XCYTE THERAPIES, INC.

As filed with the Securities and Exchange Commission on October 10, 20037, 2004
Registration No. 333-
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
XCYTE THERAPIES, INC.
(Exact name of Registrantregistrant as specified in its charter)
| Delaware | 2834 | 91-1707622 | ||
(State or incorporation or organization) | (Primary Standard Industrial | |||
Classification Code Number) | (I.R.S. Employer Identification Number) |
1124 Columbia Street, Suite 130
Seattle, Washington 98104
(206) 262-6200
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
Ronald J. Berenson, M.D.
President and Chief Executive Officer
Xcyte Therapies, Inc.
1124 Columbia Street, Suite 130
Seattle, Washington 98104
(206) 262-6200
(Name, address including zip code, and telephone number,
including area code, of agent for service)
Copies to:
| Sonya F. Erickson | Joanna | |||
| General Counsel & Vice President | ||||
| Xcyte Therapies, Inc. | ||||
| 1124 Columbia Street, Suite 130 | ||||
| Seattle, Washington 98104 | ||||
| (206) 262-6200 | Palo Alto, California 94306-2155 | |||
| (206) 447-0900 | (650) |
Approximate date of commencement of proposed sale to the public:As soon as practicable after the Registration Statement becomes effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.¨
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box.¨
CALCULATION OF REGISTRATION FEE(1)FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(2) | Amount of Registration Fee(3) | Amount to be Registered(1) | Proposed Maximum Per Share | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee | |||||||||||||||
Convertible Exchangeable Preferred Stock, par value $0.001 | 1,725,000 | $ | 10.00 | $ | 17,250,000 | $ | 2,185.58 | ||||||||||||||
Convertible Subordinated Debentures | $ | 17,250,000 | (2) | — | (3) | — | (3) | — | (3) | ||||||||||||
Common Stock, par value $0.001 | $ | 75,000,000.00 | $6,067.50 | (4) | — | (3) | — | (3) | — | (3) | |||||||||||
Common Stock, par value $0.001 | 1,000,000 | (5) | $ | 3.33 | (6) | $ | 3,333,333.33 | $ | 421.92 | ||||||||||||
| (1) |
| Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. |
| (2) | This number represents a total of $17,250,000 aggregate principal amount of Convertible Subordinated Debentures issuable if we elect to exchange all of the Convertible Exchangeable Preferred Stock for Convertible Subordinated Debentures. For purposes of estimating the number of debentures to be included upon exchange of the preferred stock, we calculated the amount of debentures issuable upon exchange based on an exchange price of $10 per share of preferred stock. |
| (3) |
| (4) | This number represents shares of common stock issuable upon conversion of the Convertible Exchangeable Preferred Stock or the Convertible Subordinated Debentures. In addition, the shares set forth in the table, pursuant to Rule 416 under the Securities Act of 1933, include an indeterminate number of shares of common stock issuable upon conversion of the preferred stock or the debentures, as these amounts may be adjusted as a result of stock splits, stock dividends and antidilution provisions, or in payment of such make-whole obligations. |
| (5) | This number represents the estimated maximum number of shares of our common stock issuable to satisfy the dividend make-whole payment and interest make-whole payment pursuant to the |
| (6) | Estimated solely for the purpose of calculating the registration fee, pursuant to Rule 457(c), based on |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
ThisThe information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we areit is not soliciting offersan offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECTTO COMPLETION, DATED OCTOBER 7, 2004
1,500,000 Shares
XCYTE THERAPIES, INC. |  |
% Convertible Exchangeable Preferred Stock
(Cumulative Dividend, Liquidation Preference $10 Per Share)
· Xcyte Therapies, Inc. is offering 1,500,000 shares of % convertible exchangeable preferred stock, which is referred to · Dividends will be cumulative from the date of original issue at the annual rate of % of the liquidation preference of the convertible preferred stock, payable quarterly on the day of , , , and , commencing , 2005. Any dividends must be declared by our board of directors and must come from funds that are legally available for dividend payments. · You may convert each share of the convertible preferred stock into shares of our common stock based on the initial conversion price of $ , subject to certain adjustments. · We may elect to automatically convert the convertible preferred stock into our common stock if the closing price of our common stock has exceeded $ , which is 150% of the conversion price of the convertible preferred stock, for at least 20 trading days during any 30-day trading period, ending within five trading days prior to notice of automatic conversion. · If we elect to automatically convert, or you elect to voluntarily convert, some or all of the convertible preferred stock into our common stock prior to , 2007, we will make an additional payment on the convertible preferred stock equal to the aggregate amount of dividends that would have been payable on the convertible preferred stock through and including , 2007, less any dividends already paid on the convertible preferred stock. | · We may elect to redeem the convertible preferred stock after , 2007 on the terms described in this prospectus. · At our option, we may exchange the convertible preferred stock in whole, but not in part, on any dividend payment date beginning on , 2005 for our % convertible subordinated debentures. If we elect to exchange the convertible preferred stock for debentures, the exchange rate will be $10 principal amount of debentures for each share of the convertible preferred stock. The debentures, if issued upon exchange of the convertible preferred stock, will mature 25 years after the exchange date and will have terms substantially similar to those of the preferred stock. · The convertible preferred stock has no maturity date and no voting rights prior to conversion into common stock, except under limited circumstances. · Shares of our common stock are listed on the Nasdaq National Market under the symbol “XCYT.” The last reported sale price of our common shares on October |
Shares

Common Stock
This is the initial public offering of our common stock. No public market currently exists for our common stock. We are selling all of the shares of common stock offered by this prospectus. We expect the public offering price to be between $ and $ per share.
We have applied to have our common stock approved for quotation on The Nasdaq National Market under the symbol “XCYT.”
Investing in our common stockinvestment involves a high degree of risk. Before buying any shares, you should read the discussion of material risks of investing in our common stock inSee ““Risk factors”Factors” beginning on page 7.9.
| Per Share | Total | |||||
Public offering price | $ | $ | ||||
Underwriting discounts and commissions | $ | $ | ||||
Proceeds, before expenses, to Xcyte Therapies, Inc. | $ | $ | ||||
The underwriters have a 30-day option to purchase up to 225,000 additional shares of convertible preferred stock from us to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracydetermined if this prospectus is truthful or adequacy of this prospectus.complete. Any representation to the contrary is a criminal offense.
| ||||||||
| ||||||||
| ||||||||
| ||||||||
The underwriters may also purchase up to an additional shares of our common stock at the public offering price, less the underwriting discounts and commissions, to cover over-allotments, if any, within 30 days from the date of this prospectus. If the underwriters exercise the option in full, the total underwriting discounts and commissions will be $ , and our total proceeds, before expenses, will be $ .
The underwriters are offering the common stock as set forth under “Underwriting.” Delivery of the shares will be made on or about , 2003.
UBS Investment Bank
U.S. Bancorp Piper Jaffray
Wells Fargo Securities, LLC
The date of this prospectus is , 2003.2004.
| Page | ||
| 1 | ||
| 9 | ||
| 27 | ||
| 28 | ||
| 28 | ||
Ratio of Earnings to Fixed Charges and Preferred Stock Dividends | 29 | |
| 29 | ||
| 30 | ||
| 31 | ||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 32 | |
| 41 | ||
| 66 | ||
| 67 | ||
| 79 | ||
| 82 | ||
| 84 | ||
| 94 | ||
| 101 | ||
| 105 | ||
| 113 | ||
| 115 | ||
| 118 | ||
| 118 | ||
| 118 | ||
| F-1 |
You should rely only on the information contained in this prospectus. We have not, and the underwriters have not, authorized anyoneany other person to provide you with information different from that contained in this prospectus. We are offeringinformation. This prospectus is not an offer to sell, andnor is it seeking offersan offer to buy, shares of our common stock onlythese securities in jurisdictionsany state where offers and sales arethe offer or sale is not permitted. The information contained in this prospectus is complete and accurate only as of the date of this prospectus, regardless ofon the time of delivery of this prospectus or of any sale of shares of our common stock.
Through and including , 2003, federal securities lawsfront cover, but the information may require all dealershave changed since that effect transactions in our common stock, whether or not participating in this offering, to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.date.
XcyteTM, Xcyte TherapiesTM, XcellerateTM and Xcellerated T CellsTM are trademarks of Xcyte Therapies, Inc. All other trademarks appearing in this prospectus are the property of their respective holders.
Prospectus summaryPROSPECTUS SUMMARY
This summary highlights selected information appearing elsewhere in this prospectus and may not contain all ofprospectus. While this summary highlights what we consider to be the information that ismost important to you. This prospectus includes information about the shares we are offering as well as information regarding our business and detailed financial data. Youus, you should carefully read this prospectus and the registration statement of which this prospectus is a part in itstheir entirety before making an investment decision, especially the risks of investing in our commonthe convertible preferred stock, which we discuss under “Risk factors”Factors” beginning on page 7,9, and our financial statements and related notes beginning on page F-1.
Unless the context requires otherwise, the words “Xcyte,” “we,” “company,” “us” and “our” refer to Xcyte Therapies, Inc.
OUR BUSINESSOur Business
We are a biotechnology company developing a new class of therapeutic products thatdesigned to enhance the body’s natural immune responses to treat cancer, infectious diseases and other medical conditions associated with weakened immune systems. We producederive our therapeutic products which consist of activated, patient-specificfrom a patient’s own T cells, usingwhich are cells of the immune system that orchestrate immune responses and can detect and eliminate cancer cells and infected cells in the body. We use our patented and proprietary Xcellerate Technology. We use blood collected from the patientTechnology to generate activated T cells, which we call Xcellerated T Cells.Cells, from blood that is collected from the patient. Activated T cells are T cells that have been stimulated to carry out immune functions. Our Xcellerate Technology is designed to rapidly activatesactivate and expandsexpand the patient’s T cells outside of the body. These Xcellerated T Cells are subsequentlythen administered to the patient.
We believe, based on clinical trials to date, that our Xcellerate Technology can produce Xcellerated T Cells in sufficient numbers to generate rapid and potent immune responses to treat a variety of medical conditions.
We believe In our ongoing clinical studies using our Xcellerate Technology, allows us to consistentlywe have observed an increase in the quantity and restorea restoration of the quality and diversity of T cells fromin patients with weakened immune systems. We have submitted the findings on the increase in quantity of T cells to the FDA and plan to submit additional data in our next annual report. We believe we can efficiently manufacture Xcellerated T Cells for therapeutic applications. In addition, based on clinical studies to date, we believe the Xcellerated T Cells are safe and generally well tolerated and can be easily administered to patients in an outpatient clinical setting. We expect Xcellerated T Cells may be used alone or in combination with other complementary treatments.
Our clinical trials and independent clinical trials using an earlier version of our technology, to date, have involved small numbers of patients and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results. We and our scientific collaboratorsother clinical investigators have completed or are conducting clinical trials in the following indications:
Chronic lymphocytic leukemia, or CLL. In our ongoing Phase I/II clinical trial in CLL, treatment with Xcellerated T Cells resulted in a 50% to 100% reduction in the size of enlarged lymph nodes in 12 of 17 (71%) patients for whom data was available as of September 27, 2004. In addition, there was a 50% or greater reduction in spleen size as measured below the rib cage by physical examination in 10 of the 13 patients (77%) with enlarged spleens. These findings were submitted to the FDA in the Information Packet for a Type B End of Phase II meeting held on September 23, 2004. At this meeting we discussed with the FDA our plans for a Phase II/III clinical trial of Xcellerated T Cells in patients with CLL who have been previously treated with chemotherapy and have failed treatment with Campath, an FDA-approved drug used to treat CLL. Based on feedback from the FDA during |
this meeting, we intend to modify our planned protocol for this Phase II/III clinical trial to provide the FDA with data we believe will address the FDA’s concerns regarding the subcutaneous route of Campath administration and the dose and schedule of Xcellerated T |
| Multiple myeloma. In our ongoing Phase I/II clinical trial, we have shown that treatment with Xcellerated T Cells |
| Non-Hodgkin’s lymphoma. In an independent clinical trial, conducted by one of our scientific founders under a physician-sponsored |
|
1
an earlier version of our proprietary technology. We have been advised that these data have been submitted to the FDA for review. The results of this study were |
OUR SOLUTION
We have developed our proprietary Xcellerate Technology, which consistently activates and grows large numbers of T cellsex vivo, or outside of the body, for multiple therapeutic applications.
Benefits of Xcellerated T Cells
We believe Xcellerated T Cells may be an effective treatment for cancer and infectious diseases and may have the following clinical benefits:
Benefits of our Xcellerate Technology
We believe our Xcellerate Technology may have the following benefits:
2
OUR STRATEGYOur Strategy
Our goal is to be a leader in the field of T cell therapy and to leverage our expertise in T cell activation to develop and commercialize products to treat patients with cancer, infectious diseases and other medical conditions associated with weakened immune systems. We willplan to initially develop Xcellerated T Cells to treat life-threatening diseases, such as cancer and HIV, which currently have inadequate treatments. Key elements of our strategy include the following:
| Maximize speed to market. |
| Expand the |
| Leverage complementary technologies and therapies. |
| Retain |
| Enhance our manufacturing capabilities. |
| Expand and enhance our intellectual property. |
OUR CORPORATE INFORMATIONRisks Associated With Our Business
We are a development stage company. We are subject to numerous risks and obstacles, and we have highlighted the most important of them in “Risk Factors” beginning on page 9. In particular, we have a limited operating history and have incurred losses in each fiscal year since our inception. We incurred net losses of approximately $18.5 million for the year ended December 31, 2003 and $24.4 million for the six months ended June 30, 2004, and our deficit accumulated during the development stage was approximately $111.0 million as of June 30, 2004. We have no commercial products for sale, and we anticipate that we will incur substantial and increasing losses over the next several years as we expand our research, development and clinical trial activities, acquire or license technologies, scale up and improve our manufacturing operations, seek regulatory approval and, if we receive FDA approval, commercialize our products. Because of the numerous risks and uncertainties associated with our product development efforts, we are unable to predict whether or when we will achieve profitability. Our clinical trials and independent clinical trials using an earlier version of our technology, to date, have involved small numbers of patients, and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. The results reported are preliminary and success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
Our Corporate Information
We were incorporated in Delaware as MolecuRx, Inc. in January 1996. We changed our name to CDR Therapeutics, Inc. in August 1996 and changed our name to Xcyte Therapies, Inc. in October 1997. Our principal executive offices are located at 1124 Columbia Street, Suite 130, Seattle, Washington 98104, and our telephone number is (206) 262-6200. Our web site address is www.xcytetherapies.com.www.xcytetherapies.com. The information contained on our web site is not incorporated by reference into and does not form any part of this prospectus.
3THE OFFERING
The offering
| 1,500,000 shares of % convertible exchangeable preferred stock, | par value $0.001 per share (1,725,000 shares |
|
|
|
| |
Dividends | Dividends will be cumulative from the date of original issue at the annual rate of % of the liquidation preference of the convertible preferred stock, payable quarterly on the day of , , , and , commencing , 2005. Any dividends must be declared by our board of directors and must come from funds which are legally available for dividend payments. | |
Conversion Rights | Unless we redeem or exchange the convertible preferred stock, the convertible preferred stock can be converted at your option at any time into shares of our common stock at an initial conversion price of $ (equivalent to a conversion rate of approximately shares of common stock for each share of convertible preferred stock). The initial conversion price with respect to the convertible preferred stock is subject to adjustment in certain events, including a non-stock fundamental change or a common stock fundamental change, which are explained in more detail under the section entitled “Description of Convertible Preferred Stock—Conversion—Conversion Price Adjustment—Merger, Consolidation or Sale of Assets.” | |
Automatic Conversion | Unless we redeem or exchange the convertible preferred stock, we may elect to automatically convert some or all of the convertible preferred stock into shares of our common stock if the closing sale price of our common stock has exceeded 150% of the conversion price for at least 20 out of 30 consecutive trading days ending within five trading days prior to the notice of automatic conversion. | |
Dividend Make-Whole Payment | If we elect to automatically convert, or you voluntarily convert, some or all of the convertible preferred stock into shares of our common stock prior to , 2007, we will make an additional payment on the convertible preferred stock equal to the aggregate amount of cumulative | |
| dividends that would have accrued and become payable on the convertible preferred stock from the date of original issue through and including , 2007, less any dividends already paid on the convertible preferred stock. This additional payment is payable by us in cash or, at our option, in shares of our common stock, or a combination of cash and shares of our common stock. | ||
Liquidation Preference | In the event of our voluntary or involuntary dissolution, liquidation or winding up, you will be entitled to be paid a liquidation preference equal to $10 per share of convertible preferred stock, plus accrued and unpaid dividends before any distribution of assets may be made to holders of capital stock ranking junior to the convertible preferred stock. | |
Optional Redemption | On or after , 2007, we may redeem the convertible preferred stock, in whole or in part, at our option at the redemption prices set forth in this prospectus, together with accrued dividends to, but excluding, the redemption date. See the section entitled “Description of Convertible Preferred Stock—Optional Redemption” below. | |
Voting Rights | Except as provided by law and in other limited situations described in this prospectus, you will not be entitled to any voting rights. However, you will, among other things, be entitled to vote as a separate class to elect two directors if we have not paid the equivalent of six or more quarterly dividends, whether or not consecutive. These voting rights will continue until we pay the full accrued but unpaid dividends on the convertible preferred stock. | |
Exchange Provisions | At our option, we may exchange the convertible preferred stock in whole, but not in part, on any dividend payment date beginning on , 2005 for our % convertible subordinated debentures. If we elect to exchange the convertible preferred stock for debentures, the exchange rate will be $10 principal amount of debentures for each share of convertible preferred stock. | |
Debentures | The debentures, if issued upon exchange of the convertible preferred stock, will have the following terms: | |
Interest Rate | The debentures will have an interest rate of % per year. Interest will be payable on and of each year, beginning on the first interest payment date after the exchange date. | |
Redemption | On or after , 2007 we may redeem the debentures at the redemption prices listed in this prospectus, plus accrued interest. | |
Maturity | The debentures will mature 25 years after the exchange date. | |
Conversion | The debentures may be converted at any time by the holder prior to maturity into shares of our common stock at the same conversion price applicable to the convertible preferred stock, subject to adjustment upon certain events. | |
Automatic Conversion | We may automatically convert the debentures into shares of our common stock at any time prior to maturity under the same terms applicable to the convertible preferred stock. | |
Interest Make-Whole Payment | If you voluntarily convert or we elect to automatically convert some or all of the debentures into shares of our common stock prior to , 2007, we will also make an additional payment on the debentures equal to the aggregate amount of interest that would have accrued and been payable from date of the original issuance of the debentures pursuant to the exchange through and including , 2007, less any interest paid with respect to such debentures. This additional payment is payable by us, in cash or, at our option, in shares of our common stock, or a combination of cash and shares of our common stock. | |
Subordination | The debentures are subordinated to all existing and future senior indebtedness and are effectively subordinated to all of the indebtedness and other liabilities (including trade and other payables, but excluding intercompany liabilities) of us and our subsidiaries. As of June 30, 2004, we had | |
| approximately $2.2 million of indebtedness outstanding that would have constituted senior indebtedness and approximately $4.6 million of indebtedness and other liabilities outstanding to which the debentures would have been effectively subordinated (including trade and other payables, but excluding intercompany liabilities). The indenture governing the debentures does not limit the amount of indebtedness, including senior indebtedness, that we and our subsidiaries may incur. See the section entitled “Description of Debentures—Subordination” below. | ||
Use of Proceeds | We expect to use the net proceeds | |
|
| |
The number of shares of our common stock outstanding at the closing of this offering is based on 8,402,636 shares of our common stock outstanding as of September 30, 2003 and excludes:
Our common stock |
Nasdaq National Market Symbol for our Convertible Preferred Stock | We will make an application to trade the convertible preferred stock on the Nasdaq National Market under the symbol “XCYTP.” | |
Listing of | ||
Debentures
Unless otherwise indicated, all information in this prospectus assumes the following:
Risk Factors | An investment in the | |
Unless we specifically state otherwise, the information in this prospectus assumes that the underwriters have not exercised their option to purchase up to additional shares of our common stock to cover over-allotments, if any.
4SUMMARY FINANCIAL DATA
Summary financial data
The following summary financial data for the years ended December 31, 19981999 through 2002 has2003 have been derived from our audited financial statements. The following summary financial data for the six-month periods ended June 30, 2003 and 2002,2004, and the summary balance sheet data as of June 30, 20032004 have been derived from our unaudited condensed financial statements. The unaudited condensed financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments we consider necessary for the fair presentation of the information. Operating results for the six months ended June 30, 20032004 are not necessarily indicative of the results that may be expected for the entire year ending December 31, 2003.2004. This information is only a summary and should be read together with the financial statements and the notes to those statements appearing elsewhere in this prospectus and the information under “Selected financial data”Financial Data” and “Management’s discussionDiscussion and analysisAnalysis of financial conditionFinancial Condition and resultsResults of operations.Operations.”
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| Statement of operations data | 1998 | 1999 | 2000 | 2001 | 2002 | 2002 | 2003 | |||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||||||||||
Total revenue | $ | — | $ | 16 | $ | 98 | $ | 30 | $ | — | $ | — | $ | 72 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 4,317 | 5,471 | 11,257 | 14,701 | 14,663 | 7,651 | 7,029 | |||||||||||||||||||||
General and administrative | 1,427 | 1,654 | 2,403 | 5,204 | 4,979 | 2,883 | 2,194 | |||||||||||||||||||||
Total operating expenses | 5,744 | 7,125 | 13,660 | 19,905 | 19,642 | 10,534 | 9,223 | |||||||||||||||||||||
Loss from operations | (5,744 | ) | (7,109 | ) | (13,562 | ) | (19,875 | ) | (19,642 | ) | (10,534 | ) | (9,151 | ) | ||||||||||||||
Other income (expense), net | 298 | 162 | 621 | 363 | 189 | 122 | (38 | ) | ||||||||||||||||||||
Net loss | (5,446 | ) | (6,947 | ) | (12,941 | ) | (19,512 | ) | (19,453 | ) | (10,412 | ) | (9,189 | ) | ||||||||||||||
Accretion of preferred stock | — | — | (8,411 | ) | (8,001 | ) | (8,001 | ) | — | |||||||||||||||||||
Net loss applicable to common stockholders | $ | (5,446 | ) | $ | (6,947 | ) | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,413 | ) | $ | (9,189 | ) | |||||||
Basic and diluted net loss per common share | $ | (0.86 | ) | $ | (1.15 | ) | $ | (2.16 | ) | $ | (4.03 | ) | $ | (3.52 | ) | $ | (2.45 | ) | $ | (1.13 | ) | |||||||
Shares used in basic and diluted net loss per share calculation | 6,355 | 6,050 | 6,003 | 6,936 | 7,809 | 7,523 | 8,144 | |||||||||||||||||||||
Pro forma net loss per common share (unaudited)(1) | $ | (0.44 | ) | $ | (0.20 | ) | ||||||||||||||||||||||
Shares used in pro forma net loss per common share calculation (unaudited)(1) | 44,550 | 45,427 | ||||||||||||||||||||||||||
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||
| (in thousands, except per share data) | (unaudited) | |||||||||||||||||||||||||||
Statement of Operations Data | ||||||||||||||||||||||||||||
Total revenue | $ | 16 | $ | 98 | $ | 30 | $ | — | $ | 170 | $ | 72 | $ | 36 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 5,471 | 11,257 | 14,701 | 14,663 | 13,685 | 7,029 | 8,601 | |||||||||||||||||||||
General and administrative | 1,654 | 2,403 | 5,204 | 4,979 | 4,322 | 2,194 | 3,297 | |||||||||||||||||||||
Total operating expenses | 7,125 | 13,660 | 19,905 | 19,642 | 18,007 | 9,223 | 11,898 | |||||||||||||||||||||
Loss from operations | (7,109 | ) | (13,562 | ) | (19,875 | ) | (19,642 | ) | (17,837 | ) | (9,151 | ) | (11,862 | ) | ||||||||||||||
Other income (expense), net | 162 | 621 | 363 | 189 | (620 | ) | (38 | ) | (12,508 | ) | ||||||||||||||||||
Net loss | (6,947 | ) | (12,941 | ) | (19,512 | ) | (19,453 | ) | (18,457 | ) | (9,189 | ) | (24,370 | ) | ||||||||||||||
Accretion of preferred stock | — | — | (8,411 | ) | (8,001 | ) | — | — | (8,973 | ) | ||||||||||||||||||
Net loss applicable to common stockholders | $ | (6,947 | ) | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,457 | ) | $ | (9,189 | ) | $ | (33,343 | ) | |||||||
Basic and diluted net loss per common share | $ | (6.32 | ) | $ | (11.86 | ) | $ | (22.14 | ) | $ | (19.34 | ) | $ | (12.40 | ) | $ | (6.21 | ) | $ | (3.66 | ) | |||||||
Shares used in basic and diluted net loss per share calculation | 1,100 | 1,091 | 1,261 | 1,420 | 1,488 | 1,481 | 9,107 | |||||||||||||||||||||
5
The following table contains a summary of our balance sheet as of June 30, 2003:2004:
| on an actual basis; |
| on |
| As of June 30, 2003 | ||||||||||
| Balance sheet data | Actual | Pro forma | Pro forma as | |||||||
| (in thousands) | ||||||||||
Cash, cash equivalents and short term investments | $ | 8,892 | $ | 22,502 | $ | |||||
Working capital | 7,252 | 20,862 | ||||||||
Total assets | 12,695 | 26,305 | ||||||||
Long-term obligations, less current portion | 1,477 | 1,477 | ||||||||
Redeemable convertible preferred stock | 64,604 | — | ||||||||
Redeemable convertible preferred stock warrants | 1,069 | — | ||||||||
Total stockholders’ equity (deficit) | (56,589 | ) | 9,957 | |||||||
Balance Sheet Data Cash, cash equivalents and short-term investments Working capital Total assets Long-term obligations, less current portion Total stockholders’ equityThe above balance sheet data is based on 8,402,636 shares of our common stock outstanding as of June 30, 2003 and excludes: As of June 30, 2004 Actual As adjusted (unaudited, in thousands) $ 33,730 $ 47,315 30,838 44,423 39,860 53,445 2,594 2,594 33,097 46,682
6
Investing in our commonthe convertible preferred stock involves a high degree of risk. You should carefully consider the risks described below with all of the other information included in this prospectus before making an investment decision.deciding to invest in the convertible preferred stock. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In this event, the market price of the convertible preferred stock and our common stock could decline and you could lose part or all of your investment. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
RISKS RELATED TO OUR BUSINESSRisks Related To Our Business
We expect to continue to incur substantial losses, and we may never achieve profitability.
We are a development stage company with limited operating history. We have incurred significant operating losses since we began operations in 1996, including net losses of approximately $9.2$18.5 million for the year ended December 31, 2003 and $24.4 million for the six months ended June 30, 2003,2004, and we may never become profitable. As of June 30, 2003,2004, we had a deficit accumulated during the development stage of approximately $77.3$111.0 million. These losses have resulted principally from costs incurred in our research and development programs and from our general and administrative expenses. We also expect to incur significant costs to renovate our leased facility for the manufacture of Xcellerated T Cells for our planned clinical trials and, if we receive FDA approval, for initial commercialization activities. To date, we have derived no revenues from product sales or royalties. We do not expect to have any significant product sales or royalty revenue for a number of years. Our operating losses have been increasing during the past several years and will continue to increase significantly in the next several years as we expand our research and development, participate in clinical trial activities, acquire or license technologies, scale up and improve our manufacturing operations, seek regulatory approvals and, if we receive FDA approval, commercialize our products. These losses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Because of the numerous risks and uncertainties associated with our product development efforts, we are unable to predict when we may become profitable, if at all. If we are unable to achieve and then maintain profitability, the market value of our common and convertible preferred stock will likely decline.
We will need to raise substantial additional capital to fund our operations, and our failure to obtain funding when needed may force us to delay, reduce or eliminate our product development programs or collaboration efforts.
Developing products and conducting clinical trials for the treatment of cancer and infectious diseases require substantial amounts of capital. To date, we have raised capital primarily through private equity financings, an initial public offering, the sale of convertible promissory notes and equipment leases. Currently, we anticipate that our cash, cash equivalents and investments will be adequate to satisfy our capital needs through at least the end of the second quarter of 2005 if we do not raise capital in this offering. If we are unable to timely obtain additional funding in a timely fashion, we may never conduct required clinical trials to demonstrate safety and clinical efficacy of Xcellerated T Cells, and we may never obtain FDA approval or commercialize any of our products. We will need to raise additional capital to, among other things:
| fund our clinical trials; |
| expand our research and development activities; |
| scale up and improve our manufacturing operations; |
| finance our general and administrative expenses; |
| acquire or license technologies; |
7
Risk factors
| prepare, file, prosecute, maintain, enforce and defend our patent and other proprietary rights; |
| pursue regulatory approval and commercialization of Xcellerated T Cells and any other products that we may develop; and |
| develop and implement sales, marketing and distribution capabilities. |
Our net cash used in operations has exceeded our cash generated from operations for each year since our inception. For example, we used approximately $8.0 million in operating activities for the six months ended June 30, 2003 and approximately $15.2 million in 2002. Based upon the current status of our product development and collaboration plans, we believe that the net proceeds of this offering, together with our cash, cash equivalents and investments, will be adequate to satisfy our capital needs through at least the next 18 months. However, changes in our business may occur that would consume available capital resources sooner than we expect. See “Management’s discussion and analysis of financial condition and results of operations—Liquidity and capital resources.” Our future funding requirements will depend on many factors, including, among other things:
| the progress, expansion and cost of our clinical trials and research and development activities; |
| any future decisions we may make about the scope and prioritization of the programs we pursue; |
| the development of new product candidates or uses for our Xcellerate Technology; |
| changes in regulatory policies or laws that affect our operations; and |
| competing technological and market developments. |
If we raise additional funds by issuing equity securities, further dilution to stockholders may result and new investors could have rights superior to holders of the shares issued in this offering.our current stockholders. In addition, debt financing, if available, may include restrictive covenants. If adequate funds are not available to us, we may have to liquidate some or all of our assets or delay, reduce the scope of or eliminate some portion or all of our development programs or clinical trials. We also may have to license to other companies our products or technologies that we would prefer to develop and commercialize ourselves.
WeDue to our limited resources and access to capital, we must prioritize our development programs and may decidechoose to pursue development programs for Xcellerated T Cells that may never receive regulatory approval or prove to be profitable.
Because we have limited resources and access to capital to fund our operations, our management must make significant prioritization decisions on which programs to pursue and how much of our resources to allocate to each program. We are currently focusing our research and development efforts on the use of Xcellerated T Cells to treat CLL, multiple myeloma, non-Hodgkin’s lymphoma kidney cancer, prostate cancer and HIV. Our management has broad discretion to suspend, scale down or discontinue any of these programs or to initiate new programs to treat other clinical indications. Xcellerated T Cells may never prove to be safe and clinically effective to treat any of these indications, and the market for these indications may never prove to be profitable even if we obtain regulatory approval for these indications. Accordingly, we cannot assure you that the programs we decide to pursue will lead to regulatory approval or will prove to be profitable.
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends in part on obtaining, maintaining and enforcing our patents and in-licensed and proprietary rights throughout the world. We believe we own, or have rights under licenses to, issued patents and pending patent applications that are necessary to commercialize Xcellerated T Cells. However, the patents on which we rely may be challenged and invalidated, and our patent applications may not result in issued patents. Moreover, our patents and patent applications may not be sufficiently broad to prevent others from practicing our technologies or developing competing products. We also face the risk that others may independently develop similar or alternative technologies or may design around our proprietary and patented technologies.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date in the United States. Furthermore, the application and enforcement of patent laws and regulations in foreign countries is even more uncertain, particularly where, as here, patent rights are co-owned with others, thus requiring their consent to ensure exclusivity in the marketplace. Accordingly, we cannot assure you that we will be able to effectively file, protect or defend our proprietary rights in the United States or in foreign jurisdictions on a consistent basis.
Third parties may successfully challenge the validity of our patents. We will only be able to protect our technologies from unauthorized use by third parties to the extent that valid and enforceable patents or other
proprietary rights cover them. Because the issuance of a patent is not conclusive of its validity or enforceability, we cannot assure you how much protection, if any, will be given to our patents if we attempt to enforce them or if others challenge their validity in court. It is possible that a competitor may successfully challenge our patents or that a challenge will result in limiting the coverage of our patents. If the outcome of litigation is adverse to us, third parties may be able to use our technologies without payment to us.
In addition, it is possible that others may infringe upon our patents or successfully avoid them through design innovation. We may initiate litigation to police unauthorized use of our proprietary rights. However, the cost of litigation to uphold the validity of our patents and to prevent infringement could be substantial, particularly where patent rights are co-owned with others, thus requiring their participation in the litigation, and the litigation will consume time and other resources. Some parties may be better able to sustain the costs of complex patent litigation because they have substantially greater resources. Moreover, if a court decides that our patents are not valid, we will not have the right to stop others from using our inventions. There is also the risk that, even if the validity of our patents were upheld, a court may refuse to stop others on the grounds that their activities do not infringe upon our patents. Because protecting our intellectual property is difficult and expensive, we may be unable to prevent misappropriation of our proprietary rights.
We also rely on certain proprietary trade secrets and know-how, especially where we believe patent protection is not appropriate or obtainable. Trade secrets and know-how, however, are difficult to protect. We have taken measures to protect our unpatented trade secrets and know-how, including the use of confidentiality and invention assignment agreements with our employees, consultants and some of our contractors. It is possible, however, that these persons may unintentionally or willingly breach the agreements or that our competitors may independently develop or otherwise discover our trade secrets and know-how.
The clinical and commercial utility of our Xcellerate Technology is uncertain and may never be realized.
Our Xcellerate Technology is based on a novel approach to treat cancer and infectious diseases and is in an early stage of development. We have limitedOur clinical data or examplestrials and independent clinical trials using an earlier version of similarour technology, to conclude that our Xcellerate Technology may be safe and clinically effective.date, have involved small numbers of patients, which, unless otherwise stated, were not designed to produce statistically significant results as to efficacy. In addition, these trials have neither been randomized nor blinded to ensure the results are due to the effect of Xcellerated T Cells. Some of the data regarding
8
Risk factors
our Xcellerate Technology were derived from independent clinical trials, including physician-sponsored trials, which we do not control. In addition, data from these independent clinical trials were derived using T cells activated with an earlier version of our proprietary technology. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results. Acceptable results in early trials may not be repeated in later trials. In addition, we may not be able to treat patients if we cannot collect a sufficient quantity of T cells that meet our minimum specifications to enable us to produce Xcellerated T Cells. Also, some patients may be unable to tolerate the required procedures for blood collection and administration of Xcellerated T Cells.
We Finally, we only have limited data on the safety and efficacyexperience in treating patients with multiple doses of Xcellerated T Cells, which may be required to treatachieve optimal therapeutic effects.
Although we have observed few serious side effects in patients infused with very weakened immune systems, such as patients with HIV. AlthoughXcellerated T Cells in clinical trials conducted to date, we may not ultimately be able to provide the FDA with satisfactory data to support a claim of clinical safety and efficacy sufficient to enable the FDA to approve Xcellerated T Cells for commercialization. This may be because later clinical trials may fail to reproduce favorable data we may have obtained in earlier clinical trials, because the FDA may disagree with how we interpret the data from these clinical trials or because the FDA may not accept these therapeutic effects as valid endpoints in pivotal trials necessary for market approval. For example, although our studies to date have indicated that our Xcellerate Technology can lead to increased T cell and lymphocyte counts, the FDA will not accept increased T cell and lymphocyte counts as a valid endpoint in pivotal studies necessary for market approval. Instead, we would be required to show that Xcellerated T Cells lead to a significant clinical benefit. We will also need to demonstrate that Xcellerated T Cells are safe. We do not have data on possible harmful long-term effects of Xcellerated T Cells and will not
have any data on long-term effects in the near future. Furthermore,We also have limited data on the data from our clinical trials may be limited because these clinical trials generally do not involve a large numbersafety and efficacy of patients.Xcellerated T Cells to treat patients with very weakened immune systems, such as patients with HIV. For these and other reasons, the clinical effectiveness and commercialibility of our Xcellerate Technology is uncertain and may never be realized.
Our ability to initiate a pivotal trial in patients with CLL on our proposed protocol and timeline is uncertain and highly dependent on the FDA.
We cannot be sure that the FDA will accept the Phase II/III clinical trial protocol we plan to submit in the fourth quarter of 2004 for Xcellerated T Cells in patients with CLL, who have been previously treated with chemotherapy and have failed treatment with Campath. The FDA may conclude that we have not adequately addressed the issues they raised in our initial meeting on September 23, 2004 or they may propose additional modifications to address new concerns they have with our protocol. If the FDA does not accept the Phase II/III clinical trial protocol we plan to submit in the fourth quarter of 2004 or if the FDA requires us to conduct a separate clinical trial to address their concerns, then our plan to initiate a pivotal trial by the end of the second quarter of 2005 could be significantly delayed. Our clinical development plan for CLL is premised upon the continued existence of an unmet medical need in this population. FDA approval of another drug or biologic to treat Campath-refractory CLL could result in the FDA requiring that we conduct larger, controlled studies in more patients.
To date, Xcellerated T Cells have been shown in CLL patients to decrease lymph nodes and spleen size, but not leukemic blood counts. We cannot be sure that the FDA will accept two of these three major measurements of tumor response as sufficient to support product approval. In addition, although the FDA has accepted tumor response as a valid clinical endpoint in disease indications where there is an unmet clinical need such as CLL, we cannot be sure that the FDA will not require us to demonstrate patient survival in a pre-approval trial rather than a post-approval confirming trial that we plan to do. The Phase II/III clinical trial we plan to conduct is not randomized or powered statistically to demonstrate patient survival. To address decreases in leukemic counts in the blood in order to achieve all three major measurements of tumor response, we are planning to enroll CLL patients in our proposed Phase II/III clinical trial who have been recently treated with Campath, a drug that leads to decreases in leukemic counts in the blood. We have not previously tested the effects of using Xcellerated T Cells after use of Campath. We cannot be sure that patients’ leukemic counts will not rise again after the use of Campath or that we will observe a similar safety profile and treatment effects of our Xcellerated T Cells in CLL patients who have received Campath as we have observed in our previous clinical trials.
We may fail to obtain or may experience delays in obtaining regulatory approvalapprovals to market Xcellerated T Cells, which will significantly harm our business.
We do not have the necessary approvalapprovals to market or sell Xcellerated T Cells in the United States or any foreign market. Before marketing Xcellerated T Cells, we must successfully complete extensive preclinical studies and clinical trials and rigorous regulatory approval procedures. We cannot assure you that we will obtain the necessary regulatory approvalapprovals to commercialize Xcellerated T Cells.
Conducting clinical trials is uncertain and expensive and often takes many years to complete. The results from preclinical testing and early clinical trials are often not predictive of results obtained in later clinical trials. In conducting clinical trials, we may fail to establish the effectiveness of Xcellerated T Cells for the targeted indication or we may discover unforeseen side effects. Moreover, clinical trials may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Clinical trials are also often subject to unanticipated delays. In addition, we are currently developing a custom bioreactor system in our manufacturing process, and we will not be able to obtain FDA approval to commercialize Xcellerated T Cells without the FDA’s acceptance of our manufacturing process using this bioreactor system. Also, patients participating in the trials may die before completion of the trial or suffer adverse medical effects unrelated to treatment with Xcellerated T Cells. This could delay or lead to termination of our clinical trials. A number of companies in the biotechnology industry have suffered significant setbacks in every stage of clinical trials, even in advanced clinical trials after positive results in earlier trials. In addition, we have developed a custom bioreactor system in our manufacturing process, and we will not be able to
obtain FDA approval to commercialize Xcellerated T Cells without the FDA’s acceptance of our manufacturing process using this bioreactor system.
To date, the FDA has approved only a few cell-based therapies for commercialization. The FDA recently formed a new division that will regulate biologic products, such as Xcellerated T Cells. The processes and requirements associated with this new division may cause delays and additional costs in obtaining regulatory approvals for our products. Because our Xcellerate Technology is novel, and cell-based therapies are relatively new, regulatory agencies may lack experience in evaluating product candidates like Xcellerated T Cells. This inexperience may lengthen the regulatory review process, increase our development costs and delay or prevent commercialization of Xcellerated T Cells.
In addition, the following factors may impede or delay our ability to obtain timely regulatory approvals, if at all:
| our limited experience in filing and pursuing the applications necessary to gain regulatory approvals; |
9
Risk factors
| any failure to satisfy efficacy, safety or quality standards; |
| any difficulty identifying, recruiting, enrolling and retaining a sufficient number of qualified patients for our clinical trials; |
| · | a decision by us or regulators to suspend or terminate our clinical trials if the participating patients are being exposed to unacceptable health risks; |
| regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials if investigators find us not to be in compliance with applicable regulatory requirements; |
| our ability to produce sufficient quantities of Xcellerated T Cells to complete our clinical trials; |
| varying interpretations of the data generated from our clinical trials; and |
| changes in governmental regulations or administrative |
Any delays in, or termination of, our clinical trials could materially and adversely affect our development and collaboration timelines, which may cause our stock price to decline. If we do not complete clinical trials for Xcellerated T Cells and obtain regulatory approvals, we will not be able to commercialize Xcellerated T Cells and we may not be able to recover any of the substantial costs we have invested in the development of Xcellerated T Cells.
We have limited manufacturing experience and may not be able to manufacture Xcellerated T Cells on a large scale or in a cost-effective manner.
We currently manufacture Xcellerated T Cells for research and development and our clinical activities in one manufacturing facility in Seattle, Washington. We have not demonstrated the ability to manufacture Xcellerated T Cells beyond quantities sufficient for research and development and limited clinical activities. We have no experience manufacturing Xcellerated T Cells at the capacity that will be necessary to support large clinical trials or commercial sales. We plan to relocate our manufacturing activities to our leased property in Bothell, Washington, which we plan to renovatehave recently renovated for the manufacture of Xcellerated T Cells for our planned clinical trials and, initial commercialization if we receive FDA approval.approval, initial commercialization. However, we may encounter difficulties in obtaining the approvals for and designing, constructing, validating and operating any newthis manufacturing facility. We may also be unable to hire the qualified personnel that we will require to accommodate the expansion of our operations and manufacturing capabilities. If we relocate our manufacturing activities to a new facility during or after a pivotal clinical trial, we will be required to demonstrate to the FDA similarity of the Xcellerated T Cells manufactured in the new facility to the Xcellerated T Cells manufactured in the prior facility to obtain FDA approval. If we cannot adequately demonstrate similarity to the FDA, we could be required to repeat clinical trials, which would be expensive and substantially delay regulatory approval.
Because our Xcellerate Technology is a patient-specific, cell-based product, the manufacture of Xcellerated T Cells is more complicated than the manufacture of most pharmaceuticals. Our present manufacturing process may not meet our initial expectations as to reproducibility, yield, purity or other measurements of performance. In addition, we have recently begunare using a custom bioreactor system in our manufacturing process and only have limited manufacturing experience using this bioreactor system to activate and expand T cells. Because this new manufacturing process is unproven, we may never successfully utilize our custom bioreactor system to commercialize our products. In addition, because some of our prior clinical trials were conducted using a prior version of the manufacturing system, which did not use the custom bioreactor, we may have to show comparability of the Xcellerated T Cells manufactured with the different versions of the manufacturing systems we have used. To show comparability, we may be required to conduct additional clinical trials. If we make additional modifications in our manufacturing process in the future, we may also have to show comparability of newer versions of the manufacturing process. We are currently negotiating a manufacturing and supply agreement with Wave Biotech LLC, the manufacturer of our bioreactor system. If we are unable to successfully negotiate this contract or are unable to procure a suitable alternative manufacturer in a timely manner, we wouldcould face a setback in the development of our manufacturing process. For these and other reasons, we may not be able to manufacture Xcellerated T Cells on a large scale or in a cost-effective manner.
We are the only manufacturer of Xcellerated T Cells. Although we are considering third partythird-party manufacturing options, we expect that we will conduct most of our manufacturing in our own facility for the next several years. Furthermore, because we are the only manufacturer of Xcellerated T Cells and we currently use only one manufacturing facility, any damage to or destruction of our manufacturing
10
Risk factors
facility or our equipment, prolonged power outage, contamination of our facility or shutdown by the
FDA or other regulatory authority could significantly impair or curtail our ability to produce Xcellerated T Cells. In addition, we store our patients’ cells in freezers at our manufacturing facility. If these cells are damaged at our facility, including by the loss or malfunction of these freezers or our back-up power systems, we would need to collect replacement patient cells, which would delay our patients’ treatments. If we are unable to collect replacement cells from our patients, we could incur liability and our business could suffer.
The government and other third-party payors may control the pricing and profitability of our products.
Our ability to commercialize Xcellerated T Cells successfully will depend in part on the extent to which governmental authorities, private health insurers and other organizations establish appropriate reimbursement levels for the cost of Xcellerated T Cells and related treatments. Increasing emphasis on managed care in the United States will continue to put pressure on the pricing of healthcare products. In addition, governmental authorities may establish pricing and reimbursement levels for some disease indications but not others, which may reduce the demand for Xcellerated T Cells and our profitability. Pricing and profitability of healthcare products are also subject to governmental control in some foreign markets. Cost control initiatives could:
| result in lower prices for Xcellerated T Cells or any future products or their exclusion from reimbursement programs; |
| reduce any future revenues we may receive from collaborators; |
| discourage physicians from delivering Xcellerated T Cells to patients in connection with clinical trials or future |
| · | limit off-label use of Xcellerated T Cells. |
We rely on third parties to conduct some of the clinical trials for Xcellerated T Cells, and their failure to timely and successfully perform their obligations to us, or their defective performance, could significantly harm our product development programs.programs and our business.
Because we rely on academic institutions, site management operationsorganizations and clinical research organizations to conduct, supervise or monitor some or all aspects of clinical trials involving our Xcellerate Technology, we have limited control over the timing and other aspects of these clinical trials. If these third parties do not successfully carry out their duties under their agreements with us, fail to inform us if these trials fail to comply with clinical
trial protocols or fail to meet expected deadlines, this may adversely affect our clinical trials and we may not be able to obtain regulatory approvals.
A third party on whom we rely to conduct clinical trials for Xcellerated T Cells could conduct those clinical trials defectively. This could lead to patients experiencing harmful side effects or could prevent us from proving that Xcellerated T Cells are effective, which may result in:
| · | our failure to obtain or maintain regulatory approval; |
| · | physicians not using or recommending our products; and |
| · | significant product liability. |
Xcellerated T Cells may never achieve market acceptance even if we obtain regulatory approvals.
We do not expect to receive regulatory approvals for the commercial sale of any products derived from our Xcellerate Technology for several years, if at all. Even if we do receive regulatory approvals, the future commercial success of Xcellerated T Cells will depend, among other things, uponon its acceptance by physicians, patients, health carehealthcare payors and other members of the medical community as a therapeutic and cost-effective alternative to commercially available products. Because only a few cell-based therapy products have been commercialized, we do not know to what extent cell-based immunotherapy products will be accepted as therapeutic alternatives. If we fail to gain market acceptance, we may not be able to earn sufficient revenues to continue our business. Market acceptance of and demand for any product that we may develop will depend on many factors, including:
| our ability to provide acceptable evidence of safety and efficacy; |
11
Risk factors
| convenience and ease of administration; |
| prevalence and severity of adverse side effects; |
| availability of alternative and competing treatments; |
| cost effectiveness; |
| effectiveness of our marketing and distribution strategy and the pricing of any product that we may develop; |
| publicity concerning our products or competitive products; and |
| our ability to obtain sufficient third-party coverage or reimbursement. |
If Xcellerated T Cells do not become widely accepted by physicians and patients, it is unlikely that we will ever become profitable.
Even if we obtain regulatory approvals for Xcellerated T Cells, those approvals and ongoing regulation of our products may limit how we manufacture and market our products, which could prevent us from realizing the full benefit of our efforts.
If we obtain regulatory approvals, Xcellerated T Cells, theour Xcellerate Technology and our manufacturing facilities will be subject to continual review, including periodic inspections, by the FDA and other USU.S. and foreign regulatory authorities. In addition, regulatory authorities may impose significant restrictions on the indicated uses or marketing of Xcellerated T Cells or other products that we may develop. These and other factors may significantly restrict our ability to successfully commercialize Xcellerated T Cells and theour Xcellerate Technology.
We and many of our vendors and suppliers are required to comply with current Good Manufacturing Practices, or cGMP, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Furthermore, our manufacturing facilities must be approved by
regulatory agencies before these facilities can be used to manufacture Xcellerated T Cells, and they will also be subject to additional regulatory inspections. Any material changes we may make to our manufacturing process may require approvalapprovals by the FDA and state or foreign regulatory authorities. Failure to comply with FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, partial or total suspension of production or withdrawal of a product from the market.
We must also report adverse events that occur when our products are used. The discovery of previously unknown problems with Xcellerated T Cells or our manufacturing facilities may result in restrictions or sanctions on our products or manufacturing facilities, including withdrawal of our products from the market. Regulatory agencies may also require us to reformulate our products, conduct additional clinical trials, make changes in the labeling of our product or obtain re-approvals. This may cause our reputation in the market place to suffer or subject us to lawsuits, including class action suits.
We rely on third parties to administer Xcellerated T Cells to patients, and our business could be harmed if these third parties administer Xcellerated T Cells incorrectly.
We rely on the expertise of physicians, nurses and other associated medical personnel to administer Xcellerated T Cells to patients. Although our Xcellerate Technology employs mostly standard medical procedures, if these medical professionalspersonnel are not properly trained to administer, or are negligent in the administration of, Xcellerated T Cells, the therapeutic effect of Xcellerated T Cells may be diminished or the patient may suffer critical injury.
12
Risk factors
In addition, third-party medical personnel must thaw Xcellerated T Cells received from us. If this thawing is not performed correctly, the patient may suffer critical injury. While we intend to provide training materials and adequate resources to these third-party medical professionals,personnel, the thawing of Xcellerated T Cells will occur outside our supervision and may not be administered properly. If, due to a third-party error, people believe that Xcellerated T Cells are ineffective or harmful, the desire to use Xcellerated T Cells may decline, which will negatively impact our ability to generate revenue. We may also face significant liability even though we aremay not be responsible for the actions of these third parties.
There are risks inherent in our business that may subject us to potential product liability suits and other claims, which may require us to engage in expensive and time-consuming litigation or pay substantial damages and may harm our reputation and reduce the demand for our product.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of biopharmaceutical products. We will face an even greater risk of product liability if we commercialize Xcellerated T Cells. An individual may bring a product liability claim against us if Xcellerated T Cells cause, or merely appear to have caused, an injury. For example,In addition, we have been named as a defendantare licensing our Xcellerate Technology in connection with a clinical trial using technology similarthe field of HIV retroviral gene therapy to ours conducted at the University of Chicago Hospital. This proceeding is currently pending. Although we intendour collaborative partner, Fresenius Biotech GmbH, or Fresenius. We may incur liability and be exposed to vigorously defend this lawsuit, because of the nature of the complaint against us, we cannot predict the probability of a favorable or unfavorable outcome or estimate the amount or range of potential loss. See “Business—Legal proceedings.”claims for products manufactured by Fresenius.
Certain aspects of how Xcellerated T Cells are processed and administered may enhanceincrease our exposure to liability. Our Xcellerate Technology requires us to activate a patient’s T cellsex vivo, or outside of the body, using blood collected from patients. Third partythe patient. Third-party physicians or other medical personnel initially collect a patient’s blood through a process called leukapheresis, which may pose risks, such as bleeding and infection. The blood that we collect from our patients may contain infectious agents that may infect medical personnel or others with whom the blood comes in contact. Medical personnel administer Xcellerated T Cells to patients intravenously in an outpatient procedure. This procedure poses risks to the patient similar to those occurring with infusions of other frozen cell products, such as stem cells, including blood clots, infection and mild to severe allergic reactions.
It is possible that we or third parties may misidentify Xcellerated T Cells and deliver them to the wrong patient. If these misidentified Xcellerated T Cells are administered to the wrong patient, the patient could suffer irreversible injury or death.
The discovery of unforeseen side effects of Xcellerated T Cells could also lead to lawsuits against us. Regardless of merit or eventual outcome, product liability or other claims may, among other things, result in:
| injury to our reputation and decreased demand for Xcellerated T Cells; |
| withdrawal of clinical trial volunteers; |
| costs of related litigation; and |
| substantial monetary awards to plaintiffs. |
We currently have clinical trial insurance that covers our clinical trials up to $5.0 million per occurrence with a $5.0 million aggregate limit, and we intend to obtain product liability coverage in the future. However, due to factors outside of our control, including the risks discussed above as well as conditions in the relevant insurance coveragemarkets, we may not be availableable to us at anrenew or obtain such coverage on acceptable cost,terms, if at all. EvenFurthermore, even if we secure coverage,
13
Risk factors
we may not be able to obtain insurance coverage that will bepolicy limits adequate to satisfy any liability that may arise. If a successful product liability or other claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover these claims and our business operations could suffer.
If Xcellerated T Cells or components of our Xcellerate Technology alone or in combination with complementary treatments cause unforeseen harmful side effects, physicians may not use our products and/or we may incur significant product liability, which will adversely affect our ability to operate our business.
Xcellerated T Cells or components of our Xcellerate Technology may cause unforeseen harmful side effects. For example, a patient receiving Xcellerated T Cells could have a severe allergic reaction or could develop an autoimmune condition. While we employ procedures to substantially remove the antibodies and beads used to generate Xcellerated T Cells, it is possible that residual antibodies or beads may be infused into patients and cause harmful effects.
In addition, we have not conducted studies on the long-term effects associated with the different types of media that we use to grow and freeze cells as part of our Xcellerate Technology. These media contain substances that have proved harmful if used in certain quantities. While we believe that we use sufficiently small quantities of these substances, harmful effects may still arise from our use of these media. As we continue to develop our Xcellerate Technology, we may encounter harmful side effects that we did not previously observe in our prior studies and clinical trials.
We believe Xcellerated T Cells may be used in combination with complementary treatments, including cancer vaccines, monoclonal antibodies, genes, cytokines or chemotherapy, and one or more of these other therapies could cause harmful side effects that could be attributed to Xcellerated T Cells. Any or all of these harmful side effects may occur at various stages of our product development, including the research stage, the development stage, the clinical stage or the commercial stage of our products. If people believe Xcellerated T Cells or any component of our Xcellerate Technology alone or in combination with complementary treatments causecauses harmful side effects, we may incur significant damages from product liability claims, which will adversely affect our ability to operate our business.
We rely on a limited number of manufacturers and suppliers for some of the key components of our Xcellerate Technology. The loss of these suppliers, or their failure to provide us with adequate quantities of these key components when needed, could delay our clinical trials and prevent or delay commercialization of Xcellerated T Cells.
We rely on third partythird-party suppliers for some of the key components used to manufacture Xcellerated T Cells. We rely on Lonza Biologics PLC, or Lonza, to develop and manufacture the antibodies that we use in our Xcellerate Technology. LonzaEither party may terminate our agreements with Lonza for breach or insolvency of the agreements we have with themother party or if we commit a breach. We are aware of few companies with the abilityLonza is unable to manufacture commercial grade antibodies.perform its obligations for scientific or technical reasons. Our current agreements with
Lonza only provide for manufacturing development and validation, and the creation and submission of materials required to obtain regulatory approval of the antibody manufacturing process. We are using the antibodies supplied by Lonza under the agreements to manufacture of these antibodies for usethe Xcellerated T Cells used in our clinical trials. We are currently negotiating an agreement with Lonza to manufacture the antibodies for commercial use. If we are unable to negotiate this contract with Lonza or are unable to procure a suitable alternative manufacturer in a timely manner and on favorable terms, if at all, we may incur significant costs and be unable to continue developing our Xcellerate Technology. We are aware of few companies with the ability to manufacture commercial-grade antibodies.
Our Xcellerate Technology also depends in part on the successful attachment of the antibodies to magnetic beads. We currently use magnetic beads developed and manufactured by Dynal S.A.A.S., or Dynal, in Oslo, Norway. Our contract with Dynal expires in August 2009, and eitherhas the right to terminate the agreement if we do not purchase a minimum quantity of beads. Either party may terminate the
14
Risk factors
contract agreement as of August 2009 for any reason, or earlier for the material breach.breach or insolvency of the other party. If the agreement is not terminated by August 2009, either party can elect to extend the term of the agreement for an additional 5 years. Otherwise, it will automatically renew on a year to year basis. We are contractually obligated to obtain our beads from Dynal as long asunless Dynal is ableunable to fill our orders.orders or certain other circumstances arise. If Dynal terminates our contract or if Dynal discontinues manufacturing our beads for any reason, we may be unable to find a suitable alternative manufacturer in a timely manner, or at all, which would delay our clinical trials and delay or prevent commercialization of Xcellerated T Cells.
Our manufacturing process currently uses a commercially available tissue culture media that is available from only one manufacturer, Cambrex Bio Science Walkersville, Inc. If Cambrex is unwilling or unable to supply us with this media, we would need to use an alternative tissue culture media, which may delay our clinical trials and harm our business. We do not have agreements with Cambrex which obligate them to provide us with any products for future clinical trials or future commercial sales.
In addition, we currently use a custom bioreactor to manufacture Xcellerated T Cells that is available from only one manufacturer, Wave Biotech LLC. There are a limited number of manufacturers that are capable of manufacturing custom bioreactors. If Wave Biotech is unwilling or unable to manufacture or supply us with custom bioreactors, we may be unable to find a suitable alternative in a timely manner, or at all, which would delay our clinical trials and delay or prevent commercialization of Xcellerated T Cells. We do not have agreements with Wave Biotech which obligate them to provide us with custom bioreactors.
We have qualified and validated commercially available disposable bags and tubing sets in our manufacturing process from only one manufacturer, Baxter International, Inc. If Baxter is unwilling or unable to supply us with the disposables, we would need to find an alternative manufacturer and qualify and validate alternative disposables, which may delay our clinical trials and harm our business. We do not have agreements with Baxter which obligate them to provide us with any products for future clinical trials or future commercial sales.
Although these and other suppliers have produced our components with acceptable quality, quantity and cost in the past, they may be unable or unwilling to timely meet our future demands. They may also increase the prices they charge us. Obtaining similar FDA-acceptable components from other suppliers and validating these components may be difficult and expensive. If we have to switch to a replacement supplier, we could face additional regulatory delays, which could interrupt the manufacture and delivery of our product for an extended period. In addition, because Lonza and Dynal are located outside the United States, we are subject to foreign import laws and customs regulations, which complicate, and could delay, shipment of components to us and delay the development and production of Xcellerated T Cells. Any delay in the development or production of Xcellerated T Cells may impact our ability to generate revenue and cause our stock price to decline.
If we or any of our third partythird-party manufacturers do not maintain high standards of manufacturing, our ability to develop and commercialize Xcellerated T Cells could be delayed or curtailed.
We and any third parties that we may use in the future to manufacture our products must continuously adhere to cGMP regulations enforced by the FDA through its facilities inspection program. If our facilities or the facilities of these third parties do not pass a pre-approval plant inspection, the FDA will not grant market approval for Xcellerated T Cells. In complying with cGMP, we and any third partythird-party manufacturers must expend significant time, money and effort in production, record-keeping and quality control to assure that each component of our Xcellerate Technology meets applicable specifications and other requirements. We or any of these third partythird-party manufacturers may also be subject to comparable or more stringent regulations of foreign regulatory authorities. If we or any of our third partythird-party manufacturers fail to comply with these requirements, we may be subject to regulatory action, which could delay or curtail our ability to develop and commercialize Xcellerated T Cells. If our component part manufacturers and suppliers fail to provide components of sufficient quality, our clinical trials or commercialization of Xcellerated T Cells could be delayed or halted and we could face product liability claims.
Our leased facilities are at risk of damage by earthquakes, and any damage to our facilities will harm our clinical trials and development programs.
We currently rely on the availability and condition of our leased Seattle, Washington facility to conduct research and development and for the manufacture of Xcellerated T Cells. This facility is located in a seismic zone, and there is the possibility of an earthquake which, depending on its magnitude, could be disruptive to our operations. Our leased facility in Bothell, Washington, where we intend to locate our initial commercial manufacturing activities, is also in a seismic area. We currently have no insurance against damage caused by earthquakes.
If third partythird-party carriers fail to ship patient samples and our products in a proper and timely manner, the treatment of patients could be delayed or prevented, our reputation may suffer and we may incur liability.
We depend on third partythird-party carriers to deliver patient-specific blood cells to us and to deliver Xcellerated T Cells back to patients in a careful and timely manner. Our Xcellerate Technology currently requires that we process each patient’s leukapheresis blood sample within 48 hours of collection. Xcellerated T Cells must currently be shipped in a frozen storage shipping container and received by the patient within six days from leaving our manufacturing facility. If the shipping containers fail to maintain the necessary
15
Risk factors
temperature, Xcellerated T Cells could be damaged. If third partythird-party carriers fail to timely deliver the leukapheresis blood sample to us or fail to timely ship Xcellerated T Cells to the clinic, or if they damage or contaminate them during shipment, the treatment of patients could be delayed or discontinued, our reputation may suffer and we may incur liability. In addition, as we expand our clinical trial sites, we may need to make modifications to the shipping process to ship internationally, such as requiring third parties to freeze the patient’s white blood cells prior to shipment to us for processing, which may reduce our control over the production of Xcellerated T Cells. Furthermore, shipping blood products internationally will subject us to foreign import laws and customs regulations, which complicate, and could delay, shipment of components to and from us and delay the development, production and infusion of Xcellerated T Cells.
We use hazardous materials and must comply with environmental, health and safety laws and regulations, which can be expensive and restrict how we do business.
Our research and development and manufacturing processes involve the controlled storage, use and disposal of hazardous materials, including biological hazardous materials. We are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. Although we believe that our safety procedures for handling and disposing of these hazardous materials comply with the standards prescribed by law and regulation, we cannot completely eliminate the risk of accidental contamination or injury from hazardous materials. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of our insurance. We may not be able to obtain insurance on acceptable terms, if at all. We could incur significant costs to comply with current or future environmental laws and regulations.
Our current commercial property insurance provides coverage up to $25,000 for pollution clean-up or removal and up to $25,000 for biological agency clean-up or removal. Additionally our business income coverage provides for up to $250,000 for extra expenses for pollution clean-up or removal to enable us to re-establish operations after a hazardous event.
In some circumstances we plan to rely on collaborators to commercialize Xcellerated T Cells. If our current collaborators do not perform as expected or if future collaborators do not commit adequate resources to their collaboration with us, our product development and potential for profitability may suffer.
We have entered into alliances with third-party collaborators to develop and market Xcellerated T Cells for diseases and markets that we are not pursuing on our own. In addition, our strategy includes substantial reliance on additional strategic collaborations for research, development, manufacturing, marketing and other commercialization activities relating to Xcellerated T Cells. If our collaborators do not prioritize and commit substantial resources to these collaborations, or if we are unable to secure successful future collaborations, we may be unable to commercialize Xcellerated T Cells for important diseases and in important markets, which would limit our ability to generate revenue and become profitable. Furthermore, disputes may arise between us and our existing or future collaborators, which could result in delays in the development and commercialization of Xcellerated T Cells.
For example, we have licensed our Xcellerate Technology and some related improvements, on an exclusive basis in the field of HIV retroviral gene therapy to Fresenius, for research, development and commercialization in Europe, with a letterright of intentfirst negotiation under some circumstances to establish an allianceexpand their territory to include North America. Our agreement with Taiwan Cellular Therapy Company, or TCTC, a company newly formed under the laws of Taiwan,Fresenius requires us to develop and marketlicense our Xcellerate Technology, including methods for manufacturing Xcellerated T Cells, in Australia and Asia, excluding Japan. The letter of intentto Fresenius. This agreement also requires us to negotiate in good faithsupply all proprietary magnetic beads, or Xcyte Dynabeads, used to manufacture Xcellerated T Cells ordered by Fresenius to support its development and commercialization efforts. If we do not supply the Xcyte Dynabeads, Fresenius has the right to manufacture such Xcyte Dynabeads on its own or through a definitivethird party, until such time that we are able to supply the quantity of Xcyte Dynabeads ordered by Fresenius. The agreement with TCTC on or before November 15, 2003. However,terminates upon the letterlast to expire of intent provides that the definitive agreementlicensed patents and is subject to amongearlier termination by Fresenius at any time if Fresenius determines it cannot develop a commercially viable product or complete a required manufacturing audit. The agreement may be terminated by Xcyte if Fresenius does not meet certain development and commercialization milestones and by either party for the material breach or insolvency of the other things, TCTC closing, or obtaining commitments for at least US$25 million in equity financing on or before December 30, 2003. TCTC’s ability to obtain the required equity financing is very uncertain. In addition,party. At Fresenius’ expense, we may not be able to negotiate mutually agreeable terms for a definitive agreement or we or TCTC may elect not to proceed with the alliance. If
we elect not to proceed with the alliance for any reason, other than for a good faith inability to negotiate a definitive agreement, and we enter into an agreement with a third party in Australia or Asia, excluding Japan, before February 22, 2004, we will be required to pay a break-up fee to TCTC.
Although there is substantial uncertainty as to whether we will complete an alliance with TCTC, we have agreed to reserve the rights to our Xcellerate Technology in Australia and Asia, excluding Japan, for TCTC until at least December 30, 2003. If we complete an alliance with TCTC, we will beare required to expend significant resources to transfer technology to TCTCFresenius and assist them in developing and
16
Risk factors
manufacturing products using our Xcellerate Technology. Even if we complete an alliance with TCTC, TCTCso, Fresenius may not have sufficient resources to fund, or may subsequently decide not to proceed with, development of our Xcellerate Technology. In this event, we may terminate the Fresenius agreement, but we may not have sufficient capital resources to develop ourthe use of Xcellerate Technology in the field of HIV retroviral gene therapy in Europe or North America on our own in Australia or Asia.own.
We may be unable to establish sales, marketing and distribution capabilities necessary to successfully commercialize our products.
We currently have only limited marketing capabilities and no direct or third-party sales or distribution capabilities. We currently plan to develop an internal sales force to serve certain North American markets and pursue strategic partnerships to obtain development and marketing support for territories outside North America. However, we may be unable to establish marketing, sales and distribution capabilities necessary to commercialize and gain market acceptance for our potential products. In addition, developing a sales force, or entering into co-promotion agreements with third parties, is expensive and time-consuming and could delay any product launch. Co-promotion or other marketing arrangements with third parties to commercialize potential products may also not be successful and could significantly limit the revenues we derive from Xcellerated T Cells.
We face competition in our industry, and many of our competitors have substantially greater experience and resources than we have.
Even if our Xcellerate Technology proves successful, we might not be able to remain competitive because of the rapid pace of technological development in the biotechnology field.
We are currently aware of several companies developingex vivo cell-based immunotherapy products as a method of treating cancer and infectious diseases. These competitors include Antigenics, Inc., CancerVax Corporation, Cell Genesys, Inc., CellExSys, Inc. (recently sold to Chromos Molecular Systems, Inc.), Dendreon Corporation, Favrille, Inc., Genitope Corporation, IDM, S.A. and Kirin Pharmaceutical. Some of our competitors have greater financial and other resources, larger research and development staffs and more experienced capabilities in researching, developing and testing products than we do. Many of these companies also have more experience in conducting clinical trials, obtaining FDA and other regulatory approvals and in manufacturing, marketing and distributing therapeutic products. Smaller companies may successfully compete with us by establishing collaborative relationships with larger pharmaceutical companies or academic institutions. In addition, large pharmaceutical companies or other companies with greater resources or experience than us may choose to forgoex vivo cell-based immunotherapy opportunities that would have otherwise been complementary to our product development and collaboration plans. Our competitors may succeed in developing, obtaining patent protection for or commercializing their products more rapidly than us. A competing company developing, or acquiring rights to, a more effective therapeutic product for the same diseases targeted by us, or one that offers significantly lower costs of treatment, could render our products noncompetitive or obsolete.
We plan significant growth, which we may not be able to effectively manage.
We will need to add a significant number of new personnel and expand our capabilities in order to successfully pursue our research, development and commercialization efforts and secure collaborations to market and distribute our products. This growth may strain our existing managerial, operational, financial and other resources. We also intend to add personnel in our research and development and manufacturing departments as we expand our clinical trial and research capabilities. Our failure to manage our growth effectively could delay or curtail our product development and commercialization efforts and harm our business.
17
Risk factors
If we lose key management or scientific personnel, our business could suffer.
Our success depends, to a significant extent, uponon the efforts and abilities of Ronald J. Berenson, M.D., our President and Chief Executive Officer, Robert L. Kirkman, M.D., our Chief Business Officer and Vice President, Stewart Craig, Ph.D., our Chief Operating Officer and Vice President, Mark Frohlich, M.D., our Medical Director and Vice President, and other members of our senior management and our scientific personnel. We do not have employment agreements with Dr. Berenson, Dr. Craig or several other members of our senior management. Additionally, any employment agreement that we may enter into will not ensure the retention of the employee. Since the pool of employees with relevant experience in immunology and biotechnology is small, replacing any of our senior management or scientific personnel would likely be costly and time-consuming. Although we maintain key person life insurance on Dr. Berenson, we do not maintain key person life insurance on any of our other officers, employees or consultants. The loss of the services of one or more of our key employees could delay or curtail our research and development and product development efforts.
We may undertake acquisitions in the future, and any difficulties from integrating suchthese acquisitions could damage our ability to attain or maintain profitability.
We may acquire additional businesses, products or product candidates that complement or augment our existing business. However, we currently have no commitments or agreements, and are not involved in any negotiations, to acquire any businesses, products or technologies. Integrating any newly acquired business or product could be expensive and time-consuming. We may not be able to integrate any acquired business or product successfully or operate any acquired business profitably. Moreover, we manymay need to raise additional funds through public or private debt or equity financing to make acquisitions, which may result in dilution to stockholders and the incurrence of indebtedness that may include restrictive covenants.
Changes in the value of the British pound and Euro relative to the USU.S. dollar may adversely affect us.
We do not engage in foreign currency hedging; however, we have entered into certain contracts denominated in foreign currencies and therefore we are exposed to currency exchange risks.
Under our agreements with Lonza to purchase antibodies, we must make payments denominated in British pounds. As a result, from time to time, we are exposed to currency exchange risks. We do not engage in currency hedging.risks related to the British pound. Accordingly, if the British pound strengthens against the USU.S. dollar, our payments to Lonza will increase in USU.S. dollar terms. We have paid a total of $4.9 million to Lonza under our agreements with them as of December 31, 2003, consisting of approximately $252,000, $1.7 million, $1.6 million and $1.3 million during the years ended December 31, 2000, 2001, 2002 and 2003, respectively. Assuming development and supply services are completed as scheduled under our agreements with Lonza, our remaining payments will be approximately $1.4 million through the end of 2005.
The terms of our license agreement with Fresenius include potential royalties on net sales as well as potential milestone payments to us denominated in the Euro. As a result, we are exposed to currency exchange risks related to the Euro. If the Euro weakens against the U.S. dollar, payments received from Fresenius will decrease in U.S. dollar terms.
If we do not achieve our projected development goals in the time frames we announce and expect, the commercialization of our products may be delayed and, as a result, our stock price may decline.
From time to time, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of scientific studies and clinical trials and the submission of regulatory filings. From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones arewill be based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in some cases for reasons beyond our control. If we do not meet these milestones as publicly announced, the commercialization of our products may be delayed and, as a result, our stock price may decline.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends in part on obtaining, maintaining and enforcing our patents and in-licensed and proprietary rights. We believe we own, or have rights under licenses to, issued patents and pending
18
Risk factors
patent applications that are necessary to commercialize Xcellerated T Cells. However, the patents on which we rely may be challenged and invalidated, and our patent applications may not result in issued patents. Moreover, our patents and patent applications may not be sufficiently broad to prevent others from practicing our technologies or developing competing products. We also face the risk that others may independently develop similar or alternative technologies or may design around our proprietary and patented technologies.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date in the United States. Furthermore, the application and enforcement of patent laws and regulations in foreign countries is even more uncertain. Accordingly, we cannot assure you that we will be able to effectively file, protect or defend our proprietary rights in the United States or in foreign jurisdictions on a consistent basis.
Third parties may successfully challenge the validity of our patents. We will only be able to protect our technologies from unauthorized use by third parties to the extent that valid and enforceable patents or other proprietary rights cover them. Because the issuance of a patent is not conclusive of its validity or enforceability, we cannot assure you how much protection, if any, will be given to our patents if we attempt to enforce them or if others challenge their validity in court. It is possible that a competitor may successfully challenge our patents or that a challenge will result in limiting the coverage of our patents. If the outcome of litigation is adverse to us, third parties may be able to use our technologies without payment to us.
In addition, it is possible that competitors may infringe upon our patents or successfully avoid them through design innovation. We may initiate litigation to police unauthorized use of our proprietary rights. However, the cost of litigation to uphold the validity of our patents and to prevent infringement could be substantial, and the litigation will consume time and other resources. Some of our competitors may be better able to sustain the costs of complex patent litigation because they have substantially greater resources. Moreover, if a court decides that our patents are not valid, we will not have the right to stop others from using our inventions. There is also the risk that, even if the validity of our patents were upheld, a court may refuse to stop others on the ground that their activities do not infringe upon our patents. Because protecting our intellectual property is difficult and expensive, we may be unable to prevent misappropriation of our proprietary rights.
We also rely on certain proprietary trade secrets and know-how, especially where we believe patent protection is not appropriate or obtainable. Trade secrets and know-how, however, are difficult to protect. We have taken measures to protect our unpatented trade secrets and know-how, including the use of confidentiality and invention assignment agreements with our employees, consultants and some of our contractors. It is possible, however, that these persons may unintentionally or willingly breach the agreements or that our competitors may independently develop or otherwise discover our trade secrets and know-how.
If the use of our technologies conflicts with the rights of others, we could be subject to expensive litigation or be required to obtain licenses from others to develop or market Xcellerated T Cells.
Our competitors or others may have or acquire patent rights that they could enforce against us. If they do so, we may be required to alter our Xcellerate Technology, pay licensing fees or cease activities. If our Xcellerate Technology conflicts with patent rights of others, third parties could bring legal action against
19
Risk factors
us or our licensees, suppliers, customers or potential collaborators, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we might have to obtain a license in order to continue to manufacture or market the affected products. A required license under the related patent may not be available on acceptable terms, if at all.
We may be unaware that the use of our technology conflicts with pending or issued patents. Because patent applications can take many years to issue, there may be currently pending applications, unknown to us, that may later result in issued patents upon which our Xcellerate Technology or Xcellerated T Cells may infringe. There could also be existing patents of which we are unaware upon which our Xcellerate Technology or Xcellerated T Cells may infringe. In addition, if third parties file patent applications or obtain patents claiming technology also claimed by us in pending applications, we may have to participate in interference proceedings in the USU.S. Patent and Trademark Office to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of the filed foreign patent applications. We may have to participate in interference proceedings involving our issued patents or our pending applications.
If a third party claims that we infringe upon its proprietary rights, any of the following may occur:
| we may become involved in time-consuming and expensive litigation, even if the claim is without merit; |
| we may become liable for substantial damages for past infringement if a court decides that our technology infringes upon a competitor’s patent; |
| a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross licenses to our patents; and |
| we may have to redesign our technology or clinical candidate so that it does not infringe upon others’ patent rights, which may not be possible or could require substantial funds or time. |
If any of these events occurs, our business will suffer and the market price of our common stock will likely decline.
Our rights to use antibodies and technologies licensed to us by third parties are not within our control, and we may not be able to implement our Xcellerate Technology without these antibodies and technologies.
We have licensed patents and other rights which are necessary to our Xcellerate Technology and Xcellerated T Cells. Our business will significantly suffer if these licenses terminate, if the licensors fail to abide by the terms of the license or fail to prevent infringement by third parties or if the licensed patents or other rights are found to be invalid.
Our Xcellerate Technology uses two monoclonal antibodies that we license from third parties. We rely on our non-exclusive license from the Fred Hutchinson Cancer Research Center in Seattle, Washington to use the monoclonal antibody that binds to the CD3 molecule and our exclusive license from Diaclone S.A., or Diaclone, in Besancon, France to use the monoclonal antibody that binds to the CD28 molecule. These antibodies are necessary components of our Xcellerate Technology. We rely on these licensors to prevent infringement of the licensed antibody. Our rights to use these antibodies depend on the licensors abiding by the terms of those licenses and not terminating them. Our license agreement with the Fred
20
Risk factors
Hutchinson Research Center is effective for 15 years following the first commercial sale of a product based on the license and may be terminated in the event of aearlier by either party for material breach. Our license agreement with Diaclone is effective for 15 years from the date of the first FDA approval, or its foreign equivalent, of a therapeutic product based oncontaining a bead coated with the licenselicensed antibody and may be terminated in the event of aearlier by either party for material breach. Our contractWith regard to our agreement with Diaclone, at the end of the relevant 15-year period, we will have a perpetual, irrevocable, fully-paid royalty-free, exclusive license. Except for certain circumstances which would permit us to obtain the monoclonal antibody from third parties or manufacture it ourselves, our agreement with Diaclone obligates us to purchase the monoclonal antibody from Diaclonethem until we begin preparing for Phase III clinical trials.trials of a product covered by this license.
In addition, we have in-licensed several T cell activation patents and patent applications from the Genetics Institute, a subsidiary of Wyeth, Inc. The technology underlying these patents creates the basis foris a critical part of our Xcellerate Technology. These licensesUnder our agreement, we have the right to enforce the licensed patents. The license from Genetics Institute terminateterminates upon the expirationend of the enforceable term of the last licensed patent or the license agreements under which Genetics Institute has sublicensed rights to Xcyte, and may also be terminated in the event of aearlier by either party for material breach. Of the fourfive in-licensed U.S. patents presently issued related to this technology, two patents expire in 2016, and two others expire in 2019.2019, and the remaining patent expires in 2020.
If we violate the terms of our licenses, or otherwise lose our rights to these antibodies, patents or patent applications, we may be unable to continue development of our Xcellerate Technology. Our licensors or others may dispute the scope of our rights under any of these licenses. Additionally, the licensors under these licenses might breach the terms of their respective agreements or fail to preventassist in the prevention of infringement of the licensed patents by third parties. Loss of any of these licenses for any reason could materially harm our financial condition and operating results.
RISKS RELATING TO THIS OFFERINGRisks Relating To This Offering
You will suffer immediate and substantial dilution.
We expect the initial public offering price of our shares to be substantially higher than the book value per share of our outstanding common stock. Accordingly, investors purchasing shares of common stock in this offering will:
To the extent outstanding stock options or warrants are exercised after this offering, there will be further dilution to new investors. See “Dilution.”
If our principal stockholders, executive officers and directors choose to act together, they may be able to control our management and operations, acting in their best interests and not necessarily those of other stockholders.
Our executive officers, directors and principal stockholders, and entities affiliated with them, will beneficially own in the aggregate approximately %44.0% of our common stock following this offering.offering, and approximately % of our common and convertible preferred stock taken together on an as-converted to common stock basis. This significant concentration of share ownership may adversely affect the trading price forof our common stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders. These stockholders, acting together, will have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets. In addition, they could dictate the management of our business and affairs. This concentration of ownership could have the effect of delaying, deferring or preventing a change in control of us or impeding a merger, or consolidation, takeover or other business combination that could be favorable to you. Since the convertible preferred stock has very limited voting rights prior to conversion, you will have little or no ability to control matters requiring approval of our stockholders.
21
Risk factors
The future sale of our common stock could negatively affect our stock price.
After this offering, based on shares outstanding as of September 27, 2004, we will have approximately 14,826,970 shares of common stock outstanding orand 1,500,000 shares if the underwriters exercise their over-allotment option in full.of convertible preferred stock outstanding that are convertible into shares of our common stock. The 1,500,000 shares of convertible preferred stock sold in this offering, or 1,725,000 shares if the underwriters exercise their over-allotment option in full, will be freely tradable without restriction under the federal securities laws unless purchased by our affiliates. The remaining shares of common and convertible preferred stock outstanding after this offering will be available for public sale subject in some cases to volume, lock-up and other limitations. See “Shares eligible for future sale.”
If our common or convertible preferred stockholders sell substantial amounts of commonour stock in the public market, or the market perceives that such sales may occur, the market price of our common and convertible preferred stock could fall. After this offering, according to the terms of our investors rights agreement, the holders of approximately 44,099,3978,992,108 shares of our common stock orand warrants to purchase shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. Furthermore, if we were to include in a company-initiated registration statement shares held by those holders pursuant to the exercise of their registrations rights, the sale of those salesshares could impair our ability to raise needed capital by depressing the price at which we could sell our common stock.
In addition, we will need to raise substantial additional capital in the future to fund our operations. If we raise additional funds by issuing equity securities, our stock price may decline and our existing stockholders may experience significant dilution.
Upon the expiration of a 90-day lock-up agreement, a substantial number of shares of our common stock will become available for sale in the public market which may cause the market price of our preferred and common stock to decline.
On , 2005, which is 90 days after the date of this offering, lock-up agreements covering approximately 5.5 million shares of our common stock held by existing stockholders will expire and those shares will become available for sale. If these stockholders sell substantial amounts of our common stock in the public market at concentrated times, the market price of our common and, in turn our convertible preferred stock, could fall. These sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price acceptable to us.
An active, liquid trading market for our commonthe convertible preferred stock and debentures may never develop.
Prior to this offering, there was no public market for our commonthe convertible preferred stock. An active trading market for our commonthe convertible preferred stock may not develop following this offering. You may not be able to sell your shares quickly or at the market price if trading in our stock is not active. The initial public offering price may not be indicative of prices that will prevail in the trading market. See “Underwriting” for more information regarding the factors considered in determining the initial public offering price. In addition, if we exchange the convertible preferred stock for debentures, we are required to list the debentures on an exchange but there can be no assurances that a market in the debentures will develop. Our ability to list and continue to list the convertible preferred stock on the Nasdaq National Market will depend on our ability to meet the Nasdaq National Market listing requirements for both the convertible preferred stock and our common stock.
Our common and convertible preferred stock may experience extreme price and volume fluctuations, which could lead to costly litigation for us and make an investment in us less appealing.
The market price of our common and convertible preferred stock may fluctuate substantially due to a variety of factors, including:
| results of our clinical trials; |
| announcements of technological innovations or new products or services by us or our competitors; |
| media reports and publications about immunotherapy; |
| announcements concerning our competitors or the biotechnology industry in general; |
| new regulatory pronouncements and changes in regulatory guidelines; |
| general and industry-specific economic conditions; |
| additions to or departures of our key personnel; |
| changes in financial estimates or recommendations by securities analysts; |
| variations in our quarterly results; |
| announcements about our collaborators or licensors; and |
| changes in accounting principles. |
22
Risk factors
The market prices of the securities of biotechnology companies, particularly companies like ours without consistent product revenues and earnings, have been highly volatile and are likely to remain highly volatile in the future. This volatility has often been unrelated to the operating performance of particular companies. In the past, companies that experience volatility in the market price of their securities have often faced securities class action litigation. Moreover, market prices for stocks of biotechnology-related and technology companies particularly following an initial public offering, frequently reach levels that bear no relationship to the operating performance of these companies. These market prices generally are not sustainable and are highly volatile. Whether or not meritorious, litigation brought against us could result in substantial costs, divert our management’s attention and resources and harm our financial condition and results of operations.
Our amended and restated certificate of incorporation and bylaws may delay or prevent a change in our management.
Our amended and restated certificate of incorporation and bylaws will contain provisions that could delay or prevent a change in our board of directors and management teams. Some of these provisions:
| authorize the issuance of preferred stock that can be created and issued by the board of directors without prior stockholder approval, commonly referred to as “blank check” preferred stock, with rights senior to those of our common stock; and |
| provide for a classified board of directors. |
These provisions could make it more difficult for commonour stockholders to replace members of the board.our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt to replace theour current management team.
We may have limited ability to pay cash dividends on the convertible preferred stock.
Delaware law may limit our ability to pay cash dividends on the convertible preferred stock. Under Delaware law, cash dividends on our capital stock may only be paid from “surplus” or, if there is no “surplus,” from the corporations net profits for the current or preceding fiscal year. Delaware law defines “surplus” as the amount by which the total assets of a corporation, after subtracting its total liabilities, exceed the corporation’s capital, as determined by its board of directors. Since we are not profitable, our ability to pay cash dividends will require the availability of adequate surplus. Even if adequate surplus is available to pay cash dividends on the convertible preferred stock, we may not have sufficient cash to pay dividends on the convertible preferred stock. We currently intend to pay cash dividends on the convertible preferred stock.
We may allocate the net proceeds from this offering in ways with which you may not agree.
We expect to use the net proceeds fromof this offering to fundfor working capital and general corporate purposes, including clinical trial, activities, manufacturing and preclinical research and development activities, and for other general corporate purposes, includingas well as capital expenditures and complementary technology acquisition and working capital.acquisitions. See “Use of proceeds.Proceeds.” Our management, however, has broad discretion in the use of the net proceeds from this offering and could spend the net proceeds in ways that do not necessarily improve our operating results or the value of our common stock.
23If we exchange the convertible preferred stock for debentures, the exchange will be taxable but we will not provide any cash to you to pay any tax liability you may incur.
If we automatically convert the convertible preferred stock, you should be aware that there is a substantial risk of fluctuation in the price of our common stock from the date we elect to automatically convert to the conversion date.
We may elect to automatically convert the convertible preferred stock on or prior to maturity if our common stock price has exceeded 150% of the conversion price for at least 20 trading days during a 30-day trading period ending within five trading days prior to the notice of automatic conversion. You should be aware that there is a risk of fluctuation in the price of our common stock between the time when we may first elect to automatically convert the preferred and the automatic conversion date.
You will suffer immediate and substantial dilution.
The offering price of the convertible preferred stock is substantially higher than the book value per share of our outstanding common stock. Accordingly, investors purchasing shares of convertible preferred stock in this offering will pay a price per share of the common stock into which such preferred stock is convertible that substantially exceeds the value of our assets after subtracting liabilities.
Special note regarding forward-looking statementsSPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections entitledtitled “Prospectus summary,Summary,” “Risk factors,Factors,” “Management’s discussionDiscussion and analysisAnalysis of financial conditionFinancial Condition and resultsResults of operations”Operations” and “Business,” contains forward-looking statements. Forward-looking statements convey our current expectations or forecasts of future events. All statements contained in this prospectus other than statements of historical fact are forward-looking statements. Forward-looking statements include statements regarding our future financial position, business strategy, budgets, projected costs, plans and objectives of management for future operations. The words “may,” “continue,” “estimate,” “intend,” “plan,” “will,” “believe,” “project,” “expect,” “anticipate” and similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking.
Any or all of our forward-looking statements in this prospectus may turn out to be inaccurate. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operation,operations, business strategy and financial needs. They may be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including the risks, uncertainties and assumptions described in “Risk factors.Factors.” In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur as contemplated, and actual results could differ materially from those anticipated or implied by the forward-looking statements.
You should not unduly rely on these forward-looking statements, which speak only as of the date of this prospectus. Unless required by law, we undertake no obligation to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this prospectus.
24 See “Where You Can Find More Information.”
Use of proceedsUSE OF PROCEEDS
We estimate that the net proceeds to us from the sale of the 1,500,000 shares of commonconvertible preferred stock we are offering will be approximately $$13.6 million, assuming an initial public offering price of $$10 per share, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate the net proceeds to us will be approximately $ million.expenses.
We have no current specific plan for the proceeds. The principal purposes of this offering include to obtain additional working capital to fund anticipated operating losses, establish a public market for our common stock and facilitate our future access to public markets. We expect to use the net proceeds of this offering for working capital and general corporate purposes, including:
| clinical trial |
| manufacturing |
| preclinical research and development activities; |
| capital |
| complementary technology |
Although we have identified some types of uses above, we have and reserve broad discretion to use the proceeds from this offering differently. When and if the opportunity arises, we may use a portion of the proceeds to acquire or invest in complementary businesses, products or technologies. We currently have no commitments or agreements, and are not involved in any negotiations, to acquire any businesses, products or technologies. Pending any ultimate use of any portion of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, investment-grade and interest-bearing instruments.
Based uponon the current status of our product development and collaboration plans, we believe that the net proceeds of this offering, together with our cash, cash equivalents and investments, will be adequate to satisfy our capital needs through at least the next 18 months.end of 2005. See “Management’s discussionDiscussion and analysisAnalysis of financial conditionFinancial Condition and resultsResults of operations—Operations—Liquidity and capital resources.Capital Resources.”
Dividend policyWe currently intend to pay cash dividends on the convertible preferred stock. Dividends on the convertible preferred stock are cumulative, meaning that if they are not paid they continue to accrue and must be paid prior to the payment of any dividends on our common stock. For a discussion of dividends payable on the convertible preferred stock, please see “Description of Convertible Preferred Stock—Dividends.”
We have never declared or paid any cash dividends on our common stock and do not currently anticipate declaring or paying cash dividends on our common stock in the foreseeable future. WeExcept for dividends payable on the convertible preferred stock, we currently intend to retain all of our future earnings, if any, to finance operations. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and other factors that our board of directors may deem relevant.
RATIO OF EARNINGS TO FIXED CHARGES AND PREFERRED STOCK DIVIDENDS
Our ratio of earnings to fixed charges and preferred stock dividends for each of the periods indicated as follows:
| Fiscal Year Ended December 31, | Six Months Ended June 30, | |||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||
Ratio of earnings to fixed charges and preferred stock dividends(1) | — | — | — | — | — | — | — | |||||||
26
| (1) | For the fiscal years ended December 31, 1999, 2000, 2001, 2002 and 2003, and for the six months ended June 30, 2003 and 2004, earnings were insufficient to cover fixed charges by $6.9 million, $12.9 million, $19.5 million, $19.5 million, $18.5 million, $9.2 million and $24.4 million, respectively. For this reason, no ratios are provided. |
CapitalizationPRICE RANGE OF COMMON STOCK
Our common stock began trading March 16, 2004 and is traded on the Nasdaq National Market under the symbol “XCYT.” The following table sets forth, for the calendar periods indicated, the high and low sale prices per share of the common stock as reported on the Nasdaq National Market: We will make an application to list the convertible preferred stock on the Nasdaq National Market under the symbol “XCYTP.”
| High | Low | |||||
2004 | ||||||
First Quarter (Beginning March 16, 2004) | $ | 8.50 | $ | 6.51 | ||
Second Quarter | $ | 7.45 | $ | 4.00 | ||
Third Quarter | $ | 5.04 | $ | 2.99 | ||
Fourth Quarter (through October 6, 2004) | $ | 3.70 | $ | 3.17 | ||
The following table sets forth our cash, cash equivalents and short term investments and capitalization as of June 30, 2003:2004:
| on an actual basis; |
| on |
| As of June 30, 2003 | ||||||||||||
| Actual | Pro forma | Pro forma as adjusted | ||||||||||
| (in thousands, except share and per share data) | ||||||||||||
Cash, cash equivalents and short-term investments | $ | 8,892 | $ | 22,502 | $ | |||||||
Long-term obligations, less current portion | 1,477 | 1,477 | 1,477 | |||||||||
Redeemable convertible preferred stock; 37,300,234 shares issued and outstanding, actual; no shares issued and outstanding, pro forma and pro forma as adjusted; $76,520 aggregate preference in liquidation, actual | 64,604 | — | — | |||||||||
Redeemable convertible preferred stock warrants | 1,069 | — | — | |||||||||
Stockholders’ equity (deficit): | ||||||||||||
Preferred stock, $0.001 par value per share; 42,000,000 shares authorized, actual; 5,000,000 shares authorized, pro forma and pro forma as adjusted; no shares issued and outstanding, actual, pro forma and pro forma as adjusted | — | — | — | |||||||||
Common stock, par value $0.001 per share; 70,000,000 shares authorized, actual; 100,000,000 shares authorized, pro forma and pro forma as adjusted; 8,402,636 shares issued and outstanding, actual; 45,702,870 shares issued and outstanding, pro forma; 58,432,844 shares issued and outstanding, pro forma as adjusted | 8 | 58 | ||||||||||
Additional paid-in capital | 21,963 | 100,307 | ||||||||||
Deferred stock compensation | (1,236 | ) | (1,236 | ) | ||||||||
Accumulated other comprehensive income | 3 | 3 | 3 | |||||||||
Deficit accumulated during the development stage | (77,327 | ) | (89,175 | ) | (89,175 | ) | ||||||
Total stockholders’ equity (deficit) | (56,589 | ) | 9,957 | |||||||||
Total capitalization | $ | 10,561 | $ | (1,287 | ) | $ | ||||||
27
Capitalization
| As of June 30, 2004 | ||||||||
| Actual | As adjusted | |||||||
(unaudited, in thousands, data) | ||||||||
Cash, cash equivalents and short-term investments | $ | 33,730 | $ | 47,315 | ||||
Long-term obligations, less current portion | $ | 2,594 | $ | 2,594 | ||||
Stockholders’ equity: | ||||||||
Preferred stock, $0.001 par value per share; 5,000,000 shares authorized; no shares issued, actual; 1,725,000 shares designated % convertible exchangeable preferred stock, as adjusted; 1,500,000 shares issued and outstanding, as adjusted | — | 2 | ||||||
Common stock, par value $0.001 per share; 70,000,000 | 15 | 15 | ||||||
Additional paid-in capital | 146,511 | 160,094 | ||||||
Deferred stock compensation | (2,404 | ) | (2,404 | ) | ||||
Accumulated other comprehensive loss | (60 | ) | (60 | ) | ||||
Deficit accumulated during the development stage | (110,965 | ) | (110,965 | ) | ||||
Total stockholders’ equity | 33,097 | 46,682 | ||||||
Total capitalization | $ | 35,691 | $ | 49,276 | ||||
The table above should be read in conjunction with our financial statements and related notes included in this prospectus. This table is based on 8,402,63614,826,573 shares of our common stock outstanding as of June 30, 20032004 and excludes:excludes the following:
| · | 46,607 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2004 at a weighted average exercise price of $7.94 per share; |
| · | 933,045 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2004 under our 1996 Stock Option Plan at a weighted average exercise price of |
28
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share you pay in this offering and the net tangible book value per share of our common stock immediately after this offering. Our net tangible book value as of June 30, 2003 was approximately $56.6 million, or $(6.73) per share of common stock. Net tangible book value per share is equal to our total tangible assets minus total liabilities, all divided by the number of shares of common stock outstanding as of June 30, 2003. Our pro forma net tangible book value per share as of June 30, 2003 was approximately $10.8 million, or $0.18 per share of common stock. Pro forma net tangible book value per share gives effect to:
After giving effect to the sale of the shares of common stock we are offering at an assumed initial public offering price of $ per share, after deducting underwriting discounts and commissions and our estimated offering expenses, our pro forma as adjusted net tangible book value would have been approximately $ million, or $ per share of common stock. This represents an immediate increase in pro forma net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors. The following table illustrates this calculation on a per share basis:
Assumed initial public offering price per share | $ | ||||||
Net tangible book value per share as of June 30, 2003 | $ | (6.73 | ) | ||||
Increase attributable to the conversion or exercise of our convertible preferred stock, exercise and conversion of warrants and issuance and conversion of convertible promissory notes | 6.91 | ||||||
Pro forma net tangible book value as of June 30, 2003 | .18 | ||||||
Pro forma increase per share attributable to the offering | |||||||
Pro forma as adjusted net tangible book value per share after this offering | |||||||
Pro forma dilution per share to new investors | $ | ||||||
If the underwriters exercise their over-allotment option in full, pro forma as adjusted net tangible book value will increase to $ per share, representing an increase to existing stockholders of $ per share, and there will be an immediate dilution of $ per share to new investors.
29
Dilution
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2003, after giving effect to this offering and the pro forma adjustments referred to above, the total number of shares of our common stock purchased from us and the total consideration and average price per share paid by existing stockholders and by new investors:
| Total shares | Total consideration | Average | ||||||||||||
| Number | % | Amount | % | |||||||||||
Existing stockholders | 58,483,844 | $ | 89,845,000 | $ | 1.54 | |||||||||
New investors | ||||||||||||||
Total | 100.0 | % | $ | 100.0 | % | |||||||||
If the underwriters exercise their over-allotment option in full, the following will occur:
The tables and calculations above are based on 8,402,636 shares of our common stock outstanding as of June 30, 2003 and exclude:
The exercise of outstanding options and warrants having an exercise price less than the offering price will increase dilution to new investors.
30
Selected financial dataSELECTED FINANCIAL DATA
This section presents our historical financial data. The following should be read with, and is qualified in its entirety by reference to, the financial statements included in this prospectus, including the notes to the financial statements, and the information under “Management’s discussionDiscussion and analysisAnalysis of financial conditionFinancial Condition and resultsResults of operations.Operations.” The statementsstatement of operations data for the years ended December 31, 2000, 2001, 2002 and 20022003 and the balance sheet data as of December 31, 20012002 and 20022003 have been derived from our audited financial statements included elsewhere in this prospectus. The statementsstatement of operations data for the years ended December 31, 19981999 and 19992000 and the balance sheet data as of December 31, 1998, 1999, 2000 and 20002001 have been derived from our audited financial statements that are not included in this prospectus. The statement of operations data for the six-month periods ended June 30, 20022003 and 20032004 and the balance sheet data as of June 30, 20032004 have been derived from our unaudited condensed financial statements included elsewhere in this prospectus. The unaudited condensed financial statements have been prepared on a basis consistent with that of our audited financial statements and include all adjustments we consider necessary for the fair presentation of the information. Operating results for the six months ended June 30, 20032004 are not necessarily indicative of the results that may be expected for the entire year ending December 31, 2003.2004.
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| Statement of operations data | 1998 | 1999 | 2000 | 2001 | 2002 | 2002 | 2003 | |||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||||||||||
Revenue: | ||||||||||||||||||||||||||||
Collaborative agreement | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 72 | ||||||||||||||
Government grant | — | 16 | 98 | 30 | — | — | — | |||||||||||||||||||||
Total revenue | — | 16 | 98 | 30 | — | — | 72 | |||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 4,317 | 5,471 | 11,257 | 14,701 | 14,663 | 7,651 | 7,029 | |||||||||||||||||||||
General and administrative | 1,427 | 1,654 | 2,403 | 5,204 | 4,979 | 2,883 | 2,194 | |||||||||||||||||||||
Total operating expenses | 5,744 | 7,125 | 13,660 | 19,905 | 19,642 | 10,534 | 9,223 | |||||||||||||||||||||
Loss from operations | (5,744 | ) | (7,109 | ) | (13,562 | ) | (19,875 | ) | (19,642 | ) | (10,534 | ) | (9,151 | ) | ||||||||||||||
Other income (expense), net | 298 | 162 | 621 | 363 | 189 | 122 | (38 | ) | ||||||||||||||||||||
Net loss | (5,446 | ) | (6,947 | ) | (12,941 | ) | (19,512 | ) | (19,453 | ) | (10,412 | ) | (9,189 | ) | ||||||||||||||
Accretion of preferred stock | — | — | — | (8,411 | ) | (8,001 | ) | (8,001 | ) | — | ||||||||||||||||||
Net loss applicable to common stockholders | $ | (5,446 | ) | $ | (6,947 | ) | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,413 | ) | $ | (9,189 | ) | |||||||
Basic and diluted net loss per common share | $ | (0.86 | ) | $ | (1.15 | ) | $ | (2.16 | ) | $ | (4.03 | ) | $ | (3.52 | ) | $ | (2.45 | ) | $ | (1.13 | ) | |||||||
Shares used in basic and diluted net loss per common share calculation | 6,355 | 6,050 | 6,003 | 6,936 | 7,809 | 7,523 | 8,144 | |||||||||||||||||||||
Pro forma net loss per common share (unaudited)(1) | $ | (0.44 | ) | $ | (0.20 | ) | ||||||||||||||||||||||
Shares used in pro forma net loss per common share calculation (unaudited)(1) | 44,550 | 45,427 | ||||||||||||||||||||||||||
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||
| (in thousands, except per share data) | (unaudited) | |||||||||||||||||||||||||||
Statement of Operations Data | ||||||||||||||||||||||||||||
Revenue: | ||||||||||||||||||||||||||||
License fee | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 12 | ||||||||||||||
Collaborative agreement | — | — | — | — | 170 | 72 | 24 | |||||||||||||||||||||
Government grant | 16 | 98 | 30 | — | — | — | — | |||||||||||||||||||||
Total revenue | 16 | 98 | 30 | — | 170 | 72 | 36 | |||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 5,471 | 11,257 | 14,701 | 14,663 | 13,685 | 7,029 | 8,601 | |||||||||||||||||||||
General and administrative | 1,654 | 2,403 | 5,204 | 4,979 | 4,322 | 2,194 | 3,297 | |||||||||||||||||||||
Total operating expenses | 7,125 | 13,660 | 19,905 | 19,642 | 18,007 | 9,223 | 11,898 | |||||||||||||||||||||
Loss from operations | (7,109 | ) | (13,562 | ) | (19,875 | ) | (19,642 | ) | (17,837 | ) | (9,151 | ) | (11,862 | ) | ||||||||||||||
Other income (expense), net | 162 | 621 | 363 | 189 | (620 | ) | (38 | ) | (12,508 | ) | ||||||||||||||||||
Net loss | (6,947 | ) | (12,941 | ) | (19,512 | ) | (19,453 | ) | (18,457 | ) | (9,189 | ) | (24,370 | ) | ||||||||||||||
Accretion of preferred stock | — | — | (8,411 | ) | (8,001 | ) | — | — | (8,973 | ) | ||||||||||||||||||
Net loss applicable to common stockholders | $ | (6,947 | ) | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,457 | ) | $ | (9,189 | ) | $ | (33,343 | ) | |||||||
Basic and diluted net loss per common share | $ | (6.32 | ) | $ | (11,86 | ) | $ | (22.14 | ) | $ | (19.34 | ) | $ | (12.40 | ) | $ | (6.21 | ) | $ | (3.66 | ) | |||||||
Shares used in basic and diluted net loss per common share calculation | 1,100 | 1,091 | 1,261 | 1,420 | 1,488 | 1,481 | 9,107 | |||||||||||||||||||||
31
| As of December 31, | As of June 30, 2004 | |||||||||||||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||||||||||||
| (in thousands) | (unaudited) | |||||||||||||||||||||||
Balance Sheet Data | ||||||||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 7,363 | $ | 23,926 | $ | 21,098 | $ | 17,344 | $ | 13,540 | $ | 33,730 | ||||||||||||
Working capital | 6,100 | 21,785 | 19,135 | 15,570 | (653 | ) | 30,838 | |||||||||||||||||
Total assets | 10,055 | 28,479 | 24,727 | 21,434 | 18,498 | 39,860 | ||||||||||||||||||
Long-term obligations, less current portion | 854 | 952 | 1,046 | 1,514 | 1,555 | 2,594 | ||||||||||||||||||
Redeemable convertible preferred stock and warrants | 23,405 | 49,053 | 57,629 | 65,673 | 67,071 | — | ||||||||||||||||||
Deficit accumulated during the development stage | (16,232 | ) | (29,173 | ) | (48,685 | ) | (68,138 | ) | (86,595 | ) | (110,965 | ) | ||||||||||||
Total stockholders’ equity (deficit) | (15,804 | ) | (25,384 | ) | (36,260 | ) | (48,125 | ) | (64,840 | ) | 33,097 | |||||||||||||
Selected financial dataMANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
| As of December 31, | As of June 30, | |||||||||||||||||||||||||
Balance sheets data | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 12,152 | $ | 7,363 | $ | 23,926 | $ | 21,098 | $ | 17,344 | $ | 8,892 | ||||||||||||||
Working capital | 11,589 | 6,100 | 21,785 | 19,135 | 15,570 | 7,252 | ||||||||||||||||||||
Total assets | 16,044 | 10,055 | 28,479 | 24,727 | 21,536 | 12,695 | ||||||||||||||||||||
Long-term obligations, less current portion | 941 | 854 | 952 | 1,046 | 1,514 | 1,477 | ||||||||||||||||||||
Redeemable convertible preferred stock and warrants | 23,390 | 23,405 | 49,053 | 57,629 | 65,673 | 65,673 | ||||||||||||||||||||
Deficit accumulated during the development stage | (9,285 | ) | (16,232 | ) | (29,173 | ) | (48,685 | ) | (68,138 | ) | (77,327 | ) | ||||||||||||||
Total stockholders’ deficit | (8,939 | ) | (15,804 | ) | (25,384 | ) | (36,260 | ) | (48,125 | ) | (56,589 | ) | ||||||||||||||
32
Management’s discussion and analysis of financial condition and results of operationsAND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes to those financial statements included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under “Risk factors”Factors” and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
OVERVIEWOverview
We are a biotechnology company developing a new class of therapeutic products thatdesigned to enhance the body’s natural immune responses to treat cancer, infectious diseases and other medical conditions associated with weakened immune systems. We producederive our therapeutic products which consist of activated, patient-specificfrom a patient’s own T cells, usingwhich are cells of the immune system that orchestrate immune responses and can detect and eliminate cancer cells and infected cells in the body. We use our patented and proprietary Xcellerate Technology. We use blood collected from the patientTechnology to generate activated T cells, which we call Xcellerated T Cells.Cells, from blood that is collected from the patient. Activated T cells are T cells that have been stimulated to carry out immune functions. Our Xcellerate Technology is designed to rapidly activatesactivate and expandsexpand the patient’s T cells outside of the body. These Xcellerated T Cells are subsequentlythen administered to the patient. We believe, based on clinical trials to date, that our Xcellerate Technology can produce Xcellerated T Cells in sufficient numbers to generate rapid and potent immune responses to treat a variety of medical conditions.
Since our inception in 1996, we have focused our activities primarily on the development of a new class ofthese therapeutic products that enhance the body’s natural immune responses for the treatment of cancer, infectious diseases and other medical conditions associated with weakened immune systems.products. We are a development-stage company and have incurred significant losses since our inception. As of June 30, 2003,2004, our deficit accumulated during the development stage was $77.3$111.0 million. Our operating expenses consist of research and development expenses and general and administrative expenses.
We have recognized revenues from inception through June 30, 20032004 of approximately $316,000$450,000 from sublicenselicense fees, payments under a collaborative agreement and income from a National Institutes of Health Phase I Small Business Innovation Research, or SBIR, grant in CLL. In the third quarter of 2003, the National Institutes of Health awarded us a $1.2 million SBIR grant in the same indication. We intend to continue to apply for other grants in the future.chronic lymphocytic leukemia. We currently do not market any products and will not for several years, if at all. Accordingly, we do not expect to have any product sales or royalty revenue for a number of years. Our net losses are primarily a result of research and development and general and administrative expenses incurred to support our operations. We anticipate incurring net losses over at least the next several years as we complete our clinical trials, apply for regulatory approvals, continue development of our technology and expand our operations.
Research and developmentDevelopment
To date, our research and development expenses have consisted primarily of costs incurred for drug discovery and research, preclinical development, clinical trials and regulatory activities. Research and development activity-related costs include:
| payroll and personnel-related expenses; |
| clinical trial and regulatory-related costs; |
| laboratory supplies; |
33
Management’s discussion and analysis of financial condition and results of operations
| contractual costs associated with developing antibodies and beads; |
| technology license costs; |
| rent and facility expenses for our laboratory and |
| scientific consulting fees. |
Our research and development efforts to date have primarily focused on the development of our proprietary Xcellerate Technology and Xcellerated T Cells. From inception through June 30, 2003,2004, we incurred research and development expenses of approximately $60.2$75.4 million, substantially all of which relate to the research and development of this technology. Currently, we are focusing our efforts on advancing our product through clinical trials. Because of the risks and uncertainties inherent in the clinical trials and regulatory process, we are unable to estimate with any certainty the length of time or expenses to continue development of Xcellerated T Cells for commercialization. However, we expect our research and development expenses to increase as we continue to improve our proprietary Xcellerate Technology and develop Xcellerated T Cells for additional clinical indications for Xcellerated T Cells.indications.
General and administrative expensesAdministrative Expenses
GeneralOur general and administrative expenses are costs associated with supporting our operations, including payroll and personnel-related expenses and professional fees. In addition, rent and facility expenses for our administrative office area and other general office support activities are also included in our general and administrative expenses.
CRITICAL ACCOUNTING POLICIESCritical Accounting Policies
We have based our discussion and analysis of our financial condition and results of operations on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from those estimates. While noteNote 1 to our financial statements summarizes each of our significant accounting policies that we believe is important to the presentation of our financial statements, we believe the following accounting policies to be critical to the estimates and assumptions used in the preparation of our financial statements.
Stock-based compensationStock-Based Compensation
We have adopted the disclosure-only provisions of Financial Accounting Standards Board (FASB) Statement of Financial Accounting Standards No. 123,Accounting for Stock-Based Compensation(SFAS 123). Accordingly, we apply Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees(APB 25), and related interpretations in accounting for stock options. Pursuant to APB 25, we recognize employee stock-based compensation expense based on the intrinsic value of the option at the date of grant. Deferred stock-based compensation includes amounts recorded when the exercise price of an option is lower than the fair value of the underlying common stock on the date of grant. We amortize deferred stock-based compensation over the vesting period of the underlying option using the graded-vestinggraded vesting method.
We record stock options granted to non-employees using the fair value approach in accordance with SFAS 123 and Emerging Issues Task Force Consensus Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. We periodically revalue the options to non-employees over their vesting terms. We determine the fair value of options granted to non-employees using the Black-Scholes option-pricing model.
34
Management’s discussion and analysis of financial condition and results of operations
We determinePrior to our initial public offering, we determined the fair value of our common stock for purposes of these calculations based on our review of the primary business factors underlying the value of our common stock on the date these option grants arewere made or revalued, viewed in light of thisour initial public offering and the expected initial public offering price per share. Subsequent to our initial public offering, the fair value is determined based on the price of the common stock as reported by the Nasdaq National Market inThe Wall Street Journal.
Revenue recognitionRecognition
To date, we have generated no revenues from sales of products. Revenues relate to fees received for licensed technology, cost reimbursement contracts and ana SBIR grant awarded to us by the National Institutes of Health.
We recognize revenue associated with up-front license fees and research and development funding payments ratably over the relevant periods specified in the agreement, which generally is the research and development period.period we are obligated to perform services. We recognize revenue under research and development cost-reimbursement agreements as the related costs are incurred. We recognize revenue related to grant agreements as the related research and development expenses are incurred.
Cash, cash equivalentsCash Equivalents and investmentsInvestments
We classify all investment securities as available-for-sale, carried at fair value. We report unrealized gains and losses as a separate component of stockholders’ deficit.equity (deficit). We include amortization, accretion, interest and dividends, realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities in interest income. Statement of Financial Accounting Standards No. 115,Accounting for Certain Investments in Debt and Equity Securities, and Securities and Exchange Commission (SEC) Staff Accounting Bulletin (SAB) 59,Accounting for Noncurrent Marketable Equity Securities, provide guidance on determining when an investment is other-than-temporarily impaired. This evaluation depends on the specific facts and circumstances. Factors that we consider in determining whether an other-than-temporary decline in value has occurred include: the market value of the security in relation to its cost basis; the financial condition of the investee; and the intent and ability to retain the investment for a sufficient period of time to allow for possible recovery in the market value of the investment.
RESULTS OF OPERATIONSResults of Operations
Six months endedMonths Ended June 30, 20032004 and 20022003
Revenue
Revenue was approximately $36,000 and $72,000 infor the six months ended June 30, 2004 and 2003, respectively. This consisted of revenue recognized related to the amortization of license fees received and reimbursements of our costs incurred under a collaboration agreement.
Research and Development
Research and development expenses represented approximately 72% and 76% of our operating expenses for the six months ended June 30, 2004 and 2003, respectively. Research and development expenses increased 22%, from $7.0 million for the six months ended June 30, 2003 to $8.6 million for the six months ended June 30, 2004. The increase was primarily the result of amounts charged to expense for contractual obligations relating to developing our bead technology, in addition to increases in clinical trial costs, laboratory supplies, salary and consistedother personnel-related expenses and non-cash stock compensation expense. Expenses associated with developing our bead technology totaled $500,000 for the six months ended June 30, 2004, with no such costs incurred for the six months ended June 30, 2003. Clinical trial and laboratory supplies costs have increased as we continue to advance and expand our clinical testing. As of June 30, 2004 we had 71 employees in research and development and manufacturing operations compared to 53 employees in research and development and manufacturing operations as of June 30, 2003. In addition, our non-cash stock compensation expense increased from $399,000 for the six months ended June 30, 2003 to $603,000 for the six months ended June 30, 2004. These increases were partially offset by a reduction of $1.2 million in contractual payments relating to developing our antibody technology. The higher level of expense in the first half of 2003, related to our antibody technology, resulted from obligations to the third-party manufacturer of the antibodies that we use in our Xcellerate Technology. Since we store these antibodies in our inventory for use when needed in clinical trials and research and development activities, the manufacture of these antibodies occurs periodically, resulting in a corresponding increase in expense from time to time. We anticipate that research and development expenses will continue to grow in the foreseeable future as we expand our research, development and clinical trial activities.
General and Administrative
General and administrative expenses represented approximately 28% and 24% of our operating expenses for the six months ended June 30, 2004 and 2003, respectively. General and administrative expenses increased 50%,
from $2.2 million for the six months ended June 30, 2003 to $3.3 million for the six months ended June 30, 2004. The rise was due primarily to increases in professional fees, insurance costs, salary and other personnel-related expenses and non-cash stock compensation expense. Non-cash stock compensation expense increased from $326,000 for the six months ended June 30, 2003 to $614,000 for the six months ended June 30, 2004. We anticipate that general and administrative expenses will increase in the foreseeable future as we support our growth and incur costs related to being a public company.
Other Income (Expense)
Other expense, comprised primarily of interest expense and interest income, totaled $38,000 for the six months ended June 30, 2003, compared to $12.5 million for the six months ended June 30, 2004. Interest income increased 57%, from $94,000 for the six months ended June 30, 2003 to $148,000 for the six months ended June 30, 2004, due to increased average cash and investment balances upon which interest is earned. Interest expense increased from $131,000 for the six months ended June 30, 2003 to $12.7 million for the six months ended June 30, 2004, due to interest expense associated with the convertible promissory notes issued in October 2003. Upon consummation of our initial public offering and conversion of the notes to common stock, we recognized $11.3 million in interest expense, which represented the beneficial conversion feature of the notes. We also recognized an additional $1.1 million in interest expense associated with the discount on the notes, representing the value of the proceeds allocated to the warrants received by the note holders.
Accretion of Preferred Stock
For the six months ended June 30, 2004, we recognized $9.0 million in accretion of preferred stock to arrive at our net loss applicable to common stockholders. No such accretion was recognized for the six months ended June 30, 2003. This accretion represented the remaining discount associated with our Series E and F preferred stock, which was recognized when the preferred stock was converted into common stock upon the closing of our initial public offering.
Years Ended December 31, 2003 and 2002
Revenue
Revenue was approximately $170,000 in the year ended December 31, 2003, consisting of funds received under a cost-reimbursement agreement. We recognized no revenue in the six monthsyear ended June 30,December 31, 2002.
Research and developmentDevelopment
Research and development expenses represented approximately 76% and 73%75% of our operating expenses for the six monthsyears ended June 30,December 31, 2003 and 2002, respectively. Research and development expenses decreased 8.1%6.7%, from $7.7$14.7 million in the six monthsyear ended June 30,December 31, 2002 to $7.0$13.7 million in the six monthsyear ended June 30,December 31, 2003. The decrease was primarily due to a reduction in technology license costs, contractual payments relating to developing our bead technology and non-cash stock compensation.compensation expense. Technology license costs totaled $729,000$829,000 in the six monthsyear ended June 30,December 31, 2002, representing the value of stock and cash paid as an initial fee for a license we obtained from an academic institution. We incurred no technology license costs in the six monthsyear ended June 30,December 31, 2003. Expenses associated with developing our bead technology totaled $500,000 in 2002, with no such costs incurred in 2003. Non-cash stock compensation expense decreased from $661,000$1.3 million in the six monthsyear ended June 30,December 31, 2002 to $399,000$884,000 in the six monthsyear ended June 30, 2003.December 31, 2003, as a result of a reduction in the number of options granted. Decreases in research and development expenses were
35
Management’s discussion and analysis of financial condition and results of operations
partially offset by an increase of $446,000$220,000 in contractual payments relating to developing our antibody technology, in addition to increases in clinical trial and laboratory supplies costs. The increase in payments related to our antibody technology resulted from the third-party manufacture of the antibodies that we use in our Xcellerate Technology. Since we store these antibodies in our inventory for use when needed in clinical trials and research and development activities, the manufacture of these antibodies occurs periodically, resulting in a corresponding increase in expense from time to time.
General and administrativeAdministrative
General and administrative expenses represented approximately 24% and 27% or25% of our operating expenses for the six monthsyears ended June 30,December 31, 2003 and 2002, respectively. General and administrative expenses decreased 24%13.2%, from $2.9$5.0 million in the six monthsyear ended June 30,December 31, 2002 to $2.2$4.3 million in the six monthsyear ended June 30, 2003,December 31, 2003. The decrease was due primarily to a decrease in non-cash stock compensation expense and the absence of expenses related to an initial public offering registration process that we initiated and terminated in the first half of 2002. Non-cash stock compensation expense decreased 62%40%, from $862,000$1.3 million in the six monthsyear ended June 30,December 31, 2002 to $326,000$783,000 in the six monthsyear ended June 30, 2003.December 31, 2003, as a result of a reduction in the number of options granted. Costs we incurred in association with the initial public offering registration process in the six monthsyear ended June 30,December 31, 2002 totaled $272,000.
Other income (expense)Income (Expense)
Other income, comprised primarily of interest income and interest expense, totaled $122,000$189,000 in the six monthsyear ended June 30,December 31, 2002, compared to other expense of $38,000$620,000 in the six monthsyear ended June 30,December 31, 2003. Interest income decreased 62%68%, from $247,000$467,000 in the six monthsyear ended June 30,December 31, 2002 to $94,000$149,000 in the six monthsyear ended June 30,December 31, 2003, due to decreased cash and investment balances upon which interest is earned and declining interest rates. Interest expense increased 6.5%,188% from $123,000$267,000 in the six monthsyear ended June 30,December 31, 2002 to $131,000$768,000 in the six monthsyear ended June 30,December 31, 2003, due primarily to higher debt balances related to equipment financings.interest expense associated with the convertible promissory notes issued in October 2003.
Years endedEnded December 31, 2002 and 2001
Revenue
Revenue was approximately $30,000 in the year ended December 31, 2001, consisting of income from a National Institutes of Health SBIR grant. We recognized no revenue in the year ended December 31, 2002.
Research and developmentDevelopment
Research and development expenses represented approximately 75% and 74% of our operating expenses for the years ended December 31, 2002 and 2001, respectively. Research and development expenses totaled $14.7 million in each of the years ended December 31, 2002 and 2001. Increases inWhile total expenses were the same for 2002 and 2001, several individual components of research and development expenses were primarily due to technologyexpense fluctuated significantly between the years. Technology license costs, contractual payments relating to developing our bead technology and an increase in salary and other personnel-related expenses.expenses increased from 2001 to 2002. Technology license costs comprised the largest increase and totaled $829,000 in the year ended December 31, 2002, representing the value of stock and cash paid for a license we obtained from an academic institution. We incurred no technology license costs in the year ended December 31, 2001. Increases in research and development expensesThese increases were offset by a reduction of $1.1 million in contractual payments relating to developing our antibody technology, in addition to reduced non-cash compensation expense. The higher level of payments in 2001 related to our antibody technology resulted from the third-party manufacture of the antibodies that we use in our Xcellerate Technology. Since we store these antibodies in our inventory for use when needed in clinical trials and research and development activities, the manufacture of these antibodies occurs periodically, resulting in a corresponding increase in expense from time to time. The reduction in non-cash compensation expense resulted primarily from a decrease in management’s estimate of the fair market value per share of common stock.
General and administrativeAdministrative
General and administrative expenses represented approximately 25% and 26% or our operating expenses for the years ended December 31, 2002 and 2001, respectively. General and administrative expenses decreased 4.3%, from $5.2 million in the year ended December 31, 2001 to $5.0 million in the year ended December 31, 2002. The decrease was due primarily to an $880,000 reduction in
36
Management’s discussion and analysis of financial condition and results of operations
professional fees related to an initial public offering that we withdrew in 2001, partially offset by a $351,000 increase in non-cash stock compensation and increases
in salary and other personnel-related expenses. The increase in non-cash stock compensation resulted from an increase in the number of options granted.
Other income (expense)Income (Expense)
Other income, comprised primarily of interest income and interest expense, decreased 48%, from $363,000 in the year ended December 31, 2001 to $189,000 in the year ended December 31, 2002. Interest income decreased 33%, from $698,000 in the year ended December 31, 2001 to $467,000 in the year ended December 31, 2002, due to decreased cash and investment balances upon which interest is earned and declining interest rates. Interest expense increased 2.7%, from $260,000 in the year ended December 31, 2001 to $267,000 in the year ended December 31, 2002, due primarily to higher debt balances related to equipment financings.
Years ended December 31, 2001 and 2000Quarterly Financial Data
For information relating to our quarterly financial data, see Note 13 “Quarterly Financial Data” in our financial statements included elsewhere in this prospectus.
RevenueStock-Based Compensation
Revenue was approximately $30,000 in the year ended December 31, 2001 and $98,000 in the year ended December 31, 2000 and consisted of income from a National Institutes of Health SBIR grant.
Research and development
Research and development expenses represented approximately 74% and 82% of our operating expenses forDuring the years ended December 31, 2003, 2002 and 2001, and 2000, respectively. Research and development expenses increased 31%, from $11.3we recorded deferred stock-based compensation totaling $2.4 million, in the year ended December 31, 2000 to $14.7$3.2 million in the year ended December 31, 2001. The $3.4 million increase was primarily due to contractual payments relating to developing our antibody technology, an increase in non-cash stock compensation, increased rent and facilities-related expenses and laboratory supplies. Contractual payments relating to developing our antibody technology increased $1.7 million, non-cash stock compensation increased $1.2 million and rent and facilities-related expenses increased $1.1 million. Increases in research and development expenses were partially offset by a $1.4 million reduction in contractual payments relating to developing our bead technology, in addition to a decrease in technology license costs.
General and administrative
General and administrative expenses represented approximately 26% and 18% or our operating expenses for the years ended December 31, 2001 and 2000, respectively. General and administrative expenses increased 117%, from $2.4 million in the year ended December 31, 2000 to $5.2 million in the year ended December 31, 2001, due primarily to $1.1 million in expenses related to an initial public offering that we subsequently withdrew, in addition to increases in non-cash stock compensation and salary and other personnel related expenses.
Other income (expense)
Other income, comprised primarily of interest income and interest expense, decreased 42%, from $621,000 in the year ended December 31, 2000 to $363,000 in the year ended December 31, 2001. Interest income decreased 20%, from $868,000 in the year ended December 31, 2000 to $698,000 in the year ended December 31, 2001, due to decreased cash balance upon which interest is earned and declining interest rates. Interest expense increased 5.3%, from $247,000 in the year ended December 31, 2000 to $260,000 in the year ended December 31, 2001, due primarily to higher debt balances related to equipment financings.
37
Management’s discussion and analysis of financial condition and results of operations
STOCK-BASED COMPENSATION
During the six months ended June 30, 2003,2004, we recorded deferred stock-based compensation totaling $86,000. During the years ended December 31, 2001 and 2002, we recorded deferred stock-based compensation totaling $1.7 million and $3.2 million, respectively.$811,000. We amortize the deferred stock-based compensation to expense onusing the graded vesting method. As of June 30, 2003,2004, there was approximately $1.2$2.4 million of deferred stock-based compensation to be amortized in future periods as follows: $442,000$978,000 for the six months ending December 31, 2003, $513,000 in 2004, $217,000$942,000 in 2005, $62,000$397,000 in 2006 and $1,000$86,000 in 2007. After June 30,During the years ended December 31, 2003, 2002 and 2001, we issued to employees additionalgranted non-employee stock options and warrants to purchase 886,50024,543, 6,363 and 71,814 shares of our common stock, at a weighted average exercise price of $1.00 per share. As a result, we expect to amortize an additional $2.0 million in deferred stock-based compensation in future periods as follows: $295,000 for the six months ending December 31, 2003, $1.0 million in 2004, $426,000 in 2005, $196,000 in 2006 and $42,000 in 2007. In 2001 and 2002, we granted non-employeerespectively. No such stock options to purchase 395,000 and 35,000 shares of common stock, respectively. Duringor warrants were granted during the six months ended June 30, 2003, we issued warrants to non-employees to purchase 75,000 shares of our common stock.2004. We determined the fair value of options and warrants granted to non-employees using the Black-Scholes option-pricing model. We will periodically measure this value as the underlying options vest. Total stock-based compensation expense for non-employees was $360,000, $65,000 and $1.1 million and $65,000 for the years ended December 31, 2003, 2002 and 2001, and 2002, respectively, and $130,000respectively. Total stock-based compensation expense for non-employees was $39,000 for the six months ended June 30, 2003.2004.
INCOME TAXESIncome Taxes
We have incurred net operating losses since inception, and we have consequently not paid any federal, state or foreign income taxes. OnAs of December 31, 2002,2003, we had net operating loss carryforwards of approximately $55.9$74 million and research and development tax credit carryforwards of approximately $3.0$3.2 million. If not utilized, the net operating loss and tax credit carryforwards will expire at various dates beginning in 2011. If we do not achieve profitability, our net operating loss carryforwards may be lost. In addition, the change-in-ownership provisions as specified under Section 382 of the Internal Revenue Code of 1986, as amended, may substantially limit utilization of net operating loss and tax credit carryforwards annually. We are currently not subject to these limitations. However, any future annual limitations may result in the expiration of our net operating loss and tax credit carryforwards before utilization.
DeferredOur deferred tax assets consist primarily of net operating loss carryforwards. Because of our history of operating losses, we do not have a sufficient basis to project that future income will be sufficient to realize the deferred tax assets during the carryforward period. As a result, we have provided a full valuation allowance on the net deferred tax assets for all periods presented. The valuation allowance has increased each fiscal year primarily due to that fiscal year’s net operating loss carryforward.
LIQUIDITY AND CAPITAL RESOURCESLiquidity and Capital Resources
As of June 30, 2003,2004, we had cash, cash equivalents and short-term investments of $8.9$33.7 million, with cash equivalents being held in highly liquid money market accounts with financial institutions. Cash, cash equivalents and short-term investments were $23.9$13.5 million, on$17.3 million and $21.1 million as of December 31, 2000, $21.1 million on December 31,2003, 2002 and 2001, and $17.3 million on December 31, 2002. respectively.
In October 2003,March 2004, we raised net proceeds of $12.7approximately $29.7 million from the sale of convertible promissory notes. These convertible promissory notes will convert into approximately 7,268,9054,200,000 shares of common stock atin our initial public offering. In connection with the initial public offering, all of our outstanding
shares of redeemable convertible preferred stock and all of our outstanding convertible promissory notes, including interest accrued thereon through the closing date of this offering.the offering, were converted into 6,781,814 and 1,357,357 shares of our common stock, respectively.
We have financed our operations since inception through private and public placements of equity securities, grant revenue, license fees, from a sublicense agreement, payments under a collaborative agreement, equipment financings and interest income earned on cash, cash equivalents and investments. From inception through June 30, 2003,2004, we have raised net proceeds of $75.6 million from private equity financing.financings, $29.7 million from our initial public offering and $12.7 million from the sale of convertible promissory notes. Since our
38
Management’s discussion and analysis of financial condition and results of operations
inception to June 30, 2004, we have received $316,000$450,000 in revenue, $5.5$6.9 million in equipment financings and $3.4$3.6 million in interest income. To date, inflation has not had a material effect on our business.
Since our inception, investing activities, other than purchases and maturities of investments, have consisted primarily of purchases of property and equipment. OnAs of June 30, 2003,2004, our investment in property and equipment was $5.2$7.5 million. We anticipate our capital expenditures will increase in the future as we construct and renovate our planned manufacturing plant and expand our current facilities.
Net cash used in operating activities was $8.3$8.0 million for each of the six monthssix-month periods ended June 30, 20022004 and $8.0 million for the six months ended June 30, 2003. Net cash used in operating activities was $11.2$15.5 million, in$15.2 million and $15.1 million for the yearyears ended December 31, 2000, $15.1 million in the year ended December 31,2003, 2002 and 2001, and $15.2 million in the year ended December 31, 2002.respectively. Expenditures in these periods were generally a result of research and development expenses and general and administrative expenses in support of our operations.
We have entered into agreements to develop bead and antibody technology that requirerequired significant cash expenditures, including an agreement with Dynal under which we have agreed to make payments totaling $3.0 million upon the accomplishment of bead development activities. Additionally, we have two agreements with Lonza under which we have agreed to make payments to develop and produce cGMP-grade antibodies totaling $4.8$6.3 million. As of June 30, 2003,2004, we have paid $2.5the entire $3.0 million to Dynal and the entire $4.8$4.9 million to Lonza. We anticipate that the remaining payments to Lonza will be made in 2005. Under our license agreement with Genetics Institute, we must spend no less than $500,000 annually on research and development activities related to product development until the first commercial sale of a product. Our license agreement with an academic institution also requires us to maintain minimum spending levels on research and development related to product development, as follows: $200,000 in the first year of the agreement, $300,000 in the second year, $400,000 in the third year, $500,000 in the fourth year and $1 million in each subsequent year.
The following summarizes our long-term contractual obligations as of December 31, 20022003 (in thousands):
| Payments due by period | Payments due by period | |||||||||||||||||||||||||||||
Contractual obligations | Total | Less than 1 year | 1 to 3 years | 4 to 5 years | After 5 years | Total | Less than 1 year | 1-3 years | 3-5 years | After 5 years | ||||||||||||||||||||
Operating leases | $ | 10,395 | $ | 1,469 | $ | 3,086 | $ | 2,474 | $ | 3,366 | $ | 9,046 | $ | 1,571 | $ | 3,010 | $ | 2,205 | $ | 2,260 | ||||||||||
Equipment financing | 1,991 | 818 | 1,052 | 121 | — | 1,923 | 845 | 1,052 | 26 | — | ||||||||||||||||||||
Total (1) | $ | 12,386 | $ | 2,287 | $ | 4,138 | $ | 2,595 | $ | 3,366 | $ | 10,969 | $ | 2,416 | $ | 4,062 | $ | 2,231 | $ | 2,260 | ||||||||||
| (1) | Does not include commitments for product development spending under the Genetics Institute |
We have financed the acquisition of laboratory and scientific equipment, furniture and fixtures, computer equipment and leasehold improvements through financing arrangements with General Electric Capital Corporation, Oxford Finance Corporation and Phoenix Leasing Incorporated. In connection with the financings, we have issued common stock warrants to these lenders. At December 31, 2003, we had two financing arrangements. Under the first arrangement, with General Electric Capital Corporation, we could borrow up to $1.7 million. At June 30, 2004, we had $728,000 available under the outstanding arrangement, which was replaced by a new financing agreement with General Electric Capital Corporation in July 2004. This new agreement provides for borrowings up to $3.0 million, and expires in July 2005 unless renewed. Under the second arrangement, with Oxford Finance Corporation, we could borrow up to $2.5 million. At June 30, 2004,
we had $1.7 million available under the outstanding arrangement, which was replaced by a new financing agreement with Oxford Finance Corporation in July 2004. This new agreement provides for borrowings up to $3.0 million, and expires in December 2005 unless renewed. Outstanding borrowings under the current and previous financing arrangements were $1.9 million and $1.8 million at years ended December 31, 2002 and 2003, respectively, and $2.3 million at June 30, 2004. Outstanding borrowings require monthly principal and interest payments and mature at various dates through 2008. Interest rates applicable to the outstanding borrowings at December 31, 2003 range from 9.18% to 14.11%. The weighted average interest rates for borrowings outstanding during the years ended December 31, 2001, 2002 and 2003 and the six months ended June 30, 2004 were 12.66%, 11.09%, 10.27% and 9.72%, respectively. Borrowings are secured by the acquired assets that have a net book value of $2.3 million at December 31, 2003. Under all agreements, we are required to comply with certain nonfinancial covenants.
We expect to use the net proceeds from this offering to fund clinical trial activities, manufacturing activities and preclinical research and development activities and for other general corporate purposes, including capital expenditures, technology acquisitionacquisitions and working capital to fund anticipated operating losses. See “Use of proceeds.Proceeds.”
Based uponon the current status of our product development and collaboration plans, we believe that the net proceeds of this offering, together with our cash, cash equivalents and investments, will be adequate to satisfy our capital needs through at least the next 18 months. However, we may needend of 2005. We will likely seek additional financing prior to that time to, among other things, support our continuing product development, manufacturing and clinical trials for Phase II or Phase III clinical trials.trials in future periods. Furthermore, we expect to require additional funding before we are able to
39
Management’s discussion and analysis of financial condition and results of operations
generate revenue, if at all, from our potential products. Additional financing may not be available on favorable terms, if at all. If we are unable to raise additional funds when we need them, we may have to delay, reduce or eliminate some or all of our development programs and some or all of our clinical trials. We also may have to license our technologies to others, including technologies that we would prefer to develop internally.internally, to raise capital.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONSCertain Relationships and Related Party Transactions
For a description of our related party transactions, see “Certain relationshipsRelationships and related party transactions.Related Party Transactions.”
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2002, the FASB issued SFAS 146,Recent Accounting for Costs Associated with Exit or Disposal Activities, which addresses accounting for restructuring, discontinued operation, plant closing or other exit or disposal activity. SFAS 146 requires companies to recognize costs associated with exit or disposal activities when they are incurred rather than at the date of a commitment to an exit or disposal plan. SFAS 146 is to be applied prospectively to exit or disposal activities initiated after December 31, 2002. We do not expect the adoption of SFAS 146 to have a material impact on our financial position or results of operations.
In November 2002, the FASB issued FIN 45,Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others, and Interpretation of FASB Statements No. 5, 57 and 107 and Rescission of FASB Interpretation No. 34. FIN 45 clarifies the requirements of SFAS 5,Accounting for Contingencies, relating to the guarantor’s accounting for, and disclosure of, the issuance of certain types of guarantees. The disclosure provisions of FIN 45 apply to financial statements for the periods ending after December 15, 2002. However, the provisions for initial recognition and measurement apply on a prospective basis to guarantees that are issued or modified after December 31, 2002. We do not expect the adoption of FIN 45 to have a material impact on our financial position or results of operations.Pronouncements
In January 2003, the FASB issued FIN 46,Consolidation of Variable Interest Entities. FIN 46 clarifies the application of Accounting Research Bulletin No. 51,Consolidated Financial Statements, to entities in which the equity investors do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. FIN 46 applies immediately to variable interest entities created after January 31, 2003 and to variable interest entities in which an enterprise obtains an interest after that date. It applies in the first fiscal year or interim period beginning after June 15, 2003 to variable interest entities in which an enterprise holds a variable interest that it acquired before February 1, 2003. FIN 46 applies to public enterprises as of the beginning of the applicable interim or annual period. We do not believe there will be a material effect on our financial condition or results of operations from the adoption of the provisions of FIN 46.
In November 2002, the Emerging Issues Task Force reached a consensus on Issue No. 00-21,Revenue Arrangements with Multiple Deliverables(EITF Issue No. 00-21). This Issue provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. We are currently evaluating the effect that the adoption of EITF Issue No. 00-21 will have on our financial statements.
40
Management’s discussion and analysis of financial condition and results of operations
In May 2003, the FASB issued SFAS 150,Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity. SFAS 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. SFAS 150 requires that an issuer classify
a financial instrument that is within itsSFAS 150’s scope as a liability by reporting the cumulative effect of a change in accounting principle. The requirements of SFAS 150 apply to the first fiscal period beginning after December 15, 2004. We are currently evaluating the impact of adopting SFAS 150.
Quantitative and Qualitative Disclosures About Market Risk
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest rate riskRate Risk
Our short-term investments atas of June 30, 2003 consist2004 consisted of $18.0 million in corporate bonds, $4.6 million in federal agency obligations, and highly rated corporate$2.3 million in municipal bonds with contractual maturities of one year or less. Due to the short-term nature of our investments, we believe that our exposure to market interest rate fluctuations is minimal. The corporate bonds in which we invest are rated “A” or better by both Moody’s and Standard and Poor’s. Our cash and cash equivalents are held primarily in highly liquid money market accounts. A hypothetical 10% change in short-term interest rates from those in effect at June 30, 20032004 would not have a significant impact on our financial position or on our expected results of operations. We do not currently hold any derivative financial instruments.
Because interest rates on our equipment financing obligations are fixed at the beginning of the repayment term, exposure to changes in interest rates is limited to new financings.
Foreign Currency Risk
We do not engage in foreign currency riskhedging; however, we have entered into certain contracts denominated in foreign currencies and therefore we are subject to currency exchange risks.
For antibody development and supply services provided by Lonza, we must make payments denominated in British pounds. As a result, from time to time, we are exposed to currency exchange risks. We do not engage in currency hedging, and, ifrisks related to the British pound. If the British pound strengthens against the USU.S. dollar, our payments to Lonza will increase in USU.S. dollar terms. AtAssuming development and supply services are completed as scheduled under our agreements with Lonza, our remaining payments will be approximately $1.4 million through the end of 2005. A hypothetical 10% change in the British pound from the rate in effect at June 30, 2003, we2004 would not have no outstanding obligationsa significant impact on our financial position or significant future contractual commitments to Lonza.our expected results of operations.
41The terms of our license agreement with Fresenius include the receipt of potential royalties on net sales as well as potential milestone payments to us denominated in Euro. As a result, we are exposed to currency exchange risks related to the Euro. If the Euro weakens against the U.S. dollar, payments received from Fresenius will decrease in U.S. dollar terms. A hypothetical 10% change in the Euro from the rate in effect at June 30, 2004 would not have a significant impact on our financial position or our expected results of operations.
OVERVIEWOverview
We are a biotechnology company developing a new class of therapeutic products thatdesigned to enhance the body’s natural immune responses to treat cancer, infectious diseases and other medical conditions associated with weakened immune systems. We producederive our therapeutic products which consist of activated, patient-specificfrom a patient’s own T cells, usingwhich are cells of the immune system that orchestrate immune responses and can detect and eliminate cancer cells and infected cells in the body. We use our patented and proprietary Xcellerate Technology. We use blood collected from the patientTechnology to generate activated T cells, which we call Xcellerated T Cells.Cells, from blood that is collected from the patient. Activated T cells are T cells that have been stimulated to carry out immune functions. Our Xcellerate Technology is designed to rapidly activatesactivate and expandsexpand the patient’s T cells outside of the body. These Xcellerated T Cells are subsequentlythen administered to the patient.
We believe, based on clinical trials to date, our Xcellerate Technology can produce Xcellerated T Cells in sufficient numbers to generate rapid and potent immune responses to treat a variety of medical conditions.
We believe In our ongoing clinical studies using our Xcellerate Technology, allows us to consistentlywe have observed an increase in the quantity and restorea restoration of the quality and diversity of T cells fromin patients with weakened immune systems. We have submitted the findings on the increase in quantity of T cells to the FDA and plan to submit additional data in our next annual report. We believe we can efficiently manufacture Xcellerated T Cells for therapeutic applications. In addition, based on clinical studies to date, we believe the Xcellerated T Cells are safe and generally well tolerated and can be easily administered to patients in an outpatient clinical setting. We expect Xcellerated T Cells may be used alone or in combination with other complementary treatments. We and our scientific collaboratorsother clinical investigators have completed or are conducting clinical trials in the following indications:
| Chronic lymphocytic leukemia, or CLL. In our ongoing Phase I/II clinical trial in CLL, treatment with Xcellerated T Cells resulted in a 50% to 100% reduction in the size of enlarged lymph nodes in 12 of 17 (71%) patients for whom data was available as of September 27, 2004. In addition, there was a 50% or greater reduction in spleen size as measured below the rib cage by physical examination in 10 of the 13 patients (77%) with enlarged spleens. These findings were submitted to the FDA in the Information Packet for a Type B End of Phase II meeting held on September 23, 2004. At this meeting we discussed with the FDA our plans for a Phase II/III clinical trial of Xcellerated T Cells in patients with CLL who have been previously treated with chemotherapy and have failed treatment with Campath, an FDA-approved drug used to treat CLL. Based on feedback from the FDA during this meeting, we intend to modify our planned protocol for this Phase II/III clinical trial to provide the FDA with data we believe will address the FDA’s concerns regarding the subcutaneous route of Campath administration and the dose and schedule of Xcellerated T |
| Multiple myeloma. In our ongoing Phase I/II clinical trial, we have shown that treatment with Xcellerated T Cells |
Non-Hodgkin’s lymphoma. In an independent clinical trial, conducted by one of our scientific founders under a physician-sponsored |
Hodgkin’s lymphoma patients undergoing high-dose chemotherapy and autologous stem cell transplantation were treated with T cells activated with an earlier version of our proprietary technology. |
42
Business
In clinical trials, treatment withwe have observed few side effects in most patients. As of September 27, 2004, in over 156 infusions of Xcellerated T Cells, we have had only two serious adverse events reportable to the FDA that were judged as possibly or probably related to the treatment. The first of these was generally well tolerateda rash that resolved following treatment. The second of these was congestive heart failure in a patient with fewpre-existing severe anemia that resolved approximately two hours following treatment. We subsequently amended our protocol to identify patients with anemia prior to administering Xcellerated T Cells. In general, side effects. Side effects were similar to those observed with infusions of other kinds of cells, such as red blood cells or frozen cell products, and typically minor, including fever, chills, increased heart rate, nausea and sweating. Our clinical trials and independent clinical trials using an earlier version of our technology, to date, have involved small numbers of patients, and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
Based on these positive clinical trial results, we believe there are several majorimportant clinical opportunities for Xcellerated T Cells. We plan to initially focus our development efforts onin those clinical indications that we believe have significant commercial opportunities and offer the most rapid path to regulatory approval. We believe hematological malignancies, including CLL, multiple myeloma and non-Hodgkin’s lymphoma, represent major potential markets for Xcellerated T Cells. In addition, these types of cancer are generally incurable, which means that Xcellerated T Cells may qualify for fast track approval by the FDA, andwhich could shorten the time to potential regulatory approval and commercialization. We plan to initiate one or more pivotal clinical trials in one of these hematological malignancies in the next 18 months.2005.
BACKGROUNDBackground
T cellsCells and the immune systemImmune System
T cells are critically important to a properly functioning immune system. The immune system is responsible for protecting the body from foreign invaders and eliminating tumor cells and pathogens, including tumor cells, bacteria, viruses and fungi. Classically, the immune system is divided into two arms, known as humoral immunity and cell-mediated immunity. Humoral immune responses are mediated by antibodies, which several biopharmaceutical companies have developed into major commercial products to treat a range of diseases, including cancer, infectious diseases and autoimmune diseases. Cell-mediated immunity also plays a critical role in fighting many of these illnesses. T cells, the most common type of lymphocyte, play the central role in cell-mediated immunity. Accordingly, weWe believe T cells may be used to treat cancer, infectious diseases and autoimmune diseases.
Healthy individuals have a few hundred billion T cells that circulate throughout the body. Upon encountering tumor cells or pathogens, T cells become activated and recognize and eliminate pathogensthem from the body. They do this by performing several important functions. First, T cells stimulate many other components of the immune system that are required for effective immune responses. For example, activated T cells control the proliferation and differentiation of another type of lymphocyte,other lymphocytes, B cells, which make antibodies that help fight infections. Additionally, activated T cells recognize and mark abnormal cells, such as tumor cells or infected cells, for destruction by the immune system. Activated T cells also participate directly in killing tumor cells and infectious agents, such as viruses. Finally, T cells also produce substances that stimulate the production of important blood cells including neutrophils and natural killer cells that may help fight infections, platelets that prevent bleeding, and red blood cells that carry oxygen to tissues.
Every T cell carries its own distinct receptor, the T cell receptor, which is capable of recognizing a specific antigen. Antigens are substances produced by tumor cells, viruses, bacteria or other pathogens that cause disease and may be distinguishable from substances produced by healthy cells. Healthy individuals have a population of T cells that expresses millions of different T cell receptors. It is this broad spectrum of T cell receptors that provides the diverse T cell repertoire that makes it possible for the immune system to recognize and respond to a wide variety of harmful pathogens that cause disease.
Activation of T cellsCells
T cells remain in a resting state until they become activated upon encountering antigens expressed by infected cells or tumor cells. Although activation depends on the specificity of binding of an antigen to a T cell receptor, all T cells display similar characteristics upon activation. For example, when T cells undergo activation, they become more sensitive to stimulation by antigens. This makes activated T cells especially effective at eradicating pathogens that would otherwise escape recognition from the immune system. In addition, upon activation, T cells rapidly multiply to large numbers in the body. Accordingly, it is the process of activation that makes T cells potent therapeutic agents.
43
Business
Two signals are required to activate T cells, Signal 1 and Signal 2, which are delivered by two molecules, CD3 and CD28, present on the surface of T cells. Signal 1 occurs when the CD3 molecule, which is tightly associated with the T cell receptor, is stimulated by engagement of the receptor by an antigen taken up, processed and presented by an antigen-presenting cell. Signal 2 occurs when the same antigen-presenting cell engages the CD28 molecule on the T cell. When the CD3 and CD28 molecules are stimulated, T cells become activated and produce an immune response. If only Signal 1 is generated, T cells are only partially activated and die quickly. If only Signal 2 is generated, no immune response occurs at all. Only the simultaneous delivery of both Signal 1 and Signal 2 generates activated T cells that can function properly in the body.body and survive for prolonged periods.
When a T cell becomes activated, it produces a number of different molecules to carry out its many functions. Some of these molecules, known as cytokines, are secreted by the T cell while other molecules are expressed on the surface of the T cell. Many of these molecules activate other cellular elements of the immune system. The activated T cell also produces several toxic substances that are responsible for directly killing pathogens. Several different molecules that a T cell produces in proper amounts work together to generate an effective immune response. Many of these molecules are extremely potent and would be extremely toxic if they were administered
intravenously or by other routes that allow them to circulate throughout the body. The activated T cell is able to control the production and site of delivery of these molecules in order to generate a safe immune response that is concentrated at the site of disease.
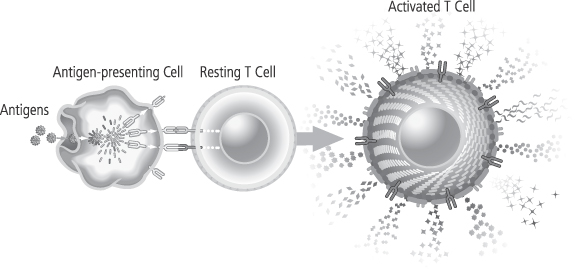
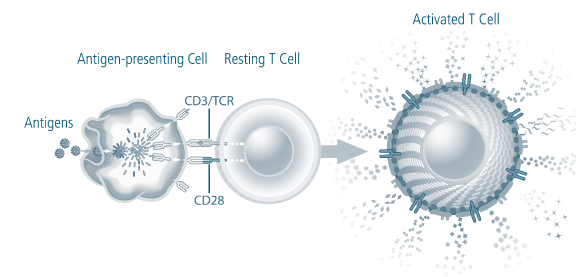
The dangersDangers of T cell deficienciesCell Deficiencies
The quantity, quality and diversity of T cells are critically important for a properly functioning immune system.
| Quantity. A variety of treatments for cancer and autoimmune diseases destroy T cells, including chemotherapy, radiation and some monoclonal antibodies. In addition, many diseases, such as HIV and several kinds of |
Quality.In many diseases, such as cancer and HIV, T cells have a reduced ability to generate effective immune responses. Many chemotherapy drugs and immunosuppressive agents also depress the activity and function of T cells. Defective T cells may not be able to respond to normal signals required for an |
44
Business
effective immune response. These T cells may produce insufficient numbers of molecules required either to mark tumor cells for destruction or to directly destroy them. |
| Diversity.A decreased diversity of T cell receptors is observed in many diseases, including cancer, HIV and autoimmune diseases. This decreased spectrum of T cell receptors narrows the ability of T cells to recognize a broad array of antigens. This may reduce a patient’s ability to respond to and eliminate cancer and infectious diseases. |
In many patients, decreases in the quantity, quality and diversity of T cells occur together. This puts patients at an increased risk of developing serious and often life-threatening infectious diseases as well as cancer. For example, patients with autoimmune diseases treated with immunosuppressive drugs have an increased risk of infections. Additionally, transplant patients treated with similar drugs have an increased risk of infections and non-Hodgkin’snon- Hodgkin’s lymphoma. Patients with HIV have an increased risk of developing non-Hodgkin’s lymphoma and multiple myeloma. Patients with certain types of congenitalprimary immunodeficiencies have an increased risk of developing infections as well as non-Hodgkin’s lymphoma and gastric cancer. In each of these medical conditions, patients often have poorly functioning T cells that are reduced in number and have limited diversity, which makes themthese patients particularly susceptible to infection and cancer.
Conversely, the presence of a sufficient number of healthy T cells is associated with improved therapeutic outcome in patients with cancer, HIV and autoimmune diseases. At the time of diagnosis, patients with non-Hodgkin’s lymphoma who have higher lymphocyte counts have better survival. Several recent independent
clinical studies have shown that cancer patients who experience more rapid and complete recovery of lymphocytes after chemotherapy have improved survival and clinical outcome. Improved prognosis has been well documented in HIV patients whose T cell counts significantly increased after anti-HIV therapy. These patients demonstrate improvements in T cell function as well as in T cell receptor repertoire diversity after successful treatment. Restoring healthy T cell diversity has also been associated with remission of disease in patients with certain autoimmune diseases.
Current approachesApproaches to activateActivate the immune systemImmune System and their limitationsTheir Limitations
There has been a major clinical focus on developing therapeutic agents to strengthen and activate a patient’s immune system. Many of these agents are used to activate the patient’s T cells inside the body. These therapeutic agents include:
| Cytokines. Cytokines, such as IL-2, are potent chemical messengers produced by the immune system that stimulate T cells and generate an immune response. Although cytokines have demonstrated therapeutic effects in cancer and infectious diseases, they are associated with serious and sometimes life-threatening side |
| Monoclonal antibodies. A variety of different monoclonal antibodies are being developed that target molecules expressed on the surface of T cells. Some of these target molecules activate T cells, while others inhibit T cell activation. By blocking the molecules that inhibit T cell activation, T cell activity can be increased. These antibodies have demonstrated limited therapeutic activity, and some of these molecules have been associated with serious side effects due to overactive T cells. |
Adjuvants. �� Other therapeutic agents known as adjuvants have also been developed to stimulate immune responses. Some of the most potent adjuvants are derived from bacteria that make a variety of |
45
Business
molecules |
| Vaccines. A number of different vaccines are under development to treat cancer and HIV. These vaccines are made up of antigens expressed by tumor cells or HIV and are often administered with adjuvants. Patients are treated with the goal of stimulating T cells to respond to antigens, so that the T cells become activated and destroy the cancer or virus. However, many patients with cancer or HIV have deficiencies in the quantity, quality or diversity of their T cells, which may limit their ability to generate an effective response to the vaccine. This may be one reason vaccines have been ineffective in treating cancer and HIV. |
| Dendritic cells. Cells of the immune system known as dendritic cells are being used to stimulate immune responses in patients with cancer. In healthy individuals, dendritic cells deliver both Signal 1 and Signal 2, which activate T cells. For most clinical applications, a patient’s own dendritic cells are grown outside of the body and then administered back to the patient. However, the ability to generate dendritic cells varies from patient to patient. Recently, it has been documented that dendritic cells under some circumstances may also make molecules that inhibit T cell responses. In addition, many patients with cancer or HIV have T cell deficiencies, which may limit their ability to respond to dendritic cells. Accordingly, dendritic cells may be limited in their ability to activate patients’ T cells and generate effective immune responses. |
Activated T cells generated using other methods. To overcome the limitations of activating T cells inside of the body, researchers have attempted to activate and grow patients’ T cellsex vivo, or outside of the body, before administering them for therapeutic applications. The development of monoclonal antibodies, which are proteins derived from a single clone of antibody-producing cells |
that bind to well-defined targets, made it possible to develop reagents that bind to the CD3 molecule and deliver Signal 1 to T cells. These antibodies are used to activate and grow T cells outside of the body. However, the process generates only one of the two signals required to activate T cells. Without Signal 2, this results in limited activity, growth and survival of T cells in the laboratory as well as after their administration into patients. Some recent approaches use antigens to target T cell receptors to generate antigen-specific T cells. However, these approaches result in a restricted T cell response that may not be effective for many clinical applications requiring broader T cell responses. |
OUR SOLUTIONOur Solution
Our therapeutic approachTherapeutic Approach
We have developed our patented and proprietary Xcellerate Technology, which can be used to consistently activate and grow large numbers of T cells outside of the body for therapeutic applications. The cells generated with this process, which we call Xcellerated T Cells, have been observed to have the broad diversity of T cell receptors that we believe may beare required to recognize and eliminate cancer and infectious diseases. These activated T cells secrete a wide spectrum of molecules, such as cytokines, and express a broad range of molecules on their cell surfaces to generate an effective immune response. In addition, T cells generated using an earlier version of our proprietary technology have been shown to survive for more than one year after infusion in patients. We believe the long-term survival of these cells may lead to sustained therapeutic responses.
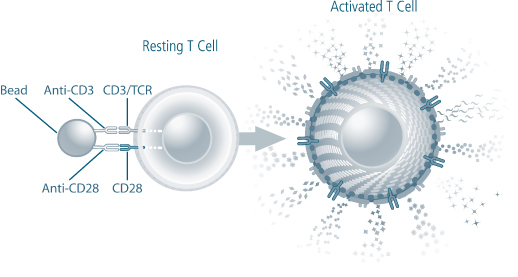
Our patented Xcellerate Technology is used in a process that employs magnetic beads, which are plastic-coated magnetic microspheres, densely covered with two monoclonal antibodies that deliver Signal 1 and
46
Business
Signal 2 to activate T cells. One of the monoclonal antibodies delivers Signal 1 to T cells by binding directly to the CD3 molecule. By targeting the CD3 molecule, our Xcellerate Technology can be used to activate and grow T cells expressing a broad diversity of T cell receptors. Our Xcellerate Technology also uses another monoclonal antibody that binds to the CD28 molecule to deliver Signal 2 to T cells. We attach both of these monoclonal antibodies to the surface of magnetic beads. When T cells bind to the monoclonal antibodies on these magnetic beads, they become activated and significantly increase in number. Accordingly, weWe believe these magnetic beads can provide the signals required to activate and grow a broad spectrum of T cells characterized by a diverse T cell receptor repertoire. These Xcellerated T Cells are then administered to the patient with the goal of restoring the health of the patient’s immune system and ability to eliminate cancer and infectious diseases.
To produce Xcellerated T Cells, white blood cells, a rich source of T cells, are first collected from a patient’s blood in an outpatient clinical setting using a standard procedure called leukapheresis. These cells are sent to our
cGMP manufacturing facility, where they are frozen and stored. When needed, the cells are thawed and processed in a closed system to avoid exposure to the outside environment, reducing the risk of microbial contamination. In this process, the patient’s white blood cells are mixed with our microscopic magnetic beads and then placed in a sterile, custom disposable bioreactor containing a solution of nutrients and a low level of IL-2 that sustains the growth of the T cells. We then add our microscopic magnetic beads. These beads are covered with our two monoclonal antibodies, which deliver Signal 1 and Signal 2 to activate the T cells in the solution. During an approximately 10-dayapproximate 10-13 day period after the application of the beads, the T cells become activated and rapidly increase in number. At the end of this period, the antibody-coated magnetic beads are substantially removed and thewith a magnetic device. The Xcellerated T Cells are then frozen for increased shelf life. We have documented that we can store the Xcellerated T Cells in a frozen state for at least 12 months without significant loss of activity. When requested by the physician, the frozen Xcellerated T Cells are shipped to the outpatient clinic where they are thawed and administered by intravenous infusion in approximately two hours.
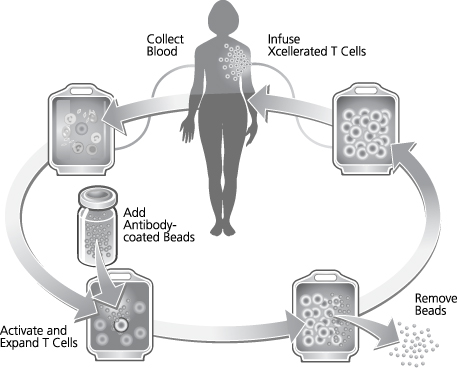
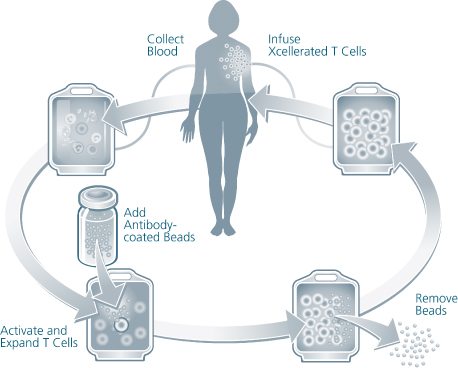
47
Business
For purposes of safety purposes and regulatory compliance, we have established procedures designed to track patients’ cells during the manufacture and shipment of Xcellerated T Cells. Each patient receives a unique identifying number that also contains a code for the clinical site where they are being treated. This unique identifying number is used to track, monitor and record all documentation, labels and materials relating to the production of the patient’s Xcellerated T Cells from blood collection through infusion of the final product. Before the product is shipped to the clinical site, we conduct quality control procedures in our laboratory. These procedures are designed to assure that Xcellerated T Cells meet strict quality control criteria such as T cell purity, dosage, potency, safety and sterility.
Benefits of Xcellerated T Cells
We believe Xcellerated T Cells may be an effective treatment for cancer and infectious diseases and may have the following clinical benefits:
Increased T cell quantity. |
during the manufacturing process. The results of this process for manufacturing Xcellerated T Cells for multiple myeloma patients and CLL patients were published in the peer-reviewedBioProcessing Journal in November 2003 and accepted for publication in the peer-reviewed journalCytotherapy in December 2004, respectively. We have submitted some of these data to the FDA and plan to submit additional data for their review. One hundred billion T cells represents approximately 25% to 30% of the total number of T cells found in healthy individuals. We believe this number of Xcellerated T Cells is sufficient to generate therapeutic effects in patients with cancer, infectious diseases and autoimmune diseases. In our ongoing Phase I/II clinical trial in multiple myeloma, we |
| Prolonged T cell survival. In |
| Improved T cell quality. We have documented that Xcellerated T Cells |
| Favorable side effect |
48
Business
Complementary to other therapies. |
caused by chemotherapy or other drugs that suppress the immune system. In addition, we believe Xcellerated T Cells may be combined with anti-viral drugs as well as therapies that activate the immune system, such as cancer vaccines. We |
Benefits of ourOur Xcellerate Technology
We believe our Xcellerate Technology may have the following benefits:
| Ex vivo process. |
| Broad clinical applications. Based on recent clinical trials, we believe our Xcellerate Technology can be applied to a variety of diseases. We have demonstrated in the laboratory as well as in our cGMP manufacturing facility that our Xcellerate Technology can be used to activate and grow T cells from patients with a variety of cancers, including kidney cancer, prostate cancer, non-Hodgkin’s lymphoma, multiple myeloma and leukemia. |
| Ease of administration. We initially collect a patient’s white blood cells, a rich source of T cells, in a standard outpatient procedure called leukapheresis. After our process is completed, Xcellerated T Cells |
| Reproducible and cost-effective manufacturing. We use the same standardized process to produce Xcellerated T Cells for all patients. Other than our proprietary components, our Xcellerate Technology incorporates commercially available products and standard clinical and blood bank supplies, which enables us to efficiently manufacture Xcellerated T Cells. |
OUR STRATEGYOur Strategy
Our goal is to be a leader in the field of T cell therapy and to leverage our expertise in T cell activation to develop and commercialize products to treat patients with cancer, infectious diseases, autoimmune diseases and compromised immune systems. Key elements of our strategy include the following:
Maximize speed to market. We plan to initiate one or more pivotal clinical trials in CLL, multiple myeloma or non-Hodgkin’s lymphoma in |
49
Business
provide the most rapid and cost-effective commercialization strategy for Xcellerated T Cells. We believe that focusing on life-threatening diseases |
| Expand the |
| Leverage complementary technologies and therapies. Xcellerated T Cells may be effective in combination with current treatments for cancer and infectious diseases, such as chemotherapy. We believe Xcellerated T Cells may help ameliorate the effects of immunosuppression associated with treatment of autoimmune diseases. We also intend to explore opportunities to combine complementary technologies and therapies, such as |
| Retain |
| Enhance our manufacturing capabilities. We have a major focus on developing an efficient and cost-effective process to manufacture Xcellerated T Cells. We currently produce T cells for clinical trials using a cost-effective process that is readily scaleable. We intend to make additional improvements to our manufacturing procedures and components, which should further reduce the costs of manufacturing. In addition, we plan to optimize our manufacturing process for other disease indications in the future. |
| Expand and enhance our intellectual property. We have a portfolio of issued patents and patent applications that we own or exclusively license, which we believe provides patent coverage for our Xcellerate Technology. As we continue to improve our Xcellerate Technology, including developing process improvements and improving the activity and the specificity of Xcellerated T Cells, we intend to file patents to protect these improvements. |
50
Business
CLINICAL APPLICATIONSClinical Applications
The table below summarizes the current status of clinical trial applications that use our proprietary technology:
Disease and | Clinical trial status | Sponsor | ||||
Cancer—Hematological malignancies | ||||||
Chronic Lymphocytic Leukemia | ||||||
| Ongoing Phase I/II | Xcyte | ||||
· Post-Campath | ||||||
| Planned Phase II/III | Xcyte | |||||
Multiple myeloma | ||||||
| Ongoing Phase I/II | Xcyte | ||||
| Ongoing Phase I/II | Physician | |||||
| Xcyte | |||||
Non-Hodgkin’s lymphoma | Completed Phase I | Physician | ||||
| �� | ||||||
| ||||||
| Xcyte | |||||
| ||||||
HIV | Completed Phase I | Physician | ||||
| Ongoing Phase I | Fresenius | |||||
| Ongoing Phase II | Physician | |||||
| Xcyte | ||||
Cancer—Solid tumors | ||||
Kidney cancer | Completed Phase I/II | Xcyte | ||
Prostate cancer | Completed Phase I/II | Xcyte |
CancerCancer—Hematological Malignancies
The American Cancer Society estimates that 1.3 million new cases of cancer will occur in the United States in 2003. Many cancer patients are treated with chemotherapy drugs, which often have limited efficacy and are associated with severe and sometimes life-threatening side effects. Physicians have recently begun to recognize the important role that the immune system may play in controlling cancer. As a result, immune-based therapeutic products, such as monoclonal antibodies, have become important drugs used to treat patients with cancer. These therapeutic products have become more widely used not only because of their efficacy, but also because they are generally better tolerated than chemotherapy.
Hematological malignancies
Hematological malignancies are cancers of the blood or bone marrow. The American Cancer Society estimates that there will be approximately 106,200110,960 new cases of hematological malignancies in the United States in 2003.2004. Hematological malignancies include leukemia, non-Hodgkin’s lymphoma, multiple myeloma and Hodgkin’s disease.lymphoma. Because hematological malignancies have usually spread throughout the body by the time of diagnosis, they typically require treatment with chemotherapy. Recently, immune-based therapeutic products have been developed to treat some hematological malignancies. Most kinds of hematological malignancies, including CLL, multiple myeloma and the vast majority of non-Hodgkin’s lymphoma,lymphomas, are cancers of lymphocytes known as B cells. In healthy individuals, T cells control the proliferation of B cells. However, in patients with B cell malignancies, T cells are abnormal, and this may contribute to uncontrolled B cell proliferation and tumor progression.
Chronic lymphocytic leukemiaLymphocytic Leukemia
According to third-party sources, approximately 75,000 patients have CLL in the United States, and there will be 8,190 new cases of CLL and 4,800 deaths due to this disease in the United States in 2004. The disease is characterized by proliferation of malignant lymphocytes in the bone marrow, lymph nodes and spleen, which leads to an increase in white blood cell counts, as well as enlarged lymph nodes and spleens in most patients. A number of chemotherapy drugs can be used to treat leukemia. Recently, the FDA approved two drugs, fludarabine, a chemotherapy agent, and Campath, a monoclonal antibody, to treat CLL. These drugs are effective in some patients but do not cure the disease. Both fludarabine and Campath are powerful drugs that destroy all lymphocytes. Consequently, patients treated with these drugs suffer from severe T cell deficiencies, which increase the risk of infection.
51In 2003, we began treating patients with CLL with a single infusion of Xcellerated T Cells with no other therapy in a Phase I/II clinical trial. We treated a minimum of three patients at each of three different dose levels of 10, 30 and 60-100 billion Xcellerated T Cells. Serious injury has sometimes occurred with other therapeutic agents used to treat CLL due to rapid destruction of leukemic cells. To reduce this risk, we started with a low dose in
Businessthis trial and have gradually increased the dose of Xcellerated T Cells. A total of 17 patients have been treated as of September 27, 2004. We have observed few side effects in most patients. As of September 27, 2004, we have reported one serious adverse event to the FDA for this trial, which involved a patient who developed an abnormal heart rhythm 17 days following treatment. In the judgment of the attending physician, the event was “unlikely related” to the therapy. In addition, we have documented a 50% to 100% reduction in the size of enlarged lymph nodes in 12 of 17 patients (71%) evaluated and a 50% or greater reductions in spleen size as measured below the ribcage by physical examination in 10 of the 13 patients (77%) with enlarged spleens. To date, we have not observed any significant decrease in leukemia counts in the blood of these patients. We have also documented increases in white blood cells including T cells, neutrophils and natural killer T cells, which may help fight infections and cancer, increases in platelets, which prevent bleeding, and increases in red blood cells as measured by hemoglobin, which carry oxygen. These findings have been submitted to the FDA in the Information Packet for a Type B End of Phase II meeting held on September 23, 2004. In July 2004, we amended the protocol for the Phase I/II clinical trial to allow patients to receive a second infusion of Xcellerated T Cells and to enroll additional patients in this trial.
Our clinical trials to date have involved small numbers of patients, and we have not designed or been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
We plan to initiate a randomized, Phase II /III clinical trial in which patients who have previously received chemotherapy and who have failed treatment with Campath will be treated with Campath with or without subsequent treatment with Xcellerated T Cells. Use of Campath is a standard treatment for CLL but increases the risk of infection in part because Campath eradicates nearly all T cells for several months following treatment. In addition, although Campath can decrease leukemic cell counts in the blood, it has less therapeutic activity in the lymph nodes and spleens of CLL patients. Accordingly, we believe there is a strong clinical rationale for combining Xcellerated T Cells with Campath. We discussed our plans for this trial with the FDA at an End of Phase II meeting on September 23, 2004. Based on feedback from the FDA during this meeting, we intend to modify our planned protocol for this Phase II/III clinical trial to provide the FDA with data we believe will address the FDA’s concerns regarding the subcutaneous route of Campath administration and the dose and schedule of Xcellerated T Cells. While we believe these modifications will be responsive to the FDA’s requests, we cannot be certain that this protocol will satisfy the FDA with respect to the issues raised at the FDA’s September 23, 2004 meeting. We have begun preparation for this Phase II/III clinical trial and expect to enroll our first patient by the end of the second quarter of 2005, subject to the FDA accepting our protocol.
Multiple myelomaMyeloma
Multiple myeloma is a form of cancer that usually originates in the bone marrow and has metastasized to multiple bone sites by the time of diagnosis. According to third-party sources, approximately 50,000 patients have multiple myeloma in the United States, approximately 15,270 new patients will be diagnosed with multiple myeloma and 11,070 patients will die of the disease in 2004. Chemotherapy has been the most common form of treatment for multiple myeloma. More recently, physicians started using drugs such as Velcade and thalidomide to treat this disease. These drugs can temporarily reduce the tumor load in patients with myeloma but only rarely eradicate the disease. The most effective therapeutic approach for treatment of multiple myeloma is high-dose chemotherapy followed by autologous stem cell transplantation. However, this therapy is not curative, and only approximately 25% of patients achieve a complete response. In addition, patients whose lymphocyte counts recover slowly after transplant have a poor clinical outcome. We believe that administering Xcellerated T Cells may be able to accelerate lymphocyte recovery and improve the clinical outcome of these patients.
We have completed treatment of all 36 of the planned patients in our ongoing Phase I/II clinical trial |
52
Business
Additionally, we and others have demonstrated that the diversity of the T cell receptor repertoire is extremely limited in patients with multiple myeloma. In our clinical trial,Patients received a single infusion of Xcellerated T Cells three days following high-dose chemotherapy and autologous stem cell transplantation. Treatment with Xcellerated T Cells has resulted in few side effects in most patients and two serious adverse events reportable to the FDA. Of these two events only one,
which involved a patient who developed a rash after treatment that subsequently resolved, was judged to be possibly or probably related to the therapy. Lymphocyte recovery and T cell receptor repertoire demonstratedrecovery in all 36 patients has been much more rapid than observed in a normal pattern in fourcomparable group of the first five evaluable patients by four weeks after transplant. In contrast, in multiple myeloma patients who dodid not receive Xcellerated T Cells it typically takes more than a year forafter stem cell transplantation. Rapid lymphocyte recovery has been correlated with improved prognosis and increased survival in previous independent clinical studies. We believe the limited T cell receptor repertoire to return to normal after transplant. The improvements in the time to lymphocyte recovery and diversity of the T cell repertoire may lead to a better clinical outcome in these patients. We are currently monitoring these patients for infections, days in hospital and other clinical parameters that may be associated with immune recovery. We are also evaluatingPreliminary results of our clinical trial show that, of the 35 patients evaluable for tumor responses, 18 patients (51%) had a greater than 90% decrease in the tumor marker used to measure disease. We have submitted these findings to the FDA and expect that preliminarywill submit additional data in our next annual report. Additional follow-up will become available duringbe required to determine the first halftherapeutic effects of 2004.Xcellerated T Cells after transplant. In independent clinical trials, a greater than 90% decrease in the tumor marker has been associated with increased survival in multiple myeloma patients.
In an ongoing independent Phase I clinical trial, one of our scientific founders and his collaborators have treated 40 multiple myeloma patients with activated T cells following high-dose chemotherapy and autologous stem cell transplantation. These patients received T cells activated using an earlier version of our proprietary technology. Administration of activated T cells resulted in few side effects in most patients and was associated with rapid lymphocyte and T cell recovery. In addition, tumor responses have been documented in a majority of these patients.
We are planning to initiateconducting a Phase II clinical trial in multiple myeloma in the non-transplant setting. Wewhich we plan to enroll approximately 30 patients who have failed prior therapies. Patients in this planned trial will beare randomized to treatment with either a single infusion of Xcellerated T Cells alone or treatment with the drug fludarabine followed by a single infusion of Xcellerated T Cells. This trial is designed to evaluate whether treatment with Xcellerated T Cells is effective without other treatment as well asa stand-alone therapy and whether fludarabine can enhance the anti-tumor effects of Xcellerated T Cells in patients with multiple myeloma. As of September 27, 2004, we have treated 18 patients in this trial. Our clinical trials to date have involved small numbers of patients and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
Non-Hodgkin’s lymphomaLymphoma
Non-Hodgkin’s lymphoma is a cancer that originates in the lymph nodes of the body. According to third-party sources, approximately 310,000 patients have non-Hodgkin’s lymphoma, and approximately 54,370 new patients will be diagnosed with this disease in the United States in 2004. About 60% of newly diagnosed patients have an |
Based on the substantial therapeutic effects that we have documented in the lymph nodes in patients with CLL, we plan to initiate a Phase II clinical trial to test whether Xcellerated T Cells can be used to treat patients with non-Hodgkin’s lymphoma. More recently, immune-based therapeutic products, such as the monoclonal antibody Rituxan, have increasingly been used alone or in combination with chemotherapy. Patients with low-grade lymphomas.lymphoma often respond to Rituxan treatment, but they cannot be cured with any form of therapy. These patients eventually become refractory to all forms of therapy and die from their disease. Patients with aggressive non-Hodgkin’s lymphoma may be cured with chemotherapy treatment. However, most patients relapse or fail to respond to therapy and have a poor prognosis. Some of these patients may be treated with high-dose chemotherapy followed by an autologous stem cell transplant, but there are few patients with long-term survival.
An independent clinical trial was conducted by one of our scientific founders under a physician-sponsored IND with the FDA in 16 non-Hodgkin’s lymphoma patients with aggressive disease and a poor prognosis. The patients were treated with high-dose chemotherapy and an autologous stem cell transplant followed by administration of a single infusion of activated T cells generated using an earlier version of our proprietary technology. As reported in the medical journalBlood in September 2003, 8 out of these 16 patients with a very poor prognosis were still alive with a median follow-up of 33 months. These data were derived from an independent clinical trial, which we did not control and which was not designed to produce statistically
significant results as to efficacy or to ensure the results were due to the effects of T cells activated using an earlier version of our proprietary technology. We have been advised that these data have been submitted to the FDA for review.
We believe administration of Xcellerated T Cells may increase the lymphocyte counts of patients with low-grade lymphoma. Recent studies have demonstrated a correlation between lymphocyte counts in patients with low-grade lymphoma and their survival. We believe administration of Xcellerated T Cells may increase the lymphocyte counts of patients withIn addition, low-grade lymphoma. Low-grade lymphomas havelymphoma has many similar characteristics to CLL. However, in contrast to CLL, tumor cells are rarely found on routine examination of the blood in patients with lymphoma. The primary site of disease in patients with low-grade lymphomaslymphoma is the lymph nodes. There is one type of low-grade lymphoma, known as small lymphocytic lymphoma, which is classified as the same disease as CLL, except for the absence of tumor cells in the blood. Because of similarities between some of these low-grade lymphomas and CLL and the effects that we have documented in the lymph nodes in patients with CLL, we have initiated a Phase II clinical trial to test whether Xcellerated T Cells can be used to treat patients with the most common forms of low-grade lymphomas, including small lymphocytic, follicular, marginal zone and mantle cell types. As of September 27, 2004, we had treated 9 patients in this clinical trial. Our clinical trials to date have involved small numbers of patients, and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerate Technology. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
53HIV
Business
According to third-party sources, there are estimated to be approximately 950,000 individuals infected with HIV in the United States. HIV patients are at increased risk of infections and cancer. In HIV, patients’ T cells become infected with the virus, leading to low numbers of T cells and an extremely narrow T cell receptor repertoire. According to independent clinical studies, it has been shown that increasing T cell count and restoring T cell repertoire are associated with improved clinical outcome. Patients with HIV are currently treated with combinations of anti-viral drugs known as highly active antiretroviral therapy, or HAART. Although HAART is effective in suppressing the virus and delaying the onset of acquired immunodeficiency syndrome, or AIDS, HAART often ceases to be effective in a significant number of patients over time. HAART is also associated with serious side effects.
Kidney cancerOne of our scientific founders independently demonstrated in the laboratory that T cells activated using an earlier version of our proprietary technology were resistant to infection with HIV. In an independent clinical trial conducted by one of our scientific founders under a physician-sponsored IND with the FDA, eight HIV patients were administered T cells activated using an earlier version of our proprietary technology. The results were published in the medical journalNature Medicine in January 2002, where it was reported that the treatment increased the average of the patient population’s T cell counts to within the normal range for at least one year following initiation of therapy. We have been advised that these data have been submitted to the FDA. In laboratory studies, the investigators also demonstrated that they were able to restore a broad T cell receptor diversity in the T cells that were produced using this technology.
We have entered into a collaboration under which Fresenius Biotech GmbH has treated HIV patients with genetically-modified T cells produced using our Xcellerate Technology. Ten patients have been enrolled in a Phase I clinical trial under this collaboration. In addition, one of our scientific founders is independently conducting clinical trials using genetically modified T cells grown using an earlier version of our proprietary technology to treat patients infected with HIV, the results of which are not yet publicly available. We do not control independent clinical trials, including physician-sponsored trials, and such trials have not been designed nor are they required to be designed to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the T cells activated by an earlier version of our proprietary technology. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results. One of our scientific founders and his collaborators conducted a preclinical study in an HIV model in monkeys in which he demonstrated that T cells activated using proprietary technology administered after one month of anti-viral drug therapy suppressed viral infection for more than a year. The results of this study were published in the medical journalBlood in January 2002. We have been advised that these data have been submitted to the FDA. Based on this study, we are conducting laboratory studies in HIV with the goal of pursuing a similar approach in HIV patients. If these laboratory studies are successful, we plan to initiate a clinical trial using Xcellerated T Cells in patients with HIV. Cancer—Solid Tumors Solid tumors are cancers that originate in organs of the body. The American Cancer Society estimates that there will be over one million new patients with solid tumors, such as breast, prostate, kidney, lung, liver and colon cancers and approximately 450,000 people will die from these types of cancers in the United States in 2004. These cancers are typically treated with surgery or radiation. Chemotherapy is used with limited success in treating solid tumors such as breast cancer, but it is generally ineffective in curing patients once the cancer has spread or metastasized. Recently, immune-based therapeutic products, including monoclonal antibodies, such as Herceptin, are being used to treat patients with solid tumors, such as breast cancer and ovarian cancer. Kidney Cancer The American Cancer Society estimates that approximately 35,710 patients will be diagnosed with kidney cancer in the United States in 2004. Approximately one-third of the patients with kidney cancer will develop metastatic disease. Once patients develop metastatic disease, they have a very poor prognosis with an average survival of approximately one year. According to third-party sources, the five-year survival for patients with metastatic kidney cancer is less than 5%, and approximately 12,000 deaths were expected to occur in the United States in 2003. The only drug currently approved by the FDA for treating metastatic kidney cancer is IL-2, a cytokine that activates T cells and increases lymphocyte counts. However, the FDA-approved regimen requires extremely high doses of IL-2, which are associated with serious and life-threatening side effects. Several recent clinical studies have demonstrated a strong correlation between the increase in lymphocyte counts that occurs with IL-2 therapy and clinical outcome in patients with metastatic kidney cancer. We believe administration of Xcellerated T Cells may improve the clinical outcome in these patients by boosting lymphocyte counts. In February 2003, we completed a Phase I/II clinical trial of Xcellerated T Cells in 25 patients with metastatic kidney cancer. In this clinical trial, patients were treated with two infusions of Xcellerated T Cells approximately four weeks apart. After each infusion of Xcellerated T Cells, patients were treated with low doses of IL-2. We observed few side effects in most patients and no serious adverse events reportable to the FDA related to the therapy. We also observed the complete elimination of detectable bone metastases in two patients. Furthermore, there was a statistically significant increase in lymphocyte counts |
We also observed evidence of therapeutic effects at sites of metastases to bone in two patients. Furthermore, lymphocyte counts significantly increased with treatment, and there was a trend to increasedan increase in post-infusion survival in patients achieving higher lymphocyte counts. MedianThe median survival in these patients was 21 months in these patients.months. Several previousindependent clinical trials have shown that the median survival in patients with metastatic kidney cancer is approximately 12 months. The results of thisour clinical trial were reported in the medical journalClinical Cancer Researchin September 2003. 2003, and have been submitted to the FDA for review.
We are evaluating plans for a pivotal trial in kidney cancer based on cost, longer time to approval and potential for finding a partnerpartnership opportunities to support further development.development of this clinical indication. Our clinical trials to date have involved small numbers of patients and we have neither designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
Prostate Cancer
Prostate cancer is the most common form of cancer in men in the United States. The American Cancer Society estimates that there will be 230,110 new cases and approximately 29,900 patients will die of prostate cancer in
the United States in 2004. Patients with prostate cancer can be cured by surgery if the disease is localized. However, once the disease spreads to other organs, it cannot be cured with current standard treatments, either hormonal therapy or chemotherapy.
In June 2003, we completed a Phase I/II clinical trial in 19 patients with hormone-refractory prostate cancer. Patients were treated with a single infusion of Xcellerated T Cells. The therapy resulted in few side effects in most |
54
Business
therapy was generally well tolerated and led to significant and sustained increases in patients’ lymphocyte counts. Two patients demonstrated significantgreater than 50% decreases in serum levels of the tumor marker, PSA. We have submitted these data to the FDA for review. In some independent clinical studies, decreases in PSA levels have been shown to correlate with improved survival in patients with prostate cancer. There was one serious adverse event reportable to the FDA involving a patient with pre-existing severe anemia who suffered congestive heart failure. The patient’s symptoms resolved approximately two hours following treatment. We subsequently amended our protocol to identify patients with anemia prior to administering Xcellerated T Cells. Our clinical trials to date have involved small numbers of patients, and we have not designed nor been required to design such trials to produce statistically significant results as to efficacy. These trials have neither been randomized nor blinded to ensure that the results are due to the effects of the Xcellerated T Cells. Success in early clinical trials neither ensures that large-scale trials will be successful nor predicts final results.
HIV
Autoimmune diseasesDiseases
An overactive immune system is believed to play a central role in a variety of illnesses classified as autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus and scleroderma. Attempts to control the disease with therapeutic agents that suppress the immune system are often effective. However, some patients have more serious forms of these diseases and do not respond to conventional therapy, while others experience serious side effects from these chronic immunosuppressive therapies. Recently, high-dose chemotherapy and/or radiation have been used with autologous stem cell transplantation to eradicate these patients’ diseased immune systems in an attempt to cure several of these diseases. Although effective in many patients, this form of therapy has been associated with serious and life-threatening toxicities. Many scientists now believe that certain populations of T cells play a central role in causing several autoimmune diseases. This is manifested by narrowing of the T cell receptor repertoire, which has been shown to return to normal when patients with some of these diseases achieve remission. Many therapeutic agents are available that can selectively eliminate T cells without causing the serious toxicities associated with the intensive regimens used with stem cell transplantation. IfWe believe that if our Xcellerate Technology can be used to generate healthy T cells from patients with autoimmune diseases, it may be possible to administer Xcellerated T Cells to restore a healthy immune system after patients are treated with drugs that eliminate T cells in the body.
55
Business
We have demonstrated in laboratory studies that our Xcellerate Technology can be used to activate and grow T cells from patients with several autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus and scleroderma. These studies have also shown that we can restore the narrow T cell repertoire characteristic of many of these patients to a more normal diverse pattern using our Xcellerate Technology. Further preclinical studies are planned that, if successful, could lead to the initiation of a clinical trial using this approach in patients with serious forms of autoimmune diseases.
RESEARCH AND DEVELOPMENTResearch and Development
As of September 30, 2003,27, 2004, we had a total of 2726 employees dedicated to research and development, including seven9 with advanced degrees. We spent approximately $47.7$14.7 million, from January 1, 2000 through$14.7 million and $13.7 million during the years ended December 31, 2001, 2002 and 2003, respectively, and $8.6 million during the six months ended June 30, 20032004 on the research and development of our Xcellerate Technology and Xcellerated T Cells. Our internal research and development efforts are focused on:
| Improving our Xcellerate Technology. We intend to continuously evaluate and improve our Xcellerate Technology. |
| Increasing the therapeutic activity of Xcellerated T Cells. We intend to continuously evaluate and improve the therapeutic activity of Xcellerated T Cells. We are currently evaluating whether other molecules of the immune system or genes could be used to improve the therapeutic activity of Xcellerated T Cells. We are working with several groups to evaluate using Xcellerated T Cells in conjunction with recently discovered antigens to specifically target cancers and infectious diseases associated with those antigens. We have conducted laboratory studies demonstrating that we can generate large numbers of antigen-specific Xcellerated T Cells with anti-tumor activity in several types of cancer, including melanoma, breast cancer, kidney cancer and lung cancer. We expect that some of our collaborators will be conducting physician-sponsored clinical trials with these approaches in the near future. |
| Developing additional clinical indications for Xcellerated T Cells. There are many medical conditions that are associated with deficiencies in T cells. We are currently studying the potential to use Xcellerated T Cells to treat these illnesses. For example, patients with autoimmune diseases are treated with immunosuppressive drugs that damage their immune systems. We have demonstrated in laboratory studies that we can activate and grow T cells and restore a normal T cell repertoire in patients with several of these diseases. In addition, we |
MANUFACTURING AND SUPPLYManufacturing and Supply
We designed, built and operate a pilotour current manufacturing facility in Seattle, Washington in accordance with cGMP. We use this facility to manufacture Xcellerated T Cells for clinical trials. We have also leasedcompleted the construction of the initial phase of an additional leased facility that we intend to develop to manufacture Xcellerated T Cells for our planned clinical trials and, initial commercialization if we obtain FDA approval. Weapproval, initial commercialization. This facility is undergoing qualification and validation, and we expect to begin manufacturing Xcellerated T Cells at this facility in the secondfirst half of 2004.2005. Except for our antibody-coated beads and custom bioreactor system, all of the components that are required to implement our Xcellerate Technology are commercially available products and standard clinical and blood bank supplies.
In August 1999, we entered into a contractan agreement with Dynal for the cGMP gradecGMP-grade manufacture of our antibody-coated beads for clinical and future commercial uses. For completed milestones,In March 2004, we amended our agreement to allow Dynal to sell a research-grade version of our antibody-coated beads. We have paid $2.5Dynal $3.0 million as of September 30, 2003 and, assumingJuly 31, 2004 for completed milestones. Dynal has the remaining milestones are completed,right to terminate the contract if we will be
56
Business
obligated to pay an additional $0.5 million. Thedo not purchase a minimum quantity of beads. Either party may terminate the agreement will terminate inas of August 2009 for any reason, or earlier upon a material breach by, or insolvency of, the other party. If the agreement is not terminated by August 2009, either party. Theparty can elect to extend the term may be extendedof the agreement for an additional five years by either party.5 years. Otherwise, it will automatically renew on a year to year basis.
In June 2000, we entered into two service agreements with Lonza, which were subsequently amended, for the cGMP gradecGMP-grade manufacture of the two monoclonal antibodies for use with our antibody-coated beads. Under the terms of these agreements, we are obligated to make certain payments to Lonza. We have paid $4.9 million as of SeptemberJune 30, 2003.2004. Assuming development and supply services under our agreements with Lonza are completed as scheduled under our agreements with Lonza, our remaining payments will be approximately $1.4 million through the end of 2005. These agreements may be terminated by either party for a material breach.breach or insolvency of the other party or in the event that the manufacturing services cannot be completed for scientific or technical reasons.
We use tissue culture media and a custom bioreactor in our manufacturing process. We currently do not have agreements with third parties to supply us with tissue culture media or bioreactors.
COMPETITIONCompetition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Many entities, including pharmaceutical and biotechnology companies, academic institutions and other research organizations are actively engaged in the discovery, research and development of products that could compete with our products under development. They may also compete with us in recruiting and retaining skilled scientific talent.
There are numerous pharmaceutical and biotechnology companies that are developing therapies for cancer and infectious disease generally, and many of these companies are focused on activating the immune system using therapeutic agents, including monoclonal antibodies, cytokines, vaccines, adjuvants, dendritic cells, nucleotides and cells. We are currently aware of several companies developingex vivocell-based immunotherapy products as a method of treating cancer and infectious diseases. These competitors include Antigenics, Inc., CancerVax Corporation, Cell Genesys, Inc., CellExSys, Inc. (recently sold to Chromos Molecular Systems, Inc.), Dendreon Corporation, Favrille, Inc., Genitope Corporation, IDM, S.A. and Kirin Pharmaceutical. Even if our Xcellerate Technology proves successful, we might not be able to remain competitive in this rapidly advancing area of technology. Some of our potential competitors may have more financial and other resources, larger research and development staffs and more experienced capabilities in researching, developing and testing products. Some of these companies also have more experience than us in conducting clinical trials, obtaining FDA and other regulatory approval,approvals and in manufacturing, marketing and distributing of medical products. Smaller companies may successfully compete with us by establishing collaborative relationships with larger pharmaceutical companies or academic institutions. Our competitors may succeed in developing, obtaining patent protection for or commercializing their products more rapidly than us. A competing company developing, or acquiring rights to, a more effective therapeutic product for the same diseases targeted by us, or one that offers significantly lower costs of treatment, could render our products noncompetitive or obsolete.
INTELLECTUAL PROPERTYIntellectual Property
We rely on a combination of patent, trademark, copyright and trade secret laws to protect our proprietary technologies and products. We aggressively seek USU.S. and international patent protection to further our business strategy and for major components of our Xcellerate Technology, including important antibody components and methods of T cell activation. We also rely uponon trade secret protection for our confidential and proprietary information. We enter into licenses to technologies we view as necessary.
We have a portfolio of issued patents and patent applications, which we believe provides patent coverage for our Xcellerate Technology. As of September 30, 2003,October 1, 2004, we owned or held exclusive rights to six issued
57
Business
patents, six allowed patent applications and numerous pending patent applications in the United States in the field of or directed toex vivoT cell stimulation. TwoThree of the issued patents relate to methods of stimulating T cells utilized by our Xcellerate Technology, two of which expire in 2019 and one of which expires in 2021, while two other issued patents, which expire in 2016, relate to a method of stimulating T cells and an antibody that we are not currently using. The final twoTwo additional issued patents expire in 2020 and are in the field of or directed to immunosuppression and the treatment and prevention of immune disorders related to T cells. These two issued patents are directed to the use of a specific compound for these applications, and immunorejectionone of transplanted material.these patents is directed specifically to compositions of matter including likely derivatives of this compound. The final issued patent expires in 2020 and relates toex vivo T cell stimulation to improve uptake of exogenous nucleic acid molecules, thus having gene therapy applications. We also have licensed numerous currently pending foreign patent applications and fiveseven issued foreign patents corresponding to our T cell stimulation technology.
In general, we apply for patent protection of methods and products relating to immunotherapy for treatment of cancer, immune deficiencies, autoimmune diseases and infectious diseases. With respect to proprietary know-how that is not patentable, we have chosen to rely on trade secret protection and confidentiality agreements to protect our interests. We have taken security measures to protect our proprietary know-how, technologies and confidential data and continue to explore further methods of protection.
We require all employees, consultants and collaborators to enter into confidentiality agreements, and all employees and most consultants enter into invention assignment agreements with us. The confidentiality agreements generally provide that all confidential information developed or made known to the individual during the course of such relationship will be kept confidential and not disclosed to third parties, except in specified circumstances. These invention agreements generally provide that all inventions conceived by the individual in the course of rendering services to us shallwill be our exclusive property. There can be no assurance,We cannot assure you, however, that these agreements will provide meaningful protection or adequate remedies for any breach or that our trade secrets will not otherwise become known or be independently discovered by our competitors. Any of these events could adversely affect our competitive position in the marketplace.
In the case of a strategic partnership or other collaborative arrangement which requires the sharing of data, our policy is to disclose to our partner, under controlled circumstances, only data that is relevant to the partnership or arrangement during the contractual term of the strategic partnership or collaborative arrangement, subject to a duty of confidentiality on the part of our partner or collaborator. Disputes may arise as to the ownership and corresponding rights in know-how and inventions resulting from research by us and our future corporate partners, licensors, scientific collaborators and consultants. We cannot assure you that we will be able to maintain our proprietary position or that third parties will not circumvent any proprietary protection we have. Our failure to maintain exclusive or other rights to these technologies could harm our competitive position.
To continue developing and commercializing our current and future products, we may license intellectual property from commercial or academic entities to obtain the rights to technology that is required for our discovery, research, development and commercialization activities.
In preparation for the commercial distribution of our products and services if we obtain FDA approval, we have filed a number of trademark applications.
Corporate collaborationsCollaborations
Part of our strategy is to establish corporate collaborations with pharmaceutical, biopharmaceutical and biotechnology companies for the development and commercialization of our Xcellerate Technology. We focus our efforts on partnering our technologies in markets and diseases that we do not plan to pursue on our own. We target collaborators that have the expertise and capability to develop, manufacture, obtain regulatory approvals for and commercialize our Xcellerate Technology. In our corporate
58
Business
collaborations, we seek to cover our research and development expenses through research funding, milestone payments and technology or license fees. We also seek to retain significant downstream participation in product sales through either profit-sharingprofit sharing or product royalties paid on annual net sales.
Fresenius Biotech GmbH
The letterIn November 2003, we licensed our Xcellerate Technology and some related improvements on an exclusive basis in the field of intent sets forth certain termsHIV retroviral gene therapy to be incorporated intoFresenius for research, development, and commercialization in Europe, with a definitiveright of first negotiation under some circumstances to expand their territory to include North America. Our agreement including our obligationwith Fresenius requires us to transferlicense our Xcellerate Technology, including methods for manufacturing capability,Xcellerated T Cells, to TCTCFresenius, transfer certain enabling technology and supply all proprietary magnetic beads, or Xcyte Dynabeads, ordered by Fresenius to provide trainingsupport its development and commercialization efforts. If we do not supply the Xcyte Dynabeads, Fresenius has the right to TCTCmanufacture such Xcyte Dynabeads on its own or through a third party, until such time that we are able to supply the usequantity of Xcyte Dynabeads ordered by Fresenius. Fresenius has agreed to reimburse us for our expenses in transferring the technology and manufacture ofpay us for the Xcyte Dynabeads on a cost-plus basis. In addition, under the agreement Fresenius has granted us a perpetual, irrevocable, non-exclusive, fully paid worldwide license to technology invented by Fresenius that directly relates to our Xcellerate Technology. The business terms of the letter of intent include issuance of 2% of TCTC’s equity to us, potentialThis agreement includes royalties on net sales and sublicensing revenue as well as up to US$105.4 million Euros in license issue fees and potential milestone payments basedto us, less applicable sublicense fees payable by us to third parties, for each product developed under this agreement. Fresenius’ obligation to pay us royalties under this
agreement terminates on clinical trials conducteda country-by-country basis upon the later of the last to expire of the licensed patents or 15 years after the first commercial sale of a product in the country. The agreement is also subject to earlier termination by TCTCFresenius at any time if Fresenius determines it cannot develop a commercially viable product or complete a required manufacturing audit, at any time by Xcyte if Fresenius does not meet certain development and us. In addition, if we enter into a definitive agreement, we are obligated to purchase US$2.5 millioncommercialization milestones and by either party for the material breach or insolvency of TCTC equity on the same terms and conditions as the other investors and,party. The agreement specifies that the termination of certain technology licenses, under certain circumstances, to pay a percentagewhich we obtained much of any revenue we receive from granting license rights to our Xcellerate Technology, in Japan. Both parties will have diligence obligationsis a breach of this agreement.
Fresenius is conducting a Phase I trial to pursuetreat HIV patients with genetically-modified T cells produced using our Xcellerate Technology in their respective territories, and TCTC will have the right to market our Xcellerate Technology in any country in which we do not seek market approval for our Xcellerate Technology within a specified period of time following US approval of our first product.
The letter of intent will terminate in the event a definitive agreement is not executed prior to November 15, 2003 or if either party elects to terminate it earlier upon material breach by the other party or in our or TCTC’s sole discretion subject to payment of a break-up fee.Technology.
Technology licensesLicenses
Where consistent with our strategy, we seek to obtain technologies that complement and expand our existing technology base. We have licensed and will continue to license technology from selected research and academic institutions, as well as other organizations. Under these license agreements, we generally seek to obtain unrestricted sublicense rights. We are generally obligated under these agreements to pursue product development make development milestone payments and pay royalties on any product sales. We have not been required to pay any royalties through September 30, 2003.27, 2004. In addition to license agreements, we seek relationships with other entities that may benefit us and support our business goals.
Diaclone S.A. In October 1999, we entered into a license agreement with |
59
Business
will have a perpetual, irrevocable, royalty-free, exclusive license. We paid |
Fred Hutchinson Cancer Research Center.In October 1999, we entered into a license agreement with the Fred Hutchinson Cancer Research Center. Under the agreement, the Fred Hutchinson Cancer Research Center granted us a non-exclusive, worldwide license to make, use and sell products or services using the monoclonal antibody that binds to the CD3 molecule for T cell stimulation forex vivo |
may be terminated earlier by either party for material breach or by Fred Hutchinson Cancer Research Center for Xcyte’s insolvency. Thereafter, our license will be fully-paid. |
| Genetics Institute.In July 1998, we entered into a license agreement with Genetics |
GOVERNMENTAL REGULATIONGovernmental Regulation
Governmental authorities in the United States and other countries extensively regulate the preclinical and clinical testing, approval, manufacturing, labeling, storage, record-keeping, reporting, advertising, promotion, import, export, marketing and distribution, among other things, of immunotherapy products and other drugs and biological products. In the United States, the FDA, under the Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal statutes and regulations, subjects pharmaceutical products to rigorous review and regulation. If we do not comply with applicable requirements, we may be fined, our products may be recalled or seized, our clinical trials may be suspended or terminated, our production may be totallypartially or partiallytotally suspended, the government may refuse to approve our marketing applications or allow us to distribute our products and we may be subject to an injunction and/or criminally prosecuted. The FDA also has the authority to revoke previously granted marketing authorizations.
In order to obtain approval of a new product from the FDA, we must, among other requirements, submit proof of safety and efficacy as well as detailed information on the manufacture, quality, composition and labeling of the product in a new drug application or a biologics license application. In most cases, this proof entails extensive laboratory tests and preclinical and clinical trials. This testing, the preparation of
60
Business
necessary applications, the processing of those applications by the FDA and review of the applications by an FDA advisory panel of outside experts are expensive and typically take many years to complete. Additionally, the FDA recently formed a new division that will regulate biologic products, such as Xcellerated T Cells. The processes and requirements associated with this new division may cause delays and additional costs in obtaining regulatory approval of our products or for regulatory authorization for our clinical trials. The FDA may not act quickly or favorably in reviewing these applications, or may deny approval altogether, and we may encounter significant difficulties or costs in our efforts to obtain FDA approval, which could delay or preclude us from marketing any products we may develop. The FDA may also require post-marketing testing and surveillance to monitor the effects of approved products or place conditions on any approval that could restrict the commercial applications of these products. The FDA may withdraw product approval if we fail to comply with regulatory standards, if we encounter problems following initial marketing or if new safety or other issues are discovered regarding our products after approval. With respect
to patented products or technologies, delays imposed by the governmental approval process may materially reduce or eliminate the period during which we will have the exclusive right to exploit the products or technologies.
In order to conduct research to obtain regulatory approval for marketing, we must submit information to the FDA on the planned research in the form of an investigational new drug application. The investigational new drug application must contain, among other things, an investigational plan for the therapy, a study protocol, information on the study investigators, preclinical data, such as toxicology data, and other known information about the investigational compound. An investigational new drug application generally must be submitted by a commercial sponsor who intends to collect data on the safety and efficacy of a new drug or biological product prior to conducting human trials and submitting an application for marketing approval. In certain circumstances, an investigational new drug application may also be submitted which allows physicians to gain an initial understanding of the compound through an expanded access program. Data from expanded access trials can generally be used to support the safety, but not the efficacy, of a product.
After an investigational new drug application becomes effective, a sponsor may commence human clinical trials. The sponsor typically conducts human clinical trials in three sequential phases, but the phases may overlap. In Phase I clinical trials, the product is generally tested in a small number of patients or healthy volunteers primarily for safety at one or more doses. In Phase II, in addition to safety, the sponsor typically evaluates the efficacy of the product in a patient population somewhat larger than Phase I clinical trials. It is customary in cancer clinical trials for the FDA to allow companies to combine Phase I and Phase II clinical trials into a Phase I/II clinical trial. Phase III clinical trials typically involve additional testing for safety and clinical efficacy in an expanded population at geographically dispersed test sites and are intended to generate the pivotal data on which a marketing application will be based. The studies must be adequate and well-controlled and otherwise conform to appropriate scientific and legal standards.
Prior to the commencement of each clinical trial, the sponsor must submit to the FDA a clinical plan, or protocol, accompanied by the approval of an institutional review board responsible for protecting the welfare of study subjects for a site participating in the trials. The sponsor must also ensure that investigators obtain informed consent from all study subjects prior to commencement of each study, and the sponsor must comply with monitoring, reporting and so-called good clinical practice requirements throughout the conduct of the study, among other legal requirements. The FDA may prevent an investigational new drug application from taking effect, or may order the temporary or permanent discontinuation of a clinical trial, at any time. An institutional review board may also prevent a study from going forward, or may temporarily or permanently discontinue a clinical trial, at any time. If a
61
Business
study is not conducted in accordance with applicable legal requirements and sound scientific standards, the data from the study may be deemed invalid and unusable.
The sponsor must submit to the FDA the results of the preclinical and clinical trials, together with, among other things, detailed information on the manufacture, quality and composition of the product, in the form of a new drug application or, in the case of a biologic, a biologics license application. The application must also contain proposed labeling for the product setting forth the proposed conditions of use for which the applicant is seeking approval and be accompanied by the payment of a significant user fee. The FDA can refuse to file an application if it is deemed not sufficiently complete to permit review, or has some other deficiency.
Because the FDA is regulating Xcellerated T Cells as a biologic, we must submit biologics license applications to the FDA to obtain approval of our products. A biologics license application requires data showing the safety, purity and potency of the product. In a process which generally takes several years or more, the FDA reviews this application and, when and if it decides that adequate data are available to show that the new compound is both safe and effective and that other applicable requirements have been met, approves the drug or biologic for marketing. Prior to issuing a denial or an approval, the FDA often will seek recommendations from one of its advisory committees of independent experts. The amount of time taken for this approval process is a function of a number of variables, including the quality of the submission and studies presented, the potential contribution that the compound will make in improving the treatment of the disease in question, the recommendations of the
FDA advisory committee and the workload at the FDA. It is possible that our Xcellerate Technology will not successfully proceed through this approval process or that the FDA will not approve our applications in any specific period of time, or at all. Any approval, if obtained, could be limited or could be made contingent on burdensome post-approval commitments or could be otherwise restricted.
Congress enacted the Food and Drug Administration Modernization Act of 1997, in part, to ensure the availability of safe and effective drugs, biologics and medical devices by expediting the FDA review process for new products. The Modernization Act establishes a statutory program for the approval of fast track products, including qualifying biologics. We may, from time to time, decide to request fast track approval for Xcellerated T Cells. A fast track product is defined as a new drug or biologic intended for the treatment of a serious or life-threateninglife- threatening disease or condition that demonstrates the potential to address unmet medical needs for this disease or condition. Under the fast track program, the sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a fast track product at any time during the clinical development of the product.
The Modernization Act specifies that the FDA must determine whether the product qualifies for fast track designation within 60 days of receipt of the sponsor’s request. The FDA can base approval of a marketing application for a fast track product on an effect on a clinical endpoint or on another “surrogate” endpoint that is reasonably likely to predict clinical benefit. The FDA may subject approval of an application for a fast track product to post-approval studies to validate the surrogate endpoint or confirm the effect on the clinical endpoint and prior review of all promotional materials. In addition, the FDA may withdraw its approval of a fast track product on an expedited basisdesignation on a number of grounds, including the sponsor’s failure to conduct any required post-approval study with due diligence.
If the FDA’s preliminary review of clinical data suggests that a fast track product may be effective, the agency may initiate review of sections of a marketing or license application for a fast track product before the sponsor completes the entire application. This rolling review may be available if the applicant provides a schedule for submission of remaining information and pays applicable user fees. However, the time periods specified under the Prescription Drug User Fee Act concerning timing goals to which the
62
Business
FDA has committed in reviewing an application do not begin until the sponsor submits the entire application.
We have requested, and may from time to time continue to request, orphan drug status for Xcellerated T Cells. Orphan drug designation may be granted to those products developed to treat diseases or conditions that affect fewer than 200,000 persons in the United States. We believe that some of our target cancer patient populations meet these criteria. Under the law, the developer of an orphan drug may be entitled to seven years of market exclusivity following the approval of the product by the FDA, exemption from user fee payments to the FDA and a 50% tax credit for the amount of money spent on human clinical trials. We cannot predict whether the FDA will grant either an orphan drug or fast track designation or whether our products will ultimately receive FDA approval or orphan drug market exclusivity. We also cannot predict the ultimate impact, if any, of the fast track process or orphan drug status on the timing, likelihood or scope of FDA approval of our immunotherapy products. Even if we are able to obtain FDA approval with orphan drug marketing exclusivity, other competing products may still be approved if they are deemed to be sufficiently different than our products, or clinically superior or under certain other circumstances. This could reduce or eliminate the value of any orphan drug marketing exclusivity.
The FDA may, during its review of a new drug application or biologics license application, ask for additional test data. If the FDA does ultimately approve the product, it may require post-marketing testing, including potentially expensive Phase IV studies, and surveillance to monitor the safety and effectiveness of the product. In addition, the FDA may in some circumstances impose restrictions on the use of the product, which may be difficult and expensive to administer, may affect whether the product is commercially viable and may require prior approval of promotional materials.
Before approving a new drug application or biologics license application, the FDA will also inspect the facilities where the product is manufactured and will not approve the product unless the manufacturing facilities are in
compliance with cGMP. In addition, the manufacture, holding and distribution of a product must remain in compliance with cGMP following approval. Manufacturers must continue to expend time, money and effort in the area of production and quality control and record keeping and reporting to ensure full compliance with those requirements.
The labeling, advertising, promotion, marketing and distribution of a drug or biologic product must be in compliance with FDA regulatory requirements. Our distribution of pharmaceutical samples to physicians must comply with the Prescription Drug Marketing Act. In addition, manufacturers are required to report adverse events and errors and accidents in the manufacturing process. Changes to an approved product, or changes to the manufacturing process, may require the filing of a supplemental application for FDA review and approval. Failure to comply with applicable requirements can lead to the FDA demanding that production and shipment cease, and, in some cases, that the manufacturer recall products or to FDA enforcement actions that can include seizures, injunctions and criminal prosecution. These failures can also lead to FDA withdrawal of approval to market the product. Where the FDA determines that there has been improper promotion or marketing, it may require corrective communications such as “Dear Doctor” letters. Even if we comply with FDA and other requirements, new information regarding the safety or effectiveness of a product, or a change in the law or regulations, could lead the FDA to modify or withdraw a product approval.
In addition to FDA requirements, our manufacturing, sales, promotion, and other activities following product approval are subject to regulation by numerous other regulatory authorities, including, in the United States, the Centers for Medicare & Medicaid Services, other divisions of the Department of Health and Human Services and state and local governments. Among other laws and requirements, our
63
Business
sales, marketing and scientific/educational programs must comply with the Federal Medicare-Medicaid anti-fraud and abuse statutes and similar state laws. Our pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
We are also subject to regulation by the Occupational Safety & Health Administration, or OSHA, and the Environmental Protection Agency, or EPA, and to various laws and regulations relating to safe working conditions, laboratory and manufacturing practices and the use and disposal of hazardous or potentially hazardous substances, including radioactive compounds used in connection with our research and development activities, and we may in the future be subject to other federal, state or local laws or regulations. OSHA, the EPA or other regulatory agencies may promulgate regulations that may affect our research and development programs. We are also subject to regulation by the Department of Transportation and to various laws and regulations relating to the shipping of cells and other similar items. We are unable to predict whether any agency will adopt any regulation that could limit or impede our operations.
Depending on the circumstances, failure to meet these other applicable regulatory requirements can result in criminal prosecution, fines or other penalties, injunctions, recall or seizure of products, totalpartial or partialtotal suspension of production, denial or withdrawal of pre-marketing product approval or refusal to allow us to enter into supply contracts, including government contracts.
Sales of pharmaceutical products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. Whether or not we have obtained FDA approval, we must obtain approval of a product by comparable regulatory authorities of foreign countries prior to the commencement of marketing the product in those countries. The time required to obtain this approval may be longer or shorter than that required for FDA approval. The foreign regulatory approval process includes all the risks associated with FDA regulation set forth above, as well as country-specific regulations, including in some countries price controls.
In May 2000, we filed our initial Phase I investigational new drug application, or IND, involving Xcellerated T Cells to treat metastatic kidney cancer. The FDA allowed us to start the trial in June 2000. The trial was
completed in February 2003. In September 2001, we amended the IND to add a Phase I study of Xcellerated T Cells to treat hormone refractory prostate cancer. The trial was completed in June 2003. In August 2002, we amended the IND to add a Phase I/II to treat multiple myeloma patients post autologous stem cell transplantation. We anticipate completion of theThis trial by June 2004.is ongoing. In November 2002, we amended the IND to add a Phase I/II study to treat CLL. We anticipate completion of the trialThis CLL study was subsequently amended in the first half of 2004.July 2004 to allow for additional patients and is still ongoing. In September 2003, we amended the IND to add a randomized Phase II study to treat multiple myeloma patients with and without fludarabine. We anticipate completion of the trial by the end of the second halfquarter of 2005. In December of 2003, we amended the IND to add a Phase II study to treat non-Hodgkin’s lymphoma patients. We anticipate completion of the trial by the end of 2005.
LEGAL PROCEEDINGSLegal Proceedings
From time to time, we may be involved in various other legal proceedings in thelitigation relating to claims rising out of our ordinary course of business. Although it isWe are not feasiblecurrently a party to predict the outcome of these proceedings or any claims made against us, we do not anticipate that the ultimate liability of these proceedings or claims will have a materially adverse effect on our financial position or results of operations.
64
Business
On July 26, 2000, Karen Lenahan filed suit against the University of Chicago, the University of Chicago Hospitals, Central DuPage Hospital and various doctors, seeking to recover damages arising out of the death of Mrs. Lenahan’s husband, Shawn Lenahan. The complaint, filed in the Circuit Court of Cook County, Illinois, alleged that the physicians committed medical malpractice. Mr. Lenahan was treated in an independent clinical trial using technology similar to ours. This trial was initiated prior to our licensing of this technology. The complaint was amended to add additional defendants, and on February 26, 2001, a second amended complaint was filed that named us as a defendant. The second amended complaint attempted to allege that we participated in an unlawful conspiracy to induce Mr. Lenahan to participate in a drug protocol for an experimental treatment for his non-Hodgkin’s lymphoma.
On May 7, 2001, we filed a motion seeking to dismiss the conspiracy claims, the only counts in the second amended complaint in which we were named as a defendant. On June 29, 2001, the court upheld the motion to dismiss. On July 27, 2001, the plaintiff filed a fourth amended complaint, which again named us as a defendant and attempted to allege that we and our co-defendants unlawfully conspired against Mr. Lenahan. On August 31, 2001, we filed a motion to dismiss the conspiracy claims against us. On February 25, 2002, the court upheld the motion to dismiss. However, the court granted the plaintiff one final chance to file an amended complaint. On March 25, 2002, the plaintiff filed a fifth amended complaint, which alleged similar claims as the fourth amended complaint. We filed a motion to dismiss the conspiracy claims and on July 22, 2002, the court granted our motion to dismiss the plaintiff’s fifth amended complaint with prejudice. On August 20, 2002, the plaintiff filed a notice of appeal from the court’s order granting our motion to dismiss. On April 7, 2003, we filed our response brief, and, on May 1, 2003, the plaintiff filed its reply brief. We cannot predict when we will have a decision on the appeal. We deny having committed any conspiracy against Mr. Lenahan and intend to vigorously defend this lawsuit. However, because of the nature of the complaint against us, we cannot predict the probability of a favorable or unfavorable outcome, or estimate the amount or range of potential loss.material legal proceedings.
EMPLOYEESEmployees
As of September 30, 2003,27, 2004, we had 6398 employees, 2726 of whom are directly involved in research and development and 2338 of whom are involved in manufacturing operations. We consider our relations with our employees to be good.
FACILITIESFacilities
We currently lease a total of approximately 62,50063,500 square feet of space at twothree facilities. We lease approximately 22,000 square feet of office and laboratory space and a pilot cellcGMP manufacturing facility in Seattle, Washington, with monthly payments of approximately $48,000.$49,000. The lease on this space expires in October 2006, and we have options to renew for two additional five-year terms. We sublease approximately 1,000 square feet of laboratory space and equipment in Seattle, Washington, with monthly payments of approximately $3,333. The sublease on this space expires in March 2005, and we have options to extend for additional six-month terms at the sublessor’s discretion. We also lease approximately 40,500 square feet of space in Bothell, Washington, with monthly payments of approximately $77,000. We plan to renovatehave renovated approximately 20,000 square feet of this facility for the manufacture Xcellerated T Cells for our planned clinical trials and, initial commercialization if we receive FDA approval.obtain regulatory approval, initial commercialization. The initial lease term on this space expires December 2010, and we have options to renew until December 2020. Under the terms of the lease, we also have rights to negotiate for further expansion space in the building.
65 We believe that this facility has sufficient space to accommodate expansion of our operations.
Scientific advisory boardSCIENTIFIC ADVISORY BOARD
Our Scientific Advisory Board is our network of medical, scientific and clinical advisors and collaborators who consult with our scientists. In addition, our Scientific Advisory Board members, none of whom are our managers or employees, advise us regarding our research and development programs, the design of our clinical trials, as well as other medical and scientific matters relating to our business. The following persons serve on our Scientific Advisory Board:
Joseph Bertino, M.D.,is the Associate Director of the Cancer Institute of New Jersey and University Professor of Medicine and Pharmacology.Pharmacology at the University of Medicine and Dentistry of New Jersey.
Jeffrey Bluestone, Ph.D.,is one of our scientific founders and is a Professor at the University of California, San Francisco and the Director of the UCSF Diabetes Center.
Edward Clark, Ph.D.,is a Professor of Immunology and a Professor of Microbiology at the University of Washington.
John Hansen, M.D.,is a Member, of Clinical Research at the Fred Hutchinson Cancer Research Center and Professor, Division of Medicine,Medical Oncology, University of Washington.
Carl June, M.D.,is one of our scientific founders and is the Vice ChairmanProfessor of the Department of MolecularPathology and Cellular EngineeringLaboratory Medicine at the University of Pennsylvania.
Hyam Levitsky, M.D., is a Professor of Oncology, Medicine and Urology at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
Ronald Levy, M.D.,is theProfessor of Medicine and Chief of the Division of Medical Oncology at the Stanford Medical Center.
Gerald Nepom, M.D., Ph.D.,is the Director, Benaroya Research Institute, at Virginia Mason.
E. Donnall Thomas, M.D.,is a Member and former Director of Clinical Research at the Fred Hutchinson Family Cancer Research Center. Dr. Thomas was awarded the 1990 Nobel Prize in Medicine.
Craig Thompson, M.D.,is one of our scientific founders and is the Scientific Director of the Abramson Family Cancer Research Institute at the University of Pennsylvania.
Robert M. Williams, Ph.D.,is a University Distinguished Professor, Department of Chemistry, at Colorado State University.
66 Dr. Williams is also a member of our board of directors.
EXECUTIVE OFFICERS AND DIRECTORSExecutive Officers and Directors
Set forth below is the name, age, position and a brief account of the business experience of each of our executive officers and directors:
Name | Age | Position(s) | ||
Ronald J. Berenson, M.D. | President, Chief Executive Officer and Director | |||
Robert L. Kirkman, M.D. | 55 | Chief Business Officer and Vice President | ||
Stewart Craig, Ph.D. | Chief Operating Officer and Vice President | |||
Mark Frohlich, M.D. | 42 | Medical Director and Vice President | ||
Lawrence A. Romel, M.S. | 47 | Vice President, Clinical Operations and Project Management | ||
Mark L. Bonyhadi, Ph.D. | Vice President of Research | |||
Kathi L. Cordova, C.P.A. | Senior Vice President of Finance and Treasurer | |||
Joanna S. Black, J.D. | General Counsel, Vice President and Secretary | |||
| Director | |||
| Director | |||
| ||||
Peter Langecker, M.D., Ph.D. | Director | |||
Robert T. Nelsen, M.B.A. | Director | |||
Daniel K. Spiegelman, M.B.A. | 46 | Director | ||
Stephen N. Wertheimer, M.M. | 53 | Director | ||
Robert M. Williams, Ph.D. | Director | |||
Ronald J. Berenson, M.D.,is our founder and has served as our President, Chief Executive Officer and as a member of our board of directors since our inception. From April 1989 until February 1995, Dr. Berenson held several positions at CellPro, Inc., a stem cell therapy company that he founded, with his last positions being Executive Vice President, and Chief Medical and Scientific Officer.Officer and Director. Dr. Berenson also served on the board of directors of CellPro, Inc. from July 1984 to March 1989, and currently serves on the board of directors of Calypso Medical Technologies, Inc.the Fred Hutchinson Cancer Research Center Foundation. Dr. Berenson was a faculty member at the Fred Hutchinson Cancer Research Center, where he last held the position of Assistant Member. Dr. Berenson is a board-certified internist and medical oncologist who completed his medical oncology fellowship training at Stanford University Medical Center. Dr. Berenson received a B.S. in biology from Stanford University and an M.D. from Yale University School of Medicine.
Robert Kirkman, M.D.,has served as our Vice President and Chief Business Officer since January 2004. Prior to joining us, Dr. Kirkman held the position of Vice President of Business Development and Corporate Communications at Protein Design Labs, Inc. from July 1998 to August 2003.Prior to that, Dr. Kirkman served as Chief of the Division of Transplantation at Brigham and Women’s Hospital, and as an Associate Professor of Surgery at Harvard Medical School. Dr. Kirkman received a B.A. in Economics from Yale University and an M.D. from Harvard Medical School. He is a Fellow of the American College of Surgeons.
Stewart Craig, Ph.D.,has served as our Chief Operating Officer and Vice President since October 1999. From July 1996 to September 1999, Dr. Craig served as Vice President of Development and Operations at Osiris Therapeutics, Inc., a stem cell therapy company. From January 1994 to June 1996, Dr. Craig served as Vice President of Product and Process Development at SyStemix Inc., a stem cell and gene therapy company. From June 1987 to December 1993, Dr. Craig held the positions of Group Leader and Senior Scientist at British Biotech, a biotechnology company. Dr. Craig received a B.Sc. in biochemistry and a Ph.D. in physical biochemistry from the University of Newcastle upon Tyne, UK.
Mark Frohlich, M.D.,has served as our Medical Director since October 2001 and has served as our Vice President since January 2002. Dr. Frohlich is a board-certified medical oncologist with an appointment as
Clinical Assistant Professor of Medicine at the University of Washington. From July 1998 to October 2001, Dr. Frohlich held the position of Assistant Adjunct Professor of Medicine at the University of California at San Francisco. From July 1994 to June 1998, Dr. Frohlich completed his fellowship in medical oncology at the University of California at San Francisco. Dr. Frohlich received a B.S. in electrical engineering and economics from Yale University and an M.D. from Harvard Medical School.
Lawrence A. Romel, M.S., has served as our Vice President of Clinical Operations and Project Management since July 2004. From June 2002 to July 2004, Mr. Romel served as Senior Director of Project Management, New Product Planning, and Clinical Operations at Cell Genesys, Inc., a cancer vaccine and oncolytic viral therapies company. From July 2000 to June 2002, Mr. Romel served as Vice President of Clinical Operations at SuperGen, Inc., an emerging pharmaceutical company. From August 1999 to July 2000, Mr. Romel served as Vice President Clinical Operations and Regulatory Affairs at Onyx Pharmaceuticals, Inc., a biotechnology company. Mr. Romel received a M.S. in Chemistry from the University of Illinois-Chicago.
Mark L. Bonyhadi, Ph.D.,has served as our Vice President of Research since January 2003. Dr. Bonyhadi previously served as our Director of Research from January 2002 to January 2003,
67
Management
Director of Strategic Scientific Development from April 2001 to December 2001 and Director of Biological Research from May 1997 to March 2001. From September 1990 to April 1997, Dr. Bonyhadi served as Senior Scientist with SyStemix, Inc., a stem cell and gene therapy company. Dr. Bonyhadi received a B.A. in biology from Reed College and a Ph.D. in immunology from the University of California at Berkeley.
Kathi L. Cordova, C.P.A.,has served as our Senior Vice President of Finance and Treasurer since September 2003. Ms. Cordova previously served as our Vice President of Finance from March 1997 to September 2003. From February 1994 to February 1997, Ms. Cordova held the position of Assistant Controller in a joint venture between American Life Insurance Company, a subsidiary of American International Group, an insurance company, and Italy’s Confederazione Italiana Sindicati dei Lavoratori, a labor union. From August 1991 to January 1994, Ms. Cordova served as Management Associate with the Life Division of American International Group, an insurance company. Ms. Cordova received a B.A. in international relations from Stanford University and an MAM.A. in international relations from The Johns Hopkins University.
Joanna S. Black, J.D.,has served as our General Counsel and Secretary since January 2002 and has served as our Vice President since September 2003. From September 1998 to January 2002, Ms. Black worked as an attorney at Venture Law Group, A Professional Corporation, a law firm. From August 1997 to August 1998, Ms. Black worked as an attorney at Wilson Sonsini Goodrich & Rosati, P.C., a law firm. Ms. Black received a B.A. in economics and public policy from Stanford University and a J.D. from Columbia University School of Law.
Robert E. Curry, Ph.D., has served as one of our directors since July 2002 and from May 2000 to January 2002. Dr. Curry has been a Venture Partner at Alliance Technology Ventures, a venture capital firm, since July 2002. Dr. Curry previously served as a General Partner of Sprout Group from May 1991 to June 2002. He currently is a director of Emerald Bio-Agricultural Corporation, a medical products company, and Tripath Imaging, Inc., a cancer therapy company. Dr. Curry received a B.S. in physics from the University of Illinois and an M.S. and Ph.D. in chemistry from Purdue University.
Jean Deleage, Ph.D.,has served as one of our directors since August 1996. Dr. Deleage ishas been a founder and managing director of Alta Partners, a venture capital firm since 1996, and was previously a founder of Burr, Egan, Deleage & Company, a venture capital fund, and Sofinnova Ventures, Inc., a venture capital fund. Dr. Deleage is a director of Crucell, Kosan Biosciences andIncorporated, Rigel Pharmaceuticals, Inc., and several private companies, all biopharmaceutical companies. Dr. Deleage received an M.S. in electrical engineering from the Ecole Supérieure d’Electricité and a Ph.D. in economics from the Sorbonne.
Dennis Henner, Ph.D.,has served as one of our directors since July 2002. Dr. Henner has been a General Partner at MPM Capital, a venture capital firm, since January 2002 and was a Venture Partner at MPM Capital from May 2001 through December 2001. From April 1996 to February 2001, Dr. Henner held the positions of Senior Vice President of Research and Vice President of Research at Genentech, Inc., a biotechnology company. Dr. Henner is currently a director of biotechnology companies Tercica, Medica, Inc., Rigel Pharmaceuticals, Inc., Synergia Pharma, Inc. and Rinat Neuroscience Corporation. Dr. Henner received his B.A. in Life Sciences and his Ph.D. from the Department of Microbiology at the University of Virginia.
Peter Langecker, M.D., Ph.D.,has served as one of our directors since December 1999.January 2000. Since October 1999, Dr. Langecker has served as Chief Medical Officer and Vice President of Clinical Affairs of BioMedicines, Inc.,
a biotechnology company. From July 1997 to September 1999, Dr. Langecker served as Vice President of Clinical Affairs and Regulatory Affairs of Sugen, Inc., a biotechnology company. From JulyMarch 1995 to July 1997, Dr. Langecker served as Vice President of Clinical ResearchAffairs of Coulter Pharmaceutical,Pharmaceuticals, Inc., a biotechnology company. Before that, Dr. Langecker held various medical positions at Ciba Geigy and
68
Management
Schering-Plough. Dr. Langecker received an M.D. and a Ph.D. in medical sciences from Ludwig Maximilians University in Munich.
Robert T. Nelsen, M.B.A.,has served as one of our directors since August 1996. Since 1992, Mr. Nelsen has served as a managing director of ARCH Venture Partners, a venture capital firm. Mr. Nelsen also serves as a director of Adolor Corporation, (ADLR), an analgesics development company, Caliper Technologies Corporation (CALP), a biochip company, and Illumina Corporation (ILMN), a biotechnology company. Mr. Nelsen received a B.S. in biology and economics from the University of Puget Sound and an M.B.A. from the University of Chicago.
Daniel K. Spiegelman , M.B.A.,has served as one of our directors since September 2004. Since September 1999, Mr. Spiegelman has served as the Senior Vice President and Chief Financial Officer for CV Therapeutics, Inc. From January 1998 to September 1999, Mr. Spiegelman served as Vice President and Chief Financial Officer for CV Therapeutics, Inc. From July 1991 until January 1998, Mr. Spiegelman was employed by Genentech, Inc., a biotechnology company, holding the position of treasurer from January 1996 to January 1998, assistant treasurer from July 1992 to December 1996, and treasury manager from July 1991 to July 1992. Mr. Spiegelman holds a B.A. in economics from Stanford University and an M.B.A. from Stanford Graduate School of Business.
Stephen N. Wertheimer, M.M., has served as one of our directors since November 2003. Mr. Wertheimer has served as a managing director of W Capital Partners, a private equity firm, since June 2001. From 1996 to June 2001, Mr. Wertheimer held the position of managing director of CRT Capital Group. Mr. Wertheimer is currently a director of El Paso Electric Company, an electric utility. Mr. Wertheimer received an M.M. from the Kellogg School, Northwestern University, and earned a B.S. in finance and economics at Indiana University.
Robert M. Williams, Ph.D.,has served as one of our directors since November 1996 and a member of our Scientific Advisory Board since November 1996.1995. Since September 1980, Professor Williams has served as a Professor of Chemistry at Colorado State University, and, in 2001, he was appointed University Distinguished Professor. During his career, Professor Williams has provided consulting services to several biotechnology and pharmaceutical companies, including Cubist Pharmaceutical Company, Microcide Pharmaceuticals, Hoffman-La Roche, G.D. Searle, W.R. Grace, NewBiotics ChemGenex, Nutrasweet and EPIX Medical, Inc. Professor Williams received a B.A. in chemistry from Syracuse University and a Ph.D. in organic chemistry from the Massachusetts Institute of Technology. Following graduate school, Professor Williams served as a postdoctoral fellow at Harvard University. He has served as a visiting professor at the University of California, Berkeley in 1990 and at Harvard University from 1994 to 1995.
BOARD COMPOSITIONBoard Composition
Our board of directors is currently comprised of seven directors and one vacancy. Following the closing of this offering, theeight directors. The board will beis divided into three classes, with each director serving a three-year term and one class being elected at each year’s annual meeting of stockholders. Dr. Langecker and Dr. Williams will be in the class of directors whose initial term expires at the 2004 annual meeting of stockholders. Drs. Deleage and Henner will be in the class of directors whose initial term expires at the 2005 annual meeting of stockholders. Directors Dr. Berenson,Deleage, Dr. CurryHenner and Mr. NelsenWertheimer will be in the class of directors whose initial term expires at the 2006 annual meeting of stockholders. Dr. Berenson, Mr. Spiegelman and Mr. Nelsen will be in the class of directors whose initial term expires at the 2007 annual meeting of stockholders.
BOARD COMMITTEESBoard Committees
Our board of directors has established an audit committee, a compensation committee, a nominating committee and a pricingstock option committee.
The audit committee currently consists of Dr. Deleage, Mr. Spiegelman and Mr. Wertheimer. Dr. Curry and Dr. Henner.Deleage serves as the chairperson of the committee. The audit committee is responsible for assuring the integrity of our financial control, audit and reporting functions and reviews with our management and our independent auditors the effectiveness of our financial controls and accounting and reporting practices and procedures. In addition, the audit committee reviews the qualifications of our independent auditors, is responsible for their appointment, compensation, retention and oversight and reviews the scope, fees and results of activities related to audit and non-audit services. Prior to the formation of the audit committee, the full board of directors conducted the responsibilities of the audit committee, which met annually with representatives of our independent auditors, including in executive sessions where members of management were excused.
The pricingcompensation committee consists of Mr. Nelsen, Dr. Berenson, Dr. Deleage, Dr. CurryLangecker and Mr. Nelsen. The pricing committee is responsible for determining the terms of this initial public offering, including, but not limited to, determining the number of shares to be sold by us and the initial public offering price per share.
69
Management
Effective upon this offering, the compensation committee will consist of Dr. Curry, Mr. Nelsen and Dr. Langecker.Spiegelman. The compensation committee’s principal responsibility is to administer our stock plans and to set the salary and incentive compensation, including stock option grants, for our Chief Executive Officer and senior staff members.other executive officers.
The nominating committee consists of Dr. Langecker, Mr. Wertheimer and Dr. Williams. The purpose of the nominating committee is to identify individuals qualified to serve as members of our Board, and recommend nominees for election.
The stock option committee consists of Dr. Berenson and Mr. Nelsen. The stock option committee’s principal responsibility is to grant stock options under the our stock plans to newly hired non-executive officers in accordance with set parameters outlined by the Board.
DIRECTOR COMPENSATIONDirector Compensation
Our sixseven outside directors are compensated with cash and options to purchase our common stock. The onlystock pursuant to our 2003 Directors’ Stock Option Plan. Non-employee directors are entitled to an annual cash compensation theyretainer of $20,000, and receive is$1,000 for each board meeting attended in person, $500 for each board meeting participated in telephonically, and $500 for each committee meeting participated, in addition to reimbursement for out-of-pocket expenses incurred in connection with attending board and committee meetings.
In November 1996, Dr. Deleage and Dr. Williams were each awarded non-statutory options for 30,0005,454 shares of our common stock. In November 1999, Dr. Langecker was awarded a non-statutory option for 30,0005,454 shares of our common stock. These shares vest over a four-year period at a rate of 25% of the total number of shares one year after the date of grant, with the remaining shares vesting monthly in equal installments over the next 36 months. In November 2003, Dr. Williams was awarded non-statutory options for 2,727 fully vested shares of our common stock in connection with his service on our Scientific Advisory Board. In September 2004, in connection with his election to our board of directors, Mr. Spiegelman was granted an option to purchase 10,000 shares of our common stock under the amended 2003 Directors’ Stock Option Plan, which option is subject to stockholder approval and is not exercisable until such approval. Directors who are our employees are eligible to participate in our 1996 Stock Option Plan, and, effective at the closing of this offering, they will also be eligible to participate in our 2003 Stock Plan and 2003 Employee Stock Purchase Plan. Until the closing of this offering, directorsDirectors who are not our employees have been eligible to participate in our 1996 Stock Option Plan. Effective at the closing of this offering, directors who are not our employees will be eligible to participate in our 2003 Directors’ Stock Option Plan as well as our 2003 Stock Plan, but will no longer be eligible to participate in our 1996 Stock Option Plan.
Pursuant to our 2003 Directors’ Stock Option Plan, each non-employee director joining our board after June 2, 2004 is automatically granted an option to purchase 10,000 shares of our common stock. In addition, on the date of each annual meeting of our stockholders, each non-employee director is granted an option to purchase 10,000 shares of common stock if, on that date, the director has served on our board of directors for at least six months. Furthermore, directors serving as the chairperson of a committee of our board, or as members of the audit committee of our board, are granted an option to purchase 2,500 shares of common stock on the date of each annual meeting of our stockholders. The total number of shares subject to options granted under this plan vests in equal monthly installments over two years. Although this plan is currently effective, prior to receiving stockholder approval, options granted under this plan will not be exercisable and will be contingent on such approval
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATIONCompensation Committee Interlocks and Insider Participation
Dr. Deleage, Mr. Nelsen, Dr. Curry and Dr. Berenson served on our compensation committee in 2002.2003. During 2002,2003, none of our executive officers served as a director or member of the compensation committee of any other entity that had any executive officer who served on our board of directors or on our compensation committee.
LIMITATIONS ON LIABILITY AND INDEMNIFICATION OF OFFICERS AND DIRECTORSLimitations on Liability and Indemnification of Officers and Directors
Our amended and restated certificate of incorporation which will be effective upon the closing of this offering, limits the liability of our directors to the maximum extent permitted by Delaware law. Delaware law provides that a corporation may eliminate the personal liability of its
directors for monetary damages for breach of their fiduciary duties as directors, except liability for any of the following acts:
| breach of their duty of loyalty to us or our stockholders; |
| acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| unlawful payments of dividends or unlawful stock repurchases or redemptions; and |
| any transaction from which the director derived an improper personal benefit. |
Our amended and restated bylaws which will be effective upon the closing of this offering, provide that we will indemnify our directors, officers, employees and other agents to the fullest extent permitted by the Delaware General Corporation Law. Our amended and restated bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent of ours for any liability arising out of his or her actions in such capacity, regardless of whether the Delaware General Corporation Law would permit a corporation to indemnify for such liability.
70
Management
We have obtained directors’ and officers’ insurance providing indemnification for all of our directors, officers and employees for certain liabilities. In addition to the indemnification provided for in our amended and restated bylaws, we have entered into agreements to indemnify our directors and executive officers. These agreements, among other things, indemnify our directors and executive officers for expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any of them in any action or proceeding arising out of his or her services as a director, officer, employee, agent or fiduciary of ours, any subsidiary of ours or any other company or enterprise to which the personhe or she provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers. At present, there is no litigation or proceeding involving any of our directors or officers in which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
EXECUTIVE COMPENSATIONExecutive Compensation
The following table summarizes the compensation paid to, awarded to or earned during the years ended December 31, 2002 and 2003 by our Chief Executive Officer and each of our four other most highly compensated executive officers whose total salary and bonus exceeded $100,000 for services rendered to us in all capacities during 2002.the years ended December 31, 2002 and 2003. The executive officers listed in the table below are referred to in this prospectus as our named executive officers.
Summary compensation tableCompensation Table
| Annual compensation for 2002 | Long-term compensation securities underlying options | All other compensation ($) | |||||||
Name and principal position(s) | Salary ($) | Bonus ($) | |||||||
Ronald J. Berenson, M.D. President and Chief Executive Officer | 239,276 | 25,051 | — | 595 | (1) | ||||
Stewart Craig, Ph.D. Chief Operating Officer and Vice President | 205,714 | 51 | — | 527 | (2) | ||||
Kathi Cordova, C.P.A. Senior Vice President of Finance and Treasurer | 139,588 | 74 | — | 391 | (3) | ||||
Mark Frohlich, M.D Vice President and Medical Director | 172,183 | 16,043 | — | 534 | (4) | ||||
Lewis Chapman Chief Business Officer | 100,403 | 40,051 | — | 312 | (5) | ||||
| Year | Annual compensation | Long-term compensation Securities underlying options | All other compensation | ||||||||||||
| Salary | Bonus | |||||||||||||||
Ronald J. Berenson, M.D. President and Chief Executive Officer | 2003 2002 | $ | 249,714 239,276 | $ | 35,000 25,051 | $ | — | $ | 286 595 | (1) (1) | ||||||
Stewart Craig, Ph.D. Chief Operating Officer and Vice President | 2003 2002 | 215,176 205,714 | — 51 | — | 284 527 | (2) (2) | ||||||||||
Kathi L. Cordova, C.P.A. Senior Vice President of Finance and Treasurer | 2003 2002 | 150,547 139,588 | — | — | 286 391 | (3) (3) | ||||||||||
Mark Frohlich, M.D Medical Director and Vice President | 2003 2002 | 181,759 172,183 | 17,447 16,043 | — | 513 534 | (4) (4) | ||||||||||
Lewis Chapman Chief Business Officer | 2003 2002 | 201,488 100,403 | 35,000 40,051 | — | 380 312 | (5) (5) | ||||||||||
Joanna S. Black, J.D. General Counsel and Vice President | 2003 2002 | (7) | 154,882 128,656 | — 51 | — | 264 377 | (6) (6) | |||||||||
Footnotes appear on following page
| (1) | Dr. Berenson received other compensation consisting of the payment of insurance premiums for group term life benefits in the amount of |
| (2) | Dr. Craig received other compensation consisting of the payment of insurance premiums for group term life benefits in the amount of |
| (3) | Ms. Cordova received other compensation consisting of the payment of insurance premiums for group term life benefits in the amount of |
| (4) | Dr. Frohlich received other compensation consisting of the payment of insurance premiums for group term life benefits in the amount of |
| (5) | Mr. Chapman received other compensation consisting of the payment of insurance premiums for group term life insurance in the amount of |
71
Management
| (6) | Ms. Black received other compensation consisting of the payment of insurance premiums for group term life benefits in the amount of $264 in 2003 and $377 in 2002. |
| (7) | Ms. Black joined Xcyte Therapies, Inc. in January 2002. |
The following table provides summary information concerning the individual grants of stock options to each of our named executive officers for the fiscal year ended December 31, 2002.2003. The exercise price per share was valued by our board of directors on the date of grant, and each option was issued at the estimated fair market value on the date of grant based upon the purchase price paid by investors for shares of our preferred stock, taking into account the liquidation preferences and other rights, privileges and preferences associated with such preferred stock.
Each option represents the right to purchase one share of our common stock. The options generally become vestedvest over four years. See “Management—Employee benefit plans”Equity Compensation Plan Information” for more details regarding these options. In 2002,2003, we granted options to purchase an aggregate of 1,960,998225,470 shares of our common stock to various officers, employees, directors and others.
The potential realizable value at assumed annual rates of stock price appreciation for the option term represents hypothetical gains that could be achieved for the respective options if exercised at the end of the option term. SEC rules specify the 0%, 5% and 10% assumed annual rates of compounded stock price appreciation, which do not represent our estimate or projection of our future common stock prices. These amounts represent assumed rates of appreciation in the value of our common stock from the initial public offering price, (assuming anbased on the initial public offering price of $$8 per share).share. Actual gains, if any, on stock option exercises depend on the future performance of our common stock and overall stock market conditions. The amounts reflected in the table may not necessarily be achieved.
Option grantsGrants in fiscal year 2002(1)Fiscal Year 2003(1)
Named executive officers | Number of securities underlying options granted | Percentage of total options granted to employees (%) | Exercise price per share ($) | Market price of underlying security on grant date ($) | Expiration date | Potential realizable value at assumed annual rates of stock appreciation for option term ($) | |||||||||||||||||||||||||||||
| 0% | 5% | 10% | |||||||||||||||||||||||||||||||||
Named executive officer | Number of securities underlying options granted | Percentage of total options granted to employees | Exercise price per share | Expiration date | Potential realizable value at assumed annual rates of stock appreciation for option term | ||||||||||||||||||||||||||||||
| 0% | 5% | 10% | |||||||||||||||||||||||||||||||||
Ronald J. Berenson, M.D. | 250,000 | 12.98 | 1.00 | 10/23/12 | 45,453 | 21.18 | % | $ | 5.50 | 09/22/13 | $ | 113,633 | $ | 342,314 | $ | 693,156 | |||||||||||||||||||
Stewart Craig, Ph.D. | 200,000 | 10.38 | 1.00 | 01/30/12 | 18,181 | 8.47 | % | 5.50 | 09/22/13 | 45,453 | 136,924 | 277,259 | |||||||||||||||||||||||
Mark Frohlich, M.D. | 160,000 | 8.30 | 1.00 | 01/30/12 | 36,363 | 16.94 | % | 5.50 | 09/22/13 | 90,908 | 273,855 | 554,534 | |||||||||||||||||||||||
Kathi Cordova, C.P.A. | 80,000 | 4.15 | 1.00 | 01/30/12 | |||||||||||||||||||||||||||||||
Lewis Chapman(2) | 400,000 | 20.76 | 1.00 | 07/23/12 | |||||||||||||||||||||||||||||||
Kathi L. Cordova, C.P.A. | 18,181 | 8.47 | % | 5.50 | 09/22/13 | 45,453 | 136,924 | 277,259 | |||||||||||||||||||||||||||
Joanna S. Black, J.D. | 13,636 | 6.35 | % | 5.50 | 09/22/13 | 34,090 | 102,695 | 207,948 | |||||||||||||||||||||||||||
Lewis Chapman | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||
| (1) These |
72
Management
The following table shows information as of December 31, 2002,2003 concerning the number and value of exercised options and unexercised options held by each of our named executive officers. There was no public trading market for our common stock as of December 31, 2002.2003. Accordingly, the value of the unexercised in-the-money options listed below has been calculated on the basis of the assumedour initial public offering price of $$8 per share, less the applicable exercise price per share multiplied by the number of shares underlying the options.
Aggregated option exercises during 2002Option Exercises During 2003 and year-end option valuesFiscal Year-End Option Values
| Shares acquired upon exercise | Value realized($) | Number of securities December 31, 2002(#) | Value of unexercised in-the-money options at December 31, 2002($) | |||||||||
Named executive officers | Exercisable | Unexercisable | Exercisable | Unexercisable | ||||||||
Ronald J. Berenson, M.D. | — | — | 158,333 | 241,667 | ||||||||
Stewart Craig, Ph.D. | — | — | 286,666 | 163,334 | ||||||||
Mark Frohlich, M.D. | — | — | 40,999 | 159,001 | ||||||||
Kathi Cordova, C.P.A. | — | — | 39,666 | 65,334 | ||||||||
Lewis Chapman(1) | — | — | — | 400,000 | ||||||||
Named executive officer | Shares acquired upon exercise | Value realized | Number of securities underlying unexercised options at December 31, 2003 | Value of unexercised in-the-money options at December 31, 2003 | |||||||||||
| Exercisable | Unexercisable | Exercisable | Unexercisable | ||||||||||||
Ronald J. Berenson, M.D. | — | $ | — | 40,150 | 78,029 | $ | 225,322 | $ | 195,073 | ||||||
Stewart Craig, Ph.D. | — | — | 60,301 | 39,696 | 347,347 | 99,240 | |||||||||
Mark Frohlich, M.D. | — | — | 16,907 | 55,818 | 42,268 | 139,545 | |||||||||
Kathi L. Cordova, C.P.A. | — | — | 11,029 | 26,242 | 48,395 | 65,605 | |||||||||
Joanna S. Black, J.D. | — | — | 8,521 | 23,295 | 21,303 | 58,238 | |||||||||
Lewis Chapman | — | — | — | — | — | — | |||||||||
EMPLOYMENT AGREEMENTSEmployment Agreements
Ms. Black’s employment agreement, dated December 31, 2001, provides for at-will employment for an unspecified term. Under this agreement, Ms. Black is entitled to an annual base salary of $150,000 per year and an initial stock option grant for 50,0009,090 shares of our common stock. This employment agreement also provides that Ms. Black will receive severance payments equal to three months of her then current base salary, paid ratably over a three-month period, and three months of continued health coverage if her employment is terminated other than for cause and she signs a standard release of any claims against us.
Mr. Chapman’s employment agreement, dated May 29, 2002, providesprovided for at-will employment for an unspecified term. Under this agreement, Mr. Chapman iswas entitled to an annual base salary of $200,000 per year, an initial stock option grant for 400,00072,727 shares of our common stock, a one-time signing bonus of $40,000 and a one-time home purchase bonus of $35,000. This employment agreement also providesprovided that Mr. Chapman willwould receive severance payments equal to six months of his then current base salary, paid ratably over a six-month period, and six months of continued health coverage if his employment iswas terminated other than for cause and he signssigned a standard release of any claims against us. Mr. Chapman’s employment with us ended in August 2003, and we are currentlyhave completed making theseall severance payments owed to him.him under this agreement.
Dr. Frohlich’s employment agreement, dated August 27, 2001, provides for at-will employment for an unspecified term. Under this agreement, Dr. Frohlich is entitled to an annual base salary of $170,000, an initial stock option grant for 40,0007,272 shares of our common stock, a one-time signing bonus of $40,000 and a loan of $50,000 for a down payment of a principal residence.residence forgiven over four years. This employment agreement also provides that Dr. Frohlich will receive severance payments equal to three months of his then current base salary, paid ratably over a three-month period, and three months of continued health coverage if his employment is terminated other than for cause and he signs a standard release of any claims against us. In this event, Dr. Frohlich’s employment agreement provides that we will forgive up to $40,000the outstanding principal of the amount loaned to him for a down payment on a principal residence.
73Dr. Kirkman’s employment agreement, dated January 15, 2004, provides for at-will employment for an unspecified term. Under this agreement, Dr. Kirkman will receive an annual base salary of $240,000, a stock option grant for 72,727 shares of our common stock, a one-time signing bonus of $85,000 and relocation assistance reimbursement up to an aggregate of $15,000. This employment agreement also provides that Dr. Kirkman will receive severance payments equal to six months of his then current base salary, paid ratably over a six-month period, and six months of continued health coverage if his employment is terminated other than for cause during the first year of his employment, provided that he signs a standard release of any claims against us at such time.
ManagementMr. Romel’s offer letter dated June 14, 2004, provides for at-will employment for an unspecified term. Under this agreement, Mr. Romel will receive an annual base salary of $198,000, a stock option grant for 30,000 shares of our common stock, a lump-sum relocation bonus of $8,250 and relocation assistance reimbursement. This offer letter also provides that Mr. Romel will receive severance payments equal to three months of his then current base salary, paid ratably over a three-month period, and three months of continued health coverage if his employment is terminated other than for cause, provided that he signs a standard release of any claims against us at such time.
EQUITY COMPENSATION PLAN INFORMATIONEquity Compensation Plan Information
2003 Stock Plan
Our 2003 Stock Plan was adopted by our board of directors in September 2003 and will be submitted for approvalwas approved by our stockholders prior to completion of this offering.in March 2004. This plan provides for the grant of incentive stock options to employees (including employee directors) and nonstatutory stock options and stock purchase rights to employees, directors (excluding non-employee directors) and consultants. The purposes of this plan are to attract and retain the best available personnel, to provide additional incentives to our employees and consultants and to promote the success of our business. A total of 3.5 million636,363 shares of common stock will beare reserved for issuance under this plan.
As of September 27, 2004:
| · | 44,880 shares of common stock were issuable upon exercise of outstanding options granted under this option plan at a weighted average exercise price of $4.27; |
| · | no shares of common stock were issued upon exercise of options; and |
| · | 591,483 shares of common stock remained available for future grants under this plan. |
The number of shares reserved for issuance under this plan will automatically increase on the first day of each fiscal year beginning in 2005 and ending in 2010 by the lesser of:
| 4% of the number of shares of our common stock outstanding on the last day of the immediately preceding fiscal year; or |
| any lesser number of shares that our board of directors determines. |
All share numbers reflected in this plan summary, as well as the exercise price or purchase price applicable to outstanding options or purchase rights, will be automatically proportionately adjusted in the event we undertake certain changes in our capital structure, such as a stock split, stock dividend or other similar transaction.
The administrator of the plan is our board of directors or a committee of our board. In the case of options and stock purchase rights intended to qualify as “performance based“performance-based compensation” within the meaning of Section 162(m) of the Internal Revenue Code of 1986, as amended, the committee will consist of two or more “outside directors” within the meaning of Section 162(m). In addition, in administering the plan, we intend to comply with other applicable legal and regulatory requirements as may apply from time to time, including any NASDAQNasdaq listing requirements. The administrator determines the terms of options and stock purchase rights granted under this plan, including the number of shares subject to the award, the exercise or purchase price and the vesting and/or exercisability of the award and any other conditions to which the award is subject. No employee, however, may receive awards for more than 1 million181,818 shares under this plan in any fiscal year. Incentive stock options granted under this plan must have an exercise price of at least 100% of the fair market value of the common stock on the date of grant. Incentive stock options granted to an employee who holds more than 10% of the total voting power of all classes of our stock or any parent or subsidiary’s stock cannot be less than 110% of the fair market value of the common stock on the date of grant. The exercise price of nonstatutory stock options and the purchase price of stock purchase rights will be the price determined by the administrator, although nonstatutory stock options and stock purchase rights granted to our Chief Executive Officer and our four other most highly compensated officers will generally equal at least 100% of the grant date fair market value if we
intend that the awards to those individuals will qualify as “performance-based compensation” within the meaning of Section 162(m) of the Internal Revenue Code. Payment of the exercise or purchase price may be made in cash or any other consideration determined by the administrator.administrator, subject to applicable legal requirements.
The administrator will determine the term of options granted under this plan, which may not exceed 10 years, or 5 years in the case of an incentive stock option granted to a holder of more than 10% of the total voting power of all classes of our stock or a parent or subsidiary’s stock. Generally, an option
74
Management
granted under this plan is nontransferable,non-transferable, other than by will or the laws of descent or distribution, and may be exercised during the lifetime of the optionee only by the optionee. However, the administrator may, in its discretion, provide for the limited transferability of nonstatutorynon-statutory stock options granted under this plan. We generally have the right to repurchase any stock issued pursuant to stock purchase rights granted under this plan upon the termination of the holder’s employment or consulting relationship with us for any reason, including death or disability. The repurchase price is the exerciseoriginal purchase price ofpaid by the stock purchase rightpurchaser or the fair market value of the shares at the date of the repurchase, whichever is less. This repurchase right will lapse at sucha rate asthat the administrator may determine.
If we sell all or substantially all of our assets or if we are acquired by another corporation, each outstanding option and stock purchase right may be assumed or an equivalent award may be substituted by the successor corporation, with appropriate adjustments made to both the price and number of shares subject to the option or purchase right. If the successor does assume the outstanding options and purchase rights, the lesser of 25% of the shares subject to an option or initially subject to repurchase or the remaining unvested shares will vest immediately prior to the closing of the transaction, and, if the holder is “involuntarily terminated” within one year after the closing, the lesser of another 25% of the shares subject to the option or initially subject to repurchase or the remaining unvested shares will vest on termination. “Involuntary termination” includes termination by Xcyteus without cause,“cause,” or voluntary resignation within 30 days following: a reduction in the optionholder’s base salary of more than 20% (except where there is a similar reduction in the base salaries of similarly situated employees) or relocation of the optionholder’s principal work site by more than 50 miles. If the successor corporation does not assume options and purchase rights or substitute equivalent options or purchase rights, then vesting of all shares subject to options will accelerate fully, all repurchase rights will lapse immediately prior to the closing of the transaction and options and purchase rights will terminate as of the closing of the transaction.
The administratorboard of directors has authority to amend or terminate this plan, but no action may be taken that impairs the rights of any holder of an outstanding option or stock purchase right without the holder’s consent. In addition, we must obtain stockholder approval of amendments to the plan as required by applicable law. Unless terminated earlier by the board of directors, this plan will terminate in 2013.
1996 Stock Option Plan
Our 1996 Stock Option Plan was adopted by our board of directors in September 1996. As of September 30, 2003:27, 2004:
The board of directors amended this plan in September 2003 to increase the number of shares reserved for issuance under the plan by an additional 2 million363,636 to 6.4 million. The1,163,636 and the amended plan will be submitted towas approved by our stockholders for approval prior to completion of this offering.in March 2004. All share numbers reflected in this plan summary, as well as the exercise price or purchase price applicable to outstanding options, or purchase rights, will be automatically proportionately adjusted in the event we make certain changes in our capital structure, such as a stock split, stock dividend or other similar transaction.
75
Management
The terms of the awards under this plan are generally the same as the terms of the awards that may be issued under the 2003 Stock Plan, except for the following features:
| only options can be granted under this plan; |
| stock options granted under this plan are |
| options granted to residents of California prior to the closing of |
If we sell all or substantially all of our assets, or if we are acquired by another corporation, each outstanding option may be assumed or an equivalent award substituted by the successor corporation, with appropriate adjustments made to both the price and number of shares subject to the option. If the successor does assumeassumes the outstanding options or substitutes equivalent options, 25% of the shares subject to each option that are unvested immediately prior to the consummation of the transaction will vest immediately prior to the closing of the transaction. If the successor corporation does not assume options or substitute equivalent options or a comparable cash incentive program based on the value of the options at the closing, then vesting of all shares subject to options will accelerate fully immediately prior to the closing of the transaction unless otherwise provided under an individual grant.
2003 Employee Stock Purchase Plan
Our 2003 Employee Stock Purchase Plan was adopted by theour board of directors in September 2003 and will be submitted for approvalwas approved by our stockholders prior to completion of this offering.in March 2004. A total of 600,000109,090 shares of common stock will beare reserved for issuance under this plan, none of which have been issued as of the date of this prospectus.September 27, 2004. The number of shares reserved for issuance under this plan will automatically increase on the first day of each of our fiscal years beginning in 2005 and ending in 2010 and equal toby the lesser of:
| 1% of the number of shares of common stock outstanding on the last day of the immediately preceding fiscal year; or |
| any lesser number of shares that our board of directors determines. |
All share numbers reflected in this plan summary, as well as the exercise price or purchase price applicable to outstanding options or purchase rights, will be automatically proportionately adjusted in the event we make certain changes in our capital structure, such as a stock split, stock dividend or other similar transaction. If approved by our stockholders, thisThis plan becomesbecame effective upon the date of thisour initial public offering. Unless terminated earlier by our board of directors, this plan terminates in 2023.
This plan, which is intended to qualify under Section 423 of the Internal Revenue Code, allows employees to purchase our common stock at a discount from the market price through payroll deductions. The plan will be implemented by a series of offering periods, each of which has a duration of approximately six months, commencing generally on May 1 and November 1 of each year. We expect theThe first offering period to commencecommenced on the effective date of the registration statement of which this prospectus is a partMarch 16, 2004 and will end on April 30,October 31, 2004. Each eligible employee will automatically be granted an option to participate in the plan and will be automatically enrolled in the first offering period. Payroll
76
Management
deductions and continued participation in the initial offering period will not be determined until after the effective date of the Form S-8 registration statement, which we intend to file to register the shares reserved for issuance under this plan, as described below. At the end of each offering period, anAn automatic purchase will be made for participants.participants on the last trading day of each offering period.
Our board of directors, or a committee appointed by the board, will administer this plan. In addition, in administering the plan, we intend to comply with other applicable legal and regulatory requirements as may apply from time to time, including any NASDAQNasdaq listing requirements. Our employees, including officers and
employee directors or employees of any majority-owned subsidiary designated by the board, are eligible to participate in this plan if they are customarily employed by us or any such subsidiary for at least 20 hours per week and more than five months per year. The plan prohibits granting an optionpurchase rights to an employee in the following circumstances:
| where, immediately after the grant, the employee would own stock and/or hold outstanding options to purchase stock equaling 5% or more of the total voting power or value of all classes of our stock or the stock of our subsidiaries; or |
| where the option would permit the employee to purchase stock under this plan at a rate that exceeds $25,000 |
This plan permits eligible employees to purchase common stock through payroll deductions of up to 15% of an employee’s eligible cash compensation, which includes salary, bonuses and other wage payments made by us to the participants. A participant may purchase a maximum of 2,500454 shares of our common stock under this plan in any one offering period.
Amounts deducted and accumulated by plan participants are used to purchase shares of our common stock at the end of each six-month offering period. The purchase price is equal to 85% of the fair market value of the common stock at the beginningfirst trading day of the offering period or at the endlast trading day of the offering period, whichever is less. Employees may end their participation in this plan at any time duringprior to the last trading day of an offering period, and participation ends automatically on termination of employment.
If we merge or consolidate with or into another corporation or sell all or substantially all of our assets, each right to purchase stock under this plan may be assumed, or an equivalent right substituted, by the successor corporation. However, if the successor corporation refuses to assume each purchase right or to substitute an equivalent right, the board of directors will shorten any ongoing offering period so that employees’ rights to purchase stock under this plan are exercised prior to the transaction. Our board of directors may extend future offering periods to up to 27 months and may increase or decrease the maximum contribution rate of an employee’s eligible cash compensation. Our board of directors has the power to amend or terminate this plan as long as the action does not adversely affect any outstanding rights to purchase stock under the plan. However, our board of directors may amend or terminate this plan or an offering period even if it would adversely affect outstanding optionspurchase rights in order to avoid our incurring adverse accounting charges or if the board of directors determines that termination of the plan or offering period is in our best interests and the best interests of our stockholders. We mustwill obtain stockholder approval for any amendment to the purchase plan to the extent required by law.
2003 Directors’ Stock Option Plan
Our 2003 Directors’ Stock Option Plan was adopted by our board of directors in September 2003 and was approved by our stockholders in March 2004. In June 2004, our board of directors amended this plan which amendments will be submitted for approval by our stockholders at our next annual meeting of our stockholders in 2005. This plan is currently effective but prior to completion of this offering. If approved by our stockholders, thisreceiving stockholders’ approval, options granted under the amended plan will become effectivenot be exercisable and will be contingent on the effective date of the registration statement of
77
Management
which this prospectus is a part.such approval. A total of 500,00090,909 shares of common stock will beare reserved for issuance under the this plan, all of which remain available for future grants as of the date of this prospectus.plan. All share numbers reflected in this plan summary, as well as the exercise price or purchase price applicable to outstanding options, or purchase rights, will be automatically proportionately adjusted in the event we make certain changes in our capital structure, such as a stock split, stock dividend or other similar transaction.
As of September 27, 2004:
| · | 10,000 shares of common stock were issuable upon exercise of outstanding options granted under this option plan at an exercise price of $4.53; |
| · | no shares of common stock were issued upon exercise of options; and |
| · | 80,909 shares of common stock remained available for future grants under this plan. |
This plan is designed to work automatically, without administration. However, to the extent administration is necessary, it will be performed by our board of directors. It is expected that any conflicts of interest that may arise will be addressed by abstention of any interested director from both deliberations and voting regarding matters in which the director has a personal interest. Unless terminated earlier by the board of directors, this plan will terminate in 2013.
This plan provides that each person who first becomes a non-employee director after the completion of this offeringJune 2, 2004 will be granted a nonstatutorynon-statutory stock option to purchase 25,00010,000 shares of our common stock on the date when the person first becomes a member of our board of directors. On the date of each annual meeting of our stockholders, each of our nonemployeenon-employee directors (including nonemployeenon-employee directors who did not receive the 25,00010,000 share grant described above) will be granted an option to purchase 10,000 shares of common stock if, on that date, the director has served on our board of directors for at least six months. In addition, directors serving as the chairperson of a committee of the board, or as members of the audit committee of the board, will be granted an option to purchase 2,500 shares of common stock on the date of each annual meeting of our stockholders. The exercise price of all stock options granted under this plan will be equal to the fair market value of the common stock on the date of grant of the option. This plan provides that one third of the total number of shares subject to each option granted to a new director will vest 12 months after the date of grant. Afterwards, the remaining sharesunder this plan will vest in equal monthly installments over the next two years so that the option will be fully vested after threetwo years. Annual options granted to directors will vest in full on the day prior to the first anniversary of the date of the grant of the option.
All options granted under this plan will have a term of 10 years and an exercise price equal to the fair market value on the date of grant. If a non-employee director ceases to serve as a director for any reason other than death or disability, he or she may, within the 90 days after the date he or she ceases to be a director, exercise options that were vested as of the date of termination. If the former director does not exercise the option within this 90-day period, the option will terminate. If a director’s service terminates as a result of his or her disability or death, or if a director dies within three months following termination, the director or his or her estate may exercise options that were vested as of the date of termination or death at any time during the 12 months after the date of termination or death. Options granted under this plan are generally non-transferable by the option holder other than by will or the laws of descent or distribution, pursuant to a qualified domestic relations order or to family members or family trusts or foundations. Generally, only the option holder or a permitted transferee may exercise the option during the lifetime of the option holder.
If we are acquired by another corporation, each option outstanding under this plan will be assumed or equivalent options will be substituted by our acquirer,acquiror, unless our acquireracquiror does not agree to this assumption or substitution. If our acquireracquiror does not agree to assume the options or substitute them, the options will terminate upon consummation of the transaction to the extent not previously exercised.transaction. In connection with an acquisition that qualifies as a change of control as defined in the option plan, the vesting of each outstanding option will accelerate in full, and each director holding options under this plan will have the right to exercise his or her options immediately before the consummation of the acquisition as to all shares underlying the options. Our board of directors may amend or terminate this plan as long as doing so does not adversely affect any outstanding option and we obtain stockholder approval for any amendment to the extent required by applicable law and the procedure for option grants are not amended more than once every 6 months, other than to the extent required by applicable law. Any such amendment or termination shall not adversely affect outstanding options granted under the plan.
78
Management
401(k) planPlan
Effective February 1, 1997, we established a tax-qualified employee savings and retirement plan, or 401(k) plan, which covers all of our employees. This plan is intended to qualify under Section 401 of the Internal Revenue Code so that contributions by us to the plan, if any, will be deductible by us when made. Under this plan, eligible employees may elect to reduce their current compensation and defer their pre-tax earnings, subject to the Internal Revenue Service’s annual contribution limits. Deferral contributions are fully vested at all times. This plan permits, but does not require, discretionary matching contributions by a percentage amount that our board of directors may annually determine. The plan also permits additional discretionary contributions by us on behalf of all participants in the plan. These additional company contributions vest 25% per year of service and will be fully vested after four years of service. The trustee under the plan invests an employee’s account balance under the plan in accordance with the employee’s written direction. To the extent an employee directs the investment of his or her account balance under the plan, the Employment Retirement Income Security Act relieves the trustee from liability for any loss resulting from the employee’s direction of the investment.
79CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Certain relationshipsDuring the last three fiscal years, there has not been any transaction or series of similar transactions to which we were or are a party in which the amount involved exceeded or exceeds $60,000 and related partyin which any of our directors or executive officers, any holder of more than 5% of any class of our voting securities or any member of the immediate family of any of these persons had or will have a direct or indirect material interest, other than the compensation arrangements described in “Management” above and the transactions described below.
We believe that we have executed all of the transactions described below on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors and principal stockholders and their affiliates are approved by a majority of our board of directors, including a majority of the independent and disinterested members of our board of directors, and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
From our inception through September 27, 2004, we issued the following securities to various investors in private placement transactions:
| · | 1,151,664 shares of Series A preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, CV Sofinnova Venture Partners and Sprout Group at a purchase price of $5.23 per share in August 1996; |
| · | 683,125 shares of Series B preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, CV Sofinnova Venture Partners and Sprout Group at a purchase price of $6.05 per share in August 1997; |
| · | 1,306,470 shares of Series C preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, CV Sofinnova Venture Partners, Falcon Technology Partners, Fluke Capital Management, TGI Fund (W Capital Partners acquired these shares from TGI Fund), Sprout Group and Vulcan Ventures at a purchase price of $9.19 per share in July 1998; |
| · | 1,838,139 shares of Series D preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, MPM Capital, Sprout Group, Vector Fund, Vulcan Ventures and TGI Fund (W Capital Partners acquired these shares from TGI Fund) at a purchase price of $15.29 per share in May 2000 and August 2000; |
| · | 863,648 shares of Series E preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, China Development Industrial Bank Inc., MPM Capital, Sprout Group, Vulcan Ventures and TGI Fund (W Capital Partners acquired these shares from TGI Fund) at a purchase price of $15.29 per share in November 2001; and |
| · | 808,040 shares of Series F preferred stock to investors including, but not limited to, entities affiliated with Alta Partners, ARCH Venture Partners, RiverVest Venture Fund, Sprout Group, Vector Fund and V-Sciences Investments Pte Ltd at a purchase price of $15.29 per share in February and March 2002. |
In addition, we issued:
| · | 545,434 shares of common stock and 95,690 shares of Series A preferred stock in exchange for all of the outstanding capital stock of CellGenEx, Inc. in August 1997 and April 1998; and |
| · | 26,522 shares of Series B preferred stock in July 1998 and 3,636 shares of common stock in June 1999 in connection with license agreements. |
In addition, as of September 27, 2004, warrants to purchase an aggregate of 46,607 shares of common stock issued since our inception remained outstanding.
Since our inception, we have engaged in transactions with our executive officers, directors and holders of more than 5% of our voting securities and their respective affiliates. The following table summarizes the number of shares of our stock purchased by our executive officers, directors and 5% stockholders and persons and entities associated with them in private placement transactions. Each share of each series of preferred stock convertsconverted automatically upon closing of this offering into one share of common stock.stock upon the closing of our initial public offering in March 2004.
Investor(1) | Common stock | Series A preferred stock | Series B preferred stock | Series C preferred stock | Series D preferred stock | Series E preferred stock | Series F preferred stock | |||||||||||||||||||||
Investor(1) | Common stock | Series A preferred stock | Series B preferred stock | Series C preferred stock | Series D preferred stock | Series E preferred stock | Series F preferred stock | |||||||||||||||||||||
Directors and executive officers | ||||||||||||||||||||||||||||
Ronald J. Berenson, M.D.(2) | 2,373,256 | 57,895 | — | — | — | — | — | |||||||||||||||||||||
Ronald J. Berenson, M.D.(2) | 431,499 | 10,526 | — | — | — | — | — | |||||||||||||||||||||
Robert M. Williams, Ph.D. | 200,000 | — | — | — | — | — | — | 36,363 | — | — | — | — | — | — | ||||||||||||||
Entities affiliated with directors | ||||||||||||||||||||||||||||
Alta Partners(3) | — | 1,894,737 | 805,281 | 971,331 | 584,547 | 351,677 | 8,035 | |||||||||||||||||||||
ARCH Venture Partners(4) | — | 789,469 | 2,045,454 | 1,119,265 | 1,321,942 | 935,251 | 899,104 | |||||||||||||||||||||
Sprout Group(5) | — | 2,631,579 | 545,454 | 1,142,937 | 323,741 | 356,079 | 3,634 | |||||||||||||||||||||
MPM Capital(6) | 483,453 | — | — | — | 4,316,547 | 719,424 | — | |||||||||||||||||||||
Five percent stockholders | ||||||||||||||||||||||||||||
Ronald J. Berenson, M.D.(2) | 2,373,256 | 57,895 | — | — | — | — | — | |||||||||||||||||||||
Alta Partners(3) | — | 1,894,737 | 805,281 | 971,331 | 584,547 | 351,677 | 8,035 | |||||||||||||||||||||
ARCH Venture Partners(4) | — | 789,469 | 2,045,454 | 1,119,265 | 1,321,942 | 935,251 | 899,104 | |||||||||||||||||||||
Sprout Group(5) | — | 2,631,579 | 545,454 | 1,142,937 | 323,741 | 356,079 | 3,634 | |||||||||||||||||||||
MPM Capital(6) | 483,453 | — | — | — | 4,316,547 | 719,424 | — | |||||||||||||||||||||
W Capital Partners Ironworks, L.P. | — | — | — | 1,796,410 | 286,022 | 301,601 | — | |||||||||||||||||||||
Alta Partners(3) | — | 344,497 | 146,414 | 176,604 | 106,280 | 63,941 | 1,460 | |||||||||||||||||||||
ARCH Venture Partners(4) | — | 143,539 | 371,900 | 203,502 | 240,352 | 170,045 | 163,473 | |||||||||||||||||||||
MPM Capital(5) | 87,899 | — | — | — | 784,825 | 130,802 | — | |||||||||||||||||||||
5% stockholders | ||||||||||||||||||||||||||||
Ronald J. Berenson, M.D.(2) | 431,499 | 10,526 | — | — | — | — | — | |||||||||||||||||||||
Alta Partners(3) | — | 344,497 | 146,414 | 176,604 | 106,280 | 63,941 | 1,460 | |||||||||||||||||||||
ARCH Venture Partners(4) | — | 143,539 | 371,900 | 203,502 | 240,352 | 170,045 | 163,473 | |||||||||||||||||||||
Sprout Group | — | 478,466 | 99,172 | 207,805 | 58,861 | 64,741 | 660 | |||||||||||||||||||||
MPM Capital(5) | 87,899 | — | — | 784,825 | 130,802 | — | ||||||||||||||||||||||
W Capital Partners Ironworks, L.P.(6) | — | — | — | 326,620 | 52,004 | 54,836 | — | |||||||||||||||||||||
Vector Fund | — | — | — | — | 719,425 | — | 1,114,889 | — | — | — | — | 130,804 | — | 202,706 | ||||||||||||||
Vulcan Ventures | 80,575 | — | — | 598,802 | 719,424 | 719,424 | — | 14,650 | — | — | 108,873 | 130,804 | 130,804 | — | ||||||||||||||
| (1) | See “Principal stockholders” for more details on shares held by these purchasers. |
| (2) | Includes shares held in trust. |
| (3) | Dr. Deleage is managing director of Alta Partners. |
| (4) | Mr. Nelsen is a managing director of entities affiliated with ARCH Venture Partners. |
| (5) |
| Dr. Henner is a general partner of MPM Capital. |
| (6) | Mr. Wertheimer is a managing director of W Capital Partners. |
In connection with our acquisition of all the outstanding capital stock of CellGenEx, we issued warrants to purchase 66,983 shares of Series A preferred stock at $5.23 per share in August 1997. In addition, in connection with our Series D preferred stock private placement, we issued warrants to purchase 205,858 shares of common stock at $1.65 per share in August 2000. In connection with our Series E preferred stock private placement, we issued warrants to purchase 470,205 shares of common stock at $0.055 per share in November 2001. In connection with our Series F preferred stock private placement, we issued warrants to purchase 439,932 shares of common stock at $0.055 per share in February and March 2002.
The following table summarizes the number of shares of common stock and preferred stock issuable pursuant toissued upon exercise of warrants granted to 5% stockholders, directors, executive officers and entities affiliated with our executive officers and directors in private placement transactions:
Investor(1) | Shares of common warrants | Shares of Series A preferred stock underlying warrants | ||
Alta Partners(2) | 261,312 | — | ||
ARCH Venture Partners(3) | 1,146,760 | 276,307 | ||
Sprout Group(4) | 232,101 | — | ||
MPM Asset Management LLC(5) | 391,686 | — | ||
W Capital Partners Ironworks, L.P. | 196,239 | — | ||
Vector Fund | 687,570 | — | ||
Vulcan Ventures | 391,686 | — |
Investor(1) | Shares of common stock issued upon exercise of warrants | |
Alta Partners(2) | 43,808 | |
ARCH Venture Partners(3) | 219,123 | |
Sprout Group | 40,589 | |
MPM Asset Management LLC(4) | 70,722 | |
W Capital Partners Ironworks, L.P.(5) | 34,271 | |
Vector Fund | 117,693 | |
Vulcan Ventures | 70,725 |
Footnotes on following page
| (1) | See “Principal stockholders” for more details on shares held by these purchasers. |
| (2) | Dr. Deleage is managing director of Alta Partners. |
| (3) | Mr. Nelsen is a managing director of entities affiliated with ARCH Venture Partners. |
| (4) |
| Dr. Henner is a general partner of MPM Capital. |
80
Certain relationships and related party transactions
| (5) | Mr. Wertheimer is a managing director of W Capital Partners. |
In July 1999, we entered into a License Agreement with Genecraft Inc.,LLC, or Genecraft, of which Dr. Jeffrey Ledbetter, our former Chief Scientific Officer and one of our scientific founders, is a principal founder. Under this agreement, in return for royalties we granted an exclusive sublicense to Genecraft for the rights to severalone pending patent applicationsapplication that we are not using in the field ofin vivoactivation of T cells.
We have entered into indemnification agreements with our officers and directors containing provisions which require us, among other things, to indemnify our officers and directors against liabilities that may arise by reason of their status or service as officers or directors (other than liabilities arising from willful and other misconduct) and to advance their expenses incurred as a result of any proceeding against them as to which they could be indemnified. See “Management— LimitationLimitations on liabilityLiability and indemnificationIndemnification of officersOfficers and directors.Directors.”
We maintain key person life insurance, under which we are the beneficiary, on Dr. Berenson in the amount of $2 million.
In connection with our acquisition of all of the outstanding capital stock of CellGenEx, Inc., we reserved an aggregate of 1,582,340287,698 shares of our common stock in a milestone pool or a Milestone Pool, for issuance to our scientific founders, Drs. Jeffrey Bluestone, Carl June, Jeffrey Ledbetter and Craig Thompson, upon the achievement of scientific milestones determined by a milestone committee. In February 2001, we entered into a settlement agreement with each of Drs. Bluestone, June and Thompson to terminate the Milestone Poolmilestone pool, and no option grants were made pursuant to the Milestone Pool. In addition, we entered into a consulting agreement with each of Drs. Bluestone, June and Thompson under which each agreed to consult with us and to continue to serve on our Scientific Advisory Board. In exchange for these services, each consultant was awarded a non-statutory stock optionoptions for 125,000an aggregate of 22,727 shares of our common stock. Forty percentstock, consisting of theseone option to purchase 9,090 shares vested uponof our common stock at an exercise price of $2.75 per share and a second option to purchase 13,636 shares of our common stock at an exercise price of $5.50 per share. The 13,636 shares vest in equal monthly installments (284 shares per month) over the execution48 month term of the consulting agreement and 1/48 of the remaining shares subject to the option vest each following month.agreement. Dr. Ledbetter, our former Chief Scientific Officer, waived his rights to the Milestone Poolmilestone pool in connection with his resignation in March 1999.
Dr. Frohlich’s employment agreement, dated August 27, 2001, provides that we will forgive over four years from the next two years up to $40,000 in loansdate of the agreement a $50,000 home loan we made to him by the Company in connection with commencement of his employment.
Pursuant to a clinical trial agreement dated November 25, 2003, James R. Berenson, M.D., a brother of our President and Chief Executive Officer, has acted as and will continue to act as a principal investigator for some of our clinical trials run by a site management organization called Oncotherapeutics.
81In October 2003, we issued and sold convertible promissory notes in an aggregate amount of approximately $12.7 million to investors, including, but not limited to, Alta Partners, ARCH Venture Partners, MPM Capital, The Sprout Group, Vector Partners, Vulcan Ventures and W Capital Partners Ironworks. These convertible promissory notes were converted into approximately 1,357,357 shares of our common stock upon completion of our initial public offering.
Principal stockholdersPRINCIPAL STOCKHOLDERS
The following table shows information known to us with respect to the beneficial ownership of our common stock as of September 30, 2003, as adjusted27, 2004 by each of our directors, each named executive officer, each person or group of affiliated persons known by us to reflect the salebeneficially own more than 5% of the shares ofour common stock, offered by:and all of our directors and executive officers as a group.
Beneficial ownership and percentage ownership are determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock underlying options and warrants that are exercisable within 60 days of September 30, 200327, 2004 are considered to be outstanding. To our knowledge, except as indicated in the footnotes to the following table and subject to community property laws where applicable, the persons named in this table have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
The following table reflects the conversion of all shares of our preferred stock outstanding as of September 30, 2003 into an aggregate of 37,300,234 shares of our common stock which will become effective at the closing of this offering. This table is based on 45,702,87014,826,970 shares of our common stock outstanding as of September 30, 2003 and27, 2004, excluding the shares outstanding immediately afterof our common stock issuable upon conversion of the convertible preferred stock offered by this offering.prospectus. The address for those individuals for which an address is not otherwise indicated is: c/o Xcyte Therapies, Inc., 1124 Columbia Street, Suite 130, Seattle, Washington 98104.
Name and address of beneficial owner | Number of shares owned | Number of shares underlying options or warrants | Percent of shares beneficially owned | ||||
Directors and named executive officers | |||||||
Ronald J. Berenson, M.D.(1) | 442,025 | 20,408 | 3.1 | % | |||
Stewart Craig, Ph.D. | — | 72,025 | * | ||||
Mark Frohlich, M.D. | — | 31,838 | * | ||||
Kathi L. Cordova, C.P.A | 13,635 | 19,119 | * | ||||
Joanna S. Black, J.D. | — | 16,179 | * | ||||
Jean Deleage, Ph.D.(2) c/o Alta Partners One Embarcadero Center Suite 4050 San Francisco, CA 94111 | 1,142,400 | 5,454 | 7.7 | ||||
Peter Langecker, M.D., Ph.D. | — | 5,454 | * | ||||
Robert T. Nelsen(3) c/o ARCH Venture Partners 8725 W. Higgins Road, Suite 290 Chicago, IL 60631 | 2,054,271 | — | 13.9 | ||||
Dennis Henner, Ph.D.(4) c/o MPM Asset Management LLC 111 Huntington Avenue 31st Floor Boston, MA 02199 | — | — | — | ||||
Stephen N. Wertheimer(5) c/o W Capital Partners 245 Park Avenue 39th Floor New York, NY 10167 | 574,363 | — | 3.9 | ||||
Robert M. Williams, Ph.D. | 44,544 | — | * | ||||
Daniel Spiegelman(6) | — | 833 | * | ||||
All executive officers and directors as a group (14 persons) | 5,452,203 | 194,071 | 37.6 | % | |||
5% stockholders | |||||||
Alta Partners(2) One Embarcadero Center Suite 4050 San Francisco, CA 94111 | 1,142,400 | 5,454 | 7.7 | ||||
Arch Venture Partners(3) 8725 W. Higgins Road, Suite 290 Chicago, IL 60631 | 2,054,271 | — | 13.9 | ||||
MPM Capital(7) c/o MPM Asset Management LLC 111 Huntington Avenue 31st Floor Boston, MA 02199 | 1,180,965 | — | 8.0 | ||||
The Sprout Group(8) 3000 Sand Hill Road Building 1, Suite 170 Menlo Park, CA 94025 | 960,964 | — | 6.5 | ||||
82Footnotes on following page
Principal stockholders
Name and address of beneficial owner | Number of shares beneficially | Number of shares underlying options or warrants | Percent of shares beneficially owned | ||||||
| Before this offering | After this offering | ||||||||
Directors and named executive officers | |||||||||
Ronald J. Berenson, M.D.(2) | 2,431,151 | 212,499 | 5.8 | % | % | ||||
Stewart Craig, Ph.D. | — | 326,666 | * | ||||||
Mark Frohlich, M.D. | — | 86,165 | * | ||||||
Kathi L. Cordova, C.P.A.(3) | 75,000 | 57,666 | * | ||||||
Jean Deleage, Ph.D.(4) | 4,615,608 | 291,312 | 10.7 | ||||||
c/o Alta Partners One Embarcadero Center Suite 4050 San Francisco, CA 94111 | |||||||||
Peter Langecker, M.D., Ph.D. | — | 30,000 | * | ||||||
Robert T. Nelsen(5) | 7,110,485 | 1,423,067 | 18.6 | ||||||
c/o ARCH Venture Partners 8725 W. Higgins Road, Suite 290 Chicago, IL 60631 | |||||||||
Robert E. Curry, Ph.D.(6) | 5,003,424 | 232,101 | 11.4 | ||||||
c/o the Sprout Group 3000 Sand Hill Road Building 1, Suite 170 Menlo Park, CA 94025 | |||||||||
Dennis Henner, Ph.D.(7) | 5,519,424 | 391,686 | 12.8 | ||||||
c/o MPM Asset Management LLC 111 Huntington Avenue 31st Floor Boston, MA 02199 | |||||||||
Robert M. Williams, Ph.D. | 200,000 | 30,000 | * | ||||||
Joanna S. Black, J.D. | — | 43,749 | * | ||||||
Mark L. Bonyhadi, Ph.D. | — | 70,333 | * | ||||||
All executive officers and directors as a group (12 persons) | 24,955,092 | 4,196,553 | 58.4 | ||||||
Five percent stockholders | |||||||||
Alta Partners(4) | 4,615,608 | 291,312 | 10.7 | ||||||
One Embarcadero Center Suite 4050 San Francisco, CA 94111 | |||||||||
Arch Venture Partners(5) | 7,110,485 | 1,423,067 | 18.6 | ||||||
8725 W. Higgins Road, Suite 290 Chicago, IL 60631 | |||||||||
Sprout Group(6) | 5,003,424 | 232,101 | 11.4 | ||||||
c/o the Sprout Group 3000 Sand Hill Road Building 1, Suite 170 Menlo Park, CA 94025 | |||||||||
MPM Capital(7) | 5,519,424 | 391,686 | 12.8 | ||||||
c/o MPM Asset Management LLC 111 Huntington Avenue 31st Floor Boston, MA 02199 | |||||||||
W Capital Partners Ironworks, L.P.(8) | 2,384,033 | 196,239 | 5.6 | ||||||
245 Park Avenue 39th Floor New York, NY 10167 | |||||||||
Ronald J. Berenson, M.D.(2) | 2,431,151 | 212,499 | 5.8 | ||||||
Vector Fund(9) | 1,834,314 | 687,570 | 5.4 | ||||||
1751 Lake Cook Road Suite 350 Deerfield, IL 60015 | |||||||||
Vulcan Ventures(10) | 2,118,225 | 391,686 | 5.4 | ||||||
505 Orion Station 505 Fifth Avenue South Suite 900 Seattle, WA 98104 | |||||||||
| * | Represents beneficial ownership of less than 1%. |
83
Principal stockholders
| (1) | Includes |
| (2) | Includes |
| (3) | Includes |
| (5) | Mr. Wertheimer is the managing director of W Capital Partners Ironworks, L.P., shares voting and dispositive power with respect to this partnership and disclaims beneficial ownership of the shares in which he has no pecuniary interest. |
| Mr. Spiegelman’s option is still subject to stockholder approval and is not exercisable until such approval. |
| (7) |
| (8) | Includes 19,216 shares of common stock held by DLJ Capital Corporation; 95,027 shares of common stock held by DLJ First ESC, L.P.; 835,950 shares of common stock held by Sprout Capital VII, L.P.; and 9,704 shares of common stock held by the Sprout CEO Fund, L.P. |
DESCRIPTION OF CONVERTIBLE PREFERRED STOCK
The following is a summary of the material terms of the convertible preferred stock. You should refer to the actual terms of the convertible preferred stock and the certificate of designations filed with the Secretary of State of the State of Delaware, a form of which is filed as an exhibit to this registration statement. As used in this description, the words “we,” “us” or “our” do not include any current or future subsidiary of Xcyte.
General
Our board of directors has the authority, without stockholder approval, to issue up to 5,000,000 shares of preferred stock in one or more series and to determine the rights, privileges and limitations of the preferred stock. The rights, preferences, powers and limitations on different series of preferred stock may differ with respect to dividend rates, amounts payable on liquidation, voting rights, conversion rights, redemption provisions, sinking fund provisions, and purchase funds and other matters.
Pursuant to its authority, our board of directors has designated 1,725,000 shares of the preferred stock that we now have authority to issue as the convertible preferred stock. The shares of convertible preferred stock, when issued and sold in the manner contemplated by this prospectus, will be duly and validly issued, fully paid and nonassessable. You will not have any preemptive rights if we issue other series of preferred stock. The convertible preferred stock is not subject to any sinking fund. We have no obligation to retire the convertible preferred stock. The convertible preferred stock has a perpetual maturity and may remain outstanding indefinitely, subject to your right to convert the convertible preferred stock and our right to cause the conversion of the convertible preferred stock and exchange or redeem the convertible preferred stock at our option. Any convertible preferred stock converted, exchanged or redeemed or acquired by us will, upon cancellation, have the status of authorized but unissued shares of convertible preferred stock. We will be able to reissue these cancelled shares of convertible preferred stock.
Dividends
When and if declared by our board of directors out of the legally available funds, you will be entitled to receive cash dividends at an annual rate of % of the liquidation preference of the convertible preferred stock. Dividends will be payable quarterly on , , and beginning , 2005. If any dividends are not declared, they will accrue and be paid at such later date, if any, as determined by our board of directors. Dividends on the convertible preferred stock will be cumulative from the issue date. Dividends will be payable to holders of record as they appear on our stock books not more than 60 days nor less than 10 days preceding the payment dates, as fixed by our board of directors. If the convertible preferred stock is called for redemption on a redemption date between the dividend record date and the dividend payment date and you do not convert the convertible preferred stock (as described below), you shall receive the dividend payment together with all other accrued and unpaid dividends on the redemption date instead of receiving the dividend on the dividend date. Dividends payable on the convertible preferred stock for any period greater or less than a full dividend period will be computed on the basis of a 360-day year consisting of twelve 30-day months. Accrued but unpaid dividends will not bear interest.
If we do not pay or set aside cumulative dividends in full on the convertible preferred stock and any other preferred stock ranking on the same basis as to dividends, all dividends declared upon shares of the convertible preferred stock and any other preferred stock will be declared on a pro rata basis until all accrued dividends are paid in full. For these purposes, “pro rata” means that the amount of dividends declared per share on the convertible preferred stock and any other preferred stock bear to each other will be the same ratio that accrued and unpaid dividends per share on the shares of the convertible preferred stock and such other preferred stock bear to each other. We will not be able to redeem, purchase or otherwise acquire any of our stock ranking on the same basis as the convertible preferred stock as to dividends or liquidation preferences unless we have paid or set aside full cumulative dividends, if any, accrued on all outstanding shares of convertible preferred stock.
Unless we have paid or set aside cumulative dividends in full on the convertible preferred stock and any other of the convertible preferred stock ranking on the same basis as to dividends:
| · | we may not declare or pay or set aside dividends on common stock or any other stock ranking junior to the convertible preferred stock as to dividends or liquidation preferences, excluding dividends or distributions of shares, options, warrants or rights to purchase common stock or other stock ranking junior to the convertible preferred stock as to dividends; or |
| · | we will not be able to redeem, purchase or otherwise acquire any of our other stock ranking junior to the convertible preferred stock as to dividends or liquidation preferences, except in very limited circumstances. |
Under Delaware law, we may only make dividends or distributions to our stockholders from:
| · | our surplus; or |
| · | the net profits for the current fiscal year or the fiscal year before which the dividend or distribution is declared under certain circumstances. |
Our ability to pay dividends and make any other distributions in the future will depend upon our financial results, liquidity and financial condition.
Conversion
Conversion Rights
You may convert the convertible preferred stock at any time into a number of shares of common stock determined by dividing the $10 liquidation preference by the conversion price of $ , subject to adjustment as described below. This conversion price is equivalent to a conversion rate of approximately shares of common stock for each share of convertible preferred stock. We will not make any adjustment to the conversion price for accrued or unpaid dividends upon conversion. We will not issue fractional shares of common stock upon conversion. However, we will instead pay cash for each fractional share based upon the market price of the common stock on the last business day prior to the conversion date. If we call the convertible preferred stock for redemption, your right to convert the convertible preferred stock will expire at the close of business on the business day immediately preceding the date fixed for redemption, unless we fail to pay the redemption price.
In order to convert your shares of convertible preferred stock, you must either:
| · | deliver your convertible preferred stock certificate at the transfer agent office and a duly signed and completed notice of conversion, or |
| · | if the convertible preferred stock is held in global form, according to the procedures established by the depositary as described below under the subsection entitled “Form and Denomination.” |
The conversion date will be the date you deliver your convertible preferred stock certificate and the duly signed and completed notice of conversion to the transfer agent. You will not be required to pay any U.S. federal, state or local issuance taxes or duties or costs incurred by us on conversion, but will be required to pay any tax or duty payable as a result of the common stock upon conversion being issued other than in your name. We will not issue common stock certificates unless all taxes and duties, if any, have been paid by the holder. If you convert your convertible preferred stock after a dividend record date and prior to the next dividend payment date, you will have to pay us an amount equal to the dividend payable on such dividend payment date unless the convertible preferred stock has been called for redemption or we have issued a notice of automatic conversion.
Automatic Conversion
Unless we redeem or exchange the convertible preferred stock, we may elect to convert some or all of the convertible preferred stock into shares of our common stock if the closing price of our common stock has exceeded 150% of the conversion price for at least 20 out of 30 consecutive trading days ending within five
trading days prior to the notice of automatic conversion. If we elect to convert less than all of the shares of convertible preferred stock, we shall select the shares to be converted by lot or pro rata or in some other equitable manner in our discretion. After , 2007, we may not elect to automatically convert the convertible preferred stock if full cumulative dividends on the convertible preferred stock for all past dividend periods have not been paid or set aside for payment.
Conversion Price Adjustment—General
The conversion price of $ will be adjusted if:
| (1) | we dividend or distribute common stock on shares of our common stock; |
| (2) | we subdivide or combine our common stock; |
| (3) | we issue to all holders of common stock certain rights or warrants to purchase our common stock at less than the current market price; |
| (4) | we dividend or distribute to all holders of our common stock shares of our capital stock or evidences of indebtedness or assets, excluding: |
| · | those rights, warrants, dividends or distributions referred to in (1) or (3), or |
| · | dividends and distributions paid in cash; |
| (5) | we make a dividend or distribution consisting of cash to all holders of common stock; |
| (6) | we purchase common stock pursuant to a tender offer made by us or any of our subsidiaries; and |
| (7) | a person other than us or any of our subsidiaries makes any payment on a tender offer or exchange offer and, as of the closing of the offer, the board of directors is not recommending rejection of the offer. We will only make this adjustment if the tender or exchange offer increases a person’s ownership to more than 25% of our outstanding common stock, and only if the payment per share of common stock exceeds the current market price of our common stock. We will not make this adjustment if the offering documents disclose our plan to engage in any consolidation, merger, or transfer of all or substantially all of our properties and if specified conditions are met. |
If we implement a stockholder rights plan, this new rights plan must provide that upon conversion of the existing convertible preferred stock the holders will receive, in addition to the common stock issuable upon such conversion, the rights under such rights plan regardless of whether the rights have separated from the common stock before the time of conversion, in which case the conversion rate will be adjusted as if we distributed to all holders of our common stock, shares of our capital stock, evidences of indebtedness or assets as described above, subject to readjustment in the event of the expiration, termination or redemption of such rights.
The occurrence and magnitude of certain of the adjustments described above is dependent upon the current market price of our common stock. For these purposes, “current market price” generally means the lesser of:
| · | the closing sale price on certain specified dates, or |
| · | the average of the closing prices of the common stock for the ten trading day period immediately prior to certain specified dates. |
We may make a temporary reduction in the conversion price of the convertible preferred stock if our board of directors determines that this decrease would be in the best interests of Xcyte. We may, at our option, reduce the conversion price if our board of directors deems it advisable to avoid or diminish any income tax to holders of common stock resulting from any dividend or distribution of stock or rights to acquire stock or from any event treated as such for income tax purposes. See the section entitled “Material Federal Income Tax Consequences” below for more information.
Conversion Price Adjustment—Merger, Consolidation or Sale of Assets
If we are involved in a transaction in which shares of our common stock are converted into the right to receive other securities, cash or other property, or a sale or transfer of all or substantially all of our assets under which the holders of our common stock shall be entitled to receive other securities, cash or other property, then appropriate provision shall be made so that your convertible preferred stock will convert into:
| (1) | if the transaction is a common stock fundamental change, as defined below, common stock of the kind received by holders of common stock as a result of common stock fundamental change in accordance with paragraph (1) below under the subsection entitled “—Fundamental Change Conversion Price Adjustments,” and |
| (2) | if the transaction is not a common stock fundamental change, and subject to funds being legally available at conversion, the kind and amount of the securities, cash or other property that would have been receivable upon the recapitalization, reclassification, consolidation, merger, sale, transfer or share exchange by a holder of the number of shares of common stock issuable upon conversion of the |
The company formed by the consolidation, merger, asset acquisition or share acquisition shall provide for this right in its organizational document. This organizational document shall also provide for adjustments so that the organizational document shall be as nearly practicably equivalent to adjustments in this section for events occurring after the effective date of the organizational document.
The following types of transactions, among others, would be covered by this adjustment:
| (1) | we recapitalize or reclassify our common stock, except for: |
| · | a change in par value, |
| · | a change from par value to no par value, |
| · | a change from no par value to par value, or |
| · | a subdivision or combination of |
| (2) | we consolidate or merge into any other person, or any merger of another person into us, except for a merger that does not result in a reclassification, conversion, exchange or cancellation of common stock, |
| (3) | we sell, transfer or lease all or substantially all of our assets and holders of our common stock become entitled to receive other securities, cash or other property, or |
| (4) | we undertake any compulsory share exchange. |
Fundamental Change Conversion Price Adjustments
If a fundamental change occurs, the conversion price will be adjusted as follows:
| (1) | in the case of a common stock fundamental change, the conversion price shall be the conversion price after giving effect to any other prior adjustments effected pursuant to the preceding paragraphs, multiplied by |
| · | 100% of the value of the consideration received by a holder of our common stock is common stock of the successor, acquiror or other third party, and cash, if any, paid with respect to any |
fractional interests in such common stock resulting from such common stock fundamental change, and |
| · | all of our common stock shall have been exchanged for, converted into or acquired for, common stock of the successor, acquiror or other third party, and any cash with respect to fractional interests, |
the conversion price shall be the conversion price in effect immediately prior to such common stock fundamental change multiplied by a fraction, the numerator of which is one (1) and the denominator of which is the number of shares of common stock of the successor, acquiror or other third party received by a holder of one share of our common stock as a result of the common stock fundamental change; and
| (2) | in the case of a non-stock fundamental change, the conversion price shall be the lower of: |
| · | the conversion price after giving effect to any other prior adjustments effected pursuant to the preceding paragraphs, and |
| · | the product of: |
| (A) | the applicable price, and |
| (B) | a fraction, the numerator of which is $10 and the denominator of which is (x) the amount of the redemption price for one share of convertible preferred stock if the redemption date were the date of the non-stock fundamental change (or if the date of such non-stock fundamental change falls within the period beginning on the first issue date of the convertible preferred stock through , 2005, or the twelve-month period commencing , 2005, the product of % or %, respectively, and $10) plus (y) any then-accrued and unpaid distributions on one share of convertible preferred stock. |
You may receive significantly different consideration upon conversion depending upon whether a fundamental change is a non-stock fundamental change or a common stock fundamental change. In the event of a non-stock fundamental change, your convertible preferred stock will convert into stock and other securities or property or assets, including cash, determined by the number of shares of common stock receivable upon conversion at the conversion price as adjusted in accordance with (2) above. In the event of a common stock fundamental change, under certain circumstances you will receive different consideration depending on whether you convert your convertible preferred stock on or after the common stock fundamental change. For example, you will only receive common stock of the successor, acquiror or other third party if you convert your convertible preferred stock following a common stock fundamental change in which less than 100% of the value of the consideration received by a holder of common stock is common stock of the successor, acquiror or other third party. However, if you had converted your convertible preferred stock prior to the common stock fundamental change, you would have received consideration in the form of such common stock as well as any other securities or assets, including cash, issuable to the holders of our common stock in connection with the common stock fundamental change.
Definitions for the Fundamental Change Adjustment Provision
“applicable price” means:
| · | in a non-stock fundamental change in which the holders of common stock |
| · | in the |
“common stock fundamental change” means any fundamental change in which more than 50% of the value, as determined in good faith by our board of directors, of the consideration received by holders of our common stock consists of common stock that, for the 10 trading days immediately prior to such fundamental change, has been admitted for listing or admitted for listing subject to notice of issuance on a national securities exchange or quoted on the Nasdaq National Market, except that a fundamental change shall not be a common stock fundamental change unless either:
| · | we continue to exist after the occurrence of |
| · | not later than the occurrence of the fundamental change, the outstanding convertible preferred stock is converted into or exchanged for shares of |
“fundamental change” means the occurrence of any transaction or event or series of transactions or events pursuant to which all or substantially all of our common stock shall be exchanged for, converted into, acquired for or shall constitute solely the right to receive cash, securities, property or other assets, whether by means of an exchange offer, liquidation, tender offer, consolidation, merger, combination, reclassification, recapitalization or otherwise. However, for purposes of adjustment of the conversion price, in the case of any series of transactions or events, the fundamental change shall be deemed to have occurred when substantially all of the common stock shall have been exchanged for, converted into or acquired for, or shall constitute solely the right to receive, such cash, securities, property or other assets, but the adjustment shall be based upon the consideration that the holders of our common stock received in the transaction or event as a result of which more than 50% of our common stock shall have been exchanged for, converted into or acquired for, or shall constitute solely the right to receive, such cash, securities, property or other assets.
84
“purchaser stock price” means the average of the daily closing price for one share of the common stock received by holders of the common stock in the common stock fundamental change during the 10 trading days immediately prior to the date fixed for the determination of the holders of the common stock entitled to receive such common stock or, if there is no such date, prior to the date upon which the holders of the common stock shall have the right to receive such common stock.
Dividend Make-Whole Payment
If we elect to automatically convert, or you voluntarily convert, some or all of the convertible preferred stock into shares of our common stock prior to , 2007, we will make an additional payment equal to the total value of the aggregate amount of cumulative dividends that would have accrued and become payable on the convertible preferred stock from the date of original issue through and including , 2007, less any dividends already paid on the convertible preferred stock. This additional payment is payable by us, in cash, or, at our option, in shares of our common stock or a combination of cash and shares of our common stock. In the event of an automatic conversion or any voluntary conversion undertaken after we provide notice of an automatic conversion, the shares of common stock issued in payment of the dividend make-whole payment will be valued at 150% of the conversion price on the effective date of the conversion. In all other circumstances, any shares of our common stock issued in payment of the dividend make-whole payment will be valued at the greater of (i) 95% of the average closing price of our common stock for the two trading days prior to the effective date of conversion or (ii) $ , which is the closing price of our common stock on the date of this prospectus. In the event of an automatic conversion, the notice of automatic conversion will specify whether we will make the dividend make-whole payment in cash, shares of our common stock or a combination of cash and shares of our common stock. We will not issue fractional shares for any additional payment upon conversion but will instead make a cash adjustment for any fractional share payment.
Liquidation Rights
In the event of our voluntary or involuntary dissolution, liquidation, or winding up, you shall receive a liquidation preference of $10 per share and all accrued and unpaid dividends through the distribution date. Holders of any class or series of preferred stock ranking on the same basis as your convertible preferred stock as to liquidation shall also be entitled to receive the full respective liquidation preferences and any accrued and unpaid dividends through the distribution date. Only after the preferred stock holders have received their liquidation preference and any accrued and unpaid dividends will we distribute assets to common stock holders or any of our other stock ranking junior to the shares of convertible preferred stock upon liquidation. If upon such dissolution, liquidation or winding up, we do not have enough assets to pay in full the amounts due on the convertible preferred stock and any other preferred stock ranking on the same basis with your convertible preferred stock as to liquidation, you and the holders of such other preferred stock will share ratably in any such distributions of our assets:
| · | first in proportion to the liquidation preferences until the preferences are paid in full, and |
| · | then in proportion to the amounts of accrued but unpaid dividends. |
After we pay any liquidation preference and accrued dividends, you will not be entitled to participate any further in the distribution of our assets. The following events will not be deemed to be a dissolution, liquidation or winding up of Xcyte:
| · | the sale of all or substantially all of the assets; |
| · | our merger or consolidation into or with any other corporation; or |
| · | our liquidation, dissolution, winding up or reorganization immediately followed by a reincorporation as another corporation. |
Optional Redemption
On or after , 2007 we may redeem the convertible preferred stock, out of legally available funds, in whole or in part, at our option, at the redemption prices listed below. The redemption price is as follows for the 12-month period beginning of the following years, beginning , 2007 and ending on , 2008 in the case of the first period:
YEAR | REDEMPTION PRICE | ||
2007 | $ | ||
2008 | |||
2009 | |||
2010 | |||
2011 | |||
2012 | |||
2013 | |||
and $10.00 at , 2014 and thereafter. In each case we will pay accrued and unpaid dividends to, but excluding, the redemption date. We are required to give notice of redemption not more than 60 and not less than 20 days before the redemption date.
If we redeem less than all of the shares of convertible preferred stock, we shall select the shares to be redeemed by lot or pro rata or in some other equitable manner in our sole discretion.
Exchange Provisions
We may exchange the convertible preferred stock in whole, but not in part, for debentures on any dividend payment date on or after , 2005 at the rate of $10 principal amount of debentures for each outstanding share of convertible preferred stock. Debentures will be issuable in denominations of $1,000 and integral multiples of $1,000. See the section entitled “Description of Debentures” below. If the exchange results in an amount of debentures that is not an integral multiple of $1,000, we will pay in cash an amount in excess of the closest integral multiple of $1,000. We will mail written notice of our intention to exchange the convertible preferred stock to each record holder not less than 30 nor more than 60 days prior to the exchange date.
We refer to the date fixed for exchange of the convertible preferred stock for debentures as the “exchange date.” On the exchange date, your rights as a stockholder of Xcyte shall cease. Your shares of convertible preferred stock will no longer be outstanding, and will only represent the right to receive the debentures and any accrued and unpaid dividends, without interest. We may not exercise our option to exchange the convertible preferred stock for the debentures if:
| · | full cumulative dividends on the convertible preferred stock to the exchange date have not been paid or set aside for payment, or |
| · | an event of default under the indenture would occur on conversion, or has occurred and is continuing. |
The exchange of convertible preferred stock for debentures will be a taxable event, since holders will be exchanging their convertible preferred stock for debt and we will not make any related cash payment to the holder. See the section entitled “Material Federal Income Tax Consequences” below.
Voting Rights
You will have no voting rights except as described below or as required by law. Shares held by us or any entity controlled by us will not have any voting rights.
If we have not paid dividends on the convertible preferred stock or on any outstanding shares of preferred stock ranking on the same basis as to dividends with the convertible preferred stock in an aggregate amount equal to at least six quarterly dividends whether or not consecutive, we will increase the size of our board of directors by two additional directors. So long as dividends remain due and unpaid, holders of the convertible preferred stock, voting separately as a class with holders of preferred stock ranking on the same basis as to dividends having like voting rights, will be entitled to elect two additional directors at any meeting of stockholders at which directors are to be elected. These directors will be appointed to classes on the board as determined by our board of directors. These voting rights will terminate when we have declared and either paid or set aside for payment all accrued and unpaid dividends. The terms of office of all directors so elected will terminate immediately upon the termination of these voting rights.
Without the vote or consent of the holders of at least a majority of the shares of convertible preferred stock, we may not:
| · | adversely change the rights, preferences and limitations of the convertible preferred stock by modifying our certificate of incorporation or bylaws, or |
| · | authorize, issue, reclassify, increase the authorized amount, or authorize or issue any convertible obligation or security or right to purchase any class of stock that ranks senior to or on the same basis with the convertible preferred stock as to dividends or distributions of assets upon liquidation, dissolution or winding up of the stock. |
In addition, without the vote or consent of the holders of at least a majority of the shares of convertible preferred stock we may not:
| · | enter into a share exchange that affects the convertible preferred stock, |
| · | consolidate with or merge into another entity, or |
| · | permit another entity to consolidate with or merge into us, |
unless the convertible preferred stock remains outstanding and its rights, privileges and preferences are unaffected or it is converted into or exchanged for convertible preferred stock of the surviving entity having rights, preferences and limitations substantially similar, but no less favorable, to the convertible preferred stock.
In determining a majority under these voting provisions, holders of convertible preferred stock will vote together with holders of any other preferred stock that rank on parity as to dividends and that have like voting rights.
Form and Denomination
Except in very limited circumstances, the shares of convertible preferred stock will be evidenced by a global certificate which will be deposited with, or on behalf of, the Depository Trust Company, or DTC, and registered in the name of Cede & Co. as DTC’s nominee. Except as set forth below, the global certificate may be transferred, in whole or in part, only to another nominee of DTC or to a successor of DTC or its nominee.
Purchasers may hold their interests in the global certificate directly through DTC or indirectly through organizations which are participants in DTC. Transfers between participants will be effected in the ordinary way in accordance with DTC rules and will be settled in clearing house funds. The laws of some states require that certain persons take physical delivery of securities in definitive form. Consequently, the ability to transfer beneficial interests in the global certificate to such persons may be limited.
Purchasers may beneficially own interests in the global certificate held by DTC only through participants, or certain banks, brokers, dealers, trust companies and other parties that clear through or maintain a custodial relationship, with a participant, either directly or indirectly through indirect participants. So long as Cede & Co., as the nominee of DTC, is the registered owner of the global certificate, Cede & Co. for all purposes will be considered the sole holder of the global certificate. Except as provided below, owners of beneficial interests in the global certificate will not be entitled to have certificates registered in their names, will not receive or be entitled to receive physical delivery of certificates in definitive form, and will not be considered the holders.
Payment of dividends on and the redemption price of the global certificate will be made to Cede & Co. by wire transfer of immediately available funds. Neither we, the trustee nor any paying agent will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in the global certificate or for maintaining, supervising or reviewing any records relating to such beneficial ownership interests.
We have been informed by DTC that, with respect to any payment of dividends on or the redemption price of the global certificate, DTC’s practice is to credit participants’ accounts on the payment date with payments in amounts proportionate to their respective beneficial interests in the convertible preferred stock represented by the global certificate as shown on the records of DTC, unless DTC has reason to believe that it will not receive payment on such payment date. Payments by participants to owners of beneficial interests in convertible preferred stock represented by the global certificate held through such participants will be the responsibility of such participants, as is now the case with securities held for the accounts of customers registered in “street name.”
If you would like to convert your convertible preferred stock into common stock pursuant to the terms of the convertible preferred stock, you should contact your broker or other direct or indirect DTC participant to obtain information on procedures, including proper forms and cut-off times, for submitting those requests.
Because DTC can only act on behalf of participants, who in turn act on behalf of indirect participants and certain banks, the ability of a person having a beneficial interest in convertible preferred stock represented by the global certificate to pledge such interest to persons or entities that do not participate in the DTC system, or otherwise take actions in respect of such interest, may be affected by the lack of a physical certificate evidencing such interest.
Neither we, the transfer agent, registrar, paying agent nor conversion agent will have any responsibility for the performance by DTC or its participants or indirect participants under the rules and procedures governing their operations. DTC has advised us that it will take any action permitted to be taken by a holder of convertible preferred stock only at the direction of one or more participants to whose account with DTC interests in the global certificate are credited and only in respect of the amount of shares of the convertible preferred stock represented by the global certificate as to which the participant has given this direction.
DTC is a limited purpose trust company organized under the laws of the State of New York, a member of the Federal Reserve System, a “clearing corporation” within the meaning of the Uniform Commercial Code and a “clearing agency” registered pursuant to the provisions of Section 17A of the Exchange Act. DTC was created to hold securities for its participants and to facilitate the clearance and settlement of securities transactions between participants through electronic book-entry changes to accounts of its participants, thereby eliminating the need for physical movement of certificates. Participants include securities brokers and dealers, banks, trust
companies and clearing corporations and may include certain other organizations such as the initial purchaser. Certain participants, together with other entities, own DTC. Indirect access to the DTC system is available to others such as banks, brokers, dealers and trust companies that clear through, or maintain a custodial relationship with, a participant, either directly or indirectly.
If DTC is at any time unwilling or unable to continue as depositary and a successor depositary is not appointed by us within 90 days, we will cause convertible preferred stock to be issued in definitive form in exchange for the global certificate.
Nasdaq National Market Listing
We will make an application to list the convertible preferred stock on the Nasdaq National Market under the symbol “XCYTP.”
Transfer Agent and Registrar
American Stock Transfer and Trust Company will act as transfer agent and registrar for the convertible preferred stock. Its address is 59 Maiden Lane, New York, NY 10038, and its telephone number is (212) 936-5100.
DescriptionDESCRIPTION OF DEBENTURES
If we elect to issue debentures in exchange for the convertible preferred stock, we will issue the debentures under an indenture between us and U.S. Bank National Association, as trustee. The following summarizes the material provisions of capitalthe indenture and the debentures. You should refer to the actual terms of the indenture and the debentures for the definitive terms and conditions that have been filed as an exhibit to this registration statement. As used in this description, the words “we,” “us” or “our” do not include any current or future subsidiary of Xcyte.
If we elect to issue debentures for convertible preferred stock, we will issue the debentures at a rate of $10 principal amount of debentures for each share of convertible preferred stock that we exchange. The debentures will be general, unsecured, subordinated obligations of Xcyte. The debentures will initially be limited to an aggregate principal amount equal to the aggregate liquidation value of the outstanding convertible preferred stock, excluding accrued and unpaid dividends payable upon liquidation. We may, without the consent of the holders, “reopen” the notes and issue additional notes under the indenture with the same terms and with the same CUSIP numbers as the notes offered hereby in an unlimited aggregate principal amount, provided that no such additional notes may be issued unless fungible with the notes offered hereby for U.S. federal income tax purposes. The debentures will mature 25 years after the exchange date, unless earlier converted by a holder or redeemed at our option.
The debentures will be issued only in fully registered form, without coupons, in denominations of $1,000 and any integral multiple of $1,000. You will not be required to pay a service charge for registration of transfer or exchange of the debentures. We may, however, require you to pay any tax or other governmental charge payable as a result of the common stock issued upon conversion being issued other than in your name.
We will maintain an office in New York, New York where payments will be made on the debentures and where transfer of debentures will be registrable. Initially, this office will be an office or agency of the trustee in New York, New York.
The debentures will be issued in the same form as the convertible preferred stock for which debentures were exchanged. Any global certificates will be replaced with one or more global debentures as described above under the section entitled “Description of Preferred Stock—Form, Denomination and Registration.” Debentures may be issued in certificated form in exchange for a global debenture under limited specified circumstances.
We are not restricted from paying dividends or repurchasing securities under the indenture. We are not subject to any financial covenants under the indenture.
Interest
The debentures will bear interest at the rate of % per year. Interest will be paid on and of each year to the record holder on the preceding and . Interest will be computed on the basis of a 360-day year consisting of twelve 30-day months. We may, at our option, pay interest in the debentures by check mailed to the holders. However, holders of more than $2,000,000 in principal amount of debentures will be paid by wire transfer in immediately available funds at the holder’s election.
Conversion Rights
Holders may convert their debentures at any time prior to maturity, subject to prior redemption, at a conversion price of $ , subject to adjustment as described under the section entitled “Description of Preferred Stock—Conversion Rights” above. Holders may convert debentures in denominations of $1,000 and multiples of $1,000. If you convert your debentures after a record date and prior to the next interest payment date, you will have to pay us interest unless the debentures have been called for redemption or we have issued a notice of an automatic conversion. We are not required to issue fractional shares of common stock upon conversion of debentures.
Instead, we will pay a cash adjustment based upon the market price of the common stock on the last business day prior to the date of conversion.
If the debentures are called for redemption, your conversion rights will expire at the close of business of the business day preceding the redemption date, unless we default in the payment of the redemption price. Except as described in this section, the conversion provisions of the debentures will be identical to the conversion provisions of the convertible preferred stock. See the section entitled “Description of Convertible Preferred Stock—Conversion—Conversion Rights” above for more information.
In order to convert your debentures, you must deliver the debenture at the specified office of a conversion agent, along with a duly signed and completed notice of conversion and any interest that may be required as described in the preceding paragraph. The conversion date shall be the date on which you deliver the debenture, the duly signed and completed notice of conversion and any required interest payments as described in the preceding paragraph.
You will not be required to pay any taxes or duties payable for the issue or delivery of common stock on conversion. You will, however, be required to pay any tax or duty payable as a result of the issuance of common stock upon conversion in a name other than your name. We will not issue or deliver common stock unless all taxes and duties, if any, have been paid by the holder.
Automatic Conversion
Unless we redeem or exchange the debentures, we may elect to automatically convert some or all of the debentures into shares of our common stock if the closing price of our common stock has exceeded 150% of the conversion price for at least 20 out of 30 consecutive trading days ending within five days prior to the notice of automatic conversion. If we elect to convert less than all of the debentures, the trustee shall select the debentures to be converted by lot or pro rata or in some other equitable manner in our discretion.
Interest Make-Whole Payment
If we elect to automatically convert, or you voluntarily convert, some or all of the debentures into shares of our common stock prior to , 2007, we will make an additional payment equal to the total value of the aggregate amount of interest that would have accrued and become payable on the debentures from the date of issuance upon the exchange through and including , 2007, less any interest already paid on the debentures. This additional payment is payable by us, in cash, or, at our option, in shares of our common stock or a combination of cash and shares of our common stock. In the event of an automatic conversion or any voluntary conversion undertaken after we provide notice of an automatic conversion, the shares of common stock issued in payment of the interest make-whole payment will be valued at 150% of the conversion price on the effective date of the conversion. In all other circumstances, any shares of our common stock issued in payment of the interest make-whole payment will be valued at the greater of (i) 95% of the average closing price of our common stock for the two trading days prior to the effective date of conversion or (ii) $ , which is the closing price of our common stock on the date of this prospectus. In the event of an automatic conversion, the notice of automatic conversion will specify whether we will make the interest make-whole payment in cash, shares of our common stock or a combination of cash and shares of our common stock. We will not issue fractional shares for any additional payment upon conversion but will instead make a cash adjustment for any fractional share payment.
Subordination
The debentures are subordinated to the prior payment in full of all senior indebtedness as provided in the indenture. Upon any distribution of our assets upon our dissolution, winding up, liquidation or reorganization, the payments on the debentures will be subordinated to the prior payment in full of all senior indebtedness. However, holders of debentures may receive securities that are subordinated at least to the same extent as the debentures are subordinated to senior indebtedness and any securities issued in exchange for senior indebtedness under the indenture.
If the debentures are accelerated as a result of an event of default, holders of all senior indebtedness will be entitled to payment in full in cash before the holders of the debentures will be entitled to receive any payment on the debentures. We are required to promptly notify holders of senior indebtedness if payment of the debentures is accelerated because of an event of default.
We may not make any payment on the debentures if:
| · | a default in the payment of senior indebtedness occurs and is continuing beyond any period of grace, or |
| · | any other default occurs and is continuing under any designated senior indebtedness that permits holders of designated senior indebtedness to accelerate its maturity, and the trustee receives a notice known as a payment blockage notice from us or any other person permitted to give such notice under the indenture. |
We may resume making payments on the debentures:
| · | in the case of a payment default, upon the date on which such default is cured or waived or ceases to exist, and |
| · | in case of any other default, the earlier of the date on which such other default is cured or waived or ceases to exist or 179 days after receipt of the payment blockage notice, unless the maturity of any senior indebtedness is accelerated. |
No new period of payment blockage arising due to a default other than a payment default may be commenced unless:
| · | 365 days have elapsed since the effectiveness of the immediately prior payment blockage notice, and |
| · | all scheduled payments on the debentures have been paid in full in cash. |
No default other than a payment default that existed or was continuing on the date of delivery of any payment blockage notice to the trustee shall be the basis for a subsequent payment blockage notice.
By reason of the subordination provisions, in the event of our bankruptcy, dissolution or reorganization, holders of senior indebtedness may receive more, and holders of the debentures may receive less, than our other creditors. These subordination provisions will not prevent the occurrence of any event of default under the indenture.
“senior indebtedness” means the principal, premium, if any, and interest on any indebtedness of Xcyte, including bankruptcy interest or any other payment on indebtedness, whether outstanding on the date of the indenture or thereafter created, incurred, assumed, guaranteed or in effect guaranteed by us including all deferrals or renewals or amendments or modifications. However, senior indebtedness does not include:
| · | indebtedness evidenced by the debentures, |
| · | any liability for federal, state, local or other taxes owed or owing by us, |
| · | our indebtedness to any of our subsidiaries, |
| · | any of our trade payables incurred in the ordinary course of business, and |
| · | any indebtedness that expressly provides that the indebtedness shall not be senior in right of payment to, or is on the same basis with, or is subordinated or junior to, the debentures. |
“indebtedness” means:
| (1) | all obligations: |
| · | for borrowed money, |
| · | evidenced by a note, debenture, bond or other written instrument, |
| · | under a lease required to be capitalized on the balance sheet of the lessee under generally accepted accounting principles, |
| · | under any lease or related document, including a purchase agreement, that provides that we are contractually obligated to purchase or cause a third party to purchase and thereby guarantee a minimum residual value of the lease property to the lessor and our obligations under this lease or related document to purchase or to cause a third party to purchase such leased property, |
| · | letters of credit, bank guarantees or bankers’ acceptances, including reimbursement obligations, |
| · | indebtedness secured by a mortgage, pledge, lien, encumbrance, charge or adverse claim affecting title in an encumbrance to which the property or assets of the person are subject, |
| · | the balance of deferred and unpaid purchase price of any property or assets, |
| · | under interest rate or currency swap agreements, cap, floor and collar agreements, spot and forward contracts and similar agreements and arrangements; |
| (2) | any obligation of others of the type described in the preceding section (1) or under section (3) below assumed by or guaranteed or in effect guaranteed through an agreement to purchase; and |
| (3) | any deferrals, renewals or amendments or modifications of section (1) and section (2) above. |
“designated senior indebtedness” means any particular senior indebtedness that expressly provides that such senior indebtedness shall be designated senior indebtedness for purposes of the indenture.
If the trustee or any holder of debentures receives any payment or distribution of our assets of any kind in contravention of the indenture, then this payment or distribution will be held by the recipient in trust for the benefit of the holders of senior indebtedness and will be immediately paid over or delivered to the holders of senior indebtedness or their representatives.
The debentures are our exclusive obligations. The payment of dividends and the making of loans and advances to us by any subsidiaries we may have may be subject to statutory or contractual restrictions, will depend upon the earnings of those subsidiaries and are subject to various business considerations.
Our right to receive assets of any of our subsidiaries upon their liquidation or reorganization (and the consequent right of the holders of the debentures to participate in those assets) is effectively subordinated to the claims of that subsidiary’s creditors (including trade creditors), except to the extent that we are recognized as a creditor of that subsidiary, in which case our claims would still be subordinate to any security interests in the assets of that subsidiary and any indebtedness of that subsidiary senior to that held by us.
As of June 30, 2004, we had approximately $2.2 million of indebtedness outstanding that would have constituted senior indebtedness, and approximately $4.6 million of indebtedness and other liabilities outstanding to which the notes would have been effectively subordinated (including trade and other payables, but excluding intercompany liabilities). The indenture will not limit the amount of additional indebtedness, including senior
indebtedness, which we can create, incur, assume or guarantee, nor will the indenture limit the amount of indebtedness or other liabilities that any subsidiary can create, incur, assume or guarantee.
Optional Redemption
On or after , 2007, we may redeem the debentures, in whole or in part, at our option, at the redemption prices listed below. The redemption prices, expressed as a percentage of the principal amount, are as follows for the 12-month periods beginning of the following years, beginning , 2007 and ending on , 2008 in the case of the first period.
YEAR | REDEMPTION PRICE | ||
2007 | $ | ||
2008 | |||
2009 | |||
2010 | |||
2011 | |||
2012 | |||
2013 | |||
and 100% at , 2014 and thereafter. In each case we will pay accrued interest to, but excluding, the redemption date. If the redemption date is an interest payment date, we will pay interest to the record holders as of the relevant record date. We are required to give notice not more than 60 and not less than 20 days before the redemption date.
If fewer than all the debentures are to be redeemed, the trustee will select the debentures to be redeemed in principal amounts of $1,000 or multiples of 1,000 by lot or, in its discretion, on a pro rata basis.
No sinking fund is provided for the debentures, which means that we are not required under the indenture to redeem or retire the debentures periodically.
Events of Default and Remedies
The following events are “events of default” under the indenture:
| · | we fail to pay the principal or premium, if any, on the debentures when due, whether or not prohibited by the subordination provisions of the indenture; |
| · | we fail to pay interest on the debentures when due and this failure continues for 30 days, whether or not prohibited by the subordination provisions of the indenture; |
| · | we fail to perform any covenant in the indenture and this failure continues for 45 days after notice is given in accordance with the indenture; |
| · | we fail to pay at maturity, including any applicable grace period, an amount of indebtedness in excess of $5.0 million and this failure continues for 30 days after notice given in accordance with the indenture; |
| · | a default by us on any indebtedness that results in the acceleration of indebtedness in an amount in excess of $5.0 million, without the indebtedness being discharged or the acceleration being rescinded or annulled for 30 days after notice given in accordance with the indenture; or |
| · | events involving our bankruptcy, insolvency or reorganization, as described in the indenture. |
The trustee is required to give notice to holders of all uncured defaults known to the trustee within 90 days after the occurrence of the default. However, the trustee may withhold this notice if it determines in good faith that it is in the best interest of the holders, except notice of:
| · | a default in the payment of the principal or premium, if any, or interest on the debentures, or |
| · | a default in the payment of any redemption obligation. |
If an event of default has occurred and is continuing, the trustee or the holders of not less than 25% in aggregate principal amount of outstanding debentures may declare the principal and premium, if any, on the debentures and accrued interest on the debentures to be immediately due and payable. However, if we cure all defaults, except payment defaults on the debentures as a result of the acceleration, and we meet certain conditions, this acceleration declaration may be canceled and past defaults may be waived by the holders of a majority in principal amount of outstanding debentures. If an event of default resulting from events of bankruptcy, insolvency or reorganization were to occur, all unpaid principal and accrued interest on outstanding debentures will become due and payable immediately without any declaration or other act on the part of the trustee or any holders of debentures, subject to certain limitations.
Holders of a majority in principal amount of the outstanding debentures may, subject to certain limitations, direct the time, method and place of conducting any proceeding for any remedy available to the trustee or exercising any trust or power conferred on the trustee. The trustee shall be entitled to receive from holders reasonable security or indemnity against any costs, expenses and liabilities incurred by the trustee. Before you may institute a proceeding which respect to the indenture, each of the following must occur:
| · | you must have given the trustee written notice of a continuing event of default; |
| · | the holders of at least 25% of the aggregate principal amount of all outstanding debentures must make a written request of the trustee to take action because of the default; |
| · | holders must have offered reasonable indemnification to the trustee against the cost, expenses and liabilities of taking action; |
| · | the trustee must not have received from the holders of a majority in aggregate principal amount of the outstanding debentures a direction inconsistent with the written request; and |
| · | the trustee must not have taken action for 60 days after the receipt of such notice and offer of indemnification. |
These limitations do not apply to a suit for the enforcement of payment of the principal of or any premium or interest on a debenture or the right to convert the debenture in accordance with the indenture.
Generally, the holders of not less than a majority of the aggregate principal amount of outstanding debentures may waive any default or event of default, except if:
| · | we fail to pay principal, premium or interest on any debenture when due; |
| · | we fail to convert any debenture into common stock; or |
| · | we fail to comply with any of the provisions of the indenture that would require the consent of the holder of each outstanding debenture affected. |
We will send the trustee annually a statement as to whether we are in default and the nature of any default under the indenture.
Limitation on Merger, Sale or Consolidation
We may not consolidate with or merge with or into another person or sell, lease, convey or transfer all or substantially all of our assets on a consolidated basis, whether in a single or series of related transactions, to another person or group of affiliated persons, unless:
| · | either (A) we are the surviving entity or (B) the resulting entity is a U.S. corporation, and expressly assumes in writing all of our obligations under the debentures and the indenture; |
| · | no default or event of default exists or shall occur immediately after giving effect to the transaction; and |
| · | other conditions specified in the indenture are satisfied. |
Modifications of the Indenture
The consent of the holders of a majority in principal amount of outstanding debentures at the time is required to modify or amend the indenture or any supplemental indenture. However, a modification or amendment would require the consent of the holder of each outstanding debenture affected if it would:
| · | extend the fixed maturity of any debenture; |
| · | reduce the rate or extend the time for payment of interest on any debenture; |
| · | reduce the principal amount or any premium of any debenture; |
| · | reduce any amount payable upon redemption of any debenture; |
| · | impair or adversely affect a holder’s right to institute suit for the payment on any debenture; |
| · | change the currency in which the debentures are payable; |
| · | impair or adversely change the right to convert the debentures; |
| · | adversely modify the subordination provisions of the debentures; or |
| · | reduce the percentage required to consent to modifications and amendments. |
Taxation of Debentures
You should read the section entitled “Material Federal Income Tax Consequences” below for a discussion of the U.S. federal income tax consequences that may apply to you as a debenture holder.
Governing Law
The indenture and the debentures will be governed by the laws of the State of New York.
Listing of Debentures
It is a condition to our ability to exchange the convertible preferred stock for debentures that the debentures be listed on one of the following markets: the Nasdaq National Market, Nasdaq SmallCap Market, American Stock Exchange, New York Stock Exchange or another national securities exchange.
Concerning the Trustee
We have accepted U.S. Bank National Association as the trustee, initial paying agent, conversion agent, registrar and custodian for the debentures. We may maintain deposit accounts and conduct other banking transactions with the trustee or its affiliates in the ordinary course of business. In addition, the trustee and its affiliates may in the future provide banking and other services to us in the ordinary course of their business. If there is an event of default under the indenture, the trustee will:
| · | exercise the rights and powers given to the trustee under the indenture and |
| · | use the same degree and care and skill in its exercise as a prudent person would exercise under the circumstances in the conduct of the person’s own affairs. |
If the trustee becomes one of our creditors, the indenture and the Trust Indenture Act of 1939 may limit the trustee from obtaining payment of claims in certain cases or realizing on certain property received by the trustee.
DESCRIPTION OF OUR OTHER CAPITAL STOCK
GENERALGeneral
Our amended and restated certificate of incorporation, which will become effective uponUpon the closing of this offering authorizes the issuanceour authorized capital stock will consist of up to 100 million100,000,000 shares of common stock, par value $0.001 per share, and 5 million5,000,000 shares of preferred stock, par value $0.001 per share. The rights and preferences of the preferred stock other than the convertible preferred stock offered by this prospectus, may thereafter be established from time to time by our board of directors. As of September 30, 2003, 8,402,63627, 2004, 14,826,970 shares of common stock were issued and outstanding and 37,300,234no shares of preferred stock convertible into 37,300,234 shares of common stock upon the completion of this offering were issued and outstanding. As of September 30, 2003,27, 2004, we had 79124 common stockholders of record and 44 preferred stockholders of record.
Immediately after the closing of this offering, we will have approximately shares of common stock outstanding, assuming no exercise of the underwriters’ over-allotment option and no exercise of options to acquire 3,985,560 additional shares of common stock or warrants to purchase 256,353 shares of preferred stock convertible into 256,353 shares of common stock that are outstanding as of September 30, 2003.
The description below gives effect to the filing of the certificate of designations amending our amended and restated certificate of incorporation andto create the adoption of our amended and restated bylaws and is qualified in its entirety by reference to these documents, copiesconvertible preferred stock, a copy of which areis filed as exhibitsan exhibit to the registration statement of which this prospectus is a part.
COMMON STOCKCommon Stock
Each holder of common stock is entitled to one vote for each share on all matters to be voted upon by the stockholders, and there are no cumulative voting rights. Subject to preferences to which holders of preferred stock issued after the sale of the common stock being offered may be entitled, holders of common stock are entitled to receive ratably those dividends, if any, that may be declared from time to time by our board of directors out of funds legally available for the payment of dividends. In the event of a liquidation, dissolution or winding up of us, holders of our common stock would be entitled to share in our assets remaining after the payment of liabilities and the satisfaction of any liquidation preference that may be granted to holders of any outstanding shares of preferred stock. Holders of our common stock have no preemptive or conversion rights or other subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. All outstanding shares of common stock are, and the shares of common stock offered by us in this offering, when issued and paid for, will be, fully paid and nonassessable. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock which we may designate in the future.
PREFERRED STOCKPreferred Stock
The convertible preferred stock is described under the heading “Description of Convertible Exchangeable Preferred Stock.”
Upon the closing of this offering, our board of directors will be authorized, subject to any limitations prescribed by law, without stockholder approval, to issue from time to time up to an aggregate of 5 million3,275,000 shares of preferred stock in one or more series. Each series of preferred stock will have the rights and preferences, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as our board of directors determines. The issuance of preferred stock could adversely affect the voting power of holders of our common stock and the likelihood that holders of our common stock will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of making it more difficult for a third party to acquire, or of
85
Description of capital stock
discouraging a third party from attempting to acquire, a majority of our outstanding voting stock. We have no present plans to issue any shares of preferred stock.
WARRANTSWarrants
As of September 30, 2003, the following27, 2004 we had outstanding warrants were outstanding:that expire between July 2006 and February 2009 to purchase at a weighted average exercise price of $7.94 per share an aggregate of 46,607 shares of common stock.
REGISTRATION RIGHTSRegistration Rights
We and the holderscertain of our preferred stock, certain holders of warrants to purchase our preferred stockexisting stockholders and certain holders of our common stockwarrantholders entered into an investor rights agreement, dated May 25, 2000, as amended on August 8, 2000, October 18, 2000, November 13, 2001, February 5, 2002, May 22,
2002 and AugustOctober 9, 2003. This investors rights agreement provides these holders with customary demand and piggyback registration rights with respect to the shares of common stock held by them and common stock to be issued upon conversion or exercise of preferred stock andissuable pursuant to warrants held by them. In addition, the holders of our preferred stock are entitled to receive quarterly and annual financial statements, subject to certain conditions and limitations.
Demand registrationRegistration
According to the terms of the investor rights agreement, the holders of 44,099,3978,992,108 shares of our common stock or warrants to purchase shares of our common stock have the right to require us to register their shares with the SEC for resale to the public. To demand such a registration, holders who hold together an aggregate of at least 50% of the shares having registration rights must request a registration statement to register shares for an aggregate offering price of at least $10 million, net of underwriting discounts and commissions. We are not required to effect more than two demand registrations. We have currently not effected, or received a request for, any demand registrations. We may defer the filing of a demand registration statement for a period of up to 90 days once in any 12-month period.
Piggyback registrationRegistration
If we file a registration statement for a public offering of any of our securities solely for cash, other than a registration statement relating solely to our stock plans, the holders of demand registration rights will have the right to include their shares in the registration statement.
Form S-3 registrationRegistration
At any time after we become eligible to file a registration statement on Form S-3, holders of shares of common stock having demand and piggyback registration rights may require us to file a Form S-3 registration statement. We are obligated to file only one Form S-3 registration statement in any six-month period. Furthermore, the aggregate offering proceeds of the requested Form S-3 registration, before deducting underwriting discounts and expenses, must be at least $500,000. We may defer one registration request for 120 days in any 12-month period.
86
Description of capital stock
These registration rights are subject to certain conditions and limitations, including the right of the underwriters of an offering to limit the number of shares of common stock to be included in the registration. We are generally required to bear the expenses of all registrations, except underwriting discounts and commissions. However, we will not pay for any expenses of any demand registration if the request is subsequently withdrawn by the holders of a majority of the securities to be registered.registered unless such holders forfeit their right to one demand registration. The investors rights agreement also contains our commitment to indemnify the holders of registration rights for losses attributable to statements or omissions by us incurred with registrations under the agreement. The registration rights terminate on March 19, 2009, which is five years after the closing of thisour initial public offering.
ANTI-TAKEOVER EFFECTS OF PROVISIONS OF OUR AMENDED AND RESTATED CERTIFICATE OF INCORPORATION AND BYLAWS AND DELAWARE AND WASHINGTON LAWAnti-Takeover Effects of Provisions of Our Amended and Restated Certificate of Incorporation and Bylaws and Delaware and Washington Law
Provisions of our amended and restated certificate of incorporation and bylaws which will become effective upon the closing of this offering, may have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, control of us. These provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. Our amended and restated bylaws and certificate of incorporation eliminate the right of stockholders to call special meetings of stockholders or to act by written consent without a meeting and require advance notice for stockholder proposals and director nominations, which may preclude stockholders from bringing matters before an annual meeting of stockholders or from making nominations for directors at an annual meeting of stockholders. The authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferringdeterring hostile takeovers or delaying changes in control of us or management of us.our management. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which, subject to certain exceptions, generally prohibits a Delaware corporation from
engaging in any business combination with any interested stockholder for a period of three years following the time that such stockholder became an interested stockholder, unless:
| prior to the business combination, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares |
| shares owned by persons who are directors and also officers; and |
| shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| at or after the time of the |
| approved by our board of directors; and |
| authorized at an annual or special meeting of our stockholders, and not by written consent, by the affirmative vote of at least 66 |
In general, the Delaware General Corporation Law defines an interested stockholder to be an entity or person that beneficially owns 15% or more of the outstanding voting stock of the corporation or any entity or person that is an affiliate or associate of such entity or person.
The Delaware General Corporation Law generally defines business combination to include the following:
87
Description of capital stock
| any merger or consolidation involving the corporation and the interested stockholder; |
| any sale, lease, exchange, mortgage, pledge, transfer or other disposition of 10% or more of the assets of the corporation or its majority-owned subsidiary that involves interested stockholder; |
| subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| subject to certain exceptions, any transaction involving the corporation that has the effect of increasing the interested stockholder’s proportionate share of the stock of any class or series of the corporation; and |
| the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
The laws of the State of Washington, where our principal executive offices are located, impose restrictions on certain transactions between certain foreign corporations and significant stockholders. Chapter 23B.19 of the Washington Business Corporation Act, or the WBCA, generally prohibits a target corporation, with certain exceptions, from engaging in certain significant business transactions with an acquiring person for a period of five years after the acquiring person first became an acquiring person, unless the transaction or the purchase of shares by the acquiring person is approved by a majority of the members of the target corporation’s board of directors prior to the time the acquiring person first became an acquiring person. An acquiring person is generally a person or group of persons who beneficially owns 10% or more of the voting securities of the target corporation. Prohibited transactions include, among other things:
| a merger or consolidation with, disposition of assets to, or issuance or redemption of stock to or from, the acquiring person; |
| termination of 5% or more of the employees of the target corporation as a result of the acquiring person’s acquisition of 10% or more of the shares of the target corporation; and |
| allowing the acquiring person to receive a disproportionate benefit as a stockholder; |
After the five-year period, a significant business transaction may take place as long as it complies with certain fair price provisions of the statute. A target corporation includes a foreign corporation if:
| the corporation has a class of voting shares registered pursuant to |
| the corporation’s principal executive office is located in Washington; |
| the corporation has either: |
| more than 10% of its stockholders of record resident in Washington; |
| more than 10% of its shares owned of record by Washington residents; or |
| 1,000 or more stockholders of record resident in Washington; |
88
Description of capital stock
| a majority of the corporation’s employees are Washington residents or more than 1,000 Washington residents are employees of the corporation; and |
| a majority of the corporation’s tangible assets are located in Washington or the corporation has more than $50 million of tangible assets located in Washington. |
Because a corporation may not opt out of this statute, we anticipate this statute will apply to us. Depending on whether we meet the definition of a target corporation, Chapter 23B.19 of the WBCA may have the effect of delaying, deferringdeterring or preventing a change in control of us.
NASDAQ NATIONAL MARKET LISTINGNasdaq National Market Listing
We have applied to have ourOur common stock has been approved for quotation on Thethe Nasdaq National Market under the symbol “XCYT.”
TRANSFER AGENT AND REGISTRARTransfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company. Its address is 59 Maiden Lane, New York, NY 10038, and its telephone number is (212) 936-5100.
89
Shares eligible for future saleMATERIAL FEDERAL INCOME TAX CONSEQUENCES
PriorThe following summary of the material federal income tax consequences of acquiring, owning and disposing of the convertible preferred stock, the debentures and the common stock is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury regulations, court decisions, and Internal Revenue Service (“IRS”) rulings and pronouncements now in effect, all of which are subject to thisdiffering interpretations and which are subject to change, possibly on a retroactive basis.
This summary assumes that the convertible preferred stock is acquired at its original offering there has been no publicat its original issue price and that the convertible preferred stock, the debentures and the common stock are held as capital assets, within the meaning of section 1221 of the Code. This summary does not address all of the tax consequences that may be relevant to particular holders in light of their personal circumstances, or to certain types of holders (such as banks, financial institutions, dealers in securities or commodities, traders in securities that elect to use a mark to market method of accounting for their holdings, insurance companies, regulated investment companies, personal holding companies, corporations subject to the alternative minimum tax, tax-exempt organizations, pension funds, certain U.S. expatriates, partnerships or other entities classified as partnerships for U.S. federal income tax purposes, certain hybrid entities and their owners, U.S. holders who have a “functional currency” other than the U.S. dollar, persons who own 10% or more of our voting stock, or persons who hold the convertible preferred stock, debentures or common stock as positions in a “straddle” or as part of a “hedging,” “conversion” or “constructive sale” transaction for United States federal income tax purposes). Also not addressed are the consequences under estate, state, local and foreign tax laws or the tax consequences to subsequent holders of the convertible preferred stock, debentures or common stock.
We have not sought and will not seek any rulings from the IRS concerning the tax consequences of the acquisition, ownership or disposition of the convertible preferred stock, the debentures or the common stock. Future salesAccordingly, the IRS may successfully challenge the tax consequences described below. Prospective purchasers are advised to consult their own tax advisors regarding the tax consequences of substantial amountsacquiring, holding, or disposing of ourthe convertible preferred stock, debentures or common stock in light of their own investment circumstances.
Characterization of Convertible Preferred Stock and Debentures
Under section 385(c) of the public market, Code, our characterization of the convertible preferred stock as “stock” and the debentures as “debt” is binding upon us and all holders of the convertible preferred stock and the debentures, other than holders who disclose on their tax returns that they are treating the convertible preferred stock and/or the perceptiondebentures in a manner inconsistent with such characterization. Although our characterization of the convertible preferred stock and the debentures is not binding upon the IRS or any court, this summary assumes that the convertible preferred stock and the debentures will be treated in a manner consistent with our characterization. Holders should be aware that if the convertible preferred stock is treated as “debt” for federal income tax purposes, the tax consequences of acquiring, holding and disposing of the convertible preferred stock will differ materially from the tax consequences described in this prospectus. Similarly, if the debentures are treated as “stock” for federal income tax purposes, the tax consequences of acquiring, holding and disposing of the debentures will differ materially from the tax consequences described in this prospectus.
Distributions on Convertible Preferred Stock and Common Stock
Distributions with respect to the convertible preferred stock and common stock will constitute dividends, to the extent that we have current or accumulated earnings and profits for federal income tax purposes as of the end of the tax year of the distribution. Dividends paid to non-corporate U.S. holders in taxable years beginning prior to January 1, 2009, will be subject to tax as net capital gain at the maximum rate of 15% if the holder has held the shares of stock for more than 60 days during the 120-day period beginning 60 days before the ex-dividend date and other requirements applicable to “qualified dividend income” are satisfied. Dividends paid to corporations will generally be eligible for the 70% dividends-received deduction under section 243 of the Code, subject to the limitations contained in sections 246 and 246A of the Code.
In general, the dividends-received deduction is available only if the stock in respect of which a dividend is paid has been held for at least 46 days during the 90-day period beginning on the date which is 45 days before the ex-dividend date, or at least 91 days during the 180-day period beginning on the date which is 90 days before the ex-dividend date in the case of a dividend paid with respect to preferred stock and which is attributable to a period or periods aggregating more than 366 days. A taxpayer’s holding period for these sales couldpurposes is reduced by periods during which the taxpayer’s risk of loss with respect to the stock is considered diminished by reason of the existence of options, contracts to sell or other similar transactions. The dividends-received deduction will not be available to the extent that the taxpayer is under an obligation to make related payments with respect to positions in substantially similar or related property. The dividends-received deduction is limited to specified percentages of the holder’s taxable income and may be reduced or eliminated if the holder has indebtedness “directly attributable to [its] investment” in the stock. Prospective corporate purchasers of convertible preferred stock should consult their own tax advisors to determine whether these limitations might apply to them. No assurance can be given that we will have sufficient earnings and profits for federal income tax purposes to cause all or even any distributions from Xcyte to be taxable as dividends. As a result, no assurance can be given that any distribution on the convertible preferred stock or common stock will be treated as a dividend for which the dividends-received deduction will be available.
If distributions with respect to the convertible preferred stock or common stock exceed our current and accumulated earnings and profits, the excess will be applied against and reduce the holder’s basis in the convertible preferred stock or common stock, as applicable. Any amount in excess of the amount of the dividend and the amount applied against basis will be treated as capital gain.
Extraordinary Dividends
If a corporate holder of convertible preferred or common stock receives an “extraordinary dividend” from Xcyte with respect to stock which it has not held for more than two years before the dividend announcement date, the basis of the stock will be reduced (but not below zero) by the portion of the dividend which is not taxable because of the dividends-received deduction. If, because of the limitation on reducing basis below zero, any amount of the non-taxable portion of an extraordinary dividend has not been applied to reduce basis, such amount will be treated as gain from the sale or exchange of stock in the taxable year in which the extraordinary dividend is received. An “extraordinary dividend” on the convertible preferred or common stock would include a dividend that (i) equals or exceeds 5%, in the case of the convertible preferred stock, or 10%, in the case of the common stock, of the holder’s adjusted basis in the stock, treating all dividends having ex-dividend dates within an 85-day period as one dividend, or (ii) exceeds 20% of the holder’s adjusted basis in the stock, treating all dividends having ex-dividend dates within a 365-day period as one dividend. A holder may elect to use the fair market value of the stock rather than its adjusted basis for purposes of applying the 5%, 10% or 20% limitation if the holder is able to establish such fair market value to the satisfaction of the IRS. An “extraordinary dividend” also includes any amount treated as a dividend in the case of a redemption of the convertible preferred stock or common stock that is not pro rata to all shareholders, irrespective of the holder’s holding period of the stock.
Special rules apply with respect to “qualified preferred dividends.” A qualified preferred dividend is any fixed dividend payable with respect to stock which (i) provides for fixed preferred dividends payable no less often than annually and (ii) is not in arrears as to dividends when acquired, provided the actual rate of return on such stock does not exceed 15%. For this purpose, the actual rate of return is determined solely by taking into account dividends during such holding period and by using the lesser of the adjusted basis or the liquidation preference in respect of such preferred stock. Where a qualified preferred dividend exceeds the 5% or 20% limitation described above, the extraordinary dividend rules will not apply if the taxpayer holds the stock for more than five years. If the taxpayer disposes of the stock before it has been held for more than five years, the aggregate reduction in basis will not exceed the excess of the qualified preferred dividends paid on such stock during the period held by the taxpayer over the qualified preferred dividends that would have been paid during such period on the basis of the stated rate of return as determined under section 1059(e)(3) of the Code. The length of time that a taxpayer is deemed to have held stock for this purpose is determined under principles similar to those applicable for purposes of the dividends-received deduction discussed above.
Any loss on the sale or exchange of stock with respect to which an individual holder receives an extraordinary dividend that is also qualified dividend income (see “Distributions on Convertible Preferred Stock and Common Stock” above) will be treated as long-term capital loss to the extent of the dividend. The deductibility of capital losses is limited.
Redemption Premium
If (i) preferred stock is, like the convertible preferred stock, redeemable only at the issuer’s option, (ii) the facts and circumstances on the issue date indicate that redemption is more likely than not to occur, could adversely affectand (iii) the redemption price of ourthe preferred stock as of the most likely redemption date exceeds the issue price (so that there is a “redemption premium”), then the redemption premium may be taxable as a constructive dividend to the extent of the issuing corporation’s current or accumulated earnings and profits over the period from issuance to the most likely redemption date. If a redemption premium is subject to the foregoing treatment, a holder of the convertible preferred stock would take the amount of the premium into income under an economic accrual method similar to the method described under “Original Issue Discount and Premiums on Debentures,” below. Under applicable Treasury regulations, a redemption premium is not subject to the foregoing treatment if it will be paid “as a result of changes in economic or market conditions over which neither the issuer nor the holder has legal or practical control” and is “solely in the nature of a penalty for premature redemption.” The Treasury regulations also provide a “safe harbor,” pursuant to which a redemption will not be treated as more likely than not to occur, as to a given holder, if: (x) the issuer and the holder are not “related” under certain tests prescribed by the Code, (y) the issuer is not effectively required or compelled by any plan, arrangement, or agreement to redeem the stock, and (z) redemption would not reduce the yield of the stock. Because the foregoing tests are based upon an evaluation of all facts and circumstances surrounding the issuance and redemption of preferred stock, the conclusion cannot be entirely certain; however, it is Xcyte’s belief that no part of the premium payable upon redemption of the convertible preferred stock will be treated as a constructive dividend to the holders of the convertible preferred stock. It is also possible that upon an actual redemption, the redemption premium would, together with the other redemption proceeds, be treated as a dividend for federal income tax purposes. See “Redemption of Convertible Preferred Stock for Cash,” below.
Redemption of Convertible Preferred Stock For Cash
A redemption of shares of convertible preferred stock by Xcyte for cash will be treated as a distribution taxable as a dividend (and, possibly, an “extraordinary dividend”) (see “Distributions on Convertible Preferred Stock and Common Stock” and “Extraordinary Dividends,” above) to redeeming shareholders to the extent of Xcyte’s current or accumulated earnings and profits unless the redemption:
| · | results in a complete termination of the shareholder’s interest in Xcyte (within the meaning of section 302(b)(3) of the Code); |
| · | is “substantially disproportionate” (within the meaning of section 302(b)(2) of the Code) with respect to the holder; or |
| · | is “not essentially equivalent to a dividend” (within the meaning of section 302(b)(1) of the Code). |
In determining whether any of these tests has been met, shares considered to be owned by the holder by reason of the constructive ownership rules set forth in section 318 of the Code, as well as shares actually owned, will be taken into account. If any of the foregoing tests is met, the redemption of shares of convertible preferred stock for cash will result in taxable gain or loss equal to the difference between the amount of cash received (except cash attributable to accrued, unpaid, declared dividends, which will be taxable as a dividend described above), and the holder’s basis in the redeemed shares. Any such gain or loss will be capital gain or loss and will be long-term capital gain or loss if the holding period exceeds one year. Long-term capital gains are taxable at a maximum rate of 15% in the case of individuals and 35% in the case of corporations. The deductibility of capital losses is subject to limitations.
Exchange For Debentures
An exchange of shares of convertible preferred stock for debentures will also be subject to the rules of section 302 of the Code described in “Redemption of Convertible Preferred Stock For Cash” above. Since a holder of debentures will be treated under the constructive ownership rules as owning the common stock into which the debentures are convertible, the exchange would not by itself satisfy the “complete termination” test or the “substantially disproportionate” test described above. The “not essentially equivalent to a dividend” test could be met only if the exchange were regarded as resulting in a meaningful reduction in the holder’s proportionate interest in Xcyte. If none of these tests is met, the fair market value of the debentures received upon the exchange will be taxable as a dividend (and, in the case of a corporate holder, as an “extraordinary dividend”—see above) to the extent of Xcyte’s current or accumulated earnings and profits and then would be treated as a return of capital to the extent of the holder’s basis in the convertible preferred stock. If the fair market value of the debentures exceeds the amounts treated as a dividend and as a return of capital, any such excess would be treated as capital gain.
In the event that receipt of the debentures is taxable as a dividend, the basis of the debentures will be equal to their fair market value as of the date of the exchange. If the holder retains any stock in Xcyte, the remaining basis in the convertible preferred stock will be transferred to such retained stock. If the holder retains no stock in Xcyte, it is unclear whether the remaining basis in the convertible preferred stock would be transferred to the debentures or would be lost. Under Proposed Treasury regulations, the remaining basis would be treated as a loss recognized on a disposition of the redeemed stock on the date of the redemption, which loss might be taken into account at a later date. These proposed Treasury regulations would only apply to transactions occurring after the date these regulations are finalized and published. For purposes of determining the recognition of gain under the extraordinary dividend basis reduction rules described above, only the basis of the shares of convertible preferred stock exchanged for the debentures would be taken into account.
Prospective purchasers should consult their own tax advisors regarding satisfaction of the section 302 tests in their particular circumstances, including the possibility that a sale of a part of the holder’s convertible preferred stock or the debentures received might be regarded as reducing the holder’s interest in Xcyte, thereby satisfying one of the tests of section 302(b); in such a case, the shareholder would recognize capital gain or loss on the exchange. For purposes of determining gain or loss, the amount realized by a shareholder would be the issue price of the debentures received (see “Original Issue Discount and Premium on Debentures”). Any such gain or loss will be capital gain or loss and will be long-term capital gain or loss if the holding period exceeds one year. Long-term capital gains are taxable at a maximum rate of 15% in the case of individuals and 35% in the case of corporations. The deductibility of capital losses is subject to limitations. The installment method will not be available for reporting such gain in the event that the convertible preferred stock, the debentures, or the common stock into which the debentures are convertible are traded or readily tradable on an established securities market.
Original Issue Discount and Premium On Debentures
Stated interest on the debentures will be includable in income in accordance with the holder’s method of accounting. There is also a risk that the debentures will be treated as having original issue discount taxable as interest income as discussed below.
If the convertible preferred stock is exchanged for debentures at a time when the stated redemption price at maturity of the debentures exceeds their issue price by an amount equal to or greater than one-fourth of one percent of the stated redemption price at maturity multiplied by the number of complete years to maturity, the debentures will be treated as having original issue discount equal to the entire amount of such excess.
Whether or not the exchange of the convertible preferred stock for debentures is treated as a dividend under the section 302 tests, the issue price of the debentures will depend upon whether the convertible preferred stock or the debentures are or will be traded on an established securities market. If the debentures are listed on an exchange or are otherwise considered, under Treasury regulations issued under section 1273 of the Code, to be
traded on an established securities market at any time during the 60-day period ending 30 days after the date of the exchange, the issue price of the debentures will be their fair market value as determined as of the date of the exchange. If the debentures are not listed on an exchange or otherwise considered to be traded on an established securities market within such time period, but the convertible preferred stock is so listed or traded, the issue price of the debentures will be the fair market value of the convertible preferred stock as of the date of the exchange. If neither the convertible preferred stock nor the debentures are listed on an exchange or otherwise considered to be traded on an established securities market within the requisite time period, the issue price of the debentures will be their stated principal amount, assuming that the debentures bear “adequate stated interest” within the meaning of section 1274 of the Code. If the debentures do not bear adequate stated interest, the issue price will be equal to their “imputed principal amount” as determined under section 1274 of the Code.
A holder of a debenture would generally be required to include in gross income (irrespective of the holder’s method of accounting) a portion of the original issue discount for each year during which it holds the debenture even though the cash to which such income is attributable would not be received until maturity or redemption of the debenture. The amount of any original issue discount included in income for each year would be calculated under a constant yield to maturity formula that would result in the allocation of less original issue discount to the early years of the term of the debenture and more original issue discount to later years.
If the convertible preferred stock is exchanged for debentures whose issue price exceeds the amount payable at maturity (or earlier call date, if appropriate), such excess (excluding the amount thereof attributable to the conversion feature) will be deductible by the holder of the debentures as amortizable bond premium over the term of the debentures (taking into account earlier call dates, as appropriate), under a yield to maturity formula, if an election by the taxpayer under section 171 of the Code is in effect or is made. Such election would apply to all obligations owned or subsequently acquired by the taxpayer during or after the taxable year in which the election is made. The amortizable bond premium will be treated as an offset to stated interest on the debentures to the extent thereof and any excess will be allowable as a deduction subject to the following limitation. The amount of any amortized bond premium deduction will be limited to the excess of the holder’s interest income inclusions on the debenture in prior accrual periods over bond premium deductions allowed the holder in such prior periods, and any amount in excess of such limitation will be carried forward as additional bond premium in the next accrual period.
If the exchange of the convertible preferred stock for debentures is treated as a dividend under the section 302 tests, the basis of the debentures will equal their fair market value as of the date of the exchange. If this basis is less than its stated redemption price at maturity, it would appear that a holder will recognize capital gain upon satisfaction of the debenture at maturity. If the basis of a debenture exceeds the amounts payable at maturity, a holder should be able to elect to amortize bond premium under the rules discussed above.
Redemption or Sale of Debentures
Generally a redemption or sale of the debentures will result in taxable gain or loss equal to the difference between the amount of cash and fair market value of other property received and the holder’s basis in the debentures. To the extent that the amount received is attributable to accrued interest, however, that amount will be taxed as ordinary income. The basis of a holder who received the debentures in exchange for shares of convertible preferred stock will generally be equal to the fair market value of the debentures at the time of exchange plus any original issue discount included in the holder’s income or minus any premium previously allowed as an offset to interest income on the debentures. Such gain or loss will be capital gain or loss and will be long-term gain or loss if the holding period for the debentures exceeds one year. Long-term capital gains are taxable at a maximum rate of 15% in the case of individuals and 35% in the case of corporations. The deductibility of capital losses is subject to limitations.
If the debentures are issued with original issue discount and Xcyte were found to have had an intention at the time the debentures were issued to call them before maturity, any gain realized on a sale, exchange or redemption of debentures prior to the maturity would be considered ordinary income to the extent of any unamortized
original issue discount for the period remaining to the stated maturity of the debentures. Xcyte cannot predict whether it would have an intention, when and if the debentures are issued, to call the debentures before their maturity.
Conversion of Convertible Preferred Stock or Debentures Into Common Stock
No gain or loss will generally be recognized upon conversion of shares of convertible preferred stock or debentures into shares of common stock, except that (i) gain or loss will be recognized to the extent of the difference between the cash paid in lieu of fractional shares of common stock and the basis of the convertible preferred stock or debentures allocable to such fractional shares, (ii) ordinary income will be recognized on the conversion of debentures to the extent of the shares of common stock attributable to accrued interest, (iii) additional payments made as dividend make-whole payments on conversion of convertible preferred stock prior to , 2007, will be treated as distributions taxable as dividends, return of capital or capital gain in the amount of the cash and/or the fair market value of the common stock (measured on the date of distribution) constituting such additional payments (see “Distributions on Convertible Preferred Stock and Common Stock” and “Extraordinary Dividends,” above), (iv) additional payments made as interest make-whole payments on conversion of debentures prior to , 2007, will be treated as payments of additional interest taxable as ordinary income when received and (v), if the conversion of convertible preferred stock takes place when there is a dividend arrearage on the convertible preferred stock and the fair market value of the common stock exceeds the issue price of the convertible preferred stock, a portion of the common stock received might be taxable as a dividend, return of capital or capital gain (see “Distributions on Convertible Preferred Stock and Common Stock” and “Extraordinary Dividends,” above). Assuming the conversion is not treated as resulting in the payment of a dividend, the basis of the common stock received upon conversion will be equal to the basis of the shares of convertible preferred stock or the debentures converted (less the amount of basis allocable to any fractional share of common stock for which cash is received), and the holding period of the common stock will include the holding period of the shares of convertible preferred stock or the debentures converted. The basis of any common stock treated as a dividend will be equal to its fair market value on the date of the distribution and its holding period will begin on the day after the conversion.
Adjustment of Conversion Price
Holders of convertible preferred stock, debentures or common stock may be deemed to have received constructive distributions where the conversion ratio or conversion price is adjusted to reflect property distributions with respect to common stock into which such convertible preferred stock or debentures are convertible. Adjustments to the conversion ratio or conversion price made pursuant to a bona fide reasonable adjustment formula which has the effect of preventing the dilution of the interest of the holders of the convertible preferred stock or debentures, however, will generally not be considered to result in a constructive distribution of stock. Certain of the possible adjustments provided in the convertible preferred stock and the debentures may not qualify as being pursuant to a bona fide reasonable adjustment formula. If such adjustments were made, the holders of convertible preferred stock or debentures might be deemed to have received constructive distributions taxable as a dividend, return of capital or capital gain in accordance with the general rules for the income tax treatment of distributions discussed above in “Distributions on Convertible Preferred Stock and Common Stock” and “Extraordinary Dividends.”
Backup Withholding
Under the backup withholding provisions of the Code and applicable Treasury regulations, a holder of convertible preferred stock, debentures or common stock may be subject to backup withholding at the rate of 28% with respect to dividends or interest (including original issue discount) paid on, or the proceeds of a sale, exchange or redemption of convertible preferred stock, debentures or common stock, unless (i) such holder is a corporation or comes within certain other exempt categories and when required demonstrates this fact or (ii) provides a taxpayer identification number, certifies as to no loss of exemption from backup withholding for interest and dividends and otherwise complies with applicable requirements of the backup withholding rules. The
amount of any backup withholding from a payment to a holder will be allowed as a credit against the holder’s federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Special Tax Rules Applicable to Foreign Holders
For purposes of the following discussion, a “Foreign Holder” is any holder who is not (i) a citizen or resident of the United States, (ii) a corporation or partnership (including any entity treated as a corporation or partnership for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any State or any political subdivision thereof, (iii) an estate the income of which is subject to United States federal income taxation regardless of source, or (iv) a trust if such trust elects to be treated as a U.S. person for U.S. federal income tax purposes, or a trust (A) over the administration of which a court within the United States is able to exercise primary supervision and (B) all substantial decisions of which one or more United States persons have the authority to control.
Income received by a Foreign Holder in the form of dividends on convertible preferred stock or common stock or interest and original issue discount on the debentures will be subject to a United States federal withholding tax at a 30% rate upon the actual payment of the dividends, interest or principal representing original issue discount except as described below and except where an applicable tax treaty provides for the reduction or elimination of such withholding tax. Dividends paid to Foreign Holders outside the United States that are subject to the withholding tax described above will generally be exempt from United States backup withholding tax but will be subject to United States information reporting requirements. Pursuant to a tax treaty or other agreement, this information may also be made available to the tax authorities in the country in which the Foreign Holder resides. A Foreign Holder generally will be taxable in the same manner as a United States person with respect to dividend, interest and original issue discount income if such income is effectively connected with the conduct of a trade or business in the United States, and if provided in a tax treaty, attributable to a permanent establishment in the United States. Such effectively connected income received by a Foreign Holder that is a corporation may in certain circumstances be subject to an additional “branch profits tax” at a 30% rate, or if applicable, a lower treaty rate. In order to claim the benefit of a tax treaty or to claim exemption from withholding because the income is effectively connected with the conduct of a trade or business in the U.S., a Foreign Holder must provide a properly executed IRS Form W-8BEN for treaty benefits or W-8ECI for effectively connected income (or such successor form as the IRS designates), prior to the payment of dividends. These forms must be periodically updated. Foreign Holders may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund. If a Foreign Holder holds preferred or common stock through a foreign partnership or a foreign intermediary, the foreign partnership or foreign intermediary will also be required to comply with certain certification requirements. The rules regarding withholding are complex, are subject to change, and vary depending on your particular situation. We suggest that you consult with your tax advisor regarding the application of such rules to your situation.
Payments of interest and principal representing original issue discount on the debentures received by a Foreign Holder will not be subject to United States federal withholding tax provided that (i) the Foreign Holder does not actually or constructively own 10% or more of the total combined voting power of all classes of stock of Xcyte entitled to vote, and (ii) the holder is not a controlled foreign corporation that is related to Xcyte through stock ownership, and in general, either (a) Xcyte or its paying agent can reliably associate the payment with documentation upon which it can rely to treat the payment as made to a foreign beneficial owner under Treasury regulations issued under section 1441 of the Code; (b) Xcyte or its paying agent can reliably associate the payment with a withholding certificate from a person claiming to be a withholding foreign partnership and the foreign partnership can reliably associate the payment with documentation upon which it can rely to treat the payment as made to a foreign beneficial owner in accordance with such Treasury regulations; (c) Xcyte or its paying agent can reliably associate the payment with a withholding certificate from a person representing to be a “qualified intermediary” that has assumed primary withholding responsibility under such Treasury regulations and the qualified intermediary can reliably associate the payment with documentation upon which it can rely to
treat the payment as made to a foreign beneficial owner in accordance with its agreement with the IRS; or (d) Xcyte or its paying agent receives a statement, under penalties of perjury from an authorized representative of a financial institution stating that the financial institution has received from the beneficial owner a withholding certificate described in such Treasury regulations or that it has received from another financial institution a similar statement that it, or another financial institution acting on behalf of the beneficial owner, has received such a withholding certificate from the beneficial owner. In general, it will not be necessary for a Foreign Holder to obtain or furnish a United States taxpayer identification number to Xcyte or its paying agent in order to claim the foregoing exemption from United States withholding tax on payments of interest and original issue discount.
Provided that Xcyte is not, and has not been, a “United States real property holding corporation” within the meaning of section 897(c) of the Code, a Foreign Holder generally will not be subject to United States federal income or withholding tax on gain realized on the sale or exchange of convertible preferred stock, common stock, or debentures unless (i) the holder is an individual who is present in the United States for 183 days or more during the taxable year and as to whom such gain is from United States sources or (ii) the gain is effectively connected with a United States trade or business of the holder, and if required by a tax treaty, attributable to a permanent establishment in the United States. Upon a redemption of the convertible preferred stock for cash or an exchange of convertible preferred stock for debentures, Xcyte may be required to withhold tax on the entire amount of the proceeds at a 30% rate or lower treaty rate applicable to dividends unless a Foreign Holder is able to demonstrate to the satisfaction of Xcyte that such redemption or exchange satisfies the section 302 tests discussed above with respect to such Foreign Holder (see “Redemption of Convertible Preferred Stock for Cash” and “Exchange for Debentures,” above). In the case of an exchange of convertible preferred stock for debentures, this would result in a Foreign Holder receiving a reduced principal amount of debentures.
The payment of the proceeds of the sale of convertible preferred stock, common stock or debentures to or through the United States office of a broker will be subject to information reporting and possible backup withholding at a rate of 28% unless the owner certifies its non-United States status under penalties of perjury or otherwise establishes an exemption in accordance with applicable Treasury regulations. The payment of the proceeds of the sale of convertible preferred stock, common stock or debentures to or through the foreign office of a foreign broker generally will not be subject to information reporting or backup withholding. In the case of the payment of proceeds from the disposition of convertible preferred stock, common stock or debentures through a foreign office of a broker that is a United States person or a “United States related person,” the applicable Treasury regulations require information reporting, but not backup withholding, on the payment unless the broker has documentary evidence in its files that the owner is a non-United States person and the broker has no actual knowledge to the contrary. For this purpose, a “United States related person” is (i) a
“controlled foreign corporation” for United States federal income tax purposes, (ii) a foreign person 50% or more of whose gross income from all sources for a specified period is derived from activities that are effectively connected with the conduct of a United States trade or business or (iii) a foreign partnership that, at any time during its taxable year, is more than 50% owned by United States persons or is engaged in the conduct of a United States trade or business. Any amounts withheld under the backup withholding rules from a payment to a Foreign Holder will be allowed as a refund or a credit against such Foreign Holder’s United States federal income tax, provided that the required information is timely furnished to the IRS.
SHARES ELIGIBLE FOR FUTURE SALE
Based on the number of shares outstanding as of September 30, 2003,27, 2004, we will have approximately 14,826,970 shares of our common stock outstanding and 1,500,000 shares of convertible preferred stock outstanding after the completion of this offering (approximately(1,725,000 shares of convertible preferred stock if the underwriters exercise their overallotment option in full). These shares of convertible preferred stock are convertible into shares of our common stock ( shares of our common stock if the underwriters exercise their overallotment option in full). Of those shares, the 1,500,000 shares of commonconvertible preferred stock sold in this offering ((1,725,000 shares if the underwriters exercise their overallotment option in full) will be freely transferable without restriction, unless purchased by our affiliates. The remaining shares of common stock to be outstanding immediately following the completion of this offering, which are “restricted securities” under Rule 144 of the Securities Act of 1933, or Rule 144, as well as any other shares held by our affiliates, may not be resold except pursuant to an effective registration statement or an applicable exemption from registration, including an exemption under Rule 144.
All of our officers and directors, and security holders holding over %certain of our outstandingsignificant stockholders, who in the aggregate hold 5,452,203 shares of our common stock shares subject to outstanding options and warrants, have entered into lock-up agreements pursuant to which they have generally agreed, subject to limitedcertain exceptions, not to offer or sell any shares of common stock or securities convertible into or exchangeable or exercisable for shares of common stock for a period of 18090 days from the date of this prospectus without the prior written consent of UBS Securities LLC.Piper Jaffray & Co. See “Underwriting.”
After the offering, the holders of 8,992,108 shares of our common stock (including shares issuable upon exercise of outstanding warrants)and warrants will be entitled to registration rights. For more information on these registration rights, see “Description of capital stock—Our Other Capital Stock—Registration rights.Rights.”
In general, under Rule 144, as currently in effect, an affiliate of ours who beneficially owns shares of our common stock that are not restricted securities, or a person who beneficially owns for more than one year shares of our common stock that are restricted securities, may generally sell, within any three-month period, a number of shares that does not exceed the greater of:
| 1% of the number of shares of our common stock then outstanding, which will equal approximately 148,270 shares immediately after this offering; and |
| the average weekly trading volume of our common stock on |
Sales under Rule 144 are also subject to requirements with respect to manner of sale, notice and the availability of current public information about us. Generally, a person who was not our affiliate at any time during the three months before the sale, and who has beneficially owned shares of our common stock that are restricted securities for at least two years, may sell those shares without regard to the volume limitations, manner of sale provisions,restrictions, notice requirements or the requirements with respect to availability of current public information about us.
Generally, an employee, officer, director or consultant who purchased shares of our common stock before March 16, 2004, the effective date of the registration statement of which this prospectus is a part,our initial public offering, or who holds options as of that date, pursuant to a written compensatory plan or contract may rely on the resale provisions of Rule 701 under the Securities Act. Under Rule 701, these persons who are not our affiliates may now generally sell their eligible securities commencing 90 days after the effective date of the registration statement of which this prospectus is a part, without having to comply with the public information,
90
Shares eligible for future sale
holding period, volume limitation or notice provisions of Rule 144. These persons who are our affiliates may generally sell their eligible securities under Rule 701, commencing 90 days after the effective date of the registration statement of which this prospectus is a part, without having to comply with Rule 144’s one-year holding period restriction.restriction but subject to other restrictions of Rule 144.
Neither Rule 144 nor Rule 701 supersedes the contractual obligations of certain of our security holders set forth in the lock-up agreements described above.
The 14,826,970 shares of our common stock that were outstanding on September 30, 200327, 2004 are or will assuming conversion of our preferred stock in connection with this initial public offeringbecome eligible for sale, pursuant to Rule 144 or Rule 701, become eligible for sale without registration approximately as follows:
| 9,708,329 shares of common stock |
| 3,753,960 shares of common stock |
| the remaining 1,364,681 shares of common stock will become eligible under Rule 144 for sale in the public market from time to time |
The above does not take into consideration the effect of the lock-up agreements described above.
STOCK OPTIONSStock Options
WeAs of September 27, 2004, we have reserved an aggregate of 6.4 million1,163,636 shares of our common stock for issuance under our 1996 Stock Option Plan, 3.5 million636,363 shares of our common stock for issuance under our 2003 Stock Plan, 500,00090,909 shares of our common stock for issuance under our 2003 Directors’ Stock Option Plan and 600,000109,090 shares of our common stock for issuance under our 2003 Employee Stock Purchase Plan. As of September 30, 2003,27, 2004, we had outstanding options under our 1996 Stock Option Plan to purchase 3,985,560949,232 shares of our common stock, outstanding options under our 2003 Stock Plan to purchase 44,880 shares of our common stock and outstanding options under our 2003 Directors’ Stock Option Plan to purchase 10,000 shares of our common stock. We intend to register theAll of these shares subject to these plans and the optionsare registered on a registration statement under the Securities Act of 1933 on Form S-8 following this offering.S-8. Subject to the lock-up agreements, the restrictions imposed under the 1996 Stock Option Plan, the 2003 Stock Plan, the 2003 Directors’ Stock Option Plan, the 2003 Employee Stock Purchase Plan and related option agreements, shares of common stock issued under these plans or agreements after the effective date of any registration statement on Form S-8 will be available for sale in the public market without restriction to the extent that they are held by persons who are not our affiliates.
91
We are offering the shares of our commonthe convertible preferred stock described in this prospectus through the underwriters named below. UBS Securities LLC, U.S. Bancorp Piper Jaffrey Inc.Jaffray & Co. and Wells FargoJMP Securities LLC are the representatives of the underwriters. We have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of commonconvertible preferred stock listed next to its name in the following table:
Underwriters | Number of shares | |
| ||
| ||
| ||
Total | �� | |
The underwriting agreement provides that the underwriters must buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Our commonThe convertible preferred stock is offered subject to a number of conditions, including:
| receipt and acceptance of |
| the underwriters’ right to reject orders in whole or in part. |
We have been advised by the representatives that the underwriters intend to make a market in our common stock but that they are not obligated to do so and may discontinue making a market at any time without notice.
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
Over-allotment optionOver-Allotment Option
We have granted the underwriters an option to buy up to an aggregate of 225,000 additional shares of our commonthe convertible preferred stock. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above.
Commissions and discountsDiscounts
Shares sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from the initial public offering price. If all the shares are not sold at the initial public offering price, the representatives may change the offering price and the other selling terms. Sales of shares made outside of the United States may be made by affiliates of the underwriters. Upon execution
92
Underwriting
of the underwriting agreement, the underwriters will be obligated to purchase the shares at the prices and upon the terms stated therein and, as a result, will thereafter bear any risk associated with changing the offering price to the public or other selling terms. The underwriters have informed us that they do not expect discretionary sales to exceed % of the shares of common stock to be offered.
The following table shows the per share and total underwriting discounts and commissions we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional 225,000 shares.
| No exercise | Full exercise | |||||
Per share | $ | $ | ||||
Total | $ | $ |
We estimate that the total expenses of this offering payable by us, not including the underwriting discounts and commissions, will be approximately .$440,000.
No salesSales of similar securitiesSimilar Securities
We, our executive officers and directors and substantially all existingcertain of our significant stockholders have entered into lock-up agreements with the underwriters. Under these agreements, we and each of these persons generally may not, without the prior written approval of UBS Securities LLC,Piper Jaffray & Co., offer, sell, contactcontract to sell or otherwise dispose of directly or indirectly or hedge our common stock or securities convertible into or exchangeable for or exercisable for our common stock.stock, subject to certain exceptions. These restrictions will be in effect for a period of 18090 days after the date of this prospectus. At any time and without public notice, UBS Securities LLCPiper Jaffray & Co. may, in theirits sole discretion, release allsome or someall of the securities from these lock-up agreements.
Indemnification and Contribution
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments the underwriters may be required to make in respect of those liabilities.
Nasdaq National Market quotationQuotation
We have appliedwill apply to have our commonthe convertible preferred stock approved for quotation on Thethe Nasdaq National Market under the trading symbol “XCYT.“XCYTP.”
Price stabilization, short positionsStabilization, Short Positions
In connection with thisorder to facilitate the offering of the preferred stock, the underwriters may engage in activitiestransactions that stabilize, maintain or otherwise affect the price of our convertible preferred stock or common stock. Specifically, the underwriters may over-allot in connection with this offering, creating a short position in our preferred stock including:
Stabilizing transactions consist of bidsfor their own account. In addition, to cover over-allotments or purchases made forto stabilize the purpose of preventing or retarding a decline in the market price of our commonthe preferred stock, while this offering is in progress. These transactions may also include making short sales of our common stock, which involve the sale by the underwriters of
93
Underwriting
a greater number of shares of commonmay bid for, and purchase, our convertible preferred stock than they are required to purchase in this offering and purchasing shares ofor common stock in the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked” shorts, which are short positions in excess of that amount.market.
The underwriters may close out any covered short position in our convertible preferred stock or common stock either by either exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option.
Naked short sales are in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of theour convertible preferred stock or common stock in the open market that could adversely affect investors who purchased in this offering.market.
The underwriters may also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares of our preferred stock sold by or for the account of that underwriter in stabilizing or short covering transactions.
As a result of these activities, the price of our common and convertible preferred stock may be higher that the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on Thethe Nasdaq National Market, in the over-the-counter market or otherwise.
Determination of Convertible Preferred Stock Terms
Prior to this offering, there has beenwas no public market for our commonthe convertible preferred stock. The initial public offeringterms and conditions of the convertible preferred stock, including the dividend rate and the conversion price will be determined by negotiation by us and the representatives of the underwriters. The principal factors to be considered in determining the initial public offering pricethese terms and conditions include:
| the market price of our common stock; |
| · | the information set forth in this prospectus and otherwise available to representatives; |
| our history and prospects and the history of, and prospects |
| our past and present financial performance and an assessment of our management; |
| our prospects for future earnings and the present state of our development; |
| the general condition of the securities markets at the time of this offering; |
| the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| other factors deemed relevant by the underwriters and us. |
At our request, certain of the underwriters have reserved up to % of the common stock being offered by this prospectus for sale to our directors, officers, employees and other individuals associated with us and members of their families at the initial offering price. The sales will be made by UBS Warburg LLC through a directed share program. We do not know if these persons will choose to purchase all or any portion of these reserved shares, but any purchases they do make will reduce the number of shares available to the general public. These persons must commit to purchase no later than the close of business on the day following the date of this prospectus. Any employees or other persons purchasing such reserved shares will be prohibited from disposing of or hedging such shares for a period of at least 180 days after the date of this prospectus.Affiliations
94
Underwriting
TheCertain of the underwriters and their affiliates have in the past provided and may from time to time provide certain commercial banking, financial advisory, and investment banking and other services for us for which they were and will be entitled to receive customaryseparate fees.
The underwriters and their affiliates may from time to time in the future engage in transactions with us and perform services for us in the ordinary course of their business.
95
The validity of the commonconvertible preferred stock we are offering will be passed upon for us by Heller Ehrman White & McAuliffe LLP, Seattle, Washington. Dewey BallantineCooley Godward LLP, East Palo Alto, California, is counsel for the underwriters in connection with this offering. Investment partnerships associatedAs of the date of this prospectus, an investment partnership affiliated with Cooley Godward LLP beneficially owns an aggregate of 4,784 shares of our common stock. Both an investment entity affiliated with Heller Ehrman White & McAuliffe LLP and individual attorneys of Heller Ehrman White & McAuliffe LLP beneficially own an aggregate of 16,188 shares of our Series D preferred stock and warrants to purchase 1,8123,209 shares of our common stock. These shares of Series D preferred stock will convert into 16,188 shares of our common stock upon completion of this offering.
The financial statements of Xcyte Therapies, Inc. at December 31, 2002 and 2001,2003, and for each of the three years in the period ended December 31, 2002,2003 and for the period from inception (January 5, 1996) to December 31, 2003, appearing in this Prospectusprospectus and Registration Statementregistration statement have been audited by Ernst & Young LLP, independent auditors,registered public accounting firm, as set forth in their report thereon (which contains an emphasisexplanatory paragraph describing conditions that adversely affectraise substantial doubt about the Company’s liquidityability to continue as a going concern as described in Note 1 to the financial statements) appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
Where you can find more informationWHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933 with respect to the shares of commonconvertible preferred stock we are offering. This prospectus does not contain all of the information in the registration statement and the exhibits to the registration statement. For further information with respect to us, and our common stock and the convertible preferred stock, we refer you to the registration statement and to the exhibits to the registration statement. Statements contained in this prospectus about the contents of any contract or any other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy the registration statement of which this prospectus is a partany document we file at the SEC’s Public Reference Room which is located at 450 Fifth Street, N.W., Washington, D.C. 20549. You can request copies of the registration statement by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s Public Reference Room. In addition, thepublic reference room. The SEC maintains an Internet website, which is located at www.sec.gov,site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at theSEC, including Xcyte Therapies, Inc. The SEC’s Internet website. Upon completion of this offering, we willsite can be subject to thefound athttp://www.sec.gov.
The information and reporting requirements of the Securities Exchange Act of 1934 currently apply to us and we will filecontinue to apply to us following this offering. We intend to furnish holders of our common and convertible preferred stock with annual reports proxy statements andcontaining, among other information, with the SEC.audited financial statements certified by an independent public accounting firm. We intend to furnish other reports as we may determine or as may be required by law.
We maintain an Internet website at www.xcytetherapies.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
96
Where you can find more information
This prospectus includes statistical data that were obtained from industry publications. These industry publications generally indicate that the authors of these publications have obtained information from sources believed to be reliable but do not guarantee the accuracy and completeness of their information. While we believe these industry publications to be reliable, we have not independently verified their data.
97
Index to financial statementsINDEX TO FINANCIAL STATEMENTS
| Page | ||
Report of Ernst & Young LLP, | F-2 | |
| F-3 | ||
| F-4 | ||
Statements of changes in stockholders’ | F-5 | |
| F-7 | ||
| F-8 |
F-1
REPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORS
REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors
Xcyte Therapies, Inc.
We have audited the accompanying balance sheets of Xcyte Therapies, Inc. (a development stage company) (the Company) as of December 31, 20012002 and 2002,2003, and the related statements of operations, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2002.2003 and for the period from inception (January 5, 1996) to December 31, 2003. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditingthe standards generally accepted inof the United States.Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
Since the date of completion of our audit of the accompanying financial statements and initial issuance of our report thereon dated April 25, 2003, the Company, as discussed in Note 14, has experienced a decrease in its cash and cash equivalents and short-term investments and continues to incur substantial operating losses that adversely affect the Company’s current results of operations and liquidity. Note 14 describes management’s plans to address these issues.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Xcyte Therapies, Inc. (a development stage company) at December 31, 20012002 and 2002,2003, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2002,2003 and for the period from inception (January 5, 1996) to December 31, 2003, in conformity with accounting principlesU.S. generally accepted in the United States.accounting principles.
As describeddiscussed in Note 1 to the financial statements, the Company has restated its balance sheets as of December 31, 2001 and 2002 and its statements ofCompany’s recurring losses from operations and stockholders’ deficit for eachnet capital deficiency raise substantial doubt about its ability to continue as a going concern. Management’s plans as to these matters are also described in Note 1. The 2003 financial statements do not include any adjustments that might result from the outcome of the three years in the period ended December 31, 2002.this uncertainty.
/s/ Ernst & Young LLP
Seattle, Washington
April 25, 2003,January 23, 2004,
except for the second and fourth paragraphsfirst paragraph of Note 1 and Note 13,8,
as to which the date is October 9, 2003
F-2March 4, 2004
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
BALANCE SHEETS
(in thousands, except share and per share data)
| December 31, | Pro forma 2003 (Note 13) | |||||||||||||||||||||||||||
| 2001 | 2002 | June 30, 2003 | December 31, | June 30, 2004 | ||||||||||||||||||||||||
| 2002 | 2003 | |||||||||||||||||||||||||||
| (restated) | (restated) | (unaudited) | ||||||||||||||||||||||||||
| (in thousands, except share and per share data) | (unaudited) | |||||||||||||||||||||||||||
Assets | ||||||||||||||||||||||||||||
Current assets: | ||||||||||||||||||||||||||||
Cash and cash equivalents | $ | 21,098 | $ | 3,728 | $ | 1,918 | $ | 3,728 | $ | 2,241 | $ | 8,802 | ||||||||||||||||
Short-term investments | — | 13,616 | 6,974 | 13,616 | 11,299 | 24,928 | ||||||||||||||||||||||
Prepaid expenses and other current assets | 349 | 699 | 494 | 598 | 519 | 1,277 | ||||||||||||||||||||||
Total current assets | 21,447 | 18,043 | 9,386 | 17,942 | 14,059 | 35,007 | ||||||||||||||||||||||
Property and equipment, net | 2,303 | 2,613 | 2,469 | 2,613 | 2,767 | 3,905 | ||||||||||||||||||||||
Deposits and other assets | 977 | 879 | 840 | 879 | 1,672 | 948 | ||||||||||||||||||||||
Total assets | $ | 24,727 | $ | 21,535 | $ | 12,695 | $ | 21,434 | $ | 18,498 | $ | 39,860 | ||||||||||||||||
Liabilities and stockholders’ equity (deficit) | ||||||||||||||||||||||||||||
Current liabilities: | ||||||||||||||||||||||||||||
Accounts payable | $ | 1,023 | $ | 595 | $ | 728 | $ | 595 | $ | 954 | $ | 2,045 | ||||||||||||||||
Accrued compensation and related benefits | 387 | 339 | 361 | 339 | 405 | 463 | ||||||||||||||||||||||
Other accrued liabilities | 259 | 721 | 241 | 721 | 856 | 640 | ||||||||||||||||||||||
Current portion of deferred revenue | — | — | 47 | |||||||||||||||||||||||||
Convertible promissory notes | — | 11,652 | — | |||||||||||||||||||||||||
Current portion of equipment financings | 643 | 818 | 804 | 717 | 845 | 974 | ||||||||||||||||||||||
Total current liabilities | 2,312 | 2,473 | 2,134 | 2,372 | 14,712 | 4,169 | ||||||||||||||||||||||
Deferred revenue, less current portion | — | — | 786 | |||||||||||||||||||||||||
Equipment financings, less current portion | 738 | 1,052 | 960 | 1,052 | 993 | 1,220 | ||||||||||||||||||||||
Other liabilities | 308 | 462 | 517 | 462 | 562 | 588 | ||||||||||||||||||||||
Commitments and contingencies | ||||||||||||||||||||||||||||
Redeemable convertible preferred stock, par value $0.001 per share Issued and outstanding—32,809,142, 37,253,393 and 37,300,234 shares as of December 31, 2001, December 31, 2002 and June 30, 2003, respectively (no shares, pro forma); aggregate preference in liquidation—$76,475 and $76,520 at December 31, 2002 and June 30, 2003, respectively |
| 56,552 |
|
| 64,540 |
|
| 64,604 |
| |||||||||||||||||||
Redeemable convertible preferred stock, Issued and outstanding—6,773,298 and 6,781,814 shares as of December 31, 2002 and December 31, 2003, respectively; none as of June 30, 2004 | ||||||||||||||||||||||||||||
Aggregate preference in liquidation—$76,475 and $76,520 at December 31, 2002 and December 31, 2003, respectively; none as of June 30, 2004 | 64,540 | 64,604 | — | |||||||||||||||||||||||||
Redeemable convertible preferred stock warrants | 1,077 | 1,133 | 1,069 | 1,133 | 2,467 | — | ||||||||||||||||||||||
Stockholders’ equity (deficit): | ||||||||||||||||||||||||||||
Preferred stock, $0.001 par value per share | ||||||||||||||||||||||||||||
Authorized—42,000,000 shares (5,000,000 shares, pro forma) Designated redeemable and convertible—41,909,976 (issued and outstanding—none, pro forma) | — | — | — | — | ||||||||||||||||||||||||
Common stock, par value $0.001 per share Authorized—70,000,000 shares (100,000,000 shares, pro forma) Issued and outstanding—7,437,380, 8,381,539 and 8,402,636 shares at December 31, 2001, December 31, 2002 and June 30, 2003, respectively (45,702,870 shares, pro forma) | 7 | 8 | 8 | 46 | ||||||||||||||||||||||||
Authorized—42,000,000 shares (5,000,000 shares as of June 30, 2004) | ||||||||||||||||||||||||||||
Designated redeemable and convertible—41,909,976 shares as of December 31, 2002 and 2003 (none as of June 30, 2004) | — | — | — | |||||||||||||||||||||||||
Common stock, par value $0.001 per share | ||||||||||||||||||||||||||||
Authorized—70,000,000 shares (100,000,000 shares as of June 30, 2004) | ||||||||||||||||||||||||||||
Issued and outstanding—1,523,867 and 1,546,624 shares as of December 31, 2002 and December 31, 2003, respectively (14,826,573 as of June 30, 2004) | 2 | 2 | 15 | |||||||||||||||||||||||||
Additional paid-in capital | 14,482 | 21,881 | 21,963 | 87,598 | 21,887 | 24,532 | 146,511 | |||||||||||||||||||||
Deferred stock compensation | (2,064 | ) | (1,880 | ) | (1,236 | ) | (1,236 | ) | (1,880 | ) | (2,774 | ) | (2,404 | ) | ||||||||||||||
Accumulated other comprehensive income | — | 4 | 3 | 3 | ||||||||||||||||||||||||
Accumulated other comprehensive income (loss) | 4 | (5 | ) | (60 | ) | |||||||||||||||||||||||
Deficit accumulated during the development stage | (48,685 | ) | (68,138 | ) | (77,327 | ) | (77,327 | ) | (68,138 | ) | (86,595 | ) | (110,965 | ) | ||||||||||||||
Total stockholders’ equity (deficit) | (36,260 | ) | (48,125 | ) | (56,589 | ) | $ | 9,084 | $ | (48,125 | ) | $ | (64,840 | ) | $ | 33,097 | ||||||||||||
Total liabilities and stockholders’ deficit | $ | 24,727 | $ | 21,535 | $ | 12,695 | ||||||||||||||||||||||
Total liabilities and stockholders’ equity (deficit) | $ | 21,434 | $ | 18,498 | $ | 39,860 | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-3
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| Year ended December 31, | Six months ended June 30, | Period from June 30, | Year Ended December 31, | Period from inception December 31, 2003 | Six Months Ended June 30, | Period from June 30, 2004 | ||||||||||||||||||||||||||||||||||||||||||||||
| 2000 | 2001 | 2002 | 2002 | 2003 | 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||||||||||||||||||||||||||||
| (in thousands, except share and per share data) | (Unaudited) | (Unaudited) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue: | (restated) | (restated) | (restated) | (unaudited) | (unaudited) | |||||||||||||||||||||||||||||||||||||||||||||||
License fee | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 100 | $ | — | $ | — | $ | — | $ | 100 | $ | — | $ | 12 | $ | 112 | ||||||||||||||||||||||||||
Collaborative agreement | — | — | — | — | 72 | 72 | — | — | 170 | 170 | 72 | 24 | 194 | |||||||||||||||||||||||||||||||||||||||
Government grant | 98 | 30 | — | — | — | 144 | 30 | — | — | 144 | — | — | 144 | |||||||||||||||||||||||||||||||||||||||
Total revenue | 98 | 30 | — | — | 72 | 316 | 30 | — | 170 | 414 | 72 | 36 | 450 | |||||||||||||||||||||||||||||||||||||||
Operating expense: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Research and development | 11,257 | 14,701 | 14,663 | 7,651 | 7,029 | 60,169 | 14,701 | 14,663 | 13,685 | 66,825 | 7,029 | 8,601 | 75,426 | |||||||||||||||||||||||||||||||||||||||
General and administrative | 2,403 | 5,204 | 4,979 | 2,883 | 2,194 | 19,323 | 5,204 | 4,979 | 4,322 | 21,451 | 2,194 | 3,297 | 24,748 | |||||||||||||||||||||||||||||||||||||||
Total operating expense | 13,660 | 19,905 | 19,642 | 10,534 | 9,223 | 79,492 | 19,905 | 19,642 | 18,007 | 88,276 | 9,223 | 11,898 | 100,174 | |||||||||||||||||||||||||||||||||||||||
Loss from operations | (13,562 | ) | (19,875 | ) | (19,642 | ) | (10,534 | ) | (9,151 | ) | (79,176 | ) | (19,875 | ) | (19,642 | ) | (17,837 | ) | (87,862 | ) | (9,151 | ) | (11,862 | ) | (99,724 | ) | ||||||||||||||||||||||||||
Other income (expense): | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Interest income | 868 | 698 | 467 | 247 | 94 | 3,417 | 698 | 467 | 149 | 3,472 | 94 | 148 | 3,620 | |||||||||||||||||||||||||||||||||||||||
Interest expense | (247 | ) | (260 | ) | (267 | ) | (123 | ) | (131 | ) | (1,373 | ) | (260 | ) | (267 | ) | (768 | ) | (2,010 | ) | (131 | ) | (12,656 | ) | (14,666 | ) | ||||||||||||||||||||||||||
Loss on sale of equipment | — | (75 | ) | (11 | ) | (2 | ) | (1 | ) | (195 | ) | (75 | ) | (11 | ) | (1 | ) | (195 | ) | (1 | ) | — | (195 | ) | ||||||||||||||||||||||||||||
Other income, net | 621 | 363 | 189 | 122 | (38 | ) | 1,849 | |||||||||||||||||||||||||||||||||||||||||||||
Other income (expense), net | 363 | 189 | (620 | ) | 1,267 | (38 | ) | (12,508 | ) | (11,241 | ) | |||||||||||||||||||||||||||||||||||||||||
Net loss | (12,941 | ) | (19,512 | ) | (19,453 | ) | (10,412 | ) | (9,189 | ) | (77,327 | ) | (19,512 | ) | (19,453 | ) | (18,457 | ) | (86,595 | ) | (9,189 | ) | (24,370 | ) | (110,965 | ) | ||||||||||||||||||||||||||
Accretion of preferred stock | — | (8,411 | ) | (8,001 | ) | (8,001 | ) | — | (16,412 | ) | (8,411 | ) | (8,001 | ) | — | (16,412 | ) | — | (8,973 | ) | (25,385 | ) | ||||||||||||||||||||||||||||||
Net loss applicable to common stockholders | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,413 | ) | $ | (9,189 | ) | $ | (93,739 | ) | ||||||||||||||||||||||||||||||||||
Net loss attributable to common stockholders | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,457 | ) | $ | (103,007 | ) | $ | (9,189 | ) | $ | (33,343 | ) | $ | (136,350 | ) | |||||||||||||||||||||||||||||||
Basic and diluted net loss per | $ | (2.16 | ) | $ | (4.03 | ) | $ | (3.52 | ) | $ | (2.45 | ) | $ | (1.13 | ) | $ | (22.14 | ) | $ | (19.34 | ) | $ | (12.40 | ) | $ | (6.21 | ) | $ | (3.66 | ) | ||||||||||||||||||||||
Shares used in computation of | 6,002,557 | 6,935,989 | 7,808,653 | 7,523,096 | 8,143,609 | 1,261,089 | 1,419,755 | 1,488,218 | 1,480,603 | 9,107,401 | ||||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-4
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICITEQUITY (DEFICIT)
(in thousands, except share data)
| Common stock | Additional paid-in capital | Deferred stock compensation | Deficit accumulated during the development stage | Accumulated other comprehensive loss | Total | ||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||
| (restated | ) | (restated | ) | ||||||||||||||||||||||||
Common stock issued upon incorporation (unaudited) | 3,374,634 | $ | 3 | $ | — | $ | — | $ | — | $ | — | $ | 3 | ||||||||||||||
Deferred stock-based compensation (unaudited) | — | — | 7 | (7 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation (unaudited) | — | — | — | 2 | — | — | 2 | ||||||||||||||||||||
Common stock issued for technology license (unaudited) | 198,609 | — | — | — | — | — | — | ||||||||||||||||||||
Net loss (unaudited) | — | — | — | (551 | ) | — | (551 | ) | |||||||||||||||||||
Balance at December 31, 1996 (unaudited) | 3,573,243 | 3 | 7 | (5 | ) | (551 | ) | — | (546 | ) | |||||||||||||||||
Common stock repurchases (unaudited) | (635,000 | ) | (1 | ) | — | — | — | — | (1 | ) | |||||||||||||||||
Common stock issued in acquisition (unaudited) | 2,999,910 | 3 | 327 | — | — | — | 330 | ||||||||||||||||||||
Deferred stock-based compensation (unaudited) | — | — | 9 | (9 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation (unaudited) | — | — | — | 4 | — | — | 4 | ||||||||||||||||||||
Common stock issued for technology license (unaudited) | 407,198 | 1 | — | — | — | — | 1 | ||||||||||||||||||||
Stock options exercised (unaudited) | 12,750 | — | 1 | — | — | — | 1 | ||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | (3,288 | ) | — | (3,288 | ) | ||||||||||||||||||
Balance at December 31, 1997 (unaudited) | 6,358,101 | 6 | 344 | (10 | ) | (3,839 | ) | — | (3,499 | ) | |||||||||||||||||
Repurchase of founder’s stock (unaudited) | (88,542 | ) | — | — | — | — | — | — | |||||||||||||||||||
Stock options exercised (unaudited) | 250 | — | — | — | — | — | — | ||||||||||||||||||||
Deferred stock-based compensation (unaudited) | — | — | 8 | (8 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation (unaudited) | — | — | — | 6 | — | — | 6 | ||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | (5,446 | ) | — | (5,446 | ) | ||||||||||||||||||
Balance at December 31, 1998 (unaudited) | 6,269,809 | 6 | 352 | (12 | ) | (9,285 | ) | — | (8,939 | ) | |||||||||||||||||
Common stock returned for technology license termination | (400,000 | ) | — | — | — | — | — | — | |||||||||||||||||||
Common stock issued for technology license | 20,000 | — | 2 | — | — | — | 2 | ||||||||||||||||||||
Deferred stock-based compensation | — | — | 720 | (720 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation | — | — | — | 93 | — | — | 93 | ||||||||||||||||||||
Stock options exercised | 53,770 | — | 5 | — | — | — | 5 | ||||||||||||||||||||
Change in unrealized loss on investments | — | — | — | — | — | (18 | ) | (18 | ) | ||||||||||||||||||
Net loss | — | — | — | — | (6,947 | ) | — | (6,947 | ) | ||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | (6,965 | ) | |||||||||||||||||||
Balance at December 31, 1999 | 5,943,579 | 6 | 1,079 | (639 | ) | (16,232 | ) | (18 | ) | (15,804 | ) | ||||||||||||||||
Common stock issued for technology license | 150,000 | — | 744 | — | — | — | 744 | ||||||||||||||||||||
Issuance of common stock warrants | — | 2,716 | — | — | — | 2,716 | |||||||||||||||||||||
Deferred stock-based compensation | — | — | 1,988 | (1,988 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation | — | — | — | 770 | — | — | 770 | ||||||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 112 | — | — | — | 112 | ||||||||||||||||||||
Stock options exercised | 709,149 | 1 | 227 | — | — | — | 228 | ||||||||||||||||||||
Change in unrealized loss on investments | — | — | — | — | — | 18 | 18 | ||||||||||||||||||||
Net loss | — | — | — | — | (12,941 | ) | — | (12,941 | ) | ||||||||||||||||||
Comprehensive loss | — | — | — | — | — | — | (12,923 | ) | |||||||||||||||||||
Balance at December 31, 2000 (restated) | 6,802,728 | 7 | 6,866 | (1,857 | ) | (29,173 | ) | — | (24,157 | ) | |||||||||||||||||
Common stock repurchased | (13,333 | ) | — | (2 | ) | — | — | — | (2 | ) | |||||||||||||||||
Warrants issued and beneficial conversion in preferred stock | — | — | 13,060 | — | — | — | 13,060 | ||||||||||||||||||||
Deferred stock-based compensation | — | — | 1,652 | (1,652 | ) | — | — | — | |||||||||||||||||||
Amortization of deferred compensation | — | — | — | 1,445 | — | — | 1,445 | ||||||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 1,122 | — | — | — | 1,122 | ||||||||||||||||||||
Stock options and warrants exercised | 647,985 | — | 195 | — | — | — | 195 | ||||||||||||||||||||
Accretion of redeemable convertible preferred stock | — | — | (8,411 | ) | — | — | — | (8,411 | ) | ||||||||||||||||||
Net loss and comprehensive loss | — | — | — | — | (19,512 | ) | — | (19,512 | ) | ||||||||||||||||||
| Common stock | Additional paid-in capital | Deferred stock compensation | Accumulated other comprehensive income (loss) | Deficit accumulated during the development stage | Total | |||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||||||||
Common stock issued upon incorporation | 613,564 | $ | 1 | $ | 2 | $ | — | $ | — | $ | — | $ | 3 | |||||||||||||
Deferred stock-based compensation | — | — | 7 | (7 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 2 | — | — | 2 | |||||||||||||||||||
Common stock issued August 1996 for technology license, valued at $0.0055 per share | 36,110 | — | — | — | — | — | — | |||||||||||||||||||
Net loss | — | — | — | — | (551 | ) | (551 | ) | ||||||||||||||||||
Balance at December 31, 1996 | 649,674 | 1 | 9 | (5 | ) | — | (551 | ) | (546 | ) | ||||||||||||||||
Common stock repurchases | (115,454 | ) | — | (1 | ) | — | — | — | (1 | ) | ||||||||||||||||
Common stock issued August 1997 in acquisition, valued at $0.61 per share | 545,434 | — | 330 | — | — | — | 330 | |||||||||||||||||||
Deferred stock-based compensation | — | — | 9 | (9 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 4 | — | — | 4 | |||||||||||||||||||
Common stock issued January 1997 for technology license, valued at $0.0055 per share | 74,033 | — | 1 | — | — | — | 1 | |||||||||||||||||||
Stock options exercised | 2,317 | — | 1 | — | — | — | 1 | |||||||||||||||||||
Net loss | — | — | — | — | — | (3,288 | ) | (3,288 | ) | |||||||||||||||||
Balance at December 31, 1997 | 1,156,004 | 1 | 349 | (10 | ) | — | (3,839 | ) | (3,499 | ) | ||||||||||||||||
Repurchase of founder’s stock | (16,098 | ) | — | — | — | — | — | — | ||||||||||||||||||
Stock options exercised | 45 | — | — | — | — | — | — | |||||||||||||||||||
Deferred stock-based compensation | — | — | 8 | (8 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 6 | — | — | 6 | |||||||||||||||||||
Net loss | — | — | — | — | — | (5,446 | ) | (5,446 | ) | |||||||||||||||||
Balance at December 31, 1998 | 1,139,951 | 1 | 357 | (12 | ) | — | (9,285 | ) | (8,939 | ) | ||||||||||||||||
Common stock returned for technology license termination | (72,726 | ) | — | — | — | — | — | — | ||||||||||||||||||
Common stock issued June 1999 for technology license, valued at $0.55 per share | 3,636 | — | 2 | — | — | — | 2 | |||||||||||||||||||
Deferred stock-based compensation | — | — | 720 | (720 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 93 | — | — | 93 | |||||||||||||||||||
Stock options exercised | 9,769 | — | 5 | — | — | — | 5 | |||||||||||||||||||
Change in unrealized loss on investments | — | — | — | — | (18 | ) | — | (18 | ) | |||||||||||||||||
Net loss | — | — | — | — | — | (6,947 | ) | (6,947 | ) | |||||||||||||||||
Comprehensive loss | (6,965 | ) | ||||||||||||||||||||||||
Balance at December 31, 1999 | 1,080,630 | 1 | 1,084 | (639 | ) | (18 | ) | (16,232 | ) | (15,804 | ) | |||||||||||||||
Common stock issued December 2000 for technology license, valued at $27.28 per share | 27,272 | — | 744 | — | — | — | 744 | |||||||||||||||||||
Issuance of common stock warrants | — | — | 2,716 | — | — | — | 2,716 | |||||||||||||||||||
Deferred stock-based compensation | — | — | 1,988 | (1,988 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 770 | — | — | 770 | |||||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 112 | — | — | — | 112 | |||||||||||||||||||
Stock options exercised | 128,922 | — | 228 | — | — | — | 228 | |||||||||||||||||||
Change in unrealized loss on investments | — | — | — | — | 18 | — | 18 | |||||||||||||||||||
Net loss | — | — | — | — | — | (12,941 | ) | (12,941 | ) | |||||||||||||||||
Comprehensive loss | (12,923 | ) | ||||||||||||||||||||||||
Balance at December 31, 2000 | 1,236,824 | 1 | 6,872 | (1,857 | ) | — | (29,173 | ) | (24,157 | ) | ||||||||||||||||
Common stock repurchased | (2,424 | ) | — | (2 | ) | — | — | — | (2 | ) | ||||||||||||||||
Warrants issued November 2001 and beneficial conversion in preferred stock | — | — | 13,060 | — | — | — | 13,060 | |||||||||||||||||||
Deferred stock-based compensation | — | — | 1,652 | (1,652 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation | — | — | — | 1,445 | — | — | 1,445 | |||||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 1,122 | — | — | — | 1,122 | |||||||||||||||||||
Stock options and warrants exercised | 117,807 | — | 195 | — | — | — | 195 | |||||||||||||||||||
Accretion of redeemable convertible preferred stock | — | — | (8,411 | ) | — | — | — | (8,411 | ) | |||||||||||||||||
Net loss and comprehensive loss | — | — | — | — | — | (19,512 | ) | (19,512 | ) | |||||||||||||||||
Balance at December 31, 2001 | 1,352,207 | $ | 1 | $ | 14,488 | $ | (2,064 | ) | $ | — | $ | (48,685 | ) | $ | (36,260 | ) | ||||||||||
The accompanying notes are an integral part of these financial statements.
XCYTE THERAPIES, INC.
F-5
Xcyte Therapies, Inc. (a(a development stage company)
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICITEQUITY (DEFICIT) (continued)
(in thousands, except share data)
| Common stock | Additional paid-in capital | Deferred stock compensation | Accumulated deficit during the development stage | Accumulated other comprehensive loss | Total | ||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||
| (restated) | (restated) | ||||||||||||||||||||||||
Balance at December 31, 2001 (restated) | 7,437,380 | $ | 7 | $ | 14,482 | $ | (2,064 | ) | $ | (48,685 | ) | $ | — | $ | (36,260 | ) | |||||||||
Common stock issued for technology license | 350,000 | — | 679 | — | — | — | 679 | ||||||||||||||||||
Warrants issued and beneficial conversion in preferred stock | — | — | 12,325 | — | — | — | 12,325 | ||||||||||||||||||
Deferred stock-based compensation | — | — | 3,188 | (3,188 | ) | — | — | — | |||||||||||||||||
Amortization of deferred compensation, net of reversal of $867 for terminated employees | — | — | (867 | ) | 3,372 | — | — | 2,505 | |||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 65 | — | — | — | 65 | ||||||||||||||||||
Stock options and warrants exercised | 594,159 | 1 | 10 | — | — | — | 11 | ||||||||||||||||||
Accretion of redeemable convertible preferred stock | — | — | (8,001 | ) | — | — | — | (8,001 | ) | ||||||||||||||||
Change in unrealized gain on investments | — | — | — | — | — | 4 | 4 | ||||||||||||||||||
Net loss | — | — | — | — | (19,453 | ) | — | (19,453 | ) | ||||||||||||||||
Comprehensive loss | (19,449 | ) | |||||||||||||||||||||||
Balance at December 31, 2002 (restated) | 8,381,539 | 8 | 21,881 | (1,880 | ) | (68,138 | ) | 4 | (48,125 | ) | |||||||||||||||
Deferred stock-based compensation (unaudited) | — | — | 86 | (86 | ) | — | — | — | |||||||||||||||||
Amortization of deferred compensation, net of reversal of $135 for terminated employees (unaudited) | — | — | (135 | ) | 730 | — | — | 595 | |||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services (unaudited) | — | — | 130 | — | — | — | 130 | ||||||||||||||||||
Stock options and warrants exercised (unaudited) | 21,097 | — | 1 | — | — | — | 1 | ||||||||||||||||||
Change in unrealized gain on investments (unaudited) | — | — | — | — | — | (1 | ) | (1 | ) | ||||||||||||||||
Net loss (unaudited) | — | — | — | — | (9,189 | ) | — | (9,189 | ) | ||||||||||||||||
Comprehensive loss (unaudited) | (9,190 | ) | |||||||||||||||||||||||
Balance at June 30, 2003 (unaudited) | 8,402,636 | $ | 8 | $ | 21,963 | $ | (1,236 | ) | $ | (77,327 | ) | $ | 3 | $ | (56,589 | ) | |||||||||
| Common stock | Additional paid-in capital | Deferred stock compensation | Accumulated other comprehensive income (loss) | Deficit stage | Total | ||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||
| (in thousands, except share data) | |||||||||||||||||||||||||
Balance at December 31, 2001 | 1,352,207 | $ | 1 | $ | 14,488 | $ | (2,064 | ) | $ | — | $ | (48,685 | ) | $ | (36,260 | ) | |||||||||
Common stock issued May 2002 for technology license, valued at $10.67 per share | 63,636 | — | 679 | — | — | — | 679 | ||||||||||||||||||
Warrants issued February and March 2002 and beneficial conversion in preferred stock | — | — | 12,325 | — | — | — | 12,325 | ||||||||||||||||||
Deferred stock-based compensation | — | — | 3,188 | (3,188 | ) | — | — | — | |||||||||||||||||
Amortization of deferred compensation, net of reversal of $867 for terminated employees | — | — | (867 | ) | 3,372 | — | — | 2,505 | |||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 65 | — | — | — | 65 | ||||||||||||||||||
Stock options and warrants exercised | 108,024 | 1 | 10 | — | — | — | 11 | ||||||||||||||||||
Accretion of redeemable convertible preferred stock | — | — | (8,001 | ) | — | — | — | (8,001 | ) | ||||||||||||||||
Change in unrealized gain on investments | — | — | — | — | 4 | — | 4 | ||||||||||||||||||
Net loss | — | — | — | — | — | (19,453 | ) | (19,453 | ) | ||||||||||||||||
Comprehensive loss | (19,449 | ) | |||||||||||||||||||||||
Balance at December 31, 2002 | 1,523,867 | 2 | 21,887 | (1,880 | ) | 4 | (68,138 | ) | (48,125 | ) | |||||||||||||||
Deferred stock-based compensation | — | — | 2,423 | (2,423 | ) | — | — | — | |||||||||||||||||
Amortization of deferred compensation, net of reversal of $222 for terminated employees | — | — | (222 | ) | 1,529 | — | — | 1,307 | |||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services | — | — | 360 | — | — | — | 360 | ||||||||||||||||||
Stock options and warrants exercised | 22,757 | — | 84 | — | — | — | 84 | ||||||||||||||||||
Change in unrealized gain on investments | — | — | — | — | (9 | ) | — | (9 | ) | ||||||||||||||||
Net loss | — | — | — | — | — | (18,457 | ) | (18,457 | ) | ||||||||||||||||
Comprehensive loss | (18,466 | ) | |||||||||||||||||||||||
Balance at December 31, 2003 | 1,546,624 | 2 | 24,532 | (2,774 | ) | (5 | ) | (86,595 | ) | (64,840 | ) | ||||||||||||||
Issuance of common stock at $8.00 per share, net of issuance costs (unaudited) | 4,200,000 | 4 | 29,696 | — | — | — | 29,700 | ||||||||||||||||||
Conversion of preferred stock and warrants into common stock and warrants (unaudited) | 6,781,814 | 7 | 76,037 | — | — | — | 76,044 | ||||||||||||||||||
Conversion of promissory notes and accrued interest into common stock (unaudited) | 1,357,357 | 1 | 13,029 | — | — | — | 13,030 | ||||||||||||||||||
Recognition of beneficial conversion on convertible promissory notes (unaudited) | — | — | 11,276 | — | — | — | 11,276 | ||||||||||||||||||
Deferred stock-based compensation (unaudited) | — | — | 811 | (811 | ) | — | — | — | |||||||||||||||||
Amortization of deferred compensation, net of reversal of $3 for terminated employees (unaudited) | — | — | (3 | ) | 1,181 | — | — | 1,178 | |||||||||||||||||
Remeasurement and issuance of stock options in exchange for consulting services (unaudited) | — | — | 39 | — | — | — | 39 | ||||||||||||||||||
Accretion of redeemable convertible preferred stock (unaudited) | — | — | (8,973 | ) | — | — | — | (8,973 | ) | ||||||||||||||||
Stock options and warrants exercised (unaudited) | 940,778 | 1 | 67 | — | — | — | 68 | ||||||||||||||||||
Change in unrealized gain on investments (unaudited) | — | — | — | — | (55 | ) | — | (55 | ) | ||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | (24,370 | ) | (24,370 | ) | ||||||||||||||||
Comprehensive loss (unaudited) | (24,425 | ) | |||||||||||||||||||||||
Balance at June 30, 2004 (unaudited) | 14,826,573 | $ | 15 | $ | 146,511 | $ | (2,404 | ) | $ | (60 | ) | $ | (110,965 | ) | $ | 33,097 | |||||||||
The accompanying notes are an integral part of these financial statements.
F-6
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
STATEMENTS OF CASH FLOWS
(in thousands, except share data)
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 2000 | 2001 | 2002 | 2002 | 2003 | Period from 2003 | Years Ended December 31, | Period from 2003 | Six Months Ended June 30, | Period from 2004 | |||||||||||||||||||||||||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (in thousands) | (Unaudited) | (Unaudited) | ||||||||||||||||||||||||||||||||||||||||||||||||
Cash flows from operating activities | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Cash flows from operating activities: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Net loss | $ | (12,941 | ) | $ | (19,512 | ) | $ | (19,453 | ) | $ | (10,412 | ) | $ | (9,189 | ) | $ | (77,327 | ) | $ | (19,512 | ) | $ | (19,453 | ) | $ | (18,457 | ) | $ | (86,595 | ) | $ | (9,189 | ) | $ | (24,370 | ) | $ | (110,965 | ) | |||||||||||||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Noncash research and development expense | 744 | — | 679 | 679 | — | 1,716 | — | 679 | — | 1,716 | — | — | 1,716 | |||||||||||||||||||||||||||||||||||||||
Amortization of investment premiums, net | — | — | 217 | 71 | 47 | 264 | ||||||||||||||||||||||||||||||||||||||||||||||
Amortization of investment premiums (discounts), net | — | 217 | 89 | 306 | 47 | 251 | 557 | |||||||||||||||||||||||||||||||||||||||||||||
Noncash stock compensation expense | 882 | 2,567 | 2,570 | 1,523 | 725 | 6,849 | 2,567 | 2,570 | 1,667 | 7,791 | 725 | 1,217 | 9,008 | |||||||||||||||||||||||||||||||||||||||
Noncash interest expense | 21 | 44 | 55 | 26 | 25 | 163 | 44 | 55 | 365 | 503 | 25 | 12,536 | 13,039 | |||||||||||||||||||||||||||||||||||||||
Noncash rent expense | — | 34 | 34 | 17 | 17 | 85 | 34 | 34 | 34 | 102 | 17 | 17 | 119 | |||||||||||||||||||||||||||||||||||||||
Depreciation and amortization | 670 | 766 | 823 | 388 | 430 | 4,281 | 766 | 823 | 840 | 4,691 | 430 | 455 | 5,146 | |||||||||||||||||||||||||||||||||||||||
Loss on sale of property and equipment | — | 75 | 11 | 2 | 1 | 195 | 75 | 11 | 1 | 195 | 1 | — | 195 | |||||||||||||||||||||||||||||||||||||||
Changes in assets and liabilities: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Increase (decrease) in accounts payable | 232 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
(Increase) decrease in prepaid expenses and other current assets | (384 | ) | 140 | (350 | ) | (317 | ) | 204 | (659 | ) | 140 | (298 | ) | 140 | (671 | ) | 204 | (793 | ) | (1,464 | ) | |||||||||||||||||||||||||||||||
(Increase) decrease in deposits and other assets | (1,242 | ) | 766 | 63 | 70 | 23 | (433 | ) | 766 | 63 | (825 | ) | (1,281 | ) | 23 | 707 | (574 | ) | ||||||||||||||||||||||||||||||||||
Increase (decrease) in accounts payable | 330 | (312 | ) | (428 | ) | (326 | ) | 134 | 729 | (312 | ) | (428 | ) | 359 | 954 | 134 | 1,091 | 2,045 | ||||||||||||||||||||||||||||||||||
Increase in accrued liabilities | 499 | 333 | 568 | 11 | (404 | ) | 1,118 | |||||||||||||||||||||||||||||||||||||||||||||
Increase (decrease) in accrued liabilities | 333 | 568 | 301 | 1,823 | (404 | ) | 876 | 2,699 | ||||||||||||||||||||||||||||||||||||||||||||
Net cash used in operating activities | (11,189 | ) | (15,099 | ) | (15,211 | ) | (8,268 | ) | (7,987 | ) | (63,019 | ) | (15,099 | ) | (15,159 | ) | (15,486 | ) | (70,466 | ) | (7,987 | ) | (8,013 | ) | (78,479 | ) | ||||||||||||||||||||||||||
Cash flows from investing activities | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Cash flows from investing activities: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Purchases of property and equipment | (799 | ) | (888 | ) | (1,144 | ) | (737 | ) | (287 | ) | (6,209 | ) | (888 | ) | (1,144 | ) | (995 | ) | (6,917 | ) | (287 | ) | (1,593 | ) | (8,510 | ) | ||||||||||||||||||||||||||
Proceeds from sale of property and equipment | — | 31 | — | — | — | 64 | 31 | — | — | 64 | — | — | 64 | |||||||||||||||||||||||||||||||||||||||
Net cash acquired in acquisition | — | — | — | — | — | 437 | — | — | — | 437 | — | — | 437 | |||||||||||||||||||||||||||||||||||||||
Purchases of investments available-for-sale | — | — | (26,975 | ) | (17,698 | ) | (16,642 | ) | (49,433 | ) | — | (26,975 | ) | (30,543 | ) | (63,334 | ) | (16,642 | ) | (43,497 | ) | (106,831 | ) | |||||||||||||||||||||||||||||
Purchases of investments held-to-maturity | — | — | — | — | — | (17,732 | ) | — | — | — | (17,732 | ) | — | — | (17,732 | ) | ||||||||||||||||||||||||||||||||||||
Proceeds from sales and maturities of investments available-for-sale | 7,257 | — | 13,146 | — | 23,236 | 54,786 | — | 13,146 | 32,761 | 64,311 | 23,236 | 29,563 | 93,874 | |||||||||||||||||||||||||||||||||||||||
Proceeds from sales and maturities of investment, held-to-maturity | — | — | — | — | — | 5,145 | ||||||||||||||||||||||||||||||||||||||||||||||
Proceeds from sales and maturities of investments held-to-maturity held-to-maturity | — | — | — | 5,145 | — | — | 5,145 | |||||||||||||||||||||||||||||||||||||||||||||
Net cash provided by (used in) investing activities | 6,458 | (857 | ) | (14,973 | ) | (18,435 | ) | 6,307 | (12,942 | ) | (857 | ) | (14,973 | ) | 1,223 | (18,026 | ) | 6,307 | (15,527 | ) | (33,553 | ) | ||||||||||||||||||||||||||||||
Cash flows from financing activities | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Net proceeds from issuance of preferred stock | 27,988 | 13,111 | 12,313 | 12,313 | — | 75,554 | ||||||||||||||||||||||||||||||||||||||||||||||
Cash flows from financing activities: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Net proceeds from issuances of preferred stock | 13,111 | 12,313 | — | 75,554 | — | — | 75,554 | |||||||||||||||||||||||||||||||||||||||||||||
Net proceeds from issuances of common stock | — | — | — | — | — | 29,700 | 29,700 | |||||||||||||||||||||||||||||||||||||||||||||
Net proceeds from issuances of convertible promissory notes | — | — | 12,660 | 12,660 | — | — | 12,660 | |||||||||||||||||||||||||||||||||||||||||||||
Common stock repurchased | — | (2 | ) | — | — | — | (3 | ) | (2 | ) | — | — | (3 | ) | — | — | (3 | ) | ||||||||||||||||||||||||||||||||||
Proceeds from stock options and warrants exercised | 228 | 195 | 11 | 8 | 1 | 440 | 195 | 11 | 83 | 522 | 1 | 68 | 590 | |||||||||||||||||||||||||||||||||||||||
Proceeds from equipment financings | 977 | 706 | 1,304 | 716 | 330 | 5,469 | 706 | 1,304 | 913 | 6,052 | 330 | 867 | 6,919 | |||||||||||||||||||||||||||||||||||||||
Principal payments on equipment financings | (660 | ) | (882 | ) | (814 | ) | (365 | ) | (461 | ) | (3,581 | ) | (882 | ) | (866 | ) | (880 | ) | (4,052 | ) | (461 | ) | (534 | ) | (4,586 | ) | ||||||||||||||||||||||||||
Net cash provided by (used in) financing activities | 28,533 | 13,128 | 12,814 | 12,672 | (130 | ) | 77,879 | 13,128 | 12,762 | 12,776 | 90,733 | (130 | ) | 30,101 | 120,834 | |||||||||||||||||||||||||||||||||||||
Net increase (decrease) in cash and cash equivalents | 23,802 | (2,828 | ) | (17,370 | ) | (14,031 | ) | (1,810 | ) | 1,918 | (2,828 | ) | (17,370 | ) | (1,487 | ) | 2,241 | (1,810 | ) | 6,561 | 8,802 | |||||||||||||||||||||||||||||||
Cash and cash equivalents at beginning of period | 124 | 23,926 | 21,098 | 21,098 | 3,728 | — | 23,926 | 21,098 | 3,728 | — | 3,728 | 2,241 | — | |||||||||||||||||||||||||||||||||||||||
Cash and cash equivalents at end of period | $ | 23,926 | $ | 21,098 | $ | 3,728 | $ | 7,067 | $ | 1,918 | $ | 1,918 | $ | 21,098 | $ | 3,728 | $ | 2,241 | $ | 2,241 | $ | 1,918 | $ | 8,802 | $ | 8,802 | ||||||||||||||||||||||||||
Supplemental cash flow information | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Supplemental cash flow information: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Interest paid | $ | 229 | $ | 216 | $ | 212 | $ | 98 | $ | 106 | $ | 1,235 | $ | 216 | $ | 212 | $ | 212 | $ | 1,341 | $ | 106 | $ | 108 | $ | 1,449 | ||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-7
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
1. Organization and significant accounting policies
Organization
Xcyte Therapies, Inc. (the Company), a development stage enterprise, operates in one business segment, developing products based on T cell activation to treat cancer, infectious diseases and other medical conditions associated with compromised immune systems. As a development stage enterprise, substantially all efforts of the Company have been devoted to performing research and experimentation, conducting clinical trials, developing and acquiring intellectual properties, raising capital and recruiting and training personnel.
Liquidity
We haveThe Company has experienced losses since ourits inception, including a net loss for the six monthsyear ended June 30,December 31, 2003. Net losses may continue for at least the next several years as we proceedthe Company proceeds with the development of ourits technologies. The size of these losses will depend on the creation of revenue from the commercialization and development of ourits technologies, if any, and on the level of ourthe Company’s expenses. OurThe Company’s cash, cash equivalents and short-term investments have decreased from $17.3 million as of December 31, 2002 to $8.9$13.5 million as of June 30,December 31, 2003. In October 2003, wethe Company issued convertible notes for net proceeds of approximately $12.7 million. The notes convert to common stock upon the closing of thisan initial public offering. These convertible notes are due in October 2004, if not converted prior to that date.or on or after April 30, 2004 should a majority of the noteholders so elect. If the notes do not convert, wethe Company will require additional funding to continue ourits business activities through at least December 31, 2004. We believeThe Company believes that sufficient additional funding will be available to meet ourits projected operating and capital requirements through December 31, 2004, and arethe Company is considering various options, including securing additional equity financing and obtaining new collaborators. If we raisethe Company raises additional capital by issuing equity or convertible debt securities, our existing stockholders may experience substantial dilution. If we requirethe Company requires additional financing, there can be no assurance that it will be available on satisfactory terms, or at all. If we arethe Company is unable to secure additional financing on reasonable terms, or areis unable to generate sufficient new sources of revenue through arrangements with customers, collaborators and licensees, wethe Company will be forced to take substantial restructuring actions, which may include significantly reducing ourthe Company’s anticipated level of expenditures, the sale of some or all of ourthe Company’s assets, or obtaining funds by entering into financing or collaborative agreements on unattractive terms, or we wellthe Company will not be able to fund operations.
On March 19, 2004, the Company completed an initial public offering which, after deducting underwriting discounts and offering-related expenses, resulted in net proceeds of approximately $29.7 million and issuance by the Company of 4,200,000 shares of common stock. In connection with the initial public offering, all of the outstanding shares of the Company’s redeemable convertible preferred stock and all of its outstanding convertible promissory notes, including interest accrued thereon through the closing date of the offering, were converted into shares of common stock. Concurrent with the initial public offering, certain warrants were converted into common stock through payment of cash and cashless exercises.
The Company’s cash, cash equivalents and short-term investments have increased from $13.5 million as of December 31, 2003 to $33.7 million as of June 30, 2004.
Unaudited interim financial information
The financial information as of June 30, 20032004 and for the six months ended June 30, 20022004 and 2003 and the period from inception (January 5, 1996) to June 30, 20032004 is unaudited. In the opinion of management, all
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the six months ended June 30, 20032004 are not necessarily indicative of results that may be expected for the entire year.
Restatement of financial information
The balance sheet as of December 31, 2001 and 2002 and the consolidated statements of operations and changes in stockholders’ deficit for each of the three years in the period ended December 31, 2002, have been restated to recognize the accretion associated with beneficial conversion feature of the Company’s Series E and F redeemable convertible preferred stock immediately upon issuance (earliest date of
F-8
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
conversion) and to reverse the accretion previously recognized on the discount associated with the Company’s Series D, E and F redeemable convertible preferred stock due to redemption not being probable. Management believes that it is unlikely that the investors would redeem the preferred stock due to the Company’s plans for an initial public offering. This restatement was the result of application of Emerging Issues Task Force Topic D-98,Classification and Measurement of Redeemable Securities, and Emerging Issues Task Force Issue No. 00-27,Application of Issue No. 98-5 to Certain Convertible Securities. The effect of this restatement is to increase (decrease) net loss applicable to common stockholders by ($614), $4,171 and ($15,421) for the years ended December 31, 2000, 2001 and 2002, respectively; to increase (decrease) additional paid-in-capital by ($614), $3,557 and ($11,688), respectively, and to increase (decrease) stockholders’ deficit and redeemable convertible preferred stock by ($614), $3,703 and ($11,688), at December 31, 2000, 2001 and 2002, respectively.
Cash, cash equivalents and investments
Cash equivalents include highly liquid investments with a maturity on the date of purchase of three months or less. The Company’s cash equivalents consist of money market securities. While cash and cash equivalents held by financial institutions may at times exceed federally insured limits, management believes that no material credit or market risk exposure exists due to the high quality of the institutions. The Company has not experienced any losses on such accounts.
All investment securities are classified as available-for-sale and are carried at fair value. Unrealized gains and losses are reported in a separate component of stockholders’ deficit. Amortization, accretion, interest and dividends, realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in interest income. The cost of securities sold is based on the specific-identification method. Investments in securities with maturities of less than one year or which management intends to use to fund current operations are classified as short-term investments.
The Company evaluates whether an investment is other-than-temporarily impaired. This evaluation is dependent on the specific facts and circumstances. Factors that are considered in determining whether an other-than-temporary decline in value has occurred include: the market value of the security in relation to its cost basis; the financial condition of the investee; and the intent and ability to retain the investment for a sufficient period of time to allow for recovery in the market value of the investment.
Property and equipment
Property and equipment is stated at cost and is depreciated using the straight-line method over the assets’ useful lives, which are six years for equipment and furniture and fixtures and three years for computer equipment. Leasehold improvements are amortized over the lesser of their estimated useful lives or the term of the lease.
Impairment of long-lived assets
In accordance with the provisions of Statement of Financial Accounting Standards No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets(SFAS 144), the Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amounts of the assets may not be fully recoverable. An
F-9
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
impairment loss will be recognized when estimated undiscounted future cash flows expected to result from the use of an asset and its eventual disposition are less than its carrying amount. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair value of the long-lived asset.
Revenue recognition
To date, the Company has generated no revenues from sales of products. Revenues relate to fees received for licensed technology, cost reimbursement contracts and a Small Business Innovation Research (SBIR) grant awarded to the Company by the National Institutes of Health. Revenue associated with up-front license fees and
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
research and development funding payments are recognized ratably over the relevant periods specified in the agreement, generally the research and development period.period the Company is obligated to perform services. Revenue under research and development cost-reimbursement agreements is recognized as the related costs are incurred. Revenue related to grant agreements is recognized as related research and development expenses are incurred.
Other comprehensive income (loss)
Other comprehensive income (loss) includes certain changes in equity that are excluded from net income (loss). The Company’s only other comprehensive income (loss) is unrealized gain (loss) on investments.
Research and development expenses
Research and development expenses are charged to expense as incurred and include, but are not limited to, personnel costs, lab supplies, depreciation, amortization and other indirect costs.
Segments
The Company has adopted Statement of Financial Accounting Standards No. 131,Disclosure about Segments of an Enterprise and Related Information (SFAS 131), and related disclosures about its products, services, geographic areas and major customers. The Company has determined that it operates in only one segment.
Stock-based compensation
The Company has adopted the disclosure-only provisions of Statement of Financial Accounting Standards No. 123,Accounting for Stock-Based Compensation(SFAS 123), as amended by SFAS No. 148,Accounting for Stock-Based Compensation—Transition and Disclosure, and applies Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees(APB 25), and related interpretations in accounting for stock options. Accordingly, employee stock-based compensation expense is recognized based on the intrinsic value of the option at the date of grant.
As required under SFAS No. 123, the pro forma effects of stock-based compensation on net loss are estimated at the date of grant using the minimum value method of the Black-Scholes option pricing model, as allowed for nonpublic companies.option-pricing model. The Black-Scholes option pricingoption-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. Because the Company’s employee stock options have characteristics significantly different
F-10
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, the existing models do not, in management’s opinion, necessarily provide a reliable single measure of the fair value of itsthe Company’s employee stock options.
The fair value of these options was estimated at the date of grant using the minimum value methodBlack-Scholes option-pricing model with the following weighted average assumptions for the years ended December 31, 2000, 2001, 2002 and 20022003 and the six months ended June 30, 20022003 and 2003:2004: risk-free interest rates of 5.61%4.5%, 4.5%5.0%, 5.0%, 5.0% and 5.0%, respectively; a dividend yield of 0% for all periods; expected volatility of 0%75% to 80% for all periods; and weighted average expected lives of the options of 3.7, 4, 4, 4 and 4 years respectively.for all periods. The estimated weighted average fair value of stock options granted during 2000, 2001, 2002 and 20022003 and the six months ended June 30, 20022003 and 20032004 was $2.71, $4.60, $2.28, $3.20$25.28, $12.55, $13.76, $6.33 and $1.15$6.56 per share of common stock, respectively.
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the vesting period of the related options. The Company’s pro forma information follows (in thousands)thousands, other than per share information):
| Year ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||||||||||||||
| 2000 | 2001 | 2002 | 2002 | 2003 | Year ended December 31, | Six months ended June 30, | ||||||||||||||||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||||||||||||||||
Net loss applicable to common stockholders; | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,413 | ) | $ | (9,189 | ) | |||||||||||||||||||||||||
Add: Employee stock-based compensation as reported | 770 | 1,445 | 2,505 | 1,522 | 595 | |||||||||||||||||||||||||||||||||||
Net loss applicable to common stockholders, as reported | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,457 | ) | $ | (9,189 | ) | $ | (33,343 | ) | |||||||||||||||||||||||||
Add: Employee stock-based compensation, as reported | 1,445 | 2,505 | 1,307 | 595 | 1,178 | |||||||||||||||||||||||||||||||||||
Deduct: Stock-based compensation determined under the fair value method | (798 | ) | (1,591 | ) | (2,879 | ) | (1,695 | ) | (805 | ) | (1,591 | ) | (2,879 | ) | (1,612 | ) | (805 | ) | (1,520 | ) | ||||||||||||||||||||
Pro forma net loss | $ | (12,969 | ) | $ | (28,069 | ) | $ | (27,828 | ) | $ | (18,586 | ) | $ | (9,399 | ) | $ | (28,069 | ) | $ | (27,828 | ) | $ | (18,762 | ) | $ | (9,399 | ) | $ | (33,685 | ) | ||||||||||
Basic and diluted pro forma net loss per share | $ | (2.16 | ) | $ | (4.05 | ) | $ | (3.56 | ) | $ | (2.47 | ) | $ | (1.15 | ) | $ | (22.26 | ) | $ | (19.60 | ) | $ | (12.61 | ) | $ | (6.35 | ) | $ | (3.70 | ) | ||||||||||
Stock options granted to nonemployeesnon-employees are recorded using the fair value approach in accordance with SFAS 123 and Emerging Issues Task Force Consensus (EITF) Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services(EITF 96-18). The options to nonemployeesnon-employees are subject to periodic revaluation over their vesting terms.
Deferred stock compensation includes amounts recorded when the exercise price of an option is lower than the fair value of the underlying common stock on the date of grant. Deferred stock-based compensation is amortized over the vesting period of the underlying option using the graded-vesting method.
Income taxes
The Company accounts for income taxes utilizing the liability method in accordance with Statement of Financial Accounting Standards No. 109,Accounting for Income Taxes(SFAS 109). Deferred tax assets or liabilities are recorded for all temporary differences between financial and tax reporting. A valuation allowance is recorded when it is more likely than not that the deferred tax asset will not be recovered.
F-11
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
Net loss per share
Basic net loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of common shares outstanding for the period. Common stock equivalents, including redeemable convertible preferred stock, stock options and warrants are excluded from the computation of diluted loss per share as their effect is anti-dilutive. For the periods presented, there is no difference between the basic and diluted net loss per share.
Financial instruments
Financial instruments, including cash and cash equivalents and payables, are recorded at cost, which approximates fair value based on the short-term maturities of these instruments. The fair value of investments is determined based on quoted market prices. Refer to Note 2 for further information on the fair value of
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
investments. Based on the borrowing rates currently available to the Company for loans with similar terms, management believes that the carrying value of equipment financing arrangements approximates fair value.
Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Recent accounting pronouncements
In June 2002, the FASB issued SFAS 146,Accounting for Costs Associated with Exit or Disposal Activities, which addresses accounting for restructuring, discontinued operation,operations, plant closingclosings or other exit or disposal activity. SFAS 146 requires companies to recognize costs associated with exit or disposal activities when they are incurred rather than at the date of a commitment to an exit or disposal plan. SFAS 146 is to be applied prospectively to exit or disposal activities initiated after December 31, 2002. The adoption of SFAS 146 had no initial impact on the Company’s financial statements.
In November 2002, the FASB issued FIN 45,Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others, and Interpretation of FASB Statements No. 5, 57 and 107 and Rescission of FASB Interpretation No. 34.34. FIN 45 clarifies the requirements of SFAS 5,Accounting for Contingencies,, relating to the guarantor’s accounting for, and disclosure of, the issuance of certain types of guarantees. The disclosure provisions of FIN 45 are effective for financial statements of periods ending after December 15, 2002. However, the provisions for initial recognition and measurement are effective on a prospective basis for guarantees that are issued or modified after December 31, 2002. The adoption of FIN 45 had no initial impact on the Company’s financial statements.
In November 2002, the Emerging Issues Task Force reached a consensus on Issue No. 00-21,Revenue Arrangements with Multiple Deliverables. EITF Issue No. 00-21 provides guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets.
F-12
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
use assets. The provisions of EITF Issue No. 00-21 will apply to revenue arrangements entered into in fiscal periods beginning after June 15, 2003. The Company is currently evaluating the effects that the adoption of EITF Issue No. 00-21 will havehad no initial impact on the Company’s financial statements.
In January 2003, the FASB issued FIN 46,Consolidation of Variable Interest Entities. FIN 46 clarifies the application of Accounting Research Bulletin No. 51,Consolidated Financial Statements, to certain entities in which the equity investors do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. FIN 46 applies immediately to variable interest entities created after January 31, 2003 and to variable interest entities in which an enterprise obtains an interest after that date. It applies in the first fiscal year or interim period beginning after JuneDecember 15, 2003 to variable interest entities in which an enterprise holds a variable interest that it acquired before February 1, 2003. FIN 46 applies to public enterprises as of the beginning of the applicable interim or annual period. The Company does not believe there will be a material effect upon its financial condition or results of operations from the adoption of the provisions of FIN 46.
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
In May 2003, the FASB issued SFAS 150,Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity. SFAS 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. SFAS 150 requires that an issuer classify a financial instrument that is within its scope as a liability by reporting the cumulative effect of a change in accounting principle. The requirements of SFAS 150 are to be applied to the first fiscal period beginning after December 15, 2004. The Company is currently evaluating the impact of adopting SFAS 150.150 and does not expect there to be a significant impact upon adoption.
Reclassifications
Certain prior year amounts have been reclassified to conform to current year presentation.
2. Investments
A summary of investments follows (in thousands):
| December 31, 2002 | |||||||||||||
| Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | ||||||||||
Federal agency obligations | $ | 1,532 | $ | 1 | $ | — | $ | 1,533 | |||||
Corporate bonds | 9,859 | 5 | (2 | ) | 9,862 | ||||||||
Municipal bonds | 2,221 | — | — | 2,221 | |||||||||
Total | $ | 13,612 | $ | 6 | $ | (2 | ) | $ | 13,616 | ||||
| June 30, 2003 | |||||||||||||||||||||||||
| Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | December 31, 2003 | |||||||||||||||||||||
| Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | ||||||||||||||||||||||
Federal agency obligations | $ | 6,006 | $ | 2 | $ | — | $ | 6,008 | $ | 770 | $ | — | $ | — | $ | 770 | |||||||||
Corporate bonds | 965 | 1 | — | 966 | 9,680 | 1 | (6 | ) | 9,675 | ||||||||||||||||
Municipal bonds | 854 | — | — | 854 | |||||||||||||||||||||
Total | $ | 6,971 | $ | 3 | $ | — | $ | 6,974 | $ | 11,304 | $ | 1 | $ | (6 | ) | $ | 11,299 | ||||||||
F-13
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
| June 30, 2004 | |||||||||||||
| Amortized cost | Gross unrealized gains | Gross unrealized losses | Fair value | ||||||||||
Federal agency obligations | $ | 4,634 | $ | — | $ | (15 | ) | $ | 4,619 | ||||
Corporate bonds | 18,027 | — | (36 | ) | 17,991 | ||||||||
Municipal bonds | 2,327 | — | (9 | ) | 2,318 | ||||||||
Total | $ | 24,988 | $ | — | $ | (60 | ) | $ | 24,928 | ||||
The Company has realized no gains or losses upon the sale of available-for-sale securities during the years ended December 31, 2000, 2001, 2002 and 20022003 and the six months ended June 30, 2003.2003 and 2004, as no investments were sold prior to maturity. The Company has evaluated the nature of the investments, the duration of the impairments
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
(all less than 1 year) and concluded that the impairments are not other-than-temporary. All investments held at December 31, 2002 and 2003 and June 30, 20032004 have contractual maturities within one year. During the year ended December 31, 2001, the Company held only cash and cash equivalents and no investments.
3. Property and equipment
Property and equipment consists of the following (in thousands):
| December 31, | June 30, 2003 | |||||||||||||||||||||||
| 2001 | 2002 | December 31, | June 30, 2004 | |||||||||||||||||||||
| 2002 | 2003 | |||||||||||||||||||||||
Equipment | $ | 2,270 | $ | 2,957 | $ | 3,194 | $ | 2,957 | $ | 3,794 | $ | 4,486 | ||||||||||||
Furniture and fixtures | 191 | 197 | 199 | 197 | 218 | 223 | ||||||||||||||||||
Leasehold improvements | 769 | 916 | 920 | 916 | 989 | 1,807 | ||||||||||||||||||
Computer equipment | 612 | 888 | 925 | 888 | 946 | 1,024 | ||||||||||||||||||
Property and equipment, gross | 3,842 | 4,958 | 5,238 | 4,958 | 5,947 | 7,540 | ||||||||||||||||||
Less accumulated amortization and depreciation | (1,539 | ) | (2,345 | ) | (2,769 | ) | (2,345 | ) | (3,180 | ) | (3,635 | ) | ||||||||||||
Property and equipment, net | $ | 2,303 | $ | 2,613 | $ | 2,469 | $ | 2,613 | $ | 2,767 | $ | 3,905 | ||||||||||||
Depreciation expense totaled $470,000, $632,000, $823,000 and $823,000$840,000 during the years ended December 31, 2000, 2001, 2002 and 2002,2003, respectively, and $430,000 and $455,000 during the six months ended June 30, 2003.2003 and 2004, respectively.
4. Employee note receivable
During the year ended December 31, 2001, the Company made a $50,000 secured loan to an employee in connection with an individual employment agreement. The loan bears interest at an annual rate of 8.24% and is repayable in equal quarterly installments over four years. The note balance of $50,000, $36,000, $24,000 and $33,000$17,000 at December 31, 20012002 and 20022003 and June 30, 2003,2004, respectively, has been classified in deposits and other assets. Interest earned on the note has been immaterial to date.
5. Significant agreements
Technology licenses
In 1998, the Company entered into a license agreement with Genetics Institute, under which the Company was granted the use ofa license under Genetics Institute’s rights to several patents for intellectual propertyand patent applications in exchange for the payment of a nonrefundable fee ofupfront license fees totaling approximately $53,000, 145,875for the issuance of 26,522 shares of Series B preferred stock and warrants to purchase 194,50035,363 shares of Series B preferred stock at $1.10$6.05 per share. The nonrefundable fee wasfees were charged to research and development expenseexpenses when paid. The Company, or sublicensee, is required to spend no less than $500,000 annually on research and development activities related to product development until the first commercial sale of thea product.
In 1999, the Company entered into a license and supply agreement with Diaclone S.A., in which the Company was granted a license to developmake, use and commercializesell certain products created with a specific antibody. In consideration for the license, the Company paid and charged to research and development expense a $75,000 nonrefundable fee.
In addition, the Company entered into a license agreement with the Fred Hutchinson Cancer Research Center in which the Company was granted a license to make, use and sell a specific antibody for certain therapeutic and
XCYTE THERAPIES, INC.
F-14
Xcyte Therapies, Inc. (a(a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
a license to develop and commercialize a specific antibody.research purposes. In consideration for the license, the Company paid nonrefundable license fees of $50,000. The Company also agreed to issue 150,00027,272 shares of common stock, valued at $744,000, to the Fred Hutchinson Cancer Research Center. The Company charged research and development expense for all nonrefundable fees paid and the value of the common stock issued.
During the year ended December 31, 2002, the Company entered into a license agreement with the Trustees of the University of Pennsylvania, whereby the Company was granted the right to use certain intellectual property in exchange for payment of nonrefundable license fees of $150,000. The Company also agreed to issue 350,00063,636 shares of common stock, valued at $679,000, to the Trustees of the University of Pennsylvania. In addition, the Company may be required to make future payments under the license agreement based on the achievement of certain milestone events, which could total $3.9 million. The Company charged research and development expense for all nonrefundable fees paid and the value of common stock issued. In October 2003, the Company notified the University of Pennsylvania that it was terminating the license agreement. This termination was effective December 30, 2003, following the 60-day notice period as required pursuant to the terms of the license agreement.
All license agreements require the payment of royalties by the Company based on sales and services. No royalty payments have been required or paid through December 31, 2002.June 30, 2004.
Manufacturing and supply contracts
The Company entered into a development and supply agreement with Dynal S.A. during the year ended December 31, 1999. The Company has agreed to make nonrefundable payments totaling $3.0 million for certain development activities conducted by Dynal S.A. As of December 31, 2002,2003, the Company had made payments totaling $2.0$2.5 million under the agreement ($2.53.0 million as of June 30, 2003)2004), which were charged to research and development expense. Of the Company’s remaining $1.0 million in payments, $500,000 was accrued at December 31, 2003 and paid in January 2003. The remaining $500,000 payment is payable upon the achievement of specified milestones per the development work plans. As estimated completion dates of the milestone activities are speculative and subject to delivery and acceptance of the product from Dynal to the Company, the Company will expense such payment in the period the products are delivered and accepted. Under the terms of the supply agreement, should the Company is required tonot buy a minimum $250,000 of beads in the first 12 months after the development phase ends and $500,000 of beads annually thereafter over the remaining term of the agreement, which expires inDynal shall have the right to terminate the agreement. Either party may terminate the agreement as of August 2009 for any reason, or earlier uponon account of the material breach or insolvency of the other party. If the agreement is not terminated by August 2009, either party. The term may be extendedparty can elect to extend the terms of the agreement for an additional five years by either party.years. Otherwise, it will automatically renew on a year to year basis. In March 2004, the Company amended the agreement to allow Dynal to sell a research-grade version of the Company’s antibody-coated beads.
During the year ended December 31, 2000, the Company entered into development and supply agreements with Lonza Biologics PLC (Lonza). Under the terms of the agreements, the Company is obligated to make payments in British pounds. Exchange rate gains and losses have been insignificant to date. The Company paid approximately $252,000, $1.7 million, $1.6 million and $1.6$1.3 million under the agreements during the years ended December 31, 2000, 2001, 2002 and 2002,2003, respectively, and $1.3 million$47,000 during the six months ended June 30, 2003,2004, all of which were charged to research and development expense. As of June 30, 2003,2004, the Company had no significant remaining contractual obligations to Lonza. In August 2004, the Company amended its agreements with Lonza, resulting in additional obligations of approximately $1.4 million, which are scheduled to be paid in 2005.
Corporate collaborationcollaborations
In MarchNovember 2003, the Company entered into an agreement withlicensed to Fresenius Biotechnology GmbH, a wholly ownedwholly-owned subsidiary of Fresenius AG. Under the terms of the agreement, which expired in August 2003, Fresenius was granted the right to useAG, the Company’s Xcellerate Technology to conduct a Phase I/IIon an exclusive basis in the field of HIV retroviral gene therapy, studyfor development and commercialization in Europe. The Company is requiredEurope with an option under certain circumstances to transfer its Xcellerate Technology, includingexpand
XCYTE THERAPIES, INC.
F-15
Xcyte Therapies, Inc. (a(a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
their rights to North America. The agreement with Fresenius requires the Company to transfer its Xcellerate Technology, including manufacturing capability, to Fresenius and supply all antibody-coated beads required by Fresenius to support the trial.its development and commercialization efforts. Fresenius is requiredhad previously agreed to reimburse the Company for its expenses associated within transferring the technology and supplyingto pay the Company for the antibody-coated beads. As of June 30,beads on a cost-plus basis. For the year ended December 31, 2003, the Company hashad recognized $72,000$170,000 as revenue related to the reimbursement of its costs.actual costs ($24,000 for the six months ended June 30, 2004). The terms of the agreement include potential royalties on net sales as well as potential milestone payments to the Company less applicable sublicense fees payable by Xcyte to third parties for each product developed. For the six months ended June 30, 2004, the Company had recognized $12,000 as revenue related to payments received. Fresenius’ obligation to pay the Company royalties under this agreement terminates on a country-by-country basis upon the later of the last to expire of the licensed patents or fifteen years after the first commercial sale of a product in the country. The agreement is also subject to earlier termination by Fresenius at any time if Fresenius determines it cannot develop a commercially viable product or complete a required manufacturing audit; by Xcyte if Fresenius does not meet development milestones; and by either party for the material breach or insolvency of the other party.
6. Redeemable convertible preferred stock and warrants
Preferred stock
A summary of redeemable convertible preferred stock follows (in thousands, except share data):
| December 31, 2002 | June 30, 2003 | |||||||||||||||||||||||||||||||||||||||
Shares authorized and designated | Issued and outstanding shares | Aggregate redemption and liquidation preference | Carrying value | Shares authorized and designated | Issued and outstanding shares | Aggregate redemption and liquidation preference | Carrying value | December 31, 2002 | December 31, 2003 | |||||||||||||||||||||||||||||||
| Shares designated | Issued and outstanding shares | Aggregate redemption and liquidation preference | Carrying value | Shares designated | Issued and outstanding shares | Aggregate redemption and liquidation preference | Carrying value | |||||||||||||||||||||||||||||||||
Series A | 7,300,080 | 6,860,512 | $ | 6,517 | $ | 6,596 | 7,300,080 | 6,907,353 | $ | 6,562 | $ | 6,660 | 7,300,080 | 1,247,354 | $ | 6,517 | $ | 6,596 | 7,300,080 | 1,255,870 | $ | 6,562 | $ | 6,660 | ||||||||||||||||
Series B | 4,097,580 | 3,903,080 | 4,293 | 4,293 | 4,097,580 | 3,903,080 | 4,293 | 4,293 | 4,097,580 | 709,647 | 4,293 | 4,293 | 4,097,580 | 709,647 | 4,293 | 4,293 | ||||||||||||||||||||||||
Series C | 7,212,316 | 7,185,630 | 12,000 | 11,976 | 7,212,316 | 7,185,630 | 12,000 | 11,976 | 7,212,316 | 1,306,470 | 12,000 | 11,976 | 7,212,316 | 1,306,470 | 12,000 | 11,976 | ||||||||||||||||||||||||
Series D | 10,300,000 | 10,109,825 | 28,105 | 25,263 | 10,300,000 | 10,109,825 | 28,105 | 25,263 | 10,300,000 | 1,838,139 | 28,105 | 25,263 | 10,300,000 | 1,838,139 | 28,105 | 25,263 | ||||||||||||||||||||||||
Series E | 6,500,000 | 4,750,095 | 13,205 | 8,411 | 6,500,000 | 4,750,095 | 13,205 | 8,411 | 6,500,000 | 863,648 | 13,205 | 8,411 | 6,500,000 | 863,648 | 13,205 | 8,411 | ||||||||||||||||||||||||
Series F | 6,500,000 | 4,444,251 | 12,355 | 8,001 | 6,500,000 | 4,444,251 | 12,355 | 8,001 | 6,500,000 | 808,040 | 12,355 | 8,001 | 6,500,000 | 808,040 | 12,355 | 8,001 | ||||||||||||||||||||||||
| 41,909,976 | 37,253,393 | $ | 76,475 | $ | 64,540 | 41,909,976 | 37,300,234 | $ | 76,520 | $ | 64,604 | 41,909,976 | 6,773,298 | $ | 76,475 | $ | 64,540 | 41,909,976 | 6,781,814 | $ | 76,520 | $ | 64,604 | |||||||||||||||||
From inception through December 31, 1999, the Company issued 6,334,2121,151,664 shares of Series A preferred stock at $0.95$5.23 per share for proceeds of $6.0 million; 3,757,205683,125 shares of Series B preferred stock at $1.10$6.05 per share for proceeds of $4.1 million; and 7,185,6301,306,470 shares of Series C preferred stock at $1.67$9.19 per share for proceeds of $12.0 million. The Company also issued an additional 526,30095,690 shares of Series A preferred stock in conjunction with a business acquisition. The value of the Series A preferred stock of $579,000 was included in the determination of the purchase price of the acquired business. The Company also issued 145,87526,522 shares of Series B preferred stock to acquire technology licenses. These shares were valued at $1.10$6.05 per share for an aggregate amount of $160,000. There were no significant costs associated with the Series A, B and C private placements.
During the year ended December 31, 2000, the Company completed a private placement of 10,109,8251,838,139 shares at $2.78$15.29 per share of Series D redeemable preferred stock for $28.0 million, net of offering costs of $117,000. In connection with the offering, holders of the Series D preferred stock received 1,132,287 warrants to purchase 205,858 shares of common stock at an exercise price of $0.30$1.65 per share. The warrants were valued at $2.7 million using
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
the Black-Scholes option pricingoption-pricing model. The warrants expire in August 2005 or upon the completion of an initial public offering of the Company’s common stock. Of the total net proceeds of $28.0 million, $2.7 million has been recorded in paid-in capital and $25.3 million has been recorded as redeemable convertible preferred stock.
During the year ended December 31, 2001, the Company completed a private placement of 4,750,095863,648 shares at $2.78$15.29 per share of Series E redeemable preferred stock for $13.1 million, net of offering costs of $145,000. In connection with the offering, holders of the Series E preferred stock received warrants to purchase 2,586,162470,205 shares of common stock at an exercise price of $0.01$0.055 per share. The warrants expire
F-16
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
in November 2006 or upon completion of an initial public offering. The net proceeds from the Series E preferred stock offering were allocated based on the relative fair values of the warrants, using the Black-Scholes option pricingoption-pricing model, and the preferred stock. The Company assigned $4.6 million to the value of the warrants and $8.4 million to the value of the preferred stock. After allocating a portion of the proceeds to the common stock warrants, the effective conversion price of the preferred stock was at a discount to the price of the common stock into which the preferred stock is convertible. The discount associated with the beneficial conversion feature is limited to the proceeds allocated to the preferred stock, or $8.4 million. Accordingly, the preferred stock was initially recorded at zero. The Company has recognized the amortization of the discount associated with the beneficial conversion of $8.4 million as a charge to additional paid-in capital (also shown as a deduction to arrive at net loss applicable to common stockholders) and a credit to preferred stock immediately upon issuance since the preferred stock may be converted into common stock at any time, at the holder’s option. The remaining discount of $4.6 million will be amortized at the time that redemption by the holders is considered probable or the preferred stock is converted into common stock. Management believes that it is unlikely that the investors would redeem the preferred stock due to the Company’s plan for an initial public offering.
During the year ended December 31, 2002, the Company completed a private placement of 4,444,251808,040 shares at $2.78$15.29 per share of Series F redeemable preferred stock for $12.3 million, net of offering costs of $30,000. In connection with the offering, holders of the Series F preferred stock received 2,419,649 warrants to purchase 439,932 shares of common stock at an exercise price of $0.01$0.055 per share. The warrants expire in February 2007 or upon completion of an initial public offering of the Company’s common stock. The net proceeds from the Series F preferred stock offering were allocated based on the relative fair values of the warrants, using the Black-Scholes option pricingoption-pricing model, and the preferred stock. The Company assigned $4.3 million to the value of the warrants and $8.0 million to the value of the preferred stock. After allocating a portion of the proceeds to the common stock warrants, the effective conversion price of the preferred stock was at a discount to the price of the common stock into which the preferred stock is convertible. The discount associated with the beneficial conversion is limited to the proceeds allocated to the preferred stock, or $8.0 million. The Company has recognized the amortization of the discount associated with the beneficial conversion of $8.0 million as a charge to additional paid-in capital (also shown as a deduction to arrive at net loss applicable to common stockholders) and a credit to preferred stock immediately upon issuance since the preferred stock may be converted into common stock at any time, at the holder’s option. The remaining discount of $4.3 million will be amortized at the time that redemption by the holders is considered probable or the preferred stock is converted into common stock. Management believes that it is unlikely that the investors would redeem the preferred stock due to the Company’s plan for an initial public offering.
Holders of Series A, B, C, D, E and F preferred stock have preferential rights to noncumulative dividends at a rate of $0.076, $0.088, $0.1336, $0.2224, $0.2224$0.418, $0.484, $0.7348, $1.2232, $1.2232 and $0.2224$1.2232 per share, respectively, when and if declared by the BoardCompany’s board of Directors.directors. The holders are entitled to the number of votes equal to the number of shares of common stock into which the preferred stock could be converted. In the event of liquidation, the holders of
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
Series A, B, C, D, E and F preferred stock have preferential rights to liquidation payments of $0.95, $1.10, $1.67, $2.78, $2.78$5.23, $6.05, $9.19, $15.29, $15.29 and $2.78$15.29 per share, respectively, plus any accrued but unpaid dividends. After the distributions to the holders of preferred stock have been made, the remaining assets of the Company available for distribution to stockholders shallwill be distributed pro rata among the holders of common stock and preferred stock.
The preferred stock can be converted, at the option of the holder, one-for-one into common stock subject to adjustment for antidilutive events. The conversion price for Series A, B, C, D, E and F preferred stock
F-17
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 is $5.23, $6.05, $9.19, $15.29, $15.29 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
is $0.95, $1.10, $1.67, $2.78, $2.78 and $2.78,$15.29, respectively. Each share of the preferred stock shallwill automatically be converted into shares of common stock upon the closing of an initial public offering, provided that the price per share is not less than $4.00$22.00 and the aggregate gross proceeds to the Company are not less than $20.0 million.
In addition, the Company has granted registration rights, preferential rights in liquidation and rights of first offer to the preferred stockholders and is precluded from carrying out certain actions without the approval of the majority of the preferred stockholders voting as a group.
As of December 31, 2002,2003, the preferred stock is redeemable at the option of the holder, upon the vote of a majority of the outstanding shares of preferred stock at any time.stock. The Series A, B, C, D, E and F redemption price is $0.95, $1.10, $1.67, $2.78, $2.78prices are $5.23, $6.05, $9.19, $15.29, $15.29 and $2.78$15.29 per share, respectively.
In connection with the initial public offering in March 2004, all of the outstanding shares of the Company’s redeemable convertible preferred stock were converted into 6,781,814 shares of common stock.
Warrants
From inception through December 31, 1999, warrants were issued to purchase 368,41066,983 shares of Series A preferred stock in connection with a business acquisition at an exercise price of $0.95$5.23 per share. The value of the warrants of $330,000 was included in the determination of the purchase price of the business. In addition, warrants to purchase 71,15812,937 shares of Series A preferred stock at $0.95$5.23 per share and warrants to purchase 12,3152,238 shares of Series C preferred stock at $1.67$9.19 per share were issued in connection with equipment financing. The estimated fair value of the warrants issued of $64,000 and $15,000, respectively, was recorded as an additional financing cost and is being amortized over the term of the loan as interest expense. The warrants to purchase 71,15812,937 shares of Series A preferred stock were exercised in March 2003 through a net exercise, resulting in the issuance of 46,8418,516 shares of Series A preferred stock. In addition, the Company issued warrants to purchase 194,50035,363 shares of Series B preferred stock as partial consideration for a technology license. The warrants were issued at an exercise price of $1.10$6.05 per share, and the estimated fair value of the warrants of $131,000 was charged to research and development expense.
During the years ended December 31, 2000 2001 and 2002,2001, the Company issued warrants to purchase 14,371, 23,741 and 23,741 shares, respectively,2,612 of Series C D and F preferred stock at an exercise price of $1.67, $2.78$9.19, and $2.78 per share,4,316 of Series D preferred stock at an exercise price of $15.29, respectively in connection with equipment financing. The estimated fair value of the warrants issued of $36,000 for Series C and $113,000 for Series D was recorded as additional financing cost and is being amortized over the term of the loan as interest expense using the effective interest method.
During the years ended December 31, 2002 and 2003, the Company issued warrants to purchase 4,316 and 1,143 of Series F stock at an exercise price of $15.29 and $15.29, respectively in connection with equipment financing.
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
The estimated fair value of the warrants issued of $56,000 respectively,and $14,000 was recorded as additional financing cost and is being amortized over the term of the loan as interest expense using the effective interest method.
During the year ended December 31, 2000, the Company issued a warrant for the purchase of 80,00014,545 shares of Series D preferred stock at an exercise price of $2.78$15.29 per share, in connection with a lease for a manufacturing facility. The estimated fair value of the warrant of $340,000 was recorded as deferred rent and is being recognized as additional rent expense over the initial term of the lease.
During the year ended December 31, 2001, the Company issued a warrant for the purchase of 10,0001,818 shares of Series E preferred stock at an exercise price of $2.78$15.29 per share for services provided in connection with the private placement of Series E redeemable preferred stock. The estimated fair value of the warrants of $48,000 was included in offering costs of the placement.
F-18
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
Warrants expire at various dates from August 2005 to February 2012, including 470,7252012. 86,727 warrants outstanding at December 31, 2002, which2003 will expire upon the closing of an initial public offering. All remaining preferred stock warrants, (327,511(46,607 at December 31, 2002 and 256,353 at June 30, 2003) that do not expire upon the closing of ana public offering, will convert to common stock warrants upon the closing of an initial public offering. The Company has valued the warrants issued during the years ended December 31, 2000, 2001, 2002 and 20022003 using the Black-Scholes option pricingoption-pricing model with the following assumptions: no dividend yields; life of 5 years to 10 years; risk-free interest rates of 4.5% to 5.42%; and volatility of 75% to 80%.
Concurrent with the closing of the initial public offering in March 2004, certain preferred stock warrants that expired upon the closing of a public offering were converted into common stock through cashless exercises, resulting in the issuance of 23,233 shares of common stock.
7. Stock option planplans
1996 Stock Option Plan
Under the Company’s Amended and Restated 1996 Stock Option Plan (1996 Plan), 4.4 million1,163,636 shares of common stock have been reserved for grants to employees, directors and consultants as of December 31, 2002.2003. In September 2003, the 1996 Plan was amended to increase common stock reserved for grants to 1,163,636 shares and certain outstanding stock options were modified to accelerate vesting for employees with a five-year vesting schedule to a four-year schedule. There was no immediate accounting impact to this change. However, if employees benefit from the change, the appropriate stock compensation charge will be recorded in the period in which there was a benefit to the employee(s) based upon the measurement of the intrinsic value of the related stock options on the date of modification. The term of the 1996 Plan is 10 years unless terminated earlier by the Board of Directors. Options granted under the 1996 Plan may be designated as incentive or nonqualified at the discretion of the 1996 Plan administrator. The vesting period, exercise price and expiration period of options are also established at the discretion of the 1996 Plan administrator. Vesting periods are typically four or five years, and incentive stock options are exercisable at no less than the fair market value at the date of grant, and nonqualified stock options are exercisable at prices determined by the 1996 Plan administrator. In no event shall the term of any incentive stock option exceed 10 years.
Shares issued upon exercise of options that are unvested are restricted and subject to repurchase by the Company at the original exercise price upon termination of employment, and such restrictions lapse over the original vesting schedule. During the year ended December 31, 2000, the Board of Directors amended the 1996 Plan to
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
allow options granted to certain executives to become exercisable immediately. Three executives elected to early exercise stock options for 513,85893,426 shares of restricted common stock in the year ended December 31, 2000. During the year ended December 31, 2001, the Company repurchased 13,3332,424 shares of restricted stock. The shares were repurchased in an amount equal to the original purchase price of the shares. At December 31, 2002,2003, there were a total of 261,08030,207 shares of restricted common stock outstanding and subject to repurchase (213,611(21,577 shares at June 30, 2003)2004). A summary of stock option activity and related information follows:
| Years ended December 31, | Six months ended June 30, | |||||||||||||||||||||||
| 2000 | 2001 | 2002 | 2003 | |||||||||||||||||||||
| Options | Weighted- average exercise price | Options | Weighted average exercise price | Options | Weighted average exercise price | Options | Weighted average exercise price | |||||||||||||||||
Outstanding at beginning of period | 1,222,125 | $ | 0.14 | 1,139,268 | $ | 0.24 | 1,880,606 | $ | 0.53 | 3,358,067 | $ | 0.77 | ||||||||||||
Granted at fair value | — | — | — | — | 697,750 | 1.00 | — | — | ||||||||||||||||
Granted at less than fair value | 999,094 | 0.38 | 820,500 | 0.91 | 1,263,248 | 1.00 | 143,250 | 1.00 | ||||||||||||||||
Canceled | (372,802 | ) | 0.16 | (66,516 | ) | 0.30 | (476,793 | ) | 0.78 | (87,142 | ) | 0.85 | ||||||||||||
Exercised | (709,149 | ) | 0.32 | (12,646 | ) | 0.17 | (6,744 | ) | 0.36 | (21,097 | ) | 0.22 | ||||||||||||
Outstanding at end of period | 1,139,268 | $ | 0.24 | 1,880,606 | $ | 0.53 | 3,358,067 | $ | 0.77 | 3,393,078 | $ | 0.78 | ||||||||||||
F-19
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
| Years ended December 31, | ||||||||||||||||||||||||
| 2001 | 2002 | 2003 | Six months ended June 30, 2004 | |||||||||||||||||||||
| Options | Weighted average | Options | Weighted average exercise price | Options | Weighted average exercise price | Options | Weighted average exercise price | |||||||||||||||||
Outstanding at beginning of period | 207,088 | $ | 1.32 | 341,858 | $ | 2.92 | 610,489 | $ | 4.24 | 717,615 | $ | 4.48 | ||||||||||||
Granted at fair value | — | — | 126,853 | 5.50 | — | — | 183,326 | 6.44 | ||||||||||||||||
Granted at less than fair value | 149,148 | 5.01 | 229,641 | 5.50 | 225,470 | 5.45 | 80,453 | 5.50 | ||||||||||||||||
Canceled | (12,083 | ) | 1.65 | (86,641 | ) | 4.29 | (95,587 | ) | 5.34 | (3,806 | ) | 5.48 | ||||||||||||
Exercised | (2,295 | ) | 0.94 | (1,222 | ) | 1.98 | (22,757 | ) | 3.69 | (44,543 | ) | 1.25 | ||||||||||||
Outstanding at end of period | 341,858 | $ | 2.92 | 610,489 | $ | 4.24 | 717,615 | $ | 4.48 | 933,045 | $ | 5.10 | ||||||||||||
The following summarizes information about stock options outstanding and exercisable at December 31, 2002:2003:
Number of options | Outstanding weighted average remaining contractual life (years) | Weighted average exercise price | Exercisable | |||||||||
Range of exercise price | Number of options | Weighted average exercise price | ||||||||||
$0.10-$0.11 | 186,416 | 4.37 | $ | 0.10 | 186,416 | $ | 0.10 | |||||
$0.17 | 434,021 | 6.87 | 0.17 | 422,311 | 0.17 | |||||||
$0.30-$0.50 | 432,931 | 7.87 | 0.43 | 331,751 | 0.44 | |||||||
$1.00 | 2,304,699 | 9.12 | 1.00 | 312,930 | 1.00 | |||||||
| 3,358,067 | 8.40 | $ | 0.77 | 1,253,408 | $ | 0.44 | ||||||
Range of exercise price | Number of options | Outstanding weighted average remaining contractual life (years) | Weighted average exercise price | Exercisable | ||||||||
| Number of options | Weighted average exercise price | |||||||||||
$0.55 - $0.61 | 32,185 | 3.37 | $ | 0.57 | 32,185 | $ | 0.57 | |||||
$0.92 | 79,687 | 6.01 | 0.92 | 79,680 | 0.92 | |||||||
$1.65 - $2.75 | 65,847 | 6.85 | 2.33 | 56,790 | 2.35 | |||||||
$5.50 | 539,896 | 8.72 | 5.50 | 160,176 | 5.50 | |||||||
| 717,615 | 8.01 | $ | 4.48 | 328,831 | $ | 3.36 | ||||||
The number of options exercisable at December 31, 2000, 2001, 2002 and 20022003 was 669,821, 1,026,385186,615, 227,892 and 1,253,408,328,831, respectively. The weighted average exercise price of options vested and exercisable at December 31, 2000, 2001, 2002 and 20022003 was $0.15, $0.30$1.65, $2.53 and $0.46,$3.36, respectively.
During the years ended December 31, 2000, 2001, 2002 and 2002,2003, the Company granted options to purchase a total of 122,500, 395,00071,814, 6,363 and 35,00010,908 shares of common stock, respectively, to consultants and Scientific Advisory Board members for services to be performed through October 2007.April 2008. No stock option grants have been made to non-employeessuch options were granted during the six months ended June 30, 2003.2004. In accordance with SFAS 123 and EITF 96-18, options granted to consultants and Scientific Advisory Board members are periodically revalued over the related service periods. The Company recorded stock compensation of $112,000, $1.1 million, $65,000 and $65,000$360,000 during the years ended December 31, 2000, 2001, 2002 and 2002,2003, respectively, and $130,000 and $39,000 during the six months ended June 30, 2003 and 2004, respectively, related to consulting services.
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
During the years ended December 31, 2000, 2001, 2002 and 20022003 and the six months ended June 30, 2003,2004, in connection with the grant of certain options to employees, the Company recorded deferred stock compensation of $2.0 million, $1.7 million, $3.2 million, $2.4 million and $86,000,$811,000, respectively, representing the difference between the exercise price and the subsequently determined fair value of the Company’s common stock on the date such stock options were granted. The subsequently determined fair value of the Company’s common stock was $5.31$29.21 during the yearsyear ended December 31, 2000 and 2001, ranged from $1.00$5.50 to $3.82$21.01 during the year ended December 31, 2002, ranged from $5.50 to $18.59 during the year ended December 31, 2003 and ranged from $1.00$4.10 to $2.51$15.57 during the six months ended June 30, 2003.2004. Deferred stock compensation is being amortized on a graded vesting method. During the years ended December 31, 2000, 2001, 2002 and 20022003 and the six months ended June 30, 2003 and 2004, the Company recorded noncashnon-cash deferred stock compensation expense related to employees of $770,000, $1.4 million, $2.5 million, $1.3 million, $595,000 and $595,000,$1.2 million, respectively.
Other stock plans
F-20In connection with the Company’s initial public offering, the Board of Directors authorized, subject to final stockholder approval, the following additional plans.
The 2003 Stock Plan (2003 Plan) provides for the grant of incentive stock options and stock purchase rights to employees (including employee directors) and non-statutory stock options to employees, directors and consultants. A total of 636,363 shares of common stock have been reserved for issuance under the 2003 Plan. The number of shares reserved for issuance under the 2003 Plan will be subject to an automatic annual increase on the first day of each fiscal year beginning in 2005 and ending in 2010 equal to the lesser of 109,090 shares, 4% of the number of outstanding shares of common stock on the last day of the immediately preceding fiscal year or such lesser number of shares as the Board of Directors determines. With respect to options granted under the 2003 Plan, the term of options may not exceed 10 years. In no event may an employee receive awards for more than one million shares under the 2003 Plan in any fiscal year.
A total of 90,909 shares of common stock have been reserved for issuance under the 2003 Directors’ Stock Option Plan, as amended in June 2004 (2003 Directors’ Plan). Under the 2003 Directors’ Plan, each non-employee director who first becomes a non-employee director after the effective date of the plan will receive an automatic initial grant of an option to purchase 10,000 shares of common stock upon becoming a member of the Board of Directors. On the date of each annual meeting of stockholders, each non-employee director will be granted an option to purchase 10,000 shares of common stock if, on such a date, the director has served on the Board of Directors for at least six months. Additionally, the chairman of each committee of the Board of Directors and each member of the audit committee will receive an additional annual option grant to purchase 2,500 shares of common stock. The 2003 Directors’ Plan provides that each option granted to a non-employee director shall vest in equal monthly installments over two years. All options granted under the 2003 Directors’ Plan have a term of 10 years and an exercise price equal to the fair market value on the date of the grant.
A total of 109,090 shares of common stock have been reserved for issuance under the 2003 Employee Stock Purchase Plan (2003 Employee Plan). The number of shares reserved for issuance under the 2003 Employee Plan will be increased on the first day of each of the fiscal years in 2005 to 2010 by the lesser of 54,545 shares, 1% of the number of outstanding shares of common stock on the last day of the immediately preceding fiscal year or such lesser number of shares as the Board of Directors determines. Unless terminated earlier by the Board of Directors, the 2003 Employee Plan will terminate in September 2023.
Xcyte Therapies, Inc. (aXCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
8. Common stock
Stock split
On March 4, 2004 the Company effected a 2 for 11 reverse stock split of the outstanding common and preferred stock and stock options and warrants. All share and per share amounts have been retroactively restated in the accompanying financial statements and notes for all periods presented to reflect the reverse stock split.
Common stock reserved for future issuance at December 31, 20022003 and June 30, 20032004 is as follows:
| Description | December 31, 2002 | June 30, 2003 | December 31, 2003 | June 30, 2004 | ||||
1996 Stock Option Plan | ||||||||
Options granted and outstanding | 3,358,067 | 3,393,078 | 717,615 | 933,045 | ||||
Options reserved for future grant | 259,957 | 203,849 | 278,691 | 21,143 | ||||
2003 Stock Plan | 636,363 | 636,363 | ||||||
2003 Directors’ Stock Option Plan | 90,909 | 90,909 | ||||||
2003 Employee Stock Purchase Plan | 109,090 | 109,090 | ||||||
Series A preferred stock | 7,300,080 | 7,300,080 | 7,300,080 | — | ||||
Series B preferred stock | 4,097,580 | 4,097,580 | 4,097,580 | — | ||||
Series C preferred stock | 7,212,316 | 7,212,316 | 7,212,316 | — | ||||
Series D preferred stock | 10,300,000 | 10,300,000 | 10,300,000 | — | ||||
Series E preferred stock | 6,500,000 | 6,500,000 | 6,500,000 | — | ||||
Series F preferred stock | 6,500,000 | 6,500,000 | 6,500,000 | — | ||||
Preferred stock warrants | 798,236 | 727,078 | 133,334 | — | ||||
Common stock warrants | 4,915,344 | 4,990,344 | 907,316 | 46,607 | ||||
| 51,241,580 | 51,224,325 | 44,783,294 | 1,837,157 | |||||
Milestone pool
Pursuant to a business acquisition prior to January 1, 1999, the Company reserved 1,582,340287,698 shares of common stock (Milestone Pool) for the Company’s possible acquisition of new technology from the scientific founders of the acquired business. During the year ended December 31, 2001, the Milestone Pool was terminated. In exchange for the termination of all rights to the remaining Milestone Pool shares, these scientific founders entered in consulting agreements and were granted options to purchase a total of 375,00068,178 shares of the Company’s common stock, of which 150,000 shares immediately vested.stock. The remaining options vest in equal monthly installments over the four-year consulting term and will be periodically revalued and recognized as expense over the related service period. During the years ended December 31, 2001, 2002 and 20022003 and the six months ended June 30, 2003 and 2004, the Company recorded stock-based compensation of $980,000, $30,000, $132,000, $63,000 and $63,000,$18,000, respectively.
Common stock warrants
During the years ended December 31, 2000, 2001 and 2002, theThe Company has issued warrants to purchase 1,132,287, 2,586,162 and 2,419,649 shares of common stock, respectively, to private investors in connection with the issuance of Series D, E and F preferred stock. During the six monthsyear ended June 30,December 31, 2003, the Company issued warrants to purchase 75,00013,635 shares of common stock in connection with a consulting arrangement. During the years endedAt December 31, 2001 and 2002,2003 warrants to purchase 635,339 and 587,415907,316 shares of common stock were exercised, respectively. No warrants were exercised duringoutstanding with a weighted average exercise price of $0.30 per share. Concurrent with the year ended December 31, 2000 orCompany’s initial public offering in March 2004, all outstanding
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003.2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
common stock warrants existing immediately prior to the closing of the offering were converted into common stock through payment of cash and cashless exercises, resulting in the issuance of 873,002 shares of common stock. Also concurrent with the initial public offering, certain preferred stock warrants that did not expire at the closing of the offering were automatically converted into common stock warrants. At June 30, 2004, warrants to purchase 46,607 shares of common stock remain outstanding with a weighted average exercise price of $7.94 per share.
9. Income taxes
At December 31, 2002,2003, the Company had operating loss carryforwards of approximately $55.9$74.0 million and research and development tax credit carryforwards of $3.0$3.2 million for federal income tax reporting purposes. The net operating losses and tax credits will expire beginning in 2011 if not previously utilized.
F-21
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
In certain circumstances, as specified inunder Section 382 of the Internal Revenue Code of 1986, as amended, due to ownership changes, the Company’s ability to utilize its net operating loss carryforwards may be limited.
Deferred income taxes reflect the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. The significant components of deferred taxes are as follows (in thousands):
| December 31, | ||||||||||||||||
| 2001 | 2002 | December 31, | ||||||||||||||
| 2002 | 2003 | |||||||||||||||
Deferred tax assets: | ||||||||||||||||
Net operating loss carryforwards | $ | 13,959 | $ | 19,008 | $ | 19,008 | $ | 25,147 | ||||||||
Research and development tax credit | 2,265 | 2,972 | 2,972 | 3,195 | ||||||||||||
License agreements | 302 | 562 | 562 | 242 | ||||||||||||
Other | 161 | 230 | 230 | 309 | ||||||||||||
| 16,687 | 22,772 | 22,772 | 28,893 | |||||||||||||
Less valuation allowance | (16,532 | ) | (22,679 | ) | (22,679 | ) | (28,743 | ) | ||||||||
Net deferred tax assets | 155 | 93 | 93 | 150 | ||||||||||||
Deferred tax liabilities: | ||||||||||||||||
Depreciation | (155 | ) | (93 | ) | (93 | ) | (150 | ) | ||||||||
Net deferred taxes | $ | — | $ | — | $ | — | $ | — | ||||||||
A valuation allowance has been recorded for deferred tax assets because realization is primarily dependent on generating sufficient taxable income prior to the expiration of net operating loss carryforwards. The valuation allowance for deferred tax assets increased $4.3 million, $6.5 million and $6.1 million during the years ended December 31, 2000, 20012002 and 2002,2003, respectively, principally due to net operating losses recorded during those periods. There have been no offsets or other deductions to the valuation allowance in any period since the Company’s inception.
10. Convertible promissory notes
In October 2003, the Company issued Convertible Promissory Notes for $12.7 million. Interest on the unpaid principal amount of the Notes accrues annually at a rate of 6 percent. Principal and any accrued but unpaid
XCYTE THERAPIES, INC.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS—(Continued)
(Information as of June 30, 2004, for the six months ended June 30, 2003 and 2004
and for the period from inception (January 5, 1996) to June 30, 2004 is unaudited)
interest under these Notes are due and payable upon demand by the holder at any time after October 2004; provided, however, that on or after April 30, 2004, the holders of at least a majority of the aggregate principal amount of the Notes may elect to accelerate the maturity to a date after April 30, 2004. The Notes (including accrued and unpaid interest) are automatically convertible into shares of the Company’s common stock, upon the closing of the Company’s initial public offering. The Notes are also convertible into shares of a subsequent private round of financing, should the holders of at least a majority of the aggregate principal amount of the Notes so elect.
In connection with the issuance of the Notes, the holders of the Notes received warrants to purchase 207,977 shares of the Company’s Series F preferred stock at $15.29 per share, exercisable after the maturity date of the Notes, through 2008. If an initial public offering occurs prior to the maturity date of the Notes and the closing of the next private financing, then the warrants will expire. If the Company completes its next private round of financing prior to the maturity date of the Notes, the warrants become exercisable at the price per share of that round. The Company allocated $1.4 million of the proceeds to the warrants based on the relative fair values of the Notes and warrants (using the Black-Scholes option pricing model). The resulting $1.4 million discount on the Notes is being amortized to interest expense over the term of the Notes.
Should the Company consummate its initial public offering, and the Notes convert to common stock, the Company will recognize $11.3 million in additional interest expense, which represents the beneficial conversion feature of the Notes. This interest expense would be in addition to recognizing interest expense associated with the unamortized discount existing on the date of conversion.
The number of shares to be issued upon conversion shall be equal to the quotient obtained by dividing (A) the entire principal amount of the Notes plus accrued but unpaid interest as of the closing by (B) $9.625, rounded to the nearest whole share.
In connection with the initial public offering in March 2004, all of the outstanding convertible promissory notes, including interest accrued thereon through the closing date of the offering, were converted into 1,357,357 shares of common stock.
11. Long-term obligations and lease obligations
The Company has commitments for noncancelable operating leases for a manufacturing facility, building space and office equipment. The building lease includes rent escalation clauses (3% annually) and has two five-year renewal options. The manufacturing facility lease contains annual rent escalations of 4.5% and an option to renew the lease for two additional five-year periods. In addition to base rent, the Company is required to pay a pro rata share of the operating costs related to the manufacturing facility and building leased space. The Company was required to provide security under the manufacturing lease agreement totaling $435,000 in the form of cash and issued a preferred stock warrant to the lessor.
The Company has financed the acquisition of laboratory and scientific equipment, furniture and fixtures, computer equipment and leasehold improvements through financing arrangements with various third parties. In connection with the financings, the Company has issued preferred stock warrants to the third parties. At December 31, 2002,2003, the Company has onehad two financing arrangements. Under the first arrangement, under which it maythe Company could borrow up to $1.7 million. At June 30, 2002, borrowings under this arrangement are limited to $500,000 until2004, the Company receives additional funding acceptable to the lender. At June 30, 2003, the Company has $170,000had $728,000 available to it under the outstanding arrangement, which expireswas replaced by a new financing agreement with the same party in January 2004 unlessJuly 2004.
XCYTE THERAPIES, INC.
F-22
Xcyte Therapies, Inc. (a(a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
This new agreement provides for borrowings up to $3.0 million, and expires in July 2005 unless renewed. Subsequent to June 30, 2003,Under the second arrangement, the Company entered into a financing arrangement with a new lender. Under the terms of the agreement, the Company maycould borrow up to $2.5 million. At June 30, 2004, the Company had $1.7 million available to financeit under the acquisition of laboratoryoutstanding arrangement, which was replaced by a new financing agreement with the same party in July 2004. This new agreement provides for borrowings up to $3.0 million, and scientific equipment, furniture and fixtures, computer equipment and leasehold improvements.expires in December 2005 unless renewed. Outstanding borrowings under the current and previous financing arrangements were $1.5$1.9 million and $2.0$1.8 million at years ended December 31, 20012002 and 2002,2003, respectively, and $1.9$2.3 million at June 30, 2003.2004. Outstanding borrowings require monthly principal and interest payments and mature at various dates through 2007.2008. Interest rates applicable to the outstanding borrowings at December 31, 20022003 range from 9.18% to 14.12%14.11%. The weighted average interest rates for borrowings outstanding during the years ended December 31, 2000, 2001, 2002 and 20022003 and the six months ended June 30, 20032004 were 13.24%, 12.66%, 11.09%, 10.27% and 10.61%9.72%, respectively. Borrowings are secured by the acquired assets that have a net book value of $2.2$2.3 million at December 31, 2002.2003. Under all agreements, the Company is required to comply with certain nonfinancial covenants.
Future minimum payments under operating leases and equipment financing arrangements at December 31, 20022003 are as follows (in thousands):
Equipment financings arrangements | Operating leases | |||||||||||||
Equipment financings arrangements | Operating leases | |||||||||||||
Year ended December 31, | ||||||||||||||
2003 | $ | 818 | $ | 1,469 | ||||||||||
2004 | 661 | 1,514 | $ | 845 | $ | 1,571 | ||||||||
2005 | 391 | 1,572 | 677 | 1,580 | ||||||||||
2006 | 121 | 1,416 | 375 | 1,430 | ||||||||||
2007 | — | 1,058 | 26 | 1,085 | ||||||||||
2008 | — | 1,120 | ||||||||||||
Thereafter | — | 3,366 | — | 2,260 | ||||||||||
| 1,991 | $ | 10,395 | 1,923 | $ | 9,046 | |||||||||
Less unamortized discount | (121 | ) | (85 | ) | ||||||||||
Less current portion | (818 | ) | (845 | ) | ||||||||||
Long-term equipment obligations | $ | 1,052 | $ | 993 | ||||||||||
Rent expense totaled $643,000, $1.6 million and $1.6 million during each of the years ended December 31, 2000, 2001, and 2002 and 2003 and $807,000 and $843,000 during the six months ended June 30, 2003 and 2004, respectively.
XCYTE THERAPIES, INC.
11. Commitments and contingencies
The Company has been named as (a defendant in a complaint involving several other parties. Due to the uncertainty of litigation, the Company’s management and external legal counsel are unable to determine the ultimate outcome of the matter or estimate the amount or range of potential loss, if any, which may arise.
F-23
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
12. Net loss per share
The calculation of basic and diluted loss per share is as followsshown on the table below (in thousands, except share and per share data):.
| Year ended December 31, | Six months ended June 30, | |||||||||||||||||||||||||||||||||||||||
| 2000 | 2001 | 2002 | 2002 | 2003 | Year ended December 31, | Six months ended June 30, | ||||||||||||||||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||||||||||||||||
Net loss | $ | (12,941 | ) | $ | (19,512 | ) | $ | (19,453 | ) | $ | (10,412 | ) | $ | (9,189 | ) | $ | (19,512 | ) | $ | (19,453 | ) | $ | (18,457 | ) | $ | (9,189 | ) | $ | (24,370 | ) | ||||||||||
Accretion of preferred stock | — | (8,411 | ) | (8,001 | ) | (8,001 | ) | — | (8,411 | ) | (8,001 | ) | — | — | (8,973 | ) | ||||||||||||||||||||||||
Net loss applicable to common stockholders | $ | (12,941 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,413 | ) | $ | (9,189 | ) | $ | (27,923 | ) | $ | (27,454 | ) | $ | (18,457 | ) | $ | (9,189 | ) | $ | (33,343 | ) | ||||||||||
Weighted average common shares | 6,067,480 | 7,405,576 | 8,121,940 | 7,860,690 | 8,384,910 | 1,346,468 | 1,476,716 | 1,527,775 | 1,524,476 | 9,134,012 | ||||||||||||||||||||||||||||||
Weighted average common shares subject to repurchase | (64,923 | ) | (469,587 | ) | (313,287 | ) | (337,594 | ) | (241,301 | ) | (85,379 | ) | (56,961 | ) | (39,557 | ) | (43,873 | ) | (26,611 | ) | ||||||||||||||||||||
Weighted average number of shares used for basic and diluted per share amounts | 6,002,557 | 6,935,989 | 7,808,653 | 7,523,096 | 8,143,609 | 1,261,089 | 1,419,755 | 1,488,218 | 1,480,603 | 9,107,401 | ||||||||||||||||||||||||||||||
Basic and diluted net loss per common share | $ | (2.16 | ) | $ | (4.03 | ) | $ | (3.52 | ) | $ | (2.45 | ) | $ | (1.13 | ) | $ | (22.14 | ) | $ | (19.34 | ) | $ | (12.40 | ) | $ | (6.21 | ) | $ | (3.66 | ) | ||||||||||
Pro forma (unaudited): | ||||||||||||||||||||||||||||||||||||||||
Weighted average shares used above | 7,808,653 | 8,143,609 | ||||||||||||||||||||||||||||||||||||||
Pro forma adjustment to reflect weighted effect of assumed conversion of redeemable convertible preferred stock | 36,741,509 | 37,283,671 | ||||||||||||||||||||||||||||||||||||||
Pro forma weighted average shares outstanding | 44,550,162 | 45,427,280 | ||||||||||||||||||||||||||||||||||||||
Pro forma basic and diluted net loss per share | $ | (0.44 | ) | $ | (0.20 | ) | ||||||||||||||||||||||||||||||||||
The Company has excluded all redeemable convertible preferred stock, redeemable convertible preferred stock warrants, convertible promissory notes, common stock warrants and outstanding stock options from the calculation of diluted net loss per common share because all securities are antidilutive for the periods presented. The total number of shares excluded from the calculations of diluted net loss per common share was 31,073,109, 38,547,3537,008,479, 8,422,596 and 46,325,0409,880,023 for the years ended December 31, 2000, 2001, 2002 and 2002,2003, respectively, and 45,832,5278,438,244 and 46,410,734979,652 for the six months ended June 30, 20022003 and 2003,2004, respectively.
XCYTE THERAPIES, INC.
13. Subsequent events
Initial public offering
In September 2003, the Company’s Board of Directors authorized the Company to file (a registration statement with the Securities and Exchange Commission for an initial public offering of its common stock (the Offering). Prior to completion of the Offering, the Company will amend and restate its Certificate of Incorporation to authorize the issuance of up to 100 million shares of common stock, par value $0.001 per share, and 5 million shares of preferred stock, par value $0.001 per share, the rights and preferences of which may be established from time to time by the Board of Directors. If the Company’s Offering is consummated, all of the outstanding redeemable convertible preferred stock will be automatically converted into common stock, and 470,725 redeemable convertible preferred stock warrants will expire upon the closing of the Offering. All remaining preferred stock warrants (256,353) which do not expire upon the closing will convert to common stock warrants upon the closing of the
F-24
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)STATEMENTS—(Continued)
(Information as of June 30, 2003,2004, for the six months ended June 30, 20022003 and 2003 2004
and for the period from inception (January 5, 1996) to June 30, 20032004 is unaudited)
Offering. Unaudited pro forma stockholders’ equity as of June 30, 2003, reflects the effect of the assumed conversion of the preferred stock.
If the Offering is consummated, all related costs will be offset against the proceeds in equity. If not consummated, the costs will be charged to expense in the period the Offering is terminated. At June 30, 2003, there were no costs deferred associated with the Offering.
1996 Stock Plan13. Quarterly financial data (unaudited)
In September 2003, the 1996 Plan was amended to increase common stock reserved for grants to 6.4 million shares.
Other stock plans
In connection with the Offering, the Board of Directors authorized, subject to final shareholder approval, the following additional plans.
The 2003 Stock Plan (2003 Plan) providesfollowing table contains selected unaudited statement of operations information for the grant of incentive stock options and stock purchase rights to employees (including employee directors) and nonstatutory stock options to employees, directors and consultants. A total of 3.5 million shares of common stock have been reserved for issuance under the 2003 Plan. The number of shares reserved for issuance under the 2003 Plan will be subject to an automatic annual increase on the first day of each fiscal year beginning in 2005 and ending in 2010 equal to the lesser of 600,000 shares, 4% of the number of outstanding shares of common stock on the last day of the immediately preceding fiscal year or such lesser number of shares as the Board of Directors determines. With respect to options granted under the 2003 Plan, the term of options may not exceed 10 years. In no event may an employee receive awards for more than 1 million shares under the 2003 Plan in any fiscal year.
A total of 500,000 shares of common stock has been reserved for issuance under the 2003 Directors’ Stock Option Plan (2003 Directors’ Plan). Under the 2003 Directors’ Plan, each non-employee director who first becomes a non-employee director after the effective date of the plan will receive an automatic initial grant of an option to purchase 25,000 shares of common stock upon becoming a member of the Board of Directors. On the date of each annual meeting of stockholders, each non-employee director will be granted an option to purchase 10,000 shares of common stock if, on such a date, the director has served on the Board of Directors for at least six months. The 2003 Directors’ Plan provides that each option granted to a new director shall vest at the rate of one-third of the total number of shares subject to such option 12 months after the date of grant, with the remaining shares vesting thereafter in equal monthly installments over the next two years so that the option will be fully vested after three years. Each annual option granted to a director vests in full at the end of one year. All options granted under the 2003 Directors’ Plan have a term of 10 years and an exercise price equal to the fair market value on the date of the grant.
A total of 600,000 shares of common stock have been reserved for issuance under the 2003 Employee Stock Purchase Plan (2003 Employee Plan). The number of shares reserved for issuance under the 2003 Employee Plan will be increased on the first day of each of the fiscal yearsquarters in 2005 to 2010 by the lesser of 300,000 shares, 1% of the number of outstanding shares of common stock on the last day of the
F-25
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003, and the first two quarters of 2004. The Company believes that the following information reflects all adjustments, consisting of only normal recurring adjustments, necessary for a fair presentation of the information for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| Quarter ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
2002 | ||||||||||||||||
Revenue | $ | — | $ | — | $ | — | $ | — | ||||||||
Net loss | $ | (4,942 | ) | $ | (5,470 | ) | $ | (4,286 | ) | $ | (4,755 | ) | ||||
Net loss attributable to common stockholders(1) | $ | (12,943 | ) | $ | (5,470 | ) | $ | (4,286 | ) | $ | (4,755 | ) | ||||
Basic and diluted net loss per common share(1) | $ | (9.93 | ) | $ | (3.82 | ) | $ | (2.92 | ) | $ | (3.23 | ) | ||||
2003 | ||||||||||||||||
Revenue | $ | 13 | $ | 59 | $ | 73 | $ | 25 | ||||||||
Net loss | $ | (3,843 | ) | $ | (5,346 | ) | $ | (3,975 | ) | $ | (5,293 | ) | ||||
Net loss attributable to common stockholders | $ | (3,843 | ) | $ | (5,346 | ) | $ | (3,975 | ) | $ | (5,293 | ) | ||||
Basic and diluted net loss per common share | $ | (2.60 | ) | $ | (3.60 | ) | $ | (2.67 | ) | $ | (3.53 | ) | ||||
2004 | ||||||||||||||||
Revenue | $ | 12 | $ | 24 | ||||||||||||
Net loss(2) | $ | (18,284 | ) | $ | (6,086 | ) | ||||||||||
Net loss attributable to common stockholders(3) | $ | (27,257 | ) | $ | (6,086 | ) | ||||||||||
Basic and diluted net loss per common share(2),(3) | $ | (7.98 | ) | $ | (0.41 | ) | ||||||||||
| (1) | Net loss attributable to common stockholders for the quarter ended March 31, 2002 includes $8.0 million in accretion of preferred stock. |
| (2) | Net loss for the quarter ended March 31, 2004 includes $12.5 million in noncash interest expense associated with the convertible promissory notes. |
| (3) | Net loss attributable to common stockholders for the quarter ended March 31, 2004 includes $9.0 million in accretion of preferred stock. |
immediately preceding fiscal year or such lesser number of shares as the Board of Directors determines. Unless terminated earlier by the Board of Directors, the 2003 Employee Plan will terminate in September 2023.
1,500,000 Shares
Stock optionsXCYTE THERAPIES, INC.
In September 2003, the Company’s Board of Directors granted 886,500 and 15,000 stock options to employees and non-employees, respectively, under the 1996 Plan. The Company will record additional deferred compensation of $2.0 million related to these option grants to employees in the third quarter of 2003, which will be amortized over the vesting period. The options to non-employees will be periodically revalued and recognized as expense over the related service period.
% Convertible Exchangeable Preferred Stock
SBIR grant(Cumulative Dividend, Liquidation Preference $10 Per Share)
In August 2003, the Company was awarded a $1.2 million SBIR grant by the National Institutes of Health to help fund the Company’s clinical trial evaluating the use of Xcellerated T Cells to treat patients with chronic lymphocytic leukemia.
Corporate collaborations
The Company has a letter of intent to establish an alliance with Taiwan Cellular Therapy Company, or TCTC, a company newly formed under the laws of Taiwan, to develop and market our Xcellerate Technology in Australia and Asia, excluding Japan. The letter of intent requires the Company to negotiate in good faith a definitive agreement with TCTC on or before November 15, 2003. The letter of intent provides that the definitive agreement is subject to, among other things, TCTC closing, or obtaining commitments for, at least $25 million in equity financing on or before December 30, 2003. If the Company elects not to proceed with the alliance for any reason, other than for a good faith inability to negotiate a definitive agreement, and the Company enters into an agreement with a third party in Australia or Asia before February 22, 2004, the Company will be required to pay a break-up fee to TCTC.
The Company has agreed to reserve the rights to its Xcellerate Technology in Australia and Asia for TCTC until at least December 30, 2003. If the Company completes an alliance with TCTC, the Company will be required to expend significant resources to transfer technology to TCTC and assist them in developing and manufacturing components of the Xcellerate Technology. The Company will also be required to invest $2.5 million in TCTC’s equity financing.
Convertible promissory notes
In October 2003, the Company issued Convertible Promissory Notes for $12.7 million. Interest on the unpaid principal amount of the Notes accrues annually at a rate of 6 percent. Principal and any accrued but unpaid interest under these Notes are due and payable upon demand by the holder at any time after October 2004; provided, however, that on or after February 1, 2004, the holders of at least a majority of the aggregate principal amount of the Notes may elect to accelerate the maturity to a date after February 1, 2004.
F-26
Xcyte Therapies, Inc. (a development stage company)
NOTES TO FINANCIAL STATEMENTS — (continued)
(Information as of June 30, 2003, for the six months ended June 30, 2002 and 2003 and for the period from inception (January 5, 1996) to June 30, 2003 is unaudited)
In connection with the issuance of the Convertible Promissory Notes, the holders of the Notes received warrants to purchase additional shares of the Company’s stock at a price defined in the warrant agreement. If an initial public offering occurs prior to the maturity date of the Notes and the closing of the next private financing, then the warrants will be null and void.
If the Company consummates its initial public offering in which all of the Company’s outstanding preferred stock converts to common stock, prior to the maturity date, the entire principal amount of and accrued but unpaid interest on these Notes will be converted into shares of the Company’s common stock with a par value per share of $0.001 simultaneously with the closing of the initial public offering.
F-27

PROSPECTUS
Part IIPiper Jaffray
JMP Securities
, 2004
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other expensesExpenses of issuanceIssuance and distributionDistribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of the convertible exchangeable preferred stock, debentures and common stock being registered. All amounts are estimates except the SEC registration fee, the NASD filing fee and The Nasdaq National Market listing fee.
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| Amount | |||
SEC registration fee | $ | 2,608 | |
NASD filing fee | 2,558 | ||
Nasdaq National Market listing fee | 5,000 | ||
Printing and engraving expenses | 50,000 | ||
Legal fees and expenses | 250,000 | ||
Accounting fees and expenses | 100,000 | ||
Blue sky qualification fees and expenses | 5,000 | ||
Transfer agent and registrar fees | 3,500 | ||
Trustee fees | 10,000 | ||
Miscellaneous fees and expenses | 11,334 | ||
Total | $ | 440,000 | |
Item 14. Indemnification of directorsDirectors and officersOfficers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation to grant, indemnity to directors and officers in terms sufficiently broad to permit indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act of 1933, as amended. Article XIV of our Amended and Restated Certificate of Incorporation (Exhibit 3.23.1 hereto) and Article VI of our Amended and Restated Bylaws (Exhibit 3.3 hereto), provide for indemnification of our directors and officers, and permits indemnification of our employees and other agents to the maximum extent permitted under the laws of Delaware. Delaware law provides that a corporation may eliminate the personal liability of its directors for monetary damages for breach of their fiduciary duties as directors, except liability for:
| breach of their duty of loyalty to the corporation or its stockholders; |
| acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| unlawful payments of dividends or unlawful stock repurchases or redemptions; and |
| any transaction from which the director derived an improper personal benefit. |
In addition, we intend to enter into indemnification agreements (Exhibit 10.1 hereto) with our officers and directors. The underwriting agreement (Exhibit 1.1 hereto) also provides for cross-indemnification among us, and the underwriters with respect to certain matters, including matters arising under the Securities Act. We maintain directors’ and officers’ liability insurance.
II-1
Item 15. Recent Sales of Unregistered Securities
Since September 30, 2000,27, 2001, we have sold and issued the following securities:
| 1. | As of |
II-1
Part II
were Ronald J. Berenson, Joanna S. Black, Mark Frohlich, Mark L. Bonyhadi, Kathi L. Cordova, Stewart Craig, Jean Deleage, Peter Langecker and Robert M. Williams. |
| 2. | As of |
| 3. |
| In November 2001, we issued |
| 4. | In November 2001, we granted and issued warrants with an expiration date of the earlier of either the closing of |
| 5. | In November 2001, we granted and issued a warrant with an expiration date of the earlier of either the closing of |
| 6. | In February and March 2002, we issued |
| 7. | In February and March 2002, we granted and issued warrants with an expiration date of the earlier of either the closing of |
| 8. | In February 2002, we granted and issued a warrant with an expiration date of February 7, 2009 to purchase |
| 9. | In May 2002, we issued |
| 10. | In April 2003, we granted and issued a warrant with an expiration date of the earlier of the closing of our initial public offering or April 1, 2008 to purchase 6,363 shares of common stock at an exercise price of $5.50 to Inkeun Lee in connection with consulting services. On March 19, 2004, this warrant was net exercised for 1,988 shares in connection with our initial public offering. |
II-2
Part II
| 11. | In April 2003, we granted and issued a warrant with an expiration date of the earlier of the closing of our initial public offering or April 1, 2008 to purchase 7,272 shares of common stock at an exercise price of $5.50 to Inkeun Lee in connection with consulting services. On March 19, 2004, this warrant was terminated in connection with our initial public offering. |
| 12. | In July 2003, we granted and issued a warrant with an expiration date of the earlier of July 17, 2010 or the closing of |
| 13. | In September 2003, we granted and issued a warrant with an expiration date of the earlier of September 5, 2010 or the closing of |
| 14. | In October 2003, we sold convertible promissory notes in an aggregate amount of approximately $12.7 million to investors, including but not limited to Alta Partners, ARCH Venture Partners, MPM Capital, Sprout Group, Vector Fund, Vulcan Ventures and W Capital Partners Ironworks, L.P. These convertible promissory notes |
| 15. | In |
| 16. | In December 2003, we granted and issued a warrant with an expiration date of the earlier of December 19, 2010 or |
| 17. | In February 2004, we granted and issued a warrant with an expiration date of the earlier of February 25, 2011 or the closing of our initial public offering |
The issuances of the above securities were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) thereof, or Regulation D, or another applicable exemption of the Securities Act, as transactions by an issuer not involving any public offering. In addition, certain issuances described in Items 1 and 2 were deemed exempt from registration under the Securities Act in reliance upon Rule 701 promulgated under the Securities Act. The recipients of securities in each such transaction represented to us their intentions to acquire the securities for investment purposes only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and warrants issued in such transactions. All recipients had adequate access, through their relationships with us and otherwise, to information about us.
II-3
Item 16. Exhibits
Exhibit number | Description | ||
| 1.1†† | Form of Underwriting Agreement. | ||
| 3.1* | Amended and Restated Certificate of Incorporation of Xcyte Therapies, Inc. | ||
| 3.2†† | Form of | ||
| 3.3* | Amended and Restated Bylaws of Xcyte Therapies, Inc. | ||
| |||
Form of Preferred Stock Certificate of Designations to be filed and effective upon completion of this offering (filed as Exhibit 3.2). | |||
| 4.3†† | Form of Indenture. | ||
| 4.4* | Specimen Common Stock Certificate. | ||
| 5.1 | Opinion of Heller Ehrman White & McAuliffe LLP. | ||
| 10.1* | Form of Indemnification Agreement between Xcyte Therapies, Inc. and each of its officers and directors. | ||
| 10.2* |
| ||
| 10.3* |
| ||
II-3
Part II
|
| ||
| 10.4* | Amended and Restated Investor Rights Agreement dated February 5, 2002. | ||
| 10.5* | Amendment to Amended and Restated Investor Rights Agreement dated May 22, 2002. | ||
Waiver of Preemptive Rights and Amendment to Amended and Restated Investor Rights Agreement dated October 9, 2003. | |||
| 10.7* | Form of Warrant to purchase Common Stock issued by Xcyte Therapies, Inc. | ||
| |||
| 10.8* | Form of Warrant to purchase Series F Preferred Stock issued by Xcyte Therapies, Inc. in favor of General Electric Capital Corporation. | ||
| |||
| |||
| |||
| 10.9* | Master Security Agreement between Xcyte Therapies, Inc. and Oxford Finance Corporation dated July 1, 2003. | ||
| |||
| |||
| 10.10* | Senior Loan and Security Agreement dated July 1, 1999 between Xcyte Therapies, Inc. and Phoenix Leasing Incorporated. | ||
| 10.11 | Master Security Agreement dated | ||
Amendment No. 1 to Master Security Agreement dated May 1, 2000 between Xcyte Therapies, Inc. and General Electric Capital Corporation. | |||
| 10.13 | Amendment No. 2 to Master Security Agreement dated August 18, 2004 between Xcyte Therapies, Inc. and General Electric Capital Corporation. | ||
| 10.14* | Facility Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
| 10.15* | First Amendment to Lease dated October 23, 2001 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
| 10.16* | Second Amendment to Lease dated March 26, 2003 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
II-4
Exhibit number | Description | ||
| 10.17 | * | Third Amendment to Lease dated November 12, 2003 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | |
| * | Facility Lease dated December 7, 2000 between Xcyte Therapies, Inc. and Hibbs/ Woodinville Associates, LLC. | ||
| * | Amended and Restated 1996 Stock Option Plan. | ||
Form of Notice of Option Grant and Agreement for 1996 Stock Option Plan | |||
| 10.21 | * | 2003 Stock Plan. | |
Form of Notice of Stock Option Grant and Agreement for 2003 Stock Plan. | |||
| 10.23 | * | 2003 Employee Stock Purchase Plan. | |
| 10.24 | Amended and Restated 2003 Directors’ Stock Option Plan. | ||
Form of Notice of Stock Option Grant and Agreement | |||
| *† | License and Supply Agreement dated October 15, 1999 between Xcyte Therapies, Inc. and Diaclone S.A., as amended. | ||
II-4
Part II
| |||
| 10.27 | *† |
| |
| *† | Development and Supply Agreement dated August 1, 1999 between Xcyte Therapies, Inc. and Dynal S.A. | ||
| **† | Amendment to Development and Supply Agreement dated March 26, 2004 between Xcyte Therapies, Inc. and Dynal, S.A. | ||
| 10.30 | *† | License Agreement dated July 8, 1998 between Xcyte Therapies, Inc. | |
| *† | First Amendment to License Agreement dated April 10, 2003 between Xcyte Therapies, Inc. and Genetics Institute, Inc. | ||
| 10.32 | *† | Non-Exclusive License Agreement dated October 20, 1999 between Xcyte Therapies, Inc. and the Fred Hutchinson Cancer Research Center, as amended. | |
| *† | Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 1 dated January 10, 2001 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 2 dated April 18, 2001 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 3 dated August 26, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 4 dated September 30, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 5 dated August 5, 2003 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| † | Amendment No. 6 dated August 2, 2004 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.40 | *† | Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | |
| *† | Amendment No. 2 dated August 26, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| *† | Amendment No. 3 dated August 5, 2003 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
II-5
Exhibit number | Description | ||
| 10.43† | Amendment No. 4 dated August 2, 2004 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
Collaboration Agreement dated November 14, 2003 between Xcyte Therapies, Inc. and Fresenius Biotech GmbH. | |||
| 10.45* | Employment Agreement between Xcyte Therapies, Inc. and Mark Frohlich, M.D. dated as of August 27, 2001. | ||
| 10.46* | Employment Agreement between Xcyte Therapies, Inc. and Joanna | ||
Employment Agreement between Xcyte Therapies, Inc. and Robert L. Kirkman dated as of January 15, 2004. | |||
| 10.48*** | Employee Offer Letter between Xcyte Therapies, Inc. and Larry Romel dated June 14, 2004. | ||
| 10.49** | Xcyte Therapies, Inc. Code of Business Conduct and Ethics. | ||
| 12.1 | Statement Regarding Computation of Ratio of Earnings to Fixed Charges and Preferred Stock Dividends | ||
| 23.1 | Consent of Ernst & Young LLP, Independent | ||
| 23.2 | Consent of Heller Ehrman White & McAuliffe LLP (included in Exhibit 5.1). | ||
| 24.1 | Power of Attorney (see page | ||
| 25.1 | Form T-1 Statement of Eligibility of Trustee | ||
| * | Previously filed as an exhibit to registrant’s registration statement on Form S-1, File No. 333-109653, originally filed with the Commission on October 10, 2003, as subsequently amended, and incorporated herein by reference. |
| ** |
| *** | Previously filed as an exhibit to registrant’s quarterly report on form 10-Q filed with the Commission on August 16, 2004. |
| † | Certain information in these exhibits has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.406. |
| †† | This document will be filed as an exhibit to an amendment to this registration statement. |
II-5II-6
Part II
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and |
| (2) | for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fideoffering thereof. |
II-6II-7
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Seattle, State of Washington on October 10, 2003.7, 2004.
| XCYTE THERAPIES, INC. | ||
| By: | /S/ RONALD J. BERENSON | |
Ronald J. Berenson, M.D. President and Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints, jointly and severally, Ronald J. Berenson, Joanna S.Lin Black and Kathi L. Cordova, and each of them, as his attorney-in-fact, with full power of substitution and re-substitution, for him in any and all capacities, to sign any and all amendments to this Registration Statement (including post-effective amendments), and any and all Registration Statements filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, in connection with or related to the offering contemplated by this Registration Statement and its amendments, if any, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming such person’s signature as it may be signed by said attorney to any and all amendments to said Registration Statement.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
Signature | Title | Date | ||
/S/ RONALD J. BERENSON Ronald J. Berenson, M.D. | President, Chief Executive Officer and Director (Principal Executive Officer) | October | ||
/S/ KATHI L. CORDOVA Kathi L. Cordova | Senior Vice President of Finance and Treasurer (Principal Financial and Accounting Officer) | October | ||
/S/
| Director | October | ||
/S/ JEAN DELEAGE Jean Deleage, Ph.D. | Director | October | ||
/S/ DENNIS HENNER Dennis Henner, Ph.D. | Director | October | ||
/S/ PETER LANGECKER Peter Langecker, M.D., Ph.D. | Director | October | ||
II-8
Signature | Title | Date | ||
/S/ ROBERT T. NELSEN Robert T. Nelsen | Director | October | ||
/S/ ROBERT M. WILLIAMS Robert M. Williams, Ph.D. | Director | October 7, 2004 | ||
/S/ DANIEL K. SPIEGELMAN Daniel K. Spiegelman | Director | October 7, 2004 | ||
II-7II-9
Exhibit indexIndex
Exhibit number | Description | ||
| 1.1†† | Form of Underwriting Agreement. | ||
| 3.1* | Amended and Restated Certificate of Incorporation of Xcyte Therapies, Inc. | ||
| 3.2†† | Form of | ||
| 3.3* | Amended and Restated Bylaws of Xcyte Therapies, Inc. | ||
| |||
Form of Preferred Stock Certificate of Designations to be filed and effective upon completion of this offering (filed as Exhibit 3.2). | |||
| 4.3†† | Form of Indenture. | ||
| 4.4* | Specimen Common Stock Certificate. | ||
| 5.1 | Opinion of Heller Ehrman White & McAuliffe LLP. | ||
| 10.1* | Form of Indemnification Agreement between Xcyte Therapies, Inc. and each of its officers and directors. | ||
| 10.2* |
| ||
| 10.3* |
| ||
| 10.4* | Amended and Restated Investor Rights Agreement dated February 5, 2002. | ||
| 10.5* | Amendment to Amended and Restated Investor Rights Agreement dated May 22, 2002. | ||
Waiver of Preemptive Rights and Amendment to Amended and Restated Investor Rights Agreement dated October 9, 2003. | |||
| 10.7* | Form of Warrant to purchase Common Stock issued by Xcyte Therapies, Inc. | ||
| |||
| 10.8* | Form of Warrant to purchase Series F Preferred Stock issued by Xcyte Therapies, Inc. in favor of General Electric Capital Corporation. | ||
| |||
| |||
| |||
| 10.9* | Master Security Agreement between Xcyte Therapies, Inc. and Oxford Finance Corporation dated July 1, 2003. | ||
| |||
| |||
| 10.10* | Senior Loan and Security Agreement dated July 1, 1999 between Xcyte Therapies, Inc. and Phoenix Leasing Incorporated. | ||
| 10.11 | Master Security Agreement dated | ||
Amendment No. 1 to Master Security Agreement dated May 1, 2000 between Xcyte Therapies, Inc. and General Electric Capital Corporation. | |||
| 10.13 | Amendment No. 2 to Master Security Agreement dated August 18, 2004 between Xcyte Therapies, Inc. and General Electric Capital Corporation. | ||
| 10.14* | Facility Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
| 10.15* | First Amendment to Lease dated October 23, 2001 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
| 10.16* | Second Amendment to Lease dated March 26, 2003 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
Exhibit number | Description | ||
| 10.17* | Third Amendment to Lease dated November 12, 2003 to Lease dated June 21, 1999 between Xcyte Therapies, Inc. and Alexandria Real Estate Equities, Inc. | ||
| 10.18* | Facility Lease dated December 7, 2000 between Xcyte Therapies, Inc. and Hibbs/ Woodinville Associates, LLC. | ||
| 10.19* | Amended and Restated 1996 Stock Option Plan. | ||
| |||
| 10.20 | Form of Notice of Option Grant and Agreement for 1996 Stock Option Plan | ||
| 10.21* | 2003 Stock Plan. | ||
Form of Notice of Stock Option Grant and Agreement for 2003 Stock Plan. | |||
| 10.23* | 2003 Employee Stock Purchase Plan. | ||
| 10.24 | Amended and Restated 2003 Directors’ Stock Option Plan. | ||
Form of Notice of Stock Option Grant and Agreement | |||
License and Supply Agreement dated October 15, 1999 between Xcyte Therapies, Inc. and Diaclone S.A., as amended. | |||
First Amendment to License and Supply Agreement dated August 15, 2000 between Xcyte Therapies, Inc. and Diaclone S.A., as amended. | |||
| 10.28*† | Development and Supply Agreement dated August 1, 1999 between Xcyte Therapies, Inc. and Dynal S.A. | ||
Amendment to Development and Supply Agreement dated March 26, 2004 between Xcyte Therapies, Inc. and Dynal, S.A. | |||
| 10.30*† | License Agreement dated July 8, 1998 between Xcyte Therapies, Inc. | ||
First Amendment to License Agreement dated April 10, 2003 between Xcyte Therapies, Inc. and Genetics Institute, Inc. | |||
| 10.32*† | Non-Exclusive License Agreement dated October 20, 1999 between Xcyte Therapies, Inc. and the Fred Hutchinson Cancer Research Center, as amended. | ||
| 10.33*† | Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.34*† | Amendment No. 1 dated January 10, 2001 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.35*† | Amendment No. 2 dated April 18, 2001 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.36*† | Amendment No. 3 dated August 26, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.37*† | Amendment No. 4 dated September 30, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.38*† | Amendment No. 5 dated August 5, 2003 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
Amendment No. 6 dated August 2, 2004 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | |||
| 10.40*† | Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.41*† | Amendment No. 2 dated August 26, 2002 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
Exhibit number | Description | ||
| 10.42*† | Amendment No. 3 dated August 5, 2003 to the Services Agreement dated June 6, 2000 between Xcyte Therapies, Inc. and Lonza Biologics PLC. | ||
| 10.43† | Amendment No. | ||
Collaboration Agreement dated November 14, 2003 between Xcyte Therapies, Inc. and Fresenius Biotech GmbH. | |||
| 10.45* | Employment Agreement between Xcyte Therapies, Inc. and Mark Frohlich, M.D. dated as of August 27, 2001. | ||
| 10.46* | Employment Agreement between Xcyte Therapies, Inc. and Joanna | ||
Employment Agreement between Xcyte Therapies, Inc. and Robert L. Kirkman dated as of January 15, 2004. | |||
| 10.48*** | Employee Offer Letter between Xcyte Therapies, Inc. and Larry Romel dated June 14, 2004. | ||
| 10.49** | Xcyte Therapies, Inc. Code of Business Conduct and Ethics. | ||
| 12.1 | Statement Regarding Computation of Ratio of Earnings to Fixed Charges and Preferred Stock Dividends | ||
| 23.1 | Consent of Ernst & Young LLP, Independent | ||
| 23.2 | Consent of Heller Ehrman White & McAuliffe LLP (included in Exhibit 5.1). | ||
| 24.1 | Power of Attorney (see page | ||
| 25.1 | Form T-1 Statement of Eligibility of Trustee | ||
| * | Previously filed as an exhibit to registrant’s registration statement on Form S-1, File No. 333-109653, originally filed with the Commission on October 10, 2003, as subsequently amended, and incorporated herein by reference. |
| ** |
| *** | Previously filed as an exhibit to registrant’s quarterly report on form 10-Q filed with the Commission on August 16, 2004. |
| † | Certain information in these exhibits has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.406. |
| †† | This document will be filed as an exhibit to an amendment to this registration statement. |