
As filed with the Securities and Exchange Commission on November 21, 2016December 1, 2021
Registration No. 333-259834
United States
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,Washington, D.C. 20549
_______________________
FORM S-1Amendment No. 2 to
Form S-1/A
REGISTRATION STATEMENT
UNDER THE
SECURITIES ACT OF 1933
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
(Exact name of Registrant as Specified in Its Charter)_______________________
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC. | ||||
(Exact name of registrant as specified in its charter) |
Nevada | 2836 | 87-0622284 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification |
2017 W Peoria Avenue
211 E Osborn Road
Phoenix, AZ 8502985012
(833) 336-7636
(602) 680-7439
(Address, including zip code, and telephone number, including area code,
of registrant'sregistrant’s principal executive offices)
Timothy Warbington CEO
Chief Executive Officer
2017 W Peoria Avenue
Creative Medical Technology Holdings, Inc.
211 E Osborn Road
Phoenix, AZ 85029
(602) 680-7439
timwarbington@yahoo.com
Arizona 85012
(833) 336-7636
(Name, address, including zip code, and telephone number,
including area code,of agent for service)
Copies of Communications to:
Copies to:
Ronald N. Vance
Vance, Higley & Associates, P.C.
Attorneys at Law
1656 Reunion Avenue
Suite 250
South Jordan, UT 84095
(801) 446-8802
(801) 446-8803 (fax)
ron@vancelaw.us
Zev M. Bomrind, Esq. Fox Rothschild LLP 101 Park Avenue New York, New York 10178 (212) 878-7951 | Robert F. Charron, Esq. Charles Phillips, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of Americas, 11th Floor New York, NY 10105 (212) 370-1300 |
Approximate date of commencement of proposed sale to thepublic: From time to timeAs soon as practicable after the effective date of this Registration Statement becomes effective.registration statement.
If any of the securities being registered on this formForm are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box.x ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
Emerging growth company | ☒ |
| Calculation of Registration Fee | ||||||||||||||||
| Title of Each Class of Securities to be Registered | Amount to be Registered | Proposed Maximum Offering Price Per Share (1) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee | ||||||||||||
| Common Stock, $.001 par value | 23,732,669 | $ | 0.49 | $ | 11,629,008 | $ | 1,347.80 | |||||||||
| TOTAL | 23,732,669 | $ | 11,629,008 | $ | 1,347.80 | |||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Exchange Act. ☐
(1) CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities to be Registered |
| Proposed Price (1) |
|
| Amount of |
| ||
Common stock, par value $0.0001 per share (2) |
| $ | 23,000,000 |
|
| $ | 2,132.10 |
|
Warrants to purchase shares of common stock |
| ________ |
|
| (3 | ) | ||
Common stock issuable upon exercise of warrants (2) |
| $ | 23,000,000 |
|
| $ | 2,132.10 |
|
Pre-funded warrants to purchase shares of common Stock |
| (4 | ) |
| ________ |
| ||
Common stock issuable upon exercise of pre-funded warrants |
| (4 | ) |
| ________ |
| ||
Representative’s Warrants to purchase common stock (3) |
| ________ |
|
| ________ |
| ||
Shares of common stock issuable upon exercise of Representative’s Warrants (5)(6) |
| $ | 2,875,000 |
|
| $ | 266.51 |
|
TOTAL |
| $ | 48,875,000 |
|
| $ | 4,530.71 | (7) |
(1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. |
(2) | Includes stock and/or warrants that may be issued upon exercise of a 45-day option granted to the underwriters to cover over-allotments, if any. |
(3) | In accordance with Rule 457(g) under the Securities Act, because the shares of the common stock underlying the Warrants and Representative’s warrants are registered hereby, no separate registration fee is required with respect to the warrants registered hereby. |
(4) | The proposed maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded warrants sold in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants to be sold in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock sold in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock and pre-funded warrants (including the common stock issuable upon exercise of the pre-funded warrants), if any, is $23,000,000, including the underwriter’s option to purchase additional shares of common stock. |
(5) | Includes shares of common stock which may be issued upon exercise of additional warrants which may be issued upon exercise of 45-day option granted to the underwriters to cover over-allotment, if any. |
(6) | These warrants are exercisable at a per share exercise price equal to 125% of the public offering price. As estimated solely for the purpose of recalculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the Representative Warrants is $2,875,000, which is equal to 125% of $2,300,000 (5% of $46,000,000). |
(7) | The fee was previously paid. |
In the event of a stock split, stock dividend, or similar transaction involving our common stock, the number of shares registered shall automatically be increased to cover the additional shares of common stock issuable pursuant to Rule 457(c) solely for416 under the purpose of calculating the amount of the registration fee.Securities Act.
The registrantRegistrant hereby amends this registration statementRegistration Statement on such date or dates as may be necessary to delay its effective date until the registrantRegistrant shall file a further amendment which specifically states that this registration statementRegistration Statement shall thereafterhereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statementRegistration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
SUBJECT TO COMPLETION, DATED NOVEMBER 21, 2016
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
SUBJECT TO COMPLETION, DATED DECEMBER __, 2021
Creative Medical Technology Holdings, Inc.
23,732,6694,842,615 Shares of Common Stock
Warrants to Purchase up to 4,842,615 Shares of Common Stock
Pre-Funded Warrants to Purchase up to 4,842,615 Shares of Common Stock
This prospectus relates to the resale by the selling stockholdersis a firm commitment public offering of up to 23,732,669 shares of our common stock. The selling stockholders acquired the shares being offered in this prospectus pursuant to the reverse merger transaction with our operating subsidiary completed on May 18, 2016.
The selling stockholders, or their pledgees, donees, transferees or other successors-in-interest, may offer the4,842,615 shares of our common stock and warrants to purchase up to 4,842,615 shares of our common stock (which we refer to as “Public Warrants”) at a combined public offering price of $4.13 per shares of common stock and accompanying Public Warrant. Each Public Warrant is immediately exercisable for resaleone share of common stock at an exercise price of $4.13 share (or 100% of the price of each share of common stock sold in the over-the-counter market,offering) and will expire five years from the date of issuance.
We are also offering to those purchasers, if any, whose purchase of common stock in isolated transactions, orthis offering would otherwise result in a combinationany such purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of such methodspurchaser, 9.99%) of sale. The selling stockholders will sell their shares at prevailing market prices or privately negotiated prices. There will be no underwriter’s discounts or commissions, except forour outstanding common stock immediately following the chargesconsummation of this offering, the opportunity to a selling shareholder for sales through a broker-dealer. All net proceeds from a sale will go to the selling shareholder and not to us. We will pay the expenses of registering these shares.
Our stock is quoted on OTCQB under the symbol “CELZ.” On November 10, 2016, the last reported sale pricepurchase pre-funded warrants in lieu of shares of our common stock onthat would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the OTCQB Marketplace was $0.51.election of such purchaser, 9.99%) of our outstanding common stock. The purchase price for each pre-funded warrant will equal the per share public offering price for the common stock in this offering less the $0.0001 per share exercise price of each such pre-funded warrant. Each pre-funded warrant will be exercisable upon issuance and will not expire prior to exercise.
We are an “emerging growth company”Our common stock is quoted for trading on the Pink Market maintained by OTC Markets Group (the “OTC Pink”) under the federal securities laws andsymbol “CELZ.” We have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “CELZ”, which listing is a condition to this offering. No assurance can be given that our application will be subjectapproved. On November 29, 2021, the last reported sales price for our common stock as quoted on the OTC Pink was $4.75 per share.
The share and per share information in this prospectus give retroactive effect to reduced public company reporting requirements. Investingthe 1-for-500 reverse split of our outstanding common stock effected on November 10, 2021.
__________________________
An investment in our common stock and warrants involves risks. Youa high degree of risk. Before buying any securities you should carefully considerread the Risk Factorsdiscussion of the material risks of investing in our common stock and warrants in “Risk Factors” beginning on page 46 of this prospectus.
__________________________
Neither the Securities and Exchange Commission nor any other state securities commission has approved or disapproved of these securities or passed upon the adequacyaccuracy or accuracyadequacy of this prospectus. Any representation to the contrary is a criminal offense.
Per Share and related Public Warrant (2) | Per Pre-Funded Warrant and related Public Warrant (2) | Total | ||||||||||
Public offering price | $ | $ | $ | |||||||||
Underwriting discounts and commissions (1) | $ | $ | $ | |||||||||
Proceeds to us before offering expenses (3) | $ | $ | $ | |||||||||
________________
(1) | Does not reflect additional compensation to the representative in the form of warrants to purchase up to 484,262 shares of common stock (assuming the over-allotment option is fully exercised) at an exercise price equal to 125% of the public offering price. We have also agreed to reimburse the representative for certain expenses. See “Underwriting” on page 67 of this prospectus for a description of these arrangements. |
(2) | The public offering price and underwriting discount corresponds to (x)(i) a public offering price per share of $ and (ii) a public offering price per Public Warrant of $ , and (y)(i) a public offering price per pre-funded warrant of $ and (ii) a public offering price per Public Warrant of $ . |
| (3) | We estimate the total expenses of this offering will be approximately $380,000. Assumes no exercise of the over-allotment option we have granted to the underwriters as described below. |
We have granted the underwriters a 45-day option to purchase up to 726,393 additional shares of common stock and/or Public Warrants to purchase up to 726,393 additional shares of common stock.
The date of this prospectus is ____________, 2016underwriters expect to deliver our shares, Public Warrants and pre-funded warrants, if any, to purchasers in the offering on or about , 2021.
Roth Capital Partners
TABLE OF CONTENTSProspectus dated [●], 2021.
TABLE OF CONTENTS
1 | |||
6 | |||
17 | |||
18 | |||
19 | |||
20 | |||
21 | |||
22 | |||
Management’s Discussion And Analysis Of Financial Condition And Results Of Operation | 35 | ||
39 | |||
42 | |||
43 | |||
Security Ownership Of Certain Beneficial Owners And Management | 45 | ||
46 | |||
47 | |||
51 | |||
52 | |||
57 | |||
64 | |||
64 | |||
64 | |||
64 | |||
F-1 |
________________________________________
MARKET AND INDUSTRY DATA
The market data and certain other statistical information used throughout this prospectus are based on independent industry publications, governmental publications, reports by market research firms or other independent sources. Some data are also based on our good faith estimates.
BASIS OF PRESENTATION
References herein to the “Company,” “Registrant,” “we,” “us,” “our” and “our company” refer to Creative Medical Technology Holdings, Inc., a Nevada corporation and its subsidiaries.
Certain monetary amounts, percentages and other figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables or charts and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
________________________________________
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS PROSPECTUS OR IN ANY FREE WRITING PROSPECTUS WE MAY AUTHORIZE TO BE DELIVERED OR MADE AVAILABLE TO YOU. WE HAVE NOT, AND THE UNDERWRITERS HAVE NOT, AUTHORIZED ANYONE TO PROVIDE YOU WITH DIFFERENT INFORMATION. WE ARE NOT MAKING AN OFFER OF THESE SECURITIES IN ANY STATE WHERE THE OFFER IS NOT PERMITTED. YOU SHOULD NOT ASSUME THAT THE INFORMATION PROVIDED IN THIS PROSPECTUS IS ACCURATE AS OF ANY DATE OTHER THAN THE DATE ON THE FRONT OF THIS PROSPECTUS.
No person is authorized in connection with this prospectus to give any information or to make any representations about us, the securities offered hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus. If any other information or representation is given or made, such information or representation may not be relied upon as having been authorized by us.
v | |
| Table of Contents |
PROSPECTUS SUMMARY This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in our securities. You should carefully read the entire prospectus, including “Risk Factors” and the financial statements and notes thereto, before making an investment decision. Corporate Overview We are a commercial stage biotechnology company focused on immunology, urology, neurology and orthopedics using adult stem cell treatments and interrelated regenerative technologies for the treatment of multiple indications. Our existing and pipeline of therapies and products include of the following:
Our subsidiary, Creative Medical Technologies, Inc. (“CMT”), was originally created to monetize U.S. Patent No. 8,372,797 and related intellectual property related to the treatment of erectile dysfunction (“ED”), which it acquired in February 2016. Subsequently, we have expanded our development and acquisition of intellectual property beyond urology to include therapeutic treatments utilizing “re-programmed” stem cells, and the treatment of neurologic disorders, lower back pain, type I diabetes, and heart, liver, kidney and other diseases using various types of stem cells through our ImmCelz, Inc., StemSpine, Inc. and AmnioStem LLC subsidiaries. However, neither ImmCelz Inc., StemSpine Inc. nor AmnioStem LLC have commenced commercial activities. We currently conduct substantially all of our commercial operations through CMT, which markets and sells our CaverStem® and FemCelz® disposable kits utilized by physicians to perform autologous procedures that treat erectile dysfunction and female sexual dysfunction, respectively. Our CaverStem® and FemCelz® kits are currently available through physicians at eight locations in the United States. In addition to our CaverStem® and FemCelz®products, we are currently in the process of recruiting clinical sites for our StemSpine® Regenerative Stem Cell Procedure for the Treatment of Degenerative Disc Disease. Our StemSpine® treatment is an autologous procedure that utilizes a patient’s own stem cells to treat lower back pain. In 2020, through our ImmCelz Inc. subsidiary, we began exploring the development of treatments that utilize a patient’s own extracted immune cells that are then “reprogrammed” by culturing them outside the patient’s body with optimized stem cells. The immune cells are then re-injected into the patient from whom they were extracted. We believe this process endows the immune cells with regenerative properties that may be suitable for the treatment of stroke victims, among other indications. In contrast to other stem cell-based approaches, the immune cells are significantly smaller in size than stem cells and are believed to more effectively penetrate areas of the damaged tissues and induce regeneration. We are currently primarily focused on expanding the commercial sale and use of our CaverStem® and FemCelz® products by physicians in the United States and Europe, and commercializing our StemSpine® treatment for lower back pain. We also recently filed an Investigational New Drug (IND) application with the FDA to treat stroke utilizing our ImmCelzTM technology. In the future, subject to the availability of capital, we will seek to further develop additional therapeutic products that utilize our proprietary intellectual property. Listing on the Nasdaq Capital Market Our common stock is currently quoted on the Pink Market maintained by OTC Markets Group (the “OTC Pink”). In connection with this offering, we have applied to list our common stock on the Nasdaq Capital Market (“Nasdaq”) under the symbol “CELZ”. We do not intend to apply to list the Public Warrants or the pre-funded warrants on Nasdaq or any nationally recognized trading system. If our listing application is approved, we expect to list our common stock on Nasdaq upon consummation of the offering, at which point our common stock will cease to be traded on the OTC Pink. No assurance can be given that our listing application will be approved. Nasdaq listing requirements include, among other things, a stock price threshold. As a result, in order to meet such requirement, on November 10, 2021, we effected a 1-for-500 reverse split of our common stock. There can be no assurance that our common stock will be listed on the Nasdaq. However, we will not complete this offering if we are not so listed. |
| 1 |
Risks Associated With our Business Our ability to execute our business strategy is subject to numerous risks, as more fully described in the section captioned “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our common stock and warrants. In particular, risks associated with our business include, but are not limited to, the following: | ||
· | We have incurred substantial losses and our future profitability is uncertain. | |
· | Even with the proceeds from this offering, we will need additional capital to fund our operations as planned. | |
· | Our future success is dependent on significantly increasing sales of our CaverStem® and FemCelz® products, and the timely and successful development and commercialization of our StemSpine® treatment for lower back pain and/or our ImmCelzTM technology for the treatment of multiple indications; if we are not successful in increasing sales of our existing products or commercializing our proposed new products, our business prospects would be significantly harmed. | |
· | We will need to obtain regulatory approval for our ImmCelzTM therapy. | |
· | The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects. | |
· | Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. | |
· | Later discovery of previously unknown problems could limit our ability to market or sell our products and therapies even those that have been approved, and can expose us to product liability claims. | |
· | We rely in part on third parties for research and clinical trials for our products. | |
· | We currently have a very limited marketing and sales organization and may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue. | |
· | We rely upon third parties for the manufacture of our CaverStem® and FemCelz® disposable kits and are dependent on their quality and effectiveness. | |
· | We face competition from well-funded companies. | |
· | We may not be able to maintain the patent rights we rely upon to protect our intellectual property, they may not be sufficient, and we may be subject to claims that we infringe the intellectual property of others; | |
· | We are subject to increasing government regulations, price controls and other restrictions on pricing, reimbursement and access to therapies and drugs, which could adversely affect our future revenues and profitability. | |
· | We are dependent on our executive officers and consultants, and we may not be able to pursue our current business strategy effectively if we lose them. | |
| Bridge Financing In August 2021, we completed the sale of 15% Original Issue Discount Senior Notes (or “Bridge Notes”) in the aggregate principal amount of $4,456,176. In connection with the sale of the Bridge Notes, holders of shares of our preferred stock exchanged such preferred stock for additional Bridge Notes in the aggregate principal amount of $690,000. The Bridge Notes mature on February 11, 2022, subject to the requirement that we redeem the Bridge Notes prior to such date with the net proceeds of any future offering of our securities, including this public offering. Accordingly, we will use a portion of the proceeds of the offering to redeem the Bridge Notes. Pursuant to the Purchase Agreement, the Company also issued to the purchasers of the Bridge Notes five-year warrants to purchase an aggregate of 363,046 shares of our common stock at an initial exercise price of $14.175 per share, subject to anti-dilution adjustment in the event of future sales of our equity below the then exercise price, stock dividends, stock splits and other specified events. | ||
Corporate and other Information We were incorporated on December 3, 1998, in the State of Nevada under the name Jolley Marketing, Inc. On May 18, 2016, we completed a reverse merger transaction under which Creative Medical Technologies, Inc. became our wholly-owned subsidiary. In connection with this merger, we changed our name to Creative Medical Technologies Holdings, Inc. to reflect our current business. Our principal offices are located at 211 E Osborn Road, Phoenix, AZ, 85012, and our telephone number is (833) 336-7636. We maintain a website at https://creativemedicaltechnology.com. Information contained on our website does not constitute part of this prospectus. Implications of Being an Emerging Growth Company We currently qualify as an “emerging growth company” under Jumpstart Our Business Act of 2012, as amended, or the JOBS Act. As a result, we rely on exemptions from certain disclosure requirements, and are not required to: | |||
· | have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; | ||
· | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); | ||
· | submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay” and “say-on-frequency;” and | ||
· | disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. | ||
We may take advantage of these provisions through 2021. For example, we have taken advantage of the reduced reporting requirements with respect to disclosure regarding our executive compensation arrangements, have presented only two years of audited financial statements, have presented reduced “Management’s Discussion and Analysis of Financial Condition and Results of | In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares of common stock held by non-affiliates does not equal or exceed $250 million as of the prior June 30th, or (2) our annual revenues did not equal or exceed $100 million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any reduced disclosure obligations, it may also make it difficult to compare our financial statements with other public companies. | ||
| Table of Contents |
The Offering | ||
Common Stock Offered by Us | 4,842,615 shares. | |
Public Warrants Offered by Us | Warrants to purchase up to 4,842,615 shares of our common stock, which will be exercisable during the period commencing on the date of their issuance and ending five years from such date at an exercise price per share of common stock equal to 100% of the combined public offering price per share of common stock and Public Warrant in this offering. | |
Pre-funded Warrants Offered by Us | We are also offering to certain purchasers whose purchase of our common stock in this offering would otherwise result in the purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase pre-funded warrants in lieu of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. Each pre-funded warrant will be exercisable for one share of common stock. The purchase price of each pre-funded warrant and the accompanying Public Warrant will equal the price at which the common stock and the accompanying Public Warrant are being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until exercised in full. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue one Public Warrant for each share of common stock and for each pre-funded warrant to purchase one share of common stock sold in this offering, the number of Public Warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. | |
Common stock outstanding prior to this offering (1) | 2,452,348 shares | |
Number of shares of common stock offered by us: | 4,842,615 shares | |
Public offering price: | $4.13 per share of Common Stock and accompanying Public Warrant, or pre-funded warrant and accompanying Public Warrant, as applicable. | |
Common stock outstanding after this offering (1) | 7,294,963 shares (assuming we sell only shares of common stock and no pre-funded warrants, and none of the warrants issued in this offering are exercised). | |
Use of proceeds | We estimate that we will receive net proceeds from this offering of approximately $18,220,000 (or $20,550,000 if the overallotment option is exercised in full) based upon an offering price of $4.13 per Unit, after deducting underwriting discounts and estimated offering expenses payable by us. We currently intend to use the net proceeds we receive from this offering to (i) redeem our Bridge Notes in the outstanding amount of $5,146,176, (ii) repurchase our Series A Preferred Stock from our Chief Executive Officer for an aggregate purchase price of approximately $195,000, (iii) hire marketing and sales personnel to support sales of our CaverStem® and FemCelz® products, (iv) proceed with a clinical study of 100 patients intended to support the safety and efficacy of our StemSpine® Regenerative Stem Cell Procedure for Treatment of Degenerative Disc Disease, (v) conduct, a Phase I clinical trial for the treatment of stroke utilizing our ImmCelzTM technology, (vi) continue to develop other products and therapies, and (vii) fund working capital and general corporate purposes using any remaining amounts. See “Use of Proceeds” on page 18. | |
Representative’s Warrants | The registration statement of which this prospectus is a part also registers for sale warrants (the “Representative’s Warrants”) to purchase 484,262 shares of our common stock (556,901 shares of common stock if the over-allotment option is exercised in full) to Roth Capital Partners (the “Representative”), as the representative of the several underwriters, as a portion of the underwriting compensation payable to the underwriters in connection with this offering. The Representative’s Warrants will be immediately exercisable at an exercise price of $5.1625 (125% of the price which shares of common stock are offered to the public pursuant to this offering) and expire on the fifth anniversary of the commencement of sales of this offering. Please see “Underwriting — Representative’s Warrants” for a description of these warrants. | |
| Table of Contents |
Over-allotment option: | The Underwriting Agreement provides that we will grant to the underwriters an option, exercisable within 45 days after the date of this prospectus, to acquire up to 726,393 additional shares of common stock and/or Public Warrants to purchase up to 726,393 additional shares of common stock, solely for the purpose of covering over-allotments. | |||
Lock-Up | Our directors, executive officers, and certain stockholders have agreed with the Representative not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock or securities convertible into common stock for a period of 6 months commencing on the date of this prospectus. | |||
Risk Factors | You should carefully read the “Risk Factors” section of this prospectus beginning on page 6 for a discussion of factors that you should consider before deciding to invest in our securities. | |||
Trading Symbol and Listing | Our common stock is presently quoted on the OTC Pink under the symbol “CELZ”. We have applied to have our common stock listed on the NASDAQ Capital Market under the symbol “CELZ”. If our listing application is not approved, we will not complete this offering. We do not intend to apply for listing of the Public Warrants or pre-funded warrants on any national securities exchange or trading system. | |||
(1) Unless we indicate otherwise, the number of shares of our common stock outstanding after this offering is based on 2,452,348 shares of common stock outstanding on November 15, 2021, and excludes the following: | ||||
· | 600,000 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan; and | |||
· | 481,351 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $13.28 per share. | |||
Unless otherwise noted, the information in this prospectus assumes: | ||||
· | no exercise of the outstanding warrants described above; | |||
· | no exercise of Public Warrants; | |||
· | no exercise of the Representative’s Warrants; and | |||
· | no exercise of the underwriters’ option to purchase additional shares and/or warrants from us in this offering. | |||
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors in addition to other information in this prospectus before purchasing our securities. The risks and uncertainties described below are those that we currently deem to be material and that we believe are specific to our company, our industry and our securities. In addition to these risks, our business may be subject to risks currently unknown to us. If any of these or other risks actually occurs, our business may be adversely affected, the trading price of our securities may decline and you may lose all or part of your investment.
Risks Related to our Financial Position and Capital Needs
We have incurred recent losses and our future profitability is uncertain.
We have incurred net losses of approximately $36.3 million and $8.4 million for the years ended December 31, 2020 and December 31, 2019, respectively, and 1,844,000 for the nine months ended September 30, 2021. While our recent losses have predominantly resulted from non-cash charges relating to the change in the fair value of derivative liabilities on our balance sheets, and these derivative liabilities were subsequently substantially extinguished, we expect our operating losses to continue until such time, if ever, that product sales, licensing fees, royalties and other sources generate sufficient revenue to fund our operations. We cannot predict when, if ever, we might achieve profitability and cannot be certain that we will be able to sustain profitability, if achieved.
Even with the proceeds from this offering, we will need additional capital to fund our operations as planned.
For the year ended December 31, 2020, our operations used approximately $435,000 in cash, and for the nine months ended September 31, 2021, our operations used approximately $1,054,000 in cash. Cash used in operations consisted primarily of cash on hand and cash raised through private placements of our securities. At September 30, 2021, we had a cash balance of approximately $2,337,930 and current liabilities exceeded current assets by $917,327. Although we expect to raise additional funds from the offering, we will need additional capital to maintain our operations, continue our research and development programs, conduct clinical trials, seek regulatory approvals and manufacture and market our products. We will seek such additional funds through public or private equity or debt financings and other sources. We cannot be certain that adequate additional funding will be available to us on acceptable terms, if at all. If we cannot raise the additional funds required for our anticipated operations, we may be required to reduce the scope of or eliminate our research and development programs, delay our clinical trials and the ability to seek regulatory approvals, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency. If we raise additional funds through future offerings of shares of our common stock or other securities, such offerings would cause dilution of current stockholders’ percentage ownership in the Company, which could be substantial. Future offerings also could have a material and adverse effect on the price of our common stock.
We have generated minimal revenues from our products. We will not achieve profitability unless we generate increased revenues from our current or proposed products or therapies.
Revenues generated from sales of our CaverStem® and FemCelz® kits were only $164,500 and $165,500 for the years ended December 31, 2020 and December 31, 2019, respectively, and $20,000 for the nine months ended September 30, 2021. To sustain our operating costs and generate profits, we will need to significantly increase revenues from our CaverStem® and FemCelz® products or from our other products or therapies that have not yet been commercialized.
We expect to continue to incur significant financial losses in the future as we seek regulatory approval for ourImmCelzTM immunotherapy platform and conduct clinical trials of our StemSpine® Regenerative Stem Cell Procedure for Treatment of Degenerative Disc Disease.
We have not authorized anyoneyet received the necessary regulatory approvals for our ImmCelzTM immunotherapy platform. In addition, we are recruiting patients for a clinical trial of StemSpine® Regenerative Stem Cell Procedure for Treatment of Degenerative Disc Disease in an effort to provide you with information differentdemonstrate the efficacy of our procedure to the medical community in order to generate demand for this procedure. We anticipate that our expenses will increase substantially as we:
● | initiate, conduct and complete ongoing, anticipated or future preclinical studies and clinical trials for our current and future product candidates; | |
● | seek marketing approvals for product candidates that successfully complete clinical trials; and | |
● | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain marketing approval. |
| 6 |
| Table of Contents |
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our auditors, Haynie & Company, have indicated in their report on our consolidated financial statements for the fiscal year ended December 31, 2020, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses from that containedoperations and significant accumulated deficit. In addition, we continue to experience negative cash flows from operations. Notwithstanding the funds we expect to raise in this prospectus.offering, our auditors may again provide a “going concern” opinion with respect to our future audited financial statements. A going concern opinion could impair our ability to finance our operations through the sale of equity. Our ability to continue as a going concern will depend upon the availability of equity financing which represents the primary source of cash flows that will permit us to meet our financial obligations as they come due and continue our research and development efforts.
Risks Related to Product Development, Regulatory Approval and Commercialization
Our product candidates’ commercial viability remain subject to current and future preclinical studies, clinical trials, regulatory approvals, and the risks generally inherent in the development of biopharmaceutical products. If we are unable to successfully advance or develop our product candidates, our business will be materially harmed.
In the near-term, failure to successfully advance the development of our proposed products may have a material adverse effect on us. To date, other than limited sales generated from our CaverStem® and FemCelz® products, we have not successfully developed or commercially marketed, distributed, or sold any product candidate. The selling stockholderssuccess of our business may depend upon our ability to successfully advance the development of our current and future product candidates through preclinical studies and clinical trials, where applicable, have the product candidates approved for sale by the FDA or regulatory authorities in other countries, and ultimately have the product candidates successfully commercialized by us or a commercial partner. We cannot assure you that the results of our ongoing preclinical studies or clinical trials will support or justify the continued development of our product candidates, or that we will receive the necessary approvals from the FDA, or similar regulatory authorities in other countries, to advance the development of our product candidates.
We may not be successful in our commercialization efforts for our proposed products and therapies, which may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
To the extent we possess or obtain the necessary regulatory approval for our proposed products and therapies, we still may not be successful in our commercialization efforts or in gaining sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. Market acceptance will require us to build and maintain strong relationships with healthcare professionals that treat the indications our therapies are offeringintended to address. A failure to build or maintain these important relationships with these healthcare professionals and treatment centers could result in lower market acceptance. Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our products and therapies may require significant resources and may never be successful. The degree of market acceptance of our products and therapies will depend on a number of factors, including:
● | their efficacy; | |
● | limitations or warnings or any restrictions on use, and the prevalence and severity of any side effects; | |
● | the availability and efficacy of alternative treatments; | |
● | the effectiveness of sales and marketing efforts and the strength of marketing and distribution support; | |
● | their cost-effectiveness compared to alternative therapies; and | |
● | availability and amount of coverage and reimbursement from government payors, managed care plans and other third-party payors. |
| 7 |
| Table of Contents |
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that any regulatory authority whose approval we will require in order to market and sell our products in any territory will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our regulatory submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including FDA approval. Our CaverStem®, FemCelz® and StemSpine®products are exempt from the FDA premarket review and approval process as these autologous therapies involve treating the patient with his or her own cells. However, we will require FDA approval of ImmCelzTM for the treatment of stroke and other indications. We have only limited experience in filing the applications necessary to gain regulatory approvals and have relied, and expect to continue to rely, in part, on consultants and third-party contract research organizations, or CROs, with expertise in this area to assist us in this process. Securing FDA approval requires the submission of extensive non-clinical and clinical data and supporting information to the FDA for each therapeutic indication to establish a product candidate’s safety and efficacy for each indication. If third parties upon whom we rely fail to perform satisfactorily, or do not adequately fulfill their obligations under the terms of our agreements with them, our efforts to secure regulatory approval of our product candidates may be delayed or prove unsuccessful.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Clinical trials are expensive and complex, can take many years and have uncertain outcomes. We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us or regulatory authorities to delay or suspend clinical trials, or delay the analysis of data from completed or ongoing clinical trials. We estimate that clinical trials of ImmCelzTM for the treatment of stroke will continue for several years, but they may take significantly longer to complete. Failure can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic candidates, including but not limited to:
· | delays in securing clinical investigators or trial sites for the clinical trials; | |
· | delays in obtaining institutional review board and other regulatory approvals to commence a clinical trial; | |
· | slower than anticipated patient recruitment and enrollment; | |
· | negative or inconclusive results from clinical trials; | |
· | unforeseen safety issues; | |
· | uncertain dosing issues; | |
· | an inability to monitor patients adequately during or after treatment; and | |
· | problems with investigator or patient compliance with the trial protocols. |
A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials, even after seeing promising results in earlier clinical trials. We do not know whether any clinical trials we conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market ImmCelzTM.
| 8 |
| Table of Contents |
Our autologous products are currently not eligible for reimbursement from public or private insurers.
Currently, our CaverStem® and FemCelz® products and related medical procedures are paid for by patients and not eligible for reimbursement from public or private insurers. As a general rule, reimbursement is available only for products and therapies that have been approved of by the FDA. Our CaverStem® and FemCelz® products were exempt from the FDA premarket review and approval process as these autologous therapies involve treating the patient with his or her own cells. While we believe that the requirement that patients directly pay the cost for our CaverStem® and FemCelz® products and procedures make these procedures more attractive to doctors, these treatments are only available to patients that can afford to pay for them. Our success and extent of our growth will depend in part on the extent to which reimbursement for the costs of our products and related treatments will be available from third party payers, such as public and private insurers and health systems.
The pharmaceutical business is subject to increasing government regulation and reform, including with respect to price controls, reimbursement and access to therapies, which could adversely affect our future revenues and profitability.
Our existing and proposed products may not be considered cost-effective, and third-party or government reimbursement might not be available or sufficient. Globally, governmental and other third-party payors are becoming increasingly aggressive in attempting to contain health care costs by strictly controlling, directly or indirectly, pricing and reimbursement and, in some cases, limiting or denying coverage altogether on the basis of a variety of justifications, and we expect pressures on pricing and reimbursement from both governments and private payors inside and outside the U.S. to continue.
Our existing and proposed products are and will be subject to substantial pricing, reimbursement, and access pressures from state Medicaid programs, private insurance programs and pharmacy benefit managers, and the implementation of U.S. health care reform legislation that is increasing these pricing pressures. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, instituted comprehensive health care reform, and includes provisions that, among other things, reduce and/or limit Medicare reimbursement, and impose new and/or increased taxes. The future of the Affordable Care Act and its constituent parts are uncertain at this time.
The continuing efforts of government and insurance companies, health maintenance organizations, and other payors of health care costs to contain or reduce costs of health care may affect our future revenues and profitability or those of our potential customers, suppliers, and collaborative partners, as well as the availability of capital.
United States federal and state privacy laws, and equivalent laws of other nations, may increase our costs of operation and expose us to civil and criminal sanctions.
Regulation of data processing is evolving, as federal, state, and foreign governments continue to adopt new, or modify existing, laws and regulations addressing data privacy and security, and the collection, processing, storage, transfer, and use of data. These new or proposed laws and regulations are subject to differing interpretations and may be inconsistent among jurisdictions, and guidance on implementation and compliance practices are often updated or otherwise revised, which adds to the complexity of processing personal data. These and other requirements could require us or our collaborators to incur additional costs to achieve compliance, limit our competitiveness, necessitate the acceptance of more onerous obligations in our contracts, restrict our ability to use, store, transfer, and process data, impact our or our collaborators’ ability to process or use data in order to support the provision of our products, affect our or our collaborators’ ability to offer our products in certain locations, or cause regulators to reject, limit or disrupt our clinical trial activities.
We and our collaborators may be subject to federal, state and foreign data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state personal information laws, state data breach notification laws, state health information privacy laws and federal and state consumer protection laws and regulations that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH. Depending on the facts and circumstances, we could be subject to civil or criminal penalties if we knowingly use or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
| 9 |
| Table of Contents |
Later discovery of previously unknown problems could limit our ability to market or sell our products or therapies, and can expose us to product liability claims.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with any third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
● | refusals or delays in the approval of applications or supplements to approved applications; | |
● | refusal of a regulatory authority to review pending market approval applications or supplements to approved applications; | |
● | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls or seizures; | |
● | fines, warning letters, or holds on clinical trials; | |
● | injunctions or the imposition of civil or criminal penalties; | |
● | restrictions on product administration, requirements for additional clinical trials, or changes to product labeling requirements; or | |
● | recommendations by regulatory authorities against entering into governmental contracts with us. |
Discovery of previously unknown problems or risks relating to our product could also subject us to potential liabilities through product liability claims.
If we do not obtain required approvals in other countries in which we aim to market our products, we will be limited in our ability to export or sell the products in those markets.
Our lack of experience in conducting clinical trials in foreign jurisdictions may negatively impact the approval process in those jurisdictions. If we are unable to obtain and maintain required approval from one or more foreign jurisdictions where we would like to sell our products or therapies, we will be unable to market products as intended, our international market opportunity will be limited and our results of operations will be harmed.
We rely in part on third parties for research and clinical trials for our products and therapies.
We rely on contract research organizations (“CROs”), academic institutions, corporate partners, and other third parties to assist us in managing, monitoring, and otherwise carrying out clinical trials and research activities. We rely or will rely heavily on these parties for the execution of our clinical studies and control only certain aspects of their activities. Accordingly, we may have less control over the timing and other aspects of these clinical trials than if we conducted them entirely on our own. Although we rely on these third parties to manage the data from clinical trials, we will be responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Our failure, or the failure of third parties on which we rely, to comply with the strict requirements relating to conducting, recording, and reporting the results of clinical trials, or to follow good clinical practices, may delay the regulatory approval process or cause us to fail to obtain regulatory approval for our proposed products and therapies.
| 10 |
| Table of Contents |
We currently have a very limited marketing and sales organization and may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our products and therapies, we may not be able to generate sufficient revenues to support our operations.
Our current sales, marketing and distribution capabilities consist of one independent contractor who promotes the sale our CaverStem® and FemCelz® products to medical doctors. To generate sufficient revenues to support our operations, we will have to seek collaborators, especially for marketing and sales outside of the United States, or invest significant amounts of financial and management resources to develop internal sales, distribution and marketing capabilities. We may not be able to enter into collaborations or hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms, or at all. In addition, our product revenues and our profitability, if any, may be lower if we rely on third parties for these functions than if we were to market, sell and distribute products that we develop ourselves. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and seeking offersmarket our products effectively. Even if we determine to buy,perform sales, marketing and distribution functions ourselves, we could face a number of additional related risks, including:
● | we may not be able to attract and build an effective marketing department or sales force; | |
● | the cost of establishing a marketing department or sales force may exceed our available financial resources and the revenue generated by our product candidates that we may develop, in-license or acquire; and | |
● | our direct sales and marketing efforts may not be successful. |
We rely upon third parties for the manufacture of our CaverStem® and FemCelz®disposable kits and are dependent on their quality and effectiveness.
We rely upon third parties for the manufacture of our CaverStem® and FemCelz® disposable kits. The failure to achieve and maintain high manufacturing standards, or to detect or control anticipated or unanticipated manufacturing errors or the frequent occurrence of such errors, could result in cost overruns, product recalls or withdrawals, patient injury or death, and other problems that could seriously hurt our business.
We may be unable to compete effectively with marketed therapies or drugs targeting similar indications to our products and therapies.
We face competition generally from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize any products that are safer, more effective, have fewer side effects or are less expensive than our products and therapies. These potential competitors may also compete with us in establishing clinical trial sites, and patient enrollment for clinical trials.
Our business and operations would suffer in the event of computer system failures or security breaches.
In the ordinary course of our business, we collect, store and transmit confidential information, including intellectual property, and proprietary business information. Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations, or CROs, and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyberattacks, natural disasters, fire, terrorism, war and telecommunication and electrical failures. Cyberattacks are increasing in their frequency, sophistication and intensity. Cyberattacks could include the deployment of harmful malware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. Significant disruptions of our information technology systems or security breaches could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property and proprietary business information and personal information), and could result in financial, legal, business and reputational harm to us. If such disruptions were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Further, the COVID-19 pandemic has resulted in a significant number of our employees and partners working remotely, which increases the risk of a data breach or issues with data and cybersecurity. To the extent that any disruption or security breach results in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our future product candidates could be delayed.
| 11 |
| Table of Contents |
We are subject to risks arising from the recent global outbreak of the COVID-19 coronavirus.
The recent outbreak of the COVID-19 coronavirus has spread across the globe and is impacting worldwide economic activity. A pandemic, including COVID-19 or other public health epidemic, poses the risk that we or our employees, CROs, suppliers, manufacturers and other partners may be prevented from conducting business activities for an indefinite period of time, including due to the spread of the disease or shutdowns that may be requested or mandated by governmental authorities. During 2020 and 2021, COVID-19 has resulted in significantly reduced revenues from our CaverStem® and FemCelz® products, as elective procedures in general have been greatly reduced throughout the United States during the pandemic. In addition, the continued spread of COVID-19 could disrupt our clinical trials, supply chain and the manufacture or shipment of our products, and other related activities, which could have a material adverse effect on our business, financial condition and results of operations. COVID-19 has also had an adverse impact on global economic conditions which could impair our ability to raise capital when needed.
Risks Related to Our Intellectual Property
We may not be able to protect our proprietary rights.
Our commercial success will depend in large part upon our ability to protect our proprietary rights. There is no assurance, for example, that any additional patents will be issued based on our or our pending applications or, if issued, that such patents will not become the subject of a re-examination, will provide us with competitive advantages, will not be challenged by any third parties, or that the patents of others will not prevent the commercialization of products and services incorporating our technology. Furthermore, there can be no guarantee that others will not independently develop similar products and services, duplicate any of our products and services, or design around any patents we obtain.
Our commercial success will also depend upon our ability to avoid infringing patents issued to others. If we were judicially determined to be infringing on any third-party patent, we could be required to pay damages, alter our products, services or processes, obtain licenses, or cease certain activities. If we are required in the future to obtain any licenses from third parties for some of our products and/or services, there can be no guarantee that we would be able to do so on commercially favorable terms, if at all. United States and foreign patent applications are not immediately made public, so we might be surprised by the grant to someone else of a patent on a technology we are actively using.
In addition to patents, we rely on unpatented trade secrets and proprietary technological expertise, and confidentiality agreements with our partners, employees, advisors, vendors, and consultants to protect our trade secrets and proprietary technological expertise. There can be no guarantee that these agreements will not be breached, or that we will have adequate remedies for any breach, or that our unpatented trade secrets and proprietary technological expertise will not otherwise become known or be independently discovered by competitors.
Failure to obtain or maintain patent protection or to protect our trade secrets could have a substantial negative effect on our results of operations and financial condition.
We are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our product candidates.
There is a substantial amount of litigation over patent and other intellectual property rights in the biotechnology industry. Whether or not a product infringes a patent involves complex legal and factual considerations, the determination of which is often uncertain. Our competitors or other parties may assert that our product candidates and the methods employed may be covered by patents held by them. If any of our products infringes a valid patent, we could be prevented from manufacturing or selling such product unless we are able to obtain a license or able to redesign the product in such a manner as to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid infringement, nor does a later redesign protect the Company from prior infringement.
We may need to initiate lawsuits to protect or enforce our intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
In order to protect or enforce our intellectual property rights, we may need to initiate patent, trademark and related litigation against third parties, such as infringement suits or requests for injunctive relief. Our ability to establish and maintain a competitive position may be achieved in part by prosecuting claims against others who we believe to be infringing its rights. Any lawsuits or administrative proceedings in patent offices that we initiate or that are initiated against us could be expensive, take significant time and divert our management’s attention from other business concerns and the outcome of litigation to enforce our intellectual property rights in patents, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, or adversely affect our ability to distribute any products that are subject to such litigation. In addition, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits or administrative proceedings that we initiate, and the damages or other remedies awarded, including attorney fees, if any, may not be commercially valuable.
| 12 |
| Table of Contents |
Risks Related to Employee Matters
We are dependent on our executive officers, and we may not be able to pursue our current business strategy effectively if we lose them.
Our success to date has largely depended on the efforts and abilities of Timothy Warbington, our Chief Executive Officer, and Drs. Thomas Ichim and Amit Patel, who were our founders and who currently serve as directors and/or consultants to us. Our ability to manage our operations and meet our business objectives could be adversely affected if, for any reason, such officers do not remain with us.
Our employees, clinical trial investigators, CROs, consultants, vendors and any potential commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
We are exposed to the risk of fraud or other misconduct by our employees, clinical trial investigators, CROs, consultants, vendors and any potential commercial partners. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) U.S. laws and regulations or those of foreign jurisdictions, including those laws that require the reporting of true, complete and accurate information, (ii) manufacturing standards, (iii) federal and state health and data privacy, security, fraud and abuse, government price reporting, transparency reporting requirements, and other healthcare laws and regulations in the United States and abroad or (iv) laws that require the true, complete and accurate reporting of financial information or data. Such misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from government funded healthcare programs, such as Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional integrity reporting and oversight obligations, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and foreign country laws, we could be subject to criminal and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent third country programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in whole or in part, by Medicare, Medicaid or any other federal healthcare program. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, many states have adopted laws similar to the federal Anti-Kickback Statute that apply to activity in those states, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
While we believe our operations will be in compliance with the federal Anti-Kickback Statute and similar state laws, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
| 13 |
| Table of Contents |
Risks Related To Our Common Stock and This Offering
Our management has broad discretion as to the use of the net proceeds from this offering.
We currently intend to use the net proceeds that we receive from this offering to (i) redeem our Bridge Notes in the outstanding amount of $5,146,176, (ii) repurchase our Series A Preferred Stock from our Chief Executive Officer for an aggregate purchase price of approximately $195,000, (iii) hire marketing and sales personnel to support sales of our CaverStem® and FemCelz® products, (iv) proceed with a clinical study of 100 patients intended to support the safety and efficacy of our StemSpine® Regenerative Stem Cell Procedure for Treatment of Degenerative Disc Disease, (v) conduct a Phase I clinical trial for the treatment of stroke utilizing our ImmCelzTM technology, (vi) continue to develop other products and therapies, and (vii) fund working capital and general corporate purposes using any remaining amounts. Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in “Use of Proceeds.” Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds. Our management may spend a portion or all of the net proceeds from this offering in ways that holders of our common stock may not desire or that may not yield a significant return or any return at all. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may also invest the net proceeds from this offering in a manner that does not produce income or that loses value.
There is no assurance that an active trading market for our common stock will be sustained, and there is no market for our Public Warrants or pre-funded warrants.
The public offering price for the securities will be determined by negotiations between us and the representatives of the underwriters and may not be indicative of prices that will prevail in the trading market. Upon closing of this offering, our common stock will be listed on the Nasdaq Capital Market, however, we cannot ensure that an active public market for our common stock will develop after this offering, or that if it does develop, it will be sustained. In addition, we do not intend to apply to list the Public Warrants or pre-funded warrants on the Nasdaq Capital Market or any nationally recognized trading system, and accordingly, there will be no trading market for such warrants. In the absence of an active public trading market:
● | you may not be able to resell your securities at or above the public offering price; | |
● | the market price of our common stock may experience more price volatility; and | |
● | there may be less efficiency in carrying out your purchase and sale orders. |
The market price of our common stock may be highly volatile, and you could lose all or part of your investment.
The trading price of our common stock and Public Warrants is likely to be volatile. This volatility may prevent you from being able to sell your securities at or above the price you paid for your securities. Our stock price and Public Warrant price could be subject to wide fluctuations in response to a variety of factors, which include:
● | whether we achieve our anticipated corporate objectives; | |
● | whether we achieve our anticipated corporate objectives; | |
● | termination of the lock-up agreement or other restrictions on the ability of our stockholders and other security holders to sell shares after this offering; and | |
● | general economic or political conditions in the United States or elsewhere. |
In addition, the stock market in general, and the stock of clinical stage biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
| 14 |
| Table of Contents |
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of 4,842,615 shares of common stock and accompanying Public Warrants in this offering at a combined public offering price of $4.13 per share of common stock and accompanying Public Warrant, after deducting underwriter discounts and commissions and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $2.12 per share. For a further description of the dilution that investors in this offering may experience, see “Dilution.”
In the past, we have issued shares of common stock and warrants in private placements of our securities, and we have issued shares of common stock as compensation to our officers and directors. Our issuance of shares of common stock in the future, and the exercise of outstanding warrants or warrants that we may issue in the future, may result in additional dilution to investors in this offering.
If, after being listing on The Nasdaq Capital Market, we are delisted and our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not maintain a listing on Nasdaq and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
If our securities are listed on Nasdaq, our failure to meet the continued listing requirements of The Nasdaq Capital Market could result in a de-listing of our securities.
If after listing we fail to satisfy the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to de-list our securities. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
We will indemnify and hold harmless our officers and directors to the maximum extent permitted by Nevada law.
Our bylaws provide that we will indemnify and hold harmless our officers and directors against claims arising from our activities, to the maximum extent permitted by Nevada law. If we were called upon to perform under our indemnification agreement, then the portion of our assets expended for such purpose would reduce the amount otherwise available for our business.
Substantial future sales of shares of our common stock only in jurisdictions where offersthe public market could cause our stock price to fall.
Except for 226,948 shares of our common stock held by our affiliates, all of our outstanding shares of common are currently freely trading or eligible for resale without restriction under Rule 144. In addition, the lock-up agreements which our officers, directors, and principal stockholders entered into with the underwriter expire six months after the date of this prospectus. Upon the expiration of those lock-up agreements, the outstanding shares of common stock covered by them become eligible for resale in the open market (subject to Rule 144 volume limitations applicable to executive officers, directors and 10% or more stockholders), resulting in more shares eligible for sale and potentially causing selling in the market to increase and our stock price to decline. Additional sales of a substantial number of our shares of our common stock in the public market, or the perception that sales could occur, could have a material adverse effect on the price of our common stock.
Because we do not expect to pay dividends for the foreseeable future, investors seeking cash dividends should not purchase shares of common stock.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do not anticipate paying any cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our Board of Directors after taking into account various factors, including but not limited to our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. Accordingly, investors seeking cash dividends should not purchase our shares.
| 15 |
| Table of Contents |
Warrants are permitted. speculative in nature.
The warrants offered pursuant to this prospectus do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our commons stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the Public Warrants may exercise their right to acquire the common stock and pay an exercise price of $4.13, prior to five years from the date of issuance, after which date any unexercised Public Warrants will expire and have no further value. Moreover, following this offering, the market value of the Public Warrants is uncertain and there can be no assurance that the market value of the Public Warrants will equal or exceed their public offering price. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the Public Warrants, and, consequently, whether it will ever be profitable for holders of the Public Warrants to exercise those warrants.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company” as defined in the Jump-Start Our Business Startups Act, or the JOBS Act, and we take advantage of some of the exemptions from reporting requirements that are applicable to other public companies that are not emerging growth companies, including:
• | not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; |
• | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
• | reduced disclosure obligations regarding executive compensation; and |
• | not being required to hold a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We currently expect that we will remain an emerging growth company until December 31, 2021.
Under Section 107(b) of the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Risks Associated with Our Reverse Stock Split
The 1-for-500 reverse stock split has caused our stock price to decline relative to its value before the split.
We effected a reverse stock split of our authorized, issued and outstanding common stock on November 10, 2021 at a ratio of 1-for-500 in order to achieve a sufficient increase in our stock price to enable us to qualify for listing on Nasdaq. Following the effectiveness of the reverse stock split, there has been a substantial decrease in our share price, and there is no assurance that the reverse split will not cause a further decline in the value of our outstanding common stock.
We cannot assure you that we will be able to continue to comply with the minimum bid price requirement of The Nasdaq Capital Market.
There can be no assurance that the market price of our common stock will remain at the level required for continuing compliance with that requirement. It is not uncommon for the market price of a company’s common stock to decline in the period following a reverse stock split. If the market price of our common stock continues to decline following the effectuation of the reverse stock split, the percentage decline may be greater than would occur in the absence of a reverse stock split. In any event, other factors unrelated to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the market price of our common stock and jeopardize our ability to meet or maintain The Nasdaq Capital Market’s minimum bid price requirement.
The reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be affected adversely by the reverse stock split given the reduced number of shares that are now outstanding following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split has increased the number of stockholders who own odd lots (less than 100 shares) of our common stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in a share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
| 16 |
| Table of Contents |
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this prospectus contains certain forward-looking statements. All statements other than statements of historical facts contained or incorporated by reference in this prospectus, including statements regarding our future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “will,” “may,” “future,” “plan,” “intend” and “expect” and similar expressions generally identify forward-looking statements. These forward-looking statements are not guarantees and are subject to known and unknown risks, uncertainties and assumptions that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. Although we believe that our plans, intentions and expectations reflected in the forward-looking statements are reasonable, we cannot be sure that they will be achieved. Particular uncertainties that could cause our actual results to be materially different than those expressed in our forward-looking statements include: our history of losses; our inability to receive regulatory approval for our products; later discovery of previously unknown problems; reliance on third parties; competition between us and other companies in the industry; delays in the development of products; our ability to raise additional capital; continued services of our executive management team; and statements of assumption underlying any of the foregoing, as well as other factors set forth under the caption “Risk Factors” on page 6 of this prospectus. All subsequent written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by the foregoing. Except as required by law, we undertake no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
| 17 |
| Table of Contents |
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $18,220,000 based on a public offering price of $4.13 per share of common stock and accompanying Public Warrant, after deducting estimated underwriting discounts and estimated offering expenses payable by us and excluding the proceeds, if any, from the exercise of the Public Warrants issued in this offering. If the underwriters’ over-allotment option is accurate onlyexercised in full, we estimate that our net proceeds will be approximately $21,010,000 after deducting estimated underwriting discounts and estimated offering expenses payable by us and excluding the proceeds, if any, from the exercise of the Public Warrants.
We currently intend to use the net proceeds we receive from this offering to (i) redeem our Bridge Notes in the outstanding amount of $5,146,176, (ii) repurchase our Series A Preferred Stock from our Chief Executive Officer for an aggregate purchase price of approximately $195,000, (iii) hire marketing and sales personnel to support sales of our CaverStem® and FemCelz® products, (iv) proceed with a clinical study of 100 patients intended to support the safety and efficacy of our StemSpine® Regenerative Stem Cell Procedure for Treatment of Degenerative Disc Disease, (v) conduct, a Phase I clinical trial for the treatment of stroke utilizing our ImmCelzTM technology, (vi) continue to develop other products and therapies, and (vii) to fund working capital and general corporate purposes using any remaining amounts.
Based on our planned use of the net proceeds, we estimate such funds, together with our existing cash and cash equivalents, will be sufficient for us to fund our operating expenses and capital expenditure requirements through at least June 2023. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we expect.
The expected use of the net proceeds from the offering represents our intentions based upon our current plans and business conditions. The amounts we actually expend in these areas, and the timing thereof, may vary significantly from our current intentions and will depend on a number of factors, including the success of research and product development efforts, cash generated from future operations and actual expenses to operate our business.
The amounts and timing of our preclinical and clinical expenditures and the extent of preclinical and clinical development may vary significantly depending on numerous factors, including the status, results and timing of our current preclinical studies and the preclinical studies and clinical trials which we may commence in the future, the product approval process with the FDA and other regulatory agencies, and any new collaborations we may enter into with third parties and any unforeseen cash needs. As a result, we cannot predict with any certainty all of the particular uses for the net proceeds or the amounts that we will actually spend on the uses set forth above. Accordingly, our management will have broad discretion in the application of the net proceeds, and investors will be relying on the judgment of our management regarding the application of the net proceeds of this offering.
The expected net proceeds of this offering will not be sufficient for us to fund any of our product candidates through regulatory approval, and we will need to raise substantial additional capital to complete the development and commercialization of our product candidates.
| 18 |
| Table of Contents |
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock at any time in the foreseeable future. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our Board and will depend on, among other factors, our financial condition, operating results, capital requirements, general business conditions, the terms of any future credit agreements and other factors that our Board may deem relevant.
| 19 |
| Table of Contents |
CAPITALIZATION
The following table sets forth our cash and cash equivalents, debt obligations, and capitalization as of September 30, 2021 (i) on an actual basis, and (ii) on a pro forma as adjusted basis to give effect to the dateissuance and sale of shares of our common stock and Public Warrants in this offering at a combined public offering price of $4.13 per share and accompanying Public Warrant, for total net proceeds of approximately $18,220,00 (assuming no sale of pre-funded warrants and no exercise of the underwriters’ over-allotment option).
|
| As of September 30, 2021 |
| |||||
|
| Actual |
|
| Pro Forma (1) |
| ||
|
|
|
|
|
|
| ||
Cash |
| $ | 2,337,930 |
|
| $ | 15,257,708 |
|
Notes payable, net of discount of $2,219,430 |
|
| 2,743,430 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
Stockholders’ (deficit) equity: |
|
| - |
|
|
| - |
|
Series A preferred stock, $0.001 par value, 3,000,000 shares authorized, 3,000,000 shares issued and outstanding actual; no shares authorized or issued and outstanding pro forma |
|
| 3,000 |
|
|
| - |
|
Series B preferred stock, $0.001 par value, 1,000 shares authorized, no shares issued and outstanding actual; no shares authorized or issued and outstanding pro forma |
|
| - |
|
|
| - |
|
Series C preferred stock, $0.001 par value, 1,000 shares authorized, no shares issued and outstanding actual; no shares authorized or issued and outstanding pro forma |
|
| - |
|
|
| - |
|
Common stock, $0.001 par value, 6,000,000,000 shares authorized; 2,458,250 and 1,537,081 issued and 2,458,242 and 1,537,073 outstanding actual; 50,000,000 shares authorized issued and outstanding pro forma |
|
| 2,458 |
|
|
| 7,301 |
|
Additional paid-in capital |
|
| 38,434192 |
|
|
| 42,474,928 |
|
Accumulated deficit |
|
| (38,802,995 | ) |
|
| (47,222,366 | ) |
Total stockholders’ equity (deficit) |
|
| (363,345 | ) |
|
| 15,262,863 |
|
Total capitalization |
| $ | 3,131,912 |
|
| $ | 16,011,690 |
|
(1) | Each $0.50 increase (decrease) in the public offering price of $4.13 per share and accompanying Public Warrant would increase (decrease) cash and cash equivalents, working capital, total assets, total liabilities, additional paid-in capital and total stockholders’ (deficit) equity by $2,589,588, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the estimated underwriting discounts and commissions. Similarly, each increase (decrease) of 500,000 shares of common stock and accompanying Public Warrants offered by us would increase (decrease) each of cash and cash equivalents, working capital, total assets, additional paid-in capital and total stockholders’ (deficit) equity by $1,920,450 assuming the public offering price of $4.13 per share and accompanying Public Warrant remains the same, and after deducting the estimated underwriting discounts and commissions. |
The foregoing pro forma as adjusted information is illustrative only, and our capitalization following the completion of this prospectus, regardlessoffering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. The foregoing table reflects the repayment in full of the timeBridge Notes we issued in August 2021 in the outstanding amount of delivery$5,146,176 which we will redeem with the proceeds of this Offering, and the repurchase our Series A Preferred Stock from our Chief Executive Officer for an aggregate purchase price of approximately $195,000. You should read this table together with our financial statements and the related notes appearing elsewhere in this prospectus and the “Selected Financial Data” and “Management’s discussion and analysis of financial condition and results of operations” sections of this prospectus.
The above discussion and table is based on 2,458,242 shares of common stock outstanding on September 30, 2021, and excludes the following:
• | 600,000 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan; | |
• | 481,351 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $13.28 per share; | |
• | 4,842,615 shares of common stock issuable upon the exercise of the Public Warrants issued pursuant to this offering (or Public Warrants to purchase up to 5,569,007 shares of common stock assuming the underwriters’ over-allotment option is fully exercised); and | |
• | 484,262 shares of common stock issuable upon the exercise of the warrants to be issued to the Representative (or warrants to purchase up to 556,901 shares of common stock assuming the underwriters’ over-allotment option is fully exercised). |
| 20 |
| Table of Contents |
DILUTION
If you invest in our securities in this offering, your interest will be diluted to the extent of the difference between the public offering price per share of common stock and accompanying Public Warrant and the pro forma as adjusted net tangible book value per share of common stock immediately after this offering.
Our net tangible book value is the amount of our total tangible assets less our total liabilities. We had a net tangible book value deficit as of September 30, 2021 of $(914,045), or of any sale$(0.37) per share of common stock.
ForPro forma as adjusted net tangible book value is our pro forma net tangible book value, plus the effect of the sale of our securities in this offering at the public offering price of $4.13 per share of common stock and accompanying Public Warrant and after deducting the underwriting discounts and commissions and other estimated offering expenses payable by us. Our pro forma as adjusted net tangible book value as of September 30, 2021 would have been approximately $14,709,163, or $2.01 per share. This amount represents an immediate increase in pro forma as adjusted net tangible book value of approximately $2.39 per share to our existing stockholders, and an immediate dilution of $2.12 per share to new investors outsideparticipating in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the U.S.: We have notpublic offering price per share paid by new investors.
The following table illustrates this per share dilution:
Assumed public offering price per share (attributing no value to the warrants) |
| $ | 4.13 |
|
Net tangible book value per share as of September 30, 2021 |
| $ | (914,045 | ) |
Increase in pro forma as adjusted net tangible book value per share after this offering |
| $ | 15,623,208 |
|
Pro forma as adjusted net tangible book value per share after giving effect to this offering |
| $ | 14,709,163 |
|
Dilution in pro forma as adjusted net tangible book value per share to new investors |
| $ | 2.12 |
|
Each $0.50 increase (decrease) in the public offering price of $4.13 per share and accompanying Public Warrant would increase (decrease) the pro forma as adjusted net tangible book value per share by $0.35, and the selling stockholders have not done anything that would permitdilution per share to new investors in this offering or possession or distributionby $0.31, assuming the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, in any jurisdiction where action for that purpose is required, other thanremains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Each increase of 500,000 in the U.S. You are required to inform yourselves about and to observe any restrictions relating to the offeringnumber of the shares of common stock and accompanying Public Warrants sold in this offering would increase (decrease) our pro forma as adjusted net tangible book value by approximately $1,920,450 and the distributiondilution per share to new investors in this offering by $0.12, assuming that the public offering price per share and possession of this prospectus outside ofaccompanying Public Warrant remains the U.S.same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Unless otherwise indicated, any referenceThe information above assumes that the Representative does not exercise its over-allotment option. If the Representative exercises its over-allotment option in full, the pro forma as adjusted net tangible book value will increase to “our company”, “we”, “us”,$2.18 per share, representing an immediate increase to existing stockholders of $0.17 per share and an immediate dilution of $1.95 per share to new investors.
The foregoing discussion and table do not take into account further dilution to new investors that could occur upon the exercise of outstanding warrants having a per share exercise or “our” refersconversion price less than the per share offering price to Creative Medical Technology Holdings, Inc.,the public in this offering.
The above discussion and as applicabletable is based on 2,458,242 shares of common stock outstanding on September 30, 2021, and excludes the following:
· | 600,000 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan; | |
· | 481,351 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $13.28 per share; | |
· | 4,842,615 shares of common stock issuable upon the exercise of the Public Warrants issued pursuant to this offering (or Public Warrants to purchase up to 5,569,007 shares of common stock assuming the underwriters’ over-allotment option is fully exercised); | |
· | the issuance of any pre-funded warrants in this offering; and | |
· | 484,262 shares of common stock issuable upon the exercise of the warrants to be issued to the Representative (or warrants to purchase up to 556,901 shares of common stock assuming the underwriters’ over-allotment option is fully exercised). |
To the extent that outstanding options and warrants are exercised, investors will experience further dilution. In addition, we may choose to its wholly ownedraise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| 21 |
| Table of Contents |
OUR BUSINESS
Overview
We are a commercial stage biotechnology company focused on immunology, urology, neurology and orthopedics using adult stem cell treatments and interrelated regenerative technologies for the treatment of multiple indications. Our existing and pipeline of therapies and products include the following:
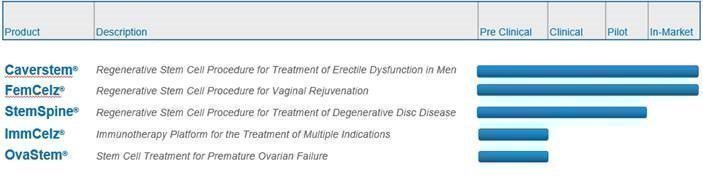
Our subsidiary, Creative Medical Technologies, Inc. (“CMT”), a Nevada corporation.
PROSPECTUS SUMMARYwas originally created to monetize U.S. Patent No. 8,372,797 and related intellectual property related to the treatment of erectile dysfunction (“ED”), which it acquired in February 2016. Subsequently, we have expanded our development and acquisition of intellectual property beyond urology to include therapeutic treatments utilizing “re-programmed” stem cells, and the treatment of neurologic disorders, lower back pain, type I diabetes, and heart, liver, kidney and other diseases using various types of stem cells through our ImmCelz, Inc., StemSpine, Inc. and AmnioStem LLC subsidiaries. However, neither ImmCelz Inc., StemSpine Inc. nor AmnioStem LLC have commenced commercial activities.
The following summary highlights material information contained in this prospectus. This summary does not containWe currently conduct substantially all the information you should consider before investingof our commercial operations through CMT, which markets and sells our CaverStem® and FemCelz® disposable kits utilized by physicians to perform autologous procedures that treat erectile dysfunction and female sexual dysfunction, respectively. Our CaverStem® and FemCelz® kits are currently available through physicians at eight locations in the securities. Before makingUnited States.
In addition to our CaverStem® and FemCelz® products, we are currently in the process of recruiting clinical sites for our StemSpine® Regenerative Stem Cell Procedure for the Treatment of Degenerative Disc Disease. Our StemSpine® treatment is an investment decision, you should readautologous procedure that utilizes a patient’s own stem cells to treat lower back pain.
In 2020, through our ImmCelz Inc. subsidiary, we began exploring the entire prospectus carefully, includingdevelopment of treatments that utilize a patient’s own extracted immune cells that are then “reprogrammed” by culturing them outside the “Risk Factors” section,patient’s body with optimized stem cells. The immune cells are then re-injected into the financial statementspatient from whom they were extracted. We believe this process endows the immune cells with regenerative properties that may be suitable for the treatment of stroke victims, among other indications. In contrast to other stem cell-based approaches, the immune cells are significantly smaller in size than stem cells and are believed to more effectively penetrate areas of the notesdamaged tissues and induce regeneration.
We are currently primarily focused on expanding the commercial sale and use of our CaverStem® and FemCelz® products by physicians in the Unites States and Europe, and commercializing our StemSpine® treatment for lower back pain. We also recently filed an Investigational New Drug (IND) application with the FDA to treat stroke utilizing our ImmCelzTM technology. In the future, subject to the financial statements.
Creative Medical Technology Holdings, Inc.availability of capital, we will seek to further develop additional therapeutic products that utilize our proprietary intellectual property.
We were incorporated on December 3, 1998, in the State of Nevada under the name Jolley Marketing, Inc., and have one wholly owned subsidiary, Creative Medical Technologies, Inc., a Nevada corporation, (“CMT”), which conducts all of our business operations. From our incorporation in 1998 through approximately June 2008, we were in the startup phase of our proposed business operations in the telecommunications and retail lighting products fields. We had only limited operations during this startup phase and on approximately June 30, 2008, we ceased all business operations because of increased competition in the industry, dwindling sales, and elevated costs associated with generating sales.
On May 18, 2016, we closed an Agreement and Plan of Merger (the “Merger Agreement”) with CMT, Steven L. White, our principal shareholder and the sole officer and director, and Jolley Acquisition Corp.,completed a Nevada corporation and wholly owned subsidiary of our company (the “Merger Sub”). As a result of the closing of the Merger Agreement, the Merger Sub was merged with and into CMT with CMT being the surviving corporation and CMTreverse merger transaction under which Creative Medical Technologies, Inc. became our wholly-owned subsidiary. Effective May 18, 2016, we filed Articles of Merger and Articles of Exchange with the Nevada Secretary of State evidencing the closing and the issuance of our shares to the shareholders of CMT. Following closing, Mr. White, who was our majority shareholder prior to the closing, sold 15,100,000 shares of our common stock to us for $5,000, after which the shares were cancelled and returned to our authorized but unissued shares of common stock.
In connection with the closing, CMT caused Creative Medical Health, Inc., a Delaware corporation and parent of CMT (“CMH”), to advance $25,000 to us for payment of certain obligations. Prior to the execution of the Merger Agreement, CMH advanced to us $8,256 for the payment of certain accounts payable and $5,000 for repayment of certain notes payable. At closing, CMT caused CMH to advance $5,000 to us for the purchase of Mr. White’s shares and the balance of the $25,000 for the payment of our remaining accounts payable. The amounts advanced by the parent of CMT are evidenced by an 8% Promissory Note dated May 18, 2016.
At closing, each share of common stock of CMT issued and outstanding immediately prior to the closing was converted into 6.4666666 shares of our common stock (97,000,000 shares), which now constitutes approximately 97%, of our issued and outstanding common stock. The equity of the Company has been retroactively restated to show the effect of the reversethis merger, on the common stock outstanding for the periods presented.
As a condition of closing, we delivered Cancellation of Indebtedness Agreements evidencing the cancellation of all prior outstanding notes payable, except for promissory notes in the aggregate amount of $20,000 which are payable upon obtaining DTC eligibility for our common stock.
At closing, Timothy Warbington, Donald Dickerson, Thomas Ichim, PhD, and Amit Patel, MD were appointed as our directors and Mr. White resigned from all positions with our company.
Effective May 18, 2016, in connection with the closing, we filed Articles of Merger with the Nevada Secretary of State evidencing the change ofchanged our name to “Creative Medical Technology Holdings, Inc.” This merger was between our company and a newly formed Nevada corporation, Creative Medical TechnologyTechnologies Holdings, Inc., which was formed solely to effectreflect our name change.current business.
| 22 |
| Table of Contents |
Our Products
CaverStem® - Erectile Dysfunction Treatment (Commercialized)
Creative Medical Technologies
CMT was created as the urological arm of CMH, to monetize the treatments or products developed or acquired by CMH prior to creation of CMT and transferred to it after its incorporation. CMT has been engaged in the regenerative medicine field of male and female sexual dysfunction and infertility using stem cells. CMT acquired a patent for its erectile dysfunction (“ED”) treatment from CMH and was granted a license by Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, a non-profit biomedical research and education institute (“LABIOMED”), for the infertility treatment. On June 9, 2016, Dr. Patel and Dr. Ichim, two of our directors, filed a patent application for the treatment of miscarriages, which has been assigned to CMT.
CMTCaverStem® is a clinical stage company in the pre-revenue stage and intends to complete the testing of its ED treatment and, if warranted, market treatment kits to physicians for use with their patients suffering from ED. CMT is currently engaged inclinically proven, patented procedure (U.S. Patent No. 8,372,797) that utilizes a 15-month clinical trial study being conducted at UCLA by LABIOMED on the efficacy and safety of the ED treatment. The study involves testing on 40 subjects and is intended to have a duration of 15 months. CMT also intends to test in the future and, if warranted, market licensed stem cell products under its infertility technology and multipotent amniotic fetalpatient’s own stem cells licenses, and to assess strategy partners for co-development of the technology to treat miscarriages.
Erectile Dysfunction Treatment
On February 3, 2016, CMT entered into a Patent Purchase Agreement with CMH pursuant to which CMH assigned to CMT its rights to US Patent No. 8,372,797, entitled “Treatment of Erectile Dysfunction by Stem Cell Therapy” which was issued to CMH by the USPTO on February 12, 2013, and related know-how and technology.erective dysfunction (ED). The closing of the Patent Purchase Agreement occurred in May 2016, and we issued the 64,666,667 shares of CMT’s common stock to CMH.
While previous studies have demonstrated that stem cells can enhance blood vessel function, this patent application was the first to demonstrate that administration of stem cells can lead to enhanced erections. Aspects of this patent have already been clinically used. In one specific example, FDA approved bone marrow extraction devices used to concentrate bone marrow and to inject stem cells into the penile bodies. This procedure has been demonstrated safe and feasibleeffective in small patient studiesclinical trials, and in a single case study performed by Dr. Ichim, one of our directors in 2013. Although management believesis geared to the results of this limited study were positive, there is no indication that such results will be achievedestimated nine million men in the Company’s current clinical trial orUnited States that suffer from ED and do not respond to PDE5 inhibitors in later trials.drugs such as Viagra and Cialis due to damage to smooth muscle and blood vessels.
Prior toManagement has determined that CaverStem® is exempt from the closingFDA premarket review and approval process under Section 361 of the Patent Purchase Agreement, CMH was inPublic Health Service Act (or PHS Act”), as the processprocedure involves the autologous treatment of commencing clinical trial studies ona patient with his own cells during the efficacy and safety ofsame surgical procedure without intervening processing steps beyond rinsing or cleansing the ED stem cell treatment. As a result of the purchase of the ED patent by us, we will now take over the clinical trial studies. After the trial is completed, and if the data supports the treatment, we intend to market the technology with complete kits and training to medical doctors, who can practice the treatment for their patients.extracted cells.
We entered into a Clinical Trial Agreement dated September 19, 2016, with LABIOMED and conducted by Dr. Jacob Rajfer with UCLA Harbor Hospital as its principal investigator. An IRB (Institutional Review Board) application has been submitted and approved. The purpose of the clinical trials is to evaluate the safety and efficacy of the ED stem cell treatment. Enrollment in the clinical trials began in December 2015 and the clinical trial began during first quarter 2016 with approximately 40 participants. On October 2015 CMH entered into a Master Services Agreement dated November 15, 2015, with Professional Research Consulting, Inc., doing business as PRC Clinical, a contract research organization, to oversee the clinical trials. We estimate that the study will take approximately 15 months to complete. Under the terms of the Patent Purchase Agreement, CMH assigned these agreements to us and we have assumed the duties and obligations under these agreements.
Procedures for use of our EDOur CaverStem® stem cell treatment consistconsists of a one-hour out-patient visit in a physician’s office. The physician would harvestharvests a patient’s stem cells from bone marrow fromin the hip using a local anesthetic and separateanesthetic. The extraction device is designed to harvest only the stem cells, using a cell separator.while filtering out the red blood cells, thereby eliminating the need for any centrifugation. The separated and cleansed stem cells wouldare then be injected into the patient’s venus cavernosacorpus cavernosum (erectile tissue) to stimulate muscle and generate blood vessel regeneration. Management believesClinical research data concludes that suchour CaverStem® treatment should resultresults in a marked increase in duration and frequency of erections and the ability to sustain erections until orgasm, with no known treatment-associated adverse events. The current
We generate revenues through the sale of disposable bone marrow aspiration kits to physicians who use the kits to perform the CaverStem® procedure. We contract with physicians to purchase kits and, in turn, provide exclusivity in their market through our patent protection, marketing support and training.
Our CaverStem® technology is protected by U.S. Patent No. 8,372,797, entitled “Treatment of Erectile Dysfunction by Stem Cell Therapy” which was issued to CMH by the United Stated Patent and Trademark Office (“USPTO”) on February 12, 2013. We acquired this patent and related know-how and technology from CMH in May 2016. CaverStem® is also a U.S. registered trademark (Reg. No. 5716528).
In August 2017, we completed recruitment on a clinical trial is beingof the CaverStem® procedure conducted to validateby the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Following completion of recruitment and treatment of the study subjects, an independent Institutional Review Board (IRB) overseeing the study validated the procedure as safe. In the same time frame, other worldwide, peer-reviewed and published clinical trials using the same procedure validated the efficacy and safety of the ED treatment. As a result of these two developments, management concluded the CaverStem® procedure is both safe and effective and commenced marketing activities in November of 2017. From November 2017, through September 2019, the data from 40 patients in the clinical trial was combined with data from a 100-patient clinical registry and analyzed. The results were then submitted to the Journal of Translational Medicine for peer-review and publication and subsequently published in its January 2020 edition. The peer-reviewed results validated 100% safety and 85% efficacy of the CaverStem® procedure. This marked the largest ever study of the safety and efficacy of bone marrow stem cells used to tread erectile dysfunction.
Male InfertilityWe plan to use a portion of the proceeds of this offering to expand our marketing and promotion of CaverStem® both to medical professional and the general public to generate increased CaverStem® sales.
FemCelz® - Female Sexual Function Treatment (Commercialized)
In September 2018, we launched our proprietary FemCelz® procedure for the treatment of the loss of genital sensitivity and dryness experienced by women. The FemCelz® procedure uses the patient’s own stem cells to improve female sexual function, and is similar to the CaverStem® procedure. Management has determined that FemCelz® is exempt from the FDA premarket review and approval process under Section 361 of the PHS Act, as the procedure involves the autologous treatment of a patient with her own cells during the same surgical procedure without intervening processing steps beyond rinsing or cleansing the extracted cells.
Our FemCelz® stem cell treatment consists of a one-hour out-patient visit in a physician’s office. The physician harvests a patient’s stem cells from bone marrow in the hip using a local anesthetic. The extraction device is designed to harvest only the stem cells, while filtering out the red blood cells, thereby eliminating the need for any centrifugation. The cells are then injected into the pubocervical fascia (peri-G-spot), skene’s glands, and around the peri-clitoral to stimulate muscle and blood vessel regeneration.
| 23 |
| Table of Contents |
We generate revenues through the sale of disposable bone marrow aspiration kits to physicians who use the kits to perform the FemCelz® procedure. We contract with physicians to purchase kits and, in turn, provide exclusivity in their market through our patent protection, marketing support and training.
FemCelz® is a U.S. registered trademark (Reg. No. 6107881).
StemSpine® - Regenerative Stem Cell Procedure for the Treatment of Degenerative Disc Disease (Clinical Trials)
Our StemSpine® procedure uses the patient’s own stem cells to reverse the effects of atherosclerosis and treat chronic lower back pain. A recent study reported that that an estimated 2.6 million patients in the U.S. will have suffered from degenerative disc disease in 2021, with the number increasing to close to four million patients in 2028.
Management has determined that StemSpine® is exempt from the FDA premarket review and approval process under Section 361 of the PHS Act, as the procedure involves the autologous treatment of a patient with his or her own cells during the same surgical procedure without intervening processing steps beyond rinsing or cleansing the extracted cells.
Our StemSpine® stem cell treatment consists of a one-hour out-patient visit in a physician’s office. The physician harvests a patient’s stem cells from bone marrow in the hip using a local anesthetic. The extraction device is designed to harvest only the stem cells, while filtering out the red blood cells, thereby eliminating the need for any centrifugation. The cells are then administered into muscles surrounding the area of lower back pain, such as the psoas major muscle to stimulate blood vessel regeneration. New blood vessels increase circulation around the disc and thus stimulates regeneration of the disc.
Lower back pain is the single leading cause of disability worldwide, affecting mobility, functionality and the emotional state. To date, treatment options have ranged from prescription medication, to physical therapy and even acupuncture. Unfortunately, in patients whose lower back pain originates from disc degeneration, existing approved treatments do not address the underlying cause, but only symptoms.
Recent U.S. clinical trials using stem cells administered directly into the disc have shown promise in regenerating injured discs, and by this means reducing pain in some patients. Companies such as Mesoblast Limited and BioRestorative Therapeutics have patient follow-ups as long as three years post injection and show some degree of pain reduction and disc regeneration without adverse effects.
A significant number of patients suffering from lower back pain have deficient circulation in the areas surrounding the discs, which is believed by some to be the initial cause of disc degeneration. Our StemSpine® technology utilizes biologicals to stimulate a process termed angiogenesis, which overcomes the deficient circulation causing disc degeneration.
In May 2017, we formed StemSpine, LLC for the purpose of using stem cells to treat back pain under a patent we acquired from CMH. In June 2017, we filed an additional patent application covering the synergy between intradiscal stem cell injection subsequent to stimulation of perispinal angiogenesis.
In October 2019 we announced the successful completion of a pilot study of 15 patients with over 12 months of data showing safety and efficacy. Evaluation of patients at 30, 60 90, 180, and 360 days revealed significant improvement in mobility and reduction in pain score. The mean pain score (on a scale of 1 to 10, with 10 being most severe), changed from 8.9 at baseline to 4.3 at 30 days, and sustained to 1.8 at 6 months and 1.3 at 12 months, with a gradual reduction in overall pain medication utilization guided by patients’ healthcare teams. No serious adverse effects were noted, with some short-term bruising in two patients at the harvest site. No long term adverse events were reported related to the procedure.
While we have not yet generated any revenues from our StemSpine® procedure, we have commenced physician recruitment in key markets to perform the procedure in a clinical trial of StemSpine® in an effort to demonstrate the efficacy of our procedure to the medical community and generate demand for this procedure. We intend to publish the results of this trial in a clinical registry similar to our data gathering efforts that we used to support our CaverStem® procedure. We will provide disposable kits at no cost to the physician or patient, and reimburse the physicians for their time and effort. In return, the physicians will track the results of the StemSpine® procedures and upload the data onto the clinical registry. We plan to commercialize StemSpine® once we have been able to publish data from the clinical registry supporting the efficacy of StemSpine®, although there can be no assurance that our trials will generate such data.
StemSpine® is a U.S. registered trademark (Reg. No. 5997521).
| 24 |
| Table of Contents |
ImmCelz™ - Universal Donor Stem Cell Therapy for Stroke Treatment (Pre-Clinical Trials)
We are developing our ImmCelz™ technology for the treatment of stroke patients. According to the National Institute of Health, stroke affects approximately 800,000 people in the United States annually alone. Very few treatments are available to stroke victims subsequent to initiation of brain damage.
ImmCelz™ utilizes a patient’s own extracted immune cells that are then “reprogrammed” by culturing them outside the patient’s body with optimized amniotic stem cells. The immune cells are then re-injected into the patient from whom they were extracted. We believe this process endows the immune cells with regenerative properties that may be suitable for the treatment of stroke victims. In contrast with other stem cell-based approaches, the immune cells are significantly smaller in size than stem cells and are believed to more effectively penetrate areas of the damaged tissues and induce regeneration.
Unlike our CaverStem®, FemCelz® and StemSpine® procedures, because the patient’s cells are cultured with amniotic cells prior to reinjection, we will require FDA approval before we can market or sell ImmCelz™. We filed an Investigational New Drug (IND) application (number 27375) with the FDA for a Phase I study of our ImmCelz™ technology to treat stroke in January 2021. The proposed study will involve treatment of four groups of eight patients per group. Treated patients will receive one intravenous injection of ImmCelz™ product at either 0.5 times 106 cells per kilogram; 1 times 106 cells per kilogram; or 2 times 106 cells per kilogram; with the fourth group serving as a control. Patients will be followed for a total of six months with both safety and efficacy being examined.
The particular immune cells we use for ImmCelz™ are “JadiCells™” which were developed by Amit Patel, one of our of our directors. Pursuant to a license agreement we entered into with Jadi Cell LLC, which is owned and controlled by Dr. Patel, we have been granted the right to exploit Jadi Cell’s patent rights (including U.S. Patent Number 9,803,176 and similar patents issued by other countries) and proprietary know how in connection with the enhancement of autologous cells.
We have filed an application with the USPTO to trademark ImmCelzTM (Ser. No. 88829362).
OvaStem™ - Stem Cell Therapy for Premature Ovarian Failure (Pre-Clinical Trials)
We are developing our OvaStem™ technology for the treatment of female infertility. Our treatment is intended to treat women suffering from infertility induced by factors such as chemotherapy and other non-natural causes, as well as age-associated infertility, and infertility with unexplained causes. In these cases, IVF treatment covers novel means for treating male infertility using stem cells. The methods claimed in this patent describe implantationmay not be appropriate as the woman’s ovaries are not able to generate eggs capable of being fertilized. Studies have shown that the introduction of stem cells into the testesdysfunctional ovaries induce fertility, reduce ovarian fibrosis, accelerate maturation of immature oocytes and restore growth factor production damaged by aging and cancer interventions. Accordingly, we believe that our OvaStem™ procedure may be a suitable treatment for these women with damaged ovaries.
The OvaStem™ stem cell treatment will consist of a mammal wherebyone-hour out-patient visit in a physician’s office. The physician will harvest a patient’s stem cells may serve to addressfrom bone marrow in the hip using a deficiency of germ cells (developmental precursors of sperm cells), Sertoli cells (somatic cells that aid in sperm development) and/or Leydig cells (testosterone-producing cells). This invention was based onlocal anesthetic. The extraction device will harvest only the discovery that bone marrow-derived stem cells, can differentiate into germwhile filtering out the red blood cells, Sertolithereby eliminating the need for any centrifugation. The cells and/or Leydig cells when transplantedwould then be administered into the testes of experimental animals.dysfunctional ovaries.
Like our CaverStem® and FemCelz® procedures, because OvaStem™ will utilize a patient’s own extracted immune cells, management has determined that OvaStem™ will be exempt from the FDA premarket review and approval process under Section 361 of the PHS Act, as the procedure involves the autologous treatment of a patient with her own cells during the same surgical procedure without intervening processing steps beyond rinsing or cleansing the extracted cells.
We believe the administration of stem cells using the methods describedBefore we can commercialize OvaStem™ we will need to conduct clinical studies that demonstrate that OvaStem™ is in this patent applicationfact effective in treating female infertility and restoring ovarian function. However, there can be usedno assurance as to improve conditions related to male infertility and/or testosterone deficiency caused by aging, disease or trauma, including individuals with cancer that have undergone irradiationthe outcome of the pelvic area or chemotherapy. such trials.
Other conditions involving abnormalities of the testes and/or low sperm counts could also be addressed using these methods. We believe there is great interest in leveraging the therapeutic potential of bone marrow-derived stem cell populations, especially to address male fertility issues. According to a study by the CDC, of an estimated 3.3-4.6 million men in the U.S. that sought medical advice for fertility issues, 18% were diagnosed with infertility, with deficiencies in sperm numbers of quality being the primary underlying causes of male infertility.Products and Services
Additional Indications
We are in the process of designing a clinical trial for the infertility treatment and intend to continue to prosecute the patent application.
Female Sexual Dysfunction Treatment
There are many types of female sexual arousal disorders, some of which manifest as mental obstacles to sex. CMT’s current patent application would focus on physical manifestations of sexual arousal disorder, as an extension of their work with stem cell therapies for ED. The technology specifically targets atherosclerotic tissue, which basically means tissue and vessels through which blood cannot pass. Sometimes, lack of blood flow to the vagina and clitoris can make sex painful or even impossible. Research has demonstrated that vaginal engorgement and clitoral erection are important facets of sexual interaction for women. The technique developed by CMT uses regenerative cells (such as stem cells) to help encourage the process. We plan to design a trial and execute that trial in the future after we pursue the male infertility procedure.
In addition, we recently acquired a license for patented use of amniotic fluid-derived stem cells which can be implemented in order to improve or repair the dysfunctional cellular pathways that contribute to disorders of sexual function and fertility.
Miscarriage Treatment
CMT scientists Drs. Amit Patel and Thomas Ichim recently filed a US Patent entitled “Adipose Derived Immunomodulatory Cells for Immunotherapy of Recurrent Spontaneous Abortions”, which coversalso exploring the use of a woman’s own fat derived stem cells for prevention of pregnancy loss. It is believed that more than 30% of all pregnancies result in a loss and that in many cases miscarriage is repetitive. Recurrent spontaneous abortion (miscarriage) is defined by the American Congress of Obstetricians and Gynecologists as two our regenerative technologies and/or more miscarriages in the first trimester. Approximately 1% to 5% of women of reproductive age suffer from recurrent spontaneous abortion.
The patent is based on animal data in which mice genetically bred to replicate human spontaneous recurrent miscarriages were shown to achieve much higher frequency of live births after administration of fat derived stem cells. Specifically, in mice prone to pregnancy failure that were treated with saline, an average of two living fetuses and six resorbed fetuses were detected per mouse. In mice receiving the fat derived stem cells, an average of six living fetus’s and one resorbed fetus were detected per mouse. The average litter size of mice is between six and eight offspring per pregnancy.
Current Operations
As a result of the closing of the Merger Agreement, we are now a company engaged in stem cell research and applications for use to treat male and female sexual dysfunction, infertility and related issues. We are a clinical stage company and intend first to complete the testing of the ED treatment and, if warranted, market the treatment under the name “Caverstem” to physicians for use with their patients suffering from ED. Following further testing, we also intend to market licensed products under our infertility technology license and the female sexual dysfunction patent application. We also intend to seek strategy partners for co-development of our miscarriage treatment technology.
Through this prospectus, certain selling stockholders are offering up to 23,732,669 shares of our common stock. Each of the selling stockholders acquired their shares in the closing of the Merger Agreement, which occurred on May 18, 2016.
Our principal executive offices are located at 2017 W Peoria Avenue, Phoenix, AZ 85029. Our telephone number is (602) 680-7439. Our website address is www.creativemedicaltechnology.com. Information contained on our website or connected thereto does not constitute a part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part.
The Offeringhave filed patents covering treatments for:
· | Preventing the rejection of | |
· | Kidney failure | |
· | Liver failure | |
· | Type 1 Diabetes | |
· | Heart attack | |
· | Parkinson’s Disease |
| Table of Contents | ||
Investment in our common stock has a high degree of risk. Before you invest you should carefully consider the risks and uncertainties described below and the other information in this prospectus. If any of the following risks actually occur, our business, operating results and financial condition could be harmed and the value of our common stock could go down. This means you could lose all or a part of your investment.
Risks Related to our Business and Industry
Because our auditors have issued a going concern opinion, there is substantial uncertainty we will continue operations in which case you could lose your investment.
In their report dated September 1, 2016, our independent registered public accounting firm, Haynie & Company, stated that our financial statements for the period ended December 31, 2015, were prepared assuming the company will continue as a going concern. This means that there is substantial doubt that we can continue as an ongoing business. For the period from inception (December 30, 2015) to September 30, 2016, we incurred a net loss of $572,106 and had generated no revenues. We will need to generate significant revenue in order to achieve consistent profitability and we may never become profitable. We estimate the company will require approximately $600,000 over the next twelve months to complete its ED clinical trials and additional funds for corporate operating expenses. The going concern paragraph in the independent auditor’s report emphasizes the uncertainty related to our business as well as the level of risk associated with an investment in our common stock. To implement our plan of operations we require further funding.
Our sole operating subsidiary has a limited operating history and has not yet generated any revenues.
CMT’s limited operating history in the ED and other treatment fields, makes evaluating our business and future prospects difficult. CMT was incorporated on December 30, 2015, and organized in February 2016. We do not anticipate generating income from CMT’s operations until at least 2017, and only then if the continued testing of our ED treatment proves successful. To date we have generated no revenues. We intend in the longer term to derive revenues from the sales of our ED treatment and other urological treatments, such as in the field of female sexual dysfunction and infertility, and our technology for treatment of miscarriages. Development of our treatments for ED and other functions will require significant investment prior to commercial introduction, and we may never be able to successfully develop or commercialize the ED or any other treatments.
We will require additional funding to develop and commercialize our ED and other prospective stem cell product candidates.If we are unable to secure additional financing on acceptable terms, or at all, we may be forced to modify our current business plan or to curtail or cease our planned operations.
We anticipate incurring significant operating losses and using significant funds for planned research and operating activities. Our existing cash resources are insufficient to finance even our immediate operations. Accordingly, we will need to secure additional sources of capital to develop our business and product candidates, as planned. We intend to seek substantial additional financing through public and/or private financing, which may include equity and/or debt financings, research grants and through other arrangements, including collaborative arrangements. As part of such efforts, we may seek loans from certain of our executive officers, directors and/or current shareholders.
If we are unable to secure additional financing in the near term, we may be forced to:
If we are forced to take any of these steps our common stock may be worthless.
Any future financing may result in ownership dilution to our existing shareholders and may grant rights to investors more favorable than the rights currently held by our shareholders.
If we raise additional capital by issuing equity, equity-related or convertible securities, the economic, voting and other rights of our existing shareholders may be diluted, and those newly-issued securities may be issued at prices that are at a significant discount to current and/or then prevailing market prices. In addition, any such newly issued securities may have rights superior to those of our common stock. If we obtain additional capital through collaborative arrangements, we may be required to relinquish greater rights to our technologies or product candidates than we might otherwise have or become subject to restrictive covenants that may affect our business.
Each of our current product candidates is in an early stage of development and we may never succeed in developing and/or commercializing them. We depend heavily on the success of our ED product candidate, which is still in clinical trials. If we are unable to commercialize our ED treatment, our infertility treatment, or any future urological product or other candidates, or if we experience significant delays in doing so, our business may fail.Virtual Physician™
We intend to investlaunch Virtual Physician™ as a significanttelehealth platform and Web site to educate, recruit and refer patients for our regenerative procedures in partnership with our national network of medical professionals that perform our regenerative procedures. As we commercialize additional products and procedures, Virtual Physician™ would be expanded to support them. In addition, to reach a wider audience, the platform would also support third-party regenerative products and procedures outside of our proprietary procedures.
Our business model for Virtual Physician™ includes:
· | Acting as a Management Service Organization. We would provide administrative services to health care providers and charge a fee per qualified patient to the providers. | |
· | Royalty for promotion of products from external partners. As the platform grows in popularity, we would include advertising on the Virtual Physician™ Web site featuring third-party products and services. | |
· | Direct sales of ancillary products: Virtual Physician™ would allow our patients to purchase specially curated ancillary medical products targeted to their underlying medical conditions. |
Marketing
We market our CaverStem® and FemCelzTM procedures using a multifaceted marketing approach that includes:
· | A robust web site for each of these products (CaverStem.com and FemCelz.com) designed to attract and educate both physicians and patients | |
· | Publishing results of clinical studies demonstrating the efficacy of our products and therapies, and building clinical registries accessible to medical professionals that include the results of such studies | |
· | Online advertising | |
· | Social Media – Twitter, Facebook | |
· | In-office flyers and banners | |
· | Patient testimonials | |
· | Informative videos |
The first product we marketed is the CaverStem® procedure to treat erectile dysfunction, which was initiated in November 2017. We subsequently initiated marketing of FemCelz® in March 2019. The two procedures are now offered in the following eight markets:
• | Arkansas – Martinsburg | |
• | California |
o | La Quinta | |
o | Oakland | |
o | Tarzana |
• | Hawaii – Honolulu | |
• | Texas |
o | Austin | |
o | San Antonio |
• | West Virginia – Fayetteville |
To-date, we have recruited physicians by partnering with independent sales representatives who represent the Company to physicians across the United States and Europe. Going forward, management plans to continue to partner with independent sales representatives.
| 26 |
| Table of Contents |
Intellectual Property
We have developed and acquired a robust intellectual property portfolio related to the utilization of stem cells to improve patient lives in the areas of urology, neurology and orthopedics. Our patent portfolio is currently composed of four issued patents and 10 pending patent applications filed in the United States with the USPTO as follows
Title |
| Application Number |
| Application Filing Date |
| Patent Type |
| Patent Number |
|
Treatment of Erectile Dysfunction by Stem Cell Therapy |
| 12305589 |
| 06/22/2007 |
| Utility |
| 8,372,797 |
|
Treatment Of Disc Degenerative Disease |
| 12301597 |
| 09/30/2009 |
| Utility |
| 9,598,673 |
|
Methods For Treatment Of Premature Ovarian Failure And Ovarian Aging Using Regenerative Cells |
| 15652213 |
| 07/17/2017 |
| Utility |
| 10,792,310 |
|
Perispinal Perfusion by Administration of T regulatory Cells Alone or in Combination with Angiogenic Cell Therapies |
| 16009982 |
| 06/15/2018 |
| Utility |
| 10,842,815 |
|
Inducing and Accelerating Post-Stroke Recovery by Administration of Amniotic Fluid Derived Stem Cells |
| 15702735 |
| 09/12/2017 |
| Utility |
|
|
|
Generation of Autologous Immune Mcratiodulatory Cells for Treatment of Neurological Conditions |
| 15987739 |
| 05/23/2018 |
| Utility |
|
|
|
Extracorporeal Shock Wave Ultrasound For Enhancement Of Regenerative Activities In Erectile Dysfunction |
| 16799656 |
| 02/24/2020 |
| Utility |
|
|
|
Induction of Infectious Tolerance by Ex Vivo Reprogrammed Immune Cells |
| 63123380 |
| 12/09/2020 |
| Provisional |
|
|
|
Treatment of Kidney Failure using Ex Vivo Reprogrammed Immune Cells |
| 63141555 |
| 01/26/2021 |
| Provisional |
|
|
|
Treatment of Liver Failure by Ex Vivo Reprogrammed Immune Cells |
| 63131261 |
| 12/28/2020 |
| Provisional |
|
|
|
Treatment of Heart Failure and/or Post Infarct Pathological Remodeling by Ex Vivo Reprogrammed Immune Cells |
| 63132472 |
| 12/30/2020 |
| Provisional |
|
|
|
Treatment of Diabetes Using Immune Cells Reprogrammed Ex Vivo by Regenerative Cells. |
| 63138776 |
| 01/18/2021 |
| Provisional |
|
|
|
Prevention and/or Treatment of Type 1 Diabetes by Augmentation of Myeloid Suppressor Cell Activity |
| 63208249 |
| 06/08/2021 |
| Provisional |
|
|
|
Therapeutic Monocytic Lineage Cells |
| 63223245 |
| 07/19/2021 |
| Provisional |
|
|
|
Patent Purchase and License Agreements
Lower Back Pain Patent Purchase. We acquired U.S. Patent No. 9,598,673 covering the use of various stem cells for the treatment of lower back pain from our affiliate CMH pursuant to a Patent Purchase Agreement dated May 17, 2017, which was amended in November 2017. The inventors of the patent were Thomas Ichim, PhD and Amit Patel, MD, directors of ours, and Annette Marleau, PhD. As amended, the Patent Purchase Agreement includes the following terms:
· | We were required to pay CMH $100,000 within 30 days of demand as an initial payment. | |
· | Upon the determination to pursue the technology via use of autologous cells, we were required to pay CMH: |
o | $100,000 upon the signing agreement with a university for the initiation of an IRB clinical trial. | |
o | $200,000, upon completion of the IRB clinical trial. | |
o | $300,000 in the event we commercialize the technology via use of autologous cells by a physician without a clinical trial. |
| 27 |
| Table of Contents |
· | In the event we determine to pursue the technology via use of allogenic cells, we are required to pay CMH: |
o | $100,000 upon filing an IND with the FDA. | |
o | $200,000 upon dosing of the first patient in a Phase 1-2 clinical trial. | |
o | $400,000 upon dosing the first patient in a Phase 3 clinical trial. |
· | Each payment may be made in cash or shares of our common at a discount of 30% to the recent trading price. | |
· | In the event our shares of common stock trade below $0.01 per share for two or more consecutive trading days, the number of any shares issuable as payment doubles. | |
· | For a period of five years from the date of the first sale of any product derived from the patent, we are required to make royalty payments of 5% from gross sales of products, and 50% of sale price or ongoing payments from third parties for licenses granted under the patent to third parties. |
We paid CMH the $100,000 obligation of the initial payment due under this agreement, by a $50,000 cash payment and the issuance of 6,667 shares of common stock on December 12, 2019. On December 31, 2019, following our announcement with respect to the clinical commercialization of the StemSpine technology, we paid CMH $50,000 of the $300,000 obligation due under this agreement through the issuance of 133 shares of common stock. On September 30, 2020 we paid CMH an additional $40,000 of the $300,000 obligation due under this agreement through the issuance of 84,656 shares of common stock, and in January 2021 we paid CMH an additional $50,000 of the $300,000 obligation due under this agreement through the issuance of 89,286 shares of common stock. The remaining portion of our effortsthe $300,000 obligation has been paid in cash.
ImmCelz™ License. On December 28, 2020, we entered into a patent license agreement with Jadi Cell, LLC, a company owned and financial resources in our ED treatment product candidatecontrolled by Dr. Amit Patel, a director of ours. The agreement provides us with an exclusive, worldwide license to U.S. Patent No. 9,803,176 “Methods and depend heavily on its success. This treatment is currentlycompositions for the clinical derivation of an allogenic cell and therapeutic uses” and the proprietary process of expanding the master cell bank of Jadi Cell LLC, in the clinical testing stagefield of development. enhancing autologous cells. The agreement includes the following terms:
· | We were required to pay an initial license fee of $250,000 either in cash or shares of our common stock at a discount of 25% of the closing price of our common stock on the date of the agreement. | |
· | Within thirty (30) days of the end of each calendar quarter, we are required to pay Jadi Cell five percent (5%) of the net income we generate from ImmCelz™ during such calendar quarter. | |
· | If we sell or dispose of the ImmCelz™ business, we will be required to pay Jadi Cell ten percent of the proceeds of the sale. | |
· | The agreement may only be terminated by Jadi Cell if we are in material breach of the agreement, in the event of our bankruptcy, if we cease to engage in the ImmCelz™ business or if we challenge the validity of the patent rights granted to us under the agreement. |
To date, we have not made any payments to Jadi Cell under this agreement, including the $250,000 initial license fee currently owing to Jadi Cell.
Trademarks
We need to devote significant additionalhave obtained trademark registration for CaverStem®, StemSpine® and FemCelz®, and have trademark applications pending for ImmCelzTM and OvaStemTM.
Competition
We compete with many pharmaceutical, biotechnology and medical device companies, as well as other private and public stem cell companies involved in the development and commercialization of cell-based medical technologies and therapies. Many of our competitors and potential competitors have substantially greater financial, technological, research and development, financial resourcesmarketing and personnel to develop additional commercially viable urological products, obtain regulatory approvals,resources than we do. We cannot forecast when or if necessary, and establish a sales and marketing infrastructure. Wethese companies are likely to encounter hurdlesbring their products and unexpected issues astherapies to market in competition with our products and therapies or those that we proceedare pursuing. Regenerative medicine is rapidly progressing, in large part through the development of our ED treatmentcell-based therapies or devices designed to isolate cells from human tissues. Most efforts involve cell sources, such as bone marrow, adipose tissue, embryonic and our other product candidates. There are many reasons that we may not succeed in our efforts to develop our product candidates, including the possibility that our product candidates will be deemed ineffective or unsafe; our product candidates will be too expensive to manufacture or market or will not achieve broad market acceptance; others will hold proprietary rights that will prevent us from marketing our product candidates; or our competitors will market products that are perceived as equivalent or superior.fetal tissue, umbilical cord and peripheral blood and skeletal muscle.
IfWhile there are a number of public and private companies that market and sell treatments for erectile dysfunction, our clinical trials on ourCaverStem® procedure is targeted at men who, due to damage to the blood vessels and smooth muscle tissue in the penis, do not respond to PDE5 inhibitors such as Viagra or Cialis. For these men, the only widely available treatment is invasive, non-reversible rod or pump implantation into the penis, or the painful injection into the penis of drugs containing alprostadil. Currently, we believe there are fewer than a dozen private clinics in the U.S. that offer autologous stem cell treatments are unsuccessfulfor erectile dysfunction. None of these firms is believed to have filed for patent protection or significantly delayed, or if we do not complete ourconducted clinical trials we may not be ableusing bone marrow to commercialize our product candidates.
Our ED treatment has been studied in a limited number of patients to date, but we have not yet completed our current large-scale trial to establish thevalidate safety and efficacy of our ED treatment. In addition,as we have not commenced clinical trials on our infertility treatment or our treatmenthave.
| 28 |
| Table of Contents |
Similarly, while there are many treatments available for female sexual dysfunction, we are not aware of any competitor for our FemCelz® procedure that uses or proposes to use autological stem cells to treat female sexual dysfunction, nor are we aware of any potential competitor for OvaStem™ that uses or proposes to use autological stem cells to treat women with damaged ovaries who do not respond to available medications.
Companies working in the area of regenerative medicine with regard to the disc and spine include, among others, Mesoblast, SpinalCyte, BioRestorative Therapies, DiscGenics and Isto Biologics. Companies working in the area of regenerative medicine to treat stroke victims include Athersys, Inc., among others.
Government Regulation
U.S. Government Regulation
The health care industry is highly regulated in the United States. The federal government, through various departments and agencies, state and local governments, and private third-party accreditation organizations, regulate and monitor the health care industry, associated products, and operations. The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, approval, manufacture, distribution and marketing of medical products, including drugs, biologics, and medical devices. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, post-approval monitoring, advertising, promotion, sampling and import and export of medical products. The following is a general overview of the laws and regulations pertaining to our business.
FDA Regulation of Stem Cell Treatment and Products
The FDA regulates the manufacture of human stem cell treatments and associated products under the authority of the Public Health Service Act (or PHS Act), and the Federal Food, Drug, and Cosmetic Act (or FDCA). Stem cells can be regulated under the FDA’s Human Cells, Tissues, and Cellular and Tissue-Based Products Regulations, referred to as HCT/Ps, or may also be subject to the FDA’s drug, biologic, or medical device regulations, each as discussed below.
Human Cells, Tissues, and Cellular and Tissue-Based Products Regulation
Under Section 361 of the PHS Act, the FDA issued specific regulations governing the use of HCT/Ps in humans. Pursuant to Part 1271 of Title 21 of the Code of Federal Regulations, or CFR, or the HCT/P Regulations, the FDA established a unified registration and listing system for establishments that manufacture and process HCT/Ps. The regulations also include provisions pertaining to donor eligibility determinations; current good tissue practices covering all stages of production, including harvesting, processing, manufacture, storage, labeling, packaging, and distribution; and other procedures to prevent the introduction, transmission, and spread of communicable diseases.
The HCT/P Regulations define HCT/Ps as articles “containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion or transfer into a human recipient.” The HCT/P Regulations strictly constrain the types of products that may be regulated solely as an HCT/P. Factors considered include the degree of manipulation, whether the product is intended for a homologous function, whether the product has been combined with noncellular or non-tissue components, and the product’s effect or dependence on the body’s metabolic function. In those instances where cells, tissues, and cellular and tissue-based products have been only minimally manipulated, are intended strictly for homologous use, have not been combined with noncellular or nontissue substances, and do not depend on or have any effect on the body’s metabolism, the manufacturer is only required to register with the FDA, submit a list of manufactured products, and adopt and implement procedures for the control of communicable diseases. If one or more of the above factors has been exceeded, the product would be regulated as a drug, biological product, or medical device rather than an HCT/P.
In addition, pursuant to the “Same Surgical Procedure Exception” under Section 1271.15(b) of the HCT/P Regulations, the FDA has provided guidance that exempts HCT/Ps from Section 361 of the PHS Act where autologous cells are removed from an individual and implanted into the same individual during a single surgical procedure without intervening processing steps beyond rinsing, cleansing, sizing, or shaping. The FDA’s rationale is that this type of procedure raise no additional risks beyond that typically associated with surgery. Management has determined that our CaverStem® and FemCelz® procedures and therapies are exempt from the FDA premarket review and approval process and other HCT/P Regulations under the Same Surgical Procedure Exception, and that our StemSpine® and OvaStem™ procedures and therapies will be similarly exempt. Conversely, because our ImmCelz™ therapy will treat a patient’s stem cells with Jadi Cells before being injected back into the patient, we will need to obtain FDA regulatory approval for ImmCelz™ in the same manner as a standard drug, as described below.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions including public warning letters, fines, consent decrees, orders of retention, recall or destruction of product, orders to cease manufacturing, and criminal prosecution. If any of these events were to occur, it could materially adversely affect us.
| 29 |
| Table of Contents |
Drug and Biological Product Regulation
An HCT/P product that does not secured a strategic partner to develop our miscarriage treatment. We may experience numerous unforeseen events during, ormeet the criteria for being solely regulated under Section 361 of the PHS Act will be regulated as a resultdrug, device or biological product under the FDCA and/or Section 351 of our currentthe PHS Act, and applicable FDA regulations. The FDA has broad regulatory authority over drugs and biologics marketed for sale in the United States. The FDA regulates the research, clinical testing, manufacturing, safety, effectiveness, labeling, storage, recordkeeping, promotion, distribution, and production of drugs and biological products. The FDA also regulates the export of drugs and biological products manufactured in the United States to international markets in certain situations.
The process required by the FDA before a drug or futurebiologic may be marketed in the United States generally involves the following:
· | completion of non-clinical laboratory tests, animal studies and formulation studies conducted according to Good Laboratory Practice (or GLP), or other applicable regulations; | |
· | submission of an IND, which allows clinical trials to begin unless the FDA objects within 30 days; | |
· | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug or biologic for its intended use or uses conducted in accordance with FDA regulations and Good Clinical Practices (or GCP), which are international ethical and scientific quality standards meant to ensure that the rights, safety and well-being of trial participants are protected and that the integrity of the data is maintained; | |
· | registration of clinical trials of FDA-regulated products and certain clinical trial information; | |
· | preparation and submission to the FDA of a new drug application (or NDA), in the case of a drug or biologics license application (or BLA) in the case of a biologic; | |
· | review of the product by an FDA advisory committee, where appropriate or if applicable; | |
· | satisfactory completion of pre-approval inspection of manufacturing facilities and clinical trial sites at which the product, or components thereof, are produced to assess compliance with Good Manufacturing Practice, or cGMP, requirements and of selected clinical trial sites to assess compliance with GCP requirements; and | |
· | FDA approval of an NDA or BLA which must occur before a drug or biologic can be marketed or sold. |
Approval of an NDA requires a showing that the drug is safe and effective for its intended use and that the methods, facilities, and controls used for the manufacturing, processing, and packaging of the drug are adequate to preserve its identity, strength, quality, and purity. To obtain a BLA, a manufacturer must show that the proposed product is safe, pure, and potent and that the facility in which the product is manufactured, processed, packed, or held meets established quality control standards.
For purposes of an NDA or BLA approval by the FDA, human clinical trials that couldare typically conducted in the following phases (which may overlap):
· | Phase 1: The investigational product is initially given to healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. These trials may also provide early evidence on effectiveness. During Phase 1 clinical trials, sufficient information about the investigational product’s pharmacokinetics and pharmacologic effects may be obtained to permit the design of well-controlled and scientifically valid Phase 2 clinical trials. | |
· | Phase 2: These clinical trials are conducted in a limited number of human subjects in the target population to identify possible adverse effects and safety risks, to determine the efficacy of the investigational product for specific targeted diseases and to determine dosage tolerance and dosage levels. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more costly Phase 3 clinical trials. | |
· | Phase 3: Phase 3 clinical trials are undertaken after Phase 2 clinical trials demonstrate that a dosage range of the investigational product appears effective and has a tolerable safety profile. The Phase 2 clinical trials must also provide sufficient information for the design of Phase 3 clinical trials. Phase 3 clinical trials are conducted to provide statistically significant evidence of clinical efficacy and to further test for safety risks in an expanded human subject population at multiple clinical trial sites. These clinical trials are intended to further evaluate dosage, effectiveness and safety, to establish the overall benefit-risk profile of the investigational product and to provide an adequate basis for product labeling and approval by the FDA. In most cases, the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of an investigational drug or biologic. |
| 30 |
| Table of Contents |
All clinical trials must be conducted in accordance with FDA regulations, GCP requirements and their protocols in order for the data to be considered reliable for regulatory purposes. Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. These government regulations may delay or prevent commercializationapproval of product candidates for a considerable period of time and impose costly procedures upon our business operations.
The FDA may require, or companies may pursue, additional clinical trials, referred to as Phase 4 clinical trials, after a product is approved. Such trials may be made a condition to be satisfied for continuing drug approval. The results of Phase 4 clinical trials can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA has authority to require sponsors to conduct post-marketing trials to specifically address safety issues identified by the agency.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, manufacturing processes or facilities, require submission and FDA approval of a new NDA or BLA, or an NDA or BLA supplement, before the change can be implemented. An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA and BLA supplements as it does in reviewing NDAs and BLAs.
Drug and biological products must also comply with applicable requirements, including monitoring and recordkeeping activities, manufacturing requirements, reporting to the applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling, or off-label use, limitations on industry-sponsored scientific and educational activities and requirements for promotional activities involving the internet. Although physicians may, in their independent professional medical judgment, prescribe legally available drugs for off-label uses, manufacturers typically may not market or promote such off-label uses. Modifications or enhancements to the product or its labeling, or changes of the site of manufacture, are often subject to the approval of the FDA and other regulators, who may or may not grant approval or may include a lengthy review process.
In the event that the FDA does not regulate our product candidates in the United States solely under the HCT/P regulation, our products and activities could be regulated as drug or biological products under the FDCA. If regulated as drug or biological products, we will need to expend significant resources to ensure regulatory compliance. If an IND and NDA or BLA are required for any of our product candidates, includingthere is no assurance as to whether or when we will receive FDA approval of the following:
Failuresproduct candidate. The process of designing, conducting, compiling and submitting the non-clinical and clinical studies required for NDA or perceived failures in our clinical trials would delayBLA approval is time-consuming, expensive and may prevent ourunpredictable. The process can take many years, depending on the product development and regulatory approval process, make it difficult for us to establish collaborations, negatively affect our reputation and competitive position and otherwise have a material adverse effect on our proposed business.the FDA’s requirements.
We dependIn addition, even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on third parties to assist ususe in the form of warnings, precautions or contraindications, or in the form of onerous risk management plans, restrictions on distribution or use, or post-marketing trial requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product, including safety labeling or imposition of a Risk Evaluation and Mitigation Strategy, or REMS, the requirement to conduct post-market studies or clinical trials or even complete withdrawal of the product from the market. Delay in obtaining, or failure to obtain, regulatory approval for our products, or obtaining approval but for significantly limited use, would harm our business. Further, we cannot predict what adverse governmental regulations may arise from future United States or foreign governmental action.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our preclinical studiesproducts, total or partial shutdown of our production, withdrawal of approvals, and clinical trials, andcriminal prosecutions. If any failure of those partiesthese events were to fulfill their obligations could result in costs and delays and prevent us from obtaining regulatory approval, if needed, or successfully commercializing our product candidates on a timely basis, if at all.
We may engage consultants and clinical research organizations (“CROs”) to help design, and to assist us in conducting, our preclinical studies and clinical trials and to collect and analyze data from those studies and trials. The consultants and contract research organizations we engage interact with clinical investigators to enroll patients in our clinical trials. As a result, we depend on these consultants and CROs to perform the studies and trials in accordance with the investigational plan and protocol for each product candidate and in compliance with regulations and standards, commonly referred to as “good clinical practice”, for conducting, recording and reporting results of clinical trials to assure that the data and results are credible and accurate and the trial participants are adequately protected. We may face delays in our commercialization efforts if these parties do not perform their obligations in a timely or competent fashion or if we are forced to change service providers. Any third parties that we hire to conduct clinical trials may also provide services to our competitors, which could compromise the performance of their obligations to us. If these third parties do not successfully carry out their duties or meet expected deadlines, or if the quality, completeness or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical trial protocols or for other reasons, our clinical trials may be extended, delayed or terminated or may otherwise prove to be unsuccessful. If there are delays or failures in clinical trials as a result of the failure to perform by third parties, our development costs will increase, and we may not be able to commercialize our product candidates. In addition, we may not be able to establish or maintain relationships with these third parties on favorable terms, if at all. If we need to enter into replacement arrangements because a third party is not performing in accordance with our expectations, we may not be able to do so without undue delays or considerable expenditures or at all.
The loss of or inability to retain key personneloccur, it could materially adversely affect our operations.us.
Our management includes a select group of experienced medical and technology professionals, particularly Dr. Patel and Dr. Ichim, who have been instrumental in developing the technology for the treatments currently held by us. The success of our operations will, in part, depend on the successful continued involvement of these individuals. If these individuals leave the employment of or engagement with us or CMH, then our ability to operate will be negatively impacted. There can be no assurance that we will be successful in retaining key personnel.
Risks Related to Our Intellectual Property
We hold certain intellectual property rights and intend to acquire additional intellectual property rights in the future. Our success will be dependent in large part on safeguarding our intellectual property rights and obtaining or retaining patent and other proprietary protection for our product candidates.
We have purchased the patent for our ED treatment and have a license to use the process for our infertility treatment and for amniotic fetal stem cells. We also hold patent applications for female sexual dysfunction and miscarriage treatments. The ED treatment is protected by a patent which expires on 2026. We hold a license for the infertility under a patent application made by the licensor and another license for multipotent amniotic fetal stem cells under an existing patent. Our two patent applications have not been granted. Our business plan is to acquire additional patent licensing rights in the urological field, or other rights which may not be protected by patents. Our commercial success will depend to a significant degree on our ability to:
| Table of |
Failure to obtain adequate patent protection for our product candidates, the failure of our licensors to protect our licensed patent rights, or the failure to protect our existing patent rights, may impair our ability to be competitive. The availability of infringing products in markets where we have patent protection, or the availability of competing products in markets where we do not have adequate patent protection, could erode the market for our product candidates, negatively impact the prices we can charge for our product candidates, and harm our reputation if infringing or competing products are manufactured to inferior standards.
Patents acquired by us may not be valid or enforceable, and may be challenged by third parties.
We cannot assure you that any patents issued or licensed to us would be held valid by a court or administrative body or that we would be able to successfully enforce our patents against infringers, including our competitors. The issuance of a patent is not conclusive as to its validity or enforceability, and the validity and enforceability of a patent is susceptible to challenge on numerous legal grounds. Challenges raised in patent infringement litigation brought by or against us may result in determinations that patents that have been issued or licensed to us or any patents that may be issued to us or our licensors in the future are invalid, unenforceable or otherwise subject to limitations. In the event of any such determinations, third parties may be able to use the discoveries or technologies claimed in these patents without paying licensing fees or royalties to us, which could significantly diminish the value of our intellectual property and our competitive advantage. Even if our patents are held to be enforceable, others may be able to design around our patents or develop products similar to our products that are not within the scope of any of our patents.
In addition, enforcing the patents that have been licensed to us and any patents that may be issued to us in the future against third parties may require significant expenditures regardless of the outcome of such efforts. Our inability to enforce our patents against infringers and competitors may impair our ability to be competitive and could have a material adverse effect on our business.
If we are not able to protect and control unpatented trade secrets, know-how and other technological innovation, we may suffer competitive harm.
We may also rely on unpatented technology, trade secrets, confidential information and proprietary know-how to protect our technology and maintain any future competitive position, especially when we do not believe that patent protection is appropriate or can be obtained. Trade secrets are difficult to protect. In order to protect proprietary technology and processes, we rely in part on confidentiality and intellectual property assignment agreements with our employees, consultants and others. These agreements generally provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of the individual’s relationship with us except in limited circumstances. These agreements generally also provide that we shall own all inventions conceived by the individual in the course of rendering services to us. These agreements may not effectively prevent disclosure of confidential information or result in the effective assignment to us of intellectual property, and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements. In addition, others may independently discover trade secrets and proprietary information that have been licensed to us or that we own, and in such case we could not assert any trade secret rights against such party.
Enforcing a claim that a party illegally obtained and is using trade secrets that have been licensed to us or that we own is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could have a material adverse effect on our business. Moreover, some of our academic institution licensors, collaborators and scientific advisors have rights to publish data and information to which we have rights. If we cannot maintain the confidentiality of our technologies and other confidential information in connection with our collaborations, our ability to protect our proprietary information or obtain patent protection in the future may be impaired, which could have a material adverse effect on our business.
Risks Related to Our Common StockFDA Expedited Review Programs
The beneficial ownership of our common stock is concentrated among existing executive officers and directors.
CMH, our executive officers, and our directors own beneficially, in the aggregate, approximately 78% of the outstanding common stock, including 64,996,667 shares (approximately 64%) owned by CMH. The outstanding shares of our common stock owned by CMH are voted by Mr. Warbington, the CEO of our company and CMH. As a result, these parties will be able to exercise a significant level of control over all matters requiring shareholder approval, including the election of directors, amendments to our Articles of Incorporation, and approval of significant corporate transactions. This control could have the effect of delaying or preventing a change of control or changes in management and will make the approval of certain transactions difficult or impossible without the support of these shareholders.
The public trading market for our common stock is volatile and will likely result in higher spreads in stock prices.
Our common stock is trading in the over-the-counter market and is quoted on the OTCQB. The over-the-counter market for securities has historically experienced extreme price and volume fluctuations during certain periods. These broad market fluctuations and other factors, such as our ability to implement our business plan, as well as economic conditions and quarterly variations in our results of operations, may adversely affect the market price of our common stock. In addition, the spreads on stock traded through the over-the-counter market are generally unregulated and higher than on stock exchanges, which means that the difference between the price at which shares could be purchased by investors on the over-the-counter market compared to the price at which they could be subsequently sold would be greater than on these exchanges. Significant spreads between the bid and asked prices of the stock could continue during any period in which a sufficient volume of trading is unavailable or if the stock is quoted by an insignificant number of market makers. We cannot insure that our trading volume will be sufficient to significantly reduce this spread, or that we will have sufficient market makers to affect this spread. These higher spreads could adversely affect investors who purchase the shares at the higher price at which the shares are sold, but subsequently sell the shares at the lower bid prices quoted by the brokers. Unless the bid price for the stock increases and exceeds the price paid for the shares by the investor, plus brokerage commissions or charges, shareholders could lose money on the sale. For higher spreads such as those on over-the-counter stocks, this is likely a much greater percentage of the price of the stock than for exchange listed stocks. There is no assurance that at the time the shareholder wishes to sell the shares, the bid price will have sufficiently increased to create a profit on the sale.
Because our shares are designated as “penny stock”, broker-dealers will be less likely to trade in our stock due to, among other items, the requirements for broker-dealers to disclose to investors the risks inherent in penny stocks and to make a determination that the investment is suitable for the purchaser.
Our shares are designated as “penny stock” as defined in Rule 3a51-1 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and thus may be more illiquid than shares not designated as penny stock. The SEC has adopted rules which regulate broker-dealer practices in connection with transactions in “penny stocks.” Penny stocks are defined generally as: non-Nasdaq equity securities with a price of less than $5.00 per share; not traded on a “recognized” national exchange; or in issuers with net tangible assets less than $2,000,000, if the issuer has been in continuous operation for at least three years, or $10,000,000, if in continuous operation for less than three years, or with average revenues of less than $6,000,000 for the last three years. The penny stock rules require a broker-dealer to deliver a standardized risk disclosure document prepared by the SEC, to provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, monthly account statements showing the market value of each penny stock held in the customer’s account, to make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a stock that is subject to the penny stock rules. Since our securities are subject to the penny stock rules, investors in the shares may find it more difficult to sell their shares. Many brokers have decided not to trade in penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. The reduction in the number of available market makers and other broker-dealers willing to trade in penny stocks may limit the ability of purchasers in this offering to sell their stock in any secondary market. These penny stock regulations, and the restrictions imposed on the resale of penny stocks by these regulations, could adversely affect our stock price.
Our Board of Directors can, without shareholder approval, cause preferred stock to be issued on terms that adversely affect common shareholders.
Under our Articles of Incorporation, our board of directorsFDA is authorized to issue upexpedite the review of NDAs and BLAs in several ways. Under the Fast Track program, the sponsor of a drug or biologic product candidate may request the FDA to 10,000,000 shares of preferred stock, none of which are issued and outstanding as ofdesignate the date of this report. Also, our board of directors, without shareholder approval, may determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares. If our board of directors causes any shares of preferred stock to be issued, the rights of the holders of our common stock could be adversely affected. Our board of directors’ ability to determine the terms of preferred stock and to cause its issuance, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of making it more difficultproduct for a third party to acquire a majority of our outstanding voting stock. Preferred shares issued by our board of directors could include voting rights, or even super voting rights, which could shift the ability to control our company to the holders of our preferred stock. Preferred shares could also have conversion rights into shares of our common stock at a discount to the market price of the common stock which could negatively affect the market for our common stock. In addition, preferred shares would have preference in the event of our liquidation, which means that the holders of preferred shares would be entitled to receive the net assets of our company distributed in liquidation before the common stock holders receive any distribution of the liquidated assets.
We have not paid, and do not intend to pay in the near future, dividends on our common shares and therefore, unless our common stock appreciates in value, our shareholders may not benefit from holding our common stock.
We have not paid any cash dividends since inception. Although we anticipate allocating funds for payment of dividends from future earnings, if any, we do not anticipate this occurring until we complete testing of our ED treatment and generating earnings from marketing of the ED treatment, of which there is no assurance. Therefore, any return on the investment made in our shares of common stock will likely be dependent initially upon the shareholder’s ability to sell our common shares in the open market, if one should develop, at prices in excess of the amount paid for our common shares and broker commissions on the sales.
Because we became public by means of a reverse merger, we may not be able to attract the attention of brokerage firms.
Additional risks may exist because we became public through a “reverse merger.” Securities analysts of brokerage firms may not provide coverage of our company since there is little incentive for brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct secondary offerings on our behalf in the future.
Shares of our common stock that have not been registered under federal securities laws are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a former “shell company.”
Prior to the closing of the Merger Agreement, we were deemed a “shell company” under applicable SEC rules and regulations because we had no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets. Pursuant to Rule 144 promulgated under the Securities Act sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which Form 10-type information reflecting our statusspecific indication as a non-shell company, is filedFast Track product concurrent with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Form 8-K reports. Without registration under the Securities Act, our shareholders will be forced to hold their shares of our common stock for at least that 12-month period after the filing of the report on Form 8-K followingIND. Drug and biologic products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the closingpotential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product candidate and the specific indication for which it is being studied.
In addition to other benefits, such as the ability to have greater interactions with the FDA, the FDA may initiate review of sections of a Fast Track NDA or BLA before the application is complete, a process known as rolling review.
Any product submitted to the FDA for marketing, including under a Fast Track program, may also be eligible for the following other types of FDA programs intended to expedite development and review:
· | Breakthrough therapy designation. To qualify for the breakthrough therapy program, product candidates must be intended to treat a serious or life-threatening disease or condition, and preliminary clinical evidence must indicate that such product candidates may demonstrate substantial improvement on one or more clinically significant endpoints over existing therapies. The FDA will seek to ensure the sponsor of a breakthrough therapy product candidate receives intensive guidance on an efficient drug development program, intensive involvement of senior managers and experienced staff on a proactive, collaborative and cross-disciplinary review, and rolling review. | |
· | Priority review. A product candidate is eligible for priority review if it treats a serious condition and, if approved, it would be a significant improvement in the safety or effectiveness of the treatment, diagnosis or prevention of a serious condition compared to marketed products. The FDA aims to complete its review of priority review applications within six months as opposed to ten months for standard review. | |
· | Accelerated approval. Drug or biologic products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval. Accelerated approval means that a product candidate may be approved on the basis of adequate and well-controlled clinical trials establishing that the product candidate has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity and prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug or biologic product candidate receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials. |
Fast Track designation, breakthrough therapy designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Further, with the passage of the 21st Century Cures Act, or the Cures Act, in December 2016, Congress authorized the FDA to accelerate review and approval of products designated as regenerative advanced therapies. A product is eligible for this designation if it is a regenerative medicine advanced therapy, or RMAT (which may include a cell therapy), that is intended to treat, modify, reverse merger beforeor cure a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such disease or condition. The benefits of a RMAT designation include early interactions with the FDA to expedite development and review, benefits available to breakthrough therapies, potential eligibility for priority review and accelerated approval based on surrogate or intermediate endpoints.
Medical Device Regulation
The FDA also has broad authority over the regulation of medical devices marketed for sale in the United States. The FDA regulates the research, clinical testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, promotion, distribution, and production of medical devices. The FDA also regulates the export of medical devices manufactured in the United States to international markets.
Under the FDCA, medical devices are classified into one of three classes, Class I, Class II, or Class III, depending upon the degree of risk associated with the medical device and the extent of control needed to ensure safety and effectiveness. Class I devices are subject to the lowest degree of regulatory scrutiny because they are eligibleconsidered low risk devices and need only comply with the FDA’s General Controls. The General Controls include compliance with the registration, listing, adverse event reporting requirements, and applicable portions of the Quality System Regulation as well as the general misbranding and adulteration prohibitions.
| 32 |
| Table of Contents |
Class II devices are subject to sell those shares pursuantthe General Controls as well as certain Special Controls such as 510(k) premarket notification. Class III devices are subject to Rule 144,the highest degree of regulatory scrutiny and even aftertypically include life supporting and life sustaining devices and implants. They are subject to the General Controls and Special Controls that 12-month period, salesinclude a premarket approval application, or PMA. “New” devices are automatically regulated as Class III devices unless they are shown to be low risk, in which case they may be subject to de novo review to be moved to Class I or Class II. Clinical research of an investigational device is subject to the FDA’s Investigational Device Exemption, or IDE, regulations. Nonsignificant risk devices are subject to abbreviated requirements that do not be made under Rule 144 unless we are in compliancerequire a submission to the FDA but must have Institutional Review Board (IRB) approval and comply with other requirements pertaining to informed consent, labeling, recordkeeping, reporting, and monitoring. Significant risk devices require the submission of Rule 144. Further,an IDE application to the FDA and the FDA’s approval of the IDE application.
The FDA premarket clearance and approval process can be lengthy, expensive and uncertain. It generally takes three to twelve months from submission to obtain 510(k) premarket clearance, although it will bemay take longer. Approval of a PMA could take one to four years, or more, difficultfrom the time the application is submitted and there is no guarantee of ultimate clearance or approval. Securing FDA clearances and approvals may require the submission of extensive clinical data and supporting information to the FDA. Additionally, the FDA actively enforces regulations prohibiting marketing and promotion of devices for us to raise funding to support our operationsindications or uses that have not been cleared or approved by the FDA. In addition, modifications or enhancements of products that could affect the safety or effectiveness or effect a major change in the intended use of a device that was either cleared through the sale of debt510(k) process or equity securities unless we agree to register such securities underapproved through the Securities Act, which could cause us to expend significant time and cash resources. The lack of liquidity of our securities as a result of the inability to sell under Rule 144 for a longer period of time than a non-former shell company could negatively affect the market price of our securities.
We are an “emerging growth company,” and will be able take advantage of reduced disclosure requirements applicable to “emerging growth companies,” which could make our common stock less attractive to investors.PMA process may require further FDA review through new 510(k) or PMA submissions.
We are an “emerging growth company,”In the event we develop processes, products or services which qualify as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and, for as long as we continuemedical devices subject to be an “emerging growth company,”FDA regulation, we intend to take advantagecomply with such regulations. If the FDA determines that our products are regulated as medical devices and we have failed to comply with applicable regulatory requirements, it can impose a variety of certain exemptionsenforcement actions from various reportingpublic warning letters, application integrity proceedings, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our products, total or partial shutdown of our production, withdrawal of approvals, and criminal prosecutions. If any of these events were to occur, it could materially adversely affect us.
Current Good Manufacturing Practices and other FDA Regulations of Cellular Therapy Products
Products that fall outside of the HCT/P regulations and are regulated as drugs, biological products, or devices must comply with applicable cGMP regulations. These cGMPs and related quality standards are designed to ensure the products that are processed at a facility meet the FDA’s applicable requirements applicablefor identity, strength, quality, sterility, purity, and safety. In the event that our domestic United States operations are subject to other public companies but not to “emerging growth companies,” including, but not limited to, not being requiredthe FDA’s drug, biological product, or device regulations, we intend to comply with the auditor attestationapplicable cGMPs and quality regulations.
If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of Section 404enforcement actions from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure of our products, total or partial shutdown of our production, withdrawal of approvals, and criminal prosecutions. If any of these events were to occur, it could materially adversely affect us.
Health Insurance Portability and Accountability Act—Protection of Patient Health Information
We may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The Health Insurance Portability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, including the Final Omnibus Rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information on certain types of individuals and organizations. In addition, certain state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other and from HIPAA in significant ways and may not have the same effect, thus complicating compliance efforts. Further, we may need to also comply with additional federal or state privacy laws and regulations that may apply to certain diagnoses, such as HIV/AIDS, to the extent that they apply to us.
The Department of Health and Human Services, or HHS, through its Office for Civil Rights, investigates breach reports and determines whether administrative or technical modifications are required and whether civil or criminal sanctions should be imposed. Companies failing to comply with HIPAA and the implementing regulations may also be subject to civil money penalties or in the case of knowing violations, potential criminal penalties, including monetary fines, imprisonment, or both. In some cases, the State Attorneys General may seek enforcement and appropriate sanctions in federal court.
| 33 |
| Table of Contents |
Other Applicable U.S. Laws
In addition to the above-described regulation by United States federal and state government, the following are other federal and state laws and regulations that could directly or indirectly affect our ability to operate the business:
• | state and local licensure, registration, and regulation of the development of pharmaceuticals and biologics; | |
• | state and local licensure of medical professionals; | |
• | state statutes and regulations related to the corporate practice of medicine; | |
• | other laws and regulations administered by the FDA; | |
• | other laws and regulations administered by HHS; | |
• | state and local laws and regulations governing human subject research and clinical trials; | |
• | the federal physician self-referral prohibition, also known as Stark Law, and any state equivalents to Stark Law; | |
• | the federal False Claims Act, or FCA; | |
• | the federal Anti-Kickback Statute, or AKS, and any state equivalent statutes and regulations; | |
• | federal and state coverage and reimbursement laws and regulations; | |
• | state and local laws and regulations for the disposal and handling of medical waste and biohazardous material; | |
• | Occupational Safety and Health Administration, or OSHA, regulations and requirements; | |
• | the Physician Payments Sunshine Act (in the event that our products are classified as drugs, biologics, devices or medical supplies and are reimbursed by Medicare, Medicaid or the Children’s Health Insurance Program); | |
• | state and other federal laws addressing the privacy of health information; and | |
• | state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare professionals and other potential referral sources, state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare professionals or marketing expenditures, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Violation of any of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensationlaws described above or any other governmental laws and regulations may result in our periodic reportspenalties, including civil and proxy statements,criminal penalties, damages, fines, the curtailment or restructuring of operations, the exclusion from participation in federal and exemptions fromstate healthcare programs and imprisonment. Furthermore, efforts to ensure that business activities and business arrangements comply with applicable healthcare laws and regulations can be costly for manufacturers of branded prescription products.
Foreign Government Regulation
In general, we will need to comply with the requirementsgovernment regulations of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years, or until the earliest of (i) the last day of the first fiscal yeareach individual country in which our annual gross revenues exceed $1 billion, (ii)products are to be distributed and sold. These regulations vary in complexity and can be as stringent, and on occasion even more stringent, than FDA regulations in the dateUnited States. Due to the fact that there are new and emerging cell therapy regulations that have recently been drafted and/or implemented in various countries around the world, the application and subsequent implementation of these new and emerging regulations have little to no precedence. Therefore, the level of complexity and stringency is not always precisely understood for each country, creating greater uncertainty for the international regulatory process. Furthermore, government regulations can change with little to no notice and may result in up-regulation of our product(s), thereby creating a greater regulatory burden for our cell processing technology products. We have not yet thoroughly explored the applicable laws and regulations that we will need to comply with in foreign jurisdictions. It is possible that we may not be permitted to expand our business into one or more foreign jurisdictions.
Employees
We currently employ three people on a full-time basis, and one consultant that is engaged on a part-time basis. None of our employees belong to a union. We believe relations with our employees are good.
Description Of Property
We do not currently own any real property. Our corporate office is located at 211 East Osborn Road, Phoenix, Arizona, which we lease on month-to-month basis. Management believes that this space is adequate to meet our current and foreseeable needs.
Legal Proceedings
From time to time, we may become a “large accelerated filer” as definedinvolved in Rule 12b-2various lawsuits and legal proceedings, which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
In April 2016, we entered into an agreement with Spencer Clarke, LLC to perform banking services. We believe that no banking services were provided under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractiveagreement, and as a result we terminated the agreement in June 2016. Spencer Clarke, LLC subsequently commenced an arbitration proceeding against us asserting that it was entitled to fees under the agreement. Thereafter, in April 2019, Spencer Clarke, LLC was awarded $600,749 by the Commercial Arbitration Tribunal of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile.the American Arbitration Association in the Matter of Arbitration, Case Number 01-18-0003-3441.
| 34 |
| Table of Contents |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This prospectus contains not only statements that are historical facts, but also statements that are forward-looking. Forward-looking statements are, by their very nature, uncertain and risky. Forward-looking statements can be identified by the use of forward-looking terminology such as the words “believes,” “intends,” “may,” “should,” “anticipates,” “expects,” “could,” “plans,” or comparable terminology. These risks and uncertainties include the following:
Although the forward-looking statements in this prospectus reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by them. Consequently, and because forward-looking statements are inherently subject to risks and uncertainties, the actual results and outcomes may differ materially from the results and outcomes discussed in the forward-looking statements. You are urged to carefully review and consider the various disclosures made by us in this prospectus as we attempt to advise interested parties of the risks and factors that may affect our business, financial condition, and results of operations and prospects.
We will not receive any proceeds from the sale of the common stock by the selling stockholders.
Market Information
Our common stock is quoted on the OTCQB under the symbol “CELZ.” The table below sets forth for the periods indicated the quarterly high and low bid prices as reported by OTC Markets. Limited trading volume has occurred during these periods. These quotations reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not necessarily represent actual transactions.
| Quarter | High | Low | ||||||||
| FISCAL YEAR ENDING DECEMBER 31, 2016 | First | $ | 0.02 | $ | 0.02 | |||||
| Second | $ | 0.40 | $ | 0.01 | ||||||
| Third | $ | 0.51 | $ | 0.33 | ||||||
| Quarter | High | Low | ||||||||
| FISCAL YEAR ENDED DECEMBER 31, 2015 | First | $ | 0.05 | $ | 0.05 | |||||
| Second | $ | 0.05 | $ | 0.01 | ||||||
| Third | $ | 0.01 | $ | 0.01 | ||||||
| Fourth | $ | 0.55 | $ | 0.01 | ||||||
| Quarter | High | Low | ||||||||
| FISCAL YEAR ENDED DECEMBER 31, 2014 | First | $ | 0.02 | $ | 0.02 | |||||
| Second | $ | 0.055 | $ | 0.02 | ||||||
| Third | $ | 0.055 | $ | 0.05 | ||||||
| Fourth | $ | 0.05 | $ | 0.05 | ||||||
Our common stock is considered to be penny stock under rules promulgated by the Securities and Exchange Commission (the “SEC”). Under these rules, broker-dealers participating in transactions in these securities must first deliver a risk disclosure document which describes risks associated with these stocks, broker-dealers’ duties, customers’ rights and remedies, market and other information, and make suitability determinations approving the customers for these stock transactions based on financial situation, investment experience and objectives. Broker-dealers must also disclose these restrictions in writing, provide monthly account statements to customers, and obtain specific written consent of each customer. With these restrictions, the likely effect of designation as a penny stock is to decrease the willingness of broker-dealers to make a market for the stock, to decrease the liquidity of the stock and increase the transaction cost of sales and purchases of these stocks compared to other securities.
We have granted registration rights only to the selling stockholders herein. We have not proposed to publicly offer any shares of our common stock in a primary offering.
Availability of Rule 144
We were a shell company prior to the filing of our report on Form 8-K on May 19, 2016. Rule 144 is not available for the resale of securities issued by companies that are, or previously were, shell companies, such as our company. Paragraph (i) of Rule 144 prohibits the use of the rule for resale of securities issued by any shell companies (other than business combination related shell companies) or any issuer that has been at any time previously a shell company, except where the following conditions are met:
As a result, our shareholders will not be able to sell their shares of our common stock under Rule 144 prior to the one year anniversary of our filing of our Current Report on Form 8-K on May 19, 2016.
Holders
As of the close of business on November 17, 2016, we had approximately 71 holders of our common stock. The number of record holders was determined from the records of our transfer agent and does not include beneficial owners of common stock whose shares are held in the names of various security brokers, dealers, and registered clearing agencies. We have appointed VStock Transfer, LLC, 18 Lafayette Pl, Woodmere, NY 11598, to act as transfer agent for the common stock.
Dividends
We have not declared or paid any cash dividends on our common stock during the two fiscal years ended December 31, 2015, or in any subsequent period. We do not anticipate or contemplate paying dividends on our common stock until we complete testing of our ED treatment and generating earnings from marketing of the ED treatment, of which there is no assurance. The only restrictions that limit the ability to pay dividends on common equity, or that are likely to do so in the future, are those restrictions imposed by law.
Securities Authorized for Issuance under Equity Compensation Plans
As of December 31, 2015, we had not adopted any compensation plans (including individual compensation arrangements) under which our equity securities were authorized for issuance.
2016 Stock Incentive Plan
Effective May 18, 2016, pursuant to the closing of the Merger Agreement, our board of directors adopted and assumed the 2016 Stock Incentive Plan, which had been approved by CMT’s board of directors prior to the closing. The purposes of the plan are (a) to enhance our ability to attract and retain the services of qualified employees, officers, directors, consultants, and other service providers upon whose judgment, initiative and efforts the successful conduct and development of our business largely depends, and (b) to provide additional incentives to such persons or entities to devote their utmost effort and skill to our advancement and betterment, by providing them an opportunity to participate in the ownership of our company and thereby have an interest in our success and increased value.
There are 2,000,000 shares of common stock authorized for nonstatutory and incentive stock options, stock grants, restricted stock units, and stock appreciation rights, which are subject to adjustment in the event of stock splits, stock dividends, and other situations. We have granted options to purchase up to 500,000 shares of common stock to two members of our scientific advisory board.
The plan is administered by our board of directors or any committee designated by our board of directors. The persons eligible to participate in the plan are as follows: (a) our employees and any employees of our subsidiaries; (b) non-employee members of the board or non-employee members of our board of directors of any of our subsidiaries; and (c) consultants and other independent advisors who provide services to us or any of our subsidiaries. Options may be granted, or shares issued, only to consultants or advisors who are natural persons and who provide bona fide services to us or one of our subsidiaries, provided that the services are not in connection with the offer or sale of securities in a capital-raising transaction, and do not directly or indirectly promote or maintain a market for our securities.
The plan will continue in effect until all of the stock available for grant or issuance has been acquired through exercise of options or grants of shares, or until May 18, 2026, whichever is earlier. The plan may also be terminated in the event of certain corporate transactions such as a merger or consolidation or the sale, transfer or other disposition of all or substantially all of our assets.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Management’s Discussion and Analysis of Financial Condition and Results of Operations, and other parts of this prospectus contain certain forward-looking statements. Historical results may not indicate future performance. Our forward-looking statements reflect our current views about future events;that involve risks and uncertainties. All forward-looking statements included in this prospectus are based on assumptionsinformation available to us on the date hereof, and are subjectwe assume no obligation to known and unknown risks and uncertainties that could causeupdate any such forward-looking statements. Our actual results tocould differ materially from those contemplated byanticipated in these statements. Factors that may cause differences between actual results and those contemplated by forward-looking statements include, butas a result of a number of factors, including those set forth in the section captioned “Risk Factors” on page 6 of this prospectus. The following should be read in conjunction with our audited financial statements included elsewhere herein.
Overview
We are not limited to, those discussed in “Risk Factors.” We undertake no obligation to publicly update or revise any forward-looking statements, including any changes that might result from any facts, events, or circumstances after the date hereof that may bear upon forward-looking statements. Furthermore, we cannot guarantee future results, events, levels of activity, performance, or achievements
Basis of Presentation
The financial information presented belowa commercial stage biotechnology company focused on immunology, urology, neurology and the following Management Discussionorthopedics using adult stem cell treatments and Analysis of the Consolidated Financial Condition, Results of Operations, Stockholders’ Equity and Cash Flowinterrelated regenerative technologies for the periods ended September 30, 2016,treatment of multiple indications. Our existing and December 31, 2015 gives effect to our acquisitionspipeline of therapies and products include the following:
Our subsidiary, Creative Medical Technologies, Inc. (“CMT”) on May 18, 2016. In accordance with the accounting reporting requirements for the recapitalization, was originally created to monetize U.S. Patent No. 8,372,797 and related intellectual property related to the “reverse merger”treatment of erectile dysfunction (“ED”), which it acquired in February 2016. Subsequently, we have expanded our development and acquisition of intellectual property beyond urology to include therapeutic treatments utilizing “re-programmed” stem cells, and the treatment of neurologic disorders, lower back pain, type I diabetes, and heart, liver, kidney and other diseases using various types of stem cells through our ImmCelz, Inc., StemSpine, Inc. and AmnioStem LLC subsidiaries. However, neither ImmCelz Inc., StemSpine Inc. nor AmnioStem LLC have commenced commercial activities.
We currently conduct substantially all of our commercial operations through CMT, the financial statements for CMT have been adjustedwhich markets and sells our CaverStem® and FemCelz® disposable kits utilized by physicians to reflect the changeperform autologous procedures that treat erectile dysfunction and female sexual dysfunction, respectively. Our CaverStem® and FemCelz® kits are currently available through physicians at eight locations in the shares outstandingUnited States.
In addition to our CaverStem® and FemCelz® products, we are currently in the par valueprocess of recruiting clinical sites for our StemSpine® Regenerative Stem Cell Procedure for the Treatment of Degenerative Disc Disease. Our StemSpine® treatment is an autologous procedure that utilizes a patient’s own stem cells to treat lower back pain.
In 2020, through our ImmCelz Inc. subsidiary, we began exploring the development of treatments that utilize a patient’s own extracted immune cells that are then “reprogrammed” by culturing them outside the patient’s body with optimized stem cells. The immune cells are then re-injected into the patient from whom they were extracted. We believe this process endows the immune cells with regenerative properties that may be suitable for the treatment of stroke victims, among other indications. In contrast to other stem cell-based approaches, the immune cells are significantly smaller in size than stem cells and are believed to more effectively penetrate areas of the common stock of CMT. Additionally, all intercompany transactions between Creative Medical Technology Holdings, Inc. (“CMTH”)damaged tissues and CMT have been eliminated.
Overview
On May 18, 2016, Creative Medical Technology Holdings, Inc., formerly known as Jolley Marketing, Inc., a Nevada corporation (the “Company”, “we”, “our”, or “us”), closed (the “Closing”) the Agreement and Plan of Merger (the “Merger Agreement”) with Creative Medical Technologies, Inc., a Nevada corporation (“CMT”), Steven L. White, the principal shareholder and the sole officer and director of the Company (“Mr. White”), and Jolley Acquisition Corp., a Nevada corporation and wholly owned subsidiary of the Company (the “Merger Sub”). As a result of the Closing of the Merger Agreement, the Merger Sub was merged with and into CMT with CMT being the surviving corporation and CMT became a wholly-owned subsidiary of the Company. Upon completion of the transaction, we acquired CMT (which is now our wholly-owned subsidiary) and became a company engaged in stem cell research and applications for use to treat ED, infertility and other issues. In June 2016, we filed a patent application containing novel data suggesting that specialized cells obtained from adipose (fat) tissue are capable of preventing recurrent miscarriages in an animal model with data supporting our entry into the field of reproductive immunology, which aims to address issues such as recurrent miscarriages and preterm labor. CMT was incorporated in the State of Nevada on December 30, 2015. All references to the Company after the Closing refer to Creative Medical Technology Holdings, Inc. and Creative Medical Technologies, Inc., collectively.induce regeneration.
We are considered to be a clinical stage company, since we are devoting substantially allcurrently primarily focused on expanding the commercial sale and use of our efforts to establishingCaverStem® and FemCelz® products by physicians in the Unites States and Europe, and commercializing our business and because our planned principal operations have not commenced. Our fiscal year end is December 31st. We have acquired the licensing rights for our infertility treatments, purchased the patent for our ED treatments, and filed patent applications for our female sexual dysfunction treatment andStemSpine® treatment for recurrent miscarriages. lower back pain. We also recently filed an Investigational New Drug (IND) application with the FDA to treat stroke utilizing our ImmCelzTM technology. In the future, subject to the availability of capital, we will seek to further develop additional therapeutic products that utilize our proprietary intellectual property.
| 35 |
|
| Table of Contents |
As of September 30, 2016, we have incurred interest expense of $3,757 arising from the related party notes of $125,000 and $2,600 arising from the related party cash advances.
Plan of Operations
We anticipate that if our clinical studies on our ED stem cell treatment are successful, we can commence marketing kits for the treatment in 2017. For the next 12 months our plan of operations is to complete these clinical trials and commence marketing of the kits. We estimate the costs to complete the clinical trials will be approximately $600,000, excluding overhead and other costs associated with maintaining our company structure. As of September 30, 2016, we had approximately $33,000 cash on hand with additional funding verbally committed over the next 90 days in our current nonpublic common stock offering. With an estimated monthly cash burn rate of approximately $40,000 based on historic trends and anticipated future expenses, management anticipates sufficient cash on hand and committed funds to meet operating expenses and costs of the current clinical trials through at least February 2017. Historically, we have met our cash flow requirements through the sale of equity securities or borrowed funds from our parent. We intend to continue to seek investments to meet our cash flow requirements, including both operating expenses and the balance of funding required to compete our ED clinical trials. The securities offered by us to potential investors have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.If we are unable to obtain further financing, we may seek alternative sources of funding or revise our business plan. We currently have no alternative sources for funding.
Results of Operations – For the Three and Nine MonthsThree-month Period Ended September 30, 2016,2021 and for the Period Ended December 31, 20152020
Because CMT was not incorporated until December 30, 2015, we had no comparable operations for the prior 2015 quarters and no comparative information is available. Prior to May 18, 2016, we were an inactive shell company with no business operations, which was recapitalized via a reverse merger. Following the Closing we incorporated the business plans and operations of CMT (a clinical stage company).
Gross Revenue.Revenue. We generated no$10,000 in gross revenue for the three-month period ended December 31, 2015 and none for the three and nine months ended September 30, 2016.2021, in comparison with $77,000 for the comparable quarter a year ago. The decrease of $67,000 or 87% reflects the ongoing negative impact of the COVID-19 pandemic.
Cost of Goods Sold. We generated $4,000 cost of goods sold for the three-month period ended September 30, 2021, in comparison with $17,600 for the comparable quarter a year ago. The decrease of $13,600 or 77% reflects the ongoing negative impact of the COVID-19 pandemic on sales.
Gross Profit/(Loss). We generated $6,000 gross profit for the three-month period ended September 30, 2021, in comparison with $59,400 for the comparable quarter a year ago. The decrease of $53,400 or 90% is due to the reduced level of sales in the quarter.
General and Administrative Expenses.Expenses. General and administrative expenses for the three and nine months ended September 30, 2016, totaled $164,732, and $493,484 respectively. For the three months ended September 30, 2016 general and administrative consisted primarily of management fees of $105,000 and professional fees of $51,036. For the nine months ended September 30, 2016, general and administrative consisted primarily of management fees of $318,000 and professional fees of $151,975. We anticipate that the expense for the three monththree-month period ended September 30, 2016, will be more indicative of future expenses. There was $500 expense2021, totaled $757,235 in comparison with $285,100, for the period ended December 31, 2015 representingcomparable quarter a year ago. The increase of $472,135 or 166% is primarily due to increases of $78,357 in marketing expenses associated with a re-energized CaverStem® marketing campaign, $383,612 in stock-based compensation associated with the costsissued service provider warrants, and an increase of incorporation.$58,697 in legal fees associated with the bridge loan and S-1 filing offset by a $80,742 reduction in commissions as a result of lower sales.
Amortization Expenses. Amortization expenses for the three-month period ended September 30, 2021, totaled $23,021 in comparison with $16,771, for the comparable quarter a year ago. The increase of $6,250 or 37% is due to the added amortization of the $250,000 Jadi Cell asset.
Research and Development Expenses. ResearchThere was $59,180 in research and development expenses for the three and nine monthsthree-month period ended September 30, 2016, totaled $45,174 and $67,566, respectively. We anticipate that2021 in comparison to no expenses for the comparable quarter a year ago. The expenses are associated with our efforts to respond to information requests from the FDA associated with our ImmCelz® IND filing for the treatment of stroke.
Other Income / Expense. Other expense for the three-month period ended September 30, 2016, will be more indicative2021, totaled $1,010,160 in comparison with a gain of future expenses. We also incurred license fees under a royalty agreement of $7,300 in the quarter ended June 30, 2016; there were no license fees incurred in the quarter ended March 31, 2016 or September 30, 2016. Pending completion of the development of the patented technology included in our license agreement, we will not have additional license fees until we have a saleable product. There was no research and development expense$637,322, for the period ended December 31, 2015.comparable quarter a year ago. The increased expense of $1,647,482, is primarily due to an increase of $1,016,163 in interest expense associated with the retirement of convertible debt identified in Note 2, a loss of $194,044 in derivative liabilities compared to a gain of $926,532 for the comparable quarter a year ago, offset by a gain of $489,157 on extinguished debt.
Net Loss.Income/Loss. For the reasons stated above, our net lossesloss for the three and nine monthsthree-month period ended September 30, 2016 were $214,5142021, totaled $1,843,596 in comparison to net income of $394,851 for the comparable quarter a year ago.
Results of Operations – For the Nine-month Period Ended September 30, 2021 and $571,606, respectively. Because our current clinical stage operations did not commence until2020
Gross Revenue. We generated gross revenue of $20,000 for the nine-month period ended JuneSeptember 30, 2016, we anticipate that2021, in comparison with $147,000 for the three-monthcomparable period will be more reflectivea year ago. The decrease of future net losses.$127,000 or 86% reflects the ongoing negative impact of the COVID-19 pandemic.
Cost of Goods Sold. We generated $8,500 cost of goods sold for the nine-month period ended September 30, 2021, in comparison with $42,596 for the comparable period a year ago. The decrease of $34,096 or 80% is due to the reduction in sales.
Gross Profit/(Loss). We generated $11,500 gross profit for the nine-month period ended September 30, 2021, in comparison with $104,404 for the comparable period a year ago. The decrease of $92,904 or 89% is due to the reduction in sales.
General and Administrative Expenses. General and administrative expenses for the nine-month period ended September 30, 2021, totaled $1,536,479 in comparison with $846,154, for the comparable period a year ago. The increase of $690,325 or 82% is primarily due to increases of $595,380 in stock-based compensation associated with recruitment of Scientific Advisory Board, service providers, and new employee members, $98,988 in legal fees associated with the bridge loan identified in
Note 2 and the S-1 filing identified in Subsequent Events, and a $68,902 increase in marketing expenses associated with a re-energized CaverStem® marketing campaign offset by $86,242 in reduced commissions as a result of lower sales.
Amortization Expenses. Amortization expenses for the nine-month period ended September 30, 2021, totaled $69,063 in comparison with $50,021, for the comparable period a year ago. The increase of $19,042 or 38% is due to the added amortization of the $250,000 Jadi Cell asset.
Research and Development Expenses. There was $59,180 in research and development expenses for the nine-month period ended September 30, 2021 in comparison to no expenses for the comparable period a year ago. The expenses are associated with our efforts to respond to information requests from the FDA associated with our ImmCelz® IND filing for the treatment of stroke.
Other Income / Expense. Other income for the nine-month period ended September 30, 2021, totaled $24,740,463 in comparison with $3,086,539, for the comparable period a year ago. The increased income of $21,653,924, or 702% is primarily due to an increased gain in the fair value of derivative liabilities of $22,033,774 combined with a gain of $585,601 associated with gains on extinguishment of convertible notes.
Net Income/Loss. For the reasons stated above, our net income for the nine-month period ended September 30, 2021, totaled $23,087,241 in comparison to $2,294,768, for the comparable period a year ago.
| 36 |
| Table of Contents |
Results of Operations – For the Year Ended December 31, 2020, and for the Year Ended December 31, 2019
Gross Revenue. We generated $164,500 in gross revenue for the year ended December 31, 20152020 in comparison with $165,500 for the comparable period a year ago. The decrease of $1,000 or 0.6% is primarily due to the effects of the COVID-19 pandemic. Prior to the pandemic, we were experiencing an upward trend is sales. However, from March through June 2020 and then September through December 2020, nearly all elective procedures were halted in the U.S. This resulted in a near cessation of CaverStem® and FemCelz® procedures in those time frames. Also, there were a number of incentives offered to new physicians that reduced the average per-unit price.
Cost of Goods Sold. We generated $50,596 in cost of goods sold for the year ended December 31, 2020 in comparison with $45,499 for the comparable period a year ago. The increase of $5,097 or 11.2% was consistent with the per-unit cost. The nearly flat cost of goods sold was primarily due to the COVID-19 pandemic. From March through June 2020 and then September through December 2020, nearly all elective procedures were halted in the U.S. This resulted in a near cessation of CaverStem® and FemCelz® procedures in those time frames.
Gross Profit/(Loss). We generated 113,904 in gross profit for the year ended December 31, 2020 in comparison with $120,001 in gross profit for the comparable period a year ago. The decrease of $6,097 or 5.1% is due to the lower sales associated with the COVID-19 pandemic and lower per-unit revenue from new physician promotions.
General and Administrative Expenses. General and administrative expenses for the year ended December 31, 2020, totaled $1,228,739, in comparison with $1,250,169 for the comparable period a year ago. The decrease of $21,430, or 1.7% is primarily due to reduced marketing and travel expenses due to the COVID-19 pandemic offset by increased amortization expenses associated with the StemSpine® patent acquisition.
Research and Development Expenses. We had no research and development expenses for the years ended December 31, 2020 and 2019. However, we expect our research and development expenses to increase substantially in the future as we seek regulatory approval of our ImmCelzTM technology to treat stroke, and seek to further develop additional therapeutic products that utilize our proprietary intellectual property.
Operating Loss. For the reasons stated above, our operating loss for the year ended December 31, 2020 was $1,114,835 in comparison with $1,130,168 for the comparable period a year ago.
Other Expense. Other expenses for the year ended December 31, 2020 totaled $35,210,395 in comparison with $7,353,505 for the comparable period a year ago. The increase of $27,856,890, or 378.8% is due to an increase of $28,888,870 in the change in fair value of derivative liabilities offset by a decrease of $665,938 in interest expense compared to a $366,042 loss for the comparable period a year ago. We incurred interest expense calculated on our promissory notes. We recorded the amortization of various debt discounts associated with our convertible promissory notes. The discounts are the result of derivative liabilities which are recorded due to the variability of the notes’ conversion prices. The derivative liabilities are required to be re-measured as of each reporting date.
Net Loss. For the reasons stated above, our net loss of $500.for the year ended December 31, 2020 was $36,325,230 in comparison with $8,483,673 for the comparable period a year ago.
Intangible license. The Company has acquired a royalty license from Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (“LABIOMED”) granting the exclusive license for any products and services we develop under the LABIOMED patent.
The license was acquired for a cash payment of $5,000, issuance of 50,000 shares of restricted common stock of the Company (valued at $500), and an agreement to reimburse LABIOMED up to $1,800 for expenses incurred by LABIOMED in reviving and defending their patent. The Company has expensed the cash paid, the value of the stock issued, and the expected reimbursement of $1,800 for a total intangible royalty expense – license fees of $7,300.
Amortization Expense. The CompanyWe acquired a patent [U.S.(U.S. Patent No. 8,372,797]8,372,797) from CMH on February 2, 2016, in exchange for 64,666,667123,333 shares of CMTHour restricted common stock valued at $100,000. The Patentpatent expires in 20252026 and the Company haswe have elected to amortize the Patentpatent over a ten yearten-year period on a straight linestraight-line basis. On August 25, 2016, we entered into a License Agreement which grants us the exclusive right to all products derived from US Patent No. 7,569,385 for multipotent amniotic fetal stem cells. Under the terms of the license agreement, we paid an initial license fee within 30 days of entering into the agreement. The patent expires in 2026 and we have elected to amortize the patent over a ten-year period on a straight-line basis. On May 17, 2017, we purchased U.S. Patent No. 9,598,673 covering use of various stem cells for treatment of lower back pain from CMH. Under the terms of the agreement, we are required to pay CMH $100,000. The agreement was modified in November 2017 to waive payment of the initial license fee, modify the fee structure and add the ability to convert the outstanding payable balance into common shares. In November 2019, we announced the commercialization of the lower back procedure using a patient’s own cells. This triggered a milestone payment due to CMH in the amount of $300,000. The patent expires in 2027, and we have elected to amortize the patent over a ten-year period on a straight-line basis. In December 2020, we entered into a Patent License Agreement with Jadi Cells, Inc. Execution of the contract triggered a milestone payment due from the Company to Jadi Cells, Inc. in the amount of $250,000. Given the contract execution date of December 28, 2020 no amortization expense was recognized.
Amortization expense of $2,521 and $6,603$66,792 was recorded for the three and nine monthsyear ended September 30, 2016, respectively,December 31, 2020, representing the amortization of the ED, Patentmultipotent amniotic fetal stem cell and lower back pain patents based upon the remaining life of the Patent (ten years).patents. There was $26,950 of amortization expense recorded for the period ended December 31, 2019.
| 37 |
| Table of Contents |
Liquidity and Capital Resources
Statements of Operations
Management believes our operations will increase in the future and will include the acquisition of additional licenses and patents, and the development, exploitation and commercialization of technologies within the stem cell field.
The following table sets forth the components of our consolidated financial statements of operations for the periods presented.
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Three Months Ended September 30, 2016 | For the Nine Months Ended September 30, 2016 | For the Period from December 30, 2015 (Inception) to December 31, 2015 | ||||||||||
| (Unaudited) | (Unaudited) | |||||||||||
| REVENUES | $ | - | $ | - | $ | - | ||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 45,174 | 67,566 | - | |||||||||
| General and administrative | 164,732 | 493,484 | 500 | |||||||||
| Amortization of patent costs | 637 | 6,719 | - | |||||||||
| TOTAL EXPENSES | 212,543 | 567,769 | 500 | |||||||||
| OTHER INCOME/(EXPENSE) | ||||||||||||
| Interest expense | (1, 971) | (3,837 | ) | - | ||||||||
| OPERATING LOSS | (214,514 | ) | (571,606 | ) | (500 | ) | ||||||
| NET LOSS | (214,514 | ) | $ | (571,606 | ) | $ | (500 | ) | ||||
| BASIC AND DILUTED LOSS PER SHARE | (0.00 | ) | (0.01 | ) | (0.00 | ) | ||||||
| WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING | 100,621,359 | 90,866,349 | 32,010,000 | |||||||||
Basis of Presentation / Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As of September 30, 2016, the Company2021, we had $33,456$2,338,930 of available cash and a working capital deficit of $412,501. Forapproximately $917,000. In comparison, as of December 31, 2020, we had approximately $98,000 of available cash and a working capital deficit of approximately $40,423,000.
Net Cash used in Operating Activities. We used cash in our operating activities due to our losses from operations. Net cash used in operating activities was $1,054,446 for the nine monthsnine-month period ended September 30, 20162021 in comparison to $491,542 for the Company had no revenue, no operating incomecomparable period a year ago, an increase of $562,904 or 115%. The increase in cash used in operations was primarily related to a $299,900 increase in payments made to CMH and a $100,669 decrease in accrued expenses.
Net cash used net cash forin operating activities of $259,830. As ofwas $434,556 for the year ended December 31, 20152020 in comparison to $1,213,093 for the Companyyear ended December 31, 2019, a decrease of $778,537 or 64%. The decrease in cash used in operations was newly formedprimarily related to an increase in change in fair value of derivatives liabilities in the year ended December 31, 2020.
Net Cash used in Investing Activities. There was a $200,000 advance to a related party and hadno investing activity during the nine-month period ended September 30, 2021 and September 30, 2020, respectively. Cash used in investing activities was $250,000 for the year ended December 31, 2020 in comparison to $0 for the year ended December 31, 2019.
Net Cash from Financing Activities. In the nine-month period ended September 30, 2021, we raised $4,784,790 through the issuance of convertible debt, preferred stock and short-term, non-convertible notes. We spent $1,256,926 on re-payment of notes and debt issuance costs. In the nine-month period ended September 30, 2020, we raised $458,600 primarily through the issuance of convertible debt. The $3,092,764 or 700% increase in cash flows from financing activities were primarily related to the proceeds from short-term, non-convertible notes, issuance of convertible notes and the sale of preferred stock. Net cash flows from financing activities was $693,920 for the year ended December 31, 2020 in comparison to $997,685 for the year ended December 31, 2019, a decrease of $303,765 or 30%.
Our principal source of liquidity has been funds received from the sale of our common and preferred stock and issuance of notes, including convertible notes. Our experience to-date indicates the lenders are most likely to convert the debt into equity prior to or in lieu of full payment at maturity. Going forward, our short-term funding needs are expected to be satisfied by equity investments, including the proceeds of this offering, funds to be loaned to us by third parties and revenues generated from our CaverStem® and FemCelz® procedures. Our long-term liquidity needs are expected to be satisfied from future offerings of our equity securities. It is possible that CMH may provide future financing for us. We do not begun operating. Thesehave any arrangements, agreements, or sources for long-term funding.
In addition to other smaller financing transactions we completed in the last two years, in August 2021, we completed the sale of 15% Original Issue Discount Senior Notes (or “Bridge Notes”) in the aggregate principal amount of $4,456,176. In connection with the sale of the Bridge Notes, holders of shares of our preferred stock exchanged such preferred stock for additional Bridge Notes in the aggregate principal amount of $690,000. The Bridge Notes mature on February 11, 2022, subject to the requirement that we redeem the Bridge Notes prior to such date with the net proceeds of any future offering of the Company’s securities, including this public offering. Accordingly, we will use a portion of the proceeds of the offering to redeem the Bridge Notes. Pursuant to the Purchase Agreement, we also issued to the purchasers of our Bridge Notes five-year warrants to purchase an aggregate of 363,046 shares of our common stock at an initial exercise price of $14.175 per share, subject to anti-dilution adjustment in the event of future sales of equity by us below the then exercise price, stock dividends, stock splits and other specified events.
We have continued to realize losses from operations. Upon the closing of this offering, we believe we will have sufficient cash to meet our anticipated operating costs and capital expenditure requirements through June 2023. However, we will need to raise additional capital to support our ongoing operations and continue our clinical trials. We expect to continue to raise additional capital through the sale of our securities from time to time for the foreseeable future to fund the development of our drug product candidates through clinical development, manufacturing and commercialization. Our ability to obtain such additional capital will likely be subject to various factors, among others, indicateincluding our overall business performance and market conditions. There can be no guarantee that the Company maywe will be unablesuccessful in its ability to raise capital to fund future operational and development initiatives. Our need for additional capital as described above raises substantial doubt about our ability to continue as a going concern for the next twelve months.concern. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amount and classification of liabilities that might be necessary shouldresult from the Company be unable to continue as a going concern. The Company’s ability to continue as a going concern is dependent upon its ability to obtain additional financing as may be required, and ultimately to attain sufficient cash flow from operations to meet its obligations on a timely basis. Management is in the processoutcome of negotiating various financing plans including access to ongoing credit facilities and possible sale of capital stock either in private of in public offerings and believes these steps may generate sufficient cash flow for the Company to continue as a going concern. If the Company is unsuccessful in these efforts, it may be required to substantially curtail or terminate its operations.uncertainties.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future material effect on our consolidated financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity capital expenditures or capital resources.
Liquidity and Capital Resources
Our principal source of liquidity has been funds received from the sale of our common stock to CMH in the amount of $79,500 and $125,000 in loans from CMH. Going forward, our short-term funding needs are expected to be satisfied by funds to be loaned to us by our parent entity. Our long-term liquidity needs are expected to be satisfied from future offerings of our equity securities. It is possible that CMH may provide future financing for us. We do not have any arrangements, agreements, or sources for long-term funding.
Our only commitments for expenditures relate to the completion of the clinical studies for the ED stem cell treatment and general and administrative costs, including reimbursements to our parent company for services performed by their executive officers on our behalf. During the next 12 months we also anticipate commencing marketing activities for our ED treatment in preparation for completion of the clinical trials.
We anticipate that if our clinical studies on our ED stem cell treatment are successful, we can commence marketing kits for the treatment in 2017. For the next 12 months our plan of operations is to complete these clinical trials and commence manufacture of the kits. We estimate the costs to complete the clinical trials will be approximately $600,000, excluding overhead and other costs associated with maintaining our company structure. We believe that our current cash on hand would meet our cash flow requirements for only a few more months. If we are unable to obtain further financing, we may seek alternative sources of funding or revise our business plan. We currently have no alternative sources for funding.
Our financial statements included with this prospectus have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. We have incurred substantial expenses and generated no revenues from operations during the periods covered by these financial statements. These factors raise substantial doubt about our ability to continue as a going concern. There is no assurance that we will be successful in meeting the continuing financial obligations of the company. Our financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles accepted in the United States. In connection with the preparation of our financial statements, we are required to make assumptions and estimates about future events and apply judgments that affect the reported amounts of assets, liabilities, revenue, expenses and the related disclosures. We base our assumptions, estimates and judgments on historical experience, current trends and other factors that management believes to be relevant at the time our consolidated financial statements are prepared. On a regular basis, we review the accounting policies, assumptions, estimates and judgments to ensure that our financial statements are presented fairly and in accordance with GAAP. However, because future events and their effects cannot be determined with certainty, actual results could differ from our assumptions and estimates, and such differences could be material.
Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Certain specified reduced reporting and other regulatory requirements that are available to public companies that are emerging growth companies. These provisions include:
| 38 |
We have elected to take advantage of the exemption from the adoption of new or revised financial accounting standards until they would apply to private companies. As a result of this election, our financial statements may not be comparable to public companies required to adopt these new requirements.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
On May 18, 2016, Heaton & Company, PLLC (“Heaton”), the principal accountant for the Company, was dismissed and the Company engaged Haynie & Company (“Haynie”), as the Company’s principal accountant for the Company’s fiscal year ending December 31, 2016, and the interim periods for 2016. The decision to change principal accountants was approved by the Company’s Board of Directors.
The report of Heaton, on the Company’s financial statements for either of the past two years did not contain an adverse opinion or disclaimer of opinion, or was not qualified or modified as to uncertainty, audit scope or accounting principles except that the report of Heaton included in the Company’s Annual Report on Form 10-K for 2015 did include a paragraph disclosing uncertainty about the Company’s ability to continue as a going concern.
There were no disagreements between the Company and Heaton, for the two most recent fiscal years and any subsequent interim period through May 18, 2016 (date of dismissal) on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to the satisfaction of Heaton, would have caused them to make reference to the subject matter of the disagreement in connection with its report. Further, Heaton has not advised the Company that:
Historical Background
We were incorporated on December 3, 1998, in the State of Nevada, and have one wholly-owned subsidiary, Creative Medical Technologies, Inc., a Nevada corporation (“CMT”), which conducts all of our business operations. On September 14, 2016, we formed a limited liability company, Amniostem LLC Inc., in Nevada for the purpose of creating and/or license intellectual property in the area of amniotic fluid derived stem cells for therapeutic applications. This entity is a wholly owned subsidiary of CMT and has not commenced any business activities. From our incorporation in 1998 through approximately June 2008, we were in the startup phase of our proposed business operations. Originally, Ronald Jolley, the initial director and Chief Executive Officer, intended to operate the company as a telecommunications company by obtaining business service agreements. He was not successful in obtaining service contracts and decided to redefine the company’s business plan to service industrial, commercial and residential lighting needs. Beginning in February 2000, we began selling lighting products for business and commercial applications in the greater metropolitan area of Utah County, Utah. In addition, we offered consulting services to help our customers achieve the optimum lighting solutions. To this end, we had agreements in place with local wholesale suppliers of electronics to purchase lamps and lighting products at wholesale prices. In turn, we sold the lighting products at a marked up cost to our customers.
In September 2002, we filed a registration statement to raise up to $75,000 for operating capital through the sale of our common stock by Mr. Jolley as selling agent for the proposed offering. Because of health problems of Mr. Jolley, the registration statement was withdrawn in November 2002 and no funds were raised.
We had only limited operations during this startup phase through June 30, 2008, at which time we ceased all business operations because of increased competition in the industry, dwindling sales, and elevated costs associated with generating sales.
From March 2005 through February 2006 we issued 4,850,000 shares for $15,000 to generate operating capital for the company.
On August 2, 2007, our board of directors authorized a reverse split of our outstanding shares of common stock at the rate of one share for each 10 shares outstanding. At the time of the reverse stock split we had 19,137,500 shares outstanding, which were reduced to 1,913,750 shares as a result of the reverse stock split. All references in this prospectus to outstanding shares give effect to this reverse stock split.
On August 30, 2007, we issued 97,000,000 shares to Mr. White for $15,000 in a transaction which changed the control of our company from Mr. Jolley to Mr. White. In connection with the stock purchase, Mr. White assumed management of our company and became the sole officer and director. The purpose of this change of control was to alter our business and permit us to seek potential operating target companies to acquire, or to be acquired by, in order to generate material business operations.
In August 2008 we completed a non-public offering of our common stock in which we issued 7,760,000 shares for gross proceeds of $30,000. The net proceeds of this offering were allocated and used for our operating costs.
Our principal executive offices are located at 2017 W Peoria Avenue, Phoenix, AZ 85029.
Creative Medical Technologies
CMT was created as the urological arm of its parent company, Creative Medical Health, Inc., a Delaware corporation incorporated on October 31, 2011 (“CMH”), to monetize the treatments or products developed or acquired by CMH prior to creation of CMT and transferred to it after its incorporation. CMT has been engaged in the regenerative medicine field of male and female sexual dysfunction and infertility, as well as the field of recurrent spontaneous abortions (miscarriages), using stem cells. CMT acquired a patent for its erectile dysfunction (“ED”) treatment from CMH and was granted a license by Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, a non-profit biomedical research and education institute (“LABIOMED”), for the infertility treatment.
CMT is a clinical stage company and intends to complete the testing of its ED treatment and, if warranted, market treatment kits to physicians for use with their patients suffering from ED. CMT is currently engaged in a 15-month clinical trial study being conducted at UCLA by LABIOMED on the efficacy and safety of the ED treatment. The study involves testing on 40 subjects and is intended to have a duration of 15 months. CMT also intends to test and, if warranted, market licensed stem cell products under its infertility technology license.
In connection with CMT’s organization, it sold 32,010,000 shares of its common stock to CMH for $49,500, which shares were subsequently distributed as a stock dividend by CMH pro rata to its shareholders. In connection with the granting of the license by LABIOMED to CMT for the infertility technology, CMT issued 323,000 shares of its common stock and also recorded an obligation to repay up to $1,800 to LABIOMED (for expenses which may be incurred in reviving and defending the patent). CMT also issued 64,666,667 shares of its common stock to CMH for the acquisition of the patent underlying the ED technology. At closing CMT had 97,000,000 shares of its common stock issued and outstanding.
CMT entered into a line of credit evidenced by a Loan Agreement dated February 2, 2016, with CMH for $50,000, which amount has been fully borrowed by CMT, and entered into a second Loan Agreement dated May 1, 2016, with CMH for an additional $50,000 has also been fully borrowed by CMT. The funds advanced under each line of credit are evidenced by separate 8% Promissory Notes dated February 2, 2016, and May 1, 2016, respectively. Each first note matures on April 30, 2017, and the second note matures on July 31, 2017.
As a result of the closing of the Merger Agreement, we are now a company engaged in stem cell research and applications for use to treat male and female sexual dysfunction, infertility, miscarriages, and related issues. We are a clinical stage company and intend to complete the testing of the ED treatment and, if warranted, market the treatment under the name “Caverstem” to physicians for use with their patients suffering from ED. Following further testing, we also intend to market licensed products under our infertility technology license and the female sexual dysfunction patent application.
Erectile Dysfunction Treatment
On February 3, 2016, CMT entered into a Patent Purchase Agreement with CMH pursuant to which CMH assigned to CMT its rights to US Patent No. 8,372,797, entitled “Treatment of Erectile Dysfunction by Stem Cell Therapy” which was issued to CMH by the USPTO on February 12, 2013, and related know-how and technology. The closing of the Patent Purchase Agreement occurred in May 2016, and we issued the 64,666,667 shares of CMT’s common stock to CMH.
While previous studies have demonstrated that stem cells can enhance blood vessel function, this patent application was the first to demonstrate that administration of stem cells can lead to enhanced erections. Aspects of this patent have already been clinically used. In one specific example, FDA approved bone marrow extraction devices used to concentrate bone marrow and to inject stem cells into the penile bodies. This procedure has been demonstrated safe and feasible in small patient studies.
Prior to the closing of the Patent Purchase Agreement, CMH was in the process of commencing clinical trial studies on the efficacy and safety of the ED stem cell treatment. As a result of the purchase of the ED patent by us, we have now taken over the clinical trial studies. After the trial is completed, and if the data supports the treatment, we intend to market the technology with complete kits and training to medical doctors, who can practice the treatment for their patients.
Prior studies on both animals and humans have been conducted which management believes would support the efficacy and safety of the treatment currently under clinical trials by the Company. Bone marrow stem cells have been used for over four decades in the area of hematopoietic stem cell transplantation. Stimulation of angiogenesis using this cell population has been performed in animal models of ischemia, as well as in clinical trials. One study used bone marrow cells that were isolated for expression of the p75 nerve growth factor receptor using magnetic activated cell sorting. They chose this population based on possible enhancement of neurogenic potential. Intracavernous administration of these cells into a rat bilateral cavernous nerve crush injury model was performed. At a four week follow up, improvement in erectile function as assessed by mean intracavernous-to-mean arterial pressure ratio and total intracavernous pressure was assessed. Significant improvements were observed in animals receiving the p75 selected cells as compared to those receiving an equal concentration of bone marrow derived multipotent stromal cells, fibroblasts, or saline. Significantly higher levels of FGF-2 were found in the cavernosum of animals receiving the p75 selected cells.
Clinical use of stem cells in treatment of ED has been reported in a study outside the U.S. which treated seven patients with diabetes associated ED which was unresponsive to medication for at least six months with an average of 1.5 × 10(7) cord blood mononuclear cells injected intracavernously. Three additional patients with similar characteristics were used as controls. No treatment associated abnormalities were reported despite the allogeneic nature of the cells in absence of immune suppression. One month after treatment, morning erections were regained in three participants. By the third month post treatment six of the seven patients had regained morning erections. In all patients rigidity increased as the result of cord blood administration, but was not sufficient for penetration. When the patients were administered PDE5 inhibitor before coitus, two achieved penetration and experienced orgasm, and maintained for more than six months; however, one participant could not achieved penetration at ninth month. An increase in sexual desire was reported in six of the seven patients. No improvements were observed in any of the three control patients.
In 2013, Dr. Thomas Ichim, one of our directors, conducted a pilot study on a single subject where a total of 60 ml of bone marrow aspirate was obtained and processed in a closed-system concentration device. Bone marrow mononuclear cells were concentrated to a volume of 2 ml, with 1 ml administered into each side of the smooth muscle of the penis using a 25 gauge syringe. A tourniquet was placed around the base of the penis during the injection procedure and held for five minutes to allow for maximal retention. No immediate injection-associated adverse events were noted. The patient reported a morning erection two days after cell administration. Although blood vessel and smooth muscle growth could not occur during this short time period, the possibility of bone marrow released nitric oxide stimulating erections via vasodilation may be postulated. Three weeks after treatment, the patient reported erection strong enough for penetration, but did not have ability to sustain the erection until orgasm. At three month follow-up the patient reported having intercourse until orgasm several times and a marked increase in morning erections. No adverse effects or ectopic tissue formation was observed.
CMH entered into a Clinical Trial Agreement dated May 18, 2015, with LABIOMED and conducted by Dr. Jacob Rajfer with UCLA Harbor Hospital as its principal investigator. An IRB (Institutional Review Board) application has been submitted and approved. The purpose of the clinical trials is to evaluate the safety and efficacy of the ED stem cell treatment. Enrolment in the clinical trials began in December 2015 and the clinical trial began during first quarter 2016 with approximately 40 participants. In October 2015 CMH entered into a Master Services Agreement dated November 15, 2015, with Professional Research Consulting, Inc., doing business as PRC Clinical, a contract research organization, to oversee the clinical trials. The primary outcome measures for these clinical trials include the following: (i) improvement in erectile function as measured by total score in the International Index of Erectile Function; (ii) change in Doppler Ultrasound (papaverine induced color duplex Doppler) for evaluating blood flow; (iii) change in dynamic infusion cavernosometry that measures veno-occlusive pressure, each of which should require approximately six months from baseline; and (iv) adverse events, which should require approximately 12 months from baseline. The secondary outcome measures includes improvement in erectile function as measured by total score in the International Index of Erectile Function, which should require approximately 12 months from baseline. Under the terms of the Patent Purchase Agreement, CMH assigned these agreements to us and we have assumed the duties and obligations under these agreements.
Procedures for use of our ED stem cell treatment consist of a one-hour out-patient visit in a physician’s office. The physician would harvest a patient’s bone marrow from the hip using local anesthetic and separate the stem cells using a cell separator. The separated and cleansed stem cells would then be injected into the patient’s venus cavernosa to stimulate muscle and generate blood vessel regeneration. Management believes that such treatment should result in a marked increase in duration and frequency of erections and the ability to sustain erections until orgasm, with no known treatment-associated adverse events. The current clinical trial is being conducted to validate the efficacy and safety of the treatment.
Management projects the completion of the study and publication of results to occur by mid-2017. If the study results are positive, management expects to commence commercialization of the Caverstem procedure to U.S. urologists in the second half of 2017.
Male Infertility Treatment
The patent application for our infertility treatment covers novel means for treating male infertility using stem cells. The methods claimed in this patent describe implantation of stem cells into the testes of a mammal whereby stem cells may serve to address a deficiency of germ cells (developmental precursors of sperm cells), Sertoli cells (somatic cells that aid in sperm development) and/or Leydig cells (testosterone-producing cells). This invention was based on the discovery that bone marrow-derived stem cells can differentiate into germ cells, Sertoli cells and/or Leydig cells when transplanted into the testes of experimental animals.
We believe the administration of stem cells using the methods described in this patent application can be used to improve conditions related to male infertility and/or testosterone deficiency caused by aging, disease or trauma, including individuals with cancer that have undergone irradiation of the pelvic area or chemotherapy. Other conditions involving abnormalities of the testes and/or low sperm counts could also be addressed using these methods. We believe there is great interest in leveraging the therapeutic potential of bone marrow-derived stem cell populations, especially to address male fertility issues. According to a study by the CDC, of an estimated 3.3-4.6 million men in the U.S. that sought medical advice for fertility issues, 18% were diagnosed with infertility, with deficiencies in sperm numbers of quality being the primary underlying causes of male infertility.
We are in the process of designing a clinical trial for the infertility treatment and intend to continue to prosecute the patent application. Management does not anticipate proceeding with clinical trains or commercialization of treatments from this technology until the patent claims are granted. While there may be some trial design or other pre-work performed, the trial will not commence until the claims are granted. Thus, the developmental timeline are dependent upon when, and if, the patent claims are granted. We anticipate that prosecuting the patent claims could require from one to five years. Following patent approval, the projected timeline for commercialization is anticipated to be between three and four years.
Female Sexual Dysfunction Treatment
There are many types of female sexual arousal disorders, some of which manifest as mental obstacles to sex. CMT’s current patent application would focus on physical manifestations of sexual arousal disorder, as an extension of their work with stem cell therapies for ED. The technology specifically targets atherosclerotic tissue, which basically means tissue and vessels through which blood cannot pass. Sometimes, lack of blood flow to the vagina and clitoris can make sex painful or even impossible. Research has demonstrated that vaginal engorgement and clitoral erection are important facets of sexual interaction for women. The technique developed by CMT uses regenerative cells (such as stem cells) to help encourage the process. We plan to design a trial and execute that trial in the future after we pursue the male infertility procedure. Management does not anticipate commercialization of treatments from this technology until the patent claims are granted. While there may be some trial design or other pre-work performed, the trial will not commence until the claims are granted. Thus, the developmental timeline are dependent upon when, and if, the patent claims are granted. We anticipate that prosecuting the patent claims could require from one to five years. Following patent approval, the projected timeline for commercialization is anticipated to be between three and four years.
Miscarriage Treatment
On June 9, 2016, CMT scientists Drs. Amit Patel and Thomas Ichim, two of our directors, filed a US Patent entitled “Adipose Derived Immunomodulatory Cells for Immunotherapy of Recurrent Spontaneous Abortions”, which covers the use of a woman’s own fat derived stem cells for prevention of pregnancy loss. It is believed that more than 30% of all pregnancies result in a loss and that in many cases miscarriage is repetitive. Recurrent spontaneous abortion (miscarriage) is defined by the American Congress of Obstetricians and Gynecologists as two or more miscarriages in the first trimester. Approximately 1% to 5% of women of reproductive age suffer from recurrent spontaneous abortion.
The patent is based on animal data in which mice genetically bred to replicate human spontaneous recurrent miscarriages were shown to achieve much higher frequency of live births after administration of fat derived stem cells. Specifically, in mice prone to pregnancy failure that were treated with saline, an average of two living fetuses and six resorbed fetuses were detected per mouse. In mice receiving the fat derived stem cells, an average of six living fetus’s and one resorbed fetus were detected per mouse. The average litter size of mice is six to eight offspring per pregnancy.
Given that use of patient’s fat derived stem cells is common practice in the U.S., management believes the potential of applying this existing procedure to recurrent pregnancy loss represents a material development opportunity for us.
Multipotent Amniotic Fetal Stem Cells
On August 25, 2016, CMT entered into a License Agreement which grants us the exclusive right to all products derived from US Patent No. 7,569,385 for multipotent amniotic fetal stem cells. This patent covers methods for identifying, isolating, expanding and differentiating a novel population of therapeutic stem cells, specifically, stem cells derived from amniotic fluid. In the scope of available stem cell technologies, this invention describes compositions of fetal-derived stem cells and methods for generating these cells that can allow for tissue regeneration without raising the ethical concerns that are inherent to embryonic/fetal-derived cell types. The source of these stem cells is amniotic fluid harvested during routine amniocentesis of pregnant women, whereby the isolated cell population is subsequently cultured and expanded to create a bank of therapeutic stem cells.
With future funding, we intend to leverage this technology to advance our proposed program for treating male and female infertility indications where there are unmet needs for new treatment options. In this context, we anticipate that amniotic fluid-derived stem cells could be implemented in order to improve or repair the dysfunctional cellular pathways that contribute to disorders of sexual function and fertility. If we are successful in securing additional funding, we intend to complete in vitro characterization and optimization of stem cell production, to initiate toxicity and dose escalation studies for a selected indication, to submit an Investigational New Drug application with the FDA, and, subsequently, to progress to clinical studies using this therapeutic cell population.
Management plans to develop both this and the miscarriage treatment technologies as a new regenerative medicine platform for use with multiple indications. Given amniotic stem cells are allogenic (someone else’s), the development of this cell platform will fall within the jurisdiction of the The Center for Biologics Evaluation and Research (“CBER”). This organization regulates products under a variety of regulatory authorities including the Public Health Service Act and the Food Drug and Cosmetic Act. CBER manages the Biologics License Application (“BLA”) process which is a request for permission to introduce, or deliver for introduction, a biologic product into interstate commerce. This process validates safety and efficacy through animal studies, first-in-human (Phase I), safety and initial efficacy (Phase II) and pivotal trials (Phase III). The projected time frame to complete this process is seven to ten years and will require significant investments or partnering with a larger firm to fully fund the trials up to and including marketing approval. Management intends to file a BLA on the amniotic stem cell with recurring miscarriages as the first indication to be commercialized.
Marketing
The first product we intend to market is the treatment for ED, which, if current clinical trials prove successful, would be ready to market approximately third quarter 2017. We intend to implement a multifaceted marketing approach which focus primarily on urologists. We anticipate attending conferences sponsored by the American Urological Association across the country beginning second quarter 2017. We also plan to attend physician seminars and training in Los Angeles, New York, Florida, and Texas. We further propose to create a print and television advertising campaign and print in-clinic handouts, posters, and white papers. During second calendar quarter of 2017 we intend to establish our marketing staff to implement our marketing strategy fully. Prior to then, our executive officers will be involved in marketing efforts. We also plan to publish the results of our clinical studies, if favorable, in prominent urological journals.
Intellectual Property
ED Patent. CMH acquired the use patent application for treatment of ED by stem cell therapy in July 2011 and prosecuted the application until the ED patent was issued in 2013. We have closed a Patent Purchase Agreement dated February 3, 2016, with CMH to acquire the ED patent and related know-how and technology for 64,666,667 shares of our common stock. The assignment documents have been filed with the U.S. Patent and Trademark Office.
Male Infertility License Agreement. Effective January 29, 2016, we entered into a License Agreement with LABIOMED granting us an exclusive license in the U.S. and its territories and possessions to make and market products or services authorized under LABIOMED’s U.S. use Patent Application 14/508,763 (filed October 7, 2014, and claiming priority back to U.S. Ser. No. 60/790,085 filed on 4/7/2006). We also have the right, with LABIOMED’s consent, to grant sublicenses. Subject to early termination provisions, the license agreement expires on the last to expire of the patents under which the license was granted. We have the right to terminate the agreement at any time upon 90 days’ prior written notice. LABIOMED has the right to terminate the agreement upon the breach of certain covenants under the agreement, including the failure to make required payments to LABIOMED, failure to obtain and maintain required insurance coverage, our failure to meet performance milestones, our insolvency or bankruptcy, underreporting or underpayment of royalties in excess of 20% for any 12-month period, our challenge to the patent rights underlying the license, or our default in the performance of any obligations under the agreement not cured within 90 days following receipt of notice. The agreement obligates us to use our commercially reasonable efforts to develop and commercialize the licensed products and to initiate human clinical trials within specified times. If we fail to meet these milestones within the designated periods, LABIOMED may terminate the license or convert it to non- exclusive. Under the terms of the agreement we paid $5,000 to LABIOMED as a non-refundable license issue royalty, agreed to reimburse them up to $1,800 for its expenses in reviving the patent application, and issued 323,333 shares of CMT’s common stock. We are subject to a 6% royalty to LABIOMED on net sales of any licensed products and 25% on any non-royalty sublicense income. Commencing three years after the date of the agreement, and each subsequent year thereafter, we are required to pay annual maintenance royalties of $20,000, unless during the prior one-year period we paid $50,000 or more in actual royalty payments. Finally, we have agreed to pay them certain milestone payments upon achieving the milestones set forth in the agreement, which in the aggregate we estimate to be approximately $300,000.
Female Sexual Dysfunction Patent Application. Drs. Patel and Ichim, two of our directors, have assigned to CMT for nominal consideration use their patent application (U.S. Patent Application 62319753) for the use of regenerative cells as a treatment option for women who experience sexual desire, but have difficulty reaching the arousal stage. The patent application was filed with the U.S. Patent and Trademark Office on April 7, 2016. This patent was assigned to CMT on August 28, 2016.
Miscarriage Treatment Patent Application. CMT scientists Drs. Patel and Ichim have also assigned to CMT for nominal consideration their U.S. use Patent entitled “Adipose Derived Immunomodulatory Cells for Immunotherapy of Recurrent Spontaneous Abortions”, (U.S. Patent Application 62/347,898) which covers the use of a woman’s own fat derived stem cells for prevention of pregnancy loss. The patent application was filed with the U.S. Patent and Trademark Office on June 9, 2016. This patent was assigned to CMT on August 28, 2016.
Multipotent Amniotic Fetal Stem Cells License Agreement. On August 25, 2016, CMT entered into a License Agreement dated August 25, 2016, with The Regents of The University of California, represented by its San Diego campus of the University of California, San Diego, Office of Innovation and Commercialization. This license agreement grants to CMT the exclusive right to all products derived from U.S. Patent No. 7,569,385 for use of multipotent amniotic fetal stem cells composition of matter throughout the world during the period ending on the expiration date of the longest-lived patent rights under the patent. The license agreement also permits CMT to grant sublicenses. Under the terms of the license agreement, CMT is required to diligently develop, manufacture, and sell any products licensed under the agreement. We are required to pay the University an initial license fee within 30 days of entering into the agreement. We are also required to pay annual license maintenance fees on each anniversary date of the agreement, which maintenance fees would be credited toward any earned royalties for any given period. The License Agreement provides for payment of various milestone payments, which in the aggregate are estimated at approximately $2,000,000, and earned royalties on the net sales of licensed products by CMT or any sublicensee of between approximately 5% and 20%. CMT is also required to reimburse the University for any future costs associated with maintaining the patent. CMT may terminate the license agreement for any reason upon 90 days��� written notice and the University may terminate the agreement in the event CMT fails to meet its obligations set forth therein, unless the breach is cured within 30 days of the notice from the University specifying the breach. CMT is also obligated to indemnify the University against claims arising due to the exercise of the license by CMT or any sublicensee. CMT is also required to maintain adequate general liability insurance.
Trademark and Trade Name.On April 14, 2015, CMH was granted a trademark by the U.S. Patent and Trademark Office for the name “Caverstem.” On February 23, 2016 CMH applied for a trademark for the name “Creative Medical Technologies.” Under the terms of the Patent Purchase Agreement CMH has assigned these trademarks to us. We are in the process of assigning these trademarks to CMT.
Government Regulation
Human cells, tissues or cellular or tissue-based products (“HCT/Ps”) intended for implantation, transplantation, infusion or transfer into a human recipient are regulated by the Center for Biologics Evaluation and Research at the Food and Drug Administration (the “FDA”) under the authority of section 361 of the Public Health Service Act (the “PHSA”) Act under which the FDA established regulations for HCT/Ps to prevent the introduction, transmission, and spread of communicable diseases. Specifically, Section 361 states that “human cells, tissues, and cellular or tissue-based products” (HCT/Ps) are regulated solely under this section if it meets all of the following criteria: (i) minimally manipulated; (ii) for homologous use only; (iii) do not involve the combination of the cells or tissues with another articles; and (iv) are for autologous use. The FDA has recently published draft guidance to the industry (Homologous Use of Human Cells, Tissues, and Cellular and Tissue-Based Products – Draft Guidance for Industry and FDA Staff 10/2015) that further clarifies the FDA’s position on what constitutes homologous use of HCT/Ps. The FDA in this guidance states, “As defined in 21 CFR 1271.3(c), homologous use means the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor”. A key function of bone marrow derived mesenchymal and endothelial stem cells such as are used in our treatments are to stimulate cellular regrowth and repair and is consistent with the repair function the cells perform as they circulate through the body. These cells not only secrete growth factors and other intercellular signals to command and coordinate the repair process, they can also replace and regenerate tissues by themselves directly.
Management believes that its stem cell treatments for ED, male infertility, and female sexual dysfunction would meet the criteria for inclusion under Section 361. Under certain circumstances, an establishment may qualify for an exception from the requirements of Section 361 the PHSA. Section 1271.15(b) provides the following exception from Section 361 regulation: “You are not required to comply with the requirements of this part if you are an establishment that removes HCT/P’s from an individual and implants such HCT/P’s into the same individual during the same surgical procedure.” For the exception to apply, an establishment must meet three criteria: (i) remove and implant the HCT/Ps into the same individual from whom they were removed; (ii) implant the HCT/Ps within the same surgical procedure; and (iii) the HCT/Ps remain in their original form, which means that they are only rinsed, cleaned, sized, or shaped in the procedure.
If the FDA were to modify its regulations or significantly change their guidance, the products mentioned above would be subject to traditional premarket and postmarket requirements arising under section 351 of the PHSA Act. “351 HCT/Ps” require approval of a Biologic License Application, and their manufacture must comply with Current Good Manufacturing Practices (“GMPs”). This would extend the research and development timelines on these products from three years to seven to ten years. Additionally, significantly greater capital resources would be required to complete the efforts.
Management expects and plans to pursue the regulatory pathway consistent with a new biological drug for our Amniotic stem cell and recurring miscarriage products. As such, we will fall under the jurisdiction of the The Center for Biologics Evaluation and Research (CBER). This organization regulates products under a variety of regulatory authorities including the Public Health Service Act and the Food Drug and Cosmetic Act. CBER manages the Biologics License Application (BLA) process which is a request for permission to introduce, or deliver for introduction, a biologic product into interstate commerce (21 CFR 601.2). This process validates safety and efficacy through animal studies, first-in-human (Phase I), Safety and initial efficacy (Phase II) and pivotal trials (Phase III).
Competition
There are a number of public and private companies engaged in stem cell research and applications for use to treat ED, infertility and other issues. Many of these have been engaged in this field for a significant period.
In the ED marketplace there are a few private companies engaged in stem cell research and applications. This research and the possible treatments are aimed at the approximately 9,000,000 men in the United States who, due to damage to the blood vessels and smooth muscle tissue in the penis, do not respond to PDE5 inhibitors such as Viagra or Cialis, do not respond to or cannot tolerate penis injections containing Alprostadil such as Averject or Edex, or are not one of approximately 25,000 men in the U.S. who elect invasive, non-reversible rod or pump implantation into the penis. Currently, management’s research has determined that there are fewer than a dozen private clinics in the U.S. that offer stem cell treatments for ED. None of these firms is believed to have filed for patent protection or conducted clinical trials using bone marrow to validate safety and efficacy. By comparison, we are conducting an IRB-approved clinical trial to validate safety and efficacy, using a patented procedure, at a leading research institution, performed by an accomplished researcher. Management believes that the combination of patent protection, a validated “standard of care” procedure, and competitively priced equipment and disposable kits will allow the company to compete successfully in this marketplace.
In the marketplaces for male infertility, female sexual dysfunction, and recurring miscarriages, management believes little competition exists. While there is a significant body of academic research in vitro and in animal models on the benefits of treating male infertility, female sexual dysfunction and recurring miscarriages with stem cells, management is not aware of any public or private U.S. firms pursuing human research or commercialization of these treatments. Based upon management’s research, there are fewer than five private clinics in the U.S. that offer any form of stem cell treatment for these indications. Therefore, it is management’s expectation that we will be the first entrant in the market space upon completion of our research and commercialization efforts.
Employees
We have no employees, but we use and pay for the services of employees of our parent, CMH. We have agreed to reimburse our parent company a flat monthly rate for the time spent by their management team on our business operations.
Properties
We use at no cost a portion of the offices of CMH as our principal executive offices. The office space used by us consists of 2,400 square feet located at 2017 W Peoria Avenue, Phoenix, Arizona. The building is owned by an entity controlled by Timothy Warbington, an executive officer and director and one of our principal shareholders, and the current lease between this entity and CMH expires in 2022. Management believes that this space is adequate to meet the Company’s current and foreseeable needs.
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
Current ManagementMANAGEMENT
The following table sets forthcontains information concerning our directorsregarding the current members of the Board of Directors and executive officers:officers. The ages of individuals are provided as of November 15, 2021:
Name | Position | Age | ||
Timothy Warbington | President, | 60 | ||
Donald Dickerson | Chief Financial Officer & Senior Vice-President and Director | 56 | ||
Thomas Ichim, PhD (5) | Director | 45 | ||
Amit Patel, MD (5) | Director | 48 | ||
Michael H. Finger (1)(2)(3)(4) | Director | 74 | ||
Susan Snow (1)(2)(3)(4) | Director | 64 | ||
Bruce S. Urdang (1)(2)(3)(4) | Director | 63 |
Directors are elected annually at(1) Member of the annual meetingaudit committee.
(2) Member of shareholders. Each director holds office until the next annual meetingcorporate governance and nominating committee.
(3) Member of shareholders at which his or her term expires and until his or her successor is elected and qualified, or until his or her earlier death, resignation or removal pursuantthe compensation committee.
(4) Appointment to our bylaws. Officers are elected by our boardBoard of directors at the annual meeting of our board of directors held each year immediately following the annual meeting of the shareholders, and each officer holds office until the next annual meeting at which officers are toDirectors will be elected and until his or her successor is elected and qualified, or until his or her earlier resignation or removal pursuant to our bylaws. Each of the above directors and officers has served in his or her office sinceeffective upon the closing of this offering.
(5) Will resign as a director of ours immediately prior to the reverse merger on May 18, 2016.consummation of this offering.
Business Experience of Executive Officers and Directors
Drs. Ichim and Patel were selected as directors because of their experience and expertise in the field of stem cell research. Dr. Ichim sits and has set on a number of scientific advisory boards, including MyoStim Pacers, San Diego (2011-present); Cromos Pharmaceuticals, Moscow (2010-present); Orcrist Inc, Edmonton, Canada, Chairman (2008-2010); and Entest Bio, La Mesa, California (2010-2011). He also served as editor for StemCellPatents.com (2007-2009). He has also published a number of medical abstracts in stem cell research and has authored or co-authored a number of peer reviewed articles on stem cell research. Dr. Patel has authored numerous articles in the medical field, including peer reviewed and non-peer reviewed professional journal articles on stem cell research. Since 2008 he has been Director of Clinical Regenerative Medicine. Messrs. Warbington and Dickerson were selected as directors because of their experience in managing biotech companies. Mr. Warbington has over 25 years of experience in managing companies and has spent the last five years in the biotech field. Mr. Dickerson has over 30 years of experience in the fields of finance and management. We believe the combination of the skills in the field of stem cell research and business operations contribute to a balance which allows these individuals to pool their skills and work collaboratively on our Board of Directors.
The information below sets forth the employment background of the above persons, and any directorships held by them during the last five years in any company with a class of securities registered pursuant to section 12 of the Exchange Act or subject to the requirements of section 15(d) of the Exchange Act or any company registered as an investment company under the Investment Company Act of 1940.
Timothy Warbington. Mr. Warbington has served as aour director and as Chief Executive Officer of CMT since February 2016 and has served as a director, Chief Executive Officer and President of CMH since October 2011. He has over 25 years of executive level management experience. Mr. Warbington received a Bachelor’s Degree in Accounting from Arizona State University in 1984. From 1993 through 2007 he owned and operated a multi-million dollar national agricultural (produce) and finance company with annual revenues of $5,000,000 to $12,000,000. Prior to that, he served as Chief Operating Officer of the U.S. subsidiary of a British firm engaged in the international food trade. For eight years, Mr. Warbington has invested in the biotechnology industry and has provided strategic and tactical advice as a consultant to a publicly traded bio-tech firm. In connection with this experience, he has built a network of scientists, physicians and executives to participate as executive officers and directors of CMH.
Dr. Thomas Ichim. Dr. Ichim has servedMr. Warbington’s experience as a director and as President of CMT since February 2016, and has served as a director and Vice-President of CMH, and as President of the Biotech Division, since October 2011. From 2007 until 2015 he served as Chief Science Officer, Chief Executive Officer, and President, and was a director, of MediStem Inc., a San Diego-based company engaged indevelopment of endometrial regenerative cellswhich was acquired in 2014 by Intrexon Corporation for $26,000,000. From 2004 until 2007 he served as program manager for biorasi LLC, a clinical research organization. He also served as a director of Regen BioPHarma, Inc., a publicly traded biotechnology company, from 2012 until 2015. In 2005 Dr. Ichim received his PhD in Immunology from University of Sciences Arts and Technology, Olveston Monserrat; in 1999 he received a MSc in Microbiology and Immunology from University of Western Ontario, London, Ontario, Canada;an executive, and in 1994 he received a BSc in Biology from the Universityparticular with respect to biotechnology companies, qualify him to serve as one of Waterloo, Waterloo, Ontario, Canada.our directors.
Donald Dickerson.Dickerson. Mr. Dickerson has served as aour director and as Chief Financial Officer and Senior Vice-President of CMT since February 2016, and has served as a director and as Vice President and Chief Operating Officer of CMH since June 2014. He received his Masters of Business Administration in Finance from the University of Southern California in May 1992. Mr. Dickerson has worked in a number of management and accounting positions and has experience with companies in the technology, manufacturing and health sciences area. From October 2003 until February 2009 he was employed as a vice-president for JP Morgan Chase in finance; from March 2009 until May 2014 he served as a director for GMT Ventures in finance and operations; and from June 2011 until May 2014 he also served as CFO for Medistem, Inc. in finance.
Dr. Annette Marleau.Thomas Ichim. Dr. MarleauIchim has served as a Senior Vice-Presidentdirector of CMTours since February 2016, and hasas a director of CMH, and as President of its Biotech Division, since October 2011. Dr. Ichim will resign as director of ours immediately prior to the consummation of this offering. From 2007 until 2015 he served as ViceChief Science Officer, Chief Executive Officer, and President, and Chief Scientific Officerwas a director, of MediStem Inc., a San Diego-based company engaged in development of endometrial regenerative cells which was acquired in 2014 by Intrexon Corporation for CMH since March 2014. Prior to joining CMH,$26,000,000. From 2004 until 2007 he served as program manager for Biorasi LLC, a clinical research organization. He also serves as a director of Therapeutic Solutions International, Inc., a publicly-traded company, and previously served as a director of Regen BioPharma, Inc., a publicly traded biotechnology company, from 2012 until 2015. Dr. Marleau was the DirectorIchim sits and has sat on a number of Tumor Immunology at Aethlon Medical Inc. from 2011 until 2014.scientific advisory boards, including MyoStim Pacers, San Diego (2011-present); Cromos Pharmaceuticals, Moscow (2010-2019); Orcrist Inc, Edmonton, Canada, Chairman (2008-2010); and Entest Bio, La Mesa, California (2010-2011). He also served as editor for StemCellPatents.com (2007-2009). He has also authored or co-authored over 120 peer reviewed articles and three medical textbooks, and has been named as an inventor on over 200 patent and patent applications.
In 2005 Dr. MarleauIchim received herhis PhD in Immunology from theUniversity of Sciences Arts and Technology, Olveston Monserrat; in 1999 he received a MSc in Microbiology and Immunology from University of Western Ontario, London, Ontario, Canada; and in 2004,1994 he received a MS in Reproductive Immunology from Ontario Veterinary Collateral, University of Guelph, Canada in 1999; and a BSBSc in Biology from the University of Waterloo, Waterloo, Ontario, Canada.
Dr. Ichim’s experience and expertise in the field of stem cell research qualify him to serve as one of our directors.
| 39 |
| Table of Contents |
Dr. Amit N. Patel. Dr. Patel has served as a director of CMTours since February 2016 and a director of CMH since October 2011. Dr. Patel will resign as director of ours immediately prior to the consummation of this offering. He has been a practicing heart surgeon since 2008. Dr. Patel received his medical degree in 1998 from Case Western Reserve University, Cleveland, Ohio. He is currently holds the following positions at the University of Utah; Associate Professor, Department of Surgery, Division of Cardiothoracic Surgerylicensed and Director Clinical Regenerative Medicine and Tissue Engineering.practicing cardio-thoracic surgeon in Florida. Dr. Patel has an MD from Case Western Reserve University. He is also a director of Jadi Cell, LLC, a research facility locatedLLC. Dr. Patel has authored numerous articles in Salt Lake City, Utah.the medical field, including peer reviewed and non-peer reviewed professional journal articles on stem cell research. Since 2008 he has been Director of Clinical Regenerative Medicine.
Legal ProceedingsDr. Patel’s medical experience and expertise, particularly in the field of stem cell research, qualify him to serve as one of our directors.
DuringMichael H. Finger has agreed to serve as a director upon the past ten years there have been no events under any bankruptcy act, no criminal proceedingsclosing of this offering.Mr.Finger is the manager and no judgments, injunctions, orders or decrees materialprincipal member of Alternative Sales Source, LLC, a real estate consulting firm that he founded in 2017. Prior to founding Alternative Sales Source, LLC, Mr. Finger was active as the evaluationfounder and principal shareholder of the abilityrelated companies, Hyland Bay Systems, of which he was Chief Financial Officer, and integrityHyland Bay Realty, both of anywhich he sold in 2016. Mr. Finger also founded Cardinal Financial Services, Inc., a national commercial real estate mortgage brokerage firm that he operated for over 20 years until its sale in 2007. Mr. Finger received his MBA in finance from Colombia University and holds a B.A. in biology from Boston University.
Mr. Finger’s business and financial experience and expertise qualify him to serve as one of the persons nominatedour directors.
Susan Snow has agreed to become directors or executive officersserve as a director upon the closing of the Merger Agreement,this offering. Since January 2018, Ms. Snow has served as Senior VP, Operations at Redhorse, a consulting firm specializing in contacts and none of these persons has been involvedrelationships with U.S. governmental agencies. Previously, from May 2009 until January 2018, she was a principal at Transitional Finance Partners. She began her professional career and earned her CPA at KPMG, where she spent 4 years before leaving for a Chief Financial Officer role in any judicial or administrative proceedings resulting from involvement in mail or wire fraud or fraud in connection with any business entity, any judicial or administrative proceedings based on violations of federal or state securities, commodities, banking or insurance laws or regulations, or any disciplinary sanctions or orders imposed by a stock, commodities or derivatives exchange or other self-regulatory organization.
Family Relationshipsprivate industry.
There are no family relationships between any director or executive officer, except for Dr. IchimMs. Snow’s financial and Dr. Marleau who are husbandcorporate experience and wife.expertise qualify her to serve as one of our directors.
Director IndependenceBruce S. Urdang, Esq. has agreed to serve as a director upon the closing of this offering. Mr. Urdang is an attorney in private practice, having represented clients in real estate and business transactions, and commercial litigation, at the Law Offices of Bruce S. Urdang, J.D. since 1989. Mr. Urdang has also been a professor at Northern Arizona University’s School of Hotel and Restaurant Management since 1989. Mr. Urdang received his J.D. from St. John’s School of Law and holds a B.A. in political science from the State University of New York, Oneonta.
We have adopted the independence standardsMr. Urdang’s legal and business experience and expertise qualify him to serve as one of the NYSE MKT LLC, to determine the independenceour directors.
Director Independence
Upon completion of directors. These standards provide that a person will be considered an independent director if he or she is not an officer of the company and is, in the view ofthis offering, our board of directors freewill consist of five directors, three of whom will be “independent” as defined under the rules of the Nasdaq Capital Market because they are not employees or executive officers of the Company, and have not been paid more than $120,000 of compensation by the Company in any relationship that would interfere withconsecutive 12-month period during the exercisepast three years. Tim Warbington, our Chief Executive Officer, is not an independent director due to his employment by us as executive officers.
Audit Committee
Upon completion of independent judgment. Under this standard,offering, our boardaudit committee will be comprised of directorsSusan Snow, Bruce Urdang and Michael Finger. Susan Snow will serve as the chairperson of our audit committee. Our Board has determined that Thomas Ichim, PhDeach member of our audit committee meets the requirements for independence and Amit Patel, MD would meet this standard,financial literacy under the applicable rules and therefore, would be consideredregulations of the SEC and the listing standards of the Nasdaq. Our Board has also determined that Susan Snow is an “audit committee financial expert” as defined in the rules of the SEC and has the requisite financial sophistication as defined under the listing standards of the Nasdaq. The responsibilities of our audit committee will include, among other things:
· | selecting and hiring the independent registered public accounting firm to audit our financial statements; | |
· | overseeing the performance of the independent registered public accounting firm and taking those actions as it deems necessary to satisfy itself that the accountants are independent of management; | |
· | reviewing financial statements and discussing with management and the independent registered public accounting firm our annual audited and quarterly financial statements, the results of the independent audit and the quarterly reviews, and the reports and certifications regarding internal control over financial reporting and disclosure controls; | |
· | preparing the audit committee report that the SEC requires to be included in our annual proxy statement; | |
· | reviewing the adequacy and effectiveness of our internal controls and disclosure controls and procedures; | |
· | overseeing our policies on risk assessment and risk management; | |
· | reviewing related party transactions; and | |
· | approving or, as required, pre-approving, all audit and all permissible non-audit services and fees to be performed by the independent registered public accounting firm. |
Our audit committee will operate under a written charter, to be independent.effective prior to the completion of this offering, which satisfies the applicable rules and regulations of the SEC and the listing standards of Nasdaq.
| 40 |
| Table of Contents |
Compensation Committee
We do not have standingUpon completion of this offering, our compensation nominating, or audit committeescommittee will be comprised of Susan Snow, Bruce Urdang and Michael Finger. Bruce Urdang will serve as the chairperson of our compensation committee. Our Board has determined that each member of our compensation committee meets the requirements for independence under the applicable rules and regulations of the boardSEC and listing standards of Nasdaq. Each member of the compensation committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Exchange Act. The purpose of our compensation committee will be to oversee our compensation policies, plans and benefit programs and to discharge the responsibilities of our Board relating to compensation of our executive officers. The responsibilities of our compensation committee will include, among other things:
· | reviewing and approving or recommending to the Board for approval compensation of our executive officers and directors; | |
· | overseeing our overall compensation philosophy and compensation policies, plans and benefit programs for service providers, including our executive officers; | |
· | reviewing, approving and making recommendations to our Board regarding incentive compensation and equity plans; and | |
· | administering our equity compensation plans. |
Our compensation committee will operate under a written charter, to be effective prior to the completion of this offering, which satisfies the applicable rules and regulations of the SEC and the listing standards of Nasdaq.
Corporate Governance and Nominating Committee
Upon completion of this offering, our corporate governance and nominating committee will be comprised of Susan Snow, Bruce Urdang and Michael Finger. Susan Snow will serve as chairperson of our corporate governance and nominating committee. Our Board has determined that all members of our nominating and corporate governance committee meet the requirements for independence under the applicable rules and regulations of the SEC and listing standards of the Nasdaq. The responsibilities of our nominating and corporate governance committee will include, among other things:
· | identifying, evaluating and selecting, or making recommendations to our Board regarding, nominees for election to our Board and its committees; | |
· | evaluating the performance of our Board and of individual directors; | |
· | considering and making recommendations to our Board regarding the composition of our Board and its committees; and | |
· | developing and making recommendations to our Board regarding corporate governance guidelines and matters. |
Our nominating and corporate governance committee will operate under a written charter, to be effective prior to the completion of this offering, which satisfies the applicable rules and regulations of the SEC and the listing standards of Nasdaq.
Code of Ethics
The Board adopted a Code of Business Conduct and Ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or committeescontroller, or persons performing similar functions.functions and agents and representatives, including consultants. Following the completion of this offering, a copy of the code of ethics and conduct will be available on our website at www.creativemedicaltechnology.com. We intend to form these committeesdisclose future amendments to such code, or any waivers of its requirements, applicable to any principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions or our directors on our website identified above. The inclusion of our website address in this prospectus does not include or incorporate by reference the near future.information on our website into this prospectus.
| 41 |
| Table of Contents |
EXECUTIVE COMPENSATION
Certain Relationships and Related Transactions
On May 18, 2016, we closed the Merger Agreement with CMT, Mr. White, and the Merger Sub. As a result of the closing, the Merger Sub was merged with and into CMT with CMT being the surviving corporation and CMT became a wholly-owned subsidiary of our company. Mr. White, who was our majority shareholder prior to the closing, sold 15,100,000 shares of our common stock owned by him to us following closing for $5,000, after which we cancelled the shares.
In connection with the reverse acquisition transaction, CMH advanced $25,000 to us for payment of certain obligations and purchase of Mr. White’s stock. Prior to the execution of the Merger Agreement, $13,256 was advanced for the payment of certain accounts payable and other obligations. At closing, CMT advanced the balance of the $25,000 to us for the purchase of Mr. White’s shares and the payment of our remaining accounts payable. The amounts advanced by CMH are evidenced by an 8% promissory note which matures on May 18, 2018. In addition, we settled all outstanding promissory notes payable, except for promissory notes not exceeding $20,000, which will be paid upon obtaining DTC eligibility for our common stock. No cash consideration was paid for cancellation of these promissory notes.
At closing, each share of common stock of CMT issued and outstanding immediately prior to the closing was converted into 6.4666666 shares of our common stock, which now constitutes approximately 97%, of our common stock. The shares of CMT exchanged in the closing were distributed to the CMT shareholders as a stock dividend by CMH, which purchased the shares from CMT for $49,500, which funds were used for operating expenses by CMT and to purchase the license from LABIOMED for the infertility treatment.
At closing, Timothy Warbington, Donald Dickerson, Thomas Ichim, PhD, and Amit Patel, MD were appointed as directors of our company and Mr. White resigned from all positions with the company. Post-closing, Mr. Warbington was appointed as President, Chief Executive Officer, and Chairman, Mr.Donald Dickerson, was appointed asour Chief Financial Officer, and Annette Marleau, PhD, was appointed as Senior Vice President.
CMT entered into a line of credit evidenced by a Loan Agreement dated February 2, 2016, with CMH, a principal shareholder of our company, for $50,000, which amount has been fully borrowed by CMT, and entered into a second Loan Agreement dated May 1, 2016, with CMH for an additional $50,000 which has also been fully borrowed by CMT. The funds advanced under each line of credit are evidenced by separate 8% Promissory Notes dated February 2, 2016, and May 1, 2016, respectively. The first note matures on April 30, 2017, and the second note matures on July 31, 2017.
Effective January 1, 2016, we agreed to reimburse CMH, our parent company, $35,000 per month for the services performed for us by our current officers and directors. Each of these officers and directors is either an employee of or consultant with our parent company.
During the quarter ended September 30, 2016, we sold 300,000 shares to CMH for gross proceeds of $30,000. In connection with the offering, we also issued warrants to purchase 30,000 shares of our common stock at $0.10 per share. The warrants are currently exercisable and expire on August 1, 2019.
Executive Compensation
Steven L. White served as our Chief Executive Officer from August 2007 until May 18, 2016.only officers during 2020 and 2019. Neither Mr. WhiteWarbington nor any other personMr. Dickerson received compensation directly from us or any subsidiary during the years ended December 31, 20152020 or 2014, which would be reportable pursuant2019, for services rendered in any capacities to this item.
Except as described below, we have not entered into any employment or compensation agreements or arrangements withus. During 2020 and 2019, each of Messrs. Warbington Ichim,and Dickerson and Patel and Mrs. Marleau for their services as officers or directors of our company. Each of these persons iswere employed by CMH and will continue to receive his or hertheir salary from CMH for services performed for CMT and our company.us. We have agreed to reimbursereimbursed CMH for the services performed for CMT and our company by CMH employees beginning January 1, 2016.at the rate of $35,000 per month. The following table sets forth the amount of monthly compensation reimbursed to CMH for our named executive officers during 2020 and 2019:
Name |
| Position |
| Monthly |
| |
Timothy Warbington |
| Chief Executive Officer, Director |
| $ | 20,000 |
|
Donald Dickerson |
| Chief Financial Officer, Director |
| $ | 15,000 |
|
Equity Awards
Warrants to purchase 20,000 shares of Common Stock were granted to each of three directors; Dr. Patel, Dr. Ichim and Mr. Dickerson for the year-ended December 31, 2020. Other than these Warrants, no equity awards were outstanding or held by our named executive officers for the year ended December 31, 2020.
Compensation of Directors
During 2020, each of Dr. Ichim and Dr. Patel were compensated for their services to us directors indirectly through CMH. The following table sets forth the amount of monthly compensation expense CMT has agreed to reimburse to CMH for its senior executive officers:
| Name | CMT Position | Monthly Reimbursement | ||||
| Timothy Warbington | Chief Executive Officer | $ | 10,000 | |||
| Donald Dickerson | Chief Financial Officer | $ | 10,000 | |||
| Annette Marleau | Senior Vice-President | $ | 5,000 | |||
In addition, CMH has a consulting agreement with Dr. Patel and commencing January 1, 2016, CMT has agreed to reimburse CMH $5,000 per month for time allocated by Dr. Patel to CMT and our company. We have alsowe agreed to reimburse CMH for our directors that do not serve as executive officers of ours during 2020:
Name |
| Position |
| Monthly |
| |
Thomas Ichim |
| Director |
| $ | 5,000 |
|
Amit Patel |
| Director |
| $ | 5,000 |
|
Effective September 16, 2021, following the termination of the Management Reimbursement Agreement described below, we entered into consulting relationships with Thomas Ichim and Amit Patel, under which we pay them annual compensation of $120,000 and $240,000, respectively.
Upon the closing of this Offering, we expect to pay our non-employee directors an annual retainer of $80,000, plus $20,000 for each committee they chair. We may pay such retainer in a combination of cash and stock.
Employment Agreements
None of our executive officers are currently a party to an employment agreement with us. Effective September 16, 2021, following the termination of the Management Reimbursement Agreement described below, we entered into employment relationships with Timothy Warbington, our Chief Executive Officer, and Donald Dickerson, our Chief Financial Officer, under which we pay them annual base salaries of $330,000 and $300,000, respectively.
| 42 |
| Table of Contents |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 2019 to which we have been a party in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets as of December 31, 2020 and 2019, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
Management Reimbursement Agreement
On November 17, 2017, we entered into a Management Reimbursement Agreement with CMH, a related party whose directors and executive officers include our executive officers and directors. Pursuant to this agreement, during 2019 and 2020, and until September 16, 2021, we reimbursed CMH an aggregate of $45,000 per month for the services of management and consultants employed by CMH (including our Chief Executive Officer and Chief Financial Officer, and our directors Dr. Patel and Dr. Ichim). The agreement provided that at the option of CMH, the reimbursable amounts may be paid from time to time in shares of common stock of the Company at a price equal to a 30% discount to the lowest closing price during the 20 trading days prior to time the notice is given. The Agreement may be terminated by either party upon 30 days’ prior written notice. This agreement was terminated effective September 15, 2021. At December 31, 2020, we owed CMH $18,782 under this agreement, and at September 30, 2021, no amounts were we owed CMH under this agreement.
Debt Settlement Agreement
On January 12, 2018, we entered into a Debt Settlement Agreement with Timothy Warbington, our Chief Executive Officer, under which we issued 3,000,000 shares of Series A Preferred Stock to Mr. Warbington in exchange for the cancellation of $150,000 of debt owed by the Company to CMH, which CMH in turn was obligated to pay Mr. Warbington. Each share of Series A Preferred Stock currently has the voting power of 2 shares of common stock (or an aggregate of 6,000,000 shares of common stock), and as such provides Mr. Warbington with substantial control over all matters subject to a vote of our shareholders. Mr. Warbington has agreed to surrender the Series A Preferred Stock to the Company immediately prior to the closing of the offering in exchange for $150,000 plus 8% interest on such amount from January 2018 until the date of surrender.
Jadi Cell License Agreement
On December 28, 2020, we entered into a patent license agreement with Jadi Cell, LLC, a company owned and controlled by Dr. Amit Patel, a director of ours. The agreement provides us with an exclusive license to U.S. Patent No. 9,803,176 “Methods and compositions for the clinical derivation of an allogenic cell and therapeutic uses” and the proprietary process of expanding the master cell bank of Jadi Cell LLC, in the field of enhancing autologous cells. The agreement includes the following terms:
· | We were required to pay an initial license fee of $250,000 either in cash or shares of our common stock at a discount of 25% of the closing price of our common stock on the date of the agreement. | |
· | Within thirty (30) days of the end of each calendar quarter, we are required to pay Jadi Cell five percent (5%) of the net income we generate from ImmCelz™ during such calendar quarter . | |
· | If we sell or dispose of the ImmCelz™ business, we will be required to pay Jadi Cell ten percent of the proceeds of the sale. | |
· | The agreement may only be terminated by Jadi Cell if we are in material breach of the agreement, in the event of our bankruptcy, if we cease to engage in the ImmCelz™ business or if we challenge the validity of the patent rights granted to us under the agreement. |
To date, we have not made any payments to Jadi Cell under this agreement, including the $250,000 initial license fee currently owing to Jadi Cell.
| 43 |
| Table of Contents |
StemSpine Patent Purchase
We acquired U.S. Patent No. 9,598,673 covering the use of various stem cells for the treatment of lower back pain from our affiliate CMH pursuant to a Patent Purchase Agreement dated May 17, 2017, which was amended in November 2017. The inventors of the patent were Thomas Ichim, PhD oneand Amit Patel, MD, directors of our directors, atours, and Annette Marleau, PhD. As amended, the rate of $5,000 per month.Patent Purchase Agreement includes the following terms:
· | We were required to pay CMH $100,000 within 30 days of demand as an initial payment. | |
· | Upon the determination to pursue the technology via use of autologous cells, we were required to pay CMH: |
Equity Awards
o | $100,000 upon the signing agreement with a university for the initiation of an IRB clinical trial. | |
o | $200,000, upon completion of the IRB clinical trial. | |
o | $300,00 in the event we commercialize the technology via use of autologous cells by a physician without a clinical trial. |
· | In the event we determine to pursue the technology via use of allogenic cells, we are required to pay CMH: |
o | $100,000 upon filing an IND with the FDA. | |
o | $200,000 upon dosing of the first patient in a Phase 1-2 clinical trial. | |
o | $400,000 upon dosing the first patient in a Phase 3 clinical trial. |
· | Each payment may be made in cash or shares of our common at a discount of 30% to the recent trading price. | |
· | In the event our shares of common stock trade below $0.01 per share for two or more consecutive trading days, the number of any shares issuable as payment doubles. | |
· | For a period of five years from the date of the first sale of any product derived from the patent, we are required to make royalty payments of 5% from gross sales of products, and 50% of sale price or ongoing payments from third parties for licenses granted under the patent to third parties. |
We paid CMH the $100,000 obligation of the initial payment due under this agreement, by a $50,000 cash payment and the issuance of 6,667 shares of common stock on December 12, 2019. On December 31, 2019, following our announcement with respect to the clinical commercialization of the StemSpine technology, we paid CMH $50,000 of the $300,000 obligation due under this agreement through the issuance of 133 shares of common stock. On September 30, 2020 we paid CMH an additional $40,000 of the $300,000 obligation due under this agreement through the issuance of 84,656 shares of common stock, and in January 2021 we paid CMH an additional $50,000 of the $300,000 obligation due under this agreement through the issuance of 89,286 shares of common stock. The remaining portion of the $300,000 obligation has been paid in cash.
| 44 |
|
| Table of Contents |
Compensation of Directors
No compensation was paid to or earned by any director during the year ended December 31, 2015.
Security Ownership of Certain Beneficial Owners and ManagementSECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table and footnotes thereto sets forth certain information regardingwith respect to the numberbeneficial ownership of our common stock as of November 30, 2021, as adjusted to reflect the sale of common stock offered by us in this offering, for:
· | each person, or group of affiliated persons, who we know to beneficially own more than 5% of our common stock; | |
· | each of our named executive officers; | |
· | each of our directors and director nominees; and | |
· | all of our executive officers and directors as a group. |
The percentage of beneficial ownership information shown in the table is based on 2,452,348 shares of common stock beneficially ownedcurrently outstanding, and assumes no participation in this offering by (i) each director and named executive officerthe parties below. The percentage of our company, (ii) each person known by us to bebeneficial ownership shown in the beneficial owner of 5% or more of its issued and outstandingtable after this offering is based upon 7,294,963 shares of common stock and (iii) all named executive officers and directorsoutstanding after the close of this offering, assuming the company as a group. In calculating any percentage in the following tablesale of common stock beneficially owned by one or more persons named therein, the following table assumes 102,113,7504,842,615 shares of common stock issuedby us in the offering, no sale of pre-funded warrants, and outstanding immediately following the closingno exercise of the transaction.underwriter’s option to purchase additional shares of our common stock in this offering.
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of our common stock issuable pursuant to the exercise of warrants that are either immediately exercisable or exercisable within 60 days of November 30, 2021. These shares are deemed to be outstanding and beneficially owned by the person holding those warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise further indicated, in the following table, the footnotes thereto and/persons or elsewhereentities identified in this prospectus, the persons and entities named in the following table have sole voting and sole investment power with respect to theall shares set forth opposite the shareholder’s name,shown as beneficially owned by them, subject to applicable community property laws, where applicable. Unless as otherwise indicated in the following table and/or the footnotes thereto, the address of each person beneficially owning in excess of 5% of the outstanding common stock named in the following table is: 2017 W Peoria Avenue, Phoenix, Arizona 85029.laws.
| Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership(1) | Percent of Class(1) | ||||||
| Named Executive Officers and Directors | ||||||||
| Timothy Warbington | 71,463,334 | (2) | 69.7 | % | ||||
| Donald Dickerson | 1,293,333 | 1.3 | % | |||||
| Thomas Ichim PhD | 2,586,667 | (3) | 2.5 | % | ||||
| Amit Patel, MD | 3,880,000 | (4) | 3.8 | % | ||||
| Steven L. White | - | - | ||||||
| Executive Officers and Directors as a Group (5 Persons) | 79,676,001 | 77.7 | % | |||||
| 5% Beneficial Holders | ||||||||
Creative Medical Health, Inc.(5) 2007 W Peoria Ave Phoenix, AZ 85029 | 64,996,667 | (6) | 63.7 | % | ||||
| Timothy Herbst 2031 W. Peoria Ave. Phoenix, AZ 85029 | 10,346,667 | 10.1 | % | |||||
Names and Address of Individual or Identity of Group(1) |
| Number of Shares Beneficially Owned |
|
| Beneficial Ownership Prior to the Offering (%) |
|
| Beneficial Ownership After the Offering (%) |
| |||
Officers and Directors |
|
|
|
|
|
|
|
|
| |||
Timothy Warbington |
|
| 227,035 | (1) |
|
| 9.3 | % |
|
| 3.11 | % |
Donald Dickerson |
|
| 30,017 | (2) |
|
| 1.2 | % |
| * |
| |
Thomas Ichim PhD |
|
| 19,094 | (3) |
| * |
|
| * |
| ||
Amit Patel, MD |
|
| 194,525 | (4) |
|
| 7.4 | % |
|
| 2.60 | % |
All Directors and Executive Officers as a Group (Four Persons) |
|
| 470,671 |
|
|
| 16.1 | % |
|
| 6.26 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
5% Holders |
|
|
|
|
|
|
|
|
|
|
|
|
Creative Medical Health, Inc. 2008 W Lupine Ave Phoenix, AZ 85029 |
|
| 226,948 | (5) |
|
| 9.1 | % |
|
| 3.11 | % |
* | Less than one |
(1) | Includes |
(2) | Includes 30,000 shares issuable under currently exercisable warrants. |
(3) | Includes 34 shares are held by Biotech Holdings LLC, a limited liability company controlled by Mr. |
(4) | Includes 30,000 share issuable under currently exercisable warrants and 164,474 shares |
(5) | Mr. Warbington, as President and CEO of Creative Medical Health, Inc. has voting and investment power over these shares, which are included in the shares beneficially owned by him above. |
SELLING STOCKHOLDERSDETERMINATION OF OFFERING PRICE
The table below sets forth information concerningoffering price for the resalesecurities offered by this prospectus and the exercise price of the warrants have been negotiated between the Representative and us. In determining the offering price of the common stock, pre-funded warrants and accompanying Public Warrants and the exercise price of the Public Warrants, the following factors were considered:
· | prevailing market conditions; | |
· | our historical performance and capital structure; | |
· | estimates of our business potential and earnings prospects; | |
· | an overall assessment of our management; and | |
· | the consideration of these factors in relation to market valuation of companies in related businesses. |
Transactions in our common stock are currently reported under the symbol “CELZ” on the OTC Pink. Any over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual transactions.
As of November 30, 2021, there were 75 record holders of our common stock.
| 46 |
| Table of Contents |
DESCRIPTION OF SECURITIES
The following description of our capital stock and provisions of our articles of incorporation and bylaws are summaries and are qualified by reference to the articles of incorporation and bylaws that have been filed with the SEC as exhibits to the registration statement of which this prospectus forms a part.
Description of Existing Securities
Common Stock
We are authorized to issue 50,000,000 shares of Common Stock, $0.001 par value per share, of which 2,452,348 are outstanding on the date of this prospectus. Holders of shares of our Common Stock are entitled to one vote per share on all matters submitted to a vote of the stockholders and are not entitled to cumulative voting rights. Our shares of our Common Stock do not carry any preemptive, conversion or subscription rights, and there are no sinking fund or redemption provisions applicable to the shares of our Common Stock. Holders of our Common Stock are entitled to receive dividends and other distributions in cash, stock or property as may be declared by our Board of Directors from time to time out of our assets or funds legally available for dividends or other distributions, subject to dividend or distribution preferences that may be applicable to any then outstanding shares of preferred stock. In the event of our voluntary or involuntary liquidation, dissolution or winding up, holders of shares of our Common Stock are entitled to share ratably in the assets legally available for distribution to stockholders after payment of all debts and other liabilities and satisfaction of the liquidation preference, if any, granted to the holders of any preferred stock then outstanding. All outstanding shares of our Common Stock are fully paid and nonassessable.
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, $0.001 par value per share, of which 3,000,000 shares of Series A Preferred Stock have been authorized and are outstanding on the date of this prospectus. Our certificate of incorporation authorizes our Board of Directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law, the authorized shares of preferred stock will be available for issuance without further action by you. Our Board of Directors is able to determine, with respect to any series of preferred stock, the powers (including voting powers), preferences and relative, participating, optional or other special rights, and the qualifications, limitations or restrictions thereof, including, without limitation:
● | the designation of the series; |
● | the number of shares of the series, which our Board of Directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding); |
● | whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series; |
● | the dates at which dividends, if any, will be payable; |
● | the redemption rights and price or prices, if any, for shares of the series; |
● | the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series; |
● | the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs; |
● | whether the shares of the series will be convertible into shares of any other class or series, or any other security, of the Company or any other corporation, and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion may be made; |
● | restrictions on the issuance of shares of the same series or of any other class or series; and |
● | the voting rights, if any, of the holders of the series. |
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our Common Stock might believe to be in their best interests or in which the holders of our Common Stock might receive a premium for your Common Stock over the market price of the Common Stock. Additionally, the issuance of preferred stock may adversely affect the holders of our Common Stock by restricting dividends on the Common Stock, diluting the voting power of the Common Stock or subordinating the liquidation rights of the Common Stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our Common Stock.
| 47 |
| Table of Contents |
Series A Preferred Stock
On January 12, 2018, we entered into a Debt Settlement Agreement with Timothy Warbington, our Chief Executive Officer, under which we issued 3,000,000 shares of Series A Preferred Stock to Mr. Warbington in exchange for the cancellation of $150,000 of debt owed by the Company to CMH, which CMH in turn was obligated to pay Mr. Warbington. Each share of Series A Preferred Stock currently has the voting power of 2 shares of common stock by the selling stockholders. We will not receive any proceeds from the resale of the common stock by the selling stockholders. None of the selling stockholders is a registered broker-dealer.
The following table also sets forth the name of each person who is offering the resale of(or an aggregate 6,000,000 shares of common stock), and as such provides Mr. Warbington with substantial control over all matters subject to a vote of our shareholders. In addition, each share of Series A Preferred Stock has a liquidation preference of $0.05 per share plus simple interest at the rate of 8% of issuance from the date of issuance, is redeemable on or after the fourth anniversary of the issuance date at a redemption price equal to the liquidation value of such shares (including accrued dividends thereon), and is not convertible into common stock. Mr. Warbington has agreed to surrender the Series A Preferred Stock to the Company immediately prior to the closing of the offering in exchange for $150.000 plus 8% interest on such amount from January 2018 until the date of surrender.
Warrants
We currently have warrants outstanding to purchase a total of 481,351 shares of our common stock, byexercisable until various dates ranging from May 2024 to August 2031 at exercise prices ranging from $11.50 per share to $17.00 per share. Our outstanding warrants include warrants to purchase 363,046 shares of common at an exercise price of $14.175 per share (“Bridge Warrants”) issued in our August 2021 Bridge Note financing. Pursuant to the anti-dilution adjustment provisions provided for in the Bridge Warrants, as result of this prospectus,offering, the exercise price of the Bridge Warrants will be reduced to $4.13 per share and the number of shares of common stock beneficiallyissuable upon exercise of the Bridge Warrants will be increased to approximately 1,246,000.
Description of Securities in this Offering
Common Stock. The material terms and provisions of our common stock are described under the caption “Description of Existing Securities”.
Public Warrants. Upon completion of this offering we expect to have 4,842,615 Public Warrants outstanding (5,569,007 if the Public Warrants reserved for the over-allotment are sold), each Public Warrant is exercisable for one share of common stock at an exercise price of $4.13 per share (100% of the price of the combined price of a share of common stock and Public Warrant sold in the offering) and is immediately exercisable for a period of five years following the date of issuance.
Pursuant to a warrant agency agreement between us and vStock Transfer LLC, as warrant agent, the Public Warrants will be issued in book-entry form and shall be initially represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
The number of Public Warrants outstanding, and the exercise price of those securities, will be adjusted proportionately in the event of a reverse or forward stock split of our common stock, a recapitalization or reclassification of our common stock, payment of dividends or distributions in common stock to our common stock holders, or similar transactions. In the event that the Company effects a rights offering to its common stock holders or a pro rata distribution of its assets among its common stock holders, then the holder of the Public Warrants will have the right to participate in such distribution and rights offering to the extent of their pro rata share of the Company’s outstanding common stock assuming they owned by each person, the number of shares of common stock issuable upon the exercise of their Public Warrants. In the event of a “Fundamental Transaction” by the Company, such as a merger or consolidation of it with another company, the sale or other disposition of all or substantially all of the Company’s assets in one or a series of related transactions, a purchase offer, tender offer or exchange offer, or any reclassification, reorganization or recapitalization of the Company’s common stock, then the Public Warrant holder will have the right to receive, for each share of common stock issuable upon the exercise of the Public Warrant, at the option of the holder, the number of shares of common stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration payable as a result of the Fundamental Transaction, that would have been issued or conveyed to the Public Warrant holder had the holder exercised the Public Warrant immediately preceding the closing of the Fundamental Transaction. In lieu of receiving such common stock and additional consideration in the Fundamental Transaction, the Public Warrant holder may elect to have the Company or the successor entity purchase the Public Warrant holder’s Public Warrant for its fair market value measured by the Black Scholes method.
The Company will promptly notify the Public Warrant holders in writing of any adjustment to the exercise price or to the number of the outstanding Public Warrants, declaration of a dividend or other distribution, a special non-recurring cash dividend on or a redemption of the common stock, the authorization of a rights offering, the approval of the stock holders required for any proposed reclassification of the common stock, a consolidation or merger by the Company, sale of all or substantially all of the assets of the Company, any compulsory share exchange, or the authorization of any voluntary or involuntary dissolution, liquidation, or winding up of the Company.
The Public Warrants contain a contractual provision stating that all questions concerning the construction, validity, enforcement and interpretation of the Public Warrants are governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law.
There is no trading market available for the Public Warrants on any securities exchange or nationally recognized trading system, and we do not intend to list the Public Warrants on any securities exchange or nationally recognized trading system.
Pre-Funded Warrants. Each pre-funded warrant will have an initial exercise price per share equal to $0.0001. The pre-funded warrants will be immediately exercisable and may be soldexercised at any time until exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and the exercise price. The pre-funded warrants will be issued separately from the accompanying Public Warrants, and may be transferred separately immediately thereafter.
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the pre-funded warrants in this offering andmay elect to deliver their exercise notice following the percentage each person will own afterpricing of the offering assuming they sell alland prior to the issuance of the pre-funded warrants at closing to have their pre-funded warrants exercised immediately upon issuance and receive shares offered.
| Name | Amount Beneficial Ownership Before Offering | Percentage of Common Stock Owned Before Offering1 | Amount to be Offered for the Security Holder’s Account | Amount to be Beneficially Owned After Offering1 | Percentage of Common Stock Owned After Offering2 | |||||||||||||||
| Michael Finger | 97,000 | * | 97,000 | 0 | 0 | % | ||||||||||||||
| Timothy Herbst | 10,346,667 | 10.1 | % | 4,138,668 | 6,207,999 | 6.1 | % | |||||||||||||
| Henry Baldenegro | 2,198,667 | 2.2 | % | 2,198,667 | 0 | 0 | % | |||||||||||||
| James Scott Walker | 711,333 | * | 711,333 | 0 | 0 | % | ||||||||||||||
| Mayar El Khatib | 905,333 | * | 905,333 | 0 | 0 | % | ||||||||||||||
| Ahmed El Khatib | 6,467 | * | 6,467 | 0 | 0 | % | ||||||||||||||
| Alia El Khatib | 25,867 | * | 25,867 | 0 | 0 | % | ||||||||||||||
| Afnan El Khatib | 135,800 | * | 135,800 | 0 | 0 | % | ||||||||||||||
| David Hanna | 1,034,667 | 1.0 | % | 1,034,667 | 0 | 0 | % | |||||||||||||
| Angela Nunez | 258,667 | * | 258,667 | 0 | 0 | % | ||||||||||||||
| Kelly Mikules | 646,667 | * | 646,667 | 0 | 0 | % | ||||||||||||||
| Lawrence Grant | 12,933 | * | 12,933 | 0 | 0 | % | ||||||||||||||
| Delores Alonso | 12,933 | * | 12,933 | 0 | 0 | % | ||||||||||||||
| Carlos Alonso | 71,133 | * | 71,133 | 0 | 0 | % | ||||||||||||||
| Melissa Moore | 109,933 | * | 109,933 | 0 | 0 | % | ||||||||||||||
| Heather Ott | 64,667 | * | 64,667 | 0 | 0 | % | ||||||||||||||
| Josh Berg | 38,800 | * | 38,800 | 0 | 0 | % | ||||||||||||||
| Aaron Gorin | 38,800 | * | 38,800 | 0 | 0 | % | ||||||||||||||
| Howard Salamon | 25,867 | * | 25,867 | 0 | 0 | % | ||||||||||||||
| Creative Medical Health3 | 64,996,667 | 63.7 | % | 12,933,334 | 52,033,333 | 51 | % | |||||||||||||
| David Brandt | 97,000 | * | 97,000 | 0 | 0 | % | ||||||||||||||
| Charles Freericks Jr. | 32,333 | * | 32,333 | 0 | 0 | % | ||||||||||||||
| Gerald Collins | 64,667 | * | 64,667 | 0 | 0 | % | ||||||||||||||
| R. Allen Lowe | 32,333 | * | 32,333 | 0 | 0 | % | ||||||||||||||
| Ronald Evjen | 38,800 | * | 38,800 | 0 | 0 | % | ||||||||||||||
| TOTAL | 82,007,001 | 23,732,669 | 58,241,332 | |||||||||||||||||
*Lessof common stock underlying the pre-funded warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 1%
1Based4.99% of the outstanding shares of common stock immediately after exercise, except that upon 102,113,750 shares outstanding.
2 Based upon 102,113,750 shares outstanding afterat least 61 days’ prior notice from the offering.
3 Shares owned by Creative Medical Health, Inc., for which Mr. Warbington serves as President and Chief Executive Officer. Mr. Warbington shares beneficialholder to us, the holder may increase the amount of ownership of these shares. Includes 30,000 shares issuable upon exercise of warrants.
Eachoutstanding stock after exercising the holder’s pre-funded warrants up to 9.99% of the selling stockholders received his, her, or itsnumber shares inof common stock outstanding immediately after giving effect to the reverse acquisition transaction which closed on May 18, 2016.
The number andexercise, as such percentage of shares beneficially ownedownership is determined in accordance with Rule 13d-3the terms of the Exchange Act andpre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the information is not necessarily indicativeissuance of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes anythe pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding shares as to which the selling shareholder has sole or shared voting power or investment power and also any shares, which the selling shareholder has the right to acquire within 60 days.
General
Our authorized capital stock consists of 600,000,000common stock. No fractional shares of common stock and 10,000,000 shares of preferred stock, par value $0.001 per share. Management intends to reduce the number of authorized shares. We have not issued any preferred shares. Our board of directors can, without shareholder approval, cause shares of preferred stock towill be issued and may determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares. If our board of directors causes any shares of preferred stock to be issued, the rights of the holders of our common stock could be adversely affected. our board of directors’ ability to determine the terms of preferred stock and to cause its issuance, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effectexercise of a pre-funded warrant. In lieu of fractional shares, we will round up to the next whole share.
| 48 |
| Table of Contents |
If, at the time a holder exercises its pre-funded warrants, then in lieu of making itthe cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more difficult for a third party to acquire a majoritythan 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting stock. Preferred shares issuedpower represented by our boardoutstanding shares of directors could include voting rights, or even super voting rights, which could shift the ability to control the company tocommon stock, the holders of the preferred stock. Preferred shares could also have conversion rights into shares of common stock at a discount to the market price of the common stock which could negatively affect the market for our common stock. In addition, preferred shares would have preference in the event of liquidation of the company, which means that the holders of preferred shares wouldpre-funded warrants will be entitled to receive the net assetsupon exercise of the company distributed in liquidation beforepre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
Representative’s Warrants. We also expect to have up to an additional 484,262 warrants outstanding (556,901 if the common stock holders receive any distributionand/or Public Warrants reserved for the over-allotment are sold), issuable to the Representative of this offering (“Representative’s Warrants”). The Representative Warrants will have substantially the same terms as the warrants being offered to investors pursuant to this prospectus, except that the Representative Warrants will have a cashless exercise provision, have an exercise price equal to 125% of the liquidated assets. We have no current planspublic offering price, and terminate five years from the commencement of sales of the offering. Pursuant to issue any shares of preferred stock.
Common Stock
We are authorized to issue up to 600,000,000 shares of $0.001 par value common stock, which we intend to reduce inFINRA Rule 5110(e), the future. The holders of common stock, including the shares offered hereby, are entitled to equal dividendsRepresentative Warrants and distributions, per share, with respect to the common stock when, as and if declared by our board of directors from funds legally available therefore. No holder of any shares of common stock hasissued upon exercise of the Representative Warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a pre-emptiveperiod of 360 days immediately following the date of commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of reorganization of the issuer; (ii) to any FINRA member firm participating in the offering and the officers, partners, registered persons or affiliates thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the Representative or related persons does not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period; (vi) if we meet the registration requirements of Forms S-3, F-3 or F-10; or (vii) back to us in a transaction exempt from registration with the SEC. The Representative Warrants and the shares of common stock underlying the Representative Warrants are registered on the registration statement of which this prospectus forms a part.
The number of Representative’s Warrants outstanding and the exercise price of those securities will be adjusted proportionately, as permitted by FINRA Rule 5110(g)(8)(E), in the event of a reverse or forward stock split of our common stock, a recapitalization or reclassification of our common stock, payment of dividends or distributions in common stock to our common stock holders, or similar transactions. In the event that the Company effects a rights offering to its common stock holders or a pro rata distribution of its assets among its common stock holders, then the holder of the Representative’s Warrants will have the right to subscribeparticipate in such distribution and rights offering to the extent of their pro rata share of the Company’s outstanding common stock assuming they owned the number of shares of common stock issuable upon the exercise of their warrants. In the event of a “Fundamental Transaction” by the Company, such as a merger or consolidation of it with another company, the sale or other disposition of all or substantially all of the Company’s assets in one or a series of related transactions, a purchase offer, tender offer or exchange offer, or any reclassification, reorganization or recapitalization of the Company’s common stock, then the warrant holder will have the right to receive, for each share of common stock issuable upon the exercise of the warrant, at the option of the holder, the number of shares of common stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration payable as a result of the Fundamental Transaction that would have been issued or conveyed to the warrant holder had the holder exercised the warrant immediately preceding the closing of the Fundamental Transaction. In lieu of receiving such common stock and additional consideration in the Fundamental Transaction, the warrant holder may elect to have the Company or the successor entity purchase the warrant holder’s warrant for its fair market value measured by the Black Scholes method.
The Company will promptly notify the holders of the Representative’s Warrants in writing of any adjustment to the exercise price or to the number of the outstanding warrants, declaration of a dividend or other distribution, a special non-recurring cash dividend on or redemption of the common stock, the authorization of a rights offering, the approval of the stock holders required for any securitiesproposed reclassification of our company nor arethe common stock, a consolidation or merger by the Company, sale of all or substantially all of the assets of the Company, any common shares subject to redemptioncompulsory share exchange, or convertible into other securitiesthe authorization of our company. Uponany voluntary or involuntary dissolution, liquidation, dissolution or winding up of the Company.
Nevada Anti-Takeover Statutes
The following provisions of the Nevada Revised Statutes (“NRS”) could, if applicable, have the effect of discouraging takeovers of our company.
Transactions with Interested Stockholders. The NRS prohibits a publicly-traded Nevada company from engaging in any business combination with an interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless, prior to that date, the board of directors of the corporation approved either the business combination itself or the transaction that resulted in the stockholder becoming an interested stockholder.
An “interested stockholder” is defined as any entity or person beneficially owning, directly or indirectly, 10% or more of the outstanding voting stock of the corporation and any entity or person affiliated with, controlling, or controlled by any of these entities or persons. The definition of “business combination” is sufficiently broad to cover virtually any type of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise benefit its own interests rather than the interests of the corporation and its stockholders.
| 49 |
| Table of Contents |
In addition, business combinations that are not approved and therefore take place after paymentthe three year waiting period may also be prohibited unless approved by the board of creditorsdirectors and preferred shareholders,stockholders or the price to be paid by the interested stockholder is equal to the highest of (i) the highest price per share paid by the interested stockholder within the 3 years immediately preceding the date of the announcement of the business combination or in the transaction in which he or she became an interested stockholder, whichever is higher; (ii) the market value per common share on the date of announcement of the business combination or the date the interested stockholder acquired the shares, whichever is higher; or (iii) if any, the assets will be divided pro-rata on a share-for-share basis amonghigher for the holders of preferred stock, the shareshighest liquidation value of commonthe preferred stock.
Each shareAcquisition of common stock is entitled to one vote with respect toa Controlling Interest. The NRS contains provisions governing the electionacquisition of any director or any other matter upon which shareholders are required or permitted to vote. Under Nevada corporate law, holders of our common stock do not have cumulative voting rights, so that the holders of more than 50% of the combined shares voting for the election of directors may elect all of the directors, if they choose to do soa “controlling interest” and in that event, the holders of the remaining shares will not be able to elect any members to our board of directors.
Nevada Anti-Takeover Laws
The Nevada Business Corporation Law contains a provision governing “Acquisition of Controlling Interest.” This law provides generally that any person or entity that acquires 20% or more of the outstanding voting shares of a publicly-heldan “issuing corporation,” defined as Nevada corporation that has 200 or more stockholders at least 100 of whom are Nevada residents (as set forth in the secondary publiccorporation’s stock ledger); and does business in Nevada directly or private marketthrough an affiliated corporation, may be denied voting rights with respect to the acquired shares, unless a majority of the disinterested shareholdersstockholder of the corporation elects to restore such voting rights in whole or in part.
The control sharestatute focuses on the acquisition act provides thatof a person or entity acquires “control shares” whenever it acquires“controlling interest” defined as the ownership of outstanding shares that,sufficient, but for the operationcontrol share law, to enable the acquiring person, directly or indirectly and individually or in association with others, to exercise (i) one-fifth or more, but less than one-third; (ii) one-third or more, but less than a majority; or (iii) a majority or more of the control share acquisition act, would bring its voting power within any of the following three ranges: (1) 20 to 33 1/3%, (2) 33 1/3 to 50%, or (3) more than 50%. A “control share acquisition” is generally defined as the direct or indirect acquisition of either ownership or voting power associated with issued and outstanding control shares. The shareholders or board of directors of a corporation may elect to exempt the stock of the corporation from the provisions of the control share acquisition act through adoption of a provision to that effect in the Articleselection of Incorporation or Bylaws of the corporation. Our Articles of Incorporation and Bylaws do not exempt our common stock from the control share acquisition act. The control share acquisition act is applicable only to shares of “Issuing Corporations” as defined by the act. An Issuing Corporation is a Nevada corporation, which; (1) has 200 or more shareholders, with at least 100 of such shareholders being both shareholders of record and residents of Nevada; and (2) does business in Nevada directly or through an affiliated corporation.directors.
At this time, we doThe question of whether or not have 100 shareholdersto confer voting rights may only be considered once by the stockholders and once a decision is made, it cannot be revisited. In addition, unless a corporation’s articles of record residentincorporation or bylaws provide otherwise (i) acquired voting securities are redeemable in whole or in part by the issuing corporation at the average price paid for the securities within 30 days if the acquiring person has not given a timely information statement to the issuing corporation or if the stockholders vote not to grant voting rights to the acquiring person’s securities; and (ii) if voting rights are granted to the acquiring person, then any stockholder who voted against the grant of Nevada. Therefore,voting rights may demand purchase from the issuing corporation, at fair value, of all or any portion of their securities.
The provisions of the control share acquisition actthis section do not apply to acquisitions made pursuant to the laws of descent and distribution, the enforcement of a judgment, or the satisfaction of a security interest, or acquisitions made in connection with certain mergers or reorganizations.
| 50 |
| Table of Contents |
Future sales of substantial amounts of our shares and will not until such time as these requirements have been met. At such time as they may apply to us, the provisions of the control share acquisition act may discourage companies or persons interested in acquiring a significant interest in or control of the company, regardless of whether such acquisition may becommon stock in the interestpublic market, including shares issued upon the exercise of outstanding options or warrants, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity securities. Except for 226,948 shares of our shareholders.
The Nevada “Combination with Interested Stockholders Statute” may also have an effectcommon stock held by our affiliates, all of delaying or making it more difficult to effect a change in control of the company. This statute prevents an “interested stockholder” and a resident domestic Nevada corporation from entering into a “combination,” unless certain conditions are met. The statute defines “combination” to include any merger or consolidation with an “interested stockholder,” or any sale, lease, exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions with an “interested stockholder” having; (1) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation; (2) an aggregate market value equal to 5% or more of the aggregate market value of allour outstanding shares of the corporation;common are currently freely trading or (3) representing 10% or more of the earning power or net income of the corporation. An “interested stockholder” means the beneficial owner of 10% or more of the voting shares of a resident domestic corporation, or an affiliate or associate thereof. A corporation affected by the statute may not engage in a “combination” within three years after the interested stockholder acquires its shares unless the combination or purchase is approved by the board of directors before the interested stockholder acquired such shares. If approval is not obtained, then after the expiration of the three-year period, the business combination may be consummated with the approval of the board of directors or a majority of the voting power held by disinterested stockholders, or if the consideration to be paid by the interested stockholder is at least equal to the highest of: (1) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which he became an interested stockholder, whichever is higher; (2) the market value per common share on the date of announcement of the combination or the date the interested stockholder acquired the shares, whichever is higher; or (3) if highereligible for the holders of preferred stock, the highest liquidation value of the preferred stock. The effect of Nevada’s business combination law is to potentially discourage parties interested in taking control of the company from doing so if it cannot obtain the approval of our board of directors.resale without restriction under Rule 144.
Currently,Upon completion of this offering we estimate that we will have no Nevada shareholders. Further, we do not do business in Nevada directly or through an affiliate corporation and we do not intend to do so. Accordingly, there are no anti-takeover provisions that have the effect of delaying or preventing a change in our control.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We intend to develop a dividend policy in the future as we complete the testing of the ED treatment and commence marketing efforts.
We are registering7,294,963 outstanding shares of our common stock, calculated as of November 15, 2021, assuming no further exercise of outstanding warrants, and no sale of shares reserved for the underwriter for over-allotment allocation, if any.
Sale of Restricted Securities
The shares of our common stock sold pursuant to permitthis offering will be registered under the Securities Act or 1933, as amended, and therefore freely transferable, except for such shares held by our affiliates. Our affiliates will be deemed to own “control” securities that are not registered for resale under the registration statement covering this prospectus. Individuals who may be considered our affiliates after the offering include individuals who control, are controlled by or are under common control with us, as those terms generally are interpreted for federal securities law purposes. These individuals may include some or all of our directors and executive officers. Individuals who are our affiliates are not permitted to resell their shares of our common stock unless such shares are separately registered under an effective registration statement under the Securities Act of 1933, as amended, or an exemption from the registration requirements of the Securities Act of 1933, as amended, is available, such as Rule 144.
Rule 144
In general, under Rule 144 as currently in effect, a person (or persons whose shares are aggregated), including an affiliate, who beneficially owns “restricted securities” (i.e. securities that are not registered by an effective registration statement) of a “reporting company” may not sell these securities until the person has beneficially owned them for at least six months. Thereafter, affiliates may not sell within any three-month period a number of shares in excess of the greater of: (i) 1% of the then outstanding shares of Common Stock as shown by the most recent report or statement published by the issuer; and (ii) the average weekly reported trading volume in such securities during the four preceding calendar weeks.
Sales under Rule 144 by our affiliates will also be subject to restrictions relating to manner of sale, notice and the availability of current public information about us and may be affected only through unsolicited brokers’ transactions.
Persons not deemed to be affiliates who have beneficially owned “restricted securities” for at least six months but for less than one year may sell these securities, provided that current public information about the Company is “available,” which means that, on the date of sale, we are current in our Exchange Act filings. After beneficially owning “restricted securities” for one year, our non-affiliates may engage in unlimited re-sales of such securities.
Shares purchased by our affiliates in this offering may be “controlled securities” rather than “restricted securities.” “Controlled securities” are subject to the same volume limitations as “restricted securities” but are not subject to holding period requirements.
| 51 |
| Table of Contents |
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of the material U.S. federal income tax considerations relating to the purchase, ownership and disposition of our common stock, Public Warrants and pre-funded warrants purchased in this offering, which we refer to collectively as our securities, but is for general information purposes only and does not purport to be a complete analysis of all the potential tax considerations. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended (the “Code”), existing and proposed Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income and estate tax consequences different from those set forth below. There can be no assurance that the Internal Revenue Service (the “IRS”) will not challenge one or more of the tax consequences described herein, and we have not obtained, and do not intend to obtain, an opinion of counsel or ruling from the IRS with respect to the U.S. federal income tax considerations relating to the purchase, ownership or disposition of our securities.
This summary does not address the tax considerations arising under the laws of any U.S. state, local or any non-U.S. jurisdiction, or under U.S. federal non-income tax laws, or the potential application of the Medicare contribution tax on net investment income. In addition, this discussion does not address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
· | banks, insurance companies, regulated investment companies, real estate investment trusts or other financial institutions; | |
· | persons subject to the alternative minimum tax; | |
· | tax-exempt organizations or governmental organizations; | |
· | controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; | |
· | brokers or dealers in securities or currencies; | |
· | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; | |
· | partnerships or other entities or arrangements classified as partnerships for U.S. federal income tax purposes or other pass-through entities (and investors therein); | |
· | persons that own, or are deemed to own, more than five percent of our common stock (except to the extent specifically set forth below); | |
· | certain former citizens or long-term residents of the United States; | |
· | persons whose functional currency is not the U.S. dollar; | |
· | persons who hold our common stock or warrants as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction or integrated investment; | |
· | persons subject to special tax accounting rules as a result of any item of gross income with respect to our common stock or warrants being taken into account in an applicable financial statement within the meaning of 451(b) of the Code; | |
· | persons who hold or receive our common stock or warrants pursuant to the exercise of any employee stock option or otherwise as compensation; | |
· | persons who hold or receive our common stock or warrants pursuant to conversion rights under convertible instruments; | |
· | persons who do not hold our common stock or warrants as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes); or | |
· | persons deemed to sell our common stock or warrants under the constructive sale provisions of the Code. |
For the purposes of this discussion, a “U.S. holder” means a beneficial owner of our common stock or warrants that is, for U.S. federal income tax purposes: (a) an individual who is a citizen or resident of the United States, (b) a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. A “non-U.S. holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock or warrants that is not a U.S. holder or an entity or arrangement treated as a partnership for U.S. federal income tax purposes.
If a partnership or entity classified as a partnership for U.S. federal income tax purposes holds our common stock or warrants, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock or warrants, and partners in such partnerships, should consult their tax advisors.
| 52 |
| Table of Contents |
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock or warrants arising under the U.S. federal estate or gift tax laws or under the laws of any state, local, non-U.S. or other taxing jurisdiction or under any applicable tax treaty. In addition, significant changes in U.S. federal income tax laws were recently enacted. You should consult with your tax advisor with respect to such changes in U.S. tax law as well as potentially conforming changes in state tax laws.
Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, we believe a pre-funded warrant should be treated as a share for U.S. federal income tax purposes and a holder of pre-funded warrants should generally be taxed in the same manner as a holder of shares of common stock, as described below. Accordingly, no gain or loss should be recognized upon the exercise of a pre-funded warrant and, upon exercise, the holding period of a pre-funded warrant should carry over to the share of common stock received. Similarly, the tax basis of the pre-funded warrant should carry over to the share of common stock received upon exercise, increased by the selling stockholders, fromexercise price of $0.0001 per share. However, our characterization is not binding on the IRS, and the IRS may treat the pre-funded warrants as warrants to acquire our shares of common stock. If so, the amount and character of your gain with respect to an investment in our pre-funded warrants could change. Accordingly, each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition of pre-funded warrants pursuant to this offering (including potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
Investment Unit
For U.S. federal income tax purposes, the shares of common stock (or pre-funded warrants) and accompanying Public Warrants acquired in this offering will be treated as an “investment unit” consisting of one share of common stock (or pre-funded warrant) and a Public Warrant. The purchase price for each investment unit will be allocated between these two components in proportion to their relative fair market values at the time the investment unit is purchased by the holder. This allocation of the purchase price for each investment unit will establish the holder’s initial tax basis for U.S. federal income tax purposes in the share of common stock (or pre-funded warrant) and the Public Warrant included in each investment unit. Each holder should consult his, her or its own tax advisor regarding the allocation of the purchase price to time afterits investment unit.
U.S. Holders
Exercise and Expiration of Warrants
In general, a U.S. holder will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a warrant. The U.S. holder will take a tax basis in the shares acquired on the exercise of a warrant equal to the exercise price of the warrant, increased by the U.S. holder’s adjusted tax basis in the warrant exercised (as determined pursuant to the rules discussed above). The U.S. holder’s holding period in the shares of our common stock acquired on exercise of the warrant will begin on the date of this prospectus. We have agreed to maintain the effectivenessexercise of the registration statementwarrant, and will not include any period for which the U.S. holder held the warrant.
In certain limited circumstances, a U.S. holder may be permitted to undertake a cashless exercise of which this prospectuswarrants into our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into our common stock is unclear, and the tax consequences of a partcashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. U.S. holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
The lapse or expiration of a warrant will be treated as if the U.S. holder sold or exchanged the warrant and recognized a capital loss equal to the U.S. holder’s tax basis in the warrant. The deductibility of capital losses is subject to limitations.
Certain Adjustments to and Distributions on Warrants
Under Section 305 of the Code, an adjustment to the number of shares of common stock issued on the exercise of the warrants or an adjustment to the exercise price of the warrants may be treated as a constructive distribution to a U.S. holder of the warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our stockholders). An adjustment made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property to the holders of warrants. In certain circumstances, if we were to make a distribution in cash or other property with respect to our common stock after the issuance of the warrants, then we may make a corresponding distribution to the holders of the warrants. The taxation of a distribution received with respect to a warrant is unclear. It is possible such a distribution would be treated as a distribution (or constructive distribution), although other treatments are possible. For more information regarding the U.S. federal income tax considerations related to distributions, see the discussion below regarding “-Distributions.” U.S. holders should consult their tax advisors regarding the proper treatment of any adjustments to the warrants and any distributions with respect to the warrants.
Distributions
As described in the section captioned “Dividend Policy,” we have never paid cash distributions on our common stock and do not anticipate doing so in the foreseeable future. In the event that we do make distributions on our common stock to a U.S. holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a U.S. holder’s adjusted tax basis in our common stock. Any remaining excess will be treated as gain realized on the sale or exchange of our common stock as described below under the section titled “- Disposition of Our Common Stock or Warrants.” Under current law, if certain requirements are met, a preferential U.S. federal income tax rate will apply to any dividends paid to a beneficial owner of our common stock who is an individual U.S. holder and meets certain holding period requirements.
Distributions constituting dividends for U.S. federal income tax purposes that are made to U.S. holders that are corporate stockholders may qualify for the selling stockholders untildividends received deduction, or DRD, which is generally available to corporate stockholders. No assurance can be given that we will have sufficient earnings and profits (as determined for U.S. federal income tax purposes) to cause any distributions to be eligible for a DRD. In addition, a DRD is available only if certain holding periods and other taxable income requirements are satisfied.
| 53 |
| Table of Contents |
Disposition of Our Common Stock or Warrants
Upon a sale or other taxable disposition of our common stock or warrants, a U.S. holder generally will recognize capital gain or loss in an amount equal to the earlier of (i)difference between the date on which all ofamount realized and the shares included for itU.S. holder’s adjusted tax basis in the registration statement may be sold pursuantcommon stock or warrants. Capital gain or loss will constitute long-term capital gain or loss if the U.S. holder’s holding period for the common stock or warrants exceeds one year. The deductibility of capital losses is subject to Rule 144 without volume restrictionscertain limitations. U.S. holders who recognize losses with respect to a disposition of our common stock or public informationwarrants should consult their own tax advisors regarding the tax treatment of such losses.
Information Reporting and Backup Withholding
Information reporting requirements generally will apply to payments of dividends (including constructive dividends) on the common stock and anywarrants and all restrictive legends have been removed from the shares, (ii) when all of the shares have been disposed of pursuant to the registration statement,proceeds of a sale or (iii) December 31, 2018. Weother disposition of common stock and warrants paid by us to a U.S. holder unless such U.S. holder is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. holder fails to provide the holder’s taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with applicable requirements to establish an exemption.
Backup withholding is not an additional tax. Rather, any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against the U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS. U.S. holders should consult their own tax advisors regarding their qualification for exemption from information reporting and backup withholding and the procedure for obtaining such exemption.
Non-U.S. Holders
Exercise and Expiration of Warrants
In general, a non-U.S. holder will not receive anyrecognize gain or loss for U.S. federal income tax purposes upon the exercise of the proceeds from the sale by the selling stockholders of suchwarrants into shares of our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into our common stock is unclear. A non-U.S. holder should consult his, her, or its own tax advisor regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
The expiration of a warrant will be treated as if the non-U.S. holder sold or exchanged the warrant and recognized a capital loss equal to the non-U.S. holder’s tax basis in the warrant. However, a non-U.S. holder will not be able to utilize a loss recognized upon expiration of a warrant against the non-U.S. holder’s U.S. federal income tax liability unless the loss is effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the non-U.S. holder is present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain Adjustments to and Distributions on Warrants
As described under “-U.S. Holders -Certain Adjustments to and Distributions on Warrants,” an adjustment to the warrants could result in a constructive distribution to a non-U.S. holder, which would be treated as described under “-Distributions” below, and the tax treatment of distributions on the warrants is unclear. Any resulting withholding tax attributable to deemed dividends would be collected from other amounts payable or distributable to the non-U.S. holder. Non-U.S. holders should consult their tax advisors regarding the proper treatment of any adjustments to and distributions on the warrants.
Distributions
As described in the section captioned “Dividend Policy,” we have never paid cash distributions on our common stock and do not anticipate doing so in the foreseeable future. However, if we do pay cash distributions on our common stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of common stock (see “Disposition of Our Common Stock or Warrants” below).
| 54 |
| Table of Contents |
Subject to the discussion below on effectively connected income, backup withholding and foreign accounts, any distribution (including constructive distributions) that is treated as a dividend paid to a non-U.S. holder generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, a non-U.S. holder generally must provide the applicable withholding agent with an IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate version of IRS Form W-8 certifying the non-U.S. holder’s entitlement to benefits under that treaty.
We generally are not required to withhold tax on dividends paid (or constructive dividends deemed paid) to a non-U.S. holder that are effectively connected with the holder’s conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment or fixed base maintained by the holder in the United States) if a properly executed IRS Form W-8ECI stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to the applicable withholding agent). Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits, subject to an applicable income tax treaty providing otherwise. In addition, a corporate non-U.S. holder receiving effectively connected dividends may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. You should consult your tax advisor regarding any applicable tax treaties that may provide for different rules.
If a non-U.S. holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will bear all feesbe required to provide appropriate documentation to such agent. The holder’s agent may then be required to provide certification to the applicable withholding agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. withholding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
Disposition of Our Common Stock or Warrants
In general, subject to the discussion below under “Backup Withholding and expenses incidentInformation Reporting,” a non-U.S. holder generally will not be subject to U.S. federal income tax or withholding tax on any gain realized upon the sale or other disposition of our obligationcommon stock or warrants unless:
· | the gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States); | |
· | the non-U.S. holder is a non-resident alien individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or | |
· | our common stock constitutes a United States real property interest by reason of our status as a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the non-U.S. holder’s disposition of, or their holding period for, our common stock. |
We believe that we are not currently and will not become a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to register these sharesthe fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, your common stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded common stock at any time during the shorter of the five-year period preceding your disposition of, or your holding period for, our common stock.
The selling stockholdersA non-U.S. holder described in the first bullet above will sell their shares at prevailing market prices or privately negotiated prices. These salesbe required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates and in the manner applicable to U.S. persons, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. A non-U.S. holder described in the second bullet above will be subject to tax at 30% (or such lower rate specified by an applicable income tax treaty) on the gain derived from the sale, which gain may be offset by U.S. source capital losses for the year (provided such holder has timely filed U.S. federal income tax returns with respect to such losses). You should consult any applicable income tax or other treaties that may provide for different rules.
| 55 |
| Table of Contents |
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of distributions (including constructive distributions) on our common stock or warrants paid to each non-U.S. holder, their name and address, and the amount of tax withheld, if any. A similar report will be sent to the applicable non-U.S. holder. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in the non-U.S. holder’s country of residence.
Payments of dividends (including constructive dividends) or of proceeds on the disposition of our common stock or warrants made to a non-U.S. holder may be subject to information reporting and backup withholding at a current rate of 24% unless the non-U.S. holder establishes an exemption, for example, by properly certifying their non-U.S. status on an IRS Form W-8BEN, IRS Form W-8BEN-E or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that a holder is a U.S. person.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds of a disposition of our common stock or warrants effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed and appropriate IRS Form W-8 or otherwise meets documentary evidence requirements for establishing non- U.S. holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the U.S. through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in transactions,fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
Backup withholding is not an additional tax; rather, the U.S. federal income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, you may be able to obtain a refund or credit from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance Act
The Foreign Account Tax Compliance Act and the rules and regulations promulgated thereunder, collectively FATCA, generally impose withholding tax at a rate of 30% on dividends (including constructive dividends) on, and gross proceeds from the sale or other disposition of, our common stock or warrants if paid to a “foreign financial institution” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to, among other things, withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. FATCA also generally imposes a U.S. federal withholding tax of 30% on dividends (including constructive dividends) on and gross proceeds from the sale or other disposition of our common stock or warrants if paid to a “non-financial foreign entity” (as specially defined under these rules) unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. The withholding provisions under FATCA generally apply to dividends (including constructive dividends) on our common stock and warrants. The Treasury Secretary has issued proposed regulations providing that the withholding provisions under FATCA do not apply with respect to payment of gross proceeds from a sale or other disposition of our common stock or warrants, which may involve crossesbe relied upon by taxpayers until final regulations are issued. Under certain circumstances, a non-U.S. holder might be eligible for refunds or block transactions,credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. You should consult your tax advisors regarding the possible implications of FACTA on your investment in our common stock and warrants.
The preceding discussion of U.S. federal tax considerations is for general information only. It is not tax advice. Each prospective investor should consult its tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock and warrants, including the consequences of any one or moreproposed change in applicable laws.
| 56 |
| Table of Contents |
UNDERWRITING
We have entered into an underwriting agreement, dated as of _________ with Roth Capital Partners, LLC as the sole representative of the underwriters (“Roth” or the “Representative”), with respect to the shares, Public Warrants and pre-funded warrants being offered. Roth is the sole book running manager for the offering. Subject to the terms and conditions of an underwriting agreement between us and the Representative, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stock, Public Warrants and pre-funded warrants listed next to its name in the following methods:table:
Name of Underwriter |
Number of Shares | Number of Pre-Funded | Number of Warrants | ||||||||||
Roth Capital Partners, LLC |
Total |
IfThe underwriters are committed to purchase all the selling stockholders effectshares of common stock, Public Warrants and pre-funded warrants offered by this prospectus if they purchase any shares of common stock, Public Warrants and pre-funded warrants. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock, Public Warrants and/or pre-funded warrants covered by the underwriters’ over-allotment option described below. The underwriters are offering the shares of common stock, Public Warrants and pre-funded warrants, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such transactionsas the receipt by sellingthe underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-Allotment Option
We have granted to the underwriters an option, exercisable no later than 45 calendar days after the date of the underwriting agreement, to purchase up to 726,393 additional shares of common stock and/or 726,393 additional Public Warrants at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option only to cover over-allotments, if any, made in connection with this offering. To the extent the option is exercised and the conditions of the underwriting agreement are satisfied, we will be obligated to sell to the underwriters, and the underwriters will be obligated to purchase, these additional shares of common stock and/or Public Warrants.
Representative’s Warrants
We have agreed to issue Representative Warrants to the representative of the underwriters to purchase a number of shares of our common stock equal to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasersfive percent (5.0%) of the shares of common stock for whom they may actissued or issuable by the Company in this offering (including shares of common stock issuable upon the exercise of any Public Warrants and pre-funded warrants issued to investors in this offering). The Representative Warrants will have substantially the same terms as agent orthe Public Warrants being offered to whom they may sell as principal (which discounts, concessions or commissions asinvestors pursuant to particular underwriters, broker-dealers or agents may be in excessthis prospectus, except that the Representative Warrants will have an exercise price equal to 125% of those customary in the typespublic offering price, and terminate five years from the commencement of transactions involved). In connection with sales of the offering. Pursuant to FINRA Rule 5110(e), the Representative Warrants and any shares of our common stock issued upon exercise of the Representative Warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 360 days immediately following the date of commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of reorganization of the issuer; (ii) to any FINRA member firm participating in the offering and the officers, partners, registered persons or affiliates thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the Representative or related persons does not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the selling stockholders may enter into hedging transactionsfund and the participating members in the aggregate do not own more than 10% of the equity in the fund; (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period; (vi) if we meet the registration requirements of Forms S-3, F-3 or F-10; or (vii) back to us in a transaction exempt from registration with broker-dealers, which may in turn engage in short sales ofthe SEC. The Representative Warrants and the shares of common stock inunderlying the course of hedging in positions they assume. The selling stockholders may also sell shares of our common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. To the knowledge of management, no selling shareholder has taken, or plans to take, a short position in our stock prior to the effectiveness of the registration statement of which this prospectus is a part. The selling stockholders may also loan or pledge shares of our common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may pledge or grant a security interest in some or all of the shares of our common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, the shares of our common stock may be sold in such states only throughRepresentative Warrants are registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling shareholder will sell any or all of the shares of common stock registered pursuant toon the registration statement of which this prospectus forms a part.
| 57 |
| Table of Contents |
Underwriting Discount, Commissions and Expenses
The selling stockholdersunderwriters propose to offer the common stock, Public Warrants and any other person participating in such distribution will be subjectpre-funded warrants offered by us to applicable provisionsthe public at the public offering price per share set forth on the cover of this prospectus. In addition, the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, whichunderwriters may limit the timing of purchases and sales of anyoffer some of the shares of common stock, Public Warrants and pre-funded warrants to other securities dealers at such price less a concession of $ per share and $ per related Public Warrant. After the initial offering, the public offering price and concession to dealers may be changed. No such change will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the sellingRepresentative of the over-allotment option.
Per Share and | Per Pre-Funded | Total | Total With | |||||||||||||
Public offering price | $ | $ | $ | |||||||||||||
Underwriting discount (7.0%)(1) | $ | $ | $ | |||||||||||||
Proceeds, before expenses, to us(2) | $ | $ | $ | |||||||||||||
__________________
(1) | In addition to the underwriting discount, we have agreed to reimburse the Representative to cover certain accountable expenses of the Representative in connection with this offering in an amount up to $125,000. We have also agreed to issue the Representative of the underwriters the Representative Warrants described above under “Representative Warrants.” | |
(2) | We estimate that the total expenses of the offering payable by us, excluding the total underwriting discounts, will be approximately $380,000. |
Lock-Up Agreements
We and each of our officers, directors, affiliates and certain existing stockholders andaggregating at least 10.0% of our outstanding shares have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any other participating person. Regulation M may also restrictoption for the abilitysale of or otherwise dispose of any person engagedshares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock for a period of six (6) months after this offering is completed without the prior written consent of Roth.
Roth may in the distributionits sole discretion, and at any time without notice, release some or all of the shares of common stocksubject to engage in market-making activities with respectlock-up agreements prior to the shares of common stock. Allexpiration of the foregoing may affectlock-up period. When determining whether or not to release shares from the marketabilitylock-up agreements, the Representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the shares of common stockrelease is being requested and market conditions at the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.time.
Right of First Refusal
We will pay all expenseshave granted Roth a right of first refusal, upon the completion of the registrationoffering, for a period of eighteen months from the termination or expiration of the sharesCompany’s engagement with the Representative, to act as the exclusive placement agent, or lead left underwriter and sole book-runner, upon certain terms, for each of common stock pursuantour future public and private equity and debt offerings, including all equity-linked financings.
Tail Fee
We shall pay the Representative the cash and warrant compensation provided above on the gross proceeds provided to us by investors that participated in this offering or were introduced to us by the registration rights provisions containedRepresentative or which we met during our engagement of the Representative in any public or private offering or capital-raising transaction within twelve months following the registration rights agreements between us andexpiration or termination of our engagement of the selling stockholders; provided, however, that a selling shareholder will pay all underwriting discounts and selling commissions, if any. Representative.
| 58 |
| Table of Contents |
Indemnification
We willhave agreed to indemnify the selling stockholdersunderwriters against liabilities, including some liabilities under the Securities Act, in accordance with the registration rights agreements, or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against civilcertain liabilities, including liabilities under the Securities Act, and to contribute to payments that may arise from any written information furnished to us by the selling stockholders specifically for use in this prospectus, in accordance with the related registration rights provisions, or weunderwriters may be entitledrequired to contribution.make for these liabilities.
OTC and Nasdaq Capital Market
Our common stock is presently quoted on the OTC Pink under the symbol “CELZ”. We have applied to have our common stock listed on The selling stockholders have advised usNasdaq Capital Market under the symbol “CELZ”. We will not consummate this offering unless our common stock is approved for listing on Nasdaq. No assurance can be given that they haveour application will be approved. Trading quotes of securities on an over-the-counter marketplace may not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the salebe indicative of the shares, nor is there an underwritermarket price of those securities on a national securities exchange. We do not intend to apply to list the Public Warrants or coordinating broker actingpre-funded warrants on any national securities exchange or other nationally recognized trading system.
Price Stabilization, Short Positions, and Penalty Bids
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. Specifically, the underwriters may over-allot in connection with this offering by selling more shares and warrants than are set forth on the proposed salecover page of the shares by the selling stockholders. If we are notified by any one or more selling stockholders that any material arrangement has been entered into withthis prospectus. This creates a broker-dealershort position in our common stock for the sale of shares throughits own account. The short position may be either a block trade, special offering, exchange distribution or secondary distributioncovered short position or a purchase bynaked short position. In a broker or dealer, we will file, or cause to be filed, a supplement to this prospectus, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such selling shareholder and of the participating broker-dealer(s), (ii)covered short position, the number of shares common stock or warrants over-allotted by the underwriters is not greater than the number of shares of common stock or warrants that they may purchase in the over-allotment option. In a naked short position, the number of shares of common stock or warrants involved (iii)is greater than the number of shares common stock or warrants in the over-allotment option. To close out a short position, the underwriters may elect to exercise all or part of the over-allotment option. The underwriters may also elect to stabilize the price at which such shares were sold, (iv)of our common stock or reduce any short position by bidding for, and purchasing, common stock in the commissions paidopen market.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or discounts ordealer repays selling concessions allowed to such broker-dealer(s), where applicable, (v)it for distributing a security in this offering because the underwriter repurchases that such broker-dealer(s) didsecurity in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, shares of our common stock in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our common stock at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not conductrequired to engage in these activities, and may discontinue any investigationof these activities at any time without notice. These transactions may be effected on Nasdaq, in the over-the-counter market, or otherwise.
In connection with this offering, the underwriters and selling group members, if any, or their affiliates may engage in passive market making transactions in our common stock immediately prior to verifythe commencement of sales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103 generally provides that:
· | a passive market maker may not effect transactions or display bids for our common stock in excess of the highest independent bid price by persons who are not passive market makers; |
· | net purchases by a passive market maker on each day are generally limited to 30% of the passive market maker’s average daily trading volume in our common stock during a specified two-month prior period or 200 shares, whichever is greater, and must be discontinued when that limit is reached; and |
· | passive market making bids must be identified as such. |
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the representatives of the underwriters and may also be made available on a website maintained by other underwriters. The underwriters may agree to allocate a number of shares to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives of the underwriters to underwriters that may make Internet distributions on the same basis as other allocations. In connection with the offering, the underwriters or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The underwriters have informed us that they do not expect to confirm sales of shares and warrants offered by this prospectus to accounts over which they exercise discretionary authority.
Other than the prospectus in electronic format, the information set outon any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of the prospectus or incorporated by reference in this prospectus, and (vi) other facts material to the transaction.
Once sold under the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be relied upon by investors.
| 59 |
| Table of Contents |
Certain Relationships
Certain of the underwriters and their affiliates may provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business, for which they may receive customary fees and commissions. Roth acted as our exclusive placement agent for our August 2021 private placement in which Roth participated and received cash and warrant compensation in connection therewith.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us, the selling stockholders or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
| 60 |
| Table of Contents |
European Economic Area
In relation to each Member State of the European Economic Area (each a “Relevant State”), no shares have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that it may make an offer to the public in that Relevant State of any shares at any time under the following exemptions under the Prospectus Regulation:
(a) | to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
(b) | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of representatives for any such offer; or |
(c) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of the shares shall require the Issuer or any Manager to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to the shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2 and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Hong Kong
The shares of common stock have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (the “SFO”) of Hong Kong and any rules made thereunder; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong) (the “CO”) or which do not constitute an offer to the public within the meaning of the CO. No advertisement, invitation or document relating to the shares of common stock willhas been or may be freely tradableissued or has been or may be in the handspossession of personsany person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than our affiliates.
The selling stockholders are not restricted aswith respect to the price or prices at which they may sell their shares. Sales of the shares may have an adverse effect on the market price of the common stock.
The validity of the shares of common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made thereunder.
| 61 |
| Table of Contents |
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered underor sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (“ISA”), nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
· | to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and | |
· | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
· | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and | |
· | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
| 62 |
| Table of Contents |
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to be distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. We may not render services relating to the securities within the United Arab Emirates, including the receipt of applications and/or the allotment or redemption of such shares.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
In relation to the United Kingdom, no shares have been offered or will be offered pursuant to this offering to the public in the United Kingdom prior to the publication of a prospectus in relation to the shares that either (i) has been approved by the Financial Conduct Authority, or (ii) is to be treated as if it had been approved by the Financial Conduct Authority in accordance with the transitional provision in Regulation 74 of the Prospectus (Amendment etc.) (EU Exit) Regulations 2019, except that offers of shares may be made to the public in the United Kingdom at any time under the following exemptions under the UK Prospectus Regulation:
(a) | to any legal entity which is a qualified investor as defined under Article 2 of the UK Prospectus Regulation; |
(b) | to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the UK Prospectus Regulation), subject to obtaining the prior consent of representatives for any such offer; or |
(c) | in any other circumstances falling within Section 86 of the Financial Services and Markets Act 2000 (the “FSMA”), |
provided that no such offer of the shares shall require the Issuer or any representative to publish a prospectus pursuant to Section 85 of the FSMA or supplement a prospectus pursuant to Article 23 of the UK Prospectus Regulation.
| 63 |
| Table of Contents |
For the purposes of this provision, the expression an “offer to the public” in relation to the shares in the United Kingdom means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018.
In addition, this prospectus is only being distributed to, and is only directed at, and any investment or investment activity to which this prospectus relates is available only to, and will be engaged in only with, persons who are outside the United Kingdom or persons in the United Kingdom (i) having professional experience in matters relating to investments who fall within the definition of “investment professionals” in Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”); or (ii) who are high net worth entities falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). Persons who are not relevant persons should not take any action on the basis of this prospectus and should not act or rely on it.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock and warrant agent is vStock Transfer LLC, at 18 Lafayette Place, Woodmere, NY 11598. The transfer agent’s telephone number is (212) 828-8436.
LEGAL MATTERS
Selected legal matters with respect to the validity of the securities offered by this prospectus will be passed upon for us by Vance, Higley & Associates, P.C., Attorneys at Law.Fox Rothschild LLP, 101 Park Avenue, New York, NY 10178.
Our financialThe audited consolidated balance sheets at December 31, 2020 and 2019 and the audited consolidated statements of operations, stockholders’ equity and cash flows for the partial yearyears ended December 31, 2015, were2020 and 2019 have been audited by Haynie & Company, and areour independent registered public accounting firm. We have included these financial statements in this registration statement in reliance upon the reports of such reportsfirm given upon thetheir authority of Haynie & Company, as experts in accounting and auditing.
ADDITIONALABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the SEC. You should rely only on the information provided in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of Common Stock. Applicable SEC rules may require us to update this prospectus in the future.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act (SEC File No. 333-_________) relating to the shares of common stock being offered by this prospectus,file annual, quarterly and reference is made to such registration statement. This prospectus constitutes the prospectus of Creative Medical Technology Holdings, Inc., filed as part of the registration statement,current reports, proxy statements and it does not contain allother information in the registration statement, as certain portions have been omitted in accordance with the rules and regulations of the SEC.
Upon the effective date of the registration statement of which this prospectus is a part, we will be required to file reports and other documents with the SEC. We do not presently intend to voluntarily furnish you with a copy of our annual report. You may read and copy any materialsreport, statement or other information that we file with the SEC at the public reference room of the SEC Public Reference Room at 100 F Street, NE.N.E., Washington, DC 20549, between the hours of 10:00 a.m. and 3:00 p.m., except federal holidays and official closings, at the Public Reference Room.D.C. 20549. You may obtain further information on the operation of the Public Reference Roomroom by calling the SEC at 1-800-SEC-0330. Our SEC filings are also available to the public at the SEC’s website at www.sec.gov, as well as our website at https://creativemedicaltechnology.com. Information contained on our website does not constitute a part of this prospectus.
This prospectus is part of a registration statement that we filed with the SEC. This prospectus and any accompanying prospectus supplement do not contain all of the information included in the registration statement, and certain statements contained in this prospectus and any accompanying prospectus supplement about the provisions or contents of any contract, agreement or any other document referred to herein are not necessarily complete. For each of these contracts, agreements or documents filed as an exhibit to the registration statement, we refer you to the actual exhibit for a more complete description of the matters involved. In addition, we have omitted certain parts of the registration statement in accordance with the rules and regulations of the SEC. To obtain all of the information that we filed with the SEC in connection herewith, we refer you to the registration statement, including its exhibits and schedules. You should assume that the information contained in this prospectus and any accompanying prospectus supplement is accurate only as of the date appearing on the Internet website forfront of the SEC at http://www.sec.gov.
INDEX TO FINANCIAL STATEMENTSprospectus or prospectus supplement, as applicable.
| 64 | |
| Table of Contents |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
Table of Contents
| F-1 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and ShareholdersCreative Medical Technology Holdings, Inc.
Phoenix, Arizona
We have audited the accompanying balance sheet of Creative Medical Technology Holdings, Inc. as of December 31, 2015, and the related statements of operations, stockholders' equity, and cash flows for the period from December 30, 2015 (inception) through December 31, 2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Creative Medical Technology Holdings, Inc. as of December 31, 2015, and the results of its operations, and its cash flows for the period from December 30, 2015 (inception) through December 31, 2015, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As disclosed in Note 1 to the financial statements, the Company has negative cash flow from operations and a net capital deficiency. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Haynie & CompanyHaynie & Company
Salt Lake City, Utah
September 1, 2016
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
| September 30, 2016 | December 31, 2015 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 33,456 | $ | 100 | ||||
| Stock subscription receivable | - | 49,500 | ||||||
| Total Current Assets | 33,456 | 49,600 | ||||||
| OTHER ASSETS | ||||||||
| ED Patent net of amortization | 103,281 | - | ||||||
| TOTAL ASSETS | $ | 136,737 | $ | 49,600 | ||||
| LIABILITIES & STOCKHOLDER'S (DEFICIT) EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | 44,849 | $ | - | ||||
| Notes payable - related party current | 100,000 | - | ||||||
| Accrued expenses | 2,822 | - | ||||||
| Management fee payable - related party | 280,000 | - | ||||||
| Notes payable | 15,686 | - | ||||||
| Advances from related party | 2,600 | 100 | ||||||
| Total Current Liabilities | 445,957 | 100 | ||||||
| LONG TERM LIABILIBTY | ||||||||
| Notes payable -related party, net of current portion | 25,000 | - | ||||||
| Accrued expenses, net of current portion | 935 | - | ||||||
| TOTAL LIABILITIES | 471,892 | 100 | ||||||
| Commitments and contingencies | ||||||||
| STOCKHOLDERS' (DEFICIT) EQUITY | ||||||||
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, no shares issued and outstanding par value, | - | - | ||||||
| Common stock, $0.001 par value, 600,000,000 shares authorized, 101,313,750 shares and 32,010,000 shares issued and outstanding, respectively | 101,314 | 32,010 | ||||||
| Additional paid in capital/(deficit) | 135,637 | 17,990 | ||||||
| Accumulated deficit | (572,106 | ) | (500 | ) | ||||
| TOTAL STOCKHOLDERS' (DEFICIT) EQUITY | (335,155 | ) | 49,500 | |||||
| TOTAL LIABILITIES & STOCKHOLDERS' (DEFICIT) EQUITY | $ | 136,737 | $ | 49,600 | ||||
|
| September 30, 2021 |
|
| December 31, 2020 |
| ||
|
|
|
|
|
|
| ||
ASSETS |
|
|
|
|
|
| ||
CURRENT ASSETS |
|
|
|
|
|
| ||
Cash |
| $ | 2,377,930 |
|
| $ | 98,012 |
|
Related party advance |
|
| 200,000 |
|
|
| 0 |
|
Total Current Assets |
|
| 2,577,930 |
|
|
| 98,012 |
|
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
|
Other assets |
|
| 3,282 |
|
|
| 0 |
|
Licenses, net of amortization |
|
| 550,700 |
|
|
| 619,763 |
|
TOTAL ASSETS |
| $ | 3,131,912 |
|
| $ | 717,775 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 454,525 |
|
| $ | 350,899 |
|
Accrued expenses |
|
| 29,920 |
|
|
| 159,771 |
|
Management fee and patent liabilities - related parties |
|
| 250,082 |
|
|
| 468,782 |
|
Convertible notes payable, net of discount of $0 and $409,649, respectively |
|
| 0 |
|
|
| 788,701 |
|
Notes payable, net of discount of $2,219,430 and $0, respectively |
|
| 2,743,430 |
|
|
| 0 |
|
Advances from related party |
|
| 17,300 |
|
|
| 10,800 |
|
Derivative liabilities |
|
| 0 |
|
|
| 38,741,832 |
|
Total Current Liabilities |
|
| 3,495,257 |
|
|
| 40,520,785 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 7,000,000 and 7,000,000 shares authorized, no shares issued and outstanding at September 30, 2021 and December 31, 2020 |
|
| 0 |
|
|
| 0 |
|
Series A preferred stock, $0.001 par value, 3,000,000 shares authorized, 3,000,000 shares issued and outstanding at September 30, 2021 and December 31, 2020 |
|
| 3,000 |
|
|
| 3,000 |
|
Series B preferred stock, $0.001 par value, 1,000 shares authorized, no shares issued and outstanding at September 30, 2021 and December 31, 2020 |
|
| - |
|
|
| 0 |
|
Series C preferred stock, $0.001 par value, 500 shares authorized, no shares issued and outstanding at September 30, 2021 and December 31, 2020 |
|
| 0 |
|
|
| 0 |
|
Common stock, $0.001 par value, 6,000,000,000 shares authorized; 2,458,250 and 1,537,081 issued and 2,458,242 and 1,537,073 outstanding at September 30, 2021 and December 31, 2020, respectively |
|
| 2,458 |
|
|
| 1,537 |
|
Additional paid-in capital |
|
| 38,434,192 |
|
|
| 22,082,689 |
|
Accumulated deficit |
|
| (38,802,995 | ) |
|
| (61,890,236 | ) |
TOTAL STOCKHOLDERS' DEFICIT |
|
| (363,345 | ) |
|
| (39,803,010 | ) |
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT |
| $ | 3,131,912 |
|
| $ | 717,775 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.statements
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Three Months Ended June September 30, 2016 | For the Nine Months Ended September 30, 2016 | For the Period from December 30, 2015 (Inception) to December 31, 2015 | ||||||||||
| (Unaudited) | (Unaudited) | |||||||||||
| REVENUES | $ | - | $ | - | $ | - | ||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 45,174 | 67,566 | - | |||||||||
| General and administrative | 164,732 | 493,484 | 500 | |||||||||
| Amortization of patent costs | 2,637 | 6,719 | - | |||||||||
| TOTAL EXPENSES | 212,543 | 567,769 | 500 | |||||||||
| OTHER INCOME/(EXPENSE) | ||||||||||||
| Interest expense | (1,971 | ) | (3,837 | ) | - | |||||||
| OPERATING LOSS | (214,514 | ) | (571,606 | ) | (500 | ) | ||||||
| NET LOSS | (214,514 | ) | $ | (571,606 | ) | $ | (500 | ) | ||||
| BASIC AND DILUTED LOSS PER SHARE | (0.00 | ) | (0.01 | ) | �� | (0.00 | ) | |||||
| WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING | 100,621,359 | 90,866,349 | 32,010,000 | |||||||||
|
| For the Three Months Ended September 30, 2021 |
|
| For the Three Months Ended September 30, 2020 |
|
| For the Nine Months Ended September 30, 2021 |
|
| For the Nine Months Ended September 30, 2020 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
Revenues |
| $ | 10,000 |
|
| $ | 77,000 |
|
| $ | 20,000 |
|
| $ | 147,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenues |
|
| 4,000 |
|
|
| 17,600 |
|
|
| 8,500 |
|
|
| 42,596 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
| 6,000 |
|
|
| 59,400 |
|
|
| 11,500 |
|
|
| 104,404 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 59,180 |
|
|
| 0 |
|
|
| 59,180 |
|
|
| 0 |
|
Selling, general and administrative |
|
| 757,235 |
|
|
| 285,100 |
|
|
| 1,536,479 |
|
|
| 846,154 |
|
Amortization of patent costs |
|
| 23,021 |
|
|
| 16,771 |
|
|
| 69,063 |
|
|
| 50,021 |
|
TOTAL EXPENSES |
|
| 839,436 |
|
|
| 301,871 |
|
|
| 1,664,722 |
|
|
| 896,175 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss |
|
| (833,436 | ) |
|
| (242,471 | ) |
|
| (1,653,222 | ) |
|
| (791,771 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME/(EXPENSE) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
| (1,305,273 | ) |
|
| (289,210 | ) |
|
| (1,875,687 | ) |
|
| (910,236 | ) |
Gain on extinguishment of convertible notes |
|
| 489,157 |
|
|
| 0 |
|
|
| 585,601 |
|
|
| 0 |
|
Change in fair value of derivatives liabilities |
|
| (194,044 | ) |
|
| 926,532 |
|
|
| 26,030,549 |
|
|
| 3,996,775 |
|
Total other income (expense) |
|
| (1,010,160 | ) |
|
| 637,322 |
|
|
| 24,740,463 |
|
|
| 3,086,539 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME (LOSS) BEFORE PROVISION FOR INCOME TAXES |
|
| (1,843,596 | ) |
|
| 394,851 |
|
|
| 23,087,241 |
|
|
| 2,294,768 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME (LOSS) |
| $ | (1,843,596 | ) |
| $ | 394,851 |
|
| $ | 23,087,241 |
|
| $ | 2,294,768 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BASIC NET INCOME (LOSS) PER SHARE |
| $ | (0.75 | ) |
| $ | 0.58 |
|
| $ | 9.98 |
|
| $ | 6.21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DILUTED NET INCOME (LOSS) PER SHARE |
| $ | (0.75 | ) |
| $ | 0.14 |
|
| $ | 9.77 |
|
| $ | 1.07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING - BASIC |
|
| 2,452,076 |
|
|
| 684,983 |
|
|
| 2,313,005 |
|
|
| 369,423 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING - DILUTED |
|
| 2,452,076 |
|
|
| 2,725,722 |
|
|
| 2,363,145 |
|
|
| 2,138,913 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.statements
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS' (DEFICIT) EQUITY
FOR THE PERIODS ENDED DECEMBER 31, 2015 AND SEPTEMBER 30, 2016 (UNAUDITED)
| Total | ||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Paid in | Accumulated | Stockholders' Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance, December 30, 2015 | - | $ | - | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Common, stock issued for subscription | - | - | 32,010,000 | 32,010 | 17,990 | - | 50,000 | |||||||||||||||||||||
| Net (loss) | - | - | - | - | - | (500 | ) | (500 | ) | |||||||||||||||||||
| Balance 12/31/15 | - | $ | - | 32,010,000 | $ | 32,010 | $ | 17,990 | $ | (500 | ) | $ | 49,500 | |||||||||||||||
| Common stock issued for cash | - | - | 1,000,000 | 1,000 | 99,000 | - | 100,000 | |||||||||||||||||||||
| Common stock issued for cash from related party | - | - | 300,000 | 300 | 29,700 | - | 30,000 | |||||||||||||||||||||
| Common stock issued for medical technology license | - | - | 323,333 | 323 | 677 | - | 1,000 | |||||||||||||||||||||
| Common stock issued for ED Patent | - | - | 64,996,667 | 64,667 | 35,333 | - | 100,000 | |||||||||||||||||||||
| Acquisition of Creative Medical Technology Holdings, Inc. as part of recapitalization | - | - | 18,113,750 | 18,114 | (62,898 | ) | - | (44,784 | ) | |||||||||||||||||||
| Cancellation of Shares Outstanding | - | - | (15,100,000 | ) | (15,100 | ) | 15,100 | - | - | |||||||||||||||||||
| Stock-based compensation | - | - | - | - | 735 | - | 735 | |||||||||||||||||||||
| Net loss for nine months ended September 30, 2016 | - | - | - | - | - | (571,606 | ) | (571,606 | ) | |||||||||||||||||||
| Balance September 30, 2016 (unaudited) | - | $ | - | 100,313,750 | $ | 100,314 | $ | 135,637 | $ | (572,106 | ) | $ | (335,155 | ) | ||||||||||||||
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Nine Months Ended September 30, 2016 | For the Period from December 30, 2015 (Date of Inception) to December 31, 2015 | |||||||
| (Unaudited) | ||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (571,606 | ) | $ | (500 | ) | ||
| Adjustment to reconcile net loss to net cash from operating activities: | ||||||||
| Stock-based compensation | 735 | |||||||
| Amortization | 6,719 | - | ||||||
| Changes in assets and liabilities: | ||||||||
| Accounts payable | 20,565 | - | ||||||
| Accrued expenses | 3,757 | - | ||||||
| Management fee payable | 280,000 | 100 | ||||||
| Net cash (used) by operating activities | (259,830 | ) | (400 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of licenses | (10,000 | ) | ||||||
| Net cash (used) by investing activities | (10,000 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Cash received from notes payable – related party | 125,000 | - | ||||||
| Cash paid on notes payable | (4,314 | ) | - | |||||
| Cash received from subscription receivable | 50,000 | - | ||||||
| Proceeds from sale of common stock | 130,000 | |||||||
| Related party advances | 2,500 | |||||||
| Contributed capital | - | 500 | ||||||
| Net cash provided by financing activities | 303,186 | 500 | ||||||
| NET INCREASE IN CASH | 33,356 | 100 | ||||||
| BEGINNING CASH BALANCE | 100 | - | ||||||
| ENDING CASH BALANCE | $ | 33,456 | 100 | |||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash payments for interest | $ | - | - | |||||
| Cash payments for income taxes | $ | - | - | |||||
| NON CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Purchase of patents by issuance of common stock | $ | 100,000 | - | |||||
| Payable assumed in reverse merger | $ | 24,284 | - | |||||
| Note payable assumed in reverse merger | $ | 20,000 | - | |||||
| Fair value of warrants issued in private placement | 8,197 | |||||||
|
| For the Nine Months Ended September 30, 2021 |
|
| For the Nine Months Ended September 30, 2020 |
| ||
|
|
|
|
|
|
| ||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
| ||
Net income |
| $ | 23,087,241 |
|
| $ | 2,294,768 |
|
Adjustments to reconcile net income to net cash from operating activities: |
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| 595,380 |
|
|
| 68,242 |
|
Amortization |
|
| 69,063 |
|
|
| 50,021 |
|
Amortization of debt discounts |
|
| 1,755,104 |
|
|
| 804,898 |
|
Change in fair value of derivatives liabilities |
|
| (26,030,549 | ) |
|
| (3,996,775 | ) |
Increase in principal and accrued interest balances due to penalty provision |
|
| 93,821 |
|
|
| 0 |
|
Gain on extinguishment of convertible notes |
|
| (585,601 | ) |
|
| 0 |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
Accounts receivable |
|
| 0 |
|
|
| 5,600 |
|
Accounts payable |
|
| 103,626 |
|
|
| 23,666 |
|
Accrued expenses |
|
| 26,169 |
|
|
| 126,838 |
|
Management fee payable |
|
| (168,700 | ) |
|
| 131,200 |
|
Net cash used in operating activities |
|
| (1,054,446 | ) |
|
| (491,542 | ) |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Advance to related party |
|
| (200,000 | ) |
|
| 0 |
|
Net cash used in investing activities |
|
| (200,000 | ) |
|
| - |
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Proceeds from note payable |
|
| 3,887,750 |
|
|
| 0 |
|
Payments on notes payable |
|
| (105,000 | ) |
|
| - |
|
Payment of debt issuance costs |
|
| (443,239 | ) |
|
| 0 |
|
Payment of deferred offering costs |
|
| (3,282 | ) |
|
| - |
|
Payments on convertible notes payable |
|
| 0 |
|
|
| (17,000 | ) |
Proceeds from convertible notes payable |
|
| 435,040 |
|
|
| 458,600 |
|
Proceeds from sale of preferred stock |
|
| 462,000 |
|
|
| 0 |
|
Related party advances |
|
| 226,500 |
|
|
| - |
|
Repayment of related party advances |
|
| (220,000 | ) |
|
| - |
|
Payments to settle convertible notes payable and warrants |
|
| (705,405 | ) |
|
| 0 |
|
Net cash provided from financing activities |
|
| 3,534,364 |
|
|
| 441,600 |
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH |
|
| 2,279,918 |
|
|
| (49,942 | ) |
BEGINNING CASH BALANCE |
|
| 98,012 |
|
|
| 88,648 |
|
ENDING CASH BALANCE |
| $ | 2,377,930 |
|
| $ | 38,706 |
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
Cash payments for interest |
| $ | 9,186 |
|
| $ | 6,000 |
|
Cash payments for income taxes |
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
NON-CASH INVESTING AND FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Accrued dividends on preferred stock |
| $ | 27,725 |
|
| $ | 0 |
|
Warrants issued with notes payable and as a service fee |
| $ | 2,097,629 |
|
| $ | 0 |
|
Conversion of notes payable, accrued interest and derivative liabilities into common stock |
| $ | 13,747,415 |
|
| $ | 2,772,149 |
|
Conversion of management fees and patent liability into common stock |
| $ | 50,000 |
|
| $ | 160,000 |
|
Discounts on convertible notes payable due to derivative liabilities |
| $ | 134,640 |
|
| $ | 0 |
|
Exchange of preferred stock for notes payable |
| $ | 572,275 |
|
| $ | 0 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 | |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENT OF STOCKHOLDERS’ DEFICIT
|
| Series A Preferred Stock |
|
| Series B Preferred Stock | Series C Preferred Stock | Common Stock | Additional Paid-in |
|
| Accumulated |
|
| Total Stockholders' |
| |||||||||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
| Capital |
|
| Deficit |
|
| Deficit |
| ||||||||||||
December 31, 2020 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 1,537,073 |
|
| $ | 1,537 |
|
| $ | 22,082,689 |
|
| $ | (61,890,236 | ) |
| $ | (39,803,010 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from sales of preferred stock |
|
| - |
|
|
| 0 |
|
|
| 350 |
|
|
| 321,000 |
|
|
| 150 |
|
|
| 141,000 |
|
|
| 4,286 |
|
|
| 4 |
|
|
| (4 | ) |
|
| 0 |
|
|
| 462,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for related party patent and management liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 89,286 |
|
|
| 89 |
|
|
| 49,911 |
|
|
| 0 |
|
|
| 50,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 789,727 |
|
|
| 790 |
|
|
| 1,382,541 |
|
|
| 0 |
|
|
| 1,383,331 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 12,364,084 |
|
|
| 0 |
|
|
| 12,364,084 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends on preferred stock |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| (27,725 | ) |
|
| 0 |
|
|
| (27,725 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cashless exercise of warrants |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 37,870 |
|
|
| 38 |
|
|
| (38 | ) |
|
| 0 |
|
|
| 0 |
|
Warrants issued with notes payable |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 2,097,629 |
|
|
| 0 |
|
|
| 2,097,629 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock redemption |
|
| - |
|
|
| 0 |
|
|
| (350 | ) |
|
| (321,000 | ) |
|
| (150 | ) |
|
| (141,000 | ) |
|
| - |
|
|
| 0 |
|
|
| (110,275 | ) |
|
| 0 |
|
|
| (572,275 | ) |
Stock-based compensation |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 595,380 |
|
|
| 0 |
|
|
| 595,380 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| 23,087,241 |
|
|
| 23,087,241 |
|
September 30, 2021 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 2,458,242 |
|
| $ | 2,458 |
|
| $ | 38,434,192 |
|
| $ | (38,802,995 | ) |
| $ | (363,345 | ) |
|
| Series A Preferred Stock |
|
| Series B Preferred Stock |
|
| Series C Preferred Stock |
|
| Common Stock |
|
| Additional Paid-in |
|
| Accumulated |
|
| Total Stockholders' |
| |||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| Deficit |
| |||||||||||
June 30, 2021 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| 350 |
|
| $ | 321,000 |
|
|
| 150 |
|
| $ | 141,000 |
|
|
| 2,440,614 |
|
| $ | 2,440 |
|
| $ | 35,777,506 |
|
| $ | (36,959,399 | ) |
| $ | (714,453 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 17,628 |
|
|
| 18 |
|
|
| 153,962 |
|
|
| 0 |
|
|
| 153,980 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 138,731 |
|
|
| 0 |
|
|
| 138,731 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends on preferred stock |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| (6,973 | ) |
|
| 0 |
|
|
| (6,973 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued with notes payable |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 2,097,629 |
|
|
| 0 |
|
|
| 2,097,629 |
|
Preferred stock redemption |
|
| - |
|
|
| 0 |
|
|
| (350 | ) |
|
| (321,000 | ) |
|
| (150 | ) |
|
| (141,000 | ) |
|
| - |
|
|
| 0 |
|
|
| (110,275 | ) |
|
| 0 |
|
|
| (572,275 | ) |
Stock-based compensation |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 383,612 |
|
|
| 0 |
|
|
| 383,612 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| (1,843,596 | ) |
|
| (1,843,596 | ) |
September 30, 2021 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 2,458,242 |
|
| $ | 2,458 |
|
| $ | 38,434,192 |
|
| $ | (38,802,995 | ) |
| $ | (363,345 | ) |
The accompanying notes are an integral part of these consolidated financial statements
| F-5 |
| Table of Contents |
|
| Series A Preferred Stock |
|
| Series B Preferred Stock |
|
| Series C Preferred Stock |
|
| Common Stock |
|
| Additional Paid-in |
|
| Accumulated |
|
| Total Stockholders' |
| |||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| Deficit |
| |||||||||||
December 31, 2019 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 44,978 |
|
| $ | 45 |
|
| $ | 17,490,462 |
|
| $ | (25,565,006 | ) |
| $ | (8,071,499 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for related party management liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 128,630 |
|
|
| 129 |
|
|
| 159,871 |
|
|
| 0 |
|
|
| 160,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 777,865 |
|
|
| 778 |
|
|
| 1,032,640 |
|
|
| 0 |
|
|
| 1,033,418 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 1,738,731 |
|
|
| 0 |
|
|
| 1,738,731 |
|
Stock-based compensation |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 68,242 |
|
|
| 0 |
|
|
| 68,242 |
|
Difference in shares from reverse stock split |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 3 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| 2,294,768 |
|
|
| 2,294,768 |
|
September 30, 2020 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 951,476 |
|
| $ | 952 |
|
| $ | 20,489,946 |
|
| $ | (23,270,238 | ) |
| $ | (2,776,340 | ) |
|
| Series A Preferred Stock |
|
| Series B Preferred Stock |
|
| Series C Preferred Stock |
|
| Common Stock |
|
| Additional Paid-in |
|
| Accumulated |
|
| Total Stockholders' |
| |||||||||||||||||||||||
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| Deficit |
| |||||||||||
June 30, 2020 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 490,644 |
|
|
| 491 |
|
| $ | 19,456,926 |
|
| $ | (23,665,089 | ) |
| $ | (4,204,672 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for related party management liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 84,656 |
|
|
| 85 |
|
|
| 39,915 |
|
|
| 0 |
|
|
| 40,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 376,176 |
|
|
| 376 |
|
|
| 427,786 |
|
|
| 0 |
|
|
| 428,162 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 497,077 |
|
|
| 0 |
|
|
| 497,077 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 68,242 |
|
|
| 0 |
|
|
| 68,242 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| 394,851 |
|
|
| 394,851 |
|
September 30, 2020 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| - |
|
| $ | 0 |
|
|
| - |
|
| $ | 0 |
|
|
| 951,476 |
|
| $ | 952 |
|
| $ | 20,489,946 |
|
| $ | (23,270,238 | ) |
| $ | (2,776,340 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015 (AUDITED) AND SEPTEMBER 30, 2016 (UNAUDITED)2021
Introductory Comment
Unless otherwise indicated, any reference to “our company”, “we”, “us”, or “our” refers to Creative Medical Technology Holdings, Inc., and as applicable to its wholly owned subsidiaries.
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization - – Creative Medical Technologies Holdings, Inc. is a commercial stage biotechnology company focused on immunology, urology, orthopedics and neurology using adult stem cell treatments.
Our subsidiary, Creative Medical Technologies, Inc. (the “Company” or “CMT”(“CMT”), was incorporatedoriginally created to monetize U.S. Patent No. 8,372,797 and related intellectual property related to the treatment of erectile dysfunction (“ED”), which it acquired in February 2016. Subsequently, we have expanded our development and acquisition of intellectual property beyond urology to include therapeutic treatments utilizing “re-programmed” stem cells, and the treatment of neurologic disorders, lower back pain, type I diabetes, and heart, liver, kidney and other diseases using various types of stem cells through our ImmCelz, Inc., StemSpine, Inc. and AmnioStem LLC subsidiaries. However, neither ImmCelz Inc., StemSpine Inc. nor AmnioStem LLC have commenced commercial activities.
We currently conduct substantially all of our commercial operations through CMT, which markets and sells our CaverStem® and FemCelz® disposable kits utilized by physicians to perform autologous procedures that treat erectile dysfunction and female sexual dysfunction, respectively. Our CaverStem® and FemCelz® kits are currently available through physicians at eight locations in the State of Nevada on December 30, 2015, and, subject to the reverse merger discussed below, elected December 31 as the Company’s year-end. The Company’s activities to date have consisted of developing a business plan, raising capital through the issuance of equity instruments and notes payable from a related party, and obtaining the rights via a license agreement to certain medical technology.United States.
On May 18, 2016,In addition to our CaverStem® and FemCelz® products, we are currently in the Company was acquired byprocess of recruiting clinical sites for our StemSpine® Regenerative Stem Cell Procedure for the Treatment of Degenerative Disc Disease. Our StemSpine® treatment is an autologous procedure that utilizes a public company, Creative Medical Technology Holdings, Inc. (formerly Jolley Marketing, Inc.) in a “reverse merger” wherein the Company was recapitalized via the common stock of Jolley.patient’s own stem cells to treat lower back pain.
Creative Medical Technology Holdings,In 2020, through our ImmCelz Inc., formerly Jolley Marketing, Inc. (the “Public Company” or “CMTH”) was incorporated on December 3, 1998, subsidiary, we began exploring the development of treatments that utilize a patient’s own extracted immune cells that are then “reprogrammed” by culturing them outside the patient’s body with optimized stem cells. The immune cells are then re-injected into the patient from whom they were extracted. We believe this process endows the immune cells with regenerative properties that may be suitable for the treatment of stroke victims, among other indications. In contrast to other stem cell-based approaches, the immune cells are significantly smaller in Nevada. On May 18, 2016, the Public Company consummated an Agreementsize than stem cells and Plan of Mergerare believed to acquire allmore effectively penetrate areas of the outstanding capital stock of CMT in a transaction which has been accounted for as a recapitalization of the Public Company as follows:damaged tissues and induce regeneration.
On September 14, 2016, CMT filed a certificate of organization for Amniostem LLC, a Nevada limited liability company and wholly owned subsidiary of CMT. Amniostem, LLC was formed to create and/or license intellectual property in the area of amniotic fluid derived stem cells for therapeutic applications. With this company management intends to address what it believes are unmet medical needs through development and commercialization of amniotic fluid stem cell based technologies. Management intends to seek in licensing opportunities as well as create intellectual property in-house for this newly created entity.
Use of Estimates - – The preparation of the financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Basis of Presentation / Liquidity - – The accompanying unaudited condensed consolidated financial statements have been prepared without audit. In the opinion of management, all adjustments (which include only normal recurring adjustments) necessary to present fairly the financial position, results of operations and cash flows at September 30, 2016,2021 and for the threethree- and nine month periodsix-month periods then ended have been made. Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been condensed or omitted. The operations for the threethree- and nine-month periodsix-month periods ended September 30, 2016,2021, are not necessarily indicative of the operating results for the full year.
Going Concern - – The accompanying unaudited condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. However, during the nine monthsnine-month period ended September 30, 2016,2021, the Company incurred a net loss of $571,606, had negative cash flows from operating activities had working capital of $49,500 at December 31, 2015$1,054,446 and had a working capital deficit of $412,501 at September 30, 2016 and had no revenue-generating activities.$917,327. These factors raise substantial doubt about the ability of the Company to continue as a going concern. In August 2021, the Company received approximately $3.7 million in senior notes. The proceeds from the senior notes were used to settle outstanding convertible notes and is being used to fund operations. The senior notes will require repayment prior to February 11, 2022 and thus a short-term liability. In this regard, management is proposing to raise any necessary additional funds not provided by operations through loans or through additional sales of equity securities. If the Company raises additional funds through offerings of shares of common stock or other securities, such offerings would cause dilution of current stockholders’ percentage ownership in the Company, which could be substantial. Future offerings could also have a material and adverse effect on the price of the Company’s common stock. On September 28, 2021, the Company filed a Registration Statement on Form S-1 with the Securities and Exchange Commission to raise capital through the offer and sale of units consisting of shares of common stock and warrants to purchase additional shares of common stock in a firm commitment underwriting to be conducted by Roth Capital Partners. However, there can be no assurance that the Company will be successful in completing the offering. The securities to be sold in the offering may not be sold nor may offers to buy be accepted prior to the time the Registration Statement becomes effective. There is no assurance that the Company will be successful in raising this additional capital or in achieving profitable operations. The unaudited condensed consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
| F-7 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
Risks and Uncertainties - On January 30, 2020, the World Health Organization declared the COVID-19 outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the COVID-19 include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and businesses. The COVID-19 and actions taken to mitigate it have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is unknown how long these conditions will last and what the complete financial effect will be to the company, to-date, the Company is experiencing a reduction in revenues due to the prioritization of medical resources to address the COVID-19 outbreak. In several of our markets, most non-essential (including elective) procedures have been placed on hold. While this has a negative financial impact to our revenues, there have been the same reductions to our costs. Additionally, since the Company maintains no inventory and require nearly all of customers to pre-pay, there is no risk to receivables or inventory write-downs. The company expects existing orders temporarily on hold and continued sales, training and patient treatments will resume once the physician’s offices are back to being fully operational.
Revenue - We have adopted the new revenue recognition standards that went into effect on January 1, 2019. All revenues reported in 2019 and beyond reflect those standards. Adoption of the standards had no effect on the Company’s revenues.
Fair Value of Financial Instruments - The Company’s financial instruments consist of cash and cash equivalents, convertible notes, and payables. The carrying amount of cash and cash equivalents and payables approximates fair value because of the short-term nature of these items.
When determining fair value, whenever possible the Company uses observable market data, and relies on unobservable inputs only when observable market data is not available. As of September 30, 2021, and December 31, 2020, the Company didn’t have any Level 1 or 2 financial instruments. The table below reflects the results of our Level 3 fair value calculations:
|
| Notes |
|
| Warrants |
|
| Total |
| |||
Derivative liability at December 31, 2020 |
| $ | 37,343,835 |
|
| $ | 1,397,997 |
|
| $ | 38,741,832 |
|
Addition of new conversion option derivatives |
|
| 1,077,757 |
|
|
| 0 |
|
|
| 1,077,757 |
|
Extinguishment/modification |
|
| (726,998 | ) |
|
| (346 | ) |
|
| (727,344 | ) |
Conversion of note derivatives |
|
| (10,494,316 | ) |
|
| (1,869,768 | ) |
|
| (12,364,084 | ) |
Change in fair value |
|
| (27,200,278 | ) |
|
| 472,117 |
|
|
| (26,728,161 | ) |
Derivative liability at September 30, 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
Basic and Diluted Loss Per Share – The Company follows Financial Accounting Standards Board (“FASB”) ASC 260 Earnings per Share to account for earnings per share. Basic earnings per share (“EPS”) calculations are determined by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share calculations are determined by dividing net income by the weighted average number of common shares and dilutive common share equivalents outstanding. Dilutive common share equivalents include the dilutive effect of in-the-money share equivalents, which are calculated, based on the average share price for each period using the treasury stock method. Under the treasury stock method, the exercise price of an award, if any, the amount of compensation cost, if any, for future service that the Company has not yet recognized, and the estimated tax benefits that would be recorded in paid-in capital, if any, when an award is settled are assumed to be used to repurchase shares in the current period. During periods when common stock equivalents, if any, are anti-dilutive they are not considered in the computation.
| F-8 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
The following is a summary of outstanding securities which have been included in the calculation of diluted net income per share and reconciliation of net income to net income available to common stockholders for the nine-months ended September 30, 2021.
|
| For the Three Months Ended September 30, 2020 |
|
| For the Nine Months Ended September 30, 2021 |
|
| For the Nine Months Ended September 30, 2020 |
| |||
Weighted average common shares outstanding used in calculating basic earnings per share |
|
| 684,983 |
|
|
| 2,313,005 |
|
|
| 369,423 |
|
Effect of Series B and C preferred stock |
|
| 0 |
|
|
| - |
|
|
| - |
|
Effect of warrants |
|
| 47,784 |
|
|
| 50,140 |
|
|
| 56,313 |
|
Effect of convertible notes payable |
|
| 1,579,107 |
|
|
| - |
|
|
| 1,307,727 |
|
Effect of convertible related party management fee and patent liabilities |
|
| 413,896 |
|
|
| - |
|
|
| 413,896 |
|
Weighted average common shares outstanding used in calculating diluted earnings per share |
|
| 2,452,076 |
|
|
| 2,363,145 |
|
|
| 2,138,913 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income as reported |
| $ | 394,851 |
|
| $ | 23,087,241 |
|
| $ | 2,294,768 |
|
Add - Interest on convertible notes payable |
|
| 28,228 |
|
|
| - |
|
|
| 105,338 |
|
Net income available to common stockholders |
| $ | 423,079 |
|
| $ | 23,087,241 |
|
| $ | 2,400,106 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted income per Share |
| $ | 0.16 |
|
| $ | 9.77 |
|
| $ | 1.12 |
|
The Company excluded 7 options and 18 warrants from the computation of diluted net income per share for the nine-months ended September 30, 2021 as their exercise prices were in excess of the average closing market price of the Company’s common stock during that period.
During the three-month period ended September 30, 2021, the Company had 7 options and 481,351 warrants to purchase common stock outstanding. The effect during the three-month period ended September 30, 2021 was anti-dilutive due to the net loss during that period.
| F-9 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
The Company excluded 7options and 17,842 warrants from the computation of diluted net income per share for the three-months ended September 30, 2020 as their exercise prices were in excess of the average closing market price of the Company’s common stock during that period. The Company excluded 7 options and 413 warrants from the computation of diluted net income per share for the nine-months ended September 30, 2020 as their exercise prices were in excess of the average closing market price of the Company’s common stock during that period.
On November 10, 2021, we effected a 1-for-500 reverse split of our authorized and issued and outstanding shares of common stock. All share references have been restated for this reverse split to the earliest period presented. As a result of the split, the authorized shares of the Company’s common stock decreased to 50,000,000 shares.
Recent Accounting Pronouncements – The Company has reviewed all recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operation, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its financial statements.
NOTE 2 – LICENSING AGREEMENTS
ED Patent – The Company acquired a patent from CMH. Amortization expense of $2,493 and $7,479 were recorded for the three- and nine-month periods ended September 30, 2021. As of September 30, 2021, and December 31, 2020, the carrying value of the patent was $43,481 and $50,960, respectively. The Company expects to amortize approximately $9,972 annually through 2026 related to the patent costs.
Multipotent Amniotic Fetal Stem Cells License Agreement - In August 2016, CMT entered into a License Agreement with a University. This license agreement grants to CMT the exclusive right to all products derived from a patent for use of multipotent amniotic fetal stem cells composition of matter throughout the world during the period ending on the expiration date of the longest-lived patent rights under the patent. CMT paid the University an initial license fee within 30 days of entering into the agreement. CMT is also required to pay annual license maintenance fees on each anniversary date of the agreement, which maintenance fees would be credited toward any earned royalties for any given period. The License Agreement provides for payment of various milestone payments and earned royalties on the net sales of licensed products by CMT or any sub licensee. CMT is also required to reimburse the University for any future costs associated with maintaining the patent. CMT may terminate the license agreement for any reason upon 90 days’ written notice and the University may terminate the agreement in the event CMT fails to meet its obligations set forth therein, unless the breach is cured within 30 days of the notice from the University specifying the breach. CMT is also obligated to indemnify the University against claims arising due to the exercise of the license by CMT or any sub licensee. As of September 30, 2021, and December 31, 2020, no amounts are currently due to the University.
The Company estimates that the patent expires in February 2026 and has elected to amortize the patent through the period of expiration on a straight-line basis. Amortization expenses of $293 and $879 were recorded for the three- and nine-month periods ended September 30, 2021. Amortization expenses of $293 and $882 was recorded for the three- and nine-month periods ended September 30, 2020. As of September 30, 2021 and December 31, 2020, the carrying values of the patent were $4,670and $5,549, respectively. The Company expects to amortize approximately $1,172 annually through 2026 related to the patent costs.
Lower Back Patent – The Company, through its subsidiary StemSpine, LLC, acquired a patent from CMH, a related company, on August 16, 2017, covering the use of various stem cells for the treatment of lower back pain from pursuant to a Patent Purchase Agreement, which was amended in November 2017. As amended, the agreement provides the following:
· | The Company is required to pay CMH $100,000 within 30 days of demand as an initial payment. | |
· | In the event the Company determines to pursue the technology via use of autologous cells, the Company will pay CMH: |
o | $100,000 upon the signing agreement with a university for the initiation of an IRB clinical trial. |
o | $200,000, upon completion of the IRB clinical trial. |
o | $300,000 in the event we commercialize the technology via use of autologous cells by a physician without a clinical trial. |
· | In the event the Company determines to pursue the technology via use of allogenic cells, the Company will pay CMH: |
o | $100,000 upon filing an IND with the FDA. |
o | $200,000 upon dosing of the first patient in a Phase 1-2 clinical trial. |
o | $400,000 upon dosing the first patient in a Phase 3 clinical trial. |
· | Payment may be made in cash or shares of our common at a discount of 30% to the recent trading price. | |
· | In the event the Company’s shares of common stock trade below $0.01 per share for two or more consecutive trading days, the number of any shares issuable as payment doubles. | |
· | For a period of five years from the date of the first sale of any product derived from the patent, the Company is required to make royalty payments of 5% from gross sales of products, and 50% of sale price or ongoing payments from third parties for licenses granted under the patent to third parties. |
| F-10 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
The patent expires on May 19, 2027 and the Company has elected to amortize the patent over a ten-year period on a straight-line basis. Amortization expenses of $2,500 and $7,500 were recorded for the three- and nine-month periods ended September 30, 2021. As of September 30, 2021, and December 31, 2020, the carrying value of the initial patent license was $57,500 and $65,000, respectively. The Company expects to amortize approximately $10,000 annually through 2027 related to the patent costs.
In November 2019, following a successful international pilot study, the Company elected to initiate commercialization of the StemSpine procedure using autologous stem cells. As a result, the Company is obligated to pay CMH $300,000 pursuant to the Patent Purchase Agreement as described above. During the nine-months ended September 30, 2021, $50,000 of this amount was converted into 89,286 shares of the Company’s common stock. As of September 30, 2021, the remaining liability balance was $0. The Company has elected to amortize the patent over a ten-year period on a straight-line basis. Amortization expense of $11,485 and $34,455 were recorded for the three- and nine-month periods ended September 30, 2021. Amortization expense of $11,485 and $34,455 were recorded for the three- and nine-month periods ended September 30, 2020, As of September 30, 2021 and December 31, 2020, the carrying value of the patent was $213,799 and $248,254, respectively. The Company expects to amortize approximately $46,000 annually through 2027 related to the patent costs.
ImmCelzTM - On December 28, 2020, ImmCelz, Inc. (“ImmCelz”), a newly formed Nevada corporation and wholly owned subsidiary of the Company, entered into a Patent License Agreement dated December 28, 2020 (the “Agreement”), with Jadi Cell, LLC. (“Jadi”), a company controlled by Dr. Amit Patel, a Board Member. The Agreement grants to ImmCelzTM the patent rights under U.S. Patent# 9,803,176 B2, “Methods and compositions for the clinical derivation of an allogenic cell and therapeutic uses”. The contract grants ImmCelzTM access to proprietary process of expanding the master cell bank of Jadi Cell LLC, as currently practiced by Licensor and as documented in standard operating procedures (SOPs) and other written documentation. The terms of the agreement are as follows:
· | Licensee shall pay Licensor a license fee of 250,000 (the “Upfront Royalty”), which can also be paid in CELZ stock at a discount of 25% of the closing price of $1.85, which is based on the date of this agreement | |
· | Within thirty (30) days of the end of each calendar quarter during the term of this Agreement, Licensee will pay Licensor five percent (5%) of the Net Income of ImmCelzTM. during such calendar quarter (the “Continuing Royalty”) | |
· | in one or a series of related transactions, of all or substantially all of the business or assets of Licensee ImmCelz, Inc. (“Sale of Assets”) will result in a one-time ten-percent allocation to the licensor, the Continuing Royalty will be calculated at five percent (5%) of the Net Income of Licensee in any calendar quarter in which the Net Income in such calendar quarter reflects the receipt of any consideration from such Sale of Assets. |
As a result, the Company is obligated to pay Jadi $250,000 pursuant to the Patent License Agreement as described above.
The Company has elected to amortize the patent over a ten-year period on a straight-line basis. Amortization expense of $6,250 and $18,750 were recorded for the three- and nine-month periods ended September 30, 2021. There was no amortization expense recorded for the three- and nine-month periods ended September 30, 2020. As of September 30, 2021, and December 31, 2020, the carrying values of the patent were $231,250 and $250,000, respectively. The Company expects to amortize approximately $25,000annually through 2030 related to the licensing costs.
| F-11 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
NOTE 3 – RELATED PARTY TRANSACTIONS
Up until September 15, 2021, the Company was a party to an agreement with CMH under which the Company was obligated to reimburse CMH for services of the Company’s executive officers and directors employed by CMH and performing services for the Company. At the option of CMH, the amounts owed to CMH under the agreement were payable from time to time in shares of common stock of the Company at a price equal to a 30% discount to the lowest closing price during the 20 trading days prior to time CMH provided notice of its exercise of this option. The agreement originally provided for a monthly reimbursement in the amount of $35,000, which amount was increased to $45,000 effective January 1, 2019. During the three months ended September 30, 2021 and 2020, the Company recorded $112,500 and $135,000, respectively in expense in connection with this agreement. Following the termination of this agreement with CMH in September 2021, the Company entered into direct employment relationships with its executive officers, and direct consulting arrangements with its non-employee directors. As of September 30, 2021, no amounts were owed to CMH under the terminated agreement. As of December 31, 2020, amounts due to CMH under the arrangement were $18,782.
On May 28, 2021, our CEO, Mr. Timothy Warbington, and Board Member, Dr. Amit Patel, advanced the company $50,000 and $150,000 respectively. The two notes were repaid in August 2021.
See Note 2 for discussion of an additional related party transaction with CMH.
NOTE 4 – DEBT
On August 11, 2021, we completed the sale of 15% Original Issue Discount Senior Notes (“Bridge Notes”) in the aggregate principal amount of $4,456,176 to a group of institutional investors (the “Purchasers”). In connection with the sale of the Bridge Notes, holders of our shares of Series B Preferred Stock and Series C Preferred Stock exchanged such preferred stock for additional Bridge Notes in the aggregate principal amount of $690,000. The Bridge Notes mature on February 11, 2022, subject to the requirement that we redeem the Bridge Notes prior to such date with the net proceeds of any future offering of our securities. The Notes do not bear interest other than upon an event of default, and are not convertible into the Company’s common stock. In addition, the Notes are subject to covenants, events of defaults and other terms and conditions customary in transactions of this nature. The Company is amortizing the on-issuance discount and financing fees totaling $758,426 to interest expense with respect to these notes.
The Company also issued to the purchasers of the Bridge Notes five-year warrants to purchase an aggregate of 363,046 shares of our common stock at an initial exercise price of $14.175 per share, subject to anti-dilution adjustment in the event of future sales of our equity below the then exercise price, stock dividends, stock splits and other specified events.
Roth Capital Partners (“Roth”), acted as sole placement agent for the offering. Pursuant to terms of an engagement letter with Roth, the Company paid Roth a placement agent fee in the amount $312,750. The Company also issued Roth a warrant to purchase 20,189 shares of common stock with the same terms as the warrants issued to the Purchasers.
During the nine-months ended September 30, 2021, we also issued $498,800 in convertible notes to accredited investors with net proceeds of $435,040, which have since been repaid in full. The notes were to mature during February and July of 2022 and bore interest at a rate of 8%. The notes were convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 71% of the average of the two lowest traded prices or the lowest trade price of the Company’s common stock during the previous 15 trading days preceding the conversion date. The Company amortized the discount due to derivative liabilities and on-issuance discount totaling $443,905 to interest expense with respect to these notes.
On May 28, 2021, Mr. Timothy Warbington, who is our CEO and Chairman; and Dr. Amit Patel, who is a director of ours, advanced the company $50,000 and $150,000 respectively. The two notes were repaid during the quarter ended September 30, 2021, did not have any conversion features, and bore interest at the rate of 5% per annum.
On June 21, 2021, we issued a $105,000, non-convertible note to an accredited investor with net proceeds of $100,000. The note was repaid during the quarter ended September 30, 2021, did not have any conversion features, and bore interest at the rate of 10% per annum.
During the nine-months ended September 30, 2021 and 2020, the Company amortized $1,755,104 and $804,898 respectively, to interest expense. As of September 30, 2021, total discounts of $0.
During the nine-months ended September 30, 2021, the Company issued an aggregate of 789,727 shares upon the conversion of $1,383,331 of outstanding principal, interest and fees on outstanding notes, and 37,870 shares upon the cashless exercise of 43,167 warrants. During the nine-months ended September 30, 2020, the Company issued an aggregate of 777,865 shares upon the conversion of $1,033,418 of outstanding principal, interest and fees on existing, outstanding notes.
During the nine-months ended September 30, 2020, the Company extinguished $23,000 of principal and interest with no pre-payment premiums.
As of September 30, 2021, future loan maturities are as follows:
For the year ended December 31, |
|
|
| |
|
|
|
| |
2021 |
|
| 0 |
|
2022 |
|
| 5,146,176 |
|
Total |
| $ | 5,146,176 |
|
| F-12 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
NOTE 5 – DERIVATIVE LIABILITIES
Derivative Liabilities
In connection with convertible notes payable, the Company records derivative liabilities for the conversion feature. The derivative liabilities are valued on the date the convertible note payable become convertible and revalued at each reporting period. During the nine-months ended September 30, 2021, the Company recorded initial derivative liabilities of $1,077,757 based upon the following Black-Scholes option pricing model average assumptions: an exercise price of $0.0106 to $0.0248 our stock price on the date of grant of $0.0340 to $0.0806, expected dividend yield of 0%, expected volatility of 75.03% to 98.14%, risk free interest rate of 0.10% and expected terms of 1.0 year. Upon initial valuation, the derivative liabilities exceeded the face values certain of the convertible notes payable by approximately $697,602, which was recorded as a day one loss in derivative liability.
In August 2021, we completed the sale of 15% Original Issue Discount Senior Notes (“Bridge Notes”) in the aggregate principal amount of $4,456,176 to a group of institutional investors (the “Purchasers”). A portion of the proceeds were used to repay the principal, accrued interest, pre-payment fees and other premiums of all the outstanding convertible notes as well as all previously outstanding warrants with re-pricing and anti-dilutive features. The result was $0 in derivative liabilities as-of the period ended September 30,2021.
NOTE 6 – WARRANTS
From January 2021 through September 2021, the Company issued 421,066 warrants in connection with a non-convertible debt issuance and incentive grants to new Scientific Advisory Board and employee members. During the nine-months ended September 30, 2021, two of these individuals exercised 43,167warrants.
The fair value of each warrant is estimated using the Black-Scholes valuation model on the date of issuance and if needed at each period end. Assumptions used in calculating the fair value during the nine months ended September 30, 2021 were as follows:
|
| Weighted Average Inputs Used |
| |
|
|
|
| |
Annual dividend yield |
| $ | 0 |
|
Expected life (years) |
| 2.7 to 10.0 |
| |
Risk-free interest rate |
| 0.23% to 0.81 | % | |
Expected volatility |
| 92.93% to 98.81 | % | |
Common stock price |
| $ | 11.5000 to $17.0000 |
|
Since the expected life of the warrants was greater than the Company’s historical stock information available, the Public Company determined the expected volatility based on price fluctuations of comparable public companies.
| F-13 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
The issuances, exercises and pricing re-sets during the nine months ended September 30, 2021, are as follows:
Outstanding at December 31, 2020 |
|
| 152,738 |
|
Issuances |
|
| 441,255 |
|
Exercises |
|
| (43,167 | ) |
Anti-Dilution/Modification |
|
| - |
|
Forfeitures/cancellations |
|
| (69,475 | ) |
Outstanding at September 30, 2021 |
|
| 481,351 |
|
Weighted Average Price at September 30, 2021 |
| $ | 13.2809 |
|
NOTE 7 – STOCKHOLDERS’ DEFICIT
Series B Convertible Preferred Stock Equity Financing
On February 11, 2021, the Board of Directors of the Corporation had authorized issuance of up to 350 shares of preferred stock, $0.001 par value per share, designated as Series B Convertible Preferred Stock. Each share of Preferred Stock shall have a par value of $0.001 per share and a stated value of $1,200, subject to increase set forth in the Certificate of Designation.
Dividends: Each share of Series B Convertible Preferred Stock shall be entitled to receive, and the Corporation shall pay, cumulative dividends of 10% per annum, payable quarterly, beginning on the Original Issuance Date and ending on the date that such share of Series B Convertible Preferred Share has been converted or redeemed (the “Dividend End Date”). Dividends may be paid in cash or in shares of Series B Convertible Preferred Stock. From and after the initial Closing Date, in addition to the payment of dividends pursuant to Section 2(a), each Holder shall be entitled to receive, and the Corporation shall pay, dividends on shares of Series B Convertible Preferred Stock equal to (on an as-if-converted-to-Common-Stock basis) and in the same form as dividends actually paid on shares of the common stock when, as and if such dividends are paid on shares of the common stock. The Corporation shall pay no dividends on shares of the common stock unless it simultaneously complies with the previous sentence.
Voting Rights: The Series B Convertible Preferred Stock will vote together with the common stock on an as converted basis subject to the Beneficial Ownership Limitations (not in excess of 4.99% conversion limitation). However, as long as any shares of Series B Convertible Preferred Stock are outstanding, the Corporation shall not, without the affirmative vote of the Holders of a majority of the then outstanding shares of the Series B Convertible Preferred Stock directly and/or indirectly (a) alter or change adversely the powers, preferences or rights given to the Series B Convertible Preferred Stock or alter or amend this Certificate of Designation, (b) authorize or create any class of stock ranking as to redemption or distribution of assets upon a Liquidation (as defined in Section 5) senior to, or otherwise pari passu with, the Series B Convertible Preferred Stock or, authorize or create any class of stock ranking as to dividends senior to, or otherwise pari passu with, the Series b Convertible Preferred Stock, (c) amend its Articles of Incorporation or other charter documents in any manner that adversely affects any rights of the Holders, (d) increase the number of authorized shares of Series B Convertible Preferred Stock, or (e) enter into any agreement with respect to any of the foregoing.
Liquidation: Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a “Liquidation”), the Holders shall be entitled to receive out of the assets, whether capital or surplus, of the Corporation an amount equal to the Stated Value, plus any accrued and unpaid dividends thereon and any other fees or liquidated damages then due and owing thereon under this Certificate of Designation, for each share of Series B Convertible Preferred Stock before any distribution or payment shall be made to the holders of any Junior Securities, and if the assets of the Corporation shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the Holders shall be ratably distributed among the Holders in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full.
Conversion: Each share of Series B Convertible Preferred Stock shall be convertible, at any time and from time to time from and after the Original Issue Date at the option of the Holder thereof, into that number of shares of common stock (subject to the limitations) determined by dividing the Stated Value of such share of Series B Convertible Preferred Stock by the Conversion Price. The Conversion Price for the Series B Convertible Preferred Stock shall be the amount equal to $0.05 per share. Following the event of a default the conversion price shall be $0.35.
| F-14 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
Redemption: The Series B Convertible Preferred Stock may be redeemed by payment of the stated value thereof, with the following premiums based on the time of the redemption.
· | 105% of the stated value if the redemption takes place within 90 days of issuance; |
· | 110% of the stated value if the redemption takes place after 90 days and within 120 days of issuance |
· | 120% of the stated value if the redemption takes place after 120 days and within 180 days of issuance |
In addition, the Series B Preferred Stock contain various redemption provisions for which are contingent upon future events including but not limited to having sufficient authorized shares, change in control, bankruptcy, etc. Upon a triggering event, the Company the redemption price is 125% of the stated value plus all unpaid dividends and liquidated damages.
Most Favored Nation Provision. From the date hereof until the date when the Holder no longer holds any shares of Series B Preferred Stock, upon any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents for cash consideration, Indebtedness or a combination of units thereof (a “Subsequent Financing”), the Holder may elect, in its sole discretion, to exchange (in lieu of conversion), if applicable, all or some of the shares of Series B Preferred Stock then held for any securities or units issued in a Subsequent Financing on a $1.00 for $1.00 basis. The Company shall provide the Holder with notice of any such Subsequent Financing in the manner set forth below. For purposes of illustration, if a Subsequent Financing were to occur whereby the Company sells and issues a convertible note with a conversion price that includes a discount to the market price of its Common Stock, the Holder will be entitled to receive the same convertible note on the exact same terms on a dollar-for-dollar basis via the exchange of the Series B Preferred Stock the Holder holds on the date of the sale and issuance of the convertible note.
On February 12, 2021, pursuant to the terms noted above, the Company entered into a new preferred equity financing agreement with BHP Capital, LLC (“BHP”) in the amount of $350,000 for 350 shares of the newly-designated Series B Convertible Preferred Stock valued at $1,200 per share for which $326,600 in proceeds were received by the Company. In connection with the closing, the Company issued an additional 3,000 shares of common stock as a service fee. The Company has accounted for the transaction with equity at the proceeds received was considered consideration for all securities issued.
In August 2021, the preferred shares were redeemed at 120% of their stated value per the terms of their designations through the issuance of the bridge note with the same terms as the bridge note described in Note 4.
Series C Convertible Preferred Stock Equity Financing
On March 30, 2021, the Board of Directors of the Corporation had authorized issuance of up to 150 shares of preferred stock, $0.001 par value per share, designated as Series C Convertible Preferred Stock. Each share of Preferred Stock shall have a par value of $0.001 per share and a stated value of $1,200, subject to increase set forth in the Certificate of Designation.
Dividends: Each share of Series C Convertible Preferred Stock shall be entitled to receive, and the Corporation shall pay, cumulative dividends of 10% per annum, payable quarterly, beginning on the Original Issuance Date and ending on the date that such share of Series C Convertible Preferred Share has been converted or redeemed (the “Dividend End Date”). Dividends may be paid in cash or in shares of Series C Convertible Preferred Stock. From and after the initial Closing Date, in addition to the payment of dividends pursuant to Section 2(a), each Holder shall be entitled to receive, and the Corporation shall pay, dividends on shares of Series C Convertible Preferred Stock equal to (on an as-if-converted-to-Common-Stock basis) and in the same form as dividends actually paid on shares of the common stock when, as and if such dividends are paid on shares of the common stock. The Corporation shall pay no dividends on shares of the common stock unless it simultaneously complies with the previous sentence.
Voting Rights: The Series C Convertible Preferred Stock will vote together with the common stock on an as converted basis subject to the Beneficial Ownership Limitations (not in excess of 4.99% conversion limitation). However, as long as any shares of Series C Convertible Preferred Stock are outstanding, the Corporation shall not, without the affirmative vote of the Holders of a majority of the then outstanding shares of the Series C Convertible Preferred Stock directly and/or indirectly (a) alter or change adversely the powers, preferences or rights given to the Series C Convertible Preferred Stock or alter or amend this Certificate of Designation, (b) authorize or create any class of stock ranking as to redemption or distribution of assets upon a Liquidation (as defined in Section 5) senior to, or otherwise pari passu with, the Series C Convertible Preferred Stock or, authorize or create any class of stock ranking as to dividends senior to, or otherwise pari passu with, the Series C Convertible Preferred Stock, (c) amend its Articles of Incorporation or other charter documents in any manner that adversely affects any rights of the Holders, (d) increase the number of authorized shares of Series C Convertible Preferred Stock, or (e) enter into any agreement with respect to any of the foregoing.
| F-15 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2021
Liquidation: Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a “Liquidation”), the Holders shall be entitled to receive out of the assets, whether capital or surplus, of the Corporation an amount equal to the Stated Value, plus any accrued and unpaid dividends thereon and any other fees or liquidated damages then due and owing thereon under this Certificate of Designation, for each share of Series C Convertible Preferred Stock before any distribution or payment shall be made to the holders of any Junior Securities, and if the assets of the Corporation shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the Holders shall be ratably distributed among the Holders in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full.
Conversion: Each share of Series C Convertible Preferred Stock shall be convertible, at any time and from time to time from and after the Original Issue Date at the option of the Holder thereof, into that number of shares of common stock (subject to the limitations) determined by dividing the Stated Value of such share of Series C Convertible Preferred Stock by the Conversion Price. The Conversion Price for the Series C Convertible Preferred Stock shall be the amount equal to $0.05 per share. Following the event of a default the conversion price shall be $0.35.
Redemption: The Series C Convertible Preferred Stock may be redeemed by payment of the stated value thereof, with the following premiums based on the time of the redemption.
· | 105% of the stated value if the redemption takes place within 90 days of issuance; |
· | 110% of the stated value if the redemption takes place after 90 days and within 120 days of issuance |
· | 120% of the stated value if the redemption takes place after 120 days and within 180 days of issuance |
In addition, the Series C Preferred Stock contain various redemption provisions for which are contingent upon future events including but not limited to having sufficient authorized shares, change in control, bankruptcy, etc. Upon a triggering event, the Company the redemption price is 125% of the stated value plus all unpaid dividends and liquidated damages.
Most Favored Nation Provision. From the date hereof until the date when the Holder no longer holds any shares of Series C Preferred Stock, upon any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents for cash consideration, Indebtedness or a combination of units thereof (a “Subsequent Financing”), the Holder may elect, in its sole discretion, to exchange (in lieu of conversion), if applicable, all or some of the shares of Series C Preferred Stock then held for any securities or units issued in a Subsequent Financing on a $1.00 for $1.00 basis. The Company shall provide the Holder with notice of any such Subsequent Financing in the manner set forth below. For purposes of illustration, if a Subsequent Financing were to occur whereby the Company sells and issues a convertible note with a conversion price that includes a discount to the market price of its Common Stock, the Holder will be entitled to receive the same convertible note on the exact same terms on a dollar-for-dollar basis via the exchange of the Series C Preferred Stock the Holder holds on the date of the sale and issuance of the convertible note.
On March 30, 2021, pursuant to the terms noted above, the Company entered into a new preferred equity financing agreement with Fourth Man, LLC (“FM”) in the amount of $150,000. The closing under the SPA consisted of 150 shares of Series C Convertible Preferred Stock, stated value $1,200 per share, issued to FM for a purchase price of $150,000, or $1,000 per share, for which $141,049 in proceeds were received by the Company. In connection with the closing, the Company issued an additional 642,857 shares of common stock as a service fee. The Company has accounted for the transaction with equity at the proceeds received was considered consideration for all securities issued.
In August 2021, the preferred shares were redeemed at 120% of their stated value per the terms of their designations through the issuance of the bridge note with the same terms as the bridge note described in Note 4.
NOTE 8 – SUBSEQUENT EVENTS
In accordance with ASC 855, management reviewed all material events through November 15, 2021, for these financial statements and there are no material subsequent events to report, except as follows:
In November, 2021, following the approval of the Company’s Board of Directors and holders of a majority the Company’s voting stock, the Company filed an amendment to its Articles of Incorporation increasing the Company’s authorized shares of Common Stock to 25 billion from 6 billion. Thereafter, on November 10, 2021, following the approval of the Board of Directors of the Company, the Company effected a 1-for-500 reverse split of the both the Company’s authorized and outstanding shares of Common Stock, by filing a Certificate of Change with the Nevada Secretary of State under Section 78.209 of the Nevada Revised Statutes. Following the stock split, the Company’s authorized common stock was reduced to 50,000,000 shares, and the Company had outstanding approximately 2,452,348 shares of Common Stock. No fractional shares will be issued, and no cash or other consideration will be paid, in connection with the reverse stock split. Instead, the Company will issue one whole share of the post-Reverse Stock Split Common Stock to any stockholder who otherwise would have received a fractional share as a result of the reverse stock split. All share references have been restated for this reverse split to the earliest period presented.
| F-16 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Creative Medical Technology Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Creative Medical Technology Holdings, Inc. (the Company) as of December 31, 2020 and 2019, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2020, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Consideration of the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has recurring net losses, negative cash flows from operations, and negative working capital. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Haynie & Company
Haynie & Company
Salt Lake City, Utah
March 16, 2021, except for the reverse stock-split retrospectively presented in these financial statements described in Note 9, as to which is dated November 23, 2021
We have been the Company’s auditor since 2016
| F-17 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
|
| December 31, 2020 |
|
| December 31, 2019 |
| ||
ASSETS |
|
|
|
|
|
| ||
CURRENT ASSETS |
|
|
|
|
|
| ||
Cash |
| $ | 98,012 |
|
| $ | 88,648 |
|
Accounts receivable |
|
| - |
|
|
| 5,600 |
|
Total Current Assets |
|
| 98,012 |
|
|
| 94,248 |
|
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
|
Licenses, net of amortization |
|
| 619,763 |
|
|
| 436,555 |
|
TOTAL ASSETS |
| $ | 717,775 |
|
| $ | 530,803 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 350,899 |
|
| $ | 320,785 |
|
Accrued expenses |
|
| 159,771 |
|
|
| 120,492 |
|
Management fee and patent liabilities - related parties |
|
| 468,782 |
|
|
| 240,082 |
|
Convertible notes payable, net of discount of $409,649 and $560,899, respectively |
|
| 788,701 |
|
|
| 1,062,266 |
|
Advances from related party |
|
| 10,800 |
|
|
| 10,800 |
|
Derivative liabilities |
|
| 38,741,832 |
|
|
| 6,847,877 |
|
Total Current Liabilities |
|
| 40,520,785 |
|
|
| 8,602,302 |
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES |
|
| 40,520,785 |
|
|
| 8,602,302 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ DEFICIT |
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 7,000,000 and 7,000,000 shares authorized, no shares issued and outstanding at December 31, 2020 and 2019 |
|
| - |
|
|
| - |
|
Series A preferred stock, $0.001 par value, 3,000,000 shares authorized, 3,000,000 shares issued and outstanding at December 31, 2020 and 2019 |
|
| 3,000 |
|
|
| 3,000 |
|
| Common stock, $0.001 par value, 12,000,000,000 shares authorized; 1,537,081 and 44,986 issued and 1,537,073 and 44,978 outstanding at December 31, 2020 and 2019, respectively |
|
| 1,537 |
|
|
| 45 |
|
Additional paid-in capital |
|
| 22,082,689 |
|
|
| 17,490,462 |
|
Accumulated deficit |
|
| (61,890,236 | ) |
|
| (25,565,006 | ) |
TOTAL STOCKHOLDERS’ DEFICIT |
|
| (39,803,010 | ) |
|
| (8,071,499 | ) |
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT |
| $ | 717,775 |
|
| $ | 530,803 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-18 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
|
| For the Year |
|
| For the Year |
| ||
|
|
|
|
|
|
| ||
Revenues |
| $ | 164,500 |
|
| $ | 165,500 |
|
|
|
|
|
|
|
|
|
|
Cost of revenues |
|
| 50,596 |
|
|
| 45,499 |
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
| 113,904 |
|
|
| 120,001 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
| 1,161,947 |
|
|
| 1,223,219 |
|
Amortization of patent costs |
|
| 66,792 |
|
|
| 26,950 |
|
TOTAL EXPENSES |
|
| 1,228,739 |
|
|
| 1,250,169 |
|
|
|
|
|
|
|
|
|
|
Operating loss |
|
| (1,114,835 | ) |
|
| (1,130,168 | ) |
|
|
|
|
|
|
|
|
|
OTHER INCOME/(EXPENSE) |
|
|
|
|
|
|
|
|
Interest expense |
|
| (1,229,590 | ) |
|
| (1,895,528 | ) |
Gain loss on extinguishment of convertible notes |
|
| 0 |
|
|
| (366,042 | ) |
Change in fair value of derivatives liabilities |
|
| (33,980,805 | ) |
|
| (5,091,935 | ) |
Total other income (expense) |
|
| (35,210,395 | ) |
|
| (7,353,505 | ) |
|
|
|
|
|
|
|
|
|
LOSS BEFORE PROVISION FOR INCOME TAXES |
|
| (36,325,230 | ) |
|
| (8,483,673 | ) |
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
NET LOSS |
| $ | (36,325,230 | ) |
| $ | (8,483,673 | ) |
|
|
|
|
|
|
|
|
|
BASIC AND DILUTED NET LOSS PER SHARE |
| $ | (62.69 | ) |
| $ | (410.12 | ) |
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING - BASIC AND DILUTED |
|
| 579,461 |
|
|
| 20,686 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-19 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
| For the Year |
|
| For the Year |
| ||
|
|
|
|
|
|
| ||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
| ||
Net loss |
| $ | (36,325,230 | ) |
| $ | (8,483,673 | ) |
Adjustments to reconcile net loss to net cash from operating activities: |
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| 170,323 |
|
|
| 0 |
|
Amortization |
|
| 66,792 |
|
|
| 26,950 |
|
Amortization of debt discounts |
|
| 979,960 |
|
|
| 1,723,451 |
|
Change in fair value of derivatives liabilities |
|
| 33,980,805 |
|
|
| 5,091,935 |
|
Loss on extinguishment of convertible notes payable |
|
| 0 |
|
|
| 266,042 |
|
Excess consideration issued in connection with relieve of management fees and patent liability |
|
| 0 |
|
|
| 100,000 |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
Accounts receivable |
|
| 5,600 |
|
|
| 4,000 |
|
Accounts payable |
|
| 30,114 |
|
|
| (11,054 | ) |
Accrued expenses |
|
| 167,029 |
|
|
| 189,256 |
|
Management fee payable |
|
| 490,051 |
|
|
| (120,000 | ) |
Net cash used in operating activities |
|
| (434,556 | ) |
|
| (1,213,093 | ) |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
| (250,000 | ) |
|
| 0 |
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Payments on notes payable to related party |
|
| 0 |
|
|
| (50,000 | ) |
Proceeds from notes payable to related party |
|
| 0 |
|
|
| 50,000 |
|
Payments on convertible notes payable |
|
| (17,000 | ) |
|
| (263,411 | ) |
Prepayment premiums paid on convertible notes payable |
|
| - |
|
|
| (41,699 | ) |
Proceeds from convertible notes payable |
|
| 710,920 |
|
|
| 1,302,795 |
|
Net cash provided from financing activities |
|
| 693,920 |
|
|
| 997,685 |
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH |
|
| 9,364 |
|
|
| (215,408 | ) |
BEGINNING CASH BALANCE |
|
| 88,648 |
|
|
| 304,056 |
|
ENDING CASH BALANCE |
| $ | 98,012 |
|
| $ | 88,648 |
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
Cash payments for interest |
| $ | 6,000 |
|
| $ | 18,177 |
|
Cash payments for income taxes |
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
NON-CASH INVESTING AND FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Related party liability incurred for additional patent costs |
| $ | 0 |
|
| $ | 300,000 |
|
Beneficial conversion feature issued with Allogenics patent liability |
| $ | 101,351 |
|
| $ | 0 |
|
Conversion of notes payable, accrued interest and derivative liabilities into common stock |
| $ | 4,162,045 |
|
| $ | 4,156,811 |
|
Conversion of management fees and patent liability into common stock |
| $ | 160,000 |
|
| $ | 138,000 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-20 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
|
| Series A Preferred Stock |
|
| Common Stock |
|
| Additional Paid-in |
|
| Accumulated |
|
| Total Stockholders' |
| |||||||||||||
|
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| Deficit |
| |||||||
Balance as of December 31, 2018 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| 12,036 |
|
| $ | 12 |
|
| $ | 13,083,684 |
|
| $ | (17,081,333 | ) |
| $ | (3,994,637 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for cashless warrant exercise |
|
| - |
|
|
| 0 |
|
|
| 1,063 |
|
|
| 1 |
|
|
| (1 | ) |
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| 23,746 |
|
|
| 24 |
|
|
| 1,501,251 |
|
|
| 0 |
|
|
| 1,501,275 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for related party management fee and patent liabilities |
|
| - |
|
|
| 0 |
|
|
| 8,133 |
|
|
| 8 |
|
|
| 249,992 |
|
|
| 0 |
|
|
| 250,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 2,655,536 |
|
|
| 0 |
|
|
| 2,655,536 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| (8,483,673 | ) |
|
| (8,483,673 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2019 |
|
| 3,000,000 |
|
|
| 3,000 |
|
|
| 44,978 |
|
|
| 45 |
|
|
| 17,490,462 |
|
|
| (25,565,006 | ) |
|
| (8,071,499 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for related party management fee and patent liabilities |
|
| - |
|
|
| 0 |
|
|
| 128,630 |
|
|
| 129 |
|
|
| 159,871 |
|
|
| 0 |
|
|
| 160,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible notes, accrued interest and derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| 1,363,463 |
|
|
| 1,363 |
|
|
| 1,365,342 |
|
|
| 0 |
|
|
| 1,366,705 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature issued with Allogenics patent liability |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 101,351 |
|
|
| 0 |
|
|
| 101,351 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 170,323 |
|
|
| 0 |
|
|
| 170,323 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Relief of derivative liabilities |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 2,795,340 |
|
|
| 0 |
|
|
| 2,795,340 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Differences in shares from reverse stock split |
|
| - |
|
|
| 0 |
|
|
| 2 |
|
|
| 0 |
|
|
| (0 | ) |
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| - |
|
|
| 0 |
|
|
| - |
|
|
| 0 |
|
|
| 0 |
|
|
| (36,325,230 | ) |
|
| (36,325,230 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2020 |
|
| 3,000,000 |
|
| $ | 3,000 |
|
|
| 1,537,073 |
|
| $ | 1,537 |
|
| $ | 22,082,689 |
|
| $ | (61,890,236 | ) |
| $ | (39,803,010 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
| F-21 |
| Table of Contents |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization - Creative Medical Technologies Holdings, Inc. (the “Company”) is a commercial stage biotechnology company focused on immunology, urology, orthopedics and neurology using adult stem cell treatments. The Company was incorporated on December 3, 1998, in the State of Nevada under the name Jolley Marketing, Inc. On May 18, 2016, the Company closed a transaction which was accounted for as a recapitalization, reverse merger, under which Creative Medical Technologies, Inc., a Nevada corporation (“CMT”) became the Company’s wholly-owned subsidiary, and Creative Medical Health, Inc. (“CMH”), which was CMT’s sole stockholder prior to the merger, became the Company’s principal stockholder. In connection with this merger, the Company changed its name to Creative Medical Technologies Holdings, Inc. to reflect its current business.
CMT was originally created on December 30, 2015 (“Inception”), as the urological arm of CMH to monetize a patent and related intellectual property related to the treatment of erectile dysfunction (“ED”), which it acquired from CMH in February 2016. Subsequently, the Company has expanded its development and acquisition of intellectual property beyond urology to include therapeutic treatments utilizing amniotic stem cells, and the treatment of neurologic disorders and lower back pain using various types of stem cells through its AmnioStem LLC, StemSpine, LLC, and ImmCelz, Inc. subsidiaries. However, neither AmnioStem LLC, StemSpine, Inc. nor ImmCelz, Inc. have commenced commercial activities.
Risks and Uncertainties - The Company has a limited operating history and has only recently started to generate revenues from its planned principal operations.
On January 30, 2020, the World Health Organization declared the COVID-19 outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the COVID-19 include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and businesses. The COVID-19 and actions taken to mitigate it have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is unknown how long these conditions will last and what the complete financial effect will be to the company, to-date, the Company is experiencing a reduction in revenues due to the prioritization of medical resources to address the COVID-19 outbreak. In several of our markets, all non-essential (including elective) procedures have been placed on hold. While this has a negative financial impact to our revenues, there have been the same reductions to our costs. Additionally, since the Company maintains no inventory and require nearly all of customers to pre-pay, there is no risk to receivables or inventory write-downs. The company expects existing orders temporarily on hold and continued sales, training and patient treatments will resume once the physician’s offices are back to being fully operational.
The Company’s business and operations are sensitive to general business and economic conditions in the U.S. and worldwide. These conditions include short-term and long-term interest rates, inflation, fluctuations in debt and equity capital markets and the general condition of the U.S. and world economy. A host of factors beyond the Company’s control could cause fluctuations in these conditions, including the political environment and acts or threats of war or terrorism. Adverse developments in these general business and economic conditions, including through recession, downturn or otherwise, could have a material adverse effect on the Company’s financial condition and the results of its operations.
The Company has only recently started to generate sales and we have limited marketing and/or distribution capabilities. The Company has limited experience in developing, training or managing a sales force and will incur substantial additional expenses if it decides to market any of its current and future products and services with an internal sales organization. Developing a marketing and sales force is also time consuming and could delay launch of its future products and services. In addition, the Company will compete with many companies that currently have extensive and well-funded marketing and sales operations. The Company’s marketing and sales efforts may be unable to compete successfully against these companies. In addition, the Company has limited capital to devote to sales and marketing.
The Company’s industry is characterized by rapid changes in technology and customer demands. As a result, the Company’s products and services may quickly become obsolete and unmarketable. The Company’s future success will depend on its ability to adapt to technological advances, anticipate customer demands, develop new products and services and enhance the Company’s current products and services on a timely and cost-effective basis. Further, the Company’s products and services must remain competitive with those of other companies with substantially greater resources. The Company may experience technical or other difficulties that could delay or prevent the development, introduction or marketing of new products and services or enhanced versions of existing products and services. Also, the Company may not be able to adapt new or enhanced products and services to emerging industry standards, and the Company’s new products and services may not be favorably received. In addition, the Company may not have the capital resources to further the development of existing and/or new ones.
| F-22 |
| Table of Contents |
Use of Estimates - The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Basis of Presentation - The consolidated financial statements and accompanying notes have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation. In the opinion of the Company’s management, the consolidated financial statements include all adjustments, which include only normal recurring adjustments, necessary for the fair presentation of the Company’s financial position for the periods presented.
Going Concern - The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the U.S., which contemplate continuation of the Company as a going concern. However, during the fiscal year ended December 31, 2020, the Company incurred a net loss of $36,325,230, had negative cash flows from operating activities of $434,556, had negative working capital of $40,422,773 at December 31, 2020 and has only generate revenues for approximately 3 years. These factors raise substantial doubt about the ability of the Company to continue as a going concern. In this regard, management expects to raise additional funds through the sale of convertible notes, preferred stock, common stock and/or other securities. There is no assurance that the Company will be successful in raising this additional capital or in achieving profitable operations. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Concentration Risks - The Federal Deposit Insurance Corporation (FDIC) insures cash deposits in most general bank accounts for up to $250,000 per institution. The Company had nomaintains its cash deposits that exceeded insured amounts during anybalances at one financial institution. As of December 31, 2020, the periods reported.Company’s balance did not exceed the limit.
Cash Equivalents - The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents.
Fair Value of Financial InstrumentsInstrument- The Company’s financial instruments consist of cash and cash equivalents, convertible notes, and payables. The carrying amount of cash and cash equivalents and payables approximates fair value because of the short-term nature of these items.
Fair value is an exit price, representing the amount that would be received from the sale of an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. Fair value measurements are required to be disclosed by level within the following fair value hierarchy:
Level 1 – Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2 – Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
Level 3 – Inputs lack observable market data to corroborate management’s estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
| F-23 |
| Table of Contents |
When determining fair value, whenever possible the Company uses observable market data, and relies on unobservable inputs only when observable market data is not available. As of December 31, 2020, the Company has level 3 fair value calculations on derivative liabilities. The table below reflects the results of our Level 3 fair value calculations:
|
| Notes |
|
| Warrants |
|
| Total |
| |||
|
|
|
|
|
|
|
|
|
| |||
Derivative liability at December 31, 2019 |
| $ | 6,659,055 |
|
| $ | 188,822 |
|
| $ | 6,847,877 |
|
Addition of new conversion option derivatives |
|
| 2,572,723 |
|
|
| 0 |
|
|
| 2,572,723 |
|
Extinguishment/modification |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Conversion of note derivatives |
|
| (2,795,340 | ) |
|
| 0 |
|
|
| (2,795,340 | ) |
Change in fair value |
|
| 30,907,397 |
|
|
| 1,209,175 |
|
|
| 32,116,572 |
|
Derivative liability at December 31, 2020 |
| $ | 37,343,835 |
|
| $ | 1,397,997 |
|
| $ | 38,741,832 |
|
Intangible Assets - Intangible assets with finite lives are amortized over their respective estimated lives and reviewed for impairment whenever events or other changes in circumstances indicate that the carrying amount may not be recoverable. The impairment testing compares carrying values to fair values and, when appropriate, the carrying value of these assets is reduced to fair value. Impairment charges, if any, are recorded in the period in which the impairment is determined.
Impairment - The Company records impairment losses when indicators of impairment are present and undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. Furthermore, the Company will make periodic assessments of technology and clinical testing to determine if it plans to continue to pursue the technology and if the license, patent or other rights have value. To date no impairment has been recorded.
RevenueDerivative Liabilities - The Company will recognize revenueA derivative is an instrument whose value is “derived” from an underlying instrument or index such as it is earned as defined by US Generally Accepted Accounting Principles. During the periods ended December 31, 2015a future, forward, swap, option contract, or other financial instrument with similar characteristics, including certain derivative instruments embedded in other contracts and September 30, 2016, there were no sales and revenue was not recognized. For the next several months, until clinical trials have successfully been completed,for hedging activities.
As a matter of policy, the Company does not anticipate there will be revenueinvest in separable financial derivatives or engage in hedging transactions. However, the Company entered into certain debt financing transactions in fiscal 2020 and 2019, as disclosed in Notes 4 and 5, containing certain conversion features that have resulted in the instruments being deemed derivatives. We evaluate such derivative instruments to report.properly classify such instruments within equity or as liabilities in our financial statements. Our policy is to settle instruments indexed to our common shares on a first-in-first-out basis.
The classification of a derivative instrument is reassessed at each reporting date. If the classification changes as a result of events during a reporting period, the instrument is reclassified as of the date of the event that caused the reclassification. There is no limit on the number of times a contract may be reclassified.
Instruments classified as derivative liabilities are remeasured using the Black-Scholes model at each reporting period (or upon reclassification), and the change in fair value is recorded on our consolidated statement of operations.
Revenue - The Company recognizes revenues in accordance with Accounting Standards Codification (“ASC”) 606, “Revenue from contracts with customers”. Revenues are recognized when control of the promised goods or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. Deferred revenue represents amounts which still have yet to be earned.
The Company generates revenue from the sale of disposable stem cell concentration kits. Revenues are recognized when control of the promised goods or services are transferred to the customer, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services, which is generally on delivery to the customer.
Payments received for which the earnings process is not yet complete are deferred. As of December 31, 2020, the Company had deferred revenue of $32,000 included within current liabilities in the accompanying consolidated balance sheet.
Research and Development - Research and development will continue to be the principala significant function of the Company. Research and development costs will be expensed as incurred. Expenses in the accompanying financial statements include certain costs which are directly associated with the Company’s research and development:
1. | Erectile Dysfunction Technology based upon the use of stem cells. These costs, which consist primarily of monies paid for clinical trial expenses, materials and supplies and compensation costs amounted to |
2. | Amniotic Fluid-based Stem Cells. Pre-clinical research costs, which consist primarily of monies paid for laboratory space, materials and supplies amounted to $0 for the |
| F-24 |
| Table of Contents |
Stock-Based Compensation –The Company accounts for its stock-based compensation in accordance with Accounting Standards Codification ("ASC"(“ASC”) 718, Compensation - Stock Compensation. The Company accounts for all stock-based compensation using a fair-value method on the grant date and recognizes the fair value of each award as an expense over the requisite vesting period. The Company recognizes stock option forfeitures as they occur as there is insufficient historical data to accurately determine future forfeitures rates.
The Company follows ASC 505-50, Equity-Based Payments to Non-Employees, for stock options and warrants issued to consultants and other non-employees. In accordance with ASC 505-50, these stock options and warrants issued as compensation for services provided to the Company are accounted for based upon the fair value of the services provided or the estimated fair market value of the option or warrant, whichever can be more clearly determined. The fair value of the equity instrument, which is revalued at each reporting period, is charged directly to compensation expense and additional paid-in capital over the period during which services are rendered.
Income Taxes – The Company has no provisionaccounts for income taxes at September 30, 2016, as it has no taxable incomeusing the asset and no recognizable tax asset.The Company will recognizeliability method, which requires the amountrecognition of income taxes payable or refundable at the end of the current year and will recognize any deferred tax assets and liabilities for operating loss carryforwards and for the expected future tax consequences attributable toof events that have been recognized in the financial statements or in the Company’s tax returns. Deferred income taxes are recognized for differences between the consolidated financial statement amountsreporting and tax bases of certain assets and liabilities and their respective tax basis. Deferred tax assets and deferred liabilities are measured usingat the enacted statutory tax rates expected to apply to taxable income in effect for the years thosein which the temporary differences are expected to be recovered or settled. Deferredreverse. The effect on deferred taxes of a change in tax assets are reduced by a valuation allowance torates is recognized in income in the extentperiod that uncertainty exists as to whetherincludes the enactment date. The Company evaluates the realizability of deferred tax assets will ultimatelyand valuation allowances are provided when necessary to reduce net deferred tax assets to the amounts expected to be realized.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such positions are then measured based on the largest benefit that has a greater than 50% likelihood of being realized upon settlement. The Company will recognize interest and penalties related to unrecognized tax benefits in the income tax provision in the accompanying statement of operations.
The Company calculates the current and deferred income tax provision based on estimates and assumptions that could differ from the actual results reflected in income tax returns filed in subsequent years. Adjustments based on filed income tax returns are recorded when identified. The amount of income taxes paid is subject to examination by U.S. federal and state tax authorities. The estimate of the potential outcome of any uncertain tax issue is subject to management’s assessment of relevant risks, facts and circumstances existing at that time. To the extent that the assessment of such tax positions change, the change in estimate is recorded in the period in which the determination is made.
Basic and Diluted Loss Per Share –The Company follows Financial Accounting Standards Board ("FASB"(“FASB”) ASC 260 Earnings per Share to account for earnings per share. Basic earnings per share (“EPS”) calculations are determined by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share calculations are determined by dividing net income by the weighted average number of common shares and dilutive common share equivalents outstanding. During loss periods when common stock equivalents, if any, are anti-dilutive they are not considered in the computation. During the three and nine month periodyear ended September 30, 2016,December 31, 2020, the Company had 500,0007 options and 130,000152,738 warrants to purchase common stock outstanding; however, the effecteffects were anti-dilutive due to the net loss. During the year ended December 31, 2019, the Company had 7 options and 10,089 warrants to purchase common stock outstanding; however, the effects were anti-dilutive due to the net loss.
Recent Accounting Pronouncements – The Company has reviewed all recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operation, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its financial statements.
NOTE 2 – LICENSING AGREEMENTS
ED Patent – The Company acquired a patent from Creative Medical Health, Inc. (“CMH”)CMH, a related company on February 2, 2016, in exchange for 64,666,667862 shares of CMTH restricted common stock valued at $100,000. CMH holds a significant amount of the Public Company'sCompany’s common stock. The patent expires in 2025 and the Company has elected to amortize the patent over a ten year-year period on a straight linestraight-line basis. Amortization expense of $2,521 and $6,603$9,972 was recorded for the threeyears ended December 31, 2020 and nine month period ended September 30, 2016, respectively.
Male Infertility License Agreement - The Company has acquired a royalty license from Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (“LABIOMED”) granting2019. As of December 31, 2020, the exclusive license to the products and services of a LABIOMED patent.
The license was acquired for a cash payment of $5,000, issuance of 50,000 shares of restricted common stock of the Company (valued at $500, which is the par value of $0.01 per share), and an agreement to reimburse LABIOMED up to $1,800 for expenses incurred by LABIOMED in reviving and defending their patent. The Company has expensed the cash paid, thecarrying value of the stock issued, andpatent was $50,960. The Company expects to amortize $9,972 annually through 2026 related to the expected reimbursement of $1,800 for a total intangible royalty expense – license fees of $7,300.patent costs.
The Company is subject to a 6% royalty payment to LABIOMED on net sales of any products under this license and 25% on any non-royalty sublicense income. Commencing three years after the date of the agreement, and each subsequent year thereafter, the Company is required to pay to LABIOMED annual maintenance royalties of $20,000, unless during the prior one-year period the Company paid $50,000 or more in actual royalty payments. Finally, the Company agreed to pay LABIOMED certain milestone payments upon achieving the milestones set forth in the agreement. As of September 30, 2016, no amounts are currently due to LABIOMED.
Multipotent Amniotic Fetal Stem Cells License Agreement-On August 25, 2016, CMT entered into a License Agreement dated August 25, 2016, with a University. This license agreement grants to CMT the exclusive right to all products derived from a patent for use of multipotent amniotic fetal stem cells composition of matter throughout the world during the period ending on the expiration date of the longest-lived patent rights under the patent. The license agreement also permits CMT to grant sublicenses. Under the terms of the license agreement, CMT is required to diligently develop, manufacture, and sell any products licensed under the agreement. CMT paid the University an initial license fee within 30 days of entering into the agreement. CMT is also required to pay annual license maintenance fees on each anniversary date of the agreement, which maintenance fees would be credited toward any earned royalties for any given period. The License Agreement provides for payment of various milestone payments and earned royalties on the net sales of licensed products by CMT or any sub licensee. CMT is also required to reimburse the University for any future costs associated with maintaining the patent. CMT may terminate the license agreement for any reason upon 90 days’ written notice and the University may terminate the agreement in the event CMT fails to meet its obligations set forth therein, unless the breach is cured within 30 days of the notice from the University specifying the breach. CMT is also obligated to indemnify the University against claims arising due to the exercise of the license by CMT or any sub licensee. As of September 30, 2016,December 31, 2020, no amounts are currently due to the University.
The Company estimates that the patent expires in February 2026 and has elected to amortize the patent through the period of expiration on a straight linestraight-line basis. Amortization expense of $116 and $116$1,172 was recorded for the threeyears ended December 31, 2020 and nine month2019. As of December 31, 2020, the carrying value of the patent was $5,256. The Company expects to amortize approximately $1,172 annually through 2026 related to the patent costs.
| F-25 |
| Table of Contents |
Lower Back Patent – The Company, through its subsidiary StemSpine, LLC, acquired a patent from CMH, a related company, on May 17, 2017, covering the use of various stem cells for the treatment of lower back pain from pursuant to a Patent Purchase Agreement, which was amended in November 2017. As amended, the agreement provides the following:
· | The Company is required to pay CMH $100,000 within 30 days of demand as an initial payment. | |
· | In the event the Company determines to pursue the technology via use of autologous cells, the Company will pay CMH: |
o | $100,000 upon the signing agreement with a university for the initiation of an IRB clinical trial. | |
o | $200,000, upon completion of the IRB clinical trial. | |
o | $300,000 in the event we commercialize the technology via use of autologous cells by a physician without a clinical trial. |
· | In the event the Company determines to pursue the technology via use of allogenic cells, the Company will pay CMH: |
o | $100,000 upon filing an IND with the FDA. | |
o | $200,000 upon dosing of the first patient in a Phase 1-2 clinical trial. | |
o | $400,000 upon dosing the first patient in a Phase 3 clinical trial. |
· | Payment may be made in cash or shares of our common at a discount of 30% to the lowest closing price within 20 business days prior to the conversion date. | |
· | In the event the Company’s shares of common stock trade below $5.00 per share for two or more consecutive trading days, the number of any shares issuable as payment doubles. | |
· | For a period of five years from the date of the first sale of any product derived from the patent, the Company is required to make royalty payments of 5% from gross sales of products, and 50% of sale price or ongoing payments from third parties for licenses granted under the patent to third parties. |
The patent expires on May 19, 2027 and the Company has elected to amortize the patent over a ten-year period on a straight-line basis. Amortization expense of $10,000 was recorded for the years ended September 30, 2016, respectively.December 31, 2020 and 2019. As of December 31, 2020, the carrying value of the initial patent license was $65,000. The Company expects to amortize approximately $10,000 annually through 2027 related to the patent costs.
In November, 2019, following a successful international pilot study, the Company elected to initiate commercialization of the StemSpine procedure using autologous stem cells. As a result, the Company is obligated to pay CMH $300,000 pursuant to the Patent Purchase Agreement as described above. The Company has elected to amortize the patent over a ten-year period on a straight-line basis. Amortization expense of $45,940 was recorded for the year ended December 31, 2020. Amortization expense of $5,806 was recorded for the year ended December 31, 2019. As of December 31, 2020, the carrying value of the patent was $248,254. The Company expects to amortize approximately $46,000 annually through 2027 related to the patent costs.
ImmCelz™ - On December 28, 2020, ImmCelz, Inc. (“ImmCelz”), a newly formed Nevada corporation and wholly owned subsidiary of the Company, entered into a Patent License Agreement dated December 28, 2020 (the “Agreement”), with Jadi Cell, LLC. (“Jadi”), a company controlled by Dr. Amit Patel, a Board Member. The Agreement grants to ImmCelz™ the patent rights under U.S. Patent# 9,803,176 B2, “Methods and compositions for the clinical derivation of an allogenic cell and therapeutic uses”. The contract grants ImmCelz™ access to proprietary process of expanding the master cell bank of Jadi Cell LLC, as currently practiced by Licensor and as documented in standard operating procedures (SOPs) and other written documentation. The terms of the agreement are as follows:
· | Licensee shall pay Licensor a license fee of $250,000 (the “Upfront Royalty”), which can also be paid in CELZ stock at a discount of 25% of the closing price of $1.85 which is based on the date of this agreement | |
· | Within thirty (30) days of the end of each calendar quarter during the term of this Agreement, Licensee will pay Licensor five percent (5%) of the Net Income of ImmCelz™. during such calendar quarter (the “Continuing Royalty”) | |
· | in one or a series of related transactions, of all or substantially all of the business or assets of Licensee ImmCelz, Inc. (“Sale of Assets”) will result in a one-time ten-percent allocation to the licensor, the Continuing Royalty will be calculated at five percent (5%) of the Net Income of Licensee in any calendar quarter in which the Net Income in such calendar quarter reflects the receipt of any consideration from such Sale of Assets. |
| F-26 |
| Table of Contents |
As a result, the Company is obligated to pay Jadi $250,000 pursuant to the Patent License Agreement as described above. Refer to exhibit 10.29 for the complete agreement.
The Company has elected to amortize the patent over a ten-year period on a straight-line basis. Given the date of the Agreement, there was no amortization expense recorded for the year ended December 31, 2020. As of December 31, 2020, the carrying value of the patent was $250,000. The Company expects to amortize approximately $25,000 annually through 2030 related to the patent costs.
As of December 31, 2020, future expected amortization of these assets is as follows:
For the year ended December 31, |
|
|
| |
|
|
|
| |
2021 |
|
| 93,327 |
|
2022 |
|
| 93,327 |
|
2023 |
|
| 93,327 |
|
2024 |
|
| 93,327 |
|
2025 |
|
| 93,327 |
|
Thereafter |
|
| 152,835 |
|
Total |
| $ | 619,470 |
|
The following is a rollforward of the Company’s licensing agreements for the year end December 31, 2020.
|
| Assets |
|
| Accumulated Amortization |
| ||
|
|
|
|
|
|
| ||
Balances at December 31, 2019 |
| $ | 510,000 |
|
| $ | (73,445 | ) |
Addition of new assets |
|
| 250,000 |
|
|
| 0 |
|
Amortization |
|
| 0 |
|
|
| (66,792 | ) |
Balances at December 31, 2020 |
| $ | 760,000 |
|
| $ | (140,237 | ) |
NOTE 3 – RELATED PARTY TRANSACTIONS
The Company has incurred $315,000a monetary obligation to a related corporation to reimburse the cost of services provided to the Company (management and consulting) through September 30, 2016.December 31, 2020. Each of the Company’s executive officers is employed by the parent company, CMH, and will continue to receive his or her salary or compensation from CMH. The Company has an agreement with CMH which obligates the Company to reimburse CMH $35,000 per month for such services beginning January 2016. On November 17, 2017, the Company entered into an amended Management Reimbursement Agreement dated November 17, 2017, with Creative Medical Technologies, Inc. (“CMT”), the wholly owned subsidiary of the Company, and with Creative Medical Health, Inc., the parent of the Company (“CMH”). The compensation paidAgreement memorializes the arrangement between the parties whereby the Company has, since January 1, 2016, reimbursed CMH $35,000 per month for the services of management and consultants employed by CMH will include an allocation ofand performing services performed for CMH and for the Company. TheCompany and CMT. At the option of CMH, the reimbursable amounts are presented as a “management fee payable - related party” onset forth in the accompanying unaudited consolidated balance sheet at September 30, 2016. The liability is non-interest bearing, unsecured, and willAgreement may be due uponpaid from time to time in shares of common stock of the Company successfully raising at least $1,000,000 througha price equal to a 30% discount to the salelowest closing price during the 20 trading days prior to time the notice is given. The Agreement may be terminated by either party upon 30 days’ prior written notice. The agreement was amended in December 2018 to increase the monthly reimbursement from $35,000 to $45,000 effective January 1, 2019 and thereafter.
| F-27 |
| Table of Contents |
On January 12, 2018, the Company entered into a Debt Settlement Agreement with Timothy Warbington, our CEO, Chairman, and principal shareholder, and Creative Medical Health, Inc., the parent of equity. the Company, whereby Mr. Warbington cancelled $150,000 of debt owed by CMH to him in return for which he would receive 3,000,000 shares of Series A Preferred Stock which CMH agreed to receive in return for cancellation of $150,000 of debt owed by us to CMH for management reimbursement costs.
As of September 30, 2016,December 31, 2020, and 2019, amounts due to CMH under the arrangement were $280,000.$18,782 and $82 respectively.
During the nine months ended September 30, 2016, the Company paid $3,000 in consulting fees to an officer of the Company for services rendered. The payment is included in general and administrative expenses.
Through September 30, 2016, the Company has entered into three note payable agreements with CMH in which the proceeds were used in operations. The notes payable were dated February 2, 2016, May 1, 2016 and May 18, 2016 and resulted in borrowings of $50,000, $50,000 and $25,000, respectively. Notes payable of $50,000 mature on April 30, 2017,2018, $50,000 on July 31, 20172018 and $25,000 on May 18, 2018. On May 4, 2017, CMT and CMH entered into a Note Extension and Limited Waiver Agreement whereby the parties extended the maturity date of the 8% Promissory Note dated February 2, 2016, in the principal amount of $50,000, from April 30, 2017, to April 30, 2018, and CMH waived the nonpayment of the Note by CMT on the original maturity date. On extension, CMT paid to CMH accrued interest related to the extended note of $4,050. On July 31, 2017, CMT and CMH entered into a Note Extension and Limited Waiver Agreement whereby the parties extended the maturity date of the 8% Promissory Note dated May 1, 2016, in the principal amount of $50,000, from July 31, 2017, toJuly 31, 2018, and CMH waived the nonpayment of the Note by CMT on the original maturity date. On extension, CMT paid to CMH accrued interest related to the extended note of $4,050. The notes incur interest at 8% per annum on the outstanding balance of the notes. As of September 30, 2016,December 31, 2020, accrued, unpaid interest was $0.
On April 11, 2018, CMH converted $136,003 of principal and accrued interest was $3,757.into 19,711 common shares. As of December 31, 2020, the Company had fulfilled all the obligations of the notes.
During the nine month ended September 30,On August 12, 2016, CMH has advanced the Company $2,000 for operations. The amount is due on demand and doesn'tdoes not incur interest.
DuringOn May 17, 2017, StemSpine, LLC (“StemSpine”), a newly formed Nevada limited liability company and wholly owned subsidiary of Creative Medical Technologies, Inc. (“CMT”), the nine months ended September 30, 2016,wholly owned subsidiary of the Company, issued 300,000entered into a Patent Purchase Agreement dated May 17, 2017 (the “Agreement”), with Creative Medical Holdings, Inc. (“CMH”).
To date, the Company has paid CMH the $100,000 obligation of the initial payment due under this agreement, by a $50,000cash payment and the issuance of 667shares of common stock on December 12, 2019. On December 31, 2019 the Company paid CMH $50,000 of the $300,000 obligation from the second payment due under this agreement through the issuance of 133 shares of common stockstock. On September 30, 2020 the Company paid CMH $40,000 of the $300,000 obligation from the second payment due under this agreement through the issuance of 84,656 shares of common stock.
On December 28, 2020, ImmCelz, Inc. (“ImmCelz”), a newly formed Nevada corporation and 30,000 warrantswholly owned subsidiary of the Company, entered into a Patent License Agreement dated December 28, 2020 (the “Agreement”), with Jadi Cell, LLC. (“Jadi”), a company controlled by Dr. Amit Patel, a Board Member. Execution of the agreement triggered a $250,000 payment obligation to CMH for $30,000 in cash proceeds, see Note 5 for additional information.Jadi.
See Note 2 for discussion of an additional related party transactiontransactions with CMH.CMH relating the purchase of our ED patent in 2016, our StemSpine® patent in 2017 and the ImmCelz™ Patent License agreement in 2020.
NOTE 4 – STOCK BASEDDEBT
During 2019, we issued $1,572,400 in convertible notes to accredited investors with net proceeds of $1,302,795. The notes matured from February through October of 2020 and bore interest rates ranging from 2% to 11%. In February 2019, we entered into three separate exchange agreements with holders of common stock purchase warrants issued by the Company in September 2018 and November 2018. Under each exchange agreement, the Company issued a convertible promissory note in the principal amount of $100,000 to the warrant holder party to such exchange agreement in exchange for the cancellation of common stock purchase warrants held by such warrant holder, initially exercisable for an aggregate of 43 shares of the Company’s common stock. The notes were convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 66% of the average of the two lowest traded prices of the Company’s common stock during the previous 15 trading days preceding the conversion date, the lowest trade price during the previous 20 trading days, or the volume weighted average price over the prior 15 trading days. The loans are evidenced by promissory notes and bore interest in a range of 8% to 11%. The loan maturity dates ranged from February 19, 2020 through October 11, 2020. The Company amortized the on-issuance discounts of $1,194,357 to interest expense using the straight-line method over the original terms of the loans. During 2019 the Company amortized $1,613,226 to interest expense. As of December 31, 2019, a discount of $374,124 remained.
During 2019, the Company issued an aggregate of 23,746 shares upon the conversion of $1,501,275 of outstanding principal, interest and fees on existing, outstanding notes and 1,062 shares upon the cashless exercise of 1,188 warrants.
| F-28 |
| Table of Contents |
During the year ended December 31, 2019, the Company incurred $41,699 in pre-payment premiums associated with the extinguishment of $313,111 in principal that was recorded as interest expense.
During 2020, we issued $831,140 in convertible notes to accredited investors with net proceeds of $710,920. The notes mature from February through December of 2021 and bear an interest rate of 8%. The notes are convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 71% of the average of the two lowest traded prices of the Company’s common stock during the previous 15 trading days preceding the conversion date, the lowest trade price during the previous 15 trading days. The Company is amortizing the on-issuance discounts of $828,710 to interest expense using the straight-line method over the original terms of the loans. During 2020 the Company amortized $979,959 to interest expense. As of December 31, 2020, a discount of $409,650 remained.
During 2020, we issued an aggregate of 1,363,463 shares upon the conversion of $1,366,705 of outstanding principal, interest and fees on existing, outstanding notes.
As of December 31, 2020, future loan maturities are as follows:
For the year ending December 31, |
|
|
| |
|
|
|
| |
2021 |
|
| 1,305,005 |
|
NOTE 5 – DERIVATIVE LIABILITIES
Derivative Liabilities
In connection with convertible notes payable and the related party management fee and patent liability, the Company records derivative liabilities for the conversion feature. In addition, the Company has warrants for which the exercise prices reset upon future events. These warrants are also considered to be derivative liabilities. The derivative liabilities are valued on the date the convertible note payable and the related party liabilities become convertible and revalued at each reporting period. The warrants are valued on the date of issuance and revalued at each reporting period. During the year ended December 31, 2020, the Company recorded initial derivative liabilities of $2,572,723 based upon the following Black-Scholes option pricing model average assumptions: an exercise price of 0.40 to $0.60our stock price on the date of grant of $1.70to $18.25, expected dividend yield of 0%, expected volatility of 103.79% to 131.89%, risk free interest rate of 0.16% to 1.62% and an expected term of 1.0 year. Upon initial valuation, the derivative liability exceeded the face value certain of the convertible note payables by approximately $1,864,233, which was recorded as a day one loss on derivative liability.
On December 31, 2020, the derivative liabilities were revalued at $38,741,832 resulting in a loss of $33,980,805 related to the change in fair market value of the derivative liabilities. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following average assumptions: an exercise price of $0.40 to $6.70, our stock price on the date of valuation $13.90, expected dividend yield of 0%, expected volatility of 98.14% to 100.94%, risk-free interest rate of 0.17%, and expected terms ranging from 0.50 to 3.57 years.
During the year ended December 31, 2019, the Company recorded initial derivative liabilities of $2,794,910 based upon the following Black-Scholes option pricing model average assumptions: an exercise price of $25.00 to $1,545, our stock price on the date of grant of $30.00to $1,390, expected dividend yield of 0%, expected volatility of 87.61% to 103.96%, risk free interest rate of 1.56% to 2.55% and expected terms ranging from 1.0 to 5.0 years. Upon initial valuation, the derivative liability exceeded the face value certain of the convertible note payables by approximately $1,215,985, which was recorded as a day one loss on derivative liability.
On December 31, 2019, the derivative liabilities were revalued at $6,847,877 resulting in a loss of $3,512,648 related to the change in fair market value of the derivative liabilities. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following average assumptions: an exercise price of $10.00to $295, our stock price on the date of valuation $25.00, expected dividend yield of 0%, expected volatility of 93.84% to 103.96%, risk-free interest rate of 1.62%, and expected terms ranging from 0.50 to 4.58 years.
Future Potential Dilution
Most of the Company’s convertible notes payable contain adjustable conversion terms with significant discounts to market. As of December 31, 2020, the Company’s convertible notes payable are potentially convertible into an aggregate of approximately 1.4 billion shares of common stock. In addition, due to the variable conversion prices on some of the Company’s convertible notes, the number of common shares issuable is dependent upon the traded price of the Company’s common stock.
| F-29 |
| Table of Contents |
NOTE 6 – STOCK-BASED COMPENSATION
The Public Company has reserved 2,000,00027 shares under its 2016 Stock Incentive Plan (the “Plan”“Plan”). The Plan was adopted by the board of directors on May 18, 2016, as a vehicle for the recruitment and retention of qualified employees and consultants. The Plan is administered by the Board of Directors. The Public Company may issue, to eligible employees or contractors, restricted common stock, options, stock appreciation rights and restricted stock units. The terms and conditions of awards under the Plan will be determined by the Board of Directors.
In July and September 2016, the Company granted 10-year options to two parties for accepting appointment to the Company’s scientific advisory board. Each award consisted of options to purchase up to 250,000500 shares at $0.175$87.50 per share. The options vest at a rate of 50,000100 on each anniversary date of the respective grants. One of the advisory board members has also agreed to furnish to the Company access to laboratory facility for 10,000 shares of restricted common stock per month; however, a formal agreement for the laboratory facility has not been agreed to. The options are accounted for as non-employee stock options and thus revalued for reporting purposes at the end of each quarter. During 2020 and 2019, the fair market value of the options was insignificant to the financial statements.
Since the expected life of the options was greater than the Company’s historical stock information available, the Company determined the expected volatility based on price fluctuations of comparable public companies.
There were no options issued during the years ended December 31, 2020 and 2019.
Option activity for the years ended December 31, 2020 and 2019 consists of the following:
|
| Stock Options |
|
| Weighted Average Exercise Price |
|
| Weighted Average Life Remaining |
| |||
Outstanding, December 31, 2018 |
|
| 7 |
|
| $ | 13,125 |
|
|
| 7.65 |
|
Issued |
|
| - |
|
|
| - |
|
|
| - |
|
Exercised |
|
| - |
|
|
| - |
|
|
| - |
|
Expired |
|
| - |
|
|
| - |
|
|
| - |
|
Outstanding, December 31, 2019 |
|
| 7 |
|
| $ | 13,125 |
|
|
| 6.65 |
|
Issued |
|
| - |
|
|
| - |
|
|
| - |
|
Exercised |
|
| - |
|
|
| - |
|
|
| - |
|
Expired |
|
| - |
|
|
| - |
|
|
| - |
|
Outstanding, December 31, 2020 |
|
| 7 |
|
| $ | 13,125 |
|
|
| 5.64 |
|
Vested, December 31, 2020 |
|
| 5 |
|
| $ | 13,125 |
|
|
| 5.64 |
|
See Note 2 for discussion related to the issuance of common stock in connection with licensing agreements. See Note 4 and 5 for discussion regarding warrants issued with a convertible note payable.
From April through September 2020, we granted 23,262 3-year warrants to a vendor for services rendered at exercise prices ranging from $1.45 to $3.50. The value of the warrants was determined to be $35,670 based upon the Black-Scholes method, see variables used below. In December 2020,we granted a total of 60,000 warrants to three of our board members at an exercise price of $2.00. The value of the warrants was determined to be $102,081 based upon the Black-Scholes method, see variables used below. As of December 31, 2020, future estimated stock-based compensation expected to be recorded was estimated to be $0.
The fair value of each optionwarrant award is estimated using the Black-Scholes valuation model. Assumptions used in calculating the fair value at September 30, 2016during the year ended December 31, 2020 were as follows:
| Weighted | ||||
| Average | ||||
| Inputs Used | ||||
| Annual dividend yield | $ | - | ||
| Expected life (years) | 7.40 | |||
| Risk-free interest rate | 1.42 | % | ||
| Expected volatility | 87.60 | % | ||
| Common stock price | $ | 0.10 | ||
Weighted | ||||
Annual dividend yield | $ | - | ||
Expected life (years) | 3.0 | |||
Risk-free interest rate | 0.11% to0.94 | % | ||
Expected volatility | 95.27% to 106.51 | % | ||
Common stock price | $ | 1.45 to 9.50 | ||
Since the expected life of the options was greater than the Public Company's historical stock information available, the Public Company determined the expected volatility based on price fluctuations of comparable public companies.See Note 7 for warrant rollforward.
| F-30 |
| Table of Contents |
NOTE 7 – STOCKHOLDERS’ DEFICIT
Stock based compensation for the three and nine months ended September 30, 2016 was $735 and $735, respectively, and included with general and administrative expenses. As of September 30, 2016, future estimated stock based compensation to be recorded was $34,756 which will be recognized through 2021.
NOTE 5 – STOCKHOLDERS’ DEFICIT
In August 2016,During 2019, the Company commenced a non-public offering of up to 10,000,000 shares of common stock at $0.10 per share, and at no additional cost, one warrantentered into convertible loan agreements with third parties that included 486 5-year warrants to purchase anothera share of common stock at $0.10 per share for each ten shares purchased ininitial prices ranging from $295 to $1,545. On the offering. The securities offered have not been and will not be registered under the Securities Actdate of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. From August 6, 2016 to September 30, 2016,issuance, the Company sold 1,300,000accounted for the conversion feature on the warrants as derivative liabilities, see Note 5. Derivative accounting applies as the number of warrants and the conversion price are variable and do not have a floor as to the number of common shares in this offering. resulting in proceeds of $130,000 andwhich could be converted. For the issuance ofyear ended December 31, 2019, outstanding warrants were increased by 9,100 to purchase 130,000 shares of common stock. Of this amount $30,000 was received from CMH, a related party. The fair valuereflect the terms of the warrants of $8,197 was estimated usingwarrant agreements. For the Black-Scholes valuation model. Theyear ended December 31, 2019, 1,188 warrants were classified as equity as they were issued in connection with a capital raise. converted into 1,062 common shares through cashless conversions. As of December 31, 2019, 10,089 warrants remained.
Assumptions used in calculating the fair value of the warrants upon issuanceissued in 2019 were as follows:
| Inputs Used | ||||
| Annual dividend yield | $ | - | ||
| Expected life (years) | 3.00 | |||
| Risk-free interest rate | 0.86 | % | ||
| Expected volatility | 102.59 | % | ||
| Common stock price | $ | 0.10 | ||
Range of | ||||
Inputs Used | ||||
Annual dividend yield | $ | - | ||
Expected life (years) | 5.00 | |||
Risk-free interest rate | 1.56 to 2.23 | % | ||
Expected volatility | 82.85% to 92.64.00 | % | ||
Common stock price | $ | 295 to 760 | ||
During 2020, the Companygranted 3-year warrants to three board members to purchase an aggregate of 60,000 shares of common stock at a price of $0.004 and 8,214,286 to a contractor for services rendered at prices ranging from $2.00 to $2.50. For the year ended December 31, 2020, outstanding warrants were increased from the initial issuance of 291 warrants to 69,063 to reflect the terms of the existing warrant agreements. For the year ended December 31, 2020 there were no warrants converted into common shares through cashless conversions. As of December 31, 2020, 152,745 warrants remained.
Assumptions used in calculating the fair value of the warrants issued in 2020 were as follows:
Range of | ||||
Inputs Used | ||||
Annual dividend yield | $ | - | ||
Expected life (years) | 3.00 | |||
Risk-free interest rate | 0.11% to 0.29 | % | ||
Expected volatility | 99.24% to 106.51 | % | ||
Common stock price | $ | 1.45 to 2.50 | ||
Warrant activity for the years ended December 31, 2020 and 2019 consists of the following:
|
| Warrants |
|
| Weighted |
|
| Weighted |
| |||
Outstanding, December 31, 2018 |
|
| 1,894 |
|
| $ | 355.00 |
|
|
| 4.22 |
|
Issued |
|
| 486 |
|
|
|
|
|
|
|
|
|
Exercises |
|
| (1,188 | ) |
|
|
|
|
|
|
|
|
Anti-Dilution Modifications |
|
| 9,100 |
|
|
|
|
|
|
|
|
|
Forfeiture/Cancellations |
|
| (203 | ) |
|
|
|
|
|
| - |
|
Outstanding, December 31, 2019 |
|
| 10,089 |
|
| $ | 45.00 |
|
|
| 3.08 |
|
Issued |
|
| 83,262 |
|
|
|
|
|
|
|
|
|
Exercises |
|
| - |
|
|
|
|
|
|
|
|
|
Anti-Dilution Modifications |
|
| 59,394 |
|
|
|
|
|
|
| - |
|
Forfeiture/Cancellations |
|
|
|
|
|
|
|
|
|
| - |
|
Outstanding, December 31, 2020 |
|
| 152,745 |
|
| $ | 2.85 |
|
|
| 2.47 |
|
Vested, December 31, 2020 |
|
| 152,745 |
|
| $ | 2.85 |
|
|
| 2.47 |
|
See Note 5 for discussion regarding anti-dilution and modifications related to warrants accounted for as derivative liabilities.
See Note 2 for discussion related to the issuance of common stock in connection with licensing agreements.
See Note 3 for discussion related to the issuance of common stock to a related party for cash.
| F-31 |
| Table of Contents |
NOTE 68 – INCOME TAXES
The provision for income tax expense consists of the following at December 31, 2020 and 2019:
|
| 2020 |
|
| 2019 |
| ||
Income tax provision attributable to: |
|
|
|
|
|
| ||
Federal |
| $ | (250,771 | ) |
| $ | (272,800 | ) |
State and local |
|
| (69,671 | ) |
|
| (75,792 | ) |
Valuation allowance |
|
| 320,442 |
|
|
| (348,592 | ) |
Net provision for income tax |
| $ | 0 |
|
| $ | 0 |
|
Deferred tax assets consist of the following at December 31, 2020 and 2019:
|
| 2020 |
|
| 2019 |
| ||
Deferred tax asset attributable to: |
|
|
|
|
|
| ||
Net operating loss carryover |
| $ | 1,593,282 |
|
| $ | 1,347,378 |
|
Accrued management fees, related party |
|
| 155,063 |
|
|
| 80,825 |
|
Valuation allowance |
|
| (1,748,345 | ) |
|
| (1,427,903 | ) |
Net deferred tax asset |
| $ | 0 |
|
| $ | 0 |
|
The primary difference between the statutory federal rate and the Company’s effective tax rate for the years ended December 31, 2020 and 2019 was due to the 100% valuation allowance. The following is a reconciliation of the statutory federal rate and the Company’s effective tax rate for the year ended December 31, 2020 and 2019:
|
| 2020 |
|
| 2019 |
| ||
|
|
|
|
|
|
| ||
Tax at federal statutory rate |
|
| 21.0 | % |
|
| 21.0 | % |
State, net of federal benefit |
|
| 0.2 | % |
|
| 0.9 | % |
Change in temporary differences |
|
| (0.0 | )% |
|
| 0.0 | )% |
Permanent differences |
|
| (20.2 | ) |
|
| (17.9 | )% |
Valuation allowance |
|
| (0.9 | )% |
|
| (4.1 | )% |
Provision for taxes |
|
| - |
|
|
|
|
|
As of December 31, 2020, the Company had federal and state gross net operating loss carryforwards of approximately $6.5 million. The federal and state net operating losses and tax credits expire in years beginning in 2036. Under Section 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income may be limited. In general, an “ownership change” will occur if there is a cumulative change in our ownership by “5-percent shareholders” that exceeds 50 percentage points over a rolling three-year period. Similar rules may apply under state tax laws. To date, the Company hasn’t experienced “ownership changes” under section 382 of the Code and comparable state tax laws. As of December 31, 2020, the Company estimates that none of the federal and state net operating losses will be limited under Section 382 of the Code.
As of December 31, 2020, and 2019, the Company maintained a full valuation allowance on its net deferred tax assets. The valuation allowance was determined in accordance with the provisions of ASC 740, Accounting for Income Taxes, which requires an assessment of both positive and negative evidence when determining whether it is more likely than not that deferred tax assets are recoverable. Such assessment is required on a jurisdiction by jurisdiction basis. The Company’s history of cumulative losses, along with expected future U.S. losses required that a full valuation allowance be recorded against all net deferred tax assets. The Company intends to maintain a full valuation allowance on net deferred tax assets until sufficient positive evidence exists to support reversal of the valuation allowance.
| F-32 |
| Table of Contents |
The applicable federal and state rates used in calculating the deferred tax provision was 21.0% and 8.9%, respectively.
The Company files income tax returns in the U.S. and Arizona. All years presented remain subject to examination for U.S. federal and state purposes. The Company is not currently under examination in federal or state jurisdictions.
NOTE 9 – SUBSEQUENT EVENTS
In accordance with ASC 855, management reviewed all material events through November 10, 2016, for these financial statements and there are no material subsequent events to report, except as follows:
Subsequent to September 30, 2016, the Company sold 800,000On January 8, 2021 shares of common stock were issued to CMH in satisfaction of a $50,000 payment owed to CMH under the offering disclosedPatent Purchase Agreement, dated May 17, 2017, as amended on November 14, 2017, between us, our wholly-owned subsidiary StemSpine, LLC, and CMH. Pursuant to the Patent Agreement, the number of shares issued was calculated based on a 30% discount to the closing trading price of the common stock of $.0016 on December 9, 2020.
In February 2020, we completed the sale of two 10% Original Issue Discount Senior Convertible Notes to two institutional investors pursuant to a Securities Purchase Agreement between the Company and the investors. The transactions were effected pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended and Rule 506(b) promulgated thereunder. Pursuant to the Purchase Agreements, for an aggregate purchase price of $144,500, the Investors purchased the notes in Note 5, resultingthe aggregate principal amount of $157.150. The notes mature in proceedsFebruary, 2021, bear interest at a rate of $80,0008% per annum, and are convertible into shares of the Company’s common stock at a conversion prices ranging from to 60% to 61% of the average of the two lowest traded price of the Company’s common stock during the 15 trading days preceding the applicable conversion date.
On February 12, 2021, we completed the sale to an institutional investor 350,000 shares of the our newly designated Series B Preferred Stock pursuant to a Securities Purchase Agreement between the Company and the Purchaser for an aggregate purchase price of $350,000. In addition, pursuant to the Purchase Agreement, we issued the Purchaser 3,000 shares of our Common Stock as “Commitment Shares” for entering into the transaction. The transaction was effected pursuant to Secti on 4(a)(2) of the Securities Act of 1933, as amended and Rule 506 of regulation (b) promulgated thereunder.
Each share of Series B Preferred Stock has a Stated Value of $1,200.00 and is convertible into Common Stock at a conversion price equal to $25. The conversion price of the Series B Preferred Stock is subject to equitable adjustment in the event of a stock split, stock dividend or similar event with respect to the Common Stock, and will be reduced to $17.50 in the event of a “Triggering Event” a defined in the Certificate of Designation of the Series B Preferred Stock (the “Certificate of Designation”).
The Series B Preferred Stock (i) carries a quarterly dividend at the rate of 10% per annum, payable in cash or additional shares of Series B Preferred Stock, at the Company’s option, and (ii) may be redeemed by us, at its option, upon the payment of an amount equal to (a) $1,200 per share of Series B Preferred Stock, plus all accrued dividends thereon and any unpaid fees or liquidated damages then due with respect to the Series B Preferred Stock pursuant to the Certificate of Designation, multiplied by (b) a premium ranging from 5% if the redemption occurs within 90 days following the issuance of the Series B Preferred Stock, to 20% if the redemption occurs between 120 and 180 days following the issuance of the Series B Preferred Stock.
On March 11, 2020, following the approval of the Board of Directors of the Company filed a warrantCertificate of Amendment to purchase 80,000the Certificate of Designation of the Company’s Series A Preferred Stock with the Secretary of State of the State of Nevada (the “Certificate of Amendment”). The Certificate of Amendment increased the voting power of the Series A Preferred Stock to 10,00 votes per share from 30 votes per share. The Company has 3,000,000 shares of Series A Preferred Stock outstanding, all of which are held by Timothy Warbington.
From January 1, 2021 through March 17, 2021, we issued 623,404 shares of common stock.stock for the conversion of $947,575 in convertible note principal, interest and fees.
[OUTSIDE BACK COVER]
Creative Medical Technology Holdings, Inc.[A Nevada Corporation]23,732,669 SharesCommon StockDuring January and February, 2021 we issued 20,111 shares to a contractor upon the conversion of 23,167 cashless warrants. The warrants were issued to the contractor for services rendered.
In November, 2021, following the approval of the Company’s Board of Directors and holders of a majority the Company’s voting stock, the Company filed an amendment to its Articles of Incorporation increasing the Company’s authorized shares of Common Stock to 25 billion from 6 billion. Thereafter, on November 10, 2021, following the approval of the Board of Directors of the Company, the Company effected a 1-for-500 reverse split of the both the Company’s authorized and outstanding shares of Common Stock, by filing a Certificate of Change with the Nevada Secretary of State under Section 78.209 of the Nevada Revised Statutes. Following the stock split, the Company’s authorized common stock was reduced to 50,000,000 shares, and the Company had outstanding approximately 2,452,348 shares of Common Stock. No fractional shares will be issued, and no cash or other consideration will be paid, in connection with the reverse stock split. Instead, the Company will issue one whole share of the post-Reverse Stock Split Common Stock to any stockholder who otherwise would have received a fractional share as a result of the reverse stock split. All share references have been restated for this reverse split to the earliest period presented.
PROSPECTUS
| F-33 |
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC.2017 W Peoria Avenue
Phoenix, AZ 85029
Telephone (602) 680-7439
_______________, 2016
4,842,615 SHARES OF COMMON STOCK
WARRANTS TO PURCHASE UP TO 4,842,615 SHARES OF COMMON STOCK
PRE-FUNDED WARRANTS TO PURCHASE UP TO 4,842,615 SHARES OF COMMON STOCK
Until , 2016, all dealers that effect transactions in our shares, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART IIP R O S P E C T U S
Roth Capital Partners
December , 2021
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
ItemITEM 13. Other Expenses of Issuance and DistributionOTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following is an itemized statement oftable sets forth the estimated amounts of allcosts and expenses payableincurred by us in connection with the registrationsale of the common stock, other than underwriting discounts and commissions.Common Stock being registered by this registration statement. All amounts shown are estimates, except for the SECSecurities and Exchange Commission (“SEC”) registration fee.
| Securities and Exchange Commission - Registration Fee | $ | 1,348 | ||
| State filing Fees | 2,000 | |||
| Edgarizing Costs | 3,000 | |||
| Accounting Fees and Expenses | 10,000 | |||
| Legal Fees and Expenses | 20,000 | |||
| Miscellaneous | 3,652 | |||
| Total | $ | 40,000 |
SEC registration fee |
| $ | 4,530.71 |
|
Accounting fees and expenses |
| $ | 25,000.00 |
|
Legal fees and expenses |
| $ | 250,000.00 |
|
Nasdaq listing fee |
| $ | 50,000.00 |
|
Miscellaneous expenses |
| $ | 50,000.00 |
|
|
|
|
|
|
Total |
| $ | 379,530.71 |
|
None of the expenses of the offering will be paid by the selling security holders.
ItemITEM 14. Indemnification of Directors and OfficersINDEMNIFICATION OF DIRECTORS AND OFFICERS
Nevada law expressly authorizesprovides that a Nevada corporation tomay indemnify its directors, officers, employees, and agents against liabilities arising out of such persons’ conduct as directors, officers, employees, or agents if they acted in good faith, in a manner they reasonably believed to be in or not opposed to the best interests of the company, and, in the case of criminal proceedings, if they had no reasonable cause to believe their conduct was unlawful. Generally, indemnification for such persons is mandatory if such person was successful, on the merits or otherwise, in the defense of any such proceeding, or in the defense of any claim, issue, or matter in the proceeding. In addition, as provided in the articles of incorporation, bylaws, or an agreement, the corporation may pay for or reimburse the reasonable expenses incurred by such a person who is a party to a proceeding in advance of final disposition if such person furnishes to the corporation an undertaking to repay such expenses if it is ultimately determined that he did not meet the requirements. In order to provide indemnification, unless ordered by a court, the corporation must determine that the person meets the requirements for indemnification. Such determination must be made by a majority of disinterested directors; by independent legal counsel; or by a majority of the shareholders.
Section 2 of Article X of our Articles of Incorporation provides that we are required to indemnify, and advance expenses as they are incurred to, any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, or criminal, administrative or investigative, other than an action by or in the right of the corporation (i.e., a “non-derivative proceeding”), by reason of the fact that such personhe or she is or was a director, officer, employee or agent of the corporation, or fiduciary of our company,is or who iswas serving at ourthe request or directionof the corporation as a director, officer, employee agent or fiduciaryagent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by such personhim in connection with the action, suit or proceeding. The advancementproceeding if he or she:
· | Is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation; or |
· | Acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. |
In addition, a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor (i.e., a “derivative proceeding”), by reason of the fact that he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in these actions is contingentsettlement and attorneys’ fees actually and reasonably incurred by him or her in connection with the defense or settlement of the action or suit if he:
· | Is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation; or |
· | Acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation. |
Under Nevada law, indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person undertakingis fairly and reasonably entitled to repayindemnity for such expenses as the amounts advanced if itcourt deems proper.
To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any non-derivative proceeding or any derivative proceeding, or in defense of any claim, issue or matter therein, the corporation is ultimately determinedobligated to indemnify him or her against expenses, including attorneys’ fees, actually and reasonably incurred in connection with the defense.
Further, Nevada law permits a Nevada corporation to purchase and maintain insurance or to make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him or her and liability and expenses incurred by him or her in his or her capacity as a director, officer, employee or agent, or arising out of his or her status as such, whether or not the corporation has the authority to indemnify him or her against such liability and expenses.
| II-1 |
Our bylaws provide that, the Company shall, to the fullest extent permitted by the laws of the State of Nevada, indemnify any person who is or was a director or officer of the Company or any predecessor of the Company or is or was serving at the Company’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, or other entity (each such person, an “Indemnitee”) against expenses, including attorneys’ fees, judgments, fines, and amounts paid in settlement, actually and reasonably incurred by the Indemnitee in connection with any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative, other than a proceeding by or in the right of the Company, to which the Indemnitee is, was, or is threatened to be made a party by reason of being an Indemnitee, if the Indemnitee either: (a) did not entitledbreach, through intentional misconduct, fraud, or a knowing violation of law, the Indemnitee’s fiduciary duties as a director or officer to indemnification. Article VIIIact in good faith and in the interests of the Company; or (b) acted in good faith and in a manner the Indemnitee reasonably believed to be in or not opposed to the best interests of the Company and, with respect to any criminal action or proceeding, had no reasonable cause to believe the Indemnitee’s conduct was unlawful.
Additionally, our Bylaws also mandates similar indemnification provisions.bylaws provide that the Company shall, to the fullest extent permitted by the laws of the State of Nevada, indemnify any Indemnitee against expenses, including attorneys’ fees and amounts paid in settlement, actually and reasonably incurred by the Indemnitee in connection with any threatened, pending, or completed suit or action by or in the right of the Corporation to which the Indemnitee is, was, or is threatened to be made a party by reason of being an Indemnitee, if the Indemnitee either: (a) did not breach, through intentional misconduct, fraud, or a knowing violation of law, the Indemnitee’s fiduciary duties as a director or officer to act in good faith and in the interests of the Company; or (b) acted in good faith and in a manner the Indemnitee reasonably believed to be in or not opposed to the best interests of the Company.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of our companythe Company pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC suchthis indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ItemITEM 15. Recent Sales of Unregistered SecuritiesRECENT SALES OF UNREGISTERED SECURITIES
On May 18, 2016, atOver the closing of the Merger Agreement, each share of common stock of CMTpast three years, we have issued and outstanding immediately prior tosold the closing was converted into 6.4666666 shares of our common stock. As a result, a total of 97,000,000 shares were issued. The sale of thesefollowing securities was made pursuant to Rule 506(b) of Regulation D promulgated by the SECwithout registration under the Securities Act. The recipients of the shares were either “accredited investor” as defined in Rule 501(a) of Regulation D or, if they were not “accredited investors,” they (or their representatives, if applicable) met the sophistication requirements of Rule 506(b). Each non-accredited investor received information required under Rule 506(b) a reasonable time prior to the closing of the merger agreement. The recipients of the shares acknowledged appropriate investment representations with respect to the issuances and consented to the imposition of restrictive legends upon the certificates representing these securities. The recipients of the shares had a significant preexisting relationship to us prior to the transaction and did not receive their shares from us as a result of any general solicitation. The recipients of the shares and their representatives were afforded the opportunity to ask questions of our management and to receive answers concerning the terms and conditions of the transaction and issuance of the securities. No selling commissions or other remuneration was paid in connection with the sale of these securities.Act:
From August through October 2016, we sold 2,100,000Current Period Ending September 30, 2021
Subsequent to June 30, 2021, the Company borrowed $341,650 under convertible notes payable issued by two institutional investors.
Subsequent to June 30, 2021, the Company issued 17,628 shares of common stock for the conversion of $153,980 in convertible notes.
On August 11, 2021, the Company completed the sale of 15% Original Issue Discount Senior Notes (“Notes”) to a group of institutional investors (the “Purchasers”), pursuant to a Securities Purchase Agreement between the Company and the Purchasers dated as of August 9, 2021 (the “Purchase Agreement”). Pursuant to the Purchase Agreement, for an aggregate purchase price of $3,787,750, the Purchasers purchased Notes in the aggregate principal amount of $4,456,176. Each Note matures on February 11, 2022, subject to the Company’s requirement to redeem the Notes prior to such date with the net proceeds of any future offering of the Company’s securities. The Notes do not bear interest other than upon an event of default, and are not convertible into the Company’s common stock. In addition, the Notes are subject to covenants, events of defaults and other terms and conditions customary in transactions of this nature. Pursuant to the Purchase Agreement, the Company also issued to the Purchasers five-year warrants (“Warrants”) to purchase an aggregate of 314,369 shares of the Company’s common stock at an initial exercise price of $14.18 per share, subject to anti-dilution adjustment in the event of future sales of equity by the Company below the then exercise price, stock dividends, stock splits and other specified events. Roth Capital Partners (“Roth”), acted as sole placement agent for the offering. Pursuant to terms of an engagement letter with Roth, the Company paid Roth a placement agent fee of 9% of the gross proceeds of $210,000, andthe offering. The Company also issued at no additional cost, three-year warrantsRoth a warrant to purchase 210,00020,189 shares at $0.10 per share. The sale of these securities was made pursuant to Rule 506(b) of Regulation D promulgated bycommon stock with the SEC undersame terms as the Securities Act. Each of the purchasers of the share was an “accredited investor” as defined in Rule 501(a) of Regulation D. The purchasers acknowledged appropriate investment representations with respectWarrants issued to the sales ofPurchasers.
Subsequent to June 30, 2021, the shares and consented to the imposition of restrictive legends upon the certificates representing the shares. Each of the purchasers had a significant preexisting relationship to us prior to the transaction and did not purchase the shares from us as a result of any general solicitation. Each purchaser was afforded the opportunity to ask questions of our management and to receive answers concerning the terms and conditions of the investment. No selling commissions or other remuneration was paidCompany issued 30,000 warrants in connection with the salesincentive grants to Scientific Advisory Board members and employees. The warrants are exercisable at $15.00 per share, vest upon issuance and exercisable for a period of these securities.ten years.
| II-2 |
Six Months Ended June 30, 2021
Item 16. ExhibitsDuring the six-months ended June 30, 2021, we issued $157,150 in convertible notes to accredited investors with net proceeds of $134,640. The notes mature during February of 2022 and Financial Statement Schedules
| Incorporated by Reference | ||||||||||||
| Exhibit Number | Exhibit Description | Form | File No. | Exhibit | Filing Date | Filed Here- with | ||||||
| 2.1 & 10.1 | Agreement and Plan of Merger, dated April 29, 2016 | 8-K | 000-53500 | 2.1 | 5/5/16 | |||||||
| 2.2 &10.2 | Name Change Merger Agreement filed May 18, 2016 with Creative Medical Technology Holdings, Inc. | 8-K | 000-53500 | 2.1 | 5/19/16 | |||||||
| 3.1 | Articles of Incorporation | X | ||||||||||
| 3.2 | Bylaws | 10 | 000-53500 | 3.2 | 11/18/08 | |||||||
| 3.3 | Articles of Exchange filed Effective May 18, 2016 | 8-K | 000-53500 | 3.1 | 5/19/16 | |||||||
| 3.4 | Articles of Merger filed Effective May 18, 2016 (Acquisition Transaction) | 8-K | 000-53500 | 3.2 | 5/19/16 | |||||||
| 3.5 | Articles of Merger filed Effective May 18, 2016 (Name Change Transaction) | 8-K | 000-53500 | 3.3 | 5/19/16 | |||||||
| 4.1 | Form of common stock Certificate | 10-Q | 000-53500 | 4.1 | 8/19/16 | |||||||
| 4.1 & 10.3 | 2016 Stock Incentive Plan | 8-K | 000-53500 | 99.17 | 5/19/16 | |||||||
| 5.1 | Opinion re Legality of Shares | X | ||||||||||
| 10.4 | 8% Promissory Note dated May 18, 2016, for $25,000 with Creative Medical Health | 8-K | 000-53500 | 99.1 | 5/19/16 | |||||||
| 10.5 | Patent Purchase Agreement dated February 3, 2016, with Creative Medical Health | 8-K | 000-53500 | 99.2 | 5/19/16 | |||||||
| 10.6 | Loan Agreement dated February 2, 2016, with Creative Medical Health | 8-K | 000-53500 | 99.3 | 5/19/16 | |||||||
| 10.7 | 8% Promissory Note dated February 2, 2016, for $50,000 with Creative Medical Health | 8-K | 000-53500 | 99.4 | 5/19/16 | |||||||
| 10.8 | License Agreement with Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center Effective January 29, 2016 | 8-K | 000-53500 | 99.5 | 5/19/16 | |||||||
| 10.9 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Lorikeet, Inc. | 8-K | 000-53500 | 99.8 | 5/19/16 | |||||||
| 10.10 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Jackie O’Reilly | 8-K | 000-53500 | 99.9 | 5/19/16 | |||||||
| Incorporated by Reference | ||||||||||||
| Exhibit Number | Exhibit Description | Form | File No. | Exhibit | Filing Date | Filed Here- with | ||||||
| 10.11 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Dassity, Inc. | 8-K | 000-53500 | 99.10 | 5/19/16 | |||||||
| 10.12 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Sugarloaf Management, LLC | 8-K | 000-53500 | 99.11 | 5/19/16 | |||||||
| 10.13 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Serenity Services, Inc. | 8-K | 000-53500 | 99.12 | 5/19/16 | |||||||
| 10.14 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with McKinley Enterprise Profit Sharing Plan, Inc. | 8-K | 000-53500 | 99.13 | 5/19/16 | |||||||
| 10.15 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with TradeCo, Inc. | 8-K | 000-53500 | 99.14 | 5/19/16 | |||||||
| 10.16 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with Bateman Dynasty | 8-K | 000-53500 | 99.15 | 5/19/16 | |||||||
| 10.17 | Cancellation of Indebtedness Agreement, dated May 6, 2016 with McKinley Capital 401K Roth Plan | 8-K | 000-53500 | 99.16 | 5/19/16 | |||||||
| 10.18 | Loan Agreement dated May 1, 2016, with Creative Medical Health | 10-Q | 000-53500 | 10.1 | 8/19/16 | |||||||
| 10.19 | 8% Promissory Note dated May 1, 2016, for $50,000 with Creative Medical Health | 10-Q | 000-53500 | 10.2 | 8/19/16 | |||||||
| 10.20 | Consulting Agreement between Creative Medical Health, Inc. and Dr. Patel | X | ||||||||||
| 10.21 | License Agreement dated August 25, 2016, between Creative Medical Technologies, Inc. and UCSD (portions of this exhibit have been omitted pursuant to a request for confidential treatment) | 10-Q | 000-53500 | 10.1 | 11/10/16 | |||||||
| 10.22 | Master Services Agreement dated November 15, 2015, with Professional Research Consulting, Inc. | 10-Q | 000-53500 | 10.2 | 11/10/16 | |||||||
| 10.23 | Clinical Trial Agreement dated September 19, 2016 between Creative Medical Technologies, Inc. and LABIOMED | 10-Q | 000-53500 | 10.3 | 11/10/16 | |||||||
| 16.1 | Letter from Heaton & Company, PLLC, dated May 18, 2016 | 8-K | 000-53500 | 16.1 | 5/19/16 | |||||||
| 21.1 | Subsidiaries | X | ||||||||||
| 23.1 | Consent of Haynie & Company, independent registered public accounting firm | X | ||||||||||
| 23.2 | Consent of Attorney (included in Exhibit 5.1) | — | ||||||||||
| 101.INS | XBRL Instance Document | X | ||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | X | ||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | X | ||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||||
Item 17. Undertakingsbear interest at rate of 8%. The notes are convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 71% of the average of the two lowest traded prices or the lowest trade price of the Company’s common stock during the previous 15 trading days preceding the conversion date.
On February 12, 2021, the Company entered into a preferred equity financing agreement with BHP Capital, LLC (“BHP”) in the amount of $350,000 for 350 shares of the newly-designated Series B Convertible Preferred Stock valued at $1,200 per share for which $326,600 in proceeds were received by the Company. In connection with the closing, the Company issued an additional 3,000 shares of common stock as a service fee.
On March 30, 2021, the Company entered into a new preferred equity financing agreement with Fourth Man, LLC (“FM”) in the amount of $150,000 under which the Company issued FM 150 shares of Series C Convertible Preferred Stock, stated value $1,200 per share, for a purchase price of $150,000, or $1,000 per share, for which $141,049 in proceeds were received by the Company. In connection with the closing, the Company issued an additional 1,286 shares of common stock as a service fee.
Year Ended December 31, 2020
During 2020, the Company entered into convertible note agreements with accredited lenders for an aggregate principal amount of $831,140 for which $710,920 of proceeds were received. The notes are convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 71% of the average of the two lowest traded prices of the Company’s common stock during the previous 15 trading days preceding the conversion date or the lowest trade price during the previous 15 trading days.
During 2020, we issued an aggregate of 1,363,463 shares upon the conversion of $1,376,005 of outstanding principal, interest and fees on existing, outstanding notes.
Year Ended December 31, 2019
During 2019, the Company entered into convertible note agreements with accredited lenders for an aggregate principal amount of $1,572,400 for which $1,302,795 of proceeds were received. The Company also entered into a warrant exchange agreement whereby the Company issued three notes for an aggregate principal amount of $300,000, in exchange for the cancellation of Common Stock Purchase Warrants held by the warrant holders, initially exercisable for an aggregate of 129 shares of the Company’s common stock. The notes are convertible into shares of the Company’s common stock at conversion prices ranging from 60% to 65% of the average of the two lowest traded prices of the Company’s common stock during the previous 15 trading days preceding the conversion date, the lowest trade price during the previous 20 trading days, or the volume weighted average price over the prior 15 trading days.
During 2019, the Company issued an aggregate of 23,746 shares upon the conversion of $1,501,275 of outstanding principal, interest and fees on existing, outstanding notes and 1,062 shares upon the cashless exercise of 1,188 warrants.
| II-3 |
ITEM 16. EXHIBITS
The following exhibits are filed as part of this registration statement:
Exhibits | ||
Agreement dated December 28, 2020, between Jadi Cell LLC and ImmCelz, Inc. | ||
101.INS | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). | |
101.SCH | Inline XBRL Taxonomy Extension Schema Document | |
101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |
104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101). |
* Filed herewith.
** To be filed by amendment.
*** Previously filed
† Management contract or compensatory plan or arrangement.
| II-4 |
ITEM 17. UNDERTAKINGS
(a) The undersigned registrant hereby undertakes:
(1) Toto file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to:statement:
(i) Includeto include any prospectus required by sectionSection 10(a)(3) of the Securities Act;
(ii) Reflectto reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high andend of the estimated maximum offering range may be reflected in the form of prospectus filed with the SECCommission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20%a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.statement; and
(iii) Includeto include any material or changed information with respect to the plan of distribution not previously disclosed in the registration statement or anany material change to such information in the registration statement.statement;
(2) That,that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) Toto remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) Eachthat, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.Provided,, however,, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 of Regulation C of the Securities Act;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SECSecurities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
| II-5 | |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Peoria, Arizona, on November 21, 2016.December 1, 2021.
CREATIVE MEDICAL TECHNOLOGY HOLDINGS, INC. | |||
By: | /s/ Timothy Warbington | ||
Timothy Warbington | |||
Chief Executive Officer | |||
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints Timothy Warbington, with full authority to act without the others, his true and lawful attorney-in-fact and agent with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this registration statement, and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates stated.indicated:
Signature | Title | Date | ||
/s/ Timothy Warbington | Chief Executive Officer, Director | December 1, 2021 | ||
Timothy Warbington | ||||
/s/ Donald Dickerson | Chief Financial Officer, Director | December 1, 2021 | ||
Donald Dickerson | ||||
/s/ Thomas Ichim, PhD | Director | December 1, 2021 | ||
Thomas Ichim, PhD | ||||
/s/ Amit Patel, MD | Director | December 1, 2021 | ||
Amit Patel, MD |