on acceptable terms, if at all.
We believe that the net proceeds from this offering, together with our current cash, cash equivalents and short-term investments, will be sufficient to meet our projected operating requirements for at least the next 12 months. Our capital requirements will depend on many factors, including:
As a result of these factors, we may need to raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms, if at all. Furthermore, if we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
16
Risks Related to Government Regulation
We must be licensed to handle and use hazardous materials and may be liable for contamination or other harm caused by hazardous materials that we use.
We use hazardous and radioactive materials in our research and development and manufacturing processes, as well as in the provision of our imaging services. We are subject to federal, state and local regulations governing use, storage, handling and disposal of these materials and waste products. We are currently licensed to handle such materials in all states in which we operate, but there can be no assurances that we will be able to retain those licenses in the future. In addition, we must become licensed in all states in which we plan to expand. Obtaining those additional licenses is an expensive and time consuming process, and in some cases we may not be able to obtain those licenses at all.
Although we believe that our procedures for use, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from hazardous materials and we may incur liability as a result of any such contamination or injury. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources and any applicable insurance.
We have also incurred and may continue to incur expenses related to compliance with environmental laws. Such future expenses or liability could have a significant negative impact on our business, financial condition and results of operations. Further, we cannot assure you that the cost of complying with these laws and regulations will not materially increase in the future.
Compliance with extensive product regulations could be expensive and time consuming, and any failure to comply with those regulations could harm our ability to sell and market our products and imaging services.
U.S. and foreign regulatory agencies, including the FDA, govern the testing, marketing and registration of new medical devices or modifications to medical devices, in addition to regulating manufacturing practices, reporting, labeling and recordkeeping procedures. The regulatory process makes it longer, harder and more costly to bring our products to market, and we cannot assure you that any of our future products will be approved. All of our planned services, products and manufacturing activities, as well as the manufacturing activities of third-party medical device manufacturers who supply components to us, are subject to these regulations. Generally, we and our third-party manufacturers are or will be required to:
Compliance with the regulations of those agencies may delay or prevent us from introducing new or improvedcapital products which could in turn affect our ability to achieve or maintain profitability. We may be subject to sanctions, including monetary fines and criminal penalties, the temporary or permanent suspension of operations, product recalls and marketing restrictions, if we fail to comply with the laws and regulations applicable to our business. Our third-party component manufacturers may also be subject to the same sanctions and, as a result, may be unable to supply components for our products. Any failure to retain governmental approvals that we currently hold or obtain additional similar approvals could prevent us from successfully marketing our products and technology and could harm our operating results. Furthermore, changesis typically lengthy, particularly in the applicable governmental regulations could prevent further commercialization of our products and technologies and could harm our business.
Even if regulatory approval or clearance of a product is granted, regulatory agencies could impose limitations on uses forhospital market, which the product may be labeled and promoted. Further, for a marketed product,
17
its manufacturer and manufacturing facilities are subject to periodic review and inspection. Later discovery of problems with a product, manufacturer or facility may result in restrictions on the product, manufacturer or facility, including withdrawal of the product from the market or other enforcement actions.
Our products are subject to reporting requirements and recalls even after receiving FDA clearance or approval, which could harm our reputation, business and financial results.
We are subject to medical device reporting regulations that requirecause us to report to FDA or similar governmental bodies in other countries if our products cause or contribute to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. In addition, the FDA and similar governmental bodies in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall would divert management attention and financial resources and harm our reputation with customers. A recall involving our product could harm the reputation of the product and our company and would be particularly harmful to our business and financial results.experience significant revenue fluctuations.
If we fail to obtain, or are significantly delayed in obtaining, FDA clearances or approvals for future products or product enhancements, or if we fail to comply with FDA's Quality System Regulation, our ability to commercially market and distribute our products will suffer.
Our products are subject to rigorous regulation by the FDA, and numerous other federal, state and foreign governmental authorities. In the U.S., the FDA regulates virtually all aspects of a medical device's testing, manufacture, safety, labeling, storage, recordkeeping, reporting, promotion and distribution. Our failure to comply with those regulations could lead to the imposition of administrative or judicial sanctions, including injunctions, suspensions or the loss of regulatory approvals, product recalls, termination of distribution, or product seizures. In the most egregious cases, criminal sanctions or closure of our manufacturing facilities are possible. The process of obtaining regulatory approvals to market a medical device, particularly from the FDA, can be costly and time consuming, and there can be no assurance that such approvals will be granted on a timely basis, if at all. In particular unless exempt, the FDA permits commercial distribution of a new medical device only after the device has received 510(k) clearance or is the subject of an approved Premarket Approval Application, or PMA. The FDA will clear marketing of a medical device through the 510(k) process if it is demonstrated that the new product is substantially equivalent to other 510(k)-cleared products. The PMA approval process is more costly, lengthy and uncertain than the 510(k) clearance process, and must be supported by extensive data, including data from preclinical studies and human clinical trials. Because we cannot assure you that any new products we develop, or any product enhancements, will be subject to the shorter 510(k) clearance process, significant delays in the introduction of any new products or product enhancements may occur. There is no assurance that the FDA will not require a new product or product enhancement go through the lengthy and expensive PMA approval process. Further, pursuant to FDA regulations, we can only market our products for approved uses. If our products are used for purposes other than those approved by the FDA, the FDA could object to such off-label uses.
Our manufacturing processes and those of our third-party manufacturers are required to comply with the FDA's Quality System Regulation, or QSR, which covers the design, testing, production processes, controls, quality assurance, labeling, packaging, storage and shipping of our devices. In addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facility and records for periodic unscheduled inspections by federal, state and foreign agencies, including the FDA. Our or our third-party manufacturers' failure to pass a QSR inspection or to comply with these and other applicable regulatory requirements could result in disruption of our operations and manufacturing delays,
18
and a failure to take adequate corrective action could result in, among other things, withdrawal of our medical device clearances, seizure or recall of our devices, or other civil or criminal enforcement actions.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we now or in the future market and sell our products in foreign countries, we may be subject to rigorous regulation by those foreign governmental authorities. In such circumstances, we would rely significantly on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our products in foreign countries.
Modifications to our products may require new 510(k) clearances or premarket approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design, or manufacture, requires a new 510(k) clearance or, possibly, approval of a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer's decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or PMA for modification of a previously cleared product for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective. Any recall or FDA requirement that we seek additional approvals or clearances could result in delays, fines, costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
We will spend considerable time and money complying with federal and state laws, regulations, and foreign regulationsother rules, and if we are unable to fully comply with such laws, regulations, and other rules, we could face substantial penalties.
We are directly, or indirectly through our clients,customers, subject to extensive regulation by both the federal government and the states and foreign countries in which we conduct our business. The laws that directly or indirectly affect our ability to operate our business, include, but are not limited to, the following:
19
We maintain a compliance program to identify and the Stark Law, which may notcorrect any compliance issues and remain in compliance with all applicable laws, to train employees, to audit and monitor our operations, and to achieve other compliance goals. Like most companies with compliance programs, we occasionally discover compliance concerns. In such cases, we take responsive action, including corrective measures when necessary. There can be limited to government reimbursed itemsno assurance that our responsive actions will insulate us from liability associated with any detected compliance concerns.
If our past or services; and
If ourpresent operations are found to be in violation of any of the laws, regulations, rules, or policies described above or the other governmentallaws or regulations to which we or our customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaidfederal or state healthcare programs, andor the curtailment or restructuring of our operations. Similarly, if our physician customers are found to be non-compliant with applicable laws, they may be subject to sanctions whichthat could also have a negative impact on us. In addition, if we are required to obtain permits or licensure under these laws that we do not already possess, we may become subject to substantial additional regulation or incur significant expense. Any penalties, damages, fines, curtailment, or restructuring of our operations wouldcould adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations, and additional legal or regulatory change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management'smanagement’s attention from the operation of our business, and damage our reputation. ForAlthough compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, regulations, rules, and policies, the risks cannot be entirely eliminated. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security, and fraud laws may prove costly.
Healthcare policy changes could have a more detailed discussion of the various state and federal regulationsmaterial adverse effect on our business.
In response to which we are subject see "Business—Government Regulations."
Legislative or regulatory reform of theperceived increases in healthcare system may affect our ability to sell our products profitably.
In both the United States and certain other foreign jurisdictions,costs in recent years, there have been and continue to be proposals by the federal government, state governments, regulators, and third-party payers to control these costs and, more generally, to reform the U.S. healthcare system. Certain of these proposals could limit the prices we are able to charge for our products or the amounts of reimbursement available for our products, and could limit the acceptance and availability of our products. The adoption of some or all of these proposals could have a numbermaterial adverse effect on our financial position and results of legislative and regulatory proposals to change the healthcare system in ways thatoperations.
Any intrusions or attacks on our information technology infrastructure could impact our ability to conduct operations and could subject us to fines, penalties, and lawsuits related to healthcare privacy laws.
The operation of our business includes use of complex information technology infrastructures, access to the information technology networks of our customers, as well as the collection of storing of patient information that is subject to HIPAA. In recent years, attacks on corporate information technology infrastructures have become more common and more sophisticated. Attacks can range from attempts that are routinely blocked by security and related infrastructure, to intrusions that disrupt activity temporarily, to extensive intrusions that severely impact or disable a network, including ransomware that holds a network hostage until the impacted company pays a fee to the attacker. Further, attacks can specifically impact patient information stored on such networks, requiring a widespread notice to the affected population, which can be very costly. Any successful attack on our network could severely impact our ability to conduct operations and could result in lost customers. Though we carry customary insurance for notification events in the event of a patient information breach under HIPAA, our coverage may not be sufficient to cover every situation, and any notification could severely impact our customer confidence and operations.
We are subject to risks associated with self-insurance related to health benefits.
To help control our overall long-term costs associated with employee health benefits, we are self-insured up to certain limits for our health plans. As such, we are subject to risks associated with self-insurance of these health plan benefits. To limit our exposure, we have third party stop-loss insurance coverage for both individual and aggregate claim costs. However, we could still experience unforeseen and potentially significant fluctuations in our healthcare costs based on a higher than expected volume of claims below these stop-loss levels. These fluctuations could have a material adverse effect on our financial position and results of operations.
A portion of our operations are located in a facility that may be at risk from fire, earthquakes, or other disasters.
Final assembly in our manufacturing process and significant portions of our inventory are located in a single facility in Poway, California, near known fire areas and earthquake fault zones. Future natural disasters could cause substantial delays in our operations and cause us to incur additional expenses. Although we have taken precautions to insure our facilities and continuing operations, as well as provide for offsite back-up of our information systems, this may not be adequate to cover our losses in any particular case. A disaster could significantly harm our business and results of operations.
The medical device industry is litigious, which could result in the diversion of our management’s time and efforts, and require us to incur expenses and pay damages that may not be covered by our insurance.
Our operations entail risks of claims or litigation relating to product liability, radioactive contamination, patent infringement, trade secret disclosure, warranty claims, vendor disputes, product recalls, property damage, misdiagnosis, breach of contract, personal injury, and death. Any litigation or claims against us, or claims we bring against others, may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of our management from our core business, and harm our reputation. We may incur significant liability in the event of any such litigation, regardless of the merit of the action. If we are unable to obtain insurance, or if our insurance is inadequate to cover claims, our cash reserves and other assets could be negatively impacted. Additionally, costs associated with maintaining our insurance could become prohibitively expensive, and our ability to become or remain profitable could be diminished.
If we cannot provide quality technical and applications support, we could lose customers and our business and prospects will suffer.
The placement of our products and the introduction of our technology at new customer sites requires the services of highly trained technical support personnel. Hiring technical support personnel is very competitive in our industry due to the limited number of people available with the necessary scientific and technical backgrounds and ability to understand our technology at a technical level. To effectively support potential new customers and the expanding needs of current customers, we will need to expand our technical support staff. If we are unable to attract, train or retain the number of highly qualified technical services personnel that our business needs, our business and prospects will suffer.
Our long-term results depend upon our ability to improve existing products and services and introduce and market new products and services successfully.
Our business is dependent on the continued improvement of our existing products and services and our development of new products and services utilizing our current or other potential future technology. As we introduce new products and services or refine, improve or upgrade versions of existing products and services, we cannot predict the level of market acceptance or the amount of market share these products and services will achieve, if any. We cannot assure you that we will not experience material delays in the introduction of new products or services in the future.
We generally sell our products and services profitably. In the United States, federalin industries that are characterized by rapid technological changes, frequent new product introductions and state lawmakers regularly proposechanging industry standards. If we do not develop new products and at times, enact new legislation establishing significant changes in the healthcare system. Recently, President Bush signed into law the Medicare Modernization Act, which contains a wide variety of reforms that impact Medicare reimbursements to hospitalsservices and physicians including changes to Medicare payment methodologies for radiopharmaceuticals and other drugs dispensed by hospital
20
outpatient departments and for drugs dispensed by physician offices and independent diagnostic testing facilities. These changes reduced payment amounts for some of the drugs used in conjunction with our imaging procedures, although the physician fee schedule payment rates applicable to nuclear cardiology increased slightly. Downward changes to Medicare reimbursement rates may adversely impact reimbursement to customers or potential customers that use or could use our cameras and services. We cannot predict the full impact that this new legislation will have nor whether new federal legislation will be enacted in the future. The potential for adoption of healthcare reform proposalsproduct enhancements based on technological innovation on a state-by-statetimely basis, could require us to develop state-specific marketing and sales approaches. In addition, we may experience pricing pressures in connection with the sale of our products and services due to additional legislative proposals or healthcare reform initiatives. Our results of operationsmay become obsolete over time and our businessrevenues, cash flow, profitability and competitive position may suffer. Our success will depend on several factors, including our ability to:
| ● | correctly identify customer needs and preferences and predict future needs and preferences; |
| ● | allocate our research and development funding to products and services with higher growth prospects; |
| ● | anticipate and respond to our competitors’ development of new products, services, and technological innovations; |
| ● | innovate and develop new technologies and applications, and acquire or obtain rights to third-party technologies that may have valuable applications in the markets we serve; |
| ● | recruit, train, retain, motivate, and integrate key personnel, including our research and development, manufacturing, and sales and marketing personnel; and |
| ● | successfully commercialize new technologies in a timely manner, price them competitively and manufacture and deliver sufficient volumes of new products of appropriate quality on time. |
Even if we successfully innovate and develop new products, services and product enhancements, we may incur substantial costs in doing so, and our profitability may suffer.
If we do not successfully manage the development and launch of new products and services, our financial results could therefore be adversely affected by future healthcare reforms.affected.
The impact
We may face risks associated with launching new products and services. If we encounter development or manufacturing challenges or discover errors during our product development cycle, the product launch dates of regulatory changes could have a negative impact on camera sales to and leases with hospitals desiring to use our camerasnew products and services in their outpatient facilities.may be delayed. The expenses or losses associated with unsuccessful product development or launch activities or lack of market acceptance of our new products and services could adversely affect our business or financial condition.
In order for hospitals to receive certain payments for their outpatient facilities as hospital outpatient services, including services that utilize
Undetected errors or defects in our products these services must be furnished in a "provider- based" organizationcould harm our reputation or facilitydecrease market acceptance of our products.
Our products may contain undetected errors or be covered services furnished "under arrangement"defects when first introduced or as new versions or new products are released. Disruptions affecting the introduction or release of, or other performance problems with, the hospital. Failure to meet these requirements may result in reduced payments to the hospitals for their services. The Medicare program has published and revised rules establishing criteria for classifying a facility as "provider-based" or a service as furnished "under arrangement." These rates require an analysis of the facts and circumstances surrounding the delivery by a hospital of a particular service, and hospitals that use our products may damage our customers’ businesses and could harm their and our reputation. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted, or DIS servicesother significant customer relations problems may arise. We may also be subject to warranty and liability claims for damages related to errors or defects in their outpatient facilities will needour products. In addition, if we do not meet industry or quality standards, if applicable, our products may be subject to determine if they meet the applicable "provider-based"recall. A material liability claim, recall, or "under arrangement" requirements. Hospitalsother occurrence that cannot obtain sufficient payments for these services may not purchase a camera from usharms our reputation or enter into arrangements with us for provisiondecreases market acceptance of services.
The application of state certificate of need regulationsour products could harm our business and financial results.
Some states currently require, or may require in the future, a certificate of need or similar regulatory approval prior to the acquisition of high-cost capital items, including diagnostic imaging systems, or provision of diagnostic imaging services by us or our clients. In many cases, a limited number of these certificates are available in a given state. If we or our clients are unable to obtain the applicable certificate or approval or additional certificates or approvals necessary to expand our operations, these regulations may limit or preclude our operations in the relevant jurisdictions.
If we fail to comply with various licensure, or certification standards, we may be subject to loss of licensure or certification, which would adversely affect our operations.
All of the states in which we operate require that the imaging technicians that operate our cameras be licensed or certified. Obtaining such licenses may take significant time as we expand into additional states. Further, we are currently enrolled by Medicare contractors, or "carriers," as an independent diagnostic testing facility, or IDTF, in nine states and are seeking such enrollment by Medicare contractors in one additional state. Enrollment is essential for us to receive payment for healthcare services directly from Medicare. There can be no assurances we will be able to maintain such enrollment or that we will be able to gain such enrollment in other states. Any lapse in our licenses or enrollment, or the licensure or certification of our technicians, could increase our costs and adversely affect our operations and financialoperating results.
In the healthcare industry, various types of organizations are accredited to facilitate meeting certain Medicare certification requirements, expedite third-party payment and fulfill state licensure requirements. Some managed care providers prefer to contract with accredited organizations. Thus far, we have not found it necessary to seek or obtain accreditation from any established accreditation agency. If it becomes necessary for us to do so in the future in order to satisfy the requirements of third-party payors or
21
regulatory agencies, there can be no assurances that we will be able to obtain or continuously maintain this accreditation.
Audits or denials of our claims, or claims submitted by our DIS customers, by government agencies or contractors could reduce our revenues or profits and expose us to claims.
Under our "mixed bill" model, we submit claims directly to and receive payments directly from the Medicare program. Therefore, we are subject to extensive government regulation, including requirements for maintaining certain documentation to support our claims. Government agencies and Medicare contractors also may conduct inspections or surveys of our facilities, payment reviews and other audits of our claims and operations. For example, as part of a national audit conducted pursuant to the 2003 work plan, the Office of the Inspector General of the U.S. Department of Health and Human Services, or the OIG, conducted a review of one of our IDTF facilities in early 2003 to review the appropriateness of Medicare payments received. This audit was concluded without any action being taken by the OIG. While we believe this audit will have no impact on us, we cannot assure you that the OIG may not take some follow-up action. We may be subject to investigations, payment reviews and audits and cannot assure you that such scrutiny will not result in material delays in payment, as well as material recoupments or denials, which could reduce our revenue or profits. Our DIS customers also submit claims to Medicare and other third-party payors, are subject to the same types of regulation and scrutiny, and may experience the same types of problems. This could adversely affect our ability to market our leases and services and to maintain existing contracts.
Risks Related to Our Intellectual Property and Potential Litigation
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products.
We rely on patent protection as well as a combination oftrademark, copyright, trade secret and trademark laws,other intellectual property rights protection and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford onlytechnologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our success depends, in part, on our ability to protect our proprietary rights to the technologies used in our products. If we fail to protect and/or maintain our intellectual property, third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, and/or we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
We do not have any pending U.S.patent applications. We cannot assure investors that we will continue to innovate and foreignfile new patent applications, or that if filed any future patent applications will result in granted patents. Further, we cannot predict how long it will take for such patents to issue, if at all. It is possible that, for any of our patents that have issued or that may issue in the future, our competitors may design their products around our patented technologies. Further, we cannot assure investors that other parties will not challenge any patents granted to us, or that courts or regulatory agencies will hold our patents to be valid, enforceable, and/or infringed. We cannot guarantee investors that we will be successful in defending challenges made against our patents and patent applications. Any successful third-party challenge or challenges to our patents could result in the unenforceability or invalidity of such patents, or such patents being interpreted narrowly and/or in a manner adverse to our interests. Our ability to establish or maintain a technological or competitive advantage over our competitors and/or market entrants may be diminished because of these uncertainties. For these and other reasons, our intellectual property may not provide us with any competitive advantage. For example:
| ● | we may not have been the first to make the inventions claimed or disclosed in our issued patents; |
| ● | we may not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings or derivation proceedings declared by the U.S. Patent and Trademark Office (“USPTO”), which could result in substantial cost to us, and could possibly result in a loss or narrowing of patent rights. No assurance can be given that our granted patents will have priority over any other patent or patent application involved in such a proceeding, or will be held valid as an outcome of the proceeding; |
| ● | other parties may independently develop similar or alternative products and technologies or duplicate any of our products and technologies, which can potentially impact our market share, revenue, and goodwill, regardless of whether intellectual property rights are successfully enforced against these other parties; |
| ● | it is possible that our issued patents may not provide intellectual property protection of commercially viable products or product features, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties, patent offices, and/or the courts; |
| ● | we may be unaware of or unfamiliar with prior art and/or interpretations of prior art that could potentially impact the validity or scope of our patents or patent applications that we may to file; |
| ● | we take efforts and enter into agreements with employees, consultants, collaborators, and advisors to confirm ownership and chain of title in intellectual property rights. However, an inventorship or ownership dispute could arise that may permit one or more third parties to practice or enforce our intellectual property rights, including possible efforts to enforce rights against us; |
| ● | we may elect not to maintain or pursue intellectual property rights that, at some point in time, may be considered relevant to or enforceable against a competitor; |
| ● | we may not develop additional proprietary products and technologies that are patentable, or we may develop additional proprietary products and technologies that are not patentable; |
| ● | the patents or other intellectual property rights of others may have an adverse effect on our business; and |
| ● | we apply for patents relating to our products and technologies and uses thereof, as we deem appropriate. However, we or our representatives or their agents may fail to apply for patents on important products and technologies in a timely fashion or at all, or we or our representatives or their agents may fail to apply for patents in potentially relevant jurisdictions. |
To the extent our intellectual property offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct or indirect competition. If our intellectual property does not provide adequate coverage of our competitors’ products, our competitive position could be adversely affected, as could our business.
The measures that we use to protect the security of our intellectual property and other proprietary rights may not be adequate, which include claimscould result in the loss of legal protection for, and thereby diminish the value of, such intellectual property and other rights.
In addition to material aspectspursuing patents on our technology, we also rely upon trademarks, trade secrets, copyrights and unfair competition laws, as well as license agreements and other contractual provisions, to protect our intellectual property and other proprietary rights. Despite these measures, any of our intellectual property rights could be challenged, invalidated, circumvented or misappropriated. In addition, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets and/or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Moreover, if a party having an agreement with us has an overlapping or conflicting obligation to a third party, our rights in and to certain intellectual property could be undermined. Monitoring unauthorized and inadvertent disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, the outcome would be unpredictable, and any remedy may be inadequate.
In addition, competitors could purchase our products and proceduresattempt to replicate and/or improve some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design their products around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property does not adequately protect our market share against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We may need to enter into license agreements in the future.
We may need or may choose to obtain licenses and/or acquire intellectual property rights from third parties to advance our research or commercialization of our current or future products, and we cannot provide any assurances that third-party patents do not exist that might be enforced against our current or future products in the absence of such a license. We may fail to obtain any of these licenses or intellectual property rights on commercially reasonable terms. Even if we are not currently protected byable to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products, which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation.
If we are sued for infringing intellectual property rights of third parties, it would be costly and time consuming, and an unfavorable outcome in that litigation could have a material adverse effect on our business.
Our success also depends on our ability to develop, manufacture, market and sell our products and perform our services without infringing the proprietary rights of third parties. Numerous U.S. issued patents and pending patent applications owned by third parties exist in the fields in which we are developing products and services. As part of a business strategy to impede our successful commercialization and entry into new markets, competitors may allege that our products and/or services infringe their intellectual property rights.
We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against claims of infringement made by third parties. Any adverse ruling by a court or administrative body, or perception of an adverse ruling, may have a material adverse impact on our ability to conduct our business and our finances. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more products or services and could result in a substantial award of damages against us. Intellectual property litigation can be very expensive, and we may not have the financial means to defend ourselves.
Because patent applications can take many years to issue, asthere may be pending applications, some of which are unknown to us, that may result in issued patents upon which our products or proprietary technologies may infringe. Moreover, we may fail to identify issued patents of relevance or incorrectly conclude that an issued patent is invalid or not infringed by our technology or any of our products. If a third-party claims that we infringe upon a third-party’s intellectual property rights, we may have to:
| ● | seek to obtain licenses that may not be available on commercially reasonable terms, if at all; |
| ● | abandon any product alleged or held to infringe, or redesign our products or processes to avoid potential assertion of infringement; |
| ● | pay substantial damages including, in exceptional cases, treble damages and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes upon or violates the third-party’s rights; |
| ● | pay substantial royalties or fees or grant cross-licenses to our technology; or |
| ● | defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
Our issued patents could be found invalid or unenforceable if challenged in court or at the Patent Office or other administrative agency, which could have a form that will be advantageous to us. material adverse impact on our business.
Any patents we have obtained or do obtain may be challenged by re-examination or otherwise invalidated.invalidated or eventually found unenforceable. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. If we were to initiate legal proceedings against a third party to enforce a patent related to one of our products or services, the defendant in such litigation could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, as are validity challenges by the defendant against the subject patent or other patents before the USPTO. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement, failure to meet the written description requirement, indefiniteness, and/or failure to claim patent eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent intentionally withheld material information from the USPTO, or made a misleading statement, during prosecution. Additional grounds for an unenforceability assertion include an allegation of misuse or anticompetitive use of patent rights, and an allegation of incorrect inventorship with deceptive intent. Third parties may also raise similar claims before the USPTO even outside the context of litigation. The outcome is unpredictable following legal assertions of invalidity and unenforceability. With respect to the validity question, for example, we cannot be certain that no invalidating prior art existed of which we and the patent examiner were unaware during prosecution. These assertions may also be based on information known to us or the Patent Office. If a defendant or third party were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the claims of the challenged patent. Such a loss of patent protection would or could have a material adverse impact on our business.
We may be involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful.
Competitors may attempt to challenge or invalidate our patents, or may be able to design alternative techniques or devices that avoid infringement of our patents that have issued or that may issue in the future, or develop products with functionalities that are comparable to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, advisors and corporate partners, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Furthermore, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States.
In the event a competitor infringes upon our patent or other intellectual property rights, litigation to enforce our intellectual property rights or to defend our patents against challenge, even if successful, could be expensive and time consuming and could require significant time and attention from our management. We may not have sufficient resources to enforce our intellectual property rights or to defend our patents against challenges from others.
We have entered into
An adverse result in any such litigation proceedings could put one or more of our patents at risk of being invalidated, being found to be unenforceable, and/or being interpreted narrowly and could impact the validity or enforceability positions of our other patents. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a royalty-bearing license for one U.S. patent with a third-party for use in nuclear imaging, which license is co-exclusive with the U.S. government. We do not believerisk that some of our current
22
products implement the licensed patent; however, the licensor does not agree. We are currently negotiating to amend the license to resolve our dispute with the licensor. If we were to terminate the license, the licensor or subsequent licensee may allege that our current product infringes the patent, or such third-party licensee may develop and commercialize a competitive photodiode for use in gamma cameras.
The medical device industry is characterized by patent litigation and we could become subject to litigation thatconfidential information could be costly, resultcompromised by disclosure during this type of litigation. In addition, an adverse outcome in the diversionsuch litigation or proceedings may expose us to loss of our management's time and efforts, andproprietary position, expose us to significant liabilities, or require us to pay damages.
The medical device industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. Our competitors may assertseek licenses that our products, their components or the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, they may claim that their patents have priority over ours because their patents were filed or invented earlier. Because patent applications can take many years to issue, there may be applications now pending of which we are unaware, which may later result in issued patents that our products may infringe. There could also be existing patents that one or more components of our products may be infringing of which we are unaware. As the number of participants in our industry increases, the possibility of patent infringement claims against us also increases.
Any litigation or claims against us may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of our management from our core business and harm our reputation. If the relevant patents were upheld as valid and enforceable and we were found to be inadvertently infringing, we could be required to pay substantial damages and/or royalties and could be prevented from selling our products unless we could obtain a license or were able to redesign our system to avoid infringement. Any such license may not be available on reasonablecommercially acceptable terms, if at all.
We may make financial investments in other businesses that may lose value.
As we look for the best ways to deploy our capital and maximize our returns for our businesses and shareholders, we may make financial investments in other businesses or processes for purposes of enhancing our supply chain, creating financial returns, strategic developments, or other purposes. These investments may be speculative in nature, and there is no guarantee that we will experience a financial return and we may lose our entire principal balance if not successful.
Our mobile healthcare fleet is highly utilized; any downtime in our assets could have a material impact on our revenues and costs.
Our Mobile Healthcare business unit utilizes a fleet of highly sophisticated imaging and related transportation assets that require nearly 100% uptime to service our customer needs. Though we utilize an array of highly competent service providers to support our imaging fleet, imaging and related transportation machines can experience unproductive downtime. Any downtime of our imaging fleet could have near term impacts on our revenues and underlying costs.
Our goodwill and other long-lived assets are subject to potential impairment that could negatively impact our earnings.
A significant portion of our assets consists of goodwill and other long-lived assets, the carrying value of which may be reduced if we determine that those assets are impaired. At March 31, 2020, goodwill and net intangible assets represented $32.1 million, or 38.3% of our total assets. In addition, net property, plant and equipment assets totaled $20.8 million, or 24.8% of our total assets. If actual results differ from the assumptions and estimates used in our goodwill and long-lived asset valuation calculations, we could incur impairment charges, which could negatively impact our earnings.
We review our reporting units for potential goodwill impairment annually or more often if events or circumstances indicate that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. In addition, we test the recoverability of long-lived assets if events or circumstances indicate the carrying values may not be recoverable. Recoverability of long-lived assets is measured by comparison of their carrying amounts to future undiscounted cash flows the assets are expected to generate. We conduct impairment testing based on our current business strategy in light of present industry and economic conditions, as well as future expectations. There are numerous risks that may cause the fair value of a reporting unit to fall below its carrying amount and/or the value of long-lived assets to not be recoverable, which could lead to the measurement and recognition of goodwill and/or long-lived asset impairment. These risks include, but are not limited to, significant negative variances between actual and expected financial results, lowered expectations of future financial results, failure to realize anticipated synergies from acquisitions, adverse changes in the business climate, and the loss of key personnel. If we failare not able to obtain any required licenses or make any necessary changesachieve projected performance levels, future impairments could be possible, which could negatively impact our earnings.
During the year ended December 31, 2018, the Company derecognized $1.1 million of goodwill related to the termination of the Philips Agreements with DMS Health effective December 31, 2017. Additionally, during the same period, the Company recorded a $0.5 million and $0.2 million goodwill impairment loss, respectively, related to Telerhythmics LLC (Telerhythmics), the Company’s cardiac event monitoring services business that was acquired on March 13, 2014. On October 31, 2018, the Company entered into a membership interest purchase agreement (the “Telerhythmics Purchase Agreement”) with G Medical Innovations USA, Inc. (“G Medical”), pursuant to which we sold all the outstanding membership interests in Telerhythmics to G Medical. On September 10, 2019, Digirad completed its acquisition of ATRM pursuant to the ATRM Merger Agreement. The $8.2 million goodwill recognized is attributable primarily to expected synergies and the assembled workforce of ATRM. As of December 31, 2019, as a result of the finalization of the purchase price allocation for the ATRM Merger, an adjustment of $3,000 was made to the recognized amounts of goodwill resulting from the acquisition of ATRM.
No other significant impairment losses on long-lived assets were recognized during the three months ended March 31, 2020 and 2019. See Note 1.Basis of Presentationand Note 4.Merger within the notes to our unaudited condensed consolidated financial statements included in our Quarterly Report on Form 10-Q for the three months ended March 31, 2020 and incorporated herein by reference for further discussion regarding goodwill and long-lived assets.
The Company is dependent on its senior management team and other key employees.
The Company’s success depends, to a significant extent, on its senior management team and other key employees and the ability of other personnel or new hires to effectively replace key employees who may retire or resign. Failure to retain its leadership team and attract and retain new leadership and other important personnel could lead to ineffective management and operations, which could materially and adversely affect its business and operating results.
ATRM’s operating results could be adversely affected by changes in the cost and availability of raw materials.
Prices and availability of raw materials used to manufacture its products or technologies, wecan change significantly due to fluctuations in supply and demand. Additionally, availability of the raw materials used to manufacture its products may be limited at times resulting in higher prices and/or the need to find alternative suppliers. Both KBS’s and EBGL’s major material components are dimensional lumber and wood sheet products, which include plywood and oriented strand board. Lumber costs are subject to market fluctuations. Furthermore, the cost of raw materials may also be influenced by transportation costs. It is not certain that any price increases can be passed on to its customers without affecting demand or that limited availability of materials will not impact its production capabilities. The state of the financial and housing markets may also impact its suppliers and affect the availability or pricing of materials. ATRM’s inability to raise the price of its products in response to increases in prices of raw materials or to maintain a proper supply of raw materials could have an adverse effect on its revenue and earnings.
If we are unable to commercialize one or more of our products.
We rely significantly on a license agreement with Segami Corporationsecure contracts for the imaging acquisitionKBS Projects or are unable to successfully complete the KBS Projects, our revenues and processing softwareresults could be adversely affected.
We are in advanced stages of negotiation for contracts to secure the KBS Projects; however, we can provide no assurance that we will be awarded the KBS Projects, that we will be able to successfully complete the KBS Projects if they are awarded to KBS, or that the anticipated revenues from the KBS Projects will be achieved. If we are not awarded the contracts for the KBS Projects, do not complete them as planned or do not receive payment for the KBS Projects as projected, then KBS’ and our digital gamma camera,revenues and results may be adversely affected.
If KBS is unable to maintain or establish its relationships with independent dealers and contractors who sell its homes, KBS revenue could decline.
KBS sells residential homes through a network of independent dealers and contractors. As is common in the modular home industry, KBS’s independent dealers may also sell homes produced by competing manufacturers and can cancel their relationships with KBS on short notice. In addition, these dealers may not remain financially solvent, as they are subject to industry, economic, demographic and seasonal trends similar to those faced by KBS. If KBS is not able to maintain good relationships with its dealers and contractors or establish relationships with new solvent dealers or contractors, KBS’s revenue could decline.
Due to the nature of ATRM’s business, many of its expenses are fixed costs and if there are decreases in demand for its products, it may adversely affect its operating results.
Many of its expenses, particularly those relating to properties, capital equipment, and certain manufacturing overhead items, are fixed in the short term. Reduced demand for its products causes its fixed production costs to be allocated across reduced production volumes, which adversely affects its gross margins and profitability.
Certain actions taken in connection with reducing operating costs may have a negative impact on ATRM’s business.
In the event of a housing downturn and a decline in its revenues, ATRM may implement cost reduction actions such as temporary factory shutdowns, workforce reductions, pay freezes and reductions, and reductions in other expenditures. In doing so, ATRM will attempt to maintain the necessary infrastructures to allow ATRM to take full advantage of subsequent improvements in market conditions. However, there can be no assurance that reductions ATRM may make in personnel and expenditure levels and the loss of the license could resultcapabilities of personnel ATRM has terminated or may terminate will not inhibit ATRM in delivery delays, lossthe timely ramp up of customers and loss of revenue.production in response to improving market conditions.
Segami Corporation, or Segami, has developed image acquisition and processing software for our camera under a non-exclusive license agreement. In
Due to the event that Segami attempts to terminate the license agreement, refuses to extend the termnature of the license or seeks to impose unreasonable pricing or terms, we would have to find an alternative software system to use in our gamma camera. To our knowledge, there are a limited number of companies that would be able to developwork the Company and implement a software system similar to what we use in our gamma camera. As a result, inits subsidiaries perform, the event that we were unable to continue to use the software under the license from Segami, we could have delays in the production of our gamma camera as we attempted to find a substitute software provider. Furthermore, we cannot guarantee that alternative software providers would be able to meet our requirements or that their software would be available to us at favorable prices, if at all. To the extent we were unable to find an alternative source for the software, we may have to develop our own software system. We cannot guarantee that we could internally develop such a software system or that such efforts would not divert resources away from the development of other features of our camera. As a result, locating an alternative software system or developing our own software system could interrupt the manufacture and delivery of our products for an extended period of time and may cause the loss of customers and revenue.
WeCompany may be subject to damages resulting fromsignificant liability claims that we, or our employees, have wrongfully used or disclosed alleged trade secrets of their former employers.and disputes.
Many
The Company and its wholly owned subsidiaries engage in services that can result in substantial injury or damages that may expose the Company to legal proceedings, investigations and disputes. For example, in the ordinary course of our employees were previously employed atits business, the Company may be involved in legal disputes regarding personal injury and wrongful death claims, employee or labor disputes, professional liability claims, and general commercial disputes, as well as other medical device companies, including our competitors or potential competitors. Although no claims against us are currently pending, weclaims. LSVM as an exempt reporting advisor may also be subject to claims that we or our employees have inadvertently or otherwise used or disclosed trade secrets
23
legal proceedings and investigations with the SEC or other proprietary information of their former employers. Litigationregulatory bodies and may be necessaryhave disputes related to defendits fiduciary duties to the funds or instruments LSVM manages. An unfavorable legal ruling against these claims. Even if we are successful in defending against these claims, litigationthe Company or its subsidiaries could result in substantial monetary damages. Although the Company has adopted a range of insurance, risk management, safety, and risk avoidance programs designed to reduce potential liabilities, there can be no assurance that such programs will protect the Company fully from all risks and liabilities. If the Company sustains liabilities that exceed its insurance coverage or for which the Company is not insured, it could have a material adverse impact on its results of operations and financial condition.
ATRM’s costs of doing business could increase as a result of changes in, expanded enforcement of, or adoption of new federal, state or local laws and regulations.
ATRM is subject to various federal, state and local laws and regulations that govern numerous aspects of its business. In recent years, a number of new laws and regulations have been adopted, and there has been expanded enforcement of certain existing laws and regulations by federal, state and local agencies. These laws and regulations, and related interpretations and enforcement activity, may change as a result of a variety of factors, including political, economic or social events. Changes in, expanded enforcement of, or adoption of new federal, state or local laws and regulations governing minimum wage or living wage requirements; the classification of exempt and non-exempt employees; the distinction between employees and contractors; other wage, labor or workplace regulations; healthcare; data protection and cybersecurity; the sale and pricing of some of its products; transportation; logistics; supply chain transparency; taxes; unclaimed property; energy costs and consumption; or environmental matters could increase its costs of doing business or impact its operations.
Risks Related to our Indebtedness
On March 29, 2019, Digirad and certain of its healthcare subsidiaries entered into a Loan and Security Agreement with Sterling National Bank (the “SNB Loan Agreement”). The SNB Loan Agreement is a five-year revolving credit facility (maturing in March 2024), which, as amended, has a maximum credit amount of $20 million (the “SNB Credit Facility”). On January 31, 2020, Digirad and certain of its Investments Subsidiaries entered into a Loan and Security Agreement with Gerber (as amended, the “Star Loan Agreement”), which provides for a credit facility with borrowing availability of up to $2.5 million and matures on January 1, 2025, unless terminated in accordance with the terms therein (the “Star Loan”). On January 31, 2020, Digirad and certain of its Construction Subsidiaries entered into a Loan and Security Agreement with Gerber (the “EBGL Loan Agreement”), which provides for a credit facility with borrowing availability of up to $3.0 million and matures on January 1, 2022, unless extended or terminated in accordance with the terms therein (the “EBGL Loan”). On January 31, 2020, Glenbrook and EdgeBuilder entered into an Extension and Modification Agreement (the “Modification Agreement”) with Premier Bank (“Premier”) that modified the terms of the loan made by Premier to Glenbrook and EdgeBuilder pursuant to that certain Revolving Credit Loan Agreement, dated June 30, 2017, by and among Glenbrook, EdgeBuilder and Premier (as amended, the “Premier Loan Agreement”). Pursuant to the Modification Agreement, the amount of indebtedness evidenced by the promissory note issued under the Premier Loan Agreement was reduced to $1.0 million. Our credit facility under the Loan and Security Agreement, dated February 23, 2016, by and among KBS, ATRM, the Company and Gerber (as amended, the “KBS Loan Agreement”) provides for a revolving credit facility of up to $4.0 million that matures on February 22, 2021, subject to automatic extension for an additional year unless terminated. The SNB Loan Agreement, Star Loan Agreement, EBGL Loan Agreement, the Premier Loan Agreement and the KBS Loan Agreement are collectively referred to as the “Company Loan Agreements.” See Note 12,Related Party Transactions, within the notes to our unaudited condensed consolidated financial statements included in our Quarterly Report on Form 10-Q for the three months ended March 31, 2020 and incorporated herein by reference for information regarding certain ATRM promissory notes that are outstanding.
Our indebtedness could restrict our operations and make us more vulnerable to adverse economic conditions.
Our indebtedness could have important consequences for us and our stockholders. For example, the SNB Loan Agreement requires a balloon payment at the termination of the facility in March 2024 and the Star Loan Agreement has a balloon payment in January 2021, which payments may require us to dedicate a substantial portion of our cash flow from operations to this future payment if we feel we cannot be a distraction to management. If we failsuccessful in defending such claims, in addition to paying money damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hinder or preclude our ability to commercializerefinance in the future, thereby reducing the availability of our products,cash flow to fund working capital, capital expenditures, and acquisitions, and for other general corporate purposes. In addition, our indebtedness could:
| ● | increase our vulnerability to adverse economic and competitive pressures in our industry; |
| ● | place us at a competitive disadvantage compared to our competitors that have less debt; |
| ● | limit our flexibility in planning for, or reacting to, changes in our business and our industry; and |
| ● | limit our ability to borrow additional funds on terms that are acceptable to us or at all. |
The Company Loan Agreements governing our indebtedness contain restrictive covenants that restrict our operating flexibility and require that we maintain specified financial ratios. If we cannot comply with these covenants, we may be in default under one or more of the Loan Agreements.
The Loan Agreements governing our indebtedness contain restrictions and limitations on our ability to engage in activities that may be in our long-term best interests. The Loan Agreement contains affirmative and negative covenants that limit and restrict, among other things, our ability to:
| ● | incur additional debt; |
| ● | sell assets; |
| ● | incur liens or other encumbrances; |
| ● | make certain restricted payments and investments; |
| ● | acquire other businesses; and |
| ● | merge or consolidate. |
The SNB Loan Agreement limits our ability to pay dividends and to redeem our equity securities if such dividend or redemption would result in our non-compliance with the financial covenants in the SNB Loan Agreement, there is insufficient borrowing availability under the SNB Loan Agreement, or if there is a default or event of default under the SNB Loan Agreement that has occurred and is continuing. In addition, the Company Loan Agreements include explicit restrictions on the payment of dividends and distributions to Digirad, which could severely harm our business.
If we become subjectlimit the Company’s ability to product liability or warranty claims, wepay dividends. The Company may, experience reduced demand for our products ortherefore be required to pay damagesreduce or eliminate its dividends, if any, including on the Series A Preferred Stock (if any outstanding), and/or may be unable to redeem shares of the Series A Preferred Stock (if any outstanding) until compliance with such financial covenants can be met.
The Loan Agreements contain various financial covenants that, exceedgoing forward, we or our insurance coverage.subsidiaries may not have the ability to meet. Due to increased financial pressure on the Company as a result of the COVID-19 pandemic, there is an increased likelihood that we may fail to comply with such financial covenants if the impacts of the pandemic persist for a significant duration. We cannot provide any assurance that any such breach of covenants will be able to be remedied or that they will not result in a default under the Loan Agreements.
The saleLoan Agreements also contain various other affirmative and supportnegative covenants regarding, among other things, the performance of our products entailsbusiness, capital allocation decisions made by the riskCompany and its subsidiaries, or events beyond our control. Our failure to comply with our covenants and other obligations under the Loan Agreements may result in an event of product liabilitydefault thereunder. A default, if not cured or warranty claims, such as those based on claims that the failure of onewaived, may permit acceleration of our products resultedindebtedness. If our indebtedness is accelerated, we cannot be certain that we will have sufficient funds available to pay the accelerated indebtedness (together with accrued interest and fees), or that we will have the ability to refinance the accelerated indebtedness on terms favorable to us or at all. This could have serious consequences to our financial condition, operating results, and business, and could cause us to become insolvent or enter bankruptcy proceedings, and stockholders may lose all or a portion of their investment because of the priority of the claims of our creditors on our assets.
��
Substantially all of our assets (including the assets of our subsidiaries) have been pledged to lenders as security for our indebtedness under the Loan Agreements.
The Loan Agreements are secured by a first-priority security interest in substantially all of the assets of the Company and its subsidiaries and a misdiagnosis,pledge of all shares and equity interests of the Company’s subsidiaries. Upon the occurrence and during the continuation of an event of default under any Loan Agreement, the applicable lender may, among other issues.things, declare the loans and all other obligations thereunder immediately due and payable and may, in certain instances, increase the interest rate at which loans and obligations bear interest. The medical device industry has been subject to significant products liability litigation. We may incur significant liabilityexercise by a lender of remedies provided under the applicable Loan Agreement in the event of any such litigation, regardless ofa default thereunder may have a material adverse effect on the merit of the action. Although we maintain product liability insurance, we cannot be sure that this coverage is adequate or that it will continue to be available on acceptable terms, if at all. We also may face warranty exposure, which could adversely affect our operating results. Any unforeseen warranty exposure or insufficient insurance could harm our business,liquidity, financial condition and results of operations. Finally, even a meritless operations of the applicable borrowers and/or unsuccessful product liability claim could harm our reputation in the industry, lead to significant legal feesCompany and could resultcause such borrowers and/or the Company to become bankrupt or insolvent. The obligations of the Company and various subsidiaries of the Company under the Loan Agreements are guaranteed by other subsidiaries of the Company and/or the Company. In the event of any bankruptcy, liquidation, dissolution, reorganization, or similar proceeding against us, the assets that are pledged as collateral securing any unpaid amounts under the applicable Loan Agreement must first be used to pay such amounts, as well as any other obligation secured by the pledged assets, in full, before making any distributions to our stockholders. In the diversionevent of management's attention from managingany of the foregoing, our business.stockholders could lose all or a part of their investment.
The inability of the Company, ATRM, KBS, or any of the Company’s other subsidiaries to comply with applicable financial covenants under the Loan Agreements could have a material adverse effect on financial condition of the Company.
As of December 31, 2019 and 2018, KBS was not in compliance with the financial covenants under the KBS Loan Agreement requiring no net annual post-tax loss for KBS or the minimum leverage ratio covenant as of these test dates. In April 2019, June 2019 and February 2020, ATRM obtained a waiver from Gerber Finance for these events. In addition, ATRM and Gerber Finance agreed to eliminate the minimum leverage ratio covenant for years after 2018.
If the Company, ATRM, KBS, or any of the Company’s other subsidiaries fail to comply with any applicable financial covenants under the Loan Agreements to which it is a party, or if there is otherwise an event of default under the Loan Agreements by a borrower, the borrowers’ obligations thereunder may (subject to any applicable cure periods) become immediately due and payable and the applicable lender(s) may demand the repayment of the credit facilities amount outstanding and any unpaid interest thereon.
We may not be entitled to forgiveness of our recently received PPP Loans, and our application for the PPP Loans could in the future be determined to have been impermissible.
During April and May, we received proceeds of $6.7 million from various loans under the PPP of the CARES Act, which we intend to use to retain current employees, maintain payroll and make lease and utility payments. The PPP Loans mature in April and May, 2022 and bear annual interest at a rate of 1.0%.
Commencing November and December, 2020, we are required to pay the lenders equal monthly payments of principal and interest as required to fully amortize by April and May, 2022 any principal amount outstanding on the PPP Loans as of October, 2020. Under the CARES Act, loan forgiveness is generally available for the sum of documented payroll costs, covered rent payments, covered mortgage interest and covered utilities during the eight week period beginning on the date the lender makes the first disbursement of the PPP Loan. We will be required to repay any portion of the outstanding principal that is not forgiven, along with accrued interest, and we cannot provide any assurance that we will be eligible for loan forgiveness, that we will ultimately apply for forgiveness equally among all business entities, or that any amount of the PPP Loans will ultimately be forgiven by the SBA.
Furthermore, in April 2020, the Secretary of the U.S. Department of the Treasury stated that the SBA will perform a full review of any PPP loan over $2.0 million before forgiving the loan. In order to apply for the PPP Loan, we were required to certify, among other things, that the current economic uncertainty made the PPP Loan request necessary to support our ongoing operations. We made this certification in good faith after analyzing, among other things, the maintenance of our entire workforce, taking into consideration certain limitations associated with the nature of our work in the healthcare business and uncertainty of the industry where healthcare clients resources may be diverted during the COVID-19 pandemic. In considering our position, the Company took into account our need for additional funding to continue operations, and our ability to access alternative forms of capital in the current market environment to offset the effects of the COVID -19 pandemic. Following this analysis, we believe that we satisfied all eligibility criteria for the PPP Loan, and that our receipt of the PPP Loan is consistent with the broad objectives of the PPP of the CARES Act. The certification described above did not contain any objective criteria and is subject to interpretation. If, despite our good-faith belief that given our circumstances we satisfied all eligible requirements for the PPP Loans, we are later determined to have violated any applicable laws or regulations or it is otherwise determined that we were ineligible to receive the PPP Loans, we may be required to repay the PPP Loans in their entirety and/or be subject to lawsuitsadditional penalties, which could also result in adverse publicity and actions brought bydamage to reputation. In addition, if these events were to transpire, they could have a material adverse effect on our employees.business, results of operations and financial condition.
We
If we are unable to generate or borrow sufficient cash to make payments on our indebtedness, our financial condition would be materially harmed, our business could fail, and stockholders may from time to time be subject to employment claims or disputes. Recently one former and three present employees have retained counsel and have claimed that they are due overtime pay because of an alleged misclassificationlose all of their positions as non-exempt rather than exempt employees. These employees have claimed damages equalinvestment.
Our ability to back paymake scheduled payments on or to refinance our obligations will depend on our financial and operating performance, which will be affected by economic, financial, competitive, business, and other factors, some of upwhich are beyond our control. We cannot assure you that our business will generate sufficient cash flow from operations to thirty days, liquidated damagesservice our indebtedness or to fund our other liquidity needs. If we are unable to meet our debt obligations or fund our other liquidity needs, we may need to restructure or refinance all or a portion of twice the amountour indebtedness on or before maturity or sell certain of overtime pay found due and attorneys' fees.our assets. We deny any wrongdoing and intend to defend against these claims vigorously. However, we cannot assure you that we will be successful,able to restructure or that additional former or present employeesrefinance any of our indebtedness on commercially reasonable terms, if at all, which could cause us to default on our debt obligations and impair our liquidity. Any refinancing of our indebtedness could be at higher interest rates and may not joinrequire us to comply with more onerous covenants, which could further restrict our business operations.
Increases in any such action. Any employment claimsinterest rates could significantly divert our management's time and attention and could materiallyadversely affect our business.results from operations and financial condition.
The SNB, Premier and Gerber Loan Agreements allow for amounts borrowed thereunder to be subject to a floating interest rate which may change with market interest rates. An increase in prevailing interest rates would have an effect on the interest rates charged on our variable rate debt, which rise and fall upon changes in interest rates. If prevailing interest rates or other factors result in higher interest rates, the increased interest expense would adversely affect our cash flow and our ability to service our indebtedness.
Risks Related to the Securities Markets and Ownership of Ourour Common Stock
ThereThe market price of our common stock may be volatile, and the value of your investment could decline significantly.
The market price of our common stock has been, no prior publicand we expect it to continue to be, volatile. The prices at which our shares of common stock trade depend upon a number of factors, including our historical and anticipated operating results, our financial situation, announcements of new products by us or our competitors, history of timely dividend payments, our ability or inability to raise the additional capital we may need and the terms on which we raise it, and general market forand economic conditions. Some of these factors are beyond our control. Broad market fluctuations may lower the market price of our common stock and an activeaffect the volume of trading in our stock, regardless of our financial condition, results of operations, business, or prospects. It is impossible to assure you that the market may not develop.
Prior to this offering, there has been no public market forprice of our common stock. An active public trading market for our common stock may not develop or be sustained after the offering. We will negotiate and determine the initial public offering price with representatives of the underwriters and this price may not be indicative of prices that will prevail in the trading market. As a result, you may not be able to sell your shares of common stock at or abovewill not fall in the offering price. An inactive market may also impairfuture.
Our common stock has a low trading volume and shares available under our ability to raise capital by selling shares and may impairequity compensation plans could affect the trading price of our ability to acquire other businesses, products or technologies by using our shares as consideration.common stock.
Future
Our common stock historically has had a low trading volume. Any significant sales of our common stock may cause volatility in our stock price to decline.
Our current stockholders hold a substantial number ofprice. We also have registered shares of our common stock that they will be able to sell in the public market in the near future. Significant portions of these shares are held by a small number of stockholders. Sales by our current stockholders of a substantial number of shares after this offering, or the expectation that such sale may occur, could significantly reduce the market price of our common stock. Moreover, after this offering, the holders of approximately shares of common stock, including shares issued upon conversion of our preferred stock and shares issued upon the exercise of certain of our warrants, will have rights, subject to some conditions, to require us to file registration statements to permit the resale of their shares in the public market or to include their shares in registration statements that we may file for ourselves or other stockholders. Although the holders of most
24
of our outstanding capital stock have agreed with the underwriters of this offering to be bound by a 180-day lock-up agreement that prohibits these holders from selling or transferring their stock, other than in specific circumstances, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities Inc., at their discretion, can waive the restrictions of the lock-up agreement at an earlier time without prior notice or announcement and allow our stockholders to sell their shares of our common stock in the public market. If the restrictions of the lock-up agreement are waived, shares of our common stock will be available for sale into the market, subject only to applicable securities rules and regulations, which may cause our stock price to decline.
We also intend to register all common stock that we may issue under our 2004 Stock Incentive Plan, 2004 Non-Employee Director Stock Option Program and 2004 Employee Stock Purchase Plan. Once we registeremployee benefit plans or from our treasury stock. Accordingly, these shares they can be freely sold in the public market upon issuance, subject to restrictions under the securities laws and the lock-up agreements described above.laws. If any of these stockholders, or other selling stockholders, cause a large number of securities to be sold in the public market without a corresponding demand, the sales could reduce the trading price of our common stock. These sales also could impedeOne or more stockholders holding a significant amount of our abilitycommon stock might be able to raise future capital.significantly influence matters requiring approval by our stockholders, possibly including the election of directors and the approval of mergers or other business combination transactions.
Our stock price may be volatile, and you may lose all or a substantial partPayment of your investment.
Following this offering, the market price fordividends on our common stock is likelyprohibited unless we have declared and paid (or set apart for payment) full accumulated dividends on the Series A Preferred Stock, which also has a significant liquidation value.
Unless full cumulative dividends on the Series A Preferred Stock have been, or contemporaneously are, declared and paid or declared and a sum sufficient for the payment thereof is set apart for payment for all past dividend periods, no dividends (other than a dividend in shares of common stock or other shares of stock ranking junior to the Series A Preferred Stock as to dividends and upon liquidation) can be volatile,declared and paid or declared and set apart for payment on our common stock, nor can any shares of common stock be redeemed, purchased or otherwise acquired for any consideration by the Company. To date no dividends have been paid on the Series A Preferred Stock and as a result, cumulative dividends will continue to accrue as part of the liquidation value of the Series A Preferred Stock, which had a liquidation value of $10.00 per share at issuance. Dividends on the Series A Preferred Stock are payable out of amounts legally available therefor at a rate equal to 10.0% per annum per $10.00 of stated liquidation preference per share, or $1.00 per share of Series A Preferred Stock per year. Dividends on the Series A Preferred Stock are only be payable in cash. As of March 31, 2020, there were 1,915,637 shares of Series A Preferred Stock issued and outstanding.
If we fail to pay dividends on our Series A Preferred Stock for six or more consecutive quarters, holders of our Series A Stock will be entitled to elect two additional directors to our board of directors.
To date no dividends have been paid on the Series A Preferred Stock and as a result, cumulative dividends will continue to accrue as part because ourof the liquidation value of the Series A Preferred Stock. Whenever dividends on any shares of Series A Preferred Stock are in arrears for six or more consecutive quarters, then the holders of those shares together with the holders of all other series of preferred stock equal in rank with the Series A Preferred Stock upon which like voting rights have not been traded publicly. In addition,conferred and are exercisable, will be entitled to vote separately as a class for the market priceelection of a total of two additional directors to Digirad’s board of directors. Holders of our common stock will not be entitled to vote no such additional directors.
Digirad may fluctuate significantlynot be able to redeem its Series A Preferred Stock upon a Change of Control Triggering Event.
Upon the occurrence of a Change of Control Triggering Event, (as defined in responsethe Certificate of Designations, Rights and Preferences of 10% Series A Cumulative Perpetual Preferred Stock of Digirad Corporation) unless the Company has exercised its option to redeem the Series A Preferred Stock after September 10, 2024, each holder of the Series A Preferred Stock will have the right to require the Company to redeem all or any part of such holder’s Series A Preferred Stock at a numberprice equal to the liquidation preference of factors, most$10.00 per share, plus an amount equal to any accumulated and unpaid dividends up to but excluding the date of which we cannot control, including:
The protective amendment contained in our Restated Certificate of Incorporation, which is intended to help preserve the value of certain income tax assets, primarily tax net operating loss carryforwards, may have unintended negative effects.
Pursuant to Internal Revenue Code Sections 382 and 383, use of our NOLs may be limited by an “ownership change” as defined under Section 382 of the Internal Revenue Code of 1986, as amended, and the Treasury Regulations thereunder. In order to protect the Company’s significant NOLs, we filed an amendment to the Restated Certificate of Incorporation of the Company (as amended and extended, the “Protective Amendment”) with the Delaware Secretary of State on May 5, 2015. The Protective Amendment was approved by the Company’s stockholders at the Company’s 2015 Annual Meeting of Stockholders held on May 1, 2015.
On April 27, 2018, we filed a Certificate of Amendment to the Company’s Restated Certificate of Incorporation with the Secretary of State of the State of Delaware, which was approved by our stockholders at our 2018 Annual Meeting (the “Extended Protective Amendment”). The Extended Protective Amendment effects a three-year extension to the provisions of the Protective Amendment. The Extended Protective Amendment leaves the Protective Amendment unchanged in all respects, other than to extend the expiration date from May 1, 2018 to May 1, 2021, and to make revisions necessary as a result of the enactment of Public Law 115-97 (commonly referred to as the Tax Cut and Jobs Act) on December 22, 2017.
The Protective Amendment is designed to assist the Company in protecting the long-term value of its accumulated NOLs by limiting certain transfers of the Company’s common stock. The Protective Amendment’s transfer restrictions generally restrict any direct or other developments with respectindirect transfers of the common stock if the effect would be to intellectual property rights;
The Protective Amendment also requires any person attempting to become a holder of 4.99% or more of our common stock including sales by our executive officers and directors;
Anti-takeover provisions in our organizational documents and Delaware law may discourageprevent or preventdelay removal of current management or a change in control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.control.
Our restated certificate of incorporation and amended and restated bylaws contain provisions that may delay or prevent a change in control, discourage bids at a premium over the market price of our common stock, and
25
adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. These provisions include:
WeDelaware corporation, we are also subject to provisionsDelaware law, including Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a Delaware corporation law that,from engaging in general, prohibit any business combination with any interested stockholder for a beneficial ownerperiod of 15%three years following the date that the stockholder became an interested stockholder unless certain specific requirements are met as set forth in Section 203. These provisions, alone or moretogether, could have the effect of deterring or delaying changes in incumbent management, proxy contests, or changes in control.
We estimate that the net proceeds to us from our issuance and sale of the common stock, for five years unless the holder's acquisition of our stock was approved in advance by our board of directors. Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
We may become involved in securities class action litigation that could divert management's attentionpre-funded warrants and harm our business.
The stock market in general, and the Nasdaq National Market and the market for medical device companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the companies in those markets. In addition to our performance, these broad market and industry factors may materially harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a particular company's securities, securities class action litigation has often been brought against that company. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management's attention and resources, which could materially harm our financial condition and results of operations.
As a new investor, you will experience immediate and substantial dilution as a result of this offering and future equity issuances and, as a result of such dilution, our stock price could decline.
The initial public offering price will be substantially higher than the pro forma net tangible book value per share of our outstanding common stock. As a result, investors purchasing common stockwarrants in this offering will incur immediate dilution of $ per share. This dilution is due in large part to earlier investors in our company having paid substantially less than the initialbe $4.44 million, based on an assumed public offering price when they purchasedof $2.37 per share and accompanying warrant, after deducting underwriting discounts and commissions and estimated offering expenses. If the over-allotment option granted to the underwriter is exercised in full, we will receive additional net proceeds of $0.7 million, after deducting underwriting discounts and commissions and estimated offering expenses. We will only receive additional proceeds from the exercise of the pre-funded warrants and/or the warrants issuable in this offering if such pre-funded warrants and/or warrants are exercised at their shares. Investors who purchase sharesrespective exercise prices of $0.01 and $[●] per share of common stock and the holders of such pre-funded warrants and warrants pay the exercise price of such pre-funded warrants and warrants in this offeringcash.
We will contribute approximately % of the total amount we have raised to fund our operations but will own only approximately % of our common stock. We believe that the net proceeds from this offering, together with our current cash, cash equivalents and short-term investments, will be sufficient to meet our projected operating requirements foruse at least the next 12 months. Because we may require additional funds to develop new products and continue to expand our business, however, we may conduct substantial future offerings of equity securities. The exercise of outstanding options and warrants and future equity issuances, including future public offerings or future private placements of equity securities and any additional shares issued in connection with acquisitions, will result in further dilution to investors.
26
If our officers, directors and principal stockholders choose to act together, they may be able to control our management and operations, acting in their best interests and not in the best interests of other stockholders.
After this offering, our officers, directors and holders of 5% or more of our outstanding common stock will beneficially own approximately % of our common stock, after giving effect to the conversion of all outstanding shares of our preferred stock, but assuming no exercise of the underwriters' over-allotment option and no exercise of outstanding options or warrants. As a result, these stockholders, acting together, will be able to significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of this group of stockholders may not always coincide with our interests or the interests of other stockholders, and they may act in a manner that advances their best interests and not necessarily those of other stockholders. As a result of their actions or inactions our stock price may decline.
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a return.
Our management will have considerable discretion in the application of the net proceeds of this offering. We expect to use a majority$3.0 million of the net proceeds from this offering to manufacturefund the KBS Projects (three modular housing projects to be constructed in New England), and market our gamma cameras, build our salesthe remainder (if any) for working capital and marketing capabilities, expand our DIS business and repay outstanding lines of credit of approximately $9.4 million. To a lesser extent,for other general corporate purposes.
We are undertaking this offering because we anticipate usingbelieve we will be able to use the remaining net proceeds of this offering for further researchthe sale of the securities to grow our business organically and development relating to our existing products and new product opportunities, to finance regulatory approval activities and for general corporate purposes. through strategic transactions.
We may also use ahave not allocated any specific portion of the net proceeds of this offering to acquire or invest in complementary businesses, products, or technologies, or to obtain the right to use such complementary technologies, although we are not currently involved in any negotiations and have no commitments with respect to any such transactions. We cannot specify with certainty how we will use the net proceeds of this offering or our existing cash balance. The net proceeds may be used for corporate purposes that do not increase our operating results or market value. Until the net proceeds are used, they may be placed in investments that do not produce income or that lose value.
27
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are based on our management's beliefs and assumptions and on information currently available to our management. The forward-looking statements are contained principally in the sections entitled "Prospectus Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Use of Proceeds" and "Business." In some cases, you can identify forward-looking statements by terms such as "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" and similar expressions intended to identify forward-looking statements.
Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading "Risk Factors." Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management's beliefs and assumptions only as of the date of this prospectus. You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
28
We estimate that the net proceeds from this offering will be approximately $ million, based upon an assumed initial public offering price of $ per share and after deducting estimated underwriting discounts and commissions and offering expenses. If the underwriters exercise their over-allotment option in full, we estimate that our net proceeds will be approximately $ million.
We expect to use a majority of the net proceeds of this offering to manufacture and market our gamma cameras, build our sales and marketing capabilities, expand our DIS business and repay outstanding lines of credit of approximately $9.4 million.
To a lesser extent, we anticipate using the remaining net proceeds of this offering:
In addition, we may use a portion of the net proceeds from this offering to acquire products, technologies or businesses that are complementary to our own, but we currently have no commitments or agreements relating to any of these types of transactions.
Of the approximately $9.4 million of net proceeds that we intend to use to repay outstanding lines of credit, we will use approximately $4.83 million to repay in full our outstanding balance as of December 31, 2003 under our secured credit facility with Silicon Valley Bank. The secured credit facility may be used to borrow against accounts receivable and fixed assetsparticular purpose, and our outstanding balance matures in October 2004. The secured credit facility bears an interest rate equalmanagement will have the discretion to allocate the lender's prime rate, plus 1.75% per annum, but in no event less than 5.75%.
Additionally, ofproceeds as it determines. Furthermore, the approximately $9.4 million of net proceeds that we intend to use to repay outstanding lines of credit, we will use approximately $4.53 million to repay in full our outstanding balance as of December 31, 2003 under our credit facility with GE Healthcare Financial Services. The total amount outstanding under the line of credit matures in December 2004 and the interest rate under such agreement is the greater of the lender's prime rate plus 1.25% per annum, or 6%.
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. The amount and timing of our actual expenditures will depend on severalnumerous factors, including the amount of revenue generated from our operations, the progress of our commercialization efforts, and the amount of cash used in or generated by our operations. Accordingly, our management will have broad discretion inoperations, future acquisitions, if any, the applicationpace of the net proceedsintegration of any acquired businesses, and investors will be relying on the judgmentlevel of our management regarding the application of the proceeds of this offering. We reserve the right to change the use of these proceeds as a result of certain contingencies such as the results of our commercialization efforts, competitive developments, opportunities to acquire products, technologies or businessessales and other factors.marketing activities.
Pending the uses described above, we plan to invest the net proceeds of this offering in short- and medium-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
We have nevernot declared or paid any cash dividends on our capital stock.common stock since our 2017 fiscal year. We currently intend to retain all available funds and any future earnings to support operationsfor the operation and finance the growth and developmentexpansion of our business and, therefore, we do not intendanticipate declaring or paying dividends on our common stick in the foreseeable future. In addition, pursuant to its certificate of designations, so long as any Series A Preferred Stock is outstanding, we are not permitted to directly or indirectly declare or pay cashany dividend on our common stock as long as any dividends due on the Series A Preferred Stock remain unpaid. We are not current in the payment of dividends on our Series A Preferred Stock, The payment of dividends on our common stock for the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors.directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements, any limitations on payment of dividends present in our future debt agreements, and other factors that our board of directors may deem relevant.
The following table sets forth our capitalization as of DecemberMarch 31, 2003:2020 as follows:
| ● | on an actual basis; and |
| ● | on a pro forma, reflecting the issuance of 2,109,704 shares of common stock offered by this prospectus, based on an assumed offering price of $2.37 per share, assuming net proceeds of approximately $4.44 million, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, but not giving effect to the exercise of the over-allotment option, and assuming no sale of any pre-funded warrants in this offering and no exercise of any accompanying warrants or the underwriter’s warrant. |
The information below should be read in conjunction with our unaudited condensed consolidated financial statements included in our Quarterly Report on an actual basis;Form 10-Q for the quarter ended March 31, 2020, which is incorporated by reference in this prospectus. These financial statements should also be read with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which is included in our 2020 Q1 10-Q and incorporated herein by reference.
| As of March 31, 2020 | ||||||||
| Actual | Pro Forma(1) | |||||||
| (in thousands) | ||||||||
| Current liabilities | $ | 22,373 | $ | 22,373 | ||||
| Long-term debt, net of current portion | 18,775 | 18,775 | ||||||
| Deferred tax liabilities | 43 | 43 | ||||||
| Operating lease liabilities, net of current portion | 3,404 | 3,404 | ||||||
| Other liabilities | 1,359 | 1,359 | ||||||
| Total liabilities | 45,954 | 45,954 | ||||||
| Preferred stock, $0.0001 par value: 10,000,000 shares authorized: 10% Series A Cumulative Perpetual Preferred Stock, 8,000,000 shares liquidation preference ($10.00 per share), 1,915,637 shares issued or outstanding at March 31, 2020 Stockholders’ equity | 20,086 | 20,086 | ||||||
| Common stock, $0.0001 par value: 30,000,000 shares authorized; 2,055,158 shares issued and outstanding (net of treasury shares), actual; 3,860,212 shares issued and outstanding, pro forma | — | — | ||||||
| Treasury stock, at cost; 258,849 shares | (5,728 | ) | (5,728 | ) | ||||
| Additional paid-in capital | 144,977 | 149,421 | ||||||
| Accumulated other comprehensive loss | — | — | ||||||
| Accumulated deficit | (121,482 | ) | (121,482 | ) | ||||
| Total stockholders’ equity | 17,767 | 22,211 | ||||||
| Total liabilities, mezzanine equity and stockholders’ equity | $ | 83,807 | $ | 88,251 | ||||
| (1) | The “Pro Forma” information (a) does not give effect to the PPP Loans and (b) gives effect to the sale of the shares of common stock by us in the offering and the application of the estimated net proceeds derived thereby. We will pay all of the expenses of the offering including underwriting discounts and commissions, legal, accounting, printing filing fees and other direct costs. If the underwriters exercise their over-allotment option in full, total stockholders’ equity will increase by $697,500. |
The table above is based on an as adjusted basis to give effect to (1) the automatic conversion of all2,055,158 shares of preferredcommon stock outstanding as of DecemberMarch 31, 2003 into 43,555,3672020, and excludes, as of such date:
| ● | 26,517 shares of common stock reserved for issuance pursuant to grants outstanding under our 2011 Inducement Award Plan; |
| ● | 40,621 shares of common stock reserved for issuance pursuant to grants outstanding under our 2014 Equity Incentive Award Plan; |
| ● | 72,989 shares of common stock reserved for future issuance under our 2018 Equity Incentive Award Plan; and |
| ● | [●] shares of common stock issuable upon the exercise of the warrant granted to the underwriter in connection with this offering. |
Except as otherwise noted, all information in this prospectus reflects and assumes (i) no sale of pre-funded warrants in this offering, which, if sold, would reduce the number of shares that we are offering on a one-for-one basis, (ii) no exercise of outstanding options issued under our equity incentive plans and (iii) no exercise of the accompanying warrants and the underwriter’s warrant described above.
If you purchase our common stock and/or pre-funded warrants in this offering, you may experience dilution to the extent of the difference between the public offering price per share or pre-funded warrant, as applicable in this offering, and our as adjusted net tangible book value per share immediately after this offering. Net tangible book value per share is equal to the amount of our total tangible assets, less total liabilities, divided by the number of outstanding shares of our common stock upon completionstock. As of this offering, (2)March 31, 2020, our net tangible book value was approximately $5.8 million, or approximately $2.82 per share.
After giving effect to the filing of our restated certificate of incorporation, which provides for authorized capital stock of 150,000,000 shares of common stock and 10,000,000 shares of preferred stock, (3) theassumed sale by us of 2,109,704 shares of our common stock in this offering at an assumed initialcombined public offering price of $$2.37 per share and the receipt(the last reported sale price of the estimated net proceeds therefrom,our common stock on The Nasdaq Global Market on May 15, 2020), after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and (4) the repaymentassuming no sale of $9.4 million of outstanding short-term lines of credit.
You should read this table together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements and accompanying notes included elsewhereany pre-funded warrants in this prospectus.
| | As of December 31, 2003 | ||||||
|---|---|---|---|---|---|---|---|
| | Actual | As Adjusted | |||||
| | (In thousands, except share and per share amount) | ||||||
| Cash and cash equivalents | $ | 7,681 | $ | ||||
| Total debt: | |||||||
| Lines of credit | $ | 9,357 | $ | ||||
| Long-term debt | 6,349 | ||||||
| Notes payable to stockholders | 735 | ||||||
| 16,441 | |||||||
| Redeemable convertible preferred stock, $0.000001 par value: | |||||||
| 46,023,000 shares authorized, 43,555,313 shares issued and outstanding, actual; no shares issued and outstanding, as adjusted | 84,278 | ||||||
| Stockholders' equity (deficit): | |||||||
| Preferred stock, $0.000001 par value: 10,000,000 shares authorized and no shares issued and outstanding, as adjusted | — | ||||||
| Common stock, $0.001 par value: 53,000,000 shares authorized, 82,486 shares issued and outstanding, actual; 150,000,000 shares authorized, shares issued and outstanding, as adjusted | — | ||||||
| Additional paid in capital | 5,031 | ||||||
| Deferred compensation | (554 | ) | |||||
| Accumulated deficit | (80,180 | ) | |||||
| Total stockholders' equity (deficit) | (75,703 | ) | |||||
| Total capitalization | $ | 25,016 | $ | ||||
30
The number of shares in the table above excludes, as of December 31, 2003:
We expect to complete a 1-for- reverse stock split prior to completion of this offering. All share amounts in this prospectus have beenour as adjusted to give effect to this stock split.
As of December 31, 2003, we had a negative net book value of $(76.2) million, or $(923.97) per share of common stock, not taking into account the conversion of our outstanding preferred stock. Net tangible book value per share is equal to our total tangible assets (total assets less intangible assets) less total liabilities, divided by the number of shares of our outstanding common stock. Our pro forma net tangible book value as of DecemberMarch 31, 2003 was approximately $8.1 million, or $0.18 per share of common stock. Pro forma net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the pro forma number of shares of common stock outstanding as of December 31, 2003. Our pro forma net tangible book value and pro forma net tangible book value per share amounts give effect to the conversion of all outstanding shares of our convertible preferred stock into shares of common stock.
Dilution in pro forma net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the pro forma net tangible book value per share of common stock immediately after the completion of this offering. After giving effect to our sale of shares of common stock in this offering at an assumed initial public offering price of $ per share and after deducting estimated underwriting discounts and commissions and offering expenses payable by us, our adjusted pro forma net tangible book value as of December 31, 20032020 would have been $approximately $10.2 million, or $approximately $2.46 per share. This amount represents an immediate increasedecrease in pro forma net tangible book value of $$0.36 per share to our existing stockholders and an immediate dilutionincrease in pro forma net tangible book value of $$0.09 per share to new investors.investors purchasing shares of our common stock in this offering. The following table illustrates this per share dilution:
| Assumed initial public offering price per share | $ | |||||
| Net tangible book value per share at December 31, 2003 | $ | (923.97 | ) | |||
| Pro forma increase in tangible book value attributable to conversion of convertible preferred stock | $ | 924.15 | ||||
| Pro forma net tangible book value per share as of December 31, 2003 | $ | 0.18 | ||||
| Increase in pro forma net tangible book value per share attributable to new investors | $ | |||||
| Pro forma as adjusted net tangible book value per share after this offering | ||||||
| Dilution per share to new investors | $ | |||||
If
| Assumed public offering price per share | $ | 2.37 | ||||||
| Net tangible book value per share as of March 31, 2020 | $ | 2.82 | ||||||
| Decrease in net tangible book value per share after this offering | (0.36 | ) | ||||||
| As adjusted net tangible book value per share after this offering | 2.46 | |||||||
| Increase in net tangible book value per share to new investors | $ | 0.09 |
A $0.25 increase in the underwriters exercise their over-allotment option to purchase additional sharesassumed combined public offering price of $2.37 per share (the last reported sale price of our common stock on The Nasdaq Global Market on May 15, 2020) would result in this offering,an increase in our as adjusted pro forma net tangible book value at December 31, 2003 will be $after this offering of approximately $10.7 million, or $approximately $2.57 per share, representing an immediate increase in pro forma net tangible book value of $and the dilution per share to our existing stockholders and an immediate dilutioninvestors purchasing common stock and/or pre-funded warrants and/or pre-funded warrants in pro forma net tangible book value of $this offering would be approximately $0.05 per share, to new investors purchasing shares in this offering.
The following table summarizes, on a pro forma basis as of December 31, 2003, after giving effect toassuming that the conversion of all outstanding shares of our convertible preferred stock into common stock, the total number of shares of common stock purchased from us, the total consideration paid to us and the average price per share paid by existing stockholders and by new investors, based on an assumed initial public
32
offering price of $ per share before deducting estimated underwriting discounts and commissions and offering expenses payable by us:
| | Shares Purchased | Total Consideration | | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Average Price Per Share | ||||||||||||
| | Number | Percent | Amount | Percent | |||||||||
| Existing stockholders | 43,637,853 | % | $ | % | $ | ||||||||
| New investors | |||||||||||||
| Total | 100.0 | % | $ | 100.0 | % | $ | |||||||
If the underwriters exercise their over-allotment option in full, our existing stockholders would own % and our new investors would own % of the total number of shares of our common stock outstandingand/or pre-funded warrants sold by us remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, and assuming no sale of any pre-funded warrants in this offering.
The above discussionoffering and tables assume no exercise of anythe accompanying warrants or the underwriter’s warrant. Similarly, a decrease of $0.25 in the assumed combined public offering price of $2.37 per share would result in a decrease in our as adjusted net tangible book value after this offering of approximately $9.8 million, or approximately $2.34 per share, and there would be an increase in net tangible book value per share to investors purchasing common stock options and/or pre-funded warrants outstanding asin this offering of December 31, 2003. As$0.22 per share, assuming that the number of December 31, 2003, there were:
We may also increase or decrease the number of shares of our common stock available for future issuance under our 1995 Stock Option/Stock Issuance Plan, our 1997 Stock Option/Stock Issuance Plan and our 1998 Stock Option/Stock Issuance Plan; and
In 2004, our board of directors approved, effective upon the completionshares sold in this offering and other terms of this offering our 2004 Stock Incentive Plan, under whichdetermined at pricing.
The discussion and table above (i) assumes no exercise of the underwriters’ option to purchase up to an additional [●] shares have been reserved for future issuance, our 2004 Non-Employee Director Stock Option Program, under which shares have been reserved for future issuanceof common stock, (ii) assumes no exercise of the warrant to be issued to the underwriter in connection with this offering, (iii) assumes no sale of any pre-funded warrants in this offering and our 2004 Employee Stock Purchase Program, under which shares have been reserved for acquisition by our employees. Tono exercise of the extentaccompanying warrants or the underwriter’s warrant (iv) and does not give effect to the PPP Loan.
The foregoing discussion and table do not take into account further dilution to new investors that anycould occur upon the exercise of outstanding options or warrants are exercised or shares acquired, there will be further dilution to new investors.
having a per share exercise price less than the public offering price per share of common stock sold in this offering. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
SELECTED CONSOLIDATED FINANCIAL DATA
The selected consolidated statement of operations data for the years ended December 31, 2001, 2002 and 2003 and the selected balance sheet data as of December 31, 2002 and 2003, are derived from the audited financial statements for such years and as of such dates, which are included elsewhere in this prospectus. The selected consolidated statement of operations data for the years ended December 31, 1999 and 2000, and the selected balance sheet data as of December 31, 1999, 2000 and 2001, are derived from audited financial statements, which have been audited by Ernst & Young LLP, our independent auditors, for such years and as of such dates, which are not included in this prospectus. Historical results are not necessarily indicative of future results. The following selected financial data should be read together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and related notes included elsewhere in this prospectus. The selected financial data in this section is not intended to replace the financial statements.
| | Years ended December 31, | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statement of Operations Data: | 1999 | 2000 | 2001 | 2002 | 2003 | |||||||||||||
| | (In thousands, except per share data amounts) | |||||||||||||||||
| Revenues: | ||||||||||||||||||
| DIS | $ | — | $ | 1,260 | $ | 10,239 | $ | 23,005 | $ | 34,849 | ||||||||
| Product | 284 | 5,815 | 18,065 | 18,527 | 21,388 | |||||||||||||
| Total revenues | 284 | 7,075 | 28,304 | 41,532 | 56,237 | |||||||||||||
| Cost of revenues: | ||||||||||||||||||
| DIS | — | 839 | 8,344 | 16,599 | 24,463 | |||||||||||||
| Product | 265 | 9,834 | 13,192 | 13,633 | 15,092 | |||||||||||||
| Total cost of revenues | 265 | 10,673 | 21,536 | 30,232 | 39,555 | |||||||||||||
| Gross profit (loss) | 19 | (3,598 | ) | 6,768 | 11,300 | 16,682 | ||||||||||||
Operating expenses: | ||||||||||||||||||
| Research and development | 10,063 | 2,372 | 3,009 | 2,967 | 2,191 | |||||||||||||
| Sales and marketing | 1,455 | 3,586 | 9,974 | 8,066 | 6,008 | |||||||||||||
| General and administrative | 1,967 | 2,878 | 8,161 | 9,497 | 8,097 | |||||||||||||
| Amortization and impairment of intangible assets | — | 194 | 991 | 1,011 | 444 | |||||||||||||
| Stock-based compensation | — | 311 | 1,579 | 606 | 226 | |||||||||||||
| Total operating expenses | 13,485 | 9,341 | 23,714 | 22,147 | 16,966 | |||||||||||||
| Loss from operations | (13,466 | ) | (12,939 | ) | (16,946 | ) | (10,847 | ) | (284 | ) | ||||||||
| Other income (expense), net | 274 | (537 | ) | (2,965 | ) | (1,925 | ) | (1,396 | ) | |||||||||
| Net loss | $ | (13,192 | ) | $ | (13,476 | ) | $ | (19,911 | ) | $ | (12,772 | ) | $ | (1,680 | ) | |||
| Net loss applicable to common stockholders | $ | (13,192 | ) | $ | (13,524 | ) | $ | (20,041 | ) | $ | (13,037 | ) | $ | (2,006 | ) | |||
| Basic and diluted net loss per share(1): | ||||||||||||||||||
| Historical | $ | (780.49 | ) | $ | (722.22 | ) | $ | (898.86 | ) | $ | (409.23 | ) | $ | (36.46 | ) | |||
| Pro forma | $ | (0.04 | ) | |||||||||||||||
| Shares used to compute basic and diluted net loss per share(1): | ||||||||||||||||||
| Historical | 17 | 19 | 22 | 32 | 55 | |||||||||||||
| Pro forma | 43,610 | |||||||||||||||||
| The composition of stock-based compensation is as follows: | ||||||||||||||||||
| Cost of product revenue | $ | 200 | $ | 72 | $ | 83 | ||||||||||||
| Cost of DIS revenue | 98 | 51 | 31 | |||||||||||||||
| Research and development | 96 | 61 | 8 | |||||||||||||||
| Sales and marketing | 541 | 228 | 18 | |||||||||||||||
| General and administrative | 644 | 194 | 86 | |||||||||||||||
| $ | 1,579 | $ | 606 | $ | 226 | |||||||||||||
34
As of December 31, | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 1999 | 2000 | 2001 | 2002 | 2003 | |||||||||||
| | (In thousands) | |||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash and cash equivalents | $ | 2,626 | $ | 6,555 | $ | 1,967 | $ | 6,988 | $ | 7,681 | ||||||
| Working capital | 801 | 5,481 | (1,668 | ) | 3,781 | 2,578 | ||||||||||
| Total assets | 5,699 | 23,050 | 29,922 | 33,119 | 35,159 | |||||||||||
| Total debt | 2,570 | 8,614 | 14,469 | 13,932 | 16,441 | |||||||||||
| Redeemable convertible preferred stock | 32,259 | 52,255 | 66,531 | 83,952 | 84,278 | |||||||||||
| Total stockholders' equity (deficit) | (31,050 | ) | (43,479 | ) | (61,835 | ) | (73,928 | ) | (75,703 | ) | ||||||
35
MANAGEMENT'S DISCUSSION AND ANALYSIS OFFINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing at the end of this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review the "Risk Factors" section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a leader in the development, manufacture and distribution of solid-state medical imaging products and services. We were the first company to develop and commercialize a solid-state medical gamma camera for the detection of cardiovascular disease and other medical conditions. Our high performance imaging systems are mobile and provide enhanced operability and reliability and improved patient comfort and utilization when compared to traditional vacuum tube cameras. The cameras and accompanying equipment fit easily into spaces as small as seven feet by eight feet and facilitate the delivery of nuclear medicine procedures directly in a physician's office, an outpatient hospital setting or within multiple departments of a hospital. As of December 31, 2003, we had an installed base of 300 gamma cameras, over 95% of which were in the United States, including 54 cameras operated by our wholly-owned subsidiary, Digirad Imaging Solutions, Inc., or DIS.
According to industry reports, the growth rates in 2002 for procedures performed in physician offices was approximately 44% and in hospitals was approximately 6%. We believe this trend is driven by the desire of cardiologists to control their patients' diagnosis and treatment and to generate revenue that would otherwise be lost if the patient were referred to a hospital or imaging center. The mobile feature of our technology also provides us with a significant advantage in the delivery of nuclear cardiology imaging services. Through DIS, we offer FlexImaging, our mobile and comprehensive leasing service for physicians who wish to perform nuclear cardiology and nuclear medicine procedures in their offices, but do not have the patient volume, capital or personnel to justify purchasing an imaging system. DIS is currently offered in 17 states. Physicians enter into annual contracts for imaging services delivered on a per-day basis. Our annual lease contracts typically provide for one day of service per week. We sell our imaging systems to physician practices, outpatient clinics and hospitals primarily in the United States and have sold a limited number of imaging systems internationally. Our product revenue consists of sales of our solid-state gamma cameras, custom designed chairs and accessories, such as printers, viewing workstations, connectivity and collimators and revenue from our maintenance contracts.
In 2000, we sold our first solid-state gamma camera and launched our DIS business. From 2000 to 2003, our consolidated revenues grew from $7.1 million to $56.2 million. DIS and product revenues accounted for 62.0% and 38.0%, respectively, of our consolidated revenues for the year ended December 31, 2003. Given the recurring contractual revenue stream from our DIS business and our strategy to continue to expand the number of areas where we offer DIS services, we expect DIS revenue to continue to grow at a higher rate than product revenue and to continue to represent a large percentage of consolidated revenues. We attribute the overall growth of our business to geographical expansion, increased market penetration, awareness and acceptance, and the shift in the delivery of nuclear cardiology imaging procedures from hospitals to physician offices.
We reduced our net loss by $11.1 million from $12.8 million in 2002 to $1.7 million in 2003. Furthermore, we have incurred substantial operating losses since our inception. As of December 31, 2003, our accumulated deficit was $80.2 million. We believe that we will achieve our first full year of profitability
36
in 2004, and intend to continue to enhance profitability through increased volume and improved margins, although we may incur losses in any given quarter.
We experience some seasonality in our DIS business as a result of winter holidays, inclement weather and summer slowdowns principally relating to vacations. Historically, these variables have had the least impact on our second quarter operating results.
We are currently in the process of transitioning our manufacturing operations from several separate facilities to a single facility in Poway, California. We believe this will consolidate our operations and improve efficiencies. We currently purchase some components from sole source providers and are qualifying or seeking second source providers in an effort to diversify our providers.
Results Of Operations
The following table sets forth our results from operations, expressed as percentages of revenues for the years ended December 31, 2001, 2002 and 2003:
| | 2001 | 2002 | 2003 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Revenues: | |||||||||
| DIS | 36.2 | % | 55.4 | % | 62.0 | % | |||
| Product | 63.8 | 44.6 | 38.0 | ||||||
| Total revenues | 100.0 | 100.0 | 100.0 | ||||||
| Cost of revenues: | |||||||||
| DIS | 29.5 | 40.0 | 43.5 | ||||||
| Product | 46.6 | 32.8 | 26.8 | ||||||
| Total cost of revenues | 76.1 | 72.8 | 70.3 | ||||||
| Gross profit | 23.9 | 27.2 | 29.7 | ||||||
| Operating expenses: | |||||||||
| Research and development | 10.6 | 7.1 | 3.9 | ||||||
| Sales and marketing | 35.3 | 19.4 | 10.7 | ||||||
| General and administrative | 28.8 | 22.9 | 14.4 | ||||||
| Amortization and impairment of intangible assets | 3.5 | 2.4 | 0.8 | ||||||
| Stock-based compensation | 5.6 | 1.5 | 0.4 | ||||||
| Total operating expenses | 83.8 | 53.3 | 30.2 | ||||||
| Loss from operations | (59.9 | ) | (26.1 | ) | (0.5 | ) | |||
| Other income (expense) | (10.5 | ) | (4.6 | ) | (2.5 | ) | |||
| Accretion of deferred issuance costs on preferred stock | (0.4 | ) | (0.7 | ) | (0.6 | ) | |||
| Net loss applicable to common stockholders | (70.8 | )% | (31.4 | )% | (3.6 | )% | |||
Comparison of Years Ended December 31, 2003 and 2002
Revenues
Consolidated. Our revenues are divided between two primary operating segments: product sales and our DIS business. Our product revenues consist primarily of selling our solid-state gamma cameras and accessories to physicians and hospitals. DIS revenues are comprised of performing our DIS services for physicians on a per day basis in accordance with a 12-month lease with annual commitment levels. Our standard lease terms provide for automatic renewals for an additional 12-month period if the lease is not terminated in writing by the customer generally 90 days or more prior to the end of the term.
Consolidated revenues in 2003 increased to $56.2 million from $41.5 million in 2002, which represents an increase of $14.7 million, or 35.4%, primarily as a result of increased demand for our DIS services and our Cardius products. We believe that this growth was due to increased customer awareness and acceptance of our products and services.
37
DIS. Our DIS revenue increased to $34.8 million in 2003 from $23.0 million in 2002, which represents an increase of $11.8 million, or 51.5%. The increase in DIS revenue was primarily attributable to an increase in the number of physicians purchasing our DIS services and increases in the amount of services purchased by existing physician customers. To respond to this increased demand, we deployed eight additional mobile systems. We anticipate that our DIS revenue will increase if we expand into new markets and continue to penetrate existing markets. DIS revenue accounted for 62.0% of total revenues in 2003 versus 55.4% in 2002. Collectively, our DIS business operated 54 mobile and fixed site systems as of December 31, 2003.
Product. Our product revenue increased to $21.4 million in 2003 from $18.5 million in 2002, which represents an increase of $2.9 million, or 15.4%. This increase was due to increased sales of our gamma cameras and maintenance contract revenue. We sold 79 cameras in 2003 compared to 74 cameras in 2002. Product revenue accounted for 38.0% of total revenues for 2003 versus 44.6% in 2002. Maintenance contract revenues were $2.1 million in 2003 and $521,000 in 2002.
Gross Profit
Consolidated. Consolidated gross profit increased to $16.7 million in 2003 from $11.3 million in 2002, which represents an increase of $5.4 million, or 47.6%. Consolidated gross profit as a percentage of revenue increased to 29.7% in 2003 from 27.2% in 2002 primarily as a result of an increase in revenue and product cost reductions.
DIS. Cost of DIS revenue consists primarily of labor, radiopharmaceuticals, equipment depreciation and other costs associated with the provision of services. Our direct headcount relating to our DIS business increased to 137 employees at the end of 2003 from 112 employees at the end of 2002. Cost of DIS revenue increased to $24.5 million in 2003 from $16.6 million in 2002, which represents an increase of $7.9 million, or 47.4%. As a result, DIS gross profit increased to $10.4 million in 2003 from $6.4 million in 2002, which represents an increase of $4.0 million, or 62.1%. DIS gross profit as a percentage of revenue increased to 29.8% in 2003 from 27.8% in 2002. The increase was primarily a result of increased volumes and improving the per unit cost of various items consumed in providing the imaging services.
Product. Cost of goods sold primarily consists of materials, labor and overhead costs associated with the manufacturing of our products. Cost of goods sold increased to $15.1 million in 2003 from $13.6 million in 2002, which represents an increase of $1.5 million, or 10.7%. As a result, product gross profit increased to $6.3 million in 2003 from $4.9 million in 2002, which represents an increase of $1.4 million, or 28.6%. Product gross profit as a percentage of revenue increased to 29.4% in 2003 from 26.4% in 2002. The increase was primarily a result of the increase in the volume of cameras produced and cost reductions in manufacturing materials and manufacturing processes due to the introduction of our third-generation camera heads in July 2003.
Operating Expenses
Research and Development. Research and development expenses consist primarily of costs associated with the design, development, testing, deployment and enhancement of our products and manufacturing capabilities. The primary costs are salaries and fringe benefits, consulting fees, facilities and overhead charges and nonrecurring engineering costs. Research and development expenses decreased to $2.2 million in 2003 from $3.0 million in 2002, which represents a decrease of $776,000, or 26.2%, primarily as a result of our efforts to develop and launch our Cardius camera product line in 2002. Research and development headcount increased to 16 employees in 2003 from 14 employees in 2002. In the future, we expect to continue to invest between approximately 10% and 12% of product revenue on research and development as we continue to improve our existing technology and innovate.
Sales and Marketing. Sales and marketing expenses consist primarily of salaries, commissions, bonuses, recruiting costs, travel, marketing and collateral materials and tradeshow costs. Sales and
38
marketing expenses decreased to $6.0 million in 2003 from $8.1 million in 2002, which represents a decrease of $2.1 million, or 25.5%. In late 2002, we restructured the management of the sales organization and modified the compensation structure, resulting in a significant reduction in sales expense both in dollars and as a percent of revenue. In 2003, sales and marketing expenses were 10.7% of total revenue versus 19.4% in 2002. We expect to increase our sales and marketing efforts in relation with any revenue growth, as we focus on increasing market awareness of our products and offerings.
General and Administrative. General and administrative expenses consist primarily of salaries and other related costs for finance and accounting, human resources and other personnel, as well as legal and other professional fees. General and administrative expenses decreased to $8.1 million in 2003 from $9.5 million in 2002, which represents a decrease of $1.4 million, or 14.7%. Reduced outside legal expenses, which were partially offset by the addition of in-house general counsel, and a reduction in headquarters headcount, all contributed to lower general and administrative expenses. General and administrative headcount was reduced by one employee by the end of 2003 to 33 employees versus 34 employees at the end of 2002. In 2003, general and administrative expenses amounted to 14.4% of total revenue versus 22.9% in 2002. If the offering contemplated by this prospectus is completed, we will be required to incur additional general and administrative costs to meet various public reporting and compliance requirements.
Amortization and Impairment of Intangible Assets. Intangible assets primarily represent customer contracts relating to our DIS business that we purchased from a third party in 2000 and capitalized patent and trademark portfolio costs, both of which are amortized over their respective useful life. Amortization and impairment of intangibles decreased to $444,000 in 2003 from $1.0 million in 2002. The significant decline from 2002 to 2003 was principally a result of impairment charges recorded in 2002 associated with these purchased contracts.
Stock-Based Compensation Charges. Deferred compensation for stock options granted has been determined as the difference between the exercise price and the fair value of our common stock on the date of grant. Options or awards issued to non-employees are recorded at their fair value in accordance with SFAS No. 123 and periodically remeasured in accordance with EITF 96-18 and recognized over the respective service or vesting period. In connection with the grant of stock options to employees, we recorded deferred stock-based compensation of $781,000 and zero for the years ended December 31, 2003 and 2002, respectively. We recorded these amounts as a component of stockholders' equity and are amortizing the amount, on an accelerated basis, as a non-cash charge to operations over the vesting period of the options. We recorded stock-based compensation of $226,000 and $606,000 for the years ended December 31, 2003 and 2002, respectively. We expect that charges to be recognized in future periods from amortization of deferred compensation related to employee stock options grants will be $318,000, $152,000, $69,000 and $16,000 for the years ending December 31, 2004, 2005, 2006 and 2007, respectively. During February and March of 2004, we granted options to purchase 879,300 shares of common stock to employees and board members. We anticipate recording deferred stock compensation of approximately $1.3 million for the difference between the original exercise price per share determined by our board of directors and the revised estimate of fair value per share.
Other Income (Expense)
Interest expense decreased to $1.4 million in 2003 from $2.0 million in 2002, which represents a decrease of $558,000, or 28.1%. The reduction is a result of a decrease in the variable interest rates on two accounts receivable credit lines and a reduction on capital leases, and $243,000 of debt discount associated with our $1.9 million bridge financing in 2002.
Interest income decreased to $35,000 in 2003 from $65,000 in 2002, which represents a decrease of $30,000, or 45.6%, primarily due to lower interest rates in 2003 on cash and cash equivalent accounts.
39
Net Loss
Net loss decreased to $1.7 million in 2003 from $12.8 million in 2002, which represents a decrease of $11.1 million, or 86.8%, as a result of the factors described above.
Comparison of Years Ended December 31, 2002 and 2001
Revenues
Consolidated. Our consolidated revenues increased to $41.5 million in 2002 from $28.3 million in 2001, which represents an increase of $13.2 million, or 46.7%. This increase was due primarily to a significant increase in DIS imaging services volume as DIS began to achieve more market acceptance.
DIS. Our DIS revenue increased to $23.0 million in 2002 compared to $10.2 million in 2001, which represents an increase of $12.8 million, or 124.7%, resulting primarily from geographical expansion and market acceptance. Our DIS revenue accounted for 55.4% of total revenues in 2002 versus 36.2% in 2001.
Product. Our product sales revenue increased to $18.5 million in 2002 from $18.1 million in 2001, which represents an increase of $462,000, or 2.6%, in 2002. The minor increase was a result of our decision to flatten the sales and marketing organization, resulting in a low product sales growth rate over the prior year. Product revenue accounted for 44.6% of total revenues in 2002 versus 63.8% in 2001.
Gross Profit
Consolidated. Consolidated gross profit increased to $11.3 million in 2002 from $6.8 million in 2001, which represents an increase of $4.5 million, or 67.0%. Consolidated gross profit as a percentage of revenue increased to 27.2% in 2002 from 23.9% in 2001, primarily as a result of a year-to-year increase in revenue and lower cost per day to perform our DIS services.
DIS. Cost of DIS revenue increased to $16.6 million in 2002 from $8.3 million in 2001, which represents an increase of $8.3 million, or 98.9%. As a result, DIS gross profit increased to $6.4 million in 2002 from $1.9 million in 2001, which represents an increase of $4.5 million, or 238.1%. DIS gross profit as a percentage of revenue increased to 27.8% in 2002 from 18.5% in 2001. The increase was primarily a result of increased volume and other servicing efficiencies as DIS expanded geographically within the United States.
Product. Cost of goods sold increased to $13.6 million in 2002 from $13.2 million in 2001, which represents an increase of $440,000, or 3.3%. As a result, product gross profit remained flat at $4.9 million from 2001 to 2002. Product gross margin as a percentage of revenue decreased to 26.4% in 2002 from 27.0% in 2001.
Operating Expenses
Research and Development. Research and development expenses were $3.0 million in both 2001 and 2002. Although we reduced the number of employees in 2002, the launch of the Cardius camera line and associated expenses offset any reductions in research and development expenses. We reduced our research and development headcount in 2002 to 14 employees from 25 employees at the end of 2001. Research and development expenses amounted to 7.1% of consolidated revenues in 2002 versus 10.6% in 2001.
Sales and Marketing. Sales and marketing expenses decreased to $8.1 million in 2002 from $10.0 million in 2001, which represents a decrease of $1.9 million, or 19.1%. The decrease in sales and marketing expense was related primarily to reductions in our sales and marketing personnel in early 2002 as we repositioned ourselves to focus on profitable growth. Sales and marketing headcount was reduced to 29 employees at the end of 2002 versus 50 employees at the end of 2001. Sales and marketing expenses amounted to 19.4% of consolidated revenues in 2002 compared to 35.3% in 2001.
40
General and Administrative. General and administrative expenses increased to $9.5 million in 2002 from $8.2 million in 2001, which represents an increase of $1.3 million, or 16.4%. The increase resulted primarily from increases in accounting, human resource and other administrative headcount expenses and settlement fees in 2002. General and administrative expenses amounted to 22.9% of consolidated revenues in 2002, compared to 28.8% in 2001.
Amortization and Impairment of Intangible Assets. Amortization of intangible assets is primarily amortization of capitalized costs associated with purchased contracts and capitalized patent and trademark costs; both are amortized over their respective useful life. Amortization and impairment of intangible assets was constant year-to-year, $1.0 million in 2002 and 2001.
Stock-Based Compensation Charges. Total stock-based compensation decreased to $606,000, or 62.7%, in 2002 from $1.6 million in 2001, which represents a decrease of $972,000, or 61.6%, as the remaining deferred compensation was recorded in 2002.
Other Income (Expense)
Interest expense increased to $2.0 million in 2002 from $1.4 million in 2001, which represents an increase of $551,000, or 38.3%. The increase was primarily attributable to increases in the accounts receivable credit line borrowings and an increase in capital equipment lease lines for DIS equipment. We also incurred $243,000 of expense in conjunction with our bridge financing in 2002.
Interest income decreased to $65,000 in 2002 from $118,000 in 2001, which represents a decrease of $53,000, or 44.9%, due to the termination of a camera lease to a customer in 2002.
Other expenses were $1.6 million in 2001, which were related to the costs incurred in connection for a proposed initial public offering which was not completed.
Net Loss
Net loss decreased to $12.8 million in 2002 from $19.9 million in 2001, which represents a decrease of $7.1 million, or 35.9%. Net loss in 2001 decreased as a result of the factors described above.
Liquidity And Capital Resources
General
We require capital principally for operating our DIS business, interest payments, working capital, debt service and capital expenditures. Our capital expenditures consist primarily of manufactured DIS cameras, computer hardware and software. Working capital is required principally to finance accounts receivable and inventory. Our working capital requirements vary from period to period depending on manufacturing volumes, the timing of deliveries and the payment cycles of our customers and payors.
We have historically funded our operations principally through private equity financings supplemented with credit lines, equipment financing arrangements and cash from operations. We completed seven private placements of preferred stock between March 1995 and June 2002, yielding aggregate net proceeds of approximately $83.5 million. At December 31, 2003, our outstanding borrowings totaled $16.4 million. Based upon our current level of expenditures, we believe proceeds from this offering, together with cash flows from operating activities, availability under our current or future revolving credit lines will be adequate to meet our anticipated cash requirements for interest payments, working capital, debt service and capital expenditures for the next 12 months.
Our preferred stock is redeemable on or after July 31, 2004 upon the request of certain preferred stock investors. We must redeem all outstanding shares of our preferred stock by paying in cash its redemption value plus declared but unpaid dividends. No dividends have been declared through December 31, 2003. If the funds of our company that are legally available for redemption are insufficient to redeem the total number of preferred shares to be redeemed, those funds which are legally available
41
must be used to redeem the maximum possible number of shares pro rata among the various series of preferred stock. If the offering contemplated by this prospectus is not completed, and the redeemable preferred shares remain outstanding, we do not anticipate having legally available funds to redeem any portion of the preferred shares in 2004.
As of December 31, 2003, cash and cash equivalents totaled $7.7 million compared to $7.0 million at December 31, 2002. We currently invest our cash reserves in money market funds.
Net cash provided by operations was $158,000 in 2003. Net cash used in operating activities amounted to approximately $9.8 million and $16.8 million for the years ended December 31, 2002 and 2001, respectively. For these periods, net cash used in operating activities resulted primarily from operating losses and net increases in accounts receivable resulting from the growth in our business.
Net cash used in investing activities amounted to approximately $2.0 million, $1.8 million and $7.8 million for the years ended December 31, 2003, 2002 and 2001 respectively. Investing activities consist primarily of capital expenditures.
��Net cash provided by financing activities amounted to approximately $2.5 million, $16.6 million and $20.0 million for the years ended December 31, 2003, 2002 and 2001, respectively. Private placements of our preferred stock and proceeds from bank borrowings, lease financings and credit line borrowings were primarily responsible for the net cash provided by financing activities.
Working Capital
We believe that DIS and product revenues will continue to increase. We believe that a majority of this increase will occur in the cardiology office market from the use of DIS, which could increase our accounts receivable due to the extended payment cycles we experience in that business. We have adopted a number of policies and procedures to reduce these extended payment cycles, and believe that we will continue to improve collection turnaround times. For example, at the end of 2003, DIS revenues grew 51.5% over 2002, whereas the receivables increased 22.7% over 2002. If accounts receivable increase, we will use available cash on hand to fund the increase. We expect that cash on hand, cash flow from operations and additional borrowings under our new revolving credit facility will be sufficient to meet our working capital needs over the next 12 months.
Debt Service
In October 2003, we renewed an agreement for a $5.0 million revolving line of credit to provide working capital for our product sales. Borrowings under this line of credit accrue interest at the bank's floating prime rate plus 1.75% and are limited based on a formula that takes into account eligible amounts of accounts receivables, inventory and other factors. We are required to make monthly interest payments on this line of credit, which expires in October 2004, with any unpaid balance due upon expiration. As of December 31, 2003, our outstanding balance under this facility was $4.8 million. We intend to repay this loan in full with proceeds from this offering.
In January 2001, we entered into a loan and security agreement for a revolving line of credit to provide working capital for our DIS business. We are authorized to draw up to $5.0 million and the borrowings under the line of credit, as amended in March 2004, accrue interest at the higher of 6.0% or prime plus 1.25%. This revolving line of credit expires in December 2004. As of December 31, 2003, our outstanding balance under this loan and security agreement totaled $4.5 million. We intend to repay this loan in full with proceeds from this offering.
In the event we are unable to complete the offering, we believe we can renew our credit lines or access alternate sources of financing based on the improvement in our operating results and our cash flow.
We have notes payable to our stockholders totaling $735,000, which bear interest at 6.35% per year. We are obligated to repay these notes equally over the 12 quarters beginning March 31, 2004.
42
As of December 31, 2003, we had capital lease obligations totaling $6.3 million. These obligations are secured by the specific equipment financed under each lease and will be repaid monthly over the lease terms, which range from 48 to 63 months. Our DIS subsidiary entered into the majority of these capital lease obligations.
We are committed to making future cash payments on notes payable to our stockholders, capital leases (including interest), operating leases and lines of credit. We have not guaranteed the debt of any other party. The following table summarizes our contractual obligations as of December 31, 2003 (dollars in thousands):
| | Payments due by period | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contractual obligations | Total | Current | 1-3 years | 3-5 years | More than 5 years | ||||||||||
| Notes payable to stockholders | $ | 735 | $ | 245 | $ | 245 | $ | 245 | $ | — | |||||
| Capital lease obligations | 7,505 | 2,741 | 4,197 | 567 | — | ||||||||||
| Operating lease obligations | 3,861 | 696 | 1,376 | 1,170 | 619 | ||||||||||
| Lines of credit | 9,357 | 9,357 | — | — | |||||||||||
| Total | $ | 21,458 | $ | 13,039 | $ | 5,818 | $ | 1,982 | $ | 619 | |||||
Quantitative And Qualitative Disclosures About Market Risk
Our exposure to market risk due to changes in interest rates relates primarily to the increase or decrease in the amount of interest income we can earn on our investment portfolio and on the increase or decrease in the amount of interest expense we must pay on our various outstanding debt instruments. Our risk associated with fluctuating interest rates is limited, however, to certain of our long-term debt and capital lease obligations, all of which have interest rates that are closely tied to market rates, and our investments in interest rate sensitive financial instruments. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. We attempt to increase the safety and preservation of our invested principal funds by limiting default risk, market risk and reinvestment risk. We mitigate default risk by investing in investment grade securities. A hypothetical 100 basis point adverse move in interest rates along the entire interest rate yield curve would not materially affect the fair value of our interest sensitive financial instruments. Declines in interest rates over time will, however, reduce our interest income while increases in interest rates over time will increase our interest expense.
Inflation
We do not believe that inflation has had a material impact on our business or operating results during the periods presented.
Related Party Transactions
For a description of our related party transactions, see the section of this prospectus entitled "Certain Relationships and Related Transactions."
Critical Accounting Policies
The Securities and Exchange Commission defines critical accounting policies as those that are, in management's opinion, very important to the portrayal of our financial condition and results of operations and require our management's most difficult, subjective or complex judgments. In preparing our financial statements in accordance with generally accepted accounting principles in the United States, we must often make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures at the date of the financial statements and during the reporting period. Some of those judgments can be subjective and complex. Consequently, actual results could differ from our
43
estimates. The accounting policies that are most subject to important estimates or assumptions include those described below.
Revenue Recognition
We recognize revenue in accordance with Staff Accounting Bulletin No. 101 when each of the following four criteria are met:
For our product revenue, these criteria are usually met upon delivery. Our DIS revenue is recorded once the services and disposables are provided and consumed, which is normally on the day of the service. Reductions to product revenue are recorded to provide for payment adjustments and credit memos. Reductions to our DIS revenue are recorded to provide for payment adjustments and credit memos. In addition, we establish reserves against our DIS revenue to allow for uncollectible items relating to patient co-payments and contractual allowances and other adjustments, based on historical collection experience.
Accounts Receivable and Allowance for Doubtful Accounts
We use a combination of factors in evaluating the collectibility of accounts receivable. We generally establish reserves for bad debt based on the length of time that the receivables are past due. Each account is reviewed and a percentage varying from zero to 100% for each account is established. We do not establish reserves for accounts with a history of payment without disputes. We generally reserve between 20% and 50% of the outstanding balance for accounts that are more than 180 days late and under dispute. We reserve 100% of the outstanding balance for accounts that we believe constitute a high risk of default based on factors such as level of dispute, payment history and our knowledge of a customer's inability to meet its obligations. We also consider bad debt write-off history. Our estimates of collectibility could be reduced by material amounts by changed circumstances, such as a higher number of defaults or material adverse changes in a payor's ability to meet its obligations.
Long-Lived Assets
We state property and equipment and purchased contracts at cost. We capitalize betterments, which extend the useful life of the equipment. We calculate depreciation on property and equipment and purchased contracts on the straight-line method over the estimated useful live (three to seven years for property and equipment and five years for purchased contracts) of the assets. We follow Financial Accounting Standards Board ("FASB")Statement of Financial Accounting Standards ("SFAS") No. 144, Accounting for Impairment or Disposal of Long-Lived Assets, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets' carrying amount. If such assets are considered to be impaired, we measure the impairment be recognized by the amount by which the carrying amount of the assets exceeds the estimated fair value of the assets. We have taken impairment charges on certain customer contracts purchased during 2000 from Nuclear Imaging Systems, Inc. and Florida Cardiology, Inc. Assets are examined for impairment annually or more frequently if events occur that may indicate a potential asset impairment.
Inventory
We state inventories at the lower of cost (first-in, first-out) or market (net realizable value). Costs include material, labor and manufacturing overhead costs. Inventory expected to be converted into equipment to be used as mobile imaging units in DIS is classified as property and equipment. We review our inventory balances monthly for excess sale products or obsolete inventory levels. Except where firm
44
orders are on-hand, we consider inventory quantities of sale products in excess of the last 12 months' demand as excess and reserve for them at levels between 20% and 50% of cost, depending on our knowledge and forecast for the product. We establish obsolescence reserves on an increasing basis from 0% for active, high-demand products, to 100% for obsolete products. We review the reserve periodically and, if necessary, make adjustments. We rely on historical information to support our reserve and utilize management's business judgment. Once the inventory is written down, we do not adjust the reserve balance until the inventory is sold.
Warranty
We provide a warranty on certain of our products and accrue the estimated cost at the time revenue is recorded. Historically, the warranty periods have ranged from up to 24 months. Since July 2002, substantially all of the warranty periods have been 12 months before customer-sponsored maintenance begins. Warranty reserves are established based on historical experience with failure rates and repair costs and the number of units at customers covered by warranty. We review warranty reserves monthly and, if necessary, make adjustments.
New Accounting Pronouncements
In November 2002, the FASB issued FIN 45,Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others. This interpretation elaborates on the disclosures required in financial statements concerning obligations under certain guarantees. We adopted the disclosure requirements of this interpretation that were effective on December 31, 2002. The recognition provisions of the interpretation are effective in 2003 and are applicable only to guarantees issued or modified after December 31, 2002. We have not issued or modified any such guarantees and accordingly the interpretation did not have a material impact on our financial position, results of operations or cash flows for the fiscal year ended December 31, 2003.
In January 2003, the FASB issued FIN No. 46,Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51. FIN No. 46 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. In December 2003, the FASB issued FIN No. 46R, a revision to FIN No. 46. FIN No. 46R provides a broad deferral of the latest date by which all public entities must apply FIN No. 46 to certain variable interest entities to the first reporting period ending after March 15, 2004. We do not expect the adoption of FIN No. 46 or FIN No. 46R to have a material impact upon our financial position, cash flows or results of operations.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity. SFAS No. 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. The adoption of SFAS No. 150 did not have a material effect on our consolidated financial statements.
45
Overview
We are a leader in the development, manufacture and distribution of solid-state medical imaging products and services for the detection of cardiovascular disease and other medical conditions. We designed and commercialized the first solid-state gamma camera. Our initial focus is nuclear cardiology imaging procedures performed with gamma cameras, which we believe generate revenue of approximately $10.0 billion annually. Our target markets are primarily physician practices and outpatient clinics, which we believe constitute approximately 25% of this total market, or $2.5 billion.
By utilizing solid-state technology rather than bulky vacuum tubes, we believe that our imaging systems maintain image quality while offering significant advantages over vacuum tube-based systems, including mobility through reduced size and weight, enhanced operability and reliability, and improved patient comfort and utilization. Due to size and other limitations of vacuum tube cameras, nuclear imaging has traditionally been confined to dedicated and customized space within a hospital or imaging center. The mobility of our imaging systems enables us to deliver nuclear imaging procedures in a wide range of clinical settings—physician offices, outpatient clinics or within multiple departments in a hospital.
We sell our imaging systems to physicians, outpatient clinics and hospitals. In addition, through our wholly-owned subsidiary, Digirad Imaging Solutions, Inc., or DIS, we also offer a comprehensive and mobile imaging leasing and services program, called FlexImaging, for physicians who wish to perform nuclear cardiology imaging procedures in their offices but do not have the patient volume, capital or resources to justify purchasing a gamma camera. DIS provides physician customers with an imaging system, certified personnel, required licensure and other support for the performance of nuclear imaging procedures under the supervision of our physician customers. Physicians enter into annual contracts for imaging services delivered on a per-day basis. DIS currently operates 23 regional hubs and seven fixed sites in 17 states.
The mobility of our imaging systems and the flexibility of our leasing service allow cardiologists to provide nuclear imaging procedures in their offices to patients that they historically had to refer to hospitals or imaging centers. As a result, we provide physicians with more control over the diagnosis and treatment of their patients and enable physicians to capture revenue from procedures that would otherwise be referred to these hospitals and imaging centers.
Nuclear imaging is a clinical diagnostic tool that has been in use for over 40 years with reimbursement codes established since 1971. According to industry sources, approximately 18.4 million nuclear imaging procedures were performed in the United States in 2002, of which 9.9 million procedures were cardiac applications, a volume that is expected to grow by approximately 25% annually over the next three years. We estimate that the growth rate in 2002 for nuclear imaging procedures performed in physician offices was approximately 44% and in hospitals was approximately 6%. We expect the mobility of our imaging systems will continue to allow us to capitalize on this shift in the delivery of nuclear cardiology imaging services from hospitals to physician offices.
The target market for our products is the approximately 30,000 cardiologists in the United States that perform nuclear cardiology procedures. To date, we have sold or provided imaging services through DIS to approximately 500 physicians. In 2003, DIS performed over 66,000 patient procedures.
We sold our first gamma camera in March 2000 and we established DIS in September 2000. We had consolidated revenues and net losses of $41.5 million and $12.8 million, respectively, in fiscal 2002 and $56.2 million and $1.7 million, respectively, in fiscal 2003. Revenue from DIS and from our camera sales constituted 62% and 38%, respectively, of our 2003 consolidated revenues. We believe DIS will continue to provide us with recurring annual contractual revenue and comprise the largest component of our consolidated revenue.
46
Market Opportunity
Nuclear Imaging
Nuclear imaging is a form of diagnostic imaging in which depictions of the internal anatomy or physiology are generated primarily through non-invasive means. Diagnostic imaging facilitates the early diagnosis of diseases and disorders, often minimizing the scope, cost and amount of care required and reducing the need for more invasive procedures. Currently, five major types of non-invasive diagnostic imaging technologies are available: x-ray; magnetic resonance imaging, or MRI; computerized tomography, or CT; ultrasound; and nuclear imaging.
Nuclear imaging measures varying degrees of physiological activity. Physicians use the images and related clinical information to determine whether to refer patients to more invasive diagnostic or therapeutic treatments. Nuclear imaging is provided through two primary technologies, gamma cameras and dedicated positron emission tomography, or PET, machines. According to industry sources, despite the improved image quality from PET machines, gamma cameras are used for a substantial majority of nuclear imaging procedures. We believe this preference is due to the lower purchase and maintenance costs, smaller physical footprint and easier service logistics of gamma cameras. The most widely used imaging acquisition technology utilized in gamma cameras is single photon emission computed tomography, or SPECT. All of our current cardiac gamma cameras utilize SPECT.
Clinical Applications for Nuclear Imaging
Nuclear imaging is used primarily in cardiovascular, oncological and neurological applications. Nuclear imaging involves the introduction of very low-level radioactive chemicals, called radiopharmaceuticals, into the patient's body. The radiopharmaceuticals are specially formulated to concentrate temporarily in the specific part of the body to be studied. A system comprised of a gamma camera detector and computer is then used to detect the radiation signal emitted by the chemicals and to convert that signal into an image of the body part or organ. Nuclear imaging, in contrast to other diagnostic imaging modalities, shows not only the anatomy or structure of an organ or body part, but also its function—including blood flow, organ function, metabolic activity and biochemical activity. According to industry sources, the following nuclear imaging procedures were performed with gamma cameras in the United States in 2002:
Nuclear Cardiology
We believe that the 9.9 million nuclear cardiology procedures performed annually in the United States with gamma cameras generate revenue of approximately $10.0 billion. Our target market for DIS services is primarily physician practices and outpatient clinics, which we believe constitute approximately 25% of this total market, or $2.5 billion. In addition, the market for gamma camera sales across all care settings in the United States is estimated to be over $450 million annually.
According to industry sources, nuclear cardiology procedures are expected to grow by approximately 25% annually over the next three years. We believe the growth of these procedures will be driven by the expected increase in coronary heart disease. According to the American Heart Association, this increase in heart disease will result from the aging of baby boomers and the record rate of obesity and diabetes in all age groups.
47
Increasingly, a nuclear cardiology procedure is the first non-invasive, diagnostic imaging procedure performed on patients with suspected heart disease. Following the imaging study, the physician will determine the need for more invasive and expensive diagnostic procedures or therapeutic treatments. These treatments may include angiography, which is an x-ray procedure by which catheters are inserted into an artery or vein to take pictures of blood vessels; angioplasty, which is a procedure by which catheters with balloon tips are used to widen narrowed arteries; or open heart surgery. Given the clinical advantages of nuclear cardiac images, many payors require patients to complete a nuclear cardiology procedure before undergoing more invasive diagnostic procedures and therapeutic treatments.
The target market for our gamma camera sales and the FlexImaging services offered by DIS are the approximately 30,000 cardiologists in the United States that perform nuclear cardiology procedures. We have sold cameras or leased our services through DIS to approximately 500 physicians. In 2003, DIS performed over 66,000 patient procedures. We sell our imaging systems and provide our FlexImaging services to hospitals that provide nuclear cardiology procedures on either an outpatient or inpatient basis, and to physicians that provide these procedures in their offices. According to industry reports, the growth rate in 2002 for procedures performed in physician offices was approximately 44%, and in hospitals was approximately 6%. We believe this trend is driven by the desire of cardiologists to control their patients' diagnosis and treatment and to capture revenues from procedures that would otherwise be referred to hospitals or imaging centers. The unique mobility of our imaging systems allows us to capitalize on this shift from hospital-based imaging to physician office-based imaging.
Competitive Strengths
We believe that our position as a market leader in the nuclear cardiac imaging market is a product of the following competitive strengths:
48
shocks during transportation. The small size and light weight of our detector heads and the modular design of our cameras also facilitate repairs and upgrades in the field, which are often accomplished by delivering replacement components overnight.
Our Technology
Conventional Vacuum Tube Technology
Most gamma cameras use a scintillation crystal, or scintillator, to convert the energy of a gamma ray photon into light. This light is then converted by means of a photodetector into an electrical signal which is reconstructed into a diagnostic image. Most traditional gamma cameras use a single crystal sheet as the scintillator and use vacuum tubes as their photodetectors, which are referred to as vacuum tube photomultipliers. This basic approach has not undergone any fundamental change in over 40 years.
Each vacuum tube is approximately the size of a soft drink can. Since a detector can consist of up to 60 vacuum tubes, the result is a camera with both a large detector enclosure and significant weight due to the lead shield that is required around the detector enclosure. In addition, vacuum tubes cannot be easily moved or used in a mobile environment because vibration may change the electrical properties of the tubes or break them. Further, vacuum tubes may lose their vacuum over time resulting in reduced reliability.
Our Solid-State Technology
We introduced the first solid-state gamma cameras to the nuclear imaging market in March 2000. Our imaging systems utilize a proprietary photodetector which incorporates a silicon semiconductor, or photodiode, that detects light and converts it into an electronic signal for reconstruction into a diagnostic image. Our photodiode replaces the vacuum tubes used in traditional gamma cameras. The size and thickness of our photodiodes is approximately that of a dime, which enables us to build detector heads that are significantly smaller and lighter than the detector heads in traditional gamma cameras. Our solid-state photodiodes are durable and do not change their electrical properties as a result of vibration associated
49
with transportation and are more reliable over time as compared with vacuum tubes. These properties allow our imaging systems to be mobile.
Although photodiodes have been used for many years in varying applications, their use in gamma cameras was previously unsuccessful because performance and functionality limitations prevented the development of a commercially viable product. When a gamma ray emitted from a patient strikes a scintillator, only a very small amount of light is generated, and an even smaller electrical signal is produced in the photodiode. Traditional photodiodes were able to detect these small electrical signals only at very low temperatures, typically less than -20° celsius, due to the electrical noise inherent in the photodiodes. The equipment and cost required to maintain this low temperature prohibited commercialization of a photodiode-based gamma camera. Our proprietary photodiode is capable of measuring these small electrical signals at near room temperature, which reduces cost and improves reliability.
Our photodiode is packaged with our segmented scintillation crystal and readout electronics into a patented detector module. The segmented scintillation crystal allows our module to achieve higher gamma ray detection rates than the single crystal sheet used in traditional gamma cameras. We believe the improved detection rates will be useful with new molecular imaging agents that we anticipate being introduced into the market. The entire module is designed so that it can be physically joined to other modules in varying sizes and shapes, allowing for the design of large field of view and application-specific imaging systems.
Our Products
We sell a line of solid-state gamma cameras and accessories offering both general medical imaging and specific clinical-application imaging. In a typical nuclear cardiology procedure, the physician acquires two images from the patient, one while the patient's heart rate is at rest and the other after the heart has been stressed. The procedure begins with the injection of a small amount of radiopharmaceutical. A patient imaged by our gamma camera sits in an imaging chair and places both arms on a shoulder-level armrest. The chair is adjusted to align the patient's heart on the axis of the chair's rotation.
Following positioning of the patient, image acquisition begins with the patient slowly rotating through a 180 degree arc in front of the camera's detector head, which also has been positioned at heart level. The duration of the acquisition is a function of the patient's body mass, whether the test is performed with the heart at rest or under stress, the amount of radiopharmaceutical and the number of camera detectors on the system.
Stress images are acquired by stressing the heart, either through exercise or the use of other pharmaceuticals, and then injecting the radiopharmaceutical at the peak stress level. The difference between a resting and stress image allows the physician to determine the level of cardiac function. At the conclusion of each image acquisition, the chair is rotated to the exit position and the patient steps out. After collecting the images, the technologist performs the image reconstruction, checks the quality of the images and further processes the images. The physician then reviews the images and determines whether more invasive diagnostic procedures or therapeutic treatments are necessary.
We currently offer the following products:
CardiusSM-2 is a stationary, dual-head gamma camera and patient chair designed for dedicated cardiology applications and high-procedure volumes. Expensive room modifications or electrical changes are generally not required to use this imaging system in an office setting. Further, the system offers the smallest footprint available today, fitting into a seven foot by eight foot room. The Cardius-2 features two proprietary third-generation detectors that accelerate the image acquisition process, resulting in higher patient throughput. The system is suited for larger cardiology practices, dedicated hospital-based cardiology systems, or imaging centers.
50
CardiusSM-1 is a stationary, single-head gamma camera and patient chair designed for dedicated cardiology applications and lower procedure volumes. A single detector head results in image acquisition times suited for physicians and hospitals with the lower patient volumes usually associated with smaller cardiology practices. The Cardius-1 also features our proprietary third-generation detector and can be upgraded in the physician's office to a dual-head Cardius-2 by using our upgrade kit. This upgrade feature allows physicians to expand imaging volume as their practices grow and imaging needs increase.
2020tc Imager® is a mobile, single-head gamma camera that is compact and lightweight. The camera is used for general purpose imaging procedures taken from a single point of view, referred to as planar, ranging from bone scans to thyroid imaging. The small pixel size in our 2020tc Imager provides improved imaging resolution over traditional planar cameras. We sell this camera as a secondary camera to hospitals to increase their capacity and flexibility to image within multiple departments using a single asset.
SPECTpak PLUS combines our 2020tc Imager and SPECTour patient chair and provides both general purpose nuclear imaging and cardiology imaging, with the added flexibility of mobility. DIS uses the SPECTpak PLUS to provide mobile imaging services to its physician customers.
Workstations, Connectivity and Accessories. We offer a line of high-performance workstations equipped with multiple software options for nuclear image interpretation. We also sell connectivity between imagers from the same or different manufacturers to physicians who wish to integrate studies from multiple imagers into one single workstation or archival. In addition, we offer a line of accessories including hot lab equipment required for the use of radiopharmaceuticals, and various other supplies.
Digirad Imaging Solutions (DIS)
DIS offers a comprehensive and mobile imaging leasing service, called FlexImaging, which includes an imaging system, certified personnel, required licensure and other logistics for the performance of nuclear imaging procedures under the supervision of physicians. DIS allows cardiologists to provide nuclear imaging procedures in their offices to patients they historically had to refer to hospitals or imaging centers. As a result, DIS provides physicians with more control over their patients' diagnosis and treatment as well as incremental revenue opportunities. Physicians can tailor their nuclear imaging expenses to their practice needs and patient volumes.
Under our FlexImaging program, we provide a mobile camera, a state-certified nuclear imaging technologist, or CNMT, a paramedic or nurse, radioactive materials and related licensure and supervision for radiation safety services, medical supplies, a quality control process, patient preparation, administrative forms and information brochures. All imaging procedures are administered under the physician's supervision. We also customize our program to allow physicians to lease only our personnel or only our imaging systems, depending on their own practice needs.
DIS currently operates in 17 states and has approximately 350 contracts with physicians, most of whom are office-based cardiologists. DIS also provides leasing services to internists, hospitals and clinics. Our DIS operations use a "hub and spoke" model in which centrally located regional hubs anchor multiple van routes in the surrounding metropolitan areas. As of December 31, 2003, we had 164 employees in our DIS business operating 23 hubs, seven fixed sites, and 54 vans that transport our equipment and personnel to physician office locations. We have invested substantial resources developing our service infrastructure, which includes radioactive materials licensing, a staff of radiation safety officers and licensed clinicians, coordinated billing services and standardized lease agreements. We believe that our service infrastructure and know-how will support additional routes and imaging modalities in the future.
DIS has policies and procedures for the handling of radioactive materials, purchasing relationships, clinical training and quality assurance that we believe maximize operational efficiency and improve customer satisfaction. We have implemented a compliance plan that requires strict adherence to applicable state and federal regulations, including Medicare regulations. We also have an active quality assurance and
51
control program designed to optimize service and follow strict radiation safety and training programs. Our management team has developed experience in hiring and training clinical staff as well as providing quality services to our customers. We utilize proprietary software management tools that monitor key performance metrics in each of our routes, hubs and regions.
At our DIS hubs, technicians load the equipment, radiopharmaceuticals and other supplies onto specially equipped vans for transport to the physician's office, where the technicians set up the equipment for the day. After quality assurance testing, and under the physician's supervision, a technician will gather patient information, inject the patient with a radiopharmaceutical and then acquire the images for review by the physician. The technicians furnish the physician with applicable paperwork and billing information for all patients and clean the utilized areas before departing.
As of December 31, 2003, we provided FlexImaging leasing services to more than 93% of our DIS customers under annual contracts for services delivered on a per-day basis. These contracts decrease our immediate and direct dependence on physician reimbursement. Under these agreements, physicians pay us a fixed amount for each day that they lease our equipment and personnel, and they commit to the scheduling of a minimum number of lease days during the one-year lease term. The same fixed payment amount is due for each day regardless of the number of patients seen or the reimbursement obtained by the physician. As of December 31, 2003, the remaining 7% of our DIS business was provided under our "mixed bill" option. Under this type of agreement, we provide the technical component of our services and bill either the physician or the patient's third-party payor, and so remain at direct risk for reimbursement. We also bill the patient for any co-payment.
We believe DIS allows us to avoid the often lengthy and sometimes unpredictable sales cycle associated with capital equipment sales in a hospital or physician practice setting, and provides us with recurring contractual revenue. Occasionally, DIS customers purchase our imaging systems. In addition, because we own the product that we lease, we are often able to translate technical camera improvements into increased margins in our DIS business.
Business Strategy
We intend to continue to expand our business, improve our market position and increase our revenues and profits by pursuing the following business strategies:
52
Sales and Marketing
Our direct domestic sales organization consists of 25 sales representatives including 12 territory managers responsible for capital equipment sales and 13 imaging professionals responsible for DIS geographic regions. We select our sales representatives based on their expertise in nuclear imaging product sales and services. Each sales representative is subject to periodic performance reviews and is required to attend periodic sales and product training. We employ sales specialists to assist territory managers with in-office or on-site camera demonstrations. We intend to increase the number of sales representatives as we launch new products and services and to increase our marketing efforts with respect to existing products.
In addition to our direct sales force, we also sell our imaging systems in five states and Puerto Rico through three distributors and one independent sales agent. We select our distributors based on their expertise in imaging systems and sales coverage. These relationships provide the distributor the right to sell our products within the sales territory, and their sales representatives typically attend the same sales and product training as our own sales representatives.
We also have distributors in Canada and in Russia and are beginning to build an international sales organization focused on camera sales. These international distribution arrangements are exclusive within the designated countries. We have hired a dedicated international sales executive to establish relationships with additional distributors.
We often service our domestic customers remotely through high-speed Internet access and dial-up connections that facilitate system diagnosis without the need for field service or repair. When repair is required, our modular part replacement capability allows our field service engineers to perform field repairs that minimize customer downtime. We also employ applications specialists and a connectivity engineer to train our customers or provide technical support on the use of our products. We plan to engage outside service firms to support our international customers.
Manufacturing
We have been manufacturing our cameras since March 2000. Our manufacturing strategy combines our internal design expertise and proprietary process technology with strategic outsourcing. The key components of our camera's mechanical and electrical systems are designed or configured by us, and include a personal computer, power supplies, cooling system, liquid crystal display, controller boards and a data acquisition and communication system. These components are either outsourced to qualified manufacturers or built internally. We perform sub-assembly tests and final system performance tests packaging and labeling at our facility.
Suppliers of critical materials, components and subassemblies undergo ongoing quality certification by us. Most components used in the product are available from multiple sources; however, we do not currently maintain alternative manufacturing sources for certain components of the detector or for the acquisition and control software. For those components for which we have only a single source supplier, we are currently qualifying or seeking secondary sources. We utilize enterprise resource planning and collaborative software to increase efficiency and security in handling of material and inventory, centralizing our purchasing procedures, monitoring our inventory supplies and streamlining our billing methods. Our outsourcing strategy is targeted at companies that meet the standards of the FDA and the International Organization for Standardization, or ISO.
53
We and our third-party manufacturers are subject to the FDA's Quality System Regulation, state regulations such as the regulations promulgated by the California Department of Health Services, and regulations promulgated by the European Union. We currently manufacture our medical devices in a number of facilities leased by us in San Diego County, California and licensed by the FDA as well as the California Food and Drug Branch, or CFDB. We are in the process of relocating and consolidating our manufacturing to a new facility in nearby Poway, California that has not yet been licensed by either the FDA or CFDB. Our facilities and the facilities of our third-party manufacturers are subject to periodic unannounced inspections by regulatory authorities, and may undergo compliance inspections conducted by the FDA and corresponding state agencies.
In late 2004, we plan to initiate our ISO-13485 quality certification program with the expectation of receiving certification in 2005. ISO-13485 is a compilation of quality standards tailored for medical device manufacturers and promulgated by the ISO. A medical device manufacturer whose quality program has been certified to ISO requirements does not have to independently test each product that it sells in the European Union. ISO certification is required to sell our products in certain countries, however, we may not ever obtain such certification.
Research and Development
Our research and development staff currently consists of 18 employees. We have a long and extensive commitment to research and development, including an established history in developing innovative solid-state gamma cameras. In March 2000, we launched the first solid-state gamma camera for medical use and, in September 2002, we released the first dual-head, solid-state camera. In July 2003, we launched our third-generation detector that improved the reliability and sensitivity of our gamma cameras, and reduced their cost. We have an established core competency in the development of silicon photodiodes and related scintillator assemblies and signaling processing electronics, which are the core of our gamma cameras.
Our research and development efforts are primarily focused in the near term on developing further enhancements to our existing products as well as developing our next-generation products. Our objective is to increase the sensitivity and reliability of our imaging systems and their clinical and economic benefit to our physician customers and their patients.
Competition
The medical device industry, including the market for nuclear imaging systems and services, is highly competitive, subject to rapid change and significantly affected by new product and service introductions and market activities of other industry participants. In selling and leasing our imaging systems, we compete against several large medical device manufacturers, including Philips Medical Systems, General Electric Healthcare, Siemens Medical Systems and Toshiba Medical Systems. All of these competitors offer a full line of imaging cameras for each diagnostic imaging technology, including x-ray, MRI, CT, ultrasound and nuclear medicine. The existing nuclear imaging systems sold by our competitors have been in use for a longer period of time than our products and are more widely recognized and used by physicians and hospitals for nuclear imaging. Many of our competitors and potential competitors enjoy significant competitive advantages over us, including:
54
We are aware of certain major medical device companies that are attempting to develop solid-state gamma cameras, and we believe these efforts will continue. However, we are currently not aware of any other solid-state cardiac gamma camera. We are also aware of a privately-held company, Gamma Medica, which is currently marketing a solid-state gamma camera for breast imaging. We do not believe that this camera can be used in a cardiac application. However, we cannot assure you that Gamma Medica will not attempt to modify its existing camera for use in the cardiac segment in the future or develop another gamma camera for cardiac applications.
In providing our mobile leasing services, we also compete against businesses employing traditional vacuum tube cameras that must be transported in large trucks and cannot be moved in and out of physician offices. Competitive fixed-site services may require extensive or dedicated space and room renovations that result in increased start-up and ongoing costs.
Because of the size of the potential market, we anticipate that companies will dedicate significant resources to developing competing products and services, including a mobile leasing service. Current or future competitors may develop technologies and products that demonstrate better image quality, ease of use or mobility than our nuclear imaging systems. Our nuclear imaging systems or leasing services may be rendered obsolete or non-competitive by technological advances developed by one or more of our competitors. Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner, receive adequate reimbursement and are safer, less invasive and less expensive than alternatives available for the same purpose.
We believe that the principal competitive factors in our market include:
Intellectual Property
We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure agreements and other measures to protect our intellectual property. We believe that in order to have a competitive advantage, we must develop and maintain the proprietary aspects of our technologies. We require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment, consulting or advisory relationships with us. We also require our employees, consultants and advisors who we expect to work on our products to agree to disclose and assign to us all inventions conceived during the work day, using our property, or which relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary.
Patents
We have developed a patent portfolio that covers our overall products, components and processes. As of March 5, 2004, we had 21 issued U.S. patents and 31 pending patent applications, including ten U.S. applications, three international Patent Cooperation Treaty, or PCT, applications and 18 foreign
55
applications seeking protection for selected patents in Japan, Canada and Russia. The issued and pending patents cover, among other things, aspects of solid-state radiation detectors including our photodiodes, signal processing, and system configuration. Our issued patents expire between December 23, 2014 and April 20, 2021. We have multiple patents covering unique aspects and improvements for many of our products. We have entered into a royalty-bearing license for one U.S. patent with a third party for exclusive use (subject to certain reservation of rights by the U.S. Government) in nuclear imaging. We do not believe that our current products implement the licensed patent and we are currently negotiating with the third-party licensor to amend the patent license.
In addition to our solid-state detector and photodiode technology patents, we hold specific patents for an alternative solid-state method using Cadmium Zinc Telluride, or CZT, that we previously pursued for use in gamma cameras. While each of our patents applies to nuclear medicine, many also apply to the construction of area detectors for other types of medical imagers and imaging methods.
The medical device industry is characterized by the existence of a large number of patents and frequent litigation based on allegations of patent infringement. Patent litigation can involve complex factual and legal questions and its outcome is uncertain. Any claim relating to infringement of patents that is successfully asserted against us may require us to pay substantial damages. Even if we were to prevail, any litigation could be costly and time consuming and would divert the attention of our management and key personnel from our business operations. Our success will also depend in part on our not infringing patents issued to others, including our competitors and potential competitors. If our products are found to infringe the patents of others, our development, manufacture and sale of such potential products could be severely restricted or prohibited. In addition, our competitors may independently develop similar technologies. Because of the importance of our patent portfolio to our business, we may lose market share to our competitors if we fail to protect our intellectual property rights.
As the number of entrants into our market increases, the possibility of a patent infringement claim against us grows. While we make an effort to ensure that our products do not infringe other parties' patents and proprietary rights, our products and methods may be covered by U.S. patents held by our competitors. In addition, our competitors may assert that future products we may market infringe their patents.
Further, a patent infringement suit brought against us may force us to stop or delay developing, manufacturing or selling potential products that are claimed to infringe a third party's intellectual property, unless that party grants us rights to use its intellectual property. In such cases, we may be required to obtain licenses to patents or proprietary rights of others in order to continue to commercialize our products. However, we may not be able to obtain any licenses required under any patents or proprietary rights of third parties on acceptable terms, or at all. Even if we were able to obtain rights to the third party's intellectual property, these rights may be non-exclusive, thereby giving our competitors access to the same intellectual property. Ultimately, we may be unable to commercialize some of our potential products or may have to cease some of our business operations as a result of patent infringement claims, which could severely harm our business.
Trademarks
We have trademark registrations in the U.S. for the following marks: 2020tc Imager®, CardiusSST®, Digirad®, Digirad Logo®, Digirad Imaging Solutions®, FlexImaging®, and SPECTour®. We have trademark applications pending in the U.S. for the following marks: CardiusSM, DigiServSM, DigiSpectSM, DigiTechSM, and SolidiumSM. We have obtained and sought trademark protection for some of these listed marks in the European Community and Japan.
56
Government Regulations
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, these laws and their interpretations are subject to change.
Both federal and state governmental agencies are continuing heightened civil and criminal enforcement efforts in the healthcare industry. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the billing practices of healthcare providers. The federal government also has increased funding in recent years to fight healthcare fraud, and various agencies, such as the United States Department of Justice, the Office of Inspector General of the Department of Health and Human Services, or OIG, and state Medicaid fraud control units, are coordinating their enforcement efforts.
We have implemented a compliance program to help assure that we remain in compliance with the healthcare laws applicable to our business, and believe that we have structured our business operations and relationships with our customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. We discuss below the statutes and regulations that are most relevant to our business and most frequently cited in enforcement actions.
Fraud and Abuse Laws
Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. The definition of "remuneration" has been broadly interpreted to include anything of value, including for example gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash and waivers of payments. Several courts have interpreted the statute's intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, some kickback allegations have been claimed to violate the Federal False Claims Act, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, the OIG has issued a series of regulations, known as the "safe harbors," beginning in July of 1991. These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Government officials have focused their enforcement efforts on marketing of healthcare services, among other activities, and recently have brought cases against sales personnel who allegedly offered
57
unlawful inducements to potential or existing customers in an attempt to procure their business. As part of our compliance program, we review our marketing materials and train our sales personnel to help assure compliance with the Anti-Kickback Statute.
In DIS, we offer lease agreements under which physicians lease our equipment and personnel, typically for one or two days a week, for a term of a year. Under this option, which comprises 93% of our DIS customers, our customers pay us the same fixed amount for each lease day regardless of the number of patients they see or the reimbursement they obtain. They also pay us for radiopharmaceuticals and pharmacological stress agents (collectively, "supplies") used in performing the tests.
Under a second contracting option, the "mixed bill" model, used by approximately 7% of our customers, we provide and are paid for services and supplies provided to physicians for their use in treating their privately insured patients. These physicians also refer Medicare patients to us, for whom we perform the technical component of nuclear imaging procedures and on whose behalf we bill the Medicare program directly. This type of arrangement, if not properly structured, could be construed to violate the Anti-Kickback Statute and also to raise issues under another Medicare statute, 42 U.S.C. Section 1320a-7(b)(6). That statute prohibits providers from charging Medicare substantially in excess of the provider's usual and customary charges unless the Secretary of Health and Human Services finds good cause.
We believe that we have structured our lease and "mixed bill" models, as well as our marketing program, to comply with the Anti-Kickback Statute and similar state laws, as well as with 42 U.S.C. Section 1320a-7(b)(6). However, we cannot rule out the possibility that the government or other third parties could interpret these laws differently and assert otherwise.
Stark Law
The Ethics in Patient Referral Act of 1989, commonly referred to as the federal physician self-referral law or Stark Law, prohibits physician referrals of Medicare patients to an entity for certain "designated health services" if the physician or an immediate family member has an indirect or direct financial relationship with the entity and no statutory or regulatory exception applies. Financial relationships include an ownership interest in, or compensation arrangement with, the entity. It also prohibits an entity receiving a prohibited referral from billing and collecting for services rendered pursuant to such referral. "Designated health services" under Stark include inpatient and outpatient hospital services, radiology services, magnetic resonance imaging, computerized axial tomography scans, ultrasound services and outpatient prescription drugs. The Health Care Financing Administration, now known as the Centers for Medicare and Medicaid Services, or CMS, indicated in a final rule issued in 2001 that nuclear medicine is not covered as a designated healthcare service under the Stark Law. CMS has also indicated that radiopharmaceuticals and pharmacological stress agents used in nuclear imaging procedures do not constitute designated healthcare services. However, it is possible that CMS may change its interpretation in the future to include nuclear imaging and/or one or both of these supplies as designated healthcare services under the Stark Law. Should that occur, we believe the financial relationships we have with our physician customers fall within one or more exceptions to the prohibition on referrals. Therefore, we do not believe the physicians would be prohibited from referring Medicare patients to us. However, we cannot rule out the possibility that the government or other third parties could interpret these laws differently and assert otherwise.
A person who engages in a scheme to circumvent the Stark Law's prohibitions may be fined up to $100,000 for each such arrangement or scheme. In addition, anyone who presents or causes to be presented a claim to the Medicare program in violation of the Stark Law is subject to monetary penalties of up to $15,000 per claim submitted, an assessment of several times the amount claimed, and possible exclusion from participation in federal healthcare programs. In addition, claims submitted in violation of
58
the Stark Law may be alleged to be subject to liability under the federal False Claims Act and its whistleblower provisions (as discussed below).
Several states in which we operate have enacted legislation that prohibits physician self-referral arrangements and/or requires physicians to disclose any financial interest they may have with a healthcare provider to their patients when referring patients to that provider. Some of these statutes cover all patients and are not limited to Medicare beneficiaries. Possible sanctions for violating state physician self-referral laws vary, but may include loss of license and civil and criminal sanctions. State laws vary from jurisdiction to jurisdiction and, in a few states, are more restrictive than the federal Stark Law. Some states have indicated they will interpret their own self-referral statutes the same way that CMS interprets the Stark Law, but it is possible the states will interpret their own laws differently in the future. We believe that we have structured our operations to comply with these state physician self-referral prohibition laws in the jurisdictions in which we operate. However, we cannot rule out the possibility that the government or other third parties could interpret these statutes differently and assert otherwise. In certain states in which we do not yet operate, these laws may add considerable expense to or limit altogether the types of business models we may successfully utilize.
HIPAA
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
In addition to creating the two new federal healthcare crimes, HIPAA also establishes uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses. Two standards have been promulgated under HIPAA with which we currently are required to comply. We must comply with the Standards for Privacy of Individually Identifiable Health Information, which restrict our use and disclosure of certain individually identifiable health information. We have been required to comply with the Privacy Standards since April 14, 2003. We must also comply with the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures. We have been required to comply with these Standards since October 16, 2003. We believe that we are in compliance with these standards. Two other standards relevant to our use of medical information have been promulgated under HIPAA, although our compliance with these standards is not yet required. The Security Standards will require us to implement certain security measures to safeguard certain electronic health information by April 21, 2005. In addition, CMS recently published a final rule, which will require us to adopt Unique Health Identifiers for use in filing and processing healthcare claims and other transactions by May 23, 2007. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, our compliance with this law may entail significant and costly changes for us. If we fail to comply with these standards, we could be subject to criminal penalties and civil sanctions.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our operations and procedures to comply with the more stringent state laws, which may entail significant and costly changes for us. We believe that we are in compliance with such
59
state laws and regulations. However, if we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Federal False Claims Act
Another trend affecting the healthcare industry is the increased use of the federal False Claims Act and, in particular, actions under the False Claims Act's "whistleblower" or "qui tam" provisions. Those provisions allow a private individual to bring actions on behalf of the government alleging that the defendant has defrauded the federal government. The government must decide whether to intervene in the lawsuit and to become the primary prosecutor. If it declines to do so, the individual may choose to pursue the case alone, although the government must be kept apprised of the progress of the lawsuit. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery. If the individual's litigation is successful, the individual is entitled to no less than 15%, but no more than 30%, of whatever amount the government recovers. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted laws modeled after the federal False Claims Act.
When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 to $11,000 for each separate false claim. There are many potential bases for liability under the federal False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. Although simple negligence should not give rise to liability, submitting a claim with reckless disregard or deliberate ignorance of its truth or falsity could result in substantial civil liability. The False Claims Act has been used to assert liability on the basis of inadequate care, improper referrals, and improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. We are unable to predict whether we could be subject to actions under the False Claims Act, or the impact of such actions. However, the costs of defending claims under the False Claims Act, as well as sanctions imposed under the Act, could significantly affect our financial performance.
Billing and Reimbursement
DIS
Reimbursement to physicians for nuclear imaging tests consists of both a "technical component" (i.e., the actual performance of the test) and a "professional component" (i.e., the interpretation of the test, sometimes referred to as a "read" of the test). Physicians may bill for the professional component if they perform and document a bona fide interpretation. Medicare and certain other payors permit providers who perform both the technical and professional components to either bill "globally" for both components of the tests, if applicable requirements are met, or to bill for the technical component and professional component separately. In our lease model, our physician customers bill globally for both the technical and professional components of the tests. Assuming they meet certain requirements, including but not limited to adequate supervision of the non-physician personnel performing the tests, they may bill and be paid by Medicare according to the Medicare Physician Fee Schedule.
Under our "mixed bill" model, we provide the technical component of nuclear imaging services and bill either the physician (who, in turn, bills the patient or third-party payor) or, if the patient is a Medicare patient, the Medicare program. For those services we bill directly, our Medicare paymentabove is based on the Medicare Physician Fee Schedule and we bill the patient for any co-payment. The physician performs and bills the payor for the professional component for all patients, including the interpretation of the test. In our lease agreement model, we derive our revenues directly and only from customer physicians. In our "mixed bill" model, we derive revenues from Medicare, as well as direct billings to physicians.
60
Medicare has delegated the functions of enrollment and payment to contractors known as the Medicare carriers, each of whose jurisdiction varies, as some carriers govern several states, some just one state and some just a portion of a state. Although federal regulations set forth uniform rules governing IDTF billing and enrollment, each carrier is free to interpret these rules to a certain extent. For example, an IDTF is required to have one or more supervising physicians, each of whom meets certain proficiency requirements; these precise proficiency requirements vary from carrier to carrier. The nature of a particular carrier's proficiency and other requirements may add expense to or limit the types of business models we may be able to utilize successfully in the carrier's jurisdiction. At present, we are licensed as independent testing facilities in nine states and perform IDTF services in five states.
Services for which we and our customer physicians bill Medicare typically are reimbursed according to the Medicare Physician Fee Schedule that assigns a specified value to each procedure or supply, which are identified according to numeric codes. Medicare revises this Physician Fee Schedule on an annual basis. Under the Medicare Modernization Act, the Physician Fee Schedule payment rates for 2004 were increased, instead of reduced as expected prior to the legislation. The payment methodology to physician practices for drugs and radiopharmaceuticals were changed, and some payment rates decreased. If the amounts payable under the Physician Fee Schedule or payments for supplies decreases under prescribed payment methodologies, we may receive less revenue from Medicare under our mixed bill model. Similarly, our physician customers may receive less revenue for the tests they perform under our lease model, which may adversely affect the amount we can charge physicians who enter into new lease agreements or renew existing agreements.
We also lease our cameras to hospitals. The payment policies implemented by state and federal reimbursement programs for hospitals affect demand for our leasing services business by hospitals. Medicare, the single largest third-party payor in the United States, which pays certain hospitals for imaging services using our products, generally pays for inpatient services under a prospective payment system, or "PPS." Under PPS, hospitals receive a fixed amount for each Medicare patient discharge for inpatient services. Each discharge is classified into one of many diagnosis related groups, or "DRGs," corresponding to the patient's condition. The payment amount assigned to each DRG reimburses the hospital for inpatient operating costs, regardless of the services actually provided or the length of the patient's stay. Hospital capital-related costs, including investments in depreciable equipment also is paid under a PPS methodology. Although there may be opportunities to obtain additional amounts for certain high-cost new technologies in the inpatient setting, under this PPS payment methodology, Medicare does not separately reimburse hospitals for services performed using our cameras, since payment for this service is included in the DRG payment amount. Many state Medicaid programs and private payors have adopted comparable payment policies.
Medicare pays for hospital outpatient services under the outpatient prospective payment system. Under this system, services and items furnished in hospital outpatient departments are reimbursed using a pre-determined amount for each ambulatory payment classification, or "APC." Each APC groups together similar services comparable both clinically and with respect to the use of resources. Certain items and services are paid based on a fee schedule, and hospitals are reimbursed additional amounts for certain drugs, biologics and new technologies. Under the Medicare Modernization Act, revisions were made to the payment methodology for radiopharmaceuticals and drugs used with our cameras, which resulted in the increase of some and decrease of other payment rates to hospitals for these supplies. We cannot predict the extent to which the payment methodology changes will have an impact on our revenue or business, if any.
We believe we have structured our DIS contracts so that physicians and hospitals are able to bill in this manner if they comply with the terms of the contracts and the requirements of applicable radioactive materials laws are met. However, if any of our customer physicians are deemed not to meet these conditions, payment to the affected physicians could be reduced, denied or recouped. If the failure to comply is deemed to be "knowing" and/or "willful," as defined in federal statutes, the government could
61
seek to impose fines or penalties under the False Claims Act and other statutes. This may require us to restructure our agreements with these physicians and/or respond to any resultant claims by physicians or the government.
Camera Sales
We currently sell cameras to physicians, physician groups or medical groups. Physicians who perform or supervise nuclear imaging procedures in their offices are reimbursed by Medicare under the Physician Fee Schedule, assuming applicable requirements are met. Physicians are also reimbursed for the supplies they use in performing these procedures. The payment policies implemented by state and federal reimbursement programs for physicians affect demand for our cameras. We also sell cameras to hospitals. The payment policies implemented by state and federal reimbursement programs for hospitals affect demand for our cameras. The same rules and regulations concerning reimbursement for inpatient and outpatient services that apply to our hospital leases also apply to our sales of cameras to hospitals.
Non-governmental third-party payor limitations
Non-governmental managed-care payors, such as health maintenance organizations, or HMOs, preferred provider organizations, or PPOs, and certain other insurers, often impose varying requirements and limitations on the ability of diagnostic test providers such as our lease services division to receive payment directly for the services they provide. For example, some payors will not reimburse a provider of nuclear imaging services for the tests it performs unless the provider has a contract with the payor, and in many instances such payors will not enter into such contracts. On the other hand, most of these payors currently will provide reimbursement on a "global" basis to a physician who has a contract with the payor and who supervises or performs the test and provides the professional interpretation. Such payor requirements and limitations restrict the types of business models we can successfully utilize for patients covered by these payors, but currently do not preclude us from successfully implementing our lease and mixed bill models. However, we cannot rule out the possibility that some of these payors will impose new requirements or limitations in the future that could adversely affect these models and require us to develop new models.
Pharmaceutical laws
Our lease services business involve administering and furnishing radiopharmaceuticals and pharmacological stress agents, which are regulated as drugs by state and federal agencies, including the FDA and state pharmacy boards. These agencies administer laws governing the manufacturing, sale, distribution, use, administration and prescribing of drugs, including the federal Food, Drug and Cosmetic Act, state food and drug laws and state pharmacy acts. Some of our activities may be deemed by relevant agencies to require permits or licensure under these laws, which would impose substantial requirements and entail significant expense. If any of these agencies deemed our activities to require such permits or licensure, we would be required to either obtain such permits or licensure, if possible, or modify the types of business models we can utilize in the affected jurisdiction(s).
Radioactive Materials Laws
The procurement, use, transfer and storage of radioactive materials is subject to comprehensive regulation under state and federal laws. In some states, the federal Nuclear Regulatory Commission, or NRC, directly regulates such use (NRC States). In other states, a state regulatory agency performs such regulation under an agreement with the federal government (Agreement States). In both Agreement and NRC States, the use of radioactive materials requires licensure and compliance with comprehensive rules governing such licensure.
62
Because our DIS business entails the use of radiopharmaceuticals in performing nuclear medicine tests, we are required to obtain and maintain licensure under radioactive materials laws, or RAM laws, and to comply with such laws. The RAM laws require, among other things, that such materials be used by, or that their use be supervised by, individuals with specified training, expertise and credentials in the type of use in question. Such individuals are known as "authorized users."
The RAM laws include specific provisions applicable to the medical use of radioactive materials. For a business such as ours, the authorized user must be a physician with training and expertise in the use of radioactive materials for diagnostic purposes. We have entered into contracts with qualified physicians in each of our regions to serve as authorized users.
In some states, the authorized user is required to participate in or oversee the selection of patients and the ordering of procedures and/or supplies. Some states also required that an authorized user perform an interpretation of the nuclear medicine tests. The authorized user need not be present at the customer physician's site to perform such functions.
Under the RAM laws, physicians who are not licensed authorized users, but who are supervised by an authorized user on behalf of a licensed entity, are permitted to use radioactive materials under the authority of such licensure, if certain conditions are met. Because our physician customers in our lease services business are not licensees and in most cases are not qualified to serve as authorized users, they perform nuclear medicine procedures as "supervised persons." To the extent required by applicable RAM laws, the authorized users perform some of the functions described above. For example, in states where an authorized user must perform an interpretation to satisfy RAM licensing laws, an authorized user does so. The physician customer reimburses the authorized user for doing so and also performs his or her own interpretation.
We believe that we have structured our operations so that they comply with applicable RAM laws in the jurisdictions in which we operate, and that the manner in which we comply with these laws is also consistent with applicable Medicare requirements. However, we cannot rule out the possibility that the government or other third parties could interpret these laws differently and assert otherwise.
Medical Device Regulation
Our products are medical devices subject to extensive regulation by the FDA and other regulatory bodies. FDA regulations govern, among other things, the following activities that we or our partners perform and will continue to perform:
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval, or PMA, from the FDA. The FDA classifies medical devices into one of three classes, depending on the degree of risk associated with
63
each medical device and the extent of control needed to ensure safety and effectiveness. Devices deemed to pose lower risk are placed in either class I or II, which requires the manufacturer to submit to the FDA a premarket notification requesting permission for commercial distribution. This process is known as 510(k) clearance. Some low-risk devices are exempted from this requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or a device deemed not substantially equivalent to a previously cleared 510(k) device, are placed in class III. In general, a class III device cannot be marketed in the United States unless the approves the device after submission of a PMA.
510(k) Clearance Pathway
When we are required to obtain a 510(k) clearance for a device which we wish to market, we must submit a premarket notification to FDA demonstrating that the device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of PMA applications. By regulation, the FDA is required to respond to a 510(k) premarket notification within 90 days of submission of the notification. As a practical matter, clearance can take significantly longer. If FDA determines that the device, or its intended use, is not substantially equivalent to a previously-cleared device or use, the FDA will place the device, or the particular use of the device, into class III.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, will require a new 510(k) clearance or could require a PMA. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer's determination. If the FDA disagrees with a manufacturer's determination that a new clearance or approval is not required for a particular modification, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA is obtained. If the FDA requires us to seek 510(k) clearance or PMA for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements to our gamma cameras that we believe do not require new 510(k) clearances.
Premarket Approval Pathway
A PMA application must be submitted if the device cannot be cleared through the 510(k) process. The PMA process is much more demanding than the 510(k) premarket notification process. A PMA application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA's satisfaction the safety and effectiveness of the device.
After a PMA application is complete, the FDA begins an in-depth review of the submitted information. The FDA, by statute and regulation, has 180 days to review an accepted PMA application, although the review generally occurs over a significantly longer period of time, and can take up to several years. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the Quality System Regulation, or QSR. New PMA applications or PMA application supplements are required for significant modifications to the manufacturing process, labeling and design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the
64
original PMA application, and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials
A clinical trial is almost always required to support a PMA application and is sometimes required for a 510(k) premarket notification. These trials generally require submission of an application for an investigational device exemption, or IDE, to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of patients, unless the product is deemed a non-significant risk device and eligible for more abbreviated investigational device exemption requirements. Clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Future clinical trials of our motion preservation designs and possibly interbody implants will likely require that we obtain an IDE from the FDA prior to commencing clinical trials. Our clinical trials must be conducted in accordance with FDA regulations. The results of clinical testing may not be sufficient to obtain approval of the product.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
We are subject to unannounced inspections by the FDA and the Food and Drug Branch of the California Department of Health Services, and these inspections may include the manufacturing facilities of our subcontractors.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ.
65
The primary regulatory environment in Europe is that of the European Union, which consists of 15 countries encompassing most of the major countries in Europe. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms with the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout Europe. CE is an abbreviation for European Compliance. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a "Notified Body." This third-party assessment may consist of an audit of the manufacturer's quality system and specific testing of the manufacturer's product. An assessment by a Notified Body in one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union. In 2001, we were certified by TUV Product Service, a Notified Body, under the European Union Medical Device Directive allowing the CE conformity marking to be applied.
Our current products are approved for market release by the FDA. We also received regulatory approval from the Japanese Ministry of Health in October 2000, which is similar to our FDA Establishment Registration. The Canadian Standards Association has approved our 2020tc/SPECTour chair for the CSA labeling in February 2002. In March 2003, we received GOST certification, the quality and safety certification system administered by the Russian committee, Gosstandart, to distribute the 2020tc/SPECTour chair in Russia.
Employees
As of December 31, 2003, we had 289 employees, of which 137 were employed in clinical and regulatory, 71 in operations, 33 in general and administrative, 32 in sales and marketing and 16 in research and development. We had 164 employees in our DIS subsidiary. None of our employees is represented by a labor union. We have not experienced any work stoppages and consider our employee relations to be good. We are, however, aware of a claim by one former employee and three current employees that they are due unpaid overtime because of an alleged misclassification of their positions as exempt rather than non-exempt employees. For a further discussion, see "Risk Factors—Risks Related to Our Intellectual Property and Potential Litigation—We may be subject to lawsuits and actions brought by our employees."
Facilities
Our operations are headquartered in an approximately 70,000 square foot facility in Poway, California that is leased to us until February 2010. We believe that our existing facility is adequate for our current needs.
Legal Proceedings
We are currently not a party to any material legal proceedings.
66
Executive Officers, Key Employees and Directors
The following table sets forth certain information regarding our executive officers, key employees and directors:
David M. Sheehan has served as our President and Chief Executive Officer since March 2002 and as a member of our board of directors since July 2002. Mr. Sheehan joined us in September 2000 as President of Digirad Imaging Solutions, Inc., our wholly owned subsidiary. From May 1999 to September 2000, Mr. Sheehan served as the President and Chief Executive Officer of Rapidcare.com, an e-health company. From May 1997 to May 1999, he served as Vice President of Sales, Marketing, and Business Development of a division at Baxter International, Inc. that provided cardiopulmonary products and services to hospitals. Prior to this, he held operations, sales and marketing positions at Haemonetics Corporation, a supplier of blood processing equipment and services. Mr. Sheehan received his B.S. in mechanical engineering from Worcester Polytechnic Institute and his M.B.A. from the Tuck School of Business at Dartmouth College.
Todd P. Clyde has served as our Chief Financial Officer since November 2002. From January 2002 to November 2002, Mr. Clyde was Chief Financial Officer at Del Mar Database, Inc., a software company developing products for the mortgage lending industry. From March 2000 to October 2001, Mr. Clyde was Vice President and Controller at Verance Corporation, a digital information tracking and security company. From October 1997 to March 2000, Mr. Clyde was Vice President and Division Controller at I-Bus/Phoenix, a division of Maxwell Technologies, Inc. which is a manufacturer of customized industrial computing. Prior to this, he was a senior auditor at Ernst & Young, LLP, an international public accounting firm. Mr. Clyde received his B.S. in accounting and his Masters of Accountancy from Brigham Young University. Mr. Clyde is a Certified Public Accountant.
Vera P. Pardee has served as our Vice President, General Counsel and Secretary since April 2003. From July 2000 to February 2002, Ms. Pardee served as Vice President, General Counsel and Secretary of Nanogen, Inc., a biotechnology company developing molecular diagnostic tests for the clinical research and
67
diagnostics markets. From January 1988 to June 2001, Ms. Pardee was in private practice as a partner and associate at Seltzer Caplan Vitek McMahon and from 1983 to 1987 as an associate at O'Melveny & Myers, LLP. Ms. Pardee received her J.D. from Southwestern University School of Law.
Diana M. Bowden has served as our Vice President of Marketing since September 2002. From June 2001 to August 2002, Ms. Bowden served as Director of Marketing with our wholly-owned subsidiary, Digirad Imaging Solutions. From August 2000 to June 2001, Ms. Bowden served as Director of Marketing at Keylime Software, Inc., a web analytics company. From May 1998 to May 2000, she served as Director of Sales and Marketing at Ultra Acquisition Corporation, an e-commerce and manufacturing company. From June 1994 to May 1998, Ms. Bowden served as Vice President, Sales and Marketing at RadNet, a radiology service provider. Prior to this she served in various product management and sales management positions at Quest Diagnostics Incorporated, a large medical reference laboratory, and in sales and marketing positions at Iolab, a former Johnson & Johnson pharmaceutical company. She received her B.A. in biological sciences from U.C. Santa Barbara and her M.B.A. in marketing from the Peter Drucker Graduate School of Management of the Claremont Graduate University.
Herbert J. Bellucci has served as our Senior Vice President, Operations since May 2003. From April 1994 to April 2003, Mr. Bellucci was Vice President of Manufacturing at Omnicell, a company that manufactures electromechanical dispensing systems for drugs and hospital supplies. Prior to this, he was Senior Vice President of Operations at Laserscope, a manufacturer of minimally invasive surgical devices, Vice President of Operations at Vidamed, a medical device company, and Manufacturing Manager at Spectra-Physics, a division of Thermo Electric Corporation which is a supplier of laser technology. Mr. Bellucci received his B.S. in engineering from Brown University and his M.B.A. from Stanford University.
Paul J. Early has served as our Vice President and Corporate Radiation Safety Officer since March 2001. Prior to joining us, Mr. Early was the President of Associates at Medical Physics, the scientific journal of the American Association of Physicists in Medicine. Mr. Early is the author of multiple books, including the nuclear medicine textbook "Textbook of Nuclear Medicine Technology." Mr. Early is a Diplomate of the American Board of Medical Physics, the American Board of Science in Nuclear Medicine and the American Board of Radiology. Mr. Early received his B.S. from St. Ambrose University and completed two years of post-graduate studies at Creighton University.
Richard L. Conwell has served as our Vice President of Advanced Research and Development and Business Development since August 2001. Prior to that, he served as our Vice President of Marketing from January 2001 to August 2001, as Vice President of Research and Development and Marketing from March 2000 to January 2001, and as Vice President of Research and Development from June 1996 to March 2000. Prior to joining us, Mr. Conwell was Vice President of Thermo Gamma Metrics, a company which develops and markets on-line, high-speed process optimization systems for raw-materials analysis, where he was responsible for the company's bulk material analyzer business. Mr. Conwell received his B.S. in physics and computer science from Ball State University.
Martin B. Shirley has served as our Regional Vice President of Sales, East since July 2002. Prior to that, Mr. Shirley served as a Regional Sales Director for us from January 2001 to January 2002, and as a Territory Manager for us from January 2000 to January 2001. From March 1999 to December 1999, he was a principal of IsoPoint, Inc., a software company, where he was responsible for sales and contracting. Prior to this, Mr. Shirley was Regional Sales Manager at SMV America, Inc., a manufacturer of gamma cameras that was purchased by General Electric, and a Territory Manager for Dupont in their radiopharmaceutical business. Prior to this, Mr. Shirley spent five years as a Certified Nuclear Technologist. Mr. Shirley received his A.S. in nuclear medicine technology from Hillsborough Community College and his A.A. in liberal arts from Santa Fe Community College.
Stephen L. Bollinger has served as our Regional Vice President of Sales, West since July 2002. From February 2002 to July 2002, Mr. Bollinger served as our Western Regional Sales Director. From
68
October 2000 to February 2002 Mr. Bollinger worked at Data Return Corporation, a company that provides managed website hosting services, as Western Regional Sales Manager. From June 1986 to September 2000, Mr. Bollinger was a West Coast Regional Sales Manager for Kodak's medical imaging products division. Mr. Bollinger received his B.S. from University of Phoenix and his M.B.A. from University of Colorado.
Timothy J. Wollaeger has served as a member of our board of directors since April 1994 and as our Chairman since January 1996. Mr. Wollaeger has been the Managing Director for the San Diego office of Sanderling Biomedical Venture Capital since April 2002. He is also a general partner of Kingsbury Associates, L.P., a venture capital firm he founded in January 1994, which focuses on investments in the healthcare industry. From May 1990 to December 1993, Mr. Wollaeger served as Senior Vice President and a director of Columbia Hospital Corporation, a hospital management company now known as HCA Healthcare Corporation. From October 1986 until July 1993, Mr. Wollaeger was a general partner of Biovest Partners, a seed venture capital firm. He is Chairman of the board of directors of Biosite Incorporated and a founder and director of several privately held medical products companies. Mr. Wollaeger received his B.A. in economics from Yale University and his M.B.A. from the Stanford University Graduate School of Business.
Raymond V. Dittamore has served as a member of our board of directors since March 2004. Mr. Dittamore is a retired audit partner of Ernst & Young, LLP, an international public accounting firm. Mr. Dittamore retired after 35 years of service, including 14 years as the managing partner of the firm's San Diego office. Mr. Dittamore is a director of Qualcomm Incorporated, Invitrogen Corporation and Gen-Probe Incorporated. Mr. Dittamore received his B.S. from San Diego State University.
Robert M. Jaffe has served as a member of our board of directors since June 2002. He is a founder and investment officer of Sorrento Associates. Prior to founding Sorrento Associates in 1985, he was an investment banker at Merrill Lynch Capital Markets. Prior to this, he was an investment banker at Salomon Brothers, Inc. and Goldman, Sachs & Co. He was also a member of the technical staff at Hughes Aircraft Company and a consultant at McKinsey & Co. Mr. Jaffe received his M.B.A. from the Harvard Business School where he was a Baker Scholar and the recipient of The Loeb Rhoades Fellowship. He received his M.S. in electrical engineering from the California Institute of Technology, and his B.S. in electrical engineering and computer science from the University of California at Berkeley.
R. King Nelson has served as a member of our board of directors since March 2004 and previously served as a director from May 2000 to April 2002. From May 1999 to December 2003, Mr. Nelson served as the President and Chief Executive Officer of VenPro Corporation, a medical device company that develops bioprosthetic implants for venous vascular and cardiovascular medicine. From January 1980 to December 1998, Mr. Nelson held various executive positions at Baxter Healthcare Corporation, most recently as President of the perfusion service business. Mr. Nelson received his B.S. from Texas Tech University and his M.B.A. in international business from the University of Miami.
Kenneth E. Olson has served as a member of our board of directors since March 1996. From June 1984 to June 1998, he served as Chairman, and from December 1990 to February 1996 and from March 1997 to June 1998, he served as Chief Executive Officer, at Proxima Corporation, a supplier of display projection systems for professional desktop computers. From 1971 to 1987, he was Chairman and Chief Executive Officer of Topaz, Inc., a designer and manufacturer of computer peripherals. Mr. Olson also serves on the board of directors for Avanir Pharmaceuticals and WD-40 Company. He studied electrical engineering at UCLA and received his M.B.A. from Pepperdine University.
Douglas Reed, M.D. has served as a member of our board of directors since August 2000. He has been a Managing Director of Vector Fund Management, a venture capital firm which focuses on investments in the life sciences and healthcare industry since June 2000. From October 1998 to January 2000, Dr. Reed served as Vice President of Business Development for GelTex Pharmaceuticals, Inc., a company that develops and markets non-absorbed polymer drugs. From April 1996 to September 1998, Dr. Reed served
69
as Vice President of Business Development at NPS Pharmaceuticals, Inc., a company which develops small molecule drugs and recombinant peptides. Prior to this, Dr. Reed served as Vice President at S.R. One, Limited, a venture capital fund focused on investments in biopharmaceuticals and the life sciences. Dr. Reed is board certified as a neuroradiologist and has held faculty positions at the University of Washington and Yale University in the department of radiology. Dr. Reed received his B.A. in biology and M.D. from the University of Missouri—Kansas City, and his M.B.A. from the Wharton School at the University of Pennsylvania.
Board Composition
Our board of directors currently consists of seven directors, each of whom has been elected to serve a one year term. Our directors hold office until their successors have been elected and qualified or until their earlier death, resignation, disqualification or removal for cause by the affirmative vote of the holders of a majority of the outstanding stock entitled to vote on election of directors.
Board Committees
Our board of directors has an audit committee, a compensation committee and a corporate governance and nominating committee.
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. The audit committee consists of Mr. Dittamore, Mr. Nelson and Mr. Olson, each of whom is an independent member of our board of directors as defined by applicable Securities and Exchange Commission, or SEC, rules and the Nasdaq National Market listing standards. The functions of this committee include, among other things:
Both our independent auditors and internal financial personnel regularly meet privately with our audit committee and have unrestricted access to this committee.
Compensation Committee
Our compensation committee consists of Mr. Nelson and Mr. Wollaeger, each of whom is a non-management member of our board of directors. The functions of this committee include, among other things:
70
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee currently consists of Mr. Dittamore, Mr. Olson and Mr. Wollaeger, each of whom is a non-management member of our board of directors. The functions of this committee include, among other things:
We currently pay our directors $4,000 for attending in-person board meetings and $500 for attending telephonic board meetings. In addition, we also currently pay our directors $1,000 for attending in-person committee meetings and $500 for attending telephonic committee meetings. In addition, directors are reimbursed for reasonable out-of-pocket expenses in connection with attending meetings of our board of directors and committees of the board of directors. In 2003, none of our non-employee directors were granted options to purchase our common stock.
Effective upon the completion of this offering, we will adopt our 2004 Non-Employee Directors' Stock Option Program to provide for the automatic grant of options to purchase shares of common stock to non-employee directors who join the board of directors after the completion of this offering, and annual grants of shares of our common stock to each of our non-employee directors, which in each case shall vest . In addition, all of our directors are eligible to participate in our 2004 Equity Incentive Award Plan, and following the completion of this offering, our employee directors will be eligible to participate in our 2004 Employee Stock Purchase Plan. For a more detailed description of these plans, see "Benefit Plans."
Compensation Committee Interlocks And Insider Participation
Except for Mr. Wollaeger's unpaid service to us as Chief Executive Officer in May 1999, no member of our compensation committee has ever been an officer or employee of ours. None of our executive officers currently serve, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
71
Executive Compensation
The following table provides information regarding the compensation earned during the fiscal year ended December 31, 2003 by our Chief Executive Officer and our other four most highly compensated executive officers. We refer to our Chief Executive Officer and these other executive officers as our "named executive officers" in this prospectus.
Summary Compensation Table
| | | | Long-Term Compensation | | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Annual Compensation | | ||||||||
| Name and Principal Position | Securities Underlying Options(#) | Other Compensation(2) | ||||||||
| Salary | Bonus(1) | |||||||||
| David M. Sheehan President, Chief Executive Officer and Director | $ | 216,538 | $ | 37,500 | — | — | ||||
| Todd P. Clyde Chief Financial Officer | 170,000 | 22,000 | — | — | ||||||
| Diana M. Bowden Vice President of Marketing | 134,251 | 12,000 | — | — | ||||||
| Martin B. Shirley Regional Vice President of Sales, East | 203,867 | — | — | — | ||||||
| Stephen L. Bollinger Regional Vice President of Sales, West | 184,287 | — | — | — | ||||||
Stock Option Grants in Last Fiscal Year
During the fiscal year ended December 31, 2003, we granted stock options to purchase 999,631 shares of our common stock under our 1998 Stock Option/Stock Issuance Plan, including grants to executive officers. No grants of stock options were made to any of the named executive officers during 2003. All options were granted at the fair market value of our common stock as determined by our board of directors or compensation committee, as applicable, on the date of grant. Generally, 25% of the shares subject to options vest one year from the date of hire and the remainder of the shares vest in equal daily installments over the three years thereafter. Options expire ten years from the date of grant.
Aggregated Option Exercises in Last Fiscal Year and Fiscal Year End Option Values
The following table sets forth the number of shares of common stock subject to exercisable and unexercisable stock options held as of December 31, 2003 by each of the named executive officers. The value of unexercised in-the-money options at December 31, 2003 is calculated based on an assumed initial public offering price of $ per share of our common stock, which is the midpoint of the range listed on the cover of this prospectus, less the per share exercise price, multiplied by the number of shares issued upon exercise of the options, without taking into account any taxes that may be payable in connection with the option exercise. Options shown as exercisable in the table below are immediately exercisable, but we
72
have the right to purchase the shares of unvested common stock underlying some of these options upon termination of the holder's employment with us.
Benefit Plans
1995 Stock Option/Stock Issuance Plan
Our 1995 Stock Option/Stock Issuance Plan, or the 1995 Plan, was approved by our board of directors and stockholders in December 1995. As of December 31, 2003, there were a total of 9,525 shares of common stock reserved for issuance under our 1995 Plan, subject to adjustment for any future stock split, or any future stock dividend or other similar change in our common stock or our capital structure. As of December 31, 2003, options to purchase 1,984 shares of our common stock had been exercised, options to purchase 2,294 shares of our common stock were outstanding and 5,247 shares of our common stock remained available for grant. As of December 31, 2003, the outstanding options were exercisable at a weighted average exercise price of approximately $112.62 per share. The foregoing share numbers reflect a 1-for-200 reverse split of our capital stock effected in October 2002.
After the consummation of this offering, no additional options will be granted under our 1995 Plan and all options granted under our 1995 Plan that expire without having been exercised or are cancelled will become available for grant under our 2004 Stock Incentive Plan.
Awards under our 1995 Plan may consist of incentive stock options, which are stock options that qualify under Section 422 of the Internal Revenue Code, nonstatutory stock options and direct issuances of common stock.
Under our 1995 Plan, our board of directors may grant incentive stock options to employees, including officers and employee directors. Nonstatutory stock options and stock issuances may be granted to employees, directors, and consultants. Our board of directors or a committee designated by our board, referred to as the plan administrator, administers our 1995 Plan, including selecting the award recipients, determining the number of shares to be subject to each award, determining the exercise or purchase price of each award and determining the vesting and exercise periods of each award. The exercise price of all incentive stock options granted under our 1995 Plan must be at least equal to the fair market value of the common stock on the date of grant. The exercise price of all nonstatutory stock options granted under our 1995 Plan shall be determined by the plan administrator, but in no event may be less than 90% of the fair market value on the date of grant. With respect to any participant who owns stock possessing more than 10% of the voting power of all our classes of stock or the stock of any parent or subsidiary of us, the exercise price of any incentive stock option must equal at least 110% of the fair market value on the grant date. The maximum term of an incentive stock option or nonstatutory stock option must not exceed ten years. The purchase price per share for direct stock issuances must be not less than 90% of the fair market value on the date of issuance.
Under our 1995 Plan, options may not be assigned or transferred in any manner other than by will or by the laws of descent or distribution and may be exercised during the lifetime of the participant only by the participant.
If an optionee's status as an employee, director or consultant terminates for any reason other than death or disability, the optionee may exercise their vested options within the three month period following the termination. In the event the optionee dies while the optionee is an employee, director or consultant of our company, the options vested as of the date of death may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's death. In the event the optionee becomes disabled while the optionee is an employee, director or consultant of our company, the options vested as of the date of disability may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's disability.
The plan administrator has discretion to provide for acceleration of vesting in connection with a corporate transaction.
Our 1995 Plan will terminate automatically in 2005 unless terminated earlier by our board of directors. Our board of directors also has the authority to amend our 1995 Plan. However, no action may be taken which will adversely affect any option previously granted under our 1995 Plan, without the optionee's consent. To the extent necessary to comply with applicable provisions of federal securities laws, state corporate and securities laws, the Internal Revenue Code, the rules of any applicable stock exchange or national market system, and the rules of any non-U.S. jurisdiction applicable to awards granted to residents therein, we shall obtain stockholder approval of any such amendment to our 1995 Plan in such a manner and to such a degree as required.
1997 Stock Option/Stock Issuance Plan
Our 1997 Stock Option/Stock Issuance Plan, or the 1997 Plan, was approved by our board of directors in September 1997 and by our stockholders in October 1997. As of December 31, 2003, there were a total of 4,146 shares of common stock reserved for issuance under our 1997 Plan, subject to adjustment for a stock split, or any future stock dividend or other similar change in our common stock or our capital structure. As of December 31, 2003, options to purchase 678 shares of common stock had been exercised, options to purchase 415 shares of common stock were outstanding and 3,055 shares of common stock remained available for grant. As of December 31, 2003, the outstanding options were exercisable at a weighted average exercise price of approximately $50.19 per share. All capital stock and option share numbers reflect a 1-for-200 reverse split of our capital stock effected in October 2002.
After the consummation of this offering, no additional options will be granted under our 1997 Plan and all options granted under our 1997 Plan that expire without having been exercised or are cancelled will become available for grant under our 2004 Stock Incentive Plan.
Awards under our 1997 Plan may consist of incentive stock options, which are stock options that qualify under Section 422 of the Internal Revenue Code, nonstatutory stock options and direct issuances of common stock.
Under our 1997 Plan, our board may grant incentive stock options to employees, including officers and employee directors. Nonstatutory stock options and stock issuances may be granted to employees, directors, and consultants. The board of directors or a committee designated by the board, referred to as the plan administrator, administers our 1997 Plan, including selecting the award recipients, determining the number of shares to be subject to each award, determining the exercise or purchase price of each award and determining the vesting and exercise periods of each award. The exercise price of all incentive stock options granted under our 1997 Plan must be at least equal to the fair market value of the common stock on the date of grant. The exercise price of all nonstatutory stock options granted under our 1997 Plan must be determined by the plan administrator, but in no event may be less than 85% of the fair market value on the date of grant. With respect to any participant who owns stock possessing more than 10% of the voting power of all our classes of stock or the stock of any parent or subsidiary of us, the exercise price of any incentive stock option must equal at least 110% of the fair market value on the grant date. The maximum term of an incentive stock option or nonstatutory stock option must not exceed ten years, provided, however, that the maximum term of any incentive stock option granted to participant who owns
74
stock possessing more than 10% of the voting power of all our classes of stock or the stock of any parent or subsidiary of us must not exceed five years. The purchase price per share for direct stock issuances must be not less than 85% of the fair market value on the date of issuance.
Under our 1997 Plan, options may not be assigned or transferred in any manner other than by will or by the laws of descent or distribution and may be exercised during the lifetime of the participant only by the participant.
If an optionee's status as an employee, director or consultant terminates for any reason other than death or disability, the optionee may exercise their vested options within the three-month period following the termination. In the event the optionee dies while the optionee is an employee, director or consultant of our company, the options vested as of the date of death may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's death. In the event the optionee becomes disabled while the optionee is an employee, director or consultant of our company, the options vested as of the date of disability may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's disability.
In the event of a corporate transaction where the acquiror does not assume or replace options granted under our 1997 Plan, such outstanding options will become fully vested and exercisable immediately prior to the consummation of the corporate transaction. In the event of a corporate transaction in which the acquiror assumes or replaces options granted under our 1997 Plan, options issued under our 1997 Plan will not be subject to accelerated vesting. However, assumed or replaced options will automatically become fully vested and exercisable if the optionee's service is terminated by reason of an involuntary termination within 24 months of the occurrence of a corporate transaction.
Under our 1997 Plan, a corporate transaction is generally defined as:
Our 1997 Plan will terminate automatically in 2007 unless terminated earlier by our board of directors. The board of directors also has the authority to amend our 1997 Plan. However, no action may be taken which will adversely affect any option previously granted under our 1997 Plan, without the optionee's consent. To the extent necessary to comply with applicable provisions of federal securities laws, state corporate and securities laws, the Internal Revenue Code, the rules of any applicable stock exchange or national market system, and the rules of any non-U.S. jurisdiction applicable to awards granted to residents therein, we shall obtain stockholder approval of any such amendment to our 1997 Plan in such a manner and to such a degree as required.
1998 Stock Option/Stock Issuance Plan
Our 1998 Stock Option/Stock Issuance Plan, or the 1998 Plan, was approved by our board of directors in December 1998 and by our stockholders in November 1999. As of December 31, 2003, there were a total of 5,876,153 shares of common stock reserved for issuance under the 1998 Plan, subject to adjustment for any future stock split, or any future stock dividend or other similar change in our common stock or our capital structure. As of December 31, 2003, options to purchase 38,298 shares of common stock had been exercised, options to purchase 4,828,429 shares of common stock were outstanding and 1,009,845 shares of common stock remained available for grant. As of December 31, 2003, the outstanding options were exercisable at a weighted average exercise price of approximately $0.47 per share. All capital stock and option share numbers reflect a 1-for-200 reverse split of our capital stock effected in October 2002.
After the completion of this offering, no additional options will be granted under our 1998 Plan and all options granted under our 1998 Plan that expire without having been exercised or are cancelled will become available for grant under our 2004 Stock Incentive Plan.
75
Awards under our 1998 Plan may consist of incentive stock options, which are stock options that qualify under Section 422 of the Internal Revenue Code, nonstatutory stock options and direct issuances of common stock.
Under the 1998 Plan, our board may grant incentive stock options to employees, including officers and employee directors. Nonstatutory stock options and stock issuances may be granted to employees, directors, and consultants. Our board of directors or a committee designated by the board, referred to as the plan administrator, administers our 1998 Plan, including selecting the award recipients, determining the number of shares to be subject to each award, determining the exercise or purchase price of each award and determining the vesting and exercise periods of each award. The exercise price of all incentive stock options granted under our 1998 Plan must be at least equal to the fair market value of the common stock on the date of grant. The exercise price of all nonstatutory stock options granted under our 1998 Plan must be determined by the plan administrator, but in no event may be less than 85% of the fair market value on the date of grant. With respect to any participant who owns stock possessing more than 10% of the voting power of all our classes of stock or the stock of any parent or subsidiary of us, the exercise price of any incentive stock option must equal at least 110% of the fair market value on the grant date. The maximum term of an incentive stock option or nonstatutory stock option must not exceed ten years, provided, however, that the maximum term of any incentive stock option granted to participant who owns stock possessing more than 10% of the voting power of all our classes of stock or the stock of any parent or subsidiary of us must not exceed five years. The purchase price per share for direct stock issuances must be not less than 85% of the fair market value on the date of issuance.
Under our 1998 Plan, options may not be assigned or transferred in any manner other than by will or by the laws of descent or distribution and may be exercised during the lifetime of the participant only by the participant.
If an optionee's status as an employee, director or consultant terminates for any reason other than death or disability, the optionee may exercise their vested options within the three-month period following the termination. In the event the optionee dies while the optionee is an employee, director or consultant of our company, the options vested as of the date of death may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's death. In the event the optionee becomes disabled while the optionee is an employee, director or consultant of our company, the options vested as of the date of disability may be exercised prior to the earlier of their expiration date or 12 months from the date of the optionee's disability.
In the event of a corporate transaction where the acquiror does not assume or replace options granted under our 1998 Plan, such outstanding options will become fully vested and exercisable immediately prior to the consummation of the corporate transaction. In the event of a corporate transaction in which the acquiror assumes or replaces options granted under our 1998 Plan, options issued under our 1998 Plan will not be subject to accelerated vesting. However, assumed or replaced options will automatically become fully vested and exercisable if the optionee's service is terminated by reason of an involuntary termination within 24 months of the occurence of a corporate transaction.
Under our 1998 Plan, a corporate transaction is generally defined as:
Our 1998 Plan will terminate automatically in 2008 unless terminated earlier by our board of directors. Our board of directors also has the authority to amend our 1998 Plan. However, no action may be taken which will adversely affect any option previously granted under our 1998 Plan, without the optionee's consent. To the extent necessary to comply with applicable provisions of federal securities laws, state corporate and securities laws, the Internal Revenue Code, the rules of any applicable stock exchange
76
or national market system, and the rules of any non-U.S. jurisdiction applicable to awards granted to residents therein, we will obtain stockholder approval of any such amendment to our 1998 Plan in such a manner and to such a degree as required.
2004 Stock Incentive Plan
Our board of directors and our stockholders approved our 2004 Stock Incentive Plan in 2004. We have reserved shares of our common stock for issuance under our 2004 Stock Incentive Plan, subject to adjustment for a stock split, or any future stock dividend or other similar change in our common stock or our capital structure. Commencing on the first business day of each calendar year beginning in 2005, the number of shares of stock reserved for issuance under the 2004 Stock Incentive Plan (including issuance as incentive stock options) will be increased annually by a number equal to the lesser of % of the total number of shares outstanding as of that date, shares, or a lesser number of shares determined by the board. No awards have yet been granted under our 2004 Stock Incentive Plan and therefore shares of common stock remain available for grant.
Our 2004 Stock Incentive Plan provides for the grant of stock options, restricted stock, restricted stock units, stock appreciation rights and dividend equivalent rights, collectively referred to as "awards." Stock options granted under the 2004 Stock Incentive Plan may be either incentive stock options under the provisions of Section 422 of the Internal Revenue Code, or non-qualified stock options. Incentive stock options may be granted only to employees. Awards other than incentive stock options may be granted to employees, directors and consultants.
Our board of directors or a committee designated by our board, referred to as the "plan administrator," will administer our 2004 Stock Incentive Plan, including selecting the optionees, determining the number of shares to be subject to each award, determining the exercise or purchase price of each award and determining the vesting and exercise periods of each award.
The exercise price of all incentive stock options granted under our 2004 Stock Incentive Plan must be at least equal to 100% of the fair market value of the common stock on the date of grant. If, however, incentive stock options are granted to an employee who owns stock possessing more than 10% of the voting power of all classes of our stock or the stock of any parent or subsidiary of us, the exercise price of any incentive stock option granted must equal at least 110% of the fair market value on the grant date and the maximum term of these incentive stock options must not exceed five years. The maximum term of an incentive stock option granted to any other participant must not exceed ten years. The plan administrator will determine the term and exercise or purchase price of all other awards granted under our 2004 Stock Incentive Plan.
Under our 2004 Stock Incentive Plan, incentive stock options may not be sold, pledged, assigned, hypothecated, transferred or disposed of in any manner other than by will or by the laws of descent or distribution and may be exercised during the lifetime of the participant only by the participant. Other awards will be transferable by will or by the laws of descent or distribution and to the extent provided in the award agreement. Our 2004 Stock Incentive Plan permits the designation of beneficiaries by holders of awards, including incentive stock options.
In the event a participant in our 2004 Stock Incentive Plan terminates service or is terminated by us without cause, any options which have become exercisable prior to the time of termination will remain exercisable for three months from the date of termination, unless a shorter or longer period of time is determined by the plan administrator. In the event a participant in our 2004 Stock Incentive Plan is terminated by us for cause, any options which have become exercisable prior to the time of termination will immediately terminate. If termination was caused by death or disability, any options which have become exercisable prior to the time of termination will remain exercisable for 12 months from the date of termination, unless a shorter or longer period of time is determined by the plan administrator. In no event may a participant exercise the option after the expiration date of the option.
77
In the event of a corporate transaction where the acquiror assumes or replaces awards granted under our 2004 Stock Incentive Plan, none of these awards will be subject to accelerated vesting. However, assumed or replaced awards will automatically become fully vested if the grantee is terminated by the acquiror without cause or terminates employment for good reason within 24 months after the occurrence of a corporate transaction. In the event of a corporate transaction where the acquiror does not assume or replace awards granted under our 2004 Stock Incentive Plan, all of these awards become fully vested immediately prior to the consummation of the corporate transaction. Under our 2004 Stock Incentive Plan, a corporate transaction is generally defined as:
Unless terminated sooner, our 2004 Stock Incentive Plan will automatically terminate in 2014. Our board of directors has the authority to amend or terminate our 2004 Stock Incentive Plan. No amendment or termination of our 2004 Stock Incentive Plan will adversely affect any rights under awards already granted to a participant unless agreed to by the affected participant. To the extent necessary to comply with applicable provisions of federal securities laws, state corporate and securities laws, the Internal Revenue Code, the rules of any applicable stock exchange or national market system, and the rules of any non-U.S. jurisdiction applicable to awards granted to residents therein, we will obtain stockholder approval of any such amendment to our 2004 Stock Incentive Plan in such a manner and to such a degree as required.
2004 Non-Employee Director Stock Option Program
Our 2004 Non-Employee Director Stock Option Program will be adopted as part of our 2004 Stock Incentive Plan and will be subject to the terms and conditions of our 2004 Stock Incentive Plan. Our 2004 Non-Employee Director Stock Option Program was approved by our board of directors in 2004. Our 2004 Non-Employee Director Stock Option Program will become effective as of the effective date of this prospectus, and no awards will be made under this program until that time.
The purpose of our 2004 Non-Employee Director Stock Option Program is to promote the success of our business by enhancing our ability to attract and retain the best available non-employee directors and to provide them additional incentives.
Our 2004 Non-Employee Director Stock Option Program will establish an automatic option grant program for the grant of awards to non-employee directors. Under this program, each then-existing non-employee director upon the effective date of this prospectus and each non-employee director first elected to our board of directors following the closing of this offering will automatically be granted an option to acquire shares of our common stock at an exercise price per share equal to the fair market value of our common stock at the date of grant. These options will vest and become exercisable in three equal installments on each anniversary of the grant date. Upon the date of each annual stockholders' meeting, each non-employee director who has been a member of our board of directors for at least eleven months prior to the date of the stockholders' meeting will receive an automatic grant of options to acquire shares of our common stock at an exercise price equal to the fair market value of our common stock at the date of grant. These options will vest and become exercisable on the first anniversary of the grant date. The term of each automatic option grant and the extent to which it will be transferable will be provided in the agreement evidencing the option.
Our 2004 Non-Employee Director Stock Option Program will be administered by the board or a committee designated by our board made up of two or more non-employee directors so that such awards would be exempt from Section 16(b) of the Exchange Act, referred to as the "program administrator." The program administrator will determine the terms and conditions of awards, and construe and interpret the
78
terms of the program and awards granted under the program. Non-employee directors may also be granted additional awards under the 2004 Stock Incentive Plan, subject to the discretion of the board or the committee.
Unless terminated sooner, our 2004 Non-Employee Director Stock Option Program will terminate automatically in 2014 when our 2004 Stock Incentive Plan terminates. Our board of directors has the authority to amend, suspend or terminate our 2004 Non-Employee Director Stock Option Program. No amendment or termination of our 2004 Non-Employee Director Stock Option Program will adversely affect any rights under options already granted to a non-employee director unless agreed to by the affected non-employee director. Our 2004 Non-Employee Director Stock Option Program was adopted by the board pursuant to its discretionary authority under our 2004 Stock Incentive Plan to make option grants to non-employee directors. Accordingly, stockholder approval is not required for the adoption or any amendment of our 2004 Non-Employee Director Stock Option Program.
2004 Employee Stock Purchase Plan
Our board of directors and our stockholders approved our 2004 Employee Stock Purchase Plan in 2004. Our 2004 Employee Stock Purchase Plan is intended to qualify as an "employee stock purchase plan" under Section 423 of the Internal Revenue Code in order to provide our employees with an opportunity to purchase our common stock through payroll deductions. An aggregate of shares of common stock will be reserved for issuance and will be available for purchase under our 2004 Employee Stock Purchase Plan, subject to adjustment for a stock split, or any future stock dividend or other similar change in our common stock or our capital structure. Commencing on the first business day of each calendar year beginning in 2005, the number of shares of stock reserved for issuance under our 2004 Employee Stock Purchase Plan will be increased annually by a number equal to the lesser of % of the total number of shares outstanding as of that date, shares, or a lesser number of shares determined by the board.
Our board of directors or a committee designated by the board, referred to as the "plan administrator," will administer our 2004 Employee Stock Purchase Plan. All of our employees who are regularly employed for more than five months in any calendar year and work more than 20 hours per week will be eligible to participate in our 2004 Employee Stock Purchase Plan and will be automatically enrolled in the initial offer period. Employees hired after the consummation of this offering will be eligible to participate in our 2004 Employee Stock Purchase Plan, subject to a five-day waiting period after hiring. Non-employee directors, consultants, and employees subject to the rules or laws of foreign jurisdictions that prohibit or make impractical their participation in an employee stock purchase plan will not be eligible to participate in our 2004 Employee Stock Purchase Plan.
Our 2004 Employee Stock Purchase Plan will designate offer periods, purchase periods and exercise dates. Offer periods will generally be overlapping periods of 24 months. The initial offer period will begin on the effective date of our 2004 Employee Stock Purchase Plan, which is the effective date of the registration statement relating to this offering, and ends on . Additional offer periods will commence each and . Purchase periods will generally be six month periods, with the initial purchase period commencing on the effective date of the registration statement relating to this offering and ending on . Thereafter, purchase periods will commence each and . Exercise dates are the last day of each purchase period. In the event we merge with or into another corporation, sell all or substantially all of our assets, or enter into other transactions in which all of our stockholders before the transaction own less than 40% of the total combined voting power of our outstanding securities following the transaction, the plan administrator may elect to shorten the offer periods then in progress.
On the first day of each offer period, a participating employee will be granted a purchase right. A purchase right is a form of option to be automatically exercised on the forthcoming exercise dates within the offer period during which authorized deductions are to be made from the pay of participants and
79
credited to their accounts under our 2004 Employee Stock Purchase Plan. When the purchase right is exercised, the participant's withheld salary is used to purchase shares of common stock. Participants in the initial offer period will be eligible to purchase shares during the first purchase period through direct payment rather than payroll deductions. The price per share at which shares of common stock are to be purchased under our 2004 Employee Stock Purchase Plan during any purchase period is the lesser of:
The participant's purchase right is exercised in this manner on each exercise date arising in the offer period unless, on the first day of any purchase period, the fair market value of our common stock is lower than the fair market value of our common stock on the first day of the offer period. If so, the participant's participation in the original offer period is terminated, and the participant is automatically enrolled in the new offer period effective the same date.
Payroll deductions may range from 1% to % in whole percentage increments of a participant's regular base pay, exclusive of bonuses, overtime, shift-premiums, commissions, reimbursements or other expense allowances. Except for the first purchase period of the initial offer period, participants may not make direct cash payments to their accounts. The maximum number of shares of our common stock that any employee may purchase under our 2004 Employee Stock Purchase Plan during a purchase period is shares. The Internal Revenue Code imposes additional limitations on the amount of common stock that may be purchased during any calendar year.
Unless terminated sooner, the 2004 Employee Stock Purchase Plan will terminate automatically in 2014. Our board of directors will have authority to amend or terminate our 2004 Employee Stock Purchase Plan. The plan administrator may terminate any offer period on any exercise date or establish a new exercise date with respect to any offer period then in progress if the plan administrator determines that the termination of the offer period is in the best interests of the Company and its stockholders. To the extent necessary to comply with applicable provisions of federal securities laws, state corporate and securities laws, the Internal Revenue Code, the rules of any applicable stock exchange or national market system, and the rules of any non-U.S. jurisdiction applicable to awards granted to residents therein, we will obtain stockholder approval of any such amendment to our 2004 Employee Stock Purchase Plan in such a manner and to such a degree as required.
Employment Arrangements and Change of Control Arrangements
We have not entered into employment agreements with any of our executive officers.
In June 2002, we entered into a letter agreement with David M. Sheehan, our President, Chief Executive Officer and director, whereby we agreed to pay him cash bonuses in the amount of $25,000 on each of June 2002, October 2002 and January 2003 in connection with his service to us as an employee. We also agreed to pay Mr. Sheehan a further cash bonus dependent upon our receipt of certain revenues and cashflow for the fiscal year ending December 31, 2002. In addition, we agreed that in the event that at any time on or before June 2004 we were acquired or substantially all of our assets were sold, Mr. Sheehan and other members of senior management would be entitled to receive an aggregate bonus in an amount not less than $400,000 and not greater than 10% of any proceeds received by us in connection with such acquisition or sale in excess of $30,000,000. We also agreed to make a further grant of options to Mr. Sheehan to purchase shares of our common stock pursuant to our 1998 Plan.
We routinely grant our executive officers stock options under our stock incentive plans. For a description of the change of control provisions applicable to such stock options, see "Management—Benefit Plans."
80
Limitation of Liability and Indemnification of Officers and Directors
As permitted by Section 102 of the Delaware General Corporation Law, we have adopted provisions in our restated certificate of incorporation and restated bylaws that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages or breach of fiduciary duty as a director, except for liability for:
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our restated certificate of incorporation also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permitted by Section 145 of the Delaware General Corporation Law, our restated bylaws provide that:
We have entered, and intend to continue to enter, into separate indemnification agreements with each of our directors and officers which may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements require us, among other things, to indemnify our officers and directors against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified.
At present, we are not aware of any pending or threatened litigation or proceeding involving a director, officer, employee or agent in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding that might result in a claim for such indemnification.
We have purchased a policy of directors' and officers' liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment in some circumstances.
81
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
All share and per share amounts have been adjusted to give effect to a 1-for-200 reverse split of our capital stock effected in October 2002 and a 1-for- reverse split of our capital stock to be effected before the completion of this offering.
Issuances of Options
From January 2001 to December 31, 2003, we granted options to purchase an aggregate of 3,054,725 shares of our common stock to our current directors and executive officers, including each of our executive officers named in the Summary Compensation Table, at an average weighted exercise price of $0.23.
Issuance of Common Stock
In January 2002, David M, Sheehan, our President, Chief Executive Officer and a director, exercised an option to purchase 100 shares at an aggregate exercise price of $10,000.
Issuances of Preferred Stock
In January, March and April 2001, we issued and sold to investors 9,694 shares of our Series E preferred stock, at a purchase price of $607.20 per share, for an aggregate purchase price of approximately $5.89 million. In April, May and June 2002, an aggregate of 9,611 of these shares of our Series E preferred stock were exchanged for shares of our Series G preferred stock. Upon completion of this offering, the 5,447 shares of our Series E preferred stock outstanding as of December 31, 2003 will convert into 5,447 shares of our common stock.
In August 2001, we issued and sold 13,092 shares of our Series F preferred stock, at a purchase price of $650.00 per share, for an aggregate purchase price of approximately $8.51 million. In April, May and June 2002, an aggregate of 12,322 of these shares were exchanged for shares of our Series G preferred stock. Upon completion of this offering, the 770 shares of our Series F preferred stock outstanding as of December 31, 2003 will convert into 824 shares of our common stock.
In April, May and June 2002, we issued and sold 31,008,401 shares of our Series G preferred stock, at a purchase price of $2.00 per share, in exchange for the conversion of outstanding shares of our Series A, Series B, Series C, Series D, Series E and Series F preferred stock having an aggregate liquidation value of approximately $62.0 million. Concurrently with such exchange, we issued and sold 12,561,706 shares of our Series H preferred stock, at a purchase price of $1.39 per share, for an aggregate purchase price of approximately $17.5 million. Following the issuance of our Series G preferred stock, holders of 24,191 shares of our Series G preferred stock elected to convert such shares into shares of our common stock. Upon completion of this offering, the 30,984,210 shares of our Series G preferred stock outstanding as of December 31, 2003 will convert into 30,984,210 shares of our common stock, and the 12,561,706 shares of our Series H preferred stock outstanding as of December 31, 2003 will convert into 12,561,706 shares of our common stock.
82
The purchasers of our Series E preferred stock, Series F preferred stock, Series G preferred stock and Series H preferred stock include, among others, the following directors and holders of more than 5% of our outstanding stock:
| | Shares of Preferred Stock | |||||||
|---|---|---|---|---|---|---|---|---|
| Name | Series E(1) | Series F(2) | Series G(3) | Series H(4) | ||||
| Entities affiliated with Kingsbury Associates(5) | 625 | 923 | 4,788,417 | 980,348 | ||||
| Entities affiliated with Sorrento Associates(6) | — | 385 | 3,870,246 | 1,220,217 | ||||
| Entities affiliated with Vector Fund Management(7) | — | 769 | 6,117,483 | 1,284,533 | ||||
| Palivacinni Partners, LLC(8) | 124 | 100 | 70,000 | 35,221 | ||||
| Entities affiliated with Merrill Lynch Ventures(9) | 4,044 | 538 | 3,398,635 | 2,443,201 | ||||
| Kenneth E. Olson Trust dated March 16, 1989(10) | — | 154 | 65,127 | 84,268 | ||||
| Linda K. Olson (11) | — | — | 30,001 | 4,498 | ||||
| GE Capital Equity Investments, Inc.(12) | — | 4,615 | 1,498,159 | 1,435,545 | ||||
| Health Care Indemnity, Inc. | 1,647 | — | 2,000,000 | 299,791 | ||||
| Entities affiliated with Sanderling Ventures(13) | — | — | — | 2,158,702 | ||||
83
have been converted to common stock) and 195,569 shares of Series H preferred stock held by Sorrento Ventures CE, L.P. Robert M. Jaffe, a member of our board of directors, is president of (a) Sorrento Growth, Inc., which is the general partner of Sorrento Equity Growth Partners I, L.P., which is the general partner of Sorrento Growth Partners I, L.P.; and (b) Sorrento Associates, Inc., which is the general partner of (i) Sorrento Equity Partners, L.P., the general partner of Sorrento Ventures II, L.P., and (ii) Sorrento Equity Partners III, L.P., the general partner of Sorrento Ventures III, L.P. and Sorrento Ventures CE, L.P. Mr. Jaffe disclaims beneficial ownership of the shares held by Sorrento Growth Partners I, L.P., Sorrento Ventures II, L.P., Sorrento Ventures III, L.P. and Sorrento Ventures CE, L.P., except to the extent of his pecuniary interests in the named fund. Mr. Jaffe shares voting and investment power with respect to the shares held by Sorrento Growth Partners I, L.P., Sorrento Ventures II, L.P., Sorrento Ventures III, L.P. and Sorrento Growth Partners CE, L.P. with the other general partners of Sorrento Growth Partners I, L.P., Sorrento Ventures II, L.P., Sorrento Ventures III, L.P. and Sorrento Growth Partners CE, L.P.
84
member of our board of directors, is a managing director of Middleton, McNeil & Mills Associates V, LLC, the general partner of Sanderling Venture Partners V, L.P., Sanderling V Biomedical, L.P., Sanderling V Limited Partnership and Sanderling V Beteiligungs GMBH & Co. KG. Mr. Wollaeger is an owner of Sanderling V Ventures Management. Mr. Wollaeger disclaims beneficial ownership of the shares held by Sanderling Venture Partners V, L.P., Sanderling V Biomedical, L.P., Sanderling V Limited Partnership, Sanderling V Beteiligungs GMBH & Co. KG and Sanderling V Ventures Management, except to the extent of his pecuniary interests in the named fund. Mr. Wollaeger shares voting and investment power with respect to the shares held by Sanderling Venture Partners V, L.P., Sanderling V Biomedical, L.P., Sanderling V Limited Partnership and Sanderling V Beteiligungs GMBH & Co. KG with the other managing directors of Middleton, McNeil & Mills Associates V, LLC. Mr. Wollaeger shares voting and investment power with respect to the shares held by Sanderling Ventures Management with the other owners.
Sales of Promissory Notes and Warrants
In January 2002, we borrowed an aggregate of approximately $1.9 million from existing stockholders and a new investor. We issued each lending party a convertible promissory note in January 2002 bearing interest at 12% per annum. In addition, we issued and sold to each of these parties a warrant to purchase a number of shares of our common stock for $0.20 per underlying share. Each warrant has an exercise price of $300.00 per share.
In April 2002, each of the convertible promissory notes issued in January 2002 was satisfied in full by converting each note into shares of our Series H preferred stock at a purchase price of $1.39.
The purchasers of our convertible promissory notes and warrants to purchase our common stock include, among others, the following directors and holders of more than 5% of our outstanding stock:
| Name | Principal Amount of Promissory Note | Shares of Series H Preferred Stock Issued Upon Conversion of Notes(1) | Shares of Common Stock Underlying Warrants | ||||
|---|---|---|---|---|---|---|---|
| Entities affiliated with Kingsbury Associates(2) | $ | 1,025,000 | 737,410 | 420 | |||
| Entities affiliated with Vector Fund Management(3) | $ | 200,000 | 143,884 | 83 | |||
| Merrill Lynch Ventures L.P., 2001 | $ | 100,000 | 71,942 | 41 | |||
| Kenneth E. Olson Trust dated March 16, 1989(4) | $ | 100,000 | 71,942 | 41 | |||
85
II, LLC, which is a general partner of Vector Later-Stage Equity Fund II, L.P. and Vector Later-Stage Equity Fund II (Q.P.), L.P. Dr. Reed disclaims beneficial ownership of the shares held by Vector Later-Stage Equity Fund II, L.P. and Vector Later-Stage Equity Fund II (Q.P.), L.P., except to the extent of his pecuniary interests in the named fund. Dr. Reed shares voting and investment power with respect to the shares held by Vector Later-Stage Equity Fund II, L.P. and Vector Later-Stage Equity Fund II (Q.P.), L.P. with the other managing directors of Vector Fund Management II, LLC.
Bonus Arrangements
In June 2002, we entered into a letter agreement with David M. Sheehan, our President, Chief Executive Officer and a director, whereby we agreed to pay him cash bonuses in connection with his service to us as an employee and in the event that our business was acquired or our assets sold. For a description of this letter agreement, see "Management—Employment Arrangements and Change of Control Arrangements."
Other Transactions
We have entered into agreements with all holders of our preferred stock, including entities affiliated with some of our directors and holders of 5% or more of our common stock, whereby we granted them registration rights with respect to their shares of common stock issuable upon conversion of their preferred stock. For more information regarding registration rights, please see "Description of Capital Stock."
We have entered into indemnification agreements with each of our executive officers and directors. The indemnification agreements require us to indemnify these individuals to the fullest extent permitted by Delaware law and may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. In addition, we have purchased a policy of directors' and officers' liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment in some circumstances.
The following table sets forth information regarding the beneficial ownership of our common stock as of February 29, 2004, for:
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and includes voting and investment power with respect to the securities. Except as indicated by footnote, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them. The number of shares of common stock used to calculate the percentage ownership of each listed person includes the shares of common stock underlying options or warrants held by such persons that are exercisable within 60 days of February 29, 2004, if any.
Percentage of beneficial ownership before the offering is based on 43,701,825 shares, consisting of 146,4582,055,158 shares of common stock outstanding as of February 29, 2004,March 31, 2020, and 43,555,367excludes, as of such date:
| ● | 26,517 shares of common stock reserved for issuance pursuant to grants outstanding under our 2011 Inducement Award Plan; |
| ● | 40,621 shares of common stock reserved for issuance pursuant to grants outstanding under our 2014 Equity Incentive Award Plan; |
| ● | 72,989 shares of common stock reserved for future issuance under our 2018 Equity Incentive Award Plan; and |
| ● | [●] shares of common stock issuable upon the exercise of the warrant granted to the underwriter in connection with this offering. |
Except as otherwise noted, all information in this prospectus reflects and assumes (i) no sale of pre-funded warrants in this offering, which, if sold, would reduce the number of shares issuable upon the conversionthat we are offering on a one-for-one basis, (ii) no exercise of outstanding options issued under our equity incentive plans and (iii) no exercise of the preferred stock. Percentage of beneficial ownership after the offering is based on shares, including shares offered by this prospectus. Unless otherwise indicated, the address for the following stockholders is c/o Digirad Corporation, 13950 Stowe Drive, Poway, California 92064.accompanying warrants or underwriter’s warrant described above.
| | | Percentage of Shares Beneficially Owned | |||||
|---|---|---|---|---|---|---|---|
| | Number of Shares Beneficially Owned | ||||||
| Name and Address of Beneficial Owner | Before Offering | After Offering | |||||
| Executive Officers and Directors: | |||||||
| David M. Sheehan(1) | 1,456,765 | 3.2 | % | ||||
| Todd P. Clyde(2) | 395,000 | * | |||||
| Diana M. Bowden(3) | 122,544 | * | |||||
| Martin B. Shirley(4) | 141,420 | * | |||||
| Stephen L. Bollinger(5) | 102,943 | * | |||||
| Timothy J. Wollaeger(6) | 7,939,975 | 18.2 | |||||
| Raymond V. Dittamore | — | * | |||||
| Robert M. Jaffe(7) | 5,095,223 | 11.7 | |||||
| R. King Nelson(8) | 275 | * | |||||
| Kenneth E. Olson(9) | 362,062 | * | |||||
| Douglas Reed, M.D.(10) | 7,514,925 | 17.2 | |||||
| 5% Stockholders: | |||||||
| Entities affiliated with Vector Fund Management(11) 1751 Lake Cook Road, Suite 350 Deerfield, IL 60015 | 7,409,704 | 17.0 | |||||
| Merrill Lynch Ventures, L.P. 2001(12) 4 World Financial Center, 22nd Floor New York, NY 10080 | 5,846,056 | 13.4 | |||||
| Entities affiliated with Kingsbury Associates(13) 3655 Nobel Drive, Suite 490 San Diego, CA 92122 | 5,775,739 | 13.2 | |||||
87
| Entities affiliated with Sorrento Associates(7) 4370 La Jolla Village Drive, Suite 1040 San Diego, CA 92122 | 5,095,223 | 11.7 | |||||
| GE Capital Equity Investments, Inc. 120 Long Ridge Road Stamford, CT 06927 | 2,935,546 | 6.7 | |||||
| Health Care Indemnity, Inc. One Park Plaza Nashville, TN 37069 | 2,299,791 | 5.3 | |||||
| All directors and executive officers as a group (15 persons) | 24,153,264 | 51.2 |
88
McNeil & Mills Associates V, LLC. Mr. Wollaeger shares voting and investment power with respect to the shares held by Sanderling Ventures Management with the other owners.
89
pecuniary interests in the named fund. Dr. Reed shares voting and investment power with respect to the shares held by Vector Later-Stage Equity Fund, L.P., Vector Later-Stage Equity Fund II, L.P. and Vector Later-Stage Equity Fund II (Q.P.), L.P. with the other managing directors of Vector Fund Management II, LLC.
90
DESCRIPTION OF OUR CAPITAL STOCK
AND SECURITIES OFFERED
The following descriptionsdescription summarizes important terms of our capital stock give effectstock. For a complete description, you should refer to the following events:
Upon completion of this offering, our authorized capital stock will consist of 150,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of undesignated preferred stock, $0.001 par value per share.
The following summary of the rights of our capital stock is not complete and is qualified in its entiretyare incorporated by reference to our restated certificate of incorporation and restated bylaws to be in effect upon the completion of this offering, copies of which are filed as exhibits to the registration statement of which this prospectus is a part, as well as the relevant portions of the Delaware law.
We are offering (i) up to [●] shares, (ii) up to [●] pre-funded warrants, each exercisable for one share of common stock, and (iii) warrants to purchase common stock accompanying each share of common stock and pre-funded warrant, each accompanying warrant exercisable for 0.5 of a share of common stock. For each pre-funded warrant we sell, the number of shares we are offering will be decreased on a one-for-one basis.
The following description summarizes certain terms of our capital stock, the warrants and pre-funded warrants we are offering and certain provisions of our certificate of incorporation and bylaws. This summary does not purport to be complete and is qualified in its entirety by the form of the warrant, the form of the pre-funded warrant and the provisions of our certificate of incorporation and bylaws, copies of which are filed with the SEC as exhibits to the Registration Statement on Form S-1 of which this prospectus forms a part, and to the applicable provisions of Delaware law.
Authorized and Outstanding Digirad Capital Stock
Digirad’s restated certificate of incorporation authorizes the issuance of 40,000,000 shares of capital stock, consisting of 30,000,000 shares of common stock, and 10,000,000 shares of preferred stock, each with a par value per share of $0.0001. 500,000 shares of preferred stock were previously designated as Series B Participating Preferred Stock. As of the date of this prospectus, there were 2,097,381 shares of our common stock outstanding, held of record by 166 holders, and 1,915,637 shares of our 10.0% Series A Cumulative Perpetual Preferred Stock (the “Series A Preferred Stock”) outstanding.
Common Stock
Outstanding Shares
Based on 146,458 shares of common stock outstanding as of February 29, 2004, the issuance of shares of common stock in this offering, the issuance of 43,555,367 shares of common stock upon conversion of all outstanding shares of our preferred stock, and no exercise of outstanding options or warrants, there will be shares of common stock outstanding upon the closing of this offering. As of the same date, there were 4,831,138 shares subject to outstanding options under our 1995 Stock Option/Stock Issuance Plan, our 1997 Stock Option/Stock Issuance Plan and our 1998 Stock Option/Stock Issuance Plan. In addition, as of the same date, there were warrants outstanding to purchase 203,457 shares of our common stock. As of December 31, 2003, we had approximately 299 holders of our common stock.
Voting Rights
Holders of ourDigirad common stock are entitled to one vote per share on all matters to be voted upon by theDigirad stockholders. The holders of Digirad common stock are not entitled to cumulate voting rights with respect to the election of directors, which means that the holders of a majority of the shares voted can elect all of the directors then standing for election.
Dividends
Subject to limitations under Delaware law and preferences that may apply to any outstanding shares of Digirad preferred stock, holders of ourDigirad common stock are entitled to receive ratably such dividends or other distribution, if any, as may be declared by ourDigirad’s board of directors out of funds legally available therefor. In addition, we are currently not permitted to pay any dividends on our common stock, because we have unpaid accrued dividends on our Series A Preferred Stock. Pursuant to the terms of the Series A Preferred Stock (as described below), until we pay all dividends that are due and payable on the Series A Preferred Stock, we will be unable to pay dividends on our common stock.
Liquidation
In the event of ourDigirad’s liquidation, dissolution or winding up, holders of ourDigirad common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to the liquidation preference of any of ourDigirad’s outstanding preferred stock.
91
Rights and Preferences
Our
Digirad’s common stock has no preemptive, conversion or other rights to subscribe for additional securities. There are no redemption or sinking fund provisions applicable to ourDigirad common stock. The rights, preferences and privileges of holders of ourDigirad common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of ourDigirad preferred stock that weDigirad may designate and issue in the future.
Fully Paid and Nonassessable
All outstanding shares of ourDigirad common stock are and all shares our common stock to be outstanding upon completion of this offering will be, validly issued, fully paid and nonassessable.
Listing
Our common stock is listed on the NASDAQ Global Market under the symbol “DRAD”.
Digirad Series A Preferred Stock
As
Digirad’s board of February 29, 2004, there were 43,555,313 shares of convertible preferred stock outstanding. We have secured consents from the requisite number of stockholdersdirectors is authorized, subject to the automatic conversion of all outstanding shares of redeemable convertiblelimitations prescribed by Delaware law, to issue preferred stock in connection with this offering. As a result, all outstanding shares of redeemable convertible preferred stock will be converted into 43,555,367 shares of our common stock in connection with this offering and such shares of redeemable convertible preferred stock will no longer be authorized, issuedone or outstanding.
In addition, as of the same date, there were warrantsmore series, to purchase 1,939 shares of our preferred stock, warrants to purchase 249 of which will expire if not exercised prior to the completion of the offering.
Upon the closing of this offering, our board of directors will be authorized, without further stockholder approval, to issueestablish from time to time one or more series of preferred stock and to fix or alter the designations, powers, preferences, rights and any qualifications, limitations or restrictions of the shares of such series, including:
Theany series of preferred stock, without any further vote or action by Digirad stockholders. Digirad’s board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of Digirad common stock or other series of preferred stock. The issuance of preferred stock, while providing flexibility in connection with possible financings, acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of Digirad.
The following summary of the terms and conditionsprovisions of the Series A Preferred Stock does not purport to be complete and is qualified in its entirety by reference to the pertinent sections of Digirad’s restated certificate of incorporation including the Certificate of Designations for the Series A Preferred Stock, which could discouragesupplements Digirad’s restated certificate of incorporation by classifying the Series A Preferred Stock.
Digirad has authorized 8,000,000 shares of Series A Preferred Stock, 1,915,637 shares of which are currently outstanding. All outstanding shares of Series A Preferred Stock are validly issued, fully paid and nonassessable. Digirad’s board of directors may, without the approval of holders of the Series A Preferred Stock or common stock, designate additional series of authorized preferred stock ranking junior to the Series A Preferred Stock or designate additional shares of the Series A Preferred Stock and authorize the issuance of such shares. Designation of preferred stock ranking senior to the Series A Preferred Stock will require approval of the holders of Series A Preferred Stock, as described below in “Voting Rights.”
No Sinking Fund
The Series A Preferred Stock is not subject to any sinking fund.
Listing
The Series A Preferred Stock is listed on the NASDAQ Global Market under the symbol “DRADP”.
Dividends
Holders of shares of the Series A Preferred Stock are entitled to receive, when, as and if, authorized by Digirad’s board of directors (or a takeoverduly authorized committee of Digirad board of directors) and declared by Digirad, out of funds legally available for the payment of dividends, preferential cumulative cash dividends at the rate of 10.0% per annum of the liquidation preference of $10.00 per share (equivalent to a fixed annual amount of $1.00 per share).
Dividends are payable quarterly, in arrears, on the last calendar day of March, June, September and December (each a “dividend payment date”); provided that if any dividend payment date is not a business day, then the dividend that would otherwise have been payable on that dividend payment date may be paid on the next succeeding business day and no interest, additional dividends or other transactionsums will accrue on the amount so payable for the period from and after that dividend payment date to that next succeeding business day. To date no dividends have been paid on the Series A Preferred Stock and as a result, cumulative dividends will continue to accrue as part of the liquidation value of the Series A Preferred Stock.
Dividends will be payable to holders of somerecord as they appear in Digirad’s stock records for the Series A Preferred Stock at the close of business on the corresponding record date, which shall be the first day of each month in which a quarterly dividend is to be paid, whether or not a business day (each, a “dividend record date”). As a result, holders of shares of Series A Preferred Stock will not be entitled to receive dividends on a dividend payment date if such shares were not issued and outstanding on the applicable dividend record date. Digirad’s board of directors will not authorize, declare, pay or set apart for payment any dividends on shares of Series A Preferred Stock at any time that the terms and provisions of any of Digirad’s agreements, including any agreement relating to Digirad’s indebtedness, prohibits that action or provides that the authorization, declaration, payment or setting apart for payment of those dividends would constitute a breach of or a majoritydefault under any such agreement, or if such action is restricted or prohibited by law.
Notwithstanding the foregoing, dividends on the Series A Preferred Stock will accumulate whether or not restrictions exist in respect thereof, whether or not we have earnings, whether or not there are funds legally available for the payment of such dividends and whether or not Digirad declares such dividends. Accumulated but unpaid dividends on the Series A Preferred Stock will not bear interest, and holders of the Series A Preferred Stock will not be entitled to any distributions in excess of full cumulative dividends described above. Except as stated in the two paragraphs below, no dividends will be declared and paid or set apart for payment on any common stock or any series or class of equity securities ranking junior to the Series A Preferred Stock (other than a dividend in shares of common stock might believe to be in their best interests or in shares of any other class of stock ranking junior to the Series A Preferred Stock as to dividends and upon liquidation) for any period unless full cumulative dividends have been or contemporaneously are declared and paid (or declared and a sum sufficient for the payment of those dividends is set apart for such payment) on the Series A Preferred Stock for all past dividend periods.
If Digirad does not declare and either pay or set apart for payment the full cumulative dividends on the Series A Preferred Stock and all shares of capital stock that are equal in rank with Series A Preferred Stock, the amount which holdersDigirad has declared will be allocated ratably to the Series A Preferred Stock and to each series of shares of capital stock equal in rank so that the amount declared for each share of Series A Preferred Stock and for each share of each series of capital stock equal in rank is proportionate to the accrued and unpaid dividends on those shares.
Except as provided in the immediately preceding paragraph, unless full cumulative dividends on the Series A Preferred Stock have been or contemporaneously are declared and paid (or declared and a sum sufficient for the payment is set apart for payment) for all past dividend periods, no dividends (other than in shares of common stock might receiveor other shares of capital stock ranking junior to the Series A Preferred Stock as to dividends and upon liquidation) shall be declared and paid or declared and set apart for payment nor shall any other distribution be declared and made upon Digirad common stock, or any of Digirad’s other capital stock ranking junior to or equal with the Series A Preferred Stock as to dividends or upon liquidation, nor shall Digirad redeem, purchase, or otherwise acquire for any consideration (or pay or make any monies available for a premiumsinking fund for their shares over the then market price.
We have no present plans to issueredemption of any such shares) any shares of ourcommon stock, or any other shares of Digirad capital stock ranking junior to or equal with the Series A Preferred Stock as to dividends or upon liquidation.
Holders of shares of the Series A Preferred Stock are not entitled to any distribution, whether payable in cash, property or shares of capital stock, in excess of full cumulative dividends on the Series A Preferred Stock as described above. Any dividend payment made on the Series A Preferred Stock will first be credited against the earliest accumulated but unpaid dividends on the Series A Preferred Stock will accumulate as of the dividend payment date on which they first become payable.
Redemption
No Mandatory Redemption
The Series A Preferred Stock is perpetual preferred stock, after completionand Digirad is not required to provide for the mandatory redemption of this offering.the Series A Preferred Stock at any time, other than upon a Change of Control Triggering Event as described below.
92
WarrantsOptional Redemption
As of February 29, 2004, there were warrants outstanding
The Series A Preferred Stock will not be redeemable prior to purchase the followingSeptember 10, 2024. On and after September 10, 2024, at Digirad’s sole option upon not less than 30 nor more than 60 days’ written notice, Digirad may redeem shares of our capital stock:
| Description | Number of Shares Before This Offering | Weighted Average Exercise Price Before This Offering | Number of Shares After This Offering | Weighted Average Exercise Price After This Offering | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Series E Preferred Stock | 1,939 | $ | 607.20 | 1,690 | $ | 607.20 | ||||
| Common Stock | 203,457 | $ | 4.58 | 203,457 | $ | 4.58 | ||||
Warrantsthe Series A Preferred Stock, in whole or in part, at any time or from time to purchase 1,477time, for cash at a redemption price of $10.00 per share, plus an amount equal to all accumulated and unpaid dividends thereon to, but excluding, the date fixed for redemption, without interest. Holders of Series A Preferred Stock to be redeemed must then surrender such Series A Preferred Stock at the place designated in the notice. Upon surrender of the Series A Preferred Stock, the holders will be entitled to the redemption price thereon to, but excluding the date fixed for redemption, without interest. If notice of redemption of any shares of our Series E preferred stock will terminate five yearsA Preferred Stock has been given and if Digirad has deposited the funds necessary for such redemption with the paying agent for the benefit of the holders of any of the shares of Series A Preferred Stock to be redeemed, then from and after the date of this offering. Warrantssuch deposit dividends will cease to purchase 249accumulate on those shares of our Series E preferred stockA Preferred Stock, those shares of Series A Preferred Stock will no longer be deemed outstanding and all rights of the holders of such shares will terminate, upon completionexcept the right to receive the redemption price. If less than all of this offeringthe outstanding Series A Preferred Stock is to be redeemed, the Series A Preferred Stock to be redeemed shall be selected ratably by lot or by any other fair and warrants to purchase 213equitable method that Digirad’s board of directors may choose.
Unless full cumulative dividends for all applicable past dividend periods on all shares of our Series E preferred stock will terminate on July 31, 2006. Warrants to purchase 3,457A Preferred Stock and any shares of our common stock will terminatethat rank on certain dates from November 14, 2005 through July 31, 2008. Additionally, warrantsparity with regards to purchase 200,000dividends and upon liquidation have been or contemporaneously are declared and paid (or declared and a sum sufficient for payment set apart for payment for all past dividend periods), no shares of our commonSeries A Preferred Stock will be redeemed. In such event, Digirad also will not purchase or otherwise acquire directly or indirectly any shares of Series A Preferred Stock (except by exchange for Digirad capital stock ranking junior to the Series A Preferred Stock as to dividends and upon liquidation). However, the foregoing shall not prevent Digirad from purchasing shares pursuant to its restated certificate of incorporation or from acquiring shares of Series A Preferred Stock pursuant to a purchase or exchange offer made on the same terms to holders of all outstanding shares of Series A Preferred Stock and any shares of stock that rank on parity with regards to dividends and upon liquidation. So long as no dividends are in arrears, Digirad will terminate on November 13, 2007.be entitled at any time and from time to time to repurchase shares of Series A Preferred Stock in open-market transactions duly authorized by the Digirad board of directors and effected in compliance with applicable laws.
Digirad will deliver a notice of redemption, by overnight delivery, by first class mail, postage prepaid or electronically to holders thereof, or request Digirad’s agent, on behalf of Digirad, to promptly do so by overnight delivery, by first class mail, postage prepaid or electronically. The notice will be provided not less than 30 nor more than 60 days prior to the date fixed for redemption in such notice. Each such notice will state: (A) the date for redemption; (B) the number of these warrants hasSeries A Preferred Stock to be redeemed; (C) the CUSIP number for the Series A Preferred Stock; (D) the applicable redemption price on a net exercise provision under which its holder may, in lieu ofper share basis; (E) if applicable, the place or places where the certificate(s) for such shares are to be surrendered for payment of the exercise price in cash, surrender the warrant and receive a net amount of shares basedfor redemption; (F) that dividends on the fair market valueSeries A Preferred Stock to be redeemed will cease to accumulate from and after such date of redemption; and (G) the applicable provisions of Digirad’s charter under which such redemption is made. If fewer than all shares held by any holder are to be redeemed, the notice delivered to such holder will also specify the number of Series A Preferred Stock to be redeemed from such holder or the method of determining such number. Digirad may provide in any such notice that such redemption is subject to one or more conditions precedent and that Digirad will not be required to affect such redemption unless each such condition has been satisfied at the time or times and in the manner specified in such notice. No defect in the notice or delivery thereof shall affect the validity of redemption proceedings, except as required by applicable law.
If a redemption date falls after a record date and prior to the corresponding dividend payment date, however, each holder of Series A Preferred Stock at the close of business on that record date shall be entitled to the dividend payable on such shares on the corresponding dividend payment date notwithstanding the redemption of such shares before the dividend payment date.
Change of Control
If a Change of Control Triggering Event occurs with respect to the Series A Preferred Stock, unless Digirad has exercised its option to redeem such Series A Preferred Stock as described above, holders of the underlying securityDigirad Preferred Stock will have the right to require Digirad to redeem (a “Change of Control Redemption”) the Series A Preferred Stock at a price equal to the liquidation preference of $10.00 per share, plus an amount equal to any accumulated and unpaid dividends up to but excluding the date of payment, but without interest (a “Change of Control Payment”). Within 30 days following any Change of Control Triggering Event or, at Digirad’s option, prior to any Change of Control Triggering Event, but after public announcement of the transaction that constitutes or may constitute the Change of Control Triggering Event, Digirad will mail a notice to holders of Series A Preferred Stock, describing the transaction that constitutes or may constitute the Change of Control Triggering Event and offering to redeem such Series A Preferred Stock on the date specified in the applicable notice, which date will be no earlier than 30 days and no later than 60 days from the date such notice is mailed (a “Change of Control Payment Date”). The notice will, if mailed prior to the date of consummation of the Change of Control Triggering Event, state that the Change of Control Redemption is conditioned on the Change of Control Triggering Event occurring on or prior to the applicable Change of Control Payment Date.
On each Change of Control Payment Date, Digirad will, to the extent lawful:
| ● | redeem all Series A Preferred Stock or portions of Series A Preferred Stock properly tendered pursuant to the applicable Change of Control Redemption; |
| ● | deposit with the paying agent an amount equal to the Change of Control Payment in respect of all Digirad Preferred Stock properly tendered; and |
| ● | deliver or cause to be delivered to the paying agent the Series A Preferred Stock properly accepted together with an officers’ certificate stating the Series A Preferred Stock being redeemed. |
Digirad will not be required to make a Change of Control Redemption upon the occurrence of a Change of Control Triggering Event if a third party makes such an offer in the manner, at the times and otherwise in compliance with the requirements for an offer made by Digirad and the third party redeems all Series A Preferred Stock properly tendered and not withdrawn under its offer.
Digirad will comply with the requirements of Rule 14e-1 under the Exchange Act, and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with the redemption of the Series A Preferred Stock as a result of a Change of Control Triggering Event. To the extent that the provisions of any such securities laws or regulations conflict with the Change of Control Redemption provisions of the Series A Preferred Stock, Digirad will comply with those securities laws and regulations and will not be deemed to have breached its obligations under the Change of Control Redemption provisions of the Series A Preferred Stock by virtue of any such conflict.
For purposes of the foregoing discussion of the redemption of the Series A Preferred Stock at the option of the holders, the following definitions are applicable.
“Capital Stock” of a corporation means the capital stock of every class whether now or hereafter authorized, regardless of whether such capital stock shall be limited to a fixed sum or percentage with respect to the rights of the holders thereof to participate in dividends and in the distribution of assets upon the voluntary or involuntary liquidation, dissolution or winding up of such corporation.
“Change of Control Triggering Event” means the occurrence of any of the following: (1) the direct or indirect sale, lease, transfer, conveyance or other disposition (other than by way of merger or consolidation), in one or more series of related transactions, of all or substantially all of Digirad’s assets and the assets of its subsidiaries, taken as a whole, to any Person, other than Digirad or one of its subsidiaries; (2) the consummation of any transaction (including, without limitation, any merger or consolidation) the result of which is that any Person becomes the beneficial owner (as defined in Rules 13d-3 and 13d-5 under the Exchange Act), directly or indirectly, of more than 50% of Digirad’s outstanding Voting Stock or other Voting Stock into which Digirad’s Voting Stock is reclassified, consolidated, exchanged or changed, measured by voting power rather than number of shares; (3) Digirad consolidates with, or merges with or into, any Person, or any Person consolidates with, or merges with or into, Digirad, in any such event pursuant to a transaction in which any of Digirad’s outstanding Voting Stock or the Voting Stock of such other Person is converted into or exchanged for cash, securities or other property, other than any such transaction where the shares of Digirad Voting Stock outstanding immediately prior to such transaction constitute, or are converted into or exchanged for, a majority of the Voting Stock of the surviving Person or any direct or indirect parent company of the surviving Person immediately after giving effect to such transaction; (4) the first day on which a majority of the members of our board of directors are not Continuing Directors; or (5) the adoption of a plan relating to Digirad’s liquidation or dissolution. Notwithstanding the foregoing, a transaction will not be deemed to involve a Change of Control Triggering Event under clause (2) above if (i) Digirad becomes a direct or indirect wholly-owned subsidiary of a holding company and (ii)(A) the direct or indirect holders of the Voting Stock of such holding company immediately following that transaction are substantially the same as the holders of Digirad’s Voting Stock immediately prior to that transaction or (B) immediately following that transaction no Person (other than a holding company satisfying the requirements of this sentence) is the beneficial owner, directly or indirectly, of more than 50% of the Voting Stock of such holding company.
“Continuing Directors” means, as of any date of determination, any member of Digirad’s board of directors who (A) was a member of such board of directors on the date the Series A Preferred Stock was issued or (B) was nominated for election, elected or appointed to such board of directors with the approval of a majority of the continuing directors who were members of such board of directors at the time of exercisesuch nomination, election or appointment (either by a specific vote or by approval of a proxy statement in which such member was named as a nominee for election as a director, without objection to such nomination).
“Person” has the meaning given thereto in Section 13(d)(3) of the warrant after deductionExchange Act.
“Voting Stock” means, with respect to any specified Person that is a corporation as of any date, the Capital Stock of such Person that is at the time entitled to vote generally in the election of the aggregate exercise price. Eachboard of directors of such Person.
Liquidation Preference
In the event of Digirad’s voluntary or involuntary liquidation, dissolution or winding up, the holders of shares of Series A Preferred Stock will be entitled to be paid, out of our assets legally available for distribution to Digirad stockholders, a liquidation preference of $10.00 per share, plus an amount equal to any accumulated and unpaid dividends to, but excluding, the date of payment, but without interest, before any distribution of assets is made to holders of Digirad common stock or any other class or series of Digirad capital stock that ranks junior to the Series A Preferred Stock as to liquidation rights. If Digirad’s assets legally available for distribution to stockholders are insufficient to pay in full the liquidation preference on the Series A Preferred Stock and the liquidation preference on any shares of preferred stock equal in rank with the Series A Preferred Stock, all assets distributed to the holders of the Series A Preferred Stock and any other series of preferred stock equal in rank with the Series A Preferred Stock will be distributed ratably so that the amount of assets distributed per share of Series A Preferred Stock and such other series of preferred stock equal in rank with the Series A Preferred Stock shall in all cases bear to each other the same ratio that the liquidation preference per share on the Series A Preferred Stock and on such other series of preferred stock bear to each other. Written notice of any such liquidation, dissolution or winding up of Digirad, stating the payment date or dates when, and the place or places where, the amounts distributable in such circumstances shall be payable, shall be given by first class mail, postage pre-paid, not less than 30 nor more than 60 days prior to the payment date stated therein, to each record holder of the Series A Preferred Stock at the respective addresses of such holders as the same shall appear on the stock transfer records of Digirad. After payment of the full amount of the liquidation preference, plus any accumulated and unpaid dividends to which they are entitled, the holders of Series A Preferred Stock will have no right or claim to any of Digirad’s remaining assets. If Digirad converts into or consolidates or merges with or into any other corporation, trust or entity, effect a statutory share exchange or sell, lease, transfer or convey all or substantially all of its property or business, Digirad will not be deemed to have liquidated, dissolved or wound up.
Ranking
The Series A Preferred Stock will rank, with respect to rights to the payment of dividends and the distribution of assets upon Digirad’s liquidation, dissolution or winding up:
| ● | senior to all classes or series of Digirad common stock and to all other equity securities issued by Digirad other than equity securities referred to in the next two bullet points below; |
| ● | on parity with all equity securities issued by Digirad with terms specifically providing that those equity securities rank on a parity with the Series A Preferred Stock with respect to rights to the payment of dividends and the distribution of assets upon Digirad’s liquidation, dissolution or winding up; |
| ● | junior to all equity securities issued by Digirad with terms specifically providing for ranking senior to the Series A Preferred Stock with respect to rights to the payment of dividends and the distribution of assets upon Digirad’s liquidation, dissolution or winding up (please see the section entitled “Voting Rights” below); and |
| ● | effectively junior to all of Digirad’s existing and future indebtedness (including indebtedness convertible to Digirad common stock or preferred stock) and to any indebtedness and other liabilities of (as well as any preferred equity interests held by others in) Digirad’s existing subsidiaries. |
Voting Rights
Holders of the Series A Preferred Stock will not have any voting rights, except as described below or otherwise required by law.
Whenever dividends on any shares of Series A Preferred Stock are in arrears for six or more consecutive quarters, then the holders of those shares together with the holders of all other series of preferred stock equal in rank with the Series A Preferred Stock upon which like voting rights have been conferred and are exercisable, will be entitled to vote separately as a class for the election of a total of two additional directors to Digirad’s board of directors.
The election of these warrants also contains provisionsdirectors will take place at a special meeting called upon the written request of the holders of record of at least 20% of the Series A Preferred Stock and the holders of record of at least 20% of any class or series of preferred stock equal in rank with the Series A Preferred Stock which like voting rights have been conferred and are exercisable (unless such request is received less than 90 days before the date fixed for the adjustmentnext annual or special meeting of Digirad stockholders in which case, such vote will be held at the earlier of the next annual or special meeting of Digirad stockholders) or at the next annual meeting of Digirad stockholders, and at each subsequent annual or special meeting until all dividends accumulated from past dividend periods and the then current dividend period have been paid (or declared and a sum sufficient for payment set apart). A quorum for any such meeting will exist if at least a majority of the total outstanding shares of Series A Preferred Stock and shares of preferred stock equal in rank with the Series A Preferred Stock entitled to like voting rights are represented in person or by proxy at that meeting. The directors elected as described above shall be elected upon the affirmative vote of a plurality of the votes cast by the holders of shares of Series A Preferred Stock and preferred stock equal in rank with the Series A Preferred Stock voting separately as a single class, present and voting in person or by proxy at a duly called and held meeting at which a quorum is present. If and when all accumulated dividends and the dividend for the then current dividend period on the Series A Preferred Stock have been paid in full or declared or set apart for payment in full the holders of the Series A Preferred Stock shall be divested of the right to elect directors and, if all dividend arrearages have been paid in full or declared and set apart for payment in full on all series of preferred stock entitled to like voting rights, the term of office of each director so elected shall terminate. Any director so elected may be removed at any time with or without cause by, and shall not be removed otherwise than by the vote of, the holders of record of a majority of the outstanding shares of the Series A Preferred Stock having the voting rights described above, voting separately as a single class with all classes or series of preferred stock entitled to like voting rights. So long as a dividend arrearage continues, any vacancy in the office of a director elected as described above may be filled by written consent of the director elected as described above who remains in office, or if none remains in office, by a vote of the holders of record of a majority of the outstanding shares of Series A Preferred Stock when they have the voting rights described above, voting separately as a single class with all classes or series of preferred stock entitled to like voting rights. These directors shall each be entitled to one vote per director on any matter.
If a special meeting is not called by Digirad within 30 days after request from the holders of Series A Preferred Stock, then the holders of record of at least 20% of the outstanding Digirad Preferred Stock may designate a holder to call the meeting at the expense of Digirad and such meeting may be called by the holder so designated upon notice similar to that required for annual meetings of stockholders and shall be held at the place designated by the holder calling such meeting. Digirad shall pay all costs and expenses of calling and holding any meeting and of electing directors as described above, including, without limitation, the cost of preparing, reproducing and mailing the notice of such meeting, the cost of renting a room for such meeting to be held, and the cost of collecting and tabulating votes.
So long as any shares of Series A Preferred Stock remain outstanding, Digirad will not, without the affirmative vote or consent of the holders of at least a majority of the shares of the Series A Preferred Stock outstanding at the time, given in person or by proxy, either in writing or at a meeting (voting separately as a class), amend, alter or repeal the provisions of Digirad’s charter, including the articles supplementary designating the Series A Preferred Stock, whether by merger, consolidation or otherwise, so as to materially and adversely affect any right, preference, privilege or voting power of the Series A Preferred Stock. However, with respect to the occurrence of any event listed above, so long as the Series A Preferred Stock remains outstanding (or shares issued by a surviving entity in substitution for the Series A Preferred Stock) with its terms materially unchanged, taking into account that upon the occurrence of such an event, Digirad may not be the surviving entity, the occurrence of any such event shall not be deemed to materially and adversely affect such rights, preferences, privileges or voting power of holders of the Series A Preferred Stock. In addition (i) any increase in the number of authorized shares of Series A Preferred Stock, (ii) any increase in the number of authorized preferred stock or the creation or issuance of any other class or series of preferred stock, or (iii) any increase in the number of authorized shares of such class or series, in each case ranking equal with or junior to the Series A Preferred Stock with respect to payment of dividends or the distribution of assets upon liquidation, dissolution or winding up, will not be deemed to materially and adversely affect such rights, preferences, privileges or voting powers.
The foregoing voting provisions will not apply if, at or prior to the time when the act with respect to which such vote would otherwise be required shall be effected, all outstanding shares of Series A Preferred Stock shall have been redeemed or called for redemption upon proper notice and sufficient funds shall have been deposited in trust to effect such redemption.
Conversion
The Series A Preferred Stock is not convertible into or exchangeable for any of Digirad’s other property or securities.
Book-Entry Procedures
The Series A Preferred Stock was initially issued in the form of global securities held in book-entry form. DTC or its nominee is the sole registered holder of the Series A Preferred Stock. Owners of beneficial interests in the Series A Preferred Stock represented by the global securities will hold their interests pursuant to the procedures and practices of DTC. As a result, beneficial interests in any such securities will be shown on, and transfers will be effected only through, records maintained by DTC and its direct and indirect participants and any such interest may not be exchanged for certificated securities, except in limited circumstances. Owners of beneficial interests must exercise any rights in respect of other interests, including any right to convert or require repurchase of their interests in the Series A Preferred Stock, in accordance with the procedures and practices of DTC. Beneficial owners are not holders and will not be entitled to any rights provided to the holders of the Series A Preferred Stock under the global securities or the articles supplementary. Digirad and any of Digirad’s agents may treat DTC as the sole holder and registered owner of the global securities.
DTC has advised Digirad as follows: DTC is a limited-purpose trust company organized under the New York Banking Law, a “banking organization” within the meaning of the New York Uniformed Commercial Code, and a “clearing agency” registered pursuant to the provisions of Section 17A of the Exchange Act. DTC facilitates the settlement of transactions amongst participants through electronic computerized book-entry changes in participants’ accounts, eliminating the need for physical movement of securities certificates. DTC’s participants include securities brokers and dealers, including the underwriters, banks, trust companies, clearing corporations and other organizations, some of whom and/or their representatives own DTC. Access to DTC’s book-entry system is also available to others, such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly.
The Series A Preferred Stock, represented by one or more global securities, is exchangeable for certificated securities with the same terms only if:
| ● | DTC is unwilling or unable to continue as depositary or if DTC ceases to be a clearing agency registered under the Exchange Act and a successor depositary is not appointed by Digirad within 90 days; or |
| ● | Digirad decides to discontinue use of the system of book-entry transfer through DTC (or any successor depositary). |
Information Rights
During any period in which Digirad is not subject to Section 13 or 15(d) of the Exchange Act and any shares of Digirad Preferred Stock are outstanding, Digirad will use its best efforts to (i) make available on its corporate investor webpage, copies of the Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q that Digirad would have been required to file with the SEC pursuant to Section 13 or 15(d) of the Exchange Act if Digirad were subject thereto (other than any exhibits that would have been required) and (ii) promptly, upon request, supply copies of such reports to any holders of Series A Preferred Stock. Digirad will use its best effort to provide the information to the holders of the Series A Preferred Stock within 15 days after the respective dates by which a periodic report on Form 10-K or Form 10-Q, as the case may be, in respect of such information would have been required to be filed with the SEC, if Digirad were subject to Section 13 or 15(d) of the Exchange Act, in each case, based on the dates on which Digirad would be required to file such periodic reports if Digirad were a “non-accelerated filer” within the meaning of the Exchange Act.
No Preemptive Rights
No holders of the Series A Preferred Stock, as holders of Series A Preferred Stock, have any preemptive rights to purchase or subscribe for Digirad common stock or any other security.
Global Clearance and Settlement Procedures
Initial settlement for the Series A Preferred Stock was made in immediately available funds. Secondary market trading among DTC’s participants occurs in the ordinary way in accordance with DTC’s rules and will be settled in immediately available funds using DTC’s Same-Day Funds Settlement System.
No Credit Rating of the Series A Preferred Stock
The Series A Preferred Stock has not been rated by any rating agency and Digirad does not intend to have the Series A Preferred Stock rated by any rating agency.
Pre-Funded Warrants
The following description of our pre-funded warrants we are offering is a summary and is qualified in its entirety by reference to the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Duration and Exercise Price
Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.01. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and the aggregate number of shares of common stock issuable upon the exercise of the warrantis subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and reclassifications and consolidations.the exercise price. The pre-funded warrants will be issued in certificate form.
We have granted registration rights pursuant
Exercisability
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly-executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the pre-funded warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the termsissuance of the pre-funded warrants at closing to have their pre-funded warrants exercised immediately upon issuance and receive shares of common stock underlying the pre-funded warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% of the outstanding shares of common stock immediately after exercise. However, any exercise of the pre-funded warrants which would result in a holder beneficially owning more than 4.99% of our amended and restated investors' rights agreement, as discussed more fully below, to a holder of warrants to purchase an aggregate of 213outstanding shares of common stock will be subject to our Series E preferred stock.consent, provided that any beneficial ownership in excess of the 4.99% threshold will not take effect until 61 days following notice to, and approval by, us. We may, in our sole discretion, waive the 4.99% ownership limitation in connection with this offering with respect to one or more potential purchasers. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will round up to the nearest whole share.
Registration Rights
Under an amended and restated investors' rights agreement,
Cashless Exercise
If at the time of exercise there is no effective registration statement registering the shares of common stock underlying the pre-funded warrants, or the prospectus contained therein is not available for the issuance of such shares then the holders of the pre-funded warrants may exercise the pre-funded warrants, in whole or in part, by means of a majority“cashless exercise” in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise and receive the net number of the shares of common stock determined according to a formula set forth in the pre-funded warrants.
Transferability
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
Exchange Listing
There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
Right as a Shareholder
Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of our shares of common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their pre-funded warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, then the successor entity will succeed to, and be substituted for us, and may exercise every right and power that we may exercise and will assume all of our obligations under the pre-funded warrants with the same effect as if such successor entity had been named in the pre-funded warrant itself. If holders of our common stock are given a choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration it receives upon any exercise of the pre-funded warrant following such fundamental transaction.
Warrants
The following summary of certain terms and provisions of the warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant agent agreement between us and American Stock Transfer & Trust Company, as warrant agent, and the form of warrant, both of which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the warrant agent agreement, including the annexes thereto, and form of warrant.
Form
Pursuant to a warrant agent agreement between us and American Stock Transfer & Trust Company, as warrant agent, the warrants will be issued in book-entry form and shall initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Exercisability
The warrants are exercisable at any time after their original issuance, expected to be [●], 2020, and at any time up to the date that is five years after their original issuance. The warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise. No fractional shares of common stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will round up to the nearest whole share.
Exercise Limitation
A holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% of the number of shares of our common stock issued uponoutstanding immediately after giving effect to the conversionexercise, as such percentage ownership is determined in accordance with the terms of the warrants. However, any exercise of the warrants which would result in a holder beneficially owning more than 4.99% of our Series A preferredoutstanding shares of common stock Series B preferredwill be subject to our consent, provided that any beneficial ownership in excess of the 4.99% threshold will not take effect until 61 days following notice to, and approval by, us. We may, in our sole discretion, waive the 4.99% ownership limitation in connection with this offering with respect to one or more potential purchasers.
Exercise Price
The warrants will have an exercise price of $[●] per share, which will be at least 100%, and up to 125%, of the public offering price per share of our common stock Series C preferredin this offering. The exercise price is subject to appropriate adjustment in the event of certain stock Series D preferreddividends and distributions, stock Series E preferredsplits, stock Series F preferred stock, Series G preferredcombinations, reclassifications or similar events affecting our common stock and Series H preferredalso upon any distributions of assets, including cash, stock haveor other property to our stockholders.
Cashless Exercise
If at the time of exercise there is no effective registration statement registering the shares of common stock underlying the warrants, or the prospectus contained therein is not available for the issuance of such shares then the holders of the warrants may exercise the warrants, in whole or in part, by means of a “cashless exercise” in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise and receive the net number of the shares of common stock determined according to a formula set forth in the warrants.
Transferability
Subject to applicable laws, the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
There is no established trading market for the warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the warrants will be limited.
Fundamental Transactions
If a fundamental transaction occurs, then the successor entity will succeed to, and be substituted for us, and may exercise every right to require us to register their sharesand power that we may exercise and will assume all of our obligations under the warrants with the Securities and Exchange Commission followingsame effect as if such successor entity had been named in the completion of this offering, so that those shares may be publicly resold, or to include their shares in any registration statement we file as follows:
Demand Registration Rights
At any time beginning one year after the completion of this offering,warrant itself. If holders of at least 25%our common stock are given a choice as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration it receives upon any exercise of the warrant following such fundamental transaction.
Rights as a Stockholder
Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, issued upon the conversionholder of a warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the warrant.
59
Digirad’s Transfer Agent
The transfer agent for the Series A preferredPreferred Stock and common stock Series B preferred stock, Series C preferred stock, Series D preferred stock, Series E preferred stock, Series F preferred stock, Series G preferred stockis American Stock Transfer & Trust Company. Its address is 6201 15th Avenue, Brooklyn, NY 11219, and Series H preferred stock haveits telephone number is (718) 921-8200.
Dividend Paying Agent
American Stock Transfer & Trust Company will act as the right to demand that we file up to two registration statements, so long as at least 20% of their registrable securities will be registered and/or the proposed aggregate offering pricedividend payment agent in respect of the securities registered, net of underwriting discounts and commissions, is at least $25,000,000, subject to specified exceptions.Series A Preferred Stock.
Form S-3 Registration Rights
If we are eligible to file a "short-form" registration statement on Securities and Exchange Commission Form S-3, stockholders with registration rights have the right to demand that we file a registration statement on Form S-3 so long as the aggregate offering price of the securities to be sold under the
93
registration statement on Form S-3, net of underwriting discounts and commissions, is at least $1,000,000, subject to specified exceptions.
"Piggyback" Registration Rights
If we register any securities for public sale solely for cash, stockholders with registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of such shares to be included in the registration statement. In this offering the underwriters have excluded any sales by existing investors.
Expenses of Registration
Other than underwriting discounts and commissions, we will pay all expenses relating to piggyback registrations and all expenses relating to demand registrations and Form S-3 registrations so long as the aggregate amount of securities to be sold under each such registration statement exceeds the threshold amounts discussed above. However, we will not pay for the expenses of any demand or Form S-3 registration if the request is subsequently withdrawn by the stockholders initiating these registration rights, subject to specified exceptions.
Expiration of Registration Rights
The registration rights described above will expire seven years after this offering is completed. The registration rights will terminate earlier for a particular stockholder at such time as that holder, following completion of this offering, can resell all of its securities in a 90-day period under Rule 144 of the Securities Act.
Anti-takeover Effects of Delaware Law andCertain Anti-Takeover Provisions of OurDigirad’s Certificate of Incorporation and BylawsBy-Laws
Delaware Takeover Statute
We are
Digirad is subject to Section 203 of the Delaware General Corporation Law. This statute regulating corporate takeovers prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for three years following the date that the stockholder became an interested stockholder, unless:
| ● | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| ● | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| ● | on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
94
| ● | any merger or consolidation involving the corporation and the interested stockholder; |
| ● | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| ● | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; or |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Certificate of Incorporation and Bylaw Provisions
Provisions of ourDigirad’s restated certificate of incorporation and amended and restated bylaws which will become effective upon the closing of this offering, may have the effect of making it more difficult for a third party to acquire, or discourage a third party from attempting to acquire, control of our companyDigirad by means of a tender offer, a proxy contest or otherwise. These provisions may also make the removal of incumbent officers and directors more difficult. These provisions are intended to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our companyDigirad to first negotiate with us.Digirad. These provisions could also limit the price that investors might be willing to pay in the future for shares of ourDigirad common stock. These provisions may make it more difficult for Digirad stockholders to take specific corporate actions and could have the effect of delaying or preventing a change in our control.control of Digirad. The amendment of any of these anti-takeover provisions would require approval by holders of at least 662/3%two-thirds of ourDigirad’s outstanding common stock entitled to vote.
In particular, ourDigirad’s restated certificate of incorporation and restated bylaws provide for the following:
No Written Consent of Stockholders
Any action to be taken by ourDigirad stockholders must be effectedtaken and given effect at a duly called annual or special meeting and may not be effectedtaken or given effect by written consent.
Special Meetings of Stockholders
Special meetings of ourDigirad stockholders may be called only by the president, chief executive officer, chairman of the Digirad board of directors, or a majority of the members of the Digirad board of directors.directors or Digirad stockholders holding not less than 20% of the total number of votes to be cast at such a meeting.
Advance Notice Requirement
Stockholder
Digirad stockholder proposals to be brought before an annual meeting of ourDigirad stockholders must comply with advance notice procedures. These advance notice procedures require timely notice and apply in several situations, including stockholder proposals relating to the nominations of persons for election to the board of directors. Generally, to be timely, notice must be received at ourDigirad’s principal executive offices not less than 90 days or more than 120 days prior to the first anniversary date of the annual meeting for the preceding year.
Amendment of Bylaws and Certificate of Incorporation
The approval of not less that 662/3%than two-thirds of the outstanding shares of ourDigirad capital stock entitled to vote is required to amend the provisions of ourDigirad’s amended and restated bylaws by stockholder action, or to amend the provisions of ourDigirad’s restated certificate of incorporation that are described in this section or that are described under "Management—Limitation of Liabilitycertain other terms as specified in Digirad’s amended and Indemnification of Officers and Directors" above.restated bylaws. These
95
provisions will make it more difficult to circumvent the anti-takeover provisions of ourDigirad’s restated certificate of incorporation and ourDigirad’s restated bylaws.
Issuance of Undesignated Preferred Stock
Our
Digirad’s board of directors is authorized to issue, without further action by the stockholders, up to 10,000,000a further 1,500,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by the Digirad board of directors. The existence of authorized but unissued shares of preferred stock enables ourDigirad’s board of directors to render more difficult or to discourage an attempt to obtain control of usDigirad by means of a merger, tender offer, proxy contest or otherwise.
Transfer Agent and RegistrarProtective Amendment
Digirad’s restated certificate of incorporation contains a protective provision (the “Protective Amendment”) to protect Digirad’s significant net operating losses (“NOLs”). The Protective Amendment was approved by Digirad’s stockholders at Digirad’s 2015 Annual Meeting of Stockholders held on May 1, 2015. The Protective Amendment is designed to assist Digirad in protecting the long-term value of its accumulated NOLs by limiting certain transfers of Digirad’s common stock. The Protective Amendment’s transfer agent and registrar for ourrestrictions generally restrict any direct or indirect transfers of the common stock is .if the effect would be to increase the direct or indirect ownership of the common stock by any person from less than 4.99% to 4.99% or more of the common stock, or increase the percentage of the common stock owned directly or indirectly by a person owning or deemed to own 4.99% or more of the common stock. Any direct or indirect transfer attempted in violation of the Protective Amendment will be void as of the date of the prohibited transfer as to the purported transferee. The Protective Amendment also requires any person attempting to become a holder of 4.99% or more of Digirad common stock to seek the approval of Digirad’s board of directors. This may have an unintended “anti-takeover” effect because Digirad’s board of directors may be able to prevent any future takeover. Similarly, any limits on the amount of Digirad common stock that a stockholder may own could have the effect of making it more difficult for Digirad stockholders to replace Digirad’s management. Additionally, because the Protective Amendment may have the effect of restricting a Digirad stockholder’s ability to dispose of or acquire Digirad common stock, the liquidity and market value of Digirad’s common stock might suffer.
On April 27, 2018, Digirad filed a Certificate of Amendment to its restated certificate of incorporation with the Secretary of State of the State of Delaware, which was approved by Digirad’s stockholders at its 2018 Annual Meeting. The Extended Protective Amendment effects a three-year extension to the provisions of the Protective Amendment. The Extended Protective Amendment leaves the Protective Amendment unchanged in all respects, other than to extend the expiration date from May 1, 2018 to May 1, 2021, and to make revisions necessary as a result of the enactment of Public Law 115-97 (commonly referred to as the Tax Cut and Jobs Act) on December 22, 2017.
Nasdaq National Market ListingRepurchases
We have applied for approval for
Digirad has begun to repurchase shares of its outstanding common stock from time to time in market or private transactions. On October 31, 2018, Digirad’s board of directors approved a stock repurchase program that enables Digirad to repurchase up to 200,000 shares of its common stock. Digirad believes that the program will help offset the dilutive impact of employee stock option exercises, maximize the value of Digirad common stock, and that the program reflects Digirad’s belief in its strategy and operations and its commitment to its stockholders.
Under the stock repurchase program, Digirad may purchase shares of its common stock through various means, including open market transactions in compliance with Rule 10b-18 under the Exchange Act, privately negotiated transactions, tender offers or any combination thereof. The number of shares repurchased and the timing of repurchases will depend on a number of factors, including, but not limited to, stock price, trading volume and quotationgeneral market conditions, along with Digirad’s working capital requirements, general business conditions and other factors. The stock repurchase program has no time limit and may be modified, suspended or terminated at any time by the Digirad board of directors. Repurchases under the stock repurchase program will be funded from Digirad’s existing cash and cash equivalents or future cash flow and equity or debt financings. As of the date of this prospectus, Digirad has repurchased zero shares of its common stock under the repurchase program approved in 2018.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO HOLDERS OF COMMON STOCK, PRE-FUNDED WARRANTS AND WARRANTS
The following is a summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our common stock and pre-funded warrants, and the acquisition, ownership, exercise, expiration or disposition of the warrants (together with our common stock and pre-funded warrants, the “Securities”). This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed or subject to differing interpretations, possibly with retroactive effect, so as to result in U.S. federal income tax consequences different from those set forth below. We have not sought and will not seek any ruling from the Internal Revenue Service (the “IRS”), with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS or a court will agree with such statements and conclusions.
This summary does not purport to be a complete analysis of all the potential tax considerations relating to the Securities. In addition, this summary does not address the tax considerations arising under the laws of any U.S. state or local or any non-U.S. jurisdiction, estate or gift tax, the 3.8% Medicare tax on net investment income or any alternative minimum tax consequences. In addition, this discussion does not address tax considerations applicable to a holder’s particular circumstances or to a holder that may be subject to special tax rules, including, without limitation:
| ● | banks, insurance companies or other financial institutions; | |
| ● | tax-exempt entities, tax-exempt or government organizations; | |
| ● | brokers or dealers in securities or currencies; | |
| ● | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; | |
| ● | persons that own, or are deemed to own, more than 5% of our capital stock; | |
| ● | certain U.S. expatriates, citizens or former long-term residents of the United States; | |
| ● | U.S. Holders (as defined below) of any Security whose “functional currency” is not the U.S. dollar; | |
| ● | persons who hold any Security as a position in a hedging transaction, “straddle,” “conversion transaction,” synthetic security, other integrated investment or other risk reduction transaction; | |
| ● | persons who do not hold any Security as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes); | |
| ● | persons deemed to sell any Security under the constructive sale provisions of the Code; | |
| ● | pension plans; | |
| ● | foreign or domestic partnerships, or other entities or arrangements treated as partnerships for U.S. federal income tax purposes, or investors in any such entities; | |
| ● | real estate investment trusts or regulated investment companies; | |
| ● | personal holding companies or grantor trusts; | |
| ● | persons for whom our stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; | |
| ● | integral parts or controlled entities of foreign sovereigns; | |
| ● | controlled foreign corporations; | |
| ● | passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; and | |
| ● | persons that acquire any Security as compensation for services. |
In addition, if a partnership, including any entity or arrangement classified as a partnership for U.S. federal income tax purposes, holds any Security, the tax treatment of a partner generally will depend on the Nasdaq National Marketstatus of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships that hold any Security, and partners in such partnerships, should consult their tax advisors regarding the U.S. federal income tax consequences to them of the acquisition, ownership, exercise, expiration and disposition of any Security, as the case may be.
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the acquisition, ownership, exercise, expiration and disposition of any Security, as the case may be, arising under the symbol "DRAD."
SHARES ELIGIBLE FOR FUTURE SALE
U.S. federal estate or gift tax rules or under the laws of any U.S. state or local or any non-U.S. or other taxing jurisdiction or under any applicable tax treaty.
Prior
Personal Holding Company Status
We could be subject to a second level of U.S. federal income tax on a portion of our income if we are determined to be a PHC for U.S. federal income tax purposes. A U.S. corporation generally will be classified as a PHC for U.S. federal income tax purposes in a given taxable year if (1) at any time during the last half of such taxable year, five or fewer individuals (without regard to their citizenship or residency and including as individuals for this purpose certain entities such as certain tax-exempt organizations, pension funds and charitable trusts) own or are deemed to own (pursuant to certain constructive ownership rules) more than 50% of the stock of the corporation by value (by reference to all common, preferred and all other classes of stock) (the “Ownership Test”) and (2) at least 60% of the corporation’s adjusted ordinary gross income, as determined for U.S. federal income tax purposes, for such taxable year consisting of PHC income (which includes, among other things, dividends, interest, certain royalties, annuities and, under certain circumstances, rents) (the “Income Test”).
Based on our knowledge, we believe we meet the Ownership Test discussed above. However, based on our present operations and income sources, we believe that we will not meet the Income Test, and as a result we should not be treated as a PHC for the foreseeable future. If we are or were to become a PHC in a given taxable year, we would be subject to an additional PHC tax, currently 20%, on our undistributed PHC income, which generally includes our taxable income (without the benefit of any federal NOL carry forward), subject to certain adjustments. We can give no assurance that we will not become a PHC in the future.
Definition of a U.S. Holder
As used herein, the term “U.S. Holder” means a beneficial owner of any Security that is, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; | |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
Treatment of Pre-funded Warrants
Although it is not entirely free from doubt, a pre-funded warrant should be treated as a share of our common stock for U.S. federal income tax purposes and a holder of pre-funded warrants should generally be taxed in the same manner as a holder of our common stock, as described below. Accordingly, no gain or loss should be recognized upon the exercise of a pre-funded warrant and, upon exercise, the holding period of a pre-funded warrants should carry over to the share of common stock received. Similarly, the tax basis of the pre-funded warrant should carry over to the share of common stock received upon exercise, increased by the exercise price of $0.01. Each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition of pre-funded warrants pursuant to this offering there has been no public market(including potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected for our common stock. If our stockholders sell substantial amounts ofU.S. federal income tax purposes and, any references to our common stock in the public market followingbalance of this offering,discussion, shall incorporate by reference and include the prevailing market pricepre-funded warrants.
Allocation of Purchase Price
For U.S.federal income tax purposes, a purchase of our common stock could decline. Furthermore, becausetogether with a warrant should be treated as an investment unit. The purchase price for each investment unit must be allocated between the Securities making up the investment unit, in proportion to their relative fair market values at the time of purchase. This allocation of the purchase price for each investment unit will establish your initial tax basis in each Security for U.S. federal income tax purposes. The separation of common stock and the warrant included in each investment unit should not be a taxable event for U.S. federal income tax purposes. You should consult your own tax advisor regarding the allocation of the purchase price for an investment unit.
Tax Consequences to U.S. Holders
Distributions on Common Stock
As discussed above under “Dividend Policy,” we do not currently expect many shares will be available for sale for 180 days after this offering as a resultto make distributions on our common stock. In the event that we do make distributions of certain contractual and legal restrictionscash or other property, distributions paid on resale described below, sales of substantial amounts of our common stock, in the public market after these restrictions lapse could adversely affect the prevailing market price and make it difficult or impossible for us to sell equity or equity-related securities in the future at a time and price we deem appropriate.
Upon completion of this offering, we will have sharesother than certain pro rata distributions of common stock, outstanding, assuming no exercisewill be treated as a dividend to the extent paid out of currently outstanding optionsour current or warrants. Of these shares,accumulated earnings and profits and will be includible in income by the shares sold in this offering, plus any additional shares sold upon exerciseU.S. Holder and taxable as ordinary income when received. If a distribution exceeds our current and accumulated earnings and profits, the excess will be first treated as a tax-free return of the underwriters' over-allotment option,U.S. Holder’s investment, up to the U.S. Holder’s tax basis in the common stock. Any remaining excess will be freely transferable without restriction under the Securities Act, unless they are held by our "affiliates"treated as that term is used under the Securities Act and the rules and regulations promulgated thereunder. The remaining shares of common stock held by existing stockholders are restricted shares. Restricted sharesa capital gain. Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be soldeligible for taxation as “qualified dividend income” and therefore may be taxable at rates applicable to long-term capital gains. U.S. Holders should consult their tax advisers regarding the availability of the reduced tax rate on dividends in the public market only if registered or if they qualify for an exemption from registration under Rules 144 or 701 promulgated under the Securities Act, which rules are summarized below.
Taking into account the lock-up agreements described below and the provisions of Rules 144 and 701, based upon our shares outstanding as of December 31, 2003, additional shares will be available for sale in the public market, subject to certain volume and other restrictions, as follows:
Constructive Dividends on the effective date of this offering;
Under Section 305 of the lock-up agreements, which will occur 180 days after the date of this prospectus; and
Lock-up Agreements
All of our directors and officers and substantially all of our stockholders and optionholders have signed lock-up agreements with respect to shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock for 180 days after the date of this prospectus. See "Underwriting" for more description of the lock-up agreements.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the date of this prospectus, a person who has beneficially owned shares of our common stock for at least one year, including the holding period of certain prior owners other than affiliates, is entitled to sell within any three-month period a number of shares that does not exceed the greater of 1% of the number of shares of our common stock then outstanding, whichthat will equal approximatelybe issued on the exercise of the warrants, or an adjustment to the exercise price of the warrants, may be treated as a constructive distribution to a U.S. Holder of the warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our stockholders). Adjustments to the number of shares immediately after the offering, or the average weekly trading volume of our common stock that will be issued on the Nasdaq National Market during the four calendar weeks preceding the filingexercise of a notice on Form 144 with respectwarrant or adjustments to the exercise price of a warrant made pursuant to a bona fide reasonable adjustment formula that sale. Sales under Rule 144 are also subject to certain manner-of-sale provisions, notice requirements andhas the availabilityeffect of current public information about us. Additionally, substantially all Rule 144 shares arepreventing dilution of the interest of the holders of the warrants should generally not result in a constructive distribution. Any constructive distributions would generally be subject to the 180-day lock-up arrangementtax treatment described above.
97
Rule 144(k)above under “–Distributions on Common Stock.”
Under Rule 144(k), a person who is not deemed to have been one
Sale or Other Disposition of our affiliates at any time duringCommon Stock
For U.S. federal income tax purposes, gain or loss realized on the three months preceding a sale and who has beneficially owned shares for at least two years, including the holding period of certain prior owners other than affiliates, is entitled to sell those shares without complying with the volume limitations, manner-of-sale provisions, notice requirements and public information provisions of Rule 144. Therefore, unless restricted under the 180-day lock-up arrangement or otherwise, Rule 144(k) shares may be sold immediately upon the closing of this offering.
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, each of our directors, officers, employees, consultants or advisors who purchased shares from us before the date of this prospectus in connection with a compensatory stock plan or other written compensatory agreement is eligible to resell such shares 90 days after the effective datedisposition of this offering in reliance on Rule 144, but without compliance with certain restrictions, including the holding period, contained in Rule 144. However, substantially all Rule 701 shares are subject to the 180-day lock-up arrangement described above.
Registration Rights
As described above in "Description of Capital Stock—Registration Rights," upon completion of this offering, the holders of 43,555,580 shares of our common stock will be entitledcapital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the common stock for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the common stock disposed of and the amount realized on the disposition. Long-term capital gains recognized by non-corporate U.S. Holders will be subject to rightsreduced tax rates. The deductibility of capital losses is subject to limitations.
Sale or Other Disposition, Exercise or Expiration of Warrants
For U.S. federal income tax purposes, gain or loss realized on the sale or other disposition of a warrant (other than by exercise) will be capital gain or loss and will be long-term capital gain or loss if the U.S. Holder held the warrant for more than one year at the time of the sale or other disposition. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the warrant disposed of and the amount realized on the disposition.
In general, a U.S. Holder will not be required to recognize income, gain or loss upon the exercise of a warrant by payment of the exercise price, except to the extent of cash paid in lieu of a fractional share. A U.S. Holder’s tax basis in a share of common stock received upon exercise will be equal to the sum of (1) the U.S. Holder’s tax basis in the warrant and (2) the exercise price of the warrant. A U.S. Holder’s holding period in the stock received upon exercise will commence on the day or the day after such U.S. Holder exercises the warrant. No discussion is provided herein regarding the U.S. federal income tax treatment on the exercise of a warrant on a cashless basis, and U.S. Holders are urged to consult their tax advisors as to the exercise of a warrant on a cashless basis.
If a warrant expires without being exercised, a U.S. Holder will recognize a capital loss in an amount equal to such U.S. Holder’s tax basis in the warrant. This loss will be long-term capital loss if, at the time of the expiration, the U.S. Holder’s holding period in the warrant is more than one year. The deductibility of capital losses is subject to limitations.
Redemption of Common Stock
In the event that a U.S. Holder’s common stock is redeemedor if wepurchase a U.S. Holder’s common stock in an open market transaction, the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale of the common stock under Section 302 of the Code. If the redemption qualifies as a sale of common stock, the U.S. Holder will be treated as described under “–Sale or Other Disposition of Common Stock” above. If the redemption does not qualify as a sale of common stock, the U.S. Holder will be treated as receiving a corporate distribution with the tax consequences described above under “—–Distributions on Common Stock.” Whether a redemption qualifies for sale treatment will depend largely on the total number of shares of our stock treated as held by the U.S. Holder relative to all of our shares outstanding both before and after the redemption. The redemption of common stock generally will be treated as a sale of the common stock (rather than as a corporate distribution) if the redemption (1) is “substantially disproportionate” with respect to the registrationU.S. Holder, (2) results in a “complete termination” of the U.S. Holder’s interest in us or (3) is “not essentially equivalent to a dividend” with respect to the U.S. Holder. These tests are explained more fully below.
In determining whether any of the foregoing tests are satisfied, a U.S. Holder takes into account not only stock actually owned by the U.S. Holder, but also shares of our stock that are constructively owned by it. A U.S. Holder may constructively own, in addition to stock owned directly, stock owned by certain related individuals and entities in which the U.S. Holder has an interest or that have an interest in such U.S. Holder, as well as any stock the U.S. Holder has a right to acquire by exercise of an option (i.e. pre-funded warrants and warrants). In order to meet the substantially disproportionate test, the percentage of our outstanding voting stock actually and constructively owned by the U.S. Holder immediately following the redemption of common stock must, among other requirements, be less than 80% of the percentage of our outstanding voting stock actually and constructively owned by the U.S. Holder immediately before the redemption. There will be a complete termination of a U.S. Holder’s interest if either (1) all of the shares of our stock actually and constructively owned by the U.S. Holder are redeemed or (2) all of the shares of our stock actually owned by the U.S. Holder are redeemed and the U.S. Holder is eligible to waive, and effectively waives in accordance with specific rules, the attribution of stock owned by certain family members and the U.S. Holder does not constructively own any other shares of our stock. The redemption of the common stock will not be essentially equivalent to a dividend if the redemption results in a “meaningful reduction” of the U.S. Holder’s proportionate interest in us. Whether the redemption will result in a meaningful reduction in a U.S. Holder’s proportionate interest in us will depend on the particular facts and circumstances. However, the IRS has indicated in a published ruling that even a small reduction in the proportionate interest of a small minority stockholder in a publicly held corporation who exercises no control over corporate affairs may constitute such a “meaningful reduction.” A U.S. Holder should consult with its own tax advisors as to the tax consequences of a redemption.
If none of the foregoing tests is satisfied, then the redemption will be treated as a corporate distribution and the tax effects will be as described under “–Distributions on Common Stock,” above. After the application of those rules, any remaining tax basis of the U.S. Holder in the redeemed common stock will be added to the U.S. Holder’s adjusted tax basis in its remaining stock.
Tax Consequences to Non-U.S. Holders
The following is a general discussion of the material U.S. federal income tax considerations applicable to “Non-U.S. Holders” (as defined herein) with respect to their sharesownership and disposition of any Security issued pursuant to this offering. All prospective Non-U.S. Holders of any Security should consult their tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the acquisition, ownership, exercise, expiration and disposition of any Security, as the case may be. In general, a Non-U.S. Holder means a beneficial owner of any Security (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; | |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust if (1) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
Distributions on Common Stock
As discussed above under “Dividend Policy,” we do not currently expect to make distributions on our common stock. In the event that we do make a distribution on our common stock, those payments will constitute dividends for U.S. federal income tax purposes to the extent we have current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our common stock, as applicable, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “–Sale or Other Disposition of Securities Act,.” Any such distributions would be subject to the 180 day lock-up arrangementdiscussions below regarding back-up withholding and the Foreign Account Tax Compliance Act (“FATCA”).
Subject to the discussion below on effectively connected income, any dividend paid to a Non-U.S. Holder generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a Non-U.S. Holder must provide us or our agent with an IRS Form W-8BEN, IRS Form W-8 BEN-E or another appropriate version of IRS Form W-8 (or a successor form), which must be updated periodically, and which, in each case, must certify qualification for the reduced rate. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and that are not eligible for relief from U.S. (net basis) income tax under an applicable income tax treaty generally are exempt from the (gross basis) withholding tax described above. RegistrationTo obtain this exemption from withholding tax, the Non-U.S. Holder must provide the applicable withholding agent with an IRS Form W-8ECI or successor form or other applicable IRS Form W-8 certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States. Such effectively connected dividends, if not eligible for relief under an income tax treaty, would not be subject to a withholding tax, but would be taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits and if, in addition, the Non-U.S. Holder is a corporation, may also be subject to a branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
If you are eligible for a reduced rate of withholding tax pursuant to an income tax treaty, you may be able to obtain a refund of any excess amounts withheld if you timely file an appropriate claim for a refund with the IRS.
Constructive Dividends on Warrants
See the discussion of the rules applicable to constructive distributions on a warrant under“���Tax Consequences to U.S. Holders –Constructive Dividends on Warrants” above. Any constructive distributions would generally be subject to the tax treatment described above under “–Distributions on Common Stock.”
Exercise or Expiration of Warrants
In general, a Non-U.S. Holder will not be required to recognize income, gain or loss upon the exercise of a warrant by payment of the exercise price, except possibly to the extent of cash paid in lieu of a fractional share. However, no discussion is provided herein regarding the U.S. federal income tax treatment on the exercise of a warrant on a cashless basis, and Non-U.S. Holders are urged to consult their tax advisors as to the exercise of a warrant on a cashless basis.
If a warrant expires without being exercised, a Non-U.S. Holder that is engaged in a U.S. trade or business to which any income from the warrant would be effectively connected or who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the expiration occurs (and certain other conditions are met) will recognize a capital loss in an amount equal to such Non-U.S. Holder’s tax basis in the warrant.
Sale or Other Disposition of Securities
Subject to the discussion below regarding backup withholding and FATCA, a Non-U.S. Holder generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of any Security unless:
| ● | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and not eligible for relief under an applicable income tax treaty, in which case the Non-U.S. Holder will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and for a Non-U.S. Holder that is a corporation, such Non-U.S. Holder may be subject to the branch profits tax at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items; | |
| ● | the Non-U.S. Holder is an individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case the Non-U.S. Holder will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses (even though the Non-U.S. Holder is not considered a resident of the United States) (subject to applicable income tax or other treaties); or | |
| ● | we are a “U.S. real property holding corporation” for U.S. federal income tax purposes, or a USRPHC, at any time within the shorter of the five-year period preceding the disposition or the Non-U.S. Holder’s holding period for any Security. We believe we are not currently and do not anticipate becoming a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property interests relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, a Non-U.S. Holder may qualify for an exemption from U.S federal income tax based on our status as a USRPHC. If no exemption is available and we are a USRPHC, a Non-U.S. Holder’s proceeds received on the disposition any Security will generally be subject to withholding at a rate of 15% and such Non-U.S. Holder will generally be taxed on any gain in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax generally will not apply. Non-U.S. Holders should consult with their tax advisors on the availability of any such exemption in the event of we become a USRPHC. |
Redemption of Our Common Stock
The characterization for U.S. federal income tax purposes of the redemption of a Non-U.S. Holder’s common stock generally will correspond to the U.S. federal income tax characterization of such a redemption of a U.S. Holder’s common stock, as described under “Tax Consequences to U.S. Holders—Redemption of Common Stock” above, and the consequences of the redemption to the Non-U.S. Holder will be as described above under “—Distributions of Common Stock” and “—Sale or Other Disposition of Securities,” as applicable.
Backup Withholding and Information Reporting
Information returns may be filed with the IRS in connection with distributions on our common stock or constructive dividends on the warrants, and the proceeds of a sale or other disposition of any Security. A non-exempt U.S. Holder may be subject to U.S. backup withholding on these payments if it fails to provide its taxpayer identification number to the withholding agent and comply with certification procedures or otherwise establish an exemption from backup withholding.
A Non-U.S. Holder may be subject to U.S. information reporting and backup withholding on these payments unless the Non-U.S. Holder complies with certification procedures to establish that it is not a U.S. person (as defined in the Code). The certification requirements generally will be satisfied if the Non-U.S. Holder provides the applicable withholding agent with a statement on the applicable IRS Form W-8BEN or IRS Form W-8BEN-E (or suitable substitute or successor form), together with all appropriate attachments, signed under penalties of perjury, stating, among other things, that such Non-U.S. Holder is not a U.S. person. Applicable Treasury Regulations provide alternative methods for satisfying this requirement. In addition, the amount of distributions on common stock or constructive dividends on warrants paid to a Non-U.S. Holder, and the amount of any U.S. federal income tax withheld therefrom, must be reported annually to the IRS and the holder. This information may be made available by the IRS under the provisions of an applicable tax treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides.
Payment of the proceeds of the sale or other disposition of any Security to or through a non-U.S. office of a U.S. broker or of a non-U.S. broker with certain specified U.S. connections generally will be subject to information reporting requirements, but not backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or an exemption otherwise applies. Payments of the proceeds of a sale or other disposition of any Security to or through a U.S. office of a broker generally will be subject to information reporting and backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or otherwise establishes an exemption.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment generally will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Account Tax Compliance Act
FATCA imposes withholding tax on certain types of payments made to foreign financial institutions and certain other non-U.S. entities. The legislation imposes a 30% withholding tax on dividends on, or, subject to the discussion of certain proposed Treasury Regulations below, gross proceeds from the sale or other disposition of any Security paid to a “foreign financial institution” or to certain “non-financial foreign entities” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Treasury requiring, among other things, that it undertakes to identify accounts held by “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. If the country in which a payee is resident has entered into an “intergovernmental agreement” with the United States regarding FATCA, that agreement may permit the payee to report to that country rather than to the U.S. Department of the Treasury. The U.S. Treasury recently released proposed Treasury Regulations which, if finalized in their present form, would eliminate the federal withholding tax of 30% applicable to the gross proceeds of a sale or other disposition of any Security. In its preamble to such proposed Treasury Regulations, the U.S. Treasury stated that taxpayers may generally rely on the proposed regulations until final regulations are issued. Prospective investors should consult their own tax advisors regarding the possible impact of these shares underrules on their investment in any Security, and the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any salespossible impact of securities by these stockholders could have a material adverse effectrules on the trading price of our common stock.
Employee Benefit Plans
We intendentities through which they hold any Security, including, without limitation, the process and deadlines for meeting the applicable requirements to file withprevent the Securities and Exchange Commission a registration statement on Form S-8 under the Securities Act covering the shares of common stock reserved for issuance under our 1995 Stock Option/Stock Issuance Plan, 1997 Stock Option/Stock Issuance Plan, 1998 Stock Option/Stock Issuance Plan, 2004 Equity Incentive Award Plan, 2004 Non-Employee Director Stock Option Program and 2004 Employee Stock Purchase Plan. The registration statement is expected to be filed and become effective as soon as practicable after the completionimposition of this offering. Accordingly, shares registered30% withholding tax under the registration statement will be available for sale in the open market, subject to Rule 144 volume limitations applicable to affiliates, and subject to any vesting restrictions and lock-up agreements applicable to these shares.
Under
THE PRECEDING DISCUSSION IS FOR GENERAL INFORMATION ONLY. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF ANY SECURITIY, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
Maxim Group LLC (“Maxim”) is acting as sole book-runner and as representative of the underwriters (the “Representative”). Subject to the terms and subject toconditions of an underwriting agreement between us and the conditions contained in a purchase agreement dated the date of this prospectus, the underwriters named below, for whom Merrill Lynch, Pierce, Fenner & Smith Incorporated, J.P. Morgan Securities Inc., Banc of America Securities LLC and William Blair & Company, L.L.C. are acting as representatives, have severally agreed to purchase, andRepresentative, we have agreed to sell to them,each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares indicated below:of common stock, pre-funded warrants and warrants, listed next to its name in the following table:
| Name of | ||||||||||||
of shares | Number of pre-funded warrants | Number of accompanying warrants | ||||||||||
| Total | ||||||||||||
The underwriters are committed to purchase all the shares of common stock and pre-funded warrants, and warrants, offered by this prospectus if they purchase any shares of common stock, pre-funded warrants and warrants. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock, pre-funded warrants or warrants, covered by the underwriters’ over-allotment option described below. The underwriters are offering the shares of common stock, subject to their acceptance of the shares from uspre-funded warrants and warrants, subject to prior sale. The purchase agreement provides that the obligations of the several underwriterssale, when, as and if issued to pay for and accept delivery of the shares of common stock offeredaccepted by this prospectus arethem, subject to the approval of specified legal matters by their counsel, and to other conditions.conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and a legal opinion. The underwriters are obligatedreserve the right to take and pay for all of the shares of common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to takewithdraw, cancel or pay for the shares covered by the underwriters' over-allotment option described below.
The underwriters initially propose to offer part of the shares of common stock directlymodify offers to the public at the public offering price listed on the cover page of this prospectus and part to certain dealers at a price that represents a concession notreject orders in excess of $ per share under the public offering price. Any underwriter may allow, and such dealers may reallow, a concession notwhole or in excess of $ per share to other underwriters or to certain dealers. After the initial offering of the shares of common stock, the offering price and other selling terms may from time to time be varied by the representatives.part.
Over-Allotment Option
We have granted to the underwriters an option, exercisable for 30no later than 45 calendar days fromafter the date of this prospectus,the underwriting agreement, to purchase up to an aggregate of additional [●] shares of common stock and/or pre-funded warrants and/or up to an additional [●] warrants, in each case, at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of coveringonly to cover over-allotments, if any, made in connection with thethis offering of theand may exercise this option to purchase additional shares of common stock offered by this prospectus.and/or pre-funded warrants and/or warrants. To the extent the option is exercised each underwriterand the conditions of the underwriting agreement are satisfied, we will becomebe obligated subject to certain conditions,sell to the underwriters, and the underwriters will be obligated to purchase, about the same percentage of thethese additional shares of common stock asand/or pre-funded warrants and/or warrants.
Discounts and Commissions
We have agreed to pay the number listed nextunderwriters a cash fee equal to seven percent (7.0%) of the underwriter's name inaggregate gross proceeds from the preceding table bears tosale of the total number of shares of common stock listed nextand pre-funded warrants and warrants.
The Representative has advised us that the underwriters propose to offer the names of all underwriters in the preceding table. If the underwriters' option is exercised in full, the total priceshares, pre-funded warrants and warrants, directly to the public would be $ ,at the total underwriters' discounts and commissions would be $ andpublic offering price set forth on the total proceeds to us would be $ .
As joint book-running managers on behalfcover of this prospectus. In addition, the representative may offer some of the underwriting syndicate, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities Inc. will be responsible for recordingshares and/or pre-funded warrants and/or warrants, to other securities dealers at such price less a listconcession of potential investors that have expressed an interest in purchasing sharesup to $[●] per share of common stock as part of this offering.
The underwriters have informed us that they do not intend sales to discretionary accounts to exceed five percent of the total number of shares of common stock offered by them.
We, each of our directors and officers and holders of substantially all of our outstanding stock have agreed that, without the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated and
99
J.P. Morgan Securities Inc., we and they will not, during the period ending 180 days after the date of this prospectus:
whether any transaction described above is to be settled by delivery of common stock or such other securities, in cash or otherwise. These restrictions do not apply to:
provided further that in the case of each of the last three transactions described above, the recipient of the shares agrees to be subject to the restrictions described in this paragraph.and/or pre-funded warrants and warrants.
The following table showssummarizes the public offering price, underwriting discountscommissions and commissions that we areproceeds before expenses to pay to the underwriters in connection with this offering. These amounts are shownus assuming both no exercise and full exercise of the underwriters'underwriters’ option to purchase additional shares of our common stock.stock or pre-funded warrants and/or warrants. The underwriting commissions are equal to the public offering price per share, pre-funded warrant and warrant, less the amount per share the underwriters pay us for the shares of common stock, pre-funded warrants and warrants.
| Per share and accompanying | |||||||||||||||||
| warrant | Per | pre-funded warrant and accompanying warrant | Total (No | Exercise) | Total (Full Exercise) | ||||||||||||
| $ | $ | $ | $ | ||||||||||||||
| Proceeds, before expenses, to us | $ | $ | $ | $ | |||||||||||||
In addition, we
We have agreed to reimburse Maxim for its out of pocket accountable expenses, including Maxim’s legal fees up to a maximum of $90,000, in connection with the offering. We have paid $25,000 to Maxim as an advance to be applied towards reasonable out-of-pocket expenses, or the Advance. Any portion of the Advance shall be returned back to us to the extent not actually incurred. We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, payable by us, in addition to thebut excluding underwriting discounts and commission,commissions, will be approximately $ .$[●], all of which are payable by us.
In order
Underwriter’s Warrant
We have agreed to facilitateissue to the underwriters, an Underwriter’s Warrant to purchase shares of our common stock which represents 2.5% of the number of shares of common stock and/or pre-funded warrants sold in this offering. The Underwriter’s Warrant will have a term of five years from the effective date of this prospectus and an exercise price per share equal to 110% of the public offering per share price and may be exercised cashlessly if at the time of exercise there is no effective registration statement registering the shares of common stock underlying the Underwriter’s Warrant, or the prospectus contained therein is not available for the issuance of such shares. Pursuant to FINRA Rule 5110(g), the Underwriter’s Warrant and any shares issued upon exercise of the Underwriter’s Warrant shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of our reorganization; (ii) to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the underwriter or related persons does not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period.
Lock-Up Agreements
We and each of our officers and directors have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock for a period of 90 days after this offering is completed without the prior written consent of Maxim.
Maxim may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
Right of First Refusal
We have granted the Representative a right of first refusal, for a period of nine (9) months from the commencement of sales of this offering, to act as sole underwriter and sole book running manager and/or sole placement agent for any and all public and private equity, equity-linked, convertible or debt offerings of the Company.
In addition, if within six (6) months following the closing of this offering, we complete any financing of equity, equity-linked, convertible or debt or other capital raising activity (other than the exercise by any person or entity of any options, warrants or other convertible securities) with any of the investors contacted by the underwriters during this offering, then we will pay to Maxim upon the closing of such other financing a cash fee equal to seven percent (7.0%) of the aggregate gross proceeds of such financing and a warrant to purchase securities which represent 2.5% of the number of securities sold in such financing exercisable at a price equal to 110.0% of the offering price.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Price Stabilization, Short Positions, and Penalty Bids
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of theour common stock.stock, warrants or pre-funded warrants. Specifically, the underwriters may sellover-allot in connection with this offering by selling more shares or pre-funded warrants and/or warrants than they are obligated to purchase underset forth on the purchase agreement, creatingcover page of this prospectus. This creates a short position. A short sale is covered if theposition in our common stock for its own account. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares common stock or pre-funded warrants over-allotted by the underwriters is nonot greater than the number of shares available forof common stock or pre-funded warrants that they may purchase by the underwriters underin the over-allotment option. The underwriters canIn a naked short position, the number of shares of common stock or pre-funded warrants involved is greater than the number of shares common stock or pre-funded warrants in the over-allotment option. To close out a covered short sale by exercising the over-allotment option or purchasing shares in the open market. In determining the source of shares to close out a covered short sale,position, the underwriters will consider, among other things, the open market pricemay elect to exercise all or part of shares compared to the price available under the over-allotment option. The underwriters may also sell shares in excess of the over-allotment option, creating a naked short position.
100
The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in this offering. In addition,elect to stabilize the price of theour common stock or pre-funded warrants or reduce any short position by bidding for, and purchasing, common stock or pre-funded warrants in the open market.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing a security in this offering because the underwriter repurchases that security in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, shares of our common stock or pre-funded warrants in the open market. Finally, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the common stock in this offering, if the syndicate repurchases previously distributed common stock inmarket making transactions, to cover syndicate short positions or to stabilize the price of the common stock. Any of theseincluding “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of theour common stock above independent market levels.or pre-funded warrants at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not required to engage in these activities, and may enddiscontinue any of these activities at any time.time without notice.
Merrill Lynch Ventures, L.P. 2001, which is an affiliate
In connection with this offering, the underwriters and selling group members, if any, or their affiliates may engage in passive market making transactions in our common stock or pre-funded warrants immediately prior to the commencement of Merrill Lynch, Pierce, Fenner & Smith Incorporated, onesales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103 generally provides that:
| ● | a passive market maker may not effect transactions or display bids for our common stock or pre-funded warrants in excess of the highest independent bid price by persons who are not passive market makers; |
| ● | net purchases by a passive market maker on each day are generally limited to 30% of the passive market maker’s average daily trading volume in our common stock or pre-funded warrants during a specified two-month prior period or 200 shares, whichever is greater, and must be discontinued when that limit is reached; and |
| ● | passive market making bids must be identified as such. |
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the representatives of the underwriters beneficially owns in the aggregate 5,846,056and may also be made available on a website maintained by other underwriters. The underwriters may agree to allocate a number of shares or 13.4%, of our common stock, assuming conversion of all of our outstanding convertible preferred stock.
Because we may be deemedpre-funded warrants to have a conflict of interest with Merrill Lynch, Pierce, Fenner & Smith Incorporated, the offering will be conducted in accordance with Conduct Rule 2720 of the National Association of Securities Dealers, Inc. This rule requires that the public offering price of any equity security be no higher than the price recommended by a qualified independent underwriter which has participated in the preparation of the registration statement and performed its usual standard of due diligence with respect to that registration statement. J.P. Morgan Securities Inc. has agreed to act as qualified independent underwriter for this offering. The price of the shares will be no higher than that recommended by J.P. Morgan Securities Inc. J.P. Morgan Securities Inc. will not receive any additional compensation for acting as qualified independent underwriter for this offering.
We have applied for quotation of our common stock on the Nasdaq National Market under the symbol "DRAD."
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
At our request, the underwriters have reserved for sale, at the initial public offering price, up to shares offered in this prospectus for sale to some of our directors, officers, employees, business associates and other persons with whom we have a relationship. The number of shares of common stock available for sale to the general publictheir online brokerage account holders. Internet distributions will be reduced toallocated by the extent these persons purchase reserved shares. Any reserved shares which are not orally confirmed for purchase within one dayrepresentatives of pricing of this offering will be offered by the underwriters to the general publicunderwriters that may make internet distributions on the same basis as other allocations. In connection with the offering, the underwriters or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The underwriters have informed us that they do not expect to confirm sales of shares or pre-funded warrants offered by this prospectus.prospectus to accounts over which they exercise discretionary authority.
Prior to
Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of the prospectus or the registration statement of which this offering, thereprospectus forms a part, has not been no public market for our common stock. The initial public offering price willapproved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be determinedrelied upon by negotiations between us and the representatives. Among the factors to be considered in determining the initial public offering price will be our future prospects and those of our industry in general, our revenues, earnings and other financial operating information in recent periods, and the price-earnings ratios, price-sales ratios, market prices of securities and financial and operating information of companies engaged in activities similar to ours. The estimated initial public offering price range set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors. Any active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the market above the initial offering price.investors.
The validity of the shares of common stock offered in this prospectushereby will be passed upon for us by MorrisonOlshan Frome Wolosky LLP, New York, New York. Loeb & FoersterLoeb LLP, San Diego, California. Certain legal mattersNew York, New York is representing the underwriter in connection with the offering will be passed upon for the underwriters by Latham & Watkins LLP, San Diego, California.
Ernst & Young LLP, independent auditors, have audited our
The consolidated financial statements atas of December 31, 20022019 and 2003,2018 and for each of the three years then ended incorporated by reference in the period ended December 31, 2003, as set forth in their report. We have included our financial statements in thethis prospectus and elsewhere in the registration statement have been so incorporated in reliance on Ernst & Young LLP'sthe report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference, given on theirthe authority of said firm as experts in accountingauditing and auditing.accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission
This prospectus constitutes a part of a registration statement on Form S-1 filed by us with the SEC under the Securities Act with respect to the shares ofour common stock, beingwarrants and pre-funded warrants offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. We have omitted certain parts of the registration statement, as allowed by the rules and regulations of the SEC. You may wish to inspect the registration statement and its exhibits. Forthe exhibits to that registration statement for further information aboutwith respect to us and theour common stock, warrants and pre-funded warrants offered by this prospectus, we refer you toprospectus. Copies of the registration statement and its exhibits.the exhibits to such registration statement are on file at the offices of the SEC and may be obtained upon payment of the prescribed fee or may be examined without charge at the public reference facilities of the SEC described below. Statements contained or incorporated by reference in this prospectus as toconcerning the contentsprovisions of any contract or any other document referred tocertain documents are not necessarily complete,summaries of the material provisions of such documents, and each statement is qualified in each instance, we refer youits entirety by reference to the copy of the contract or otherapplicable document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our Securities and Exchange Commission filings, including the registration statement of which this prospectus is a part, over the Internet at the Securities and Exchange Commission's website atwww.sec.gov. You may also read and copy any document we file with the SecuritiesSEC.
We file annual reports, quarterly and Exchange Commission at its public reference facilities at 450 Fifth Street, N.W., Washington, D.C. 20549. You may also obtain copies of the document at prescribed rates by writing to the Public Reference Section of the Securities and Exchange Commission at 450 Fifth Street, N.W., Washington, D.C. 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act and we will filecurrent reports, proxy statements and other information with the SecuritiesSEC. The public may read and Exchange Commission. We also intend to furnish our stockholderscopy any materials that we file with annualthe SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet website that contains reports, containing our financialproxy and information statements, audited by an independent public accounting firm and quarterly reports containing our unaudited financial information. other information regarding issuers that file electronically with the SEC at www.sec.gov.
We maintain aan Internet website atwww.digirad.com. Upon completion www.digirad.com. All of this offering, you may access our annual reportreports filed with the SEC (including Annual Reports on Form 10-K, quarterly reportsQuarterly Reports on Form 10-Q, current reportsCurrent Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d)proxy statements) are accessible through the Investor Relations section of the Exchange Act with the Securities and Exchange Commissionour website, free of charge, at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the Securities and Exchange Commission.electronic filing. The reference to our webwebsite in this prospectus is an inactive textual reference only and is not a hyperlink. The contents of our website are not part of this prospectus, and you should not consider the contents of our website in making an investment decision with respect to our securities.
INCORPORATION OF INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus information contained in documents that we file with it. This means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. The documents we are incorporating by reference as of their respective dates of filing are:
| ● | our Annual Report on Form 10-K for the year ended December 31, 2019 filed with the SEC on March 9, 2020, and the amendment to our Annual Report on Form 10-K/A filed with the SEC on April 17, 2020; |
| ● | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020 filed with the SEC on May 15, 2020; and |
| ● | our Current Reports on Form 8-K filed with the SEC on February 6, 2020, March 10, 2020, April 7, 2020, April 9, 2020, May 1, 2020 and May 6, 2020. |
Any statement incorporated by reference in this prospectus from an earlier dated document that is inconsistent with a statement contained in this prospectus or in any other document filed after the date of the earlier dated document, but prior to the date hereof, which also is incorporated by reference into this prospectus, shall be deemed to be modified or superseded for purposes of this prospectus by such statement contained in this prospectus or in any other document filed after the date of the earlier dated document, but prior to the date hereof, which also is incorporated by reference into this prospectus.
Any person, including any beneficial owner, to whom this prospectus is delivered may request copies of this prospectus and any of the documents incorporated by reference into this prospectus, without charge, by written request directed to Digirad Corporation, 1048 Industrial Court, Suwanee, Georgia 30024, or via the investor relation’s section of our website at http://ir.digirad.com/, or from the SEC through the SEC’s internet website at the address does not constitute incorporationprovided under “Where You Can Find More Information.” Documents incorporated by reference into this prospectus are available without charge, excluding any exhibits to those documents unless the exhibit is specifically incorporated by reference into those documents.
Except as expressly provided above, no other information, including none of the information contained at this site.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Ernst & Young LLP, Independent Auditors
The Board of Directors and StockholdersDigirad Corporation
We have audited the accompanying consolidated balance sheets of Digirad Corporation as of December 31, 2002 and 2003, and the related statements of operations, changes in stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2003. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.website, is incorporated by reference into this prospectus.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
DISCLOSURE OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Digirad Corporation at December 31, 2002 and 2003, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2003, in conformity with accounting principles generally accepted in the United States.
San Diego, CaliforniaMarch 12, 2004
F-2
Digirad CorporationConsolidated Balance Sheets
| | | | Pro forma redeemable convertible preferred stock and stockholders' equity at December 31, 2003 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | December 31, | ||||||||||
| | 2002 | 2003 | |||||||||
| | | | (unaudited) | ||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | 6,987,666 | $ | 7,681,407 | |||||||
| Accounts receivable, net | 7,868,234 | 12,195,031 | |||||||||
| Inventories, net | 5,752,123 | 3,709,321 | |||||||||
| Other assets | 502,805 | 854,170 | |||||||||
| Total current assets | 21,110,828 | 24,439,929 | |||||||||
Property and equipment, net | 11,113,884 | 10,087,030 | |||||||||
| Intangibles, net | 894,528 | 511,832 | |||||||||
| Restricted cash | — | 120,000 | |||||||||
| Total assets | $ | 33,119,240 | $ | 35,158,791 | |||||||
| Liabilities and stockholders' equity (deficit) | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 2,150,724 | $ | 3,036,209 | |||||||
| Accrued compensation | 1,721,107 | 1,893,336 | |||||||||
| Accrued warranty | 857,830 | 1,051,242 | |||||||||
| Other accrued liabilities | 3,102,109 | 2,647,741 | |||||||||
| Deferred revenue | 1,331,462 | 1,514,488 | |||||||||
| Current portion of notes payable to stockholders | — | 245,000 | |||||||||
| Current portion of debt | 8,166,421 | 11,473,619 | |||||||||
| �� | |||||||||||
| Total current liabilities | 17,329,653 | 21,861,635 | |||||||||
| Notes payable to stockholders, net of current portion | 735,000 | 490,000 | |||||||||
| Long-term debt, net of current portion | 5,030,327 | 4,232,071 | |||||||||
Commitments and contingencies | |||||||||||
| Redeemable convertible preferred stock, $0.000001 par value: 46,023,000 shares authorized at December 31, 2002 and 2003; 43,555,313 shares issued and outstanding at December 31, 2002 and 2003, none pro forma; liquidation value—$119,512,154 at December 31, 2002 and 2003, none pro forma (unaudited) | 83,952,228 | 84,277,992 | $ | — | |||||||
Stockholders' equity (deficit): | |||||||||||
| Common stock, $0.001 par value: 213,692 and 53,000,000 shares authorized at December 31, 2002 and 2003, respectively; 47,471 and 82,486 shares issued and outstanding at December 31, 2002 and 2003, respectively, 43,637,853 outstanding pro forma (unaudited) | 47 | 82 | 43,638 | ||||||||
| Additional paid-in capital | 4,246,342 | 5,031,811 | 89,266,247 | ||||||||
| Deferred compensation | — | (554,375 | ) | (554,375 | ) | ||||||
| Accumulated deficit | (78,174,357 | ) | (80,180,425 | ) | (80,180,425 | ) | |||||
| Total stockholders' equity (deficit) | (73,927,968 | ) | (75,702,907 | ) | $ | 8,575,085 | |||||
| Total liabilities and stockholders' equity (deficit) | $ | 33,119,240 | $ | 35,158,791 | |||||||
See accompanying notes.
F-3
Digirad CorporationConsolidated Statements of Operations
| | Years ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | ||||||||
| Revenues: | |||||||||||
| DIS | $ | 10,239,256 | $ | 23,005,004 | $ | 34,848,641 | |||||
| Product | 18,065,131 | 18,526,651 | 21,387,729 | ||||||||
| Total revenues | 28,304,387 | 41,531,655 | 56,236,370 | ||||||||
Cost of revenues: | |||||||||||
| DIS | 8,344,742 | 16,599,230 | 24,463,028 | ||||||||
| Product | 13,192,140 | 13,632,437 | 15,091,721 | ||||||||
| Total cost of revenues | 21,536,882 | 30,231,667 | 39,554,749 | ||||||||
| Gross profit | 6,767,505 | 11,299,988 | 16,681,621 | ||||||||
Operating expenses: | |||||||||||
| Research and development | 3,008,651 | 2,967,055 | 2,190,570 | ||||||||
| Sales and marketing | 9,974,027 | 8,065,497 | 6,007,858 | ||||||||
| General and administrative | 8,160,558 | 9,496,794 | 8,097,349 | ||||||||
| Amortization and impairment of intangible assets | 991,229 | 1,011,371 | 443,784 | ||||||||
| Stock-based compensation | 1,578,666 | 606,169 | 226,227 | ||||||||
| Total operating expenses | 23,713,131 | 22,146,886 | 16,965,788 | ||||||||
| Loss from operations | (16,945,626 | ) | (10,846,898 | ) | (284,167 | ) | |||||
Other income (expense): | |||||||||||
| Interest income | 118,174 | 65,078 | 35,412 | ||||||||
| Interest expense | (1,438,787 | ) | (1,989,907 | ) | (1,431,549 | ) | |||||
| Other expense | (1,644,542 | ) | — | — | |||||||
| Total other income (expense) | (2,965,155 | ) | (1,924,829 | ) | (1,396,137 | ) | |||||
| Net loss | (19,910,781 | ) | (12,771,727 | ) | (1,680,304 | ) | |||||
| Accretion of deferred issuance costs on preferred stock | (130,274 | ) | (265,146 | ) | (325,764 | ) | |||||
| Net loss applicable to common stockholders | $ | (20,041,055 | ) | $ | (13,036,873 | ) | $ | (2,006,068 | ) | ||
| Basic and diluted net loss per share | $ | (898.86 | ) | $ | (409.23 | ) | $ | (36.46 | ) | ||
| Shares used in computing basic and diluted net loss per share | 22,296 | 31,857 | 55,017 | ||||||||
| Pro forma basic and diluted net loss per share | $ | (0.04 | ) | ||||||||
| Pro forma shares used to compute basic and diluted net loss per share | 43,610,384 | ||||||||||
| The composition of stock-based compensation is as follows: | |||||||||||
| Cost of product revenue | $ | 200,365 | $ | 72,000 | $ | 82,529 | |||||
| Cost of DIS revenue | 97,568 | 51,588 | 31,039 | ||||||||
| Research and development | 96,335 | 60,622 | 8,200 | ||||||||
| Sales and marketing | 540,402 | 228,057 | 18,211 | ||||||||
| General and administrative | 643,996 | 193,902 | 86,248 | ||||||||
| $ | 1,578,666 | $ | 606,169 | $ | 226,227 | ||||||
See accompanying notes.
F-4
Digirad CorporationConsolidated Statements of Changes in Stockholders' Equity (Deficit)Years ended December 31, 2001, 2002 and 2003
| | Common stock | | | Notes receivable from stockholders | | | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Additional paid-in capital | Deferred compensation | Accumulated deficit | Total stockholders' equity (deficit) | ||||||||||||||||||
| | Shares | Amount | ||||||||||||||||||||
| Balance at December 31, 2000 | 21,820 | $ | 22 | $ | 2,239,711 | $ | (536,820 | ) | $ | (85,919 | ) | $ | (45,096,429 | ) | $ | (43,479,435 | ) | |||||
| Repayment of note receivable from stockholder | — | — | — | — | 14,312 | — | 14,312 | |||||||||||||||
| Exercise of common stock options | 1,182 | 1 | 97,793 | — | (5,312 | ) | — | 92,482 | ||||||||||||||
| Issuance of options, warrants and other equity instruments to non-employees | — | — | 192,652 | — | — | — | 192,652 | |||||||||||||||
| Deferred compensation | — | — | 1,715,521 | (1,715,521 | ) | — | — | — | ||||||||||||||
| Amortization of deferred compensation | — | — | — | 1,386,014 | — | — | 1,386,014 | |||||||||||||||
| Net loss | — | — | — | — | — | (19,910,781 | ) | (19,910,781 | ) | |||||||||||||
| Accretion of deferred issuance costs on preferred stock | — | — | — | — | — | (130,274 | ) | (130,274 | ) | |||||||||||||
| Balance at December 31, 2001 | 23,002 | 23 | 4,245,677 | (866,327 | ) | (76,919 | ) | (65,137,484 | ) | (61,835,030 | ) | |||||||||||
| Conversion of preferred stock to common stock | 24,191 | 24 | 48,358 | — | — | — | 48,382 | |||||||||||||||
| Exercise of common stock options | 246 | — | 46,332 | — | — | — | 46,332 | |||||||||||||||
| Issuance of common stock for fractional shares following 1-to- 200 stock split | 32 | — | — | — | — | — | — | |||||||||||||||
| Issuance of warrants to non- employees | — | — | 16,921 | — | — | — | 16,921 | |||||||||||||||
| Issuance of warrants in connection with bridge financing | — | — | 243,052 | — | — | — | 243,052 | |||||||||||||||
| Deferred compensation | — | — | (353,998 | ) | 353,998 | — | — | — | ||||||||||||||
| Amortization of deferred compensation | — | — | — | 512,329 | — | — | 512,329 | |||||||||||||||
| Forfeiture/reserve of notes receivable from shareholders | — | — | — | — | 76,919 | — | 76,919 | |||||||||||||||
| Net loss | — | — | — | — | — | (12,771,727 | ) | (12,771,727 | ) | |||||||||||||
| Accretion of deferred issuance costs on preferred stock | — | — | — | — | — | (265,146 | ) | (265,146 | ) | |||||||||||||
| Balance at December 31, 2002 | 47,471 | 47 | 4,246,342 | — | — | (78,174,357 | ) | (73,927,968 | ) | |||||||||||||
| Exercise of common stock options | 35,015 | 35 | 4,867 | — | — | — | 4,902 | |||||||||||||||
| Deferred compensation | — | — | 780,602 | (780,602 | ) | — | — | — | ||||||||||||||
| Amortization of deferred compensation | — | — | — | 226,227 | — | — | 226,227 | |||||||||||||||
| Net loss | — | — | — | — | — | (1,680,304 | ) | (1,680,304 | ) | |||||||||||||
| Accretion of deferred issuance costs on preferred stock | — | — | — | — | — | (325,764 | ) | (325,764 | ) | |||||||||||||
| Balance at December 31, 2003 | 82,486 | $ | 82 | $ | 5,031,811 | $ | (554,375 | ) | $ | — | $ | (80,180,425 | ) | $ | (75,702,907 | ) | ||||||
See accompanying notes.
F-5
Digirad CorporationConsolidated Statements of Cash Flows
| | Years ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | |||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (19,910,781 | ) | $ | (12,771,727 | ) | $ | (1,680,304 | ) | |||
| Adjustments to reconcile net loss to net cash used by operating activities: | ||||||||||||
| Depreciation | 1,941,637 | 2,648,410 | 2,811,204 | |||||||||
| Loss on disposal of assets | — | — | 8,020 | |||||||||
| Amortization and impairment of intangibles | 991,229 | 966,765 | 443,784 | |||||||||
| Stock-based compensation | 1,386,014 | 589,248 | 226,227 | |||||||||
| Amortization of debt discount related to warrants issued in conjunction with debt | 110,954 | 335,477 | — | |||||||||
| Options, warrants and other equity instruments issued to non- employees | 192,652 | 16,921 | — | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | (1,748,827 | ) | (3,065,386 | ) | (4,326,797 | ) | ||||||
| Inventories | (4,749,603 | ) | 2,873,441 | 2,042,802 | ||||||||
| Other assets | (79,243 | ) | 167,082 | (346,384 | ) | |||||||
| Accounts payable | 1,840,790 | (2,312,844 | ) | 885,485 | ||||||||
| Accrued compensation | 1,026,763 | (384,173 | ) | 172,229 | ||||||||
| Accrued warranty and other accrued liabilities | 1,899,894 | 100,907 | (260,956 | ) | ||||||||
| Deferred revenue | 329,959 | 1,001,503 | 183,026 | |||||||||
| Net cash provided by (used in) operating activities | (16,768,562 | ) | (9,834,376 | ) | 158,336 | |||||||
Investing activities | ||||||||||||
| Purchases of property and equipment | (7,742,297 | ) | (1,653,667 | ) | (1,797,351 | ) | ||||||
| Patents and other assets | (73,878 | ) | (112,776 | ) | (181,088 | ) | ||||||
| Net cash used by investing activities | (7,816,175 | ) | (1,766,443 | ) | (1,978,439 | ) | ||||||
Financing activities | ||||||||||||
| Net issuances of common stock | 92,482 | 46,332 | 4,902 | |||||||||
| Net borrowings under lines of credit | 2,731,490 | 2,697,739 | 3,139,151 | |||||||||
| Proceeds from issuance of notes payable | — | 2,154,656 | — | |||||||||
| Repayment of obligation under notes payable | (1,536,024 | ) | (2,105,936 | ) | — | |||||||
| Net proceeds from sale of preferred stock | 14,145,810 | 15,549,982 | — | |||||||||
| Proceeds from capital lease financing | 5,363,920 | — | 1,531,028 | |||||||||
| Repayment of obligations under capital leases | (815,567 | ) | (1,721,255 | ) | (2,161,237 | ) | ||||||
| Repayment of notes receivable from stockholders | 14,312 | — | — | |||||||||
| Net cash provided by financing activities | 19,996,423 | 16,621,518 | 2,513,844 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (4,588,314 | ) | 5,020,699 | 693,741 | ||||||||
| Cash and cash equivalents at beginning of year | 6,555,281 | 1,966,967 | 6,987,666 | |||||||||
| Cash and cash equivalents at end of year | $ | 1,966,967 | $ | 6,987,666 | $ | 7,681,407 | ||||||
| Supplemental information: | ||||||||||||
| Cash paid during the year for interest | $ | 1,485,467 | $ | 1,503,546 | $ | 1,325,975 | ||||||
| Conversion of bridge notes into preferred stock | $ | — | $ | 1,575,000 | $ | — | ||||||
| Conversion of preferred stock to common stock | $ | — | $ | 48,382 | $ | — | ||||||
See accompanying notes.
F-6
Digirad CorporationNotes to Consolidated Financial StatementsDecember 31, 2003
1. The Company and Summary of Significant Accounting Policies
The Company
Digirad Corporation (the "Company"), a Delaware corporation, designs, develops, manufactures, markets, and services solid-state digital gamma cameras for use in nuclear medicine and provides, through two subsidiaries, Digirad Imaging Solutions, Inc. and Digirad Imaging Systems, Inc., collectively "DIS," in-office services for physicians, offering experienced licensed personnel and equipment that travel to the physician's office on a per day, contractual basis.
Basis of Presentation
The accompanying consolidated financial statements include the operations of DIS. Intercompany accounts have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and disclosures made in the accompanying notes to the consolidated financial statements. The Company's significant estimates include the reserve for doubtful accounts, contractual allowances and revenue adjustments, the reserves for excess and obsolete inventories, the reserve for warranty costs and the valuation allowance for deferred tax assets. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all investments with an original maturity of three months or less when purchased to be cash equivalents. Cash equivalents primarily represent funds invested in money market funds whose cost equals fair market value.
Concentration of Credit Risk
The Company has primarily sold its products to customers in the United States and its possessions. Limited sales have also been made to customers in Japan and Russia. For the years ended December 31, 2001, 2002 and 2003, no product or DIS customer accounted for 10% or more of consolidated revenues.
The percentage of the Company's net DIS revenue derived from governmental agencies, such as Medicare, has continued to decline each year since services were initiated in 2000 to less than 5% of consolidated revenue in the year ended December 31, 2003. Management believes that there are minimal credit risks associated with transactions and balances with these governmental agencies. However, there is a potential risk that reimbursement rates can be reduced in the future.
The Company maintains reserves for potential credit losses and contractual allowances, which historically have been within management's estimates.
Inventories
Inventories are stated at the lower of cost or market, cost being determined on a first-in, first-out basis.
F-7
Property and Equipment
Depreciation and amortization of property and equipment, including assets recorded under capital leases, are provided using the straight-line method over the shorter of the estimated useful lives of the related assets, which is three to seven years, or the lease term, if applicable.
Intangibles
Intangibles include patents, trademarks and acquired customer contracts and are recorded at cost. Patents are amortized over the lesser of their estimated useful or legal lives (up to 20 years). Trademarks are amortized over 10 years. Acquired customer contracts are amortized over their estimated useful lives, which is generally five years.
Impairment of Long-Lived Assets
The Company follows Financial Accounting Standards Board ("FASB") Statement of Financial Accounting Standards ("SFAS") No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets. The scope of SFAS No. 144 includes long-lived assets, or groups of assets, to be held and used as well as those which are to be disposed of by sale or by other method, but excludes a number of long-lived assets such as goodwill and intangible assets not being amortized under the application of SFAS No. 142,Goodwill and Other Intangible Assets. SFAS No. 144 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets' carrying amount. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the estimated fair value of the assets.
During 2002 and 2003, the Company recorded $566,057 and $228,177, respectively, for impairment on customer contracts acquired for the imaging services business.
Shipping and Handling Fees and Costs
The Company records all shipping and handling billings to a customer in a sales transaction as revenue earned for the goods provided in accordance with the Emerging Issues Task Force ("EITF") Issue 00-10,Accounting for Shipping and Handling Fees and Costs. The Company's revenues related to shipping and handling for all periods presented are immaterial. Shipping and handling costs are included in cost of revenues and were $300,133, $229,462 and $251,536 for 2001, 2002 and 2003, respectively.
Revenue Recognition
The Company recognizes revenue when all four of the following criteria are met: (i) persuasive evidence that an arrangement exists; (ii) delivery of the products and/or services has occurred; (iii) the selling price is fixed or determinable; and (iv) collectibility is reasonably assured. In addition, the Company complies with SEC Staff Accounting Bulletin No. 101,Revenue Recognition in Financial Statements ("SAB 101"). SAB 101 sets forth guidelines on the timing of revenue recognition based upon factors such as passage of title, installation, payment terms and customer acceptance.
The Company has two primary sources of revenue: 1) product sales, which includes the associated sale of maintenance services and 2) mobile in-office nuclear imaging services. Product revenues consist of
F-8
revenues from the sales of gamma cameras and accessories and the Company recognizes revenue upon delivery to customers. The Company also provides installation and training for camera sales in the United States. Installation and training for sales outside of the United States is the responsibility of the distributors. Neither service is essential to the functionality of the product. Both services are performed shortly after delivery and represent an insignificant cost, which the Company accrues at the time revenue is recognized. The Company also sells or provides maintenance services beyond the first year following the purchase by the customer. Revenue from these contracts is deferred and recognized ratably over the period of the obligation and is included in product sales in the accompanying consolidated statements of operations.
DIS revenue is derived from the Company's mobile in-office nuclear imaging services. Revenue related to mobile imaging services is recognized at the time services are performed and collection is reasonably assured. DIS services are generally billed on a per-day basis under annual contracts which specify the number of days of service to be provided. The Company is compensated for mobile imaging services provided to patients directly from the physicians under contract or, on a smaller scale, from certain programs administered by governmental agencies and private insurance companies.
Unaudited Pro Forma Stockholders' Equity Presentation
The unaudited pro forma stockholders' equity information in the accompanying consolidated balance sheet assumes the conversion of the outstanding shares of redeemable convertible preferred stock into 43,555,367 shares of common stock as though the completion of the initial public offering had occurred on December 31, 2003. Common shares issued in such initial public offering and any related estimated net proceeds are excluded from such pro forma information.
Stock-Based Compensation
The Company has elected to follow Accounting Principles Board ("APB") Opinion No. 25,Accounting for Stock Issued to Employees, and related Interpretations in accounting for its employee stock options as permitted by SFAS No. 123,Accounting for Stock-Based Compensation. Under APB 25, if the exercise price of the Company's employee stock options is not less than the fair value of the underlying stock on the date of grant, no compensation expense is recognized. In conjunction with certain events that occurred in 2001, the Company reviewed its exercise prices and arrived at a revised fair value for certain stock options granted in 2001 and recorded deferred stock compensation of $1,715,521, for the difference between the original exercise price per share determined by the Board of Directors and the revised estimate of fair value per share at the respective grant date. In conjunction with the Company's initial public offering contemplated by this prospectus, the Company reviewed its exercise prices and arrived at a revised fair value for certain stock options granted in 2003. The Company recorded deferred stock compensation of
F-9
$780,602 for the difference between the original exercise price per share determined by the Board of Directors and the revised estimate of fair value per share at the respective grant date.
The approximate weighted average exercise price and approximate weighted average revised fair value per share for the 8,290 options granted during the year ended December 31, 2001 was $254.00 and $300.00, respectively. The approximate weighted average exercise price and approximate weighted average revised fair value per share for the 999,631 options granted during the year ended December 31, 2003 was $0.14 and $0.93, respectively. Deferred stock compensation is recognized and amortized on an accelerated basis in accordance with FASB Interpretation ("FIN") No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans, over the vesting period of the related options, generally four years. Deferred compensation for stock options and warrants granted to non-employees is recorded at fair value as determined in accordance with SFAS No. 123, and EITF No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring or in Conjunction with Selling Goods or Services. The fair value of the unvested options, warrants, and other equity instruments is periodically remeasured and the related amortization is adjusted as necessary. Compensation expense related to stock options, warrants, and other equity instruments to acquire common stock issued to non-employees was $192,652 and $138,447 for the years ended December 31, 2001 and 2002, respectively. No material amounts of non-employee stock-based compensation were recorded in 2003.
The expected future amortization expense for deferred compensation as of December 31, 2003 is $318,112 in 2004, $152,487 in 2005, $68,571 in 2006, and $15,205 in 2007 for a total of $554,375.
Pro forma information regarding net loss is required by SFAS No.123, and has been determined as if the Company had accounted for all of its employee stock options under the fair value method of that statement. The fair value for these options was estimated at the date of grant using the Minimum Value pricing model with the following weighted average assumptions for 2001, 2002, and 2003: risk-free interest rates of 4%, 3.8% and 3% respectively; a dividend yield of 0%; and a life of the options of six, five and five years, respectively.
For purposes of disclosures required by SFAS No. 123, the estimated fair value of the options is amortized on an accelerated basis in accordance with FIN No. 28 over the vesting period. The Company's adjusted net loss information is as follows:
| | Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | |||||||
| Net loss as reported | $ | (19,910,781 | ) | $ | (12,771,727 | ) | $ | (1,680,304 | ) | |
| Add: total stock-based employee compensation included in reported net loss | 1,386,014 | 512,329 | 226,227 | |||||||
| Less: total stock-based employee compensation determined under the fair value method for all awards | (1,671,812 | ) | (1,288,485 | ) | (270,581 | ) | ||||
| Adjusted net loss | $ | (20,196,579 | ) | $ | (13,547,883 | ) | $ | (1,724,658 | ) | |
| Basic and diluted net loss per share as reported | $ | (898.86 | ) | $ | (409.23 | ) | $ | (36.46 | ) | |
| Adjusted basic and diluted net loss per share | $ | (905.84 | ) | $ | (425.27 | ) | $ | (31.35 | ) | |
F-10
The above results are not likely to be representative of the effects of applying SFAS No.123 on reported net income or loss for future years.
Warranty
We provide a warranty on certain of our products and accrue the estimated cost at the time revenue is recorded. Historically, the warranty periods have ranged from up to 24 months. Since July 2002, substantially all of the warranty periods have been 12 months before customer-sponsored maintenance begins. Warranty reserves are established based on historical experience with failure rates and repair costs and the number of units at customers covered by warranty. We review warranty reserves monthly and, if necessary, make adjustments.
Research and Development
Research and development costs are expensed as incurred.
Advertising Costs
Advertising costs are expensed as incurred. Total advertising costs for the years ended December 31, 2001, 2002 and 2003, were $411,940, $240,646 and $231,617, respectively.
Other Expense
In 2001, the Company recorded expense of $1,644,542 related to costs incurred in connection with a proposed public offering of common stock which was not completed.
Comprehensive Income
SFAS No. 130,Reporting Comprehensive Income, requires that all components of comprehensive income, including net income, be reported in the financial statements in the period in which they are recognized. Comprehensive income is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments and foreign currency translation adjustments. The Company's comprehensive loss is the same as the reported net loss for all periods.
Net Loss Per Share
The Company calculated net loss per share in accordance with SFAS No. 128,Earnings Per Share, and SAB No. 98. Basic earnings per share ("EPS") is calculated by dividing the net income or loss available to common stockholders by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted EPS is computed by dividing the net income available to common stockholders by the weighted average number of common shares outstanding for the period and the weighted average number of dilutive common stock equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, common stock subject to repurchase by the Company, convertible preferred stock, options, and warrants are considered to be common stock equivalents and are only included in the calculation of diluted earnings per share when their effect is dilutive. Under the provisions of SAB No. 98, common shares issued for nominal consideration (as
F-11
defined), if any, would be included in the per share calculations as if they were outstanding for all periods presented. No common shares have been issued for nominal consideration.
Potentially dilutive securities totaling 181,766, 48,534,380 and 48,591,847 for the years ended December 31, 2001, 2002 and 2003, respectively, were excluded from historical basic and diluted earnings per share because of their anti-dilutive effect.
The unaudited pro forma basic and diluted net loss per common share and pro forma basic and diluted weighted average common shares outstanding give effect to the conversion of all outstanding shares of redeemable convertible preferred stock upon the completion of the Company's proposed initial public offering (using the as if-converted method).
| | Years ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | ||||||||
| Historical: | |||||||||||
| Numerator: | |||||||||||
| Net loss | $ | (19,910,781 | ) | $ | (12,771,727 | ) | $ | (1,680,304 | ) | ||
| Accretion of deferred issuance costs on preferred stock | (130,274 | ) | (265,146 | ) | (325,764 | ) | |||||
| Net loss applicable to common stockholders | $ | (20,041,055 | ) | $ | (13,036,873 | ) | $ | (2,006,068 | ) | ||
| Denominator: | |||||||||||
| Weighted average common shares | 22,726 | 31,857 | 55,017 | ||||||||
| Weighted average unvested common shares subject to repurchase | (430 | ) | — | — | |||||||
| Denominator for basic and diluted earnings per share | 22,296 | 31,857 | 55,017 | ||||||||
| Basic and diluted net loss per share | $ | (898.86 | ) | $ | (409.23 | ) | $ | (36.46 | ) | ||
| Pro forma: | |||||||||||
| Net loss | $ | (1,680,304 | ) | ||||||||
| Pro forma basic and diluted net loss per share (unaudited) | $ | (0.04 | ) | ||||||||
| Shares used above | 55,017 | ||||||||||
| Pro forma adjustments to reflect weighted average effect of assumed conversion of preferred stock (unaudited) | 43,555,367 | ||||||||||
| Pro forma shares used to compute basic and diluted net loss per share (unaudited) | 43,610,384 | ||||||||||
Recently Issued Accounting Standards
In November 2002, the FASB issued FIN No. 45,Guarantor's Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others. This interpretation elaborates on the disclosures required in financial statements concerning obligations under certain guarantees. The
F-12
recognition provisions of the interpretation are effective in 2003 and are applicable only to guarantees issued or modified after December 31, 2002. The Company has not issued or modified any such guarantees and accordingly the interpretation did not have a material impact on our financial position, results of operations or cash flows for the fiscal year ended December 31, 2003.
In January 2003, the FASB issued FIN No. 46,Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51. FIN No. 46 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. In December 2003, the FASB issued FIN No. 46R, a revision to FIN No. 46. FIN No. 46R provides a broad deferral of the latest date by which all public entities must apply FIN No. 46 to certain variable interest entities to the first reporting period ending after March 15, 2004. We do not expect the adoption of FIN No. 46 or FIN No. 46R to have a material impact upon our financial position, cash flows or results of operations.
In May 2003, the FASB issued SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity. SFAS No. 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. The adoption of SFAS No. 150 did not have a material effect on our consolidated financial statements.
2. Financial Statement Details
The composition of certain balance sheet accounts is as follows:
Accounts Receivable
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | |||||
| Accounts receivable | $ | 8,338,151 | $ | 12,746,333 | |||
| Less reserves and allowance for doubtful accounts | (469,917 | ) | (551,302 | ) | |||
| $ | 7,868,234 | $ | 12,195,031 | ||||
Inventories
| | December 31, | |||||
|---|---|---|---|---|---|---|
| | 2002 | 2003 | ||||
| Raw materials | $ | 1,612,586 | $ | 2,470,587 | ||
| Work-in-progress | 3,328,060 | 772,089 | ||||
| Finished goods | 811,477 | 466,645 | ||||
| $ | 5,752,123 | $ | 3,709,321 | |||
F-13
Property and Equipment
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | |||||
| Machinery and equipment | $ | 14,885,708 | $ | 16,063,473 | |||
| Furniture and fixtures | 261,875 | 241,989 | |||||
| Computers and software | 2,006,555 | 2,326,609 | |||||
| Leasehold improvements | 939,585 | 940,085 | |||||
| Construction in process | 52,482 | 135,680 | |||||
| 18,146,205 | 19,707,836 | ||||||
| Less accumulated depreciation and amortization | (7,032,321 | ) | (9,620,806 | ) | |||
| $ | 11,113,884 | $ | 10,087,030 | ||||
During 2000, 2001 and 2003, the Company entered into a series of financing transactions structured as capital leases. The equipment, consisting of vans equipped with the Company's mobile gamma cameras, is used by DIS to provide mobile nuclear imaging services. The initial terms of these leases range from 36 to 63 months. The cost of the equipment financed was $6,082,148 ($1,816,149 of accumulated depreciation) at December 31, 2002 and $6,484,719 ($2,582,288 of accumulated depreciation) at December 31, 2003.
Intangibles
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | |||||
| Acquired customer contracts | $ | 842,447 | $ | 244,921 | |||
| Patents and trademarks | 503,027 | 482,900 | |||||
| 1,345,474 | 727,821 | ||||||
| Less accumulated amortization | (450,946 | ) | (215,989 | ) | |||
| $ | 894,528 | $ | 511,832 | ||||
Other Accrued Liabilities
| | December 31, | |||||
|---|---|---|---|---|---|---|
| | 2002 | 2003 | ||||
| Sales tax payable | $ | 657,353 | $ | 511,794 | ||
| Pharmaceuticals | — | 606,176 | ||||
| License fees | 115,066 | 263,603 | ||||
| Customer deposits | 832,676 | 294,550 | ||||
| Interest | 122,122 | 109,272 | ||||
| Legal costs | 797,954 | 121,000 | ||||
| Other accrued liabilities | 576,938 | 741,346 | ||||
| $ | 3,102,109 | $ | 2,647,741 | |||
F-14
3. Debt
The composition of the Company's debt balance is as follows:
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | |||||
| Lines of credit | $ | 6,217,576 | $ | 9,356,727 | |||
| Capital lease obligations (Note 4) | 6,979,172 | 6,348,963 | |||||
| 13,196,748 | 15,705,690 | ||||||
| Current portion of debt | (8,166,421 | ) | (11,473,619 | ) | |||
| Long-term debt, less current portion | $ | 5,030,327 | $ | 4,232,071 | |||
Lines of Credit
Since December 2001, the Company has had a $5,000,000 line of credit which accrues interest at the bank's floating prime rate plus 1.75% (5.75% at December 31, 2003). The Company is required to make monthly interest payments. The revolving line of credit expires October 15, 2004 with any unpaid balance due upon expiration. $4,825,000 was outstanding as of December 31, 2003.
In 2001, in conjunction with the amended line of credit, the Company issued the lender a warrant to purchase 213 shares of Series E preferred stock at a price of $607.20. The warrant is exercisable immediately and expires five years from the date of issuance. The fair value of the warrant was determined to be insignificant as calculated using the Black-Scholes option pricing model with the following assumptions: dividend yield of 0%; expected volatility of 75%; risk-free interest rate of 3%; and a life of three years.
In January 2001, the Company entered into a three year loan and security agreement related to DIS for a revolving line of credit. The Company can draw up to $5,000,000. The borrowings under the line of credit are limited to 85% of Qualified Accounts (as defined) and accrue interest at the higher of prime plus 1.25% or 8.25%. The revolving credit line expires December 31, 2004. $4,531,727 was outstanding as of December 31, 2003. In March 2004, the borrowings under the line of credit were revised to accrue interest at the higher of prime plus 1.25% or 6%.
Notes Payable to Stockholders
The Company has notes payable to stockholders totaling $735,000 that bear interest at 6.35% per year. The notes are due in twelve equal quarterly installments starting on March 31, 2004. Accordingly, $245,000 is included as current portion of notes payable to stockholders at December 31, 2003 in the accompanying balance sheet.
In January 2002, the Company issued and sold convertible promissory notes in the aggregate principal amount of $1,925,000 bearing an annual interest rate of 12%. On May 7, 2002, holders of $1,425,000 of the convertible promissory notes elected to convert the principal balance and outstanding interest on the notes into Series H preferred stock. The remaining convertible promissory note balance of $500,000, plus accrued interest was repaid in June 2002. In consideration for the bridge loans, the Company issued to the noteholders warrants to purchase 790 shares of the Company's common stock at an exercise price of $300.00 per share (See Note 5).
F-15
In March 2002, the Company borrowed $150,000 from one of its stockholders under the terms of a secured loan bearing interest at 8% per annum. The loan plus accrued interest was converted into Series H preferred stock in June 2002.
The Company's borrowings are generally subject to financial and other restrictive covenants. The Company is in compliance with all covenants at December 31, 2003. Substantially all of the Company's assets have been pledged as collateral.
4. Commitments and Contingencies
Leases
The Company leases its facilities under non-cancelable operating leases that expire through 2010. Rent expense was $726,237, $887,340 and $1,028,895 (including common area charges) for the years ended December 31, 2001, 2002 and 2003, respectively. Annual future minimum lease payments as of December 31, 2003 are as follows:
| | Operating Leases | Capital Leases | |||||
|---|---|---|---|---|---|---|---|
| 2004 | $ | 695,584 | $ | 2,741,210 | |||
| 2005 | 708,433 | 2,662,690 | |||||
| 2006 | 668,063 | 1,533,943 | |||||
| 2007 | 614,907 | 421,305 | |||||
| 2008 | 554,412 | 145,523 | |||||
| Thereafter | 619,400 | — | |||||
| Total minimum lease payments | $ | 3,860,799 | 7,504,671 | ||||
| Less amount representing interest | (1,155,708 | ) | |||||
| Present value of future minimum capital lease obligations | 6,348,963 | ||||||
| Less amounts due in one year | (2,116,892 | ) | |||||
| Long-term portion of capital lease obligations | $ | 4,232,071 | |||||
Litigation
In 2001, a complaint was filed in the United States District Court for the Eastern District of Pennsylvania. The complaint alleged, among other things, breach of the terms of certain agreements. The Company agreed to settle the claim for $500,000, which was recorded in 2002 as a general and administrative expense in the statement of operations.
In addition, the Company is involved from time to time in litigation relating to claims arising in the normal course of its business. The Company faces risks from third-party claims of infringement and potential litigation.
F-16
5. Redeemable Convertible Preferred Stock and Stockholders' Equity
Reverse Stock Split
In October 2002, the Board of Directors and stockholders approved a 1:200 reverse split of the Company's common stock and preferred stock. All share and per share information in the accompanying consolidated financial statements and notes thereto have been restated to reflect the stock split.
Redeemable Convertible Preferred Stock
At December 31, 2003, the various series of preferred stock outstanding are as follows:
| Date issued | Series | Price per share | Number of shares | Redemption and liquidation value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| March 1995 | A | $ | 200.00 | 250 | $ | 50,000 | |||||
| December 1995 | B | $ | 220.00 | — | — | ||||||
| August 1997 | C | $ | 250.00 | 800 | 200,000 | ||||||
| August 1997 | D | $ | 461.46 | 2,130 | 982,910 | ||||||
| June 1998 through April 2001 | E | $ | 607.20 | 5,447 | 3,307,418 | ||||||
| August 2001 | F | $ | 650.00 | 770 | 500,500 | ||||||
| April, May, and June 2002 | G | $ | 2.00 | 30,984,210 | 61,968,420 | ||||||
| April, May, and June 2002 | H | $ | 1.39 | 12,561,706 | 52,502,906 | ||||||
| 43,555,313 | 119,512,154 | ||||||||||
| Unamortized deferred issuance costs | (213,512 | ) | |||||||||
| $ | 119,298,642 | ||||||||||
On April 23, 2002, the stockholders agreed to recapitalize the Company and entered into a Stock Purchase and Exchange Agreement under which the Company sold Series H preferred stock and exchanged shares of Series A, B, C, D, E and F preferred stock for Series G preferred stock for those Existing Stockholders (as defined) that purchased their pro-rata amount of Series H preferred stock. The Company received $15,846,149 in cash and $1,654,656 from the conversion of bridge notes and related accrued interest as consideration. The Company incurred $346,168 of offering costs related to the financing. The Company issued 12,561,706 Series H preferred shares and on conversion of 139,343 Series A, B, C, D, E, and F preferred shares, the Company issued 30,984,210 Series G preferred shares.
Deferred issuance costs through December 31, 2002 and 2003 for all series of preferred stock totaled $982,043 and are being accreted up to the redemption value through July 31, 2004 (the earliest redemption date).
The preferred stock is redeemable on or after July 31, 2004, upon the request of at least half in number of the Major Investors (as defined). The Company shall redeem all outstanding shares of preferred stock by paying in cash its redemption value plus declared but unpaid dividends. No dividends have been declared through December 31, 2003.
If the funds of the Company legally available for redemption are insufficient to redeem the total number of preferred shares to be redeemed, those funds which are legally available will be used to redeem the maximum possible ratably over the various series of preferred stock. If the offering contemplated by this prospectus is not completed, and the redeemable preferred shares remain outstanding, the Company does not anticipate having legally available funds to redeem any portion of these preferred shares in 2004.
F-17
The preferred stock will automatically be converted into shares of common stock upon the closing of a sale of the Company's common stock in a public offering registered under the Securities Act of 1933 which results in aggregate gross proceeds equal to or exceeding $25,000,000 at a price equal to or exceeding $4.1796 per share of common stock, or with the approval of at least half in number of Major Investors (as defined) and holders of a majority in interest of the then outstanding voting power of the Series H preferred stock. Each share of preferred stock is convertible, at the option of the holder, into one share of common stock, except for Series F which is convertible into 1.07 shares of common stock, subject to certain antidilution adjustments for certain equity issuances after April 23, 2002.
Holders of the Series A, B, C, D, E, F, G, and H preferred stock are entitled to receive non-cumulative dividends, if and when declared by the Board of Directors, at a rate of $20.00, $22.00, $25.00, $46.146, $60.72, $65.00, $0.20, and $0.13932 per share per annum, respectively. The holder of Series G and H preferred stock are entitled to receive dividends prior and in preference to any declaration or payment of dividends (payable other than in common stock) on series A, B, C, D, E, or F preferred stock, with series H preferred stock having prior preference to Series G preferred stock. The holder of each share of preferred stock is entitled to the number of votes equal to the number of shares of common stock into which the preferred stock could be converted. The Company is subject to certain covenants under the agreements that require the vote or written consent by both (a) half in number of the Major Investors and (b) the holders of a majority of the then outstanding voting power of the Series H preferred stock. The stockholders also have certain antidilutive rights.
The Series H preferred stockholders, voting as a separate class, are entitled to elect three members of the board of directors; Series G preferred stock holders, voting as a separate class, are entitled to elect two members of the board of directors; and any additional member of the board of directors shall be elected by the holders of Series A, B, C, D, E, and F and common stockholders, voting as a separate class.
In the event of any liquidation, dissolution or winding up of the Company, the holders of preferred stock are entitled to receive their liquidation value prior and in preference to any distribution of the assets or surplus funds of the Company to the holders of common stock. If, upon the occurrence of such event, the assets and funds distributed among the holders of preferred stock are insufficient to permit full payment, the entire assets and funds of the Company would be distributed among the preferred shareholders in proportion to the product of the liquidation preference of each such share and the number of such shares owned by each such holder.
Warrants
During the year ended December 31, 2001, in conjunction with various sales and marketing arrangements, the Company issued warrants to purchase 1,059 shares of the Company's common stock at prices ranging from $200.00 to $608.00 per share. Warrants for 759 shares of common stock are exercisable immediately and expire five years from the date of issuance. The remaining 300 warrants vest 100 warrants per year beginning July 2002 and expire in July 2006. The fair value of the warrants was $144,100.
During the year ended December 31, 2002, in conjunction with sales and marketing arrangements, the Company issued warrants to purchase 200,000 shares of the Company's common stock at $1.40 per share. In conjunction with consulting agreements, the Company issued warrants to purchase 55 shares of the Company's common stock at $600.00 per share.
F-18
The warrants are exercisable immediately, and expire five years from the date of issuance. The fair value of the warrants was $16,921.
During the year ended December 31, 2003, in conjunction with sales and marketing arrangements, the Company issued warrants to purchase 1,500 shares of the Company's common stock at $0.14 per share. The warrants are exercisable immediately and expire five years from the date of issuance. The fair value of the warrants is not material.
All of the warrants were valued using the Black-Scholes option pricing model with the following assumptions: dividend yield of 0%; expected volatility of 75%; risk-free interest rates ranging from 3% to 6%; and a term of three years.
Stock Options
In December 1998, the Company's 1997 Stock Option/Stock Issuance Plan was replaced with the 1998 Stock Option/Stock Issuance Plan ("1998 Plan") under which 1,000,000 shares of common stock were reserved for issuance upon exercise of options granted by the Company. Under all stock option plans, the Company is authorized to issue an aggregate of 5,889,824 shares of common stock. Terms of the stock option agreements, including vesting requirements (which is generally four years), are determined by the Board of Directors. Upon grant, the options are exercisable immediately; however any exercised but unvested shares are subject to repurchase by the Company at the original exercise price. Options granted have a term of up to ten years.
The following table summarizes option activity under the stock option plans:
| | Shares | Weighted average exercise price | ||||
|---|---|---|---|---|---|---|
| Outstanding at December 31, 2000 | 23,146 | $ | 84.20 | |||
| Granted | 10,521 | $ | 260.01 | |||
| Cancelled | (2,793 | ) | $ | 211.95 | ||
| Exercised | (1,438 | ) | $ | 82.54 | ||
| Outstanding at December 31, 2001 | 29,436 | $ | 134.79 | |||
| Granted | 5,118,060 | $ | 0.20 | |||
| Cancelled | (371,978 | ) | $ | 4.76 | ||
| Exercised | (347 | ) | $ | 179.83 | ||
| Outstanding at December 31, 2002 | 4,775,171 | $ | 0.66 | |||
| Granted | 999,631 | $ | 0.14 | |||
| Cancelled | (908,649 | ) | $ | 0.81 | ||
| Exercised | (35,015 | ) | $ | 0.14 | ||
| Outstanding at December 31, 2003 | 4,831,138 | $ | 0.52 | |||
As of December 31, 2001, 2002 and 2003, 1,226,753, 1,109,130 and 1,018,147 shares, respectively, were available for future grants.
F-19
Following is a further breakdown of the options outstanding as of December 31, 2003:
| Exercise price | Options Outstanding | Weighted average contractual life in years | Weighted average exercise price of options outstanding | Vested options | Weighted average exercise price of vested options | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| $0.14 | 4,820,264 | 8.6 | $ | 0.14 | 2,968,476 | $ | 0.14 | |||||
| $42 - $70 | 2,199 | 3.8 | $ | 56.15 | 2,199 | $ | 56.15 | |||||
| $100 - $150 | 4,844 | 5.2 | $ | 115.48 | 4,844 | $ | 115.48 | |||||
| $200 | 1,085 | 6.0 | $ | 200.00 | 1,085 | $ | 200.00 | |||||
| $300 | 2,327 | 7.1 | $ | 300.00 | 2,327 | $ | 300.00 | |||||
| $400 | 75 | 7.5 | $ | 400.00 | 75 | $ | 400.00 | |||||
| $600 - $608 | 140 | 7.1 | $ | 605.71 | 140 | $ | 605.71 | |||||
| $700 | 204 | 6.4 | $ | 700.00 | 204 | $ | 700.00 | |||||
| 4,831,138 | 8.6 | $ | 0.52 | 2,979,350 | $ | 0.76 | ||||||
The weighted average fair values of options granted in 2001, 2002 and 2003 were $398.47, $0.02 and $0.83, respectively.
Bridge Notes
On January 25, 2002, the Company executed bridge loans in the form of Convertible Promissory Notes and associated Warrant Purchase Agreements with various investors for total gross proceeds of $1,925,000. The notes bore interest at 12% per annum and ultimately were converted into Series H Preferred Stock. The warrants allowed the investors to purchase 790 shares of the Company's common stock over the next five years at $300.00 per share. The proceeds from the financing were allocated to the carrying values of the notes and the warrants on the basis of their relative fair values at the date of issuance and which also created a beneficial conversion feature equal to the fair value of the warrants. The separate fair value of the notes was equal to their face values on the basis of their terms. The separate fair value of the warrants and the separate value of the beneficial conversion feature was each determined to be $121,526 using the Black-Scholes option pricing model with the following assumptions: expected dividend yield of 0%, expected volatility of 75%, risk-free interest rate of 3.8% and expected life of three years. The resulting discount on the notes of $243,052 was amortized to interest expense over the period the notes were outstanding. With the exception of $500,000 that was repaid in cash, the notes and accrued interest were converted into Series H preferred stock over the three closing dates of the Series H preferred stock between April 23, 2002 and June 17, 2002.
Notes Receivable from Stockholders
At December 31, 2001, the Company had notes receivable from employee stockholders of $76,919. The notes relate to the exercise of common stock options, are full recourse and bear interest at 6% per year. The notes are due on the earlier of (i) the date on which the employee ceases to be employed by the Company, (ii) 90 days after an initial public offering of the Company's common stock; or (iii) May 15, 2010. During 2002, in conjunction with a recapitalization, the Company wrote-off the value of the notes receivable since the underlying shares had little or no value and collection of the notes was unlikely. In the future, if the Company provides financing for employees to purchase stock options, the Company will
F-20
account for options under variable plan accounting in accordance with EITF Issue No. 95-16,Accounting for Stock Compensation Arrangements with Employer Loan Features Under APB Opinion No. 25.
Common Shares Reserved for Issuance
The following table summarizes common shares reserved for future issuance at December 31, 2003:
6. Income Taxes
As of December 31, 2003, the Company had federal and California income tax net operating loss carryforwards of approximately $71,600,000 and $38,300,000, respectively. The difference between the federal and California tax loss carryforwards is primarily attributable to the limitation in the utilization of California net operating loss carryforwards, which ranges from 50% to 60% during the period from 1996 to 2003. The federal tax loss carryforwards will begin expiring in 2006 unless previously utilized. The California tax loss carryforwards will begin to expire in 2004 unless previously utilized. The Company also has federal and California research and development and other credit carryforwards of approximately $1,900,000 and $1,300,000, respectively. The federal research and development and other credit carryforwards begin to expire in 2005 unless previously utilized.
Pursuant to Internal Revenue Code Sections 382 and 383, use of the Company's net operating loss and credit carryforwards may be limited because of a cumulative change in ownership of more than 50%, which may have occurred.
Significant components of the Company's deferred tax assets are shown below. A valuation allowance has been recognized to offset the deferred tax assets, as realization of such assets has not met the "more likely than not" threshold required under SFAS No. 109.
| | December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| | 2002 | 2003 | ||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 26,832,000 | $ | 27,254,000 | ||||
| Research and development and other credits | 3,187,000 | 3,037,000 | ||||||
| Reserves | 741,000 | 856,000 | ||||||
| Capitalized research expense | 279,000 | 181,000 | ||||||
| Capitalized inventory costs | 238,000 | 117,000 | ||||||
| Other, net | 1,179,000 | 1,164,000 | ||||||
| Total deferred tax assets | 32,456,000 | 32,609,000 | ||||||
| Deferred tax liabilities—depreciation and amortization | (949,000 | ) | (1,567,000 | ) | ||||
| Valuation allowance for deferred tax assets | (31,507,000 | ) | (31,042,000 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
F-21
7. Segments
The Company's reporting segments have been determined based on the nature of the products and/or services offered to customers or the nature of their function in the organization. The Company evaluates performance based on the operating income contributed by each segment. The accounting policies of the reportable segments are the same as those described in the summary of significant accounting policies.
| | Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2001 | 2002 | 2003 | |||||||
| Revenues by segment: | ||||||||||
| DIS | $ | 10,239,256 | $ | 23,005,004 | $ | 34,848,641 | ||||
| Product | 18,065,131 | 18,526,651 | 21,387,729 | |||||||
| Consolidated revenues | $ | 28,304,387 | $ | 41,531,655 | $ | 56,236,370 | ||||
Gross profit by segment: | ||||||||||
| DIS | $ | 1,894,514 | $ | 6,405,774 | $ | 10,385,613 | ||||
| Product | 4,872,991 | 4,894,214 | 6,296,008 | |||||||
| Consolidated gross profit | $ | 6,767,505 | $ | 11,299,988 | $ | 16,681,621 | ||||
Net loss by segment: | ||||||||||
| Loss from operations | ||||||||||
| DIS | $ | (5,444,866 | ) | $ | (4,827,140 | ) | $ | 2,086,882 | ||
| Product | (11,500,760 | ) | (6,019,758 | ) | (2,371,049 | ) | ||||
| Consolidated income (loss) from operations | (16,945,626 | ) | (10,846,898 | ) | (284,167 | ) | ||||
| Reconciling items | ||||||||||
| Interest income | 118,174 | 65,078 | 35,412 | |||||||
| Interest expense | (1,438,787 | ) | (1,989,907 | ) | (1,431,549 | ) | ||||
| Other income (expense) | (1,644,542 | ) | — | — | ||||||
| Consolidated net loss | $ | (19,910,781 | ) | $ | (12,771,727 | ) | $ | (1,680,304 | ) | |
Depreciation, amortization and impairment of intangible assets by segment: | ||||||||||
| DIS | $ | 1,767,170 | $ | 2,451,557 | $ | 2,151,731 | ||||
| Product | 1,165,696 | 1,163,618 | 1,103,257 | |||||||
| Consolidated depreciation and amortization | $ | 2,932,866 | $ | 3,615,175 | $ | 3,254,988 | ||||
Identifiable assets by segment: | ||||||||||
| DIS | $ | 13,586,502 | $ | 14,710,088 | $ | 16,016,201 | ||||
| Product | 16,335,908 | 18,409,152 | 19,142,590 | |||||||
| Consolidated assets | $ | 29,922,410 | $ | 33,119,240 | $ | 35,158,791 | ||||
Sales to a distributor in Japan represented 2.2% of total revenues for the year ended December 31, 2001, sales to a customer in Puerto Rico represented less than 1% of total revenues for the years ended December 31, 2002 and 2003 and sales to a customer in Russia represented less than 3% of total revenues for the year ended December 31, 2003.
8. Employee Retirement Plan
The Company has a 401(k) retirement plan (the "Plan"), under which all full-time employees may contribute up to 20% of their annual salary, within limits. The Company may elect to make discretionary contributions upon the approval of the Board of Directors. Through December 31, 2003, the Company had not contributed to the Plan.
Shares

Common Stock
P R O S P E C T U S
Merrill Lynch & Co.
JPMorgan
Banc of America Securities LLC
William Blair & Company
, 2004
Through and including , 2004 (the 25th day after commencement of this offering), federal securities law may require all dealers selling our common stock, whether or not participating in this offering, to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
PART II—INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than the underwriting discount, payable by the registrant in connection with the sale of common stock being registered. All amounts are estimates except the Securities and Exchange Commission registration fee and the National Association of Securities Dealers Inc. filing fee.
| SEC registration fee | $ | 10,928 | |
| NASD filing fee | 9,125 | ||
| Nasdaq National Market application fee | 5,000 | ||
| Nasdaq National Market entry fee | 95,000 | ||
| Nasdaq National Market annual fee (prorated for 2004) | * | ||
| Accounting fees and expenses | * | ||
| Legal fees and expenses | * | ||
| Printing and engraving expenses | * | ||
| Blue sky fees and expenses | * | ||
| Transfer agent and registrar fees and expenses | * | ||
| Miscellaneous | * | ||
Total | $ | 120,053 |
Item 14. Indemnification of Directors and Officers
As permitted by Section 102 of the Delaware General Corporation Law, we have adopted provisions in our restated certificate of incorporation and restated bylaws, which will become effective following the completion of this offering, that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages or breach of fiduciary duty as a director, except for liability for:
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our restated certificate of incorporation also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permittedofficers are indemnified as provided by Section 145 of the Delaware General Corporation Law our restated bylaws provide that:
II-1
Our restated certificate of incorporation, attached as Exhibit 3.1 hereto, and our amended and restated bylaws, attached as Exhibit 3.2 hereto, provide for the indemnification provisions described above and elsewhere herein. In addition, webylaws. We have entered into separate indemnification agreements, a formagreed to indemnify each of which is attached as Exhibit 10.21 hereto, with our directors and officers which may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements require us, among other things, to indemnify our officers and directors against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified. In addition, we have purchased a policy of directors' and officers' liability insurance that insures our directors andcertain officers against the cost of defense, settlement or payment of a judgment in some circumstances. These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and directors forcertain liabilities, including reimbursement of expenses incurred, arisingliabilities under the Securities Act.
Reference is made to the following documents filed as exhibits to this registration statement regarding relevant indemnification provisions described above and elsewhere in this prospectus:
Item 15. Recent Sales of Unregistered Securities
The following list sets forth information regarding all securities we have sold since January 2001. All share amounts and per share prices reflect a 1-for-200 reverse stock split that was effected in October 2002 and a 1-for- reverse stock split to be effected prior to completion of this offering.
II-2
II-3
The offers, sales, and issuances of the securities described in paragraphs (1), (3) - (13) and (15) were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act because the issuance of securities to the recipients did not involve a public offering. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and warrants issued in such transactions. Each of the recipients of securities in the transactions described in paragraphs (1), (3) - (13) and (15) were accredited or sophisticated persons and had adequate access, through employment, business or other relationships, to information about us.
The offers, sales and issuances of the options and common stock described in paragraphs (2), (14) and (16) - (18) were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 because the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such options and common stock were our employees, directors or bona fide consultants and received the securities under our compensatory benefit plans or a contract relating to compensation. Appropriate legends were affixed to the share certificates issued in such transactions. Each of these recipients had adequate access, through employment or other relationships, to information about us.
There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
II-4
Item 16. Exhibits and Financial Statement Schedules
II-5
No financial statement schedules are provided because the information called for is not required or is shown either in the consolidated financial statements or the notes thereto.
II-6
The undersigned hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the DGCL, our restated certificate of incorporation or our restated bylaws, the underwriting agreementprovisions described above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than theour payment by us of expenses incurred or paid by one of our directors, officers,director, officer or controlling personsperson in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, hereunder, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
We hereby undertake that:

Digirad Corporation
Up to [●] Shares Common Stock
Up to [●] Pre-funded Warrants
Up to [●] Shares of Common Stock Underlying the Pre-funded Warrants
Common Warrants to Purchase up to [●] Shares of Common Stock and
Up to [●] Shares of Common Stock underlying the Common Warrants
PROSPECTUS
Sole Book-Running Manager
Maxim Group LLC
[●], 2020
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The estimated expenses payable by us in connection with the offering described in this registration statement (other than the underwriting discount and commissions) will be as follows:
| SEC Registration Fee | $ | 1,233 | ||
| FINRA filing fee | 1,386 | |||
| Accounting fees and expenses | 55,000 | |||
| Nasdaq listing fees | 45,000 | |||
| Printing and engraving expenses | 5,000 | |||
| Legal fees and expenses | 100,000 | |||
| Total | $ | 207,619 |
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Our certificate of incorporation provides that, to the fullest extent permitted by law, a director of Digirad Corporation (the “Corporation”) shall not be personally liable to the Corporation or to its stockholders for monetary damages for any breach of fiduciary duty as a director.
Article V of our certificate of incorporation also provides:
“(A) EXCULPATION. A director of the Corporation (each, a “Director” and collectively, the “Directors”) shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a Director, except for liability (i) for any breach of the Director’s duty of loyalty to the Corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the Delaware General Corporation Law or (iv) for any transaction from which the Director derived any improper personal benefit. If the Delaware General Corporation Law is hereafter amended to further reduce or to authorize, with the approval of the Corporation’s stockholders, further reductions in the liability of the Directors for breach of fiduciary duty, then a Director shall not be liable for any such breach to the fullest extent permitted by the Delaware General Corporation Law as so amended.
(B) INDEMNIFICATION. To the extent permitted by applicable law, the Corporation is also authorized to provide indemnification of (and advancement of expenses to) such agents (and any other persons to which Delaware law permits the Corporation to provide indemnification) through bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested Directors or otherwise, in excess of the indemnification and advancement otherwise permitted by Section 145 of the Delaware General Corporation Law, subject only to limits created by applicable Delaware law (statutory or non-statutory), with respect to actions for breach of duty to the Corporation, its stockholders and others.
(C) EFFECT OF REPEAL OR MODIFICATION. Any repeal or modification of any of the foregoing provisions of this Article V shall be prospective and shall not adversely affect any right or protection of a Director, officer, agent or other person existing at the time of, or increase the liability of any Director with respect to any acts or omissions of such Director occurring prior to, such repeal or modification.”
II-1
Furthermore, our bylaws provide (A) for indemnification of Directors as set forth above, and (B) indemnification of officers of the Corporation to the fullest extent permitted by the Delaware General Corporation Law.
Section 145 of the Delaware General Corporation Law also provides for indemnification of officers, directors, employees, and agents of Delaware corporations. It is set forth below:
“Section 145. Indemnification of officers, directors, employees and agents; insurance.
| (a) | A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that the person’s conduct was unlawful. |
| (b) | A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper. |
| (c) | To the extent that a present or former director or officer of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in subsections (a) and (b) of this section, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith. |
| (d) | Any indemnification under subsections (a) and (b) of this section (unless ordered by a court) shall be made by the corporation only as authorized in the specific case upon a determination that indemnification of the present or former director, officer, employee or agent is proper in the circumstances because the person has met the applicable standard of conduct set forth in subsections (a) and (b) of this section. Such determination shall be made, with respect to a person who is a director or officer of the corporation at the time of such determination: |
(1) For purposesBy a majority vote of determiningthe directors who are not parties to such action, suit or proceeding, even though less than a quorum; or
(2) By a committee of such directors designated by majority vote of such directors, even though less than a quorum; or
(3) If there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion; or
II-2
(4) By the stockholders.
| (e) | Expenses (including attorneys’ fees) incurred by an officer or director of the corporation in defending any civil, criminal, administrative or investigative action, suit or proceeding may be paid by the corporation in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such director or officer to repay such amount if it shall ultimately be determined that such person is not entitled to be indemnified by the corporation as authorized in this section. Such expenses (including attorneys’ fees) incurred by former directors and officers or other employees and agents of the corporation or by persons serving at the request of the corporation as directors, officers, employees or agents of another corporation, partnership, joint venture, trust or other enterprise may be so paid upon such terms and conditions, if any, as the corporation deems appropriate. |
| (f) | The indemnification and advancement of expenses provided by, or granted pursuant to, the other subsections of this section shall not be deemed exclusive of any other rights to which those seeking indemnification or advancement of expenses may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in such person’s official capacity and as to action in another capacity while holding such office. A right to indemnification or to advancement of expenses arising under a provision of the certificate of incorporation or a bylaw shall not be eliminated or impaired by an amendment to the certificate of incorporation or the bylaws after the occurrence of the act or omission that is the subject of the civil, criminal, administrative or investigative action, suit or proceeding for which indemnification or advancement of expenses is sought, unless the provision in effect at the time of such act or omission explicitly authorizes such elimination or impairment after such action or omission has occurred. |
| (g) | A corporation shall have power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s status as such, whether or not the corporation would have the power to indemnify such person against such liability under this section. |
| (h) | For purposes of this section, references to “the corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, shall stand in the same position under this section with respect to the resulting or surviving corporation as such person would have with respect to such constituent corporation if its separate existence had continued. |
| (i) | For purposes of this section, references to “other enterprises” shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to any employee benefit plan; and references to “serving at the request of the corporation” shall include any service as a director, officer, employee or agent of the corporation which imposes duties on, or involves services by, such director, officer, employee or agent with respect to an employee benefit plan, its participants or beneficiaries; and a person who acted in good faith and in a manner such person reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this section. |
| (j) | The indemnification and advancement of expenses provided by, or granted pursuant to, this section shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs, executors and administrators of such a person. |
| (k) | The Court of Chancery is hereby vested with exclusive jurisdiction to hear and determine all actions for advancement of expenses or indemnification brought under this section or under any bylaw, agreement, vote of stockholders or disinterested directors, or otherwise. The Court of Chancery may summarily determine a corporation’s obligation to advance expenses (including attorneys’ fees). |
II-3
We have entered into, and intend to continue to enter into, separate indemnification agreements with our directors, executive officers, and other key employees, in addition to the indemnification provided for in our certificate of incorporation and bylaws. We also have directors and officers insurance which includes insurance for claims against these persons brought under securities laws.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
On September 10, 2019, we issued 300,000 shares of our 10.0% Series A Cumulative Perpetual Preferred Stock, par value $0.0001 per share (“Series A Preferred Stock”) in a private placement (the “Private Placement”) to Lone Star Value Investors, LP for a price of $10 per share for total proceeds to us of $3 million. The Private Placement was made pursuant to the terms of a Stock Purchase Agreement, dated as of September 10, 2019 (the “SPA”). We used the proceeds from the Private Placement for the repayment of debt owed by a wholly-owned subsidiary. Lone Star Value Investors, LP is a significant holder of our common stock and our Series A Preferred Stock.
No placement agent or other financial intermediary was engaged or compensated in connection with the Private Placement. The issuance of shares of Series A Preferred Stock in the Private Placement was exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as amended (the “Act”), as sales by an issuer not involving a public offering. The foregoing issuance was not registered under the Act, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration afforded by Section 4(a)(2) and corresponding provisions of state securities laws, which exempts transactions by an issuer not involving any public offering. In each case, the issuance was made, without any general solicitation or advertising, to a limited number of sophisticated investors with knowledge and experience of financial and business matters related to an investment in our securities. In addition, the securities issued in the foregoing issuance are restricted securities bearing transfer restrictions and the recipient acquired such securities for its own account without a view to resell or distribute them. Such securities may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements and certificates evidencing such shares contain a legend stating the same. Accordingly, the foregoing issuance was subject to the private placement exemption from registration provided by Section 4(a)(2) of the Act.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
| (a) | The following exhibits are filed as part of this Registration Statement: |
| EXHIBIT INDEX |
II-4
II-5
II-6
II-7
II-8
II-9
II-10
II-11
ITEM 17. UNDERTAKINGS.
| (a) | The undersigned registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that paragraphs (i), (ii) and (iii) do not apply if the information omitted fromrequired to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the formSEC by the registrant pursuant to Section 13 or Section 15(d) of prospectus filed as partthe Securities Exchange Act of this1934 that are incorporated by reference in the registration statement, in reliance upon Rule 430A andor is contained in a form of prospectus filed by us pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be that is part of this Registration Statement as of the time it was declared effective; and
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initialbona fide offering thereof.
II-7
II-12
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (5) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| (A) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| (B) | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
| (6) | That for the purpose of determining any liability under the Securities Act of 1933 in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
II-13
| (b) | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (h) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
| (i) | The undersigned registrant hereby undertakes that: |
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fide offering thereof. |
II-14
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrantregistrant has duly caused this Registration Statementregistration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in San Diego, California,Suwanee, Georgia on thisthe 19th day of March, 2004.May, 2020.
| DIGIRAD CORPORATION | ||||
| Name: | Matthew G. Molchan | |||
| Title: | President and Chief Executive Officer | |||
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints David M. Sheehan, President and Chief Executive Officer, and Todd P. Clyde, Chief Financial Officer, and each of them, his true and lawful attorneys-in-fact and agents, with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:indicated.
Name |
| Date | ||||||||
| By: | /s/ Matthew G. Molchan | |||||||||
| Matthew G. Molchan | President, Chief Executive Officer and Director (Principal Executive Officer) | |||||||||
| By: | /s/ David J. Noble | |||||||||
| David J. Noble | Chief Financial Officer and Chief Operating Officer (Principal Financial and Accounting Officer) | |||||||||
II-8
/s/ Jeffrey E. Eberwein* | |||||
Jeffrey E. Eberwein | Chairman | ||||
II-9
Dimitrios J. Angelis | Director | May 19, 2020 | |||
| By: | /s/ Michael A. Cunnion* | ||||
| Michael A. Cunnion | Director | May 19, 2020 |
II-15
| By: | /s/ John W. Sayward* | ||||
| John W. Sayward | Director | May 19, 2020 | |||
| By: | /s/ Mitch I. Quain* | ||||
| Mitch I. Quain | Director | May 19, 2020 | |||
| *By: | /s/ Matthew G. Molchan | May 19, 2020 | |||
Matthew G. Molchan | |||||
Attorney-in-fact |
II-16