| Large accelerated filer | Accelerated filer | ||
| Non-accelerated filer | Smaller reporting company | ☒ | |
| (do not check if a smaller reporting company) | Emerging Growth Company | ☐ | |
________________________
| Title of Each Class of Securities to be Registered (1) | Proposed Maximum Aggregate Offering Price (2) | Amount of Registration Fee (3) | |||||
| Series D preferred stock, par value $0.001 | $ | $ | |||||
| Warrants to purchase shares of common stock (4) | $ | $ | |||||
| Shares of common stock issuable upon conversion of Series D preferred stock | $ | $ | |||||
| Shares of common stock issuable upon exercise of the Warrants | $ | $ | |||||
| Total: | $ | 5,000,000 | $ | 503.50 | |||
Title of Each Class of securities to be registered | Numbert of shares of common stock to be registered (1) | Proposed Maximum Offering Price Per Share (2) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee (3) |
| Common Stock | 70,000,000 | $0.0075 | $525,000 | $65.36 |
| (1) | In |
| (2) | Based on the average of the lowest two (2) volume weighted average trading prices of the Company’s common stock during the fifteen (15) consecutive trading day period immediately preceding the filing of this Registration Statement of approximately $0.0075. The shares offered, hereunder, may be sold by the selling stockholder from time to time in the open market, through privately negotiated transactions, or a combination of these methods at market prices prevailing at the time of sale or at negotiated prices. |
| (3) | The fee is calculated by multiplying the aggregate offering amount by ..00012450, pursuant to Section 6(b) of the Securities Act of 1933. |
The registrant
PRELIMINARY PROSPECTUS
Subject to completion, dated July 1, 2016
$5,000,000

5,000
Warrants to Purchaseshares of its common stock, which will consist of up to _________ Shares of Common Stock
_________ Shares of Common Stock Underlying the Series D Preferred Stock
_________ Shares of Common Stock Underlying the Warrants
________________________
We are offering up to 5,000 shares of our Series D convertible preferred stock, together with warrants to purchase __________70,000,000 shares of common stock atto be sold by GHS Investments LLC (“GHS”) pursuant to an Equity Financing Agreement (the “Financing Agreement”) dated March 1, 2018. If issued presently, the 70,000,000 of common stock registered for resale by GHS would represent 24.89% of our issued and outstanding shares of common stock as of June 1, 2018.
Subject to certain ownership limitations, the Series D preferred stock will be convertible at any timeprevailing market prices at the holder’s option intotime of sale, at varying prices, or at negotiated prices.
be underwriting commissions or discounts under the Securities Act of 1933.
| Per Share/Warrant | Total | ||||||
| Offering Price | $ | 1,000 | $ | 5,000,000 | |||
| Placement Agent’s Fees (1) | $ | 90 | $ | 450,000 | |||
| Offering Proceeds, Before Expenses | $ | 910 | $ | 4,550,000 | |||
_____________________________
We have retained Ladenburg Thalmann & Co., Inc. to act as our exclusive placement agent in connection with this offering, and to use its “best efforts” to solicit offers to purchase thethere has been a very limited market for our securities. The placement agent is not required to sell any specific number or dollar amount of securities but will use its best efforts to sell the securities offered. This best-efforts offering does not have a minimum purchase requirement and therefore is not certain to raise any specific amount.
Investing inWhile our common stock involvesis on the OTC Markets, there has been negligible trading volume. There is no guarantee that an active trading market will develop in our securities.
5. Neither the Securities and Exchange Commission or SEC, nor any state securities commission has approved or disapproved of these securities or passed upon the securities that may be offered underaccuracy or adequacy of this prospectus, nor have any of these organizations determined if this prospectus is truthful or complete.prospectus. Any representation to the contrary is a criminal offense.
Ladenburg Thalmann

TABLE OF CONTENTS
| 5 | |
| 22 | |
| | |
| Item 6. Dilution | 22 |
| Item 7. Selling Security Holder | 22 |
| Item 8. Plan of Distribution | 24 |
| Item 9. Description of Securities to be Registered | 25 |
| Item 10. Interests of Named Experts and Counsel | 27 |
| Item 11. Information with Respect to the Registrant | 27 |
| Item 12. Incorporation of Certain Information by Reference. | 43 |
| Item 13. Other Expenses of Issuance and Distribution | 99 |
| Item 14. Indemnification of Officers and Directors | 99 |
| Item 15. Recent Sales of Unregistered Securities | 99 |
| Item 16. Exhibits and Financial Statement Schedules. | 100 |
| Item 17. Undertakings. | 103 |
| Financial Statements | 46 |
ABOUT THIS PROSPECTUS
changed since those dates. The termsselling stockholders are offering to sell and seeking offers to buy shares of our common stock only in jurisdictions where offers and sales are permitted.
FORWARD-LOOKING STATEMENTS
Statements in this prospectus, which express “belief,” “anticipation” or “expectation,” as well as other statements that are not historical facts, are forward-looking statements. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from historical results or anticipated results, including those identified, a Delaware corporation.
Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by which, such performance or results will be achieved. Forward-looking information is based on information available at the time and/or management’s good faith belief with respect to future events, and is subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in the statements.
Forward-looking statements speak only as of the date the statements are made. We assume no obligation to update forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting forward-looking information except to the extent required by applicable securities laws. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect thereto or with respect to other forward-looking statements.
SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and may not contain all of the information that may be important to you. We urge you to read the entire prospectus, carefully, including the ‘‘Risk Factors’’ section, beforefinancial statements and their explanatory notes under the Financial Statements prior to making an investment decision. Unless otherwise noted, all share and per share data in this prospectus give effect to the 1:___ reverse stock split of our common stock implemented on ___________, 2016, and is based on 67,901,547 pre-reverse split shares outstanding as of June 15, 2016. For more information about our reverse stock split, see “Recent Developments.”
Our Company
Our single-use cervical guides are manufactured by a vendor that specializes in injection molding of plastic medical products. On January 22, 2017, we entered into a license agreement with Shandong Yaohua Medical Instrument Corporation (“SMI”) pursuant to which we granted SMI an exclusive global license to manufacture the LuViva device and related disposables (subject to a carve-out for manufacture in Turkey).
2016, respectively.
Since our inception, we have raised capital through the public and private sale of debt and equity, funding from collaborative arrangements, and grants.
Our prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. We have experienced operating losses since our inception and, as of March 31, 2016 we have an accumulated deficit of about $122.9 million. To date, we have engaged primarily in research and development efforts and the early stages of marketing our products. We do not have significant experience in manufacturing, marketing or selling our products. We may not be successful in growing sales for our products. Moreover, required regulatory clearances or approvals may not be obtained in a timely manner, or at all. Our products may not ever gain market acceptance and we may not ever generate significant revenues or achieve profitability. The development and commercialization of our products requires substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We expect our operating losses to continue through at least the end of 2016 as we continue to expend substantial resources to complete commercialization of our products, obtain regulatory clearances or approvals, build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development.
Our product revenues to date have been limited. In 2015 and 2014, the majority of our revenues were from the sale of LuViva devices and disposables, as well as some revenue from grants from the NIH and licensing agreement fees received. We expect that the majority of our revenue in 2016 will be derived from revenue from the sale of LuViva devices and disposables.
Recent Developments
Reverse Stock Split. On May 18, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a reverse stock split by a ratio of not less than 1:10
Warrant Restructuring.Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 115,237,788 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), we issued or are obligated to issue 13,517,342 shares of common stock and new warrants that, if exercised as of June 14, 2016, would be exercisable for an aggregate of 214,189,622 shares of common stock.
In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), we and the holder further agreed that we will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.
The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volume of the common stock for that trading day. The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of this offering. The new warrants will have an initial exercise price equal to the exercise price of the surrendered warrants as of immediately prior to consummation of this offering, subject to customary “downside price protection” for as long as our common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.
| Shares currently outstanding: | 211,291,990 | |
| |
Shenghuo License Agreement.On June 5, 2016, we entered into a license agreement with Shenghuo Medical, LLC pursuant to which we granted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. Under the terms of the license agreement, once Shenghuo is capable of manufacturing LuViva in accordance with ISO 13485 for medical devices, Shenghuo will pay us a royalty equal to $2.00 or 20% of the distributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories.
In connection with the license grant, Shenghuo will underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also will provide up to $1.0 million in furtherance of our efforts to secure regulatory approval for LuViva from the U.S. Food and Drug Administration, in exchange for the right to receive payments equal to 2% of our future sales in the United States, up to an aggregate of $4.0 million.
Pursuant to the license agreement, Shenghuo has the option, after our next annual meeting of stockholders, to have a designee appointed to our board of directors.
As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of our common stock at a conversion price per share of $0.017402, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. We will also issue Shenghuo a five-year warrant exercisable immediately for approximately 13.8 million shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment.
Short Term Promissory Notes.On May 4, 2016 and May 26, 2016, Aquarius Opportunity Fund advanced us a total of $87,500 for 2% simple interest notes due the earlier of December 31, 2016 or consummation of this offering. Also on May 26, 2016, GPB Debt Holdings II LLC, holder of our outstanding senior secured convertible note, advanced as an additional $87,500, on the same terms as their note. We intend to offer each of them the opportunity to participate in the offering at least up to the extent of the outstanding principal and interest on these cash advances, by extinguishing all or a portion of the debt on a dollar-for-dollar basis.
Series C Exchanges.Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock.
The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.” See “Description of Securities” for a description of the material terms of the Series C1 preferred stock.
Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 722,211 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, Aquarius, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described in “—Recent Developments.”
| Shares being offered: | 70,000,000 | |
| |
The Offering
| Offering | The selling stockholders may sell all or a portion of | |
| Use of | We will not receive any proceeds from the | |
| OTC Markets Symbol: | GTHP | |
| Risk | See “Risk Factors” beginning on page |
| stock. |
Year Ended March 31, 2018 | Year Ended December 31, 2017 | Year Ended December 31, 2016 | |
| Cash | $76 | $1 | $14 |
| Total Assets | 527 | 489 | 1,492 |
| Total Liabilities | 18,465 | 19,891 | 10,758 |
| Total Stockholder’s Equity (Deficit) | (17,938) | (19,402) | (9,266) |
Three Months Ended March 31, 2018 | Three Months Ended March 31, 2017 | Year Ended December 31, 2017 | Year Ended December 31, 2016 | |
| Revenue | 4 | 21 | 244 | 605 |
| Total Expenses | 380 | 535 | 3,365 | 4,425 |
| Total Other income (expense) | 1,458 | 407 | (7,575) | (150) |
| Net Income (Loss) for the Period | 1,082 | (107) | (10,696) | (3,970) |
| Net Loss per Share | 0.012 | (0.22) | (1.29) | (24.62) |
Your
Risks RelatedPrivate Securities Litigation Reform Act of 1995 is not available to this Offering
As a new investor, you will incur substantial dilutionus as a result of this offering and future equity issuances.
Our net tangible book value deficiency as of March 31, 2016 was approximately $7.3 million, or $1.37 per share of common stock. Net tangible book value per share represents total tangible assets less total liabilities, divided by the number of shares of common stock outstanding. On a pro form basis after giving effect to the assumed sale of 5,000 shares of Series D preferred stock in this offering at a public offering price of $1,000 per share, and assuming the conversion of all the shares of Series D preferred stock sold in the offering at an assumed conversion price of $0.01 per share, which was the last reported sale price of our common stock on June 15, 2016 (and excluding shares of common stock issuable upon exercisenon- reporting issuer. Further, Section 27A(b)(2)(D) of the warrants),Securities Act and after deducting estimated placement agent fees and estimated offering expenses payable by us, if you purchase securities in this offering, you will suffer immediate and substantial dilution of approximately $0.006 per share in the net tangible book valueSection 21E(b)(2)(D) of the common stock underlyingSecurities Exchange Act expressly state that the Series D preferred stock that you acquire. See “Dilution”safe harbor for a more detailed discussion of the dilution you will incur if you participateforward looking statements does not apply to statements made in thisconnection with an initial public offering. In addition to this offering, subject to market conditions and other factors, it is likely that we will pursue additional capital to finance our operations. Accordingly, we may conduct substantial future offerings of equity securities. The exercise of outstanding options and warrants and future equity issuances, including future public offerings or future private placements of equity securities, would result in further dilution to investors.
The actual offering amount, the offering price and the net proceeds to us, if any, in this offering may be substantially less than the amounts set forth above.
Because there is no minimum offering amount or offering price required as a condition to closing in this offering, the actual public offering amount, offering price and net proceeds to us, if any, in this offering are not presently determinable and may be substantially less than the amounts set forth above. We are not required to sell any specific number or dollar amount of the securities offered in this offering, but the placement agent will use its best efforts to sell the securities offered.
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock.
If our stockholders (including those persons who may become stockholders upon conversion of outstanding convertible securities or exercise of outstanding warrants or options) sell substantial amounts of our common stock, or the public market perceives that stockholders might sell substantial amounts of our common stock, the market price of our common stock could decline significantly. Such sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that our management deems appropriate.
We will have broad discretion over the use of the proceeds of this offering and may not realize a return.
We will have considerable discretion in the application of the net cash proceeds of this offering. We intend to use the net cash proceeds to increase inventory of LuViva to meet current demand for the product, and to support general working capital and operations. However, we will retain broad discretion over the use of the net proceeds and may use the money for other corporate purposes. We may use the net cash proceeds for purposes that do not yield a significant return, if any, for our stockholders.
There is no public market for the Series D preferred stock or warrants being offered in this offering.
There is no established public trading market for the Series D preferred stock or warrants being offered in this offering. We do not expect a market to develop for the Series D preferred stock or the warrants. In addition, we do not intend to apply for listing of the Series D preferred stock or warrants on any securities exchange or expect the Series D preferred stock to trade on the OTCQB marketplace, although we will use our best efforts to have the warrants quoted on the OTCQB marketplace on or before the closing. Without an active market, the liquidity of the Series D preferred stock and warrants will be limited.
If we cannot obtain additional funds or achieve profitability, we may not be able to continue as a going concern.
| Accumulated deficit, from inception to 12/31/ | $ | |
| Preferred dividends | $ | |
| Net Loss for fiscal year | $ | |
| Accumulated deficit, from inception to 12/31/ | $ | |
| Preferred dividends | $ | |
| Net Loss for | $ | |
| Accumulated deficit, from inception to 12/31/ | $138.5 million | |
| Preferred dividends | ||
| Net Income for | $ | |
| Accumulated deficit, from inception to 3/31/ | $ |
2018. 2018. split. However, please refer to Footnote 11 - CONVERTIBLE DEBT IN DEFAULT in the paragraph: Debt Restructuring for more information regarding our warrants. common stock, which consists of shares of common stock to be sold by GHS pursuant to the Financing Agreement. If issued presently, the shares of common stock registered for resale by GHS would represent 24.89% of our issued and outstanding shares of common stock as of June 1, 2018. Expected % of Net Cash Proceeds Warrants Exercise Price Per Share Expiration Date We are a medical technology company focused on developing innovative medical devices that have the potential to improve healthcare. Our primary focus is the sales and marketing of our LuViva® Advanced Cervical Scan non-invasive cervical cancer detection device. The underlying technology of LuViva primarily relates to the use of biophotonics for the non-invasive detection of cancers. LuViva is designed to identify cervical cancers and precancers painlessly, non-invasively and at the point of care by scanning the cervix with light, then analyzing the reflected and fluorescent light. London in 2015 and at the Indonesian National Obstetrics and Gynecology (POGI) Meeting in Solo in 2016. 2018. On January 22, 2017, we entered into a license agreement with Shandong Yaohua Medical Instrument Corporation (“SMI”) pursuant to which we granted SMI an exclusive global license to manufacture the LuViva device and related disposables (subject to a carve-out for manufacture in Turkey). CFDA. questions. 30092. 1, 2018 was 148. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. Plan category Number of securities to be issued upon exercise of outstanding options, warrants and rights Weighted-average exercise price of outstanding options, warrants and rights Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) 104,356 $45.00 26,616 - - - Capital Resources 2017. $5,214, unamortized and debt issuance costs of $11,160 for the period ended December 31, 2017. the period ended December 31, 2017. 2018, we had a net debt of $76,626, including unamortized debt issuance costs of $11,672 and unamortized discount of $6,890. Based on discussions with our distributors, we expect to generate purchase orders for approximately $2 million in LuViva devices and disposables in 2018, and expect those purchase orders to result in actual sales of $1.5 million in 2018, representing what we view as current demand for our products. We cannot be assured that we will generate all or any of these additional purchase orders, or that existing orders will not be canceled by the distributors or that parts to build product will be available to meet demand, such that existing orders will result in actual sales. Because we have a short history of sales of our products, we cannot confidently predict future sales of our products beyond this time frame, and cannot be assured of any particular amount of sales. Accordingly, we have not identified any particular trends with regard to sales of our products. statements contained in our annual report on Form 10-K for the year ended December 31, 2017. PROMOTERS, AND CONTROL PERSONS If we amend our code of ethics, other than a technical, administrative or non-substantive amendment, or we grant any waiver, including any implicit waiver, from a provision of the code that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, we will disclose the nature of the amendment or waiver on our website, www.guidedinc.com, under the “Investor Relations” tab under the tab “About Us.” Also, we may elect to disclose the amendment or waiver in a report on Form 8-K filed with the Securities and Exchange Commission. Name and Principal Position Year Salary ($) Bonus ($) Option Awards ($)(1) Total ($) Gene S. Cartwright, Ph.D. President, CEO, Acting CFO and Director (2) Mark Faupel, Ph.D. Former President, CEO and Acting CFO(3) Richard Fowler, Senior Vice President of Engineering 2015 2014 243,000 203,000 - - $30,880 17,000 273,880 220,000 Name and Principal Position Number of Securities Underlying Options Exercisable (#)(1) Number of Securities Underlying Options Un-exercisable (#) Equity Incentive Plan Awards: Number of Securities Under- lying Unexercised Unearned Options (#) Option Exercise Price ($)(2) Option Expiration Date Gene S. Cartwright, Ph.D. President, CEO, Acting CFO and Director Mark Faupel, Ph.D. Former President, CEO & Acting CFO Richard Fowler Senior Vice President of Engineering Option Awards (#) Exercise Price ($) Name and Address of Beneficial Owner Percent of Class(2) PERSONS Based on the definition of independence of the NASDAQ Stock Market, the board has determined that Mr. James and directors. We BASIC AND DILUTED NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS (post 1:100 reverse stock split) Preferred Stock Series B Preferred Stock Series C Common Stock Additional Paid-In Treasury Accumulated Preferred Stock Series B Preferred Stock Series C Common Stock Additional Paid-In Treasury Accumulated BASIS OF PRESENTATION 2017. 2018. SIGNIFICANT ACCOUNTING POLICIES The Company uses the Monte Carlo simulations and binomial calculations in the calculation of the fair value of the warrant liabilities and the valuation of embedded conversion options and freestanding warrants. All intercompany transactions are eliminated. guidance. the balance sheet as a direct deduction from the carrying amount of the debt liability consistent with the debt discount. As of December 31, presented under the new revenue standard, while prior period amounts are not adjusted and continue to be reported in accordance with our historic accounting under Topic 605. 2018. During the three months ended March 31, 2017, there were revenues from one distributor, that totaled approximately $21,000. As of March 31, 2018, and December 31, 2017, the Company has received prepayments for devices and disposables recorded as deferred revenue in the amount of $28,000 and $21,000, respectively. 2017: December 31, 2015 December 31, 2014 December 31, 2014 Fair Value Measurements Using Significant Unobservable Inputs (Level 3) December 2014 Public Offering ExchangedWarrants Series C Warrants STOCKHOLDERS’ DEFICIT respectively. Between June 13, 2016 and June 14, 2016, the Company entered into various agreements with holders of the Company’s “Series B Tranche B” warrants, pursuant to which each holder separately agreed to exchange the warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. $640. issued during the period ended March 31, 2018. Weighted Average Exercise Warrants (Underlying Shares) Exercise Price Per Share Expiration Date customary anti-dilution adjustment. On Year Ended December 31, Year Ended December 31, promissory note for an aggregate purchase price of $135,000 (representing a $15,000 original issue discount). On March 20, 2018, the Company issued the note to Auctus. Pursuant to the purchase agreement, the Company also issued to Auctus a warrant exercisable to purchase an aggregate of 3,409,090 shares of the Company’s common stock. The warrant is exercisable at any time, at an exercise price per share equal to $0.00228 (110% of the closing price of the common stock on the day prior to issuance), subject to certain customary adjustments and price-protection provisions contained in the warrant. The warrant has a five-year term. The note matures nine months from the date of issuance and, in addition to the original issue discount, accrues interest at a rate of 12% per year. The Company could have prepaid the note, in whole or in part, for 115% of outstanding principal and interest until 30 days from issuance, for 125% of outstanding principal and interest at any time from 31 to 60 days from issuance, and for 130% of outstanding principal and interest at any time from 61 days from issuance to 180 days from issuance. After six months from the date of issuance, Auctus may convert the note, at any time, in whole or in part, into shares of the Company’s common stock, at a conversion price equal to the lower of the price offered in the Company’s next public offering or a 40% discount to the average of the two lowest trading prices of the common stock during the 20 trading days prior to the conversion, subject to certain customary adjustments and price-protection provisions contained in the note. The note includes customary events of default provisions and a default interest rate of 24% per year. Upon the occurrence of an event of default, Auctus may require the Company to redeem the note (or convert it into shares of common stock) at 150% of the outstanding principal balance plus accrued and unpaid interest. As of March 31, 2018, the Company has net debt of $99,000 including unamortized debt issuance costs of $31,500 and reduction related to the allocated value of the warrants of $19,500. GUIDED THERAPEUTICS INC. AND SUBSIDIARY FOR THE THREE MONTHS ENDED MARCH 31, PREFERRED STOCK DIVIDENDS NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS GUIDED THERAPEUTICS INC. AND SUBSIDIARY FOR THE THREE MONTHS ENDED MARCH 31, March 31, 2016 Fair Value at December 31, 2015 Fair Value Measurements Using Significant Unobservable Inputs (Level 3) $22,000. As of March 31, Warrants Exercise Price Expiration Date Weighted Average Exercise IN DEFAULT accredited investors of its portion of the note of to a third accredited investor. The balance was reduced by $306,863 as part of a debt restructuring on December 7, 2016. GPB. year. At December 31, 2017, there were 4,312 shares outstanding with a conversion price of $0.017 per share, such that each share of Series C preferred stock would convert into approximately 58,824 shares of the Company’s common stock. As of December 31, 2017, the Company had issued 14,766 shares of common stock and rights to common stock shares for 2,131. In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volume of the common stock for that trading day. The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of case. EXHIBIT NO. DESCRIPTION June Chairman of the Board and Director June Directorapprovals,approvals; build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development. The further development and commercialization of our products will require substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We have only generated limited revenues from product sales. Our accumulated deficit was approximately $122.9$137.5 million at March 31, 2016.maintainundergo an inspection and re-file for ISO 13485:2003 certification and the CE mark certification,Mark, which is an international symbol of quality and compliance with applicable European medical device directives. Failure to maintain ISO 13485:2003 certification or CE mark certification or other international regulatory approvals would prevent us from selling in some countries in the European Union.our ability to sellus from selling our products domestically.·we, or any collaborative partner, will make timely filings with the FDA;·the FDA will act favorably or quickly on these submissions;·we will not be required to submit additional information or perform additional clinical studies; or·we will not face other significant difficulties and costs necessary to obtain FDA clearance or approval. 7customersdistributors and any reduction, delay or cancellation of an order from these customersdistributors or the loss of any of these customersdistributors could cause our revenue to decline.customersdistributors that have accounted for substantially all of our limited revenues. As a result, the termination of a purchase order with any one of these customersdistributors may result in the loss of substantially all of our revenues. We are constantly working to develop new relationships with existing or new customers,distributors, but despite these efforts we may not be successful at generating new orders to maintain similar revenues as current purchase orders are filled. In addition, since a significant portion of our revenues is derived from a relatively few customers,distributors, any financial difficulties experienced by any one of these customers,distributors, or any delay in receiving payments from any one of these customers,distributors, could have a material adverse effect on our business, results of operations, financial condition and cash flows.customersdistributors outside of the United States. We expect that substantially all of our business will continue to come from sales in foreign markets, through increased penetration in countries where we currently sell LuViva, combined with expansion into new international markets. However, international sales are subject to a number of risks, including:customers,distributors, and if these distributors terminate their relationships with us or under-perform, we may be unable to maintain or increase our level of international revenue. We will also need to engage additional international distributors to grow our business and expand the territories in which we sell LuViva. Distributors may not commit the necessary resources to market, sell and service LuViva to the level of our expectations. If current or future distributors do not perform adequately, or if we are unable to engage distributors in particular geographic areas, our revenue from international operations will be adversely affected 82016,2018, we have been issued, or have rights to, 24 U.S. patents (including those under license). In addition, we have filed for, or have rights to, six U.S. patents (including those under license) that are still pending. There are additional international patents and pending applications. One or more of the patents we hold directly or license from third parties, including those for our cervical cancer detection products, may be successfully challenged, invalidated or circumvented, or we may otherwise be unable to rely on these patents. These risks are also present for the process we use or will use for manufacturing our products. In addition, our competitors, many of whom have substantial resources and have made substantial investments in competing technologies, may apply for and obtain patents that prevent, limit or interfere with our ability to make, use and sell our products, either in the United States or in international markets. 9 10includingwhich is considered ordinary course accounts payablepayables and accrued payroll liabilities, was $4.0$5.1 million at March 31, 2016. We currently are required to make approximately $21,000 in monthly payments of interest and, beginning August 2016, $239,583 in quarterly payments of principal on our outstanding senior secured convertible note. The outstanding principal and accrued interest on our secured promissory note is due on August 31, 2016. In connection with the Shenghuo license agreement, we have agreed to issue Shenghuo a convertible note in principal amount of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016.·we may be unable to obtain additional financing to fund working capital, operating losses, capital expenditures or acquisitions on terms acceptable to us, or at all;·the amount of our interest expense may increase if we are unable to make payments when due;·our assets might be subject to foreclosure if we default on our secured debt (see “—We have outstanding debt that is collateralized by a general security interest in all of our assets, including our intellectual property. If we were to fail to repay the debt when due, the holders would have the right to foreclose on these assets.”);·our vendors or employees may, and some have, instituted proceedings to collect on amounts owed them;·we have to use a substantial portion of our cash flows from operations to repay our indebtedness, including ordinary course accounts payable and accrued payroll liabilities, which reduces the amount of money we have for future operations, working capital, inventory, expansion, or general corporate or other business activities; and·we may be unable to refinance our indebtedness on terms acceptable to us, or at all.15, 2016,1, 2018, we had notes outstanding that are collateralized by a security interest in our current and future inventory and accounts receivable. We also had a note outstanding that is collateralized by a security interest in all of our assets, including our intellectual property. When the debt is repaid, the holders’ security interests on our assets will be extinguished. However, if an event of default occurs under the notes prior to their repayment, the holders may exercise their rights to foreclose on these secured assets for the payment of these obligations. Under “cross-default” provisions in each of the notes, an event of default under one note is automatically an event of default under the other notes. Any such default and resulting foreclosure would have a material adverse effect on our business, financial condition and results of operations.Only our Chief Executive Officer and our Senior Vice President of Engineering have employment contracts with us, and none of our employees are covered by key person or similar insurance. In addition, if we are able to successfully develop and commercialize our products, we will need to hire additional scientific, technical, marketing, managerial and finance personnel. We face intense competition for qualified personnel in these areas, many of whom are often subject to competing employment offers. 11We are significantly influenced by our directors, executive officers and their affiliated entities.Our directors, executive officers and entities affiliated with them controlled 15.31 %, of the voting power of our outstanding common stock as of June 15, 2016. These stockholders, acting together, would be able to exert significant influence on substantially all matters requiring approval by our stockholders, including the election of directors and the approval of mergers and other business combination transactions.9.05.0 million shares of preferred stock. Our undesignated shares of preferred stock may be issued in one or more series, the terms of which may be determined by the board without further stockholder action. These terms may include, among other terms, voting rights, including the right to vote as a series on particular matters, preferences as to liquidation and dividends, repurchase rights, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of our common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with or sell assets to a third party. The ability of our board to issue preferred stock could make it more difficult, delay, discourage, prevent or make it more costly to acquire or effect a change in control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock.Our board of directors has asked our stockholders to approveproposal to authorize our board to effect a1:800 reverse stock split of all of our issued and outstanding common stock.stock was implemented. There are risks associated with a reverse stock split, if it is effected.May 18,November 7, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a 1:800 reverse stock split byof all of our issued and outstanding common stock was implemented. As a ratioresult of not less than 1:10 and not more than 1:400, with no change in the number of authorizedreverse stock split, every 800 shares of ourissued and outstanding common stock and allwere converted into 1 share of common stock. All fractional shares created by the reverse stock split to bewere rounded to the nearest whole share. On May 26, 2016, our largest stockholder, John Imhoff, who is also oneThe number of our directors, agreed to vote hisauthorized shares of common stock in favor of the reverse stock split. As of the record date for the 2016 annual meeting, Dr. Imhoff held 10,361,179 shares, or 19.26%, of the outstanding shares of common stock.We intend to effect the reverse stock split prior to the consummation of this offering; however, there are no assurances that the reverse stock split will be implemented. If the reverse stock split is effected, theredid not change.·We would have additional authorized shares of common stock that the board could issue in future without stockholder approval, and such additional shares could be issued, among other purposes, in financing transactions or to resist or frustrate a third-party transaction that is favored by a majority of the independent stockholders. This could have an anti-takeover effect, in that additional shares could be issued, within the limits imposed by applicable law, in one or more transactions that could make a change in control or takeover of us more difficult.·There can be no assurance that the reverse stock split, if completed, will achieve the benefits that we hope it will achieve. The total market capitalization of our common stock after the reverse stock split may be lower than the total market capitalization before the reverse stock split.will bewere outstanding immediately following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increasehave increased the number of stockholders who own odd lots of our common stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales. 12(excluding the convertible stock or warrants in this offering) is substantial.15, 2016,1, 2018, our outstanding convertible debt was convertible into an aggregate of 151,583,8431,240,341,381 shares of our common stock, and the outstanding shares of our Series C and Series C1 preferred stock were convertible into an aggregate of 433,385,611747,804,361 shares of common stock. Also, as of that date we had warrants outstanding that were exercisable for an aggregate of 233,615,051667,513,881 shares, contractual obligations to issue 5,205,1012,132 shares, and outstanding options to purchase 104,356116 shares. The shares of common stock issuable upon conversion or exercise of these securities would have constituted approximately 91.8 %92.6% of the total number of shares of common stock then issued and outstanding.(including the convertible stock and warrants in this offering), will dilute the ownership interests of our existing stockholders.”for a portion of our convertible debt orand preferred stock, and the exercise price for certain of our Series C preferred stockwarrants, will dilute the ownership interests of our existing stockholders. and the preferred stock part of this offering, any dividends we choose to pay in shares of our common stock will be calculated based on the then-current market price of our common stock. Accordingly, if the market price of our common stock decreases, the number of shares of our common stock issuable upon conversion of the convertible debt or upon payment of dividends on our outstanding Series C preferred stock and preferred stock part of this offering will increase, and may result in the issuance of a significant number of additional shares of our common stock.(including the preferred stock part of this offering) and certain of our convertible notes and outstanding warrants, the conversion price or exercise price will be lowered if we issue common stock at a per share price below the then conversionthen-conversion price or then-exercise price for those securities. Reductions in the conversion price or exercise price would result in the issuance of a significant number of additional shares of our common stock upon conversion or exercise, which would result in dilution in the value of the shares of our outstanding common stock and the voting power represented thereby.·we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume; and·stock analysts, stock brokers and institutional investors may be risk-averse and be reluctant to follow a company such as ours that faces substantial doubt about its ability to continue as a going concern or to purchase or recommend the purchase of our shares until such time as we became more viable. 13customersdistributors or accredited investors. Penny stocks are generally defined to be an equity security that has a market price of less than $5.00 per share. For transactions covered by the rule, the broker-dealer must make a special suitability determination for the purchaser and receive the purchaser’s written agreement to the transaction prior to the sale. Consequently, the rule may affect the ability of broker-dealers to sell our securities and also may affect the ability of our stockholders to sell their securities in any market that might develop.·control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer;·manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases;·“boiler room” practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons;·excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and·the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. 14We estimate thatnet cash proceeds to us from the sale of the securities offered by this prospectus, excludingShares for general corporate and working capital purposes and acquisitions or assets, businesses or operations or for other purposes that the proceeds, if any, fromBoard of Directors, in good faith deem to be in the exercisebest interest of the warrants included in the offering, will be approximately $4.26 million, assuming the sale by us of 5,000 shares of Series D preferred stock and warrants atCompany. assumed public offering price of $1,000 per share and after deductingfor the estimated placement agent fees and estimated offering expenses payable by us. However, this is a best efforts offering with no minimum, and weshares registered hereunder, as the only shares being registered are those sold pursuant to the GHS Financing Agreement. GHS may not sell all or anya portion of the securities; as a result, weshares being offered pursuant to this prospectus at fixed prices and prevailing market prices at the time of sale, at varying prices or at negotiated prices.receive significantly less in net cash proceeds,offer and the net cash proceeds received may not be sufficient to continue to operate our business. In addition, expected net proceeds include a non-cash benefit for the extinguishment ofsell up to $175,000 in outstanding principal and interest on simple interest notes from certain70,000,000 shares of our investors.intendmay require the selling stockholder to apply any proceeds received in connection withsuspend the offering to increase inventory of LuViva to meet current demand for the product, and to support general working capital and operations. However, we will retain broad discretion over the usesales of the net proceeds and may useshares of our common stock being offered pursuant to this prospectus upon the money for other corporate purposes.The following table summarizes our currently estimated and intended useoccurrence of net cash proceeds if we receive the maximum amount of proceeds to be potentially obtainedany event that makes any statement in this offering, which we expect would provide funding for our operations for approximately 24 months. prospectus or the related registration statement untrue in any material respect or that requires the changing of statements in those documents in order to make statements in those documents not misleading.dataselling stockholder identified in the table set forth below excludesmay from time to time offer and sell under this prospectus any proceeds we could receive from the exerciseor all of the warrantsshares of common stock described under the column “Shares of Common Stock Being Offered” in the table below.issued in this offering.Expected Use for Net Cash Proceeds Expected Amount of Net Cash Proceeds
($ in 000s) Increase inventory of LuViva advanced cervical device $1,750,000 41% General working capital and operations 2,510,000 59% Total $4,260,000 100% Ifan underwriter within the net cash proceeds of this offering are less than the maximum, we except to divide the proceeds for purposes listed above on a similar percentage basis.If a warrant holder elects to exercise the warrants issued in this offering, we may also receive proceeds from the exercisemeaning of the warrants.Securities Act. Any profits realized by such selling stockholder may be deemed to be underwriting commissions.predict when or ifgive an estimate as to the warrants will be exercised. It is possible that the warrants may expire and may never be exercised.DILUTIONOur net tangible book value deficiency as of March 31, 2016 was approximately $7.3 million, or $1.37 per share of common stock. “Net tangible book value” represents total tangible assets less total liabilities. “Net tangible book value per share” represents net tangible book value divided by the total number of shares of common stock outstanding.After giving effect tothat will actually be held by the assumed saleselling stockholder upon termination of 5,000this offering, because the selling stockholders may offer some or all of the common stock under the offering contemplated by this prospectus or acquire additional shares of Series D preferred stock and accompanying warrantscommon stock. The total number of shares that may be sold, hereunder, will not exceed the number of shares offered, hereby. Please read the section entitled “Plan of Distribution” in this offering at a public offering price of $1,000 per share, and assumingprospectus.conversion of all theselling stockholder acquired or will acquire shares of Series D preferred stock sold in the offering at an assumed conversion price of $0.01, which was the last reported sale price of our common stock on June 15, 2016 (and excluding shares of common stock issuable upon exercise of warrants), and after deducting estimated placement agent fees and estimated offering expenses payable by us, our pro forma net tangible book value deficiency as of March 31, 2016 would have been approximately $5.8 million, or $0.01 per share of common stock. This represents an immediate increase in net tangible book value of $1.36 per share to our existing stockholders and an immediate dilution in net tangible book value of $(0.02) per share to investors participating in this offering. is discussed below under “The Offering.”illustrates this dilution per share to investors participating in this offering:Assumed Series D Conversion Price $ 0.01 Net tangible book value per share as of March 31, 2016 $ (1.37 ) Increase in net tangible book value per share attributable to this offering $ 1.36 Pro forma net tangible book value per share after giving effect to the offering $ (0.01 ) Dilution per share to new investors in this offering $ 0.02 The above discussion and table are based on 5,322,003 sharessets forth the name of common stock outstanding as of March 31, 2016, which does not include the following, all as of June 15, 2016: 15·104,356 shares of common stock reserved for future issuance under our 1995 Stock Plan;·433,385,611 shares of common stock reserved for issuance upon conversion of, or issuance as dividends on, our Series C and Series C1 convertible preferred stock, which we assume will be exchanged for shares of Series D preferred stock and warrants as part of this offering;·151,583,843 shares reserved for issuance upon conversion of our outstanding convertible debt;·233,615,051 shares of common stock issuable upon the exercise of outstanding warrants or warrants to be exchanged for outstanding warrants·5,205,101 shares reserved for issuance pursuant to contractual obligations; and·up to ______ shares of common stock issuable upon exercise of warrants sold as part of this offering, including up to ______ shares of common stock issuable upon exercise of warrants to be issued to the placement agentA $0.001 increase (decrease) in the assumed Series D preferred stock conversion price of $0.01 per share would not change the pro forma as adjusted net tangible book value deficiency resulting from the offering ($5.8 million), and our as adjusted net tangible book value deficiency per share by approximately $0.01, but would increase (decrease) the dilution per share to new investors by approximately $0.001, assuming the assumed number of shares of Series D preferred stock sold remains the same. A 10% increase (decrease) in the number of shares sold would would not change the pro forma as adjusted net tangible book value deficiency resulting from the offering ($5.8 million), and our as adjusted net tangible book value deficiency per share by approximately $0.01, but would increase (decrease) the dilution per share to new investors by approximately $0.001, assuming the assumed number of shares of Series D preferred stock sold remains the same.Certain of our outstanding indebtedness and our outstanding warrants are subject to customary “downside price protection” provisions in the event we sell any common stock at a price lower than the then-conversion price of these securities. The Series D preferred stock offered pursuant to this prospectus will also be subject to similar adjustment. These provisions are further described under the captions “Description of Securities” and “Description of Securities We Are Offering.” 16CAPITALIZATIONThe following table shows our cash and cash equivalents and our capitalization as of March 31, 2016:·on an actual basis;·as adjusted to give effect to transactions described in “Summary—Recent Developments”; and·as further adjusted to give effect to this offering.You should read this table together with the information under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited annual consolidated financial statements and the related notes and other financial information incorporated by reference into this prospectus from our annual report on Form 10-K for the fiscal year ended December 31, 2015 and our quarterly report on Form 10-Q for the quarterly period ended March 31, 2016. As of March 31, 2016 Actual As Adjusted(a) As Further Adjusted(b) (in thousands) Cash and cash equivalents $ 56 431 4,691 Short-term debt 259 434 434 Short-term convertible note 591 677 86 Convertible notes payable – current portion 479 479 479 Long-term convertible notes payable, net 197 197 197 Outstanding warrants, at fair value 1,588 1,310 185 Total liabilities 3,114 3,097 1,381 Series C preferred stock 1,817 1,116 — Series C1 preferred stock — 701 — Series D preferred stock — — 6,668 Common stock 297 329 329 Additional paid-in capital 115,471 115,831 116,956 Treasury stock, at cost (132 ) (132 ) (132 ) Accumulated deficit (122,903 ) (122,903 ) (122,903 ) Total stockholders’ surplus (deficit) (5,450 ) (5,058 ) 918 Total capitalization $ (8,564 ) (8,155 ) (463 ) (a)Reflects the following, each further described under “Summary—Recent Developments”:·the April-May 2016 issuances of an aggregate 4,311 shares of Series C1 preferred stock and 18,396,800 shares common stock in exchange for 1,916 then-outstanding shares of Series C preferred stock;·the cancelation of warrants held by Aquarius Opportunity Fund that were exercisable for an aggregate of 7,148,813 shares of common stock;·the May 2016 cash advances of $175,000 by Aquarius Opportunity Fund and GPB Debt Holdings II LLC;·the June 2016 obligation to issue a convertible note and warrant in connection with the Shenghuo license agreement; and·the June 2016 issuances of an aggregate of 13,517,342 shares of common stock and rights, which includes 8,312,241 common stock shares that were issued and 5,205,101 rights, in exchange for warrants previously exercisable for 8,142,977 shares of common stock.(b)Assumes sale of all of the securities offered, except (i) shares of common stock that could be issued upon conversion of the Series D preferred stock or upon exercise of the warrants sold (or issued to the placement agent) as part of this offering. Reflects receipt of the net cash proceeds from this offering prior to the application thereof. Also reflects the following, each of which is contingent upon consummation of the offering, as further described under “Summary—Recent Developments”:·the extinguishment of indebtedness represented by the May 2016 short-term cash advances as a result of the participation by Aquarius Opportunity Fund and GPB Debt Holdings II LLC in this offering at least to the extent of the principal and interest on the cash advances;·the exchange, on a dollar-for-dollar basis, of all outstanding shares Series C and Series C1 preferred stock, as well as any remaining principal and accrued interest on our convertible promissory note held by Aquarius Opportunity Fund, for shares of Series D preferred stock and warrants;·the exchange of certain warrants, exercisable for 115,237,788 shares of common stock, for new warrants, exercisable for 214,189,622 shares of common stock. 17PLAN OF DISTRIBUTIONWe are offering up to 5,000 shares of our Series D convertible preferred stock, together with warrants to purchase __________ shares of common stock, at a purchase price of $1,000 (and the shares issuable from time to time upon conversion of the Series D preferred stock and the exercise of the warrants) pursuant to this prospectus. The shares of Series D preferred stock and warrants are immediately separable and will be separately issued. There is no minimum offering amount required as a condition to closing and we may sell significantly fewer securities in the offering. The offering will terminate on ___________, unless the offering is fully subscribed before that date or we decide to terminate the offering prior to that date.In determining the offering price of the securities offered hereby, we will consider a number of factors including, but not limited to, the current market price of our common stock, trading prices of our common stock over time, the illiquidity and volatility of our common stock, our current financial condition and the prospects for our future cash flows and earnings, and market and economic conditions at the time of the offering. Once the offering price is determined, the offering price for the securities will remain fixed for the duration of the offering.Ladenburg Thalmann & Co. Inc. has agreed to act as our exclusive placement agent in connection with this offering, subject to the terms and conditions of the placement agent agreement dated _______________. Ladenburg is not purchasing or selling any securities offered by this prospectus, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities, but has agreed to use its best efforts to arrange for the sale of all of the shares of Series D preferred stock and warrants offered hereby. Therefore, we will enter into purchase agreements directly with investors in connection with this offering. Ladenburg may retain other brokers or dealers to act as sub-agents or selected-dealers on its behalf in connection with the offering. Offers will only be made to, and subscriptions accepted from, institutional investors within the meaning of state securities laws of the state in which such investor is domiciled.All cash funds received in payment for securities sold in this offering will be required to be submitted by subscribers to a non-interest bearing escrow account, and will be held by the escrow agent for such account until we and the placement agent notify the escrow agent that the offering has closed. The closing will occur, as to all subscriptions duly received and accepted by us, in one closing; we do not intend to hold multiple closings in the offering. In the event we do not accept the subscriptions and do not close the offering, escrowed funds will be promptly returned to subscribers without interest or offset.We have agreed to pay Ladenburg a placement agent’s fee of 9% of the aggregate cash purchase price of the securities sold in this offering. We will also reimburse Ladenburg for its reasonable out-of-pocket expenses, including, without limitation, fees and expenses of its counsel.The following table shows the per share and total placement agent’s fees that we will pay to Ladenburg in connection with the sale of the securities offered pursuant to this prospectus, assuming the purchase of all of the shares of Series D preferred stock and accompanying warrants offered hereby.Per share placement agent’s fees . . . . .$90Maximum offering total placement agent’s fees . . . . .$450,000Because there is no minimum amount required as a condition to the closing in this offering, the actual total offering commissions, if any, are not presently determinable and may be substantially less than the maximum amount set forth above.We have also agreed to issue warrants to Ladenburg or it designees (as permitted by FINRA Rule 5110(g)) to purchasestockholder, the number of shares of our common stock equivalentbeneficially owned by such stockholder before this offering, the number of shares to 5%be offered for such stockholder’s account and the number and (if one percent or more) the percentage of the class to be beneficially owned by such stockholder after completion of the offering. The number of shares owned are those beneficially owned, as determined under the rules of the SEC, and such information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares of our common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within 60 days, through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement, and such shares are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. Beneficial ownership percentages are calculated based on 211,291,990 shares of our common stock outstanding as of June 1, 2018.issuable upon conversionshown as beneficially owned before the offering is based on information furnished to us or otherwise based on information available to us at the timing of the Series D preferred stock (subject to reduction if required by FINRA Rule 5110), at an exercise price equal to 125% of the conversion price per share at the offering. The placement agent warrants will contain a cashless exercise provision, and a piggyback registration rights provision for the life of the warrants, but shall not contain any price-based anti-dilution provisions. The placement agent warrants will have a term expiring five years from the effective datefiling of the registration statement of which this prospectus forms a part. Pursuant Name of Selling Stockholder GHS Investments LLC (3) FINRA Rule 5110(g), the placement agentshares of common stock. Shares of common stock subject to options, warrants and anyconvertible debentures currently exercisable or convertible, or exercisable or convertible within 60 days, are counted as outstanding. The actual number of shares issuedof common stock issuable upon exercisethe conversion of the placement agent warrantsconvertible debentures is subject to adjustment depending on, among other factors, the future market price of our common stock, and could be materially less or more than the number estimated in the table.sold, transferred, assigned, pledged, or hypothecated, orsufficient, dependent upon the share price, to allow us to access the full amount contemplated under the Financing Agreement. If the bid/ask spread remains the same we will not be able to place a put for the subject of any hedging, short sale, derivative, put, or call transaction that would result infull commitment under the effective economic dispositionFinancing Agreement. Based on the average of the securities by any person for atwo (2) lowest volume weighted average prices of our common stock during the fifteen (15) consecutive trading day period preceding June 1, 2018 of 180 daysapproximately $0.0056, the registration statement covers the offer and possible sale of $392,000 worth of our shares.followingpreceding the date on which the Company delivers a put notice to GHS. In addition, there is an ownership limit for GHS of effectiveness or commencement9.99%.this offering, except the transferour common stock by GHS after delivery of any security:·by operation of law or by reason of our reorganization;·to any FINRA member firm participating in the offering and the officers or partners thereof, as long as all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period;
a put notice of such number of shares reasonably expected to be purchased by GHS under a put will not be deemed a short sale. 18·if the aggregate amount of our securities held by the holder of the placement agent warrants or related persons do not exceed 1% of the securities being offered;·that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or·the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period.have granted a right of first refusalmust deliver the other required documents, instruments and writings required. GHS is not required to purchase the put shares unless:placementresale of the shares of common stock delivered in connection with the applicable put shall have been declared effective;pursuantbut may position and resell a portion of the block as principal to which it hasfacilitate the righttransaction;act assell a specified number of such shares at a stipulated price per share;lead managerselling stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or underwriter or agent, as applicable, if we or our subsidiary raise capital through a public or private offering of equity or debt securities at any time prior to eighteen monthsdiscounts from the dateselling stockholders (or, if any broker-dealer acts as agent for the purchaser of closing of this offering.Our obligationsshares, from the purchaser) in amounts to issue and sell securities to the purchasers is subject to the conditionsbe negotiated, but, except as set forth in the securities purchase agreement, which may be waived by us at our discretion. A purchaser’s obligationa supplement to purchase securities is subject to the conditions set forththis prospectus, in the securities purchase agreement as well, which may also be waived bycase of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the purchasercase of a principal transaction a markup or markdown in its discretion.We estimate the total offering expenses in this offering that will be payable by us, excluding the placement agent’s fees, will be approximately $290,000, which includes legal, accounting and printing costs, various other fees and reimbursement of the placement agent’s expenses.We have also agreed to indemnify the placement agent for certain liabilities under the Securities Act.The foregoing does not purport to be a complete statement of the terms and conditions of the placement agent agreement or the securities purchase agreement. A copy of the placement agent agreement and the form of securities purchase agreementcompliance with investors are included as exhibits to the registration statement of which this prospectus forms a part.LadenburgFINRA IM-2440.Section 2(a)(11)the Securities Act of 1933 and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act andof 1933 in connection with such sales. In such event, any commissions received by itsuch broker-dealers or agents and any profit realized on the resale of the securities soldshares purchased by it while acting as principal mightthem may be deemed to be underwriting discountscommissions or commissionsdiscounts under the Securities Act. As an underwriter, Ladenburg is requiredAct of 1933. GHS has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to comply withdistribute the common stock of our company. Pursuant to a requirement by FINRA, the maximum commission or discount to be received by any FINRA member or independent broker-dealer may not be greater than 8% of the gross proceeds received by us for the sale of any securities being registered pursuant to Rule 415 promulgated under the Securities Act of 1933.including Rule 10b-5 andany person engaged in the distribution of the resale shares may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, underprior to the commencement of the distribution. In addition, the selling stockholders will be subject to applicable provisions of the Securities Exchange Act. TheseAct of 1934 and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of our securitiesshares of the common stock by Ladenburg acting as principal. Under these rules and regulations, Ladenburg:·may not engage in any stabilization activity in connection with our securities; and·may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.Pursuantthe selling stockholders or any other person. We will make copies of this prospectus available to the purchase agreement to be entered into in connection with this offering, we will be prohibited from issuing, entering into any agreementselling stockholders.or announcing the issuance or proposed issuancean aggregate of anyone billion (1,000,000,000) shares of common stock, or common stock equivalents (subject to certain exceptions) for a period of 90 days from the closing of this offering. 19DESCRIPTION OF SECURITIES WE ARE OFFERINGWe are offering up to 5,000 shares of our Series D convertible preferred stock, convertible into an aggregate of _________ shares of our common stock, and five-year warrants exercisable for an aggregate of ____________ shares of our common stock. Each share of Series D preferred stock we sell in the offering will be accompanied by a warrant to purchase _____ of a share of common stock, and will be sold at a combined price of $1,000.The shares of Series D preferred stock and the warrants are immediately separable and will be issued separately. Subject to certain ownership limitations, the Series D preferred stock is convertible at any time at the holder’s option into shares of our common stock at an initial conversion price of $0.01 per share, and the warrants are immediately exercisable at an initial exercise price of $_____ per share. This prospectus also covers the shares of common stock issuable upon conversion of the Series D preferred stock and upon exercise of the warrants. The material terms and provisions of our common stock, which underlies the Series D preferred stock and the warrants, and each other class of securities which qualifies or limits the foregoing, are described under the caption “Description of Securities.”Series D Preferred StockThe material terms and provisions of the Series D preferred stock being offered pursuant to this prospectus are described below. The following description is qualified in its entirety by reference to the form of certificate of designation filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions of the form of the certificate of designation for a complete description of the terms and conditions of the Series D preferred stock.Our Board has designated 5,000 shares of preferred stock as Series D Convertible Preferred Stock,$0.001 par value $0.001 per share. As of June 15, 2016, there were no shares of Series D preferred stock outstanding.Voting Rights.Except as required by law, holders of the Series D preferred stock do not have rights to vote on any matters, questions or proceedings, including the election of directors. However, as long as any shares of Series D preferred stock are outstanding,1, 2018, we will not, without the affirmative vote of the holders of a majority of the then-outstanding shares of the Series D preferred stock, (1) alter or change adversely the powers, preferences or rights given to the Series D preferred stock or alter or amend the certificate of designation, (2) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon liquidation senior to, or otherwise pari passu with, the Series D preferred stock, (3) amend our certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series D preferred stock, (4) increase the number of authorized shares of Series D preferred stock, or (5) enter into any agreement with respect to any of the foregoing.Liquidation Preference.The Series D preferred stock ranks, with respect to rights upon liquidation, winding-up or dissolution, (1) senior to common stock, (2) senior to any series of preferred stock ranked junior to the Series D preferred stock, (3) junior to our Series C preferred stock, and (4) junior to all of our existing and future indebtedness.Redemption.We have the right to redeem the Series D preferred stock for a cash payment equal to 120% of the stated value of the Series D preferred stock. Holders of Series D preferred stock will receive 20 trading days prior notice of any redemption and will have the ability to convert the Series D preferred stock into common stock during this notice period.Conversion; Beneficial Ownership Limitation.Subject to certain ownership limitations as described below, the Series D preferred stock is convertible at any time at the option of the holder into shares of our common stock at a conversion ratio determined by dividing the $1,000 stated value of the Series D preferred stock by a conversion price of $____ per share. The conversion price is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions. In addition, until our common stock is listed on a national securities exchange, if we sell or grant any right to purchase or sell any common stock or common stock equivalents entitling any person to acquirehad 211,291,990 shares of common stock (subject to exceptions for certain exempt issuances, including issuances pursuant to equity compensation plans, certain issuances to consultants, issuances in connection with exercise or exchangeoutstanding.equivalents already outstanding and issuances pursuant to acquisitions or strategic transactions or arrangements) at an effective priceshall have one (1) vote per share that is lower than the then-conversion price of the Series D preferred stock, then the conversion price will be further reduced to equal such lower price per share.Subject to limited exceptions, a holder of shares of Series D preferred stock will not have the right to convert any portion of its Series D preferred stock if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our Our common stock outstanding immediately after giving effect to its conversion; provided, however, that upon 61 days’ prior notice to us, the holder may increase such percentage to 9.99%. We have the right to force thedoes not provide a preemptive, subscription or conversion of the Series D preferred stock if our common stock meets certain minimum price and trading volume thresholds. 20Dividends.The Series D preferred stock is entitled to receive dividends (on an “as converted to common stock” basis) upon and in the same form as dividends actually paid on shares of our common stock. No other dividends will be paid on shares of Series D preferred stock.Liquidation. Upon our liquidation, dissolution or winding up after payment or provision for payment of our debts and other liabilities, but before any distribution or payment is made to the holders of any junior securities, the holders of Series D preferred stock will be entitled to be paid out of our assets available for distribution to our stockholders in an amount equal to $1,000 per share, after which any of our remaining assets will be distributed among the holders of junior classes of our capital stock in accordance with our certificate of incorporation.WarrantsThe material terms and provisions of the warrants being offered pursuant to this prospectus are described below. The following description is qualified in its entirety by reference to the form of warrant filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions of the form of the warrant for a complete description of the terms and conditions of the warrants.Duration and Exercise Price.The warrants offered hereby (including those to be issued to the placement agent, which have substantially similar terms except as indicated below, but also have certain provisions required by FINRA and an exercise price of 125% of the combined public offering price per share and warrant) will entitle the holders thereof to purchase up to an aggregate of ____________ shares of our common stock at an exercise price of $____ per share (assuming we offer ____________ shares of Series D preferred stock at an assumed conversion price of $____ per share), commencing immediately on the issuance date and will expire five years following the issuance date. The warrants will be issued separately from the Series D preferred stock offered, and may be transferred separately immediately thereafter. No warrants exercisable for a fractional amount of shares of common stock will be issued. If an investor would otherwise be entitled to receive a fractional warrant, the number of shares issuable upon exercise of the warrant will be rounded up to the nearest whole warrant. The warrant holders must pay the exercise price in cash upon exercise of the warrants. After the close of business on the expiration date, unexercised warrants will become void.Anti-Dilution Protection.The exercise price of the warrants is subject to appropriate adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock, and also upon any distributions of assets, including cash, stock or other property to our stockholders.Fundamental Transactions. In the event of any fundamental transaction, as described in the warrants and generally including any merger with another entity, the sale, transfer or other disposition of all or substantially all of our assets to another entity, or the acquisition by a person of more than 50% of our common stock, then, subject to the agreement of the counterparty to the fundamental transaction, the holders of the warrants will thereafter have the right to receive upon exercise of the warrants such shares of stock, securities or assets as would have been issuable or payable with respect to or in exchange for a number of shares of our common stock equal to the number of shares of our common stock issuable upon exercise of the warrants immediately prior to the fundamental transaction, had the fundamental transaction not taken place, and appropriate provision will be made so that the provisions of the warrants (including, for example, provisions relating to the adjustment of the exercise price) will thereafter be applicable, as nearly equivalent as may be practicable in relation to any share of stock, securities or assets deliverable upon the exercise of the warrants after the fundamental transaction.Transferability. The warrants may be transferred at the option of the holder upon surrender of the warrants with the appropriate instruments of transfer.Right as a Stockholder. Except by virtue of a holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.Exercisability.The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise. Subject to limited exceptions, a holder of will not have the right to exercise any portion of its warrant if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to its exercise; provided, however, that upon 61 days’ prior notice to us, the holder may increase such percentage to 9.99%. 21Waivers and Amendments.Subject to certain exceptions, any term of the warrants may be amended or waived with our written consent and the written consent of each holder of the then-outstanding warrants.DESCRIPTION OF SECURITIESWe are authorized to issue 1,005,000,000 shares of stock, in two classes: 1,000,000 shares of common stock, par value $.001 per share, and 5,000,000 million shares of preferred stock. As of June 15, 2016, there were 67,901,547 shares of common stock outstanding, which were held of record by 209 stockholders, and 6,679 shares of preferred stock outstanding, consisting of 2,368 shares of Series C preferred stock, which were held of record by 3 stockholders, and 4,311 shares of Series C1 preferred stock, which were held of record by 6 stockholders. Upon consummation of the offering, all outstanding shares of Series C and Series C1 preferred stock will be exchanged for shares of the Series D preferred stock and warrants as part of this offering.Common StockThe holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Subject to preferences that may be applicable to any outstanding preferred stock, holders of common stock are entitled to receive ratably such dividends as may be declared by the board out of funds legally available therefor and in liquidation proceedings. Holders of common stock have no preemptive or subscription rights and there are no redemption rightsor sinking fund provisions or rights. Our common stock holders are not entitled to cumulative voting for election of Board of Directors.respect to such shares.Preferred StockOura $.001 par value. The board is authorized, without further stockholder action,of directors has the authority to issue preferred stock in one or more seriesthese shares and to fix theset dividends, voting rights, liquidation preferences, dividend rights, repurchase rights,and conversion rights, redemption rightsprovisions, liquidation preferences, and terms, including sinking fund provisions, and certain other rights and preferences,restrictions. The board of the preferred stock. Our boarddirectors designated 525,000 shares of preferred stock as redeemable convertible preferred stock, none of which remain outstanding; 3,00033,000 shares of preferred stock as Series B Convertible Preferred Stock, none of which remain outstanding;remained outstanding, 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 2,368970 and 1,643 were issued and outstanding as of June 15, 2016;at December 31, 2017 and 2016, respectively, and 20,250 shares of preferred stock as Series C1 Convertible Preferred Stock, of which 4,311686 and 970 were issued and outstanding as of June 15, 2016;at March 31, 2018 and 5,000December 31, 2017, respectively, and 20,250 shares of preferred stock as Series DC1 Convertible Preferred Stock, none of which 4,312 were issued and outstanding asat March 31, 2018 and December 31, 2017.June 15, 2016. The material terms and provisionsthe Delaware General Corporation Law, a publicly held Delaware corporation may not engage in a broad range of our Series D preferred stock are described underbusiness combinations or other extraordinary corporate transactions with an interested shareholder without the caption “Description of Securities We Are Offering.”Although there is no current intention to do so, our board may, without stockholder approval issue additional shares of preferred stock with voting and conversion rights that could adversely affect the voting power or dividend rights of the holders of commontwo-thirds of the voting shares of the corporation (excluding shares held by the interested shareholder), unless:mayalso officers; and acquired directly from the corporation in a transaction not approved by a majority of the disinterested directors; andeffectright to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offerdelaying, deferringa corporation’s outstanding voting shares. We have not made an election in our amended Articles of Incorporation to opt out of Section 203.preventingthe transaction is otherwise exempt;changecustomer’s account, the account’s value, and information regarding the limited market in control.Series C Convertible Preferred StockDividends. Frompenny stocks; andoriginal issue date until 42 months thereafter,penny stock is a suitable investment for the holderspurchaser and receive the purchaser’s written agreement to the transaction, prior to conducting any penny stock transaction in the customer’s account.Series C preferred stock are entitledthese regulations, broker-dealers may encounter difficulties in their attempt to receive quarterly cumulative dividends at a rate per share (as a percentage of stated value per share) of 12% per year per share payable quarterly on each January 1, April 1, July 1 and October 1 during such period (beginning October 1, 2015) insell shares of our common stock, (subjectwhich may affect the ability of selling shareholders or other holders to certain conditions) or, atsell their shares in the secondary market, and have the effect of reducing the level of trading activity in the secondary market. These additional sales practice and disclosure requirements could impede the sale of our option, in cash.securities, if our securities become publicly traded. In addition, upon the conversion ofliquidity for our securities may be decreased, with a corresponding decrease in the Series C preferred stock (other than a forced conversion, described below) during the such period, we must pay the converting holder a “make-whole payment” in cash or, at our option (subject to certain conditions), shares of our common stock with respect to the converted shares of Series C preferred stock in an amount equal to $420 per $1,000 of stated value, less the amount of any dividends already paid on such shares of Series C preferred stock. To the extent we choose to pay any dividends or make-whole payments in shares of our common stock, such shares will be valued at 80% of then-current market price (calculated as the average daily volume weighted average price of our commonsecurities. Our shares in all probability will be subject to such penny stock rules and our shareholders will, in all likelihood, find it difficult to sell their securities.five consecutive trading days priorCompany as of December 31, 2017 and 2016 and for the years then ended included in this prospectus have been audited by UHY LLP, an independent registered public accounting firm, to payment). After the dividend payment period, holdersextent and for the periods set forth in our report and are incorporated herein in reliance upon such report given upon the authority of Series C preferred stock are only entitled to receive dividends on an as-if-converted basis) with holder of our common stock (other than dividends paidsaid firm as experts in additional shares of common stock).Voting. Except as otherwise proved by law or in the Series C designations, the Series C preferred stock has no voting rights. We may not, without the consent of the holders of a majorityauditing and accounting.of Series C preferred stock then outstanding, alter or change adversely the powers, preferences or rights given to the Series C preferred stock or alter or amend the Series C designations, create any class of stock with a liquidation preference equal or senior to the Series C preferred stock, amend our charter in any manner that adversely affects any rights of the holders of Series C preferred stock, increase the number of authorized shares of Series C preferred stock, or enter into any agreement with respect to any of the foregoing.
offered under this registration statement is being passed upon by Brunson Chandler, & Jones, PLLC. 22Liquidation. In the event of our liquidation, dissolution or winding-up, whether voluntary or involuntary, the holders of our Series C preferred stock will be entitled to receive out of our assets an amount equal to the stated value of their shares, plus any other fees, liquidated damages or dividends then due and owing on their shares, before any distribution or payment to the holders of any junior securities.Conversion. The Series C preferred stock is convertible at any time, at the option of the holder. In addition, if the market price of our common stock for each trading day for 20 of any 30 consecutive trading-day period exceeds 250% of the highest conversion price in effect during such period, we may force each holder to convert all or part of such holder’s shares into shares of common stock.Each share of Series C preferred stock is convertible into the number of shares of common stock equal to the quotient obtained by dividing (1) the sum of the stated value plus all accrued but unpaid dividends on such share, by (2) the per share conversion price. The initial per share conversion price was $0.095 (pre-stock split), but has been reset pursuant to the Series C designations to $0.017824 as of June 15, 2016. The conversion price will automatically adjust downward to 80% of the then-current market price of our common stock 15 trading days after any subsequent reverse stock split of the common stock, and 5 trading days after any conversions of our outstanding convertible debt. The conversion price is subject to further adjustment under certain circumstances to protect the holders of Series C preferred stock from dilution relative to certain issuances of common stock, or securities convertible into or exercisable for shares of common stock. Subject to certain exceptions, if we issue shares of common stock, or such other securities, at a price per share less than the then-effective conversion price, the conversion price will be adjusted to equal such lower per share consideration.Series C1 Convertible Preferred StockThe Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.”Secured Promissory NoteAs of June 15, 2016, we have issued and outstanding $507,614 in aggregate principal amount of secured promissory notes that, as amended, provide the holders the right to convert the outstanding balance into shares of our common stock at a conversion price per share equal to the lower of (1) $25.00 and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion price at the time of any conversion request would be lower than $15.00 per share, we have the option of delivering the conversion amount in cash in lieu of shares of our common stock. The note is secured by a lien on our inventory and accounts receivables.Senior Secured Convertible NoteAs of June 15, 2016, we have issued and outstanding $1,525,000 million in aggregate principal amount of a senior secured convertible note that is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.014224 per share, subject to certain customary adjustments and anti-dilution provisions. The note is secured by a lien on all of our assets.Convertible NoteAs of June 15, 2016, we have issued and outstanding $240,000 in aggregate principal amount of a convertible note that is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.017402 per share, subject to certain customary adjustments and anti-dilution provisions.Warrants and OptionsWe have issued warrants to purchase our common stock from time to time in connection with certain financing arrangements. As of June 15, 2016, there are warrants exercisable for an aggregate of 191,815,878 shares of common stock outstanding, as follows:
Item 11. INFORMATION WITH RESPECT TO THE REGISTRANT 23
(Underlying Shares)2,852 (1) $105.00 November 20, 2016 18,581 (2) $10.46 May 23, 2018 12,066,450 (3) $0.09375 May 23, 2018 2,000 (4) $50.00 April 23, 2019 5,618 (5) $45.00 May 22, 2019 1,842 (5) $38.00 September 10, 2019 3,255 (6) $46.081 September 27, 2019 7,553 (7) $28.13 December 2, 2019 83,927 (8) $9.00 December 2, 2020 83,927 (8) $11.00 December 2, 2020 20,000 (9) $25.50 March 30, 2018 17,547 (10) $11.88 June 29, 2020 120,000 (11) $1.03 June 29, 2020 115,789 (12) $1.03 September 4, 2020 131,842 (13) $1.03 September 21, 2020 5,163 (14) $11.88 September 4, 2020 157,895 (15) $1.03 October 23, 2020 5,163 (16) $11.88 October 23, 2020 202,123,172 (17) $0.01422 June 6, 2021 4,850,956 (18) $0.01422 February 12, 2021 13,791,518 (19) $0.017402 June 6, 2021 233,615,051 (1)Issued as part of a November 2011 private placement.(2)Issued in June 2015 in exchange for warrants originally issued in a May 2013 private placement.(3)Issued in June 2015 in exchange for warrants originally issued in a May 2013 private placement.(4)Issued to a placement agent in conjunction with an April 2014 private placement.(5)Issued to a placement agent in conjunction with a September 2014 private placement.(6)Issued as part of a September 2014 Regulation S offering.(7)Issued to a placement agent in conjunction with a 2014 public offering.(8)Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering.(9)Issued as part of a March 2015 private placement.(10)Issued to a placement agent in conjunction with a June 2015 private placement.(11)Issued as part of a June 2015 private placement.(12)Issued as part of a June 2015 private placement.(13)Issued as part of a June 2015 private placement.(14) Issued to a placement agent in conjunction with a June 2015 private placement.(15)Issued as part of a June 2015 private placement.(16)Issued to a placement agent in conjunction with a June 2015 private placement.(17)Issued as part of a February 2016 private placement.(18)Issued to a placement agent in conjunction with a February 2016 private placement.(19)Contractually obligated to be issued pursuant to a strategic license agreement.All outstanding warrant agreements provide for anti-dilution adjustments in the event of certain mergers, consolidations, reorganizations, recapitalizations, stock dividends, stock splits or other changes in our corporate structure. In addition, warrants subject to footnotes (3) and (11)-(19) in the table above are subject to “lower price issuance” anti-dilution provisions that automatically reduce the exercise price of the warrants (and, in the cases of warrants subject to footnote (3) in the table above and footnote (17), increase the number of shares of common stock issuable upon exercise), to the offering price in a subsequent issuance of our common stock, unless such subsequent issuance is exempt under the terms of the warrants.The warrants subject to footnote (3) are subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require exercise of such warrants at any time following (a) the date that is the 30th day after the later of our receipt of an approvable letter from the FDA for LuViva and the date on which the common stock achieves an average market price for 20 consecutive trading days of at least $1.30 with an average daily trading volume during such 20 consecutive trading days of at least 250 shares, or (b) the date on which the average market price of the common stock for 20 consecutive trading days immediately prior to the date we deliver a notice demanding exercise is at least $162.00 and the average daily trading volume of the common stock exceeds 250 shares for such 20 consecutive trading days. If these warrants are not timely exercised upon demand, they will expire. Upon the occurrence of certain events, we also may be required to repurchase these warrants, as well as the warrants subject to footnote (2) in the table above.
Overview 24The warrants subject to footnote (6) in the table above are also subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require the exercise of such warrants should the average trading price of our common stock over any 30 consecutive day trading period exceed $92.16.The warrants subject to footnote (8) in the table above are also subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require exercise of 50% of the then-outstanding warrants if the trading price of our common stock is at least two times the initial warrant exercise price for any 20-day trading period. Further, in the event that the trading price of our common stock is at least 2.5 times the initial warrant exercise price for any 20-day trading period, we will have the right to require the immediate exercise of 50% of the then-outstanding warrants. Any warrants not exercised within the prescribed time periods will be canceled to the extent of the number of shares subject to mandatory exercise.The holders of the warrants subject to footnote (3) in the table above have agreed to surrender the warrants upon consummation of this offering for new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants.As of June 15, 2016, we have issued options to purchase a total of 104,356 shares of our common stock pursuant to our equity incentive plan, at a weighted average exercise price of $45.46 per share. Recommendations for option grants under our equity incentive plans are made by the compensation committee of our board, subject to ratification by the full board. The compensation committee may issue options with varying vesting schedules, but all options granted pursuant to our equity incentive plans must be exercised within ten years from the date of grant. 25OUR BUSINESS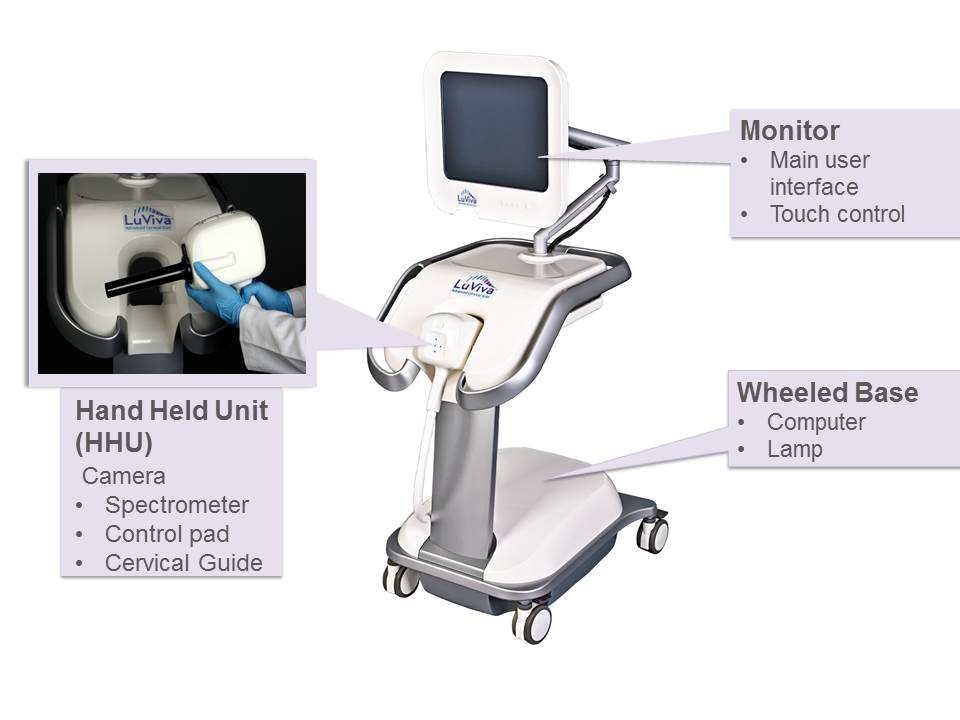
pampillomaviruspapillomavirus (HPV)—every woman essentially becomes “at risk” for cervical cancer simply after becoming sexually active. In the developing world, there are approximately 2.0 billion women aged 15 and older who are potentially eligible for screening with LuViva. Guidelines for screening intervals vary across the world, but U.S. guidelines call for screening every three years. Traditionally, the Pap smear screening test, or Pap test, is the primary cervical cancer screening methodology in the developed world. However, in developing countries, cancer screening using Pap tests is expensive and requires infrastructure and skill not currently existing, and not likely to be developed in the near future, in these countries.are able tocan provide immediate access to cervical cancer detection to large segments of a nation’s population as part of national or regional governmental healthcare programs, eliminating the need to develop expensive and resource-intensive infrastructures. 26Manufacturing, Sales Marketing and DistributionWe manufacture LuViva at our Norcross, Georgia facility. Most of the components of LuViva are custom made for us by third-party manufacturers. We adhere to ISO 13485:2003 quality standards in our manufacturing processes.Research, Development and EngineeringWe have been engaged primarily in the research, development and testing of our LuViva non-invasive cervical cancer detection product and our core biophotonic technology. Since 2013, we have incurred about $7.0 million in research and development expenses, net of about $927,000 reimbursed through collaborative arrangements and government grants. Research and development costs were approximately $1.5 million and $2.8 million in 2015 and 2014, respectivelyPatentsWe have pursued a course of developing and acquiring patents and patent rights and licensing technology. Our success depends in large part on our ability to establish and maintain the proprietary nature of our technology through the patent process and to license from others patents and patent applications necessary to develop our products. As of December 31, 2015, we have 24 granted U.S. patents relating to our biophotonic cancer detection technology and six pending U.S. patent applications. We also have three granted patents that apply to our interstitial fluid analysis system.The Need for LuVivaactually warranted.platform.platform. Early market analyses of our biophotonic technology indicated that skin cancer detection was also promising, but currently we are focused primarily on the large-scale commercialization of LuViva.
27270 000270,000 women died from cervical cancer; more than 85% of these deaths occurring in low- and middle-income countries.
28placesplace that enable women to get screened, making most pre-cancerous lesions identifiable at stages when they can easily be treated. Early treatment prevents up to 80% of cervical cancers in these countries. In developing countries, however, limited access to effective screening means that the disease is often not identified until it is further advanced and symptoms develop. In addition, prospects for treatment of such late-stage disease may be poor, resulting in a higher rate of death from cervical cancer in these countries.Bangladesh, Indonesia, Kenya and Nigeria. The number of screening candidates in those countries is approximately 246131 million and represents 3Indonesia and Nigeria represent 2 of the 10 most populous countries in the world.
29inon the cervix. This method looks for visual changes attributable to cancer. There are about two million colposcope examinations annually in the United States and Europe. In 2003, the average cost of a stand-alone colposcope examination in the United States was $185 and the average cost of a colposcopy with biopsy was $277.the most recent being presentedincluding at the International Federation of GynaecologyGynecology and Obstetrics Congress in 2015.2016.7, 2016,31, 2018, we have sold 93138 LuViva devices and approximately 35,00072,000 single-use-disposable cervical guides to international distributors. 30$7.0$7.5 million in research and development expenses, net of about $927,000 reimbursed through collaborative arrangements and government grants. Research and development costs were approximately $1.5 million$0.3 and $2.8$0.7 million in 20152017 and 2014,2016, respectively.third partythird-party funding. Because our research and clinical development programs for other cancers are at a very early stage, substantial additional research and development and clinical trials will be necessary before we can produce commercial prototypes of other cancer detection products.othersother’s patents and patent applications necessary to develop our products. As of DecemberMarch 31, 2015,2018, we have 24 granted U.S. patents relating to our biophotonic cancer detection technology and six pending U.S. patent applications. We also have three granted patents that apply to our interstitial fluid analysis system. 31WeDuring 2017 we were unable to pay the annual registration fees to maintain our ISO 13485:2003 certification which since 2014and our CE Mark. Once our financing is completed, we will make the required payments and reobtain both certifications.allowed usa regulatory regime similar to issue our Edition 3 CE Mark and sell LuViva inthat of the European Union, but due to interaction with the U.S. regulatory regime, the CFDA also shares some similarities with its U.S. counterpart. Devices are classified by the CFDA’s Center for Medical Device Evaluation (CMDE) into three categories based on medical risk, with the level of regulatory oversight determined by degree of risk and other markets.Distributorsinvasiveness. CMDE’s device classifications and definitions are as follows:beconduct electrical, mechanical and electromagnetic emission safety testing for medical devices similar to those required tofor the CE Mark. As is the case with the U.S. FDA, manufacturers in China undergo periodic inspections and must comply with other foreign regulatory agencies, and we orinternational quality standards such as ISO 13485 for medical devices. As part of our distributors currently have marketingagreement with SMI, SMI will underwrite the cost of securing approval forof LuViva from Health Canada, COFEPRIS in Mexico,with the Ministry of Health in Kenya, and the Singapore Health Sciences Authority. The time required to obtain these foreign approvals to market our products may be longer or shorter than that required in the United States, and requirements for those approvals may differ from those required by the FDA.on November 18, 2010,after the U.S. FDA requested two-years of follow-up data for patients enrolled in the study, the U.S. FDA accepted our completed PMA application on November 18, 2010, effective September 23, 2010, for substantive review. On March 7, 2011, we announced that the U.S. FDA had inspected two clinical trial sites and audited our clinical trial data base systems as part of its review process and raised no formal compliance issues. On January 20, 2012, we announced our intent to seek an independent panel review of our PMA application after receiving a “not-approvable” letter from the U.S. FDA. On November 14, 2012 we filed an amended PMA with the U.S. FDA. On September 6, 2013, we received a letter from the U.S. FDA with additional questions and met with the U.S. FDA on May 8, 2014 to discuss our response. On July 25, 2014, we announced that we had responded to the U.S. FDA’s most recent questions2016.2018, although we intend to pursue FDA approval and start studies in 2018 once funds are available. We remain committed to obtaining U.S. FDA approval, but we are focused on international sales growth, where we believe the commercial opportunities are larger and the clinical need is more significant. 32We will2016,2018, we had tennine regular employees and two consultantsone consultant to provide services to us on a full- or part-time basis. Of the 12 peopleten-people employed or engaged by us, 32 are engaged in engineering, manufacturing and development, 34 are engaged in sales and marketing activities, 1 is engaged in clinical testing and regulatory affairs, 1 are engaged in research and development activities, and 43 are engaged in administration and accounting. No employees are covered by collective bargaining agreements, and we believe we maintain good relations with our employees. 33PROPERTIEScorporate offices, which also comprise our administrative, researchprincipal executive and development, marketing and production facilities, areoperations facility is located at 5835 Peachtree Corners East, Suite D,B, Norcross, Georgia 30092, where we lease approximately 23,000 square feet under a lease that expires in June 2017.LEGAL PROCEEDINGSWe are subject to claims and legal actions that arise in the ordinary course of business. However, we are not currently subject to any claims or actions that we believe would have a material adverse effect on our financial position or results of operations.FOR OURPRICE OF THE REGISTRANT’S COMMON STOCKEQUITY AND RELATED STOCKHOLDER MATTERSquotedlisted on the OTC Bulletin Board (OTCBB) and the OTCQB quotation systems under the ticker symbol “GTHP.” The number of record holders of our common stock at June 15, 20161, 2018 was 209. On February 24, 2016, we implemented a210.100800 reverse stock split of all of our issued and outstanding common stock.stock was implemented on November 7, 2016. As a result of the reverse stock split, every 100800 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change.salescommon stock share prices for the first and second quarter of 20162018 and calendar years 20152017 and 2014,2016, as reported by the OTCBB, are as set forth in the following table. All share prices set forth in the table have been retroactively adjusted to reflect the 1:100 reverse stock split (as discussed above) for all periods presented. First Quarter Second Quarter* Third Quarter Fourth Quarter stock. 2016 2015 2014 High Low High Low High Low First Quarter $ 1.690 $ 0.107 $ 23.00 $ 14.00 $ 60.00 $ 46.00 Second Quarter* $ 0.175 $ 0.010 $ 25.00 $ 8.00 $ 60.00 $ 40.00 Third Quarter $ 12.00 $ 5.00 $ 50.00 $ 31.00 Fourth Quarter $ 6.00 $ 1.00 $ 34.00 $ 21.00 *Throughstock at June 15, 2016.Dividend Policynotnever declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any dividends sincefuture earnings for use in the operation of our inceptionbusiness and do not intend to payanticipate paying any dividends on our common stock in the foreseeable future.Securities Authorized for Issuance Under Equity Compensation PlansAllfuture, if at all. Any future determination to declare dividends will be made at the securities we have provideddiscretion of our employees,board of directors and consultants have been issued underwill depend on our stock option plans, which are approved byfinancial condition, results of operations, capital requirements, general business conditions and other factors that our stockholders. We have issued common stock to other individuals that are not employees orboard of directors in lieu of cash payments, that are not part of any plan approved by our stockholders.Securities authorized for issuance under equity compensation plans, as of June 15, 2016: (a) (b) (c) Equity compensation plans approved by security holders Equity compensation plans not approved by security holders TOTAL 104,356 $45.00 26,616
may deem relevant. 34
AND RESULTS OF OPERATIONSTheOPERATIONshould be readof our financial condition and results of operations in conjunction with our financial statements and notes thereto accompanyingincluded elsewhere in this prospectus.OverviewWe The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in the section labeled “Risk Factors.”a medical technology company focusedoften identified by words like “believe,” “expect,” “estimate,” “anticipate,” “intend,” “project,” and similar expressions, or words that, by their nature, refer to future events. You should not place undue certainty on developing innovative medicalthese forward-looking statements, which apply only as of the date of this prospectus. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from historical results or our predictions.that haveand disposables for the potentialthree months ended March 31, 2018 and 2017 were $4,000 and $21,000, respectively. Revenues decreased by approximately $17,000, or 83% from the same period in 2017. The decrease was due to improve healthcare. Our primary focus is theless activity in sales orders being shipped in 2018 and lack of funding to support sales and marketing efforts. Related costs of our LuViva® Advanced Cervical Scan non-invasive cervical cancer detection device.sales were approximately $3,000 and $16,000 for the three months ended March 31, 2018 and 2017, respectively. Costs of sales for the three months ended March 31, 2018 were approximately $13,000, or 82% lower than the same period in 2017. This resulted in a gross profit of approximately $1,000 on the sales of devices and disposables for the three months ended March 31, 2018, compared with a gross profit of approximately $5,000 for the same period in 2017.underlying technologydecrease, of approximately $22,000, or 24%, was primarily due to decreases in payroll expenses.primarily relatesdevices for 2017 and 2016 were approximately $244,000 and $605,000, respectively. Revenues in 2017 were approximately, $361,000 or 60% lower when compared to the usesame period in 2016, due to lack of biophotonicsfunding to support sales and marketing efforts. In addition, revenues were lower for 2017 and 2016 due to the repurchase of inventory in the amount of $83,000 and $92,000, respectively. Related costs of sales were approximately $530,000 and $493,000 in 2017 and 2016, respectively. Costs of sales in 2017, were approximately, $37,000 or 8% higher when compared to the same period in 2016, due to an increase in the inventory allowance. This resulted in a gross loss of approximately $286,000 on the sales of devices and disposables for 2017 compared with a gross profit of approximately $112,000 for the non-invasive detectionsame period in 2016.cancers. LuViva is designed$399,000, or 54%, was primarily due to identify cervical cancerscost reduction plans in research and precancers painlessly, non-invasivelydevelopment payroll expenses.atMarketing Expenses: Sales and marketing expenses for 2017, decreased to approximately $245,000, compared to $393,000 in 2016. The decrease, of approximately $148,000, or 38% was primarily due to Company-wide expense reduction and cost savings efforts.pointsame period in 2016. The decrease of care by scanningapproximately $550,000, or 20%, was primarily related to lower compensation and option expenses incurred during the cervix with light, then analyzing the reflectedsame period. For 2017, general and fluorescent light.LuViva provides a less invasiveadministrative expenses consisted primarily of professional fees, insurance, allowance for doubtful accounts, and painless alternativepaid and accrued compensation costs.conventional tests for cervical cancer screening and detection. Additionally, LuViva improves patient well-being not only because it eliminates pain, but also because it is convenient to use and provides rapid results at the point of care. We focus on two primary applications for LuViva: first, as a cancer screening tool$68,000 in the developing world, where infrastructuresame period in 2016, a decrease of $50,000 or 73%.support traditional cancer-screening methods is limitedapproximately $1,106,000, compared to $1,895,000 for the same period in 2016. The decrease of approximately $789,000, or non-existent,42%, was primarily related to amortization expense of debt issuance cost and second, as a triage following traditional screeningpenalty on event default of convertible debt that were higher in 2016.developed world, where a high numberfiscal year ended December 31, 2017.false positive results cause a high rate of unnecessary$5,979,000, or 120% was for reasons outlined above.ultimately costly follow-up tests.We are a Delaware corporation, originally incorporated in 1992 under the name “SpectRx, Inc.,” and, on February 22, 2008, changed our name to Guided Therapeutics, Inc. At the same time, we renamed our wholly owned subsidiary, InterScan, which originally had been incorporated as “Guided Therapeutics.”Our prospects must be considered in light At March 31, 2018, we had cash of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competitionapproximately $76,000 and a high failure rate. We have experienced operating losses since our inception and, asnegative working capital of approximately $11.7 million.2016 we have an accumulated deficit2018 consisted of about $122.9 million. To date, we have engaged primarily in research and development efforts andcash out-flows of $395,000 from operations, including approximately $1,082,000 of net income, ($1,688,000 from a gain for the early stages of marketing our products. We do not have significant experience in manufacturing, marketing or selling our products. We may not be successful in growing sales for our products. Moreover, required regulatory clearances or approvals may not be obtained in a timely manner, or at all. Our products may not ever gain market acceptance and we may not ever generate significant revenues or achieve profitability. The development and commercialization of our products requires substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We expect our operating losses to continue through at least the end of 2016 as we continue to expend substantial resources to complete commercialization of our products, obtain regulatory clearances or approvals, build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development.Our product revenues to date have been limited. In 2015, the majority of our revenues were from the sale of LuViva devices and disposables, as well as some revenue from grants from the NIH and licensing agreement fees received. We expect that the majority of our revenue in 2016 will be derived from revenue from the sale of LuViva devices and disposables.Recent DevelopmentsReverse Stock Split.On May 18, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a reverse stock split by a ratio of not less than 1:10 and not more than 1:400, with no change in the numberfair value of authorizedwarrants), and a net change from financing activities of $470,000, which primarily represented the proceeds received from proceeds from debt financing.stock, and all fractional shares created by the reverse stock splitstock. The warrant is exercisable at any time, at an exercise price per share equal to be rounded to the nearest whole share. On May 26, 2016, our largest stockholder, John Imhoff, who is also one of our directors, agreed to vote his shares of common stock in favor$0.00514 (110% of the reverse stock split. As of the record date for the 2016 annual meeting, Dr. Imhoff held 19.26% of the outstanding shares of common stock. The reverse stock split will not be effective unless and until the board files an amendment to our certificate of incorporation, which must occur prior to the first anniversary of the 2016 annual meeting. It is our intent to effect the reverse stock split prior to the closing of this offering. 35Warrant Restructuring. Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 115,237,788 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), we issued or are obligated to issue 13,517,342 shares of common stock and new warrants that, if exercised as of June 14, 2016, would be exercisable for an aggregate of 214,189,622 shares of common stock.In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), we and the holder further agreed that we will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volumeprice of the common stock for that trading day.The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of this offering. The new warrants will have an initial exercise price equal toon the exercise price of the surrendered warrants as of immediatelyday prior to consummation of this offering,issuance), subject to certain customary “downside price protection” for as long as our common stock is not listed onadjustments and price-protection provisions contained in the warrant. The warrant has a national securities exchange, and will expire five yearsfive-year term. The note matured nine months from the date of issuance.Shenghuo License Agreement.On June 5, 2016, we entered intoissuance and, in addition to the original issue discount, accrues interest at a license agreement with Shenghuo Medical, LLC pursuantrate of 12% per year. We could have prepaid the note, in whole or in part, for 115% of outstanding principal and interest until 30 days from issuance, for 125% of outstanding principal and interest at any time from 31 to which we granted Shenghuo an exclusive license60 days from issuance, and for 130% of outstanding principal and interest at any time from 61 days from issuance to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. Under the terms of the license agreement, once Shenghuo is capable of manufacturing LuViva in accordance with ISO 13485 for medical devices, Shenghuo will pay us a royalty equal to $2.00 or 20% of the distributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories.In connection with the license grant, Shenghuo will underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also will provide up to $1.0 million in furtherance of our efforts to secure regulatory approval for LuViva180 days from issuance. After six months from the U.S. Food and Drug Administration,date of issuance, Auctus may convert the note, at any time, in exchange for the right to receive payments equal to 2% of our future saleswhole or in the United States, up to an aggregate of $4.0 million.Pursuant to the license agreement, Shenghuo has the option, after our next annual meeting of stockholders, to have a designee appointed to our board of directors.As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertiblepart, into shares of our common stock, at a conversion price per shareequal to the lower of $0.017402,the price offered in our next public offering or a 40% discount to the average of the two lowest trading prices of the common stock during the 20 trading days prior to the conversion, subject to certain customary anti-dilution adjustment.adjustments and price-protection provisions contained in the note. The note will be unsecured, and is expected to provide forincludes customary events of default. We will also issue Shenghuodefault provisions and a five-year warrant exercisable immediatelydefault interest rate of 24% per year. Upon the occurrence of an event of default, Auctus may require us to redeem the note (or convert it into shares of common stock) at 150% of the outstanding principal balance plus accrued and unpaid interest. In connection with the transaction, we agreed to reimburse Auctus for approximately 13.8 million$30,000 in legal and diligence fees, of which we paid $10,000 in cash and $20,000 in restricted shares of common stock, valued at an exercise price equal$0.40 per share (a 42.86% discount to the conversionclosing price of the note, subject to customary anti-dilution adjustment.Short Term Promissory Notes.On May 4, 2016 and May 26, 2016, Aquarius Opportunity Fund advanced us a total of $87,500 for 2% simple interest notes due the earlier of December 31, 2016 or consummation of this offering. Also on May 26, 2016, GPB Debt Holdings II LLC, holder of our outstanding senior secured convertible note, advanced as an additional $87,500,common stock on the same terms as the note.day prior to issuance). We intend to offer eachallocated proceeds of them the opportunity to participate in the offering at least up$90,000 to the extent of the outstanding principalwarrants and interest on these cash advances, by extinguishing all or a portion of the debt on a dollar-for-dollar basis.Series C Exchanges. Series C Exchanges.Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock. 36The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.” See “Description of Securities” for a description of the material terms of the Series C1 preferred stock.Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 7,148,813 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreementissued in connection with the financing. Finally, Aquarius, as the holderAs of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described in “—Recent Developments.”Critical Accounting PoliciesOur material accounting policies, which we believe are the most critical to an investors understanding of our financial results and condition, are discussed below. Because we are still early in our enterprise development, the number of these policies requiring explanation is limited. As we begin to generate increased revenue from different sources, we expect that the number of applicable policies and complexity of the judgments required will increase.Revenue Recognition: We recognize revenue from contracts on a straight line basis, over the terms of the contract. We recognize revenue from grants based on the grant agreement, at the time the expenses are incurred. Revenue from the sale of the Company’s products is recognized upon shipment of such products to its customers.Valuation of Deferred Taxes: We account for income taxes in accordance with the liability method. Under the liability method, we recognize deferred assets and liabilities based upon anticipated future tax consequences attributable to differences between financial statement carrying amounts of assets and liabilities and their respective tax bases. We establish a valuation allowance to the extent that it is more likely than not that deferred tax assets will not be utilized against future taxable income.Valuation of Equity Instruments Granted to Employee, Service Providers and Investors: On the date of issuance, the instruments are recorded at their fair value as determined using either the Black-Scholes valuation model or Monte Carlo Simulation model. See Note 4 to the consolidated financial statements accompanying this report for the assumptions used in the Black-Scholes valuation.Allowance for Accounts Receivable: We estimate losses from the inability of our customers to make required payments and periodically review the payment history of each of our customers, as well as their financial condition, and revise our reserves as a result.Inventory Valuation: All inventories are stated at lower of cost or market, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased.Reverse Stock Split: On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change. 37Results of Operations (except where indicated, all per share amounts reported on a post-February 2016 reverse stock split basis, but on a pre-__________ 2016 reverse stock split basis)Comparison of the Three Months Ended March 31, 20162018, we had net debt and 2015Sales Revenue, Costinterest of Sales and Gross Loss from Devices and Disposables: Sales revenue from the sale of LuViva devices and disposables for the three months ended March 31, 2016, was $262,000, a 106% increase compared to the same period in 2015. Related costs of sales and net realizable value expenses were approximately $68,000, which resulted in a gross profit of approximately $194,000. Sales revenue for the three months ended March 31, 2015, was approximately $127,000. Related costs of sales were approximately $107,000, which resulted in a gross loss on the device and disposables of approximately $20,000. The increase was related to additional sales of devices and disposables with the Company’s primary distributor, which carry a higher profit margin than unit sales.Research and Development Expenses: Research and development expenses decreased to approximately $290,000 for the three months ended March 31, 2016, compared to $373,000 for the same period in 2015. The decrease, of approximately $83,000, was primarily due to a slight decrease in payroll expenses.Sales and Marketing Expenses: Sales and marketing expenses were approximately $117,000 during the three months ended March 31, 2016, compared to $172,000 for the same period in 2015. The decrease was primarily due to the Company-wide expense reduction and cost savings efforts.General and Administrative Expenses: General and administrative expenses decreased to approximately $917,000 during the three months ended March 31, 2016, compared to approximately $963,000 for the same period in 2015. The decrease of approximately $46,000, or 5.0%, is primarily related to lower compensation and option expenses incurred during the same period.Other Income: Other income for the three months ended March 31, 2016, was approximately $23,000, compared to other income of approximately $21,000 for the three months ended March 31, 2015.Interest Expense: Interest expense decreased to approximately $158,000 for the three months ended March 31, 2016,$30,800 as compared to approximately $492,000net debt and interest of $76,664 for the same period in 2015, primarily due to amortization of debt discount and debt issuance costs that were higher for the same period in 2015.Fair Value of Warrants Expense: Fair value of warrants expense recovery was approximately $1,395,000 for the three months ended March 31, 2016, as compared to approximately $714,000 for the same period in 2015.Net income was approximately $130,000 during the three months ended March 31, 2016, compared to a net loss of $1,245,000 for the same period in 2015, for the reasons outlined above.Comparison of 2015 and 2014General:Net loss attributable to common stockholders deceased to approximately $9.5 million, or $7.42 per share, in 2015, from $10.0 million, or $13.02 per share, in 2014.Sales Revenue, Cost of Sales and Gross Loss from Devices and Disposables:Revenues from the sale of LuViva devices for 2015 and 2014 were approximately $564,000 and $758,000, respectively. Related costs of sales and valuation allowances on the net realizable values were approximately $537,000 and $891,000 in 2015 and 2014, respectively, which resulted in a gross profit of approximately $27,000 on the sales of devices and disposables for 2015 and a gross loss of approximately $133,000 for 2014.Revenue from Grants and other Agreements: Revenue from grants and other agreements decreased to approximately $42,000 in 2015, from $65,000 in 2014, primarily due to fewer royalty receipts from certain agreements in 2015. There were no costs of sales associated with this revenue in 2015 and 2014.Research and Development Expenses: Research and development expenses decreased to approximately $1.5 million for 2015, from approximately $2.8 million in 2014. The decrease was primarily due to reduction in research and development expenses due to product launch.Sales and Marketing Expenses: Sales and marketing expenses decreased to approximately $718,000 in 2015, compared to approximately $1.2 million in 2014, due to an ongoing expense reduction program. 38General and Administrative Expense:General and administrative expense decreased to approximately $4.1 million in 2015, from about $4.6 million in 2014. The decrease, of approximately 548,000 or 11.8%, was primarily due to an overall reduction in operating expenses in 2015.Other Income: Other income was approximately $74,000 in 2015, compared to $25,000 in 2014. The increase was primarily related to income from a distribution agreement entered into in December 31, 2015.Interest Expense:Interest expense increased to approximately $1.3 million for 2015, as compared to approximately $979,000 for 2014. The increase was primarily related to amortization expense of debt issuance cost and amortization of debt discount, in conjunction with our financing efforts in 2015.Loss on Extinguishment of Debt: Loss on extinguishment of debt was zero for 2015, compared to approximately $325,000 for 2014. There was no debt extinguished in the year ended December 31, 2015.Fair Value of Warrants Expense:Fair value of warrants recovery was approximately $568,000 for 2015, as compared to recovery of $65,000 for 2014. The change in fair value of warrants was primarily due to the significant changes in warrant conversion prices, in the fiscal year ended December 31, 2015.There was no income tax benefit recorded for 2015 or 2014, due to recurring net operating losses.Liquidity and Capital ResourcesSince our inception, we have raised capital through the private sale of preferred stock and debt securities, public and private sales of common stock, funding from collaborative arrangements, and grants. At March 31, 2016, we had cash of approximately $56,000 and working capital deficit of approximately $4.0 million.Our major cash flows in the quarter ended March 31, 2016, consisted of cash out-flows of approximately $955,000 from operations, no cash inflow nor outflow from investing activities and a net change from financing activities of $976,000, which primarily represents the proceeds received from the issuance of $1,437,500 in principal amount of a 17.0% senior secured convertible note, which resulted in net cash proceeds to the Company of $1,029,000.Our major cash flows for the year ended December 31, 2015 consisted of cash out-flows of $4.0 million from operations, including approximately $6.9 million of net loss, cash outflows of $8,000 from investing activities and a net change from financing activities of $3.9 million, which primarily represented the proceeds received from issuance of common stock and warrants, proceeds from debt financing, as well as exercise of outstanding warrants and options.February 20, 2014, our President and CEO, Gene Cartwright, advanced us $50,000, in cash, for a 10% simple interest note. On October 23, 2014, August 4, 2014, and May 21, 2014, he advanced us $30,000, $200,000, and $100,000, respectively, in cash for 5% simple interest notes. On December 30, 2015, he advanced us $10,000 in cash for a 6% simple interest note. On October 24, 2014, October 7, 2014, and August 26, 2014, our Senior Vice President of Engineering, Richard Fowler, advanced us $6,100, $20,000 and $75,000, respectively, in cash for 6% simple interest notes. On October 7, 2014, our Director of Marketing advanced us $10,000 in cash for a 6% simple interest note. On November 4, 2014, Richard Blumberg, one of our stockholders, advanced us $100,000 in cash for a note for $106,500 in aggregate principal and interest due November 30, 2014. On February 20, 2014, directors Michael James, Linda Rosenstock, and John Imhoff advanced us $50,000, $50,000, $50,000, and $25,000 in cash, respectively, for 10% simple interest notes.On April 23, 2014,17, 2017, we entered into a securities purchase agreement with an accredited investorEagle Equities, LLC, providing for the issuance and salepurchase by Eagle of a 6% seniortwo convertible note with an initialredeemable notes in the aggregate principal amount of $1.5 million$88,000, with the first note being in the amount of $44,000, and an 18-month term, forthe second note being in the amount of $44,000. The first note was fully funded on May 19, 2017, upon which we received $40,000 of net proceeds (net of a purchase price of $1.0 million (an approximately 33.3%10% original issue discount). Additionally, pursuantThe second note was issued on December 21, 2017 and was initially paid for by the issuance of an offsetting $40,000 secured note issued by Eagle. Eagle was required to pay the purchase agreement, the investor purchased on May 23, 2014 an additional 6% senior convertible note with a principal amount of $2.0 millionits secured note in cash and in full prior to executing any conversions under the second note we issued. The notes bear an 18-month term, for a fixed purchase priceinterest rate of $2.0 million. On July 1, 2015, we repaid the entire outstanding balance.On September 2, 2014, we entered into a subscription agreement with a Turkish corporation, pursuant to which, on September 27, 2014, we sold 1,651,042 shares of our common stock (pre-stock split) and a warrant to purchase an additional 325,521 shares (pre-stock split), for an aggregate purchase price of $200,000. The warrant is immediately exercisable, has an exercise price per share of $0.4608 (pre-stock split)8%, and expires five years from the date of issuance. 39On September 10, 2014, we entered into a note purchase agreement with an accredited investor pursuant to which we sold a secured promissory note with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). Weare due and payable on May 17, 2018. The notes may prepay the notebe converted by Eagle at any time. The note is secured by our current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the note purchase agreement. As a result of a series of amendments to the note (since assigned to two accredited investors), the maturity date currently is August 31, 2016, and has been accruing interest at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. Pursuant to the amendments, the holders may convert the outstanding balancetime after five months from issuance into shares of our common stock at a conversion price per share equal to(as determined in the lower of (1) $0.25 (pre-stock split) and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion pricenotes) calculated at the time of any conversion, request wouldexcept for the second note, which also requires full payment by Eagle of the secured note it issued to us before conversions may be lower than $0.15(pre-stock split) per share,made. The conversion price of the notes will be equal to 60% of the lowest trading price of the common stock for the 20 prior trading days including the day upon which we havereceive a notice of conversion. The notes may be prepaid in accordance with the terms set forth in the notes. The notes also contain certain representations, warranties, covenants and events of default including if we are delinquent in our periodic report filings with the SEC, and increases in the amount of the principal and interest rates under the notes in the event of such defaults. In the event of default, at Eagle’s option and in its sole discretion, Eagle may consider the notes immediately due and payable. As of deliveringMarch 31, 2018, the conversion amount in cash in lieunotes had been converted and no balance remained outstanding, as compared to net debt of shares$41,322, including unamortized original issue discount of our common stock.November 26, 2014,May 17, 2017, we entered into a securities purchase agreement with certain accredited investorsAdar Bays, LLC, providing for the issuancepurchase by Adar of two convertible redeemable notes in the aggregate principal amount of $88,000, with the first note being in the amount of $44,000, and salethe second note being in the amount of $44,000. The first note was fully funded on May 19, 2017, upon which we received $40,000 of net proceeds (net of a public offering10% original issue discount). The second note was issued on December 21, 2017 and was initially paid for by the issuance of an aggregateoffsetting $40,000 secured note issued by Adar. Adar was required to pay the principal amount of 16,785,415its secured note in cash and in full prior to executing any conversions under the second note we issued. The notes bear an interest rate of 8%, and are due and payable on May 17, 2018. The notes may be converted by Adar at any time after five months from issuance into shares of our common stock (pre-stock split) and five-year warrants(as determined in the notes) calculated at the time of conversion, except for the second note, which also requires full payment by Adar of the secured note it issued to purchase an aggregate of 8,392,708 additional shares (pre-stock split) at a purchaseus before conversions may be made. The conversion price of $0.225 per share (pre-stock split),the notes will be equal to 60% of the lowest trading price of the common stock for aggregate gross proceeds (including non-cash extinguishment of debt) of approximately $3.8 million. We consummated the public offering on December 2, 2014.On March 16, 2015 and March 19, 2015, we entered into subscription agreements with certain accredited investors, pursuant to20 prior trading days including the day upon which we agreedreceive a notice of conversion. The notes may be prepaid in accordance with the terms set forth in the notes. The notes also contain certain representations, warranties, covenants and events of default including if we are delinquent in our periodic report filings with the SEC, and increases in the amount of the principal and interest rates under the notes in the event of such defaults. In the event of default, at Adar’s option and in its sole discretion, Adar may consider the notes immediately due and payable. As of March 31, 2018, the notes had been converted and no balance remained outstanding. As of March 31, 2018, the notes had been converted and no balance remained outstanding, as compared to sell an aggregatenet debt of 4.0 million shares$42,216, including unamortized original issue discount of our common stock (pre-stock split)$5,214, unamortized and three-year warrants to purchase an additional 2.0 million shares (pre-stock split) with an exercise price per sharedebt issuance costs of $0.255 (pre-stock split),$11,160 for an aggregate purchase price of $720,000.June 29, 2015,May 18, 2017, we entered into a securities purchase agreement with certain accredited investorsGHS Investments, LLC, an existing investor, providing for the issuance and salepurchase by GHS of an aggregate of 6,737 shares of our Series C preferred stock, at a purchase price of $750 per share and an initial conversion price of $0.095 per share (pre-stock split), and five-year warrants exercisable to purchase an aggregate of approximately 106.4 million shares of our common stock (pre-stock split) at an initial exercise price of $0.095 per share (pre-stock split), subject to certain customary adjustments and anti-dilution provisions. On September 3, 2015 we entered into an interim agreement amending the securities purchase agreement to provide for certain of the investors to purchase an additional aggregate of $550,000 in shares of Series C preferred stock and warrants on the same terms as set forth in the original agreement.On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change.On February 11, 2016, we consented to an assignment of our outstanding securedconvertible promissory note to two accredited investors. In connection within the assignment, the holders waived an ongoing event of default under the notes related to our minimum market capitalization, and agreed to eliminate the requirement going forward. On March 7, 2016, we further amended the notes to eliminate the volume limitations on sales of common stock issued or issuable upon conversion of the notes.On February 11, 2016, we entered into a securities purchase agreement with an accredited investor for the issuance and sale on February 12, 2016 of approximately $1.4 million in aggregate principal amount of $66,000, for $60,000 in net proceeds (representing a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20%10% original issue discount). The transaction closed on May 19, 2017. The note matures onupon the second anniversaryearlier of issuanceour receipt of $100,000 from revenues, loans, investments, or any other means (other than the Eagle and inAdar bridge financings) and December 31, 2017. In addition to the 20%10% original issue discount, the note accrues interest at a rate of 17%8% per year. SubjectWe may prepay the note, in whole or in part, for 110% of outstanding principal and interest until 30 days from issuance, for 120% of outstanding principal and interest at any time from 31 to certain restrictions, it is60 days from issuance and for 140% of outstanding principal and interest at any time from 61 days to 180 days from issuance. The note may not be prepaid after 180 days. After six months from the date of issuance, the note will become convertible, at any time thereafter, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.8060% of the lowest trading price during the 25 trading days prior to conversion. The note includes customary event of default provisions and a default interest rate of the lesser of 20% per share, subjectyear or the maximum amount permitted by law. Upon the occurrence of an event of default, the holder of the note may require us to redeem the note (or convert it into shares of common stock) at 150% of the outstanding principal balance. As of March 31, 2018, we had net debt of $66,000 and interest of $6,793, as compared to net debt of $66,000 for the period ended December 31, 2017.customary adjustmentsrepresentations, warranties, covenants and anti-dilution provisions.events of default, including if we are delinquent in its periodic report filings with the SEC, and provides for increases in principal and interest in the event of such defaults As of March 31, 2018, the notes had been converted and no balance remained outstanding as compared to a net debt of $46,405, including unamortized debt issuance costs of $6,595 for the period ended December 31, 2017.addition,consideration for the investor receivedforbearance, we agreed to waive, release, and discharge GPB from all claims against GPB based on facts existing on or before the date of the forbearance agreement in connection with the note, or the dealings between we and GPB, or our equity holders and GPB, in connection with the note. Pursuant to the forbearance agreement, we have reaffirmed its obligations under the note and related documents and executed a five-yearconfession of judgment regarding the amount due under the note, which GPB may file upon any future event of default by us. During the forbearance period, we must continue to comply will all the terms, covenants, and provisions of the note and related documents.approximately 1.79 million3,409,090 shares of our common stock withstock. The warrant is exercisable at any time, at an exercise price of $0.80 per share equal to $0.00228 (110% of the closing price of the common stock on the day prior to issuance), subject to certain customary adjustments and anti-dilution provisions.In connection withprice-protection provisions contained in the transaction, on Februarywarrant. The warrant has a five-year term. The note matured nine months from the date of issuance and, in addition to the original issue discount, accrues interest at a rate of 12% per year. We could have prepaid the note, in whole or in part, for 115% of outstanding principal and interest until 30 days from issuance, for 125% of outstanding principal and interest at any time from 31 to 60 days from issuance, and for 130% of outstanding principal and interest at any time from 61 days from issuance to 180 days from issuance. After six months from the date of issuance, Auctus may convert the note, at any time, in whole or in part, into shares of our common stock, at a conversion price equal to the lower of the price offered in our next public offering or a 40% discount to the average of the two lowest trading prices of the common stock during the 20 trading days prior to the conversion, subject to certain customary adjustments and price-protection provisions contained in the note. The note includes customary events of default provisions and a default interest rate of 24% per year. Upon the occurrence of an event of default, Auctus may require us to redeem the note (or convert it into shares of common stock) at 150% of the outstanding principal balance plus accrued and unpaid interest. As of March 31, 2018, we had net debt of $118,500 including unamortized debt issuance costs of $31,000.2016,2018, we and the investor entered into a four-year consultingsecurities purchase agreement pursuant towith Eagle Equities, LLC, providing for the purchase by Eagle of a convertible redeemable note in the principal amount of $66,667. The note was fully funded on March 14, 2018, upon which we received $51,000 of net proceeds (net of a 10% original issue discount and other expenses). The note bears an interest rate of 8%, and are due and payable on May 12, 2019. The note may be converted by Eagle at any time after twelve months from issuance into shares of our common stock (as determined in the investor will provide management consulting servicesnotes) calculated at the time of conversion, except for the second note, which also requires full payment by Eagle of the secured note it issued to us in exchange for a royalty payment, payable quarterly,before conversions may be made. The conversion price of the notes will be equal to 3.5%60% of the lowest trading price of the common stock for the 20 prior trading days including the day upon which we receive a notice of conversion. The notes may be prepaid in accordance with the terms set forth in the notes. The notes also contain certain representations, warranties, covenants and events of default including if we are delinquent in our periodic report filings with the SEC, and increases in the amount of the principal and interest rates under the notes in the event of such defaults. In the event of default, at Eagle’s option and in its sole discretion, Eagle may consider the notes immediately due and payable. As of March 31, 2018, the outstanding balance was $51,816, including unamortized debt issuance costs of $8,532 and unamortized discount of $6,319.revenues fromcommon stock (as determined in the salenotes) calculated at the time of products.See “—Recent Developments”conversion, except for information regarding capital-raising activities sincethe second note, which also requires full payment by Adar of the secured note it issued to us before conversions may be made. The conversion price of the notes will be equal to 60% of the lowest trading price of the common stock for the 20 prior trading days including the day upon which we receive a notice of conversion. The notes may be prepaid in accordance with the terms set forth in the notes. The notes also contain certain representations, warranties, covenants and events of default including if we are delinquent in our periodic report filings with the SEC, and increases in the amount of the principal and interest rates under the notes in the event of such defaults. In the event of default, at Adar’s option and in its sole discretion, Adar may consider the notes immediately due and payable. As of March 31, 2016.the second quarter of 2016.2018. We are evaluating various options to further reduce our cash requirements to operate at a reduced rate, as well as options to raise additional funds, including loans. 40Substantialstatements. 41 AND EXECUTIVE OFFICERS,officers as of June 15, 2016:officers:Name Age Position with Guided Therapeutics Gene S. Cartwright, Ph.D. 6263 Chief ExecuteExecutive Officer, President, Acting Chief Financial Officer and DirectorMark Faupel, Ph.D. 62 Chief Operating Officer and Director Richard L. Fowler 5961 Senior Vice President of Engineering Richard P. Blumberg 61 Director John E. Imhoff, M.D. 6768 Director Michael C. James 5759 Chairman and Director Jonathan M. Niloff, M.D. 62Director Linda Rosenstock, M.D.65Director On May 5, 2016 and May 9, 2016 respectively, Drs. Niloff and Rosenstock formally announced their decisions not to stand for reelection to our board of directors at our 2016 annual meeting of stockholders. Both of their decisions to resign were for personal reasons and were not a result of any disagreement with the Company on any matter relating to our operations, policies or practices.31,11, 2014. His most recent position was with Omnyx, LLC, a Joint Venture between GE Healthcare and the University of Pittsburgh Medical Center, where, as CEO for over four years he founded and managed the successful development of products for the field of Digital Pathology. Prior to his work with Omnyx, LLC, he was President of Molecular Diagnostics for GE Healthcare. Prior to GE, Dr. Cartwright was Divisional Vice President/General Manager for Abbott Diagnostics’ Molecular Diagnostics business. In his 24 year career at Abbott, he also served as Divisional Vice President for U.S. Marketing for five years. He received a Masters of Management degree from Northwestern’s Kellogg School of Management and also holds a Ph.D. in chemistry from Stanford University and an AB from Dartmouth College. Mr. Fowler, Senior Vice President of Engineering is an accomplished Executive with significant experience in the management of businesses that sell, market, produce and develop sophisticated medical devices and instrumentation. Mr. Fowler’s 25 plus years of experience includes assembling and managing teams, leading businesses and negotiating contracts, conducting litigation, and developing ISO, CE, FDA QSR, GMP and GCP compliant processes and products. He is adept at providing product life cycle management through effective process definition and communication - from requirements gathering, R&D feasibility, product development, product launch, production startup and support. Mr. Fowler combines outstanding analytical, out-of-the-box, and strategic thinking with strong leadership, technical, and communication skills and he excels in dynamic, demanding environments while remaining pragmatic and focused. He is able to deliver high risk projects on time and under budget as well as enhance operational effectiveness through outstanding cross-functional team leadership (R&D, marketing, product development, operations, quality assurance, sales, service, and finance). In addition, Mr. Fowler is well versed in global medical device regulatory and product compliance requirements. 42 15, 2013. Mr. James is also the Managing Partner of Kuekenhof Capital Management, LLC, a private investment management company, Chief Executive Officer and the Chief Financial Officer of Inergetics, Inc., a nutraceutical supplements company and also the Chief Financial Officer of Terra Tech Corporation, which is a hydroponic and agricultural company. He also holds the position of Managing Director of Kuekenhof Equity Fund, L.P. and Kuekenhof Partners, L.P. Mr. James currently sits on the Board of Directors of Inergetics; Inc. Mr. James was Chief Executive Officer of Nestor, Inc. from January 2009 to September 2009 and served on their Board of Directors from July 2006 to June 2009. He was employed by Moore Capital Management, Inc., a private investment management company from 1995 to 1999 and held position of Partner. He was employed by Buffalo Partners, L.P., a private investment management company from 1991 to 1994 and held the position of Chief Financial and Administrative Officer. He began his career in 1980 as a staff accountant with Eisner LLP. Mr. James received a B.S. degree in Accounting from Farleigh Dickinson University in 1980.Jonathan M. Niloff, M.D.was electeddirector in April 2010. Dr. Niloff was Vice President and Chief Medical Officer at McKesson Technology Solutions, a medical software company until May 6, 2016. Prior to that, Dr. Niloff was the Founder, Chairmanresult of growth of the Board and Chief Medical Officer of MedVentive Inc. Prior to joining MedVentive, Dr. Niloff servedCompany or as President of the Beth Israel Deaconess Physicians Organization, Medical Director for Obstetrics and Gynecology for its Affiliated Physicians Group, and Chief of Gynecology at New England Deaconess Hospital. He served as an Associate Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School. He has deep expertise in all aspects of medical cost and quality improvement, and has published extensively on the topic of gynecologic oncology including the development of the CA125 test for ovarian cancer. Dr. Niloff received his undergraduate education at The Johns Hopkins University, an M.D. degree from McGill University, and an MBA degree from Boston University.Dr. Niloff is uniquely qualified to assist the Board and management because he combines his clinical background as a Harvard Ob-Gyn with his business acumen developed through an MBA degree and as CMO of MedVentive. Dr. Niloff has specific experience in evaluating new medical technology (e.g., CA125) and its implications to cost containment and reimbursement. Furthermore, Dr. Niloff has numerous professional contacts in the Ob-Gyn community that can aid in our development and marketing of our cervical cancer detection technology.Linda Rosenstock, M.D.was appointed to the Board in April 2012. Dr. Linda Rosenstock is Dean Emeritus (served as Dean from 2000 - 2012) of the University of California, Los Angeles (UCLA) Fielding School of Public Health. She holds appointments at UCLA as Professor of Health Policy and Management, Medicine and Environmental Health Sciences and is a recognized authority in broad areas ofmandated by public health and science policy. Internationally, Dr. Rosenstock has been active in teaching and research in many developing countries and has served as an advisor to the World Health Organization. Dr. Rosenstock also chaired the United Auto Workers/General Motors Occupational Health Advisory Board. She is an Honorary Fellow of the Royal College of Physicians and an elected member of the National Academy of Sciences' Institute of Medicine where she has served as a member of their Board on Health Sciences Policy and Chair of the Committee for Preventive Services for Women. In January 2011, she was appointed by President Obama to the Advisory Group on Prevention, Health Promotion and Integrative and Public Health. She has served on the Board of Directors for Skilled Health Care since 2009.Before coming to UCLA in 2000, Dr. Rosenstock served as Director of the National Institute for Occupational Safety and Health (NIOSH) for nearly seven years. As Director of NIOSH, Dr. Rosenstock led the only federal agency with a mandate to undertake research and prevention activities in occupational safety and health. During her tenure, she was instrumental in creating the National Occupational Research Agenda, a framework for guiding occupational safety and health research, and in expanding the agency's responsibilities. In recognition of her efforts, Dr. Rosenstock received the Presidential Distinguished Executive Rank Award, the highest executive service award in the government and was also the James P. Keogh Award Winner for 2011 in appreciation of a lifetime of extraordinary leadership in occupational health and safety. Dr. Rosenstock received her M.D. and M.P.H. from The Johns Hopkins University. She conducted her advanced training at the University of Washington, where she was Chief Resident in Primary Care Internal Medicine and a Robert Wood Johnson Clinical Scholar.Dr. Rosenstock is uniquely qualified as a Board Member for Guided Therapeutics. First, as a trained physician who has also chaired the Preventive Services for Women Committee of the National Academies’ Institute of Medicine, she has been directly involved in setting institutional and government policy for breast and cervical cancer screening, which is directly relevant to our LuViva cervical cancer detection device. Secondly, she brings a wealth of international experience in developing countries, which is a focus of our product distribution effort in cancer detection. Thirdly, she has demonstrated a lifetime of extraordinary leadership and her international recognition as an expert in health policy will provide outstanding credibility to Guided Therapeutics as a leading innovator in women’s healthcare. 43CORPORATE GOVERNANCEBoard Meetings and CommitteesOur boarddirectors held fourteen meetings in 2015. No director attended fewer than 75%Ethicsthe meetings of the board of directors or the committees on which he or she served during 2015. We encourage our directorsethics that applies to attend the annual meeting of stockholders. In 2015, all of our directors, attendedofficers and employees. To obtain a copy without charge, contact our annual meeting. All of the members of the board of directors, with the exception of our Chief Executive Officer, serve on the audit, compensation and nomination committees. Although we are not subject to the listing standards of any national securities exchange or inter-dealer quotation system, based on the definition of independence in the NASDAQ listing standards, Dr. Imhoff, Mr. James, Dr. Niloff and Dr. Rosenstock are independent directors. The board works with its members and management to identify new board members, and will consider nominees recommended by stockholders. Any recommendation should be addressed in writing to the Board of Directors, c/o Corporate Secretary, Guided Therapeutics, Inc., 5835 Peachtree Corners East, Suite D,B, Norcross, Georgia 30092.audit committee selectsfollowing table lists specified compensation we paid or accrued during each of the fiscal years ended December 31, 2017 and engages2016 to the independent registered public accounting firmChief Executive Officer and our two other most highly compensated executive officers, collectively referred to audit our annualas the “named executive officers,” in 2016:President, CEO, Acting CFO and Director (2) 2016
Mark Faupel, Ph.D. 2017
Richard Fowler, Senior Vice President of Engineering and pre-approves all allowable auditthat accompany this prospectus.and any special assignments given to the accountants. The audit committee also determines the planned scope of the annual audit, any changes in accounting principles, the effectiveness and efficiency of our internal accounting staff and the independence of our external auditors. The audit committee met fourteen times in 2015. The audit committee currently consists of Dr. Imhoff, Mr. James and Drs. Niloff, and Rosenstock. Each member of the audit committee is independent in accordance with the NASDAQ listing standards for audit committee independence and applicable SEC regulations. None of the members of the audit committee has participated in the preparation of our financial statements at any time during the past three years. The board has also determined that Mr. James and Drs. Niloff, Imhoff, and Rosenstock meet the criteria specified under applicable SEC regulations forus on an “audit committee financial expert” and thatas-needed basis. On December 8, 2016, the committee members are financially sophisticated.The board of directors appointed Dr. Faupel as our new COO and director.consultation with our Chief Executive Officer, sets2016, Dr. Cartwright agreed to reduce his base salary compensation to $75,000 from $300,000. The board-granted performance bonus remained the same at $150,000 for both years, and he received usual customary company benefits. During 2015, he also received 20,000 performance-based restricted shares of common stock, which will vest as follows: (1) seven shares will vest if the stock price closes at or above $1,200 for 30 consecutive trading days, and an additional seven will vest on the first anniversary of such vesting date, in each case subject to continuous employment through the applicable vesting date; and (2) seven shares will vest if the stock price closes at or above $200,000 for 30 consecutive trading days, and an additional seven will vest on the first anniversary of such vesting date, in each case subject to continuous employment through the applicable vesting date. As of December 31, 2017, Dr. Cartwright’s deferred salary plus interest was $383,039 and his deferred bonus was $600,000.our officers, reviews management organizationpast un-paid salary.development, reviews significant employee benefit programs and establishes and administers executiveagreed to reduce his base salary compensation programs. The compensation committee currently consists of Dr. Imhoff, Mr. James, Drs. Niloff and Rosenstock, each of whom is independent under NASDAQ listing standards. The compensation committee met fourteen timesto $107,500 from $243,000 in 2015.The board of directors, in consultation with our Chief Executive Officer, reviews For both years he received the usual and recommends individuals to be nominated as directors. Our board has historically evaluated all candidates based upon, among other factors, a candidate’s financial literacy, knowledge of our industry or other background relevant to our needs, status as a stakeholder, independence, and willingness, ability and availability for service. Other than the foregoing, there have beencustomary company benefits. He received no stated minimum criteria for director nominees, although our board has considered such other factors as it has deemed to bebonus in the best interestsyears ended December 31, 2017 and 2016. In 2015, he received options to purchase 1,930 shares of us and our stockholders. The board has considered diversity as it has deemed appropriate in this context (without having a formal diversity policy), given current needs and the current needscommon stock, which vest over 48 months. As of the boardDecember 31, 2017, Mr. Fowler’s total deferred salary plus interest was approximately $429,053.maintain a balance of knowledge, experience and capability. When considering diversity, the board has considered diversity as one factor, of no greater or lesser importance than other factors and has considered diversity in a broad context of race, gender, age, business experience, skills, international experience, education, other board experience and other relevant factors.The audit committee and the compensation committee have each adopted charters, which are availableOfficers at December 31, 2017 12/31/2024 12/31/2024 12/31/2024 our web site,all outstanding options.www.guidedinc.com/Investors.htm. The nomination committee currently operates without a charter.Board Leadership Structure and Role in December 31, 2017 Name and Principal Position Ronald W. Hart, Ph.D., Director (resigned as of December 11, 2015) John E. Imhoff, M.D., Director Michael C. James, Chairman and Director Jonathan Niloff, M.D., former Director Linda Rosenstock, M.D., former Director Dr. Cartwright, our President and Chief Executive Officer, also serves as a director; our board is led by the Chairman, Mr. James, one of our independent directors. ourthe Company’s management regularly communicates with the board to discuss important risks for their review and oversight, including regulatory risk, and risks stemming from periodic litigation or other legal matters in which we are involved. Given the small size of the board, the board feels that this structure for risk oversight is appropriate (except for those risks that require risk oversight by independent directors only). 44The board of directors is specifically charged with discussing risk management (primarily financial and internal control risk), and receives regular reports from management, independent auditors, internal audit and outside legal counsel on risks related to, among others, our financial controls and reporting. The board of directors reviews risks related to compensation and makes recommendations to the board with respect to whether our compensation policies are properly aligned to discourage inappropriate risk-taking, and is regularly advised by management and, as deemed appropriate, outside legal counsel.Communication with DirectorsAny stockholder is welcome to communicate with any director or the board of directors by writing to a director or the board as a whole, c/o Corporate Secretary, 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092.Director CompensationNone of our directors received any compensation or reimbursement in cash in 2015; however, they did receive stock options or shares, in lieu of cash, for 2015 and 2014, respectively, in connection with their services as members of the board of directors and their service on board committees.Director Compensation Table, for year ended December 31, 2015Name and Principal PositionStock OptionAwards (#)Michael C. James, Chairman and Director6,000John E. Imhoff, M.D., Director6,000Jonathan M. Niloff, M.D., Director6,000Linda Rosenstock, M.D., Director6,000EXECUTIVE COMPENSATIONThe purpose of our compensation philosophy, policies and practices is to attract and retain experienced, highly qualified executives critical to our long-term success and enhancement of stockholder value. We rely on a combination of base salary and customary benefits along with long-term equity incentive compensation to achieve these objectives. In establishing compensation packages for our executive officers, the compensation committee believes that we provide our executive officers with the opportunity to earn a competitive annual base salary, and that options grants reward our executive officers for meaningful performance that contributes to enhanced long-term stockholder value and our general long-term financial health, and helps to align the interests of our executive officers with those of our stockholders.Summary Compensation TableThe following table lists specified compensation we paid or accrued during each of the fiscal years ended December 31, 2015 and 2014 to the Chief Executive Officer and our two other most highly compensated executive officers, collectively referred to as the “named executive officers,” in 2015:2015 and 2014 Summary Compensation Table2015 300,000 150,000 - $450,000 2014 300,000 150,000 $731,000 $1,046,000 2015 198,073 - $30,400 228,473 2014 264,097 13,600 17,000 294,697 (1)See Note 4 to the audited consolidated financial statements that accompany this prospectus.(2)All amounts reported as accrued. Dr. Cartwright has elected to get paid partial salary, due to the Company’s cash position.(3)Dr. Faupel is no longer employed by the Company, but instead provides consulting services to the Company. 45Dr. Cartwright’s 20152014 compensation consisted of a base salary of $300,000, with $150,000 performance condition related bonus,Management and the usual customary company benefits. He also received 20,000 both market and performance condition restricted shares of common stock (10,000 shares if the stock price closes at/above $1.50 for 30 consecutive trading days (the “Tier 1 Vesting Date”); subject to the Executive’s continuous employment with the Company through the applicable vesting date; (i) 5,000 shares will vest on the Tier 1 Vesting Date; and (ii) 5,000 shares will vest on the first anniversary of the Tier 1 Vesting Date. 10,000 GT stock price closes at/above $250.00 for 30 consecutive trading days (the “Tier 2 Vesting Date”); subject to the Executive’s continuous employment with the Company through the applicable vesting date: (i) 5,000 shares will vest on the Tier 2 Vesting Date; and (ii) 5,000 shares will vest on the first anniversary of the Tier 2 Vesting Date). Dr. Cartwright was also issued 350 and 2,500 of stock options that vest over 48 months in 2014. As of December 31, 2015, Dr. Cartwright’s deferred salary plus interest was $282,734 and his deferred bonus was $300,000.Dr. Faupel’s 2015 and 2014 compensation consisted of a base salary of $198,073 and $264,097, respectively, and usual and customary company benefits. He received no bonus in the years ended December 31, 2015 and 2014. He received 1,900 and 350 shares of stock options, which vest over 48 months in 20154 and 2014, respectively. As of December 31, 2015, Dr. Faupel’s remaining deferred salary plus interest and bonus was $53,433. He also holds a promissory note of $250,512 for past un-paid salary.Mr. Fowler’s 2015 and 2014 compensation consisted of a base salary of $243,000 and $203,000, respectively, and usual and customary company benefits. He received no bonus in the years ended December 31, 2015 and 2014. He received 1,930 and 350 shares of stock options, which vest over 48 months in 2015 and 2014, respectively. As of December 31, 2015, Mr. Fowler’s total deferred salary plus interest was approximately $247,469.Outstanding Equity Awards to Officers at December 31, 2015 Option Awards 756 - 2,094 27.00 12/31/2024 21,058 - 1,842 72.00 12/31/2024 6,173 - 1,977 59.00 12/31/2024 (1)Represents fully vested options.(2)Based on all outstanding options.Outstanding Equity Awards to Directors at December 31, 2015 Option Awards Name and Principal Position Ronald W. Hart, Ph.D., Director (resigned as of December 11, 2015) 10,925 22.00 John E. Imhoff, M.D., Director 9,038 33.00 Michael C. James, Chairman and Director 7,075 20.00 Jonathan Niloff, M.D., Director 7,429 22.00 Linda Rosenstock, M.D., Director 7,250 21.00
Related Stockholder Matters 46SHARE OWNERSHIP OF DIRECTORS, OFFICERS AND CERTAIN BENEFICIAL OWNERScommon stockequity securities as of June 15, 20161, 2018 by (1) each person whom we know to beneficially own more than 5% of the outstanding shares of our common stock, (2) each director, (3) each officer named in the summary compensation table above,below, and (4) all directors and executive officers as a group. Unless otherwise indicated, the address of each officer and director is 5835 Peachtree Corners East, Suite D.B, Norcross, Georgia 30092. Amount and Nature of Beneficial Ownership(1) John E. Imhoff (3) 186,727,185 76.44 % Michael C. James / Kuekenhof Equity Fund, LLP (4) 16,884 * Gene Cartwright (5) 26,379 * Richard L. Fowler (6) 7,224 * Linda Rosenstock (7) 10,123 * Jonathan Niloff (8) 10,828 * All directors and executive officers as a group (6 persons) (9) 186,798,623 76.46 % John E. Imhoff (5) Lynne Imhoff (6) Michael C. James/Kuekenhof Equity Fund, LLP (7) Gene Cartwright (8) Richard L. Fowler (9) Richard P. Blumberg (10) Mark Faupel (11) All directors and executive officers as a group (4 persons) (12) (*) Less than 1%. (1) Except as otherwise indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock. (2) Percentage ownership is based on 67,901,547211,291,990 shares of common stock outstanding as of the record date.June 1, 2018. Beneficial ownership is determined in accordance with the rules of the SEC, based on factors that include voting and investment power with respect to shares. Shares of common stock subject to convertible securities convertible or exercisable within 60 days after the record date, are deemed outstanding for purposes of computing the percentage ownership of the person holding those securities, but are not deemed outstanding for purposes of computing the percentage ownership of any other person.(3)Consists Note that certain of 10,361,179our outstanding securities, including certain warrants and the shares of Series C1 preferred stock held by the persons listed in this table, have anti-dilution “ratchet” or “price-protection” provisions that, when triggered, will increase the number of shares of common stock 168,781,637underlying such securities. Subject to customary exceptions, these provisions are triggered anytime we issue shares of common stock to third parties at a price lower than the then-current conversion price or exercise price of the subject securities. As a result, the beneficial ownership reported in this table is only as of the date presented, and the beneficial ownership amounts of the persons in this table may increase on a future date, even though such persons have not actually acquired any additional shares of common stock.(3) As of June 1 , 2018, there were 451 shares of Series C preferred stock outstanding, and each such share was convertible into approximately 137,061 shares of common stock. (4) As of June 1, 2018, there were 4,312.50 shares of Series C1 preferred stock outstanding, and each such share was convertible into approximately 137,061 shares of common stock. (5) Shares of common stock consist of 12,952 shares of common stock directly held, 1,754,912 shares issuable upon exercise of warrants, 16 shares subject to options, and 329,050,164 shares issuable upon conversion of 2,400.75 shares of Series C1 preferred stock, 7,575,331 shares issuable upon exercise of warrants, and 9,038 shares subject to options. As of the record date,stock. Dr. Imhoff is on the board of directors.(4)(6)ConsistsShares of 7,451common stock consist of 3,612 shares of common stock 2,357directly held, 973 shares issuable upon exercise of warrants, and 7,07692,516,447 shares issuable upon conversion of 675.00 shares of Series C1 preferred stock.(7) Shares of commons stock consist of 10 shares of common stock directly held, 4 shares issuable upon exercise of warrants, and 14 shares subject to options. As of the record date, Mr. James is on the board of directors.(5)(8)ConsistsShares of 22,732commons stock consist of 29 shares of common stock 2,357directly held, 4 shares issuable upon exercise of warrants, and 1,2905 shares subject to options. As of the record date, Mr. Dr. Cartwright is the CEO and on the board of directors.(6)(9)ConsistsShares of 981commons stock consist of 2 shares of common stock directly held and 6,24314 shares subject to options.(7)(10)Consists of 2,872 shares Shares of common stock and 7,251 shares subject to options. As consist of the record date,Dr. Rosenstock is on the board of directors.(8)Consists of 3,39923 shares of common stock directly held and 7,429700,014 shares subject to options.Asissuable upon exercise of the record date,Dr. Niloff is on the board of directors.warrants.(9)(11)ConsistsShares of 10,398,614common stock consist of 1,600 shares of common stock 168,781,637 shares issuable upon conversion of 2,400.75 shares of Series C1 preferred stock, 7,580,046directly held, 46 shares issuable upon exercise of warrants, and 38,32727 shares subject to options.options, and 41,117,786 shares issuable upon conversion of 300.00 shares of Series C1 preferred stock. Dr. Faupel is the COO and on the board of directors.(12) Shares of commons stock consists of 14,616 shares of common stock directly held, 2,454,980 shares issuable upon exercise of warrants, 49 shares subject to options, and 370,167,951 shares issuable upon conversion of 2,700.75 shares of Series C1 preferred stock. CERTAIN RELATIONSHIPS ANDTRANSACTIONSAND DIRECTOR INDEPENDENCEDrs. Niloff,Dr. Imhoff and Rosenstock are independent directors.16,847,368211 shares of common stock. Dr. Imhoff participated on terms equal to those of other Series C investors. As a result of these transactions, Dr. Imhoff’s beneficial ownership of our common stock increased from approximately 14% immediately prior to his first acquisition of shares of Series C preferred stock, to 25% immediately afterward.2400.752,400.75 shares of Series C1 preferred stock and 10,243,20012,804 shares of common stock. See “Summary—Recent Developments.”
Dr. Imhoff participated on terms equal to those of other Series C1 investors. As a result of this transaction, Dr. Imhoff’s beneficial ownership of our common stock increased from approximately 25% immediately prior to the transaction, to 77% immediately afterward. 47LEGAL MATTERSJones Day, Atlanta, Georgia, passedvalidityexercise price of the surrendered warrants as of immediately prior to consummation of the financing, subject to customary “downside price protection” for as long as our common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.that may be offered by this prospectus. Ellenoff Grossman & Schole LLP, New York, New York, acted as counselat an exercise price equal to the placement agent.EXPERTSOur consolidated financial statements as of December 31, 2015 and 2014, and for the years then ended have been audited by UHY LLP, an independent registered public accounting firm, as set forth in its report, included in this prospectus. Our financial statements and the related independent registered public accounting firm report thereon have been included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing.WHERE YOU CAN GET MORE INFORMATIONWe have filed with the SEC under the Securities Act a registration statement on Form S-1 of which this prospectus forms a part. This prospectus does not contain allconversion price of the information containednote, subject to customary anti-dilution adjustment.registration statement and its exhibits. past five fiscal years.strongly encourage youare not subject to read carefully the registration statement and its exhibits.Any statement made in this prospectus concerning the contentslisting requirements of any contract, agreementnational securities exchange or other document is onlynational securities association and, as a summaryresult, we are not at this time required to have a majority of the actual contract, agreement or other document. If we have filed any contract, agreement or other document as an exhibit to the registration statement, you should read the exhibit for a more complete understanding“independent directors” on our board of the document or matter involved.file annual, quarterly and current reports; proxy statements and other information with the SEC. You may read and copydo not believe that any of this information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for information on the operation of the Public Reference Room. The SEC also maintains an Internet website that contains reports, proxy statements and other information regarding issuers, including us, who file electronically with the SEC. The address of that site is http://www.sec.gov. The information contained on the SEC’s website is expressly not incorporated by reference into this prospectus. 48INDEX TO FINANCIAL STATEMENTSAnnual Consolidated Financial Statements Report of Independent Registered Public Accounting FirmConsolidated Financial Statements (Unaudited)F-2 Consolidated Balance Sheets (Unaudited) as of March 31, 2018 and December 31, 2015 and 20142017F-347 Consolidated Statements of Operations (Unaudited) for the YearsThree Months Ended DecemberMarch 31, 20152018 and 20142017F-4Consolidated Statements of Stockholders’ Deficit for the Years Ended December 31, 2015 and 2014F-548 Consolidated Statements of Cash Flows (Unaudited) for the YearsThree Months Ended DecemberMarch 31, 20152018 and 20142017F-649 Notes to Consolidated Financial Statements F-8Unaudited Condensed Consolidated Financial StatementsCondensed Consolidated Balance Sheets as of March 31, 2016 (unaudited) and December 31, 2015F-3Condensed Consolidated Statements of Operations (unaudited) for the Three Months Ended March 31, 2016 and 2015F-4Condensed Consolidated Statements of Cash Flows (unaudited) for the Three Months Ended March 31, 2016 and 2015F-6Notes to Condensed Consolidated Financial StatementsF-8Report of Independent Registered Public Accounting FirmTo the Board of Directors andStockholders of Guided Therapeutics, Inc.We have audited the accompanying consolidated balance sheets of Guided Therapeutics, Inc. and Subsidiary (the “Company”) as of December 31, 2015 and 2014, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for the years then ended. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Guided Therapeutics, Inc. and Subsidiary as of December 31, 2015 and 2014, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.As described in Note 1 to the consolidated financial statements, the accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company’s recurring losses from operations and accumulated deficit raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also discussed in Note 1 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty./s/ UHY LLPUHY LLPSterling Heights, MichiganMarch 15, 2016GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONSOLIDATED BALANCE SHEETS AS OF DECEMBER 31, 2015 AND 2014 (In Thousands) ASSETS 2015 2014 CURRENT ASSETS: Cash and cash equivalents $ 35 $ 162 Accounts receivable, net of allowance for doubtful accounts of $95 and $76 at
December 31, 2015 and 2014, respectively 190 338 Inventory, net of reserves of $118 and $144 at December 31, 2015 and 2014, respectively 1,119 1,180 Other current assets 780 99 Total current assets 2,124 1,779 Property and equipment, net 318 587 Debt issuance costs 48 564 Other assets 73 101 Total noncurrent assets 806 1,252 TOTAL ASSETS 2,563 $ 3,031 LIABILITIES AND STOCKHOLDERS’ DEFICIT CURRENT LIABILITIES: Short-term notes payable, including related parties current portion of long term debt 752 646 Note payable, in default 133 123 Short-term convertible notes payable, net of discount 686 1,062 Accounts payable 1,824 1,733 Accrued liabilities 1,907 1,015 Deferred revenue 217 24 Total current liabilities 5,519 4,603 Warrants, at fair value 2,606 2,070 Long-term debt, net — 40 Convertible note, net of discount — 783 Total long-term liabilities 2,606 2,893 TOTAL LIABILITIES 8,125 7,496 COMMITMENTS & CONTINGENCIES (Note 6) STOCKHOLDERS’ DEFICIT: Series B convertible preferred stock, $.001 par value; 3 shares authorized, zero and 1.2 shares issued and outstanding as of December 31, 2015 and 2014, respectively (liquidation
preference of none in December 31, 2015 and $1,200 at December 31, 2014, respectively) — 678 Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 5.6 shares issued and outstanding as of December 31, 2015 and none authorized or issued and outstanding at December 31, 2014. (Liquidation preference of $5,555 at December 31, 2015 and none at December 31, 2014). 2,052 — Common stock, $.001 par value; 1,000,000 and 195,000 shares authorized, 2,371 and 969 shares issued and outstanding as of December 31, 2015 and 2014, respectively 236 97 Additional paid-in capital 114,845 107,952 Treasury stock, at cost (132 ) (132 ) Accumulated deficit (122,563 ) (113,060 ) TOTAL GUIDED THERAPEUTICS STOCKHOLDERS’ DEFICIT (5,562 ) (4,465 ) TOTAL STOCKHOLDERS’ DEFICIT (5,562 ) (4,465 ) TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT $ 2,563 $ 3,031 The accompanying notes are an integral part of these consolidated statements. F-350 GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONSOLIDATED STATEMENTS OF OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 (In Thousands ) 2015 2014 REVENUE: Sales – devices and disposables $ 564 $ 758 Cost of goods sold 537 891 Gross profit (loss) 27 (133 ) Contract and grant revenue 42 65 OPERATING EXPENSES: Research and development 1,477 2,788 Sales and marketing 718 1,164 General and administrative 4,101 4,649 Total operating expenses 6,296 8,601 Operating loss (6,227 ) (8,669 ) OTHER INCOME (EXPENSES): Other income 74 25 Interest expense (1,317 ) (979 ) Loss on extinguishment of debt — (325 ) Change in fair value of warrants 568 65 Total other expenses (675 ) (1,214 ) LOSS FROM OPERATIONS (6,902 ) (9,883 ) PROVISION FOR INCOME TAXES — — NET LOSS (6,902 ) (9,883 ) DEEMED DIVIDENDS (1,263 ) — PREFERRED STOCK DIVIDENDS (1,338 ) (152 ) NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS $ (9,503 ) $ (10,035 ) $ (7.42 ) $ (13.02 ) WEIGHTED AVERAGE SHARES OUTSTANDING 1,280 771 The accompanying notes are an integral part of these consolidated statements. F-4STATEMENTS OF STOCKHOLDERS’ DEFICITFOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 (InBALANCE SHEETS Unaudited (in Thousands) Shares Amount Shares Amount Shares Amount Capital Stock Deficit TOTAL BALANCE, January 1, 2014 2 $ 1,139 — $ — 705 $ — $ 101,840 $ (132 ) $ (103,025 ) $ (107 ) Preferred dividends — — — — — — — — (152 ) (152 ) Conversion of preferred stock (1 ) (461 ) — — 23 2 459 — — — Issuance of common stock and warrants — — — — 209 21 3,348 — — 3,369 Exercise of warrants and options into common stock — — — — 4 — 96 — — 96 Conversion of debt into common stock — — — — 28 3 890 — — 893 Issuance of warrants and options — — — — — — 433 — — 433 Stock-based compensation expense — — — — — — 886 — — 886 Net Loss — — — — — — — — (9,883 ) (9,883 ) BALANCE, December 31, 2014 1 $ 678 — — 969 97 107,952 (132 ) (113,060 ) (4,465 ) Shares Amount Shares Amount Shares Amount Capital Stock Deficit TOTAL BALANCE, January 1, 2015 1 $ 678 — $ — 969 $ 97 $ 107,952 $ (132 ) $ (113,060 ) $ (4,465 ) Preferred dividends — — — — — — — — (352 ) (352 ) Conversion of Series C preferred stock to common stock — — (2 ) (840 ) 990 99 1,727 — (986 ) — Issuance of common stock and warrants — — — — 106 11 1,327 — — 1,338 Exercise of warrants and options for common stock — — — — 111 11 132 — — 143 Conversion of debt into common stock — — — — 168 15 999 — — 1,014 December 2014 public offering warrants exchange and common shares issuance — — — — 27 3 1,368 — (1,049 ) 322 Series B, Tranche A, warrant price adjustment — — — — — — 64 — (64 ) — Series C preferred stock and warrant issuance (1 ) (678 ) 8 2,892 — — 268 — (150 ) 2,332 Stock-based compensation — — — — 1,008 — — 1,008 Net Loss — — — — — — (6,902 ) (6,902 ) BALANCE, December 31, 2015 — $ — 6 $ 2,052 2,371 $ 236 $ 114,845 $ (132 ) $ (122,563 ) $ (5,562 ) ASSETS CURRENT ASSETS: Cash and cash equivalents Accounts receivable, net of allowance for doubtful accounts of $244 and $160 at March 31, 2018 and December 31, 2017, respectively Inventory, net of reserves of $716, at March 31, 2018 and December 31, 2017 Other current assets Total current assets Property and equipment, net Other assets Total noncurrent assets TOTAL ASSETS LIABILITIES AND STOCKHOLDERS’ DEFICIT CURRENT LIABILITIES: Notes payable in default, including related parties Short-term note payable, including related parties Convertible notes in default Convertible notes payable, net Accounts payable Accrued liabilities Deferred revenue Total current liabilities Warrants at fair value TOTAL LIABILITIES Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 0.7 and 0.9 shares issued and outstanding as of March 31, 2018 and December 31, 2017, (Liquidation preference of $686 and $970 at March 31, 2018 and December 31, 2017) Series C1 convertible preferred stock, $.001 par value; 20.3 shares authorized, 4.3 shares issued and outstanding as of March 31, 2018 and December 31, 2017, respectively (Liquidation preference of $4,312 at March 31, 2018 and December 31, 2017, respectively) Common stock, $.001 Par value; 1,000,000 shares authorized, 138,315 and 49,563 shares issued and outstanding as of March 31, 2018 and December 31, 2017 Additional paid-in capital Treasury stock, at cost Accumulated deficit TOTAL STOCKHOLDERS’ DEFICIT TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 (In Thousands) 2015 2014 CASH FLOWS FROM OPERATING ACTIVITIES: Net loss $ (6,902 ) $ (9,883 ) Adjustments to reconcile net loss to net cash used in operating activities: Bad debt expense 19 63 Depreciation and Amortization 1,054 1,240 Stock-based compensation 1,008 886 Non-employee stock based compensation 400 — Change in fair value of warrants (568 ) (65 ) Changes in operating assets and liabilities: Accounts receivable 129 (267 ) Inventory 61 14 Other current assets (681 ) 2 Other assets 28 254 Accounts payable 91 842 Deferred revenue 193 10 Accrued liabilities 1,125 296 Total adjustments 2,859 3,600 Net cash used in operating activities (4,043 ) (6,283 ) CASH FLOWS FROM INVESTING ACTIVITIES: Additions to fixed assets (8 ) (150 ) Net cash used in investing activities (8 ) (150 ) CASH FLOWS FROM FINANCING ACTIVITIES: Net proceeds from issuance of preferred stock and warrants, net 3,698 — Net proceed from issuance of common stock and warrants 720 1,730 Proceeds from debt financing, net of discount 425 5,263 Payment for debt issuance costs (48 ) (452 ) Payments on notes (1,014 ) (656 ) Proceeds from options and warrants exercised 143 97 Net cash provided by financing activities 3,924 5,982 NET CHANGE IN CASH AND CASH EQUIVALENTS (127 ) (451 ) CASH AND CASH EQUIVALENTS, beginning of year 162 613 CASH AND CASH EQUIVALENTS, end of year $ 35 $ 162 SUPPLEMENTAL SCHEDULE OF: Cash paid for: Interest $ 76 $ 33 NONCASH INVESTING AND FINANCING ACTIVITIES: Deemed dividend on beneficial conversion features of Series C Preferred stock $ 150 $ — Deemed dividend on price changes for Series B preferred stock warrants $ 64 $ — Deemed dividend on December 2014 public offering warrants $ 1,049 $ — Term changes on Series B preferred stock and December 2014 public offering warrants resulting in transfer to equity $ 324 $ — Issuance of common stock as debt repayment $ 1,014 $ — Repayment of deferred compensation via issuance of preferred stock $ 100 $ — Dividends on preferred stock $ 1,338 $ 152 Conversion of accrued expenses into common stock $ — $ 207 Payment of debt issuance costs via warrants and common stock $ — $ 522 Conversion of convertible debt into common stock $ — $ 893 Repayment of debt via issuance of common stock and warrants $ — $ 1,697 Issuance of common stock as board compensation $ — $ 355 The accompanying notes are an integral part of these consolidated statements. F-6
GUIDED THERAPEUTICS INC. AND SUBSIDIARY REVENUE: Sales – devices and disposables Cost of goods sold Gross profit OPERATING EXPENSES: Research and development Sales and marketing General and administrative Total operating expenses Loss from operations OTHER INCOME (EXPENSE): Other income Interest expense Change in fair value of warrants Total other income INCOME (LOSS) BEFORE INCOME TAXES INCOME TAXES NET INCOME (LOSS) PREFERRED STOCK DIVIDENDS NET INCOME (LOSS) ATTRIBUTABLE TO COMMON STOCKHOLDERS NET INCOME (LOSS) PER SHARE (BASIC) NET INCOME (LOSS) PER SHARE (DILUTED) WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING: BASIC DILUTED CASH FLOWS FROM OPERATING ACTIVITIES: Net income (loss) Adjustments to reconcile net income (loss) to net cash used in operating activities: Bad debt expense (recovery) Depreciation Stock based compensation Amortization of debt issuance costs and discount Change in fair value of warrants Changes in operating assets and liabilities: Inventory Accounts receivable Other current assets Other assets Accounts payable Deferred revenue Accrued liabilities Total adjustments Net cash used in operating activities CASH FLOWS FROM FINANCING ACTIVITIES: Net proceeds from issuance of common stock and warrants, net Proceeds from debt financing, net of discounts and debt issuance costs Payments made on notes payable Net cash provided by financing activities NET CHANGE IN CASH AND CASH EQUIVALENTS CASH AND CASH EQUIVALENTS, beginning of year CASH AND CASH EQUIVALENTS, end of period SUPPLEMENTAL SCHEDULE OF: Cash paid for: Interest NONCASH INVESTING AND FINANCING ACTIVITIES: Issuance of common stock as debt repayment Dividends on preferred stock DECEMBER 31, 2015 (UNAUDITED)20141. Organization, Background, and Basis of PresentationBasis of PresentationDecemberMarch 31, 2015,2018, it had an accumulated deficit of approximately $122.6$137.5 million. Through December 31, 2015,To date, the Company has devoted substantial resources toengaged primarily in research and development efforts. The Company first generated revenue from product sales in 1998, but does not have significant experience in manufacturing,efforts and the early stages of marketing or selling its products. The Company’s development efforts may not result in commercially viable products and itCompany may not be successful in growing sales for its products. Moreover, required regulatory clearances or approvals may not be obtained.obtained in a timely manner, or at all. The Company’s products may not ever gain market acceptance and the Company may not ever achieve levels of revenue to sustain further development costs and support ongoing operationsgenerate significant revenues or achieve profitability. The development and continued commercialization of the Company’s products will requirerequires substantial development, regulatory, sales and marketing, manufacturing and other expenditures. The Company expects operating losses to continue throughfor the foreseeable future as it continues to expend substantial resources to complete development of its products, obtain regulatory clearances or approvals, build its marketing, sales, manufacturing and finance capabilities, and conduct further research and development.On February 24, 2016,Company implementedinstructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all information and footnotes required by GAAP for complete financial statements. These statements reflect adjustments, all of which are of a 1:100 reverse stock splitnormal, recurring nature, and which are, in the opinion of its issued and outstanding common stock. The reverse stock split decreasedmanagement, necessary to present fairly the Company’s issued and outstanding shares of common stock from 237,101,702 shares of Common Stock to 2,371,007 sharesfinancial position as of March 31, 2018, results of operations for the three months ended March 31, 2018 and 2017, and cash flows for the three months ended March 31, 2018 and 2017. The results of operations for the three months ended March 31, 2018 are not necessarily indicative of the results for a full fiscal year. Preparing financial statements requires the Company’s management to make estimates and assumptions that date. See Note 12, Subsequent Events. Unless otherwise specified, all per shareaffect the reported amounts are reportedof assets, liabilities, revenues and expenses and disclosure of contingent assets and liabilities. Actual results could differ from those estimates. These financial statements should be read in conjunction with the financial statements and notes thereto included in the Company’s annual report on a post-stock split basis, as ofForm 10-K for the year ended December 31, 2015.DecemberMarch 31, 2015,2018, the Company had a negative working capital of approximately $3.4$11.7 million, accumulated deficit of $122.6$137.5 million, and incurred a net lossincome of $6.9$1.1 million for the yearquarter then ended.ended (net income for the quarter for the period ended March 31, 2018, related to the gain for the fair value of warrants of $1.7 million). Stockholders’ deficit totaled approximately $5.6$17.9 million at DecemberMarch 31, 2015, primarily due to recurring net losses from operations, deemed dividends on warrants and preferred stock, offset by proceeds from the exercise of options and warrants and proceeds from sales of stock.2016,2018, the Company haswill continue its plans to curtailof curtailing operations by reducing discretionary spending and staffing levels, and attempting to operate by only pursuing activities for which it has external financial support. However, there can be no assurance that such external financial support will be sufficient to maintain even limited operations or that the Company will be able to raise additional funds on acceptable terms, or at all. In such a case, the Company might be required to enter into unfavorable agreements or, if that is not possible, be unable to continue operations, and to the extent practicable, liquidate and/or file for bankruptcy protection.2.8666.7 million shares of its common stock outstanding at DecemberMarch 31, 2015,2018, with exercise prices ranging between $1.03$0.001 and $105$40,000 per share. Exercises of these warrants would generate a total of approximately $6.7$4.8 million in cash, assuming full exercise, although the Company cannot be assured that holders will exercise any warrants. Management may obtain additional funds through the public or private sale of debt or equity, and grants, if available.
However, please refer to Footnote 10 - CONVERTIBLE DEBT IN DEFAULT in the paragraph: Debt Restructuring for more information regarding our warrants.F-7Summary of Significant Accounting PoliciesFASBFinancial Accounting Standards Board (“FASB”) issued ASU 2014-09, “Revenue from Contracts with Customers,Distributors (Topic 606),” (“ASU 2014-09”). ASU 2014-09 outlines a new, single comprehensive model for entities to use in accounting for revenue arising from contracts with customersdistributors and supersedes most current revenue recognition guidance, including industry-specific guidance. This new revenue recognition model provides a five stepfive-step analysis in determining when and how revenue is recognized. The new model will requirerequires revenue recognition to depict the transfer of promised goods or services to customersdistributors in an amount that reflects the consideration a company expects to receive in exchange for those goods or services.receive. ASU 2014-09 isalso requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from distributor contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. In August 2015, the FASB issued ASU 2015-14, “Deferral of the Effective Date”, which amends ASU 2014-09. As a result, the effective for reporting periods beginning Januarydate will be the first quarter of fiscal year 2018 with early adoption permitted in the first quarter of fiscal year 2017. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.In June 2014,Subsequently, the FASB has issued the following standards related to ASU 2014-12, “Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period,2014-09: ASU 2016-08, “Revenue from Contracts with Distributors (Topic 606), Principal versus Agent Considerations (Reporting Revenue Gross versus Net),” (“ASU 2014-12”2016-08”).; ASU 2014-12 requires that a performance target that affects vesting,2016-10, “Revenue from Contracts with Distributors (Topic 606), Identifying Performance Obligations and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. Licensing,” (“ASU 2014-12 is effective for the reporting periods beginning after December 15, 2015. Early adoption is permitted.2016-10”); ASU 2016-12, “Revenue from Contracts with Distributors (Topic 606) Narrow-Scope Improvements and Practical Expedients,” (“ASU 2016-12”); and ASU 2016-20, “Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Distributors,” (“ASU 2016-20”), which are intended to provide additional guidance and clarity to ASU 2014-09. The Company is evaluatingmust adopt ASU 2016-08, ASU 2016-10, ASU 2016-12 and ASU 2016-20 along with ASU 2014-09 (collectively, the impact that adoption“New Revenue Standards”). The New Revenue Standards may be applied using one of this guidance will have on the determination or reporting of its financial results.In August 2014, the FASB issued ASU 2014-15, “Disclosure of Uncertainties about an Entity’s Ability to Continue astwo retrospective application methods: (1) a Going Concern,” (“ASU 2014-15”). ASU 2014-15 requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern for a one year period subsequent to the date of the financial statements, as entity must provide certain disclosures if conditions or events raise substantial doubt about the entity’s ability to continue as a going concern. The guidance is effectivefull retrospective approach for all entities for the first annual period ending after December 15, 2016 and interim periods thereafter, with early adoption permitted. The Company is evaluating the impactpresented, or (2) a modified retrospective approach that adoption of this guidance will have on the determination or reporting of its financial results.In April 2015, the FASB issued ASU 2015-03, “Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs,” (“ASU 2015-03”). ASU 2015-03 requires that debt issuance costs related topresents a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The guidance is effective for reporting periods beginning after December 15, 2015 and interim periods within those fiscal years with early adoption permitted. ASU 2015-03 should be applied on a retrospective basis, wherein the balance sheet of each period presented should be adjusted to reflect the effects of adoption. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.In July 2015, the FASB issued ASU 2015-11, “Simplifying the Measurement of Inventory,” (“ASU 2015-11”). ASU 2015-11 requires inventory be measured at the lower of cost and net realizable value and options that currently exist for market value be eliminated. ASU 2015-11 defines net realizable value as estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The guidance is effective for reporting periods beginning after December 15, 2016 and interim periods within those fiscal years with early adoption permitted. ASU 2015-11 should be applied prospectively. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.F-8In August 2015, the FASB issued ASU 2015-15, “Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements,” which amends ASC 835-30, “Interest - Imputation of Interest”. The ASU clarifies the presentation and subsequent measurement of debt issuance costs associated with lines of credit. These costs may be presented as an asset and amortized ratably over the term of the line of credit arrangement, regardless of whether there are outstanding borrowings on the arrangement. The effective date will be the first quarter of fiscal year 2016 and will be applied retrospectively. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.In September 2015, the FASB issued ASU 2015-16, “Business Combinations: Simplifying the Accounting for Measurement-Period Adjustments.” This ASU requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The effective date will be the first quarter of fiscal year 2016. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.In November 2015, the FASB issued ASU 2015-17, “Income Taxes (Topic 740) Balance Sheet Classification of Deferred Taxes.” The amendments in ASU 2015-17 seek to simplify the presentation of deferred income taxes and require that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. ASU 2015-17 is effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods, with early application permitted for all entitiescumulative effect as of the beginning of an interim or annual reporting period.adoption date and additional required disclosures. The Company is evaluatingadopted this standard on January 1, 2018, using the modified retrospective method, with no impact adoptionon its 2017 financial statements. The cumulative effect of thisinitially applying the new guidance will havehad no impact on determination or reporting of its financial results.In January 2016, the FASB issued ASU 2016-01, "Financial Instruments-Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities." The amendmentsstatements. However, additional disclosures will be included in ASU 2016-01, among other things, require equity investments (except those accounted for under the equity method of accounting, or those that resultfuture reporting periods in consolidationaccordance with requirements of the investee) to be measured at fair value with changes in fair value recognized in net income; requires public business entities to use the exit price notion when measuring fair value of financial instruments for disclosure purposes; requires separate presentation of financial assets and financial liabilities by measurement category and form of financial asset (i.e., securities or loans and receivables); and eliminates the requirement for public business entities to disclose the method(s) and significant assumptions used to estimate fair value that is required to be disclosed for financial instruments measured at amortized cost. The effective date will be the first quarter of fiscal year 2018. The Company is evaluating the impact the adoption of this new standard will have on its consolidated financial statements.recognizebe recognized on the balance sheet with the assets and liabilities associated with the rights and obligations created by those leases. Under the new guidance, a lessee will be required to recognize assets and liabilities for leases with lease terms of more than 12 months. Consistent with current U.S. GAAP, the recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee primarily will depend on its classification as finance or operating lease. The update is effective for reporting periods beginning after December 15, 2018. Early adoption is permitted. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.customers’distributors’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.F-9customers’distributors’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.market,net realizable value, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased.incurred. At DecemberMarch 31, 20152018 and December 31, 2014,2017, our inventories were as follows (in thousands): Year Ended December 31, 2015 2014 Raw materials $ 686 $ 884 Work in process 186 304 Finished goods 365 136 Inventory reserve (118 ) (144 ) Total $ 1,119 $ 1,180 Raw materials Work in process Finished goods Consigned inventory Inventory reserve Total depreciatedamortized at the shorter of the useful life of the asset or the remaining lease term. Depreciation and amortization expense is included in general and administrative expense on the statement of operations. Expenditures for repairs and maintenance are expensed as incurred. Property and equipment are summarized as follows at March 31, 2018 and December 31, 2015 and 20142017 (in thousands): Year Ended December 31, 2015 2014 Equipment $ 1,377 $ 1,391 Software 740 737 Furniture and fixtures 124 124 Leasehold Improvement 199 180 2,440 2,432 Less accumulated depreciation (2,122 ) (1,845 ) Total $ 318 $ 587 Equipment Software Furniture and fixtures Leasehold Improvement Less accumulated depreciation and amortization Total incurred in securing the Company’s financing arrangements are capitalized and amortized over the term of the associated debt. Deferred financingDebt issuance costs are includedpresented in other long term assets.shortshort- and long-term deposits for various tooling inventory that are being constructed for the Company. At December 31, 2015 and 2014, such balances were approximately $413,000 and $72,000, respectively.$47,000$6,000 and $50,000 in 2015$15,000 as of March 31, 2018 and 2014,December 31, 2017, respectively.F-10at December 31, 2015 and 2014 (in thousands): 2015 2014 Accrued compensation $ 1,235 $ 447 Accrued professional fees 154 203 Deferred rent 36 54 Accrued warranty 82 119 Accrued vacation 177 144 Other accrued expenses 223 48 Total $ 1,907 $ 1,015
2017Accrued compensation Accrued professional fees Accrued interest Accrued warranty Accrued vacation Accrued dividends Stock subscription Accrued expenses for licensee Other accrued expenses Total customers.distributors. The Company recognizes revenue from contracts on a straight linestraight-line basis, over the terms of the contracts. Contracts generally are for shipment of devices and disposables and revenue is recognized once it is transferred to a third-party shipper this is identified in the contract as the performance obligation that has been met.recognizesadopted a new revenue from grants basedstandard on January 1, 2018, using the modified retrospective method with no impact on our financial statements. The cumulative effect of initially adopting the new guidance had no impact on the grant agreements, atopening balance of retained earnings as of January 1, 2018. There was no material impact on the timecondensed consolidated balance sheets as of March 31, 2018 or on the expensescondensed consolidated statements of income for the three months ended March 31, 2018. Results for reporting periods beginning after January 1, 2018 are incurred.CustomersIn 2015 and 2014,Distributorsmajority ofthree months ended March 31, 2018, all the Company’s revenues were from fourtwo distributors and two customers, respectively.for extended warranty. Revenue from these customersdistributors totaled approximately $280,000 or 73% and approximately $414,000 or 50% of total revenue$4,000 for the yearperiod ended DecemberMarch 31, 2015 and 2014, respectively.2018. Accounts receivable due from those customersthese distributors represents 62% and 17% as100% of Decemberthe balance for the period ended March 31, 2015 and 2014, respectively.straight linestraight-line basis, over the terms of the contract.Effective January 1, 2007 theadopted ASC guidance regarding accounting for uncertainty in income taxes. This guidance clarifies the accounting for income taxes by prescribing the minimum recognition threshold an income tax position is required to meet before being recognized in the financial statements and applies to all income tax positions. Eachassesses each income tax position is assessed using a two-step process. A determination is first made as to whether it is more likely than not that the income tax position will be sustained, based upon technical merits, upon examination by the taxing authorities. If the income tax position is expected to meet the more likely than not criteria, the benefit recorded in the financial statements equals the largest amount that is greater than 50% likely to be realized upon its ultimate settlement. At March 31, 2018 and December 31, 2015 and 2014,2017, there were no uncertain tax positions.F-11The Company is current with its federal and applicable state tax returns filings. Although we have been experiencing recurring losses, we are obligated to file tax returns for compliance with Internal Revenue Service (“IRS”) regulations and that of applicable state jurisdictions. As of December 31, 2015, the Company has approximately $27.8 million of net operating loss eligible to be carried forward for tax purposes at federal and applicable states level.None of the Company’s federal or state income tax returns are currently under examination by the IRS or state authorities.period of time.period. The Company records equity instruments including warrants issued to non-employees based on the fair value at the date of issue. The fair value of warrants classified as equity instruments at the date of issuance is estimated using the Black-Scholes Model. The fair value of warrants classified as liabilities at the date of issuance is estimated using the Monte Carlo Simulation or Binomial model.yearsthree months ended DecemberMarch 31, 20152018 and 2014,2017, share-based compensation for options attributable to employees, officers and Board members were approximately $1,008,000$13,000 and $886,000,$19,000, respectively. These amounts have been included in the Company’s statements of operations. Compensation costs for stock options which vest over time are recognized over the vesting period. As of DecemberMarch 31, 2015,2018, the Company had noapproximately $39,000 of unrecognized compensation costs related to granted stock options to be recognized over the remaining vesting period of approximately threetwo years., establishes the authoritative definition of fair value, sets out a framework for measuring fair value, and outlines the required disclosures regarding fair value measurements. Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. The Company uses a three-tier fair value hierarchy based upon observable and non-observable inputs as follow:·●Level 1 – Quoted market prices in active markets for identical assets and liabilities; ·●Level 2 – Inputs, other than level 1 inputs, either directly or indirectly observable; and ·●Level 3 – Unobservable inputs developed using internal estimates and assumptions (there is little or no market date) which reflect those that market participants would use. DecemberMarch 31, 2015.2018. The fair value of the warrants was estimated using the Monte CarloBinomial Simulation model. Gains and losses from derivative contracts are included in net gain (loss) from derivative contracts in the statement of operations. The fair value of the Company’s derivative warrants is classified as a Level 3 measurement, since unobservable inputs are used in the valuation.2015 and 2014:( In(In Thousands)Description Level 1 Level 2 Level 3 Total Date Warrants issued in connection with the issuance of Series C preferred stock $ — $ — $ (1,145 ) $ (1,145 ) Warrants issued in connection with the issuance of Series B preferred stock $ (1,461 ) $ (1,461 ) December 31, 2015 Total long-term liabilities at fair value $ — $ — $ (2,606 ) $ (2,606 ) December 31, 2015 F-12Description Level 1 Level 2 Level 3 Total Date Warrants issued in connection with the December 2014 public offering of common stock $ — $ — $ (587 ) $ (587 ) Warrants issued in connection with the issuance of Series B preferred stock $ — $ — $ (1,483 ) $ (1,483 ) Total long-term liabilities at fair value $ — $ — $ (2,070 ) $ (2,070 ) Series B Warrants Total Balance, December 31, 2014 $ (587 ) $ (1,483 ) $ — $ (2,070 ) Warrants issued during the period — — (1,428 ) (1,428 ) Change in fair value during the period 263 22 283 568 Transfer to equity as a result of changes
to provisions 324 — — 324 Balance, December 31, 2015 $ — $ (1,461 ) $ (1,145 ) $ (2,606 ) Warrants issued in connection with Distributor Debt Warrants issued in connection with Short-Term Loans Warrants issued in connection with Senior Secured Debt Total long-term liabilities at fair value Warrants issued in connection with Distributor Debt Warrants issued in connection with Short-Term Loans Warrants issued in connection with Senior Secured Debt Total long-term liabilities at fair value Balance, December 31, 2017 Change in fair value during the period Balance, March 31, 2018 Stockholders’ Equity2,371,017138,315,085 were issued and outstanding as of March 31, 2018. As of December 31, 2015. For the year ended December 31, 2014,2017, there were 195,000,0001,000,000,000 authorized shares of common stock, of which 96,88949,562,810 were issued and outstanding.yearthree months ended DecemberMarch 31, 2015,2018, the Company issued 1,402,12888,752,275 shares of common stock as listed below:Sales of unregistered securities - Issuance - For CashSeries C Preferred Stock Conversions40,000Issuance - For Debt Repayment152,117Series C ConversionPreferred Stock Dividends1,006,777Series C DividendsConvertible Debt Conversions18,562Series B Dividends11,086Option Exercised1,786Warrant Exercised - Cashless95,413Warrant Exercised – For Cash13,500Issued for December 2014 public offering warrant exchange26,190Issued for Consulting Services36,697Total 1,402,1289,000,0005,000,000 shares of preferred stock with a $.001 par value. The board of directors has the authority to issue these shares and to set dividends, voting and conversion rights, redemption provisions, liquidation preferences, and other rights and restrictions. The board of directors designated 525,000 shares of preferred stock as redeemable convertible preferred stock, none of which remain outstanding; 3,00033,000 shares of preferred stock as Series B Convertible Preferred Stock, none of which none and 1,277 shares were issued andremained outstanding, at December 31, 2015 and December 31, 2014, respectively; and 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 5,555686 and 0 shares970 were issued and outstanding at DecemberMarch 31, 20152018 and December 31, 2014, respectively.
2017, respectively, and 20,250 shares of Series C1 Convertible Preferred Stock, of which 4,312 were issued and outstanding at March 31, 2018 and December 31, 2017.F-13Pursuant to the terms of the Series B Preferred Stock set forth in the Series B designations, shares of Series B Preferred Stock were convertible into common stock by their holder at any time, and were mandatorily convertible upon the achievement of certain conditions, including the receipt of certain approvals from the U.S. Food and Drug Administration and the achievement by the Company of specified average trading prices and volumes for the common stock. Preferred dividends totaled approximately $352,000 and $152,000 for 2015 and 2014, respectively. Dividends were paid via issuance of common stock.was originallywere issued with Tranche A warrants to purchase 18,58124 shares of common stock and Tranche B warrants purchasing 18,5817,539 shares of common stock, both at an exercise price of $108.00 per share.On June 30, 2015, as consideration for obtaining consents to an amendment to the Series B designations, the Company reduced the exercise price of the Tranche A warrants from $108.00 to $104.55$8,364 and $75 per share, and the exercise price of the Tranche B warrants from $10.455 to $9.00 per share. The change in exercise price of these warrants resulted in a deemed dividend totaling $64,000 that has been recorded as an increase to additional paid-in capital with an offsetting charge to retained earnings. The deemed dividend has been subtracted from income (added to the loss) in computing loss per common stockholder.1,292,8191 shares of common stock. These warrants arewere re-measured based upon their fair value each reporting period and classified as a liability on the Balance Sheet. In connection with the transaction, the Company issued five-year warrants exercisable for an aggregate of 1,247,737 shares of common stock at an exercise price of $9.50 per share.DecemberMarch 31, 2015,2018, there were 5,555686 shares outstanding with a conversion price of $1.03$0.007296 per share, such that each share of Series C preferred stock would convert into approximately 97,324137,061 shares of the Company’s common stock, subject to customary adjustments, including for any accrued but unpaid dividends and pursuant to certain anti-dilution provisions, as set forth in the Series C certificate of designations. The conversion price will automatically adjust downward to 80% of the then-current market price of the Company’s common stock 15 trading days after any reverse stock split of the Company’s common stock, and 5 trading days after any conversions of the Company’s outstanding convertible debt.Preferred Stockpreferred stock prior to the Dividend End Date, the Company will also pay to the converting holder a “make-whole payment” equal to the amount of unpaid dividends through the Dividend End Date on the converted shares. At March 31, 2018, the “make-whole payment” for a converted share of Series C preferred stock would convert to 57,566 shares of the Company’s common stock. The Series C preferred stock generally has no voting rights except as required by Delaware law. Upon the Company’s liquidation or sale to or merger with another corporation, each share will be entitled to a liquidation preference of $1,000, plus any accrued but unpaid dividends.The Company has provisionally allocated net proceeds totaling $935,200 to the fair value of the Series C preferred stock. The effective conversion price of $935,000 allocated to the Series C preferred stock resulted in an associated beneficial conversion feature totaling $148,000 that has been recorded as an increase to additional paid-in capital with an offsetting charge to retained earnings representing a deemed dividend. The deemed dividend has been subtracted from income (added to the loss) in computing loss per common stockholder.F-14106.4 million150 shares of Company’s common stock. The warrants contain anti-dilution adjustments in the event that the Company issues shares of common stock, or securities exercisable for or convertible into shares of common stock, at prices below the exercise price of such warrants. As a result of the anti-dilution protection, the Company is required to account for the warrants as a liability recorded at fair value each reporting period. The Company has valued the warrants using a Binomial model and allocated $1,349,000 to the fair value of the warrants. At DecemberMarch 31, 2015,2018, the exercise price per share was $1.03. OptionsUnderOutstanding, January 1, 2018 Issuances Canceled / Expired Exercised Outstanding, March 31, 2018
(Underlying Shares) 24 (1) $8,368.00 per share May 23, 2018 7,542 (2) $75.00 per share June 14, 2021 3 (3) $40,000.00 per share April 23, 2019 8 (4) $36,000.00 per share May 22, 2019 3 (5) $30,400.00 per share September 10, 2019 5 (6) $36,864.80 per share September 27, 2019 10 (7) $22,504.00 per share December 2, 2019 105 (8) $7,200.00 per share December 2, 2020 105 (9) $8,800.00 per share December 2, 2020 25 (11) $20,400.00 per share March 30, 2018 22 (12) $9,504.00 per share June 29, 2020 659 (10) $640.00 per share June 29, 2020 343 (11) $640.00 per share September 4, 2020 362 (12) $640.00 per share September 21, 2020 7 (13) $9,504.00 per share September 4, 2020 198 (14) $640.00 per share October 23, 2020 7 (15) $9,504.00 per share October 23, 2020 630,482,456 (16) $0.00228 per share June 14, 2021 30,263,158 (17) $0.00228 per share February 21, 2021 17,239 (18) $13.92 per share June 6, 2021 200,000 (19) $0.00228 per share February 13, 2022 20,000 (20) $0.18 per share May 16, 2022 550,000 (21) $0.019 per share November 16, 2020 200,000 (22) $0.029 per share December 28, 2020 1,500,000 (23) $0.0201 per share January 10, 2021 60,000 (24) $0.011 per share March 19, 2021 3,409,090 (25) $0.00228 per share March 20, 2021 (1) Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. (2) Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. (3) Issued to a placement agent in conjunction with an April 2014 private placement. (4) Issued to a placement agent in conjunction with a September 2014 private placement (5) Issued as part of a September 2014 Regulation S offering. (6) Issued to a placement agent in conjunction with a 2014 public offering. (7) Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering. (8) Issued as part of a March 2015 private placement. (9) Issued to a placement agent in conjunction with a June 2015 private placement (10) Issued as part of a June 2015 private placement. (11) Issued as part of a June 2015 private placement. (12) Issued as part of a June 2015 private placement. (13) Issued to a placement agent in conjunction with a June 2015 private placement (14) Issued as part of a June 2015 private placement. (15) Issued to a placement agent in conjunction with a June 2015 private placement (16) Issued as part of a February 2016 private placement. (17) Issued to a placement agent in conjunction with a February 2016 private placement (18) Issued pursuant to a strategic license agreement. (19) Issued as part of a February 2017 private placement. (20) Issued as part of a May 2017 private placement. (21) Issued to investors for a loan in November 2017. (22) Issued to investors for a loan in December 2017. (23) Issued to investors for a loan in January 2018. (24) Issued to investors for a loan in March 2018. (25) Issued to investors for a loan in March 2018. , a total of 26,616 has expired pursuant to its terms, so zero shares remained available for issuance at DecemberMarch 31, 2015 and 105,936 shares were subject to stock options outstanding as of that date, bringing the total number of shares subject to stock options outstanding and those remaining available for issue to 132,552 shares of common stock as of December 31, 2014.2018. The Plan allowsallowed for the issuance of incentive stock options, nonqualified stock options, and stock purchase rights. The exercise price of options iswas determined by the Company’s board of directors, but incentive stock options must bewere granted at an exercise price equal to the fair market value of the Company’s common stock as of the grant date. Options historically granted have generally become exercisable overafter four years and expire ten years from the date of grant.granted in 2015 and 2014 wereis estimated using the Black-Scholes option pricing model. A summary of the assumptions used in determining the fair value of options follows: 2015 2014 Expected volatility 152.32 % 157.70 % Expected option life in years 9.98 9.98 Expected dividend yield 0.00 % 0.00 % Risk-free interest rate 1.33 % 2.55 % Weighted average fair value per option at grant date $ 0.49 $ 0.40 Application of the Black-Scholes option pricing model involves assumptions that are judgmental and affect compensation expense. Historical information is the primary basis for the selection of expected volatility, expected option life and expected dividend yield. Expected volatility is based on the most recent historical period equal to the expected life of the option. The risk-free interest rate is based on yields of U.S. Treasury zero-coupon issues with a term equal to the expected life of the option on the date the stockNo options were granted.each of the two years ended DecemberMarch 31, 2015,2018 as follows: 2015 2014 Weighted
Average
Exercise Shares Price Shares Price Outstanding at beginning of year 69,404 $ 66.00 65,312 $ 68.00 Options granted 42,140 $ 12.00 7,548 $ 40.00 Options exercised (1,786 ) $ 48.00 (2,424 ) $ 32.00 Options expired/forfeited (3,822 ) $ 43.00 (1,031 ) $ 68.00 Outstanding at end of year 105,936 $ 45.00 69,404 $ 66.00 Options vested and exercisable at year-end 90,411 $ 49.00 59,881 $ 66.00 Options available for grant at year-end 26,616 63,148 Aggregate intrinsic value – options exercised $ — $ 497 Aggregate intrinsic value – options outstanding $ — $ 4,941 Aggregate intrinsic value – options vested and
exercisable $ — $ 6,129 Options unvested, balance at beginning of year(1) 9,523 10,672 $ 112.00 Options granted (1) 42,140 $ 40.00 7,548 $ 40.00 Vested (1) (34,383 ) $ 66.00 (7,666 ) $ 66.00 Cancelled/Forfeited (2,455 ) $ 68.00 (1,031 $ 68.00 Balance, end of period (1) 14,825 9,523 — (1) Includes awards not captured in valuation fragments F-15 Outstanding at beginning of year Options granted Options exercised Options expired/forfeited Outstanding at end of the period The Company estimates the fair value of stock options using a Black-Scholes valuation model. Key input assumptions used to estimate the fair value of stock options include the expected term, expected volatility of the Company’s common stock, the risk free interest rate, option forfeiture rates, and dividends, if any. The expected term of the options is based upon the historical term until exercise or expiration of all granted options. The expected volatility is derived from the historical volatility of the Company’s stock on the OTCBB market for a period that matches the expected term of the option. The risk-free interest rate is the constant maturity rate published by the U.S. Federal Reserve Board that corresponds to the expected term of the option.WarrantsThe following table summarizes transactions involving the Company’s outstanding warrants to purchase common stock for the year ended December 31, 2015:Warrants(Underlying Shares)Outstanding, January 1, 2015297,961Issuances2,751,872Canceled / Expired(35,973)Exercised(211,476)Outstanding, December 31, 20152,802,384The Company had the following shares reserved for the warrants as of December 31, 2015:4,399 (1) $68.00 March 31, 2016 2,852 (2) $105.00 November 20, 2016 18,581 (3) $10.46 May 23, 2018 1,292,820 (3) $1.03 May 23, 2018 2,000 (4) $50.00 April 23, 2019 5,618 (4) $45.00 May 22, 2019 1,842 (5) 38.00 September 10, 2019 3,255 (6) $46.08 September 27, 2019 7,553 (7) $28.13 December 2, 2019 83,927 (8) $9.00 December 2, 2020 83,927 (8) $11.00 December 2, 2020 20,000 (9) $25.50 March 30, 2018 17,547 (10) $11.88 June 29, 2020 526,421 (11) $1.03 June 29, 2020 273,684 (12) $1.03 September 4, 2020 289,737 (13) $1.03 September 21, 2020 5,163 (14) $11.88 September 4,2020 157,895 (15) $1.03 October 23, 2020 5,163 (16) $11.88 October 23,2020 2,802,384 (1)Issued in February 2014 as part of a buy-back of a minority interest in Interscan in December 2012.(2)Issued as part of a November 2011 private placement.(3)Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement.(4)Issued to a placement agent in conjunction with an April 2014 private placement.(5)Issued to a placement agent in conjunction with a September 2014 private placement.(6)Issued as part of a September 2014 Regulation S offering.(7)Issued to a placement agent in conjunction with a 2014 public offering.(8)Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering.(9)Issued as part of a March 2015 private placement.(10)Issued to a placement agent in conjunction with a June 2015 private placement.(11) Issued as part of a June 2015 private placement.(12)Issued as part of a June 2015 private placement.(13)Issued as part of a June 2015 private placement.(14)Issued to a placement agent in conjunction with a June 2015 private placement.(15) Issued as part of a June 2015 private placement.(16)Issued to a placement agent in conjunction with a June 2015 private placement.
6. LITIGATION AND CLAIMSF-165. Income TaxesThe Company has incurred net operating losses (“NOLs”) since inception. As of December 31, 2015, the Company had NOL carryforwards available through 2034 of approximately $73.0 million to offset its future income tax liability. The NOL carryforwards began to expire in 2008. The Company has recorded a valuation allowance for all deferred tax assets related to the NOLs. Utilization of existing NOL carry forwards may be limited in future years based on significant ownership changes. The Company is in the process of analyzing its NOLs and has not determined if it is subject to any restrictions in the Internal Revenue Code that could limit the future use of NOL.Components of deferred taxes are as follows at December 31 (in thousands): 2015 2014 Deferred tax assets $ 626 $ 466 Net operating loss carry forwards 27,770 25,994 Deferred tax liabilities — — 28,396 26,460 Valuation allowance (28,396 ) (26,460 ) $ 0 $ 0 The following is a summary of the items that caused recorded income taxes to differ from taxes computed using the statutory federal income tax rate for the years ended December 31: 2015 2014 Statutory federal tax rate 34 % 34 % State taxes, net of federal benefit 4 4 Nondeductible expenses — — Valuation allowance (38 ) (38 ) 0 % 0 % 6. Commitments and ContingenciesOperating LeasesIn December 2009, the Company moved its offices, which comprise its administrative, research and development, marketing and production facilities to 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092. The Company leases approximately 23,000 square feet under a lease that expires in June 2017. The fixed monthly lease expense is approximately $15,000 plus common charges. The Company also leases office and equipment under operating lease agreements with monthly payments of approximately $2,000. These leases expire at various dates through April 2016. Future minimum rental payments at December 31, 2015 under non-cancellable operating leases for office space and equipment are as follows (in thousands): Year Amount 2016 $ 201 2017 98 Total $ 299 Rental expense was approximately $170, 000 in 2015 and 2014.Litigation and ClaimsDecemberMarch 31, 20152018, and December 31, 2014,2017, there was no accrual recorded for any potential losses related to pending litigation.F-17ContractsAt31, 2015 and 2014,2009, the Company hadmoved its offices, which comprise its administrative, research and development, marketing and production facilities to 5835 Peachtree Corners East, Suite B, Norcross, Georgia 30092. The Company leased approximately 23,000 square feet under a royaltylease that expired in June 2017. In July 2017, the Company leased the offices on a month to month basis. On February 23, 2018, the Company modified its lease to reduce its occupancy to 12,835 square feet. The fixed monthly lease expense will be: $13,859 each month for the period beginning January 1, 2018 and ending March 31, 2018; $8,022 each month for the period beginning April 1, 2018 and ending March 31, 2019; $8,268 each month for the period beginning April 1, 2019 and ending March 31, 2020; and $8,514 each month for the period beginning April 1, 2020 and ending March 31, 2021. The Company recognizes rent expense on a straight-line basis over the estimated lease term. Future minimum rental payments at March 31, 2018 under non-cancellable operating leases for office space and equipment are as follows (in thousands):Year 2018 2019 2020 2021 Freedom Meditech, entered into on August 26, 2008, inShenghuo Medical, LLC pursuant to which the Company receives quarterly royalty payments on Freedom Meditech’s sales of products using technology licensed fromgranted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already the Company. The royalty payment equals 3% of net sales of licensed products covered by a valid claim. The aggregate royalties payable are capped at $4,000,000. Freedom Meditech’s obligationCompany’s exclusive distributor in China, Macau and Hong Kong, and the license extended to pay royalties with respect to salesmanufacturing in a particular country untilthose countries as well. Under the earlierterms of the datelicense agreement, once Shenghuo was capable of expirationmanufacturing LuViva in accordance with ISO 13485 for medical devices, Shenghuo would pay the Company a royalty equal to $2.00 or 20% of the last valid claimdistributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories. In connection with the license grant, Shenghuo was to underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also would provide up to $1.0 million in such country or the aggregate royalty cap is reached. For the years ended December 31, 2015 and 2014, the Company received approximately $36,000 and 40,000 in royalties, respectively.7. License and Technology AgreementsAs partfurtherance of the Company’s efforts to conduct research and development activities and to commercialize potential products, the Company, from time to time, enters into agreements with certain organizations and individuals that further those efforts but also obligate the Company to make future minimum payments or to remit royalties ranging from 1% to 3% of revenuesecure regulatory approval for LuViva from the sale of commercial products developed from the research. The Company generally is required to make minimum royalty paymentsU.S. Food and Drug Administration, in exchange for the exclusive licenseright to develop certain technology.8. Notes PayableShort Term Notes PayableAt December 31, 2015 and 2014, the Company maintained notes payablereceive payments equal to Premium Assignment Corporation, an insurance premium financing company,2% of approximately $172,000 and $100,000, respectively. These notes are 10 month straight-line amortizing loan dated June 24, 2015 and June 24, 2014. The notes carry annual interest of 5.2% and 4.6%, respectively. The balance due to on the Premium Assignment note was approximately $70,000 and $37,000 at December 31, 2015 and December 31, 2014, respectively.Note Payable in DefaultAt December 31, 2015, the Company maintained a note payable totaling approximately $133,000 of principal and accrued interest. The note accrues interest at 9% with a 16% default rate and requires monthly payments of $10,000. As of December 31, 2015 the note is accruing interest at the default rate and is past due. As of December 31, 2014, the balance was $163,000 and was not in default.Short Term Convertible Note PayableOn September 10, 2014, the Company sold a secured promissory note an accredited investor with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). The Company may prepay the note at any time. The note is secured by the Company’s current and future accounts receivable and inventory, pursuantsales in the United States, up to a security agreement entered into in connection with the sale. On March 10, 2015, May 4, 2015, June 1, 2015, June 16, 2015, June 29, 2015, January 21, January 29 and February 12, 2016 the Company amended the termsan aggregate of the note to extend the maturity ultimately until August 31, 2016. During the extension, interest accrues on the note at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law.$4.0 million. Pursuant to the termslicense agreement, Shenghuo had the option to have a designee appointed to the Company’s board of directors (director Richard Blumberg is that designee). As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the amendedparties, the Company agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $300,000, expected to be due the holder may convertearlier of 90 days from issuance and consummation of any capital raising transaction by the outstanding balanceCompany with net cash proceeds of at least $1.0 million. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of the Company’s common stock at a conversion price per share of $13.92, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. The Company will also issue Shenghuo a five-year warrant exercisable immediately for approximately 21,549 shares of common stock at an exercise price equal to the lower of (1) $25.0 or (2) 75% of the lowest daily volume weighted averageconversion price of the common stock during the five days priornote, subject to conversion. If the conversion price at the time of any conversion is lower than $15.00, the Company has the option of delivering the conversion amount in cash in lieu of shares of common stock. As of December 31, 2015, the holder had converted $5,395 in outstanding principal and accrued interest into 90,240 shares of common stock. The Company paid a total of $65,000 in loan modification fees. At December 31, 2015 and 2014, the balance on the note was approximately $686,000 and $1,062,000, respectively. The original issue discount of $560,000 was fully amortized as of June 30, 2015.Convertible DebtApril 23, 2014,January 22, 2017, the Company entered into a securities purchaselicense agreement with Shandong Yaohua Medical Instrument Corporation, or SMI, pursuant to which the Company granted SMI an accredited investor,exclusive global license to manufacture the LuViva device and related disposables (subject to a carve-out for manufacture in Turkey) and exclusive distribution rights in the Peoples Republic of China, Macau, Hong Kong and Taiwan. In order to facilitate the SMI agreement, immediately prior to its execution the Company entered into an agreement with Shenghuo Medical, LLC, regarding its previous license to Shenghuo. Under the terms of the new agreement, Shenghuo agreed to relinquish its manufacturing license and its distribution rights in SMI’s territories, and to waive its rights under the original Shenghuo agreement, all for as long as SMI performs under the SMI agreement.6% senior convertible note withroyalty of future sales of single-use cervical guides for LuViva. Under the terms of the royalty agreement, and for consideration of $50,000, the Company will pay them an initial principal amount of $1.5 millionaggregate perpetual royalty initially equal to $0.10, and an 18-month term,from and after October 2, 2016, equal to $0.20, for each disposable that the Company sells (or that is sold by a purchase price of $1.0 million (an approximately 33.3% original issue discount). Additionally,third party pursuant to a licensing arrangement with the purchase agreement, on May 23, 2014 the investor purchased an additional senior convertible note with an initial principal amount of $2.0 millionCompany). an 18-month term, for a fixed purchase price of $2.0 million. As of July 1, 2015, the notes were repaid in full. The balance at December 31, 2014 was $783,000.F-18The Company paid the investor a commitment fee for entering into the purchase agreement in the form of 3,218 shares of common stock. The Company also paid $50,000 of attorneys’ fees and expenses incurred by Magna in connection with the transaction. Total debt issuance costs incurred on the senior convertible note was approximately $844,000. This amount was fully amortized at December 31, 2015. Total amortization expense for the year ended December 31, 2015 and 2014 was $516,000 and $328,000, respectively.In connection with the sale of the convertible notes, the Company issued its placement agent warrants exercisable for 2,000 shares of common stock at $50.00 per share with an expiration date of April 23, 2019, and warrants exercisable for 5,618 shares of common stock at $45.00 per share with an expiration date of May 22, 2019.Prior to repayment in full, the Company had issued a total of 252,804 shares of common stock in conjunction with conversions of the senior convertible notes.Loss on Extinguishment of DebtAs part of the Company’s December 2014 public offering of common stock, the Company repaid approximately $1.4 million of debt via the issuance of 77,005 shares of common stock and five-year warrants to purchase an additional 3,850,252 shares at an exercise price of $22.50 per share. Pursuant to the senior convertible note purchase agreement, the Company had to pay a prepayment penalty of 25% of the amount prepaid. The penalty on the transaction was approximately $325,000 and was charged to loss on extinguishment of debt on the statement of operations for the year ended December 31, 2014. There was no loss on extinguishment of debt in the year ended December 31, 2015.9. Related Party TransactionsAs of December 31, 2015 and 2014,2017, the Company maintained notes payable and accrued interest to both related and non-related parties totaling approximately $682,000$1,114,000 and $609,000,$1,091,000, respectively. These notes are short term, straight-line amortizing notes. The notes carry an annual interest rate ofrates between 5% and 10% and have default rates as high a 18.0%. Included inThe Company is accruing interest at the short-termdefault rate of 18.0%.accompanying Balance Sheet.CertainCompany entered into a license agreement with a distributor pursuant to which the Company granted the distributor an exclusive license to manufacture, sell and distribute the Company’s LuViva Advanced Cervical Cancer device and related disposables in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. The distributor was already the Company’s exclusive distributor in China, Macau and Hong Kong, and the license will extend to manufacturing in those countries as well.directorscommon stock at a conversion price per share of $13.92, subject to customary anti-dilution adjustment. The note is unsecured, and officers investedis expected to provide for customary events of default. The Company will also issue the distributor a total of $182,603 in the December 2014 public offering and received 8,116five-year warrant exercisable immediately for 17,239 shares of common stock as well as five-year warrantsat an exercise price equal to purchase an additional 4,058 shares at $22.50 per share.Directors and Officers holding the Company’s Series B preferred stock participated in the issuanceconversion price of the Company’s Series C preferred stock in the second half of 2015 by exchanging a total of $525,000 in liquidation value of Series B preferred stock and $400,000 in cash, and received 1,233 shares of Series C preferred stock and five-year warrantsnote, subject to purchase an additional 131,526 shares.10. Valuation and Qualifying AccountsAllowance for Doubtful AccountsThe Company has the following allowances for doubtful accounts (in thousands): 2015 2014 Beginning balance $ 76 $ 18 Additions / (Adjustments) 19 58 Balance $ 95 $ 76
customary anti-dilution adjustment.F-19Inventory ReservesThe Company has the following reserves for inventory balance (in thousands): 2015 2014 Beginning balance $ 144 $ 184 Additions / (Adjustments) (26 ) (40 ) Balance $ 118 $ 144 11. Loss Per Common ShareBasic net loss per share attributable to common stockholders amounts are computed by dividing the net loss plus preferred stock dividends and deemed dividends on preferred stock by the weighted average number of shares outstanding during the period.12. Subsequent Events11, 2016,13, 2017, the Company entered into a securities purchase agreement with an accredited investorAuctus Fund, LLC for the issuance and sale on February 12, 2016to Auctus of $1.4375 million$170,000 in aggregate principal amount of a senior secured12% convertible promissory note for an aggregate purchase price of $1.15 million (a 20%$156,400 (representing a $13,600 original issue discount). In addition,On February 13, 2017, the investor receivedCompany issued the note to Auctus. Pursuant to the purchase agreement, the Company also issued to Auctus a warrant exercisable to purchase an aggregate of approximately 179.7 million200,000 shares of the Company’s common stock. The convertible note matureswarrant is exercisable at any time, at an exercise price per share equal to $0.00228 (110% of the closing price of the common stock on the second anniversaryday prior to issuance), subject to certain customary adjustments and price-protection provisions contained in the warrant. The warrant has a five-year term. The note matured nine months from the date of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17%12% per year. The Company will pay monthly interest coupons and, beginning six months after issuance, will pay amortized quarterly principal payments. Ifcould have prepaid the Company does not receive, on or before the first anniversary after issuance, an aggregate of at least $3.0 million from future equity or debt financings or non-dilutive grants, then the holder will have the option of accelerating the maturity date to the first anniversary of issuance. The Company may prepay the convertible note, in whole or in part, without penalty, upon 20 days’ prior written notice. Subjectfor 115% of outstanding principal and interest until 30 days from issuance, for 125% of outstanding principal and interest at any time from 31 to resale restrictions under Federal securities laws60 days from issuance, and for 130% of outstanding principal and interest at any time from 61 days from issuance to 180 days from issuance. After six months from the availabilitydate of sufficient authorized but unissued shares ofissuance, Auctus converted the Company’s common stock, the convertible note, is convertible at any time, in whole or in part, at the holder’s option, into shares of the Company’s common stock, at a conversion price equal to the lesser of $0.008 per share (pre-stock split) or 70%lower of the price offered in the Company’s next public offering or a 40% discount to the average closing price per share forof the fivetwo lowest trading prices of the common stock during the 20 trading days prior to issuance,the conversion, subject to certain customary adjustments and anti-dilutionprice-protection provisions contained in the note. The note includes customary events of default provisions and a default interest rate of 24% per year. Upon the occurrence of an event of default, Auctus required the Company to redeem the note (or convert it into shares of common stock) at 150% of the outstanding principal balance plus accrued and unpaid interest. In connection with the transaction, the Company agreed to reimburse Auctus for $30,000 in legal and diligence fees, of which we paid $10,000 in cash and $20,000 in restricted shares of common stock, valued at $0.40 per share (a 42.86% discount to the closing price of the common stock on the day prior to issuance). The Company allocated proceeds of $90,000 to the warrants and common stock issued in connection with the financing. As of March 31, 2018, the Company has net debt and interest of $30,800 as compared to net debt and interest of $76,664 for the period ended December 31, 2017.note.19%20% per year or the maximum amount permitted by law. Upon the occurrence of an event of default, the holder of the note may require the Companyus to redeem the convertible note (or convert it into shares of common stock) at 120%150% of the outstanding principal balance. TheAs of March 31, 2018, the Company has net debt of $66,000 and interest of $6,793 as compared to net debt of $66,000 for the period ended December 31, 2017.secureddue and payable on May 19, 2018. The note may be converted by a lien on all of the Company’s assets, including its intellectual property, pursuant to a security agreement entered into by the Company and GPB in connection with the transaction.The warrant is exercisablePower Up at any time pending availabilityafter 180 days from issuance into shares of sufficient authorized but unissuedCompany’s common stock at a conversion price equal to 58% of the average of the lowest two-day trading prices of the common stock during the 15 trading days prior to conversion. The note may be prepaid in accordance with its terms, at premiums ranging from 15% to 40%, depending on the time of prepayment. The note contains certain representations, warranties, covenants and events of default, including if the Company is delinquent in its periodic report filings with the SEC, and provides for increases in principal and interest in the event of such defaults. As of March 31, 2018, the notes had been converted and no balance remained outstanding as compared to a net debt of $46,405, including unamortized debt issuance costs of $6,595 of December 31, 2017.an exercisea conversion price per share equal to 60% of the conversionlowest volume weighted average price of our common stock during the convertible note,20 trading days prior to conversion, subject to certain customary adjustments and anti-dilution provisions contained in the warrant. The warrant has a five-year term.The Company used a placement agent in connection with the transaction. For its services, the placement agent received a cash placement fee equal to 4% of the aggregate gross proceeds from the transaction and a warrant to purchase shares of common stock equal to an aggregate of 6% of the total number of shares underlying the securities sold in the transaction, at an exercise price equal to, and terms otherwise identical to, the warrant issued to the investor. Finally, the Company agreed to reimburse the placement agent for its reasonable out-of-pocket expenses.In connection with the transaction, on February 12, 2016, the Company and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to the Company in exchange for a royalty payment, payable quarterly, equal to 3.5% of the Company’s revenues from the sale of products.On February 24, 2016, the Company implemented a 1:100 reverse stock split of all of its issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock of the Company were converted into 1 share. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The reverse stock split decreased the Company’s issued and outstanding shares of common stock from 287,729,113 shares to 2,877,291 shares. The number of the authorized shares did not change.F-20GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONDENSED CONSOLIDATED BALANCE SHEETS Unaudited (in Thousands) ASSETS March 31, 2016 December 31, 2015 CURRENT ASSETS: Cash and cash equivalents $ 56 $ 35 Accounts receivable, net of allowance for doubtful accounts of $75 and $95 at March 31, 2016 and December 31, 2015, respectively 230 190 Inventory, net of reserves of $156 and $118, at March 31, 2016 and December 31, 2015, respectively 1,253 1,119 Prepaid expenses and deposits 509 780 Total current assets 2,048 2,124 Property and equipment, net 264 318 Other assets 54 121 Total noncurrent assets 318 439 TOTAL ASSETS $ 2,366 $ 2,563 LIABILITIES AND STOCKHOLDERS’ DEFICIT CURRENT LIABILITIES: Short-term notes payable, including related parties $ 259 $ 752 Note payable in default, including related parties 590 133 Convertible note – current portion 479 — Short-term convertible notes payable, net 591 686 Accounts payable 1,977 1,824 Accrued liabilities 2,069 1,907 Deferred revenue 66 217 Total current liabilities 6,031 5,519 Warrants, at fair value 1,588 2,606 Long-term convertible notes payable, net 197 — Total long-term liabilities 1,785 2,606 TOTAL LIABILITIES 7,816 8,125 STOCKHOLDERS’ DEFICIT: Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 5.0 and 5.6 shares issued and outstanding as of March 31, 2016 and December 31, 2015, (Liquidation preference of $4,966 and $5,555 at March 31, 2016 and December 31, 2015) 1,817 2,052 Common stock, $.001 Par value; 1,000,000 shares authorized, 5,322 and 2,371 shares issued and outstanding as of March, 31 2016 and December 31, 2015 297 236 Additional paid-in capital 115,471 114,845 Treasury stock, at cost (132 ) (132 ) Accumulated deficit (122,903 ) (122,563 ) TOTAL STOCKHOLDERS’ DEFICIT (5,450 ) (5,562 ) TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT $ 2,366 $ 2,563 The accompanying notes are an integral part of these condensed consolidated financial statements. F-21CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited, in Thousands) 2016 2015 REVENUE: Sales – devices and disposables $ 262 $ 127 Cost of goods sold 68 107 Gross profit 194 20 OPERATING EXPENSES: Research and development 290 373 Sales and marketing 117 172 General and administrative 917 963 Total operating expenses 1,324 1,508 Operating loss (1,130 ) (1,488 ) OTHER INCOME (EXPENSES): Other income 23 21 Interest expense (158 ) (492 ) Change in fair value of warrants 1,395 714 Total other income 1,260 243 INCOME (LOSS) FROM OPERATIONS 130 (1,245 ) PROVISION FOR INCOME TAXES — — NET INCOME (LOSS) $ 130 $ (1,245 ) (470 ) (31 ) $ (340 ) $ (1,276 ) NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS BASIC $ (0.11 ) $ (1.31 ) DILUTED $ (0.00 ) $ (1.31 ) WEIGHTED AVERAGE SHARES OUTSTANDING BASIC 3,084 973 DILUTED 109,899 973 The accompanying notes are an integral part of these condensed consolidated financial statements.F-22CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (Unaudited, in Thousands) 2016 2015 CASH FLOWS FROM OPERATING ACTIVITIES: Net income (loss) $ 130 $ (1,245 ) Adjustments to reconcile net income (loss) to net cash used in operating activities: Bad debt expense 17 — Depreciation 54 72 Amortization 54 402 Stock based compensation 30 192 Change in fair value of warrants (1,395 ) (714 ) Changes in operating assets and liabilities: Inventory (135 ) (20 ) Accounts receivable (56 ) 31 Other current assets 271 47 Other assets 18 8 Accounts payable 153 (169 ) Deferred revenue (151 ) — Accrued liabilities 55 508 Other long-term liabilities — 262 Total adjustments (1,085 ) 619 Net cash used in operating activities (955 ) (626 ) CASH FLOWS FROM FINANCING ACTIVITIES: Net proceeds from issuance of common stock and warrants, net — 451 Proceeds from debt financing, net of discounts and debt issuance costs 1,029 62 Payments made on notes payable (53 ) (31 ) Net cash provided by financing activities 976 482 NET CHANGE IN CASH AND CASH EQUIVALENTS 21 (144 ) CASH AND CASH EQUIVALENTS, beginning of year 35 162 CASH AND CASH EQUIVALENTS, end of period $ 56 $ 18 SUPPLEMENTAL SCHEDULE OF: Cash paid for: Interest $ 1 $ 22 NONCASH INVESTING AND FINANCING ACTIVITIES: Issuance of common stock as debt repayment $ 125 $ 50 Dividends on preferred stock $ 470 $ 31 The accompanying notes are an integral part of these condensed consolidated financial statements.F-23GUIDED THERAPEUTICS, INC. AND SUBSIDIARYNOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)1. BASIS OF PRESENTATIONThe accompanying unaudited condensed consolidated financial statements included herein have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) for interim financial reporting and with the instructions to Form 10-Q and Article 10 of Regulation S-X by Guided Therapeutics, Inc. (formerly SpectRx, Inc.), together with its wholly owned subsidiary InterScan, Inc., (“Interscan”) (formerly Guided Therapeutics, Inc.), collectively referred to herein as the “Company”. Accordingly, they do not include all information and footnotes required by GAAP for complete financial statements. These statements reflect adjustments, all of which are of a normal, recurring nature, and which are, in the opinion of management, necessary to present fairly the Company’s financial position as of March 31, 2016, results of operations for the three months ended March 31, 2016 and 2015, and cash flows for the three months ended March 31, 2016 and 2015. The results of operations for the three months ended March 31, 2016 are not necessarily indicative of the results for a full fiscal year. Preparing financial statements requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and disclosure of contingent assets and liabilities. Actual results could differ from those estimates. These financial statements should be read in conjunction with the financial statements and notes thereto included in the Company’s annual report on Form 10-K for the year ended December 31, 2015.On February 24, 2016, the Company implemented a 1:100 reverse stock split of its issued and outstanding common stock. The reverse stock split decreased the Company’s issued and outstanding shares of common stock from 237,101,702 shares of Common Stock to 2,371,007 shares as of that date. See Note 4, Stockholders’ Deficit. Unless otherwise specified, all per share amounts are reported on a post-stock split basis, as of March 31, 2016.The Company's prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. The Company has experienced net losses since its inception and, as of March 31, 2016, it had an accumulated deficit of approximately $122.9 million. Through March 31, 2016, the Company has devoted substantial resources to research and development efforts. The Company does not have significant experience in manufacturing, marketing or selling its products. The Company's development efforts may not result in commercially viable products and it may not be successful in introducing its products. Moreover, required regulatory clearances or approvals may not be obtained. The Company's products may not ever gain market acceptance and the Company may not ever achieve levels of revenue to sustain further development costs and support ongoing operations or achieve profitability. The development and commercialization of the Company's products will require substantial development, regulatory, sales and marketing, manufacturing and other expenditures. The Company expects operating losses to continue through the foreseeable future as it continues to expend substantial resources to complete development of its products, obtain regulatory clearances or approvals and conduct further research and development.Going ConcernThe Company’s consolidated financial statements have been prepared and presented on a basis assuming it will continue as a going concern. The factors below raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might be necessary from the outcome of this uncertainty. If the Company is unable to continue operations, it may have to the extent practicable, liquidate and/or file for bankruptcy protection.LiquidityAt March 31, 2016, the Company had a working capital deficit of approximately $4.0 million and the stockholders’ deficit was approximately $5.5 million, primarily due to recurring net losses from operations and dividends on preferred stock, offset by proceeds from the exercise of options and warrants and proceeds from the sales of stock.The Company’s plans with respect to its liquidity management include the following:The Company has curtailed operations and reduced discretionary spending and staffing levels.The Company is only pursuing activities where it has financial support. However, there can be no assurance that such external financial support will be sufficient to maintain even limited operations.The Company is seeking additional capital in the private and/or public equity markets to continue operations and build sales, marketing, and distribution. The Company is currently evaluating additional equity and debt financing opportunities and may execute them when appropriate. However, there can be no assurances that the Company can consummate such a transaction, or consummate a transaction at favorable pricing.F-24The Company is seeking additional sources of cash flow with strategic businesses.The Company had warrants exercisable for approximately 31.8 million shares of its common stock outstanding at March 31, 2016, with exercise prices of $0.09375 to $105.00 per share. Exercises of these warrants would generate a total of approximately $7.1 million in cash, assuming full exercise, although the Company cannot be assured that holders will exercise any warrants2. SIGNIFICANT ACCOUNTING POLICIESThe Company’s significant accounting policies were set forth in the audited financial statements and notes thereto for the year ended December 31, 2015 included in its annual report on Form 10-K, filed with the Securities and Exchange Commission (“SEC”).Use of EstimatesThe preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant areas where estimates are used include the allowance for doubtful accounts, inventory valuation and input variables for Black-Scholes, Monte Carlo simulations and binomial calculations.Principles of ConsolidationThe accompanying consolidated financial statements include the accounts of Guided Therapeutics, Inc. and its wholly owned subsidiary.Accounting Standard UpdatesIn April 2015, the FASB issued Accounting Standards Update No. 2015-03, Interest - Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, or ASU 2015-03. ASU 2015-03 amends current presentation guidance by requiring that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. Prior to the issuance of ASU 2015-03 debt issuance costs were required to be presented as an asset in the balance sheet. On January 1, 2016, the Company adopted the provisions of ASU 2015-03 and prior period amounts have been reclassified to conform to the current period presentationAccounts ReceivableThe Company performs periodic credit evaluations of its customers’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.Revenue RecognitionRevenue from the sale of the Company’s products is recognized upon shipment of such products to its customers. The Company recognizes revenue from contracts on a straight line basis, over the terms of the contract. The Company recognizes revenue from grants based on the grant agreement, at the time the expenses are incurred. During the three months ended March 31, 2016, the majority of the Company’s revenues were from four customers. Revenue from these customers totaled approximately $262,000 or 100% for the period ended March 31, 2016 and approximately $127,000 or 73% of total revenue for the period ended March 31, 2015. Accounts receivable due from those customers represents 59% for the period ended March 31, 2016.Deferred RevenueThe Company defers payments received as revenue until earned based on the related contracts on a straight line basis, over the terms of the contract.F-25Inventory ValuationAll inventories are stated at lower of cost or market, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased. At March 31, 2016 and December 31, 2015, our inventories were as follows (in thousands): March 31, December 31, 2016 2015 Raw materials $ 827 $ 686 Work in process 256 186 Finished goods 326 365 Inventory reserve (156 ) (118 ) Total $ 1,253 $ 1,119 Property and EquipmentProperty and equipment are recorded at cost. Depreciation is computed using the straight-line method over estimated useful lives of three to seven years. Leasehold improvements are depreciated at the shorter of the useful life of the asset or the remaining lease term. Depreciation expense is included in general and administrative expense on the statement of operations. Expenditures for repairs and maintenance are expensed as incurred. Property and equipment are summarized as follows at March 31, 2016 and December 31, 2015 (in thousands): March 31, December 31, 2016 2015 Equipment $ 1,377 $ 1,377 Software 740 740 Furniture and fixtures 124 124 Leasehold Improvement 199 199 2,440 2,440 Less accumulated depreciation (2,174 ) (2,122 ) Total $ 264 $ 318 Accrued LiabilitiesAccrued liabilities are summarized as follows (in thousands): December 31,
2014Accrued compensation $ 1,249 $ 1,235 Accrued professional fees 71 154 Deferred rent 31 36 Accrued warranty 70 82 Accrued vacation 190 177 Accrued interest 33 — Accrued dividends 332 167 Other accrued expenses 93 56 Total $ 2,069 $ 1,907 F-26Significant CustomersResearch and DevelopmentResearch and development expenses consist of expenditures for research conducted by the Company and payments made under contracts with consultants or other outside parties and costs associated with internal and contracted clinical trials. All research and development costs are expensed as incurred.Income TaxesThe Company uses the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Management provides valuation allowances against the deferred tax assets for amounts that are not considered more likely than not to be realized.The Company is current with its federal and applicable state tax returns filings. Although we have been experiencing recurring losses, we are obligated to file tax returns for compliance with Internal Revenue Service (“IRS”) regulations and that of applicable state jurisdictions. As December 31, 2015, the Company has approximately $28.0 million of net operating loss eligible to be carried forward for tax purposes at federal and applicable states level. A full valuation allowance has been recorded related the deferred tax assets generated from the net operating losses.None of the Company’s federal or state income tax returns are currently under examination by the IRS or state authorities.Uncertain Tax PositionsEffective January 1, 2007 the Company adopted ASC guidance regarding accounting for uncertainty in income taxes. This guidance clarifies the accounting for income taxes by prescribing the minimum recognition threshold an income tax position is required to meet before being recognized in the financial statements and applies to all income tax positions. Each income tax position is assessed using a two-step process. A determination is first made as to whether it is more likely than not that the income tax position will be sustained, based upon technical merits, upon examination by the taxing authorities. If the income tax position is expected to meet the more likely than not criteria, the benefit recorded in the financial statements equals the largest amount that is greater than 50% likely to be realized upon its ultimate settlement. At March 31, 2016 and December 31, 2015 there were no uncertain tax positions.WarrantsThe Company has issued warrants, which allow the warrant holder to purchase one share of stock at a specified price for a specified period of time. The Company records equity instruments including warrants issued to non-employees based on the fair value at the date of issue. The fair value of warrants classified as equity instruments at the date of issuance is estimated using the Black-Scholes Model. The fair value of warrants classified as liabilities at the date of issuance is estimated using the Monte Carlo Simulation or Binomial model.Stock Based CompensationThe Company records compensation expense related to options granted to non-employees based on the fair value of the award.Compensation cost is recorded as earned for all unvested stock options outstanding at the beginning of the first year based upon the grant date fair value estimates, and for compensation cost for all share-based payments granted or modified subsequently based on fair value estimates.For the three ended March 31, 2016 and 2015 share-based compensation for options attributable to employees, officers and Board members were approximately $30,053 and $191,570. These amounts have been included in the Company’s statements of operations. Compensation costs for stock options which vest over time are recognized over the vesting period.note. As of March 31, 2016,2018, the Company had approximately $202,000has fully amortized debt issuance costs $30,000 and original issue discount of unrecognized compensation costs related to granted stock options that will be recognized over the remaining vesting period of approximately three years.3. FAIR VALUE OF FINANCIAL INSTRUMENTSThe guidance for fair value measurements, ASC820,Fair Value Measurements and Disclosures, establishes the authoritative definition of fair value, sets out a framework for measuring fair value, and outlines the required disclosures regarding fair value measurements. Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. The Company uses a three-tier fair value hierarchy based upon observable and non-observable inputs as follow:F-27·Level 1 – Quoted market prices in active markets for identical assets and liabilities;·Level 2 – Inputs, other than level 1 inputs, either directly or indirectly observable; and·Level 3 – Unobservable inputs developed using internal estimates and assumptions (there is little or no market date) which reflect those that market participants would use.The Company records its derivative activities at fair value, which consisted of warrants as of March 31, 2016. The fair value of the warrants was estimated using the Binomial Simulation model. Gains and losses from derivative contracts are included in net gain (loss) from derivative contracts in the statement of operations. The fair value of the Company’s derivative warrants is classified as a Level 3 measurement, since unobservable inputs are used in the valuation.The following table presents the fair value for those liabilities measured on a recurring basis as of March 31, 2016 and December 31, 2015:FAIR VALUE MEASUREMENTS (In Thousands)The following is summary of items that the Company measures at fair value on a recurring basis: Fair Value at March 31, 2016 Level 1 Level 2 Level 3 Total Warrants issued in connection with the issuance of Series C preferred stock $ - $ - $ (70 ) $ (70 ) Warrants issued in connection with the issuance of Series B preferred stock - - (612 ) (612 ) Warrants issued in connection with Senior Secured Debt - - (906 ) (906 ) Total long-term liabilities at fair value $ - $ - $ (1,588 ) $ (1,588 ) Level 1 Level 2 Level 3 Total Warrants issued in connection with the issuance of Series C preferred stock $ — $ — $ (1,145 ) $ (1,145 ) Warrants issued in connection with the issuance of Series B preferred stock — — (1,461 ) (1,461 ) Total long-term liabilities at fair value $ — $ — $ (2,606 ) $ (2,606 ) The following is a summary of changes to Level 3 instruments during the three months ended March 31, 2016: Senior Secured Debt Series B Warrants Series C Warrants Total Balance, December 31, 2015 $ — $ (1,461 ) $ (1,145 ) $ (2,606 ) Warrants issued during the period (377 ) — — (377 ) Change in fair value during the period (529 849 1,075 1,395 Balance, March 31, 2016 $ (906 $ (612 ) $ (70 ) $ (1,588 ) F-282016,2018, the fair value of warrants was approximately $1.6 million. A net change of approximately $1.4 million has been recorded to the accompanying statement of operations for the three months ended.4. STOCKHOLDERS’ DEFICITCommon StockThe Company has authorized 1,000,000,000 shares of common stock with $0.001 par value, of which 5,322,003 were issued and outstanding as of March 31, 2016. For the year ended December 31, 2015, there were 1,000,000,000 authorized shares of common stock, of which 2,371,017 were issued and outstanding.On February 24, 2016, the Company implemented a 1:100 reverse stock split of all of its issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock of the Company were converted into 1 share. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of the authorized shares did not change.For the three months ended March 31, 2016, the Company issued 2,950,986 shares of common stock as listed below:Series C Preferred Stock Conversions1,511,350Series C Preferred Stock Dividends631,047Convertible Debt Conversions808,589 Total2,950,986Preferred StockThe Company has authorized 5,000,000 shares of preferred stock with a $.001 par value. The board of directors has the authority to issue these shares and to set dividends, voting and conversion rights, redemption provisions, liquidation preferences, and other rights and restrictions. The board of directors designated 525,000 shares of preferred stock redeemable convertible preferred stock, none of which remain outstanding, 3,000 shares of preferred stock as Series B Preferred Stock, of which zero shares were issued and outstanding at both March 31, 2016 and December 31, 2015 and 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 4,966 and 5,555 were issued and outstanding at March 31, 2016 and December 31, 2015, respectively.On May 3, 2016, the board of directors designated 20,250 shares of preferred stock as Series C1 Convertible Preferred Stock. See Note 10, Subsequent Events.Series C Convertible Preferred StockOn June 29, 2015, the Company entered into a securities purchase agreement with certain accredited investors for the issuance and sale of an aggregate of 6,737 shares of Series C convertible preferred stock, at a purchase price of $750 per share and a stated value of $1,000 per share. On September 3, 2015 the Company entered into an interim agreement amending the securities purchase agreement to provide for certain of the investors to purchase an additional aggregate of 1,166 shares. Total cash and non-cash expenses were valued at $853,000, resulting in net proceeds of $3,698,000.Pursuant to the Series C certificate of designations, shares of Series C preferred stock are convertible into common stock by their holder at any time, and may be mandatorily convertible upon the achievement of specified average trading prices for the Company’s common stock. At March 31, 2016, there were 4,966 shares outstanding with a conversion price of $0.09375 per share, such that each share of Series C preferred stock would convert into approximately 52,977,774 shares of the Company’s common stock, subject to customary adjustments, including for any accrued but unpaid dividends and pursuant to certain anti-dilution provisions, as set forth in the Series C certificate of designations. The conversion price will automatically adjust downward to 80% of the then-current market price of the Company’s common stock 15 trading days after any reverse stock split of the Company’s common stock, and 5 trading days after any conversions of the Company’s outstanding convertible debt.F-29Holders of the Series C preferred stock are entitled to quarterly cumulative dividends at an annual rate of 12.0% until 42 months after the original issuance date (the “Dividend End Date”), payable in cash or, subject to certain conditions, the Company’s common stock. In addition, upon conversion of the Series C Preferred Stock prior to the Dividend End Date, the Company will also pay to the converting holder a “make-whole payment” equal to the amount of unpaid dividends through the Dividend End Date on the converted shares. The Series C preferred stock generally has no voting rights except as required by Delaware law. Upon the Company’s liquidation or sale to or merger with another corporation, each share will be entitled to a liquidation preference of $1,000, plus any accrued but unpaid dividends.In addition, the purchasers of the Series C preferred stock received, on a pro rata basis, warrants exercisable to purchase an aggregate of approximately 1,247,737 shares of Company’s common stock. The warrants contain anti-dilution adjustments in the event that the Company issues shares of common stock, or securities exercisable or convertible into shares of common stock, at prices below the exercise price of such warrants. As a result of the anti-dilution protection, the Company is required to account for the warrants as a liability recorded at fair value each reporting period. At March 31, 2016, the exercise price per share was $0.09375.WarrantsThe following table summarizes transactions involving the Company’s outstanding warrants to purchase common stock for the quarter ended March 31, 2016:Warrants(Underlying Shares)Outstanding, January 1, 20162,802,384Issuances28,952,716Canceled / Expired— Exercised— Outstanding, March 31, 201631,755,100The Company had the following shares reserved for the warrants as of March 31, 2016:
(Underlying Shares) 4,399 (1) $68.00 per share April 1, 2016 2,852 (2) $105.00 per share November 20, 2016 18,581 (3) $10.46 per share May 23, 2018 14,176,202 (3) $0.09375 per share May 23, 2018 2,000 (4) $50.00 per share April 23, 2019 5,618 (4) $45.00 per share May 22, 2019 1,842 (5) $38.00 per share September 10, 2019 3,255 (6) $46.081 per share September 27, 2019 7,553 (7) $28.13 per share December 2, 2019 83,927 (8) $9.00 per share December 2, 2020 83,927 (8) $11.00 per share December 2, 2020 20,000 (9) $25.50 per share March 30, 2018 17,547 (10) $11.88 per share June 29, 2020 526,421 (11) $1.03 per share June 29, 2020 273,684 (12) $1.03 per share September 4, 2020 289,737 (13) $1.03 per share September 21, 2020 5,163 (14) $11.88 per share September 4, 2020 157,895 (15) $1.03 per share October 23, 2020 5,163 (16) $11.88 per share October 23, 2020 16,069,333 (17) $0.09375 per share February 12, 2021 31,755,100 (1)Issued in February 2014 as part of a buy-back of a minority interest in Interscan in December 2012.(2)Issued as part of a November 2011 private placement.F-30(3)Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement.These warrants have anti-dilution and price protection and adjust periodically as described later in the notes.(4)Issued to a placement agent in conjunction with an April 2014 private placement.(5)Issued to a placement agent in conjunction with a September 2014 private placement.(6)Issued as part of a September 2014 Regulation S offering.(7)Issued to a placement agent in conjunction with a 2014 public offering.(8)Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering.(9)Issued as part of a March 2015 private placement.(10)Issued to a placement agent in conjunction with a June 2015 private placement.(11) Issued as part of a June 2015 private placement.(12)Issued as part of a June 2015 private placement.(13)Issued as part of a June 2015 private placement.(14)Issued to a placement agent in conjunction with a June 2015 private placement.(15) Issued as part of a June 2015 private placement.(16)Issued to a placement agent in conjunction with a June 2015 private placement.(17)Issued to a placement agent and a senior secured convertible note holder.These warrants have anti-dilution and price protection and adjust periodically as described later in the notes.5. STOCK OPTIONSUnder the Company’s 1995 Stock Plan (the “Plan”), a total of 26,616 shares remained available at March 31, 2016 and 98,451 shares were subject to stock options outstanding as of that date, bringing the total number of shares subject to stock options outstanding and those remaining available for issue to 132,552 shares of common stock as of March 31, 2016. The Plan allows the issuance of incentive stock options, nonqualified stock options, and stock purchase rights. The exercise price of options is determined by the Company’s board of directors, but incentive stock options must be granted at an exercise price equal to the fair market value of the Company’s common stock as of the grant date. Options historically granted have generally become exercisable over four years and expire ten years from the date of grant.The fair value of stock options granted in the period ended March 31, 2015 were estimated using the Black-Scholes option pricing model. No options were issued during the period ended March 31, 2016. Stock option activity for March 31, 2016 as follows: 2016 Shares Price Outstanding at beginning of year 105,936 $ 45.00 Options granted — $ — Options exercised — $ — Options expired/forfeited (7,485 ) $ 43.00 Outstanding at end of year 98,451 $ 45.00 6. LITIGATION AND CLAIMSFrom time to time, the Company may be involved in various legal proceedings and claims arising in the ordinary course of business. Management believes that the dispositions of these matters, individually or in the aggregate, are not expected to have a material adverse effect on the Company’s financial condition. However, depending on the amount and timing of such disposition, an unfavorable resolution of some or all of these matters could materially affect the future results of operations or cash flows in a particular period.As of March 31, 2016 and December 31, 2015, there was no accrual recorded for any potential losses related to pending litigation.7. NOTES PAYABLEF-31Short Term Notes PayableAt March 31, 2016 and December 31, 2015, the Company maintained notes payable and accrued interest to related parties totaling $241,000 and $682,000, respectively. These notes are short term, straight-line amortizing notes. The notes carry an annual interest rate of between 5% and 10%.At March 31, 2016, the Company maintained a note payable to Premium Assignment Corporation, an insurance premium financing company, of approximately $17,500. This note is 10 month straight-line amortizing loan dated June 24, 2015. The note carries annual interest of 5.2%. The balance due to on the Premium Assignmentinvestor for the December 28, 2016 note, was approximately $17,500 and $70,000 at March 31, 2016 and December 31, 2015, respectively.Notes Payable in DefaultAt March 31, 2016, and December 31, 2015 the Company maintained notes payable and accrued interest to related and unrelated parties totaling $590,000 and $133,000, respectively. The notes carry interest at 5% to 10% and have default rates as high a 16.5%. As of March 31, 2016 the notes are accruing interest at the default rate.8.is zero.notesnote to eliminate the volume limitations on sales of common stock issued or issuable upon conversionconversion. On July 13, 2016, the Company consented to the assignment by one of the notes.related to thison the note was $591,244$159,698 and $685,864$184,245 at March 31, 20162018 and December 31, 2015,2017, respectively.waswere approximately $130,000. This amount was being amortized over sixnine months and iswas fully amortized as of MarchDecember 31, 2015. Total amortized expense for the three months ended March 31, 2015 was approximately $49,000. For the three months ended March 31, 2015, the Company recorded amortization of approximately $213,000 on the discount. The original issue discount of $560,000 was fully amortized as of MarchDecember 31, 2015.an accredited investorGPB Debt Holdings II LLC for the issuance and sale on February 12, 2016 of $1.4375 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount of $287,500) and a discount for debt issuance costs paid at closing of $121,000 for a total of $408,500. In addition, the investorGPB received a warrant exercisable to purchase an aggregate of approximately 1,796,875 million2,246 shares of the Company’s common stock. The Company allocated proceeds totaling 359,555$359,555 to the fair value of the warrants at issuance. This was recorded as an additional discount on the debt. The convertible note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. The Company willis required to pay monthly interest coupons and beginning sixnine months after issuance, willthe Company is required to pay amortized quarterly principal payments. If the Company does not receive, on or before the first anniversary after issuance, an aggregate of at least $3.0 million from future equity or debt financings or non-dilutive grants, then the holder will have the option of accelerating the maturity date to the first anniversary of issuance. The Company may prepay the convertible note, in whole or in part, without penalty, upon 20 days’ prior written notice. Subject to resale restrictions under Federal securities laws and the availability of sufficient authorized but unissued shares of the Company’s common stock, the convertible note is convertible at any time, in whole or in part, at the holder’s option, into shares of the Company’s common stock, at a conversion price equal to the lesser of $0.80 per share or 70% of the average closing price per share for the five trading days prior to issuance, subject to certain customary adjustments and anti-dilution provisions contained in the convertible note. As of March 31,On May 28, 2016, in exchange for an additional $87,500 in cash from GPB to the Company, the principal balance was increased by the same amount. The Company is currently in default as they are past due on the convertible debt was $676,110 (net of debt issuance costs of $44,540 and debt discount of $716,850).F-32The convertible note includes customaryrequired monthly interest payments. In the event of default, provisions andthe Company shall accrue interest at a default interest rate of the lesser of 22% or the maximum amount permitted by law. The Company has accrued $117,000 for past due interest payments at December 31, 2016. Upon the occurrence of an event of default, the holder may require the Company to redeem the convertible note at 120% of the outstanding principal balance.balance (but as of March 31, 2018, had not done so). As of March 31, 2018, the balance due on the convertible debt was $2,136,863 as the Company has fully amortized debt issuance costs of $47,675 and the debt discount of $768,055 and recorded a 20% penalty totaling $305,000. In addition, the Company has accrued $451,031 of interest expense. The convertible note is secured by a lien on all of the Company’s assets, including its intellectual property, pursuant to a security agreement entered into by the Company and GPB in connection with the transaction.2016,2018, the exercise price had been adjusted to $0.09375$0.00228 and the number of common stock shares exchangeable for was 15,333,333.630,482,456. As of March 31, 2016,2018, the effective interest rate considering debt costs was 29%.the investorGPB entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to the Company in exchange for a royalty payment, payable quarterly, equal to 3.5% of the Company’s revenues from the sale of products. As of March 31, 2016 the investor2018, GPB had earned approximately $3,000$29,000 in royalties.royalties.9.senior secured convertible note originally issued to GPB on February 11, 2016, and the $306,863 in outstanding principal amount of the Company’s secured promissory note that GPB holds (see “—Secured Promissory Note”). Pursuant to the exchange agreement, upon completion of the next financing resulting in at least $1 million in cash proceeds, GPB will exchange both securities for a new convertible note in principal amount of $1,831,863. The new convertible note will mature on the second anniversary of issuance and will accrue interest at a rate of 19% per year. The Company will pay monthly interest coupons and, beginning one year after issuance, will pay amortized quarterly principal payments. Subject to resale restrictions under Federal securities laws and the availability of sufficient authorized but unissued shares of the Company’s common stock, the new convertible note will be convertible at any time, in whole or in part, at the holder’s option, into shares of common stock, at a conversion price equal to the price offered in the qualifying financing that triggers the exchange, subject to certain customary adjustments and anti-dilution provisions contained in the new convertible note. The new convertible note will include customary event of default provisions and a default interest rate of the lesser of 21% or the maximum amount permitted by law. Upon the occurrence of an event of default, GPB will be entitled to require the Company to redeem the new convertible note at 120% of the outstanding principal balance. The new convertible note will be secured by a lien on all the Company’s assets, including its intellectual property, pursuant to the security agreement entered into by the Company and GPB in connection with the issuance of the original senior secured convertible note. Additionally, the Company further agreed to amend the warrant issued with the original senior secured convertible note, to adjust the number of shares issuable upon exercise of the warrant to equal the number of shares that will initially be issuable upon conversion of the new convertible note (without giving effect to any beneficial ownership limitations set forth in the terms of the new convertible note). As an inducement to GPB to enter into these transactions, the Company agreed to increase the royalty payable to GPB pursuant to its consulting agreement with us on December 7, 2016 from 3.5% to 3.85% of revenues from the sales of the Company’s products.period.period,year, plus Series C convertible preferred stock, convertible debt, convertible preferred dividends and warrants convertible into common stock shares.10. Net Income (loss) Basic weighted average number of shares outstanding Net income (loss) per share (basic) Diluted weighted average number of shares outstanding Net income (loss) per share (diluted) Dilutive equity instruments (number of equivalent units): Stock options Preferred stock Convertible debt Warrants Total Dilutive instruments Report of Independent Registered Public Accounting Firm 72 Consolidated Financial Statements Consolidated Balance Sheets as of December 31, 2017 and December 31, 2016 73 Consolidated Statements of Operations for the Years Ended December 31, 2017 and 2016 74 Consolidated Statements of Cash Flows for the Years Ended December 31, 2017 and 2016 75 Consolidated Statement of Stockholders’ equity (deficit) for the Years Ended December 31, 2017 and 2016 76 Notes to Consolidated Financial Statements 77 Sterling Heights, Michigan April 17, 2018 GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONDENSED CONSOLIDATED BALANCE SHEETS (in thousands) AS OF DECEMBER 31, ASSETS CURRENT ASSETS: Cash and cash equivalents Accounts receivable, net of allowance for doubtful accounts of $160 and $279 at December 31, 2017 and 2016, respectively Inventory, net of reserves of $716 and $278 at December 31, 2017 and 2016, respectively Other current assets Total current assets Property and equipment, net Other assets Total noncurrent assets TOTAL ASSETS LIABILITIES AND STOCKHOLDERS’ DEFICIT CURRENT LIABILITIES: Notes payable in default, including related parties Short-term notes payable, including related parties Convertible notes in default Convertible notes payable, net Accounts payable Accrued liabilities Deferred revenue Total current liabilities Warrants, at fair value TOTAL LIABILITIES COMMITMENTS & CONTINGENCIES (Note 8) STOCKHOLDERS’ DEFICIT: Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 0.9 and 1.6 shares issued and outstanding as of December 31, 2017 and 2016, respectively. (Liquidation preference of $970 and $1,643 at December 31, 2017 and 2016, respectively). Series C1 convertible preferred stock, $.001 par value; 20.3 shares authorized, 4.3 shares issued and outstanding as of December 31, 2017 and 2016, respectively. (Liquidation preference of $4,312 at December 31, 2017 and 2016, respectively). Common stock, $.001 par value; 1,000,000 shares authorized, 49,563 and 669 shares issued and outstanding as of December 31, 2017 and 2016, respectively Additional paid-in capital Treasury stock, at cost Accumulated deficit TOTAL STOCKHOLDERS’ DEFICIT TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONSOLIDATED STATEMENTS OF OPERATIONS (in thousands) FOR THE YEARS ENDED DECEMBER 31, REVENUE: Sales – devices and disposables, net Cost of goods sold Gross (loss) profit OPERATING EXPENSES: Research and development Sales and marketing General and administrative Total operating expenses Operating loss OTHER INCOME (EXPENSES): Other income Interest expense Change in fair value of warrants Total other income (expenses) LOSS FROM OPERATIONS PROVISION FOR INCOME TAXES NET LOSS DEEMED DIVIDENDS PREFERRED STOCK DIVIDENDS NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS BASIC AND DILUTED NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS WEIGHTED AVERAGE SHARES OUTSTANDING BALANCE, January 1, 2016 Preferred dividends Issuance of common stock and warrants Conversion of Series C preferred stock to common stock Conversion of debt into common stock Issuance of common stock due to Series B, Tranche B warrants exchanged for shares and rights to shares Series C preferred stock exchanged for Series C1 preferred stock Issuance of common stock for cash Stock-based compensation Net Loss Preferred dividends Conversion of Series C preferred stock to common stock GUIDED THERAPEUTICS, INC. AND SUBSIDIARY CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE YEARS ENDED DECEMBER 31, (In Thousands) CASH FLOWS FROM OPERATING ACTIVITIES: Net loss Adjustments to reconcile net loss to net cash used in operating activities: Bad debt expense Depreciation and amortization Stock-based compensation Change in fair value of warrants Changes in operating assets and liabilities: Accounts receivable Inventory Other current assets Other assets Accounts payable Deferred revenue Accrued liabilities Total adjustments Net cash used in operating activities CASH FLOWS FROM FINANCING ACTIVITIES: Net proceeds from issuance of common stock and warrants Proceeds from debt financing, net of discount and debt issuance costs Payments on notes Net cash provided by financing activities NET CHANGE IN CASH AND CASH EQUIVALENTS CASH AND CASH EQUIVALENTS, beginning of year CASH AND CASH EQUIVALENTS, end of year SUPPLEMENTAL SCHEDULE OF: Cash paid for: Interest NONCASH INVESTING AND FINANCING ACTIVITIES: Issuance of common stock as debt repayment Dividends on preferred stock Raw materials Work in process Finished goods Consigned inventory Inventory reserve Total Equipment Software Furniture and fixtures Leasehold Improvement Less accumulated depreciation Total Accrued compensation Accrued professional fees Accrued interest Deferred rent Accrued warranty Accrued vacation Accrued dividends Stock subscription Accrued expenses for licensee Other accrued expenses Total Warrants issued in connection with Distributor Debt Warrants issued in connection with Short-Term Loans Warrants issued in connection with Senior Secured Debt Total long-term liabilities at fair value Warrants issued in connection with Distributor Debt Warrants issued in connection with Senior Secured Debt Total long-term liabilities at fair value Balance, December 31, 2016 Warrants issued during the year Change in fair value during the year Balance, December 31, 2017 Series C Preferred Stock Conversions Series C Preferred Stock Dividends Convertible Debt Conversions Issuance of shares for note agreement Total the chairman of the Company’s board of directors,and Mark Faupel, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of the Company’s newly created Series C1 preferred stock and 9,60012 (9,600 pre-split) shares of the Company’s common stock (the “Series C Exchanges”). In connection with the Series C Exchanges, each holder also agreed to roll over the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock into the next qualifying financing undertaken by the Company on a dollar-for-dollar basis and, except in the event of an additional $50,000 cash investment in the Company by the holder, to execute a customary “lockup” agreement in connection with the financing. In total, for 1,916 shares of Series C preferred stock surrendered, the Company issued 4,312 shares of Series C1 preferred stock and 18,396,80022,996 shares of common stock.payments.” Separately, on April 27, 2016,payments” and, while it has the Company entered into a Rollover and Amendment Agreement with another holder ofsame anti-dilution protections afforded the Series C preferred stock, pursuant to which the holder agreed to roll over any sharesit does not automatically reset in connection with a reverse stock split or conversion of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest onour outstanding convertible debt.convertible promissory note the holder holds, into the Company’s next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with the holder. The holder also agreedoutstanding warrants to return to the Company for cancelation warrants exercisable for 7,148,813 shares of the Company’spurchase common stock that it holds. Exceptfor the year ended December 31, 2017:Outstanding, January 1, 2017 Issuances Exercised Canceled / Expired Outstanding, December 31, 2017 Warrants(Underlying Shares) Exercise Price Expiration Date 23 (1) $8,368.00 per share May 23, 2018 7,538 (2) $75.00 per share June 14, 2021 3 (3) $40,000.00 per share April 23, 2019 7 (4) $36,000.00 per share May 22, 2019 4 (5) $30,400.00 per share September 10, 2019 2 (6) $36,864.80 per share September 27, 2019 9 (7) $22,504.00 per share December 2, 2019 105 (8) $7,200.00 per share December 2, 2020 105 (9) $8,800.00 per share December 2, 2020 25 (11) $20,400.00 per share March 30, 2018 22 (12) $9,504.00 per share June 29, 2020 145 (10) $640.00 per share June 29, 2020 150 (11) $640.00 per share September 4, 2020 362 (12) $640.00 per share September 21, 2020 6 (13) $9,504.00 per share September 4, 2020 1 (14) $640.00 per share October 23, 2020 6 (15) $9,504.00 per share October 23, 2020 279,669,261 (16) $0.00514 per share June 14, 2021 13,424,125 (17) $0.00514 per share February 21, 2021 17,239 (18) $13.92 per share June 6, 2021 200,000 (19) $0.00514 per share February 13, 2022 20,000 (20) $0.18 per share May 16, 2022 550,000 (21) $0.019 per share November 16, 2020 200,000 (22) $0.029 per share December 28, 2020 294,089,138* (1) Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. (2) Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. (3) Issued to a placement agent in conjunction with an April 2014 private placement. (4) Issued to a placement agent in conjunction with a September 2014 private placement. (5) Issued as part of a September 2014 Regulation S offering. (6) Issued to a placement agent in conjunction with a 2014 public offering. (7) Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering. (8) Issued as part of a March 2015 private placement. (9) Issued to a placement agent in conjunction with a June 2015 private placement. (10) Issued as part of a June 2015 private placement. (11) Issued as part of a June 2015 private placement. (12) Issued as part of a June 2015 private placement. (13) Issued to a placement agent in conjunction with a June 2015 private placement. (14) Issued as part of a June 2015 private placement. (15) Issued to a placement agent in conjunction with a June 2015 private placement. (16) Issued as part of a February 2016 private placement. (17) Issued to a placement agent in conjunction with a February 2016 private placement. (20) Issued as part of a May 2017 private placement. (21) Issued to investors for a loan in November 2017. (22) Issued to investors for a loan in December 2017. an additional $50,000 cash investment by the holdercertain mergers, consolidations, reorganizations, recapitalizations, stock dividends, stock splits or other changes in the Company’s next financing,corporate structure; except for (9). In addition, warrants subject to footnotes (2) and (10)-(12), (14), and (16) – (22) in the holder has agreedtable above are subject to execute a customary “lockup” agreement in connection with the financing. Finally, the holder, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain“lower price issuance” anti-dilution provisions ofthat automatically reduce the Series C that would otherwise be triggered by the transactions. F-33On May 5, 2016 and May 9, 2016 respectively, Jonathan Niloff, age 62, and Linda Rosenstock, age 65, formally announced their decisions not to stand for reelection to the Company’s board of directors at the Company’s 2016 annual meeting of stockholders. Dr. Niloff was appointed to the board in 2010 and Dr. Rosenstock was appointed in 2012. Both of their decisions to resign were for personal reasons and were not a result of any disagreement with the Company on any matter relating to the Company’s operations, policies or practices. F-34PART IIINFORMATION NOT REQUIRED IN PROSPECTUSITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTIONThe following table sets forth the various expenses and costs incurred or to be incurred by us in connection with the sale of the shares of common stock offered hereby, other than selling commissions, which will be borne by the selling stockholders. All the amounts shown are estimated except the SEC registration fee.Expense Dollar Amount SEC filing fee $ 504 Legal fees and expenses 250,000 Accounting fees and expenses 25,000 Blue sky and related expenses 5,800 Miscellaneous 8,696 Total $ 290,000 ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERSSection 145 of the Delaware General Corporation Law permits a corporation to include in its charter documents, and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by the current law. Article VII of our certificate of incorporation provides for the indemnification of directors to the fullest extent permissible under Delaware law. Article VII of our bylaws provides for the indemnification of officers, directors and third parties acting on our behalf if such person acted in good faith and in a manner reasonably believed to be in and not opposed to our best interest and, with respect to any criminal action or proceeding, if the indemnified party had no reason to believe his conduct was unlawful.ITEM 15. RECENT SALES OF UNREGISTERED SECURITIESOn February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. In this Item 15, all share and per share amounts for transactions occurring prior to this date are reported on a pre-stock split basis.November 2013 Warrant Exchange Program. In November 2013, we completed a warrant exchange program pursuant to which we exchanged warrants exercisable for a total of 3,573,691 shares of common stock, or 99.5%exercise price of the warrants eligible(and, in the cases of warrants subject to participate, for new warrants exercisable forfootnote (2), (16) and (17) in the sametable above, increase the number of shares of common stock but with a reduced exercise price of $0.40 per share and a shortened exercise period ending on November 27, 2013.Simple Interest Notes. On February 20, 2014, our President and CEO, Gene Cartwright, advanced us $50,000, in cash, for a 10% simple interest note. On October 23, 2014, August 4, 2014, and May 21, 2014, he advanced us $30,000, $200,000, and $100,000, respectively, in cash for 5% simple interest notes. On December 30, 2015, he advanced us $10,000 in cash for a 6% simple interest note. On October 24, 2014, October 7, 2014, and August 26, 2014, our Senior Vice President of Engineering, Richard Fowler, advanced us $6,100, $20,000 and $75,000, respectively, in cash for 6% simple interest notes. On October 7, 2014, our Director of Marketing advanced us $10,000 in cash for a 6% simple interest note. On November 4, 2014, Richard Blumberg, one of our stockholders, advanced us $100,000 in cash for a note for $106,500 in aggregate principal and interest due November 30, 2014. On February 20, 2014, directors Michael James, Linda Rosenstock, and John Imhoff advanced us $50,000, $50,000, $50,000, and $25,000 in cash, respectively, for 10% simple interest notes.April 2014 Senior Convertible Note Financing. On April 23, 2014, we entered into a securities purchase agreement with Magna Equities II, LLC (formerly known as Hanover Holdings I, LLC). Pursuantissuable upon exercise), to the purchase agreement, we sold Magnaoffering price in a subsequent issuance of the Company’s common stock, unless such subsequent issuance is exempt under the terms of the warrants.with a principal amountto adjust the number of $1.5 million, for a purchase price of $1.0 million (an approximately 33.3% original issue discount). As partshares issuable upon exercise of the sale, we also issued Magna 321,820warrant to equal the number of shares that will initially be issuable upon conversion of common stock. Additionally, pursuant to the purchase agreement, Magna purchased on May 23, 2014 an additional seniornew convertible note with(without giving effect to any beneficial ownership limitations set forth in the terms of the new convertible note).principal amountmandatory exercise provision. This provision permits the Company, subject to certain limitations, to require exercise of $2.0 million and an 18-month term, for a fixed purchase price of $2.0 million. We had the rightsuch warrants at any time to redeem all or a portionfollowing (a) the date that is the 30th day after the later of the total outstanding amount then remainingCompany’s receipt of an approvable letter from the U.S. FDA for LuViva and the date on which the notes, at a 25% premium. As of June 30, 2015, the notes are no longer outstanding. The notes accrued interest at an annual rate of 6.0% and were convertible into shares of our common stock achieves an average market price for 20 consecutive trading days of at a conversionleast $1,040.00 with an average daily trading volume during such 20 consecutive trading days of at least 250 shares, or (b) the date on which the average market price equalof the common stock for 20 consecutive trading days immediately prior to the lesserdate the Company delivers a notice demanding exercise is at least $129,600 and the average daily trading volume of $0.55 per share andthe common stock exceeds 250 shares for such 20 consecutive trading days. If these warrants are not timely exercised upon demand, they will expire. Upon the occurrence of certain events, the Company may be required to repurchase these warrants, as well as the warrants subject to footnote (2) in the table above.discount frommandatory exercise provision. This provision permits the lowest daily volume-weightedCompany, subject to certain limitations; to require the exercise of such warrants should the average trading price of ourits common stock over any 30 consecutive day trading period exceed $92.16.fivetable above are also subject to a mandatory exercise provision. This provision permits the Company, subject to certain limitations, to require exercise of 50% of the then-outstanding warrants if the trading days prior to conversion.II-1Regulation S Offering. On September 2, 2014, we accepted a subscription agreement with ITEM Medikal Teknolojileri LTD STI, a Turkish corporation, referred to as ITEM, pursuant to we sold 651,042 sharesprice of ourits common stock and a warrant to purchase an additional 325,521 shares, for an aggregate purchase price of $200,000. The warrant is immediately exercisable, has an exercise price per share of $0.4608, and expires five years fromat least two times the date of issuance.Secured Note Offering. On September 10, 2014, we entered into a note purchase agreement with Tonaquint, Inc., pursuant to which we sold a secured promissory note with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). We may prepay the note at any time. The note is secured by our current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the note purchase agreement. As a result of a series of amendments to the note (since assigned to two accredited investors), the maturity date currently is August 31, 2016, and has been accruing interest at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. Pursuant to the amendments, the holders may convert the outstanding balance into shares of our common stock, at a conversion price per share equal to the lower of (1) $0.25 and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion price at the time of any conversion request would be lower than $0.15 per share, we have the option of delivering the conversion amount in cash in lieu of shares of our common stock.March 2015 Private Placement. In March 2015, we entered into subscription agreements with certain accredited investors, pursuant to which we sold an aggregate of 4.0 million shares of our common stock and warrants to purchase an additional 2.0 million shares, for an aggregate purchase price of $720,000.2015 Series B Warrant Exchanges. In order to secure the consent to an amendment to the terms of our Series B preferred stock, effective June 19, 2015 we agreed with each Series B holder to reduce the exercise price on certain “Tranche A” and “Tranche B” warrants, originally issued to the Series B holders on May 21, 2013. We reduced the “Tranche A” warrant exercise price per share from $1.08 to $0.10455, andfor any 20-day trading period. Further, in the “Tranche B”event that the trading price of the Company’s common stock is at least 2.5 times the initial warrant exercise price per share from $0.10455 to $0.09. We further agreed to grantfor any 20-day trading period, the Series B holdersCompany will have the right to participaterequire the immediate exercise of 50% of the then-outstanding warrants. Any warrants not exercised within the prescribed time periods will be canceled to the extent of the number of shares subject to mandatory exercise.our next capital-raising transaction by exchanging theirthe table above have agreed to surrender the warrants, upon consummation of a qualified public financing, for new warrants exercisable for 200% of the number of shares of underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants.preferred stock forTranche B Warrantssecurities to be offered in any such transaction. Separately, we informed all other holders of outstanding “Tranche A” and “Tranche B” warrants that we had lowered the exercise prices per share for those warrants to $0.10455 and $0.09, respectively.Public Offering Warrant Exchanges. On June 25 and 26, 2015, weCompany entered into various agreements with holders of the Company’s “Series B Tranche B” warrants, originally issued in December 2014, pursuant to which each holder separately agreed to exchange its warrant for two new warrants that, unlike the original warrant, do not contain any price- or share-reset provisions. Each new warrant is exercisable for the same number of shares of our common stock as the original warrant, at any time beginning December 2, 2015 and ending December 2, 2020. The exercise price of the first new warrant is $0.09 per share and the second new warrant is $0.11 per share but, aside from the exercise price, the new warrants are identical in terms to each other. As additional consideration, we agreed to issue an aggregate of approximately 3.1 million shares of our common stock to the holders, pro rata based on the amount of shares underlying their original warrants. The holders further agreed to amend to the securities purchase agreement, dated December 2, 2014 that governed (among other things) the issuance of the original warrants.In connection with the agreement with one of the holders, Magna Equities II, LLC, on June 25, 2015 we also agreed to exchange an outstanding senior convertible note held by Magna for a new note, in stated principal amount equal to the then-outstanding principal on the original note. The new note had the same terms as the original note, except that, among other things, Magna was temporarily prohibited from converting any of the balance into shares of our common stock. On June 30, 2015, we paid in full the entire balance of the new note.Series C Financing. On June 29, 2015, we entered into a securities purchase agreement with certain accredited investors, amended on September 3, 2015, for the issuance and sale of shares of an aggregate of 7,903 shares of our Series C preferred stock, at a purchase price of $750 per share and an initial conversion price of $0.095 per share, and five-year warrants exercisable to purchase an aggregate of approximately 124.8 million shares of our common stock at an initial exercise price of $0.095 per share, subject to certain customary adjustments and anti-dilution provisions.II-2Senior Secured Convertible Note. On February 11, 2016, we entered into a securities purchase agreement with GPB Debt Holdings II LLC for the issuance and sale on February 12, 2016 of approximately $1.4 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount). The note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. Subject to certain restrictions, it is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.80 per share, subject to certain customary adjustments and anti-dilution provisions. In addition, the investor received a five-year warrant exercisable to purchase an aggregate of approximately 1.79 million shares of our common stock with an exercise price of $0.80 per share, subject to certain customary adjustments and anti-dilution provisions. On March 17, 2016, our placement agent for the transaction received a warrant with similar terms exercisable for 86,250 shares. In connection with the transaction, on February 12, 2016, we and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to us in exchange for a royalty payment, payable quarterly, equal to 3.5% of our revenues from the sale of products.Series C Exchanges.Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock. The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.”Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 7,148,813 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, Aquarius, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described above.Shenghuo License Agreement.On June 5, 2016, we entered into a license agreement with Shenghuo Medical, LLC pursuant to which we granted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of our common stock at a conversion price per share of $0.017402, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. We will also issue Shenghuo a five-year warrant exercisable immediately for approximately 13.7 million shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment.Warrant Restructuring. Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 94,828,8881,185,357 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), wethe Company issued or areis obligated to issue 13,517,34216,897 shares of common stock and new warrants that, if exercised as of June 14, 2016,the date hereof, would be exercisable for an aggregate of 214,189,622216,707 shares of common stock.II-3wethe Company and the holder further agreed that wethe Company will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.this offering.the Company’s next financing resulting in net cash proceeds to the Company of at least $1 million. The new warrants will have an initial exercise price equal to the exercise price of the surrendered warrants as of immediately prior to consummation of this offering,the financing, subject to customary “downside price protection” for as long as ourthe Company’s common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.saleCompany has incurred net operating losses ("NOLs") since inception. As of securitiesDecember 31, 2017, the company had NOL carryforwards available through 2036 of approximately $82.9 million to ITEMoffset its future income tax liability. The NOL carryforwards began to expire in 2008. The company has recorded deferred tax assets but reserved against, due to uncertainties related to utilization of NOLs as well as calculation of effective tax rate. Utilization of existing NOL carryforwards may be limited in future years based on significant ownership changes. The company is in the process of analyzing their NOL and has not determined if the company has had any change of control issues that could limit the future use of NOL. Deferred tax assets: Warrant liability Accrued executive compensation Reserves and other Net operating loss carryforwards Valuation allowance Statutory federal tax rate State taxes, net of federal benefit Nondeductible expenses Valuation allowance Current Deferred Deferred provision Impact of change in enacted tax rates Change in valuation allowance Total provision for income taxes madedetermined by the Company’s board of directors, but incentive stock options were granted at an exercise price equal to the fair market value of the Company’s common stock as of the grant date. Options historically granted have generally become exercisable over four years and expire ten years from the date of grant.non-U.S. persontotal of 116 shares of common stock pursuant to the Plan, at a weighted average exercise price of $37,090 per share. Outstanding at beginning of year Options granted Options exercised Options expired/forfeited Outstanding at end of year Options available for issue Outstanding at beginning of year Options granted Options exercised Options expired/forfeited Outstanding at end of year Options available for issue Options Vested as of December 31, 2016 Options vested in 2017 Options vested as of December 31, 2017 Weighted Average Options Unvested as of December 31, 2016 Options vested in 2017 Options expired/forfeited in 2017 Options Unvested as of December 31, 2017 offshore offeringunfavorable resolution of some or all of these matters could materially affect the future results of operations or cash flows in a particular year.Year Amount 2018 114 2019 98 2020 101 2021 26 Regulation SISO 13485 for medical devices, Shenghuo would pay the Company a royalty equal to $2.00 or 20% of the distributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories. In connection with the license grant, Shenghuo was to underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also would provide up to $1.0 million in furtherance of the Company’s efforts to secure regulatory approval for LuViva from the U.S. Food and Drug Administration, in exchange for the right to receive payments equal to 2% of the Company’s future sales in the United States, up to an aggregate of $4.0 million. Pursuant to the license agreement, Shenghuo had the option to have a designee appointed to the Company’s board of directors (director Richard Blumberg is that designee). As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, the Company agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $300,000, expected to be due the earlier of 90 days from issuance and consummation of any capital raising transaction by the Company with net cash proceeds of at least $1.0 million. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of the Company’s common stock at a conversion price per share of $13.92, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. The Company will also issue Shenghuo a five-year warrant exercisable immediately for approximately 21,549 shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment. On January 22, 2017, the Company entered into a license agreement with Shandong Yaohua Medical Instrument Corporation, or SMI, pursuant to which the Company granted SMI an exclusive global license to manufacture the LuViva device and related disposables (subject to a carve-out for manufacture in Turkey) and exclusive distribution rights in the Peoples Republic of China, Macau, Hong Kong and Taiwan. In order to facilitate the SMI agreement, immediately prior to its execution the Company entered into an agreement with Shenghuo Medical, LLC, regarding its previous license to Shenghuo. Under the terms of the new agreement, Shenghuo agreed to relinquish its manufacturing license and its distribution rights in SMI’s territories, and to waive its rights under the Securities Act.original Shenghuo agreement, all for as long as SMI performs under the SMI agreement.issuancesnotes carry annual interest rates between 5% and 10% and have default rates as high a 16.5%. The Company is accruing interest at the default rate of 16.5%.November 2013security agreement entered into by the Company and GPB in connection with the issuance of the original senior secured convertible note.Additionally, the Company further agreed to amend the warrant exchange program,issued with the Series Boriginal senior secured convertible note, to adjust the number of shares issuable upon exercise of the warrant exchanges,to equal the public offering warrant exchanges,number of shares that will initially be issuable upon conversion of the new convertible note (without giving effect to any beneficial ownership limitations set forth in the terms of the new convertible note). As an inducement to GPB to enter into these transactions, the Company agreed to increase the royalty payable to GPB pursuant to its consulting agreement with us from 3.5% to 3.85% of revenues from the sales of the Company’s products.exchangesconvertible preferred stock, convertible debt, convertible preferred dividends and warrants convertible into common stock shares. Audit fees Audit related fees Tax fees Total Fees warrant restructuring described above were exempt from registration underfees for the services performed to date. The Audit Committee may also pre-approve particular services on a case-by-case basis.reliance upon Section 3(a)(9)the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the common shares being registered, we will, unless in the opinion of our counsel the matter has been settled by a controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by us is against public policy as expressed in the Securities Act of 1933, as exchanges with existing securities holders exclusively where no commission or other remuneration was paid or given directly or indirectly for solicitingamended, and will be governed by the final adjudication of such exchanges. The remaining issuances of securities described above (as well as the issuances of common stock upon exercise of warrants received in the warrant exchange programs) were pursuant to private placements to accredited investors, and therefore were exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act as transactions by an issuer not involving a public offering. The securities described above are restricted securities for the purpose of the Securities Act. Any certificates representing the securities bear a restrictive legend providing that the securities have not been registered under the Securities Act and cannot be sold or otherwise transferred without an effective registration or an exemption therefrom. All cash proceeds from these issuances were used in product development, working capital and other general corporate purposes.(a)Exhibits 3.1 Restated Certificate of Incorporation, as amended through MayNovember 3, 2016 (incorporated by reference to Exhibit 3.1 to the quarterly report on Form 10-Q for the quarter ended March 31, 2016, filed May 19, 2016)Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the current report on Form 8-K, filed March 23, 2012) 3.3*Form of Certificate of Designations (Series D)4.1 Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the amended registration statement on Form S-1/A (No. 333-22429) filed April 24, 1997) Secured Promissory Note, dated September 10, 2014 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed September 10, 2014) Amendment #1 to Secured Promissory Note, dated March 10, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 19, 2015) Amendment #2 to Secured Promissory Note, dated May 4, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 7, 2015) Amendment #3 to Secured Promissory Note, dated June 1, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 5, 2015) Amendment #4 to Secured Promissory Note, dated June 16, 2015 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed June 30, 2015) Amendment #5 to Secured Promissory Note, dated June 29, 2015 (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed June 30, 2015) Amendment #6 to Secured Promissory Note, dated January 20, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed February 16, 2016) Amendment #7 to Secured Promissory Note, dated February 11, 2016 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed February 16, 2016) Amendment #8 to Secured Promissory Note, dated March 7, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 7, 2016) II-44.11Senior Secured Convertible Note, dated February 12, 2016 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed February 12,16, 2016)Form of Exchange Note (GPB) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed December 7, 2016) 10% OID Convertible Promissory Note (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed December 30, 2016) Convertible Promissory Note (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed February 16, 2017) Form of Warrant (Standard Form) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 14, 2010) 4.13Form of Warrant (InterScan) (incorporated by reference to Exhibit 4.13 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) 4.14Form of Warrant (November 2011 Private Placement) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K/A, filed November 28, 2011) 4.15Form of Warrant (Series B-Tranche A) (incorporated by reference to Exhibit 10.2 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) 4.16Form of Warrant (Series B-Tranche B) (incorporated by reference to Exhibit 10.3 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) 4.17Form of Warrant (Regulation S) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 8, 2014) 4.18Form of Warrant (2014 Public Offering Placement Agent) (incorporated by reference to Exhibit 4.2 to the current report on Form 8-K filed December 4, 2014) 4.19Form of Warrant (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 30, 2015) 4.20Form of Warrant (Series C) (incorporated by reference to Exhibit 4.3 to the current report on Form 8-K filed June 30, 2015) 4.21Form of Warrant (Senior Secured Convertible Note) (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed February 12, 2016) 4.22Form of Warrant (Series B-Tranche B Exchanges; GPB Exchange) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 14, 2016) 4.25*Common Stock Purchase Warrant (Convertible Promissory Note) (incorporated by reference to Exhibit 4.2 to the current report on Form of Warrant (Series D)8-K filed February 16, 2017)5.1*Opinion of Brunson Chandler & Jones, DayPLLC regarding validitylegality10.1 1995 Stock Plan and form of Stock Option Agreement (Incorporated(incorporated by reference to Exhibit 10.2 to the registration statement on Form S-1 (No. 333-22429333-22429) filed February 27, 1997)2005 Amendment to 1995 Stock Plan (incorporated by reference to Appendix 1 to the proxy statement on Schedule 14A, filed May 10, 2005) 2010 Amendment to 1995 Stock Plan (incorporated by reference to Exhibit 10.3 to the registration statement on Form S-8 (File No. 333-178261), filed December 1, 2011) 2012 Amendment to 1995 Stock Plan (incorporated by reference to Annex 1 to the proxy statement on Schedule 14A, filed April 30, 2012) Agreement and Release, dated August 30, 2011 (incorporated by reference to 10.2 to the current report on Form 8-K, filed September 2, 2011) Employment Agreement between the Company and Mark Faupel dated March 24, 2013 (incorporated by reference to Exhibit 10.10 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) Employment Agreement between the Company and Gene Cartwright, dated January 6, 2014 (incorporated by reference to Exhibit 10.11 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014). Employment Agreement between the Company and Rick L. Fowler, automatically renewed on May 9, 2013 (incorporated by reference to Exhibit 10.12 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) Consulting Agreement between the Company and GPB Debt Holdings II LLC, dated February 12, 2016 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed February 12, 2016) Securities Purchase Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014). 10.11Registration Rights Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014)Standstill Agreement (Magna Note), dated as of November 6, 2014, by and between the Company and Magna Equities II, LLC (incorporated by reference to Exhibit 1910.19 to the registration statement on Form S-1 (No. 333-198733) filed November 10, 2014)Exchange Agreement (Magna Note), dated as of June 25, 2015 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed June 30, 2015) Subscription Agreement (Regulation S), accepted September 2, 2014 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 8, 2014) II-510.15Form of Registration Rights Agreement (Regulation S), dated September 8, 2014 by and between the Company and the investor party thereto (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 8, 2014) Note Purchase Agreement (Secured Promissory Note), dated as of September 10, 2014, by and between the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 10, 2014) Security Agreement (Secured Promissory Note), dated as of September 10, 2014, by the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 10, 2014) Form of Securities Purchase Agreement (2014 Public Offering) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 4, 2014) Placement Agent Agreement (2014 Public Offering), by and between the Company and Olympus Securities, LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed December 4, 2014) Amendment to Securities Purchase Agreement (2014 Public Offering), dated as of June 26, 2015 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 30, 2015) Form of Letter Agreement (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 30, 2015) Securities Purchase Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed June 30, 2015) Registration Rights Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.7 to the current report on Form 8-K filed June 30, 2015) Form of Joinder Agreement (Series C) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed July 13, 2015) Interim Securities Purchase Agreement (Series C), dated September 3, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed September 3, 2015) Securities Purchase Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed February 12, 2016) Security Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed February 12, 2016) Rollover and Amendment Agreement, dated April 27, 2016, by and between the Company and Aquarius Opportunity Fund (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed May 3, 2016) Form of Letter Agreement (Series C Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 3, 2016) License Agreement, dated June 5, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 8, 2016) Form of Warrant Exchange Agreement (Warrant-for-Shares) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 14, 2016) Form of Warrant Exchange Agreement (Warrant-for-Warrant) (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 14, 2016) 10.33**Royalty Agreement, dated September 6, 2016, between the Company and Imhoff and Maloof (incorporated by reference to Exhibit 10.1 to the current report on Form of 8-K filed September 8, 2016)Lockup and Exchange Agreement, dated November 2, 2016, by the Company and GHS Investments, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed November 4, 2016) Exchange Agreement, dated December 7, 2016, between the Company and GPB Debt Holdings II LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 7, 2016) Amendment to Consulting Agreement, dated December 7, 2016, between the Company and GPB Debt Holdings II LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed December 7, 2016) Securities Purchase Agreement, dated December 28, 2016, between the Company and RedDiamond (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 30, 2016) 10.34**Placement Agent Agreement between Shandong Yaohua Medical Instrument Corporation and Guided Therapeutics, Inc., Confidential, Final 22 January 2017 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed January 26, 2017)Guided Therapeutics-Shenghuo Medical Agreement, 22 Jan 2017 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed January 26, 2017) Securities Purchase Agreement, dated as of February 13, 2017, by and between Guided Therapeutics, Inc. and Auctus Fund, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed February 16, 2017) Securities Purchase Agreement, dated as of March 17, 2017, by and between Guided Therapeutics, Inc. and Eagle Equities LLC and Adar Bays LLC and on May 18, 2017 with GHS Investments LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed May 24, 2017) Forbearance Agreement, dated as of August 8, 2017, by and between Guided Therapeutics, Inc. and GPB Debt Holdings II LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed August 14, 2017) Securities Purchase Agreement, dated as of August 18, 2017, by and between Guided Therapeutics, Inc. and Power Up Lending Group LTD (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed August 24, 2017) Securities Purchase Agreement, dated as of October 12, 2017, by and between Guided Therapeutics, Inc. and Power Up Lending Group LTD (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed October 25, 2017) Equity Financing Agreement dated March 1, 2018 by and between the Company and Ladenburg Thalmann & Co.GHS Investments LLCForm of Registration Rights Agreement dated March 8, 2018 by and between the Company and GHS Investments LLC Subsidiaries (incorporated by reference to Exhibit 21.1 to the registration statement on Form S-1 (No. 333-169755) filed October 5, 2010) 23.1*Consent of UHY LLP.23.2**Consent of Jones Day (included in Exhibit 5.1).24.1Powers of Attorney (included at signature page).LLP101.1* Interactive Data File *Filed herewith**To be filed via pre-effective amendmentII-6(a)undertakes:(1)To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:(i)To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;(ii)To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;(iii)To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.(2)That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fide offering thereof.(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.(4)That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:(i)Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;(ii)Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;(iii)The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and(iv)Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.(b)1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: i. To include any Prospectus required by section 10(a)(3) of the Securities Act of 1933; ii. iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; 2. 3. To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. 4. That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: i. ii. Any free writing Prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; iii. iv. Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. 5. That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: Each Prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than Prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or Prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or Prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or Prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. of the registrant pursuant to the foregoing provisions, or otherwise, the registrant haswe have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrantus of expenses incurred or paid by a director, officer or controlling person of the registrantcorporation in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrantwe will, unless in the opinion of itsour counsel the matter has been settled by a controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by itus is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
case.II-7in the City of Norcross, State of Georgia, on June 28, 2016.5, 2018. GUIDED THERAPEUTICS, INC. By: /s/Gene S. Cartwright /s/ Gene Cartwright Chief Executive OfficerCEO and ActingChief Financial OfficerKNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Gene S. Cartwright full power of substitution and resubstitution, as attorney-in-fact of the undersigned, for him and in his name, place and stead, to execute and file with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933 any and all amendments, supplements and exhibits to this Registration Statement (including pre-effective and post-effective amendments and supplements), to execute and file any and all other applications or other documents to be filed with the Commission, such attorney to have full power to act with or without the others, and to have full power and authority to do and perform, in the name and on behalf of the undersigned, every act whatsoever necessary, advisable or appropriate to be done in the premises as fully and to all intents and purposes as the undersigned might or could do in person, hereby ratifying and approving the act of said attorney and any such substitute. Pursuant to the requirements of the Securities Act of 1933, this registration statement has beenwas signed by the following persons in the capacities and on the dates indicated:DATE SIGNATURE TITLE June 28, 20165, 2018 /s/ Gene S. Cartwright President, Chief Executive Officer, Acting Chief Financial Gene S. Cartwright Officer (Principal Executive Officer and Principal Financial and Accounting Officer) Gene S. Cartwright 28, 2016 /s/ Michael C. James Michael C. James 28, 2016 /s//s/ John E. Imhoff John E. Imhoff June 28, 2016 /s/ Jonathan M. Niloff June 5, 2018 /s/ Richard P. Blumberg Director Jonathan M. NiloffRichard P. Blumberg June 28, 2016 /s/ Linda Rosenstock June 5, 2018 /s/ Mark Faupel Chief Operating Officer and Director Linda Rosenstock Mark Faupel II-8 EXHIBIT INDEXEXHIBIT NO.DESCRIPTION3.1Restated Certificate of Incorporation, as amended through May 3, 2016 (incorporated by reference to Exhibit 3.1 to the quarterly report on Form 10-Q for the quarter ended March 31, 2016, filed May 19, 2016)3.2Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the current report on Form 8-K, filed March 23, 2012)3.3*Form of Certificate of Designations (Series D)4.1Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the amended registration statement on Form S-1/A (No. 333-22429) filed April 24, 1997)4.2Secured Promissory Note, dated September 10, 2014 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed September 10, 2014)4.3Amendment #1 to Secured Promissory Note, dated March 10, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 19, 2015)4.4Amendment #2 to Secured Promissory Note, dated May 4, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 7, 2015)4.5Amendment #3 to Secured Promissory Note, dated June 1, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 5, 2015)4.6Amendment #4 to Secured Promissory Note, dated June 16, 2015 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed June 30, 2015)4.7Amendment #5 to Secured Promissory Note, dated June 29, 2015 (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed June 30, 2015)4.8Amendment #6 to Secured Promissory Note, dated January 20, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed February 16, 2016)4.9Amendment #7 to Secured Promissory Note, dated February 11, 2016 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed February 16, 2016)4.10Amendment #8 to Secured Promissory Note, dated March 7, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 7, 2016)4.11Senior Secured Convertible Note, dated February 12, 2016 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed February 12, 2016)4.12Form of Warrant (Standard Form) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 14, 2010)4.13Form of Warrant (InterScan) (incorporated by reference to Exhibit 4.13 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014)4.14Form of Warrant (November 2011 Private Placement) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K/A, filed November 28, 2011)4.15Form of Warrant (Series B-Tranche A) (incorporated by reference to Exhibit 10.2 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013)4.16Form of Warrant (Series B-Tranche B) (incorporated by reference to Exhibit 10.3 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013)4.17Form of Warrant (Regulation S) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 8, 2014)4.18Form of Warrant (2014 Public Offering Placement Agent) (incorporated by reference to Exhibit 4.2 to the current report on Form 8-K filed December 4, 2014)4.19Form of Warrant (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 30, 2015)4.20Form of Warrant (Series C) (incorporated by reference to Exhibit 4.3 to the current report on Form 8-K filed June 30, 2015)4.21Form of Warrant (Senior Secured Convertible Note) (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed February 12, 2016)4.22Form of Warrant (Series B-Tranche B Exchanges; GPB Exchange) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 14, 2016)4.25*Form of Warrant (Series D)5.1**Opinion of Jones Day regarding validity10.11995 Stock Plan and form of Stock Option Agreement (incorporated by reference to Exhibit 10.2 to the registration statement on Form S-1 (No. 333-22429) filed February 27, 1997)10.22005 Amendment to 1995 Stock Plan (incorporated by reference to Appendix 1 to the proxy statement on Schedule 14A, filed May 10, 2005)10.32010 Amendment to 1995 Stock Plan (incorporated by reference to Exhibit 10.3 to the registration statement on Form S-8 (File No. 333-178261), filed December 1, 2011)10.42012 Amendment to 1995 Stock Plan (incorporated by reference to Annex 1 to the proxy statement on Schedule 14A, filed April 30, 2012)10.5Agreement and Release, dated August 30, 2011 (incorporated by reference to 10.2 to the current report on Form 8-K, filed September 2, 2011)10.6Employment Agreement between the Company and Mark Faupel dated March 24, 2013 (incorporated by reference to Exhibit 10.10 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014)10.7Employment Agreement between the Company and Gene Cartwright, dated January 6, 2014 (incorporated by reference to Exhibit 10.11 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014).10.8Employment Agreement between the Company and Rick L. Fowler, automatically renewed on May 9, 2013 (incorporated by reference to Exhibit 10.12 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014)10.9Consulting Agreement between the Company and GPB Debt Holdings II LLC, dated February 12, 2016 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed February 12, 2016)10.10Securities Purchase Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014).10.11Registration Rights Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014)10.12Standstill Agreement (Magna Note), dated as of November 6, 2014, by and between the Company and Magna Equities II, LLC (incorporated by reference to Exhibit 19 to the registration statement on Form S-1 (No. 333-198733) filed November 10, 2014)10.13Exchange Agreement (Magna Note), dated as of June 25, 2015 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed June 30, 2015)10.14Subscription Agreement (Regulation S), accepted September 2, 2014 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 8, 2014)10.15Form of Registration Rights Agreement (Regulation S), dated September 8, 2014 by and between the Company and the investor party thereto (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 8, 2014)10.16Note Purchase Agreement (Secured Promissory Note), dated as of September 10, 2014, by and between the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 10, 2014)10.17Security Agreement (Secured Promissory Note), dated as of September 10, 2014, by the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 10, 2014)10.18Form of Securities Purchase Agreement (2014 Public Offering) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 4, 2014)10.19Placement Agent Agreement (2014 Public Offering), by and between the Company and Olympus Securities, LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed December 4, 2014)10.20Amendment to Securities Purchase Agreement (2014 Public Offering), dated as of June 26, 2015 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 30, 2015)10.21Form of Letter Agreement (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 30, 2015)10.22Securities Purchase Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed June 30, 2015)10.23Registration Rights Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.7 to the current report on Form 8-K filed June 30, 2015)10.24Form of Joinder Agreement (Series C) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed July 13, 2015)10.25Interim Securities Purchase Agreement (Series C), dated September 3, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed September 3, 2015)10.26Securities Purchase Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed February 12, 2016)10.27Security Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed February 12, 2016)10.28Rollover and Amendment Agreement, dated April 27, 2016, by and between the Company and Aquarius Opportunity Fund (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed May 3, 2016)10.29Form of Letter Agreement (Series C Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 3, 2016)10.30License Agreement, dated June 5, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 8, 2016)10.31Form of Warrant Exchange Agreement (Warrant-for-Shares) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 14, 2016)10.32Form of Warrant Exchange Agreement (Warrant-for-Warrant) (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 14, 2016)10.33**Form of Securities Purchase Agreement10.34**Placement Agent Agreement, by and between the Company and Ladenburg Thalmann & Co.21.1Subsidiaries (incorporated by reference to Exhibit 21.1 to the registration statement on Form S-1 (No. 333-169755) filed October 5, 2010)23.1*Consent of UHY LLP.23.2**Consent of Jones Day (included in Exhibit 5.1).24.1Powers of Attorney (included at signature page).101.1*Interactive Data File*Filed herewith**To be filed via pre-effective amendment